
LIMONENE
First draft prepared by
Dr K.B. Ekelman and Dr D. Benz
US Food and Drug Administration
Washington, DC, USA
1. EXPLANATION
Limonene has not been previously evaluated by the Committee.
The active isomer is designated as d-Limonene in this monograph.
In the report (Annex 1, reference 101) it is designated as
(+)-limonene.
d-Limonene is a liquid with a pleasant, lemon-like odour and
a fresh citrus taste. It is a natural constituent of a variety of
foods and beverages and is especially prevalent in citrus fruits.
Extracted d-limonene is used primarily as a lemon fragrance
in soaps, detergents, creams, lotions and perfumes, and as a
flavouring agent in foods, beverages and chewing gum (NTP, 1990). It
is found in non-alcoholic beverages (31 ppm), ice cream and ices (68
ppm), candy (49 ppm), baked goods (120 ppm), gelatins and puddings
(48-400 ppm), and chewing gum (2300 ppm) (NTP, 1990).
Human exposure to d-limonene is through consumption of foods
and beverages, both those in which it naturally occurs and those to
which it has been added.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
d-Limonene was rapidly absorbed (43 min) through the intact,
shaved abdominal skin of mice (Meyer & Meyer, 1959).
Twelve Long-Evans male rats were administered single topical
doses of 5 mg/kg bw 14C-limonene; the treated area was then
occluded for 3 h (2 males) or 6 h (10 males). Following occlusion,
the residual dose was removed and the treated area was re-occluded.
Pairs of treated rats were killed at 3, 6, 24, 48, and 72 h; urine
and faeces were collected from rats killed at 24, 48, and 72 h, and
plasma and tissue samples were taken at all time points. Authors
reported that peak concentrations of radioactivity in tissue samples
were measured 3-6 h after dosing in the gastrointestinal tract
(0.1-0.4% dose/g), livers and kidneys (0.08-0.2% dose/g), and
thyroid and fat (0.02-0.06% dose/g); except for the gastrointestinal
tract, concentrations of radioactivity in all tissues were
appreciably lower at 24 h. After 6 h of exposure, 48% of the
radioactivity was recovered in the skin; at the 24-72 h sampling
times, 8-12% was excreted in urine, 1-3% was excreted in faeces, and
14-18% was expired in air. Total mean recovery of radioactivity was
reported to be approximately 76%.
Following oral administration, the highest concentration of
d-limonene or its metabolites in rats was found in the serum
fraction of blood after 2 h. The other major organs containing
metabolites of d-limonene were the liver and kidney, with peaks
1-2 h after ingestion. After 48 h, negligible amounts of
d-limonene metabolites remained in the body. Approximately 60% of
d-limonene was excreted in the urine, 5% in the faeces, and 2% was
expired (Igimi et al., 1974).
Kodama et al. (1974) reported that 72% of d-limonene
metabolites were excreted in male rabbit urine 72 h after oral
administration, while 7% was found in the faeces.
2.1.2 Biotransformation
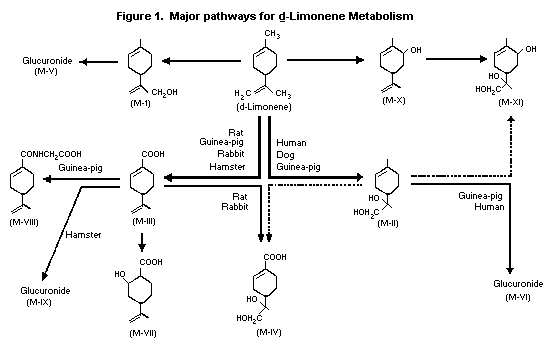
Kodama et al. (1974) identified the metabolites of orally
administered d-limonene (also known as p-mentha-1,8-diene) in male
rabbit urine as p-mentha-1,8-dien-10-ol (M-I),
p-menth-1-ene-8,9-diol (M-II), perillic acid (M-III), perillic acid
8,9-diol (M-IV), p-mentha-1,8-dien-10-yl-beta-D-glucopyranosiduronic
acid (M-V) and 8-hydroxy-p-menth-1-en-9-yl-beta-D-
glucopyranosiduronic acid (M-VI).
The list of metabolites was expanded based on a study done with
male rats, hamsters, guinea-pigs, rabbits, dogs and humans (Kodama
et al., 1976b). Five additional metabolites of d-limonene were
identified: 2-hydroxy-p-menth-8-en-7-oic acid (M-VII),
perillylglycine (M-VIII), perillyl-beta-D-glucopyrano-siduronic acid
(M-IX), p-mentha-1,8-dien-6-ol (M-X) and p-menth-1-ene-6,8,9-triol
(M-XI). The major metabolite in rats and rabbits was identified as
M-IV, while the major metabolite in hamsters was found to be M-IX.
In dogs, the major metabolite was M-II, but in guinea pigs and
humans, it was M-VI. The possible metabolic pathways used by the
various species are shown in Figure 1 below.
 Wade et al. (1966) reported that dietary limonene gives rise
to uroterpenol (M-II: p-menth-1-ene-8,9-diol) in the urine of
humans.
2.1.3 Effects on enzymes and other biochemical parameters
Groups of control (6 animals) and experimental (4 animals) male
Wistar rats were administered single gavage doses of 0, 200, 400,
600, 800, or 1200 mg/kg bw d-limonene in 2% tragacanth solution in
a total volume of 4 ml/kg. Authors reported that no effects were
observed on liver triglycerides, microsomal proteins, cytochrome b5
and drug metabolizing enzymes.
In the same experiment, male Wistar rats were treated with
gavage doses of 0 or 400 mg/kg bw d-limonene in 2% tragacanth
solution in a total volume of 4 ml/kg for 2, 3, 15 or 30 days;
animals were killed 24 h following the last dose. Authors reported
that, following repeated treatment for 30 days, relative liver
weight and hepatic phospholipid content were slightly increased, and
liver and serum cholesterol were decreased 49% and 8%, respectively.
In addition, palmitic, linoleic and arachidonic acids were
increased, and stearic acid was decreased in the liver; aminopyrine
demethylase and aniline hydroxylase were increased 26% and 22%,
respectively, and cytochrome P-450 and b5 were increased by 31% and
30%, respectively (Ariyoshi et al., 1975).
d-Limonene was reported to enhance bile flow in Wistar rats
and mongrel dogs in a dose-related manner, and to decrease the ratio
of biliary bile salts and phospholipids to cholesterol (Kodama
et al., 1976). d-Limonene was reported to be an effective
gallstone solubilizer in animals and humans (Igimi et al., 1976;
Schenk et al., 1980), and to have no effect on liver weights nor
levels of serum lipids when fed to male Wistar rats at 0.5 and 1% in
the diet (Imaizumi et al., 1985).
d-Limonene was reported to significantly damage and increase
permeability of membranes of human lung fibroblasts (Thelestam
et al., 1980; Curvall et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Table 1: Summary of acute toxicity studies with d-limonene
Species Sex Route LD50 Reference
Mouse M&F oral, in 6.3 ml/kg (M) Tsuji et
juice, 7d 8.1 ml/kg (F) al., 1974
Mouse M&F ip, in 3.7 ml/kg (M) Tsuji et
juice, 3d 3.6 ml/kg (F) al., 1974
Mouse M&F ip, in 0.7 ml/kg (M) Tsuji et
juice, 10d 0.6 ml/kg (F) al., 1974
Mouse M&F sc, in >25.6 ml/kg (M) Tsuji et
juice, 7d >25.6 ml/kg (F) al., 1974
Mouse M&F oral 5600 mg/kg (M) Tsuji et
6600 mg/kg (F) al., 1975a
Mouse M&F ip 1300 mg/kg (M) Tsuji et
1300 mg/kg (F) al., 1975a
Mouse M&F sc >41500 mg/kg (M) Tsuji et
>41500 mg/kg (F) al., 1975a
Rat M&F oral 4400 mg/kg (M) Tsuji et
5100 mg/kg (F) al., 1975a
Rat M&F ip 3600 mg/kg (M) Tsuji et
4500 mg/kg (F) al., 1975a
Rat M&F sc >20200 mg/kg (M) Tsuji et
>20200 mg/kg (F) al., 1975a
An oral dose of 3 ml d-limonene (in juice)/kg decreased
spontaneous motor activities in rats and mice and potentiated
hexobarbital-induced sleeping and hypothermia in mice. Authors also
reported that nicotine-induced convulsion and death (but not maximum
electroshock-, pentetrazol-, strychnine-, nor picrotoxin-induced
convulsions) were inhibited by d-limonene in mice. Intravenous
administration of > 0.005 mg/kg d-limonene lowered the blood
pressure of rabbits and dogs, and > 0.1-0.3 mg/kg killed those
animals. Oral administration of 3 ml/kg d-limonene did not
decrease the blood pressure of rats, however. Authors also reported
that isolated smooth muscles of the intestine, vas deferens, uterus,
and peripheral vessel were constricted by d-limonene (Tsuji
et al., 1974). Single high s.c. doses of d-limonene produced
scratch behaviour and single high i.v. doses of d-limonene
produced stretch behaviour in mice and rats (Tsuji et al., 1975a).
2.2.2 Short-term studies
2.2.2.1 Mice
Groups of 5 B6C3F1 male and female mice were given daily
gavage doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg
bw in 10 ml/kg bw corn oil 5 days/week over a 16 day period (12
total dosings). All mice (5/5) exposed to 6600 mg/kg bw/day, 4/5
males and 5/5 females exposed to 3300 mg/kg bw/day, and 1/5 males
and 1/5 females exposed to 1650 mg/kg bw/day died before the end of
the study. The one female that died at the latter dose level was
reported to have been killed by a gavage error. Authors concluded
that no compound-related clinical signs were observed in mice that
received 1650 mg d-limonene/kg bw and lived to the end of the
study, and that no compound-related histopathologic effects were
seen (NTP, 1990).
Groups of 10 B6C3F1 mice of each sex were given daily
gavage exposures of 0, 125, 250, 500, 1000 or 2000 mg
d-limonene/kg bw in 10 ml/kg bw corn oil 5 days/week for 13 weeks.
One male exposed to 2000 mg/kg bw/day, two females exposed to
2000 mg/kg bw/day, and one female exposed to 500 mg/kg bw/day, died
during the course of the study. In addition, one female exposed to
125 mg/kg bw/day and five males (one each from the groups exposed to
250 and 1000 mg/kg bw/day, and three from the group exposed to
500 mg/kg bw/day) died prior to the end of the study, but their
deaths were attributed by the authors to accidents related to the
gavage procedure used. At the two highest dose levels, mice were
seen with rough hair coats and decreased activity. An alveolar cell
adenoma was observed in the lung of one female mouse that had
received 2000 mg d-limonene/kg bw/day (NTP, 1990).
2.2.2.2 Rats
Groups of 5 F344/N rats of each sex were given daily gavage
doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg bw in
10 ml/kg bw corn oil 5 days/week over a 16 day period (12 total
doses). All rats receiving 6600 mg d-limonene/kg bw/day as well as
5/5 male and 3/5 female rats that were exposed to 3300 mg/bw/day
died. The authors reported that no clinical signs were seen in rats
receiving 1650 mg d-limonene/kg bw/day or lower, and that no
compound-related histopathologic effects were seen in any rats (NTP,
1990).
Groups of 5 male Fischer-344 rats were given single daily
gavage doses containing 75, 150 or 300 mg d-limonene/kg bw in corn
oil (5 ml/kg bw) 5 days/week for 5 or 20 doses, primarily to follow
the development of renal alterations. The authors reported that no
signs of gross toxicity were observed nor was there any effect on
weight gain or feed consumption over the course of this experiment.
However, after only 5 days of exposure, liver and kidney weights
showed a dose-related increase and there was a dose-related increase
in the number and size of hyaline droplets and accumulation of
alpha2u-globulin in the proximal convoluted tubule epithelial
cells of the kidneys. In addition, after 20 doses, granular casts in
the outer zone of the medulla and multiple cortical changes
(classified as chronic nephrosis) were also seen in dose-related
severity (Kanerva et al., 1987a).
In a subacute study, groups of 5 male and female rats were
exposed daily to 0, 277, 554, 1385 or 2770 mg d-limonene/kg bw
orally for 30 days. A general decrease in food intake and a
dose-related decrease in body weight were seen in groups of exposed
males, but little or no effect on organ weights nor relative organ
weights was observed. No significant changes were seen in
urinalysis, haematology or biochemical values. The following tissues
were examined histopathologically: adrenals, duodenum, heart,
kidneys, liver, lungs, lymph nodes, pancreas, pituitary, spleen,
stomach, testes/ovaries, thymus and thyroids. Authors reported that
no significant changes were noted except that granular casts were
seen in the kidneys of most exposed male rats (0/5, 3/5, 5/5, 5/5 or
4/5 animals exposed to 0, 277, 554, 1385 or 2770 mg/kg bw/day
respectively) (Tsuji et al., 1975a).
Groups of 10 F334/N rats of each sex were given daily gavage
doses of 0, 150, 300, 600, 1200 or 2400 mg d-limonene/kg bw in
5 ml/kg bw corn oil 5 days/week for 13 weeks. Five male and nine
female rats exposed to 2400 mg d-limonene/kg bw/day died during
the course of the study. In all rats receiving 1200 or 2400 mg/kg
bw/day, rough hair coats, lethargy and excessive lacrimation were
observed. Authors reported that nephropathy was seen in all groups
of male rats, with a dose-related increase in severity. The
nephropathy was reported to be characterized by degeneration of
epithelium in the convoluted tubules, granular casts within tubular
lumens (primarily in the outer stripe of the outer medulla), and
regeneration of the tubular epithelium. In all groups of male rats
(including control animals), hyaline droplets were observed in the
epithelium of proximal convoluted tubules in at least some animals.
An attempt to determine by "blind" evaluation if the incidence of
these droplets was dose-related was reported to have resulted in an
equivocal conclusion.
The slides containing the renal sections made from male rats
exposed to d-limonene in this 13 week study were reviewed. They
reported that hyaline droplet accumulation within the cytoplasm of
proximal convoluted tubule (PCT) epithelial cells was uniform among
all groups, including controls. However, chronic nephrosis, although
seen in all animals (including control animals), appeared with a
dose-related increase in severity. The authors attributed the
uniformity of hyaline droplet accumulation to the fact that the
animals were not exposed to d-limonene for 4-5 days before
necropsy and examination, giving the animals time to rid themselves
of some of the droplets, although more extensive damage to the
kidneys (chronic nephrosis) was not repaired (Kanerva & Alden 1987).
Chronic nephrosis consisted of cytoplasmic basophilia of the
PCT epithelial cells, tubular hyperplasia or atrophy, fibrosis of
Bowman's capsule and an interstitial fibrolymphocytic response.
Atrophic tubules were characterized by thickened basement membranes
and a decrease in tubular cross-sectional diameter due to a decrease
in cell size, whereas tubules considered to be hyperplastic showed
increases in cell size and number and an increase in cross-sectional
diameter compared with the surrounding unaffected tubules. In
addition, occasional foci of PCT epithelial cell
necrosis/degeneration were observed in kidneys from all
d-limonene-treated animals but not in control animals. All treated
animals, but not controls, also showed a dose-related increase in
the number of granular casts found in the outer stripe of the
medulla. Granular casts were markedly dilated and had attenuated or
non-existent epithelium. In addition, animals exposed to the highest
dose level (2400 mg/kg bw/day) were reported to show multifocal
hyaline casts and tubular dilation in the cortex and medulla (NTP,
1990).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
To assess the pulmonary tumour carcinogenicity of d-limonene,
groups of 15 male or female A/He mice were injected i.p. with
d-limonene 3 times weekly for 8 weeks (4.8 or 24 g/kg bw total
dose) and then necropsied 24 weeks after the first injection. No
significant induction of lung tumours was seen (Stoner et al.,
1973).
Groups of 50 8-9 week old male B6C3F1 mice were given
daily gavage doses of 0, 250, or 500 mg d-limonene/kg bw and
groups of 50 8-9 week old female B6C3F1 mice were given 0,
500, or 1000 mg d-limonene/kg bw in 10 ml/kg bw corn oil
5 days/week for 103 weeks. Mice were observed twice a day and
weighed weekly for the first 12 weeks, after which they were weighed
monthly. Clinical signs were recorded at least monthly. Moribund
animals were killed and a necropsy was performed on all available
animals. The tissues of all mice that died during the study and
animals with grossly visible lesions, as well as all animals in the
control and high dose groups, were examined for histopathology.
The authors reported that no clinical signs were associated
with administration of the test chemical. The survival of male mice
exposed to 250 mg d-limonene/kg bw/day was lower than that of
controls at the end of the study (controls: 33/50; 250 mg/kg bw/day:
24/50; 500 mg/kg bw/day: 38/50). No other differences in survival
were seen among exposed groups of either sex. Mean body weights of
exposed and control male mice were generally comparable. Weights of
female mice in the 1000 mg d-limonene/kg bw/day group, however,
were 5-15% lower than control animals after week 28.
Multinucleated liver hepatocytes and cells with cytomegaly
occurred at increased incidence in male mice exposed to 500 mg
d-limonene/kg bw/day (multinucleated hepatocytes: controls - 8/49;
250 mg/kg bw/day - 4/36 [incomplete sampling]; 500 mg/kg bw/day -
32/50) (hepatocytes with cytomegaly: controls - 23/49; 250 mg/kg
bw/day - 11/36 [incomplete sampling]; 500 mg/kg bw/day - 38/50). The
combined incidence of hepatocellular adenomas and carcinomas,
however, did not differ significantly from their incidence in
control mice (controls: 22/49; 250 mg/kg bw/day: 14/36 [incomplete
sampling]; 500 mg/kg bw/day: 15/50). No significant differences were
observed in female mice for any of these endpoints.
The incidence of adenomas or the combined incidence of adenomas
and carcinomas in the anterior pituitary gland of female mice
exposed to 1000 mg d-limonene/kg bw/day were significantly lower
than controls. The incidence of hyperplasia did not differ
significantly from controls, however (hyperplasia: controls - 16/49;
500 mg/kg bw/day - 0/8 [incomplete sampling]; 1000 mg/kg bw/day -
17/48) (adenomas: controls - 12/49; 500 mg/kg bw/day - 5/8
[incomplete sampling]; 1000 mg/kg bw/day - 1/48) (combined adenoma
and carcinoma: controls - 12/49; 500 mg/kg bw/day - 5/8 [incomplete
sampling]; 500 mg/kg - 2/48) (NTP, 1990).
2.2.3.2 Rats
Groups of fifty 7-8 week old male F344/N rats were given daily
gavage doses of 0, 75, or 150 mg d-limonene/kg bw and groups of 50
7-8 week old female F344/N rats were given 0, 300, or 600 mg
d-limonene/kg bw in 5 ml/kg bw corn oil 5 days/week for 103 weeks.
Rats were observed twice a day; they were weighed weekly for the
first 12 weeks, and monthly thereafter. Clinical signs were recorded
at least monthly. Moribund animals were killed; necropsies were
performed on all available animals. The tissues of all rats that
died during the study, animals with grossly visible lesions, and all
animals in the control and high dose groups were examined for
histopathology.
No compound-related clinical signs were reported. The survival
of the female animals exposed to 600 mg/kg bw was lower than that of
control animals after week 39, while the survival of male rats
exposed. Mean body weights of male rats exposed to 150 mg/kg bw were
generally 4%-7% lower than controls from week 28.
Cataracts were observed at increased incidence in male rats
exposed to 150 and female rats exposed to 300 or 600 mg
d-limonene/kg bw/day, and retinal degeneration was seen in all
dosed animals. The authors pointed out, however, that the rats of
each sex that were exposed to their respective highest-dose levels
were housed in the top rows of the cage racks, the animals exposed
to the low-dose levels were below them, and the controls were housed
in the lowest rows for all but the last 10 weeks of the study.
Authors conclude that these effects were due to variations in the
proximity of animals in different dose groups to the light source.
Subcutaneous tissue fibromas in male rats occurred with a
negative dose-response trend (controls: 8/50; 75 mg/kg bw/day: 2/50;
150 mg/kg bw/day: 3/50). However, the combined incidence of fibromas
and fibrosarcomas was not significantly different when dosed males
were compared to controls (controls: 8/50; 75 mg/kg bw/day: 4/50;
150 mg/kg bw/day: 3/50).
Combined squamous cell papillomas or carcinomas in the skin of
male rats showed a positive dose-response trend (controls: 0/50;
75 mg/kg bw/day: 0/50; 150 mg/kg bw/day: 3/50). However, the
incidence of these neoplasms in dosed male rats was within the range
of incidences for NTP historical control rats, and did not differ
significantly from the incidence in control animals. The authors
concluded that the positive dose-response trend was not related to
d-limonene exposure.
Mononuclear cell leukaemia occurred in male rats with a
positive dose-response trend by the incidental tumour test
(controls: 10/50; 75 mg/kg bw/day: 10/50; 150 mg/kg bw/day: 19/50).
The incidences in dosed male rats were not significantly different
from the incidences in controls, however. The authors concluded that
the positive dose-response trend was not related to d-limonene
administration.
There was a significant positive dose-response trend in the
incidence of interstitial cell tumours in the testes of male rats,
and the incidences of these tumours in dosed groups were
significantly higher than the incidences in control animals
(control: 37/50; 75 mg/kg bw/day: 47/49; 150 mg/kg bw/day: 48/50).
The authors argued, however, that since historically this type of
tumour occurs in almost all aged F344 rats, this result should not
be considered to be related to d-limonene exposure, but rather was
an artifact caused by the low survival of the control animals in
this experiment (see above).
The incidence of uterine endometrial stromal polyps in females
exposed to 300 mg/kg bw/day was increased when compared to controls.
However, the incidence in the control animals was well below the NTP
historical incidence of this tumour. For this reason, and because
there was no positive dose-response trend, the authors argued that
this was not a compound-related effect.
d-Limonene exposure of male rats was associated with
dose-related increases in the incidences of mineralization and
epithelial hyperplasia in the kidneys (mineralization: controls -
7/50; 75 mg/kg bw/day - 43/50; 150 mg/kg bw/day - 48/50) (epithelial
hyperplasia: controls - 0/50; 75 mg/kg bw/day - 35/50; 150 mg/kg
bw/day - 43/50). The lesions consisted of linear deposits of mineral
in the medulla (renal papilla) and focal hyperplasia in the
transitional epithelium overlying the papilla. Hyperplasia was
frequently located near the fornices of the renal pelvis and was
occasionally bilateral. The severity of nephropathy, which occurs
spontaneously in aged male rats, was also positively dose-related in
this experiment (severity of nephropathy, rated on a scale from 0
[not present] to 4 [marked]: control mean - 1.5; 75 mg/kg bw/day
mean - 1.8; 150 mg/kg bw/day mean - 2.2). This nephropathy was
characterized by degeneration and atrophy of the tubular epithelium,
dilation of tubules with formation of hyaline droplets and granular
casts, regeneration of tubular epithelium, glomerulosclerosis, and
interstitial inflammation and fibrosis.
Kidney tubular cell hyperplasia and neoplasms were also
increased in dosed male rats (hyperplasia: controls - 0/50; 75 mg/kg
bw/day - 4/50; 150 mg/kg bw/day - 7/50) (adenomas: controls - 0/50;
75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 8/50) (adenocarcinomas:
controls - 0/50; 75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 3/50)
(combined adenomas and carcinomas: controls - 0/50; 75 mg/kg bw/day
- 8/50; 150 mg/kg bw/day - 11/50). Incidences of tubular cell
adenomas and the combined incidences of adenomas and
adeno-carcinomas in male rats showed significant positive
dose-response trends. High-dose males had a significantly higher
incidence than control males of both categories of tumours, while
low-dose males had a significantly higher incidence of the combined
category than control males. These neoplasms are historically rare
and none were seen in control males nor in any group of females.
The authors stated that kidney tubular cell hyperplasia,
adenomas, and adenocarcinomas were part of a morphologic spectrum.
Proliferative lesions diagnosed as hyperplasia generally consisted
of one to three adjacent cross-sections of enlarged tubules with
stratification of the epithelium. In some lesions, the epithelial
cells appeared to completely fill the lumen. The tubular cell
neoplasms varied in diameter from less than 1 mm to 15 mm. They
exhibited varied patterns of growth and were either solid, cystic,
or papillary. The solid and cystic neoplasms showed little evidence
of tubular structures and the cells were arranged in solid sheets or
small solid nests separated by a delicate vascular stroma. Some
neoplasms were reported to consist of layers of epithelium lining a
fibrous connective tissue stroma and were arranged in complex
branching papillary formations. The neoplasms were classified as
adenocarcinomas primarily if they exhibited cellular pleomorphism
and anaplasia, or if they were larger than 10 mm (NTP, 1990).
2.2.3.3 Dogs
Groups of 3 male or female Japanese beagle dogs were exposed
orally to 0, 0.4, 1.2 or 3.6 ml d-limonene/kg bw daily for 6
months. Dose-related occurrences of frequent vomiting and nausea
were seen in some animals. At doses of at least 1.2 ml/kg bw/day for
females and 3.6 ml/kg bw/day for males, a decrease in body weight
gain was noted compared to controls, but little or no effect on food
intake, organ weights or relative organ weights was reported. No
significant changes were seen in urinalysis, haematology nor
biochemical values, except that in animals at the 3.6 ml/kg bw/day
level, total cholesterol and blood sugar were lowered. The following
tissues were examined histopathologically: adrenals, bile duct,
duodenum, heart, kidneys, liver, lungs, pancreas, pituitary, rectum,
spleen, stomach, testes/ovaries, thymus and thyroids. No significant
changes were noted except that granular casts were seen in the
kidneys of all male dogs exposed to 3.6 ml/kg bw/day and all females
exposed to 0.4 ml/kg bw/day or more (1/3, 1/3, 2/3 or 3/3 for males;
1/3, 2/2, 3/3 or 3/3 for females exposed to 0, 0.4, 1.2 or 3.6 ml/kg
bw, respectively) (Tsuji et al., 1975b).
In a more recent study, groups of five male and female beagle
dogs were gavaged twice daily for six months with 0, 0.12, or
1.2 ml/kg bw/day (0, 10, or 1000 mg/kg bw/day) d-limonene. The
highest dose was reported to be near the maximum tolerated dose for
emesis. The authors stated that food consumption and body weight
were not affected by treatment. Although there was a positive
dose-related trend for absolute and relative female kidney weights
and for relative male kidney weight, authors reported that there
were no histo-pathological findings in the kidneys that could be
associated with the organ weight changes. In addition, there was no
hyaline droplet accumulation nor other indication of nephropathy
such as those associated with d-limonene consumption in male rats
(Webb et al., 1990)
2.2.3.4 Cocarcinogenicity/tumour promotion studies
Two publications have reported the results of experiments in
which 0.25 ml oil of sweet orange, composed of > 90% d-limonene,
was applied to the skin of 10 male and 10 female "101" mice weekly
for 42 weeks, starting 3 weeks after an application of 300 µg
7,12-dimethyl-1,2-benzanthracene (DMBA) in 0.2 ml acetone. While no
effects in the area of the treated skin were seen in mice exposed to
d-limonene or to DMBA alone, mice exposed to DMBA followed by
exposure to d-limonene began to develop papillomas during the
twelfth week of d-limonene exposure. After 33 weeks of treatment,
13/18 treated mice showed a total of 39 papillomas. No malignant
tumours were seen in the exposed area, however.
In a second study, mice were exposed to d-limonene following
exposure to: 1) 4 skin applications of 60 mg urethane in 0.3 ml
acetone at 3 day intervals, or 2) 4 i.p. injections of 16 mg
urethane in 0.1 ml distilled water at 3 day intervals. Papillomas
appeared on 3/13 mice in group 1 and 2/13 mice in group 2 by the
fourteenth week of exposure.
In a final experiment, the terpene fraction of oil of sweet
orange (containing d-limonene) was isolated and tested with DMBA
as described for the first experiment. Papillomas in mice exposed to
both DMBA and the terpene fraction of oil of sweet orange began to
appear in the eleventh week of treatment and were visible in 8/15
mice by the thirty-third week of treatment. Although pure
d-limonene was not tested in these experiments, the authors
concluded that the promoting effects seen were probably caused by
this chemical since it was the primary constituent of the test agent
(Roe, 1959, Roe & Peirce, 1960).
A group of 23 male and female albino mice were given single
doses of 50 µg benzo(a)pyrene (BAP) by stomach tube followed by 40
weekly doses of 0.05 ml d-limonene; seventeen male and female mice
received BAP only, 15 male and female mice received d-limonene
only and 18 male and female mice served as untreated controls. While
no tumours of the forestomach epithelium were seen in control
animals, 2 animals treated with BAP alone (12%), 2 animals treated
with d-limonene alone (13%), and 5 animals treated with both BAP
and d-limonene (22%), developed 0, 2, 3, and 8 tumours,
respectively, although none was judged to be a carcinoma. The
authors concluded that although d-limonene was weakly carcinogenic
for mouse forestomach epithelium it did not increase the tumour
yield caused by pretreatment with BAP and, therefore, was not a
promoter (Field & Roe, 1965).
Cells of embryos taken from F344 rats were infected with
Rauscher murine leukaemia virus and then treated with 0.05 µg/ml
3-methylcholanthrene (3-MCA) followed by 15 µg/ml d-limonene.
Following treatment with 3-MCA and d-limonene, colony formation in
semisolid medium was observed and was considered to denote cell
transformation. Authors reported that d-limonene alone failed to
transform cells and 3-MCA alone was only effective at concentrations
exceeding 0.2 µg/ml (Roe, 1959; Roe & Peirce, 1960; Traul et al.,
1981)).
Elegbede and co-workers attempted to repeat the 1960 study of
Roe and Peirce (see above) using purified d-limonene as well as
oil of sweet orange. The skin of groups of 24 female CD-1 mice were
treated once with 51.2 µg DMBA in 0.2 ml acetone followed 14 days
later by twice weekly applications of 0.1 ml d-limonene or oil of
sweet orange mixed with 0.1 ml acetone. Other groups of mice were
similarly initiated with DMBA, but beginning 7 days later were given
a diet containing 1% d-limonene or oil of sweet orange. In all
cases, administration of d-limonene or oil of sweet orange
continued for 40 weeks after DMBA treatment. Topical (but not
ingested) d-limonene was found to slightly increase the number of
tumours induced by DMBA alone, the increase becoming significant
only after 34 weeks of treatment. When oil of sweet orange (95%
d-limonene content) was applied to the skin, authors reported that
a promoting effect was seen after 7 weeks of treatment; promotion
was not reported, however, when oil of sweet orange was incorporated
into the diet. The authors concluded that a component of orange oil
other than d-limonene was responsible for the promoting activity
observed and that the very small promoting activity seen with
d-limonene may be due to its contamination by this unidentified
component (Elegbede et al., 1986b).
