
ACESULFAME POTASSIUM
Explanation
Acesulfame K (synonyms: Acesulfam K; Potassium salt of
6-methyl-1, 2,3-oxathiazine-4(3H)-one-2,3-dioxide; Potassium salt of
3,4-dihydro-6-methyl-1,2,3-oxathiazine-4-one-2,2-dioxide; Acetosulfam)
is an artificial sweetener, approximately 200 times sweeter than
sugar. It is a white, odourless, crystalline powder with the following
chemical structure:
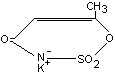 It is proposed for use as a table-top sweetener, and for use in
soft drinks, fruit preparations, desserts, breakfast cereals, chewing
gum, and other food applications appear to be possible. Based on data
for food consumption in the Federal Republic of Germany, and estimated
levels of use in these specified foods, the probable mean daily intake
has been estimated as 470 mg/day (Anon., 1980a).
Acesulfame K is stable in foods, beverages and cosmetic
preparations under normal storage conditions. Under extreme conditions
of pH and temperature, detectable decomposition may occur leading to
the formation of acetone, CO2, and ammonium hydrogen sulfate, or
amido-sulfate, as final decomposition products; under acid (pH 2.5)
conditions, minute quantities of acetoacetamide and acetoacetamide
N-sulfonic acid are formed as unstable intermediate decomposition
products, while under alkaline (pH 3-10.5) conditions, acetoacetic
acid and acetoacetamide N-sulfonic acid can be detected.
This artificial sweetener was assessed for the first time by the
Joint Expert Committee on Food Additives.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg 14C-acesulfame K/kg bw given to rats
and dogs were rapidly absorbed. Maximum blood levels reached were
0.75 ± 0.2 µg/ml in rats, 0.5 h after dosing, and 6.56 ± 2.08 µg/ml in
dogs, 1-1.5 h after dosing. In rats, 82-100% of the dose, and in dogs,
85-100% of the dose was excreted in the urine; in both species,
97-100% of their total radioactivity was excreted in faeces, and total
recovery approximated to 100%.
Rats given 10 consecutive daily doses of 10 mg/kg orally did not
show evidence of accumulation. Three days after dosing, the
concentration in the organs and plasma was 0.4 nMol/g in liver, and
less than 0.2 nMol/g in other tissues. Seven days after dosing, the
concentration in dogs was less than 0.2 nMol/g in all tissues examined
(Kellner & Christ, 1975a).
After intravenous administration of a single dose of 10 mg
14C-acesulfame K/kg bw to rats, the radioactivity was excreted
quantitatively in urine and the plasma half-life was 0.23 h (Kellner &
Christ, 1975a).
Following oral administration of a single dose of 3.6-4.5 mg
14C-acesulfame K/kg bw to pigs, maximum blood levels ranged between
0.35-0.72 µg/ml between 1-2 h after dosing and fell to undetectable
levels within 48 h. Excretion occurred mainly in the urine (Kellner &
Christ, 1975b).
Bio-transformation
The metabolism of Acesulfame K was investigated in the urine and
faeces of rats and dogs which had received single oral doses of
10 mg/kg bw, and in the urine and bile of pigs dosed orally with
5 mg/kg bw. The analytical methods used (thin-layer chromatography,
mass spectrometry and isotope dilution) detected only the original
substance in these samples (Volz, 1975).
Effects on enzymes
In vitro studies on acetoacetamide, a possible minor breakdown
product of Acesulfame K, showed that it did not function as a
substrate for thiolase, ß-hydroxyacyl-CoA-dehydrogenase, and
ß-hydroxybutyrate-dehydrogenase indicating that in vivo formation of
acetamide is not probable (Anon., 1980b).
Effects on bacteria
Acesulfame K was without antibacterial activity against 12
bacterial strains in vitro, and did not show antibacterial activity
in experimental septicaemia in the mouse with Streptococcus pyogenes
A77 or Salmonella typhimurium. Long-term culture (30 daily passages)
of Staphylococcus aureus and E. coli with a range of
concentrations of Acesulfame did not affect growth characteristics nor
sensitivity towards antibiotics, ampicillin, cephalothin, tetracycline
or gentiamycin (Schrinner & Limbert, 1977).
Nitrosation of Acesulfame K
In vitro studies were performed to investigate whether
Acesulfame K could form N-nitroso derivatives. Nitrosation was carried
out using N2O3 in glacial acetic acid, or excess NaNO2, at pH 3 and
pH 1. An N-nitroso compound was detected in low yield with N2O3 or
with nitrite at pH 1 but not at pH 3. The yield at pH 1 was estimated
to be 0.4 × 10-3% (Eisenbrand, 1979).
TOXICOLOGICAL STUDIES
Special studies on acetoacetamide - acute toxity and mutagenicity
The oral LD50 of acetoacetamide in female rats was greater than
15 g/kg bw (Anon., 1977).
Acetoacetamide was non-mutagenic in bacterial assays using four
strains of Salmonella typhimurium, with and without metabolic
activation (Gericke, 1977), did not induce point mutations in cultured
V79 Chinese hamster cells, and was negative in the cell transformation
assay using M2 mouse fibroblasts (Marquardt, 1978).
Special studies on ß-hydroxybutyramide - mutagenicity
ß-Hydroxybutyramide, a metabolite of acetamide in some mammalian
species, was non-mutagenic in the Ames' test against four strains of
Salmonella typhimurium, with and without metabolic activation
(Engelbart, 1979).
Special study on caecal enlargement
These studies were performed to investigate the possible
reversibility of caecal enlargement observed in short-term and
long-term studies.
Groups of 10 juvenile female Wistar rats, body weight
approximately 115 g, received Acesulfame K at dietary concentrations
of 0, 3.0, or 10.0% for treatment periods of 45, 49, and 90 days. One
group from each dose level was sacrificed at the end of the treatment
periods of 45 and 90 days; in addition one group from each dose level
was sacrificed after recovery periods of 41 days' treatment, and 14,
56 and 127 days following 90 days treatment. Food and water intake,
and body weight were measured weekly. At termination, the weights of
caeca with and without contents, as well as moisture content of the
contents, were determined.
At the 10% feeding level, there was an increase in food and water
intake and a reduction in body weight gain which was reversible during
the post-treatment period. At this treatment level, filled caecal
weight relative to body weight was approximately doubled after 45 and
90 days while at the 3% dietary level, a significant increase of about
30% was observed after 90 days only. A significant increase in the
water content of caecal contents was observed only in the highest dose
group.
The changes in the caecal weights after 90 days' exposure to 3%
Acesulfame K in the diet were reversible within 14 days. After 90
days' treatment at the 10% dietary level, the water content in the
caecum returned to normal within 14 days but the filled caecal weight
remained significantly increased (by approximately 30%) even after a
recovery period of 127 days (Mayer et al., 1978b).
A similar study was performed with adult female Wistar rats,
body weight approximately 220 g. Initially, animals receiving 10%
Acesulfame K in the diet experienced anorexia followed by an increased
food consumption after two weeks. Water consumption was increased
during the treatment period in both dose groups.
Filled and empty caecal weights were increased by 80% and 33%
respectively in animals receiving 10% Acesulfame for 45 days.
Increased caecal weights were observed in both dose groups after 91
days' treatment. Caecal water content was significantly increased in
both dose groups after 45 days but not after 91 days' treatment.
All the changes were reversible in animals of both dose groups
treated for 49 days followed by a 42 day recovery period. After 91
days' treatment at the 3% dietary level, all changes were reversible
within 14 days; at the 10% dietary level, caecal water content
returned to normal within 14 days but filled caecal weights were still
significantly increased (by about 30%) after a recovery period of 127
days (Mayer et al., 1978c).
These experiments did not reveal any significant differences
between juvenile and adult animals with regard to induction or
reversibility of caecal enlargement. Complete reversibility was
demonstrated at the 3% dietary level, but not at 10% in the diet. The
authors note that the coincidence of increased water intake with
increased caecal water content and the reversibility of both of these
parameters after withdrawal of Acesulfame K probably indicates that
the changes are of osmotic origin.
Special studies on carcinogenicity
Four groups of 100 male and 100 female Swiss mice were fed diets
containing 0, 0.3, 1.0, or 3.0% Acesulfame K for 80 weeks. All
survivors were sacrificed and autopsied, and weights of livers and
kidneys were recorded. All tumours and tissues showing gross lesions
suspected of being tumours and the livers of all animals were examined
microscopically (haematoxylin and eosin sections).
The feeding of Acesulfame K did not cause adverse effects on
general appearance, behaviour or survival at any of the dietary levels
but body weights were slightly decreased at the 3.0% dose level in
both sexes. The relative liver weight was decreased at all dose levels
in males only but there was no evidence of a dose-related response.
Deaths occurring during the course of the study were attributed to
chronic nephropathy, severe liver degeneration, respiratory infections
and lung tumours.
Gross and microscopic examination revealed a variety of tumours
in both test and control animals, but evaluation of the data on type
of tumour, location and incidence did not indicate that the test
compound was carcinogenic to mice at dietary levels up to 3% for 80
weeks (Beems & Til, 1976).
See also Long-term studies in rats.
Special studies on mutagenicity
An oral mutagenicity study (micronucleus test) was carried out in
which Acesulfame K was administered orally to male and female NMR1
mice at doses of 450, 1500, and 4500 mg/kg on two consecutive days.
The animals were killed six hours after the second application, bone
marrow was taken from the femur and 2000 polychromatic erythrocytes
per animal counted and scored for micronuclei. The mutagenic index did
not differ significantly from 1 (Baeder & Horstmann, 1977).
In a dominant lethal assay, Acesulfame K was administered to male
Wistar rats at dietary levels of 1.0 or 3.0% for five consecutive days
prior to mating. At day 1, 8 and 15 post-treatment each male was caged
with two untreated females for seven days. Pregnant females were
sacrificed mid-term and scored for pregnancy rate and total and dead
implants. No adverse effects were observed and it was concluded that
Acesulfame K at these dietary levels did not induce dominant lethal
mutations in spermatozoa of vas deferens and epididymis, testicular
sperm or late spermatids (Willems, 1974).
Cytogenetic investigations were conducted on bone marrow cells of
Chinese hamsters treated orally with five consecutive daily doses of
Acesulfame K of 0, 450, 1500, or 4500 mg/kg bw. The ratios of
chromosomal abnormalities in control and treated animals were low, and
values normally found in this strain were not exceeded (Mayer et al.,
1978a).
The ability o£ Acesulfame K and of acetoacetamide to induce
mutations in V79 Chinese hamster cells in vitro (8-azaguanine
susceptibility to resistance) was investigated at concentrations of
10-10 000 µg/ml. Neither of the compounds was mutagenic in this
system; acetoacetamide was slightly cytotoxic at the highest
concentration. The compounds were also screened for induction of
transformations in M2 mouse fibroblasts at similar concentrations.
Acesulfame K did not cause any transformations, but reduced plating
efficiency (cytotoxicity) was observed at concentrations of 5000 and
10 000 µg/ml; one (out of 14) transformed focus was observed with
acetoacetamide at a concentration of 5000 µg/ml but not at the higher
concentration, and this was not considered to be evidence of potential
oncogenic activity. In addition, Acesulfame K was not mutagenic nor
cytotoxic against four strains of Salmonella typhimurium at
concentrations up to 100 mg/plate (Marquardt, 1978).
Acesulfame K was neither mutagenic nor cytotoxic in the Ames'
test using four strains of Salmonella typhimurium (detecting both
base-pair and frame-shift mutations) either with or without activation
by rat microsomes (Rohrborn, 1976).
Special studies on pharmacological aspects
Dosages of Acesulfame K of 400 m/kg i.p., 500 m/kg orally, or
320 mg/kg subcutaneously, did not depress motor activity of mice
excited by Pervitin. The hexobarbital sleeping time in mice was not
changed by pretreatment with Acesulfame K at doses of 500 mg/kg
per os or 160 mg/kg subcutaneously. Metrazol-induced convulsions in
mice were not influenced by Acesulfame K at doses of 500 mg/kg
per os, 300 mg/kg i.p., or 320 mg/kg subcutaneously; anti-convulsant
activity can thus be excluded. Administration of Acesulfame K
(200 mg/kg i.p.) to mice was without effect on tetrabenazine-induced
ptosis and catalepsy, thus the compound is without anti-depressant
properties. Acesulfame K (200 mg/kg i.p., 160 mg/kg s.c., or 500 m/kg
per os) was without effect on compulsive gnawing behaviour induced by
the combined application of apomorphine and imipramine, therefore
antidepressant and anticholinergic effects are unlikely. At doses of
320 mg/kg s.c. or 500 mg/kg orally, Acesulfame K had no analgesic
effect on mice.
Predosing of rats with Acesulfame K (500 mg/kg orally or
160 mg/kg s.c.) was without anti-inflammatory effect on Aerosil-
induced paw oedema, and similar doses had no antipyretic effect in
rats with yeast-induced fever. Eight daily doses of Acesulfame K
(0-100 mg/kg) per os had no effect on serum cholesterol, total
glycerol, free glycerol or glucose levels; relative liver weights were
unchanged. In acute tests, Acesulfame K had no effect on blood sugar
levels in rats given 100 mg/kg orally, guinea-pigs given a similar
dose i.p., or rabbits receiving 100 or 500 mg/kg orally or 50 mg/kg
i.v. Acesulfame K had no diuretic effect in rats and dogs at oral
dose levels of 50 mg/kg and 20 mg/kg respectively.
Acesulfame K given intravenously to guinea-pigs at a dose level
of 24 mg/kg reduced digoxin toxicity. This effect was due to the
potassium content and not to antiarrhythmic activity of the compound.
Intravenous administration of 1-5 mg Acesulfame K/kg to anaesthetised
guinea-pigs one minute before treatment with histamine was without
effect on the bronchial musculature.
Cardiovascular experiments in anaesthetized dogs showed that
intravenous administration of Acesulfame K was without effect up to a
dose of 6 mg/kg; doses of 12 and 24 mg/kg caused a decrease in
contractility of the heart with a transient reduction of blood
pressure and peripheral blood flow. The changes were compensated for
in 3-5 minutes. Intraduodenal administration of Acesulfame K to
anaesthetized dogs at dose levels of 0-1000 mg/kg induced a slight
reduction of blood pressure at 500 mg/kg and this was accompanied by a
reduction of cardiac contractility of about 20% at 1 g/kg. The effect
was reversed in 50-80 minutes and other cardiovascular parameters were
unchanged. In the conscious dog, after a five day treatment with
Acesulfame K at 100 mg/kg per os daily, no change in blood pressure
or cardiac activity could be detected. Acesulfame K at doses of
0-24 mg/kg i.v. had no anti-arrhythmic effect on anaesthetised dogs
poisoned with K-strophanthin. Daily oral administration of Acesulfame
K (1 g/kg) to dogs for 14 days was without effect on thromboplastin
time, thrombin time, recalcification time and thromboelastography of
plasma samples.
No functional changes were detected after application of 50 µg
Acesulfame K to isolated guinea-pig heart using the Langendorff
techniques; the compound did not show antiarrhythmic activity
in isolated, perfused guinea-pig heart with aconitine and
digitoxin-induced fibrillation. In the isolated ileum of guinea-pig,
Acesulfame K at a concentration of 10 µg/ml had no neurotropic or
spasmolytic effect on smooth muscle.
Addition of Acesulfame K to dog plasma in vitro was without
effect on thrombin time, thromboplastin time or recalcification time.
Investigation of the carbonic anhydrase-inhibiting effect
in vitro showed that Acesulfame K had virtually no effect,
concentrations of 180 mg/ml being required for 50% inhibition (Vogel &
Alpermann, 1974).
Special studies on the short-term toxicity of potassium chloride
Feeding studies in rats with potassium chloride were conducted to
elucidate the possible involvement of the potassium ion in Acesulfame
K in changes observed in toxicological studies. Three groups of 20
male and 20 female weanling Wistar rats were fed for 90 days on diets
containing 0, 12 000 or 37 000 ppm (0, 1.2 or 3.7%) KCl, equivalent to
the potassium content of diets containing 0, 3, or 10% Acesulfame K.
Regular measurements were made of body weight, food and water
consumption, urinary volume and potassium content. After the animals
had been sacrificed, the caeca with and without contents were weighed
and the water content of the caecal contents were determined.
There was a dose-related increase in water consumption in both
sexes throughout the study and, in the first few weeks, the food
consumption of treated animals was slightly lower than that of
controls. Body weight gains were depressed in males of both
concentration groups throughout the 90 day period but in females
statistically significant differences were obtained only up to the
twenty-ninth day. Dose-related increases in urine volumes and urinary
potassium were observed. Filled caecal weights were increased by about
10% in males and 20% in females of the top dose group, but these
differences were not statistically significant; no differences were
observed in empty caecal weights, nor in water content of the caecal
contents. It was concluded that the potassium content of Acesulfame K
could be responsible for some adverse effects seen in toxicological
studies, particularly depressed body weight gain (Mayer et al.,
1978d).
Special studies on reproduction
A multigeneration study in rats was carried out, in which males
and females received Acesulfame K at dietary levels of 0, 0.3, 1.0 and
3.0% for three successive generations, each comprising two consecutive
litters. A teratogenicity study was conducted with 15 females per
group of the F2b and F3a generations. Rats from the F3b generation
were submitted to clinical and pathological examination. Pups from the
F1a litters were used for a chronic toxicity/carcinogenicity study at
the same dietary levels of Acesulfame K as the parents (see Long-term
studies).
Fertility, number of young per litter, birth weight, growth rate
and mortality during the lactation period were not adversely affected
and there were no indications of increased mortality in utero.
Growth rate was slightly decreased in the top dose group of the
F0 and F1 generations, and the mid-dose group of the F0 generation.
