
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
SUBSTANCES STRUCTURALLY RELATED TO MENTHOL
First draft prepared by
M.F.A. Wouters, M.E. van Apeldoom, and G.J.A. Speijers
National Institute of Public Health and the Environment
Center of Substances and Risk Assessment
Bilthoven, The Netherlands
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety Evaluation
of Flavouring Agents
Consideration of combined intake from use as
flavouring agents
Conclusions
Relevant background information
Explanation
Intake
Biological data
Absorption and metabolism
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
and carcinogenicity
Genotoxicity
References
1. EVALUATION
1.1 Introduction
The Committee evaluated menthol and 13 structurally related
substances (Table 1) using the Procedure for the Safety Evaluation of
Flavouring Agents (Figure 1, p. 222, and Annex 1, reference 131).
Menthol was first evaluated at the eleventh meeting of the
Committee (Annex 1, reference 14), when it was allocated an
unconditional ADI of 0-0.2 mg/kg bw and a conditional ADI of 0.2-2
mg/kg bw. At the eighteenth meeting, an ADI of 0-0.2 mg/kg bw was
established (Annex 1, reference 35). The Committee reevaluated menthol
at its twentieth meeting (Annex 1, reference 41), when the ADI was
maintained. At the present meeting, the Committee allocated an ADI of
0-4 mg/kg bw to menthol (see monograph, this volume).
1.2 Estimated daily per capita intake
The total annual production volume of the 14 menthyl derivatives
is approximately 140 000 kg in Europe (International Organization of
the Flavor Industry, 1995) and 79 000 kg in the United States (US
National Academy of Sciences, 1987). Menthol (No. 427) and menthone
(No. 429) account for 97% of the total annual volume in Europe and 85%
in the United States.
Menthol and some structurally related substances occur naturally
in a wide variety of foods, including spearmint oil, cornmint oil,
peppermint oil, raspberries, rum, nutmeg, and cocoa (Maarse et al.,
1994). Menthol (10-70%) and menthone (7-40%) are the principal
constituents of peppermint oil. Eight of the substances in this group
have been reported to occur naturally in foods (Nos 427, 428, 429,
430, 431, 432, 434, and 435); six of the remaining substances are
esters of menthol (Nos 433, 443, 444, and 447) or ketals of menthone
(Nos 445 and 446). Quantitative data have been reported on the natural
occurrence and consumption ratios of five of these substances, which
indicate that they are consumed predominantly in traditional foods
(i.e. consumption ratio, > 1) (Stofberg & Kirschman, 1985; Stofberg &
Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The esters in this group (Nos 427, 432, 433, and 447) would be
expected to be readily hydrolysed to menthol and their respective
carboxylic acids; the latter are endogenous in humans. Carbonate (-)
esters (Nos 443 and 444) can be expected to be hydrolysed to menthol
(No. 427) and carbonate and either ethylene glycol or propylene
glycol. The ketals (Nos 445 and 446) are hydrolysed in vitro to
yield (-)- or (±)-menthone and simple glycols. The ketones (Nos 429,
430 and 435) in this group would be reduced to their corresponding
secondary alcohols, which, like menthol, would be conjugated with
glucuronic acid and then excreted in the urine. See also 'General
aspects of metabolism', p. 223.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. Menthol (No. 427), (+)- neo-menthol (No. 428), menthyl
acetate (No. 431), menthyl iso-valerate (No. 432),
(-)-menthyllactate (No. 433), menth-1-en-3-ol (No. 434),
(-)-menthol ethylene glycol carbonate (No. 443), (-)-menthol
1- and 2-propylene glycol carbonate (No. 444), and
mono-menthyl succinate (No. 447) are classified in
structural class I (Cramer et al., 1978). Menthone (No. 3),
(±)- iso-menthone (No. 430), (+)-piperitone (No. 435),
(-)-menthone 1,2-glycerol ketal (No. 445), and (±)-menthone
1,2-glycerol ketal (No. 446) are classified in structural
class II.
Step 2. At current levels of intake, the 14 substances would not be
expected to saturate the metabolic pathways, and all of the
substances are predicted to be metabolized to innocuous
products. Evaluation of substances in the group of
flavouring agents that includes menthol and structurally
related substances was based on data on the metabolic fate
of menthol and menthone, which are metabolized by oxidation
and conjugation. The extensive conjugation of menthol with
glucuronic acid and its rapid elimination in urine and bile,
combined with its simple chemical structure, gave assurance
about the innocuous nature of the products of metabolism.
The metabolites of (+)-piperitone (an alpha,ß-unsaturated
ketone) were considered to be innocuous by comparison with
the metabolic fate and toxicity of menthone (saturated) and
carvone (alpha,ß-unsaturated). Isomers of menthone
1,2-glycerol ketal were predicted to be converted to
menthone and can thus be evaluated for safety with menthone
and its metabolite menthol. This conclusion was supported by
the available data on the toxicity of these compounds.
Step A3. The daily per capita intake of all of the substances in
this group in class I, with the exception of menthol (No.
427), is below the human intake threshold for class I (1800
µg/person per day) in both Europe and the United States,
indicating that they pose no safety concern at current
levels of estimated intake as flavouring agents. Intake of
menthol (No. 427) in Europe (18 000 µg/person per day) and
the United States (10 000 µg/person per day) is greater than
the human intake threshold for class I.
Total intake of menthol (No. 427) in Europe and the
United States from its use and use of its esters (Nos 431,
432, 433, 443, 444, and 447) as flavouring agents is above
the human intake threshold for class I: 19 000 µg/day in
Europe and 12 000 µg/day in the United States.
The daily per capita intake of all of the substances
in this group in class II, with the exception of menthone
(No. 429), is below the human intake threshold for class II
(540 µg/person per day) in both Europe and the United
States. Intake of menthone (No. 429) in Europe (1000
µg/person per day) and in the United States (2500 µg/person
per day) is greater than the human intake threshold for
class II.
Total intake of menthone (No. 429) in Europe and the
United States from its use and use of its ketals (Nos 445
and 446) as flavouring agents is above the human exposure
threshold for class II: 1000 µg/day in Europe and 2900
µg/day in the United States.
Step A4. Menthol (No. 427) and menthone (No. 429) are not endogenous
in humans.
Step A5. An ADI of 0-4 mg/kg bw was allocated to menthol at the
present meeting (see monograph, this volume). For menthone,
a NOEL of 400 mg/kg bw per day was reported in a 28-day
study of toxicity in rats (Madsen et al., 1986). There is a
safety margin of > 1000 between this NOEL and the daily
per capita intake of menthone itself (42 or 17 µg/kg bw)
and the total daily per capita intake of menthone
including its derivatives (46 or 17 µg/kg bw). This
information indicates that neither menthone nor menthol
would be expected to be of safety concern.
The stepwise evaluations of menthol and 13 structurally related
substances used as flavouring agents are summarized in Table 1.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that menthol (class I) and menthone (class
II) were consumed concomitantly on a daily basis with the other 12
structurally related substances, the estimated combined intake would
exceed the human intake threshold for class II. All 14 substances are,
however, expected to be efficiently metabolized and would not saturate
metabolic pathways. On the basis of the evaluation of the collective
data, the combined intake was judged by the Committee not to raise
safety concern.
1.6 Conclusions
Menthol and the 13 structurally related substances evaluated do
not pose a safety concern at current levels of intake as flavouring
agents. The Committee noted that the available data on their toxicity
were consistent with the results of the safety evaluations using the
procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to the safety
evaluation of 13 substances structurally related to menthol (Table 1).
A separate monograph was prepared on menthol.
The substances in this group are structurally related because
they are alicyclic terpenoid ketones, secondary alcohols, related
esters, and ketals with a 3-menthyl carbon skeleton. They therefore
have similar metabolic and toxicological profiles. The group of
menthyl derivatives includes three ketones (Nos 429, 430, and 435),
one of which is a stereoisomer (No. 430), and an unsaturated analogue
(No. 435) of menthone (No. 429); three secondary alcohols (Nos 427,
428, and 434), which include a stereoisomer (No. 428) and an
unsaturated analogue (No. 434) of menthol (No. 427); two carbonate
esters (Nos 443 and 444) of (-)-menthol and either ethylene glycol or
propylene glycol, respectively; two ketals (Nos 445 and 446) of either
(-)-menthone or (±)-menthone, respectively, and glycerol; and four
esters (Nos 431, 432, 433, and 447) of menthol and either acetic acid,
isovaleric acid, lactic acid, or succinic acid. The four carboxylic
acids are endogenous.
Table 1. Summary of safety evaluation of substances structurally related to menthol
The human intake threshold is 1800 µg/day for class I and 540 µg/day for class II substances.
Step 2: All of the substances in this group are metabolized to innocuous products.
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL for based on
class intake, exceed substance substance or related current
Europe/USA intake or its substance? intake
(µg/day) threshold? metabolites
endogenous?
Menthol 427 89-78-1 I 18 000/10 000 Yes No Yes.The dose of 375 mg/kg bw No safety
per day that produced no adverse concerna
effects (US National Cancer
Institute, 1979) is > 1000 times
the daily per capita intake
of 173 mg/kg bw per day and
305 mg/kg bw per day from use
as a flavouring agent
 (+)-neo-Menthol 428 2216-52-6 I 3/27 No N/R N/R No safety
concern
(+)-neo-Menthol 428 2216-52-6 I 3/27 No N/R N/R No safety
concern
 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL for based on
class intake, exceed substance substance or related current
Europe/USA intake or its substance? intake
(µg/day) threshold? metabolites
endogenous?
Menthone 429 89-80-5 II 1000/2500 Yes No Yes. The dose of 400 mg/kg bw No safety
per day that produced no adverse concern
effects (Madsen, 1986] is > 1000
times the daily per capita intake
of 42 mg/kg bw per day and 17
mg /kg bw per day from use as a
flavouring agent
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL for based on
class intake, exceed substance substance or related current
Europe/USA intake or its substance? intake
(µg/day) threshold? metabolites
endogenous?
