
BROWN FK
EXPLANATION
Brown FK is prepared by coupling diazotised sulfanilic acid with
a mixture of m-phenylenediamine and tolylene-2,4-diamine. The
product contains 6 major coloured components, viz.:
I 1,3-diamino-4-(4'-sulfophenylazo)-benzene
II 2,4-diamino-5-(4'-sulfophenylazo)-toluene
III 1,3-diamino-4,6-bis(4'-sulfophenylazo)-benzene
IV 1,3-diamino-2,4-bis(4'-sulfophenylazo)-benzene
V 2,4-diamino-3,5-bis(4'-sulfophenylazo)-toluene
VI 1,3-diamino-2,4,6-tris(4'-sulfophenylazo)-benzene
Brown FK was evaluated at the twenty-first meeting of the
Committee (Annex 1, reference 44), at which time it was noted that, in
long-term studies in mice, Brown FK produced hepatic nodules and
tissue pigmentation. Some of the metabolites are cardiotoxic. The
reproduction/teratogenicity studies that had been performed were
inadequate and no ADI could be established. A toxicology monograph was
prepared.
Since the previous evaluation, additional data have become
available and are summarized and discussed in the following monograph.
The previously-published monograph has been expanded and is reproduced
in its entirety below.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and bio-transformation
After an i.p. dose to a rat of 1.5 g/kg b.w. the extremities
became orange in 60 minutes, and the animal sluggish. After 24 hours
the animal was normal but the urine was deep orange-yellow (Goldblatt
& Frodsham, 1952).
On incubation with the contents of rat ileum and caecum, Brown FK
and its coloured components underwent azo-reductive fission with
formation of sulfanilic acid, a phenazine-like material (P), and ill-
defined products that could be separated chromatographically. Brown FK
also underwent azo-reductive fission when incubated with rat-liver
homogenate, but P was not detected among the products. Oral
administration of Brown FK to rats, guinea-pigs, rabbits, and pigs
resulted in the excretion of sulfanilic acid in urine and faeces;
P was detectable in trace amounts in the faeces, but was mainly
present in caecal contents, predominantly during the first 6 hours
after dosing. A "blue material" was excreted in urine. On i.p.
administration to rats, Brown FK initially gave rise to brown
colouring in bile; later, sulfanilic acid and the "blue material"
appeared in the urine. P was not found in faeces or in caecal contents
(Fore & Walker, 1967; Fore, et al., 1967).
As formed in vitro from Brown FK, P was found to consist of
2 main components, P1 and P2, which were identified as
1,4,7-triaminophenazine and 8-methyl-1,4,7-triaminophenazine,
respectively. The "blue material" was tentatively identified as an
indamine, which is an intermediate in the formation of P from the
amines produced by azo-reduction of the monoazo components of Brown
FK. Since 1,2,4-triaminobenzene oxidizes spontaneously in air to give
the indamine and P1, it is possible that the "blue material" and P
arose from aerial oxidation of the amines formed by azo-reduction of
components of Brown FK; this oxidation may have occurred during the
extraction and separation of caecal contents and faeces, or in the
urine after excretion (Fore et al., 1967; Walker, 1968).
In view of the complexity of Brown FK and the final mixture of
metabolites, investigations have been conducted on individual
components. The metabolism of 1,3-diamino-4-(4'-sulfophenylazo)-
benzene and 2,4-diamino-5-(4'-sulfophenylazo)-toluene were found to be
qualitatively similar, a proportion being excreted unchanged, but the
bulk reductively cleaved to sulfanilic acid and the corresponding
amine (the latter being acetylated before excretion). The authors
expected that the metabolism of other Brown FK components would not be
fundamentally different, and that the primary metabolic reactions
would be products of cleavage of the azo linkages (Howes, 1969; Munday
& Kirby, 1969).
Incubation of 4 individual components of Brown FK (2 mono-,
1 bis-, and 1 tris-azo-component) with rat caecal contents confirmed
that azo-reduction occurred in all cases (Walker, 1968).
A summary of the products of azo reduction of the 6 major
components of Brown FK is shown below:
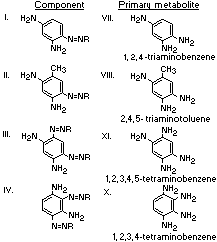
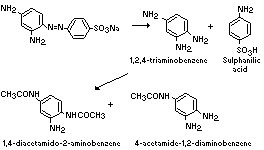
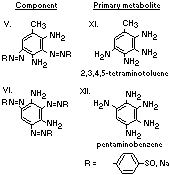 The metabolism of 1,3-diamino-4-(4'-sulfophenylazo)-benzene
(component I) in the rat is summarized below (Howes, 1969; Munday &
Kirby, 1969):
The metabolism of 1,3-diamino-4-(4'-sulfophenylazo)-benzene
(component I) in the rat is summarized below (Howes, 1969; Munday &
Kirby, 1969):
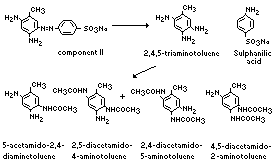
 Preliminary examination of urine from rats fed component II
showed the presence of sulfanilic acid and small quantities of
unchanged dye. Examination of an extract of the urine revealed the
presence of 5-acetamido-2,4-diaminotoluene (the major metabolite),
2,5-diacetamido-4-aminotoluene, 2,4-diacetamido-5-amino-toluene, and
4,5-diacetamido-2-amino-toluene. Unchanged dye was identified in
faecal extracts; no other dye-derived compounds were detected.
The metabolism of 2,4-diamino-5-(4'-sulfophenylazo)-toluene
(component II) in the rat is summarized below (Munday, 1969):
Preliminary examination of urine from rats fed component II
showed the presence of sulfanilic acid and small quantities of
unchanged dye. Examination of an extract of the urine revealed the
presence of 5-acetamido-2,4-diaminotoluene (the major metabolite),
2,5-diacetamido-4-aminotoluene, 2,4-diacetamido-5-amino-toluene, and
4,5-diacetamido-2-amino-toluene. Unchanged dye was identified in
faecal extracts; no other dye-derived compounds were detected.
The metabolism of 2,4-diamino-5-(4'-sulfophenylazo)-toluene
(component II) in the rat is summarized below (Munday, 1969):
 An attempt was made to determine whether 1,3-diamino-4
(4'-sulfophenylazo)-benzene (component I) is reductively cleaved in
humans, as in rats. Reduction of the closely-related compound,
prontosil rubrum, has been shown to occur in human subjects (Fuller,
1937).
An attempt was made to determine whether 1,3-diamino-4
(4'-sulfophenylazo)-benzene (component I) is reductively cleaved in
humans, as in rats. Reduction of the closely-related compound,
prontosil rubrum, has been shown to occur in human subjects (Fuller,
1937).
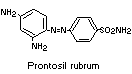 Administration of component I to human subjects led to no
detectable unchanged dye in the urine and no appreciable urinary
excretion of sulfanilic acid. It can be inferred from these results
that component I is not absorbed from the intestine as such, but no
information was given on the possible reduction of this compound
in vivo, since it was shown that orally-administered sulfanilic acid
is not absorbed in man. Sulfanilic acid, if formed from the dye, would
therefore be excreted in the faeces; the experimental confirmation of
this was not provided and these studies were not pursued further
(Jenkins & Favell, 1971).
1,2,4-triaminobenzene and 2,4,5-triaminotoluene have been shown
to uncouple oxidative phosphorylation in vitro, interfering with ATP
production in the muscle cell, and to ionic imbalance with cell death
(Munday, 1971).
Toxicological studies
Special studies on carcinogenicity
Rats
A carcinogenicity study on Brown FK was performed in CD rats with
an in utero exposure phase. Animals of the F0 generation (390 of
each sex) were allocated to 6 groups; 2 groups were untreated
(controls), 3 groups received diets containing Brown FK at constant
dietary concentrations of 160, 530, or 2630 ppm (expressed as
"coloured components of Brown FK"), and 1 group received sodium
chloride at an amount equivalent to the amount of sodium salts
received by the highest Brown FK-dose group. The animals received
these diets for 14 days prior to pairing and during pregnancy and
lactation. At weaning (28-31 days post-partum), 60 male and 60 female
animals of the F1 generation were selected from each dose group for
the long-term study. Thereafter, the Brown FK concentration in the
diet of the treated groups was adjusted to maintain constant dosages
of Brown FK of 15, 50, or 250 mg/kg b.w./day (expressed in terms of
coloured components); 2 groups served as untreated controls and one
group (salt control) received sodium chloride equivalent to the amount
of sodium salts received by the highest Brown FK-dose group. The study
was terminated when mortality in any treatment group exceeded 75%,
males and females being considered separately. Accordingly, terminal
sacrifices were initiated 105 weeks and 110 weeks after weaning of
males and females, respectively.
During the carcinogenicity study, animals were inspected twice
daily and palpated once weekly. Moribund animals were sacrificed and a
complete necropsy was performed on these rats and on those which died
during the course of the study. Ophthalmoscopic examinations were
performed on 20 males and 20 females from 1 control group and the
top-dose group after 26, 52, 77, and 102 weeks; the same animals were
examined on all 4 occasions and animals that died or were killed were
not replaced. Urinalysis was carried out on 10 rats of each sex from
each dose group after 103 weeks (males) or 105 weeks (females).
Haematological examinations and clinical chemistry investigations were
performed on 10 males and 10 females of each group after 104 weeks
(males) or 106 weeks (females).
Rats killed in extremis or at termination were subjected to a
complete necropsy and the following organ weights were recorded:
adrenals, brain, heart, kidneys, liver, ovaries/testes, pituitary,
spleen, and thyroid. Histopathological examinations were performed on
rats of both sexes from 1 control group, the top-dose group, the salt-
control group, and on any organs from the other groups that displayed
gross abnormalities. The tissues examined histopathologically
included; adrenals, aortic arch, bone, bone marrow, brain (3 levels),
caecum, colon, diaphragm, duodenum, epididymides, eyes, heart, ileum,
jejunum, kidneys, liver (2 lobes), lungs, lymph nodes (cervical and
mesenteric), mammary gland, nasal cavities, oesophagus, ovaries,
pancreas, parathyroids, pituitary, prostate, salivary gland, sciatic
nerve, seminal vesicles, skeletal muscle, skin, spinal cord
(2 levels), spleen, stomach, testes, thymus, thyroid, tongue, trachea,
urinary bladder, and uterus (including cervix).
In the reproductive phase of the study, there were no treatment-
related effects on general condition, food intake, body-weight gain,
mating performance, conception rate, or length of gestation. Litter
size, growth, and viability of the offspring were unaffected by
treatment with Brown FK. In the carcinogenicity phase, body-weight
gains in rats of either sex receiving Brown FK at a dose of 250 mg/kg
b.w./day were lower than body-weight gains in the combined control
groups; at termination the weight decrements were 14 and 10% for males
and females, respectively. Food consumption was not affected by
treatment, but water intakes of rats receiving 250 mg/kg b.w./day of
Brown FK and of rats in the salt-control groups were greater than of
untreated controls. No treatment-related effects were seen in
ophthalmic or haematological examinations, nor in urine composition.
Clinical chemistry investigations revealed higher creatine
phosphokinase, lactate dehydrogenase, and hydroxybutyrate
dehydrogenase activities in female rats of the top-dose group compared
with untreated controls, but not compared with salt controls.
Isocitrate dehydrogenase activities were higher among rats of both
sexes receiving the highest dose of Brown FK than among controls; no
effects were seen at the lower doses. In males, but not females, of
the highest-dose group, plasma albumin and T4 concentrations were
significantly elevated.
A total of 252 male and 236 female animals died or were killed
in extremis during the treatment period, but the mortality
distribution was unrelated to treatment. A total of 277 males and 308
females had palpable swellings during the treatment period but the
distribution, frequency, and time of onset were unaffected by
treatment.
No treatment-related differences were observed in absolute or
relative organ weights at necropsy, but the incidence of dark thyroid
glands among animals of the top-dose group was higher than controls.
On histopathological examination, rats exposed to the highest-dose
level of Brown FK exhibited deposition of brown pigment at particular
sites (heart, skeletal muscle, diaphragm, tongue, thyroid, caecum, and
hepatic kupffer cells) but this was not associated with any tissue
reaction and was not observed at dose levels of 15 or 50 mg/kg
b.w./day of Brown FK. Treatment with Brown FK was not associated with
enhancement of neoplasia at any site (there was a reduced incidence of
tumours in animals of the top-dose group compared with controls).
Chronic myocarditis occurred with high frequency in all groups,
but the incidence and severity were no greater in treated animals than
in controls. In females, but not males, exposure to the highest-dose
level of Brown FK was associated with an increased incidence of pelvic
nephrocalcinosis; this effect was not seen at lower-dose levels. There
was an increase in cystic distension of the follicles of the thyroid
in high-dose group females, where the lesion was seen in 40.7% of the
animals, compared with 17.2% of the controls; there was no evidence of
any effect at lower-dose levels.
The authors concluded that Brown FK was not carcinogenic in rats
under the conditions of the experiment and that the no-effect level
was 50 mg/kg b.w./day (Tesh et al., 1980; Amyes et al., 1983; Roe,
1983).
Special studies on mutagenicity
Brown FK and its constituents were assayed for mutagenicity in
Salmonella typhimurium TA1535, TA1537, and TA1538 when activated by
a rat-liver supernatant fraction. Mutagenicity was linearly dose-
dependent in the range 0-3 mg/plate, with activities ranging from 22
to 50 times the spontaneous mutation frequency. One sample of Brown FK
was mutagenic in the absence of metabolic activation, producing a
16-fold increase in mutation at 4 mg/plate. Two major constituents
of Broom FK, 2,4-diamino-5-(4'-sulfophenylazo)toluene (II) and
1,3-diamino-4-(4'-sulfophenylazo)benzene (I), each present at about
18% in the complete colour, were mutagenic in TA1538. Mutagenicity was
linearly dose-related in the range 0-1 Ámol/plate, with slopes of
1.5 mutants/nmol for compound I and 0.35 mutants/nmol for compound II.
This activity was dependent on metabolic activation. Four other major
constituents were inactive, as was sulfanilic acid, the major
excretion product. The combined effects of compounds I and II could
largely account for the mutagenicity of Brown FK (Venitt & Bushell,
1976).
Special studies on reproduction
Rats
A multigeneration reproduction study was carried out in rats in
which groups of 24 of each sex were given Brown FK in the diet at a
level of 300 ppm for 5 weeks post-weaning, then at 600 ppm for 3
successive generations; control animals received stock diet. After
weaning, the parental animals and offspring not selected for breeding
in the succeeding generation were culled. Haematological and clinical
chemistry studies were performed on 10 animals of each sex from the
parents and offspring, and autopsies carried out. Selected organ
weights were recorded at autopsy on the parents and on 30 animals of
each sex per group from the offspring. Histopathology was performed on
F3 weanlings, 10 animals of each sex per group.
Fertility, number of young per litter, birth weights, growth
rates, gestation indices, viability indices, and lactation indices
were unaffected by treatment; there was no indication of increased
mortality in utero. No treatment-related lesions were observed at
autopsy nor on histopathological examination of F3 weanlings. In
clinical observations (plasma biochemistry/haematology) and in organ
weights, occasional statistically-significant differences were seen;
these were not consistent and, in the absence of pathological lesions,
were not considered to be of toxicological significance. Under the
conditions of the study, Brown FK was without adverse effect on
reproductive performance (Wilson et al., 1983b).
Special studies on teratogenicity
Rats
Groups of 35 virgin female Wistar rats were mated with virgin
males and were fed diets containing 0, 0.03, 0.15, or 0.6% Brown FK
from day 0 to day 19 post coitus. Additional groups received 0.6%
sodium chloride (salt control) or aspirin (250 mg/kg b.w./day).
Successful pregnancies were achieved in 32-35 animals per dose group.
Five pregnant animals per group were allowed to litter normally and
then raise their offspring to weaning. The remaining animals were
sacrificed on day 21 of gestation and the foetuses removed by
Caesarian section. Two-thirds of the foetuses were examined for gross
soft-tissue abnormalities, then cleared and stained with Alizarin red
for examination for skeletal defects. The remaining one-third were
examined for soft tissue defects using Wilson's technique. No
treatment-related abnormalities were observed in any of the groups
receiving Brown FK, and this was confirmed in the groups which were
examined at weaning. Aspirin used as a positive control induced
teratological defects in foetuses and in young reared to 21 days
post partum (Unilever, 1978).
Special studies on pigment deposition
After feeding Brown FK to rats and mice, pigment was found in
heart, skeletal muscle, tongue, diaphragm, thyroids, brain, liver,
kidneys, spleen, lungs, pancreas, bladder, testes, ovary, uterus,
skin, stomach, duodenum, ileum, brown fat, and bone marrow. In
addition, a pigment has been detected in the plasma of rats.
Staining tests commonly used to identify lipofuscin were negative
with the exception of the test for metachromasia with toluidine blue.
The tests applied were as follows;
Usual response of Response of Brown
known lipofuscin FK-induced pigment
Test for iron negative negative
Sudan fat stains positive negative
Reduction of ferric salts positive negative
Reduction of ammoniacal
silver salts positive negative
Basophilic properties positive negative
Periodic acid - schiff
reaction positive negative
Acid fastness acid fast negative
Toluidine blue at pH3 stains greenish
metachromatically
green
Two further histochemical tests clearly differentiated between
lipofuscin and the Brown FK-induced pigment:
- Potassium permanganate/oxalic acid bleached lipofuscin, but not
the Brown FK-induced pigment.
- Sodium dithionite bleached both lipofuscin and the Brown FK-
induced pigment. However, after rinsing and allowing to stand in
air, the Brown FK-induced pigment reappeared; lipofuscin was
permanently bleached.
The Brown FK-induced pigment does not fluoresce in ultra-violet
light. On the other hand, all samples of lipofuscin which Brown FK
have been examined were fluorescent. Pigment has been found in the
thyroid, brown fat, and bone marrow; these tissues have not been
recorded as being sites for lipofuscin deposition. Furthermore, a
coloured substance has been demonstrated in the plasma, which has
never been found with lipofuscin.
The speed at which the Brown FK-induced pigment is deposited is
uncharacteristic of lipofuscin information. In acute studies, pigment
has been seen in the intestinal wall and villi within 24 hours of
feeding the dye, and in the kidney after 5 days. Pigment masses
produced in macrophages either in vivo after the intraperitoneal
injection of Brown FK into mice, or in vitro, when Brown FK was
incorporated in the macrophage culture medium, appeared identical.
Tests for lipofuscin proved negative; the pigment in the macrophages
closely resembled that seen in macrophages in stained sections of
tissues from rats and mice fed Brown FK.
Electron microscope studies have identified differences in
morphology between lipofuscin and the Brown FK-induced pigment. In
aged rats and mice fed Brown FK, conjugate forms were observed in
which induced pigment and control lysosomal material appeared in the
same membrane-limited body (Hope, 1971).
