
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
SAFETY EVALUATION OF ALIPHATIC ACYCLIC AND ALICYCLIC alpha-DIKETONES
AND RELATED alpha-HYDROXYKETONES
Mrs M.F.A. Wouters and Dr G.J.A. Speijers
National Institute of Public Health and the Environment, Center of
Substances and Risk Assessment, Bilthoven, The Netherlands
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety Evaluation
of Flavouring Agents
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Explanation
Intake
Biological data
Absorption and metabolism
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
and carcinogenicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 22 flavouring agents (Table 1)
that includes aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones, using the Procedure for the Safety
Evaluation of Flavouring Agents (Figure 1, p. 222, and Annex 1,
reference 131). One member of the group, diacetyl, was evaluated at
the eleventh meeting of the Committee; however, because of lack of
data, no ADI was allocated (Annex 1, reference 14).
1.2 Estimated daily per capita intake
In the United States, aliphatic acyclic and alicyclic
alpha-diketones and alpha-hydroxyketones are generally used as
flavouring agents up to average maximum levels of 200 ppm. The total
annual volume of the 22 substances in this group is approximately 44
000 kg in Europe (International Organization of the Flavor Industry,
1995) and 56 000 kg in the United States (US National Academy of
Sciences, 1970, 1982, 1987). In both Europe and the United States,
more than 95% of the total annual volume is accounted for by three
substances: acetoin (No. 405: 19 000 kg/year in Europe and 9200
kg/year in the United States), diacetyl (No. 408: 18 000 kg/year in
Europe and 42 000 kg/year in the United States), and
methylcyclopentenolone (No. 418: 4700 kg/year in Europe and 3700
kg/year in the United States). Two of these, acetoin and diacetyl,
account for more than 90% of the total annual volume in the United
States (US National Academy of Sciences, 1987).
Nineteen of the 22 aliphatic acyclic and alicyclic
alpha-diketones and alpha-hydroxyketones have been identified as
natural components of a variety of foods, including fruits,
vegetables, cocoa, and coffee (Maarse et al., 1994). Quantitative data
have been reported for the natural occurrence of six of these
substances, which indicate that the intake as natural components of
food is greater than that from their use as flavouring agents
(Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987), with one
exception (diacetyl).
1.3 Absorption, metabolism, and elimination
In rats and mice, orally administered aliphatic alpha-diketones
are rapidly absorbed from the gastrointestinal tract (Gabriel et al.,
1972). It is anticipated that at low levels of exposure, humans will
metabolize aliphatic acyclic alpha-diketone principally by
alpha-hydroxylation and subsequent oxidation of the terminal methyl
group to yield the corresponding ketocarboxylic acid. The acid may
undergo oxidative decarboxylation to yield carbon dioxide and a simple
aliphatic carboxylic acid, which may be completely metabolized in the
fatty acid pathway and citric acid cycle. At high concentrations,
another detoxification pathway is used which involves reduction to the
diol and subsequent conjugation with glucuronic acid (Westerfeld &
Berg, 1943; Williams, 1959; Gabriel et al., 1972; Otsuka et al.,
1996). Acyclic alpha-diketones and alpha-hydroxyketones without a
terminal methyl group and alicyclic diketones and hydroxyketones are
mainly metabolized by reduction to the corresponding diol, followed by
glucuronic acid conjugation and excretion (Mills & Walker, 1990; Ong
et al., 1991).
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), all 22 alpha-diketones
and related hydroxyketones are in class II.
Step 2. Studies in vitro and in vivo have demonstrated two major
routes of metabolism for diacetyl(2,3-butadione) and
acetoin, involving complete oxidation to carbon dioxide and
reduction to a diol, but data were not available on the
higher linear homologues or cyclic analogues. Alcohol
dehydrogenases, aldehyde reductase, and carbonyl reductase
are widely distributed enzymes with broad substrate
specificities; studies conducted in vitro show that
1,2-cyclo-hexandione is a better substrate than diacetyl for
aldehyde reductase and carbonyl reductase. In consequence,
it was consi-dered that the data on the extensive reduction
of acetoin can be extrapolated to other members of the
group. The alpha-diketone group is polar, and it would be
expected that all members of the group evaluated would be
eliminated by a combination of oxidation when the carbonyl
group is adjacent to a methyl group, reduction of the
carbonyl group, and excretion of the parent compound and
metabo-lites in urine. The products of these metabolic
pathways are not of concern.
Step A3. The daily per capita intakes ('eaters only') of the
substances in Europe and the United States are below the
human intake threshold for class II (540 µg/person per day),
indicating that they pose no safety concern when used at
current levels of estimated intake as flavouring agents. The
intakes of acetoin (2800 µg/person per day in Europe; 1800
µg/person per day in the United States), diacetyl (3300
µg/person per day in Europe; 8000 µg/person per day in the
United States), and methylcyclopentenolone (890 µg/person
per day in Europe; 710 µg/person per day in the United
States) are, however, greater than 540 µg/person per day.
Step A4. Acetoin and diacetyl occur endogenously in humans (Kawano,
1959; Zlatkis & Sivetz, 1960; Gabriel et al., 1972), but
methyl-cyclopentenolone does not.
Step A5. A NOEL of 500 mg/kg bw per day was reported for
methylcyclo-pentenolone in a six-month study of toxicity in
rats (Dow Chemical Co., 1953). A safety margin of > 10 000
exists between this NOEL and the estimated daily per
capita intake ('eaters only') of 15 µg/kg bw. In addition,
methylcyclo-pentenolone gave negative results in tests for
genotoxicity (reverse mutation and unscheduled DNA
synthesis; Heck et al., 1989). This information indicates
that methylcyclo-pentenolone would not be expected to be of
safety concern.
The stepwise evaluations of the 22 aliphatic acyclic and
alicyclic alpha-diketones and related alpha-hydroxyketones used as
flavouring agents are summarized in Table 1.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 22 aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones were consumed
simultaneously on a daily basis, the estimated combined intake would
exceed the human intake threshold for class II; however, all 22
substances are expected to be efficiently metabolized and would not
saturate the detoxification pathways. On the basis of the evaluation
of the collective data, there is no safety concern about combined
intake.
Table 1. Summary of results of safety evaluations on aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones
Step 1: All of the substances in the group are in structural class II. The human intake threshold for class II is 540 µg/day.
Step 2: All of the substances in this group are metabolized to innocuous products.
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Acetoin 405 513-86-0 2800/1800 Yes Yes N/R1 No safety
concern
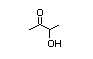 2-Acetoxy-3-butanone 406 4906-24-5 0.03/23 No N/R2 N/R2 No safety
concern
2-Acetoxy-3-butanone 406 4906-24-5 0.03/23 No N/R2 N/R2 No safety
concern
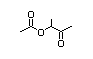 Butan-3-one-2-yl-butanoate 407 84642-61-5 0.02/0.95 No N/R2 N/R2 No safety
concern
Butan-3-one-2-yl-butanoate 407 84642-61-5 0.02/0.95 No N/R2 N/R2 No safety
concern
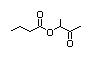 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Diacetyl 408 431-03-8 3300/8000 Yes Yes N/R1 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Diacetyl 408 431-03-8 3300/8000 Yes Yes N/R1 No safety
concern
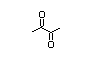 3-Hydroxy-2-pentanone 409 3142-66-3 ND/0.10 No N/R2 N/R2 No safety
concern
3-Hydroxy-2-pentanone 409 3142-66-3 ND/0.10 No N/R2 N/R2 No safety
concern
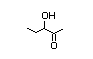 2,3-Pentanedione 410 600-14-6 220/80 No N/R2 N/R2 No safety
concern
2,3-Pentanedione 410 600-14-6 220/80 No N/R2 N/R2 No safety
concern
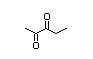 4-Methyl-2,3-pentadione 411 7493-58-5 0.48/2 No N/R2 N/R2 No safety
concern
4-Methyl-2,3-pentadione 411 7493-58-5 0.48/2 No N/R2 N/R2 No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
2,3-Hexadione 412 3848-24-6 13/10 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
2,3-Hexadione 412 3848-24-6 13/10 No N/R2 N/R2 No safety
concern
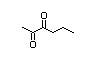 3,4-Hexadione 413 4437-51-8 33/0.76 No N/R2 N/R2 No safety
concern
3,4-Hexadione 413 4437-51-8 33/0.76 No N/R2 N/R2 No safety
concern
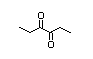 5-Methyl-2,3-hexadione 414 13706-86-0 2/6 No N/R2 N/R2 No safety
concern
5-Methyl-2,3-hexadione 414 13706-86-0 2/6 No N/R2 N/R2 No safety
concern
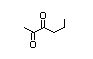 2,3-Heptanedione 415 96-04-8 2/5 No N/R2 N/R2 No safety
concern
2,3-Heptanedione 415 96-04-8 2/5 No N/R2 N/R2 No safety
concern
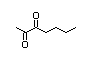 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Hydroxy-4-octanone 416 496-77-5 0.02/0.76 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Hydroxy-4-octanone 416 496-77-5 0.02/0.76 No N/R2 N/R2 No safety
concern
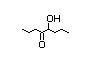 2,3-Undecadione 417 7493-59-6 0.02/0.01 No N/R2 N/R2 No safety
concern
2,3-Undecadione 417 7493-59-6 0.02/0.01 No N/R2 N/R2 No safety
concern
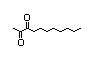 Methylcyclopentenolone 418 80-71-7 890/710 Yes No Yes. The dose of No safety
500 mg/kg bw per concern
day that had no
adverse effects
(Dow Chemical Co.,
1953) is > 10 000
times the intake.
Methylcyclopentenolone 418 80-71-7 890/710 Yes No Yes. The dose of No safety
500 mg/kg bw per concern
day that had no
adverse effects
(Dow Chemical Co.,
1953) is > 10 000
times the intake.
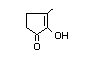 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Ethylcyclopentenolone 419 21835-01-8 50/23 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Ethylcyclopentenolone 419 21835-01-8 50/23 No N/R2 N/R2 No safety
concern
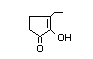 3,4-Dimethyl-1,2-cyclopentadione 420 13494-06-9 47/2 No N/R2 N/R2 No safety
concern
3,4-Dimethyl-1,2-cyclopentadione 420 13494-06-9 47/2 No N/R2 N/R2 No safety
concern
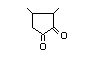 3,5-Dimethyl-1,2-cyclopentadione 421 13494-07-0 55/29 No N/R2 N/R2 No safety
concern
3,5-Dimethyl-1,2-cyclopentadione 421 13494-07-0 55/29 No N/R2 N/R2 No safety
concern
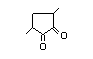 3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one 422 42348-12-9 ND/0.17 No N/R2 N/R2 No safety
concern
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one 422 42348-12-9 ND/0.17 No N/R2 N/R2 No safety
concern
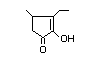 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one 423 53263-58-4 ND/0.38 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one 423 53263-58-4 ND/0.38 No N/R2 N/R2 No safety
concern
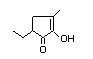 2-Hydroxy-2-cyclohexen-1-one 424 10316-66-2 0.08/0.76 No N/R2 N/R2 No safety
concern
2-Hydroxy-2-cyclohexen-1-one 424 10316-66-2 0.08/0.76 No N/R2 N/R2 No safety
concern
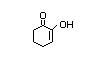 1-Methyl-2,3-cyclohexadione 425 3008-43-3 2/8 No N/R2 N/R2 No safety
concern
1-Methyl-2,3-cyclohexadione 425 3008-43-3 2/8 No N/R2 N/R2 No safety
concern
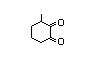 2-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one 426 4883-60-7 2/2 No N/R2 N/R2 No safety
concern
2-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one 426 4883-60-7 2/2 No N/R2 N/R2 No safety
concern
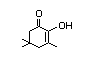 N/R1, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A4 of the procedure.
N/R2, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure.
N/D, no intake data reported.
1.6 Conclusions
The 22 aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones evaluated do not pose a safety concern at
current levels of intake as flavouring agents. No data on toxicity
were required for application of the procedure to 21 of the aliphatic
acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones. The Committee noted that the available data on
toxicity were consistent with the results of the safety evaluations
using the procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This group of substances was selected on the basis of the
criteria that all members of the group are aliphatic and contain
functional groups (alpha-diketones, alpha-hydroxyketones, and esters
of a methylcyclopentenolone hydroxyketones) which have similar
chemical reactivity and participate in common pathways of metabolic
detoxification. Acyclic and alicyclic alpha-diketones exist to varying
degrees in equilibrium with unsaturated alpha-hydroxyketones (i.e. the
ketoenolic form). Therefore, 17 of the 22 substances in this group
exist as mixtures of alpha-diketone and unsaturated
alpha-hydroxyketone. Three (Nos 405, 409, and 416) other members of
the group are aliphatic alpha-hydroxyketones which may be formed
in vivo by simple reduction of one of the two ketone functions in
the corresponding alpha-diketone. The two remaining members of the
group (Nos 406 and 407) are aliphatic esters of the
alpha-hydroxyketone acetoin (No. 405). Hydrolysis of these esters
in vivo yields acetoin (No. 405) and simple aliphatic carboxylic
acids. Consequently, it can be assumed that all of these flavouring
agents either are, or can readily form, alpha-diketones or
alpha-hydroxyketones (see Figure 1). In view of the close chemical and
biochemical relationships between these substances and the consistent
data on toxicity, they are evaluated here as a group of structurally
related compounds.
2.2 Intake
The total annual production volume of the 22 substances in this
group is approximately 44 000 kg in Europe (International Organization
of the Flavor Industry, 1995) and 56 000 kg in the United States (US
National Academy of Sciences, 1970, 1982, 1987). Production volumes
and intake values for each substance are reported in Table 2. On the
basis of the annual volumes reported in Europe and the United States,
the total estimated daily per capita intake ('eaters only') of the
22 flavouring agents in this group is 120 µg/kg bw per day in Europe
and 180 µg/kg bw per day in the United States.
N/R1, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A4 of the procedure.
N/R2, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure.
N/D, no intake data reported.
1.6 Conclusions
The 22 aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones evaluated do not pose a safety concern at
current levels of intake as flavouring agents. No data on toxicity
were required for application of the procedure to 21 of the aliphatic
acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones. The Committee noted that the available data on
toxicity were consistent with the results of the safety evaluations
using the procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This group of substances was selected on the basis of the
criteria that all members of the group are aliphatic and contain
functional groups (alpha-diketones, alpha-hydroxyketones, and esters
of a methylcyclopentenolone hydroxyketones) which have similar
chemical reactivity and participate in common pathways of metabolic
detoxification. Acyclic and alicyclic alpha-diketones exist to varying
degrees in equilibrium with unsaturated alpha-hydroxyketones (i.e. the
ketoenolic form). Therefore, 17 of the 22 substances in this group
exist as mixtures of alpha-diketone and unsaturated
alpha-hydroxyketone. Three (Nos 405, 409, and 416) other members of
the group are aliphatic alpha-hydroxyketones which may be formed
in vivo by simple reduction of one of the two ketone functions in
the corresponding alpha-diketone. The two remaining members of the
group (Nos 406 and 407) are aliphatic esters of the
alpha-hydroxyketone acetoin (No. 405). Hydrolysis of these esters
in vivo yields acetoin (No. 405) and simple aliphatic carboxylic
acids. Consequently, it can be assumed that all of these flavouring
agents either are, or can readily form, alpha-diketones or
alpha-hydroxyketones (see Figure 1). In view of the close chemical and
biochemical relationships between these substances and the consistent
data on toxicity, they are evaluated here as a group of structurally
related compounds.
2.2 Intake
The total annual production volume of the 22 substances in this
group is approximately 44 000 kg in Europe (International Organization
of the Flavor Industry, 1995) and 56 000 kg in the United States (US
National Academy of Sciences, 1970, 1982, 1987). Production volumes
and intake values for each substance are reported in Table 2. On the
basis of the annual volumes reported in Europe and the United States,
the total estimated daily per capita intake ('eaters only') of the
22 flavouring agents in this group is 120 µg/kg bw per day in Europe
and 180 µg/kg bw per day in the United States.
 The vast majority of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones have been reported to
occur in traditional foods (Maarse et al., 1994). Quantitative data on
the natural occurrence of these substances, with the exception of
diacetyl, demonstrate that they are consumed predominantly from
traditional foods (i.e. consumption ratio > 1) (Stofberg & Kirschman,
1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
Aliphatic acyclic and alicyclic alpha-diketones participate in an
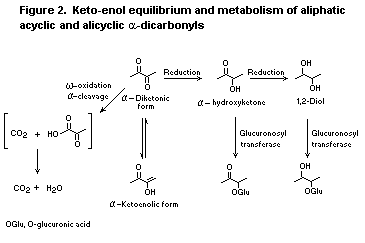
enol-keto equilibrium with the corresponding ketoenol (see Figure 1).
The enolic form predominates in alicyclic diketones, especially
cyclopentyl alpha-diketones (Gordon & Ford, 1972), essentially 100% of
which exist in the ketoenolic form; 50-80% of cyclohexyl
alpha-diketones exist in the ketoenolic form at equilibrium.
Increasing pH would be expected to shift the equilibrium in favour of
the ketoenolic form (i.e. alpha-hydroxyketone). Therefore, at
physiological pH, an increase in the alpha-hydroxyketone (i.e. enol)
form is expected.
In rats and mice, orally administered aliphatic alpha-diketones
are rapidly absorbed from the gastrointestinal tract (Gabriel et al.,
1972). Carbon dioxide produced primarily from methyl-substituted
diketones (e.g. diacetyl) is eliminated in expired air. Thus, after
injection of [2,3-14C]-acetoin to whole albino rats, 14C-carbon
dioxide appeared in expired air, with an average 12-h production of
15% (Gabriel et al., 1972). In rats, 54-82% of orally administered
radiolabelled 2,3-butanedione was excreted as carbon dioxide. At
increasing doses, the percent of the dose excreted as carbon dioxide
decreased, while urinary excretion of radiolabel increased, suggesting
that saturation occurs at higher doses. Chromatographic analysis of
urine samples yielded three major 14C-labelled components, one of
which co-eluted with uric acid. Analysis with ß-glucuronidase or
sulfatase decreased the amount of radiolabel present in one peak, with
a corresponding increase in the amount present in another major
component. Identification of urinary metabolites was not pursued owing
to the extensive excretion of 14C as carbon dioxide (Dix & Jeffcoat,
1997). In general, esters are hydrolysed to their corresponding
alcohols and carboxylic acids. Hydrolysis is catalysed by classes of
enzymes recognized as carboxylesterases or esterases, the most
important of which are the B-esterases. Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). These enzymes
occur in most mammalian tissues (Heymann, 1980; Anders, 1989) but
predominate in hepatocytes (Heymann, 1980). 2-Acetoxy-3-butanone (No.
406) and butanon-3-one-2-yl butanoate (No. 407) are expected to be
metabolized in humans to acetic acid and butanoic acid, respectively,
and to acetoin.
Aliphatic acyclic diketones
The metabolic fate of acyclic aliphatic diketones depends
primarily on the position of the carbonyl function and on chain
length. Aliphatic acyclic diketones and alpha-hydroxyketones that
contain a carbonyl function at the 2 position (i.e. methyl ketones)
may undergo alpha-hydroxylation and subsequent oxidation of the
terminal methyl group to yield corresponding ketocarboxylic acids. The
ketoacids are intermediary metabolites (e.g. alpha-ketoacids), which
may undergo oxidative decarboxylation to yield carbon dioxide and a
simple aliphatic carboxylic acid. The acid may be completely
metabolized in the fatty acid pathway and citric acid cycle.
Alternatively, the methyl-substituted diketones may be
successively reduced to the corresponding hydroxyketones and diols,
which are excreted in the urine as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain ketones. If the carbonyl function is located elsewhere on
the chain, reduction is the predominant detoxification pathway.
alpha-Hydroxyketones and their diol metabolites may be excreted as
glucuronic acid conjugates. For example, the glucuronic acid conjugate
of cyclohexanol was detected in the urine of humans exposed to
cyclohexanone (Ong et al., 1991), indicating that alpha-hydroxylation
followed by reduction of the ketone function occurs in humans.
Acetoin is metabolized primarily via oxidation at low
concentrations in vivo and by reduction to 2,3-butanediol at high
concentrations. It has been estimated that 1 g of rat liver can
oxidize 86 µg (1 µmol) acetoin per day (Gabriel et al., 1972).
Production of carbon dioxide at low levels and of 2,3-butanediol at
high levels is associated with the slower rate of ketone reduction
(Williams, 1959). Oxidation of the terminal methyl group may result in
formation of an alpha-ketoacid, which undergoes cleavage to yield
carbon dioxide and a carboxylic acid fragment. Alternatively, methyl
group oxidation may yield a ß-ketoacid which undergoes ß-cleavage to
yield two-carbon fragments. To a minor extent, these two-carbon
fragments can act as acetyl donors for acetylation of
para-aminobenzoic acid (Westerfeld & Berg, 1943).
A total dose of 78 g of acetoin was administered to a dog over
two months, both orally in a 3-4% solution and subcutaneously in a 20%
solution. Urine was collected under toluene from the beginning of
treatment up to 40 h after the last dose. 2,3-Butanediol was the major
urinary excretion product, representing 5-25% of the dose. The
remainder of the dose was completely metabolized (Westerfeld & Berg,
1943).
In liver preparations obtained from rats and rabbits, more than
95% of the radiolabel of [2,3-14C]-acetoin was detected as a mixture
of stereoisomers of 2,3-butanediol (Gabriel et al., 1971). Although
reduction of diacetyl and acetoin has been observed in animals
in vivo and in animal tissue preparations in vitro at high
concentrations, it appears that oxidation of diacetyl is a major
endogenous metabolic pathway.
Reduction of ketones is mediated by alcohol dehydrogenase and
NADPH-dependent cytosolic carbonyl reductases (Bosron & Li, 1980).