Menthone 429 89-80-5 II 1000/2500 Yes No Yes. The dose of 400 mg/kg bw No safety
per day that produced no adverse concern
effects (Madsen, 1986] is > 1000
times the daily per capita intake
of 42 mg/kg bw per day and 17
mg /kg bw per day from use as a
flavouring agent
 (±)-iso-Menthone 430 491-07-6 II 200/0.1 No N/R N/R No safety
concern
(±)-iso-Menthone 430 491-07-6 II 200/0.1 No N/R N/R No safety
concern
 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Menthyl acetate 431 16409-45-3 I 420/560 No N/R N/R No safety
concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Menthyl acetate 431 16409-45-3 I 420/560 No N/R N/R No safety
concern
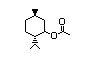 Menthyl isovalerate 432 16409-46-4 I 9/27 No N/R N/R No safety
concern
Menthyl isovalerate 432 16409-46-4 I 9/27 No N/R N/R No safety
concern
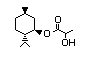
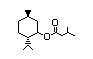 (-)-Menthyl lactate 433 59259-38-0 I 26/0.1 No N/R N/R No safety
concern
(-)-Menthyl lactate 433 59259-38-0 I 26/0.1 No N/R N/R No safety
concern
 para-Menth-1-en-3-ol 434 491-04-3 I 0.02/0.02 No N/R N/R No safety
concern
para-Menth-1-en-3-ol 434 491-04-3 I 0.02/0.02 No N/R N/R No safety
concern
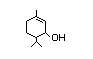 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Piperitone 435 6091-50-5 II 51/10 No N/R N/R No safety
concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Piperitone 435 6091-50-5 II 51/10 No N/R N/R No safety
concern
 (-)-Menthol ethylene glycol 443 156324-78-6 I N/D/760 No N/R N/R No safety
carbonate concern
(-)-Menthol ethylene glycol 443 156324-78-6 I N/D/760 No N/R N/R No safety
carbonate concern
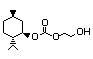 (-)-Menthol 1- and 2-propylene 444 156329-82-2 I N/D/380 No N/R N/R No safety
glycol carbonate concern
(-)-Menthol 1- and 2-propylene 444 156329-82-2 I N/D/380 No N/R N/R No safety
glycol carbonate concern
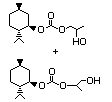 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
(-)-Menthone 1,2-glycerol 445 563187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
(-)-Menthone 1,2-glycerol 445 563187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
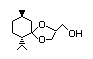 (±)-Menthone 1,2-glycerol 446 63187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
(±)-Menthone 1,2-glycerol 446 63187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
 mono-Menthyl succinate 447 77341-67-4 I N/D/22 No N/R N/R No safety
concern
mono-Menthyl succinate 447 77341-67-4 I N/D/22 No N/R N/R No safety
concern
 N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a An ADI of 0-4 mg/kg bw was established for menthol at the present meeting.
2.2 Intake
The total annual production volume of the 14 menthyl derivatives
is about 140 000 kg in Europe (International Organization of the
Flavor Industry, 1995) and 79 000 kg in The United States (US National
Academy of Sciences, 1987). The production volumes and intakes of each
substance are reported in Table 2. Menthol (No. 427) and menthone (No.
429) account for about 97% of the total annual volume in Europe and
85% in the United States.
Eight of the substances in this group (Nos 427-432, 434, and 435)
have been reported to occur naturally in foods (Maarse & Visscher,
1994). Six of the remaining substances are esters of menthol (Nos 433,
443, 444, and 447) or ketals of menthone (Nos 445 and 446), which are
hydrolysed to menthol or menthone in vivo. Menthol (10-70%) and
menthone (7-40%) are primary constituents of peppermint oil.
Quantitative data have been reported on the natural occurrence and
consumption ratios of five of these substances (see Table 2);
according to the authors, these data indicate that these substances
are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, menthyl esters are hydrolysed to menthol and their
respective carboxylic acids. The carbonate esters in this group are
hydrolysed to menthol and carbonate and either ethylene glycol or
propylene glycol. The ketals in this group are hydrolysed to menthone
and their respective alcohols. Ester hydrolysis is catalysed by
classes of enzymes recognized as carboxylesterases or esterases
(Heymann, 1980), the most important of which are the B-esterases
(Heymann, 1980; Anders, 1989). Acetyl esterases are the preferred
substrates of C-esterases (Heymann, 1980). These enzymes occur in most
mammalian tissues (Heymann, 1980; Anders, 1989) but predominate in
hepatocytes (Heymann, 1980). Esters (Nos 431-433 and 447) and
carbonate esters (Nos 443 and 444) of menthol are expected to be
hydrolysed in humans to yield menthol and their respective saturated
aliphatic carboxylic acids or alcohols.
In two studies, (-)-menthol ethylene glycol carbonate (No. 443)
and (-)-menthol propylene glycol carbonate (No. 444) were hydrolysed
after incubation with rat liver homogenate in vitro, the mean values
for hydrolysis to menthol being 85% for the ethylene glycol carbonate
and 75% for the propylene glycol carbonate (Emberger, 1994a,b). More
than 80% of radiolabelled cyclandelate (Figure 1), a structurally
related cyclohexyl ester, was hydrolysed after 20 min of incubation
with rat hepatic microsomes (White et al., 1990).
Table 2. Most recent annual usage volume of menthol and related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Menthol (427)
Europe 128 000 18 000 300
United States 54 000 10 000 170
(+)-neo-Menthol (428)
Europe 15 3 0.05
United States 140 27 0.4
Menthone (429)
Europe 7 300 1 000 17
United States 13 300 2 500 42
(±)-iso-Menthone (430)
Europe 1 100 200 3
United States 0.5 0.1 0.002
Menthyl acetate (431)
Europe 2 200 420 7
United States 2 900 560 9
Menthyl isovalerate (432)
Europe 48 9 0.2
United States 140 27 17
(-)-Menthyl lactate (433)
Europe 140 26 0.4
United States 0.5 0.1 0.002
para-Menth-1-en-3-ol (434)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003
Piperitone (435)
Europe 270 51 0
United States 50 10 0.2
(-)-Menthol ethylene gylcol carbonate (443)
Europe NR ND ND
United States 4 000 760 13
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
(-)-Menthol 1- and 2-propylene gylcol carbonate (444)
Europe NR ND ND
United States 2 000 381 6
(-)-Menthone1,2-glycerol ketal (445)
Europe NR ND ND
United States 1 000 190 3
(±)-Menthone1,2-glycerol ketal (446)
Europe NR ND ND
United States 1 000 190 3
mono-Menthyl succinate (447)
Europe NR ND ND
United States 110 22 0.4
Total
Europe 140 000
United States 79 000
Total menthol
Europe NA 12 000 190
United States NA 19 000 310
Total menthone
Europe NA 2 800 46
United States NA 1 000 17
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only')
= 32 × 106 for Europe and 32 × 106 for the United States; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows:
[(µg/day)/body weight], where body weight = 60 kg. Slight variation
may occur from rounding off.
N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a An ADI of 0-4 mg/kg bw was established for menthol at the present meeting.
2.2 Intake
The total annual production volume of the 14 menthyl derivatives
is about 140 000 kg in Europe (International Organization of the
Flavor Industry, 1995) and 79 000 kg in The United States (US National
Academy of Sciences, 1987). The production volumes and intakes of each
substance are reported in Table 2. Menthol (No. 427) and menthone (No.
429) account for about 97% of the total annual volume in Europe and
85% in the United States.
Eight of the substances in this group (Nos 427-432, 434, and 435)
have been reported to occur naturally in foods (Maarse & Visscher,
1994). Six of the remaining substances are esters of menthol (Nos 433,
443, 444, and 447) or ketals of menthone (Nos 445 and 446), which are
hydrolysed to menthol or menthone in vivo. Menthol (10-70%) and
menthone (7-40%) are primary constituents of peppermint oil.
Quantitative data have been reported on the natural occurrence and
consumption ratios of five of these substances (see Table 2);
according to the authors, these data indicate that these substances
are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, menthyl esters are hydrolysed to menthol and their
respective carboxylic acids. The carbonate esters in this group are
hydrolysed to menthol and carbonate and either ethylene glycol or
propylene glycol. The ketals in this group are hydrolysed to menthone
and their respective alcohols. Ester hydrolysis is catalysed by
classes of enzymes recognized as carboxylesterases or esterases
(Heymann, 1980), the most important of which are the B-esterases
(Heymann, 1980; Anders, 1989). Acetyl esterases are the preferred
substrates of C-esterases (Heymann, 1980). These enzymes occur in most
mammalian tissues (Heymann, 1980; Anders, 1989) but predominate in
hepatocytes (Heymann, 1980). Esters (Nos 431-433 and 447) and
carbonate esters (Nos 443 and 444) of menthol are expected to be
hydrolysed in humans to yield menthol and their respective saturated
aliphatic carboxylic acids or alcohols.
In two studies, (-)-menthol ethylene glycol carbonate (No. 443)
and (-)-menthol propylene glycol carbonate (No. 444) were hydrolysed
after incubation with rat liver homogenate in vitro, the mean values
for hydrolysis to menthol being 85% for the ethylene glycol carbonate
and 75% for the propylene glycol carbonate (Emberger, 1994a,b). More
than 80% of radiolabelled cyclandelate (Figure 1), a structurally
related cyclohexyl ester, was hydrolysed after 20 min of incubation
with rat hepatic microsomes (White et al., 1990).
Table 2. Most recent annual usage volume of menthol and related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Menthol (427)
Europe 128 000 18 000 300
United States 54 000 10 000 170
(+)-neo-Menthol (428)
Europe 15 3 0.05
United States 140 27 0.4
Menthone (429)
Europe 7 300 1 000 17
United States 13 300 2 500 42
(±)-iso-Menthone (430)
Europe 1 100 200 3
United States 0.5 0.1 0.002
Menthyl acetate (431)
Europe 2 200 420 7
United States 2 900 560 9
Menthyl isovalerate (432)
Europe 48 9 0.2
United States 140 27 17
(-)-Menthyl lactate (433)
Europe 140 26 0.4
United States 0.5 0.1 0.002
para-Menth-1-en-3-ol (434)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003
Piperitone (435)
Europe 270 51 0
United States 50 10 0.2
(-)-Menthol ethylene gylcol carbonate (443)
Europe NR ND ND
United States 4 000 760 13
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
(-)-Menthol 1- and 2-propylene gylcol carbonate (444)
Europe NR ND ND
United States 2 000 381 6
(-)-Menthone1,2-glycerol ketal (445)
Europe NR ND ND
United States 1 000 190 3
(±)-Menthone1,2-glycerol ketal (446)
Europe NR ND ND
United States 1 000 190 3
mono-Menthyl succinate (447)
Europe NR ND ND
United States 110 22 0.4
Total
Europe 140 000
United States 79 000
Total menthol
Europe NA 12 000 190
United States NA 19 000 310
Total menthone
Europe NA 2 800 46
United States NA 1 000 17
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only')
= 32 × 106 for Europe and 32 × 106 for the United States; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows:
[(µg/day)/body weight], where body weight = 60 kg. Slight variation
may occur from rounding off.