In the teratogenicity studies, no adverse effects were seen in
appearance, food consumption, autopsy of the dams, organ weights, or
litter data; no visceral or skeletal abnormalities attributable to the
treatment were observed.
In a four-week feeding study on rats of the F3b generation, body
weights and food efficiency figures were slightly decreased in males
at the highest dose level. The relative weights of the caecum were
slightly increased in both sexes of the high-dose group and in males
of the mid-dose group. Gross and microscopic examination did not
reveal any treatment-related pathological changes (Sinkeldam et al.,
1976).
In a separate study, Acesulfame K was fed to pregnant rats at
dietary levels of 0, 0.3, 1.0, or 3.0% from day 6 up to and including
day 15 of pregnancy; a positive control group received 75 000 i.u.
vitamin A/rat/day during the same period. An increase in food
consumption was observed at all three dose levels of Acesulfame K,
most pronounced in the 0.3% group. Mean foetal weight showed a slight,
dose-related increase in the test groups but skeletal and visceral
examination of the foetuses revealed no teratogenic effects
attributable to the feeding of Acesulfame K. A wide range of
abnormalities was induced by teratogenic doses of vitamin A in
positive controls (Koeter, 1975).
A reproduction study was carried out in which male and female
rats were fed diets containing 0, 0.3, 1.0, or 3.0% Acesulfame K for
12 weeks prior to mating; the dams received the same diet throughout
pregnancy and lactation. Observations were made on the fertility
of the females, number of young per litter, sex rates, gross
abnormalities, mortality, body weight, and resorption quotient. Growth
rate was slightly decreased in parent rats of the top dose group and
in the mid-dose group females. No dose-related effects were seen in
any of the observations made on the offspring, and there were no
indications of increased mortality in utero.
At weaning, 60 animals of each sex were selected from the litters
for a two-year feeding study (see below, Long-term studies)
(Sinkeldam, 1976).
An embryotoxicity study with Acesulfame K was carried out in
which female rabbits received doses of 0, 100, 300 or 900 m/kg bw by
gastric intubation from the seventh to the nineteenth day after
mating. On the twenty-ninth day of pregnancy, foetuses were delivered
by Caesarean section; live and dead foetuses, resorptions and
placentas were counted, weighed and examined macroscopically. The
24-hour survival was determined by incubation and half of the foetuses
examined for skeletal abnormalities and the remaining half for
visceral changes. One dam from the 300 mg/kg group had a premature
birth. All other observations were within the range of control values
and there was no evidence of compound-related malformations (Baeder &
Horstmann, 1977).
Acute toxicity
Species Route LC50 (mg/l) Reference
Zebra Fish Water >1 000 Markert & Weigand, 1979a
Golden Orfe Water >1 000 Markert & Weigand, 1979b
LD50 (mg/kg)
Rat p.o. 7 430 Anon., 1973
i.p. 2 240 Mayer & Weigand, 1977
Short-term studies
Rat
Four groups of 10 male and 10 female weanling Wistar-derived rats
were given diets containing 0, 1.0, 3.0, or 10% Acesulfame K for 90
days. Body weights were recorded weekly, food intake was determined
during the first four weeks and in weeks 11 and 12. In week 13, the
animals were bled from the tip of the tail and blood samples were
examined for haemoglobin content, haematocrit, RBC and total and
differential white cell counts. Pooled urine samples from each group
were collected in week 13 and examined for appearance, pH, glucose,
protein, occult blood, ketones and microscopy of the sediment. At
autopsy, blood samples were examined for SGPT, SGOT, alkaline
phosphatase, total serum protein and serum albumin. Organ weights were
recorded for heart, kidneys, liver, spleen, brain, testes/ovaries,
thymus, thyroid, adrenals and caecum (filled and empty). Histological
examination was carried out on haematoxylin/eosin sections of the
weighed organs and of lung, salivary glands, trachea, aorta, skeletal
muscle, axillary and mesenteric lymph nodes, pancreas, bladder,
prostate, epididymis, uterus, mammary gland, oesophagus, stomach,
duodenum, ileum and colon.
Food consumption of rats fed Acesulfame K at the 10% level was
depressed during the first two to three weeks and body weight gain as
markedly lowered during the first four weeks; slight diarrhoea and
increased faecal water content occurred at this dose level. A slight
increase in haemoglobin concentration was observed in males of the top
dose group only, and total serum protein was slightly decreased in
females only. Caecal enlargement was observed in both sexes receiving
10% Acesulfame K and in females receiving 3%. The relative weights of
the liver and kidneys were slightly elevated in females of the 10%
group and relative spleen weights were slightly depressed in all dose
groups.
Urinalysis, serum enzyme levels and serum albumin were not
affected by the treatment, no gross pathological changes were detected
and no dose-related abnormalities were observed histologically
(Sinkeldam, Til & Willems, 1974).
These workers considered that the caecal enlargement was a
physiological response to the presence of osmotically-active material
in the gut and that, since liver, kidney and spleen weights were
within the normal range of the strain of rat used, and no histological
changes occurred, the no toxic effect level is conservatively placed
at 3% in the diet; this is equivalent to 1.5 g/kg/day in rats.
Dog
Four groups of four female and four male beagle dogs, initially
17-21 weeks old, were fed diets containing 0, 0.3, 1.0, or 3.0%
Acesulfame K for two years. Body weight was recorded weekly for the
first 12 weeks and at four-weekly intervals thereafter. Urinalysis,
haematological examination and clinical chemistry were performed after
12, 26, 52, 78 and 104 weeks. Urinalysis included specific gravity,
pH, sugar, protein, occult blood, ketone and microscopic examination
of sediment; haematology comprised sedimentation rate, clotting time,
haemoglobin, PCV, RBC count, WBC count and differential leucocyte
count; clinical chemical investigations included blood sugar, urea,
SGOT, SGPT, serum alkaline phosphatase, total serum protein and serum
albumin. Liver function tests (bromosulfophthalein clearance) and
kidney function tests (phenol red excretion) were performed on control
and top dose group animals after 26, 52 and 104 weeks. At termination,
gross pathological examinations were performed and the following
organs weighed: heart, kidneys, spleen, liver, lungs, testes/ovaries,
thyroids, adrenals and brain. Histological examinations were performed
on the weighed organs and also on the following tissues: spinal cord,
sciatic nerve, salivary glands, skeletal muscle, thoracic aorta, skin,
tonsils, axillary, superficial, cervical and mesenteric lymph nodes,
bladder, oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon,
pancreas, trachea, circumanai glands, eyes, epididymis, prostate,
uterus, gall bladder, tongue and thymus. A marrow smear (rib bone) was
also examined. General appearance, condition, behaviour and survival
were not affected by the treatment. None of the examinations performed
revealed adverse effects related to the feeding of Acesulfame K.
The no-toxic effect level was found to be higher than 3% in the
diet corresponding to an intake of 900 mg/kg/day in dogs (Reuzel & van
der Heijden, 1977).
Long-term studies
Rat
A combined chronic toxicity and carcinogenicity study was
performed on Wistar rats (CIVO strain) which were obtained from the
F1a generation in a multigeneration study (see Special studies on
reproduction above). Four groups of 60 male and 60 female weanling
rats were given diets containing 0, 0.3, 1.0, or 3% Acesulfame K for
two years. The rats were derived from parents which had been
maintained on the same diet since weaning. Periodic observations were
made of appearance, behaviour growth and food intake. Haematological
examinations were carried out after 13, 26, 52, 78 and 104 weeks,
clinical chemical tests were performed on blood samples after 26, 52
and 104 weeks and urinalysis was done after 26, 52, 78 and 102 weeks.
At termination, survivors were autopsied and organ weights recorded
for heart, kidneys, spleen, liver, brain, gonads, thyroid, adrenals
and caecum (filled and empty). Tissue samples from 20 male and 20
female rats of the control and top dose groups only were subjected to
comprehensive histological examinations; histology on other animals
was limited to liver, spleen, adrenals, thyroid, parathyroid,
pituitary and ovaries, and to grossly visible lesions suspected of
being tumours.
Body weight gain was decreased in both sexes of the top dose
group during the first 44 weeks of the study but not significantly
thereafter. Death-rates of males fed 1.0 or 3% Acesulfame K and of
females fed 0.3% Acesulfame K were higher than controls but it was
considered that there was no evidence of mortality being increased by
treatment, and the mortality of control rats was low for the strain of
rat used. Interim deaths were mainly due to chronic respiratory
disease and lymphoreticular malignancies of pulmonary lymphoid tissue.
The incidence of pulmonary lymphoreticular tumours was relatively high
in both males and females of the top dose group but only achieved
statistical significance in females; there was also some evidence that
these tumours appeared rather earlier in males of the mid and top dose
groups. The results of haematological, clinical, chemical and
urinalysis investigations were essentially normal in all dose groups.
The relative weights of liver, kidneys, caecum and adrenals were
increased in both sexes of the high dose group but the differences
only reached statistical significance in the cases of liver and empty
caecal weight in males, and kidneys and caecal weight in females.
Gross and histopathology did not reveal any treatment-related effects
(Sinkeldam et al., 1977).
In commenting on these results, the authors pointed out the
problems of inter-group comparisons in multigeneration studies where
the animals in the different dose groups are not randomised. They
stated that the increased death rate in test animals was still within
the normal range for the strain of rats used and the mortality in
controls was lower than usual. Pulmonary lymphoreticular tumours are a
common cause of death in the strain of rats used, with very variable
incidence, and the frequency in the test groups was within the normal
range. It was concluded that the "higher" incidences and earlier
appearance in this study were fortuitous findings and did not suggest
that Acesulfame K possessed carcinogenic properties (Sinkeldam et al.,
1977).
A second combined chronic toxicity and carcinogenicity study was
carried out on a different rat strain with a lower incidence of
pulmonary tumours in untreated animals. Four groups of 60 male and 60
female SPF-Wistar rats received diets containing 0, 0.3, 1.0 or 3.0%
Acesulfame K for 120-123 weeks. The rats used were progeny from
parents which had been maintained on the same test diets since
weaning. No adverse effects, other than decreased body weight in the
top dose group were observed in this study. In particular there was no
increased mortality nor tumour incidence in the treatment groups. It
was concluded that Acesulfame K failed to show carcinogenic or other
effects of toxicological significances when fed to rats at levels of
up to 3.0% for 120 weeks (Sinkeldam et al., 1979).
OBSERVATIONS IN MAN
Three human volunteers, body weight 70-80 kg, were given a single
oral dose of 30 mg 14C-Acesulfame K in peppermint tea. Absorption was
rapid and virtually complete, maximum blood concentrations of
0.28 µg/ml occurring after 1 to 1-1/2 h. Elimination occurred rapidly
with a plasma half-life of 2-1/2 h, over 99% of the dose being
excreted in urine and less than 1% in faeces; 98% of the activity was
eliminated in the first 24 h. From the pharmacokinetic data it was
calculated that repeated doses of 30 mg at 3 h intervals would
increase the maximum serum levels 1.7-fold and at 2 h intervals
maximum serum levels would increase 2.4-fold relative to a single dose
(Christ & Rupp, 1976).
The metabolism of Acesulfame K was studied in serum and urine
from human volunteers following a single oral dose of 30 mg per
individual. Only the original substance was detected in all samples
(Volz, 1976).
Comments
Some shortcomings were apparent in the long-term/carcinogenicity
studies. The mouse carcinogenicity study was not considered to meet
current requirements in that detailed histopathology was performed
only on the livers and on others showing gross lesions suspected of
being tumours; in the second long-term feeding study in rats, only a
small proportion of the animals in the control and top dose groups
were examined histopathologically in detail. Further clarification is
needed of the report of an increased incidence of lymphomas restricted
to the lung in the first long-term rat study.
EVALUATION
No ADI allocated.
REFERENCES
Anon. (1973) 3,4-Dihydro-6-methyl-1,2,3-oxothiazine-4-one-2, 2-dioxide
potassium. Determination of acute oral toxicity in female
SPF-Wistar rat. Unpublished report submitted to WHO by Hoechst
A.G.
Anon. (1977) Acute oral toxicity of acetoacetamide in female SPF
Wistar rats. Report No. 427/77. Unpublished report submitted to
WHO by Hoechst A.G.
Anon. (1980a) Acesulfame K. A new non-nutritive sweetener. Unpublished
data supplied to WHO by Hoechst A.G.
Anon. (1980b) Investigating the efficacy of acetoacetamide as a
substrate of ß-hydroxyacyl-CoA-dehydrogenase and
ß-hydroxy-butyrate-dehydrogenase. Unpublished report submitted to
WHO by Hoechst A.G.
Baeder & Horstmann (1977) Oral embryotoxicity study of Acesulfam in
rabbits, stock Hoe:HIMK (SPF Wiga) Report No. 317/77. Unpublished
report submitted to WHO by Hoechst A.G.
Baeder, Horstmann & Weigand (1977) Oral mutagenicity study
(micronucleus test) of Acesulfam in NMRI mice. Report No. 591/77.
Unpublished report submitted by Hoechst A.G.
Beems, R. B. & Til, H. P. (1976) Carcinogenicity study with Hoe 0-95K
in mice. Report No. R5058. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Christ, O. & Rupp (1976) Human experiments with Acetosulfam-14C.
Pharmacokinetics after oral administration of 30 mg to three
healthy male probands. Unpublished report No. 01-L42-0176-76
submitted to WHO by Hoechst A.G.
Eisenbrand, G. (1979) Experiments aimed at the nitrosation of
Acetosulfam. Unpublished report submitted to WHO by Hoechst A.G.
Engelbart, K. (1979) Ames' test ß-Hydroxybutyramid Substanz 90/79.
Bericht Nr. 390/79A. Unpublished report submitted to WHO by
Hoechst A.G.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
submitted to WHO by Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975a) Re: Absorption, distribution and
elimination after administration of Acetosulfam-14C to rats and
dogs. Dr. Kn/tr 5547. Unpublished report submitted to WHO by
Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975b) Pharmacokinetic studies of
Acetosulfam-14C after oral administration to pigs. Unpublished
report submitted to WHO by Hoechst A.G.
Koeter, H. B. W. M. (1975) Effect of Hoe 0-95K on pregnancy of the
rat. Report No. R4854. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Leegwater, D. C., De Groot, A. P. & van Kalmthout-Kuyper, M. (1974)
The aetiology of caecal enlargement in the rat, Fd. Cosmet.
Toxicol., 12: 687-697
Markert, U. & Weigand, W. (1979a) Akute Toxizitat von Acesulfam-K am
Zebrabarbling (Brachydanio rerio) Bericht Nr. 754/79. Unpublished
report submitted to WHO by Hoechst A.G.
Markert, U. & Weigand, W. (1979b) Akute Toxizitat von Acesulfam-K an
Goldorfen (Leuciscus Idus f. melanotus). Bericht Nr. 755/79.
Unpublished report submitted to WHO by Hoechst A.G.
Marquardt, H. W. J. (1978) Mutagenicity and oncogenicity
of Acetosulfam-K. Studies with mammalian cells in vitro.
Unpublished report of Memorial Sloan-Kettering Cancer Centre,
N.Y. Submitted to WHO by Hoechst A.G.
Mayer, D. & Weigand, W. (1977) Acute intraperitoneal toxicity of
Acesulfam-K, batch CIVO 4, in female SPF Wistar rats. Report No.
285/77. Unpublished report prepared for Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978a) Acesulfame K. Cytogenetic
study in the Chinese hamster. Report No. 575/78. Unpublished
report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978b) Report on investigations
of the caecum-enlarging action of Acesulfam-K salt in juvenile
female rats. Report No. 416/78. Unpublished report submitted to
WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978c) A report on the
experimental studies of the effect of Acesulfam K salt in causing
caecal enlargement in adult female rats. Report No. 417/78.
Unpublished report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978d) Report on studies of the
subchronic effects on rats of high doses of potassium chloride in
the food. Report No. 418/78. Unpublished report submitted to WHO
by Hoechst A.G.
Reuzel, P. G. J. & van der Heijden, C. A. (1977) Long-term (two-year)
oral toxicity study with Hoe 0-95K in beagle dogs. Report No.
5280. Unpublished report of CIVO TNO Zeist. Submitted to WHO by
Hoechst A.G.
Rohrborn, G. (1976) Expert opinion on the mutagenicity of Acetosulfam.
Unpublished report of Universitat Dusseldorf. Submitted to WHO by
Hoechst A.G.
Schrinner & Limbert (1977) Report on investigations of the action of
the sweetener Acesulfam against bacteria. Unpublished report
submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. (1976) Reproduction study with Hoe 0-95K in rats.
Report No. R5187. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. et al. (1976) Multigeneration study with Hoe 0-95K in
rats. Report No. 5156. Unpublished report of CIVO TNO, Zeizt.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Kuper, C. F. & Beems, R. B. (1979) Combined chronic
toxicity and carcinogenicity study with Hoe 0-95K in rats
pretreated in utero. Report No. R6100. Unpublished report of
CIVO TNO, Zeist. Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Til, H. P. & Willems, M. I. (1974) Sub-chronic
(90 day) toxicity study with Hoe 0-95K in albino rats.
Unpublished report of CIVO TNO, Zeist. Submitted to WHO by
Hoechst A.G.
Sinkeldam, E. J., van der Heijden, C. A. & Koeter, H. B. W. M. (1977)
Combined chronic toxicity and carcinogenicity study with Hoe
0-95K in rats. Report No. R5229, Unpublished report of CIVO TNO,
Zeist. Submitted to WHO by Hoechst A.G.
Vogel, H. P. & Alpermann, H.-G. (1974) Sweetener 0-95K (H 73 3293).
Pharmacological investigations. Unpublished report submitted to
WHO by Hoechst A.G.
Volz, M. (1975) Research report RCL Dr. Vz/Tr No. 01-L42-0143-75.
Unpublished report submitted to WHO by Hoechst A.G.
