
ALBENDAZOLE
1. EXPLANATION
Albendazole belongs to the benzimidazole class of anthelmintic.
Other members of this group most closely related to albendazole are
fenbendazole and oxfendazole. Albendazole is currently used in a
number of countries as a human and veterinary anthelmintic. This is
the first occasion on which albendazole has been evaluated by the
Joint FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
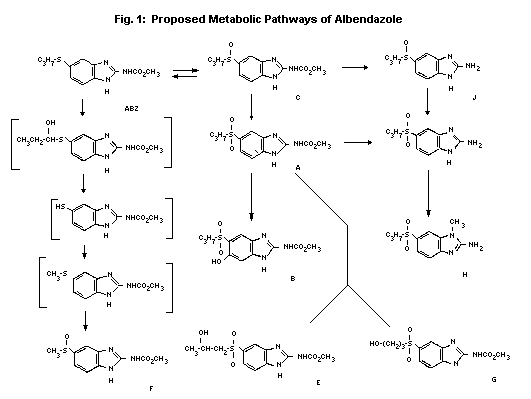
The degradation of albendazole was shown to follow similar
pathways in rats, mice, humans, cattle and sheep. See Fig. 1 for the
proposed metabolic pathways.
Male Charles River CD mice were given a single gavage dose of
14C-ring labelled albendazole. The dose of 13.2 mg/kg bw was
suspended in 1% carboxymethylcellulose. Over a 72 h period, 20.5% of
the administered radioactivity was recovered in the urine. Using TLC
and autoradiography it was revealed that the sulfoxide (C) and
metabolites G and E accounted for 81% of the label. Low levels of
parent drug (ABZ), the sulfone (A), and metabolites F, I and J were
also detected (see Figure 1) (Parish et al., 1979a).
The parent compound was virtually undetectable in the plasma of
Sprague Dawley males and females given a single gavage dose of 10.6
mg/kg bw albendazole in an aqueous suspension. Rapid metabolism let to
the appearance of the sulfoxide and subsequently the sulfone
derivatives in plasma. Both metabolites decreased to very low levels
at 18 h (Delatour et al., 1984; Souhaili-el Amri et al., 1988).
Daily dosing at 10.6 mg/kg bw in males for a period of 10 days
resulted in lower plasma levels of sulfoxide and higher levels of the
sulfone. Albendazole induces certain hepatic drug-metabolising
enzymes, which may be responsible for enhancing the degradation of
sulfoxide to sulfone following repeated administration (Souhaili-el
Amri et al., 1988).
Female Sprague Dawley rats were given a single gavage dose of
14C-ring labelled albendazole. The dose was 13.25 mg/kg bw suspended
in 1% carboxymethylcellulose. Over a 72 h period, 31% of the
administered radioactivity was recovered in the urine. Using TLC and
autoradiography it was shown that the sulfoxide, metabolite G, the
2-amino sulfone and metabolite E accounted for 89% of the radiolabel.
Low levels of the parent drug, the sulfone, and metabolites D, F, H
and J were also found. Similar doses of the sulfoxide and sulfone
derivatives resulted in the urinary excretion of 73.0 and 42.7% of the
dose, respectively. The urinary metabolites were qualitatively similar
to those observed after albendazole administration (Parish & Gyurik,
1979).
 Sheep bearing permanent ruminal and abomasal cannulae were given
a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation.
Albendazole was absorbed unchanged from the rumen. Once in the body it
was rapidly degraded, and sulfone metabolites were detected in plasma,
the former achieving the greater level. All 3 compounds were present
in the abomasum. Presumably albendazole was passed through the
stomachs while the metabolites were secreted or diffused into this
organ. Non-detectable levels of all 3 compounds were reached in plasma
and rumen at 96 h and in abomasum at 120 h (Marriner & Bogan, 1980).
Sheep were given a single oral dose of 10 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1, 2, 4, 6 or 8 days after
treatment. On day 1, the label in liver was mainly in the form of the
sulfoxide and sulfone metabolites. These compounds were progressively
converted to the 2-amino sulfone which was the primary residue on day
8. Low levels of albendazole were detected up to day 2; other
degradation products were metabolites E, G and J. Over 72 h, pooled
urine samples, the sulfoxide and 2-amino sulfone accounted for 60-70%
of the urinary radioactivity. Low levels of albendazole, the sulfone
and 6 other metabolites were also found (Parish et al., 1977a;
Colman et al., 1977).
Steers were given a single oral dose of 7.5 mg/kg bw of an
albendazole formulation. Albendazole could not be detected in plasma.
Metabolism to the sulfoxide and sulfone was rapid; these compounds
were present in plasma for up to 40 h (Prichard et al., 1985).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1 to 12 days after
treatment. In liver obtained on day l, the radioactivity was mainly as
albendazole, the sulfoxide and sulfone. Albendazole disappeared by day
6 while the sulfoxide and sulfone were progressively converted to
2-amino sulfone through day 12. Low levels of metabolites G and J were
also found. A limited examination of kidney tissue revealed a similar
metabolic profile (Kraeer et al., 1977).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Over a 7 day period, 47% of the administered
radioactivity was recovered in the urine. The sulfoxide, sulfone and
2-amino sulfone accounted for 70% of this radiolabel. Low levels of
albendazole, metabolites B, D, E, G, H and J were also found (Parish
et al., 1977b; 1979b).
Human volunteers were given a single oral 400 mg dose of
albendazole. The parent sulfide was not detected in blood by HPLC.
Peak sulfoxide concentrations were achieved after 2.4 h and decayed
biphasically with a long terminal half-life. Analysis of 24 h urine by
TLC revealed the presence of the sulfoxide, the sulfone and their
amino derivatives and metabolites B, E and G (Rossingnol &
Maisonneuve, 1984).
The above findings were confirmed in other similar studies.
Additionally, it was demonstrated that urinary recovery over a 24 hour
period accounted for up to 0.88% of the dose. Limited information
suggested biliary excretion was very low. Thus it appears that the
oral absorption of the albendazole is somewhere around 1%. When
ingested with a high fat content meal the absorption of albendazole
was shown to be 4.6 times higher, on average, in 6 volunteers
(Marriner et al., 1986; Lange et al., 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Findings in dead rats included urinary staining of abdomen, bloody
discharge around nose, chromodacryorrhea and intestinal hemorrhage.
Necropsy of dead rabbits showed intestines containing fluid and
dilated with gas. Toxic signs in other species were not reported.
2.2.2 Short-term studies
2.2.2.1 Mice
In 2 separate experiments, groups of 10 male and 10 female
Charles River CD1 mice were fed diets containing albendazole for 90
days. Drug levels in food were adjusted so that animals received
doses of 0, 5, 10, 20, 40 or 80 mg/kg bw/d in study 1 and 0, 200, 400,
800 or 1600 mg/kg bw/d in study 2.
All females and 5/10 males of the 1600 mg/kg group died
spontaneously or were killed in a moribund condition. From week 9, ear
lesions involving thickening and/or encrustation of the tips were
observed in 2/10 males and 2/9 females at 800 mg/kg and 5/5 males in
the 1600 mg/kg group. Food consumption was generally decreased in
males given 400 mg/kg or more but weight gain was depressed only at
1600 mg/kg.
Hematology was measured at the end of the study. Hemoglobin,
hematocrit and erythrocyte levels were reduced at 800 mg/kg or more
and a decreased leucocyte counts was observed in males of these
groups. Gross post mortem examinations revealed increases in absolute
and relative liver weights from 40 mg/kg and greater in males and 80
mg/kg and greater in females (Daly & Rinehart, 1980a, 1980b).
2.2.2.2 Rats
Groups of 15 to 20 male and female Charles River Sprague Dawley
rats were given gavage doses of 0, 4, 25, 48 or 168 mg/kg bw/d of
albendazole in 0.5% Tween 80 for 4 weeks.
Species Sex Vehicle LD50 (mg/kg bw) Reference
Mouse M 0.75% methocel over 3000 Macko et al.,
1975a
Rat M&F 2% tragacanth 1320 Walker, 1976a
Rat M&F 1% methocel 2400 Braun & Killeen,
1975
Hamster M&F 2% tragacanth over 10000 Walker, 1976b
Guinea-pig M&F 2% tragacanth 900 Walker, 1976c
Rabbit M&F 2% tragacanth 500-1250 Walker, 1976d
Toxic signs were produced in the 2 highest dose groups which
included diarrhea, piloerection, nasal swelling with blood stained
nasal discharge and death (7/30 rats at 48 mg/kg and 39/40 rats at 168
mg/kg died). Body weight gain was depressed at 48 mg/kg with weight
loss at 168 mg/kg while food consumption was markedly reduced at 168
mg/kg and slightly reduced at 48 mg/kg.
Hematology, blood chemistry and urinalysis were measured after 1
and 4 weeks of treatment, except in the high dose rats which exhibited
marked overt toxicity. Hemoglobin, hematocrit, erythrocyte and
leucocyte counts were reduced at 48 mg/kg.
At autopsy, adrenal size was increased at 48 and 168 mg/kg,
particularly in females. Testes were soft with reduced size and weight
in 48 mg/kg males which survived 4 weeks. Testes size was not affected
at the highest dose presumably due to early death of these males.
Histopathology examination revealed hypoplasia in testes, bone marrow,
spleen and lymph nodes in 48 and 168 mg/kg groups.
An additional group of 5 males and 5 females was given 48 mg/kg
bw/d of albendazole for 4 weeks, followed by withdrawal of treatment
for 4 weeks. All drug-related changes were reduced in severity during
the latter period indicating that the effects were reversible (Simon,
1979a).
Groups of 20 male and 20 female Long Evans rats were fed diets
containing albendazole for 91 days. Drug levels in food were adjusted
so that animals received doses of 0, 2, 10 or 30 mg/kg bw/d. The
control and high dose groups included an additional 10 males and 10
females for laboratory studies.
There were no signs of toxicity and no effects on body weight,
food consumption and ophthalmology parameters. Hematology, blood
chemistry and urinalysis were studied after 1 and 3 months of
treatment. Gross pathology and organ weights were examined in all rats
while histopathology was carried out on 15 males and 15 females from
control and high dose groups. Meaningful changes related to treatment
were not observed (Killeen & Rapp, 1975a).
Groups of 100 male and female Charles River CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then throughout mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 5, 30 or 45 mg/kg bw/day. The treatment was intended to be
for 2 years but excessive mortality necessitated termination of the
study after 26 weeks.
There were no untoward effects in F0 rats. In the F1 animals,
92/100 males and 99/100 females of the 45 mg/kg bw/day group died by
week 25. Prior to death these rats developed swollen cervical glands,
followed by swelling of paws and the genital area, scabbing in the
cervical area and emaciation (reduced weight gain and food intake).
Hematology was measured after 3 and 6 months of treatment.
Hemoglobin, hematocrit, erythocyte and leucocyte counts were decreased
and reticulocytes increased in 45 mg/kg rats at 3 months. At 6 months,
similar but slight hematological changes were seen in 30 mg/kg bw/day
rats. Segmented neutrophils were particularly affected and this was
confirmed by differential bone marrow counts in control and 30 mg/kg
rats. Blood chemistry and urinalysis were studied after 3 months of
treatment. In the 45 mg/kg bw/day group, plasma cholesterol was
increased in males and females, potassium was increased in males, and
albumin, plasma and erythrocyte cholinesterase were decreased in
females. Urinary protein was increased in 30 and 45 mg/kg bw/day
males.
Post mortems were carried out on all unscheduled deaths and
approximately 60% of animals surviving to 26 weeks. Numerous gross
alterations were noted in the high dose group including discoloration
and/or nodules in the lungs, heart, lymph nodes, spleen, pancreas,
liver, adrenal and kidney. Some of these organs were also enlarged or
showed adhesions. Additionally, thymic tissue was often absent and
testes were small and flaccid.
Histopathology, performed on selected tissues in 5 males and 5
females from 0, 30 and 45 mg/kg bw/day groups, identified a number of
instances where bacterial colonization in lungs, spleen, kidneys and
heart was associated with necrosis without the usual acute
inflammatory response. The liver showed centrilobular cloudy swelling,
vacuolation or necrosis and lymphoid tissues such as bone marrow,
spleen and thymus showed hypocellularity or other changes suggestive
of atrophy. All the above lesions were seen at 45 mg/kg while, at 30
mg/kg, only the thymic and minor hepatic effects were noted (Daly &
Hogan, 1982).
The remaining rats were used to further evaluate effects on
hematological parameters. Control and 5 mg/kg group animals were
continued on the same treatment level while rats from the 30 mg/kg
group were now given 0 or 20 mg/kg bw/d albendazole. Group sizes were
20 to 25 males and females and dosing was continued for 4 months.
Hematology was studied monthly. The 5 mg/kg rats were still
unaffected. The red and white cell effects induced at 30 mg/kg were
essentially normalized within a month of reducing the dose to 0 or 20
mg/kg. However, differential bone marrow counts at day 81 revealed a
persistent depression of the myeloid line in the 20 mg/kg group. Gross
post mortem examination of all animals showed no remarkable effects
(Daly & Hogan, 1981).
2.2.2.3 Dogs
Groups of 4 to 5 male and female beagle dogs were given gavage
doses of 0, 4, 16, 48 or 168 mg/kg bw/d of albendazole in 2% Tween 80
for 4 weeks. Toxic signs, in a few dogs at the 2 highest dose levels,
included diarrhea and vomiting with cardio-pulmonary disturbances in
1 dog. Death ensued in 1/10 dogs at 48 mg/kg and 6/10 dogs at 168
mg/kg, mainly in females. Heart rates showed marked increases in some
168 mg/kg females prior to death. Food intake was reduced at 48 mg/kg
and above and weight gain was depressed at 16 mg/kg and above.
Hematology, blood chemistry, urinalysis and ophthalmology were
studied after 1 and 4 weeks of treatment. Leucocyte counts were
decreased in a few dogs at 48 and 168 mg/kg and alkaline phosphatase
was increased from 16 mg/kg. Necropsy revealed lower absolute
testicular weight in 48 and 168 mg/kg males but no pathological
alterations (Simon, 1979b).
Groups of 4 male and female beagle dogs were given 0, 2, 10 or 39
mg/kg bw/d albendazole in capsules for 91 days. Ophthalmology,
hematology, blood chemistry and unrinalysis were studied after 1 and
3 months; organ weights, gross and histopathology were examined in all
dogs. There were no toxic signs or effects on body weight and food
intake. No treatment-related effects were found (Killeen & Rapp,
1975b).
Groups of 6 male and 6 female beagle dogs were given 0, 5, 30 or
60 mg/kg bw/d albendazole in capsules for 6 months.
Food intake was decreased in 30 and 60 mg/kg females, while
weight gain was lower in 60 mg/kg males and females. Laboratory
studies included hematology and blood chemistry monthly, urinalysis
bimonthly and ophthalmology at termination. Hemoglobin, hematocrit and
erythrocyte counts were slightly reduced at 60 mg/kg and leucocyte
counts, particularly neutrophils, were reduced at 30 and 60 mg/kg.
Autopsy on all dogs revealed decreased absolute and relative
testes and uterine weights and slight increases in relative liver and
kidney weights at 60 mg/kg. The incidences of small nodules in the
stomach were increased in all treated groups but microscopically they
were shown to be normal, submucosal lymphoid follicles. Sternal bone
marrow showed hypocellularity in 4/6 females in the 60 mg/kg group.
The NOEL was 5 mg/kg bw/day. (Daly & Hogan, 1980).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 100 male and 100 female Charles River CD-1 mice were
fed diets containing albendazole for 25 months. Drug levels were
adjusted to provide doses of 0, 25, 100 or 400 mg/kg bw/d. Additional
groups of 25 males and 25 females were given control and high dose
treatments and used for hematology measurements.
There were no toxic signs or effects on food intake and body
weight. Hematology was studied after 3, 6 , 12, 18 and 24 months in
the main groups and monthly in the ancillary groups. Erythrocyte and
leucocyte counts were decreased and platelets were increased at 400
mg/kg, particularly in females.
A complete gross post-mortem examination was carried out on all
mice. Full histopathology was undertaken on control and high dose
mice. In intermediate groups, 6 major organs and grossly abnormal
tissues were examined routinely. Flaccid or small testes, testicular
tubular degeneration, oligospermia, and aspermia in epididymides were
increased in 400 mg/kg males. Centrilobular hepatocytic vacuolation
was increased in groups given 100 and 400 mg/kg. Eye opacities were
noted in all groups; however, microscopically, cataracts were slightly
increased only in 400 mg/kg males. The relationship of these ocular
findings to treatment is questionable as the bulk of the cataracts
were unilateral and such abnormalities are commonly obtained following
repeated blood collection from the orbital sinus.
The incidence of endometrial stromal polyps appeared to be
increased over concurrent controls (Table 1) but a statistical
evaluation of the results did not reveal significant differences
between groups, and all incidences were within the historical control
range for this laboratory based on 2 studies with 2 control groups in
each. The NOEL was 25 mg/kg bw/d. (Daly & Knezevich, 1987a; Sauer,
1985, 1987b; Selwyn 1987).
Table 1: Tumor incidence in mice
Dose (mg/kg bw/d) 0 0 25 100 400 Historical Controls
Endometrial stromal tumors
Polyps 3/98 5/99 3/98 5/98 7/99 Range 0/55-8/47
Sarcomas 0/98 0/99 1/98 2/98 0/99 Cumulative 29/780
Total 3/98 5/99 4/98 7/98 7/99
2.2.3.2 Rats
Groups of 100 male and 100 female Sprague Dawley CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then through mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 3.5, 7 or 20 mg/kg bw/d for 28 months. Additional groups
of 25 males and 25 females were given control and high dose treatments
and used for hematology measurements. An interim sacrifice of 10 males
and females per group was made after 12 months.
Treatment-related effects were not observed in F0 animals. In the
F1 animals, mortality was slightly increased after 24 months in 20
mg/kg males. There were no other toxic signs or effects on body weight
and food consumption. Ophthalmology, hematology, blood chemistry and
urinalysis parameters were studied after 3, 6, 12, 18 and 24 months of
treatment. In the 20 mg/kg group, total leucocyte and neutrophil
counts were decreased at 24 months, serum cholesterol was increased in
females throughout the study and in males at some sampling times.
A complete gross post-mortem examination was carried out on all
rats. Full histopathology was undertaken on control and high dose
rats. In intermediate groups, 8 major organs and grossly abnormal
tissue were examined routinely. The 20 mg/kg animals showed increased
incidences of flaccid testes, degeneration/atrophy of germinal and
relative liver weights in males and hepatic fatty metamorphosis in
males and females.
Compared to concurrent controls, the incidences of
endometrial/cervical tumors and skin histiocytic sarcomas showed an
apparent increase in certain groups of rats (see Table 2 below).
Statistical evaluation of the findings did not reveal significant
differences between groups and in fact all frequencies were within the
historical control range for this laboratory. The NOEL was 7 mg/kg/d.
(Daly & Knezevich, 1987b; Sauer, 1985, 1987a; Selwyn, 1987).
Table 2: Tumor incidence in rats
Dose (mg/kg/d) 0 0 3.5 7 20 Historical Controls
Endometrial/cervical tumors
Polyps 3/99 5/99 9/98 9/99 10/91 Range 1/69-7/58*
Sarcomas 0/99 3/99 0/98 4/99 3/91 Cumulative 120/1864
Total 3/99 8/99 9/98 11/99 13/91
Skin histiocytic sarcomas
Males 1/100 2/100 4/98 4/100 6/100 Range 0/116-6/110**
Cumulative 32/1190
Females 0/100 4/100 0/100 1/96 5/100 Range 1/112-11/119
Cumulative 40/1188
* based on 18 studies
** based on 14 studies.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Long Evans rats were fed diets containing 0, 30, 75 and
150 ppm albendazole for 3 successive generations, commencing 64 d
before the initial mating. Each parental group consisted of 12 males
and 24 females, which were bred to produce 2 litters each. Offspring
from the second litters were selected to serve as parents of the
subsequent generations. Drug intake was calculated to be, on average,
2.3, 5.8 and 11.6 mg/kg bw/d.
There were no toxic signs or effects on body weight, food
consumption, mating, fertility, pregnancy rates, gestation length,
litter size and weight. During lactation, pup survival and/or weight
gain was depressed, but only in F1a and F2a litters given 11.6 mg/kg.
The NOEL was 5.8 mg/kg bw/d. (Schroeder & Rinehart, 1980).
Groups of 20 male Sprague Dawley CD rats were given gavage doses
of 0, 1, 10 or 30 mg/kg bw/d albendozale in 0.5% gum tragacanth, from
60 days prior to mating to the end of the breeding period. Males were
mated 1 to 1 with untreated females. Half the dams were killed on
gestation day 13, the remainder were allowed to deliver naturally and
rear pups to weaning.
In males treated with 30 mg/kg, weight gain was lower and 4
animals died or were killed moribund. Toxic signs were piloerection
and bloody nasal discharge at 30 mg/kg and dried blood around the nose
at 10 mg/kg. The ability to impregnate females was unaffected, despite
the finding in the 30 mg/kg group of reduced testicular size, together
with focal testicular hypoplasia in 8/10 rats. In the 10 mg/kg group,
there were a few hypoplastic seminiferous ducts reported in 4 of the
5 rats examined.
Uterine examinations on gestation day 13 showed fewer
implantations (not significant) at 30 mg/kg with no effect on
resorptions. In females allowed to deliver, weight gain was lower
during gestation in 30 mg/kg dams, probably reflecting the reduced
litter size and weight. Postnatal growth, physical and behavioral
development were unremarkable. The NOEL was l mg/kg bw/day. (Boutemy,
1980).
2.2.5 Special studies on genotoxicity
Table 3: Results of genotoxicity assays on albendazole
Test system Test object Concentration Result Reference
Ames test (1) S.typhimurium 1-10,000 µg/ Negative Jagannath,
TA1535, TA1537 plate 1980a
TA1538, TA98,
TA100
Ames test (2) As above As above Negative Jagannath,
1980b
Ames test (2, 3) As above As above Negative Jagannath,
1981
Ames test (1) S.typhimurium 0.5-1000 µg/ Negative Mourot,
TA97a, TA98, plate 1988
TA100, TA102
CHO chromosome Chinese hamster 0.047-1.5µg/ml Negative Galloway,
aberration ovary cells 1981
assay
Transformation BALB/3T3 mouse 10-100 µg/ml Negative Schechtman,
assay cells 1982
(1)With and without rat liver S-9 fraction
(2)With and without calf and rat liver S-9 fraction
(3)Substance tested was 2-amino sulphone metabolite.
2.2.6 Special studies on eye and skin irritation
Groups of rabbits had 100 mg albendazole powder instilled into
the conjunctival sac or 500 mg albendazole applied, under occlusion,
to abraded and non-abraded skin. There were no primary irritant
effects at any site (Macko et al., 1975b).
2.2.7 Special studies on teratogenicity
2.2.7.1 Mice
Groups of 21 to 26 pregnant Charles River CD-1 mice were given
gavage doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in 0.5%
methylcellulose. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 18.
There was no overt maternal toxicity or effect on resorption
incidence, fetal weight and external, visceral and skeletal
development of fetuses (Killeen & Rapp, 1975c).
