
STYRENE
Explanation
Styrene has not been previously evaluated for an acceptable daily
intake for man by the Joint FAO/WHO Expert Committee on Food
Additives.
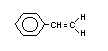 Styrene is also known as ethenylbenzene, vinylbenzene,
vinylbenzol, styrolene, styrol, styropol, styropor, styrone,
cinnamene, cinnamol, phenethylene, phenylethylene and phenylethane. It
is a colourless, viscous liquid with a melting point of -30.6°C and a
boiling point of 145.2°C. It is insoluble in water, soluble in
ethanol, ether and acetone and very soluble in benzene and petroleum
ether. Its uses include intermediate in the manufacture of plastics,
elastomers and resins which in turn are used in packaging foods, food
products and various other processes in the food industry. It is also
used as a synthetic flavouring substance and adjuvant.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies with laboratory animals have shown that styrene is
readily absorbed from the gastrointestinal tract following oral
administration (Plotnick & Weigel, 1979; Sauerhoff et al., 1976).
The distribution and excretion of a single oral dose of 20 mg
14C-styrene/kg b.w. was studied in male and female Charles-River
rats. The peak tissue levels occurred four hours after administration,
with the kidneys exhibiting the highest concentration of 14C per unit
weight, followed by the liver and pancreas. The primary route of
elimination was via urinary excretion with 90% of the dosage detected
in the urine within 24 hours, while less than 2% was detected in the
feces (Plotnick & Weigel, 1979).
The disposition of orally administered styrene was studied in
mature Sprague-Dawley rats (Spartan substrain) in which doses of 50 or
500 mg/kg 14C-styrene were administered via gavage. Approximately 95
and 90% of the 50 and 500 mg/kg b.w. doses, respectively, were
eliminated in the urine as styrene metabolites. The pulmonary route of
elimination accounted for 1.3% of the lower dosage and 8.9% of the
higher dosage. There was a distinct sex difference in the pulmonary
elimination of styrene with males expiring twice as much styrene as
the females. The fecal route of elimination accounted for only about
4% of the administered dose (Sauerhoff et al., 1976).
Metabolism
A number of metabolic studies in laboratory animals which have
utilized a variety of routes of administration have shown that styrene
is metabolized by the mixed function oxidase (MFO) system to
styrene-7, 8-oxide, and that this occurs not only in the liver but in
a number of other tissue types and organs (Leibman & Ortiz, 1970;
Salmona et al., 1976; Cantoni et al., 1978). The metabolic activation
by the microsomal system depends on the presence of necessary
cofactors for a NADPH generating system and oxygen (Kappus et al.,
1971; Bartsch et al., 1975; Salmona et al, 1976). The oxide can be
metabolized further to styrene glycol by the action of epoxide
hydrolase (Leibman & Ortiz, 1969 and 1970; Ryan & Bend, 1977).
Generally the greatest activity for the MFO and hydrolase have been
detected in the liver with the activity of the latter being greater
than that of the former (Cantoni et al., 1978). The glycol in turn can
be converted to mandelic acid, phenylglyoxylic acid and finally to
hippuric acid or it can be conjugated to glucuronic acid (Ryan & Bend,
1977; El Masri et al., 1958; Sauerhoff et al., 1976; James & White,
1967; Ikeda et al., 1974; Astrand et al., 1974; Gotell et al., 1972).
The oxide can also be conjugated with glutathione to form three
mercapturic acid derivatives, N-acetyl-s-(1-phenyl-2-hydroxyethyl)
cysteine and N-acetyl-s-(phenylacetyl) cysteine. Other metabolites
which have been identified include mercapturic acids, benzoic acid,
4-vinylphenol, 2-phenylethanol, phenaceturic acid and phenylglycol
all of which may be excreted as conjugates of glucuronic acid or
glutathione (Sauerhoff et al., 1976; Bakke & Scheline, 1970;
Delbrassine et al., 1981; El Masri et al., 1958). Studies in rats have
shown that the opening of the epoxide ring of styrene by glutathione
5-transferase is stereospecific with a preference for the R-isomer
(Delbrassine et al., 1981).
There has also been evidence generated that styrene is converted
to an arene oxide, since an intermediate, styrene-3,4-oxide, undergoes
isomerization to 4-vinylphenol which has been isolated in the urine of
both animals and men who have been exposed to styrene (Pantarotto et
al., 1978; Bakke & Scheline, 1970; Pfäffli et al., 1981; James &
White, 1967; Pachecka et al., 1979). However, this route of metabolism
is considered to be a minor one, since much more mandelic acid is
detected in the urine as compared to the 4-vinylphenol.
In vitro preparations of human erythrocytes and lymphocytes
have been shown to catalyze the oxidation of styrene to styrene oxide.
This reaction is inhibited by the presence of CO, but not by
superoxide dismutase, catalase and scavengers of hydroxyl radicals,
and it required the presence of oxygen. These data indicate that
oxyhemoglobin and not free oxygen radicals can be involved in styrene
oxidation (Tursi et al, 1983; Belvedere & Tursi, 1981).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Carcinogenicity data on styrene and styrene oxide were reviewed
by the International Agency for Research on Cancer (IARC) in 1979.
Mouse
A lifetime study following in utero exposure was conducted with
C57B1 black mice. Fifteen dams were administered a single dose of
300 mg of styrene/kg b.w. dissolved in olive oil via intubation on the
17th day of gestation. A vehicle (olive oil) treated group of 57 males
and 49 females served as a control group. Styrene was then given
weekly at the same dose level and via the same route to 27 male and 27
female offspring from weaning up to 120 weeks of age. A group of 12
males and 13 females which were the offspring of five dams treated
with olive oil on the 17th day of gestation received olive oil on a
weekly basis after weaning. The incidence of neonatal mortality of the
styrene-treated group was 35%. In the parental generation lymphomas
were noted in 10 of 12 styrene-treated animals as compared to 3 of 5
of the vehicle treated animals. In the male progeny hepatocellular
carcinomas were found in 3 of 4 animals. This compares to an occurence
of 1 to 12 of vehicle-treated controls and 1 of 47 untreated controls
(P>0.05). The incidences of all other tumor-types were similar in the
styrene-treated and control animals (Ponomarkov & Tomatis, 1978).
A group of 29 pregnant O20 mice received a single dose of
1350 mg/kg b.w. of styrene by gavage (dissolved in olive oil) on the
17th day of gestation, while a control group received olive oil alone.
This same dosage of styrene was administered to the offspring
(39 females and 45 males) from weaning to 16 weeks of age. Treatment
was terminated at this point because of excessive mortality. The most
frequently observed lesions in the styrene-treated offspring which
expired before the twentieth week were multiple centrilobular necrosis
of the liver, hypoplasia of the spleen and severe congestion of
the lungs. In those animals that expired after the forty-fifth
week multiple abscess-like cavities in the liver filled with
polymorphonuclear leukocytes were noted. The study was terminated when
the last animal had expired at 100 weeks. There were no differences
in tumor incidences between the dams treated with styrene and those
treated with olive oil. In the progeny pulmonary adenomas and
adenoearcinomas were noted in 20/23 males and 32/32 females treated
with styrene and in 8/19 males and 14/21 females in these animals
treated with olive oil alone (P>0.01, P<0.01). However, in untreated
controls 34/53 males and 25/42 females were found to also have
pulmonary adenomas and adenocarcinomas (P<0.05, P<0.01). The average
age at death of the pulmonary tumor-bearing, styrene-treated mice was
32 and 49 weeks for the males and females, respectively. In contrast,
the olive oil-treated males and females exhibited an average age of 88
and 85 weeks, while the average age of mortality of the untreated
control males and females was 94 and 99 weeks, respectively. No other
differences in tumor incidences were noted between the various groups
(Ponomarkov & Tomatis, 1978).
Groups of 50 male and 50 female B6C3F1 mice were dosed daily by
gavage five days per week with styrene dissolved in corn oil at dose
levels equivalent to 150 and 300 mg/kg b.w. Treatment was for 78 weeks
and was followed by a 13 week observation period. A group of 20
animals of each sex served as a vehicle corn oil control group. In the
males, but not females, there was a significant positive relationship
between mortality and dosage. A slight, dose-related mean body weight
depression was noted in the females, but not the males, while no other
clinical annormalities were noted. There were adequate numbers of both
males and females at risk for late-developing tumors. In the low
dosage group 92 percent (46/50) and 80 percent (40/50) of the males
and females, respectively, survived on test until the termination of
the study. The percentages of survival for the high dosage group was
78 and 76 for the males and females, respectively, and for the
controls 100 and 90 percent for the respective sexes.
During the course of the study, five low dose females were found
missing. A variety of neoplastic lesions were found in both the
styrene-treated and control animals, however, with the exception of
pulmonary tumors, the incidences neoplastic lesions were unrelated to
the administration of styrene. There was a significant increase in the
incidence of a combination of pulmonary adenomas and carcinomas in the
styrene-treated male mice with 0/20, 6/45 and 9/49 of the control, low
dosage and high dosage animals demonstrating these lesions (P = 0.024
for Fischer exact test comparing high dose and control). However, a
definitive conclusion on the carcinogenicity of styrene could not be
drawn because of the high variability of the incidence of these
neoplasia in historical control mice at the laboratory which performed
the study. The historical incidence of the combination of alveolar/
bronchiolar adenomas and aleolar/bronchiolar carcinomas in male mice
in this laboratory is 12 percent (32/271). The authors concluded that
there was no convincing evidence for carcinogenicity of styrene in the
mouse study. (National Cancer Institute, 1978)
Rat
A group of 21 BD IV rats were given 1350 mg of styrene/kg b.w.
in olive oil orally on the 17th day of gestation. Their offspring (73
males and 71 females) then received 500 mg/kg b.w. styrene weekly from
weaning for 120 weeks. Ten dams and their offsprings (36 males and 39
females) received olive oil and served as controls. The average litter
sizes of the different groups were similar. The mortality of the
offspring of styrene-treated dams (10%) was greater than that of
the controls (2.5%), but not significantly. There was no evidence
of a difference in body weight gain and survival between the
styrene-treated and control offspring. The incidence of survival were
8/73 and 20/71 of the male and female, styrene-treated animals and
14/36 and 18/39 male and female control animals. The incidence of
tumor-bearing dams was greater in the styrene-treated group than
the control group, but the difference was not significant. Three
neurogenic and three stomach tumors were observed in the
styrene-treated progeny that were not observed in the controls
(Ponomarkov & Tomatis, 1978).
A study was carried out with Fischer 344 rats in which groups of
50 males and 50 females were administered styrene dissolved in corn
oil five days per week by gavage at doses equivalent to 500, 1000 and
2000 mg/kg b.w. Treatment was for 103 weeks for the low dosage group
and 78 weeks for the medium and high dosage groups. This was followed
by an observation period of one week for the low dosage groups and
twenty-seven weeks for the medium and high dosage groups. A group of
40 animals of each sex served as vehicle controls (corn oil). The
highest dosage resulted in a significantly earlier and higher
mortality in both sexes, wherein, an additional dosed group of each
sex was included in the bioassay. The survival of the other dosage
groups were not adversely affected by styrene administration. By week
53 only 12 percent (6/50) of the males had survived, while only 14
percent (7/50) of the females had survived by week 70. The survival
incidences for the other groups were 88 percent (44/59), 94 percent
(47/50) for the low and medium dose males and 92 percent (46/50) for
the low and medium dose females. The various control groups
demonstrated survival incidences which varied from 85 to 90 percent
and 75 to 90 percent for the male and female controls. Adequate
numbers of animals were at risk in the low and medium dosage groups
from late-developing tumors. A dose-related reduction in the mean body
weight was observed in the treated males, however, the relevance of
this observation is questionable considering the decreased survival in
the high dosage group. No other compound-related clinical signs were
observed. None of the statistical tests for any site of tumors in rats
of either sex showed a significant positive association between the
administration of styrene and an increased tumor incidence (National
Cancer Institute, 1978).
In a two-year study in Sprague-Dawley rats styrene was
administered in the drinking water at intended concentrations of
125 and 250 ppm which resulted in approximate intakes of 7.7 and
14 mg/kg b.w./day for the males and 12 and 21 mg/kg b.w./day for the
females. Each treatment group was initially comprised of 50 males and
70 females, and a group of 76 males and 106 females served as
controls. The following parameters were assessed during the course of
the study; body weight gain, food and water intake, hemograms,
clinical chemistry, urinanalysis, clinical signs, mortality and gross
necropsy and histological examination at interim and terminal
sacrifices and of those animals which died during the course of the
study or were sacrificed in a moribund condition. Styrene did not
produce any changes in the above parameters with the exception that
the terminal bodyweights of the high dosage females were less than
that of the controls. Mortality throughout the study was no different
in the three groups with 34 (control), 23 (low dose) and 19 (high) of
the males and 46 (control), 30 (low dose) and 26 (high dose) of the
females expiring before termination of the study. Styrene did not
produce either gross or histological changes, nor was there an
apparent styrene-related increase in tumor incidence. All tumors noted
were common, spontaneously occuring tumors for this strain of rat or
rare tumors that occurred without regard to treatment group (Chemical
Manufacturers Association, 1980).
The long-term effects of styrene were assessed in two groups of
Wistar rats. Groups each consisted of 15 male and 15 male Wistar rats
and received a fatty solution of the monomer in dosages of either 1 or
5 mg/kg b.w. by mouth for a period of 10 months. The treatment was
then discontinued and the animals were periodically followed for up to
10 months. A postmortum examination was performed on all animals which
expired during the study and on those animals which were sacrificed at
the end of the 20th months. Two tumors were identified in the inquinal
region in females which received the highest dosage of styrene with
one tumor appearing at five months post-exposure and the other at ten
months post-exposure. Histological evaluation of the tumors revealed
that they were well developed fibroadenomas. The authors concluded
that the appearance of the tumors was associated with administration
of the styrene (Radeva & Krustev, 1982).
Groups of 40 male and 40 female Sprague-Dawley rats were
administered styrene oxide in olive oil by gavage at dosages
equivalent to 0, 50 and 250 mg/kg b.w. four or five days per week for
52 weeks, whereupon, the treatment was terminated and the animals were
permitted to live until their deaths. The numbers of males which
survived until the fifty-first week of the study were 37/40, 31/40 and
28/40 of the control, low and high dosage groups. The incidences of
survival for the females were 28/40, 31/40 and 30/40 of the control,
low and high dosage groups. The histological analysis revealed that
both dosages of the oxide elicited a higher, dose-related incidence of
papillomas and carcinomas of the forestomach than the control animals
with 19.3% of the low dosage animals and 50% of the high dosage
animals demonstrating these lesions. Many of the carcinomas were
observed to have metastasized to the liver, and in addition, many of
the oxide-treated animals were noted to have precursor lesions
(Maltoni et al., 1979).
Special studies on mutagenicity
Styrene and styrene epoxide were studied in spot tests and plate
incorporation assays for activity agains S. typhimurium straing
TA-98, 100, 1535, 1537 and 1538 with and without hepatic metabolizing
systems from Arochlor 1254 - pretreated rats and hamsters. Styrene was
not mutagenic for any of the tester strains, whereas, the epoxide was
active in strains TA-100 and 1535 in the spot and plate assays without
activation. Activity in these strains indicate that the epoxide acted
as a base substituted mutagen (Stoltz & Withey, 1977; Busk, 1979).
In other studies styrene was shown to be mutagenic to strains
TA-100 and 1535, but only after metabolic activation. Styrene oxide in
these studies was mutagenic, with and without metabolic activities
(Vainio et al., 1976; de Meester et al., 1977; Watabe et al., 1978a,
1978b; Loprieno et al., 1978; Milvy & Garro, 1976; Greim et al.,
1977).
The putative styrene intermediate, 1-vinylbenzene-3,4-oxide,
which has a half-life of 4.3 seconds at pH 7.4 in aqueous solution
proved to be a potent mutagen toward S. typhimurium, TA-100, but not
TA-98. This intermediate also demonstrated potent cytotoxicity to both
His + revertant colonies. The final metabolite, 4-vinylphenol, which
is the metabolite to which the oxide is converted proved to be neither
mutagenic or cytotoxic in these studies (Watabe et al., 1982).
The two enantiomers of styrene 7,8-oxide and various thioether
conjugates of racemic styrene 7,8-oxide were tested for mutagenic
activity in S. typhimurium TA-100. The results of these studies
suggested that the (R) enantiomer is more mutagenic than the (S)
enantiomer, with the racemic mixture demonstrating intermediate
activity and the conjugates exhibiting no activity (Pagano et al.,
1982).
The activities of epoxide hydrolase and monoxygenase were studied
in incubation mixtures for liver microsomal assays with S-9 metabolic
activation in the absence or presence of styrene from rats and mice.
The results of this study showed that the activity of the hydrolase is
more stable than that of the monoxygenase. This is further evidence
that in the S. typhimurium mutagenic assay system styrene shows
equivocal results because the styrene epoxide is being hydrolyzed at a
fairly constant rate while its formation from styrene is occurring at
decreasing rates (Bauer et al., 1980).
In a metabolizing, D7 strain of Saccharomyces cerevisiae
styrene induced an increase in the frequency of cross-over, gene
conversion and point mutation (Del Carratore et al., 1983). In a
series of mutagenic assays which measured forward mutations in
Schizosaccharomyces pombe and V-29 Chinese hamster cells and gene
conversions in S. cerevisiae in the diploid strain D4, styrene oxide
was active in the forward mutation experiments with yeast and Chinese
hamster cells and the production of gene-conversion in yeast. In
contrast, styrene was inactive in these assays, even in the presence
of purified mouse - liver microsomes. In a host-mediated assay which
utilized S. pombe both compounds were active in the production of
gene conversion, but not for forward mutation (Loprieno et al., 1976).
In subsequent studies styrene oxide, but not styrene, was shown to
have mutagenic activity in unscheduled DNA synthesis (UDS) studies in
a human heterploid cell line and in the induction of chromosomal
aberrations in the bone marrow of CD-1 mice treated by gavage with a
0.5 ml of solution 66% in olive oil. The UDS studies also utilized a
mouse liver homogenate, but styrene still failed to demonstrate
activity (Loprieno et al., 1978).
Styrene oxide in Chinese hamster ovary cells was a potent inducer
of sister chromatid exchanges (SCF), while styrene did not increase
the number of SCF per metaphase. The effect of the oxide was
diminished by the presence of a rat S9 metabolic activation system,
whereas, it had no effect on the lack of activity of styrene. In
contrast, when an inhibitor of epoxide hydrolase, cyclohexene oxide,
was used the induction of SCF by styrene with metabolic activation
became evident (de Raat, 1978).
Styrene oxide was tested in a mutagenic test screening programme
which involved a sperm abnormality test and dominant lethal test in
male mice exposed to atmospheric concentrations of 15 or 100 ppm for
7 hours per day for 5 days, a cytogenic test in bone marrow celles of
male and female rats exposed in a manner as described previously or a
single exposure of 7 hour duration and a sex-linked recessive lethal
test in Drosophila melanogaster with exposure to atmosphere of
100 ppm for 150 minutes. The results of these studies showed that
styrene oxide had no activity on the frequency of chromosomal
aberrations and sex-linked recessive lethal mutation frequency in
D. melanogaster. In the dominant lethal test a small reduction in
pregnancy frequency and total number of implantations was attributed
to styrene oxide. The highest dosage of the oxide, also resulted in a
slight increase in the frequency of sperm abnormality (McGregor,
1983).
Additional studies with Drosophila melanogaster showed that
both styrene and styrene oxide induce recessive lethal mutations and
that the frequency of their occurence is increased by pretreatment
with either sodium phenobarbitone or trichloropropane oxide (Donner et
al., 1979).
The mutagenic potential of styrene and its 7,8-oxide were studied
in the isolated perfused rat liver as the metabolizing system and
Chinese hamster V79 cells as genetic target cells. The 7,8-oxide was
rapidly metabolized by the liver, and therefore, failed to elicit a
mutagenic effect. In contrast, styrene produced an increase in V79
mutants which seemed not to be due to the formation of the 7,8-oxide,
since simultaneous analysis of its concentration in the perfusion
medium did not correlate with the mutagenic effect (Beije & Jenssen,
1982).
The in vivo exposure of Chinese hamsters to atmospheric
concentrations of styrene oxide from 25 to 100 ppm did not affect the
incidence of chromosomal aberrations and sister chromatid exchanges in
bone marrow cells. To alter the rate of chromosomal aberrations
required the administration of lethal concentrations of the oxide by
the intraperitoneal route (Norppa et al., 1979, 1983).
The daily oral administration of 500 mg of styrene kg/b.w. for
four days or 200 mg of styrene/kg b.w. failed to induce chromosomal
aberrations in the bone marrow cells of CD-1 male mice. Concurrent
pharmacokinetic studies performed on the same animals failed to show
the presence of styrene-7,8-oxide in the blood (at the nanogram level)
(Sbrana et al., 1983).
Styrene produced chromosomal breaks in in vitro preparations of
human lymphocytes, whereas, the oxide was responsible for the
formation of micronuclei and nuclear birdges (Linnainmaa et al.,
1978). In a mutagenic assay in human diploid fibroblasts styrene oxide
failed to elicit an increase in unscheduled DNA synthesis (McGregor,
1983).
Cultures of periphenal lymphocytes of workers employed in
industries with styrene exposure levels which ranged from 30 to
400 mg/mc showed significantly greater frequencies of chromosomal
aberrations than those of a matched (sex, age, age and smoking habit)
control group ( Camurri et al., 1983).
A micronucleus test based on the analysis of lymphocytes with
preserved cytoplasm revealed an increased frequency of micronuclei in
workers exposed to a time-weight average of styrene concentration
between 1 and 36 ppm. These ambient concentrations correlated with low
urinary levels of mandelic acid (Hogstedt et al., 1983).
Styrene and styrene-7,8-oxide induce pronounced dose response
increases in the occurences in sister chromatid exchanges in human
lymphocytes and whole blood cultures. The effect of styrene was
greater in the presence of erythrocytes indicating that styrene must
be converted to the 7,8-oxide for its capability to induce an increase
in the incidence of sister chromatid exchanges (Norppa et al., 1980a,
1983).
Special studies on teratogenicity
The toxicity of styrene and styrene oxide chicken embryos was
studied by injecting from 2 to 100 umol of either compound in 50 ml
of an olive oil-ethanol mixture into the air sacs of eggs from
White-Leghorn SK12 chickens. Malformations were noted in 15% of the
styrene embryos and 7% of the styrene oxide treated embryos. The
embryos were most susceptible on the day of, and the day after the
beginning of incubation (Vainio et al., 1977).
Sprague-Dawley rats in groups of 39, 30 and 29 animals were given
0, 180 and 300 mg styrene/kg b.w./day by gavage from days 6 through 15
of gestation. The styrene was administered as a peanut oil solution at
a volume of 2 mg/kg b.w. as twice daily doses of 90 or 150 mg/kg b.w.
There were no significant differences between the treated-animals and
the controls during gestation except for a diminished weight gain on
days 6 through 9 of gestation that was attributed to a reduction in
food intake. Dams were sacrificed prior to parturition and the fetuses
were examined. The incidence of external visceral and skeletal
malformations in the treated animals did not differ from either the
matched or historical controls. A compound-related effect on the
embryo and fetus was not observed, as was no teratogenic effect.
Styrene at both dosages did induce a decrease in body weight gain and
decreased food consumption in the dams (Murry et al., 1978).
Special studies on reproduction
Water and milk extracts of CNP-2p grade plastics which were
subjected to a vacuum process and contained 80 to 110 ppm styrene were
given every 24 hours to 741 rats in a multi-generation study which was
22 months in duration. A group of animals which received water and
milk served as controls. The parental generation received the extracts
from 1.5 to 13 months of age, while the F1 and F2 generations
were given the extracts from weaning to 8 and 2 months-of-age,
respectively. The administration of the extracts continued throughout
pregnancy and lactation. The animals which received the extracts
demonstrated a statistically reliable decrease in the number of red
blood cells and activity of cholinesterase after a two day fast. A
histological analysis revealed that the extract-treated animals
demonstrated dystrophic changes in the superficial, cortical renal
tubules and a catarrhal state in the duodenum. No other hematological
or histological parameters were found to be adversely altered by the
administration of the extracts. A large number of still-born, poorly
developed and deformed pups were noted to have been born to the
parental and F1 dams which were treated with the extracts as compared
to the control animals. In addition, it was noted that there was a
delay in the growth of fur in the F1 and F2 generations which were
treated with the extracts (Chernova, 1971).
A three-generation reproduction study was conducted as part of a
combined chronic two-year reproduction study in which styrene was
administered in the drinking water of rats. From each group (125 and
250 ppm groups) of the chronic study at least 10 males and 20 females
were mated to produce F1 pups and then subsequent F2 and F3
generations were produced. The following reproductive and
toxicological indices monitored in this study; mean litter size,
live-to-total pup ratios, pup survival indices at intervals from birth
to weaning were evaluated as well as liver and kidney weights of
representative pups necropsied at weaning, cytogenetic evaluation of
bone marrow samples of other weanlings, gross necropsy of F1 and F2
parents, organ weigths and histopathologic examinations of liver and
kidneys of weanlings and of tissues of representative F1 and F2
parents. Styrene had no deleterious effects on the reproductive
capacity of rats through the three generations and on other
toxicological parameters measured (Chemical Manufacturers Association,
1980).
Special studies on enzyme systems
The effect of styrene on carbohydrate balance was studied in
Wistar rats which received a dosage by gavage of 2.5 g/kg b.w. which
was equivalent to 1/2 the LD50. The results indicated that glucose
absorption and hepatic glycogen content were reduced after styrene
administration (Delag et al., 1976).
The effect of orally administered styrene on hepatic mixed
function oxidase enzyme activities, glutathione-S-transferase activity
and glutathione content was studied in male rats. Doses of 250, 450
and 900 mg/kg b.w. were administered for seven days to groups of 15
animals. The two highest dosages enhanced the activity of aryl
hydrocarbon hydroxylase and aniline hydroxylase, while the highest
dosage alone, significantly lowered the activity of glutathione-
S-transferease and glutathione content. In a subsequent study in which
an identical protocol was followed with the exception that only the
two highest dosages were used (450 and 900 mg/kg b.w.) effects on
renal MFO activity and glutathione activity were noted. An increase in
MFO activity was assessed as an increase in the activity of ary
hydrocarbon hydroxylase, aniline hydroxylase ethylmorphine-N-
demethylase and benzphetamine N-demethylase. In addition, the activity
of glutathione-S-transferase was decreased along with the levels of
renal glutathione (Das et al., 1981, 1983).
The intubation of 1 ml/kg b.w. of styrene daily for 15 days to
adult male albino rats elicited a significant increase in serotonin
and noradrenalin levels in the brain without a change in dopamine
content. These changes coincided with a significant decrease in the
activity of monoamine oxidase, however, no change in acetyl
cholinesterase activity was observed (Husain et al., 1980).
Groups of male rats were administered styrene in ground nut oil
by gavage at dosages of 200 and 400 mg/kg b.w. six days per week for
100 days. A dose-dependent increase in hepatic benzo(a)pyrene
hydroxylase and aminopyrine-N-demethylase activities were noted, while
a decrease in the activity of glutathione-S-transferase, mitochondrial
succinic dehydrogenase and B-glucoronidase and no change in glucose-6-
phosphatase were observed (Srivastava et al., 1982).
Special studies on immune function
The effects of styrene on immunological function were studied in
a series of three experiments in 36 rabbits. The animals used in the
study were immunized with lamb erythrocytes for a period of 7 to 8
days beginning with the initiation of dosing with styrene. Styrene was
administered 5 times per week in the form of an emulsion (vegetable
oil and potato starch) according to three different dosing schedules.
