
THUJONE
Explanation
Thujone is a terpenoid ketone which exists in two stereolsomeric
forms, viz:
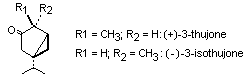 (+)-3-thujone is also known as alpha-thujone and (-)-3-isothujone
as ▀-thujone. The two isomers occur widely in essential oils, most
notably in Artemisia spp., Salvia spp., Juniperus, Tanacetum (tansy),
Thuja spp. and Cedris spp. in varying proportions.
Studies on the pharmacological and toxicological properties of
the thujones are complicated by the fact that many experiments
involved the use of ill-defined mixtures of the two isomers. Since the
isomers differ markedly in toxicity and convulsant activity,
quantitative data on mixtures of unspecified composition have to be
interpreted with caution.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Thujone (isomer not specified) was a weak inhibitor of
acyl-CoA:lysophosphatidylcholine acyltransferase activity in mouse
brain synaptosomes compared to psychoactive cannabinoids (Greenberg et
al., 1978).
Thujone was without effect on respiratory activity of the
cerebral cortex of rats pretreated with single oral doses of
10-100 mg/kg or repeated daily doses of 10 mg/kg for up to 15 days
(Pinto-Scognamiglio, 1968).
TOXICOLOGICAL STUDIES
Special studies on oil of tansy (Tanacetum vulgare)
The principal constituents of tansy oil are alpha- and ▀-thujone,
camphor and borneol, the average content of thujones is approximately
50%.
The acute oral LD50 in rats was reported as 1.15 g/kg and the
acute dermal LD50 in rabbits as >5 g/kg. When tested on dogs with
intestinal helminths, tansy oil was toxic at oral doses of 300 mg/kg.
Oil of tansy causes convulsions and signs of poisoning (stated to be
due to thujone) include vomiting, gastroenteritis, flushing, cramps,
loss of consciousness, rapid breathing, cardiac arrhythmia, enteric
bleeding and hepatitis. Death occurs from circulatory or respiratory
arrest and degenerative organ changes, and human fatalities have been
reported.
Undiluted tansy oil was not a skin irritant in hairless mice or
swine, was slightly irritating to rabbit skin in a 24-hour patch test
but was non-irritant in a 48-hour patch test in human subjects. No
sensitization reactions were seen in a maximization test on 25
volunteers and no phototoxic effects were observed in mice and swine
(Opdyke, 1976).
Special studies on oil of wormwood (Artemisia absinthium)
Artemisia oil contains mainly thujone (Guenther, 1952). The acute
oral LD50 in rats was reported as 960 mg/kg; the acute dermal LD50 in
rabbits exceeded 5 g/kg. Undiluted oil was not a skin irritant to
hairless mice, was mildly irritating to rabbit in a 24-hour patch test
and produced no irritation in a 48-hour patch test on human subjects.
No sensitization reactions were observed in a maximization test on 25
volunteers and no phototoxic effects were reported in hairless mice
and swine (Opdyke, 1975).
Special studies on pharmacological activity
The convulsant properties of thujone, and of thujone-containing
plant extracts, have been recognized for a long time (see review by
Pinto-Scognamiglio, 1967). The doses required to produce convulsions
are tabulated below:
Product Convulsive dose
Animal administered Route (mg/kg bw) Reference
Mouse (alpha + ▀) i.p. 590 Wenzel & Ross,
thujone 1957
▀-thujone i.p.a 260 Le Bourhis &
i.p.b 72 Soenen, 1973
p.o.b 250
Rat (alpha + ▀) i.p. 100 Sampson &
thujone Fernandez, 1939
Rabbit ▀-thujone i.v. 4 Keith & Stavaky,
1935
Cat ▀-thujone i.v. 7 Keith & Stavaky,
1935
Opper, 1939
▀-thujone i.v. 20 Keith & Stavaky,
1935
a Administered as a solution in olive oil.
b Administered as an aqueous emulsion in 1% Tween 20.
The thujone-induced convulsions are epileptiform in character and
are preceded by general vaso-dilation, fall in blood pressure, slowing
of cardiac rhythm and augmentation of respiratory amplitude (Pinto-
Scognamiglio, 1967).
(-)-3-isothujone and (+)-3-thujone were examined for
antinociceptive activity using the hot-plate and Nilsen tests. In the
hot-plate test, (-)-3-isothujone was found to be codeine-like (ED50 =
6.5 mg/kg) and equipotent with (-)delta-tetrahydrocannabinol while the
racemic mixture was essentially half as potent as isothujone;
(+)-3-thujone was inactive in both tests. Less antinociceptive
activity was observed in the Nilsen test (ED50 = 14.1 mg/kg) than in
the hot-plate assay (Rice & Wilson, 1976).
(+)-3-thujone was examined for psychotropic activity in mice
using a series of coordination and behavioural tests, and for
analgesic and hypnotic properties. At low, sub-convulsive doses,
thujone produced a slight augmentation of motility at a dose of
3 mg/kg i.p. and a depression of activity and exploratory behaviour at
24 mg/kg i.p. Thujone did not reinforce the convulsant activity of
pentetrazol or electric shocks and had no effect on barbiturate
sleeping time. On the other hand, barbiturates (10 mg/kg) or
trans-anethole (200-300 mg/kg i.p.) protected mice against the
convulsant action of thujone (150 mg/kg i.p.) (Le Bourhis & Soenen,
1973).
Pinto-Scognamiglio (1968) studied the effects of repeated oral
doses of thujone (10 mg/kg daily for 25 days) on spontaneous activity
and ability to learn conditioned behaviour of rats. Thujone did not
qualitatively modify either spontaneous activity or conditioned
behaviour but produced an improvement in coordination. Previous work
had indicated that thujone given to rats at doses of 50 mg/kg s.c.
produced a marked increase in activity equivalent to that seen after a
dose of 2 mg amphetamine/kg bw (cited from Pinto-Scognamiglio, 1967).
It was suggested (Castillo et al., 1975) that thujone and
tetrahydrocannabinol exert psychomimetic effects by interacting with a
common receptor but this hypothesis is not supported by the work of
Greenberg et al. (1978).
