
CHLORAMPHENICOL
EXPLANATION
Chloramphenicol is an antibiotic originally isolated from the
soil bacterium Streptomyces venezuelae. It has the following
chemical structure:
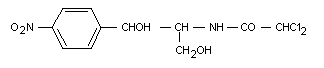 Chloramphenicol was previously considered at the twelfth meeting
of the Committee (Annex 1, reference 17), at which time it was
determined that acceptable levels in food could not be established.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
In dogs, chloramphenicol is rapidly and extensively absorbed
after oral administration of 50 mg/kg b.w., giving plasma levels of
16.5 g/l 2 hours after dosing (Watson, 1972; Watson 1977a). Similar
findings have been observed in rabbits given oral doses of 16 mg/kg
chloramphenicol (Cid et al., 1983). Dietary bran markedly
increased the rate and extent of absorption of orally administered
chloramphenicol palmitate in pigs, but it is not known if the
absorption of chloramphenicol itself would be affected in this way
(Bueno et al., 1984). Experiments with everted mouse small intestine
sacs resulted in absorption of chloramphenicol at similar rates over
different regions of the small intestine. Various chemicals and
metallic ions had no effect on the rate of absorption, and penetration
of the serosal and mucosal surfaces was similar indicating passive
absorption of chloramphenicol from the gut (Chakrabarti & Banerjee,
1977).
In adult human beings, absorption of chloramphenicol is rapid and
extensive after an oral dose. Serum levels were 20 - 40 mg/l after a
2 g dose (29 mg/kg b.w.) and 40 - 60 mg/l after a 4 g dose (57 mg/kg
b.w.) (Yunis, 1973a).
Chloramphenicol is also well absorbed by infants and neonates
after oral administration. Serum (peak) concentrations of 20 - 24 mg/l
were noted after oral doses of 40 mg/kg b.w. in neonates. Infants
given 26 mg/kg b.w. were found to have peak concentrations of 14 mg/l
(Mulhall et al., 1983). Available evidence and theoretical
considerations suggest that chloramphenicol may be percutaneously
absorbed in human beings (Guy et al., 1985a, b).
Chloramphenicol is distributed to most major organs in newborn
pigs. After i.v. administration of 0.52 mg/kg b.w. 14C-chloram-
phenicol, many tissues showed higher levels of chloramphenicol than
were found in the blood as soon as 5 minutes after administration.
These tissues included the lungs, liver, kidneys, adrenal cortex,
myocardium, pancreas, thyroid, spleen, and skeletal muscle. The
levels remained higher than in blood for up to 8 hours after dosing.
At 4 and 8 hours after administration, levels in the brain were
higher than in blood. However, there was no apparent affinity for
chloramphenicol in the bone marrow over the period of the experiment
(8 hours), where levels did not reach those noted in serum (Appelgren
et al., 1982).
Chloramphenicol in human beings, regardless of the route of
administration, extensively distributes, although the levels in
tissues depend on the route, the levels being the highest after oral
or i.v. administration. The compound has been found in the heart,
lungs, kidneys, liver, spleen, pleural fluid, seminal fluid, ascitic
fluid, and saliva. (Ambrose, 1984; Yunis 1973a; Gray, 1955). It binds
extensively to proteins in both adults and neonates, although binding
in the latter is less than in the former (Kurz et al., 1977).
Chloramphenicol can cross the placenta in human beings (Ambrose,
1984). After oral doses of 1 or 2 g chloramphenicol to pregnant women,
chloramphenicol was detected in the placenta after 1.5 - 2.5 hours,
indicating some potential for the drug to reach the fetus (Ross
et al., 1950).
In human beings with normal renal/hepatic function the volume of
distribution is around 0.7 1.4 1/kg. These values do not deviate
markedly in patients with hepatic dysfunction or with renal
impairment. Overall, these values suggest extensive distribution in
body tissues. (Ambrose, 1984; Burke et al., 1980; Slaughter
et al., 1980). Similar values were noted with infants and young
children given the sodium succinate derivative of chloramphenicol,
which is converted in vivo to chloramphenicol (Sack et al., 1980).
Chloramphenicol and its metabolites are excreted in the urine of
rats after oral dosing; up to 70% of an oral dose may he excreted in
this way (Glazko et al., 1949; Glazko et al., 1952). Limited data
suggest that chloramphenicol may be excreted in the bile of rats
following administration; around 0.4% of an i.m. dose of 40 mg/kg was
detected in the bile after 4 hours (Kunii et al., 1983). There are
no readily available data for biliary excretion after oral
administration. In new-born pigs, the majority of an i.v. dose of
chloramphenicol was excreted in the urine, while a small quantity was
excreted in the bile (Appelgren et al., 1982). Liver damage may
delay body-clearance of chloramphenicol, at least in the mini-pig
(Kroker, 1985). Following t.v. administration to goats, 69% of the
dose was excreted in the urine after 12 hours (Javed et al., 1984).
Chloramphenicol has been found to be excreted in other body
fluids after administration to animals. It has been detected at levels
up to 6 mg/l in the tears of cattle given i.v. doses of 50 mg/kg
chloramphenicol; it was detected in the tears as soon as 1 hour after
dosing (Punch et al., 1985). Chloramphenicol was detected in the
milk of goats after an i.v. dose of 100 mg/kg, with maximum levels
1 hour after administration. (Roy et al., 1986). Administration of
10 mg/kg chloramphenicol i.m. to cattle resulted in peak levels in
milk of around 1 mg/l 6 hours after injection. However, after oral
administration, no chloramphenicol was detected in milk.
(De Corte-Baeten & Debackere, 1976).
Nine patients with evidence of chloramphenicol-induced bone
marrow suppression showed greatly reduced plasma clearance times
compared with 9 others who showed no evidence of toxicity. In the
"toxic" group, 5 had liver disease, 2 had pyelonephritis, and 2 showed
neither liver disease nor renal disease. In the "non-toxic" group, 1
had liver disease, 2 had renal disease, while 6 had neither. Six hours
after an i.v. dose of 500 mg chloramphenicol succinate, the blood
level in the "toxic" group was 4.5 µg/ml (2.8 - 6.9 µg/ml), while in
the "non-toxic" group the mean level was 1.2 µg/ml (0 - 2.3 ug/ml).
Similarly, after 8 hours the mean level in the "toxic" group was
3.5 µg/ml (2.1 - 5.2 ug/ml), while in the "non-toxic" group a mean
level of 0.7 µg/ml (0 - 2.5 µg/ml) was noted. Such findings suggest
that patients susceptible to the bone-marrow effects of
chloramphenicol may clear the drug from the blood at a slower rate
than those who are not susceptible (Shurland & Weisburger, 1969).
Chloramphenicol administered to human beings is excreted
primarily in the urine (90%), up to 15% as the parent compound and the
remainder as metabolites, including conjugated derivatives
(Burke et al., 1980; Ambrose, 1984; Yunis, 1973a). Glomerular
excretion is thought to be the major mechanism of excretion
(Glazko et al., 1949).
Renal clearance varies depending on age. In one study, clearance
in neonates (less than 6 months of age) was 0.46 - 9.76 1/hour, while
in infants aged 6 months to 2.5 years values in the range 1.8 - 2.1
1/hour were reported. In two subjects aged over 2.5 years, values of
6.03 and 9.59 1/hour were noted (Burckart et al., 1983). Similar
variations with age have been noted in other studies (Mulhall et al.,
1983; Sack et al., 1980; Kauffman et al., 1981; Burckart et al.,
1982; Rajchgot et al., 1983). Renal clearance takes longer in
patients with renal insufficiency than in normal patients (Brasfield
et al., 1983). However, these differences are not marked (Smith &
Weber, 1983). It has been recommended that dosage adjustments need not
be made in patients with renal insufficiency or in anephric subjects
(Van Scoy & Wilson, 1983).
As with experimental animals, chloramphenicol is excreted in
human milk. Up to 1.3% of an administered dose may be excreted in this
way. (Vorherr, 1974). After a single, oral dose of chloramphenicol, a
peak milk concentration of 3 mg/l has been reported. This peak was
reached around 2 hours after administration, with a drop to nearly
pre-dosing levels by 8 hours post-dose (Plomp et al., 1983). Other
workers have reported similar findings (Knowles, 1965; Matsuda, 1984).
Biotransformation
Early studies indicate that the major metabolite of
chloramphenicol in the rat was the glucoronide conjugate, which was
found along with free chloramphenicol after oral dosing
(Glazko et al., 1950; Glazko et al., 1952). In in vitro studies
it has been demonstrated that the glucuronide is the main metabolic
product in isolated rat hepatocytes exposed to chloramphenicol
(Siliciano et al., 1978). Glucuronidation of chloramphenicol was
elevated in vitro in hepatocytes obtained from phenobarbital-
pretreated rats. This increase correlated with differential induction
of UDP-glucuronyltransferase in hepatocytes of rats pretreated with
phenobarbital (Ullrich & Bock, 1984).
Several metabolites of chloramphenicol have been identified in
rat urine. In addition to free chloramphenicol (1) and the glucoronide
(2), the oxamic acid (3), alcohol (4), and base (5) derivatives have
been noted in the urine of rats given i.m. doses of [3H]-chlo-
ramphenicol (1R,2R-1- p-nitrophenyl(2,2-dichloroacetamido-
1,3-(1-3H)-propandediol). The acetylarylamine (6) and arylamine
metabolites (7) have also been detected. These metabolites are shown
in Table 1.
Table 1 Metabolites of chloramphenicol in the rat
Compound R1 R2 R3
1. chloramphenicol NO2 COCHCl2 OH
2. glucuronide conjugate NO2 COCHCl2 C6H9O7
3. oxamic acid derivative NO2 COCHO2H OH
4. alcohol derivative NO2 COCH2OH OH
5. base derivative NO2 H OH
6. acetylarylamine derivative NHCOCH3 COCHCl2 OH
7. arylamine derivative NH2 COCHCl2 OH
Based upon recovered radioactivity, the major metabolites
appeared to be chloramphenicol base (approx. 26%) and the
acetylarylamine derivative (19.1%). The other metabolites were
recovered in the 8-15% range, except for the arylamine derivative,
which represented approximately 4% of the recovered radioactivity
(total identified, 93.4% of administered radioactivity; total
collected, 95.9% of administered radioactivity (Bories et al.,
1983).
An in vitro study using perfused rat liver and rat liver
microsomes indicated that the arylamine derivative may undergo
N-oxidation to form nitrosochloramphenicol by way of the N-hydroxy
derivative, which may then be conjugated with glutathione
(Ascherl et al., 1985).
There are few data available on the metabolism of chloramphenicol
in other species. In dogs, chloramphenicol, chloramphenicol base, and
chloramphenicol glucuronide conjugate appeared to be the major
metabolites (Glazko et al., 1950). Chloramphenicol, the glucuronide
conjugate, and the oxamic acid, acetylarylamine, arylamine, and base
derivatives were noted in the urine of goats given i.m. injections of
chloramphenicol (Bories et al., 1983). In vitro studies with pig
liver showed the activity of UDP-glucuronyltransferase in this species
to be similar to that in rats, suggesting that glucuronidation may be
a significant pathway of biotransformation of chloramphenicol in pigs.
The same experiments with sheep and cattle liver preparations showed
that they have much lower glucuronyl transfer activities (25% and 14%,
respectively). This may indicate that glucuronidation plays a less
important role in these species (Smith et al., 1984).
In human beings, 93% of an oral dose of chloramphenicol was
excreted in the urine within 24 hours, and it seems likely that the
major excretion product was the glucuronide conjugate. Approximately
48% of the chloramphenicol excreted in the urine within 8 hours after
oral dosing was the glucuronide conjugate; only 6% was excreted as the
parent compound and 4% as the base derivative (Baselt, 1982;
Nakagawa et al., 1975). The alcohol derivative has been detected in
the urine of neonates (Dill et al., 1960). More recent experiments
have confirmed the presence of the glucuronide conjugate and base
derivative as the major metabolites after an oral dose of 500 mg
chloramphenicol (Bories et al., 1983).
Human liver has the potential to reduce chloramphenicol. In 10
livers studied, nitro reductase activity was observed, which was
dependent on NADPH. Thus, human livers may have the capacity to
convert the nitro group of chloramphenicol to the amine, with the
further possibility of nitroso formation. Esters of chloramphenicol,
for example, the succinate, are converted to chloramphenicol in vivo
(Salem et al., 1981).
Effects on enzymes and other biochemical parameters
Chloramphenicol is known to increase the plasma levels of certain
drugs (e.g., paracetamol and phenytoin) and to prolong barbiturate
sleeping time, which is indicative of an effect on drug metabolizing
enzymes (Halpert & Neal, 1981; Nair et al., 1981; Aravindaksham &
Cherian, 1984).
The major contribution to these effects may arise from
chloramphenicol's ability to act as a suicide substrate in the
inactivation of cytochrome P-450, possibly by the binding of the
oxamic acid derivative to lysine residues of the cytochrome molecule
(Halpert & Neal, 1981; Halpert, 1981; Halpert, 1982). Of the various
isozymes of cytochrome P-450, those induced by phenobarbital appear to
be the most sensitive to chloramphenicol. Rats were pretreated with
various inducers of cytochrome P-450 (phenobarbital, ß-naphthoflavone,
pregnenolone, 16-x-carbonitrile, and clofibrate) and were then
injected i.p. with 300 mg/kg b.w. chloramphenicol. The isozymes of
cytochrome P-450 induced by phenobarbital were inhibited to some
degree by chloramphenicol administration, but those forms induced by
the other compounds investigated were not affected. Cytochrome
P-450-c, the form present in non-pretreated rats, was the most
susceptible isozyme in vivo and in vitro (Halpert et al., 1985).
Following i.v. or i.p. administration of 100 mg/kg b.w.
chloramphenicol to rats, inhibition of the conversion of n-hexane to
2-hexanol by rat liver or lung microsomes occurred. There was no
effect on the conversion of n-hexane to l-hexanol, while only liver
microsomes were markedly inhibited in the formation of 3-hexanol
(Naesland & Halpert, 1984; Naesland et al., 1983).
Chloramphenicol prevented the methanol-potentiated toxicity of
carbon tetrachloride in rats, possibly by deactivation of cytochrome
P-450 (Brabec et al., 1982). Phenobarbital-induced cytochrome P-450
appears to be responsible for the dechlorination of chloramphenicol
and the formation of chloramphenicol aldehyde in rat liver microsomes.
The dechlorination may be enhanced by glutathione. The significance of
these findings is not clear at the present time (Morris et al.,
1982; Morris et al., 1983).
Subcutaneous administration of up to 100 mg/kg b.w. chloram-
phenicol to rats inhibited hepatic N-demethylase, glucose-6-
phosphate dehydrogenase, and carboxylesterase (Hapke et al., 1977).
However, no effects were observed on monoamine oxidase in the liver,
brain, or heart of rabbits given 60 mg/kg b.w. chloramphenicol i.m.
for 5 days. Similarly, no effect on monoamine oxidase activity was
seen in in vitro experiments where rabbit liver preparations were
preincubated with chloramphenicol (Ali, 1985).
Several studies have demonstrated an effect of chloramphenicol on
mitrochondrial protein synthesis. In vitro, chloramphenicol
inhibited mitochondrial protein synthesis in rat liver and rabbit bone
marrow. The effect was similar to that noted with tetracycline (Summ
et al., 1976). Nitrosochloramphenicol inhibited rat mitochondrial
DNA polymerase in vitro, whereas the arylamine derivative and
chloramphenicol itself did not (Lim et al., 1984). Nitroschlor-
amphenicol inhibited the transport of NAD-linked substrates into
mitochondria, but it had no effect on FAD-linked substrates. It
inhibited ATP formation and completely blocked the transport of
protons out of the mitochondria (Abou-Khalil et al., 1982).
Chloramphenicol inhibited the incorporation of leucine into protein in
mitochondria from erythroid and myeloid rumour cells and in cells from
normal, myeloid, and erythroid hyperplastic bone marrow of rabbits
in vitro (Abou-Khalil et al., 1980, 1981). In mice given
chloramphenicol, the so-called megamitochondria that were produced
were deficient in cytochrome oxidase, ATP synthetase, and cytochrome b
activities, which is indicative of inhibited protein synthesis
(Wagner & Rafel, 1977). Subcutaneous administration of 500 mg/kg b.w.
chloramphenicol succinate to partially hepatectomized rats did not
significantly inhibit protein synthesis. However, liver cytochrome c
oxidase was strongly inhibited in these rats (Kroon & Vries, 1969).
Intramuscular administration of chloramphenicol to rats inhibited
mitochondrial monoamine oxidase activity in the liver, brain, and
kidney (Banerjee & Basu, 1978). When chloramphenicol was given daily
for 6 days at doses of 100 mg/kg b.w. i.p., marked reductions in the
activities of liver kynurenine hydrolase, knynurenine amino-
transferase, ß-glucuronidase, and acid ribonuclease were observed,
indicating possible effects on drug metabolizing enzymes and on
protein biosynthesis. Pyridoxal phosphokinase activity was increased
(Akhnoukh et al., 1982).
In human blood, nitrosochloramphenicol is bound to albumin
in vitro. In red blood cells, it rapidly forms adducts with
glutathione and also with the -SH groups of haemoglobin. Moreover, it
is rapidly reduced to the N-hydroxy compound, although further
reduction to the amine is very slow. Overall, the data suggest that
the nitroso derivative would be rapidly detoxified in the blood before
reaching the bone marrow (Eyer et al., 1984). Nitrosochloramphenicol,
but not chloramphenicol, induced methaemoglobinaemia in haemolyzed
human blood in vitro (Lim & Yunis, 1982).
To summarize, chloramphenicol is well absorbed in both animals
and human beings after oral administration and widespread distribution
occurs. Urinary excretion is rapid in animals and man, a major
metabolite being the glucuronide conjugate. Animals and human beings
produce a range of urinary metabolites; in vitro experiments suggest
that nitrosochloramphenicol may be an important metabolite in human
beings. It is not known if this compound is produced in vivo in man
or other species.
Toxicological studies
Special studies on carcinogenicity
In 1982 and 1987, IARC concluded that adequate tests for
carcinogenicity of chloramphenicol in animals were not available
(IARC, 1982; IARC, 1987). These conclusions confirmed an earlier
opinion by IARC workers (Tomatis et al., 1978). The two studies
below by Robin et al., and by Sanguineti et al., were included in
the IARC evaluations.
Four groups of Balb/c × AF1 male mice, 45 per group, were given
i.p. injections of either 0.5 mg busulphan on days 1, 15, 29 and 43
(2 groups) or vehicle (acetone plus distilled water, 2 groups. After
20 weeks, by which time 78 mice remained alive in the busulphan-
treated groups and 88 in the vehicle groups (the rest having
died of injection complications), one of the busulphan groups and one
of the vehicle groups was selected for treatment with chloramphenicol,
while the others served as controls and were given vehicle only (0.9%
sodium chloride). Mice in the treatment groups received i.p.
injections of 2.5 mg chloramphenicol 5 days per week for 5 weeks.
Sacrifice of the mice followed a complicated schedule depending on the
appearance of lymphomas, but all animals had been sacrificed by day
350. The following incidences of lymphoma were noted: busulphan/
chloramphenicol, 13/37; busulphan/vehicle, 4/35; chloramphenicol/
vehicle 2/42; vehicle/vehicle, 0/41. The authors thought that this
suggested that busulphan and chloramphenicol increased the frequency
and accelerated the onset of lymphomas. It also provided some
evidence that chloramphenicol alone might induce lymphomas in this
animal model, but the duration of the experiment along with other
limitations of the experimental design, particularly the dosing
regime, prevent any other conclusions from being drawn (Robin
et al., 1981).
In a study which was reported in abstract form only, in which
chloramphenicol was administered in the drinking water, an increased
incidence of lymphomas in two strains of mice and of hepatocellular
carcinoma in one strain were noted (Sanguineti et al., 1983).
Another study investigated the effect of chloramphenicol on
cirrhosis and hepatocellular carcinoma induced by the carcinogen
N-2-fluorenyldiacetamide in rats. A group of 25 male Wistar rats was
given a diet containing 0.05% N-2-fluorenyldiacetamide (equivalent to
25 mg/kg b.w./day) plus 2% chloramphenicol (equivalent to 1000 mg/kg
b.w./day) for 4 weeks. Another group of 20 rats received a diet
containing only the 0.05% N-2-fluorenyldiacetamide. A "rest" week was
then followed by another 4 weeks on the respective diets, followed by
another "rest" week and then 6 weeks of dietary administration of the
substances. After this the animals given the carcinogen only were
returned to the control diet, but those given the combined regime were
continued on this diet for another 2 weeks. Animals were sacrificed at
46 (carcinogen only) or 46-55 weeks (carcinogen plus chloramphenicol).
Of 12 animals examined which were given the carcinogen-only diet, 100%
had cirrhosis and 75% had hepatocellular carcinoma, whereas of 22
animals examined which were given the combination treatment, only one
had cirrhosis and hepatic rumours. Thus, chloramphenicol had a
protective effect on the induction of hepatic rumours by
N-2-fluorenyldiacetamide (Puron & Firminger, 1965).
Special studies on haematological effects
Groups of 18-21 57B1/10ScSnPh mice were given 4.78 Gy of
X-irradiation and were then treated three times daily with 160 or
320 mg/kg b.w. chloramphenicol succinate by s.c. injection over 3 or 5
days, beginning 10 days after the X-ray treatment. Two groups of
control animals were given chloramphenicol and no irradiation or
irradiation and no chloramphenicol. Animals were examined 4, 8, and 21
days after initiation of chloramphenicol treatment (14, 18, and 21
days after irradiation). No effects on the level of erythrocytes in
non-irradiated mice occurred; those given chloramphenicol showed
similar values to non-treated controls. The level of erythrocytes in
irradiated animals was significantly lower than in non-irradiated
animals (30% reduction 14 days after irradiation) but there was
improvement with time (26% reduction 18 days and 15% reduction 21 days
after irradiation), while irradiated animals given chloramphenicol
showed lower erythrocyte levels than those that were irradiated only;
at 14, 18, and 21 days post irradiation, irradiated mice given
chloramphenicol had erythrocyte levels 8%, 17%, and 4.5% lower,
respectively, than animals given irradiation alone, indicating that
chloramphenicol had a deleterious effect on bone marrow recovery after
X-irradiation (Vacha et al., 1981).
Normal mice and those with residual bone marrow damage following
busulphan administration were investigated for the effects of
chloramphenicol. Female Balb/c mice were injected with busulphan to
create bone marrow damage using a method that had been validated
previously by the authors. Groups of mice, some having busulphan-
induced damage and others untreated, were then given drinking water
containing 0.5 g/dl chloramphenicol succinate; groups of 5 mice were
then killed at various intervals up to and including 150 days after
chloramphenicol treatment. No effects were seen in the bone marrow
of mice given chloramphenicol alone nor in mice pretreated with
busulfan. However, animals given both busulphan and chloramphenicol
displayed a progressive fall in the number of pluripotential stem
cells and granulocytic precursor cells (Morley et al., 1976).
Similar effects were not seen in another study where busulphan-
treated mice were given drinking water containing 0.5 g/dl
chloramphenicol for six weeks. There was no effect on the colony-
forming ability of bone marrow or spleen cells. This study used
an identical busulphan regime to that described above (Pazdernik &
Corbett, 1980).
In cells of lethally X-irradiated mice given 10 mg
chloramphenicol i.p. on days 2-8 or on days 7-12 after irradiation,
mitochondrial swelling was noted in early erythroblasts, but not in
intermediate or late types. The mitochondria showed reductions in the
number of cristae (Miura et al., 1980).
A similar investigation in mice given 4.78 Gy X-irradiation and
300 mg/kg b.w. of chloramphenicol succinate showed that dividing bone
marrow cells had decreased entry into S-phase of the cell cycle. The
affected cells were mainly of the erythroid type. The same types of
effect were also noted in non-irradiated mice given chloramphenicol
(Benes et al., 1980).
The femora of mice given 500 mg/kg b.w. chloramphenicol for 6
days by injection (route unspecified) which were then implanted into
untreated syngeneic mice showed greatly decreased colony formation
when the bone marrow was subsequently injected into lethally
irradiated mice (Nara et al., 1982).
No haematological effects, including aplastic anaemia, were seen
in mice given 40 mg/kg nitrosochloramphenicol for 10 days followed by
sacrifice 6 weeks after the last injection (Siegel & Krishna, 1980;
abstract only).
Groups of 6 male Sprague-Dawley rats were each given 50 mg/kg
b.w. chloramphenicol succinate by i.v. infusion. Half of the animals
were subjected to liver resection 15 minutes after infusion, while the
others were sacrificed at this time. Bleeding time and blood loss were
significantly increased in resected animals given chloramphenicol
compared with controls (bleeding time: 500 seconds in treated animals,
300 seconds in controls; blood loss 2.2 g in treated animals, 0.9 g in
controls). No effects on haemoglobin or haematocrit were observed
following infusion or liver resection (Bengmark et al., 1981).
Four cats were given daily i.m. injections of 50 mg/kg b.w.
chloramphenicol for 21 days. Two untreated cats served as controls;
all 6 animals had recently recovered from experimental infectious
feline enteritis. Animals given chloramphenicol became very ill, with
severe loss of appetite developing within 7 days. All four developed
diarrhoea toward the end of the experiment (they were sacrificed on
day 21); one was killed in extremis. Marrow examination was carried
out, which revealed vacuolation of the myeloid and erythroid
precursors and of some lymphocytes. There were no significant changes
in peripheral red cell numbers, but white cell counts were much
reduced (Penny et al., 1967).
A group of 6 cats was given chloramphenicol orally at a dose of
125 mg/kg b.w./day for 14 days and then observed for another 3 weeks.
Another group, formerly intended as controls, were dosed with 60 mg/kg
b.w./day chloramphenicol in the same manner. Signs of toxicity
included CNS depression, dehydradation, loss of appetite, body weight
loss, diarrhoea, and vomiting. Blood and bone marrow samples were
obtained both prior to and after chloramphenicol treatment. The major
findings related to chloramphenicol administration were severe bone
marrow suppression with marrow hypoplasia, prevention of maturation of
erythroid cells, and inhibition of mitosis in the marrow. Vacuolation
of lymphocytes, and early myeloid and erythroid cells occurred. The
effects were most severe in cats given 120 mg/kg b.w. chloramphenicol.
On cessation of treatment the bone marrow suppression resolved (Watson
& Middleton, 1978).
In a similar study, 5 cats were given 50 mg/kg b.w./day
chloramphenicol orally for 21 days. CNS depression, appetite loss, and
weight loss were noted. Examination of peripheral blood was conducted
both before and after dosing. This revealed lower platelet numbers
after 1 week, and a lower neutrophil count after 3 weeks of
administration. One animal developed lymphocytopenia after 1 week and
neutropenia after 3 weeks. At the end of treatment, the bone marrow
was found to have vacuolated early myeloid cells and lymphocytes, with
reduced myeloid maturation and reduced marrow cellularity. No test for
recovery was conducted (Watson, 1980).
Twenty dogs were given oral doses of chloramphenicol for 14 days.
They were dosed in the following manner: 6 dogs, 225 mg/kg b.w./day;
4 dogs each, 175 or 125 mg/kg b.w./day; and 3 dogs each at 275 or
75 mg/kg b.w. day. Signs of toxicity included a decline in the rate of
weight gain and hypophagia. No changes in erythrocyte counts,
reticulocyte counts, haemoglobin concentration, packed cell volume, or
differential leukocyte counts occurred, but bone marrow examination of
the dogs given 225 or 275 mg/kg/b.w./day revealed suppression of
erythropoiesis. In dogs given 275 mg/kg b.w./day chloramphenicol,
decreased mitotic activity and a reduced rate of granulocyte formation
was also evident. No dogs showed bone marrow vacuolation
(Watson, 1977).
After i.v. administration of a single dose of 50 mg/kg b.w.
chloramphenicol succinate to five mongrel dogs, platelets were
obtained from blood at 0, 30, 60, 120, 180, and 240 minutes and at 24
hours after dosing. Protein synthesis as determined by the rate of
incorporation of 3H-leucine was inhibited, with maximum depression
(940% of control values) occurring at 30 - 240 minutes after dosing
(Agam et al., 1976).
Neonatal Holstein calves (1 day of age at the beginning of the
experiment) given "an adequate" intake of colostrum were given
chloramphenicol by several methods. These were, i.v. as a 25 mg/kg
b.w. bolus at 1, 7, 14, and 28 days of age, i.v. as a 25 mg/kg b.w.
injection every 12 hours until 150 mg/kg b.w. had been delivered, and
as a 25 mg/kg b.w. bolus i.v., i.m., or s.c. alternately, with 1 week
between the doses. No effects on haematological parameters were
observed, and bone marrow aspirates showed no evidence of suppression
or toxicity (Burrows et al., 1984).
A similar lack of effect on the bone marrow was noted in a study
of cross-breed calves given doses of 9, 20, or 60 mg/kg b.w.
chloramphenicol daily for 6 weeks (Mitema, 1982).
In another study in Holstein calves in which chloramphenicol was
given orally at a dose of 100 mg/kg b.w./day over 10 days, bone marrow
suppression did occur. Similar results were noted in over 50 calves
investigated over a period of time. Changes included partial aplasia
to almost complete aplasia of the marrow, with loss in cellularity of
erythrocytes, white cells, and megakaryocytes. Occasionally only fat
deposits were noted with lymphocytic infiltrations. Lower blood levels
of chloramphenicol were attained after oral intake than following i.v.
injection, but the toxic effects on the marrow were greater after oral
dosing (Krishna et al., 1981).
In in vitro experiments, chloramphenicol and its postulated
metabolite, nitrosochloramphenicol, have shown adverse effects on bone
marrow cells. Chloramphenicol caused dose-related inhibition of
erythroid and granulocytic colony forming units obtained from LAF1
mice. The lowest concentration used (5 µg/ml) caused some degree of
inhibition of erythroid cells, while the highest concentration
(60 µg/ml) produced complete inhibition (Yunis, 1977). Similar effects
were noted in a separate study (Hara et al., 1978).
Chloramphenicol and nitrosochloramphenicol inhibited DNA
synthesis in rat bone marrow cells in vitro. This was reversible
with chloramphenicol, but not with the nitroso compound. Similarly,
the nitroso compound, but not chloramphenicol, bound irreversibly to
bone marrow cells (Gross et al., 1982). However, in another
in vitro study, chloramphenicol and nitrosochloramphenicol had no
effects on mouse haematopoietic precursor cells (Pazdernik & Corbett,
1979).
Special studies on mutagenicity
The antibiotic activity of chloramphenicol is not thought to
involve any type of reaction with bacterial genetic material. It
appears to inhibit protein synthesis by binding to the 50S subunit of
the 70S ribosome, inhibiting the formation of the peptide bond during
protein synthesis (Smith & Weber, 1983, Gilman et al., 1985).
Chloramphenicol has been shown to cause DNA strand breaks in
bacterial cells and to inhibit DNA synthesis in lymphocytes and in a
phage of E. coli (Amati, 1970; Dewse, 1977; Jackson et al., 1977).
However, chloramphenicol provided resistence to UV-induced damage in
E. coli B/r (Doudney & Rinaldi, 1985).
Nitrosochloramphenicol, which is a potent inducer of DNA strand
breaks in bacterial cells, resulted in strand breakage and loss of
helix, bringing about rapid degradation of isolated E. coli DNA
in of helix, bringing about rapid degradation of isolated E. coli
DNA in vitro (Skolimowski et al., 1981, 1983). Nitrosochlor-
amphenicol, but not chloramphenicol, produced inhibition of DNA
synthesis and caused DNA strand breaks in E. coli. (Murray
et al., 1982; Yunis, 1984).
Chloramphenicol has been tested in a variety of assays for
mutagenic activity. Most of these assays are well established using
well validated methods, but some, such as colicine induction, the
induction of tandem genetic duplications in S. typhimurium, and
tests using snails, are less well known and are unvalidated. The
results of these tests are presented in Table 2.
In general chloramphenicol gave negative results in bacterial
reverse mutation assays, in assays for DNA repair in bacteria, in the
dominant lethal test in rodents and D. melanogaster, in the
CHO/HGPRT test, in a test for sister chromatid exchange in human
lymphocytes, in the micronucleus test, and in a test for DNA binding.
Tests for inhibition of growth in E. coli were also negative and,
moreover, chloramphenicol has sometimes been used as a negative
control in this assay (McCoy et al., 1980a; McCoy et al., 1980b;
Rosenkranz, 1977; Rosenkranz et al., 1974; Braun et al., 1977;
Braun et al., 1982). There were isolated exceptions to these
negative results. However, the only type of test that gave
consistently positive results was the test for induction of
chromosomal aberrations. Chromosomal anomalies were noted in human
lymphocytes treated in vitro with chloramphenicol, in mouse bone
marrow in animals given chloramphenicol at doses of 50 mg/kg i.p. or
i.m., and in F1-generation mouse liver.
The failure of chloramphenicol to give positive results in the
Ames test has been attributed by one group of authors to its bacterial
toxicity. In their study, the D(-)-threo isomer of chloramphenicol
gave negative results in the Ames test, as observed previously in
several other studies; it was also toxic to the bacterial tester
strains used, TA100 and TA1535. However, the L(+)-threo isomer,
which is not used therapeutically, was much less toxic and could be
tested at higher concentrations; with the isomer, a dose-related
mutagenic response was noted in both tester strains (Jackson et al.,
1977).
The failure of chloramphenicol to give positive results in most
types of commonly used mutagenicity tests, with the exception of those
examining the induction of chromosome aberrations, has been recognized
by other reviewers (Waters et al., 1983; Garrett et al., 1984), as
has the failure of the substance to produce mutations in vivo
(Holden, 1982).
Chloramphenicol has been reported to enhance the mutagenicity of
N-methyl-N-nitro-N-nitrosoguanidine in bacterial assays (Sklar &
Strauss, 1980; Baltz & Stonesifer, 1985).
Special study on ocular toxicity
No toxic effects were seen in groups of three rabbits following
vitrectomy when solutions containing 10 or 20 µg/ml chloramphenicol
were infused into the eyes as vitreous replacements. Histological and
electroretinographical examinations yielded normal results 2 weeks
after infusion. Following an infusion of a 50 µg/ml solution,
electroretinography was normal after 2 weeks, but abnormal retinal
histology, which was not described but was said to be widespread and
generalized, was noted (Stainer et al., 1977).
Special studies on ototoxicity
Solutions of 0.5% chloramphenicol introduced into the bullae of
the ears of guinea pigs produced no effects on hearing, but solutions
containing 1 - 5% chloramphenicol produced moderate hearing loss at a
variety of frequencies. Similar findings were noted when the
electrical responses of the hair cells were measured after
chloramphenicol administration into the bullae. Intratympanic
administration to guinea pigs of a 1% solution of chloramphenicol
produced a moderate degree of hair cell loss in the organ of Corti,
with severe inflammation of the mucosa of the middle ear. Introduction
of a solution containing 8 or 16 mg chloramphenicol through a hole in
the bullae of the ears of guinea pigs followed by sacrifice 3, 6, 9,
or 24 hours later resulted in severe destruction of hair cells and
supporting cells in the basal turns of the organ of Corti. The effects
were similar regardless of the dose or the time of sacrifice after
dosing (Proud et al., 1968; D'Angelo et al., 1967; Patterson &
Gulick, 1963; Morizono & Johnstone, 1975; Parker & James, 1978).
Table 2. Results of mutagenicity assays with chloramphenicol
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 30 µg/plate - Brem et al.,
TA1530, TA1535 1974
TA1538
S. typhimurium 0.17-24 µg/ml + Mitchell et al.,
TA98 1980
S. typhimurium Not given - Heddle & Bruce,
TA1535, TA1537 1977
S. typhimurium 30 µg/plate - Rosenkranz et al.,
TA98, TA100 1976
S. typhimurium < 4.5 nmole - McCann et al.,
TA98, TA1535 1975
TA1538
Bacterial E. coli 27 µg/ml + Mitchell &
mutation assay CM891 Gilbert, 1984
CHO/HGPRT Chinese hamster Not given Augustine et al.,
mutation assay ovary cells 1982
Gene mutation D. melanogaster Not given +a Narda & Gupta,
1972
(abstract only)
Dominant lethal D. melanogaster Not - Nasrat et al.,
1977
Mouse (101×C3H)F1 2×1.5 g/kg - Ehling, 1971
Mouse (ICR/Ha 333 mg/kg - Epstein & Shafner,
Swiss) 1968
Mouse (ICR/Ha 333 & 666 mg/kg - Epstein et al.,
Swiss) 1972
DNA repair B. subtilis 2.5×10-3 mg/ - Sekizawa &
H17, M45 disc Shibamoto, 1982
B. subtilis Not given - Suter & Jaeger,
H17, M45 1982
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
E. coli Not given + Suter & Jaeger,
AB1157/JC5547 1982
AB1157/JC2921
AB1157/JC2926
AB1157/JC5517
E. coli WP2 Mitchell et al.,
uvrA+recA+,uvrA- Not given - 1980
recA-
trp-/trp+ Not given -
A2Cs/A2Cr 3-48 µg/ml +
E. coli B/r 100 µg/ml - Masek, 1977
E. coli K12 > 30 µg/ml - Mamber et al.,
(SOS chromotest) 1986
Preferential E. coli K12 5-20 µg/ - Hyman et al.,
inhibition plate 1980
E. coli 30 µg/plate - Brem et al.,
Pol A+, Pol A- 1974
E. coli 10 µg/plate - McCoy et al.,
Pol A+, Pol A1- 1980a,b
E. coli 30 µg/plate - Longnecker et al.,
Pol A+, Pol A- 1974
E. coli 30 µg/disc - Slater et al.,
Pol A+, Pol A- 1971
Gene conversion S. cerevisiae Not given - Mitchell et al.,
D4 1980
Sister chromatid Human lymphocytes 200 µg - Pant et al., 1976
exchange (in vitro)
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Chromosomal Zea mays 30 µg/ml - Verma & Lin, 1978
aberrations
Human lymphocytes 10-40 µg/ml + Mitus & Coleman,
(in vitro) 1970
Human lymphocytes Not given + Goh, 1979
(in vitro)
Human lymphocytes 200 µg + Pant et al., 1976
Mouse, bone 50 mg/kg b.w. + Manna & Bardham,
marrow 3×50 mg/kg + 1977
b.w., 8h
intervals
Mouse, F1 liverb 50 mg/kg b.w. + Manna & Roy, 1979
Micronucleus Tradescantia 0.1 - 5mM - Ma et al., 1984
test paludosa
Mouse Not given (5 - Heddle & Bruce,
(CH3×C57)F1 daily doses) 1977
DNA binding E. coli 100-1000 µM - Kubinski et al.,
assay 1981
Enhancement of Syrian hamster 0.7-5mM + Hatch et al.,
SA7 virus cell embryo cells/ 1986
transformation simian adenovirus
SA7
Aneuploidy Hordeum vulgare 300 µg/ml + Yoshida et al.,
induction 1972
Colicine S. typhimurium 0.1-60 µg/ - Ben-Gurion, 1978
induction TA1537 REN plate
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Increased Snail Not given - Xie, 1985
embryonal length
Induction of S. typhimurium 0.155-3.1 µM - Pall & Hunter,
tandem genetic TR4179, TT1984 1985
duplication
a Very weak positive response.
b One male mouse was given 50 mg/kg chloramphenicol i.m. and then was mated
with 4 untreated females. Mice (3) were sacrificed on days 12, 16, and 18
of gestation. The remaining mouse was allowed to litter normally and the
young were sacrificed when 7 days old. Livers of fetuses and neonates were
removed and examined.
When groups of 3 - 9 female Sprague-Dawley rats were given
80 mg/kg b.w. chloramphenicol in the drinking water for 10 days with
or without exposure to short duration high intensity noise,
ototoxicity was noted as revealed by reductions in the electrical
output of the cochlea. Noise exposure alone also reduced the output,
but noise and chloramphenicol together resulted in a severe effect
(Henley et al., 1984). Similar effects were reported in abstract
form in another study in rats (Henley, 1985).
Chloramphenicol was given to guinea pigs (number unspecified) as
a single i.v. dose of 400 mg/kg b.w. The threshold for the Preyer
reflex was measured after administration and at 10, 20, and 30
minutes, 1, 2, 3, 4, and 5 hours, and then daily for 7 days. There was
no change in the Preyer reflex with noise of 1 and 8 KHz and no loss
of hair cells, indicating no ototoxic response in this study
(Beaugard et al., 1979; Beaugard et al., 1981).
Special study on effects on sleep
Oral doses of 160 250 mg/kg b.w. chloramphenicol suppressed
paradoxical sleep in cats. After a dose of 330 mg/kg b.w. paradoxical
sleep was suppressed for 24 hours, at which time there was also a
depression of slow wave sleep (Petitjean et al., 1975).
Special study on spermatogenesis
A group of male rats was treated daily with 100 mg/kg b.w.
chloramphenicol succinate for 8 days (route and animal numbers not
stated), after which they were sacrificed and the testes examined
histologically. Examination revealed total or incomplete inhibition of
spermatogonial divisions with "perturbed meiosis". No other details
were provided (Timmermans, 1974).
Special studies on teratogenicity
Monkeys
Chloramphenicol at doses of up to 200 mg per monkey
(10 mg/kg b.w.) had no effect on the development of Macaca mulatta
when given for 6 to 17 days at various intervals between the 65th and
95th days of gestation (Courtney et al., 1967; Courtney & Valerio,
1974).
Rabbits
Groups of 5 - 8 pregnant mixed breed rabbits were given 500,
1000, or 2000 mg/kg b.w./day chloramphenicol by garage on days 6 - 15,
6 - 9, or 8 -11 of gestation, respectively. Historical control data
collected over the previous 4 year period, using 192 rabbits, were
used for comparison. Excess fetal deaths did not occur in rabbits
given 500 mg/kg b.w./day chloramphenicol, but in the mid- and
high-dose groups 25% and 58% fetal deaths were noted, respectively,
compared with 10% in the historical controls. There were no excess
incidences of fetal malformations, but delayed ossification was noted
in fetuses from dams given chloramphenicol (Fritz & Hess, 1971).
Chicks
Chick eggs, less than 3 days old, were treated with 0.1 ml of
chloramphenicol solution in distilled water. The chloramphenicol, at
doses of 0.5 or 1.0 mg/egg, was injected into the albumen via the
airsac. The major anomaly observed was vesiculation of the heart and
trunk resulting from the inhibition of differentiation of the
splanchnopleure; this effect was most severe following 16 - 19 hours
of incubation (36 - 57% in the 0.5 mg/egg group and 23 - 47% in the
1.0 mg/egg group). Unfortunately, no control data were provided
(Blackwell, 1962).
Chloramphenicol also had adverse effects on development in a
separate study in which fertilized eggs with embryos at the 14- or
20-somite stages were explanted and exposed to chloramphenicol at
concentrations of 0, 200, or 300 µg/ml for 22 - 24 hours. The major
defects noted were those of the neural tube (failure to close) and
forebrain. There was also evidence of an inhibition of haemoglobin
formation (Billet et al., 1965).
Rats
Pregnant Sprague-Dawley rats (5 - 15 per group) were given gavage
doses of 500, 1000, 1500, or 2000 mg/kg b.w./day chloramphenicol over
various stages of gestation. In addition, single gavage doses of
2000 mg/kg b.w. were given to pregnant rats on days 5, 6, 7, 8, 9, or
10 of gestation. A group of 553 historical control rats was used for
comparison. Even the lowest daily dose of chloramphenicol on days 5 -
15 of gestation resulted in significant embryo/fetal deaths (63%) when
compared with historical controls (23%), whereas doses of 2000 mg/kg
b.w./day on days 15 - 17 or 2000 mg/kg b.w. on days 5, 6, or 7 had no
effect. Single doses of 2000 mg/kg b.w. on days 8, 9, or 10 of
gestation resulted in 45% fetolethality, but the most sensitive period
appeared to be days 9 - 15. For example, fetal deaths occurred 100% of
the time when 2000 mg/kg b.w./day was given over days 9 - 11 of
gestation. The highest incidences of anomalies, umbilical hernia, were
noted at 2000 mg/kg b.w./day when given over days 6 - 8 of gestation
(36%) and at 2000 mg/kg b.w. on day 8 (11%). High incidences of
delayed ossification were seen in fetuses from dams given 1000 mg/kg
b.w./day chloramphenicol on days 7 - 12 or 2000 mg/kg b.w./day on days
11 - 13 (Fritz & Hess, 1971).
The effects of chloramphenicol on pre-implantation embryos in the
rat were investigated by treating groups of 7 pregnant Sprague-Dawley
rats with 250 mg/kg b.w. chloramphenicol i.p. on either day 3 or 5 of
gestation. The animals were killed on day 5 of pregnancy and the
uterine contents examined. No effects on the number of blastocysts per
female were noted, but administration on day 3 of pregnancy
significantly reduced the number of cells per blastocyst. A reduction
was also seen when the compound was given on day 14, but these results
were not statistically significant (Giavini et al., 1979).
Pregnant Sprague-Dawley rats (number unspecified) were given 3%
dietary chloramphenicol, equivalent to 1500 mg/kg b.w./day, from days
0 to 20. The number of resorptions (% of total implants) was elevated
in rats treated with chloramphenicol (31.4 - 57.0%) compared with
controls (4.7%), while fetal weight in treated rats was only 50% of
controls. Placental weight was also much reduced, while the number of
live fetuses was greatly reduced by chloramphenicol. The authors then
examined the effects of chloramphenicol when given on specific days of
pregnancy (0 - 2, 0 - 3, 0 - 4, etc., up to 0 - 12). The major effects
on implantations, resorptions, number of live fetuses, and fetal
weights as described above were seen in the 0 - 8 to the 0 - 12
feeding schedules, suggesting an effect on implantation (or effects on
embryos soon after implantation). A large proportion of fetuses (71%)
had edema and there was an increased incidence of wavy ribs (7%) and
fused ribs (7%) in chloramphenicol-treated groups compared with
controls (zero incidences in all cases in controls) (Hackler et al.,
1975).
Chloramphenicol was investigated for its effects on avoidance
learning in rats. Four groups of 15 pregnant Wistar rats each were
treated as follows: in one group 50 mg/kg b.w. chloramphenicol
succinate was given s.c. on days 7 - 21 of gestation. In two other
groups, 50 or 100 mg/kg b.w. was given s.c. to pups for the first 3
days after birth; the fourth group served as controls. No effects on
pregnancy, litter size, fetal weight, post-natal weight gain, or
incidence of gross malformations were seen. When 60 days old, animals
were selected for conditioned-learning study and were then examined
for avoidance learning at days 5, 10, 15, and 20 from the start of the
conditioning procedure. Pups from mothers given chloramphenicol
succinate and those given the substance as neonates showed a marked
and statistically significant impairment of avoidance learning at all
four times. The effects were generally worse in pups given the
substance post-natally than in those from dams administered it during
gestation, but the differences were only slight (Bertolini & Poggioli,
1981).
Mice
Groups of 8 pregnant albino mice were given oral chloramphenicol
at doses of 25, 50, 100, or 200 mg/kg b.w. in 10 ml of distilled water
over the third trimester of pregnancy for 5 - 7 days. Animals were
allowed to give birth and the young were tested for conditioned
avoidance response, electroshock seizure threshold, and performance in
open-field tests at days 30, 38, and 42. No gross abnormalities were
seen in any of the progeny. Dose-related effects were seen in all
three elements of the test, with progeny from chloramphenicol-treated
dams showing a reduced learning ability, higher brain seizure
threshold, and poorer performance in the open-field test (Al Hachin &
Al-Baker, 1974).
Groups of 7 - 19 pregnant CD1 mice were given by garage 500,
1000, or 2000 mg/kg b.w./day chloramphenicol on days 5 - 15, 6 - 12,
or 8 - 10 of gestation, respectively. Historical control data,
collected over the previous four-year period using 307 mice, were used
for comparison. In the 1000 and 2000 mg/kg b.w./day dose groups, 71
and 100% embryo/fetal deaths occurred, respectively, compared with 24%
in controls and 31% in mice given 500 mg/kg b.w./day chloramphenicol.
The only defects observed were a low incidence of fused sternebrae and
elevated incidences of delayed ossification in fetuses from dams given
1000 mg/kg b.w./day chloramphenicol (Fritz & Hess, 1971).
In vitro
Chloramphenicol and several other chemicals were tested in a
mouse embryo limb bud cell culture test system which itself was being
validated as part of the study. In all, 22 known mouse teratogens and
5 non-teratogens were investigated in the system, which makes use of
high-density cultures of mouse embryo limb bud cells that can
differentiate and synthesize an extracellular matrix of sulfated
proteoglycans. The end-point of the test considers incorporation of
radiolabel (3H-thymidine) and the growth and synthesis of ocular
protein and cartilage proteoglycan. Chloramphenicol gave a positive
result in this test, with the maximum active concentration being
5 µg/ml. [The test was around 89% predictive; no false positives were
seen and the false negative rate was about 15% (Guntakatta et al.,
1984).]
Another in vitro test made use of the differentiation
characteristics of rat embryo midbrain and limb bud cells, and the
inhibition of differentiation by teratogens. Chloramphenicol gave a
weak response, resulting in inhibition at concentrations in excess of
50 µg/l, compared with 10 µg/l or less seen with several known
teratogens, e.g., captan, colchicine, and parbendazole (Flint & Orton,
1984).
Acute toxicity
Four groups of pregnant and non-pregnant mice were given i.v.
doses (unspecified) of chloramphenicol. No signs of toxicity were
reported. The LD50 value for non-pregnant mice was calculated as
1530 (1260 - 1840) mg/kg b.w., while that for pregnant mice was
1210 mg/kg b.w. (no confidence limits cited) (Beliles, 1972).
Short-term studies
No information available.
Long-term studies
See "Special studies on carcinogenicity".
Observations in human beings
Chloramphenicol is known to produce two major adverse effects in
human beings. One of these is a generally irreversible and often fatal
aplastic anaemia and the other is a reversible bone marrow
suppression.
Aplastic anaemia
Aplastic anaemia is the most dangerous effect produced by
chloramphenicol, although its occurrence is rare. It is usually fatal
(Benestad, 1979). Numerous publications have appeared, most of them
case reports describing the development of aplastic anaemia.
An investigation into the incidence of chloramphenicol-induced
aplastic anaemia in Hamburg suggested that the incidence was 1/11500
with a death rate 1/18500. In the period 1965 - 70, 29 cases were
reported, while in 1971, 3 cases were reported. Total doses in 18
cases were in the range of 10 to 100 g, with most individuals having
received 11 - 30 g. Onset was from 14 days (rare) to 4 - 6 months
after chloramphenicol therapy (Hausmann & Skrandies, 1974).
In a study in Israel in 1985, aplastic anaemia incidence was
7.1/106 in males and 8.7/106 in females. Chloramphenicol was
thought to account for up to 25% of cases. Aplastic anaemia usually
took up to 12 months to develop after chloramphenicol treatment
(Modan et al., 1975).
In 1969 in California, aplastic anaemia in chloramphenicol-
treated patients was said to be 13 times more frequent than in
the general population. Most individuals had been given oral
doses, but in some, aplastic anaemia had occurred after i.m.
administration. Doses were often on the order of 250 mg thrice daily
to a total of 3 g or 250 mg four times daily to a total of 5 g. The
majority of patients were in the 50 - 80 age group, but it was
observed in a 15-year-old boy given a total of 3 g chloramphenicol and
in a 37-year-old female given a total of 6 g over a month. In both
cases onset occurred 3 - 4 months after treatment ended
(Wallerstein et al., 1969).
A series of reports from Sweden in the 1970s suggested that the
incidence of aplastic anaemia was around 80 in 1.2 million. Only 4 or
5 of these were thought to be due to chloramphenicol treatment,
placing the risk at 1 in 20000 (Bottiger & Westerholm, 1972;
Bottiger & Westerholm, 1973; Bottiger, 1978; Bottiger, 1979).
Of 108 cases of aplastic anaemia reported in Istanbul, 4 were
thought to be due to chloramphenicol (Aksoy et al., 1984).
A total of 380 cases of aplastic anaemia were seen at a hospital
in Paris in the years 1971 - 1983; 194 adult cases were considered to
be due to chemical agents. In the period 1971 - 1977, 18/104 cases
were attributed to chloramphenicol, whereas chloramphenicol accounted
for 2/36 and 2/52 cases of aplastic anaemia in the periods 1977 - 1980
and 1980 - 1983, respectively, suggesting a decreasing incidence, at
least in the area of France under investigation (Najean & Baumelon,
1984).
In the period 1975 - 1980, 9 children with aplastic anaemia were
seen at the Medical School, Gadjah Mada University (Yogyakarta). Two
were idiopathic, but at least 3 could be attributed to treatment with
chloramphenicol (Widayat et al., 1983).
A study of 40 cases of aplastic anaemia by members of the
Association of Clinical Pathologists in the USA revealed that 27 were
probably due to chloramphenicol. Of these, 18 had exceeded 10 g or
more (up to 250 g) in total dosage, while 8 had received 10 g or less.
One, an infant, had received less than 2 g chloramphenicol. Onset was
usually 1 - 3 months after drug cessation. Route of administration and
its duration were not specified (Sharp, 1963).
In 1954, of 539 cases of aplastic anaemia from 37 states in the
USA, 55 were attributable to chloramphenicol. In general there was a
female preponderance and usually the effect developed 1 - 6 months
after the drug was withdrawn (Welch et al., 1954).
In a study in Iraq, however, a male preponderance of 3:1 over
females was noted. Of 60 patients with aplastic anaemia at the
University of Baghdad Teaching Hospital, 12 were associated with
chloramphenicol treatment (Al-Moudhiry, 1978).
One study in the Netherlands examined cases of blood dyscrasias
taken from the literature along with unpublished cases from adverse
drug reaction reporting systems in the United Kingdom, Denmark,
Sweden, and the Netherlands. To these were added cases from hospitals
in Northeast Switzerland. A total of 641 cases of chloramphenicol-
induced blood dyscrasias were identified. These included:
thrombocytopenia (21), agranulocytosis (51), hypoplastic anaemia (39),
bone marrow suppression (39), acute leukaemia (27), and aplastic
anaemia (464). Of the 464 cases of aplastic anaemia, 335 (72%) had
proved fatal. There appeared to be a female preponderance (261 cases;
56%). However, because this cannot be related to the total number
treated and therefore to those who did not acquire the condition, the
incidence cannot be determined (Meyler et al., 1974). The results
confirmed those of earlier work (Polak et al., 1972).
In Italy, it has been claimed that in view of the ease of
availability of chloramphenicol, the incidence of aplastic anaemia
appears to be low. For example, in 1971 there were 10 reports of fatal
side effects due to antibiotics; in 1972 there were 3. None were
attributable to chloramphenicol. During the period 1973 - 1975 there
were no reports of fatal cases (Preziosi et al., 1981).
For the period 1959 - 1969, 172 cases of aplastic anaemia were
reported in 15 hospitals in Northeast Switzerland, and 44 of these
individuals had been treated with chloramphenicol. The smallest total
dose incriminated was 3 g, while the highest was 315 g
(Keiser & Bucheggar, 1973).
In children aged 0 - 14 years in Denmark, the total number of
registered cases of aplastic anaemia was 39 for the years 1967 - 1982,
giving an annual incidence of 22/106. Probable causes were
identified for 21 of the 39 cases, and 2 of these were attributed to
chloramphenicol treatment (Clausen, 1986).
It was stated in 1983 that the probable overall incidence of
chloramphenicol-induced aplastic anaemia is somewhere between 1 in
20000 and 1 in 40000 (Venning, 1983).
In Japan, chloramphenicol appeared to be more dangerous to the
elderly population than to other groups. However, the investigation
was probably biased in that only fatal cases were examined
(Mizuno et al., 1982). Similar findings were made in an earlier
study (Shimizu et al., 1979).
For the years 1961 - 1965, 35 cases of aplastic anaemia were
reported in Colombia, and of these 10 had had past exposure to
chloramphenicol. Several others were said to have had a "strong
suspicion" of exposure to the drug. Mortality was 60% (Sarasti, 1970).
An age preponderance was noted in an analysis of 21 patients with
aplastic anaemia, 8 of whom had been treated with chloramphenicol
(Perez et al., 1981).
The minimum dose of chloramphenicol associated with the
development of aplastic anaemia is not known with certainty. The
literature citations often state only the total dose. Where cases
developed after several doses, it is not possible to say if aplastic
anaemia would also have occurred had only a single dose been given. Of
15 cases reviewed in a report in 1974, total doses of 4.5 to 80 g had
been given, with the usual levels being 8 to 14 g. As an example, a
46-year-old woman was given 15 g of chloramphenicol over 10 days.
Aplastic anaemia developed after 2 months (Hellriegel & Gross, 1974).
Total doses of 6.5 to 60 g chloramphenicol had been given to 7
individuals with aplastic anaemia reported in 1971 (Hodgkinson, 1971).
A 27-year-old woman given 30 g chloramphenicol i.v. over 12 days
developed aplastic anaemia 3 months later (Alavi, 1983).
One individual with aplastic anaemia had undergone previous
treatment 19 years earlier with a 500 mg initial dose followed by
250 mg chloramphenicol 4 times daily. At the age of 23 years, he was
given 750 mg chloramphenicol succinate (62 mg/kg b.w./day) i.v. every
6 hours for 12 days for a brain abscess. Bone marrow suppression
ensued and by the twelfth day of drug administration aplastic anaemia
had developed. The patient eventually died (Daum et al., 1979).
A 26-year-old woman was diagnosed as being anaemic during the
fifth month of pregnancy and in the sixth month she developed a skin
infection. She was given 8 g chloramphenicol. She developed aplastic
anaemia and died 8 days after giving birth. Bone marrow aspiration
confirmed aplastic anaemia. (Suda et al., 1978).
Several cases of aplastic anaemia have been reported in children.
One 7-year-old boy had been given chloramphenicol palmitate in June
and August of 1959. The dose or route of administration were not
specified. He developed aplastic anaemia 4 - 5 months later and died
(Leiken et al., 1961). A 6-year-old girl was given 25 mg/kg b.w./day
chloramphenicol for 10 days by an unspecified route. She developed
aplastic anaemia, apparently immediately after treatment and then
acute myeloblastic leukaemia (Awaad et al., 1975). A 4-year-old girl
was given oral chloramphenicol (dose not stated) for 1 week. After 2
months she developed aplastic anaemia (Young et al., 1979). Another
case of a 4-year-old girl given chloramphenicol was reported more
recently. Chloramphenicol was given i.v. for 1 week followed by oral
therapy for 8 weeks. The initial dose was 75 mg/kg b.w./day given
every 6 hours, but this was adjusted after 3 weeks to 37 mg/kg
b.w./day. Some months later she developed aplastic anaemia
(Lepow, 1986). In one recent case the patient was a neonate born at 30
weeks gestation weighing 1.7 kg. At day 20 after birth she developed
suspected meningitis and was given 4 doses of 50 mg/kg b.w./day
chloramphenicol. She developed aplastic anaemia; epidermal necrolysis
of the skin and biliary cholestasis were found at necropsy
(White et al., 1986).
A 72-year-old male patient was given a 3-week course of
chloramphenicol (dose and route unspecified) and aplastic anaemia
ensued within 4 months (Howell et al., 1975).
Aplastic anaemia is usually associated with oral intake
(Matthews et al., 1980). It has been reported that in 149 cases of
chloramphenicol-induced aplastic anaemia, 85% followed oral dosing,
14% followed parental administration, and 3% occurred after rectal
administration (Plaut & Best, 1982).
Topical administration of chloramphenicol has been followed by
aplastic anaemia. In one case, a 73-year-old woman died of aplastic
anaemia less than 2 months after beginning ophthalmic chloramphenicol
treatment with a 0.5% solution (3 - 4 times daily) and a 1% ointment
(once per day to the right eye) (Fraunfelder & Bagby, 1982). Several
other similar cases have been reported (Abrams et al., 1980;
Rosenthal & Blackman, 1965; Carpenter, 1975; Issaragrisil &
Piankijagum, 1985; Korting & Kifle, 1985). In one case the total dose
was estimated to be 32 mg of chloramphenicol (Carpenter, 1975).
One occupational case probably involved inhalation and skin
contact. It occurred in a shepherd applying a chloramphenicol spray to
the feet of sheep for treatment of foot rot. He had treated the
animals twice a week with the spray, which contained 10 g of the drug
in 100 ml of solution, for two years. Each dose contained 10 mg
(Del Giacco et al., 1981).
A link between the administration of chloramphenicol and the
development of liver disease and aplastic anaemia is known to exist.
After five patients aged 4 to 63 years were given chloramphenicol,
liver disease (as evidenced by jaundice, icterus, and elevated serum
enzymes and bilirubin) was subsequently noted. Aplastic anaemia
eventually developed (Hodgkinson, 1973). A similar case has been
reported in a 15-year-old boy given 250 mg chloramphenicol i.v. every
6 hours. Liver enzymes became elevated after 18 days and the liver
itself was tender. Aplastic anaemia developed (Caslae et al., 1982).
The mechanism of chloramphenicol-induced aplastic anaemia is not
understood. There may be a genetic element involved, as the effect has
been seen in families and in identical twins exposed to chloramphenicol
(Flach, 1982; Nagao & Maner, 1969; Silver & Zuckerman, 1980; Yunis,
1978c). The main target may be the haemopoietic pluripotential stem
cells of the marrow (Vincent, 1986; Silver & Zuckerman, 1980;
Benestad, 1974; Appelbaum & Fefer, 1981). There may also be the
possibility of initial damage to the marrow micro-environment (Camitta
et al., 1982). Failure to note this effect in human beings with
drug-induced aplastic anaemia makes the latter unlikely (Samson
et al., 1972; Vincent, 1986). In vitro studies with human bone
marrow suggest that both the erythroid and granulocytic series are
sensitive to chloramphenicol (Nara et al., 1982; Hara et al.,
1978; Yunis, 1977). The erythroid series in particular seemed sensitive
to chloramphenicol, with inhibition occurring at 10 mg/l compared with
50 mg/l for the granulocytic series (Yunis, 1977).
The problem as to whether bone marrow cells are more sensitive to
chloramphenicol in patients with aplastic anaemia induced by the drug
is not clear, as reports on the subject are conflicting (Yunis et al.,
1973a; Howell et al., 1975; Kern et al., 1975). It has been claimed,
based on the results of animal studies, that individuals who are
sensitive to chloramphenicol-induced aplastic anaemia may have
residual bone marrow damage brought about by exposure to other agents
(Morley et al., 1976).
The metabolite or metabolites responsible for the induction of
aplastic anaemia in human beings is unknown, but nitroso-
chloramphenicol has been implicated (Murray & Yunis, 1981; Nagai &
Kanamuru, 1978). Nitrosochloramphenicol can be formed by the
reduction of chloramphenicol in human liver in vitro (Salem et al.,
1981). This substance is known to be toxic to human bone marrow cells
in vitro and, moreover, is more toxic than chloramphenicol itself
(Yunis et al., 1980a, b). However, nitrosochloramphenicol is not
myelotoxic to mice in vivo (Krishna et al., 1981). It does,
however, cause DNA strand breakage in vitro, and inhibit DNA
synthesis (Gross et al., 1982; Skolimowski et al., 1983). Both
chloramphenicol and nitrosochloramphenicol are taken up rapidly by
cells, at least as demonstrated by a human transformed lymphoblastoid
cell line (Raji cells), but the nitroso-compound covalently binds to
these cells and to bone marrow cells 15 times more tightly than does
chloramphenicol (Hurray & Yunis, 1981). Photochemical decomposition of
chloramphenicol may result in potentially myeloblastic derivatives,
which may be hazardous in ophthalmic solutions (de Vries et al.,
1984). Another possible mechanism involves the immune system (and even
autoimmune damage), but there are no convincing data to support this
hypothesis. Chloramphenicol in vitro inhibited lymphocyte
transformation in human material (Burgio et al., 1974) and
chloramphenicol reduction products, including nitrosochloramphenicol,
were suppressive to antigen-reactive cells in mice (Pazdernik &
Corbett, 1980).
In summary, chloramphenicol induces aplastic anaemia in
susceptible individuals, but no dose-response relationship has been
identified. The mechanism may involve nitrosochloramphenicol, but this
has not been proven. The nature of the mechanism is unknown.
Bone marrow suppression
Reversible bone marrow suppression has been reported in patients
given chloramphenicol by a number of routes. The effect is thought to
be an unlikely event at plasma levels under 20 mg/l, and it is said to
occur in most patients at levels in excess of 25 mg/l. Generally, it
occurs within days of administration (Lery et al., 1978; Benestad,
1979). One study showed that oral doses likely to result in
suppression were usually of the order of 2 - 3 g/day or 30 - 50 mg/kg
b.w./day; durations of dosing were generally 1 - 17 days, most being 5
- 10 days. Plasma levels of chloramphenicol at 2 - 3 hours and 6 - 8
hours varied, most being in excess of 25 mg/l at 2 - 3 hours and in
excess of 30 mg/l at 6 - 8 hours. However, some were below 15 mg/l at
both times. Of the 17 patients studied, 11 had liver disease, while 3
of the remainder had renal disease. Bone marrow suppression in a
female of 21 years of age was attributed to the administration of
1.5 g/day of chloramphenicol for 18 days. The condition resolved on
cessation of therapy (Parashar et al., 1972). In 6 anaemic subjects
given up to 60 mg/kg b.w./day chloramphenicol, reticulocyte response
to vitamin B12 or iron dextran was halted and delayed (Saidi
et al., 1961).
Maturation arrest and cytoplasmic valuation of erythroid elements
were seen in the marrow (Rosenbach et al., 1960; Bartlett, 1982).
One reported case of bone marrow suppression was in a
three-year-old girl with cystic fibrosis given 70 mg/kg b.w./day
chloramphenicol for 2.5 months. The suppression was accompanied by
physical growth depression and hair loss. All these conditions
resolved on cessation of treatment (Kapp et al., 1977).