These were 250 mg/kg b.w. for 58 days, 5 mg/kg b.w. for 216 days and
0.5 mg/kg b.w. for 202 days. The results of these studies established
that the higher dosages of styrene produced acute variations in the
titer of complement and antibodies. The phagocytic activity of the
leucocytes were also observed to be depressed. These changes were
noted to have occured prior to other indications of styrene
intoxication at the highest dosage. These included a reduction of
appetite and hyperemia of the conjunctiva of the eye (Sinitskii,
1969).
Acute toxicity
Species Route LD50 Reference
Rat oral 5.0 g/kg b.w. Wolf et al., 1956
Rat oral 5.5 g/kg b.w. Ogleznev, 1963
Short-term studies
Mouse
A subchronic study was performed as a range finding study for the
NCI chronic mouse bioassay. Five males and five females were placed in
groups which received dosages of 0, 147, 215, 316, 464 and 681 mg of
styrene/kg b.w. which was administered as a corn-oil gavage five days
per week for seven weeks. A reduced mean body weight gain was noted in
the groups which received 316 and 464 mg/kg b.w., but not the highest
dosage of 681 mg/kg b.w. No other clinical abnormalities were noted to
be related to the exposure to styrene (National Cancer Institute,
1978).
In a one month study in 75 mice styrene at a dosage of 1/10 of
the LD50, 0.55 g/kg b.w., had no effect on the growth of the treated
animals. No other details of the study were provided (Ogleznev, 1963).
Rat
Rats were dosed by gavage with dosages of 0.1, 0.5, 1.0 and 2.0 g
of styrene/kg b.w. emulsified in olive oil with gum arabic. Dosing was
five days per week for 4 weeks. There was an increase in mortality and
pronounced esophageal and gastric irritation in the groups which
received the two highest dosages. Slight esophageal and gastric
irritation were also observed at the next lowest dosage of 0.5 g/kg
b.w., along with poor weigth gains in the five males which survived
the 28-day testing period. The animals which received the lowest
dosage of 0.1 g/kg b.w. were found to be in generally good condition
(Spencer et al., 1942).
Matched groups of ten female rats were dosed by gavage with
dosages of 0, 66.7, 133, 400 and 667 mg of styrene/kg b.w./day five
days per week for six months. The test compound was administered as an
olive-oil solution. The two lowest dosages had no effects on the
animals. However, the two higher dosages did produce, in a
dose-related manner, a slight reduction in the rate of growth and
slight depressions in hepatic and renal weights. Although blood cell
counts were made after 20, 40, 80 and 130 doses no compound related
effects were noted (Wolf et al., 1956).
Groups of five male and five female Fischer 344 rats received
styrene in corn-oil via gavage five days per week for seven weeks at
dosages of 0, 681, 1000, 1470, 2150 and 3160 mg/kg b.w. This was
followed by a one week observation period. The treated males,
particularly at the higher dosages, tended to have lower mean body
weight gains than the controls. At the conclusion of the study no
other compound-related clinical abnormalities were demonstrated
(National Cancer Institue, 1978).
Groups of adult, male rats were administered (six days/week)
styrene in ground nut oil by gavage dosages of 200 or 400 mg/kg b.w.
for a period of 100 days. No overt signs of toxicity were noted in the
treated animals. Weight gain and absolute and relative liver weights
were not different in the control and treated animals. The highest
dose of 400 mg/kg b.w. was associated with tiny areas of focal
necrosis in the liver which consisted of degenerated hepatocytes and
inflammatory cells (Srivastava et al., 1982).
Dog
Groups of four female and male Beagle hounds were administered
styrene by gavage at dosages of 0, 200, 400 or 600 mg/kg b.w./day for
up to 561 days. The styrene was provided as a 50/50 mixture in peanut
oil. The two highest dosages elicited Heinz bodies in the erythrocytes
of the males and occasionally in the females on the lowest dosage.
There were other sporadic changes in various hematological parameters
(decreased packed cell volume, erythrocyte counts and sedimentation
rates and hemoblogin levels and increased incidence of anisocytosis
and hypochromia of erythrocytes, hemosiderin in hepatic
reticuloendothelial cells and numbers of hepatocellular intranuclear
acidophilic crystalline inclusions) which quickly disappeared when the
administration of styrene was terminated. Although body weights, food
consumption, clinical chemistry determinations and organ weights
(brain, heart, liver, kidneys and testes) were studied none were
adversely effected by the administration of styrene (Quast et al.,
1978).
OBSERVATIONS IN MAN
Epidemiological and clinical studies
Experimental exposures to concentrations of styrene from 50 to
800 ppm for varying periods of time have shown depression of the
central nervous (CNS) system. These include reports of listlessness,
drowsiness, incoordination, decrements of manual dexterity, feeling of
intoxication and changes in visual evoked response and EEG amplitude
(Carpenter et al., 1944; Gamberale & Hultengren, 1974; Oltramare et
al., 1974). In contrast other studies have shown that styrene exposure
of from 50 to 350 ppm and again for varying periods of time did not
have effects on CNS function (Stewart et al., 1968; Gamberale &
Hultengren, 1974; Oltramare et al., 1974). In the workplace exposure
to styrene has been attributed to cause increased reaction times,
abnormal EEGs, headache, fatigue, malaise and dizziness (Seppalainen &
Harkonen, 1976; Harkonen et al., 1978; Nicholson et al., 1978; Lorimer
et al., 1976; Rosen et al., 1978; Cherry et al., 1980). There have
also been reports of styrene - induced peripheral neuropathy in the
workplace. In several studies reductions in nerve condition velocites
and mild sensory neuropathy have been noted (Lilis et al., 1978; Rosen
et al., 1978).
Styrene exposure has also resulted in irritation of the eyes,
respiratory tract, throat, and epidermis (Stewart et al., 1968; Gotell
et al., 1972; Araki et al., 1971; McLaughlin, 1946; Rosen et al.,
1978; Oltramare et al., 1974; Lorimer et al., 1976).
There are studies which suggest that styrene exposure in the
workplace has adverse effects on hepatic function. These changes have
included elevated serum enzyme activities, serum uric acid and glucose
tolerance (Lorimer et al., 1976; Axelson & Gustavson, 1978; Chmieleski
et al., 1973; Chmielewski, 1976). This link between styrene exposure
and hepatic dysfunction, however has not been definitively
established. The issue of styrene-induced reproductive and/or
teratogenic effects was raised in a study of congenital defects in
children whose mothers were occupationally exposed to styrene
(Holmberg, 1977) and in a study in which styrene was measured in
umbilical cord blood suggesting the ability of styrene to cross the
placenta (Dowty et al., 1976). In addition, an increased rate of
spontaneous abortions was noted in a study of styrene workers
(Hemminki, 1980). In contrast to these observations another study of
occupationally exposed women failed to establish a link between the
incidence of spontaneous abortions and styrene exposure (Harkonen &
Holmberg, 1982).
Several studies have examined whether styrene can induce a
carcinogenic response in humans. Several mortality studies of
occupationally exposed individuals have failed to show an excess of
deaths due to cancer (Ott et al., 1980; Frentzel-Beyme et al., 1978;
Nicholson et al., 1978). In a proportionate mortality study of 560
styrene-polystyrene polymerization workers over an approximately 15
year period showed a deficit of deaths as compared to the general
population was noted. Among the deaths of the workers were one
leukemia, one lymphoma and an additional death accompanied by leukemia
(Nicholson et al., 1978). There have been only limited epidemiological
studies performed on the possible styrene-induced carcinogenicity in
humans. A mortality study of employees engaged in the development or
manufacture of styrene-based products revealed a statistically
significant increase in the number of leukemias in comparison to other
company employees, however, when the comparison was made with national
statistics the difference was not statistically significant (Ott et
al., 1980). In a study of styrene and polystyrene plant workers in
which 24 deaths certificates were examined there was no evidence of an
association between exposure to styrene and an excess of cancer
(Frentzel-Beyme et al., 1978).
Comments
Styrene readily crosses epithelial surfaces and therefore is
absorbed from the gastrointestinal tract. Once absorbed styrene can be
eliminated unchanged via expiration or it can be converted to a number
of metabolites, including mandelic acid, phenylglyooxylic acid and
hippiuric acid, which in turn are excreted via the urine. The proposed
intermediate in this metabolic scheme is an epoxide, styrene oxide,
however its existence has been established solely in in vitro
experimentation and has never been isolated in vivo.
In studies in laboratory animals styrene has generally been shown
to elicit adverse effects only at high dose levels. These effects
include reductions in body weight gains, generation of Heinz bodies in
erythrocytes, sporadic changes in several hematological parameters,
variations in the titer of complement and antibodies and focal
necrosis in the liver. It is reported to have no teratogenic effect
nor to have adverse consequences on reproductive performance.
Mutagenic studies have generally shown styrene to be inactive,
although under some test conditions chromosomal effects were produced.
However, its metabolite, styrene oxide, has pronounced mutagenic
activity. Lifetime studies in rats have failed to demonstrate a
styrene-induced carcinogenic effect. One in three studies in the mouse
involving in utero exposure provided "limited evidence" of a
carcinogenesis. In contrast, one preliminary study has shown that
styrene oxide has carcinogenic potential.
In humans, styrene has been reported to have depressive effects
on CNS function and is an irritant to epithelial surfaces. There has
been some evidence generated in clinical studies that indicate that
styrene is hepatotoxic, however, the evidence is not conclusive. Data
from human studies have presented suggestive evidence that styrene is
teratogenic, however, the animal studies have not borne this out.
Human clinical and epidemiological studies performed to data have
generally shown that styrene is not carcinogenic and failed to
establish a link between styrene exposure and carcinogenesis.
EVALUATION
Level causing no toxicological effects
Rat: 125 ppm in drinking water, equal to 7.7 mg/kg b.w./day for 2
years.
Estimate of a provisional maximum tolerable daily intake for man
0.04 mg/kg bw.
REFERENCES
ANDERSON, N.C., TRANBERG, F.A., UGGLA, A.H., & ZETTERBERG, G. (1980)
Chromosomal aberrations and sister-chromatid exchanges in lymphocytes
of men occupationally exposed to styrene in a plastic-boat factory.
Mutat. Res., 73(2): 387-401.
ARAKI, S., ABE, A., USHIO, K., & FUJINO, M. (1971) A case of skin
atrophy, neurogenic muscular atrophy and anxiety following long
exposure to styrene. Jpn. J. Ind. Health, 13(5): 527-31.
ASTRAND, I., KILBOM, A., OVRUM, P., WAHLBERG, I., & VESTERBERG, O.
(1974) Exposure to styrene. I. Concentration in alveolar air and blood
at rest and during exercise and metabolism. Work Environ. Health,
11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ. Health,
4(Suppl.2): 215-19.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. Appl. Pharmacol., 16: 691-700.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in S.
typhimurium strains. Int. J. Cancer, 15: 429-437.
BAUER, D., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R., &
TONARELLI, S. (1980) The problem of negative results for styrene in
the in vitro mutagenesis test with metabolic activation (microsomal
assay). 2. Behaviour of epoxide hydrolase in the incubation mixtures.
Boll. Soc. Ital. Biol. Sper., 56(21): 2200-5.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in the liver
perfusion/cell culture systemœ No indication of styrene-7,8-oxide as
the principle mutagenic metabolite produced by the intact rat liver.
Chem. Biol. Interactions, 39(1): 57-76œ
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene oxide in
human blood erythrocytes and lumphocytesœ Res. Commun. Chem.
Pharmacol., 33(2): 273-82œ
BUSK, L. (1979) Mutagenic effects of styrene and styrene oxide.
Mutat. Res., 67(3): 201-208.
CAMURRI, L., CODELUPPI, S., SCARDUELLI, L., & PEDRONI, C. (1983)
Chromosomal aberrations and sister chromatid exchanges in workers
exposed to styrene. Mutat. Res., 119(3-4): 361-370.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., & BELVEDERE
G. (1978) Hepatic and extrahepatic formation and hydration of styrene
oxide in vitro in animals of different species and sex. Toxicol.
Lett., 2: 179-86.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F. Jr. (1944)
Studies on the inhalation of 1,3-butadiene; with a comparison of its
narcotic effect with benzol, toluol, and styrene and a note on the
elimination of styrene by the human. J. Ind. Hyg. Toxicol., 26(3):
69-78œ
CHEMICAL MANUFACTURERS ASSOCIATION (1980) Toxicological study on
styrene incorporated in drinking water of rats for two years in
conjunction with a three-generation reproduction study. Litton
Bionetics.
CHERNOVA, T. (1971) Evaluation of articles made on CNP-2P grade
plastics intended for packaging of food-stuffs. Vopr Pitan, 30:
56-58.
CHERRY, N., WALDRON, H.A., WELLS; G.G., WILKINSON, R.T., WILSON, H.K.,
& JONES, S. (1980) An investigation of the acute behavioural effects
of styrene on factory workers. Brit. J. Ind. Med. 37: 234-40.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R. (1973) Rating
of the exposure to styrene of persons working at the production of
polyesteric laminates. Bull. Inst. Marit. Med. Gdynia, 24:
203-209.
CHMIELEWSKI, J. (1976) Clinical and experimental research into the
pathogenesis of toxic effect of styrene. Part V. Impact of styrene on
carbohydrate balance in people in the course of their work. Bull
Inst. Marit. Trop. Med. Gdynia, 27: 177-84.
DAS, M., DIXIT, R., MUSHTAQ, M., SRIVASTAVA, S.P., & SETH, P.K. (1981)
Effect of styrene on hepatic mixed function oxydases, glutathione
content and glutathione-S-transferase activity in rats. Drug Chem.
Toxicol., 4(3): 219-27.
DAS, M., SRIVASTAVA, S.P., & SETH, P.K. (1983) Effect of styrene on
glutathione content and some xenobiotic metabolizing enzymes of rat
kidney. Acta Pharmacol. Toxicol., 52(1): 47-50.
DELAG, G., CHMIELEWSKI, J., MIKULSKI, P., & WIGLUSZ, R. (1976)
Clinical and experimental research into the pathogenesis of toxic
effect of styrene. Part VI. The effect of styrene on carbohydrate
balance, experimental research. Bull. Inst. Marit. Trop. Med.
Gdynia, 27: 185-91.
DELBRASSINE, I.P., VAN BLADEREN, P.J., SMEETS, F.L., & SEUTTEN-
BERLAGE, F. (1981) Stereoselective oxidation of styrene to styrene
oxide in rats as measured by mercapturic acid excretion.
Xenobiotica, 11(9): 589-94.
DEL CARRATORE, R., GIAGONI, P., BAUER, C., BRONZETTI, G., CORSI, C.,
NIERI, R., & PAOLINI, M. (9183) Mutagenicity of styrene on
metabolizing D7 strain of Saccharomyces cerevisiae. Boll. Soc.,
Ital., Biol. Sper., 59(2): 233-8.
DE MEESTER, C., PONCELET, F., ROBERFROLD, M., RONDELET, J., & MERCIER,
M. (1977) Mutagenicity of sltyrene and styrene oxide. Mut. Res.,
56: 147-52.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M.,
MERCIER, M., & PONCELET, F. (1981) Mutagenicity of styrene in the
Salmonella typhimurium test system. Mutat. Res., Dec. 90:
443-50.
DE RAAT, W.K. (1978) Induction of sister chromatid exchanges by
styrene and its presumed metabolite styrene oxide in the presence of
rat liver homogenate. Chem. Biol. Interactions, 20: 163-70.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals induced
by styrene and styrene oxide in Drosophila melanogaster. Mutat.
Res., 67: 373-6.
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic constituents.
Pediatr. Res., 10: 696-701.
EL MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies in
detoxication. Part 73. The metabolism of alkylbenzenes-phenylacetylene
and phenylethylene (styrene). Biochem. J., 68: 199-20.
FRENTZEL-BEYME, R., THIESS, A.M., & WIELAND, R. (1978) Survey of
mortality among employees engaged in the manufacture of styrene and
polystyrene at the BASF Ludwigshafen works. Scan. J. Work Environ.
Health, 4(Suppl. 2): 231-39.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene. II.
Psychological functions. Work Environ. Health, 11: 86-93.
GOTELL, P., AXELSON, O., & LINDELOF, B. (1972) Field studies on human
styrene exposure. Work Environ. Health, 9(2): 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMANN, W., & KRAMER, M. (1977)
Mutagenicity and chromosomal aberrations as an analytical tool for
in vitro detection of mammalian enzyme-mediated formation of
reactive metabolites. ARch. Toxicol., 39: 159-69.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., & HERNBERG,
S. (1978) Exposure-response relationship between styrene exposure and
central nervous functions. Scand. J. Work Environ. Health, 4:
53-59.
HARKONEN, H. & HOLMBERG, P.C. (1982) Obstetric histories of women
occupationally exposed to styrene. Scand. J. Work Environ. Health,
8: 74-77.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980) Spontaneous abortions
among female chemical workers in Finland. Int. Arch. Occup. Environ.
Health, 45: 123-26.
HINZ, G., GOHLKE, R., & BURCK, D. (1980) The effect of simultaneous
oral application of ethanol and styrene. [1. Acute and subacute
experiments on rats.] J. Hyg. Epidemial. Microbiol. Immunol.,
24(3): 262-270.
HOGSTEDT, B., AKESSON, B., AXELL, K., GULLBERG, B., MITELMAN, F.,
PERO, R.W., SKERFVING, S., & WELINDER, H. (1983) Increased frequency
of lymphocyte micronuclei in workers producing reinforced polyester
resin with low exposure to styrene. Scand. J. Work Environ. Health,
9(3): 241-6.
HOLMBERG, P.C. (1977) Central nervous defects in two children and
mothers exposed to chemicals in the reinforced plastics. Chance or a
causal relation? Scand. J. Work Environ. Health, 3: 212-14.
HUSAIN, R., SRIVASTAVA, S.P., MUSHTAG, M., & SETH, P.K. (1980) Effect
of styrene on levels of serotonin, noradrenaline, dopamine and
activity of acetyl cholinesterase and monamine oxidase in rat brain.
Toxicol. Lett. Nov., 7(1): 47-50.
IARC (1979) Styrene and polymers. IARC Monographs on the evaluation of
the carcinogenic risk of chemicals to humans. Volume 19: 231-274.
IARC (1979) Styrene oxide. IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Volume 19: 275-283.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I. (1974)
Evaluation of hippuric, phenylglyoxylic and mandelic acids in urine as
indices of styrene exposure. Int. Arch. Arbeitsmed., 32(1-2):
93-101.
JAMES, S.P. & WHITE, D.A. (1967) The metabolism of phenethyl bromide,
styrene and styrene oxide in the rabbit and rat. Biochem. J., 104:
914-21.
KAPPUS, H., BOLT, H., BUCHTER, A., & BOLT, W. (1976) Liver microsomal
uptake of 14C vinyl chloride and transformation to protein alkylating
metabolites in vitro. Tox. Appl. Pharm., 37: 461-471.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in liver
microsomes. Biochem. Pharmacol., 18(2): 552-54.
LEIBMAN, K.C. & ORTIZ, E. (1970) Epoxide intermediates in microsomal
oxidation of olefins to glycols. J. Pharmacol. Exp. Ther.,
173(2): 242-46.
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization workers.
Env. Res., 15:133-38.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978)
Cytogenetic effects of styrene and styrene oxide in human lymphocytes
and Allium cepa. Scand. J. Work Environ. Health, 4(Suppl. 2):
156-62.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S., BONATTI,
S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., corti, G., FREZZA, D.,
LEPORINI, C., MAZZACCARO, A., NIERI, R., ROSSELLINI, D., & ROSSI, A.M.
(1976) Mutagenicity of industrial compounds-styrene and its possible
metabolite styrene oxide. Mut. Res., 40: 317-24.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H., FISCHBEIN,
A., DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J. (1976) Clinical
studies of styrene workers. Initial findings. Environ. Health
Perspect.; 17: 171-81
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First experimental
demonstration of the carcinogenic effects of styrene oxide. Long term
bioassays on Sprague-Dawley rats by oral administration. Med.
Lavoro, 5: 358-62.
McGREGOR, D.B. (1983) Tier II Mutagenic Screening of 13 NIOSH Priority
Compounds; Individual Compound Report; Styrene oxide. Govt. Reports
Announcements & Index (GRA&I), Issue 05.
McLAUGHLIN, R.S. (1946) Chemical burns of the human cornea. Amr.
J.Ophthalmol., 29(11): 1353-62.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene oxide
(1,2-epoxyethylbenzene), a presumed styrene metabolikte. Mut. Res.,
40: 15-18.
MURRAY, F.J., JOHN, J.A., BALMER, M.F., & SCHWETZ, B.A. (1978)
Teratologic evaluation of styrene given to rats and rabbits by
inhalation or by gavage. Toxicology, 11: 335-343.
NATIONAL CANCER INSTITUTE (1978) Carcinogenesis Technical Report
Series. Bioassay of styrene for possible carcinogenicity. No. 185.
NICHOLSON, W., SELIKOFF, I., & SEIDMAN, H. (1978) Mortality experience
of styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4(suppl. 2); 247-252.
NORPPA, H., FLOVAARA, F., HUSGAFVEL-PURSLAINEN, K., SORSA, M., &
VAINIO, H. (1979) Effects of styrene oxide on chromosome aberrations,
sister chromatid exchange and hepatic drug biotransformation in
Chinese hamsters in vivo. Chem. Biol. Interactions, 26: 305-15.
NORPPA, H., SORSA, M., PFAEFFLI, P., & VAINIO, H. (1980) Styrene and
styrene oxide induce SCEs and are metabolised in human lymphocyte
cultures. Carcinogenesis, 1(4): 357-61.
NORPPA, H., SORSA, M., & VAINIO, H. (1980) Chromosomal aberrations in
bone marrow of Chinese hamsters exposed to styrene and ethanol.
Toxicol. Lett., 5: 241-44.
NORPPA, H., VAINIO, H., & SORSA, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res., Aug. 43(8):
3579-82.
NORPPA, H. & VAINIO, H. (1983) Genetic toxicity of styrene and some of
its derivatives. Scand. J. Work Environ. Health, 9(2): 108-14.
OGLEZNEV, G. (1963) Comparative characteristics of the toxic action of
aqueous solution of methylostyrene and styrene. Tr. Omskogo Med.
Inst., 48: 164-174.
OLTRAMARE, M., DESBAUMES, F., IMHOFF, C., & MICHEIELS, W. (1974)
Toxicology of monomeric styrene. Experimental and clinical studies on
man. Geneva, Editions Médecine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J., &
VENABLE, J.R. (1980) A mortality survey of employees engaged in the
development of manufacture of styrene-based products. J. Occup.
Med., 22(7): 445-60.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G., MUSSINI, E.,
& SALMONA, M. (1979) Isolation and structure determination of
enzymatically formed styrene oxide glutathione conjugates. Chem.
Biol. Interactions, 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O., BEND, J.R., & ZEIGER, E.
(1982) Mutagenicity of (R) and (S) styrene 7,8-oxide and the
intermediary mercapturic acid metabolites formed from styrene
7,8-oxide. Environ. Mutagenesis, 4: 575-584.
PANTAROTTO, C., FANELLI, R., BIDOLI, F., MORAZZONI, P., SOMMONA, M., &
SZCZAURINSKA, K. (1978) Arene oxides in styrene metabolksm, a new
perspective in styrene toxicity? Scand. J. Work Environ. Health,
4(suppl. 2): 67-77.
PFAEFFLI, P., HESSO, A., VAINIO, H., & HYVOENEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in workers
occupationally exposed to styrene. Toxicol. Appl. Pharmacol.,
60(1): 85-90.
PLOTNICK, H.B. & WEIGEL, W.W. (1979) Tissue distribution and excretion
of 14C-styrene in male and female rats. Res. Commun. Chem. Pathol.
Pharmacol., 24(3): 515-524.
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work Environ.
Health, 4(suppl. 2): 127-135.
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G., MURRAY, F.J.,
JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a toxicity study in dogs
and teratogenicity studies in rabbits and rats administered monomeric
styrene. Toxicol. Appl. Pharmacol., 45: 293-4.
RADEVA, M. & KRUSTEV, L. (1982) Studies on the effect of styrene
monomer on albino rats after long-term oral administration. Khig.
Zdraveopaz., vol. 25. ISS 5: 464-8.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H. (1978)
Neurophysiological observations after chronic styrene exposure.
Scand. J. Work Environ. Health, 4(suppl. 2); 184-94.
RYAN, A.J. & BEND, J.R. (1977) The metabolism of styrene oxide in the
isolated perfused rat liver. Drug Metab. Dispose., 5(4): 363-7.
SALMONA, M., PACKECKA, J., CANTONI, L., BELVEDERE, G., MUSSINI, E., &
GARATTINI, S. (1976) Microsomal styrene monoxygenase and styrene
epoxide hydrase activities in rats. Xenobiotica, 6(10): 585-91.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate of orally
administered styrene in rats. Midland, MI, Dow Chemical USA, December,
42 pp.
SBRANA, I. LASCIALFARI, D., ROSSI, A.M., LOPRIENO, N., BIANCHI, M.,
TORTORETO; M., & PANTAROTTO, C, (1983) Bone marrow cell chromosomal
aberrations and styrene biotransformation in mice given styrene on a
repeated oral schedule. Chem. Biol. Interact., Aug. 1, 45(3):
349-57.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological findings
among workers occupationally exposed to styrene. Scan. J. Work
Environ. Health, 3: 140-46.
SINITSKII, V (1969) Indexes of immunological reactivity in rabbits
during prolonged exposure to styrene in small doses. Gig. Premen,
Polem. Mater. Igdelii Nikk No. 1: 394-8.
SPENCER, H., IRISH, D., ADAMS, E., & ROWE, V. (1942) The response of
laboratory animals to monomenic styrene. J. Ind. Hyg. Tox., 24:
295-301.
SRIVESTAVA, S., DAS, M., MUSHTAG, M., SHANDRA, S., & SETH, P. (1982)
Hepatic effects of orally administered styrene in rats. J. Appl.
Tox., 2: 219-222.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W. (1968)
Human exposure to styrene vapor. Arch. Environ. Health, 16:
656-62.
STOLTZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of styrene and
styrene epoxide in Salmonella typhimurium. Bull. Environ. Contam.
Toxicol., 17(6): 739-42.
TURSI, F., SAMAIA, M., SALMONA, M., & BELVEDERE, G. (1983) Styrene
oxidation to styrene oxide in human erythrocytes is catalysed by
oxyhemoglobin. Experimentia, vol. 39, ISS 6, 593-4.
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., & PELKONEN, O.
(1976) A study on the mutagenic activity of styrene and styrene oxide.
Scand. J. Work Environ. Health, 3: 147-51.
VAINIO, H., HEMMINKI, K., & FLOVAARA, E. (1977) Toxicity of styrene
and styrene oxide on chick embryos. Toxicology, 8: 319-25.
WATABE, T., ISOBE, M. SAWAHATA, T., YOSHIKAWA, K., YAMADA, S., &
TAKABATAKE, E. (1978a) Metabolism and mutagenicity of styrene.
Scand. J. Work Environ. Health, 4(suppl. 2): 142-55.
WATABE, T., ISOBE, M,, YOSHIKAWA, K, & TAKABATAKE, E., (1978b) Studies
on metabolism and toxicity of styrene. Part I. Biotransformation of
styrene to styrene glycol via styrene oxide by rat liver microsomes.
J. Pharmacobio. Dyn., 1:(2): 98-104.
WATABE, T., AIZAWA, T., SAWAHATA, T., OZAWA, N., ISOBE, M.,
TAKABATAKE, E., HIRATASUKA, A. (1982) Metabolism and toxicity of
styrene: 4.1-vinylbenzene 3,4-oxide, a potent mutagen formed as a
possible intermediate in the metabolism in vivo os styrene to
4-vinylpenol. Mutat. Res., 93(1): 45-56.
WOLF, M.A., ROWE, V.K., McCOLLISTER, D.D., HOLLINGSWORTH, R.L., &
OYEN, F. (1956) Toxicological studies of certain alkylated benzenes
and benzene-experiments on laboratory animals. AMA Arch. In. Health,
14: 387-98.