2.2.3.5 Anticarcinogenicity studies
A group of 50 male C57Bl/6J mice were injected s.c. with 25 µg
dibenzpyrene (DBP) followed 24 h later by an injection of 0.15 ml
d-limonene. After 34 weeks of observation, the authors reported
that d-limonene significantly delayed the appearance of lung
adenomas induced by DBP and reduced the total number of tumours
seen. At 30 weeks post-DBP exposure without subsequent exposure to
d-limonene, lung adenomas were seen in approximately 70% of the
animals; with exposure to d-limonene, tumours were seen in
approximately 30% of the animals.
In a second experiment reported in the same paper, a group of
50 female A/J mice were injected s.c. with DBP followed by 16 weekly
injections into the tail vein of 1% v/v suspensions of 1 mg of
d-limonene. The incidence of lung adenomas was reduced from 75% of
animals exposed to DBP alone to 40% of animals exposed to DBP and
injected with d-limonene. Control incidence of lung adenomas in
this experiment was 27%. Treatment of mice with d-limonene alone
reduced this background incidence to approximately 7% (Homburger
et al., 1971).
Simultaneously, 5 µg of BAP and 10 mg d-limonene were applied
to the skin of groups of 50 female ICR/Ha Swiss mice three times a
week for 440 days. d-Limonene was found to partially inhibit BAP
carcinogenicity (Van Duuren & Goldschmidt, 1976).
Female Sprague-Dawley rats were fed diets containing 0, 1000,
or 10 000 ppm d-limonene for 1 wk, at which time they were given a
single gavage dose of 65 mg DMBA/kg bw. The d-limonene-containing
diet was then continued for 27 weeks. Authors reported that
d-limonene delayed the appearance of DMBA-induced mammary tumours
in a dose-related manner, reduced the incidence of tumours, and
caused increased regression of tumours that did appear (Elegbede
et al., 1984a,b).
Female (W/Fu x F344)F2 rats were fed a diet containing 10%
d-limonene for 80 days beginning after the first appearance of
tumours induced by intubation of rats with 130 mg DMBA/kg bw.
Significant tumour regressive action by d-limonene was observed,
and the development of subsequent tumours was also reduced
significantly (Elegbede et al., 1986a).
Following initiation with DMBA (single gavage dose of 65 mg/kg
in sesame oil), groups of female Sprague-Dawley rats were (1) fed a
basal cereal diet one week before and 24 weeks following initiation
with DMBA (controls), (2) fed a diet containing 5% d-limonene one
week before and one week after treatment with DMBA, then fed the
control diet for 24 weeks (initiation group), or (3) fed the control
diet for one week before and one week following DMBA initiation,
then fed the 5% d-limonene diet for 24 weeks
(promotion/progression group). d-Limonene was reported to be
effective in reducing the average number of rat mammary carcinomas
in DMBA-initiated rats when fed during the initiation phase or
during the initiation/progression phase of carcinogenesis. Time to
appearance of the first tumour was extended only when d-limonene
was fed during the initiation phase, however. Authors concluded that
these effects could not be attributed to changes in mammary-relevant
endocrine functions (serum levels of prolactin and duration of
estrus cycle) (Elson et al., 1988).
Female Wistar-Furth rats were (1) placed on diets containing 5%
d-limonene or 5% orange oil (source of d-limonene) for 2 weeks
prior to initiation with nitrosomethylurea (NMU; single i.v. dose of
50 mg/kg bw), then continued on these diets for 23 weeks; (2) fed
diets containing 5% d-limonene for two weeks before and one week
following initiation with NMU, then fed a basal diet for 22 weeks
(initiation group); or (3) fed basal diets prior to and for 1 week
following initiation with NMU, then fed diets containing 5%
d-limonene for 22 weeks (promotion/progression group). Both orange
oil and d-limonene decreased mammary tumour incidence (controls:
80%; d-limonene: 45%; orange oil: 47%) and the average number of
mammary tumours per rat (controls: 1.8; d-limonene: 0.4; orange
oil: 0.8) when present in the diet for two weeks before and 23 weeks
after NMU administration. d-Limonene decreased tumour incidence
and decreased the numbers of tumours per rat (by approximately 50%)
when fed during the promotion/progression experiment but not the
during the initiation experiment (Maltzman et al., 1989).
2.2.3.6 Studies on the mechanism of d-limonene carcinogenicity
This section discusses (1) spontaneously occurring nephropathy
in mature male rats and (2) the current proposed mechanism of
carcinogenicity of d-limonene and other chemicals/mixtures that
exacerbate this condition and lead to alpha2u-globulin-associated
male rat nephropathy.
(1) Mature male rat nephropathy
Normal male rats at least 30 days old exhibited marked
increased protein excretion in their urine not seen in younger males
nor in females of any age. This proteinuria reached a maximum level
of approximately 2.5-3 times that seen in females at 90 days of age.
Microscopically, the authors also observed intracellular droplets in
the epithelial cells of the upper two-thirds of the proximal
convoluted tubules of kidneys of male rats at least 60 days old. The
occurrence of these hyaline droplets consisted of a few groups of
small droplets in occasional cells in 60 day old animals. The number
of cells having droplets and the number and size of droplets per
cell increased with age; from 90 days of age most proximal tubule
cells contained them. In contrast, at 120 and 180 days of age, only
a small number of females showed occasional droplets in a few cells
of the proximal tubules.
These authors further demonstrated the sex-relatedness of this
phenomenon by castrating male rats at 30 days of age and injecting
female rats with 5 mg testosterone every other day from 50 days of
age. When examined at 120 days of age, the castrated male rats
showed proteinuria at a level equal to control females and hyaline
droplets were only seen in 4/20 animals. Testosterone-treated
females, on the other hand, had proteinuria at a level approximately
midway between control males and females. No hyaline droplets were
seen in the proximal tubule cells of these females, however, showing
that the addition of testosterone alone was insufficient to cause
them to appear (Logothetopoulos & Weinbren, 1955).
Numerous authors have since reported detailed descriptions of
spontaneously occurring nephropathy in mature male rats which is a
consequence of this hyaline droplet accumulation. The sequence of
events appears to be that: (1) protein accumulates in the lysosomes
in the cytoplasm of epithelial cells of the P2 segment of the
proximal convoluted tubules in the kidney cortex; (2) the
accumulated material becomes so abundant that it crystallizes,
forming the microscopically visible hyaline droplets; (3) the
continued build-up of material eventually leads to the death of
epithelial cells with a concomitant thinning of the epithelial layer
(although some regeneration is usually seen); and (4) the dead cell
debris becomes lodged in the outer strip of the outer medulla where
the tubules narrow, forming granular casts and causing tubule
dilation with pressure necrosis of the cells of the tubule walls.
There is also an increase in the relative weight of the kidneys
accompanying this phenomenon.
A steadily increasing number of agents have been identified
that appear to exacerbate this hyaline droplet formation and its
consequences in mature male rats. With some, noticeable changes
occur within days of exposure. These agents include complex mixtures
of hydrocarbons such as mineral spirits (Carpenter et al., 1975a;
Phillips & Cockrell, 1984), "60" solvent (Carpenter et al.,
1975b), "high naphthenic solvent" (Carpenter et al., 1977), the
petroleum-derived or shale-derived jet fuel JP-5 (MacEwen & Vernot,
1978a; Parker et al., 1981; Bruner, 1984; MacNaughton & Uddin,
1984), petroleum-derived and shale-derived diesel fuel marine (DFM),
the jet fuels JP-4, JP-TS and JP-7, and the missile propellants
JP-10 and RJ-5 (Bruner, 1984; MacNaughton & Uddin, 1984), C/10-C/11
isoparaffin (Phillips & Egan, 1984; Phillips & Cockrell, 1984), five
petroleum naphtha mixtures (Halder et al., 1984) and unleaded
gasoline (MacFarland et al., 1984; Busey & Cockrell, 1984; Halder
et al., 1984; Kitchen, 1984; Garg et al., 1988a,b, 1989; Short &
Swenberg, 1988; Short et al., 1989a). Also included on the list
are the individual chemicals decahydro-naphthalene (decalin, MacEwen
& Vernot, 1978b; Gaworski et al., 1980, 1981; Bruner, 1984; Alden
et al., 1984; Olson et al., 1986; Stone et al., 1987a,b;
Kanerva et al., 1987b,c; Read, 1988), pentachloroethane (NTP,
1983; Goldsworthy et al., 1988), 1,4-dichlorobenzene (NTP, 1987a;
Charbonneau et al., 1989), perchloroethylene (Goldsworthy et al.,
1988), dimethyl methylphosphonate (NTP, 1987b),
2,2,4-trimethylpentane (Stonard et al., 1986; Short et al.,
1987; Charbonneau et al., 1987; Lock et al., 1987; Loury
et al., 1987; Short et al., 1989a), the chemically unrelated
agents 3-methylamino-1-(3-trifluoromethyl-phenyl)-2-pyrazoline,
cis/trans-2-(4'-t-butylcyclohexyl)-3-hydroxy-1,4-naphthoquinone
and 2,3,5,6-tetrahycro-6-phenylimidazo-(2,1-b) thiazole (Read
et al., 1988) and d-limonene (Kanerva et al., 1987a; NTP,
1990).
There is no obvious common structure among the specifically
identified chemicals that cause an increased accumulation of hyaline
droplets in mature male rat kidneys. And there are examples of
agents that do have similar mixtures/structures to those listed
above that do not cause this phenomenon. These include "70" solvent
(Carpenter et al., 1975c), two other petroleum naphtha mixtures
(Halder et al., 1984), 1,2-dichlorobenzene (Charbonneau et al.,
1989) and an analog of d-limonene, 4-vinylcyclohexene (NTP, 1986).
Recently, however, Miller et al. (1989) reported that they have
identified correlations between structure and binding affinity to
alpha2u-globulin, but no specific details were given in their
published report.
While exposure to unleaded gasoline for 72 h has been shown to
cause effects that are reversible (Garg et al., 1988a), with
chronic exposure to several of these mixtures/chemicals the kidney
nephrosis has been reported to progress to hyperplasia, adenoma and
even adenocarcinoma (MacFarland et al., 1984; Kitchen, 1984;
MacNaughton & Uddin, 1984; NTP, 1987a,b,c; NTP, 1990).
(2) Mechanism for alpha2u-globulin-associated male rat
nephropathy
The mechanism proposed for alpha2u-globulin-associated male
rat nephrosis has recently been thoroughly reviewed by the US
Environmental Protection Agency (US EPA, 1991). Their review
incorporated the results of many studies, including Charbonneau &
Swenberg (1988), Swenberg et al. (1989), Flamm & Lehman-McKeeman
(1991), Lehman-McKeeman et al. (1989), Lehman-McKeeman et al.
(1990a,b), Short et al. (1987), Short et al. (1989a,b), Dietrich
& Swenberg (1991), Murty et al. (1988), Ridder et al. (1990),
and Webb et al. (1989). The review concluded that:
"Of the eight model substances tested in chronic animal
bioassays (decalin, dimethyl methyl phosphonate, JP-4 jet fuel,
JP-5 shale-derived jet fuel, d-limonene, methyl isobutyl
ketone, pentachloroethane, and unleaded gasoline), all invoked
a specific type of protein droplet nephropathy in male rats and
also produced renal tumours in male rats but not in other
species tested. It has been proposed that such renal tumours
are the end product in the following sequence of functional
changes in the epithelial cells of proximal tubules:
* Excessive accumulation of hyaline droplets in proximal
tubules, representing lysosomal overload, cell loss, and
regenerative cellular proliferation.
* Cell debris in the form of granular casts accumulates at
the 'corticomedullary' junction with associated dilation
of the affected tubule segment and more distally,
mineralization of tubules within the renal medulla.
* Single-cell necrosis accompanied by compensatory cell
proliferation and exacerbation of the chronic progressive
nephropathy characteristically found in aging rats occurs.
* Renal tubule hyperplasia and neoplasia develop
subsequently.
According to this hypothesis, the increased proliferative
response caused by the chemically induced cytotoxicity results
in clonal expansion of spontaneously initiated renal tubule
cells and increased incidence of renal tumour formation (Trump
et al., 1984a; Alden, 1989; Swenberg et al., 1989). This
line of reasoning leads supporters of the hypothesis to
conclude that the acute and chronic renal effects induced in
male rats by such chemicals will be unlikely to occur in any
species not producing alpha2u-globulin, or a very closely
related protein, in the large quantities typically seen in the
male rat (Alden, 1989; Borghoff et al., 1990; Green et al.,
1990; Olson et al., 1990; Flamm & Lehman-McKeeman, 1991;
Swenberg, 1991)."
The following paragraphs summarize some of the data that
support this hypothesis:
Contents and catabolism of hyaline droplets/
phagolysosomes
The composition of the hyaline droplet inclusions has been
identified by Kanerva et al. (1987c) using two-dimensional gel
electrophoresis and immunological binding as being four species of
the low molecular weight protein alpha2u-globulin. As expected,
alpha2u-globulin was found to be absent in immature male rats and
female rats of all ages. It was present, however, in female rats
that had been ovariectomized and repeatedly injected with
testosterone. More recently, groups have confirmed that
alpha2u-globulin is the major constituent of the renal
phagolysosomal inclusions in male rats exposed to unleaded gasoline
(Garg et al., 1988b), and perchloroethylene and penta-chloroethane
(Goldsworthy et al., 1988). Finally, Dietrich and Swenberg (1991)
reported that male NCI-Black-Reiter rats, which do not produce
alpha2u-globulin, are not susceptible to nephropathy produced by
2,2,4-trimethylpentane, 1,4-dichloro-benzene, isophorone, PS-6
unleaded gasoline, or d-limonene.
A sex-specific protein has been identified in human urine
(Bernard et al., 1989), but the concentration (up to 108 µg/l) is
about 10 000 times less than that of alpha2u-globulin in male rats
(about 40 mg/24 h) (Roy & Neuhaus, 1967). Therefore assuming that
the protein in human urine shows the same binding characteristics as
alpha2u-globulin, it is reasonable to conclude that humans would
be comparably less sensitive than the male rat.
Alpha2u-globulin is synthesized in the liver of rats of both
sexes. This protein is filtered by the kidney glomeruli and
reabsorbed by the epithelial cells of the proximal convoluted
tubules by the formation of phagosomes at the lumenal surface.
Normally, the phagosomes then combine with cellular lysosomes and
the protein is degraded. In mature male rats, however, this
catabolism is slowed in some way under hormonal influence leading to
the intracellular accumulation of the protein which becomes
manifested as hyaline droplets. The degradation is slowed even more
by exposure to at least some of the agents listed in the previous
section (Stonard et al., 1986; Short et al., 1987; Kanerva
et al., 1987c; Charbonneau et al., 1987). A time- and
dose-dependent progressive increase in the number and size, and
alterations in the morphology of phagolysosomes in proximal
convoluted tubule epithelial cells have been demonstrated with
exposure to unleaded gasoline (Garg et al,. 1989). The ability of
unleaded gasoline to induce these effects declines with the age of
the male rat, in proportion to the declining production of
alpha2u-globulin (Murty et al., 1988).
Binding of chemicals to alpha2u-globulin
1,4-Dichlorobenzene as well as its major metabolite,
2,5-dichlorophenol, were both found to be reversibly bound (and
1,4-dichlorobenzene also covalently bound) to alpha2u-globulin in
the kidneys of treated male rats (Charbonneau et al., 1989).
Similarly, two groups have attempted to identify the specific
binding between one of these agents (2,2,4-tri-methylpentane) and
alpha2u-globulin. Loury et al. (1987) reported that no covalent
binding could be found, nor did there appear to be formation of a
Schiff base. Lock et al. (1987), however, detected reversible
binding of the metabolite 2,4,4-trimethyl-2-pentanol to
alpha2u-globulin. This specific metabolite was found in liver
cells of male rats only.
Likewise with decalin, an alcohol metabolite has been
identified that is unique to male rats (Olson et al., 1986). While
both male and female rats synthesize cis,cis-2-decalol and
trans,cis-2-decalol from cis-decalin in their livers,
cis,cis-1-decalol was found only in males and
cis,trans-1-decalol was produced in much higher concentrations
than in females. In similar fashion, with trans-decalin as the
substrate trans,trans-1-decalol was seen only in livers of male
rats. When kidneys were examined, 2-decalone (cis or trans,
depending on the original substrate) was found in the males while no
decalin metabolites whatever were seen in female kidneys. Whether
these unique male decalin metabolites react with alpha2u-globulin
has not yet been tested.
More recently, Lehman-McKeeman et al. (1989) have reported
that d-limonene and d-limonene-1,2-oxide, a major metabolite of
d-limonene, were found reversibly bound to alpha2u-globulin
(identified by amino acid sequencing) in the kidneys of treated
male, but not female, rats.
Inhibition of alpha2u-globulin catabolism
Charbonneau et al. (1988) reported that alpha2u-globulin
bound in kidney cytosol was not digested by purified proteases or
proteinase.
Treating rats with leupeptin, an inhibitor of cathepsin B, a
major lysosomal peptidase, mimics the ability of unleaded gasoline
to cause the accumulation of alpha2u-globulin in kidney
phagolysosomes. Authors concluded that the mechanism of
nephrotoxicity involves inhibition of renal phagolysosomal
proteolysis (Olsen et al., 1988).
Induction of cell proliferation
Short et al. (1989a) have shown that proliferation of
epithelial cells in the P2 proximal tubule segment in male rats is
associated with exposure of the animals to either
2,2,4-trimethylpentane or unleaded gasoline. They noted that this
proliferation closely paralleled the extent and severity of
detectable alpha2u-globulin in the same cells. The authors
speculated that this proliferation leads to the nephrocarcinogenic
effects caused by these agents.
Proliferation was also noted by Charbonneau et al. (1989) in
male rats treated with 1,4-dichlorobenzene. Short et al. (1989b)
observed increased numbers of atypical cell foci and renal cell
tumours in male, but not female, rats initiated with
N-ethyl-N-hydroxyethylnitrosamine and subsequently exposed to
unleaded gasoline or 2,2,4-trimethylpentane. The extent of the
promotion was reported to be dose-related and to parallel the
effects of these compounds on chronic cell proliferation.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity
d-Limonene was reported to increase the number of abnormal
chick embryos to approximately 50% when a single dose (25 µM/embryo)
in olive oil was injected suprablastodermically (Abramovici &
Rachmuth-Roizman, 1983).
Pregnant mice were given oral doses of 0, 591, or 2363 mg/kg bw
d-limonene from days 7 through 12 of gestation. Authors reported
significant decreases in body weight gain of dams in the high-dose
group; fetuses of dams exposed to the high-dose showed increased
incidences of lumbar rib, fused rib, delayed ossification, and
decreased body weight gain relative to fetuses of control dams
(Kodama et al., 1977a).
Pregnant Japanese white rabbits were given oral doses of 0,
250, 500, or 1000 mg/kg bw d-limonene from day 6 to day 18 of
gestation. Significant decreases in body weight gain in dams given
250 or 500 mg/kg bw/day d-limonene were observed, and survival in
dams given 1000 mg/kg bw/day was significantly reduced (40%
survival). From examination of the fetuses, authors concluded that
d-limonene was not teratogenic in rabbits (Kodama et al.,
1977b).
2.2.6 Special studies on genotoxicity
Table 2: Genotoxicity data on d-limonene
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium .03-3 µmol/plate neg Florin et
(+&-activ) TA98, TA100, al., 1980
TA1535, TA1537
Yeast S. cerevisiae .1-100 mM/5x107 (1) Fahrig,
(-activ) MP1 strain cells 1984
Mammalian Mouse embryo 0-215 mg/kg (2) Fahrig,
(-activ) system 1984
Ames test S. typhimurium 0-20 µM/plate neg Watabe
(+&-activ) TA1535, TA100, et al.,
TA1537, TA1538, 1980
TA98
Ames test S. typhimurium 0-3333 µg/plate neg Haworth
(+&-activ) TA98, TA100, et al.,
TA1535, TA1537 1983
Ames test S. typhimurium 0-3333 µg/plate neg NTP,
(+&-activ) TA100, TA1535, 1990
TA1537, TA98
Mammalian Mouse L5178Y neg NTP,
(+&-activ) cells in vitro 1990
Sister Chinese hamster 0-162 µg/ml neg NTP,
chromatid ovary cells 1990
exchange in vitro
(+&-activ)
Chromosomal Chinese hamster 0-500 µg/ml (3) neg NTP,
aberrations ovary cells 0-100 µg/ml (4) neg 1990
(+&-activ) in vitro
Table 2 cont'd
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium 150 mg/plate neg Heck et
(+&-activ) TA1535, TA1537, al., 1989
TA1538, TA98,
TA100
Mammalian Mouse L5178Y 100 µg/ml neg Heck et
(+&-activ) cells in vitro al., 1989
(1) Author reported that results indicated that d-limonene was a
co-recombinant and an anti-mutagen.
(2) Author reported that results indicated that d-limonene is inactive
when given alone, but reduces the mutagenic effect of ethylnitrosourea
when the two are co-administered.
(3) Activated: addition of S9 from the livers of Aroclor 1254-induced
male Sprague Dawley rats.
(4) Not activated
2.2.7 Special studies on immune responses
Limonene has been identified as a primary skin irritant in
humans (Pirila et al., 1964; Klecak et al., 1977), rabbits
(research Institute for the Development of Fragrance Materials,
Inc., 1984; Research Institute for Fragrance Materials Inc., 1985),
guinea-pigs (Klecak et al., 1977), and mice (Gad et al., 1986).
d-Limonene was reported both to elicit (De Groot et al., 1985)
and not to elicit (Greif, 1967) allergic reactions in human skin and
to elicit allergic reactions in mouse skin (Gad et al., 1986).
d-Limonene was reported to eliminate human skin sensitization to
several aldehydes when they were co-administered with d-limonene
(Opdyke, 1975), and d-limonene was reported to reduce the
sensitivity and delayed hypersensitivity of guinea-pig skin to
citral (Hanau et al., 1983; Barbier & Benezra, 1983). Gozsy and
Kato (1957) reported that local endothelial phagocytosis is induced
in rat skin by d-limonene, and that d-limonene increases
phagocytosis in guinea-pig skin in vivo and in mouse skin
in vitro (Gozsy & Kato, 1958) and increases capillary permeability
in the guinea-pig (Kato & Gozsy, 1958).
Male BALB/c mice were treated intragastrically with 0, 0.002,
0.008, 0.033, 0.133, 0.531 or 2.125 mg/ml d-limonene daily. After
8 weeks, but not after 4 weeks, the mitogenic responses of spleen
cells taken from the treated animals and exposed in vitro to
concanavalin A and phytohaemagglutinin, which stimulate T-cells, and
lipopolysaccharide, which affects B-cells, were found to be
suppressed by the d-limonene treatment.
Other animals in groups of 10 were given these same doses of
d-limonene daily for 7 days prior to, or 8 days following,
immunization with keyhole limpet haemocyanin (KLH). Primary effects
on T- and B-cell responses were determined 10 days later.
Secondary effects were determined by reinoculating the animals
with KLH 21 days after the first inoculation and then assaying on
day 24. The primary and secondary responses were reported to be
suppressed by d-limonene if the animals were exposed to KLH first,
but stimulated in treated mice if exposed to KLH after d-limonene
exposure.
Histopathological examination of secondary lymphoreticular
tissue taken from animals exposed to d-limonene showed significant
secondary follicle development and prominent lymphoid nodules and
aggregates in the pancreas and intestinal mucosa, which were evident
in animals which had received the highest dose of d-limonene
(Evans et al., 1987).
2.3 Observations in humans
Numerous prospective and retrospective cohort epidemiological
studies have been done on populations of refinery, chemical plant,
and service station workers, exposed to mixtures of chemicals that
have been associated with male rat alpha2u-globulin nephropathy
(Hanis et al., 1979; Schottenfeld et al., 1981; Hanis et al.,
1982; Thomas et al., 1982; Austin & Schnatter, 1983; Higginson
et al., 1984; Raabe, 1984; Wen et al., 1984; Wong & Raabe, 1989;
Page & Mehlman, 1989). No statistically significant evidence was
found in any study to link incidence of cancer of the kidney to
work-place exposure to mixtures of complex hydrocarbons.
Igimi et al. (1976) reported that d-limonene was a safe and
effective gallstone solubilizer in animals and humans; side effects
in humans were reported to be pain and tenderness radiating from the
upper abdomen to the anterior chest, nausea and vomiting, and
diarrhoea.
3. COMMENTS
d-Limonene has been shown to reduce body weights
significantly in male and female mice and rats and in female
rabbits. The NOEL for this effect was 150 mg of d-limonene kg
bw/day (administered by gavage) in a 2-year study in male rats.
Oral administration of d-limonene (400 mg/kg bw/day) to rats
for 20 days slightly increased liver weight and phospholipid
content, decreased liver and serum cholesterol levels, increased the
concentration of cytochromes P450 and B5, and increased the
activities of aminopyrine demethylase and aniline hydroxylase.
Although liver lesions were not associated with the administration
of d-limonene in a 2-year study in rats (doses up to 150 mg/kg
bw/day for males; doses up to 600 mg/kg bw/day for females), a daily
gavage dose of 500 mg d-limonene/kg bw/day for 2 years was
associated with an increased incidence of multinucleated liver
hepatocytes and cytomegaly in male mice. The NOEL for these effects
was 250 mg/kg bw/day administered by gavage to male mice for 2
years.
Dermal application of d-limonene has been reported to cause
irritation and immune-mediated skin reactions in a number of
species, including humans. Oral administration of d-limonene to
mice was reported to have immunological effects, including
suppression of the in vitro mitogenic response by mouse T-cells
and B-cells and both suppression and stimulation of antigenic
responses. However, the biological significance of these in vitro
changes is not known.
Results of teratogenicity studies in mice suggest that maternal
consumption of 2400 mg of d-limonene/kg bw/day but not of
600 mg/kg bw/day, affected the development of fetuses (decreased
body weight gain, increased incidences of lumbar rib and fused rib,
and delayed ossification). However, d-limonene was reported to be
non-teratogenic in rabbits at doses ranging from 250 to 1000 mg/kg
bw/day (although the highest dose significantly reduced survival of
the dams).
d-Limonene consistently produced negative results in
genotoxicity studies, has been reported to inhibit the activity of
other mutagens and carcinogens, and gave mixed responses in
tumour-promotion studies.
Data clearly demonstrate that d-limonene exacerbates the
spontaneously occurring nephropathy in mature male rats which, after
prolonged exposure, is associated with an increase in the incidence
of renal tumours that are not commonly observed in control male
rats. However, d-limonene is not the only substance that has this
effect: various hydrocarbon mixtures and several other chemicals
have also been reported to exacerbate spontaneous nephropathy and
cause renal tumours in male rats. Although, in studies designed to
elucidate the mechanism of this carcinogenic process, chemicals
other than d-limonene were generally used as test substances, such
studies have shown that the following mechanism is likely to be
applicable to d-limonene-induced nephropathy and renal tumours in
male rats: (1) d-limonene and d-limonene-1,2-oxide (a metabolite
of d-limonene) bind reversibly to alpha2u-globulin in the
kidneys of d-limonene-treated male rats; (2) this binding further
slows down the normally slow processing of reabsorbed
alpha2u-globulin; and (3) as a result, alpha2u-globulin
accumulates in the phagolysosomes (hyaline droplets) of epithelial
cells of the proximal convoluted tubules of the kidney, leading to
necrosis, accumulation of cell debris, and regenerative hyperplasia.
The postulated mechanism of action for some non-genotoxic
carcinogens suggests that regenerative hyperplasia may increase
clonal expansion of spontaneously initiated cells and lead to the
development of the renal tumours observed.
A NOEL for the d-limonene-associated increase in
alpha2u-globulin levels in male rat kidney has not been
identified, but the lowest-observed-effect level from a 21-day
gavage study of d-limonene in male rats was 75 mg/kg bw/day. A
sex-specific protein with molecular features similar to
alpha2u-globulin has been identified in human urine, but the
concentration is at least four orders of magnitude less than that of
alpha2u-globulin in male rats.
The Committee concluded that the postulated mechanism for
d-limonene-induced nephropathy and renal tumours in the male rat
was probably not relevant to humans, and that toxic end points
associated with this effect were not appropriate bases for the
derivation of an ADI for d-limonene.
4. EVALUATION
Based on the significant decreases in body weight gain
associated with administration of d-limonene to male and female
mice and rats and female rabbits, an ADI of 0-1.5 mg/kg bw was
established for this substance. The Committee considered the known
natural occurrence and food additive uses of d-limonene, and
concluded that only a small proportion of total intake is likely to
be derived from direct additive use. The Committee therefore
recommended that food additive intake be restricted to 75 µg/kg
bw/day, which represents 5% of the maximum ADI for d-limonene.
5. REFERENCES
ABRAMOVICI, A. & RACHMUTH-ROIZMAN, P. (1983). Molecular
structure-teratogenicity relationships of some fragrance additives.
Toxicology, 29: 143-156.
ALDEN, C.L., KANERVA, R.L., RIDDER, G. & STONE, L.C. (1984). Chapter
VIII: The pathogenesis of the nephrotoxicity of volatile
hydrocarbons in the male rat. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 107-120, Princeton Scientific, Princeton, NJ.
ALDEN, C.L. (1989) Male rat specific alpha2u-globulin nephropathy
and renal tumorigenesis. In: Bach, P.H. & Lock, E.A. (eds.),
Handbook of Toxicologic Pathology. Academic Press, New York. In
press (cited in US EPA 1991).
ARIYOSHI, T., ARAKAKI, M., IDEGUCHI, K., ISHIZUKA, Y., NODA, K. &
IDE, H. (1975). Studies on the metabolism of d-limonene
(p-mentha-1,8-diene); III. Effects of d-limonene on the lipids and
drug metabolizing enzymes in rat livers. Xenobiotica, 5: 33-38.
AUSTIN, S.G. & SCHNATTER, A.R. (1983). A cohort mortality study of
petrochemical workers. Journal of Occupational Medicine, 25:
304-312.
BARBIER, P. & BENEZRA, C. (1983) The influence on induced
hypersensitivity to citral in guinea-pigs; II. Label distribution in
the skin of 14C-labelled citral. Acta Dermatovener (Stockholm),
63: 93-96.