Volz, M. (1976) Research report No. 01-L42-0177-76. Unpublished report
submitted to WHO by Hoechst A.G.
Willems, M. I. (1974) Dominant lethalassay with Hoe 0-95K in male
albino rats. Report No. R4472. Unpublished report of CIVO TNO.
Submitted to WHO by Hoechst A.G.
It is proposed for use as a table-top sweetener, and for use in
soft drinks, fruit preparations, desserts, breakfast cereals, chewing
gum, and other food applications appear to be possible. Based on data
for food consumption in the Federal Republic of Germany, and estimated
levels of use in these specified foods, the probable mean daily intake
has been estimated as 470 mg/day (Anon., 1980a).
Acesulfame K is stable in foods, beverages and cosmetic
preparations under normal storage conditions. Under extreme conditions
of pH and temperature, detectable decomposition may occur leading to
the formation of acetone, CO2, and ammonium hydrogen sulfate, or
amido-sulfate, as final decomposition products; under acid (pH 2.5)
conditions, minute quantities of acetoacetamide and acetoacetamide
N-sulfonic acid are formed as unstable intermediate decomposition
products, while under alkaline (pH 3-10.5) conditions, acetoacetic
acid and acetoacetamide N-sulfonic acid can be detected.
This artificial sweetener was assessed for the first time by the
Joint Expert Committee on Food Additives.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg 14C-acesulfame K/kg bw given to rats
and dogs were rapidly absorbed. Maximum blood levels reached were
0.75 ± 0.2 µg/ml in rats, 0.5 h after dosing, and 6.56 ± 2.08 µg/ml in
dogs, 1-1.5 h after dosing. In rats, 82-100% of the dose, and in dogs,
85-100% of the dose was excreted in the urine; in both species,
97-100% of their total radioactivity was excreted in faeces, and total
recovery approximated to 100%.
Rats given 10 consecutive daily doses of 10 mg/kg orally did not
show evidence of accumulation. Three days after dosing, the
concentration in the organs and plasma was 0.4 nMol/g in liver, and
less than 0.2 nMol/g in other tissues. Seven days after dosing, the
concentration in dogs was less than 0.2 nMol/g in all tissues examined
(Kellner & Christ, 1975a).
After intravenous administration of a single dose of 10 mg
14C-acesulfame K/kg bw to rats, the radioactivity was excreted
quantitatively in urine and the plasma half-life was 0.23 h (Kellner &
Christ, 1975a).
Following oral administration of a single dose of 3.6-4.5 mg
14C-acesulfame K/kg bw to pigs, maximum blood levels ranged between
0.35-0.72 µg/ml between 1-2 h after dosing and fell to undetectable
levels within 48 h. Excretion occurred mainly in the urine (Kellner &
Christ, 1975b).
Bio-transformation
The metabolism of Acesulfame K was investigated in the urine and
faeces of rats and dogs which had received single oral doses of
10 mg/kg bw, and in the urine and bile of pigs dosed orally with
5 mg/kg bw. The analytical methods used (thin-layer chromatography,
mass spectrometry and isotope dilution) detected only the original
substance in these samples (Volz, 1975).
Effects on enzymes
In vitro studies on acetoacetamide, a possible minor breakdown
product of Acesulfame K, showed that it did not function as a
substrate for thiolase, ß-hydroxyacyl-CoA-dehydrogenase, and
ß-hydroxybutyrate-dehydrogenase indicating that in vivo formation of
acetamide is not probable (Anon., 1980b).
Effects on bacteria
Acesulfame K was without antibacterial activity against 12
bacterial strains in vitro, and did not show antibacterial activity
in experimental septicaemia in the mouse with Streptococcus pyogenes
A77 or Salmonella typhimurium. Long-term culture (30 daily passages)
of Staphylococcus aureus and E. coli with a range of
concentrations of Acesulfame did not affect growth characteristics nor
sensitivity towards antibiotics, ampicillin, cephalothin, tetracycline
or gentiamycin (Schrinner & Limbert, 1977).
Nitrosation of Acesulfame K
In vitro studies were performed to investigate whether
Acesulfame K could form N-nitroso derivatives. Nitrosation was carried
out using N2O3 in glacial acetic acid, or excess NaNO2, at pH 3 and
pH 1. An N-nitroso compound was detected in low yield with N2O3 or
with nitrite at pH 1 but not at pH 3. The yield at pH 1 was estimated
to be 0.4 × 10-3% (Eisenbrand, 1979).
TOXICOLOGICAL STUDIES
Special studies on acetoacetamide - acute toxity and mutagenicity
The oral LD50 of acetoacetamide in female rats was greater than
15 g/kg bw (Anon., 1977).
Acetoacetamide was non-mutagenic in bacterial assays using four
strains of Salmonella typhimurium, with and without metabolic
activation (Gericke, 1977), did not induce point mutations in cultured
V79 Chinese hamster cells, and was negative in the cell transformation
assay using M2 mouse fibroblasts (Marquardt, 1978).
Special studies on ß-hydroxybutyramide - mutagenicity
ß-Hydroxybutyramide, a metabolite of acetamide in some mammalian
species, was non-mutagenic in the Ames' test against four strains of
Salmonella typhimurium, with and without metabolic activation
(Engelbart, 1979).
Special study on caecal enlargement
These studies were performed to investigate the possible
reversibility of caecal enlargement observed in short-term and
long-term studies.
Groups of 10 juvenile female Wistar rats, body weight
approximately 115 g, received Acesulfame K at dietary concentrations
of 0, 3.0, or 10.0% for treatment periods of 45, 49, and 90 days. One
group from each dose level was sacrificed at the end of the treatment
periods of 45 and 90 days; in addition one group from each dose level
was sacrificed after recovery periods of 41 days' treatment, and 14,
56 and 127 days following 90 days treatment. Food and water intake,
and body weight were measured weekly. At termination, the weights of
caeca with and without contents, as well as moisture content of the
contents, were determined.
At the 10% feeding level, there was an increase in food and water
intake and a reduction in body weight gain which was reversible during
the post-treatment period. At this treatment level, filled caecal
weight relative to body weight was approximately doubled after 45 and
90 days while at the 3% dietary level, a significant increase of about
30% was observed after 90 days only. A significant increase in the
water content of caecal contents was observed only in the highest dose
group.
The changes in the caecal weights after 90 days' exposure to 3%
Acesulfame K in the diet were reversible within 14 days. After 90
days' treatment at the 10% dietary level, the water content in the
caecum returned to normal within 14 days but the filled caecal weight
remained significantly increased (by approximately 30%) even after a
recovery period of 127 days (Mayer et al., 1978b).
A similar study was performed with adult female Wistar rats,
body weight approximately 220 g. Initially, animals receiving 10%
Acesulfame K in the diet experienced anorexia followed by an increased
food consumption after two weeks. Water consumption was increased
during the treatment period in both dose groups.
Filled and empty caecal weights were increased by 80% and 33%
respectively in animals receiving 10% Acesulfame for 45 days.
Increased caecal weights were observed in both dose groups after 91
days' treatment. Caecal water content was significantly increased in
both dose groups after 45 days but not after 91 days' treatment.
All the changes were reversible in animals of both dose groups
treated for 49 days followed by a 42 day recovery period. After 91
days' treatment at the 3% dietary level, all changes were reversible
within 14 days; at the 10% dietary level, caecal water content
returned to normal within 14 days but filled caecal weights were still
significantly increased (by about 30%) after a recovery period of 127
days (Mayer et al., 1978c).
These experiments did not reveal any significant differences
between juvenile and adult animals with regard to induction or
reversibility of caecal enlargement. Complete reversibility was
demonstrated at the 3% dietary level, but not at 10% in the diet. The
authors note that the coincidence of increased water intake with
increased caecal water content and the reversibility of both of these
parameters after withdrawal of Acesulfame K probably indicates that
the changes are of osmotic origin.
Special studies on carcinogenicity
Four groups of 100 male and 100 female Swiss mice were fed diets
containing 0, 0.3, 1.0, or 3.0% Acesulfame K for 80 weeks. All
survivors were sacrificed and autopsied, and weights of livers and
kidneys were recorded. All tumours and tissues showing gross lesions
suspected of being tumours and the livers of all animals were examined
microscopically (haematoxylin and eosin sections).
The feeding of Acesulfame K did not cause adverse effects on
general appearance, behaviour or survival at any of the dietary levels
but body weights were slightly decreased at the 3.0% dose level in
both sexes. The relative liver weight was decreased at all dose levels
in males only but there was no evidence of a dose-related response.
Deaths occurring during the course of the study were attributed to
chronic nephropathy, severe liver degeneration, respiratory infections
and lung tumours.
Gross and microscopic examination revealed a variety of tumours
in both test and control animals, but evaluation of the data on type
of tumour, location and incidence did not indicate that the test
compound was carcinogenic to mice at dietary levels up to 3% for 80
weeks (Beems & Til, 1976).
See also Long-term studies in rats.
Special studies on mutagenicity
An oral mutagenicity study (micronucleus test) was carried out in
which Acesulfame K was administered orally to male and female NMR1
mice at doses of 450, 1500, and 4500 mg/kg on two consecutive days.
The animals were killed six hours after the second application, bone
marrow was taken from the femur and 2000 polychromatic erythrocytes
per animal counted and scored for micronuclei. The mutagenic index did
not differ significantly from 1 (Baeder & Horstmann, 1977).
In a dominant lethal assay, Acesulfame K was administered to male
Wistar rats at dietary levels of 1.0 or 3.0% for five consecutive days
prior to mating. At day 1, 8 and 15 post-treatment each male was caged
with two untreated females for seven days. Pregnant females were
sacrificed mid-term and scored for pregnancy rate and total and dead
implants. No adverse effects were observed and it was concluded that
Acesulfame K at these dietary levels did not induce dominant lethal
mutations in spermatozoa of vas deferens and epididymis, testicular
sperm or late spermatids (Willems, 1974).
Cytogenetic investigations were conducted on bone marrow cells of
Chinese hamsters treated orally with five consecutive daily doses of
Acesulfame K of 0, 450, 1500, or 4500 mg/kg bw. The ratios of
chromosomal abnormalities in control and treated animals were low, and
values normally found in this strain were not exceeded (Mayer et al.,
1978a).
The ability o£ Acesulfame K and of acetoacetamide to induce
mutations in V79 Chinese hamster cells in vitro (8-azaguanine
susceptibility to resistance) was investigated at concentrations of
10-10 000 µg/ml. Neither of the compounds was mutagenic in this
system; acetoacetamide was slightly cytotoxic at the highest
concentration. The compounds were also screened for induction of
transformations in M2 mouse fibroblasts at similar concentrations.
Acesulfame K did not cause any transformations, but reduced plating
efficiency (cytotoxicity) was observed at concentrations of 5000 and
10 000 µg/ml; one (out of 14) transformed focus was observed with
acetoacetamide at a concentration of 5000 µg/ml but not at the higher
concentration, and this was not considered to be evidence of potential
oncogenic activity. In addition, Acesulfame K was not mutagenic nor
cytotoxic against four strains of Salmonella typhimurium at
concentrations up to 100 mg/plate (Marquardt, 1978).
Acesulfame K was neither mutagenic nor cytotoxic in the Ames'
test using four strains of Salmonella typhimurium (detecting both
base-pair and frame-shift mutations) either with or without activation
by rat microsomes (Rohrborn, 1976).
Special studies on pharmacological aspects
Dosages of Acesulfame K of 400 m/kg i.p., 500 m/kg orally, or
320 mg/kg subcutaneously, did not depress motor activity of mice
excited by Pervitin. The hexobarbital sleeping time in mice was not
changed by pretreatment with Acesulfame K at doses of 500 mg/kg
per os or 160 mg/kg subcutaneously. Metrazol-induced convulsions in
mice were not influenced by Acesulfame K at doses of 500 mg/kg
per os, 300 mg/kg i.p., or 320 mg/kg subcutaneously; anti-convulsant
activity can thus be excluded. Administration of Acesulfame K
(200 mg/kg i.p.) to mice was without effect on tetrabenazine-induced
ptosis and catalepsy, thus the compound is without anti-depressant
properties. Acesulfame K (200 mg/kg i.p., 160 mg/kg s.c., or 500 m/kg
per os) was without effect on compulsive gnawing behaviour induced by
the combined application of apomorphine and imipramine, therefore
antidepressant and anticholinergic effects are unlikely. At doses of
320 mg/kg s.c. or 500 mg/kg orally, Acesulfame K had no analgesic
effect on mice.
Predosing of rats with Acesulfame K (500 mg/kg orally or
160 mg/kg s.c.) was without anti-inflammatory effect on Aerosil-
induced paw oedema, and similar doses had no antipyretic effect in
rats with yeast-induced fever. Eight daily doses of Acesulfame K
(0-100 mg/kg) per os had no effect on serum cholesterol, total
glycerol, free glycerol or glucose levels; relative liver weights were
unchanged. In acute tests, Acesulfame K had no effect on blood sugar
levels in rats given 100 mg/kg orally, guinea-pigs given a similar
dose i.p., or rabbits receiving 100 or 500 mg/kg orally or 50 mg/kg
i.v. Acesulfame K had no diuretic effect in rats and dogs at oral
dose levels of 50 mg/kg and 20 mg/kg respectively.
Acesulfame K given intravenously to guinea-pigs at a dose level
of 24 mg/kg reduced digoxin toxicity. This effect was due to the
potassium content and not to antiarrhythmic activity of the compound.
Intravenous administration of 1-5 mg Acesulfame K/kg to anaesthetised
guinea-pigs one minute before treatment with histamine was without
effect on the bronchial musculature.
Cardiovascular experiments in anaesthetized dogs showed that
intravenous administration of Acesulfame K was without effect up to a
dose of 6 mg/kg; doses of 12 and 24 mg/kg caused a decrease in
contractility of the heart with a transient reduction of blood
pressure and peripheral blood flow. The changes were compensated for
in 3-5 minutes. Intraduodenal administration of Acesulfame K to
anaesthetized dogs at dose levels of 0-1000 mg/kg induced a slight
reduction of blood pressure at 500 mg/kg and this was accompanied by a
reduction of cardiac contractility of about 20% at 1 g/kg. The effect
was reversed in 50-80 minutes and other cardiovascular parameters were
unchanged. In the conscious dog, after a five day treatment with
Acesulfame K at 100 mg/kg per os daily, no change in blood pressure
or cardiac activity could be detected. Acesulfame K at doses of
0-24 mg/kg i.v. had no anti-arrhythmic effect on anaesthetised dogs
poisoned with K-strophanthin. Daily oral administration of Acesulfame
K (1 g/kg) to dogs for 14 days was without effect on thromboplastin
time, thrombin time, recalcification time and thromboelastography of
plasma samples.
No functional changes were detected after application of 50 µg
Acesulfame K to isolated guinea-pig heart using the Langendorff
techniques; the compound did not show antiarrhythmic activity
in isolated, perfused guinea-pig heart with aconitine and
digitoxin-induced fibrillation. In the isolated ileum of guinea-pig,
Acesulfame K at a concentration of 10 µg/ml had no neurotropic or
spasmolytic effect on smooth muscle.
Addition of Acesulfame K to dog plasma in vitro was without
effect on thrombin time, thromboplastin time or recalcification time.
Investigation of the carbonic anhydrase-inhibiting effect
in vitro showed that Acesulfame K had virtually no effect,
concentrations of 180 mg/ml being required for 50% inhibition (Vogel &
Alpermann, 1974).
Special studies on the short-term toxicity of potassium chloride
Feeding studies in rats with potassium chloride were conducted to
elucidate the possible involvement of the potassium ion in Acesulfame
K in changes observed in toxicological studies. Three groups of 20
male and 20 female weanling Wistar rats were fed for 90 days on diets
containing 0, 12 000 or 37 000 ppm (0, 1.2 or 3.7%) KCl, equivalent to
the potassium content of diets containing 0, 3, or 10% Acesulfame K.
Regular measurements were made of body weight, food and water
consumption, urinary volume and potassium content. After the animals
had been sacrificed, the caeca with and without contents were weighed
and the water content of the caecal contents were determined.
There was a dose-related increase in water consumption in both
sexes throughout the study and, in the first few weeks, the food
consumption of treated animals was slightly lower than that of
controls. Body weight gains were depressed in males of both
concentration groups throughout the 90 day period but in females
statistically significant differences were obtained only up to the
twenty-ninth day. Dose-related increases in urine volumes and urinary
potassium were observed. Filled caecal weights were increased by about
10% in males and 20% in females of the top dose group, but these
differences were not statistically significant; no differences were
observed in empty caecal weights, nor in water content of the caecal
contents. It was concluded that the potassium content of Acesulfame K
could be responsible for some adverse effects seen in toxicological
studies, particularly depressed body weight gain (Mayer et al.,
1978d).
Special studies on reproduction
A multigeneration study in rats was carried out, in which males
and females received Acesulfame K at dietary levels of 0, 0.3, 1.0 and
3.0% for three successive generations, each comprising two consecutive
litters. A teratogenicity study was conducted with 15 females per
group of the F2b and F3a generations. Rats from the F3b generation
were submitted to clinical and pathological examination. Pups from the
F1a litters were used for a chronic toxicity/carcinogenicity study at
the same dietary levels of Acesulfame K as the parents (see Long-term
studies).
Fertility, number of young per litter, birth weight, growth rate
and mortality during the lactation period were not adversely affected
and there were no indications of increased mortality in utero.
Growth rate was slightly decreased in the top dose group of the
F0 and F1 generations, and the mid-dose group of the F0 generation.
In the teratogenicity studies, no adverse effects were seen in
appearance, food consumption, autopsy of the dams, organ weights, or
litter data; no visceral or skeletal abnormalities attributable to the
treatment were observed.