Styrene is also known as ethenylbenzene, vinylbenzene,
vinylbenzol, styrolene, styrol, styropol, styropor, styrone,
cinnamene, cinnamol, phenethylene, phenylethylene and phenylethane. It
is a colourless, viscous liquid with a melting point of -30.6°C and a
boiling point of 145.2°C. It is insoluble in water, soluble in
ethanol, ether and acetone and very soluble in benzene and petroleum
ether. Its uses include intermediate in the manufacture of plastics,
elastomers and resins which in turn are used in packaging foods, food
products and various other processes in the food industry. It is also
used as a synthetic flavouring substance and adjuvant.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies with laboratory animals have shown that styrene is
readily absorbed from the gastrointestinal tract following oral
administration (Plotnick & Weigel, 1979; Sauerhoff et al., 1976).
The distribution and excretion of a single oral dose of 20 mg
14C-styrene/kg b.w. was studied in male and female Charles-River
rats. The peak tissue levels occurred four hours after administration,
with the kidneys exhibiting the highest concentration of 14C per unit
weight, followed by the liver and pancreas. The primary route of
elimination was via urinary excretion with 90% of the dosage detected
in the urine within 24 hours, while less than 2% was detected in the
feces (Plotnick & Weigel, 1979).
The disposition of orally administered styrene was studied in
mature Sprague-Dawley rats (Spartan substrain) in which doses of 50 or
500 mg/kg 14C-styrene were administered via gavage. Approximately 95
and 90% of the 50 and 500 mg/kg b.w. doses, respectively, were
eliminated in the urine as styrene metabolites. The pulmonary route of
elimination accounted for 1.3% of the lower dosage and 8.9% of the
higher dosage. There was a distinct sex difference in the pulmonary
elimination of styrene with males expiring twice as much styrene as
the females. The fecal route of elimination accounted for only about
4% of the administered dose (Sauerhoff et al., 1976).
Metabolism
A number of metabolic studies in laboratory animals which have
utilized a variety of routes of administration have shown that styrene
is metabolized by the mixed function oxidase (MFO) system to
styrene-7, 8-oxide, and that this occurs not only in the liver but in
a number of other tissue types and organs (Leibman & Ortiz, 1970;
Salmona et al., 1976; Cantoni et al., 1978). The metabolic activation
by the microsomal system depends on the presence of necessary
cofactors for a NADPH generating system and oxygen (Kappus et al.,
1971; Bartsch et al., 1975; Salmona et al, 1976). The oxide can be
metabolized further to styrene glycol by the action of epoxide
hydrolase (Leibman & Ortiz, 1969 and 1970; Ryan & Bend, 1977).
Generally the greatest activity for the MFO and hydrolase have been
detected in the liver with the activity of the latter being greater
than that of the former (Cantoni et al., 1978). The glycol in turn can
be converted to mandelic acid, phenylglyoxylic acid and finally to
hippuric acid or it can be conjugated to glucuronic acid (Ryan & Bend,
1977; El Masri et al., 1958; Sauerhoff et al., 1976; James & White,
1967; Ikeda et al., 1974; Astrand et al., 1974; Gotell et al., 1972).
The oxide can also be conjugated with glutathione to form three
mercapturic acid derivatives, N-acetyl-s-(1-phenyl-2-hydroxyethyl)
cysteine and N-acetyl-s-(phenylacetyl) cysteine. Other metabolites
which have been identified include mercapturic acids, benzoic acid,
4-vinylphenol, 2-phenylethanol, phenaceturic acid and phenylglycol
all of which may be excreted as conjugates of glucuronic acid or
glutathione (Sauerhoff et al., 1976; Bakke & Scheline, 1970;
Delbrassine et al., 1981; El Masri et al., 1958). Studies in rats have
shown that the opening of the epoxide ring of styrene by glutathione
5-transferase is stereospecific with a preference for the R-isomer
(Delbrassine et al., 1981).
There has also been evidence generated that styrene is converted
to an arene oxide, since an intermediate, styrene-3,4-oxide, undergoes
isomerization to 4-vinylphenol which has been isolated in the urine of
both animals and men who have been exposed to styrene (Pantarotto et
al., 1978; Bakke & Scheline, 1970; Pfäffli et al., 1981; James &
White, 1967; Pachecka et al., 1979). However, this route of metabolism
is considered to be a minor one, since much more mandelic acid is
detected in the urine as compared to the 4-vinylphenol.
In vitro preparations of human erythrocytes and lymphocytes
have been shown to catalyze the oxidation of styrene to styrene oxide.
This reaction is inhibited by the presence of CO, but not by
superoxide dismutase, catalase and scavengers of hydroxyl radicals,
and it required the presence of oxygen. These data indicate that
oxyhemoglobin and not free oxygen radicals can be involved in styrene
oxidation (Tursi et al, 1983; Belvedere & Tursi, 1981).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Carcinogenicity data on styrene and styrene oxide were reviewed
by the International Agency for Research on Cancer (IARC) in 1979.
Mouse
A lifetime study following in utero exposure was conducted with
C57B1 black mice. Fifteen dams were administered a single dose of
300 mg of styrene/kg b.w. dissolved in olive oil via intubation on the
17th day of gestation. A vehicle (olive oil) treated group of 57 males
and 49 females served as a control group. Styrene was then given
weekly at the same dose level and via the same route to 27 male and 27
female offspring from weaning up to 120 weeks of age. A group of 12
males and 13 females which were the offspring of five dams treated
with olive oil on the 17th day of gestation received olive oil on a
weekly basis after weaning. The incidence of neonatal mortality of the
styrene-treated group was 35%. In the parental generation lymphomas
were noted in 10 of 12 styrene-treated animals as compared to 3 of 5
of the vehicle treated animals. In the male progeny hepatocellular
carcinomas were found in 3 of 4 animals. This compares to an occurence
of 1 to 12 of vehicle-treated controls and 1 of 47 untreated controls
(P>0.05). The incidences of all other tumor-types were similar in the
styrene-treated and control animals (Ponomarkov & Tomatis, 1978).
A group of 29 pregnant O20 mice received a single dose of
1350 mg/kg b.w. of styrene by gavage (dissolved in olive oil) on the
17th day of gestation, while a control group received olive oil alone.
This same dosage of styrene was administered to the offspring
(39 females and 45 males) from weaning to 16 weeks of age. Treatment
was terminated at this point because of excessive mortality. The most
frequently observed lesions in the styrene-treated offspring which
expired before the twentieth week were multiple centrilobular necrosis
of the liver, hypoplasia of the spleen and severe congestion of
the lungs. In those animals that expired after the forty-fifth
week multiple abscess-like cavities in the liver filled with
polymorphonuclear leukocytes were noted. The study was terminated when
the last animal had expired at 100 weeks. There were no differences
in tumor incidences between the dams treated with styrene and those
treated with olive oil. In the progeny pulmonary adenomas and
adenoearcinomas were noted in 20/23 males and 32/32 females treated
with styrene and in 8/19 males and 14/21 females in these animals
treated with olive oil alone (P>0.01, P<0.01). However, in untreated
controls 34/53 males and 25/42 females were found to also have
pulmonary adenomas and adenocarcinomas (P<0.05, P<0.01). The average
age at death of the pulmonary tumor-bearing, styrene-treated mice was
32 and 49 weeks for the males and females, respectively. In contrast,
the olive oil-treated males and females exhibited an average age of 88
and 85 weeks, while the average age of mortality of the untreated
control males and females was 94 and 99 weeks, respectively. No other
differences in tumor incidences were noted between the various groups
(Ponomarkov & Tomatis, 1978).
Groups of 50 male and 50 female B6C3F1 mice were dosed daily by
gavage five days per week with styrene dissolved in corn oil at dose
levels equivalent to 150 and 300 mg/kg b.w. Treatment was for 78 weeks
and was followed by a 13 week observation period. A group of 20
animals of each sex served as a vehicle corn oil control group. In the
males, but not females, there was a significant positive relationship
between mortality and dosage. A slight, dose-related mean body weight
depression was noted in the females, but not the males, while no other
clinical annormalities were noted. There were adequate numbers of both
males and females at risk for late-developing tumors. In the low
dosage group 92 percent (46/50) and 80 percent (40/50) of the males
and females, respectively, survived on test until the termination of
the study. The percentages of survival for the high dosage group was
78 and 76 for the males and females, respectively, and for the
controls 100 and 90 percent for the respective sexes.
During the course of the study, five low dose females were found
missing. A variety of neoplastic lesions were found in both the
styrene-treated and control animals, however, with the exception of
pulmonary tumors, the incidences neoplastic lesions were unrelated to
the administration of styrene. There was a significant increase in the
incidence of a combination of pulmonary adenomas and carcinomas in the
styrene-treated male mice with 0/20, 6/45 and 9/49 of the control, low
dosage and high dosage animals demonstrating these lesions (P = 0.024
for Fischer exact test comparing high dose and control). However, a
definitive conclusion on the carcinogenicity of styrene could not be
drawn because of the high variability of the incidence of these
neoplasia in historical control mice at the laboratory which performed
the study. The historical incidence of the combination of alveolar/
bronchiolar adenomas and aleolar/bronchiolar carcinomas in male mice
in this laboratory is 12 percent (32/271). The authors concluded that
there was no convincing evidence for carcinogenicity of styrene in the
mouse study. (National Cancer Institute, 1978)
Rat
A group of 21 BD IV rats were given 1350 mg of styrene/kg b.w.
in olive oil orally on the 17th day of gestation. Their offspring (73
males and 71 females) then received 500 mg/kg b.w. styrene weekly from
weaning for 120 weeks. Ten dams and their offsprings (36 males and 39
females) received olive oil and served as controls. The average litter
sizes of the different groups were similar. The mortality of the
offspring of styrene-treated dams (10%) was greater than that of
the controls (2.5%), but not significantly. There was no evidence
of a difference in body weight gain and survival between the
styrene-treated and control offspring. The incidence of survival were
8/73 and 20/71 of the male and female, styrene-treated animals and
14/36 and 18/39 male and female control animals. The incidence of
tumor-bearing dams was greater in the styrene-treated group than
the control group, but the difference was not significant. Three
neurogenic and three stomach tumors were observed in the
styrene-treated progeny that were not observed in the controls
(Ponomarkov & Tomatis, 1978).
A study was carried out with Fischer 344 rats in which groups of
50 males and 50 females were administered styrene dissolved in corn
oil five days per week by gavage at doses equivalent to 500, 1000 and
2000 mg/kg b.w. Treatment was for 103 weeks for the low dosage group
and 78 weeks for the medium and high dosage groups. This was followed
by an observation period of one week for the low dosage groups and
twenty-seven weeks for the medium and high dosage groups. A group of
40 animals of each sex served as vehicle controls (corn oil). The
highest dosage resulted in a significantly earlier and higher
mortality in both sexes, wherein, an additional dosed group of each
sex was included in the bioassay. The survival of the other dosage
groups were not adversely affected by styrene administration. By week
53 only 12 percent (6/50) of the males had survived, while only 14
percent (7/50) of the females had survived by week 70. The survival
incidences for the other groups were 88 percent (44/59), 94 percent
(47/50) for the low and medium dose males and 92 percent (46/50) for
the low and medium dose females. The various control groups
demonstrated survival incidences which varied from 85 to 90 percent
and 75 to 90 percent for the male and female controls. Adequate
numbers of animals were at risk in the low and medium dosage groups
from late-developing tumors. A dose-related reduction in the mean body
weight was observed in the treated males, however, the relevance of
this observation is questionable considering the decreased survival in
the high dosage group. No other compound-related clinical signs were
observed. None of the statistical tests for any site of tumors in rats
of either sex showed a significant positive association between the
administration of styrene and an increased tumor incidence (National
Cancer Institute, 1978).
In a two-year study in Sprague-Dawley rats styrene was
administered in the drinking water at intended concentrations of
125 and 250 ppm which resulted in approximate intakes of 7.7 and
14 mg/kg b.w./day for the males and 12 and 21 mg/kg b.w./day for the
females. Each treatment group was initially comprised of 50 males and
70 females, and a group of 76 males and 106 females served as
controls. The following parameters were assessed during the course of
the study; body weight gain, food and water intake, hemograms,
clinical chemistry, urinanalysis, clinical signs, mortality and gross
necropsy and histological examination at interim and terminal
sacrifices and of those animals which died during the course of the
study or were sacrificed in a moribund condition. Styrene did not
produce any changes in the above parameters with the exception that
the terminal bodyweights of the high dosage females were less than
that of the controls. Mortality throughout the study was no different
in the three groups with 34 (control), 23 (low dose) and 19 (high) of
the males and 46 (control), 30 (low dose) and 26 (high dose) of the
females expiring before termination of the study. Styrene did not
produce either gross or histological changes, nor was there an
apparent styrene-related increase in tumor incidence. All tumors noted
were common, spontaneously occuring tumors for this strain of rat or
rare tumors that occurred without regard to treatment group (Chemical
Manufacturers Association, 1980).
The long-term effects of styrene were assessed in two groups of
Wistar rats. Groups each consisted of 15 male and 15 male Wistar rats
and received a fatty solution of the monomer in dosages of either 1 or
5 mg/kg b.w. by mouth for a period of 10 months. The treatment was
then discontinued and the animals were periodically followed for up to
10 months. A postmortum examination was performed on all animals which
expired during the study and on those animals which were sacrificed at
the end of the 20th months. Two tumors were identified in the inquinal
region in females which received the highest dosage of styrene with
one tumor appearing at five months post-exposure and the other at ten
months post-exposure. Histological evaluation of the tumors revealed
that they were well developed fibroadenomas. The authors concluded
that the appearance of the tumors was associated with administration
of the styrene (Radeva & Krustev, 1982).
Groups of 40 male and 40 female Sprague-Dawley rats were
administered styrene oxide in olive oil by gavage at dosages
equivalent to 0, 50 and 250 mg/kg b.w. four or five days per week for
52 weeks, whereupon, the treatment was terminated and the animals were
permitted to live until their deaths. The numbers of males which
survived until the fifty-first week of the study were 37/40, 31/40 and
28/40 of the control, low and high dosage groups. The incidences of
survival for the females were 28/40, 31/40 and 30/40 of the control,
low and high dosage groups. The histological analysis revealed that
both dosages of the oxide elicited a higher, dose-related incidence of
papillomas and carcinomas of the forestomach than the control animals
with 19.3% of the low dosage animals and 50% of the high dosage
animals demonstrating these lesions. Many of the carcinomas were
observed to have metastasized to the liver, and in addition, many of
the oxide-treated animals were noted to have precursor lesions
(Maltoni et al., 1979).
Special studies on mutagenicity
Styrene and styrene epoxide were studied in spot tests and plate
incorporation assays for activity agains S. typhimurium straing
TA-98, 100, 1535, 1537 and 1538 with and without hepatic metabolizing
systems from Arochlor 1254 - pretreated rats and hamsters. Styrene was
not mutagenic for any of the tester strains, whereas, the epoxide was
active in strains TA-100 and 1535 in the spot and plate assays without
activation. Activity in these strains indicate that the epoxide acted
as a base substituted mutagen (Stoltz & Withey, 1977; Busk, 1979).
In other studies styrene was shown to be mutagenic to strains
TA-100 and 1535, but only after metabolic activation. Styrene oxide in
these studies was mutagenic, with and without metabolic activities
(Vainio et al., 1976; de Meester et al., 1977; Watabe et al., 1978a,
1978b; Loprieno et al., 1978; Milvy & Garro, 1976; Greim et al.,
1977).
The putative styrene intermediate, 1-vinylbenzene-3,4-oxide,
which has a half-life of 4.3 seconds at pH 7.4 in aqueous solution
proved to be a potent mutagen toward S. typhimurium, TA-100, but not
TA-98. This intermediate also demonstrated potent cytotoxicity to both
His + revertant colonies. The final metabolite, 4-vinylphenol, which
is the metabolite to which the oxide is converted proved to be neither
mutagenic or cytotoxic in these studies (Watabe et al., 1982).
The two enantiomers of styrene 7,8-oxide and various thioether
conjugates of racemic styrene 7,8-oxide were tested for mutagenic
activity in S. typhimurium TA-100. The results of these studies
suggested that the (R) enantiomer is more mutagenic than the (S)
enantiomer, with the racemic mixture demonstrating intermediate
activity and the conjugates exhibiting no activity (Pagano et al.,
1982).
The activities of epoxide hydrolase and monoxygenase were studied
in incubation mixtures for liver microsomal assays with S-9 metabolic
activation in the absence or presence of styrene from rats and mice.
The results of this study showed that the activity of the hydrolase is
more stable than that of the monoxygenase. This is further evidence
that in the S. typhimurium mutagenic assay system styrene shows
equivocal results because the styrene epoxide is being hydrolyzed at a
fairly constant rate while its formation from styrene is occurring at
decreasing rates (Bauer et al., 1980).
In a metabolizing, D7 strain of Saccharomyces cerevisiae
styrene induced an increase in the frequency of cross-over, gene
conversion and point mutation (Del Carratore et al., 1983). In a
series of mutagenic assays which measured forward mutations in
Schizosaccharomyces pombe and V-29 Chinese hamster cells and gene
conversions in S. cerevisiae in the diploid strain D4, styrene oxide
was active in the forward mutation experiments with yeast and Chinese
hamster cells and the production of gene-conversion in yeast. In
contrast, styrene was inactive in these assays, even in the presence
of purified mouse - liver microsomes. In a host-mediated assay which
utilized S. pombe both compounds were active in the production of
gene conversion, but not for forward mutation (Loprieno et al., 1976).
In subsequent studies styrene oxide, but not styrene, was shown to
have mutagenic activity in unscheduled DNA synthesis (UDS) studies in
a human heterploid cell line and in the induction of chromosomal
aberrations in the bone marrow of CD-1 mice treated by gavage with a
0.5 ml of solution 66% in olive oil. The UDS studies also utilized a
mouse liver homogenate, but styrene still failed to demonstrate
activity (Loprieno et al., 1978).
Styrene oxide in Chinese hamster ovary cells was a potent inducer
of sister chromatid exchanges (SCF), while styrene did not increase
the number of SCF per metaphase. The effect of the oxide was
diminished by the presence of a rat S9 metabolic activation system,
whereas, it had no effect on the lack of activity of styrene. In
contrast, when an inhibitor of epoxide hydrolase, cyclohexene oxide,
was used the induction of SCF by styrene with metabolic activation
became evident (de Raat, 1978).
Styrene oxide was tested in a mutagenic test screening programme
which involved a sperm abnormality test and dominant lethal test in
male mice exposed to atmospheric concentrations of 15 or 100 ppm for
7 hours per day for 5 days, a cytogenic test in bone marrow celles of
male and female rats exposed in a manner as described previously or a
single exposure of 7 hour duration and a sex-linked recessive lethal
test in Drosophila melanogaster with exposure to atmosphere of
100 ppm for 150 minutes. The results of these studies showed that
styrene oxide had no activity on the frequency of chromosomal
aberrations and sex-linked recessive lethal mutation frequency in
D. melanogaster. In the dominant lethal test a small reduction in
pregnancy frequency and total number of implantations was attributed
to styrene oxide. The highest dosage of the oxide, also resulted in a
slight increase in the frequency of sperm abnormality (McGregor,
1983).
Additional studies with Drosophila melanogaster showed that
both styrene and styrene oxide induce recessive lethal mutations and
that the frequency of their occurence is increased by pretreatment
with either sodium phenobarbitone or trichloropropane oxide (Donner et
al., 1979).
The mutagenic potential of styrene and its 7,8-oxide were studied
in the isolated perfused rat liver as the metabolizing system and
Chinese hamster V79 cells as genetic target cells. The 7,8-oxide was
rapidly metabolized by the liver, and therefore, failed to elicit a
mutagenic effect. In contrast, styrene produced an increase in V79
mutants which seemed not to be due to the formation of the 7,8-oxide,
since simultaneous analysis of its concentration in the perfusion
medium did not correlate with the mutagenic effect (Beije & Jenssen,
1982).
The in vivo exposure of Chinese hamsters to atmospheric
concentrations of styrene oxide from 25 to 100 ppm did not affect the
incidence of chromosomal aberrations and sister chromatid exchanges in
bone marrow cells. To alter the rate of chromosomal aberrations
required the administration of lethal concentrations of the oxide by
the intraperitoneal route (Norppa et al., 1979, 1983).
The daily oral administration of 500 mg of styrene kg/b.w. for
four days or 200 mg of styrene/kg b.w. failed to induce chromosomal
aberrations in the bone marrow cells of CD-1 male mice. Concurrent
pharmacokinetic studies performed on the same animals failed to show
the presence of styrene-7,8-oxide in the blood (at the nanogram level)
(Sbrana et al., 1983).
Styrene produced chromosomal breaks in in vitro preparations of
human lymphocytes, whereas, the oxide was responsible for the
formation of micronuclei and nuclear birdges (Linnainmaa et al.,
1978). In a mutagenic assay in human diploid fibroblasts styrene oxide
failed to elicit an increase in unscheduled DNA synthesis (McGregor,
1983).
Cultures of periphenal lymphocytes of workers employed in
industries with styrene exposure levels which ranged from 30 to
400 mg/mc showed significantly greater frequencies of chromosomal
aberrations than those of a matched (sex, age, age and smoking habit)
control group ( Camurri et al., 1983).
A micronucleus test based on the analysis of lymphocytes with
preserved cytoplasm revealed an increased frequency of micronuclei in
workers exposed to a time-weight average of styrene concentration
between 1 and 36 ppm. These ambient concentrations correlated with low
urinary levels of mandelic acid (Hogstedt et al., 1983).
Styrene and styrene-7,8-oxide induce pronounced dose response
increases in the occurences in sister chromatid exchanges in human
lymphocytes and whole blood cultures. The effect of styrene was
greater in the presence of erythrocytes indicating that styrene must
be converted to the 7,8-oxide for its capability to induce an increase
in the incidence of sister chromatid exchanges (Norppa et al., 1980a,
1983).
Special studies on teratogenicity
The toxicity of styrene and styrene oxide chicken embryos was
studied by injecting from 2 to 100 umol of either compound in 50 ml
of an olive oil-ethanol mixture into the air sacs of eggs from
White-Leghorn SK12 chickens. Malformations were noted in 15% of the
styrene embryos and 7% of the styrene oxide treated embryos. The
embryos were most susceptible on the day of, and the day after the
beginning of incubation (Vainio et al., 1977).
Sprague-Dawley rats in groups of 39, 30 and 29 animals were given
0, 180 and 300 mg styrene/kg b.w./day by gavage from days 6 through 15
of gestation. The styrene was administered as a peanut oil solution at
a volume of 2 mg/kg b.w. as twice daily doses of 90 or 150 mg/kg b.w.
There were no significant differences between the treated-animals and
the controls during gestation except for a diminished weight gain on
days 6 through 9 of gestation that was attributed to a reduction in
food intake. Dams were sacrificed prior to parturition and the fetuses
were examined. The incidence of external visceral and skeletal
malformations in the treated animals did not differ from either the
matched or historical controls. A compound-related effect on the
embryo and fetus was not observed, as was no teratogenic effect.
Styrene at both dosages did induce a decrease in body weight gain and
decreased food consumption in the dams (Murry et al., 1978).
Special studies on reproduction
Water and milk extracts of CNP-2p grade plastics which were
subjected to a vacuum process and contained 80 to 110 ppm styrene were
given every 24 hours to 741 rats in a multi-generation study which was
22 months in duration. A group of animals which received water and
milk served as controls. The parental generation received the extracts
from 1.5 to 13 months of age, while the F1 and F2 generations
were given the extracts from weaning to 8 and 2 months-of-age,
respectively. The administration of the extracts continued throughout
pregnancy and lactation. The animals which received the extracts
demonstrated a statistically reliable decrease in the number of red
blood cells and activity of cholinesterase after a two day fast. A
histological analysis revealed that the extract-treated animals
demonstrated dystrophic changes in the superficial, cortical renal
tubules and a catarrhal state in the duodenum. No other hematological
or histological parameters were found to be adversely altered by the
administration of the extracts. A large number of still-born, poorly
developed and deformed pups were noted to have been born to the
parental and F1 dams which were treated with the extracts as compared
to the control animals. In addition, it was noted that there was a
delay in the growth of fur in the F1 and F2 generations which were
treated with the extracts (Chernova, 1971).
A three-generation reproduction study was conducted as part of a
combined chronic two-year reproduction study in which styrene was
administered in the drinking water of rats. From each group (125 and
250 ppm groups) of the chronic study at least 10 males and 20 females
were mated to produce F1 pups and then subsequent F2 and F3
generations were produced. The following reproductive and
toxicological indices monitored in this study; mean litter size,
live-to-total pup ratios, pup survival indices at intervals from birth
to weaning were evaluated as well as liver and kidney weights of
representative pups necropsied at weaning, cytogenetic evaluation of
bone marrow samples of other weanlings, gross necropsy of F1 and F2
parents, organ weigths and histopathologic examinations of liver and
kidneys of weanlings and of tissues of representative F1 and F2
parents. Styrene had no deleterious effects on the reproductive
capacity of rats through the three generations and on other
toxicological parameters measured (Chemical Manufacturers Association,
1980).
Special studies on enzyme systems
The effect of styrene on carbohydrate balance was studied in
Wistar rats which received a dosage by gavage of 2.5 g/kg b.w. which
was equivalent to 1/2 the LD50. The results indicated that glucose
absorption and hepatic glycogen content were reduced after styrene
administration (Delag et al., 1976).
The effect of orally administered styrene on hepatic mixed
function oxidase enzyme activities, glutathione-S-transferase activity
and glutathione content was studied in male rats. Doses of 250, 450
and 900 mg/kg b.w. were administered for seven days to groups of 15
animals. The two highest dosages enhanced the activity of aryl
hydrocarbon hydroxylase and aniline hydroxylase, while the highest
dosage alone, significantly lowered the activity of glutathione-
S-transferease and glutathione content. In a subsequent study in which
an identical protocol was followed with the exception that only the
two highest dosages were used (450 and 900 mg/kg b.w.) effects on
renal MFO activity and glutathione activity were noted. An increase in
MFO activity was assessed as an increase in the activity of ary
hydrocarbon hydroxylase, aniline hydroxylase ethylmorphine-N-
demethylase and benzphetamine N-demethylase. In addition, the activity
of glutathione-S-transferase was decreased along with the levels of
renal glutathione (Das et al., 1981, 1983).
The intubation of 1 ml/kg b.w. of styrene daily for 15 days to
adult male albino rats elicited a significant increase in serotonin
and noradrenalin levels in the brain without a change in dopamine
content. These changes coincided with a significant decrease in the
activity of monoamine oxidase, however, no change in acetyl
cholinesterase activity was observed (Husain et al., 1980).
Groups of male rats were administered styrene in ground nut oil
by gavage at dosages of 200 and 400 mg/kg b.w. six days per week for
100 days. A dose-dependent increase in hepatic benzo(a)pyrene
hydroxylase and aminopyrine-N-demethylase activities were noted, while
a decrease in the activity of glutathione-S-transferase, mitochondrial
succinic dehydrogenase and B-glucoronidase and no change in glucose-6-
phosphatase were observed (Srivastava et al., 1982).
Special studies on immune function
The effects of styrene on immunological function were studied in
a series of three experiments in 36 rabbits. The animals used in the
study were immunized with lamb erythrocytes for a period of 7 to 8
days beginning with the initiation of dosing with styrene. Styrene was
administered 5 times per week in the form of an emulsion (vegetable
oil and potato starch) according to three different dosing schedules.
These were 250 mg/kg b.w. for 58 days, 5 mg/kg b.w. for 216 days and
0.5 mg/kg b.w. for 202 days. The results of these studies established
that the higher dosages of styrene produced acute variations in the
titer of complement and antibodies. The phagocytic activity of the
leucocytes were also observed to be depressed. These changes were
noted to have occured prior to other indications of styrene
intoxication at the highest dosage. These included a reduction of
appetite and hyperemia of the conjunctiva of the eye (Sinitskii,
1969).