BERNARD, A.M., LAUWERYS, R.R., NOEL, A., VANDELEENE, B. & LAMBERT,
A. (1989). Urine protein I: a sex-dependent marker of tubular or
glomerular dysfunction. Clin. Chem., 35: 2141-2.
BORGHOFF, S.J., SHORT, B.G., & SWENBERG, J.A. (1990) Biochemical
mechanisms and pathobiology of alpha2u-globulin nephropathy.
Ann. Rev. Pharmacol. Toxicol., 30: 349-367.
BRUNER, R.H. (1984). Chapter X: Pathologic findings in laboratory
animals exposed to hydrocarbon fuels of military interest. In:
Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 133-140,
Princeton Scientific, Princeton, NJ.
BUSEY, W.M. & COCKRELL, B.Y. (1984). Chapter IV: Non-neoplastic
exposure-related renal lesions in rats following inhalation of
unleaded gasoline vapors. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 57-64, Princeton Scientific, Princeton, NJ.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975a). Petroleum hydrocarbon toxicity studies: III.
Animal and human response to vapors of Stoddard solvent. Toxicology
and Applied Pharmacology, 32: 282-297.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975b). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "60 solvent." Toxicology
and Applied Pharmacology, 32: 374-394.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975c). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "70 solvent." Toxicology
and Applied Pharmacology, 34: 395-412.
CARPENTER, C.P., GEARY, JR., D.L., MYERS, R.C., NACHREINER, D.J.,
SULLIVAN, L.J. & KING, J.M. (1977). Petroleum hydrocarbon toxicity
studies: XV. Animal and human responses to vapors of "high
naphthenic solvent." Toxicology and Applied Pharmacology, 41:
251-260.
CHARBONNEAU, M., LOCK, E.A., STRASSER, J., COX, M.G., TURNER, M.J. &
BUS, J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: I.
Metabolic disposition of TMP in male and female Fischer 344 rats.
Toxicology and Applied Pharmacology, 91: 171-181.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J. & SWENBERG,
J.A. (1989). Involvement of reversible binding to alpha2u-globulin
in 1,4-dichlorobenzene-induced nephrotoxicity. Toxicology and
Applied Pharmacology, 99: 122-132.
CHARBONNEAU, M. & SWENBERG, J.A. (1988). Studies on the bio-chemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT
Activities, 8: 1-5.
CURVALL, M., ENZELL, C. & PETTERSSON, B. (1984). An evaluation of
the utility of four in vitro short-term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke.
Cell Biology and Toxicology, 1: 173-193.
DIETRICH, D.R. & SWENBERG, J.A. (1991). NCI-Black-Reiter (NBR) male
rats fail to develop renal disease following exposure to agents that
induce alpha2u-globulin nephropathy. Fundamentals of Applied
Toxicology, 16: 749-762.
DE GROOT, A.C., LIEM, D.H., NATER, J.P. & VAN KETEL, W.G. (1985).
Patch tests with fragrance materials and preservatives. Contact
Dermatitis, 12: 87-92.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984a). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Carcinogenesis, 5: 661-664.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984b). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Proceedings of the American Association
of Cancer Researchers, 25: 127A.
ELEGBEDE, J.A., ELSON, C.E., TANNER, M.A., QURESHI, A. & GOULD, M.N.
(1986a). Regression of rat primary mammary tumours following dietary
d-limonene. Journal of the National Cancer Institute, 76:
323-325.
ELEGBEDE, J.A., MALTZMAN, T.H., VERMA, A.K., TANNER, M.A., ELSON,
C.E. & GOULD, M.N. (1986b). Mouse skin tumour promoting activity of
orange peel oil and d-limonene: a re-evaluation. Carcinogenesis,
7: 2047-2049.
ELSON, C.E., MALTZMAN, T.H., BOSTON, J.L., TANNER, M.A. & GOULD,
M.N. (1988). Anti-carcinogenic activity of d-limonene during the
initiation and promotion/progression stages of DMBA-induced rat
mammary carcinogenesis. Carcinogenesis, 9: 331-332.
FAHRIG, R. (1984). Genetic mode of action of cocarcinogens and
promoters in yeast and mice. Molecular and General Genetics, 194:
7-14.
FLAMM, W.G. & LEHMAN-McKEEMAN, L. (1991). The human relevance of the
renal tumour-inducing potential of d-limonene in male rats:
Implications for risk assessment. Regulatory Toxicology and
Pharmacology, 13: 70-86.
FLORIN, I., RUTBERG, L., CURVALL, M. & ENZELL, C. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18: 219-232.
GAD, S.C., DUNN, B.J., DOBBS, D.W., REILLY, C. & WALSH R.D. (1986).
Development and validation of an alternative dermal sensitization
test: the mouse ear swelling test (MEST). Toxicology and Applied
Pharmacology, 84: 93-114.
GARG, B.D., OLSON, M.J., DEMYAN, W.F. & ROY, A.K. (1988a). Rapid
post-exposure decay of alpha2u-globulin and hyaline droplets in
the kidneys of gasoline-treated male rats. Journal of Toxicology
and Environmental Health, 24: 145-160.
GARG, B.D., OLSON, M.J., MANCINI, M.A. & ROY, A.K. (1988b).
Localization of alpha2u-globulin in hyaline droplets
(phagolysosomes) of the male rat renal cortex by immunohistochemical
techniques. Journal of Histochemistry and Cytochemistry, 36: 960.
GARG, B.D., OLSON, M.J., LI, L.C. & ROY, A.K. (1989). Phagolysosomal
alterations induced by unleaded gasoline in epithelial cells of the
proximal convoluted tubules of male rats: Effect of dose and
treatment duration. Journal of Toxicology and Environmental Health,
26: 101-118.
GAWORSKI, C.L., HAUN, C.C. & MacEWEN, J.D. (1981). A ninety-day
inhalation toxicity study of decalin. The Toxicologist, 1, 76.
GAWORSKI, C.L., LEAHY, H.F. & BRUNER, H.F. (1980). Subchronic
inhalation toxicity of decalin, Proceedings of the Tenth Conference
on Environmental Toxicology, AFAMRL-TR-79-121 (ADA086341) Aerospace
Medical Research Laboratory, Wright-Patterson Air Force Base, OH.
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L. & POPP, J.A. (1988).
Potential role of alpha2u-globulin, protein droplet accumulation,
and cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicology and Applied Pharmacology, 96: 367-379.
GOZSY, B. & KATO, L. (1957). Action of reticulo-endothelial-system
stimulators on the histamine depleted rats. Arch. Int. Pharmacodyn,
CXII: 235-246.
GOZSY, B. & KATO, L. (1958). Reticulo-endothelial system stimulating
compounds and their mechanism of action. Can. J. Biochem. Physiol.,
36: 319-326.
GREEN, T., ODUM, J., NASH, J.A. & FOSTER, J.R. (1990).
Perchloroethylene-induced rat kidney tumours: An investigation of
the mechanisms involved and their relevance to humans. Toxicol.
Appl. Pharmacol., 102: 77-89.
GREIF, N. (1967). Cutaneous safety of fragrance material as measured
by the Maximization Test. American Perfumer and Cosmetics, 82:
54-57.
HALDER, C.A., WARNE, T.M. & HATOUM, N.S. (1984). Chapter VI: Renal
toxicity of gasoline and related petroleum naphthas in male rats.
In: Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 73-88, Princeton
Scientific, Princeton, NJ.
HANAU, D., GROSSHANS, E., BARBIER, P. & BENEZRA, C. (1983). The
influence of limonene on induced delayed hypersensitivity to citral
in guinea-pigs; I. Histology study. Acta Dermatovener (Stockholm)
63: 1-7.
HANIS, N.M., HOLMES, T.M., SHALLENBERGER, L.G. & JONES, K.E. (1982).
Epidemiologic study of refinery and chemical plant workers. Journal
of Occupational Medicine, 24: 203-212.
HANIS, N.M., STRAVRAKY, K.M. & FOWLER, J.L. (1979). Cancer mortality
in oil refinery workers. Journal of Occupational Medicine, 21:
167-174.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W. & ZEIGER, E.
(1983). Salmonella mutagenicity test results for 250 chemicals.
Environmental Mutagenesis Supplement, 1: 3-142.
HECK, J.D., VOLLMUTH, T.A., CIFONE, M.A., JAGANNATH, D.R., MYHR, B.
& CURREN, R.D. (1989). An evaluation of food flavoring ingredients
in a genetic toxicity screening battery. The Toxicologist, 1: 257.
HIGGINSON, J., MUIR, C. & BUFFLER, P.A. (1984). Chapter XV: The
epidemiology of renal carcinoma in humans with a note on the effect
of exposure to gasoline. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 203-226, Princeton Scientific, Princeton, NJ.
HOMBURGER, F., TREGER, A. & BOGER, E. (1971). Inhibition of murine
subcutaneous and intravenous benzo(rst)pentaphene carcinogenesis by
sweet orange oils and d-limonene. Oncology, 25: 1-10.
IGIMI, H., HISATSUGU, T. & NISHIMURA, M. (1976). The use of
d-limonene preparation as a dissolving agent of gallstones.
Digestive Diseases, 21: 926-939.
IGIMI, H., NISHIMURA, M., KODAMA, R. & IDE, H. (1974). Studies on
the metabolism of d-limonene (p-mentha-1,8-diene): I. The
absorption, distribution and excretion of d-limonene in rats.
Xenobiotica, 4: 77-84.
IMAIZUMI, K. HANADA, K., MAWATARI, K. & SUGANO, M. (1985). Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49: 2795-2796.
KANERVA, R.L. & ALDEN, C.L. (1987). Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and Chemical Toxicology, 25: 355-358.
KANERVA, R.L., RIDDER, G.M., LEFEVER, F.R. & ALDEN, C.L. (1987a).
Comparison of short-term renal effects due to oral administration of
decalin or d-limonene in young adult male Fischer-344 rats.
Food and Chemical Toxicology, 25: 345-353.
KANERVA, R.L., MCCRACKEN, M.S., ALDEN, C.L. & STONE, L.C. (1987b).
Morphogenesis of decalin-induced renal alterations in the male rat.
Food and Chemical Toxicology, 25: 53-61.
KANERVA, R.L., RIDDER, G.M., STONE, L.C. & ALDEN, C.L. (1987c).
Characterization of spontaneous and decalin-induced hyaline droplets
in kidneys of adult male rats. Food and Chemical Toxicology, 25:
63-82.
KATO, L. & GOZSY, B. (1958). Treatment of experimental tuberculosis
of the guinea-pig with dihydrostreptomycin and simultaneously with
substances acting on the host. Arch. Int. Pharmacodyn, CXVII:
52-62.
KITCHEN D.N. (1984). Chapter V: Neoplastic renal effects of unleaded
gasoline in Fischer 344 rats. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 65-88, Princeton Scientific, Princeton, NJ.
KLECAK, G., GELEICK, H. & FREY, R. (1977). Screening of fragrance
materials for allergenicity in the guinea-pig; comparison of four
testing methods. J. Soc. Cosmet. Chem., 28: 53-64.
KODAMA, R., NODA, K. & IDE, H. (1974). Studies on the metabolism of
d-limonene (p-mentha-1,8-diene): II. The metabolic fate of
d-limonene in rabbits. Xenobiotica, 4: 85-95.
KODAMA, R., INOUE, H., NODA, K., IDE, H., YAMAMOTO, H., YOSHIHARA,
S. & YOSHIMURA, H. (1976a). Effect of d-limonene and related
compounds on bile flow and biliary lipid composition in rats and
dogs. Life Sciences, 19: 1559-1568.
KODAMA, R., YANO, T., FURUKAWA, K., NODA, K. & IDE, H. (1976b).
Studies on the metabolism of d-limonene (p-mentha-1,8-diene): IV.
Isolation and characterization of new metabolites and species
differences in metabolism. Xenobiotica, 6: 377-389.
KODAMA, R., OKUBO, A., ARAKI, E., NODA, K., IDE, H. & IKEDA, T.
(1977a). Studies on d-limonene as a gallstone solubilizer; VII.
Effects on development of mouse fetuses and offspring. Oyo Yakuri
(Pharmacometrics) 13: 863-873.
KODAMA, R., OKUBO, A., SATO, K., ARAKI, E., NODA, K., IDE, H. &
IKEDA, T. (1977b). Studies on d-limonene as a gallstone
solubilizer; IX. Effects on development of rabbit fetuses and
offspring. Oyo Yakuri (Pharmacometrics), 13: 885-898.
LEHMAN-McKEEMAN, L.D., CAUDILL, D., TAKIJIKU, R., SCHNEIDER, R.E. &
YOUNG, J.A. (1990a). Comparative disposition of d-limonene in rats
and mice: Relevance to male-rat-specific nephrotoxicity. Toxicol.
Lett., 53: 193-195.
LEHMAN-McKEEMAN, L.D., RIVERA-TORRES, M.I. & CAUDILL, D. (1990b).
Lysosomal degradation of alpha2u-globulin and
alpha2u-globulin-xenobiotic conjugates. Toxicology and Applied
Pharmacology, 103: 539-548.
LEHMAN-McKEEMAN, L.D., RODRIGUEZ, P.A., TAKIGIKU, R., CAUDILL, D. &
FEY, M.L. (1989). d-Limonene-induced male rat-specific
nephrotoxicity: Evaluation of the association between d-limonene
and alpha2u-globulin. Toxicology and Applied Pharmacology, 99:
250-259.
LOCK, E.A., CHARBONNEAU, M., STRASSER, J., SWENBERG, J.A. & BUS,
J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: II. The
reversible binding of a TMP metabolite to a renal protein fraction
containing alpha/2u-globulin. Toxicology and Applied
Pharmacology, 91: 182-192.
LOGOTHETOPOULOS, J. & WEINBREN, K. (1955). Naturally occurring
protein droplets in the proximal tubule of the rat's kidney.
British Journal of Experimental Pathology, 36: 402-406.
LOURY, D.J., SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987). Assessment
of the covalent binding potential of 2,2,4-trimethylpentane to rat
alpha2u-globulin. Toxicology and Applied Pharmacology, 88:
44-56.
MacEWEN, J.D. & VERNOT, E. (1978a). Evaluation of toxic effects of
90-day continuous exposure to conventional JP-5 jet fuel. In: Toxic
Hazards Research Unit Annual Report, AFAMRL-TR-78-55 (ADA062138)
Aerospace Medical Research Laboratory, Wright-Patterson Air Force
Base, OH.
MacEWEN, J.D. & VERNOT, E. (1978b). The effects of subchronic
exposure of rodents to inhaled decalin. In: Toxic Hazards Research
Unit Annual Report, AFAMRL-TR-78-55 (ADA062138) Aerospace Medical
Research Laboratory, Wright-Patterson Air Force Base, OH.
MacFARLAND, H.N., ULRICH, C.E., HOLDSWORTH, C.E., KITCHEN, D.N.,
HALLIWELL, W.H. & BLUM S.C. (1984). A chronic inhalation study with
unleaded gasoline vapor. Journal of the American College of
Toxicology, 3: 231-248.
MacNAUGHTON, M.G. & UDDIN, D.E. (1984). Chapter IX: Toxicology of
mixed distillate and high-energy synthetic fuels. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 121-132, Princeton
Scientific, Princeton, NJ.
MALTZMAN, T.H., HURT, L.M., ELSON, C.E., TANNER, M.A. & GOULD, M.N.
(1989). The prevention of nitrosomethylurea-induced mammary tumours
by d-limonene and orange oil. Carcinogenesis, 10: 781-783.
MEYER, F. & MEYER, E. (1959). Absorption of ethereal oils and
substances contained in them through the skin.
Arzneimittelforschung (Drug Research), 9: 516-519.
MILLER, A.B., BOWEN, J.P., BORGHOFF, S.J. & SWENBERG, J.A. (1989).
Computational and molecular modeling studies of alpha2u-globulin.
Abstract papers of the American Chemical Society, 198: Medi-39.
MURTY, C.V.R., OLSON, M.J., GARG, B.D. & ROY, A.K. (1988).
Hydrocarbon-induced hyaline droplet nephropathy in male rats during
senescence. Toxicology and Applied Pharmacology, 96: 380-392.
NTP (1983). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of pentachloroethane (CAS No.
76-01-7) in F344/N rats and B6C3F1 mice (Gavage Studies), NTP
TR 232, NIH Publication No. 83-1788.
NTP (1986). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 4-vinyl-cyclohexene (CAS
No. 100-40-3) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 303, NIH Publication No. 86-2559.
NTP (1987a). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 1,4-di-chlorobenzene (CAS
No. 106-46-7) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 319, NIH Publication No. 87-2570.
NTP (1987b). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of dimethylmethylphosphonate
(CAS No. 756-79-6) in F344/N rats and B6C3F1 mice (Gavage
Studies), NTP TR 323, NIH Publication No. 88-2579.
NTP (1990). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of d-limonene (CAS No.
5989-27-5) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 347, NIH Publication No. 90-2802.
OLSON, C.T., YU, K.O. & SERVE, M.P. (1986). Metabolism of
nephrotoxic cis- and trans-decalin in Fischer-344 rats. Journal
of Toxicology and Environmental Health, 18: 285-292.
OLSON, M.J., JOHNSON, J.T. & REIDY, C.A. (1990). A comparison of
male rat and human urinary proteins: Implications for human
resistance to hyaline droplet nephropathy. Toxicol. Appl.
Pharmacol., 102: 524-536.
OLSON, M.J., MANCINI, M.A., GARG, B.D. & ROY, A.K. (1988).
Leupeptin-mediated alteration of renal phagolysosomes: Similarity to
hyaline droplet nephropathy of male rats exposed to unleaded
gasoline. Toxicol. Lett. 41: 245-254.
OPDYKE, D.L.J. (1975). d-Limonene. Monograph on fragrance raw
materials. Food and Cosmetics Toxicology, 13(suppl: 683-923):
825-826.
PAGE, N.P. & MEHLMAN, M. (1989). Health effects of gasoline
refueling vapors and measured exposures at service stations.
Toxicology and Industrial Health, 5: 869-890.
PARKER, G.A., BOGO, V. & YOUNG, R.W. (1981). Acute toxicity of
conventional versus shale-derived JP5 jet fuel: Light microscopic,
hematologic, and serum chemistry studies. Toxicology and Applied
Pharmacology, 57: 302-317.
PHILLIPS, R.D. & COCKRELL, B.Y. (1984). Chapter VII: Effect of
certain light hydrocarbons on kidney function and structure in male
rats. In: Advances in Modern Environmental Toxicology, Renal
Effects of Petroleum Hydrocarbons, M.A. Mehlman, editor, pp.
89-105, Princeton Scientific, Princeton, NJ.
PHILLIPS, R.D. & EGAN, G.F. (1984). Effect of C10-11 isoparaffinic
solvent on kidney function in Fischer 344 rats during eight weeks of
inhalation. Toxicology and Applied Pharmacology, 73: 500-510.
PIRILA, V., SILTANEN, E. & PIRILA, L. (1964). On the chemical nature
of the eczematogenic agent in oil of turpentine. Dermatologica,
128: 16-21.
RAABE, G.K. (1984). Chapter XVIII: Kidney cancer epidemiology in
petroleum related studies. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 259-271, Princeton Scientific, Princeton, NJ.
READ, N.G., ASTBURY, P.J., MORGAN, R.J.I., PARSONS, D.N. & PORT,
C.J. (1988). Induction and exacerbation of hyaline droplet formation
in the proximal tubular cells of the kidneys from male rats
receiving a variety of pharmacological agents. Toxicology, 52:
81-101.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1985). Acute
dermal irritation study; RIFM 1-32.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1990). The
absorption, distribution, and excretion of radioactivity after
topical application of 14C-limonene to rats.
RESEARCH INSTITUTE FOR THE DEVELOPMENT OF FRAGRANCE MATERIALS, INC.
(1984). Acute dermal irritation study; Ref. Nos. 307-338/8403.
RIDDER, G.M., VON BARGEN, E.C., ALDEN, C.L. & PARKER, R.D. (1990).
Increased hyaline droplet formation in male rats exposed to decalin
is dependent on the presence of alpha2u-globulin. Fundamental and
Applied Toxicology, 15: 732-743.
ROE, F.J.C. (1959). Oil of sweet orange: A possible role in
carcinogenesis. British Journal of Cancer, 13: 92-93.
ROE, F.J.C. & Peirce, W.E.H. (1960). Tumour promotion by citrus
oils: Tumours of the skin and urethral orifice in mice. Journal of
the National Cancer Institute, 24: 1389-1403.
ROY, A.K. & NEUHAUS, A.W. (1967) Androgenic control of a
sex-dependent protein in the rat. Nature, 214: 618-20.
SCHENK, J. DOBRONTE, Z., KOCH, H. & STOLTE, M. (1980).
Untersuchungen zur gewebevertraglichkeit von d-limonen als einem
losungsmittel cholesterineicher gallenstein. Gastroenterologie,
18: 389-394.
SCHOTTENFELD, D., WARSHAUER, M.E., ZAUBER, A.G., MEIKLE, J.G. &
HART, B.T. (1981). A prospective study of morbidity and mortality in
petroleum industry employees in the United States - a preliminary
report. In: Banbury Report 9: Quantification of Occupational
Cancer, R. Peto & M. Schneiderman, editors, pp. 247-265. Cold
Spring Harbor Laboratory, Cold Spring Harbor, NY.
SHORT, G.B., BURNETT, V.L., COX, M.G., BUS, J.S. & SWENBERG, J.A.
(1987). Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Laboratory
Investigation, 57: 564-577.
SHORT, G.B., BURNETT, V.L. & SWENBERG, J.A. (1989a). Elevated
proliferation of proximal tubule cells and localized accumulated
alpha2u-globulin in F344 rats during chronic exposure to unleaded
gasoline or 2,2,4-trimethylpentane. Toxicology and Applied
Pharmacology, 101: 414-431.
SHORT, G.B., STEINHAGEN, W.H. & SWENBERG, J.A. (1989b). Promoting
effects of unleaded gasoline and 2,2,4-trimethylpentane on the
development of atypical cell foci and renal tubular cell tumours in
rats exposed to n-ethyl-n-hydroxyethylnitrosamine. Cancer Research,
49: 6369-6378.
SHORT, G.B. & SWENBERG, J.A. (1988). Pathologic Investigations of
the mechanism of unleaded gasoline-induced renal tumours in rats.
CIIT Activities, 8: 1-6.
STONARD, M.C., PHILLIPS, P.G.N., FOSTER, J.R., SIMPSON, M.G. & LOCK,
E.A. (1986). Alpha2u-globulin: Measurement in rat kidney following
administration of 2,2,4-trimethylpentane, Toxicology, 41: 161-168.
STONE, L.C., KANERVA, R.L., BURNS, J.L. & ALDEN, C.L. (1987a).
Decalin-induced nephrotoxicity: Light and electron microscopic
examination of the effects of oral dosing on the development of
kidney lesions in the rat. Food and Chemical Toxicology, 25:
43-52.
STONE, L.C., McCRACKEN, M.S., KANERVA, R.L. & ALDEN, C.L. (1987b).
Development of a short-term model of decalin inhalation
nephrotoxicity in the male rat. Food and Chemical Toxicology, 25:
35-41.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumour
response in strain A mice. Cancer Research, 33: 3069-3085.
SWENBERG, J.A. (1991). Editorial. Risk assessment of chemicals
causing alpha2u-globulin nephropathy. Reg. Toxicol. Pharmacol.,
13: 1-2.
SWENBERG, J.A., SHORT, B., BORGHOFF, S., STRASSER, J. & CHARBONNEAU,
M. (1989). The comparative pathobiology of alpha2u-globulin
nephropathy. Toxicology and Applied Pharmacology, 97: 35-46.
THELESTAM, M., CURVALL, M. & ENZELL, C. (1980). Effect of tobacco
smoke compounds on the plasma membrane of cultured human lung
fibroblasts. Toxicology, 15: 203-217.
THOMAS, T.L., WAXWEILER, R.J., MOURE-ERASO, R., ITAYA, S. &
FRAUMENI, Jr., J.F. (1982). Mortality patterns among workers in
three Texas oil refineries. Journal of Occupational Medicine, 24:
135-141.
TRUMP, B.F., BEREZESKY, I.K., SATO, K. & SAIKO, K.U. (1984). Cell
calcium, cell injury, and cell death. Environ.Hlth. Revs., 57:
281-287.
TSUJI, M., FUJISAKI, Y., YAMACHIKA, K. NAKAGAMI, K., FUJISAKI, F.,
MITO, M., AOKI, T., KINOSHITA, S., OKUBO, A. & WATANABE, I. (1974).
Studies on d-limonene as gallstone solubilizer; I. General
pharmacological studies. Oyo Yakuri (Pharmacometrics), 8:
1439-1459.
TSUJI, M., FUJISAKI, Y., ARIKAWA, Y., MASUDA, S., KINOSHITA, S.,
OKUBO, A., NODA, K., IDE, H. & IWANAGA, Y. (1975a). Studies on
d-limonene, as gallstone solubilizer (II): Acute and subacute
toxicities. Oyo Yakuri (Pharmacometrics), 9: 387-401.
US EPA (1991). Alpha2u-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Risk
Assessment Forum, US Environmental Protection Agency, Washington,
DC, USA, EPA/625/3-91/019F.
VAN DUUREN, B.L. & GOLDSCHMIDT, B.M. (1976). Co-carcinogenic and
tumour-promoting agents in tobacco carcinogenesis. Journal of the
National Cancer Institute, 56: 1237-1242.
WADE, A.P., WILKINSON, G.S., DEAN, F.M. & PRICE, A.W. (1966). The
isolation, characterization and structure of uroterpenol, a
monoterpene from human urine. Biochem. J., 101: 727-734.
WATABE, T., HIRATSUKA, A., ISOBE, M. & OZAWA, N. (1980). Metabolism
of d-limonene by hepatic microsomes to non-mutagenic epoxides
toward Salmonella typhimurium. Biochemical Pharmacology, 29:
1068-1071.
WEBB, D.R., KANERVA, R.L., HYSELL, D.K., ALDEN, C.L. &
LEHMAN-MCKEEMAN, L.D. (1990). Assessment of the subchronic oral
toxicity of d-limonene in dogs. Fd. Chem. Toxic., 28: 669-675.
WEBB, D.R., RIDDER, G.M. & ALDEN, C.L. (1989). Acute and subacute
nephrotoxicity of d-limonene in Fischer 344 rats. Fd. Chem.
Toxicol., 27: 639-649.
WEN, C.P., ISAI, S.P., MOFFITT, K.B., BONDY, M. & GIBSON, R.L.
(1984). Chapter XVII: Epidemiologic studies of the role of gasoline
(hydrocarbon) exposure in kidney cancer risk. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 245-257, Princeton
Scientific, Princeton, NJ.
WONG, O. & RAABE, G. (1989). Critical review of cancer epidemiology
in petroleum industry employees, with a quantitative meta-analysis
by cancer site. American Journal of Industrial Medicine, 15:
283-310.
Wade et al. (1966) reported that dietary limonene gives rise
to uroterpenol (M-II: p-menth-1-ene-8,9-diol) in the urine of
humans.
2.1.3 Effects on enzymes and other biochemical parameters
Groups of control (6 animals) and experimental (4 animals) male
Wistar rats were administered single gavage doses of 0, 200, 400,
600, 800, or 1200 mg/kg bw d-limonene in 2% tragacanth solution in
a total volume of 4 ml/kg. Authors reported that no effects were
observed on liver triglycerides, microsomal proteins, cytochrome b5
and drug metabolizing enzymes.
In the same experiment, male Wistar rats were treated with
gavage doses of 0 or 400 mg/kg bw d-limonene in 2% tragacanth
solution in a total volume of 4 ml/kg for 2, 3, 15 or 30 days;
animals were killed 24 h following the last dose. Authors reported
that, following repeated treatment for 30 days, relative liver
weight and hepatic phospholipid content were slightly increased, and
liver and serum cholesterol were decreased 49% and 8%, respectively.
In addition, palmitic, linoleic and arachidonic acids were
increased, and stearic acid was decreased in the liver; aminopyrine
demethylase and aniline hydroxylase were increased 26% and 22%,
respectively, and cytochrome P-450 and b5 were increased by 31% and
30%, respectively (Ariyoshi et al., 1975).
d-Limonene was reported to enhance bile flow in Wistar rats
and mongrel dogs in a dose-related manner, and to decrease the ratio
of biliary bile salts and phospholipids to cholesterol (Kodama
et al., 1976). d-Limonene was reported to be an effective
gallstone solubilizer in animals and humans (Igimi et al., 1976;
Schenk et al., 1980), and to have no effect on liver weights nor
levels of serum lipids when fed to male Wistar rats at 0.5 and 1% in
the diet (Imaizumi et al., 1985).
d-Limonene was reported to significantly damage and increase
permeability of membranes of human lung fibroblasts (Thelestam
et al., 1980; Curvall et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Table 1: Summary of acute toxicity studies with d-limonene
Species Sex Route LD50 Reference
Mouse M&F oral, in 6.3 ml/kg (M) Tsuji et
juice, 7d 8.1 ml/kg (F) al., 1974
Mouse M&F ip, in 3.7 ml/kg (M) Tsuji et
juice, 3d 3.6 ml/kg (F) al., 1974
Mouse M&F ip, in 0.7 ml/kg (M) Tsuji et
juice, 10d 0.6 ml/kg (F) al., 1974
Mouse M&F sc, in >25.6 ml/kg (M) Tsuji et
juice, 7d >25.6 ml/kg (F) al., 1974
Mouse M&F oral 5600 mg/kg (M) Tsuji et
6600 mg/kg (F) al., 1975a
Mouse M&F ip 1300 mg/kg (M) Tsuji et
1300 mg/kg (F) al., 1975a
Mouse M&F sc >41500 mg/kg (M) Tsuji et
>41500 mg/kg (F) al., 1975a
Rat M&F oral 4400 mg/kg (M) Tsuji et
5100 mg/kg (F) al., 1975a
Rat M&F ip 3600 mg/kg (M) Tsuji et
4500 mg/kg (F) al., 1975a
Rat M&F sc >20200 mg/kg (M) Tsuji et
>20200 mg/kg (F) al., 1975a
An oral dose of 3 ml d-limonene (in juice)/kg decreased
spontaneous motor activities in rats and mice and potentiated
hexobarbital-induced sleeping and hypothermia in mice. Authors also
reported that nicotine-induced convulsion and death (but not maximum
electroshock-, pentetrazol-, strychnine-, nor picrotoxin-induced
convulsions) were inhibited by d-limonene in mice. Intravenous
administration of > 0.005 mg/kg d-limonene lowered the blood
pressure of rabbits and dogs, and > 0.1-0.3 mg/kg killed those
animals. Oral administration of 3 ml/kg d-limonene did not
decrease the blood pressure of rats, however. Authors also reported
that isolated smooth muscles of the intestine, vas deferens, uterus,
and peripheral vessel were constricted by d-limonene (Tsuji
et al., 1974). Single high s.c. doses of d-limonene produced
scratch behaviour and single high i.v. doses of d-limonene
produced stretch behaviour in mice and rats (Tsuji et al., 1975a).