The toxic effects of chloramphenicol on the bone marrow were
lessened in one group of patients by co-administration of
phenylalanine (Ingall et al., 1965). The reason for this, and its
relationship to the pathogenesis of bone marrow suppression, is
unknown. The mechanism of suppression is thought to involve inhibition
of mitrochondrial protein biosynthesis in bone marrow cells. In vitro
studies showed that 10 mg/l chloramphenicol severely inhibited protein
synthesis in bone marrow cells, although ten times this concentration
was required to inhibit mitochondrial respiratory functions (Yunis,
1973a, b; Yunis, 1978a, b; Martelo et al., 1969). As a result of
mitochondrial dysfunction, ferrochelatase activity is suppressed in
erythroid precursors. In vitro studies show inhibition of haem
synthesis with concentrations of chloramphenicol of 10 mg/l
(Becker et al., 1974). It seems likely that the major effect of
chloramphenicol in the pathogenesis of reversible bone marrow
suppression is a block on hame biosynthesis arising as a result of
mitochondrial dysfunction (Yunis & Salem, 1980). Consequently,
co-administration of phenylalaninine may in some way compensate for
the inhibitory effects of chloramphenicol on protein synthesis.
Effects on platelets
In in vitro experiments, chloramphenicol was shown to decrease
human platelet aggregation. Unfortunately, no in vivo studies are
available (Cronber et al., 1984, Djaldetti, 1983; Agam et al.,
1976).
Carcinogenicity
IARC concluded in 1982 and 1987 that there was limited evidence
for the carcinogenicity of chloramphenicol to human beings based on
studies describing cases of leukaemia (IARC, 1982; IARC, 1987).
One study described a 24-year-old male given chloramphenicol for
typhoid fever. Leukaemia followed aplastic anaemia. A chromosome
translocation (t 1:7) was discovered on karyotyping. No other details
were provided, except that similar translocations were noted in 6
other leukaemia patients known to have had exposure to leukaemogenic
substances or radiation (Scheres et al., 1985).
Acute myeloid leukaemia developed in a 6-year-old girl who
developed aplastic anaemia after being given 25 mg/kg b.w./day
chloramphenicol for 10 days. The leukaemia developed 6 months after
the aplastic anaemia (Awaad et al., 1975).
Leukaemia developed in a 38-year-old woman given a total of 8 g
of chloramphenicol 8 years before which resulted in aplastic anaemia.
A 57-year-old man who had taken chloramphenicol for 8 years (about
175 g total dose) developed aplastic anaemia followed by leukaemia
within a year, while a 61-year-old man who was treated with
chloramphenicol developed aplastic anaemia and leukaemia (Brauer &
Dameshek, 1967).
An 80-year-old man with a bacterial infection of the feet was
given orally 250 mg/day chloramphenicol, for 7 days. He developed
acute leukaemia 5 months later. The only other drugs given for his
infection were penicillin and a topical zinc ointment (Humphries,
1968).
A 14-year-old girl given 250 mg chloramphenicol for 4.5 days
developed aplastic anaemia after approximately 1 year followed by
acute leukaemia 18 months later (Seaman, 1969).
Aplastic anaemia and leukaemia may occur together at diagnosis. A
28-year-old man developed reversible bone marrow depression after
being given approximately 31 g of chloramphenicol. Five years later he
was found to have aplastic anaemia and acute leukaemia (Schmitt-Graft,
1981). Other similar cases have been reported (Adamson & Seiber, 1981;
Forni & Vigliani, 1974; Meyer & Boxer, 1973).
Of 641 cases of chloramphenicol-induced blood dyscrasias, 464
cases of aplastic anaemia and 27 cases of leukaemia were identified
(Meyler et al., 1974).
From 151 cases of blood dyscrasias induced by drugs, 3 cases of
leukaemia attributable to chloramphenicol were noted (Fraumeni, 1967).
The majority of cases of leukaemia associated with chlo-
ramphenicol therapy were acute myeloid leukaemia (Godner et al.,
1973).
The role of chloramphenicol in leukaemogenesis is not known. Some
studies have revealed abnormal karyotypes in affected patients
(Scheres et al., 1985; Goh, 1971; Cohen & Huang, 1973), but it is
not known if these abnormalities were induced directly by
chloramphenicol or were merely a feature of the disease. Aplastic
anaemia, whether induced by chemicals or idiopathic, is known to be
followed by leukaemia in some cases (Milner & Geary, 1979).
Cytogenetic abnormalities are common. Fanconi's anaemia, for example,
is often followed by leukaemia and cytogenetic abnormalities are seen
in this state. Chloramphenicol is known to induce cytogenetic
abnormalities in in vitro test systems, but it is not known if those
seen in leukaemia occurring after chloramphenicol-induced aplastic
anaemia are due to the same effects.
Cardiovascular effects
Chloramphenicol is known to induce a state often referred to as
the "Grey Baby Syndrome" or "Grey Syndrome". In general, this is a
state of cardiovascular collapse that commences on days 2 to 9
following the beginning of chloramphenicol administration. The main
features include failure to feed, vomiting, abdominal distension,
cyanosis, flaccidity, shock, and a fall in body temperature. Death is
said to occur in 60% of cases, and the syndrome generally occurs where
the dose of chloramphenicol exceeds 25 mg/kg b.w./day (Meyler &
Herkeimer, 1968). Metabolic acidosis may be a presenting feature, and
plasma levels of chloramphenicol in excess of 30 µg/ml are usually
associated with the development of the syndrome (Evans & Kleiman,
1986; Mulhall et al., 1983; Craft et al., 1974). In several cases
the doses producing the syndrome were 50 mg/kg b.w./day (Fripp
et al., 1983; Kraskinski et al., 1982; Biancaniello et al.,
1981; Haile, 1977) and in one case a daily dose of 100 mg/kg b.w. was
given (Stevens et al., 1981). A grey-type syndrome has been reported
in a 16-year-old girl given 1 g chloramphenicol i.v. every 6 hours
(95 mg/kg b.w./day) for what was thought to be Rocky Mountain spotted
fever. This dose was later changed to 3.75 g/day i.v. The syndrome
began to develop after the second day of this regime, but it resolved
with drug withdrawal and supportive treatment (Brown, 1982). The
mechanism is unknown, but experiments with isolated pig hearts suggest
that effects on mitochondria may be important (Werner et al., 1985).
Allergenic contact dermatitis
Allergenic contact dermititis has been reported in case report
following the use of chloramphenicol topical preparation (Rudzki et al.,
1976; Schewach-Millet & Shapiro, 1985; Van Joost et al.,1986;
Strick, 1983; Fraki et al., 1985).
Two cases of occupational dermatitis in oculists were attributed
to the use of chloramphenicol-containing preparations (Rebandel &
Rudzki, 1986). In one study of 330 patients with contact allergy, 10%
showed positive patch tests to chloramphenicol, while in another
investigation of 620 patients, 1.7% save positive reactions
(Blondeel et al., 1978; Rudzki & Kelniewska, 1970). Others have
reported similar findings (Forck, 1971).
Ocular toxicity
Several cases of ocular toxicity following prolonged treatment
with chloramphenicol have been reported. This has often been described
as an optic neuritis with scotomata and failing vision. Retrobulbar
neuritis may be observed. It has been seen in cases of cystic
fibrosis, although this may reflect the choice of chloramphenicol as a
treatment in this condition rather than a specific sensitivity in this
group. Total doses are often in the 80 - 250 g range given over
several months. One patient developed bilateral optic neuritis after
being given 6 g chloramphenicol per day for 6 weeks (approximately
250 g total). Peripheral neuritis may accompany the ocular effects
(Wilson, 1962; Steidl, 1965; Malbrel et al., 1977; Joy et al.,
1960; Lasky et al., 1953; Charache et al., 1977; Chang et al.,
1966; Cocke et al., 1966; Godel et al., 1980; Wallenstein &
Snyder, 1952; Huang et al., 1966; Walker, 1961; Fraunfelder & Meyer,
1984).
Ototoxicity
Deafness following chloramphenicol therapy has occasionally been
noted, although the reports have been complicated by administration of
other potentially ototoxic drugs. In one case, a 2.5-year-old boy was
given 125 mg/kg b.w./day chloramphenicol for 26 days with no other
drug treatment. He developed deafness, which persisted beyond
cessation of drug administration (Gargye & Dutta, 1959; Ajodhia & Dix,
1976; Nilges & Norther, 1971; Jones & Hanson, 1977).
Effects on testes
A 61-year-old man with pneumonia was treated with ampicillin and
chloramphenicol. He later developed aplastic anaemia after 12 days and
died of widespread sepsis 10 days after drug withdrawal. Necropsy
revealed loss of germinal epithelium, with only Sertoli cells
remaining. The testes were of normal size. As the patient was
unmarried and also exposed to ampicillin, the authors concluded that
there was no direct proof of chloramphenicol-induced testicular
toxicity. However, in view of the normal gross appearance of the
testes and the low toxicity of ampicillin, chloramphenicol seems the
most likely cause of the effects noted in this case. No dose levels
were quoted (Sheehan & Sweeny, 1982).
Teratogenicity
Chloramphenicol and/or tetracyclines have been implicated in the
genesis of cleft-lip in a study of 599 affected children. However,
because of the nature of the epidemiological study, any effects of
tetracyclines cannot be separated from those of chloramphenicol and it
cannot be stated with any certainty that chloramphenicol is a human
teratogen (Saxen, 1975).
COMMENTS
Human exposure to chloramphenicol can give rise to aplastic
anaemia, a rare but often fatal condition. The Committee concluded
that no dose-response relationship could be established for this
effect. The mechanism for the pathogenesis of aplastic anaemia is
unknown, and no suitable animal model exists.
EVALUATION
Because a no-effect level for aplastic anaemia could not be
established, and therefore it was not possible to give an assurance
that residues in foods of animal origin would be safe for sensitive
subjects, an ADI could not be allocated for chloramphenicol.
REFERENCES
Abou-Khalil, S., Salem, Z., & Yunis, A.A. (1980). Mitochondrial
metabolism in normal, myeloid, and erythroid hyperplastic rabbit
bone-marrow: Effect of chloramphenicol. Am. J. Haematol., 8, 71-79.
Abou-Khalil, S., Salem, Z., Abou-Khalil, W.H., & Yunis, A.A. (1981).
On the mechanism of erythroid cell sensitivity to chloramphenicol:
Studies on mitochondria isolated from erythroid and myeloid tumours.
Arch. Biochem. Biophys., 206, 242-248.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1982). Inhibition
by nitrosochloramphenicol of the proton translocation in mitochondria.
Biochem. Pharmacol., 31, 3823-3830.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1985).
Identification of a mitochondrial rho-dinitrobenzene reductase
activity in rat liver. Pharmacol., 31, 301-308.
Abrams, S.M., Degnan, T.J., & Vinciguerra, V. (1980). Marrow aplasia
following topical application of chloramphenicol eye ointment.
Arch. Inter. Med., 140, 576-577.
Adamson, R.H. & Seiber, S.M. (1981). Chemically induced leukemia in
humans. Environ. Health Perspect., 39, 93-103.
Agam, G., Gasner, S., Bessler, H., Fishman, P., & Djaldetti, M.
(1976). Chloramphenicol induced inhibition of platelet protein
synthesis: In vitro and in vivo studies. Brit. J. Haematol.,
33, 53-59.
Ajodhia, J.M. & Dix, M.R. (1976). Drug-induced deafness and its
treatment. Practitioner, 216, 561-570.
Akhnoukh, S., El-Shazly, N., Sallaim, N., El-Melegy, S., El-Sewedy,
S.M., Mostafa, M.H., & El-Bassioni, E.A. (1982). In vivo effect of
chloramphenicol and thiamphenicol on some enzymes of normal mouse
liver. Biochem. Pharmacol., 31, 55-57.
Aksoy, M., Erdem, S., Dincol, G., Bakioglu, I., & Kutlar, A. (1984).
Aplastic anaemia due to chemicals and drugs: A study of 108 patients.
Sex Trans. Dis., 11, 347-350.
Alavi, J.B. (1983). Aplastic anaemia associated with intravenous
chloramphenicol. Am. J. Hematol., 15: 375-379.
Al-Hachim, G.M. & Al-Baker, A. (1974). The prenatal effect of
chloramphenicol on the postnatal development of mice.
Neuropharmacol., 13, 233-237.
Ali, B.H. (1985). Lack of effect of chloramphenicol on monoamine,
diamine and plasma amine oxidase activities. Clin. Exp. Pharmacol.
Physiol., 12, 1193-5.
Al-Moudhiry, H.A.B. (1978). Aplastic anaemia in Iraq. A prospective
study. Haematologia, 12, 159-164.
Amati, P. (1970). Chloramphenicol: Effect of DNA synthesis during
phage development in Escherichia coli. Science, 168, 1226-1228.
Ambrose, P.J. (1984). Clinical pharmacokinetics of chloramphenicol and
chloramphenicol succinate. Clin. Pharmacokinetics, 9, 222-238.
Applebaum, F.R. & Fefer, A. (1981). The pathogenesis of aplastic
anaemia. Sem. Haematol., 18, 241-257.
Appelgren, L.-E., Eberhardson, B., Martin, K., & Slanina, P. (1982).
The distribution and fate of 14C-chloramphenicol in the new-born
pig. Acta pharmacol. Toxicol., 51, 345-350.
Aravindaksham, C.M. & Cherian, Z. (1984). Effect of chloramphenicol on
thiopentone sodium anesthesia in dogs. Indian Vet. J., 61, 569-573.
Ascherl, M., Eyer, P., & Kaupffmeyer, H. (1985). Formation and
disposition of nitrosochloramphenicol in rat liver. Biochem.
Pharmacol., 34, 3755-3763.
Augustine, M.L., Poulsen, N.K., & Heinze, J.E. (1982). Evaluation of
the CHO/HGPRT cell mutation test using 12 compounds. Env. Mutagen.,
4, 389-390.
Awaad, S., Khalifa, A.S., & Kamel, K. (1975). Vacuolization of
leukocytes and bone-marrow aplasia due to chloramphenicol toxicity.
Clin. Pediatr., 14, 499-506.
Baltz, R.H., & Stonesifer, J. (1985). Adaptive response and
enhancement of N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis by
chloramphenicol in Streptomyces fradiae. J. Bacteriol.,
164, 944-946.
Banerjee, S. & Basu, P.S. (1978). Inhibition of rat tissue
mitrochondrial monoamine oxidase by chloramphenicol and its
intermediates. Indian J. Biochem. Biophys., 15, 479-482.
Bartlett, J.G. (1982). Chloramphenicol. Med. Clin. N. Amer.,
66, 91-102.
Baselt, R.C. (1982). Disposition of toxic drugs in man. 2nd Edition,
Davis, California, Biomedical Publications, 136-139.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1979). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Surg. Forum, 30, 512-514.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1981). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Arch. Otolaryngol., 107, 104-109.
Becker, D., Metz, J., & Katz, J. (1974). Comparative effects of
thiamphenicol and chloramphenicol on haemopoiesis and lymphocyte
transformation in vitro in man. Postgrad. Med. J.,
50, (Suppl. 5), 88-94.
Beliles, R.P. (1972). The influence of pregnancy on the acute toxicity
of various compounds in mice. Toxicol. Appl. Pharmacol., 23, 537-540.
Benes, L., Rotreklova, E., & Pospisil, V.V.M. (1980). Inhibition of
bone marrow cell proliferation and DNA replication induced by
chloramphenicol. Folia Biol., 26, 408-414.
Benestad, H.B. (1974). Aplastic anaemia: Considerations on the
pathogenesis. Acta med. Scand., 196, 255-262.
Benestad, H.B. (1979). Drug mechanism in marrow aplasia.
In: Geary, C.G., (ed.) Aplastic anaemia, London, Balliere Tindall,
pp. 26-42.
Bengmark, S., Goransson, G., & Soucas, E. (1981). Defects in
hemostasis produced by antibiotics. An in vivo study in the rat.
Eur. Surg. Res., 13, 290-298.
Ben-Gurion, R. (1978). A simple plate test for screening colicine-
inducing substances as a tool for the detection of potential
carcinogens. Mutat. Res., 54, 289-295.
Bertolini, A. & Poggioli, R. (1981). Chloramphenicol administration
during brain development: Impairment of avoidance learning in
adulthood. Science, 21, 238-239.
Biancaniello, T., Meyer, R.A., & Kaplan, S. (1981). Chloramphenicol
and cardiotoxicity. J. Pediatr., 98, 828-830.
Billet, F.S., Collini, R., & Hamilton, L. (1965). The effects of D-and
L-threo-chloramphenicol on the early development of the chick embryo.
J.Embryol. Exp. Morph., 13, 341-356.
Blackwell, U.B. (1962). The changing inhibition of early
differentiation and general development in the chick embryo by
2-ethyl-5-methyl-benzimidazole and chloramphenicol. J. Embryo. Exp.
Morph., 10, 315-337.
Blondeel, A., Oleffe, J., & Achten, G. (1978). Contact allergy in 330
dermatological patients. Contact Derm., 4, 270-276.
Bories, G.F., Peleran, J.C., Wal, J.M., & Corpet, D.E. (1983). Simple
and ion-pair high performance liquid chromatography as an improved
analytical tool for chloramphenicol metabolic profiling. Drug Met.
Disp., 11, 249-254.
Bottiger, L.E. & Westerholm, B. (1972). Aplastic anaemia. I. Incidence
and aetiology. Acta med. Scand., 192, 315-318.
Bottiger, L.E. & Westerholm, B. (1973). Drug-induced blood dyscrasias
in Sweden. Brit. Med. J., 3, 339-343.
Bottiger, L.E. (1978). Prevelance and etiology of aplastic anaemia in
Sweden. In: Hibino, S., Takaku, F., & Shahidi, N.T. (eds.) Aplastic
Anemia. Tokyo, University Park Press, pp. 171-180.
Bottiger, L.E. (1979). Epidemiology and aetiology of aplastic anaemia.
Haematol. Blut. Transfus., 24, 27-37.
Brabec, M.J., Owens, J.B., Kenel, M., Sorscher, D., & Cornish, H.H.
(1982). Modification of methanol potentiation of carbon tetrachloride
toxicity in rats by chloramphenicol and salicylate. Drug Chem.
Toxicol., 5, 143-154.
Brasfield, J.H., Record, K.E., Griffen, W.O., & Mattingly, S. (1983).
Chloramphenicol succinate levels in patients with renal impairment.
Clin. Pharmacol., 1, 355-358.
Brauer, M.J. & Dameshek, W. (1967). Hypoplastic anemia and
myeloblastic leukemia following chloramphenicol therapy. Report of
three cases. New Engl. J. Med., 277, 1003-1005.
Braun, R., Fischer, G.W., & Schoneich, J. (1977). The mutagenicity and
DNA-damaging activity of cyclic aliphatic sulfuric acid esters.
Chem-Biol. Interact., 19, 241-252.
Braun, R., Schoneich, J., Weissflog, L., & Dedek, W. (1982). Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair direct alkylation vs. metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem-Biol. Interact.,
39, 339-350.
Brem, H., Stein, A.B., & Rosenkranz, H.S. (1974). The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res., 34, 2576-2579.
Brown, R.T. (1982). Chloramphenicol toxicity in an adolescent.
J. Adol. Health Care, 3, 53-55.
Bueno, L., Fioramonti, J., Alvinerie, M., & Bardon, T. (1984).
Influence of bran on bioavailability of chloramphenicol administered
orally to pigs. Can. J. Physiol. Pharmacol., 62, 208-211.
Burckart, G.J., Barrett, F.F., Stranghn, A.B., & Ternullo, S.R.
(1982). Chloramphenicol clearance in infants. J. Clin. Pharmacol.,
22, 49-52.
Burckart, G.J., Barett, F.F., Valle, R.D., & Mayer, M.C. (1983).
Chloramphenicol damage and pharmacokinetics in infants and children.
J. Clin. Pharmacol,, 23, 106-112.
Burke, J.T., Wargin, W.A., & Blum, M.R. (1980). High-pressure liquid
chromatographic assay for chloramphenicol, chloramphenicol-3-
monosuccinate and chloramphenicol-l-monosuccinate. J. Pharm. Sci.,
69, 909-912.
Burrows, G.E., Tyler, R.D., Craigmill, A.L., & Barto, P.B. (1984).
Chloramphenicol and the neonatal calf. Am. J. Vet. Res.,
45, 1586-1591.
Camitta, B.M., Storb, R., & Thomas, E.D. (1982). Aplastic anaemia.
Pathogenesis, diagnosis, treatment and prognosis. New Engl. J. Med.,
306, 645-652.
Carpenter, G. (1975). Chloramphenicol eye-drops and marrow aplasia.
Lancet, ii, 326-327.
Casale, T.B., Macher, A.M., & Fauci, A.S. (1982). Complete hematologic
and hepatic recovery in a patient with chloramphenicol hepatitis-
pancytopenia syndrome. J. Pediatr., 101, 1025-1027.
Chakrabarti, K. & Banerjee, S. (1977). Mechanism of intestinal
absorption of chloramphenicol. Indian J. Exp. Biol., 15, 302-303.
Chang, N., Giles, C.L., & Gregy, R.H. (1966). Optic neuritis and
chloramphenicol. Am. J. Dis. Child., 112, 46-48.
Charache, S., Finkelstein, D., Lietman, P.S., & Scott, J. (1977).
Peripheral and optic neuritis in a patient with hemoglobin SC disease
during treatment of Salmonella osteomyelitis with chloramphenicol.
Johns Hopkins Med. J., 140, 121-124.
Cid, F., Mella, F., Gonzalez, X., & Gouzalez, R. (1983). Effects of
anti-inflammatory drugs on the pharmacokinetics of chloramphenicol.
An. Real Acad. Farm., 49, 49-60.
Clausen, N. (1986). A population study of severe aplastic anemia
children. Acta paediatr. Scand., 75, 58-63.
Cocke, J.G., Brown, R.E., & Geppert, L.J. (1966). Optic neuritis with
prolonged use of chloramphenicol. J. Pediatr., 68, 27-31.
Cohen, T. & Creger, W.P. (1967). Acute myeloid leukemia following
seven years of aplastic anemia induced by chloramphenicol.
Am. J. Med., 43, 762-770.
Cohen, H.J. & Huang, A.T.-F. (1973). A marker chromosome abnormality.
Occurrence in chloramphenicol-associated acute leukaemia.
Arch. Intern. Med., 132, p. 440.
Courtney, K.D., Valerio, D.A., & Palletta, A.J. (1967). Experimental
teratology in the monkey. Toxicol. Appl. Pharmacol., 10, p. 378.
Courtney, K.D., & Valerio, D.A. (1974). Teratology in the
Macaca mulatta. Teratology, 1, 163-172.
Craft, A.W., Brocklebank, J.T., Itey, E.N., & Jackson, R.H. (1974).
The "grey-toddler". Chloramphenicol toxicity. Arch. Dis. Child.,
49, 235-237.
Cronberg, S., Wallmark, E., & Soderberg, I. (1984). Investigations on
the effect of antimicrobial drugs on platelet aggregation in vitro
and in vivo. Folia Haematol., 111, 725-734.
D'Angelo, E.P., Patterson, W.C., & Morrow, R.C. (1967). Chloramphenicol.
Topical application in the middle ear. Arch. Otolaryngol., 85,
126-128.
Daum, R.S., Cohen, D.L., & Smith, A.L. (1979). Fatal aplastic anaemia
following apparent "dose-related" chloramphenicol toxicity.
J. Pediatr., 94, 403-406.
De Corte-Baeten, K. & Debackere, M. (1976). Excretion of
chloramphenicol in the milk of lactating cows after oral and
parenteral application. Dtsch. Tieraerztl. Wochenschr., 83, 231-233.
Del Giacco, G.S., Petrini, M.T., Janelli, S., & Carcassi, U. (1981).
Fatal bone-marrow hypoplasia in a shepherd using chloramphenicol
spray. Lancet, i, p. 945.
de Vries, H., Beijersbergen van Henegouwen, G.M.J., & Huf, F.A.
(1984). Photochemical decomposition of chloramphenicol in a 0.25%
eyedrop and in a therapeutic intraocular concentration. Internat. J.
Pharmaceut., 20, 265-271.
Dewse, C.D. (1977). Variable action of chloramphenicol on DNA
synthesis in phytohaemagglutinin-stimulated human lymphocytes.
ICRS Med. Sci., 5, p.150.
Dill, W.A., Thompson, E.M., Tisken, R.A., & Glazko, A.J. (1960). A new
metabolite of chloramphenicol. Nature, 185, 535-537.
Djaldetti, M. (1983). Drug vulnerability of peripheral blood
platelets. Arch. Toxicol. (Suppl.), 6, 21-32.
Doudney, C.O. & Rinaldi, C.N. (1985). Chloramphenicol-promoted
increase in resistance to UV damage in Escherichia coli
B/r trp E65: Development of the capacity for successful repair of
otherwise mutagenic or lethal lesions in DNA. Mutat. Res.
143, 29-34.
Ehling, U.H. (1971). Comparison of radiation- and chemically-induced
dominant lethal mutations in male mice. Mutat. Res., 11, 35-44.
Epstein, S.S. & Shafner, H. (1968). Chemical mutagens in the human
environment. Nature, 219, 385-387.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972).
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
Evans, L.S. & Kleiman, M.B. (1986). Acidosis as a presenting feature
of chloramphenicol toxicity. J. Pediatr., 108, 475-477.
Eyer, F., Lierheimer, E., & Schneller, M. (1984). Reactions of
nitrosochloramphenicol in blood. Biochem. Pharmacol., 33, 2299-2308.
Flach, A.J. (1982). Chloramphenicol and aplastic anemia. Am. J.
Ophthalmol., 93, 664-665.
Flint, O.P. & Orton, T.C. (1984). An in vitro assay for teratogens
with cultures of rat embryo midbrain and limb bud cells. Toxicol.
Appl. Pharmacol., 76, 383-395.
Forck, G. (1971). Incidence and significance of chloramphenicol
allergies. Dtsch. Med. Wochen, 96, 161-165.
Forni, A. & Vigilant, E.C. (1974). Chemical leukemogenesis in man.
Ser. Haematol., 7, 211-223.
Fraki, J.E., Kalimo, K., Tuohimaa, P., & Aantaa, E. (1985). Contact
allergy to various components of topical preparations for treatment of
external otitis. Acta Otolaryngol., 100, 414-418.
Fraunfelder, F.T. & Bagby, G.C. (1982). Fatal aplastic anaemia
following topical administration of ophthalmic chloramphenicol.
Am. J. Ophthalmol., 93, 356-360.
Fraunfelder, E.T. & Meyer, S.M. (1984). Ocular toxicology update.
Austral. J. Ophthalmol., 12, 391-394.
Fraumeni, J.F. (1967). Bone-marrow depression induced by
chloramphenicol or phenylbutazone. J. Am. Med. Assoc., 201, 150-156.
Fripp, R.R., Carter, M.C., Werner, J.C., Schuler, H.G., Rannels, A.M.,
Whitman, V., & Nelson, N.M. (1983). Cardiac function and acute
chloramphenicol toxicity. J. Pediatr., 103, 487-490.
Fritz, H. & Hess, R. (1971). The effects of chloramphenicol on the
prenatal development of rats, mice and rabbits. Toxicol. Appl.
Pharmacol., 19, 667-674.
Gadner, H., Gethmann, U., Jessenberger, K., & Riehm, H. (1973). Acute
leukaemia following exposure to chloramphenicol? Three case reports
and review of the literature. Mschr. Kinderheilk, 121, 590-594.
Garrett, N.B., Stack, H.F., Gross, M.R., & Waters, M.D. (1984). An
analysis of the spectra of genetic activity produced by known or
suspected human carcinogens. Mutat. Res., 134, 89-111.
Gargye, A.K. & Dutta, D.V. (1959). Nerve deafness following
chloromycetin therapy. Indian J. Pediatr., 26, 265-266.
Glavini, E., Prati, M., & Vismara, C. (1979). The effects of
actinomycin D and chloramphenicol on rat preimplantation embryos.
Experientia, 35, 1649-1650.
Gilman, A.G., Goodman, L.S., Rall, T.W., & Murad, F. (eds.) (1985).
Goodman and Gilman's The Pharmacological Basis of Therapeutics.
7th ed. New York, MacMillan Publishing Co., pp. 1179-1184.
Glazko, A.J., Wolf, L.M., Dill, W.A., & Bratton, A.C. (1949).
Biochemical studies on chloramphenicol (chloromycetin). II. Tissue
distribution and excretion studies. J. Pharmacol. Exp. Ther.,
96, 445-459.
Glazko, A.J., Dill, W.A., & Rebstock, M.C. (1950). Biochemical studies
on chloramphenicol (chlormycetin). III. Isolation and identification
of metabolic products in urine. J. Biol. Chem., 183, 679-691.
Glazko, A.d., Dill, W.A. & Wolf, L.M. (1952). Observations on the
metabolic disposition of chloramphenicol (chloromycetin) in the rat.
J. Pharmacol. Exp. Ther., 104, 452-458.
Godel, V., Nemet, P., & Lazar, M. (1980). Chloramphenicol optic
neuropathy. Arch. Ophthalmol., 98, 1417-1421.
Goh, K. (1971). Chloramphenicol, acute leukemia and chromosomal
vacuolation. Southern Med.J., 64, p. 815.
Goh, K.-O. (1979). Chloramphenicol and chromosomal morphology.
J.Med., 10, 159-166.
Gray, J.D. (1955). The concentration of chloramphenicol in human
tissues. Canad. Med. Assoc. J., 72, 778-779.
Gross, B.J., Branchflower, R.V., Burke, T.R., Lees, D.E., & Pohl, L.R.
(1982). Bone-marrow toxicity in vitro of chloramphenicol and its
metabolites. Toxicol. Appl. Pharmacol., 64, 557-565.
Guntakatta, M., Matthews, E.J., & Rundell, J.O. (1984). Development of
a mouse embryo limb bud cell culture system for the estimation of
chemical teratogenic potential. Terat. Carcinogen. Mutat.,
44, 349-364.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985a). Percutaneous
absorption in man: A kinetic approach. Toxicol. Appl. Pharmacol.,
78, 123-129.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985b). Transdermal
absorption kinetics: A physiochemical approach. ACS Symp. Ser.,
273, 19-31.
Haile, C.A. (1977). Chloramphenicol toxicity. Southern Med. J.,
70, 479-480.
Halpert, J. (1981). Covalent modification of lysine during the suicide
inactivation of rat liver cytochrome P-450 by chloramphenicol.
Biochem. Pharmacol., 30, 875-881.
Halpert, J. & Neal, R.A. (1981). Inactivation of rat liver cytochrome
P-450 by the suicide substrates parathion and chloramphenicol. Drug
Met. Rev., 12, 239-259.
Halpert, J. (1982). Further studies of the suicide inactivation of
purified rat liver cytochrome P-450 by chloramphenicol.
Mol. Pharmacol., 21, 166-172.
Halpert, J., Balfour, C., Miller, N.E., Morgan, E.T., & Danbar, D.
(1985). Isozyme selectivity of the inhibition of rat liver cytochrome
P-450 by chloramphenicol in vivo. Mol. Pharmacol., 28, 290-296.
Hapke, H.J., Abel, J., Ghosch, H., Tachampas, S., & Youssef, S.
(1977). Effects of chloramphenicol on drug-degrading enzyme systems.
Zentrabl. Veterinaermed., 24, 701-714.
Hara, H., Kohsaki, M., Noguchi, K., & Nagai, K. (1978). Effect of
chloramphenicol on colony formation from erythrocytic precursors.
Am. J. Haematol., 5, 123-130.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W., &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by interlaboratory
testing of diverse chemicals. Environ. Mutagen., 8, 515-531.
Hausman, K. & Skrandies, G. (1974). Aplastic anaemia following
chloramphenicol therapy in Hamburg and surrounding districts.
Postgrad. Med. J., 50, 136-142.
Heddle, J.A. & Bruce, W.R. (1977). Comparisons of test for
mutagenicity or carcinogenicity using assays for sperm abnormalities,
formation of micronuclei, and mutations in Salmonella: In: Hiatt,
H.H., Watson, J.D., & Winsten, J.A. (eds.) Origins of Human
Cancer, Book C, Risk Assessment, Cold Spring Harbour Laboratory,
pp. 1549-1557.
Hellriegel, K.P., & Gross, R. (1974). Follow-up studies in
chloramphenicol-induced aplastic anaemia. Postgrad. Med. J.,
50 (Suppl.5), 136-142.
Henley, C.M., Brown, R.D., Penny, J.E., Kupetz, S.A., Hodges, K.B., &
Jobe, P.C. (1984). Impairment of cochlear function produced by
chloramphenicol and noise. Neuropharmacol., 23, 197-202.
Henley, C.M. (1985). Comparison of the ototoxicity of chloramphenicol
and gentamycin in noise exposed rats. Diss. Abs., 46, 1523-B.
Hodgkinson, R. (1971). Infectious hepatitis and aplastic anaemia.
Lancet, i, p. 1014.
Hodgkinson, R. (1973). The chloramphenicol-hepatitis-aplastic anaemia
syndrome. Med. J. Austral., 1, 939-940.
Holden, H.E. (1982). Comparison of somatic and germ cell models for
cytogenetic screening. J. Appl. Toxicol., 2, 196-200.
Howell, A., Andrews, T.M., & Watts, R.W.E. (1975). Bone-marrow cells
resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet, i, 65-69.
Huang, N.M., Harley, R.D., Promadhattavedi, V., & Sproul, A. (1966).
Visual disturbances in cystic fibrosis following chloramphenicol
administration. J. Pediatr., 68, 32-44.
Humphries, K.R. (1968). Acute myelomonocytic leukaemia following
chloramphenicol therapy. N. Z. Med. J., 68, 248-249.
Hyman, J., Leifer, Z., & Rosenkranz, H.S. (1980). The E. coli
Pol A1 assay. A quantitative procedure for diffusable and
non-diffusable chemicals. Mutat. Res., 74, 107-111.
IARC (1982). IARC (International Agency for Research on Cancer)
monographs on the evaluation of the carcinogenic risk of chemicals to
humans: Chemicals, industrial processes and industries associated
with cancer in humans. 1-29, (Suppl. 4), 79-80.
IARC (1987, in press). IARC (International Agency for Research on
Cancer) monographs on the evaluation of carcinogenic risks to humans.
Overall evaluations of carcinogenicity: an update of IARC monographs
volumes 1-42 (Suppl. 7).
Ingall, D., Sherman, J.D., Cockburn, F. & Klein, R. (1965).
Amelioration by ingestion of phenylalanine of toxic effects of
chloramphenicol on bone-marrow. New Eng. J. Med., 272, 180-185.
Issaragrisil, S. & Piankijagum, A. (1985). Aplastic anemia following
topical administration of ophthalmic chloramphenicol: Report of a case
and review of the literature. J. Med. Ass. Thailand, 88, 309-312.
Jackson, A.F., Wentzell, B.R., McCalla, D.R., & Freeman, K.B. (1977).
Chloramphenicol damages bacterial DNA. Biochem. Biophys. Res.
Commun., 78, 151-157.
Javed, I., Nawaz, M., Ahmed, M., Rehman, Z.U., & Shah, B.H. (1984).
Pharmacokinetics, renal clearance and urinary excretion of
chloramphenicol in goats. Pakis. Vet., 4, 151-157.
Jones, F.E. & Hanson, D.R. (1977). H. Influenzae meningitis treated
with ampicillin or chloramphenicol, and subsequent hearing loss.
Dev. Med. Child. Neurol., 19, 593-597.
Joy, R.J.T., Scalettar, R., & Sodee, D.B. (1960). Optic and peripheral
neuritis. Probable effects of prolonged chloramphenicol therapy.
J. Amer. Med. Assoc., 173, 1731-1734.
Kapp, J.P., Avora, B., & Sibinga, M.S. (1977). Depression of
erythropoiesis, physical growth, and hair growth by chloramphenicol in
a three-year-old child with cystic fibrosis. Clin. Pediatr.,
16, 64-66.
Kauffman, R.E., Miceli, J.N., Strebel, L., Buckley, J.A., & Dore, A.K.
(1981). Pharmacokinetics of chloramphenicol and chloramphenicol
succinate in infants and children. J. Pediatr., 98, 315-320.
Keiser, G. & Bucheggar, U. (1973). Hematological side effects of
chloramphenicol and thiamphenicol. Helv. Med. Acta, 37, 265-278.
Kern, P., Heimpel, H., Heit, W., & Kubanek, B. (1975). Bone-marrow
cells resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet i, p. 1190.
Knowles, J.A. (1965). Excretion of drugs in milk - a review.
J. Pediatr., 66, 1068-1082.
Korting, G.W. & Kifle, H. (1985). Systemic side-effects from external
application of chloramphenicol. Der Hautarzt., 36, 181-183.
Krasinski, K., Perkin, R., & Rutledge, J. (1982). Gray baby syndrome
revisited. Clin. Pediatr., 21, 571-572.
Krishna, G., Aykac, I., & Siegel, D. (1981). Recent studies on the
mechanisms of chloramphenicol activation responsible for aplastic
anemia. In: NaJean Y. (ed.) Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy. New York, Raven Press,
pp. 5-16.
Kroker, R. (1985). The pharmacokinetic behavior of chloramphenicol in
liver-damaged mini-pigs. J. Vet. Pharmacol. Ther., 8, 82-87.
Kroon, A.M. & de Vries, H. (1969). The effect of chloramphenicol on
the biogenesis of mitochondria of rat liver in vivo. Fed. Eur.
Biochem. Soc. Letters, 3, 208-210.
Kubinski, H., Gutzke, G.E., & Kubinski, Z.O. (1981). DNA-cell binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89, 95-136.
Kunii, O., Komatsu, T., & Nishiya, H. (1983). Biliary excretion of
antibiotics in rats. Jap. J. Exp. Med., 53, 51-58.
Kurz, H., Manser-Ganshorn, A., & Stickel, H.H. (1977). Differences in
the binding of drugs to plasma proteins from newborn and adult man.
I. Euro. J. Clin. Pharmacol., 11, 463-467.
Lasky, M.A., Pincus, M.H., & Katlan, N.R. (1953). Bilateral optic
neuritis following chloramphenicol therapy. J. Amer. Med. Assoc.,
151, 1403-1404.
Leiken, S.L., Welch, H., & Guin, G.H. (1961). Aplastic anaemia due to
chloramphenicol. Clin. Proc. Child Hosp., 17, 171-180.
Lepow, M. (1986). Aplastic anaemia following chloramphenicol therapy
still happens! Pediatr., 77, 932-933.
Lery, N., Descoted, J., & Evreux, J.Cl. (1978). A review of
chloramphenicol-induced blood disorders. Vet. Human Toxicol.,
20, 177-181.
Lim, L.O. & Yunis, A.A. (1982). Induction of methemoglobin formation
by nitrosochloramphenicol in hemolysed blood. Res. Commun. Chem.
Path. Pharmacol., 38, 497-500.
Lim, L.O., Abou-Khalil, W.H., & Yunis, A.A. (1984). The effect of
nitrosochloramphenicol on mitochondrial DNA polymerase activity.
J. Lab. Clin. Med., 104, 213-222.
Ling, G.V., Conzelman, G.M., Franti, C.E., & Ruby, A.L. (1980). Urine
concentration of chloramphenicol, tetracycline, and sulfisoxazole
after oral administration to healthy adult dogs. Am. J. Vet. Res.,
41, 950-952.
Longnecker, D.S., Curphey, T.J., James, S.T., Daniel, D.S., &
Jacobs, N.J. (1974). Trial of a bacterial screening system for rapid
detection of mutagens and carcinogens. Cancer Res., 34, 1658-1663.
Ma, T.-H., Harris, M.M., Anderson, V.A., Ahmed, I., Mohammad, K.,
Bare, J.L., & Lin, G. (1984). Tradescantia-micronucleus (Trad-MCN) on
140 health-related agents. Mutat. Res., 138, 157-167.
Hackler, B., Grace, R., Tippit, D., Lemire, R.J., Shepard, H., &
Kelly, V.C. (1975). Studies of the development of congenital anomalies
in rats III. Effects of inhibition of mitochondrial energy systems on
embryonic development. Teratology, 12, 291-296.
Malbrel, C., Talmud, M., Dupou, D., & Rose, J.P. (1977). Concerning a
new case of optic neuritis by chloramphenicol in the infant.
Bull. Soc. Ophthalmol. France, 77, 999-1001.
Mamber, S.W., Okasinki, W.A., Printer, C.D., & Tunac, J.B. (1986). The
Escherichia coli K-12 SOS Chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutat. Res., 171, 83-90.
Manna, G.K. & Bardham, S. (1977). Some aspects of chloramphenicol
induced bone marrow chromosome aberrations in mice. J. Cytol. Genet.,
12, 10-17.
Manna, G.K. & Roy, P.P. (1979). Chromosome aberrations in F1 embryos
and litters of chloramphenicol treated male parent mice. Natl. Acad.
Sci. Letters (India), 2, 460-462.
Martelo, O.J., Manyan, D.R., Smith, U.S., & Yunis, A.A. (1969).
Chloramphenicol and bone marrow mitochondria. J. Lab. Clin. Med.,
74, 927-940.
Masek, F. (1977). Effect of some drugs on excision repair in E. coli
cells. Chem-Biol. Interact., 17, 121-127.
Matsuda, S. (1984). Transfer of antibiotics into maternal milk.
Biol. Res. Preg. Perinatol., 5, 57-60.
Matthews, S.J. & Shawver-Matthews, S. (1980). Chloramphenicol toxicity
- oral vs. intravenous administration. J. Paediatr., 97, p. 869.
McCann, J., Choi, E., Yamasaki, E., & Ames, B.N. (1975). Detection of
carcinogens and mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc. Natl. Acad. Sci. USA, 72, 5133-5139.
McCoy, E., Hyman, J., & Rosenbranz, H.S. (1980a). A new mutagenic and
genotoxic response of the flame retardant tris (2, 3-dibromopropyl)
phosphate. Activation by singlet oxygen. Mutat. Res., 77, 209-214.
McCoy, E.C., Petrullo, L.A., & Rosenkranz, H.S. (1980b). Non-mutagenic
genotoxicants: Novobiocin and naladixic acid, 2 inhibitors of DNA
gyrase. Mutat. Res., 71, 33-43.
McCurdy, P.R. (1963). Plasma concentration of chloramphenicol and bone
marrow suppression. Blood, 21, 363-372.
Meyer, J.S. & Boxer, M. (1973). Leukemic cellular thrombi in pulmonary
blood vessels. Subleukemic myelogenous leukemia following
chloramphenicol-induced aplastic anemia. Cancer 32, 712-721.
Meyler, L. & Herkeimer, A. (1968). Side effects of drugs. 6,
Amsterdam, Excerpta Medica, pp. 286-314.
Meyler, L., Polak, B.C.P., Schut, D., Wesseling, H., & Herxheimer, A.
(1974). Blood dyscrasias attributed to chloramphenicol. A review of
641 published and unpublished cases. Postgrad. Med. J., 50
(Suppl. 5), 123-126.
Milner, G.R. & Geary, C.G. (1979). The aplasia-leukaemia syndrome. In:
Geary, C.G. (ed.) Aplastic Anaemia. London, Balliere Tindall,
pp. 230-243.
Mitchell, I. de G., Dixon, P.A., Gilbert, P.J., & White, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Problems of
evaluation. Mutat. Res., 79, 91-105.
Mitchell, I. de G. & Gilbert, P.J. (1984). The effect of pretreatment
of Escherichia coli CM891 with ethylenediamine-tetraacetate on
sensitivity to a variety of standard mutagens. Mutat. Res.,
140, 13-19.
Mitema, E.S. (1982). Effect of chloramphenicol administration on the
bovine hematology and bone marrow. Vet. Human Toxicol.,
Suppl., 162-166.
Mitus, W.J. & Coleman, N. (1970). In vitro effect of chloramphenicol
on chromosomes. Blood, 35, 689-674.
Mizuno, S., Aoki, K., Ohno, Y., Sasaki, R., & Hamajima, N. (1982).
Time series analysis of age-sex specific death rates from aplastic
anemia and the trend in production amount of chloramphenicol.
Nagoya J. Med. Sci., 44, 103-115.
Modan, B., Segal, S., Sham, M., & Sheba, C. (1975). Aplastic anemia in
Israel: Evaluation of the etiological role of chloramphenicol on a
community-wide basis. Am. J. Med. Sci., 270, 441-445.
Morizono, T. & Johnstone, B.M. (1975). Ototoxicity of chloramphenicol
ear drops with propylene glycol as solvent. Med. J. Austral.,
2, 634-638.
Morley, A., Trainor, K., & Remes, J. (1976). Residual marrow damage:
Possible explanation for idiosyncrasy to chloramphenicol. Brit. J.
Haematol., 32, 525-531.
Morris, P.L., Burke, T.R., George, J.W., & Pohl, L.R. (1982). A new
pathway for the oxidative metabolism of chloramphenicol by rat liver
microsomes. Drug Met. Disp., 10, 439-445.
Morris, P.L., Burke, T.R., & Pohl, L.R. (1983). Reductive
dechlorination of chloramphenicol by rat liver microsomes. Drug Met.
Disp., 11, 126-130.
Mulhall, A., de Louvois, J., & Hurley, R. (1983). Chloramphenicol
toxicity in neonates: Its incidence and prevention. Brit. Med. J.,
287, 1424-1427.
Murray, T. & Yunis, A.A. (1981). The cellular uptake and covalent
binding of nitrosochloramphenicol. J. Lab. Clin. Med., 98, 396-401.
Murray, T., Downey, K.M., & Yunis, A.A. (1982). Degradation of
isolated deoxyribonucleic acid mediated by nitrosochloramphenicol.
Possible role in chloramphenicol-induced aplastic anaemia. Biochem.
Pharmacol., 31, 2291-2296.
Naesland, B., Betner, I., & Halpert, J. (1983). Inhibition of rat lung
cytochrome P-450 by chloramphenicol. Extrahepatic Drug Met. Chem.
Carcinogen, Proc. Int. Mtg., 251-252.
Naesland, B. & Halpert, J. (1984). Selective inhibition by
chloramphenicol of cytochrome P-450 isozymes in rat lung and liver
involved in the hydroxylation of n-hexane. J. Pharmacol. Exp. Ther.,
231, 16-22.
Nagai, K. & Kanamaru, A. (1978). Drug-induced aplastic anaemia: Effect
of chloramphenicol on hemopoietic stems cells. In: Hibino, S., Takaku,
F., & Shakidi, N.T. (eds.) Tokyo, University Park Press, pp. 343-353.
Nagao, T. & Maner, A.M. (1969). Concordance for drug-induced aplastic
anemia in identical twins. New Engl. J. Med., 281, 7-10.
Nair, C.R., Goel, A., & Rahman, A. (1981). Inhibition of hepatic drug
metabolism by chloramphenicol-paracetamol interaction - an
experimental study. Bull. Postgrad. Inst. Med. Educ. Res.
(Chandrigarh), 15, 31-35.
Najean, Y. & Baumelon, E. (1984). Toxic etiology of aplastic anemia.
Sex Trans. Dis., 11, 343-346.
Nakagawa, T., Masada, M., & Uno, T. (1975). Gas chromatographic
determination and gas chromatographic-mass spectrometric analysis of
chloramphenicol, thiamphenicol and their metabolites. J. Chromatogr.,
111, 355-364.
Nara, N., Besshe, M., Hirashima, K., & Momoi, H. (1982). Effects of
chloramphenicol on hematopoietic inductive micro environment.
Exp. Hematol., 10, 20-25.
Narda, R.D. & Gupta, R.K. (1972). Mutation studies in
D. melanogaster. Drosoph. Inf. Serv., 48, p. 105
Nasrat, G.E., Ahmed, K.A., Nafei, H.A., & Abdel-Rahman, A.H. (1977).
Mutagenic action of certain therapeutic drugs on Drosophila
melanogaster. Bull. Fac. Agric. Univ. Cairo, 28, 697-717.
Nilges, T.C. & Norther, J.L. (1971). Iatrogenic ototoxic hearing loss.
Ann. Surg., 173, 281-289.
Pall, M.L. & Hunter, B.J. (1985). Carcinogens induce genetic tandem
duplications in Salmonella. Mutat. Res., 152, 131-145.
Pant, G.S., Kamada, N., & Tanaka, R. (1976). Sister chromatid
exchanges in peripheral lymphocytes of atomic bomb survivors and of
normal individuals exposed to radiation and chemical agents.
Hiroshima J. Med. Sci., 25, 99-105.
Parashar, S., Rao, R., Tikare, S.K., & Tikare, S.S. (1972).
Chloramphenicol induced reversible bone marrow suppression.
J. Postgrad. Med., 18, 90-92.
Parker, F.L. & James, G.W.L. (1978). The effect of various topical
antibiotic and antibacterial agents on the middle and inner ear of the
guinea-pig. J. Pharm. Pharmacol., 30, 236-239.
Patterson, W.C. & Gulick, W.L. (1963). The effects of chloramphenicol
upon electrical activity of the ear. Ann. Otol. Rhinol. Laryngol.,
72, 50-55.
Pazdernik, T.L. & Corbett, M.D. (1979). Effects of chloramphenicol
reduction products or hemopoietic precursor cells in vitro.
Pharmacol., 19, 191-195.
Pazdernik, T.L. & Corbett, M.D. (1980). Role of chloramphenicol
reduction products in aplastic anemia. Pharmacol., 20, 87-94.
Penny, R.H.C., Carlisle, C.H., Prescott, C.W., & Davidson, H.A.
(1967). Effects of chloramphenicol on the haemopoietic system of the
cat. Brit. Vet. J., 123, 145-153.
Perez, R.P., Puig, M.L., Sanchez, J.L.A., & Torres, E.L. (1981).
Aplastic anemia and drugs as causal factors. Rev. Cub. Farm.,
15, 97-103.
Petitjean, F., Sastre, J.P., Bertrand, N., Cointy, C., & Jouvet, M.
(1975). Suppression of paradoxical sleep by chloramphenicol in the
cat. Absence of effect of thiamphenicol. C. R. Seances Biol. (Paris),
169, 1236-1239.
Plaut, M.E. & Best, W.R. (1982). Aplastic anemia after parenteral
chloramphenicol: Warning renewed. New Engl. J. Med., 306, p. 1486.
Plomp, T.A., Thiery, M., & Maes, R.A.A. (1983). The passage of
thiamphenicol and chloramphenicol into human milk after single and
repeated oral administration. Vet. Human Toxicol., 25, 167-172.
Polak, B.C.P., Wesseling, H., Schut, D., Herxheimer, A., & Meyler, L.
(1972). Blood dyscrasias attributed to chloramphenicol. A review of
576 published and unpublished cases. Acta med. Scand., 192, 409-414.
Preziosi, P., Basile, V., Vacca, M., & de Natale, G. (1981). On the
correlation between regional utilization patterns of chloramphenicol
and its adverse reactions with emphasis on aplastic anaemia.
Int. J. Clin. Pharmacol.Ther. Toxicol., 19, 547-551.
Proud, G.O., Mittelman, H., & Seiden, G.D. (1968). Ototoxicity of
topically applied chloramphenicol. Arch. Otolaryngol., 87, 580-587.
Punch, P.I., Costa, N.D., Chambers, E.D., Slatter, D.H., &
Wilcox, G.E. (1985). Plasma and tear concentrations of antibiotics
administered parenterally to cattle. Res. Vet. Sci., 39, 179-187.
Puron, R. & Firminger, H.I. (1965). Protection against induced
cirrhosis and hepatocellular carcinoma in rats by chloramphenicol.
J. Natl. Cancer Inst., 85, 29-37.
Rajchgot, P., Prober, C., Soldin, S., Golas, C., Good, F., Harding, L.
& MacLeod, M. (1983). Chloramphenicol pharmacokinetics in the newborn.
Dev. Pharmacol. Ther., 6, 305-314.
Rebandel, P. & Rudzki, E. (1986). Occupational contact sensitivity in
oculists. Contact Derm., 15, p. 92.
Robin, E., Berman, M., Bhooplan, N., Cohen, H., & Fried, W. (1981).
Induction of lymphomas in mice by busulfan and chloramphenicol.
Cancer Res., 41, 3478-3482.
Rosenbach, L.M., Cavites, A.P., & Mitus, W.J. (1960). New Engl. J.
Med., 263, 724-728.
Rosenkranz, S., Carr, M.S., & Rosenkrans, H.S. (1974). 2-Haloethanols:
Mutagenicity and reactivity with DNA. Mutat. Res., 26, 367-370.
Rosenkranz, H.S., Gutter, B., & Speck, W.T. (1976). Mutagenicity and
DNA-modifying activity: A comparison of two microbial assays.
Mutat. Res., 41, 61-70.
Rosenkranz, H.S. (1977). Mutagenicity of halogenated alkanes and their
derivatives. Environ. Health Perspect., 21, 79-84.
Rosenthal, R.L. & Blackman, A. (1965). Bone marrow hypoplasia
following use of chloramphenicol eye drops. J. Am. Med. Assoc.,
191, 148-149.
Ross, S., Burke, F.G., Sites, J., Rice, E.C., & Washington, J.A.
(1950). Placental transmission of chloramphenicol (chloromycetin).
J. Am. Med. Assoc., 142, p. 1361.
Roy, B.K., Banerjee, N.C., & Pandy, S.N. (1986). Distribution of
chloramphenicol in goat blood and milk after intravenous injection.
Indian. J. Anim. Health, 25, 33-35.
Rudzki, E. & Kleniewska, D. (1970). The epidemiology of contact
dermatitis in Poland. Brit. J. Derm., 83, 543-545.
Rudzki, E., Grzywa, Z., & Maciejowska, E. (1976). Occupational contact
sensitivity in oculists. Contact Derm., 2, 181.
Sack, C.M., Kaup, J.R., & Smith, A.L. (1980). Chloramphenicol
pharmacokinetics in infants and young children. Pediatr.,
66, 579-584.
Saidi, P., Wallerstein, R.O., & Aggeler, P.M. (1961). Effect of
chloramphenicol on erythropoiesis. J. Lab. Clin. Med., 75, 247-256.
Salem, Z., Murray, T., & Yunis, A.A. (1981). The nitroreduction of
chloramphenicol by human liver tissue. J. Lab. Clin. Med.,
97, 881-886.
Samson, J.P., Hulstaert, C.E., Molenaar, I., & Nieweg, H.O. (1972).
Fine structure of the bone marrow sinusoidal wall in idiopathic and
drug induced panmyelopathy. Acta haematol., 48, 218-226.
Sanguineti, M., Rossi, L., Ognio, E., & Sauti, L. (1983). Tumours
induced in Balb/C and C57B1/6N mice following chronic administration
of chloramphenicol. In: Proceedings of a National Meeting on
Experimental and Clinical Oncology, Parma, 23-25 November,
Abstract No. 50.
Sarasti, H. (1970). Chloramphenicol in South America. New Engl.
J. Med., 282, p. 813.
Saxen, I. (1975). Associations between oral clefts and drugs taken
during pregnancy. Int. J. Epidemiol., 4, 37-44.
Scheres, J.M.J.C., Hustinx, T.W.J., Geraedts, J.P.M., Leeksma, C.H.W.,
& Meltzer, P.S. (1985). Translocation 1:7 in hematologic disorders. A
brief review of 22 cases. Cancer Gen. Cytogen., 18, 207-213.
Schewach-Millet, M. & Shapiro, D. (1985). Urticaria and angioedema due
to topically applied chloramphenicol ointment. Arch. Dermatol.,
121, p. 587.
Schmitt-Graff, A. (1981). Chloramphenicol-induced aplastic anemia
terminating with acute nonlymphocytic leukemia. Acta haematol.,
66, 267-268.
Seaman, A.J. (1969). Sequels to chloramphenicol aplastic anemia: Acute
leukaemia and paroxysmal nocturnal hemoglobinuria. Northwest Med.,
68, 831-834.
Sekizawa, J. & Shibamoto, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
Sharp, A.A. (1963). Chloramphenicol-induced blood dyscrasias: Analysis
of 40 cases. Brit. Med. J., 1, 735-736.
Sheehan, J. & Sweeney, E.C. (1982). Drugs and sperm. Brit. Med. J.,
284, p. 1331.
Shimizu, H., Kuroishi, T., Tominaga, S., Okada, H., & Aoki, K. (1979).
Production amount of chloramphenicol and mortality rate of aplastic
anaemia in Japan. Acta haem. Jap., 42, 689-696.
Siegel, D. & Krishna, G. (1980). Nitrosochloramphenicol does not
induce aplastic anaemia in mice. Pharmacologist, 22, p. 244.
Siliciano, R.F., Margolis, S., & Lietman, P.S. (1978). Chloramphenicol
metabolism in isolated rat hepatocytes. Biochem. Pharmacol.,
27, 2759-2762.
Silver, B.J. & Zuckerman, K.S. (1980). Aplastic anaemia. Recent
advances in pathogenesis and treatment. Med. Clin. North Amer.,
64, 607-627.
Sklar, R. & Strause, B. (1980). Role of the uvrE gene product and of
inducible D6-methylguanine removal in the induction of mutations by
N-methyl-N-nitro-N-nitrosoguanidine in Escherichia coli.
Mol. Biol., 143, 343-362.
Skolimowski, I.M., Rowley, D.A., Knight, R.C., & Edwards, D.I. (1981).
Reduced chloramphenicol-induced damage to DNA. J. Antimicrob.
Chemother., 7, 593-597.
Skolimowski, I.M., Knight, R.C., & Edwards, D.I. (1983). Molecular
basis of chloramphenicol and thiamphenicol toxicity to DNA in vitro.
J. Antimicrob. Chemother., 12, 535-542.
Slater, E.E., Anderson, M.D., & Rosenkranz, H.S. (1971). Rapid
detection of mutagens and carcinogens. Cancer Res., 31, 970-973.
Slaughter, R.L., Pieper, J.A., Carra, F.B., Brodsky, B., & Koup, T.R.
(1980). Chloramphenicol sodium succinate kinetics in critically ill
patients. Clin. Pharmacol. Ther., 28, 69-77.
Smith, A.L. & Weber, A. (1983). Pharmacology of chloramphenicol.
Pediatr. Clin. North Amer., 30, 209-236.
Smith, G.S., Watkins, J.B., Thompson, T.N., Rozman, K., &
Klaasen, C.D. (1984). Oxidative and conjugative metabolism of
xenobiotics by livers of cattle, sheep, swine and rats.
J. Anim. Sci., 58, 386-395.
Stainer, G.A., Peyman, B.S., Meisels, H., & Fishman, G. (1977).
Toxicity of selected antibiotics in vitreous replacement fluid.
Ann. Ophthalmol., 9, 615-618.
Steidl, P.E.-M. (1965). Bilateral optic neuritis caused by the
prolonged therapeutic use of chloramphenicol. Union Med. Canada,
94, 1061-1062.
Stevens, D,C., Kleiman, M.B., & Lietman, P.S. (1981). Exchange
transfusion in acute chloramphenicol toxicity. J. Pediatr.,
99, 651-653.
Strick, R.A. (1983). Lack of cross-reaction between DNCB and
chloramphenicol. Contact Derm., 9, 484-487.
Suda, T., Omine, M., Tsuchiya, J., & Maekawa, T. (1978). Prognostic
aspects of aplastic anaemia in pregnancy. Experience on six cases and
review of the literature. Blut., 36, 285-298.
Suhrland, L.G. & Weisberger, A.S. (1969). Delayed clearance of
chloramphenicol from serum in patients with hematologic toxicity.
Blood, 34, 466-471.
Summ, H.D., Draeger, E., & von Wasielowski, E. (1976). On the
inhibitory effect of chloramphenicol on mitochondrial protein
synthesis as a possible cause of its selective toxic side effects.
Arzneim. Forsch., 26, 28-32.
Suter, W. & Jaeger, I. (1982). Comparative evaluation of different
pairs of DNA repair-deficient and DNA repair-proficient bacterial
tester strains for rapid detection of chemical mutagens and
carcinogens. Mutat. Res., 97, 1-18.
Timmermans, L. (1974). Influence of antibiotics on spermatogenesis.
J. Urol., 112, 348-349.
Tomatis, L., Agthe, C., Bartsch, H., Huff, J., Montesano, R., Saracci,
R., Walker, E., & Wilbourn, J. (1978). Evaluation of the
carcinogenicity of chemicals: A review of the monograph program of
International Agency for Research on Cancer. Cancer Res.,
38, 877-885.
Ullrich, D. & Bock, K.W. (1984). Glucuronide formation of various
drugs in liver microsomes and in isolated hepatocytes from
phenobarbital - and 3-methylcholanthrene - treated rats. Biochem.
Pharmacol., 33, 97-101.
Vacha, J., Pospisil, M., & Velcovsky, V. (1981). The toxic effect of
chloramphenicol on erythropoiesis in X-irradiated mice. Chemother.,
27, 131-138.
Van Joost, Th., Dikland, W., Stolz, E., & Prens, E. (1986).
Sensitisation to chloramphenicol; a persistent problem. Contact
Derm., 14, 176-178.
Van Scoy, R.E. & Wilson, W.R. (1983). Antimicrobial agents in patients
with renal insufficiency. Mayo Clin. Proc., 58, 246-248.
Venning, G.R. (1983). Identification of adverse reactions to new
drugs. II - How were 18 important adverse reactions discovered and
with what delays? Brit. Med. J., 286, 289-292.
Verma, R.S. & Lin, M.S. (1978). Chemically induced alterations of the
nuclear cycle and chromosomes in root meristem cells of maize.
J. Hered., 69, 285-294.
Vincent, P.C. (1986). Drug-induced aplastic anaemia and
agranulocytosis. Incidence and mechanisms. Drugs, 31, 52-63.
Vorherr, H. (1974). Drug excretion in breast milk. Postgrad. Med.,
56, 97-104.
Wagner, T. & Rafael, J. (1977). Biochemical properties of liver
magamitochondria induced by chloramphenicol or cuprizone. Exp. Cell
Res., 107, 1-14.
Walker, G.F. (1961). Blindness during streptomycin and chloramphenicol
therapy. Brit. J. Ophthalmol., 45, 555-557.
Wallenstein, L. & Snyder, J. (1952). Neurotoxic reaction to
chloromycetin. Ann. Intern. Med., 36, 1526-1528.
Wallerstein, R.O., Candit, P.K., Kasper, C.K., Brown, J.W., &
Morrison, F.R. (1969). Statewide study of chloramphenicol therapy and
fatal aplastic anemia: J. Am. Med. Assoc., 208, 2045-2050.
Waters, M.D., Garrett, N.E., Covone-de Serres, C.M., Howard, B.E., &
Stack, H.F. (1983). Genetic toxicology of some known or suspected
human carcinogens. In: de Serres, F.J. (ed.) Chemical Mutagens.
Principles and methods for their detection. 8, New York, Plenum
Press, pp. 261-341.
Watson, A.D.J. (1972). Chloramphenicol plasma levels in the dog: A
comparison of oral subcutaneous and intramuscular administration.
J. Small Anim. Pract., 13, 147-151.
Watson, A.D.J. (1977a). Effect of concurrent drug therapy and of
feeding or chloramphenicol levels after oral administration of
chloramphenicol in dogs. Res. Vet. Sci., 22, 68-71.
Watson, A.D.J. (1977b). Chloramphenicol toxicity in dogs.
Res. Vet. Sci., 23, 66-69.
Watson, A.D.J. & Middleton, D.J. (1978). Chloramphenicol toxicosis in
cats. Am. J. Vet. Res., 39, 1199-1203.
Watson, A.D.J. (1980). Further observations on chloramphenicol
toxicosis in cats. Am. J. Vet. Res., 41, 293-294.
Welch, H., Lewis, C.N. & Kerlan, I. (1954). Blood dyscrasias. A
nationwide survey. Antibiotics Chemother., 4, 607-623.
Werner, J.C., Whitman, V., Schuler, H.G., Fripp, R.R., Rannels, A.M.,
Kasales, C.J., & La Noue, K.F. (1985). Acute myocardial effects of
chloramphenicol in newborn pigs: A possible insight into Gray Baby
syndrome. J. Infect. Dis., 152, 344-350.
White, M.P., Haboush, H.9., & Alroomi, L.G. (1986). Fatal bone marrow
aplasia due to chloramphenicol in a baby. Lancet i, 555-556.
Widayat, Sunarto, Sutaryo, Zurghiban, M., & Ismangoen (1983). Aplastic
anaemia in children. Paediatrica Indonesiana, 23, 183-191.
Wilson, W. (1962). Toxic amblyopia due to chloramphenicol. Scot. Med.
J., 7, 90-95.
Xie, L. (1985). A simple model for detection of mutagenic
(carcinogenic) substances. Huanjing Kexue Xuebao, 5, 228-233.
Yoshida, H., Yamamoto, K., & Yamaguchi, H. (1972). Fragmentation and
non-disjunction of barley chromosomes after the treatment of
chloramphenicol and cycloheximide. Cytologia, 37, 697-707.
Young, L.W., Mitnick, J.S., Yankus, W.A., & Genieser, N.B. (1979).
Radiological case of the month. Am. J. Dis. Child., 133, 545-546.
Yunis, A.A. (1973a). Chloramphenicol toxicity. In: Blood Disorders
Due to Drugs and other Agents, Amsterdam, Excerpta Medica,
pp. 107-126.
Yunis, A.A. (1973b). Chloramphenicol-induced bone marrow suppression.
Semin. Haematol., 10, 225-234.
Yunis, A.A. (1977). Differential in vitro sensitivity of marrow
erythroid and granulocytic colony forming cells to chloramphenicol.
Am. J. Haemtol., 2, 355-363.
Yunis, A.A. (1978a). Mechanisms underlying marrow toxicity from
chloramphenicol and thiamphenicol. In: Silber, R., Lo Bue, J.,
Gordon, A. (eds.) The year of hematology, New York, Plenum,
pp. 143-170.
Yunis, A.A. (1978b). Pathogenetic mechanisms in bone marrow
suppression for chloramphenicol and thiamphenicol. In: Hibino, S.,
Takaku, F., & Shahidi, N.T. (eds.) Tokyo, University Park Press,
pp. 321-334.
Yunis, A.A. (1978c). Drug-induced bone marrow aplasia. Pesq. Med.
Biol., 11, 287-296.
Yunis, A.A., Miller, A.M., Salem, Z., & Arimura, G.K. (1980a).
Chloramphenicol toxicity: Pathogenetic mechanisms and the role of the
rho-NO2 in aplastic anaemia. Clin. Toxicol., 17, 359-373.
Yunis, A.A., Miller, A.M. Salem, Z., Corbett, M.D., & Arimura, G.K.
(1980b). Nitrosochloramphenicol: Possible mediator in
chloramphenicol-induced aplastic anemia. J. Lab. Clin. Med.,
96, 34-46.
Yunis, A.A. & Salem, Z. (1980). Drug induced mitochondrial damage and
sideroblastic change. Clinics in Haematol., 9, 607-619.
Yunis, A.A. (1984). Differential in vitro toxicity of chloram-
phenicol, nitrosochloramphenicol, and thiamphenicol. Sex Trans. Dis.,
11, 340-342.