In a four-week feeding study on rats of the F3b generation, body
weights and food efficiency figures were slightly decreased in males
at the highest dose level. The relative weights of the caecum were
slightly increased in both sexes of the high-dose group and in males
of the mid-dose group. Gross and microscopic examination did not
reveal any treatment-related pathological changes (Sinkeldam et al.,
1976).
In a separate study, Acesulfame K was fed to pregnant rats at
dietary levels of 0, 0.3, 1.0, or 3.0% from day 6 up to and including
day 15 of pregnancy; a positive control group received 75 000 i.u.
vitamin A/rat/day during the same period. An increase in food
consumption was observed at all three dose levels of Acesulfame K,
most pronounced in the 0.3% group. Mean foetal weight showed a slight,
dose-related increase in the test groups but skeletal and visceral
examination of the foetuses revealed no teratogenic effects
attributable to the feeding of Acesulfame K. A wide range of
abnormalities was induced by teratogenic doses of vitamin A in
positive controls (Koeter, 1975).
A reproduction study was carried out in which male and female
rats were fed diets containing 0, 0.3, 1.0, or 3.0% Acesulfame K for
12 weeks prior to mating; the dams received the same diet throughout
pregnancy and lactation. Observations were made on the fertility
of the females, number of young per litter, sex rates, gross
abnormalities, mortality, body weight, and resorption quotient. Growth
rate was slightly decreased in parent rats of the top dose group and
in the mid-dose group females. No dose-related effects were seen in
any of the observations made on the offspring, and there were no
indications of increased mortality in utero.
At weaning, 60 animals of each sex were selected from the litters
for a two-year feeding study (see below, Long-term studies)
(Sinkeldam, 1976).
An embryotoxicity study with Acesulfame K was carried out in
which female rabbits received doses of 0, 100, 300 or 900 m/kg bw by
gastric intubation from the seventh to the nineteenth day after
mating. On the twenty-ninth day of pregnancy, foetuses were delivered
by Caesarean section; live and dead foetuses, resorptions and
placentas were counted, weighed and examined macroscopically. The
24-hour survival was determined by incubation and half of the foetuses
examined for skeletal abnormalities and the remaining half for
visceral changes. One dam from the 300 mg/kg group had a premature
birth. All other observations were within the range of control values
and there was no evidence of compound-related malformations (Baeder &
Horstmann, 1977).
Acute toxicity
Species Route LC50 (mg/l) Reference
Zebra Fish Water >1 000 Markert & Weigand, 1979a
Golden Orfe Water >1 000 Markert & Weigand, 1979b
LD50 (mg/kg)
Rat p.o. 7 430 Anon., 1973
i.p. 2 240 Mayer & Weigand, 1977
Short-term studies
Rat
Four groups of 10 male and 10 female weanling Wistar-derived rats
were given diets containing 0, 1.0, 3.0, or 10% Acesulfame K for 90
days. Body weights were recorded weekly, food intake was determined
during the first four weeks and in weeks 11 and 12. In week 13, the
animals were bled from the tip of the tail and blood samples were
examined for haemoglobin content, haematocrit, RBC and total and
differential white cell counts. Pooled urine samples from each group
were collected in week 13 and examined for appearance, pH, glucose,
protein, occult blood, ketones and microscopy of the sediment. At
autopsy, blood samples were examined for SGPT, SGOT, alkaline
phosphatase, total serum protein and serum albumin. Organ weights were
recorded for heart, kidneys, liver, spleen, brain, testes/ovaries,
thymus, thyroid, adrenals and caecum (filled and empty). Histological
examination was carried out on haematoxylin/eosin sections of the
weighed organs and of lung, salivary glands, trachea, aorta, skeletal
muscle, axillary and mesenteric lymph nodes, pancreas, bladder,
prostate, epididymis, uterus, mammary gland, oesophagus, stomach,
duodenum, ileum and colon.
Food consumption of rats fed Acesulfame K at the 10% level was
depressed during the first two to three weeks and body weight gain as
markedly lowered during the first four weeks; slight diarrhoea and
increased faecal water content occurred at this dose level. A slight
increase in haemoglobin concentration was observed in males of the top
dose group only, and total serum protein was slightly decreased in
females only. Caecal enlargement was observed in both sexes receiving
10% Acesulfame K and in females receiving 3%. The relative weights of
the liver and kidneys were slightly elevated in females of the 10%
group and relative spleen weights were slightly depressed in all dose
groups.
Urinalysis, serum enzyme levels and serum albumin were not
affected by the treatment, no gross pathological changes were detected
and no dose-related abnormalities were observed histologically
(Sinkeldam, Til & Willems, 1974).
These workers considered that the caecal enlargement was a
physiological response to the presence of osmotically-active material
in the gut and that, since liver, kidney and spleen weights were
within the normal range of the strain of rat used, and no histological
changes occurred, the no toxic effect level is conservatively placed
at 3% in the diet; this is equivalent to 1.5 g/kg/day in rats.
Dog
Four groups of four female and four male beagle dogs, initially
17-21 weeks old, were fed diets containing 0, 0.3, 1.0, or 3.0%
Acesulfame K for two years. Body weight was recorded weekly for the
first 12 weeks and at four-weekly intervals thereafter. Urinalysis,
haematological examination and clinical chemistry were performed after
12, 26, 52, 78 and 104 weeks. Urinalysis included specific gravity,
pH, sugar, protein, occult blood, ketone and microscopic examination
of sediment; haematology comprised sedimentation rate, clotting time,
haemoglobin, PCV, RBC count, WBC count and differential leucocyte
count; clinical chemical investigations included blood sugar, urea,
SGOT, SGPT, serum alkaline phosphatase, total serum protein and serum
albumin. Liver function tests (bromosulfophthalein clearance) and
kidney function tests (phenol red excretion) were performed on control
and top dose group animals after 26, 52 and 104 weeks. At termination,
gross pathological examinations were performed and the following
organs weighed: heart, kidneys, spleen, liver, lungs, testes/ovaries,
thyroids, adrenals and brain. Histological examinations were performed
on the weighed organs and also on the following tissues: spinal cord,
sciatic nerve, salivary glands, skeletal muscle, thoracic aorta, skin,
tonsils, axillary, superficial, cervical and mesenteric lymph nodes,
bladder, oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon,
pancreas, trachea, circumanai glands, eyes, epididymis, prostate,
uterus, gall bladder, tongue and thymus. A marrow smear (rib bone) was
also examined. General appearance, condition, behaviour and survival
were not affected by the treatment. None of the examinations performed
revealed adverse effects related to the feeding of Acesulfame K.
The no-toxic effect level was found to be higher than 3% in the
diet corresponding to an intake of 900 mg/kg/day in dogs (Reuzel & van
der Heijden, 1977).
Long-term studies
Rat
A combined chronic toxicity and carcinogenicity study was
performed on Wistar rats (CIVO strain) which were obtained from the
F1a generation in a multigeneration study (see Special studies on
reproduction above). Four groups of 60 male and 60 female weanling
rats were given diets containing 0, 0.3, 1.0, or 3% Acesulfame K for
two years. The rats were derived from parents which had been
maintained on the same diet since weaning. Periodic observations were
made of appearance, behaviour growth and food intake. Haematological
examinations were carried out after 13, 26, 52, 78 and 104 weeks,
clinical chemical tests were performed on blood samples after 26, 52
and 104 weeks and urinalysis was done after 26, 52, 78 and 102 weeks.
At termination, survivors were autopsied and organ weights recorded
for heart, kidneys, spleen, liver, brain, gonads, thyroid, adrenals
and caecum (filled and empty). Tissue samples from 20 male and 20
female rats of the control and top dose groups only were subjected to
comprehensive histological examinations; histology on other animals
was limited to liver, spleen, adrenals, thyroid, parathyroid,
pituitary and ovaries, and to grossly visible lesions suspected of
being tumours.
Body weight gain was decreased in both sexes of the top dose
group during the first 44 weeks of the study but not significantly
thereafter. Death-rates of males fed 1.0 or 3% Acesulfame K and of
females fed 0.3% Acesulfame K were higher than controls but it was
considered that there was no evidence of mortality being increased by
treatment, and the mortality of control rats was low for the strain of
rat used. Interim deaths were mainly due to chronic respiratory
disease and lymphoreticular malignancies of pulmonary lymphoid tissue.
The incidence of pulmonary lymphoreticular tumours was relatively high
in both males and females of the top dose group but only achieved
statistical significance in females; there was also some evidence that
these tumours appeared rather earlier in males of the mid and top dose
groups. The results of haematological, clinical, chemical and
urinalysis investigations were essentially normal in all dose groups.
The relative weights of liver, kidneys, caecum and adrenals were
increased in both sexes of the high dose group but the differences
only reached statistical significance in the cases of liver and empty
caecal weight in males, and kidneys and caecal weight in females.
Gross and histopathology did not reveal any treatment-related effects
(Sinkeldam et al., 1977).
In commenting on these results, the authors pointed out the
problems of inter-group comparisons in multigeneration studies where
the animals in the different dose groups are not randomised. They
stated that the increased death rate in test animals was still within
the normal range for the strain of rats used and the mortality in
controls was lower than usual. Pulmonary lymphoreticular tumours are a
common cause of death in the strain of rats used, with very variable
incidence, and the frequency in the test groups was within the normal
range. It was concluded that the "higher" incidences and earlier
appearance in this study were fortuitous findings and did not suggest
that Acesulfame K possessed carcinogenic properties (Sinkeldam et al.,
1977).
A second combined chronic toxicity and carcinogenicity study was
carried out on a different rat strain with a lower incidence of
pulmonary tumours in untreated animals. Four groups of 60 male and 60
female SPF-Wistar rats received diets containing 0, 0.3, 1.0 or 3.0%
Acesulfame K for 120-123 weeks. The rats used were progeny from
parents which had been maintained on the same test diets since
weaning. No adverse effects, other than decreased body weight in the
top dose group were observed in this study. In particular there was no
increased mortality nor tumour incidence in the treatment groups. It
was concluded that Acesulfame K failed to show carcinogenic or other
effects of toxicological significances when fed to rats at levels of
up to 3.0% for 120 weeks (Sinkeldam et al., 1979).
OBSERVATIONS IN MAN
Three human volunteers, body weight 70-80 kg, were given a single
oral dose of 30 mg 14C-Acesulfame K in peppermint tea. Absorption was
rapid and virtually complete, maximum blood concentrations of
0.28 µg/ml occurring after 1 to 1-1/2 h. Elimination occurred rapidly
with a plasma half-life of 2-1/2 h, over 99% of the dose being
excreted in urine and less than 1% in faeces; 98% of the activity was
eliminated in the first 24 h. From the pharmacokinetic data it was
calculated that repeated doses of 30 mg at 3 h intervals would
increase the maximum serum levels 1.7-fold and at 2 h intervals
maximum serum levels would increase 2.4-fold relative to a single dose
(Christ & Rupp, 1976).
The metabolism of Acesulfame K was studied in serum and urine
from human volunteers following a single oral dose of 30 mg per
individual. Only the original substance was detected in all samples
(Volz, 1976).
Comments
Some shortcomings were apparent in the long-term/carcinogenicity
studies. The mouse carcinogenicity study was not considered to meet
current requirements in that detailed histopathology was performed
only on the livers and on others showing gross lesions suspected of
being tumours; in the second long-term feeding study in rats, only a
small proportion of the animals in the control and top dose groups
were examined histopathologically in detail. Further clarification is
needed of the report of an increased incidence of lymphomas restricted
to the lung in the first long-term rat study.
EVALUATION
No ADI allocated.
REFERENCES
Anon. (1973) 3,4-Dihydro-6-methyl-1,2,3-oxothiazine-4-one-2, 2-dioxide
potassium. Determination of acute oral toxicity in female
SPF-Wistar rat. Unpublished report submitted to WHO by Hoechst
A.G.
Anon. (1977) Acute oral toxicity of acetoacetamide in female SPF
Wistar rats. Report No. 427/77. Unpublished report submitted to
WHO by Hoechst A.G.
Anon. (1980a) Acesulfame K. A new non-nutritive sweetener. Unpublished
data supplied to WHO by Hoechst A.G.
Anon. (1980b) Investigating the efficacy of acetoacetamide as a
substrate of ß-hydroxyacyl-CoA-dehydrogenase and
ß-hydroxy-butyrate-dehydrogenase. Unpublished report submitted to
WHO by Hoechst A.G.
Baeder & Horstmann (1977) Oral embryotoxicity study of Acesulfam in
rabbits, stock Hoe:HIMK (SPF Wiga) Report No. 317/77. Unpublished
report submitted to WHO by Hoechst A.G.
Baeder, Horstmann & Weigand (1977) Oral mutagenicity study
(micronucleus test) of Acesulfam in NMRI mice. Report No. 591/77.
Unpublished report submitted by Hoechst A.G.
Beems, R. B. & Til, H. P. (1976) Carcinogenicity study with Hoe 0-95K
in mice. Report No. R5058. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Christ, O. & Rupp (1976) Human experiments with Acetosulfam-14C.
Pharmacokinetics after oral administration of 30 mg to three
healthy male probands. Unpublished report No. 01-L42-0176-76
submitted to WHO by Hoechst A.G.
Eisenbrand, G. (1979) Experiments aimed at the nitrosation of
Acetosulfam. Unpublished report submitted to WHO by Hoechst A.G.
Engelbart, K. (1979) Ames' test ß-Hydroxybutyramid Substanz 90/79.
Bericht Nr. 390/79A. Unpublished report submitted to WHO by
Hoechst A.G.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
submitted to WHO by Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975a) Re: Absorption, distribution and
elimination after administration of Acetosulfam-14C to rats and
dogs. Dr. Kn/tr 5547. Unpublished report submitted to WHO by
Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975b) Pharmacokinetic studies of
Acetosulfam-14C after oral administration to pigs. Unpublished
report submitted to WHO by Hoechst A.G.
Koeter, H. B. W. M. (1975) Effect of Hoe 0-95K on pregnancy of the
rat. Report No. R4854. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Leegwater, D. C., De Groot, A. P. & van Kalmthout-Kuyper, M. (1974)
The aetiology of caecal enlargement in the rat, Fd. Cosmet.
Toxicol., 12: 687-697
Markert, U. & Weigand, W. (1979a) Akute Toxizitat von Acesulfam-K am
Zebrabarbling (Brachydanio rerio) Bericht Nr. 754/79. Unpublished
report submitted to WHO by Hoechst A.G.
Markert, U. & Weigand, W. (1979b) Akute Toxizitat von Acesulfam-K an
Goldorfen (Leuciscus Idus f. melanotus). Bericht Nr. 755/79.
Unpublished report submitted to WHO by Hoechst A.G.
Marquardt, H. W. J. (1978) Mutagenicity and oncogenicity
of Acetosulfam-K. Studies with mammalian cells in vitro.
Unpublished report of Memorial Sloan-Kettering Cancer Centre,
N.Y. Submitted to WHO by Hoechst A.G.
Mayer, D. & Weigand, W. (1977) Acute intraperitoneal toxicity of
Acesulfam-K, batch CIVO 4, in female SPF Wistar rats. Report No.
285/77. Unpublished report prepared for Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978a) Acesulfame K. Cytogenetic
study in the Chinese hamster. Report No. 575/78. Unpublished
report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978b) Report on investigations
of the caecum-enlarging action of Acesulfam-K salt in juvenile
female rats. Report No. 416/78. Unpublished report submitted to
WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978c) A report on the
experimental studies of the effect of Acesulfam K salt in causing
caecal enlargement in adult female rats. Report No. 417/78.
Unpublished report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978d) Report on studies of the
subchronic effects on rats of high doses of potassium chloride in
the food. Report No. 418/78. Unpublished report submitted to WHO
by Hoechst A.G.
Reuzel, P. G. J. & van der Heijden, C. A. (1977) Long-term (two-year)
oral toxicity study with Hoe 0-95K in beagle dogs. Report No.
5280. Unpublished report of CIVO TNO Zeist. Submitted to WHO by
Hoechst A.G.
Rohrborn, G. (1976) Expert opinion on the mutagenicity of Acetosulfam.
Unpublished report of Universitat Dusseldorf. Submitted to WHO by
Hoechst A.G.
Schrinner & Limbert (1977) Report on investigations of the action of
the sweetener Acesulfam against bacteria. Unpublished report
submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. (1976) Reproduction study with Hoe 0-95K in rats.
Report No. R5187. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. et al. (1976) Multigeneration study with Hoe 0-95K in
rats. Report No. 5156. Unpublished report of CIVO TNO, Zeizt.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Kuper, C. F. & Beems, R. B. (1979) Combined chronic
toxicity and carcinogenicity study with Hoe 0-95K in rats
pretreated in utero. Report No. R6100. Unpublished report of
CIVO TNO, Zeist. Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Til, H. P. & Willems, M. I. (1974) Sub-chronic
(90 day) toxicity study with Hoe 0-95K in albino rats.
Unpublished report of CIVO TNO, Zeist. Submitted to WHO by
Hoechst A.G.
Sinkeldam, E. J., van der Heijden, C. A. & Koeter, H. B. W. M. (1977)
Combined chronic toxicity and carcinogenicity study with Hoe
0-95K in rats. Report No. R5229, Unpublished report of CIVO TNO,
Zeist. Submitted to WHO by Hoechst A.G.
Vogel, H. P. & Alpermann, H.-G. (1974) Sweetener 0-95K (H 73 3293).
Pharmacological investigations. Unpublished report submitted to
WHO by Hoechst A.G.
Volz, M. (1975) Research report RCL Dr. Vz/Tr No. 01-L42-0143-75.
Unpublished report submitted to WHO by Hoechst A.G.
Volz, M. (1976) Research report No. 01-L42-0177-76. Unpublished report
submitted to WHO by Hoechst A.G.
Willems, M. I. (1974) Dominant lethalassay with Hoe 0-95K in male
albino rats. Report No. R4472. Unpublished report of CIVO TNO.
Submitted to WHO by Hoechst A.G.