Acute toxicity
LD50
Animal Isomer Route (mg/kg bw) Reference
Mouse Not specified Oral 230 Margaria, 1963
(+)-3-thujone Oral 250 Le Bourhis &
Soenen, 1975
(+)-3-thujone i.p. 260a Le Bourhis &
Soenen, 1973
72b
(+)-3-thujone s.c. 442 Rice et al.,
1976
(-)-3-isothujone s.c. 134
Rat Not specified Oral 192 Margaria, 1963
i.p. 140 Sampson &
Fernandez, 1939
Guinea-pig Not specified Oral 396 Margaria, 1963
Dog (-)-3-isothujone Oral 250 Ionescu et al.,
1958
a Administered as a solution in olive oil.
b Administered as a suspension in aqueous 1% Tween 20.
Short-term studies
Rat
Four groups of 10 male and 10 female rats received thujone in
doses of 0, 5, 10 or 20 mg/kg by gavage on six days per week for 14
weeks. The isomeric composition of the material was not specified.
Convulsions were observed after dosing in many instances in nine
female and six male animals in the top dose group; only one female
animal from the 10 mg/kg dose group had one convulsion on the thirty-
eighth day. The ED50 for both sexes lay between 10 and 20 mg/kg daily
for three months. Three female and one male rat of the top dose group
died in convulsions.
At termination, no significant differences were observed between
groups with respect to weight gain, haematology (Hb, RBC, WBC), or
weights of heart, liver, spleen, kidney and adrenals. No treatment-
dependent gross pathological or histopathological lesions were
observed. The no-effect level was 5 mg/kg/day for females and
10 mg/kg/day for males (Margaria, 1963).
A commercial mixture of alpha- and ▀-thujone was administered by
gavage to groups of 20 male and 20 female weanling rats at doses of 0,
12.5, 15.0 and 50.0 mg/kg/day for 13 weeks. The dose was given in five
increments daily as a suspension in aqueous agar. Five rats (four male
and one female) died during acclimatization and three others (one male
from each of the low and middle dose groups; one female control) died
during treatment from a viral infection.
No dose-related deaths occurred in the rats receiving 12.5 or
25.0 mg/kg bw but 37% of the males and 60% of the females in the
50.0 mg/kg dose group died under test. Post-treatment convulsions were
frequently observed and the number of animals affected was as follows:
Animals affected
Dose
(mg/kg/day) Sex Months
1 2 3
12.5 Male 0/18 0/16 0/16
Female 0/20 1/20 0/20
25.0 Male 0/18 3/18 0/18
Female 2/20 6/20 7/18
50.0 Male 7/19 13/17 10/12
Female 11/15 8/10 7/8
The convulsive ED50 was estimated as 35.5 mg/kg/day for males
and 26.3 mg/kg/day for females. No correlation was seen between the
number of convulsions in an animal and death; in the extreme, one
survivor had 10 convulsions and one animal died without convulsions.
No effects were observed on body weight gain, or haematology, and
histopathological examination at termination did not reveal any dose-
related lesions.
The no-effect level for males was 12.5 mg/kg/day; a no-effect
level cannot be established for female rats since one rat in the
lowest dose group displayed convulsions on two occasions.
Comments
No data from metabolic, reproductive or long-term studies were
available. Much of the information reported related to unspecified
isomers or mixtures thereof. It appears that ▀-thujone is
significantly more toxic than the alpha-isomer and that female animals
are more sensitive to the toxic effects than males. It is not possible
to establish an ADI for man on the information available.
REFERENCES
Castillo, J. del, Anderson, M. & Rubottom, G. M. (1975) Marijuana,
absinthe and the central nervous system, Nature (Lond.), 253,
365-366
Greenberg, J. H., Mellors, A. & McGowan, J. C. (1978) Molar volume
relationships and the specific inhibition of a synaptosomal
enzyme by psychoactive cannabinoids, J. Med. Chem., 21, 1208
Guenther, E. (1962) The essential oils, Vol. V, p. 487, Van Nostrand:
Princeton, New Jersey
Ionescu, C. N. et al. (1958) Extragerea principiilor activi din
Tanacetum vulgare, Communie Acad. Rep. pop. rom., 8, 279
(cited by Opdyke, 1976)
Keith, H. M. & Stavaky, G. W. (1935) Experimental convulsions induced
by administration of thujone. A pharmacologic study of the
influence of the autonomic nervous system on these convulsions,
Arch. Neurol. Psych., 34, 1022-1040
Le Bourhis, B. & Soenen, A.-M. (1973) Recherches sur l'action
psychotrope de quelques substances aromatiques utilisÚes en
alimentation, Fd. Cosmet. Toxicol., 11, 1-9
Margaria, R. (1963) Acute and sub-acute toxicity study on thujone.
Unpublished report of Istito di Fisiologia, UniversitÓ di Milano
Opdyke, D. L. J. (1975) Monographs on fragrance raw materials -
artemisia oil (wormwood), Fd. Cosmet. Toxicol., 13 (suppl.),
721-722
Opdyke, D. L. J. (1976) Monograph on fragrance raw materials - tansy
oil, Fd. Cosmet. Toxicol., 14 (suppl.), 869-871
Opper, L. (1939) Pathologic picture of thujone and monobrominated
camphor convulsions: comparison with pathologic picture of human
epilepsy, Arch. Neurol. Psych., 41, 460
Pinto-Scognamiglio, W. (1967) Connaissances actuelles sur l'activitÚ
pharmacodynamique de la thujone, aromatisant naturel d'un emploi
entendu, Boll. chim. farm., 106, 292-300
Pinto-Scognamiglio, W. (1968) Effeti del tujone sull'attivitÓ
spontanea e sul comportamento condizionato del ratto, Boll.
chim. farm., 107, 780-791
Rice, K. C. & Wilson, R. S. (1976) (-)-3-isothujone, a small non-
nitrogenous molecule with antinociceptive activity in mice,
J. Med. Chem., 19, 1054-1057
Sampson, W. L. & Fernandez, L. (1939) Experimental convulsions in the
rat, J. Pharm. exptl. Therap., 65, 275
Wenzel, D. G. & Ross, C. R. (1957) Central stimulating properties of
some terpenones, J. Amer. pharm. Ass., 46, 77
(+)-3-thujone is also known as alpha-thujone and (-)-3-isothujone
as ▀-thujone. The two isomers occur widely in essential oils, most
notably in Artemisia spp., Salvia spp., Juniperus, Tanacetum (tansy),
Thuja spp. and Cedris spp. in varying proportions.