Chloramphenicol was previously considered at the twelfth meeting
of the Committee (Annex 1, reference 17), at which time it was
determined that acceptable levels in food could not be established.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
In dogs, chloramphenicol is rapidly and extensively absorbed
after oral administration of 50 mg/kg b.w., giving plasma levels of
16.5 g/l 2 hours after dosing (Watson, 1972; Watson 1977a). Similar
findings have been observed in rabbits given oral doses of 16 mg/kg
chloramphenicol (Cid et al., 1983). Dietary bran markedly
increased the rate and extent of absorption of orally administered
chloramphenicol palmitate in pigs, but it is not known if the
absorption of chloramphenicol itself would be affected in this way
(Bueno et al., 1984). Experiments with everted mouse small intestine
sacs resulted in absorption of chloramphenicol at similar rates over
different regions of the small intestine. Various chemicals and
metallic ions had no effect on the rate of absorption, and penetration
of the serosal and mucosal surfaces was similar indicating passive
absorption of chloramphenicol from the gut (Chakrabarti & Banerjee,
1977).
In adult human beings, absorption of chloramphenicol is rapid and
extensive after an oral dose. Serum levels were 20 - 40 mg/l after a
2 g dose (29 mg/kg b.w.) and 40 - 60 mg/l after a 4 g dose (57 mg/kg
b.w.) (Yunis, 1973a).
Chloramphenicol is also well absorbed by infants and neonates
after oral administration. Serum (peak) concentrations of 20 - 24 mg/l
were noted after oral doses of 40 mg/kg b.w. in neonates. Infants
given 26 mg/kg b.w. were found to have peak concentrations of 14 mg/l
(Mulhall et al., 1983). Available evidence and theoretical
considerations suggest that chloramphenicol may be percutaneously
absorbed in human beings (Guy et al., 1985a, b).
Chloramphenicol is distributed to most major organs in newborn
pigs. After i.v. administration of 0.52 mg/kg b.w. 14C-chloram-
phenicol, many tissues showed higher levels of chloramphenicol than
were found in the blood as soon as 5 minutes after administration.
These tissues included the lungs, liver, kidneys, adrenal cortex,
myocardium, pancreas, thyroid, spleen, and skeletal muscle. The
levels remained higher than in blood for up to 8 hours after dosing.
At 4 and 8 hours after administration, levels in the brain were
higher than in blood. However, there was no apparent affinity for
chloramphenicol in the bone marrow over the period of the experiment
(8 hours), where levels did not reach those noted in serum (Appelgren
et al., 1982).
Chloramphenicol in human beings, regardless of the route of
administration, extensively distributes, although the levels in
tissues depend on the route, the levels being the highest after oral
or i.v. administration. The compound has been found in the heart,
lungs, kidneys, liver, spleen, pleural fluid, seminal fluid, ascitic
fluid, and saliva. (Ambrose, 1984; Yunis 1973a; Gray, 1955). It binds
extensively to proteins in both adults and neonates, although binding
in the latter is less than in the former (Kurz et al., 1977).
Chloramphenicol can cross the placenta in human beings (Ambrose,
1984). After oral doses of 1 or 2 g chloramphenicol to pregnant women,
chloramphenicol was detected in the placenta after 1.5 - 2.5 hours,
indicating some potential for the drug to reach the fetus (Ross
et al., 1950).
In human beings with normal renal/hepatic function the volume of
distribution is around 0.7 1.4 1/kg. These values do not deviate
markedly in patients with hepatic dysfunction or with renal
impairment. Overall, these values suggest extensive distribution in
body tissues. (Ambrose, 1984; Burke et al., 1980; Slaughter
et al., 1980). Similar values were noted with infants and young
children given the sodium succinate derivative of chloramphenicol,
which is converted in vivo to chloramphenicol (Sack et al., 1980).
Chloramphenicol and its metabolites are excreted in the urine of
rats after oral dosing; up to 70% of an oral dose may he excreted in
this way (Glazko et al., 1949; Glazko et al., 1952). Limited data
suggest that chloramphenicol may be excreted in the bile of rats
following administration; around 0.4% of an i.m. dose of 40 mg/kg was
detected in the bile after 4 hours (Kunii et al., 1983). There are
no readily available data for biliary excretion after oral
administration. In new-born pigs, the majority of an i.v. dose of
chloramphenicol was excreted in the urine, while a small quantity was
excreted in the bile (Appelgren et al., 1982). Liver damage may
delay body-clearance of chloramphenicol, at least in the mini-pig
(Kroker, 1985). Following t.v. administration to goats, 69% of the
dose was excreted in the urine after 12 hours (Javed et al., 1984).
Chloramphenicol has been found to be excreted in other body
fluids after administration to animals. It has been detected at levels
up to 6 mg/l in the tears of cattle given i.v. doses of 50 mg/kg
chloramphenicol; it was detected in the tears as soon as 1 hour after
dosing (Punch et al., 1985). Chloramphenicol was detected in the
milk of goats after an i.v. dose of 100 mg/kg, with maximum levels
1 hour after administration. (Roy et al., 1986). Administration of
10 mg/kg chloramphenicol i.m. to cattle resulted in peak levels in
milk of around 1 mg/l 6 hours after injection. However, after oral
administration, no chloramphenicol was detected in milk.
(De Corte-Baeten & Debackere, 1976).
Nine patients with evidence of chloramphenicol-induced bone
marrow suppression showed greatly reduced plasma clearance times
compared with 9 others who showed no evidence of toxicity. In the
"toxic" group, 5 had liver disease, 2 had pyelonephritis, and 2 showed
neither liver disease nor renal disease. In the "non-toxic" group, 1
had liver disease, 2 had renal disease, while 6 had neither. Six hours
after an i.v. dose of 500 mg chloramphenicol succinate, the blood
level in the "toxic" group was 4.5 µg/ml (2.8 - 6.9 µg/ml), while in
the "non-toxic" group the mean level was 1.2 µg/ml (0 - 2.3 ug/ml).
Similarly, after 8 hours the mean level in the "toxic" group was
3.5 µg/ml (2.1 - 5.2 ug/ml), while in the "non-toxic" group a mean
level of 0.7 µg/ml (0 - 2.5 µg/ml) was noted. Such findings suggest
that patients susceptible to the bone-marrow effects of
chloramphenicol may clear the drug from the blood at a slower rate
than those who are not susceptible (Shurland & Weisburger, 1969).
Chloramphenicol administered to human beings is excreted
primarily in the urine (90%), up to 15% as the parent compound and the
remainder as metabolites, including conjugated derivatives
(Burke et al., 1980; Ambrose, 1984; Yunis, 1973a). Glomerular
excretion is thought to be the major mechanism of excretion
(Glazko et al., 1949).
Renal clearance varies depending on age. In one study, clearance
in neonates (less than 6 months of age) was 0.46 - 9.76 1/hour, while
in infants aged 6 months to 2.5 years values in the range 1.8 - 2.1
1/hour were reported. In two subjects aged over 2.5 years, values of
6.03 and 9.59 1/hour were noted (Burckart et al., 1983). Similar
variations with age have been noted in other studies (Mulhall et al.,
1983; Sack et al., 1980; Kauffman et al., 1981; Burckart et al.,
1982; Rajchgot et al., 1983). Renal clearance takes longer in
patients with renal insufficiency than in normal patients (Brasfield
et al., 1983). However, these differences are not marked (Smith &
Weber, 1983). It has been recommended that dosage adjustments need not
be made in patients with renal insufficiency or in anephric subjects
(Van Scoy & Wilson, 1983).
As with experimental animals, chloramphenicol is excreted in
human milk. Up to 1.3% of an administered dose may be excreted in this
way. (Vorherr, 1974). After a single, oral dose of chloramphenicol, a
peak milk concentration of 3 mg/l has been reported. This peak was
reached around 2 hours after administration, with a drop to nearly
pre-dosing levels by 8 hours post-dose (Plomp et al., 1983). Other
workers have reported similar findings (Knowles, 1965; Matsuda, 1984).
Biotransformation
Early studies indicate that the major metabolite of
chloramphenicol in the rat was the glucoronide conjugate, which was
found along with free chloramphenicol after oral dosing
(Glazko et al., 1950; Glazko et al., 1952). In in vitro studies
it has been demonstrated that the glucuronide is the main metabolic
product in isolated rat hepatocytes exposed to chloramphenicol
(Siliciano et al., 1978). Glucuronidation of chloramphenicol was
elevated in vitro in hepatocytes obtained from phenobarbital-
pretreated rats. This increase correlated with differential induction
of UDP-glucuronyltransferase in hepatocytes of rats pretreated with
phenobarbital (Ullrich & Bock, 1984).
Several metabolites of chloramphenicol have been identified in
rat urine. In addition to free chloramphenicol (1) and the glucoronide
(2), the oxamic acid (3), alcohol (4), and base (5) derivatives have
been noted in the urine of rats given i.m. doses of [3H]-chlo-
ramphenicol (1R,2R-1- p-nitrophenyl(2,2-dichloroacetamido-
1,3-(1-3H)-propandediol). The acetylarylamine (6) and arylamine
metabolites (7) have also been detected. These metabolites are shown
in Table 1.
Table 1 Metabolites of chloramphenicol in the rat
Compound R1 R2 R3
1. chloramphenicol NO2 COCHCl2 OH
2. glucuronide conjugate NO2 COCHCl2 C6H9O7
3. oxamic acid derivative NO2 COCHO2H OH
4. alcohol derivative NO2 COCH2OH OH
5. base derivative NO2 H OH
6. acetylarylamine derivative NHCOCH3 COCHCl2 OH
7. arylamine derivative NH2 COCHCl2 OH
Based upon recovered radioactivity, the major metabolites
appeared to be chloramphenicol base (approx. 26%) and the
acetylarylamine derivative (19.1%). The other metabolites were
recovered in the 8-15% range, except for the arylamine derivative,
which represented approximately 4% of the recovered radioactivity
(total identified, 93.4% of administered radioactivity; total
collected, 95.9% of administered radioactivity (Bories et al.,
1983).
An in vitro study using perfused rat liver and rat liver
microsomes indicated that the arylamine derivative may undergo
N-oxidation to form nitrosochloramphenicol by way of the N-hydroxy
derivative, which may then be conjugated with glutathione
(Ascherl et al., 1985).
There are few data available on the metabolism of chloramphenicol
in other species. In dogs, chloramphenicol, chloramphenicol base, and
chloramphenicol glucuronide conjugate appeared to be the major
metabolites (Glazko et al., 1950). Chloramphenicol, the glucuronide
conjugate, and the oxamic acid, acetylarylamine, arylamine, and base
derivatives were noted in the urine of goats given i.m. injections of
chloramphenicol (Bories et al., 1983). In vitro studies with pig
liver showed the activity of UDP-glucuronyltransferase in this species
to be similar to that in rats, suggesting that glucuronidation may be
a significant pathway of biotransformation of chloramphenicol in pigs.
The same experiments with sheep and cattle liver preparations showed
that they have much lower glucuronyl transfer activities (25% and 14%,
respectively). This may indicate that glucuronidation plays a less
important role in these species (Smith et al., 1984).
In human beings, 93% of an oral dose of chloramphenicol was
excreted in the urine within 24 hours, and it seems likely that the
major excretion product was the glucuronide conjugate. Approximately
48% of the chloramphenicol excreted in the urine within 8 hours after
oral dosing was the glucuronide conjugate; only 6% was excreted as the
parent compound and 4% as the base derivative (Baselt, 1982;
Nakagawa et al., 1975). The alcohol derivative has been detected in
the urine of neonates (Dill et al., 1960). More recent experiments
have confirmed the presence of the glucuronide conjugate and base
derivative as the major metabolites after an oral dose of 500 mg
chloramphenicol (Bories et al., 1983).
Human liver has the potential to reduce chloramphenicol. In 10
livers studied, nitro reductase activity was observed, which was
dependent on NADPH. Thus, human livers may have the capacity to
convert the nitro group of chloramphenicol to the amine, with the
further possibility of nitroso formation. Esters of chloramphenicol,
for example, the succinate, are converted to chloramphenicol in vivo
(Salem et al., 1981).
Effects on enzymes and other biochemical parameters
Chloramphenicol is known to increase the plasma levels of certain
drugs (e.g., paracetamol and phenytoin) and to prolong barbiturate
sleeping time, which is indicative of an effect on drug metabolizing
enzymes (Halpert & Neal, 1981; Nair et al., 1981; Aravindaksham &
Cherian, 1984).
The major contribution to these effects may arise from
chloramphenicol's ability to act as a suicide substrate in the
inactivation of cytochrome P-450, possibly by the binding of the
oxamic acid derivative to lysine residues of the cytochrome molecule
(Halpert & Neal, 1981; Halpert, 1981; Halpert, 1982). Of the various
isozymes of cytochrome P-450, those induced by phenobarbital appear to
be the most sensitive to chloramphenicol. Rats were pretreated with
various inducers of cytochrome P-450 (phenobarbital, ß-naphthoflavone,
pregnenolone, 16-x-carbonitrile, and clofibrate) and were then
injected i.p. with 300 mg/kg b.w. chloramphenicol. The isozymes of
cytochrome P-450 induced by phenobarbital were inhibited to some
degree by chloramphenicol administration, but those forms induced by
the other compounds investigated were not affected. Cytochrome
P-450-c, the form present in non-pretreated rats, was the most
susceptible isozyme in vivo and in vitro (Halpert et al., 1985).
Following i.v. or i.p. administration of 100 mg/kg b.w.
chloramphenicol to rats, inhibition of the conversion of n-hexane to
2-hexanol by rat liver or lung microsomes occurred. There was no
effect on the conversion of n-hexane to l-hexanol, while only liver
microsomes were markedly inhibited in the formation of 3-hexanol
(Naesland & Halpert, 1984; Naesland et al., 1983).
Chloramphenicol prevented the methanol-potentiated toxicity of
carbon tetrachloride in rats, possibly by deactivation of cytochrome
P-450 (Brabec et al., 1982). Phenobarbital-induced cytochrome P-450
appears to be responsible for the dechlorination of chloramphenicol
and the formation of chloramphenicol aldehyde in rat liver microsomes.
The dechlorination may be enhanced by glutathione. The significance of
these findings is not clear at the present time (Morris et al.,
1982; Morris et al., 1983).
Subcutaneous administration of up to 100 mg/kg b.w. chloram-
phenicol to rats inhibited hepatic N-demethylase, glucose-6-
phosphate dehydrogenase, and carboxylesterase (Hapke et al., 1977).
However, no effects were observed on monoamine oxidase in the liver,
brain, or heart of rabbits given 60 mg/kg b.w. chloramphenicol i.m.
for 5 days. Similarly, no effect on monoamine oxidase activity was
seen in in vitro experiments where rabbit liver preparations were
preincubated with chloramphenicol (Ali, 1985).
Several studies have demonstrated an effect of chloramphenicol on
mitrochondrial protein synthesis. In vitro, chloramphenicol
inhibited mitochondrial protein synthesis in rat liver and rabbit bone
marrow. The effect was similar to that noted with tetracycline (Summ
et al., 1976). Nitrosochloramphenicol inhibited rat mitochondrial
DNA polymerase in vitro, whereas the arylamine derivative and
chloramphenicol itself did not (Lim et al., 1984). Nitroschlor-
amphenicol inhibited the transport of NAD-linked substrates into
mitochondria, but it had no effect on FAD-linked substrates. It
inhibited ATP formation and completely blocked the transport of
protons out of the mitochondria (Abou-Khalil et al., 1982).
Chloramphenicol inhibited the incorporation of leucine into protein in
mitochondria from erythroid and myeloid rumour cells and in cells from
normal, myeloid, and erythroid hyperplastic bone marrow of rabbits
in vitro (Abou-Khalil et al., 1980, 1981). In mice given
chloramphenicol, the so-called megamitochondria that were produced
were deficient in cytochrome oxidase, ATP synthetase, and cytochrome b
activities, which is indicative of inhibited protein synthesis
(Wagner & Rafel, 1977). Subcutaneous administration of 500 mg/kg b.w.
chloramphenicol succinate to partially hepatectomized rats did not
significantly inhibit protein synthesis. However, liver cytochrome c
oxidase was strongly inhibited in these rats (Kroon & Vries, 1969).
Intramuscular administration of chloramphenicol to rats inhibited
mitochondrial monoamine oxidase activity in the liver, brain, and
kidney (Banerjee & Basu, 1978). When chloramphenicol was given daily
for 6 days at doses of 100 mg/kg b.w. i.p., marked reductions in the
activities of liver kynurenine hydrolase, knynurenine amino-
transferase, ß-glucuronidase, and acid ribonuclease were observed,
indicating possible effects on drug metabolizing enzymes and on
protein biosynthesis. Pyridoxal phosphokinase activity was increased
(Akhnoukh et al., 1982).
In human blood, nitrosochloramphenicol is bound to albumin
in vitro. In red blood cells, it rapidly forms adducts with
glutathione and also with the -SH groups of haemoglobin. Moreover, it
is rapidly reduced to the N-hydroxy compound, although further
reduction to the amine is very slow. Overall, the data suggest that
the nitroso derivative would be rapidly detoxified in the blood before
reaching the bone marrow (Eyer et al., 1984). Nitrosochloramphenicol,
but not chloramphenicol, induced methaemoglobinaemia in haemolyzed
human blood in vitro (Lim & Yunis, 1982).
To summarize, chloramphenicol is well absorbed in both animals
and human beings after oral administration and widespread distribution
occurs. Urinary excretion is rapid in animals and man, a major
metabolite being the glucuronide conjugate. Animals and human beings
produce a range of urinary metabolites; in vitro experiments suggest
that nitrosochloramphenicol may be an important metabolite in human
beings. It is not known if this compound is produced in vivo in man
or other species.
Toxicological studies
Special studies on carcinogenicity
In 1982 and 1987, IARC concluded that adequate tests for
carcinogenicity of chloramphenicol in animals were not available
(IARC, 1982; IARC, 1987). These conclusions confirmed an earlier
opinion by IARC workers (Tomatis et al., 1978). The two studies
below by Robin et al., and by Sanguineti et al., were included in
the IARC evaluations.
Four groups of Balb/c × AF1 male mice, 45 per group, were given
i.p. injections of either 0.5 mg busulphan on days 1, 15, 29 and 43
(2 groups) or vehicle (acetone plus distilled water, 2 groups. After
20 weeks, by which time 78 mice remained alive in the busulphan-
treated groups and 88 in the vehicle groups (the rest having
died of injection complications), one of the busulphan groups and one
of the vehicle groups was selected for treatment with chloramphenicol,
while the others served as controls and were given vehicle only (0.9%
sodium chloride). Mice in the treatment groups received i.p.
injections of 2.5 mg chloramphenicol 5 days per week for 5 weeks.
Sacrifice of the mice followed a complicated schedule depending on the
appearance of lymphomas, but all animals had been sacrificed by day
350. The following incidences of lymphoma were noted: busulphan/
chloramphenicol, 13/37; busulphan/vehicle, 4/35; chloramphenicol/
vehicle 2/42; vehicle/vehicle, 0/41. The authors thought that this
suggested that busulphan and chloramphenicol increased the frequency
and accelerated the onset of lymphomas. It also provided some
evidence that chloramphenicol alone might induce lymphomas in this
animal model, but the duration of the experiment along with other
limitations of the experimental design, particularly the dosing
regime, prevent any other conclusions from being drawn (Robin
et al., 1981).
In a study which was reported in abstract form only, in which
chloramphenicol was administered in the drinking water, an increased
incidence of lymphomas in two strains of mice and of hepatocellular
carcinoma in one strain were noted (Sanguineti et al., 1983).
Another study investigated the effect of chloramphenicol on
cirrhosis and hepatocellular carcinoma induced by the carcinogen
N-2-fluorenyldiacetamide in rats. A group of 25 male Wistar rats was
given a diet containing 0.05% N-2-fluorenyldiacetamide (equivalent to
25 mg/kg b.w./day) plus 2% chloramphenicol (equivalent to 1000 mg/kg
b.w./day) for 4 weeks. Another group of 20 rats received a diet
containing only the 0.05% N-2-fluorenyldiacetamide. A "rest" week was
then followed by another 4 weeks on the respective diets, followed by
another "rest" week and then 6 weeks of dietary administration of the
substances. After this the animals given the carcinogen only were
returned to the control diet, but those given the combined regime were
continued on this diet for another 2 weeks. Animals were sacrificed at
46 (carcinogen only) or 46-55 weeks (carcinogen plus chloramphenicol).
Of 12 animals examined which were given the carcinogen-only diet, 100%
had cirrhosis and 75% had hepatocellular carcinoma, whereas of 22
animals examined which were given the combination treatment, only one
had cirrhosis and hepatic rumours. Thus, chloramphenicol had a
protective effect on the induction of hepatic rumours by
N-2-fluorenyldiacetamide (Puron & Firminger, 1965).
Special studies on haematological effects
Groups of 18-21 57B1/10ScSnPh mice were given 4.78 Gy of
X-irradiation and were then treated three times daily with 160 or
320 mg/kg b.w. chloramphenicol succinate by s.c. injection over 3 or 5
days, beginning 10 days after the X-ray treatment. Two groups of
control animals were given chloramphenicol and no irradiation or
irradiation and no chloramphenicol. Animals were examined 4, 8, and 21
days after initiation of chloramphenicol treatment (14, 18, and 21
days after irradiation). No effects on the level of erythrocytes in
non-irradiated mice occurred; those given chloramphenicol showed
similar values to non-treated controls. The level of erythrocytes in
irradiated animals was significantly lower than in non-irradiated
animals (30% reduction 14 days after irradiation) but there was
improvement with time (26% reduction 18 days and 15% reduction 21 days
after irradiation), while irradiated animals given chloramphenicol
showed lower erythrocyte levels than those that were irradiated only;
at 14, 18, and 21 days post irradiation, irradiated mice given
chloramphenicol had erythrocyte levels 8%, 17%, and 4.5% lower,
respectively, than animals given irradiation alone, indicating that
chloramphenicol had a deleterious effect on bone marrow recovery after
X-irradiation (Vacha et al., 1981).
Normal mice and those with residual bone marrow damage following
busulphan administration were investigated for the effects of
chloramphenicol. Female Balb/c mice were injected with busulphan to
create bone marrow damage using a method that had been validated
previously by the authors. Groups of mice, some having busulphan-
induced damage and others untreated, were then given drinking water
containing 0.5 g/dl chloramphenicol succinate; groups of 5 mice were
then killed at various intervals up to and including 150 days after
chloramphenicol treatment. No effects were seen in the bone marrow
of mice given chloramphenicol alone nor in mice pretreated with
busulfan. However, animals given both busulphan and chloramphenicol
displayed a progressive fall in the number of pluripotential stem
cells and granulocytic precursor cells (Morley et al., 1976).
Similar effects were not seen in another study where busulphan-
treated mice were given drinking water containing 0.5 g/dl
chloramphenicol for six weeks. There was no effect on the colony-
forming ability of bone marrow or spleen cells. This study used
an identical busulphan regime to that described above (Pazdernik &
Corbett, 1980).
In cells of lethally X-irradiated mice given 10 mg
chloramphenicol i.p. on days 2-8 or on days 7-12 after irradiation,
mitochondrial swelling was noted in early erythroblasts, but not in
intermediate or late types. The mitochondria showed reductions in the
number of cristae (Miura et al., 1980).
A similar investigation in mice given 4.78 Gy X-irradiation and
300 mg/kg b.w. of chloramphenicol succinate showed that dividing bone
marrow cells had decreased entry into S-phase of the cell cycle. The
affected cells were mainly of the erythroid type. The same types of
effect were also noted in non-irradiated mice given chloramphenicol
(Benes et al., 1980).
The femora of mice given 500 mg/kg b.w. chloramphenicol for 6
days by injection (route unspecified) which were then implanted into
untreated syngeneic mice showed greatly decreased colony formation
when the bone marrow was subsequently injected into lethally
irradiated mice (Nara et al., 1982).
No haematological effects, including aplastic anaemia, were seen
in mice given 40 mg/kg nitrosochloramphenicol for 10 days followed by
sacrifice 6 weeks after the last injection (Siegel & Krishna, 1980;
abstract only).
Groups of 6 male Sprague-Dawley rats were each given 50 mg/kg
b.w. chloramphenicol succinate by i.v. infusion. Half of the animals
were subjected to liver resection 15 minutes after infusion, while the
others were sacrificed at this time. Bleeding time and blood loss were
significantly increased in resected animals given chloramphenicol
compared with controls (bleeding time: 500 seconds in treated animals,
300 seconds in controls; blood loss 2.2 g in treated animals, 0.9 g in
controls). No effects on haemoglobin or haematocrit were observed
following infusion or liver resection (Bengmark et al., 1981).
Four cats were given daily i.m. injections of 50 mg/kg b.w.
chloramphenicol for 21 days. Two untreated cats served as controls;
all 6 animals had recently recovered from experimental infectious
feline enteritis. Animals given chloramphenicol became very ill, with
severe loss of appetite developing within 7 days. All four developed
diarrhoea toward the end of the experiment (they were sacrificed on
day 21); one was killed in extremis. Marrow examination was carried
out, which revealed vacuolation of the myeloid and erythroid
precursors and of some lymphocytes. There were no significant changes
in peripheral red cell numbers, but white cell counts were much
reduced (Penny et al., 1967).
A group of 6 cats was given chloramphenicol orally at a dose of
125 mg/kg b.w./day for 14 days and then observed for another 3 weeks.
Another group, formerly intended as controls, were dosed with 60 mg/kg
b.w./day chloramphenicol in the same manner. Signs of toxicity
included CNS depression, dehydradation, loss of appetite, body weight
loss, diarrhoea, and vomiting. Blood and bone marrow samples were
obtained both prior to and after chloramphenicol treatment. The major
findings related to chloramphenicol administration were severe bone
marrow suppression with marrow hypoplasia, prevention of maturation of
erythroid cells, and inhibition of mitosis in the marrow. Vacuolation
of lymphocytes, and early myeloid and erythroid cells occurred. The
effects were most severe in cats given 120 mg/kg b.w. chloramphenicol.
On cessation of treatment the bone marrow suppression resolved (Watson
& Middleton, 1978).
In a similar study, 5 cats were given 50 mg/kg b.w./day
chloramphenicol orally for 21 days. CNS depression, appetite loss, and
weight loss were noted. Examination of peripheral blood was conducted
both before and after dosing. This revealed lower platelet numbers
after 1 week, and a lower neutrophil count after 3 weeks of
administration. One animal developed lymphocytopenia after 1 week and
neutropenia after 3 weeks. At the end of treatment, the bone marrow
was found to have vacuolated early myeloid cells and lymphocytes, with
reduced myeloid maturation and reduced marrow cellularity. No test for
recovery was conducted (Watson, 1980).
Twenty dogs were given oral doses of chloramphenicol for 14 days.
They were dosed in the following manner: 6 dogs, 225 mg/kg b.w./day;
4 dogs each, 175 or 125 mg/kg b.w./day; and 3 dogs each at 275 or
75 mg/kg b.w. day. Signs of toxicity included a decline in the rate of
weight gain and hypophagia. No changes in erythrocyte counts,
reticulocyte counts, haemoglobin concentration, packed cell volume, or
differential leukocyte counts occurred, but bone marrow examination of
the dogs given 225 or 275 mg/kg/b.w./day revealed suppression of
erythropoiesis. In dogs given 275 mg/kg b.w./day chloramphenicol,
decreased mitotic activity and a reduced rate of granulocyte formation
was also evident. No dogs showed bone marrow vacuolation
(Watson, 1977).
After i.v. administration of a single dose of 50 mg/kg b.w.
chloramphenicol succinate to five mongrel dogs, platelets were
obtained from blood at 0, 30, 60, 120, 180, and 240 minutes and at 24
hours after dosing. Protein synthesis as determined by the rate of
incorporation of 3H-leucine was inhibited, with maximum depression
(940% of control values) occurring at 30 - 240 minutes after dosing
(Agam et al., 1976).
Neonatal Holstein calves (1 day of age at the beginning of the
experiment) given "an adequate" intake of colostrum were given
chloramphenicol by several methods. These were, i.v. as a 25 mg/kg
b.w. bolus at 1, 7, 14, and 28 days of age, i.v. as a 25 mg/kg b.w.
injection every 12 hours until 150 mg/kg b.w. had been delivered, and
as a 25 mg/kg b.w. bolus i.v., i.m., or s.c. alternately, with 1 week
between the doses. No effects on haematological parameters were
observed, and bone marrow aspirates showed no evidence of suppression
or toxicity (Burrows et al., 1984).
A similar lack of effect on the bone marrow was noted in a study
of cross-breed calves given doses of 9, 20, or 60 mg/kg b.w.
chloramphenicol daily for 6 weeks (Mitema, 1982).
In another study in Holstein calves in which chloramphenicol was
given orally at a dose of 100 mg/kg b.w./day over 10 days, bone marrow
suppression did occur. Similar results were noted in over 50 calves
investigated over a period of time. Changes included partial aplasia
to almost complete aplasia of the marrow, with loss in cellularity of
erythrocytes, white cells, and megakaryocytes. Occasionally only fat
deposits were noted with lymphocytic infiltrations. Lower blood levels
of chloramphenicol were attained after oral intake than following i.v.
injection, but the toxic effects on the marrow were greater after oral
dosing (Krishna et al., 1981).
In in vitro experiments, chloramphenicol and its postulated
metabolite, nitrosochloramphenicol, have shown adverse effects on bone
marrow cells. Chloramphenicol caused dose-related inhibition of
erythroid and granulocytic colony forming units obtained from LAF1
mice. The lowest concentration used (5 µg/ml) caused some degree of
inhibition of erythroid cells, while the highest concentration
(60 µg/ml) produced complete inhibition (Yunis, 1977). Similar effects
were noted in a separate study (Hara et al., 1978).
Chloramphenicol and nitrosochloramphenicol inhibited DNA
synthesis in rat bone marrow cells in vitro. This was reversible
with chloramphenicol, but not with the nitroso compound. Similarly,
the nitroso compound, but not chloramphenicol, bound irreversibly to
bone marrow cells (Gross et al., 1982). However, in another
in vitro study, chloramphenicol and nitrosochloramphenicol had no
effects on mouse haematopoietic precursor cells (Pazdernik & Corbett,
1979).
Special studies on mutagenicity
The antibiotic activity of chloramphenicol is not thought to
involve any type of reaction with bacterial genetic material. It
appears to inhibit protein synthesis by binding to the 50S subunit of
the 70S ribosome, inhibiting the formation of the peptide bond during
protein synthesis (Smith & Weber, 1983, Gilman et al., 1985).
Chloramphenicol has been shown to cause DNA strand breaks in
bacterial cells and to inhibit DNA synthesis in lymphocytes and in a
phage of E. coli (Amati, 1970; Dewse, 1977; Jackson et al., 1977).
However, chloramphenicol provided resistence to UV-induced damage in
E. coli B/r (Doudney & Rinaldi, 1985).
Nitrosochloramphenicol, which is a potent inducer of DNA strand
breaks in bacterial cells, resulted in strand breakage and loss of
helix, bringing about rapid degradation of isolated E. coli DNA
in of helix, bringing about rapid degradation of isolated E. coli
DNA in vitro (Skolimowski et al., 1981, 1983). Nitrosochlor-
amphenicol, but not chloramphenicol, produced inhibition of DNA
synthesis and caused DNA strand breaks in E. coli. (Murray
et al., 1982; Yunis, 1984).
Chloramphenicol has been tested in a variety of assays for
mutagenic activity. Most of these assays are well established using
well validated methods, but some, such as colicine induction, the
induction of tandem genetic duplications in S. typhimurium, and
tests using snails, are less well known and are unvalidated. The
results of these tests are presented in Table 2.
In general chloramphenicol gave negative results in bacterial
reverse mutation assays, in assays for DNA repair in bacteria, in the
dominant lethal test in rodents and D. melanogaster, in the
CHO/HGPRT test, in a test for sister chromatid exchange in human
lymphocytes, in the micronucleus test, and in a test for DNA binding.
Tests for inhibition of growth in E. coli were also negative and,
moreover, chloramphenicol has sometimes been used as a negative
control in this assay (McCoy et al., 1980a; McCoy et al., 1980b;
Rosenkranz, 1977; Rosenkranz et al., 1974; Braun et al., 1977;
Braun et al., 1982). There were isolated exceptions to these
negative results. However, the only type of test that gave
consistently positive results was the test for induction of
chromosomal aberrations. Chromosomal anomalies were noted in human
lymphocytes treated in vitro with chloramphenicol, in mouse bone
marrow in animals given chloramphenicol at doses of 50 mg/kg i.p. or
i.m., and in F1-generation mouse liver.
The failure of chloramphenicol to give positive results in the
Ames test has been attributed by one group of authors to its bacterial
toxicity. In their study, the D(-)-threo isomer of chloramphenicol
gave negative results in the Ames test, as observed previously in
several other studies; it was also toxic to the bacterial tester
strains used, TA100 and TA1535. However, the L(+)-threo isomer,
which is not used therapeutically, was much less toxic and could be
tested at higher concentrations; with the isomer, a dose-related
mutagenic response was noted in both tester strains (Jackson et al.,
1977).
The failure of chloramphenicol to give positive results in most
types of commonly used mutagenicity tests, with the exception of those
examining the induction of chromosome aberrations, has been recognized
by other reviewers (Waters et al., 1983; Garrett et al., 1984), as
has the failure of the substance to produce mutations in vivo
(Holden, 1982).
Chloramphenicol has been reported to enhance the mutagenicity of
N-methyl-N-nitro-N-nitrosoguanidine in bacterial assays (Sklar &
Strauss, 1980; Baltz & Stonesifer, 1985).
Special study on ocular toxicity
No toxic effects were seen in groups of three rabbits following
vitrectomy when solutions containing 10 or 20 µg/ml chloramphenicol
were infused into the eyes as vitreous replacements. Histological and
electroretinographical examinations yielded normal results 2 weeks
after infusion. Following an infusion of a 50 µg/ml solution,
electroretinography was normal after 2 weeks, but abnormal retinal
histology, which was not described but was said to be widespread and
generalized, was noted (Stainer et al., 1977).
Special studies on ototoxicity
Solutions of 0.5% chloramphenicol introduced into the bullae of
the ears of guinea pigs produced no effects on hearing, but solutions
containing 1 - 5% chloramphenicol produced moderate hearing loss at a
variety of frequencies. Similar findings were noted when the
electrical responses of the hair cells were measured after
chloramphenicol administration into the bullae. Intratympanic
administration to guinea pigs of a 1% solution of chloramphenicol
produced a moderate degree of hair cell loss in the organ of Corti,
with severe inflammation of the mucosa of the middle ear. Introduction
of a solution containing 8 or 16 mg chloramphenicol through a hole in
the bullae of the ears of guinea pigs followed by sacrifice 3, 6, 9,
or 24 hours later resulted in severe destruction of hair cells and
supporting cells in the basal turns of the organ of Corti. The effects
were similar regardless of the dose or the time of sacrifice after
dosing (Proud et al., 1968; D'Angelo et al., 1967; Patterson &
Gulick, 1963; Morizono & Johnstone, 1975; Parker & James, 1978).
Table 2. Results of mutagenicity assays with chloramphenicol
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 30 µg/plate - Brem et al.,
TA1530, TA1535 1974
TA1538
S. typhimurium 0.17-24 µg/ml + Mitchell et al.,
TA98 1980
S. typhimurium Not given - Heddle & Bruce,
TA1535, TA1537 1977
S. typhimurium 30 µg/plate - Rosenkranz et al.,
TA98, TA100 1976
S. typhimurium < 4.5 nmole - McCann et al.,
TA98, TA1535 1975
TA1538
Bacterial E. coli 27 µg/ml + Mitchell &
mutation assay CM891 Gilbert, 1984
CHO/HGPRT Chinese hamster Not given Augustine et al.,
mutation assay ovary cells 1982
Gene mutation D. melanogaster Not given +a Narda & Gupta,
1972
(abstract only)
Dominant lethal D. melanogaster Not - Nasrat et al.,
1977
Mouse (101×C3H)F1 2×1.5 g/kg - Ehling, 1971
Mouse (ICR/Ha 333 mg/kg - Epstein & Shafner,
Swiss) 1968
Mouse (ICR/Ha 333 & 666 mg/kg - Epstein et al.,
Swiss) 1972
DNA repair B. subtilis 2.5×10-3 mg/ - Sekizawa &
H17, M45 disc Shibamoto, 1982
B. subtilis Not given - Suter & Jaeger,
H17, M45 1982
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
E. coli Not given + Suter & Jaeger,
AB1157/JC5547 1982
AB1157/JC2921
AB1157/JC2926
AB1157/JC5517
E. coli WP2 Mitchell et al.,
uvrA+recA+,uvrA- Not given - 1980
recA-
trp-/trp+ Not given -
A2Cs/A2Cr 3-48 µg/ml +
E. coli B/r 100 µg/ml - Masek, 1977
E. coli K12 > 30 µg/ml - Mamber et al.,
(SOS chromotest) 1986
Preferential E. coli K12 5-20 µg/ - Hyman et al.,
inhibition plate 1980
E. coli 30 µg/plate - Brem et al.,
Pol A+, Pol A- 1974
E. coli 10 µg/plate - McCoy et al.,
Pol A+, Pol A1- 1980a,b
E. coli 30 µg/plate - Longnecker et al.,
Pol A+, Pol A- 1974
E. coli 30 µg/disc - Slater et al.,
Pol A+, Pol A- 1971
Gene conversion S. cerevisiae Not given - Mitchell et al.,
D4 1980
Sister chromatid Human lymphocytes 200 µg - Pant et al., 1976
exchange (in vitro)
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Chromosomal Zea mays 30 µg/ml - Verma & Lin, 1978
aberrations
Human lymphocytes 10-40 µg/ml + Mitus & Coleman,
(in vitro) 1970
Human lymphocytes Not given + Goh, 1979
(in vitro)
Human lymphocytes 200 µg + Pant et al., 1976
Mouse, bone 50 mg/kg b.w. + Manna & Bardham,
marrow 3×50 mg/kg + 1977
b.w., 8h
intervals
Mouse, F1 liverb 50 mg/kg b.w. + Manna & Roy, 1979
Micronucleus Tradescantia 0.1 - 5mM - Ma et al., 1984
test paludosa
Mouse Not given (5 - Heddle & Bruce,
(CH3×C57)F1 daily doses) 1977
DNA binding E. coli 100-1000 µM - Kubinski et al.,
assay 1981
Enhancement of Syrian hamster 0.7-5mM + Hatch et al.,
SA7 virus cell embryo cells/ 1986
transformation simian adenovirus
SA7
Aneuploidy Hordeum vulgare 300 µg/ml + Yoshida et al.,
induction 1972
Colicine S. typhimurium 0.1-60 µg/ - Ben-Gurion, 1978
induction TA1537 REN plate
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Increased Snail Not given - Xie, 1985
embryonal length
Induction of S. typhimurium 0.155-3.1 µM - Pall & Hunter,
tandem genetic TR4179, TT1984 1985
duplication
a Very weak positive response.
b One male mouse was given 50 mg/kg chloramphenicol i.m. and then was mated
with 4 untreated females. Mice (3) were sacrificed on days 12, 16, and 18
of gestation. The remaining mouse was allowed to litter normally and the
young were sacrificed when 7 days old. Livers of fetuses and neonates were
removed and examined.
When groups of 3 - 9 female Sprague-Dawley rats were given
80 mg/kg b.w. chloramphenicol in the drinking water for 10 days with
or without exposure to short duration high intensity noise,
ototoxicity was noted as revealed by reductions in the electrical
output of the cochlea. Noise exposure alone also reduced the output,
but noise and chloramphenicol together resulted in a severe effect
(Henley et al., 1984). Similar effects were reported in abstract
form in another study in rats (Henley, 1985).
Chloramphenicol was given to guinea pigs (number unspecified) as
a single i.v. dose of 400 mg/kg b.w. The threshold for the Preyer
reflex was measured after administration and at 10, 20, and 30
minutes, 1, 2, 3, 4, and 5 hours, and then daily for 7 days. There was
no change in the Preyer reflex with noise of 1 and 8 KHz and no loss
of hair cells, indicating no ototoxic response in this study
(Beaugard et al., 1979; Beaugard et al., 1981).
Special study on effects on sleep
Oral doses of 160 250 mg/kg b.w. chloramphenicol suppressed
paradoxical sleep in cats. After a dose of 330 mg/kg b.w. paradoxical
sleep was suppressed for 24 hours, at which time there was also a
depression of slow wave sleep (Petitjean et al., 1975).
Special study on spermatogenesis
A group of male rats was treated daily with 100 mg/kg b.w.
chloramphenicol succinate for 8 days (route and animal numbers not
stated), after which they were sacrificed and the testes examined
histologically. Examination revealed total or incomplete inhibition of
spermatogonial divisions with "perturbed meiosis". No other details
were provided (Timmermans, 1974).
Special studies on teratogenicity
Monkeys
Chloramphenicol at doses of up to 200 mg per monkey
(10 mg/kg b.w.) had no effect on the development of Macaca mulatta
when given for 6 to 17 days at various intervals between the 65th and
95th days of gestation (Courtney et al., 1967; Courtney & Valerio,
1974).
Rabbits
Groups of 5 - 8 pregnant mixed breed rabbits were given 500,
1000, or 2000 mg/kg b.w./day chloramphenicol by garage on days 6 - 15,
6 - 9, or 8 -11 of gestation, respectively. Historical control data
collected over the previous 4 year period, using 192 rabbits, were
used for comparison. Excess fetal deaths did not occur in rabbits
given 500 mg/kg b.w./day chloramphenicol, but in the mid- and
high-dose groups 25% and 58% fetal deaths were noted, respectively,
compared with 10% in the historical controls. There were no excess
incidences of fetal malformations, but delayed ossification was noted
in fetuses from dams given chloramphenicol (Fritz & Hess, 1971).
Chicks
Chick eggs, less than 3 days old, were treated with 0.1 ml of
chloramphenicol solution in distilled water. The chloramphenicol, at
doses of 0.5 or 1.0 mg/egg, was injected into the albumen via the
airsac. The major anomaly observed was vesiculation of the heart and
trunk resulting from the inhibition of differentiation of the
splanchnopleure; this effect was most severe following 16 - 19 hours
of incubation (36 - 57% in the 0.5 mg/egg group and 23 - 47% in the
1.0 mg/egg group). Unfortunately, no control data were provided
(Blackwell, 1962).
Chloramphenicol also had adverse effects on development in a
separate study in which fertilized eggs with embryos at the 14- or
20-somite stages were explanted and exposed to chloramphenicol at
concentrations of 0, 200, or 300 µg/ml for 22 - 24 hours. The major
defects noted were those of the neural tube (failure to close) and
forebrain. There was also evidence of an inhibition of haemoglobin
formation (Billet et al., 1965).
Rats
Pregnant Sprague-Dawley rats (5 - 15 per group) were given gavage
doses of 500, 1000, 1500, or 2000 mg/kg b.w./day chloramphenicol over
various stages of gestation. In addition, single gavage doses of
2000 mg/kg b.w. were given to pregnant rats on days 5, 6, 7, 8, 9, or
10 of gestation. A group of 553 historical control rats was used for
comparison. Even the lowest daily dose of chloramphenicol on days 5 -
15 of gestation resulted in significant embryo/fetal deaths (63%) when
compared with historical controls (23%), whereas doses of 2000 mg/kg
b.w./day on days 15 - 17 or 2000 mg/kg b.w. on days 5, 6, or 7 had no
effect. Single doses of 2000 mg/kg b.w. on days 8, 9, or 10 of
gestation resulted in 45% fetolethality, but the most sensitive period
appeared to be days 9 - 15. For example, fetal deaths occurred 100% of
the time when 2000 mg/kg b.w./day was given over days 9 - 11 of
gestation. The highest incidences of anomalies, umbilical hernia, were
noted at 2000 mg/kg b.w./day when given over days 6 - 8 of gestation
(36%) and at 2000 mg/kg b.w. on day 8 (11%). High incidences of
delayed ossification were seen in fetuses from dams given 1000 mg/kg
b.w./day chloramphenicol on days 7 - 12 or 2000 mg/kg b.w./day on days
11 - 13 (Fritz & Hess, 1971).
The effects of chloramphenicol on pre-implantation embryos in the
rat were investigated by treating groups of 7 pregnant Sprague-Dawley
rats with 250 mg/kg b.w. chloramphenicol i.p. on either day 3 or 5 of
gestation. The animals were killed on day 5 of pregnancy and the
uterine contents examined. No effects on the number of blastocysts per
female were noted, but administration on day 3 of pregnancy
significantly reduced the number of cells per blastocyst. A reduction
was also seen when the compound was given on day 14, but these results
were not statistically significant (Giavini et al., 1979).
Pregnant Sprague-Dawley rats (number unspecified) were given 3%
dietary chloramphenicol, equivalent to 1500 mg/kg b.w./day, from days
0 to 20. The number of resorptions (% of total implants) was elevated
in rats treated with chloramphenicol (31.4 - 57.0%) compared with
controls (4.7%), while fetal weight in treated rats was only 50% of
controls. Placental weight was also much reduced, while the number of
live fetuses was greatly reduced by chloramphenicol. The authors then
examined the effects of chloramphenicol when given on specific days of
pregnancy (0 - 2, 0 - 3, 0 - 4, etc., up to 0 - 12). The major effects
on implantations, resorptions, number of live fetuses, and fetal
weights as described above were seen in the 0 - 8 to the 0 - 12
feeding schedules, suggesting an effect on implantation (or effects on
embryos soon after implantation). A large proportion of fetuses (71%)
had edema and there was an increased incidence of wavy ribs (7%) and
fused ribs (7%) in chloramphenicol-treated groups compared with
controls (zero incidences in all cases in controls) (Hackler et al.,
1975).
Chloramphenicol was investigated for its effects on avoidance
learning in rats. Four groups of 15 pregnant Wistar rats each were
treated as follows: in one group 50 mg/kg b.w. chloramphenicol
succinate was given s.c. on days 7 - 21 of gestation. In two other
groups, 50 or 100 mg/kg b.w. was given s.c. to pups for the first 3
days after birth; the fourth group served as controls. No effects on
pregnancy, litter size, fetal weight, post-natal weight gain, or
incidence of gross malformations were seen. When 60 days old, animals
were selected for conditioned-learning study and were then examined
for avoidance learning at days 5, 10, 15, and 20 from the start of the
conditioning procedure. Pups from mothers given chloramphenicol
succinate and those given the substance as neonates showed a marked
and statistically significant impairment of avoidance learning at all
four times. The effects were generally worse in pups given the
substance post-natally than in those from dams administered it during
gestation, but the differences were only slight (Bertolini & Poggioli,
1981).
Mice
Groups of 8 pregnant albino mice were given oral chloramphenicol
at doses of 25, 50, 100, or 200 mg/kg b.w. in 10 ml of distilled water
over the third trimester of pregnancy for 5 - 7 days. Animals were
allowed to give birth and the young were tested for conditioned
avoidance response, electroshock seizure threshold, and performance in
open-field tests at days 30, 38, and 42. No gross abnormalities were
seen in any of the progeny. Dose-related effects were seen in all
three elements of the test, with progeny from chloramphenicol-treated
dams showing a reduced learning ability, higher brain seizure
threshold, and poorer performance in the open-field test (Al Hachin &
Al-Baker, 1974).
Groups of 7 - 19 pregnant CD1 mice were given by garage 500,
1000, or 2000 mg/kg b.w./day chloramphenicol on days 5 - 15, 6 - 12,
or 8 - 10 of gestation, respectively. Historical control data,
collected over the previous four-year period using 307 mice, were used
for comparison. In the 1000 and 2000 mg/kg b.w./day dose groups, 71
and 100% embryo/fetal deaths occurred, respectively, compared with 24%
in controls and 31% in mice given 500 mg/kg b.w./day chloramphenicol.
The only defects observed were a low incidence of fused sternebrae and
elevated incidences of delayed ossification in fetuses from dams given
1000 mg/kg b.w./day chloramphenicol (Fritz & Hess, 1971).
In vitro
Chloramphenicol and several other chemicals were tested in a
mouse embryo limb bud cell culture test system which itself was being
validated as part of the study. In all, 22 known mouse teratogens and
5 non-teratogens were investigated in the system, which makes use of
high-density cultures of mouse embryo limb bud cells that can
differentiate and synthesize an extracellular matrix of sulfated
proteoglycans. The end-point of the test considers incorporation of
radiolabel (3H-thymidine) and the growth and synthesis of ocular
protein and cartilage proteoglycan. Chloramphenicol gave a positive
result in this test, with the maximum active concentration being
5 µg/ml. [The test was around 89% predictive; no false positives were
seen and the false negative rate was about 15% (Guntakatta et al.,
1984).]
Another in vitro test made use of the differentiation
characteristics of rat embryo midbrain and limb bud cells, and the
inhibition of differentiation by teratogens. Chloramphenicol gave a
weak response, resulting in inhibition at concentrations in excess of
50 µg/l, compared with 10 µg/l or less seen with several known
teratogens, e.g., captan, colchicine, and parbendazole (Flint & Orton,
1984).
Acute toxicity
Four groups of pregnant and non-pregnant mice were given i.v.
doses (unspecified) of chloramphenicol. No signs of toxicity were
reported. The LD50 value for non-pregnant mice was calculated as
1530 (1260 - 1840) mg/kg b.w., while that for pregnant mice was
1210 mg/kg b.w. (no confidence limits cited) (Beliles, 1972).
Short-term studies
No information available.
Long-term studies
See "Special studies on carcinogenicity".
Observations in human beings
Chloramphenicol is known to produce two major adverse effects in
human beings. One of these is a generally irreversible and often fatal
aplastic anaemia and the other is a reversible bone marrow
suppression.
Aplastic anaemia
Aplastic anaemia is the most dangerous effect produced by
chloramphenicol, although its occurrence is rare. It is usually fatal
(Benestad, 1979). Numerous publications have appeared, most of them
case reports describing the development of aplastic anaemia.
An investigation into the incidence of chloramphenicol-induced
aplastic anaemia in Hamburg suggested that the incidence was 1/11500
with a death rate 1/18500. In the period 1965 - 70, 29 cases were
reported, while in 1971, 3 cases were reported. Total doses in 18
cases were in the range of 10 to 100 g, with most individuals having
received 11 - 30 g. Onset was from 14 days (rare) to 4 - 6 months
after chloramphenicol therapy (Hausmann & Skrandies, 1974).
In a study in Israel in 1985, aplastic anaemia incidence was
7.1/106 in males and 8.7/106 in females. Chloramphenicol was
thought to account for up to 25% of cases. Aplastic anaemia usually
took up to 12 months to develop after chloramphenicol treatment
(Modan et al., 1975).
In 1969 in California, aplastic anaemia in chloramphenicol-
treated patients was said to be 13 times more frequent than in
the general population. Most individuals had been given oral
doses, but in some, aplastic anaemia had occurred after i.m.
administration. Doses were often on the order of 250 mg thrice daily
to a total of 3 g or 250 mg four times daily to a total of 5 g. The
majority of patients were in the 50 - 80 age group, but it was
observed in a 15-year-old boy given a total of 3 g chloramphenicol and
in a 37-year-old female given a total of 6 g over a month. In both
cases onset occurred 3 - 4 months after treatment ended
(Wallerstein et al., 1969).
A series of reports from Sweden in the 1970s suggested that the
incidence of aplastic anaemia was around 80 in 1.2 million. Only 4 or
5 of these were thought to be due to chloramphenicol treatment,
placing the risk at 1 in 20000 (Bottiger & Westerholm, 1972;
Bottiger & Westerholm, 1973; Bottiger, 1978; Bottiger, 1979).
Of 108 cases of aplastic anaemia reported in Istanbul, 4 were
thought to be due to chloramphenicol (Aksoy et al., 1984).
A total of 380 cases of aplastic anaemia were seen at a hospital
in Paris in the years 1971 - 1983; 194 adult cases were considered to
be due to chemical agents. In the period 1971 - 1977, 18/104 cases
were attributed to chloramphenicol, whereas chloramphenicol accounted
for 2/36 and 2/52 cases of aplastic anaemia in the periods 1977 - 1980
and 1980 - 1983, respectively, suggesting a decreasing incidence, at
least in the area of France under investigation (Najean & Baumelon,
1984).
In the period 1975 - 1980, 9 children with aplastic anaemia were
seen at the Medical School, Gadjah Mada University (Yogyakarta). Two
were idiopathic, but at least 3 could be attributed to treatment with
chloramphenicol (Widayat et al., 1983).
A study of 40 cases of aplastic anaemia by members of the
Association of Clinical Pathologists in the USA revealed that 27 were
probably due to chloramphenicol. Of these, 18 had exceeded 10 g or
more (up to 250 g) in total dosage, while 8 had received 10 g or less.
One, an infant, had received less than 2 g chloramphenicol. Onset was
usually 1 - 3 months after drug cessation. Route of administration and
its duration were not specified (Sharp, 1963).
In 1954, of 539 cases of aplastic anaemia from 37 states in the
USA, 55 were attributable to chloramphenicol. In general there was a
female preponderance and usually the effect developed 1 - 6 months
after the drug was withdrawn (Welch et al., 1954).
In a study in Iraq, however, a male preponderance of 3:1 over
females was noted. Of 60 patients with aplastic anaemia at the
University of Baghdad Teaching Hospital, 12 were associated with
chloramphenicol treatment (Al-Moudhiry, 1978).
One study in the Netherlands examined cases of blood dyscrasias
taken from the literature along with unpublished cases from adverse
drug reaction reporting systems in the United Kingdom, Denmark,
Sweden, and the Netherlands. To these were added cases from hospitals
in Northeast Switzerland. A total of 641 cases of chloramphenicol-
induced blood dyscrasias were identified. These included:
thrombocytopenia (21), agranulocytosis (51), hypoplastic anaemia (39),
bone marrow suppression (39), acute leukaemia (27), and aplastic
anaemia (464). Of the 464 cases of aplastic anaemia, 335 (72%) had
proved fatal. There appeared to be a female preponderance (261 cases;
56%). However, because this cannot be related to the total number
treated and therefore to those who did not acquire the condition, the
incidence cannot be determined (Meyler et al., 1974). The results
confirmed those of earlier work (Polak et al., 1972).
In Italy, it has been claimed that in view of the ease of
availability of chloramphenicol, the incidence of aplastic anaemia
appears to be low. For example, in 1971 there were 10 reports of fatal
side effects due to antibiotics; in 1972 there were 3. None were
attributable to chloramphenicol. During the period 1973 - 1975 there
were no reports of fatal cases (Preziosi et al., 1981).
For the period 1959 - 1969, 172 cases of aplastic anaemia were
reported in 15 hospitals in Northeast Switzerland, and 44 of these
individuals had been treated with chloramphenicol. The smallest total
dose incriminated was 3 g, while the highest was 315 g
(Keiser & Bucheggar, 1973).
In children aged 0 - 14 years in Denmark, the total number of
registered cases of aplastic anaemia was 39 for the years 1967 - 1982,
giving an annual incidence of 22/106. Probable causes were
identified for 21 of the 39 cases, and 2 of these were attributed to
chloramphenicol treatment (Clausen, 1986).
It was stated in 1983 that the probable overall incidence of
chloramphenicol-induced aplastic anaemia is somewhere between 1 in
20000 and 1 in 40000 (Venning, 1983).
In Japan, chloramphenicol appeared to be more dangerous to the
elderly population than to other groups. However, the investigation
was probably biased in that only fatal cases were examined
(Mizuno et al., 1982). Similar findings were made in an earlier
study (Shimizu et al., 1979).
For the years 1961 - 1965, 35 cases of aplastic anaemia were
reported in Colombia, and of these 10 had had past exposure to
chloramphenicol. Several others were said to have had a "strong
suspicion" of exposure to the drug. Mortality was 60% (Sarasti, 1970).
An age preponderance was noted in an analysis of 21 patients with
aplastic anaemia, 8 of whom had been treated with chloramphenicol
(Perez et al., 1981).
The minimum dose of chloramphenicol associated with the
development of aplastic anaemia is not known with certainty. The
literature citations often state only the total dose. Where cases
developed after several doses, it is not possible to say if aplastic
anaemia would also have occurred had only a single dose been given. Of
15 cases reviewed in a report in 1974, total doses of 4.5 to 80 g had
been given, with the usual levels being 8 to 14 g. As an example, a
46-year-old woman was given 15 g of chloramphenicol over 10 days.
Aplastic anaemia developed after 2 months (Hellriegel & Gross, 1974).
Total doses of 6.5 to 60 g chloramphenicol had been given to 7
individuals with aplastic anaemia reported in 1971 (Hodgkinson, 1971).
A 27-year-old woman given 30 g chloramphenicol i.v. over 12 days
developed aplastic anaemia 3 months later (Alavi, 1983).
One individual with aplastic anaemia had undergone previous
treatment 19 years earlier with a 500 mg initial dose followed by
250 mg chloramphenicol 4 times daily. At the age of 23 years, he was
given 750 mg chloramphenicol succinate (62 mg/kg b.w./day) i.v. every
6 hours for 12 days for a brain abscess. Bone marrow suppression
ensued and by the twelfth day of drug administration aplastic anaemia
had developed. The patient eventually died (Daum et al., 1979).
A 26-year-old woman was diagnosed as being anaemic during the
fifth month of pregnancy and in the sixth month she developed a skin
infection. She was given 8 g chloramphenicol. She developed aplastic
anaemia and died 8 days after giving birth. Bone marrow aspiration
confirmed aplastic anaemia. (Suda et al., 1978).
Several cases of aplastic anaemia have been reported in children.
One 7-year-old boy had been given chloramphenicol palmitate in June
and August of 1959. The dose or route of administration were not
specified. He developed aplastic anaemia 4 - 5 months later and died
(Leiken et al., 1961). A 6-year-old girl was given 25 mg/kg b.w./day
chloramphenicol for 10 days by an unspecified route. She developed
aplastic anaemia, apparently immediately after treatment and then
acute myeloblastic leukaemia (Awaad et al., 1975). A 4-year-old girl
was given oral chloramphenicol (dose not stated) for 1 week. After 2
months she developed aplastic anaemia (Young et al., 1979). Another
case of a 4-year-old girl given chloramphenicol was reported more
recently. Chloramphenicol was given i.v. for 1 week followed by oral
therapy for 8 weeks. The initial dose was 75 mg/kg b.w./day given
every 6 hours, but this was adjusted after 3 weeks to 37 mg/kg
b.w./day. Some months later she developed aplastic anaemia
(Lepow, 1986). In one recent case the patient was a neonate born at 30
weeks gestation weighing 1.7 kg. At day 20 after birth she developed
suspected meningitis and was given 4 doses of 50 mg/kg b.w./day
chloramphenicol. She developed aplastic anaemia; epidermal necrolysis
of the skin and biliary cholestasis were found at necropsy
(White et al., 1986).
A 72-year-old male patient was given a 3-week course of
chloramphenicol (dose and route unspecified) and aplastic anaemia
ensued within 4 months (Howell et al., 1975).
Aplastic anaemia is usually associated with oral intake
(Matthews et al., 1980). It has been reported that in 149 cases of
chloramphenicol-induced aplastic anaemia, 85% followed oral dosing,
14% followed parental administration, and 3% occurred after rectal
administration (Plaut & Best, 1982).
Topical administration of chloramphenicol has been followed by
aplastic anaemia. In one case, a 73-year-old woman died of aplastic
anaemia less than 2 months after beginning ophthalmic chloramphenicol
treatment with a 0.5% solution (3 - 4 times daily) and a 1% ointment
(once per day to the right eye) (Fraunfelder & Bagby, 1982). Several
other similar cases have been reported (Abrams et al., 1980;
Rosenthal & Blackman, 1965; Carpenter, 1975; Issaragrisil &
Piankijagum, 1985; Korting & Kifle, 1985). In one case the total dose
was estimated to be 32 mg of chloramphenicol (Carpenter, 1975).
One occupational case probably involved inhalation and skin
contact. It occurred in a shepherd applying a chloramphenicol spray to
the feet of sheep for treatment of foot rot. He had treated the
animals twice a week with the spray, which contained 10 g of the drug
in 100 ml of solution, for two years. Each dose contained 10 mg
(Del Giacco et al., 1981).
A link between the administration of chloramphenicol and the
development of liver disease and aplastic anaemia is known to exist.
After five patients aged 4 to 63 years were given chloramphenicol,
liver disease (as evidenced by jaundice, icterus, and elevated serum
enzymes and bilirubin) was subsequently noted. Aplastic anaemia
eventually developed (Hodgkinson, 1973). A similar case has been
reported in a 15-year-old boy given 250 mg chloramphenicol i.v. every
6 hours. Liver enzymes became elevated after 18 days and the liver
itself was tender. Aplastic anaemia developed (Caslae et al., 1982).
The mechanism of chloramphenicol-induced aplastic anaemia is not
understood. There may be a genetic element involved, as the effect has
been seen in families and in identical twins exposed to chloramphenicol
(Flach, 1982; Nagao & Maner, 1969; Silver & Zuckerman, 1980; Yunis,
1978c). The main target may be the haemopoietic pluripotential stem
cells of the marrow (Vincent, 1986; Silver & Zuckerman, 1980;
Benestad, 1974; Appelbaum & Fefer, 1981). There may also be the
possibility of initial damage to the marrow micro-environment (Camitta
et al., 1982). Failure to note this effect in human beings with
drug-induced aplastic anaemia makes the latter unlikely (Samson
et al., 1972; Vincent, 1986). In vitro studies with human bone
marrow suggest that both the erythroid and granulocytic series are
sensitive to chloramphenicol (Nara et al., 1982; Hara et al.,
1978; Yunis, 1977). The erythroid series in particular seemed sensitive
to chloramphenicol, with inhibition occurring at 10 mg/l compared with
50 mg/l for the granulocytic series (Yunis, 1977).
The problem as to whether bone marrow cells are more sensitive to
chloramphenicol in patients with aplastic anaemia induced by the drug
is not clear, as reports on the subject are conflicting (Yunis et al.,
1973a; Howell et al., 1975; Kern et al., 1975). It has been claimed,
based on the results of animal studies, that individuals who are
sensitive to chloramphenicol-induced aplastic anaemia may have
residual bone marrow damage brought about by exposure to other agents
(Morley et al., 1976).
The metabolite or metabolites responsible for the induction of
aplastic anaemia in human beings is unknown, but nitroso-
chloramphenicol has been implicated (Murray & Yunis, 1981; Nagai &
Kanamuru, 1978). Nitrosochloramphenicol can be formed by the
reduction of chloramphenicol in human liver in vitro (Salem et al.,
1981). This substance is known to be toxic to human bone marrow cells
in vitro and, moreover, is more toxic than chloramphenicol itself
(Yunis et al., 1980a, b). However, nitrosochloramphenicol is not
myelotoxic to mice in vivo (Krishna et al., 1981). It does,
however, cause DNA strand breakage in vitro, and inhibit DNA
synthesis (Gross et al., 1982; Skolimowski et al., 1983). Both
chloramphenicol and nitrosochloramphenicol are taken up rapidly by
cells, at least as demonstrated by a human transformed lymphoblastoid
cell line (Raji cells), but the nitroso-compound covalently binds to
these cells and to bone marrow cells 15 times more tightly than does
chloramphenicol (Hurray & Yunis, 1981). Photochemical decomposition of
chloramphenicol may result in potentially myeloblastic derivatives,
which may be hazardous in ophthalmic solutions (de Vries et al.,
1984). Another possible mechanism involves the immune system (and even
autoimmune damage), but there are no convincing data to support this
hypothesis. Chloramphenicol in vitro inhibited lymphocyte
transformation in human material (Burgio et al., 1974) and
chloramphenicol reduction products, including nitrosochloramphenicol,
were suppressive to antigen-reactive cells in mice (Pazdernik &
Corbett, 1980).
In summary, chloramphenicol induces aplastic anaemia in
susceptible individuals, but no dose-response relationship has been
identified. The mechanism may involve nitrosochloramphenicol, but this
has not been proven. The nature of the mechanism is unknown.
Bone marrow suppression
Reversible bone marrow suppression has been reported in patients
given chloramphenicol by a number of routes. The effect is thought to
be an unlikely event at plasma levels under 20 mg/l, and it is said to
occur in most patients at levels in excess of 25 mg/l. Generally, it
occurs within days of administration (Lery et al., 1978; Benestad,
1979). One study showed that oral doses likely to result in
suppression were usually of the order of 2 - 3 g/day or 30 - 50 mg/kg
b.w./day; durations of dosing were generally 1 - 17 days, most being 5
- 10 days. Plasma levels of chloramphenicol at 2 - 3 hours and 6 - 8
hours varied, most being in excess of 25 mg/l at 2 - 3 hours and in
excess of 30 mg/l at 6 - 8 hours. However, some were below 15 mg/l at
both times. Of the 17 patients studied, 11 had liver disease, while 3
of the remainder had renal disease. Bone marrow suppression in a
female of 21 years of age was attributed to the administration of
1.5 g/day of chloramphenicol for 18 days. The condition resolved on
cessation of therapy (Parashar et al., 1972). In 6 anaemic subjects
given up to 60 mg/kg b.w./day chloramphenicol, reticulocyte response
to vitamin B12 or iron dextran was halted and delayed (Saidi
et al., 1961).
Maturation arrest and cytoplasmic valuation of erythroid elements
were seen in the marrow (Rosenbach et al., 1960; Bartlett, 1982).
One reported case of bone marrow suppression was in a
three-year-old girl with cystic fibrosis given 70 mg/kg b.w./day
chloramphenicol for 2.5 months. The suppression was accompanied by
physical growth depression and hair loss. All these conditions
resolved on cessation of treatment (Kapp et al., 1977).
The toxic effects of chloramphenicol on the bone marrow were
lessened in one group of patients by co-administration of
phenylalanine (Ingall et al., 1965). The reason for this, and its
relationship to the pathogenesis of bone marrow suppression, is
unknown. The mechanism of suppression is thought to involve inhibition
of mitrochondrial protein biosynthesis in bone marrow cells. In vitro
studies showed that 10 mg/l chloramphenicol severely inhibited protein
synthesis in bone marrow cells, although ten times this concentration
was required to inhibit mitochondrial respiratory functions (Yunis,
1973a, b; Yunis, 1978a, b; Martelo et al., 1969). As a result of
mitochondrial dysfunction, ferrochelatase activity is suppressed in
erythroid precursors. In vitro studies show inhibition of haem
synthesis with concentrations of chloramphenicol of 10 mg/l
(Becker et al., 1974). It seems likely that the major effect of
chloramphenicol in the pathogenesis of reversible bone marrow
suppression is a block on hame biosynthesis arising as a result of
mitochondrial dysfunction (Yunis & Salem, 1980). Consequently,
co-administration of phenylalaninine may in some way compensate for
the inhibitory effects of chloramphenicol on protein synthesis.