It is likely that a component of Brown FK is oxidized within the
cell to 1,4,7-triaminophenazine:
Administration of component I to human subjects led to no
detectable unchanged dye in the urine and no appreciable urinary
excretion of sulfanilic acid. It can be inferred from these results
that component I is not absorbed from the intestine as such, but no
information was given on the possible reduction of this compound
in vivo, since it was shown that orally-administered sulfanilic acid
is not absorbed in man. Sulfanilic acid, if formed from the dye, would
therefore be excreted in the faeces; the experimental confirmation of
this was not provided and these studies were not pursued further
(Jenkins & Favell, 1971).
1,2,4-triaminobenzene and 2,4,5-triaminotoluene have been shown
to uncouple oxidative phosphorylation in vitro, interfering with ATP
production in the muscle cell, and to ionic imbalance with cell death
(Munday, 1971).
Toxicological studies
Special studies on carcinogenicity
Rats
A carcinogenicity study on Brown FK was performed in CD rats with
an in utero exposure phase. Animals of the F0 generation (390 of
each sex) were allocated to 6 groups; 2 groups were untreated
(controls), 3 groups received diets containing Brown FK at constant
dietary concentrations of 160, 530, or 2630 ppm (expressed as
"coloured components of Brown FK"), and 1 group received sodium
chloride at an amount equivalent to the amount of sodium salts
received by the highest Brown FK-dose group. The animals received
these diets for 14 days prior to pairing and during pregnancy and
lactation. At weaning (28-31 days post-partum), 60 male and 60 female
animals of the F1 generation were selected from each dose group for
the long-term study. Thereafter, the Brown FK concentration in the
diet of the treated groups was adjusted to maintain constant dosages
of Brown FK of 15, 50, or 250 mg/kg b.w./day (expressed in terms of
coloured components); 2 groups served as untreated controls and one
group (salt control) received sodium chloride equivalent to the amount
of sodium salts received by the highest Brown FK-dose group. The study
was terminated when mortality in any treatment group exceeded 75%,
males and females being considered separately. Accordingly, terminal
sacrifices were initiated 105 weeks and 110 weeks after weaning of
males and females, respectively.
During the carcinogenicity study, animals were inspected twice
daily and palpated once weekly. Moribund animals were sacrificed and a
complete necropsy was performed on these rats and on those which died
during the course of the study. Ophthalmoscopic examinations were
performed on 20 males and 20 females from 1 control group and the
top-dose group after 26, 52, 77, and 102 weeks; the same animals were
examined on all 4 occasions and animals that died or were killed were
not replaced. Urinalysis was carried out on 10 rats of each sex from
each dose group after 103 weeks (males) or 105 weeks (females).
Haematological examinations and clinical chemistry investigations were
performed on 10 males and 10 females of each group after 104 weeks
(males) or 106 weeks (females).
Rats killed in extremis or at termination were subjected to a
complete necropsy and the following organ weights were recorded:
adrenals, brain, heart, kidneys, liver, ovaries/testes, pituitary,
spleen, and thyroid. Histopathological examinations were performed on
rats of both sexes from 1 control group, the top-dose group, the salt-
control group, and on any organs from the other groups that displayed
gross abnormalities. The tissues examined histopathologically
included; adrenals, aortic arch, bone, bone marrow, brain (3 levels),
caecum, colon, diaphragm, duodenum, epididymides, eyes, heart, ileum,
jejunum, kidneys, liver (2 lobes), lungs, lymph nodes (cervical and
mesenteric), mammary gland, nasal cavities, oesophagus, ovaries,
pancreas, parathyroids, pituitary, prostate, salivary gland, sciatic
nerve, seminal vesicles, skeletal muscle, skin, spinal cord
(2 levels), spleen, stomach, testes, thymus, thyroid, tongue, trachea,
urinary bladder, and uterus (including cervix).
In the reproductive phase of the study, there were no treatment-
related effects on general condition, food intake, body-weight gain,
mating performance, conception rate, or length of gestation. Litter
size, growth, and viability of the offspring were unaffected by
treatment with Brown FK. In the carcinogenicity phase, body-weight
gains in rats of either sex receiving Brown FK at a dose of 250 mg/kg
b.w./day were lower than body-weight gains in the combined control
groups; at termination the weight decrements were 14 and 10% for males
and females, respectively. Food consumption was not affected by
treatment, but water intakes of rats receiving 250 mg/kg b.w./day of
Brown FK and of rats in the salt-control groups were greater than of
untreated controls. No treatment-related effects were seen in
ophthalmic or haematological examinations, nor in urine composition.
Clinical chemistry investigations revealed higher creatine
phosphokinase, lactate dehydrogenase, and hydroxybutyrate
dehydrogenase activities in female rats of the top-dose group compared
with untreated controls, but not compared with salt controls.
Isocitrate dehydrogenase activities were higher among rats of both
sexes receiving the highest dose of Brown FK than among controls; no
effects were seen at the lower doses. In males, but not females, of
the highest-dose group, plasma albumin and T4 concentrations were
significantly elevated.
A total of 252 male and 236 female animals died or were killed
in extremis during the treatment period, but the mortality
distribution was unrelated to treatment. A total of 277 males and 308
females had palpable swellings during the treatment period but the
distribution, frequency, and time of onset were unaffected by
treatment.
No treatment-related differences were observed in absolute or
relative organ weights at necropsy, but the incidence of dark thyroid
glands among animals of the top-dose group was higher than controls.
On histopathological examination, rats exposed to the highest-dose
level of Brown FK exhibited deposition of brown pigment at particular
sites (heart, skeletal muscle, diaphragm, tongue, thyroid, caecum, and
hepatic kupffer cells) but this was not associated with any tissue
reaction and was not observed at dose levels of 15 or 50 mg/kg
b.w./day of Brown FK. Treatment with Brown FK was not associated with
enhancement of neoplasia at any site (there was a reduced incidence of
tumours in animals of the top-dose group compared with controls).
Chronic myocarditis occurred with high frequency in all groups,
but the incidence and severity were no greater in treated animals than
in controls. In females, but not males, exposure to the highest-dose
level of Brown FK was associated with an increased incidence of pelvic
nephrocalcinosis; this effect was not seen at lower-dose levels. There
was an increase in cystic distension of the follicles of the thyroid
in high-dose group females, where the lesion was seen in 40.7% of the
animals, compared with 17.2% of the controls; there was no evidence of
any effect at lower-dose levels.
The authors concluded that Brown FK was not carcinogenic in rats
under the conditions of the experiment and that the no-effect level
was 50 mg/kg b.w./day (Tesh et al., 1980; Amyes et al., 1983; Roe,
1983).
Special studies on mutagenicity
Brown FK and its constituents were assayed for mutagenicity in
Salmonella typhimurium TA1535, TA1537, and TA1538 when activated by
a rat-liver supernatant fraction. Mutagenicity was linearly dose-
dependent in the range 0-3 mg/plate, with activities ranging from 22
to 50 times the spontaneous mutation frequency. One sample of Brown FK
was mutagenic in the absence of metabolic activation, producing a
16-fold increase in mutation at 4 mg/plate. Two major constituents
of Broom FK, 2,4-diamino-5-(4'-sulfophenylazo)toluene (II) and
1,3-diamino-4-(4'-sulfophenylazo)benzene (I), each present at about
18% in the complete colour, were mutagenic in TA1538. Mutagenicity was
linearly dose-related in the range 0-1 Ámol/plate, with slopes of
1.5 mutants/nmol for compound I and 0.35 mutants/nmol for compound II.
This activity was dependent on metabolic activation. Four other major
constituents were inactive, as was sulfanilic acid, the major
excretion product. The combined effects of compounds I and II could
largely account for the mutagenicity of Brown FK (Venitt & Bushell,
1976).
Special studies on reproduction
Rats
A multigeneration reproduction study was carried out in rats in
which groups of 24 of each sex were given Brown FK in the diet at a
level of 300 ppm for 5 weeks post-weaning, then at 600 ppm for 3
successive generations; control animals received stock diet. After
weaning, the parental animals and offspring not selected for breeding
in the succeeding generation were culled. Haematological and clinical
chemistry studies were performed on 10 animals of each sex from the
parents and offspring, and autopsies carried out. Selected organ
weights were recorded at autopsy on the parents and on 30 animals of
each sex per group from the offspring. Histopathology was performed on
F3 weanlings, 10 animals of each sex per group.
Fertility, number of young per litter, birth weights, growth
rates, gestation indices, viability indices, and lactation indices
were unaffected by treatment; there was no indication of increased
mortality in utero. No treatment-related lesions were observed at
autopsy nor on histopathological examination of F3 weanlings. In
clinical observations (plasma biochemistry/haematology) and in organ
weights, occasional statistically-significant differences were seen;
these were not consistent and, in the absence of pathological lesions,
were not considered to be of toxicological significance. Under the
conditions of the study, Brown FK was without adverse effect on
reproductive performance (Wilson et al., 1983b).
Special studies on teratogenicity
Rats
Groups of 35 virgin female Wistar rats were mated with virgin
males and were fed diets containing 0, 0.03, 0.15, or 0.6% Brown FK
from day 0 to day 19 post coitus. Additional groups received 0.6%
sodium chloride (salt control) or aspirin (250 mg/kg b.w./day).
Successful pregnancies were achieved in 32-35 animals per dose group.
Five pregnant animals per group were allowed to litter normally and
then raise their offspring to weaning. The remaining animals were
sacrificed on day 21 of gestation and the foetuses removed by
Caesarian section. Two-thirds of the foetuses were examined for gross
soft-tissue abnormalities, then cleared and stained with Alizarin red
for examination for skeletal defects. The remaining one-third were
examined for soft tissue defects using Wilson's technique. No
treatment-related abnormalities were observed in any of the groups
receiving Brown FK, and this was confirmed in the groups which were
examined at weaning. Aspirin used as a positive control induced
teratological defects in foetuses and in young reared to 21 days
post partum (Unilever, 1978).
Special studies on pigment deposition
After feeding Brown FK to rats and mice, pigment was found in
heart, skeletal muscle, tongue, diaphragm, thyroids, brain, liver,
kidneys, spleen, lungs, pancreas, bladder, testes, ovary, uterus,
skin, stomach, duodenum, ileum, brown fat, and bone marrow. In
addition, a pigment has been detected in the plasma of rats.
Staining tests commonly used to identify lipofuscin were negative
with the exception of the test for metachromasia with toluidine blue.
The tests applied were as follows;
Usual response of Response of Brown
known lipofuscin FK-induced pigment
Test for iron negative negative
Sudan fat stains positive negative
Reduction of ferric salts positive negative
Reduction of ammoniacal
silver salts positive negative
Basophilic properties positive negative
Periodic acid - schiff
reaction positive negative
Acid fastness acid fast negative
Toluidine blue at pH3 stains greenish
metachromatically
green
Two further histochemical tests clearly differentiated between
lipofuscin and the Brown FK-induced pigment:
- Potassium permanganate/oxalic acid bleached lipofuscin, but not
the Brown FK-induced pigment.
- Sodium dithionite bleached both lipofuscin and the Brown FK-
induced pigment. However, after rinsing and allowing to stand in
air, the Brown FK-induced pigment reappeared; lipofuscin was
permanently bleached.
The Brown FK-induced pigment does not fluoresce in ultra-violet
light. On the other hand, all samples of lipofuscin which Brown FK
have been examined were fluorescent. Pigment has been found in the
thyroid, brown fat, and bone marrow; these tissues have not been
recorded as being sites for lipofuscin deposition. Furthermore, a
coloured substance has been demonstrated in the plasma, which has
never been found with lipofuscin.
The speed at which the Brown FK-induced pigment is deposited is
uncharacteristic of lipofuscin information. In acute studies, pigment
has been seen in the intestinal wall and villi within 24 hours of
feeding the dye, and in the kidney after 5 days. Pigment masses
produced in macrophages either in vivo after the intraperitoneal
injection of Brown FK into mice, or in vitro, when Brown FK was
incorporated in the macrophage culture medium, appeared identical.
Tests for lipofuscin proved negative; the pigment in the macrophages
closely resembled that seen in macrophages in stained sections of
tissues from rats and mice fed Brown FK.
Electron microscope studies have identified differences in
morphology between lipofuscin and the Brown FK-induced pigment. In
aged rats and mice fed Brown FK, conjugate forms were observed in
which induced pigment and control lysosomal material appeared in the
same membrane-limited body (Hope, 1971).
It is likely that a component of Brown FK is oxidized within the
cell to 1,4,7-triaminophenazine:
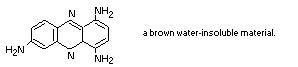 This would explain the behaviour of the pigment with sodium
dithionite, which reduces the phenazine ring to the 5,10-dihydro
derivative, which is probably colourless. After exposure to air
re-oxidation would occur. Thus, the pigment may not represent evidence
of sub-lethal cell damage, but is, instead, an insoluble oxidation
product of a dye metabolite.
1,2,4-triaminobenzene was very rapidly oxidized to
1,4,7-triaminophenazine by a mitochondrial suspension; no
phenazine derivatives were detected with triaminotoluene under
the same circumstances (Kirby, 1968b).
1,4,7-triaminophenazine is a brown, water-insoluble, material
which is very readily formed from 1,2,4-triaminobenzene (Muller,
1889).
Special studies on pigment in tissues
Histological tests were applied to sections of hearts and livers
from female rats fed Brown FK at doses of 0, 15, 50, or 250 mg/kg
b.w./day for 106-108 weeks. These tissues were obtained from animals
used in the carcinogenicity study (see above). In addition, several
tissues taken from weanling rats discarded at the end of the in
utero phase were screened to determine if any pigment was present at
the start of the long-term study due to transplacental transfer of
Broom FK or through exposure during lactation/creep feeding.
Rats fed Brown FK for over 2 years at a dose-level of
250 mg/kg b.w./day displayed substantial pigment deposition in the
heart and liver, but no effects were seen in these organs at the lower
dose-levels. Brown FK-associated pigment was not found in tissues from
weanling rats exposed to Brown FK in utero. The no-effect level for
pigment accumulation in the long-term study was 50 mg/kg b.w./day.
Differential tests showed that the pigment was not formalin pigment or
haemosiderin, but tests aimed at differentiating between lipofuscin
and Brown FK-induced pigment gave inconclusive results (Wilson et
al., 1983a).
Special studies on components I and II of Brown FK
Mice
Groups of 3 male and 3 female C57Bl mice were fed diets
containing 0, 0.5, or 1.0% of the "azobenzene" or "azotoluene"
components of Brown FK (components I & II, respectively) for 6 weeks.
With both components, the thyroids were dark and the intestines and
squamous portions of the stomach were stained salmon-pink. Heart
lesions were seen in all mice fed 1% component II, but not in those
given component I. More pigment was seen in mice fed component I, less
in those fed component II (Kirby, 1968a).
Rats
Groups of 3 male and 3 female Colworth-Wistar rats were fed
component I or component II at dietary levels of 0, 0.5, or 1% for 6
weeks. The thyroids of rats receiving component I were dark and the
hearts, muscles, and brains were stained. However, the intestines were
stained only slightly. Pale hearts and meningeal haemorrhage were seen
with component II, otherwise pigmentation was as with component I.
One-sixth of the rats treated with component I and four-fifths of the
rats treated with component II had heart lesions. More pigment was
seen histologically in component I-treated rats, less in component
II-treated animals (Kirkby, 1968a).
Special studies on amine metabolites of Brown FK
Amines derived from Brown FK and from its 2 myotoxic components,
components I and II, were injected i.v. into rats in single doses of
3.13-25 mg/kg. The mixture of amines from Brown FK was also injected
into mice in the same range of doses. Cardiac and muscular lesions
were produced by the amines in both species. These amines are
biological degradation products in the intestine. The finding that
orally-administered Brown FK is myotoxic in rats but not in mice is
probably due to differences in the intestinal flora in the 2 species
(Walker et al., 1970).
In another study, 1,2,4-triaminobenzene was given to groups of
6-7 rats orally 5 days/week for 2 weeks at 50, 60, 75, or 100 mg/kg
b.w./day. Six rats out of 7 receiving 100 mg/kg/day died after 3 doses
with severe heart lesions; 7/11 on 75 mg/kg/day also died after 3
doses. Heart pigmentation occurred after 5 days' treatment or longer.
Animals on lower doses showed both extensive heart pigmentation and
cardiac necrosis (Mulky et al., 1969).
1,2,4,5-Tetraaminobenzene was given to groups of 6 rats orally
5 days/week for 2 weeks at 150 or 200 mg/kg b.w./day. 1,2,3,4-
Tetraaminobenzene was given to groups of 6 rats orally 5 days/week for
2 weeks at 125 or 166 mg/kg b.w./day. No frank heart lesions and only
instances of diffuse increase in interstitial cells in the heart were
observed. No heart or thyroid pigment deposition was observed (Mulky
et al., 1969).
Acute toxicity
LD50
Species Route (mg/kg b.w.) Reference
Mouse oral >2,000 (with salt) Grasso et al., 1968a
oral 1,100-2,250 (with salt) Edwards & Wilson, 1966
oral 960-1,149 (no salt) Edwards & Wilson, 1966
i.p. 1,500-2,000 (with salt) Grasso et al., 1968a
i.p. 960-1,720 (with salt) Edwards & Wilson, 1966
i.p. 840-880 (no salt) Edwards & Wilson, 1966
Rat oral >8,000 (with salt) Grasso et al., 1968a
oral 900-1,910 (with salt) Edwards & Wilson, 1966
oral 780-970 (no salt) Edwards & Wilson, 1966
i.p. 750-1,150 (with salt) Grasso et al., 1968a
i.p. 1,100-2,250 (with salt) Edwards & Wilson, 1966
i.p. 960-1,150 (no salt) Edwards & Wilson, 1966
Guinea-pig oral 3,000 (with salt) Edwards & Wilson, 1966
oral 2,610 (no salt) Edwards & Wilson, 1966
i.p. 900 (with salt) Edwards & Wilson, 1966
i.p. 780 (with salt) Edwards & Wilson, 1966
Rabbit oral 450-680 (with salt) Edwards & Wilson, 1966
oral 390-590 (no salt) Edwards & Wilson, 1966
Chicken oral >10,000 (with salt) Edwards & Wilson, 1966
oral >8,700 (no salt) Edwards & Wilson, 1966
For all species, animals dying did so from within a few minutes
to 96 hours. Many animals, after either oral or i.p. treatment, showed
lack of coordination, hypersensitivity, and hyperactivity; convulsions
usually preceded death (Edwards & Wilson, 1966).
Meningeal congestion or haemorrhage was seen at post-mortem
examination in rats and mice which died following both oral and i.p.
treatment with 3.4 g/kg Brown FK as a 10% solution. This was the
highest dose administered, and the condition may have been present to
a lesser degree in animals treated with lower levels of Brown FK, but
post-mortem identification of the lesion was made difficult by tissue
colouration. The meningeal congestion/haemorrhage was probably caused
by the sodium chloride in the dye solution, since this lesion is
observed after administration of hypertonic solutions of sodium
chloride to rats. In this instance, the lowest dose levels at which
the meningeal lesion was observed were 6.0 g/kg of sodium chloride
orally as a 10% solution and 4.0 g/kg i.p. as a 5% solution (Edwards &
Wilson, 1966).