2.2.2 Short-term studies
2.2.2.1 Mice
Groups of 5 B6C3F1 male and female mice were given daily
gavage doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg
bw in 10 ml/kg bw corn oil 5 days/week over a 16 day period (12
total dosings). All mice (5/5) exposed to 6600 mg/kg bw/day, 4/5
males and 5/5 females exposed to 3300 mg/kg bw/day, and 1/5 males
and 1/5 females exposed to 1650 mg/kg bw/day died before the end of
the study. The one female that died at the latter dose level was
reported to have been killed by a gavage error. Authors concluded
that no compound-related clinical signs were observed in mice that
received 1650 mg d-limonene/kg bw and lived to the end of the
study, and that no compound-related histopathologic effects were
seen (NTP, 1990).
Groups of 10 B6C3F1 mice of each sex were given daily
gavage exposures of 0, 125, 250, 500, 1000 or 2000 mg
d-limonene/kg bw in 10 ml/kg bw corn oil 5 days/week for 13 weeks.
One male exposed to 2000 mg/kg bw/day, two females exposed to
2000 mg/kg bw/day, and one female exposed to 500 mg/kg bw/day, died
during the course of the study. In addition, one female exposed to
125 mg/kg bw/day and five males (one each from the groups exposed to
250 and 1000 mg/kg bw/day, and three from the group exposed to
500 mg/kg bw/day) died prior to the end of the study, but their
deaths were attributed by the authors to accidents related to the
gavage procedure used. At the two highest dose levels, mice were
seen with rough hair coats and decreased activity. An alveolar cell
adenoma was observed in the lung of one female mouse that had
received 2000 mg d-limonene/kg bw/day (NTP, 1990).
2.2.2.2 Rats
Groups of 5 F344/N rats of each sex were given daily gavage
doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg bw in
10 ml/kg bw corn oil 5 days/week over a 16 day period (12 total
doses). All rats receiving 6600 mg d-limonene/kg bw/day as well as
5/5 male and 3/5 female rats that were exposed to 3300 mg/bw/day
died. The authors reported that no clinical signs were seen in rats
receiving 1650 mg d-limonene/kg bw/day or lower, and that no
compound-related histopathologic effects were seen in any rats (NTP,
1990).
Groups of 5 male Fischer-344 rats were given single daily
gavage doses containing 75, 150 or 300 mg d-limonene/kg bw in corn
oil (5 ml/kg bw) 5 days/week for 5 or 20 doses, primarily to follow
the development of renal alterations. The authors reported that no
signs of gross toxicity were observed nor was there any effect on
weight gain or feed consumption over the course of this experiment.
However, after only 5 days of exposure, liver and kidney weights
showed a dose-related increase and there was a dose-related increase
in the number and size of hyaline droplets and accumulation of
alpha2u-globulin in the proximal convoluted tubule epithelial
cells of the kidneys. In addition, after 20 doses, granular casts in
the outer zone of the medulla and multiple cortical changes
(classified as chronic nephrosis) were also seen in dose-related
severity (Kanerva et al., 1987a).
In a subacute study, groups of 5 male and female rats were
exposed daily to 0, 277, 554, 1385 or 2770 mg d-limonene/kg bw
orally for 30 days. A general decrease in food intake and a
dose-related decrease in body weight were seen in groups of exposed
males, but little or no effect on organ weights nor relative organ
weights was observed. No significant changes were seen in
urinalysis, haematology or biochemical values. The following tissues
were examined histopathologically: adrenals, duodenum, heart,
kidneys, liver, lungs, lymph nodes, pancreas, pituitary, spleen,
stomach, testes/ovaries, thymus and thyroids. Authors reported that
no significant changes were noted except that granular casts were
seen in the kidneys of most exposed male rats (0/5, 3/5, 5/5, 5/5 or
4/5 animals exposed to 0, 277, 554, 1385 or 2770 mg/kg bw/day
respectively) (Tsuji et al., 1975a).
Groups of 10 F334/N rats of each sex were given daily gavage
doses of 0, 150, 300, 600, 1200 or 2400 mg d-limonene/kg bw in
5 ml/kg bw corn oil 5 days/week for 13 weeks. Five male and nine
female rats exposed to 2400 mg d-limonene/kg bw/day died during
the course of the study. In all rats receiving 1200 or 2400 mg/kg
bw/day, rough hair coats, lethargy and excessive lacrimation were
observed. Authors reported that nephropathy was seen in all groups
of male rats, with a dose-related increase in severity. The
nephropathy was reported to be characterized by degeneration of
epithelium in the convoluted tubules, granular casts within tubular
lumens (primarily in the outer stripe of the outer medulla), and
regeneration of the tubular epithelium. In all groups of male rats
(including control animals), hyaline droplets were observed in the
epithelium of proximal convoluted tubules in at least some animals.
An attempt to determine by "blind" evaluation if the incidence of
these droplets was dose-related was reported to have resulted in an
equivocal conclusion.
The slides containing the renal sections made from male rats
exposed to d-limonene in this 13 week study were reviewed. They
reported that hyaline droplet accumulation within the cytoplasm of
proximal convoluted tubule (PCT) epithelial cells was uniform among
all groups, including controls. However, chronic nephrosis, although
seen in all animals (including control animals), appeared with a
dose-related increase in severity. The authors attributed the
uniformity of hyaline droplet accumulation to the fact that the
animals were not exposed to d-limonene for 4-5 days before
necropsy and examination, giving the animals time to rid themselves
of some of the droplets, although more extensive damage to the
kidneys (chronic nephrosis) was not repaired (Kanerva & Alden 1987).
Chronic nephrosis consisted of cytoplasmic basophilia of the
PCT epithelial cells, tubular hyperplasia or atrophy, fibrosis of
Bowman's capsule and an interstitial fibrolymphocytic response.
Atrophic tubules were characterized by thickened basement membranes
and a decrease in tubular cross-sectional diameter due to a decrease
in cell size, whereas tubules considered to be hyperplastic showed
increases in cell size and number and an increase in cross-sectional
diameter compared with the surrounding unaffected tubules. In
addition, occasional foci of PCT epithelial cell
necrosis/degeneration were observed in kidneys from all
d-limonene-treated animals but not in control animals. All treated
animals, but not controls, also showed a dose-related increase in
the number of granular casts found in the outer stripe of the
medulla. Granular casts were markedly dilated and had attenuated or
non-existent epithelium. In addition, animals exposed to the highest
dose level (2400 mg/kg bw/day) were reported to show multifocal
hyaline casts and tubular dilation in the cortex and medulla (NTP,
1990).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
To assess the pulmonary tumour carcinogenicity of d-limonene,
groups of 15 male or female A/He mice were injected i.p. with
d-limonene 3 times weekly for 8 weeks (4.8 or 24 g/kg bw total
dose) and then necropsied 24 weeks after the first injection. No
significant induction of lung tumours was seen (Stoner et al.,
1973).
Groups of 50 8-9 week old male B6C3F1 mice were given
daily gavage doses of 0, 250, or 500 mg d-limonene/kg bw and
groups of 50 8-9 week old female B6C3F1 mice were given 0,
500, or 1000 mg d-limonene/kg bw in 10 ml/kg bw corn oil
5 days/week for 103 weeks. Mice were observed twice a day and
weighed weekly for the first 12 weeks, after which they were weighed
monthly. Clinical signs were recorded at least monthly. Moribund
animals were killed and a necropsy was performed on all available
animals. The tissues of all mice that died during the study and
animals with grossly visible lesions, as well as all animals in the
control and high dose groups, were examined for histopathology.
The authors reported that no clinical signs were associated
with administration of the test chemical. The survival of male mice
exposed to 250 mg d-limonene/kg bw/day was lower than that of
controls at the end of the study (controls: 33/50; 250 mg/kg bw/day:
24/50; 500 mg/kg bw/day: 38/50). No other differences in survival
were seen among exposed groups of either sex. Mean body weights of
exposed and control male mice were generally comparable. Weights of
female mice in the 1000 mg d-limonene/kg bw/day group, however,
were 5-15% lower than control animals after week 28.
Multinucleated liver hepatocytes and cells with cytomegaly
occurred at increased incidence in male mice exposed to 500 mg
d-limonene/kg bw/day (multinucleated hepatocytes: controls - 8/49;
250 mg/kg bw/day - 4/36 [incomplete sampling]; 500 mg/kg bw/day -
32/50) (hepatocytes with cytomegaly: controls - 23/49; 250 mg/kg
bw/day - 11/36 [incomplete sampling]; 500 mg/kg bw/day - 38/50). The
combined incidence of hepatocellular adenomas and carcinomas,
however, did not differ significantly from their incidence in
control mice (controls: 22/49; 250 mg/kg bw/day: 14/36 [incomplete
sampling]; 500 mg/kg bw/day: 15/50). No significant differences were
observed in female mice for any of these endpoints.
The incidence of adenomas or the combined incidence of adenomas
and carcinomas in the anterior pituitary gland of female mice
exposed to 1000 mg d-limonene/kg bw/day were significantly lower
than controls. The incidence of hyperplasia did not differ
significantly from controls, however (hyperplasia: controls - 16/49;
500 mg/kg bw/day - 0/8 [incomplete sampling]; 1000 mg/kg bw/day -
17/48) (adenomas: controls - 12/49; 500 mg/kg bw/day - 5/8
[incomplete sampling]; 1000 mg/kg bw/day - 1/48) (combined adenoma
and carcinoma: controls - 12/49; 500 mg/kg bw/day - 5/8 [incomplete
sampling]; 500 mg/kg - 2/48) (NTP, 1990).
2.2.3.2 Rats
Groups of fifty 7-8 week old male F344/N rats were given daily
gavage doses of 0, 75, or 150 mg d-limonene/kg bw and groups of 50
7-8 week old female F344/N rats were given 0, 300, or 600 mg
d-limonene/kg bw in 5 ml/kg bw corn oil 5 days/week for 103 weeks.
Rats were observed twice a day; they were weighed weekly for the
first 12 weeks, and monthly thereafter. Clinical signs were recorded
at least monthly. Moribund animals were killed; necropsies were
performed on all available animals. The tissues of all rats that
died during the study, animals with grossly visible lesions, and all
animals in the control and high dose groups were examined for
histopathology.
No compound-related clinical signs were reported. The survival
of the female animals exposed to 600 mg/kg bw was lower than that of
control animals after week 39, while the survival of male rats
exposed. Mean body weights of male rats exposed to 150 mg/kg bw were
generally 4%-7% lower than controls from week 28.
Cataracts were observed at increased incidence in male rats
exposed to 150 and female rats exposed to 300 or 600 mg
d-limonene/kg bw/day, and retinal degeneration was seen in all
dosed animals. The authors pointed out, however, that the rats of
each sex that were exposed to their respective highest-dose levels
were housed in the top rows of the cage racks, the animals exposed
to the low-dose levels were below them, and the controls were housed
in the lowest rows for all but the last 10 weeks of the study.
Authors conclude that these effects were due to variations in the
proximity of animals in different dose groups to the light source.
Subcutaneous tissue fibromas in male rats occurred with a
negative dose-response trend (controls: 8/50; 75 mg/kg bw/day: 2/50;
150 mg/kg bw/day: 3/50). However, the combined incidence of fibromas
and fibrosarcomas was not significantly different when dosed males
were compared to controls (controls: 8/50; 75 mg/kg bw/day: 4/50;
150 mg/kg bw/day: 3/50).
Combined squamous cell papillomas or carcinomas in the skin of
male rats showed a positive dose-response trend (controls: 0/50;
75 mg/kg bw/day: 0/50; 150 mg/kg bw/day: 3/50). However, the
incidence of these neoplasms in dosed male rats was within the range
of incidences for NTP historical control rats, and did not differ
significantly from the incidence in control animals. The authors
concluded that the positive dose-response trend was not related to
d-limonene exposure.
Mononuclear cell leukaemia occurred in male rats with a
positive dose-response trend by the incidental tumour test
(controls: 10/50; 75 mg/kg bw/day: 10/50; 150 mg/kg bw/day: 19/50).
The incidences in dosed male rats were not significantly different
from the incidences in controls, however. The authors concluded that
the positive dose-response trend was not related to d-limonene
administration.
There was a significant positive dose-response trend in the
incidence of interstitial cell tumours in the testes of male rats,
and the incidences of these tumours in dosed groups were
significantly higher than the incidences in control animals
(control: 37/50; 75 mg/kg bw/day: 47/49; 150 mg/kg bw/day: 48/50).
The authors argued, however, that since historically this type of
tumour occurs in almost all aged F344 rats, this result should not
be considered to be related to d-limonene exposure, but rather was
an artifact caused by the low survival of the control animals in
this experiment (see above).
The incidence of uterine endometrial stromal polyps in females
exposed to 300 mg/kg bw/day was increased when compared to controls.
However, the incidence in the control animals was well below the NTP
historical incidence of this tumour. For this reason, and because
there was no positive dose-response trend, the authors argued that
this was not a compound-related effect.
d-Limonene exposure of male rats was associated with
dose-related increases in the incidences of mineralization and
epithelial hyperplasia in the kidneys (mineralization: controls -
7/50; 75 mg/kg bw/day - 43/50; 150 mg/kg bw/day - 48/50) (epithelial
hyperplasia: controls - 0/50; 75 mg/kg bw/day - 35/50; 150 mg/kg
bw/day - 43/50). The lesions consisted of linear deposits of mineral
in the medulla (renal papilla) and focal hyperplasia in the
transitional epithelium overlying the papilla. Hyperplasia was
frequently located near the fornices of the renal pelvis and was
occasionally bilateral. The severity of nephropathy, which occurs
spontaneously in aged male rats, was also positively dose-related in
this experiment (severity of nephropathy, rated on a scale from 0
[not present] to 4 [marked]: control mean - 1.5; 75 mg/kg bw/day
mean - 1.8; 150 mg/kg bw/day mean - 2.2). This nephropathy was
characterized by degeneration and atrophy of the tubular epithelium,
dilation of tubules with formation of hyaline droplets and granular
casts, regeneration of tubular epithelium, glomerulosclerosis, and
interstitial inflammation and fibrosis.
Kidney tubular cell hyperplasia and neoplasms were also
increased in dosed male rats (hyperplasia: controls - 0/50; 75 mg/kg
bw/day - 4/50; 150 mg/kg bw/day - 7/50) (adenomas: controls - 0/50;
75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 8/50) (adenocarcinomas:
controls - 0/50; 75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 3/50)
(combined adenomas and carcinomas: controls - 0/50; 75 mg/kg bw/day
- 8/50; 150 mg/kg bw/day - 11/50). Incidences of tubular cell
adenomas and the combined incidences of adenomas and
adeno-carcinomas in male rats showed significant positive
dose-response trends. High-dose males had a significantly higher
incidence than control males of both categories of tumours, while
low-dose males had a significantly higher incidence of the combined
category than control males. These neoplasms are historically rare
and none were seen in control males nor in any group of females.
The authors stated that kidney tubular cell hyperplasia,
adenomas, and adenocarcinomas were part of a morphologic spectrum.
Proliferative lesions diagnosed as hyperplasia generally consisted
of one to three adjacent cross-sections of enlarged tubules with
stratification of the epithelium. In some lesions, the epithelial
cells appeared to completely fill the lumen. The tubular cell
neoplasms varied in diameter from less than 1 mm to 15 mm. They
exhibited varied patterns of growth and were either solid, cystic,
or papillary. The solid and cystic neoplasms showed little evidence
of tubular structures and the cells were arranged in solid sheets or
small solid nests separated by a delicate vascular stroma. Some
neoplasms were reported to consist of layers of epithelium lining a
fibrous connective tissue stroma and were arranged in complex
branching papillary formations. The neoplasms were classified as
adenocarcinomas primarily if they exhibited cellular pleomorphism
and anaplasia, or if they were larger than 10 mm (NTP, 1990).
2.2.3.3 Dogs
Groups of 3 male or female Japanese beagle dogs were exposed
orally to 0, 0.4, 1.2 or 3.6 ml d-limonene/kg bw daily for 6
months. Dose-related occurrences of frequent vomiting and nausea
were seen in some animals. At doses of at least 1.2 ml/kg bw/day for
females and 3.6 ml/kg bw/day for males, a decrease in body weight
gain was noted compared to controls, but little or no effect on food
intake, organ weights or relative organ weights was reported. No
significant changes were seen in urinalysis, haematology nor
biochemical values, except that in animals at the 3.6 ml/kg bw/day
level, total cholesterol and blood sugar were lowered. The following
tissues were examined histopathologically: adrenals, bile duct,
duodenum, heart, kidneys, liver, lungs, pancreas, pituitary, rectum,
spleen, stomach, testes/ovaries, thymus and thyroids. No significant
changes were noted except that granular casts were seen in the
kidneys of all male dogs exposed to 3.6 ml/kg bw/day and all females
exposed to 0.4 ml/kg bw/day or more (1/3, 1/3, 2/3 or 3/3 for males;
1/3, 2/2, 3/3 or 3/3 for females exposed to 0, 0.4, 1.2 or 3.6 ml/kg
bw, respectively) (Tsuji et al., 1975b).
In a more recent study, groups of five male and female beagle
dogs were gavaged twice daily for six months with 0, 0.12, or
1.2 ml/kg bw/day (0, 10, or 1000 mg/kg bw/day) d-limonene. The
highest dose was reported to be near the maximum tolerated dose for
emesis. The authors stated that food consumption and body weight
were not affected by treatment. Although there was a positive
dose-related trend for absolute and relative female kidney weights
and for relative male kidney weight, authors reported that there
were no histo-pathological findings in the kidneys that could be
associated with the organ weight changes. In addition, there was no
hyaline droplet accumulation nor other indication of nephropathy
such as those associated with d-limonene consumption in male rats
(Webb et al., 1990)
2.2.3.4 Cocarcinogenicity/tumour promotion studies
Two publications have reported the results of experiments in
which 0.25 ml oil of sweet orange, composed of > 90% d-limonene,
was applied to the skin of 10 male and 10 female "101" mice weekly
for 42 weeks, starting 3 weeks after an application of 300 µg
7,12-dimethyl-1,2-benzanthracene (DMBA) in 0.2 ml acetone. While no
effects in the area of the treated skin were seen in mice exposed to
d-limonene or to DMBA alone, mice exposed to DMBA followed by
exposure to d-limonene began to develop papillomas during the
twelfth week of d-limonene exposure. After 33 weeks of treatment,
13/18 treated mice showed a total of 39 papillomas. No malignant
tumours were seen in the exposed area, however.
In a second study, mice were exposed to d-limonene following
exposure to: 1) 4 skin applications of 60 mg urethane in 0.3 ml
acetone at 3 day intervals, or 2) 4 i.p. injections of 16 mg
urethane in 0.1 ml distilled water at 3 day intervals. Papillomas
appeared on 3/13 mice in group 1 and 2/13 mice in group 2 by the
fourteenth week of exposure.
In a final experiment, the terpene fraction of oil of sweet
orange (containing d-limonene) was isolated and tested with DMBA
as described for the first experiment. Papillomas in mice exposed to
both DMBA and the terpene fraction of oil of sweet orange began to
appear in the eleventh week of treatment and were visible in 8/15
mice by the thirty-third week of treatment. Although pure
d-limonene was not tested in these experiments, the authors
concluded that the promoting effects seen were probably caused by
this chemical since it was the primary constituent of the test agent
(Roe, 1959, Roe & Peirce, 1960).
A group of 23 male and female albino mice were given single
doses of 50 µg benzo(a)pyrene (BAP) by stomach tube followed by 40
weekly doses of 0.05 ml d-limonene; seventeen male and female mice
received BAP only, 15 male and female mice received d-limonene
only and 18 male and female mice served as untreated controls. While
no tumours of the forestomach epithelium were seen in control
animals, 2 animals treated with BAP alone (12%), 2 animals treated
with d-limonene alone (13%), and 5 animals treated with both BAP
and d-limonene (22%), developed 0, 2, 3, and 8 tumours,
respectively, although none was judged to be a carcinoma. The
authors concluded that although d-limonene was weakly carcinogenic
for mouse forestomach epithelium it did not increase the tumour
yield caused by pretreatment with BAP and, therefore, was not a
promoter (Field & Roe, 1965).
Cells of embryos taken from F344 rats were infected with
Rauscher murine leukaemia virus and then treated with 0.05 µg/ml
3-methylcholanthrene (3-MCA) followed by 15 µg/ml d-limonene.
Following treatment with 3-MCA and d-limonene, colony formation in
semisolid medium was observed and was considered to denote cell
transformation. Authors reported that d-limonene alone failed to
transform cells and 3-MCA alone was only effective at concentrations
exceeding 0.2 µg/ml (Roe, 1959; Roe & Peirce, 1960; Traul et al.,
1981)).
Elegbede and co-workers attempted to repeat the 1960 study of
Roe and Peirce (see above) using purified d-limonene as well as
oil of sweet orange. The skin of groups of 24 female CD-1 mice were
treated once with 51.2 µg DMBA in 0.2 ml acetone followed 14 days
later by twice weekly applications of 0.1 ml d-limonene or oil of
sweet orange mixed with 0.1 ml acetone. Other groups of mice were
similarly initiated with DMBA, but beginning 7 days later were given
a diet containing 1% d-limonene or oil of sweet orange. In all
cases, administration of d-limonene or oil of sweet orange
continued for 40 weeks after DMBA treatment. Topical (but not
ingested) d-limonene was found to slightly increase the number of
tumours induced by DMBA alone, the increase becoming significant
only after 34 weeks of treatment. When oil of sweet orange (95%
d-limonene content) was applied to the skin, authors reported that
a promoting effect was seen after 7 weeks of treatment; promotion
was not reported, however, when oil of sweet orange was incorporated
into the diet. The authors concluded that a component of orange oil
other than d-limonene was responsible for the promoting activity
observed and that the very small promoting activity seen with
d-limonene may be due to its contamination by this unidentified
component (Elegbede et al., 1986b).
2.2.3.5 Anticarcinogenicity studies
A group of 50 male C57Bl/6J mice were injected s.c. with 25 µg
dibenzpyrene (DBP) followed 24 h later by an injection of 0.15 ml
d-limonene. After 34 weeks of observation, the authors reported
that d-limonene significantly delayed the appearance of lung
adenomas induced by DBP and reduced the total number of tumours
seen. At 30 weeks post-DBP exposure without subsequent exposure to
d-limonene, lung adenomas were seen in approximately 70% of the
animals; with exposure to d-limonene, tumours were seen in
approximately 30% of the animals.
In a second experiment reported in the same paper, a group of
50 female A/J mice were injected s.c. with DBP followed by 16 weekly
injections into the tail vein of 1% v/v suspensions of 1 mg of
d-limonene. The incidence of lung adenomas was reduced from 75% of
animals exposed to DBP alone to 40% of animals exposed to DBP and
injected with d-limonene. Control incidence of lung adenomas in
this experiment was 27%. Treatment of mice with d-limonene alone
reduced this background incidence to approximately 7% (Homburger
et al., 1971).
Simultaneously, 5 µg of BAP and 10 mg d-limonene were applied
to the skin of groups of 50 female ICR/Ha Swiss mice three times a
week for 440 days. d-Limonene was found to partially inhibit BAP
carcinogenicity (Van Duuren & Goldschmidt, 1976).
Female Sprague-Dawley rats were fed diets containing 0, 1000,
or 10 000 ppm d-limonene for 1 wk, at which time they were given a
single gavage dose of 65 mg DMBA/kg bw. The d-limonene-containing
diet was then continued for 27 weeks. Authors reported that
d-limonene delayed the appearance of DMBA-induced mammary tumours
in a dose-related manner, reduced the incidence of tumours, and
caused increased regression of tumours that did appear (Elegbede
et al., 1984a,b).
Female (W/Fu x F344)F2 rats were fed a diet containing 10%
d-limonene for 80 days beginning after the first appearance of
tumours induced by intubation of rats with 130 mg DMBA/kg bw.
Significant tumour regressive action by d-limonene was observed,
and the development of subsequent tumours was also reduced
significantly (Elegbede et al., 1986a).
Following initiation with DMBA (single gavage dose of 65 mg/kg
in sesame oil), groups of female Sprague-Dawley rats were (1) fed a
basal cereal diet one week before and 24 weeks following initiation
with DMBA (controls), (2) fed a diet containing 5% d-limonene one
week before and one week after treatment with DMBA, then fed the
control diet for 24 weeks (initiation group), or (3) fed the control
diet for one week before and one week following DMBA initiation,
then fed the 5% d-limonene diet for 24 weeks
(promotion/progression group). d-Limonene was reported to be
effective in reducing the average number of rat mammary carcinomas
in DMBA-initiated rats when fed during the initiation phase or
during the initiation/progression phase of carcinogenesis. Time to
appearance of the first tumour was extended only when d-limonene
was fed during the initiation phase, however. Authors concluded that
these effects could not be attributed to changes in mammary-relevant
endocrine functions (serum levels of prolactin and duration of
estrus cycle) (Elson et al., 1988).
Female Wistar-Furth rats were (1) placed on diets containing 5%
d-limonene or 5% orange oil (source of d-limonene) for 2 weeks
prior to initiation with nitrosomethylurea (NMU; single i.v. dose of
50 mg/kg bw), then continued on these diets for 23 weeks; (2) fed
diets containing 5% d-limonene for two weeks before and one week
following initiation with NMU, then fed a basal diet for 22 weeks
(initiation group); or (3) fed basal diets prior to and for 1 week
following initiation with NMU, then fed diets containing 5%
d-limonene for 22 weeks (promotion/progression group). Both orange
oil and d-limonene decreased mammary tumour incidence (controls:
80%; d-limonene: 45%; orange oil: 47%) and the average number of
mammary tumours per rat (controls: 1.8; d-limonene: 0.4; orange
oil: 0.8) when present in the diet for two weeks before and 23 weeks
after NMU administration. d-Limonene decreased tumour incidence
and decreased the numbers of tumours per rat (by approximately 50%)
when fed during the promotion/progression experiment but not the
during the initiation experiment (Maltzman et al., 1989).
2.2.3.6 Studies on the mechanism of d-limonene carcinogenicity
This section discusses (1) spontaneously occurring nephropathy
in mature male rats and (2) the current proposed mechanism of
carcinogenicity of d-limonene and other chemicals/mixtures that
exacerbate this condition and lead to alpha2u-globulin-associated
male rat nephropathy.
(1) Mature male rat nephropathy
Normal male rats at least 30 days old exhibited marked
increased protein excretion in their urine not seen in younger males
nor in females of any age. This proteinuria reached a maximum level
of approximately 2.5-3 times that seen in females at 90 days of age.
Microscopically, the authors also observed intracellular droplets in
the epithelial cells of the upper two-thirds of the proximal
convoluted tubules of kidneys of male rats at least 60 days old. The
occurrence of these hyaline droplets consisted of a few groups of
small droplets in occasional cells in 60 day old animals. The number
of cells having droplets and the number and size of droplets per
cell increased with age; from 90 days of age most proximal tubule
cells contained them. In contrast, at 120 and 180 days of age, only
a small number of females showed occasional droplets in a few cells
of the proximal tubules.
These authors further demonstrated the sex-relatedness of this
phenomenon by castrating male rats at 30 days of age and injecting
female rats with 5 mg testosterone every other day from 50 days of
age. When examined at 120 days of age, the castrated male rats
showed proteinuria at a level equal to control females and hyaline
droplets were only seen in 4/20 animals. Testosterone-treated
females, on the other hand, had proteinuria at a level approximately
midway between control males and females. No hyaline droplets were
seen in the proximal tubule cells of these females, however, showing
that the addition of testosterone alone was insufficient to cause
them to appear (Logothetopoulos & Weinbren, 1955).
Numerous authors have since reported detailed descriptions of
spontaneously occurring nephropathy in mature male rats which is a
consequence of this hyaline droplet accumulation. The sequence of
events appears to be that: (1) protein accumulates in the lysosomes
in the cytoplasm of epithelial cells of the P2 segment of the
proximal convoluted tubules in the kidney cortex; (2) the
accumulated material becomes so abundant that it crystallizes,
forming the microscopically visible hyaline droplets; (3) the
continued build-up of material eventually leads to the death of
epithelial cells with a concomitant thinning of the epithelial layer
(although some regeneration is usually seen); and (4) the dead cell
debris becomes lodged in the outer strip of the outer medulla where
the tubules narrow, forming granular casts and causing tubule
dilation with pressure necrosis of the cells of the tubule walls.
There is also an increase in the relative weight of the kidneys
accompanying this phenomenon.
A steadily increasing number of agents have been identified
that appear to exacerbate this hyaline droplet formation and its
consequences in mature male rats. With some, noticeable changes
occur within days of exposure. These agents include complex mixtures
of hydrocarbons such as mineral spirits (Carpenter et al., 1975a;
Phillips & Cockrell, 1984), "60" solvent (Carpenter et al.,
1975b), "high naphthenic solvent" (Carpenter et al., 1977), the
petroleum-derived or shale-derived jet fuel JP-5 (MacEwen & Vernot,
1978a; Parker et al., 1981; Bruner, 1984; MacNaughton & Uddin,
1984), petroleum-derived and shale-derived diesel fuel marine (DFM),
the jet fuels JP-4, JP-TS and JP-7, and the missile propellants
JP-10 and RJ-5 (Bruner, 1984; MacNaughton & Uddin, 1984), C/10-C/11
isoparaffin (Phillips & Egan, 1984; Phillips & Cockrell, 1984), five
petroleum naphtha mixtures (Halder et al., 1984) and unleaded
gasoline (MacFarland et al., 1984; Busey & Cockrell, 1984; Halder
et al., 1984; Kitchen, 1984; Garg et al., 1988a,b, 1989; Short &
Swenberg, 1988; Short et al., 1989a). Also included on the list
are the individual chemicals decahydro-naphthalene (decalin, MacEwen
& Vernot, 1978b; Gaworski et al., 1980, 1981; Bruner, 1984; Alden
et al., 1984; Olson et al., 1986; Stone et al., 1987a,b;
Kanerva et al., 1987b,c; Read, 1988), pentachloroethane (NTP,
1983; Goldsworthy et al., 1988), 1,4-dichlorobenzene (NTP, 1987a;
Charbonneau et al., 1989), perchloroethylene (Goldsworthy et al.,
1988), dimethyl methylphosphonate (NTP, 1987b),
2,2,4-trimethylpentane (Stonard et al., 1986; Short et al.,
1987; Charbonneau et al., 1987; Lock et al., 1987; Loury
et al., 1987; Short et al., 1989a), the chemically unrelated
agents 3-methylamino-1-(3-trifluoromethyl-phenyl)-2-pyrazoline,
cis/trans-2-(4'-t-butylcyclohexyl)-3-hydroxy-1,4-naphthoquinone
and 2,3,5,6-tetrahycro-6-phenylimidazo-(2,1-b) thiazole (Read
et al., 1988) and d-limonene (Kanerva et al., 1987a; NTP,
1990).