Acute toxicity
Species Route LD50 Reference
Rat oral 5.0 g/kg b.w. Wolf et al., 1956
Rat oral 5.5 g/kg b.w. Ogleznev, 1963
Short-term studies
Mouse
A subchronic study was performed as a range finding study for the
NCI chronic mouse bioassay. Five males and five females were placed in
groups which received dosages of 0, 147, 215, 316, 464 and 681 mg of
styrene/kg b.w. which was administered as a corn-oil gavage five days
per week for seven weeks. A reduced mean body weight gain was noted in
the groups which received 316 and 464 mg/kg b.w., but not the highest
dosage of 681 mg/kg b.w. No other clinical abnormalities were noted to
be related to the exposure to styrene (National Cancer Institute,
1978).
In a one month study in 75 mice styrene at a dosage of 1/10 of
the LD50, 0.55 g/kg b.w., had no effect on the growth of the treated
animals. No other details of the study were provided (Ogleznev, 1963).
Rat
Rats were dosed by gavage with dosages of 0.1, 0.5, 1.0 and 2.0 g
of styrene/kg b.w. emulsified in olive oil with gum arabic. Dosing was
five days per week for 4 weeks. There was an increase in mortality and
pronounced esophageal and gastric irritation in the groups which
received the two highest dosages. Slight esophageal and gastric
irritation were also observed at the next lowest dosage of 0.5 g/kg
b.w., along with poor weigth gains in the five males which survived
the 28-day testing period. The animals which received the lowest
dosage of 0.1 g/kg b.w. were found to be in generally good condition
(Spencer et al., 1942).
Matched groups of ten female rats were dosed by gavage with
dosages of 0, 66.7, 133, 400 and 667 mg of styrene/kg b.w./day five
days per week for six months. The test compound was administered as an
olive-oil solution. The two lowest dosages had no effects on the
animals. However, the two higher dosages did produce, in a
dose-related manner, a slight reduction in the rate of growth and
slight depressions in hepatic and renal weights. Although blood cell
counts were made after 20, 40, 80 and 130 doses no compound related
effects were noted (Wolf et al., 1956).
Groups of five male and five female Fischer 344 rats received
styrene in corn-oil via gavage five days per week for seven weeks at
dosages of 0, 681, 1000, 1470, 2150 and 3160 mg/kg b.w. This was
followed by a one week observation period. The treated males,
particularly at the higher dosages, tended to have lower mean body
weight gains than the controls. At the conclusion of the study no
other compound-related clinical abnormalities were demonstrated
(National Cancer Institue, 1978).
Groups of adult, male rats were administered (six days/week)
styrene in ground nut oil by gavage dosages of 200 or 400 mg/kg b.w.
for a period of 100 days. No overt signs of toxicity were noted in the
treated animals. Weight gain and absolute and relative liver weights
were not different in the control and treated animals. The highest
dose of 400 mg/kg b.w. was associated with tiny areas of focal
necrosis in the liver which consisted of degenerated hepatocytes and
inflammatory cells (Srivastava et al., 1982).
Dog
Groups of four female and male Beagle hounds were administered
styrene by gavage at dosages of 0, 200, 400 or 600 mg/kg b.w./day for
up to 561 days. The styrene was provided as a 50/50 mixture in peanut
oil. The two highest dosages elicited Heinz bodies in the erythrocytes
of the males and occasionally in the females on the lowest dosage.
There were other sporadic changes in various hematological parameters
(decreased packed cell volume, erythrocyte counts and sedimentation
rates and hemoblogin levels and increased incidence of anisocytosis
and hypochromia of erythrocytes, hemosiderin in hepatic
reticuloendothelial cells and numbers of hepatocellular intranuclear
acidophilic crystalline inclusions) which quickly disappeared when the
administration of styrene was terminated. Although body weights, food
consumption, clinical chemistry determinations and organ weights
(brain, heart, liver, kidneys and testes) were studied none were
adversely effected by the administration of styrene (Quast et al.,
1978).
OBSERVATIONS IN MAN
Epidemiological and clinical studies
Experimental exposures to concentrations of styrene from 50 to
800 ppm for varying periods of time have shown depression of the
central nervous (CNS) system. These include reports of listlessness,
drowsiness, incoordination, decrements of manual dexterity, feeling of
intoxication and changes in visual evoked response and EEG amplitude
(Carpenter et al., 1944; Gamberale & Hultengren, 1974; Oltramare et
al., 1974). In contrast other studies have shown that styrene exposure
of from 50 to 350 ppm and again for varying periods of time did not
have effects on CNS function (Stewart et al., 1968; Gamberale &
Hultengren, 1974; Oltramare et al., 1974). In the workplace exposure
to styrene has been attributed to cause increased reaction times,
abnormal EEGs, headache, fatigue, malaise and dizziness (Seppalainen &
Harkonen, 1976; Harkonen et al., 1978; Nicholson et al., 1978; Lorimer
et al., 1976; Rosen et al., 1978; Cherry et al., 1980). There have
also been reports of styrene - induced peripheral neuropathy in the
workplace. In several studies reductions in nerve condition velocites
and mild sensory neuropathy have been noted (Lilis et al., 1978; Rosen
et al., 1978).
Styrene exposure has also resulted in irritation of the eyes,
respiratory tract, throat, and epidermis (Stewart et al., 1968; Gotell
et al., 1972; Araki et al., 1971; McLaughlin, 1946; Rosen et al.,
1978; Oltramare et al., 1974; Lorimer et al., 1976).
There are studies which suggest that styrene exposure in the
workplace has adverse effects on hepatic function. These changes have
included elevated serum enzyme activities, serum uric acid and glucose
tolerance (Lorimer et al., 1976; Axelson & Gustavson, 1978; Chmieleski
et al., 1973; Chmielewski, 1976). This link between styrene exposure
and hepatic dysfunction, however has not been definitively
established. The issue of styrene-induced reproductive and/or
teratogenic effects was raised in a study of congenital defects in
children whose mothers were occupationally exposed to styrene
(Holmberg, 1977) and in a study in which styrene was measured in
umbilical cord blood suggesting the ability of styrene to cross the
placenta (Dowty et al., 1976). In addition, an increased rate of
spontaneous abortions was noted in a study of styrene workers
(Hemminki, 1980). In contrast to these observations another study of
occupationally exposed women failed to establish a link between the
incidence of spontaneous abortions and styrene exposure (Harkonen &
Holmberg, 1982).
Several studies have examined whether styrene can induce a
carcinogenic response in humans. Several mortality studies of
occupationally exposed individuals have failed to show an excess of
deaths due to cancer (Ott et al., 1980; Frentzel-Beyme et al., 1978;
Nicholson et al., 1978). In a proportionate mortality study of 560
styrene-polystyrene polymerization workers over an approximately 15
year period showed a deficit of deaths as compared to the general
population was noted. Among the deaths of the workers were one
leukemia, one lymphoma and an additional death accompanied by leukemia
(Nicholson et al., 1978). There have been only limited epidemiological
studies performed on the possible styrene-induced carcinogenicity in
humans. A mortality study of employees engaged in the development or
manufacture of styrene-based products revealed a statistically
significant increase in the number of leukemias in comparison to other
company employees, however, when the comparison was made with national
statistics the difference was not statistically significant (Ott et
al., 1980). In a study of styrene and polystyrene plant workers in
which 24 deaths certificates were examined there was no evidence of an
association between exposure to styrene and an excess of cancer
(Frentzel-Beyme et al., 1978).
Comments
Styrene readily crosses epithelial surfaces and therefore is
absorbed from the gastrointestinal tract. Once absorbed styrene can be
eliminated unchanged via expiration or it can be converted to a number
of metabolites, including mandelic acid, phenylglyooxylic acid and
hippiuric acid, which in turn are excreted via the urine. The proposed
intermediate in this metabolic scheme is an epoxide, styrene oxide,
however its existence has been established solely in in vitro
experimentation and has never been isolated in vivo.
In studies in laboratory animals styrene has generally been shown
to elicit adverse effects only at high dose levels. These effects
include reductions in body weight gains, generation of Heinz bodies in
erythrocytes, sporadic changes in several hematological parameters,
variations in the titer of complement and antibodies and focal
necrosis in the liver. It is reported to have no teratogenic effect
nor to have adverse consequences on reproductive performance.
Mutagenic studies have generally shown styrene to be inactive,
although under some test conditions chromosomal effects were produced.
However, its metabolite, styrene oxide, has pronounced mutagenic
activity. Lifetime studies in rats have failed to demonstrate a
styrene-induced carcinogenic effect. One in three studies in the mouse
involving in utero exposure provided "limited evidence" of a
carcinogenesis. In contrast, one preliminary study has shown that
styrene oxide has carcinogenic potential.
In humans, styrene has been reported to have depressive effects
on CNS function and is an irritant to epithelial surfaces. There has
been some evidence generated in clinical studies that indicate that
styrene is hepatotoxic, however, the evidence is not conclusive. Data
from human studies have presented suggestive evidence that styrene is
teratogenic, however, the animal studies have not borne this out.
Human clinical and epidemiological studies performed to data have
generally shown that styrene is not carcinogenic and failed to
establish a link between styrene exposure and carcinogenesis.
EVALUATION
Level causing no toxicological effects
Rat: 125 ppm in drinking water, equal to 7.7 mg/kg b.w./day for 2
years.
Estimate of a provisional maximum tolerable daily intake for man
0.04 mg/kg bw.
REFERENCES
ANDERSON, N.C., TRANBERG, F.A., UGGLA, A.H., & ZETTERBERG, G. (1980)
Chromosomal aberrations and sister-chromatid exchanges in lymphocytes
of men occupationally exposed to styrene in a plastic-boat factory.
Mutat. Res., 73(2): 387-401.
ARAKI, S., ABE, A., USHIO, K., & FUJINO, M. (1971) A case of skin
atrophy, neurogenic muscular atrophy and anxiety following long
exposure to styrene. Jpn. J. Ind. Health, 13(5): 527-31.
ASTRAND, I., KILBOM, A., OVRUM, P., WAHLBERG, I., & VESTERBERG, O.
(1974) Exposure to styrene. I. Concentration in alveolar air and blood
at rest and during exercise and metabolism. Work Environ. Health,
11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ. Health,
4(Suppl.2): 215-19.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. Appl. Pharmacol., 16: 691-700.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in S.
typhimurium strains. Int. J. Cancer, 15: 429-437.
BAUER, D., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R., &
TONARELLI, S. (1980) The problem of negative results for styrene in
the in vitro mutagenesis test with metabolic activation (microsomal
assay). 2. Behaviour of epoxide hydrolase in the incubation mixtures.
Boll. Soc. Ital. Biol. Sper., 56(21): 2200-5.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in the liver
perfusion/cell culture systemœ No indication of styrene-7,8-oxide as
the principle mutagenic metabolite produced by the intact rat liver.
Chem. Biol. Interactions, 39(1): 57-76œ
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene oxide in
human blood erythrocytes and lumphocytesœ Res. Commun. Chem.
Pharmacol., 33(2): 273-82œ
BUSK, L. (1979) Mutagenic effects of styrene and styrene oxide.
Mutat. Res., 67(3): 201-208.
CAMURRI, L., CODELUPPI, S., SCARDUELLI, L., & PEDRONI, C. (1983)
Chromosomal aberrations and sister chromatid exchanges in workers
exposed to styrene. Mutat. Res., 119(3-4): 361-370.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., & BELVEDERE
G. (1978) Hepatic and extrahepatic formation and hydration of styrene
oxide in vitro in animals of different species and sex. Toxicol.
Lett., 2: 179-86.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F. Jr. (1944)
Studies on the inhalation of 1,3-butadiene; with a comparison of its
narcotic effect with benzol, toluol, and styrene and a note on the
elimination of styrene by the human. J. Ind. Hyg. Toxicol., 26(3):
69-78œ
CHEMICAL MANUFACTURERS ASSOCIATION (1980) Toxicological study on
styrene incorporated in drinking water of rats for two years in
conjunction with a three-generation reproduction study. Litton
Bionetics.
CHERNOVA, T. (1971) Evaluation of articles made on CNP-2P grade
plastics intended for packaging of food-stuffs. Vopr Pitan, 30:
56-58.
CHERRY, N., WALDRON, H.A., WELLS; G.G., WILKINSON, R.T., WILSON, H.K.,
& JONES, S. (1980) An investigation of the acute behavioural effects
of styrene on factory workers. Brit. J. Ind. Med. 37: 234-40.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R. (1973) Rating
of the exposure to styrene of persons working at the production of
polyesteric laminates. Bull. Inst. Marit. Med. Gdynia, 24:
203-209.
CHMIELEWSKI, J. (1976) Clinical and experimental research into the
pathogenesis of toxic effect of styrene. Part V. Impact of styrene on
carbohydrate balance in people in the course of their work. Bull
Inst. Marit. Trop. Med. Gdynia, 27: 177-84.
DAS, M., DIXIT, R., MUSHTAQ, M., SRIVASTAVA, S.P., & SETH, P.K. (1981)
Effect of styrene on hepatic mixed function oxydases, glutathione
content and glutathione-S-transferase activity in rats. Drug Chem.
Toxicol., 4(3): 219-27.
DAS, M., SRIVASTAVA, S.P., & SETH, P.K. (1983) Effect of styrene on
glutathione content and some xenobiotic metabolizing enzymes of rat
kidney. Acta Pharmacol. Toxicol., 52(1): 47-50.
DELAG, G., CHMIELEWSKI, J., MIKULSKI, P., & WIGLUSZ, R. (1976)
Clinical and experimental research into the pathogenesis of toxic
effect of styrene. Part VI. The effect of styrene on carbohydrate
balance, experimental research. Bull. Inst. Marit. Trop. Med.
Gdynia, 27: 185-91.
DELBRASSINE, I.P., VAN BLADEREN, P.J., SMEETS, F.L., & SEUTTEN-
BERLAGE, F. (1981) Stereoselective oxidation of styrene to styrene
oxide in rats as measured by mercapturic acid excretion.
Xenobiotica, 11(9): 589-94.
DEL CARRATORE, R., GIAGONI, P., BAUER, C., BRONZETTI, G., CORSI, C.,
NIERI, R., & PAOLINI, M. (9183) Mutagenicity of styrene on
metabolizing D7 strain of Saccharomyces cerevisiae. Boll. Soc.,
Ital., Biol. Sper., 59(2): 233-8.
DE MEESTER, C., PONCELET, F., ROBERFROLD, M., RONDELET, J., & MERCIER,
M. (1977) Mutagenicity of sltyrene and styrene oxide. Mut. Res.,
56: 147-52.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M.,
MERCIER, M., & PONCELET, F. (1981) Mutagenicity of styrene in the
Salmonella typhimurium test system. Mutat. Res., Dec. 90:
443-50.
DE RAAT, W.K. (1978) Induction of sister chromatid exchanges by
styrene and its presumed metabolite styrene oxide in the presence of
rat liver homogenate. Chem. Biol. Interactions, 20: 163-70.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals induced
by styrene and styrene oxide in Drosophila melanogaster. Mutat.
Res., 67: 373-6.
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic constituents.
Pediatr. Res., 10: 696-701.
EL MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies in
detoxication. Part 73. The metabolism of alkylbenzenes-phenylacetylene
and phenylethylene (styrene). Biochem. J., 68: 199-20.
FRENTZEL-BEYME, R., THIESS, A.M., & WIELAND, R. (1978) Survey of
mortality among employees engaged in the manufacture of styrene and
polystyrene at the BASF Ludwigshafen works. Scan. J. Work Environ.
Health, 4(Suppl. 2): 231-39.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene. II.
Psychological functions. Work Environ. Health, 11: 86-93.
GOTELL, P., AXELSON, O., & LINDELOF, B. (1972) Field studies on human
styrene exposure. Work Environ. Health, 9(2): 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMANN, W., & KRAMER, M. (1977)
Mutagenicity and chromosomal aberrations as an analytical tool for
in vitro detection of mammalian enzyme-mediated formation of
reactive metabolites. ARch. Toxicol., 39: 159-69.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., & HERNBERG,
S. (1978) Exposure-response relationship between styrene exposure and
central nervous functions. Scand. J. Work Environ. Health, 4:
53-59.
HARKONEN, H. & HOLMBERG, P.C. (1982) Obstetric histories of women
occupationally exposed to styrene. Scand. J. Work Environ. Health,
8: 74-77.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980) Spontaneous abortions
among female chemical workers in Finland. Int. Arch. Occup. Environ.
Health, 45: 123-26.
HINZ, G., GOHLKE, R., & BURCK, D. (1980) The effect of simultaneous
oral application of ethanol and styrene. [1. Acute and subacute
experiments on rats.] J. Hyg. Epidemial. Microbiol. Immunol.,
24(3): 262-270.
HOGSTEDT, B., AKESSON, B., AXELL, K., GULLBERG, B., MITELMAN, F.,
PERO, R.W., SKERFVING, S., & WELINDER, H. (1983) Increased frequency
of lymphocyte micronuclei in workers producing reinforced polyester
resin with low exposure to styrene. Scand. J. Work Environ. Health,
9(3): 241-6.
HOLMBERG, P.C. (1977) Central nervous defects in two children and
mothers exposed to chemicals in the reinforced plastics. Chance or a
causal relation? Scand. J. Work Environ. Health, 3: 212-14.
HUSAIN, R., SRIVASTAVA, S.P., MUSHTAG, M., & SETH, P.K. (1980) Effect
of styrene on levels of serotonin, noradrenaline, dopamine and
activity of acetyl cholinesterase and monamine oxidase in rat brain.
Toxicol. Lett. Nov., 7(1): 47-50.
IARC (1979) Styrene and polymers. IARC Monographs on the evaluation of
the carcinogenic risk of chemicals to humans. Volume 19: 231-274.
IARC (1979) Styrene oxide. IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Volume 19: 275-283.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I. (1974)
Evaluation of hippuric, phenylglyoxylic and mandelic acids in urine as
indices of styrene exposure. Int. Arch. Arbeitsmed., 32(1-2):
93-101.
JAMES, S.P. & WHITE, D.A. (1967) The metabolism of phenethyl bromide,
styrene and styrene oxide in the rabbit and rat. Biochem. J., 104:
914-21.
KAPPUS, H., BOLT, H., BUCHTER, A., & BOLT, W. (1976) Liver microsomal
uptake of 14C vinyl chloride and transformation to protein alkylating
metabolites in vitro. Tox. Appl. Pharm., 37: 461-471.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in liver
microsomes. Biochem. Pharmacol., 18(2): 552-54.
LEIBMAN, K.C. & ORTIZ, E. (1970) Epoxide intermediates in microsomal
oxidation of olefins to glycols. J. Pharmacol. Exp. Ther.,
173(2): 242-46.
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization workers.
Env. Res., 15:133-38.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978)
Cytogenetic effects of styrene and styrene oxide in human lymphocytes
and Allium cepa. Scand. J. Work Environ. Health, 4(Suppl. 2):
156-62.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S., BONATTI,
S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., corti, G., FREZZA, D.,
LEPORINI, C., MAZZACCARO, A., NIERI, R., ROSSELLINI, D., & ROSSI, A.M.
(1976) Mutagenicity of industrial compounds-styrene and its possible
metabolite styrene oxide. Mut. Res., 40: 317-24.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H., FISCHBEIN,
A., DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J. (1976) Clinical
studies of styrene workers. Initial findings. Environ. Health
Perspect.; 17: 171-81
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First experimental
demonstration of the carcinogenic effects of styrene oxide. Long term
bioassays on Sprague-Dawley rats by oral administration. Med.
Lavoro, 5: 358-62.
McGREGOR, D.B. (1983) Tier II Mutagenic Screening of 13 NIOSH Priority
Compounds; Individual Compound Report; Styrene oxide. Govt. Reports
Announcements & Index (GRA&I), Issue 05.
McLAUGHLIN, R.S. (1946) Chemical burns of the human cornea. Amr.
J.Ophthalmol., 29(11): 1353-62.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene oxide
(1,2-epoxyethylbenzene), a presumed styrene metabolikte. Mut. Res.,
40: 15-18.
MURRAY, F.J., JOHN, J.A., BALMER, M.F., & SCHWETZ, B.A. (1978)
Teratologic evaluation of styrene given to rats and rabbits by
inhalation or by gavage. Toxicology, 11: 335-343.
NATIONAL CANCER INSTITUTE (1978) Carcinogenesis Technical Report
Series. Bioassay of styrene for possible carcinogenicity. No. 185.
NICHOLSON, W., SELIKOFF, I., & SEIDMAN, H. (1978) Mortality experience
of styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4(suppl. 2); 247-252.
NORPPA, H., FLOVAARA, F., HUSGAFVEL-PURSLAINEN, K., SORSA, M., &
VAINIO, H. (1979) Effects of styrene oxide on chromosome aberrations,
sister chromatid exchange and hepatic drug biotransformation in
Chinese hamsters in vivo. Chem. Biol. Interactions, 26: 305-15.
NORPPA, H., SORSA, M., PFAEFFLI, P., & VAINIO, H. (1980) Styrene and
styrene oxide induce SCEs and are metabolised in human lymphocyte
cultures. Carcinogenesis, 1(4): 357-61.
NORPPA, H., SORSA, M., & VAINIO, H. (1980) Chromosomal aberrations in
bone marrow of Chinese hamsters exposed to styrene and ethanol.
Toxicol. Lett., 5: 241-44.
NORPPA, H., VAINIO, H., & SORSA, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res., Aug. 43(8):
3579-82.
NORPPA, H. & VAINIO, H. (1983) Genetic toxicity of styrene and some of
its derivatives. Scand. J. Work Environ. Health, 9(2): 108-14.
OGLEZNEV, G. (1963) Comparative characteristics of the toxic action of
aqueous solution of methylostyrene and styrene. Tr. Omskogo Med.
Inst., 48: 164-174.
OLTRAMARE, M., DESBAUMES, F., IMHOFF, C., & MICHEIELS, W. (1974)
Toxicology of monomeric styrene. Experimental and clinical studies on
man. Geneva, Editions Médecine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J., &
VENABLE, J.R. (1980) A mortality survey of employees engaged in the
development of manufacture of styrene-based products. J. Occup.
Med., 22(7): 445-60.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G., MUSSINI, E.,
& SALMONA, M. (1979) Isolation and structure determination of
enzymatically formed styrene oxide glutathione conjugates. Chem.
Biol. Interactions, 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O., BEND, J.R., & ZEIGER, E.
(1982) Mutagenicity of (R) and (S) styrene 7,8-oxide and the
intermediary mercapturic acid metabolites formed from styrene
7,8-oxide. Environ. Mutagenesis, 4: 575-584.
PANTAROTTO, C., FANELLI, R., BIDOLI, F., MORAZZONI, P., SOMMONA, M., &
SZCZAURINSKA, K. (1978) Arene oxides in styrene metabolksm, a new
perspective in styrene toxicity? Scand. J. Work Environ. Health,
4(suppl. 2): 67-77.
PFAEFFLI, P., HESSO, A., VAINIO, H., & HYVOENEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in workers
occupationally exposed to styrene. Toxicol. Appl. Pharmacol.,
60(1): 85-90.
PLOTNICK, H.B. & WEIGEL, W.W. (1979) Tissue distribution and excretion
of 14C-styrene in male and female rats. Res. Commun. Chem. Pathol.
Pharmacol., 24(3): 515-524.
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work Environ.
Health, 4(suppl. 2): 127-135.
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G., MURRAY, F.J.,
JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a toxicity study in dogs
and teratogenicity studies in rabbits and rats administered monomeric
styrene. Toxicol. Appl. Pharmacol., 45: 293-4.
RADEVA, M. & KRUSTEV, L. (1982) Studies on the effect of styrene
monomer on albino rats after long-term oral administration. Khig.
Zdraveopaz., vol. 25. ISS 5: 464-8.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H. (1978)
Neurophysiological observations after chronic styrene exposure.
Scand. J. Work Environ. Health, 4(suppl. 2); 184-94.
RYAN, A.J. & BEND, J.R. (1977) The metabolism of styrene oxide in the
isolated perfused rat liver. Drug Metab. Dispose., 5(4): 363-7.
SALMONA, M., PACKECKA, J., CANTONI, L., BELVEDERE, G., MUSSINI, E., &
GARATTINI, S. (1976) Microsomal styrene monoxygenase and styrene
epoxide hydrase activities in rats. Xenobiotica, 6(10): 585-91.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate of orally
administered styrene in rats. Midland, MI, Dow Chemical USA, December,
42 pp.
SBRANA, I. LASCIALFARI, D., ROSSI, A.M., LOPRIENO, N., BIANCHI, M.,
TORTORETO; M., & PANTAROTTO, C, (1983) Bone marrow cell chromosomal
aberrations and styrene biotransformation in mice given styrene on a
repeated oral schedule. Chem. Biol. Interact., Aug. 1, 45(3):
349-57.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological findings
among workers occupationally exposed to styrene. Scan. J. Work
Environ. Health, 3: 140-46.
SINITSKII, V (1969) Indexes of immunological reactivity in rabbits
during prolonged exposure to styrene in small doses. Gig. Premen,
Polem. Mater. Igdelii Nikk No. 1: 394-8.
SPENCER, H., IRISH, D., ADAMS, E., & ROWE, V. (1942) The response of
laboratory animals to monomenic styrene. J. Ind. Hyg. Tox., 24:
295-301.
SRIVESTAVA, S., DAS, M., MUSHTAG, M., SHANDRA, S., & SETH, P. (1982)
Hepatic effects of orally administered styrene in rats. J. Appl.
Tox., 2: 219-222.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W. (1968)
Human exposure to styrene vapor. Arch. Environ. Health, 16:
656-62.
STOLTZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of styrene and
styrene epoxide in Salmonella typhimurium. Bull. Environ. Contam.
Toxicol., 17(6): 739-42.
TURSI, F., SAMAIA, M., SALMONA, M., & BELVEDERE, G. (1983) Styrene
oxidation to styrene oxide in human erythrocytes is catalysed by
oxyhemoglobin. Experimentia, vol. 39, ISS 6, 593-4.
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., & PELKONEN, O.
(1976) A study on the mutagenic activity of styrene and styrene oxide.
Scand. J. Work Environ. Health, 3: 147-51.
VAINIO, H., HEMMINKI, K., & FLOVAARA, E. (1977) Toxicity of styrene
and styrene oxide on chick embryos. Toxicology, 8: 319-25.
WATABE, T., ISOBE, M. SAWAHATA, T., YOSHIKAWA, K., YAMADA, S., &
TAKABATAKE, E. (1978a) Metabolism and mutagenicity of styrene.
Scand. J. Work Environ. Health, 4(suppl. 2): 142-55.
WATABE, T., ISOBE, M,, YOSHIKAWA, K, & TAKABATAKE, E., (1978b) Studies
on metabolism and toxicity of styrene. Part I. Biotransformation of
styrene to styrene glycol via styrene oxide by rat liver microsomes.
J. Pharmacobio. Dyn., 1:(2): 98-104.
WATABE, T., AIZAWA, T., SAWAHATA, T., OZAWA, N., ISOBE, M.,
TAKABATAKE, E., HIRATASUKA, A. (1982) Metabolism and toxicity of
styrene: 4.1-vinylbenzene 3,4-oxide, a potent mutagen formed as a
possible intermediate in the metabolism in vivo os styrene to
4-vinylpenol. Mutat. Res., 93(1): 45-56.
WOLF, M.A., ROWE, V.K., McCOLLISTER, D.D., HOLLINGSWORTH, R.L., &
OYEN, F. (1956) Toxicological studies of certain alkylated benzenes
and benzene-experiments on laboratory animals. AMA Arch. In. Health,
14: 387-98.