Following oral intubation, external tissue colouration was
apparent after several hours in rats and guinea-pigs. No colouration
of the tissues was seen in rabbits and chickens. After i.p. injection,
external tissue colouration was apparent and intense after a few
minutes in rats and guinea-pigs. Colour was seen in the faeces of
rats, mice, rabbits, and guinea-pigs up to 24 hours after oral
treatment; it was also excreted in the urine of rats, mice, guinea-
pigs, and rabbits within 15 minutes of either oral or i.p. treatment
(Edwards & Wilson, 1966).
Hearts from some rats and mice surviving for 21 days after
treatment were examined histologically. Degenerative lesions were
found in 15% of rats given orally 1-2.5 g/kg b.w. Brown FK, but not in
rats given 3.37 g/kg b.w. Brown FK. The same lesions were found in 50%
of mice given orally 0.9 g/kg b.w. Brown FK, but not when given 0.6,
1.35, or 2.03 g/kg b.w. Brown FK. When given i.p., 25-60% of mice
showed lesions at 0.75 and 1.03 g/kg b.w. Brown FK (Edwards & Wilson,
1966).
Short-term studies
Mice
Groups of 10 male or 10 female mice received the colour (either
fresh or stored) at a level of 1 g/kg daily for 3 weeks. A significant
reduction in body-weight gain was noted in the mice receiving the
stored solution, but not in those receiving fresh solution. One male
and 1 female receiving the fresh solution showed cardiac lesions
(BIBRA, 1964).
Daily oral or i.p. doses up to 2 g/kg or 1 g/kg, respectively,
for 43 days to groups of 10 or 12 mice were well-tolerated (Grasso
et al., 1968a).
Groups of 10 male and 10 female mice (Colworth C57Bl strain,
initially 6 weeks old) were fed for 90 days on a synthetic diet
containing 0, 0.05, 0.075, 0.10, 0.25, 0.50, 0.75, 1.0, or 2.0% Brown
FK (equivalent to 0, 0.025, 0.0375, 0.05, 0.125, 0.25, 0.375, 0.50, or
1.0% Brown FK-coloured components) containing 51% dye component and
47% salt. A further group of 20 mice were fed synthetic diet
containing added 1.0% sodium chloride as a control for the additional
dietary salt derived from the Brown FK. At the 0.125% dietary colour
level, pigment deposition occurred in tissues. At 0.25% and above
there was splenic enlargement, at 0.50% the liver and heart were
enlarged, and at 1.0% there was reduced growth, poor food utilization,
liver, spleen, heart, and testicular enlargement, and histological
evidence of degenerative heart lesions. The thyroids, muscle,
intestine and squamous part of the stomach were pigmented (Ashmole
et al., 1958).
Rats
Groups of animals received the colour at a level of 0.5 g Brown
FK per kg b.w. for 3 weeks, orally or i.p. Twenty rats were dosed
orally, of which 6 animals died after having been administered between
5 and 11 doses. Post-mortem examination of rats dying during the test
or killed at the end revealed general tissue-staining in 5 rats. Of 18
hearts examined histologically, 8 showed degenerative lesions, and a
brown pigment was observed in small amounts in 9 hearts after 3 weeks.
In the multiple-dose i.p. test, 8 rats were treated and none died
during the treatment period. General organ-staining was observed in
all animals at post-mortem examination. Hearts from 7 rats were
examined microscopically and degenerative lesions were found in 1
heart and small amounts of pigment in 3 hearts after 3 weeks (Kirkby,
1968a).
No ill-effects were seen in 3 weanling rats given a 0.1% solution
Brown FK for 28 days, the intake being equivalent to 15 mg/day
(Goldblatt & Frodsham, 1952).
Administration of 2 or 3 oral doses of 1 g Brown FK/kg b.w.
to rats induced a myopathy in cardiac and skeletal muscles
characterized by multiple vacuoles about 1-2 micrometers in diameter.
Ultrastructurally, these were shown to consist of areas of
fibrollolysis. Histochemically, the myopathy was accompanied by a
moderate increase in acid phosphatase activity and by a loss of
phosphorylase activity. Subsequently, complete lysis of the affected
fibres ensued. In the heart, lysis was followed by macrophage invasion
and fibroblastic proliferation, and in skeletal muscle by
regeneration. The occurrence of lipofuscin in muscle fibres and in
macrophages was scanty and erratic. When Brown FK was given in the
diet at a level of 2%, fibrillolysis and an increase in the number and
electron-density of lysosomes was observed ultrastructurally during
weeks 2 to 3 of the test. These changes were accompanied by a marked
elevation of histochemically-demonstrable acid phosphatase.
Progressive deposition of lipofuscin was the principal pathological
feature during weeks 3 to 12 (Grasso et al., 1968b).
Daily oral doses of up to 2 g/kg Brown FK for 43 days to groups
of 10 rats induced rapid loss of weight and death, with severe damage
to cardiac and skeletal muscle, characterized by vacuolar myopathy and
lipofuscin deposition. Of 3 pure components of Brown FK studied,
component II, and to a lesser extent component I, produced similar,
but not identical lesions to those induced by the parent colour after
repeated oral doses of 0.5 g/kg. Ultrastructural studies confirmed an
extensive loss of myofibrillar elements, and histochemical studies
revealed a loss in the activity of mitochondrial enzymes. Similar i.p.
injections in doses up to 1.0 g/kg for 43 days to groups of 10 or 12
rats did not have any effect on the heart or skeletal muscle (Grasso
et al., 1968b).
Experiments were performed using groups of 10-12 rats receiving
the colour at levels of 0.1 or 1 g/kg orally or 0.1, 0.25, or 1 g/kg
i.p. daily for up to a maximum of 43 doses. A specific cardiac lesion
was identified at the oral dose of 1 g/kg. There were large areas of
myocardial necrosis and replacement by large mononuclears, with
involvement of the sub-pericardial region and endocardium. Some
myocardial cells had lost their stainable cytoplasm and appeared only
as empty sheaths. When administered i.p., the colour produced little
or no cardiac damage at any dose tested. At these high doses of
1 g/kg, most animals showed congestion, fatty change, or necrosis of
the liver with hydropic degeneration of the kidney. There was no
obvious splenomegaly. Daily doses of 100 mg/kg by stomach tube
produced 2 pericardial and 1 sub-pericardial lesions. In addition,
early hydroponic degeneration of the kidney was seen in 2 rats, with
1 of these animals also showing fatty change in the liver (BIBRA,
1964).
Administration of Brown FK (purity 80.0%) at dietary levels of 0,
0.001, 0.01, 0.1, or 1.0% for 150 days showed no adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. One male rat at the 1.0% level showed
typical myocardial changes; other rats showed deposits of lipofuscin,
especially in females. The no-effect level was 0.1% (Gaunt et al.,
1968).
Groups of 12 male and 12 female rats (Colworth-Wistar strain,
initially 3-4 weeks old) were fed for 112 days on a commercial stock
diet containing 0, 0.05, 0.1, 0.5, 1.0, or 2.0% Brown FK (0, 0.025,
0.05, 0.25, 0.5, or 1.0% Brown FK-coloured components) containing 51%
dye component and 47% salt. A further group of 24 rats were fed the
commercial stock diet containing added 1.0% sodium chloride as a
control for the additional dietary salt derived from the Brown FK. In
addition, to eliminate damage to the heart from cardiac puncture in
any rat kept to 16 weeks, groups of 6 male and 6 female rats were fed
6 weeks on powdered stock diet containing 0, 0.05, 0.5, or 2.0% Brown
FK (0, 0.025, 0.25, or 1.0% Brown FK-coloured components). A group of
12 rats also received 1.0% sodium chloride added to the basic diet.
All these rats were used for biochemical tests during weeks 0-6 after
which they were killed. At the 0.25% level tissue pigmentation
appeared, at 0.5% liver enlargement occurred, and at the 1% level
there was reduced growth, poor food utilization, enlargement of the
liver, testes, and thyroid, histological evidence of degenerative
heart lesions, increased urinary indican excretion, and elevation of
SGOT. The intestine, squamous portion of the stomach, and the thyroid
were stained. The no-effect level was 0.05% (Ashmole et al., 1966).
Pigs
Groups of female and male pigs were given doses of 0, 100, 250,
or 500 mg Brown FK/kg/day for 24 weeks without adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. Lipofuscin was widely distributed in
animals of both sexes at all dose levels in one or more organs. The
liver was particularly affected in that lipofuscan deposition was
accompanied by increased lysosomal enzyme activity, which was more
marked at the higher dose-levels. It was also seen in the heart in
males, where it was associated with an increased acid phosphatase
activity, and in the kidneys at the highest-dose level in females and
at all levels in males. A no-effect level could not be determined in
this study (Gaunt et al., 1968).
Long-term studies
Mice
Groups of 40 male and 40 female Colworth C57Bl mice were fed for
80 weeks on a synthetic diet containing 0, 0.0125, 0.0375, 0.075,
0.125, or 0.625% Brown FK-coloured components (the Brown FK used in
this study contained 62.5% coloured components). Only at the 0.625%
level was there reduced growth and food utilization and increased
mortality among females. There were increased liver, kidney, spleen,
brain, and testes weights, evidence of splenic haemopoisis, and
increased myocardial fibrosis. Heart weights were increased at the
0.125% level. Increased hepatic nodules were seen at 0.075% and higher
levels and pigment deposition at 0.0375% and higher levels. At
termination, after 80 weeks, the number of animals with nodules at the
different dose levels was 26, 23, 27, 56, 42, and 64, respectively.
Increased hepatic nodules were observed at 0.075% and higher levels.
The number of mice with hepatocellular carcinoma were 3, 2, 0, 5, 6,
and 2 at the various dose levels, respectively. Pigment deposition was
observed at dose levels of 0.0375% and higher (Wilson et al., 1970).
Rats
Groups of 32 male or 36 female Colworth-Wistar rats were fed for
2 years on a synthetic diet containing 0, 0.01, 0.03, 0.06, 0.1, or
0.5% Brown FK-coloured components (Brown FK used in this study
contained 54.2% coloured components). Only at the 0.5% level was there
increased splenic weight and hepatic granulomata. Pigment deposition
was seen at dose levels of 0.06% and higher. The no-effect level for
pigment deposition was 0.03%; it was 0.06% when based on toxicity
evidence (Wilson et al., 1971).
Observations in man
No information available.
Comments
The carcinogenicity study did not reveal any increase in the
incidence of tumours, nor did the reproduction and teratogenicity
studies show any adverse effects on reproductive function.
The myopathy seen in rats given high doses of Brown FK in short-
term studies affected all striated muscle, was accompanied by pigment
deposition, and was dose-dependent with a high threshold. In the long-
term/carcinogenicity study in rats, the observed myocarditis was not
dose-related and there was no pigmentation in animals of the low-dose
group nor controls. However, deficiencies in histopathological
examination of tissues from the low- and intermediate-dose groups
hindered the establishment of a no-effect level in this study.
In earlier long-term studies, the no-effect level (with respect
to pigment deposition) was 0.03% in the diet, equivalent to 15 mg/kg
b.w./day, based on the coloured components of Brown FK.
EVALUATION
Level causing no toxicological effect
Mouse: 0.0125% in the diet, equivalent to 19 mg/kg b.w./day.
Rat: 0.03% in the diet, equivalent to 15 mg/kg b.w./day.
Estimate of temporary acceptable daily intake for man
0-0.075 mg/kg b.w. (based on colour components)
Further work or information
Required by 1986
A complete histopathological examination of tissues from the low-
and intermediate-dose groups in the long-term/carcinogenicity study in
rats.
REFERENCES
Amyes, S.J., McSheehy, T.W., & Whitney, J.C. (1983). Brown FK: Life
span combined toxicity and oncogenicity study in rats pre-exposed
in utero. 2. Toxicity and oncogenicity phase. Unpublished
report No. 82/URL012/573 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Ashmole, R.T., Campbell, P., Kirkby, W.W., & Wilson, R. (1966).
Effects of feeding dietary Brown FK to rats for six and 16 weeks.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Ashmole, R.T., Kirkby, W.W., & Wilson, R. (1958). Thirteen week mouse
feeding trial. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
BIBRA (1964). Unpublished research report No. 5/1964 from British
Industrial Biological Research Association, Carshalton, Surrey,
England. Submitted to WHO.
Edwards, K.B. & Wilson, R. (1966). Acute toxicity of Brown FK in rats,
mice, guinea-pigs, rabbits and chickens. Unpublished report from
Unilever Research Laboratories.
Fore, H. & Walker, R. (1967). Studies on Brown FK. I. Composition and
synthesis of components. Fd. Cosmet. Toxicol., 5, 1-9.
Fore, H., Walker, R., & Golberg, L. (1967). Studies on Brown FK. II.
Degradative changes undergone in vitro and in vivo. Fd.
Cosmet. Toxicol., 5, 459-473.
Fuller, A.T. (1937). Is rho-aminobenzene sulphonamide active agent in
prontosil therapy? Lancet, 1, 194-198.
Gaunt, I.F., Hall, D.E., Grasso, P., & Golberg, L. (1968). Studies on
Brown FK. V. Short-term feeding studies in the rat and pig. Fd.
Cosmet. Toxicol., 6, 301-312.
Goldblatt & Frodsham (1952). Private communication from ICI
(unpublished report).
Grasso, P., Gaunt, I.F., Hall, D.E., Golberg, L., & Batstone, E.
(1968a). Studies on Brown FK. III. Administration of high doses
to rats and mice. Fd. Cosmet. Toxicol., 6, 1-11.
Grasso, P., Muir, A., Golberg, L., & Batstone, E. (1968b). Cytopathic
effects of Brown FK on cardiac and skeletal muscle in the rat.
Fd. Cosmet. Toxicol., 6, 13-24.
Hope, J. (1971). Ultrastructure of the pigment induced in various
tissues of the rat by long-term feeding of the dye Brown FK.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Howes, D. (1969). Metabolism of 14C labelled 1,3-diamino-4-(rho
sulphophenylazo)benzene, a component of the dye Brown FK, in the
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Jenkins, F.P. & Favell, D.J. (1971). Metabolism of the
"monoazobenzene" component of Brown FK in human subjects.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Kirkby, W.W. (1968a). Effects of Brown FK and two of its constituents
on pigment deposition and lesions in rats and mice. Unpublished
report from Unilever Research Laboratories. Submitted to WHO by
Unilever Ltd.
Kirkby, W.W. (1968b). Nature of the pigment induced in tissues of rats
and mice fed Brown FK. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Mulky, M.J., Mundy, R., Ashmole, R.T., & Kirkby, W.W. (1969).
Evaluation of the terminal causative agent in Brown FK induced
myopathy and pigment deposition. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Muller, E. (1889). Chem. Ber., 22, 856.
Munday, R. (1969). Metabolism of 2,4-diamino-5-(rho-sulphophenylazo)
toluene. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Munday, R. (1971). Uncoupling of oxidative phosphorylation by Brown FK
metabolites. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Munday, R. & Kirkby, W.W. (1969). Metabolism of 1,3-diamino-4-(rho
sulphophenylazo)benzene. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Roe, F.J.C. (1983). Histopathological evaluation of sections derived
from URL/12/BFK lifespan combined toxicity and oncogenicity study
in rats pre-exposed in utero. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Tesh, J.M., McSheehy, T.W., McAnulty, P.A., & Collier, M.J. (1980).
Brown FK: Lifespan combined toxicity and oncongenicity study in
rats pre-exposed in utero. 1. Reproductive phase. Unpublished
report No. 80/URL012/051 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Unilever (1978). Teratology study on Brown FK in the Colworth-Wistar
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Venitt, S. & Bushell, C.T. (1976). Mutagenicity of the food colour
Brown FK and constituents in Salmonella typhimurium. Mutation
Research, 40, 309-316.
Walker, R. (1968). Intestinal degradation of azo food colours with
particular reference to Brown FK. Ph.D. Thesis, University of
Reading, U.K.
Walker, R., Grasso, P., & Gaunt, I.F. (1970). Myotoxicity of amine
metabolites from Brown FK. Fd. Cosmet Toxicol., 8, 539-542.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1970).
Biological evaluation of Brown FK; 80-week mouse feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1971).
Biological evaluation of Brown FK: 2-year rat feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Hague, P.H., & Hardy, W.S. (1983a). Brown FK: Lifespan
combined toxicity and oncogenicity study in rats pre-exposed in
utero: Examination of selected tissues for Brown FK associated
pigment. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Wilson, R., McCormick, S.G., Cook, H.J., Norris, L., Robinson, J.A., &
Williams, T.C. (1983b). Evaluation of the effects of food colour
Brown FK on the fertility and reproductive performance of rats.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
This would explain the behaviour of the pigment with sodium
dithionite, which reduces the phenazine ring to the 5,10-dihydro
derivative, which is probably colourless. After exposure to air
re-oxidation would occur. Thus, the pigment may not represent evidence
of sub-lethal cell damage, but is, instead, an insoluble oxidation
product of a dye metabolite.
1,2,4-triaminobenzene was very rapidly oxidized to
1,4,7-triaminophenazine by a mitochondrial suspension; no
phenazine derivatives were detected with triaminotoluene under
the same circumstances (Kirby, 1968b).
1,4,7-triaminophenazine is a brown, water-insoluble, material
which is very readily formed from 1,2,4-triaminobenzene (Muller,
1889).
Special studies on pigment in tissues
Histological tests were applied to sections of hearts and livers
from female rats fed Brown FK at doses of 0, 15, 50, or 250 mg/kg
b.w./day for 106-108 weeks. These tissues were obtained from animals
used in the carcinogenicity study (see above). In addition, several
tissues taken from weanling rats discarded at the end of the in
utero phase were screened to determine if any pigment was present at
the start of the long-term study due to transplacental transfer of
Broom FK or through exposure during lactation/creep feeding.
Rats fed Brown FK for over 2 years at a dose-level of
250 mg/kg b.w./day displayed substantial pigment deposition in the
heart and liver, but no effects were seen in these organs at the lower
dose-levels. Brown FK-associated pigment was not found in tissues from
weanling rats exposed to Brown FK in utero. The no-effect level for
pigment accumulation in the long-term study was 50 mg/kg b.w./day.
Differential tests showed that the pigment was not formalin pigment or
haemosiderin, but tests aimed at differentiating between lipofuscin
and Brown FK-induced pigment gave inconclusive results (Wilson et
al., 1983a).
Special studies on components I and II of Brown FK
Mice
Groups of 3 male and 3 female C57Bl mice were fed diets
containing 0, 0.5, or 1.0% of the "azobenzene" or "azotoluene"
components of Brown FK (components I & II, respectively) for 6 weeks.
With both components, the thyroids were dark and the intestines and
squamous portions of the stomach were stained salmon-pink. Heart
lesions were seen in all mice fed 1% component II, but not in those
given component I. More pigment was seen in mice fed component I, less
in those fed component II (Kirby, 1968a).