See Also:
Toxicological Abbreviations
Acesulfame potassium (WHO Food Additives Series 18)
Acesulfame potassium (WHO Food Additives Series 28)
ACESULFAME POTASSIUM (JECFA Evaluation)
 It is proposed for use as a table-top sweetener, and for use in
soft drinks, fruit preparations, desserts, breakfast cereals, chewing
gum, and other food applications appear to be possible. Based on data
for food consumption in the Federal Republic of Germany, and estimated
levels of use in these specified foods, the probable mean daily intake
has been estimated as 470 mg/day (Anon., 1980a).
Acesulfame K is stable in foods, beverages and cosmetic
preparations under normal storage conditions. Under extreme conditions
of pH and temperature, detectable decomposition may occur leading to
the formation of acetone, CO2, and ammonium hydrogen sulfate, or
amido-sulfate, as final decomposition products; under acid (pH 2.5)
conditions, minute quantities of acetoacetamide and acetoacetamide
N-sulfonic acid are formed as unstable intermediate decomposition
products, while under alkaline (pH 3-10.5) conditions, acetoacetic
acid and acetoacetamide N-sulfonic acid can be detected.
This artificial sweetener was assessed for the first time by the
Joint Expert Committee on Food Additives.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg 14C-acesulfame K/kg bw given to rats
and dogs were rapidly absorbed. Maximum blood levels reached were
0.75 ± 0.2 µg/ml in rats, 0.5 h after dosing, and 6.56 ± 2.08 µg/ml in
dogs, 1-1.5 h after dosing. In rats, 82-100% of the dose, and in dogs,
85-100% of the dose was excreted in the urine; in both species,
97-100% of their total radioactivity was excreted in faeces, and total
recovery approximated to 100%.
Rats given 10 consecutive daily doses of 10 mg/kg orally did not
show evidence of accumulation. Three days after dosing, the
concentration in the organs and plasma was 0.4 nMol/g in liver, and
less than 0.2 nMol/g in other tissues. Seven days after dosing, the
concentration in dogs was less than 0.2 nMol/g in all tissues examined
(Kellner & Christ, 1975a).
After intravenous administration of a single dose of 10 mg
14C-acesulfame K/kg bw to rats, the radioactivity was excreted
quantitatively in urine and the plasma half-life was 0.23 h (Kellner &
Christ, 1975a).
Following oral administration of a single dose of 3.6-4.5 mg
14C-acesulfame K/kg bw to pigs, maximum blood levels ranged between
0.35-0.72 µg/ml between 1-2 h after dosing and fell to undetectable
levels within 48 h. Excretion occurred mainly in the urine (Kellner &
Christ, 1975b).
Bio-transformation
The metabolism of Acesulfame K was investigated in the urine and
faeces of rats and dogs which had received single oral doses of
10 mg/kg bw, and in the urine and bile of pigs dosed orally with
5 mg/kg bw. The analytical methods used (thin-layer chromatography,
mass spectrometry and isotope dilution) detected only the original
substance in these samples (Volz, 1975).
Effects on enzymes
In vitro studies on acetoacetamide, a possible minor breakdown
product of Acesulfame K, showed that it did not function as a
substrate for thiolase, ß-hydroxyacyl-CoA-dehydrogenase, and
ß-hydroxybutyrate-dehydrogenase indicating that in vivo formation of
acetamide is not probable (Anon., 1980b).
Effects on bacteria
Acesulfame K was without antibacterial activity against 12
bacterial strains in vitro, and did not show antibacterial activity
in experimental septicaemia in the mouse with Streptococcus pyogenes
A77 or Salmonella typhimurium. Long-term culture (30 daily passages)
of Staphylococcus aureus and E. coli with a range of
concentrations of Acesulfame did not affect growth characteristics nor
sensitivity towards antibiotics, ampicillin, cephalothin, tetracycline
or gentiamycin (Schrinner & Limbert, 1977).
Nitrosation of Acesulfame K
In vitro studies were performed to investigate whether
Acesulfame K could form N-nitroso derivatives. Nitrosation was carried
out using N2O3 in glacial acetic acid, or excess NaNO2, at pH 3 and
pH 1. An N-nitroso compound was detected in low yield with N2O3 or
with nitrite at pH 1 but not at pH 3. The yield at pH 1 was estimated
to be 0.4 × 10-3% (Eisenbrand, 1979).
TOXICOLOGICAL STUDIES
Special studies on acetoacetamide - acute toxity and mutagenicity
The oral LD50 of acetoacetamide in female rats was greater than
15 g/kg bw (Anon., 1977).
Acetoacetamide was non-mutagenic in bacterial assays using four
strains of Salmonella typhimurium, with and without metabolic
activation (Gericke, 1977), did not induce point mutations in cultured
V79 Chinese hamster cells, and was negative in the cell transformation
assay using M2 mouse fibroblasts (Marquardt, 1978).
Special studies on ß-hydroxybutyramide - mutagenicity
ß-Hydroxybutyramide, a metabolite of acetamide in some mammalian
species, was non-mutagenic in the Ames' test against four strains of
Salmonella typhimurium, with and without metabolic activation
(Engelbart, 1979).
Special study on caecal enlargement
These studies were performed to investigate the possible
reversibility of caecal enlargement observed in short-term and
long-term studies.
Groups of 10 juvenile female Wistar rats, body weight
approximately 115 g, received Acesulfame K at dietary concentrations
of 0, 3.0, or 10.0% for treatment periods of 45, 49, and 90 days. One
group from each dose level was sacrificed at the end of the treatment
periods of 45 and 90 days; in addition one group from each dose level
was sacrificed after recovery periods of 41 days' treatment, and 14,
56 and 127 days following 90 days treatment. Food and water intake,
and body weight were measured weekly. At termination, the weights of
caeca with and without contents, as well as moisture content of the
contents, were determined.
At the 10% feeding level, there was an increase in food and water
intake and a reduction in body weight gain which was reversible during
the post-treatment period. At this treatment level, filled caecal
weight relative to body weight was approximately doubled after 45 and
90 days while at the 3% dietary level, a significant increase of about
30% was observed after 90 days only. A significant increase in the
water content of caecal contents was observed only in the highest dose
group.
The changes in the caecal weights after 90 days' exposure to 3%
Acesulfame K in the diet were reversible within 14 days. After 90
days' treatment at the 10% dietary level, the water content in the
caecum returned to normal within 14 days but the filled caecal weight
remained significantly increased (by approximately 30%) even after a
recovery period of 127 days (Mayer et al., 1978b).
A similar study was performed with adult female Wistar rats,
body weight approximately 220 g. Initially, animals receiving 10%
Acesulfame K in the diet experienced anorexia followed by an increased
food consumption after two weeks. Water consumption was increased
during the treatment period in both dose groups.
Filled and empty caecal weights were increased by 80% and 33%
respectively in animals receiving 10% Acesulfame for 45 days.
Increased caecal weights were observed in both dose groups after 91
days' treatment. Caecal water content was significantly increased in
both dose groups after 45 days but not after 91 days' treatment.
All the changes were reversible in animals of both dose groups
treated for 49 days followed by a 42 day recovery period. After 91
days' treatment at the 3% dietary level, all changes were reversible
within 14 days; at the 10% dietary level, caecal water content
returned to normal within 14 days but filled caecal weights were still
significantly increased (by about 30%) after a recovery period of 127
days (Mayer et al., 1978c).
These experiments did not reveal any significant differences
between juvenile and adult animals with regard to induction or
reversibility of caecal enlargement. Complete reversibility was
demonstrated at the 3% dietary level, but not at 10% in the diet. The
authors note that the coincidence of increased water intake with
increased caecal water content and the reversibility of both of these
parameters after withdrawal of Acesulfame K probably indicates that
the changes are of osmotic origin.
Special studies on carcinogenicity
Four groups of 100 male and 100 female Swiss mice were fed diets
containing 0, 0.3, 1.0, or 3.0% Acesulfame K for 80 weeks. All
survivors were sacrificed and autopsied, and weights of livers and
kidneys were recorded. All tumours and tissues showing gross lesions
suspected of being tumours and the livers of all animals were examined
microscopically (haematoxylin and eosin sections).
The feeding of Acesulfame K did not cause adverse effects on
general appearance, behaviour or survival at any of the dietary levels
but body weights were slightly decreased at the 3.0% dose level in
both sexes. The relative liver weight was decreased at all dose levels
in males only but there was no evidence of a dose-related response.
Deaths occurring during the course of the study were attributed to
chronic nephropathy, severe liver degeneration, respiratory infections
and lung tumours.
Gross and microscopic examination revealed a variety of tumours
in both test and control animals, but evaluation of the data on type
of tumour, location and incidence did not indicate that the test
compound was carcinogenic to mice at dietary levels up to 3% for 80
weeks (Beems & Til, 1976).
See also Long-term studies in rats.
Special studies on mutagenicity
An oral mutagenicity study (micronucleus test) was carried out in
which Acesulfame K was administered orally to male and female NMR1
mice at doses of 450, 1500, and 4500 mg/kg on two consecutive days.
The animals were killed six hours after the second application, bone
marrow was taken from the femur and 2000 polychromatic erythrocytes
per animal counted and scored for micronuclei. The mutagenic index did
not differ significantly from 1 (Baeder & Horstmann, 1977).
In a dominant lethal assay, Acesulfame K was administered to male
Wistar rats at dietary levels of 1.0 or 3.0% for five consecutive days
prior to mating. At day 1, 8 and 15 post-treatment each male was caged
with two untreated females for seven days. Pregnant females were
sacrificed mid-term and scored for pregnancy rate and total and dead
implants. No adverse effects were observed and it was concluded that
Acesulfame K at these dietary levels did not induce dominant lethal
mutations in spermatozoa of vas deferens and epididymis, testicular
sperm or late spermatids (Willems, 1974).
Cytogenetic investigations were conducted on bone marrow cells of
Chinese hamsters treated orally with five consecutive daily doses of
Acesulfame K of 0, 450, 1500, or 4500 mg/kg bw. The ratios of
chromosomal abnormalities in control and treated animals were low, and
values normally found in this strain were not exceeded (Mayer et al.,
1978a).
The ability o£ Acesulfame K and of acetoacetamide to induce
mutations in V79 Chinese hamster cells in vitro (8-azaguanine
susceptibility to resistance) was investigated at concentrations of
10-10 000 µg/ml. Neither of the compounds was mutagenic in this
system; acetoacetamide was slightly cytotoxic at the highest
concentration. The compounds were also screened for induction of
transformations in M2 mouse fibroblasts at similar concentrations.
Acesulfame K did not cause any transformations, but reduced plating
efficiency (cytotoxicity) was observed at concentrations of 5000 and
10 000 µg/ml; one (out of 14) transformed focus was observed with
acetoacetamide at a concentration of 5000 µg/ml but not at the higher
concentration, and this was not considered to be evidence of potential
oncogenic activity. In addition, Acesulfame K was not mutagenic nor
cytotoxic against four strains of Salmonella typhimurium at
concentrations up to 100 mg/plate (Marquardt, 1978).
Acesulfame K was neither mutagenic nor cytotoxic in the Ames'
test using four strains of Salmonella typhimurium (detecting both
base-pair and frame-shift mutations) either with or without activation
by rat microsomes (Rohrborn, 1976).
Special studies on pharmacological aspects
Dosages of Acesulfame K of 400 m/kg i.p., 500 m/kg orally, or
320 mg/kg subcutaneously, did not depress motor activity of mice
excited by Pervitin. The hexobarbital sleeping time in mice was not
changed by pretreatment with Acesulfame K at doses of 500 mg/kg
per os or 160 mg/kg subcutaneously. Metrazol-induced convulsions in
mice were not influenced by Acesulfame K at doses of 500 mg/kg
per os, 300 mg/kg i.p., or 320 mg/kg subcutaneously; anti-convulsant
activity can thus be excluded. Administration of Acesulfame K
(200 mg/kg i.p.) to mice was without effect on tetrabenazine-induced
ptosis and catalepsy, thus the compound is without anti-depressant
properties. Acesulfame K (200 mg/kg i.p., 160 mg/kg s.c., or 500 m/kg
per os) was without effect on compulsive gnawing behaviour induced by
the combined application of apomorphine and imipramine, therefore
antidepressant and anticholinergic effects are unlikely. At doses of
320 mg/kg s.c. or 500 mg/kg orally, Acesulfame K had no analgesic
effect on mice.
Predosing of rats with Acesulfame K (500 mg/kg orally or
160 mg/kg s.c.) was without anti-inflammatory effect on Aerosil-
induced paw oedema, and similar doses had no antipyretic effect in
rats with yeast-induced fever. Eight daily doses of Acesulfame K
(0-100 mg/kg) per os had no effect on serum cholesterol, total
glycerol, free glycerol or glucose levels; relative liver weights were
unchanged. In acute tests, Acesulfame K had no effect on blood sugar
levels in rats given 100 mg/kg orally, guinea-pigs given a similar
dose i.p., or rabbits receiving 100 or 500 mg/kg orally or 50 mg/kg
i.v. Acesulfame K had no diuretic effect in rats and dogs at oral
dose levels of 50 mg/kg and 20 mg/kg respectively.
Acesulfame K given intravenously to guinea-pigs at a dose level
of 24 mg/kg reduced digoxin toxicity. This effect was due to the
potassium content and not to antiarrhythmic activity of the compound.
Intravenous administration of 1-5 mg Acesulfame K/kg to anaesthetised
guinea-pigs one minute before treatment with histamine was without
effect on the bronchial musculature.
Cardiovascular experiments in anaesthetized dogs showed that
intravenous administration of Acesulfame K was without effect up to a
dose of 6 mg/kg; doses of 12 and 24 mg/kg caused a decrease in
contractility of the heart with a transient reduction of blood
pressure and peripheral blood flow. The changes were compensated for
in 3-5 minutes. Intraduodenal administration of Acesulfame K to
anaesthetized dogs at dose levels of 0-1000 mg/kg induced a slight
reduction of blood pressure at 500 mg/kg and this was accompanied by a
reduction of cardiac contractility of about 20% at 1 g/kg. The effect
was reversed in 50-80 minutes and other cardiovascular parameters were
unchanged. In the conscious dog, after a five day treatment with
Acesulfame K at 100 mg/kg per os daily, no change in blood pressure
or cardiac activity could be detected. Acesulfame K at doses of
0-24 mg/kg i.v. had no anti-arrhythmic effect on anaesthetised dogs
poisoned with K-strophanthin. Daily oral administration of Acesulfame
K (1 g/kg) to dogs for 14 days was without effect on thromboplastin
time, thrombin time, recalcification time and thromboelastography of
plasma samples.
No functional changes were detected after application of 50 µg
Acesulfame K to isolated guinea-pig heart using the Langendorff
techniques; the compound did not show antiarrhythmic activity
in isolated, perfused guinea-pig heart with aconitine and
digitoxin-induced fibrillation. In the isolated ileum of guinea-pig,
Acesulfame K at a concentration of 10 µg/ml had no neurotropic or
spasmolytic effect on smooth muscle.
Addition of Acesulfame K to dog plasma in vitro was without
effect on thrombin time, thromboplastin time or recalcification time.
Investigation of the carbonic anhydrase-inhibiting effect
in vitro showed that Acesulfame K had virtually no effect,
concentrations of 180 mg/ml being required for 50% inhibition (Vogel &
Alpermann, 1974).
Special studies on the short-term toxicity of potassium chloride
Feeding studies in rats with potassium chloride were conducted to
elucidate the possible involvement of the potassium ion in Acesulfame
K in changes observed in toxicological studies. Three groups of 20
male and 20 female weanling Wistar rats were fed for 90 days on diets
containing 0, 12 000 or 37 000 ppm (0, 1.2 or 3.7%) KCl, equivalent to
the potassium content of diets containing 0, 3, or 10% Acesulfame K.
Regular measurements were made of body weight, food and water
consumption, urinary volume and potassium content. After the animals
had been sacrificed, the caeca with and without contents were weighed
and the water content of the caecal contents were determined.
There was a dose-related increase in water consumption in both
sexes throughout the study and, in the first few weeks, the food
consumption of treated animals was slightly lower than that of
controls. Body weight gains were depressed in males of both
concentration groups throughout the 90 day period but in females
statistically significant differences were obtained only up to the
twenty-ninth day. Dose-related increases in urine volumes and urinary
potassium were observed. Filled caecal weights were increased by about
10% in males and 20% in females of the top dose group, but these
differences were not statistically significant; no differences were
observed in empty caecal weights, nor in water content of the caecal
contents. It was concluded that the potassium content of Acesulfame K
could be responsible for some adverse effects seen in toxicological
studies, particularly depressed body weight gain (Mayer et al.,
1978d).
Special studies on reproduction
A multigeneration study in rats was carried out, in which males
and females received Acesulfame K at dietary levels of 0, 0.3, 1.0 and
3.0% for three successive generations, each comprising two consecutive
litters. A teratogenicity study was conducted with 15 females per
group of the F2b and F3a generations. Rats from the F3b generation
were submitted to clinical and pathological examination. Pups from the
F1a litters were used for a chronic toxicity/carcinogenicity study at
the same dietary levels of Acesulfame K as the parents (see Long-term
studies).
Fertility, number of young per litter, birth weight, growth rate
and mortality during the lactation period were not adversely affected
and there were no indications of increased mortality in utero.
Growth rate was slightly decreased in the top dose group of the
F0 and F1 generations, and the mid-dose group of the F0 generation.
In the teratogenicity studies, no adverse effects were seen in
appearance, food consumption, autopsy of the dams, organ weights, or
litter data; no visceral or skeletal abnormalities attributable to the
treatment were observed.
In a four-week feeding study on rats of the F3b generation, body
weights and food efficiency figures were slightly decreased in males
at the highest dose level. The relative weights of the caecum were
slightly increased in both sexes of the high-dose group and in males
of the mid-dose group. Gross and microscopic examination did not
reveal any treatment-related pathological changes (Sinkeldam et al.,
1976).