See Also:
Toxicological Abbreviations
Styrene (EHC 26, 1983)
Styrene (ICSC)
STYRENE (JECFA Evaluation)
Styrene (PIM 509)
Styrene (IARC Summary & Evaluation, Volume 60, 1994)
Styrene (IARC Summary & Evaluation, Volume 82, 2002)
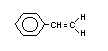 Styrene is also known as ethenylbenzene, vinylbenzene,
vinylbenzol, styrolene, styrol, styropol, styropor, styrone,
cinnamene, cinnamol, phenethylene, phenylethylene and phenylethane. It
is a colourless, viscous liquid with a melting point of -30.6°C and a
boiling point of 145.2°C. It is insoluble in water, soluble in
ethanol, ether and acetone and very soluble in benzene and petroleum
ether. Its uses include intermediate in the manufacture of plastics,
elastomers and resins which in turn are used in packaging foods, food
products and various other processes in the food industry. It is also
used as a synthetic flavouring substance and adjuvant.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies with laboratory animals have shown that styrene is
readily absorbed from the gastrointestinal tract following oral
administration (Plotnick & Weigel, 1979; Sauerhoff et al., 1976).
The distribution and excretion of a single oral dose of 20 mg
14C-styrene/kg b.w. was studied in male and female Charles-River
rats. The peak tissue levels occurred four hours after administration,
with the kidneys exhibiting the highest concentration of 14C per unit
weight, followed by the liver and pancreas. The primary route of
elimination was via urinary excretion with 90% of the dosage detected
in the urine within 24 hours, while less than 2% was detected in the
feces (Plotnick & Weigel, 1979).
The disposition of orally administered styrene was studied in
mature Sprague-Dawley rats (Spartan substrain) in which doses of 50 or
500 mg/kg 14C-styrene were administered via gavage. Approximately 95
and 90% of the 50 and 500 mg/kg b.w. doses, respectively, were
eliminated in the urine as styrene metabolites. The pulmonary route of
elimination accounted for 1.3% of the lower dosage and 8.9% of the
higher dosage. There was a distinct sex difference in the pulmonary
elimination of styrene with males expiring twice as much styrene as
the females. The fecal route of elimination accounted for only about
4% of the administered dose (Sauerhoff et al., 1976).
Metabolism
A number of metabolic studies in laboratory animals which have
utilized a variety of routes of administration have shown that styrene
is metabolized by the mixed function oxidase (MFO) system to
styrene-7, 8-oxide, and that this occurs not only in the liver but in
a number of other tissue types and organs (Leibman & Ortiz, 1970;
Salmona et al., 1976; Cantoni et al., 1978). The metabolic activation
by the microsomal system depends on the presence of necessary
cofactors for a NADPH generating system and oxygen (Kappus et al.,
1971; Bartsch et al., 1975; Salmona et al, 1976). The oxide can be
metabolized further to styrene glycol by the action of epoxide
hydrolase (Leibman & Ortiz, 1969 and 1970; Ryan & Bend, 1977).
Generally the greatest activity for the MFO and hydrolase have been
detected in the liver with the activity of the latter being greater
than that of the former (Cantoni et al., 1978). The glycol in turn can
be converted to mandelic acid, phenylglyoxylic acid and finally to
hippuric acid or it can be conjugated to glucuronic acid (Ryan & Bend,
1977; El Masri et al., 1958; Sauerhoff et al., 1976; James & White,
1967; Ikeda et al., 1974; Astrand et al., 1974; Gotell et al., 1972).
The oxide can also be conjugated with glutathione to form three
mercapturic acid derivatives, N-acetyl-s-(1-phenyl-2-hydroxyethyl)
cysteine and N-acetyl-s-(phenylacetyl) cysteine. Other metabolites
which have been identified include mercapturic acids, benzoic acid,
4-vinylphenol, 2-phenylethanol, phenaceturic acid and phenylglycol
all of which may be excreted as conjugates of glucuronic acid or
glutathione (Sauerhoff et al., 1976; Bakke & Scheline, 1970;
Delbrassine et al., 1981; El Masri et al., 1958). Studies in rats have
shown that the opening of the epoxide ring of styrene by glutathione
5-transferase is stereospecific with a preference for the R-isomer
(Delbrassine et al., 1981).
There has also been evidence generated that styrene is converted
to an arene oxide, since an intermediate, styrene-3,4-oxide, undergoes
isomerization to 4-vinylphenol which has been isolated in the urine of
both animals and men who have been exposed to styrene (Pantarotto et
al., 1978; Bakke & Scheline, 1970; Pfäffli et al., 1981; James &
White, 1967; Pachecka et al., 1979). However, this route of metabolism
is considered to be a minor one, since much more mandelic acid is
detected in the urine as compared to the 4-vinylphenol.
In vitro preparations of human erythrocytes and lymphocytes
have been shown to catalyze the oxidation of styrene to styrene oxide.
This reaction is inhibited by the presence of CO, but not by
superoxide dismutase, catalase and scavengers of hydroxyl radicals,
and it required the presence of oxygen. These data indicate that
oxyhemoglobin and not free oxygen radicals can be involved in styrene
oxidation (Tursi et al, 1983; Belvedere & Tursi, 1981).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Carcinogenicity data on styrene and styrene oxide were reviewed
by the International Agency for Research on Cancer (IARC) in 1979.
Mouse
A lifetime study following in utero exposure was conducted with
C57B1 black mice. Fifteen dams were administered a single dose of
300 mg of styrene/kg b.w. dissolved in olive oil via intubation on the
17th day of gestation. A vehicle (olive oil) treated group of 57 males
and 49 females served as a control group. Styrene was then given
weekly at the same dose level and via the same route to 27 male and 27
female offspring from weaning up to 120 weeks of age. A group of 12
males and 13 females which were the offspring of five dams treated
with olive oil on the 17th day of gestation received olive oil on a
weekly basis after weaning. The incidence of neonatal mortality of the
styrene-treated group was 35%. In the parental generation lymphomas
were noted in 10 of 12 styrene-treated animals as compared to 3 of 5
of the vehicle treated animals. In the male progeny hepatocellular
carcinomas were found in 3 of 4 animals. This compares to an occurence
of 1 to 12 of vehicle-treated controls and 1 of 47 untreated controls
(P>0.05). The incidences of all other tumor-types were similar in the
styrene-treated and control animals (Ponomarkov & Tomatis, 1978).
A group of 29 pregnant O20 mice received a single dose of
1350 mg/kg b.w. of styrene by gavage (dissolved in olive oil) on the
17th day of gestation, while a control group received olive oil alone.
This same dosage of styrene was administered to the offspring
(39 females and 45 males) from weaning to 16 weeks of age. Treatment
was terminated at this point because of excessive mortality. The most
frequently observed lesions in the styrene-treated offspring which
expired before the twentieth week were multiple centrilobular necrosis
of the liver, hypoplasia of the spleen and severe congestion of
the lungs. In those animals that expired after the forty-fifth
week multiple abscess-like cavities in the liver filled with
polymorphonuclear leukocytes were noted. The study was terminated when
the last animal had expired at 100 weeks. There were no differences
in tumor incidences between the dams treated with styrene and those
treated with olive oil. In the progeny pulmonary adenomas and
adenoearcinomas were noted in 20/23 males and 32/32 females treated
with styrene and in 8/19 males and 14/21 females in these animals
treated with olive oil alone (P>0.01, P<0.01). However, in untreated
controls 34/53 males and 25/42 females were found to also have
pulmonary adenomas and adenocarcinomas (P<0.05, P<0.01). The average
age at death of the pulmonary tumor-bearing, styrene-treated mice was
32 and 49 weeks for the males and females, respectively. In contrast,
the olive oil-treated males and females exhibited an average age of 88
and 85 weeks, while the average age of mortality of the untreated
control males and females was 94 and 99 weeks, respectively. No other
differences in tumor incidences were noted between the various groups
(Ponomarkov & Tomatis, 1978).
Groups of 50 male and 50 female B6C3F1 mice were dosed daily by
gavage five days per week with styrene dissolved in corn oil at dose
levels equivalent to 150 and 300 mg/kg b.w. Treatment was for 78 weeks
and was followed by a 13 week observation period. A group of 20
animals of each sex served as a vehicle corn oil control group. In the
males, but not females, there was a significant positive relationship
between mortality and dosage. A slight, dose-related mean body weight
depression was noted in the females, but not the males, while no other
clinical annormalities were noted. There were adequate numbers of both
males and females at risk for late-developing tumors. In the low
dosage group 92 percent (46/50) and 80 percent (40/50) of the males
and females, respectively, survived on test until the termination of
the study. The percentages of survival for the high dosage group was
78 and 76 for the males and females, respectively, and for the
controls 100 and 90 percent for the respective sexes.
During the course of the study, five low dose females were found
missing. A variety of neoplastic lesions were found in both the
styrene-treated and control animals, however, with the exception of
pulmonary tumors, the incidences neoplastic lesions were unrelated to
the administration of styrene. There was a significant increase in the
incidence of a combination of pulmonary adenomas and carcinomas in the
styrene-treated male mice with 0/20, 6/45 and 9/49 of the control, low
dosage and high dosage animals demonstrating these lesions (P = 0.024
for Fischer exact test comparing high dose and control). However, a
definitive conclusion on the carcinogenicity of styrene could not be
drawn because of the high variability of the incidence of these
neoplasia in historical control mice at the laboratory which performed
the study. The historical incidence of the combination of alveolar/
bronchiolar adenomas and aleolar/bronchiolar carcinomas in male mice
in this laboratory is 12 percent (32/271). The authors concluded that
there was no convincing evidence for carcinogenicity of styrene in the
mouse study. (National Cancer Institute, 1978)
Rat
A group of 21 BD IV rats were given 1350 mg of styrene/kg b.w.
in olive oil orally on the 17th day of gestation. Their offspring (73
males and 71 females) then received 500 mg/kg b.w. styrene weekly from
weaning for 120 weeks. Ten dams and their offsprings (36 males and 39
females) received olive oil and served as controls. The average litter
sizes of the different groups were similar. The mortality of the
offspring of styrene-treated dams (10%) was greater than that of
the controls (2.5%), but not significantly. There was no evidence
of a difference in body weight gain and survival between the
styrene-treated and control offspring. The incidence of survival were
8/73 and 20/71 of the male and female, styrene-treated animals and
14/36 and 18/39 male and female control animals. The incidence of
tumor-bearing dams was greater in the styrene-treated group than
the control group, but the difference was not significant. Three
neurogenic and three stomach tumors were observed in the
styrene-treated progeny that were not observed in the controls
(Ponomarkov & Tomatis, 1978).
A study was carried out with Fischer 344 rats in which groups of
50 males and 50 females were administered styrene dissolved in corn
oil five days per week by gavage at doses equivalent to 500, 1000 and
2000 mg/kg b.w. Treatment was for 103 weeks for the low dosage group
and 78 weeks for the medium and high dosage groups. This was followed
by an observation period of one week for the low dosage groups and
twenty-seven weeks for the medium and high dosage groups. A group of
40 animals of each sex served as vehicle controls (corn oil). The
highest dosage resulted in a significantly earlier and higher
mortality in both sexes, wherein, an additional dosed group of each
sex was included in the bioassay. The survival of the other dosage
groups were not adversely affected by styrene administration. By week
53 only 12 percent (6/50) of the males had survived, while only 14
percent (7/50) of the females had survived by week 70. The survival
incidences for the other groups were 88 percent (44/59), 94 percent
(47/50) for the low and medium dose males and 92 percent (46/50) for
the low and medium dose females. The various control groups
demonstrated survival incidences which varied from 85 to 90 percent
and 75 to 90 percent for the male and female controls. Adequate
numbers of animals were at risk in the low and medium dosage groups
from late-developing tumors. A dose-related reduction in the mean body
weight was observed in the treated males, however, the relevance of
this observation is questionable considering the decreased survival in
the high dosage group. No other compound-related clinical signs were
observed. None of the statistical tests for any site of tumors in rats
of either sex showed a significant positive association between the
administration of styrene and an increased tumor incidence (National
Cancer Institute, 1978).
In a two-year study in Sprague-Dawley rats styrene was
administered in the drinking water at intended concentrations of
125 and 250 ppm which resulted in approximate intakes of 7.7 and
14 mg/kg b.w./day for the males and 12 and 21 mg/kg b.w./day for the
females. Each treatment group was initially comprised of 50 males and
70 females, and a group of 76 males and 106 females served as
controls. The following parameters were assessed during the course of
the study; body weight gain, food and water intake, hemograms,
clinical chemistry, urinanalysis, clinical signs, mortality and gross
necropsy and histological examination at interim and terminal
sacrifices and of those animals which died during the course of the
study or were sacrificed in a moribund condition. Styrene did not
produce any changes in the above parameters with the exception that
the terminal bodyweights of the high dosage females were less than
that of the controls. Mortality throughout the study was no different
in the three groups with 34 (control), 23 (low dose) and 19 (high) of
the males and 46 (control), 30 (low dose) and 26 (high dose) of the
females expiring before termination of the study. Styrene did not
produce either gross or histological changes, nor was there an
apparent styrene-related increase in tumor incidence. All tumors noted
were common, spontaneously occuring tumors for this strain of rat or
rare tumors that occurred without regard to treatment group (Chemical
Manufacturers Association, 1980).
The long-term effects of styrene were assessed in two groups of
Wistar rats. Groups each consisted of 15 male and 15 male Wistar rats
and received a fatty solution of the monomer in dosages of either 1 or
5 mg/kg b.w. by mouth for a period of 10 months. The treatment was
then discontinued and the animals were periodically followed for up to
10 months. A postmortum examination was performed on all animals which
expired during the study and on those animals which were sacrificed at
the end of the 20th months. Two tumors were identified in the inquinal
region in females which received the highest dosage of styrene with
one tumor appearing at five months post-exposure and the other at ten
months post-exposure. Histological evaluation of the tumors revealed
that they were well developed fibroadenomas. The authors concluded
that the appearance of the tumors was associated with administration
of the styrene (Radeva & Krustev, 1982).
Groups of 40 male and 40 female Sprague-Dawley rats were
administered styrene oxide in olive oil by gavage at dosages
equivalent to 0, 50 and 250 mg/kg b.w. four or five days per week for
52 weeks, whereupon, the treatment was terminated and the animals were
permitted to live until their deaths. The numbers of males which
survived until the fifty-first week of the study were 37/40, 31/40 and
28/40 of the control, low and high dosage groups. The incidences of
survival for the females were 28/40, 31/40 and 30/40 of the control,
low and high dosage groups. The histological analysis revealed that
both dosages of the oxide elicited a higher, dose-related incidence of
papillomas and carcinomas of the forestomach than the control animals
with 19.3% of the low dosage animals and 50% of the high dosage
animals demonstrating these lesions. Many of the carcinomas were
observed to have metastasized to the liver, and in addition, many of
the oxide-treated animals were noted to have precursor lesions
(Maltoni et al., 1979).
Special studies on mutagenicity
Styrene and styrene epoxide were studied in spot tests and plate
incorporation assays for activity agains S. typhimurium straing
TA-98, 100, 1535, 1537 and 1538 with and without hepatic metabolizing
systems from Arochlor 1254 - pretreated rats and hamsters. Styrene was
not mutagenic for any of the tester strains, whereas, the epoxide was
active in strains TA-100 and 1535 in the spot and plate assays without
activation. Activity in these strains indicate that the epoxide acted
as a base substituted mutagen (Stoltz & Withey, 1977; Busk, 1979).
In other studies styrene was shown to be mutagenic to strains
TA-100 and 1535, but only after metabolic activation. Styrene oxide in
these studies was mutagenic, with and without metabolic activities
(Vainio et al., 1976; de Meester et al., 1977; Watabe et al., 1978a,
1978b; Loprieno et al., 1978; Milvy & Garro, 1976; Greim et al.,
1977).
The putative styrene intermediate, 1-vinylbenzene-3,4-oxide,
which has a half-life of 4.3 seconds at pH 7.4 in aqueous solution
proved to be a potent mutagen toward S. typhimurium, TA-100, but not
TA-98. This intermediate also demonstrated potent cytotoxicity to both
His + revertant colonies. The final metabolite, 4-vinylphenol, which
is the metabolite to which the oxide is converted proved to be neither
mutagenic or cytotoxic in these studies (Watabe et al., 1982).
The two enantiomers of styrene 7,8-oxide and various thioether
conjugates of racemic styrene 7,8-oxide were tested for mutagenic
activity in S. typhimurium TA-100. The results of these studies
suggested that the (R) enantiomer is more mutagenic than the (S)
enantiomer, with the racemic mixture demonstrating intermediate
activity and the conjugates exhibiting no activity (Pagano et al.,
1982).
The activities of epoxide hydrolase and monoxygenase were studied
in incubation mixtures for liver microsomal assays with S-9 metabolic
activation in the absence or presence of styrene from rats and mice.
The results of this study showed that the activity of the hydrolase is
more stable than that of the monoxygenase. This is further evidence
that in the S. typhimurium mutagenic assay system styrene shows
equivocal results because the styrene epoxide is being hydrolyzed at a
fairly constant rate while its formation from styrene is occurring at
decreasing rates (Bauer et al., 1980).
In a metabolizing, D7 strain of Saccharomyces cerevisiae
styrene induced an increase in the frequency of cross-over, gene
conversion and point mutation (Del Carratore et al., 1983). In a
series of mutagenic assays which measured forward mutations in
Schizosaccharomyces pombe and V-29 Chinese hamster cells and gene
conversions in S. cerevisiae in the diploid strain D4, styrene oxide
was active in the forward mutation experiments with yeast and Chinese
hamster cells and the production of gene-conversion in yeast. In
contrast, styrene was inactive in these assays, even in the presence
of purified mouse - liver microsomes. In a host-mediated assay which
utilized S. pombe both compounds were active in the production of
gene conversion, but not for forward mutation (Loprieno et al., 1976).
In subsequent studies styrene oxide, but not styrene, was shown to
have mutagenic activity in unscheduled DNA synthesis (UDS) studies in
a human heterploid cell line and in the induction of chromosomal
aberrations in the bone marrow of CD-1 mice treated by gavage with a
0.5 ml of solution 66% in olive oil. The UDS studies also utilized a
mouse liver homogenate, but styrene still failed to demonstrate
activity (Loprieno et al., 1978).
Styrene oxide in Chinese hamster ovary cells was a potent inducer
of sister chromatid exchanges (SCF), while styrene did not increase
the number of SCF per metaphase. The effect of the oxide was
diminished by the presence of a rat S9 metabolic activation system,
whereas, it had no effect on the lack of activity of styrene. In
contrast, when an inhibitor of epoxide hydrolase, cyclohexene oxide,
was used the induction of SCF by styrene with metabolic activation
became evident (de Raat, 1978).
Styrene oxide was tested in a mutagenic test screening programme
which involved a sperm abnormality test and dominant lethal test in
male mice exposed to atmospheric concentrations of 15 or 100 ppm for
7 hours per day for 5 days, a cytogenic test in bone marrow celles of
male and female rats exposed in a manner as described previously or a
single exposure of 7 hour duration and a sex-linked recessive lethal
test in Drosophila melanogaster with exposure to atmosphere of
100 ppm for 150 minutes. The results of these studies showed that
styrene oxide had no activity on the frequency of chromosomal
aberrations and sex-linked recessive lethal mutation frequency in
D. melanogaster. In the dominant lethal test a small reduction in
pregnancy frequency and total number of implantations was attributed
to styrene oxide. The highest dosage of the oxide, also resulted in a
slight increase in the frequency of sperm abnormality (McGregor,
1983).
Additional studies with Drosophila melanogaster showed that
both styrene and styrene oxide induce recessive lethal mutations and
that the frequency of their occurence is increased by pretreatment
with either sodium phenobarbitone or trichloropropane oxide (Donner et
al., 1979).
The mutagenic potential of styrene and its 7,8-oxide were studied
in the isolated perfused rat liver as the metabolizing system and
Chinese hamster V79 cells as genetic target cells. The 7,8-oxide was
rapidly metabolized by the liver, and therefore, failed to elicit a
mutagenic effect. In contrast, styrene produced an increase in V79
mutants which seemed not to be due to the formation of the 7,8-oxide,
since simultaneous analysis of its concentration in the perfusion
medium did not correlate with the mutagenic effect (Beije & Jenssen,
1982).
The in vivo exposure of Chinese hamsters to atmospheric
concentrations of styrene oxide from 25 to 100 ppm did not affect the
incidence of chromosomal aberrations and sister chromatid exchanges in
bone marrow cells. To alter the rate of chromosomal aberrations
required the administration of lethal concentrations of the oxide by
the intraperitoneal route (Norppa et al., 1979, 1983).
The daily oral administration of 500 mg of styrene kg/b.w. for
four days or 200 mg of styrene/kg b.w. failed to induce chromosomal
aberrations in the bone marrow cells of CD-1 male mice. Concurrent
pharmacokinetic studies performed on the same animals failed to show
the presence of styrene-7,8-oxide in the blood (at the nanogram level)
(Sbrana et al., 1983).
Styrene produced chromosomal breaks in in vitro preparations of
human lymphocytes, whereas, the oxide was responsible for the
formation of micronuclei and nuclear birdges (Linnainmaa et al.,
1978). In a mutagenic assay in human diploid fibroblasts styrene oxide
failed to elicit an increase in unscheduled DNA synthesis (McGregor,
1983).
Cultures of periphenal lymphocytes of workers employed in
industries with styrene exposure levels which ranged from 30 to
400 mg/mc showed significantly greater frequencies of chromosomal
aberrations than those of a matched (sex, age, age and smoking habit)
control group ( Camurri et al., 1983).
A micronucleus test based on the analysis of lymphocytes with
preserved cytoplasm revealed an increased frequency of micronuclei in
workers exposed to a time-weight average of styrene concentration
between 1 and 36 ppm. These ambient concentrations correlated with low
urinary levels of mandelic acid (Hogstedt et al., 1983).
Styrene and styrene-7,8-oxide induce pronounced dose response
increases in the occurences in sister chromatid exchanges in human
lymphocytes and whole blood cultures. The effect of styrene was
greater in the presence of erythrocytes indicating that styrene must
be converted to the 7,8-oxide for its capability to induce an increase
in the incidence of sister chromatid exchanges (Norppa et al., 1980a,
1983).
Special studies on teratogenicity
The toxicity of styrene and styrene oxide chicken embryos was
studied by injecting from 2 to 100 umol of either compound in 50 ml
of an olive oil-ethanol mixture into the air sacs of eggs from
White-Leghorn SK12 chickens. Malformations were noted in 15% of the
styrene embryos and 7% of the styrene oxide treated embryos. The
embryos were most susceptible on the day of, and the day after the
beginning of incubation (Vainio et al., 1977).
Sprague-Dawley rats in groups of 39, 30 and 29 animals were given
0, 180 and 300 mg styrene/kg b.w./day by gavage from days 6 through 15
of gestation. The styrene was administered as a peanut oil solution at
a volume of 2 mg/kg b.w. as twice daily doses of 90 or 150 mg/kg b.w.
There were no significant differences between the treated-animals and
the controls during gestation except for a diminished weight gain on
days 6 through 9 of gestation that was attributed to a reduction in
food intake. Dams were sacrificed prior to parturition and the fetuses
were examined. The incidence of external visceral and skeletal
malformations in the treated animals did not differ from either the
matched or historical controls. A compound-related effect on the
embryo and fetus was not observed, as was no teratogenic effect.
Styrene at both dosages did induce a decrease in body weight gain and
decreased food consumption in the dams (Murry et al., 1978).
Special studies on reproduction
Water and milk extracts of CNP-2p grade plastics which were
subjected to a vacuum process and contained 80 to 110 ppm styrene were
given every 24 hours to 741 rats in a multi-generation study which was
22 months in duration. A group of animals which received water and
milk served as controls. The parental generation received the extracts
from 1.5 to 13 months of age, while the F1 and F2 generations
were given the extracts from weaning to 8 and 2 months-of-age,
respectively. The administration of the extracts continued throughout
pregnancy and lactation. The animals which received the extracts
demonstrated a statistically reliable decrease in the number of red
blood cells and activity of cholinesterase after a two day fast. A
histological analysis revealed that the extract-treated animals
demonstrated dystrophic changes in the superficial, cortical renal
tubules and a catarrhal state in the duodenum. No other hematological
or histological parameters were found to be adversely altered by the
administration of the extracts. A large number of still-born, poorly
developed and deformed pups were noted to have been born to the
parental and F1 dams which were treated with the extracts as compared
to the control animals. In addition, it was noted that there was a
delay in the growth of fur in the F1 and F2 generations which were
treated with the extracts (Chernova, 1971).
A three-generation reproduction study was conducted as part of a
combined chronic two-year reproduction study in which styrene was
administered in the drinking water of rats. From each group (125 and
250 ppm groups) of the chronic study at least 10 males and 20 females
were mated to produce F1 pups and then subsequent F2 and F3
generations were produced. The following reproductive and
toxicological indices monitored in this study; mean litter size,
live-to-total pup ratios, pup survival indices at intervals from birth
to weaning were evaluated as well as liver and kidney weights of
representative pups necropsied at weaning, cytogenetic evaluation of
bone marrow samples of other weanlings, gross necropsy of F1 and F2
parents, organ weigths and histopathologic examinations of liver and
kidneys of weanlings and of tissues of representative F1 and F2
parents. Styrene had no deleterious effects on the reproductive
capacity of rats through the three generations and on other
toxicological parameters measured (Chemical Manufacturers Association,
1980).
Special studies on enzyme systems
The effect of styrene on carbohydrate balance was studied in
Wistar rats which received a dosage by gavage of 2.5 g/kg b.w. which
was equivalent to 1/2 the LD50. The results indicated that glucose
absorption and hepatic glycogen content were reduced after styrene
administration (Delag et al., 1976).
The effect of orally administered styrene on hepatic mixed
function oxidase enzyme activities, glutathione-S-transferase activity
and glutathione content was studied in male rats. Doses of 250, 450
and 900 mg/kg b.w. were administered for seven days to groups of 15
animals. The two highest dosages enhanced the activity of aryl
hydrocarbon hydroxylase and aniline hydroxylase, while the highest
dosage alone, significantly lowered the activity of glutathione-
S-transferease and glutathione content. In a subsequent study in which
an identical protocol was followed with the exception that only the
two highest dosages were used (450 and 900 mg/kg b.w.) effects on
renal MFO activity and glutathione activity were noted. An increase in
MFO activity was assessed as an increase in the activity of ary
hydrocarbon hydroxylase, aniline hydroxylase ethylmorphine-N-
demethylase and benzphetamine N-demethylase. In addition, the activity
of glutathione-S-transferase was decreased along with the levels of
renal glutathione (Das et al., 1981, 1983).
The intubation of 1 ml/kg b.w. of styrene daily for 15 days to
adult male albino rats elicited a significant increase in serotonin
and noradrenalin levels in the brain without a change in dopamine
content. These changes coincided with a significant decrease in the
activity of monoamine oxidase, however, no change in acetyl
cholinesterase activity was observed (Husain et al., 1980).
Groups of male rats were administered styrene in ground nut oil
by gavage at dosages of 200 and 400 mg/kg b.w. six days per week for
100 days. A dose-dependent increase in hepatic benzo(a)pyrene
hydroxylase and aminopyrine-N-demethylase activities were noted, while
a decrease in the activity of glutathione-S-transferase, mitochondrial
succinic dehydrogenase and B-glucoronidase and no change in glucose-6-
phosphatase were observed (Srivastava et al., 1982).
Special studies on immune function
The effects of styrene on immunological function were studied in
a series of three experiments in 36 rabbits. The animals used in the
study were immunized with lamb erythrocytes for a period of 7 to 8
days beginning with the initiation of dosing with styrene. Styrene was
administered 5 times per week in the form of an emulsion (vegetable
oil and potato starch) according to three different dosing schedules.