Rats
Groups of 3 male and 3 female Colworth-Wistar rats were fed
component I or component II at dietary levels of 0, 0.5, or 1% for 6
weeks. The thyroids of rats receiving component I were dark and the
hearts, muscles, and brains were stained. However, the intestines were
stained only slightly. Pale hearts and meningeal haemorrhage were seen
with component II, otherwise pigmentation was as with component I.
One-sixth of the rats treated with component I and four-fifths of the
rats treated with component II had heart lesions. More pigment was
seen histologically in component I-treated rats, less in component
II-treated animals (Kirkby, 1968a).
Special studies on amine metabolites of Brown FK
Amines derived from Brown FK and from its 2 myotoxic components,
components I and II, were injected i.v. into rats in single doses of
3.13-25 mg/kg. The mixture of amines from Brown FK was also injected
into mice in the same range of doses. Cardiac and muscular lesions
were produced by the amines in both species. These amines are
biological degradation products in the intestine. The finding that
orally-administered Brown FK is myotoxic in rats but not in mice is
probably due to differences in the intestinal flora in the 2 species
(Walker et al., 1970).
In another study, 1,2,4-triaminobenzene was given to groups of
6-7 rats orally 5 days/week for 2 weeks at 50, 60, 75, or 100 mg/kg
b.w./day. Six rats out of 7 receiving 100 mg/kg/day died after 3 doses
with severe heart lesions; 7/11 on 75 mg/kg/day also died after 3
doses. Heart pigmentation occurred after 5 days' treatment or longer.
Animals on lower doses showed both extensive heart pigmentation and
cardiac necrosis (Mulky et al., 1969).
1,2,4,5-Tetraaminobenzene was given to groups of 6 rats orally
5 days/week for 2 weeks at 150 or 200 mg/kg b.w./day. 1,2,3,4-
Tetraaminobenzene was given to groups of 6 rats orally 5 days/week for
2 weeks at 125 or 166 mg/kg b.w./day. No frank heart lesions and only
instances of diffuse increase in interstitial cells in the heart were
observed. No heart or thyroid pigment deposition was observed (Mulky
et al., 1969).
Acute toxicity
LD50
Species Route (mg/kg b.w.) Reference
Mouse oral >2,000 (with salt) Grasso et al., 1968a
oral 1,100-2,250 (with salt) Edwards & Wilson, 1966
oral 960-1,149 (no salt) Edwards & Wilson, 1966
i.p. 1,500-2,000 (with salt) Grasso et al., 1968a
i.p. 960-1,720 (with salt) Edwards & Wilson, 1966
i.p. 840-880 (no salt) Edwards & Wilson, 1966
Rat oral >8,000 (with salt) Grasso et al., 1968a
oral 900-1,910 (with salt) Edwards & Wilson, 1966
oral 780-970 (no salt) Edwards & Wilson, 1966
i.p. 750-1,150 (with salt) Grasso et al., 1968a
i.p. 1,100-2,250 (with salt) Edwards & Wilson, 1966
i.p. 960-1,150 (no salt) Edwards & Wilson, 1966
Guinea-pig oral 3,000 (with salt) Edwards & Wilson, 1966
oral 2,610 (no salt) Edwards & Wilson, 1966
i.p. 900 (with salt) Edwards & Wilson, 1966
i.p. 780 (with salt) Edwards & Wilson, 1966
Rabbit oral 450-680 (with salt) Edwards & Wilson, 1966
oral 390-590 (no salt) Edwards & Wilson, 1966
Chicken oral >10,000 (with salt) Edwards & Wilson, 1966
oral >8,700 (no salt) Edwards & Wilson, 1966
For all species, animals dying did so from within a few minutes
to 96 hours. Many animals, after either oral or i.p. treatment, showed
lack of coordination, hypersensitivity, and hyperactivity; convulsions
usually preceded death (Edwards & Wilson, 1966).
Meningeal congestion or haemorrhage was seen at post-mortem
examination in rats and mice which died following both oral and i.p.
treatment with 3.4 g/kg Brown FK as a 10% solution. This was the
highest dose administered, and the condition may have been present to
a lesser degree in animals treated with lower levels of Brown FK, but
post-mortem identification of the lesion was made difficult by tissue
colouration. The meningeal congestion/haemorrhage was probably caused
by the sodium chloride in the dye solution, since this lesion is
observed after administration of hypertonic solutions of sodium
chloride to rats. In this instance, the lowest dose levels at which
the meningeal lesion was observed were 6.0 g/kg of sodium chloride
orally as a 10% solution and 4.0 g/kg i.p. as a 5% solution (Edwards &
Wilson, 1966).
Following oral intubation, external tissue colouration was
apparent after several hours in rats and guinea-pigs. No colouration
of the tissues was seen in rabbits and chickens. After i.p. injection,
external tissue colouration was apparent and intense after a few
minutes in rats and guinea-pigs. Colour was seen in the faeces of
rats, mice, rabbits, and guinea-pigs up to 24 hours after oral
treatment; it was also excreted in the urine of rats, mice, guinea-
pigs, and rabbits within 15 minutes of either oral or i.p. treatment
(Edwards & Wilson, 1966).
Hearts from some rats and mice surviving for 21 days after
treatment were examined histologically. Degenerative lesions were
found in 15% of rats given orally 1-2.5 g/kg b.w. Brown FK, but not in
rats given 3.37 g/kg b.w. Brown FK. The same lesions were found in 50%
of mice given orally 0.9 g/kg b.w. Brown FK, but not when given 0.6,
1.35, or 2.03 g/kg b.w. Brown FK. When given i.p., 25-60% of mice
showed lesions at 0.75 and 1.03 g/kg b.w. Brown FK (Edwards & Wilson,
1966).
Short-term studies
Mice
Groups of 10 male or 10 female mice received the colour (either
fresh or stored) at a level of 1 g/kg daily for 3 weeks. A significant
reduction in body-weight gain was noted in the mice receiving the
stored solution, but not in those receiving fresh solution. One male
and 1 female receiving the fresh solution showed cardiac lesions
(BIBRA, 1964).
Daily oral or i.p. doses up to 2 g/kg or 1 g/kg, respectively,
for 43 days to groups of 10 or 12 mice were well-tolerated (Grasso
et al., 1968a).
Groups of 10 male and 10 female mice (Colworth C57Bl strain,
initially 6 weeks old) were fed for 90 days on a synthetic diet
containing 0, 0.05, 0.075, 0.10, 0.25, 0.50, 0.75, 1.0, or 2.0% Brown
FK (equivalent to 0, 0.025, 0.0375, 0.05, 0.125, 0.25, 0.375, 0.50, or
1.0% Brown FK-coloured components) containing 51% dye component and
47% salt. A further group of 20 mice were fed synthetic diet
containing added 1.0% sodium chloride as a control for the additional
dietary salt derived from the Brown FK. At the 0.125% dietary colour
level, pigment deposition occurred in tissues. At 0.25% and above
there was splenic enlargement, at 0.50% the liver and heart were
enlarged, and at 1.0% there was reduced growth, poor food utilization,
liver, spleen, heart, and testicular enlargement, and histological
evidence of degenerative heart lesions. The thyroids, muscle,
intestine and squamous part of the stomach were pigmented (Ashmole
et al., 1958).
Rats
Groups of animals received the colour at a level of 0.5 g Brown
FK per kg b.w. for 3 weeks, orally or i.p. Twenty rats were dosed
orally, of which 6 animals died after having been administered between
5 and 11 doses. Post-mortem examination of rats dying during the test
or killed at the end revealed general tissue-staining in 5 rats. Of 18
hearts examined histologically, 8 showed degenerative lesions, and a
brown pigment was observed in small amounts in 9 hearts after 3 weeks.
In the multiple-dose i.p. test, 8 rats were treated and none died
during the treatment period. General organ-staining was observed in
all animals at post-mortem examination. Hearts from 7 rats were
examined microscopically and degenerative lesions were found in 1
heart and small amounts of pigment in 3 hearts after 3 weeks (Kirkby,
1968a).
No ill-effects were seen in 3 weanling rats given a 0.1% solution
Brown FK for 28 days, the intake being equivalent to 15 mg/day
(Goldblatt & Frodsham, 1952).
Administration of 2 or 3 oral doses of 1 g Brown FK/kg b.w.
to rats induced a myopathy in cardiac and skeletal muscles
characterized by multiple vacuoles about 1-2 micrometers in diameter.
Ultrastructurally, these were shown to consist of areas of
fibrollolysis. Histochemically, the myopathy was accompanied by a
moderate increase in acid phosphatase activity and by a loss of
phosphorylase activity. Subsequently, complete lysis of the affected
fibres ensued. In the heart, lysis was followed by macrophage invasion
and fibroblastic proliferation, and in skeletal muscle by
regeneration. The occurrence of lipofuscin in muscle fibres and in
macrophages was scanty and erratic. When Brown FK was given in the
diet at a level of 2%, fibrillolysis and an increase in the number and
electron-density of lysosomes was observed ultrastructurally during
weeks 2 to 3 of the test. These changes were accompanied by a marked
elevation of histochemically-demonstrable acid phosphatase.
Progressive deposition of lipofuscin was the principal pathological
feature during weeks 3 to 12 (Grasso et al., 1968b).
Daily oral doses of up to 2 g/kg Brown FK for 43 days to groups
of 10 rats induced rapid loss of weight and death, with severe damage
to cardiac and skeletal muscle, characterized by vacuolar myopathy and
lipofuscin deposition. Of 3 pure components of Brown FK studied,
component II, and to a lesser extent component I, produced similar,
but not identical lesions to those induced by the parent colour after
repeated oral doses of 0.5 g/kg. Ultrastructural studies confirmed an
extensive loss of myofibrillar elements, and histochemical studies
revealed a loss in the activity of mitochondrial enzymes. Similar i.p.
injections in doses up to 1.0 g/kg for 43 days to groups of 10 or 12
rats did not have any effect on the heart or skeletal muscle (Grasso
et al., 1968b).
Experiments were performed using groups of 10-12 rats receiving
the colour at levels of 0.1 or 1 g/kg orally or 0.1, 0.25, or 1 g/kg
i.p. daily for up to a maximum of 43 doses. A specific cardiac lesion
was identified at the oral dose of 1 g/kg. There were large areas of
myocardial necrosis and replacement by large mononuclears, with
involvement of the sub-pericardial region and endocardium. Some
myocardial cells had lost their stainable cytoplasm and appeared only
as empty sheaths. When administered i.p., the colour produced little
or no cardiac damage at any dose tested. At these high doses of
1 g/kg, most animals showed congestion, fatty change, or necrosis of
the liver with hydropic degeneration of the kidney. There was no
obvious splenomegaly. Daily doses of 100 mg/kg by stomach tube
produced 2 pericardial and 1 sub-pericardial lesions. In addition,
early hydroponic degeneration of the kidney was seen in 2 rats, with
1 of these animals also showing fatty change in the liver (BIBRA,
1964).
Administration of Brown FK (purity 80.0%) at dietary levels of 0,
0.001, 0.01, 0.1, or 1.0% for 150 days showed no adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. One male rat at the 1.0% level showed
typical myocardial changes; other rats showed deposits of lipofuscin,
especially in females. The no-effect level was 0.1% (Gaunt et al.,
1968).
Groups of 12 male and 12 female rats (Colworth-Wistar strain,
initially 3-4 weeks old) were fed for 112 days on a commercial stock
diet containing 0, 0.05, 0.1, 0.5, 1.0, or 2.0% Brown FK (0, 0.025,
0.05, 0.25, 0.5, or 1.0% Brown FK-coloured components) containing 51%
dye component and 47% salt. A further group of 24 rats were fed the
commercial stock diet containing added 1.0% sodium chloride as a
control for the additional dietary salt derived from the Brown FK. In
addition, to eliminate damage to the heart from cardiac puncture in
any rat kept to 16 weeks, groups of 6 male and 6 female rats were fed
6 weeks on powdered stock diet containing 0, 0.05, 0.5, or 2.0% Brown
FK (0, 0.025, 0.25, or 1.0% Brown FK-coloured components). A group of
12 rats also received 1.0% sodium chloride added to the basic diet.
All these rats were used for biochemical tests during weeks 0-6 after
which they were killed. At the 0.25% level tissue pigmentation
appeared, at 0.5% liver enlargement occurred, and at the 1% level
there was reduced growth, poor food utilization, enlargement of the
liver, testes, and thyroid, histological evidence of degenerative
heart lesions, increased urinary indican excretion, and elevation of
SGOT. The intestine, squamous portion of the stomach, and the thyroid
were stained. The no-effect level was 0.05% (Ashmole et al., 1966).
Pigs
Groups of female and male pigs were given doses of 0, 100, 250,
or 500 mg Brown FK/kg/day for 24 weeks without adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. Lipofuscin was widely distributed in
animals of both sexes at all dose levels in one or more organs. The
liver was particularly affected in that lipofuscan deposition was
accompanied by increased lysosomal enzyme activity, which was more
marked at the higher dose-levels. It was also seen in the heart in
males, where it was associated with an increased acid phosphatase
activity, and in the kidneys at the highest-dose level in females and
at all levels in males. A no-effect level could not be determined in
this study (Gaunt et al., 1968).
Long-term studies
Mice
Groups of 40 male and 40 female Colworth C57Bl mice were fed for
80 weeks on a synthetic diet containing 0, 0.0125, 0.0375, 0.075,
0.125, or 0.625% Brown FK-coloured components (the Brown FK used in
this study contained 62.5% coloured components). Only at the 0.625%
level was there reduced growth and food utilization and increased
mortality among females. There were increased liver, kidney, spleen,
brain, and testes weights, evidence of splenic haemopoisis, and
increased myocardial fibrosis. Heart weights were increased at the
0.125% level. Increased hepatic nodules were seen at 0.075% and higher
levels and pigment deposition at 0.0375% and higher levels. At
termination, after 80 weeks, the number of animals with nodules at the
different dose levels was 26, 23, 27, 56, 42, and 64, respectively.
Increased hepatic nodules were observed at 0.075% and higher levels.
The number of mice with hepatocellular carcinoma were 3, 2, 0, 5, 6,
and 2 at the various dose levels, respectively. Pigment deposition was
observed at dose levels of 0.0375% and higher (Wilson et al., 1970).
Rats
Groups of 32 male or 36 female Colworth-Wistar rats were fed for
2 years on a synthetic diet containing 0, 0.01, 0.03, 0.06, 0.1, or
0.5% Brown FK-coloured components (Brown FK used in this study
contained 54.2% coloured components). Only at the 0.5% level was there
increased splenic weight and hepatic granulomata. Pigment deposition
was seen at dose levels of 0.06% and higher. The no-effect level for
pigment deposition was 0.03%; it was 0.06% when based on toxicity
evidence (Wilson et al., 1971).
Observations in man
No information available.
Comments
The carcinogenicity study did not reveal any increase in the
incidence of tumours, nor did the reproduction and teratogenicity
studies show any adverse effects on reproductive function.
The myopathy seen in rats given high doses of Brown FK in short-
term studies affected all striated muscle, was accompanied by pigment
deposition, and was dose-dependent with a high threshold. In the long-
term/carcinogenicity study in rats, the observed myocarditis was not
dose-related and there was no pigmentation in animals of the low-dose
group nor controls. However, deficiencies in histopathological
examination of tissues from the low- and intermediate-dose groups
hindered the establishment of a no-effect level in this study.
In earlier long-term studies, the no-effect level (with respect
to pigment deposition) was 0.03% in the diet, equivalent to 15 mg/kg
b.w./day, based on the coloured components of Brown FK.
EVALUATION
Level causing no toxicological effect
Mouse: 0.0125% in the diet, equivalent to 19 mg/kg b.w./day.
Rat: 0.03% in the diet, equivalent to 15 mg/kg b.w./day.
Estimate of temporary acceptable daily intake for man
0-0.075 mg/kg b.w. (based on colour components)
Further work or information
Required by 1986
A complete histopathological examination of tissues from the low-
and intermediate-dose groups in the long-term/carcinogenicity study in
rats.
REFERENCES
Amyes, S.J., McSheehy, T.W., & Whitney, J.C. (1983). Brown FK: Life
span combined toxicity and oncogenicity study in rats pre-exposed
in utero. 2. Toxicity and oncogenicity phase. Unpublished
report No. 82/URL012/573 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Ashmole, R.T., Campbell, P., Kirkby, W.W., & Wilson, R. (1966).
Effects of feeding dietary Brown FK to rats for six and 16 weeks.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Ashmole, R.T., Kirkby, W.W., & Wilson, R. (1958). Thirteen week mouse
feeding trial. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
BIBRA (1964). Unpublished research report No. 5/1964 from British
Industrial Biological Research Association, Carshalton, Surrey,
England. Submitted to WHO.
Edwards, K.B. & Wilson, R. (1966). Acute toxicity of Brown FK in rats,
mice, guinea-pigs, rabbits and chickens. Unpublished report from
Unilever Research Laboratories.
Fore, H. & Walker, R. (1967). Studies on Brown FK. I. Composition and
synthesis of components. Fd. Cosmet. Toxicol., 5, 1-9.
Fore, H., Walker, R., & Golberg, L. (1967). Studies on Brown FK. II.
Degradative changes undergone in vitro and in vivo. Fd.
Cosmet. Toxicol., 5, 459-473.
Fuller, A.T. (1937). Is rho-aminobenzene sulphonamide active agent in
prontosil therapy? Lancet, 1, 194-198.
Gaunt, I.F., Hall, D.E., Grasso, P., & Golberg, L. (1968). Studies on
Brown FK. V. Short-term feeding studies in the rat and pig. Fd.
Cosmet. Toxicol., 6, 301-312.
Goldblatt & Frodsham (1952). Private communication from ICI
(unpublished report).
Grasso, P., Gaunt, I.F., Hall, D.E., Golberg, L., & Batstone, E.
(1968a). Studies on Brown FK. III. Administration of high doses
to rats and mice. Fd. Cosmet. Toxicol., 6, 1-11.
Grasso, P., Muir, A., Golberg, L., & Batstone, E. (1968b). Cytopathic
effects of Brown FK on cardiac and skeletal muscle in the rat.
Fd. Cosmet. Toxicol., 6, 13-24.
Hope, J. (1971). Ultrastructure of the pigment induced in various
tissues of the rat by long-term feeding of the dye Brown FK.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Howes, D. (1969). Metabolism of 14C labelled 1,3-diamino-4-(rho
sulphophenylazo)benzene, a component of the dye Brown FK, in the
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Jenkins, F.P. & Favell, D.J. (1971). Metabolism of the
"monoazobenzene" component of Brown FK in human subjects.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Kirkby, W.W. (1968a). Effects of Brown FK and two of its constituents
on pigment deposition and lesions in rats and mice. Unpublished
report from Unilever Research Laboratories. Submitted to WHO by
Unilever Ltd.
Kirkby, W.W. (1968b). Nature of the pigment induced in tissues of rats
and mice fed Brown FK. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Mulky, M.J., Mundy, R., Ashmole, R.T., & Kirkby, W.W. (1969).
