
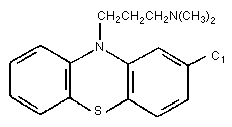
CHLORPROMAZINE
First Draft prepared by
Dr. Radovan Fuchs, Institute for Medical Research
and Occupational Health, University of Zagreb, Yugoslavia
1. EXPLANATION
Chloropromazine belongs to the group of phenothiazine
derivatives. It is widely used in human medicine in the therapy of
schizophrenia, organic psychoses and the manic phase of
manic-depressive illness. In veterinary medicine it is used as a
tranquillizer and antiemetic agent. This compound has not been
previously evaluated by the Joint FAO/WHO Expert Committee on Food
Additives.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Intestinal absorption of chlorpromazine is complete but it is
variably metabolized during its passage through the intestinal wall.
More than 90% of the drug in plasma is bound to proteins. It is
metabolized mainly in liver, and there is an indication that it may
accelerate its own hepatic metabolism or conjugation; in humans,
after several weeks of treatment, the concentration of
chlorpromazine in blood is lower with level dosage. After being
absorbed the drug is widely distributed in the body and its
lipophilicity allows it to achieve sufficient intra-membrane
concentration to influence the stability or fluidity of the cell
membrane (Baldessarini, 1975).
The major metabolic pathways of chlorpromazine are
hydroxylation and conjugation with glucuronic acid. Oxidative
processes, as well, play a very important role in biotransformation
of the drug, and the sulfoxide form possesses about one-eighth the
sedative action of the parent drug in dogs (Booth, 1988). Among 10
to 12 metabolites occurring in man (Baldessarini, 1975) N-oxide
metabolites undergo significant reduction back to the parent
compound in a number of species including man (Jaworski et al.,
1990).
The biological half-life of chlorpromazine is about 6 hours in
dogs. After intravenous administration of 2.5 mg/kg b.w. of
chlorpromazine to the goat the plasma elimination half-life is 1.51
± 0.48 hours (Nawaz, 1981). In the goat, the concentration of the
drug is higher in milk than in plasma (Nawaz & Rasmussen 1979).
After the drug was administered intravenously or orally to horses
its metabolites were detected in urine for up to 96 hours. Only 10%
or 27% of the dose, respectively, is recovered in the urine of
horses following an intravenous or oral administration (Booth,
1988).
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Acute toxicity of chlorpromazine
Species Sex Vehicle Route LD50 Reference
(mg/kg b.w.)
Mouse * * p.o. 135 Nagai et al., 1976
* * i.p. 136 Fujimori & Cobb,
1965
* * i.p. 115 Herr et al., 1985
* * i.v. 51 Domenjoz &
Theobald, 1959
M&F * i.v. 20 Casagrande et al.,
1971
Rat * * p.o. 210 Irwin et al., 1959
* * i.p. 71
* * i.v. 49 Domenjoz &
Theobald, 1959
* * i.v. 23 Irwin et al. 1959
Rabbit * * i.v. 16 Domenjoz &
Theobald, 1959
Dog * * i.v. 30 Irwin et al. 1959
* Details not reported
2.2.2 Short-term studies
2.2.2.1 Guinea pigs
Groups of 8 male and female guinea pigs were injected
intraperitioneally with chlorpromazine dissolved in physiological
saline at a dose of 30 mg/kg b.w./day for 7 consecutive days. The
animals were killed by decapitation on the 8th day after first
dosing. Necropsies were performed and tissue samples were taken from
ileum, colon and caecum only.
In 7 out of 8 animals numerous delicate fibrous adhesions at
the peritoneal surface were seen. Focal areas of haemorrhage were
found on the peritoneal surface of the caecum. Histopathologically,
occasional fibrous adhesions without other changes were observed in
ileum and colon. In 4 out of 8 animals the caecum showed marked
submucosal oedema. In some areas inflammatory changes and
haemorrhages were observed (Szanto et al., 1988).
2.2.3 Long-term/carcinogenicity studies
Data not available.
2.2.4 Reproduction studies
2.2.4.1 Mice
Chlorpromazine was given orally at a dose of 16 mg/kg b.w./day
throughout the pregnancy period of C57BL10 mice. A decreased number
of pregnancies, increase in days between mating and birth and
reduced weight gain throughout pregnancy were noted. Maternal brain
weight, liver glycogen and serum cholesterol were reported to be
changed after chlorpromazine treatment. Statistically significant
differences were observed in mean litter weights, brain, liver,
heart, organ to body weight ratios, and serum and organ biochemistry
of offspring between chlorpromazine-treated and control groups. The
details were not given in the report (Rolsten, 1967).
Forty-four newborn LACA strain mice were injected
subcutaneously with a single dose of 20 mg/kg b.w. chlorpromazine
disolved in distilled water on days 4, 6, 7, 8, 9, and 10 after
birth. The animals were killed at 30 days of age. Testes and seminal
vesicles were removed, weighed and examined histopathologically. A
group of 7 untreated mice was used as control. A general increase in
the proportion of tubules which contained spermatids, spermatozoa,
and luminal spermatozoa was observed. The most prominent effect was
seen in animals injected at day 7. In this group of animals a
greater testis weight and seminal vesicle weight was noticed as
well. Generally it was shown that the single injection of
chlorpromazine at a dose of 20 mg/kg b.w., when administered up to
the 10th day of life, accelerated sexual maturation of male mice
(Hogarth & Chalmers, 1973).
Two groups of 20 female inbred C5BL/10 mice received orally 4
or 16 mg/kg b.w./day of chlorpromazine throughout the period of
pregnancy. Control groups consisting of the corresponding number of
animals were treated with the same volume of placebo. The treatment
with chlorpromazine started on the 6th day after mating. All the
animals were tested daily in the open field test for exploration and
activity level. Effects of the drug on delays between mating and
birth, drug-induced changes in maternal weight, and litter size and
weight were recorded.
There was no statistically significant difference in body
weight of dams among the groups at the time of birth. No difference
was observed in behaviour between the animals of the 4 mg/kg
b.w./day group compared to the controls. In the 16 mg/kg b.w./day
group frequent sedation lasting 1 to 5 hours was noticed after
treatment. In the high-dose group, animals showed a statistically
significant delay between mating and birth, and treated animals had
a significantly lower number of offspring. When the two dose levels
were combined, the mean litter weights were also significantly lower
than the mean weight of litters from control dams (Orday et al.,
1963).
2.2.4.2 Rats
A group of 24 male albino rats (Rattus norvegicus) was given
chlorpromazine i.m. at a dose of 1 mg/animal/day, equivalent to
5 mg/kg b.w./day (calculated on a rat weight of 200 g), for 7 or 15
consecutive days. Groups consisting of 12 rats served as controls.
On day 8 or 16 animals were killed and necropsied. Testes, caput and
cauda epididymis were excised, blotted free of blood, weighed and
used for biochemical studies.
Significant decreases in the weight of testes, caput and cauda
epididymides were observed as well as altered activity of some
androgen dependent enzymes. An overall decrease in the level of free
ascorbic acid, succinate dehydrogenase, alkaline phosphatase and
increases of acid phosphatase and cholesterol level in testis and
epididymides were seen (Clinoy & Seethalakshmi, 1977).
Chlorpromazine was given i.m. to female rats on the 4th day of
pregnancy at a dose of 20 mg/kg b.w. It was found that the drug
disturbed the late stage of pregnancy. Details were not reported
(Bovet-Natti & Bovet, 1959).
A separate study testing sexual behaviour was performed on
twelve 150-day-old Sprague-Dawley male rats. Chlorpromazine was
injected intraperitoneally at a single dose of 2.5 mg/kg b.w.
Placebo consisted of equivalent volume of distilled water injected
to twelve control animals. It was found that treatment with
chlorpromazine reduced the number of copulations preceding
ejaculation. The number of copulations/minute, or rate of
copulations, was significantly reduced (Gillett, 1960).
2.2.5 Special studies on genotoxicity
Table 2. Results of genotoxicity assays on chlorpromazine
Test system Test object Concentration Result Reference
Chromosomal Human 0.24-2.0 µg/ml positive Jin-fu et al.
aberration lymphocytes 1988
assay
SRCE Human 0.25-2.0 µg/ml positive Jin-fu et al.
lymphocytes 1988
Ames test1 S. typhimurium 5-10 µg/ml positive Obaseiki-Ebor
TA97 his, TA & Akerele,
102 his, EE97, 1988
EE102
transconjugates)
Fluctuation E. coli 2-4 µg/ml positive Obaseiki-Ebor
test & Akerele,
1988
1. With rat liver S9 fraction
2.2.6 Special studies on embryotoxicity and teratogenicity
2.2.6.1 Mice
Groups of 10 pregnant 3-month-old mice were given
chlorpromazine at a dose of 1.8 and 9.2 mg/kg b.w./day. The drug was
injected intraperitoneally once daily from the 6th to the 16th day
of gestation. The mice were killed 2 or 3 days before parturition.
The average growth weight, increased body weight and fetal
malformations were recorded. A negative control group receiving
0.3 ml saline and a positive control group receiving 0.3 ml of
cod-liver oil containing vitamins A and D were injected with
corresponding solutions in the same way and at the same gestation
days as drug-treated groups.
Incidence of abnormal mouse fetuses was significantly higher
in treated groups and positive controls when compared to the
negative control group. Mean body weight of fetuses from treated
dams was lower. The percentage of malformed fetuses was 38.5% in the
low-dose and 42.9% in the high-dose group, 0% in the negative
control and 28.6% in the positive control group. A description of
the malformations was not given (Jin-fu et al., 1988).
2.2.6.2 Rats
Female CF rats were given chlorpromazine intraperitoneally at a
single dose of 100 mg/kg b.w. on the 14th day of gestation. The
controls were injected with a corresponding volume of physiologic
saline. The number of treated animals was not given. Between the
16th and the 20th day of gestation the fetuses were collected by
uterotomy. Only living fetuses and intact preparations were used in
this study.
It was found that ossification was delayed by 1 to 3 days in
the long bones of the extremities, by 1 day in scapulae and by 2 to
3 days in the ileum. Ischium and pubis remained unossified until the
20th day of gestation. Ossification of the skull bones was also
delayed. The sternebrae were found to be most affected (Singh &
Padmanabhan, 1979).
Six compounds have been studied for teratogenic potential on
Wistar/H-Riop pregnant rats. Groups of 5 pregnant rats were given
single oral equimolar doses of 3.7 x 10-4M/kg b.w. of perphenzine;
chlorpromazine; chlorcyclizine; thenalidine; haloanisone and
haloperidol. The dose of chlorpromazine coresponded to 0.585 mg/kg
b.w. The drugs were given to the animals on the 13th, 14th or 15th
day of gestation respectively. Mothers were killed on the 21st day
of pregnancy. Resorption, living and dead fetuses, fetal weight and
external malformations were recorded.
Higher fetal mortality (P<0.01) was observed in the
chlorpromazine treated group compared to controls. The weight of
fetuses was significantly lower (P<0.01) as well. The data indicate
an embryotoxic effect of the drug in rats (Druga et al., 1980).
Groups composed of 19-20 pregnant CAW; CFE (SD) rats were
treated with chlorpromazine from day 6 through day 15 of gestation.
Tablets of the drug were pulverized and administered to the animals
orally in 2.5% aqueous Tween solution. The dose was adjusted so that
each group received 5, 25 or 35 mg/kg b.w./day chlorpromazine. On
day 21 of pregnancy the dams were killed and the offspring removed.
The numbers of live fetuses, resorptions and nidations, sex and
weight of each live fetus were recorded.
The average litter size in the high-dose group was
significantly lower (P<0.05), and the percentage of resorption in
the intermediate and high-dose groups was higher (P<0.01), than in
controls. Compared to the controls the body weight of pups was found
to be lower than of low- and intermediate-dose groups (P<0.01),
but not of the high-dose group. In one pup of the low-dose group
malformations were recognized: absence of tail, and 3 posterior
lumbar vertebrae, and disturbance in ossification of vertebrae
(Beall, 1972).
Groups of Charles River CD female rats consisting of 20 mated
rats each were given 1, 3 or 9 mg/kg b.w./day chlorpromazine orally
by gavage from day 6 through 15 of gestation. Two identical groups
receiving 0.5% aqueous methylcellulose served as control. One half
of the animals were killed on day 21 of gestation and fetuses were
examined for external abnormalities. Remaining females were allowed
to deliver. Two male and two female pups in each litter were
randomly selected for evaluation of physical development, behaviour
and reproduction performance. The remaining animals were autopsied
at 15 or 16 weeks of age.
In females dosed with 9 mg/kg b.w./day decreased activity was
observed 2 to 4 hours postdosing. There were no changes in
reproductive status of females at caesarian section, or in average
fetal weight. No teratogenic effects were observed. In females which
were allowed to deliver no alterations were observed in the length
of gestation and number of live and dead pups per litter on day 1
postpartum. Statistically significant decreases were found in
average pup weight of 3 and 9 mg/kg b.w./day groups but no
dose-response relationship was observed. There was no
treatment-related change in the postnatal development of pups.
Average organ weights of F1 offspring were comparable among the
groups including controls. No effect on mating performance,
reproductive status of females at term, number of live offspring and
average pup weight was reported. Significantly increased activity in
an open field test was observed in the 9 mg/kg b.w./day group in
week 7 postpartum. The same was observed in the 3 mg/kg b.w./day
group in the 13th week postpartum. There was a significant decrease
in latency time in intermediate and high-dose groups in weeks 3 and
13 postpartum. No histopathological changes were observed in the
brains of treated rats. The NOEL for teratogenicity in this study
was 9 mg/kg b.w./day (Robertson et al., 1980).