Studies on the pharmacological and toxicological properties of
the thujones are complicated by the fact that many experiments
involved the use of ill-defined mixtures of the two isomers. Since the
isomers differ markedly in toxicity and convulsant activity,
quantitative data on mixtures of unspecified composition have to be
interpreted with caution.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Thujone (isomer not specified) was a weak inhibitor of
acyl-CoA:lysophosphatidylcholine acyltransferase activity in mouse
brain synaptosomes compared to psychoactive cannabinoids (Greenberg et
al., 1978).
Thujone was without effect on respiratory activity of the
cerebral cortex of rats pretreated with single oral doses of
10-100 mg/kg or repeated daily doses of 10 mg/kg for up to 15 days
(Pinto-Scognamiglio, 1968).
TOXICOLOGICAL STUDIES
Special studies on oil of tansy (Tanacetum vulgare)
The principal constituents of tansy oil are alpha- and ▀-thujone,
camphor and borneol, the average content of thujones is approximately
50%.
The acute oral LD50 in rats was reported as 1.15 g/kg and the
acute dermal LD50 in rabbits as >5 g/kg. When tested on dogs with
intestinal helminths, tansy oil was toxic at oral doses of 300 mg/kg.
Oil of tansy causes convulsions and signs of poisoning (stated to be
due to thujone) include vomiting, gastroenteritis, flushing, cramps,
loss of consciousness, rapid breathing, cardiac arrhythmia, enteric
bleeding and hepatitis. Death occurs from circulatory or respiratory
arrest and degenerative organ changes, and human fatalities have been
reported.
Undiluted tansy oil was not a skin irritant in hairless mice or
swine, was slightly irritating to rabbit skin in a 24-hour patch test
but was non-irritant in a 48-hour patch test in human subjects. No
sensitization reactions were seen in a maximization test on 25
volunteers and no phototoxic effects were observed in mice and swine
(Opdyke, 1976).
Special studies on oil of wormwood (Artemisia absinthium)
Artemisia oil contains mainly thujone (Guenther, 1952). The acute
oral LD50 in rats was reported as 960 mg/kg; the acute dermal LD50 in
rabbits exceeded 5 g/kg. Undiluted oil was not a skin irritant to
hairless mice, was mildly irritating to rabbit in a 24-hour patch test
and produced no irritation in a 48-hour patch test on human subjects.
No sensitization reactions were observed in a maximization test on 25
volunteers and no phototoxic effects were reported in hairless mice
and swine (Opdyke, 1975).
Special studies on pharmacological activity
The convulsant properties of thujone, and of thujone-containing
plant extracts, have been recognized for a long time (see review by
Pinto-Scognamiglio, 1967). The doses required to produce convulsions
are tabulated below:
Product Convulsive dose
Animal administered Route (mg/kg bw) Reference
Mouse (alpha + ▀) i.p. 590 Wenzel & Ross,
thujone 1957
▀-thujone i.p.a 260 Le Bourhis &
i.p.b 72 Soenen, 1973
p.o.b 250
Rat (alpha + ▀) i.p. 100 Sampson &
thujone Fernandez, 1939
Rabbit ▀-thujone i.v. 4 Keith & Stavaky,
1935
Cat ▀-thujone i.v. 7 Keith & Stavaky,
1935
Opper, 1939
▀-thujone i.v. 20 Keith & Stavaky,
1935
a Administered as a solution in olive oil.
b Administered as an aqueous emulsion in 1% Tween 20.
The thujone-induced convulsions are epileptiform in character and
are preceded by general vaso-dilation, fall in blood pressure, slowing
of cardiac rhythm and augmentation of respiratory amplitude (Pinto-
Scognamiglio, 1967).
(-)-3-isothujone and (+)-3-thujone were examined for
antinociceptive activity using the hot-plate and Nilsen tests. In the
hot-plate test, (-)-3-isothujone was found to be codeine-like (ED50 =
6.5 mg/kg) and equipotent with (-)delta-tetrahydrocannabinol while the
racemic mixture was essentially half as potent as isothujone;
(+)-3-thujone was inactive in both tests. Less antinociceptive
activity was observed in the Nilsen test (ED50 = 14.1 mg/kg) than in
the hot-plate assay (Rice & Wilson, 1976).
(+)-3-thujone was examined for psychotropic activity in mice
using a series of coordination and behavioural tests, and for
analgesic and hypnotic properties. At low, sub-convulsive doses,
thujone produced a slight augmentation of motility at a dose of
3 mg/kg i.p. and a depression of activity and exploratory behaviour at
24 mg/kg i.p. Thujone did not reinforce the convulsant activity of
pentetrazol or electric shocks and had no effect on barbiturate
sleeping time. On the other hand, barbiturates (10 mg/kg) or
trans-anethole (200-300 mg/kg i.p.) protected mice against the
convulsant action of thujone (150 mg/kg i.p.) (Le Bourhis & Soenen,
1973).
Pinto-Scognamiglio (1968) studied the effects of repeated oral
doses of thujone (10 mg/kg daily for 25 days) on spontaneous activity
and ability to learn conditioned behaviour of rats. Thujone did not
qualitatively modify either spontaneous activity or conditioned
behaviour but produced an improvement in coordination. Previous work
had indicated that thujone given to rats at doses of 50 mg/kg s.c.
produced a marked increase in activity equivalent to that seen after a
dose of 2 mg amphetamine/kg bw (cited from Pinto-Scognamiglio, 1967).
It was suggested (Castillo et al., 1975) that thujone and
tetrahydrocannabinol exert psychomimetic effects by interacting with a
common receptor but this hypothesis is not supported by the work of
Greenberg et al. (1978).