Effects on platelets
In in vitro experiments, chloramphenicol was shown to decrease
human platelet aggregation. Unfortunately, no in vivo studies are
available (Cronber et al., 1984, Djaldetti, 1983; Agam et al.,
1976).
Carcinogenicity
IARC concluded in 1982 and 1987 that there was limited evidence
for the carcinogenicity of chloramphenicol to human beings based on
studies describing cases of leukaemia (IARC, 1982; IARC, 1987).
One study described a 24-year-old male given chloramphenicol for
typhoid fever. Leukaemia followed aplastic anaemia. A chromosome
translocation (t 1:7) was discovered on karyotyping. No other details
were provided, except that similar translocations were noted in 6
other leukaemia patients known to have had exposure to leukaemogenic
substances or radiation (Scheres et al., 1985).
Acute myeloid leukaemia developed in a 6-year-old girl who
developed aplastic anaemia after being given 25 mg/kg b.w./day
chloramphenicol for 10 days. The leukaemia developed 6 months after
the aplastic anaemia (Awaad et al., 1975).
Leukaemia developed in a 38-year-old woman given a total of 8 g
of chloramphenicol 8 years before which resulted in aplastic anaemia.
A 57-year-old man who had taken chloramphenicol for 8 years (about
175 g total dose) developed aplastic anaemia followed by leukaemia
within a year, while a 61-year-old man who was treated with
chloramphenicol developed aplastic anaemia and leukaemia (Brauer &
Dameshek, 1967).
An 80-year-old man with a bacterial infection of the feet was
given orally 250 mg/day chloramphenicol, for 7 days. He developed
acute leukaemia 5 months later. The only other drugs given for his
infection were penicillin and a topical zinc ointment (Humphries,
1968).
A 14-year-old girl given 250 mg chloramphenicol for 4.5 days
developed aplastic anaemia after approximately 1 year followed by
acute leukaemia 18 months later (Seaman, 1969).
Aplastic anaemia and leukaemia may occur together at diagnosis. A
28-year-old man developed reversible bone marrow depression after
being given approximately 31 g of chloramphenicol. Five years later he
was found to have aplastic anaemia and acute leukaemia (Schmitt-Graft,
1981). Other similar cases have been reported (Adamson & Seiber, 1981;
Forni & Vigliani, 1974; Meyer & Boxer, 1973).
Of 641 cases of chloramphenicol-induced blood dyscrasias, 464
cases of aplastic anaemia and 27 cases of leukaemia were identified
(Meyler et al., 1974).
From 151 cases of blood dyscrasias induced by drugs, 3 cases of
leukaemia attributable to chloramphenicol were noted (Fraumeni, 1967).
The majority of cases of leukaemia associated with chlo-
ramphenicol therapy were acute myeloid leukaemia (Godner et al.,
1973).
The role of chloramphenicol in leukaemogenesis is not known. Some
studies have revealed abnormal karyotypes in affected patients
(Scheres et al., 1985; Goh, 1971; Cohen & Huang, 1973), but it is
not known if these abnormalities were induced directly by
chloramphenicol or were merely a feature of the disease. Aplastic
anaemia, whether induced by chemicals or idiopathic, is known to be
followed by leukaemia in some cases (Milner & Geary, 1979).
Cytogenetic abnormalities are common. Fanconi's anaemia, for example,
is often followed by leukaemia and cytogenetic abnormalities are seen
in this state. Chloramphenicol is known to induce cytogenetic
abnormalities in in vitro test systems, but it is not known if those
seen in leukaemia occurring after chloramphenicol-induced aplastic
anaemia are due to the same effects.
Cardiovascular effects
Chloramphenicol is known to induce a state often referred to as
the "Grey Baby Syndrome" or "Grey Syndrome". In general, this is a
state of cardiovascular collapse that commences on days 2 to 9
following the beginning of chloramphenicol administration. The main
features include failure to feed, vomiting, abdominal distension,
cyanosis, flaccidity, shock, and a fall in body temperature. Death is
said to occur in 60% of cases, and the syndrome generally occurs where
the dose of chloramphenicol exceeds 25 mg/kg b.w./day (Meyler &
Herkeimer, 1968). Metabolic acidosis may be a presenting feature, and
plasma levels of chloramphenicol in excess of 30 µg/ml are usually
associated with the development of the syndrome (Evans & Kleiman,
1986; Mulhall et al., 1983; Craft et al., 1974). In several cases
the doses producing the syndrome were 50 mg/kg b.w./day (Fripp
et al., 1983; Kraskinski et al., 1982; Biancaniello et al.,
1981; Haile, 1977) and in one case a daily dose of 100 mg/kg b.w. was
given (Stevens et al., 1981). A grey-type syndrome has been reported
in a 16-year-old girl given 1 g chloramphenicol i.v. every 6 hours
(95 mg/kg b.w./day) for what was thought to be Rocky Mountain spotted
fever. This dose was later changed to 3.75 g/day i.v. The syndrome
began to develop after the second day of this regime, but it resolved
with drug withdrawal and supportive treatment (Brown, 1982). The
mechanism is unknown, but experiments with isolated pig hearts suggest
that effects on mitochondria may be important (Werner et al., 1985).
Allergenic contact dermatitis
Allergenic contact dermititis has been reported in case report
following the use of chloramphenicol topical preparation (Rudzki et al.,
1976; Schewach-Millet & Shapiro, 1985; Van Joost et al.,1986;
Strick, 1983; Fraki et al., 1985).
Two cases of occupational dermatitis in oculists were attributed
to the use of chloramphenicol-containing preparations (Rebandel &
Rudzki, 1986). In one study of 330 patients with contact allergy, 10%
showed positive patch tests to chloramphenicol, while in another
investigation of 620 patients, 1.7% save positive reactions
(Blondeel et al., 1978; Rudzki & Kelniewska, 1970). Others have
reported similar findings (Forck, 1971).
Ocular toxicity
Several cases of ocular toxicity following prolonged treatment
with chloramphenicol have been reported. This has often been described
as an optic neuritis with scotomata and failing vision. Retrobulbar
neuritis may be observed. It has been seen in cases of cystic
fibrosis, although this may reflect the choice of chloramphenicol as a
treatment in this condition rather than a specific sensitivity in this
group. Total doses are often in the 80 - 250 g range given over
several months. One patient developed bilateral optic neuritis after
being given 6 g chloramphenicol per day for 6 weeks (approximately
250 g total). Peripheral neuritis may accompany the ocular effects
(Wilson, 1962; Steidl, 1965; Malbrel et al., 1977; Joy et al.,
1960; Lasky et al., 1953; Charache et al., 1977; Chang et al.,
1966; Cocke et al., 1966; Godel et al., 1980; Wallenstein &
Snyder, 1952; Huang et al., 1966; Walker, 1961; Fraunfelder & Meyer,
1984).
Ototoxicity
Deafness following chloramphenicol therapy has occasionally been
noted, although the reports have been complicated by administration of
other potentially ototoxic drugs. In one case, a 2.5-year-old boy was
given 125 mg/kg b.w./day chloramphenicol for 26 days with no other
drug treatment. He developed deafness, which persisted beyond
cessation of drug administration (Gargye & Dutta, 1959; Ajodhia & Dix,
1976; Nilges & Norther, 1971; Jones & Hanson, 1977).
Effects on testes
A 61-year-old man with pneumonia was treated with ampicillin and
chloramphenicol. He later developed aplastic anaemia after 12 days and
died of widespread sepsis 10 days after drug withdrawal. Necropsy
revealed loss of germinal epithelium, with only Sertoli cells
remaining. The testes were of normal size. As the patient was
unmarried and also exposed to ampicillin, the authors concluded that
there was no direct proof of chloramphenicol-induced testicular
toxicity. However, in view of the normal gross appearance of the
testes and the low toxicity of ampicillin, chloramphenicol seems the
most likely cause of the effects noted in this case. No dose levels
were quoted (Sheehan & Sweeny, 1982).
Teratogenicity
Chloramphenicol and/or tetracyclines have been implicated in the
genesis of cleft-lip in a study of 599 affected children. However,
because of the nature of the epidemiological study, any effects of
tetracyclines cannot be separated from those of chloramphenicol and it
cannot be stated with any certainty that chloramphenicol is a human
teratogen (Saxen, 1975).
COMMENTS
Human exposure to chloramphenicol can give rise to aplastic
anaemia, a rare but often fatal condition. The Committee concluded
that no dose-response relationship could be established for this
effect. The mechanism for the pathogenesis of aplastic anaemia is
unknown, and no suitable animal model exists.
EVALUATION
Because a no-effect level for aplastic anaemia could not be
established, and therefore it was not possible to give an assurance
that residues in foods of animal origin would be safe for sensitive
subjects, an ADI could not be allocated for chloramphenicol.
REFERENCES
Abou-Khalil, S., Salem, Z., & Yunis, A.A. (1980). Mitochondrial
metabolism in normal, myeloid, and erythroid hyperplastic rabbit
bone-marrow: Effect of chloramphenicol. Am. J. Haematol., 8, 71-79.
Abou-Khalil, S., Salem, Z., Abou-Khalil, W.H., & Yunis, A.A. (1981).
On the mechanism of erythroid cell sensitivity to chloramphenicol:
Studies on mitochondria isolated from erythroid and myeloid tumours.
Arch. Biochem. Biophys., 206, 242-248.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1982). Inhibition
by nitrosochloramphenicol of the proton translocation in mitochondria.
Biochem. Pharmacol., 31, 3823-3830.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1985).
Identification of a mitochondrial rho-dinitrobenzene reductase
activity in rat liver. Pharmacol., 31, 301-308.
Abrams, S.M., Degnan, T.J., & Vinciguerra, V. (1980). Marrow aplasia
following topical application of chloramphenicol eye ointment.
Arch. Inter. Med., 140, 576-577.
Adamson, R.H. & Seiber, S.M. (1981). Chemically induced leukemia in
humans. Environ. Health Perspect., 39, 93-103.
Agam, G., Gasner, S., Bessler, H., Fishman, P., & Djaldetti, M.
(1976). Chloramphenicol induced inhibition of platelet protein
synthesis: In vitro and in vivo studies. Brit. J. Haematol.,
33, 53-59.
Ajodhia, J.M. & Dix, M.R. (1976). Drug-induced deafness and its
treatment. Practitioner, 216, 561-570.
Akhnoukh, S., El-Shazly, N., Sallaim, N., El-Melegy, S., El-Sewedy,
S.M., Mostafa, M.H., & El-Bassioni, E.A. (1982). In vivo effect of
chloramphenicol and thiamphenicol on some enzymes of normal mouse
liver. Biochem. Pharmacol., 31, 55-57.
Aksoy, M., Erdem, S., Dincol, G., Bakioglu, I., & Kutlar, A. (1984).
Aplastic anaemia due to chemicals and drugs: A study of 108 patients.
Sex Trans. Dis., 11, 347-350.
Alavi, J.B. (1983). Aplastic anaemia associated with intravenous
chloramphenicol. Am. J. Hematol., 15: 375-379.
Al-Hachim, G.M. & Al-Baker, A. (1974). The prenatal effect of
chloramphenicol on the postnatal development of mice.
Neuropharmacol., 13, 233-237.
Ali, B.H. (1985). Lack of effect of chloramphenicol on monoamine,
diamine and plasma amine oxidase activities. Clin. Exp. Pharmacol.
Physiol., 12, 1193-5.
Al-Moudhiry, H.A.B. (1978). Aplastic anaemia in Iraq. A prospective
study. Haematologia, 12, 159-164.
Amati, P. (1970). Chloramphenicol: Effect of DNA synthesis during
phage development in Escherichia coli. Science, 168, 1226-1228.
Ambrose, P.J. (1984). Clinical pharmacokinetics of chloramphenicol and
chloramphenicol succinate. Clin. Pharmacokinetics, 9, 222-238.
Applebaum, F.R. & Fefer, A. (1981). The pathogenesis of aplastic
anaemia. Sem. Haematol., 18, 241-257.
Appelgren, L.-E., Eberhardson, B., Martin, K., & Slanina, P. (1982).
The distribution and fate of 14C-chloramphenicol in the new-born
pig. Acta pharmacol. Toxicol., 51, 345-350.
Aravindaksham, C.M. & Cherian, Z. (1984). Effect of chloramphenicol on
thiopentone sodium anesthesia in dogs. Indian Vet. J., 61, 569-573.
Ascherl, M., Eyer, P., & Kaupffmeyer, H. (1985). Formation and
disposition of nitrosochloramphenicol in rat liver. Biochem.
Pharmacol., 34, 3755-3763.
Augustine, M.L., Poulsen, N.K., & Heinze, J.E. (1982). Evaluation of
the CHO/HGPRT cell mutation test using 12 compounds. Env. Mutagen.,
4, 389-390.
Awaad, S., Khalifa, A.S., & Kamel, K. (1975). Vacuolization of
leukocytes and bone-marrow aplasia due to chloramphenicol toxicity.
Clin. Pediatr., 14, 499-506.
Baltz, R.H., & Stonesifer, J. (1985). Adaptive response and
enhancement of N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis by
chloramphenicol in Streptomyces fradiae. J. Bacteriol.,
164, 944-946.
Banerjee, S. & Basu, P.S. (1978). Inhibition of rat tissue
mitrochondrial monoamine oxidase by chloramphenicol and its
intermediates. Indian J. Biochem. Biophys., 15, 479-482.
Bartlett, J.G. (1982). Chloramphenicol. Med. Clin. N. Amer.,
66, 91-102.
Baselt, R.C. (1982). Disposition of toxic drugs in man. 2nd Edition,
Davis, California, Biomedical Publications, 136-139.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1979). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Surg. Forum, 30, 512-514.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1981). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Arch. Otolaryngol., 107, 104-109.
Becker, D., Metz, J., & Katz, J. (1974). Comparative effects of
thiamphenicol and chloramphenicol on haemopoiesis and lymphocyte
transformation in vitro in man. Postgrad. Med. J.,
50, (Suppl. 5), 88-94.
Beliles, R.P. (1972). The influence of pregnancy on the acute toxicity
of various compounds in mice. Toxicol. Appl. Pharmacol., 23, 537-540.
Benes, L., Rotreklova, E., & Pospisil, V.V.M. (1980). Inhibition of
bone marrow cell proliferation and DNA replication induced by
chloramphenicol. Folia Biol., 26, 408-414.
Benestad, H.B. (1974). Aplastic anaemia: Considerations on the
pathogenesis. Acta med. Scand., 196, 255-262.
Benestad, H.B. (1979). Drug mechanism in marrow aplasia.
In: Geary, C.G., (ed.) Aplastic anaemia, London, Balliere Tindall,
pp. 26-42.
Bengmark, S., Goransson, G., & Soucas, E. (1981). Defects in
hemostasis produced by antibiotics. An in vivo study in the rat.
Eur. Surg. Res., 13, 290-298.
Ben-Gurion, R. (1978). A simple plate test for screening colicine-
inducing substances as a tool for the detection of potential
carcinogens. Mutat. Res., 54, 289-295.
Bertolini, A. & Poggioli, R. (1981). Chloramphenicol administration
during brain development: Impairment of avoidance learning in
adulthood. Science, 21, 238-239.
Biancaniello, T., Meyer, R.A., & Kaplan, S. (1981). Chloramphenicol
and cardiotoxicity. J. Pediatr., 98, 828-830.
Billet, F.S., Collini, R., & Hamilton, L. (1965). The effects of D-and
L-threo-chloramphenicol on the early development of the chick embryo.
J.Embryol. Exp. Morph., 13, 341-356.
Blackwell, U.B. (1962). The changing inhibition of early
differentiation and general development in the chick embryo by
2-ethyl-5-methyl-benzimidazole and chloramphenicol. J. Embryo. Exp.
Morph., 10, 315-337.
Blondeel, A., Oleffe, J., & Achten, G. (1978). Contact allergy in 330
dermatological patients. Contact Derm., 4, 270-276.
Bories, G.F., Peleran, J.C., Wal, J.M., & Corpet, D.E. (1983). Simple
and ion-pair high performance liquid chromatography as an improved
analytical tool for chloramphenicol metabolic profiling. Drug Met.
Disp., 11, 249-254.
Bottiger, L.E. & Westerholm, B. (1972). Aplastic anaemia. I. Incidence
and aetiology. Acta med. Scand., 192, 315-318.
Bottiger, L.E. & Westerholm, B. (1973). Drug-induced blood dyscrasias
in Sweden. Brit. Med. J., 3, 339-343.
Bottiger, L.E. (1978). Prevelance and etiology of aplastic anaemia in
Sweden. In: Hibino, S., Takaku, F., & Shahidi, N.T. (eds.) Aplastic
Anemia. Tokyo, University Park Press, pp. 171-180.
Bottiger, L.E. (1979). Epidemiology and aetiology of aplastic anaemia.
Haematol. Blut. Transfus., 24, 27-37.
Brabec, M.J., Owens, J.B., Kenel, M., Sorscher, D., & Cornish, H.H.
(1982). Modification of methanol potentiation of carbon tetrachloride
toxicity in rats by chloramphenicol and salicylate. Drug Chem.
Toxicol., 5, 143-154.
Brasfield, J.H., Record, K.E., Griffen, W.O., & Mattingly, S. (1983).
Chloramphenicol succinate levels in patients with renal impairment.
Clin. Pharmacol., 1, 355-358.
Brauer, M.J. & Dameshek, W. (1967). Hypoplastic anemia and
myeloblastic leukemia following chloramphenicol therapy. Report of
three cases. New Engl. J. Med., 277, 1003-1005.
Braun, R., Fischer, G.W., & Schoneich, J. (1977). The mutagenicity and
DNA-damaging activity of cyclic aliphatic sulfuric acid esters.
Chem-Biol. Interact., 19, 241-252.
Braun, R., Schoneich, J., Weissflog, L., & Dedek, W. (1982). Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair direct alkylation vs. metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem-Biol. Interact.,
39, 339-350.
Brem, H., Stein, A.B., & Rosenkranz, H.S. (1974). The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res., 34, 2576-2579.
Brown, R.T. (1982). Chloramphenicol toxicity in an adolescent.
J. Adol. Health Care, 3, 53-55.
Bueno, L., Fioramonti, J., Alvinerie, M., & Bardon, T. (1984).
Influence of bran on bioavailability of chloramphenicol administered
orally to pigs. Can. J. Physiol. Pharmacol., 62, 208-211.
Burckart, G.J., Barrett, F.F., Stranghn, A.B., & Ternullo, S.R.
(1982). Chloramphenicol clearance in infants. J. Clin. Pharmacol.,
22, 49-52.
Burckart, G.J., Barett, F.F., Valle, R.D., & Mayer, M.C. (1983).
Chloramphenicol damage and pharmacokinetics in infants and children.
J. Clin. Pharmacol,, 23, 106-112.
Burke, J.T., Wargin, W.A., & Blum, M.R. (1980). High-pressure liquid
chromatographic assay for chloramphenicol, chloramphenicol-3-
monosuccinate and chloramphenicol-l-monosuccinate. J. Pharm. Sci.,
69, 909-912.
Burrows, G.E., Tyler, R.D., Craigmill, A.L., & Barto, P.B. (1984).
Chloramphenicol and the neonatal calf. Am. J. Vet. Res.,
45, 1586-1591.
Camitta, B.M., Storb, R., & Thomas, E.D. (1982). Aplastic anaemia.
Pathogenesis, diagnosis, treatment and prognosis. New Engl. J. Med.,
306, 645-652.
Carpenter, G. (1975). Chloramphenicol eye-drops and marrow aplasia.
Lancet, ii, 326-327.
Casale, T.B., Macher, A.M., & Fauci, A.S. (1982). Complete hematologic
and hepatic recovery in a patient with chloramphenicol hepatitis-
pancytopenia syndrome. J. Pediatr., 101, 1025-1027.
Chakrabarti, K. & Banerjee, S. (1977). Mechanism of intestinal
absorption of chloramphenicol. Indian J. Exp. Biol., 15, 302-303.
Chang, N., Giles, C.L., & Gregy, R.H. (1966). Optic neuritis and
chloramphenicol. Am. J. Dis. Child., 112, 46-48.
Charache, S., Finkelstein, D., Lietman, P.S., & Scott, J. (1977).
Peripheral and optic neuritis in a patient with hemoglobin SC disease
during treatment of Salmonella osteomyelitis with chloramphenicol.
Johns Hopkins Med. J., 140, 121-124.
Cid, F., Mella, F., Gonzalez, X., & Gouzalez, R. (1983). Effects of
anti-inflammatory drugs on the pharmacokinetics of chloramphenicol.
An. Real Acad. Farm., 49, 49-60.
Clausen, N. (1986). A population study of severe aplastic anemia
children. Acta paediatr. Scand., 75, 58-63.
Cocke, J.G., Brown, R.E., & Geppert, L.J. (1966). Optic neuritis with
prolonged use of chloramphenicol. J. Pediatr., 68, 27-31.
Cohen, T. & Creger, W.P. (1967). Acute myeloid leukemia following
seven years of aplastic anemia induced by chloramphenicol.
Am. J. Med., 43, 762-770.
Cohen, H.J. & Huang, A.T.-F. (1973). A marker chromosome abnormality.
Occurrence in chloramphenicol-associated acute leukaemia.
Arch. Intern. Med., 132, p. 440.
Courtney, K.D., Valerio, D.A., & Palletta, A.J. (1967). Experimental
teratology in the monkey. Toxicol. Appl. Pharmacol., 10, p. 378.
Courtney, K.D., & Valerio, D.A. (1974). Teratology in the
Macaca mulatta. Teratology, 1, 163-172.
Craft, A.W., Brocklebank, J.T., Itey, E.N., & Jackson, R.H. (1974).
The "grey-toddler". Chloramphenicol toxicity. Arch. Dis. Child.,
49, 235-237.
Cronberg, S., Wallmark, E., & Soderberg, I. (1984). Investigations on
the effect of antimicrobial drugs on platelet aggregation in vitro
and in vivo. Folia Haematol., 111, 725-734.
D'Angelo, E.P., Patterson, W.C., & Morrow, R.C. (1967). Chloramphenicol.
Topical application in the middle ear. Arch. Otolaryngol., 85,
126-128.
Daum, R.S., Cohen, D.L., & Smith, A.L. (1979). Fatal aplastic anaemia
following apparent "dose-related" chloramphenicol toxicity.
J. Pediatr., 94, 403-406.
De Corte-Baeten, K. & Debackere, M. (1976). Excretion of
chloramphenicol in the milk of lactating cows after oral and
parenteral application. Dtsch. Tieraerztl. Wochenschr., 83, 231-233.
Del Giacco, G.S., Petrini, M.T., Janelli, S., & Carcassi, U. (1981).
Fatal bone-marrow hypoplasia in a shepherd using chloramphenicol
spray. Lancet, i, p. 945.
de Vries, H., Beijersbergen van Henegouwen, G.M.J., & Huf, F.A.
(1984). Photochemical decomposition of chloramphenicol in a 0.25%
eyedrop and in a therapeutic intraocular concentration. Internat. J.
Pharmaceut., 20, 265-271.
Dewse, C.D. (1977). Variable action of chloramphenicol on DNA
synthesis in phytohaemagglutinin-stimulated human lymphocytes.
ICRS Med. Sci., 5, p.150.
Dill, W.A., Thompson, E.M., Tisken, R.A., & Glazko, A.J. (1960). A new
metabolite of chloramphenicol. Nature, 185, 535-537.
Djaldetti, M. (1983). Drug vulnerability of peripheral blood
platelets. Arch. Toxicol. (Suppl.), 6, 21-32.
Doudney, C.O. & Rinaldi, C.N. (1985). Chloramphenicol-promoted
increase in resistance to UV damage in Escherichia coli
B/r trp E65: Development of the capacity for successful repair of
otherwise mutagenic or lethal lesions in DNA. Mutat. Res.
143, 29-34.
Ehling, U.H. (1971). Comparison of radiation- and chemically-induced
dominant lethal mutations in male mice. Mutat. Res., 11, 35-44.
Epstein, S.S. & Shafner, H. (1968). Chemical mutagens in the human
environment. Nature, 219, 385-387.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972).
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
Evans, L.S. & Kleiman, M.B. (1986). Acidosis as a presenting feature
of chloramphenicol toxicity. J. Pediatr., 108, 475-477.
Eyer, F., Lierheimer, E., & Schneller, M. (1984). Reactions of
nitrosochloramphenicol in blood. Biochem. Pharmacol., 33, 2299-2308.
Flach, A.J. (1982). Chloramphenicol and aplastic anemia. Am. J.
Ophthalmol., 93, 664-665.
Flint, O.P. & Orton, T.C. (1984). An in vitro assay for teratogens
with cultures of rat embryo midbrain and limb bud cells. Toxicol.
Appl. Pharmacol., 76, 383-395.
Forck, G. (1971). Incidence and significance of chloramphenicol
allergies. Dtsch. Med. Wochen, 96, 161-165.
Forni, A. & Vigilant, E.C. (1974). Chemical leukemogenesis in man.
Ser. Haematol., 7, 211-223.
Fraki, J.E., Kalimo, K., Tuohimaa, P., & Aantaa, E. (1985). Contact
allergy to various components of topical preparations for treatment of
external otitis. Acta Otolaryngol., 100, 414-418.
Fraunfelder, F.T. & Bagby, G.C. (1982). Fatal aplastic anaemia
following topical administration of ophthalmic chloramphenicol.
Am. J. Ophthalmol., 93, 356-360.
Fraunfelder, E.T. & Meyer, S.M. (1984). Ocular toxicology update.
Austral. J. Ophthalmol., 12, 391-394.
Fraumeni, J.F. (1967). Bone-marrow depression induced by
chloramphenicol or phenylbutazone. J. Am. Med. Assoc., 201, 150-156.
Fripp, R.R., Carter, M.C., Werner, J.C., Schuler, H.G., Rannels, A.M.,
Whitman, V., & Nelson, N.M. (1983). Cardiac function and acute
chloramphenicol toxicity. J. Pediatr., 103, 487-490.
Fritz, H. & Hess, R. (1971). The effects of chloramphenicol on the
prenatal development of rats, mice and rabbits. Toxicol. Appl.
Pharmacol., 19, 667-674.
Gadner, H., Gethmann, U., Jessenberger, K., & Riehm, H. (1973). Acute
leukaemia following exposure to chloramphenicol? Three case reports
and review of the literature. Mschr. Kinderheilk, 121, 590-594.
Garrett, N.B., Stack, H.F., Gross, M.R., & Waters, M.D. (1984). An
analysis of the spectra of genetic activity produced by known or
suspected human carcinogens. Mutat. Res., 134, 89-111.
Gargye, A.K. & Dutta, D.V. (1959). Nerve deafness following
chloromycetin therapy. Indian J. Pediatr., 26, 265-266.
Glavini, E., Prati, M., & Vismara, C. (1979). The effects of
actinomycin D and chloramphenicol on rat preimplantation embryos.
Experientia, 35, 1649-1650.
Gilman, A.G., Goodman, L.S., Rall, T.W., & Murad, F. (eds.) (1985).
Goodman and Gilman's The Pharmacological Basis of Therapeutics.
7th ed. New York, MacMillan Publishing Co., pp. 1179-1184.
Glazko, A.J., Wolf, L.M., Dill, W.A., & Bratton, A.C. (1949).
Biochemical studies on chloramphenicol (chloromycetin). II. Tissue
distribution and excretion studies. J. Pharmacol. Exp. Ther.,
96, 445-459.
Glazko, A.J., Dill, W.A., & Rebstock, M.C. (1950). Biochemical studies
on chloramphenicol (chlormycetin). III. Isolation and identification
of metabolic products in urine. J. Biol. Chem., 183, 679-691.
Glazko, A.d., Dill, W.A. & Wolf, L.M. (1952). Observations on the
metabolic disposition of chloramphenicol (chloromycetin) in the rat.
J. Pharmacol. Exp. Ther., 104, 452-458.
Godel, V., Nemet, P., & Lazar, M. (1980). Chloramphenicol optic
neuropathy. Arch. Ophthalmol., 98, 1417-1421.
Goh, K. (1971). Chloramphenicol, acute leukemia and chromosomal
vacuolation. Southern Med.J., 64, p. 815.
Goh, K.-O. (1979). Chloramphenicol and chromosomal morphology.
J.Med., 10, 159-166.
Gray, J.D. (1955). The concentration of chloramphenicol in human
tissues. Canad. Med. Assoc. J., 72, 778-779.
Gross, B.J., Branchflower, R.V., Burke, T.R., Lees, D.E., & Pohl, L.R.
(1982). Bone-marrow toxicity in vitro of chloramphenicol and its
metabolites. Toxicol. Appl. Pharmacol., 64, 557-565.
Guntakatta, M., Matthews, E.J., & Rundell, J.O. (1984). Development of
a mouse embryo limb bud cell culture system for the estimation of
chemical teratogenic potential. Terat. Carcinogen. Mutat.,
44, 349-364.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985a). Percutaneous
absorption in man: A kinetic approach. Toxicol. Appl. Pharmacol.,
78, 123-129.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985b). Transdermal
absorption kinetics: A physiochemical approach. ACS Symp. Ser.,
273, 19-31.
Haile, C.A. (1977). Chloramphenicol toxicity. Southern Med. J.,
70, 479-480.
Halpert, J. (1981). Covalent modification of lysine during the suicide
inactivation of rat liver cytochrome P-450 by chloramphenicol.
Biochem. Pharmacol., 30, 875-881.
Halpert, J. & Neal, R.A. (1981). Inactivation of rat liver cytochrome
P-450 by the suicide substrates parathion and chloramphenicol. Drug
Met. Rev., 12, 239-259.
Halpert, J. (1982). Further studies of the suicide inactivation of
purified rat liver cytochrome P-450 by chloramphenicol.
Mol. Pharmacol., 21, 166-172.
Halpert, J., Balfour, C., Miller, N.E., Morgan, E.T., & Danbar, D.
(1985). Isozyme selectivity of the inhibition of rat liver cytochrome
P-450 by chloramphenicol in vivo. Mol. Pharmacol., 28, 290-296.
Hapke, H.J., Abel, J., Ghosch, H., Tachampas, S., & Youssef, S.
(1977). Effects of chloramphenicol on drug-degrading enzyme systems.
Zentrabl. Veterinaermed., 24, 701-714.
Hara, H., Kohsaki, M., Noguchi, K., & Nagai, K. (1978). Effect of
chloramphenicol on colony formation from erythrocytic precursors.
Am. J. Haematol., 5, 123-130.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W., &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by interlaboratory
testing of diverse chemicals. Environ. Mutagen., 8, 515-531.
Hausman, K. & Skrandies, G. (1974). Aplastic anaemia following
chloramphenicol therapy in Hamburg and surrounding districts.
Postgrad. Med. J., 50, 136-142.
Heddle, J.A. & Bruce, W.R. (1977). Comparisons of test for
mutagenicity or carcinogenicity using assays for sperm abnormalities,
formation of micronuclei, and mutations in Salmonella: In: Hiatt,
H.H., Watson, J.D., & Winsten, J.A. (eds.) Origins of Human
Cancer, Book C, Risk Assessment, Cold Spring Harbour Laboratory,
pp. 1549-1557.
Hellriegel, K.P., & Gross, R. (1974). Follow-up studies in
chloramphenicol-induced aplastic anaemia. Postgrad. Med. J.,
50 (Suppl.5), 136-142.
Henley, C.M., Brown, R.D., Penny, J.E., Kupetz, S.A., Hodges, K.B., &
Jobe, P.C. (1984). Impairment of cochlear function produced by
chloramphenicol and noise. Neuropharmacol., 23, 197-202.
Henley, C.M. (1985). Comparison of the ototoxicity of chloramphenicol
and gentamycin in noise exposed rats. Diss. Abs., 46, 1523-B.
Hodgkinson, R. (1971). Infectious hepatitis and aplastic anaemia.
Lancet, i, p. 1014.
Hodgkinson, R. (1973). The chloramphenicol-hepatitis-aplastic anaemia
syndrome. Med. J. Austral., 1, 939-940.
Holden, H.E. (1982). Comparison of somatic and germ cell models for
cytogenetic screening. J. Appl. Toxicol., 2, 196-200.
Howell, A., Andrews, T.M., & Watts, R.W.E. (1975). Bone-marrow cells
resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet, i, 65-69.
Huang, N.M., Harley, R.D., Promadhattavedi, V., & Sproul, A. (1966).
Visual disturbances in cystic fibrosis following chloramphenicol
administration. J. Pediatr., 68, 32-44.
Humphries, K.R. (1968). Acute myelomonocytic leukaemia following
chloramphenicol therapy. N. Z. Med. J., 68, 248-249.
Hyman, J., Leifer, Z., & Rosenkranz, H.S. (1980). The E. coli
Pol A1 assay. A quantitative procedure for diffusable and
non-diffusable chemicals. Mutat. Res., 74, 107-111.
IARC (1982). IARC (International Agency for Research on Cancer)
monographs on the evaluation of the carcinogenic risk of chemicals to
humans: Chemicals, industrial processes and industries associated
with cancer in humans. 1-29, (Suppl. 4), 79-80.
IARC (1987, in press). IARC (International Agency for Research on
Cancer) monographs on the evaluation of carcinogenic risks to humans.
Overall evaluations of carcinogenicity: an update of IARC monographs
volumes 1-42 (Suppl. 7).
Ingall, D., Sherman, J.D., Cockburn, F. & Klein, R. (1965).
Amelioration by ingestion of phenylalanine of toxic effects of
chloramphenicol on bone-marrow. New Eng. J. Med., 272, 180-185.
Issaragrisil, S. & Piankijagum, A. (1985). Aplastic anemia following
topical administration of ophthalmic chloramphenicol: Report of a case
and review of the literature. J. Med. Ass. Thailand, 88, 309-312.
Jackson, A.F., Wentzell, B.R., McCalla, D.R., & Freeman, K.B. (1977).
Chloramphenicol damages bacterial DNA. Biochem. Biophys. Res.
Commun., 78, 151-157.
Javed, I., Nawaz, M., Ahmed, M., Rehman, Z.U., & Shah, B.H. (1984).
Pharmacokinetics, renal clearance and urinary excretion of
chloramphenicol in goats. Pakis. Vet., 4, 151-157.
Jones, F.E. & Hanson, D.R. (1977). H. Influenzae meningitis treated
with ampicillin or chloramphenicol, and subsequent hearing loss.
Dev. Med. Child. Neurol., 19, 593-597.
Joy, R.J.T., Scalettar, R., & Sodee, D.B. (1960). Optic and peripheral
neuritis. Probable effects of prolonged chloramphenicol therapy.
J. Amer. Med. Assoc., 173, 1731-1734.
Kapp, J.P., Avora, B., & Sibinga, M.S. (1977). Depression of
erythropoiesis, physical growth, and hair growth by chloramphenicol in
a three-year-old child with cystic fibrosis. Clin. Pediatr.,
16, 64-66.
Kauffman, R.E., Miceli, J.N., Strebel, L., Buckley, J.A., & Dore, A.K.
(1981). Pharmacokinetics of chloramphenicol and chloramphenicol
succinate in infants and children. J. Pediatr., 98, 315-320.
Keiser, G. & Bucheggar, U. (1973). Hematological side effects of
chloramphenicol and thiamphenicol. Helv. Med. Acta, 37, 265-278.
Kern, P., Heimpel, H., Heit, W., & Kubanek, B. (1975). Bone-marrow
cells resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet i, p. 1190.
Knowles, J.A. (1965). Excretion of drugs in milk - a review.
J. Pediatr., 66, 1068-1082.
Korting, G.W. & Kifle, H. (1985). Systemic side-effects from external
application of chloramphenicol. Der Hautarzt., 36, 181-183.
Krasinski, K., Perkin, R., & Rutledge, J. (1982). Gray baby syndrome
revisited. Clin. Pediatr., 21, 571-572.
Krishna, G., Aykac, I., & Siegel, D. (1981). Recent studies on the
mechanisms of chloramphenicol activation responsible for aplastic
anemia. In: NaJean Y. (ed.) Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy. New York, Raven Press,
pp. 5-16.
Kroker, R. (1985). The pharmacokinetic behavior of chloramphenicol in
liver-damaged mini-pigs. J. Vet. Pharmacol. Ther., 8, 82-87.
Kroon, A.M. & de Vries, H. (1969). The effect of chloramphenicol on
the biogenesis of mitochondria of rat liver in vivo. Fed. Eur.
Biochem. Soc. Letters, 3, 208-210.
Kubinski, H., Gutzke, G.E., & Kubinski, Z.O. (1981). DNA-cell binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89, 95-136.
Kunii, O., Komatsu, T., & Nishiya, H. (1983). Biliary excretion of
antibiotics in rats. Jap. J. Exp. Med., 53, 51-58.
Kurz, H., Manser-Ganshorn, A., & Stickel, H.H. (1977). Differences in
the binding of drugs to plasma proteins from newborn and adult man.
I. Euro. J. Clin. Pharmacol., 11, 463-467.
Lasky, M.A., Pincus, M.H., & Katlan, N.R. (1953). Bilateral optic
neuritis following chloramphenicol therapy. J. Amer. Med. Assoc.,
151, 1403-1404.
Leiken, S.L., Welch, H., & Guin, G.H. (1961). Aplastic anaemia due to
chloramphenicol. Clin. Proc. Child Hosp., 17, 171-180.
Lepow, M. (1986). Aplastic anaemia following chloramphenicol therapy
still happens! Pediatr., 77, 932-933.
Lery, N., Descoted, J., & Evreux, J.Cl. (1978). A review of
chloramphenicol-induced blood disorders. Vet. Human Toxicol.,
20, 177-181.
Lim, L.O. & Yunis, A.A. (1982). Induction of methemoglobin formation
by nitrosochloramphenicol in hemolysed blood. Res. Commun. Chem.
Path. Pharmacol., 38, 497-500.
Lim, L.O., Abou-Khalil, W.H., & Yunis, A.A. (1984). The effect of
nitrosochloramphenicol on mitochondrial DNA polymerase activity.
J. Lab. Clin. Med., 104, 213-222.
Ling, G.V., Conzelman, G.M., Franti, C.E., & Ruby, A.L. (1980). Urine
concentration of chloramphenicol, tetracycline, and sulfisoxazole
after oral administration to healthy adult dogs. Am. J. Vet. Res.,
41, 950-952.
Longnecker, D.S., Curphey, T.J., James, S.T., Daniel, D.S., &
Jacobs, N.J. (1974). Trial of a bacterial screening system for rapid
detection of mutagens and carcinogens. Cancer Res., 34, 1658-1663.
Ma, T.-H., Harris, M.M., Anderson, V.A., Ahmed, I., Mohammad, K.,
Bare, J.L., & Lin, G. (1984). Tradescantia-micronucleus (Trad-MCN) on
140 health-related agents. Mutat. Res., 138, 157-167.
Hackler, B., Grace, R., Tippit, D., Lemire, R.J., Shepard, H., &
Kelly, V.C. (1975). Studies of the development of congenital anomalies
in rats III. Effects of inhibition of mitochondrial energy systems on
embryonic development. Teratology, 12, 291-296.
Malbrel, C., Talmud, M., Dupou, D., & Rose, J.P. (1977). Concerning a
new case of optic neuritis by chloramphenicol in the infant.
Bull. Soc. Ophthalmol. France, 77, 999-1001.
Mamber, S.W., Okasinki, W.A., Printer, C.D., & Tunac, J.B. (1986). The
Escherichia coli K-12 SOS Chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutat. Res., 171, 83-90.
Manna, G.K. & Bardham, S. (1977). Some aspects of chloramphenicol
induced bone marrow chromosome aberrations in mice. J. Cytol. Genet.,
12, 10-17.
Manna, G.K. & Roy, P.P. (1979). Chromosome aberrations in F1 embryos
and litters of chloramphenicol treated male parent mice. Natl. Acad.
Sci. Letters (India), 2, 460-462.
Martelo, O.J., Manyan, D.R., Smith, U.S., & Yunis, A.A. (1969).
Chloramphenicol and bone marrow mitochondria. J. Lab. Clin. Med.,
74, 927-940.
Masek, F. (1977). Effect of some drugs on excision repair in E. coli
cells. Chem-Biol. Interact., 17, 121-127.
Matsuda, S. (1984). Transfer of antibiotics into maternal milk.
Biol. Res. Preg. Perinatol., 5, 57-60.
Matthews, S.J. & Shawver-Matthews, S. (1980). Chloramphenicol toxicity
- oral vs. intravenous administration. J. Paediatr., 97, p. 869.
McCann, J., Choi, E., Yamasaki, E., & Ames, B.N. (1975). Detection of
carcinogens and mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc. Natl. Acad. Sci. USA, 72, 5133-5139.
McCoy, E., Hyman, J., & Rosenbranz, H.S. (1980a). A new mutagenic and
genotoxic response of the flame retardant tris (2, 3-dibromopropyl)
phosphate. Activation by singlet oxygen. Mutat. Res., 77, 209-214.
McCoy, E.C., Petrullo, L.A., & Rosenkranz, H.S. (1980b). Non-mutagenic
genotoxicants: Novobiocin and naladixic acid, 2 inhibitors of DNA
gyrase. Mutat. Res., 71, 33-43.
McCurdy, P.R. (1963). Plasma concentration of chloramphenicol and bone
marrow suppression. Blood, 21, 363-372.
Meyer, J.S. & Boxer, M. (1973). Leukemic cellular thrombi in pulmonary
blood vessels. Subleukemic myelogenous leukemia following
chloramphenicol-induced aplastic anemia. Cancer 32, 712-721.
Meyler, L. & Herkeimer, A. (1968). Side effects of drugs. 6,
Amsterdam, Excerpta Medica, pp. 286-314.
Meyler, L., Polak, B.C.P., Schut, D., Wesseling, H., & Herxheimer, A.
(1974). Blood dyscrasias attributed to chloramphenicol. A review of
641 published and unpublished cases. Postgrad. Med. J., 50
(Suppl. 5), 123-126.
Milner, G.R. & Geary, C.G. (1979). The aplasia-leukaemia syndrome. In:
Geary, C.G. (ed.) Aplastic Anaemia. London, Balliere Tindall,
pp. 230-243.
Mitchell, I. de G., Dixon, P.A., Gilbert, P.J., & White, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Problems of
evaluation. Mutat. Res., 79, 91-105.
Mitchell, I. de G. & Gilbert, P.J. (1984). The effect of pretreatment
of Escherichia coli CM891 with ethylenediamine-tetraacetate on
sensitivity to a variety of standard mutagens. Mutat. Res.,
140, 13-19.
Mitema, E.S. (1982). Effect of chloramphenicol administration on the
bovine hematology and bone marrow. Vet. Human Toxicol.,
Suppl., 162-166.
Mitus, W.J. & Coleman, N. (1970). In vitro effect of chloramphenicol
on chromosomes. Blood, 35, 689-674.
Mizuno, S., Aoki, K., Ohno, Y., Sasaki, R., & Hamajima, N. (1982).
Time series analysis of age-sex specific death rates from aplastic
anemia and the trend in production amount of chloramphenicol.
Nagoya J. Med. Sci., 44, 103-115.
Modan, B., Segal, S., Sham, M., & Sheba, C. (1975). Aplastic anemia in
Israel: Evaluation of the etiological role of chloramphenicol on a
community-wide basis. Am. J. Med. Sci., 270, 441-445.
Morizono, T. & Johnstone, B.M. (1975). Ototoxicity of chloramphenicol
ear drops with propylene glycol as solvent. Med. J. Austral.,
2, 634-638.
Morley, A., Trainor, K., & Remes, J. (1976). Residual marrow damage:
Possible explanation for idiosyncrasy to chloramphenicol. Brit. J.
Haematol., 32, 525-531.
Morris, P.L., Burke, T.R., George, J.W., & Pohl, L.R. (1982). A new
pathway for the oxidative metabolism of chloramphenicol by rat liver
microsomes. Drug Met. Disp., 10, 439-445.
Morris, P.L., Burke, T.R., & Pohl, L.R. (1983). Reductive
dechlorination of chloramphenicol by rat liver microsomes. Drug Met.
Disp., 11, 126-130.
Mulhall, A., de Louvois, J., & Hurley, R. (1983). Chloramphenicol
toxicity in neonates: Its incidence and prevention. Brit. Med. J.,
287, 1424-1427.
Murray, T. & Yunis, A.A. (1981). The cellular uptake and covalent
binding of nitrosochloramphenicol. J. Lab. Clin. Med., 98, 396-401.
Murray, T., Downey, K.M., & Yunis, A.A. (1982). Degradation of
isolated deoxyribonucleic acid mediated by nitrosochloramphenicol.
Possible role in chloramphenicol-induced aplastic anaemia. Biochem.
Pharmacol., 31, 2291-2296.
Naesland, B., Betner, I., & Halpert, J. (1983). Inhibition of rat lung
cytochrome P-450 by chloramphenicol. Extrahepatic Drug Met. Chem.
Carcinogen, Proc. Int. Mtg., 251-252.
Naesland, B. & Halpert, J. (1984). Selective inhibition by
chloramphenicol of cytochrome P-450 isozymes in rat lung and liver
involved in the hydroxylation of n-hexane. J. Pharmacol. Exp. Ther.,
231, 16-22.
Nagai, K. & Kanamaru, A. (1978). Drug-induced aplastic anaemia: Effect
of chloramphenicol on hemopoietic stems cells. In: Hibino, S., Takaku,
F., & Shakidi, N.T. (eds.) Tokyo, University Park Press, pp. 343-353.
Nagao, T. & Maner, A.M. (1969). Concordance for drug-induced aplastic
anemia in identical twins. New Engl. J. Med., 281, 7-10.
Nair, C.R., Goel, A., & Rahman, A. (1981). Inhibition of hepatic drug
metabolism by chloramphenicol-paracetamol interaction - an
experimental study. Bull. Postgrad. Inst. Med. Educ. Res.
(Chandrigarh), 15, 31-35.
Najean, Y. & Baumelon, E. (1984). Toxic etiology of aplastic anemia.
Sex Trans. Dis., 11, 343-346.
Nakagawa, T., Masada, M., & Uno, T. (1975). Gas chromatographic
determination and gas chromatographic-mass spectrometric analysis of
chloramphenicol, thiamphenicol and their metabolites. J. Chromatogr.,
111, 355-364.
Nara, N., Besshe, M., Hirashima, K., & Momoi, H. (1982). Effects of
chloramphenicol on hematopoietic inductive micro environment.
Exp. Hematol., 10, 20-25.
Narda, R.D. & Gupta, R.K. (1972). Mutation studies in
D. melanogaster. Drosoph. Inf. Serv., 48, p. 105
Nasrat, G.E., Ahmed, K.A., Nafei, H.A., & Abdel-Rahman, A.H. (1977).
Mutagenic action of certain therapeutic drugs on Drosophila
melanogaster. Bull. Fac. Agric. Univ. Cairo, 28, 697-717.
Nilges, T.C. & Norther, J.L. (1971). Iatrogenic ototoxic hearing loss.
Ann. Surg., 173, 281-289.
Pall, M.L. & Hunter, B.J. (1985). Carcinogens induce genetic tandem
duplications in Salmonella. Mutat. Res., 152, 131-145.
Pant, G.S., Kamada, N., & Tanaka, R. (1976). Sister chromatid
exchanges in peripheral lymphocytes of atomic bomb survivors and of
normal individuals exposed to radiation and chemical agents.
Hiroshima J. Med. Sci., 25, 99-105.
Parashar, S., Rao, R., Tikare, S.K., & Tikare, S.S. (1972).
Chloramphenicol induced reversible bone marrow suppression.
J. Postgrad. Med., 18, 90-92.
Parker, F.L. & James, G.W.L. (1978). The effect of various topical
antibiotic and antibacterial agents on the middle and inner ear of the
guinea-pig. J. Pharm. Pharmacol., 30, 236-239.
Patterson, W.C. & Gulick, W.L. (1963). The effects of chloramphenicol
upon electrical activity of the ear. Ann. Otol. Rhinol. Laryngol.,
72, 50-55.
Pazdernik, T.L. & Corbett, M.D. (1979). Effects of chloramphenicol
reduction products or hemopoietic precursor cells in vitro.
Pharmacol., 19, 191-195.
Pazdernik, T.L. & Corbett, M.D. (1980). Role of chloramphenicol
reduction products in aplastic anemia. Pharmacol., 20, 87-94.
Penny, R.H.C., Carlisle, C.H., Prescott, C.W., & Davidson, H.A.
(1967). Effects of chloramphenicol on the haemopoietic system of the
cat. Brit. Vet. J., 123, 145-153.
Perez, R.P., Puig, M.L., Sanchez, J.L.A., & Torres, E.L. (1981).
Aplastic anemia and drugs as causal factors. Rev. Cub. Farm.,
15, 97-103.
Petitjean, F., Sastre, J.P., Bertrand, N., Cointy, C., & Jouvet, M.
(1975). Suppression of paradoxical sleep by chloramphenicol in the
cat. Absence of effect of thiamphenicol. C. R. Seances Biol. (Paris),
169, 1236-1239.
Plaut, M.E. & Best, W.R. (1982). Aplastic anemia after parenteral
chloramphenicol: Warning renewed. New Engl. J. Med., 306, p. 1486.
Plomp, T.A., Thiery, M., & Maes, R.A.A. (1983). The passage of
thiamphenicol and chloramphenicol into human milk after single and
repeated oral administration. Vet. Human Toxicol., 25, 167-172.
Polak, B.C.P., Wesseling, H., Schut, D., Herxheimer, A., & Meyler, L.
(1972). Blood dyscrasias attributed to chloramphenicol. A review of
576 published and unpublished cases. Acta med. Scand., 192, 409-414.
Preziosi, P., Basile, V., Vacca, M., & de Natale, G. (1981). On the
correlation between regional utilization patterns of chloramphenicol
and its adverse reactions with emphasis on aplastic anaemia.
Int. J. Clin. Pharmacol.Ther. Toxicol., 19, 547-551.
Proud, G.O., Mittelman, H., & Seiden, G.D. (1968). Ototoxicity of
topically applied chloramphenicol. Arch. Otolaryngol., 87, 580-587.
Punch, P.I., Costa, N.D., Chambers, E.D., Slatter, D.H., &
Wilcox, G.E. (1985). Plasma and tear concentrations of antibiotics
administered parenterally to cattle. Res. Vet. Sci., 39, 179-187.
Puron, R. & Firminger, H.I. (1965). Protection against induced
cirrhosis and hepatocellular carcinoma in rats by chloramphenicol.
J. Natl. Cancer Inst., 85, 29-37.
Rajchgot, P., Prober, C., Soldin, S., Golas, C., Good, F., Harding, L.
& MacLeod, M. (1983). Chloramphenicol pharmacokinetics in the newborn.
Dev. Pharmacol. Ther., 6, 305-314.
Rebandel, P. & Rudzki, E. (1986). Occupational contact sensitivity in
oculists. Contact Derm., 15, p. 92.
Robin, E., Berman, M., Bhooplan, N., Cohen, H., & Fried, W. (1981).
Induction of lymphomas in mice by busulfan and chloramphenicol.
Cancer Res., 41, 3478-3482.
Rosenbach, L.M., Cavites, A.P., & Mitus, W.J. (1960). New Engl. J.
Med., 263, 724-728.
Rosenkranz, S., Carr, M.S., & Rosenkrans, H.S. (1974). 2-Haloethanols:
Mutagenicity and reactivity with DNA. Mutat. Res., 26, 367-370.
Rosenkranz, H.S., Gutter, B., & Speck, W.T. (1976). Mutagenicity and
DNA-modifying activity: A comparison of two microbial assays.
Mutat. Res., 41, 61-70.
Rosenkranz, H.S. (1977). Mutagenicity of halogenated alkanes and their
derivatives. Environ. Health Perspect., 21, 79-84.
Rosenthal, R.L. & Blackman, A. (1965). Bone marrow hypoplasia
following use of chloramphenicol eye drops. J. Am. Med. Assoc.,
191, 148-149.
Ross, S., Burke, F.G., Sites, J., Rice, E.C., & Washington, J.A.
(1950). Placental transmission of chloramphenicol (chloromycetin).
J. Am. Med. Assoc., 142, p. 1361.
Roy, B.K., Banerjee, N.C., & Pandy, S.N. (1986). Distribution of
chloramphenicol in goat blood and milk after intravenous injection.
Indian. J. Anim. Health, 25, 33-35.
Rudzki, E. & Kleniewska, D. (1970). The epidemiology of contact
dermatitis in Poland. Brit. J. Derm., 83, 543-545.
Rudzki, E., Grzywa, Z., & Maciejowska, E. (1976). Occupational contact
sensitivity in oculists. Contact Derm., 2, 181.
Sack, C.M., Kaup, J.R., & Smith, A.L. (1980). Chloramphenicol
pharmacokinetics in infants and young children. Pediatr.,
66, 579-584.
Saidi, P., Wallerstein, R.O., & Aggeler, P.M. (1961). Effect of
chloramphenicol on erythropoiesis. J. Lab. Clin. Med., 75, 247-256.
Salem, Z., Murray, T., & Yunis, A.A. (1981). The nitroreduction of
chloramphenicol by human liver tissue. J. Lab. Clin. Med.,
97, 881-886.
Samson, J.P., Hulstaert, C.E., Molenaar, I., & Nieweg, H.O. (1972).
Fine structure of the bone marrow sinusoidal wall in idiopathic and
drug induced panmyelopathy. Acta haematol., 48, 218-226.
Sanguineti, M., Rossi, L., Ognio, E., & Sauti, L. (1983). Tumours
induced in Balb/C and C57B1/6N mice following chronic administration
of chloramphenicol. In: Proceedings of a National Meeting on
Experimental and Clinical Oncology, Parma, 23-25 November,
Abstract No. 50.
Sarasti, H. (1970). Chloramphenicol in South America. New Engl.
J. Med., 282, p. 813.
Saxen, I. (1975). Associations between oral clefts and drugs taken
during pregnancy. Int. J. Epidemiol., 4, 37-44.
Scheres, J.M.J.C., Hustinx, T.W.J., Geraedts, J.P.M., Leeksma, C.H.W.,
& Meltzer, P.S. (1985). Translocation 1:7 in hematologic disorders. A
brief review of 22 cases. Cancer Gen. Cytogen., 18, 207-213.
Schewach-Millet, M. & Shapiro, D. (1985). Urticaria and angioedema due
to topically applied chloramphenicol ointment. Arch. Dermatol.,
121, p. 587.
Schmitt-Graff, A. (1981). Chloramphenicol-induced aplastic anemia
terminating with acute nonlymphocytic leukemia. Acta haematol.,
66, 267-268.
Seaman, A.J. (1969). Sequels to chloramphenicol aplastic anemia: Acute
leukaemia and paroxysmal nocturnal hemoglobinuria. Northwest Med.,
68, 831-834.
Sekizawa, J. & Shibamoto, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
Sharp, A.A. (1963). Chloramphenicol-induced blood dyscrasias: Analysis
of 40 cases. Brit. Med. J., 1, 735-736.
Sheehan, J. & Sweeney, E.C. (1982). Drugs and sperm. Brit. Med. J.,
284, p. 1331.
Shimizu, H., Kuroishi, T., Tominaga, S., Okada, H., & Aoki, K. (1979).
Production amount of chloramphenicol and mortality rate of aplastic
anaemia in Japan. Acta haem. Jap., 42, 689-696.
Siegel, D. & Krishna, G. (1980). Nitrosochloramphenicol does not
induce aplastic anaemia in mice. Pharmacologist, 22, p. 244.
Siliciano, R.F., Margolis, S., & Lietman, P.S. (1978). Chloramphenicol
metabolism in isolated rat hepatocytes. Biochem. Pharmacol.,
27, 2759-2762.
Silver, B.J. & Zuckerman, K.S. (1980). Aplastic anaemia. Recent
advances in pathogenesis and treatment. Med. Clin. North Amer.,
64, 607-627.
Sklar, R. & Strause, B. (1980). Role of the uvrE gene product and of
inducible D6-methylguanine removal in the induction of mutations by
N-methyl-N-nitro-N-nitrosoguanidine in Escherichia coli.
Mol. Biol., 143, 343-362.
Skolimowski, I.M., Rowley, D.A., Knight, R.C., & Edwards, D.I. (1981).
Reduced chloramphenicol-induced damage to DNA. J. Antimicrob.
Chemother., 7, 593-597.
Skolimowski, I.M., Knight, R.C., & Edwards, D.I. (1983). Molecular
basis of chloramphenicol and thiamphenicol toxicity to DNA in vitro.
J. Antimicrob. Chemother., 12, 535-542.
Slater, E.E., Anderson, M.D., & Rosenkranz, H.S. (1971). Rapid
detection of mutagens and carcinogens. Cancer Res., 31, 970-973.
Slaughter, R.L., Pieper, J.A., Carra, F.B., Brodsky, B., & Koup, T.R.
(1980). Chloramphenicol sodium succinate kinetics in critically ill
patients. Clin. Pharmacol. Ther., 28, 69-77.
Smith, A.L. & Weber, A. (1983). Pharmacology of chloramphenicol.
Pediatr. Clin. North Amer., 30, 209-236.
Smith, G.S., Watkins, J.B., Thompson, T.N., Rozman, K., &
Klaasen, C.D. (1984). Oxidative and conjugative metabolism of
xenobiotics by livers of cattle, sheep, swine and rats.
J. Anim. Sci., 58, 386-395.
Stainer, G.A., Peyman, B.S., Meisels, H., & Fishman, G. (1977).
Toxicity of selected antibiotics in vitreous replacement fluid.
Ann. Ophthalmol., 9, 615-618.
Steidl, P.E.-M. (1965). Bilateral optic neuritis caused by the
prolonged therapeutic use of chloramphenicol. Union Med. Canada,
94, 1061-1062.
Stevens, D,C., Kleiman, M.B., & Lietman, P.S. (1981). Exchange
transfusion in acute chloramphenicol toxicity. J. Pediatr.,
99, 651-653.
Strick, R.A. (1983). Lack of cross-reaction between DNCB and
chloramphenicol. Contact Derm., 9, 484-487.
Suda, T., Omine, M., Tsuchiya, J., & Maekawa, T. (1978). Prognostic
aspects of aplastic anaemia in pregnancy. Experience on six cases and
review of the literature. Blut., 36, 285-298.
Suhrland, L.G. & Weisberger, A.S. (1969). Delayed clearance of
chloramphenicol from serum in patients with hematologic toxicity.
Blood, 34, 466-471.
Summ, H.D., Draeger, E., & von Wasielowski, E. (1976). On the
inhibitory effect of chloramphenicol on mitochondrial protein
synthesis as a possible cause of its selective toxic side effects.
Arzneim. Forsch., 26, 28-32.
Suter, W. & Jaeger, I. (1982). Comparative evaluation of different
pairs of DNA repair-deficient and DNA repair-proficient bacterial
tester strains for rapid detection of chemical mutagens and
carcinogens. Mutat. Res., 97, 1-18.
Timmermans, L. (1974). Influence of antibiotics on spermatogenesis.
J. Urol., 112, 348-349.
Tomatis, L., Agthe, C., Bartsch, H., Huff, J., Montesano, R., Saracci,
R., Walker, E., & Wilbourn, J. (1978). Evaluation of the
carcinogenicity of chemicals: A review of the monograph program of
International Agency for Research on Cancer. Cancer Res.,
38, 877-885.
Ullrich, D. & Bock, K.W. (1984). Glucuronide formation of various
drugs in liver microsomes and in isolated hepatocytes from
phenobarbital - and 3-methylcholanthrene - treated rats. Biochem.
Pharmacol., 33, 97-101.
Vacha, J., Pospisil, M., & Velcovsky, V. (1981). The toxic effect of
chloramphenicol on erythropoiesis in X-irradiated mice. Chemother.,
27, 131-138.
Van Joost, Th., Dikland, W., Stolz, E., & Prens, E. (1986).
Sensitisation to chloramphenicol; a persistent problem. Contact
Derm., 14, 176-178.
Van Scoy, R.E. & Wilson, W.R. (1983). Antimicrobial agents in patients
with renal insufficiency. Mayo Clin. Proc., 58, 246-248.
Venning, G.R. (1983). Identification of adverse reactions to new
drugs. II - How were 18 important adverse reactions discovered and
with what delays? Brit. Med. J., 286, 289-292.
Verma, R.S. & Lin, M.S. (1978). Chemically induced alterations of the
nuclear cycle and chromosomes in root meristem cells of maize.
J. Hered., 69, 285-294.
Vincent, P.C. (1986). Drug-induced aplastic anaemia and
agranulocytosis. Incidence and mechanisms. Drugs, 31, 52-63.
Vorherr, H. (1974). Drug excretion in breast milk. Postgrad. Med.,
56, 97-104.
Wagner, T. & Rafael, J. (1977). Biochemical properties of liver
magamitochondria induced by chloramphenicol or cuprizone. Exp. Cell
Res., 107, 1-14.
Walker, G.F. (1961). Blindness during streptomycin and chloramphenicol
therapy. Brit. J. Ophthalmol., 45, 555-557.
Wallenstein, L. & Snyder, J. (1952). Neurotoxic reaction to
chloromycetin. Ann. Intern. Med., 36, 1526-1528.
Wallerstein, R.O., Candit, P.K., Kasper, C.K., Brown, J.W., &
Morrison, F.R. (1969). Statewide study of chloramphenicol therapy and
fatal aplastic anemia: J. Am. Med. Assoc., 208, 2045-2050.
Waters, M.D., Garrett, N.E., Covone-de Serres, C.M., Howard, B.E., &
Stack, H.F. (1983). Genetic toxicology of some known or suspected
human carcinogens. In: de Serres, F.J. (ed.) Chemical Mutagens.
Principles and methods for their detection. 8, New York, Plenum
Press, pp. 261-341.
Watson, A.D.J. (1972). Chloramphenicol plasma levels in the dog: A
comparison of oral subcutaneous and intramuscular administration.
J. Small Anim. Pract., 13, 147-151.
Watson, A.D.J. (1977a). Effect of concurrent drug therapy and of
feeding or chloramphenicol levels after oral administration of
chloramphenicol in dogs. Res. Vet. Sci., 22, 68-71.
Watson, A.D.J. (1977b). Chloramphenicol toxicity in dogs.
Res. Vet. Sci., 23, 66-69.
Watson, A.D.J. & Middleton, D.J. (1978). Chloramphenicol toxicosis in
cats. Am. J. Vet. Res., 39, 1199-1203.
Watson, A.D.J. (1980). Further observations on chloramphenicol
toxicosis in cats. Am. J. Vet. Res., 41, 293-294.
Welch, H., Lewis, C.N. & Kerlan, I. (1954). Blood dyscrasias. A
nationwide survey. Antibiotics Chemother., 4, 607-623.
Werner, J.C., Whitman, V., Schuler, H.G., Fripp, R.R., Rannels, A.M.,
Kasales, C.J., & La Noue, K.F. (1985). Acute myocardial effects of
chloramphenicol in newborn pigs: A possible insight into Gray Baby
syndrome. J. Infect. Dis., 152, 344-350.
White, M.P., Haboush, H.9., & Alroomi, L.G. (1986). Fatal bone marrow
aplasia due to chloramphenicol in a baby. Lancet i, 555-556.
Widayat, Sunarto, Sutaryo, Zurghiban, M., & Ismangoen (1983). Aplastic
anaemia in children. Paediatrica Indonesiana, 23, 183-191.
Wilson, W. (1962). Toxic amblyopia due to chloramphenicol. Scot. Med.
J., 7, 90-95.
Xie, L. (1985). A simple model for detection of mutagenic
(carcinogenic) substances. Huanjing Kexue Xuebao, 5, 228-233.
Yoshida, H., Yamamoto, K., & Yamaguchi, H. (1972). Fragmentation and
non-disjunction of barley chromosomes after the treatment of
chloramphenicol and cycloheximide. Cytologia, 37, 697-707.
Young, L.W., Mitnick, J.S., Yankus, W.A., & Genieser, N.B. (1979).
Radiological case of the month. Am. J. Dis. Child., 133, 545-546.
Yunis, A.A. (1973a). Chloramphenicol toxicity. In: Blood Disorders
Due to Drugs and other Agents, Amsterdam, Excerpta Medica,
pp. 107-126.
Yunis, A.A. (1973b). Chloramphenicol-induced bone marrow suppression.
Semin. Haematol., 10, 225-234.
Yunis, A.A. (1977). Differential in vitro sensitivity of marrow
erythroid and granulocytic colony forming cells to chloramphenicol.
Am. J. Haemtol., 2, 355-363.
Yunis, A.A. (1978a). Mechanisms underlying marrow toxicity from
chloramphenicol and thiamphenicol. In: Silber, R., Lo Bue, J.,
Gordon, A. (eds.) The year of hematology, New York, Plenum,
pp. 143-170.
Yunis, A.A. (1978b). Pathogenetic mechanisms in bone marrow
suppression for chloramphenicol and thiamphenicol. In: Hibino, S.,
Takaku, F., & Shahidi, N.T. (eds.) Tokyo, University Park Press,
pp. 321-334.
Yunis, A.A. (1978c). Drug-induced bone marrow aplasia. Pesq. Med.
Biol., 11, 287-296.
Yunis, A.A., Miller, A.M., Salem, Z., & Arimura, G.K. (1980a).
Chloramphenicol toxicity: Pathogenetic mechanisms and the role of the
rho-NO2 in aplastic anaemia. Clin. Toxicol., 17, 359-373.
Yunis, A.A., Miller, A.M. Salem, Z., Corbett, M.D., & Arimura, G.K.
(1980b). Nitrosochloramphenicol: Possible mediator in
chloramphenicol-induced aplastic anemia. J. Lab. Clin. Med.,
96, 34-46.
Yunis, A.A. & Salem, Z. (1980). Drug induced mitochondrial damage and
sideroblastic change. Clinics in Haematol., 9, 607-619.
Yunis, A.A. (1984). Differential in vitro toxicity of chloram-
phenicol, nitrosochloramphenicol, and thiamphenicol. Sex Trans. Dis.,
11, 340-342.