Sprague-Dawley pregnant rats were given orally 20 mg/kg
b.w./day of chlorpromazine hydrochloride from day 6 to day 20 of
gestation. Control group animals received the same amount of vehicle
(saline) in the same manner. Dams were weighed on day 0 and every 3
days from day 6 of gestation to day 21 of gestation. Data concerning
length of gestation, litter size, sex distribution, weight and
number of dead or malformed offspring were recorded. Behavioural
testing was performed on all offspring.
No significant effect was observed on maternal weight,
gestation length, litter size, sex distribution within litters, or
offspring mortality. External examination did not reveal any
malformations of offspring. There were no significant differences
between the treated and control group in the measurements of
physical parameters. The chlorpromazine treated group showed
significant enhancement at day 6 in the righting reflex (P<0.01).
Swimming angle development was improved in the treated group on days
6 (P<0.05) and 8 (P<0.01). In the negative geotaxis test no
significant effect was observed. Ambulation in chlorpromazine
treated females was increased (P<0.05) on postnatal day 35. On day
22 the rotorod performance of males was significantly lower
(P<0.05) but not that of females. No difference was observed among
the groups in water maze, pupil contraction and auditory startle
responses. Among the animals from the treated group, when compared
to controls, significantly lower nocturnal activity (P<0.01) was
seen. Biochemical results revealed no differences in noradrenalin or
dopamine contents, but there was a significant reduction in whole
brain DNA concentration. Histopathologically, no changes in the
brains of treated rats were reported (Saillenfait & Vannier, 1988).
Eleven pregnant Sprague-Dawley rats were injected with
chlorpromazine subcutaneously on days 4 through 7 of gestation. The
drug was dissolved in distilled water and injected three times a day
at a total daily dose of 6 mg/kg b.w./day. A control group of 11
animals received distilled water only. No significant differences in
litter size were seen. In the chlorpromazine-treated group
significantly more deaths occurred compared to controls. Offspring
from treated animals showed decreased motor activity and increased
audiogenic seizure. No histomorphological changes in brains of
animals were observed (Jewett & Norton, 1966).
2.2.7 Special studies on immune responses
A group of male Wistar rats was preimmunized using
chlorpromazine-haemocyanin conjugate, precipitated with 2% aluminium
hydroxide gel, injected at a dose of 3 mg into the Payer's patches
of the small intestine. Two to seven days after injection the
animals were fed diet containing chlorpromazine hydro-chloride at
25 mg/kg b.w./day. A second group of nonimmunized rats received the
same diet and the control group the diet without chlorpromazine. All
rats were fed the chlorpromazine diet for 65, 75, or 90 days and
were returned to a normal diet 3 days before killing. Blood, bile,
liver and in some cases other organs were collected and analyzed.
Elevated IgA antibodies in bile were found in 7 out of 10
immunized animals. Anti-chlorpromazine antibodies were also found in
serum but the class of antibodies was not determined. No significant
gross pathology was observed. Histopathologically in the livers of
some animals fed chlorpromazine diet periportal loss of glycogen,
focal fatty changes and increased cellularity were seen (Mullock et
al., 1983).
2.3 Observations in man
Therapeutic doses of chloropromazine may cause orthostatic
hypotension in humans which may result in syncope. Obstructive type
of jaundice was observed, at an incidence of 2-4%. Biopsies showed
centrolobular cholestasis, with mild inflammatory response.
Eosinophilia and eosinophilic infiltrations of the liver were
frequently observed. During the chlorpromazine treatment
leukocytosis and leukopenia have been observed, but in not more than
1 out of 10 000 patients. This complication was more frequently
observed during the first 6 weeks of treatment and more often in
older women than in men.
In patients receiving chlorpromazine therapy dermatological
reactions were frequently observed. Urticaria or dermatitis was
detected in about 5% of patients and 3 types of skin disorders were
generally observed: hypersensitivity reaction, contact dermatitis
and photosensitivity. Hypersensitivity reaction that may be
urticarial, maculopopular, petechial and oedematous occurred usually
between the first and eighth weeks of treatment. Contact dermatitis
could be seen in personnel handling chlorpromazine but there is a
possibility of cross-sensitivity to other phenothiazines. During
long-term therapy of schizoprenic patients chlorpromazine can induce
abnormal pigmentation of the skin which is manifested as gray-blue
pigmentation in regions exposed to sunlight. Epithelial keratopathy
and opacities in the cornea and in the lens of the eye were also
noted (reviewed in Baldessarini, 1975; Davies, 1985).
During chlorpromazine therapy interference of the drug with
human female pituitary-gonadal function as evidence by development
of lactorrhea and amenorrhea was reported as well.
This effect was associated primarily with the use of large
doses of chlorpromazine (Rudel & Kind, 1966).
3. COMMENTS
Chlorpromazine has a broad spectrum of pharmacological
activity. It produces behavioural changes and blocks many cell
membrane receptors, notably those for dopamine and norpinephrine.
Besides its tranquillizing and sedative actions, it has a number of
other pharmacological effects and shows synergism with other classes
of central nervous system depressants. Chlorpromazine appears to be
variably absorbed but is metabolized in the gut as well as in the
liver, where it can accelerate its own metabolism or conjugation.
After being absorbed, the drug is widely distributed in the body and
its lipophilicity allows it to achieve a high enough intra-membrane
concentration to influence the stability or fluidity of cell
membranes. In the blood, more than 90% of the drug is bound to
plasma proteins. It is metabolized by oxidation, demethylation, and
hydroxylation, together with conjugation with glucuronic acid,
leading to the formation of a sulfoxide, which was found to possess
about one-eighth of the sedative action of the parent drug in the
dog. N-Oxide metabolites on the other hand, undergo significant
reduction in a number of species including humans to produce the
parent compound again. In humans, chlorpromazine and its metabolites
can be detected in urine for 6-18 months after termination of
treatment.
Although the drug was introduced into clinical use in the
1950s, and a number of papers on it have been published since, there
was a general lack of relevant toxicological data for evaluation.
The intravenous LD50 (median lethal dose) of chlorpromazine
was 20, 23, 16, and 30 mg/kg b.w. in mice, rats, rabbits and dogs
respectively.
Data from short-term, long-term, and carcinogenicity studies
were not available to the Committee. Limited recent studies suggest
that chlorpromazine may be genotoxic, as shown by microbial
genotoxicity tests and by tests in human lymphocytes in culture. In
addition, it has been established that certain reactive metabolic
intermediates are capable of binding to macromolecules, including
DNA.
The Committee noted that there were a number of published
reports, often containing contradictory results, on the effects of
chlorpromazine on reproduction and fetal development in experimental
animals, and on its behavioural effects on pups whose mothers had
been treated during fetal development. While the design of most of
these studies makes them inappropriate for evaluation, the concerns
to which they give rise cannot be ignored.
Since chlorpromazine has been in use for such a long period of
time a number of published reports are available on the toxicity and
side-effects of the drug in humans. Therapeutic doses may cause a
number of side-effects in the circulatory and nervous systems, and
adverse effects on blood cells, the skin, and the eye. Interference
with human pituitary and gonadal function results in galactorrhoea
and amenorrhoea.
4. EVALUATION
In view of the lack of relevant toxicological data, the
long-term persistence of chlorpromazine in humans, the spectrum of
additional effects of the drug, and the probability that even small
doses can cause behavioural change, the Committee was unable to
establish an ADI. Furthermore the Committee suggested that
chlorpromazine should not be used in food producing animals.
5. REFERENCES
BALDESSARINI, R.J. (1975) Drugs and the treatment of psychiatric
disorders. In: Goodman, L.S. & Gilman, A. (eds.), Goodman and
Gilman's The Pharmacological Basis of Therapeutics. Macmillan
Publishing Co., Inc.New York, Toronto, London.pp. 391-447.
BEALL, J.R. (1971) A teratogenic study of chlorpromazine,
orphenadine, perphenazine, and LSD-25 in rats. Toxicol. Appl.
Pharmacol., 21, 230-236.
BOOTH, N.H. (1988) Psychotropic agents. In: Booth, N.H. & McDonald,
L.E. (eds.), Veterinary Pharmacology and Therapeutics. Iowa State
University Press/Ames pp. 363-395.
BOVET-NITTI, F. & BOVET, D. (1959) Action of some sympatholytic
agents on pregnancy in the rat. P.S.E.B.M. 100: 555-557.
CASAGRANDE, C., GALLI, A., FERRINI, R. & MIRAGOLI, G. (1971)
Neuroleptic phenothiazones with 1,4-Diazabicyclo-(4.4.0)decane and
- (4.3.0)monane ring system. Arzneim. Forsch., 21: 308-811.
CHINOY, N.J. & SEETHALAKSHMI, L. (1977) Effects of drugs on the
reproductive physiology of male albino rats: Part I- Central
depressants & analgesic-antipyretics. Ind. J. Exp. Biol., 16:
316-322.
DAVIES, D.M. (ed.) (1985) Textbook of Adverse Drug Reactions. Oxford
University Press 1985, New York, Toronto.
DOMENJOZ, R. & THEOBALD, W. (1959). Zur Pharmakologie des Tofranil
(N-(3-Dimethylaminopropyl) - Iminodibenzyl - Hydrochlorid). Arch.
Int. Pharmacodyn., 120: 450-489.
DRUGA, A., NYITRAY, M. & SZASZOVSZKY, E. (1980) Experimental
teratogenicity of structurally similar compounds with or without
piperazine-ring: A preliminary report. Pol. J. Pharmacol. Pharm.,
32: 199-204.
FUJIMORI, H. & COBB, D.P. (1965) Central nervous system depressant
activity of MA1337, 3-(3-(4-m-chlorophenyl-1-piperazyl)-2.4(1H,3H)
quinazolinedione hydrochloride. J. Pharm. Exp. Ther., 148:
151-157.
GILLETT, E. (1960) Effects of chlorpromazine and D-Lysergic acid
diethylamine on sex behavior of male rats. P.S.E.B.M., 103:
392-394.
HERR, F., STEWART, J. & CHAREST, M-P. (1961). Tranquilizers and
antidepressants: a pharmacological comparison. Arch. Int.
Pharmacodyn., CXXIV: 328-324.
HOGARTH, P.J. & CHALMERS, P. (1973) Effect of the neonatal
administration of a single dose of chlorpromazine on the subsequent
sexual development of male mice. J. Reprod. Fert., 34: 539-541.
IRWIN, S., SLABOK, M., DEBIASE, P.L. & GOVIER, W.M. (1959)
Perhrnazine (Trilafon), a new potent tranquilizer and antiemetic:
I.Behavior profile, acute toxicity and behavioural mode of action.
Arch. Int. Pharmacodyn., CXVIII: 358-374.
JAWORSKI, T.J., HAWES, E.M., MCKAY, G. & MIDHA, K.K. (1990) The
metabolism of chlorpromazine N-oxide in man and dog. Xenobiotica,
20: 107-115.
JEWETT, R.E. & NORTON, S. (1966) Effect of tranquillizing drugs on
postnatal behaviour. Experiment. Neurolog., 14: 33-43.
JIN-FUN, J., YI-SHOU, Y., WEI-YU, W., GUI-XIAN, X. & MINGSHENG, C.
(1988) Mutagenicity and teratogenicity of chlorpromazine and
scopolamine. Chinese Med. J., 101: 339-345.
MULLOCK, B., HALL, D.E., SHAW, L.J. & HINTON, R.H. (1983)
Immunoresponses to chlorpromazine in rats detection and relation to
hepatoxicity. Biochem. Pharmacol., 32: 2733-2738.
NAGAI, Y., MAKI, A., KANDA, H., NATSUKA, K., & UMEMOTO, S. (1976)
Studies on psychotic agents. I Synthesis of
3,8-Disubstituted-1-oxa-3, 8-diazaspiro(4,5)decan-2,4-dione
derivatives. Chem. Pharm. Bull., 24: 11179-1188.
NAWAZ, M. (1981) Pharmacokinetics and dosage of chlorpromazine in
goats. J. Vet. Pharm. Therap., 2: 39-45.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1988) The mutagenic activity of
chlorpromazine. Mutation Res., 208: 33-39.
ORDY, J.M., LATANICK, A., JOHANSON, R. & MASSOPUST, L.C. (1963)
Chlorpromazine effects on pregnancy and offspring in mice.
P.S.E.B.M., 113: 833-836.
ROBERTSON, R.T., MAJKA, J.A., PETER. C.P. & BOKELMAN, D.L. (1980)
Effect of prenatal exposure to chlorpromazine on postnatal
development and behavior of rats. Tox. Appl. Pharm., 53: 541-549.
ROLSTEN (1967) Effects of chlorpromazine and psilocin on pregnancy
of C57BL/10 mice and their offspring at birth. Anat. Res., 157:
311.
RUDEL, H.W. & KIND, F.A. (1966) The biology of anti-fertility
steroids. Acta Endocrinolog. Suplementum., 105: 7-45.
SAILLENFAIT, A.M. & VANNIER, B. (1988) Methodological proposal in
behavioural teratogenicity testing: Assessment of propoxyphene,
chlorpromazine and vitamin A as positive controls. Teratology, 37:
185-199.