In a separate study, Acesulfame K was fed to pregnant rats at
dietary levels of 0, 0.3, 1.0, or 3.0% from day 6 up to and including
day 15 of pregnancy; a positive control group received 75 000 i.u.
vitamin A/rat/day during the same period. An increase in food
consumption was observed at all three dose levels of Acesulfame K,
most pronounced in the 0.3% group. Mean foetal weight showed a slight,
dose-related increase in the test groups but skeletal and visceral
examination of the foetuses revealed no teratogenic effects
attributable to the feeding of Acesulfame K. A wide range of
abnormalities was induced by teratogenic doses of vitamin A in
positive controls (Koeter, 1975).
A reproduction study was carried out in which male and female
rats were fed diets containing 0, 0.3, 1.0, or 3.0% Acesulfame K for
12 weeks prior to mating; the dams received the same diet throughout
pregnancy and lactation. Observations were made on the fertility
of the females, number of young per litter, sex rates, gross
abnormalities, mortality, body weight, and resorption quotient. Growth
rate was slightly decreased in parent rats of the top dose group and
in the mid-dose group females. No dose-related effects were seen in
any of the observations made on the offspring, and there were no
indications of increased mortality in utero.
At weaning, 60 animals of each sex were selected from the litters
for a two-year feeding study (see below, Long-term studies)
(Sinkeldam, 1976).
An embryotoxicity study with Acesulfame K was carried out in
which female rabbits received doses of 0, 100, 300 or 900 m/kg bw by
gastric intubation from the seventh to the nineteenth day after
mating. On the twenty-ninth day of pregnancy, foetuses were delivered
by Caesarean section; live and dead foetuses, resorptions and
placentas were counted, weighed and examined macroscopically. The
24-hour survival was determined by incubation and half of the foetuses
examined for skeletal abnormalities and the remaining half for
visceral changes. One dam from the 300 mg/kg group had a premature
birth. All other observations were within the range of control values
and there was no evidence of compound-related malformations (Baeder &
Horstmann, 1977).
Acute toxicity
Species Route LC50 (mg/l) Reference
Zebra Fish Water >1 000 Markert & Weigand, 1979a
Golden Orfe Water >1 000 Markert & Weigand, 1979b
LD50 (mg/kg)
Rat p.o. 7 430 Anon., 1973
i.p. 2 240 Mayer & Weigand, 1977
Short-term studies
Rat
Four groups of 10 male and 10 female weanling Wistar-derived rats
were given diets containing 0, 1.0, 3.0, or 10% Acesulfame K for 90
days. Body weights were recorded weekly, food intake was determined
during the first four weeks and in weeks 11 and 12. In week 13, the
animals were bled from the tip of the tail and blood samples were
examined for haemoglobin content, haematocrit, RBC and total and
differential white cell counts. Pooled urine samples from each group
were collected in week 13 and examined for appearance, pH, glucose,
protein, occult blood, ketones and microscopy of the sediment. At
autopsy, blood samples were examined for SGPT, SGOT, alkaline
phosphatase, total serum protein and serum albumin. Organ weights were
recorded for heart, kidneys, liver, spleen, brain, testes/ovaries,
thymus, thyroid, adrenals and caecum (filled and empty). Histological
examination was carried out on haematoxylin/eosin sections of the
weighed organs and of lung, salivary glands, trachea, aorta, skeletal
muscle, axillary and mesenteric lymph nodes, pancreas, bladder,
prostate, epididymis, uterus, mammary gland, oesophagus, stomach,
duodenum, ileum and colon.
Food consumption of rats fed Acesulfame K at the 10% level was
depressed during the first two to three weeks and body weight gain as
markedly lowered during the first four weeks; slight diarrhoea and
increased faecal water content occurred at this dose level. A slight
increase in haemoglobin concentration was observed in males of the top
dose group only, and total serum protein was slightly decreased in
females only. Caecal enlargement was observed in both sexes receiving
10% Acesulfame K and in females receiving 3%. The relative weights of
the liver and kidneys were slightly elevated in females of the 10%
group and relative spleen weights were slightly depressed in all dose
groups.
Urinalysis, serum enzyme levels and serum albumin were not
affected by the treatment, no gross pathological changes were detected
and no dose-related abnormalities were observed histologically
(Sinkeldam, Til & Willems, 1974).
These workers considered that the caecal enlargement was a
physiological response to the presence of osmotically-active material
in the gut and that, since liver, kidney and spleen weights were
within the normal range of the strain of rat used, and no histological
changes occurred, the no toxic effect level is conservatively placed
at 3% in the diet; this is equivalent to 1.5 g/kg/day in rats.
Dog
Four groups of four female and four male beagle dogs, initially
17-21 weeks old, were fed diets containing 0, 0.3, 1.0, or 3.0%
Acesulfame K for two years. Body weight was recorded weekly for the
first 12 weeks and at four-weekly intervals thereafter. Urinalysis,
haematological examination and clinical chemistry were performed after
12, 26, 52, 78 and 104 weeks. Urinalysis included specific gravity,
pH, sugar, protein, occult blood, ketone and microscopic examination
of sediment; haematology comprised sedimentation rate, clotting time,
haemoglobin, PCV, RBC count, WBC count and differential leucocyte
count; clinical chemical investigations included blood sugar, urea,
SGOT, SGPT, serum alkaline phosphatase, total serum protein and serum
albumin. Liver function tests (bromosulfophthalein clearance) and
kidney function tests (phenol red excretion) were performed on control
and top dose group animals after 26, 52 and 104 weeks. At termination,
gross pathological examinations were performed and the following
organs weighed: heart, kidneys, spleen, liver, lungs, testes/ovaries,
thyroids, adrenals and brain. Histological examinations were performed
on the weighed organs and also on the following tissues: spinal cord,
sciatic nerve, salivary glands, skeletal muscle, thoracic aorta, skin,
tonsils, axillary, superficial, cervical and mesenteric lymph nodes,
bladder, oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon,
pancreas, trachea, circumanai glands, eyes, epididymis, prostate,
uterus, gall bladder, tongue and thymus. A marrow smear (rib bone) was
also examined. General appearance, condition, behaviour and survival
were not affected by the treatment. None of the examinations performed
revealed adverse effects related to the feeding of Acesulfame K.
The no-toxic effect level was found to be higher than 3% in the
diet corresponding to an intake of 900 mg/kg/day in dogs (Reuzel & van
der Heijden, 1977).
Long-term studies
Rat
A combined chronic toxicity and carcinogenicity study was
performed on Wistar rats (CIVO strain) which were obtained from the
F1a generation in a multigeneration study (see Special studies on
reproduction above). Four groups of 60 male and 60 female weanling
rats were given diets containing 0, 0.3, 1.0, or 3% Acesulfame K for
two years. The rats were derived from parents which had been
maintained on the same diet since weaning. Periodic observations were
made of appearance, behaviour growth and food intake. Haematological
examinations were carried out after 13, 26, 52, 78 and 104 weeks,
clinical chemical tests were performed on blood samples after 26, 52
and 104 weeks and urinalysis was done after 26, 52, 78 and 102 weeks.
At termination, survivors were autopsied and organ weights recorded
for heart, kidneys, spleen, liver, brain, gonads, thyroid, adrenals
and caecum (filled and empty). Tissue samples from 20 male and 20
female rats of the control and top dose groups only were subjected to
comprehensive histological examinations; histology on other animals
was limited to liver, spleen, adrenals, thyroid, parathyroid,
pituitary and ovaries, and to grossly visible lesions suspected of
being tumours.
Body weight gain was decreased in both sexes of the top dose
group during the first 44 weeks of the study but not significantly
thereafter. Death-rates of males fed 1.0 or 3% Acesulfame K and of
females fed 0.3% Acesulfame K were higher than controls but it was
considered that there was no evidence of mortality being increased by
treatment, and the mortality of control rats was low for the strain of
rat used. Interim deaths were mainly due to chronic respiratory
disease and lymphoreticular malignancies of pulmonary lymphoid tissue.
The incidence of pulmonary lymphoreticular tumours was relatively high
in both males and females of the top dose group but only achieved
statistical significance in females; there was also some evidence that
these tumours appeared rather earlier in males of the mid and top dose
groups. The results of haematological, clinical, chemical and
urinalysis investigations were essentially normal in all dose groups.
The relative weights of liver, kidneys, caecum and adrenals were
increased in both sexes of the high dose group but the differences
only reached statistical significance in the cases of liver and empty
caecal weight in males, and kidneys and caecal weight in females.
Gross and histopathology did not reveal any treatment-related effects
(Sinkeldam et al., 1977).
In commenting on these results, the authors pointed out the
problems of inter-group comparisons in multigeneration studies where
the animals in the different dose groups are not randomised. They
stated that the increased death rate in test animals was still within
the normal range for the strain of rats used and the mortality in
controls was lower than usual. Pulmonary lymphoreticular tumours are a
common cause of death in the strain of rats used, with very variable
incidence, and the frequency in the test groups was within the normal
range. It was concluded that the "higher" incidences and earlier
appearance in this study were fortuitous findings and did not suggest
that Acesulfame K possessed carcinogenic properties (Sinkeldam et al.,
1977).
A second combined chronic toxicity and carcinogenicity study was
carried out on a different rat strain with a lower incidence of
pulmonary tumours in untreated animals. Four groups of 60 male and 60
female SPF-Wistar rats received diets containing 0, 0.3, 1.0 or 3.0%
Acesulfame K for 120-123 weeks. The rats used were progeny from
parents which had been maintained on the same test diets since
weaning. No adverse effects, other than decreased body weight in the
top dose group were observed in this study. In particular there was no
increased mortality nor tumour incidence in the treatment groups. It
was concluded that Acesulfame K failed to show carcinogenic or other
effects of toxicological significances when fed to rats at levels of
up to 3.0% for 120 weeks (Sinkeldam et al., 1979).
OBSERVATIONS IN MAN
Three human volunteers, body weight 70-80 kg, were given a single
oral dose of 30 mg 14C-Acesulfame K in peppermint tea. Absorption was
rapid and virtually complete, maximum blood concentrations of
0.28 µg/ml occurring after 1 to 1-1/2 h. Elimination occurred rapidly
with a plasma half-life of 2-1/2 h, over 99% of the dose being
excreted in urine and less than 1% in faeces; 98% of the activity was
eliminated in the first 24 h. From the pharmacokinetic data it was
calculated that repeated doses of 30 mg at 3 h intervals would
increase the maximum serum levels 1.7-fold and at 2 h intervals
maximum serum levels would increase 2.4-fold relative to a single dose
(Christ & Rupp, 1976).
The metabolism of Acesulfame K was studied in serum and urine
from human volunteers following a single oral dose of 30 mg per
individual. Only the original substance was detected in all samples
(Volz, 1976).
Comments
Some shortcomings were apparent in the long-term/carcinogenicity
studies. The mouse carcinogenicity study was not considered to meet
current requirements in that detailed histopathology was performed
only on the livers and on others showing gross lesions suspected of
being tumours; in the second long-term feeding study in rats, only a
small proportion of the animals in the control and top dose groups
were examined histopathologically in detail. Further clarification is
needed of the report of an increased incidence of lymphomas restricted
to the lung in the first long-term rat study.
EVALUATION
No ADI allocated.
REFERENCES
Anon. (1973) 3,4-Dihydro-6-methyl-1,2,3-oxothiazine-4-one-2, 2-dioxide
potassium. Determination of acute oral toxicity in female
SPF-Wistar rat. Unpublished report submitted to WHO by Hoechst
A.G.
Anon. (1977) Acute oral toxicity of acetoacetamide in female SPF
Wistar rats. Report No. 427/77. Unpublished report submitted to
WHO by Hoechst A.G.
Anon. (1980a) Acesulfame K. A new non-nutritive sweetener. Unpublished
data supplied to WHO by Hoechst A.G.
Anon. (1980b) Investigating the efficacy of acetoacetamide as a
substrate of ß-hydroxyacyl-CoA-dehydrogenase and
ß-hydroxy-butyrate-dehydrogenase. Unpublished report submitted to
WHO by Hoechst A.G.
Baeder & Horstmann (1977) Oral embryotoxicity study of Acesulfam in
rabbits, stock Hoe:HIMK (SPF Wiga) Report No. 317/77. Unpublished
report submitted to WHO by Hoechst A.G.
Baeder, Horstmann & Weigand (1977) Oral mutagenicity study
(micronucleus test) of Acesulfam in NMRI mice. Report No. 591/77.
Unpublished report submitted by Hoechst A.G.
Beems, R. B. & Til, H. P. (1976) Carcinogenicity study with Hoe 0-95K
in mice. Report No. R5058. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Christ, O. & Rupp (1976) Human experiments with Acetosulfam-14C.
Pharmacokinetics after oral administration of 30 mg to three
healthy male probands. Unpublished report No. 01-L42-0176-76
submitted to WHO by Hoechst A.G.
Eisenbrand, G. (1979) Experiments aimed at the nitrosation of
Acetosulfam. Unpublished report submitted to WHO by Hoechst A.G.
Engelbart, K. (1979) Ames' test ß-Hydroxybutyramid Substanz 90/79.
Bericht Nr. 390/79A. Unpublished report submitted to WHO by
Hoechst A.G.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
submitted to WHO by Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975a) Re: Absorption, distribution and
elimination after administration of Acetosulfam-14C to rats and
dogs. Dr. Kn/tr 5547. Unpublished report submitted to WHO by
Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975b) Pharmacokinetic studies of
Acetosulfam-14C after oral administration to pigs. Unpublished
report submitted to WHO by Hoechst A.G.
Koeter, H. B. W. M. (1975) Effect of Hoe 0-95K on pregnancy of the
rat. Report No. R4854. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Leegwater, D. C., De Groot, A. P. & van Kalmthout-Kuyper, M. (1974)
The aetiology of caecal enlargement in the rat, Fd. Cosmet.
Toxicol., 12: 687-697
Markert, U. & Weigand, W. (1979a) Akute Toxizitat von Acesulfam-K am
Zebrabarbling (Brachydanio rerio) Bericht Nr. 754/79. Unpublished
report submitted to WHO by Hoechst A.G.
Markert, U. & Weigand, W. (1979b) Akute Toxizitat von Acesulfam-K an
Goldorfen (Leuciscus Idus f. melanotus). Bericht Nr. 755/79.
Unpublished report submitted to WHO by Hoechst A.G.
Marquardt, H. W. J. (1978) Mutagenicity and oncogenicity
of Acetosulfam-K. Studies with mammalian cells in vitro.
Unpublished report of Memorial Sloan-Kettering Cancer Centre,
N.Y. Submitted to WHO by Hoechst A.G.
Mayer, D. & Weigand, W. (1977) Acute intraperitoneal toxicity of
Acesulfam-K, batch CIVO 4, in female SPF Wistar rats. Report No.
285/77. Unpublished report prepared for Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978a) Acesulfame K. Cytogenetic
study in the Chinese hamster. Report No. 575/78. Unpublished
report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978b) Report on investigations
of the caecum-enlarging action of Acesulfam-K salt in juvenile
female rats. Report No. 416/78. Unpublished report submitted to
WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978c) A report on the
experimental studies of the effect of Acesulfam K salt in causing
caecal enlargement in adult female rats. Report No. 417/78.
Unpublished report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978d) Report on studies of the
subchronic effects on rats of high doses of potassium chloride in
the food. Report No. 418/78. Unpublished report submitted to WHO
by Hoechst A.G.
Reuzel, P. G. J. & van der Heijden, C. A. (1977) Long-term (two-year)
oral toxicity study with Hoe 0-95K in beagle dogs. Report No.
5280. Unpublished report of CIVO TNO Zeist. Submitted to WHO by
Hoechst A.G.
Rohrborn, G. (1976) Expert opinion on the mutagenicity of Acetosulfam.
Unpublished report of Universitat Dusseldorf. Submitted to WHO by
Hoechst A.G.
Schrinner & Limbert (1977) Report on investigations of the action of
the sweetener Acesulfam against bacteria. Unpublished report
submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. (1976) Reproduction study with Hoe 0-95K in rats.
Report No. R5187. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. et al. (1976) Multigeneration study with Hoe 0-95K in
rats. Report No. 5156. Unpublished report of CIVO TNO, Zeizt.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Kuper, C. F. & Beems, R. B. (1979) Combined chronic
toxicity and carcinogenicity study with Hoe 0-95K in rats
pretreated in utero. Report No. R6100. Unpublished report of
CIVO TNO, Zeist. Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Til, H. P. & Willems, M. I. (1974) Sub-chronic
(90 day) toxicity study with Hoe 0-95K in albino rats.
Unpublished report of CIVO TNO, Zeist. Submitted to WHO by
Hoechst A.G.
Sinkeldam, E. J., van der Heijden, C. A. & Koeter, H. B. W. M. (1977)
Combined chronic toxicity and carcinogenicity study with Hoe
0-95K in rats. Report No. R5229, Unpublished report of CIVO TNO,
Zeist. Submitted to WHO by Hoechst A.G.
Vogel, H. P. & Alpermann, H.-G. (1974) Sweetener 0-95K (H 73 3293).
Pharmacological investigations. Unpublished report submitted to
WHO by Hoechst A.G.
Volz, M. (1975) Research report RCL Dr. Vz/Tr No. 01-L42-0143-75.
Unpublished report submitted to WHO by Hoechst A.G.
Volz, M. (1976) Research report No. 01-L42-0177-76. Unpublished report
submitted to WHO by Hoechst A.G.
Willems, M. I. (1974) Dominant lethalassay with Hoe 0-95K in male
albino rats. Report No. R4472. Unpublished report of CIVO TNO.
Submitted to WHO by Hoechst A.G.