See Also:
Toxicological Abbreviations
Chloramphenicol (WHO Food Additives Series 53)
Chloramphenicol (WHO Food Additives Series 33)
CHLORAMPHENICOL (JECFA Evaluation)
Chloramphenicol (IARC Summary & Evaluation, Volume 50, 1990)
 Chloramphenicol was previously considered at the twelfth meeting
of the Committee (Annex 1, reference 17), at which time it was
determined that acceptable levels in food could not be established.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
In dogs, chloramphenicol is rapidly and extensively absorbed
after oral administration of 50 mg/kg b.w., giving plasma levels of
16.5 g/l 2 hours after dosing (Watson, 1972; Watson 1977a). Similar
findings have been observed in rabbits given oral doses of 16 mg/kg
chloramphenicol (Cid et al., 1983). Dietary bran markedly
increased the rate and extent of absorption of orally administered
chloramphenicol palmitate in pigs, but it is not known if the
absorption of chloramphenicol itself would be affected in this way
(Bueno et al., 1984). Experiments with everted mouse small intestine
sacs resulted in absorption of chloramphenicol at similar rates over
different regions of the small intestine. Various chemicals and
metallic ions had no effect on the rate of absorption, and penetration
of the serosal and mucosal surfaces was similar indicating passive
absorption of chloramphenicol from the gut (Chakrabarti & Banerjee,
1977).
In adult human beings, absorption of chloramphenicol is rapid and
extensive after an oral dose. Serum levels were 20 - 40 mg/l after a
2 g dose (29 mg/kg b.w.) and 40 - 60 mg/l after a 4 g dose (57 mg/kg
b.w.) (Yunis, 1973a).
Chloramphenicol is also well absorbed by infants and neonates
after oral administration. Serum (peak) concentrations of 20 - 24 mg/l
were noted after oral doses of 40 mg/kg b.w. in neonates. Infants
given 26 mg/kg b.w. were found to have peak concentrations of 14 mg/l
(Mulhall et al., 1983). Available evidence and theoretical
considerations suggest that chloramphenicol may be percutaneously
absorbed in human beings (Guy et al., 1985a, b).
Chloramphenicol is distributed to most major organs in newborn
pigs. After i.v. administration of 0.52 mg/kg b.w. 14C-chloram-
phenicol, many tissues showed higher levels of chloramphenicol than
were found in the blood as soon as 5 minutes after administration.
These tissues included the lungs, liver, kidneys, adrenal cortex,
myocardium, pancreas, thyroid, spleen, and skeletal muscle. The
levels remained higher than in blood for up to 8 hours after dosing.
At 4 and 8 hours after administration, levels in the brain were
higher than in blood. However, there was no apparent affinity for
chloramphenicol in the bone marrow over the period of the experiment
(8 hours), where levels did not reach those noted in serum (Appelgren
et al., 1982).
Chloramphenicol in human beings, regardless of the route of
administration, extensively distributes, although the levels in
tissues depend on the route, the levels being the highest after oral
or i.v. administration. The compound has been found in the heart,
lungs, kidneys, liver, spleen, pleural fluid, seminal fluid, ascitic
fluid, and saliva. (Ambrose, 1984; Yunis 1973a; Gray, 1955). It binds
extensively to proteins in both adults and neonates, although binding
in the latter is less than in the former (Kurz et al., 1977).
Chloramphenicol can cross the placenta in human beings (Ambrose,
1984). After oral doses of 1 or 2 g chloramphenicol to pregnant women,
chloramphenicol was detected in the placenta after 1.5 - 2.5 hours,
indicating some potential for the drug to reach the fetus (Ross
et al., 1950).
In human beings with normal renal/hepatic function the volume of
distribution is around 0.7 1.4 1/kg. These values do not deviate
markedly in patients with hepatic dysfunction or with renal
impairment. Overall, these values suggest extensive distribution in
body tissues. (Ambrose, 1984; Burke et al., 1980; Slaughter
et al., 1980). Similar values were noted with infants and young
children given the sodium succinate derivative of chloramphenicol,
which is converted in vivo to chloramphenicol (Sack et al., 1980).
Chloramphenicol and its metabolites are excreted in the urine of
rats after oral dosing; up to 70% of an oral dose may he excreted in
this way (Glazko et al., 1949; Glazko et al., 1952). Limited data
suggest that chloramphenicol may be excreted in the bile of rats
following administration; around 0.4% of an i.m. dose of 40 mg/kg was
detected in the bile after 4 hours (Kunii et al., 1983). There are
no readily available data for biliary excretion after oral
administration. In new-born pigs, the majority of an i.v. dose of
chloramphenicol was excreted in the urine, while a small quantity was
excreted in the bile (Appelgren et al., 1982). Liver damage may
delay body-clearance of chloramphenicol, at least in the mini-pig
(Kroker, 1985). Following t.v. administration to goats, 69% of the
dose was excreted in the urine after 12 hours (Javed et al., 1984).
Chloramphenicol has been found to be excreted in other body
fluids after administration to animals. It has been detected at levels
up to 6 mg/l in the tears of cattle given i.v. doses of 50 mg/kg
chloramphenicol; it was detected in the tears as soon as 1 hour after
dosing (Punch et al., 1985). Chloramphenicol was detected in the
milk of goats after an i.v. dose of 100 mg/kg, with maximum levels
1 hour after administration. (Roy et al., 1986). Administration of
10 mg/kg chloramphenicol i.m. to cattle resulted in peak levels in
milk of around 1 mg/l 6 hours after injection. However, after oral
administration, no chloramphenicol was detected in milk.
(De Corte-Baeten & Debackere, 1976).
Nine patients with evidence of chloramphenicol-induced bone
marrow suppression showed greatly reduced plasma clearance times
compared with 9 others who showed no evidence of toxicity. In the
"toxic" group, 5 had liver disease, 2 had pyelonephritis, and 2 showed
neither liver disease nor renal disease. In the "non-toxic" group, 1
had liver disease, 2 had renal disease, while 6 had neither. Six hours
after an i.v. dose of 500 mg chloramphenicol succinate, the blood
level in the "toxic" group was 4.5 µg/ml (2.8 - 6.9 µg/ml), while in
the "non-toxic" group the mean level was 1.2 µg/ml (0 - 2.3 ug/ml).
Similarly, after 8 hours the mean level in the "toxic" group was
3.5 µg/ml (2.1 - 5.2 ug/ml), while in the "non-toxic" group a mean
level of 0.7 µg/ml (0 - 2.5 µg/ml) was noted. Such findings suggest
that patients susceptible to the bone-marrow effects of
chloramphenicol may clear the drug from the blood at a slower rate
than those who are not susceptible (Shurland & Weisburger, 1969).
Chloramphenicol administered to human beings is excreted
primarily in the urine (90%), up to 15% as the parent compound and the
remainder as metabolites, including conjugated derivatives
(Burke et al., 1980; Ambrose, 1984; Yunis, 1973a). Glomerular
excretion is thought to be the major mechanism of excretion
(Glazko et al., 1949).
Renal clearance varies depending on age. In one study, clearance
in neonates (less than 6 months of age) was 0.46 - 9.76 1/hour, while
in infants aged 6 months to 2.5 years values in the range 1.8 - 2.1
1/hour were reported. In two subjects aged over 2.5 years, values of
6.03 and 9.59 1/hour were noted (Burckart et al., 1983). Similar
variations with age have been noted in other studies (Mulhall et al.,
1983; Sack et al., 1980; Kauffman et al., 1981; Burckart et al.,
1982; Rajchgot et al., 1983). Renal clearance takes longer in
patients with renal insufficiency than in normal patients (Brasfield
et al., 1983). However, these differences are not marked (Smith &
Weber, 1983). It has been recommended that dosage adjustments need not
be made in patients with renal insufficiency or in anephric subjects
(Van Scoy & Wilson, 1983).
As with experimental animals, chloramphenicol is excreted in
human milk. Up to 1.3% of an administered dose may be excreted in this
way. (Vorherr, 1974). After a single, oral dose of chloramphenicol, a
peak milk concentration of 3 mg/l has been reported. This peak was
reached around 2 hours after administration, with a drop to nearly
pre-dosing levels by 8 hours post-dose (Plomp et al., 1983). Other
workers have reported similar findings (Knowles, 1965; Matsuda, 1984).
Biotransformation
Early studies indicate that the major metabolite of
chloramphenicol in the rat was the glucoronide conjugate, which was
found along with free chloramphenicol after oral dosing
(Glazko et al., 1950; Glazko et al., 1952). In in vitro studies
it has been demonstrated that the glucuronide is the main metabolic
product in isolated rat hepatocytes exposed to chloramphenicol
(Siliciano et al., 1978). Glucuronidation of chloramphenicol was
elevated in vitro in hepatocytes obtained from phenobarbital-
pretreated rats. This increase correlated with differential induction
of UDP-glucuronyltransferase in hepatocytes of rats pretreated with
phenobarbital (Ullrich & Bock, 1984).
Several metabolites of chloramphenicol have been identified in
rat urine. In addition to free chloramphenicol (1) and the glucoronide
(2), the oxamic acid (3), alcohol (4), and base (5) derivatives have
been noted in the urine of rats given i.m. doses of [3H]-chlo-
ramphenicol (1R,2R-1- p-nitrophenyl(2,2-dichloroacetamido-
1,3-(1-3H)-propandediol). The acetylarylamine (6) and arylamine
metabolites (7) have also been detected. These metabolites are shown
in Table 1.
Table 1 Metabolites of chloramphenicol in the rat
Compound R1 R2 R3
1. chloramphenicol NO2 COCHCl2 OH
2. glucuronide conjugate NO2 COCHCl2 C6H9O7
3. oxamic acid derivative NO2 COCHO2H OH
4. alcohol derivative NO2 COCH2OH OH
5. base derivative NO2 H OH
6. acetylarylamine derivative NHCOCH3 COCHCl2 OH
7. arylamine derivative NH2 COCHCl2 OH
Based upon recovered radioactivity, the major metabolites
appeared to be chloramphenicol base (approx. 26%) and the
acetylarylamine derivative (19.1%). The other metabolites were
recovered in the 8-15% range, except for the arylamine derivative,
which represented approximately 4% of the recovered radioactivity
(total identified, 93.4% of administered radioactivity; total
collected, 95.9% of administered radioactivity (Bories et al.,
1983).
An in vitro study using perfused rat liver and rat liver
microsomes indicated that the arylamine derivative may undergo
N-oxidation to form nitrosochloramphenicol by way of the N-hydroxy
derivative, which may then be conjugated with glutathione
(Ascherl et al., 1985).
There are few data available on the metabolism of chloramphenicol
in other species. In dogs, chloramphenicol, chloramphenicol base, and
chloramphenicol glucuronide conjugate appeared to be the major
metabolites (Glazko et al., 1950). Chloramphenicol, the glucuronide
conjugate, and the oxamic acid, acetylarylamine, arylamine, and base
derivatives were noted in the urine of goats given i.m. injections of
chloramphenicol (Bories et al., 1983). In vitro studies with pig
liver showed the activity of UDP-glucuronyltransferase in this species
to be similar to that in rats, suggesting that glucuronidation may be
a significant pathway of biotransformation of chloramphenicol in pigs.
The same experiments with sheep and cattle liver preparations showed
that they have much lower glucuronyl transfer activities (25% and 14%,
respectively). This may indicate that glucuronidation plays a less
important role in these species (Smith et al., 1984).
In human beings, 93% of an oral dose of chloramphenicol was
excreted in the urine within 24 hours, and it seems likely that the
major excretion product was the glucuronide conjugate. Approximately
48% of the chloramphenicol excreted in the urine within 8 hours after
oral dosing was the glucuronide conjugate; only 6% was excreted as the
parent compound and 4% as the base derivative (Baselt, 1982;
Nakagawa et al., 1975). The alcohol derivative has been detected in
the urine of neonates (Dill et al., 1960). More recent experiments
have confirmed the presence of the glucuronide conjugate and base
derivative as the major metabolites after an oral dose of 500 mg
chloramphenicol (Bories et al., 1983).
Human liver has the potential to reduce chloramphenicol. In 10
livers studied, nitro reductase activity was observed, which was
dependent on NADPH. Thus, human livers may have the capacity to
convert the nitro group of chloramphenicol to the amine, with the
further possibility of nitroso formation. Esters of chloramphenicol,
for example, the succinate, are converted to chloramphenicol in vivo
(Salem et al., 1981).
Effects on enzymes and other biochemical parameters
Chloramphenicol is known to increase the plasma levels of certain
drugs (e.g., paracetamol and phenytoin) and to prolong barbiturate
sleeping time, which is indicative of an effect on drug metabolizing
enzymes (Halpert & Neal, 1981; Nair et al., 1981; Aravindaksham &
Cherian, 1984).
The major contribution to these effects may arise from
chloramphenicol's ability to act as a suicide substrate in the
inactivation of cytochrome P-450, possibly by the binding of the
oxamic acid derivative to lysine residues of the cytochrome molecule
(Halpert & Neal, 1981; Halpert, 1981; Halpert, 1982). Of the various
isozymes of cytochrome P-450, those induced by phenobarbital appear to
be the most sensitive to chloramphenicol. Rats were pretreated with
various inducers of cytochrome P-450 (phenobarbital, ß-naphthoflavone,
pregnenolone, 16-x-carbonitrile, and clofibrate) and were then
injected i.p. with 300 mg/kg b.w. chloramphenicol. The isozymes of
cytochrome P-450 induced by phenobarbital were inhibited to some
degree by chloramphenicol administration, but those forms induced by
the other compounds investigated were not affected. Cytochrome
P-450-c, the form present in non-pretreated rats, was the most
susceptible isozyme in vivo and in vitro (Halpert et al., 1985).
Following i.v. or i.p. administration of 100 mg/kg b.w.
chloramphenicol to rats, inhibition of the conversion of n-hexane to
2-hexanol by rat liver or lung microsomes occurred. There was no
effect on the conversion of n-hexane to l-hexanol, while only liver
microsomes were markedly inhibited in the formation of 3-hexanol
(Naesland & Halpert, 1984; Naesland et al., 1983).
Chloramphenicol prevented the methanol-potentiated toxicity of
carbon tetrachloride in rats, possibly by deactivation of cytochrome
P-450 (Brabec et al., 1982). Phenobarbital-induced cytochrome P-450
appears to be responsible for the dechlorination of chloramphenicol
and the formation of chloramphenicol aldehyde in rat liver microsomes.
The dechlorination may be enhanced by glutathione. The significance of
these findings is not clear at the present time (Morris et al.,
1982; Morris et al., 1983).
Subcutaneous administration of up to 100 mg/kg b.w. chloram-
phenicol to rats inhibited hepatic N-demethylase, glucose-6-
phosphate dehydrogenase, and carboxylesterase (Hapke et al., 1977).
However, no effects were observed on monoamine oxidase in the liver,
brain, or heart of rabbits given 60 mg/kg b.w. chloramphenicol i.m.
for 5 days. Similarly, no effect on monoamine oxidase activity was
seen in in vitro experiments where rabbit liver preparations were
preincubated with chloramphenicol (Ali, 1985).
Several studies have demonstrated an effect of chloramphenicol on
mitrochondrial protein synthesis. In vitro, chloramphenicol
inhibited mitochondrial protein synthesis in rat liver and rabbit bone
marrow. The effect was similar to that noted with tetracycline (Summ
et al., 1976). Nitrosochloramphenicol inhibited rat mitochondrial
DNA polymerase in vitro, whereas the arylamine derivative and
chloramphenicol itself did not (Lim et al., 1984). Nitroschlor-
amphenicol inhibited the transport of NAD-linked substrates into
mitochondria, but it had no effect on FAD-linked substrates. It
inhibited ATP formation and completely blocked the transport of
protons out of the mitochondria (Abou-Khalil et al., 1982).
Chloramphenicol inhibited the incorporation of leucine into protein in
mitochondria from erythroid and myeloid rumour cells and in cells from
normal, myeloid, and erythroid hyperplastic bone marrow of rabbits
in vitro (Abou-Khalil et al., 1980, 1981). In mice given
chloramphenicol, the so-called megamitochondria that were produced
were deficient in cytochrome oxidase, ATP synthetase, and cytochrome b
activities, which is indicative of inhibited protein synthesis
(Wagner & Rafel, 1977). Subcutaneous administration of 500 mg/kg b.w.
chloramphenicol succinate to partially hepatectomized rats did not
significantly inhibit protein synthesis. However, liver cytochrome c
oxidase was strongly inhibited in these rats (Kroon & Vries, 1969).
Intramuscular administration of chloramphenicol to rats inhibited
mitochondrial monoamine oxidase activity in the liver, brain, and
kidney (Banerjee & Basu, 1978). When chloramphenicol was given daily
for 6 days at doses of 100 mg/kg b.w. i.p., marked reductions in the
activities of liver kynurenine hydrolase, knynurenine amino-
transferase, ß-glucuronidase, and acid ribonuclease were observed,
indicating possible effects on drug metabolizing enzymes and on
protein biosynthesis. Pyridoxal phosphokinase activity was increased
(Akhnoukh et al., 1982).
In human blood, nitrosochloramphenicol is bound to albumin
in vitro. In red blood cells, it rapidly forms adducts with
glutathione and also with the -SH groups of haemoglobin. Moreover, it
is rapidly reduced to the N-hydroxy compound, although further
reduction to the amine is very slow. Overall, the data suggest that
the nitroso derivative would be rapidly detoxified in the blood before
reaching the bone marrow (Eyer et al., 1984). Nitrosochloramphenicol,
but not chloramphenicol, induced methaemoglobinaemia in haemolyzed
human blood in vitro (Lim & Yunis, 1982).
To summarize, chloramphenicol is well absorbed in both animals
and human beings after oral administration and widespread distribution
occurs. Urinary excretion is rapid in animals and man, a major
metabolite being the glucuronide conjugate. Animals and human beings
produce a range of urinary metabolites; in vitro experiments suggest
that nitrosochloramphenicol may be an important metabolite in human
beings. It is not known if this compound is produced in vivo in man
or other species.
Toxicological studies
Special studies on carcinogenicity
In 1982 and 1987, IARC concluded that adequate tests for
carcinogenicity of chloramphenicol in animals were not available
(IARC, 1982; IARC, 1987). These conclusions confirmed an earlier
opinion by IARC workers (Tomatis et al., 1978). The two studies
below by Robin et al., and by Sanguineti et al., were included in
the IARC evaluations.
Four groups of Balb/c × AF1 male mice, 45 per group, were given
i.p. injections of either 0.5 mg busulphan on days 1, 15, 29 and 43
(2 groups) or vehicle (acetone plus distilled water, 2 groups. After
20 weeks, by which time 78 mice remained alive in the busulphan-
treated groups and 88 in the vehicle groups (the rest having
died of injection complications), one of the busulphan groups and one
of the vehicle groups was selected for treatment with chloramphenicol,
while the others served as controls and were given vehicle only (0.9%
sodium chloride). Mice in the treatment groups received i.p.
injections of 2.5 mg chloramphenicol 5 days per week for 5 weeks.
Sacrifice of the mice followed a complicated schedule depending on the
appearance of lymphomas, but all animals had been sacrificed by day
350. The following incidences of lymphoma were noted: busulphan/
chloramphenicol, 13/37; busulphan/vehicle, 4/35; chloramphenicol/
vehicle 2/42; vehicle/vehicle, 0/41. The authors thought that this
suggested that busulphan and chloramphenicol increased the frequency
and accelerated the onset of lymphomas. It also provided some
evidence that chloramphenicol alone might induce lymphomas in this
animal model, but the duration of the experiment along with other
limitations of the experimental design, particularly the dosing
regime, prevent any other conclusions from being drawn (Robin
et al., 1981).
In a study which was reported in abstract form only, in which
chloramphenicol was administered in the drinking water, an increased
incidence of lymphomas in two strains of mice and of hepatocellular
carcinoma in one strain were noted (Sanguineti et al., 1983).
Another study investigated the effect of chloramphenicol on
cirrhosis and hepatocellular carcinoma induced by the carcinogen
N-2-fluorenyldiacetamide in rats. A group of 25 male Wistar rats was
given a diet containing 0.05% N-2-fluorenyldiacetamide (equivalent to
25 mg/kg b.w./day) plus 2% chloramphenicol (equivalent to 1000 mg/kg
b.w./day) for 4 weeks. Another group of 20 rats received a diet
containing only the 0.05% N-2-fluorenyldiacetamide. A "rest" week was
then followed by another 4 weeks on the respective diets, followed by
another "rest" week and then 6 weeks of dietary administration of the
substances. After this the animals given the carcinogen only were
returned to the control diet, but those given the combined regime were
continued on this diet for another 2 weeks. Animals were sacrificed at
46 (carcinogen only) or 46-55 weeks (carcinogen plus chloramphenicol).
Of 12 animals examined which were given the carcinogen-only diet, 100%
had cirrhosis and 75% had hepatocellular carcinoma, whereas of 22
animals examined which were given the combination treatment, only one
had cirrhosis and hepatic rumours. Thus, chloramphenicol had a
protective effect on the induction of hepatic rumours by
N-2-fluorenyldiacetamide (Puron & Firminger, 1965).
Special studies on haematological effects
Groups of 18-21 57B1/10ScSnPh mice were given 4.78 Gy of
X-irradiation and were then treated three times daily with 160 or
320 mg/kg b.w. chloramphenicol succinate by s.c. injection over 3 or 5
days, beginning 10 days after the X-ray treatment. Two groups of
control animals were given chloramphenicol and no irradiation or
irradiation and no chloramphenicol. Animals were examined 4, 8, and 21
days after initiation of chloramphenicol treatment (14, 18, and 21
days after irradiation). No effects on the level of erythrocytes in
non-irradiated mice occurred; those given chloramphenicol showed
similar values to non-treated controls. The level of erythrocytes in
irradiated animals was significantly lower than in non-irradiated
animals (30% reduction 14 days after irradiation) but there was
improvement with time (26% reduction 18 days and 15% reduction 21 days
after irradiation), while irradiated animals given chloramphenicol
showed lower erythrocyte levels than those that were irradiated only;
at 14, 18, and 21 days post irradiation, irradiated mice given
chloramphenicol had erythrocyte levels 8%, 17%, and 4.5% lower,
respectively, than animals given irradiation alone, indicating that
chloramphenicol had a deleterious effect on bone marrow recovery after
X-irradiation (Vacha et al., 1981).
Normal mice and those with residual bone marrow damage following
busulphan administration were investigated for the effects of
chloramphenicol. Female Balb/c mice were injected with busulphan to
create bone marrow damage using a method that had been validated
previously by the authors. Groups of mice, some having busulphan-
induced damage and others untreated, were then given drinking water
containing 0.5 g/dl chloramphenicol succinate; groups of 5 mice were
then killed at various intervals up to and including 150 days after
chloramphenicol treatment. No effects were seen in the bone marrow
of mice given chloramphenicol alone nor in mice pretreated with
busulfan. However, animals given both busulphan and chloramphenicol
displayed a progressive fall in the number of pluripotential stem
cells and granulocytic precursor cells (Morley et al., 1976).
Similar effects were not seen in another study where busulphan-
treated mice were given drinking water containing 0.5 g/dl
chloramphenicol for six weeks. There was no effect on the colony-
forming ability of bone marrow or spleen cells. This study used
an identical busulphan regime to that described above (Pazdernik &
Corbett, 1980).
In cells of lethally X-irradiated mice given 10 mg
chloramphenicol i.p. on days 2-8 or on days 7-12 after irradiation,
mitochondrial swelling was noted in early erythroblasts, but not in
intermediate or late types. The mitochondria showed reductions in the
number of cristae (Miura et al., 1980).
A similar investigation in mice given 4.78 Gy X-irradiation and
300 mg/kg b.w. of chloramphenicol succinate showed that dividing bone
marrow cells had decreased entry into S-phase of the cell cycle. The
affected cells were mainly of the erythroid type. The same types of
effect were also noted in non-irradiated mice given chloramphenicol
(Benes et al., 1980).
The femora of mice given 500 mg/kg b.w. chloramphenicol for 6
days by injection (route unspecified) which were then implanted into
untreated syngeneic mice showed greatly decreased colony formation
when the bone marrow was subsequently injected into lethally
irradiated mice (Nara et al., 1982).
No haematological effects, including aplastic anaemia, were seen
in mice given 40 mg/kg nitrosochloramphenicol for 10 days followed by
sacrifice 6 weeks after the last injection (Siegel & Krishna, 1980;
abstract only).
Groups of 6 male Sprague-Dawley rats were each given 50 mg/kg
b.w. chloramphenicol succinate by i.v. infusion. Half of the animals
were subjected to liver resection 15 minutes after infusion, while the
others were sacrificed at this time. Bleeding time and blood loss were
significantly increased in resected animals given chloramphenicol
compared with controls (bleeding time: 500 seconds in treated animals,
300 seconds in controls; blood loss 2.2 g in treated animals, 0.9 g in
controls). No effects on haemoglobin or haematocrit were observed
following infusion or liver resection (Bengmark et al., 1981).
Four cats were given daily i.m. injections of 50 mg/kg b.w.
chloramphenicol for 21 days. Two untreated cats served as controls;
all 6 animals had recently recovered from experimental infectious
feline enteritis. Animals given chloramphenicol became very ill, with
severe loss of appetite developing within 7 days. All four developed
diarrhoea toward the end of the experiment (they were sacrificed on
day 21); one was killed in extremis. Marrow examination was carried
out, which revealed vacuolation of the myeloid and erythroid
precursors and of some lymphocytes. There were no significant changes
in peripheral red cell numbers, but white cell counts were much
reduced (Penny et al., 1967).
A group of 6 cats was given chloramphenicol orally at a dose of
125 mg/kg b.w./day for 14 days and then observed for another 3 weeks.
Another group, formerly intended as controls, were dosed with 60 mg/kg
b.w./day chloramphenicol in the same manner. Signs of toxicity
included CNS depression, dehydradation, loss of appetite, body weight
loss, diarrhoea, and vomiting. Blood and bone marrow samples were
obtained both prior to and after chloramphenicol treatment. The major
findings related to chloramphenicol administration were severe bone
marrow suppression with marrow hypoplasia, prevention of maturation of
erythroid cells, and inhibition of mitosis in the marrow. Vacuolation
of lymphocytes, and early myeloid and erythroid cells occurred. The
effects were most severe in cats given 120 mg/kg b.w. chloramphenicol.
On cessation of treatment the bone marrow suppression resolved (Watson
& Middleton, 1978).
In a similar study, 5 cats were given 50 mg/kg b.w./day
chloramphenicol orally for 21 days. CNS depression, appetite loss, and
weight loss were noted. Examination of peripheral blood was conducted
both before and after dosing. This revealed lower platelet numbers
after 1 week, and a lower neutrophil count after 3 weeks of
administration. One animal developed lymphocytopenia after 1 week and
neutropenia after 3 weeks. At the end of treatment, the bone marrow
was found to have vacuolated early myeloid cells and lymphocytes, with
reduced myeloid maturation and reduced marrow cellularity. No test for
recovery was conducted (Watson, 1980).
Twenty dogs were given oral doses of chloramphenicol for 14 days.
They were dosed in the following manner: 6 dogs, 225 mg/kg b.w./day;
4 dogs each, 175 or 125 mg/kg b.w./day; and 3 dogs each at 275 or
75 mg/kg b.w. day. Signs of toxicity included a decline in the rate of
weight gain and hypophagia. No changes in erythrocyte counts,
reticulocyte counts, haemoglobin concentration, packed cell volume, or
differential leukocyte counts occurred, but bone marrow examination of
the dogs given 225 or 275 mg/kg/b.w./day revealed suppression of
erythropoiesis. In dogs given 275 mg/kg b.w./day chloramphenicol,
decreased mitotic activity and a reduced rate of granulocyte formation
was also evident. No dogs showed bone marrow vacuolation
(Watson, 1977).
After i.v. administration of a single dose of 50 mg/kg b.w.
chloramphenicol succinate to five mongrel dogs, platelets were
obtained from blood at 0, 30, 60, 120, 180, and 240 minutes and at 24
hours after dosing. Protein synthesis as determined by the rate of
incorporation of 3H-leucine was inhibited, with maximum depression
(940% of control values) occurring at 30 - 240 minutes after dosing
(Agam et al., 1976).
Neonatal Holstein calves (1 day of age at the beginning of the
experiment) given "an adequate" intake of colostrum were given
chloramphenicol by several methods. These were, i.v. as a 25 mg/kg
b.w. bolus at 1, 7, 14, and 28 days of age, i.v. as a 25 mg/kg b.w.
injection every 12 hours until 150 mg/kg b.w. had been delivered, and
as a 25 mg/kg b.w. bolus i.v., i.m., or s.c. alternately, with 1 week
between the doses. No effects on haematological parameters were
observed, and bone marrow aspirates showed no evidence of suppression
or toxicity (Burrows et al., 1984).
A similar lack of effect on the bone marrow was noted in a study
of cross-breed calves given doses of 9, 20, or 60 mg/kg b.w.
chloramphenicol daily for 6 weeks (Mitema, 1982).
In another study in Holstein calves in which chloramphenicol was
given orally at a dose of 100 mg/kg b.w./day over 10 days, bone marrow
suppression did occur. Similar results were noted in over 50 calves
investigated over a period of time. Changes included partial aplasia
to almost complete aplasia of the marrow, with loss in cellularity of
erythrocytes, white cells, and megakaryocytes. Occasionally only fat
deposits were noted with lymphocytic infiltrations. Lower blood levels
of chloramphenicol were attained after oral intake than following i.v.
injection, but the toxic effects on the marrow were greater after oral
dosing (Krishna et al., 1981).
In in vitro experiments, chloramphenicol and its postulated
metabolite, nitrosochloramphenicol, have shown adverse effects on bone
marrow cells. Chloramphenicol caused dose-related inhibition of
erythroid and granulocytic colony forming units obtained from LAF1
mice. The lowest concentration used (5 µg/ml) caused some degree of
inhibition of erythroid cells, while the highest concentration
(60 µg/ml) produced complete inhibition (Yunis, 1977). Similar effects
were noted in a separate study (Hara et al., 1978).
Chloramphenicol and nitrosochloramphenicol inhibited DNA
synthesis in rat bone marrow cells in vitro. This was reversible
with chloramphenicol, but not with the nitroso compound. Similarly,
the nitroso compound, but not chloramphenicol, bound irreversibly to
bone marrow cells (Gross et al., 1982). However, in another
in vitro study, chloramphenicol and nitrosochloramphenicol had no
effects on mouse haematopoietic precursor cells (Pazdernik & Corbett,
1979).
Special studies on mutagenicity
The antibiotic activity of chloramphenicol is not thought to
involve any type of reaction with bacterial genetic material. It
appears to inhibit protein synthesis by binding to the 50S subunit of
the 70S ribosome, inhibiting the formation of the peptide bond during
protein synthesis (Smith & Weber, 1983, Gilman et al., 1985).
Chloramphenicol has been shown to cause DNA strand breaks in
bacterial cells and to inhibit DNA synthesis in lymphocytes and in a
phage of E. coli (Amati, 1970; Dewse, 1977; Jackson et al., 1977).
However, chloramphenicol provided resistence to UV-induced damage in
E. coli B/r (Doudney & Rinaldi, 1985).
Nitrosochloramphenicol, which is a potent inducer of DNA strand
breaks in bacterial cells, resulted in strand breakage and loss of
helix, bringing about rapid degradation of isolated E. coli DNA
in of helix, bringing about rapid degradation of isolated E. coli
DNA in vitro (Skolimowski et al., 1981, 1983). Nitrosochlor-
amphenicol, but not chloramphenicol, produced inhibition of DNA
synthesis and caused DNA strand breaks in E. coli. (Murray
et al., 1982; Yunis, 1984).
Chloramphenicol has been tested in a variety of assays for
mutagenic activity. Most of these assays are well established using
well validated methods, but some, such as colicine induction, the
induction of tandem genetic duplications in S. typhimurium, and
tests using snails, are less well known and are unvalidated. The
results of these tests are presented in Table 2.
In general chloramphenicol gave negative results in bacterial
reverse mutation assays, in assays for DNA repair in bacteria, in the
dominant lethal test in rodents and D. melanogaster, in the
CHO/HGPRT test, in a test for sister chromatid exchange in human
lymphocytes, in the micronucleus test, and in a test for DNA binding.
Tests for inhibition of growth in E. coli were also negative and,
moreover, chloramphenicol has sometimes been used as a negative
control in this assay (McCoy et al., 1980a; McCoy et al., 1980b;
Rosenkranz, 1977; Rosenkranz et al., 1974; Braun et al., 1977;
Braun et al., 1982). There were isolated exceptions to these
negative results. However, the only type of test that gave
consistently positive results was the test for induction of
chromosomal aberrations. Chromosomal anomalies were noted in human
lymphocytes treated in vitro with chloramphenicol, in mouse bone
marrow in animals given chloramphenicol at doses of 50 mg/kg i.p. or
i.m., and in F1-generation mouse liver.
The failure of chloramphenicol to give positive results in the
Ames test has been attributed by one group of authors to its bacterial
toxicity. In their study, the D(-)-threo isomer of chloramphenicol
gave negative results in the Ames test, as observed previously in
several other studies; it was also toxic to the bacterial tester
strains used, TA100 and TA1535. However, the L(+)-threo isomer,
which is not used therapeutically, was much less toxic and could be
tested at higher concentrations; with the isomer, a dose-related
mutagenic response was noted in both tester strains (Jackson et al.,
1977).
The failure of chloramphenicol to give positive results in most
types of commonly used mutagenicity tests, with the exception of those
examining the induction of chromosome aberrations, has been recognized
by other reviewers (Waters et al., 1983; Garrett et al., 1984), as
has the failure of the substance to produce mutations in vivo
(Holden, 1982).
Chloramphenicol has been reported to enhance the mutagenicity of
N-methyl-N-nitro-N-nitrosoguanidine in bacterial assays (Sklar &
Strauss, 1980; Baltz & Stonesifer, 1985).
Special study on ocular toxicity
No toxic effects were seen in groups of three rabbits following
vitrectomy when solutions containing 10 or 20 µg/ml chloramphenicol
were infused into the eyes as vitreous replacements. Histological and
electroretinographical examinations yielded normal results 2 weeks
after infusion. Following an infusion of a 50 µg/ml solution,
electroretinography was normal after 2 weeks, but abnormal retinal
histology, which was not described but was said to be widespread and
generalized, was noted (Stainer et al., 1977).
Special studies on ototoxicity
Solutions of 0.5% chloramphenicol introduced into the bullae of
the ears of guinea pigs produced no effects on hearing, but solutions
containing 1 - 5% chloramphenicol produced moderate hearing loss at a
variety of frequencies. Similar findings were noted when the
electrical responses of the hair cells were measured after
chloramphenicol administration into the bullae. Intratympanic
administration to guinea pigs of a 1% solution of chloramphenicol
produced a moderate degree of hair cell loss in the organ of Corti,
with severe inflammation of the mucosa of the middle ear. Introduction
of a solution containing 8 or 16 mg chloramphenicol through a hole in
the bullae of the ears of guinea pigs followed by sacrifice 3, 6, 9,
or 24 hours later resulted in severe destruction of hair cells and
supporting cells in the basal turns of the organ of Corti. The effects
were similar regardless of the dose or the time of sacrifice after
dosing (Proud et al., 1968; D'Angelo et al., 1967; Patterson &
Gulick, 1963; Morizono & Johnstone, 1975; Parker & James, 1978).
Table 2. Results of mutagenicity assays with chloramphenicol
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 30 µg/plate - Brem et al.,
TA1530, TA1535 1974
TA1538
S. typhimurium 0.17-24 µg/ml + Mitchell et al.,
TA98 1980
S. typhimurium Not given - Heddle & Bruce,
TA1535, TA1537 1977
S. typhimurium 30 µg/plate - Rosenkranz et al.,
TA98, TA100 1976
S. typhimurium < 4.5 nmole - McCann et al.,
TA98, TA1535 1975
TA1538
Bacterial E. coli 27 µg/ml + Mitchell &
mutation assay CM891 Gilbert, 1984
CHO/HGPRT Chinese hamster Not given Augustine et al.,
mutation assay ovary cells 1982
Gene mutation D. melanogaster Not given +a Narda & Gupta,
1972
(abstract only)
Dominant lethal D. melanogaster Not - Nasrat et al.,
1977
Mouse (101×C3H)F1 2×1.5 g/kg - Ehling, 1971
Mouse (ICR/Ha 333 mg/kg - Epstein & Shafner,
Swiss) 1968
Mouse (ICR/Ha 333 & 666 mg/kg - Epstein et al.,
Swiss) 1972
DNA repair B. subtilis 2.5×10-3 mg/ - Sekizawa &
H17, M45 disc Shibamoto, 1982
B. subtilis Not given - Suter & Jaeger,
H17, M45 1982
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
E. coli Not given + Suter & Jaeger,
AB1157/JC5547 1982
AB1157/JC2921
AB1157/JC2926
AB1157/JC5517
E. coli WP2 Mitchell et al.,
uvrA+recA+,uvrA- Not given - 1980
recA-
trp-/trp+ Not given -
A2Cs/A2Cr 3-48 µg/ml +
E. coli B/r 100 µg/ml - Masek, 1977
E. coli K12 > 30 µg/ml - Mamber et al.,
(SOS chromotest) 1986
Preferential E. coli K12 5-20 µg/ - Hyman et al.,
inhibition plate 1980
E. coli 30 µg/plate - Brem et al.,
Pol A+, Pol A- 1974
E. coli 10 µg/plate - McCoy et al.,
Pol A+, Pol A1- 1980a,b
E. coli 30 µg/plate - Longnecker et al.,
Pol A+, Pol A- 1974
E. coli 30 µg/disc - Slater et al.,
Pol A+, Pol A- 1971
Gene conversion S. cerevisiae Not given - Mitchell et al.,
D4 1980
Sister chromatid Human lymphocytes 200 µg - Pant et al., 1976
exchange (in vitro)
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Chromosomal Zea mays 30 µg/ml - Verma & Lin, 1978
aberrations
Human lymphocytes 10-40 µg/ml + Mitus & Coleman,
(in vitro) 1970
Human lymphocytes Not given + Goh, 1979
(in vitro)
Human lymphocytes 200 µg + Pant et al., 1976
Mouse, bone 50 mg/kg b.w. + Manna & Bardham,
marrow 3×50 mg/kg + 1977
b.w., 8h
intervals
Mouse, F1 liverb 50 mg/kg b.w. + Manna & Roy, 1979
Micronucleus Tradescantia 0.1 - 5mM - Ma et al., 1984
test paludosa
Mouse Not given (5 - Heddle & Bruce,
(CH3×C57)F1 daily doses) 1977
DNA binding E. coli 100-1000 µM - Kubinski et al.,
assay 1981
Enhancement of Syrian hamster 0.7-5mM + Hatch et al.,
SA7 virus cell embryo cells/ 1986
transformation simian adenovirus
SA7
Aneuploidy Hordeum vulgare 300 µg/ml + Yoshida et al.,
induction 1972
Colicine S. typhimurium 0.1-60 µg/ - Ben-Gurion, 1978
induction TA1537 REN plate
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Increased Snail Not given - Xie, 1985
embryonal length
Induction of S. typhimurium 0.155-3.1 µM - Pall & Hunter,
tandem genetic TR4179, TT1984 1985
duplication
a Very weak positive response.
b One male mouse was given 50 mg/kg chloramphenicol i.m. and then was mated
with 4 untreated females. Mice (3) were sacrificed on days 12, 16, and 18
of gestation. The remaining mouse was allowed to litter normally and the
young were sacrificed when 7 days old. Livers of fetuses and neonates were
removed and examined.
When groups of 3 - 9 female Sprague-Dawley rats were given
80 mg/kg b.w. chloramphenicol in the drinking water for 10 days with
or without exposure to short duration high intensity noise,
ototoxicity was noted as revealed by reductions in the electrical
output of the cochlea. Noise exposure alone also reduced the output,
but noise and chloramphenicol together resulted in a severe effect
(Henley et al., 1984). Similar effects were reported in abstract
form in another study in rats (Henley, 1985).
Chloramphenicol was given to guinea pigs (number unspecified) as
a single i.v. dose of 400 mg/kg b.w. The threshold for the Preyer
reflex was measured after administration and at 10, 20, and 30
minutes, 1, 2, 3, 4, and 5 hours, and then daily for 7 days. There was
no change in the Preyer reflex with noise of 1 and 8 KHz and no loss
of hair cells, indicating no ototoxic response in this study
(Beaugard et al., 1979; Beaugard et al., 1981).
Special study on effects on sleep
Oral doses of 160 250 mg/kg b.w. chloramphenicol suppressed
paradoxical sleep in cats. After a dose of 330 mg/kg b.w. paradoxical
sleep was suppressed for 24 hours, at which time there was also a
depression of slow wave sleep (Petitjean et al., 1975).
Special study on spermatogenesis
A group of male rats was treated daily with 100 mg/kg b.w.
chloramphenicol succinate for 8 days (route and animal numbers not
stated), after which they were sacrificed and the testes examined
histologically. Examination revealed total or incomplete inhibition of
spermatogonial divisions with "perturbed meiosis". No other details
were provided (Timmermans, 1974).
Special studies on teratogenicity
Monkeys
Chloramphenicol at doses of up to 200 mg per monkey
(10 mg/kg b.w.) had no effect on the development of Macaca mulatta
when given for 6 to 17 days at various intervals between the 65th and
95th days of gestation (Courtney et al., 1967; Courtney & Valerio,
1974).
Rabbits
Groups of 5 - 8 pregnant mixed breed rabbits were given 500,
1000, or 2000 mg/kg b.w./day chloramphenicol by garage on days 6 - 15,
6 - 9, or 8 -11 of gestation, respectively. Historical control data
collected over the previous 4 year period, using 192 rabbits, were
used for comparison. Excess fetal deaths did not occur in rabbits
given 500 mg/kg b.w./day chloramphenicol, but in the mid- and
high-dose groups 25% and 58% fetal deaths were noted, respectively,
compared with 10% in the historical controls. There were no excess
incidences of fetal malformations, but delayed ossification was noted
in fetuses from dams given chloramphenicol (Fritz & Hess, 1971).
Chicks
Chick eggs, less than 3 days old, were treated with 0.1 ml of
chloramphenicol solution in distilled water. The chloramphenicol, at
doses of 0.5 or 1.0 mg/egg, was injected into the albumen via the
airsac. The major anomaly observed was vesiculation of the heart and
trunk resulting from the inhibition of differentiation of the
splanchnopleure; this effect was most severe following 16 - 19 hours
of incubation (36 - 57% in the 0.5 mg/egg group and 23 - 47% in the
1.0 mg/egg group). Unfortunately, no control data were provided
(Blackwell, 1962).
Chloramphenicol also had adverse effects on development in a
separate study in which fertilized eggs with embryos at the 14- or
20-somite stages were explanted and exposed to chloramphenicol at
concentrations of 0, 200, or 300 µg/ml for 22 - 24 hours. The major
defects noted were those of the neural tube (failure to close) and
forebrain. There was also evidence of an inhibition of haemoglobin
formation (Billet et al., 1965).
Rats
Pregnant Sprague-Dawley rats (5 - 15 per group) were given gavage
doses of 500, 1000, 1500, or 2000 mg/kg b.w./day chloramphenicol over
various stages of gestation. In addition, single gavage doses of
2000 mg/kg b.w. were given to pregnant rats on days 5, 6, 7, 8, 9, or
10 of gestation. A group of 553 historical control rats was used for
comparison. Even the lowest daily dose of chloramphenicol on days 5 -
15 of gestation resulted in significant embryo/fetal deaths (63%) when
compared with historical controls (23%), whereas doses of 2000 mg/kg
b.w./day on days 15 - 17 or 2000 mg/kg b.w. on days 5, 6, or 7 had no
effect. Single doses of 2000 mg/kg b.w. on days 8, 9, or 10 of
gestation resulted in 45% fetolethality, but the most sensitive period
appeared to be days 9 - 15. For example, fetal deaths occurred 100% of
the time when 2000 mg/kg b.w./day was given over days 9 - 11 of
gestation. The highest incidences of anomalies, umbilical hernia, were
noted at 2000 mg/kg b.w./day when given over days 6 - 8 of gestation
(36%) and at 2000 mg/kg b.w. on day 8 (11%). High incidences of
delayed ossification were seen in fetuses from dams given 1000 mg/kg
b.w./day chloramphenicol on days 7 - 12 or 2000 mg/kg b.w./day on days
11 - 13 (Fritz & Hess, 1971).
The effects of chloramphenicol on pre-implantation embryos in the
rat were investigated by treating groups of 7 pregnant Sprague-Dawley
rats with 250 mg/kg b.w. chloramphenicol i.p. on either day 3 or 5 of
gestation. The animals were killed on day 5 of pregnancy and the
uterine contents examined. No effects on the number of blastocysts per
female were noted, but administration on day 3 of pregnancy
significantly reduced the number of cells per blastocyst. A reduction
was also seen when the compound was given on day 14, but these results
were not statistically significant (Giavini et al., 1979).
Pregnant Sprague-Dawley rats (number unspecified) were given 3%
dietary chloramphenicol, equivalent to 1500 mg/kg b.w./day, from days
0 to 20. The number of resorptions (% of total implants) was elevated
in rats treated with chloramphenicol (31.4 - 57.0%) compared with
controls (4.7%), while fetal weight in treated rats was only 50% of
controls. Placental weight was also much reduced, while the number of
live fetuses was greatly reduced by chloramphenicol. The authors then
examined the effects of chloramphenicol when given on specific days of
pregnancy (0 - 2, 0 - 3, 0 - 4, etc., up to 0 - 12). The major effects
on implantations, resorptions, number of live fetuses, and fetal
weights as described above were seen in the 0 - 8 to the 0 - 12
feeding schedules, suggesting an effect on implantation (or effects on
embryos soon after implantation). A large proportion of fetuses (71%)
had edema and there was an increased incidence of wavy ribs (7%) and
fused ribs (7%) in chloramphenicol-treated groups compared with
controls (zero incidences in all cases in controls) (Hackler et al.,
1975).
Chloramphenicol was investigated for its effects on avoidance
learning in rats. Four groups of 15 pregnant Wistar rats each were
treated as follows: in one group 50 mg/kg b.w. chloramphenicol
succinate was given s.c. on days 7 - 21 of gestation. In two other
groups, 50 or 100 mg/kg b.w. was given s.c. to pups for the first 3
days after birth; the fourth group served as controls. No effects on
pregnancy, litter size, fetal weight, post-natal weight gain, or
incidence of gross malformations were seen. When 60 days old, animals
were selected for conditioned-learning study and were then examined
for avoidance learning at days 5, 10, 15, and 20 from the start of the
conditioning procedure. Pups from mothers given chloramphenicol
succinate and those given the substance as neonates showed a marked
and statistically significant impairment of avoidance learning at all
four times. The effects were generally worse in pups given the
substance post-natally than in those from dams administered it during
gestation, but the differences were only slight (Bertolini & Poggioli,
1981).
Mice
Groups of 8 pregnant albino mice were given oral chloramphenicol
at doses of 25, 50, 100, or 200 mg/kg b.w. in 10 ml of distilled water
over the third trimester of pregnancy for 5 - 7 days. Animals were
allowed to give birth and the young were tested for conditioned
avoidance response, electroshock seizure threshold, and performance in
open-field tests at days 30, 38, and 42. No gross abnormalities were
seen in any of the progeny. Dose-related effects were seen in all
three elements of the test, with progeny from chloramphenicol-treated
dams showing a reduced learning ability, higher brain seizure
threshold, and poorer performance in the open-field test (Al Hachin &
Al-Baker, 1974).
Groups of 7 - 19 pregnant CD1 mice were given by garage 500,
1000, or 2000 mg/kg b.w./day chloramphenicol on days 5 - 15, 6 - 12,
or 8 - 10 of gestation, respectively. Historical control data,
collected over the previous four-year period using 307 mice, were used
for comparison. In the 1000 and 2000 mg/kg b.w./day dose groups, 71
and 100% embryo/fetal deaths occurred, respectively, compared with 24%
in controls and 31% in mice given 500 mg/kg b.w./day chloramphenicol.
The only defects observed were a low incidence of fused sternebrae and
elevated incidences of delayed ossification in fetuses from dams given
1000 mg/kg b.w./day chloramphenicol (Fritz & Hess, 1971).
In vitro
Chloramphenicol and several other chemicals were tested in a
mouse embryo limb bud cell culture test system which itself was being
validated as part of the study. In all, 22 known mouse teratogens and
5 non-teratogens were investigated in the system, which makes use of
high-density cultures of mouse embryo limb bud cells that can
differentiate and synthesize an extracellular matrix of sulfated
proteoglycans. The end-point of the test considers incorporation of
radiolabel (3H-thymidine) and the growth and synthesis of ocular
protein and cartilage proteoglycan. Chloramphenicol gave a positive
result in this test, with the maximum active concentration being
5 µg/ml. [The test was around 89% predictive; no false positives were
seen and the false negative rate was about 15% (Guntakatta et al.,
1984).]
Another in vitro test made use of the differentiation
characteristics of rat embryo midbrain and limb bud cells, and the
inhibition of differentiation by teratogens. Chloramphenicol gave a
weak response, resulting in inhibition at concentrations in excess of
50 µg/l, compared with 10 µg/l or less seen with several known
teratogens, e.g., captan, colchicine, and parbendazole (Flint & Orton,
1984).
Acute toxicity
Four groups of pregnant and non-pregnant mice were given i.v.
doses (unspecified) of chloramphenicol. No signs of toxicity were
reported. The LD50 value for non-pregnant mice was calculated as
1530 (1260 - 1840) mg/kg b.w., while that for pregnant mice was
1210 mg/kg b.w. (no confidence limits cited) (Beliles, 1972).
Short-term studies
No information available.
Long-term studies
See "Special studies on carcinogenicity".
Observations in human beings
Chloramphenicol is known to produce two major adverse effects in
human beings. One of these is a generally irreversible and often fatal
aplastic anaemia and the other is a reversible bone marrow
suppression.
Aplastic anaemia
Aplastic anaemia is the most dangerous effect produced by
chloramphenicol, although its occurrence is rare. It is usually fatal
(Benestad, 1979). Numerous publications have appeared, most of them
case reports describing the development of aplastic anaemia.
An investigation into the incidence of chloramphenicol-induced
aplastic anaemia in Hamburg suggested that the incidence was 1/11500
with a death rate 1/18500. In the period 1965 - 70, 29 cases were
reported, while in 1971, 3 cases were reported. Total doses in 18
cases were in the range of 10 to 100 g, with most individuals having
received 11 - 30 g. Onset was from 14 days (rare) to 4 - 6 months
after chloramphenicol therapy (Hausmann & Skrandies, 1974).
In a study in Israel in 1985, aplastic anaemia incidence was
7.1/106 in males and 8.7/106 in females. Chloramphenicol was
thought to account for up to 25% of cases. Aplastic anaemia usually
took up to 12 months to develop after chloramphenicol treatment
(Modan et al., 1975).
In 1969 in California, aplastic anaemia in chloramphenicol-
treated patients was said to be 13 times more frequent than in
the general population. Most individuals had been given oral
doses, but in some, aplastic anaemia had occurred after i.m.
administration. Doses were often on the order of 250 mg thrice daily
to a total of 3 g or 250 mg four times daily to a total of 5 g. The
majority of patients were in the 50 - 80 age group, but it was
observed in a 15-year-old boy given a total of 3 g chloramphenicol and
in a 37-year-old female given a total of 6 g over a month. In both
cases onset occurred 3 - 4 months after treatment ended
(Wallerstein et al., 1969).
A series of reports from Sweden in the 1970s suggested that the
incidence of aplastic anaemia was around 80 in 1.2 million. Only 4 or
5 of these were thought to be due to chloramphenicol treatment,
placing the risk at 1 in 20000 (Bottiger & Westerholm, 1972;
Bottiger & Westerholm, 1973; Bottiger, 1978; Bottiger, 1979).
Of 108 cases of aplastic anaemia reported in Istanbul, 4 were
thought to be due to chloramphenicol (Aksoy et al., 1984).
A total of 380 cases of aplastic anaemia were seen at a hospital
in Paris in the years 1971 - 1983; 194 adult cases were considered to
be due to chemical agents. In the period 1971 - 1977, 18/104 cases
were attributed to chloramphenicol, whereas chloramphenicol accounted
for 2/36 and 2/52 cases of aplastic anaemia in the periods 1977 - 1980
and 1980 - 1983, respectively, suggesting a decreasing incidence, at
least in the area of France under investigation (Najean & Baumelon,
1984).
In the period 1975 - 1980, 9 children with aplastic anaemia were
seen at the Medical School, Gadjah Mada University (Yogyakarta). Two
were idiopathic, but at least 3 could be attributed to treatment with
chloramphenicol (Widayat et al., 1983).
A study of 40 cases of aplastic anaemia by members of the
Association of Clinical Pathologists in the USA revealed that 27 were
probably due to chloramphenicol. Of these, 18 had exceeded 10 g or
more (up to 250 g) in total dosage, while 8 had received 10 g or less.
One, an infant, had received less than 2 g chloramphenicol. Onset was
usually 1 - 3 months after drug cessation. Route of administration and
its duration were not specified (Sharp, 1963).
In 1954, of 539 cases of aplastic anaemia from 37 states in the
USA, 55 were attributable to chloramphenicol. In general there was a
female preponderance and usually the effect developed 1 - 6 months
after the drug was withdrawn (Welch et al., 1954).
In a study in Iraq, however, a male preponderance of 3:1 over
females was noted. Of 60 patients with aplastic anaemia at the
University of Baghdad Teaching Hospital, 12 were associated with
chloramphenicol treatment (Al-Moudhiry, 1978).
One study in the Netherlands examined cases of blood dyscrasias
taken from the literature along with unpublished cases from adverse
drug reaction reporting systems in the United Kingdom, Denmark,
Sweden, and the Netherlands. To these were added cases from hospitals
in Northeast Switzerland. A total of 641 cases of chloramphenicol-
induced blood dyscrasias were identified. These included:
thrombocytopenia (21), agranulocytosis (51), hypoplastic anaemia (39),
bone marrow suppression (39), acute leukaemia (27), and aplastic
anaemia (464). Of the 464 cases of aplastic anaemia, 335 (72%) had
proved fatal. There appeared to be a female preponderance (261 cases;
56%). However, because this cannot be related to the total number
treated and therefore to those who did not acquire the condition, the
incidence cannot be determined (Meyler et al., 1974). The results
confirmed those of earlier work (Polak et al., 1972).
In Italy, it has been claimed that in view of the ease of
availability of chloramphenicol, the incidence of aplastic anaemia
appears to be low. For example, in 1971 there were 10 reports of fatal
side effects due to antibiotics; in 1972 there were 3. None were
attributable to chloramphenicol. During the period 1973 - 1975 there
were no reports of fatal cases (Preziosi et al., 1981).
For the period 1959 - 1969, 172 cases of aplastic anaemia were
reported in 15 hospitals in Northeast Switzerland, and 44 of these
individuals had been treated with chloramphenicol. The smallest total
dose incriminated was 3 g, while the highest was 315 g
(Keiser & Bucheggar, 1973).
In children aged 0 - 14 years in Denmark, the total number of
registered cases of aplastic anaemia was 39 for the years 1967 - 1982,
giving an annual incidence of 22/106. Probable causes were
identified for 21 of the 39 cases, and 2 of these were attributed to
chloramphenicol treatment (Clausen, 1986).
It was stated in 1983 that the probable overall incidence of
chloramphenicol-induced aplastic anaemia is somewhere between 1 in
20000 and 1 in 40000 (Venning, 1983).
In Japan, chloramphenicol appeared to be more dangerous to the
elderly population than to other groups. However, the investigation
was probably biased in that only fatal cases were examined
(Mizuno et al., 1982). Similar findings were made in an earlier
study (Shimizu et al., 1979).
For the years 1961 - 1965, 35 cases of aplastic anaemia were
reported in Colombia, and of these 10 had had past exposure to
chloramphenicol. Several others were said to have had a "strong
suspicion" of exposure to the drug. Mortality was 60% (Sarasti, 1970).
An age preponderance was noted in an analysis of 21 patients with
aplastic anaemia, 8 of whom had been treated with chloramphenicol
(Perez et al., 1981).
The minimum dose of chloramphenicol associated with the
development of aplastic anaemia is not known with certainty. The
literature citations often state only the total dose. Where cases
developed after several doses, it is not possible to say if aplastic
anaemia would also have occurred had only a single dose been given. Of
15 cases reviewed in a report in 1974, total doses of 4.5 to 80 g had
been given, with the usual levels being 8 to 14 g. As an example, a
46-year-old woman was given 15 g of chloramphenicol over 10 days.
Aplastic anaemia developed after 2 months (Hellriegel & Gross, 1974).
Total doses of 6.5 to 60 g chloramphenicol had been given to 7
individuals with aplastic anaemia reported in 1971 (Hodgkinson, 1971).
A 27-year-old woman given 30 g chloramphenicol i.v. over 12 days
developed aplastic anaemia 3 months later (Alavi, 1983).
One individual with aplastic anaemia had undergone previous
treatment 19 years earlier with a 500 mg initial dose followed by
250 mg chloramphenicol 4 times daily. At the age of 23 years, he was
given 750 mg chloramphenicol succinate (62 mg/kg b.w./day) i.v. every
6 hours for 12 days for a brain abscess. Bone marrow suppression
ensued and by the twelfth day of drug administration aplastic anaemia
had developed. The patient eventually died (Daum et al., 1979).
A 26-year-old woman was diagnosed as being anaemic during the
fifth month of pregnancy and in the sixth month she developed a skin
infection. She was given 8 g chloramphenicol. She developed aplastic
anaemia and died 8 days after giving birth. Bone marrow aspiration
confirmed aplastic anaemia. (Suda et al., 1978).
Several cases of aplastic anaemia have been reported in children.
One 7-year-old boy had been given chloramphenicol palmitate in June
and August of 1959. The dose or route of administration were not
specified. He developed aplastic anaemia 4 - 5 months later and died
(Leiken et al., 1961). A 6-year-old girl was given 25 mg/kg b.w./day
chloramphenicol for 10 days by an unspecified route. She developed
aplastic anaemia, apparently immediately after treatment and then
acute myeloblastic leukaemia (Awaad et al., 1975). A 4-year-old girl
was given oral chloramphenicol (dose not stated) for 1 week. After 2
months she developed aplastic anaemia (Young et al., 1979). Another
case of a 4-year-old girl given chloramphenicol was reported more
recently. Chloramphenicol was given i.v. for 1 week followed by oral
therapy for 8 weeks. The initial dose was 75 mg/kg b.w./day given
every 6 hours, but this was adjusted after 3 weeks to 37 mg/kg
b.w./day. Some months later she developed aplastic anaemia
(Lepow, 1986). In one recent case the patient was a neonate born at 30
weeks gestation weighing 1.7 kg. At day 20 after birth she developed
suspected meningitis and was given 4 doses of 50 mg/kg b.w./day
chloramphenicol. She developed aplastic anaemia; epidermal necrolysis
of the skin and biliary cholestasis were found at necropsy
(White et al., 1986).
A 72-year-old male patient was given a 3-week course of
chloramphenicol (dose and route unspecified) and aplastic anaemia
ensued within 4 months (Howell et al., 1975).
Aplastic anaemia is usually associated with oral intake
(Matthews et al., 1980). It has been reported that in 149 cases of
chloramphenicol-induced aplastic anaemia, 85% followed oral dosing,
14% followed parental administration, and 3% occurred after rectal
administration (Plaut & Best, 1982).
Topical administration of chloramphenicol has been followed by
aplastic anaemia. In one case, a 73-year-old woman died of aplastic
anaemia less than 2 months after beginning ophthalmic chloramphenicol
treatment with a 0.5% solution (3 - 4 times daily) and a 1% ointment
(once per day to the right eye) (Fraunfelder & Bagby, 1982). Several
other similar cases have been reported (Abrams et al., 1980;
Rosenthal & Blackman, 1965; Carpenter, 1975; Issaragrisil &
Piankijagum, 1985; Korting & Kifle, 1985). In one case the total dose
was estimated to be 32 mg of chloramphenicol (Carpenter, 1975).
One occupational case probably involved inhalation and skin
contact. It occurred in a shepherd applying a chloramphenicol spray to
the feet of sheep for treatment of foot rot. He had treated the
animals twice a week with the spray, which contained 10 g of the drug
in 100 ml of solution, for two years. Each dose contained 10 mg
(Del Giacco et al., 1981).
A link between the administration of chloramphenicol and the
development of liver disease and aplastic anaemia is known to exist.
After five patients aged 4 to 63 years were given chloramphenicol,
liver disease (as evidenced by jaundice, icterus, and elevated serum
enzymes and bilirubin) was subsequently noted. Aplastic anaemia
eventually developed (Hodgkinson, 1973). A similar case has been
reported in a 15-year-old boy given 250 mg chloramphenicol i.v. every
6 hours. Liver enzymes became elevated after 18 days and the liver
itself was tender. Aplastic anaemia developed (Caslae et al., 1982).
The mechanism of chloramphenicol-induced aplastic anaemia is not
understood. There may be a genetic element involved, as the effect has
been seen in families and in identical twins exposed to chloramphenicol
(Flach, 1982; Nagao & Maner, 1969; Silver & Zuckerman, 1980; Yunis,
1978c). The main target may be the haemopoietic pluripotential stem
cells of the marrow (Vincent, 1986; Silver & Zuckerman, 1980;
Benestad, 1974; Appelbaum & Fefer, 1981). There may also be the
possibility of initial damage to the marrow micro-environment (Camitta
et al., 1982). Failure to note this effect in human beings with
drug-induced aplastic anaemia makes the latter unlikely (Samson
et al., 1972; Vincent, 1986). In vitro studies with human bone
marrow suggest that both the erythroid and granulocytic series are
sensitive to chloramphenicol (Nara et al., 1982; Hara et al.,
1978; Yunis, 1977). The erythroid series in particular seemed sensitive
to chloramphenicol, with inhibition occurring at 10 mg/l compared with
50 mg/l for the granulocytic series (Yunis, 1977).
The problem as to whether bone marrow cells are more sensitive to
chloramphenicol in patients with aplastic anaemia induced by the drug
is not clear, as reports on the subject are conflicting (Yunis et al.,
1973a; Howell et al., 1975; Kern et al., 1975). It has been claimed,
based on the results of animal studies, that individuals who are
sensitive to chloramphenicol-induced aplastic anaemia may have
residual bone marrow damage brought about by exposure to other agents
(Morley et al., 1976).
The metabolite or metabolites responsible for the induction of
aplastic anaemia in human beings is unknown, but nitroso-
chloramphenicol has been implicated (Murray & Yunis, 1981; Nagai &
Kanamuru, 1978). Nitrosochloramphenicol can be formed by the
reduction of chloramphenicol in human liver in vitro (Salem et al.,
1981). This substance is known to be toxic to human bone marrow cells
in vitro and, moreover, is more toxic than chloramphenicol itself
(Yunis et al., 1980a, b). However, nitrosochloramphenicol is not
myelotoxic to mice in vivo (Krishna et al., 1981). It does,
however, cause DNA strand breakage in vitro, and inhibit DNA
synthesis (Gross et al., 1982; Skolimowski et al., 1983). Both
chloramphenicol and nitrosochloramphenicol are taken up rapidly by
cells, at least as demonstrated by a human transformed lymphoblastoid
cell line (Raji cells), but the nitroso-compound covalently binds to
these cells and to bone marrow cells 15 times more tightly than does
chloramphenicol (Hurray & Yunis, 1981). Photochemical decomposition of
chloramphenicol may result in potentially myeloblastic derivatives,
which may be hazardous in ophthalmic solutions (de Vries et al.,
1984). Another possible mechanism involves the immune system (and even
autoimmune damage), but there are no convincing data to support this
hypothesis. Chloramphenicol in vitro inhibited lymphocyte
transformation in human material (Burgio et al., 1974) and
chloramphenicol reduction products, including nitrosochloramphenicol,
were suppressive to antigen-reactive cells in mice (Pazdernik &
Corbett, 1980).
In summary, chloramphenicol induces aplastic anaemia in
susceptible individuals, but no dose-response relationship has been
identified. The mechanism may involve nitrosochloramphenicol, but this
has not been proven. The nature of the mechanism is unknown.
Bone marrow suppression
Reversible bone marrow suppression has been reported in patients
given chloramphenicol by a number of routes. The effect is thought to
be an unlikely event at plasma levels under 20 mg/l, and it is said to
occur in most patients at levels in excess of 25 mg/l. Generally, it
occurs within days of administration (Lery et al., 1978; Benestad,
1979). One study showed that oral doses likely to result in
suppression were usually of the order of 2 - 3 g/day or 30 - 50 mg/kg
b.w./day; durations of dosing were generally 1 - 17 days, most being 5
- 10 days. Plasma levels of chloramphenicol at 2 - 3 hours and 6 - 8
hours varied, most being in excess of 25 mg/l at 2 - 3 hours and in
excess of 30 mg/l at 6 - 8 hours. However, some were below 15 mg/l at
both times. Of the 17 patients studied, 11 had liver disease, while 3
of the remainder had renal disease. Bone marrow suppression in a
female of 21 years of age was attributed to the administration of
1.5 g/day of chloramphenicol for 18 days. The condition resolved on
cessation of therapy (Parashar et al., 1972). In 6 anaemic subjects
given up to 60 mg/kg b.w./day chloramphenicol, reticulocyte response
to vitamin B12 or iron dextran was halted and delayed (Saidi
et al., 1961).
Maturation arrest and cytoplasmic valuation of erythroid elements
were seen in the marrow (Rosenbach et al., 1960; Bartlett, 1982).
One reported case of bone marrow suppression was in a
three-year-old girl with cystic fibrosis given 70 mg/kg b.w./day
chloramphenicol for 2.5 months. The suppression was accompanied by
physical growth depression and hair loss. All these conditions
resolved on cessation of treatment (Kapp et al., 1977).
The toxic effects of chloramphenicol on the bone marrow were
lessened in one group of patients by co-administration of
phenylalanine (Ingall et al., 1965). The reason for this, and its
relationship to the pathogenesis of bone marrow suppression, is
unknown. The mechanism of suppression is thought to involve inhibition
of mitrochondrial protein biosynthesis in bone marrow cells. In vitro
studies showed that 10 mg/l chloramphenicol severely inhibited protein
synthesis in bone marrow cells, although ten times this concentration
was required to inhibit mitochondrial respiratory functions (Yunis,
1973a, b; Yunis, 1978a, b; Martelo et al., 1969). As a result of
mitochondrial dysfunction, ferrochelatase activity is suppressed in
erythroid precursors. In vitro studies show inhibition of haem
synthesis with concentrations of chloramphenicol of 10 mg/l
(Becker et al., 1974). It seems likely that the major effect of
chloramphenicol in the pathogenesis of reversible bone marrow
suppression is a block on hame biosynthesis arising as a result of
mitochondrial dysfunction (Yunis & Salem, 1980). Consequently,
co-administration of phenylalaninine may in some way compensate for
the inhibitory effects of chloramphenicol on protein synthesis.