SINGH, S. & PADMANABHAN, R. (1979) Effect of chlorpromazine on
skeletogenesis. Acta Orthop. Scand., 50: 151-159.
SZANTO, P., EHRENPREIS, E., KRAKOW, M., RUBINSTEIN, O. & EHRENPREIS,
S. (1988) Histologic changes in the guinea pig gastrointestinal
tract following 1 week's administration of chlorpromazine,
haloperidol or atropine. Psychopharmacol., 95: 351-355.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Intestinal absorption of chlorpromazine is complete but it is
variably metabolized during its passage through the intestinal wall.
More than 90% of the drug in plasma is bound to proteins. It is
metabolized mainly in liver, and there is an indication that it may
accelerate its own hepatic metabolism or conjugation; in humans,
after several weeks of treatment, the concentration of
chlorpromazine in blood is lower with level dosage. After being
absorbed the drug is widely distributed in the body and its
lipophilicity allows it to achieve sufficient intra-membrane
concentration to influence the stability or fluidity of the cell
membrane (Baldessarini, 1975).
The major metabolic pathways of chlorpromazine are
hydroxylation and conjugation with glucuronic acid. Oxidative
processes, as well, play a very important role in biotransformation
of the drug, and the sulfoxide form possesses about one-eighth the
sedative action of the parent drug in dogs (Booth, 1988). Among 10
to 12 metabolites occurring in man (Baldessarini, 1975) N-oxide
metabolites undergo significant reduction back to the parent
compound in a number of species including man (Jaworski et al.,
1990).
The biological half-life of chlorpromazine is about 6 hours in
dogs. After intravenous administration of 2.5 mg/kg b.w. of
chlorpromazine to the goat the plasma elimination half-life is 1.51
± 0.48 hours (Nawaz, 1981). In the goat, the concentration of the
drug is higher in milk than in plasma (Nawaz & Rasmussen 1979).
After the drug was administered intravenously or orally to horses
its metabolites were detected in urine for up to 96 hours. Only 10%
or 27% of the dose, respectively, is recovered in the urine of
horses following an intravenous or oral administration (Booth,
1988).
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Acute toxicity of chlorpromazine
Species Sex Vehicle Route LD50 Reference
(mg/kg b.w.)
Mouse * * p.o. 135 Nagai et al., 1976
* * i.p. 136 Fujimori & Cobb,
1965
* * i.p. 115 Herr et al., 1985
* * i.v. 51 Domenjoz &
Theobald, 1959
M&F * i.v. 20 Casagrande et al.,
1971
Rat * * p.o. 210 Irwin et al., 1959
* * i.p. 71
* * i.v. 49 Domenjoz &
Theobald, 1959
* * i.v. 23 Irwin et al. 1959
Rabbit * * i.v. 16 Domenjoz &
Theobald, 1959
Dog * * i.v. 30 Irwin et al. 1959
* Details not reported
2.2.2 Short-term studies
2.2.2.1 Guinea pigs
Groups of 8 male and female guinea pigs were injected
intraperitioneally with chlorpromazine dissolved in physiological
saline at a dose of 30 mg/kg b.w./day for 7 consecutive days. The
animals were killed by decapitation on the 8th day after first
dosing. Necropsies were performed and tissue samples were taken from
ileum, colon and caecum only.
In 7 out of 8 animals numerous delicate fibrous adhesions at
the peritoneal surface were seen. Focal areas of haemorrhage were
found on the peritoneal surface of the caecum. Histopathologically,
occasional fibrous adhesions without other changes were observed in
ileum and colon. In 4 out of 8 animals the caecum showed marked
submucosal oedema. In some areas inflammatory changes and
haemorrhages were observed (Szanto et al., 1988).
2.2.3 Long-term/carcinogenicity studies
Data not available.
2.2.4 Reproduction studies
2.2.4.1 Mice
Chlorpromazine was given orally at a dose of 16 mg/kg b.w./day
throughout the pregnancy period of C57BL10 mice. A decreased number
of pregnancies, increase in days between mating and birth and
reduced weight gain throughout pregnancy were noted. Maternal brain
weight, liver glycogen and serum cholesterol were reported to be
changed after chlorpromazine treatment. Statistically significant
differences were observed in mean litter weights, brain, liver,
heart, organ to body weight ratios, and serum and organ biochemistry
of offspring between chlorpromazine-treated and control groups. The
details were not given in the report (Rolsten, 1967).
Forty-four newborn LACA strain mice were injected
subcutaneously with a single dose of 20 mg/kg b.w. chlorpromazine
disolved in distilled water on days 4, 6, 7, 8, 9, and 10 after
birth. The animals were killed at 30 days of age. Testes and seminal
vesicles were removed, weighed and examined histopathologically. A
group of 7 untreated mice was used as control. A general increase in
the proportion of tubules which contained spermatids, spermatozoa,
and luminal spermatozoa was observed. The most prominent effect was
seen in animals injected at day 7. In this group of animals a
greater testis weight and seminal vesicle weight was noticed as
well. Generally it was shown that the single injection of
chlorpromazine at a dose of 20 mg/kg b.w., when administered up to
the 10th day of life, accelerated sexual maturation of male mice
(Hogarth & Chalmers, 1973).
Two groups of 20 female inbred C5BL/10 mice received orally 4
or 16 mg/kg b.w./day of chlorpromazine throughout the period of
pregnancy. Control groups consisting of the corresponding number of
animals were treated with the same volume of placebo. The treatment
with chlorpromazine started on the 6th day after mating. All the
animals were tested daily in the open field test for exploration and
activity level. Effects of the drug on delays between mating and
birth, drug-induced changes in maternal weight, and litter size and
weight were recorded.
There was no statistically significant difference in body
weight of dams among the groups at the time of birth. No difference
was observed in behaviour between the animals of the 4 mg/kg
b.w./day group compared to the controls. In the 16 mg/kg b.w./day
group frequent sedation lasting 1 to 5 hours was noticed after
treatment. In the high-dose group, animals showed a statistically
significant delay between mating and birth, and treated animals had
a significantly lower number of offspring. When the two dose levels
were combined, the mean litter weights were also significantly lower
than the mean weight of litters from control dams (Orday et al.,
1963).
2.2.4.2 Rats
A group of 24 male albino rats (Rattus norvegicus) was given
chlorpromazine i.m. at a dose of 1 mg/animal/day, equivalent to
5 mg/kg b.w./day (calculated on a rat weight of 200 g), for 7 or 15
consecutive days. Groups consisting of 12 rats served as controls.
On day 8 or 16 animals were killed and necropsied. Testes, caput and
cauda epididymis were excised, blotted free of blood, weighed and
used for biochemical studies.
Significant decreases in the weight of testes, caput and cauda
epididymides were observed as well as altered activity of some
androgen dependent enzymes. An overall decrease in the level of free
ascorbic acid, succinate dehydrogenase, alkaline phosphatase and
increases of acid phosphatase and cholesterol level in testis and
epididymides were seen (Clinoy & Seethalakshmi, 1977).
Chlorpromazine was given i.m. to female rats on the 4th day of
pregnancy at a dose of 20 mg/kg b.w. It was found that the drug
disturbed the late stage of pregnancy. Details were not reported
(Bovet-Natti & Bovet, 1959).
A separate study testing sexual behaviour was performed on
twelve 150-day-old Sprague-Dawley male rats. Chlorpromazine was
injected intraperitoneally at a single dose of 2.5 mg/kg b.w.
Placebo consisted of equivalent volume of distilled water injected
to twelve control animals. It was found that treatment with
chlorpromazine reduced the number of copulations preceding
ejaculation. The number of copulations/minute, or rate of
copulations, was significantly reduced (Gillett, 1960).
2.2.5 Special studies on genotoxicity
Table 2. Results of genotoxicity assays on chlorpromazine
Test system Test object Concentration Result Reference
Chromosomal Human 0.24-2.0 µg/ml positive Jin-fu et al.
aberration lymphocytes 1988
assay
SRCE Human 0.25-2.0 µg/ml positive Jin-fu et al.
lymphocytes 1988
Ames test1 S. typhimurium 5-10 µg/ml positive Obaseiki-Ebor
TA97 his, TA & Akerele,
102 his, EE97, 1988
EE102
transconjugates)
Fluctuation E. coli 2-4 µg/ml positive Obaseiki-Ebor
test & Akerele,
1988
1. With rat liver S9 fraction
2.2.6 Special studies on embryotoxicity and teratogenicity
2.2.6.1 Mice
Groups of 10 pregnant 3-month-old mice were given
chlorpromazine at a dose of 1.8 and 9.2 mg/kg b.w./day. The drug was
injected intraperitoneally once daily from the 6th to the 16th day
of gestation. The mice were killed 2 or 3 days before parturition.
The average growth weight, increased body weight and fetal
malformations were recorded. A negative control group receiving
0.3 ml saline and a positive control group receiving 0.3 ml of
cod-liver oil containing vitamins A and D were injected with
corresponding solutions in the same way and at the same gestation
days as drug-treated groups.
Incidence of abnormal mouse fetuses was significantly higher
in treated groups and positive controls when compared to the
negative control group. Mean body weight of fetuses from treated
dams was lower. The percentage of malformed fetuses was 38.5% in the
low-dose and 42.9% in the high-dose group, 0% in the negative
control and 28.6% in the positive control group. A description of
the malformations was not given (Jin-fu et al., 1988).
2.2.6.2 Rats
Female CF rats were given chlorpromazine intraperitoneally at a
single dose of 100 mg/kg b.w. on the 14th day of gestation. The
controls were injected with a corresponding volume of physiologic
saline. The number of treated animals was not given. Between the
16th and the 20th day of gestation the fetuses were collected by
uterotomy. Only living fetuses and intact preparations were used in
this study.
It was found that ossification was delayed by 1 to 3 days in
the long bones of the extremities, by 1 day in scapulae and by 2 to
3 days in the ileum. Ischium and pubis remained unossified until the
20th day of gestation. Ossification of the skull bones was also
delayed. The sternebrae were found to be most affected (Singh &
Padmanabhan, 1979).
Six compounds have been studied for teratogenic potential on
Wistar/H-Riop pregnant rats. Groups of 5 pregnant rats were given
single oral equimolar doses of 3.7 x 10-4M/kg b.w. of perphenzine;
chlorpromazine; chlorcyclizine; thenalidine; haloanisone and
haloperidol. The dose of chlorpromazine coresponded to 0.585 mg/kg
b.w. The drugs were given to the animals on the 13th, 14th or 15th
day of gestation respectively. Mothers were killed on the 21st day
of pregnancy. Resorption, living and dead fetuses, fetal weight and
external malformations were recorded.
Higher fetal mortality (P<0.01) was observed in the
chlorpromazine treated group compared to controls. The weight of
fetuses was significantly lower (P<0.01) as well. The data indicate
an embryotoxic effect of the drug in rats (Druga et al., 1980).
Groups composed of 19-20 pregnant CAW; CFE (SD) rats were
treated with chlorpromazine from day 6 through day 15 of gestation.
Tablets of the drug were pulverized and administered to the animals
orally in 2.5% aqueous Tween solution. The dose was adjusted so that
each group received 5, 25 or 35 mg/kg b.w./day chlorpromazine. On
day 21 of pregnancy the dams were killed and the offspring removed.
The numbers of live fetuses, resorptions and nidations, sex and
weight of each live fetus were recorded.
The average litter size in the high-dose group was
significantly lower (P<0.05), and the percentage of resorption in
the intermediate and high-dose groups was higher (P<0.01), than in
controls. Compared to the controls the body weight of pups was found
to be lower than of low- and intermediate-dose groups (P<0.01),
but not of the high-dose group. In one pup of the low-dose group
malformations were recognized: absence of tail, and 3 posterior
lumbar vertebrae, and disturbance in ossification of vertebrae
(Beall, 1972).
Groups of Charles River CD female rats consisting of 20 mated
rats each were given 1, 3 or 9 mg/kg b.w./day chlorpromazine orally
by gavage from day 6 through 15 of gestation. Two identical groups
receiving 0.5% aqueous methylcellulose served as control. One half
of the animals were killed on day 21 of gestation and fetuses were
examined for external abnormalities. Remaining females were allowed
to deliver. Two male and two female pups in each litter were
randomly selected for evaluation of physical development, behaviour
and reproduction performance. The remaining animals were autopsied
at 15 or 16 weeks of age.
In females dosed with 9 mg/kg b.w./day decreased activity was
observed 2 to 4 hours postdosing. There were no changes in
reproductive status of females at caesarian section, or in average
fetal weight. No teratogenic effects were observed. In females which
were allowed to deliver no alterations were observed in the length
of gestation and number of live and dead pups per litter on day 1
postpartum. Statistically significant decreases were found in
average pup weight of 3 and 9 mg/kg b.w./day groups but no
dose-response relationship was observed. There was no
treatment-related change in the postnatal development of pups.
Average organ weights of F1 offspring were comparable among the
groups including controls. No effect on mating performance,
reproductive status of females at term, number of live offspring and
average pup weight was reported. Significantly increased activity in
an open field test was observed in the 9 mg/kg b.w./day group in
week 7 postpartum. The same was observed in the 3 mg/kg b.w./day
group in the 13th week postpartum. There was a significant decrease
in latency time in intermediate and high-dose groups in weeks 3 and
13 postpartum. No histopathological changes were observed in the
brains of treated rats. The NOEL for teratogenicity in this study
was 9 mg/kg b.w./day (Robertson et al., 1980).