Reduction of the endogenous substances acetoin (No. 405) and diacetyl
(No. 408) is catalysed by the substrate-specific enzymes diacetyl
reductase and acetoin reductase, respectively. In rat liver slices,
diacetyl, acetoin, and 2,3-butanediol are interconvertible (Gabriel et
al., 1972).
In male Wistar albino rats, a single oral dose of diacetyl at
5 mmol/kg bw (430 mg/kg bw) was metabolized by reduction to acetoin,
which was present at high concentrations in major organs 1 h after
dosing. The subsequent reduction product, 2,3-butanediol, was detected
in liver, kidney, and brain (Otsuka et al., 1996). Only a 10-min
incubation is required to convert 10 nmol (9 × 10-4 mg) diacetyl to
3.7 nmol (3 × 10-4 mg) acetoin and 6.3 nmol (6 × 10-4 mg)
2,3-butanediol in rat liver homogenate. Diacetyl was reduced by NADH-
and NADPH-dependent diacetyl reductase isolated from a homogenate of
male Wistar rat liver. The organ-specific reductase activity was
greatest in the liver and least in the brain (Otsuka et al., 1996).
Acetoin was given either orally in a 3-4% solution or
subcutaneously in a 20% solution to rats, multiple doses being spaced
equally throughout each day. The acetoin used was obtained as the
polymer (H, 15), which apparently reverts to an optically inactive
monomer in aqueous solution. No diacetyl was detected in the urine of
the rats; acetoin was not excreted to any appreciable extent in the
urine, and the major excretion product was 2,3-butanediol (Westerfeld
& Berg, 1943). In dogs, acetoin is excreted in the urine, in part as
2,3-butanediol. Most of an oral dose disappeared and was presumed to
be completely metabolized (Westerfeld & Berg, 1943). In another study,
2,3-butanediol readily conjugated glucuronic acid (Neuberg &
Gottshchalk, 1971).
Diacetyl and acetoin are endogenous in humans (Kawano, 1959;
Zlatkis & Sivetz, 1960). They are formed when pyruvate is converted to
acetoin and diacetyl by pyruvate decarboxylase (Gabriel et al.,
1972). Mean fasting blood concentrations of approximately 100 µg
acetoin per 100 ml blood have been reported (Dawson & Hullin, 1954).
Pyruvate also forms diacetyl in vitro in rat liver preparations
(Järnefelt, 1955) and in microorganisms (Juni & Heym, 1956).
Alicyclic alpha-diketones and alicyclic dicarbonyls
In general, alicyclic alpha-diketones are metabolized via a
reduction pathway (Williams, 1959). In humans, structurally related
alicyclic monoketones are reduced to the corresponding alcohols or
undergo alpha-hydroxylation and reduction to yield diols, which are
excreted as the glucuronic acid conjugates. For example, the
glucuronic acid conjugate of cyclohexanol is present in the urine of
humans exposed to atmospheres containing cyclohexanone at
concentrations of 2-30 ppm (Ong et al., 1991). A mixture of diols,
including cis- and trans-1,2-cyclohexanediol,1,3-cyclohexanediol,
and 1,4-cyclohexanediol, was detected in the urine of infants exposed
to cyclohexanone present in dextrose solutions used for intravenous
feeding. Presumably, the cyclohexanone undergoes alpha-hydroxylation
and then reduction of the ketone function to yield the corresponding
alpha-cyclohexanediols (Mills & Walker, 1990).
On the basis of the studies described above, it is anticipated
that humans will metabolize low concentrations of aliphatic acyclic
methyl ketones principally by oxidation of the terminal methyl group.
At higher concentrations, reduction to the diol and subsequent
conjugation with glucuronic acid form a competing detoxification
pathway. Other aliphatic acyclic alpha-diketones and
alpha-hydroxyketones and alicyclic diketones and hydroxyketones are
reduced, conjugated with glucuronic acid, and excreted.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
The results of studies of the acute toxicity of seven of the 22
diketones, hydroxyketones, and alicyclic dicarbonyls have been
reported and are summarized in Table 3. The low acute toxicity of the
group is demonstrated by oral LD50 values in the range 990 to > 8000
mg/kg bw.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones as flavouring substances
in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Acetoin (405)
Europe 19 000 2800 46
United States 9 000 1800 29
2-Acetoxy-3-butanone (406)
Europe 0.2 0.03 0.0005
United States 120 23 0.4
Butan-3-one-2-yl-butanoate (407)
Europe 0.1 0.02 0.0003
United States 5 0.95 0.02
Diacetyl (408)
Europe 18 000 3300 56
United States 42 000 8000 133
3-Hydroxy-2-pentanone (409)
Europe NR ND 0
United States 0.5 0.10 0.002
2,3-Pentanedione (410)
Europe 1 100 220 4
United States 420 80 1
4-Methyl-2,3-pentadione (411)
Europe 2.5 0.48 0.01
United States 12 2 0.04
2,3-Hexanedione (412)
Europe 70 13 0.22
United States 50 10 0.2
3,4-Hexanedione (413)
Europe 170 33 0.5
United States 4 0.76 0.01
5-Methyl-2,3-hexanedione (414)
Europe 9 2 0.03
United States 30 6 0.10
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2,3-Heptanedione (415)
Europe 8 2 0.03
United States 26 5 0.08
5-Hydroxy-4-octanone (416)
Europe 0.1 0.02 0.0003
United States 4 0.76 0.01
2,3-Undecadione (417)
Europe 0.03 0.01 0.0001
United States 0.1 0.02 0.0003
Methylcyclopentenolone (418)
Europe 4 700 890 15
United States 3 700 710 12
3-Ethylcyclopentenolone (419)
Europe 260 50 0.8
United States 120 23 0.4
3,4-Dimethyl-1,2-cyclopentanedione (420)
Europe 250 47 0.8
United States 13 2 0.04
3,5-Dimethyl-1,2-cyclopentanedione (421)
Europe 290 55 0.9
United States 150 29 0.5
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one (422)
Europe NR ND ND
United States 0.9 0.17 0.003
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one (423)
Europe NR ND ND
United States 2 0.38 0.01
2-Hydroxy-2-cyclohexen-1-one (424)
Europe 0.4 0.08 0.001
United States 4 0.76 0.01
1-Methyl-2,3-cyclohexadione (425)
Europe 11 2 0.03
United States 44 8 0.1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2-Hydroxy-3,5,5-trimethyl-2-cyclohexenone (426)
Europe 10 2 0.03
United States 8 2 0.03
Total
Europe 44 000
United States 56 000
NR, not reported; ND, not determined
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only') = 32 × 106 for Europe and
32 × 106 for the United States; 0.6 represents the assumption that
only 60% of the flavour volume was reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight],
where body weight = 60 kg. Slight variation may occur from rounding off.
Table 3. Studies of acute toxicity with aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones used as flavouring substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
Acetoin 405 Rat NR Oral > 5000 Moreno (1977)
Butan-3-one-2-yl 407 Mouse, rat NR Oral > 8000 Pellmont (1969)
butanoate
Diacetyl 408 Rat M Gavage 3400 Colley et al.(1969)
F 3000
Rat NR Gavage 1580 Jenner et al.(1964)
Guinea-pig NR Gavage 990 Jenner et al.(1964)
2,3-Pentanedione 410 Rat NR Oral 3000 Moreno (1977)
2,3-Hexanedione 412 Rat NR Oral > 5000 Moreno (1977)
5-Methyl-2,3-hexanedione 414 Rat NR Oral > 5000 Moreno (1979)
Methylcyclopentenolone 418 Mouse NR Gavage 1350 Givadaun Corp.(1952)
Rat NR Oral 1850 Moreno (1976)
Guinea-pig NR Gavage 1400 Dow Chemical Co.
(1953)
Mouse NR Oral 1350 Leberco Labs (1952)
NR, not reported; M, male; F, female
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The toxicity of nine of the 22 substances has been studied. The
results are summarized in Table 4 and described below.
Acetoin (No. 405)
Groups of 15 male and 15 female CFE rats were given acetoin in
their drinking-water at concentrations of 0 (control), 750, 3000, or
12 000 mg/kg (equivalent to 0, 85, 330, or 1300 mg/kg bw per day (US
Food & Drug Administration, 1993)). No animals died during the study,
and their condition and appearance were normal. The body weights of
males at 12 000 mg/kg in drinking-water decreased significantly from
week 5, and at weeks 2, 6, and 13 the relative weight of the liver was
statistically significantly greater in these animals than in controls.
A similar effect was seen in female rats, but only after 13 weeks.
Haematological examination conducted at 13 weeks showed a small (4-8%)
but statistically significant (p < 0.05) decrease in haemoglobin
concentration and erythrocyte counts in animals of each sex at the
high dose, but these changes were not accompanied by a decrease in
haematocrit. Urinalysis and blood chemical determinations performed at
the end of the study on all animals revealed no statistically
significant differences between treated and control groups.
Histopathological examination also revealed no adverse effects. The
authors suggested that the increased relative liver weights were a
reaction of the liver to an increased metabolic load resulting from
the high intake of acetoin. The NOEL was 3000 ppm, equivalent to
330 mg/kg bw per day (Gaunt et al., 1972), which is greater than
10 000 the daily per capita intake ('eaters only') of 46 and 29
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
Diacetyl (No. 408)
Groups of 15 male and 15 female weanling specific
pathogen-free-derived CFE rats were given 5 ml/kg bw of an aqueous
solution containing 0, 0.2, 0.6, 1.8, or 11% diacetyl by gavage for 90
days, providing daily doses of diacetyl calculated to be 0, 10, 30,
90, and 540 mg/kg bw. Body weight, food intake, and water consumption
were recorded weekly. Growth retardation and increased water
consumption were observed in animals at the high dose, the effects
being more pronounced in males. Haematological and urinary parameters
and enzyme activities were measured. Anaemia and increased
polymorphonuclear leukocytosis were observed in rats at the high dose
but were attributed to haemorrhage, infections, and ulcers of the
stomach. The relative weights of the liver, kidney, and pituitary and
adrenal glands were increased in these animals, and the increases were
greater than could be accounted for by the reduction in body weight.
Macroscopic and microscopic examination of all major organs revealed
ulcers in the squamous and glandular regions of the gastric mucosa.
The lesions observed in the squamous part of the stomach were
associated with hypertrophy or intercellular oedema. No histological
changes were seen in animals treated at lower doses. No other
significant differences were observed between treated and control
animals. The NOEL was 90 mg/kg bw per day (Colley et al., 1969), which
is 1500 times the daily per capita intake ('eaters only') of 56
µg/kg bw from its use as a flavouring agent in Europe and 500 times
the intake of 130 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Hexanedione (No. 413)
Groups of 10-16 male and female Charles River CD rats were housed
in pairs of the same sex and fed for 90 days on a basal diet alone or
supplemented with 3,4-hexanedione for an average daily intake of
17 mg/kg bw. Body weight and food consumption were measured daily and
were considered normal. Haematological examinations and blood urea
determinations were performed on 50% of the animals at week 7 and at
the end of treatment: no significant differences were seen between
treated and control animals. At necropsy, the liver and kidney weights
were normal, and gross and histological examination performed on a
wide range of organs revealed no dose-related lesions. The NOEL was 17
mg/kg bw per day (Posternak et al., 1969), which is more than 30 000
the daily per capita intake ('eaters only') of 0.5 and 0.01 µg/kg bw
from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
Methylcyclopentenolone (No. 418)
Groups of 15 male and 15 female rats aged four to five weeks
(strain not specified) were placed on a diet containing 0 or 1%
methylcyclo-pentenolone, equivalent to a daily intake of 0 or 500
mg/kg bw, for six months. Each animal was weighed twice weekly and
observed frequently for gross appearance and behaviour. The tissues of
most animals that died or were killed during the course of the study
and of all those kiled at the end of the study were examined grossly.
Tissues of representative animals from the control and treated groups
(numbers unspecified) were examined microscopically, and the weights
of the lungs, heart, liver, kidneys, spleen, and testes were recorded.
Haematological parameters were measured in representative animals from
the control and treated groups (numbers not specified) at the end of
the experiment. There were no statistically significant differences
between treated and control animals in any of the parameters measured.
The NOEL was 500 mg/kg bw per day (Dow Chemical Co., 1953), which is
more than 30 000 the daily per capita intake ('eaters only') of 15
and 12 µg/kg bw from its use as a flavouring agent in Europe and the
United States, respectively (see Table 2).
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
Groups of 10 male and 10 female Charles River CD rats were fed
diets providing 3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses of 0,
100, 200, or 400 mg/kg bw per day for 91 days. No adverse effects were
seen on behaviour, body weight, food consumption, haematological,
urinary, or ophthalmological parameters, or gross or histopathological
appearance. The NOEL was 400 mg/kg bw per day (King et al., 1979),
which is more than 50 000 the daily per capita intake ('eaters
only') of 0.8 and 0.4 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
In a combined study of developmental toxicity and
carcinogenicity, three successive generations of male and
female Charles River CD-COBS rats received
3-ethyl-2-hydroxy-2-cyclopenten-1-one in the basal diet at doses of 0
(untreated control), 0 (propylene glycol control), 30, 80, or
200 mg/kg bw per day. The F0 generation was entered into the study
after weaning and was mated 64 days later. These animals were treated
for 12 months, during which time the females were given a supplemental
diet throughout gestation and lactation. The F1 generation was
initially exposed in utero, subsequently via the dams' milk until
weaning, and then treated for two years and bred twice (at days 99 and
155) to produce F2a and F3a generations. The F0 and F2 generations
consisted of 40 animals of each sex in the untreated control group and
20 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups. In the F1
generation, there were 100 animals of each sex in the untreated
control group and 50 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups.
Survival, clinical symptoms, food consumption, reproductive
perfor-mance, and haematological and clinical chemical parameters were
not adversely affected in the F0 or F1 generations. Slightly
depressed growth was reported in F1 females receiving the highest
dose, but the effect was not significant when compared with the growth
of controls receiving propylene glycol. Gross pathological and
histopathological examination of animals of these generations revealed
no significant treatment-related effects. An increased incidence of
bile-duct hyperplasia observed in these groups was not statistically
significant when compared with the incidence of in controls. The
incidence of benign or malignant tumours in treated animals was
similar to that in controls. The NOEL was 200 mg/kg bw per day (King
et al., 1979), which is more than 200 000 the daily per capita
intake ('eaters only') of 0.8 and 0.4 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
In a three-generation study of toxicity in Charles River
CD-COBS rats with 3-ethyl-2-hydroxy-2-cyclopenten-1-one, 120
F0 animals were entered into the study just after weaning and were
mated after 64 days, providing the F1 generation. Animals of the
F0 generation were selected at random to be maintained as controls
or were given 3-ethyl-2-hydroxy-2-cyclopenten-1-one in the diet.
The F1 generation was maintained on the diet for 755 days, during
which time they were mated twice (at days 99 and 155). The first
mating provided the rats for the F2 generation, and the second mating
provided rats which were sacrificed after weaning. The F2 generation
was treated for 84 days and then mated to produce the F3 generation.
Rats of all generations were maintained on a basal diet with
3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses equal to daily intakes
of 0, 30, 80, or 200 mg/kg bw. Interim clinical chemical analyses were
performed on the F0 generation at months 4 and 6. Ophthalmological,
clinical chemical, and haematological examinations were performed on
the F1 generation at months 6, 12, 18, and 24. At termination, both
the F0 and the F1 generations were submitted to clinical chemical,
haematological, and histopathological examinations. F2 adult animals
were killed without necropsy at the time of weaning of their
offspring.
No evidence of treatment-related effects was found, and the
general health of the rats was unaffected throughout treatment.
Mammary swellings in females and other subcutaneous nodules were
considered not to be related to treatment because they were equally
distributed among control and treated groups. No effects were seen on
food consumption, weight gain, or clinical chemical or haematological
parameters. In the F0 generation, there were no pathological findings
at the 12-month necropsy or at histopathological examination. In the
F1 generation, there were no macroscopic observations that could be
related to the treatment. Histologically, bile-duct hyperplasia was
seen in all groups, including controls; however, there was no
statistically significant difference between treated and control
groups and the findings were considered not to be related to
treatment. Only tumours usually found in rats of this strain and age
were observed, indicating the absence of treatment-related effects.
The NOEL in this study was 200 mg/kg bw per day (King et al., 1979),
which is more than 100 000 the daily per capita intake ('eaters
only') of 0.4 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Dimethylcyclopentane-1,2-dione (No. 420)
Groups of 10 male Charles River CD rats were fed diets containing
3,4-dimethyl-1,2-cyclopentadione at concentrations of 0, 400, 4000, or
13 000 mg/kg (equivalent to 0, 20, 200, and 640 mg/kg bw per day) for
90 days. Food consumption, body-weight gain, haematological
parameters, organ weights, and gross and microscopic appearance were
observed throughout the study. Animals at the intermediate and high
doses showed a moderate but consistent depression in food intake
throughout the experiment, and their final mean body weights were
about 10% less than those of controls. There were no consistent
indications of a profound disturbance of food conversion efficiency,
although minor increases in the food conversion ratio were seen in
some animals at the high dose. Haematological parameters, organ
weights, and gross and microscopic appearance were similar in treated
and control groups. The authors attributed the decreased body weight
observed at higher doses to the unpalatability of the test substance.
The NOEL was 200 mg/kg bw per day (Wheldon & Krajkeman, 1967), which
is more than 200 000 the daily per capita intake ('eaters only') of
0.8 and 0.04 µg/kg bw from its use as a flavouring agent in Europe and
the United States, respectively (see Table 2).
3,5-Dimethylcyclopentane-1,2-dione (No. 421)
Groups of 10 male Charles River CD rats were fed
3,5-dimethyl-1,2-cyclopenthexadione in the diet for 13 weeks at
concentrations of 0, 1000, or 10 000 mg/kg diet (equivalent to 50 and
500 mg/kg bw per day). A fourth group was fed a diet initially
containing 12 000 ppm (equivalent to 610 mg/kg bw per day), but the
level was increased to 24 000 mg/kg diet (equivalent to
1200 mg/kg bw per day) during week 6 through to the end of experiment.
Haematological examinations were performed on five animals from each
group just before the end of the 13-week period; organ weight analysis
and gross and histopathological examinations at the end of the study
revealed no significant differences between control and treated
animals. The food consumption of animals at the two highest doses was
reduced throughout the experiment, accompanied by decreased weight
gain, which resulted in mean body weights that were 10-17% lower than
those of controls at the end of the study. The authors attributed the
decrease to the unpalatability of the test substance. The NOEL was 50
mg/kg bw per day (Wheldon & Krajkeman, 1967), which is more than
50 000 the daily per capita intake ('eaters only') of 0.9 and 0.5
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
2-Hydroxy-2-cyclohexen-1-one (No. 424)
2-Hydroxy-2-cyclohexen-1-one was added to the diet of 15 male and
15 female albino Wistar rats for 90 days at a concentration calculated
to result in an average daily intake of 5 mg/kg bw. Detailed
measurements of haematological parameters, blood chemistry, urine,
gross and histopathological appearance, organ weights, body weight,
and food consumption revealed no statistically significant difference
between treated and control groups (Morgareidge et al., 1974). The
NOEL was 5 mg/kg bw per day (Cox, 1974), which is more than 500 000
the daily per capita intake ('eaters only') of 0.001 and 0.01 µg/kg
bw from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
1-Methyl-2,3-cyclohexadione (No. 425)
Groups of 10 male Charles River CD rats were fed
1-methyl-2,3-cyclohexadione in the diet at concentrations of 0, 100,
or 1000 mg/kg (equivalent to 0, 5, and 50 mg/kg bw per day) for 13
weeks. A fourth group was fed a diet initially containing 13 500 mg/kg
diet (equivalent to 675 mg/kg bw per day), which was raised to 27 000
mg/kg bw (equivalent to 1350 mg/kg bw per day) during week
6. Observation of haematological parameters, organ weights, and gross
and histopathological appearance revealed no difference between
treated and control groups. The food consumption of rats at the high
dose was reduced throughout the experiment, accompanied by decreased
body-weight gain, and the effect was more marked when the dietary
concentration was raised. The NOEL was 50 mg/kg bw per day (Wheldon &
Krajkeman, 1967), which is more than 500 000 the daily per capita
intake ('eaters only') of 0.03 and 0.1 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
2.3.2.3 Genotoxicity
The available studies of genotoxicity are summarized in Table 5.
Most of the studies were carried out with Salmonella typhimurium in
the Ames test. Acetoin, diacetyl, and 1,2-cyclohexanedione showed some
mtagenicity in strains TA100 and TA104. As the mutation frequencies
were low and the positive results were always accompanied by negative
results, the overall conclusion was that this group of substances does
not induce gene mutation in bacteria in vitro. Diacetyl did not
induce mutation in Saccharomyces cerevisiae (US Food & Drug
Administration, 1974). Methylcyclopentenolone did not induce
unscheduled DNA synthesis in rat hepatocytes (Heck et al., 1989).
2.3.2.4 Other relevant studies
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
In the three-generation, 23-month study of toxicity described
above, treatment had no effect on clinical signs, weight gain, food
consumption, copulation rates, or mating behaviour of males or
females, fertility index, length of gestation, parturition, litter
size, or incidence of stillbirth. The growth and survival rates of
pups during lactation were normal. Gross examination of all offspring
from birth to weaning revealed no significant treatment-related
malformations or lesions (King et al., 1979).
Diacetyl (No. 408)
Groups of 25-27 Syrian golden hamsters, 21-24 CD-1 mice, and
21-23 albino Wistar rats were given a solution containing 90%
diacetyl by gavage on days 6-10 of gestation for hamsters and days
6-15 of gestation for mice and rats. The doses for all species were
16, 74, 345, or 1600 mg/kg bw per day. No effects were seen on
maternal survival, weight, or reproductive parameters or on fetal
survival or microscopic appearance of external, skeletal, or soft
tissues (US Food & Drug Administration, 1973).
4. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon,
R. (1989) Contribution of coffee aroma constituents to the
mutagenicity of coffee. Food Chem. Toxicol., 27, 227-232.