It is proposed for use as a table-top sweetener, and for use in
soft drinks, fruit preparations, desserts, breakfast cereals, chewing
gum, and other food applications appear to be possible. Based on data
for food consumption in the Federal Republic of Germany, and estimated
levels of use in these specified foods, the probable mean daily intake
has been estimated as 470 mg/day (Anon., 1980a).
Acesulfame K is stable in foods, beverages and cosmetic
preparations under normal storage conditions. Under extreme conditions
of pH and temperature, detectable decomposition may occur leading to
the formation of acetone, CO2, and ammonium hydrogen sulfate, or
amido-sulfate, as final decomposition products; under acid (pH 2.5)
conditions, minute quantities of acetoacetamide and acetoacetamide
N-sulfonic acid are formed as unstable intermediate decomposition
products, while under alkaline (pH 3-10.5) conditions, acetoacetic
acid and acetoacetamide N-sulfonic acid can be detected.
This artificial sweetener was assessed for the first time by the
Joint Expert Committee on Food Additives.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg 14C-acesulfame K/kg bw given to rats
and dogs were rapidly absorbed. Maximum blood levels reached were
0.75 ± 0.2 µg/ml in rats, 0.5 h after dosing, and 6.56 ± 2.08 µg/ml in
dogs, 1-1.5 h after dosing. In rats, 82-100% of the dose, and in dogs,
85-100% of the dose was excreted in the urine; in both species,
97-100% of their total radioactivity was excreted in faeces, and total
recovery approximated to 100%.
Rats given 10 consecutive daily doses of 10 mg/kg orally did not
show evidence of accumulation. Three days after dosing, the
concentration in the organs and plasma was 0.4 nMol/g in liver, and
less than 0.2 nMol/g in other tissues. Seven days after dosing, the
concentration in dogs was less than 0.2 nMol/g in all tissues examined
(Kellner & Christ, 1975a).
After intravenous administration of a single dose of 10 mg
14C-acesulfame K/kg bw to rats, the radioactivity was excreted
quantitatively in urine and the plasma half-life was 0.23 h (Kellner &
Christ, 1975a).
Following oral administration of a single dose of 3.6-4.5 mg
14C-acesulfame K/kg bw to pigs, maximum blood levels ranged between
0.35-0.72 µg/ml between 1-2 h after dosing and fell to undetectable
levels within 48 h. Excretion occurred mainly in the urine (Kellner &
Christ, 1975b).
Bio-transformation
The metabolism of Acesulfame K was investigated in the urine and
faeces of rats and dogs which had received single oral doses of
10 mg/kg bw, and in the urine and bile of pigs dosed orally with
5 mg/kg bw. The analytical methods used (thin-layer chromatography,
mass spectrometry and isotope dilution) detected only the original
substance in these samples (Volz, 1975).
Effects on enzymes
In vitro studies on acetoacetamide, a possible minor breakdown
product of Acesulfame K, showed that it did not function as a
substrate for thiolase, ß-hydroxyacyl-CoA-dehydrogenase, and
ß-hydroxybutyrate-dehydrogenase indicating that in vivo formation of
acetamide is not probable (Anon., 1980b).
Effects on bacteria
Acesulfame K was without antibacterial activity against 12
bacterial strains in vitro, and did not show antibacterial activity
in experimental septicaemia in the mouse with Streptococcus pyogenes
A77 or Salmonella typhimurium. Long-term culture (30 daily passages)
of Staphylococcus aureus and E. coli with a range of
concentrations of Acesulfame did not affect growth characteristics nor
sensitivity towards antibiotics, ampicillin, cephalothin, tetracycline
or gentiamycin (Schrinner & Limbert, 1977).
Nitrosation of Acesulfame K
In vitro studies were performed to investigate whether
Acesulfame K could form N-nitroso derivatives. Nitrosation was carried
out using N2O3 in glacial acetic acid, or excess NaNO2, at pH 3 and
pH 1. An N-nitroso compound was detected in low yield with N2O3 or
with nitrite at pH 1 but not at pH 3. The yield at pH 1 was estimated
to be 0.4 × 10-3% (Eisenbrand, 1979).
TOXICOLOGICAL STUDIES
Special studies on acetoacetamide - acute toxity and mutagenicity
The oral LD50 of acetoacetamide in female rats was greater than
15 g/kg bw (Anon., 1977).
Acetoacetamide was non-mutagenic in bacterial assays using four
strains of Salmonella typhimurium, with and without metabolic
activation (Gericke, 1977), did not induce point mutations in cultured
V79 Chinese hamster cells, and was negative in the cell transformation
assay using M2 mouse fibroblasts (Marquardt, 1978).
Special studies on ß-hydroxybutyramide - mutagenicity
ß-Hydroxybutyramide, a metabolite of acetamide in some mammalian
species, was non-mutagenic in the Ames' test against four strains of
Salmonella typhimurium, with and without metabolic activation
(Engelbart, 1979).
Special study on caecal enlargement
These studies were performed to investigate the possible
reversibility of caecal enlargement observed in short-term and
long-term studies.
Groups of 10 juvenile female Wistar rats, body weight
approximately 115 g, received Acesulfame K at dietary concentrations
of 0, 3.0, or 10.0% for treatment periods of 45, 49, and 90 days. One
group from each dose level was sacrificed at the end of the treatment
periods of 45 and 90 days; in addition one group from each dose level
was sacrificed after recovery periods of 41 days' treatment, and 14,
56 and 127 days following 90 days treatment. Food and water intake,
and body weight were measured weekly. At termination, the weights of
caeca with and without contents, as well as moisture content of the
contents, were determined.
At the 10% feeding level, there was an increase in food and water
intake and a reduction in body weight gain which was reversible during
the post-treatment period. At this treatment level, filled caecal
weight relative to body weight was approximately doubled after 45 and
90 days while at the 3% dietary level, a significant increase of about
30% was observed after 90 days only. A significant increase in the
water content of caecal contents was observed only in the highest dose
group.
The changes in the caecal weights after 90 days' exposure to 3%
Acesulfame K in the diet were reversible within 14 days. After 90
days' treatment at the 10% dietary level, the water content in the
caecum returned to normal within 14 days but the filled caecal weight
remained significantly increased (by approximately 30%) even after a
recovery period of 127 days (Mayer et al., 1978b).
A similar study was performed with adult female Wistar rats,
body weight approximately 220 g. Initially, animals receiving 10%
Acesulfame K in the diet experienced anorexia followed by an increased
food consumption after two weeks. Water consumption was increased
during the treatment period in both dose groups.
Filled and empty caecal weights were increased by 80% and 33%
respectively in animals receiving 10% Acesulfame for 45 days.
Increased caecal weights were observed in both dose groups after 91
days' treatment. Caecal water content was significantly increased in
both dose groups after 45 days but not after 91 days' treatment.
All the changes were reversible in animals of both dose groups
treated for 49 days followed by a 42 day recovery period. After 91
days' treatment at the 3% dietary level, all changes were reversible
within 14 days; at the 10% dietary level, caecal water content
returned to normal within 14 days but filled caecal weights were still
significantly increased (by about 30%) after a recovery period of 127
days (Mayer et al., 1978c).
These experiments did not reveal any significant differences
between juvenile and adult animals with regard to induction or
reversibility of caecal enlargement. Complete reversibility was
demonstrated at the 3% dietary level, but not at 10% in the diet. The
authors note that the coincidence of increased water intake with
increased caecal water content and the reversibility of both of these
parameters after withdrawal of Acesulfame K probably indicates that
the changes are of osmotic origin.
Special studies on carcinogenicity
Four groups of 100 male and 100 female Swiss mice were fed diets
containing 0, 0.3, 1.0, or 3.0% Acesulfame K for 80 weeks. All
survivors were sacrificed and autopsied, and weights of livers and
kidneys were recorded. All tumours and tissues showing gross lesions
suspected of being tumours and the livers of all animals were examined
microscopically (haematoxylin and eosin sections).
The feeding of Acesulfame K did not cause adverse effects on
general appearance, behaviour or survival at any of the dietary levels
but body weights were slightly decreased at the 3.0% dose level in
both sexes. The relative liver weight was decreased at all dose levels
in males only but there was no evidence of a dose-related response.
Deaths occurring during the course of the study were attributed to
chronic nephropathy, severe liver degeneration, respiratory infections
and lung tumours.
Gross and microscopic examination revealed a variety of tumours
in both test and control animals, but evaluation of the data on type
of tumour, location and incidence did not indicate that the test
compound was carcinogenic to mice at dietary levels up to 3% for 80
weeks (Beems & Til, 1976).
See also Long-term studies in rats.
Special studies on mutagenicity
An oral mutagenicity study (micronucleus test) was carried out in
which Acesulfame K was administered orally to male and female NMR1
mice at doses of 450, 1500, and 4500 mg/kg on two consecutive days.
The animals were killed six hours after the second application, bone
marrow was taken from the femur and 2000 polychromatic erythrocytes
per animal counted and scored for micronuclei. The mutagenic index did
not differ significantly from 1 (Baeder & Horstmann, 1977).
In a dominant lethal assay, Acesulfame K was administered to male
Wistar rats at dietary levels of 1.0 or 3.0% for five consecutive days
prior to mating. At day 1, 8 and 15 post-treatment each male was caged
with two untreated females for seven days. Pregnant females were
sacrificed mid-term and scored for pregnancy rate and total and dead
implants. No adverse effects were observed and it was concluded that
Acesulfame K at these dietary levels did not induce dominant lethal
mutations in spermatozoa of vas deferens and epididymis, testicular
sperm or late spermatids (Willems, 1974).
Cytogenetic investigations were conducted on bone marrow cells of
Chinese hamsters treated orally with five consecutive daily doses of
Acesulfame K of 0, 450, 1500, or 4500 mg/kg bw. The ratios of
chromosomal abnormalities in control and treated animals were low, and
values normally found in this strain were not exceeded (Mayer et al.,
1978a).
The ability o£ Acesulfame K and of acetoacetamide to induce
mutations in V79 Chinese hamster cells in vitro (8-azaguanine
susceptibility to resistance) was investigated at concentrations of
10-10 000 µg/ml. Neither of the compounds was mutagenic in this
system; acetoacetamide was slightly cytotoxic at the highest
concentration. The compounds were also screened for induction of
transformations in M2 mouse fibroblasts at similar concentrations.
Acesulfame K did not cause any transformations, but reduced plating
efficiency (cytotoxicity) was observed at concentrations of 5000 and
10 000 µg/ml; one (out of 14) transformed focus was observed with
acetoacetamide at a concentration of 5000 µg/ml but not at the higher
concentration, and this was not considered to be evidence of potential
oncogenic activity. In addition, Acesulfame K was not mutagenic nor
cytotoxic against four strains of Salmonella typhimurium at
concentrations up to 100 mg/plate (Marquardt, 1978).
Acesulfame K was neither mutagenic nor cytotoxic in the Ames'
test using four strains of Salmonella typhimurium (detecting both
base-pair and frame-shift mutations) either with or without activation
by rat microsomes (Rohrborn, 1976).
Special studies on pharmacological aspects
Dosages of Acesulfame K of 400 m/kg i.p., 500 m/kg orally, or
320 mg/kg subcutaneously, did not depress motor activity of mice
excited by Pervitin. The hexobarbital sleeping time in mice was not
changed by pretreatment with Acesulfame K at doses of 500 mg/kg
per os or 160 mg/kg subcutaneously. Metrazol-induced convulsions in
mice were not influenced by Acesulfame K at doses of 500 mg/kg
per os, 300 mg/kg i.p., or 320 mg/kg subcutaneously; anti-convulsant
activity can thus be excluded. Administration of Acesulfame K
(200 mg/kg i.p.) to mice was without effect on tetrabenazine-induced
ptosis and catalepsy, thus the compound is without anti-depressant
properties. Acesulfame K (200 mg/kg i.p., 160 mg/kg s.c., or 500 m/kg
per os) was without effect on compulsive gnawing behaviour induced by
the combined application of apomorphine and imipramine, therefore
antidepressant and anticholinergic effects are unlikely. At doses of
320 mg/kg s.c. or 500 mg/kg orally, Acesulfame K had no analgesic
effect on mice.
Predosing of rats with Acesulfame K (500 mg/kg orally or
160 mg/kg s.c.) was without anti-inflammatory effect on Aerosil-
induced paw oedema, and similar doses had no antipyretic effect in
rats with yeast-induced fever. Eight daily doses of Acesulfame K
(0-100 mg/kg) per os had no effect on serum cholesterol, total
glycerol, free glycerol or glucose levels; relative liver weights were
unchanged. In acute tests, Acesulfame K had no effect on blood sugar
levels in rats given 100 mg/kg orally, guinea-pigs given a similar
dose i.p., or rabbits receiving 100 or 500 mg/kg orally or 50 mg/kg
i.v. Acesulfame K had no diuretic effect in rats and dogs at oral
dose levels of 50 mg/kg and 20 mg/kg respectively.
Acesulfame K given intravenously to guinea-pigs at a dose level
of 24 mg/kg reduced digoxin toxicity. This effect was due to the
potassium content and not to antiarrhythmic activity of the compound.
Intravenous administration of 1-5 mg Acesulfame K/kg to anaesthetised
guinea-pigs one minute before treatment with histamine was without
effect on the bronchial musculature.
Cardiovascular experiments in anaesthetized dogs showed that
intravenous administration of Acesulfame K was without effect up to a
dose of 6 mg/kg; doses of 12 and 24 mg/kg caused a decrease in
contractility of the heart with a transient reduction of blood
pressure and peripheral blood flow. The changes were compensated for
in 3-5 minutes. Intraduodenal administration of Acesulfame K to
anaesthetized dogs at dose levels of 0-1000 mg/kg induced a slight
reduction of blood pressure at 500 mg/kg and this was accompanied by a
reduction of cardiac contractility of about 20% at 1 g/kg. The effect
was reversed in 50-80 minutes and other cardiovascular parameters were
unchanged. In the conscious dog, after a five day treatment with
Acesulfame K at 100 mg/kg per os daily, no change in blood pressure
or cardiac activity could be detected. Acesulfame K at doses of
0-24 mg/kg i.v. had no anti-arrhythmic effect on anaesthetised dogs
poisoned with K-strophanthin. Daily oral administration of Acesulfame
K (1 g/kg) to dogs for 14 days was without effect on thromboplastin
time, thrombin time, recalcification time and thromboelastography of
plasma samples.
No functional changes were detected after application of 50 µg
Acesulfame K to isolated guinea-pig heart using the Langendorff
techniques; the compound did not show antiarrhythmic activity
in isolated, perfused guinea-pig heart with aconitine and
digitoxin-induced fibrillation. In the isolated ileum of guinea-pig,
Acesulfame K at a concentration of 10 µg/ml had no neurotropic or
spasmolytic effect on smooth muscle.
Addition of Acesulfame K to dog plasma in vitro was without
effect on thrombin time, thromboplastin time or recalcification time.
Investigation of the carbonic anhydrase-inhibiting effect
in vitro showed that Acesulfame K had virtually no effect,
concentrations of 180 mg/ml being required for 50% inhibition (Vogel &
Alpermann, 1974).
Special studies on the short-term toxicity of potassium chloride
Feeding studies in rats with potassium chloride were conducted to
elucidate the possible involvement of the potassium ion in Acesulfame
K in changes observed in toxicological studies. Three groups of 20
male and 20 female weanling Wistar rats were fed for 90 days on diets
containing 0, 12 000 or 37 000 ppm (0, 1.2 or 3.7%) KCl, equivalent to
the potassium content of diets containing 0, 3, or 10% Acesulfame K.
Regular measurements were made of body weight, food and water
consumption, urinary volume and potassium content. After the animals
had been sacrificed, the caeca with and without contents were weighed
and the water content of the caecal contents were determined.
There was a dose-related increase in water consumption in both
sexes throughout the study and, in the first few weeks, the food
consumption of treated animals was slightly lower than that of
controls. Body weight gains were depressed in males of both
concentration groups throughout the 90 day period but in females
statistically significant differences were obtained only up to the
twenty-ninth day. Dose-related increases in urine volumes and urinary
potassium were observed. Filled caecal weights were increased by about
10% in males and 20% in females of the top dose group, but these
differences were not statistically significant; no differences were
observed in empty caecal weights, nor in water content of the caecal
contents. It was concluded that the potassium content of Acesulfame K
could be responsible for some adverse effects seen in toxicological
studies, particularly depressed body weight gain (Mayer et al.,
1978d).
Special studies on reproduction
A multigeneration study in rats was carried out, in which males
and females received Acesulfame K at dietary levels of 0, 0.3, 1.0 and
3.0% for three successive generations, each comprising two consecutive
litters. A teratogenicity study was conducted with 15 females per
group of the F2b and F3a generations. Rats from the F3b generation
were submitted to clinical and pathological examination. Pups from the
F1a litters were used for a chronic toxicity/carcinogenicity study at
the same dietary levels of Acesulfame K as the parents (see Long-term
studies).
Fertility, number of young per litter, birth weight, growth rate
and mortality during the lactation period were not adversely affected
and there were no indications of increased mortality in utero.
Growth rate was slightly decreased in the top dose group of the
F0 and F1 generations, and the mid-dose group of the F0 generation.
In the teratogenicity studies, no adverse effects were seen in
appearance, food consumption, autopsy of the dams, organ weights, or
litter data; no visceral or skeletal abnormalities attributable to the
treatment were observed.
In a four-week feeding study on rats of the F3b generation, body
weights and food efficiency figures were slightly decreased in males
at the highest dose level. The relative weights of the caecum were
slightly increased in both sexes of the high-dose group and in males
of the mid-dose group. Gross and microscopic examination did not
reveal any treatment-related pathological changes (Sinkeldam et al.,
1976).