Sprague-Dawley pregnant rats were given orally 20 mg/kg
b.w./day of chlorpromazine hydrochloride from day 6 to day 20 of
gestation. Control group animals received the same amount of vehicle
(saline) in the same manner. Dams were weighed on day 0 and every 3
days from day 6 of gestation to day 21 of gestation. Data concerning
length of gestation, litter size, sex distribution, weight and
number of dead or malformed offspring were recorded. Behavioural
testing was performed on all offspring.
No significant effect was observed on maternal weight,
gestation length, litter size, sex distribution within litters, or
offspring mortality. External examination did not reveal any
malformations of offspring. There were no significant differences
between the treated and control group in the measurements of
physical parameters. The chlorpromazine treated group showed
significant enhancement at day 6 in the righting reflex (P<0.01).
Swimming angle development was improved in the treated group on days
6 (P<0.05) and 8 (P<0.01). In the negative geotaxis test no
significant effect was observed. Ambulation in chlorpromazine
treated females was increased (P<0.05) on postnatal day 35. On day
22 the rotorod performance of males was significantly lower
(P<0.05) but not that of females. No difference was observed among
the groups in water maze, pupil contraction and auditory startle
responses. Among the animals from the treated group, when compared
to controls, significantly lower nocturnal activity (P<0.01) was
seen. Biochemical results revealed no differences in noradrenalin or
dopamine contents, but there was a significant reduction in whole
brain DNA concentration. Histopathologically, no changes in the
brains of treated rats were reported (Saillenfait & Vannier, 1988).
Eleven pregnant Sprague-Dawley rats were injected with
chlorpromazine subcutaneously on days 4 through 7 of gestation. The
drug was dissolved in distilled water and injected three times a day
at a total daily dose of 6 mg/kg b.w./day. A control group of 11
animals received distilled water only. No significant differences in
litter size were seen. In the chlorpromazine-treated group
significantly more deaths occurred compared to controls. Offspring
from treated animals showed decreased motor activity and increased
audiogenic seizure. No histomorphological changes in brains of
animals were observed (Jewett & Norton, 1966).
2.2.7 Special studies on immune responses
A group of male Wistar rats was preimmunized using
chlorpromazine-haemocyanin conjugate, precipitated with 2% aluminium
hydroxide gel, injected at a dose of 3 mg into the Payer's patches
of the small intestine. Two to seven days after injection the
animals were fed diet containing chlorpromazine hydro-chloride at
25 mg/kg b.w./day. A second group of nonimmunized rats received the
same diet and the control group the diet without chlorpromazine. All
rats were fed the chlorpromazine diet for 65, 75, or 90 days and
were returned to a normal diet 3 days before killing. Blood, bile,
liver and in some cases other organs were collected and analyzed.
Elevated IgA antibodies in bile were found in 7 out of 10
immunized animals. Anti-chlorpromazine antibodies were also found in
serum but the class of antibodies was not determined. No significant
gross pathology was observed. Histopathologically in the livers of
some animals fed chlorpromazine diet periportal loss of glycogen,
focal fatty changes and increased cellularity were seen (Mullock et
al., 1983).
2.3 Observations in man
Therapeutic doses of chloropromazine may cause orthostatic
hypotension in humans which may result in syncope. Obstructive type
of jaundice was observed, at an incidence of 2-4%. Biopsies showed
centrolobular cholestasis, with mild inflammatory response.
Eosinophilia and eosinophilic infiltrations of the liver were
frequently observed. During the chlorpromazine treatment
leukocytosis and leukopenia have been observed, but in not more than
1 out of 10 000 patients. This complication was more frequently
observed during the first 6 weeks of treatment and more often in
older women than in men.
In patients receiving chlorpromazine therapy dermatological
reactions were frequently observed. Urticaria or dermatitis was
detected in about 5% of patients and 3 types of skin disorders were
generally observed: hypersensitivity reaction, contact dermatitis
and photosensitivity. Hypersensitivity reaction that may be
urticarial, maculopopular, petechial and oedematous occurred usually
between the first and eighth weeks of treatment. Contact dermatitis
could be seen in personnel handling chlorpromazine but there is a
possibility of cross-sensitivity to other phenothiazines. During
long-term therapy of schizoprenic patients chlorpromazine can induce
abnormal pigmentation of the skin which is manifested as gray-blue
pigmentation in regions exposed to sunlight. Epithelial keratopathy
and opacities in the cornea and in the lens of the eye were also
noted (reviewed in Baldessarini, 1975; Davies, 1985).
During chlorpromazine therapy interference of the drug with
human female pituitary-gonadal function as evidence by development
of lactorrhea and amenorrhea was reported as well.
This effect was associated primarily with the use of large
doses of chlorpromazine (Rudel & Kind, 1966).
3. COMMENTS
Chlorpromazine has a broad spectrum of pharmacological
activity. It produces behavioural changes and blocks many cell
membrane receptors, notably those for dopamine and norpinephrine.
Besides its tranquillizing and sedative actions, it has a number of
other pharmacological effects and shows synergism with other classes
of central nervous system depressants. Chlorpromazine appears to be
variably absorbed but is metabolized in the gut as well as in the
liver, where it can accelerate its own metabolism or conjugation.
After being absorbed, the drug is widely distributed in the body and
its lipophilicity allows it to achieve a high enough intra-membrane
concentration to influence the stability or fluidity of cell
membranes. In the blood, more than 90% of the drug is bound to
plasma proteins. It is metabolized by oxidation, demethylation, and
hydroxylation, together with conjugation with glucuronic acid,
leading to the formation of a sulfoxide, which was found to possess
about one-eighth of the sedative action of the parent drug in the
dog. N-Oxide metabolites on the other hand, undergo significant
reduction in a number of species including humans to produce the
parent compound again. In humans, chlorpromazine and its metabolites
can be detected in urine for 6-18 months after termination of
treatment.
Although the drug was introduced into clinical use in the
1950s, and a number of papers on it have been published since, there
was a general lack of relevant toxicological data for evaluation.
The intravenous LD50 (median lethal dose) of chlorpromazine
was 20, 23, 16, and 30 mg/kg b.w. in mice, rats, rabbits and dogs
respectively.
Data from short-term, long-term, and carcinogenicity studies
were not available to the Committee. Limited recent studies suggest
that chlorpromazine may be genotoxic, as shown by microbial
genotoxicity tests and by tests in human lymphocytes in culture. In
addition, it has been established that certain reactive metabolic
intermediates are capable of binding to macromolecules, including
DNA.
The Committee noted that there were a number of published
reports, often containing contradictory results, on the effects of
chlorpromazine on reproduction and fetal development in experimental
animals, and on its behavioural effects on pups whose mothers had
been treated during fetal development. While the design of most of
these studies makes them inappropriate for evaluation, the concerns
to which they give rise cannot be ignored.
Since chlorpromazine has been in use for such a long period of
time a number of published reports are available on the toxicity and
side-effects of the drug in humans. Therapeutic doses may cause a
number of side-effects in the circulatory and nervous systems, and
adverse effects on blood cells, the skin, and the eye. Interference
with human pituitary and gonadal function results in galactorrhoea
and amenorrhoea.
4. EVALUATION
In view of the lack of relevant toxicological data, the
long-term persistence of chlorpromazine in humans, the spectrum of
additional effects of the drug, and the probability that even small
doses can cause behavioural change, the Committee was unable to
establish an ADI. Furthermore the Committee suggested that
chlorpromazine should not be used in food producing animals.
5. REFERENCES
BALDESSARINI, R.J. (1975) Drugs and the treatment of psychiatric
disorders. In: Goodman, L.S. & Gilman, A. (eds.), Goodman and
Gilman's The Pharmacological Basis of Therapeutics. Macmillan
Publishing Co., Inc.New York, Toronto, London.pp. 391-447.
BEALL, J.R. (1971) A teratogenic study of chlorpromazine,
orphenadine, perphenazine, and LSD-25 in rats. Toxicol. Appl.
Pharmacol., 21, 230-236.
BOOTH, N.H. (1988) Psychotropic agents. In: Booth, N.H. & McDonald,
L.E. (eds.), Veterinary Pharmacology and Therapeutics. Iowa State
University Press/Ames pp. 363-395.
BOVET-NITTI, F. & BOVET, D. (1959) Action of some sympatholytic
agents on pregnancy in the rat. P.S.E.B.M. 100: 555-557.
CASAGRANDE, C., GALLI, A., FERRINI, R. & MIRAGOLI, G. (1971)
Neuroleptic phenothiazones with 1,4-Diazabicyclo-(4.4.0)decane and
- (4.3.0)monane ring system. Arzneim. Forsch., 21: 308-811.
CHINOY, N.J. & SEETHALAKSHMI, L. (1977) Effects of drugs on the
reproductive physiology of male albino rats: Part I- Central
depressants & analgesic-antipyretics. Ind. J. Exp. Biol., 16:
316-322.
DAVIES, D.M. (ed.) (1985) Textbook of Adverse Drug Reactions. Oxford
University Press 1985, New York, Toronto.
DOMENJOZ, R. & THEOBALD, W. (1959). Zur Pharmakologie des Tofranil
(N-(3-Dimethylaminopropyl) - Iminodibenzyl - Hydrochlorid). Arch.
Int. Pharmacodyn., 120: 450-489.
DRUGA, A., NYITRAY, M. & SZASZOVSZKY, E. (1980) Experimental
teratogenicity of structurally similar compounds with or without
piperazine-ring: A preliminary report. Pol. J. Pharmacol. Pharm.,
32: 199-204.
FUJIMORI, H. & COBB, D.P. (1965) Central nervous system depressant
activity of MA1337, 3-(3-(4-m-chlorophenyl-1-piperazyl)-2.4(1H,3H)
quinazolinedione hydrochloride. J. Pharm. Exp. Ther., 148:
151-157.
GILLETT, E. (1960) Effects of chlorpromazine and D-Lysergic acid
diethylamine on sex behavior of male rats. P.S.E.B.M., 103:
392-394.
HERR, F., STEWART, J. & CHAREST, M-P. (1961). Tranquilizers and
antidepressants: a pharmacological comparison. Arch. Int.
Pharmacodyn., CXXIV: 328-324.
HOGARTH, P.J. & CHALMERS, P. (1973) Effect of the neonatal
administration of a single dose of chlorpromazine on the subsequent
sexual development of male mice. J. Reprod. Fert., 34: 539-541.
IRWIN, S., SLABOK, M., DEBIASE, P.L. & GOVIER, W.M. (1959)
Perhrnazine (Trilafon), a new potent tranquilizer and antiemetic:
I.Behavior profile, acute toxicity and behavioural mode of action.
Arch. Int. Pharmacodyn., CXVIII: 358-374.
JAWORSKI, T.J., HAWES, E.M., MCKAY, G. & MIDHA, K.K. (1990) The
metabolism of chlorpromazine N-oxide in man and dog. Xenobiotica,
20: 107-115.
JEWETT, R.E. & NORTON, S. (1966) Effect of tranquillizing drugs on
postnatal behaviour. Experiment. Neurolog., 14: 33-43.
JIN-FUN, J., YI-SHOU, Y., WEI-YU, W., GUI-XIAN, X. & MINGSHENG, C.
(1988) Mutagenicity and teratogenicity of chlorpromazine and
scopolamine. Chinese Med. J., 101: 339-345.
MULLOCK, B., HALL, D.E., SHAW, L.J. & HINTON, R.H. (1983)
Immunoresponses to chlorpromazine in rats detection and relation to
hepatoxicity. Biochem. Pharmacol., 32: 2733-2738.
NAGAI, Y., MAKI, A., KANDA, H., NATSUKA, K., & UMEMOTO, S. (1976)
Studies on psychotic agents. I Synthesis of
3,8-Disubstituted-1-oxa-3, 8-diazaspiro(4,5)decan-2,4-dione
derivatives. Chem. Pharm. Bull., 24: 11179-1188.
NAWAZ, M. (1981) Pharmacokinetics and dosage of chlorpromazine in
goats. J. Vet. Pharm. Therap., 2: 39-45.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1988) The mutagenic activity of
chlorpromazine. Mutation Res., 208: 33-39.
ORDY, J.M., LATANICK, A., JOHANSON, R. & MASSOPUST, L.C. (1963)
Chlorpromazine effects on pregnancy and offspring in mice.
P.S.E.B.M., 113: 833-836.
ROBERTSON, R.T., MAJKA, J.A., PETER. C.P. & BOKELMAN, D.L. (1980)
Effect of prenatal exposure to chlorpromazine on postnatal
development and behavior of rats. Tox. Appl. Pharm., 53: 541-549.
ROLSTEN (1967) Effects of chlorpromazine and psilocin on pregnancy
of C57BL/10 mice and their offspring at birth. Anat. Res., 157:
311.
RUDEL, H.W. & KIND, F.A. (1966) The biology of anti-fertility
steroids. Acta Endocrinolog. Suplementum., 105: 7-45.
SAILLENFAIT, A.M. & VANNIER, B. (1988) Methodological proposal in
behavioural teratogenicity testing: Assessment of propoxyphene,
chlorpromazine and vitamin A as positive controls. Teratology, 37:
185-199.
SINGH, S. & PADMANABHAN, R. (1979) Effect of chlorpromazine on
skeletogenesis. Acta Orthop. Scand., 50: 151-159.