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Table 4. Short-term and long-term studies of toxicity in rats with aliphatic acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones used as flavouring agents
Substance No. Sex No. groups/ Route Time NOEL Reference
no. per group (days) (mg/kg bw
per day)
Acetoin 405 M/F 3/30 Drinking-water 90 330 Gaunt et al. (1972)
Diacetyl 408 M/F 4/30 Gavage 90 90 Colley et al. (1969)
3,4-Hexanedione 413 M/F 2/10-16 Diet 90 17a Posternak et al. (1969)
Methylcyclopentenolone 418 M/F 1/30 Diet 185 500a Dow Chemical Co. (1953)
3-Ethyl-2-hydroxy-2-cyclopenten-1-one 419 M/F 3/20 Diet 91 400a King et al. (1979)
M/F 3/100 Diet 730 200 King et al. (1979)
3,4-Dimethyl-1,2-cyclopentadione 420 M 3/10 Diet 90 645 Wheldon & Krajkeman (1967)
3,5-Dimethyl-1,2-cyclopentadione 421 M 3/10 Diet 90 610a Wheldon & Krajkeman (1967)
2-Hydroxy-2-cyclohexen-1-one 424 M/F 1/30 Diet 90 5a Morgareidge et al. (1974)
1-Methyl-2,3-cyclohexadione 425 M 3/10 Diet 90 675-1350a Wheldon & Krajkeman (1967)
M, male; F, female
a The study was performed at a single or multiple doses that had no adverse effects; therefore, no NOEL was determined.
The NOEL is probably higher than the dose reported to have no adverse effects.
Table 5. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones
used as flavouring agents
Substance No. End-point Test object Dose Results Reference
Acetoin 405 Reverse mutation S. typhimurium TA100 < 4500 mg/plate Negativea Garst et al. (1983)
Positiveb
Reverse mutation S. typhimurium TA100 420 mg/plate Negativeb Kim et al. (1987)
Diacetyl 408 Reverse mutation S. typhimurium TA100, Not reported Positivea,c Kato et al. (1989)
(modified test) TA104; E. coli
Reverse mutation S. typhimurium TA104 530 µg/plate Positived Marnett et al. (1985)
(modified test)
Reverse mutation S. typhimurium TA100 90 µg/plate Negativee Kim et al. (1987)
Reverse mutation S. typhimurium TA104 5-500 µg/platef Positivea,c Shane et al. (1988)
Negativeb
Reverse mutation S. typhimurium TA102, 5-500 µg/platef Negativee Shane et al. (1988)
TA100
Reverse mutation S. typhimurium TA100 152-950 µg/plate Negativeb Dorado et al. (1992)
Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA1535, 1% Negativee US Food & Drug
(suspension test) TA1537, TA1538 Administration (1974)
Reverse mutation S. typhimurium TA102 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
Reverse mutation S. typhimurium TA98, 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
TA100
Reverse mutation S. typhimurium TA100 2 or 4 mmol/plate Positiveg Suwa et al. (1982)
Mutation S. cerevisiae Not reported Negativee US Food & Drug
Administration (1974)
2,3-Pentanedione 410 Reverse mutation S. typhimurium TA100 105 µg/plate Negativeb Kim et al. (1987)
Reverse mutation S. typhimurium TA100, 0.9-90 000 µg/plate Negativee Aeschbacher et al. (1989)
3,4-Hexanedione 413 Reverse mutation S. typhimurium TA100 228-4900 µg/plate Negativeb Dorado et al. (1992)
Table 5. (continued)
Substance No. End-point Test object Dose Results Reference
Methylcyclopentenolone 418 Reverse mutation S. typhimurium TA1535, 10 000 µg/plate Negativee Heck et al. (1989)
TA1537, TA1538, TA98,
TA100
Unscheduled DNA Rat hepatocytes 500 µg/plate Negativea Heck et al. (1989)
synthesis
2-Hydroxy-2-cyclohexen-1-one 424 Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA100 112-1000 µg/plate Negativeb Dorado et al. (1992)
a With metabolic activation
b Without metabolic activation
c Mutation frequency, 2-3
d Result based on authors' criterion for significant mutagenic effect, i.e. mutation frequency > 1.5
e With and without metabolic activation
f Estimated from graph
g Mutation frequency, < 2.5
Bjeldanes, L.F. & Chew, H. (1979) Mutagenicity of 1,2-dicarbonyl
compounds: Maltol, kojic acid, diacetyl and related substances.
Mutat. Res., 67, 367-371.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxication, New York, Academic
Press, Vol. I, pp. 231-248.
Colley, J., Gaunt, I.F., Lansdown, A.B.G., Grasso, P. & Gangolli, S.D.
(1969) Acute and short-term toxicity of diacetyl in rats. Food Chem.
Toxicol., 7, 571-580.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, J. & Hullin, R.P. (1954) Metabolism of acetoin. Formation and
utilization of acetoin and 2,3-butanediol in the decerebrated cat.
Biochem. J., 57, 177-180.
Dix & Jeffcoat (1997) Disposition and excretion of
[14C]2,3-butanedione in male rats following oral administration.
Unpublished report from Research Triangle Institute (NIEHS contract
No. NO1-ES-75407). Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Dorado, L., Montoya, M.R. & Rodriguez-Mellado, J.M. (1992) A
contribution to the study of the structure-mutagenicity relationship
for alpha-dicarbonyl compounds using the Ames test. Mutat. Res.,
269, 301-306.
Dow Chemical Co. (1953) Toxicity of cyclotene--Summary. Unpublished
report from Biochemical Research Department, Midland, Michigan, 15
July. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Gabriel, M.A., Jabara, H. & Al-Khalidi, A.A. (1971) Metabolism of
acetoin in mammalian liver slices and extracts. Biochem. J., 124,
793-800.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Garst, J., Stapleton, P. & Johnson, J. (1983) Mutagenicity of
alpha-hydroxy ketones may involve superoxide anion radical. Oxy
Radicals and Their Scavenger Systems, Amsterdam, Elsevier Science
Publishing, Vol. 2, pp. 125-130.
Gaunt, I.F., Branton, P.G., Kiss, I.S., Grasso, P. & Gangolli, S.D.
(1972) Short-term toxicity of acetoin (acetyl methylcarbinol) in rats.
Food Cosmet. Toxicol., 10, 131-141.
Givaudan Corp. (1952) Acute oral toxicity study of
methylcyclopentenolone in mice. Unpublished report to the Research
Institute of Fragrance Materials. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Gordon, A.J. & Ford, R.A. (1972) The Chemist's Companion, New York,
John Wiley & Sons, pp. 49-51.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Järnefelt, J. (1955) Studies on the enzymatic synthesis and breakdown
of acetoin in the animal organism. Ann. Acad. Sci. Fenn. Ser.
A. V. Med Anthropol., 57, 7-78.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Juni, E. & Heym, G.A. (1956) A cyclic pathway for the bacterial
dissimilation of 2,3-butanediol, acetyl methyl carbinol, and diacetyl.
J. Bacteriol., 71, 425-432.
Kato, F., Araki, A., Nozaki, K. & Matsushi, T. (1989) Mutagenicity of
aldehydes and diketones. Mutat. Res., 216, 366-367.
Kawano, T. (1959) Relation of acetoin and pantothenic acid. Fukuoka
Igaku Zasshi, 50, 2939-2953.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic
heterocyclic amines. Mutat. Res., 177, 9-15.
King, T., Faccini, J.M., Jachbaur, J., Perraud, J. & Monro, A.M.
(1979) 3-Generation and chronic toxicity study in rats. Unpublished
report from Pfizer Central Research. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Leberco Labs (1952) Acute toxicity of methylcyclopentenolone.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mills, G.A. & Walker, V. (1990) Urinary excretion of cyclohexanediol,
a metabolite of the solvent cyclohexanone, by infants in a special
care unit. Clin. Chem., 36, 870-874.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1977) Acute toxicity studies in rats, rabbits and guinea
pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1979) Acute toxicity studies in rats, rabbits and
guinea-pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974) 90 Day feeding study
with 2-hydroxy-2-cyclohexen-1-one in rats. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Ong, C.N., Sia, G.L., Chia, S.E., Phoon, W.H. & Tan, K.T. (1991)
Determination of cyclohexanol in urine and its use in environmental
monitoring of cyclohexanone exposure. J. Anal. Toxicol., 15, 13-16.
Otsuka, M., Mine, T., Ohuchi, K. & Ohmori, S. (1996) A detoxication
route for acetaldehyde: Metabolism of diacetyl, acetoin, and
2,3-butanediol in liver homogenate and perfused liver of rats.
J. Biochem., 119, 246-251.
Pellmont, D. (1969) Studies with rats and mice on substance No.
R01-3801 (Toxikologisches Labor 256, Bau 69). Unpublished report to
the Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological test on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102 and TA104. Environ. Mol. Mutag., 11,
195-206.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Suwa, Y., Nagao, M., Kosugi, A. & Sugimura, T. (1982) Sulfite
suppresses the mutagenic property of coffee. Mutat. Res., 102,
383-391.
US Food & Drug Administration (1973) Teratologic Evaluation of FDA
71-73 (Starter Distillate, Hansen) (NTIS PB-223-833;
FDABF-GRAS-152), Washington DC, United States.
US Food & Drug Administration (1974) Mutagenic Evaluation of
Compound FDA 71-73 (Starter Distillate) (NTIS PB-245-431;
FDABF-GRAS-275), Washington DC, United States.
US Food & Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1970) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1982) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
Westerfeld, W.W. & Berg, R.L. (1943) Observations on the metabolism of
acetoin. J. Biol. Chem., 148, 523-528.
Wheldon, G.H. & Krajkeman, A.J. (1967) The effects of ten
food-flavoring additives administered to rats over a period of
thirteen weeks. Unpublished report from Huntington Research Center.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Williams, T.R. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 62, 78.
Zlatkis, A. & Sivetz, M. (1960) Analysis of coffee volatiles by gas
chromatography. Food Res., 25, 395-398.
The vast majority of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones have been reported to
occur in traditional foods (Maarse et al., 1994). Quantitative data on
the natural occurrence of these substances, with the exception of
diacetyl, demonstrate that they are consumed predominantly from
traditional foods (i.e. consumption ratio > 1) (Stofberg & Kirschman,
1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
Aliphatic acyclic and alicyclic alpha-diketones participate in an
enol-keto equilibrium with the corresponding ketoenol (see Figure 1).
The enolic form predominates in alicyclic diketones, especially
cyclopentyl alpha-diketones (Gordon & Ford, 1972), essentially 100% of
which exist in the ketoenolic form; 50-80% of cyclohexyl
alpha-diketones exist in the ketoenolic form at equilibrium.
Increasing pH would be expected to shift the equilibrium in favour of
the ketoenolic form (i.e. alpha-hydroxyketone). Therefore, at
physiological pH, an increase in the alpha-hydroxyketone (i.e. enol)
form is expected.
In rats and mice, orally administered aliphatic alpha-diketones
are rapidly absorbed from the gastrointestinal tract (Gabriel et al.,
1972). Carbon dioxide produced primarily from methyl-substituted
diketones (e.g. diacetyl) is eliminated in expired air. Thus, after
injection of [2,3-14C]-acetoin to whole albino rats, 14C-carbon
dioxide appeared in expired air, with an average 12-h production of
15% (Gabriel et al., 1972). In rats, 54-82% of orally administered
radiolabelled 2,3-butanedione was excreted as carbon dioxide. At
increasing doses, the percent of the dose excreted as carbon dioxide
decreased, while urinary excretion of radiolabel increased, suggesting
that saturation occurs at higher doses. Chromatographic analysis of
urine samples yielded three major 14C-labelled components, one of
which co-eluted with uric acid. Analysis with ß-glucuronidase or
sulfatase decreased the amount of radiolabel present in one peak, with
a corresponding increase in the amount present in another major
component. Identification of urinary metabolites was not pursued owing
to the extensive excretion of 14C as carbon dioxide (Dix & Jeffcoat,
1997). In general, esters are hydrolysed to their corresponding
alcohols and carboxylic acids. Hydrolysis is catalysed by classes of
enzymes recognized as carboxylesterases or esterases, the most
important of which are the B-esterases. Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). These enzymes
occur in most mammalian tissues (Heymann, 1980; Anders, 1989) but
predominate in hepatocytes (Heymann, 1980). 2-Acetoxy-3-butanone (No.
406) and butanon-3-one-2-yl butanoate (No. 407) are expected to be
metabolized in humans to acetic acid and butanoic acid, respectively,
and to acetoin.
Aliphatic acyclic diketones
The metabolic fate of acyclic aliphatic diketones depends
primarily on the position of the carbonyl function and on chain
length. Aliphatic acyclic diketones and alpha-hydroxyketones that
contain a carbonyl function at the 2 position (i.e. methyl ketones)
may undergo alpha-hydroxylation and subsequent oxidation of the
terminal methyl group to yield corresponding ketocarboxylic acids. The
ketoacids are intermediary metabolites (e.g. alpha-ketoacids), which
may undergo oxidative decarboxylation to yield carbon dioxide and a
simple aliphatic carboxylic acid. The acid may be completely
metabolized in the fatty acid pathway and citric acid cycle.
Alternatively, the methyl-substituted diketones may be
successively reduced to the corresponding hydroxyketones and diols,
which are excreted in the urine as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain ketones. If the carbonyl function is located elsewhere on
the chain, reduction is the predominant detoxification pathway.
alpha-Hydroxyketones and their diol metabolites may be excreted as
glucuronic acid conjugates. For example, the glucuronic acid conjugate
of cyclohexanol was detected in the urine of humans exposed to
cyclohexanone (Ong et al., 1991), indicating that alpha-hydroxylation
followed by reduction of the ketone function occurs in humans.
Acetoin is metabolized primarily via oxidation at low
concentrations in vivo and by reduction to 2,3-butanediol at high
concentrations. It has been estimated that 1 g of rat liver can
oxidize 86 µg (1 µmol) acetoin per day (Gabriel et al., 1972).
Production of carbon dioxide at low levels and of 2,3-butanediol at
high levels is associated with the slower rate of ketone reduction
(Williams, 1959). Oxidation of the terminal methyl group may result in
formation of an alpha-ketoacid, which undergoes cleavage to yield
carbon dioxide and a carboxylic acid fragment. Alternatively, methyl
group oxidation may yield a ß-ketoacid which undergoes ß-cleavage to
yield two-carbon fragments. To a minor extent, these two-carbon
fragments can act as acetyl donors for acetylation of
para-aminobenzoic acid (Westerfeld & Berg, 1943).
A total dose of 78 g of acetoin was administered to a dog over
two months, both orally in a 3-4% solution and subcutaneously in a 20%
solution. Urine was collected under toluene from the beginning of
treatment up to 40 h after the last dose. 2,3-Butanediol was the major
urinary excretion product, representing 5-25% of the dose. The
remainder of the dose was completely metabolized (Westerfeld & Berg,
1943).
In liver preparations obtained from rats and rabbits, more than
95% of the radiolabel of [2,3-14C]-acetoin was detected as a mixture
of stereoisomers of 2,3-butanediol (Gabriel et al., 1971). Although
reduction of diacetyl and acetoin has been observed in animals
in vivo and in animal tissue preparations in vitro at high
concentrations, it appears that oxidation of diacetyl is a major
endogenous metabolic pathway.
Reduction of ketones is mediated by alcohol dehydrogenase and
NADPH-dependent cytosolic carbonyl reductases (Bosron & Li, 1980).
Reduction of the endogenous substances acetoin (No. 405) and diacetyl
(No. 408) is catalysed by the substrate-specific enzymes diacetyl
reductase and acetoin reductase, respectively. In rat liver slices,
diacetyl, acetoin, and 2,3-butanediol are interconvertible (Gabriel et
al., 1972).
In male Wistar albino rats, a single oral dose of diacetyl at
5 mmol/kg bw (430 mg/kg bw) was metabolized by reduction to acetoin,
which was present at high concentrations in major organs 1 h after
dosing. The subsequent reduction product, 2,3-butanediol, was detected
in liver, kidney, and brain (Otsuka et al., 1996). Only a 10-min
incubation is required to convert 10 nmol (9 × 10-4 mg) diacetyl to
3.7 nmol (3 × 10-4 mg) acetoin and 6.3 nmol (6 × 10-4 mg)
2,3-butanediol in rat liver homogenate. Diacetyl was reduced by NADH-
and NADPH-dependent diacetyl reductase isolated from a homogenate of
male Wistar rat liver. The organ-specific reductase activity was
greatest in the liver and least in the brain (Otsuka et al., 1996).
Acetoin was given either orally in a 3-4% solution or
subcutaneously in a 20% solution to rats, multiple doses being spaced
equally throughout each day. The acetoin used was obtained as the
polymer (H, 15), which apparently reverts to an optically inactive
monomer in aqueous solution. No diacetyl was detected in the urine of
the rats; acetoin was not excreted to any appreciable extent in the
urine, and the major excretion product was 2,3-butanediol (Westerfeld
& Berg, 1943). In dogs, acetoin is excreted in the urine, in part as
2,3-butanediol. Most of an oral dose disappeared and was presumed to
be completely metabolized (Westerfeld & Berg, 1943). In another study,
2,3-butanediol readily conjugated glucuronic acid (Neuberg &
Gottshchalk, 1971).
Diacetyl and acetoin are endogenous in humans (Kawano, 1959;
Zlatkis & Sivetz, 1960). They are formed when pyruvate is converted to
acetoin and diacetyl by pyruvate decarboxylase (Gabriel et al.,
1972). Mean fasting blood concentrations of approximately 100 µg
acetoin per 100 ml blood have been reported (Dawson & Hullin, 1954).
Pyruvate also forms diacetyl in vitro in rat liver preparations
(Järnefelt, 1955) and in microorganisms (Juni & Heym, 1956).
Alicyclic alpha-diketones and alicyclic dicarbonyls
In general, alicyclic alpha-diketones are metabolized via a
reduction pathway (Williams, 1959). In humans, structurally related
alicyclic monoketones are reduced to the corresponding alcohols or
undergo alpha-hydroxylation and reduction to yield diols, which are
excreted as the glucuronic acid conjugates. For example, the
glucuronic acid conjugate of cyclohexanol is present in the urine of
humans exposed to atmospheres containing cyclohexanone at
concentrations of 2-30 ppm (Ong et al., 1991). A mixture of diols,
including cis- and trans-1,2-cyclohexanediol,1,3-cyclohexanediol,
and 1,4-cyclohexanediol, was detected in the urine of infants exposed
to cyclohexanone present in dextrose solutions used for intravenous
feeding. Presumably, the cyclohexanone undergoes alpha-hydroxylation
and then reduction of the ketone function to yield the corresponding
alpha-cyclohexanediols (Mills & Walker, 1990).
On the basis of the studies described above, it is anticipated
that humans will metabolize low concentrations of aliphatic acyclic
methyl ketones principally by oxidation of the terminal methyl group.
At higher concentrations, reduction to the diol and subsequent
conjugation with glucuronic acid form a competing detoxification
pathway. Other aliphatic acyclic alpha-diketones and
alpha-hydroxyketones and alicyclic diketones and hydroxyketones are
reduced, conjugated with glucuronic acid, and excreted.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
The results of studies of the acute toxicity of seven of the 22
diketones, hydroxyketones, and alicyclic dicarbonyls have been
reported and are summarized in Table 3. The low acute toxicity of the
group is demonstrated by oral LD50 values in the range 990 to > 8000
mg/kg bw.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones as flavouring substances
in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Acetoin (405)
Europe 19 000 2800 46
United States 9 000 1800 29
2-Acetoxy-3-butanone (406)
Europe 0.2 0.03 0.0005
United States 120 23 0.4
Butan-3-one-2-yl-butanoate (407)
Europe 0.1 0.02 0.0003
United States 5 0.95 0.02
Diacetyl (408)
Europe 18 000 3300 56
United States 42 000 8000 133
3-Hydroxy-2-pentanone (409)
Europe NR ND 0
United States 0.5 0.10 0.002
2,3-Pentanedione (410)
Europe 1 100 220 4
United States 420 80 1
4-Methyl-2,3-pentadione (411)
Europe 2.5 0.48 0.01
United States 12 2 0.04
2,3-Hexanedione (412)
Europe 70 13 0.22
United States 50 10 0.2
3,4-Hexanedione (413)
Europe 170 33 0.5
United States 4 0.76 0.01
5-Methyl-2,3-hexanedione (414)
Europe 9 2 0.03
United States 30 6 0.10
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2,3-Heptanedione (415)
Europe 8 2 0.03
United States 26 5 0.08
5-Hydroxy-4-octanone (416)
Europe 0.1 0.02 0.0003
United States 4 0.76 0.01
2,3-Undecadione (417)
Europe 0.03 0.01 0.0001
United States 0.1 0.02 0.0003
Methylcyclopentenolone (418)
Europe 4 700 890 15
United States 3 700 710 12
3-Ethylcyclopentenolone (419)
Europe 260 50 0.8
United States 120 23 0.4
3,4-Dimethyl-1,2-cyclopentanedione (420)
Europe 250 47 0.8
United States 13 2 0.04
3,5-Dimethyl-1,2-cyclopentanedione (421)
Europe 290 55 0.9
United States 150 29 0.5
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one (422)
Europe NR ND ND
United States 0.9 0.17 0.003
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one (423)
Europe NR ND ND
United States 2 0.38 0.01
2-Hydroxy-2-cyclohexen-1-one (424)
Europe 0.4 0.08 0.001
United States 4 0.76 0.01
1-Methyl-2,3-cyclohexadione (425)
Europe 11 2 0.03
United States 44 8 0.1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2-Hydroxy-3,5,5-trimethyl-2-cyclohexenone (426)
Europe 10 2 0.03
United States 8 2 0.03
Total
Europe 44 000
United States 56 000
NR, not reported; ND, not determined
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only') = 32 × 106 for Europe and
32 × 106 for the United States; 0.6 represents the assumption that
only 60% of the flavour volume was reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight],
where body weight = 60 kg. Slight variation may occur from rounding off.