These were 250 mg/kg b.w. for 58 days, 5 mg/kg b.w. for 216 days and
0.5 mg/kg b.w. for 202 days. The results of these studies established
that the higher dosages of styrene produced acute variations in the
titer of complement and antibodies. The phagocytic activity of the
leucocytes were also observed to be depressed. These changes were
noted to have occured prior to other indications of styrene
intoxication at the highest dosage. These included a reduction of
appetite and hyperemia of the conjunctiva of the eye (Sinitskii,
1969).
Acute toxicity
Species Route LD50 Reference
Rat oral 5.0 g/kg b.w. Wolf et al., 1956
Rat oral 5.5 g/kg b.w. Ogleznev, 1963
Short-term studies
Mouse
A subchronic study was performed as a range finding study for the
NCI chronic mouse bioassay. Five males and five females were placed in
groups which received dosages of 0, 147, 215, 316, 464 and 681 mg of
styrene/kg b.w. which was administered as a corn-oil gavage five days
per week for seven weeks. A reduced mean body weight gain was noted in
the groups which received 316 and 464 mg/kg b.w., but not the highest
dosage of 681 mg/kg b.w. No other clinical abnormalities were noted to
be related to the exposure to styrene (National Cancer Institute,
1978).
In a one month study in 75 mice styrene at a dosage of 1/10 of
the LD50, 0.55 g/kg b.w., had no effect on the growth of the treated
animals. No other details of the study were provided (Ogleznev, 1963).
Rat
Rats were dosed by gavage with dosages of 0.1, 0.5, 1.0 and 2.0 g
of styrene/kg b.w. emulsified in olive oil with gum arabic. Dosing was
five days per week for 4 weeks. There was an increase in mortality and
pronounced esophageal and gastric irritation in the groups which
received the two highest dosages. Slight esophageal and gastric
irritation were also observed at the next lowest dosage of 0.5 g/kg
b.w., along with poor weigth gains in the five males which survived
the 28-day testing period. The animals which received the lowest
dosage of 0.1 g/kg b.w. were found to be in generally good condition
(Spencer et al., 1942).
Matched groups of ten female rats were dosed by gavage with
dosages of 0, 66.7, 133, 400 and 667 mg of styrene/kg b.w./day five
days per week for six months. The test compound was administered as an
olive-oil solution. The two lowest dosages had no effects on the
animals. However, the two higher dosages did produce, in a
dose-related manner, a slight reduction in the rate of growth and
slight depressions in hepatic and renal weights. Although blood cell
counts were made after 20, 40, 80 and 130 doses no compound related
effects were noted (Wolf et al., 1956).
Groups of five male and five female Fischer 344 rats received
styrene in corn-oil via gavage five days per week for seven weeks at
dosages of 0, 681, 1000, 1470, 2150 and 3160 mg/kg b.w. This was
followed by a one week observation period. The treated males,
particularly at the higher dosages, tended to have lower mean body
weight gains than the controls. At the conclusion of the study no
other compound-related clinical abnormalities were demonstrated
(National Cancer Institue, 1978).
Groups of adult, male rats were administered (six days/week)
styrene in ground nut oil by gavage dosages of 200 or 400 mg/kg b.w.
for a period of 100 days. No overt signs of toxicity were noted in the
treated animals. Weight gain and absolute and relative liver weights
were not different in the control and treated animals. The highest
dose of 400 mg/kg b.w. was associated with tiny areas of focal
necrosis in the liver which consisted of degenerated hepatocytes and
inflammatory cells (Srivastava et al., 1982).
Dog
Groups of four female and male Beagle hounds were administered
styrene by gavage at dosages of 0, 200, 400 or 600 mg/kg b.w./day for
up to 561 days. The styrene was provided as a 50/50 mixture in peanut
oil. The two highest dosages elicited Heinz bodies in the erythrocytes
of the males and occasionally in the females on the lowest dosage.
There were other sporadic changes in various hematological parameters
(decreased packed cell volume, erythrocyte counts and sedimentation
rates and hemoblogin levels and increased incidence of anisocytosis
and hypochromia of erythrocytes, hemosiderin in hepatic
reticuloendothelial cells and numbers of hepatocellular intranuclear
acidophilic crystalline inclusions) which quickly disappeared when the
administration of styrene was terminated. Although body weights, food
consumption, clinical chemistry determinations and organ weights
(brain, heart, liver, kidneys and testes) were studied none were
adversely effected by the administration of styrene (Quast et al.,
1978).
OBSERVATIONS IN MAN
Epidemiological and clinical studies
Experimental exposures to concentrations of styrene from 50 to
800 ppm for varying periods of time have shown depression of the
central nervous (CNS) system. These include reports of listlessness,
drowsiness, incoordination, decrements of manual dexterity, feeling of
intoxication and changes in visual evoked response and EEG amplitude
(Carpenter et al., 1944; Gamberale & Hultengren, 1974; Oltramare et
al., 1974). In contrast other studies have shown that styrene exposure
of from 50 to 350 ppm and again for varying periods of time did not
have effects on CNS function (Stewart et al., 1968; Gamberale &
Hultengren, 1974; Oltramare et al., 1974). In the workplace exposure
to styrene has been attributed to cause increased reaction times,
abnormal EEGs, headache, fatigue, malaise and dizziness (Seppalainen &
Harkonen, 1976; Harkonen et al., 1978; Nicholson et al., 1978; Lorimer
et al., 1976; Rosen et al., 1978; Cherry et al., 1980). There have
also been reports of styrene - induced peripheral neuropathy in the
workplace. In several studies reductions in nerve condition velocites
and mild sensory neuropathy have been noted (Lilis et al., 1978; Rosen
et al., 1978).
Styrene exposure has also resulted in irritation of the eyes,
respiratory tract, throat, and epidermis (Stewart et al., 1968; Gotell
et al., 1972; Araki et al., 1971; McLaughlin, 1946; Rosen et al.,
1978; Oltramare et al., 1974; Lorimer et al., 1976).
There are studies which suggest that styrene exposure in the
workplace has adverse effects on hepatic function. These changes have
included elevated serum enzyme activities, serum uric acid and glucose
tolerance (Lorimer et al., 1976; Axelson & Gustavson, 1978; Chmieleski
et al., 1973; Chmielewski, 1976). This link between styrene exposure
and hepatic dysfunction, however has not been definitively
established. The issue of styrene-induced reproductive and/or
teratogenic effects was raised in a study of congenital defects in
children whose mothers were occupationally exposed to styrene
(Holmberg, 1977) and in a study in which styrene was measured in
umbilical cord blood suggesting the ability of styrene to cross the
placenta (Dowty et al., 1976). In addition, an increased rate of
spontaneous abortions was noted in a study of styrene workers
(Hemminki, 1980). In contrast to these observations another study of
occupationally exposed women failed to establish a link between the
incidence of spontaneous abortions and styrene exposure (Harkonen &
Holmberg, 1982).
Several studies have examined whether styrene can induce a
carcinogenic response in humans. Several mortality studies of
occupationally exposed individuals have failed to show an excess of
deaths due to cancer (Ott et al., 1980; Frentzel-Beyme et al., 1978;
Nicholson et al., 1978). In a proportionate mortality study of 560
styrene-polystyrene polymerization workers over an approximately 15
year period showed a deficit of deaths as compared to the general
population was noted. Among the deaths of the workers were one
leukemia, one lymphoma and an additional death accompanied by leukemia
(Nicholson et al., 1978). There have been only limited epidemiological
studies performed on the possible styrene-induced carcinogenicity in
humans. A mortality study of employees engaged in the development or
manufacture of styrene-based products revealed a statistically
significant increase in the number of leukemias in comparison to other
company employees, however, when the comparison was made with national
statistics the difference was not statistically significant (Ott et
al., 1980). In a study of styrene and polystyrene plant workers in
which 24 deaths certificates were examined there was no evidence of an
association between exposure to styrene and an excess of cancer
(Frentzel-Beyme et al., 1978).
Comments
Styrene readily crosses epithelial surfaces and therefore is
absorbed from the gastrointestinal tract. Once absorbed styrene can be
eliminated unchanged via expiration or it can be converted to a number
of metabolites, including mandelic acid, phenylglyooxylic acid and
hippiuric acid, which in turn are excreted via the urine. The proposed
intermediate in this metabolic scheme is an epoxide, styrene oxide,
however its existence has been established solely in in vitro
experimentation and has never been isolated in vivo.
In studies in laboratory animals styrene has generally been shown
to elicit adverse effects only at high dose levels. These effects
include reductions in body weight gains, generation of Heinz bodies in
erythrocytes, sporadic changes in several hematological parameters,
variations in the titer of complement and antibodies and focal
necrosis in the liver. It is reported to have no teratogenic effect
nor to have adverse consequences on reproductive performance.
Mutagenic studies have generally shown styrene to be inactive,
although under some test conditions chromosomal effects were produced.
However, its metabolite, styrene oxide, has pronounced mutagenic
activity. Lifetime studies in rats have failed to demonstrate a
styrene-induced carcinogenic effect. One in three studies in the mouse
involving in utero exposure provided "limited evidence" of a
carcinogenesis. In contrast, one preliminary study has shown that
styrene oxide has carcinogenic potential.
In humans, styrene has been reported to have depressive effects
on CNS function and is an irritant to epithelial surfaces. There has
been some evidence generated in clinical studies that indicate that
styrene is hepatotoxic, however, the evidence is not conclusive. Data
from human studies have presented suggestive evidence that styrene is
teratogenic, however, the animal studies have not borne this out.
Human clinical and epidemiological studies performed to data have
generally shown that styrene is not carcinogenic and failed to
establish a link between styrene exposure and carcinogenesis.
EVALUATION
Level causing no toxicological effects
Rat: 125 ppm in drinking water, equal to 7.7 mg/kg b.w./day for 2
years.
Estimate of a provisional maximum tolerable daily intake for man
0.04 mg/kg bw.
REFERENCES
ANDERSON, N.C., TRANBERG, F.A., UGGLA, A.H., & ZETTERBERG, G. (1980)
Chromosomal aberrations and sister-chromatid exchanges in lymphocytes
of men occupationally exposed to styrene in a plastic-boat factory.
Mutat. Res., 73(2): 387-401.
ARAKI, S., ABE, A., USHIO, K., & FUJINO, M. (1971) A case of skin
atrophy, neurogenic muscular atrophy and anxiety following long
exposure to styrene. Jpn. J. Ind. Health, 13(5): 527-31.
ASTRAND, I., KILBOM, A., OVRUM, P., WAHLBERG, I., & VESTERBERG, O.
(1974) Exposure to styrene. I. Concentration in alveolar air and blood
at rest and during exercise and metabolism. Work Environ. Health,
11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ. Health,
4(Suppl.2): 215-19.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. Appl. Pharmacol., 16: 691-700.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in S.
typhimurium strains. Int. J. Cancer, 15: 429-437.
BAUER, D., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R., &
TONARELLI, S. (1980) The problem of negative results for styrene in
the in vitro mutagenesis test with metabolic activation (microsomal
assay). 2. Behaviour of epoxide hydrolase in the incubation mixtures.
Boll. Soc. Ital. Biol. Sper., 56(21): 2200-5.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in the liver
perfusion/cell culture systemœ No indication of styrene-7,8-oxide as
the principle mutagenic metabolite produced by the intact rat liver.
Chem. Biol. Interactions, 39(1): 57-76œ
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene oxide in
human blood erythrocytes and lumphocytesœ Res. Commun. Chem.
Pharmacol., 33(2): 273-82œ
BUSK, L. (1979) Mutagenic effects of styrene and styrene oxide.
Mutat. Res., 67(3): 201-208.
CAMURRI, L., CODELUPPI, S., SCARDUELLI, L., & PEDRONI, C. (1983)
Chromosomal aberrations and sister chromatid exchanges in workers
exposed to styrene. Mutat. Res., 119(3-4): 361-370.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., & BELVEDERE
G. (1978) Hepatic and extrahepatic formation and hydration of styrene
oxide in vitro in animals of different species and sex. Toxicol.
Lett., 2: 179-86.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F. Jr. (1944)
Studies on the inhalation of 1,3-butadiene; with a comparison of its
narcotic effect with benzol, toluol, and styrene and a note on the
elimination of styrene by the human. J. Ind. Hyg. Toxicol., 26(3):
69-78œ
CHEMICAL MANUFACTURERS ASSOCIATION (1980) Toxicological study on
styrene incorporated in drinking water of rats for two years in
conjunction with a three-generation reproduction study. Litton
Bionetics.
CHERNOVA, T. (1971) Evaluation of articles made on CNP-2P grade
plastics intended for packaging of food-stuffs. Vopr Pitan, 30:
56-58.
CHERRY, N., WALDRON, H.A., WELLS; G.G., WILKINSON, R.T., WILSON, H.K.,
& JONES, S. (1980) An investigation of the acute behavioural effects
of styrene on factory workers. Brit. J. Ind. Med. 37: 234-40.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R. (1973) Rating
of the exposure to styrene of persons working at the production of
polyesteric laminates. Bull. Inst. Marit. Med. Gdynia, 24:
203-209.
CHMIELEWSKI, J. (1976) Clinical and experimental research into the
pathogenesis of toxic effect of styrene. Part V. Impact of styrene on
carbohydrate balance in people in the course of their work. Bull
Inst. Marit. Trop. Med. Gdynia, 27: 177-84.
DAS, M., DIXIT, R., MUSHTAQ, M., SRIVASTAVA, S.P., & SETH, P.K. (1981)
Effect of styrene on hepatic mixed function oxydases, glutathione
content and glutathione-S-transferase activity in rats. Drug Chem.
Toxicol., 4(3): 219-27.
DAS, M., SRIVASTAVA, S.P., & SETH, P.K. (1983) Effect of styrene on
glutathione content and some xenobiotic metabolizing enzymes of rat
kidney. Acta Pharmacol. Toxicol., 52(1): 47-50.
DELAG, G., CHMIELEWSKI, J., MIKULSKI, P., & WIGLUSZ, R. (1976)
Clinical and experimental research into the pathogenesis of toxic
effect of styrene. Part VI. The effect of styrene on carbohydrate
balance, experimental research. Bull. Inst. Marit. Trop. Med.
Gdynia, 27: 185-91.
DELBRASSINE, I.P., VAN BLADEREN, P.J., SMEETS, F.L., & SEUTTEN-
BERLAGE, F. (1981) Stereoselective oxidation of styrene to styrene
oxide in rats as measured by mercapturic acid excretion.
Xenobiotica, 11(9): 589-94.
DEL CARRATORE, R., GIAGONI, P., BAUER, C., BRONZETTI, G., CORSI, C.,
NIERI, R., & PAOLINI, M. (9183) Mutagenicity of styrene on
metabolizing D7 strain of Saccharomyces cerevisiae. Boll. Soc.,
Ital., Biol. Sper., 59(2): 233-8.
DE MEESTER, C., PONCELET, F., ROBERFROLD, M., RONDELET, J., & MERCIER,
M. (1977) Mutagenicity of sltyrene and styrene oxide. Mut. Res.,
56: 147-52.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M.,
MERCIER, M., & PONCELET, F. (1981) Mutagenicity of styrene in the
Salmonella typhimurium test system. Mutat. Res., Dec. 90:
443-50.
DE RAAT, W.K. (1978) Induction of sister chromatid exchanges by
styrene and its presumed metabolite styrene oxide in the presence of
rat liver homogenate. Chem. Biol. Interactions, 20: 163-70.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals induced
by styrene and styrene oxide in Drosophila melanogaster. Mutat.
Res., 67: 373-6.
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic constituents.
Pediatr. Res., 10: 696-701.
EL MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies in
detoxication. Part 73. The metabolism of alkylbenzenes-phenylacetylene
and phenylethylene (styrene). Biochem. J., 68: 199-20.
FRENTZEL-BEYME, R., THIESS, A.M., & WIELAND, R. (1978) Survey of
mortality among employees engaged in the manufacture of styrene and
polystyrene at the BASF Ludwigshafen works. Scan. J. Work Environ.
Health, 4(Suppl. 2): 231-39.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene. II.
Psychological functions. Work Environ. Health, 11: 86-93.
GOTELL, P., AXELSON, O., & LINDELOF, B. (1972) Field studies on human
styrene exposure. Work Environ. Health, 9(2): 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMANN, W., & KRAMER, M. (1977)
Mutagenicity and chromosomal aberrations as an analytical tool for
in vitro detection of mammalian enzyme-mediated formation of
reactive metabolites. ARch. Toxicol., 39: 159-69.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., & HERNBERG,
S. (1978) Exposure-response relationship between styrene exposure and
central nervous functions. Scand. J. Work Environ. Health, 4:
53-59.
HARKONEN, H. & HOLMBERG, P.C. (1982) Obstetric histories of women
occupationally exposed to styrene. Scand. J. Work Environ. Health,
8: 74-77.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980) Spontaneous abortions
among female chemical workers in Finland. Int. Arch. Occup. Environ.
Health, 45: 123-26.
HINZ, G., GOHLKE, R., & BURCK, D. (1980) The effect of simultaneous
oral application of ethanol and styrene. [1. Acute and subacute
experiments on rats.] J. Hyg. Epidemial. Microbiol. Immunol.,
24(3): 262-270.
HOGSTEDT, B., AKESSON, B., AXELL, K., GULLBERG, B., MITELMAN, F.,
PERO, R.W., SKERFVING, S., & WELINDER, H. (1983) Increased frequency
of lymphocyte micronuclei in workers producing reinforced polyester
resin with low exposure to styrene. Scand. J. Work Environ. Health,
9(3): 241-6.
HOLMBERG, P.C. (1977) Central nervous defects in two children and
mothers exposed to chemicals in the reinforced plastics. Chance or a
causal relation? Scand. J. Work Environ. Health, 3: 212-14.
HUSAIN, R., SRIVASTAVA, S.P., MUSHTAG, M., & SETH, P.K. (1980) Effect
of styrene on levels of serotonin, noradrenaline, dopamine and
activity of acetyl cholinesterase and monamine oxidase in rat brain.
Toxicol. Lett. Nov., 7(1): 47-50.
IARC (1979) Styrene and polymers. IARC Monographs on the evaluation of
the carcinogenic risk of chemicals to humans. Volume 19: 231-274.
IARC (1979) Styrene oxide. IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Volume 19: 275-283.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I. (1974)
Evaluation of hippuric, phenylglyoxylic and mandelic acids in urine as
indices of styrene exposure. Int. Arch. Arbeitsmed., 32(1-2):
93-101.
JAMES, S.P. & WHITE, D.A. (1967) The metabolism of phenethyl bromide,
styrene and styrene oxide in the rabbit and rat. Biochem. J., 104:
914-21.
KAPPUS, H., BOLT, H., BUCHTER, A., & BOLT, W. (1976) Liver microsomal
uptake of 14C vinyl chloride and transformation to protein alkylating
metabolites in vitro. Tox. Appl. Pharm., 37: 461-471.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in liver
microsomes. Biochem. Pharmacol., 18(2): 552-54.
LEIBMAN, K.C. & ORTIZ, E. (1970) Epoxide intermediates in microsomal
oxidation of olefins to glycols. J. Pharmacol. Exp. Ther.,
173(2): 242-46.
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization workers.
Env. Res., 15:133-38.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978)
Cytogenetic effects of styrene and styrene oxide in human lymphocytes
and Allium cepa. Scand. J. Work Environ. Health, 4(Suppl. 2):
156-62.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S., BONATTI,
S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., corti, G., FREZZA, D.,
LEPORINI, C., MAZZACCARO, A., NIERI, R., ROSSELLINI, D., & ROSSI, A.M.
(1976) Mutagenicity of industrial compounds-styrene and its possible
metabolite styrene oxide. Mut. Res., 40: 317-24.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H., FISCHBEIN,
A., DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J. (1976) Clinical
studies of styrene workers. Initial findings. Environ. Health
Perspect.; 17: 171-81
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First experimental
demonstration of the carcinogenic effects of styrene oxide. Long term
bioassays on Sprague-Dawley rats by oral administration. Med.
Lavoro, 5: 358-62.
McGREGOR, D.B. (1983) Tier II Mutagenic Screening of 13 NIOSH Priority
Compounds; Individual Compound Report; Styrene oxide. Govt. Reports
Announcements & Index (GRA&I), Issue 05.
McLAUGHLIN, R.S. (1946) Chemical burns of the human cornea. Amr.
J.Ophthalmol., 29(11): 1353-62.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene oxide
(1,2-epoxyethylbenzene), a presumed styrene metabolikte. Mut. Res.,
40: 15-18.
MURRAY, F.J., JOHN, J.A., BALMER, M.F., & SCHWETZ, B.A. (1978)
Teratologic evaluation of styrene given to rats and rabbits by
inhalation or by gavage. Toxicology, 11: 335-343.
NATIONAL CANCER INSTITUTE (1978) Carcinogenesis Technical Report
Series. Bioassay of styrene for possible carcinogenicity. No. 185.
NICHOLSON, W., SELIKOFF, I., & SEIDMAN, H. (1978) Mortality experience
of styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4(suppl. 2); 247-252.
NORPPA, H., FLOVAARA, F., HUSGAFVEL-PURSLAINEN, K., SORSA, M., &
VAINIO, H. (1979) Effects of styrene oxide on chromosome aberrations,
sister chromatid exchange and hepatic drug biotransformation in
Chinese hamsters in vivo. Chem. Biol. Interactions, 26: 305-15.
NORPPA, H., SORSA, M., PFAEFFLI, P., & VAINIO, H. (1980) Styrene and
styrene oxide induce SCEs and are metabolised in human lymphocyte
cultures. Carcinogenesis, 1(4): 357-61.
NORPPA, H., SORSA, M., & VAINIO, H. (1980) Chromosomal aberrations in
bone marrow of Chinese hamsters exposed to styrene and ethanol.
Toxicol. Lett., 5: 241-44.
NORPPA, H., VAINIO, H., & SORSA, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res., Aug. 43(8):
3579-82.
NORPPA, H. & VAINIO, H. (1983) Genetic toxicity of styrene and some of
its derivatives. Scand. J. Work Environ. Health, 9(2): 108-14.
OGLEZNEV, G. (1963) Comparative characteristics of the toxic action of
aqueous solution of methylostyrene and styrene. Tr. Omskogo Med.
Inst., 48: 164-174.
OLTRAMARE, M., DESBAUMES, F., IMHOFF, C., & MICHEIELS, W. (1974)
Toxicology of monomeric styrene. Experimental and clinical studies on
man. Geneva, Editions Médecine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J., &
VENABLE, J.R. (1980) A mortality survey of employees engaged in the
development of manufacture of styrene-based products. J. Occup.
Med., 22(7): 445-60.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G., MUSSINI, E.,
& SALMONA, M. (1979) Isolation and structure determination of
enzymatically formed styrene oxide glutathione conjugates. Chem.
Biol. Interactions, 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O., BEND, J.R., & ZEIGER, E.
(1982) Mutagenicity of (R) and (S) styrene 7,8-oxide and the
intermediary mercapturic acid metabolites formed from styrene
7,8-oxide. Environ. Mutagenesis, 4: 575-584.
PANTAROTTO, C., FANELLI, R., BIDOLI, F., MORAZZONI, P., SOMMONA, M., &
SZCZAURINSKA, K. (1978) Arene oxides in styrene metabolksm, a new
perspective in styrene toxicity? Scand. J. Work Environ. Health,
4(suppl. 2): 67-77.
PFAEFFLI, P., HESSO, A., VAINIO, H., & HYVOENEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in workers
occupationally exposed to styrene. Toxicol. Appl. Pharmacol.,
60(1): 85-90.
PLOTNICK, H.B. & WEIGEL, W.W. (1979) Tissue distribution and excretion
of 14C-styrene in male and female rats. Res. Commun. Chem. Pathol.
Pharmacol., 24(3): 515-524.
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work Environ.
Health, 4(suppl. 2): 127-135.
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G., MURRAY, F.J.,
JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a toxicity study in dogs
and teratogenicity studies in rabbits and rats administered monomeric
styrene. Toxicol. Appl. Pharmacol., 45: 293-4.
RADEVA, M. & KRUSTEV, L. (1982) Studies on the effect of styrene
monomer on albino rats after long-term oral administration. Khig.
Zdraveopaz., vol. 25. ISS 5: 464-8.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H. (1978)
Neurophysiological observations after chronic styrene exposure.
Scand. J. Work Environ. Health, 4(suppl. 2); 184-94.
RYAN, A.J. & BEND, J.R. (1977) The metabolism of styrene oxide in the
isolated perfused rat liver. Drug Metab. Dispose., 5(4): 363-7.
SALMONA, M., PACKECKA, J., CANTONI, L., BELVEDERE, G., MUSSINI, E., &
GARATTINI, S. (1976) Microsomal styrene monoxygenase and styrene
epoxide hydrase activities in rats. Xenobiotica, 6(10): 585-91.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate of orally
administered styrene in rats. Midland, MI, Dow Chemical USA, December,
42 pp.
SBRANA, I. LASCIALFARI, D., ROSSI, A.M., LOPRIENO, N., BIANCHI, M.,
TORTORETO; M., & PANTAROTTO, C, (1983) Bone marrow cell chromosomal
aberrations and styrene biotransformation in mice given styrene on a
repeated oral schedule. Chem. Biol. Interact., Aug. 1, 45(3):
349-57.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological findings
among workers occupationally exposed to styrene. Scan. J. Work
Environ. Health, 3: 140-46.
SINITSKII, V (1969) Indexes of immunological reactivity in rabbits
during prolonged exposure to styrene in small doses. Gig. Premen,
Polem. Mater. Igdelii Nikk No. 1: 394-8.
SPENCER, H., IRISH, D., ADAMS, E., & ROWE, V. (1942) The response of
laboratory animals to monomenic styrene. J. Ind. Hyg. Tox., 24:
295-301.
SRIVESTAVA, S., DAS, M., MUSHTAG, M., SHANDRA, S., & SETH, P. (1982)
Hepatic effects of orally administered styrene in rats. J. Appl.
Tox., 2: 219-222.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W. (1968)
Human exposure to styrene vapor. Arch. Environ. Health, 16:
656-62.
STOLTZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of styrene and
styrene epoxide in Salmonella typhimurium. Bull. Environ. Contam.
Toxicol., 17(6): 739-42.
TURSI, F., SAMAIA, M., SALMONA, M., & BELVEDERE, G. (1983) Styrene
oxidation to styrene oxide in human erythrocytes is catalysed by
oxyhemoglobin. Experimentia, vol. 39, ISS 6, 593-4.
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., & PELKONEN, O.
(1976) A study on the mutagenic activity of styrene and styrene oxide.
Scand. J. Work Environ. Health, 3: 147-51.
VAINIO, H., HEMMINKI, K., & FLOVAARA, E. (1977) Toxicity of styrene
and styrene oxide on chick embryos. Toxicology, 8: 319-25.
WATABE, T., ISOBE, M. SAWAHATA, T., YOSHIKAWA, K., YAMADA, S., &
TAKABATAKE, E. (1978a) Metabolism and mutagenicity of styrene.
Scand. J. Work Environ. Health, 4(suppl. 2): 142-55.
WATABE, T., ISOBE, M,, YOSHIKAWA, K, & TAKABATAKE, E., (1978b) Studies
on metabolism and toxicity of styrene. Part I. Biotransformation of
styrene to styrene glycol via styrene oxide by rat liver microsomes.
J. Pharmacobio. Dyn., 1:(2): 98-104.
WATABE, T., AIZAWA, T., SAWAHATA, T., OZAWA, N., ISOBE, M.,
TAKABATAKE, E., HIRATASUKA, A. (1982) Metabolism and toxicity of
styrene: 4.1-vinylbenzene 3,4-oxide, a potent mutagen formed as a
possible intermediate in the metabolism in vivo os styrene to
4-vinylpenol. Mutat. Res., 93(1): 45-56.
WOLF, M.A., ROWE, V.K., McCOLLISTER, D.D., HOLLINGSWORTH, R.L., &
OYEN, F. (1956) Toxicological studies of certain alkylated benzenes
and benzene-experiments on laboratory animals. AMA Arch. In. Health,
14: 387-98.