In a separate study, Acesulfame K was fed to pregnant rats at
dietary levels of 0, 0.3, 1.0, or 3.0% from day 6 up to and including
day 15 of pregnancy; a positive control group received 75 000 i.u.
vitamin A/rat/day during the same period. An increase in food
consumption was observed at all three dose levels of Acesulfame K,
most pronounced in the 0.3% group. Mean foetal weight showed a slight,
dose-related increase in the test groups but skeletal and visceral
examination of the foetuses revealed no teratogenic effects
attributable to the feeding of Acesulfame K. A wide range of
abnormalities was induced by teratogenic doses of vitamin A in
positive controls (Koeter, 1975).
A reproduction study was carried out in which male and female
rats were fed diets containing 0, 0.3, 1.0, or 3.0% Acesulfame K for
12 weeks prior to mating; the dams received the same diet throughout
pregnancy and lactation. Observations were made on the fertility
of the females, number of young per litter, sex rates, gross
abnormalities, mortality, body weight, and resorption quotient. Growth
rate was slightly decreased in parent rats of the top dose group and
in the mid-dose group females. No dose-related effects were seen in
any of the observations made on the offspring, and there were no
indications of increased mortality in utero.
At weaning, 60 animals of each sex were selected from the litters
for a two-year feeding study (see below, Long-term studies)
(Sinkeldam, 1976).
An embryotoxicity study with Acesulfame K was carried out in
which female rabbits received doses of 0, 100, 300 or 900 m/kg bw by
gastric intubation from the seventh to the nineteenth day after
mating. On the twenty-ninth day of pregnancy, foetuses were delivered
by Caesarean section; live and dead foetuses, resorptions and
placentas were counted, weighed and examined macroscopically. The
24-hour survival was determined by incubation and half of the foetuses
examined for skeletal abnormalities and the remaining half for
visceral changes. One dam from the 300 mg/kg group had a premature
birth. All other observations were within the range of control values
and there was no evidence of compound-related malformations (Baeder &
Horstmann, 1977).
Acute toxicity
Species Route LC50 (mg/l) Reference
Zebra Fish Water >1 000 Markert & Weigand, 1979a
Golden Orfe Water >1 000 Markert & Weigand, 1979b
LD50 (mg/kg)
Rat p.o. 7 430 Anon., 1973
i.p. 2 240 Mayer & Weigand, 1977
Short-term studies
Rat
Four groups of 10 male and 10 female weanling Wistar-derived rats
were given diets containing 0, 1.0, 3.0, or 10% Acesulfame K for 90
days. Body weights were recorded weekly, food intake was determined
during the first four weeks and in weeks 11 and 12. In week 13, the
animals were bled from the tip of the tail and blood samples were
examined for haemoglobin content, haematocrit, RBC and total and
differential white cell counts. Pooled urine samples from each group
were collected in week 13 and examined for appearance, pH, glucose,
protein, occult blood, ketones and microscopy of the sediment. At
autopsy, blood samples were examined for SGPT, SGOT, alkaline
phosphatase, total serum protein and serum albumin. Organ weights were
recorded for heart, kidneys, liver, spleen, brain, testes/ovaries,
thymus, thyroid, adrenals and caecum (filled and empty). Histological
examination was carried out on haematoxylin/eosin sections of the
weighed organs and of lung, salivary glands, trachea, aorta, skeletal
muscle, axillary and mesenteric lymph nodes, pancreas, bladder,
prostate, epididymis, uterus, mammary gland, oesophagus, stomach,
duodenum, ileum and colon.
Food consumption of rats fed Acesulfame K at the 10% level was
depressed during the first two to three weeks and body weight gain as
markedly lowered during the first four weeks; slight diarrhoea and
increased faecal water content occurred at this dose level. A slight
increase in haemoglobin concentration was observed in males of the top
dose group only, and total serum protein was slightly decreased in
females only. Caecal enlargement was observed in both sexes receiving
10% Acesulfame K and in females receiving 3%. The relative weights of
the liver and kidneys were slightly elevated in females of the 10%
group and relative spleen weights were slightly depressed in all dose
groups.
Urinalysis, serum enzyme levels and serum albumin were not
affected by the treatment, no gross pathological changes were detected
and no dose-related abnormalities were observed histologically
(Sinkeldam, Til & Willems, 1974).
These workers considered that the caecal enlargement was a
physiological response to the presence of osmotically-active material
in the gut and that, since liver, kidney and spleen weights were
within the normal range of the strain of rat used, and no histological
changes occurred, the no toxic effect level is conservatively placed
at 3% in the diet; this is equivalent to 1.5 g/kg/day in rats.
Dog
Four groups of four female and four male beagle dogs, initially
17-21 weeks old, were fed diets containing 0, 0.3, 1.0, or 3.0%
Acesulfame K for two years. Body weight was recorded weekly for the
first 12 weeks and at four-weekly intervals thereafter. Urinalysis,
haematological examination and clinical chemistry were performed after
12, 26, 52, 78 and 104 weeks. Urinalysis included specific gravity,
pH, sugar, protein, occult blood, ketone and microscopic examination
of sediment; haematology comprised sedimentation rate, clotting time,
haemoglobin, PCV, RBC count, WBC count and differential leucocyte
count; clinical chemical investigations included blood sugar, urea,
SGOT, SGPT, serum alkaline phosphatase, total serum protein and serum
albumin. Liver function tests (bromosulfophthalein clearance) and
kidney function tests (phenol red excretion) were performed on control
and top dose group animals after 26, 52 and 104 weeks. At termination,
gross pathological examinations were performed and the following
organs weighed: heart, kidneys, spleen, liver, lungs, testes/ovaries,
thyroids, adrenals and brain. Histological examinations were performed
on the weighed organs and also on the following tissues: spinal cord,
sciatic nerve, salivary glands, skeletal muscle, thoracic aorta, skin,
tonsils, axillary, superficial, cervical and mesenteric lymph nodes,
bladder, oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon,
pancreas, trachea, circumanai glands, eyes, epididymis, prostate,
uterus, gall bladder, tongue and thymus. A marrow smear (rib bone) was
also examined. General appearance, condition, behaviour and survival
were not affected by the treatment. None of the examinations performed
revealed adverse effects related to the feeding of Acesulfame K.
The no-toxic effect level was found to be higher than 3% in the
diet corresponding to an intake of 900 mg/kg/day in dogs (Reuzel & van
der Heijden, 1977).
Long-term studies
Rat
A combined chronic toxicity and carcinogenicity study was
performed on Wistar rats (CIVO strain) which were obtained from the
F1a generation in a multigeneration study (see Special studies on
reproduction above). Four groups of 60 male and 60 female weanling
rats were given diets containing 0, 0.3, 1.0, or 3% Acesulfame K for
two years. The rats were derived from parents which had been
maintained on the same diet since weaning. Periodic observations were
made of appearance, behaviour growth and food intake. Haematological
examinations were carried out after 13, 26, 52, 78 and 104 weeks,
clinical chemical tests were performed on blood samples after 26, 52
and 104 weeks and urinalysis was done after 26, 52, 78 and 102 weeks.
At termination, survivors were autopsied and organ weights recorded
for heart, kidneys, spleen, liver, brain, gonads, thyroid, adrenals
and caecum (filled and empty). Tissue samples from 20 male and 20
female rats of the control and top dose groups only were subjected to
comprehensive histological examinations; histology on other animals
was limited to liver, spleen, adrenals, thyroid, parathyroid,
pituitary and ovaries, and to grossly visible lesions suspected of
being tumours.
Body weight gain was decreased in both sexes of the top dose
group during the first 44 weeks of the study but not significantly
thereafter. Death-rates of males fed 1.0 or 3% Acesulfame K and of
females fed 0.3% Acesulfame K were higher than controls but it was
considered that there was no evidence of mortality being increased by
treatment, and the mortality of control rats was low for the strain of
rat used. Interim deaths were mainly due to chronic respiratory
disease and lymphoreticular malignancies of pulmonary lymphoid tissue.
The incidence of pulmonary lymphoreticular tumours was relatively high
in both males and females of the top dose group but only achieved
statistical significance in females; there was also some evidence that
these tumours appeared rather earlier in males of the mid and top dose
groups. The results of haematological, clinical, chemical and
urinalysis investigations were essentially normal in all dose groups.
The relative weights of liver, kidneys, caecum and adrenals were
increased in both sexes of the high dose group but the differences
only reached statistical significance in the cases of liver and empty
caecal weight in males, and kidneys and caecal weight in females.
Gross and histopathology did not reveal any treatment-related effects
(Sinkeldam et al., 1977).
In commenting on these results, the authors pointed out the
problems of inter-group comparisons in multigeneration studies where
the animals in the different dose groups are not randomised. They
stated that the increased death rate in test animals was still within
the normal range for the strain of rats used and the mortality in
controls was lower than usual. Pulmonary lymphoreticular tumours are a
common cause of death in the strain of rats used, with very variable
incidence, and the frequency in the test groups was within the normal
range. It was concluded that the "higher" incidences and earlier
appearance in this study were fortuitous findings and did not suggest
that Acesulfame K possessed carcinogenic properties (Sinkeldam et al.,
1977).
A second combined chronic toxicity and carcinogenicity study was
carried out on a different rat strain with a lower incidence of
pulmonary tumours in untreated animals. Four groups of 60 male and 60
female SPF-Wistar rats received diets containing 0, 0.3, 1.0 or 3.0%
Acesulfame K for 120-123 weeks. The rats used were progeny from
parents which had been maintained on the same test diets since
weaning. No adverse effects, other than decreased body weight in the
top dose group were observed in this study. In particular there was no
increased mortality nor tumour incidence in the treatment groups. It
was concluded that Acesulfame K failed to show carcinogenic or other
effects of toxicological significances when fed to rats at levels of
up to 3.0% for 120 weeks (Sinkeldam et al., 1979).
OBSERVATIONS IN MAN
Three human volunteers, body weight 70-80 kg, were given a single
oral dose of 30 mg 14C-Acesulfame K in peppermint tea. Absorption was
rapid and virtually complete, maximum blood concentrations of
0.28 µg/ml occurring after 1 to 1-1/2 h. Elimination occurred rapidly
with a plasma half-life of 2-1/2 h, over 99% of the dose being
excreted in urine and less than 1% in faeces; 98% of the activity was
eliminated in the first 24 h. From the pharmacokinetic data it was
calculated that repeated doses of 30 mg at 3 h intervals would
increase the maximum serum levels 1.7-fold and at 2 h intervals
maximum serum levels would increase 2.4-fold relative to a single dose
(Christ & Rupp, 1976).
The metabolism of Acesulfame K was studied in serum and urine
from human volunteers following a single oral dose of 30 mg per
individual. Only the original substance was detected in all samples
(Volz, 1976).
Comments
Some shortcomings were apparent in the long-term/carcinogenicity
studies. The mouse carcinogenicity study was not considered to meet
current requirements in that detailed histopathology was performed
only on the livers and on others showing gross lesions suspected of
being tumours; in the second long-term feeding study in rats, only a
small proportion of the animals in the control and top dose groups
were examined histopathologically in detail. Further clarification is
needed of the report of an increased incidence of lymphomas restricted
to the lung in the first long-term rat study.
EVALUATION
No ADI allocated.
REFERENCES
Anon. (1973) 3,4-Dihydro-6-methyl-1,2,3-oxothiazine-4-one-2, 2-dioxide
potassium. Determination of acute oral toxicity in female
SPF-Wistar rat. Unpublished report submitted to WHO by Hoechst
A.G.
Anon. (1977) Acute oral toxicity of acetoacetamide in female SPF
Wistar rats. Report No. 427/77. Unpublished report submitted to
WHO by Hoechst A.G.
Anon. (1980a) Acesulfame K. A new non-nutritive sweetener. Unpublished
data supplied to WHO by Hoechst A.G.
Anon. (1980b) Investigating the efficacy of acetoacetamide as a
substrate of ß-hydroxyacyl-CoA-dehydrogenase and
ß-hydroxy-butyrate-dehydrogenase. Unpublished report submitted to
WHO by Hoechst A.G.
Baeder & Horstmann (1977) Oral embryotoxicity study of Acesulfam in
rabbits, stock Hoe:HIMK (SPF Wiga) Report No. 317/77. Unpublished
report submitted to WHO by Hoechst A.G.
Baeder, Horstmann & Weigand (1977) Oral mutagenicity study
(micronucleus test) of Acesulfam in NMRI mice. Report No. 591/77.
Unpublished report submitted by Hoechst A.G.
Beems, R. B. & Til, H. P. (1976) Carcinogenicity study with Hoe 0-95K
in mice. Report No. R5058. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Christ, O. & Rupp (1976) Human experiments with Acetosulfam-14C.
Pharmacokinetics after oral administration of 30 mg to three
healthy male probands. Unpublished report No. 01-L42-0176-76
submitted to WHO by Hoechst A.G.
Eisenbrand, G. (1979) Experiments aimed at the nitrosation of
Acetosulfam. Unpublished report submitted to WHO by Hoechst A.G.
Engelbart, K. (1979) Ames' test ß-Hydroxybutyramid Substanz 90/79.
Bericht Nr. 390/79A. Unpublished report submitted to WHO by
Hoechst A.G.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
submitted to WHO by Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975a) Re: Absorption, distribution and
elimination after administration of Acetosulfam-14C to rats and
dogs. Dr. Kn/tr 5547. Unpublished report submitted to WHO by
Hoechst A.G.
Kellner, H.-M. & Christ, O. (1975b) Pharmacokinetic studies of
Acetosulfam-14C after oral administration to pigs. Unpublished
report submitted to WHO by Hoechst A.G.
Koeter, H. B. W. M. (1975) Effect of Hoe 0-95K on pregnancy of the
rat. Report No. R4854. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Leegwater, D. C., De Groot, A. P. & van Kalmthout-Kuyper, M. (1974)
The aetiology of caecal enlargement in the rat, Fd. Cosmet.
Toxicol., 12: 687-697
Markert, U. & Weigand, W. (1979a) Akute Toxizitat von Acesulfam-K am
Zebrabarbling (Brachydanio rerio) Bericht Nr. 754/79. Unpublished
report submitted to WHO by Hoechst A.G.
Markert, U. & Weigand, W. (1979b) Akute Toxizitat von Acesulfam-K an
Goldorfen (Leuciscus Idus f. melanotus). Bericht Nr. 755/79.
Unpublished report submitted to WHO by Hoechst A.G.
Marquardt, H. W. J. (1978) Mutagenicity and oncogenicity
of Acetosulfam-K. Studies with mammalian cells in vitro.
Unpublished report of Memorial Sloan-Kettering Cancer Centre,
N.Y. Submitted to WHO by Hoechst A.G.
Mayer, D. & Weigand, W. (1977) Acute intraperitoneal toxicity of
Acesulfam-K, batch CIVO 4, in female SPF Wistar rats. Report No.
285/77. Unpublished report prepared for Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978a) Acesulfame K. Cytogenetic
study in the Chinese hamster. Report No. 575/78. Unpublished
report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978b) Report on investigations
of the caecum-enlarging action of Acesulfam-K salt in juvenile
female rats. Report No. 416/78. Unpublished report submitted to
WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978c) A report on the
experimental studies of the effect of Acesulfam K salt in causing
caecal enlargement in adult female rats. Report No. 417/78.
Unpublished report submitted to WHO by Hoechst A.G.
Mayer, D., Weigand, W. & Kramer, M. (1978d) Report on studies of the
subchronic effects on rats of high doses of potassium chloride in
the food. Report No. 418/78. Unpublished report submitted to WHO
by Hoechst A.G.
Reuzel, P. G. J. & van der Heijden, C. A. (1977) Long-term (two-year)
oral toxicity study with Hoe 0-95K in beagle dogs. Report No.
5280. Unpublished report of CIVO TNO Zeist. Submitted to WHO by
Hoechst A.G.
Rohrborn, G. (1976) Expert opinion on the mutagenicity of Acetosulfam.
Unpublished report of Universitat Dusseldorf. Submitted to WHO by
Hoechst A.G.
Schrinner & Limbert (1977) Report on investigations of the action of
the sweetener Acesulfam against bacteria. Unpublished report
submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. (1976) Reproduction study with Hoe 0-95K in rats.
Report No. R5187. Unpublished report of CIVO TNO, Zeist.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J. et al. (1976) Multigeneration study with Hoe 0-95K in
rats. Report No. 5156. Unpublished report of CIVO TNO, Zeizt.
Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Kuper, C. F. & Beems, R. B. (1979) Combined chronic
toxicity and carcinogenicity study with Hoe 0-95K in rats
pretreated in utero. Report No. R6100. Unpublished report of
CIVO TNO, Zeist. Submitted to WHO by Hoechst A.G.
Sinkeldam, E. J., Til, H. P. & Willems, M. I. (1974) Sub-chronic
(90 day) toxicity study with Hoe 0-95K in albino rats.
Unpublished report of CIVO TNO, Zeist. Submitted to WHO by
Hoechst A.G.
Sinkeldam, E. J., van der Heijden, C. A. & Koeter, H. B. W. M. (1977)
Combined chronic toxicity and carcinogenicity study with Hoe
0-95K in rats. Report No. R5229, Unpublished report of CIVO TNO,
Zeist. Submitted to WHO by Hoechst A.G.
Vogel, H. P. & Alpermann, H.-G. (1974) Sweetener 0-95K (H 73 3293).
Pharmacological investigations. Unpublished report submitted to
WHO by Hoechst A.G.
Volz, M. (1975) Research report RCL Dr. Vz/Tr No. 01-L42-0143-75.
Unpublished report submitted to WHO by Hoechst A.G.
Volz, M. (1976) Research report No. 01-L42-0177-76. Unpublished report
submitted to WHO by Hoechst A.G.
Willems, M. I. (1974) Dominant lethalassay with Hoe 0-95K in male
albino rats. Report No. R4472. Unpublished report of CIVO TNO.
Submitted to WHO by Hoechst A.G.