Table 3. Studies of acute toxicity with aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones used as flavouring substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
Acetoin 405 Rat NR Oral > 5000 Moreno (1977)
Butan-3-one-2-yl 407 Mouse, rat NR Oral > 8000 Pellmont (1969)
butanoate
Diacetyl 408 Rat M Gavage 3400 Colley et al.(1969)
F 3000
Rat NR Gavage 1580 Jenner et al.(1964)
Guinea-pig NR Gavage 990 Jenner et al.(1964)
2,3-Pentanedione 410 Rat NR Oral 3000 Moreno (1977)
2,3-Hexanedione 412 Rat NR Oral > 5000 Moreno (1977)
5-Methyl-2,3-hexanedione 414 Rat NR Oral > 5000 Moreno (1979)
Methylcyclopentenolone 418 Mouse NR Gavage 1350 Givadaun Corp.(1952)
Rat NR Oral 1850 Moreno (1976)
Guinea-pig NR Gavage 1400 Dow Chemical Co.
(1953)
Mouse NR Oral 1350 Leberco Labs (1952)
NR, not reported; M, male; F, female
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The toxicity of nine of the 22 substances has been studied. The
results are summarized in Table 4 and described below.
Acetoin (No. 405)
Groups of 15 male and 15 female CFE rats were given acetoin in
their drinking-water at concentrations of 0 (control), 750, 3000, or
12 000 mg/kg (equivalent to 0, 85, 330, or 1300 mg/kg bw per day (US
Food & Drug Administration, 1993)). No animals died during the study,
and their condition and appearance were normal. The body weights of
males at 12 000 mg/kg in drinking-water decreased significantly from
week 5, and at weeks 2, 6, and 13 the relative weight of the liver was
statistically significantly greater in these animals than in controls.
A similar effect was seen in female rats, but only after 13 weeks.
Haematological examination conducted at 13 weeks showed a small (4-8%)
but statistically significant (p < 0.05) decrease in haemoglobin
concentration and erythrocyte counts in animals of each sex at the
high dose, but these changes were not accompanied by a decrease in
haematocrit. Urinalysis and blood chemical determinations performed at
the end of the study on all animals revealed no statistically
significant differences between treated and control groups.
Histopathological examination also revealed no adverse effects. The
authors suggested that the increased relative liver weights were a
reaction of the liver to an increased metabolic load resulting from
the high intake of acetoin. The NOEL was 3000 ppm, equivalent to
330 mg/kg bw per day (Gaunt et al., 1972), which is greater than
10 000 the daily per capita intake ('eaters only') of 46 and 29
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
Diacetyl (No. 408)
Groups of 15 male and 15 female weanling specific
pathogen-free-derived CFE rats were given 5 ml/kg bw of an aqueous
solution containing 0, 0.2, 0.6, 1.8, or 11% diacetyl by gavage for 90
days, providing daily doses of diacetyl calculated to be 0, 10, 30,
90, and 540 mg/kg bw. Body weight, food intake, and water consumption
were recorded weekly. Growth retardation and increased water
consumption were observed in animals at the high dose, the effects
being more pronounced in males. Haematological and urinary parameters
and enzyme activities were measured. Anaemia and increased
polymorphonuclear leukocytosis were observed in rats at the high dose
but were attributed to haemorrhage, infections, and ulcers of the
stomach. The relative weights of the liver, kidney, and pituitary and
adrenal glands were increased in these animals, and the increases were
greater than could be accounted for by the reduction in body weight.
Macroscopic and microscopic examination of all major organs revealed
ulcers in the squamous and glandular regions of the gastric mucosa.
The lesions observed in the squamous part of the stomach were
associated with hypertrophy or intercellular oedema. No histological
changes were seen in animals treated at lower doses. No other
significant differences were observed between treated and control
animals. The NOEL was 90 mg/kg bw per day (Colley et al., 1969), which
is 1500 times the daily per capita intake ('eaters only') of 56
µg/kg bw from its use as a flavouring agent in Europe and 500 times
the intake of 130 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Hexanedione (No. 413)
Groups of 10-16 male and female Charles River CD rats were housed
in pairs of the same sex and fed for 90 days on a basal diet alone or
supplemented with 3,4-hexanedione for an average daily intake of
17 mg/kg bw. Body weight and food consumption were measured daily and
were considered normal. Haematological examinations and blood urea
determinations were performed on 50% of the animals at week 7 and at
the end of treatment: no significant differences were seen between
treated and control animals. At necropsy, the liver and kidney weights
were normal, and gross and histological examination performed on a
wide range of organs revealed no dose-related lesions. The NOEL was 17
mg/kg bw per day (Posternak et al., 1969), which is more than 30 000
the daily per capita intake ('eaters only') of 0.5 and 0.01 µg/kg bw
from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
Methylcyclopentenolone (No. 418)
Groups of 15 male and 15 female rats aged four to five weeks
(strain not specified) were placed on a diet containing 0 or 1%
methylcyclo-pentenolone, equivalent to a daily intake of 0 or 500
mg/kg bw, for six months. Each animal was weighed twice weekly and
observed frequently for gross appearance and behaviour. The tissues of
most animals that died or were killed during the course of the study
and of all those kiled at the end of the study were examined grossly.
Tissues of representative animals from the control and treated groups
(numbers unspecified) were examined microscopically, and the weights
of the lungs, heart, liver, kidneys, spleen, and testes were recorded.
Haematological parameters were measured in representative animals from
the control and treated groups (numbers not specified) at the end of
the experiment. There were no statistically significant differences
between treated and control animals in any of the parameters measured.
The NOEL was 500 mg/kg bw per day (Dow Chemical Co., 1953), which is
more than 30 000 the daily per capita intake ('eaters only') of 15
and 12 µg/kg bw from its use as a flavouring agent in Europe and the
United States, respectively (see Table 2).
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
Groups of 10 male and 10 female Charles River CD rats were fed
diets providing 3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses of 0,
100, 200, or 400 mg/kg bw per day for 91 days. No adverse effects were
seen on behaviour, body weight, food consumption, haematological,
urinary, or ophthalmological parameters, or gross or histopathological
appearance. The NOEL was 400 mg/kg bw per day (King et al., 1979),
which is more than 50 000 the daily per capita intake ('eaters
only') of 0.8 and 0.4 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
In a combined study of developmental toxicity and
carcinogenicity, three successive generations of male and
female Charles River CD-COBS rats received
3-ethyl-2-hydroxy-2-cyclopenten-1-one in the basal diet at doses of 0
(untreated control), 0 (propylene glycol control), 30, 80, or
200 mg/kg bw per day. The F0 generation was entered into the study
after weaning and was mated 64 days later. These animals were treated
for 12 months, during which time the females were given a supplemental
diet throughout gestation and lactation. The F1 generation was
initially exposed in utero, subsequently via the dams' milk until
weaning, and then treated for two years and bred twice (at days 99 and
155) to produce F2a and F3a generations. The F0 and F2 generations
consisted of 40 animals of each sex in the untreated control group and
20 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups. In the F1
generation, there were 100 animals of each sex in the untreated
control group and 50 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups.
Survival, clinical symptoms, food consumption, reproductive
perfor-mance, and haematological and clinical chemical parameters were
not adversely affected in the F0 or F1 generations. Slightly
depressed growth was reported in F1 females receiving the highest
dose, but the effect was not significant when compared with the growth
of controls receiving propylene glycol. Gross pathological and
histopathological examination of animals of these generations revealed
no significant treatment-related effects. An increased incidence of
bile-duct hyperplasia observed in these groups was not statistically
significant when compared with the incidence of in controls. The
incidence of benign or malignant tumours in treated animals was
similar to that in controls. The NOEL was 200 mg/kg bw per day (King
et al., 1979), which is more than 200 000 the daily per capita
intake ('eaters only') of 0.8 and 0.4 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
In a three-generation study of toxicity in Charles River
CD-COBS rats with 3-ethyl-2-hydroxy-2-cyclopenten-1-one, 120
F0 animals were entered into the study just after weaning and were
mated after 64 days, providing the F1 generation. Animals of the
F0 generation were selected at random to be maintained as controls
or were given 3-ethyl-2-hydroxy-2-cyclopenten-1-one in the diet.
The F1 generation was maintained on the diet for 755 days, during
which time they were mated twice (at days 99 and 155). The first
mating provided the rats for the F2 generation, and the second mating
provided rats which were sacrificed after weaning. The F2 generation
was treated for 84 days and then mated to produce the F3 generation.
Rats of all generations were maintained on a basal diet with
3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses equal to daily intakes
of 0, 30, 80, or 200 mg/kg bw. Interim clinical chemical analyses were
performed on the F0 generation at months 4 and 6. Ophthalmological,
clinical chemical, and haematological examinations were performed on
the F1 generation at months 6, 12, 18, and 24. At termination, both
the F0 and the F1 generations were submitted to clinical chemical,
haematological, and histopathological examinations. F2 adult animals
were killed without necropsy at the time of weaning of their
offspring.
No evidence of treatment-related effects was found, and the
general health of the rats was unaffected throughout treatment.
Mammary swellings in females and other subcutaneous nodules were
considered not to be related to treatment because they were equally
distributed among control and treated groups. No effects were seen on
food consumption, weight gain, or clinical chemical or haematological
parameters. In the F0 generation, there were no pathological findings
at the 12-month necropsy or at histopathological examination. In the
F1 generation, there were no macroscopic observations that could be
related to the treatment. Histologically, bile-duct hyperplasia was
seen in all groups, including controls; however, there was no
statistically significant difference between treated and control
groups and the findings were considered not to be related to
treatment. Only tumours usually found in rats of this strain and age
were observed, indicating the absence of treatment-related effects.
The NOEL in this study was 200 mg/kg bw per day (King et al., 1979),
which is more than 100 000 the daily per capita intake ('eaters
only') of 0.4 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Dimethylcyclopentane-1,2-dione (No. 420)
Groups of 10 male Charles River CD rats were fed diets containing
3,4-dimethyl-1,2-cyclopentadione at concentrations of 0, 400, 4000, or
13 000 mg/kg (equivalent to 0, 20, 200, and 640 mg/kg bw per day) for
90 days. Food consumption, body-weight gain, haematological
parameters, organ weights, and gross and microscopic appearance were
observed throughout the study. Animals at the intermediate and high
doses showed a moderate but consistent depression in food intake
throughout the experiment, and their final mean body weights were
about 10% less than those of controls. There were no consistent
indications of a profound disturbance of food conversion efficiency,
although minor increases in the food conversion ratio were seen in
some animals at the high dose. Haematological parameters, organ
weights, and gross and microscopic appearance were similar in treated
and control groups. The authors attributed the decreased body weight
observed at higher doses to the unpalatability of the test substance.
The NOEL was 200 mg/kg bw per day (Wheldon & Krajkeman, 1967), which
is more than 200 000 the daily per capita intake ('eaters only') of
0.8 and 0.04 µg/kg bw from its use as a flavouring agent in Europe and
the United States, respectively (see Table 2).
3,5-Dimethylcyclopentane-1,2-dione (No. 421)
Groups of 10 male Charles River CD rats were fed
3,5-dimethyl-1,2-cyclopenthexadione in the diet for 13 weeks at
concentrations of 0, 1000, or 10 000 mg/kg diet (equivalent to 50 and
500 mg/kg bw per day). A fourth group was fed a diet initially
containing 12 000 ppm (equivalent to 610 mg/kg bw per day), but the
level was increased to 24 000 mg/kg diet (equivalent to
1200 mg/kg bw per day) during week 6 through to the end of experiment.
Haematological examinations were performed on five animals from each
group just before the end of the 13-week period; organ weight analysis
and gross and histopathological examinations at the end of the study
revealed no significant differences between control and treated
animals. The food consumption of animals at the two highest doses was
reduced throughout the experiment, accompanied by decreased weight
gain, which resulted in mean body weights that were 10-17% lower than
those of controls at the end of the study. The authors attributed the
decrease to the unpalatability of the test substance. The NOEL was 50
mg/kg bw per day (Wheldon & Krajkeman, 1967), which is more than
50 000 the daily per capita intake ('eaters only') of 0.9 and 0.5
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
2-Hydroxy-2-cyclohexen-1-one (No. 424)
2-Hydroxy-2-cyclohexen-1-one was added to the diet of 15 male and
15 female albino Wistar rats for 90 days at a concentration calculated
to result in an average daily intake of 5 mg/kg bw. Detailed
measurements of haematological parameters, blood chemistry, urine,
gross and histopathological appearance, organ weights, body weight,
and food consumption revealed no statistically significant difference
between treated and control groups (Morgareidge et al., 1974). The
NOEL was 5 mg/kg bw per day (Cox, 1974), which is more than 500 000
the daily per capita intake ('eaters only') of 0.001 and 0.01 µg/kg
bw from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
1-Methyl-2,3-cyclohexadione (No. 425)
Groups of 10 male Charles River CD rats were fed
1-methyl-2,3-cyclohexadione in the diet at concentrations of 0, 100,
or 1000 mg/kg (equivalent to 0, 5, and 50 mg/kg bw per day) for 13
weeks. A fourth group was fed a diet initially containing 13 500 mg/kg
diet (equivalent to 675 mg/kg bw per day), which was raised to 27 000
mg/kg bw (equivalent to 1350 mg/kg bw per day) during week
6. Observation of haematological parameters, organ weights, and gross
and histopathological appearance revealed no difference between
treated and control groups. The food consumption of rats at the high
dose was reduced throughout the experiment, accompanied by decreased
body-weight gain, and the effect was more marked when the dietary
concentration was raised. The NOEL was 50 mg/kg bw per day (Wheldon &
Krajkeman, 1967), which is more than 500 000 the daily per capita
intake ('eaters only') of 0.03 and 0.1 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
2.3.2.3 Genotoxicity
The available studies of genotoxicity are summarized in Table 5.
Most of the studies were carried out with Salmonella typhimurium in
the Ames test. Acetoin, diacetyl, and 1,2-cyclohexanedione showed some
mtagenicity in strains TA100 and TA104. As the mutation frequencies
were low and the positive results were always accompanied by negative
results, the overall conclusion was that this group of substances does
not induce gene mutation in bacteria in vitro. Diacetyl did not
induce mutation in Saccharomyces cerevisiae (US Food & Drug
Administration, 1974). Methylcyclopentenolone did not induce
unscheduled DNA synthesis in rat hepatocytes (Heck et al., 1989).
2.3.2.4 Other relevant studies
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
In the three-generation, 23-month study of toxicity described
above, treatment had no effect on clinical signs, weight gain, food
consumption, copulation rates, or mating behaviour of males or
females, fertility index, length of gestation, parturition, litter
size, or incidence of stillbirth. The growth and survival rates of
pups during lactation were normal. Gross examination of all offspring
from birth to weaning revealed no significant treatment-related
malformations or lesions (King et al., 1979).
Diacetyl (No. 408)
Groups of 25-27 Syrian golden hamsters, 21-24 CD-1 mice, and
21-23 albino Wistar rats were given a solution containing 90%
diacetyl by gavage on days 6-10 of gestation for hamsters and days
6-15 of gestation for mice and rats. The doses for all species were
16, 74, 345, or 1600 mg/kg bw per day. No effects were seen on
maternal survival, weight, or reproductive parameters or on fetal
survival or microscopic appearance of external, skeletal, or soft
tissues (US Food & Drug Administration, 1973).
4. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon,
R. (1989) Contribution of coffee aroma constituents to the
mutagenicity of coffee. Food Chem. Toxicol., 27, 227-232.
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Table 4. Short-term and long-term studies of toxicity in rats with aliphatic acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones used as flavouring agents
Substance No. Sex No. groups/ Route Time NOEL Reference
no. per group (days) (mg/kg bw
per day)
Acetoin 405 M/F 3/30 Drinking-water 90 330 Gaunt et al. (1972)
Diacetyl 408 M/F 4/30 Gavage 90 90 Colley et al. (1969)
3,4-Hexanedione 413 M/F 2/10-16 Diet 90 17a Posternak et al. (1969)
Methylcyclopentenolone 418 M/F 1/30 Diet 185 500a Dow Chemical Co. (1953)
3-Ethyl-2-hydroxy-2-cyclopenten-1-one 419 M/F 3/20 Diet 91 400a King et al. (1979)
M/F 3/100 Diet 730 200 King et al. (1979)
3,4-Dimethyl-1,2-cyclopentadione 420 M 3/10 Diet 90 645 Wheldon & Krajkeman (1967)
3,5-Dimethyl-1,2-cyclopentadione 421 M 3/10 Diet 90 610a Wheldon & Krajkeman (1967)
2-Hydroxy-2-cyclohexen-1-one 424 M/F 1/30 Diet 90 5a Morgareidge et al. (1974)
1-Methyl-2,3-cyclohexadione 425 M 3/10 Diet 90 675-1350a Wheldon & Krajkeman (1967)
M, male; F, female
a The study was performed at a single or multiple doses that had no adverse effects; therefore, no NOEL was determined.
The NOEL is probably higher than the dose reported to have no adverse effects.
Table 5. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones
used as flavouring agents
Substance No. End-point Test object Dose Results Reference
Acetoin 405 Reverse mutation S. typhimurium TA100 < 4500 mg/plate Negativea Garst et al. (1983)
Positiveb
Reverse mutation S. typhimurium TA100 420 mg/plate Negativeb Kim et al. (1987)
Diacetyl 408 Reverse mutation S. typhimurium TA100, Not reported Positivea,c Kato et al. (1989)
(modified test) TA104; E. coli
Reverse mutation S. typhimurium TA104 530 µg/plate Positived Marnett et al. (1985)
(modified test)
Reverse mutation S. typhimurium TA100 90 µg/plate Negativee Kim et al. (1987)
Reverse mutation S. typhimurium TA104 5-500 µg/platef Positivea,c Shane et al. (1988)
Negativeb
Reverse mutation S. typhimurium TA102, 5-500 µg/platef Negativee Shane et al. (1988)
TA100
Reverse mutation S. typhimurium TA100 152-950 µg/plate Negativeb Dorado et al. (1992)
Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA1535, 1% Negativee US Food & Drug
(suspension test) TA1537, TA1538 Administration (1974)
Reverse mutation S. typhimurium TA102 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
Reverse mutation S. typhimurium TA98, 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
TA100
Reverse mutation S. typhimurium TA100 2 or 4 mmol/plate Positiveg Suwa et al. (1982)
Mutation S. cerevisiae Not reported Negativee US Food & Drug
Administration (1974)
2,3-Pentanedione 410 Reverse mutation S. typhimurium TA100 105 µg/plate Negativeb Kim et al. (1987)
Reverse mutation S. typhimurium TA100, 0.9-90 000 µg/plate Negativee Aeschbacher et al. (1989)
3,4-Hexanedione 413 Reverse mutation S. typhimurium TA100 228-4900 µg/plate Negativeb Dorado et al. (1992)
Table 5. (continued)
Substance No. End-point Test object Dose Results Reference
Methylcyclopentenolone 418 Reverse mutation S. typhimurium TA1535, 10 000 µg/plate Negativee Heck et al. (1989)
TA1537, TA1538, TA98,
TA100
Unscheduled DNA Rat hepatocytes 500 µg/plate Negativea Heck et al. (1989)
synthesis
2-Hydroxy-2-cyclohexen-1-one 424 Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA100 112-1000 µg/plate Negativeb Dorado et al. (1992)
a With metabolic activation
b Without metabolic activation
c Mutation frequency, 2-3
d Result based on authors' criterion for significant mutagenic effect, i.e. mutation frequency > 1.5
e With and without metabolic activation
f Estimated from graph
g Mutation frequency, < 2.5
Bjeldanes, L.F. & Chew, H. (1979) Mutagenicity of 1,2-dicarbonyl
compounds: Maltol, kojic acid, diacetyl and related substances.
Mutat. Res., 67, 367-371.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxication, New York, Academic
Press, Vol. I, pp. 231-248.
Colley, J., Gaunt, I.F., Lansdown, A.B.G., Grasso, P. & Gangolli, S.D.
(1969) Acute and short-term toxicity of diacetyl in rats. Food Chem.
Toxicol., 7, 571-580.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, J. & Hullin, R.P. (1954) Metabolism of acetoin. Formation and
utilization of acetoin and 2,3-butanediol in the decerebrated cat.
Biochem. J., 57, 177-180.
Dix & Jeffcoat (1997) Disposition and excretion of
[14C]2,3-butanedione in male rats following oral administration.
Unpublished report from Research Triangle Institute (NIEHS contract
No. NO1-ES-75407). Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Dorado, L., Montoya, M.R. & Rodriguez-Mellado, J.M. (1992) A
contribution to the study of the structure-mutagenicity relationship
for alpha-dicarbonyl compounds using the Ames test. Mutat. Res.,
269, 301-306.
Dow Chemical Co. (1953) Toxicity of cyclotene--Summary. Unpublished
report from Biochemical Research Department, Midland, Michigan, 15
July. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Gabriel, M.A., Jabara, H. & Al-Khalidi, A.A. (1971) Metabolism of
acetoin in mammalian liver slices and extracts. Biochem. J., 124,
793-800.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Garst, J., Stapleton, P. & Johnson, J. (1983) Mutagenicity of
alpha-hydroxy ketones may involve superoxide anion radical. Oxy
Radicals and Their Scavenger Systems, Amsterdam, Elsevier Science
Publishing, Vol. 2, pp. 125-130.
Gaunt, I.F., Branton, P.G., Kiss, I.S., Grasso, P. & Gangolli, S.D.
(1972) Short-term toxicity of acetoin (acetyl methylcarbinol) in rats.
Food Cosmet. Toxicol., 10, 131-141.
Givaudan Corp. (1952) Acute oral toxicity study of
methylcyclopentenolone in mice. Unpublished report to the Research
Institute of Fragrance Materials. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Gordon, A.J. & Ford, R.A. (1972) The Chemist's Companion, New York,
John Wiley & Sons, pp. 49-51.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Järnefelt, J. (1955) Studies on the enzymatic synthesis and breakdown
of acetoin in the animal organism. Ann. Acad. Sci. Fenn. Ser.
A. V. Med Anthropol., 57, 7-78.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Juni, E. & Heym, G.A. (1956) A cyclic pathway for the bacterial
dissimilation of 2,3-butanediol, acetyl methyl carbinol, and diacetyl.
J. Bacteriol., 71, 425-432.
Kato, F., Araki, A., Nozaki, K. & Matsushi, T. (1989) Mutagenicity of
aldehydes and diketones. Mutat. Res., 216, 366-367.