2.2.7.2 Rats
Groups of 25 pregnant Charles River CD rats were given gavage
doses of 0, 5, 20 or 40 mg/kg bw/d albendazole in 0.5%
methylcellulose. In each group 19 rats were treated on gestation days
16 to 20 and 6 were treated from gestation day 16 to lactation day 20.
All dams were allowed to litter naturally.
There was no maternal toxicity or effect on gestation or
parturition. At 40 mg/kg, litter size and weight were reduced at birth
and remained depressed during lactation. A total of 6, 17, 4 and 55
pups died in the 0, 5, 20 and 40 mg/kg groups, respectively. Small
lungs and kidneys and anasarca in the 40 mg/kg pups were possibly
related to treatment, but an unequivocal conclusion was not possible
in view of the low number of control pups examined. The authors
concluded that developmental and behavioral characteristics were
unrelated to albendazole treatment, but detailed supporting data were
not provided. The NOEL was 20 mg/kg bw/day. (Johnson, 1981).
A series of studies was carried out at Biodynamics in Long Evans
rats. A similar protocol was used in each study, which included dosing
on gestation days 6 to 15; sacrifice of dams on gestation day 20;
maternal observations for overt toxicity, weight gain and uterine
parameters; fetal examination for size, weight, and external, visceral
and skeletal abnormalities.
Study A Groups of 20 pregnant rats were given gavage doses of 0, 2,
5, 10 or 30 mg/kg bw/d albendazole in 0.5% methylcellulose. Maternal
weight gain and survival were lower at 30 mg/kg. At this dose, there
was a high incidence of resorptions, surviving fetuses showed reduced
size, weight and multiple gross, visceral and skeletal malformations.
Limb abnormalities such as micromelia, ectromelia, curved femur and
microfetalis were also seen in other treated groups. However, the
incidences were low, they were not dose-related and they were observed
only in 1 or 2 litters per group (Killeen & Rapp, 1975e; Christian,
1984, 1987a.).
Study B Groups of 19-20 pregnant rats were given gavage doses of 0,
0.5, 2, 5 or 10 mg/kg bw/d albendazole in 0.5% methylcellulose.
Fetuses of the 10 mg/kg group revealed reduced size and weight,
retarded skeletal ossification and increased incidences of micromelia
and microfetalis (which included shortened long bones in fore and hind
limbs) (Killeen & Rapp, 1976; Christian, 1984, 1987a).
Study C Groups of 30 to 60 pregnant rats were fed diets containing
29% freeze dried liver, obtained from cattle 48 hours after a single
oral dose of 0 or 27.5 mg/kg bw albendazole. Drug intake was
calculated to represent 0.42 mg/kg bw/d albendazole equivalents. The
only possible treatment-related observation was shortened limb bones
in 2/248 treated fetuses from 2 different litters. These effects were
not seen in 460 controls and the authors noted that they were an
infrequent finding at Biodynamics (Hogan & Rinehart, 1977).
A re-evaluation of these affected fetuses failed to confirm the
above description. The apparent defects were described as artifacts
caused by poor staining (Christian, 1987b).
Study D Groups of 20 to 22 pregnant rats were fed diets containing
10, 20 or 30% freeze dried liver, obtained from cattle 12 days after
a single oral dose of 0 or 16.5 mg/kg bw albendazole. Drug intake was
calculated to be approximately 0.02, 0.04 and 0.06 mg/kg bw/d
albendazole equivalents. In the group given 30% exposed liver,
resorptions were increased, 9 of which were in 1 female. If data from
this rat were eliminated there were no significant effects. The
overall NOEL for studies A to D was 5 mg/kg bw/day. (Schroeder &
Rinehart, 1978).
Groups of pregnant Sprague Dawley rats were given gavage doses of
0, 5.3, 6.0, 6.62, 8.83, 10.6 or 13.25 mg/kg bw/d albendazole or 9 of
its animal metabolites at equimolar or higher doses. Treatments were
on gestation days 8 to 15 and dams were killed on gestation day 21.
The results were presented in summary form only.
Skeletal abnormalities were increased at dose levels of 6.62
mg/kg and greater with increases in resorptions and external
malformations and decreased fetal weight at 8.83 mg/kg albendazole and
above. The major malformations were craniofacial and bone defects.
Qualitatively similar findings were obtained with equimolar amounts of
albendazole sulfoxide while the other metabolites A, B, E, F, G, J, I
and H (section 2.1) were all ineffective. The concomitant
administration of SKF-525-A, a microsomal oxidation inhibitor, almost
completely suppressed the embryotoxic and developmental effects of
albendazole. The NOEL was 6 mg/kg bw/day. (Martin, 1980).
Groups of pregnant Sprague Dawley rats were given diets
containing albendazole or 40% freeze dried liver, obtained from cattle
24, 48 or 96 hours after a single oral dose of 0 or 20 mg/kg bw
albendazole. Drug levels in food were adjusted to provide 0, 12, 24 or
36 mg/kg bw/d but doses given through incorporation of liver could not
be estimated. Treatment of rats was on gestation days 8 to 15 and dams
were killed on gestation day 21. Albendazole doses of 24 and 36 mg/kg
produced virtually 100% embryolethality, the single live fetus was
small and had skeletal abnormalities. There were no effects at other
exposure levels. The NOEL was 12 mg/kg bw/day. (Grannec, 1980).
2.2.7.3 Rabbits
Groups of 15 pregnant New Zealand White rabbits were given gavage
doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in methylcellulose.
Treatment was on gestation days 7 to 19 and does were killed on
gestation day 30.
Maternal mortality was increased at 30 mg/kg but body weight
comparisons were not meaningful due to wide variation within groups.
The 30mg/kg group showed a reduction in implants, due largely to 2
does with corpora lutea but no implants, and increases in resorptions
and ectrodactyly. Fetal size and weight were depressed at 10 and 30
mg/kg. The NOEL was 5 mg/kg bw/day. (Killeen & Rapp, 1975d).
2.2.7.4 Sheep
In two separate experiments, groups of 15 to 44 Dorset Horn Cross
and Clun mated ewes were given a single dose of 0, 7.5, 10, 15 or 20
mg/kg bw albendazole by oral drench. Overall, there were 71, 43, 44,
43 and 42 animals in the 0, 7.5, 10, 15 and 20 mg/kg groups,
respectively. Treatment was on gestation day 17 and ewes were allowed
to deliver naturally.
There was no overt maternal toxicity but premature delivery was
noted in more ewes of the 20 mg/kg group than in the others. All
premature lambs in this group were stillborn, consequently the number
of live lambs was reduced at 20 mg/kg, as was survival of the lambs to
post partum day 55. Some lambs were sacrificed in extremis or because
they were considered commercially unviable, thus the total post partum
loss was 22/123, 4/67, 11/73, 12/73 and 39/61 in the 0, 7.5, 10, 15
and 20 mg/kg groups, respectively. Post-mortem examination of these
lambs revealed increased incidences of prognathia, scoliosis, spina
bifida and reduced tail at 20 mg/kg and displaced, poorly developed or
absent kidneys at 15 and 20 mg/kg. The NOEL was 10 mg/kg bw/d. (Tesh
& Harper, 1977).
The data shown in Table 4 indicates a relationship between
teratogenesis and peak plasma concentration of albendaxole sulfoxide.
Data from Bogan & Marriner (1984).
Table 4. Relationship between plasma albendazole sulfoxide
and teratogenesis
Species Albendazole Dose Peak Plasma Teratogenic
(mg/kg bw) Albendazole
(mg/ml)
Sheep 10 2.50 Yes
Cattle 10 0.57 No(1)
Rabbit 30 8.82 Yes
Rat 10 6.6 Yes
Mouse 30 n.a.(3) No
Man 400 mg/person 0.16 No(2)
(1) Embryotoxic but not teratogenic
(2) No intentional studies performed
(3) Not available
2.2.8 Special studies on mode of action of benzimadoles
Benzimadazoles bind with tubulin and inhibit its polymertisation
to microtubules. A comparison of inhibitory concentrations for some
benzimadazoles in nematodes and mammalian cells reveals a selective
action (reviewed in Sharma & Abuzar, 1983).
2.3 Observations in man
Albendazole has been used in 80 countries for 6 years to treat
gastrointestinal parasitic infections in man at a recommended dose
rate of 400 mg/person. There are a number of published reports on the
use of albendazole in man (see Marriner et al., 1986; Lange et al.,
1988).
In field trials in Nigeria, it was reported that 17 nulliparous
women aged between 16 and 18 years were inadvertently given a single
dose of 400 mg albendazole in the first trimester of pregnancy without
any adverse effects on the mother or child (A.B.C. Nwosu, 1985).
3. COMMENTS
Comprehensive toxicological data on albendazole were submitted
including the results of studies on its metabolism, carcinogenicity,
genotoxicity, effects on reproduction and teratogenicity, and
short-term studies.
Pharmacokinetic studies, although not specifically designed to
measure the extent of absorption, suggested that about 20-30% of
ingested albendazole was absorbed in mice and rats, about 1% in humans
and 50% in cattle. In all the species studied, oral dosing produced
very low plasma levels of unchanged drug, because of rapid first-pass
metabolism in the liver. The primary metabolic reactions were the
oxidation of the sulfide moiety of albendazole to the sulfoxide and
sulfone, followed by cleavage of the carbamate moiety to form the
2-amino-sulfone. This last compound was found to be the main residue
in the livers of sheep and cattle. The degradation of albendazole
followed similar pathways in rats, mice, cattle, sheep and humans.
In a study in mice in which albendazole was administered in the
diet for 25 months, anemia, leukopenia and testicular degeneration
were noted at 400 mg/kg bw/day. Hepatocellular vacuolation was
produced at 100 and 400 mg/kg bw/day, endometrial stromal polyps was
slightly higher than in concurrent controls, but statistical
significance was not achieved and all incidences were within the
laboratory historical control range. The NOEL was 25 mg/kg bw/day.
In a 28-month study in the rat in which the compound was given
in the diet, the highest dose of 20 mg/kg bw/day caused mortality,
neutropenia, hypercholesterolemia, testicular degeneration and
hepatic fatty metamorphosis. The incidence of endometrial/cervical
tumors and histiocytic carcomas in the skin showed an apparent
increase in certain treated groups. However, when compared with
those for the controls, these findings were not statistically
significantly different and were within the laboratory historical
control range. The NOEL was 7 mg/kg bw/day.
Questions had been raised regarding the statistical analyses of
the mouse and rat carcinogenicity studies and the use of historical
controls in the examination of the tumor incidence. The studies were
reviewed and were determined to be satisfactory. The statistical
analysis of both rodent carcinogenicity studies was also reviewed and
found to have been conducted in accordance with currently acceptable
procedures.
Albendazole did not produce bacterial mutations, chromosomal
aberrations or morphological transformations in cultured mammalian
cells. The 2-aminosulphone metabolite did not produce bacterial
mutations.
A three-generation reproduction study in which the compound was
administered in the diet was conducted in rats. There were no effects
on fertility or reproduction indices. The only findings were
reductions in pup postnatal survival and growth at 11.6 mg/kg bw/day.
The NOEL was 5.8 mg/kg bw/day.
Male rats were treated by gavage in a fertility study. Overt
toxic effects and testicular hypoplasia were noted at 10 and 30 mg/kg
bw/day. Fertility indices were not affected. Litter size and weight
were reduced at 30 mg/kg bw/day, the former effect being probably due
to a lower implantation rate. The NOEL was 1 mg/kg bw/day.
In a perinatal and postnatal study in rats in which albendazole
was administered by gavage, there was decreased survival and growth
in utero and during lactation in the pups of females given 40 mg/kg
bw/day group. There was also some evidence of retarded organ
development in the offspring of this group. The NOEL was 20 mg/kg
bw/day.
In a teratology study in mice in which albendazole was
administered by gavage, there were no adverse effects at dose levels
up to 30 mg/kg bw/day.
Several teratology studies were conducted in the rat in which the
compound was administered either by gavage or by dietary exposure.
Embryotoxic, fetotoxic effects and external malformations were
produced at doses of 8.8 mg/kg bw/day and above. Skeletal
malformations, in particular limb defects, which were seen at doses of
6.62 mg/kg bw/day, constituted the most sensitive indicator of
developmental toxicity. The NOEL was 5 mg/kg bw/day. When administered
in equimolar amounts, qualitatively similar findings were obtained
with albendazole sulphoxide, while eight other metabolites of
albendazole did not show any effects.
In rabbits, the toxic dose to the dams of 30 mg/kg bw/day was
associated with embryotoxicity and partially or totally missing
digits. Fetal growth retardation was observed at doses of 10 mg/kg
bw/day and above. The NOEL was 5 mg/kg bw/day.
A teratology study was conducted in sheep using a single oral
dose of albendazole on day 17 of pregnancy. Premature delivery,
fetotoxic effects and postnatal death were produced at 20 mg/kg bw and
malformations were increased at 15 and 20 mg/kg. The NOEL was 10 mg/kg
bw/day.
Dogs were dosed for six months by the administration of the
compound in capsules. They showed neutropenia at 30 mg/kg bw/day and
above. At 60 mg/kg bw/day there was also anemia, decreased body,
testicular and uterine weights, and bone marrow hypocellularity. The
NOEL in this study was 5 mg/kg bw/day.
Albendazole has been used in 80 countries to treat
gastrointestinal parasitic infections in humans at a dose rate of 400
mg/person, and there are a number of published reports on its use. In
field trials in Nigeria, an unpublished report noted that 17
nulliparous women aged between 16 and 18 years were inadvertently
given a single dose of 400 mg albendazole during the first trimester
of pregnancy without any apparent adverse effects on the mother or the
neonate.
Some of the effects observed in general toxicity studies with
albendazole may be explained by one of the biological actions of the
benzimadazoles, which is to interfere with tubulin polymerization and
thus inhibit spindle formation and mitosis.
The most significant toxicological manifestation resulting from
treatment with albendazole was its teratogenic activity, limb defects
in the rat being the most sensitive indicator of developmental
toxicity.
A NOEL of 5 mg/kg bw/day was reported in several studies in rats,
rabbits and dogs. Although for males in the rat fertility study the
figure was 1 mg/kg bw/day, it was noted that the next highest dose
used in the study was 10 mg/kg bw/day. In addition, in the
multigeneration reproduction study in rats, there was no effect on
fertility at 11.6 mg/kg bw/day, which was the highest dose used, and
the reported NOEL was 5.8 mg/kg bw/day, based on depression of weight
gain in pups.
An ADI of 0-0.05 mg/kg bw was established for albendazole based
on a NOEL of 5 mg/kg bw/day and using a safety factor of 100.
A safety factor of 100 was chosen for this compound after taking
into consideration poor absorption in humans, rapid metabolism, the
lack of teratogenic potential of most metabolites, the use of the drug
in humans and the identity of residues in food.
4. EVALUATION
Level causing no toxicological effect
Rat, rabbit, dog equal to 5 mg/kg bw/day.
Estimate of acceptable daily intake
0-0.05 mg/kg bw.
5. REFERENCES
BOGAN, J.A. & MARRINER, S.E. (1984). Pharmacodynamic and toxicological
aspects of albendazole in man and animals. In: Fock, M. (Ed.).
Albendazole in helminthiasis. Royal Society of Medicine International
Congress and Symposium Series No. 61. London, Royal Society of
Medicine, pp. 13-21.
BOUTEMY, C. (1980). Expert study of the action of the compound
albendazole on the fertility of the male rat, per os. Unpublished
study No. 2477 RSR from IFM Research Center. Submitted to WHO by
SmithKline and French.
BRAUN, W.G. & KILLEEN, J.C. (1975). Acute oral toxicity in rats,
compound No. SK&F 62979. Unpublished project No. 2605-75 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1984). Review of albendazole rat teratogenicity
studies. Unpublished report from Argus International. Submitted to WHO
by SmithKline and French.
CHRISTIAN, M.S. (1987a). Review of reported "micropodia" in
albendazole rat teratogenicity studies. Unpublished report from Argus
International. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1987b). Review of developmental toxicity
(embryo-fetal toxicity/teratogenicity) and reproductive toxicity
studies of albendazole in mice, rabbits and rats. Unpublished report
from Argus International. Submitted to WHO by SmithKline and French.
COLMAN, W., TOWNER, C., TOWNER, D. & SOKOLEK, J. (1977). Metabolic
fate of albendazole in sheep tissues. Unpublished report from
Applebrook Research Centre. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1980). A six-month oral toxicity study with
albendaxole in dogs. Unpublished project No. 790-2371 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1981). A toxicity study of albendazole in
rats. Unpublished project No. 80-2449 from Biodynamics Incorporated.
Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1982). A long-term oral dietary toxicity
carcinogenciity study of albendazole in rats. Unpublished project No.
79-2383 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987a). A long-term oral (dietary)
carcinogenicity study of albendazole in mice. Unpublished project No.
80-2480 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987b) A long-term oral
toxicity/carcinogenicity study of albendazole in rats. Unpublished
project No. 80/2448 from Biodynamics Incorporated. Submitted to WHO by
SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980a). A three month dose range finding
study with albendazole in mice. Unpublished project No. 79-2368 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980b). A three month feeding study of
albendazole in mice. Unpublished project No. 79-2423 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DELATOUR, P., GARNIER, F., BENOIT, E. & LONGIN, Ch. (1984). A
correlation of toxicity of albendazole and oxfendazole with their free
metabolites and bound residues. J.Vet.Pharmacol.Therap., 7, 139-145.
GALLOWAY, S.M. (1981). Mutagenicity evaluation of albendazole (SK&F
62979) in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished project No. 22000 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
GRANNEC, C. (1980). Albendazole: relay embryotoxicity. Thesis for
D.Vet.Sci. Claude Bernard University, Lyons. Submitted to WHO by
SmithKline and French.
GYURIK, R.J. et al., (1981). Metabolism of albendazole in cattle,
sheep, rats and mice. Drug Metabolism and Disposition 9, 503.
HOGAN, G.R. & RINEHART, W.E. (1977). A segment II teratologic relay
toxicity study in rats of liver obtained from calves treated with
albendazole. Unpublished project No. 76-1443A from Biodynamics
incorporated. Submitted to WHO by SmithKline and French.
JAGANNATH, D.R. (1980a). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome plate test. Unpublished
project No. 20988 from Litton Bionetics. Submitted to WHO by
SmithKline and French.
JAGANNATH, D.R. (1980b). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome preincubation plate test.
Unpublished project No. 20998 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
JAGANNATH, D.R. (1981). Mutagenicity evaluation of SK&F 81038 in the
Ames Salmonella/microsome plate test. Unpublished project No. 20988
from Litton Bionetics. Submitted to WHO by SmithKline and French.
JOHNSON, D.E. (1981). Perinatal and postnatal study in rats.
Unpublished report No. 483-001 from International Research and
Development Corporation. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975a). A three month oral toxicity study
of SK&F 62979 in rats. Unpublished project No. 75-1109 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. 6 RAPP, W.R. (1975b). A three month oral toxicity study
of SK&F 62979 in dogs. Unpublished project No. 75-1110 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975c). A segment II mouse teratology
study of SK&F 62979. Unpublished project No. 74-1096 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975d). A teratology study of SK&F 62979
in rabbits. Unpublished project No. 75-11234 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP. W.R. (1975e). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 74-1947 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1976). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 75-1274 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KRAEER, P., TOWNER, D. & SOKOLEK, J. (1977). Metabolic fate of
albendazole in cattle tissues. Unpublished report from Applebrook
Research Center. Submitted to WHO by SmithKline and French.
LANGE, H., EGGERS, R. & BIRCHER, J. (1988). Increased systemic
availability of albendazole when taken with a fatty meal.
Eur.J.Clin.Pharmacol., 34, 315-317.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975a). Local
irritation, skin sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975b). Local
irritation, skin, sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MARRINER, S.E. & BOGAN, J.A. (1980). Pharmacokinetics of albendazole
in sheep. Am.J.Vet.Res., 41, 1126-1129.
MARRINER, S.E., MORRIS, D.L., DICKSON, B. & BOGAN, J.A. (1986).
Pharmacokinetics of albendazole in man. Eur.J.Clin.Pharmacol., 30,
705-708.
MARTIN, D. (1980). Albendazole: embryotoxic study of ten metabolites.
Thesis for D.Vet.Sci. Claude Bernard University, Lyons. Submitted to
WHO by SmithKline and French.
MOUROT, D. (1988). Albendazole: Ames test with and without metabolic
activation. Unpublished report from Laboratoire National des
Médicaments Vétérinaires. Submitted to WHO by SmithKline and French.
NWOSU, A.B.C. (1985). Albendazole field trials in Nigeria. Unpublished
report submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977a). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W. & GYURIK, R.J. (1977b). The isolation and
characterization of radioactive metabolites from the urine of steers
treated orally with 14°C; labelled SK&F 62979 (Albendazole).
Unpublished report from Applebrook Research Center. Submitted to WHO
by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977c). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C. & GYURIK, R. (1979). Urinary excretion and identification
of metabolites from urine of Sprague-Dawley rats orally dosed with
albendazole - 14°C albendazole sulfoxide - 14°C and albendazole
sulfone - 14°C. Unpublished report No. A-4003-78 from Applebrook
Research Centre. Submitted to WHO by SmithKline and French.
PARISH, R.C., GYURIK, R. & BRUNER, E. (1979a). The isolation and
quantitation of metabolites from urine of Charles River mice treated
orally with 14°C-labelled SK&F 62979 (Albendazole). Unpublished report
No. A-4006-78 from Applebrook Research Center. Submitted to WHO by
SmithKline and French.
PARISH, R.C., GYURIK, R.J. & BRUNER, E. (1979b). The isolation and
quantitation of radioactive metabolites from the urine of steers
treated orally with 14°C-labelled SK&F 62979. Unpublished report from
Applebrook Research Center. Submitted to WHO by SmithKline and French.
PRICHARD, R.K., HENNESSY, D.R., STEEL, J.W. & LACEY, E. (1985).
Metabolite concentrations in plasma following treatment of cattle with
five anthelmintics. Res.Vet.Sci., 39, 173-178.
ROSSIGNOL, J.F. & MAISONNEUVE, H. (1984). Albendazole: a new concept
in the control of intestinal helminthiasis. Gastroenterol.Clin.Biol.,
8, 569-576.
SAUER, R.M. (1985). Pathology Working Group report on albendazole in
Sprague-Dawley rats and CD-1 mice. Unpublished report from Pathco
Incorporated. Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987a). Pathology Working Group report on albendazole in
Sprague-Dawley rats. Unpublished report from Pathco Incorporated.
Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987b). Pathology Working Group report on albendazole in
CD-1 mice. Unpublished report from Pathco Incorporated. Submitted to
WHO by SmithKline and French.
SCHECHTMAN, L.M. (1982). Activity of T1709 in the in vitro mammalian
cell transformation assay in the presence of exogenous metabolic
activation. Unpublished project No. T1709.109 from Microbiolobical
Associates. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E., & RINEHART, W.E. (1978). A segment II teratologic
relay toxicity study in rats on liver obtained from cattle treated
with albendazole. Unpublished project No. 77-1825 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E. & RINEHART, W.E. (1980). A three-generation
reproduction study with albendazole in rats. Unpublished project No.