Acute toxicity
LD50
Animal Isomer Route (mg/kg bw) Reference
Mouse Not specified Oral 230 Margaria, 1963
(+)-3-thujone Oral 250 Le Bourhis &
Soenen, 1975
(+)-3-thujone i.p. 260a Le Bourhis &
Soenen, 1973
72b
(+)-3-thujone s.c. 442 Rice et al.,
1976
(-)-3-isothujone s.c. 134
Rat Not specified Oral 192 Margaria, 1963
i.p. 140 Sampson &
Fernandez, 1939
Guinea-pig Not specified Oral 396 Margaria, 1963
Dog (-)-3-isothujone Oral 250 Ionescu et al.,
1958
a Administered as a solution in olive oil.
b Administered as a suspension in aqueous 1% Tween 20.
Short-term studies
Rat
Four groups of 10 male and 10 female rats received thujone in
doses of 0, 5, 10 or 20 mg/kg by gavage on six days per week for 14
weeks. The isomeric composition of the material was not specified.
Convulsions were observed after dosing in many instances in nine
female and six male animals in the top dose group; only one female
animal from the 10 mg/kg dose group had one convulsion on the thirty-
eighth day. The ED50 for both sexes lay between 10 and 20 mg/kg daily
for three months. Three female and one male rat of the top dose group
died in convulsions.
At termination, no significant differences were observed between
groups with respect to weight gain, haematology (Hb, RBC, WBC), or
weights of heart, liver, spleen, kidney and adrenals. No treatment-
dependent gross pathological or histopathological lesions were
observed. The no-effect level was 5 mg/kg/day for females and
10 mg/kg/day for males (Margaria, 1963).
A commercial mixture of alpha- and ▀-thujone was administered by
gavage to groups of 20 male and 20 female weanling rats at doses of 0,
12.5, 15.0 and 50.0 mg/kg/day for 13 weeks. The dose was given in five
increments daily as a suspension in aqueous agar. Five rats (four male
and one female) died during acclimatization and three others (one male
from each of the low and middle dose groups; one female control) died
during treatment from a viral infection.
No dose-related deaths occurred in the rats receiving 12.5 or
25.0 mg/kg bw but 37% of the males and 60% of the females in the
50.0 mg/kg dose group died under test. Post-treatment convulsions were
frequently observed and the number of animals affected was as follows:
Animals affected
Dose
(mg/kg/day) Sex Months
1 2 3
12.5 Male 0/18 0/16 0/16
Female 0/20 1/20 0/20
25.0 Male 0/18 3/18 0/18
Female 2/20 6/20 7/18
50.0 Male 7/19 13/17 10/12
Female 11/15 8/10 7/8
The convulsive ED50 was estimated as 35.5 mg/kg/day for males
and 26.3 mg/kg/day for females. No correlation was seen between the
number of convulsions in an animal and death; in the extreme, one
survivor had 10 convulsions and one animal died without convulsions.
No effects were observed on body weight gain, or haematology, and
histopathological examination at termination did not reveal any dose-
related lesions.
The no-effect level for males was 12.5 mg/kg/day; a no-effect
level cannot be established for female rats since one rat in the
lowest dose group displayed convulsions on two occasions.
Comments
No data from metabolic, reproductive or long-term studies were
available. Much of the information reported related to unspecified
isomers or mixtures thereof. It appears that ▀-thujone is
significantly more toxic than the alpha-isomer and that female animals
are more sensitive to the toxic effects than males. It is not possible
to establish an ADI for man on the information available.
REFERENCES
Castillo, J. del, Anderson, M. & Rubottom, G. M. (1975) Marijuana,
absinthe and the central nervous system, Nature (Lond.), 253,
365-366
Greenberg, J. H., Mellors, A. & McGowan, J. C. (1978) Molar volume
relationships and the specific inhibition of a synaptosomal
enzyme by psychoactive cannabinoids, J. Med. Chem., 21, 1208
Guenther, E. (1962) The essential oils, Vol. V, p. 487, Van Nostrand:
Princeton, New Jersey
Ionescu, C. N. et al. (1958) Extragerea principiilor activi din
Tanacetum vulgare, Communie Acad. Rep. pop. rom., 8, 279
(cited by Opdyke, 1976)
Keith, H. M. & Stavaky, G. W. (1935) Experimental convulsions induced
by administration of thujone. A pharmacologic study of the
influence of the autonomic nervous system on these convulsions,
Arch. Neurol. Psych., 34, 1022-1040
Le Bourhis, B. & Soenen, A.-M. (1973) Recherches sur l'action
psychotrope de quelques substances aromatiques utilisÚes en
alimentation, Fd. Cosmet. Toxicol., 11, 1-9
Margaria, R. (1963) Acute and sub-acute toxicity study on thujone.
Unpublished report of Istito di Fisiologia, UniversitÓ di Milano
Opdyke, D. L. J. (1975) Monographs on fragrance raw materials -
artemisia oil (wormwood), Fd. Cosmet. Toxicol., 13 (suppl.),
721-722
Opdyke, D. L. J. (1976) Monograph on fragrance raw materials - tansy
oil, Fd. Cosmet. Toxicol., 14 (suppl.), 869-871
Opper, L. (1939) Pathologic picture of thujone and monobrominated
camphor convulsions: comparison with pathologic picture of human
epilepsy, Arch. Neurol. Psych., 41, 460
Pinto-Scognamiglio, W. (1967) Connaissances actuelles sur l'activitÚ
pharmacodynamique de la thujone, aromatisant naturel d'un emploi
entendu, Boll. chim. farm., 106, 292-300
Pinto-Scognamiglio, W. (1968) Effeti del tujone sull'attivitÓ
spontanea e sul comportamento condizionato del ratto, Boll.
chim. farm., 107, 780-791
Rice, K. C. & Wilson, R. S. (1976) (-)-3-isothujone, a small non-
nitrogenous molecule with antinociceptive activity in mice,
J. Med. Chem., 19, 1054-1057
Sampson, W. L. & Fernandez, L. (1939) Experimental convulsions in the
rat, J. Pharm. exptl. Therap., 65, 275
Wenzel, D. G. & Ross, C. R. (1957) Central stimulating properties of
some terpenones, J. Amer. pharm. Ass., 46, 77
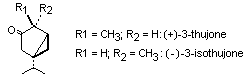 (+)-3-thujone is also known as alpha-thujone and (-)-3-isothujone
as ▀-thujone. The two isomers occur widely in essential oils, most
notably in Artemisia spp., Salvia spp., Juniperus, Tanacetum (tansy),
Thuja spp. and Cedris spp. in varying proportions.