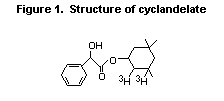 Menthyl derivatives are metabolized like other alicyclic ketones
and secondary alcohols. Ketones are reduced to their corresponding
secondary alcohols and conjugated mainly with glucuronic acid (Quick,
1928; Williams, 1940; Atzl et al., 1972). The metabolites of menthol
are eliminated in the urine or faeces either unchanged or conjugated
with glucuronic acid (Yamaguchi et al., 1994). The parent ketone,
menthone, is primarily reduced to the correspon-ding secondary
alcohol, neo-menthol, which is metabolized and eliminated by
pathways similar to those of its stereoisomer, menthol (Williams,
1940).
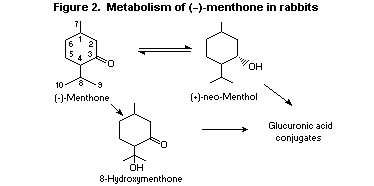
(-)-Menthone given to rabbits at a dose of 1000 mg/kg bw was
stereoselectively reduced to (+)- neo-menthol (Figure 2). Similarly,
the stereoisomer (+)- iso-menthone was reduced to (+)- iso-menthol.
About 67% of a 1000-mg/kg bw dose of (+)- neo-menthol given to
rabbits by stomach tube was eliminated in the urine as the glucuronic
acid conjugate (Williams, 1940). Like other alicyclic terpenoids such
as menthol, (±)- iso-menthone may also undergo omega-oxidation of the
alkyl ring substituents, to yield the corresponding hydroxyketones.
Data on structurally related alicyclic terpenoids suggest that
oxidation occurs preferentially on the isopropyl or methyl substituent
and not on the cyclohexane ring (Yamaguchi et al., 1994).
Menthyl derivatives are metabolized like other alicyclic ketones
and secondary alcohols. Ketones are reduced to their corresponding
secondary alcohols and conjugated mainly with glucuronic acid (Quick,
1928; Williams, 1940; Atzl et al., 1972). The metabolites of menthol
are eliminated in the urine or faeces either unchanged or conjugated
with glucuronic acid (Yamaguchi et al., 1994). The parent ketone,
menthone, is primarily reduced to the correspon-ding secondary
alcohol, neo-menthol, which is metabolized and eliminated by
pathways similar to those of its stereoisomer, menthol (Williams,
1940).
(-)-Menthone given to rabbits at a dose of 1000 mg/kg bw was
stereoselectively reduced to (+)- neo-menthol (Figure 2). Similarly,
the stereoisomer (+)- iso-menthone was reduced to (+)- iso-menthol.
About 67% of a 1000-mg/kg bw dose of (+)- neo-menthol given to
rabbits by stomach tube was eliminated in the urine as the glucuronic
acid conjugate (Williams, 1940). Like other alicyclic terpenoids such
as menthol, (±)- iso-menthone may also undergo omega-oxidation of the
alkyl ring substituents, to yield the corresponding hydroxyketones.
Data on structurally related alicyclic terpenoids suggest that
oxidation occurs preferentially on the isopropyl or methyl substituent
and not on the cyclohexane ring (Yamaguchi et al., 1994).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 11 of the 14 substances
(Table 3). The values are in the range 940-7300 mg/kg bw, indicating
that the acute toxicity of orally administered menthol and related
substances is low. The lowest reported value was not, however,
supported by other studies in the same species.
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of short-term studies and of long-term studies of
toxicity and carcinogenicity on substances related to menthol are
summarized in Table 4 and described below. Studies on menthol are
summarized in the separate monograph on that substance.
Menthone (No. 429)
Three short-term studies on peppermint oil and peppermint oil
components, i.e. menthone, pulegone, and menthol, from the same
laboratory showed 'cyst-like spaces' in the white matter of the
cerebellum of rats. Groups of male and female rats were given menthone
at doses of 0, 200, 400, or 800 mg/kg bw per day (Madsen et al.,
1986); peppermint oil at 0, 10, 40, or 100 mg/kg bw per day (Thorup et
al., 1983a); pulegone at 0, 20, 80, or 160 mg/kg bw per day (Thorup et
al., 1983b); or menthol at 0, 200, 400, or 800 mg/kg bw per day
(Thorup et al., 1983b) by gavage daily for 28 days. Cyst-like spaces
in the white cerebellar matter were reported at the two highest doses
of peppermint oil and pulegone and at all doses of menthone. A similar
effect was not observed with menthol.
The slides of the brains of the animals in these studies were
subsequently reviewed independently (Smith et al., 1996). Three
conclusions were reached:
(1) No cellular reaction was seen in tissue adjacent to the cyst-like
spaces in the white matter of the cerbellum, either in the three
studies or in a subsequent 90-day study in rats given peppermint
oil at a dose of 0, 10, 40, or 100 mg/kg bw per day (Spindler &
Madsen, 1992). The appearance and extent of the cyst-like spaces
were no different in the last, longer study than in the first
three.
(2) The cyst-like spaces in cerebellar tissue were not seen in
five-week studies in which rats of the same strain were given
peppermint oil at doses of 150 or 500 mg/kg bw per day and dogs
were given peppermint oil in gelatin capsules daily at a dose of
25 or 125 mg/kg bw per day (Mengs & Stotzem, 1989).
Table 3. Studies of the acute toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
(+)-neo-Menthol 428 Mouse NR Gavage 4000 Wokes (1932)
Menthone 429 Rat M/F Oral 1600-1950 Levenstein (1973);
Igmi & Ide (1974)
Menthyl acetate 431 Rat M/F Gavage > 7000 Levenstein (1973)
Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
Menthyl iso-valerate 432 Rat NR Oral > 5000 Moreno (1976)
(-)-Menthyl lactate 433 Rat M/F Oral 7257 Reagan & Becci (1984)
(-)-Menthol ethylene glycol carbonate 443 Rat M/F Oral > 2000 Tuffnell (1992)
(-)-Menthol 1- and 2-propylene glycol carbonate 444 Rat M/F Oral > 2000 Driscoll (1993)
(±)-Menthone 1,2-glycerol ketal 445 Rat M/F Oral 5716 Reijnders (1991a)
Rat M/F Gavage > 2000 Skydsgaard (1991)
Monomenthyl succinate 447 Rat M/F Oral > 5000 Mercier (1994)
NR, not reported; M, male; F, female
Table 4. Short-term and long-term studies of the toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex No. of groups/ Route Duration NOEL Reference
no. per group (mg/kg
bw per day)
Menthone 429 Rats M/F 3/20 Gavage 28 weeks 400 Madsen (1986)
Mice F 2/NR Intraperitoneal 24 weeks --a Stoner et al. (1973)
(±)-Menthone 1,2-glycerol ketal 446 Rats M/F 3/10 Gavage 28 days 50 Reijnders (1991b)
M, male; F, female; NR, not reported
a No NOEL was determined.
(3) No cyst-like spaces were seen in cerebellar tissue when the
brains of rats were perfused with peppermint oil (Olsen, 1994).
The authors of the independent review concluded that the
cyst-like spaces found after treatment of rats with peppermint oil,
pulegone, or menthone were artefacts arising from inadequate
preparation and fixation of the cerebellar tissue (Adams et al., 1996;
Smith et al., 1996).
Other reported effects in the groups of 10 male and 10 female
rats receiving menthone at doses of 0, 200, 400, or 800 mg/kg bw per
day by gavage for 28 days included a signficant reduction in food
consumption among males receiving the high dose, among females at all
doses during the first two weeks, and among females at the high dose
during week 3 (Madsen et al., 1986). The reduction was attributed to
the unpalatibility of the diet and is similar to that observed in
other studies with menthone (Mengs & Stotzem, 1989). It was further
reported that the relative weights of the kidney, spleen, liver, and
brain in females and of the spleen, liver, and brain in males were
statistically significantly increased at all doses. Furthermore, the
bilirubin concentration and alkaline phosphatase activity in plasma
were increased in all treated animals. A further statistical review
showed, however, that the increases in organ weights and clinical
chemical parameters occurred only at the high dose. The NOEL for
menthone was therefore 400 mg/kg bw per day (Madsen et al., 1986),
which is more than 10 000 times the daily per capita intake ('eaters
only') of 17 and 42 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
Groups of female A/He mice were given intraperitoneal injections
of menthone at doses of 1900 or 4750 mg/kg bw three times weekly for
eight weeks. The animals were observed for an additional 16 weeks;
20-45% of the treated animals and 15% of the controls died before the
end of the study. No increases were seen in the incidences of
non-neoplastic or neoplastic lesions in the lung, liver, kidney,
spleen, thymus, intestine, or salivary or endocrine glands of treated
animals. The authors reported that the vehicle used, tricaprylin, had
caused a 3-4 g loss of weight in control animals during the first week
of the study, high mortality rates, and higher mean incidences of
tumours (Stoner et al., 1973).
(±)-Menthone 1,2-glycerol ketal (No. 446)
Four groups of five male and five female Wistar rat were given
(±)-menthone 1,2-glycerol ketal at doses of 0, 50, 200, or 800 mg/kg
bw per day by gavage for 28 days and were observed daily throughout
the study. The body weights and food consumption of the animals were
measured weekly and on the day before necropsy, when macroscopic
observations and organ weights were recorded. The adrenal glands,
heart, kidney, liver, and stomach were examined histologically.
Decreased serum glucose concentrations and increased kidney weights
were noted in males at the two highest doses. Periportal
hepatocellular hypertrophy was reported in these animals and in
females at the highest dose. The hypertrophy in the males was
accompanied by fine hepatocellular vacuolation. Increased liver
weights were reported in males and females at the highest dose. The
NOEL was 50 mg/kg bw per day (Reijnders, 1991b), which is more than
10 000 times the daily per capita intake ('eaters only') of 3 µg/kg
bw from its use as a flavouring agent in the United States (see Table
2).
2.3.2.3 Genotoxicity
The results of tests for genotoxicity with five representative
menthyl derivatives are shown in Table 5.
Menthone (No. 429) was not mutagenic in the standard Ames test
with Salmonella typhimurium strains TA98, TA100, or TA1535 when
tested at concentrations of up to 800 µg/plate, either with or without
metabolic activation; however, it induced reverse mutation in
S. typhimurium TA97 at concentrations up to 160 µg/plate in the
presence of metabolic activation and at concentrations up to 800
µg/plate in the absence of activation. It was also mutagenic in strain
TA1537 at concentrations of 32 and 6.4 µg/plate (Andersen & Jensen,
1984).