77-2019 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
SELWYN, M.R. (1987). Statistical analysis for oral carcinogenicity
studies of albendazole in mice and rats. Unpublished study from
Statistics Unlimited Inc. Submitted to WHO by SmithKline and French.
SHARMA, S. & ABUZAR, S. (1983). The benzimadozole anthelmintics -
chemistry and biological activity. Prog. Drug Res., 27, 85-161.
SIMON, J.C. (1979a). 4-week sub-acute oral toxicity study in rats.
Unpublished study No. 2206 TSR from IFM Research Center. Submitted to
WHO by SmithKline and French.
SIMON, J.C. (1979b). Sub-acute toxicity study in dogs. Unpublished
study No. 2204 TSC from IFM Research Center. Submitted to WHO by
SmithKline and French.
SOUHAILI-EL AMRI, H., FARGETTON, X., BENOIT, E., TOTIS, M. & BATT,
A-M. (1988). Inducing effect of albendazole on rat liver
drug-metabolising enzymes and metabolite pharmacokinetics.
Toxicol.App.Pharmacol., 92, 141-149.
TESH, J.M. & HARPER, J.A. (1977). Albendazole (SK&F 62979): effects of
single oral administration upon pregnancy in sheep. Unpublished report
No. 77/SAF210/205 from Life Science Research. Submitted to WHO by
SmithKline and French.
WALKER, T.F. (1976a). The acute oral toxicity of SK&F 62979
(Albendazole) in the rat. Unpublished report No. B 335A from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976b). The acute oral toxicity of SK&F 62979
(Albendazole) in the hamster. Unpublished report No. B 335C from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976c). The acute oral toxicity of SK&F 62979
(Albendazole) in the guinea pig. Unpublished report No. B 335B from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976d). The acute oral toxicity of SK&F 62979
(Albendazole) in the rabbit. Unpublished report No. B 335D from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
Sheep bearing permanent ruminal and abomasal cannulae were given
a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation.
Albendazole was absorbed unchanged from the rumen. Once in the body it
was rapidly degraded, and sulfone metabolites were detected in plasma,
the former achieving the greater level. All 3 compounds were present
in the abomasum. Presumably albendazole was passed through the
stomachs while the metabolites were secreted or diffused into this
organ. Non-detectable levels of all 3 compounds were reached in plasma
and rumen at 96 h and in abomasum at 120 h (Marriner & Bogan, 1980).
Sheep were given a single oral dose of 10 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1, 2, 4, 6 or 8 days after
treatment. On day 1, the label in liver was mainly in the form of the
sulfoxide and sulfone metabolites. These compounds were progressively
converted to the 2-amino sulfone which was the primary residue on day
8. Low levels of albendazole were detected up to day 2; other
degradation products were metabolites E, G and J. Over 72 h, pooled
urine samples, the sulfoxide and 2-amino sulfone accounted for 60-70%
of the urinary radioactivity. Low levels of albendazole, the sulfone
and 6 other metabolites were also found (Parish et al., 1977a;
Colman et al., 1977).
Steers were given a single oral dose of 7.5 mg/kg bw of an
albendazole formulation. Albendazole could not be detected in plasma.
Metabolism to the sulfoxide and sulfone was rapid; these compounds
were present in plasma for up to 40 h (Prichard et al., 1985).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1 to 12 days after
treatment. In liver obtained on day l, the radioactivity was mainly as
albendazole, the sulfoxide and sulfone. Albendazole disappeared by day
6 while the sulfoxide and sulfone were progressively converted to
2-amino sulfone through day 12. Low levels of metabolites G and J were
also found. A limited examination of kidney tissue revealed a similar
metabolic profile (Kraeer et al., 1977).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Over a 7 day period, 47% of the administered
radioactivity was recovered in the urine. The sulfoxide, sulfone and
2-amino sulfone accounted for 70% of this radiolabel. Low levels of
albendazole, metabolites B, D, E, G, H and J were also found (Parish
et al., 1977b; 1979b).
Human volunteers were given a single oral 400 mg dose of
albendazole. The parent sulfide was not detected in blood by HPLC.
Peak sulfoxide concentrations were achieved after 2.4 h and decayed
biphasically with a long terminal half-life. Analysis of 24 h urine by
TLC revealed the presence of the sulfoxide, the sulfone and their
amino derivatives and metabolites B, E and G (Rossingnol &
Maisonneuve, 1984).
The above findings were confirmed in other similar studies.
Additionally, it was demonstrated that urinary recovery over a 24 hour
period accounted for up to 0.88% of the dose. Limited information
suggested biliary excretion was very low. Thus it appears that the
oral absorption of the albendazole is somewhere around 1%. When
ingested with a high fat content meal the absorption of albendazole
was shown to be 4.6 times higher, on average, in 6 volunteers
(Marriner et al., 1986; Lange et al., 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Findings in dead rats included urinary staining of abdomen, bloody
discharge around nose, chromodacryorrhea and intestinal hemorrhage.
Necropsy of dead rabbits showed intestines containing fluid and
dilated with gas. Toxic signs in other species were not reported.
2.2.2 Short-term studies
2.2.2.1 Mice
In 2 separate experiments, groups of 10 male and 10 female
Charles River CD1 mice were fed diets containing albendazole for 90
days. Drug levels in food were adjusted so that animals received
doses of 0, 5, 10, 20, 40 or 80 mg/kg bw/d in study 1 and 0, 200, 400,
800 or 1600 mg/kg bw/d in study 2.
All females and 5/10 males of the 1600 mg/kg group died
spontaneously or were killed in a moribund condition. From week 9, ear
lesions involving thickening and/or encrustation of the tips were
observed in 2/10 males and 2/9 females at 800 mg/kg and 5/5 males in
the 1600 mg/kg group. Food consumption was generally decreased in
males given 400 mg/kg or more but weight gain was depressed only at
1600 mg/kg.
Hematology was measured at the end of the study. Hemoglobin,
hematocrit and erythrocyte levels were reduced at 800 mg/kg or more
and a decreased leucocyte counts was observed in males of these
groups. Gross post mortem examinations revealed increases in absolute
and relative liver weights from 40 mg/kg and greater in males and 80
mg/kg and greater in females (Daly & Rinehart, 1980a, 1980b).
2.2.2.2 Rats
Groups of 15 to 20 male and female Charles River Sprague Dawley
rats were given gavage doses of 0, 4, 25, 48 or 168 mg/kg bw/d of
albendazole in 0.5% Tween 80 for 4 weeks.
Species Sex Vehicle LD50 (mg/kg bw) Reference
Mouse M 0.75% methocel over 3000 Macko et al.,
1975a
Rat M&F 2% tragacanth 1320 Walker, 1976a
Rat M&F 1% methocel 2400 Braun & Killeen,
1975
Hamster M&F 2% tragacanth over 10000 Walker, 1976b
Guinea-pig M&F 2% tragacanth 900 Walker, 1976c
Rabbit M&F 2% tragacanth 500-1250 Walker, 1976d
Toxic signs were produced in the 2 highest dose groups which
included diarrhea, piloerection, nasal swelling with blood stained
nasal discharge and death (7/30 rats at 48 mg/kg and 39/40 rats at 168
mg/kg died). Body weight gain was depressed at 48 mg/kg with weight
loss at 168 mg/kg while food consumption was markedly reduced at 168
mg/kg and slightly reduced at 48 mg/kg.
Hematology, blood chemistry and urinalysis were measured after 1
and 4 weeks of treatment, except in the high dose rats which exhibited
marked overt toxicity. Hemoglobin, hematocrit, erythrocyte and
leucocyte counts were reduced at 48 mg/kg.
At autopsy, adrenal size was increased at 48 and 168 mg/kg,
particularly in females. Testes were soft with reduced size and weight
in 48 mg/kg males which survived 4 weeks. Testes size was not affected
at the highest dose presumably due to early death of these males.
Histopathology examination revealed hypoplasia in testes, bone marrow,
spleen and lymph nodes in 48 and 168 mg/kg groups.
An additional group of 5 males and 5 females was given 48 mg/kg
bw/d of albendazole for 4 weeks, followed by withdrawal of treatment
for 4 weeks. All drug-related changes were reduced in severity during
the latter period indicating that the effects were reversible (Simon,
1979a).
Groups of 20 male and 20 female Long Evans rats were fed diets
containing albendazole for 91 days. Drug levels in food were adjusted
so that animals received doses of 0, 2, 10 or 30 mg/kg bw/d. The
control and high dose groups included an additional 10 males and 10
females for laboratory studies.
There were no signs of toxicity and no effects on body weight,
food consumption and ophthalmology parameters. Hematology, blood
chemistry and urinalysis were studied after 1 and 3 months of
treatment. Gross pathology and organ weights were examined in all rats
while histopathology was carried out on 15 males and 15 females from
control and high dose groups. Meaningful changes related to treatment
were not observed (Killeen & Rapp, 1975a).
Groups of 100 male and female Charles River CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then throughout mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 5, 30 or 45 mg/kg bw/day. The treatment was intended to be
for 2 years but excessive mortality necessitated termination of the
study after 26 weeks.
There were no untoward effects in F0 rats. In the F1 animals,
92/100 males and 99/100 females of the 45 mg/kg bw/day group died by
week 25. Prior to death these rats developed swollen cervical glands,
followed by swelling of paws and the genital area, scabbing in the
cervical area and emaciation (reduced weight gain and food intake).
Hematology was measured after 3 and 6 months of treatment.
Hemoglobin, hematocrit, erythocyte and leucocyte counts were decreased
and reticulocytes increased in 45 mg/kg rats at 3 months. At 6 months,
similar but slight hematological changes were seen in 30 mg/kg bw/day
rats. Segmented neutrophils were particularly affected and this was
confirmed by differential bone marrow counts in control and 30 mg/kg
rats. Blood chemistry and urinalysis were studied after 3 months of
treatment. In the 45 mg/kg bw/day group, plasma cholesterol was
increased in males and females, potassium was increased in males, and
albumin, plasma and erythrocyte cholinesterase were decreased in
females. Urinary protein was increased in 30 and 45 mg/kg bw/day
males.
Post mortems were carried out on all unscheduled deaths and
approximately 60% of animals surviving to 26 weeks. Numerous gross
alterations were noted in the high dose group including discoloration
and/or nodules in the lungs, heart, lymph nodes, spleen, pancreas,
liver, adrenal and kidney. Some of these organs were also enlarged or
showed adhesions. Additionally, thymic tissue was often absent and
testes were small and flaccid.
Histopathology, performed on selected tissues in 5 males and 5
females from 0, 30 and 45 mg/kg bw/day groups, identified a number of
instances where bacterial colonization in lungs, spleen, kidneys and
heart was associated with necrosis without the usual acute
inflammatory response. The liver showed centrilobular cloudy swelling,
vacuolation or necrosis and lymphoid tissues such as bone marrow,
spleen and thymus showed hypocellularity or other changes suggestive
of atrophy. All the above lesions were seen at 45 mg/kg while, at 30
mg/kg, only the thymic and minor hepatic effects were noted (Daly &
Hogan, 1982).
The remaining rats were used to further evaluate effects on
hematological parameters. Control and 5 mg/kg group animals were
continued on the same treatment level while rats from the 30 mg/kg
group were now given 0 or 20 mg/kg bw/d albendazole. Group sizes were
20 to 25 males and females and dosing was continued for 4 months.
Hematology was studied monthly. The 5 mg/kg rats were still
unaffected. The red and white cell effects induced at 30 mg/kg were
essentially normalized within a month of reducing the dose to 0 or 20
mg/kg. However, differential bone marrow counts at day 81 revealed a
persistent depression of the myeloid line in the 20 mg/kg group. Gross
post mortem examination of all animals showed no remarkable effects
(Daly & Hogan, 1981).
2.2.2.3 Dogs
Groups of 4 to 5 male and female beagle dogs were given gavage
doses of 0, 4, 16, 48 or 168 mg/kg bw/d of albendazole in 2% Tween 80
for 4 weeks. Toxic signs, in a few dogs at the 2 highest dose levels,
included diarrhea and vomiting with cardio-pulmonary disturbances in
1 dog. Death ensued in 1/10 dogs at 48 mg/kg and 6/10 dogs at 168
mg/kg, mainly in females. Heart rates showed marked increases in some
168 mg/kg females prior to death. Food intake was reduced at 48 mg/kg
and above and weight gain was depressed at 16 mg/kg and above.
Hematology, blood chemistry, urinalysis and ophthalmology were
studied after 1 and 4 weeks of treatment. Leucocyte counts were
decreased in a few dogs at 48 and 168 mg/kg and alkaline phosphatase
was increased from 16 mg/kg. Necropsy revealed lower absolute
testicular weight in 48 and 168 mg/kg males but no pathological
alterations (Simon, 1979b).
Groups of 4 male and female beagle dogs were given 0, 2, 10 or 39
mg/kg bw/d albendazole in capsules for 91 days. Ophthalmology,
hematology, blood chemistry and unrinalysis were studied after 1 and
3 months; organ weights, gross and histopathology were examined in all
dogs. There were no toxic signs or effects on body weight and food
intake. No treatment-related effects were found (Killeen & Rapp,
1975b).
Groups of 6 male and 6 female beagle dogs were given 0, 5, 30 or
60 mg/kg bw/d albendazole in capsules for 6 months.
Food intake was decreased in 30 and 60 mg/kg females, while
weight gain was lower in 60 mg/kg males and females. Laboratory
studies included hematology and blood chemistry monthly, urinalysis
bimonthly and ophthalmology at termination. Hemoglobin, hematocrit and
erythrocyte counts were slightly reduced at 60 mg/kg and leucocyte
counts, particularly neutrophils, were reduced at 30 and 60 mg/kg.
Autopsy on all dogs revealed decreased absolute and relative
testes and uterine weights and slight increases in relative liver and
kidney weights at 60 mg/kg. The incidences of small nodules in the
stomach were increased in all treated groups but microscopically they
were shown to be normal, submucosal lymphoid follicles. Sternal bone
marrow showed hypocellularity in 4/6 females in the 60 mg/kg group.
The NOEL was 5 mg/kg bw/day. (Daly & Hogan, 1980).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 100 male and 100 female Charles River CD-1 mice were
fed diets containing albendazole for 25 months. Drug levels were
adjusted to provide doses of 0, 25, 100 or 400 mg/kg bw/d. Additional
groups of 25 males and 25 females were given control and high dose
treatments and used for hematology measurements.
There were no toxic signs or effects on food intake and body
weight. Hematology was studied after 3, 6 , 12, 18 and 24 months in
the main groups and monthly in the ancillary groups. Erythrocyte and
leucocyte counts were decreased and platelets were increased at 400
mg/kg, particularly in females.
A complete gross post-mortem examination was carried out on all
mice. Full histopathology was undertaken on control and high dose
mice. In intermediate groups, 6 major organs and grossly abnormal
tissues were examined routinely. Flaccid or small testes, testicular
tubular degeneration, oligospermia, and aspermia in epididymides were
increased in 400 mg/kg males. Centrilobular hepatocytic vacuolation
was increased in groups given 100 and 400 mg/kg. Eye opacities were
noted in all groups; however, microscopically, cataracts were slightly
increased only in 400 mg/kg males. The relationship of these ocular
findings to treatment is questionable as the bulk of the cataracts
were unilateral and such abnormalities are commonly obtained following
repeated blood collection from the orbital sinus.
The incidence of endometrial stromal polyps appeared to be
increased over concurrent controls (Table 1) but a statistical
evaluation of the results did not reveal significant differences
between groups, and all incidences were within the historical control
range for this laboratory based on 2 studies with 2 control groups in
each. The NOEL was 25 mg/kg bw/d. (Daly & Knezevich, 1987a; Sauer,
1985, 1987b; Selwyn 1987).
Table 1: Tumor incidence in mice
Dose (mg/kg bw/d) 0 0 25 100 400 Historical Controls
Endometrial stromal tumors
Polyps 3/98 5/99 3/98 5/98 7/99 Range 0/55-8/47
Sarcomas 0/98 0/99 1/98 2/98 0/99 Cumulative 29/780
Total 3/98 5/99 4/98 7/98 7/99
2.2.3.2 Rats
Groups of 100 male and 100 female Sprague Dawley CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then through mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 3.5, 7 or 20 mg/kg bw/d for 28 months. Additional groups
of 25 males and 25 females were given control and high dose treatments
and used for hematology measurements. An interim sacrifice of 10 males
and females per group was made after 12 months.
Treatment-related effects were not observed in F0 animals. In the
F1 animals, mortality was slightly increased after 24 months in 20
mg/kg males. There were no other toxic signs or effects on body weight
and food consumption. Ophthalmology, hematology, blood chemistry and
urinalysis parameters were studied after 3, 6, 12, 18 and 24 months of
treatment. In the 20 mg/kg group, total leucocyte and neutrophil
counts were decreased at 24 months, serum cholesterol was increased in
females throughout the study and in males at some sampling times.
A complete gross post-mortem examination was carried out on all
rats. Full histopathology was undertaken on control and high dose
rats. In intermediate groups, 8 major organs and grossly abnormal
tissue were examined routinely. The 20 mg/kg animals showed increased
incidences of flaccid testes, degeneration/atrophy of germinal and
relative liver weights in males and hepatic fatty metamorphosis in
males and females.
Compared to concurrent controls, the incidences of
endometrial/cervical tumors and skin histiocytic sarcomas showed an
apparent increase in certain groups of rats (see Table 2 below).
Statistical evaluation of the findings did not reveal significant
differences between groups and in fact all frequencies were within the
historical control range for this laboratory. The NOEL was 7 mg/kg/d.
(Daly & Knezevich, 1987b; Sauer, 1985, 1987a; Selwyn, 1987).
Table 2: Tumor incidence in rats
Dose (mg/kg/d) 0 0 3.5 7 20 Historical Controls
Endometrial/cervical tumors
Polyps 3/99 5/99 9/98 9/99 10/91 Range 1/69-7/58*
Sarcomas 0/99 3/99 0/98 4/99 3/91 Cumulative 120/1864
Total 3/99 8/99 9/98 11/99 13/91
Skin histiocytic sarcomas
Males 1/100 2/100 4/98 4/100 6/100 Range 0/116-6/110**
Cumulative 32/1190
Females 0/100 4/100 0/100 1/96 5/100 Range 1/112-11/119
Cumulative 40/1188
* based on 18 studies
** based on 14 studies.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Long Evans rats were fed diets containing 0, 30, 75 and
150 ppm albendazole for 3 successive generations, commencing 64 d
before the initial mating. Each parental group consisted of 12 males
and 24 females, which were bred to produce 2 litters each. Offspring
from the second litters were selected to serve as parents of the
subsequent generations. Drug intake was calculated to be, on average,
2.3, 5.8 and 11.6 mg/kg bw/d.
There were no toxic signs or effects on body weight, food
consumption, mating, fertility, pregnancy rates, gestation length,
litter size and weight. During lactation, pup survival and/or weight
gain was depressed, but only in F1a and F2a litters given 11.6 mg/kg.
The NOEL was 5.8 mg/kg bw/d. (Schroeder & Rinehart, 1980).
Groups of 20 male Sprague Dawley CD rats were given gavage doses
of 0, 1, 10 or 30 mg/kg bw/d albendozale in 0.5% gum tragacanth, from
60 days prior to mating to the end of the breeding period. Males were
mated 1 to 1 with untreated females. Half the dams were killed on
gestation day 13, the remainder were allowed to deliver naturally and
rear pups to weaning.
In males treated with 30 mg/kg, weight gain was lower and 4
animals died or were killed moribund. Toxic signs were piloerection
and bloody nasal discharge at 30 mg/kg and dried blood around the nose
at 10 mg/kg. The ability to impregnate females was unaffected, despite
the finding in the 30 mg/kg group of reduced testicular size, together
with focal testicular hypoplasia in 8/10 rats. In the 10 mg/kg group,
there were a few hypoplastic seminiferous ducts reported in 4 of the
5 rats examined.
Uterine examinations on gestation day 13 showed fewer
implantations (not significant) at 30 mg/kg with no effect on
resorptions. In females allowed to deliver, weight gain was lower
during gestation in 30 mg/kg dams, probably reflecting the reduced
litter size and weight. Postnatal growth, physical and behavioral
development were unremarkable. The NOEL was l mg/kg bw/day. (Boutemy,
1980).
2.2.5 Special studies on genotoxicity
Table 3: Results of genotoxicity assays on albendazole
Test system Test object Concentration Result Reference
Ames test (1) S.typhimurium 1-10,000 µg/ Negative Jagannath,
TA1535, TA1537 plate 1980a
TA1538, TA98,
TA100
Ames test (2) As above As above Negative Jagannath,
1980b
Ames test (2, 3) As above As above Negative Jagannath,
1981
Ames test (1) S.typhimurium 0.5-1000 µg/ Negative Mourot,
TA97a, TA98, plate 1988
TA100, TA102
CHO chromosome Chinese hamster 0.047-1.5µg/ml Negative Galloway,
aberration ovary cells 1981
assay
Transformation BALB/3T3 mouse 10-100 µg/ml Negative Schechtman,
assay cells 1982
(1)With and without rat liver S-9 fraction
(2)With and without calf and rat liver S-9 fraction
(3)Substance tested was 2-amino sulphone metabolite.
2.2.6 Special studies on eye and skin irritation
Groups of rabbits had 100 mg albendazole powder instilled into
the conjunctival sac or 500 mg albendazole applied, under occlusion,
to abraded and non-abraded skin. There were no primary irritant
effects at any site (Macko et al., 1975b).
2.2.7 Special studies on teratogenicity
2.2.7.1 Mice
Groups of 21 to 26 pregnant Charles River CD-1 mice were given
gavage doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in 0.5%
methylcellulose. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 18.
There was no overt maternal toxicity or effect on resorption
incidence, fetal weight and external, visceral and skeletal
development of fetuses (Killeen & Rapp, 1975c).
2.2.7.2 Rats
Groups of 25 pregnant Charles River CD rats were given gavage
doses of 0, 5, 20 or 40 mg/kg bw/d albendazole in 0.5%
methylcellulose. In each group 19 rats were treated on gestation days
16 to 20 and 6 were treated from gestation day 16 to lactation day 20.
All dams were allowed to litter naturally.
There was no maternal toxicity or effect on gestation or
parturition. At 40 mg/kg, litter size and weight were reduced at birth
and remained depressed during lactation. A total of 6, 17, 4 and 55
pups died in the 0, 5, 20 and 40 mg/kg groups, respectively. Small
lungs and kidneys and anasarca in the 40 mg/kg pups were possibly
related to treatment, but an unequivocal conclusion was not possible
in view of the low number of control pups examined. The authors
concluded that developmental and behavioral characteristics were
unrelated to albendazole treatment, but detailed supporting data were
not provided. The NOEL was 20 mg/kg bw/day. (Johnson, 1981).
A series of studies was carried out at Biodynamics in Long Evans
rats. A similar protocol was used in each study, which included dosing
on gestation days 6 to 15; sacrifice of dams on gestation day 20;
maternal observations for overt toxicity, weight gain and uterine
parameters; fetal examination for size, weight, and external, visceral
and skeletal abnormalities.