Evaluation of the terminal causative agent in Brown FK induced
myopathy and pigment deposition. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Muller, E. (1889). Chem. Ber., 22, 856.
Munday, R. (1969). Metabolism of 2,4-diamino-5-(rho-sulphophenylazo)
toluene. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Munday, R. (1971). Uncoupling of oxidative phosphorylation by Brown FK
metabolites. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Munday, R. & Kirkby, W.W. (1969). Metabolism of 1,3-diamino-4-(rho
sulphophenylazo)benzene. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Roe, F.J.C. (1983). Histopathological evaluation of sections derived
from URL/12/BFK lifespan combined toxicity and oncogenicity study
in rats pre-exposed in utero. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Tesh, J.M., McSheehy, T.W., McAnulty, P.A., & Collier, M.J. (1980).
Brown FK: Lifespan combined toxicity and oncongenicity study in
rats pre-exposed in utero. 1. Reproductive phase. Unpublished
report No. 80/URL012/051 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Unilever (1978). Teratology study on Brown FK in the Colworth-Wistar
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Venitt, S. & Bushell, C.T. (1976). Mutagenicity of the food colour
Brown FK and constituents in Salmonella typhimurium. Mutation
Research, 40, 309-316.
Walker, R. (1968). Intestinal degradation of azo food colours with
particular reference to Brown FK. Ph.D. Thesis, University of
Reading, U.K.
Walker, R., Grasso, P., & Gaunt, I.F. (1970). Myotoxicity of amine
metabolites from Brown FK. Fd. Cosmet Toxicol., 8, 539-542.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1970).
Biological evaluation of Brown FK; 80-week mouse feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1971).
Biological evaluation of Brown FK: 2-year rat feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Hague, P.H., & Hardy, W.S. (1983a). Brown FK: Lifespan
combined toxicity and oncogenicity study in rats pre-exposed in
utero: Examination of selected tissues for Brown FK associated
pigment. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Wilson, R., McCormick, S.G., Cook, H.J., Norris, L., Robinson, J.A., &
Williams, T.C. (1983b). Evaluation of the effects of food colour
Brown FK on the fertility and reproductive performance of rats.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.

 The metabolism of 1,3-diamino-4-(4'-sulfophenylazo)-benzene
(component I) in the rat is summarized below (Howes, 1969; Munday &
Kirby, 1969):
The metabolism of 1,3-diamino-4-(4'-sulfophenylazo)-benzene
(component I) in the rat is summarized below (Howes, 1969; Munday &
Kirby, 1969):
 Preliminary examination of urine from rats fed component II
showed the presence of sulfanilic acid and small quantities of
unchanged dye. Examination of an extract of the urine revealed the
presence of 5-acetamido-2,4-diaminotoluene (the major metabolite),
2,5-diacetamido-4-aminotoluene, 2,4-diacetamido-5-amino-toluene, and
4,5-diacetamido-2-amino-toluene. Unchanged dye was identified in
faecal extracts; no other dye-derived compounds were detected.
The metabolism of 2,4-diamino-5-(4'-sulfophenylazo)-toluene
(component II) in the rat is summarized below (Munday, 1969):
Preliminary examination of urine from rats fed component II
showed the presence of sulfanilic acid and small quantities of
unchanged dye. Examination of an extract of the urine revealed the
presence of 5-acetamido-2,4-diaminotoluene (the major metabolite),
2,5-diacetamido-4-aminotoluene, 2,4-diacetamido-5-amino-toluene, and
4,5-diacetamido-2-amino-toluene. Unchanged dye was identified in
faecal extracts; no other dye-derived compounds were detected.
The metabolism of 2,4-diamino-5-(4'-sulfophenylazo)-toluene
(component II) in the rat is summarized below (Munday, 1969):
 An attempt was made to determine whether 1,3-diamino-4
(4'-sulfophenylazo)-benzene (component I) is reductively cleaved in
humans, as in rats. Reduction of the closely-related compound,
prontosil rubrum, has been shown to occur in human subjects (Fuller,
1937).
An attempt was made to determine whether 1,3-diamino-4
(4'-sulfophenylazo)-benzene (component I) is reductively cleaved in
humans, as in rats. Reduction of the closely-related compound,
prontosil rubrum, has been shown to occur in human subjects (Fuller,
1937).
 Administration of component I to human subjects led to no
detectable unchanged dye in the urine and no appreciable urinary
excretion of sulfanilic acid. It can be inferred from these results
that component I is not absorbed from the intestine as such, but no
information was given on the possible reduction of this compound
in vivo, since it was shown that orally-administered sulfanilic acid
is not absorbed in man. Sulfanilic acid, if formed from the dye, would
therefore be excreted in the faeces; the experimental confirmation of
this was not provided and these studies were not pursued further
(Jenkins & Favell, 1971).
1,2,4-triaminobenzene and 2,4,5-triaminotoluene have been shown
to uncouple oxidative phosphorylation in vitro, interfering with ATP
production in the muscle cell, and to ionic imbalance with cell death
(Munday, 1971).
Toxicological studies
Special studies on carcinogenicity
Rats
A carcinogenicity study on Brown FK was performed in CD rats with
an in utero exposure phase. Animals of the F0 generation (390 of
each sex) were allocated to 6 groups; 2 groups were untreated
(controls), 3 groups received diets containing Brown FK at constant
dietary concentrations of 160, 530, or 2630 ppm (expressed as
"coloured components of Brown FK"), and 1 group received sodium
chloride at an amount equivalent to the amount of sodium salts
received by the highest Brown FK-dose group. The animals received
these diets for 14 days prior to pairing and during pregnancy and
lactation. At weaning (28-31 days post-partum), 60 male and 60 female
animals of the F1 generation were selected from each dose group for
the long-term study. Thereafter, the Brown FK concentration in the
diet of the treated groups was adjusted to maintain constant dosages
of Brown FK of 15, 50, or 250 mg/kg b.w./day (expressed in terms of
coloured components); 2 groups served as untreated controls and one
group (salt control) received sodium chloride equivalent to the amount
of sodium salts received by the highest Brown FK-dose group. The study
was terminated when mortality in any treatment group exceeded 75%,
males and females being considered separately. Accordingly, terminal
sacrifices were initiated 105 weeks and 110 weeks after weaning of
males and females, respectively.
During the carcinogenicity study, animals were inspected twice
daily and palpated once weekly. Moribund animals were sacrificed and a
complete necropsy was performed on these rats and on those which died
during the course of the study. Ophthalmoscopic examinations were
performed on 20 males and 20 females from 1 control group and the
top-dose group after 26, 52, 77, and 102 weeks; the same animals were
examined on all 4 occasions and animals that died or were killed were
not replaced. Urinalysis was carried out on 10 rats of each sex from
each dose group after 103 weeks (males) or 105 weeks (females).
Haematological examinations and clinical chemistry investigations were
performed on 10 males and 10 females of each group after 104 weeks
(males) or 106 weeks (females).
Rats killed in extremis or at termination were subjected to a
complete necropsy and the following organ weights were recorded:
adrenals, brain, heart, kidneys, liver, ovaries/testes, pituitary,
spleen, and thyroid. Histopathological examinations were performed on
rats of both sexes from 1 control group, the top-dose group, the salt-
control group, and on any organs from the other groups that displayed
gross abnormalities. The tissues examined histopathologically
included; adrenals, aortic arch, bone, bone marrow, brain (3 levels),
caecum, colon, diaphragm, duodenum, epididymides, eyes, heart, ileum,
jejunum, kidneys, liver (2 lobes), lungs, lymph nodes (cervical and
mesenteric), mammary gland, nasal cavities, oesophagus, ovaries,
pancreas, parathyroids, pituitary, prostate, salivary gland, sciatic
nerve, seminal vesicles, skeletal muscle, skin, spinal cord
(2 levels), spleen, stomach, testes, thymus, thyroid, tongue, trachea,
urinary bladder, and uterus (including cervix).
In the reproductive phase of the study, there were no treatment-
related effects on general condition, food intake, body-weight gain,
mating performance, conception rate, or length of gestation. Litter
size, growth, and viability of the offspring were unaffected by
treatment with Brown FK. In the carcinogenicity phase, body-weight
gains in rats of either sex receiving Brown FK at a dose of 250 mg/kg
b.w./day were lower than body-weight gains in the combined control
groups; at termination the weight decrements were 14 and 10% for males
and females, respectively. Food consumption was not affected by
treatment, but water intakes of rats receiving 250 mg/kg b.w./day of
Brown FK and of rats in the salt-control groups were greater than of
untreated controls. No treatment-related effects were seen in
ophthalmic or haematological examinations, nor in urine composition.
Clinical chemistry investigations revealed higher creatine
phosphokinase, lactate dehydrogenase, and hydroxybutyrate
dehydrogenase activities in female rats of the top-dose group compared
with untreated controls, but not compared with salt controls.
Isocitrate dehydrogenase activities were higher among rats of both
sexes receiving the highest dose of Brown FK than among controls; no
effects were seen at the lower doses. In males, but not females, of
the highest-dose group, plasma albumin and T4 concentrations were
significantly elevated.
A total of 252 male and 236 female animals died or were killed
in extremis during the treatment period, but the mortality
distribution was unrelated to treatment. A total of 277 males and 308
females had palpable swellings during the treatment period but the
distribution, frequency, and time of onset were unaffected by
treatment.
No treatment-related differences were observed in absolute or
relative organ weights at necropsy, but the incidence of dark thyroid
glands among animals of the top-dose group was higher than controls.
On histopathological examination, rats exposed to the highest-dose
level of Brown FK exhibited deposition of brown pigment at particular
sites (heart, skeletal muscle, diaphragm, tongue, thyroid, caecum, and
hepatic kupffer cells) but this was not associated with any tissue
reaction and was not observed at dose levels of 15 or 50 mg/kg
b.w./day of Brown FK. Treatment with Brown FK was not associated with
enhancement of neoplasia at any site (there was a reduced incidence of
tumours in animals of the top-dose group compared with controls).
Chronic myocarditis occurred with high frequency in all groups,
but the incidence and severity were no greater in treated animals than
in controls. In females, but not males, exposure to the highest-dose
level of Brown FK was associated with an increased incidence of pelvic
nephrocalcinosis; this effect was not seen at lower-dose levels. There
was an increase in cystic distension of the follicles of the thyroid
in high-dose group females, where the lesion was seen in 40.7% of the
animals, compared with 17.2% of the controls; there was no evidence of
any effect at lower-dose levels.
The authors concluded that Brown FK was not carcinogenic in rats
under the conditions of the experiment and that the no-effect level
was 50 mg/kg b.w./day (Tesh et al., 1980; Amyes et al., 1983; Roe,
1983).
Special studies on mutagenicity
Brown FK and its constituents were assayed for mutagenicity in
Salmonella typhimurium TA1535, TA1537, and TA1538 when activated by
a rat-liver supernatant fraction. Mutagenicity was linearly dose-
dependent in the range 0-3 mg/plate, with activities ranging from 22
to 50 times the spontaneous mutation frequency. One sample of Brown FK
was mutagenic in the absence of metabolic activation, producing a
16-fold increase in mutation at 4 mg/plate. Two major constituents
of Broom FK, 2,4-diamino-5-(4'-sulfophenylazo)toluene (II) and
1,3-diamino-4-(4'-sulfophenylazo)benzene (I), each present at about
18% in the complete colour, were mutagenic in TA1538. Mutagenicity was
linearly dose-related in the range 0-1 Ámol/plate, with slopes of
1.5 mutants/nmol for compound I and 0.35 mutants/nmol for compound II.
This activity was dependent on metabolic activation. Four other major
constituents were inactive, as was sulfanilic acid, the major
excretion product. The combined effects of compounds I and II could
largely account for the mutagenicity of Brown FK (Venitt & Bushell,
1976).
Special studies on reproduction
Rats
A multigeneration reproduction study was carried out in rats in
which groups of 24 of each sex were given Brown FK in the diet at a
level of 300 ppm for 5 weeks post-weaning, then at 600 ppm for 3
successive generations; control animals received stock diet. After
weaning, the parental animals and offspring not selected for breeding
in the succeeding generation were culled. Haematological and clinical
chemistry studies were performed on 10 animals of each sex from the
parents and offspring, and autopsies carried out. Selected organ
weights were recorded at autopsy on the parents and on 30 animals of
each sex per group from the offspring. Histopathology was performed on
F3 weanlings, 10 animals of each sex per group.
Fertility, number of young per litter, birth weights, growth
rates, gestation indices, viability indices, and lactation indices
were unaffected by treatment; there was no indication of increased
mortality in utero. No treatment-related lesions were observed at
autopsy nor on histopathological examination of F3 weanlings. In
clinical observations (plasma biochemistry/haematology) and in organ
weights, occasional statistically-significant differences were seen;
these were not consistent and, in the absence of pathological lesions,
were not considered to be of toxicological significance. Under the
conditions of the study, Brown FK was without adverse effect on
reproductive performance (Wilson et al., 1983b).
Special studies on teratogenicity
Rats
Groups of 35 virgin female Wistar rats were mated with virgin
males and were fed diets containing 0, 0.03, 0.15, or 0.6% Brown FK
from day 0 to day 19 post coitus. Additional groups received 0.6%
sodium chloride (salt control) or aspirin (250 mg/kg b.w./day).
Successful pregnancies were achieved in 32-35 animals per dose group.
Five pregnant animals per group were allowed to litter normally and
then raise their offspring to weaning. The remaining animals were
sacrificed on day 21 of gestation and the foetuses removed by
Caesarian section. Two-thirds of the foetuses were examined for gross
soft-tissue abnormalities, then cleared and stained with Alizarin red
for examination for skeletal defects. The remaining one-third were
examined for soft tissue defects using Wilson's technique. No
treatment-related abnormalities were observed in any of the groups
receiving Brown FK, and this was confirmed in the groups which were
examined at weaning. Aspirin used as a positive control induced
teratological defects in foetuses and in young reared to 21 days
post partum (Unilever, 1978).
Special studies on pigment deposition
After feeding Brown FK to rats and mice, pigment was found in
heart, skeletal muscle, tongue, diaphragm, thyroids, brain, liver,
kidneys, spleen, lungs, pancreas, bladder, testes, ovary, uterus,
skin, stomach, duodenum, ileum, brown fat, and bone marrow. In
addition, a pigment has been detected in the plasma of rats.
Staining tests commonly used to identify lipofuscin were negative
with the exception of the test for metachromasia with toluidine blue.
The tests applied were as follows;
Usual response of Response of Brown
known lipofuscin FK-induced pigment
Test for iron negative negative
Sudan fat stains positive negative
Reduction of ferric salts positive negative
Reduction of ammoniacal
silver salts positive negative
Basophilic properties positive negative
Periodic acid - schiff
reaction positive negative
Acid fastness acid fast negative
Toluidine blue at pH3 stains greenish
metachromatically
green
Two further histochemical tests clearly differentiated between
lipofuscin and the Brown FK-induced pigment:
- Potassium permanganate/oxalic acid bleached lipofuscin, but not
the Brown FK-induced pigment.
- Sodium dithionite bleached both lipofuscin and the Brown FK-
induced pigment. However, after rinsing and allowing to stand in
air, the Brown FK-induced pigment reappeared; lipofuscin was
permanently bleached.
The Brown FK-induced pigment does not fluoresce in ultra-violet
light. On the other hand, all samples of lipofuscin which Brown FK
have been examined were fluorescent. Pigment has been found in the
thyroid, brown fat, and bone marrow; these tissues have not been
recorded as being sites for lipofuscin deposition. Furthermore, a
coloured substance has been demonstrated in the plasma, which has
never been found with lipofuscin.
The speed at which the Brown FK-induced pigment is deposited is
uncharacteristic of lipofuscin information. In acute studies, pigment
has been seen in the intestinal wall and villi within 24 hours of
feeding the dye, and in the kidney after 5 days. Pigment masses
produced in macrophages either in vivo after the intraperitoneal
injection of Brown FK into mice, or in vitro, when Brown FK was
incorporated in the macrophage culture medium, appeared identical.
Tests for lipofuscin proved negative; the pigment in the macrophages
closely resembled that seen in macrophages in stained sections of
tissues from rats and mice fed Brown FK.
Electron microscope studies have identified differences in
morphology between lipofuscin and the Brown FK-induced pigment. In
aged rats and mice fed Brown FK, conjugate forms were observed in
which induced pigment and control lysosomal material appeared in the
same membrane-limited body (Hope, 1971).
It is likely that a component of Brown FK is oxidized within the
cell to 1,4,7-triaminophenazine:
Administration of component I to human subjects led to no
detectable unchanged dye in the urine and no appreciable urinary
excretion of sulfanilic acid. It can be inferred from these results
that component I is not absorbed from the intestine as such, but no
information was given on the possible reduction of this compound
in vivo, since it was shown that orally-administered sulfanilic acid
is not absorbed in man. Sulfanilic acid, if formed from the dye, would
therefore be excreted in the faeces; the experimental confirmation of
this was not provided and these studies were not pursued further
(Jenkins & Favell, 1971).
1,2,4-triaminobenzene and 2,4,5-triaminotoluene have been shown
to uncouple oxidative phosphorylation in vitro, interfering with ATP
production in the muscle cell, and to ionic imbalance with cell death
(Munday, 1971).
Toxicological studies
Special studies on carcinogenicity
Rats
A carcinogenicity study on Brown FK was performed in CD rats with
an in utero exposure phase. Animals of the F0 generation (390 of
each sex) were allocated to 6 groups; 2 groups were untreated
(controls), 3 groups received diets containing Brown FK at constant
dietary concentrations of 160, 530, or 2630 ppm (expressed as
"coloured components of Brown FK"), and 1 group received sodium
chloride at an amount equivalent to the amount of sodium salts
received by the highest Brown FK-dose group. The animals received
these diets for 14 days prior to pairing and during pregnancy and
lactation. At weaning (28-31 days post-partum), 60 male and 60 female
animals of the F1 generation were selected from each dose group for
the long-term study. Thereafter, the Brown FK concentration in the
diet of the treated groups was adjusted to maintain constant dosages
of Brown FK of 15, 50, or 250 mg/kg b.w./day (expressed in terms of
coloured components); 2 groups served as untreated controls and one
group (salt control) received sodium chloride equivalent to the amount
of sodium salts received by the highest Brown FK-dose group. The study
was terminated when mortality in any treatment group exceeded 75%,
males and females being considered separately. Accordingly, terminal
sacrifices were initiated 105 weeks and 110 weeks after weaning of
males and females, respectively.
During the carcinogenicity study, animals were inspected twice
daily and palpated once weekly. Moribund animals were sacrificed and a
complete necropsy was performed on these rats and on those which died
during the course of the study. Ophthalmoscopic examinations were
performed on 20 males and 20 females from 1 control group and the
top-dose group after 26, 52, 77, and 102 weeks; the same animals were
examined on all 4 occasions and animals that died or were killed were
not replaced. Urinalysis was carried out on 10 rats of each sex from
each dose group after 103 weeks (males) or 105 weeks (females).
Haematological examinations and clinical chemistry investigations were
performed on 10 males and 10 females of each group after 104 weeks
(males) or 106 weeks (females).