Effects on platelets
In in vitro experiments, chloramphenicol was shown to decrease
human platelet aggregation. Unfortunately, no in vivo studies are
available (Cronber et al., 1984, Djaldetti, 1983; Agam et al.,
1976).
Carcinogenicity
IARC concluded in 1982 and 1987 that there was limited evidence
for the carcinogenicity of chloramphenicol to human beings based on
studies describing cases of leukaemia (IARC, 1982; IARC, 1987).
One study described a 24-year-old male given chloramphenicol for
typhoid fever. Leukaemia followed aplastic anaemia. A chromosome
translocation (t 1:7) was discovered on karyotyping. No other details
were provided, except that similar translocations were noted in 6
other leukaemia patients known to have had exposure to leukaemogenic
substances or radiation (Scheres et al., 1985).
Acute myeloid leukaemia developed in a 6-year-old girl who
developed aplastic anaemia after being given 25 mg/kg b.w./day
chloramphenicol for 10 days. The leukaemia developed 6 months after
the aplastic anaemia (Awaad et al., 1975).
Leukaemia developed in a 38-year-old woman given a total of 8 g
of chloramphenicol 8 years before which resulted in aplastic anaemia.
A 57-year-old man who had taken chloramphenicol for 8 years (about
175 g total dose) developed aplastic anaemia followed by leukaemia
within a year, while a 61-year-old man who was treated with
chloramphenicol developed aplastic anaemia and leukaemia (Brauer &
Dameshek, 1967).
An 80-year-old man with a bacterial infection of the feet was
given orally 250 mg/day chloramphenicol, for 7 days. He developed
acute leukaemia 5 months later. The only other drugs given for his
infection were penicillin and a topical zinc ointment (Humphries,
1968).
A 14-year-old girl given 250 mg chloramphenicol for 4.5 days
developed aplastic anaemia after approximately 1 year followed by
acute leukaemia 18 months later (Seaman, 1969).
Aplastic anaemia and leukaemia may occur together at diagnosis. A
28-year-old man developed reversible bone marrow depression after
being given approximately 31 g of chloramphenicol. Five years later he
was found to have aplastic anaemia and acute leukaemia (Schmitt-Graft,
1981). Other similar cases have been reported (Adamson & Seiber, 1981;
Forni & Vigliani, 1974; Meyer & Boxer, 1973).
Of 641 cases of chloramphenicol-induced blood dyscrasias, 464
cases of aplastic anaemia and 27 cases of leukaemia were identified
(Meyler et al., 1974).
From 151 cases of blood dyscrasias induced by drugs, 3 cases of
leukaemia attributable to chloramphenicol were noted (Fraumeni, 1967).
The majority of cases of leukaemia associated with chlo-
ramphenicol therapy were acute myeloid leukaemia (Godner et al.,
1973).
The role of chloramphenicol in leukaemogenesis is not known. Some
studies have revealed abnormal karyotypes in affected patients
(Scheres et al., 1985; Goh, 1971; Cohen & Huang, 1973), but it is
not known if these abnormalities were induced directly by
chloramphenicol or were merely a feature of the disease. Aplastic
anaemia, whether induced by chemicals or idiopathic, is known to be
followed by leukaemia in some cases (Milner & Geary, 1979).
Cytogenetic abnormalities are common. Fanconi's anaemia, for example,
is often followed by leukaemia and cytogenetic abnormalities are seen
in this state. Chloramphenicol is known to induce cytogenetic
abnormalities in in vitro test systems, but it is not known if those
seen in leukaemia occurring after chloramphenicol-induced aplastic
anaemia are due to the same effects.
Cardiovascular effects
Chloramphenicol is known to induce a state often referred to as
the "Grey Baby Syndrome" or "Grey Syndrome". In general, this is a
state of cardiovascular collapse that commences on days 2 to 9
following the beginning of chloramphenicol administration. The main
features include failure to feed, vomiting, abdominal distension,
cyanosis, flaccidity, shock, and a fall in body temperature. Death is
said to occur in 60% of cases, and the syndrome generally occurs where
the dose of chloramphenicol exceeds 25 mg/kg b.w./day (Meyler &
Herkeimer, 1968). Metabolic acidosis may be a presenting feature, and
plasma levels of chloramphenicol in excess of 30 µg/ml are usually
associated with the development of the syndrome (Evans & Kleiman,
1986; Mulhall et al., 1983; Craft et al., 1974). In several cases
the doses producing the syndrome were 50 mg/kg b.w./day (Fripp
et al., 1983; Kraskinski et al., 1982; Biancaniello et al.,
1981; Haile, 1977) and in one case a daily dose of 100 mg/kg b.w. was
given (Stevens et al., 1981). A grey-type syndrome has been reported
in a 16-year-old girl given 1 g chloramphenicol i.v. every 6 hours
(95 mg/kg b.w./day) for what was thought to be Rocky Mountain spotted
fever. This dose was later changed to 3.75 g/day i.v. The syndrome
began to develop after the second day of this regime, but it resolved
with drug withdrawal and supportive treatment (Brown, 1982). The
mechanism is unknown, but experiments with isolated pig hearts suggest
that effects on mitochondria may be important (Werner et al., 1985).
Allergenic contact dermatitis
Allergenic contact dermititis has been reported in case report
following the use of chloramphenicol topical preparation (Rudzki et al.,
1976; Schewach-Millet & Shapiro, 1985; Van Joost et al.,1986;
Strick, 1983; Fraki et al., 1985).
Two cases of occupational dermatitis in oculists were attributed
to the use of chloramphenicol-containing preparations (Rebandel &
Rudzki, 1986). In one study of 330 patients with contact allergy, 10%
showed positive patch tests to chloramphenicol, while in another
investigation of 620 patients, 1.7% save positive reactions
(Blondeel et al., 1978; Rudzki & Kelniewska, 1970). Others have
reported similar findings (Forck, 1971).
Ocular toxicity
Several cases of ocular toxicity following prolonged treatment
with chloramphenicol have been reported. This has often been described
as an optic neuritis with scotomata and failing vision. Retrobulbar
neuritis may be observed. It has been seen in cases of cystic
fibrosis, although this may reflect the choice of chloramphenicol as a
treatment in this condition rather than a specific sensitivity in this
group. Total doses are often in the 80 - 250 g range given over
several months. One patient developed bilateral optic neuritis after
being given 6 g chloramphenicol per day for 6 weeks (approximately
250 g total). Peripheral neuritis may accompany the ocular effects
(Wilson, 1962; Steidl, 1965; Malbrel et al., 1977; Joy et al.,
1960; Lasky et al., 1953; Charache et al., 1977; Chang et al.,
1966; Cocke et al., 1966; Godel et al., 1980; Wallenstein &
Snyder, 1952; Huang et al., 1966; Walker, 1961; Fraunfelder & Meyer,
1984).
Ototoxicity
Deafness following chloramphenicol therapy has occasionally been
noted, although the reports have been complicated by administration of
other potentially ototoxic drugs. In one case, a 2.5-year-old boy was
given 125 mg/kg b.w./day chloramphenicol for 26 days with no other
drug treatment. He developed deafness, which persisted beyond
cessation of drug administration (Gargye & Dutta, 1959; Ajodhia & Dix,
1976; Nilges & Norther, 1971; Jones & Hanson, 1977).
Effects on testes
A 61-year-old man with pneumonia was treated with ampicillin and
chloramphenicol. He later developed aplastic anaemia after 12 days and
died of widespread sepsis 10 days after drug withdrawal. Necropsy
revealed loss of germinal epithelium, with only Sertoli cells
remaining. The testes were of normal size. As the patient was
unmarried and also exposed to ampicillin, the authors concluded that
there was no direct proof of chloramphenicol-induced testicular
toxicity. However, in view of the normal gross appearance of the
testes and the low toxicity of ampicillin, chloramphenicol seems the
most likely cause of the effects noted in this case. No dose levels
were quoted (Sheehan & Sweeny, 1982).
Teratogenicity
Chloramphenicol and/or tetracyclines have been implicated in the
genesis of cleft-lip in a study of 599 affected children. However,
because of the nature of the epidemiological study, any effects of
tetracyclines cannot be separated from those of chloramphenicol and it
cannot be stated with any certainty that chloramphenicol is a human
teratogen (Saxen, 1975).
COMMENTS
Human exposure to chloramphenicol can give rise to aplastic
anaemia, a rare but often fatal condition. The Committee concluded
that no dose-response relationship could be established for this
effect. The mechanism for the pathogenesis of aplastic anaemia is
unknown, and no suitable animal model exists.
EVALUATION
Because a no-effect level for aplastic anaemia could not be
established, and therefore it was not possible to give an assurance
that residues in foods of animal origin would be safe for sensitive
subjects, an ADI could not be allocated for chloramphenicol.
REFERENCES
Abou-Khalil, S., Salem, Z., & Yunis, A.A. (1980). Mitochondrial
metabolism in normal, myeloid, and erythroid hyperplastic rabbit
bone-marrow: Effect of chloramphenicol. Am. J. Haematol., 8, 71-79.
Abou-Khalil, S., Salem, Z., Abou-Khalil, W.H., & Yunis, A.A. (1981).
On the mechanism of erythroid cell sensitivity to chloramphenicol:
Studies on mitochondria isolated from erythroid and myeloid tumours.
Arch. Biochem. Biophys., 206, 242-248.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1982). Inhibition
by nitrosochloramphenicol of the proton translocation in mitochondria.
Biochem. Pharmacol., 31, 3823-3830.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1985).
Identification of a mitochondrial rho-dinitrobenzene reductase
activity in rat liver. Pharmacol., 31, 301-308.
Abrams, S.M., Degnan, T.J., & Vinciguerra, V. (1980). Marrow aplasia
following topical application of chloramphenicol eye ointment.
Arch. Inter. Med., 140, 576-577.
Adamson, R.H. & Seiber, S.M. (1981). Chemically induced leukemia in
humans. Environ. Health Perspect., 39, 93-103.
Agam, G., Gasner, S., Bessler, H., Fishman, P., & Djaldetti, M.
(1976). Chloramphenicol induced inhibition of platelet protein
synthesis: In vitro and in vivo studies. Brit. J. Haematol.,
33, 53-59.
Ajodhia, J.M. & Dix, M.R. (1976). Drug-induced deafness and its
treatment. Practitioner, 216, 561-570.
Akhnoukh, S., El-Shazly, N., Sallaim, N., El-Melegy, S., El-Sewedy,
S.M., Mostafa, M.H., & El-Bassioni, E.A. (1982). In vivo effect of
chloramphenicol and thiamphenicol on some enzymes of normal mouse
liver. Biochem. Pharmacol., 31, 55-57.
Aksoy, M., Erdem, S., Dincol, G., Bakioglu, I., & Kutlar, A. (1984).
Aplastic anaemia due to chemicals and drugs: A study of 108 patients.
Sex Trans. Dis., 11, 347-350.
Alavi, J.B. (1983). Aplastic anaemia associated with intravenous
chloramphenicol. Am. J. Hematol., 15: 375-379.
Al-Hachim, G.M. & Al-Baker, A. (1974). The prenatal effect of
chloramphenicol on the postnatal development of mice.
Neuropharmacol., 13, 233-237.
Ali, B.H. (1985). Lack of effect of chloramphenicol on monoamine,
diamine and plasma amine oxidase activities. Clin. Exp. Pharmacol.
Physiol., 12, 1193-5.
Al-Moudhiry, H.A.B. (1978). Aplastic anaemia in Iraq. A prospective
study. Haematologia, 12, 159-164.
Amati, P. (1970). Chloramphenicol: Effect of DNA synthesis during
phage development in Escherichia coli. Science, 168, 1226-1228.
Ambrose, P.J. (1984). Clinical pharmacokinetics of chloramphenicol and
chloramphenicol succinate. Clin. Pharmacokinetics, 9, 222-238.
Applebaum, F.R. & Fefer, A. (1981). The pathogenesis of aplastic
anaemia. Sem. Haematol., 18, 241-257.
Appelgren, L.-E., Eberhardson, B., Martin, K., & Slanina, P. (1982).
The distribution and fate of 14C-chloramphenicol in the new-born
pig. Acta pharmacol. Toxicol., 51, 345-350.
Aravindaksham, C.M. & Cherian, Z. (1984). Effect of chloramphenicol on
thiopentone sodium anesthesia in dogs. Indian Vet. J., 61, 569-573.
Ascherl, M., Eyer, P., & Kaupffmeyer, H. (1985). Formation and
disposition of nitrosochloramphenicol in rat liver. Biochem.
Pharmacol., 34, 3755-3763.
Augustine, M.L., Poulsen, N.K., & Heinze, J.E. (1982). Evaluation of
the CHO/HGPRT cell mutation test using 12 compounds. Env. Mutagen.,
4, 389-390.
Awaad, S., Khalifa, A.S., & Kamel, K. (1975). Vacuolization of
leukocytes and bone-marrow aplasia due to chloramphenicol toxicity.
Clin. Pediatr., 14, 499-506.
Baltz, R.H., & Stonesifer, J. (1985). Adaptive response and
enhancement of N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis by
chloramphenicol in Streptomyces fradiae. J. Bacteriol.,
164, 944-946.
Banerjee, S. & Basu, P.S. (1978). Inhibition of rat tissue
mitrochondrial monoamine oxidase by chloramphenicol and its
intermediates. Indian J. Biochem. Biophys., 15, 479-482.
Bartlett, J.G. (1982). Chloramphenicol. Med. Clin. N. Amer.,
66, 91-102.
Baselt, R.C. (1982). Disposition of toxic drugs in man. 2nd Edition,
Davis, California, Biomedical Publications, 136-139.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1979). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Surg. Forum, 30, 512-514.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1981). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Arch. Otolaryngol., 107, 104-109.
Becker, D., Metz, J., & Katz, J. (1974). Comparative effects of
thiamphenicol and chloramphenicol on haemopoiesis and lymphocyte
transformation in vitro in man. Postgrad. Med. J.,
50, (Suppl. 5), 88-94.
Beliles, R.P. (1972). The influence of pregnancy on the acute toxicity
of various compounds in mice. Toxicol. Appl. Pharmacol., 23, 537-540.
Benes, L., Rotreklova, E., & Pospisil, V.V.M. (1980). Inhibition of
bone marrow cell proliferation and DNA replication induced by
chloramphenicol. Folia Biol., 26, 408-414.
Benestad, H.B. (1974). Aplastic anaemia: Considerations on the
pathogenesis. Acta med. Scand., 196, 255-262.
Benestad, H.B. (1979). Drug mechanism in marrow aplasia.
In: Geary, C.G., (ed.) Aplastic anaemia, London, Balliere Tindall,
pp. 26-42.
Bengmark, S., Goransson, G., & Soucas, E. (1981). Defects in
hemostasis produced by antibiotics. An in vivo study in the rat.
Eur. Surg. Res., 13, 290-298.
Ben-Gurion, R. (1978). A simple plate test for screening colicine-
inducing substances as a tool for the detection of potential
carcinogens. Mutat. Res., 54, 289-295.
Bertolini, A. & Poggioli, R. (1981). Chloramphenicol administration
during brain development: Impairment of avoidance learning in
adulthood. Science, 21, 238-239.
Biancaniello, T., Meyer, R.A., & Kaplan, S. (1981). Chloramphenicol
and cardiotoxicity. J. Pediatr., 98, 828-830.
Billet, F.S., Collini, R., & Hamilton, L. (1965). The effects of D-and
L-threo-chloramphenicol on the early development of the chick embryo.
J.Embryol. Exp. Morph., 13, 341-356.
Blackwell, U.B. (1962). The changing inhibition of early
differentiation and general development in the chick embryo by
2-ethyl-5-methyl-benzimidazole and chloramphenicol. J. Embryo. Exp.
Morph., 10, 315-337.
Blondeel, A., Oleffe, J., & Achten, G. (1978). Contact allergy in 330
dermatological patients. Contact Derm., 4, 270-276.
Bories, G.F., Peleran, J.C., Wal, J.M., & Corpet, D.E. (1983). Simple
and ion-pair high performance liquid chromatography as an improved
analytical tool for chloramphenicol metabolic profiling. Drug Met.
Disp., 11, 249-254.
Bottiger, L.E. & Westerholm, B. (1972). Aplastic anaemia. I. Incidence
and aetiology. Acta med. Scand., 192, 315-318.
Bottiger, L.E. & Westerholm, B. (1973). Drug-induced blood dyscrasias
in Sweden. Brit. Med. J., 3, 339-343.
Bottiger, L.E. (1978). Prevelance and etiology of aplastic anaemia in
Sweden. In: Hibino, S., Takaku, F., & Shahidi, N.T. (eds.) Aplastic
Anemia. Tokyo, University Park Press, pp. 171-180.
Bottiger, L.E. (1979). Epidemiology and aetiology of aplastic anaemia.
Haematol. Blut. Transfus., 24, 27-37.
Brabec, M.J., Owens, J.B., Kenel, M., Sorscher, D., & Cornish, H.H.
(1982). Modification of methanol potentiation of carbon tetrachloride
toxicity in rats by chloramphenicol and salicylate. Drug Chem.
Toxicol., 5, 143-154.
Brasfield, J.H., Record, K.E., Griffen, W.O., & Mattingly, S. (1983).
Chloramphenicol succinate levels in patients with renal impairment.
Clin. Pharmacol., 1, 355-358.
Brauer, M.J. & Dameshek, W. (1967). Hypoplastic anemia and
myeloblastic leukemia following chloramphenicol therapy. Report of
three cases. New Engl. J. Med., 277, 1003-1005.
Braun, R., Fischer, G.W., & Schoneich, J. (1977). The mutagenicity and
DNA-damaging activity of cyclic aliphatic sulfuric acid esters.
Chem-Biol. Interact., 19, 241-252.
Braun, R., Schoneich, J., Weissflog, L., & Dedek, W. (1982). Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair direct alkylation vs. metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem-Biol. Interact.,
39, 339-350.
Brem, H., Stein, A.B., & Rosenkranz, H.S. (1974). The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res., 34, 2576-2579.
Brown, R.T. (1982). Chloramphenicol toxicity in an adolescent.
J. Adol. Health Care, 3, 53-55.
Bueno, L., Fioramonti, J., Alvinerie, M., & Bardon, T. (1984).
Influence of bran on bioavailability of chloramphenicol administered
orally to pigs. Can. J. Physiol. Pharmacol., 62, 208-211.
Burckart, G.J., Barrett, F.F., Stranghn, A.B., & Ternullo, S.R.
(1982). Chloramphenicol clearance in infants. J. Clin. Pharmacol.,
22, 49-52.
Burckart, G.J., Barett, F.F., Valle, R.D., & Mayer, M.C. (1983).
Chloramphenicol damage and pharmacokinetics in infants and children.
J. Clin. Pharmacol,, 23, 106-112.
Burke, J.T., Wargin, W.A., & Blum, M.R. (1980). High-pressure liquid
chromatographic assay for chloramphenicol, chloramphenicol-3-
monosuccinate and chloramphenicol-l-monosuccinate. J. Pharm. Sci.,
69, 909-912.
Burrows, G.E., Tyler, R.D., Craigmill, A.L., & Barto, P.B. (1984).
Chloramphenicol and the neonatal calf. Am. J. Vet. Res.,
45, 1586-1591.
Camitta, B.M., Storb, R., & Thomas, E.D. (1982). Aplastic anaemia.
Pathogenesis, diagnosis, treatment and prognosis. New Engl. J. Med.,
306, 645-652.
Carpenter, G. (1975). Chloramphenicol eye-drops and marrow aplasia.
Lancet, ii, 326-327.
Casale, T.B., Macher, A.M., & Fauci, A.S. (1982). Complete hematologic
and hepatic recovery in a patient with chloramphenicol hepatitis-
pancytopenia syndrome. J. Pediatr., 101, 1025-1027.
Chakrabarti, K. & Banerjee, S. (1977). Mechanism of intestinal
absorption of chloramphenicol. Indian J. Exp. Biol., 15, 302-303.
Chang, N., Giles, C.L., & Gregy, R.H. (1966). Optic neuritis and
chloramphenicol. Am. J. Dis. Child., 112, 46-48.
Charache, S., Finkelstein, D., Lietman, P.S., & Scott, J. (1977).
Peripheral and optic neuritis in a patient with hemoglobin SC disease
during treatment of Salmonella osteomyelitis with chloramphenicol.
Johns Hopkins Med. J., 140, 121-124.
Cid, F., Mella, F., Gonzalez, X., & Gouzalez, R. (1983). Effects of
anti-inflammatory drugs on the pharmacokinetics of chloramphenicol.
An. Real Acad. Farm., 49, 49-60.
Clausen, N. (1986). A population study of severe aplastic anemia
children. Acta paediatr. Scand., 75, 58-63.
Cocke, J.G., Brown, R.E., & Geppert, L.J. (1966). Optic neuritis with
prolonged use of chloramphenicol. J. Pediatr., 68, 27-31.
Cohen, T. & Creger, W.P. (1967). Acute myeloid leukemia following
seven years of aplastic anemia induced by chloramphenicol.
Am. J. Med., 43, 762-770.
Cohen, H.J. & Huang, A.T.-F. (1973). A marker chromosome abnormality.
Occurrence in chloramphenicol-associated acute leukaemia.
Arch. Intern. Med., 132, p. 440.
Courtney, K.D., Valerio, D.A., & Palletta, A.J. (1967). Experimental
teratology in the monkey. Toxicol. Appl. Pharmacol., 10, p. 378.
Courtney, K.D., & Valerio, D.A. (1974). Teratology in the
Macaca mulatta. Teratology, 1, 163-172.
Craft, A.W., Brocklebank, J.T., Itey, E.N., & Jackson, R.H. (1974).
The "grey-toddler". Chloramphenicol toxicity. Arch. Dis. Child.,
49, 235-237.
Cronberg, S., Wallmark, E., & Soderberg, I. (1984). Investigations on
the effect of antimicrobial drugs on platelet aggregation in vitro
and in vivo. Folia Haematol., 111, 725-734.
D'Angelo, E.P., Patterson, W.C., & Morrow, R.C. (1967). Chloramphenicol.
Topical application in the middle ear. Arch. Otolaryngol., 85,
126-128.
Daum, R.S., Cohen, D.L., & Smith, A.L. (1979). Fatal aplastic anaemia
following apparent "dose-related" chloramphenicol toxicity.
J. Pediatr., 94, 403-406.
De Corte-Baeten, K. & Debackere, M. (1976). Excretion of
chloramphenicol in the milk of lactating cows after oral and
parenteral application. Dtsch. Tieraerztl. Wochenschr., 83, 231-233.
Del Giacco, G.S., Petrini, M.T., Janelli, S., & Carcassi, U. (1981).
Fatal bone-marrow hypoplasia in a shepherd using chloramphenicol
spray. Lancet, i, p. 945.
de Vries, H., Beijersbergen van Henegouwen, G.M.J., & Huf, F.A.
(1984). Photochemical decomposition of chloramphenicol in a 0.25%
eyedrop and in a therapeutic intraocular concentration. Internat. J.
Pharmaceut., 20, 265-271.
Dewse, C.D. (1977). Variable action of chloramphenicol on DNA
synthesis in phytohaemagglutinin-stimulated human lymphocytes.
ICRS Med. Sci., 5, p.150.
Dill, W.A., Thompson, E.M., Tisken, R.A., & Glazko, A.J. (1960). A new
metabolite of chloramphenicol. Nature, 185, 535-537.
Djaldetti, M. (1983). Drug vulnerability of peripheral blood
platelets. Arch. Toxicol. (Suppl.), 6, 21-32.
Doudney, C.O. & Rinaldi, C.N. (1985). Chloramphenicol-promoted
increase in resistance to UV damage in Escherichia coli
B/r trp E65: Development of the capacity for successful repair of
otherwise mutagenic or lethal lesions in DNA. Mutat. Res.
143, 29-34.
Ehling, U.H. (1971). Comparison of radiation- and chemically-induced
dominant lethal mutations in male mice. Mutat. Res., 11, 35-44.
Epstein, S.S. & Shafner, H. (1968). Chemical mutagens in the human
environment. Nature, 219, 385-387.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972).
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
Evans, L.S. & Kleiman, M.B. (1986). Acidosis as a presenting feature
of chloramphenicol toxicity. J. Pediatr., 108, 475-477.
Eyer, F., Lierheimer, E., & Schneller, M. (1984). Reactions of
nitrosochloramphenicol in blood. Biochem. Pharmacol., 33, 2299-2308.
Flach, A.J. (1982). Chloramphenicol and aplastic anemia. Am. J.
Ophthalmol., 93, 664-665.
Flint, O.P. & Orton, T.C. (1984). An in vitro assay for teratogens
with cultures of rat embryo midbrain and limb bud cells. Toxicol.
Appl. Pharmacol., 76, 383-395.
Forck, G. (1971). Incidence and significance of chloramphenicol
allergies. Dtsch. Med. Wochen, 96, 161-165.
Forni, A. & Vigilant, E.C. (1974). Chemical leukemogenesis in man.
Ser. Haematol., 7, 211-223.
Fraki, J.E., Kalimo, K., Tuohimaa, P., & Aantaa, E. (1985). Contact
allergy to various components of topical preparations for treatment of
external otitis. Acta Otolaryngol., 100, 414-418.
Fraunfelder, F.T. & Bagby, G.C. (1982). Fatal aplastic anaemia
following topical administration of ophthalmic chloramphenicol.
Am. J. Ophthalmol., 93, 356-360.
Fraunfelder, E.T. & Meyer, S.M. (1984). Ocular toxicology update.
Austral. J. Ophthalmol., 12, 391-394.
Fraumeni, J.F. (1967). Bone-marrow depression induced by
chloramphenicol or phenylbutazone. J. Am. Med. Assoc., 201, 150-156.
Fripp, R.R., Carter, M.C., Werner, J.C., Schuler, H.G., Rannels, A.M.,
Whitman, V., & Nelson, N.M. (1983). Cardiac function and acute
chloramphenicol toxicity. J. Pediatr., 103, 487-490.
Fritz, H. & Hess, R. (1971). The effects of chloramphenicol on the
prenatal development of rats, mice and rabbits. Toxicol. Appl.
Pharmacol., 19, 667-674.
Gadner, H., Gethmann, U., Jessenberger, K., & Riehm, H. (1973). Acute
leukaemia following exposure to chloramphenicol? Three case reports
and review of the literature. Mschr. Kinderheilk, 121, 590-594.
Garrett, N.B., Stack, H.F., Gross, M.R., & Waters, M.D. (1984). An
analysis of the spectra of genetic activity produced by known or
suspected human carcinogens. Mutat. Res., 134, 89-111.
Gargye, A.K. & Dutta, D.V. (1959). Nerve deafness following
chloromycetin therapy. Indian J. Pediatr., 26, 265-266.
Glavini, E., Prati, M., & Vismara, C. (1979). The effects of
actinomycin D and chloramphenicol on rat preimplantation embryos.
Experientia, 35, 1649-1650.
Gilman, A.G., Goodman, L.S., Rall, T.W., & Murad, F. (eds.) (1985).
Goodman and Gilman's The Pharmacological Basis of Therapeutics.
7th ed. New York, MacMillan Publishing Co., pp. 1179-1184.
Glazko, A.J., Wolf, L.M., Dill, W.A., & Bratton, A.C. (1949).
Biochemical studies on chloramphenicol (chloromycetin). II. Tissue
distribution and excretion studies. J. Pharmacol. Exp. Ther.,
96, 445-459.
Glazko, A.J., Dill, W.A., & Rebstock, M.C. (1950). Biochemical studies
on chloramphenicol (chlormycetin). III. Isolation and identification
of metabolic products in urine. J. Biol. Chem., 183, 679-691.
Glazko, A.d., Dill, W.A. & Wolf, L.M. (1952). Observations on the
metabolic disposition of chloramphenicol (chloromycetin) in the rat.
J. Pharmacol. Exp. Ther., 104, 452-458.
Godel, V., Nemet, P., & Lazar, M. (1980). Chloramphenicol optic
neuropathy. Arch. Ophthalmol., 98, 1417-1421.
Goh, K. (1971). Chloramphenicol, acute leukemia and chromosomal
vacuolation. Southern Med.J., 64, p. 815.
Goh, K.-O. (1979). Chloramphenicol and chromosomal morphology.
J.Med., 10, 159-166.
Gray, J.D. (1955). The concentration of chloramphenicol in human
tissues. Canad. Med. Assoc. J., 72, 778-779.
Gross, B.J., Branchflower, R.V., Burke, T.R., Lees, D.E., & Pohl, L.R.
(1982). Bone-marrow toxicity in vitro of chloramphenicol and its
metabolites. Toxicol. Appl. Pharmacol., 64, 557-565.
Guntakatta, M., Matthews, E.J., & Rundell, J.O. (1984). Development of
a mouse embryo limb bud cell culture system for the estimation of
chemical teratogenic potential. Terat. Carcinogen. Mutat.,
44, 349-364.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985a). Percutaneous
absorption in man: A kinetic approach. Toxicol. Appl. Pharmacol.,
78, 123-129.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985b). Transdermal
absorption kinetics: A physiochemical approach. ACS Symp. Ser.,
273, 19-31.
Haile, C.A. (1977). Chloramphenicol toxicity. Southern Med. J.,
70, 479-480.
Halpert, J. (1981). Covalent modification of lysine during the suicide
inactivation of rat liver cytochrome P-450 by chloramphenicol.
Biochem. Pharmacol., 30, 875-881.
Halpert, J. & Neal, R.A. (1981). Inactivation of rat liver cytochrome
P-450 by the suicide substrates parathion and chloramphenicol. Drug
Met. Rev., 12, 239-259.
Halpert, J. (1982). Further studies of the suicide inactivation of
purified rat liver cytochrome P-450 by chloramphenicol.
Mol. Pharmacol., 21, 166-172.
Halpert, J., Balfour, C., Miller, N.E., Morgan, E.T., & Danbar, D.
(1985). Isozyme selectivity of the inhibition of rat liver cytochrome
P-450 by chloramphenicol in vivo. Mol. Pharmacol., 28, 290-296.
Hapke, H.J., Abel, J., Ghosch, H., Tachampas, S., & Youssef, S.
(1977). Effects of chloramphenicol on drug-degrading enzyme systems.
Zentrabl. Veterinaermed., 24, 701-714.
Hara, H., Kohsaki, M., Noguchi, K., & Nagai, K. (1978). Effect of
chloramphenicol on colony formation from erythrocytic precursors.
Am. J. Haematol., 5, 123-130.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W., &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by interlaboratory
testing of diverse chemicals. Environ. Mutagen., 8, 515-531.
Hausman, K. & Skrandies, G. (1974). Aplastic anaemia following
chloramphenicol therapy in Hamburg and surrounding districts.
Postgrad. Med. J., 50, 136-142.
Heddle, J.A. & Bruce, W.R. (1977). Comparisons of test for
mutagenicity or carcinogenicity using assays for sperm abnormalities,
formation of micronuclei, and mutations in Salmonella: In: Hiatt,
H.H., Watson, J.D., & Winsten, J.A. (eds.) Origins of Human
Cancer, Book C, Risk Assessment, Cold Spring Harbour Laboratory,
pp. 1549-1557.
Hellriegel, K.P., & Gross, R. (1974). Follow-up studies in
chloramphenicol-induced aplastic anaemia. Postgrad. Med. J.,
50 (Suppl.5), 136-142.
Henley, C.M., Brown, R.D., Penny, J.E., Kupetz, S.A., Hodges, K.B., &
Jobe, P.C. (1984). Impairment of cochlear function produced by
chloramphenicol and noise. Neuropharmacol., 23, 197-202.
Henley, C.M. (1985). Comparison of the ototoxicity of chloramphenicol
and gentamycin in noise exposed rats. Diss. Abs., 46, 1523-B.
Hodgkinson, R. (1971). Infectious hepatitis and aplastic anaemia.
Lancet, i, p. 1014.
Hodgkinson, R. (1973). The chloramphenicol-hepatitis-aplastic anaemia
syndrome. Med. J. Austral., 1, 939-940.
Holden, H.E. (1982). Comparison of somatic and germ cell models for
cytogenetic screening. J. Appl. Toxicol., 2, 196-200.
Howell, A., Andrews, T.M., & Watts, R.W.E. (1975). Bone-marrow cells
resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet, i, 65-69.
Huang, N.M., Harley, R.D., Promadhattavedi, V., & Sproul, A. (1966).
Visual disturbances in cystic fibrosis following chloramphenicol
administration. J. Pediatr., 68, 32-44.
Humphries, K.R. (1968). Acute myelomonocytic leukaemia following
chloramphenicol therapy. N. Z. Med. J., 68, 248-249.
Hyman, J., Leifer, Z., & Rosenkranz, H.S. (1980). The E. coli
Pol A1 assay. A quantitative procedure for diffusable and
non-diffusable chemicals. Mutat. Res., 74, 107-111.
IARC (1982). IARC (International Agency for Research on Cancer)
monographs on the evaluation of the carcinogenic risk of chemicals to
humans: Chemicals, industrial processes and industries associated
with cancer in humans. 1-29, (Suppl. 4), 79-80.
IARC (1987, in press). IARC (International Agency for Research on
Cancer) monographs on the evaluation of carcinogenic risks to humans.
Overall evaluations of carcinogenicity: an update of IARC monographs
volumes 1-42 (Suppl. 7).
Ingall, D., Sherman, J.D., Cockburn, F. & Klein, R. (1965).
Amelioration by ingestion of phenylalanine of toxic effects of
chloramphenicol on bone-marrow. New Eng. J. Med., 272, 180-185.
Issaragrisil, S. & Piankijagum, A. (1985). Aplastic anemia following
topical administration of ophthalmic chloramphenicol: Report of a case
and review of the literature. J. Med. Ass. Thailand, 88, 309-312.
Jackson, A.F., Wentzell, B.R., McCalla, D.R., & Freeman, K.B. (1977).
Chloramphenicol damages bacterial DNA. Biochem. Biophys. Res.
Commun., 78, 151-157.
Javed, I., Nawaz, M., Ahmed, M., Rehman, Z.U., & Shah, B.H. (1984).
Pharmacokinetics, renal clearance and urinary excretion of
chloramphenicol in goats. Pakis. Vet., 4, 151-157.
Jones, F.E. & Hanson, D.R. (1977). H. Influenzae meningitis treated
with ampicillin or chloramphenicol, and subsequent hearing loss.
Dev. Med. Child. Neurol., 19, 593-597.
Joy, R.J.T., Scalettar, R., & Sodee, D.B. (1960). Optic and peripheral
neuritis. Probable effects of prolonged chloramphenicol therapy.
J. Amer. Med. Assoc., 173, 1731-1734.
Kapp, J.P., Avora, B., & Sibinga, M.S. (1977). Depression of
erythropoiesis, physical growth, and hair growth by chloramphenicol in
a three-year-old child with cystic fibrosis. Clin. Pediatr.,
16, 64-66.
Kauffman, R.E., Miceli, J.N., Strebel, L., Buckley, J.A., & Dore, A.K.
(1981). Pharmacokinetics of chloramphenicol and chloramphenicol
succinate in infants and children. J. Pediatr., 98, 315-320.
Keiser, G. & Bucheggar, U. (1973). Hematological side effects of
chloramphenicol and thiamphenicol. Helv. Med. Acta, 37, 265-278.
Kern, P., Heimpel, H., Heit, W., & Kubanek, B. (1975). Bone-marrow
cells resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet i, p. 1190.
Knowles, J.A. (1965). Excretion of drugs in milk - a review.
J. Pediatr., 66, 1068-1082.
Korting, G.W. & Kifle, H. (1985). Systemic side-effects from external
application of chloramphenicol. Der Hautarzt., 36, 181-183.
Krasinski, K., Perkin, R., & Rutledge, J. (1982). Gray baby syndrome
revisited. Clin. Pediatr., 21, 571-572.
Krishna, G., Aykac, I., & Siegel, D. (1981). Recent studies on the
mechanisms of chloramphenicol activation responsible for aplastic
anemia. In: NaJean Y. (ed.) Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy. New York, Raven Press,
pp. 5-16.
Kroker, R. (1985). The pharmacokinetic behavior of chloramphenicol in
liver-damaged mini-pigs. J. Vet. Pharmacol. Ther., 8, 82-87.
Kroon, A.M. & de Vries, H. (1969). The effect of chloramphenicol on
the biogenesis of mitochondria of rat liver in vivo. Fed. Eur.
Biochem. Soc. Letters, 3, 208-210.
Kubinski, H., Gutzke, G.E., & Kubinski, Z.O. (1981). DNA-cell binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89, 95-136.
Kunii, O., Komatsu, T., & Nishiya, H. (1983). Biliary excretion of
antibiotics in rats. Jap. J. Exp. Med., 53, 51-58.
Kurz, H., Manser-Ganshorn, A., & Stickel, H.H. (1977). Differences in
the binding of drugs to plasma proteins from newborn and adult man.
I. Euro. J. Clin. Pharmacol., 11, 463-467.
Lasky, M.A., Pincus, M.H., & Katlan, N.R. (1953). Bilateral optic
neuritis following chloramphenicol therapy. J. Amer. Med. Assoc.,
151, 1403-1404.
Leiken, S.L., Welch, H., & Guin, G.H. (1961). Aplastic anaemia due to
chloramphenicol. Clin. Proc. Child Hosp., 17, 171-180.
Lepow, M. (1986). Aplastic anaemia following chloramphenicol therapy
still happens! Pediatr., 77, 932-933.
Lery, N., Descoted, J., & Evreux, J.Cl. (1978). A review of
chloramphenicol-induced blood disorders. Vet. Human Toxicol.,
20, 177-181.
Lim, L.O. & Yunis, A.A. (1982). Induction of methemoglobin formation
by nitrosochloramphenicol in hemolysed blood. Res. Commun. Chem.
Path. Pharmacol., 38, 497-500.
Lim, L.O., Abou-Khalil, W.H., & Yunis, A.A. (1984). The effect of
nitrosochloramphenicol on mitochondrial DNA polymerase activity.
J. Lab. Clin. Med., 104, 213-222.
Ling, G.V., Conzelman, G.M., Franti, C.E., & Ruby, A.L. (1980). Urine
concentration of chloramphenicol, tetracycline, and sulfisoxazole
after oral administration to healthy adult dogs. Am. J. Vet. Res.,
41, 950-952.
Longnecker, D.S., Curphey, T.J., James, S.T., Daniel, D.S., &
Jacobs, N.J. (1974). Trial of a bacterial screening system for rapid
detection of mutagens and carcinogens. Cancer Res., 34, 1658-1663.
Ma, T.-H., Harris, M.M., Anderson, V.A., Ahmed, I., Mohammad, K.,
Bare, J.L., & Lin, G. (1984). Tradescantia-micronucleus (Trad-MCN) on
140 health-related agents. Mutat. Res., 138, 157-167.
Hackler, B., Grace, R., Tippit, D., Lemire, R.J., Shepard, H., &
Kelly, V.C. (1975). Studies of the development of congenital anomalies
in rats III. Effects of inhibition of mitochondrial energy systems on
embryonic development. Teratology, 12, 291-296.
Malbrel, C., Talmud, M., Dupou, D., & Rose, J.P. (1977). Concerning a
new case of optic neuritis by chloramphenicol in the infant.
Bull. Soc. Ophthalmol. France, 77, 999-1001.
Mamber, S.W., Okasinki, W.A., Printer, C.D., & Tunac, J.B. (1986). The
Escherichia coli K-12 SOS Chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutat. Res., 171, 83-90.
Manna, G.K. & Bardham, S. (1977). Some aspects of chloramphenicol
induced bone marrow chromosome aberrations in mice. J. Cytol. Genet.,
12, 10-17.
Manna, G.K. & Roy, P.P. (1979). Chromosome aberrations in F1 embryos
and litters of chloramphenicol treated male parent mice. Natl. Acad.
Sci. Letters (India), 2, 460-462.
Martelo, O.J., Manyan, D.R., Smith, U.S., & Yunis, A.A. (1969).
Chloramphenicol and bone marrow mitochondria. J. Lab. Clin. Med.,
74, 927-940.
Masek, F. (1977). Effect of some drugs on excision repair in E. coli
cells. Chem-Biol. Interact., 17, 121-127.
Matsuda, S. (1984). Transfer of antibiotics into maternal milk.
Biol. Res. Preg. Perinatol., 5, 57-60.
Matthews, S.J. & Shawver-Matthews, S. (1980). Chloramphenicol toxicity
- oral vs. intravenous administration. J. Paediatr., 97, p. 869.
McCann, J., Choi, E., Yamasaki, E., & Ames, B.N. (1975). Detection of
carcinogens and mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc. Natl. Acad. Sci. USA, 72, 5133-5139.
McCoy, E., Hyman, J., & Rosenbranz, H.S. (1980a). A new mutagenic and
genotoxic response of the flame retardant tris (2, 3-dibromopropyl)
phosphate. Activation by singlet oxygen. Mutat. Res., 77, 209-214.
McCoy, E.C., Petrullo, L.A., & Rosenkranz, H.S. (1980b). Non-mutagenic
genotoxicants: Novobiocin and naladixic acid, 2 inhibitors of DNA
gyrase. Mutat. Res., 71, 33-43.
McCurdy, P.R. (1963). Plasma concentration of chloramphenicol and bone
marrow suppression. Blood, 21, 363-372.
Meyer, J.S. & Boxer, M. (1973). Leukemic cellular thrombi in pulmonary
blood vessels. Subleukemic myelogenous leukemia following
chloramphenicol-induced aplastic anemia. Cancer 32, 712-721.
Meyler, L. & Herkeimer, A. (1968). Side effects of drugs. 6,
Amsterdam, Excerpta Medica, pp. 286-314.
Meyler, L., Polak, B.C.P., Schut, D., Wesseling, H., & Herxheimer, A.
(1974). Blood dyscrasias attributed to chloramphenicol. A review of
641 published and unpublished cases. Postgrad. Med. J., 50
(Suppl. 5), 123-126.
Milner, G.R. & Geary, C.G. (1979). The aplasia-leukaemia syndrome. In:
Geary, C.G. (ed.) Aplastic Anaemia. London, Balliere Tindall,
pp. 230-243.
Mitchell, I. de G., Dixon, P.A., Gilbert, P.J., & White, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Problems of
evaluation. Mutat. Res., 79, 91-105.
Mitchell, I. de G. & Gilbert, P.J. (1984). The effect of pretreatment
of Escherichia coli CM891 with ethylenediamine-tetraacetate on
sensitivity to a variety of standard mutagens. Mutat. Res.,
140, 13-19.
Mitema, E.S. (1982). Effect of chloramphenicol administration on the
bovine hematology and bone marrow. Vet. Human Toxicol.,
Suppl., 162-166.
Mitus, W.J. & Coleman, N. (1970). In vitro effect of chloramphenicol
on chromosomes. Blood, 35, 689-674.
Mizuno, S., Aoki, K., Ohno, Y., Sasaki, R., & Hamajima, N. (1982).
Time series analysis of age-sex specific death rates from aplastic
anemia and the trend in production amount of chloramphenicol.
Nagoya J. Med. Sci., 44, 103-115.
Modan, B., Segal, S., Sham, M., & Sheba, C. (1975). Aplastic anemia in
Israel: Evaluation of the etiological role of chloramphenicol on a
community-wide basis. Am. J. Med. Sci., 270, 441-445.
Morizono, T. & Johnstone, B.M. (1975). Ototoxicity of chloramphenicol
ear drops with propylene glycol as solvent. Med. J. Austral.,
2, 634-638.
Morley, A., Trainor, K., & Remes, J. (1976). Residual marrow damage:
Possible explanation for idiosyncrasy to chloramphenicol. Brit. J.
Haematol., 32, 525-531.
Morris, P.L., Burke, T.R., George, J.W., & Pohl, L.R. (1982). A new
pathway for the oxidative metabolism of chloramphenicol by rat liver
microsomes. Drug Met. Disp., 10, 439-445.
Morris, P.L., Burke, T.R., & Pohl, L.R. (1983). Reductive
dechlorination of chloramphenicol by rat liver microsomes. Drug Met.
Disp., 11, 126-130.
Mulhall, A., de Louvois, J., & Hurley, R. (1983). Chloramphenicol
toxicity in neonates: Its incidence and prevention. Brit. Med. J.,
287, 1424-1427.
Murray, T. & Yunis, A.A. (1981). The cellular uptake and covalent
binding of nitrosochloramphenicol. J. Lab. Clin. Med., 98, 396-401.
Murray, T., Downey, K.M., & Yunis, A.A. (1982). Degradation of
isolated deoxyribonucleic acid mediated by nitrosochloramphenicol.
Possible role in chloramphenicol-induced aplastic anaemia. Biochem.
Pharmacol., 31, 2291-2296.
Naesland, B., Betner, I., & Halpert, J. (1983). Inhibition of rat lung
cytochrome P-450 by chloramphenicol. Extrahepatic Drug Met. Chem.
Carcinogen, Proc. Int. Mtg., 251-252.
Naesland, B. & Halpert, J. (1984). Selective inhibition by
chloramphenicol of cytochrome P-450 isozymes in rat lung and liver
involved in the hydroxylation of n-hexane. J. Pharmacol. Exp. Ther.,
231, 16-22.
Nagai, K. & Kanamaru, A. (1978). Drug-induced aplastic anaemia: Effect
of chloramphenicol on hemopoietic stems cells. In: Hibino, S., Takaku,
F., & Shakidi, N.T. (eds.) Tokyo, University Park Press, pp. 343-353.
Nagao, T. & Maner, A.M. (1969). Concordance for drug-induced aplastic
anemia in identical twins. New Engl. J. Med., 281, 7-10.
Nair, C.R., Goel, A., & Rahman, A. (1981). Inhibition of hepatic drug
metabolism by chloramphenicol-paracetamol interaction - an
experimental study. Bull. Postgrad. Inst. Med. Educ. Res.
(Chandrigarh), 15, 31-35.
Najean, Y. & Baumelon, E. (1984). Toxic etiology of aplastic anemia.
Sex Trans. Dis., 11, 343-346.
Nakagawa, T., Masada, M., & Uno, T. (1975). Gas chromatographic
determination and gas chromatographic-mass spectrometric analysis of
chloramphenicol, thiamphenicol and their metabolites. J. Chromatogr.,
111, 355-364.
Nara, N., Besshe, M., Hirashima, K., & Momoi, H. (1982). Effects of
chloramphenicol on hematopoietic inductive micro environment.
Exp. Hematol., 10, 20-25.
Narda, R.D. & Gupta, R.K. (1972). Mutation studies in
D. melanogaster. Drosoph. Inf. Serv., 48, p. 105
Nasrat, G.E., Ahmed, K.A., Nafei, H.A., & Abdel-Rahman, A.H. (1977).
Mutagenic action of certain therapeutic drugs on Drosophila
melanogaster. Bull. Fac. Agric. Univ. Cairo, 28, 697-717.
Nilges, T.C. & Norther, J.L. (1971). Iatrogenic ototoxic hearing loss.
Ann. Surg., 173, 281-289.
Pall, M.L. & Hunter, B.J. (1985). Carcinogens induce genetic tandem
duplications in Salmonella. Mutat. Res., 152, 131-145.
Pant, G.S., Kamada, N., & Tanaka, R. (1976). Sister chromatid
exchanges in peripheral lymphocytes of atomic bomb survivors and of
normal individuals exposed to radiation and chemical agents.
Hiroshima J. Med. Sci., 25, 99-105.
Parashar, S., Rao, R., Tikare, S.K., & Tikare, S.S. (1972).
Chloramphenicol induced reversible bone marrow suppression.
J. Postgrad. Med., 18, 90-92.
Parker, F.L. & James, G.W.L. (1978). The effect of various topical
antibiotic and antibacterial agents on the middle and inner ear of the
guinea-pig. J. Pharm. Pharmacol., 30, 236-239.
Patterson, W.C. & Gulick, W.L. (1963). The effects of chloramphenicol
upon electrical activity of the ear. Ann. Otol. Rhinol. Laryngol.,
72, 50-55.
Pazdernik, T.L. & Corbett, M.D. (1979). Effects of chloramphenicol
reduction products or hemopoietic precursor cells in vitro.
Pharmacol., 19, 191-195.
Pazdernik, T.L. & Corbett, M.D. (1980). Role of chloramphenicol
reduction products in aplastic anemia. Pharmacol., 20, 87-94.
Penny, R.H.C., Carlisle, C.H., Prescott, C.W., & Davidson, H.A.
(1967). Effects of chloramphenicol on the haemopoietic system of the
cat. Brit. Vet. J., 123, 145-153.
Perez, R.P., Puig, M.L., Sanchez, J.L.A., & Torres, E.L. (1981).
Aplastic anemia and drugs as causal factors. Rev. Cub. Farm.,
15, 97-103.
Petitjean, F., Sastre, J.P., Bertrand, N., Cointy, C., & Jouvet, M.
(1975). Suppression of paradoxical sleep by chloramphenicol in the
cat. Absence of effect of thiamphenicol. C. R. Seances Biol. (Paris),
169, 1236-1239.
Plaut, M.E. & Best, W.R. (1982). Aplastic anemia after parenteral
chloramphenicol: Warning renewed. New Engl. J. Med., 306, p. 1486.
Plomp, T.A., Thiery, M., & Maes, R.A.A. (1983). The passage of
thiamphenicol and chloramphenicol into human milk after single and
repeated oral administration. Vet. Human Toxicol., 25, 167-172.
Polak, B.C.P., Wesseling, H., Schut, D., Herxheimer, A., & Meyler, L.
(1972). Blood dyscrasias attributed to chloramphenicol. A review of
576 published and unpublished cases. Acta med. Scand., 192, 409-414.
Preziosi, P., Basile, V., Vacca, M., & de Natale, G. (1981). On the
correlation between regional utilization patterns of chloramphenicol
and its adverse reactions with emphasis on aplastic anaemia.
Int. J. Clin. Pharmacol.Ther. Toxicol., 19, 547-551.
Proud, G.O., Mittelman, H., & Seiden, G.D. (1968). Ototoxicity of
topically applied chloramphenicol. Arch. Otolaryngol., 87, 580-587.
Punch, P.I., Costa, N.D., Chambers, E.D., Slatter, D.H., &
Wilcox, G.E. (1985). Plasma and tear concentrations of antibiotics
administered parenterally to cattle. Res. Vet. Sci., 39, 179-187.
Puron, R. & Firminger, H.I. (1965). Protection against induced
cirrhosis and hepatocellular carcinoma in rats by chloramphenicol.
J. Natl. Cancer Inst., 85, 29-37.
Rajchgot, P., Prober, C., Soldin, S., Golas, C., Good, F., Harding, L.
& MacLeod, M. (1983). Chloramphenicol pharmacokinetics in the newborn.
Dev. Pharmacol. Ther., 6, 305-314.
Rebandel, P. & Rudzki, E. (1986). Occupational contact sensitivity in
oculists. Contact Derm., 15, p. 92.
Robin, E., Berman, M., Bhooplan, N., Cohen, H., & Fried, W. (1981).
Induction of lymphomas in mice by busulfan and chloramphenicol.
Cancer Res., 41, 3478-3482.
Rosenbach, L.M., Cavites, A.P., & Mitus, W.J. (1960). New Engl. J.
Med., 263, 724-728.
Rosenkranz, S., Carr, M.S., & Rosenkrans, H.S. (1974). 2-Haloethanols:
Mutagenicity and reactivity with DNA. Mutat. Res., 26, 367-370.
Rosenkranz, H.S., Gutter, B., & Speck, W.T. (1976). Mutagenicity and
DNA-modifying activity: A comparison of two microbial assays.
Mutat. Res., 41, 61-70.
Rosenkranz, H.S. (1977). Mutagenicity of halogenated alkanes and their
derivatives. Environ. Health Perspect., 21, 79-84.
Rosenthal, R.L. & Blackman, A. (1965). Bone marrow hypoplasia
following use of chloramphenicol eye drops. J. Am. Med. Assoc.,
191, 148-149.
Ross, S., Burke, F.G., Sites, J., Rice, E.C., & Washington, J.A.
(1950). Placental transmission of chloramphenicol (chloromycetin).
J. Am. Med. Assoc., 142, p. 1361.
Roy, B.K., Banerjee, N.C., & Pandy, S.N. (1986). Distribution of
chloramphenicol in goat blood and milk after intravenous injection.
Indian. J. Anim. Health, 25, 33-35.
Rudzki, E. & Kleniewska, D. (1970). The epidemiology of contact
dermatitis in Poland. Brit. J. Derm., 83, 543-545.
Rudzki, E., Grzywa, Z., & Maciejowska, E. (1976). Occupational contact
sensitivity in oculists. Contact Derm., 2, 181.
Sack, C.M., Kaup, J.R., & Smith, A.L. (1980). Chloramphenicol
pharmacokinetics in infants and young children. Pediatr.,
66, 579-584.
Saidi, P., Wallerstein, R.O., & Aggeler, P.M. (1961). Effect of
chloramphenicol on erythropoiesis. J. Lab. Clin. Med., 75, 247-256.
Salem, Z., Murray, T., & Yunis, A.A. (1981). The nitroreduction of
chloramphenicol by human liver tissue. J. Lab. Clin. Med.,
97, 881-886.
Samson, J.P., Hulstaert, C.E., Molenaar, I., & Nieweg, H.O. (1972).
Fine structure of the bone marrow sinusoidal wall in idiopathic and
drug induced panmyelopathy. Acta haematol., 48, 218-226.
Sanguineti, M., Rossi, L., Ognio, E., & Sauti, L. (1983). Tumours
induced in Balb/C and C57B1/6N mice following chronic administration
of chloramphenicol. In: Proceedings of a National Meeting on
Experimental and Clinical Oncology, Parma, 23-25 November,
Abstract No. 50.
Sarasti, H. (1970). Chloramphenicol in South America. New Engl.
J. Med., 282, p. 813.
Saxen, I. (1975). Associations between oral clefts and drugs taken
during pregnancy. Int. J. Epidemiol., 4, 37-44.
Scheres, J.M.J.C., Hustinx, T.W.J., Geraedts, J.P.M., Leeksma, C.H.W.,
& Meltzer, P.S. (1985). Translocation 1:7 in hematologic disorders. A
brief review of 22 cases. Cancer Gen. Cytogen., 18, 207-213.
Schewach-Millet, M. & Shapiro, D. (1985). Urticaria and angioedema due
to topically applied chloramphenicol ointment. Arch. Dermatol.,
121, p. 587.
Schmitt-Graff, A. (1981). Chloramphenicol-induced aplastic anemia
terminating with acute nonlymphocytic leukemia. Acta haematol.,
66, 267-268.
Seaman, A.J. (1969). Sequels to chloramphenicol aplastic anemia: Acute
leukaemia and paroxysmal nocturnal hemoglobinuria. Northwest Med.,
68, 831-834.
Sekizawa, J. & Shibamoto, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
Sharp, A.A. (1963). Chloramphenicol-induced blood dyscrasias: Analysis
of 40 cases. Brit. Med. J., 1, 735-736.
Sheehan, J. & Sweeney, E.C. (1982). Drugs and sperm. Brit. Med. J.,
284, p. 1331.
Shimizu, H., Kuroishi, T., Tominaga, S., Okada, H., & Aoki, K. (1979).
Production amount of chloramphenicol and mortality rate of aplastic
anaemia in Japan. Acta haem. Jap., 42, 689-696.
Siegel, D. & Krishna, G. (1980). Nitrosochloramphenicol does not
induce aplastic anaemia in mice. Pharmacologist, 22, p. 244.
Siliciano, R.F., Margolis, S., & Lietman, P.S. (1978). Chloramphenicol
metabolism in isolated rat hepatocytes. Biochem. Pharmacol.,
27, 2759-2762.
Silver, B.J. & Zuckerman, K.S. (1980). Aplastic anaemia. Recent
advances in pathogenesis and treatment. Med. Clin. North Amer.,
64, 607-627.
Sklar, R. & Strause, B. (1980). Role of the uvrE gene product and of
inducible D6-methylguanine removal in the induction of mutations by
N-methyl-N-nitro-N-nitrosoguanidine in Escherichia coli.
Mol. Biol., 143, 343-362.
Skolimowski, I.M., Rowley, D.A., Knight, R.C., & Edwards, D.I. (1981).
Reduced chloramphenicol-induced damage to DNA. J. Antimicrob.
Chemother., 7, 593-597.
Skolimowski, I.M., Knight, R.C., & Edwards, D.I. (1983). Molecular
basis of chloramphenicol and thiamphenicol toxicity to DNA in vitro.
J. Antimicrob. Chemother., 12, 535-542.
Slater, E.E., Anderson, M.D., & Rosenkranz, H.S. (1971). Rapid
detection of mutagens and carcinogens. Cancer Res., 31, 970-973.
Slaughter, R.L., Pieper, J.A., Carra, F.B., Brodsky, B., & Koup, T.R.
(1980). Chloramphenicol sodium succinate kinetics in critically ill
patients. Clin. Pharmacol. Ther., 28, 69-77.
Smith, A.L. & Weber, A. (1983). Pharmacology of chloramphenicol.
Pediatr. Clin. North Amer., 30, 209-236.
Smith, G.S., Watkins, J.B., Thompson, T.N., Rozman, K., &
Klaasen, C.D. (1984). Oxidative and conjugative metabolism of
xenobiotics by livers of cattle, sheep, swine and rats.
J. Anim. Sci., 58, 386-395.
Stainer, G.A., Peyman, B.S., Meisels, H., & Fishman, G. (1977).
Toxicity of selected antibiotics in vitreous replacement fluid.
Ann. Ophthalmol., 9, 615-618.
Steidl, P.E.-M. (1965). Bilateral optic neuritis caused by the
prolonged therapeutic use of chloramphenicol. Union Med. Canada,
94, 1061-1062.
Stevens, D,C., Kleiman, M.B., & Lietman, P.S. (1981). Exchange
transfusion in acute chloramphenicol toxicity. J. Pediatr.,
99, 651-653.
Strick, R.A. (1983). Lack of cross-reaction between DNCB and
chloramphenicol. Contact Derm., 9, 484-487.
Suda, T., Omine, M., Tsuchiya, J., & Maekawa, T. (1978). Prognostic
aspects of aplastic anaemia in pregnancy. Experience on six cases and
review of the literature. Blut., 36, 285-298.
Suhrland, L.G. & Weisberger, A.S. (1969). Delayed clearance of
chloramphenicol from serum in patients with hematologic toxicity.
Blood, 34, 466-471.
Summ, H.D., Draeger, E., & von Wasielowski, E. (1976). On the
inhibitory effect of chloramphenicol on mitochondrial protein
synthesis as a possible cause of its selective toxic side effects.
Arzneim. Forsch., 26, 28-32.
Suter, W. & Jaeger, I. (1982). Comparative evaluation of different
pairs of DNA repair-deficient and DNA repair-proficient bacterial
tester strains for rapid detection of chemical mutagens and
carcinogens. Mutat. Res., 97, 1-18.
Timmermans, L. (1974). Influence of antibiotics on spermatogenesis.
J. Urol., 112, 348-349.
Tomatis, L., Agthe, C., Bartsch, H., Huff, J., Montesano, R., Saracci,
R., Walker, E., & Wilbourn, J. (1978). Evaluation of the
carcinogenicity of chemicals: A review of the monograph program of
International Agency for Research on Cancer. Cancer Res.,
38, 877-885.
Ullrich, D. & Bock, K.W. (1984). Glucuronide formation of various
drugs in liver microsomes and in isolated hepatocytes from
phenobarbital - and 3-methylcholanthrene - treated rats. Biochem.
Pharmacol., 33, 97-101.
Vacha, J., Pospisil, M., & Velcovsky, V. (1981). The toxic effect of
chloramphenicol on erythropoiesis in X-irradiated mice. Chemother.,
27, 131-138.
Van Joost, Th., Dikland, W., Stolz, E., & Prens, E. (1986).
Sensitisation to chloramphenicol; a persistent problem. Contact
Derm., 14, 176-178.
Van Scoy, R.E. & Wilson, W.R. (1983). Antimicrobial agents in patients
with renal insufficiency. Mayo Clin. Proc., 58, 246-248.
Venning, G.R. (1983). Identification of adverse reactions to new
drugs. II - How were 18 important adverse reactions discovered and
with what delays? Brit. Med. J., 286, 289-292.
Verma, R.S. & Lin, M.S. (1978). Chemically induced alterations of the
nuclear cycle and chromosomes in root meristem cells of maize.
J. Hered., 69, 285-294.
Vincent, P.C. (1986). Drug-induced aplastic anaemia and
agranulocytosis. Incidence and mechanisms. Drugs, 31, 52-63.
Vorherr, H. (1974). Drug excretion in breast milk. Postgrad. Med.,
56, 97-104.
Wagner, T. & Rafael, J. (1977). Biochemical properties of liver
magamitochondria induced by chloramphenicol or cuprizone. Exp. Cell
Res., 107, 1-14.
Walker, G.F. (1961). Blindness during streptomycin and chloramphenicol
therapy. Brit. J. Ophthalmol., 45, 555-557.
Wallenstein, L. & Snyder, J. (1952). Neurotoxic reaction to
chloromycetin. Ann. Intern. Med., 36, 1526-1528.
Wallerstein, R.O., Candit, P.K., Kasper, C.K., Brown, J.W., &
Morrison, F.R. (1969). Statewide study of chloramphenicol therapy and
fatal aplastic anemia: J. Am. Med. Assoc., 208, 2045-2050.
Waters, M.D., Garrett, N.E., Covone-de Serres, C.M., Howard, B.E., &
Stack, H.F. (1983). Genetic toxicology of some known or suspected
human carcinogens. In: de Serres, F.J. (ed.) Chemical Mutagens.
Principles and methods for their detection. 8, New York, Plenum
Press, pp. 261-341.
Watson, A.D.J. (1972). Chloramphenicol plasma levels in the dog: A
comparison of oral subcutaneous and intramuscular administration.
J. Small Anim. Pract., 13, 147-151.
Watson, A.D.J. (1977a). Effect of concurrent drug therapy and of
feeding or chloramphenicol levels after oral administration of
chloramphenicol in dogs. Res. Vet. Sci., 22, 68-71.
Watson, A.D.J. (1977b). Chloramphenicol toxicity in dogs.
Res. Vet. Sci., 23, 66-69.
Watson, A.D.J. & Middleton, D.J. (1978). Chloramphenicol toxicosis in
cats. Am. J. Vet. Res., 39, 1199-1203.
Watson, A.D.J. (1980). Further observations on chloramphenicol
toxicosis in cats. Am. J. Vet. Res., 41, 293-294.
Welch, H., Lewis, C.N. & Kerlan, I. (1954). Blood dyscrasias. A
nationwide survey. Antibiotics Chemother., 4, 607-623.
Werner, J.C., Whitman, V., Schuler, H.G., Fripp, R.R., Rannels, A.M.,
Kasales, C.J., & La Noue, K.F. (1985). Acute myocardial effects of
chloramphenicol in newborn pigs: A possible insight into Gray Baby
syndrome. J. Infect. Dis., 152, 344-350.
White, M.P., Haboush, H.9., & Alroomi, L.G. (1986). Fatal bone marrow
aplasia due to chloramphenicol in a baby. Lancet i, 555-556.
Widayat, Sunarto, Sutaryo, Zurghiban, M., & Ismangoen (1983). Aplastic
anaemia in children. Paediatrica Indonesiana, 23, 183-191.
Wilson, W. (1962). Toxic amblyopia due to chloramphenicol. Scot. Med.
J., 7, 90-95.
Xie, L. (1985). A simple model for detection of mutagenic
(carcinogenic) substances. Huanjing Kexue Xuebao, 5, 228-233.
Yoshida, H., Yamamoto, K., & Yamaguchi, H. (1972). Fragmentation and
non-disjunction of barley chromosomes after the treatment of
chloramphenicol and cycloheximide. Cytologia, 37, 697-707.
Young, L.W., Mitnick, J.S., Yankus, W.A., & Genieser, N.B. (1979).
Radiological case of the month. Am. J. Dis. Child., 133, 545-546.
Yunis, A.A. (1973a). Chloramphenicol toxicity. In: Blood Disorders
Due to Drugs and other Agents, Amsterdam, Excerpta Medica,
pp. 107-126.
Yunis, A.A. (1973b). Chloramphenicol-induced bone marrow suppression.
Semin. Haematol., 10, 225-234.
Yunis, A.A. (1977). Differential in vitro sensitivity of marrow
erythroid and granulocytic colony forming cells to chloramphenicol.
Am. J. Haemtol., 2, 355-363.
Yunis, A.A. (1978a). Mechanisms underlying marrow toxicity from
chloramphenicol and thiamphenicol. In: Silber, R., Lo Bue, J.,
Gordon, A. (eds.) The year of hematology, New York, Plenum,
pp. 143-170.
Yunis, A.A. (1978b). Pathogenetic mechanisms in bone marrow
suppression for chloramphenicol and thiamphenicol. In: Hibino, S.,
Takaku, F., & Shahidi, N.T. (eds.) Tokyo, University Park Press,
pp. 321-334.
Yunis, A.A. (1978c). Drug-induced bone marrow aplasia. Pesq. Med.
Biol., 11, 287-296.
Yunis, A.A., Miller, A.M., Salem, Z., & Arimura, G.K. (1980a).
Chloramphenicol toxicity: Pathogenetic mechanisms and the role of the
rho-NO2 in aplastic anaemia. Clin. Toxicol., 17, 359-373.
Yunis, A.A., Miller, A.M. Salem, Z., Corbett, M.D., & Arimura, G.K.
(1980b). Nitrosochloramphenicol: Possible mediator in
chloramphenicol-induced aplastic anemia. J. Lab. Clin. Med.,
96, 34-46.
Yunis, A.A. & Salem, Z. (1980). Drug induced mitochondrial damage and
sideroblastic change. Clinics in Haematol., 9, 607-619.
Yunis, A.A. (1984). Differential in vitro toxicity of chloram-
phenicol, nitrosochloramphenicol, and thiamphenicol. Sex Trans. Dis.,
11, 340-342.