Study A Groups of 20 pregnant rats were given gavage doses of 0, 2,
5, 10 or 30 mg/kg bw/d albendazole in 0.5% methylcellulose. Maternal
weight gain and survival were lower at 30 mg/kg. At this dose, there
was a high incidence of resorptions, surviving fetuses showed reduced
size, weight and multiple gross, visceral and skeletal malformations.
Limb abnormalities such as micromelia, ectromelia, curved femur and
microfetalis were also seen in other treated groups. However, the
incidences were low, they were not dose-related and they were observed
only in 1 or 2 litters per group (Killeen & Rapp, 1975e; Christian,
1984, 1987a.).
Study B Groups of 19-20 pregnant rats were given gavage doses of 0,
0.5, 2, 5 or 10 mg/kg bw/d albendazole in 0.5% methylcellulose.
Fetuses of the 10 mg/kg group revealed reduced size and weight,
retarded skeletal ossification and increased incidences of micromelia
and microfetalis (which included shortened long bones in fore and hind
limbs) (Killeen & Rapp, 1976; Christian, 1984, 1987a).
Study C Groups of 30 to 60 pregnant rats were fed diets containing
29% freeze dried liver, obtained from cattle 48 hours after a single
oral dose of 0 or 27.5 mg/kg bw albendazole. Drug intake was
calculated to represent 0.42 mg/kg bw/d albendazole equivalents. The
only possible treatment-related observation was shortened limb bones
in 2/248 treated fetuses from 2 different litters. These effects were
not seen in 460 controls and the authors noted that they were an
infrequent finding at Biodynamics (Hogan & Rinehart, 1977).
A re-evaluation of these affected fetuses failed to confirm the
above description. The apparent defects were described as artifacts
caused by poor staining (Christian, 1987b).
Study D Groups of 20 to 22 pregnant rats were fed diets containing
10, 20 or 30% freeze dried liver, obtained from cattle 12 days after
a single oral dose of 0 or 16.5 mg/kg bw albendazole. Drug intake was
calculated to be approximately 0.02, 0.04 and 0.06 mg/kg bw/d
albendazole equivalents. In the group given 30% exposed liver,
resorptions were increased, 9 of which were in 1 female. If data from
this rat were eliminated there were no significant effects. The
overall NOEL for studies A to D was 5 mg/kg bw/day. (Schroeder &
Rinehart, 1978).
Groups of pregnant Sprague Dawley rats were given gavage doses of
0, 5.3, 6.0, 6.62, 8.83, 10.6 or 13.25 mg/kg bw/d albendazole or 9 of
its animal metabolites at equimolar or higher doses. Treatments were
on gestation days 8 to 15 and dams were killed on gestation day 21.
The results were presented in summary form only.
Skeletal abnormalities were increased at dose levels of 6.62
mg/kg and greater with increases in resorptions and external
malformations and decreased fetal weight at 8.83 mg/kg albendazole and
above. The major malformations were craniofacial and bone defects.
Qualitatively similar findings were obtained with equimolar amounts of
albendazole sulfoxide while the other metabolites A, B, E, F, G, J, I
and H (section 2.1) were all ineffective. The concomitant
administration of SKF-525-A, a microsomal oxidation inhibitor, almost
completely suppressed the embryotoxic and developmental effects of
albendazole. The NOEL was 6 mg/kg bw/day. (Martin, 1980).
Groups of pregnant Sprague Dawley rats were given diets
containing albendazole or 40% freeze dried liver, obtained from cattle
24, 48 or 96 hours after a single oral dose of 0 or 20 mg/kg bw
albendazole. Drug levels in food were adjusted to provide 0, 12, 24 or
36 mg/kg bw/d but doses given through incorporation of liver could not
be estimated. Treatment of rats was on gestation days 8 to 15 and dams
were killed on gestation day 21. Albendazole doses of 24 and 36 mg/kg
produced virtually 100% embryolethality, the single live fetus was
small and had skeletal abnormalities. There were no effects at other
exposure levels. The NOEL was 12 mg/kg bw/day. (Grannec, 1980).
2.2.7.3 Rabbits
Groups of 15 pregnant New Zealand White rabbits were given gavage
doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in methylcellulose.
Treatment was on gestation days 7 to 19 and does were killed on
gestation day 30.
Maternal mortality was increased at 30 mg/kg but body weight
comparisons were not meaningful due to wide variation within groups.
The 30mg/kg group showed a reduction in implants, due largely to 2
does with corpora lutea but no implants, and increases in resorptions
and ectrodactyly. Fetal size and weight were depressed at 10 and 30
mg/kg. The NOEL was 5 mg/kg bw/day. (Killeen & Rapp, 1975d).
2.2.7.4 Sheep
In two separate experiments, groups of 15 to 44 Dorset Horn Cross
and Clun mated ewes were given a single dose of 0, 7.5, 10, 15 or 20
mg/kg bw albendazole by oral drench. Overall, there were 71, 43, 44,
43 and 42 animals in the 0, 7.5, 10, 15 and 20 mg/kg groups,
respectively. Treatment was on gestation day 17 and ewes were allowed
to deliver naturally.
There was no overt maternal toxicity but premature delivery was
noted in more ewes of the 20 mg/kg group than in the others. All
premature lambs in this group were stillborn, consequently the number
of live lambs was reduced at 20 mg/kg, as was survival of the lambs to
post partum day 55. Some lambs were sacrificed in extremis or because
they were considered commercially unviable, thus the total post partum
loss was 22/123, 4/67, 11/73, 12/73 and 39/61 in the 0, 7.5, 10, 15
and 20 mg/kg groups, respectively. Post-mortem examination of these
lambs revealed increased incidences of prognathia, scoliosis, spina
bifida and reduced tail at 20 mg/kg and displaced, poorly developed or
absent kidneys at 15 and 20 mg/kg. The NOEL was 10 mg/kg bw/d. (Tesh
& Harper, 1977).
The data shown in Table 4 indicates a relationship between
teratogenesis and peak plasma concentration of albendaxole sulfoxide.
Data from Bogan & Marriner (1984).
Table 4. Relationship between plasma albendazole sulfoxide
and teratogenesis
Species Albendazole Dose Peak Plasma Teratogenic
(mg/kg bw) Albendazole
(mg/ml)
Sheep 10 2.50 Yes
Cattle 10 0.57 No(1)
Rabbit 30 8.82 Yes
Rat 10 6.6 Yes
Mouse 30 n.a.(3) No
Man 400 mg/person 0.16 No(2)
(1) Embryotoxic but not teratogenic
(2) No intentional studies performed
(3) Not available
2.2.8 Special studies on mode of action of benzimadoles
Benzimadazoles bind with tubulin and inhibit its polymertisation
to microtubules. A comparison of inhibitory concentrations for some
benzimadazoles in nematodes and mammalian cells reveals a selective
action (reviewed in Sharma & Abuzar, 1983).
2.3 Observations in man
Albendazole has been used in 80 countries for 6 years to treat
gastrointestinal parasitic infections in man at a recommended dose
rate of 400 mg/person. There are a number of published reports on the
use of albendazole in man (see Marriner et al., 1986; Lange et al.,
1988).
In field trials in Nigeria, it was reported that 17 nulliparous
women aged between 16 and 18 years were inadvertently given a single
dose of 400 mg albendazole in the first trimester of pregnancy without
any adverse effects on the mother or child (A.B.C. Nwosu, 1985).
3. COMMENTS
Comprehensive toxicological data on albendazole were submitted
including the results of studies on its metabolism, carcinogenicity,
genotoxicity, effects on reproduction and teratogenicity, and
short-term studies.
Pharmacokinetic studies, although not specifically designed to
measure the extent of absorption, suggested that about 20-30% of
ingested albendazole was absorbed in mice and rats, about 1% in humans
and 50% in cattle. In all the species studied, oral dosing produced
very low plasma levels of unchanged drug, because of rapid first-pass
metabolism in the liver. The primary metabolic reactions were the
oxidation of the sulfide moiety of albendazole to the sulfoxide and
sulfone, followed by cleavage of the carbamate moiety to form the
2-amino-sulfone. This last compound was found to be the main residue
in the livers of sheep and cattle. The degradation of albendazole
followed similar pathways in rats, mice, cattle, sheep and humans.
In a study in mice in which albendazole was administered in the
diet for 25 months, anemia, leukopenia and testicular degeneration
were noted at 400 mg/kg bw/day. Hepatocellular vacuolation was
produced at 100 and 400 mg/kg bw/day, endometrial stromal polyps was
slightly higher than in concurrent controls, but statistical
significance was not achieved and all incidences were within the
laboratory historical control range. The NOEL was 25 mg/kg bw/day.
In a 28-month study in the rat in which the compound was given
in the diet, the highest dose of 20 mg/kg bw/day caused mortality,
neutropenia, hypercholesterolemia, testicular degeneration and
hepatic fatty metamorphosis. The incidence of endometrial/cervical
tumors and histiocytic carcomas in the skin showed an apparent
increase in certain treated groups. However, when compared with
those for the controls, these findings were not statistically
significantly different and were within the laboratory historical
control range. The NOEL was 7 mg/kg bw/day.
Questions had been raised regarding the statistical analyses of
the mouse and rat carcinogenicity studies and the use of historical
controls in the examination of the tumor incidence. The studies were
reviewed and were determined to be satisfactory. The statistical
analysis of both rodent carcinogenicity studies was also reviewed and
found to have been conducted in accordance with currently acceptable
procedures.
Albendazole did not produce bacterial mutations, chromosomal
aberrations or morphological transformations in cultured mammalian
cells. The 2-aminosulphone metabolite did not produce bacterial
mutations.
A three-generation reproduction study in which the compound was
administered in the diet was conducted in rats. There were no effects
on fertility or reproduction indices. The only findings were
reductions in pup postnatal survival and growth at 11.6 mg/kg bw/day.
The NOEL was 5.8 mg/kg bw/day.
Male rats were treated by gavage in a fertility study. Overt
toxic effects and testicular hypoplasia were noted at 10 and 30 mg/kg
bw/day. Fertility indices were not affected. Litter size and weight
were reduced at 30 mg/kg bw/day, the former effect being probably due
to a lower implantation rate. The NOEL was 1 mg/kg bw/day.
In a perinatal and postnatal study in rats in which albendazole
was administered by gavage, there was decreased survival and growth
in utero and during lactation in the pups of females given 40 mg/kg
bw/day group. There was also some evidence of retarded organ
development in the offspring of this group. The NOEL was 20 mg/kg
bw/day.
In a teratology study in mice in which albendazole was
administered by gavage, there were no adverse effects at dose levels
up to 30 mg/kg bw/day.
Several teratology studies were conducted in the rat in which the
compound was administered either by gavage or by dietary exposure.
Embryotoxic, fetotoxic effects and external malformations were
produced at doses of 8.8 mg/kg bw/day and above. Skeletal
malformations, in particular limb defects, which were seen at doses of
6.62 mg/kg bw/day, constituted the most sensitive indicator of
developmental toxicity. The NOEL was 5 mg/kg bw/day. When administered
in equimolar amounts, qualitatively similar findings were obtained
with albendazole sulphoxide, while eight other metabolites of
albendazole did not show any effects.
In rabbits, the toxic dose to the dams of 30 mg/kg bw/day was
associated with embryotoxicity and partially or totally missing
digits. Fetal growth retardation was observed at doses of 10 mg/kg
bw/day and above. The NOEL was 5 mg/kg bw/day.
A teratology study was conducted in sheep using a single oral
dose of albendazole on day 17 of pregnancy. Premature delivery,
fetotoxic effects and postnatal death were produced at 20 mg/kg bw and
malformations were increased at 15 and 20 mg/kg. The NOEL was 10 mg/kg
bw/day.
Dogs were dosed for six months by the administration of the
compound in capsules. They showed neutropenia at 30 mg/kg bw/day and
above. At 60 mg/kg bw/day there was also anemia, decreased body,
testicular and uterine weights, and bone marrow hypocellularity. The
NOEL in this study was 5 mg/kg bw/day.
Albendazole has been used in 80 countries to treat
gastrointestinal parasitic infections in humans at a dose rate of 400
mg/person, and there are a number of published reports on its use. In
field trials in Nigeria, an unpublished report noted that 17
nulliparous women aged between 16 and 18 years were inadvertently
given a single dose of 400 mg albendazole during the first trimester
of pregnancy without any apparent adverse effects on the mother or the
neonate.
Some of the effects observed in general toxicity studies with
albendazole may be explained by one of the biological actions of the
benzimadazoles, which is to interfere with tubulin polymerization and
thus inhibit spindle formation and mitosis.
The most significant toxicological manifestation resulting from
treatment with albendazole was its teratogenic activity, limb defects
in the rat being the most sensitive indicator of developmental
toxicity.
A NOEL of 5 mg/kg bw/day was reported in several studies in rats,
rabbits and dogs. Although for males in the rat fertility study the
figure was 1 mg/kg bw/day, it was noted that the next highest dose
used in the study was 10 mg/kg bw/day. In addition, in the
multigeneration reproduction study in rats, there was no effect on
fertility at 11.6 mg/kg bw/day, which was the highest dose used, and
the reported NOEL was 5.8 mg/kg bw/day, based on depression of weight
gain in pups.
An ADI of 0-0.05 mg/kg bw was established for albendazole based
on a NOEL of 5 mg/kg bw/day and using a safety factor of 100.
A safety factor of 100 was chosen for this compound after taking
into consideration poor absorption in humans, rapid metabolism, the
lack of teratogenic potential of most metabolites, the use of the drug
in humans and the identity of residues in food.
4. EVALUATION
Level causing no toxicological effect
Rat, rabbit, dog equal to 5 mg/kg bw/day.
Estimate of acceptable daily intake
0-0.05 mg/kg bw.
5. REFERENCES
BOGAN, J.A. & MARRINER, S.E. (1984). Pharmacodynamic and toxicological
aspects of albendazole in man and animals. In: Fock, M. (Ed.).
Albendazole in helminthiasis. Royal Society of Medicine International
Congress and Symposium Series No. 61. London, Royal Society of
Medicine, pp. 13-21.
BOUTEMY, C. (1980). Expert study of the action of the compound
albendazole on the fertility of the male rat, per os. Unpublished
study No. 2477 RSR from IFM Research Center. Submitted to WHO by
SmithKline and French.
BRAUN, W.G. & KILLEEN, J.C. (1975). Acute oral toxicity in rats,
compound No. SK&F 62979. Unpublished project No. 2605-75 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1984). Review of albendazole rat teratogenicity
studies. Unpublished report from Argus International. Submitted to WHO
by SmithKline and French.
CHRISTIAN, M.S. (1987a). Review of reported "micropodia" in
albendazole rat teratogenicity studies. Unpublished report from Argus
International. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1987b). Review of developmental toxicity
(embryo-fetal toxicity/teratogenicity) and reproductive toxicity
studies of albendazole in mice, rabbits and rats. Unpublished report
from Argus International. Submitted to WHO by SmithKline and French.
COLMAN, W., TOWNER, C., TOWNER, D. & SOKOLEK, J. (1977). Metabolic
fate of albendazole in sheep tissues. Unpublished report from
Applebrook Research Centre. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1980). A six-month oral toxicity study with
albendaxole in dogs. Unpublished project No. 790-2371 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1981). A toxicity study of albendazole in
rats. Unpublished project No. 80-2449 from Biodynamics Incorporated.
Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1982). A long-term oral dietary toxicity
carcinogenciity study of albendazole in rats. Unpublished project No.
79-2383 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987a). A long-term oral (dietary)
carcinogenicity study of albendazole in mice. Unpublished project No.
80-2480 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987b) A long-term oral
toxicity/carcinogenicity study of albendazole in rats. Unpublished
project No. 80/2448 from Biodynamics Incorporated. Submitted to WHO by
SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980a). A three month dose range finding
study with albendazole in mice. Unpublished project No. 79-2368 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980b). A three month feeding study of
albendazole in mice. Unpublished project No. 79-2423 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DELATOUR, P., GARNIER, F., BENOIT, E. & LONGIN, Ch. (1984). A
correlation of toxicity of albendazole and oxfendazole with their free
metabolites and bound residues. J.Vet.Pharmacol.Therap., 7, 139-145.
GALLOWAY, S.M. (1981). Mutagenicity evaluation of albendazole (SK&F
62979) in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished project No. 22000 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
GRANNEC, C. (1980). Albendazole: relay embryotoxicity. Thesis for
D.Vet.Sci. Claude Bernard University, Lyons. Submitted to WHO by
SmithKline and French.
GYURIK, R.J. et al., (1981). Metabolism of albendazole in cattle,
sheep, rats and mice. Drug Metabolism and Disposition 9, 503.
HOGAN, G.R. & RINEHART, W.E. (1977). A segment II teratologic relay
toxicity study in rats of liver obtained from calves treated with
albendazole. Unpublished project No. 76-1443A from Biodynamics
incorporated. Submitted to WHO by SmithKline and French.
JAGANNATH, D.R. (1980a). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome plate test. Unpublished
project No. 20988 from Litton Bionetics. Submitted to WHO by
SmithKline and French.
JAGANNATH, D.R. (1980b). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome preincubation plate test.
Unpublished project No. 20998 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
JAGANNATH, D.R. (1981). Mutagenicity evaluation of SK&F 81038 in the
Ames Salmonella/microsome plate test. Unpublished project No. 20988
from Litton Bionetics. Submitted to WHO by SmithKline and French.
JOHNSON, D.E. (1981). Perinatal and postnatal study in rats.
Unpublished report No. 483-001 from International Research and
Development Corporation. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975a). A three month oral toxicity study
of SK&F 62979 in rats. Unpublished project No. 75-1109 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. 6 RAPP, W.R. (1975b). A three month oral toxicity study
of SK&F 62979 in dogs. Unpublished project No. 75-1110 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975c). A segment II mouse teratology
study of SK&F 62979. Unpublished project No. 74-1096 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975d). A teratology study of SK&F 62979
in rabbits. Unpublished project No. 75-11234 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP. W.R. (1975e). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 74-1947 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1976). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 75-1274 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KRAEER, P., TOWNER, D. & SOKOLEK, J. (1977). Metabolic fate of
albendazole in cattle tissues. Unpublished report from Applebrook
Research Center. Submitted to WHO by SmithKline and French.
LANGE, H., EGGERS, R. & BIRCHER, J. (1988). Increased systemic
availability of albendazole when taken with a fatty meal.
Eur.J.Clin.Pharmacol., 34, 315-317.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975a). Local
irritation, skin sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975b). Local
irritation, skin, sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MARRINER, S.E. & BOGAN, J.A. (1980). Pharmacokinetics of albendazole
in sheep. Am.J.Vet.Res., 41, 1126-1129.
MARRINER, S.E., MORRIS, D.L., DICKSON, B. & BOGAN, J.A. (1986).
Pharmacokinetics of albendazole in man. Eur.J.Clin.Pharmacol., 30,
705-708.
MARTIN, D. (1980). Albendazole: embryotoxic study of ten metabolites.
Thesis for D.Vet.Sci. Claude Bernard University, Lyons. Submitted to
WHO by SmithKline and French.
MOUROT, D. (1988). Albendazole: Ames test with and without metabolic
activation. Unpublished report from Laboratoire National des
Médicaments Vétérinaires. Submitted to WHO by SmithKline and French.
NWOSU, A.B.C. (1985). Albendazole field trials in Nigeria. Unpublished
report submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977a). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W. & GYURIK, R.J. (1977b). The isolation and
characterization of radioactive metabolites from the urine of steers
treated orally with 14°C; labelled SK&F 62979 (Albendazole).
Unpublished report from Applebrook Research Center. Submitted to WHO
by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977c). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C. & GYURIK, R. (1979). Urinary excretion and identification
of metabolites from urine of Sprague-Dawley rats orally dosed with
albendazole - 14°C albendazole sulfoxide - 14°C and albendazole
sulfone - 14°C. Unpublished report No. A-4003-78 from Applebrook
Research Centre. Submitted to WHO by SmithKline and French.
PARISH, R.C., GYURIK, R. & BRUNER, E. (1979a). The isolation and
quantitation of metabolites from urine of Charles River mice treated
orally with 14°C-labelled SK&F 62979 (Albendazole). Unpublished report
No. A-4006-78 from Applebrook Research Center. Submitted to WHO by
SmithKline and French.
PARISH, R.C., GYURIK, R.J. & BRUNER, E. (1979b). The isolation and
quantitation of radioactive metabolites from the urine of steers
treated orally with 14°C-labelled SK&F 62979. Unpublished report from
Applebrook Research Center. Submitted to WHO by SmithKline and French.
PRICHARD, R.K., HENNESSY, D.R., STEEL, J.W. & LACEY, E. (1985).
Metabolite concentrations in plasma following treatment of cattle with
five anthelmintics. Res.Vet.Sci., 39, 173-178.
ROSSIGNOL, J.F. & MAISONNEUVE, H. (1984). Albendazole: a new concept
in the control of intestinal helminthiasis. Gastroenterol.Clin.Biol.,
8, 569-576.
SAUER, R.M. (1985). Pathology Working Group report on albendazole in
Sprague-Dawley rats and CD-1 mice. Unpublished report from Pathco
Incorporated. Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987a). Pathology Working Group report on albendazole in
Sprague-Dawley rats. Unpublished report from Pathco Incorporated.
Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987b). Pathology Working Group report on albendazole in
CD-1 mice. Unpublished report from Pathco Incorporated. Submitted to
WHO by SmithKline and French.
SCHECHTMAN, L.M. (1982). Activity of T1709 in the in vitro mammalian
cell transformation assay in the presence of exogenous metabolic
activation. Unpublished project No. T1709.109 from Microbiolobical
Associates. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E., & RINEHART, W.E. (1978). A segment II teratologic
relay toxicity study in rats on liver obtained from cattle treated
with albendazole. Unpublished project No. 77-1825 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E. & RINEHART, W.E. (1980). A three-generation
reproduction study with albendazole in rats. Unpublished project No.
77-2019 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
SELWYN, M.R. (1987). Statistical analysis for oral carcinogenicity
studies of albendazole in mice and rats. Unpublished study from
Statistics Unlimited Inc. Submitted to WHO by SmithKline and French.
SHARMA, S. & ABUZAR, S. (1983). The benzimadozole anthelmintics -
chemistry and biological activity. Prog. Drug Res., 27, 85-161.
SIMON, J.C. (1979a). 4-week sub-acute oral toxicity study in rats.
Unpublished study No. 2206 TSR from IFM Research Center. Submitted to
WHO by SmithKline and French.
SIMON, J.C. (1979b). Sub-acute toxicity study in dogs. Unpublished
study No. 2204 TSC from IFM Research Center. Submitted to WHO by
SmithKline and French.
SOUHAILI-EL AMRI, H., FARGETTON, X., BENOIT, E., TOTIS, M. & BATT,
A-M. (1988). Inducing effect of albendazole on rat liver
drug-metabolising enzymes and metabolite pharmacokinetics.
Toxicol.App.Pharmacol., 92, 141-149.
TESH, J.M. & HARPER, J.A. (1977). Albendazole (SK&F 62979): effects of
single oral administration upon pregnancy in sheep. Unpublished report
No. 77/SAF210/205 from Life Science Research. Submitted to WHO by
SmithKline and French.