Studies on the pharmacological and toxicological properties of
the thujones are complicated by the fact that many experiments
involved the use of ill-defined mixtures of the two isomers. Since the
isomers differ markedly in toxicity and convulsant activity,
quantitative data on mixtures of unspecified composition have to be
interpreted with caution.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Thujone (isomer not specified) was a weak inhibitor of
acyl-CoA:lysophosphatidylcholine acyltransferase activity in mouse
brain synaptosomes compared to psychoactive cannabinoids (Greenberg et
al., 1978).
Thujone was without effect on respiratory activity of the
cerebral cortex of rats pretreated with single oral doses of
10-100 mg/kg or repeated daily doses of 10 mg/kg for up to 15 days
(Pinto-Scognamiglio, 1968).
TOXICOLOGICAL STUDIES
Special studies on oil of tansy (Tanacetum vulgare)
The principal constituents of tansy oil are alpha- and ▀-thujone,
camphor and borneol, the average content of thujones is approximately
50%.
The acute oral LD50 in rats was reported as 1.15 g/kg and the
acute dermal LD50 in rabbits as >5 g/kg. When tested on dogs with
intestinal helminths, tansy oil was toxic at oral doses of 300 mg/kg.
Oil of tansy causes convulsions and signs of poisoning (stated to be
due to thujone) include vomiting, gastroenteritis, flushing, cramps,
loss of consciousness, rapid breathing, cardiac arrhythmia, enteric
bleeding and hepatitis. Death occurs from circulatory or respiratory
arrest and degenerative organ changes, and human fatalities have been
reported.
Undiluted tansy oil was not a skin irritant in hairless mice or
swine, was slightly irritating to rabbit skin in a 24-hour patch test
but was non-irritant in a 48-hour patch test in human subjects. No
sensitization reactions were seen in a maximization test on 25
volunteers and no phototoxic effects were observed in mice and swine
(Opdyke, 1976).
Special studies on oil of wormwood (Artemisia absinthium)
Artemisia oil contains mainly thujone (Guenther, 1952). The acute
oral LD50 in rats was reported as 960 mg/kg; the acute dermal LD50 in
rabbits exceeded 5 g/kg. Undiluted oil was not a skin irritant to
hairless mice, was mildly irritating to rabbit in a 24-hour patch test
and produced no irritation in a 48-hour patch test on human subjects.
No sensitization reactions were observed in a maximization test on 25
volunteers and no phototoxic effects were reported in hairless mice
and swine (Opdyke, 1975).
Special studies on pharmacological activity
The convulsant properties of thujone, and of thujone-containing
plant extracts, have been recognized for a long time (see review by
Pinto-Scognamiglio, 1967). The doses required to produce convulsions
are tabulated below:
Product Convulsive dose
Animal administered Route (mg/kg bw) Reference
Mouse (alpha + ▀) i.p. 590 Wenzel & Ross,
thujone 1957
▀-thujone i.p.a 260 Le Bourhis &
i.p.b 72 Soenen, 1973
p.o.b 250
Rat (alpha + ▀) i.p. 100 Sampson &
thujone Fernandez, 1939
Rabbit ▀-thujone i.v. 4 Keith & Stavaky,
1935
Cat ▀-thujone i.v. 7 Keith & Stavaky,
1935
Opper, 1939
▀-thujone i.v. 20 Keith & Stavaky,
1935
a Administered as a solution in olive oil.
b Administered as an aqueous emulsion in 1% Tween 20.
The thujone-induced convulsions are epileptiform in character and
are preceded by general vaso-dilation, fall in blood pressure, slowing
of cardiac rhythm and augmentation of respiratory amplitude (Pinto-
Scognamiglio, 1967).
(-)-3-isothujone and (+)-3-thujone were examined for
antinociceptive activity using the hot-plate and Nilsen tests. In the
hot-plate test, (-)-3-isothujone was found to be codeine-like (ED50 =
6.5 mg/kg) and equipotent with (-)delta-tetrahydrocannabinol while the
racemic mixture was essentially half as potent as isothujone;
(+)-3-thujone was inactive in both tests. Less antinociceptive
activity was observed in the Nilsen test (ED50 = 14.1 mg/kg) than in
the hot-plate assay (Rice & Wilson, 1976).
(+)-3-thujone was examined for psychotropic activity in mice
using a series of coordination and behavioural tests, and for
analgesic and hypnotic properties. At low, sub-convulsive doses,
thujone produced a slight augmentation of motility at a dose of
3 mg/kg i.p. and a depression of activity and exploratory behaviour at
24 mg/kg i.p. Thujone did not reinforce the convulsant activity of
pentetrazol or electric shocks and had no effect on barbiturate
sleeping time. On the other hand, barbiturates (10 mg/kg) or
trans-anethole (200-300 mg/kg i.p.) protected mice against the
convulsant action of thujone (150 mg/kg i.p.) (Le Bourhis & Soenen,
1973).
Pinto-Scognamiglio (1968) studied the effects of repeated oral
doses of thujone (10 mg/kg daily for 25 days) on spontaneous activity
and ability to learn conditioned behaviour of rats. Thujone did not
qualitatively modify either spontaneous activity or conditioned
behaviour but produced an improvement in coordination. Previous work
had indicated that thujone given to rats at doses of 50 mg/kg s.c.
produced a marked increase in activity equivalent to that seen after a
dose of 2 mg amphetamine/kg bw (cited from Pinto-Scognamiglio, 1967).
It was suggested (Castillo et al., 1975) that thujone and
tetrahydrocannabinol exert psychomimetic effects by interacting with a
common receptor but this hypothesis is not supported by the work of
Greenberg et al. (1978).