There is no obvious common structure among the specifically
identified chemicals that cause an increased accumulation of hyaline
droplets in mature male rat kidneys. And there are examples of
agents that do have similar mixtures/structures to those listed
above that do not cause this phenomenon. These include "70" solvent
(Carpenter et al., 1975c), two other petroleum naphtha mixtures
(Halder et al., 1984), 1,2-dichlorobenzene (Charbonneau et al.,
1989) and an analog of d-limonene, 4-vinylcyclohexene (NTP, 1986).
Recently, however, Miller et al. (1989) reported that they have
identified correlations between structure and binding affinity to
alpha2u-globulin, but no specific details were given in their
published report.
While exposure to unleaded gasoline for 72 h has been shown to
cause effects that are reversible (Garg et al., 1988a), with
chronic exposure to several of these mixtures/chemicals the kidney
nephrosis has been reported to progress to hyperplasia, adenoma and
even adenocarcinoma (MacFarland et al., 1984; Kitchen, 1984;
MacNaughton & Uddin, 1984; NTP, 1987a,b,c; NTP, 1990).
(2) Mechanism for alpha2u-globulin-associated male rat
nephropathy
The mechanism proposed for alpha2u-globulin-associated male
rat nephrosis has recently been thoroughly reviewed by the US
Environmental Protection Agency (US EPA, 1991). Their review
incorporated the results of many studies, including Charbonneau &
Swenberg (1988), Swenberg et al. (1989), Flamm & Lehman-McKeeman
(1991), Lehman-McKeeman et al. (1989), Lehman-McKeeman et al.
(1990a,b), Short et al. (1987), Short et al. (1989a,b), Dietrich
& Swenberg (1991), Murty et al. (1988), Ridder et al. (1990),
and Webb et al. (1989). The review concluded that:
"Of the eight model substances tested in chronic animal
bioassays (decalin, dimethyl methyl phosphonate, JP-4 jet fuel,
JP-5 shale-derived jet fuel, d-limonene, methyl isobutyl
ketone, pentachloroethane, and unleaded gasoline), all invoked
a specific type of protein droplet nephropathy in male rats and
also produced renal tumours in male rats but not in other
species tested. It has been proposed that such renal tumours
are the end product in the following sequence of functional
changes in the epithelial cells of proximal tubules:
* Excessive accumulation of hyaline droplets in proximal
tubules, representing lysosomal overload, cell loss, and
regenerative cellular proliferation.
* Cell debris in the form of granular casts accumulates at
the 'corticomedullary' junction with associated dilation
of the affected tubule segment and more distally,
mineralization of tubules within the renal medulla.
* Single-cell necrosis accompanied by compensatory cell
proliferation and exacerbation of the chronic progressive
nephropathy characteristically found in aging rats occurs.
* Renal tubule hyperplasia and neoplasia develop
subsequently.
According to this hypothesis, the increased proliferative
response caused by the chemically induced cytotoxicity results
in clonal expansion of spontaneously initiated renal tubule
cells and increased incidence of renal tumour formation (Trump
et al., 1984a; Alden, 1989; Swenberg et al., 1989). This
line of reasoning leads supporters of the hypothesis to
conclude that the acute and chronic renal effects induced in
male rats by such chemicals will be unlikely to occur in any
species not producing alpha2u-globulin, or a very closely
related protein, in the large quantities typically seen in the
male rat (Alden, 1989; Borghoff et al., 1990; Green et al.,
1990; Olson et al., 1990; Flamm & Lehman-McKeeman, 1991;
Swenberg, 1991)."
The following paragraphs summarize some of the data that
support this hypothesis:
Contents and catabolism of hyaline droplets/
phagolysosomes
The composition of the hyaline droplet inclusions has been
identified by Kanerva et al. (1987c) using two-dimensional gel
electrophoresis and immunological binding as being four species of
the low molecular weight protein alpha2u-globulin. As expected,
alpha2u-globulin was found to be absent in immature male rats and
female rats of all ages. It was present, however, in female rats
that had been ovariectomized and repeatedly injected with
testosterone. More recently, groups have confirmed that
alpha2u-globulin is the major constituent of the renal
phagolysosomal inclusions in male rats exposed to unleaded gasoline
(Garg et al., 1988b), and perchloroethylene and penta-chloroethane
(Goldsworthy et al., 1988). Finally, Dietrich and Swenberg (1991)
reported that male NCI-Black-Reiter rats, which do not produce
alpha2u-globulin, are not susceptible to nephropathy produced by
2,2,4-trimethylpentane, 1,4-dichloro-benzene, isophorone, PS-6
unleaded gasoline, or d-limonene.
A sex-specific protein has been identified in human urine
(Bernard et al., 1989), but the concentration (up to 108 µg/l) is
about 10 000 times less than that of alpha2u-globulin in male rats
(about 40 mg/24 h) (Roy & Neuhaus, 1967). Therefore assuming that
the protein in human urine shows the same binding characteristics as
alpha2u-globulin, it is reasonable to conclude that humans would
be comparably less sensitive than the male rat.
Alpha2u-globulin is synthesized in the liver of rats of both
sexes. This protein is filtered by the kidney glomeruli and
reabsorbed by the epithelial cells of the proximal convoluted
tubules by the formation of phagosomes at the lumenal surface.
Normally, the phagosomes then combine with cellular lysosomes and
the protein is degraded. In mature male rats, however, this
catabolism is slowed in some way under hormonal influence leading to
the intracellular accumulation of the protein which becomes
manifested as hyaline droplets. The degradation is slowed even more
by exposure to at least some of the agents listed in the previous
section (Stonard et al., 1986; Short et al., 1987; Kanerva
et al., 1987c; Charbonneau et al., 1987). A time- and
dose-dependent progressive increase in the number and size, and
alterations in the morphology of phagolysosomes in proximal
convoluted tubule epithelial cells have been demonstrated with
exposure to unleaded gasoline (Garg et al,. 1989). The ability of
unleaded gasoline to induce these effects declines with the age of
the male rat, in proportion to the declining production of
alpha2u-globulin (Murty et al., 1988).
Binding of chemicals to alpha2u-globulin
1,4-Dichlorobenzene as well as its major metabolite,
2,5-dichlorophenol, were both found to be reversibly bound (and
1,4-dichlorobenzene also covalently bound) to alpha2u-globulin in
the kidneys of treated male rats (Charbonneau et al., 1989).
Similarly, two groups have attempted to identify the specific
binding between one of these agents (2,2,4-tri-methylpentane) and
alpha2u-globulin. Loury et al. (1987) reported that no covalent
binding could be found, nor did there appear to be formation of a
Schiff base. Lock et al. (1987), however, detected reversible
binding of the metabolite 2,4,4-trimethyl-2-pentanol to
alpha2u-globulin. This specific metabolite was found in liver
cells of male rats only.
Likewise with decalin, an alcohol metabolite has been
identified that is unique to male rats (Olson et al., 1986). While
both male and female rats synthesize cis,cis-2-decalol and
trans,cis-2-decalol from cis-decalin in their livers,
cis,cis-1-decalol was found only in males and
cis,trans-1-decalol was produced in much higher concentrations
than in females. In similar fashion, with trans-decalin as the
substrate trans,trans-1-decalol was seen only in livers of male
rats. When kidneys were examined, 2-decalone (cis or trans,
depending on the original substrate) was found in the males while no
decalin metabolites whatever were seen in female kidneys. Whether
these unique male decalin metabolites react with alpha2u-globulin
has not yet been tested.
More recently, Lehman-McKeeman et al. (1989) have reported
that d-limonene and d-limonene-1,2-oxide, a major metabolite of
d-limonene, were found reversibly bound to alpha2u-globulin
(identified by amino acid sequencing) in the kidneys of treated
male, but not female, rats.
Inhibition of alpha2u-globulin catabolism
Charbonneau et al. (1988) reported that alpha2u-globulin
bound in kidney cytosol was not digested by purified proteases or
proteinase.
Treating rats with leupeptin, an inhibitor of cathepsin B, a
major lysosomal peptidase, mimics the ability of unleaded gasoline
to cause the accumulation of alpha2u-globulin in kidney
phagolysosomes. Authors concluded that the mechanism of
nephrotoxicity involves inhibition of renal phagolysosomal
proteolysis (Olsen et al., 1988).
Induction of cell proliferation
Short et al. (1989a) have shown that proliferation of
epithelial cells in the P2 proximal tubule segment in male rats is
associated with exposure of the animals to either
2,2,4-trimethylpentane or unleaded gasoline. They noted that this
proliferation closely paralleled the extent and severity of
detectable alpha2u-globulin in the same cells. The authors
speculated that this proliferation leads to the nephrocarcinogenic
effects caused by these agents.
Proliferation was also noted by Charbonneau et al. (1989) in
male rats treated with 1,4-dichlorobenzene. Short et al. (1989b)
observed increased numbers of atypical cell foci and renal cell
tumours in male, but not female, rats initiated with
N-ethyl-N-hydroxyethylnitrosamine and subsequently exposed to
unleaded gasoline or 2,2,4-trimethylpentane. The extent of the
promotion was reported to be dose-related and to parallel the
effects of these compounds on chronic cell proliferation.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity
d-Limonene was reported to increase the number of abnormal
chick embryos to approximately 50% when a single dose (25 µM/embryo)
in olive oil was injected suprablastodermically (Abramovici &
Rachmuth-Roizman, 1983).
Pregnant mice were given oral doses of 0, 591, or 2363 mg/kg bw
d-limonene from days 7 through 12 of gestation. Authors reported
significant decreases in body weight gain of dams in the high-dose
group; fetuses of dams exposed to the high-dose showed increased
incidences of lumbar rib, fused rib, delayed ossification, and
decreased body weight gain relative to fetuses of control dams
(Kodama et al., 1977a).
Pregnant Japanese white rabbits were given oral doses of 0,
250, 500, or 1000 mg/kg bw d-limonene from day 6 to day 18 of
gestation. Significant decreases in body weight gain in dams given
250 or 500 mg/kg bw/day d-limonene were observed, and survival in
dams given 1000 mg/kg bw/day was significantly reduced (40%
survival). From examination of the fetuses, authors concluded that
d-limonene was not teratogenic in rabbits (Kodama et al.,
1977b).
2.2.6 Special studies on genotoxicity
Table 2: Genotoxicity data on d-limonene
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium .03-3 µmol/plate neg Florin et
(+&-activ) TA98, TA100, al., 1980
TA1535, TA1537
Yeast S. cerevisiae .1-100 mM/5x107 (1) Fahrig,
(-activ) MP1 strain cells 1984
Mammalian Mouse embryo 0-215 mg/kg (2) Fahrig,
(-activ) system 1984
Ames test S. typhimurium 0-20 µM/plate neg Watabe
(+&-activ) TA1535, TA100, et al.,
TA1537, TA1538, 1980
TA98
Ames test S. typhimurium 0-3333 µg/plate neg Haworth
(+&-activ) TA98, TA100, et al.,
TA1535, TA1537 1983
Ames test S. typhimurium 0-3333 µg/plate neg NTP,
(+&-activ) TA100, TA1535, 1990
TA1537, TA98
Mammalian Mouse L5178Y neg NTP,
(+&-activ) cells in vitro 1990
Sister Chinese hamster 0-162 µg/ml neg NTP,
chromatid ovary cells 1990
exchange in vitro
(+&-activ)
Chromosomal Chinese hamster 0-500 µg/ml (3) neg NTP,
aberrations ovary cells 0-100 µg/ml (4) neg 1990
(+&-activ) in vitro
Table 2 cont'd
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium 150 mg/plate neg Heck et
(+&-activ) TA1535, TA1537, al., 1989
TA1538, TA98,
TA100
Mammalian Mouse L5178Y 100 µg/ml neg Heck et
(+&-activ) cells in vitro al., 1989
(1) Author reported that results indicated that d-limonene was a
co-recombinant and an anti-mutagen.
(2) Author reported that results indicated that d-limonene is inactive
when given alone, but reduces the mutagenic effect of ethylnitrosourea
when the two are co-administered.
(3) Activated: addition of S9 from the livers of Aroclor 1254-induced
male Sprague Dawley rats.
(4) Not activated
2.2.7 Special studies on immune responses
Limonene has been identified as a primary skin irritant in
humans (Pirila et al., 1964; Klecak et al., 1977), rabbits
(research Institute for the Development of Fragrance Materials,
Inc., 1984; Research Institute for Fragrance Materials Inc., 1985),
guinea-pigs (Klecak et al., 1977), and mice (Gad et al., 1986).
d-Limonene was reported both to elicit (De Groot et al., 1985)
and not to elicit (Greif, 1967) allergic reactions in human skin and
to elicit allergic reactions in mouse skin (Gad et al., 1986).
d-Limonene was reported to eliminate human skin sensitization to
several aldehydes when they were co-administered with d-limonene
(Opdyke, 1975), and d-limonene was reported to reduce the
sensitivity and delayed hypersensitivity of guinea-pig skin to
citral (Hanau et al., 1983; Barbier & Benezra, 1983). Gozsy and
Kato (1957) reported that local endothelial phagocytosis is induced
in rat skin by d-limonene, and that d-limonene increases
phagocytosis in guinea-pig skin in vivo and in mouse skin
in vitro (Gozsy & Kato, 1958) and increases capillary permeability
in the guinea-pig (Kato & Gozsy, 1958).
Male BALB/c mice were treated intragastrically with 0, 0.002,
0.008, 0.033, 0.133, 0.531 or 2.125 mg/ml d-limonene daily. After
8 weeks, but not after 4 weeks, the mitogenic responses of spleen
cells taken from the treated animals and exposed in vitro to
concanavalin A and phytohaemagglutinin, which stimulate T-cells, and
lipopolysaccharide, which affects B-cells, were found to be
suppressed by the d-limonene treatment.
Other animals in groups of 10 were given these same doses of
d-limonene daily for 7 days prior to, or 8 days following,
immunization with keyhole limpet haemocyanin (KLH). Primary effects
on T- and B-cell responses were determined 10 days later.
Secondary effects were determined by reinoculating the animals
with KLH 21 days after the first inoculation and then assaying on
day 24. The primary and secondary responses were reported to be
suppressed by d-limonene if the animals were exposed to KLH first,
but stimulated in treated mice if exposed to KLH after d-limonene
exposure.
Histopathological examination of secondary lymphoreticular
tissue taken from animals exposed to d-limonene showed significant
secondary follicle development and prominent lymphoid nodules and
aggregates in the pancreas and intestinal mucosa, which were evident
in animals which had received the highest dose of d-limonene
(Evans et al., 1987).
2.3 Observations in humans
Numerous prospective and retrospective cohort epidemiological
studies have been done on populations of refinery, chemical plant,
and service station workers, exposed to mixtures of chemicals that
have been associated with male rat alpha2u-globulin nephropathy
(Hanis et al., 1979; Schottenfeld et al., 1981; Hanis et al.,
1982; Thomas et al., 1982; Austin & Schnatter, 1983; Higginson
et al., 1984; Raabe, 1984; Wen et al., 1984; Wong & Raabe, 1989;
Page & Mehlman, 1989). No statistically significant evidence was
found in any study to link incidence of cancer of the kidney to
work-place exposure to mixtures of complex hydrocarbons.
Igimi et al. (1976) reported that d-limonene was a safe and
effective gallstone solubilizer in animals and humans; side effects
in humans were reported to be pain and tenderness radiating from the
upper abdomen to the anterior chest, nausea and vomiting, and
diarrhoea.
3. COMMENTS
d-Limonene has been shown to reduce body weights
significantly in male and female mice and rats and in female
rabbits. The NOEL for this effect was 150 mg of d-limonene kg
bw/day (administered by gavage) in a 2-year study in male rats.
Oral administration of d-limonene (400 mg/kg bw/day) to rats
for 20 days slightly increased liver weight and phospholipid
content, decreased liver and serum cholesterol levels, increased the
concentration of cytochromes P450 and B5, and increased the
activities of aminopyrine demethylase and aniline hydroxylase.
Although liver lesions were not associated with the administration
of d-limonene in a 2-year study in rats (doses up to 150 mg/kg
bw/day for males; doses up to 600 mg/kg bw/day for females), a daily
gavage dose of 500 mg d-limonene/kg bw/day for 2 years was
associated with an increased incidence of multinucleated liver
hepatocytes and cytomegaly in male mice. The NOEL for these effects
was 250 mg/kg bw/day administered by gavage to male mice for 2
years.
Dermal application of d-limonene has been reported to cause
irritation and immune-mediated skin reactions in a number of
species, including humans. Oral administration of d-limonene to
mice was reported to have immunological effects, including
suppression of the in vitro mitogenic response by mouse T-cells
and B-cells and both suppression and stimulation of antigenic
responses. However, the biological significance of these in vitro
changes is not known.
Results of teratogenicity studies in mice suggest that maternal
consumption of 2400 mg of d-limonene/kg bw/day but not of
600 mg/kg bw/day, affected the development of fetuses (decreased
body weight gain, increased incidences of lumbar rib and fused rib,
and delayed ossification). However, d-limonene was reported to be
non-teratogenic in rabbits at doses ranging from 250 to 1000 mg/kg
bw/day (although the highest dose significantly reduced survival of
the dams).
d-Limonene consistently produced negative results in
genotoxicity studies, has been reported to inhibit the activity of
other mutagens and carcinogens, and gave mixed responses in
tumour-promotion studies.
Data clearly demonstrate that d-limonene exacerbates the
spontaneously occurring nephropathy in mature male rats which, after
prolonged exposure, is associated with an increase in the incidence
of renal tumours that are not commonly observed in control male
rats. However, d-limonene is not the only substance that has this
effect: various hydrocarbon mixtures and several other chemicals
have also been reported to exacerbate spontaneous nephropathy and
cause renal tumours in male rats. Although, in studies designed to
elucidate the mechanism of this carcinogenic process, chemicals
other than d-limonene were generally used as test substances, such
studies have shown that the following mechanism is likely to be
applicable to d-limonene-induced nephropathy and renal tumours in
male rats: (1) d-limonene and d-limonene-1,2-oxide (a metabolite
of d-limonene) bind reversibly to alpha2u-globulin in the
kidneys of d-limonene-treated male rats; (2) this binding further
slows down the normally slow processing of reabsorbed
alpha2u-globulin; and (3) as a result, alpha2u-globulin
accumulates in the phagolysosomes (hyaline droplets) of epithelial
cells of the proximal convoluted tubules of the kidney, leading to
necrosis, accumulation of cell debris, and regenerative hyperplasia.
The postulated mechanism of action for some non-genotoxic
carcinogens suggests that regenerative hyperplasia may increase
clonal expansion of spontaneously initiated cells and lead to the
development of the renal tumours observed.
A NOEL for the d-limonene-associated increase in
alpha2u-globulin levels in male rat kidney has not been
identified, but the lowest-observed-effect level from a 21-day
gavage study of d-limonene in male rats was 75 mg/kg bw/day. A
sex-specific protein with molecular features similar to
alpha2u-globulin has been identified in human urine, but the
concentration is at least four orders of magnitude less than that of
alpha2u-globulin in male rats.
The Committee concluded that the postulated mechanism for
d-limonene-induced nephropathy and renal tumours in the male rat
was probably not relevant to humans, and that toxic end points
associated with this effect were not appropriate bases for the
derivation of an ADI for d-limonene.
4. EVALUATION
Based on the significant decreases in body weight gain
associated with administration of d-limonene to male and female
mice and rats and female rabbits, an ADI of 0-1.5 mg/kg bw was
established for this substance. The Committee considered the known
natural occurrence and food additive uses of d-limonene, and
concluded that only a small proportion of total intake is likely to
be derived from direct additive use. The Committee therefore
recommended that food additive intake be restricted to 75 µg/kg
bw/day, which represents 5% of the maximum ADI for d-limonene.
5. REFERENCES
ABRAMOVICI, A. & RACHMUTH-ROIZMAN, P. (1983). Molecular
structure-teratogenicity relationships of some fragrance additives.
Toxicology, 29: 143-156.
ALDEN, C.L., KANERVA, R.L., RIDDER, G. & STONE, L.C. (1984). Chapter
VIII: The pathogenesis of the nephrotoxicity of volatile
hydrocarbons in the male rat. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 107-120, Princeton Scientific, Princeton, NJ.
ALDEN, C.L. (1989) Male rat specific alpha2u-globulin nephropathy
and renal tumorigenesis. In: Bach, P.H. & Lock, E.A. (eds.),
Handbook of Toxicologic Pathology. Academic Press, New York. In
press (cited in US EPA 1991).
ARIYOSHI, T., ARAKAKI, M., IDEGUCHI, K., ISHIZUKA, Y., NODA, K. &
IDE, H. (1975). Studies on the metabolism of d-limonene
(p-mentha-1,8-diene); III. Effects of d-limonene on the lipids and
drug metabolizing enzymes in rat livers. Xenobiotica, 5: 33-38.
AUSTIN, S.G. & SCHNATTER, A.R. (1983). A cohort mortality study of
petrochemical workers. Journal of Occupational Medicine, 25:
304-312.
BARBIER, P. & BENEZRA, C. (1983) The influence on induced
hypersensitivity to citral in guinea-pigs; II. Label distribution in
the skin of 14C-labelled citral. Acta Dermatovener (Stockholm),
63: 93-96.
BERNARD, A.M., LAUWERYS, R.R., NOEL, A., VANDELEENE, B. & LAMBERT,
A. (1989). Urine protein I: a sex-dependent marker of tubular or
glomerular dysfunction. Clin. Chem., 35: 2141-2.
BORGHOFF, S.J., SHORT, B.G., & SWENBERG, J.A. (1990) Biochemical
mechanisms and pathobiology of alpha2u-globulin nephropathy.
Ann. Rev. Pharmacol. Toxicol., 30: 349-367.
BRUNER, R.H. (1984). Chapter X: Pathologic findings in laboratory
animals exposed to hydrocarbon fuels of military interest. In:
Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 133-140,
Princeton Scientific, Princeton, NJ.
BUSEY, W.M. & COCKRELL, B.Y. (1984). Chapter IV: Non-neoplastic
exposure-related renal lesions in rats following inhalation of
unleaded gasoline vapors. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 57-64, Princeton Scientific, Princeton, NJ.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975a). Petroleum hydrocarbon toxicity studies: III.
Animal and human response to vapors of Stoddard solvent. Toxicology
and Applied Pharmacology, 32: 282-297.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975b). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "60 solvent." Toxicology
and Applied Pharmacology, 32: 374-394.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975c). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "70 solvent." Toxicology
and Applied Pharmacology, 34: 395-412.
CARPENTER, C.P., GEARY, JR., D.L., MYERS, R.C., NACHREINER, D.J.,
SULLIVAN, L.J. & KING, J.M. (1977). Petroleum hydrocarbon toxicity
studies: XV. Animal and human responses to vapors of "high
naphthenic solvent." Toxicology and Applied Pharmacology, 41:
251-260.
CHARBONNEAU, M., LOCK, E.A., STRASSER, J., COX, M.G., TURNER, M.J. &
BUS, J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: I.
Metabolic disposition of TMP in male and female Fischer 344 rats.
Toxicology and Applied Pharmacology, 91: 171-181.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J. & SWENBERG,
J.A. (1989). Involvement of reversible binding to alpha2u-globulin
in 1,4-dichlorobenzene-induced nephrotoxicity. Toxicology and
Applied Pharmacology, 99: 122-132.
CHARBONNEAU, M. & SWENBERG, J.A. (1988). Studies on the bio-chemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT
Activities, 8: 1-5.
CURVALL, M., ENZELL, C. & PETTERSSON, B. (1984). An evaluation of
the utility of four in vitro short-term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke.
Cell Biology and Toxicology, 1: 173-193.
DIETRICH, D.R. & SWENBERG, J.A. (1991). NCI-Black-Reiter (NBR) male
rats fail to develop renal disease following exposure to agents that
induce alpha2u-globulin nephropathy. Fundamentals of Applied
Toxicology, 16: 749-762.
DE GROOT, A.C., LIEM, D.H., NATER, J.P. & VAN KETEL, W.G. (1985).
Patch tests with fragrance materials and preservatives. Contact
Dermatitis, 12: 87-92.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984a). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Carcinogenesis, 5: 661-664.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984b). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Proceedings of the American Association
of Cancer Researchers, 25: 127A.
ELEGBEDE, J.A., ELSON, C.E., TANNER, M.A., QURESHI, A. & GOULD, M.N.
(1986a). Regression of rat primary mammary tumours following dietary
d-limonene. Journal of the National Cancer Institute, 76:
323-325.
ELEGBEDE, J.A., MALTZMAN, T.H., VERMA, A.K., TANNER, M.A., ELSON,
C.E. & GOULD, M.N. (1986b). Mouse skin tumour promoting activity of
orange peel oil and d-limonene: a re-evaluation. Carcinogenesis,
7: 2047-2049.
ELSON, C.E., MALTZMAN, T.H., BOSTON, J.L., TANNER, M.A. & GOULD,
M.N. (1988). Anti-carcinogenic activity of d-limonene during the
initiation and promotion/progression stages of DMBA-induced rat
mammary carcinogenesis. Carcinogenesis, 9: 331-332.
FAHRIG, R. (1984). Genetic mode of action of cocarcinogens and
promoters in yeast and mice. Molecular and General Genetics, 194:
7-14.
FLAMM, W.G. & LEHMAN-McKEEMAN, L. (1991). The human relevance of the
renal tumour-inducing potential of d-limonene in male rats:
Implications for risk assessment. Regulatory Toxicology and
Pharmacology, 13: 70-86.
FLORIN, I., RUTBERG, L., CURVALL, M. & ENZELL, C. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18: 219-232.
GAD, S.C., DUNN, B.J., DOBBS, D.W., REILLY, C. & WALSH R.D. (1986).
Development and validation of an alternative dermal sensitization
test: the mouse ear swelling test (MEST). Toxicology and Applied
Pharmacology, 84: 93-114.
GARG, B.D., OLSON, M.J., DEMYAN, W.F. & ROY, A.K. (1988a). Rapid
post-exposure decay of alpha2u-globulin and hyaline droplets in
the kidneys of gasoline-treated male rats. Journal of Toxicology
and Environmental Health, 24: 145-160.
GARG, B.D., OLSON, M.J., MANCINI, M.A. & ROY, A.K. (1988b).
Localization of alpha2u-globulin in hyaline droplets
(phagolysosomes) of the male rat renal cortex by immunohistochemical
techniques. Journal of Histochemistry and Cytochemistry, 36: 960.
GARG, B.D., OLSON, M.J., LI, L.C. & ROY, A.K. (1989). Phagolysosomal
alterations induced by unleaded gasoline in epithelial cells of the
proximal convoluted tubules of male rats: Effect of dose and
treatment duration. Journal of Toxicology and Environmental Health,
26: 101-118.
GAWORSKI, C.L., HAUN, C.C. & MacEWEN, J.D. (1981). A ninety-day
inhalation toxicity study of decalin. The Toxicologist, 1, 76.
GAWORSKI, C.L., LEAHY, H.F. & BRUNER, H.F. (1980). Subchronic
inhalation toxicity of decalin, Proceedings of the Tenth Conference
on Environmental Toxicology, AFAMRL-TR-79-121 (ADA086341) Aerospace
Medical Research Laboratory, Wright-Patterson Air Force Base, OH.
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L. & POPP, J.A. (1988).
Potential role of alpha2u-globulin, protein droplet accumulation,
and cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicology and Applied Pharmacology, 96: 367-379.
GOZSY, B. & KATO, L. (1957). Action of reticulo-endothelial-system
stimulators on the histamine depleted rats. Arch. Int. Pharmacodyn,
CXII: 235-246.
GOZSY, B. & KATO, L. (1958). Reticulo-endothelial system stimulating
compounds and their mechanism of action. Can. J. Biochem. Physiol.,
36: 319-326.
GREEN, T., ODUM, J., NASH, J.A. & FOSTER, J.R. (1990).
Perchloroethylene-induced rat kidney tumours: An investigation of
the mechanisms involved and their relevance to humans. Toxicol.
Appl. Pharmacol., 102: 77-89.
GREIF, N. (1967). Cutaneous safety of fragrance material as measured
by the Maximization Test. American Perfumer and Cosmetics, 82:
54-57.
HALDER, C.A., WARNE, T.M. & HATOUM, N.S. (1984). Chapter VI: Renal
toxicity of gasoline and related petroleum naphthas in male rats.
In: Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 73-88, Princeton
Scientific, Princeton, NJ.
HANAU, D., GROSSHANS, E., BARBIER, P. & BENEZRA, C. (1983). The
influence of limonene on induced delayed hypersensitivity to citral
in guinea-pigs; I. Histology study. Acta Dermatovener (Stockholm)
63: 1-7.
HANIS, N.M., HOLMES, T.M., SHALLENBERGER, L.G. & JONES, K.E. (1982).
Epidemiologic study of refinery and chemical plant workers. Journal
of Occupational Medicine, 24: 203-212.
HANIS, N.M., STRAVRAKY, K.M. & FOWLER, J.L. (1979). Cancer mortality
in oil refinery workers. Journal of Occupational Medicine, 21:
167-174.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W. & ZEIGER, E.
(1983). Salmonella mutagenicity test results for 250 chemicals.
Environmental Mutagenesis Supplement, 1: 3-142.
HECK, J.D., VOLLMUTH, T.A., CIFONE, M.A., JAGANNATH, D.R., MYHR, B.
& CURREN, R.D. (1989). An evaluation of food flavoring ingredients
in a genetic toxicity screening battery. The Toxicologist, 1: 257.
HIGGINSON, J., MUIR, C. & BUFFLER, P.A. (1984). Chapter XV: The
epidemiology of renal carcinoma in humans with a note on the effect
of exposure to gasoline. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 203-226, Princeton Scientific, Princeton, NJ.