WALKER, T.F. (1976a). The acute oral toxicity of SK&F 62979
(Albendazole) in the rat. Unpublished report No. B 335A from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976b). The acute oral toxicity of SK&F 62979
(Albendazole) in the hamster. Unpublished report No. B 335C from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976c). The acute oral toxicity of SK&F 62979
(Albendazole) in the guinea pig. Unpublished report No. B 335B from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976d). The acute oral toxicity of SK&F 62979
(Albendazole) in the rabbit. Unpublished report No. B 335D from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
See Also:
Toxicological Abbreviations
ALBENDAZOLE (JECFA Evaluation)
 Sheep bearing permanent ruminal and abomasal cannulae were given
a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation.
Albendazole was absorbed unchanged from the rumen. Once in the body it
was rapidly degraded, and sulfone metabolites were detected in plasma,
the former achieving the greater level. All 3 compounds were present
in the abomasum. Presumably albendazole was passed through the
stomachs while the metabolites were secreted or diffused into this
organ. Non-detectable levels of all 3 compounds were reached in plasma
and rumen at 96 h and in abomasum at 120 h (Marriner & Bogan, 1980).
Sheep were given a single oral dose of 10 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1, 2, 4, 6 or 8 days after
treatment. On day 1, the label in liver was mainly in the form of the
sulfoxide and sulfone metabolites. These compounds were progressively
converted to the 2-amino sulfone which was the primary residue on day
8. Low levels of albendazole were detected up to day 2; other
degradation products were metabolites E, G and J. Over 72 h, pooled
urine samples, the sulfoxide and 2-amino sulfone accounted for 60-70%
of the urinary radioactivity. Low levels of albendazole, the sulfone
and 6 other metabolites were also found (Parish et al., 1977a;
Colman et al., 1977).
Steers were given a single oral dose of 7.5 mg/kg bw of an
albendazole formulation. Albendazole could not be detected in plasma.
Metabolism to the sulfoxide and sulfone was rapid; these compounds
were present in plasma for up to 40 h (Prichard et al., 1985).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1 to 12 days after
treatment. In liver obtained on day l, the radioactivity was mainly as
albendazole, the sulfoxide and sulfone. Albendazole disappeared by day
6 while the sulfoxide and sulfone were progressively converted to
2-amino sulfone through day 12. Low levels of metabolites G and J were
also found. A limited examination of kidney tissue revealed a similar
metabolic profile (Kraeer et al., 1977).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Over a 7 day period, 47% of the administered
radioactivity was recovered in the urine. The sulfoxide, sulfone and
2-amino sulfone accounted for 70% of this radiolabel. Low levels of
albendazole, metabolites B, D, E, G, H and J were also found (Parish
et al., 1977b; 1979b).
Human volunteers were given a single oral 400 mg dose of
albendazole. The parent sulfide was not detected in blood by HPLC.
Peak sulfoxide concentrations were achieved after 2.4 h and decayed
biphasically with a long terminal half-life. Analysis of 24 h urine by
TLC revealed the presence of the sulfoxide, the sulfone and their
amino derivatives and metabolites B, E and G (Rossingnol &
Maisonneuve, 1984).
The above findings were confirmed in other similar studies.
Additionally, it was demonstrated that urinary recovery over a 24 hour
period accounted for up to 0.88% of the dose. Limited information
suggested biliary excretion was very low. Thus it appears that the
oral absorption of the albendazole is somewhere around 1%. When
ingested with a high fat content meal the absorption of albendazole
was shown to be 4.6 times higher, on average, in 6 volunteers
(Marriner et al., 1986; Lange et al., 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Findings in dead rats included urinary staining of abdomen, bloody
discharge around nose, chromodacryorrhea and intestinal hemorrhage.
Necropsy of dead rabbits showed intestines containing fluid and
dilated with gas. Toxic signs in other species were not reported.
2.2.2 Short-term studies
2.2.2.1 Mice
In 2 separate experiments, groups of 10 male and 10 female
Charles River CD1 mice were fed diets containing albendazole for 90
days. Drug levels in food were adjusted so that animals received
doses of 0, 5, 10, 20, 40 or 80 mg/kg bw/d in study 1 and 0, 200, 400,
800 or 1600 mg/kg bw/d in study 2.
All females and 5/10 males of the 1600 mg/kg group died
spontaneously or were killed in a moribund condition. From week 9, ear
lesions involving thickening and/or encrustation of the tips were
observed in 2/10 males and 2/9 females at 800 mg/kg and 5/5 males in
the 1600 mg/kg group. Food consumption was generally decreased in
males given 400 mg/kg or more but weight gain was depressed only at
1600 mg/kg.
Hematology was measured at the end of the study. Hemoglobin,
hematocrit and erythrocyte levels were reduced at 800 mg/kg or more
and a decreased leucocyte counts was observed in males of these
groups. Gross post mortem examinations revealed increases in absolute
and relative liver weights from 40 mg/kg and greater in males and 80
mg/kg and greater in females (Daly & Rinehart, 1980a, 1980b).
2.2.2.2 Rats
Groups of 15 to 20 male and female Charles River Sprague Dawley
rats were given gavage doses of 0, 4, 25, 48 or 168 mg/kg bw/d of
albendazole in 0.5% Tween 80 for 4 weeks.
Species Sex Vehicle LD50 (mg/kg bw) Reference
Mouse M 0.75% methocel over 3000 Macko et al.,
1975a
Rat M&F 2% tragacanth 1320 Walker, 1976a
Rat M&F 1% methocel 2400 Braun & Killeen,
1975
Hamster M&F 2% tragacanth over 10000 Walker, 1976b
Guinea-pig M&F 2% tragacanth 900 Walker, 1976c
Rabbit M&F 2% tragacanth 500-1250 Walker, 1976d
Toxic signs were produced in the 2 highest dose groups which
included diarrhea, piloerection, nasal swelling with blood stained
nasal discharge and death (7/30 rats at 48 mg/kg and 39/40 rats at 168
mg/kg died). Body weight gain was depressed at 48 mg/kg with weight
loss at 168 mg/kg while food consumption was markedly reduced at 168
mg/kg and slightly reduced at 48 mg/kg.
Hematology, blood chemistry and urinalysis were measured after 1
and 4 weeks of treatment, except in the high dose rats which exhibited
marked overt toxicity. Hemoglobin, hematocrit, erythrocyte and
leucocyte counts were reduced at 48 mg/kg.
At autopsy, adrenal size was increased at 48 and 168 mg/kg,
particularly in females. Testes were soft with reduced size and weight
in 48 mg/kg males which survived 4 weeks. Testes size was not affected
at the highest dose presumably due to early death of these males.
Histopathology examination revealed hypoplasia in testes, bone marrow,
spleen and lymph nodes in 48 and 168 mg/kg groups.
An additional group of 5 males and 5 females was given 48 mg/kg
bw/d of albendazole for 4 weeks, followed by withdrawal of treatment
for 4 weeks. All drug-related changes were reduced in severity during
the latter period indicating that the effects were reversible (Simon,
1979a).
Groups of 20 male and 20 female Long Evans rats were fed diets
containing albendazole for 91 days. Drug levels in food were adjusted
so that animals received doses of 0, 2, 10 or 30 mg/kg bw/d. The
control and high dose groups included an additional 10 males and 10
females for laboratory studies.
There were no signs of toxicity and no effects on body weight,
food consumption and ophthalmology parameters. Hematology, blood
chemistry and urinalysis were studied after 1 and 3 months of
treatment. Gross pathology and organ weights were examined in all rats
while histopathology was carried out on 15 males and 15 females from
control and high dose groups. Meaningful changes related to treatment
were not observed (Killeen & Rapp, 1975a).
Groups of 100 male and female Charles River CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then throughout mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 5, 30 or 45 mg/kg bw/day. The treatment was intended to be
for 2 years but excessive mortality necessitated termination of the
study after 26 weeks.
There were no untoward effects in F0 rats. In the F1 animals,
92/100 males and 99/100 females of the 45 mg/kg bw/day group died by
week 25. Prior to death these rats developed swollen cervical glands,
followed by swelling of paws and the genital area, scabbing in the
cervical area and emaciation (reduced weight gain and food intake).
Hematology was measured after 3 and 6 months of treatment.
Hemoglobin, hematocrit, erythocyte and leucocyte counts were decreased
and reticulocytes increased in 45 mg/kg rats at 3 months. At 6 months,
similar but slight hematological changes were seen in 30 mg/kg bw/day
rats. Segmented neutrophils were particularly affected and this was
confirmed by differential bone marrow counts in control and 30 mg/kg
rats. Blood chemistry and urinalysis were studied after 3 months of
treatment. In the 45 mg/kg bw/day group, plasma cholesterol was
increased in males and females, potassium was increased in males, and
albumin, plasma and erythrocyte cholinesterase were decreased in
females. Urinary protein was increased in 30 and 45 mg/kg bw/day
males.
Post mortems were carried out on all unscheduled deaths and
approximately 60% of animals surviving to 26 weeks. Numerous gross
alterations were noted in the high dose group including discoloration
and/or nodules in the lungs, heart, lymph nodes, spleen, pancreas,
liver, adrenal and kidney. Some of these organs were also enlarged or
showed adhesions. Additionally, thymic tissue was often absent and
testes were small and flaccid.
Histopathology, performed on selected tissues in 5 males and 5
females from 0, 30 and 45 mg/kg bw/day groups, identified a number of
instances where bacterial colonization in lungs, spleen, kidneys and
heart was associated with necrosis without the usual acute
inflammatory response. The liver showed centrilobular cloudy swelling,
vacuolation or necrosis and lymphoid tissues such as bone marrow,
spleen and thymus showed hypocellularity or other changes suggestive
of atrophy. All the above lesions were seen at 45 mg/kg while, at 30
mg/kg, only the thymic and minor hepatic effects were noted (Daly &
Hogan, 1982).
The remaining rats were used to further evaluate effects on
hematological parameters. Control and 5 mg/kg group animals were
continued on the same treatment level while rats from the 30 mg/kg
group were now given 0 or 20 mg/kg bw/d albendazole. Group sizes were
20 to 25 males and females and dosing was continued for 4 months.
Hematology was studied monthly. The 5 mg/kg rats were still
unaffected. The red and white cell effects induced at 30 mg/kg were
essentially normalized within a month of reducing the dose to 0 or 20
mg/kg. However, differential bone marrow counts at day 81 revealed a
persistent depression of the myeloid line in the 20 mg/kg group. Gross
post mortem examination of all animals showed no remarkable effects
(Daly & Hogan, 1981).
2.2.2.3 Dogs
Groups of 4 to 5 male and female beagle dogs were given gavage
doses of 0, 4, 16, 48 or 168 mg/kg bw/d of albendazole in 2% Tween 80
for 4 weeks. Toxic signs, in a few dogs at the 2 highest dose levels,
included diarrhea and vomiting with cardio-pulmonary disturbances in
1 dog. Death ensued in 1/10 dogs at 48 mg/kg and 6/10 dogs at 168
mg/kg, mainly in females. Heart rates showed marked increases in some
168 mg/kg females prior to death. Food intake was reduced at 48 mg/kg
and above and weight gain was depressed at 16 mg/kg and above.
Hematology, blood chemistry, urinalysis and ophthalmology were
studied after 1 and 4 weeks of treatment. Leucocyte counts were
decreased in a few dogs at 48 and 168 mg/kg and alkaline phosphatase
was increased from 16 mg/kg. Necropsy revealed lower absolute
testicular weight in 48 and 168 mg/kg males but no pathological
alterations (Simon, 1979b).
Groups of 4 male and female beagle dogs were given 0, 2, 10 or 39
mg/kg bw/d albendazole in capsules for 91 days. Ophthalmology,
hematology, blood chemistry and unrinalysis were studied after 1 and
3 months; organ weights, gross and histopathology were examined in all
dogs. There were no toxic signs or effects on body weight and food
intake. No treatment-related effects were found (Killeen & Rapp,
1975b).
Groups of 6 male and 6 female beagle dogs were given 0, 5, 30 or
60 mg/kg bw/d albendazole in capsules for 6 months.
Food intake was decreased in 30 and 60 mg/kg females, while
weight gain was lower in 60 mg/kg males and females. Laboratory
studies included hematology and blood chemistry monthly, urinalysis
bimonthly and ophthalmology at termination. Hemoglobin, hematocrit and
erythrocyte counts were slightly reduced at 60 mg/kg and leucocyte
counts, particularly neutrophils, were reduced at 30 and 60 mg/kg.
Autopsy on all dogs revealed decreased absolute and relative
testes and uterine weights and slight increases in relative liver and
kidney weights at 60 mg/kg. The incidences of small nodules in the
stomach were increased in all treated groups but microscopically they
were shown to be normal, submucosal lymphoid follicles. Sternal bone
marrow showed hypocellularity in 4/6 females in the 60 mg/kg group.
The NOEL was 5 mg/kg bw/day. (Daly & Hogan, 1980).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 100 male and 100 female Charles River CD-1 mice were
fed diets containing albendazole for 25 months. Drug levels were
adjusted to provide doses of 0, 25, 100 or 400 mg/kg bw/d. Additional
groups of 25 males and 25 females were given control and high dose
treatments and used for hematology measurements.
There were no toxic signs or effects on food intake and body
weight. Hematology was studied after 3, 6 , 12, 18 and 24 months in
the main groups and monthly in the ancillary groups. Erythrocyte and
leucocyte counts were decreased and platelets were increased at 400
mg/kg, particularly in females.
A complete gross post-mortem examination was carried out on all
mice. Full histopathology was undertaken on control and high dose
mice. In intermediate groups, 6 major organs and grossly abnormal
tissues were examined routinely. Flaccid or small testes, testicular
tubular degeneration, oligospermia, and aspermia in epididymides were
increased in 400 mg/kg males. Centrilobular hepatocytic vacuolation
was increased in groups given 100 and 400 mg/kg. Eye opacities were
noted in all groups; however, microscopically, cataracts were slightly
increased only in 400 mg/kg males. The relationship of these ocular
findings to treatment is questionable as the bulk of the cataracts
were unilateral and such abnormalities are commonly obtained following
repeated blood collection from the orbital sinus.
The incidence of endometrial stromal polyps appeared to be
increased over concurrent controls (Table 1) but a statistical
evaluation of the results did not reveal significant differences
between groups, and all incidences were within the historical control
range for this laboratory based on 2 studies with 2 control groups in
each. The NOEL was 25 mg/kg bw/d. (Daly & Knezevich, 1987a; Sauer,
1985, 1987b; Selwyn 1987).
Table 1: Tumor incidence in mice
Dose (mg/kg bw/d) 0 0 25 100 400 Historical Controls
Endometrial stromal tumors
Polyps 3/98 5/99 3/98 5/98 7/99 Range 0/55-8/47
Sarcomas 0/98 0/99 1/98 2/98 0/99 Cumulative 29/780
Total 3/98 5/99 4/98 7/98 7/99
2.2.3.2 Rats
Groups of 100 male and 100 female Sprague Dawley CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then through mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 3.5, 7 or 20 mg/kg bw/d for 28 months. Additional groups
of 25 males and 25 females were given control and high dose treatments
and used for hematology measurements. An interim sacrifice of 10 males
and females per group was made after 12 months.
Treatment-related effects were not observed in F0 animals. In the
F1 animals, mortality was slightly increased after 24 months in 20
mg/kg males. There were no other toxic signs or effects on body weight
and food consumption. Ophthalmology, hematology, blood chemistry and
urinalysis parameters were studied after 3, 6, 12, 18 and 24 months of
treatment. In the 20 mg/kg group, total leucocyte and neutrophil
counts were decreased at 24 months, serum cholesterol was increased in
females throughout the study and in males at some sampling times.
A complete gross post-mortem examination was carried out on all
rats. Full histopathology was undertaken on control and high dose
rats. In intermediate groups, 8 major organs and grossly abnormal
tissue were examined routinely. The 20 mg/kg animals showed increased
incidences of flaccid testes, degeneration/atrophy of germinal and
relative liver weights in males and hepatic fatty metamorphosis in
males and females.
Compared to concurrent controls, the incidences of
endometrial/cervical tumors and skin histiocytic sarcomas showed an
apparent increase in certain groups of rats (see Table 2 below).
Statistical evaluation of the findings did not reveal significant
differences between groups and in fact all frequencies were within the
historical control range for this laboratory. The NOEL was 7 mg/kg/d.
(Daly & Knezevich, 1987b; Sauer, 1985, 1987a; Selwyn, 1987).
Table 2: Tumor incidence in rats
Dose (mg/kg/d) 0 0 3.5 7 20 Historical Controls
Endometrial/cervical tumors
Polyps 3/99 5/99 9/98 9/99 10/91 Range 1/69-7/58*
Sarcomas 0/99 3/99 0/98 4/99 3/91 Cumulative 120/1864
Total 3/99 8/99 9/98 11/99 13/91
Skin histiocytic sarcomas
Males 1/100 2/100 4/98 4/100 6/100 Range 0/116-6/110**
Cumulative 32/1190
Females 0/100 4/100 0/100 1/96 5/100 Range 1/112-11/119
Cumulative 40/1188
* based on 18 studies
** based on 14 studies.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Long Evans rats were fed diets containing 0, 30, 75 and
150 ppm albendazole for 3 successive generations, commencing 64 d
before the initial mating. Each parental group consisted of 12 males
and 24 females, which were bred to produce 2 litters each. Offspring
from the second litters were selected to serve as parents of the
subsequent generations. Drug intake was calculated to be, on average,
2.3, 5.8 and 11.6 mg/kg bw/d.
There were no toxic signs or effects on body weight, food
consumption, mating, fertility, pregnancy rates, gestation length,
litter size and weight. During lactation, pup survival and/or weight
gain was depressed, but only in F1a and F2a litters given 11.6 mg/kg.
The NOEL was 5.8 mg/kg bw/d. (Schroeder & Rinehart, 1980).
Groups of 20 male Sprague Dawley CD rats were given gavage doses
of 0, 1, 10 or 30 mg/kg bw/d albendozale in 0.5% gum tragacanth, from
60 days prior to mating to the end of the breeding period. Males were
mated 1 to 1 with untreated females. Half the dams were killed on
gestation day 13, the remainder were allowed to deliver naturally and
rear pups to weaning.
In males treated with 30 mg/kg, weight gain was lower and 4
animals died or were killed moribund. Toxic signs were piloerection
and bloody nasal discharge at 30 mg/kg and dried blood around the nose
at 10 mg/kg. The ability to impregnate females was unaffected, despite
the finding in the 30 mg/kg group of reduced testicular size, together
with focal testicular hypoplasia in 8/10 rats. In the 10 mg/kg group,
there were a few hypoplastic seminiferous ducts reported in 4 of the
5 rats examined.
Uterine examinations on gestation day 13 showed fewer
implantations (not significant) at 30 mg/kg with no effect on
resorptions. In females allowed to deliver, weight gain was lower
during gestation in 30 mg/kg dams, probably reflecting the reduced
litter size and weight. Postnatal growth, physical and behavioral
development were unremarkable. The NOEL was l mg/kg bw/day. (Boutemy,
1980).
2.2.5 Special studies on genotoxicity
Table 3: Results of genotoxicity assays on albendazole
Test system Test object Concentration Result Reference
Ames test (1) S.typhimurium 1-10,000 µg/ Negative Jagannath,
TA1535, TA1537 plate 1980a
TA1538, TA98,
TA100
Ames test (2) As above As above Negative Jagannath,
1980b
Ames test (2, 3) As above As above Negative Jagannath,
1981
Ames test (1) S.typhimurium 0.5-1000 µg/ Negative Mourot,
TA97a, TA98, plate 1988
TA100, TA102
CHO chromosome Chinese hamster 0.047-1.5µg/ml Negative Galloway,
aberration ovary cells 1981
assay
Transformation BALB/3T3 mouse 10-100 µg/ml Negative Schechtman,
assay cells 1982
(1)With and without rat liver S-9 fraction
(2)With and without calf and rat liver S-9 fraction
(3)Substance tested was 2-amino sulphone metabolite.
2.2.6 Special studies on eye and skin irritation
Groups of rabbits had 100 mg albendazole powder instilled into
the conjunctival sac or 500 mg albendazole applied, under occlusion,
to abraded and non-abraded skin. There were no primary irritant
effects at any site (Macko et al., 1975b).
2.2.7 Special studies on teratogenicity
2.2.7.1 Mice
Groups of 21 to 26 pregnant Charles River CD-1 mice were given
gavage doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in 0.5%
methylcellulose. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 18.
There was no overt maternal toxicity or effect on resorption
incidence, fetal weight and external, visceral and skeletal
development of fetuses (Killeen & Rapp, 1975c).
2.2.7.2 Rats
Groups of 25 pregnant Charles River CD rats were given gavage
doses of 0, 5, 20 or 40 mg/kg bw/d albendazole in 0.5%
methylcellulose. In each group 19 rats were treated on gestation days
16 to 20 and 6 were treated from gestation day 16 to lactation day 20.
All dams were allowed to litter naturally.
There was no maternal toxicity or effect on gestation or
parturition. At 40 mg/kg, litter size and weight were reduced at birth
and remained depressed during lactation. A total of 6, 17, 4 and 55
pups died in the 0, 5, 20 and 40 mg/kg groups, respectively. Small
lungs and kidneys and anasarca in the 40 mg/kg pups were possibly
related to treatment, but an unequivocal conclusion was not possible
in view of the low number of control pups examined. The authors
concluded that developmental and behavioral characteristics were
unrelated to albendazole treatment, but detailed supporting data were
not provided. The NOEL was 20 mg/kg bw/day. (Johnson, 1981).
A series of studies was carried out at Biodynamics in Long Evans
rats. A similar protocol was used in each study, which included dosing
on gestation days 6 to 15; sacrifice of dams on gestation day 20;
maternal observations for overt toxicity, weight gain and uterine
parameters; fetal examination for size, weight, and external, visceral
and skeletal abnormalities.
Study A Groups of 20 pregnant rats were given gavage doses of 0, 2,
5, 10 or 30 mg/kg bw/d albendazole in 0.5% methylcellulose. Maternal
weight gain and survival were lower at 30 mg/kg. At this dose, there
was a high incidence of resorptions, surviving fetuses showed reduced
size, weight and multiple gross, visceral and skeletal malformations.
Limb abnormalities such as micromelia, ectromelia, curved femur and
microfetalis were also seen in other treated groups. However, the
incidences were low, they were not dose-related and they were observed
only in 1 or 2 litters per group (Killeen & Rapp, 1975e; Christian,
1984, 1987a.).
Study B Groups of 19-20 pregnant rats were given gavage doses of 0,
0.5, 2, 5 or 10 mg/kg bw/d albendazole in 0.5% methylcellulose.
Fetuses of the 10 mg/kg group revealed reduced size and weight,
retarded skeletal ossification and increased incidences of micromelia
and microfetalis (which included shortened long bones in fore and hind
limbs) (Killeen & Rapp, 1976; Christian, 1984, 1987a).