Acute toxicity
LD50
Animal Isomer Route (mg/kg bw) Reference
Mouse Not specified Oral 230 Margaria, 1963
(+)-3-thujone Oral 250 Le Bourhis &
Soenen, 1975
(+)-3-thujone i.p. 260a Le Bourhis &
Soenen, 1973
72b
(+)-3-thujone s.c. 442 Rice et al.,
1976
(-)-3-isothujone s.c. 134
Rat Not specified Oral 192 Margaria, 1963
i.p. 140 Sampson &
Fernandez, 1939
Guinea-pig Not specified Oral 396 Margaria, 1963
Dog (-)-3-isothujone Oral 250 Ionescu et al.,
1958
a Administered as a solution in olive oil.
b Administered as a suspension in aqueous 1% Tween 20.
Short-term studies
Rat
Four groups of 10 male and 10 female rats received thujone in
doses of 0, 5, 10 or 20 mg/kg by gavage on six days per week for 14
weeks. The isomeric composition of the material was not specified.
Convulsions were observed after dosing in many instances in nine
female and six male animals in the top dose group; only one female
animal from the 10 mg/kg dose group had one convulsion on the thirty-
eighth day. The ED50 for both sexes lay between 10 and 20 mg/kg daily
for three months. Three female and one male rat of the top dose group
died in convulsions.
At termination, no significant differences were observed between
groups with respect to weight gain, haematology (Hb, RBC, WBC), or
weights of heart, liver, spleen, kidney and adrenals. No treatment-
dependent gross pathological or histopathological lesions were
observed. The no-effect level was 5 mg/kg/day for females and
10 mg/kg/day for males (Margaria, 1963).
A commercial mixture of alpha- and ▀-thujone was administered by
gavage to groups of 20 male and 20 female weanling rats at doses of 0,
12.5, 15.0 and 50.0 mg/kg/day for 13 weeks. The dose was given in five
increments daily as a suspension in aqueous agar. Five rats (four male
and one female) died during acclimatization and three others (one male
from each of the low and middle dose groups; one female control) died
during treatment from a viral infection.
No dose-related deaths occurred in the rats receiving 12.5 or
25.0 mg/kg bw but 37% of the males and 60% of the females in the
50.0 mg/kg dose group died under test. Post-treatment convulsions were
frequently observed and the number of animals affected was as follows:
Animals affected
Dose
(mg/kg/day) Sex Months
1 2 3
12.5 Male 0/18 0/16 0/16
Female 0/20 1/20 0/20
25.0 Male 0/18 3/18 0/18
Female 2/20 6/20 7/18
50.0 Male 7/19 13/17 10/12
Female 11/15 8/10 7/8
The convulsive ED50 was estimated as 35.5 mg/kg/day for males
and 26.3 mg/kg/day for females. No correlation was seen between the
number of convulsions in an animal and death; in the extreme, one
survivor had 10 convulsions and one animal died without convulsions.
No effects were observed on body weight gain, or haematology, and
histopathological examination at termination did not reveal any dose-
related lesions.
The no-effect level for males was 12.5 mg/kg/day; a no-effect
level cannot be established for female rats since one rat in the
lowest dose group displayed convulsions on two occasions.
Comments
No data from metabolic, reproductive or long-term studies were
available. Much of the information reported related to unspecified
isomers or mixtures thereof. It appears that ▀-thujone is
significantly more toxic than the alpha-isomer and that female animals
are more sensitive to the toxic effects than males. It is not possible
to establish an ADI for man on the information available.
REFERENCES
Castillo, J. del, Anderson, M. & Rubottom, G. M. (1975) Marijuana,
absinthe and the central nervous system, Nature (Lond.), 253,
365-366
Greenberg, J. H., Mellors, A. & McGowan, J. C. (1978) Molar volume
relationships and the specific inhibition of a synaptosomal
enzyme by psychoactive cannabinoids, J. Med. Chem., 21, 1208
Guenther, E. (1962) The essential oils, Vol. V, p. 487, Van Nostrand:
Princeton, New Jersey
Ionescu, C. N. et al. (1958) Extragerea principiilor activi din
Tanacetum vulgare, Communie Acad. Rep. pop. rom., 8, 279
(cited by Opdyke, 1976)
Keith, H. M. & Stavaky, G. W. (1935) Experimental convulsions induced
by administration of thujone. A pharmacologic study of the
influence of the autonomic nervous system on these convulsions,
Arch. Neurol. Psych., 34, 1022-1040
Le Bourhis, B. & Soenen, A.-M. (1973) Recherches sur l'action
psychotrope de quelques substances aromatiques utilisÚes en
alimentation, Fd. Cosmet. Toxicol., 11, 1-9
Margaria, R. (1963) Acute and sub-acute toxicity study on thujone.
Unpublished report of Istito di Fisiologia, UniversitÓ di Milano
Opdyke, D. L. J. (1975) Monographs on fragrance raw materials -
artemisia oil (wormwood), Fd. Cosmet. Toxicol., 13 (suppl.),
721-722
Opdyke, D. L. J. (1976) Monograph on fragrance raw materials - tansy
oil, Fd. Cosmet. Toxicol., 14 (suppl.), 869-871
Opper, L. (1939) Pathologic picture of thujone and monobrominated
camphor convulsions: comparison with pathologic picture of human
epilepsy, Arch. Neurol. Psych., 41, 460
Pinto-Scognamiglio, W. (1967) Connaissances actuelles sur l'activitÚ
pharmacodynamique de la thujone, aromatisant naturel d'un emploi
entendu, Boll. chim. farm., 106, 292-300
Pinto-Scognamiglio, W. (1968) Effeti del tujone sull'attivitÓ
spontanea e sul comportamento condizionato del ratto, Boll.
chim. farm., 107, 780-791
Rice, K. C. & Wilson, R. S. (1976) (-)-3-isothujone, a small non-
nitrogenous molecule with antinociceptive activity in mice,
J. Med. Chem., 19, 1054-1057
Sampson, W. L. & Fernandez, L. (1939) Experimental convulsions in the
rat, J. Pharm. exptl. Therap., 65, 275
Wenzel, D. G. & Ross, C. R. (1957) Central stimulating properties of
some terpenones, J. Amer. pharm. Ass., 46, 77
(+)-3-thujone is also known as alpha-thujone and (-)-3-isothujone
as ▀-thujone. The two isomers occur widely in essential oils, most
notably in Artemisia spp., Salvia spp., Juniperus, Tanacetum (tansy),
Thuja spp. and Cedris spp. in varying proportions.