SZANTO, P., EHRENPREIS, E., KRAKOW, M., RUBINSTEIN, O. & EHRENPREIS,
S. (1988) Histologic changes in the guinea pig gastrointestinal
tract following 1 week's administration of chlorpromazine,
haloperidol or atropine. Psychopharmacol., 95: 351-355.
See Also:
Toxicological Abbreviations
CHLORPROMAZINE (JECFA Evaluation)
Chlorpromazine (PIM 125)
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Intestinal absorption of chlorpromazine is complete but it is
variably metabolized during its passage through the intestinal wall.
More than 90% of the drug in plasma is bound to proteins. It is
metabolized mainly in liver, and there is an indication that it may
accelerate its own hepatic metabolism or conjugation; in humans,
after several weeks of treatment, the concentration of
chlorpromazine in blood is lower with level dosage. After being
absorbed the drug is widely distributed in the body and its
lipophilicity allows it to achieve sufficient intra-membrane
concentration to influence the stability or fluidity of the cell
membrane (Baldessarini, 1975).
The major metabolic pathways of chlorpromazine are
hydroxylation and conjugation with glucuronic acid. Oxidative
processes, as well, play a very important role in biotransformation
of the drug, and the sulfoxide form possesses about one-eighth the
sedative action of the parent drug in dogs (Booth, 1988). Among 10
to 12 metabolites occurring in man (Baldessarini, 1975) N-oxide
metabolites undergo significant reduction back to the parent
compound in a number of species including man (Jaworski et al.,
1990).
The biological half-life of chlorpromazine is about 6 hours in
dogs. After intravenous administration of 2.5 mg/kg b.w. of
chlorpromazine to the goat the plasma elimination half-life is 1.51
± 0.48 hours (Nawaz, 1981). In the goat, the concentration of the
drug is higher in milk than in plasma (Nawaz & Rasmussen 1979).
After the drug was administered intravenously or orally to horses
its metabolites were detected in urine for up to 96 hours. Only 10%
or 27% of the dose, respectively, is recovered in the urine of
horses following an intravenous or oral administration (Booth,
1988).
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Acute toxicity of chlorpromazine
Species Sex Vehicle Route LD50 Reference
(mg/kg b.w.)
Mouse * * p.o. 135 Nagai et al., 1976
* * i.p. 136 Fujimori & Cobb,
1965
* * i.p. 115 Herr et al., 1985
* * i.v. 51 Domenjoz &
Theobald, 1959
M&F * i.v. 20 Casagrande et al.,
1971
Rat * * p.o. 210 Irwin et al., 1959
* * i.p. 71
* * i.v. 49 Domenjoz &
Theobald, 1959
* * i.v. 23 Irwin et al. 1959
Rabbit * * i.v. 16 Domenjoz &
Theobald, 1959
Dog * * i.v. 30 Irwin et al. 1959
* Details not reported
2.2.2 Short-term studies
2.2.2.1 Guinea pigs
Groups of 8 male and female guinea pigs were injected
intraperitioneally with chlorpromazine dissolved in physiological
saline at a dose of 30 mg/kg b.w./day for 7 consecutive days. The
animals were killed by decapitation on the 8th day after first
dosing. Necropsies were performed and tissue samples were taken from
ileum, colon and caecum only.
In 7 out of 8 animals numerous delicate fibrous adhesions at
the peritoneal surface were seen. Focal areas of haemorrhage were
found on the peritoneal surface of the caecum. Histopathologically,
occasional fibrous adhesions without other changes were observed in
ileum and colon. In 4 out of 8 animals the caecum showed marked
submucosal oedema. In some areas inflammatory changes and
haemorrhages were observed (Szanto et al., 1988).
2.2.3 Long-term/carcinogenicity studies
Data not available.
2.2.4 Reproduction studies
2.2.4.1 Mice
Chlorpromazine was given orally at a dose of 16 mg/kg b.w./day
throughout the pregnancy period of C57BL10 mice. A decreased number
of pregnancies, increase in days between mating and birth and
reduced weight gain throughout pregnancy were noted. Maternal brain
weight, liver glycogen and serum cholesterol were reported to be
changed after chlorpromazine treatment. Statistically significant
differences were observed in mean litter weights, brain, liver,
heart, organ to body weight ratios, and serum and organ biochemistry
of offspring between chlorpromazine-treated and control groups. The
details were not given in the report (Rolsten, 1967).
Forty-four newborn LACA strain mice were injected
subcutaneously with a single dose of 20 mg/kg b.w. chlorpromazine
disolved in distilled water on days 4, 6, 7, 8, 9, and 10 after
birth. The animals were killed at 30 days of age. Testes and seminal
vesicles were removed, weighed and examined histopathologically. A
group of 7 untreated mice was used as control. A general increase in
the proportion of tubules which contained spermatids, spermatozoa,
and luminal spermatozoa was observed. The most prominent effect was
seen in animals injected at day 7. In this group of animals a
greater testis weight and seminal vesicle weight was noticed as
well. Generally it was shown that the single injection of
chlorpromazine at a dose of 20 mg/kg b.w., when administered up to
the 10th day of life, accelerated sexual maturation of male mice
(Hogarth & Chalmers, 1973).
Two groups of 20 female inbred C5BL/10 mice received orally 4
or 16 mg/kg b.w./day of chlorpromazine throughout the period of
pregnancy. Control groups consisting of the corresponding number of
animals were treated with the same volume of placebo. The treatment
with chlorpromazine started on the 6th day after mating. All the
animals were tested daily in the open field test for exploration and
activity level. Effects of the drug on delays between mating and
birth, drug-induced changes in maternal weight, and litter size and
weight were recorded.
There was no statistically significant difference in body
weight of dams among the groups at the time of birth. No difference
was observed in behaviour between the animals of the 4 mg/kg
b.w./day group compared to the controls. In the 16 mg/kg b.w./day
group frequent sedation lasting 1 to 5 hours was noticed after
treatment. In the high-dose group, animals showed a statistically
significant delay between mating and birth, and treated animals had
a significantly lower number of offspring. When the two dose levels
were combined, the mean litter weights were also significantly lower
than the mean weight of litters from control dams (Orday et al.,
1963).
2.2.4.2 Rats
A group of 24 male albino rats (Rattus norvegicus) was given
chlorpromazine i.m. at a dose of 1 mg/animal/day, equivalent to
5 mg/kg b.w./day (calculated on a rat weight of 200 g), for 7 or 15
consecutive days. Groups consisting of 12 rats served as controls.
On day 8 or 16 animals were killed and necropsied. Testes, caput and
cauda epididymis were excised, blotted free of blood, weighed and
used for biochemical studies.
Significant decreases in the weight of testes, caput and cauda
epididymides were observed as well as altered activity of some
androgen dependent enzymes. An overall decrease in the level of free
ascorbic acid, succinate dehydrogenase, alkaline phosphatase and
increases of acid phosphatase and cholesterol level in testis and
epididymides were seen (Clinoy & Seethalakshmi, 1977).
Chlorpromazine was given i.m. to female rats on the 4th day of
pregnancy at a dose of 20 mg/kg b.w. It was found that the drug
disturbed the late stage of pregnancy. Details were not reported
(Bovet-Natti & Bovet, 1959).
A separate study testing sexual behaviour was performed on
twelve 150-day-old Sprague-Dawley male rats. Chlorpromazine was
injected intraperitoneally at a single dose of 2.5 mg/kg b.w.
Placebo consisted of equivalent volume of distilled water injected
to twelve control animals. It was found that treatment with
chlorpromazine reduced the number of copulations preceding
ejaculation. The number of copulations/minute, or rate of
copulations, was significantly reduced (Gillett, 1960).
2.2.5 Special studies on genotoxicity
Table 2. Results of genotoxicity assays on chlorpromazine
Test system Test object Concentration Result Reference
Chromosomal Human 0.24-2.0 µg/ml positive Jin-fu et al.
aberration lymphocytes 1988
assay
SRCE Human 0.25-2.0 µg/ml positive Jin-fu et al.
lymphocytes 1988
Ames test1 S. typhimurium 5-10 µg/ml positive Obaseiki-Ebor
TA97 his, TA & Akerele,
102 his, EE97, 1988
EE102
transconjugates)
Fluctuation E. coli 2-4 µg/ml positive Obaseiki-Ebor
test & Akerele,
1988
1. With rat liver S9 fraction
2.2.6 Special studies on embryotoxicity and teratogenicity
2.2.6.1 Mice
Groups of 10 pregnant 3-month-old mice were given
chlorpromazine at a dose of 1.8 and 9.2 mg/kg b.w./day. The drug was
injected intraperitoneally once daily from the 6th to the 16th day
of gestation. The mice were killed 2 or 3 days before parturition.
The average growth weight, increased body weight and fetal
malformations were recorded. A negative control group receiving
0.3 ml saline and a positive control group receiving 0.3 ml of
cod-liver oil containing vitamins A and D were injected with
corresponding solutions in the same way and at the same gestation
days as drug-treated groups.
Incidence of abnormal mouse fetuses was significantly higher
in treated groups and positive controls when compared to the
negative control group. Mean body weight of fetuses from treated
dams was lower. The percentage of malformed fetuses was 38.5% in the
low-dose and 42.9% in the high-dose group, 0% in the negative
control and 28.6% in the positive control group. A description of
the malformations was not given (Jin-fu et al., 1988).
2.2.6.2 Rats
Female CF rats were given chlorpromazine intraperitoneally at a
single dose of 100 mg/kg b.w. on the 14th day of gestation. The
controls were injected with a corresponding volume of physiologic
saline. The number of treated animals was not given. Between the
16th and the 20th day of gestation the fetuses were collected by
uterotomy. Only living fetuses and intact preparations were used in
this study.
It was found that ossification was delayed by 1 to 3 days in
the long bones of the extremities, by 1 day in scapulae and by 2 to
3 days in the ileum. Ischium and pubis remained unossified until the
20th day of gestation. Ossification of the skull bones was also
delayed. The sternebrae were found to be most affected (Singh &
Padmanabhan, 1979).
Six compounds have been studied for teratogenic potential on
Wistar/H-Riop pregnant rats. Groups of 5 pregnant rats were given
single oral equimolar doses of 3.7 x 10-4M/kg b.w. of perphenzine;
chlorpromazine; chlorcyclizine; thenalidine; haloanisone and
haloperidol. The dose of chlorpromazine coresponded to 0.585 mg/kg
b.w. The drugs were given to the animals on the 13th, 14th or 15th
day of gestation respectively. Mothers were killed on the 21st day
of pregnancy. Resorption, living and dead fetuses, fetal weight and
external malformations were recorded.
Higher fetal mortality (P<0.01) was observed in the
chlorpromazine treated group compared to controls. The weight of
fetuses was significantly lower (P<0.01) as well. The data indicate
an embryotoxic effect of the drug in rats (Druga et al., 1980).
Groups composed of 19-20 pregnant CAW; CFE (SD) rats were
treated with chlorpromazine from day 6 through day 15 of gestation.
Tablets of the drug were pulverized and administered to the animals
orally in 2.5% aqueous Tween solution. The dose was adjusted so that
each group received 5, 25 or 35 mg/kg b.w./day chlorpromazine. On
day 21 of pregnancy the dams were killed and the offspring removed.
The numbers of live fetuses, resorptions and nidations, sex and
weight of each live fetus were recorded.
The average litter size in the high-dose group was
significantly lower (P<0.05), and the percentage of resorption in
the intermediate and high-dose groups was higher (P<0.01), than in
controls. Compared to the controls the body weight of pups was found
to be lower than of low- and intermediate-dose groups (P<0.01),
but not of the high-dose group. In one pup of the low-dose group
malformations were recognized: absence of tail, and 3 posterior
lumbar vertebrae, and disturbance in ossification of vertebrae
(Beall, 1972).
Groups of Charles River CD female rats consisting of 20 mated
rats each were given 1, 3 or 9 mg/kg b.w./day chlorpromazine orally
by gavage from day 6 through 15 of gestation. Two identical groups
receiving 0.5% aqueous methylcellulose served as control. One half
of the animals were killed on day 21 of gestation and fetuses were
examined for external abnormalities. Remaining females were allowed
to deliver. Two male and two female pups in each litter were
randomly selected for evaluation of physical development, behaviour
and reproduction performance. The remaining animals were autopsied
at 15 or 16 weeks of age.
In females dosed with 9 mg/kg b.w./day decreased activity was
observed 2 to 4 hours postdosing. There were no changes in
reproductive status of females at caesarian section, or in average
fetal weight. No teratogenic effects were observed. In females which
were allowed to deliver no alterations were observed in the length
of gestation and number of live and dead pups per litter on day 1
postpartum. Statistically significant decreases were found in
average pup weight of 3 and 9 mg/kg b.w./day groups but no
dose-response relationship was observed. There was no
treatment-related change in the postnatal development of pups.
Average organ weights of F1 offspring were comparable among the
groups including controls. No effect on mating performance,
reproductive status of females at term, number of live offspring and
average pup weight was reported. Significantly increased activity in
an open field test was observed in the 9 mg/kg b.w./day group in
week 7 postpartum. The same was observed in the 3 mg/kg b.w./day
group in the 13th week postpartum. There was a significant decrease
in latency time in intermediate and high-dose groups in weeks 3 and
13 postpartum. No histopathological changes were observed in the
brains of treated rats. The NOEL for teratogenicity in this study
was 9 mg/kg b.w./day (Robertson et al., 1980).