Study C Groups of 30 to 60 pregnant rats were fed diets containing
29% freeze dried liver, obtained from cattle 48 hours after a single
oral dose of 0 or 27.5 mg/kg bw albendazole. Drug intake was
calculated to represent 0.42 mg/kg bw/d albendazole equivalents. The
only possible treatment-related observation was shortened limb bones
in 2/248 treated fetuses from 2 different litters. These effects were
not seen in 460 controls and the authors noted that they were an
infrequent finding at Biodynamics (Hogan & Rinehart, 1977).
A re-evaluation of these affected fetuses failed to confirm the
above description. The apparent defects were described as artifacts
caused by poor staining (Christian, 1987b).
Study D Groups of 20 to 22 pregnant rats were fed diets containing
10, 20 or 30% freeze dried liver, obtained from cattle 12 days after
a single oral dose of 0 or 16.5 mg/kg bw albendazole. Drug intake was
calculated to be approximately 0.02, 0.04 and 0.06 mg/kg bw/d
albendazole equivalents. In the group given 30% exposed liver,
resorptions were increased, 9 of which were in 1 female. If data from
this rat were eliminated there were no significant effects. The
overall NOEL for studies A to D was 5 mg/kg bw/day. (Schroeder &
Rinehart, 1978).
Groups of pregnant Sprague Dawley rats were given gavage doses of
0, 5.3, 6.0, 6.62, 8.83, 10.6 or 13.25 mg/kg bw/d albendazole or 9 of
its animal metabolites at equimolar or higher doses. Treatments were
on gestation days 8 to 15 and dams were killed on gestation day 21.
The results were presented in summary form only.
Skeletal abnormalities were increased at dose levels of 6.62
mg/kg and greater with increases in resorptions and external
malformations and decreased fetal weight at 8.83 mg/kg albendazole and
above. The major malformations were craniofacial and bone defects.
Qualitatively similar findings were obtained with equimolar amounts of
albendazole sulfoxide while the other metabolites A, B, E, F, G, J, I
and H (section 2.1) were all ineffective. The concomitant
administration of SKF-525-A, a microsomal oxidation inhibitor, almost
completely suppressed the embryotoxic and developmental effects of
albendazole. The NOEL was 6 mg/kg bw/day. (Martin, 1980).
Groups of pregnant Sprague Dawley rats were given diets
containing albendazole or 40% freeze dried liver, obtained from cattle
24, 48 or 96 hours after a single oral dose of 0 or 20 mg/kg bw
albendazole. Drug levels in food were adjusted to provide 0, 12, 24 or
36 mg/kg bw/d but doses given through incorporation of liver could not
be estimated. Treatment of rats was on gestation days 8 to 15 and dams
were killed on gestation day 21. Albendazole doses of 24 and 36 mg/kg
produced virtually 100% embryolethality, the single live fetus was
small and had skeletal abnormalities. There were no effects at other
exposure levels. The NOEL was 12 mg/kg bw/day. (Grannec, 1980).
2.2.7.3 Rabbits
Groups of 15 pregnant New Zealand White rabbits were given gavage
doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in methylcellulose.
Treatment was on gestation days 7 to 19 and does were killed on
gestation day 30.
Maternal mortality was increased at 30 mg/kg but body weight
comparisons were not meaningful due to wide variation within groups.
The 30mg/kg group showed a reduction in implants, due largely to 2
does with corpora lutea but no implants, and increases in resorptions
and ectrodactyly. Fetal size and weight were depressed at 10 and 30
mg/kg. The NOEL was 5 mg/kg bw/day. (Killeen & Rapp, 1975d).
2.2.7.4 Sheep
In two separate experiments, groups of 15 to 44 Dorset Horn Cross
and Clun mated ewes were given a single dose of 0, 7.5, 10, 15 or 20
mg/kg bw albendazole by oral drench. Overall, there were 71, 43, 44,
43 and 42 animals in the 0, 7.5, 10, 15 and 20 mg/kg groups,
respectively. Treatment was on gestation day 17 and ewes were allowed
to deliver naturally.
There was no overt maternal toxicity but premature delivery was
noted in more ewes of the 20 mg/kg group than in the others. All
premature lambs in this group were stillborn, consequently the number
of live lambs was reduced at 20 mg/kg, as was survival of the lambs to
post partum day 55. Some lambs were sacrificed in extremis or because
they were considered commercially unviable, thus the total post partum
loss was 22/123, 4/67, 11/73, 12/73 and 39/61 in the 0, 7.5, 10, 15
and 20 mg/kg groups, respectively. Post-mortem examination of these
lambs revealed increased incidences of prognathia, scoliosis, spina
bifida and reduced tail at 20 mg/kg and displaced, poorly developed or
absent kidneys at 15 and 20 mg/kg. The NOEL was 10 mg/kg bw/d. (Tesh
& Harper, 1977).
The data shown in Table 4 indicates a relationship between
teratogenesis and peak plasma concentration of albendaxole sulfoxide.
Data from Bogan & Marriner (1984).
Table 4. Relationship between plasma albendazole sulfoxide
and teratogenesis
Species Albendazole Dose Peak Plasma Teratogenic
(mg/kg bw) Albendazole
(mg/ml)
Sheep 10 2.50 Yes
Cattle 10 0.57 No(1)
Rabbit 30 8.82 Yes
Rat 10 6.6 Yes
Mouse 30 n.a.(3) No
Man 400 mg/person 0.16 No(2)
(1) Embryotoxic but not teratogenic
(2) No intentional studies performed
(3) Not available
2.2.8 Special studies on mode of action of benzimadoles
Benzimadazoles bind with tubulin and inhibit its polymertisation
to microtubules. A comparison of inhibitory concentrations for some
benzimadazoles in nematodes and mammalian cells reveals a selective
action (reviewed in Sharma & Abuzar, 1983).
2.3 Observations in man
Albendazole has been used in 80 countries for 6 years to treat
gastrointestinal parasitic infections in man at a recommended dose
rate of 400 mg/person. There are a number of published reports on the
use of albendazole in man (see Marriner et al., 1986; Lange et al.,
1988).
In field trials in Nigeria, it was reported that 17 nulliparous
women aged between 16 and 18 years were inadvertently given a single
dose of 400 mg albendazole in the first trimester of pregnancy without
any adverse effects on the mother or child (A.B.C. Nwosu, 1985).
3. COMMENTS
Comprehensive toxicological data on albendazole were submitted
including the results of studies on its metabolism, carcinogenicity,
genotoxicity, effects on reproduction and teratogenicity, and
short-term studies.
Pharmacokinetic studies, although not specifically designed to
measure the extent of absorption, suggested that about 20-30% of
ingested albendazole was absorbed in mice and rats, about 1% in humans
and 50% in cattle. In all the species studied, oral dosing produced
very low plasma levels of unchanged drug, because of rapid first-pass
metabolism in the liver. The primary metabolic reactions were the
oxidation of the sulfide moiety of albendazole to the sulfoxide and
sulfone, followed by cleavage of the carbamate moiety to form the
2-amino-sulfone. This last compound was found to be the main residue
in the livers of sheep and cattle. The degradation of albendazole
followed similar pathways in rats, mice, cattle, sheep and humans.
In a study in mice in which albendazole was administered in the
diet for 25 months, anemia, leukopenia and testicular degeneration
were noted at 400 mg/kg bw/day. Hepatocellular vacuolation was
produced at 100 and 400 mg/kg bw/day, endometrial stromal polyps was
slightly higher than in concurrent controls, but statistical
significance was not achieved and all incidences were within the
laboratory historical control range. The NOEL was 25 mg/kg bw/day.
In a 28-month study in the rat in which the compound was given
in the diet, the highest dose of 20 mg/kg bw/day caused mortality,
neutropenia, hypercholesterolemia, testicular degeneration and
hepatic fatty metamorphosis. The incidence of endometrial/cervical
tumors and histiocytic carcomas in the skin showed an apparent
increase in certain treated groups. However, when compared with
those for the controls, these findings were not statistically
significantly different and were within the laboratory historical
control range. The NOEL was 7 mg/kg bw/day.
Questions had been raised regarding the statistical analyses of
the mouse and rat carcinogenicity studies and the use of historical
controls in the examination of the tumor incidence. The studies were
reviewed and were determined to be satisfactory. The statistical
analysis of both rodent carcinogenicity studies was also reviewed and
found to have been conducted in accordance with currently acceptable
procedures.
Albendazole did not produce bacterial mutations, chromosomal
aberrations or morphological transformations in cultured mammalian
cells. The 2-aminosulphone metabolite did not produce bacterial
mutations.
A three-generation reproduction study in which the compound was
administered in the diet was conducted in rats. There were no effects
on fertility or reproduction indices. The only findings were
reductions in pup postnatal survival and growth at 11.6 mg/kg bw/day.
The NOEL was 5.8 mg/kg bw/day.
Male rats were treated by gavage in a fertility study. Overt
toxic effects and testicular hypoplasia were noted at 10 and 30 mg/kg
bw/day. Fertility indices were not affected. Litter size and weight
were reduced at 30 mg/kg bw/day, the former effect being probably due
to a lower implantation rate. The NOEL was 1 mg/kg bw/day.
In a perinatal and postnatal study in rats in which albendazole
was administered by gavage, there was decreased survival and growth
in utero and during lactation in the pups of females given 40 mg/kg
bw/day group. There was also some evidence of retarded organ
development in the offspring of this group. The NOEL was 20 mg/kg
bw/day.
In a teratology study in mice in which albendazole was
administered by gavage, there were no adverse effects at dose levels
up to 30 mg/kg bw/day.
Several teratology studies were conducted in the rat in which the
compound was administered either by gavage or by dietary exposure.
Embryotoxic, fetotoxic effects and external malformations were
produced at doses of 8.8 mg/kg bw/day and above. Skeletal
malformations, in particular limb defects, which were seen at doses of
6.62 mg/kg bw/day, constituted the most sensitive indicator of
developmental toxicity. The NOEL was 5 mg/kg bw/day. When administered
in equimolar amounts, qualitatively similar findings were obtained
with albendazole sulphoxide, while eight other metabolites of
albendazole did not show any effects.
In rabbits, the toxic dose to the dams of 30 mg/kg bw/day was
associated with embryotoxicity and partially or totally missing
digits. Fetal growth retardation was observed at doses of 10 mg/kg
bw/day and above. The NOEL was 5 mg/kg bw/day.
A teratology study was conducted in sheep using a single oral
dose of albendazole on day 17 of pregnancy. Premature delivery,
fetotoxic effects and postnatal death were produced at 20 mg/kg bw and
malformations were increased at 15 and 20 mg/kg. The NOEL was 10 mg/kg
bw/day.
Dogs were dosed for six months by the administration of the
compound in capsules. They showed neutropenia at 30 mg/kg bw/day and
above. At 60 mg/kg bw/day there was also anemia, decreased body,
testicular and uterine weights, and bone marrow hypocellularity. The
NOEL in this study was 5 mg/kg bw/day.
Albendazole has been used in 80 countries to treat
gastrointestinal parasitic infections in humans at a dose rate of 400
mg/person, and there are a number of published reports on its use. In
field trials in Nigeria, an unpublished report noted that 17
nulliparous women aged between 16 and 18 years were inadvertently
given a single dose of 400 mg albendazole during the first trimester
of pregnancy without any apparent adverse effects on the mother or the
neonate.
Some of the effects observed in general toxicity studies with
albendazole may be explained by one of the biological actions of the
benzimadazoles, which is to interfere with tubulin polymerization and
thus inhibit spindle formation and mitosis.
The most significant toxicological manifestation resulting from
treatment with albendazole was its teratogenic activity, limb defects
in the rat being the most sensitive indicator of developmental
toxicity.
A NOEL of 5 mg/kg bw/day was reported in several studies in rats,
rabbits and dogs. Although for males in the rat fertility study the
figure was 1 mg/kg bw/day, it was noted that the next highest dose
used in the study was 10 mg/kg bw/day. In addition, in the
multigeneration reproduction study in rats, there was no effect on
fertility at 11.6 mg/kg bw/day, which was the highest dose used, and
the reported NOEL was 5.8 mg/kg bw/day, based on depression of weight
gain in pups.
An ADI of 0-0.05 mg/kg bw was established for albendazole based
on a NOEL of 5 mg/kg bw/day and using a safety factor of 100.
A safety factor of 100 was chosen for this compound after taking
into consideration poor absorption in humans, rapid metabolism, the
lack of teratogenic potential of most metabolites, the use of the drug
in humans and the identity of residues in food.
4. EVALUATION
Level causing no toxicological effect
Rat, rabbit, dog equal to 5 mg/kg bw/day.
Estimate of acceptable daily intake
0-0.05 mg/kg bw.
5. REFERENCES
BOGAN, J.A. & MARRINER, S.E. (1984). Pharmacodynamic and toxicological
aspects of albendazole in man and animals. In: Fock, M. (Ed.).
Albendazole in helminthiasis. Royal Society of Medicine International
Congress and Symposium Series No. 61. London, Royal Society of
Medicine, pp. 13-21.
BOUTEMY, C. (1980). Expert study of the action of the compound
albendazole on the fertility of the male rat, per os. Unpublished
study No. 2477 RSR from IFM Research Center. Submitted to WHO by
SmithKline and French.
BRAUN, W.G. & KILLEEN, J.C. (1975). Acute oral toxicity in rats,
compound No. SK&F 62979. Unpublished project No. 2605-75 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1984). Review of albendazole rat teratogenicity
studies. Unpublished report from Argus International. Submitted to WHO
by SmithKline and French.
CHRISTIAN, M.S. (1987a). Review of reported "micropodia" in
albendazole rat teratogenicity studies. Unpublished report from Argus
International. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1987b). Review of developmental toxicity
(embryo-fetal toxicity/teratogenicity) and reproductive toxicity
studies of albendazole in mice, rabbits and rats. Unpublished report
from Argus International. Submitted to WHO by SmithKline and French.
COLMAN, W., TOWNER, C., TOWNER, D. & SOKOLEK, J. (1977). Metabolic
fate of albendazole in sheep tissues. Unpublished report from
Applebrook Research Centre. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1980). A six-month oral toxicity study with
albendaxole in dogs. Unpublished project No. 790-2371 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1981). A toxicity study of albendazole in
rats. Unpublished project No. 80-2449 from Biodynamics Incorporated.
Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1982). A long-term oral dietary toxicity
carcinogenciity study of albendazole in rats. Unpublished project No.
79-2383 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987a). A long-term oral (dietary)
carcinogenicity study of albendazole in mice. Unpublished project No.
80-2480 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987b) A long-term oral
toxicity/carcinogenicity study of albendazole in rats. Unpublished
project No. 80/2448 from Biodynamics Incorporated. Submitted to WHO by
SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980a). A three month dose range finding
study with albendazole in mice. Unpublished project No. 79-2368 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980b). A three month feeding study of
albendazole in mice. Unpublished project No. 79-2423 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DELATOUR, P., GARNIER, F., BENOIT, E. & LONGIN, Ch. (1984). A
correlation of toxicity of albendazole and oxfendazole with their free
metabolites and bound residues. J.Vet.Pharmacol.Therap., 7, 139-145.
GALLOWAY, S.M. (1981). Mutagenicity evaluation of albendazole (SK&F
62979) in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished project No. 22000 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
GRANNEC, C. (1980). Albendazole: relay embryotoxicity. Thesis for
D.Vet.Sci. Claude Bernard University, Lyons. Submitted to WHO by
SmithKline and French.
GYURIK, R.J. et al., (1981). Metabolism of albendazole in cattle,
sheep, rats and mice. Drug Metabolism and Disposition 9, 503.
HOGAN, G.R. & RINEHART, W.E. (1977). A segment II teratologic relay
toxicity study in rats of liver obtained from calves treated with
albendazole. Unpublished project No. 76-1443A from Biodynamics
incorporated. Submitted to WHO by SmithKline and French.
JAGANNATH, D.R. (1980a). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome plate test. Unpublished
project No. 20988 from Litton Bionetics. Submitted to WHO by
SmithKline and French.
JAGANNATH, D.R. (1980b). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome preincubation plate test.
Unpublished project No. 20998 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
JAGANNATH, D.R. (1981). Mutagenicity evaluation of SK&F 81038 in the
Ames Salmonella/microsome plate test. Unpublished project No. 20988
from Litton Bionetics. Submitted to WHO by SmithKline and French.
JOHNSON, D.E. (1981). Perinatal and postnatal study in rats.
Unpublished report No. 483-001 from International Research and
Development Corporation. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975a). A three month oral toxicity study
of SK&F 62979 in rats. Unpublished project No. 75-1109 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. 6 RAPP, W.R. (1975b). A three month oral toxicity study
of SK&F 62979 in dogs. Unpublished project No. 75-1110 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975c). A segment II mouse teratology
study of SK&F 62979. Unpublished project No. 74-1096 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975d). A teratology study of SK&F 62979
in rabbits. Unpublished project No. 75-11234 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP. W.R. (1975e). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 74-1947 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1976). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 75-1274 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KRAEER, P., TOWNER, D. & SOKOLEK, J. (1977). Metabolic fate of
albendazole in cattle tissues. Unpublished report from Applebrook
Research Center. Submitted to WHO by SmithKline and French.
LANGE, H., EGGERS, R. & BIRCHER, J. (1988). Increased systemic
availability of albendazole when taken with a fatty meal.
Eur.J.Clin.Pharmacol., 34, 315-317.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975a). Local
irritation, skin sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975b). Local
irritation, skin, sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MARRINER, S.E. & BOGAN, J.A. (1980). Pharmacokinetics of albendazole
in sheep. Am.J.Vet.Res., 41, 1126-1129.
MARRINER, S.E., MORRIS, D.L., DICKSON, B. & BOGAN, J.A. (1986).
Pharmacokinetics of albendazole in man. Eur.J.Clin.Pharmacol., 30,
705-708.
MARTIN, D. (1980). Albendazole: embryotoxic study of ten metabolites.
Thesis for D.Vet.Sci. Claude Bernard University, Lyons. Submitted to
WHO by SmithKline and French.
MOUROT, D. (1988). Albendazole: Ames test with and without metabolic
activation. Unpublished report from Laboratoire National des
Médicaments Vétérinaires. Submitted to WHO by SmithKline and French.
NWOSU, A.B.C. (1985). Albendazole field trials in Nigeria. Unpublished
report submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977a). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W. & GYURIK, R.J. (1977b). The isolation and
characterization of radioactive metabolites from the urine of steers
treated orally with 14°C; labelled SK&F 62979 (Albendazole).
Unpublished report from Applebrook Research Center. Submitted to WHO
by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977c). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C. & GYURIK, R. (1979). Urinary excretion and identification
of metabolites from urine of Sprague-Dawley rats orally dosed with
albendazole - 14°C albendazole sulfoxide - 14°C and albendazole
sulfone - 14°C. Unpublished report No. A-4003-78 from Applebrook
Research Centre. Submitted to WHO by SmithKline and French.
PARISH, R.C., GYURIK, R. & BRUNER, E. (1979a). The isolation and
quantitation of metabolites from urine of Charles River mice treated
orally with 14°C-labelled SK&F 62979 (Albendazole). Unpublished report
No. A-4006-78 from Applebrook Research Center. Submitted to WHO by
SmithKline and French.
PARISH, R.C., GYURIK, R.J. & BRUNER, E. (1979b). The isolation and
quantitation of radioactive metabolites from the urine of steers
treated orally with 14°C-labelled SK&F 62979. Unpublished report from
Applebrook Research Center. Submitted to WHO by SmithKline and French.
PRICHARD, R.K., HENNESSY, D.R., STEEL, J.W. & LACEY, E. (1985).
Metabolite concentrations in plasma following treatment of cattle with
five anthelmintics. Res.Vet.Sci., 39, 173-178.
ROSSIGNOL, J.F. & MAISONNEUVE, H. (1984). Albendazole: a new concept
in the control of intestinal helminthiasis. Gastroenterol.Clin.Biol.,
8, 569-576.
SAUER, R.M. (1985). Pathology Working Group report on albendazole in
Sprague-Dawley rats and CD-1 mice. Unpublished report from Pathco
Incorporated. Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987a). Pathology Working Group report on albendazole in
Sprague-Dawley rats. Unpublished report from Pathco Incorporated.
Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987b). Pathology Working Group report on albendazole in
CD-1 mice. Unpublished report from Pathco Incorporated. Submitted to
WHO by SmithKline and French.
SCHECHTMAN, L.M. (1982). Activity of T1709 in the in vitro mammalian
cell transformation assay in the presence of exogenous metabolic
activation. Unpublished project No. T1709.109 from Microbiolobical
Associates. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E., & RINEHART, W.E. (1978). A segment II teratologic
relay toxicity study in rats on liver obtained from cattle treated
with albendazole. Unpublished project No. 77-1825 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E. & RINEHART, W.E. (1980). A three-generation
reproduction study with albendazole in rats. Unpublished project No.
77-2019 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
SELWYN, M.R. (1987). Statistical analysis for oral carcinogenicity
studies of albendazole in mice and rats. Unpublished study from
Statistics Unlimited Inc. Submitted to WHO by SmithKline and French.
SHARMA, S. & ABUZAR, S. (1983). The benzimadozole anthelmintics -
chemistry and biological activity. Prog. Drug Res., 27, 85-161.
SIMON, J.C. (1979a). 4-week sub-acute oral toxicity study in rats.
Unpublished study No. 2206 TSR from IFM Research Center. Submitted to
WHO by SmithKline and French.
SIMON, J.C. (1979b). Sub-acute toxicity study in dogs. Unpublished
study No. 2204 TSC from IFM Research Center. Submitted to WHO by
SmithKline and French.
SOUHAILI-EL AMRI, H., FARGETTON, X., BENOIT, E., TOTIS, M. & BATT,
A-M. (1988). Inducing effect of albendazole on rat liver
drug-metabolising enzymes and metabolite pharmacokinetics.
Toxicol.App.Pharmacol., 92, 141-149.
TESH, J.M. & HARPER, J.A. (1977). Albendazole (SK&F 62979): effects of
single oral administration upon pregnancy in sheep. Unpublished report
No. 77/SAF210/205 from Life Science Research. Submitted to WHO by
SmithKline and French.
WALKER, T.F. (1976a). The acute oral toxicity of SK&F 62979
(Albendazole) in the rat. Unpublished report No. B 335A from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976b). The acute oral toxicity of SK&F 62979
(Albendazole) in the hamster. Unpublished report No. B 335C from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976c). The acute oral toxicity of SK&F 62979
(Albendazole) in the guinea pig. Unpublished report No. B 335B from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976d). The acute oral toxicity of SK&F 62979
(Albendazole) in the rabbit. Unpublished report No. B 335D from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
Sheep bearing permanent ruminal and abomasal cannulae were given
a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation.
Albendazole was absorbed unchanged from the rumen. Once in the body it
was rapidly degraded, and sulfone metabolites were detected in plasma,
the former achieving the greater level. All 3 compounds were present
in the abomasum. Presumably albendazole was passed through the
stomachs while the metabolites were secreted or diffused into this
organ. Non-detectable levels of all 3 compounds were reached in plasma
and rumen at 96 h and in abomasum at 120 h (Marriner & Bogan, 1980).