Kawano, T. (1959) Relation of acetoin and pantothenic acid. Fukuoka
Igaku Zasshi, 50, 2939-2953.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic
heterocyclic amines. Mutat. Res., 177, 9-15.
King, T., Faccini, J.M., Jachbaur, J., Perraud, J. & Monro, A.M.
(1979) 3-Generation and chronic toxicity study in rats. Unpublished
report from Pfizer Central Research. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Leberco Labs (1952) Acute toxicity of methylcyclopentenolone.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mills, G.A. & Walker, V. (1990) Urinary excretion of cyclohexanediol,
a metabolite of the solvent cyclohexanone, by infants in a special
care unit. Clin. Chem., 36, 870-874.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1977) Acute toxicity studies in rats, rabbits and guinea
pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1979) Acute toxicity studies in rats, rabbits and
guinea-pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974) 90 Day feeding study
with 2-hydroxy-2-cyclohexen-1-one in rats. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Ong, C.N., Sia, G.L., Chia, S.E., Phoon, W.H. & Tan, K.T. (1991)
Determination of cyclohexanol in urine and its use in environmental
monitoring of cyclohexanone exposure. J. Anal. Toxicol., 15, 13-16.
Otsuka, M., Mine, T., Ohuchi, K. & Ohmori, S. (1996) A detoxication
route for acetaldehyde: Metabolism of diacetyl, acetoin, and
2,3-butanediol in liver homogenate and perfused liver of rats.
J. Biochem., 119, 246-251.
Pellmont, D. (1969) Studies with rats and mice on substance No.
R01-3801 (Toxikologisches Labor 256, Bau 69). Unpublished report to
the Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological test on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102 and TA104. Environ. Mol. Mutag., 11,
195-206.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Suwa, Y., Nagao, M., Kosugi, A. & Sugimura, T. (1982) Sulfite
suppresses the mutagenic property of coffee. Mutat. Res., 102,
383-391.
US Food & Drug Administration (1973) Teratologic Evaluation of FDA
71-73 (Starter Distillate, Hansen) (NTIS PB-223-833;
FDABF-GRAS-152), Washington DC, United States.
US Food & Drug Administration (1974) Mutagenic Evaluation of
Compound FDA 71-73 (Starter Distillate) (NTIS PB-245-431;
FDABF-GRAS-275), Washington DC, United States.
US Food & Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1970) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1982) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
Westerfeld, W.W. & Berg, R.L. (1943) Observations on the metabolism of
acetoin. J. Biol. Chem., 148, 523-528.
Wheldon, G.H. & Krajkeman, A.J. (1967) The effects of ten
food-flavoring additives administered to rats over a period of
thirteen weeks. Unpublished report from Huntington Research Center.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Williams, T.R. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 62, 78.
Zlatkis, A. & Sivetz, M. (1960) Analysis of coffee volatiles by gas
chromatography. Food Res., 25, 395-398.
See Also:
Toxicological Abbreviations
 2-Acetoxy-3-butanone 406 4906-24-5 0.03/23 No N/R2 N/R2 No safety
concern
2-Acetoxy-3-butanone 406 4906-24-5 0.03/23 No N/R2 N/R2 No safety
concern
 Butan-3-one-2-yl-butanoate 407 84642-61-5 0.02/0.95 No N/R2 N/R2 No safety
concern
Butan-3-one-2-yl-butanoate 407 84642-61-5 0.02/0.95 No N/R2 N/R2 No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Diacetyl 408 431-03-8 3300/8000 Yes Yes N/R1 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Diacetyl 408 431-03-8 3300/8000 Yes Yes N/R1 No safety
concern
 3-Hydroxy-2-pentanone 409 3142-66-3 ND/0.10 No N/R2 N/R2 No safety
concern
3-Hydroxy-2-pentanone 409 3142-66-3 ND/0.10 No N/R2 N/R2 No safety
concern
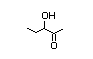 2,3-Pentanedione 410 600-14-6 220/80 No N/R2 N/R2 No safety
concern
2,3-Pentanedione 410 600-14-6 220/80 No N/R2 N/R2 No safety
concern
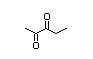 4-Methyl-2,3-pentadione 411 7493-58-5 0.48/2 No N/R2 N/R2 No safety
concern
4-Methyl-2,3-pentadione 411 7493-58-5 0.48/2 No N/R2 N/R2 No safety
concern
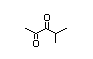 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
2,3-Hexadione 412 3848-24-6 13/10 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
2,3-Hexadione 412 3848-24-6 13/10 No N/R2 N/R2 No safety
concern
 3,4-Hexadione 413 4437-51-8 33/0.76 No N/R2 N/R2 No safety
concern
3,4-Hexadione 413 4437-51-8 33/0.76 No N/R2 N/R2 No safety
concern
 5-Methyl-2,3-hexadione 414 13706-86-0 2/6 No N/R2 N/R2 No safety
concern
5-Methyl-2,3-hexadione 414 13706-86-0 2/6 No N/R2 N/R2 No safety
concern
 2,3-Heptanedione 415 96-04-8 2/5 No N/R2 N/R2 No safety
concern
2,3-Heptanedione 415 96-04-8 2/5 No N/R2 N/R2 No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Hydroxy-4-octanone 416 496-77-5 0.02/0.76 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Hydroxy-4-octanone 416 496-77-5 0.02/0.76 No N/R2 N/R2 No safety
concern
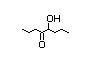 2,3-Undecadione 417 7493-59-6 0.02/0.01 No N/R2 N/R2 No safety
concern
2,3-Undecadione 417 7493-59-6 0.02/0.01 No N/R2 N/R2 No safety
concern
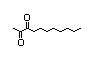 Methylcyclopentenolone 418 80-71-7 890/710 Yes No Yes. The dose of No safety
500 mg/kg bw per concern
day that had no
adverse effects
(Dow Chemical Co.,
1953) is > 10 000
times the intake.
Methylcyclopentenolone 418 80-71-7 890/710 Yes No Yes. The dose of No safety
500 mg/kg bw per concern
day that had no
adverse effects
(Dow Chemical Co.,
1953) is > 10 000
times the intake.
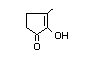 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Ethylcyclopentenolone 419 21835-01-8 50/23 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
Ethylcyclopentenolone 419 21835-01-8 50/23 No N/R2 N/R2 No safety
concern
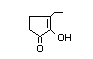 3,4-Dimethyl-1,2-cyclopentadione 420 13494-06-9 47/2 No N/R2 N/R2 No safety
concern
3,4-Dimethyl-1,2-cyclopentadione 420 13494-06-9 47/2 No N/R2 N/R2 No safety
concern
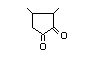 3,5-Dimethyl-1,2-cyclopentadione 421 13494-07-0 55/29 No N/R2 N/R2 No safety
concern
3,5-Dimethyl-1,2-cyclopentadione 421 13494-07-0 55/29 No N/R2 N/R2 No safety
concern
 3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one 422 42348-12-9 ND/0.17 No N/R2 N/R2 No safety
concern
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one 422 42348-12-9 ND/0.17 No N/R2 N/R2 No safety
concern
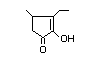 Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one 423 53263-58-4 ND/0.38 No N/R2 N/R2 No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated Step A3 Step A4 Step A5 Conclusion
per capita Does intake Endogenous? Adequate based on
intake exceed NOAEL for current
Europe/USA intake substance intake
(µg/day) threshold? or related
substance?
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one 423 53263-58-4 ND/0.38 No N/R2 N/R2 No safety
concern
 2-Hydroxy-2-cyclohexen-1-one 424 10316-66-2 0.08/0.76 No N/R2 N/R2 No safety
concern
2-Hydroxy-2-cyclohexen-1-one 424 10316-66-2 0.08/0.76 No N/R2 N/R2 No safety
concern
 1-Methyl-2,3-cyclohexadione 425 3008-43-3 2/8 No N/R2 N/R2 No safety
concern
1-Methyl-2,3-cyclohexadione 425 3008-43-3 2/8 No N/R2 N/R2 No safety
concern
 2-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one 426 4883-60-7 2/2 No N/R2 N/R2 No safety
concern
2-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one 426 4883-60-7 2/2 No N/R2 N/R2 No safety
concern
 N/R1, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A4 of the procedure.
N/R2, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure.
N/D, no intake data reported.
1.6 Conclusions
The 22 aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones evaluated do not pose a safety concern at
current levels of intake as flavouring agents. No data on toxicity
were required for application of the procedure to 21 of the aliphatic
acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones. The Committee noted that the available data on
toxicity were consistent with the results of the safety evaluations
using the procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This group of substances was selected on the basis of the
criteria that all members of the group are aliphatic and contain
functional groups (alpha-diketones, alpha-hydroxyketones, and esters
of a methylcyclopentenolone hydroxyketones) which have similar
chemical reactivity and participate in common pathways of metabolic
detoxification. Acyclic and alicyclic alpha-diketones exist to varying
degrees in equilibrium with unsaturated alpha-hydroxyketones (i.e. the
ketoenolic form). Therefore, 17 of the 22 substances in this group
exist as mixtures of alpha-diketone and unsaturated
alpha-hydroxyketone. Three (Nos 405, 409, and 416) other members of
the group are aliphatic alpha-hydroxyketones which may be formed
in vivo by simple reduction of one of the two ketone functions in
the corresponding alpha-diketone. The two remaining members of the
group (Nos 406 and 407) are aliphatic esters of the
alpha-hydroxyketone acetoin (No. 405). Hydrolysis of these esters
in vivo yields acetoin (No. 405) and simple aliphatic carboxylic
acids. Consequently, it can be assumed that all of these flavouring
agents either are, or can readily form, alpha-diketones or
alpha-hydroxyketones (see Figure 1). In view of the close chemical and
biochemical relationships between these substances and the consistent
data on toxicity, they are evaluated here as a group of structurally
related compounds.
2.2 Intake
The total annual production volume of the 22 substances in this
group is approximately 44 000 kg in Europe (International Organization
of the Flavor Industry, 1995) and 56 000 kg in the United States (US
National Academy of Sciences, 1970, 1982, 1987). Production volumes
and intake values for each substance are reported in Table 2. On the
basis of the annual volumes reported in Europe and the United States,
the total estimated daily per capita intake ('eaters only') of the
22 flavouring agents in this group is 120 µg/kg bw per day in Europe
and 180 µg/kg bw per day in the United States.
N/R1, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A4 of the procedure.
N/R2, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure.
N/D, no intake data reported.
1.6 Conclusions
The 22 aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones evaluated do not pose a safety concern at
current levels of intake as flavouring agents. No data on toxicity
were required for application of the procedure to 21 of the aliphatic
acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones. The Committee noted that the available data on
toxicity were consistent with the results of the safety evaluations
using the procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This group of substances was selected on the basis of the
criteria that all members of the group are aliphatic and contain
functional groups (alpha-diketones, alpha-hydroxyketones, and esters
of a methylcyclopentenolone hydroxyketones) which have similar
chemical reactivity and participate in common pathways of metabolic
detoxification. Acyclic and alicyclic alpha-diketones exist to varying
degrees in equilibrium with unsaturated alpha-hydroxyketones (i.e. the
ketoenolic form). Therefore, 17 of the 22 substances in this group
exist as mixtures of alpha-diketone and unsaturated
alpha-hydroxyketone. Three (Nos 405, 409, and 416) other members of
the group are aliphatic alpha-hydroxyketones which may be formed
in vivo by simple reduction of one of the two ketone functions in
the corresponding alpha-diketone. The two remaining members of the
group (Nos 406 and 407) are aliphatic esters of the
alpha-hydroxyketone acetoin (No. 405). Hydrolysis of these esters
in vivo yields acetoin (No. 405) and simple aliphatic carboxylic
acids. Consequently, it can be assumed that all of these flavouring
agents either are, or can readily form, alpha-diketones or
alpha-hydroxyketones (see Figure 1). In view of the close chemical and
biochemical relationships between these substances and the consistent
data on toxicity, they are evaluated here as a group of structurally
related compounds.
2.2 Intake
The total annual production volume of the 22 substances in this
group is approximately 44 000 kg in Europe (International Organization
of the Flavor Industry, 1995) and 56 000 kg in the United States (US
National Academy of Sciences, 1970, 1982, 1987). Production volumes
and intake values for each substance are reported in Table 2. On the
basis of the annual volumes reported in Europe and the United States,
the total estimated daily per capita intake ('eaters only') of the
22 flavouring agents in this group is 120 µg/kg bw per day in Europe
and 180 µg/kg bw per day in the United States.
 The vast majority of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones have been reported to
occur in traditional foods (Maarse et al., 1994). Quantitative data on
the natural occurrence of these substances, with the exception of
diacetyl, demonstrate that they are consumed predominantly from
traditional foods (i.e. consumption ratio > 1) (Stofberg & Kirschman,
1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
Aliphatic acyclic and alicyclic alpha-diketones participate in an
enol-keto equilibrium with the corresponding ketoenol (see Figure 1).
The enolic form predominates in alicyclic diketones, especially
cyclopentyl alpha-diketones (Gordon & Ford, 1972), essentially 100% of
which exist in the ketoenolic form; 50-80% of cyclohexyl
alpha-diketones exist in the ketoenolic form at equilibrium.
Increasing pH would be expected to shift the equilibrium in favour of
the ketoenolic form (i.e. alpha-hydroxyketone). Therefore, at
physiological pH, an increase in the alpha-hydroxyketone (i.e. enol)
form is expected.
In rats and mice, orally administered aliphatic alpha-diketones
are rapidly absorbed from the gastrointestinal tract (Gabriel et al.,
1972). Carbon dioxide produced primarily from methyl-substituted
diketones (e.g. diacetyl) is eliminated in expired air. Thus, after
injection of [2,3-14C]-acetoin to whole albino rats, 14C-carbon
dioxide appeared in expired air, with an average 12-h production of
15% (Gabriel et al., 1972). In rats, 54-82% of orally administered
radiolabelled 2,3-butanedione was excreted as carbon dioxide. At
increasing doses, the percent of the dose excreted as carbon dioxide
decreased, while urinary excretion of radiolabel increased, suggesting
that saturation occurs at higher doses. Chromatographic analysis of
urine samples yielded three major 14C-labelled components, one of
which co-eluted with uric acid. Analysis with ß-glucuronidase or
sulfatase decreased the amount of radiolabel present in one peak, with
a corresponding increase in the amount present in another major
component. Identification of urinary metabolites was not pursued owing
to the extensive excretion of 14C as carbon dioxide (Dix & Jeffcoat,
1997). In general, esters are hydrolysed to their corresponding
alcohols and carboxylic acids. Hydrolysis is catalysed by classes of
enzymes recognized as carboxylesterases or esterases, the most
important of which are the B-esterases. Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). These enzymes
occur in most mammalian tissues (Heymann, 1980; Anders, 1989) but
predominate in hepatocytes (Heymann, 1980). 2-Acetoxy-3-butanone (No.
406) and butanon-3-one-2-yl butanoate (No. 407) are expected to be
metabolized in humans to acetic acid and butanoic acid, respectively,
and to acetoin.
Aliphatic acyclic diketones
The metabolic fate of acyclic aliphatic diketones depends
primarily on the position of the carbonyl function and on chain
length. Aliphatic acyclic diketones and alpha-hydroxyketones that
contain a carbonyl function at the 2 position (i.e. methyl ketones)
may undergo alpha-hydroxylation and subsequent oxidation of the
terminal methyl group to yield corresponding ketocarboxylic acids. The
ketoacids are intermediary metabolites (e.g. alpha-ketoacids), which
may undergo oxidative decarboxylation to yield carbon dioxide and a
simple aliphatic carboxylic acid. The acid may be completely
metabolized in the fatty acid pathway and citric acid cycle.
Alternatively, the methyl-substituted diketones may be
successively reduced to the corresponding hydroxyketones and diols,
which are excreted in the urine as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain ketones. If the carbonyl function is located elsewhere on
the chain, reduction is the predominant detoxification pathway.
alpha-Hydroxyketones and their diol metabolites may be excreted as
glucuronic acid conjugates. For example, the glucuronic acid conjugate
of cyclohexanol was detected in the urine of humans exposed to
cyclohexanone (Ong et al., 1991), indicating that alpha-hydroxylation
followed by reduction of the ketone function occurs in humans.
Acetoin is metabolized primarily via oxidation at low
concentrations in vivo and by reduction to 2,3-butanediol at high
concentrations. It has been estimated that 1 g of rat liver can
oxidize 86 µg (1 µmol) acetoin per day (Gabriel et al., 1972).
Production of carbon dioxide at low levels and of 2,3-butanediol at
high levels is associated with the slower rate of ketone reduction
(Williams, 1959). Oxidation of the terminal methyl group may result in
formation of an alpha-ketoacid, which undergoes cleavage to yield
carbon dioxide and a carboxylic acid fragment. Alternatively, methyl
group oxidation may yield a ß-ketoacid which undergoes ß-cleavage to
yield two-carbon fragments. To a minor extent, these two-carbon
fragments can act as acetyl donors for acetylation of
para-aminobenzoic acid (Westerfeld & Berg, 1943).
A total dose of 78 g of acetoin was administered to a dog over
two months, both orally in a 3-4% solution and subcutaneously in a 20%
solution. Urine was collected under toluene from the beginning of
treatment up to 40 h after the last dose. 2,3-Butanediol was the major
urinary excretion product, representing 5-25% of the dose. The
remainder of the dose was completely metabolized (Westerfeld & Berg,
1943).
In liver preparations obtained from rats and rabbits, more than
95% of the radiolabel of [2,3-14C]-acetoin was detected as a mixture
of stereoisomers of 2,3-butanediol (Gabriel et al., 1971). Although
reduction of diacetyl and acetoin has been observed in animals
in vivo and in animal tissue preparations in vitro at high
concentrations, it appears that oxidation of diacetyl is a major
endogenous metabolic pathway.
Reduction of ketones is mediated by alcohol dehydrogenase and
NADPH-dependent cytosolic carbonyl reductases (Bosron & Li, 1980).
Reduction of the endogenous substances acetoin (No. 405) and diacetyl
(No. 408) is catalysed by the substrate-specific enzymes diacetyl
reductase and acetoin reductase, respectively. In rat liver slices,
diacetyl, acetoin, and 2,3-butanediol are interconvertible (Gabriel et
al., 1972).
In male Wistar albino rats, a single oral dose of diacetyl at
5 mmol/kg bw (430 mg/kg bw) was metabolized by reduction to acetoin,
which was present at high concentrations in major organs 1 h after
dosing. The subsequent reduction product, 2,3-butanediol, was detected
in liver, kidney, and brain (Otsuka et al., 1996). Only a 10-min
incubation is required to convert 10 nmol (9 × 10-4 mg) diacetyl to
3.7 nmol (3 × 10-4 mg) acetoin and 6.3 nmol (6 × 10-4 mg)
2,3-butanediol in rat liver homogenate. Diacetyl was reduced by NADH-
and NADPH-dependent diacetyl reductase isolated from a homogenate of
male Wistar rat liver. The organ-specific reductase activity was
greatest in the liver and least in the brain (Otsuka et al., 1996).
Acetoin was given either orally in a 3-4% solution or
subcutaneously in a 20% solution to rats, multiple doses being spaced
equally throughout each day. The acetoin used was obtained as the
polymer (H, 15), which apparently reverts to an optically inactive
monomer in aqueous solution. No diacetyl was detected in the urine of
the rats; acetoin was not excreted to any appreciable extent in the
urine, and the major excretion product was 2,3-butanediol (Westerfeld
& Berg, 1943). In dogs, acetoin is excreted in the urine, in part as
2,3-butanediol. Most of an oral dose disappeared and was presumed to
be completely metabolized (Westerfeld & Berg, 1943). In another study,
2,3-butanediol readily conjugated glucuronic acid (Neuberg &
Gottshchalk, 1971).
Diacetyl and acetoin are endogenous in humans (Kawano, 1959;
Zlatkis & Sivetz, 1960). They are formed when pyruvate is converted to
acetoin and diacetyl by pyruvate decarboxylase (Gabriel et al.,
1972). Mean fasting blood concentrations of approximately 100 µg
acetoin per 100 ml blood have been reported (Dawson & Hullin, 1954).
Pyruvate also forms diacetyl in vitro in rat liver preparations
(Järnefelt, 1955) and in microorganisms (Juni & Heym, 1956).
Alicyclic alpha-diketones and alicyclic dicarbonyls
In general, alicyclic alpha-diketones are metabolized via a
reduction pathway (Williams, 1959). In humans, structurally related
alicyclic monoketones are reduced to the corresponding alcohols or
undergo alpha-hydroxylation and reduction to yield diols, which are
excreted as the glucuronic acid conjugates. For example, the
glucuronic acid conjugate of cyclohexanol is present in the urine of
humans exposed to atmospheres containing cyclohexanone at
concentrations of 2-30 ppm (Ong et al., 1991). A mixture of diols,
including cis- and trans-1,2-cyclohexanediol,1,3-cyclohexanediol,
and 1,4-cyclohexanediol, was detected in the urine of infants exposed
to cyclohexanone present in dextrose solutions used for intravenous
feeding. Presumably, the cyclohexanone undergoes alpha-hydroxylation
and then reduction of the ketone function to yield the corresponding
alpha-cyclohexanediols (Mills & Walker, 1990).
On the basis of the studies described above, it is anticipated
that humans will metabolize low concentrations of aliphatic acyclic
methyl ketones principally by oxidation of the terminal methyl group.