Studies on the pharmacological and toxicological properties of
the thujones are complicated by the fact that many experiments
involved the use of ill-defined mixtures of the two isomers. Since the
isomers differ markedly in toxicity and convulsant activity,
quantitative data on mixtures of unspecified composition have to be
interpreted with caution.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Thujone (isomer not specified) was a weak inhibitor of
acyl-CoA:lysophosphatidylcholine acyltransferase activity in mouse
brain synaptosomes compared to psychoactive cannabinoids (Greenberg et
al., 1978).
Thujone was without effect on respiratory activity of the
cerebral cortex of rats pretreated with single oral doses of
10-100 mg/kg or repeated daily doses of 10 mg/kg for up to 15 days
(Pinto-Scognamiglio, 1968).
TOXICOLOGICAL STUDIES
Special studies on oil of tansy (Tanacetum vulgare)
The principal constituents of tansy oil are alpha- and ▀-thujone,
camphor and borneol, the average content of thujones is approximately
50%.
The acute oral LD50 in rats was reported as 1.15 g/kg and the
acute dermal LD50 in rabbits as >5 g/kg. When tested on dogs with
intestinal helminths, tansy oil was toxic at oral doses of 300 mg/kg.
Oil of tansy causes convulsions and signs of poisoning (stated to be
due to thujone) include vomiting, gastroenteritis, flushing, cramps,
loss of consciousness, rapid breathing, cardiac arrhythmia, enteric
bleeding and hepatitis. Death occurs from circulatory or respiratory
arrest and degenerative organ changes, and human fatalities have been
reported.
Undiluted tansy oil was not a skin irritant in hairless mice or
swine, was slightly irritating to rabbit skin in a 24-hour patch test
but was non-irritant in a 48-hour patch test in human subjects. No
sensitization reactions were seen in a maximization test on 25
volunteers and no phototoxic effects were observed in mice and swine
(Opdyke, 1976).
Special studies on oil of wormwood (Artemisia absinthium)
Artemisia oil contains mainly thujone (Guenther, 1952). The acute
oral LD50 in rats was reported as 960 mg/kg; the acute dermal LD50 in
rabbits exceeded 5 g/kg. Undiluted oil was not a skin irritant to
hairless mice, was mildly irritating to rabbit in a 24-hour patch test
and produced no irritation in a 48-hour patch test on human subjects.
No sensitization reactions were observed in a maximization test on 25
volunteers and no phototoxic effects were reported in hairless mice
and swine (Opdyke, 1975).
Special studies on pharmacological activity
The convulsant properties of thujone, and of thujone-containing
plant extracts, have been recognized for a long time (see review by
Pinto-Scognamiglio, 1967). The doses required to produce convulsions
are tabulated below:
Product Convulsive dose
Animal administered Route (mg/kg bw) Reference
Mouse (alpha + ▀) i.p. 590 Wenzel & Ross,
thujone 1957
▀-thujone i.p.a 260 Le Bourhis &
i.p.b 72 Soenen, 1973
p.o.b 250
Rat (alpha + ▀) i.p. 100 Sampson &
thujone Fernandez, 1939
Rabbit ▀-thujone i.v. 4 Keith & Stavaky,
1935
Cat ▀-thujone i.v. 7 Keith & Stavaky,
1935
Opper, 1939
▀-thujone i.v. 20 Keith & Stavaky,
1935
a Administered as a solution in olive oil.
b Administered as an aqueous emulsion in 1% Tween 20.
The thujone-induced convulsions are epileptiform in character and
are preceded by general vaso-dilation, fall in blood pressure, slowing
of cardiac rhythm and augmentation of respiratory amplitude (Pinto-
Scognamiglio, 1967).
(-)-3-isothujone and (+)-3-thujone were examined for
antinociceptive activity using the hot-plate and Nilsen tests. In the
hot-plate test, (-)-3-isothujone was found to be codeine-like (ED50 =
6.5 mg/kg) and equipotent with (-)delta-tetrahydrocannabinol while the
racemic mixture was essentially half as potent as isothujone;
(+)-3-thujone was inactive in both tests. Less antinociceptive
activity was observed in the Nilsen test (ED50 = 14.1 mg/kg) than in
the hot-plate assay (Rice & Wilson, 1976).
(+)-3-thujone was examined for psychotropic activity in mice
using a series of coordination and behavioural tests, and for
analgesic and hypnotic properties. At low, sub-convulsive doses,
thujone produced a slight augmentation of motility at a dose of
3 mg/kg i.p. and a depression of activity and exploratory behaviour at
24 mg/kg i.p. Thujone did not reinforce the convulsant activity of
pentetrazol or electric shocks and had no effect on barbiturate
sleeping time. On the other hand, barbiturates (10 mg/kg) or
trans-anethole (200-300 mg/kg i.p.) protected mice against the
convulsant action of thujone (150 mg/kg i.p.) (Le Bourhis & Soenen,
1973).
Pinto-Scognamiglio (1968) studied the effects of repeated oral
doses of thujone (10 mg/kg daily for 25 days) on spontaneous activity
and ability to learn conditioned behaviour of rats. Thujone did not
qualitatively modify either spontaneous activity or conditioned
behaviour but produced an improvement in coordination. Previous work
had indicated that thujone given to rats at doses of 50 mg/kg s.c.
produced a marked increase in activity equivalent to that seen after a
dose of 2 mg amphetamine/kg bw (cited from Pinto-Scognamiglio, 1967).
It was suggested (Castillo et al., 1975) that thujone and
tetrahydrocannabinol exert psychomimetic effects by interacting with a
common receptor but this hypothesis is not supported by the work of
Greenberg et al. (1978).