Sprague-Dawley pregnant rats were given orally 20 mg/kg
b.w./day of chlorpromazine hydrochloride from day 6 to day 20 of
gestation. Control group animals received the same amount of vehicle
(saline) in the same manner. Dams were weighed on day 0 and every 3
days from day 6 of gestation to day 21 of gestation. Data concerning
length of gestation, litter size, sex distribution, weight and
number of dead or malformed offspring were recorded. Behavioural
testing was performed on all offspring.
No significant effect was observed on maternal weight,
gestation length, litter size, sex distribution within litters, or
offspring mortality. External examination did not reveal any
malformations of offspring. There were no significant differences
between the treated and control group in the measurements of
physical parameters. The chlorpromazine treated group showed
significant enhancement at day 6 in the righting reflex (P<0.01).
Swimming angle development was improved in the treated group on days
6 (P<0.05) and 8 (P<0.01). In the negative geotaxis test no
significant effect was observed. Ambulation in chlorpromazine
treated females was increased (P<0.05) on postnatal day 35. On day
22 the rotorod performance of males was significantly lower
(P<0.05) but not that of females. No difference was observed among
the groups in water maze, pupil contraction and auditory startle
responses. Among the animals from the treated group, when compared
to controls, significantly lower nocturnal activity (P<0.01) was
seen. Biochemical results revealed no differences in noradrenalin or
dopamine contents, but there was a significant reduction in whole
brain DNA concentration. Histopathologically, no changes in the
brains of treated rats were reported (Saillenfait & Vannier, 1988).
Eleven pregnant Sprague-Dawley rats were injected with
chlorpromazine subcutaneously on days 4 through 7 of gestation. The
drug was dissolved in distilled water and injected three times a day
at a total daily dose of 6 mg/kg b.w./day. A control group of 11
animals received distilled water only. No significant differences in
litter size were seen. In the chlorpromazine-treated group
significantly more deaths occurred compared to controls. Offspring
from treated animals showed decreased motor activity and increased
audiogenic seizure. No histomorphological changes in brains of
animals were observed (Jewett & Norton, 1966).
2.2.7 Special studies on immune responses
A group of male Wistar rats was preimmunized using
chlorpromazine-haemocyanin conjugate, precipitated with 2% aluminium
hydroxide gel, injected at a dose of 3 mg into the Payer's patches
of the small intestine. Two to seven days after injection the
animals were fed diet containing chlorpromazine hydro-chloride at
25 mg/kg b.w./day. A second group of nonimmunized rats received the
same diet and the control group the diet without chlorpromazine. All
rats were fed the chlorpromazine diet for 65, 75, or 90 days and
were returned to a normal diet 3 days before killing. Blood, bile,
liver and in some cases other organs were collected and analyzed.
Elevated IgA antibodies in bile were found in 7 out of 10
immunized animals. Anti-chlorpromazine antibodies were also found in
serum but the class of antibodies was not determined. No significant
gross pathology was observed. Histopathologically in the livers of
some animals fed chlorpromazine diet periportal loss of glycogen,
focal fatty changes and increased cellularity were seen (Mullock et
al., 1983).
2.3 Observations in man
Therapeutic doses of chloropromazine may cause orthostatic
hypotension in humans which may result in syncope. Obstructive type
of jaundice was observed, at an incidence of 2-4%. Biopsies showed
centrolobular cholestasis, with mild inflammatory response.
Eosinophilia and eosinophilic infiltrations of the liver were
frequently observed. During the chlorpromazine treatment
leukocytosis and leukopenia have been observed, but in not more than
1 out of 10 000 patients. This complication was more frequently
observed during the first 6 weeks of treatment and more often in
older women than in men.
In patients receiving chlorpromazine therapy dermatological
reactions were frequently observed. Urticaria or dermatitis was
detected in about 5% of patients and 3 types of skin disorders were
generally observed: hypersensitivity reaction, contact dermatitis
and photosensitivity. Hypersensitivity reaction that may be
urticarial, maculopopular, petechial and oedematous occurred usually
between the first and eighth weeks of treatment. Contact dermatitis
could be seen in personnel handling chlorpromazine but there is a
possibility of cross-sensitivity to other phenothiazines. During
long-term therapy of schizoprenic patients chlorpromazine can induce
abnormal pigmentation of the skin which is manifested as gray-blue
pigmentation in regions exposed to sunlight. Epithelial keratopathy
and opacities in the cornea and in the lens of the eye were also
noted (reviewed in Baldessarini, 1975; Davies, 1985).
During chlorpromazine therapy interference of the drug with
human female pituitary-gonadal function as evidence by development
of lactorrhea and amenorrhea was reported as well.
This effect was associated primarily with the use of large
doses of chlorpromazine (Rudel & Kind, 1966).
3. COMMENTS
Chlorpromazine has a broad spectrum of pharmacological
activity. It produces behavioural changes and blocks many cell
membrane receptors, notably those for dopamine and norpinephrine.
Besides its tranquillizing and sedative actions, it has a number of
other pharmacological effects and shows synergism with other classes
of central nervous system depressants. Chlorpromazine appears to be
variably absorbed but is metabolized in the gut as well as in the
liver, where it can accelerate its own metabolism or conjugation.
After being absorbed, the drug is widely distributed in the body and
its lipophilicity allows it to achieve a high enough intra-membrane
concentration to influence the stability or fluidity of cell
membranes. In the blood, more than 90% of the drug is bound to
plasma proteins. It is metabolized by oxidation, demethylation, and
hydroxylation, together with conjugation with glucuronic acid,
leading to the formation of a sulfoxide, which was found to possess
about one-eighth of the sedative action of the parent drug in the
dog. N-Oxide metabolites on the other hand, undergo significant
reduction in a number of species including humans to produce the
parent compound again. In humans, chlorpromazine and its metabolites
can be detected in urine for 6-18 months after termination of
treatment.
Although the drug was introduced into clinical use in the
1950s, and a number of papers on it have been published since, there
was a general lack of relevant toxicological data for evaluation.
The intravenous LD50 (median lethal dose) of chlorpromazine
was 20, 23, 16, and 30 mg/kg b.w. in mice, rats, rabbits and dogs
respectively.
Data from short-term, long-term, and carcinogenicity studies
were not available to the Committee. Limited recent studies suggest
that chlorpromazine may be genotoxic, as shown by microbial
genotoxicity tests and by tests in human lymphocytes in culture. In
addition, it has been established that certain reactive metabolic
intermediates are capable of binding to macromolecules, including
DNA.
The Committee noted that there were a number of published
reports, often containing contradictory results, on the effects of
chlorpromazine on reproduction and fetal development in experimental
animals, and on its behavioural effects on pups whose mothers had
been treated during fetal development. While the design of most of
these studies makes them inappropriate for evaluation, the concerns
to which they give rise cannot be ignored.
Since chlorpromazine has been in use for such a long period of
time a number of published reports are available on the toxicity and
side-effects of the drug in humans. Therapeutic doses may cause a
number of side-effects in the circulatory and nervous systems, and
adverse effects on blood cells, the skin, and the eye. Interference
with human pituitary and gonadal function results in galactorrhoea
and amenorrhoea.
4. EVALUATION
In view of the lack of relevant toxicological data, the
long-term persistence of chlorpromazine in humans, the spectrum of
additional effects of the drug, and the probability that even small
doses can cause behavioural change, the Committee was unable to
establish an ADI. Furthermore the Committee suggested that
chlorpromazine should not be used in food producing animals.
5. REFERENCES
BALDESSARINI, R.J. (1975) Drugs and the treatment of psychiatric
disorders. In: Goodman, L.S. & Gilman, A. (eds.), Goodman and
Gilman's The Pharmacological Basis of Therapeutics. Macmillan
Publishing Co., Inc.New York, Toronto, London.pp. 391-447.
BEALL, J.R. (1971) A teratogenic study of chlorpromazine,
orphenadine, perphenazine, and LSD-25 in rats. Toxicol. Appl.
Pharmacol., 21, 230-236.
BOOTH, N.H. (1988) Psychotropic agents. In: Booth, N.H. & McDonald,
L.E. (eds.), Veterinary Pharmacology and Therapeutics. Iowa State
University Press/Ames pp. 363-395.
BOVET-NITTI, F. & BOVET, D. (1959) Action of some sympatholytic
agents on pregnancy in the rat. P.S.E.B.M. 100: 555-557.
CASAGRANDE, C., GALLI, A., FERRINI, R. & MIRAGOLI, G. (1971)
Neuroleptic phenothiazones with 1,4-Diazabicyclo-(4.4.0)decane and
- (4.3.0)monane ring system. Arzneim. Forsch., 21: 308-811.
CHINOY, N.J. & SEETHALAKSHMI, L. (1977) Effects of drugs on the
reproductive physiology of male albino rats: Part I- Central
depressants & analgesic-antipyretics. Ind. J. Exp. Biol., 16:
316-322.
DAVIES, D.M. (ed.) (1985) Textbook of Adverse Drug Reactions. Oxford
University Press 1985, New York, Toronto.
DOMENJOZ, R. & THEOBALD, W. (1959). Zur Pharmakologie des Tofranil
(N-(3-Dimethylaminopropyl) - Iminodibenzyl - Hydrochlorid). Arch.
Int. Pharmacodyn., 120: 450-489.
DRUGA, A., NYITRAY, M. & SZASZOVSZKY, E. (1980) Experimental
teratogenicity of structurally similar compounds with or without
piperazine-ring: A preliminary report. Pol. J. Pharmacol. Pharm.,
32: 199-204.
FUJIMORI, H. & COBB, D.P. (1965) Central nervous system depressant
activity of MA1337, 3-(3-(4-m-chlorophenyl-1-piperazyl)-2.4(1H,3H)
quinazolinedione hydrochloride. J. Pharm. Exp. Ther., 148:
151-157.
GILLETT, E. (1960) Effects of chlorpromazine and D-Lysergic acid
diethylamine on sex behavior of male rats. P.S.E.B.M., 103:
392-394.
HERR, F., STEWART, J. & CHAREST, M-P. (1961). Tranquilizers and
antidepressants: a pharmacological comparison. Arch. Int.
Pharmacodyn., CXXIV: 328-324.
HOGARTH, P.J. & CHALMERS, P. (1973) Effect of the neonatal
administration of a single dose of chlorpromazine on the subsequent
sexual development of male mice. J. Reprod. Fert., 34: 539-541.
IRWIN, S., SLABOK, M., DEBIASE, P.L. & GOVIER, W.M. (1959)
Perhrnazine (Trilafon), a new potent tranquilizer and antiemetic:
I.Behavior profile, acute toxicity and behavioural mode of action.
Arch. Int. Pharmacodyn., CXVIII: 358-374.
JAWORSKI, T.J., HAWES, E.M., MCKAY, G. & MIDHA, K.K. (1990) The
metabolism of chlorpromazine N-oxide in man and dog. Xenobiotica,
20: 107-115.
JEWETT, R.E. & NORTON, S. (1966) Effect of tranquillizing drugs on
postnatal behaviour. Experiment. Neurolog., 14: 33-43.
JIN-FUN, J., YI-SHOU, Y., WEI-YU, W., GUI-XIAN, X. & MINGSHENG, C.
(1988) Mutagenicity and teratogenicity of chlorpromazine and
scopolamine. Chinese Med. J., 101: 339-345.
MULLOCK, B., HALL, D.E., SHAW, L.J. & HINTON, R.H. (1983)
Immunoresponses to chlorpromazine in rats detection and relation to
hepatoxicity. Biochem. Pharmacol., 32: 2733-2738.
NAGAI, Y., MAKI, A., KANDA, H., NATSUKA, K., & UMEMOTO, S. (1976)
Studies on psychotic agents. I Synthesis of
3,8-Disubstituted-1-oxa-3, 8-diazaspiro(4,5)decan-2,4-dione
derivatives. Chem. Pharm. Bull., 24: 11179-1188.
NAWAZ, M. (1981) Pharmacokinetics and dosage of chlorpromazine in
goats. J. Vet. Pharm. Therap., 2: 39-45.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1988) The mutagenic activity of
chlorpromazine. Mutation Res., 208: 33-39.
ORDY, J.M., LATANICK, A., JOHANSON, R. & MASSOPUST, L.C. (1963)
Chlorpromazine effects on pregnancy and offspring in mice.
P.S.E.B.M., 113: 833-836.
ROBERTSON, R.T., MAJKA, J.A., PETER. C.P. & BOKELMAN, D.L. (1980)
Effect of prenatal exposure to chlorpromazine on postnatal
development and behavior of rats. Tox. Appl. Pharm., 53: 541-549.
ROLSTEN (1967) Effects of chlorpromazine and psilocin on pregnancy
of C57BL/10 mice and their offspring at birth. Anat. Res., 157:
311.
RUDEL, H.W. & KIND, F.A. (1966) The biology of anti-fertility
steroids. Acta Endocrinolog. Suplementum., 105: 7-45.
SAILLENFAIT, A.M. & VANNIER, B. (1988) Methodological proposal in
behavioural teratogenicity testing: Assessment of propoxyphene,
chlorpromazine and vitamin A as positive controls. Teratology, 37:
185-199.
SINGH, S. & PADMANABHAN, R. (1979) Effect of chlorpromazine on
skeletogenesis. Acta Orthop. Scand., 50: 151-159.