Chloramphenicol was previously considered at the twelfth meeting
of the Committee (Annex 1, reference 17), at which time it was
determined that acceptable levels in food could not be established.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
In dogs, chloramphenicol is rapidly and extensively absorbed
after oral administration of 50 mg/kg b.w., giving plasma levels of
16.5 g/l 2 hours after dosing (Watson, 1972; Watson 1977a). Similar
findings have been observed in rabbits given oral doses of 16 mg/kg
chloramphenicol (Cid et al., 1983). Dietary bran markedly
increased the rate and extent of absorption of orally administered
chloramphenicol palmitate in pigs, but it is not known if the
absorption of chloramphenicol itself would be affected in this way
(Bueno et al., 1984). Experiments with everted mouse small intestine
sacs resulted in absorption of chloramphenicol at similar rates over
different regions of the small intestine. Various chemicals and
metallic ions had no effect on the rate of absorption, and penetration
of the serosal and mucosal surfaces was similar indicating passive
absorption of chloramphenicol from the gut (Chakrabarti & Banerjee,
1977).
In adult human beings, absorption of chloramphenicol is rapid and
extensive after an oral dose. Serum levels were 20 - 40 mg/l after a
2 g dose (29 mg/kg b.w.) and 40 - 60 mg/l after a 4 g dose (57 mg/kg
b.w.) (Yunis, 1973a).
Chloramphenicol is also well absorbed by infants and neonates
after oral administration. Serum (peak) concentrations of 20 - 24 mg/l
were noted after oral doses of 40 mg/kg b.w. in neonates. Infants
given 26 mg/kg b.w. were found to have peak concentrations of 14 mg/l
(Mulhall et al., 1983). Available evidence and theoretical
considerations suggest that chloramphenicol may be percutaneously
absorbed in human beings (Guy et al., 1985a, b).
Chloramphenicol is distributed to most major organs in newborn
pigs. After i.v. administration of 0.52 mg/kg b.w. 14C-chloram-
phenicol, many tissues showed higher levels of chloramphenicol than
were found in the blood as soon as 5 minutes after administration.
These tissues included the lungs, liver, kidneys, adrenal cortex,
myocardium, pancreas, thyroid, spleen, and skeletal muscle. The
levels remained higher than in blood for up to 8 hours after dosing.
At 4 and 8 hours after administration, levels in the brain were
higher than in blood. However, there was no apparent affinity for
chloramphenicol in the bone marrow over the period of the experiment
(8 hours), where levels did not reach those noted in serum (Appelgren
et al., 1982).
Chloramphenicol in human beings, regardless of the route of
administration, extensively distributes, although the levels in
tissues depend on the route, the levels being the highest after oral
or i.v. administration. The compound has been found in the heart,
lungs, kidneys, liver, spleen, pleural fluid, seminal fluid, ascitic
fluid, and saliva. (Ambrose, 1984; Yunis 1973a; Gray, 1955). It binds
extensively to proteins in both adults and neonates, although binding
in the latter is less than in the former (Kurz et al., 1977).
Chloramphenicol can cross the placenta in human beings (Ambrose,
1984). After oral doses of 1 or 2 g chloramphenicol to pregnant women,
chloramphenicol was detected in the placenta after 1.5 - 2.5 hours,
indicating some potential for the drug to reach the fetus (Ross
et al., 1950).
In human beings with normal renal/hepatic function the volume of
distribution is around 0.7 1.4 1/kg. These values do not deviate
markedly in patients with hepatic dysfunction or with renal
impairment. Overall, these values suggest extensive distribution in
body tissues. (Ambrose, 1984; Burke et al., 1980; Slaughter
et al., 1980). Similar values were noted with infants and young
children given the sodium succinate derivative of chloramphenicol,
which is converted in vivo to chloramphenicol (Sack et al., 1980).
Chloramphenicol and its metabolites are excreted in the urine of
rats after oral dosing; up to 70% of an oral dose may he excreted in
this way (Glazko et al., 1949; Glazko et al., 1952). Limited data
suggest that chloramphenicol may be excreted in the bile of rats
following administration; around 0.4% of an i.m. dose of 40 mg/kg was
detected in the bile after 4 hours (Kunii et al., 1983). There are
no readily available data for biliary excretion after oral
administration. In new-born pigs, the majority of an i.v. dose of
chloramphenicol was excreted in the urine, while a small quantity was
excreted in the bile (Appelgren et al., 1982). Liver damage may
delay body-clearance of chloramphenicol, at least in the mini-pig
(Kroker, 1985). Following t.v. administration to goats, 69% of the
dose was excreted in the urine after 12 hours (Javed et al., 1984).
Chloramphenicol has been found to be excreted in other body
fluids after administration to animals. It has been detected at levels
up to 6 mg/l in the tears of cattle given i.v. doses of 50 mg/kg
chloramphenicol; it was detected in the tears as soon as 1 hour after
dosing (Punch et al., 1985). Chloramphenicol was detected in the
milk of goats after an i.v. dose of 100 mg/kg, with maximum levels
1 hour after administration. (Roy et al., 1986). Administration of
10 mg/kg chloramphenicol i.m. to cattle resulted in peak levels in
milk of around 1 mg/l 6 hours after injection. However, after oral
administration, no chloramphenicol was detected in milk.
(De Corte-Baeten & Debackere, 1976).
Nine patients with evidence of chloramphenicol-induced bone
marrow suppression showed greatly reduced plasma clearance times
compared with 9 others who showed no evidence of toxicity. In the
"toxic" group, 5 had liver disease, 2 had pyelonephritis, and 2 showed
neither liver disease nor renal disease. In the "non-toxic" group, 1
had liver disease, 2 had renal disease, while 6 had neither. Six hours
after an i.v. dose of 500 mg chloramphenicol succinate, the blood
level in the "toxic" group was 4.5 µg/ml (2.8 - 6.9 µg/ml), while in
the "non-toxic" group the mean level was 1.2 µg/ml (0 - 2.3 ug/ml).
Similarly, after 8 hours the mean level in the "toxic" group was
3.5 µg/ml (2.1 - 5.2 ug/ml), while in the "non-toxic" group a mean
level of 0.7 µg/ml (0 - 2.5 µg/ml) was noted. Such findings suggest
that patients susceptible to the bone-marrow effects of
chloramphenicol may clear the drug from the blood at a slower rate
than those who are not susceptible (Shurland & Weisburger, 1969).
Chloramphenicol administered to human beings is excreted
primarily in the urine (90%), up to 15% as the parent compound and the
remainder as metabolites, including conjugated derivatives
(Burke et al., 1980; Ambrose, 1984; Yunis, 1973a). Glomerular
excretion is thought to be the major mechanism of excretion
(Glazko et al., 1949).
Renal clearance varies depending on age. In one study, clearance
in neonates (less than 6 months of age) was 0.46 - 9.76 1/hour, while
in infants aged 6 months to 2.5 years values in the range 1.8 - 2.1
1/hour were reported. In two subjects aged over 2.5 years, values of
6.03 and 9.59 1/hour were noted (Burckart et al., 1983). Similar
variations with age have been noted in other studies (Mulhall et al.,
1983; Sack et al., 1980; Kauffman et al., 1981; Burckart et al.,
1982; Rajchgot et al., 1983). Renal clearance takes longer in
patients with renal insufficiency than in normal patients (Brasfield
et al., 1983). However, these differences are not marked (Smith &
Weber, 1983). It has been recommended that dosage adjustments need not
be made in patients with renal insufficiency or in anephric subjects
(Van Scoy & Wilson, 1983).
As with experimental animals, chloramphenicol is excreted in
human milk. Up to 1.3% of an administered dose may be excreted in this
way. (Vorherr, 1974). After a single, oral dose of chloramphenicol, a
peak milk concentration of 3 mg/l has been reported. This peak was
reached around 2 hours after administration, with a drop to nearly
pre-dosing levels by 8 hours post-dose (Plomp et al., 1983). Other
workers have reported similar findings (Knowles, 1965; Matsuda, 1984).
Biotransformation
Early studies indicate that the major metabolite of
chloramphenicol in the rat was the glucoronide conjugate, which was
found along with free chloramphenicol after oral dosing
(Glazko et al., 1950; Glazko et al., 1952). In in vitro studies
it has been demonstrated that the glucuronide is the main metabolic
product in isolated rat hepatocytes exposed to chloramphenicol
(Siliciano et al., 1978). Glucuronidation of chloramphenicol was
elevated in vitro in hepatocytes obtained from phenobarbital-
pretreated rats. This increase correlated with differential induction
of UDP-glucuronyltransferase in hepatocytes of rats pretreated with
phenobarbital (Ullrich & Bock, 1984).
Several metabolites of chloramphenicol have been identified in
rat urine. In addition to free chloramphenicol (1) and the glucoronide
(2), the oxamic acid (3), alcohol (4), and base (5) derivatives have
been noted in the urine of rats given i.m. doses of [3H]-chlo-
ramphenicol (1R,2R-1- p-nitrophenyl(2,2-dichloroacetamido-
1,3-(1-3H)-propandediol). The acetylarylamine (6) and arylamine
metabolites (7) have also been detected. These metabolites are shown
in Table 1.
Table 1 Metabolites of chloramphenicol in the rat
Compound R1 R2 R3
1. chloramphenicol NO2 COCHCl2 OH
2. glucuronide conjugate NO2 COCHCl2 C6H9O7
3. oxamic acid derivative NO2 COCHO2H OH
4. alcohol derivative NO2 COCH2OH OH
5. base derivative NO2 H OH
6. acetylarylamine derivative NHCOCH3 COCHCl2 OH
7. arylamine derivative NH2 COCHCl2 OH
Based upon recovered radioactivity, the major metabolites
appeared to be chloramphenicol base (approx. 26%) and the
acetylarylamine derivative (19.1%). The other metabolites were
recovered in the 8-15% range, except for the arylamine derivative,
which represented approximately 4% of the recovered radioactivity
(total identified, 93.4% of administered radioactivity; total
collected, 95.9% of administered radioactivity (Bories et al.,
1983).
An in vitro study using perfused rat liver and rat liver
microsomes indicated that the arylamine derivative may undergo
N-oxidation to form nitrosochloramphenicol by way of the N-hydroxy
derivative, which may then be conjugated with glutathione
(Ascherl et al., 1985).
There are few data available on the metabolism of chloramphenicol
in other species. In dogs, chloramphenicol, chloramphenicol base, and
chloramphenicol glucuronide conjugate appeared to be the major
metabolites (Glazko et al., 1950). Chloramphenicol, the glucuronide
conjugate, and the oxamic acid, acetylarylamine, arylamine, and base
derivatives were noted in the urine of goats given i.m. injections of
chloramphenicol (Bories et al., 1983). In vitro studies with pig
liver showed the activity of UDP-glucuronyltransferase in this species
to be similar to that in rats, suggesting that glucuronidation may be
a significant pathway of biotransformation of chloramphenicol in pigs.
The same experiments with sheep and cattle liver preparations showed
that they have much lower glucuronyl transfer activities (25% and 14%,
respectively). This may indicate that glucuronidation plays a less
important role in these species (Smith et al., 1984).
In human beings, 93% of an oral dose of chloramphenicol was
excreted in the urine within 24 hours, and it seems likely that the
major excretion product was the glucuronide conjugate. Approximately
48% of the chloramphenicol excreted in the urine within 8 hours after
oral dosing was the glucuronide conjugate; only 6% was excreted as the
parent compound and 4% as the base derivative (Baselt, 1982;
Nakagawa et al., 1975). The alcohol derivative has been detected in
the urine of neonates (Dill et al., 1960). More recent experiments
have confirmed the presence of the glucuronide conjugate and base
derivative as the major metabolites after an oral dose of 500 mg
chloramphenicol (Bories et al., 1983).
Human liver has the potential to reduce chloramphenicol. In 10
livers studied, nitro reductase activity was observed, which was
dependent on NADPH. Thus, human livers may have the capacity to
convert the nitro group of chloramphenicol to the amine, with the
further possibility of nitroso formation. Esters of chloramphenicol,
for example, the succinate, are converted to chloramphenicol in vivo
(Salem et al., 1981).
Effects on enzymes and other biochemical parameters
Chloramphenicol is known to increase the plasma levels of certain
drugs (e.g., paracetamol and phenytoin) and to prolong barbiturate
sleeping time, which is indicative of an effect on drug metabolizing
enzymes (Halpert & Neal, 1981; Nair et al., 1981; Aravindaksham &
Cherian, 1984).
The major contribution to these effects may arise from
chloramphenicol's ability to act as a suicide substrate in the
inactivation of cytochrome P-450, possibly by the binding of the
oxamic acid derivative to lysine residues of the cytochrome molecule
(Halpert & Neal, 1981; Halpert, 1981; Halpert, 1982). Of the various
isozymes of cytochrome P-450, those induced by phenobarbital appear to
be the most sensitive to chloramphenicol. Rats were pretreated with
various inducers of cytochrome P-450 (phenobarbital, ß-naphthoflavone,
pregnenolone, 16-x-carbonitrile, and clofibrate) and were then
injected i.p. with 300 mg/kg b.w. chloramphenicol. The isozymes of
cytochrome P-450 induced by phenobarbital were inhibited to some
degree by chloramphenicol administration, but those forms induced by
the other compounds investigated were not affected. Cytochrome
P-450-c, the form present in non-pretreated rats, was the most
susceptible isozyme in vivo and in vitro (Halpert et al., 1985).
Following i.v. or i.p. administration of 100 mg/kg b.w.
chloramphenicol to rats, inhibition of the conversion of n-hexane to
2-hexanol by rat liver or lung microsomes occurred. There was no
effect on the conversion of n-hexane to l-hexanol, while only liver
microsomes were markedly inhibited in the formation of 3-hexanol
(Naesland & Halpert, 1984; Naesland et al., 1983).
Chloramphenicol prevented the methanol-potentiated toxicity of
carbon tetrachloride in rats, possibly by deactivation of cytochrome
P-450 (Brabec et al., 1982). Phenobarbital-induced cytochrome P-450
appears to be responsible for the dechlorination of chloramphenicol
and the formation of chloramphenicol aldehyde in rat liver microsomes.
The dechlorination may be enhanced by glutathione. The significance of
these findings is not clear at the present time (Morris et al.,
1982; Morris et al., 1983).
Subcutaneous administration of up to 100 mg/kg b.w. chloram-
phenicol to rats inhibited hepatic N-demethylase, glucose-6-
phosphate dehydrogenase, and carboxylesterase (Hapke et al., 1977).
However, no effects were observed on monoamine oxidase in the liver,
brain, or heart of rabbits given 60 mg/kg b.w. chloramphenicol i.m.
for 5 days. Similarly, no effect on monoamine oxidase activity was
seen in in vitro experiments where rabbit liver preparations were
preincubated with chloramphenicol (Ali, 1985).
Several studies have demonstrated an effect of chloramphenicol on
mitrochondrial protein synthesis. In vitro, chloramphenicol
inhibited mitochondrial protein synthesis in rat liver and rabbit bone
marrow. The effect was similar to that noted with tetracycline (Summ
et al., 1976). Nitrosochloramphenicol inhibited rat mitochondrial
DNA polymerase in vitro, whereas the arylamine derivative and
chloramphenicol itself did not (Lim et al., 1984). Nitroschlor-
amphenicol inhibited the transport of NAD-linked substrates into
mitochondria, but it had no effect on FAD-linked substrates. It
inhibited ATP formation and completely blocked the transport of
protons out of the mitochondria (Abou-Khalil et al., 1982).
Chloramphenicol inhibited the incorporation of leucine into protein in
mitochondria from erythroid and myeloid rumour cells and in cells from
normal, myeloid, and erythroid hyperplastic bone marrow of rabbits
in vitro (Abou-Khalil et al., 1980, 1981). In mice given
chloramphenicol, the so-called megamitochondria that were produced
were deficient in cytochrome oxidase, ATP synthetase, and cytochrome b
activities, which is indicative of inhibited protein synthesis
(Wagner & Rafel, 1977). Subcutaneous administration of 500 mg/kg b.w.
chloramphenicol succinate to partially hepatectomized rats did not
significantly inhibit protein synthesis. However, liver cytochrome c
oxidase was strongly inhibited in these rats (Kroon & Vries, 1969).
Intramuscular administration of chloramphenicol to rats inhibited
mitochondrial monoamine oxidase activity in the liver, brain, and
kidney (Banerjee & Basu, 1978). When chloramphenicol was given daily
for 6 days at doses of 100 mg/kg b.w. i.p., marked reductions in the
activities of liver kynurenine hydrolase, knynurenine amino-
transferase, ß-glucuronidase, and acid ribonuclease were observed,
indicating possible effects on drug metabolizing enzymes and on
protein biosynthesis. Pyridoxal phosphokinase activity was increased
(Akhnoukh et al., 1982).
In human blood, nitrosochloramphenicol is bound to albumin
in vitro. In red blood cells, it rapidly forms adducts with
glutathione and also with the -SH groups of haemoglobin. Moreover, it
is rapidly reduced to the N-hydroxy compound, although further
reduction to the amine is very slow. Overall, the data suggest that
the nitroso derivative would be rapidly detoxified in the blood before
reaching the bone marrow (Eyer et al., 1984). Nitrosochloramphenicol,
but not chloramphenicol, induced methaemoglobinaemia in haemolyzed
human blood in vitro (Lim & Yunis, 1982).
To summarize, chloramphenicol is well absorbed in both animals
and human beings after oral administration and widespread distribution
occurs. Urinary excretion is rapid in animals and man, a major
metabolite being the glucuronide conjugate. Animals and human beings
produce a range of urinary metabolites; in vitro experiments suggest
that nitrosochloramphenicol may be an important metabolite in human
beings. It is not known if this compound is produced in vivo in man
or other species.
Toxicological studies
Special studies on carcinogenicity
In 1982 and 1987, IARC concluded that adequate tests for
carcinogenicity of chloramphenicol in animals were not available
(IARC, 1982; IARC, 1987). These conclusions confirmed an earlier
opinion by IARC workers (Tomatis et al., 1978). The two studies
below by Robin et al., and by Sanguineti et al., were included in
the IARC evaluations.
Four groups of Balb/c × AF1 male mice, 45 per group, were given
i.p. injections of either 0.5 mg busulphan on days 1, 15, 29 and 43
(2 groups) or vehicle (acetone plus distilled water, 2 groups. After
20 weeks, by which time 78 mice remained alive in the busulphan-
treated groups and 88 in the vehicle groups (the rest having
died of injection complications), one of the busulphan groups and one
of the vehicle groups was selected for treatment with chloramphenicol,
while the others served as controls and were given vehicle only (0.9%
sodium chloride). Mice in the treatment groups received i.p.
injections of 2.5 mg chloramphenicol 5 days per week for 5 weeks.
Sacrifice of the mice followed a complicated schedule depending on the
appearance of lymphomas, but all animals had been sacrificed by day
350. The following incidences of lymphoma were noted: busulphan/
chloramphenicol, 13/37; busulphan/vehicle, 4/35; chloramphenicol/
vehicle 2/42; vehicle/vehicle, 0/41. The authors thought that this
suggested that busulphan and chloramphenicol increased the frequency
and accelerated the onset of lymphomas. It also provided some
evidence that chloramphenicol alone might induce lymphomas in this
animal model, but the duration of the experiment along with other
limitations of the experimental design, particularly the dosing
regime, prevent any other conclusions from being drawn (Robin
et al., 1981).
In a study which was reported in abstract form only, in which
chloramphenicol was administered in the drinking water, an increased
incidence of lymphomas in two strains of mice and of hepatocellular
carcinoma in one strain were noted (Sanguineti et al., 1983).
Another study investigated the effect of chloramphenicol on
cirrhosis and hepatocellular carcinoma induced by the carcinogen
N-2-fluorenyldiacetamide in rats. A group of 25 male Wistar rats was
given a diet containing 0.05% N-2-fluorenyldiacetamide (equivalent to
25 mg/kg b.w./day) plus 2% chloramphenicol (equivalent to 1000 mg/kg
b.w./day) for 4 weeks. Another group of 20 rats received a diet
containing only the 0.05% N-2-fluorenyldiacetamide. A "rest" week was
then followed by another 4 weeks on the respective diets, followed by
another "rest" week and then 6 weeks of dietary administration of the
substances. After this the animals given the carcinogen only were
returned to the control diet, but those given the combined regime were
continued on this diet for another 2 weeks. Animals were sacrificed at
46 (carcinogen only) or 46-55 weeks (carcinogen plus chloramphenicol).
Of 12 animals examined which were given the carcinogen-only diet, 100%
had cirrhosis and 75% had hepatocellular carcinoma, whereas of 22
animals examined which were given the combination treatment, only one
had cirrhosis and hepatic rumours. Thus, chloramphenicol had a
protective effect on the induction of hepatic rumours by
N-2-fluorenyldiacetamide (Puron & Firminger, 1965).
Special studies on haematological effects
Groups of 18-21 57B1/10ScSnPh mice were given 4.78 Gy of
X-irradiation and were then treated three times daily with 160 or
320 mg/kg b.w. chloramphenicol succinate by s.c. injection over 3 or 5
days, beginning 10 days after the X-ray treatment. Two groups of
control animals were given chloramphenicol and no irradiation or
irradiation and no chloramphenicol. Animals were examined 4, 8, and 21
days after initiation of chloramphenicol treatment (14, 18, and 21
days after irradiation). No effects on the level of erythrocytes in
non-irradiated mice occurred; those given chloramphenicol showed
similar values to non-treated controls. The level of erythrocytes in
irradiated animals was significantly lower than in non-irradiated
animals (30% reduction 14 days after irradiation) but there was
improvement with time (26% reduction 18 days and 15% reduction 21 days
after irradiation), while irradiated animals given chloramphenicol
showed lower erythrocyte levels than those that were irradiated only;
at 14, 18, and 21 days post irradiation, irradiated mice given
chloramphenicol had erythrocyte levels 8%, 17%, and 4.5% lower,
respectively, than animals given irradiation alone, indicating that
chloramphenicol had a deleterious effect on bone marrow recovery after
X-irradiation (Vacha et al., 1981).
Normal mice and those with residual bone marrow damage following
busulphan administration were investigated for the effects of
chloramphenicol. Female Balb/c mice were injected with busulphan to
create bone marrow damage using a method that had been validated
previously by the authors. Groups of mice, some having busulphan-
induced damage and others untreated, were then given drinking water
containing 0.5 g/dl chloramphenicol succinate; groups of 5 mice were
then killed at various intervals up to and including 150 days after
chloramphenicol treatment. No effects were seen in the bone marrow
of mice given chloramphenicol alone nor in mice pretreated with
busulfan. However, animals given both busulphan and chloramphenicol
displayed a progressive fall in the number of pluripotential stem
cells and granulocytic precursor cells (Morley et al., 1976).
Similar effects were not seen in another study where busulphan-
treated mice were given drinking water containing 0.5 g/dl
chloramphenicol for six weeks. There was no effect on the colony-
forming ability of bone marrow or spleen cells. This study used
an identical busulphan regime to that described above (Pazdernik &
Corbett, 1980).
In cells of lethally X-irradiated mice given 10 mg
chloramphenicol i.p. on days 2-8 or on days 7-12 after irradiation,
mitochondrial swelling was noted in early erythroblasts, but not in
intermediate or late types. The mitochondria showed reductions in the
number of cristae (Miura et al., 1980).
A similar investigation in mice given 4.78 Gy X-irradiation and
300 mg/kg b.w. of chloramphenicol succinate showed that dividing bone
marrow cells had decreased entry into S-phase of the cell cycle. The
affected cells were mainly of the erythroid type. The same types of
effect were also noted in non-irradiated mice given chloramphenicol
(Benes et al., 1980).
The femora of mice given 500 mg/kg b.w. chloramphenicol for 6
days by injection (route unspecified) which were then implanted into
untreated syngeneic mice showed greatly decreased colony formation
when the bone marrow was subsequently injected into lethally
irradiated mice (Nara et al., 1982).
No haematological effects, including aplastic anaemia, were seen
in mice given 40 mg/kg nitrosochloramphenicol for 10 days followed by
sacrifice 6 weeks after the last injection (Siegel & Krishna, 1980;
abstract only).
Groups of 6 male Sprague-Dawley rats were each given 50 mg/kg
b.w. chloramphenicol succinate by i.v. infusion. Half of the animals
were subjected to liver resection 15 minutes after infusion, while the
others were sacrificed at this time. Bleeding time and blood loss were
significantly increased in resected animals given chloramphenicol
compared with controls (bleeding time: 500 seconds in treated animals,
300 seconds in controls; blood loss 2.2 g in treated animals, 0.9 g in
controls). No effects on haemoglobin or haematocrit were observed
following infusion or liver resection (Bengmark et al., 1981).
Four cats were given daily i.m. injections of 50 mg/kg b.w.
chloramphenicol for 21 days. Two untreated cats served as controls;
all 6 animals had recently recovered from experimental infectious
feline enteritis. Animals given chloramphenicol became very ill, with
severe loss of appetite developing within 7 days. All four developed
diarrhoea toward the end of the experiment (they were sacrificed on
day 21); one was killed in extremis. Marrow examination was carried
out, which revealed vacuolation of the myeloid and erythroid
precursors and of some lymphocytes. There were no significant changes
in peripheral red cell numbers, but white cell counts were much
reduced (Penny et al., 1967).
A group of 6 cats was given chloramphenicol orally at a dose of
125 mg/kg b.w./day for 14 days and then observed for another 3 weeks.
Another group, formerly intended as controls, were dosed with 60 mg/kg
b.w./day chloramphenicol in the same manner. Signs of toxicity
included CNS depression, dehydradation, loss of appetite, body weight
loss, diarrhoea, and vomiting. Blood and bone marrow samples were
obtained both prior to and after chloramphenicol treatment. The major
findings related to chloramphenicol administration were severe bone
marrow suppression with marrow hypoplasia, prevention of maturation of
erythroid cells, and inhibition of mitosis in the marrow. Vacuolation
of lymphocytes, and early myeloid and erythroid cells occurred. The
effects were most severe in cats given 120 mg/kg b.w. chloramphenicol.
On cessation of treatment the bone marrow suppression resolved (Watson
& Middleton, 1978).
In a similar study, 5 cats were given 50 mg/kg b.w./day
chloramphenicol orally for 21 days. CNS depression, appetite loss, and
weight loss were noted. Examination of peripheral blood was conducted
both before and after dosing. This revealed lower platelet numbers
after 1 week, and a lower neutrophil count after 3 weeks of
administration. One animal developed lymphocytopenia after 1 week and
neutropenia after 3 weeks. At the end of treatment, the bone marrow
was found to have vacuolated early myeloid cells and lymphocytes, with
reduced myeloid maturation and reduced marrow cellularity. No test for
recovery was conducted (Watson, 1980).
Twenty dogs were given oral doses of chloramphenicol for 14 days.
They were dosed in the following manner: 6 dogs, 225 mg/kg b.w./day;
4 dogs each, 175 or 125 mg/kg b.w./day; and 3 dogs each at 275 or
75 mg/kg b.w. day. Signs of toxicity included a decline in the rate of
weight gain and hypophagia. No changes in erythrocyte counts,
reticulocyte counts, haemoglobin concentration, packed cell volume, or
differential leukocyte counts occurred, but bone marrow examination of
the dogs given 225 or 275 mg/kg/b.w./day revealed suppression of
erythropoiesis. In dogs given 275 mg/kg b.w./day chloramphenicol,
decreased mitotic activity and a reduced rate of granulocyte formation
was also evident. No dogs showed bone marrow vacuolation
(Watson, 1977).
After i.v. administration of a single dose of 50 mg/kg b.w.
chloramphenicol succinate to five mongrel dogs, platelets were
obtained from blood at 0, 30, 60, 120, 180, and 240 minutes and at 24
hours after dosing. Protein synthesis as determined by the rate of
incorporation of 3H-leucine was inhibited, with maximum depression
(940% of control values) occurring at 30 - 240 minutes after dosing
(Agam et al., 1976).
Neonatal Holstein calves (1 day of age at the beginning of the
experiment) given "an adequate" intake of colostrum were given
chloramphenicol by several methods. These were, i.v. as a 25 mg/kg
b.w. bolus at 1, 7, 14, and 28 days of age, i.v. as a 25 mg/kg b.w.
injection every 12 hours until 150 mg/kg b.w. had been delivered, and
as a 25 mg/kg b.w. bolus i.v., i.m., or s.c. alternately, with 1 week
between the doses. No effects on haematological parameters were
observed, and bone marrow aspirates showed no evidence of suppression
or toxicity (Burrows et al., 1984).
A similar lack of effect on the bone marrow was noted in a study
of cross-breed calves given doses of 9, 20, or 60 mg/kg b.w.
chloramphenicol daily for 6 weeks (Mitema, 1982).
In another study in Holstein calves in which chloramphenicol was
given orally at a dose of 100 mg/kg b.w./day over 10 days, bone marrow
suppression did occur. Similar results were noted in over 50 calves
investigated over a period of time. Changes included partial aplasia
to almost complete aplasia of the marrow, with loss in cellularity of
erythrocytes, white cells, and megakaryocytes. Occasionally only fat
deposits were noted with lymphocytic infiltrations. Lower blood levels
of chloramphenicol were attained after oral intake than following i.v.
injection, but the toxic effects on the marrow were greater after oral
dosing (Krishna et al., 1981).
In in vitro experiments, chloramphenicol and its postulated
metabolite, nitrosochloramphenicol, have shown adverse effects on bone
marrow cells. Chloramphenicol caused dose-related inhibition of
erythroid and granulocytic colony forming units obtained from LAF1
mice. The lowest concentration used (5 µg/ml) caused some degree of
inhibition of erythroid cells, while the highest concentration
(60 µg/ml) produced complete inhibition (Yunis, 1977). Similar effects
were noted in a separate study (Hara et al., 1978).
Chloramphenicol and nitrosochloramphenicol inhibited DNA
synthesis in rat bone marrow cells in vitro. This was reversible
with chloramphenicol, but not with the nitroso compound. Similarly,
the nitroso compound, but not chloramphenicol, bound irreversibly to
bone marrow cells (Gross et al., 1982). However, in another
in vitro study, chloramphenicol and nitrosochloramphenicol had no
effects on mouse haematopoietic precursor cells (Pazdernik & Corbett,
1979).
Special studies on mutagenicity
The antibiotic activity of chloramphenicol is not thought to
involve any type of reaction with bacterial genetic material. It
appears to inhibit protein synthesis by binding to the 50S subunit of
the 70S ribosome, inhibiting the formation of the peptide bond during
protein synthesis (Smith & Weber, 1983, Gilman et al., 1985).
Chloramphenicol has been shown to cause DNA strand breaks in
bacterial cells and to inhibit DNA synthesis in lymphocytes and in a
phage of E. coli (Amati, 1970; Dewse, 1977; Jackson et al., 1977).
However, chloramphenicol provided resistence to UV-induced damage in
E. coli B/r (Doudney & Rinaldi, 1985).
Nitrosochloramphenicol, which is a potent inducer of DNA strand
breaks in bacterial cells, resulted in strand breakage and loss of
helix, bringing about rapid degradation of isolated E. coli DNA
in of helix, bringing about rapid degradation of isolated E. coli
DNA in vitro (Skolimowski et al., 1981, 1983). Nitrosochlor-
amphenicol, but not chloramphenicol, produced inhibition of DNA
synthesis and caused DNA strand breaks in E. coli. (Murray
et al., 1982; Yunis, 1984).
Chloramphenicol has been tested in a variety of assays for
mutagenic activity. Most of these assays are well established using
well validated methods, but some, such as colicine induction, the
induction of tandem genetic duplications in S. typhimurium, and
tests using snails, are less well known and are unvalidated. The
results of these tests are presented in Table 2.
In general chloramphenicol gave negative results in bacterial
reverse mutation assays, in assays for DNA repair in bacteria, in the
dominant lethal test in rodents and D. melanogaster, in the
CHO/HGPRT test, in a test for sister chromatid exchange in human
lymphocytes, in the micronucleus test, and in a test for DNA binding.
Tests for inhibition of growth in E. coli were also negative and,
moreover, chloramphenicol has sometimes been used as a negative
control in this assay (McCoy et al., 1980a; McCoy et al., 1980b;
Rosenkranz, 1977; Rosenkranz et al., 1974; Braun et al., 1977;
Braun et al., 1982). There were isolated exceptions to these
negative results. However, the only type of test that gave
consistently positive results was the test for induction of
chromosomal aberrations. Chromosomal anomalies were noted in human
lymphocytes treated in vitro with chloramphenicol, in mouse bone
marrow in animals given chloramphenicol at doses of 50 mg/kg i.p. or
i.m., and in F1-generation mouse liver.
The failure of chloramphenicol to give positive results in the
Ames test has been attributed by one group of authors to its bacterial
toxicity. In their study, the D(-)-threo isomer of chloramphenicol
gave negative results in the Ames test, as observed previously in
several other studies; it was also toxic to the bacterial tester
strains used, TA100 and TA1535. However, the L(+)-threo isomer,
which is not used therapeutically, was much less toxic and could be
tested at higher concentrations; with the isomer, a dose-related
mutagenic response was noted in both tester strains (Jackson et al.,
1977).
The failure of chloramphenicol to give positive results in most
types of commonly used mutagenicity tests, with the exception of those
examining the induction of chromosome aberrations, has been recognized
by other reviewers (Waters et al., 1983; Garrett et al., 1984), as
has the failure of the substance to produce mutations in vivo
(Holden, 1982).
Chloramphenicol has been reported to enhance the mutagenicity of
N-methyl-N-nitro-N-nitrosoguanidine in bacterial assays (Sklar &
Strauss, 1980; Baltz & Stonesifer, 1985).
Special study on ocular toxicity
No toxic effects were seen in groups of three rabbits following
vitrectomy when solutions containing 10 or 20 µg/ml chloramphenicol
were infused into the eyes as vitreous replacements. Histological and
electroretinographical examinations yielded normal results 2 weeks
after infusion. Following an infusion of a 50 µg/ml solution,
electroretinography was normal after 2 weeks, but abnormal retinal
histology, which was not described but was said to be widespread and
generalized, was noted (Stainer et al., 1977).
Special studies on ototoxicity
Solutions of 0.5% chloramphenicol introduced into the bullae of
the ears of guinea pigs produced no effects on hearing, but solutions
containing 1 - 5% chloramphenicol produced moderate hearing loss at a
variety of frequencies. Similar findings were noted when the
electrical responses of the hair cells were measured after
chloramphenicol administration into the bullae. Intratympanic
administration to guinea pigs of a 1% solution of chloramphenicol
produced a moderate degree of hair cell loss in the organ of Corti,
with severe inflammation of the mucosa of the middle ear. Introduction
of a solution containing 8 or 16 mg chloramphenicol through a hole in
the bullae of the ears of guinea pigs followed by sacrifice 3, 6, 9,
or 24 hours later resulted in severe destruction of hair cells and
supporting cells in the basal turns of the organ of Corti. The effects
were similar regardless of the dose or the time of sacrifice after
dosing (Proud et al., 1968; D'Angelo et al., 1967; Patterson &
Gulick, 1963; Morizono & Johnstone, 1975; Parker & James, 1978).
Table 2. Results of mutagenicity assays with chloramphenicol
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 30 µg/plate - Brem et al.,
TA1530, TA1535 1974
TA1538
S. typhimurium 0.17-24 µg/ml + Mitchell et al.,
TA98 1980
S. typhimurium Not given - Heddle & Bruce,
TA1535, TA1537 1977
S. typhimurium 30 µg/plate - Rosenkranz et al.,
TA98, TA100 1976
S. typhimurium < 4.5 nmole - McCann et al.,
TA98, TA1535 1975
TA1538
Bacterial E. coli 27 µg/ml + Mitchell &
mutation assay CM891 Gilbert, 1984
CHO/HGPRT Chinese hamster Not given Augustine et al.,
mutation assay ovary cells 1982
Gene mutation D. melanogaster Not given +a Narda & Gupta,
1972
(abstract only)
Dominant lethal D. melanogaster Not - Nasrat et al.,
1977
Mouse (101×C3H)F1 2×1.5 g/kg - Ehling, 1971
Mouse (ICR/Ha 333 mg/kg - Epstein & Shafner,
Swiss) 1968
Mouse (ICR/Ha 333 & 666 mg/kg - Epstein et al.,
Swiss) 1972
DNA repair B. subtilis 2.5×10-3 mg/ - Sekizawa &
H17, M45 disc Shibamoto, 1982
B. subtilis Not given - Suter & Jaeger,
H17, M45 1982
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
E. coli Not given + Suter & Jaeger,
AB1157/JC5547 1982
AB1157/JC2921
AB1157/JC2926
AB1157/JC5517
E. coli WP2 Mitchell et al.,
uvrA+recA+,uvrA- Not given - 1980
recA-
trp-/trp+ Not given -
A2Cs/A2Cr 3-48 µg/ml +
E. coli B/r 100 µg/ml - Masek, 1977
E. coli K12 > 30 µg/ml - Mamber et al.,
(SOS chromotest) 1986
Preferential E. coli K12 5-20 µg/ - Hyman et al.,
inhibition plate 1980
E. coli 30 µg/plate - Brem et al.,
Pol A+, Pol A- 1974
E. coli 10 µg/plate - McCoy et al.,
Pol A+, Pol A1- 1980a,b
E. coli 30 µg/plate - Longnecker et al.,
Pol A+, Pol A- 1974
E. coli 30 µg/disc - Slater et al.,
Pol A+, Pol A- 1971
Gene conversion S. cerevisiae Not given - Mitchell et al.,
D4 1980
Sister chromatid Human lymphocytes 200 µg - Pant et al., 1976
exchange (in vitro)
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Chromosomal Zea mays 30 µg/ml - Verma & Lin, 1978
aberrations
Human lymphocytes 10-40 µg/ml + Mitus & Coleman,
(in vitro) 1970
Human lymphocytes Not given + Goh, 1979
(in vitro)
Human lymphocytes 200 µg + Pant et al., 1976
Mouse, bone 50 mg/kg b.w. + Manna & Bardham,
marrow 3×50 mg/kg + 1977
b.w., 8h
intervals
Mouse, F1 liverb 50 mg/kg b.w. + Manna & Roy, 1979
Micronucleus Tradescantia 0.1 - 5mM - Ma et al., 1984
test paludosa
Mouse Not given (5 - Heddle & Bruce,
(CH3×C57)F1 daily doses) 1977
DNA binding E. coli 100-1000 µM - Kubinski et al.,
assay 1981
Enhancement of Syrian hamster 0.7-5mM + Hatch et al.,
SA7 virus cell embryo cells/ 1986
transformation simian adenovirus
SA7
Aneuploidy Hordeum vulgare 300 µg/ml + Yoshida et al.,
induction 1972
Colicine S. typhimurium 0.1-60 µg/ - Ben-Gurion, 1978
induction TA1537 REN plate
Table 2. Results of mutagenicity assays with chloramphenicol (cont'd)
Test System Test Object Concentration Results Reference
Increased Snail Not given - Xie, 1985
embryonal length
Induction of S. typhimurium 0.155-3.1 µM - Pall & Hunter,
tandem genetic TR4179, TT1984 1985
duplication
a Very weak positive response.
b One male mouse was given 50 mg/kg chloramphenicol i.m. and then was mated
with 4 untreated females. Mice (3) were sacrificed on days 12, 16, and 18
of gestation. The remaining mouse was allowed to litter normally and the
young were sacrificed when 7 days old. Livers of fetuses and neonates were
removed and examined.
When groups of 3 - 9 female Sprague-Dawley rats were given
80 mg/kg b.w. chloramphenicol in the drinking water for 10 days with
or without exposure to short duration high intensity noise,
ototoxicity was noted as revealed by reductions in the electrical
output of the cochlea. Noise exposure alone also reduced the output,
but noise and chloramphenicol together resulted in a severe effect
(Henley et al., 1984). Similar effects were reported in abstract
form in another study in rats (Henley, 1985).
Chloramphenicol was given to guinea pigs (number unspecified) as
a single i.v. dose of 400 mg/kg b.w. The threshold for the Preyer
reflex was measured after administration and at 10, 20, and 30
minutes, 1, 2, 3, 4, and 5 hours, and then daily for 7 days. There was
no change in the Preyer reflex with noise of 1 and 8 KHz and no loss
of hair cells, indicating no ototoxic response in this study
(Beaugard et al., 1979; Beaugard et al., 1981).
Special study on effects on sleep
Oral doses of 160 250 mg/kg b.w. chloramphenicol suppressed
paradoxical sleep in cats. After a dose of 330 mg/kg b.w. paradoxical
sleep was suppressed for 24 hours, at which time there was also a
depression of slow wave sleep (Petitjean et al., 1975).
Special study on spermatogenesis
A group of male rats was treated daily with 100 mg/kg b.w.
chloramphenicol succinate for 8 days (route and animal numbers not
stated), after which they were sacrificed and the testes examined
histologically. Examination revealed total or incomplete inhibition of
spermatogonial divisions with "perturbed meiosis". No other details
were provided (Timmermans, 1974).
Special studies on teratogenicity
Monkeys
Chloramphenicol at doses of up to 200 mg per monkey
(10 mg/kg b.w.) had no effect on the development of Macaca mulatta
when given for 6 to 17 days at various intervals between the 65th and
95th days of gestation (Courtney et al., 1967; Courtney & Valerio,
1974).
Rabbits
Groups of 5 - 8 pregnant mixed breed rabbits were given 500,
1000, or 2000 mg/kg b.w./day chloramphenicol by garage on days 6 - 15,
6 - 9, or 8 -11 of gestation, respectively. Historical control data
collected over the previous 4 year period, using 192 rabbits, were
used for comparison. Excess fetal deaths did not occur in rabbits
given 500 mg/kg b.w./day chloramphenicol, but in the mid- and
high-dose groups 25% and 58% fetal deaths were noted, respectively,
compared with 10% in the historical controls. There were no excess
incidences of fetal malformations, but delayed ossification was noted
in fetuses from dams given chloramphenicol (Fritz & Hess, 1971).
Chicks
Chick eggs, less than 3 days old, were treated with 0.1 ml of
chloramphenicol solution in distilled water. The chloramphenicol, at
doses of 0.5 or 1.0 mg/egg, was injected into the albumen via the
airsac. The major anomaly observed was vesiculation of the heart and
trunk resulting from the inhibition of differentiation of the
splanchnopleure; this effect was most severe following 16 - 19 hours
of incubation (36 - 57% in the 0.5 mg/egg group and 23 - 47% in the
1.0 mg/egg group). Unfortunately, no control data were provided
(Blackwell, 1962).
Chloramphenicol also had adverse effects on development in a
separate study in which fertilized eggs with embryos at the 14- or
20-somite stages were explanted and exposed to chloramphenicol at
concentrations of 0, 200, or 300 µg/ml for 22 - 24 hours. The major
defects noted were those of the neural tube (failure to close) and
forebrain. There was also evidence of an inhibition of haemoglobin
formation (Billet et al., 1965).
Rats
Pregnant Sprague-Dawley rats (5 - 15 per group) were given gavage
doses of 500, 1000, 1500, or 2000 mg/kg b.w./day chloramphenicol over
various stages of gestation. In addition, single gavage doses of
2000 mg/kg b.w. were given to pregnant rats on days 5, 6, 7, 8, 9, or
10 of gestation. A group of 553 historical control rats was used for
comparison. Even the lowest daily dose of chloramphenicol on days 5 -
15 of gestation resulted in significant embryo/fetal deaths (63%) when
compared with historical controls (23%), whereas doses of 2000 mg/kg
b.w./day on days 15 - 17 or 2000 mg/kg b.w. on days 5, 6, or 7 had no
effect. Single doses of 2000 mg/kg b.w. on days 8, 9, or 10 of
gestation resulted in 45% fetolethality, but the most sensitive period
appeared to be days 9 - 15. For example, fetal deaths occurred 100% of
the time when 2000 mg/kg b.w./day was given over days 9 - 11 of
gestation. The highest incidences of anomalies, umbilical hernia, were
noted at 2000 mg/kg b.w./day when given over days 6 - 8 of gestation
(36%) and at 2000 mg/kg b.w. on day 8 (11%). High incidences of
delayed ossification were seen in fetuses from dams given 1000 mg/kg
b.w./day chloramphenicol on days 7 - 12 or 2000 mg/kg b.w./day on days
11 - 13 (Fritz & Hess, 1971).
The effects of chloramphenicol on pre-implantation embryos in the
rat were investigated by treating groups of 7 pregnant Sprague-Dawley
rats with 250 mg/kg b.w. chloramphenicol i.p. on either day 3 or 5 of
gestation. The animals were killed on day 5 of pregnancy and the
uterine contents examined. No effects on the number of blastocysts per
female were noted, but administration on day 3 of pregnancy
significantly reduced the number of cells per blastocyst. A reduction
was also seen when the compound was given on day 14, but these results
were not statistically significant (Giavini et al., 1979).
Pregnant Sprague-Dawley rats (number unspecified) were given 3%
dietary chloramphenicol, equivalent to 1500 mg/kg b.w./day, from days
0 to 20. The number of resorptions (% of total implants) was elevated
in rats treated with chloramphenicol (31.4 - 57.0%) compared with
controls (4.7%), while fetal weight in treated rats was only 50% of
controls. Placental weight was also much reduced, while the number of
live fetuses was greatly reduced by chloramphenicol. The authors then
examined the effects of chloramphenicol when given on specific days of
pregnancy (0 - 2, 0 - 3, 0 - 4, etc., up to 0 - 12). The major effects
on implantations, resorptions, number of live fetuses, and fetal
weights as described above were seen in the 0 - 8 to the 0 - 12
feeding schedules, suggesting an effect on implantation (or effects on
embryos soon after implantation). A large proportion of fetuses (71%)
had edema and there was an increased incidence of wavy ribs (7%) and
fused ribs (7%) in chloramphenicol-treated groups compared with
controls (zero incidences in all cases in controls) (Hackler et al.,
1975).
Chloramphenicol was investigated for its effects on avoidance
learning in rats. Four groups of 15 pregnant Wistar rats each were
treated as follows: in one group 50 mg/kg b.w. chloramphenicol
succinate was given s.c. on days 7 - 21 of gestation. In two other
groups, 50 or 100 mg/kg b.w. was given s.c. to pups for the first 3
days after birth; the fourth group served as controls. No effects on
pregnancy, litter size, fetal weight, post-natal weight gain, or
incidence of gross malformations were seen. When 60 days old, animals
were selected for conditioned-learning study and were then examined
for avoidance learning at days 5, 10, 15, and 20 from the start of the
conditioning procedure. Pups from mothers given chloramphenicol
succinate and those given the substance as neonates showed a marked
and statistically significant impairment of avoidance learning at all
four times. The effects were generally worse in pups given the
substance post-natally than in those from dams administered it during
gestation, but the differences were only slight (Bertolini & Poggioli,
1981).
Mice
Groups of 8 pregnant albino mice were given oral chloramphenicol
at doses of 25, 50, 100, or 200 mg/kg b.w. in 10 ml of distilled water
over the third trimester of pregnancy for 5 - 7 days. Animals were
allowed to give birth and the young were tested for conditioned
avoidance response, electroshock seizure threshold, and performance in
open-field tests at days 30, 38, and 42. No gross abnormalities were
seen in any of the progeny. Dose-related effects were seen in all
three elements of the test, with progeny from chloramphenicol-treated
dams showing a reduced learning ability, higher brain seizure
threshold, and poorer performance in the open-field test (Al Hachin &
Al-Baker, 1974).
Groups of 7 - 19 pregnant CD1 mice were given by garage 500,
1000, or 2000 mg/kg b.w./day chloramphenicol on days 5 - 15, 6 - 12,
or 8 - 10 of gestation, respectively. Historical control data,
collected over the previous four-year period using 307 mice, were used
for comparison. In the 1000 and 2000 mg/kg b.w./day dose groups, 71
and 100% embryo/fetal deaths occurred, respectively, compared with 24%
in controls and 31% in mice given 500 mg/kg b.w./day chloramphenicol.
The only defects observed were a low incidence of fused sternebrae and
elevated incidences of delayed ossification in fetuses from dams given
1000 mg/kg b.w./day chloramphenicol (Fritz & Hess, 1971).
In vitro
Chloramphenicol and several other chemicals were tested in a
mouse embryo limb bud cell culture test system which itself was being
validated as part of the study. In all, 22 known mouse teratogens and
5 non-teratogens were investigated in the system, which makes use of
high-density cultures of mouse embryo limb bud cells that can
differentiate and synthesize an extracellular matrix of sulfated
proteoglycans. The end-point of the test considers incorporation of
radiolabel (3H-thymidine) and the growth and synthesis of ocular
protein and cartilage proteoglycan. Chloramphenicol gave a positive
result in this test, with the maximum active concentration being
5 µg/ml. [The test was around 89% predictive; no false positives were
seen and the false negative rate was about 15% (Guntakatta et al.,
1984).]
Another in vitro test made use of the differentiation
characteristics of rat embryo midbrain and limb bud cells, and the
inhibition of differentiation by teratogens. Chloramphenicol gave a
weak response, resulting in inhibition at concentrations in excess of
50 µg/l, compared with 10 µg/l or less seen with several known
teratogens, e.g., captan, colchicine, and parbendazole (Flint & Orton,
1984).
Acute toxicity
Four groups of pregnant and non-pregnant mice were given i.v.
doses (unspecified) of chloramphenicol. No signs of toxicity were
reported. The LD50 value for non-pregnant mice was calculated as
1530 (1260 - 1840) mg/kg b.w., while that for pregnant mice was
1210 mg/kg b.w. (no confidence limits cited) (Beliles, 1972).
Short-term studies
No information available.
Long-term studies
See "Special studies on carcinogenicity".
Observations in human beings
Chloramphenicol is known to produce two major adverse effects in
human beings. One of these is a generally irreversible and often fatal
aplastic anaemia and the other is a reversible bone marrow
suppression.
Aplastic anaemia
Aplastic anaemia is the most dangerous effect produced by
chloramphenicol, although its occurrence is rare. It is usually fatal
(Benestad, 1979). Numerous publications have appeared, most of them
case reports describing the development of aplastic anaemia.
An investigation into the incidence of chloramphenicol-induced
aplastic anaemia in Hamburg suggested that the incidence was 1/11500
with a death rate 1/18500. In the period 1965 - 70, 29 cases were
reported, while in 1971, 3 cases were reported. Total doses in 18
cases were in the range of 10 to 100 g, with most individuals having
received 11 - 30 g. Onset was from 14 days (rare) to 4 - 6 months
after chloramphenicol therapy (Hausmann & Skrandies, 1974).
In a study in Israel in 1985, aplastic anaemia incidence was
7.1/106 in males and 8.7/106 in females. Chloramphenicol was
thought to account for up to 25% of cases. Aplastic anaemia usually
took up to 12 months to develop after chloramphenicol treatment
(Modan et al., 1975).
In 1969 in California, aplastic anaemia in chloramphenicol-
treated patients was said to be 13 times more frequent than in
the general population. Most individuals had been given oral
doses, but in some, aplastic anaemia had occurred after i.m.
administration. Doses were often on the order of 250 mg thrice daily
to a total of 3 g or 250 mg four times daily to a total of 5 g. The
majority of patients were in the 50 - 80 age group, but it was
observed in a 15-year-old boy given a total of 3 g chloramphenicol and
in a 37-year-old female given a total of 6 g over a month. In both
cases onset occurred 3 - 4 months after treatment ended
(Wallerstein et al., 1969).
A series of reports from Sweden in the 1970s suggested that the
incidence of aplastic anaemia was around 80 in 1.2 million. Only 4 or
5 of these were thought to be due to chloramphenicol treatment,
placing the risk at 1 in 20000 (Bottiger & Westerholm, 1972;
Bottiger & Westerholm, 1973; Bottiger, 1978; Bottiger, 1979).
Of 108 cases of aplastic anaemia reported in Istanbul, 4 were
thought to be due to chloramphenicol (Aksoy et al., 1984).
A total of 380 cases of aplastic anaemia were seen at a hospital
in Paris in the years 1971 - 1983; 194 adult cases were considered to
be due to chemical agents. In the period 1971 - 1977, 18/104 cases
were attributed to chloramphenicol, whereas chloramphenicol accounted
for 2/36 and 2/52 cases of aplastic anaemia in the periods 1977 - 1980
and 1980 - 1983, respectively, suggesting a decreasing incidence, at
least in the area of France under investigation (Najean & Baumelon,
1984).
In the period 1975 - 1980, 9 children with aplastic anaemia were
seen at the Medical School, Gadjah Mada University (Yogyakarta). Two
were idiopathic, but at least 3 could be attributed to treatment with
chloramphenicol (Widayat et al., 1983).
A study of 40 cases of aplastic anaemia by members of the
Association of Clinical Pathologists in the USA revealed that 27 were
probably due to chloramphenicol. Of these, 18 had exceeded 10 g or
more (up to 250 g) in total dosage, while 8 had received 10 g or less.
One, an infant, had received less than 2 g chloramphenicol. Onset was
usually 1 - 3 months after drug cessation. Route of administration and
its duration were not specified (Sharp, 1963).
In 1954, of 539 cases of aplastic anaemia from 37 states in the
USA, 55 were attributable to chloramphenicol. In general there was a
female preponderance and usually the effect developed 1 - 6 months
after the drug was withdrawn (Welch et al., 1954).
In a study in Iraq, however, a male preponderance of 3:1 over
females was noted. Of 60 patients with aplastic anaemia at the
University of Baghdad Teaching Hospital, 12 were associated with
chloramphenicol treatment (Al-Moudhiry, 1978).
One study in the Netherlands examined cases of blood dyscrasias
taken from the literature along with unpublished cases from adverse
drug reaction reporting systems in the United Kingdom, Denmark,
Sweden, and the Netherlands. To these were added cases from hospitals
in Northeast Switzerland. A total of 641 cases of chloramphenicol-
induced blood dyscrasias were identified. These included:
thrombocytopenia (21), agranulocytosis (51), hypoplastic anaemia (39),
bone marrow suppression (39), acute leukaemia (27), and aplastic
anaemia (464). Of the 464 cases of aplastic anaemia, 335 (72%) had
proved fatal. There appeared to be a female preponderance (261 cases;
56%). However, because this cannot be related to the total number
treated and therefore to those who did not acquire the condition, the
incidence cannot be determined (Meyler et al., 1974). The results
confirmed those of earlier work (Polak et al., 1972).
In Italy, it has been claimed that in view of the ease of
availability of chloramphenicol, the incidence of aplastic anaemia
appears to be low. For example, in 1971 there were 10 reports of fatal
side effects due to antibiotics; in 1972 there were 3. None were
attributable to chloramphenicol. During the period 1973 - 1975 there
were no reports of fatal cases (Preziosi et al., 1981).
For the period 1959 - 1969, 172 cases of aplastic anaemia were
reported in 15 hospitals in Northeast Switzerland, and 44 of these
individuals had been treated with chloramphenicol. The smallest total
dose incriminated was 3 g, while the highest was 315 g
(Keiser & Bucheggar, 1973).
In children aged 0 - 14 years in Denmark, the total number of
registered cases of aplastic anaemia was 39 for the years 1967 - 1982,
giving an annual incidence of 22/106. Probable causes were
identified for 21 of the 39 cases, and 2 of these were attributed to
chloramphenicol treatment (Clausen, 1986).
It was stated in 1983 that the probable overall incidence of
chloramphenicol-induced aplastic anaemia is somewhere between 1 in
20000 and 1 in 40000 (Venning, 1983).
In Japan, chloramphenicol appeared to be more dangerous to the
elderly population than to other groups. However, the investigation
was probably biased in that only fatal cases were examined
(Mizuno et al., 1982). Similar findings were made in an earlier
study (Shimizu et al., 1979).
For the years 1961 - 1965, 35 cases of aplastic anaemia were
reported in Colombia, and of these 10 had had past exposure to
chloramphenicol. Several others were said to have had a "strong
suspicion" of exposure to the drug. Mortality was 60% (Sarasti, 1970).
An age preponderance was noted in an analysis of 21 patients with
aplastic anaemia, 8 of whom had been treated with chloramphenicol
(Perez et al., 1981).
The minimum dose of chloramphenicol associated with the
development of aplastic anaemia is not known with certainty. The
literature citations often state only the total dose. Where cases
developed after several doses, it is not possible to say if aplastic
anaemia would also have occurred had only a single dose been given. Of
15 cases reviewed in a report in 1974, total doses of 4.5 to 80 g had
been given, with the usual levels being 8 to 14 g. As an example, a
46-year-old woman was given 15 g of chloramphenicol over 10 days.
Aplastic anaemia developed after 2 months (Hellriegel & Gross, 1974).
Total doses of 6.5 to 60 g chloramphenicol had been given to 7
individuals with aplastic anaemia reported in 1971 (Hodgkinson, 1971).
A 27-year-old woman given 30 g chloramphenicol i.v. over 12 days
developed aplastic anaemia 3 months later (Alavi, 1983).
One individual with aplastic anaemia had undergone previous
treatment 19 years earlier with a 500 mg initial dose followed by
250 mg chloramphenicol 4 times daily. At the age of 23 years, he was
given 750 mg chloramphenicol succinate (62 mg/kg b.w./day) i.v. every
6 hours for 12 days for a brain abscess. Bone marrow suppression
ensued and by the twelfth day of drug administration aplastic anaemia
had developed. The patient eventually died (Daum et al., 1979).
A 26-year-old woman was diagnosed as being anaemic during the
fifth month of pregnancy and in the sixth month she developed a skin
infection. She was given 8 g chloramphenicol. She developed aplastic
anaemia and died 8 days after giving birth. Bone marrow aspiration
confirmed aplastic anaemia. (Suda et al., 1978).
Several cases of aplastic anaemia have been reported in children.
One 7-year-old boy had been given chloramphenicol palmitate in June
and August of 1959. The dose or route of administration were not
specified. He developed aplastic anaemia 4 - 5 months later and died
(Leiken et al., 1961). A 6-year-old girl was given 25 mg/kg b.w./day
chloramphenicol for 10 days by an unspecified route. She developed
aplastic anaemia, apparently immediately after treatment and then
acute myeloblastic leukaemia (Awaad et al., 1975). A 4-year-old girl
was given oral chloramphenicol (dose not stated) for 1 week. After 2
months she developed aplastic anaemia (Young et al., 1979). Another
case of a 4-year-old girl given chloramphenicol was reported more
recently. Chloramphenicol was given i.v. for 1 week followed by oral
therapy for 8 weeks. The initial dose was 75 mg/kg b.w./day given
every 6 hours, but this was adjusted after 3 weeks to 37 mg/kg
b.w./day. Some months later she developed aplastic anaemia
(Lepow, 1986). In one recent case the patient was a neonate born at 30
weeks gestation weighing 1.7 kg. At day 20 after birth she developed
suspected meningitis and was given 4 doses of 50 mg/kg b.w./day
chloramphenicol. She developed aplastic anaemia; epidermal necrolysis
of the skin and biliary cholestasis were found at necropsy
(White et al., 1986).
A 72-year-old male patient was given a 3-week course of
chloramphenicol (dose and route unspecified) and aplastic anaemia
ensued within 4 months (Howell et al., 1975).
Aplastic anaemia is usually associated with oral intake
(Matthews et al., 1980). It has been reported that in 149 cases of
chloramphenicol-induced aplastic anaemia, 85% followed oral dosing,
14% followed parental administration, and 3% occurred after rectal
administration (Plaut & Best, 1982).
Topical administration of chloramphenicol has been followed by
aplastic anaemia. In one case, a 73-year-old woman died of aplastic
anaemia less than 2 months after beginning ophthalmic chloramphenicol
treatment with a 0.5% solution (3 - 4 times daily) and a 1% ointment
(once per day to the right eye) (Fraunfelder & Bagby, 1982). Several
other similar cases have been reported (Abrams et al., 1980;
Rosenthal & Blackman, 1965; Carpenter, 1975; Issaragrisil &
Piankijagum, 1985; Korting & Kifle, 1985). In one case the total dose
was estimated to be 32 mg of chloramphenicol (Carpenter, 1975).
One occupational case probably involved inhalation and skin
contact. It occurred in a shepherd applying a chloramphenicol spray to
the feet of sheep for treatment of foot rot. He had treated the
animals twice a week with the spray, which contained 10 g of the drug
in 100 ml of solution, for two years. Each dose contained 10 mg
(Del Giacco et al., 1981).
A link between the administration of chloramphenicol and the
development of liver disease and aplastic anaemia is known to exist.
After five patients aged 4 to 63 years were given chloramphenicol,
liver disease (as evidenced by jaundice, icterus, and elevated serum
enzymes and bilirubin) was subsequently noted. Aplastic anaemia
eventually developed (Hodgkinson, 1973). A similar case has been
reported in a 15-year-old boy given 250 mg chloramphenicol i.v. every
6 hours. Liver enzymes became elevated after 18 days and the liver
itself was tender. Aplastic anaemia developed (Caslae et al., 1982).
The mechanism of chloramphenicol-induced aplastic anaemia is not
understood. There may be a genetic element involved, as the effect has
been seen in families and in identical twins exposed to chloramphenicol
(Flach, 1982; Nagao & Maner, 1969; Silver & Zuckerman, 1980; Yunis,
1978c). The main target may be the haemopoietic pluripotential stem
cells of the marrow (Vincent, 1986; Silver & Zuckerman, 1980;
Benestad, 1974; Appelbaum & Fefer, 1981). There may also be the
possibility of initial damage to the marrow micro-environment (Camitta
et al., 1982). Failure to note this effect in human beings with
drug-induced aplastic anaemia makes the latter unlikely (Samson
et al., 1972; Vincent, 1986). In vitro studies with human bone
marrow suggest that both the erythroid and granulocytic series are
sensitive to chloramphenicol (Nara et al., 1982; Hara et al.,
1978; Yunis, 1977). The erythroid series in particular seemed sensitive
to chloramphenicol, with inhibition occurring at 10 mg/l compared with
50 mg/l for the granulocytic series (Yunis, 1977).
The problem as to whether bone marrow cells are more sensitive to
chloramphenicol in patients with aplastic anaemia induced by the drug
is not clear, as reports on the subject are conflicting (Yunis et al.,
1973a; Howell et al., 1975; Kern et al., 1975). It has been claimed,
based on the results of animal studies, that individuals who are
sensitive to chloramphenicol-induced aplastic anaemia may have
residual bone marrow damage brought about by exposure to other agents
(Morley et al., 1976).
The metabolite or metabolites responsible for the induction of
aplastic anaemia in human beings is unknown, but nitroso-
chloramphenicol has been implicated (Murray & Yunis, 1981; Nagai &
Kanamuru, 1978). Nitrosochloramphenicol can be formed by the
reduction of chloramphenicol in human liver in vitro (Salem et al.,
1981). This substance is known to be toxic to human bone marrow cells
in vitro and, moreover, is more toxic than chloramphenicol itself
(Yunis et al., 1980a, b). However, nitrosochloramphenicol is not
myelotoxic to mice in vivo (Krishna et al., 1981). It does,
however, cause DNA strand breakage in vitro, and inhibit DNA
synthesis (Gross et al., 1982; Skolimowski et al., 1983). Both
chloramphenicol and nitrosochloramphenicol are taken up rapidly by
cells, at least as demonstrated by a human transformed lymphoblastoid
cell line (Raji cells), but the nitroso-compound covalently binds to
these cells and to bone marrow cells 15 times more tightly than does
chloramphenicol (Hurray & Yunis, 1981). Photochemical decomposition of
chloramphenicol may result in potentially myeloblastic derivatives,
which may be hazardous in ophthalmic solutions (de Vries et al.,
1984). Another possible mechanism involves the immune system (and even
autoimmune damage), but there are no convincing data to support this
hypothesis. Chloramphenicol in vitro inhibited lymphocyte
transformation in human material (Burgio et al., 1974) and
chloramphenicol reduction products, including nitrosochloramphenicol,
were suppressive to antigen-reactive cells in mice (Pazdernik &
Corbett, 1980).
In summary, chloramphenicol induces aplastic anaemia in
susceptible individuals, but no dose-response relationship has been
identified. The mechanism may involve nitrosochloramphenicol, but this
has not been proven. The nature of the mechanism is unknown.
Bone marrow suppression
Reversible bone marrow suppression has been reported in patients
given chloramphenicol by a number of routes. The effect is thought to
be an unlikely event at plasma levels under 20 mg/l, and it is said to
occur in most patients at levels in excess of 25 mg/l. Generally, it
occurs within days of administration (Lery et al., 1978; Benestad,
1979). One study showed that oral doses likely to result in
suppression were usually of the order of 2 - 3 g/day or 30 - 50 mg/kg
b.w./day; durations of dosing were generally 1 - 17 days, most being 5
- 10 days. Plasma levels of chloramphenicol at 2 - 3 hours and 6 - 8
hours varied, most being in excess of 25 mg/l at 2 - 3 hours and in
excess of 30 mg/l at 6 - 8 hours. However, some were below 15 mg/l at
both times. Of the 17 patients studied, 11 had liver disease, while 3
of the remainder had renal disease. Bone marrow suppression in a
female of 21 years of age was attributed to the administration of
1.5 g/day of chloramphenicol for 18 days. The condition resolved on
cessation of therapy (Parashar et al., 1972). In 6 anaemic subjects
given up to 60 mg/kg b.w./day chloramphenicol, reticulocyte response
to vitamin B12 or iron dextran was halted and delayed (Saidi
et al., 1961).
Maturation arrest and cytoplasmic valuation of erythroid elements
were seen in the marrow (Rosenbach et al., 1960; Bartlett, 1982).
One reported case of bone marrow suppression was in a
three-year-old girl with cystic fibrosis given 70 mg/kg b.w./day
chloramphenicol for 2.5 months. The suppression was accompanied by
physical growth depression and hair loss. All these conditions
resolved on cessation of treatment (Kapp et al., 1977).
The toxic effects of chloramphenicol on the bone marrow were
lessened in one group of patients by co-administration of
phenylalanine (Ingall et al., 1965). The reason for this, and its
relationship to the pathogenesis of bone marrow suppression, is
unknown. The mechanism of suppression is thought to involve inhibition
of mitrochondrial protein biosynthesis in bone marrow cells. In vitro
studies showed that 10 mg/l chloramphenicol severely inhibited protein
synthesis in bone marrow cells, although ten times this concentration
was required to inhibit mitochondrial respiratory functions (Yunis,
1973a, b; Yunis, 1978a, b; Martelo et al., 1969). As a result of
mitochondrial dysfunction, ferrochelatase activity is suppressed in
erythroid precursors. In vitro studies show inhibition of haem
synthesis with concentrations of chloramphenicol of 10 mg/l
(Becker et al., 1974). It seems likely that the major effect of
chloramphenicol in the pathogenesis of reversible bone marrow
suppression is a block on hame biosynthesis arising as a result of
mitochondrial dysfunction (Yunis & Salem, 1980). Consequently,
co-administration of phenylalaninine may in some way compensate for
the inhibitory effects of chloramphenicol on protein synthesis.