HOMBURGER, F., TREGER, A. & BOGER, E. (1971). Inhibition of murine
subcutaneous and intravenous benzo(rst)pentaphene carcinogenesis by
sweet orange oils and d-limonene. Oncology, 25: 1-10.
IGIMI, H., HISATSUGU, T. & NISHIMURA, M. (1976). The use of
d-limonene preparation as a dissolving agent of gallstones.
Digestive Diseases, 21: 926-939.
IGIMI, H., NISHIMURA, M., KODAMA, R. & IDE, H. (1974). Studies on
the metabolism of d-limonene (p-mentha-1,8-diene): I. The
absorption, distribution and excretion of d-limonene in rats.
Xenobiotica, 4: 77-84.
IMAIZUMI, K. HANADA, K., MAWATARI, K. & SUGANO, M. (1985). Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49: 2795-2796.
KANERVA, R.L. & ALDEN, C.L. (1987). Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and Chemical Toxicology, 25: 355-358.
KANERVA, R.L., RIDDER, G.M., LEFEVER, F.R. & ALDEN, C.L. (1987a).
Comparison of short-term renal effects due to oral administration of
decalin or d-limonene in young adult male Fischer-344 rats.
Food and Chemical Toxicology, 25: 345-353.
KANERVA, R.L., MCCRACKEN, M.S., ALDEN, C.L. & STONE, L.C. (1987b).
Morphogenesis of decalin-induced renal alterations in the male rat.
Food and Chemical Toxicology, 25: 53-61.
KANERVA, R.L., RIDDER, G.M., STONE, L.C. & ALDEN, C.L. (1987c).
Characterization of spontaneous and decalin-induced hyaline droplets
in kidneys of adult male rats. Food and Chemical Toxicology, 25:
63-82.
KATO, L. & GOZSY, B. (1958). Treatment of experimental tuberculosis
of the guinea-pig with dihydrostreptomycin and simultaneously with
substances acting on the host. Arch. Int. Pharmacodyn, CXVII:
52-62.
KITCHEN D.N. (1984). Chapter V: Neoplastic renal effects of unleaded
gasoline in Fischer 344 rats. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 65-88, Princeton Scientific, Princeton, NJ.
KLECAK, G., GELEICK, H. & FREY, R. (1977). Screening of fragrance
materials for allergenicity in the guinea-pig; comparison of four
testing methods. J. Soc. Cosmet. Chem., 28: 53-64.
KODAMA, R., NODA, K. & IDE, H. (1974). Studies on the metabolism of
d-limonene (p-mentha-1,8-diene): II. The metabolic fate of
d-limonene in rabbits. Xenobiotica, 4: 85-95.
KODAMA, R., INOUE, H., NODA, K., IDE, H., YAMAMOTO, H., YOSHIHARA,
S. & YOSHIMURA, H. (1976a). Effect of d-limonene and related
compounds on bile flow and biliary lipid composition in rats and
dogs. Life Sciences, 19: 1559-1568.
KODAMA, R., YANO, T., FURUKAWA, K., NODA, K. & IDE, H. (1976b).
Studies on the metabolism of d-limonene (p-mentha-1,8-diene): IV.
Isolation and characterization of new metabolites and species
differences in metabolism. Xenobiotica, 6: 377-389.
KODAMA, R., OKUBO, A., ARAKI, E., NODA, K., IDE, H. & IKEDA, T.
(1977a). Studies on d-limonene as a gallstone solubilizer; VII.
Effects on development of mouse fetuses and offspring. Oyo Yakuri
(Pharmacometrics) 13: 863-873.
KODAMA, R., OKUBO, A., SATO, K., ARAKI, E., NODA, K., IDE, H. &
IKEDA, T. (1977b). Studies on d-limonene as a gallstone
solubilizer; IX. Effects on development of rabbit fetuses and
offspring. Oyo Yakuri (Pharmacometrics), 13: 885-898.
LEHMAN-McKEEMAN, L.D., CAUDILL, D., TAKIJIKU, R., SCHNEIDER, R.E. &
YOUNG, J.A. (1990a). Comparative disposition of d-limonene in rats
and mice: Relevance to male-rat-specific nephrotoxicity. Toxicol.
Lett., 53: 193-195.
LEHMAN-McKEEMAN, L.D., RIVERA-TORRES, M.I. & CAUDILL, D. (1990b).
Lysosomal degradation of alpha2u-globulin and
alpha2u-globulin-xenobiotic conjugates. Toxicology and Applied
Pharmacology, 103: 539-548.
LEHMAN-McKEEMAN, L.D., RODRIGUEZ, P.A., TAKIGIKU, R., CAUDILL, D. &
FEY, M.L. (1989). d-Limonene-induced male rat-specific
nephrotoxicity: Evaluation of the association between d-limonene
and alpha2u-globulin. Toxicology and Applied Pharmacology, 99:
250-259.
LOCK, E.A., CHARBONNEAU, M., STRASSER, J., SWENBERG, J.A. & BUS,
J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: II. The
reversible binding of a TMP metabolite to a renal protein fraction
containing alpha/2u-globulin. Toxicology and Applied
Pharmacology, 91: 182-192.
LOGOTHETOPOULOS, J. & WEINBREN, K. (1955). Naturally occurring
protein droplets in the proximal tubule of the rat's kidney.
British Journal of Experimental Pathology, 36: 402-406.
LOURY, D.J., SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987). Assessment
of the covalent binding potential of 2,2,4-trimethylpentane to rat
alpha2u-globulin. Toxicology and Applied Pharmacology, 88:
44-56.
MacEWEN, J.D. & VERNOT, E. (1978a). Evaluation of toxic effects of
90-day continuous exposure to conventional JP-5 jet fuel. In: Toxic
Hazards Research Unit Annual Report, AFAMRL-TR-78-55 (ADA062138)
Aerospace Medical Research Laboratory, Wright-Patterson Air Force
Base, OH.
MacEWEN, J.D. & VERNOT, E. (1978b). The effects of subchronic
exposure of rodents to inhaled decalin. In: Toxic Hazards Research
Unit Annual Report, AFAMRL-TR-78-55 (ADA062138) Aerospace Medical
Research Laboratory, Wright-Patterson Air Force Base, OH.
MacFARLAND, H.N., ULRICH, C.E., HOLDSWORTH, C.E., KITCHEN, D.N.,
HALLIWELL, W.H. & BLUM S.C. (1984). A chronic inhalation study with
unleaded gasoline vapor. Journal of the American College of
Toxicology, 3: 231-248.
MacNAUGHTON, M.G. & UDDIN, D.E. (1984). Chapter IX: Toxicology of
mixed distillate and high-energy synthetic fuels. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 121-132, Princeton
Scientific, Princeton, NJ.
MALTZMAN, T.H., HURT, L.M., ELSON, C.E., TANNER, M.A. & GOULD, M.N.
(1989). The prevention of nitrosomethylurea-induced mammary tumours
by d-limonene and orange oil. Carcinogenesis, 10: 781-783.
MEYER, F. & MEYER, E. (1959). Absorption of ethereal oils and
substances contained in them through the skin.
Arzneimittelforschung (Drug Research), 9: 516-519.
MILLER, A.B., BOWEN, J.P., BORGHOFF, S.J. & SWENBERG, J.A. (1989).
Computational and molecular modeling studies of alpha2u-globulin.
Abstract papers of the American Chemical Society, 198: Medi-39.
MURTY, C.V.R., OLSON, M.J., GARG, B.D. & ROY, A.K. (1988).
Hydrocarbon-induced hyaline droplet nephropathy in male rats during
senescence. Toxicology and Applied Pharmacology, 96: 380-392.
NTP (1983). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of pentachloroethane (CAS No.
76-01-7) in F344/N rats and B6C3F1 mice (Gavage Studies), NTP
TR 232, NIH Publication No. 83-1788.
NTP (1986). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 4-vinyl-cyclohexene (CAS
No. 100-40-3) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 303, NIH Publication No. 86-2559.
NTP (1987a). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 1,4-di-chlorobenzene (CAS
No. 106-46-7) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 319, NIH Publication No. 87-2570.
NTP (1987b). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of dimethylmethylphosphonate
(CAS No. 756-79-6) in F344/N rats and B6C3F1 mice (Gavage
Studies), NTP TR 323, NIH Publication No. 88-2579.
NTP (1990). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of d-limonene (CAS No.
5989-27-5) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 347, NIH Publication No. 90-2802.
OLSON, C.T., YU, K.O. & SERVE, M.P. (1986). Metabolism of
nephrotoxic cis- and trans-decalin in Fischer-344 rats. Journal
of Toxicology and Environmental Health, 18: 285-292.
OLSON, M.J., JOHNSON, J.T. & REIDY, C.A. (1990). A comparison of
male rat and human urinary proteins: Implications for human
resistance to hyaline droplet nephropathy. Toxicol. Appl.
Pharmacol., 102: 524-536.
OLSON, M.J., MANCINI, M.A., GARG, B.D. & ROY, A.K. (1988).
Leupeptin-mediated alteration of renal phagolysosomes: Similarity to
hyaline droplet nephropathy of male rats exposed to unleaded
gasoline. Toxicol. Lett. 41: 245-254.
OPDYKE, D.L.J. (1975). d-Limonene. Monograph on fragrance raw
materials. Food and Cosmetics Toxicology, 13(suppl: 683-923):
825-826.
PAGE, N.P. & MEHLMAN, M. (1989). Health effects of gasoline
refueling vapors and measured exposures at service stations.
Toxicology and Industrial Health, 5: 869-890.
PARKER, G.A., BOGO, V. & YOUNG, R.W. (1981). Acute toxicity of
conventional versus shale-derived JP5 jet fuel: Light microscopic,
hematologic, and serum chemistry studies. Toxicology and Applied
Pharmacology, 57: 302-317.
PHILLIPS, R.D. & COCKRELL, B.Y. (1984). Chapter VII: Effect of
certain light hydrocarbons on kidney function and structure in male
rats. In: Advances in Modern Environmental Toxicology, Renal
Effects of Petroleum Hydrocarbons, M.A. Mehlman, editor, pp.
89-105, Princeton Scientific, Princeton, NJ.
PHILLIPS, R.D. & EGAN, G.F. (1984). Effect of C10-11 isoparaffinic
solvent on kidney function in Fischer 344 rats during eight weeks of
inhalation. Toxicology and Applied Pharmacology, 73: 500-510.
PIRILA, V., SILTANEN, E. & PIRILA, L. (1964). On the chemical nature
of the eczematogenic agent in oil of turpentine. Dermatologica,
128: 16-21.
RAABE, G.K. (1984). Chapter XVIII: Kidney cancer epidemiology in
petroleum related studies. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 259-271, Princeton Scientific, Princeton, NJ.
READ, N.G., ASTBURY, P.J., MORGAN, R.J.I., PARSONS, D.N. & PORT,
C.J. (1988). Induction and exacerbation of hyaline droplet formation
in the proximal tubular cells of the kidneys from male rats
receiving a variety of pharmacological agents. Toxicology, 52:
81-101.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1985). Acute
dermal irritation study; RIFM 1-32.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1990). The
absorption, distribution, and excretion of radioactivity after
topical application of 14C-limonene to rats.
RESEARCH INSTITUTE FOR THE DEVELOPMENT OF FRAGRANCE MATERIALS, INC.
(1984). Acute dermal irritation study; Ref. Nos. 307-338/8403.
RIDDER, G.M., VON BARGEN, E.C., ALDEN, C.L. & PARKER, R.D. (1990).
Increased hyaline droplet formation in male rats exposed to decalin
is dependent on the presence of alpha2u-globulin. Fundamental and
Applied Toxicology, 15: 732-743.
ROE, F.J.C. (1959). Oil of sweet orange: A possible role in
carcinogenesis. British Journal of Cancer, 13: 92-93.
ROE, F.J.C. & Peirce, W.E.H. (1960). Tumour promotion by citrus
oils: Tumours of the skin and urethral orifice in mice. Journal of
the National Cancer Institute, 24: 1389-1403.
ROY, A.K. & NEUHAUS, A.W. (1967) Androgenic control of a
sex-dependent protein in the rat. Nature, 214: 618-20.
SCHENK, J. DOBRONTE, Z., KOCH, H. & STOLTE, M. (1980).
Untersuchungen zur gewebevertraglichkeit von d-limonen als einem
losungsmittel cholesterineicher gallenstein. Gastroenterologie,
18: 389-394.
SCHOTTENFELD, D., WARSHAUER, M.E., ZAUBER, A.G., MEIKLE, J.G. &
HART, B.T. (1981). A prospective study of morbidity and mortality in
petroleum industry employees in the United States - a preliminary
report. In: Banbury Report 9: Quantification of Occupational
Cancer, R. Peto & M. Schneiderman, editors, pp. 247-265. Cold
Spring Harbor Laboratory, Cold Spring Harbor, NY.
SHORT, G.B., BURNETT, V.L., COX, M.G., BUS, J.S. & SWENBERG, J.A.
(1987). Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Laboratory
Investigation, 57: 564-577.
SHORT, G.B., BURNETT, V.L. & SWENBERG, J.A. (1989a). Elevated
proliferation of proximal tubule cells and localized accumulated
alpha2u-globulin in F344 rats during chronic exposure to unleaded
gasoline or 2,2,4-trimethylpentane. Toxicology and Applied
Pharmacology, 101: 414-431.
SHORT, G.B., STEINHAGEN, W.H. & SWENBERG, J.A. (1989b). Promoting
effects of unleaded gasoline and 2,2,4-trimethylpentane on the
development of atypical cell foci and renal tubular cell tumours in
rats exposed to n-ethyl-n-hydroxyethylnitrosamine. Cancer Research,
49: 6369-6378.
SHORT, G.B. & SWENBERG, J.A. (1988). Pathologic Investigations of
the mechanism of unleaded gasoline-induced renal tumours in rats.
CIIT Activities, 8: 1-6.
STONARD, M.C., PHILLIPS, P.G.N., FOSTER, J.R., SIMPSON, M.G. & LOCK,
E.A. (1986). Alpha2u-globulin: Measurement in rat kidney following
administration of 2,2,4-trimethylpentane, Toxicology, 41: 161-168.
STONE, L.C., KANERVA, R.L., BURNS, J.L. & ALDEN, C.L. (1987a).
Decalin-induced nephrotoxicity: Light and electron microscopic
examination of the effects of oral dosing on the development of
kidney lesions in the rat. Food and Chemical Toxicology, 25:
43-52.
STONE, L.C., McCRACKEN, M.S., KANERVA, R.L. & ALDEN, C.L. (1987b).
Development of a short-term model of decalin inhalation
nephrotoxicity in the male rat. Food and Chemical Toxicology, 25:
35-41.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumour
response in strain A mice. Cancer Research, 33: 3069-3085.
SWENBERG, J.A. (1991). Editorial. Risk assessment of chemicals
causing alpha2u-globulin nephropathy. Reg. Toxicol. Pharmacol.,
13: 1-2.
SWENBERG, J.A., SHORT, B., BORGHOFF, S., STRASSER, J. & CHARBONNEAU,
M. (1989). The comparative pathobiology of alpha2u-globulin
nephropathy. Toxicology and Applied Pharmacology, 97: 35-46.
THELESTAM, M., CURVALL, M. & ENZELL, C. (1980). Effect of tobacco
smoke compounds on the plasma membrane of cultured human lung
fibroblasts. Toxicology, 15: 203-217.
THOMAS, T.L., WAXWEILER, R.J., MOURE-ERASO, R., ITAYA, S. &
FRAUMENI, Jr., J.F. (1982). Mortality patterns among workers in
three Texas oil refineries. Journal of Occupational Medicine, 24:
135-141.
TRUMP, B.F., BEREZESKY, I.K., SATO, K. & SAIKO, K.U. (1984). Cell
calcium, cell injury, and cell death. Environ.Hlth. Revs., 57:
281-287.
TSUJI, M., FUJISAKI, Y., YAMACHIKA, K. NAKAGAMI, K., FUJISAKI, F.,
MITO, M., AOKI, T., KINOSHITA, S., OKUBO, A. & WATANABE, I. (1974).
Studies on d-limonene as gallstone solubilizer; I. General
pharmacological studies. Oyo Yakuri (Pharmacometrics), 8:
1439-1459.
TSUJI, M., FUJISAKI, Y., ARIKAWA, Y., MASUDA, S., KINOSHITA, S.,
OKUBO, A., NODA, K., IDE, H. & IWANAGA, Y. (1975a). Studies on
d-limonene, as gallstone solubilizer (II): Acute and subacute
toxicities. Oyo Yakuri (Pharmacometrics), 9: 387-401.
US EPA (1991). Alpha2u-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Risk
Assessment Forum, US Environmental Protection Agency, Washington,
DC, USA, EPA/625/3-91/019F.
VAN DUUREN, B.L. & GOLDSCHMIDT, B.M. (1976). Co-carcinogenic and
tumour-promoting agents in tobacco carcinogenesis. Journal of the
National Cancer Institute, 56: 1237-1242.
WADE, A.P., WILKINSON, G.S., DEAN, F.M. & PRICE, A.W. (1966). The
isolation, characterization and structure of uroterpenol, a
monoterpene from human urine. Biochem. J., 101: 727-734.
WATABE, T., HIRATSUKA, A., ISOBE, M. & OZAWA, N. (1980). Metabolism
of d-limonene by hepatic microsomes to non-mutagenic epoxides
toward Salmonella typhimurium. Biochemical Pharmacology, 29:
1068-1071.
WEBB, D.R., KANERVA, R.L., HYSELL, D.K., ALDEN, C.L. &
LEHMAN-MCKEEMAN, L.D. (1990). Assessment of the subchronic oral
toxicity of d-limonene in dogs. Fd. Chem. Toxic., 28: 669-675.
WEBB, D.R., RIDDER, G.M. & ALDEN, C.L. (1989). Acute and subacute
nephrotoxicity of d-limonene in Fischer 344 rats. Fd. Chem.
Toxicol., 27: 639-649.
WEN, C.P., ISAI, S.P., MOFFITT, K.B., BONDY, M. & GIBSON, R.L.
(1984). Chapter XVII: Epidemiologic studies of the role of gasoline
(hydrocarbon) exposure in kidney cancer risk. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 245-257, Princeton
Scientific, Princeton, NJ.
WONG, O. & RAABE, G. (1989). Critical review of cancer epidemiology
in petroleum industry employees, with a quantitative meta-analysis
by cancer site. American Journal of Industrial Medicine, 15:
283-310.
See Also:
Toxicological Abbreviations
Limonene (CICADS 5, 1998)
 Wade et al. (1966) reported that dietary limonene gives rise
to uroterpenol (M-II: p-menth-1-ene-8,9-diol) in the urine of
humans.
2.1.3 Effects on enzymes and other biochemical parameters
Groups of control (6 animals) and experimental (4 animals) male
Wistar rats were administered single gavage doses of 0, 200, 400,
600, 800, or 1200 mg/kg bw d-limonene in 2% tragacanth solution in
a total volume of 4 ml/kg. Authors reported that no effects were
observed on liver triglycerides, microsomal proteins, cytochrome b5
and drug metabolizing enzymes.
In the same experiment, male Wistar rats were treated with
gavage doses of 0 or 400 mg/kg bw d-limonene in 2% tragacanth
solution in a total volume of 4 ml/kg for 2, 3, 15 or 30 days;
animals were killed 24 h following the last dose. Authors reported
that, following repeated treatment for 30 days, relative liver
weight and hepatic phospholipid content were slightly increased, and
liver and serum cholesterol were decreased 49% and 8%, respectively.
In addition, palmitic, linoleic and arachidonic acids were
increased, and stearic acid was decreased in the liver; aminopyrine
demethylase and aniline hydroxylase were increased 26% and 22%,
respectively, and cytochrome P-450 and b5 were increased by 31% and
30%, respectively (Ariyoshi et al., 1975).
d-Limonene was reported to enhance bile flow in Wistar rats
and mongrel dogs in a dose-related manner, and to decrease the ratio
of biliary bile salts and phospholipids to cholesterol (Kodama
et al., 1976). d-Limonene was reported to be an effective
gallstone solubilizer in animals and humans (Igimi et al., 1976;
Schenk et al., 1980), and to have no effect on liver weights nor
levels of serum lipids when fed to male Wistar rats at 0.5 and 1% in
the diet (Imaizumi et al., 1985).
d-Limonene was reported to significantly damage and increase
permeability of membranes of human lung fibroblasts (Thelestam
et al., 1980; Curvall et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Table 1: Summary of acute toxicity studies with d-limonene
Species Sex Route LD50 Reference
Mouse M&F oral, in 6.3 ml/kg (M) Tsuji et
juice, 7d 8.1 ml/kg (F) al., 1974
Mouse M&F ip, in 3.7 ml/kg (M) Tsuji et
juice, 3d 3.6 ml/kg (F) al., 1974
Mouse M&F ip, in 0.7 ml/kg (M) Tsuji et
juice, 10d 0.6 ml/kg (F) al., 1974
Mouse M&F sc, in >25.6 ml/kg (M) Tsuji et
juice, 7d >25.6 ml/kg (F) al., 1974
Mouse M&F oral 5600 mg/kg (M) Tsuji et
6600 mg/kg (F) al., 1975a
Mouse M&F ip 1300 mg/kg (M) Tsuji et
1300 mg/kg (F) al., 1975a
Mouse M&F sc >41500 mg/kg (M) Tsuji et
>41500 mg/kg (F) al., 1975a
Rat M&F oral 4400 mg/kg (M) Tsuji et
5100 mg/kg (F) al., 1975a
Rat M&F ip 3600 mg/kg (M) Tsuji et
4500 mg/kg (F) al., 1975a
Rat M&F sc >20200 mg/kg (M) Tsuji et
>20200 mg/kg (F) al., 1975a
An oral dose of 3 ml d-limonene (in juice)/kg decreased
spontaneous motor activities in rats and mice and potentiated
hexobarbital-induced sleeping and hypothermia in mice. Authors also
reported that nicotine-induced convulsion and death (but not maximum
electroshock-, pentetrazol-, strychnine-, nor picrotoxin-induced
convulsions) were inhibited by d-limonene in mice. Intravenous
administration of > 0.005 mg/kg d-limonene lowered the blood
pressure of rabbits and dogs, and > 0.1-0.3 mg/kg killed those
animals. Oral administration of 3 ml/kg d-limonene did not
decrease the blood pressure of rats, however. Authors also reported
that isolated smooth muscles of the intestine, vas deferens, uterus,
and peripheral vessel were constricted by d-limonene (Tsuji
et al., 1974). Single high s.c. doses of d-limonene produced
scratch behaviour and single high i.v. doses of d-limonene
produced stretch behaviour in mice and rats (Tsuji et al., 1975a).
2.2.2 Short-term studies
2.2.2.1 Mice
Groups of 5 B6C3F1 male and female mice were given daily
gavage doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg
bw in 10 ml/kg bw corn oil 5 days/week over a 16 day period (12
total dosings). All mice (5/5) exposed to 6600 mg/kg bw/day, 4/5
males and 5/5 females exposed to 3300 mg/kg bw/day, and 1/5 males
and 1/5 females exposed to 1650 mg/kg bw/day died before the end of
the study. The one female that died at the latter dose level was
reported to have been killed by a gavage error. Authors concluded
that no compound-related clinical signs were observed in mice that
received 1650 mg d-limonene/kg bw and lived to the end of the
study, and that no compound-related histopathologic effects were
seen (NTP, 1990).
Groups of 10 B6C3F1 mice of each sex were given daily
gavage exposures of 0, 125, 250, 500, 1000 or 2000 mg
d-limonene/kg bw in 10 ml/kg bw corn oil 5 days/week for 13 weeks.
One male exposed to 2000 mg/kg bw/day, two females exposed to
2000 mg/kg bw/day, and one female exposed to 500 mg/kg bw/day, died
during the course of the study. In addition, one female exposed to
125 mg/kg bw/day and five males (one each from the groups exposed to
250 and 1000 mg/kg bw/day, and three from the group exposed to
500 mg/kg bw/day) died prior to the end of the study, but their
deaths were attributed by the authors to accidents related to the
gavage procedure used. At the two highest dose levels, mice were
seen with rough hair coats and decreased activity. An alveolar cell
adenoma was observed in the lung of one female mouse that had
received 2000 mg d-limonene/kg bw/day (NTP, 1990).
2.2.2.2 Rats
Groups of 5 F344/N rats of each sex were given daily gavage
doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg bw in
10 ml/kg bw corn oil 5 days/week over a 16 day period (12 total
doses). All rats receiving 6600 mg d-limonene/kg bw/day as well as
5/5 male and 3/5 female rats that were exposed to 3300 mg/bw/day
died. The authors reported that no clinical signs were seen in rats
receiving 1650 mg d-limonene/kg bw/day or lower, and that no
compound-related histopathologic effects were seen in any rats (NTP,
1990).
Groups of 5 male Fischer-344 rats were given single daily
gavage doses containing 75, 150 or 300 mg d-limonene/kg bw in corn
oil (5 ml/kg bw) 5 days/week for 5 or 20 doses, primarily to follow
the development of renal alterations. The authors reported that no
signs of gross toxicity were observed nor was there any effect on
weight gain or feed consumption over the course of this experiment.
However, after only 5 days of exposure, liver and kidney weights
showed a dose-related increase and there was a dose-related increase
in the number and size of hyaline droplets and accumulation of
alpha2u-globulin in the proximal convoluted tubule epithelial
cells of the kidneys. In addition, after 20 doses, granular casts in
the outer zone of the medulla and multiple cortical changes
(classified as chronic nephrosis) were also seen in dose-related
severity (Kanerva et al., 1987a).
In a subacute study, groups of 5 male and female rats were
exposed daily to 0, 277, 554, 1385 or 2770 mg d-limonene/kg bw
orally for 30 days. A general decrease in food intake and a
dose-related decrease in body weight were seen in groups of exposed
males, but little or no effect on organ weights nor relative organ
weights was observed. No significant changes were seen in
urinalysis, haematology or biochemical values. The following tissues
were examined histopathologically: adrenals, duodenum, heart,
kidneys, liver, lungs, lymph nodes, pancreas, pituitary, spleen,
stomach, testes/ovaries, thymus and thyroids. Authors reported that
no significant changes were noted except that granular casts were
seen in the kidneys of most exposed male rats (0/5, 3/5, 5/5, 5/5 or
4/5 animals exposed to 0, 277, 554, 1385 or 2770 mg/kg bw/day
respectively) (Tsuji et al., 1975a).
Groups of 10 F334/N rats of each sex were given daily gavage
doses of 0, 150, 300, 600, 1200 or 2400 mg d-limonene/kg bw in
5 ml/kg bw corn oil 5 days/week for 13 weeks. Five male and nine
female rats exposed to 2400 mg d-limonene/kg bw/day died during
the course of the study. In all rats receiving 1200 or 2400 mg/kg
bw/day, rough hair coats, lethargy and excessive lacrimation were
observed. Authors reported that nephropathy was seen in all groups
of male rats, with a dose-related increase in severity. The
nephropathy was reported to be characterized by degeneration of
epithelium in the convoluted tubules, granular casts within tubular
lumens (primarily in the outer stripe of the outer medulla), and
regeneration of the tubular epithelium. In all groups of male rats
(including control animals), hyaline droplets were observed in the
epithelium of proximal convoluted tubules in at least some animals.
An attempt to determine by "blind" evaluation if the incidence of
these droplets was dose-related was reported to have resulted in an
equivocal conclusion.
The slides containing the renal sections made from male rats
exposed to d-limonene in this 13 week study were reviewed. They
reported that hyaline droplet accumulation within the cytoplasm of
proximal convoluted tubule (PCT) epithelial cells was uniform among
all groups, including controls. However, chronic nephrosis, although
seen in all animals (including control animals), appeared with a
dose-related increase in severity. The authors attributed the
uniformity of hyaline droplet accumulation to the fact that the
animals were not exposed to d-limonene for 4-5 days before
necropsy and examination, giving the animals time to rid themselves
of some of the droplets, although more extensive damage to the
kidneys (chronic nephrosis) was not repaired (Kanerva & Alden 1987).
Chronic nephrosis consisted of cytoplasmic basophilia of the
PCT epithelial cells, tubular hyperplasia or atrophy, fibrosis of
Bowman's capsule and an interstitial fibrolymphocytic response.
Atrophic tubules were characterized by thickened basement membranes
and a decrease in tubular cross-sectional diameter due to a decrease
in cell size, whereas tubules considered to be hyperplastic showed
increases in cell size and number and an increase in cross-sectional
diameter compared with the surrounding unaffected tubules. In
addition, occasional foci of PCT epithelial cell
necrosis/degeneration were observed in kidneys from all
d-limonene-treated animals but not in control animals. All treated
animals, but not controls, also showed a dose-related increase in
the number of granular casts found in the outer stripe of the
medulla. Granular casts were markedly dilated and had attenuated or
non-existent epithelium. In addition, animals exposed to the highest
dose level (2400 mg/kg bw/day) were reported to show multifocal
hyaline casts and tubular dilation in the cortex and medulla (NTP,
1990).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
To assess the pulmonary tumour carcinogenicity of d-limonene,
groups of 15 male or female A/He mice were injected i.p. with
d-limonene 3 times weekly for 8 weeks (4.8 or 24 g/kg bw total
dose) and then necropsied 24 weeks after the first injection. No
significant induction of lung tumours was seen (Stoner et al.,
1973).
Groups of 50 8-9 week old male B6C3F1 mice were given
daily gavage doses of 0, 250, or 500 mg d-limonene/kg bw and
groups of 50 8-9 week old female B6C3F1 mice were given 0,
500, or 1000 mg d-limonene/kg bw in 10 ml/kg bw corn oil
5 days/week for 103 weeks. Mice were observed twice a day and
weighed weekly for the first 12 weeks, after which they were weighed
monthly. Clinical signs were recorded at least monthly. Moribund
animals were killed and a necropsy was performed on all available
animals. The tissues of all mice that died during the study and
animals with grossly visible lesions, as well as all animals in the
control and high dose groups, were examined for histopathology.
The authors reported that no clinical signs were associated
with administration of the test chemical. The survival of male mice
exposed to 250 mg d-limonene/kg bw/day was lower than that of
controls at the end of the study (controls: 33/50; 250 mg/kg bw/day:
24/50; 500 mg/kg bw/day: 38/50). No other differences in survival
were seen among exposed groups of either sex. Mean body weights of
exposed and control male mice were generally comparable. Weights of
female mice in the 1000 mg d-limonene/kg bw/day group, however,
were 5-15% lower than control animals after week 28.