Styrene is also known as ethenylbenzene, vinylbenzene,
vinylbenzol, styrolene, styrol, styropol, styropor, styrone,
cinnamene, cinnamol, phenethylene, phenylethylene and phenylethane. It
is a colourless, viscous liquid with a melting point of -30.6°C and a
boiling point of 145.2°C. It is insoluble in water, soluble in
ethanol, ether and acetone and very soluble in benzene and petroleum
ether. Its uses include intermediate in the manufacture of plastics,
elastomers and resins which in turn are used in packaging foods, food
products and various other processes in the food industry. It is also
used as a synthetic flavouring substance and adjuvant.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies with laboratory animals have shown that styrene is
readily absorbed from the gastrointestinal tract following oral
administration (Plotnick & Weigel, 1979; Sauerhoff et al., 1976).
The distribution and excretion of a single oral dose of 20 mg
14C-styrene/kg b.w. was studied in male and female Charles-River
rats. The peak tissue levels occurred four hours after administration,
with the kidneys exhibiting the highest concentration of 14C per unit
weight, followed by the liver and pancreas. The primary route of
elimination was via urinary excretion with 90% of the dosage detected
in the urine within 24 hours, while less than 2% was detected in the
feces (Plotnick & Weigel, 1979).
The disposition of orally administered styrene was studied in
mature Sprague-Dawley rats (Spartan substrain) in which doses of 50 or
500 mg/kg 14C-styrene were administered via gavage. Approximately 95
and 90% of the 50 and 500 mg/kg b.w. doses, respectively, were
eliminated in the urine as styrene metabolites. The pulmonary route of
elimination accounted for 1.3% of the lower dosage and 8.9% of the
higher dosage. There was a distinct sex difference in the pulmonary
elimination of styrene with males expiring twice as much styrene as
the females. The fecal route of elimination accounted for only about
4% of the administered dose (Sauerhoff et al., 1976).
Metabolism
A number of metabolic studies in laboratory animals which have
utilized a variety of routes of administration have shown that styrene
is metabolized by the mixed function oxidase (MFO) system to
styrene-7, 8-oxide, and that this occurs not only in the liver but in
a number of other tissue types and organs (Leibman & Ortiz, 1970;
Salmona et al., 1976; Cantoni et al., 1978). The metabolic activation
by the microsomal system depends on the presence of necessary
cofactors for a NADPH generating system and oxygen (Kappus et al.,
1971; Bartsch et al., 1975; Salmona et al, 1976). The oxide can be
metabolized further to styrene glycol by the action of epoxide
hydrolase (Leibman & Ortiz, 1969 and 1970; Ryan & Bend, 1977).
Generally the greatest activity for the MFO and hydrolase have been
detected in the liver with the activity of the latter being greater
than that of the former (Cantoni et al., 1978). The glycol in turn can
be converted to mandelic acid, phenylglyoxylic acid and finally to
hippuric acid or it can be conjugated to glucuronic acid (Ryan & Bend,
1977; El Masri et al., 1958; Sauerhoff et al., 1976; James & White,
1967; Ikeda et al., 1974; Astrand et al., 1974; Gotell et al., 1972).
The oxide can also be conjugated with glutathione to form three
mercapturic acid derivatives, N-acetyl-s-(1-phenyl-2-hydroxyethyl)
cysteine and N-acetyl-s-(phenylacetyl) cysteine. Other metabolites
which have been identified include mercapturic acids, benzoic acid,
4-vinylphenol, 2-phenylethanol, phenaceturic acid and phenylglycol
all of which may be excreted as conjugates of glucuronic acid or
glutathione (Sauerhoff et al., 1976; Bakke & Scheline, 1970;
Delbrassine et al., 1981; El Masri et al., 1958). Studies in rats have
shown that the opening of the epoxide ring of styrene by glutathione
5-transferase is stereospecific with a preference for the R-isomer
(Delbrassine et al., 1981).
There has also been evidence generated that styrene is converted
to an arene oxide, since an intermediate, styrene-3,4-oxide, undergoes
isomerization to 4-vinylphenol which has been isolated in the urine of
both animals and men who have been exposed to styrene (Pantarotto et
al., 1978; Bakke & Scheline, 1970; Pfäffli et al., 1981; James &
White, 1967; Pachecka et al., 1979). However, this route of metabolism
is considered to be a minor one, since much more mandelic acid is
detected in the urine as compared to the 4-vinylphenol.
In vitro preparations of human erythrocytes and lymphocytes
have been shown to catalyze the oxidation of styrene to styrene oxide.
This reaction is inhibited by the presence of CO, but not by
superoxide dismutase, catalase and scavengers of hydroxyl radicals,
and it required the presence of oxygen. These data indicate that
oxyhemoglobin and not free oxygen radicals can be involved in styrene
oxidation (Tursi et al, 1983; Belvedere & Tursi, 1981).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Carcinogenicity data on styrene and styrene oxide were reviewed
by the International Agency for Research on Cancer (IARC) in 1979.
Mouse
A lifetime study following in utero exposure was conducted with
C57B1 black mice. Fifteen dams were administered a single dose of
300 mg of styrene/kg b.w. dissolved in olive oil via intubation on the
17th day of gestation. A vehicle (olive oil) treated group of 57 males
and 49 females served as a control group. Styrene was then given
weekly at the same dose level and via the same route to 27 male and 27
female offspring from weaning up to 120 weeks of age. A group of 12
males and 13 females which were the offspring of five dams treated
with olive oil on the 17th day of gestation received olive oil on a
weekly basis after weaning. The incidence of neonatal mortality of the
styrene-treated group was 35%. In the parental generation lymphomas
were noted in 10 of 12 styrene-treated animals as compared to 3 of 5
of the vehicle treated animals. In the male progeny hepatocellular
carcinomas were found in 3 of 4 animals. This compares to an occurence
of 1 to 12 of vehicle-treated controls and 1 of 47 untreated controls
(P>0.05). The incidences of all other tumor-types were similar in the
styrene-treated and control animals (Ponomarkov & Tomatis, 1978).
A group of 29 pregnant O20 mice received a single dose of
1350 mg/kg b.w. of styrene by gavage (dissolved in olive oil) on the
17th day of gestation, while a control group received olive oil alone.
This same dosage of styrene was administered to the offspring
(39 females and 45 males) from weaning to 16 weeks of age. Treatment
was terminated at this point because of excessive mortality. The most
frequently observed lesions in the styrene-treated offspring which
expired before the twentieth week were multiple centrilobular necrosis
of the liver, hypoplasia of the spleen and severe congestion of
the lungs. In those animals that expired after the forty-fifth
week multiple abscess-like cavities in the liver filled with
polymorphonuclear leukocytes were noted. The study was terminated when
the last animal had expired at 100 weeks. There were no differences
in tumor incidences between the dams treated with styrene and those
treated with olive oil. In the progeny pulmonary adenomas and
adenoearcinomas were noted in 20/23 males and 32/32 females treated
with styrene and in 8/19 males and 14/21 females in these animals
treated with olive oil alone (P>0.01, P<0.01). However, in untreated
controls 34/53 males and 25/42 females were found to also have
pulmonary adenomas and adenocarcinomas (P<0.05, P<0.01). The average
age at death of the pulmonary tumor-bearing, styrene-treated mice was
32 and 49 weeks for the males and females, respectively. In contrast,
the olive oil-treated males and females exhibited an average age of 88
and 85 weeks, while the average age of mortality of the untreated
control males and females was 94 and 99 weeks, respectively. No other
differences in tumor incidences were noted between the various groups
(Ponomarkov & Tomatis, 1978).
Groups of 50 male and 50 female B6C3F1 mice were dosed daily by
gavage five days per week with styrene dissolved in corn oil at dose
levels equivalent to 150 and 300 mg/kg b.w. Treatment was for 78 weeks
and was followed by a 13 week observation period. A group of 20
animals of each sex served as a vehicle corn oil control group. In the
males, but not females, there was a significant positive relationship
between mortality and dosage. A slight, dose-related mean body weight
depression was noted in the females, but not the males, while no other
clinical annormalities were noted. There were adequate numbers of both
males and females at risk for late-developing tumors. In the low
dosage group 92 percent (46/50) and 80 percent (40/50) of the males
and females, respectively, survived on test until the termination of
the study. The percentages of survival for the high dosage group was
78 and 76 for the males and females, respectively, and for the
controls 100 and 90 percent for the respective sexes.
During the course of the study, five low dose females were found
missing. A variety of neoplastic lesions were found in both the
styrene-treated and control animals, however, with the exception of
pulmonary tumors, the incidences neoplastic lesions were unrelated to
the administration of styrene. There was a significant increase in the
incidence of a combination of pulmonary adenomas and carcinomas in the
styrene-treated male mice with 0/20, 6/45 and 9/49 of the control, low
dosage and high dosage animals demonstrating these lesions (P = 0.024
for Fischer exact test comparing high dose and control). However, a
definitive conclusion on the carcinogenicity of styrene could not be
drawn because of the high variability of the incidence of these
neoplasia in historical control mice at the laboratory which performed
the study. The historical incidence of the combination of alveolar/
bronchiolar adenomas and aleolar/bronchiolar carcinomas in male mice
in this laboratory is 12 percent (32/271). The authors concluded that
there was no convincing evidence for carcinogenicity of styrene in the
mouse study. (National Cancer Institute, 1978)
Rat
A group of 21 BD IV rats were given 1350 mg of styrene/kg b.w.
in olive oil orally on the 17th day of gestation. Their offspring (73
males and 71 females) then received 500 mg/kg b.w. styrene weekly from
weaning for 120 weeks. Ten dams and their offsprings (36 males and 39
females) received olive oil and served as controls. The average litter
sizes of the different groups were similar. The mortality of the
offspring of styrene-treated dams (10%) was greater than that of
the controls (2.5%), but not significantly. There was no evidence
of a difference in body weight gain and survival between the
styrene-treated and control offspring. The incidence of survival were
8/73 and 20/71 of the male and female, styrene-treated animals and
14/36 and 18/39 male and female control animals. The incidence of
tumor-bearing dams was greater in the styrene-treated group than
the control group, but the difference was not significant. Three
neurogenic and three stomach tumors were observed in the
styrene-treated progeny that were not observed in the controls
(Ponomarkov & Tomatis, 1978).
A study was carried out with Fischer 344 rats in which groups of
50 males and 50 females were administered styrene dissolved in corn
oil five days per week by gavage at doses equivalent to 500, 1000 and
2000 mg/kg b.w. Treatment was for 103 weeks for the low dosage group
and 78 weeks for the medium and high dosage groups. This was followed
by an observation period of one week for the low dosage groups and
twenty-seven weeks for the medium and high dosage groups. A group of
40 animals of each sex served as vehicle controls (corn oil). The
highest dosage resulted in a significantly earlier and higher
mortality in both sexes, wherein, an additional dosed group of each
sex was included in the bioassay. The survival of the other dosage
groups were not adversely affected by styrene administration. By week
53 only 12 percent (6/50) of the males had survived, while only 14
percent (7/50) of the females had survived by week 70. The survival
incidences for the other groups were 88 percent (44/59), 94 percent
(47/50) for the low and medium dose males and 92 percent (46/50) for
the low and medium dose females. The various control groups
demonstrated survival incidences which varied from 85 to 90 percent
and 75 to 90 percent for the male and female controls. Adequate
numbers of animals were at risk in the low and medium dosage groups
from late-developing tumors. A dose-related reduction in the mean body
weight was observed in the treated males, however, the relevance of
this observation is questionable considering the decreased survival in
the high dosage group. No other compound-related clinical signs were
observed. None of the statistical tests for any site of tumors in rats
of either sex showed a significant positive association between the
administration of styrene and an increased tumor incidence (National
Cancer Institute, 1978).
In a two-year study in Sprague-Dawley rats styrene was
administered in the drinking water at intended concentrations of
125 and 250 ppm which resulted in approximate intakes of 7.7 and
14 mg/kg b.w./day for the males and 12 and 21 mg/kg b.w./day for the
females. Each treatment group was initially comprised of 50 males and
70 females, and a group of 76 males and 106 females served as
controls. The following parameters were assessed during the course of
the study; body weight gain, food and water intake, hemograms,
clinical chemistry, urinanalysis, clinical signs, mortality and gross
necropsy and histological examination at interim and terminal
sacrifices and of those animals which died during the course of the
study or were sacrificed in a moribund condition. Styrene did not
produce any changes in the above parameters with the exception that
the terminal bodyweights of the high dosage females were less than
that of the controls. Mortality throughout the study was no different
in the three groups with 34 (control), 23 (low dose) and 19 (high) of
the males and 46 (control), 30 (low dose) and 26 (high dose) of the
females expiring before termination of the study. Styrene did not
produce either gross or histological changes, nor was there an
apparent styrene-related increase in tumor incidence. All tumors noted
were common, spontaneously occuring tumors for this strain of rat or
rare tumors that occurred without regard to treatment group (Chemical
Manufacturers Association, 1980).
The long-term effects of styrene were assessed in two groups of
Wistar rats. Groups each consisted of 15 male and 15 male Wistar rats
and received a fatty solution of the monomer in dosages of either 1 or
5 mg/kg b.w. by mouth for a period of 10 months. The treatment was
then discontinued and the animals were periodically followed for up to
10 months. A postmortum examination was performed on all animals which
expired during the study and on those animals which were sacrificed at
the end of the 20th months. Two tumors were identified in the inquinal
region in females which received the highest dosage of styrene with
one tumor appearing at five months post-exposure and the other at ten
months post-exposure. Histological evaluation of the tumors revealed
that they were well developed fibroadenomas. The authors concluded
that the appearance of the tumors was associated with administration
of the styrene (Radeva & Krustev, 1982).
Groups of 40 male and 40 female Sprague-Dawley rats were
administered styrene oxide in olive oil by gavage at dosages
equivalent to 0, 50 and 250 mg/kg b.w. four or five days per week for
52 weeks, whereupon, the treatment was terminated and the animals were
permitted to live until their deaths. The numbers of males which
survived until the fifty-first week of the study were 37/40, 31/40 and
28/40 of the control, low and high dosage groups. The incidences of
survival for the females were 28/40, 31/40 and 30/40 of the control,
low and high dosage groups. The histological analysis revealed that
both dosages of the oxide elicited a higher, dose-related incidence of
papillomas and carcinomas of the forestomach than the control animals
with 19.3% of the low dosage animals and 50% of the high dosage
animals demonstrating these lesions. Many of the carcinomas were
observed to have metastasized to the liver, and in addition, many of
the oxide-treated animals were noted to have precursor lesions
(Maltoni et al., 1979).
Special studies on mutagenicity
Styrene and styrene epoxide were studied in spot tests and plate
incorporation assays for activity agains S. typhimurium straing
TA-98, 100, 1535, 1537 and 1538 with and without hepatic metabolizing
systems from Arochlor 1254 - pretreated rats and hamsters. Styrene was
not mutagenic for any of the tester strains, whereas, the epoxide was
active in strains TA-100 and 1535 in the spot and plate assays without
activation. Activity in these strains indicate that the epoxide acted
as a base substituted mutagen (Stoltz & Withey, 1977; Busk, 1979).
In other studies styrene was shown to be mutagenic to strains
TA-100 and 1535, but only after metabolic activation. Styrene oxide in
these studies was mutagenic, with and without metabolic activities
(Vainio et al., 1976; de Meester et al., 1977; Watabe et al., 1978a,
1978b; Loprieno et al., 1978; Milvy & Garro, 1976; Greim et al.,
1977).
The putative styrene intermediate, 1-vinylbenzene-3,4-oxide,
which has a half-life of 4.3 seconds at pH 7.4 in aqueous solution
proved to be a potent mutagen toward S. typhimurium, TA-100, but not
TA-98. This intermediate also demonstrated potent cytotoxicity to both
His + revertant colonies. The final metabolite, 4-vinylphenol, which
is the metabolite to which the oxide is converted proved to be neither
mutagenic or cytotoxic in these studies (Watabe et al., 1982).
The two enantiomers of styrene 7,8-oxide and various thioether
conjugates of racemic styrene 7,8-oxide were tested for mutagenic
activity in S. typhimurium TA-100. The results of these studies
suggested that the (R) enantiomer is more mutagenic than the (S)
enantiomer, with the racemic mixture demonstrating intermediate
activity and the conjugates exhibiting no activity (Pagano et al.,
1982).
The activities of epoxide hydrolase and monoxygenase were studied
in incubation mixtures for liver microsomal assays with S-9 metabolic
activation in the absence or presence of styrene from rats and mice.
The results of this study showed that the activity of the hydrolase is
more stable than that of the monoxygenase. This is further evidence
that in the S. typhimurium mutagenic assay system styrene shows
equivocal results because the styrene epoxide is being hydrolyzed at a
fairly constant rate while its formation from styrene is occurring at
decreasing rates (Bauer et al., 1980).
In a metabolizing, D7 strain of Saccharomyces cerevisiae
styrene induced an increase in the frequency of cross-over, gene
conversion and point mutation (Del Carratore et al., 1983). In a
series of mutagenic assays which measured forward mutations in
Schizosaccharomyces pombe and V-29 Chinese hamster cells and gene
conversions in S. cerevisiae in the diploid strain D4, styrene oxide
was active in the forward mutation experiments with yeast and Chinese
hamster cells and the production of gene-conversion in yeast. In
contrast, styrene was inactive in these assays, even in the presence
of purified mouse - liver microsomes. In a host-mediated assay which
utilized S. pombe both compounds were active in the production of
gene conversion, but not for forward mutation (Loprieno et al., 1976).
In subsequent studies styrene oxide, but not styrene, was shown to
have mutagenic activity in unscheduled DNA synthesis (UDS) studies in
a human heterploid cell line and in the induction of chromosomal
aberrations in the bone marrow of CD-1 mice treated by gavage with a
0.5 ml of solution 66% in olive oil. The UDS studies also utilized a
mouse liver homogenate, but styrene still failed to demonstrate
activity (Loprieno et al., 1978).
Styrene oxide in Chinese hamster ovary cells was a potent inducer
of sister chromatid exchanges (SCF), while styrene did not increase
the number of SCF per metaphase. The effect of the oxide was
diminished by the presence of a rat S9 metabolic activation system,
whereas, it had no effect on the lack of activity of styrene. In
contrast, when an inhibitor of epoxide hydrolase, cyclohexene oxide,
was used the induction of SCF by styrene with metabolic activation
became evident (de Raat, 1978).
Styrene oxide was tested in a mutagenic test screening programme
which involved a sperm abnormality test and dominant lethal test in
male mice exposed to atmospheric concentrations of 15 or 100 ppm for
7 hours per day for 5 days, a cytogenic test in bone marrow celles of
male and female rats exposed in a manner as described previously or a
single exposure of 7 hour duration and a sex-linked recessive lethal
test in Drosophila melanogaster with exposure to atmosphere of
100 ppm for 150 minutes. The results of these studies showed that
styrene oxide had no activity on the frequency of chromosomal
aberrations and sex-linked recessive lethal mutation frequency in
D. melanogaster. In the dominant lethal test a small reduction in
pregnancy frequency and total number of implantations was attributed
to styrene oxide. The highest dosage of the oxide, also resulted in a
slight increase in the frequency of sperm abnormality (McGregor,
1983).
Additional studies with Drosophila melanogaster showed that
both styrene and styrene oxide induce recessive lethal mutations and
that the frequency of their occurence is increased by pretreatment
with either sodium phenobarbitone or trichloropropane oxide (Donner et
al., 1979).
The mutagenic potential of styrene and its 7,8-oxide were studied
in the isolated perfused rat liver as the metabolizing system and
Chinese hamster V79 cells as genetic target cells. The 7,8-oxide was
rapidly metabolized by the liver, and therefore, failed to elicit a
mutagenic effect. In contrast, styrene produced an increase in V79
mutants which seemed not to be due to the formation of the 7,8-oxide,
since simultaneous analysis of its concentration in the perfusion
medium did not correlate with the mutagenic effect (Beije & Jenssen,
1982).
The in vivo exposure of Chinese hamsters to atmospheric
concentrations of styrene oxide from 25 to 100 ppm did not affect the
incidence of chromosomal aberrations and sister chromatid exchanges in
bone marrow cells. To alter the rate of chromosomal aberrations
required the administration of lethal concentrations of the oxide by
the intraperitoneal route (Norppa et al., 1979, 1983).
The daily oral administration of 500 mg of styrene kg/b.w. for
four days or 200 mg of styrene/kg b.w. failed to induce chromosomal
aberrations in the bone marrow cells of CD-1 male mice. Concurrent
pharmacokinetic studies performed on the same animals failed to show
the presence of styrene-7,8-oxide in the blood (at the nanogram level)
(Sbrana et al., 1983).
Styrene produced chromosomal breaks in in vitro preparations of
human lymphocytes, whereas, the oxide was responsible for the
formation of micronuclei and nuclear birdges (Linnainmaa et al.,
1978). In a mutagenic assay in human diploid fibroblasts styrene oxide
failed to elicit an increase in unscheduled DNA synthesis (McGregor,
1983).
Cultures of periphenal lymphocytes of workers employed in
industries with styrene exposure levels which ranged from 30 to
400 mg/mc showed significantly greater frequencies of chromosomal
aberrations than those of a matched (sex, age, age and smoking habit)
control group ( Camurri et al., 1983).
A micronucleus test based on the analysis of lymphocytes with
preserved cytoplasm revealed an increased frequency of micronuclei in
workers exposed to a time-weight average of styrene concentration
between 1 and 36 ppm. These ambient concentrations correlated with low
urinary levels of mandelic acid (Hogstedt et al., 1983).
Styrene and styrene-7,8-oxide induce pronounced dose response
increases in the occurences in sister chromatid exchanges in human
lymphocytes and whole blood cultures. The effect of styrene was
greater in the presence of erythrocytes indicating that styrene must
be converted to the 7,8-oxide for its capability to induce an increase
in the incidence of sister chromatid exchanges (Norppa et al., 1980a,
1983).
Special studies on teratogenicity
The toxicity of styrene and styrene oxide chicken embryos was
studied by injecting from 2 to 100 umol of either compound in 50 ml
of an olive oil-ethanol mixture into the air sacs of eggs from
White-Leghorn SK12 chickens. Malformations were noted in 15% of the
styrene embryos and 7% of the styrene oxide treated embryos. The
embryos were most susceptible on the day of, and the day after the
beginning of incubation (Vainio et al., 1977).
Sprague-Dawley rats in groups of 39, 30 and 29 animals were given
0, 180 and 300 mg styrene/kg b.w./day by gavage from days 6 through 15
of gestation. The styrene was administered as a peanut oil solution at
a volume of 2 mg/kg b.w. as twice daily doses of 90 or 150 mg/kg b.w.
There were no significant differences between the treated-animals and
the controls during gestation except for a diminished weight gain on
days 6 through 9 of gestation that was attributed to a reduction in
food intake. Dams were sacrificed prior to parturition and the fetuses
were examined. The incidence of external visceral and skeletal
malformations in the treated animals did not differ from either the
matched or historical controls. A compound-related effect on the
embryo and fetus was not observed, as was no teratogenic effect.
Styrene at both dosages did induce a decrease in body weight gain and
decreased food consumption in the dams (Murry et al., 1978).
Special studies on reproduction
Water and milk extracts of CNP-2p grade plastics which were
subjected to a vacuum process and contained 80 to 110 ppm styrene were
given every 24 hours to 741 rats in a multi-generation study which was
22 months in duration. A group of animals which received water and
milk served as controls. The parental generation received the extracts
from 1.5 to 13 months of age, while the F1 and F2 generations
were given the extracts from weaning to 8 and 2 months-of-age,
respectively. The administration of the extracts continued throughout
pregnancy and lactation. The animals which received the extracts
demonstrated a statistically reliable decrease in the number of red
blood cells and activity of cholinesterase after a two day fast. A
histological analysis revealed that the extract-treated animals
demonstrated dystrophic changes in the superficial, cortical renal
tubules and a catarrhal state in the duodenum. No other hematological
or histological parameters were found to be adversely altered by the
administration of the extracts. A large number of still-born, poorly
developed and deformed pups were noted to have been born to the
parental and F1 dams which were treated with the extracts as compared
to the control animals. In addition, it was noted that there was a
delay in the growth of fur in the F1 and F2 generations which were
treated with the extracts (Chernova, 1971).
A three-generation reproduction study was conducted as part of a
combined chronic two-year reproduction study in which styrene was
administered in the drinking water of rats. From each group (125 and
250 ppm groups) of the chronic study at least 10 males and 20 females
were mated to produce F1 pups and then subsequent F2 and F3
generations were produced. The following reproductive and
toxicological indices monitored in this study; mean litter size,
live-to-total pup ratios, pup survival indices at intervals from birth
to weaning were evaluated as well as liver and kidney weights of
representative pups necropsied at weaning, cytogenetic evaluation of
bone marrow samples of other weanlings, gross necropsy of F1 and F2
parents, organ weigths and histopathologic examinations of liver and
kidneys of weanlings and of tissues of representative F1 and F2
parents. Styrene had no deleterious effects on the reproductive
capacity of rats through the three generations and on other
toxicological parameters measured (Chemical Manufacturers Association,
1980).
Special studies on enzyme systems
The effect of styrene on carbohydrate balance was studied in
Wistar rats which received a dosage by gavage of 2.5 g/kg b.w. which
was equivalent to 1/2 the LD50. The results indicated that glucose
absorption and hepatic glycogen content were reduced after styrene
administration (Delag et al., 1976).
The effect of orally administered styrene on hepatic mixed
function oxidase enzyme activities, glutathione-S-transferase activity
and glutathione content was studied in male rats. Doses of 250, 450
and 900 mg/kg b.w. were administered for seven days to groups of 15
animals. The two highest dosages enhanced the activity of aryl
hydrocarbon hydroxylase and aniline hydroxylase, while the highest
dosage alone, significantly lowered the activity of glutathione-
S-transferease and glutathione content. In a subsequent study in which
an identical protocol was followed with the exception that only the
two highest dosages were used (450 and 900 mg/kg b.w.) effects on
renal MFO activity and glutathione activity were noted. An increase in
MFO activity was assessed as an increase in the activity of ary
hydrocarbon hydroxylase, aniline hydroxylase ethylmorphine-N-
demethylase and benzphetamine N-demethylase. In addition, the activity
of glutathione-S-transferase was decreased along with the levels of
renal glutathione (Das et al., 1981, 1983).
The intubation of 1 ml/kg b.w. of styrene daily for 15 days to
adult male albino rats elicited a significant increase in serotonin
and noradrenalin levels in the brain without a change in dopamine
content. These changes coincided with a significant decrease in the
activity of monoamine oxidase, however, no change in acetyl
cholinesterase activity was observed (Husain et al., 1980).
Groups of male rats were administered styrene in ground nut oil
by gavage at dosages of 200 and 400 mg/kg b.w. six days per week for
100 days. A dose-dependent increase in hepatic benzo(a)pyrene
hydroxylase and aminopyrine-N-demethylase activities were noted, while
a decrease in the activity of glutathione-S-transferase, mitochondrial
succinic dehydrogenase and B-glucoronidase and no change in glucose-6-
phosphatase were observed (Srivastava et al., 1982).
Special studies on immune function
The effects of styrene on immunological function were studied in
a series of three experiments in 36 rabbits. The animals used in the
study were immunized with lamb erythrocytes for a period of 7 to 8
days beginning with the initiation of dosing with styrene. Styrene was
administered 5 times per week in the form of an emulsion (vegetable
oil and potato starch) according to three different dosing schedules.
These were 250 mg/kg b.w. for 58 days, 5 mg/kg b.w. for 216 days and
0.5 mg/kg b.w. for 202 days. The results of these studies established
that the higher dosages of styrene produced acute variations in the
titer of complement and antibodies. The phagocytic activity of the
leucocytes were also observed to be depressed. These changes were
noted to have occured prior to other indications of styrene
intoxication at the highest dosage. These included a reduction of
appetite and hyperemia of the conjunctiva of the eye (Sinitskii,
1969).