Rats killed in extremis or at termination were subjected to a
complete necropsy and the following organ weights were recorded:
adrenals, brain, heart, kidneys, liver, ovaries/testes, pituitary,
spleen, and thyroid. Histopathological examinations were performed on
rats of both sexes from 1 control group, the top-dose group, the salt-
control group, and on any organs from the other groups that displayed
gross abnormalities. The tissues examined histopathologically
included; adrenals, aortic arch, bone, bone marrow, brain (3 levels),
caecum, colon, diaphragm, duodenum, epididymides, eyes, heart, ileum,
jejunum, kidneys, liver (2 lobes), lungs, lymph nodes (cervical and
mesenteric), mammary gland, nasal cavities, oesophagus, ovaries,
pancreas, parathyroids, pituitary, prostate, salivary gland, sciatic
nerve, seminal vesicles, skeletal muscle, skin, spinal cord
(2 levels), spleen, stomach, testes, thymus, thyroid, tongue, trachea,
urinary bladder, and uterus (including cervix).
In the reproductive phase of the study, there were no treatment-
related effects on general condition, food intake, body-weight gain,
mating performance, conception rate, or length of gestation. Litter
size, growth, and viability of the offspring were unaffected by
treatment with Brown FK. In the carcinogenicity phase, body-weight
gains in rats of either sex receiving Brown FK at a dose of 250 mg/kg
b.w./day were lower than body-weight gains in the combined control
groups; at termination the weight decrements were 14 and 10% for males
and females, respectively. Food consumption was not affected by
treatment, but water intakes of rats receiving 250 mg/kg b.w./day of
Brown FK and of rats in the salt-control groups were greater than of
untreated controls. No treatment-related effects were seen in
ophthalmic or haematological examinations, nor in urine composition.
Clinical chemistry investigations revealed higher creatine
phosphokinase, lactate dehydrogenase, and hydroxybutyrate
dehydrogenase activities in female rats of the top-dose group compared
with untreated controls, but not compared with salt controls.
Isocitrate dehydrogenase activities were higher among rats of both
sexes receiving the highest dose of Brown FK than among controls; no
effects were seen at the lower doses. In males, but not females, of
the highest-dose group, plasma albumin and T4 concentrations were
significantly elevated.
A total of 252 male and 236 female animals died or were killed
in extremis during the treatment period, but the mortality
distribution was unrelated to treatment. A total of 277 males and 308
females had palpable swellings during the treatment period but the
distribution, frequency, and time of onset were unaffected by
treatment.
No treatment-related differences were observed in absolute or
relative organ weights at necropsy, but the incidence of dark thyroid
glands among animals of the top-dose group was higher than controls.
On histopathological examination, rats exposed to the highest-dose
level of Brown FK exhibited deposition of brown pigment at particular
sites (heart, skeletal muscle, diaphragm, tongue, thyroid, caecum, and
hepatic kupffer cells) but this was not associated with any tissue
reaction and was not observed at dose levels of 15 or 50 mg/kg
b.w./day of Brown FK. Treatment with Brown FK was not associated with
enhancement of neoplasia at any site (there was a reduced incidence of
tumours in animals of the top-dose group compared with controls).
Chronic myocarditis occurred with high frequency in all groups,
but the incidence and severity were no greater in treated animals than
in controls. In females, but not males, exposure to the highest-dose
level of Brown FK was associated with an increased incidence of pelvic
nephrocalcinosis; this effect was not seen at lower-dose levels. There
was an increase in cystic distension of the follicles of the thyroid
in high-dose group females, where the lesion was seen in 40.7% of the
animals, compared with 17.2% of the controls; there was no evidence of
any effect at lower-dose levels.
The authors concluded that Brown FK was not carcinogenic in rats
under the conditions of the experiment and that the no-effect level
was 50 mg/kg b.w./day (Tesh et al., 1980; Amyes et al., 1983; Roe,
1983).
Special studies on mutagenicity
Brown FK and its constituents were assayed for mutagenicity in
Salmonella typhimurium TA1535, TA1537, and TA1538 when activated by
a rat-liver supernatant fraction. Mutagenicity was linearly dose-
dependent in the range 0-3 mg/plate, with activities ranging from 22
to 50 times the spontaneous mutation frequency. One sample of Brown FK
was mutagenic in the absence of metabolic activation, producing a
16-fold increase in mutation at 4 mg/plate. Two major constituents
of Broom FK, 2,4-diamino-5-(4'-sulfophenylazo)toluene (II) and
1,3-diamino-4-(4'-sulfophenylazo)benzene (I), each present at about
18% in the complete colour, were mutagenic in TA1538. Mutagenicity was
linearly dose-related in the range 0-1 Ámol/plate, with slopes of
1.5 mutants/nmol for compound I and 0.35 mutants/nmol for compound II.
This activity was dependent on metabolic activation. Four other major
constituents were inactive, as was sulfanilic acid, the major
excretion product. The combined effects of compounds I and II could
largely account for the mutagenicity of Brown FK (Venitt & Bushell,
1976).
Special studies on reproduction
Rats
A multigeneration reproduction study was carried out in rats in
which groups of 24 of each sex were given Brown FK in the diet at a
level of 300 ppm for 5 weeks post-weaning, then at 600 ppm for 3
successive generations; control animals received stock diet. After
weaning, the parental animals and offspring not selected for breeding
in the succeeding generation were culled. Haematological and clinical
chemistry studies were performed on 10 animals of each sex from the
parents and offspring, and autopsies carried out. Selected organ
weights were recorded at autopsy on the parents and on 30 animals of
each sex per group from the offspring. Histopathology was performed on
F3 weanlings, 10 animals of each sex per group.
Fertility, number of young per litter, birth weights, growth
rates, gestation indices, viability indices, and lactation indices
were unaffected by treatment; there was no indication of increased
mortality in utero. No treatment-related lesions were observed at
autopsy nor on histopathological examination of F3 weanlings. In
clinical observations (plasma biochemistry/haematology) and in organ
weights, occasional statistically-significant differences were seen;
these were not consistent and, in the absence of pathological lesions,
were not considered to be of toxicological significance. Under the
conditions of the study, Brown FK was without adverse effect on
reproductive performance (Wilson et al., 1983b).
Special studies on teratogenicity
Rats
Groups of 35 virgin female Wistar rats were mated with virgin
males and were fed diets containing 0, 0.03, 0.15, or 0.6% Brown FK
from day 0 to day 19 post coitus. Additional groups received 0.6%
sodium chloride (salt control) or aspirin (250 mg/kg b.w./day).
Successful pregnancies were achieved in 32-35 animals per dose group.
Five pregnant animals per group were allowed to litter normally and
then raise their offspring to weaning. The remaining animals were
sacrificed on day 21 of gestation and the foetuses removed by
Caesarian section. Two-thirds of the foetuses were examined for gross
soft-tissue abnormalities, then cleared and stained with Alizarin red
for examination for skeletal defects. The remaining one-third were
examined for soft tissue defects using Wilson's technique. No
treatment-related abnormalities were observed in any of the groups
receiving Brown FK, and this was confirmed in the groups which were
examined at weaning. Aspirin used as a positive control induced
teratological defects in foetuses and in young reared to 21 days
post partum (Unilever, 1978).
Special studies on pigment deposition
After feeding Brown FK to rats and mice, pigment was found in
heart, skeletal muscle, tongue, diaphragm, thyroids, brain, liver,
kidneys, spleen, lungs, pancreas, bladder, testes, ovary, uterus,
skin, stomach, duodenum, ileum, brown fat, and bone marrow. In
addition, a pigment has been detected in the plasma of rats.
Staining tests commonly used to identify lipofuscin were negative
with the exception of the test for metachromasia with toluidine blue.
The tests applied were as follows;
Usual response of Response of Brown
known lipofuscin FK-induced pigment
Test for iron negative negative
Sudan fat stains positive negative
Reduction of ferric salts positive negative
Reduction of ammoniacal
silver salts positive negative
Basophilic properties positive negative
Periodic acid - schiff
reaction positive negative
Acid fastness acid fast negative
Toluidine blue at pH3 stains greenish
metachromatically
green
Two further histochemical tests clearly differentiated between
lipofuscin and the Brown FK-induced pigment:
- Potassium permanganate/oxalic acid bleached lipofuscin, but not
the Brown FK-induced pigment.
- Sodium dithionite bleached both lipofuscin and the Brown FK-
induced pigment. However, after rinsing and allowing to stand in
air, the Brown FK-induced pigment reappeared; lipofuscin was
permanently bleached.
The Brown FK-induced pigment does not fluoresce in ultra-violet
light. On the other hand, all samples of lipofuscin which Brown FK
have been examined were fluorescent. Pigment has been found in the
thyroid, brown fat, and bone marrow; these tissues have not been
recorded as being sites for lipofuscin deposition. Furthermore, a
coloured substance has been demonstrated in the plasma, which has
never been found with lipofuscin.
The speed at which the Brown FK-induced pigment is deposited is
uncharacteristic of lipofuscin information. In acute studies, pigment
has been seen in the intestinal wall and villi within 24 hours of
feeding the dye, and in the kidney after 5 days. Pigment masses
produced in macrophages either in vivo after the intraperitoneal
injection of Brown FK into mice, or in vitro, when Brown FK was
incorporated in the macrophage culture medium, appeared identical.
Tests for lipofuscin proved negative; the pigment in the macrophages
closely resembled that seen in macrophages in stained sections of
tissues from rats and mice fed Brown FK.
Electron microscope studies have identified differences in
morphology between lipofuscin and the Brown FK-induced pigment. In
aged rats and mice fed Brown FK, conjugate forms were observed in
which induced pigment and control lysosomal material appeared in the
same membrane-limited body (Hope, 1971).
It is likely that a component of Brown FK is oxidized within the
cell to 1,4,7-triaminophenazine:
 This would explain the behaviour of the pigment with sodium
dithionite, which reduces the phenazine ring to the 5,10-dihydro
derivative, which is probably colourless. After exposure to air
re-oxidation would occur. Thus, the pigment may not represent evidence
of sub-lethal cell damage, but is, instead, an insoluble oxidation
product of a dye metabolite.
1,2,4-triaminobenzene was very rapidly oxidized to
1,4,7-triaminophenazine by a mitochondrial suspension; no
phenazine derivatives were detected with triaminotoluene under
the same circumstances (Kirby, 1968b).
1,4,7-triaminophenazine is a brown, water-insoluble, material
which is very readily formed from 1,2,4-triaminobenzene (Muller,
1889).
Special studies on pigment in tissues
Histological tests were applied to sections of hearts and livers
from female rats fed Brown FK at doses of 0, 15, 50, or 250 mg/kg
b.w./day for 106-108 weeks. These tissues were obtained from animals
used in the carcinogenicity study (see above). In addition, several
tissues taken from weanling rats discarded at the end of the in
utero phase were screened to determine if any pigment was present at
the start of the long-term study due to transplacental transfer of
Broom FK or through exposure during lactation/creep feeding.
Rats fed Brown FK for over 2 years at a dose-level of
250 mg/kg b.w./day displayed substantial pigment deposition in the
heart and liver, but no effects were seen in these organs at the lower
dose-levels. Brown FK-associated pigment was not found in tissues from
weanling rats exposed to Brown FK in utero. The no-effect level for
pigment accumulation in the long-term study was 50 mg/kg b.w./day.
Differential tests showed that the pigment was not formalin pigment or
haemosiderin, but tests aimed at differentiating between lipofuscin
and Brown FK-induced pigment gave inconclusive results (Wilson et
al., 1983a).
Special studies on components I and II of Brown FK
Mice
Groups of 3 male and 3 female C57Bl mice were fed diets
containing 0, 0.5, or 1.0% of the "azobenzene" or "azotoluene"
components of Brown FK (components I & II, respectively) for 6 weeks.
With both components, the thyroids were dark and the intestines and
squamous portions of the stomach were stained salmon-pink. Heart
lesions were seen in all mice fed 1% component II, but not in those
given component I. More pigment was seen in mice fed component I, less
in those fed component II (Kirby, 1968a).
Rats
Groups of 3 male and 3 female Colworth-Wistar rats were fed
component I or component II at dietary levels of 0, 0.5, or 1% for 6
weeks. The thyroids of rats receiving component I were dark and the
hearts, muscles, and brains were stained. However, the intestines were
stained only slightly. Pale hearts and meningeal haemorrhage were seen
with component II, otherwise pigmentation was as with component I.
One-sixth of the rats treated with component I and four-fifths of the
rats treated with component II had heart lesions. More pigment was
seen histologically in component I-treated rats, less in component
II-treated animals (Kirkby, 1968a).
Special studies on amine metabolites of Brown FK
Amines derived from Brown FK and from its 2 myotoxic components,
components I and II, were injected i.v. into rats in single doses of
3.13-25 mg/kg. The mixture of amines from Brown FK was also injected
into mice in the same range of doses. Cardiac and muscular lesions
were produced by the amines in both species. These amines are
biological degradation products in the intestine. The finding that
orally-administered Brown FK is myotoxic in rats but not in mice is
probably due to differences in the intestinal flora in the 2 species
(Walker et al., 1970).
In another study, 1,2,4-triaminobenzene was given to groups of
6-7 rats orally 5 days/week for 2 weeks at 50, 60, 75, or 100 mg/kg
b.w./day. Six rats out of 7 receiving 100 mg/kg/day died after 3 doses
with severe heart lesions; 7/11 on 75 mg/kg/day also died after 3
doses. Heart pigmentation occurred after 5 days' treatment or longer.
Animals on lower doses showed both extensive heart pigmentation and
cardiac necrosis (Mulky et al., 1969).
1,2,4,5-Tetraaminobenzene was given to groups of 6 rats orally
5 days/week for 2 weeks at 150 or 200 mg/kg b.w./day. 1,2,3,4-
Tetraaminobenzene was given to groups of 6 rats orally 5 days/week for
2 weeks at 125 or 166 mg/kg b.w./day. No frank heart lesions and only
instances of diffuse increase in interstitial cells in the heart were
observed. No heart or thyroid pigment deposition was observed (Mulky
et al., 1969).
Acute toxicity
LD50
Species Route (mg/kg b.w.) Reference
Mouse oral >2,000 (with salt) Grasso et al., 1968a
oral 1,100-2,250 (with salt) Edwards & Wilson, 1966
oral 960-1,149 (no salt) Edwards & Wilson, 1966
i.p. 1,500-2,000 (with salt) Grasso et al., 1968a
i.p. 960-1,720 (with salt) Edwards & Wilson, 1966
i.p. 840-880 (no salt) Edwards & Wilson, 1966
Rat oral >8,000 (with salt) Grasso et al., 1968a
oral 900-1,910 (with salt) Edwards & Wilson, 1966
oral 780-970 (no salt) Edwards & Wilson, 1966
i.p. 750-1,150 (with salt) Grasso et al., 1968a
i.p. 1,100-2,250 (with salt) Edwards & Wilson, 1966
i.p. 960-1,150 (no salt) Edwards & Wilson, 1966
Guinea-pig oral 3,000 (with salt) Edwards & Wilson, 1966
oral 2,610 (no salt) Edwards & Wilson, 1966
i.p. 900 (with salt) Edwards & Wilson, 1966
i.p. 780 (with salt) Edwards & Wilson, 1966
Rabbit oral 450-680 (with salt) Edwards & Wilson, 1966
oral 390-590 (no salt) Edwards & Wilson, 1966
Chicken oral >10,000 (with salt) Edwards & Wilson, 1966
oral >8,700 (no salt) Edwards & Wilson, 1966
For all species, animals dying did so from within a few minutes
to 96 hours. Many animals, after either oral or i.p. treatment, showed
lack of coordination, hypersensitivity, and hyperactivity; convulsions
usually preceded death (Edwards & Wilson, 1966).
Meningeal congestion or haemorrhage was seen at post-mortem
examination in rats and mice which died following both oral and i.p.
treatment with 3.4 g/kg Brown FK as a 10% solution. This was the
highest dose administered, and the condition may have been present to
a lesser degree in animals treated with lower levels of Brown FK, but
post-mortem identification of the lesion was made difficult by tissue
colouration. The meningeal congestion/haemorrhage was probably caused
by the sodium chloride in the dye solution, since this lesion is
observed after administration of hypertonic solutions of sodium
chloride to rats. In this instance, the lowest dose levels at which
the meningeal lesion was observed were 6.0 g/kg of sodium chloride
orally as a 10% solution and 4.0 g/kg i.p. as a 5% solution (Edwards &
Wilson, 1966).
Following oral intubation, external tissue colouration was
apparent after several hours in rats and guinea-pigs. No colouration
of the tissues was seen in rabbits and chickens. After i.p. injection,
external tissue colouration was apparent and intense after a few
minutes in rats and guinea-pigs. Colour was seen in the faeces of
rats, mice, rabbits, and guinea-pigs up to 24 hours after oral
treatment; it was also excreted in the urine of rats, mice, guinea-
pigs, and rabbits within 15 minutes of either oral or i.p. treatment
(Edwards & Wilson, 1966).
Hearts from some rats and mice surviving for 21 days after
treatment were examined histologically. Degenerative lesions were
found in 15% of rats given orally 1-2.5 g/kg b.w. Brown FK, but not in
rats given 3.37 g/kg b.w. Brown FK. The same lesions were found in 50%
of mice given orally 0.9 g/kg b.w. Brown FK, but not when given 0.6,
1.35, or 2.03 g/kg b.w. Brown FK. When given i.p., 25-60% of mice
showed lesions at 0.75 and 1.03 g/kg b.w. Brown FK (Edwards & Wilson,
1966).
Short-term studies
Mice
Groups of 10 male or 10 female mice received the colour (either
fresh or stored) at a level of 1 g/kg daily for 3 weeks. A significant
reduction in body-weight gain was noted in the mice receiving the
stored solution, but not in those receiving fresh solution. One male
and 1 female receiving the fresh solution showed cardiac lesions
(BIBRA, 1964).
Daily oral or i.p. doses up to 2 g/kg or 1 g/kg, respectively,
for 43 days to groups of 10 or 12 mice were well-tolerated (Grasso
et al., 1968a).
Groups of 10 male and 10 female mice (Colworth C57Bl strain,
initially 6 weeks old) were fed for 90 days on a synthetic diet
containing 0, 0.05, 0.075, 0.10, 0.25, 0.50, 0.75, 1.0, or 2.0% Brown
FK (equivalent to 0, 0.025, 0.0375, 0.05, 0.125, 0.25, 0.375, 0.50, or
1.0% Brown FK-coloured components) containing 51% dye component and
47% salt. A further group of 20 mice were fed synthetic diet
containing added 1.0% sodium chloride as a control for the additional
dietary salt derived from the Brown FK. At the 0.125% dietary colour
level, pigment deposition occurred in tissues. At 0.25% and above
there was splenic enlargement, at 0.50% the liver and heart were
enlarged, and at 1.0% there was reduced growth, poor food utilization,
liver, spleen, heart, and testicular enlargement, and histological
evidence of degenerative heart lesions. The thyroids, muscle,
intestine and squamous part of the stomach were pigmented (Ashmole
et al., 1958).