Acute toxicity
LD50
Animal Isomer Route (mg/kg bw) Reference
Mouse Not specified Oral 230 Margaria, 1963
(+)-3-thujone Oral 250 Le Bourhis &
Soenen, 1975
(+)-3-thujone i.p. 260a Le Bourhis &
Soenen, 1973
72b
(+)-3-thujone s.c. 442 Rice et al.,
1976
(-)-3-isothujone s.c. 134
Rat Not specified Oral 192 Margaria, 1963
i.p. 140 Sampson &
Fernandez, 1939
Guinea-pig Not specified Oral 396 Margaria, 1963
Dog (-)-3-isothujone Oral 250 Ionescu et al.,
1958
a Administered as a solution in olive oil.
b Administered as a suspension in aqueous 1% Tween 20.
Short-term studies
Rat
Four groups of 10 male and 10 female rats received thujone in
doses of 0, 5, 10 or 20 mg/kg by gavage on six days per week for 14
weeks. The isomeric composition of the material was not specified.
Convulsions were observed after dosing in many instances in nine
female and six male animals in the top dose group; only one female
animal from the 10 mg/kg dose group had one convulsion on the thirty-
eighth day. The ED50 for both sexes lay between 10 and 20 mg/kg daily
for three months. Three female and one male rat of the top dose group
died in convulsions.
At termination, no significant differences were observed between
groups with respect to weight gain, haematology (Hb, RBC, WBC), or
weights of heart, liver, spleen, kidney and adrenals. No treatment-
dependent gross pathological or histopathological lesions were
observed. The no-effect level was 5 mg/kg/day for females and
10 mg/kg/day for males (Margaria, 1963).
A commercial mixture of alpha- and ▀-thujone was administered by
gavage to groups of 20 male and 20 female weanling rats at doses of 0,
12.5, 15.0 and 50.0 mg/kg/day for 13 weeks. The dose was given in five
increments daily as a suspension in aqueous agar. Five rats (four male
and one female) died during acclimatization and three others (one male
from each of the low and middle dose groups; one female control) died
during treatment from a viral infection.
No dose-related deaths occurred in the rats receiving 12.5 or
25.0 mg/kg bw but 37% of the males and 60% of the females in the
50.0 mg/kg dose group died under test. Post-treatment convulsions were
frequently observed and the number of animals affected was as follows:
Animals affected
Dose
(mg/kg/day) Sex Months
1 2 3
12.5 Male 0/18 0/16 0/16
Female 0/20 1/20 0/20
25.0 Male 0/18 3/18 0/18
Female 2/20 6/20 7/18
50.0 Male 7/19 13/17 10/12
Female 11/15 8/10 7/8
The convulsive ED50 was estimated as 35.5 mg/kg/day for males
and 26.3 mg/kg/day for females. No correlation was seen between the
number of convulsions in an animal and death; in the extreme, one
survivor had 10 convulsions and one animal died without convulsions.
No effects were observed on body weight gain, or haematology, and
histopathological examination at termination did not reveal any dose-
related lesions.
The no-effect level for males was 12.5 mg/kg/day; a no-effect
level cannot be established for female rats since one rat in the
lowest dose group displayed convulsions on two occasions.
Comments
No data from metabolic, reproductive or long-term studies were
available. Much of the information reported related to unspecified
isomers or mixtures thereof. It appears that ▀-thujone is
significantly more toxic than the alpha-isomer and that female animals
are more sensitive to the toxic effects than males. It is not possible
to establish an ADI for man on the information available.
REFERENCES
Castillo, J. del, Anderson, M. & Rubottom, G. M. (1975) Marijuana,
absinthe and the central nervous system, Nature (Lond.), 253,
365-366
Greenberg, J. H., Mellors, A. & McGowan, J. C. (1978) Molar volume
relationships and the specific inhibition of a synaptosomal
enzyme by psychoactive cannabinoids, J. Med. Chem., 21, 1208
Guenther, E. (1962) The essential oils, Vol. V, p. 487, Van Nostrand:
Princeton, New Jersey
Ionescu, C. N. et al. (1958) Extragerea principiilor activi din
Tanacetum vulgare, Communie Acad. Rep. pop. rom., 8, 279
(cited by Opdyke, 1976)
Keith, H. M. & Stavaky, G. W. (1935) Experimental convulsions induced
by administration of thujone. A pharmacologic study of the
influence of the autonomic nervous system on these convulsions,
Arch. Neurol. Psych., 34, 1022-1040
Le Bourhis, B. & Soenen, A.-M. (1973) Recherches sur l'action
psychotrope de quelques substances aromatiques utilisÚes en
alimentation, Fd. Cosmet. Toxicol., 11, 1-9
Margaria, R. (1963) Acute and sub-acute toxicity study on thujone.
Unpublished report of Istito di Fisiologia, UniversitÓ di Milano
Opdyke, D. L. J. (1975) Monographs on fragrance raw materials -
artemisia oil (wormwood), Fd. Cosmet. Toxicol., 13 (suppl.),
721-722
Opdyke, D. L. J. (1976) Monograph on fragrance raw materials - tansy
oil, Fd. Cosmet. Toxicol., 14 (suppl.), 869-871
Opper, L. (1939) Pathologic picture of thujone and monobrominated
camphor convulsions: comparison with pathologic picture of human
epilepsy, Arch. Neurol. Psych., 41, 460
Pinto-Scognamiglio, W. (1967) Connaissances actuelles sur l'activitÚ
pharmacodynamique de la thujone, aromatisant naturel d'un emploi
entendu, Boll. chim. farm., 106, 292-300
Pinto-Scognamiglio, W. (1968) Effeti del tujone sull'attivitÓ
spontanea e sul comportamento condizionato del ratto, Boll.
chim. farm., 107, 780-791
Rice, K. C. & Wilson, R. S. (1976) (-)-3-isothujone, a small non-
nitrogenous molecule with antinociceptive activity in mice,
J. Med. Chem., 19, 1054-1057
Sampson, W. L. & Fernandez, L. (1939) Experimental convulsions in the
rat, J. Pharm. exptl. Therap., 65, 275
Wenzel, D. G. & Ross, C. R. (1957) Central stimulating properties of
some terpenones, J. Amer. pharm. Ass., 46, 77