At higher concentrations, reduction to the diol and subsequent
conjugation with glucuronic acid form a competing detoxification
pathway. Other aliphatic acyclic alpha-diketones and
alpha-hydroxyketones and alicyclic diketones and hydroxyketones are
reduced, conjugated with glucuronic acid, and excreted.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
The results of studies of the acute toxicity of seven of the 22
diketones, hydroxyketones, and alicyclic dicarbonyls have been
reported and are summarized in Table 3. The low acute toxicity of the
group is demonstrated by oral LD50 values in the range 990 to > 8000
mg/kg bw.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones as flavouring substances
in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Acetoin (405)
Europe 19 000 2800 46
United States 9 000 1800 29
2-Acetoxy-3-butanone (406)
Europe 0.2 0.03 0.0005
United States 120 23 0.4
Butan-3-one-2-yl-butanoate (407)
Europe 0.1 0.02 0.0003
United States 5 0.95 0.02
Diacetyl (408)
Europe 18 000 3300 56
United States 42 000 8000 133
3-Hydroxy-2-pentanone (409)
Europe NR ND 0
United States 0.5 0.10 0.002
2,3-Pentanedione (410)
Europe 1 100 220 4
United States 420 80 1
4-Methyl-2,3-pentadione (411)
Europe 2.5 0.48 0.01
United States 12 2 0.04
2,3-Hexanedione (412)
Europe 70 13 0.22
United States 50 10 0.2
3,4-Hexanedione (413)
Europe 170 33 0.5
United States 4 0.76 0.01
5-Methyl-2,3-hexanedione (414)
Europe 9 2 0.03
United States 30 6 0.10
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2,3-Heptanedione (415)
Europe 8 2 0.03
United States 26 5 0.08
5-Hydroxy-4-octanone (416)
Europe 0.1 0.02 0.0003
United States 4 0.76 0.01
2,3-Undecadione (417)
Europe 0.03 0.01 0.0001
United States 0.1 0.02 0.0003
Methylcyclopentenolone (418)
Europe 4 700 890 15
United States 3 700 710 12
3-Ethylcyclopentenolone (419)
Europe 260 50 0.8
United States 120 23 0.4
3,4-Dimethyl-1,2-cyclopentanedione (420)
Europe 250 47 0.8
United States 13 2 0.04
3,5-Dimethyl-1,2-cyclopentanedione (421)
Europe 290 55 0.9
United States 150 29 0.5
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one (422)
Europe NR ND ND
United States 0.9 0.17 0.003
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one (423)
Europe NR ND ND
United States 2 0.38 0.01
2-Hydroxy-2-cyclohexen-1-one (424)
Europe 0.4 0.08 0.001
United States 4 0.76 0.01
1-Methyl-2,3-cyclohexadione (425)
Europe 11 2 0.03
United States 44 8 0.1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2-Hydroxy-3,5,5-trimethyl-2-cyclohexenone (426)
Europe 10 2 0.03
United States 8 2 0.03
Total
Europe 44 000
United States 56 000
NR, not reported; ND, not determined
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only') = 32 × 106 for Europe and
32 × 106 for the United States; 0.6 represents the assumption that
only 60% of the flavour volume was reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight],
where body weight = 60 kg. Slight variation may occur from rounding off.
Table 3. Studies of acute toxicity with aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones used as flavouring substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
Acetoin 405 Rat NR Oral > 5000 Moreno (1977)
Butan-3-one-2-yl 407 Mouse, rat NR Oral > 8000 Pellmont (1969)
butanoate
Diacetyl 408 Rat M Gavage 3400 Colley et al.(1969)
F 3000
Rat NR Gavage 1580 Jenner et al.(1964)
Guinea-pig NR Gavage 990 Jenner et al.(1964)
2,3-Pentanedione 410 Rat NR Oral 3000 Moreno (1977)
2,3-Hexanedione 412 Rat NR Oral > 5000 Moreno (1977)
5-Methyl-2,3-hexanedione 414 Rat NR Oral > 5000 Moreno (1979)
Methylcyclopentenolone 418 Mouse NR Gavage 1350 Givadaun Corp.(1952)
Rat NR Oral 1850 Moreno (1976)
Guinea-pig NR Gavage 1400 Dow Chemical Co.
(1953)
Mouse NR Oral 1350 Leberco Labs (1952)
NR, not reported; M, male; F, female
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The toxicity of nine of the 22 substances has been studied. The
results are summarized in Table 4 and described below.
Acetoin (No. 405)
Groups of 15 male and 15 female CFE rats were given acetoin in
their drinking-water at concentrations of 0 (control), 750, 3000, or
12 000 mg/kg (equivalent to 0, 85, 330, or 1300 mg/kg bw per day (US
Food & Drug Administration, 1993)). No animals died during the study,
and their condition and appearance were normal. The body weights of
males at 12 000 mg/kg in drinking-water decreased significantly from
week 5, and at weeks 2, 6, and 13 the relative weight of the liver was
statistically significantly greater in these animals than in controls.
A similar effect was seen in female rats, but only after 13 weeks.
Haematological examination conducted at 13 weeks showed a small (4-8%)
but statistically significant (p < 0.05) decrease in haemoglobin
concentration and erythrocyte counts in animals of each sex at the
high dose, but these changes were not accompanied by a decrease in
haematocrit. Urinalysis and blood chemical determinations performed at
the end of the study on all animals revealed no statistically
significant differences between treated and control groups.
Histopathological examination also revealed no adverse effects. The
authors suggested that the increased relative liver weights were a
reaction of the liver to an increased metabolic load resulting from
the high intake of acetoin. The NOEL was 3000 ppm, equivalent to
330 mg/kg bw per day (Gaunt et al., 1972), which is greater than
10 000 the daily per capita intake ('eaters only') of 46 and 29
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
Diacetyl (No. 408)
Groups of 15 male and 15 female weanling specific
pathogen-free-derived CFE rats were given 5 ml/kg bw of an aqueous
solution containing 0, 0.2, 0.6, 1.8, or 11% diacetyl by gavage for 90
days, providing daily doses of diacetyl calculated to be 0, 10, 30,
90, and 540 mg/kg bw. Body weight, food intake, and water consumption
were recorded weekly. Growth retardation and increased water
consumption were observed in animals at the high dose, the effects
being more pronounced in males. Haematological and urinary parameters
and enzyme activities were measured. Anaemia and increased
polymorphonuclear leukocytosis were observed in rats at the high dose
but were attributed to haemorrhage, infections, and ulcers of the
stomach. The relative weights of the liver, kidney, and pituitary and
adrenal glands were increased in these animals, and the increases were
greater than could be accounted for by the reduction in body weight.
Macroscopic and microscopic examination of all major organs revealed
ulcers in the squamous and glandular regions of the gastric mucosa.
The lesions observed in the squamous part of the stomach were
associated with hypertrophy or intercellular oedema. No histological
changes were seen in animals treated at lower doses. No other
significant differences were observed between treated and control
animals. The NOEL was 90 mg/kg bw per day (Colley et al., 1969), which
is 1500 times the daily per capita intake ('eaters only') of 56
µg/kg bw from its use as a flavouring agent in Europe and 500 times
the intake of 130 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Hexanedione (No. 413)
Groups of 10-16 male and female Charles River CD rats were housed
in pairs of the same sex and fed for 90 days on a basal diet alone or
supplemented with 3,4-hexanedione for an average daily intake of
17 mg/kg bw. Body weight and food consumption were measured daily and
were considered normal. Haematological examinations and blood urea
determinations were performed on 50% of the animals at week 7 and at
the end of treatment: no significant differences were seen between
treated and control animals. At necropsy, the liver and kidney weights
were normal, and gross and histological examination performed on a
wide range of organs revealed no dose-related lesions. The NOEL was 17
mg/kg bw per day (Posternak et al., 1969), which is more than 30 000
the daily per capita intake ('eaters only') of 0.5 and 0.01 µg/kg bw
from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
Methylcyclopentenolone (No. 418)
Groups of 15 male and 15 female rats aged four to five weeks
(strain not specified) were placed on a diet containing 0 or 1%
methylcyclo-pentenolone, equivalent to a daily intake of 0 or 500
mg/kg bw, for six months. Each animal was weighed twice weekly and
observed frequently for gross appearance and behaviour. The tissues of
most animals that died or were killed during the course of the study
and of all those kiled at the end of the study were examined grossly.
Tissues of representative animals from the control and treated groups
(numbers unspecified) were examined microscopically, and the weights
of the lungs, heart, liver, kidneys, spleen, and testes were recorded.
Haematological parameters were measured in representative animals from
the control and treated groups (numbers not specified) at the end of
the experiment. There were no statistically significant differences
between treated and control animals in any of the parameters measured.
The NOEL was 500 mg/kg bw per day (Dow Chemical Co., 1953), which is
more than 30 000 the daily per capita intake ('eaters only') of 15
and 12 µg/kg bw from its use as a flavouring agent in Europe and the
United States, respectively (see Table 2).
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
Groups of 10 male and 10 female Charles River CD rats were fed
diets providing 3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses of 0,
100, 200, or 400 mg/kg bw per day for 91 days. No adverse effects were
seen on behaviour, body weight, food consumption, haematological,
urinary, or ophthalmological parameters, or gross or histopathological
appearance. The NOEL was 400 mg/kg bw per day (King et al., 1979),
which is more than 50 000 the daily per capita intake ('eaters
only') of 0.8 and 0.4 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
In a combined study of developmental toxicity and
carcinogenicity, three successive generations of male and
female Charles River CD-COBS rats received
3-ethyl-2-hydroxy-2-cyclopenten-1-one in the basal diet at doses of 0
(untreated control), 0 (propylene glycol control), 30, 80, or
200 mg/kg bw per day. The F0 generation was entered into the study
after weaning and was mated 64 days later. These animals were treated
for 12 months, during which time the females were given a supplemental
diet throughout gestation and lactation. The F1 generation was
initially exposed in utero, subsequently via the dams' milk until
weaning, and then treated for two years and bred twice (at days 99 and
155) to produce F2a and F3a generations. The F0 and F2 generations
consisted of 40 animals of each sex in the untreated control group and
20 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups. In the F1
generation, there were 100 animals of each sex in the untreated
control group and 50 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups.
Survival, clinical symptoms, food consumption, reproductive
perfor-mance, and haematological and clinical chemical parameters were
not adversely affected in the F0 or F1 generations. Slightly
depressed growth was reported in F1 females receiving the highest
dose, but the effect was not significant when compared with the growth
of controls receiving propylene glycol. Gross pathological and
histopathological examination of animals of these generations revealed
no significant treatment-related effects. An increased incidence of
bile-duct hyperplasia observed in these groups was not statistically
significant when compared with the incidence of in controls. The
incidence of benign or malignant tumours in treated animals was
similar to that in controls. The NOEL was 200 mg/kg bw per day (King
et al., 1979), which is more than 200 000 the daily per capita
intake ('eaters only') of 0.8 and 0.4 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
In a three-generation study of toxicity in Charles River
CD-COBS rats with 3-ethyl-2-hydroxy-2-cyclopenten-1-one, 120
F0 animals were entered into the study just after weaning and were
mated after 64 days, providing the F1 generation. Animals of the
F0 generation were selected at random to be maintained as controls
or were given 3-ethyl-2-hydroxy-2-cyclopenten-1-one in the diet.
The F1 generation was maintained on the diet for 755 days, during
which time they were mated twice (at days 99 and 155). The first
mating provided the rats for the F2 generation, and the second mating
provided rats which were sacrificed after weaning. The F2 generation
was treated for 84 days and then mated to produce the F3 generation.
Rats of all generations were maintained on a basal diet with
3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses equal to daily intakes
of 0, 30, 80, or 200 mg/kg bw. Interim clinical chemical analyses were
performed on the F0 generation at months 4 and 6. Ophthalmological,
clinical chemical, and haematological examinations were performed on
the F1 generation at months 6, 12, 18, and 24. At termination, both
the F0 and the F1 generations were submitted to clinical chemical,
haematological, and histopathological examinations. F2 adult animals
were killed without necropsy at the time of weaning of their
offspring.
No evidence of treatment-related effects was found, and the
general health of the rats was unaffected throughout treatment.
Mammary swellings in females and other subcutaneous nodules were
considered not to be related to treatment because they were equally
distributed among control and treated groups. No effects were seen on
food consumption, weight gain, or clinical chemical or haematological
parameters. In the F0 generation, there were no pathological findings
at the 12-month necropsy or at histopathological examination. In the
F1 generation, there were no macroscopic observations that could be
related to the treatment. Histologically, bile-duct hyperplasia was
seen in all groups, including controls; however, there was no
statistically significant difference between treated and control
groups and the findings were considered not to be related to
treatment. Only tumours usually found in rats of this strain and age
were observed, indicating the absence of treatment-related effects.
The NOEL in this study was 200 mg/kg bw per day (King et al., 1979),
which is more than 100 000 the daily per capita intake ('eaters
only') of 0.4 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Dimethylcyclopentane-1,2-dione (No. 420)
Groups of 10 male Charles River CD rats were fed diets containing
3,4-dimethyl-1,2-cyclopentadione at concentrations of 0, 400, 4000, or
13 000 mg/kg (equivalent to 0, 20, 200, and 640 mg/kg bw per day) for
90 days. Food consumption, body-weight gain, haematological
parameters, organ weights, and gross and microscopic appearance were
observed throughout the study. Animals at the intermediate and high
doses showed a moderate but consistent depression in food intake
throughout the experiment, and their final mean body weights were
about 10% less than those of controls. There were no consistent
indications of a profound disturbance of food conversion efficiency,
although minor increases in the food conversion ratio were seen in
some animals at the high dose. Haematological parameters, organ
weights, and gross and microscopic appearance were similar in treated
and control groups. The authors attributed the decreased body weight
observed at higher doses to the unpalatability of the test substance.
The NOEL was 200 mg/kg bw per day (Wheldon & Krajkeman, 1967), which
is more than 200 000 the daily per capita intake ('eaters only') of
0.8 and 0.04 µg/kg bw from its use as a flavouring agent in Europe and
the United States, respectively (see Table 2).
3,5-Dimethylcyclopentane-1,2-dione (No. 421)
Groups of 10 male Charles River CD rats were fed
3,5-dimethyl-1,2-cyclopenthexadione in the diet for 13 weeks at
concentrations of 0, 1000, or 10 000 mg/kg diet (equivalent to 50 and
500 mg/kg bw per day). A fourth group was fed a diet initially
containing 12 000 ppm (equivalent to 610 mg/kg bw per day), but the
level was increased to 24 000 mg/kg diet (equivalent to
1200 mg/kg bw per day) during week 6 through to the end of experiment.
Haematological examinations were performed on five animals from each
group just before the end of the 13-week period; organ weight analysis
and gross and histopathological examinations at the end of the study
revealed no significant differences between control and treated
animals. The food consumption of animals at the two highest doses was
reduced throughout the experiment, accompanied by decreased weight
gain, which resulted in mean body weights that were 10-17% lower than
those of controls at the end of the study. The authors attributed the
decrease to the unpalatability of the test substance. The NOEL was 50
mg/kg bw per day (Wheldon & Krajkeman, 1967), which is more than
50 000 the daily per capita intake ('eaters only') of 0.9 and 0.5
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
2-Hydroxy-2-cyclohexen-1-one (No. 424)
2-Hydroxy-2-cyclohexen-1-one was added to the diet of 15 male and
15 female albino Wistar rats for 90 days at a concentration calculated
to result in an average daily intake of 5 mg/kg bw. Detailed
measurements of haematological parameters, blood chemistry, urine,
gross and histopathological appearance, organ weights, body weight,
and food consumption revealed no statistically significant difference
between treated and control groups (Morgareidge et al., 1974). The
NOEL was 5 mg/kg bw per day (Cox, 1974), which is more than 500 000
the daily per capita intake ('eaters only') of 0.001 and 0.01 µg/kg
bw from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
1-Methyl-2,3-cyclohexadione (No. 425)
Groups of 10 male Charles River CD rats were fed
1-methyl-2,3-cyclohexadione in the diet at concentrations of 0, 100,
or 1000 mg/kg (equivalent to 0, 5, and 50 mg/kg bw per day) for 13
weeks. A fourth group was fed a diet initially containing 13 500 mg/kg
diet (equivalent to 675 mg/kg bw per day), which was raised to 27 000
mg/kg bw (equivalent to 1350 mg/kg bw per day) during week
6. Observation of haematological parameters, organ weights, and gross
and histopathological appearance revealed no difference between
treated and control groups. The food consumption of rats at the high
dose was reduced throughout the experiment, accompanied by decreased
body-weight gain, and the effect was more marked when the dietary
concentration was raised. The NOEL was 50 mg/kg bw per day (Wheldon &
Krajkeman, 1967), which is more than 500 000 the daily per capita
intake ('eaters only') of 0.03 and 0.1 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
2.3.2.3 Genotoxicity
The available studies of genotoxicity are summarized in Table 5.
Most of the studies were carried out with Salmonella typhimurium in
the Ames test. Acetoin, diacetyl, and 1,2-cyclohexanedione showed some
mtagenicity in strains TA100 and TA104. As the mutation frequencies
were low and the positive results were always accompanied by negative
results, the overall conclusion was that this group of substances does
not induce gene mutation in bacteria in vitro. Diacetyl did not
induce mutation in Saccharomyces cerevisiae (US Food & Drug
Administration, 1974). Methylcyclopentenolone did not induce
unscheduled DNA synthesis in rat hepatocytes (Heck et al., 1989).
2.3.2.4 Other relevant studies
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
In the three-generation, 23-month study of toxicity described
above, treatment had no effect on clinical signs, weight gain, food
consumption, copulation rates, or mating behaviour of males or
females, fertility index, length of gestation, parturition, litter
size, or incidence of stillbirth. The growth and survival rates of
pups during lactation were normal. Gross examination of all offspring
from birth to weaning revealed no significant treatment-related
malformations or lesions (King et al., 1979).
Diacetyl (No. 408)
Groups of 25-27 Syrian golden hamsters, 21-24 CD-1 mice, and
21-23 albino Wistar rats were given a solution containing 90%
diacetyl by gavage on days 6-10 of gestation for hamsters and days
6-15 of gestation for mice and rats. The doses for all species were
16, 74, 345, or 1600 mg/kg bw per day. No effects were seen on
maternal survival, weight, or reproductive parameters or on fetal
survival or microscopic appearance of external, skeletal, or soft
tissues (US Food & Drug Administration, 1973).
4. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon,
R. (1989) Contribution of coffee aroma constituents to the
mutagenicity of coffee. Food Chem. Toxicol., 27, 227-232.
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Table 4. Short-term and long-term studies of toxicity in rats with aliphatic acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones used as flavouring agents
Substance No. Sex No. groups/ Route Time NOEL Reference
no. per group (days) (mg/kg bw
per day)
Acetoin 405 M/F 3/30 Drinking-water 90 330 Gaunt et al. (1972)
Diacetyl 408 M/F 4/30 Gavage 90 90 Colley et al. (1969)
3,4-Hexanedione 413 M/F 2/10-16 Diet 90 17a Posternak et al. (1969)
Methylcyclopentenolone 418 M/F 1/30 Diet 185 500a Dow Chemical Co. (1953)
3-Ethyl-2-hydroxy-2-cyclopenten-1-one 419 M/F 3/20 Diet 91 400a King et al. (1979)
M/F 3/100 Diet 730 200 King et al. (1979)
3,4-Dimethyl-1,2-cyclopentadione 420 M 3/10 Diet 90 645 Wheldon & Krajkeman (1967)
3,5-Dimethyl-1,2-cyclopentadione 421 M 3/10 Diet 90 610a Wheldon & Krajkeman (1967)
2-Hydroxy-2-cyclohexen-1-one 424 M/F 1/30 Diet 90 5a Morgareidge et al. (1974)
1-Methyl-2,3-cyclohexadione 425 M 3/10 Diet 90 675-1350a Wheldon & Krajkeman (1967)
M, male; F, female
a The study was performed at a single or multiple doses that had no adverse effects; therefore, no NOEL was determined.
The NOEL is probably higher than the dose reported to have no adverse effects.
Table 5. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones
used as flavouring agents
Substance No. End-point Test object Dose Results Reference
Acetoin 405 Reverse mutation S. typhimurium TA100 < 4500 mg/plate Negativea Garst et al. (1983)
Positiveb
Reverse mutation S. typhimurium TA100 420 mg/plate Negativeb Kim et al. (1987)
Diacetyl 408 Reverse mutation S. typhimurium TA100, Not reported Positivea,c Kato et al. (1989)
(modified test) TA104; E. coli
Reverse mutation S. typhimurium TA104 530 µg/plate Positived Marnett et al. (1985)
(modified test)
Reverse mutation S. typhimurium TA100 90 µg/plate Negativee Kim et al. (1987)
Reverse mutation S. typhimurium TA104 5-500 µg/platef Positivea,c Shane et al. (1988)
Negativeb
Reverse mutation S. typhimurium TA102, 5-500 µg/platef Negativee Shane et al. (1988)
TA100
Reverse mutation S. typhimurium TA100 152-950 µg/plate Negativeb Dorado et al. (1992)
Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA1535, 1% Negativee US Food & Drug
(suspension test) TA1537, TA1538 Administration (1974)
Reverse mutation S. typhimurium TA102 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
Reverse mutation S. typhimurium TA98, 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
TA100
Reverse mutation S. typhimurium TA100 2 or 4 mmol/plate Positiveg Suwa et al. (1982)
Mutation S. cerevisiae Not reported Negativee US Food & Drug
Administration (1974)
2,3-Pentanedione 410 Reverse mutation S. typhimurium TA100 105 µg/plate Negativeb Kim et al. (1987)
Reverse mutation S. typhimurium TA100, 0.9-90 000 µg/plate Negativee Aeschbacher et al. (1989)
3,4-Hexanedione 413 Reverse mutation S. typhimurium TA100 228-4900 µg/plate Negativeb Dorado et al. (1992)
Table 5. (continued)
Substance No. End-point Test object Dose Results Reference
Methylcyclopentenolone 418 Reverse mutation S. typhimurium TA1535, 10 000 µg/plate Negativee Heck et al. (1989)
TA1537, TA1538, TA98,
TA100
Unscheduled DNA Rat hepatocytes 500 µg/plate Negativea Heck et al. (1989)
synthesis
2-Hydroxy-2-cyclohexen-1-one 424 Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA100 112-1000 µg/plate Negativeb Dorado et al. (1992)
a With metabolic activation
b Without metabolic activation
c Mutation frequency, 2-3
d Result based on authors' criterion for significant mutagenic effect, i.e. mutation frequency > 1.5
e With and without metabolic activation
f Estimated from graph
g Mutation frequency, < 2.5
Bjeldanes, L.F. & Chew, H. (1979) Mutagenicity of 1,2-dicarbonyl
compounds: Maltol, kojic acid, diacetyl and related substances.