Rats
Groups of animals received the colour at a level of 0.5 g Brown
FK per kg b.w. for 3 weeks, orally or i.p. Twenty rats were dosed
orally, of which 6 animals died after having been administered between
5 and 11 doses. Post-mortem examination of rats dying during the test
or killed at the end revealed general tissue-staining in 5 rats. Of 18
hearts examined histologically, 8 showed degenerative lesions, and a
brown pigment was observed in small amounts in 9 hearts after 3 weeks.
In the multiple-dose i.p. test, 8 rats were treated and none died
during the treatment period. General organ-staining was observed in
all animals at post-mortem examination. Hearts from 7 rats were
examined microscopically and degenerative lesions were found in 1
heart and small amounts of pigment in 3 hearts after 3 weeks (Kirkby,
1968a).
No ill-effects were seen in 3 weanling rats given a 0.1% solution
Brown FK for 28 days, the intake being equivalent to 15 mg/day
(Goldblatt & Frodsham, 1952).
Administration of 2 or 3 oral doses of 1 g Brown FK/kg b.w.
to rats induced a myopathy in cardiac and skeletal muscles
characterized by multiple vacuoles about 1-2 micrometers in diameter.
Ultrastructurally, these were shown to consist of areas of
fibrollolysis. Histochemically, the myopathy was accompanied by a
moderate increase in acid phosphatase activity and by a loss of
phosphorylase activity. Subsequently, complete lysis of the affected
fibres ensued. In the heart, lysis was followed by macrophage invasion
and fibroblastic proliferation, and in skeletal muscle by
regeneration. The occurrence of lipofuscin in muscle fibres and in
macrophages was scanty and erratic. When Brown FK was given in the
diet at a level of 2%, fibrillolysis and an increase in the number and
electron-density of lysosomes was observed ultrastructurally during
weeks 2 to 3 of the test. These changes were accompanied by a marked
elevation of histochemically-demonstrable acid phosphatase.
Progressive deposition of lipofuscin was the principal pathological
feature during weeks 3 to 12 (Grasso et al., 1968b).
Daily oral doses of up to 2 g/kg Brown FK for 43 days to groups
of 10 rats induced rapid loss of weight and death, with severe damage
to cardiac and skeletal muscle, characterized by vacuolar myopathy and
lipofuscin deposition. Of 3 pure components of Brown FK studied,
component II, and to a lesser extent component I, produced similar,
but not identical lesions to those induced by the parent colour after
repeated oral doses of 0.5 g/kg. Ultrastructural studies confirmed an
extensive loss of myofibrillar elements, and histochemical studies
revealed a loss in the activity of mitochondrial enzymes. Similar i.p.
injections in doses up to 1.0 g/kg for 43 days to groups of 10 or 12
rats did not have any effect on the heart or skeletal muscle (Grasso
et al., 1968b).
Experiments were performed using groups of 10-12 rats receiving
the colour at levels of 0.1 or 1 g/kg orally or 0.1, 0.25, or 1 g/kg
i.p. daily for up to a maximum of 43 doses. A specific cardiac lesion
was identified at the oral dose of 1 g/kg. There were large areas of
myocardial necrosis and replacement by large mononuclears, with
involvement of the sub-pericardial region and endocardium. Some
myocardial cells had lost their stainable cytoplasm and appeared only
as empty sheaths. When administered i.p., the colour produced little
or no cardiac damage at any dose tested. At these high doses of
1 g/kg, most animals showed congestion, fatty change, or necrosis of
the liver with hydropic degeneration of the kidney. There was no
obvious splenomegaly. Daily doses of 100 mg/kg by stomach tube
produced 2 pericardial and 1 sub-pericardial lesions. In addition,
early hydroponic degeneration of the kidney was seen in 2 rats, with
1 of these animals also showing fatty change in the liver (BIBRA,
1964).
Administration of Brown FK (purity 80.0%) at dietary levels of 0,
0.001, 0.01, 0.1, or 1.0% for 150 days showed no adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. One male rat at the 1.0% level showed
typical myocardial changes; other rats showed deposits of lipofuscin,
especially in females. The no-effect level was 0.1% (Gaunt et al.,
1968).
Groups of 12 male and 12 female rats (Colworth-Wistar strain,
initially 3-4 weeks old) were fed for 112 days on a commercial stock
diet containing 0, 0.05, 0.1, 0.5, 1.0, or 2.0% Brown FK (0, 0.025,
0.05, 0.25, 0.5, or 1.0% Brown FK-coloured components) containing 51%
dye component and 47% salt. A further group of 24 rats were fed the
commercial stock diet containing added 1.0% sodium chloride as a
control for the additional dietary salt derived from the Brown FK. In
addition, to eliminate damage to the heart from cardiac puncture in
any rat kept to 16 weeks, groups of 6 male and 6 female rats were fed
6 weeks on powdered stock diet containing 0, 0.05, 0.5, or 2.0% Brown
FK (0, 0.025, 0.25, or 1.0% Brown FK-coloured components). A group of
12 rats also received 1.0% sodium chloride added to the basic diet.
All these rats were used for biochemical tests during weeks 0-6 after
which they were killed. At the 0.25% level tissue pigmentation
appeared, at 0.5% liver enlargement occurred, and at the 1% level
there was reduced growth, poor food utilization, enlargement of the
liver, testes, and thyroid, histological evidence of degenerative
heart lesions, increased urinary indican excretion, and elevation of
SGOT. The intestine, squamous portion of the stomach, and the thyroid
were stained. The no-effect level was 0.05% (Ashmole et al., 1966).
Pigs
Groups of female and male pigs were given doses of 0, 100, 250,
or 500 mg Brown FK/kg/day for 24 weeks without adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. Lipofuscin was widely distributed in
animals of both sexes at all dose levels in one or more organs. The
liver was particularly affected in that lipofuscan deposition was
accompanied by increased lysosomal enzyme activity, which was more
marked at the higher dose-levels. It was also seen in the heart in
males, where it was associated with an increased acid phosphatase
activity, and in the kidneys at the highest-dose level in females and
at all levels in males. A no-effect level could not be determined in
this study (Gaunt et al., 1968).
Long-term studies
Mice
Groups of 40 male and 40 female Colworth C57Bl mice were fed for
80 weeks on a synthetic diet containing 0, 0.0125, 0.0375, 0.075,
0.125, or 0.625% Brown FK-coloured components (the Brown FK used in
this study contained 62.5% coloured components). Only at the 0.625%
level was there reduced growth and food utilization and increased
mortality among females. There were increased liver, kidney, spleen,
brain, and testes weights, evidence of splenic haemopoisis, and
increased myocardial fibrosis. Heart weights were increased at the
0.125% level. Increased hepatic nodules were seen at 0.075% and higher
levels and pigment deposition at 0.0375% and higher levels. At
termination, after 80 weeks, the number of animals with nodules at the
different dose levels was 26, 23, 27, 56, 42, and 64, respectively.
Increased hepatic nodules were observed at 0.075% and higher levels.
The number of mice with hepatocellular carcinoma were 3, 2, 0, 5, 6,
and 2 at the various dose levels, respectively. Pigment deposition was
observed at dose levels of 0.0375% and higher (Wilson et al., 1970).
Rats
Groups of 32 male or 36 female Colworth-Wistar rats were fed for
2 years on a synthetic diet containing 0, 0.01, 0.03, 0.06, 0.1, or
0.5% Brown FK-coloured components (Brown FK used in this study
contained 54.2% coloured components). Only at the 0.5% level was there
increased splenic weight and hepatic granulomata. Pigment deposition
was seen at dose levels of 0.06% and higher. The no-effect level for
pigment deposition was 0.03%; it was 0.06% when based on toxicity
evidence (Wilson et al., 1971).
Observations in man
No information available.
Comments
The carcinogenicity study did not reveal any increase in the
incidence of tumours, nor did the reproduction and teratogenicity
studies show any adverse effects on reproductive function.
The myopathy seen in rats given high doses of Brown FK in short-
term studies affected all striated muscle, was accompanied by pigment
deposition, and was dose-dependent with a high threshold. In the long-
term/carcinogenicity study in rats, the observed myocarditis was not
dose-related and there was no pigmentation in animals of the low-dose
group nor controls. However, deficiencies in histopathological
examination of tissues from the low- and intermediate-dose groups
hindered the establishment of a no-effect level in this study.
In earlier long-term studies, the no-effect level (with respect
to pigment deposition) was 0.03% in the diet, equivalent to 15 mg/kg
b.w./day, based on the coloured components of Brown FK.
EVALUATION
Level causing no toxicological effect
Mouse: 0.0125% in the diet, equivalent to 19 mg/kg b.w./day.
Rat: 0.03% in the diet, equivalent to 15 mg/kg b.w./day.
Estimate of temporary acceptable daily intake for man
0-0.075 mg/kg b.w. (based on colour components)
Further work or information
Required by 1986
A complete histopathological examination of tissues from the low-
and intermediate-dose groups in the long-term/carcinogenicity study in
rats.
REFERENCES
Amyes, S.J., McSheehy, T.W., & Whitney, J.C. (1983). Brown FK: Life
span combined toxicity and oncogenicity study in rats pre-exposed
in utero. 2. Toxicity and oncogenicity phase. Unpublished
report No. 82/URL012/573 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Ashmole, R.T., Campbell, P., Kirkby, W.W., & Wilson, R. (1966).
Effects of feeding dietary Brown FK to rats for six and 16 weeks.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Ashmole, R.T., Kirkby, W.W., & Wilson, R. (1958). Thirteen week mouse
feeding trial. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
BIBRA (1964). Unpublished research report No. 5/1964 from British
Industrial Biological Research Association, Carshalton, Surrey,
England. Submitted to WHO.
Edwards, K.B. & Wilson, R. (1966). Acute toxicity of Brown FK in rats,
mice, guinea-pigs, rabbits and chickens. Unpublished report from
Unilever Research Laboratories.
Fore, H. & Walker, R. (1967). Studies on Brown FK. I. Composition and
synthesis of components. Fd. Cosmet. Toxicol., 5, 1-9.
Fore, H., Walker, R., & Golberg, L. (1967). Studies on Brown FK. II.
Degradative changes undergone in vitro and in vivo. Fd.
Cosmet. Toxicol., 5, 459-473.
Fuller, A.T. (1937). Is rho-aminobenzene sulphonamide active agent in
prontosil therapy? Lancet, 1, 194-198.
Gaunt, I.F., Hall, D.E., Grasso, P., & Golberg, L. (1968). Studies on
Brown FK. V. Short-term feeding studies in the rat and pig. Fd.
Cosmet. Toxicol., 6, 301-312.
Goldblatt & Frodsham (1952). Private communication from ICI
(unpublished report).
Grasso, P., Gaunt, I.F., Hall, D.E., Golberg, L., & Batstone, E.
(1968a). Studies on Brown FK. III. Administration of high doses
to rats and mice. Fd. Cosmet. Toxicol., 6, 1-11.
Grasso, P., Muir, A., Golberg, L., & Batstone, E. (1968b). Cytopathic
effects of Brown FK on cardiac and skeletal muscle in the rat.
Fd. Cosmet. Toxicol., 6, 13-24.
Hope, J. (1971). Ultrastructure of the pigment induced in various
tissues of the rat by long-term feeding of the dye Brown FK.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Howes, D. (1969). Metabolism of 14C labelled 1,3-diamino-4-(rho
sulphophenylazo)benzene, a component of the dye Brown FK, in the
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Jenkins, F.P. & Favell, D.J. (1971). Metabolism of the
"monoazobenzene" component of Brown FK in human subjects.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Kirkby, W.W. (1968a). Effects of Brown FK and two of its constituents
on pigment deposition and lesions in rats and mice. Unpublished
report from Unilever Research Laboratories. Submitted to WHO by
Unilever Ltd.
Kirkby, W.W. (1968b). Nature of the pigment induced in tissues of rats
and mice fed Brown FK. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Mulky, M.J., Mundy, R., Ashmole, R.T., & Kirkby, W.W. (1969).
Evaluation of the terminal causative agent in Brown FK induced
myopathy and pigment deposition. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Muller, E. (1889). Chem. Ber., 22, 856.
Munday, R. (1969). Metabolism of 2,4-diamino-5-(rho-sulphophenylazo)
toluene. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Munday, R. (1971). Uncoupling of oxidative phosphorylation by Brown FK
metabolites. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Munday, R. & Kirkby, W.W. (1969). Metabolism of 1,3-diamino-4-(rho
sulphophenylazo)benzene. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Roe, F.J.C. (1983). Histopathological evaluation of sections derived
from URL/12/BFK lifespan combined toxicity and oncogenicity study
in rats pre-exposed in utero. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Tesh, J.M., McSheehy, T.W., McAnulty, P.A., & Collier, M.J. (1980).
Brown FK: Lifespan combined toxicity and oncongenicity study in
rats pre-exposed in utero. 1. Reproductive phase. Unpublished
report No. 80/URL012/051 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Unilever (1978). Teratology study on Brown FK in the Colworth-Wistar
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Venitt, S. & Bushell, C.T. (1976). Mutagenicity of the food colour
Brown FK and constituents in Salmonella typhimurium. Mutation
Research, 40, 309-316.
Walker, R. (1968). Intestinal degradation of azo food colours with
particular reference to Brown FK. Ph.D. Thesis, University of
Reading, U.K.
Walker, R., Grasso, P., & Gaunt, I.F. (1970). Myotoxicity of amine
metabolites from Brown FK. Fd. Cosmet Toxicol., 8, 539-542.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1970).
Biological evaluation of Brown FK; 80-week mouse feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1971).
Biological evaluation of Brown FK: 2-year rat feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Hague, P.H., & Hardy, W.S. (1983a). Brown FK: Lifespan
combined toxicity and oncogenicity study in rats pre-exposed in
utero: Examination of selected tissues for Brown FK associated
pigment. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Wilson, R., McCormick, S.G., Cook, H.J., Norris, L., Robinson, J.A., &
Williams, T.C. (1983b). Evaluation of the effects of food colour
Brown FK on the fertility and reproductive performance of rats.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
This would explain the behaviour of the pigment with sodium
dithionite, which reduces the phenazine ring to the 5,10-dihydro
derivative, which is probably colourless. After exposure to air
re-oxidation would occur. Thus, the pigment may not represent evidence
of sub-lethal cell damage, but is, instead, an insoluble oxidation
product of a dye metabolite.
1,2,4-triaminobenzene was very rapidly oxidized to
1,4,7-triaminophenazine by a mitochondrial suspension; no
phenazine derivatives were detected with triaminotoluene under
the same circumstances (Kirby, 1968b).
1,4,7-triaminophenazine is a brown, water-insoluble, material
which is very readily formed from 1,2,4-triaminobenzene (Muller,
1889).
Special studies on pigment in tissues
Histological tests were applied to sections of hearts and livers
from female rats fed Brown FK at doses of 0, 15, 50, or 250 mg/kg
b.w./day for 106-108 weeks. These tissues were obtained from animals
used in the carcinogenicity study (see above). In addition, several
tissues taken from weanling rats discarded at the end of the in
utero phase were screened to determine if any pigment was present at
the start of the long-term study due to transplacental transfer of
Broom FK or through exposure during lactation/creep feeding.
Rats fed Brown FK for over 2 years at a dose-level of
250 mg/kg b.w./day displayed substantial pigment deposition in the
heart and liver, but no effects were seen in these organs at the lower
dose-levels. Brown FK-associated pigment was not found in tissues from
weanling rats exposed to Brown FK in utero. The no-effect level for
pigment accumulation in the long-term study was 50 mg/kg b.w./day.
Differential tests showed that the pigment was not formalin pigment or
haemosiderin, but tests aimed at differentiating between lipofuscin
and Brown FK-induced pigment gave inconclusive results (Wilson et
al., 1983a).
Special studies on components I and II of Brown FK
Mice
Groups of 3 male and 3 female C57Bl mice were fed diets
containing 0, 0.5, or 1.0% of the "azobenzene" or "azotoluene"
components of Brown FK (components I & II, respectively) for 6 weeks.
With both components, the thyroids were dark and the intestines and
squamous portions of the stomach were stained salmon-pink. Heart
lesions were seen in all mice fed 1% component II, but not in those
given component I. More pigment was seen in mice fed component I, less
in those fed component II (Kirby, 1968a).
Rats
Groups of 3 male and 3 female Colworth-Wistar rats were fed
component I or component II at dietary levels of 0, 0.5, or 1% for 6
weeks. The thyroids of rats receiving component I were dark and the
hearts, muscles, and brains were stained. However, the intestines were
stained only slightly. Pale hearts and meningeal haemorrhage were seen
with component II, otherwise pigmentation was as with component I.
One-sixth of the rats treated with component I and four-fifths of the
rats treated with component II had heart lesions. More pigment was
seen histologically in component I-treated rats, less in component
II-treated animals (Kirkby, 1968a).
Special studies on amine metabolites of Brown FK
Amines derived from Brown FK and from its 2 myotoxic components,
components I and II, were injected i.v. into rats in single doses of
3.13-25 mg/kg. The mixture of amines from Brown FK was also injected
into mice in the same range of doses. Cardiac and muscular lesions
were produced by the amines in both species. These amines are
biological degradation products in the intestine. The finding that
orally-administered Brown FK is myotoxic in rats but not in mice is
probably due to differences in the intestinal flora in the 2 species
(Walker et al., 1970).
In another study, 1,2,4-triaminobenzene was given to groups of
6-7 rats orally 5 days/week for 2 weeks at 50, 60, 75, or 100 mg/kg
b.w./day. Six rats out of 7 receiving 100 mg/kg/day died after 3 doses
with severe heart lesions; 7/11 on 75 mg/kg/day also died after 3
doses. Heart pigmentation occurred after 5 days' treatment or longer.
Animals on lower doses showed both extensive heart pigmentation and
cardiac necrosis (Mulky et al., 1969).
1,2,4,5-Tetraaminobenzene was given to groups of 6 rats orally
5 days/week for 2 weeks at 150 or 200 mg/kg b.w./day. 1,2,3,4-
Tetraaminobenzene was given to groups of 6 rats orally 5 days/week for
2 weeks at 125 or 166 mg/kg b.w./day. No frank heart lesions and only
instances of diffuse increase in interstitial cells in the heart were
observed. No heart or thyroid pigment deposition was observed (Mulky
et al., 1969).