Sheep were given a single oral dose of 10 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1, 2, 4, 6 or 8 days after
treatment. On day 1, the label in liver was mainly in the form of the
sulfoxide and sulfone metabolites. These compounds were progressively
converted to the 2-amino sulfone which was the primary residue on day
8. Low levels of albendazole were detected up to day 2; other
degradation products were metabolites E, G and J. Over 72 h, pooled
urine samples, the sulfoxide and 2-amino sulfone accounted for 60-70%
of the urinary radioactivity. Low levels of albendazole, the sulfone
and 6 other metabolites were also found (Parish et al., 1977a;
Colman et al., 1977).
Steers were given a single oral dose of 7.5 mg/kg bw of an
albendazole formulation. Albendazole could not be detected in plasma.
Metabolism to the sulfoxide and sulfone was rapid; these compounds
were present in plasma for up to 40 h (Prichard et al., 1985).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Animals were killed 1 to 12 days after
treatment. In liver obtained on day l, the radioactivity was mainly as
albendazole, the sulfoxide and sulfone. Albendazole disappeared by day
6 while the sulfoxide and sulfone were progressively converted to
2-amino sulfone through day 12. Low levels of metabolites G and J were
also found. A limited examination of kidney tissue revealed a similar
metabolic profile (Kraeer et al., 1977).
Calves were given a single oral dose of 20 mg/kg bw 14C-ring
labelled albendazole. Over a 7 day period, 47% of the administered
radioactivity was recovered in the urine. The sulfoxide, sulfone and
2-amino sulfone accounted for 70% of this radiolabel. Low levels of
albendazole, metabolites B, D, E, G, H and J were also found (Parish
et al., 1977b; 1979b).
Human volunteers were given a single oral 400 mg dose of
albendazole. The parent sulfide was not detected in blood by HPLC.
Peak sulfoxide concentrations were achieved after 2.4 h and decayed
biphasically with a long terminal half-life. Analysis of 24 h urine by
TLC revealed the presence of the sulfoxide, the sulfone and their
amino derivatives and metabolites B, E and G (Rossingnol &
Maisonneuve, 1984).
The above findings were confirmed in other similar studies.
Additionally, it was demonstrated that urinary recovery over a 24 hour
period accounted for up to 0.88% of the dose. Limited information
suggested biliary excretion was very low. Thus it appears that the
oral absorption of the albendazole is somewhere around 1%. When
ingested with a high fat content meal the absorption of albendazole
was shown to be 4.6 times higher, on average, in 6 volunteers
(Marriner et al., 1986; Lange et al., 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Findings in dead rats included urinary staining of abdomen, bloody
discharge around nose, chromodacryorrhea and intestinal hemorrhage.
Necropsy of dead rabbits showed intestines containing fluid and
dilated with gas. Toxic signs in other species were not reported.
2.2.2 Short-term studies
2.2.2.1 Mice
In 2 separate experiments, groups of 10 male and 10 female
Charles River CD1 mice were fed diets containing albendazole for 90
days. Drug levels in food were adjusted so that animals received
doses of 0, 5, 10, 20, 40 or 80 mg/kg bw/d in study 1 and 0, 200, 400,
800 or 1600 mg/kg bw/d in study 2.
All females and 5/10 males of the 1600 mg/kg group died
spontaneously or were killed in a moribund condition. From week 9, ear
lesions involving thickening and/or encrustation of the tips were
observed in 2/10 males and 2/9 females at 800 mg/kg and 5/5 males in
the 1600 mg/kg group. Food consumption was generally decreased in
males given 400 mg/kg or more but weight gain was depressed only at
1600 mg/kg.
Hematology was measured at the end of the study. Hemoglobin,
hematocrit and erythrocyte levels were reduced at 800 mg/kg or more
and a decreased leucocyte counts was observed in males of these
groups. Gross post mortem examinations revealed increases in absolute
and relative liver weights from 40 mg/kg and greater in males and 80
mg/kg and greater in females (Daly & Rinehart, 1980a, 1980b).
2.2.2.2 Rats
Groups of 15 to 20 male and female Charles River Sprague Dawley
rats were given gavage doses of 0, 4, 25, 48 or 168 mg/kg bw/d of
albendazole in 0.5% Tween 80 for 4 weeks.
Species Sex Vehicle LD50 (mg/kg bw) Reference
Mouse M 0.75% methocel over 3000 Macko et al.,
1975a
Rat M&F 2% tragacanth 1320 Walker, 1976a
Rat M&F 1% methocel 2400 Braun & Killeen,
1975
Hamster M&F 2% tragacanth over 10000 Walker, 1976b
Guinea-pig M&F 2% tragacanth 900 Walker, 1976c
Rabbit M&F 2% tragacanth 500-1250 Walker, 1976d
Toxic signs were produced in the 2 highest dose groups which
included diarrhea, piloerection, nasal swelling with blood stained
nasal discharge and death (7/30 rats at 48 mg/kg and 39/40 rats at 168
mg/kg died). Body weight gain was depressed at 48 mg/kg with weight
loss at 168 mg/kg while food consumption was markedly reduced at 168
mg/kg and slightly reduced at 48 mg/kg.
Hematology, blood chemistry and urinalysis were measured after 1
and 4 weeks of treatment, except in the high dose rats which exhibited
marked overt toxicity. Hemoglobin, hematocrit, erythrocyte and
leucocyte counts were reduced at 48 mg/kg.
At autopsy, adrenal size was increased at 48 and 168 mg/kg,
particularly in females. Testes were soft with reduced size and weight
in 48 mg/kg males which survived 4 weeks. Testes size was not affected
at the highest dose presumably due to early death of these males.
Histopathology examination revealed hypoplasia in testes, bone marrow,
spleen and lymph nodes in 48 and 168 mg/kg groups.
An additional group of 5 males and 5 females was given 48 mg/kg
bw/d of albendazole for 4 weeks, followed by withdrawal of treatment
for 4 weeks. All drug-related changes were reduced in severity during
the latter period indicating that the effects were reversible (Simon,
1979a).
Groups of 20 male and 20 female Long Evans rats were fed diets
containing albendazole for 91 days. Drug levels in food were adjusted
so that animals received doses of 0, 2, 10 or 30 mg/kg bw/d. The
control and high dose groups included an additional 10 males and 10
females for laboratory studies.
There were no signs of toxicity and no effects on body weight,
food consumption and ophthalmology parameters. Hematology, blood
chemistry and urinalysis were studied after 1 and 3 months of
treatment. Gross pathology and organ weights were examined in all rats
while histopathology was carried out on 15 males and 15 females from
control and high dose groups. Meaningful changes related to treatment
were not observed (Killeen & Rapp, 1975a).
Groups of 100 male and female Charles River CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then throughout mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 5, 30 or 45 mg/kg bw/day. The treatment was intended to be
for 2 years but excessive mortality necessitated termination of the
study after 26 weeks.
There were no untoward effects in F0 rats. In the F1 animals,
92/100 males and 99/100 females of the 45 mg/kg bw/day group died by
week 25. Prior to death these rats developed swollen cervical glands,
followed by swelling of paws and the genital area, scabbing in the
cervical area and emaciation (reduced weight gain and food intake).
Hematology was measured after 3 and 6 months of treatment.
Hemoglobin, hematocrit, erythocyte and leucocyte counts were decreased
and reticulocytes increased in 45 mg/kg rats at 3 months. At 6 months,
similar but slight hematological changes were seen in 30 mg/kg bw/day
rats. Segmented neutrophils were particularly affected and this was
confirmed by differential bone marrow counts in control and 30 mg/kg
rats. Blood chemistry and urinalysis were studied after 3 months of
treatment. In the 45 mg/kg bw/day group, plasma cholesterol was
increased in males and females, potassium was increased in males, and
albumin, plasma and erythrocyte cholinesterase were decreased in
females. Urinary protein was increased in 30 and 45 mg/kg bw/day
males.
Post mortems were carried out on all unscheduled deaths and
approximately 60% of animals surviving to 26 weeks. Numerous gross
alterations were noted in the high dose group including discoloration
and/or nodules in the lungs, heart, lymph nodes, spleen, pancreas,
liver, adrenal and kidney. Some of these organs were also enlarged or
showed adhesions. Additionally, thymic tissue was often absent and
testes were small and flaccid.
Histopathology, performed on selected tissues in 5 males and 5
females from 0, 30 and 45 mg/kg bw/day groups, identified a number of
instances where bacterial colonization in lungs, spleen, kidneys and
heart was associated with necrosis without the usual acute
inflammatory response. The liver showed centrilobular cloudy swelling,
vacuolation or necrosis and lymphoid tissues such as bone marrow,
spleen and thymus showed hypocellularity or other changes suggestive
of atrophy. All the above lesions were seen at 45 mg/kg while, at 30
mg/kg, only the thymic and minor hepatic effects were noted (Daly &
Hogan, 1982).
The remaining rats were used to further evaluate effects on
hematological parameters. Control and 5 mg/kg group animals were
continued on the same treatment level while rats from the 30 mg/kg
group were now given 0 or 20 mg/kg bw/d albendazole. Group sizes were
20 to 25 males and females and dosing was continued for 4 months.
Hematology was studied monthly. The 5 mg/kg rats were still
unaffected. The red and white cell effects induced at 30 mg/kg were
essentially normalized within a month of reducing the dose to 0 or 20
mg/kg. However, differential bone marrow counts at day 81 revealed a
persistent depression of the myeloid line in the 20 mg/kg group. Gross
post mortem examination of all animals showed no remarkable effects
(Daly & Hogan, 1981).
2.2.2.3 Dogs
Groups of 4 to 5 male and female beagle dogs were given gavage
doses of 0, 4, 16, 48 or 168 mg/kg bw/d of albendazole in 2% Tween 80
for 4 weeks. Toxic signs, in a few dogs at the 2 highest dose levels,
included diarrhea and vomiting with cardio-pulmonary disturbances in
1 dog. Death ensued in 1/10 dogs at 48 mg/kg and 6/10 dogs at 168
mg/kg, mainly in females. Heart rates showed marked increases in some
168 mg/kg females prior to death. Food intake was reduced at 48 mg/kg
and above and weight gain was depressed at 16 mg/kg and above.
Hematology, blood chemistry, urinalysis and ophthalmology were
studied after 1 and 4 weeks of treatment. Leucocyte counts were
decreased in a few dogs at 48 and 168 mg/kg and alkaline phosphatase
was increased from 16 mg/kg. Necropsy revealed lower absolute
testicular weight in 48 and 168 mg/kg males but no pathological
alterations (Simon, 1979b).
Groups of 4 male and female beagle dogs were given 0, 2, 10 or 39
mg/kg bw/d albendazole in capsules for 91 days. Ophthalmology,
hematology, blood chemistry and unrinalysis were studied after 1 and
3 months; organ weights, gross and histopathology were examined in all
dogs. There were no toxic signs or effects on body weight and food
intake. No treatment-related effects were found (Killeen & Rapp,
1975b).
Groups of 6 male and 6 female beagle dogs were given 0, 5, 30 or
60 mg/kg bw/d albendazole in capsules for 6 months.
Food intake was decreased in 30 and 60 mg/kg females, while
weight gain was lower in 60 mg/kg males and females. Laboratory
studies included hematology and blood chemistry monthly, urinalysis
bimonthly and ophthalmology at termination. Hemoglobin, hematocrit and
erythrocyte counts were slightly reduced at 60 mg/kg and leucocyte
counts, particularly neutrophils, were reduced at 30 and 60 mg/kg.
Autopsy on all dogs revealed decreased absolute and relative
testes and uterine weights and slight increases in relative liver and
kidney weights at 60 mg/kg. The incidences of small nodules in the
stomach were increased in all treated groups but microscopically they
were shown to be normal, submucosal lymphoid follicles. Sternal bone
marrow showed hypocellularity in 4/6 females in the 60 mg/kg group.
The NOEL was 5 mg/kg bw/day. (Daly & Hogan, 1980).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 100 male and 100 female Charles River CD-1 mice were
fed diets containing albendazole for 25 months. Drug levels were
adjusted to provide doses of 0, 25, 100 or 400 mg/kg bw/d. Additional
groups of 25 males and 25 females were given control and high dose
treatments and used for hematology measurements.
There were no toxic signs or effects on food intake and body
weight. Hematology was studied after 3, 6 , 12, 18 and 24 months in
the main groups and monthly in the ancillary groups. Erythrocyte and
leucocyte counts were decreased and platelets were increased at 400
mg/kg, particularly in females.
A complete gross post-mortem examination was carried out on all
mice. Full histopathology was undertaken on control and high dose
mice. In intermediate groups, 6 major organs and grossly abnormal
tissues were examined routinely. Flaccid or small testes, testicular
tubular degeneration, oligospermia, and aspermia in epididymides were
increased in 400 mg/kg males. Centrilobular hepatocytic vacuolation
was increased in groups given 100 and 400 mg/kg. Eye opacities were
noted in all groups; however, microscopically, cataracts were slightly
increased only in 400 mg/kg males. The relationship of these ocular
findings to treatment is questionable as the bulk of the cataracts
were unilateral and such abnormalities are commonly obtained following
repeated blood collection from the orbital sinus.
The incidence of endometrial stromal polyps appeared to be
increased over concurrent controls (Table 1) but a statistical
evaluation of the results did not reveal significant differences
between groups, and all incidences were within the historical control
range for this laboratory based on 2 studies with 2 control groups in
each. The NOEL was 25 mg/kg bw/d. (Daly & Knezevich, 1987a; Sauer,
1985, 1987b; Selwyn 1987).
Table 1: Tumor incidence in mice
Dose (mg/kg bw/d) 0 0 25 100 400 Historical Controls
Endometrial stromal tumors
Polyps 3/98 5/99 3/98 5/98 7/99 Range 0/55-8/47
Sarcomas 0/98 0/99 1/98 2/98 0/99 Cumulative 29/780
Total 3/98 5/99 4/98 7/98 7/99
2.2.3.2 Rats
Groups of 100 male and 100 female Sprague Dawley CD rats were fed
diets containing albendazole. The initial groups (F0) received doses
of 0, 1, 2.5 or 5 mg/kg bw/d for 60 days and then through mating,
gestation and post-natal periods. Similar size groups of F1 animals
received 0, 3.5, 7 or 20 mg/kg bw/d for 28 months. Additional groups
of 25 males and 25 females were given control and high dose treatments
and used for hematology measurements. An interim sacrifice of 10 males
and females per group was made after 12 months.
Treatment-related effects were not observed in F0 animals. In the
F1 animals, mortality was slightly increased after 24 months in 20
mg/kg males. There were no other toxic signs or effects on body weight
and food consumption. Ophthalmology, hematology, blood chemistry and
urinalysis parameters were studied after 3, 6, 12, 18 and 24 months of
treatment. In the 20 mg/kg group, total leucocyte and neutrophil
counts were decreased at 24 months, serum cholesterol was increased in
females throughout the study and in males at some sampling times.
A complete gross post-mortem examination was carried out on all
rats. Full histopathology was undertaken on control and high dose
rats. In intermediate groups, 8 major organs and grossly abnormal
tissue were examined routinely. The 20 mg/kg animals showed increased
incidences of flaccid testes, degeneration/atrophy of germinal and
relative liver weights in males and hepatic fatty metamorphosis in
males and females.
Compared to concurrent controls, the incidences of
endometrial/cervical tumors and skin histiocytic sarcomas showed an
apparent increase in certain groups of rats (see Table 2 below).
Statistical evaluation of the findings did not reveal significant
differences between groups and in fact all frequencies were within the
historical control range for this laboratory. The NOEL was 7 mg/kg/d.
(Daly & Knezevich, 1987b; Sauer, 1985, 1987a; Selwyn, 1987).
Table 2: Tumor incidence in rats
Dose (mg/kg/d) 0 0 3.5 7 20 Historical Controls
Endometrial/cervical tumors
Polyps 3/99 5/99 9/98 9/99 10/91 Range 1/69-7/58*
Sarcomas 0/99 3/99 0/98 4/99 3/91 Cumulative 120/1864
Total 3/99 8/99 9/98 11/99 13/91
Skin histiocytic sarcomas
Males 1/100 2/100 4/98 4/100 6/100 Range 0/116-6/110**
Cumulative 32/1190
Females 0/100 4/100 0/100 1/96 5/100 Range 1/112-11/119
Cumulative 40/1188
* based on 18 studies
** based on 14 studies.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Long Evans rats were fed diets containing 0, 30, 75 and
150 ppm albendazole for 3 successive generations, commencing 64 d
before the initial mating. Each parental group consisted of 12 males
and 24 females, which were bred to produce 2 litters each. Offspring
from the second litters were selected to serve as parents of the
subsequent generations. Drug intake was calculated to be, on average,
2.3, 5.8 and 11.6 mg/kg bw/d.
There were no toxic signs or effects on body weight, food
consumption, mating, fertility, pregnancy rates, gestation length,
litter size and weight. During lactation, pup survival and/or weight
gain was depressed, but only in F1a and F2a litters given 11.6 mg/kg.
The NOEL was 5.8 mg/kg bw/d. (Schroeder & Rinehart, 1980).
Groups of 20 male Sprague Dawley CD rats were given gavage doses
of 0, 1, 10 or 30 mg/kg bw/d albendozale in 0.5% gum tragacanth, from
60 days prior to mating to the end of the breeding period. Males were
mated 1 to 1 with untreated females. Half the dams were killed on
gestation day 13, the remainder were allowed to deliver naturally and
rear pups to weaning.
In males treated with 30 mg/kg, weight gain was lower and 4
animals died or were killed moribund. Toxic signs were piloerection
and bloody nasal discharge at 30 mg/kg and dried blood around the nose
at 10 mg/kg. The ability to impregnate females was unaffected, despite
the finding in the 30 mg/kg group of reduced testicular size, together
with focal testicular hypoplasia in 8/10 rats. In the 10 mg/kg group,
there were a few hypoplastic seminiferous ducts reported in 4 of the
5 rats examined.
Uterine examinations on gestation day 13 showed fewer
implantations (not significant) at 30 mg/kg with no effect on
resorptions. In females allowed to deliver, weight gain was lower
during gestation in 30 mg/kg dams, probably reflecting the reduced
litter size and weight. Postnatal growth, physical and behavioral
development were unremarkable. The NOEL was l mg/kg bw/day. (Boutemy,
1980).
2.2.5 Special studies on genotoxicity
Table 3: Results of genotoxicity assays on albendazole
Test system Test object Concentration Result Reference
Ames test (1) S.typhimurium 1-10,000 µg/ Negative Jagannath,
TA1535, TA1537 plate 1980a
TA1538, TA98,
TA100
Ames test (2) As above As above Negative Jagannath,
1980b
Ames test (2, 3) As above As above Negative Jagannath,
1981
Ames test (1) S.typhimurium 0.5-1000 µg/ Negative Mourot,
TA97a, TA98, plate 1988
TA100, TA102
CHO chromosome Chinese hamster 0.047-1.5µg/ml Negative Galloway,
aberration ovary cells 1981
assay
Transformation BALB/3T3 mouse 10-100 µg/ml Negative Schechtman,
assay cells 1982
(1)With and without rat liver S-9 fraction
(2)With and without calf and rat liver S-9 fraction
(3)Substance tested was 2-amino sulphone metabolite.
2.2.6 Special studies on eye and skin irritation
Groups of rabbits had 100 mg albendazole powder instilled into
the conjunctival sac or 500 mg albendazole applied, under occlusion,
to abraded and non-abraded skin. There were no primary irritant
effects at any site (Macko et al., 1975b).
2.2.7 Special studies on teratogenicity
2.2.7.1 Mice
Groups of 21 to 26 pregnant Charles River CD-1 mice were given
gavage doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in 0.5%
methylcellulose. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 18.
There was no overt maternal toxicity or effect on resorption
incidence, fetal weight and external, visceral and skeletal
development of fetuses (Killeen & Rapp, 1975c).
2.2.7.2 Rats
Groups of 25 pregnant Charles River CD rats were given gavage
doses of 0, 5, 20 or 40 mg/kg bw/d albendazole in 0.5%
methylcellulose. In each group 19 rats were treated on gestation days
16 to 20 and 6 were treated from gestation day 16 to lactation day 20.
All dams were allowed to litter naturally.
There was no maternal toxicity or effect on gestation or
parturition. At 40 mg/kg, litter size and weight were reduced at birth
and remained depressed during lactation. A total of 6, 17, 4 and 55
pups died in the 0, 5, 20 and 40 mg/kg groups, respectively. Small
lungs and kidneys and anasarca in the 40 mg/kg pups were possibly
related to treatment, but an unequivocal conclusion was not possible
in view of the low number of control pups examined. The authors
concluded that developmental and behavioral characteristics were
unrelated to albendazole treatment, but detailed supporting data were
not provided. The NOEL was 20 mg/kg bw/day. (Johnson, 1981).
A series of studies was carried out at Biodynamics in Long Evans
rats. A similar protocol was used in each study, which included dosing
on gestation days 6 to 15; sacrifice of dams on gestation day 20;
maternal observations for overt toxicity, weight gain and uterine
parameters; fetal examination for size, weight, and external, visceral
and skeletal abnormalities.
Study A Groups of 20 pregnant rats were given gavage doses of 0, 2,
5, 10 or 30 mg/kg bw/d albendazole in 0.5% methylcellulose. Maternal
weight gain and survival were lower at 30 mg/kg. At this dose, there
was a high incidence of resorptions, surviving fetuses showed reduced
size, weight and multiple gross, visceral and skeletal malformations.
Limb abnormalities such as micromelia, ectromelia, curved femur and
microfetalis were also seen in other treated groups. However, the
incidences were low, they were not dose-related and they were observed
only in 1 or 2 litters per group (Killeen & Rapp, 1975e; Christian,
1984, 1987a.).
Study B Groups of 19-20 pregnant rats were given gavage doses of 0,
0.5, 2, 5 or 10 mg/kg bw/d albendazole in 0.5% methylcellulose.
Fetuses of the 10 mg/kg group revealed reduced size and weight,
retarded skeletal ossification and increased incidences of micromelia
and microfetalis (which included shortened long bones in fore and hind
limbs) (Killeen & Rapp, 1976; Christian, 1984, 1987a).
Study C Groups of 30 to 60 pregnant rats were fed diets containing
29% freeze dried liver, obtained from cattle 48 hours after a single
oral dose of 0 or 27.5 mg/kg bw albendazole. Drug intake was
calculated to represent 0.42 mg/kg bw/d albendazole equivalents. The
only possible treatment-related observation was shortened limb bones
in 2/248 treated fetuses from 2 different litters. These effects were
not seen in 460 controls and the authors noted that they were an
infrequent finding at Biodynamics (Hogan & Rinehart, 1977).
A re-evaluation of these affected fetuses failed to confirm the
above description. The apparent defects were described as artifacts
caused by poor staining (Christian, 1987b).
Study D Groups of 20 to 22 pregnant rats were fed diets containing
10, 20 or 30% freeze dried liver, obtained from cattle 12 days after
a single oral dose of 0 or 16.5 mg/kg bw albendazole. Drug intake was
calculated to be approximately 0.02, 0.04 and 0.06 mg/kg bw/d
albendazole equivalents. In the group given 30% exposed liver,
resorptions were increased, 9 of which were in 1 female. If data from
this rat were eliminated there were no significant effects. The
overall NOEL for studies A to D was 5 mg/kg bw/day. (Schroeder &
Rinehart, 1978).