SZANTO, P., EHRENPREIS, E., KRAKOW, M., RUBINSTEIN, O. & EHRENPREIS,
S. (1988) Histologic changes in the guinea pig gastrointestinal
tract following 1 week's administration of chlorpromazine,
haloperidol or atropine. Psychopharmacol., 95: 351-355.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Intestinal absorption of chlorpromazine is complete but it is
variably metabolized during its passage through the intestinal wall.
More than 90% of the drug in plasma is bound to proteins. It is
metabolized mainly in liver, and there is an indication that it may
accelerate its own hepatic metabolism or conjugation; in humans,
after several weeks of treatment, the concentration of
chlorpromazine in blood is lower with level dosage. After being
absorbed the drug is widely distributed in the body and its
lipophilicity allows it to achieve sufficient intra-membrane
concentration to influence the stability or fluidity of the cell
membrane (Baldessarini, 1975).
The major metabolic pathways of chlorpromazine are
hydroxylation and conjugation with glucuronic acid. Oxidative
processes, as well, play a very important role in biotransformation
of the drug, and the sulfoxide form possesses about one-eighth the
sedative action of the parent drug in dogs (Booth, 1988). Among 10
to 12 metabolites occurring in man (Baldessarini, 1975) N-oxide
metabolites undergo significant reduction back to the parent
compound in a number of species including man (Jaworski et al.,
1990).
The biological half-life of chlorpromazine is about 6 hours in
dogs. After intravenous administration of 2.5 mg/kg b.w. of
chlorpromazine to the goat the plasma elimination half-life is 1.51
± 0.48 hours (Nawaz, 1981). In the goat, the concentration of the
drug is higher in milk than in plasma (Nawaz & Rasmussen 1979).
After the drug was administered intravenously or orally to horses
its metabolites were detected in urine for up to 96 hours. Only 10%
or 27% of the dose, respectively, is recovered in the urine of
horses following an intravenous or oral administration (Booth,
1988).
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Acute toxicity of chlorpromazine
Species Sex Vehicle Route LD50 Reference
(mg/kg b.w.)
Mouse * * p.o. 135 Nagai et al., 1976
* * i.p. 136 Fujimori & Cobb,
1965
* * i.p. 115 Herr et al., 1985
* * i.v. 51 Domenjoz &
Theobald, 1959
M&F * i.v. 20 Casagrande et al.,
1971
Rat * * p.o. 210 Irwin et al., 1959
* * i.p. 71
* * i.v. 49 Domenjoz &
Theobald, 1959
* * i.v. 23 Irwin et al. 1959
Rabbit * * i.v. 16 Domenjoz &
Theobald, 1959
Dog * * i.v. 30 Irwin et al. 1959
* Details not reported
2.2.2 Short-term studies
2.2.2.1 Guinea pigs
Groups of 8 male and female guinea pigs were injected
intraperitioneally with chlorpromazine dissolved in physiological
saline at a dose of 30 mg/kg b.w./day for 7 consecutive days. The
animals were killed by decapitation on the 8th day after first
dosing. Necropsies were performed and tissue samples were taken from
ileum, colon and caecum only.
In 7 out of 8 animals numerous delicate fibrous adhesions at
the peritoneal surface were seen. Focal areas of haemorrhage were
found on the peritoneal surface of the caecum. Histopathologically,
occasional fibrous adhesions without other changes were observed in
ileum and colon. In 4 out of 8 animals the caecum showed marked
submucosal oedema. In some areas inflammatory changes and
haemorrhages were observed (Szanto et al., 1988).
2.2.3 Long-term/carcinogenicity studies
Data not available.
2.2.4 Reproduction studies
2.2.4.1 Mice
Chlorpromazine was given orally at a dose of 16 mg/kg b.w./day
throughout the pregnancy period of C57BL10 mice. A decreased number
of pregnancies, increase in days between mating and birth and
reduced weight gain throughout pregnancy were noted. Maternal brain
weight, liver glycogen and serum cholesterol were reported to be
changed after chlorpromazine treatment. Statistically significant
differences were observed in mean litter weights, brain, liver,
heart, organ to body weight ratios, and serum and organ biochemistry
of offspring between chlorpromazine-treated and control groups. The
details were not given in the report (Rolsten, 1967).
Forty-four newborn LACA strain mice were injected
subcutaneously with a single dose of 20 mg/kg b.w. chlorpromazine
disolved in distilled water on days 4, 6, 7, 8, 9, and 10 after
birth. The animals were killed at 30 days of age. Testes and seminal
vesicles were removed, weighed and examined histopathologically. A
group of 7 untreated mice was used as control. A general increase in
the proportion of tubules which contained spermatids, spermatozoa,
and luminal spermatozoa was observed. The most prominent effect was
seen in animals injected at day 7. In this group of animals a
greater testis weight and seminal vesicle weight was noticed as
well. Generally it was shown that the single injection of
chlorpromazine at a dose of 20 mg/kg b.w., when administered up to
the 10th day of life, accelerated sexual maturation of male mice
(Hogarth & Chalmers, 1973).
Two groups of 20 female inbred C5BL/10 mice received orally 4
or 16 mg/kg b.w./day of chlorpromazine throughout the period of
pregnancy. Control groups consisting of the corresponding number of
animals were treated with the same volume of placebo. The treatment
with chlorpromazine started on the 6th day after mating. All the
animals were tested daily in the open field test for exploration and
activity level. Effects of the drug on delays between mating and
birth, drug-induced changes in maternal weight, and litter size and
weight were recorded.
There was no statistically significant difference in body
weight of dams among the groups at the time of birth. No difference
was observed in behaviour between the animals of the 4 mg/kg
b.w./day group compared to the controls. In the 16 mg/kg b.w./day
group frequent sedation lasting 1 to 5 hours was noticed after
treatment. In the high-dose group, animals showed a statistically
significant delay between mating and birth, and treated animals had
a significantly lower number of offspring. When the two dose levels
were combined, the mean litter weights were also significantly lower
than the mean weight of litters from control dams (Orday et al.,
1963).
2.2.4.2 Rats
A group of 24 male albino rats (Rattus norvegicus) was given
chlorpromazine i.m. at a dose of 1 mg/animal/day, equivalent to
5 mg/kg b.w./day (calculated on a rat weight of 200 g), for 7 or 15
consecutive days. Groups consisting of 12 rats served as controls.
On day 8 or 16 animals were killed and necropsied. Testes, caput and
cauda epididymis were excised, blotted free of blood, weighed and
used for biochemical studies.
Significant decreases in the weight of testes, caput and cauda
epididymides were observed as well as altered activity of some
androgen dependent enzymes. An overall decrease in the level of free
ascorbic acid, succinate dehydrogenase, alkaline phosphatase and
increases of acid phosphatase and cholesterol level in testis and
epididymides were seen (Clinoy & Seethalakshmi, 1977).
Chlorpromazine was given i.m. to female rats on the 4th day of
pregnancy at a dose of 20 mg/kg b.w. It was found that the drug
disturbed the late stage of pregnancy. Details were not reported
(Bovet-Natti & Bovet, 1959).
A separate study testing sexual behaviour was performed on
twelve 150-day-old Sprague-Dawley male rats. Chlorpromazine was
injected intraperitoneally at a single dose of 2.5 mg/kg b.w.
Placebo consisted of equivalent volume of distilled water injected
to twelve control animals. It was found that treatment with
chlorpromazine reduced the number of copulations preceding
ejaculation. The number of copulations/minute, or rate of
copulations, was significantly reduced (Gillett, 1960).
2.2.5 Special studies on genotoxicity
Table 2. Results of genotoxicity assays on chlorpromazine
Test system Test object Concentration Result Reference
Chromosomal Human 0.24-2.0 µg/ml positive Jin-fu et al.
aberration lymphocytes 1988
assay
SRCE Human 0.25-2.0 µg/ml positive Jin-fu et al.
lymphocytes 1988
Ames test1 S. typhimurium 5-10 µg/ml positive Obaseiki-Ebor
TA97 his, TA & Akerele,
102 his, EE97, 1988
EE102
transconjugates)
Fluctuation E. coli 2-4 µg/ml positive Obaseiki-Ebor
test & Akerele,
1988
1. With rat liver S9 fraction
2.2.6 Special studies on embryotoxicity and teratogenicity
2.2.6.1 Mice
Groups of 10 pregnant 3-month-old mice were given
chlorpromazine at a dose of 1.8 and 9.2 mg/kg b.w./day. The drug was
injected intraperitoneally once daily from the 6th to the 16th day
of gestation. The mice were killed 2 or 3 days before parturition.
The average growth weight, increased body weight and fetal
malformations were recorded. A negative control group receiving
0.3 ml saline and a positive control group receiving 0.3 ml of
cod-liver oil containing vitamins A and D were injected with
corresponding solutions in the same way and at the same gestation
days as drug-treated groups.
Incidence of abnormal mouse fetuses was significantly higher
in treated groups and positive controls when compared to the
negative control group. Mean body weight of fetuses from treated
dams was lower. The percentage of malformed fetuses was 38.5% in the
low-dose and 42.9% in the high-dose group, 0% in the negative
control and 28.6% in the positive control group. A description of
the malformations was not given (Jin-fu et al., 1988).
2.2.6.2 Rats
Female CF rats were given chlorpromazine intraperitoneally at a
single dose of 100 mg/kg b.w. on the 14th day of gestation. The
controls were injected with a corresponding volume of physiologic
saline. The number of treated animals was not given. Between the
16th and the 20th day of gestation the fetuses were collected by
uterotomy. Only living fetuses and intact preparations were used in
this study.
It was found that ossification was delayed by 1 to 3 days in
the long bones of the extremities, by 1 day in scapulae and by 2 to
3 days in the ileum. Ischium and pubis remained unossified until the
20th day of gestation. Ossification of the skull bones was also
delayed. The sternebrae were found to be most affected (Singh &
Padmanabhan, 1979).
Six compounds have been studied for teratogenic potential on
Wistar/H-Riop pregnant rats. Groups of 5 pregnant rats were given
single oral equimolar doses of 3.7 x 10-4M/kg b.w. of perphenzine;
chlorpromazine; chlorcyclizine; thenalidine; haloanisone and
haloperidol. The dose of chlorpromazine coresponded to 0.585 mg/kg
b.w. The drugs were given to the animals on the 13th, 14th or 15th
day of gestation respectively. Mothers were killed on the 21st day
of pregnancy. Resorption, living and dead fetuses, fetal weight and
external malformations were recorded.
Higher fetal mortality (P<0.01) was observed in the
chlorpromazine treated group compared to controls. The weight of
fetuses was significantly lower (P<0.01) as well. The data indicate
an embryotoxic effect of the drug in rats (Druga et al., 1980).
Groups composed of 19-20 pregnant CAW; CFE (SD) rats were
treated with chlorpromazine from day 6 through day 15 of gestation.
Tablets of the drug were pulverized and administered to the animals
orally in 2.5% aqueous Tween solution. The dose was adjusted so that
each group received 5, 25 or 35 mg/kg b.w./day chlorpromazine. On
day 21 of pregnancy the dams were killed and the offspring removed.
The numbers of live fetuses, resorptions and nidations, sex and
weight of each live fetus were recorded.
The average litter size in the high-dose group was
significantly lower (P<0.05), and the percentage of resorption in
the intermediate and high-dose groups was higher (P<0.01), than in
controls. Compared to the controls the body weight of pups was found
to be lower than of low- and intermediate-dose groups (P<0.01),
but not of the high-dose group. In one pup of the low-dose group
malformations were recognized: absence of tail, and 3 posterior
lumbar vertebrae, and disturbance in ossification of vertebrae
(Beall, 1972).
Groups of Charles River CD female rats consisting of 20 mated
rats each were given 1, 3 or 9 mg/kg b.w./day chlorpromazine orally
by gavage from day 6 through 15 of gestation. Two identical groups
receiving 0.5% aqueous methylcellulose served as control. One half
of the animals were killed on day 21 of gestation and fetuses were
examined for external abnormalities. Remaining females were allowed
to deliver. Two male and two female pups in each litter were
randomly selected for evaluation of physical development, behaviour
and reproduction performance. The remaining animals were autopsied
at 15 or 16 weeks of age.
In females dosed with 9 mg/kg b.w./day decreased activity was
observed 2 to 4 hours postdosing. There were no changes in
reproductive status of females at caesarian section, or in average
fetal weight. No teratogenic effects were observed. In females which
were allowed to deliver no alterations were observed in the length
of gestation and number of live and dead pups per litter on day 1
postpartum. Statistically significant decreases were found in
average pup weight of 3 and 9 mg/kg b.w./day groups but no
dose-response relationship was observed. There was no
treatment-related change in the postnatal development of pups.
Average organ weights of F1 offspring were comparable among the
groups including controls. No effect on mating performance,
reproductive status of females at term, number of live offspring and
average pup weight was reported. Significantly increased activity in
an open field test was observed in the 9 mg/kg b.w./day group in
week 7 postpartum. The same was observed in the 3 mg/kg b.w./day
group in the 13th week postpartum. There was a significant decrease
in latency time in intermediate and high-dose groups in weeks 3 and
13 postpartum. No histopathological changes were observed in the
brains of treated rats. The NOEL for teratogenicity in this study
was 9 mg/kg b.w./day (Robertson et al., 1980).