Mutat. Res., 67, 367-371.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxication, New York, Academic
Press, Vol. I, pp. 231-248.
Colley, J., Gaunt, I.F., Lansdown, A.B.G., Grasso, P. & Gangolli, S.D.
(1969) Acute and short-term toxicity of diacetyl in rats. Food Chem.
Toxicol., 7, 571-580.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, J. & Hullin, R.P. (1954) Metabolism of acetoin. Formation and
utilization of acetoin and 2,3-butanediol in the decerebrated cat.
Biochem. J., 57, 177-180.
Dix & Jeffcoat (1997) Disposition and excretion of
[14C]2,3-butanedione in male rats following oral administration.
Unpublished report from Research Triangle Institute (NIEHS contract
No. NO1-ES-75407). Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Dorado, L., Montoya, M.R. & Rodriguez-Mellado, J.M. (1992) A
contribution to the study of the structure-mutagenicity relationship
for alpha-dicarbonyl compounds using the Ames test. Mutat. Res.,
269, 301-306.
Dow Chemical Co. (1953) Toxicity of cyclotene--Summary. Unpublished
report from Biochemical Research Department, Midland, Michigan, 15
July. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Gabriel, M.A., Jabara, H. & Al-Khalidi, A.A. (1971) Metabolism of
acetoin in mammalian liver slices and extracts. Biochem. J., 124,
793-800.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Garst, J., Stapleton, P. & Johnson, J. (1983) Mutagenicity of
alpha-hydroxy ketones may involve superoxide anion radical. Oxy
Radicals and Their Scavenger Systems, Amsterdam, Elsevier Science
Publishing, Vol. 2, pp. 125-130.
Gaunt, I.F., Branton, P.G., Kiss, I.S., Grasso, P. & Gangolli, S.D.
(1972) Short-term toxicity of acetoin (acetyl methylcarbinol) in rats.
Food Cosmet. Toxicol., 10, 131-141.
Givaudan Corp. (1952) Acute oral toxicity study of
methylcyclopentenolone in mice. Unpublished report to the Research
Institute of Fragrance Materials. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Gordon, A.J. & Ford, R.A. (1972) The Chemist's Companion, New York,
John Wiley & Sons, pp. 49-51.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Järnefelt, J. (1955) Studies on the enzymatic synthesis and breakdown
of acetoin in the animal organism. Ann. Acad. Sci. Fenn. Ser.
A. V. Med Anthropol., 57, 7-78.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Juni, E. & Heym, G.A. (1956) A cyclic pathway for the bacterial
dissimilation of 2,3-butanediol, acetyl methyl carbinol, and diacetyl.
J. Bacteriol., 71, 425-432.
Kato, F., Araki, A., Nozaki, K. & Matsushi, T. (1989) Mutagenicity of
aldehydes and diketones. Mutat. Res., 216, 366-367.
Kawano, T. (1959) Relation of acetoin and pantothenic acid. Fukuoka
Igaku Zasshi, 50, 2939-2953.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic
heterocyclic amines. Mutat. Res., 177, 9-15.
King, T., Faccini, J.M., Jachbaur, J., Perraud, J. & Monro, A.M.
(1979) 3-Generation and chronic toxicity study in rats. Unpublished
report from Pfizer Central Research. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Leberco Labs (1952) Acute toxicity of methylcyclopentenolone.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mills, G.A. & Walker, V. (1990) Urinary excretion of cyclohexanediol,
a metabolite of the solvent cyclohexanone, by infants in a special
care unit. Clin. Chem., 36, 870-874.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1977) Acute toxicity studies in rats, rabbits and guinea
pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1979) Acute toxicity studies in rats, rabbits and
guinea-pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974) 90 Day feeding study
with 2-hydroxy-2-cyclohexen-1-one in rats. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Ong, C.N., Sia, G.L., Chia, S.E., Phoon, W.H. & Tan, K.T. (1991)
Determination of cyclohexanol in urine and its use in environmental
monitoring of cyclohexanone exposure. J. Anal. Toxicol., 15, 13-16.
Otsuka, M., Mine, T., Ohuchi, K. & Ohmori, S. (1996) A detoxication
route for acetaldehyde: Metabolism of diacetyl, acetoin, and
2,3-butanediol in liver homogenate and perfused liver of rats.
J. Biochem., 119, 246-251.
Pellmont, D. (1969) Studies with rats and mice on substance No.
R01-3801 (Toxikologisches Labor 256, Bau 69). Unpublished report to
the Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological test on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102 and TA104. Environ. Mol. Mutag., 11,
195-206.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Suwa, Y., Nagao, M., Kosugi, A. & Sugimura, T. (1982) Sulfite
suppresses the mutagenic property of coffee. Mutat. Res., 102,
383-391.
US Food & Drug Administration (1973) Teratologic Evaluation of FDA
71-73 (Starter Distillate, Hansen) (NTIS PB-223-833;
FDABF-GRAS-152), Washington DC, United States.
US Food & Drug Administration (1974) Mutagenic Evaluation of
Compound FDA 71-73 (Starter Distillate) (NTIS PB-245-431;
FDABF-GRAS-275), Washington DC, United States.
US Food & Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1970) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1982) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
Westerfeld, W.W. & Berg, R.L. (1943) Observations on the metabolism of
acetoin. J. Biol. Chem., 148, 523-528.
Wheldon, G.H. & Krajkeman, A.J. (1967) The effects of ten
food-flavoring additives administered to rats over a period of
thirteen weeks. Unpublished report from Huntington Research Center.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Williams, T.R. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 62, 78.
Zlatkis, A. & Sivetz, M. (1960) Analysis of coffee volatiles by gas
chromatography. Food Res., 25, 395-398.
The vast majority of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones have been reported to
occur in traditional foods (Maarse et al., 1994). Quantitative data on
the natural occurrence of these substances, with the exception of
diacetyl, demonstrate that they are consumed predominantly from
traditional foods (i.e. consumption ratio > 1) (Stofberg & Kirschman,
1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
Aliphatic acyclic and alicyclic alpha-diketones participate in an
enol-keto equilibrium with the corresponding ketoenol (see Figure 1).
The enolic form predominates in alicyclic diketones, especially
cyclopentyl alpha-diketones (Gordon & Ford, 1972), essentially 100% of
which exist in the ketoenolic form; 50-80% of cyclohexyl
alpha-diketones exist in the ketoenolic form at equilibrium.
Increasing pH would be expected to shift the equilibrium in favour of
the ketoenolic form (i.e. alpha-hydroxyketone). Therefore, at
physiological pH, an increase in the alpha-hydroxyketone (i.e. enol)
form is expected.
In rats and mice, orally administered aliphatic alpha-diketones
are rapidly absorbed from the gastrointestinal tract (Gabriel et al.,
1972). Carbon dioxide produced primarily from methyl-substituted
diketones (e.g. diacetyl) is eliminated in expired air. Thus, after
injection of [2,3-14C]-acetoin to whole albino rats, 14C-carbon
dioxide appeared in expired air, with an average 12-h production of
15% (Gabriel et al., 1972). In rats, 54-82% of orally administered
radiolabelled 2,3-butanedione was excreted as carbon dioxide. At
increasing doses, the percent of the dose excreted as carbon dioxide
decreased, while urinary excretion of radiolabel increased, suggesting
that saturation occurs at higher doses. Chromatographic analysis of
urine samples yielded three major 14C-labelled components, one of
which co-eluted with uric acid. Analysis with ß-glucuronidase or
sulfatase decreased the amount of radiolabel present in one peak, with
a corresponding increase in the amount present in another major
component. Identification of urinary metabolites was not pursued owing
to the extensive excretion of 14C as carbon dioxide (Dix & Jeffcoat,
1997). In general, esters are hydrolysed to their corresponding
alcohols and carboxylic acids. Hydrolysis is catalysed by classes of
enzymes recognized as carboxylesterases or esterases, the most
important of which are the B-esterases. Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). These enzymes
occur in most mammalian tissues (Heymann, 1980; Anders, 1989) but
predominate in hepatocytes (Heymann, 1980). 2-Acetoxy-3-butanone (No.
406) and butanon-3-one-2-yl butanoate (No. 407) are expected to be
metabolized in humans to acetic acid and butanoic acid, respectively,
and to acetoin.
Aliphatic acyclic diketones
The metabolic fate of acyclic aliphatic diketones depends
primarily on the position of the carbonyl function and on chain
length. Aliphatic acyclic diketones and alpha-hydroxyketones that
contain a carbonyl function at the 2 position (i.e. methyl ketones)
may undergo alpha-hydroxylation and subsequent oxidation of the
terminal methyl group to yield corresponding ketocarboxylic acids. The
ketoacids are intermediary metabolites (e.g. alpha-ketoacids), which
may undergo oxidative decarboxylation to yield carbon dioxide and a
simple aliphatic carboxylic acid. The acid may be completely
metabolized in the fatty acid pathway and citric acid cycle.
Alternatively, the methyl-substituted diketones may be
successively reduced to the corresponding hydroxyketones and diols,
which are excreted in the urine as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain ketones. If the carbonyl function is located elsewhere on
the chain, reduction is the predominant detoxification pathway.
alpha-Hydroxyketones and their diol metabolites may be excreted as
glucuronic acid conjugates. For example, the glucuronic acid conjugate
of cyclohexanol was detected in the urine of humans exposed to
cyclohexanone (Ong et al., 1991), indicating that alpha-hydroxylation
followed by reduction of the ketone function occurs in humans.
Acetoin is metabolized primarily via oxidation at low
concentrations in vivo and by reduction to 2,3-butanediol at high
concentrations. It has been estimated that 1 g of rat liver can
oxidize 86 µg (1 µmol) acetoin per day (Gabriel et al., 1972).
Production of carbon dioxide at low levels and of 2,3-butanediol at
high levels is associated with the slower rate of ketone reduction
(Williams, 1959). Oxidation of the terminal methyl group may result in
formation of an alpha-ketoacid, which undergoes cleavage to yield
carbon dioxide and a carboxylic acid fragment. Alternatively, methyl
group oxidation may yield a ß-ketoacid which undergoes ß-cleavage to
yield two-carbon fragments. To a minor extent, these two-carbon
fragments can act as acetyl donors for acetylation of
para-aminobenzoic acid (Westerfeld & Berg, 1943).
A total dose of 78 g of acetoin was administered to a dog over
two months, both orally in a 3-4% solution and subcutaneously in a 20%
solution. Urine was collected under toluene from the beginning of
treatment up to 40 h after the last dose. 2,3-Butanediol was the major
urinary excretion product, representing 5-25% of the dose. The
remainder of the dose was completely metabolized (Westerfeld & Berg,
1943).
In liver preparations obtained from rats and rabbits, more than
95% of the radiolabel of [2,3-14C]-acetoin was detected as a mixture
of stereoisomers of 2,3-butanediol (Gabriel et al., 1971). Although
reduction of diacetyl and acetoin has been observed in animals
in vivo and in animal tissue preparations in vitro at high
concentrations, it appears that oxidation of diacetyl is a major
endogenous metabolic pathway.
Reduction of ketones is mediated by alcohol dehydrogenase and
NADPH-dependent cytosolic carbonyl reductases (Bosron & Li, 1980).
Reduction of the endogenous substances acetoin (No. 405) and diacetyl
(No. 408) is catalysed by the substrate-specific enzymes diacetyl
reductase and acetoin reductase, respectively. In rat liver slices,
diacetyl, acetoin, and 2,3-butanediol are interconvertible (Gabriel et
al., 1972).
In male Wistar albino rats, a single oral dose of diacetyl at
5 mmol/kg bw (430 mg/kg bw) was metabolized by reduction to acetoin,
which was present at high concentrations in major organs 1 h after
dosing. The subsequent reduction product, 2,3-butanediol, was detected
in liver, kidney, and brain (Otsuka et al., 1996). Only a 10-min
incubation is required to convert 10 nmol (9 × 10-4 mg) diacetyl to
3.7 nmol (3 × 10-4 mg) acetoin and 6.3 nmol (6 × 10-4 mg)
2,3-butanediol in rat liver homogenate. Diacetyl was reduced by NADH-
and NADPH-dependent diacetyl reductase isolated from a homogenate of
male Wistar rat liver. The organ-specific reductase activity was
greatest in the liver and least in the brain (Otsuka et al., 1996).
Acetoin was given either orally in a 3-4% solution or
subcutaneously in a 20% solution to rats, multiple doses being spaced
equally throughout each day. The acetoin used was obtained as the
polymer (H, 15), which apparently reverts to an optically inactive
monomer in aqueous solution. No diacetyl was detected in the urine of
the rats; acetoin was not excreted to any appreciable extent in the
urine, and the major excretion product was 2,3-butanediol (Westerfeld
& Berg, 1943). In dogs, acetoin is excreted in the urine, in part as
2,3-butanediol. Most of an oral dose disappeared and was presumed to
be completely metabolized (Westerfeld & Berg, 1943). In another study,
2,3-butanediol readily conjugated glucuronic acid (Neuberg &
Gottshchalk, 1971).
Diacetyl and acetoin are endogenous in humans (Kawano, 1959;
Zlatkis & Sivetz, 1960). They are formed when pyruvate is converted to
acetoin and diacetyl by pyruvate decarboxylase (Gabriel et al.,
1972). Mean fasting blood concentrations of approximately 100 µg
acetoin per 100 ml blood have been reported (Dawson & Hullin, 1954).
Pyruvate also forms diacetyl in vitro in rat liver preparations
(Järnefelt, 1955) and in microorganisms (Juni & Heym, 1956).
Alicyclic alpha-diketones and alicyclic dicarbonyls
In general, alicyclic alpha-diketones are metabolized via a
reduction pathway (Williams, 1959). In humans, structurally related
alicyclic monoketones are reduced to the corresponding alcohols or
undergo alpha-hydroxylation and reduction to yield diols, which are
excreted as the glucuronic acid conjugates. For example, the
glucuronic acid conjugate of cyclohexanol is present in the urine of
humans exposed to atmospheres containing cyclohexanone at
concentrations of 2-30 ppm (Ong et al., 1991). A mixture of diols,
including cis- and trans-1,2-cyclohexanediol,1,3-cyclohexanediol,
and 1,4-cyclohexanediol, was detected in the urine of infants exposed
to cyclohexanone present in dextrose solutions used for intravenous
feeding. Presumably, the cyclohexanone undergoes alpha-hydroxylation
and then reduction of the ketone function to yield the corresponding
alpha-cyclohexanediols (Mills & Walker, 1990).
On the basis of the studies described above, it is anticipated
that humans will metabolize low concentrations of aliphatic acyclic
methyl ketones principally by oxidation of the terminal methyl group.
At higher concentrations, reduction to the diol and subsequent
conjugation with glucuronic acid form a competing detoxification
pathway. Other aliphatic acyclic alpha-diketones and
alpha-hydroxyketones and alicyclic diketones and hydroxyketones are
reduced, conjugated with glucuronic acid, and excreted.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
The results of studies of the acute toxicity of seven of the 22
diketones, hydroxyketones, and alicyclic dicarbonyls have been
reported and are summarized in Table 3. The low acute toxicity of the
group is demonstrated by oral LD50 values in the range 990 to > 8000
mg/kg bw.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic
alpha-diketones and related alpha-hydroxyketones as flavouring substances
in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Acetoin (405)
Europe 19 000 2800 46
United States 9 000 1800 29
2-Acetoxy-3-butanone (406)
Europe 0.2 0.03 0.0005
United States 120 23 0.4
Butan-3-one-2-yl-butanoate (407)
Europe 0.1 0.02 0.0003
United States 5 0.95 0.02
Diacetyl (408)
Europe 18 000 3300 56
United States 42 000 8000 133
3-Hydroxy-2-pentanone (409)
Europe NR ND 0
United States 0.5 0.10 0.002
2,3-Pentanedione (410)
Europe 1 100 220 4
United States 420 80 1
4-Methyl-2,3-pentadione (411)
Europe 2.5 0.48 0.01
United States 12 2 0.04
2,3-Hexanedione (412)
Europe 70 13 0.22
United States 50 10 0.2
3,4-Hexanedione (413)
Europe 170 33 0.5
United States 4 0.76 0.01
5-Methyl-2,3-hexanedione (414)
Europe 9 2 0.03
United States 30 6 0.10
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2,3-Heptanedione (415)
Europe 8 2 0.03
United States 26 5 0.08
5-Hydroxy-4-octanone (416)
Europe 0.1 0.02 0.0003
United States 4 0.76 0.01
2,3-Undecadione (417)
Europe 0.03 0.01 0.0001
United States 0.1 0.02 0.0003
Methylcyclopentenolone (418)
Europe 4 700 890 15
United States 3 700 710 12
3-Ethylcyclopentenolone (419)
Europe 260 50 0.8
United States 120 23 0.4
3,4-Dimethyl-1,2-cyclopentanedione (420)
Europe 250 47 0.8
United States 13 2 0.04
3,5-Dimethyl-1,2-cyclopentanedione (421)
Europe 290 55 0.9
United States 150 29 0.5
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one (422)
Europe NR ND ND
United States 0.9 0.17 0.003
5-Ethyl-2-hydroxy-3-methylcyclopent-2-en-1-one (423)
Europe NR ND ND
United States 2 0.38 0.01
2-Hydroxy-2-cyclohexen-1-one (424)
Europe 0.4 0.08 0.001
United States 4 0.76 0.01
1-Methyl-2,3-cyclohexadione (425)
Europe 11 2 0.03
United States 44 8 0.1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
2-Hydroxy-3,5,5-trimethyl-2-cyclohexenone (426)
Europe 10 2 0.03
United States 8 2 0.03
Total
Europe 44 000
United States 56 000
NR, not reported; ND, not determined
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only') = 32 × 106 for Europe and
32 × 106 for the United States; 0.6 represents the assumption that
only 60% of the flavour volume was reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight],
where body weight = 60 kg. Slight variation may occur from rounding off.
Table 3. Studies of acute toxicity with aliphatic acyclic and alicyclic alpha-diketones and
related alpha-hydroxyketones used as flavouring substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
Acetoin 405 Rat NR Oral > 5000 Moreno (1977)
Butan-3-one-2-yl 407 Mouse, rat NR Oral > 8000 Pellmont (1969)
butanoate
Diacetyl 408 Rat M Gavage 3400 Colley et al.(1969)
F 3000
Rat NR Gavage 1580 Jenner et al.(1964)
Guinea-pig NR Gavage 990 Jenner et al.(1964)
2,3-Pentanedione 410 Rat NR Oral 3000 Moreno (1977)
2,3-Hexanedione 412 Rat NR Oral > 5000 Moreno (1977)
5-Methyl-2,3-hexanedione 414 Rat NR Oral > 5000 Moreno (1979)
Methylcyclopentenolone 418 Mouse NR Gavage 1350 Givadaun Corp.(1952)
Rat NR Oral 1850 Moreno (1976)
Guinea-pig NR Gavage 1400 Dow Chemical Co.
(1953)
Mouse NR Oral 1350 Leberco Labs (1952)
NR, not reported; M, male; F, female
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The toxicity of nine of the 22 substances has been studied. The
results are summarized in Table 4 and described below.
Acetoin (No. 405)
Groups of 15 male and 15 female CFE rats were given acetoin in
their drinking-water at concentrations of 0 (control), 750, 3000, or
12 000 mg/kg (equivalent to 0, 85, 330, or 1300 mg/kg bw per day (US
Food & Drug Administration, 1993)). No animals died during the study,
and their condition and appearance were normal. The body weights of
males at 12 000 mg/kg in drinking-water decreased significantly from
week 5, and at weeks 2, 6, and 13 the relative weight of the liver was
statistically significantly greater in these animals than in controls.
A similar effect was seen in female rats, but only after 13 weeks.
Haematological examination conducted at 13 weeks showed a small (4-8%)
but statistically significant (p < 0.05) decrease in haemoglobin
concentration and erythrocyte counts in animals of each sex at the
high dose, but these changes were not accompanied by a decrease in
haematocrit. Urinalysis and blood chemical determinations performed at
the end of the study on all animals revealed no statistically
significant differences between treated and control groups.
Histopathological examination also revealed no adverse effects. The
authors suggested that the increased relative liver weights were a
reaction of the liver to an increased metabolic load resulting from
the high intake of acetoin. The NOEL was 3000 ppm, equivalent to
330 mg/kg bw per day (Gaunt et al., 1972), which is greater than
10 000 the daily per capita intake ('eaters only') of 46 and 29
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
Diacetyl (No. 408)
Groups of 15 male and 15 female weanling specific
pathogen-free-derived CFE rats were given 5 ml/kg bw of an aqueous
solution containing 0, 0.2, 0.6, 1.8, or 11% diacetyl by gavage for 90
days, providing daily doses of diacetyl calculated to be 0, 10, 30,
90, and 540 mg/kg bw. Body weight, food intake, and water consumption
were recorded weekly. Growth retardation and increased water
consumption were observed in animals at the high dose, the effects
being more pronounced in males. Haematological and urinary parameters
and enzyme activities were measured. Anaemia and increased
polymorphonuclear leukocytosis were observed in rats at the high dose
but were attributed to haemorrhage, infections, and ulcers of the
stomach. The relative weights of the liver, kidney, and pituitary and
adrenal glands were increased in these animals, and the increases were
greater than could be accounted for by the reduction in body weight.
Macroscopic and microscopic examination of all major organs revealed
ulcers in the squamous and glandular regions of the gastric mucosa.