(-)-Menthol ethylene glycol carbonate (No. 443), (-)-menthol
1,2-propylene glycol carbonate (No. 444), and (±)-menthone
1,2-glycerol ketal (No. 446) were not mutagenic in the standard Ames
test or in the preincubation protocol with S. typhimurium strains
TA98, TA100, TA1535, TA1537, and TA1538 at concentrations up to 5000
µg/plate, either with or without metabolic activation (King, 1991;
Poth, 1991; King, 1992, 1993). (-)-Menthol ethylene glycol carbonate
(No. 443) and (-)-menthol 1,2-propylene glycol carbonate (No. 444) did
not induce chromosomal aberrations in human peripheral blood
lymphocytes when tested at concentrations up to 300 µg/ml (King,
1994a,b). (±)-Menthone 1,2-glycerol ketal (No. 446) given orally to
NWRI mice at doses up to 2500 mg/kg bw did not induce micronuclei in
bone-marrow cells (Völkner, 1991).
4. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York, Taylor &
Francis, pp. 81-97.
Table 5. Results of assays for the genotoxicity of substances structurally related to menthol
Substance No. End-point Test object Dose Result Reference
In vitro
Menthone 429 Reverse mutation S. typhimurium TA 1535, 800 µg/plate Negativea Anderson &
TA 100, TA 98 Jensen (1984)
Reverse mutation S. typhimurium TA 1537, 6.4-800 µg/plate Positivea Andersen &
TA 97 Jensen (1984)
(-)-Menthol ethylene 443 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1992)
glycol TA 1537, TA 98, TA 100,
TA 1538
Chromosomal Human peripheral blood 300 µg/ml Negativea King (1994a)
aberration lymphocytes
(-)-Menthol 1- and 444 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1993)
2-propylene glycol TA 1537, TA 1538, TA 98,
carbonate TA 100
Chromosomal Human peripheral blood 300 µg/plate Negativea King (1994b)
aberration lymphocytes
(±)-Menthone 1,2-glycerol 446 Reverse mutation S. typhimurium TA 1535, 1500 µg/plate Negativea King (1991)
ketal TA 1537, TA 98, TA 100,
TA 1538
Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea Poth (1991)
TA 1537, TA 98, TA 100,
TA 1538
In vivo
(±)-Menthone 1,2-glycerol 446 Micronucleus NMRI mice 2500 mg/kg bw Negative Volkner (1991)
ketal formation
a With and without metabolic activation
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Driscoll, R. (1993) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Emberger (1994a) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Emberger (1994b) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxification, 2nd Ed., New York, Academic
Press, pp. 291-323.
Igmi, H. & Ide, H. (1974) Improvements in or Relating to Substances
for Use in the Treatment of Gallstones, Patet 1343561, Appl.
13606/772, A61k27/00, 23 March 1972.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
King, M.T. (1991) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
King, M.T (1992) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1993) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1994a) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
King, M.T. (1994b) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Levenstein, I. (1973) Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Mercier, O. (1994) Acute oral toxicity. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States
Olsen, P. (1994) Oral presentation to the FEMA Expert Panel.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Poth, A. (1991) Salmonella typhimurium reverse mutation assay with
91/917 247. Unpublished report from Cytotest Cell Research. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of
frescolate/l-methyl lactate in Sprague-Dawley rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Reijnders, J.B. (1991a) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Reijnders, J.B. (1991b) Subacute 28-day oral toxicity. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United State, Washington DC, United States.
Shelanski, M.V. & Moldovan, M. (1972) Unpublished report to the
Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Skydsgaard, K. (1991) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B. &
the FEMA Expert Panel (1996) Recent progress in the consideration of
flavoring ingredients under the Food Additives Amendment. 17. GRAS
substances. Food Technol., 50, 72-78, 80-81.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Tuffnell, P.P. (1992) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 11 of the 14 substances
(Table 3). The values are in the range 940-7300 mg/kg bw, indicating
that the acute toxicity of orally administered menthol and related
substances is low. The lowest reported value was not, however,
supported by other studies in the same species.
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of short-term studies and of long-term studies of
toxicity and carcinogenicity on substances related to menthol are
summarized in Table 4 and described below. Studies on menthol are
summarized in the separate monograph on that substance.
Menthone (No. 429)
Three short-term studies on peppermint oil and peppermint oil
components, i.e. menthone, pulegone, and menthol, from the same
laboratory showed 'cyst-like spaces' in the white matter of the
cerebellum of rats. Groups of male and female rats were given menthone
at doses of 0, 200, 400, or 800 mg/kg bw per day (Madsen et al.,
1986); peppermint oil at 0, 10, 40, or 100 mg/kg bw per day (Thorup et
al., 1983a); pulegone at 0, 20, 80, or 160 mg/kg bw per day (Thorup et
al., 1983b); or menthol at 0, 200, 400, or 800 mg/kg bw per day
(Thorup et al., 1983b) by gavage daily for 28 days. Cyst-like spaces
in the white cerebellar matter were reported at the two highest doses
of peppermint oil and pulegone and at all doses of menthone. A similar
effect was not observed with menthol.
The slides of the brains of the animals in these studies were
subsequently reviewed independently (Smith et al., 1996). Three
conclusions were reached:
(1) No cellular reaction was seen in tissue adjacent to the cyst-like
spaces in the white matter of the cerbellum, either in the three
studies or in a subsequent 90-day study in rats given peppermint
oil at a dose of 0, 10, 40, or 100 mg/kg bw per day (Spindler &
Madsen, 1992). The appearance and extent of the cyst-like spaces
were no different in the last, longer study than in the first
three.
(2) The cyst-like spaces in cerebellar tissue were not seen in
five-week studies in which rats of the same strain were given
peppermint oil at doses of 150 or 500 mg/kg bw per day and dogs
were given peppermint oil in gelatin capsules daily at a dose of
25 or 125 mg/kg bw per day (Mengs & Stotzem, 1989).
Table 3. Studies of the acute toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
(+)-neo-Menthol 428 Mouse NR Gavage 4000 Wokes (1932)
Menthone 429 Rat M/F Oral 1600-1950 Levenstein (1973);
Igmi & Ide (1974)
Menthyl acetate 431 Rat M/F Gavage > 7000 Levenstein (1973)
Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
Menthyl iso-valerate 432 Rat NR Oral > 5000 Moreno (1976)
(-)-Menthyl lactate 433 Rat M/F Oral 7257 Reagan & Becci (1984)
(-)-Menthol ethylene glycol carbonate 443 Rat M/F Oral > 2000 Tuffnell (1992)
(-)-Menthol 1- and 2-propylene glycol carbonate 444 Rat M/F Oral > 2000 Driscoll (1993)
(±)-Menthone 1,2-glycerol ketal 445 Rat M/F Oral 5716 Reijnders (1991a)
Rat M/F Gavage > 2000 Skydsgaard (1991)
Monomenthyl succinate 447 Rat M/F Oral > 5000 Mercier (1994)
NR, not reported; M, male; F, female
Table 4. Short-term and long-term studies of the toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex No. of groups/ Route Duration NOEL Reference
no. per group (mg/kg
bw per day)
Menthone 429 Rats M/F 3/20 Gavage 28 weeks 400 Madsen (1986)
Mice F 2/NR Intraperitoneal 24 weeks --a Stoner et al. (1973)
(±)-Menthone 1,2-glycerol ketal 446 Rats M/F 3/10 Gavage 28 days 50 Reijnders (1991b)
M, male; F, female; NR, not reported
a No NOEL was determined.
(3) No cyst-like spaces were seen in cerebellar tissue when the
brains of rats were perfused with peppermint oil (Olsen, 1994).
The authors of the independent review concluded that the
cyst-like spaces found after treatment of rats with peppermint oil,
pulegone, or menthone were artefacts arising from inadequate
preparation and fixation of the cerebellar tissue (Adams et al., 1996;
Smith et al., 1996).
Other reported effects in the groups of 10 male and 10 female
rats receiving menthone at doses of 0, 200, 400, or 800 mg/kg bw per
day by gavage for 28 days included a signficant reduction in food
consumption among males receiving the high dose, among females at all
doses during the first two weeks, and among females at the high dose
during week 3 (Madsen et al., 1986). The reduction was attributed to
the unpalatibility of the diet and is similar to that observed in
other studies with menthone (Mengs & Stotzem, 1989). It was further
reported that the relative weights of the kidney, spleen, liver, and
brain in females and of the spleen, liver, and brain in males were
statistically significantly increased at all doses. Furthermore, the
bilirubin concentration and alkaline phosphatase activity in plasma
were increased in all treated animals. A further statistical review
showed, however, that the increases in organ weights and clinical
chemical parameters occurred only at the high dose. The NOEL for
menthone was therefore 400 mg/kg bw per day (Madsen et al., 1986),
which is more than 10 000 times the daily per capita intake ('eaters
only') of 17 and 42 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
Groups of female A/He mice were given intraperitoneal injections
of menthone at doses of 1900 or 4750 mg/kg bw three times weekly for
eight weeks. The animals were observed for an additional 16 weeks;
20-45% of the treated animals and 15% of the controls died before the
end of the study. No increases were seen in the incidences of
non-neoplastic or neoplastic lesions in the lung, liver, kidney,
spleen, thymus, intestine, or salivary or endocrine glands of treated
animals. The authors reported that the vehicle used, tricaprylin, had
caused a 3-4 g loss of weight in control animals during the first week
of the study, high mortality rates, and higher mean incidences of
tumours (Stoner et al., 1973).
(±)-Menthone 1,2-glycerol ketal (No. 446)
Four groups of five male and five female Wistar rat were given
(±)-menthone 1,2-glycerol ketal at doses of 0, 50, 200, or 800 mg/kg
bw per day by gavage for 28 days and were observed daily throughout
the study. The body weights and food consumption of the animals were
measured weekly and on the day before necropsy, when macroscopic
observations and organ weights were recorded. The adrenal glands,
heart, kidney, liver, and stomach were examined histologically.
Decreased serum glucose concentrations and increased kidney weights
were noted in males at the two highest doses. Periportal
hepatocellular hypertrophy was reported in these animals and in
females at the highest dose. The hypertrophy in the males was
accompanied by fine hepatocellular vacuolation. Increased liver
weights were reported in males and females at the highest dose. The
NOEL was 50 mg/kg bw per day (Reijnders, 1991b), which is more than
10 000 times the daily per capita intake ('eaters only') of 3 µg/kg
bw from its use as a flavouring agent in the United States (see Table
2).
2.3.2.3 Genotoxicity
The results of tests for genotoxicity with five representative
menthyl derivatives are shown in Table 5.