Acute toxicity
LD50
Species Route (mg/kg b.w.) Reference
Mouse oral >2,000 (with salt) Grasso et al., 1968a
oral 1,100-2,250 (with salt) Edwards & Wilson, 1966
oral 960-1,149 (no salt) Edwards & Wilson, 1966
i.p. 1,500-2,000 (with salt) Grasso et al., 1968a
i.p. 960-1,720 (with salt) Edwards & Wilson, 1966
i.p. 840-880 (no salt) Edwards & Wilson, 1966
Rat oral >8,000 (with salt) Grasso et al., 1968a
oral 900-1,910 (with salt) Edwards & Wilson, 1966
oral 780-970 (no salt) Edwards & Wilson, 1966
i.p. 750-1,150 (with salt) Grasso et al., 1968a
i.p. 1,100-2,250 (with salt) Edwards & Wilson, 1966
i.p. 960-1,150 (no salt) Edwards & Wilson, 1966
Guinea-pig oral 3,000 (with salt) Edwards & Wilson, 1966
oral 2,610 (no salt) Edwards & Wilson, 1966
i.p. 900 (with salt) Edwards & Wilson, 1966
i.p. 780 (with salt) Edwards & Wilson, 1966
Rabbit oral 450-680 (with salt) Edwards & Wilson, 1966
oral 390-590 (no salt) Edwards & Wilson, 1966
Chicken oral >10,000 (with salt) Edwards & Wilson, 1966
oral >8,700 (no salt) Edwards & Wilson, 1966
For all species, animals dying did so from within a few minutes
to 96 hours. Many animals, after either oral or i.p. treatment, showed
lack of coordination, hypersensitivity, and hyperactivity; convulsions
usually preceded death (Edwards & Wilson, 1966).
Meningeal congestion or haemorrhage was seen at post-mortem
examination in rats and mice which died following both oral and i.p.
treatment with 3.4 g/kg Brown FK as a 10% solution. This was the
highest dose administered, and the condition may have been present to
a lesser degree in animals treated with lower levels of Brown FK, but
post-mortem identification of the lesion was made difficult by tissue
colouration. The meningeal congestion/haemorrhage was probably caused
by the sodium chloride in the dye solution, since this lesion is
observed after administration of hypertonic solutions of sodium
chloride to rats. In this instance, the lowest dose levels at which
the meningeal lesion was observed were 6.0 g/kg of sodium chloride
orally as a 10% solution and 4.0 g/kg i.p. as a 5% solution (Edwards &
Wilson, 1966).
Following oral intubation, external tissue colouration was
apparent after several hours in rats and guinea-pigs. No colouration
of the tissues was seen in rabbits and chickens. After i.p. injection,
external tissue colouration was apparent and intense after a few
minutes in rats and guinea-pigs. Colour was seen in the faeces of
rats, mice, rabbits, and guinea-pigs up to 24 hours after oral
treatment; it was also excreted in the urine of rats, mice, guinea-
pigs, and rabbits within 15 minutes of either oral or i.p. treatment
(Edwards & Wilson, 1966).
Hearts from some rats and mice surviving for 21 days after
treatment were examined histologically. Degenerative lesions were
found in 15% of rats given orally 1-2.5 g/kg b.w. Brown FK, but not in
rats given 3.37 g/kg b.w. Brown FK. The same lesions were found in 50%
of mice given orally 0.9 g/kg b.w. Brown FK, but not when given 0.6,
1.35, or 2.03 g/kg b.w. Brown FK. When given i.p., 25-60% of mice
showed lesions at 0.75 and 1.03 g/kg b.w. Brown FK (Edwards & Wilson,
1966).
Short-term studies
Mice
Groups of 10 male or 10 female mice received the colour (either
fresh or stored) at a level of 1 g/kg daily for 3 weeks. A significant
reduction in body-weight gain was noted in the mice receiving the
stored solution, but not in those receiving fresh solution. One male
and 1 female receiving the fresh solution showed cardiac lesions
(BIBRA, 1964).
Daily oral or i.p. doses up to 2 g/kg or 1 g/kg, respectively,
for 43 days to groups of 10 or 12 mice were well-tolerated (Grasso
et al., 1968a).
Groups of 10 male and 10 female mice (Colworth C57Bl strain,
initially 6 weeks old) were fed for 90 days on a synthetic diet
containing 0, 0.05, 0.075, 0.10, 0.25, 0.50, 0.75, 1.0, or 2.0% Brown
FK (equivalent to 0, 0.025, 0.0375, 0.05, 0.125, 0.25, 0.375, 0.50, or
1.0% Brown FK-coloured components) containing 51% dye component and
47% salt. A further group of 20 mice were fed synthetic diet
containing added 1.0% sodium chloride as a control for the additional
dietary salt derived from the Brown FK. At the 0.125% dietary colour
level, pigment deposition occurred in tissues. At 0.25% and above
there was splenic enlargement, at 0.50% the liver and heart were
enlarged, and at 1.0% there was reduced growth, poor food utilization,
liver, spleen, heart, and testicular enlargement, and histological
evidence of degenerative heart lesions. The thyroids, muscle,
intestine and squamous part of the stomach were pigmented (Ashmole
et al., 1958).
Rats
Groups of animals received the colour at a level of 0.5 g Brown
FK per kg b.w. for 3 weeks, orally or i.p. Twenty rats were dosed
orally, of which 6 animals died after having been administered between
5 and 11 doses. Post-mortem examination of rats dying during the test
or killed at the end revealed general tissue-staining in 5 rats. Of 18
hearts examined histologically, 8 showed degenerative lesions, and a
brown pigment was observed in small amounts in 9 hearts after 3 weeks.
In the multiple-dose i.p. test, 8 rats were treated and none died
during the treatment period. General organ-staining was observed in
all animals at post-mortem examination. Hearts from 7 rats were
examined microscopically and degenerative lesions were found in 1
heart and small amounts of pigment in 3 hearts after 3 weeks (Kirkby,
1968a).
No ill-effects were seen in 3 weanling rats given a 0.1% solution
Brown FK for 28 days, the intake being equivalent to 15 mg/day
(Goldblatt & Frodsham, 1952).
Administration of 2 or 3 oral doses of 1 g Brown FK/kg b.w.
to rats induced a myopathy in cardiac and skeletal muscles
characterized by multiple vacuoles about 1-2 micrometers in diameter.
Ultrastructurally, these were shown to consist of areas of
fibrollolysis. Histochemically, the myopathy was accompanied by a
moderate increase in acid phosphatase activity and by a loss of
phosphorylase activity. Subsequently, complete lysis of the affected
fibres ensued. In the heart, lysis was followed by macrophage invasion
and fibroblastic proliferation, and in skeletal muscle by
regeneration. The occurrence of lipofuscin in muscle fibres and in
macrophages was scanty and erratic. When Brown FK was given in the
diet at a level of 2%, fibrillolysis and an increase in the number and
electron-density of lysosomes was observed ultrastructurally during
weeks 2 to 3 of the test. These changes were accompanied by a marked
elevation of histochemically-demonstrable acid phosphatase.
Progressive deposition of lipofuscin was the principal pathological
feature during weeks 3 to 12 (Grasso et al., 1968b).
Daily oral doses of up to 2 g/kg Brown FK for 43 days to groups
of 10 rats induced rapid loss of weight and death, with severe damage
to cardiac and skeletal muscle, characterized by vacuolar myopathy and
lipofuscin deposition. Of 3 pure components of Brown FK studied,
component II, and to a lesser extent component I, produced similar,
but not identical lesions to those induced by the parent colour after
repeated oral doses of 0.5 g/kg. Ultrastructural studies confirmed an
extensive loss of myofibrillar elements, and histochemical studies
revealed a loss in the activity of mitochondrial enzymes. Similar i.p.
injections in doses up to 1.0 g/kg for 43 days to groups of 10 or 12
rats did not have any effect on the heart or skeletal muscle (Grasso
et al., 1968b).
Experiments were performed using groups of 10-12 rats receiving
the colour at levels of 0.1 or 1 g/kg orally or 0.1, 0.25, or 1 g/kg
i.p. daily for up to a maximum of 43 doses. A specific cardiac lesion
was identified at the oral dose of 1 g/kg. There were large areas of
myocardial necrosis and replacement by large mononuclears, with
involvement of the sub-pericardial region and endocardium. Some
myocardial cells had lost their stainable cytoplasm and appeared only
as empty sheaths. When administered i.p., the colour produced little
or no cardiac damage at any dose tested. At these high doses of
1 g/kg, most animals showed congestion, fatty change, or necrosis of
the liver with hydropic degeneration of the kidney. There was no
obvious splenomegaly. Daily doses of 100 mg/kg by stomach tube
produced 2 pericardial and 1 sub-pericardial lesions. In addition,
early hydroponic degeneration of the kidney was seen in 2 rats, with
1 of these animals also showing fatty change in the liver (BIBRA,
1964).
Administration of Brown FK (purity 80.0%) at dietary levels of 0,
0.001, 0.01, 0.1, or 1.0% for 150 days showed no adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. One male rat at the 1.0% level showed
typical myocardial changes; other rats showed deposits of lipofuscin,
especially in females. The no-effect level was 0.1% (Gaunt et al.,
1968).
Groups of 12 male and 12 female rats (Colworth-Wistar strain,
initially 3-4 weeks old) were fed for 112 days on a commercial stock
diet containing 0, 0.05, 0.1, 0.5, 1.0, or 2.0% Brown FK (0, 0.025,
0.05, 0.25, 0.5, or 1.0% Brown FK-coloured components) containing 51%
dye component and 47% salt. A further group of 24 rats were fed the
commercial stock diet containing added 1.0% sodium chloride as a
control for the additional dietary salt derived from the Brown FK. In
addition, to eliminate damage to the heart from cardiac puncture in
any rat kept to 16 weeks, groups of 6 male and 6 female rats were fed
6 weeks on powdered stock diet containing 0, 0.05, 0.5, or 2.0% Brown
FK (0, 0.025, 0.25, or 1.0% Brown FK-coloured components). A group of
12 rats also received 1.0% sodium chloride added to the basic diet.
All these rats were used for biochemical tests during weeks 0-6 after
which they were killed. At the 0.25% level tissue pigmentation
appeared, at 0.5% liver enlargement occurred, and at the 1% level
there was reduced growth, poor food utilization, enlargement of the
liver, testes, and thyroid, histological evidence of degenerative
heart lesions, increased urinary indican excretion, and elevation of
SGOT. The intestine, squamous portion of the stomach, and the thyroid
were stained. The no-effect level was 0.05% (Ashmole et al., 1966).
Pigs
Groups of female and male pigs were given doses of 0, 100, 250,
or 500 mg Brown FK/kg/day for 24 weeks without adverse effects on
growth, food consumption, haematological indices, liver and kidney
function, or organ weights. Lipofuscin was widely distributed in
animals of both sexes at all dose levels in one or more organs. The
liver was particularly affected in that lipofuscan deposition was
accompanied by increased lysosomal enzyme activity, which was more
marked at the higher dose-levels. It was also seen in the heart in
males, where it was associated with an increased acid phosphatase
activity, and in the kidneys at the highest-dose level in females and
at all levels in males. A no-effect level could not be determined in
this study (Gaunt et al., 1968).
Long-term studies
Mice
Groups of 40 male and 40 female Colworth C57Bl mice were fed for
80 weeks on a synthetic diet containing 0, 0.0125, 0.0375, 0.075,
0.125, or 0.625% Brown FK-coloured components (the Brown FK used in
this study contained 62.5% coloured components). Only at the 0.625%
level was there reduced growth and food utilization and increased
mortality among females. There were increased liver, kidney, spleen,
brain, and testes weights, evidence of splenic haemopoisis, and
increased myocardial fibrosis. Heart weights were increased at the
0.125% level. Increased hepatic nodules were seen at 0.075% and higher
levels and pigment deposition at 0.0375% and higher levels. At
termination, after 80 weeks, the number of animals with nodules at the
different dose levels was 26, 23, 27, 56, 42, and 64, respectively.
Increased hepatic nodules were observed at 0.075% and higher levels.
The number of mice with hepatocellular carcinoma were 3, 2, 0, 5, 6,
and 2 at the various dose levels, respectively. Pigment deposition was
observed at dose levels of 0.0375% and higher (Wilson et al., 1970).
Rats
Groups of 32 male or 36 female Colworth-Wistar rats were fed for
2 years on a synthetic diet containing 0, 0.01, 0.03, 0.06, 0.1, or
0.5% Brown FK-coloured components (Brown FK used in this study
contained 54.2% coloured components). Only at the 0.5% level was there
increased splenic weight and hepatic granulomata. Pigment deposition
was seen at dose levels of 0.06% and higher. The no-effect level for
pigment deposition was 0.03%; it was 0.06% when based on toxicity
evidence (Wilson et al., 1971).
Observations in man
No information available.
Comments
The carcinogenicity study did not reveal any increase in the
incidence of tumours, nor did the reproduction and teratogenicity
studies show any adverse effects on reproductive function.
The myopathy seen in rats given high doses of Brown FK in short-
term studies affected all striated muscle, was accompanied by pigment
deposition, and was dose-dependent with a high threshold. In the long-
term/carcinogenicity study in rats, the observed myocarditis was not
dose-related and there was no pigmentation in animals of the low-dose
group nor controls. However, deficiencies in histopathological
examination of tissues from the low- and intermediate-dose groups
hindered the establishment of a no-effect level in this study.
In earlier long-term studies, the no-effect level (with respect
to pigment deposition) was 0.03% in the diet, equivalent to 15 mg/kg
b.w./day, based on the coloured components of Brown FK.
EVALUATION
Level causing no toxicological effect
Mouse: 0.0125% in the diet, equivalent to 19 mg/kg b.w./day.
Rat: 0.03% in the diet, equivalent to 15 mg/kg b.w./day.
Estimate of temporary acceptable daily intake for man
0-0.075 mg/kg b.w. (based on colour components)
Further work or information
Required by 1986
A complete histopathological examination of tissues from the low-
and intermediate-dose groups in the long-term/carcinogenicity study in
rats.
REFERENCES
Amyes, S.J., McSheehy, T.W., & Whitney, J.C. (1983). Brown FK: Life
span combined toxicity and oncogenicity study in rats pre-exposed
in utero. 2. Toxicity and oncogenicity phase. Unpublished
report No. 82/URL012/573 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Ashmole, R.T., Campbell, P., Kirkby, W.W., & Wilson, R. (1966).
Effects of feeding dietary Brown FK to rats for six and 16 weeks.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Ashmole, R.T., Kirkby, W.W., & Wilson, R. (1958). Thirteen week mouse
feeding trial. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
BIBRA (1964). Unpublished research report No. 5/1964 from British
Industrial Biological Research Association, Carshalton, Surrey,
England. Submitted to WHO.
Edwards, K.B. & Wilson, R. (1966). Acute toxicity of Brown FK in rats,
mice, guinea-pigs, rabbits and chickens. Unpublished report from
Unilever Research Laboratories.
Fore, H. & Walker, R. (1967). Studies on Brown FK. I. Composition and
synthesis of components. Fd. Cosmet. Toxicol., 5, 1-9.
Fore, H., Walker, R., & Golberg, L. (1967). Studies on Brown FK. II.
Degradative changes undergone in vitro and in vivo. Fd.
Cosmet. Toxicol., 5, 459-473.
Fuller, A.T. (1937). Is rho-aminobenzene sulphonamide active agent in
prontosil therapy? Lancet, 1, 194-198.
Gaunt, I.F., Hall, D.E., Grasso, P., & Golberg, L. (1968). Studies on
Brown FK. V. Short-term feeding studies in the rat and pig. Fd.
Cosmet. Toxicol., 6, 301-312.
Goldblatt & Frodsham (1952). Private communication from ICI
(unpublished report).
Grasso, P., Gaunt, I.F., Hall, D.E., Golberg, L., & Batstone, E.
(1968a). Studies on Brown FK. III. Administration of high doses
to rats and mice. Fd. Cosmet. Toxicol., 6, 1-11.
Grasso, P., Muir, A., Golberg, L., & Batstone, E. (1968b). Cytopathic
effects of Brown FK on cardiac and skeletal muscle in the rat.
Fd. Cosmet. Toxicol., 6, 13-24.
Hope, J. (1971). Ultrastructure of the pigment induced in various
tissues of the rat by long-term feeding of the dye Brown FK.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Howes, D. (1969). Metabolism of 14C labelled 1,3-diamino-4-(rho
sulphophenylazo)benzene, a component of the dye Brown FK, in the
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Jenkins, F.P. & Favell, D.J. (1971). Metabolism of the
"monoazobenzene" component of Brown FK in human subjects.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Kirkby, W.W. (1968a). Effects of Brown FK and two of its constituents
on pigment deposition and lesions in rats and mice. Unpublished
report from Unilever Research Laboratories. Submitted to WHO by
Unilever Ltd.
Kirkby, W.W. (1968b). Nature of the pigment induced in tissues of rats
and mice fed Brown FK. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Mulky, M.J., Mundy, R., Ashmole, R.T., & Kirkby, W.W. (1969).
Evaluation of the terminal causative agent in Brown FK induced
myopathy and pigment deposition. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Muller, E. (1889). Chem. Ber., 22, 856.
Munday, R. (1969). Metabolism of 2,4-diamino-5-(rho-sulphophenylazo)
toluene. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Munday, R. (1971). Uncoupling of oxidative phosphorylation by Brown FK
metabolites. Unpublished report from Unilever Research
Laboratories. Submitted to WHO by Unilever Ltd.
Munday, R. & Kirkby, W.W. (1969). Metabolism of 1,3-diamino-4-(rho
sulphophenylazo)benzene. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Roe, F.J.C. (1983). Histopathological evaluation of sections derived
from URL/12/BFK lifespan combined toxicity and oncogenicity study
in rats pre-exposed in utero. Unpublished report from Unilever
Research Laboratories. Submitted to WHO by Unilever Ltd.
Tesh, J.M., McSheehy, T.W., McAnulty, P.A., & Collier, M.J. (1980).
Brown FK: Lifespan combined toxicity and oncongenicity study in
rats pre-exposed in utero. 1. Reproductive phase. Unpublished
report No. 80/URL012/051 from Life Science Research, Essex, U.K.
Submitted to WHO by Unilever Ltd.
Unilever (1978). Teratology study on Brown FK in the Colworth-Wistar
rat. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Venitt, S. & Bushell, C.T. (1976). Mutagenicity of the food colour
Brown FK and constituents in Salmonella typhimurium. Mutation
Research, 40, 309-316.
Walker, R. (1968). Intestinal degradation of azo food colours with
particular reference to Brown FK. Ph.D. Thesis, University of
Reading, U.K.
Walker, R., Grasso, P., & Gaunt, I.F. (1970). Myotoxicity of amine
metabolites from Brown FK. Fd. Cosmet Toxicol., 8, 539-542.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1970).
Biological evaluation of Brown FK; 80-week mouse feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Gellatly, J.B.M., Kirkby, W.W., & Ashmole, R.T. (1971).
Biological evaluation of Brown FK: 2-year rat feeding trial.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
Wilson, R., Hague, P.H., & Hardy, W.S. (1983a). Brown FK: Lifespan
combined toxicity and oncogenicity study in rats pre-exposed in
utero: Examination of selected tissues for Brown FK associated
pigment. Unpublished report from Unilever Research Laboratories.
Submitted to WHO by Unilever Ltd.
Wilson, R., McCormick, S.G., Cook, H.J., Norris, L., Robinson, J.A., &
Williams, T.C. (1983b). Evaluation of the effects of food colour
Brown FK on the fertility and reproductive performance of rats.
Unpublished report from Unilever Research Laboratories. Submitted
to WHO by Unilever Ltd.