Sprague-Dawley pregnant rats were given orally 20 mg/kg
b.w./day of chlorpromazine hydrochloride from day 6 to day 20 of
gestation. Control group animals received the same amount of vehicle
(saline) in the same manner. Dams were weighed on day 0 and every 3
days from day 6 of gestation to day 21 of gestation. Data concerning
length of gestation, litter size, sex distribution, weight and
number of dead or malformed offspring were recorded. Behavioural
testing was performed on all offspring.
No significant effect was observed on maternal weight,
gestation length, litter size, sex distribution within litters, or
offspring mortality. External examination did not reveal any
malformations of offspring. There were no significant differences
between the treated and control group in the measurements of
physical parameters. The chlorpromazine treated group showed
significant enhancement at day 6 in the righting reflex (P<0.01).
Swimming angle development was improved in the treated group on days
6 (P<0.05) and 8 (P<0.01). In the negative geotaxis test no
significant effect was observed. Ambulation in chlorpromazine
treated females was increased (P<0.05) on postnatal day 35. On day
22 the rotorod performance of males was significantly lower
(P<0.05) but not that of females. No difference was observed among
the groups in water maze, pupil contraction and auditory startle
responses. Among the animals from the treated group, when compared
to controls, significantly lower nocturnal activity (P<0.01) was
seen. Biochemical results revealed no differences in noradrenalin or
dopamine contents, but there was a significant reduction in whole
brain DNA concentration. Histopathologically, no changes in the
brains of treated rats were reported (Saillenfait & Vannier, 1988).
Eleven pregnant Sprague-Dawley rats were injected with
chlorpromazine subcutaneously on days 4 through 7 of gestation. The
drug was dissolved in distilled water and injected three times a day
at a total daily dose of 6 mg/kg b.w./day. A control group of 11
animals received distilled water only. No significant differences in
litter size were seen. In the chlorpromazine-treated group
significantly more deaths occurred compared to controls. Offspring
from treated animals showed decreased motor activity and increased
audiogenic seizure. No histomorphological changes in brains of
animals were observed (Jewett & Norton, 1966).
2.2.7 Special studies on immune responses
A group of male Wistar rats was preimmunized using
chlorpromazine-haemocyanin conjugate, precipitated with 2% aluminium
hydroxide gel, injected at a dose of 3 mg into the Payer's patches
of the small intestine. Two to seven days after injection the
animals were fed diet containing chlorpromazine hydro-chloride at
25 mg/kg b.w./day. A second group of nonimmunized rats received the
same diet and the control group the diet without chlorpromazine. All
rats were fed the chlorpromazine diet for 65, 75, or 90 days and
were returned to a normal diet 3 days before killing. Blood, bile,
liver and in some cases other organs were collected and analyzed.
Elevated IgA antibodies in bile were found in 7 out of 10
immunized animals. Anti-chlorpromazine antibodies were also found in
serum but the class of antibodies was not determined. No significant
gross pathology was observed. Histopathologically in the livers of
some animals fed chlorpromazine diet periportal loss of glycogen,
focal fatty changes and increased cellularity were seen (Mullock et
al., 1983).
2.3 Observations in man
Therapeutic doses of chloropromazine may cause orthostatic
hypotension in humans which may result in syncope. Obstructive type
of jaundice was observed, at an incidence of 2-4%. Biopsies showed
centrolobular cholestasis, with mild inflammatory response.
Eosinophilia and eosinophilic infiltrations of the liver were
frequently observed. During the chlorpromazine treatment
leukocytosis and leukopenia have been observed, but in not more than
1 out of 10 000 patients. This complication was more frequently
observed during the first 6 weeks of treatment and more often in
older women than in men.
In patients receiving chlorpromazine therapy dermatological
reactions were frequently observed. Urticaria or dermatitis was
detected in about 5% of patients and 3 types of skin disorders were
generally observed: hypersensitivity reaction, contact dermatitis
and photosensitivity. Hypersensitivity reaction that may be
urticarial, maculopopular, petechial and oedematous occurred usually
between the first and eighth weeks of treatment. Contact dermatitis
could be seen in personnel handling chlorpromazine but there is a
possibility of cross-sensitivity to other phenothiazines. During
long-term therapy of schizoprenic patients chlorpromazine can induce
abnormal pigmentation of the skin which is manifested as gray-blue
pigmentation in regions exposed to sunlight. Epithelial keratopathy
and opacities in the cornea and in the lens of the eye were also
noted (reviewed in Baldessarini, 1975; Davies, 1985).
During chlorpromazine therapy interference of the drug with
human female pituitary-gonadal function as evidence by development
of lactorrhea and amenorrhea was reported as well.
This effect was associated primarily with the use of large
doses of chlorpromazine (Rudel & Kind, 1966).
3. COMMENTS
Chlorpromazine has a broad spectrum of pharmacological
activity. It produces behavioural changes and blocks many cell
membrane receptors, notably those for dopamine and norpinephrine.
Besides its tranquillizing and sedative actions, it has a number of
other pharmacological effects and shows synergism with other classes
of central nervous system depressants. Chlorpromazine appears to be
variably absorbed but is metabolized in the gut as well as in the
liver, where it can accelerate its own metabolism or conjugation.
After being absorbed, the drug is widely distributed in the body and
its lipophilicity allows it to achieve a high enough intra-membrane
concentration to influence the stability or fluidity of cell
membranes. In the blood, more than 90% of the drug is bound to
plasma proteins. It is metabolized by oxidation, demethylation, and
hydroxylation, together with conjugation with glucuronic acid,
leading to the formation of a sulfoxide, which was found to possess
about one-eighth of the sedative action of the parent drug in the
dog. N-Oxide metabolites on the other hand, undergo significant
reduction in a number of species including humans to produce the
parent compound again. In humans, chlorpromazine and its metabolites
can be detected in urine for 6-18 months after termination of
treatment.
Although the drug was introduced into clinical use in the
1950s, and a number of papers on it have been published since, there
was a general lack of relevant toxicological data for evaluation.
The intravenous LD50 (median lethal dose) of chlorpromazine
was 20, 23, 16, and 30 mg/kg b.w. in mice, rats, rabbits and dogs
respectively.
Data from short-term, long-term, and carcinogenicity studies
were not available to the Committee. Limited recent studies suggest
that chlorpromazine may be genotoxic, as shown by microbial
genotoxicity tests and by tests in human lymphocytes in culture. In
addition, it has been established that certain reactive metabolic
intermediates are capable of binding to macromolecules, including
DNA.
The Committee noted that there were a number of published
reports, often containing contradictory results, on the effects of
chlorpromazine on reproduction and fetal development in experimental
animals, and on its behavioural effects on pups whose mothers had
been treated during fetal development. While the design of most of
these studies makes them inappropriate for evaluation, the concerns
to which they give rise cannot be ignored.
Since chlorpromazine has been in use for such a long period of
time a number of published reports are available on the toxicity and
side-effects of the drug in humans. Therapeutic doses may cause a
number of side-effects in the circulatory and nervous systems, and
adverse effects on blood cells, the skin, and the eye. Interference
with human pituitary and gonadal function results in galactorrhoea
and amenorrhoea.
4. EVALUATION
In view of the lack of relevant toxicological data, the
long-term persistence of chlorpromazine in humans, the spectrum of
additional effects of the drug, and the probability that even small
doses can cause behavioural change, the Committee was unable to
establish an ADI. Furthermore the Committee suggested that
chlorpromazine should not be used in food producing animals.
5. REFERENCES
BALDESSARINI, R.J. (1975) Drugs and the treatment of psychiatric
disorders. In: Goodman, L.S. & Gilman, A. (eds.), Goodman and
Gilman's The Pharmacological Basis of Therapeutics. Macmillan
Publishing Co., Inc.New York, Toronto, London.pp. 391-447.
BEALL, J.R. (1971) A teratogenic study of chlorpromazine,
orphenadine, perphenazine, and LSD-25 in rats. Toxicol. Appl.
Pharmacol., 21, 230-236.
BOOTH, N.H. (1988) Psychotropic agents. In: Booth, N.H. & McDonald,
L.E. (eds.), Veterinary Pharmacology and Therapeutics. Iowa State
University Press/Ames pp. 363-395.
BOVET-NITTI, F. & BOVET, D. (1959) Action of some sympatholytic
agents on pregnancy in the rat. P.S.E.B.M. 100: 555-557.
CASAGRANDE, C., GALLI, A., FERRINI, R. & MIRAGOLI, G. (1971)
Neuroleptic phenothiazones with 1,4-Diazabicyclo-(4.4.0)decane and
- (4.3.0)monane ring system. Arzneim. Forsch., 21: 308-811.
CHINOY, N.J. & SEETHALAKSHMI, L. (1977) Effects of drugs on the
reproductive physiology of male albino rats: Part I- Central
depressants & analgesic-antipyretics. Ind. J. Exp. Biol., 16:
316-322.
DAVIES, D.M. (ed.) (1985) Textbook of Adverse Drug Reactions. Oxford
University Press 1985, New York, Toronto.
DOMENJOZ, R. & THEOBALD, W. (1959). Zur Pharmakologie des Tofranil
(N-(3-Dimethylaminopropyl) - Iminodibenzyl - Hydrochlorid). Arch.
Int. Pharmacodyn., 120: 450-489.
DRUGA, A., NYITRAY, M. & SZASZOVSZKY, E. (1980) Experimental
teratogenicity of structurally similar compounds with or without
piperazine-ring: A preliminary report. Pol. J. Pharmacol. Pharm.,
32: 199-204.
FUJIMORI, H. & COBB, D.P. (1965) Central nervous system depressant
activity of MA1337, 3-(3-(4-m-chlorophenyl-1-piperazyl)-2.4(1H,3H)
quinazolinedione hydrochloride. J. Pharm. Exp. Ther., 148:
151-157.
GILLETT, E. (1960) Effects of chlorpromazine and D-Lysergic acid
diethylamine on sex behavior of male rats. P.S.E.B.M., 103:
392-394.
HERR, F., STEWART, J. & CHAREST, M-P. (1961). Tranquilizers and
antidepressants: a pharmacological comparison. Arch. Int.
Pharmacodyn., CXXIV: 328-324.
HOGARTH, P.J. & CHALMERS, P. (1973) Effect of the neonatal
administration of a single dose of chlorpromazine on the subsequent
sexual development of male mice. J. Reprod. Fert., 34: 539-541.
IRWIN, S., SLABOK, M., DEBIASE, P.L. & GOVIER, W.M. (1959)
Perhrnazine (Trilafon), a new potent tranquilizer and antiemetic:
I.Behavior profile, acute toxicity and behavioural mode of action.
Arch. Int. Pharmacodyn., CXVIII: 358-374.
JAWORSKI, T.J., HAWES, E.M., MCKAY, G. & MIDHA, K.K. (1990) The
metabolism of chlorpromazine N-oxide in man and dog. Xenobiotica,
20: 107-115.
JEWETT, R.E. & NORTON, S. (1966) Effect of tranquillizing drugs on
postnatal behaviour. Experiment. Neurolog., 14: 33-43.
JIN-FUN, J., YI-SHOU, Y., WEI-YU, W., GUI-XIAN, X. & MINGSHENG, C.
(1988) Mutagenicity and teratogenicity of chlorpromazine and
scopolamine. Chinese Med. J., 101: 339-345.
MULLOCK, B., HALL, D.E., SHAW, L.J. & HINTON, R.H. (1983)
Immunoresponses to chlorpromazine in rats detection and relation to
hepatoxicity. Biochem. Pharmacol., 32: 2733-2738.
NAGAI, Y., MAKI, A., KANDA, H., NATSUKA, K., & UMEMOTO, S. (1976)
Studies on psychotic agents. I Synthesis of
3,8-Disubstituted-1-oxa-3, 8-diazaspiro(4,5)decan-2,4-dione
derivatives. Chem. Pharm. Bull., 24: 11179-1188.
NAWAZ, M. (1981) Pharmacokinetics and dosage of chlorpromazine in
goats. J. Vet. Pharm. Therap., 2: 39-45.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1988) The mutagenic activity of
chlorpromazine. Mutation Res., 208: 33-39.
ORDY, J.M., LATANICK, A., JOHANSON, R. & MASSOPUST, L.C. (1963)
Chlorpromazine effects on pregnancy and offspring in mice.
P.S.E.B.M., 113: 833-836.
ROBERTSON, R.T., MAJKA, J.A., PETER. C.P. & BOKELMAN, D.L. (1980)
Effect of prenatal exposure to chlorpromazine on postnatal
development and behavior of rats. Tox. Appl. Pharm., 53: 541-549.
ROLSTEN (1967) Effects of chlorpromazine and psilocin on pregnancy
of C57BL/10 mice and their offspring at birth. Anat. Res., 157:
311.
RUDEL, H.W. & KIND, F.A. (1966) The biology of anti-fertility
steroids. Acta Endocrinolog. Suplementum., 105: 7-45.
SAILLENFAIT, A.M. & VANNIER, B. (1988) Methodological proposal in
behavioural teratogenicity testing: Assessment of propoxyphene,
chlorpromazine and vitamin A as positive controls. Teratology, 37:
185-199.
SINGH, S. & PADMANABHAN, R. (1979) Effect of chlorpromazine on
skeletogenesis. Acta Orthop. Scand., 50: 151-159.
SZANTO, P., EHRENPREIS, E., KRAKOW, M., RUBINSTEIN, O. & EHRENPREIS,
S. (1988) Histologic changes in the guinea pig gastrointestinal
tract following 1 week's administration of chlorpromazine,
haloperidol or atropine. Psychopharmacol., 95: 351-355.