Menthone (No. 429) was not mutagenic in the standard Ames test
with Salmonella typhimurium strains TA98, TA100, or TA1535 when
tested at concentrations of up to 800 µg/plate, either with or without
metabolic activation; however, it induced reverse mutation in
S. typhimurium TA97 at concentrations up to 160 µg/plate in the
presence of metabolic activation and at concentrations up to 800
µg/plate in the absence of activation. It was also mutagenic in strain
TA1537 at concentrations of 32 and 6.4 µg/plate (Andersen & Jensen,
1984).
(-)-Menthol ethylene glycol carbonate (No. 443), (-)-menthol
1,2-propylene glycol carbonate (No. 444), and (±)-menthone
1,2-glycerol ketal (No. 446) were not mutagenic in the standard Ames
test or in the preincubation protocol with S. typhimurium strains
TA98, TA100, TA1535, TA1537, and TA1538 at concentrations up to 5000
µg/plate, either with or without metabolic activation (King, 1991;
Poth, 1991; King, 1992, 1993). (-)-Menthol ethylene glycol carbonate
(No. 443) and (-)-menthol 1,2-propylene glycol carbonate (No. 444) did
not induce chromosomal aberrations in human peripheral blood
lymphocytes when tested at concentrations up to 300 µg/ml (King,
1994a,b). (±)-Menthone 1,2-glycerol ketal (No. 446) given orally to
NWRI mice at doses up to 2500 mg/kg bw did not induce micronuclei in
bone-marrow cells (Völkner, 1991).
4. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York, Taylor &
Francis, pp. 81-97.
Table 5. Results of assays for the genotoxicity of substances structurally related to menthol
Substance No. End-point Test object Dose Result Reference
In vitro
Menthone 429 Reverse mutation S. typhimurium TA 1535, 800 µg/plate Negativea Anderson &
TA 100, TA 98 Jensen (1984)
Reverse mutation S. typhimurium TA 1537, 6.4-800 µg/plate Positivea Andersen &
TA 97 Jensen (1984)
(-)-Menthol ethylene 443 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1992)
glycol TA 1537, TA 98, TA 100,
TA 1538
Chromosomal Human peripheral blood 300 µg/ml Negativea King (1994a)
aberration lymphocytes
(-)-Menthol 1- and 444 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1993)
2-propylene glycol TA 1537, TA 1538, TA 98,
carbonate TA 100
Chromosomal Human peripheral blood 300 µg/plate Negativea King (1994b)
aberration lymphocytes
(±)-Menthone 1,2-glycerol 446 Reverse mutation S. typhimurium TA 1535, 1500 µg/plate Negativea King (1991)
ketal TA 1537, TA 98, TA 100,
TA 1538
Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea Poth (1991)
TA 1537, TA 98, TA 100,
TA 1538
In vivo
(±)-Menthone 1,2-glycerol 446 Micronucleus NMRI mice 2500 mg/kg bw Negative Volkner (1991)
ketal formation
a With and without metabolic activation
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Driscoll, R. (1993) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Emberger (1994a) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Emberger (1994b) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxification, 2nd Ed., New York, Academic
Press, pp. 291-323.
Igmi, H. & Ide, H. (1974) Improvements in or Relating to Substances
for Use in the Treatment of Gallstones, Patet 1343561, Appl.
13606/772, A61k27/00, 23 March 1972.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
King, M.T. (1991) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
King, M.T (1992) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1993) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1994a) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
King, M.T. (1994b) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Levenstein, I. (1973) Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Mercier, O. (1994) Acute oral toxicity. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States
Olsen, P. (1994) Oral presentation to the FEMA Expert Panel.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Poth, A. (1991) Salmonella typhimurium reverse mutation assay with
91/917 247. Unpublished report from Cytotest Cell Research. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of
frescolate/l-methyl lactate in Sprague-Dawley rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Reijnders, J.B. (1991a) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Reijnders, J.B. (1991b) Subacute 28-day oral toxicity. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United State, Washington DC, United States.
Shelanski, M.V. & Moldovan, M. (1972) Unpublished report to the
Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Skydsgaard, K. (1991) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B. &
the FEMA Expert Panel (1996) Recent progress in the consideration of
flavoring ingredients under the Food Additives Amendment. 17. GRAS
substances. Food Technol., 50, 72-78, 80-81.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Tuffnell, P.P. (1992) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
See Also:
Toxicological Abbreviations
 (+)-neo-Menthol 428 2216-52-6 I 3/27 No N/R N/R No safety
concern
(+)-neo-Menthol 428 2216-52-6 I 3/27 No N/R N/R No safety
concern
 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL for based on
class intake, exceed substance substance or related current
Europe/USA intake or its substance? intake
(µg/day) threshold? metabolites
endogenous?
Menthone 429 89-80-5 II 1000/2500 Yes No Yes. The dose of 400 mg/kg bw No safety
per day that produced no adverse concern
effects (Madsen, 1986] is > 1000
times the daily per capita intake
of 42 mg/kg bw per day and 17
mg /kg bw per day from use as a
flavouring agent
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL for based on
class intake, exceed substance substance or related current
Europe/USA intake or its substance? intake
(µg/day) threshold? metabolites
endogenous?
Menthone 429 89-80-5 II 1000/2500 Yes No Yes. The dose of 400 mg/kg bw No safety
per day that produced no adverse concern
effects (Madsen, 1986] is > 1000
times the daily per capita intake
of 42 mg/kg bw per day and 17
mg /kg bw per day from use as a
flavouring agent
 (±)-iso-Menthone 430 491-07-6 II 200/0.1 No N/R N/R No safety
concern
(±)-iso-Menthone 430 491-07-6 II 200/0.1 No N/R N/R No safety
concern
 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Menthyl acetate 431 16409-45-3 I 420/560 No N/R N/R No safety
concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Menthyl acetate 431 16409-45-3 I 420/560 No N/R N/R No safety
concern
 Menthyl isovalerate 432 16409-46-4 I 9/27 No N/R N/R No safety
concern
Menthyl isovalerate 432 16409-46-4 I 9/27 No N/R N/R No safety
concern
 (-)-Menthyl lactate 433 59259-38-0 I 26/0.1 No N/R N/R No safety
concern
(-)-Menthyl lactate 433 59259-38-0 I 26/0.1 No N/R N/R No safety
concern
 para-Menth-1-en-3-ol 434 491-04-3 I 0.02/0.02 No N/R N/R No safety
concern
para-Menth-1-en-3-ol 434 491-04-3 I 0.02/0.02 No N/R N/R No safety
concern
 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Piperitone 435 6091-50-5 II 51/10 No N/R N/R No safety
concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
Piperitone 435 6091-50-5 II 51/10 No N/R N/R No safety
concern
 (-)-Menthol ethylene glycol 443 156324-78-6 I N/D/760 No N/R N/R No safety
carbonate concern
(-)-Menthol ethylene glycol 443 156324-78-6 I N/D/760 No N/R N/R No safety
carbonate concern
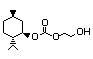 (-)-Menthol 1- and 2-propylene 444 156329-82-2 I N/D/380 No N/R N/R No safety
glycol carbonate concern
(-)-Menthol 1- and 2-propylene 444 156329-82-2 I N/D/380 No N/R N/R No safety
glycol carbonate concern
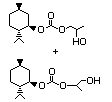 Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
(-)-Menthone 1,2-glycerol 445 563187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
Table 1. (continued)
Substance No. CAS No. Step 1 Estimated Step A3 Step A4 Step A5 Conclusion
Structural per capita Does intake Is the Adequate NOEL based on
class intake, exceed substance for substance current
Europe/USA intake or its or related intake
(µg/day) threshold? metabolites substance?
endogenous?
(-)-Menthone 1,2-glycerol 445 563187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
 (±)-Menthone 1,2-glycerol 446 63187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
(±)-Menthone 1,2-glycerol 446 63187-91-7 II N/D/190 No N/R N/R No safety
ketal concern
 mono-Menthyl succinate 447 77341-67-4 I N/D/22 No N/R N/R No safety
concern
mono-Menthyl succinate 447 77341-67-4 I N/D/22 No N/R N/R No safety
concern
 N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a An ADI of 0-4 mg/kg bw was established for menthol at the present meeting.
2.2 Intake
The total annual production volume of the 14 menthyl derivatives
is about 140 000 kg in Europe (International Organization of the
Flavor Industry, 1995) and 79 000 kg in The United States (US National
Academy of Sciences, 1987). The production volumes and intakes of each
substance are reported in Table 2. Menthol (No. 427) and menthone (No.
429) account for about 97% of the total annual volume in Europe and
85% in the United States.
Eight of the substances in this group (Nos 427-432, 434, and 435)
have been reported to occur naturally in foods (Maarse & Visscher,
1994). Six of the remaining substances are esters of menthol (Nos 433,
443, 444, and 447) or ketals of menthone (Nos 445 and 446), which are
hydrolysed to menthol or menthone in vivo. Menthol (10-70%) and
menthone (7-40%) are primary constituents of peppermint oil.
Quantitative data have been reported on the natural occurrence and
consumption ratios of five of these substances (see Table 2);
according to the authors, these data indicate that these substances
are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, menthyl esters are hydrolysed to menthol and their
respective carboxylic acids. The carbonate esters in this group are
hydrolysed to menthol and carbonate and either ethylene glycol or
propylene glycol. The ketals in this group are hydrolysed to menthone
and their respective alcohols. Ester hydrolysis is catalysed by
classes of enzymes recognized as carboxylesterases or esterases
(Heymann, 1980), the most important of which are the B-esterases
(Heymann, 1980; Anders, 1989). Acetyl esterases are the preferred
substrates of C-esterases (Heymann, 1980). These enzymes occur in most
mammalian tissues (Heymann, 1980; Anders, 1989) but predominate in
hepatocytes (Heymann, 1980). Esters (Nos 431-433 and 447) and
carbonate esters (Nos 443 and 444) of menthol are expected to be
hydrolysed in humans to yield menthol and their respective saturated
aliphatic carboxylic acids or alcohols.
In two studies, (-)-menthol ethylene glycol carbonate (No. 443)
and (-)-menthol propylene glycol carbonate (No. 444) were hydrolysed
after incubation with rat liver homogenate in vitro, the mean values
for hydrolysis to menthol being 85% for the ethylene glycol carbonate
and 75% for the propylene glycol carbonate (Emberger, 1994a,b). More
than 80% of radiolabelled cyclandelate (Figure 1), a structurally
related cyclohexyl ester, was hydrolysed after 20 min of incubation
with rat hepatic microsomes (White et al., 1990).