Multinucleated liver hepatocytes and cells with cytomegaly
occurred at increased incidence in male mice exposed to 500 mg
d-limonene/kg bw/day (multinucleated hepatocytes: controls - 8/49;
250 mg/kg bw/day - 4/36 [incomplete sampling]; 500 mg/kg bw/day -
32/50) (hepatocytes with cytomegaly: controls - 23/49; 250 mg/kg
bw/day - 11/36 [incomplete sampling]; 500 mg/kg bw/day - 38/50). The
combined incidence of hepatocellular adenomas and carcinomas,
however, did not differ significantly from their incidence in
control mice (controls: 22/49; 250 mg/kg bw/day: 14/36 [incomplete
sampling]; 500 mg/kg bw/day: 15/50). No significant differences were
observed in female mice for any of these endpoints.
The incidence of adenomas or the combined incidence of adenomas
and carcinomas in the anterior pituitary gland of female mice
exposed to 1000 mg d-limonene/kg bw/day were significantly lower
than controls. The incidence of hyperplasia did not differ
significantly from controls, however (hyperplasia: controls - 16/49;
500 mg/kg bw/day - 0/8 [incomplete sampling]; 1000 mg/kg bw/day -
17/48) (adenomas: controls - 12/49; 500 mg/kg bw/day - 5/8
[incomplete sampling]; 1000 mg/kg bw/day - 1/48) (combined adenoma
and carcinoma: controls - 12/49; 500 mg/kg bw/day - 5/8 [incomplete
sampling]; 500 mg/kg - 2/48) (NTP, 1990).
2.2.3.2 Rats
Groups of fifty 7-8 week old male F344/N rats were given daily
gavage doses of 0, 75, or 150 mg d-limonene/kg bw and groups of 50
7-8 week old female F344/N rats were given 0, 300, or 600 mg
d-limonene/kg bw in 5 ml/kg bw corn oil 5 days/week for 103 weeks.
Rats were observed twice a day; they were weighed weekly for the
first 12 weeks, and monthly thereafter. Clinical signs were recorded
at least monthly. Moribund animals were killed; necropsies were
performed on all available animals. The tissues of all rats that
died during the study, animals with grossly visible lesions, and all
animals in the control and high dose groups were examined for
histopathology.
No compound-related clinical signs were reported. The survival
of the female animals exposed to 600 mg/kg bw was lower than that of
control animals after week 39, while the survival of male rats
exposed. Mean body weights of male rats exposed to 150 mg/kg bw were
generally 4%-7% lower than controls from week 28.
Cataracts were observed at increased incidence in male rats
exposed to 150 and female rats exposed to 300 or 600 mg
d-limonene/kg bw/day, and retinal degeneration was seen in all
dosed animals. The authors pointed out, however, that the rats of
each sex that were exposed to their respective highest-dose levels
were housed in the top rows of the cage racks, the animals exposed
to the low-dose levels were below them, and the controls were housed
in the lowest rows for all but the last 10 weeks of the study.
Authors conclude that these effects were due to variations in the
proximity of animals in different dose groups to the light source.
Subcutaneous tissue fibromas in male rats occurred with a
negative dose-response trend (controls: 8/50; 75 mg/kg bw/day: 2/50;
150 mg/kg bw/day: 3/50). However, the combined incidence of fibromas
and fibrosarcomas was not significantly different when dosed males
were compared to controls (controls: 8/50; 75 mg/kg bw/day: 4/50;
150 mg/kg bw/day: 3/50).
Combined squamous cell papillomas or carcinomas in the skin of
male rats showed a positive dose-response trend (controls: 0/50;
75 mg/kg bw/day: 0/50; 150 mg/kg bw/day: 3/50). However, the
incidence of these neoplasms in dosed male rats was within the range
of incidences for NTP historical control rats, and did not differ
significantly from the incidence in control animals. The authors
concluded that the positive dose-response trend was not related to
d-limonene exposure.
Mononuclear cell leukaemia occurred in male rats with a
positive dose-response trend by the incidental tumour test
(controls: 10/50; 75 mg/kg bw/day: 10/50; 150 mg/kg bw/day: 19/50).
The incidences in dosed male rats were not significantly different
from the incidences in controls, however. The authors concluded that
the positive dose-response trend was not related to d-limonene
administration.
There was a significant positive dose-response trend in the
incidence of interstitial cell tumours in the testes of male rats,
and the incidences of these tumours in dosed groups were
significantly higher than the incidences in control animals
(control: 37/50; 75 mg/kg bw/day: 47/49; 150 mg/kg bw/day: 48/50).
The authors argued, however, that since historically this type of
tumour occurs in almost all aged F344 rats, this result should not
be considered to be related to d-limonene exposure, but rather was
an artifact caused by the low survival of the control animals in
this experiment (see above).
The incidence of uterine endometrial stromal polyps in females
exposed to 300 mg/kg bw/day was increased when compared to controls.
However, the incidence in the control animals was well below the NTP
historical incidence of this tumour. For this reason, and because
there was no positive dose-response trend, the authors argued that
this was not a compound-related effect.
d-Limonene exposure of male rats was associated with
dose-related increases in the incidences of mineralization and
epithelial hyperplasia in the kidneys (mineralization: controls -
7/50; 75 mg/kg bw/day - 43/50; 150 mg/kg bw/day - 48/50) (epithelial
hyperplasia: controls - 0/50; 75 mg/kg bw/day - 35/50; 150 mg/kg
bw/day - 43/50). The lesions consisted of linear deposits of mineral
in the medulla (renal papilla) and focal hyperplasia in the
transitional epithelium overlying the papilla. Hyperplasia was
frequently located near the fornices of the renal pelvis and was
occasionally bilateral. The severity of nephropathy, which occurs
spontaneously in aged male rats, was also positively dose-related in
this experiment (severity of nephropathy, rated on a scale from 0
[not present] to 4 [marked]: control mean - 1.5; 75 mg/kg bw/day
mean - 1.8; 150 mg/kg bw/day mean - 2.2). This nephropathy was
characterized by degeneration and atrophy of the tubular epithelium,
dilation of tubules with formation of hyaline droplets and granular
casts, regeneration of tubular epithelium, glomerulosclerosis, and
interstitial inflammation and fibrosis.
Kidney tubular cell hyperplasia and neoplasms were also
increased in dosed male rats (hyperplasia: controls - 0/50; 75 mg/kg
bw/day - 4/50; 150 mg/kg bw/day - 7/50) (adenomas: controls - 0/50;
75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 8/50) (adenocarcinomas:
controls - 0/50; 75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 3/50)
(combined adenomas and carcinomas: controls - 0/50; 75 mg/kg bw/day
- 8/50; 150 mg/kg bw/day - 11/50). Incidences of tubular cell
adenomas and the combined incidences of adenomas and
adeno-carcinomas in male rats showed significant positive
dose-response trends. High-dose males had a significantly higher
incidence than control males of both categories of tumours, while
low-dose males had a significantly higher incidence of the combined
category than control males. These neoplasms are historically rare
and none were seen in control males nor in any group of females.
The authors stated that kidney tubular cell hyperplasia,
adenomas, and adenocarcinomas were part of a morphologic spectrum.
Proliferative lesions diagnosed as hyperplasia generally consisted
of one to three adjacent cross-sections of enlarged tubules with
stratification of the epithelium. In some lesions, the epithelial
cells appeared to completely fill the lumen. The tubular cell
neoplasms varied in diameter from less than 1 mm to 15 mm. They
exhibited varied patterns of growth and were either solid, cystic,
or papillary. The solid and cystic neoplasms showed little evidence
of tubular structures and the cells were arranged in solid sheets or
small solid nests separated by a delicate vascular stroma. Some
neoplasms were reported to consist of layers of epithelium lining a
fibrous connective tissue stroma and were arranged in complex
branching papillary formations. The neoplasms were classified as
adenocarcinomas primarily if they exhibited cellular pleomorphism
and anaplasia, or if they were larger than 10 mm (NTP, 1990).
2.2.3.3 Dogs
Groups of 3 male or female Japanese beagle dogs were exposed
orally to 0, 0.4, 1.2 or 3.6 ml d-limonene/kg bw daily for 6
months. Dose-related occurrences of frequent vomiting and nausea
were seen in some animals. At doses of at least 1.2 ml/kg bw/day for
females and 3.6 ml/kg bw/day for males, a decrease in body weight
gain was noted compared to controls, but little or no effect on food
intake, organ weights or relative organ weights was reported. No
significant changes were seen in urinalysis, haematology nor
biochemical values, except that in animals at the 3.6 ml/kg bw/day
level, total cholesterol and blood sugar were lowered. The following
tissues were examined histopathologically: adrenals, bile duct,
duodenum, heart, kidneys, liver, lungs, pancreas, pituitary, rectum,
spleen, stomach, testes/ovaries, thymus and thyroids. No significant
changes were noted except that granular casts were seen in the
kidneys of all male dogs exposed to 3.6 ml/kg bw/day and all females
exposed to 0.4 ml/kg bw/day or more (1/3, 1/3, 2/3 or 3/3 for males;
1/3, 2/2, 3/3 or 3/3 for females exposed to 0, 0.4, 1.2 or 3.6 ml/kg
bw, respectively) (Tsuji et al., 1975b).
In a more recent study, groups of five male and female beagle
dogs were gavaged twice daily for six months with 0, 0.12, or
1.2 ml/kg bw/day (0, 10, or 1000 mg/kg bw/day) d-limonene. The
highest dose was reported to be near the maximum tolerated dose for
emesis. The authors stated that food consumption and body weight
were not affected by treatment. Although there was a positive
dose-related trend for absolute and relative female kidney weights
and for relative male kidney weight, authors reported that there
were no histo-pathological findings in the kidneys that could be
associated with the organ weight changes. In addition, there was no
hyaline droplet accumulation nor other indication of nephropathy
such as those associated with d-limonene consumption in male rats
(Webb et al., 1990)
2.2.3.4 Cocarcinogenicity/tumour promotion studies
Two publications have reported the results of experiments in
which 0.25 ml oil of sweet orange, composed of > 90% d-limonene,
was applied to the skin of 10 male and 10 female "101" mice weekly
for 42 weeks, starting 3 weeks after an application of 300 µg
7,12-dimethyl-1,2-benzanthracene (DMBA) in 0.2 ml acetone. While no
effects in the area of the treated skin were seen in mice exposed to
d-limonene or to DMBA alone, mice exposed to DMBA followed by
exposure to d-limonene began to develop papillomas during the
twelfth week of d-limonene exposure. After 33 weeks of treatment,
13/18 treated mice showed a total of 39 papillomas. No malignant
tumours were seen in the exposed area, however.
In a second study, mice were exposed to d-limonene following
exposure to: 1) 4 skin applications of 60 mg urethane in 0.3 ml
acetone at 3 day intervals, or 2) 4 i.p. injections of 16 mg
urethane in 0.1 ml distilled water at 3 day intervals. Papillomas
appeared on 3/13 mice in group 1 and 2/13 mice in group 2 by the
fourteenth week of exposure.
In a final experiment, the terpene fraction of oil of sweet
orange (containing d-limonene) was isolated and tested with DMBA
as described for the first experiment. Papillomas in mice exposed to
both DMBA and the terpene fraction of oil of sweet orange began to
appear in the eleventh week of treatment and were visible in 8/15
mice by the thirty-third week of treatment. Although pure
d-limonene was not tested in these experiments, the authors
concluded that the promoting effects seen were probably caused by
this chemical since it was the primary constituent of the test agent
(Roe, 1959, Roe & Peirce, 1960).
A group of 23 male and female albino mice were given single
doses of 50 µg benzo(a)pyrene (BAP) by stomach tube followed by 40
weekly doses of 0.05 ml d-limonene; seventeen male and female mice
received BAP only, 15 male and female mice received d-limonene
only and 18 male and female mice served as untreated controls. While
no tumours of the forestomach epithelium were seen in control
animals, 2 animals treated with BAP alone (12%), 2 animals treated
with d-limonene alone (13%), and 5 animals treated with both BAP
and d-limonene (22%), developed 0, 2, 3, and 8 tumours,
respectively, although none was judged to be a carcinoma. The
authors concluded that although d-limonene was weakly carcinogenic
for mouse forestomach epithelium it did not increase the tumour
yield caused by pretreatment with BAP and, therefore, was not a
promoter (Field & Roe, 1965).
Cells of embryos taken from F344 rats were infected with
Rauscher murine leukaemia virus and then treated with 0.05 µg/ml
3-methylcholanthrene (3-MCA) followed by 15 µg/ml d-limonene.
Following treatment with 3-MCA and d-limonene, colony formation in
semisolid medium was observed and was considered to denote cell
transformation. Authors reported that d-limonene alone failed to
transform cells and 3-MCA alone was only effective at concentrations
exceeding 0.2 µg/ml (Roe, 1959; Roe & Peirce, 1960; Traul et al.,
1981)).
Elegbede and co-workers attempted to repeat the 1960 study of
Roe and Peirce (see above) using purified d-limonene as well as
oil of sweet orange. The skin of groups of 24 female CD-1 mice were
treated once with 51.2 µg DMBA in 0.2 ml acetone followed 14 days
later by twice weekly applications of 0.1 ml d-limonene or oil of
sweet orange mixed with 0.1 ml acetone. Other groups of mice were
similarly initiated with DMBA, but beginning 7 days later were given
a diet containing 1% d-limonene or oil of sweet orange. In all
cases, administration of d-limonene or oil of sweet orange
continued for 40 weeks after DMBA treatment. Topical (but not
ingested) d-limonene was found to slightly increase the number of
tumours induced by DMBA alone, the increase becoming significant
only after 34 weeks of treatment. When oil of sweet orange (95%
d-limonene content) was applied to the skin, authors reported that
a promoting effect was seen after 7 weeks of treatment; promotion
was not reported, however, when oil of sweet orange was incorporated
into the diet. The authors concluded that a component of orange oil
other than d-limonene was responsible for the promoting activity
observed and that the very small promoting activity seen with
d-limonene may be due to its contamination by this unidentified
component (Elegbede et al., 1986b).
2.2.3.5 Anticarcinogenicity studies
A group of 50 male C57Bl/6J mice were injected s.c. with 25 µg
dibenzpyrene (DBP) followed 24 h later by an injection of 0.15 ml
d-limonene. After 34 weeks of observation, the authors reported
that d-limonene significantly delayed the appearance of lung
adenomas induced by DBP and reduced the total number of tumours
seen. At 30 weeks post-DBP exposure without subsequent exposure to
d-limonene, lung adenomas were seen in approximately 70% of the
animals; with exposure to d-limonene, tumours were seen in
approximately 30% of the animals.
In a second experiment reported in the same paper, a group of
50 female A/J mice were injected s.c. with DBP followed by 16 weekly
injections into the tail vein of 1% v/v suspensions of 1 mg of
d-limonene. The incidence of lung adenomas was reduced from 75% of
animals exposed to DBP alone to 40% of animals exposed to DBP and
injected with d-limonene. Control incidence of lung adenomas in
this experiment was 27%. Treatment of mice with d-limonene alone
reduced this background incidence to approximately 7% (Homburger
et al., 1971).
Simultaneously, 5 µg of BAP and 10 mg d-limonene were applied
to the skin of groups of 50 female ICR/Ha Swiss mice three times a
week for 440 days. d-Limonene was found to partially inhibit BAP
carcinogenicity (Van Duuren & Goldschmidt, 1976).
Female Sprague-Dawley rats were fed diets containing 0, 1000,
or 10 000 ppm d-limonene for 1 wk, at which time they were given a
single gavage dose of 65 mg DMBA/kg bw. The d-limonene-containing
diet was then continued for 27 weeks. Authors reported that
d-limonene delayed the appearance of DMBA-induced mammary tumours
in a dose-related manner, reduced the incidence of tumours, and
caused increased regression of tumours that did appear (Elegbede
et al., 1984a,b).
Female (W/Fu x F344)F2 rats were fed a diet containing 10%
d-limonene for 80 days beginning after the first appearance of
tumours induced by intubation of rats with 130 mg DMBA/kg bw.
Significant tumour regressive action by d-limonene was observed,
and the development of subsequent tumours was also reduced
significantly (Elegbede et al., 1986a).
Following initiation with DMBA (single gavage dose of 65 mg/kg
in sesame oil), groups of female Sprague-Dawley rats were (1) fed a
basal cereal diet one week before and 24 weeks following initiation
with DMBA (controls), (2) fed a diet containing 5% d-limonene one
week before and one week after treatment with DMBA, then fed the
control diet for 24 weeks (initiation group), or (3) fed the control
diet for one week before and one week following DMBA initiation,
then fed the 5% d-limonene diet for 24 weeks
(promotion/progression group). d-Limonene was reported to be
effective in reducing the average number of rat mammary carcinomas
in DMBA-initiated rats when fed during the initiation phase or
during the initiation/progression phase of carcinogenesis. Time to
appearance of the first tumour was extended only when d-limonene
was fed during the initiation phase, however. Authors concluded that
these effects could not be attributed to changes in mammary-relevant
endocrine functions (serum levels of prolactin and duration of
estrus cycle) (Elson et al., 1988).
Female Wistar-Furth rats were (1) placed on diets containing 5%
d-limonene or 5% orange oil (source of d-limonene) for 2 weeks
prior to initiation with nitrosomethylurea (NMU; single i.v. dose of
50 mg/kg bw), then continued on these diets for 23 weeks; (2) fed
diets containing 5% d-limonene for two weeks before and one week
following initiation with NMU, then fed a basal diet for 22 weeks
(initiation group); or (3) fed basal diets prior to and for 1 week
following initiation with NMU, then fed diets containing 5%
d-limonene for 22 weeks (promotion/progression group). Both orange
oil and d-limonene decreased mammary tumour incidence (controls:
80%; d-limonene: 45%; orange oil: 47%) and the average number of
mammary tumours per rat (controls: 1.8; d-limonene: 0.4; orange
oil: 0.8) when present in the diet for two weeks before and 23 weeks
after NMU administration. d-Limonene decreased tumour incidence
and decreased the numbers of tumours per rat (by approximately 50%)
when fed during the promotion/progression experiment but not the
during the initiation experiment (Maltzman et al., 1989).
2.2.3.6 Studies on the mechanism of d-limonene carcinogenicity
This section discusses (1) spontaneously occurring nephropathy
in mature male rats and (2) the current proposed mechanism of
carcinogenicity of d-limonene and other chemicals/mixtures that
exacerbate this condition and lead to alpha2u-globulin-associated
male rat nephropathy.
(1) Mature male rat nephropathy
Normal male rats at least 30 days old exhibited marked
increased protein excretion in their urine not seen in younger males
nor in females of any age. This proteinuria reached a maximum level
of approximately 2.5-3 times that seen in females at 90 days of age.
Microscopically, the authors also observed intracellular droplets in
the epithelial cells of the upper two-thirds of the proximal
convoluted tubules of kidneys of male rats at least 60 days old. The
occurrence of these hyaline droplets consisted of a few groups of
small droplets in occasional cells in 60 day old animals. The number
of cells having droplets and the number and size of droplets per
cell increased with age; from 90 days of age most proximal tubule
cells contained them. In contrast, at 120 and 180 days of age, only
a small number of females showed occasional droplets in a few cells
of the proximal tubules.
These authors further demonstrated the sex-relatedness of this
phenomenon by castrating male rats at 30 days of age and injecting
female rats with 5 mg testosterone every other day from 50 days of
age. When examined at 120 days of age, the castrated male rats
showed proteinuria at a level equal to control females and hyaline
droplets were only seen in 4/20 animals. Testosterone-treated
females, on the other hand, had proteinuria at a level approximately
midway between control males and females. No hyaline droplets were
seen in the proximal tubule cells of these females, however, showing
that the addition of testosterone alone was insufficient to cause
them to appear (Logothetopoulos & Weinbren, 1955).
Numerous authors have since reported detailed descriptions of
spontaneously occurring nephropathy in mature male rats which is a
consequence of this hyaline droplet accumulation. The sequence of
events appears to be that: (1) protein accumulates in the lysosomes
in the cytoplasm of epithelial cells of the P2 segment of the
proximal convoluted tubules in the kidney cortex; (2) the
accumulated material becomes so abundant that it crystallizes,
forming the microscopically visible hyaline droplets; (3) the
continued build-up of material eventually leads to the death of
epithelial cells with a concomitant thinning of the epithelial layer
(although some regeneration is usually seen); and (4) the dead cell
debris becomes lodged in the outer strip of the outer medulla where
the tubules narrow, forming granular casts and causing tubule
dilation with pressure necrosis of the cells of the tubule walls.
There is also an increase in the relative weight of the kidneys
accompanying this phenomenon.
A steadily increasing number of agents have been identified
that appear to exacerbate this hyaline droplet formation and its
consequences in mature male rats. With some, noticeable changes
occur within days of exposure. These agents include complex mixtures
of hydrocarbons such as mineral spirits (Carpenter et al., 1975a;
Phillips & Cockrell, 1984), "60" solvent (Carpenter et al.,
1975b), "high naphthenic solvent" (Carpenter et al., 1977), the
petroleum-derived or shale-derived jet fuel JP-5 (MacEwen & Vernot,
1978a; Parker et al., 1981; Bruner, 1984; MacNaughton & Uddin,
1984), petroleum-derived and shale-derived diesel fuel marine (DFM),
the jet fuels JP-4, JP-TS and JP-7, and the missile propellants
JP-10 and RJ-5 (Bruner, 1984; MacNaughton & Uddin, 1984), C/10-C/11
isoparaffin (Phillips & Egan, 1984; Phillips & Cockrell, 1984), five
petroleum naphtha mixtures (Halder et al., 1984) and unleaded
gasoline (MacFarland et al., 1984; Busey & Cockrell, 1984; Halder
et al., 1984; Kitchen, 1984; Garg et al., 1988a,b, 1989; Short &
Swenberg, 1988; Short et al., 1989a). Also included on the list
are the individual chemicals decahydro-naphthalene (decalin, MacEwen
& Vernot, 1978b; Gaworski et al., 1980, 1981; Bruner, 1984; Alden
et al., 1984; Olson et al., 1986; Stone et al., 1987a,b;
Kanerva et al., 1987b,c; Read, 1988), pentachloroethane (NTP,
1983; Goldsworthy et al., 1988), 1,4-dichlorobenzene (NTP, 1987a;
Charbonneau et al., 1989), perchloroethylene (Goldsworthy et al.,
1988), dimethyl methylphosphonate (NTP, 1987b),
2,2,4-trimethylpentane (Stonard et al., 1986; Short et al.,
1987; Charbonneau et al., 1987; Lock et al., 1987; Loury
et al., 1987; Short et al., 1989a), the chemically unrelated
agents 3-methylamino-1-(3-trifluoromethyl-phenyl)-2-pyrazoline,
cis/trans-2-(4'-t-butylcyclohexyl)-3-hydroxy-1,4-naphthoquinone
and 2,3,5,6-tetrahycro-6-phenylimidazo-(2,1-b) thiazole (Read
et al., 1988) and d-limonene (Kanerva et al., 1987a; NTP,
1990).
There is no obvious common structure among the specifically
identified chemicals that cause an increased accumulation of hyaline
droplets in mature male rat kidneys. And there are examples of
agents that do have similar mixtures/structures to those listed
above that do not cause this phenomenon. These include "70" solvent
(Carpenter et al., 1975c), two other petroleum naphtha mixtures
(Halder et al., 1984), 1,2-dichlorobenzene (Charbonneau et al.,
1989) and an analog of d-limonene, 4-vinylcyclohexene (NTP, 1986).
Recently, however, Miller et al. (1989) reported that they have
identified correlations between structure and binding affinity to
alpha2u-globulin, but no specific details were given in their
published report.
While exposure to unleaded gasoline for 72 h has been shown to
cause effects that are reversible (Garg et al., 1988a), with
chronic exposure to several of these mixtures/chemicals the kidney
nephrosis has been reported to progress to hyperplasia, adenoma and
even adenocarcinoma (MacFarland et al., 1984; Kitchen, 1984;
MacNaughton & Uddin, 1984; NTP, 1987a,b,c; NTP, 1990).
(2) Mechanism for alpha2u-globulin-associated male rat
nephropathy
The mechanism proposed for alpha2u-globulin-associated male
rat nephrosis has recently been thoroughly reviewed by the US
Environmental Protection Agency (US EPA, 1991). Their review
incorporated the results of many studies, including Charbonneau &
Swenberg (1988), Swenberg et al. (1989), Flamm & Lehman-McKeeman
(1991), Lehman-McKeeman et al. (1989), Lehman-McKeeman et al.
(1990a,b), Short et al. (1987), Short et al. (1989a,b), Dietrich
& Swenberg (1991), Murty et al. (1988), Ridder et al. (1990),
and Webb et al. (1989). The review concluded that:
"Of the eight model substances tested in chronic animal
bioassays (decalin, dimethyl methyl phosphonate, JP-4 jet fuel,
JP-5 shale-derived jet fuel, d-limonene, methyl isobutyl
ketone, pentachloroethane, and unleaded gasoline), all invoked
a specific type of protein droplet nephropathy in male rats and
also produced renal tumours in male rats but not in other
species tested. It has been proposed that such renal tumours
are the end product in the following sequence of functional
changes in the epithelial cells of proximal tubules:
* Excessive accumulation of hyaline droplets in proximal
tubules, representing lysosomal overload, cell loss, and
regenerative cellular proliferation.
* Cell debris in the form of granular casts accumulates at
the 'corticomedullary' junction with associated dilation
of the affected tubule segment and more distally,
mineralization of tubules within the renal medulla.
* Single-cell necrosis accompanied by compensatory cell
proliferation and exacerbation of the chronic progressive
nephropathy characteristically found in aging rats occurs.
* Renal tubule hyperplasia and neoplasia develop
subsequently.
According to this hypothesis, the increased proliferative
response caused by the chemically induced cytotoxicity results
in clonal expansion of spontaneously initiated renal tubule
cells and increased incidence of renal tumour formation (Trump
et al., 1984a; Alden, 1989; Swenberg et al., 1989). This
line of reasoning leads supporters of the hypothesis to
conclude that the acute and chronic renal effects induced in
male rats by such chemicals will be unlikely to occur in any
species not producing alpha2u-globulin, or a very closely
related protein, in the large quantities typically seen in the
male rat (Alden, 1989; Borghoff et al., 1990; Green et al.,
1990; Olson et al., 1990; Flamm & Lehman-McKeeman, 1991;
Swenberg, 1991)."
The following paragraphs summarize some of the data that
support this hypothesis:
Contents and catabolism of hyaline droplets/
phagolysosomes
The composition of the hyaline droplet inclusions has been
identified by Kanerva et al. (1987c) using two-dimensional gel
electrophoresis and immunological binding as being four species of
the low molecular weight protein alpha2u-globulin. As expected,
alpha2u-globulin was found to be absent in immature male rats and
female rats of all ages. It was present, however, in female rats
that had been ovariectomized and repeatedly injected with
testosterone. More recently, groups have confirmed that
alpha2u-globulin is the major constituent of the renal
phagolysosomal inclusions in male rats exposed to unleaded gasoline
(Garg et al., 1988b), and perchloroethylene and penta-chloroethane
(Goldsworthy et al., 1988). Finally, Dietrich and Swenberg (1991)
reported that male NCI-Black-Reiter rats, which do not produce
alpha2u-globulin, are not susceptible to nephropathy produced by
2,2,4-trimethylpentane, 1,4-dichloro-benzene, isophorone, PS-6
unleaded gasoline, or d-limonene.
A sex-specific protein has been identified in human urine
(Bernard et al., 1989), but the concentration (up to 108 µg/l) is
about 10 000 times less than that of alpha2u-globulin in male rats
(about 40 mg/24 h) (Roy & Neuhaus, 1967). Therefore assuming that
the protein in human urine shows the same binding characteristics as
alpha2u-globulin, it is reasonable to conclude that humans would
be comparably less sensitive than the male rat.
Alpha2u-globulin is synthesized in the liver of rats of both
sexes. This protein is filtered by the kidney glomeruli and
reabsorbed by the epithelial cells of the proximal convoluted
tubules by the formation of phagosomes at the lumenal surface.
Normally, the phagosomes then combine with cellular lysosomes and
the protein is degraded. In mature male rats, however, this
catabolism is slowed in some way under hormonal influence leading to
the intracellular accumulation of the protein which becomes
manifested as hyaline droplets. The degradation is slowed even more
by exposure to at least some of the agents listed in the previous
section (Stonard et al., 1986; Short et al., 1987; Kanerva
et al., 1987c; Charbonneau et al., 1987). A time- and
dose-dependent progressive increase in the number and size, and
alterations in the morphology of phagolysosomes in proximal
convoluted tubule epithelial cells have been demonstrated with
exposure to unleaded gasoline (Garg et al,. 1989). The ability of
unleaded gasoline to induce these effects declines with the age of
the male rat, in proportion to the declining production of
alpha2u-globulin (Murty et al., 1988).
Binding of chemicals to alpha2u-globulin
1,4-Dichlorobenzene as well as its major metabolite,
2,5-dichlorophenol, were both found to be reversibly bound (and
1,4-dichlorobenzene also covalently bound) to alpha2u-globulin in
the kidneys of treated male rats (Charbonneau et al., 1989).
Similarly, two groups have attempted to identify the specific
binding between one of these agents (2,2,4-tri-methylpentane) and
alpha2u-globulin. Loury et al. (1987) reported that no covalent
binding could be found, nor did there appear to be formation of a
Schiff base. Lock et al. (1987), however, detected reversible
binding of the metabolite 2,4,4-trimethyl-2-pentanol to
alpha2u-globulin. This specific metabolite was found in liver
cells of male rats only.
Likewise with decalin, an alcohol metabolite has been
identified that is unique to male rats (Olson et al., 1986). While
both male and female rats synthesize cis,cis-2-decalol and
trans,cis-2-decalol from cis-decalin in their livers,
cis,cis-1-decalol was found only in males and
cis,trans-1-decalol was produced in much higher concentrations
than in females. In similar fashion, with trans-decalin as the
substrate trans,trans-1-decalol was seen only in livers of male
rats. When kidneys were examined, 2-decalone (cis or trans,
depending on the original substrate) was found in the males while no
decalin metabolites whatever were seen in female kidneys. Whether
these unique male decalin metabolites react with alpha2u-globulin
has not yet been tested.
More recently, Lehman-McKeeman et al. (1989) have reported
that d-limonene and d-limonene-1,2-oxide, a major metabolite of
d-limonene, were found reversibly bound to alpha2u-globulin
(identified by amino acid sequencing) in the kidneys of treated
male, but not female, rats.
Inhibition of alpha2u-globulin catabolism
Charbonneau et al. (1988) reported that alpha2u-globulin
bound in kidney cytosol was not digested by purified proteases or
proteinase.