The lesions observed in the squamous part of the stomach were
associated with hypertrophy or intercellular oedema. No histological
changes were seen in animals treated at lower doses. No other
significant differences were observed between treated and control
animals. The NOEL was 90 mg/kg bw per day (Colley et al., 1969), which
is 1500 times the daily per capita intake ('eaters only') of 56
µg/kg bw from its use as a flavouring agent in Europe and 500 times
the intake of 130 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Hexanedione (No. 413)
Groups of 10-16 male and female Charles River CD rats were housed
in pairs of the same sex and fed for 90 days on a basal diet alone or
supplemented with 3,4-hexanedione for an average daily intake of
17 mg/kg bw. Body weight and food consumption were measured daily and
were considered normal. Haematological examinations and blood urea
determinations were performed on 50% of the animals at week 7 and at
the end of treatment: no significant differences were seen between
treated and control animals. At necropsy, the liver and kidney weights
were normal, and gross and histological examination performed on a
wide range of organs revealed no dose-related lesions. The NOEL was 17
mg/kg bw per day (Posternak et al., 1969), which is more than 30 000
the daily per capita intake ('eaters only') of 0.5 and 0.01 µg/kg bw
from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
Methylcyclopentenolone (No. 418)
Groups of 15 male and 15 female rats aged four to five weeks
(strain not specified) were placed on a diet containing 0 or 1%
methylcyclo-pentenolone, equivalent to a daily intake of 0 or 500
mg/kg bw, for six months. Each animal was weighed twice weekly and
observed frequently for gross appearance and behaviour. The tissues of
most animals that died or were killed during the course of the study
and of all those kiled at the end of the study were examined grossly.
Tissues of representative animals from the control and treated groups
(numbers unspecified) were examined microscopically, and the weights
of the lungs, heart, liver, kidneys, spleen, and testes were recorded.
Haematological parameters were measured in representative animals from
the control and treated groups (numbers not specified) at the end of
the experiment. There were no statistically significant differences
between treated and control animals in any of the parameters measured.
The NOEL was 500 mg/kg bw per day (Dow Chemical Co., 1953), which is
more than 30 000 the daily per capita intake ('eaters only') of 15
and 12 µg/kg bw from its use as a flavouring agent in Europe and the
United States, respectively (see Table 2).
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
Groups of 10 male and 10 female Charles River CD rats were fed
diets providing 3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses of 0,
100, 200, or 400 mg/kg bw per day for 91 days. No adverse effects were
seen on behaviour, body weight, food consumption, haematological,
urinary, or ophthalmological parameters, or gross or histopathological
appearance. The NOEL was 400 mg/kg bw per day (King et al., 1979),
which is more than 50 000 the daily per capita intake ('eaters
only') of 0.8 and 0.4 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
In a combined study of developmental toxicity and
carcinogenicity, three successive generations of male and
female Charles River CD-COBS rats received
3-ethyl-2-hydroxy-2-cyclopenten-1-one in the basal diet at doses of 0
(untreated control), 0 (propylene glycol control), 30, 80, or
200 mg/kg bw per day. The F0 generation was entered into the study
after weaning and was mated 64 days later. These animals were treated
for 12 months, during which time the females were given a supplemental
diet throughout gestation and lactation. The F1 generation was
initially exposed in utero, subsequently via the dams' milk until
weaning, and then treated for two years and bred twice (at days 99 and
155) to produce F2a and F3a generations. The F0 and F2 generations
consisted of 40 animals of each sex in the untreated control group and
20 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups. In the F1
generation, there were 100 animals of each sex in the untreated
control group and 50 of each sex in the propylene glycol control and
3-ethyl-2-hydroxy-2-cyclopenten-1-one-treated groups.
Survival, clinical symptoms, food consumption, reproductive
perfor-mance, and haematological and clinical chemical parameters were
not adversely affected in the F0 or F1 generations. Slightly
depressed growth was reported in F1 females receiving the highest
dose, but the effect was not significant when compared with the growth
of controls receiving propylene glycol. Gross pathological and
histopathological examination of animals of these generations revealed
no significant treatment-related effects. An increased incidence of
bile-duct hyperplasia observed in these groups was not statistically
significant when compared with the incidence of in controls. The
incidence of benign or malignant tumours in treated animals was
similar to that in controls. The NOEL was 200 mg/kg bw per day (King
et al., 1979), which is more than 200 000 the daily per capita
intake ('eaters only') of 0.8 and 0.4 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
In a three-generation study of toxicity in Charles River
CD-COBS rats with 3-ethyl-2-hydroxy-2-cyclopenten-1-one, 120
F0 animals were entered into the study just after weaning and were
mated after 64 days, providing the F1 generation. Animals of the
F0 generation were selected at random to be maintained as controls
or were given 3-ethyl-2-hydroxy-2-cyclopenten-1-one in the diet.
The F1 generation was maintained on the diet for 755 days, during
which time they were mated twice (at days 99 and 155). The first
mating provided the rats for the F2 generation, and the second mating
provided rats which were sacrificed after weaning. The F2 generation
was treated for 84 days and then mated to produce the F3 generation.
Rats of all generations were maintained on a basal diet with
3-ethyl-2-hydroxy-2-cyclopenten-1-one at doses equal to daily intakes
of 0, 30, 80, or 200 mg/kg bw. Interim clinical chemical analyses were
performed on the F0 generation at months 4 and 6. Ophthalmological,
clinical chemical, and haematological examinations were performed on
the F1 generation at months 6, 12, 18, and 24. At termination, both
the F0 and the F1 generations were submitted to clinical chemical,
haematological, and histopathological examinations. F2 adult animals
were killed without necropsy at the time of weaning of their
offspring.
No evidence of treatment-related effects was found, and the
general health of the rats was unaffected throughout treatment.
Mammary swellings in females and other subcutaneous nodules were
considered not to be related to treatment because they were equally
distributed among control and treated groups. No effects were seen on
food consumption, weight gain, or clinical chemical or haematological
parameters. In the F0 generation, there were no pathological findings
at the 12-month necropsy or at histopathological examination. In the
F1 generation, there were no macroscopic observations that could be
related to the treatment. Histologically, bile-duct hyperplasia was
seen in all groups, including controls; however, there was no
statistically significant difference between treated and control
groups and the findings were considered not to be related to
treatment. Only tumours usually found in rats of this strain and age
were observed, indicating the absence of treatment-related effects.
The NOEL in this study was 200 mg/kg bw per day (King et al., 1979),
which is more than 100 000 the daily per capita intake ('eaters
only') of 0.4 µg/kg bw from its use as a flavouring agent in the
United States (see Table 2).
3,4-Dimethylcyclopentane-1,2-dione (No. 420)
Groups of 10 male Charles River CD rats were fed diets containing
3,4-dimethyl-1,2-cyclopentadione at concentrations of 0, 400, 4000, or
13 000 mg/kg (equivalent to 0, 20, 200, and 640 mg/kg bw per day) for
90 days. Food consumption, body-weight gain, haematological
parameters, organ weights, and gross and microscopic appearance were
observed throughout the study. Animals at the intermediate and high
doses showed a moderate but consistent depression in food intake
throughout the experiment, and their final mean body weights were
about 10% less than those of controls. There were no consistent
indications of a profound disturbance of food conversion efficiency,
although minor increases in the food conversion ratio were seen in
some animals at the high dose. Haematological parameters, organ
weights, and gross and microscopic appearance were similar in treated
and control groups. The authors attributed the decreased body weight
observed at higher doses to the unpalatability of the test substance.
The NOEL was 200 mg/kg bw per day (Wheldon & Krajkeman, 1967), which
is more than 200 000 the daily per capita intake ('eaters only') of
0.8 and 0.04 µg/kg bw from its use as a flavouring agent in Europe and
the United States, respectively (see Table 2).
3,5-Dimethylcyclopentane-1,2-dione (No. 421)
Groups of 10 male Charles River CD rats were fed
3,5-dimethyl-1,2-cyclopenthexadione in the diet for 13 weeks at
concentrations of 0, 1000, or 10 000 mg/kg diet (equivalent to 50 and
500 mg/kg bw per day). A fourth group was fed a diet initially
containing 12 000 ppm (equivalent to 610 mg/kg bw per day), but the
level was increased to 24 000 mg/kg diet (equivalent to
1200 mg/kg bw per day) during week 6 through to the end of experiment.
Haematological examinations were performed on five animals from each
group just before the end of the 13-week period; organ weight analysis
and gross and histopathological examinations at the end of the study
revealed no significant differences between control and treated
animals. The food consumption of animals at the two highest doses was
reduced throughout the experiment, accompanied by decreased weight
gain, which resulted in mean body weights that were 10-17% lower than
those of controls at the end of the study. The authors attributed the
decrease to the unpalatability of the test substance. The NOEL was 50
mg/kg bw per day (Wheldon & Krajkeman, 1967), which is more than
50 000 the daily per capita intake ('eaters only') of 0.9 and 0.5
µg/kg bw from its use as a flavouring agent in Europe and the United
States, respectively (see Table 2).
2-Hydroxy-2-cyclohexen-1-one (No. 424)
2-Hydroxy-2-cyclohexen-1-one was added to the diet of 15 male and
15 female albino Wistar rats for 90 days at a concentration calculated
to result in an average daily intake of 5 mg/kg bw. Detailed
measurements of haematological parameters, blood chemistry, urine,
gross and histopathological appearance, organ weights, body weight,
and food consumption revealed no statistically significant difference
between treated and control groups (Morgareidge et al., 1974). The
NOEL was 5 mg/kg bw per day (Cox, 1974), which is more than 500 000
the daily per capita intake ('eaters only') of 0.001 and 0.01 µg/kg
bw from its use as a flavouring agent in Europe and the United States,
respectively (see Table 2).
1-Methyl-2,3-cyclohexadione (No. 425)
Groups of 10 male Charles River CD rats were fed
1-methyl-2,3-cyclohexadione in the diet at concentrations of 0, 100,
or 1000 mg/kg (equivalent to 0, 5, and 50 mg/kg bw per day) for 13
weeks. A fourth group was fed a diet initially containing 13 500 mg/kg
diet (equivalent to 675 mg/kg bw per day), which was raised to 27 000
mg/kg bw (equivalent to 1350 mg/kg bw per day) during week
6. Observation of haematological parameters, organ weights, and gross
and histopathological appearance revealed no difference between
treated and control groups. The food consumption of rats at the high
dose was reduced throughout the experiment, accompanied by decreased
body-weight gain, and the effect was more marked when the dietary
concentration was raised. The NOEL was 50 mg/kg bw per day (Wheldon &
Krajkeman, 1967), which is more than 500 000 the daily per capita
intake ('eaters only') of 0.03 and 0.1 µg/kg bw from its use as a
flavouring agent in Europe and the United States, respectively (see
Table 2).
2.3.2.3 Genotoxicity
The available studies of genotoxicity are summarized in Table 5.
Most of the studies were carried out with Salmonella typhimurium in
the Ames test. Acetoin, diacetyl, and 1,2-cyclohexanedione showed some
mtagenicity in strains TA100 and TA104. As the mutation frequencies
were low and the positive results were always accompanied by negative
results, the overall conclusion was that this group of substances does
not induce gene mutation in bacteria in vitro. Diacetyl did not
induce mutation in Saccharomyces cerevisiae (US Food & Drug
Administration, 1974). Methylcyclopentenolone did not induce
unscheduled DNA synthesis in rat hepatocytes (Heck et al., 1989).
2.3.2.4 Other relevant studies
3-Ethyl-2-hydroxy-2-cyclopenten-1-one (No. 419)
In the three-generation, 23-month study of toxicity described
above, treatment had no effect on clinical signs, weight gain, food
consumption, copulation rates, or mating behaviour of males or
females, fertility index, length of gestation, parturition, litter
size, or incidence of stillbirth. The growth and survival rates of
pups during lactation were normal. Gross examination of all offspring
from birth to weaning revealed no significant treatment-related
malformations or lesions (King et al., 1979).
Diacetyl (No. 408)
Groups of 25-27 Syrian golden hamsters, 21-24 CD-1 mice, and
21-23 albino Wistar rats were given a solution containing 90%
diacetyl by gavage on days 6-10 of gestation for hamsters and days
6-15 of gestation for mice and rats. The doses for all species were
16, 74, 345, or 1600 mg/kg bw per day. No effects were seen on
maternal survival, weight, or reproductive parameters or on fetal
survival or microscopic appearance of external, skeletal, or soft
tissues (US Food & Drug Administration, 1973).
4. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon,
R. (1989) Contribution of coffee aroma constituents to the
mutagenicity of coffee. Food Chem. Toxicol., 27, 227-232.
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Table 4. Short-term and long-term studies of toxicity in rats with aliphatic acyclic and alicyclic alpha-diketones and related
alpha-hydroxyketones used as flavouring agents
Substance No. Sex No. groups/ Route Time NOEL Reference
no. per group (days) (mg/kg bw
per day)
Acetoin 405 M/F 3/30 Drinking-water 90 330 Gaunt et al. (1972)
Diacetyl 408 M/F 4/30 Gavage 90 90 Colley et al. (1969)
3,4-Hexanedione 413 M/F 2/10-16 Diet 90 17a Posternak et al. (1969)
Methylcyclopentenolone 418 M/F 1/30 Diet 185 500a Dow Chemical Co. (1953)
3-Ethyl-2-hydroxy-2-cyclopenten-1-one 419 M/F 3/20 Diet 91 400a King et al. (1979)
M/F 3/100 Diet 730 200 King et al. (1979)
3,4-Dimethyl-1,2-cyclopentadione 420 M 3/10 Diet 90 645 Wheldon & Krajkeman (1967)
3,5-Dimethyl-1,2-cyclopentadione 421 M 3/10 Diet 90 610a Wheldon & Krajkeman (1967)
2-Hydroxy-2-cyclohexen-1-one 424 M/F 1/30 Diet 90 5a Morgareidge et al. (1974)
1-Methyl-2,3-cyclohexadione 425 M 3/10 Diet 90 675-1350a Wheldon & Krajkeman (1967)
M, male; F, female
a The study was performed at a single or multiple doses that had no adverse effects; therefore, no NOEL was determined.
The NOEL is probably higher than the dose reported to have no adverse effects.
Table 5. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones
used as flavouring agents
Substance No. End-point Test object Dose Results Reference
Acetoin 405 Reverse mutation S. typhimurium TA100 < 4500 mg/plate Negativea Garst et al. (1983)
Positiveb
Reverse mutation S. typhimurium TA100 420 mg/plate Negativeb Kim et al. (1987)
Diacetyl 408 Reverse mutation S. typhimurium TA100, Not reported Positivea,c Kato et al. (1989)
(modified test) TA104; E. coli
Reverse mutation S. typhimurium TA104 530 µg/plate Positived Marnett et al. (1985)
(modified test)
Reverse mutation S. typhimurium TA100 90 µg/plate Negativee Kim et al. (1987)
Reverse mutation S. typhimurium TA104 5-500 µg/platef Positivea,c Shane et al. (1988)
Negativeb
Reverse mutation S. typhimurium TA102, 5-500 µg/platef Negativee Shane et al. (1988)
TA100
Reverse mutation S. typhimurium TA100 152-950 µg/plate Negativeb Dorado et al. (1992)
Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA1535, 1% Negativee US Food & Drug
(suspension test) TA1537, TA1538 Administration (1974)
Reverse mutation S. typhimurium TA102 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
Reverse mutation S. typhimurium TA98, 0.17-17 200 µg/plate Negativee Aeschbacher et al. (1989)
TA100
Reverse mutation S. typhimurium TA100 2 or 4 mmol/plate Positiveg Suwa et al. (1982)
Mutation S. cerevisiae Not reported Negativee US Food & Drug
Administration (1974)
2,3-Pentanedione 410 Reverse mutation S. typhimurium TA100 105 µg/plate Negativeb Kim et al. (1987)
Reverse mutation S. typhimurium TA100, 0.9-90 000 µg/plate Negativee Aeschbacher et al. (1989)
3,4-Hexanedione 413 Reverse mutation S. typhimurium TA100 228-4900 µg/plate Negativeb Dorado et al. (1992)
Table 5. (continued)
Substance No. End-point Test object Dose Results Reference
Methylcyclopentenolone 418 Reverse mutation S. typhimurium TA1535, 10 000 µg/plate Negativee Heck et al. (1989)
TA1537, TA1538, TA98,
TA100
Unscheduled DNA Rat hepatocytes 500 µg/plate Negativea Heck et al. (1989)
synthesis
2-Hydroxy-2-cyclohexen-1-one 424 Reverse mutation S. typhimurium TA100 10, 100, 1000, Positivee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA98 10, 100, 1000, Negativee Bjeldanes & Chew (1979)
10 000 µg/plate
Reverse mutation S. typhimurium TA100 112-1000 µg/plate Negativeb Dorado et al. (1992)
a With metabolic activation
b Without metabolic activation
c Mutation frequency, 2-3
d Result based on authors' criterion for significant mutagenic effect, i.e. mutation frequency > 1.5
e With and without metabolic activation
f Estimated from graph
g Mutation frequency, < 2.5
Bjeldanes, L.F. & Chew, H. (1979) Mutagenicity of 1,2-dicarbonyl
compounds: Maltol, kojic acid, diacetyl and related substances.
Mutat. Res., 67, 367-371.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxication, New York, Academic
Press, Vol. I, pp. 231-248.
Colley, J., Gaunt, I.F., Lansdown, A.B.G., Grasso, P. & Gangolli, S.D.
(1969) Acute and short-term toxicity of diacetyl in rats. Food Chem.
Toxicol., 7, 571-580.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, J. & Hullin, R.P. (1954) Metabolism of acetoin. Formation and
utilization of acetoin and 2,3-butanediol in the decerebrated cat.
Biochem. J., 57, 177-180.
Dix & Jeffcoat (1997) Disposition and excretion of
[14C]2,3-butanedione in male rats following oral administration.
Unpublished report from Research Triangle Institute (NIEHS contract
No. NO1-ES-75407). Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Dorado, L., Montoya, M.R. & Rodriguez-Mellado, J.M. (1992) A
contribution to the study of the structure-mutagenicity relationship
for alpha-dicarbonyl compounds using the Ames test. Mutat. Res.,
269, 301-306.
Dow Chemical Co. (1953) Toxicity of cyclotene--Summary. Unpublished
report from Biochemical Research Department, Midland, Michigan, 15
July. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Gabriel, M.A., Jabara, H. & Al-Khalidi, A.A. (1971) Metabolism of
acetoin in mammalian liver slices and extracts. Biochem. J., 124,
793-800.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Garst, J., Stapleton, P. & Johnson, J. (1983) Mutagenicity of
alpha-hydroxy ketones may involve superoxide anion radical. Oxy
Radicals and Their Scavenger Systems, Amsterdam, Elsevier Science
Publishing, Vol. 2, pp. 125-130.
Gaunt, I.F., Branton, P.G., Kiss, I.S., Grasso, P. & Gangolli, S.D.
(1972) Short-term toxicity of acetoin (acetyl methylcarbinol) in rats.
Food Cosmet. Toxicol., 10, 131-141.
Givaudan Corp. (1952) Acute oral toxicity study of
methylcyclopentenolone in mice. Unpublished report to the Research
Institute of Fragrance Materials. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Gordon, A.J. & Ford, R.A. (1972) The Chemist's Companion, New York,
John Wiley & Sons, pp. 49-51.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Järnefelt, J. (1955) Studies on the enzymatic synthesis and breakdown
of acetoin in the animal organism. Ann. Acad. Sci. Fenn. Ser.
A. V. Med Anthropol., 57, 7-78.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Juni, E. & Heym, G.A. (1956) A cyclic pathway for the bacterial
dissimilation of 2,3-butanediol, acetyl methyl carbinol, and diacetyl.
J. Bacteriol., 71, 425-432.
Kato, F., Araki, A., Nozaki, K. & Matsushi, T. (1989) Mutagenicity of
aldehydes and diketones. Mutat. Res., 216, 366-367.
Kawano, T. (1959) Relation of acetoin and pantothenic acid. Fukuoka
Igaku Zasshi, 50, 2939-2953.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic
heterocyclic amines. Mutat. Res., 177, 9-15.
King, T., Faccini, J.M., Jachbaur, J., Perraud, J. & Monro, A.M.
(1979) 3-Generation and chronic toxicity study in rats. Unpublished
report from Pfizer Central Research. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Leberco Labs (1952) Acute toxicity of methylcyclopentenolone.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mills, G.A. & Walker, V. (1990) Urinary excretion of cyclohexanediol,
a metabolite of the solvent cyclohexanone, by infants in a special
care unit. Clin. Chem., 36, 870-874.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1977) Acute toxicity studies in rats, rabbits and guinea
pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Moreno, O.M. (1979) Acute toxicity studies in rats, rabbits and
guinea-pigs. Unpublished report to the Research Institute of Fragrance
Materials. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974) 90 Day feeding study
with 2-hydroxy-2-cyclohexen-1-one in rats. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Ong, C.N., Sia, G.L., Chia, S.E., Phoon, W.H. & Tan, K.T. (1991)
Determination of cyclohexanol in urine and its use in environmental
monitoring of cyclohexanone exposure. J. Anal. Toxicol., 15, 13-16.
Otsuka, M., Mine, T., Ohuchi, K. & Ohmori, S. (1996) A detoxication
route for acetaldehyde: Metabolism of diacetyl, acetoin, and
2,3-butanediol in liver homogenate and perfused liver of rats.
J. Biochem., 119, 246-251.
Pellmont, D. (1969) Studies with rats and mice on substance No.
R01-3801 (Toxikologisches Labor 256, Bau 69). Unpublished report to
the Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological test on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102 and TA104. Environ. Mol. Mutag., 11,
195-206.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Suwa, Y., Nagao, M., Kosugi, A. & Sugimura, T. (1982) Sulfite
suppresses the mutagenic property of coffee. Mutat. Res., 102,
383-391.
US Food & Drug Administration (1973) Teratologic Evaluation of FDA
71-73 (Starter Distillate, Hansen) (NTIS PB-223-833;
FDABF-GRAS-152), Washington DC, United States.
US Food & Drug Administration (1974) Mutagenic Evaluation of
Compound FDA 71-73 (Starter Distillate) (NTIS PB-245-431;
FDABF-GRAS-275), Washington DC, United States.
US Food & Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1970) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1982) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
Westerfeld, W.W. & Berg, R.L. (1943) Observations on the metabolism of
acetoin. J. Biol. Chem., 148, 523-528.
Wheldon, G.H. & Krajkeman, A.J. (1967) The effects of ten
food-flavoring additives administered to rats over a period of
thirteen weeks. Unpublished report from Huntington Research Center.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Williams, T.R. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 62, 78.
Zlatkis, A. & Sivetz, M. (1960) Analysis of coffee volatiles by gas
chromatography. Food Res., 25, 395-398.