Table 2. Most recent annual usage volume of menthol and related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Menthol (427)
Europe 128 000 18 000 300
United States 54 000 10 000 170
(+)-neo-Menthol (428)
Europe 15 3 0.05
United States 140 27 0.4
Menthone (429)
Europe 7 300 1 000 17
United States 13 300 2 500 42
(±)-iso-Menthone (430)
Europe 1 100 200 3
United States 0.5 0.1 0.002
Menthyl acetate (431)
Europe 2 200 420 7
United States 2 900 560 9
Menthyl isovalerate (432)
Europe 48 9 0.2
United States 140 27 17
(-)-Menthyl lactate (433)
Europe 140 26 0.4
United States 0.5 0.1 0.002
para-Menth-1-en-3-ol (434)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003
Piperitone (435)
Europe 270 51 0
United States 50 10 0.2
(-)-Menthol ethylene gylcol carbonate (443)
Europe NR ND ND
United States 4 000 760 13
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
(-)-Menthol 1- and 2-propylene gylcol carbonate (444)
Europe NR ND ND
United States 2 000 381 6
(-)-Menthone1,2-glycerol ketal (445)
Europe NR ND ND
United States 1 000 190 3
(±)-Menthone1,2-glycerol ketal (446)
Europe NR ND ND
United States 1 000 190 3
mono-Menthyl succinate (447)
Europe NR ND ND
United States 110 22 0.4
Total
Europe 140 000
United States 79 000
Total menthol
Europe NA 12 000 190
United States NA 19 000 310
Total menthone
Europe NA 2 800 46
United States NA 1 000 17
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only')
= 32 × 106 for Europe and 32 × 106 for the United States; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows:
[(µg/day)/body weight], where body weight = 60 kg. Slight variation
may occur from rounding off.
N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a An ADI of 0-4 mg/kg bw was established for menthol at the present meeting.
2.2 Intake
The total annual production volume of the 14 menthyl derivatives
is about 140 000 kg in Europe (International Organization of the
Flavor Industry, 1995) and 79 000 kg in The United States (US National
Academy of Sciences, 1987). The production volumes and intakes of each
substance are reported in Table 2. Menthol (No. 427) and menthone (No.
429) account for about 97% of the total annual volume in Europe and
85% in the United States.
Eight of the substances in this group (Nos 427-432, 434, and 435)
have been reported to occur naturally in foods (Maarse & Visscher,
1994). Six of the remaining substances are esters of menthol (Nos 433,
443, 444, and 447) or ketals of menthone (Nos 445 and 446), which are
hydrolysed to menthol or menthone in vivo. Menthol (10-70%) and
menthone (7-40%) are primary constituents of peppermint oil.
Quantitative data have been reported on the natural occurrence and
consumption ratios of five of these substances (see Table 2);
according to the authors, these data indicate that these substances
are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, menthyl esters are hydrolysed to menthol and their
respective carboxylic acids. The carbonate esters in this group are
hydrolysed to menthol and carbonate and either ethylene glycol or
propylene glycol. The ketals in this group are hydrolysed to menthone
and their respective alcohols. Ester hydrolysis is catalysed by
classes of enzymes recognized as carboxylesterases or esterases
(Heymann, 1980), the most important of which are the B-esterases
(Heymann, 1980; Anders, 1989). Acetyl esterases are the preferred
substrates of C-esterases (Heymann, 1980). These enzymes occur in most
mammalian tissues (Heymann, 1980; Anders, 1989) but predominate in
hepatocytes (Heymann, 1980). Esters (Nos 431-433 and 447) and
carbonate esters (Nos 443 and 444) of menthol are expected to be
hydrolysed in humans to yield menthol and their respective saturated
aliphatic carboxylic acids or alcohols.
In two studies, (-)-menthol ethylene glycol carbonate (No. 443)
and (-)-menthol propylene glycol carbonate (No. 444) were hydrolysed
after incubation with rat liver homogenate in vitro, the mean values
for hydrolysis to menthol being 85% for the ethylene glycol carbonate
and 75% for the propylene glycol carbonate (Emberger, 1994a,b). More
than 80% of radiolabelled cyclandelate (Figure 1), a structurally
related cyclohexyl ester, was hydrolysed after 20 min of incubation
with rat hepatic microsomes (White et al., 1990).
Table 2. Most recent annual usage volume of menthol and related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Menthol (427)
Europe 128 000 18 000 300
United States 54 000 10 000 170
(+)-neo-Menthol (428)
Europe 15 3 0.05
United States 140 27 0.4
Menthone (429)
Europe 7 300 1 000 17
United States 13 300 2 500 42
(±)-iso-Menthone (430)
Europe 1 100 200 3
United States 0.5 0.1 0.002
Menthyl acetate (431)
Europe 2 200 420 7
United States 2 900 560 9
Menthyl isovalerate (432)
Europe 48 9 0.2
United States 140 27 17
(-)-Menthyl lactate (433)
Europe 140 26 0.4
United States 0.5 0.1 0.002
para-Menth-1-en-3-ol (434)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003
Piperitone (435)
Europe 270 51 0
United States 50 10 0.2
(-)-Menthol ethylene gylcol carbonate (443)
Europe NR ND ND
United States 4 000 760 13
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
(-)-Menthol 1- and 2-propylene gylcol carbonate (444)
Europe NR ND ND
United States 2 000 381 6
(-)-Menthone1,2-glycerol ketal (445)
Europe NR ND ND
United States 1 000 190 3
(±)-Menthone1,2-glycerol ketal (446)
Europe NR ND ND
United States 1 000 190 3
mono-Menthyl succinate (447)
Europe NR ND ND
United States 110 22 0.4
Total
Europe 140 000
United States 79 000
Total menthol
Europe NA 12 000 190
United States NA 19 000 310
Total menthone
Europe NA 2 800 46
United States NA 1 000 17
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days],
where population (10%, 'eaters only')
= 32 × 106 for Europe and 32 × 106 for the United States; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the surveys
(US National Academy of Sciences, 1970, 1982, 1987; International
Organization of the Flavor Industry, 1995).
Intake (µg/kg bw per day) calculated as follows:
[(µg/day)/body weight], where body weight = 60 kg. Slight variation
may occur from rounding off.
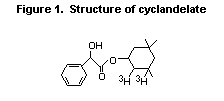 Menthyl derivatives are metabolized like other alicyclic ketones
and secondary alcohols. Ketones are reduced to their corresponding
secondary alcohols and conjugated mainly with glucuronic acid (Quick,
1928; Williams, 1940; Atzl et al., 1972). The metabolites of menthol
are eliminated in the urine or faeces either unchanged or conjugated
with glucuronic acid (Yamaguchi et al., 1994). The parent ketone,
menthone, is primarily reduced to the correspon-ding secondary
alcohol, neo-menthol, which is metabolized and eliminated by
pathways similar to those of its stereoisomer, menthol (Williams,
1940).
(-)-Menthone given to rabbits at a dose of 1000 mg/kg bw was
stereoselectively reduced to (+)- neo-menthol (Figure 2). Similarly,
the stereoisomer (+)- iso-menthone was reduced to (+)- iso-menthol.
About 67% of a 1000-mg/kg bw dose of (+)- neo-menthol given to
rabbits by stomach tube was eliminated in the urine as the glucuronic
acid conjugate (Williams, 1940). Like other alicyclic terpenoids such
as menthol, (±)- iso-menthone may also undergo omega-oxidation of the
alkyl ring substituents, to yield the corresponding hydroxyketones.
Data on structurally related alicyclic terpenoids suggest that
oxidation occurs preferentially on the isopropyl or methyl substituent
and not on the cyclohexane ring (Yamaguchi et al., 1994).
Menthyl derivatives are metabolized like other alicyclic ketones
and secondary alcohols. Ketones are reduced to their corresponding
secondary alcohols and conjugated mainly with glucuronic acid (Quick,
1928; Williams, 1940; Atzl et al., 1972). The metabolites of menthol
are eliminated in the urine or faeces either unchanged or conjugated
with glucuronic acid (Yamaguchi et al., 1994). The parent ketone,
menthone, is primarily reduced to the correspon-ding secondary
alcohol, neo-menthol, which is metabolized and eliminated by
pathways similar to those of its stereoisomer, menthol (Williams,
1940).
(-)-Menthone given to rabbits at a dose of 1000 mg/kg bw was
stereoselectively reduced to (+)- neo-menthol (Figure 2). Similarly,
the stereoisomer (+)- iso-menthone was reduced to (+)- iso-menthol.
About 67% of a 1000-mg/kg bw dose of (+)- neo-menthol given to
rabbits by stomach tube was eliminated in the urine as the glucuronic
acid conjugate (Williams, 1940). Like other alicyclic terpenoids such
as menthol, (±)- iso-menthone may also undergo omega-oxidation of the
alkyl ring substituents, to yield the corresponding hydroxyketones.
Data on structurally related alicyclic terpenoids suggest that
oxidation occurs preferentially on the isopropyl or methyl substituent
and not on the cyclohexane ring (Yamaguchi et al., 1994).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 11 of the 14 substances
(Table 3). The values are in the range 940-7300 mg/kg bw, indicating
that the acute toxicity of orally administered menthol and related
substances is low. The lowest reported value was not, however,
supported by other studies in the same species.
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of short-term studies and of long-term studies of
toxicity and carcinogenicity on substances related to menthol are
summarized in Table 4 and described below. Studies on menthol are
summarized in the separate monograph on that substance.
Menthone (No. 429)
Three short-term studies on peppermint oil and peppermint oil
components, i.e. menthone, pulegone, and menthol, from the same
laboratory showed 'cyst-like spaces' in the white matter of the
cerebellum of rats. Groups of male and female rats were given menthone
at doses of 0, 200, 400, or 800 mg/kg bw per day (Madsen et al.,
1986); peppermint oil at 0, 10, 40, or 100 mg/kg bw per day (Thorup et
al., 1983a); pulegone at 0, 20, 80, or 160 mg/kg bw per day (Thorup et
al., 1983b); or menthol at 0, 200, 400, or 800 mg/kg bw per day
(Thorup et al., 1983b) by gavage daily for 28 days. Cyst-like spaces
in the white cerebellar matter were reported at the two highest doses
of peppermint oil and pulegone and at all doses of menthone. A similar
effect was not observed with menthol.
The slides of the brains of the animals in these studies were
subsequently reviewed independently (Smith et al., 1996). Three
conclusions were reached:
(1) No cellular reaction was seen in tissue adjacent to the cyst-like
spaces in the white matter of the cerbellum, either in the three
studies or in a subsequent 90-day study in rats given peppermint
oil at a dose of 0, 10, 40, or 100 mg/kg bw per day (Spindler &
Madsen, 1992). The appearance and extent of the cyst-like spaces
were no different in the last, longer study than in the first
three.