Acute toxicity
Species Route LD50 Reference
Rat oral 5.0 g/kg b.w. Wolf et al., 1956
Rat oral 5.5 g/kg b.w. Ogleznev, 1963
Short-term studies
Mouse
A subchronic study was performed as a range finding study for the
NCI chronic mouse bioassay. Five males and five females were placed in
groups which received dosages of 0, 147, 215, 316, 464 and 681 mg of
styrene/kg b.w. which was administered as a corn-oil gavage five days
per week for seven weeks. A reduced mean body weight gain was noted in
the groups which received 316 and 464 mg/kg b.w., but not the highest
dosage of 681 mg/kg b.w. No other clinical abnormalities were noted to
be related to the exposure to styrene (National Cancer Institute,
1978).
In a one month study in 75 mice styrene at a dosage of 1/10 of
the LD50, 0.55 g/kg b.w., had no effect on the growth of the treated
animals. No other details of the study were provided (Ogleznev, 1963).
Rat
Rats were dosed by gavage with dosages of 0.1, 0.5, 1.0 and 2.0 g
of styrene/kg b.w. emulsified in olive oil with gum arabic. Dosing was
five days per week for 4 weeks. There was an increase in mortality and
pronounced esophageal and gastric irritation in the groups which
received the two highest dosages. Slight esophageal and gastric
irritation were also observed at the next lowest dosage of 0.5 g/kg
b.w., along with poor weigth gains in the five males which survived
the 28-day testing period. The animals which received the lowest
dosage of 0.1 g/kg b.w. were found to be in generally good condition
(Spencer et al., 1942).
Matched groups of ten female rats were dosed by gavage with
dosages of 0, 66.7, 133, 400 and 667 mg of styrene/kg b.w./day five
days per week for six months. The test compound was administered as an
olive-oil solution. The two lowest dosages had no effects on the
animals. However, the two higher dosages did produce, in a
dose-related manner, a slight reduction in the rate of growth and
slight depressions in hepatic and renal weights. Although blood cell
counts were made after 20, 40, 80 and 130 doses no compound related
effects were noted (Wolf et al., 1956).
Groups of five male and five female Fischer 344 rats received
styrene in corn-oil via gavage five days per week for seven weeks at
dosages of 0, 681, 1000, 1470, 2150 and 3160 mg/kg b.w. This was
followed by a one week observation period. The treated males,
particularly at the higher dosages, tended to have lower mean body
weight gains than the controls. At the conclusion of the study no
other compound-related clinical abnormalities were demonstrated
(National Cancer Institue, 1978).
Groups of adult, male rats were administered (six days/week)
styrene in ground nut oil by gavage dosages of 200 or 400 mg/kg b.w.
for a period of 100 days. No overt signs of toxicity were noted in the
treated animals. Weight gain and absolute and relative liver weights
were not different in the control and treated animals. The highest
dose of 400 mg/kg b.w. was associated with tiny areas of focal
necrosis in the liver which consisted of degenerated hepatocytes and
inflammatory cells (Srivastava et al., 1982).
Dog
Groups of four female and male Beagle hounds were administered
styrene by gavage at dosages of 0, 200, 400 or 600 mg/kg b.w./day for
up to 561 days. The styrene was provided as a 50/50 mixture in peanut
oil. The two highest dosages elicited Heinz bodies in the erythrocytes
of the males and occasionally in the females on the lowest dosage.
There were other sporadic changes in various hematological parameters
(decreased packed cell volume, erythrocyte counts and sedimentation
rates and hemoblogin levels and increased incidence of anisocytosis
and hypochromia of erythrocytes, hemosiderin in hepatic
reticuloendothelial cells and numbers of hepatocellular intranuclear
acidophilic crystalline inclusions) which quickly disappeared when the
administration of styrene was terminated. Although body weights, food
consumption, clinical chemistry determinations and organ weights
(brain, heart, liver, kidneys and testes) were studied none were
adversely effected by the administration of styrene (Quast et al.,
1978).
OBSERVATIONS IN MAN
Epidemiological and clinical studies
Experimental exposures to concentrations of styrene from 50 to
800 ppm for varying periods of time have shown depression of the
central nervous (CNS) system. These include reports of listlessness,
drowsiness, incoordination, decrements of manual dexterity, feeling of
intoxication and changes in visual evoked response and EEG amplitude
(Carpenter et al., 1944; Gamberale & Hultengren, 1974; Oltramare et
al., 1974). In contrast other studies have shown that styrene exposure
of from 50 to 350 ppm and again for varying periods of time did not
have effects on CNS function (Stewart et al., 1968; Gamberale &
Hultengren, 1974; Oltramare et al., 1974). In the workplace exposure
to styrene has been attributed to cause increased reaction times,
abnormal EEGs, headache, fatigue, malaise and dizziness (Seppalainen &
Harkonen, 1976; Harkonen et al., 1978; Nicholson et al., 1978; Lorimer
et al., 1976; Rosen et al., 1978; Cherry et al., 1980). There have
also been reports of styrene - induced peripheral neuropathy in the
workplace. In several studies reductions in nerve condition velocites
and mild sensory neuropathy have been noted (Lilis et al., 1978; Rosen
et al., 1978).
Styrene exposure has also resulted in irritation of the eyes,
respiratory tract, throat, and epidermis (Stewart et al., 1968; Gotell
et al., 1972; Araki et al., 1971; McLaughlin, 1946; Rosen et al.,
1978; Oltramare et al., 1974; Lorimer et al., 1976).
There are studies which suggest that styrene exposure in the
workplace has adverse effects on hepatic function. These changes have
included elevated serum enzyme activities, serum uric acid and glucose
tolerance (Lorimer et al., 1976; Axelson & Gustavson, 1978; Chmieleski
et al., 1973; Chmielewski, 1976). This link between styrene exposure
and hepatic dysfunction, however has not been definitively
established. The issue of styrene-induced reproductive and/or
teratogenic effects was raised in a study of congenital defects in
children whose mothers were occupationally exposed to styrene
(Holmberg, 1977) and in a study in which styrene was measured in
umbilical cord blood suggesting the ability of styrene to cross the
placenta (Dowty et al., 1976). In addition, an increased rate of
spontaneous abortions was noted in a study of styrene workers
(Hemminki, 1980). In contrast to these observations another study of
occupationally exposed women failed to establish a link between the
incidence of spontaneous abortions and styrene exposure (Harkonen &
Holmberg, 1982).
Several studies have examined whether styrene can induce a
carcinogenic response in humans. Several mortality studies of
occupationally exposed individuals have failed to show an excess of
deaths due to cancer (Ott et al., 1980; Frentzel-Beyme et al., 1978;
Nicholson et al., 1978). In a proportionate mortality study of 560
styrene-polystyrene polymerization workers over an approximately 15
year period showed a deficit of deaths as compared to the general
population was noted. Among the deaths of the workers were one
leukemia, one lymphoma and an additional death accompanied by leukemia
(Nicholson et al., 1978). There have been only limited epidemiological
studies performed on the possible styrene-induced carcinogenicity in
humans. A mortality study of employees engaged in the development or
manufacture of styrene-based products revealed a statistically
significant increase in the number of leukemias in comparison to other
company employees, however, when the comparison was made with national
statistics the difference was not statistically significant (Ott et
al., 1980). In a study of styrene and polystyrene plant workers in
which 24 deaths certificates were examined there was no evidence of an
association between exposure to styrene and an excess of cancer
(Frentzel-Beyme et al., 1978).
Comments
Styrene readily crosses epithelial surfaces and therefore is
absorbed from the gastrointestinal tract. Once absorbed styrene can be
eliminated unchanged via expiration or it can be converted to a number
of metabolites, including mandelic acid, phenylglyooxylic acid and
hippiuric acid, which in turn are excreted via the urine. The proposed
intermediate in this metabolic scheme is an epoxide, styrene oxide,
however its existence has been established solely in in vitro
experimentation and has never been isolated in vivo.
In studies in laboratory animals styrene has generally been shown
to elicit adverse effects only at high dose levels. These effects
include reductions in body weight gains, generation of Heinz bodies in
erythrocytes, sporadic changes in several hematological parameters,
variations in the titer of complement and antibodies and focal
necrosis in the liver. It is reported to have no teratogenic effect
nor to have adverse consequences on reproductive performance.
Mutagenic studies have generally shown styrene to be inactive,
although under some test conditions chromosomal effects were produced.
However, its metabolite, styrene oxide, has pronounced mutagenic
activity. Lifetime studies in rats have failed to demonstrate a
styrene-induced carcinogenic effect. One in three studies in the mouse
involving in utero exposure provided "limited evidence" of a
carcinogenesis. In contrast, one preliminary study has shown that
styrene oxide has carcinogenic potential.
In humans, styrene has been reported to have depressive effects
on CNS function and is an irritant to epithelial surfaces. There has
been some evidence generated in clinical studies that indicate that
styrene is hepatotoxic, however, the evidence is not conclusive. Data
from human studies have presented suggestive evidence that styrene is
teratogenic, however, the animal studies have not borne this out.
Human clinical and epidemiological studies performed to data have
generally shown that styrene is not carcinogenic and failed to
establish a link between styrene exposure and carcinogenesis.
EVALUATION
Level causing no toxicological effects
Rat: 125 ppm in drinking water, equal to 7.7 mg/kg b.w./day for 2
years.
Estimate of a provisional maximum tolerable daily intake for man
0.04 mg/kg bw.
REFERENCES
ANDERSON, N.C., TRANBERG, F.A., UGGLA, A.H., & ZETTERBERG, G. (1980)
Chromosomal aberrations and sister-chromatid exchanges in lymphocytes
of men occupationally exposed to styrene in a plastic-boat factory.
Mutat. Res., 73(2): 387-401.
ARAKI, S., ABE, A., USHIO, K., & FUJINO, M. (1971) A case of skin
atrophy, neurogenic muscular atrophy and anxiety following long
exposure to styrene. Jpn. J. Ind. Health, 13(5): 527-31.
ASTRAND, I., KILBOM, A., OVRUM, P., WAHLBERG, I., & VESTERBERG, O.
(1974) Exposure to styrene. I. Concentration in alveolar air and blood
at rest and during exercise and metabolism. Work Environ. Health,
11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ. Health,
4(Suppl.2): 215-19.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. Appl. Pharmacol., 16: 691-700.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in S.
typhimurium strains. Int. J. Cancer, 15: 429-437.
BAUER, D., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R., &
TONARELLI, S. (1980) The problem of negative results for styrene in
the in vitro mutagenesis test with metabolic activation (microsomal
assay). 2. Behaviour of epoxide hydrolase in the incubation mixtures.
Boll. Soc. Ital. Biol. Sper., 56(21): 2200-5.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in the liver
perfusion/cell culture systemœ No indication of styrene-7,8-oxide as
the principle mutagenic metabolite produced by the intact rat liver.
Chem. Biol. Interactions, 39(1): 57-76œ
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene oxide in
human blood erythrocytes and lumphocytesœ Res. Commun. Chem.
Pharmacol., 33(2): 273-82œ
BUSK, L. (1979) Mutagenic effects of styrene and styrene oxide.
Mutat. Res., 67(3): 201-208.
CAMURRI, L., CODELUPPI, S., SCARDUELLI, L., & PEDRONI, C. (1983)
Chromosomal aberrations and sister chromatid exchanges in workers
exposed to styrene. Mutat. Res., 119(3-4): 361-370.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., & BELVEDERE
G. (1978) Hepatic and extrahepatic formation and hydration of styrene
oxide in vitro in animals of different species and sex. Toxicol.
Lett., 2: 179-86.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F. Jr. (1944)
Studies on the inhalation of 1,3-butadiene; with a comparison of its
narcotic effect with benzol, toluol, and styrene and a note on the
elimination of styrene by the human. J. Ind. Hyg. Toxicol., 26(3):
69-78œ
CHEMICAL MANUFACTURERS ASSOCIATION (1980) Toxicological study on
styrene incorporated in drinking water of rats for two years in
conjunction with a three-generation reproduction study. Litton
Bionetics.
CHERNOVA, T. (1971) Evaluation of articles made on CNP-2P grade
plastics intended for packaging of food-stuffs. Vopr Pitan, 30:
56-58.
CHERRY, N., WALDRON, H.A., WELLS; G.G., WILKINSON, R.T., WILSON, H.K.,
& JONES, S. (1980) An investigation of the acute behavioural effects
of styrene on factory workers. Brit. J. Ind. Med. 37: 234-40.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R. (1973) Rating
of the exposure to styrene of persons working at the production of
polyesteric laminates. Bull. Inst. Marit. Med. Gdynia, 24:
203-209.
CHMIELEWSKI, J. (1976) Clinical and experimental research into the
pathogenesis of toxic effect of styrene. Part V. Impact of styrene on
carbohydrate balance in people in the course of their work. Bull
Inst. Marit. Trop. Med. Gdynia, 27: 177-84.
DAS, M., DIXIT, R., MUSHTAQ, M., SRIVASTAVA, S.P., & SETH, P.K. (1981)
Effect of styrene on hepatic mixed function oxydases, glutathione
content and glutathione-S-transferase activity in rats. Drug Chem.
Toxicol., 4(3): 219-27.
DAS, M., SRIVASTAVA, S.P., & SETH, P.K. (1983) Effect of styrene on
glutathione content and some xenobiotic metabolizing enzymes of rat
kidney. Acta Pharmacol. Toxicol., 52(1): 47-50.
DELAG, G., CHMIELEWSKI, J., MIKULSKI, P., & WIGLUSZ, R. (1976)
Clinical and experimental research into the pathogenesis of toxic
effect of styrene. Part VI. The effect of styrene on carbohydrate
balance, experimental research. Bull. Inst. Marit. Trop. Med.
Gdynia, 27: 185-91.
DELBRASSINE, I.P., VAN BLADEREN, P.J., SMEETS, F.L., & SEUTTEN-
BERLAGE, F. (1981) Stereoselective oxidation of styrene to styrene
oxide in rats as measured by mercapturic acid excretion.
Xenobiotica, 11(9): 589-94.
DEL CARRATORE, R., GIAGONI, P., BAUER, C., BRONZETTI, G., CORSI, C.,
NIERI, R., & PAOLINI, M. (9183) Mutagenicity of styrene on
metabolizing D7 strain of Saccharomyces cerevisiae. Boll. Soc.,
Ital., Biol. Sper., 59(2): 233-8.
DE MEESTER, C., PONCELET, F., ROBERFROLD, M., RONDELET, J., & MERCIER,
M. (1977) Mutagenicity of sltyrene and styrene oxide. Mut. Res.,
56: 147-52.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M.,
MERCIER, M., & PONCELET, F. (1981) Mutagenicity of styrene in the
Salmonella typhimurium test system. Mutat. Res., Dec. 90:
443-50.
DE RAAT, W.K. (1978) Induction of sister chromatid exchanges by
styrene and its presumed metabolite styrene oxide in the presence of
rat liver homogenate. Chem. Biol. Interactions, 20: 163-70.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals induced
by styrene and styrene oxide in Drosophila melanogaster. Mutat.
Res., 67: 373-6.
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic constituents.
Pediatr. Res., 10: 696-701.
EL MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies in
detoxication. Part 73. The metabolism of alkylbenzenes-phenylacetylene
and phenylethylene (styrene). Biochem. J., 68: 199-20.
FRENTZEL-BEYME, R., THIESS, A.M., & WIELAND, R. (1978) Survey of
mortality among employees engaged in the manufacture of styrene and
polystyrene at the BASF Ludwigshafen works. Scan. J. Work Environ.
Health, 4(Suppl. 2): 231-39.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene. II.
Psychological functions. Work Environ. Health, 11: 86-93.
GOTELL, P., AXELSON, O., & LINDELOF, B. (1972) Field studies on human
styrene exposure. Work Environ. Health, 9(2): 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMANN, W., & KRAMER, M. (1977)
Mutagenicity and chromosomal aberrations as an analytical tool for
in vitro detection of mammalian enzyme-mediated formation of
reactive metabolites. ARch. Toxicol., 39: 159-69.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., & HERNBERG,
S. (1978) Exposure-response relationship between styrene exposure and
central nervous functions. Scand. J. Work Environ. Health, 4:
53-59.
HARKONEN, H. & HOLMBERG, P.C. (1982) Obstetric histories of women
occupationally exposed to styrene. Scand. J. Work Environ. Health,
8: 74-77.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980) Spontaneous abortions
among female chemical workers in Finland. Int. Arch. Occup. Environ.
Health, 45: 123-26.
HINZ, G., GOHLKE, R., & BURCK, D. (1980) The effect of simultaneous
oral application of ethanol and styrene. [1. Acute and subacute
experiments on rats.] J. Hyg. Epidemial. Microbiol. Immunol.,
24(3): 262-270.
HOGSTEDT, B., AKESSON, B., AXELL, K., GULLBERG, B., MITELMAN, F.,
PERO, R.W., SKERFVING, S., & WELINDER, H. (1983) Increased frequency
of lymphocyte micronuclei in workers producing reinforced polyester
resin with low exposure to styrene. Scand. J. Work Environ. Health,
9(3): 241-6.
HOLMBERG, P.C. (1977) Central nervous defects in two children and
mothers exposed to chemicals in the reinforced plastics. Chance or a
causal relation? Scand. J. Work Environ. Health, 3: 212-14.
HUSAIN, R., SRIVASTAVA, S.P., MUSHTAG, M., & SETH, P.K. (1980) Effect
of styrene on levels of serotonin, noradrenaline, dopamine and
activity of acetyl cholinesterase and monamine oxidase in rat brain.
Toxicol. Lett. Nov., 7(1): 47-50.
IARC (1979) Styrene and polymers. IARC Monographs on the evaluation of
the carcinogenic risk of chemicals to humans. Volume 19: 231-274.
IARC (1979) Styrene oxide. IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Volume 19: 275-283.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I. (1974)
Evaluation of hippuric, phenylglyoxylic and mandelic acids in urine as
indices of styrene exposure. Int. Arch. Arbeitsmed., 32(1-2):
93-101.
JAMES, S.P. & WHITE, D.A. (1967) The metabolism of phenethyl bromide,
styrene and styrene oxide in the rabbit and rat. Biochem. J., 104:
914-21.
KAPPUS, H., BOLT, H., BUCHTER, A., & BOLT, W. (1976) Liver microsomal
uptake of 14C vinyl chloride and transformation to protein alkylating
metabolites in vitro. Tox. Appl. Pharm., 37: 461-471.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in liver
microsomes. Biochem. Pharmacol., 18(2): 552-54.
LEIBMAN, K.C. & ORTIZ, E. (1970) Epoxide intermediates in microsomal
oxidation of olefins to glycols. J. Pharmacol. Exp. Ther.,
173(2): 242-46.
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization workers.
Env. Res., 15:133-38.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978)
Cytogenetic effects of styrene and styrene oxide in human lymphocytes
and Allium cepa. Scand. J. Work Environ. Health, 4(Suppl. 2):
156-62.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S., BONATTI,
S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., corti, G., FREZZA, D.,
LEPORINI, C., MAZZACCARO, A., NIERI, R., ROSSELLINI, D., & ROSSI, A.M.
(1976) Mutagenicity of industrial compounds-styrene and its possible
metabolite styrene oxide. Mut. Res., 40: 317-24.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H., FISCHBEIN,
A., DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J. (1976) Clinical
studies of styrene workers. Initial findings. Environ. Health
Perspect.; 17: 171-81
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First experimental
demonstration of the carcinogenic effects of styrene oxide. Long term
bioassays on Sprague-Dawley rats by oral administration. Med.
Lavoro, 5: 358-62.
McGREGOR, D.B. (1983) Tier II Mutagenic Screening of 13 NIOSH Priority
Compounds; Individual Compound Report; Styrene oxide. Govt. Reports
Announcements & Index (GRA&I), Issue 05.
McLAUGHLIN, R.S. (1946) Chemical burns of the human cornea. Amr.
J.Ophthalmol., 29(11): 1353-62.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene oxide
(1,2-epoxyethylbenzene), a presumed styrene metabolikte. Mut. Res.,
40: 15-18.
MURRAY, F.J., JOHN, J.A., BALMER, M.F., & SCHWETZ, B.A. (1978)
Teratologic evaluation of styrene given to rats and rabbits by
inhalation or by gavage. Toxicology, 11: 335-343.
NATIONAL CANCER INSTITUTE (1978) Carcinogenesis Technical Report
Series. Bioassay of styrene for possible carcinogenicity. No. 185.
NICHOLSON, W., SELIKOFF, I., & SEIDMAN, H. (1978) Mortality experience
of styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4(suppl. 2); 247-252.
NORPPA, H., FLOVAARA, F., HUSGAFVEL-PURSLAINEN, K., SORSA, M., &
VAINIO, H. (1979) Effects of styrene oxide on chromosome aberrations,
sister chromatid exchange and hepatic drug biotransformation in
Chinese hamsters in vivo. Chem. Biol. Interactions, 26: 305-15.
NORPPA, H., SORSA, M., PFAEFFLI, P., & VAINIO, H. (1980) Styrene and
styrene oxide induce SCEs and are metabolised in human lymphocyte
cultures. Carcinogenesis, 1(4): 357-61.
NORPPA, H., SORSA, M., & VAINIO, H. (1980) Chromosomal aberrations in
bone marrow of Chinese hamsters exposed to styrene and ethanol.
Toxicol. Lett., 5: 241-44.
NORPPA, H., VAINIO, H., & SORSA, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res., Aug. 43(8):
3579-82.
NORPPA, H. & VAINIO, H. (1983) Genetic toxicity of styrene and some of
its derivatives. Scand. J. Work Environ. Health, 9(2): 108-14.
OGLEZNEV, G. (1963) Comparative characteristics of the toxic action of
aqueous solution of methylostyrene and styrene. Tr. Omskogo Med.
Inst., 48: 164-174.
OLTRAMARE, M., DESBAUMES, F., IMHOFF, C., & MICHEIELS, W. (1974)
Toxicology of monomeric styrene. Experimental and clinical studies on
man. Geneva, Editions Médecine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J., &
VENABLE, J.R. (1980) A mortality survey of employees engaged in the
development of manufacture of styrene-based products. J. Occup.
Med., 22(7): 445-60.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G., MUSSINI, E.,
& SALMONA, M. (1979) Isolation and structure determination of
enzymatically formed styrene oxide glutathione conjugates. Chem.
Biol. Interactions, 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O., BEND, J.R., & ZEIGER, E.
(1982) Mutagenicity of (R) and (S) styrene 7,8-oxide and the
intermediary mercapturic acid metabolites formed from styrene
7,8-oxide. Environ. Mutagenesis, 4: 575-584.
PANTAROTTO, C., FANELLI, R., BIDOLI, F., MORAZZONI, P., SOMMONA, M., &
SZCZAURINSKA, K. (1978) Arene oxides in styrene metabolksm, a new
perspective in styrene toxicity? Scand. J. Work Environ. Health,
4(suppl. 2): 67-77.
PFAEFFLI, P., HESSO, A., VAINIO, H., & HYVOENEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in workers
occupationally exposed to styrene. Toxicol. Appl. Pharmacol.,
60(1): 85-90.
PLOTNICK, H.B. & WEIGEL, W.W. (1979) Tissue distribution and excretion
of 14C-styrene in male and female rats. Res. Commun. Chem. Pathol.
Pharmacol., 24(3): 515-524.
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work Environ.
Health, 4(suppl. 2): 127-135.
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G., MURRAY, F.J.,
JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a toxicity study in dogs
and teratogenicity studies in rabbits and rats administered monomeric
styrene. Toxicol. Appl. Pharmacol., 45: 293-4.
RADEVA, M. & KRUSTEV, L. (1982) Studies on the effect of styrene
monomer on albino rats after long-term oral administration. Khig.
Zdraveopaz., vol. 25. ISS 5: 464-8.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H. (1978)
Neurophysiological observations after chronic styrene exposure.
Scand. J. Work Environ. Health, 4(suppl. 2); 184-94.
RYAN, A.J. & BEND, J.R. (1977) The metabolism of styrene oxide in the
isolated perfused rat liver. Drug Metab. Dispose., 5(4): 363-7.
SALMONA, M., PACKECKA, J., CANTONI, L., BELVEDERE, G., MUSSINI, E., &
GARATTINI, S. (1976) Microsomal styrene monoxygenase and styrene
epoxide hydrase activities in rats. Xenobiotica, 6(10): 585-91.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate of orally
administered styrene in rats. Midland, MI, Dow Chemical USA, December,
42 pp.
SBRANA, I. LASCIALFARI, D., ROSSI, A.M., LOPRIENO, N., BIANCHI, M.,
TORTORETO; M., & PANTAROTTO, C, (1983) Bone marrow cell chromosomal
aberrations and styrene biotransformation in mice given styrene on a
repeated oral schedule. Chem. Biol. Interact., Aug. 1, 45(3):
349-57.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological findings
among workers occupationally exposed to styrene. Scan. J. Work
Environ. Health, 3: 140-46.
SINITSKII, V (1969) Indexes of immunological reactivity in rabbits
during prolonged exposure to styrene in small doses. Gig. Premen,
Polem. Mater. Igdelii Nikk No. 1: 394-8.
SPENCER, H., IRISH, D., ADAMS, E., & ROWE, V. (1942) The response of
laboratory animals to monomenic styrene. J. Ind. Hyg. Tox., 24:
295-301.
SRIVESTAVA, S., DAS, M., MUSHTAG, M., SHANDRA, S., & SETH, P. (1982)
Hepatic effects of orally administered styrene in rats. J. Appl.
Tox., 2: 219-222.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W. (1968)
Human exposure to styrene vapor. Arch. Environ. Health, 16:
656-62.
STOLTZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of styrene and
styrene epoxide in Salmonella typhimurium. Bull. Environ. Contam.
Toxicol., 17(6): 739-42.
TURSI, F., SAMAIA, M., SALMONA, M., & BELVEDERE, G. (1983) Styrene
oxidation to styrene oxide in human erythrocytes is catalysed by
oxyhemoglobin. Experimentia, vol. 39, ISS 6, 593-4.
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., & PELKONEN, O.
(1976) A study on the mutagenic activity of styrene and styrene oxide.
Scand. J. Work Environ. Health, 3: 147-51.
VAINIO, H., HEMMINKI, K., & FLOVAARA, E. (1977) Toxicity of styrene
and styrene oxide on chick embryos. Toxicology, 8: 319-25.
WATABE, T., ISOBE, M. SAWAHATA, T., YOSHIKAWA, K., YAMADA, S., &
TAKABATAKE, E. (1978a) Metabolism and mutagenicity of styrene.
Scand. J. Work Environ. Health, 4(suppl. 2): 142-55.
WATABE, T., ISOBE, M,, YOSHIKAWA, K, & TAKABATAKE, E., (1978b) Studies
on metabolism and toxicity of styrene. Part I. Biotransformation of
styrene to styrene glycol via styrene oxide by rat liver microsomes.
J. Pharmacobio. Dyn., 1:(2): 98-104.
WATABE, T., AIZAWA, T., SAWAHATA, T., OZAWA, N., ISOBE, M.,
TAKABATAKE, E., HIRATASUKA, A. (1982) Metabolism and toxicity of
styrene: 4.1-vinylbenzene 3,4-oxide, a potent mutagen formed as a
possible intermediate in the metabolism in vivo os styrene to
4-vinylpenol. Mutat. Res., 93(1): 45-56.
WOLF, M.A., ROWE, V.K., McCOLLISTER, D.D., HOLLINGSWORTH, R.L., &
OYEN, F. (1956) Toxicological studies of certain alkylated benzenes
and benzene-experiments on laboratory animals. AMA Arch. In. Health,
14: 387-98.