Groups of pregnant Sprague Dawley rats were given gavage doses of
0, 5.3, 6.0, 6.62, 8.83, 10.6 or 13.25 mg/kg bw/d albendazole or 9 of
its animal metabolites at equimolar or higher doses. Treatments were
on gestation days 8 to 15 and dams were killed on gestation day 21.
The results were presented in summary form only.
Skeletal abnormalities were increased at dose levels of 6.62
mg/kg and greater with increases in resorptions and external
malformations and decreased fetal weight at 8.83 mg/kg albendazole and
above. The major malformations were craniofacial and bone defects.
Qualitatively similar findings were obtained with equimolar amounts of
albendazole sulfoxide while the other metabolites A, B, E, F, G, J, I
and H (section 2.1) were all ineffective. The concomitant
administration of SKF-525-A, a microsomal oxidation inhibitor, almost
completely suppressed the embryotoxic and developmental effects of
albendazole. The NOEL was 6 mg/kg bw/day. (Martin, 1980).
Groups of pregnant Sprague Dawley rats were given diets
containing albendazole or 40% freeze dried liver, obtained from cattle
24, 48 or 96 hours after a single oral dose of 0 or 20 mg/kg bw
albendazole. Drug levels in food were adjusted to provide 0, 12, 24 or
36 mg/kg bw/d but doses given through incorporation of liver could not
be estimated. Treatment of rats was on gestation days 8 to 15 and dams
were killed on gestation day 21. Albendazole doses of 24 and 36 mg/kg
produced virtually 100% embryolethality, the single live fetus was
small and had skeletal abnormalities. There were no effects at other
exposure levels. The NOEL was 12 mg/kg bw/day. (Grannec, 1980).
2.2.7.3 Rabbits
Groups of 15 pregnant New Zealand White rabbits were given gavage
doses of 0, 2, 5, 10 or 30 mg/kg bw/d albendazole in methylcellulose.
Treatment was on gestation days 7 to 19 and does were killed on
gestation day 30.
Maternal mortality was increased at 30 mg/kg but body weight
comparisons were not meaningful due to wide variation within groups.
The 30mg/kg group showed a reduction in implants, due largely to 2
does with corpora lutea but no implants, and increases in resorptions
and ectrodactyly. Fetal size and weight were depressed at 10 and 30
mg/kg. The NOEL was 5 mg/kg bw/day. (Killeen & Rapp, 1975d).
2.2.7.4 Sheep
In two separate experiments, groups of 15 to 44 Dorset Horn Cross
and Clun mated ewes were given a single dose of 0, 7.5, 10, 15 or 20
mg/kg bw albendazole by oral drench. Overall, there were 71, 43, 44,
43 and 42 animals in the 0, 7.5, 10, 15 and 20 mg/kg groups,
respectively. Treatment was on gestation day 17 and ewes were allowed
to deliver naturally.
There was no overt maternal toxicity but premature delivery was
noted in more ewes of the 20 mg/kg group than in the others. All
premature lambs in this group were stillborn, consequently the number
of live lambs was reduced at 20 mg/kg, as was survival of the lambs to
post partum day 55. Some lambs were sacrificed in extremis or because
they were considered commercially unviable, thus the total post partum
loss was 22/123, 4/67, 11/73, 12/73 and 39/61 in the 0, 7.5, 10, 15
and 20 mg/kg groups, respectively. Post-mortem examination of these
lambs revealed increased incidences of prognathia, scoliosis, spina
bifida and reduced tail at 20 mg/kg and displaced, poorly developed or
absent kidneys at 15 and 20 mg/kg. The NOEL was 10 mg/kg bw/d. (Tesh
& Harper, 1977).
The data shown in Table 4 indicates a relationship between
teratogenesis and peak plasma concentration of albendaxole sulfoxide.
Data from Bogan & Marriner (1984).
Table 4. Relationship between plasma albendazole sulfoxide
and teratogenesis
Species Albendazole Dose Peak Plasma Teratogenic
(mg/kg bw) Albendazole
(mg/ml)
Sheep 10 2.50 Yes
Cattle 10 0.57 No(1)
Rabbit 30 8.82 Yes
Rat 10 6.6 Yes
Mouse 30 n.a.(3) No
Man 400 mg/person 0.16 No(2)
(1) Embryotoxic but not teratogenic
(2) No intentional studies performed
(3) Not available
2.2.8 Special studies on mode of action of benzimadoles
Benzimadazoles bind with tubulin and inhibit its polymertisation
to microtubules. A comparison of inhibitory concentrations for some
benzimadazoles in nematodes and mammalian cells reveals a selective
action (reviewed in Sharma & Abuzar, 1983).
2.3 Observations in man
Albendazole has been used in 80 countries for 6 years to treat
gastrointestinal parasitic infections in man at a recommended dose
rate of 400 mg/person. There are a number of published reports on the
use of albendazole in man (see Marriner et al., 1986; Lange et al.,
1988).
In field trials in Nigeria, it was reported that 17 nulliparous
women aged between 16 and 18 years were inadvertently given a single
dose of 400 mg albendazole in the first trimester of pregnancy without
any adverse effects on the mother or child (A.B.C. Nwosu, 1985).
3. COMMENTS
Comprehensive toxicological data on albendazole were submitted
including the results of studies on its metabolism, carcinogenicity,
genotoxicity, effects on reproduction and teratogenicity, and
short-term studies.
Pharmacokinetic studies, although not specifically designed to
measure the extent of absorption, suggested that about 20-30% of
ingested albendazole was absorbed in mice and rats, about 1% in humans
and 50% in cattle. In all the species studied, oral dosing produced
very low plasma levels of unchanged drug, because of rapid first-pass
metabolism in the liver. The primary metabolic reactions were the
oxidation of the sulfide moiety of albendazole to the sulfoxide and
sulfone, followed by cleavage of the carbamate moiety to form the
2-amino-sulfone. This last compound was found to be the main residue
in the livers of sheep and cattle. The degradation of albendazole
followed similar pathways in rats, mice, cattle, sheep and humans.
In a study in mice in which albendazole was administered in the
diet for 25 months, anemia, leukopenia and testicular degeneration
were noted at 400 mg/kg bw/day. Hepatocellular vacuolation was
produced at 100 and 400 mg/kg bw/day, endometrial stromal polyps was
slightly higher than in concurrent controls, but statistical
significance was not achieved and all incidences were within the
laboratory historical control range. The NOEL was 25 mg/kg bw/day.
In a 28-month study in the rat in which the compound was given
in the diet, the highest dose of 20 mg/kg bw/day caused mortality,
neutropenia, hypercholesterolemia, testicular degeneration and
hepatic fatty metamorphosis. The incidence of endometrial/cervical
tumors and histiocytic carcomas in the skin showed an apparent
increase in certain treated groups. However, when compared with
those for the controls, these findings were not statistically
significantly different and were within the laboratory historical
control range. The NOEL was 7 mg/kg bw/day.
Questions had been raised regarding the statistical analyses of
the mouse and rat carcinogenicity studies and the use of historical
controls in the examination of the tumor incidence. The studies were
reviewed and were determined to be satisfactory. The statistical
analysis of both rodent carcinogenicity studies was also reviewed and
found to have been conducted in accordance with currently acceptable
procedures.
Albendazole did not produce bacterial mutations, chromosomal
aberrations or morphological transformations in cultured mammalian
cells. The 2-aminosulphone metabolite did not produce bacterial
mutations.
A three-generation reproduction study in which the compound was
administered in the diet was conducted in rats. There were no effects
on fertility or reproduction indices. The only findings were
reductions in pup postnatal survival and growth at 11.6 mg/kg bw/day.
The NOEL was 5.8 mg/kg bw/day.
Male rats were treated by gavage in a fertility study. Overt
toxic effects and testicular hypoplasia were noted at 10 and 30 mg/kg
bw/day. Fertility indices were not affected. Litter size and weight
were reduced at 30 mg/kg bw/day, the former effect being probably due
to a lower implantation rate. The NOEL was 1 mg/kg bw/day.
In a perinatal and postnatal study in rats in which albendazole
was administered by gavage, there was decreased survival and growth
in utero and during lactation in the pups of females given 40 mg/kg
bw/day group. There was also some evidence of retarded organ
development in the offspring of this group. The NOEL was 20 mg/kg
bw/day.
In a teratology study in mice in which albendazole was
administered by gavage, there were no adverse effects at dose levels
up to 30 mg/kg bw/day.
Several teratology studies were conducted in the rat in which the
compound was administered either by gavage or by dietary exposure.
Embryotoxic, fetotoxic effects and external malformations were
produced at doses of 8.8 mg/kg bw/day and above. Skeletal
malformations, in particular limb defects, which were seen at doses of
6.62 mg/kg bw/day, constituted the most sensitive indicator of
developmental toxicity. The NOEL was 5 mg/kg bw/day. When administered
in equimolar amounts, qualitatively similar findings were obtained
with albendazole sulphoxide, while eight other metabolites of
albendazole did not show any effects.
In rabbits, the toxic dose to the dams of 30 mg/kg bw/day was
associated with embryotoxicity and partially or totally missing
digits. Fetal growth retardation was observed at doses of 10 mg/kg
bw/day and above. The NOEL was 5 mg/kg bw/day.
A teratology study was conducted in sheep using a single oral
dose of albendazole on day 17 of pregnancy. Premature delivery,
fetotoxic effects and postnatal death were produced at 20 mg/kg bw and
malformations were increased at 15 and 20 mg/kg. The NOEL was 10 mg/kg
bw/day.
Dogs were dosed for six months by the administration of the
compound in capsules. They showed neutropenia at 30 mg/kg bw/day and
above. At 60 mg/kg bw/day there was also anemia, decreased body,
testicular and uterine weights, and bone marrow hypocellularity. The
NOEL in this study was 5 mg/kg bw/day.
Albendazole has been used in 80 countries to treat
gastrointestinal parasitic infections in humans at a dose rate of 400
mg/person, and there are a number of published reports on its use. In
field trials in Nigeria, an unpublished report noted that 17
nulliparous women aged between 16 and 18 years were inadvertently
given a single dose of 400 mg albendazole during the first trimester
of pregnancy without any apparent adverse effects on the mother or the
neonate.
Some of the effects observed in general toxicity studies with
albendazole may be explained by one of the biological actions of the
benzimadazoles, which is to interfere with tubulin polymerization and
thus inhibit spindle formation and mitosis.
The most significant toxicological manifestation resulting from
treatment with albendazole was its teratogenic activity, limb defects
in the rat being the most sensitive indicator of developmental
toxicity.
A NOEL of 5 mg/kg bw/day was reported in several studies in rats,
rabbits and dogs. Although for males in the rat fertility study the
figure was 1 mg/kg bw/day, it was noted that the next highest dose
used in the study was 10 mg/kg bw/day. In addition, in the
multigeneration reproduction study in rats, there was no effect on
fertility at 11.6 mg/kg bw/day, which was the highest dose used, and
the reported NOEL was 5.8 mg/kg bw/day, based on depression of weight
gain in pups.
An ADI of 0-0.05 mg/kg bw was established for albendazole based
on a NOEL of 5 mg/kg bw/day and using a safety factor of 100.
A safety factor of 100 was chosen for this compound after taking
into consideration poor absorption in humans, rapid metabolism, the
lack of teratogenic potential of most metabolites, the use of the drug
in humans and the identity of residues in food.
4. EVALUATION
Level causing no toxicological effect
Rat, rabbit, dog equal to 5 mg/kg bw/day.
Estimate of acceptable daily intake
0-0.05 mg/kg bw.
5. REFERENCES
BOGAN, J.A. & MARRINER, S.E. (1984). Pharmacodynamic and toxicological
aspects of albendazole in man and animals. In: Fock, M. (Ed.).
Albendazole in helminthiasis. Royal Society of Medicine International
Congress and Symposium Series No. 61. London, Royal Society of
Medicine, pp. 13-21.
BOUTEMY, C. (1980). Expert study of the action of the compound
albendazole on the fertility of the male rat, per os. Unpublished
study No. 2477 RSR from IFM Research Center. Submitted to WHO by
SmithKline and French.
BRAUN, W.G. & KILLEEN, J.C. (1975). Acute oral toxicity in rats,
compound No. SK&F 62979. Unpublished project No. 2605-75 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1984). Review of albendazole rat teratogenicity
studies. Unpublished report from Argus International. Submitted to WHO
by SmithKline and French.
CHRISTIAN, M.S. (1987a). Review of reported "micropodia" in
albendazole rat teratogenicity studies. Unpublished report from Argus
International. Submitted to WHO by SmithKline and French.
CHRISTIAN, M.S. (1987b). Review of developmental toxicity
(embryo-fetal toxicity/teratogenicity) and reproductive toxicity
studies of albendazole in mice, rabbits and rats. Unpublished report
from Argus International. Submitted to WHO by SmithKline and French.
COLMAN, W., TOWNER, C., TOWNER, D. & SOKOLEK, J. (1977). Metabolic
fate of albendazole in sheep tissues. Unpublished report from
Applebrook Research Centre. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1980). A six-month oral toxicity study with
albendaxole in dogs. Unpublished project No. 790-2371 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1981). A toxicity study of albendazole in
rats. Unpublished project No. 80-2449 from Biodynamics Incorporated.
Submitted to WHO by SmithKline and French.
DALY, I.W. & HOGAN, G.K. (1982). A long-term oral dietary toxicity
carcinogenciity study of albendazole in rats. Unpublished project No.
79-2383 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987a). A long-term oral (dietary)
carcinogenicity study of albendazole in mice. Unpublished project No.
80-2480 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
DALY, I.W. & KNEZEVICH, A.L. (1987b) A long-term oral
toxicity/carcinogenicity study of albendazole in rats. Unpublished
project No. 80/2448 from Biodynamics Incorporated. Submitted to WHO by
SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980a). A three month dose range finding
study with albendazole in mice. Unpublished project No. 79-2368 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
DALY, I.W. & RINEHART, W.E. (1980b). A three month feeding study of
albendazole in mice. Unpublished project No. 79-2423 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
DELATOUR, P., GARNIER, F., BENOIT, E. & LONGIN, Ch. (1984). A
correlation of toxicity of albendazole and oxfendazole with their free
metabolites and bound residues. J.Vet.Pharmacol.Therap., 7, 139-145.
GALLOWAY, S.M. (1981). Mutagenicity evaluation of albendazole (SK&F
62979) in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished project No. 22000 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
GRANNEC, C. (1980). Albendazole: relay embryotoxicity. Thesis for
D.Vet.Sci. Claude Bernard University, Lyons. Submitted to WHO by
SmithKline and French.
GYURIK, R.J. et al., (1981). Metabolism of albendazole in cattle,
sheep, rats and mice. Drug Metabolism and Disposition 9, 503.
HOGAN, G.R. & RINEHART, W.E. (1977). A segment II teratologic relay
toxicity study in rats of liver obtained from calves treated with
albendazole. Unpublished project No. 76-1443A from Biodynamics
incorporated. Submitted to WHO by SmithKline and French.
JAGANNATH, D.R. (1980a). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome plate test. Unpublished
project No. 20988 from Litton Bionetics. Submitted to WHO by
SmithKline and French.
JAGANNATH, D.R. (1980b). Mutagenicity evaluation of albendazole (SK&F
62979) in the Ames Salmonella/microsome preincubation plate test.
Unpublished project No. 20998 from Litton Bionetics. Submitted to WHO
by SmithKline and French.
JAGANNATH, D.R. (1981). Mutagenicity evaluation of SK&F 81038 in the
Ames Salmonella/microsome plate test. Unpublished project No. 20988
from Litton Bionetics. Submitted to WHO by SmithKline and French.
JOHNSON, D.E. (1981). Perinatal and postnatal study in rats.
Unpublished report No. 483-001 from International Research and
Development Corporation. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975a). A three month oral toxicity study
of SK&F 62979 in rats. Unpublished project No. 75-1109 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. 6 RAPP, W.R. (1975b). A three month oral toxicity study
of SK&F 62979 in dogs. Unpublished project No. 75-1110 from
Biodynamics Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975c). A segment II mouse teratology
study of SK&F 62979. Unpublished project No. 74-1096 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1975d). A teratology study of SK&F 62979
in rabbits. Unpublished project No. 75-11234 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP. W.R. (1975e). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 74-1947 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KILLEEN, J.C. & RAPP, W.R. (1976). A segment II rat teratology study
of SK&F 62979. Unpublished project No. 75-1274 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
KRAEER, P., TOWNER, D. & SOKOLEK, J. (1977). Metabolic fate of
albendazole in cattle tissues. Unpublished report from Applebrook
Research Center. Submitted to WHO by SmithKline and French.
LANGE, H., EGGERS, R. & BIRCHER, J. (1988). Increased systemic
availability of albendazole when taken with a fatty meal.
Eur.J.Clin.Pharmacol., 34, 315-317.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975a). Local
irritation, skin sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MACKO, E., HORNER, E., DeVAN, J. & WHITE, R. (1975b). Local
irritation, skin, sensitivity and acute toxicity studies using SK&F
62979. Unpublished report from SmithKline and French Laboratories.
Submitted to WHO by SmithKline and French.
MARRINER, S.E. & BOGAN, J.A. (1980). Pharmacokinetics of albendazole
in sheep. Am.J.Vet.Res., 41, 1126-1129.
MARRINER, S.E., MORRIS, D.L., DICKSON, B. & BOGAN, J.A. (1986).
Pharmacokinetics of albendazole in man. Eur.J.Clin.Pharmacol., 30,
705-708.
MARTIN, D. (1980). Albendazole: embryotoxic study of ten metabolites.
Thesis for D.Vet.Sci. Claude Bernard University, Lyons. Submitted to
WHO by SmithKline and French.
MOUROT, D. (1988). Albendazole: Ames test with and without metabolic
activation. Unpublished report from Laboratoire National des
Médicaments Vétérinaires. Submitted to WHO by SmithKline and French.
NWOSU, A.B.C. (1985). Albendazole field trials in Nigeria. Unpublished
report submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977a). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C., CHOW, A.W. & GYURIK, R.J. (1977b). The isolation and
characterization of radioactive metabolites from the urine of steers
treated orally with 14°C; labelled SK&F 62979 (Albendazole).
Unpublished report from Applebrook Research Center. Submitted to WHO
by SmithKline and French.
PARISH, R.C., CHOW, A.W., GYURIK, R.J. & ZABER, B. (1977c). The
isolation and characterization of radioactive metabolites from the
urine of sheep treated orally with 14°C-labelled SK&F 62979
(Albendazole). Unpublished report from Applebrook Research Center.
Submitted to WHO by SmithKline and French.
PARISH, R.C. & GYURIK, R. (1979). Urinary excretion and identification
of metabolites from urine of Sprague-Dawley rats orally dosed with
albendazole - 14°C albendazole sulfoxide - 14°C and albendazole
sulfone - 14°C. Unpublished report No. A-4003-78 from Applebrook
Research Centre. Submitted to WHO by SmithKline and French.
PARISH, R.C., GYURIK, R. & BRUNER, E. (1979a). The isolation and
quantitation of metabolites from urine of Charles River mice treated
orally with 14°C-labelled SK&F 62979 (Albendazole). Unpublished report
No. A-4006-78 from Applebrook Research Center. Submitted to WHO by
SmithKline and French.
PARISH, R.C., GYURIK, R.J. & BRUNER, E. (1979b). The isolation and
quantitation of radioactive metabolites from the urine of steers
treated orally with 14°C-labelled SK&F 62979. Unpublished report from
Applebrook Research Center. Submitted to WHO by SmithKline and French.
PRICHARD, R.K., HENNESSY, D.R., STEEL, J.W. & LACEY, E. (1985).
Metabolite concentrations in plasma following treatment of cattle with
five anthelmintics. Res.Vet.Sci., 39, 173-178.
ROSSIGNOL, J.F. & MAISONNEUVE, H. (1984). Albendazole: a new concept
in the control of intestinal helminthiasis. Gastroenterol.Clin.Biol.,
8, 569-576.
SAUER, R.M. (1985). Pathology Working Group report on albendazole in
Sprague-Dawley rats and CD-1 mice. Unpublished report from Pathco
Incorporated. Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987a). Pathology Working Group report on albendazole in
Sprague-Dawley rats. Unpublished report from Pathco Incorporated.
Submitted to WHO by SmithKline and French.
SAUER, R.M. (1987b). Pathology Working Group report on albendazole in
CD-1 mice. Unpublished report from Pathco Incorporated. Submitted to
WHO by SmithKline and French.
SCHECHTMAN, L.M. (1982). Activity of T1709 in the in vitro mammalian
cell transformation assay in the presence of exogenous metabolic
activation. Unpublished project No. T1709.109 from Microbiolobical
Associates. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E., & RINEHART, W.E. (1978). A segment II teratologic
relay toxicity study in rats on liver obtained from cattle treated
with albendazole. Unpublished project No. 77-1825 from Biodynamics
Incorporated. Submitted to WHO by SmithKline and French.
SCHROEDER, R.E. & RINEHART, W.E. (1980). A three-generation
reproduction study with albendazole in rats. Unpublished project No.
77-2019 from Biodynamics Incorporated. Submitted to WHO by SmithKline
and French.
SELWYN, M.R. (1987). Statistical analysis for oral carcinogenicity
studies of albendazole in mice and rats. Unpublished study from
Statistics Unlimited Inc. Submitted to WHO by SmithKline and French.
SHARMA, S. & ABUZAR, S. (1983). The benzimadozole anthelmintics -
chemistry and biological activity. Prog. Drug Res., 27, 85-161.
SIMON, J.C. (1979a). 4-week sub-acute oral toxicity study in rats.
Unpublished study No. 2206 TSR from IFM Research Center. Submitted to
WHO by SmithKline and French.
SIMON, J.C. (1979b). Sub-acute toxicity study in dogs. Unpublished
study No. 2204 TSC from IFM Research Center. Submitted to WHO by
SmithKline and French.
SOUHAILI-EL AMRI, H., FARGETTON, X., BENOIT, E., TOTIS, M. & BATT,
A-M. (1988). Inducing effect of albendazole on rat liver
drug-metabolising enzymes and metabolite pharmacokinetics.
Toxicol.App.Pharmacol., 92, 141-149.
TESH, J.M. & HARPER, J.A. (1977). Albendazole (SK&F 62979): effects of
single oral administration upon pregnancy in sheep. Unpublished report
No. 77/SAF210/205 from Life Science Research. Submitted to WHO by
SmithKline and French.
WALKER, T.F. (1976a). The acute oral toxicity of SK&F 62979
(Albendazole) in the rat. Unpublished report No. B 335A from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976b). The acute oral toxicity of SK&F 62979
(Albendazole) in the hamster. Unpublished report No. B 335C from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976c). The acute oral toxicity of SK&F 62979
(Albendazole) in the guinea pig. Unpublished report No. B 335B from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
WALKER, T.F. (1976d). The acute oral toxicity of SK&F 62979
(Albendazole) in the rabbit. Unpublished report No. B 335D from
SmithKline and French Laboratories. Submitted to WHO by SmithKline and
French.