(2) The cyst-like spaces in cerebellar tissue were not seen in
five-week studies in which rats of the same strain were given
peppermint oil at doses of 150 or 500 mg/kg bw per day and dogs
were given peppermint oil in gelatin capsules daily at a dose of
25 or 125 mg/kg bw per day (Mengs & Stotzem, 1989).
Table 3. Studies of the acute toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
(+)-neo-Menthol 428 Mouse NR Gavage 4000 Wokes (1932)
Menthone 429 Rat M/F Oral 1600-1950 Levenstein (1973);
Igmi & Ide (1974)
Menthyl acetate 431 Rat M/F Gavage > 7000 Levenstein (1973)
Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
Menthyl iso-valerate 432 Rat NR Oral > 5000 Moreno (1976)
(-)-Menthyl lactate 433 Rat M/F Oral 7257 Reagan & Becci (1984)
(-)-Menthol ethylene glycol carbonate 443 Rat M/F Oral > 2000 Tuffnell (1992)
(-)-Menthol 1- and 2-propylene glycol carbonate 444 Rat M/F Oral > 2000 Driscoll (1993)
(±)-Menthone 1,2-glycerol ketal 445 Rat M/F Oral 5716 Reijnders (1991a)
Rat M/F Gavage > 2000 Skydsgaard (1991)
Monomenthyl succinate 447 Rat M/F Oral > 5000 Mercier (1994)
NR, not reported; M, male; F, female
Table 4. Short-term and long-term studies of the toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex No. of groups/ Route Duration NOEL Reference
no. per group (mg/kg
bw per day)
Menthone 429 Rats M/F 3/20 Gavage 28 weeks 400 Madsen (1986)
Mice F 2/NR Intraperitoneal 24 weeks --a Stoner et al. (1973)
(±)-Menthone 1,2-glycerol ketal 446 Rats M/F 3/10 Gavage 28 days 50 Reijnders (1991b)
M, male; F, female; NR, not reported
a No NOEL was determined.
(3) No cyst-like spaces were seen in cerebellar tissue when the
brains of rats were perfused with peppermint oil (Olsen, 1994).
The authors of the independent review concluded that the
cyst-like spaces found after treatment of rats with peppermint oil,
pulegone, or menthone were artefacts arising from inadequate
preparation and fixation of the cerebellar tissue (Adams et al., 1996;
Smith et al., 1996).
Other reported effects in the groups of 10 male and 10 female
rats receiving menthone at doses of 0, 200, 400, or 800 mg/kg bw per
day by gavage for 28 days included a signficant reduction in food
consumption among males receiving the high dose, among females at all
doses during the first two weeks, and among females at the high dose
during week 3 (Madsen et al., 1986). The reduction was attributed to
the unpalatibility of the diet and is similar to that observed in
other studies with menthone (Mengs & Stotzem, 1989). It was further
reported that the relative weights of the kidney, spleen, liver, and
brain in females and of the spleen, liver, and brain in males were
statistically significantly increased at all doses. Furthermore, the
bilirubin concentration and alkaline phosphatase activity in plasma
were increased in all treated animals. A further statistical review
showed, however, that the increases in organ weights and clinical
chemical parameters occurred only at the high dose. The NOEL for
menthone was therefore 400 mg/kg bw per day (Madsen et al., 1986),
which is more than 10 000 times the daily per capita intake ('eaters
only') of 17 and 42 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
Groups of female A/He mice were given intraperitoneal injections
of menthone at doses of 1900 or 4750 mg/kg bw three times weekly for
eight weeks. The animals were observed for an additional 16 weeks;
20-45% of the treated animals and 15% of the controls died before the
end of the study. No increases were seen in the incidences of
non-neoplastic or neoplastic lesions in the lung, liver, kidney,
spleen, thymus, intestine, or salivary or endocrine glands of treated
animals. The authors reported that the vehicle used, tricaprylin, had
caused a 3-4 g loss of weight in control animals during the first week
of the study, high mortality rates, and higher mean incidences of
tumours (Stoner et al., 1973).
(±)-Menthone 1,2-glycerol ketal (No. 446)
Four groups of five male and five female Wistar rat were given
(±)-menthone 1,2-glycerol ketal at doses of 0, 50, 200, or 800 mg/kg
bw per day by gavage for 28 days and were observed daily throughout
the study. The body weights and food consumption of the animals were
measured weekly and on the day before necropsy, when macroscopic
observations and organ weights were recorded. The adrenal glands,
heart, kidney, liver, and stomach were examined histologically.
Decreased serum glucose concentrations and increased kidney weights
were noted in males at the two highest doses. Periportal
hepatocellular hypertrophy was reported in these animals and in
females at the highest dose. The hypertrophy in the males was
accompanied by fine hepatocellular vacuolation. Increased liver
weights were reported in males and females at the highest dose. The
NOEL was 50 mg/kg bw per day (Reijnders, 1991b), which is more than
10 000 times the daily per capita intake ('eaters only') of 3 µg/kg
bw from its use as a flavouring agent in the United States (see Table
2).
2.3.2.3 Genotoxicity
The results of tests for genotoxicity with five representative
menthyl derivatives are shown in Table 5.
Menthone (No. 429) was not mutagenic in the standard Ames test
with Salmonella typhimurium strains TA98, TA100, or TA1535 when
tested at concentrations of up to 800 µg/plate, either with or without
metabolic activation; however, it induced reverse mutation in
S. typhimurium TA97 at concentrations up to 160 µg/plate in the
presence of metabolic activation and at concentrations up to 800
µg/plate in the absence of activation. It was also mutagenic in strain
TA1537 at concentrations of 32 and 6.4 µg/plate (Andersen & Jensen,
1984).
(-)-Menthol ethylene glycol carbonate (No. 443), (-)-menthol
1,2-propylene glycol carbonate (No. 444), and (±)-menthone
1,2-glycerol ketal (No. 446) were not mutagenic in the standard Ames
test or in the preincubation protocol with S. typhimurium strains
TA98, TA100, TA1535, TA1537, and TA1538 at concentrations up to 5000
µg/plate, either with or without metabolic activation (King, 1991;
Poth, 1991; King, 1992, 1993). (-)-Menthol ethylene glycol carbonate
(No. 443) and (-)-menthol 1,2-propylene glycol carbonate (No. 444) did
not induce chromosomal aberrations in human peripheral blood
lymphocytes when tested at concentrations up to 300 µg/ml (King,
1994a,b). (±)-Menthone 1,2-glycerol ketal (No. 446) given orally to
NWRI mice at doses up to 2500 mg/kg bw did not induce micronuclei in
bone-marrow cells (Völkner, 1991).
4. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York, Taylor &
Francis, pp. 81-97.
Table 5. Results of assays for the genotoxicity of substances structurally related to menthol
Substance No. End-point Test object Dose Result Reference
In vitro
Menthone 429 Reverse mutation S. typhimurium TA 1535, 800 µg/plate Negativea Anderson &
TA 100, TA 98 Jensen (1984)
Reverse mutation S. typhimurium TA 1537, 6.4-800 µg/plate Positivea Andersen &
TA 97 Jensen (1984)
(-)-Menthol ethylene 443 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1992)
glycol TA 1537, TA 98, TA 100,
TA 1538
Chromosomal Human peripheral blood 300 µg/ml Negativea King (1994a)
aberration lymphocytes
(-)-Menthol 1- and 444 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1993)
2-propylene glycol TA 1537, TA 1538, TA 98,
carbonate TA 100
Chromosomal Human peripheral blood 300 µg/plate Negativea King (1994b)
aberration lymphocytes
(±)-Menthone 1,2-glycerol 446 Reverse mutation S. typhimurium TA 1535, 1500 µg/plate Negativea King (1991)
ketal TA 1537, TA 98, TA 100,
TA 1538
Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea Poth (1991)
TA 1537, TA 98, TA 100,
TA 1538
In vivo
(±)-Menthone 1,2-glycerol 446 Micronucleus NMRI mice 2500 mg/kg bw Negative Volkner (1991)
ketal formation
a With and without metabolic activation
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Driscoll, R. (1993) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Emberger (1994a) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Emberger (1994b) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxification, 2nd Ed., New York, Academic
Press, pp. 291-323.
Igmi, H. & Ide, H. (1974) Improvements in or Relating to Substances
for Use in the Treatment of Gallstones, Patet 1343561, Appl.
13606/772, A61k27/00, 23 March 1972.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
King, M.T. (1991) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
King, M.T (1992) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1993) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1994a) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
King, M.T. (1994b) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Levenstein, I. (1973) Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Mercier, O. (1994) Acute oral toxicity. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States
Olsen, P. (1994) Oral presentation to the FEMA Expert Panel.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Poth, A. (1991) Salmonella typhimurium reverse mutation assay with
91/917 247. Unpublished report from Cytotest Cell Research. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of
frescolate/l-methyl lactate in Sprague-Dawley rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Reijnders, J.B. (1991a) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Reijnders, J.B. (1991b) Subacute 28-day oral toxicity. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United State, Washington DC, United States.
Shelanski, M.V. & Moldovan, M. (1972) Unpublished report to the
Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Skydsgaard, K. (1991) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B. &
the FEMA Expert Panel (1996) Recent progress in the consideration of
flavoring ingredients under the Food Additives Amendment. 17. GRAS
substances. Food Technol., 50, 72-78, 80-81.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Tuffnell, P.P. (1992) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 11 of the 14 substances
(Table 3). The values are in the range 940-7300 mg/kg bw, indicating
that the acute toxicity of orally administered menthol and related
substances is low. The lowest reported value was not, however,
supported by other studies in the same species.
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of short-term studies and of long-term studies of
toxicity and carcinogenicity on substances related to menthol are
summarized in Table 4 and described below. Studies on menthol are
summarized in the separate monograph on that substance.
Menthone (No. 429)
Three short-term studies on peppermint oil and peppermint oil
components, i.e. menthone, pulegone, and menthol, from the same
laboratory showed 'cyst-like spaces' in the white matter of the
cerebellum of rats. Groups of male and female rats were given menthone
at doses of 0, 200, 400, or 800 mg/kg bw per day (Madsen et al.,
1986); peppermint oil at 0, 10, 40, or 100 mg/kg bw per day (Thorup et
al., 1983a); pulegone at 0, 20, 80, or 160 mg/kg bw per day (Thorup et
al., 1983b); or menthol at 0, 200, 400, or 800 mg/kg bw per day
(Thorup et al., 1983b) by gavage daily for 28 days. Cyst-like spaces
in the white cerebellar matter were reported at the two highest doses
of peppermint oil and pulegone and at all doses of menthone. A similar
effect was not observed with menthol.