Effects on platelets
In in vitro experiments, chloramphenicol was shown to decrease
human platelet aggregation. Unfortunately, no in vivo studies are
available (Cronber et al., 1984, Djaldetti, 1983; Agam et al.,
1976).
Carcinogenicity
IARC concluded in 1982 and 1987 that there was limited evidence
for the carcinogenicity of chloramphenicol to human beings based on
studies describing cases of leukaemia (IARC, 1982; IARC, 1987).
One study described a 24-year-old male given chloramphenicol for
typhoid fever. Leukaemia followed aplastic anaemia. A chromosome
translocation (t 1:7) was discovered on karyotyping. No other details
were provided, except that similar translocations were noted in 6
other leukaemia patients known to have had exposure to leukaemogenic
substances or radiation (Scheres et al., 1985).
Acute myeloid leukaemia developed in a 6-year-old girl who
developed aplastic anaemia after being given 25 mg/kg b.w./day
chloramphenicol for 10 days. The leukaemia developed 6 months after
the aplastic anaemia (Awaad et al., 1975).
Leukaemia developed in a 38-year-old woman given a total of 8 g
of chloramphenicol 8 years before which resulted in aplastic anaemia.
A 57-year-old man who had taken chloramphenicol for 8 years (about
175 g total dose) developed aplastic anaemia followed by leukaemia
within a year, while a 61-year-old man who was treated with
chloramphenicol developed aplastic anaemia and leukaemia (Brauer &
Dameshek, 1967).
An 80-year-old man with a bacterial infection of the feet was
given orally 250 mg/day chloramphenicol, for 7 days. He developed
acute leukaemia 5 months later. The only other drugs given for his
infection were penicillin and a topical zinc ointment (Humphries,
1968).
A 14-year-old girl given 250 mg chloramphenicol for 4.5 days
developed aplastic anaemia after approximately 1 year followed by
acute leukaemia 18 months later (Seaman, 1969).
Aplastic anaemia and leukaemia may occur together at diagnosis. A
28-year-old man developed reversible bone marrow depression after
being given approximately 31 g of chloramphenicol. Five years later he
was found to have aplastic anaemia and acute leukaemia (Schmitt-Graft,
1981). Other similar cases have been reported (Adamson & Seiber, 1981;
Forni & Vigliani, 1974; Meyer & Boxer, 1973).
Of 641 cases of chloramphenicol-induced blood dyscrasias, 464
cases of aplastic anaemia and 27 cases of leukaemia were identified
(Meyler et al., 1974).
From 151 cases of blood dyscrasias induced by drugs, 3 cases of
leukaemia attributable to chloramphenicol were noted (Fraumeni, 1967).
The majority of cases of leukaemia associated with chlo-
ramphenicol therapy were acute myeloid leukaemia (Godner et al.,
1973).
The role of chloramphenicol in leukaemogenesis is not known. Some
studies have revealed abnormal karyotypes in affected patients
(Scheres et al., 1985; Goh, 1971; Cohen & Huang, 1973), but it is
not known if these abnormalities were induced directly by
chloramphenicol or were merely a feature of the disease. Aplastic
anaemia, whether induced by chemicals or idiopathic, is known to be
followed by leukaemia in some cases (Milner & Geary, 1979).
Cytogenetic abnormalities are common. Fanconi's anaemia, for example,
is often followed by leukaemia and cytogenetic abnormalities are seen
in this state. Chloramphenicol is known to induce cytogenetic
abnormalities in in vitro test systems, but it is not known if those
seen in leukaemia occurring after chloramphenicol-induced aplastic
anaemia are due to the same effects.
Cardiovascular effects
Chloramphenicol is known to induce a state often referred to as
the "Grey Baby Syndrome" or "Grey Syndrome". In general, this is a
state of cardiovascular collapse that commences on days 2 to 9
following the beginning of chloramphenicol administration. The main
features include failure to feed, vomiting, abdominal distension,
cyanosis, flaccidity, shock, and a fall in body temperature. Death is
said to occur in 60% of cases, and the syndrome generally occurs where
the dose of chloramphenicol exceeds 25 mg/kg b.w./day (Meyler &
Herkeimer, 1968). Metabolic acidosis may be a presenting feature, and
plasma levels of chloramphenicol in excess of 30 µg/ml are usually
associated with the development of the syndrome (Evans & Kleiman,
1986; Mulhall et al., 1983; Craft et al., 1974). In several cases
the doses producing the syndrome were 50 mg/kg b.w./day (Fripp
et al., 1983; Kraskinski et al., 1982; Biancaniello et al.,
1981; Haile, 1977) and in one case a daily dose of 100 mg/kg b.w. was
given (Stevens et al., 1981). A grey-type syndrome has been reported
in a 16-year-old girl given 1 g chloramphenicol i.v. every 6 hours
(95 mg/kg b.w./day) for what was thought to be Rocky Mountain spotted
fever. This dose was later changed to 3.75 g/day i.v. The syndrome
began to develop after the second day of this regime, but it resolved
with drug withdrawal and supportive treatment (Brown, 1982). The
mechanism is unknown, but experiments with isolated pig hearts suggest
that effects on mitochondria may be important (Werner et al., 1985).
Allergenic contact dermatitis
Allergenic contact dermititis has been reported in case report
following the use of chloramphenicol topical preparation (Rudzki et al.,
1976; Schewach-Millet & Shapiro, 1985; Van Joost et al.,1986;
Strick, 1983; Fraki et al., 1985).
Two cases of occupational dermatitis in oculists were attributed
to the use of chloramphenicol-containing preparations (Rebandel &
Rudzki, 1986). In one study of 330 patients with contact allergy, 10%
showed positive patch tests to chloramphenicol, while in another
investigation of 620 patients, 1.7% save positive reactions
(Blondeel et al., 1978; Rudzki & Kelniewska, 1970). Others have
reported similar findings (Forck, 1971).
Ocular toxicity
Several cases of ocular toxicity following prolonged treatment
with chloramphenicol have been reported. This has often been described
as an optic neuritis with scotomata and failing vision. Retrobulbar
neuritis may be observed. It has been seen in cases of cystic
fibrosis, although this may reflect the choice of chloramphenicol as a
treatment in this condition rather than a specific sensitivity in this
group. Total doses are often in the 80 - 250 g range given over
several months. One patient developed bilateral optic neuritis after
being given 6 g chloramphenicol per day for 6 weeks (approximately
250 g total). Peripheral neuritis may accompany the ocular effects
(Wilson, 1962; Steidl, 1965; Malbrel et al., 1977; Joy et al.,
1960; Lasky et al., 1953; Charache et al., 1977; Chang et al.,
1966; Cocke et al., 1966; Godel et al., 1980; Wallenstein &
Snyder, 1952; Huang et al., 1966; Walker, 1961; Fraunfelder & Meyer,
1984).
Ototoxicity
Deafness following chloramphenicol therapy has occasionally been
noted, although the reports have been complicated by administration of
other potentially ototoxic drugs. In one case, a 2.5-year-old boy was
given 125 mg/kg b.w./day chloramphenicol for 26 days with no other
drug treatment. He developed deafness, which persisted beyond
cessation of drug administration (Gargye & Dutta, 1959; Ajodhia & Dix,
1976; Nilges & Norther, 1971; Jones & Hanson, 1977).
Effects on testes
A 61-year-old man with pneumonia was treated with ampicillin and
chloramphenicol. He later developed aplastic anaemia after 12 days and
died of widespread sepsis 10 days after drug withdrawal. Necropsy
revealed loss of germinal epithelium, with only Sertoli cells
remaining. The testes were of normal size. As the patient was
unmarried and also exposed to ampicillin, the authors concluded that
there was no direct proof of chloramphenicol-induced testicular
toxicity. However, in view of the normal gross appearance of the
testes and the low toxicity of ampicillin, chloramphenicol seems the
most likely cause of the effects noted in this case. No dose levels
were quoted (Sheehan & Sweeny, 1982).
Teratogenicity
Chloramphenicol and/or tetracyclines have been implicated in the
genesis of cleft-lip in a study of 599 affected children. However,
because of the nature of the epidemiological study, any effects of
tetracyclines cannot be separated from those of chloramphenicol and it
cannot be stated with any certainty that chloramphenicol is a human
teratogen (Saxen, 1975).
COMMENTS
Human exposure to chloramphenicol can give rise to aplastic
anaemia, a rare but often fatal condition. The Committee concluded
that no dose-response relationship could be established for this
effect. The mechanism for the pathogenesis of aplastic anaemia is
unknown, and no suitable animal model exists.
EVALUATION
Because a no-effect level for aplastic anaemia could not be
established, and therefore it was not possible to give an assurance
that residues in foods of animal origin would be safe for sensitive
subjects, an ADI could not be allocated for chloramphenicol.
REFERENCES
Abou-Khalil, S., Salem, Z., & Yunis, A.A. (1980). Mitochondrial
metabolism in normal, myeloid, and erythroid hyperplastic rabbit
bone-marrow: Effect of chloramphenicol. Am. J. Haematol., 8, 71-79.
Abou-Khalil, S., Salem, Z., Abou-Khalil, W.H., & Yunis, A.A. (1981).
On the mechanism of erythroid cell sensitivity to chloramphenicol:
Studies on mitochondria isolated from erythroid and myeloid tumours.
Arch. Biochem. Biophys., 206, 242-248.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1982). Inhibition
by nitrosochloramphenicol of the proton translocation in mitochondria.
Biochem. Pharmacol., 31, 3823-3830.
Abou-Khalil, S., Abou-Khalil, W.H., & Yunis, A.A. (1985).
Identification of a mitochondrial rho-dinitrobenzene reductase
activity in rat liver. Pharmacol., 31, 301-308.
Abrams, S.M., Degnan, T.J., & Vinciguerra, V. (1980). Marrow aplasia
following topical application of chloramphenicol eye ointment.
Arch. Inter. Med., 140, 576-577.
Adamson, R.H. & Seiber, S.M. (1981). Chemically induced leukemia in
humans. Environ. Health Perspect., 39, 93-103.
Agam, G., Gasner, S., Bessler, H., Fishman, P., & Djaldetti, M.
(1976). Chloramphenicol induced inhibition of platelet protein
synthesis: In vitro and in vivo studies. Brit. J. Haematol.,
33, 53-59.
Ajodhia, J.M. & Dix, M.R. (1976). Drug-induced deafness and its
treatment. Practitioner, 216, 561-570.
Akhnoukh, S., El-Shazly, N., Sallaim, N., El-Melegy, S., El-Sewedy,
S.M., Mostafa, M.H., & El-Bassioni, E.A. (1982). In vivo effect of
chloramphenicol and thiamphenicol on some enzymes of normal mouse
liver. Biochem. Pharmacol., 31, 55-57.
Aksoy, M., Erdem, S., Dincol, G., Bakioglu, I., & Kutlar, A. (1984).
Aplastic anaemia due to chemicals and drugs: A study of 108 patients.
Sex Trans. Dis., 11, 347-350.
Alavi, J.B. (1983). Aplastic anaemia associated with intravenous
chloramphenicol. Am. J. Hematol., 15: 375-379.
Al-Hachim, G.M. & Al-Baker, A. (1974). The prenatal effect of
chloramphenicol on the postnatal development of mice.
Neuropharmacol., 13, 233-237.
Ali, B.H. (1985). Lack of effect of chloramphenicol on monoamine,
diamine and plasma amine oxidase activities. Clin. Exp. Pharmacol.
Physiol., 12, 1193-5.
Al-Moudhiry, H.A.B. (1978). Aplastic anaemia in Iraq. A prospective
study. Haematologia, 12, 159-164.
Amati, P. (1970). Chloramphenicol: Effect of DNA synthesis during
phage development in Escherichia coli. Science, 168, 1226-1228.
Ambrose, P.J. (1984). Clinical pharmacokinetics of chloramphenicol and
chloramphenicol succinate. Clin. Pharmacokinetics, 9, 222-238.
Applebaum, F.R. & Fefer, A. (1981). The pathogenesis of aplastic
anaemia. Sem. Haematol., 18, 241-257.
Appelgren, L.-E., Eberhardson, B., Martin, K., & Slanina, P. (1982).
The distribution and fate of 14C-chloramphenicol in the new-born
pig. Acta pharmacol. Toxicol., 51, 345-350.
Aravindaksham, C.M. & Cherian, Z. (1984). Effect of chloramphenicol on
thiopentone sodium anesthesia in dogs. Indian Vet. J., 61, 569-573.
Ascherl, M., Eyer, P., & Kaupffmeyer, H. (1985). Formation and
disposition of nitrosochloramphenicol in rat liver. Biochem.
Pharmacol., 34, 3755-3763.
Augustine, M.L., Poulsen, N.K., & Heinze, J.E. (1982). Evaluation of
the CHO/HGPRT cell mutation test using 12 compounds. Env. Mutagen.,
4, 389-390.
Awaad, S., Khalifa, A.S., & Kamel, K. (1975). Vacuolization of
leukocytes and bone-marrow aplasia due to chloramphenicol toxicity.
Clin. Pediatr., 14, 499-506.
Baltz, R.H., & Stonesifer, J. (1985). Adaptive response and
enhancement of N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis by
chloramphenicol in Streptomyces fradiae. J. Bacteriol.,
164, 944-946.
Banerjee, S. & Basu, P.S. (1978). Inhibition of rat tissue
mitrochondrial monoamine oxidase by chloramphenicol and its
intermediates. Indian J. Biochem. Biophys., 15, 479-482.
Bartlett, J.G. (1982). Chloramphenicol. Med. Clin. N. Amer.,
66, 91-102.
Baselt, R.C. (1982). Disposition of toxic drugs in man. 2nd Edition,
Davis, California, Biomedical Publications, 136-139.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1979). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Surg. Forum, 30, 512-514.
Beaugard, M.E., Asakuma, S., & Snow, J.B. (1981). Comparative
ototoxicity of chloramphenicol and kanamycin with ethacrynic acid.
Arch. Otolaryngol., 107, 104-109.
Becker, D., Metz, J., & Katz, J. (1974). Comparative effects of
thiamphenicol and chloramphenicol on haemopoiesis and lymphocyte
transformation in vitro in man. Postgrad. Med. J.,
50, (Suppl. 5), 88-94.
Beliles, R.P. (1972). The influence of pregnancy on the acute toxicity
of various compounds in mice. Toxicol. Appl. Pharmacol., 23, 537-540.
Benes, L., Rotreklova, E., & Pospisil, V.V.M. (1980). Inhibition of
bone marrow cell proliferation and DNA replication induced by
chloramphenicol. Folia Biol., 26, 408-414.
Benestad, H.B. (1974). Aplastic anaemia: Considerations on the
pathogenesis. Acta med. Scand., 196, 255-262.
Benestad, H.B. (1979). Drug mechanism in marrow aplasia.
In: Geary, C.G., (ed.) Aplastic anaemia, London, Balliere Tindall,
pp. 26-42.
Bengmark, S., Goransson, G., & Soucas, E. (1981). Defects in
hemostasis produced by antibiotics. An in vivo study in the rat.
Eur. Surg. Res., 13, 290-298.
Ben-Gurion, R. (1978). A simple plate test for screening colicine-
inducing substances as a tool for the detection of potential
carcinogens. Mutat. Res., 54, 289-295.
Bertolini, A. & Poggioli, R. (1981). Chloramphenicol administration
during brain development: Impairment of avoidance learning in
adulthood. Science, 21, 238-239.
Biancaniello, T., Meyer, R.A., & Kaplan, S. (1981). Chloramphenicol
and cardiotoxicity. J. Pediatr., 98, 828-830.
Billet, F.S., Collini, R., & Hamilton, L. (1965). The effects of D-and
L-threo-chloramphenicol on the early development of the chick embryo.
J.Embryol. Exp. Morph., 13, 341-356.
Blackwell, U.B. (1962). The changing inhibition of early
differentiation and general development in the chick embryo by
2-ethyl-5-methyl-benzimidazole and chloramphenicol. J. Embryo. Exp.
Morph., 10, 315-337.
Blondeel, A., Oleffe, J., & Achten, G. (1978). Contact allergy in 330
dermatological patients. Contact Derm., 4, 270-276.
Bories, G.F., Peleran, J.C., Wal, J.M., & Corpet, D.E. (1983). Simple
and ion-pair high performance liquid chromatography as an improved
analytical tool for chloramphenicol metabolic profiling. Drug Met.
Disp., 11, 249-254.
Bottiger, L.E. & Westerholm, B. (1972). Aplastic anaemia. I. Incidence
and aetiology. Acta med. Scand., 192, 315-318.
Bottiger, L.E. & Westerholm, B. (1973). Drug-induced blood dyscrasias
in Sweden. Brit. Med. J., 3, 339-343.
Bottiger, L.E. (1978). Prevelance and etiology of aplastic anaemia in
Sweden. In: Hibino, S., Takaku, F., & Shahidi, N.T. (eds.) Aplastic
Anemia. Tokyo, University Park Press, pp. 171-180.
Bottiger, L.E. (1979). Epidemiology and aetiology of aplastic anaemia.
Haematol. Blut. Transfus., 24, 27-37.
Brabec, M.J., Owens, J.B., Kenel, M., Sorscher, D., & Cornish, H.H.
(1982). Modification of methanol potentiation of carbon tetrachloride
toxicity in rats by chloramphenicol and salicylate. Drug Chem.
Toxicol., 5, 143-154.
Brasfield, J.H., Record, K.E., Griffen, W.O., & Mattingly, S. (1983).
Chloramphenicol succinate levels in patients with renal impairment.
Clin. Pharmacol., 1, 355-358.
Brauer, M.J. & Dameshek, W. (1967). Hypoplastic anemia and
myeloblastic leukemia following chloramphenicol therapy. Report of
three cases. New Engl. J. Med., 277, 1003-1005.
Braun, R., Fischer, G.W., & Schoneich, J. (1977). The mutagenicity and
DNA-damaging activity of cyclic aliphatic sulfuric acid esters.
Chem-Biol. Interact., 19, 241-252.
Braun, R., Schoneich, J., Weissflog, L., & Dedek, W. (1982). Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair direct alkylation vs. metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem-Biol. Interact.,
39, 339-350.
Brem, H., Stein, A.B., & Rosenkranz, H.S. (1974). The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res., 34, 2576-2579.
Brown, R.T. (1982). Chloramphenicol toxicity in an adolescent.
J. Adol. Health Care, 3, 53-55.
Bueno, L., Fioramonti, J., Alvinerie, M., & Bardon, T. (1984).
Influence of bran on bioavailability of chloramphenicol administered
orally to pigs. Can. J. Physiol. Pharmacol., 62, 208-211.
Burckart, G.J., Barrett, F.F., Stranghn, A.B., & Ternullo, S.R.
(1982). Chloramphenicol clearance in infants. J. Clin. Pharmacol.,
22, 49-52.
Burckart, G.J., Barett, F.F., Valle, R.D., & Mayer, M.C. (1983).
Chloramphenicol damage and pharmacokinetics in infants and children.
J. Clin. Pharmacol,, 23, 106-112.
Burke, J.T., Wargin, W.A., & Blum, M.R. (1980). High-pressure liquid
chromatographic assay for chloramphenicol, chloramphenicol-3-
monosuccinate and chloramphenicol-l-monosuccinate. J. Pharm. Sci.,
69, 909-912.
Burrows, G.E., Tyler, R.D., Craigmill, A.L., & Barto, P.B. (1984).
Chloramphenicol and the neonatal calf. Am. J. Vet. Res.,
45, 1586-1591.
Camitta, B.M., Storb, R., & Thomas, E.D. (1982). Aplastic anaemia.
Pathogenesis, diagnosis, treatment and prognosis. New Engl. J. Med.,
306, 645-652.
Carpenter, G. (1975). Chloramphenicol eye-drops and marrow aplasia.
Lancet, ii, 326-327.
Casale, T.B., Macher, A.M., & Fauci, A.S. (1982). Complete hematologic
and hepatic recovery in a patient with chloramphenicol hepatitis-
pancytopenia syndrome. J. Pediatr., 101, 1025-1027.
Chakrabarti, K. & Banerjee, S. (1977). Mechanism of intestinal
absorption of chloramphenicol. Indian J. Exp. Biol., 15, 302-303.
Chang, N., Giles, C.L., & Gregy, R.H. (1966). Optic neuritis and
chloramphenicol. Am. J. Dis. Child., 112, 46-48.
Charache, S., Finkelstein, D., Lietman, P.S., & Scott, J. (1977).
Peripheral and optic neuritis in a patient with hemoglobin SC disease
during treatment of Salmonella osteomyelitis with chloramphenicol.
Johns Hopkins Med. J., 140, 121-124.
Cid, F., Mella, F., Gonzalez, X., & Gouzalez, R. (1983). Effects of
anti-inflammatory drugs on the pharmacokinetics of chloramphenicol.
An. Real Acad. Farm., 49, 49-60.
Clausen, N. (1986). A population study of severe aplastic anemia
children. Acta paediatr. Scand., 75, 58-63.
Cocke, J.G., Brown, R.E., & Geppert, L.J. (1966). Optic neuritis with
prolonged use of chloramphenicol. J. Pediatr., 68, 27-31.
Cohen, T. & Creger, W.P. (1967). Acute myeloid leukemia following
seven years of aplastic anemia induced by chloramphenicol.
Am. J. Med., 43, 762-770.
Cohen, H.J. & Huang, A.T.-F. (1973). A marker chromosome abnormality.
Occurrence in chloramphenicol-associated acute leukaemia.
Arch. Intern. Med., 132, p. 440.
Courtney, K.D., Valerio, D.A., & Palletta, A.J. (1967). Experimental
teratology in the monkey. Toxicol. Appl. Pharmacol., 10, p. 378.
Courtney, K.D., & Valerio, D.A. (1974). Teratology in the
Macaca mulatta. Teratology, 1, 163-172.
Craft, A.W., Brocklebank, J.T., Itey, E.N., & Jackson, R.H. (1974).
The "grey-toddler". Chloramphenicol toxicity. Arch. Dis. Child.,
49, 235-237.
Cronberg, S., Wallmark, E., & Soderberg, I. (1984). Investigations on
the effect of antimicrobial drugs on platelet aggregation in vitro
and in vivo. Folia Haematol., 111, 725-734.
D'Angelo, E.P., Patterson, W.C., & Morrow, R.C. (1967). Chloramphenicol.
Topical application in the middle ear. Arch. Otolaryngol., 85,
126-128.
Daum, R.S., Cohen, D.L., & Smith, A.L. (1979). Fatal aplastic anaemia
following apparent "dose-related" chloramphenicol toxicity.
J. Pediatr., 94, 403-406.
De Corte-Baeten, K. & Debackere, M. (1976). Excretion of
chloramphenicol in the milk of lactating cows after oral and
parenteral application. Dtsch. Tieraerztl. Wochenschr., 83, 231-233.
Del Giacco, G.S., Petrini, M.T., Janelli, S., & Carcassi, U. (1981).
Fatal bone-marrow hypoplasia in a shepherd using chloramphenicol
spray. Lancet, i, p. 945.
de Vries, H., Beijersbergen van Henegouwen, G.M.J., & Huf, F.A.
(1984). Photochemical decomposition of chloramphenicol in a 0.25%
eyedrop and in a therapeutic intraocular concentration. Internat. J.
Pharmaceut., 20, 265-271.
Dewse, C.D. (1977). Variable action of chloramphenicol on DNA
synthesis in phytohaemagglutinin-stimulated human lymphocytes.
ICRS Med. Sci., 5, p.150.
Dill, W.A., Thompson, E.M., Tisken, R.A., & Glazko, A.J. (1960). A new
metabolite of chloramphenicol. Nature, 185, 535-537.
Djaldetti, M. (1983). Drug vulnerability of peripheral blood
platelets. Arch. Toxicol. (Suppl.), 6, 21-32.
Doudney, C.O. & Rinaldi, C.N. (1985). Chloramphenicol-promoted
increase in resistance to UV damage in Escherichia coli
B/r trp E65: Development of the capacity for successful repair of
otherwise mutagenic or lethal lesions in DNA. Mutat. Res.
143, 29-34.
Ehling, U.H. (1971). Comparison of radiation- and chemically-induced
dominant lethal mutations in male mice. Mutat. Res., 11, 35-44.
Epstein, S.S. & Shafner, H. (1968). Chemical mutagens in the human
environment. Nature, 219, 385-387.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972).
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol., 23, 288-325.
Evans, L.S. & Kleiman, M.B. (1986). Acidosis as a presenting feature
of chloramphenicol toxicity. J. Pediatr., 108, 475-477.
Eyer, F., Lierheimer, E., & Schneller, M. (1984). Reactions of
nitrosochloramphenicol in blood. Biochem. Pharmacol., 33, 2299-2308.
Flach, A.J. (1982). Chloramphenicol and aplastic anemia. Am. J.
Ophthalmol., 93, 664-665.
Flint, O.P. & Orton, T.C. (1984). An in vitro assay for teratogens
with cultures of rat embryo midbrain and limb bud cells. Toxicol.
Appl. Pharmacol., 76, 383-395.
Forck, G. (1971). Incidence and significance of chloramphenicol
allergies. Dtsch. Med. Wochen, 96, 161-165.
Forni, A. & Vigilant, E.C. (1974). Chemical leukemogenesis in man.
Ser. Haematol., 7, 211-223.
Fraki, J.E., Kalimo, K., Tuohimaa, P., & Aantaa, E. (1985). Contact
allergy to various components of topical preparations for treatment of
external otitis. Acta Otolaryngol., 100, 414-418.
Fraunfelder, F.T. & Bagby, G.C. (1982). Fatal aplastic anaemia
following topical administration of ophthalmic chloramphenicol.
Am. J. Ophthalmol., 93, 356-360.
Fraunfelder, E.T. & Meyer, S.M. (1984). Ocular toxicology update.
Austral. J. Ophthalmol., 12, 391-394.
Fraumeni, J.F. (1967). Bone-marrow depression induced by
chloramphenicol or phenylbutazone. J. Am. Med. Assoc., 201, 150-156.
Fripp, R.R., Carter, M.C., Werner, J.C., Schuler, H.G., Rannels, A.M.,
Whitman, V., & Nelson, N.M. (1983). Cardiac function and acute
chloramphenicol toxicity. J. Pediatr., 103, 487-490.
Fritz, H. & Hess, R. (1971). The effects of chloramphenicol on the
prenatal development of rats, mice and rabbits. Toxicol. Appl.
Pharmacol., 19, 667-674.
Gadner, H., Gethmann, U., Jessenberger, K., & Riehm, H. (1973). Acute
leukaemia following exposure to chloramphenicol? Three case reports
and review of the literature. Mschr. Kinderheilk, 121, 590-594.
Garrett, N.B., Stack, H.F., Gross, M.R., & Waters, M.D. (1984). An
analysis of the spectra of genetic activity produced by known or
suspected human carcinogens. Mutat. Res., 134, 89-111.
Gargye, A.K. & Dutta, D.V. (1959). Nerve deafness following
chloromycetin therapy. Indian J. Pediatr., 26, 265-266.
Glavini, E., Prati, M., & Vismara, C. (1979). The effects of
actinomycin D and chloramphenicol on rat preimplantation embryos.
Experientia, 35, 1649-1650.
Gilman, A.G., Goodman, L.S., Rall, T.W., & Murad, F. (eds.) (1985).
Goodman and Gilman's The Pharmacological Basis of Therapeutics.
7th ed. New York, MacMillan Publishing Co., pp. 1179-1184.
Glazko, A.J., Wolf, L.M., Dill, W.A., & Bratton, A.C. (1949).
Biochemical studies on chloramphenicol (chloromycetin). II. Tissue
distribution and excretion studies. J. Pharmacol. Exp. Ther.,
96, 445-459.
Glazko, A.J., Dill, W.A., & Rebstock, M.C. (1950). Biochemical studies
on chloramphenicol (chlormycetin). III. Isolation and identification
of metabolic products in urine. J. Biol. Chem., 183, 679-691.
Glazko, A.d., Dill, W.A. & Wolf, L.M. (1952). Observations on the
metabolic disposition of chloramphenicol (chloromycetin) in the rat.
J. Pharmacol. Exp. Ther., 104, 452-458.
Godel, V., Nemet, P., & Lazar, M. (1980). Chloramphenicol optic
neuropathy. Arch. Ophthalmol., 98, 1417-1421.
Goh, K. (1971). Chloramphenicol, acute leukemia and chromosomal
vacuolation. Southern Med.J., 64, p. 815.
Goh, K.-O. (1979). Chloramphenicol and chromosomal morphology.
J.Med., 10, 159-166.
Gray, J.D. (1955). The concentration of chloramphenicol in human
tissues. Canad. Med. Assoc. J., 72, 778-779.
Gross, B.J., Branchflower, R.V., Burke, T.R., Lees, D.E., & Pohl, L.R.
(1982). Bone-marrow toxicity in vitro of chloramphenicol and its
metabolites. Toxicol. Appl. Pharmacol., 64, 557-565.
Guntakatta, M., Matthews, E.J., & Rundell, J.O. (1984). Development of
a mouse embryo limb bud cell culture system for the estimation of
chemical teratogenic potential. Terat. Carcinogen. Mutat.,
44, 349-364.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985a). Percutaneous
absorption in man: A kinetic approach. Toxicol. Appl. Pharmacol.,
78, 123-129.
Guy, R.H., Hadgraft, J., & Maibach, H.I. (1985b). Transdermal
absorption kinetics: A physiochemical approach. ACS Symp. Ser.,
273, 19-31.
Haile, C.A. (1977). Chloramphenicol toxicity. Southern Med. J.,
70, 479-480.
Halpert, J. (1981). Covalent modification of lysine during the suicide
inactivation of rat liver cytochrome P-450 by chloramphenicol.
Biochem. Pharmacol., 30, 875-881.
Halpert, J. & Neal, R.A. (1981). Inactivation of rat liver cytochrome
P-450 by the suicide substrates parathion and chloramphenicol. Drug
Met. Rev., 12, 239-259.
Halpert, J. (1982). Further studies of the suicide inactivation of
purified rat liver cytochrome P-450 by chloramphenicol.
Mol. Pharmacol., 21, 166-172.
Halpert, J., Balfour, C., Miller, N.E., Morgan, E.T., & Danbar, D.
(1985). Isozyme selectivity of the inhibition of rat liver cytochrome
P-450 by chloramphenicol in vivo. Mol. Pharmacol., 28, 290-296.
Hapke, H.J., Abel, J., Ghosch, H., Tachampas, S., & Youssef, S.
(1977). Effects of chloramphenicol on drug-degrading enzyme systems.
Zentrabl. Veterinaermed., 24, 701-714.
Hara, H., Kohsaki, M., Noguchi, K., & Nagai, K. (1978). Effect of
chloramphenicol on colony formation from erythrocytic precursors.
Am. J. Haematol., 5, 123-130.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W., &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by interlaboratory
testing of diverse chemicals. Environ. Mutagen., 8, 515-531.
Hausman, K. & Skrandies, G. (1974). Aplastic anaemia following
chloramphenicol therapy in Hamburg and surrounding districts.
Postgrad. Med. J., 50, 136-142.
Heddle, J.A. & Bruce, W.R. (1977). Comparisons of test for
mutagenicity or carcinogenicity using assays for sperm abnormalities,
formation of micronuclei, and mutations in Salmonella: In: Hiatt,
H.H., Watson, J.D., & Winsten, J.A. (eds.) Origins of Human
Cancer, Book C, Risk Assessment, Cold Spring Harbour Laboratory,
pp. 1549-1557.
Hellriegel, K.P., & Gross, R. (1974). Follow-up studies in
chloramphenicol-induced aplastic anaemia. Postgrad. Med. J.,
50 (Suppl.5), 136-142.
Henley, C.M., Brown, R.D., Penny, J.E., Kupetz, S.A., Hodges, K.B., &
Jobe, P.C. (1984). Impairment of cochlear function produced by
chloramphenicol and noise. Neuropharmacol., 23, 197-202.
Henley, C.M. (1985). Comparison of the ototoxicity of chloramphenicol
and gentamycin in noise exposed rats. Diss. Abs., 46, 1523-B.
Hodgkinson, R. (1971). Infectious hepatitis and aplastic anaemia.
Lancet, i, p. 1014.
Hodgkinson, R. (1973). The chloramphenicol-hepatitis-aplastic anaemia
syndrome. Med. J. Austral., 1, 939-940.
Holden, H.E. (1982). Comparison of somatic and germ cell models for
cytogenetic screening. J. Appl. Toxicol., 2, 196-200.
Howell, A., Andrews, T.M., & Watts, R.W.E. (1975). Bone-marrow cells
resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet, i, 65-69.
Huang, N.M., Harley, R.D., Promadhattavedi, V., & Sproul, A. (1966).
Visual disturbances in cystic fibrosis following chloramphenicol
administration. J. Pediatr., 68, 32-44.
Humphries, K.R. (1968). Acute myelomonocytic leukaemia following
chloramphenicol therapy. N. Z. Med. J., 68, 248-249.
Hyman, J., Leifer, Z., & Rosenkranz, H.S. (1980). The E. coli
Pol A1 assay. A quantitative procedure for diffusable and
non-diffusable chemicals. Mutat. Res., 74, 107-111.
IARC (1982). IARC (International Agency for Research on Cancer)
monographs on the evaluation of the carcinogenic risk of chemicals to
humans: Chemicals, industrial processes and industries associated
with cancer in humans. 1-29, (Suppl. 4), 79-80.
IARC (1987, in press). IARC (International Agency for Research on
Cancer) monographs on the evaluation of carcinogenic risks to humans.
Overall evaluations of carcinogenicity: an update of IARC monographs
volumes 1-42 (Suppl. 7).
Ingall, D., Sherman, J.D., Cockburn, F. & Klein, R. (1965).
Amelioration by ingestion of phenylalanine of toxic effects of
chloramphenicol on bone-marrow. New Eng. J. Med., 272, 180-185.
Issaragrisil, S. & Piankijagum, A. (1985). Aplastic anemia following
topical administration of ophthalmic chloramphenicol: Report of a case
and review of the literature. J. Med. Ass. Thailand, 88, 309-312.
Jackson, A.F., Wentzell, B.R., McCalla, D.R., & Freeman, K.B. (1977).
Chloramphenicol damages bacterial DNA. Biochem. Biophys. Res.
Commun., 78, 151-157.
Javed, I., Nawaz, M., Ahmed, M., Rehman, Z.U., & Shah, B.H. (1984).
Pharmacokinetics, renal clearance and urinary excretion of
chloramphenicol in goats. Pakis. Vet., 4, 151-157.
Jones, F.E. & Hanson, D.R. (1977). H. Influenzae meningitis treated
with ampicillin or chloramphenicol, and subsequent hearing loss.
Dev. Med. Child. Neurol., 19, 593-597.
Joy, R.J.T., Scalettar, R., & Sodee, D.B. (1960). Optic and peripheral
neuritis. Probable effects of prolonged chloramphenicol therapy.
J. Amer. Med. Assoc., 173, 1731-1734.
Kapp, J.P., Avora, B., & Sibinga, M.S. (1977). Depression of
erythropoiesis, physical growth, and hair growth by chloramphenicol in
a three-year-old child with cystic fibrosis. Clin. Pediatr.,
16, 64-66.
Kauffman, R.E., Miceli, J.N., Strebel, L., Buckley, J.A., & Dore, A.K.
(1981). Pharmacokinetics of chloramphenicol and chloramphenicol
succinate in infants and children. J. Pediatr., 98, 315-320.
Keiser, G. & Bucheggar, U. (1973). Hematological side effects of
chloramphenicol and thiamphenicol. Helv. Med. Acta, 37, 265-278.
Kern, P., Heimpel, H., Heit, W., & Kubanek, B. (1975). Bone-marrow
cells resistant to chloramphenicol in chloramphenicol-induced aplastic
anaemia. Lancet i, p. 1190.
Knowles, J.A. (1965). Excretion of drugs in milk - a review.
J. Pediatr., 66, 1068-1082.
Korting, G.W. & Kifle, H. (1985). Systemic side-effects from external
application of chloramphenicol. Der Hautarzt., 36, 181-183.
Krasinski, K., Perkin, R., & Rutledge, J. (1982). Gray baby syndrome
revisited. Clin. Pediatr., 21, 571-572.
Krishna, G., Aykac, I., & Siegel, D. (1981). Recent studies on the
mechanisms of chloramphenicol activation responsible for aplastic
anemia. In: NaJean Y. (ed.) Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy. New York, Raven Press,
pp. 5-16.
Kroker, R. (1985). The pharmacokinetic behavior of chloramphenicol in
liver-damaged mini-pigs. J. Vet. Pharmacol. Ther., 8, 82-87.
Kroon, A.M. & de Vries, H. (1969). The effect of chloramphenicol on
the biogenesis of mitochondria of rat liver in vivo. Fed. Eur.
Biochem. Soc. Letters, 3, 208-210.
Kubinski, H., Gutzke, G.E., & Kubinski, Z.O. (1981). DNA-cell binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89, 95-136.
Kunii, O., Komatsu, T., & Nishiya, H. (1983). Biliary excretion of
antibiotics in rats. Jap. J. Exp. Med., 53, 51-58.
Kurz, H., Manser-Ganshorn, A., & Stickel, H.H. (1977). Differences in
the binding of drugs to plasma proteins from newborn and adult man.
I. Euro. J. Clin. Pharmacol., 11, 463-467.
Lasky, M.A., Pincus, M.H., & Katlan, N.R. (1953). Bilateral optic
neuritis following chloramphenicol therapy. J. Amer. Med. Assoc.,
151, 1403-1404.
Leiken, S.L., Welch, H., & Guin, G.H. (1961). Aplastic anaemia due to
chloramphenicol. Clin. Proc. Child Hosp., 17, 171-180.
Lepow, M. (1986). Aplastic anaemia following chloramphenicol therapy
still happens! Pediatr., 77, 932-933.
Lery, N., Descoted, J., & Evreux, J.Cl. (1978). A review of
chloramphenicol-induced blood disorders. Vet. Human Toxicol.,
20, 177-181.
Lim, L.O. & Yunis, A.A. (1982). Induction of methemoglobin formation
by nitrosochloramphenicol in hemolysed blood. Res. Commun. Chem.
Path. Pharmacol., 38, 497-500.
Lim, L.O., Abou-Khalil, W.H., & Yunis, A.A. (1984). The effect of
nitrosochloramphenicol on mitochondrial DNA polymerase activity.
J. Lab. Clin. Med., 104, 213-222.
Ling, G.V., Conzelman, G.M., Franti, C.E., & Ruby, A.L. (1980). Urine
concentration of chloramphenicol, tetracycline, and sulfisoxazole
after oral administration to healthy adult dogs. Am. J. Vet. Res.,
41, 950-952.
Longnecker, D.S., Curphey, T.J., James, S.T., Daniel, D.S., &
Jacobs, N.J. (1974). Trial of a bacterial screening system for rapid
detection of mutagens and carcinogens. Cancer Res., 34, 1658-1663.
Ma, T.-H., Harris, M.M., Anderson, V.A., Ahmed, I., Mohammad, K.,
Bare, J.L., & Lin, G. (1984). Tradescantia-micronucleus (Trad-MCN) on
140 health-related agents. Mutat. Res., 138, 157-167.
Hackler, B., Grace, R., Tippit, D., Lemire, R.J., Shepard, H., &
Kelly, V.C. (1975). Studies of the development of congenital anomalies
in rats III. Effects of inhibition of mitochondrial energy systems on
embryonic development. Teratology, 12, 291-296.
Malbrel, C., Talmud, M., Dupou, D., & Rose, J.P. (1977). Concerning a
new case of optic neuritis by chloramphenicol in the infant.
Bull. Soc. Ophthalmol. France, 77, 999-1001.
Mamber, S.W., Okasinki, W.A., Printer, C.D., & Tunac, J.B. (1986). The
Escherichia coli K-12 SOS Chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutat. Res., 171, 83-90.
Manna, G.K. & Bardham, S. (1977). Some aspects of chloramphenicol
induced bone marrow chromosome aberrations in mice. J. Cytol. Genet.,
12, 10-17.
Manna, G.K. & Roy, P.P. (1979). Chromosome aberrations in F1 embryos
and litters of chloramphenicol treated male parent mice. Natl. Acad.
Sci. Letters (India), 2, 460-462.
Martelo, O.J., Manyan, D.R., Smith, U.S., & Yunis, A.A. (1969).
Chloramphenicol and bone marrow mitochondria. J. Lab. Clin. Med.,
74, 927-940.
Masek, F. (1977). Effect of some drugs on excision repair in E. coli
cells. Chem-Biol. Interact., 17, 121-127.
Matsuda, S. (1984). Transfer of antibiotics into maternal milk.
Biol. Res. Preg. Perinatol., 5, 57-60.
Matthews, S.J. & Shawver-Matthews, S. (1980). Chloramphenicol toxicity
- oral vs. intravenous administration. J. Paediatr., 97, p. 869.
McCann, J., Choi, E., Yamasaki, E., & Ames, B.N. (1975). Detection of
carcinogens and mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc. Natl. Acad. Sci. USA, 72, 5133-5139.
McCoy, E., Hyman, J., & Rosenbranz, H.S. (1980a). A new mutagenic and
genotoxic response of the flame retardant tris (2, 3-dibromopropyl)
phosphate. Activation by singlet oxygen. Mutat. Res., 77, 209-214.
McCoy, E.C., Petrullo, L.A., & Rosenkranz, H.S. (1980b). Non-mutagenic
genotoxicants: Novobiocin and naladixic acid, 2 inhibitors of DNA
gyrase. Mutat. Res., 71, 33-43.
McCurdy, P.R. (1963). Plasma concentration of chloramphenicol and bone
marrow suppression. Blood, 21, 363-372.
Meyer, J.S. & Boxer, M. (1973). Leukemic cellular thrombi in pulmonary
blood vessels. Subleukemic myelogenous leukemia following
chloramphenicol-induced aplastic anemia. Cancer 32, 712-721.
Meyler, L. & Herkeimer, A. (1968). Side effects of drugs. 6,
Amsterdam, Excerpta Medica, pp. 286-314.
Meyler, L., Polak, B.C.P., Schut, D., Wesseling, H., & Herxheimer, A.
(1974). Blood dyscrasias attributed to chloramphenicol. A review of
641 published and unpublished cases. Postgrad. Med. J., 50
(Suppl. 5), 123-126.
Milner, G.R. & Geary, C.G. (1979). The aplasia-leukaemia syndrome. In:
Geary, C.G. (ed.) Aplastic Anaemia. London, Balliere Tindall,
pp. 230-243.
Mitchell, I. de G., Dixon, P.A., Gilbert, P.J., & White, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Problems of
evaluation. Mutat. Res., 79, 91-105.
Mitchell, I. de G. & Gilbert, P.J. (1984). The effect of pretreatment
of Escherichia coli CM891 with ethylenediamine-tetraacetate on
sensitivity to a variety of standard mutagens. Mutat. Res.,
140, 13-19.
Mitema, E.S. (1982). Effect of chloramphenicol administration on the
bovine hematology and bone marrow. Vet. Human Toxicol.,
Suppl., 162-166.
Mitus, W.J. & Coleman, N. (1970). In vitro effect of chloramphenicol
on chromosomes. Blood, 35, 689-674.
Mizuno, S., Aoki, K., Ohno, Y., Sasaki, R., & Hamajima, N. (1982).
Time series analysis of age-sex specific death rates from aplastic
anemia and the trend in production amount of chloramphenicol.
Nagoya J. Med. Sci., 44, 103-115.
Modan, B., Segal, S., Sham, M., & Sheba, C. (1975). Aplastic anemia in
Israel: Evaluation of the etiological role of chloramphenicol on a
community-wide basis. Am. J. Med. Sci., 270, 441-445.
Morizono, T. & Johnstone, B.M. (1975). Ototoxicity of chloramphenicol
ear drops with propylene glycol as solvent. Med. J. Austral.,
2, 634-638.
Morley, A., Trainor, K., & Remes, J. (1976). Residual marrow damage:
Possible explanation for idiosyncrasy to chloramphenicol. Brit. J.
Haematol., 32, 525-531.
Morris, P.L., Burke, T.R., George, J.W., & Pohl, L.R. (1982). A new
pathway for the oxidative metabolism of chloramphenicol by rat liver
microsomes. Drug Met. Disp., 10, 439-445.
Morris, P.L., Burke, T.R., & Pohl, L.R. (1983). Reductive
dechlorination of chloramphenicol by rat liver microsomes. Drug Met.
Disp., 11, 126-130.
Mulhall, A., de Louvois, J., & Hurley, R. (1983). Chloramphenicol
toxicity in neonates: Its incidence and prevention. Brit. Med. J.,
287, 1424-1427.
Murray, T. & Yunis, A.A. (1981). The cellular uptake and covalent
binding of nitrosochloramphenicol. J. Lab. Clin. Med., 98, 396-401.
Murray, T., Downey, K.M., & Yunis, A.A. (1982). Degradation of
isolated deoxyribonucleic acid mediated by nitrosochloramphenicol.
Possible role in chloramphenicol-induced aplastic anaemia. Biochem.
Pharmacol., 31, 2291-2296.
Naesland, B., Betner, I., & Halpert, J. (1983). Inhibition of rat lung
cytochrome P-450 by chloramphenicol. Extrahepatic Drug Met. Chem.
Carcinogen, Proc. Int. Mtg., 251-252.
Naesland, B. & Halpert, J. (1984). Selective inhibition by
chloramphenicol of cytochrome P-450 isozymes in rat lung and liver
involved in the hydroxylation of n-hexane. J. Pharmacol. Exp. Ther.,
231, 16-22.
Nagai, K. & Kanamaru, A. (1978). Drug-induced aplastic anaemia: Effect
of chloramphenicol on hemopoietic stems cells. In: Hibino, S., Takaku,
F., & Shakidi, N.T. (eds.) Tokyo, University Park Press, pp. 343-353.
Nagao, T. & Maner, A.M. (1969). Concordance for drug-induced aplastic
anemia in identical twins. New Engl. J. Med., 281, 7-10.
Nair, C.R., Goel, A., & Rahman, A. (1981). Inhibition of hepatic drug
metabolism by chloramphenicol-paracetamol interaction - an
experimental study. Bull. Postgrad. Inst. Med. Educ. Res.
(Chandrigarh), 15, 31-35.
Najean, Y. & Baumelon, E. (1984). Toxic etiology of aplastic anemia.
Sex Trans. Dis., 11, 343-346.
Nakagawa, T., Masada, M., & Uno, T. (1975). Gas chromatographic
determination and gas chromatographic-mass spectrometric analysis of
chloramphenicol, thiamphenicol and their metabolites. J. Chromatogr.,
111, 355-364.
Nara, N., Besshe, M., Hirashima, K., & Momoi, H. (1982). Effects of
chloramphenicol on hematopoietic inductive micro environment.
Exp. Hematol., 10, 20-25.
Narda, R.D. & Gupta, R.K. (1972). Mutation studies in
D. melanogaster. Drosoph. Inf. Serv., 48, p. 105
Nasrat, G.E., Ahmed, K.A., Nafei, H.A., & Abdel-Rahman, A.H. (1977).
Mutagenic action of certain therapeutic drugs on Drosophila
melanogaster. Bull. Fac. Agric. Univ. Cairo, 28, 697-717.
Nilges, T.C. & Norther, J.L. (1971). Iatrogenic ototoxic hearing loss.
Ann. Surg., 173, 281-289.
Pall, M.L. & Hunter, B.J. (1985). Carcinogens induce genetic tandem
duplications in Salmonella. Mutat. Res., 152, 131-145.
Pant, G.S., Kamada, N., & Tanaka, R. (1976). Sister chromatid
exchanges in peripheral lymphocytes of atomic bomb survivors and of
normal individuals exposed to radiation and chemical agents.
Hiroshima J. Med. Sci., 25, 99-105.
Parashar, S., Rao, R., Tikare, S.K., & Tikare, S.S. (1972).
Chloramphenicol induced reversible bone marrow suppression.
J. Postgrad. Med., 18, 90-92.
Parker, F.L. & James, G.W.L. (1978). The effect of various topical
antibiotic and antibacterial agents on the middle and inner ear of the
guinea-pig. J. Pharm. Pharmacol., 30, 236-239.
Patterson, W.C. & Gulick, W.L. (1963). The effects of chloramphenicol
upon electrical activity of the ear. Ann. Otol. Rhinol. Laryngol.,
72, 50-55.
Pazdernik, T.L. & Corbett, M.D. (1979). Effects of chloramphenicol
reduction products or hemopoietic precursor cells in vitro.
Pharmacol., 19, 191-195.
Pazdernik, T.L. & Corbett, M.D. (1980). Role of chloramphenicol
reduction products in aplastic anemia. Pharmacol., 20, 87-94.
Penny, R.H.C., Carlisle, C.H., Prescott, C.W., & Davidson, H.A.
(1967). Effects of chloramphenicol on the haemopoietic system of the
cat. Brit. Vet. J., 123, 145-153.
Perez, R.P., Puig, M.L., Sanchez, J.L.A., & Torres, E.L. (1981).
Aplastic anemia and drugs as causal factors. Rev. Cub. Farm.,
15, 97-103.
Petitjean, F., Sastre, J.P., Bertrand, N., Cointy, C., & Jouvet, M.
(1975). Suppression of paradoxical sleep by chloramphenicol in the
cat. Absence of effect of thiamphenicol. C. R. Seances Biol. (Paris),
169, 1236-1239.
Plaut, M.E. & Best, W.R. (1982). Aplastic anemia after parenteral
chloramphenicol: Warning renewed. New Engl. J. Med., 306, p. 1486.
Plomp, T.A., Thiery, M., & Maes, R.A.A. (1983). The passage of
thiamphenicol and chloramphenicol into human milk after single and
repeated oral administration. Vet. Human Toxicol., 25, 167-172.
Polak, B.C.P., Wesseling, H., Schut, D., Herxheimer, A., & Meyler, L.
(1972). Blood dyscrasias attributed to chloramphenicol. A review of
576 published and unpublished cases. Acta med. Scand., 192, 409-414.
Preziosi, P., Basile, V., Vacca, M., & de Natale, G. (1981). On the
correlation between regional utilization patterns of chloramphenicol
and its adverse reactions with emphasis on aplastic anaemia.
Int. J. Clin. Pharmacol.Ther. Toxicol., 19, 547-551.
Proud, G.O., Mittelman, H., & Seiden, G.D. (1968). Ototoxicity of
topically applied chloramphenicol. Arch. Otolaryngol., 87, 580-587.
Punch, P.I., Costa, N.D., Chambers, E.D., Slatter, D.H., &
Wilcox, G.E. (1985). Plasma and tear concentrations of antibiotics
administered parenterally to cattle. Res. Vet. Sci., 39, 179-187.
Puron, R. & Firminger, H.I. (1965). Protection against induced
cirrhosis and hepatocellular carcinoma in rats by chloramphenicol.
J. Natl. Cancer Inst., 85, 29-37.
Rajchgot, P., Prober, C., Soldin, S., Golas, C., Good, F., Harding, L.
& MacLeod, M. (1983). Chloramphenicol pharmacokinetics in the newborn.
Dev. Pharmacol. Ther., 6, 305-314.
Rebandel, P. & Rudzki, E. (1986). Occupational contact sensitivity in
oculists. Contact Derm., 15, p. 92.
Robin, E., Berman, M., Bhooplan, N., Cohen, H., & Fried, W. (1981).
Induction of lymphomas in mice by busulfan and chloramphenicol.
Cancer Res., 41, 3478-3482.
Rosenbach, L.M., Cavites, A.P., & Mitus, W.J. (1960). New Engl. J.
Med., 263, 724-728.
Rosenkranz, S., Carr, M.S., & Rosenkrans, H.S. (1974). 2-Haloethanols:
Mutagenicity and reactivity with DNA. Mutat. Res., 26, 367-370.
Rosenkranz, H.S., Gutter, B., & Speck, W.T. (1976). Mutagenicity and
DNA-modifying activity: A comparison of two microbial assays.
Mutat. Res., 41, 61-70.
Rosenkranz, H.S. (1977). Mutagenicity of halogenated alkanes and their
derivatives. Environ. Health Perspect., 21, 79-84.
Rosenthal, R.L. & Blackman, A. (1965). Bone marrow hypoplasia
following use of chloramphenicol eye drops. J. Am. Med. Assoc.,
191, 148-149.
Ross, S., Burke, F.G., Sites, J., Rice, E.C., & Washington, J.A.
(1950). Placental transmission of chloramphenicol (chloromycetin).
J. Am. Med. Assoc., 142, p. 1361.
Roy, B.K., Banerjee, N.C., & Pandy, S.N. (1986). Distribution of
chloramphenicol in goat blood and milk after intravenous injection.
Indian. J. Anim. Health, 25, 33-35.
Rudzki, E. & Kleniewska, D. (1970). The epidemiology of contact
dermatitis in Poland. Brit. J. Derm., 83, 543-545.
Rudzki, E., Grzywa, Z., & Maciejowska, E. (1976). Occupational contact
sensitivity in oculists. Contact Derm., 2, 181.
Sack, C.M., Kaup, J.R., & Smith, A.L. (1980). Chloramphenicol
pharmacokinetics in infants and young children. Pediatr.,
66, 579-584.
Saidi, P., Wallerstein, R.O., & Aggeler, P.M. (1961). Effect of
chloramphenicol on erythropoiesis. J. Lab. Clin. Med., 75, 247-256.
Salem, Z., Murray, T., & Yunis, A.A. (1981). The nitroreduction of
chloramphenicol by human liver tissue. J. Lab. Clin. Med.,
97, 881-886.
Samson, J.P., Hulstaert, C.E., Molenaar, I., & Nieweg, H.O. (1972).
Fine structure of the bone marrow sinusoidal wall in idiopathic and
drug induced panmyelopathy. Acta haematol., 48, 218-226.
Sanguineti, M., Rossi, L., Ognio, E., & Sauti, L. (1983). Tumours
induced in Balb/C and C57B1/6N mice following chronic administration
of chloramphenicol. In: Proceedings of a National Meeting on
Experimental and Clinical Oncology, Parma, 23-25 November,
Abstract No. 50.
Sarasti, H. (1970). Chloramphenicol in South America. New Engl.
J. Med., 282, p. 813.
Saxen, I. (1975). Associations between oral clefts and drugs taken
during pregnancy. Int. J. Epidemiol., 4, 37-44.
Scheres, J.M.J.C., Hustinx, T.W.J., Geraedts, J.P.M., Leeksma, C.H.W.,
& Meltzer, P.S. (1985). Translocation 1:7 in hematologic disorders. A
brief review of 22 cases. Cancer Gen. Cytogen., 18, 207-213.
Schewach-Millet, M. & Shapiro, D. (1985). Urticaria and angioedema due
to topically applied chloramphenicol ointment. Arch. Dermatol.,
121, p. 587.
Schmitt-Graff, A. (1981). Chloramphenicol-induced aplastic anemia
terminating with acute nonlymphocytic leukemia. Acta haematol.,
66, 267-268.
Seaman, A.J. (1969). Sequels to chloramphenicol aplastic anemia: Acute
leukaemia and paroxysmal nocturnal hemoglobinuria. Northwest Med.,
68, 831-834.
Sekizawa, J. & Shibamoto, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
Sharp, A.A. (1963). Chloramphenicol-induced blood dyscrasias: Analysis
of 40 cases. Brit. Med. J., 1, 735-736.
Sheehan, J. & Sweeney, E.C. (1982). Drugs and sperm. Brit. Med. J.,
284, p. 1331.
Shimizu, H., Kuroishi, T., Tominaga, S., Okada, H., & Aoki, K. (1979).
Production amount of chloramphenicol and mortality rate of aplastic
anaemia in Japan. Acta haem. Jap., 42, 689-696.
Siegel, D. & Krishna, G. (1980). Nitrosochloramphenicol does not
induce aplastic anaemia in mice. Pharmacologist, 22, p. 244.
Siliciano, R.F., Margolis, S., & Lietman, P.S. (1978). Chloramphenicol
metabolism in isolated rat hepatocytes. Biochem. Pharmacol.,
27, 2759-2762.
Silver, B.J. & Zuckerman, K.S. (1980). Aplastic anaemia. Recent
advances in pathogenesis and treatment. Med. Clin. North Amer.,
64, 607-627.
Sklar, R. & Strause, B. (1980). Role of the uvrE gene product and of
inducible D6-methylguanine removal in the induction of mutations by
N-methyl-N-nitro-N-nitrosoguanidine in Escherichia coli.
Mol. Biol., 143, 343-362.
Skolimowski, I.M., Rowley, D.A., Knight, R.C., & Edwards, D.I. (1981).
Reduced chloramphenicol-induced damage to DNA. J. Antimicrob.
Chemother., 7, 593-597.
Skolimowski, I.M., Knight, R.C., & Edwards, D.I. (1983). Molecular
basis of chloramphenicol and thiamphenicol toxicity to DNA in vitro.
J. Antimicrob. Chemother., 12, 535-542.
Slater, E.E., Anderson, M.D., & Rosenkranz, H.S. (1971). Rapid
detection of mutagens and carcinogens. Cancer Res., 31, 970-973.
Slaughter, R.L., Pieper, J.A., Carra, F.B., Brodsky, B., & Koup, T.R.
(1980). Chloramphenicol sodium succinate kinetics in critically ill
patients. Clin. Pharmacol. Ther., 28, 69-77.
Smith, A.L. & Weber, A. (1983). Pharmacology of chloramphenicol.
Pediatr. Clin. North Amer., 30, 209-236.
Smith, G.S., Watkins, J.B., Thompson, T.N., Rozman, K., &
Klaasen, C.D. (1984). Oxidative and conjugative metabolism of
xenobiotics by livers of cattle, sheep, swine and rats.
J. Anim. Sci., 58, 386-395.
Stainer, G.A., Peyman, B.S., Meisels, H., & Fishman, G. (1977).
Toxicity of selected antibiotics in vitreous replacement fluid.
Ann. Ophthalmol., 9, 615-618.
Steidl, P.E.-M. (1965). Bilateral optic neuritis caused by the
prolonged therapeutic use of chloramphenicol. Union Med. Canada,
94, 1061-1062.
Stevens, D,C., Kleiman, M.B., & Lietman, P.S. (1981). Exchange
transfusion in acute chloramphenicol toxicity. J. Pediatr.,
99, 651-653.
Strick, R.A. (1983). Lack of cross-reaction between DNCB and
chloramphenicol. Contact Derm., 9, 484-487.
Suda, T., Omine, M., Tsuchiya, J., & Maekawa, T. (1978). Prognostic
aspects of aplastic anaemia in pregnancy. Experience on six cases and
review of the literature. Blut., 36, 285-298.
Suhrland, L.G. & Weisberger, A.S. (1969). Delayed clearance of
chloramphenicol from serum in patients with hematologic toxicity.
Blood, 34, 466-471.
Summ, H.D., Draeger, E., & von Wasielowski, E. (1976). On the
inhibitory effect of chloramphenicol on mitochondrial protein
synthesis as a possible cause of its selective toxic side effects.
Arzneim. Forsch., 26, 28-32.
Suter, W. & Jaeger, I. (1982). Comparative evaluation of different
pairs of DNA repair-deficient and DNA repair-proficient bacterial
tester strains for rapid detection of chemical mutagens and
carcinogens. Mutat. Res., 97, 1-18.
Timmermans, L. (1974). Influence of antibiotics on spermatogenesis.
J. Urol., 112, 348-349.
Tomatis, L., Agthe, C., Bartsch, H., Huff, J., Montesano, R., Saracci,
R., Walker, E., & Wilbourn, J. (1978). Evaluation of the
carcinogenicity of chemicals: A review of the monograph program of
International Agency for Research on Cancer. Cancer Res.,
38, 877-885.
Ullrich, D. & Bock, K.W. (1984). Glucuronide formation of various
drugs in liver microsomes and in isolated hepatocytes from
phenobarbital - and 3-methylcholanthrene - treated rats. Biochem.
Pharmacol., 33, 97-101.
Vacha, J., Pospisil, M., & Velcovsky, V. (1981). The toxic effect of
chloramphenicol on erythropoiesis in X-irradiated mice. Chemother.,
27, 131-138.
Van Joost, Th., Dikland, W., Stolz, E., & Prens, E. (1986).
Sensitisation to chloramphenicol; a persistent problem. Contact
Derm., 14, 176-178.
Van Scoy, R.E. & Wilson, W.R. (1983). Antimicrobial agents in patients
with renal insufficiency. Mayo Clin. Proc., 58, 246-248.
Venning, G.R. (1983). Identification of adverse reactions to new
drugs. II - How were 18 important adverse reactions discovered and
with what delays? Brit. Med. J., 286, 289-292.
Verma, R.S. & Lin, M.S. (1978). Chemically induced alterations of the
nuclear cycle and chromosomes in root meristem cells of maize.
J. Hered., 69, 285-294.
Vincent, P.C. (1986). Drug-induced aplastic anaemia and
agranulocytosis. Incidence and mechanisms. Drugs, 31, 52-63.
Vorherr, H. (1974). Drug excretion in breast milk. Postgrad. Med.,
56, 97-104.
Wagner, T. & Rafael, J. (1977). Biochemical properties of liver
magamitochondria induced by chloramphenicol or cuprizone. Exp. Cell
Res., 107, 1-14.
Walker, G.F. (1961). Blindness during streptomycin and chloramphenicol
therapy. Brit. J. Ophthalmol., 45, 555-557.
Wallenstein, L. & Snyder, J. (1952). Neurotoxic reaction to
chloromycetin. Ann. Intern. Med., 36, 1526-1528.
Wallerstein, R.O., Candit, P.K., Kasper, C.K., Brown, J.W., &
Morrison, F.R. (1969). Statewide study of chloramphenicol therapy and
fatal aplastic anemia: J. Am. Med. Assoc., 208, 2045-2050.
Waters, M.D., Garrett, N.E., Covone-de Serres, C.M., Howard, B.E., &
Stack, H.F. (1983). Genetic toxicology of some known or suspected
human carcinogens. In: de Serres, F.J. (ed.) Chemical Mutagens.
Principles and methods for their detection. 8, New York, Plenum
Press, pp. 261-341.
Watson, A.D.J. (1972). Chloramphenicol plasma levels in the dog: A
comparison of oral subcutaneous and intramuscular administration.
J. Small Anim. Pract., 13, 147-151.
Watson, A.D.J. (1977a). Effect of concurrent drug therapy and of
feeding or chloramphenicol levels after oral administration of
chloramphenicol in dogs. Res. Vet. Sci., 22, 68-71.
Watson, A.D.J. (1977b). Chloramphenicol toxicity in dogs.
Res. Vet. Sci., 23, 66-69.
Watson, A.D.J. & Middleton, D.J. (1978). Chloramphenicol toxicosis in
cats. Am. J. Vet. Res., 39, 1199-1203.
Watson, A.D.J. (1980). Further observations on chloramphenicol
toxicosis in cats. Am. J. Vet. Res., 41, 293-294.
Welch, H., Lewis, C.N. & Kerlan, I. (1954). Blood dyscrasias. A
nationwide survey. Antibiotics Chemother., 4, 607-623.
Werner, J.C., Whitman, V., Schuler, H.G., Fripp, R.R., Rannels, A.M.,
Kasales, C.J., & La Noue, K.F. (1985). Acute myocardial effects of
chloramphenicol in newborn pigs: A possible insight into Gray Baby
syndrome. J. Infect. Dis., 152, 344-350.
White, M.P., Haboush, H.9., & Alroomi, L.G. (1986). Fatal bone marrow
aplasia due to chloramphenicol in a baby. Lancet i, 555-556.
Widayat, Sunarto, Sutaryo, Zurghiban, M., & Ismangoen (1983). Aplastic
anaemia in children. Paediatrica Indonesiana, 23, 183-191.
Wilson, W. (1962). Toxic amblyopia due to chloramphenicol. Scot. Med.
J., 7, 90-95.
Xie, L. (1985). A simple model for detection of mutagenic
(carcinogenic) substances. Huanjing Kexue Xuebao, 5, 228-233.
Yoshida, H., Yamamoto, K., & Yamaguchi, H. (1972). Fragmentation and
non-disjunction of barley chromosomes after the treatment of
chloramphenicol and cycloheximide. Cytologia, 37, 697-707.
Young, L.W., Mitnick, J.S., Yankus, W.A., & Genieser, N.B. (1979).
Radiological case of the month. Am. J. Dis. Child., 133, 545-546.
Yunis, A.A. (1973a). Chloramphenicol toxicity. In: Blood Disorders
Due to Drugs and other Agents, Amsterdam, Excerpta Medica,
pp. 107-126.
Yunis, A.A. (1973b). Chloramphenicol-induced bone marrow suppression.
Semin. Haematol., 10, 225-234.
Yunis, A.A. (1977). Differential in vitro sensitivity of marrow
erythroid and granulocytic colony forming cells to chloramphenicol.
Am. J. Haemtol., 2, 355-363.
Yunis, A.A. (1978a). Mechanisms underlying marrow toxicity from
chloramphenicol and thiamphenicol. In: Silber, R., Lo Bue, J.,
Gordon, A. (eds.) The year of hematology, New York, Plenum,
pp. 143-170.
Yunis, A.A. (1978b). Pathogenetic mechanisms in bone marrow
suppression for chloramphenicol and thiamphenicol. In: Hibino, S.,
Takaku, F., & Shahidi, N.T. (eds.) Tokyo, University Park Press,
pp. 321-334.
Yunis, A.A. (1978c). Drug-induced bone marrow aplasia. Pesq. Med.
Biol., 11, 287-296.
Yunis, A.A., Miller, A.M., Salem, Z., & Arimura, G.K. (1980a).
Chloramphenicol toxicity: Pathogenetic mechanisms and the role of the
rho-NO2 in aplastic anaemia. Clin. Toxicol., 17, 359-373.
Yunis, A.A., Miller, A.M. Salem, Z., Corbett, M.D., & Arimura, G.K.
(1980b). Nitrosochloramphenicol: Possible mediator in
chloramphenicol-induced aplastic anemia. J. Lab. Clin. Med.,
96, 34-46.
Yunis, A.A. & Salem, Z. (1980). Drug induced mitochondrial damage and
sideroblastic change. Clinics in Haematol., 9, 607-619.
Yunis, A.A. (1984). Differential in vitro toxicity of chloram-
phenicol, nitrosochloramphenicol, and thiamphenicol. Sex Trans. Dis.,
11, 340-342.