The slides of the brains of the animals in these studies were
subsequently reviewed independently (Smith et al., 1996). Three
conclusions were reached:
(1) No cellular reaction was seen in tissue adjacent to the cyst-like
spaces in the white matter of the cerbellum, either in the three
studies or in a subsequent 90-day study in rats given peppermint
oil at a dose of 0, 10, 40, or 100 mg/kg bw per day (Spindler &
Madsen, 1992). The appearance and extent of the cyst-like spaces
were no different in the last, longer study than in the first
three.
(2) The cyst-like spaces in cerebellar tissue were not seen in
five-week studies in which rats of the same strain were given
peppermint oil at doses of 150 or 500 mg/kg bw per day and dogs
were given peppermint oil in gelatin capsules daily at a dose of
25 or 125 mg/kg bw per day (Mengs & Stotzem, 1989).
Table 3. Studies of the acute toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
(+)-neo-Menthol 428 Mouse NR Gavage 4000 Wokes (1932)
Menthone 429 Rat M/F Oral 1600-1950 Levenstein (1973);
Igmi & Ide (1974)
Menthyl acetate 431 Rat M/F Gavage > 7000 Levenstein (1973)
Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
Menthyl iso-valerate 432 Rat NR Oral > 5000 Moreno (1976)
(-)-Menthyl lactate 433 Rat M/F Oral 7257 Reagan & Becci (1984)
(-)-Menthol ethylene glycol carbonate 443 Rat M/F Oral > 2000 Tuffnell (1992)
(-)-Menthol 1- and 2-propylene glycol carbonate 444 Rat M/F Oral > 2000 Driscoll (1993)
(±)-Menthone 1,2-glycerol ketal 445 Rat M/F Oral 5716 Reijnders (1991a)
Rat M/F Gavage > 2000 Skydsgaard (1991)
Monomenthyl succinate 447 Rat M/F Oral > 5000 Mercier (1994)
NR, not reported; M, male; F, female
Table 4. Short-term and long-term studies of the toxicity of substances structurally related to menthol used as flavouring agents
Substance No. Species Sex No. of groups/ Route Duration NOEL Reference
no. per group (mg/kg
bw per day)
Menthone 429 Rats M/F 3/20 Gavage 28 weeks 400 Madsen (1986)
Mice F 2/NR Intraperitoneal 24 weeks --a Stoner et al. (1973)
(±)-Menthone 1,2-glycerol ketal 446 Rats M/F 3/10 Gavage 28 days 50 Reijnders (1991b)
M, male; F, female; NR, not reported
a No NOEL was determined.
(3) No cyst-like spaces were seen in cerebellar tissue when the
brains of rats were perfused with peppermint oil (Olsen, 1994).
The authors of the independent review concluded that the
cyst-like spaces found after treatment of rats with peppermint oil,
pulegone, or menthone were artefacts arising from inadequate
preparation and fixation of the cerebellar tissue (Adams et al., 1996;
Smith et al., 1996).
Other reported effects in the groups of 10 male and 10 female
rats receiving menthone at doses of 0, 200, 400, or 800 mg/kg bw per
day by gavage for 28 days included a signficant reduction in food
consumption among males receiving the high dose, among females at all
doses during the first two weeks, and among females at the high dose
during week 3 (Madsen et al., 1986). The reduction was attributed to
the unpalatibility of the diet and is similar to that observed in
other studies with menthone (Mengs & Stotzem, 1989). It was further
reported that the relative weights of the kidney, spleen, liver, and
brain in females and of the spleen, liver, and brain in males were
statistically significantly increased at all doses. Furthermore, the
bilirubin concentration and alkaline phosphatase activity in plasma
were increased in all treated animals. A further statistical review
showed, however, that the increases in organ weights and clinical
chemical parameters occurred only at the high dose. The NOEL for
menthone was therefore 400 mg/kg bw per day (Madsen et al., 1986),
which is more than 10 000 times the daily per capita intake ('eaters
only') of 17 and 42 µg/kg bw from its use as a flavouring agent in
Europe and the United States, respectively (see Table 2).
Groups of female A/He mice were given intraperitoneal injections
of menthone at doses of 1900 or 4750 mg/kg bw three times weekly for
eight weeks. The animals were observed for an additional 16 weeks;
20-45% of the treated animals and 15% of the controls died before the
end of the study. No increases were seen in the incidences of
non-neoplastic or neoplastic lesions in the lung, liver, kidney,
spleen, thymus, intestine, or salivary or endocrine glands of treated
animals. The authors reported that the vehicle used, tricaprylin, had
caused a 3-4 g loss of weight in control animals during the first week
of the study, high mortality rates, and higher mean incidences of
tumours (Stoner et al., 1973).
(±)-Menthone 1,2-glycerol ketal (No. 446)
Four groups of five male and five female Wistar rat were given
(±)-menthone 1,2-glycerol ketal at doses of 0, 50, 200, or 800 mg/kg
bw per day by gavage for 28 days and were observed daily throughout
the study. The body weights and food consumption of the animals were
measured weekly and on the day before necropsy, when macroscopic
observations and organ weights were recorded. The adrenal glands,
heart, kidney, liver, and stomach were examined histologically.
Decreased serum glucose concentrations and increased kidney weights
were noted in males at the two highest doses. Periportal
hepatocellular hypertrophy was reported in these animals and in
females at the highest dose. The hypertrophy in the males was
accompanied by fine hepatocellular vacuolation. Increased liver
weights were reported in males and females at the highest dose. The
NOEL was 50 mg/kg bw per day (Reijnders, 1991b), which is more than
10 000 times the daily per capita intake ('eaters only') of 3 µg/kg
bw from its use as a flavouring agent in the United States (see Table
2).
2.3.2.3 Genotoxicity
The results of tests for genotoxicity with five representative
menthyl derivatives are shown in Table 5.
Menthone (No. 429) was not mutagenic in the standard Ames test
with Salmonella typhimurium strains TA98, TA100, or TA1535 when
tested at concentrations of up to 800 µg/plate, either with or without
metabolic activation; however, it induced reverse mutation in
S. typhimurium TA97 at concentrations up to 160 µg/plate in the
presence of metabolic activation and at concentrations up to 800
µg/plate in the absence of activation. It was also mutagenic in strain
TA1537 at concentrations of 32 and 6.4 µg/plate (Andersen & Jensen,
1984).
(-)-Menthol ethylene glycol carbonate (No. 443), (-)-menthol
1,2-propylene glycol carbonate (No. 444), and (±)-menthone
1,2-glycerol ketal (No. 446) were not mutagenic in the standard Ames
test or in the preincubation protocol with S. typhimurium strains
TA98, TA100, TA1535, TA1537, and TA1538 at concentrations up to 5000
µg/plate, either with or without metabolic activation (King, 1991;
Poth, 1991; King, 1992, 1993). (-)-Menthol ethylene glycol carbonate
(No. 443) and (-)-menthol 1,2-propylene glycol carbonate (No. 444) did
not induce chromosomal aberrations in human peripheral blood
lymphocytes when tested at concentrations up to 300 µg/ml (King,
1994a,b). (±)-Menthone 1,2-glycerol ketal (No. 446) given orally to
NWRI mice at doses up to 2500 mg/kg bw did not induce micronuclei in
bone-marrow cells (Völkner, 1991).
4. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York, Taylor &
Francis, pp. 81-97.
Table 5. Results of assays for the genotoxicity of substances structurally related to menthol
Substance No. End-point Test object Dose Result Reference
In vitro
Menthone 429 Reverse mutation S. typhimurium TA 1535, 800 µg/plate Negativea Anderson &
TA 100, TA 98 Jensen (1984)
Reverse mutation S. typhimurium TA 1537, 6.4-800 µg/plate Positivea Andersen &
TA 97 Jensen (1984)
(-)-Menthol ethylene 443 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1992)
glycol TA 1537, TA 98, TA 100,
TA 1538
Chromosomal Human peripheral blood 300 µg/ml Negativea King (1994a)
aberration lymphocytes
(-)-Menthol 1- and 444 Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea King (1993)
2-propylene glycol TA 1537, TA 1538, TA 98,
carbonate TA 100
Chromosomal Human peripheral blood 300 µg/plate Negativea King (1994b)
aberration lymphocytes
(±)-Menthone 1,2-glycerol 446 Reverse mutation S. typhimurium TA 1535, 1500 µg/plate Negativea King (1991)
ketal TA 1537, TA 98, TA 100,
TA 1538
Reverse mutation S. typhimurium TA 1535, 5000 µg/plate Negativea Poth (1991)
TA 1537, TA 98, TA 100,
TA 1538
In vivo
(±)-Menthone 1,2-glycerol 446 Micronucleus NMRI mice 2500 mg/kg bw Negative Volkner (1991)
ketal formation
a With and without metabolic activation
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Driscoll, R. (1993) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States.
Emberger (1994a) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Emberger (1994b) Unpublished report. Submitted to WHO by the Flavor
and Extract Manufacturers' Association of the United States,
Washington DC, United States
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxification, 2nd Ed., New York, Academic
Press, pp. 291-323.
Igmi, H. & Ide, H. (1974) Improvements in or Relating to Substances
for Use in the Treatment of Gallstones, Patet 1343561, Appl.
13606/772, A61k27/00, 23 March 1972.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
King, M.T. (1991) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
King, M.T (1992) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1993) Mutagenicity study in the Salmonella
typhimurium/mammalian microsome reverse mutation assay (Ames-Test).
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
King, M.T. (1994a) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
King, M.T. (1994b) Mutagenicity study in the chromosome aberration
tests with human peripheral blood lymphocytes in vitro. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Levenstein, I. (1973) Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Mercier, O. (1994) Acute oral toxicity. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report. Submitted to WHO by the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States
Olsen, P. (1994) Oral presentation to the FEMA Expert Panel.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Poth, A. (1991) Salmonella typhimurium reverse mutation assay with
91/917 247. Unpublished report from Cytotest Cell Research. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of
frescolate/l-methyl lactate in Sprague-Dawley rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States
Reijnders, J.B. (1991a) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Reijnders, J.B. (1991b) Subacute 28-day oral toxicity. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United State, Washington DC, United States.
Shelanski, M.V. & Moldovan, M. (1972) Unpublished report to the
Research Institute of Fragrance Materials. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States
Skydsgaard, K. (1991) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B. &
the FEMA Expert Panel (1996) Recent progress in the consideration of
flavoring ingredients under the Food Additives Amendment. 17. GRAS
substances. Food Technol., 50, 72-78, 80-81.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Tuffnell, P.P. (1992) Acute oral toxicity. Unpublished report.
Submitted to WHO by the Flavor and Extract Manufacturers' Association
of the United States, Washington DC, United States
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