Treating rats with leupeptin, an inhibitor of cathepsin B, a
major lysosomal peptidase, mimics the ability of unleaded gasoline
to cause the accumulation of alpha2u-globulin in kidney
phagolysosomes. Authors concluded that the mechanism of
nephrotoxicity involves inhibition of renal phagolysosomal
proteolysis (Olsen et al., 1988).
Induction of cell proliferation
Short et al. (1989a) have shown that proliferation of
epithelial cells in the P2 proximal tubule segment in male rats is
associated with exposure of the animals to either
2,2,4-trimethylpentane or unleaded gasoline. They noted that this
proliferation closely paralleled the extent and severity of
detectable alpha2u-globulin in the same cells. The authors
speculated that this proliferation leads to the nephrocarcinogenic
effects caused by these agents.
Proliferation was also noted by Charbonneau et al. (1989) in
male rats treated with 1,4-dichlorobenzene. Short et al. (1989b)
observed increased numbers of atypical cell foci and renal cell
tumours in male, but not female, rats initiated with
N-ethyl-N-hydroxyethylnitrosamine and subsequently exposed to
unleaded gasoline or 2,2,4-trimethylpentane. The extent of the
promotion was reported to be dose-related and to parallel the
effects of these compounds on chronic cell proliferation.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity
d-Limonene was reported to increase the number of abnormal
chick embryos to approximately 50% when a single dose (25 µM/embryo)
in olive oil was injected suprablastodermically (Abramovici &
Rachmuth-Roizman, 1983).
Pregnant mice were given oral doses of 0, 591, or 2363 mg/kg bw
d-limonene from days 7 through 12 of gestation. Authors reported
significant decreases in body weight gain of dams in the high-dose
group; fetuses of dams exposed to the high-dose showed increased
incidences of lumbar rib, fused rib, delayed ossification, and
decreased body weight gain relative to fetuses of control dams
(Kodama et al., 1977a).
Pregnant Japanese white rabbits were given oral doses of 0,
250, 500, or 1000 mg/kg bw d-limonene from day 6 to day 18 of
gestation. Significant decreases in body weight gain in dams given
250 or 500 mg/kg bw/day d-limonene were observed, and survival in
dams given 1000 mg/kg bw/day was significantly reduced (40%
survival). From examination of the fetuses, authors concluded that
d-limonene was not teratogenic in rabbits (Kodama et al.,
1977b).
2.2.6 Special studies on genotoxicity
Table 2: Genotoxicity data on d-limonene
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium .03-3 µmol/plate neg Florin et
(+&-activ) TA98, TA100, al., 1980
TA1535, TA1537
Yeast S. cerevisiae .1-100 mM/5x107 (1) Fahrig,
(-activ) MP1 strain cells 1984
Mammalian Mouse embryo 0-215 mg/kg (2) Fahrig,
(-activ) system 1984
Ames test S. typhimurium 0-20 µM/plate neg Watabe
(+&-activ) TA1535, TA100, et al.,
TA1537, TA1538, 1980
TA98
Ames test S. typhimurium 0-3333 µg/plate neg Haworth
(+&-activ) TA98, TA100, et al.,
TA1535, TA1537 1983
Ames test S. typhimurium 0-3333 µg/plate neg NTP,
(+&-activ) TA100, TA1535, 1990
TA1537, TA98
Mammalian Mouse L5178Y neg NTP,
(+&-activ) cells in vitro 1990
Sister Chinese hamster 0-162 µg/ml neg NTP,
chromatid ovary cells 1990
exchange in vitro
(+&-activ)
Chromosomal Chinese hamster 0-500 µg/ml (3) neg NTP,
aberrations ovary cells 0-100 µg/ml (4) neg 1990
(+&-activ) in vitro
Table 2 cont'd
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium 150 mg/plate neg Heck et
(+&-activ) TA1535, TA1537, al., 1989
TA1538, TA98,
TA100
Mammalian Mouse L5178Y 100 µg/ml neg Heck et
(+&-activ) cells in vitro al., 1989
(1) Author reported that results indicated that d-limonene was a
co-recombinant and an anti-mutagen.
(2) Author reported that results indicated that d-limonene is inactive
when given alone, but reduces the mutagenic effect of ethylnitrosourea
when the two are co-administered.
(3) Activated: addition of S9 from the livers of Aroclor 1254-induced
male Sprague Dawley rats.
(4) Not activated
2.2.7 Special studies on immune responses
Limonene has been identified as a primary skin irritant in
humans (Pirila et al., 1964; Klecak et al., 1977), rabbits
(research Institute for the Development of Fragrance Materials,
Inc., 1984; Research Institute for Fragrance Materials Inc., 1985),
guinea-pigs (Klecak et al., 1977), and mice (Gad et al., 1986).
d-Limonene was reported both to elicit (De Groot et al., 1985)
and not to elicit (Greif, 1967) allergic reactions in human skin and
to elicit allergic reactions in mouse skin (Gad et al., 1986).
d-Limonene was reported to eliminate human skin sensitization to
several aldehydes when they were co-administered with d-limonene
(Opdyke, 1975), and d-limonene was reported to reduce the
sensitivity and delayed hypersensitivity of guinea-pig skin to
citral (Hanau et al., 1983; Barbier & Benezra, 1983). Gozsy and
Kato (1957) reported that local endothelial phagocytosis is induced
in rat skin by d-limonene, and that d-limonene increases
phagocytosis in guinea-pig skin in vivo and in mouse skin
in vitro (Gozsy & Kato, 1958) and increases capillary permeability
in the guinea-pig (Kato & Gozsy, 1958).
Male BALB/c mice were treated intragastrically with 0, 0.002,
0.008, 0.033, 0.133, 0.531 or 2.125 mg/ml d-limonene daily. After
8 weeks, but not after 4 weeks, the mitogenic responses of spleen
cells taken from the treated animals and exposed in vitro to
concanavalin A and phytohaemagglutinin, which stimulate T-cells, and
lipopolysaccharide, which affects B-cells, were found to be
suppressed by the d-limonene treatment.
Other animals in groups of 10 were given these same doses of
d-limonene daily for 7 days prior to, or 8 days following,
immunization with keyhole limpet haemocyanin (KLH). Primary effects
on T- and B-cell responses were determined 10 days later.
Secondary effects were determined by reinoculating the animals
with KLH 21 days after the first inoculation and then assaying on
day 24. The primary and secondary responses were reported to be
suppressed by d-limonene if the animals were exposed to KLH first,
but stimulated in treated mice if exposed to KLH after d-limonene
exposure.
Histopathological examination of secondary lymphoreticular
tissue taken from animals exposed to d-limonene showed significant
secondary follicle development and prominent lymphoid nodules and
aggregates in the pancreas and intestinal mucosa, which were evident
in animals which had received the highest dose of d-limonene
(Evans et al., 1987).
2.3 Observations in humans
Numerous prospective and retrospective cohort epidemiological
studies have been done on populations of refinery, chemical plant,
and service station workers, exposed to mixtures of chemicals that
have been associated with male rat alpha2u-globulin nephropathy
(Hanis et al., 1979; Schottenfeld et al., 1981; Hanis et al.,
1982; Thomas et al., 1982; Austin & Schnatter, 1983; Higginson
et al., 1984; Raabe, 1984; Wen et al., 1984; Wong & Raabe, 1989;
Page & Mehlman, 1989). No statistically significant evidence was
found in any study to link incidence of cancer of the kidney to
work-place exposure to mixtures of complex hydrocarbons.
Igimi et al. (1976) reported that d-limonene was a safe and
effective gallstone solubilizer in animals and humans; side effects
in humans were reported to be pain and tenderness radiating from the
upper abdomen to the anterior chest, nausea and vomiting, and
diarrhoea.
3. COMMENTS
d-Limonene has been shown to reduce body weights
significantly in male and female mice and rats and in female
rabbits. The NOEL for this effect was 150 mg of d-limonene kg
bw/day (administered by gavage) in a 2-year study in male rats.
Oral administration of d-limonene (400 mg/kg bw/day) to rats
for 20 days slightly increased liver weight and phospholipid
content, decreased liver and serum cholesterol levels, increased the
concentration of cytochromes P450 and B5, and increased the
activities of aminopyrine demethylase and aniline hydroxylase.
Although liver lesions were not associated with the administration
of d-limonene in a 2-year study in rats (doses up to 150 mg/kg
bw/day for males; doses up to 600 mg/kg bw/day for females), a daily
gavage dose of 500 mg d-limonene/kg bw/day for 2 years was
associated with an increased incidence of multinucleated liver
hepatocytes and cytomegaly in male mice. The NOEL for these effects
was 250 mg/kg bw/day administered by gavage to male mice for 2
years.
Dermal application of d-limonene has been reported to cause
irritation and immune-mediated skin reactions in a number of
species, including humans. Oral administration of d-limonene to
mice was reported to have immunological effects, including
suppression of the in vitro mitogenic response by mouse T-cells
and B-cells and both suppression and stimulation of antigenic
responses. However, the biological significance of these in vitro
changes is not known.
Results of teratogenicity studies in mice suggest that maternal
consumption of 2400 mg of d-limonene/kg bw/day but not of
600 mg/kg bw/day, affected the development of fetuses (decreased
body weight gain, increased incidences of lumbar rib and fused rib,
and delayed ossification). However, d-limonene was reported to be
non-teratogenic in rabbits at doses ranging from 250 to 1000 mg/kg
bw/day (although the highest dose significantly reduced survival of
the dams).
d-Limonene consistently produced negative results in
genotoxicity studies, has been reported to inhibit the activity of
other mutagens and carcinogens, and gave mixed responses in
tumour-promotion studies.
Data clearly demonstrate that d-limonene exacerbates the
spontaneously occurring nephropathy in mature male rats which, after
prolonged exposure, is associated with an increase in the incidence
of renal tumours that are not commonly observed in control male
rats. However, d-limonene is not the only substance that has this
effect: various hydrocarbon mixtures and several other chemicals
have also been reported to exacerbate spontaneous nephropathy and
cause renal tumours in male rats. Although, in studies designed to
elucidate the mechanism of this carcinogenic process, chemicals
other than d-limonene were generally used as test substances, such
studies have shown that the following mechanism is likely to be
applicable to d-limonene-induced nephropathy and renal tumours in
male rats: (1) d-limonene and d-limonene-1,2-oxide (a metabolite
of d-limonene) bind reversibly to alpha2u-globulin in the
kidneys of d-limonene-treated male rats; (2) this binding further
slows down the normally slow processing of reabsorbed
alpha2u-globulin; and (3) as a result, alpha2u-globulin
accumulates in the phagolysosomes (hyaline droplets) of epithelial
cells of the proximal convoluted tubules of the kidney, leading to
necrosis, accumulation of cell debris, and regenerative hyperplasia.
The postulated mechanism of action for some non-genotoxic
carcinogens suggests that regenerative hyperplasia may increase
clonal expansion of spontaneously initiated cells and lead to the
development of the renal tumours observed.
A NOEL for the d-limonene-associated increase in
alpha2u-globulin levels in male rat kidney has not been
identified, but the lowest-observed-effect level from a 21-day
gavage study of d-limonene in male rats was 75 mg/kg bw/day. A
sex-specific protein with molecular features similar to
alpha2u-globulin has been identified in human urine, but the
concentration is at least four orders of magnitude less than that of
alpha2u-globulin in male rats.
The Committee concluded that the postulated mechanism for
d-limonene-induced nephropathy and renal tumours in the male rat
was probably not relevant to humans, and that toxic end points
associated with this effect were not appropriate bases for the
derivation of an ADI for d-limonene.
4. EVALUATION
Based on the significant decreases in body weight gain
associated with administration of d-limonene to male and female
mice and rats and female rabbits, an ADI of 0-1.5 mg/kg bw was
established for this substance. The Committee considered the known
natural occurrence and food additive uses of d-limonene, and
concluded that only a small proportion of total intake is likely to
be derived from direct additive use. The Committee therefore
recommended that food additive intake be restricted to 75 µg/kg
bw/day, which represents 5% of the maximum ADI for d-limonene.
5. REFERENCES
ABRAMOVICI, A. & RACHMUTH-ROIZMAN, P. (1983). Molecular
structure-teratogenicity relationships of some fragrance additives.
Toxicology, 29: 143-156.
ALDEN, C.L., KANERVA, R.L., RIDDER, G. & STONE, L.C. (1984). Chapter
VIII: The pathogenesis of the nephrotoxicity of volatile
hydrocarbons in the male rat. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 107-120, Princeton Scientific, Princeton, NJ.
ALDEN, C.L. (1989) Male rat specific alpha2u-globulin nephropathy
and renal tumorigenesis. In: Bach, P.H. & Lock, E.A. (eds.),
Handbook of Toxicologic Pathology. Academic Press, New York. In
press (cited in US EPA 1991).
ARIYOSHI, T., ARAKAKI, M., IDEGUCHI, K., ISHIZUKA, Y., NODA, K. &
IDE, H. (1975). Studies on the metabolism of d-limonene
(p-mentha-1,8-diene); III. Effects of d-limonene on the lipids and
drug metabolizing enzymes in rat livers. Xenobiotica, 5: 33-38.
AUSTIN, S.G. & SCHNATTER, A.R. (1983). A cohort mortality study of
petrochemical workers. Journal of Occupational Medicine, 25:
304-312.
BARBIER, P. & BENEZRA, C. (1983) The influence on induced
hypersensitivity to citral in guinea-pigs; II. Label distribution in
the skin of 14C-labelled citral. Acta Dermatovener (Stockholm),
63: 93-96.
BERNARD, A.M., LAUWERYS, R.R., NOEL, A., VANDELEENE, B. & LAMBERT,
A. (1989). Urine protein I: a sex-dependent marker of tubular or
glomerular dysfunction. Clin. Chem., 35: 2141-2.
BORGHOFF, S.J., SHORT, B.G., & SWENBERG, J.A. (1990) Biochemical
mechanisms and pathobiology of alpha2u-globulin nephropathy.
Ann. Rev. Pharmacol. Toxicol., 30: 349-367.
BRUNER, R.H. (1984). Chapter X: Pathologic findings in laboratory
animals exposed to hydrocarbon fuels of military interest. In:
Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 133-140,
Princeton Scientific, Princeton, NJ.
BUSEY, W.M. & COCKRELL, B.Y. (1984). Chapter IV: Non-neoplastic
exposure-related renal lesions in rats following inhalation of
unleaded gasoline vapors. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 57-64, Princeton Scientific, Princeton, NJ.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975a). Petroleum hydrocarbon toxicity studies: III.
Animal and human response to vapors of Stoddard solvent. Toxicology
and Applied Pharmacology, 32: 282-297.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975b). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "60 solvent." Toxicology
and Applied Pharmacology, 32: 374-394.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975c). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "70 solvent." Toxicology
and Applied Pharmacology, 34: 395-412.
CARPENTER, C.P., GEARY, JR., D.L., MYERS, R.C., NACHREINER, D.J.,
SULLIVAN, L.J. & KING, J.M. (1977). Petroleum hydrocarbon toxicity
studies: XV. Animal and human responses to vapors of "high
naphthenic solvent." Toxicology and Applied Pharmacology, 41:
251-260.
CHARBONNEAU, M., LOCK, E.A., STRASSER, J., COX, M.G., TURNER, M.J. &
BUS, J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: I.
Metabolic disposition of TMP in male and female Fischer 344 rats.
Toxicology and Applied Pharmacology, 91: 171-181.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J. & SWENBERG,
J.A. (1989). Involvement of reversible binding to alpha2u-globulin
in 1,4-dichlorobenzene-induced nephrotoxicity. Toxicology and
Applied Pharmacology, 99: 122-132.
CHARBONNEAU, M. & SWENBERG, J.A. (1988). Studies on the bio-chemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT
Activities, 8: 1-5.
CURVALL, M., ENZELL, C. & PETTERSSON, B. (1984). An evaluation of
the utility of four in vitro short-term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke.
Cell Biology and Toxicology, 1: 173-193.
DIETRICH, D.R. & SWENBERG, J.A. (1991). NCI-Black-Reiter (NBR) male
rats fail to develop renal disease following exposure to agents that
induce alpha2u-globulin nephropathy. Fundamentals of Applied
Toxicology, 16: 749-762.
DE GROOT, A.C., LIEM, D.H., NATER, J.P. & VAN KETEL, W.G. (1985).
Patch tests with fragrance materials and preservatives. Contact
Dermatitis, 12: 87-92.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984a). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Carcinogenesis, 5: 661-664.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984b). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Proceedings of the American Association
of Cancer Researchers, 25: 127A.
ELEGBEDE, J.A., ELSON, C.E., TANNER, M.A., QURESHI, A. & GOULD, M.N.
(1986a). Regression of rat primary mammary tumours following dietary
d-limonene. Journal of the National Cancer Institute, 76:
323-325.
ELEGBEDE, J.A., MALTZMAN, T.H., VERMA, A.K., TANNER, M.A., ELSON,
C.E. & GOULD, M.N. (1986b). Mouse skin tumour promoting activity of
orange peel oil and d-limonene: a re-evaluation. Carcinogenesis,
7: 2047-2049.
ELSON, C.E., MALTZMAN, T.H., BOSTON, J.L., TANNER, M.A. & GOULD,
M.N. (1988). Anti-carcinogenic activity of d-limonene during the
initiation and promotion/progression stages of DMBA-induced rat
mammary carcinogenesis. Carcinogenesis, 9: 331-332.
FAHRIG, R. (1984). Genetic mode of action of cocarcinogens and
promoters in yeast and mice. Molecular and General Genetics, 194:
7-14.
FLAMM, W.G. & LEHMAN-McKEEMAN, L. (1991). The human relevance of the
renal tumour-inducing potential of d-limonene in male rats:
Implications for risk assessment. Regulatory Toxicology and
Pharmacology, 13: 70-86.
FLORIN, I., RUTBERG, L., CURVALL, M. & ENZELL, C. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18: 219-232.
GAD, S.C., DUNN, B.J., DOBBS, D.W., REILLY, C. & WALSH R.D. (1986).
Development and validation of an alternative dermal sensitization
test: the mouse ear swelling test (MEST). Toxicology and Applied
Pharmacology, 84: 93-114.
GARG, B.D., OLSON, M.J., DEMYAN, W.F. & ROY, A.K. (1988a). Rapid
post-exposure decay of alpha2u-globulin and hyaline droplets in
the kidneys of gasoline-treated male rats. Journal of Toxicology
and Environmental Health, 24: 145-160.
GARG, B.D., OLSON, M.J., MANCINI, M.A. & ROY, A.K. (1988b).
Localization of alpha2u-globulin in hyaline droplets
(phagolysosomes) of the male rat renal cortex by immunohistochemical
techniques. Journal of Histochemistry and Cytochemistry, 36: 960.
GARG, B.D., OLSON, M.J., LI, L.C. & ROY, A.K. (1989). Phagolysosomal
alterations induced by unleaded gasoline in epithelial cells of the
proximal convoluted tubules of male rats: Effect of dose and
treatment duration. Journal of Toxicology and Environmental Health,
26: 101-118.
GAWORSKI, C.L., HAUN, C.C. & MacEWEN, J.D. (1981). A ninety-day
inhalation toxicity study of decalin. The Toxicologist, 1, 76.
GAWORSKI, C.L., LEAHY, H.F. & BRUNER, H.F. (1980). Subchronic
inhalation toxicity of decalin, Proceedings of the Tenth Conference
on Environmental Toxicology, AFAMRL-TR-79-121 (ADA086341) Aerospace
Medical Research Laboratory, Wright-Patterson Air Force Base, OH.
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L. & POPP, J.A. (1988).
Potential role of alpha2u-globulin, protein droplet accumulation,
and cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicology and Applied Pharmacology, 96: 367-379.
GOZSY, B. & KATO, L. (1957). Action of reticulo-endothelial-system
stimulators on the histamine depleted rats. Arch. Int. Pharmacodyn,
CXII: 235-246.
GOZSY, B. & KATO, L. (1958). Reticulo-endothelial system stimulating
compounds and their mechanism of action. Can. J. Biochem. Physiol.,
36: 319-326.
GREEN, T., ODUM, J., NASH, J.A. & FOSTER, J.R. (1990).
Perchloroethylene-induced rat kidney tumours: An investigation of
the mechanisms involved and their relevance to humans. Toxicol.
Appl. Pharmacol., 102: 77-89.
GREIF, N. (1967). Cutaneous safety of fragrance material as measured
by the Maximization Test. American Perfumer and Cosmetics, 82:
54-57.
HALDER, C.A., WARNE, T.M. & HATOUM, N.S. (1984). Chapter VI: Renal
toxicity of gasoline and related petroleum naphthas in male rats.
In: Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 73-88, Princeton
Scientific, Princeton, NJ.
HANAU, D., GROSSHANS, E., BARBIER, P. & BENEZRA, C. (1983). The
influence of limonene on induced delayed hypersensitivity to citral
in guinea-pigs; I. Histology study. Acta Dermatovener (Stockholm)
63: 1-7.
HANIS, N.M., HOLMES, T.M., SHALLENBERGER, L.G. & JONES, K.E. (1982).
Epidemiologic study of refinery and chemical plant workers. Journal
of Occupational Medicine, 24: 203-212.
HANIS, N.M., STRAVRAKY, K.M. & FOWLER, J.L. (1979). Cancer mortality
in oil refinery workers. Journal of Occupational Medicine, 21:
167-174.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W. & ZEIGER, E.
(1983). Salmonella mutagenicity test results for 250 chemicals.
Environmental Mutagenesis Supplement, 1: 3-142.
HECK, J.D., VOLLMUTH, T.A., CIFONE, M.A., JAGANNATH, D.R., MYHR, B.
& CURREN, R.D. (1989). An evaluation of food flavoring ingredients
in a genetic toxicity screening battery. The Toxicologist, 1: 257.
HIGGINSON, J., MUIR, C. & BUFFLER, P.A. (1984). Chapter XV: The
epidemiology of renal carcinoma in humans with a note on the effect
of exposure to gasoline. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 203-226, Princeton Scientific, Princeton, NJ.
HOMBURGER, F., TREGER, A. & BOGER, E. (1971). Inhibition of murine
subcutaneous and intravenous benzo(rst)pentaphene carcinogenesis by
sweet orange oils and d-limonene. Oncology, 25: 1-10.
IGIMI, H., HISATSUGU, T. & NISHIMURA, M. (1976). The use of
d-limonene preparation as a dissolving agent of gallstones.
Digestive Diseases, 21: 926-939.
IGIMI, H., NISHIMURA, M., KODAMA, R. & IDE, H. (1974). Studies on
the metabolism of d-limonene (p-mentha-1,8-diene): I. The
absorption, distribution and excretion of d-limonene in rats.
Xenobiotica, 4: 77-84.
IMAIZUMI, K. HANADA, K., MAWATARI, K. & SUGANO, M. (1985). Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49: 2795-2796.
KANERVA, R.L. & ALDEN, C.L. (1987). Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and Chemical Toxicology, 25: 355-358.
KANERVA, R.L., RIDDER, G.M., LEFEVER, F.R. & ALDEN, C.L. (1987a).
Comparison of short-term renal effects due to oral administration of
decalin or d-limonene in young adult male Fischer-344 rats.
Food and Chemical Toxicology, 25: 345-353.
KANERVA, R.L., MCCRACKEN, M.S., ALDEN, C.L. & STONE, L.C. (1987b).
Morphogenesis of decalin-induced renal alterations in the male rat.
Food and Chemical Toxicology, 25: 53-61.
KANERVA, R.L., RIDDER, G.M., STONE, L.C. & ALDEN, C.L. (1987c).
Characterization of spontaneous and decalin-induced hyaline droplets
in kidneys of adult male rats. Food and Chemical Toxicology, 25:
63-82.
KATO, L. & GOZSY, B. (1958). Treatment of experimental tuberculosis
of the guinea-pig with dihydrostreptomycin and simultaneously with
substances acting on the host. Arch. Int. Pharmacodyn, CXVII:
52-62.
KITCHEN D.N. (1984). Chapter V: Neoplastic renal effects of unleaded
gasoline in Fischer 344 rats. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 65-88, Princeton Scientific, Princeton, NJ.
KLECAK, G., GELEICK, H. & FREY, R. (1977). Screening of fragrance
materials for allergenicity in the guinea-pig; comparison of four
testing methods. J. Soc. Cosmet. Chem., 28: 53-64.
KODAMA, R., NODA, K. & IDE, H. (1974). Studies on the metabolism of
d-limonene (p-mentha-1,8-diene): II. The metabolic fate of
d-limonene in rabbits. Xenobiotica, 4: 85-95.
KODAMA, R., INOUE, H., NODA, K., IDE, H., YAMAMOTO, H., YOSHIHARA,
S. & YOSHIMURA, H. (1976a). Effect of d-limonene and related
compounds on bile flow and biliary lipid composition in rats and
dogs. Life Sciences, 19: 1559-1568.
KODAMA, R., YANO, T., FURUKAWA, K., NODA, K. & IDE, H. (1976b).
Studies on the metabolism of d-limonene (p-mentha-1,8-diene): IV.
Isolation and characterization of new metabolites and species
differences in metabolism. Xenobiotica, 6: 377-389.
KODAMA, R., OKUBO, A., ARAKI, E., NODA, K., IDE, H. & IKEDA, T.
(1977a). Studies on d-limonene as a gallstone solubilizer; VII.
Effects on development of mouse fetuses and offspring. Oyo Yakuri
(Pharmacometrics) 13: 863-873.
KODAMA, R., OKUBO, A., SATO, K., ARAKI, E., NODA, K., IDE, H. &
IKEDA, T. (1977b). Studies on d-limonene as a gallstone
solubilizer; IX. Effects on development of rabbit fetuses and
offspring. Oyo Yakuri (Pharmacometrics), 13: 885-898.
LEHMAN-McKEEMAN, L.D., CAUDILL, D., TAKIJIKU, R., SCHNEIDER, R.E. &
YOUNG, J.A. (1990a). Comparative disposition of d-limonene in rats
and mice: Relevance to male-rat-specific nephrotoxicity. Toxicol.
Lett., 53: 193-195.
LEHMAN-McKEEMAN, L.D., RIVERA-TORRES, M.I. & CAUDILL, D. (1990b).
Lysosomal degradation of alpha2u-globulin and
alpha2u-globulin-xenobiotic conjugates. Toxicology and Applied
Pharmacology, 103: 539-548.
LEHMAN-McKEEMAN, L.D., RODRIGUEZ, P.A., TAKIGIKU, R., CAUDILL, D. &
FEY, M.L. (1989). d-Limonene-induced male rat-specific
nephrotoxicity: Evaluation of the association between d-limonene
and alpha2u-globulin. Toxicology and Applied Pharmacology, 99:
250-259.
LOCK, E.A., CHARBONNEAU, M., STRASSER, J., SWENBERG, J.A. & BUS,
J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: II. The
reversible binding of a TMP metabolite to a renal protein fraction
containing alpha/2u-globulin. Toxicology and Applied
Pharmacology, 91: 182-192.
LOGOTHETOPOULOS, J. & WEINBREN, K. (1955). Naturally occurring
protein droplets in the proximal tubule of the rat's kidney.
British Journal of Experimental Pathology, 36: 402-406.
LOURY, D.J., SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987). Assessment
of the covalent binding potential of 2,2,4-trimethylpentane to rat
alpha2u-globulin. Toxicology and Applied Pharmacology, 88:
44-56.
MacEWEN, J.D. & VERNOT, E. (1978a). Evaluation of toxic effects of
90-day continuous exposure to conventional JP-5 jet fuel. In: Toxic
Hazards Research Unit Annual Report, AFAMRL-TR-78-55 (ADA062138)
Aerospace Medical Research Laboratory, Wright-Patterson Air Force
Base, OH.
MacEWEN, J.D. & VERNOT, E. (1978b). The effects of subchronic
exposure of rodents to inhaled decalin. In: Toxic Hazards Research
Unit Annual Report, AFAMRL-TR-78-55 (ADA062138) Aerospace Medical
Research Laboratory, Wright-Patterson Air Force Base, OH.
MacFARLAND, H.N., ULRICH, C.E., HOLDSWORTH, C.E., KITCHEN, D.N.,
HALLIWELL, W.H. & BLUM S.C. (1984). A chronic inhalation study with
unleaded gasoline vapor. Journal of the American College of
Toxicology, 3: 231-248.
MacNAUGHTON, M.G. & UDDIN, D.E. (1984). Chapter IX: Toxicology of
mixed distillate and high-energy synthetic fuels. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 121-132, Princeton
Scientific, Princeton, NJ.
MALTZMAN, T.H., HURT, L.M., ELSON, C.E., TANNER, M.A. & GOULD, M.N.
(1989). The prevention of nitrosomethylurea-induced mammary tumours
by d-limonene and orange oil. Carcinogenesis, 10: 781-783.
MEYER, F. & MEYER, E. (1959). Absorption of ethereal oils and
substances contained in them through the skin.
Arzneimittelforschung (Drug Research), 9: 516-519.
MILLER, A.B., BOWEN, J.P., BORGHOFF, S.J. & SWENBERG, J.A. (1989).
Computational and molecular modeling studies of alpha2u-globulin.
Abstract papers of the American Chemical Society, 198: Medi-39.
MURTY, C.V.R., OLSON, M.J., GARG, B.D. & ROY, A.K. (1988).
Hydrocarbon-induced hyaline droplet nephropathy in male rats during
senescence. Toxicology and Applied Pharmacology, 96: 380-392.
NTP (1983). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of pentachloroethane (CAS No.
76-01-7) in F344/N rats and B6C3F1 mice (Gavage Studies), NTP
TR 232, NIH Publication No. 83-1788.
NTP (1986). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 4-vinyl-cyclohexene (CAS
No. 100-40-3) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 303, NIH Publication No. 86-2559.
NTP (1987a). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 1,4-di-chlorobenzene (CAS
No. 106-46-7) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 319, NIH Publication No. 87-2570.
NTP (1987b). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of dimethylmethylphosphonate
(CAS No. 756-79-6) in F344/N rats and B6C3F1 mice (Gavage
Studies), NTP TR 323, NIH Publication No. 88-2579.
NTP (1990). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of d-limonene (CAS No.
5989-27-5) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 347, NIH Publication No. 90-2802.
OLSON, C.T., YU, K.O. & SERVE, M.P. (1986). Metabolism of
nephrotoxic cis- and trans-decalin in Fischer-344 rats. Journal
of Toxicology and Environmental Health, 18: 285-292.
OLSON, M.J., JOHNSON, J.T. & REIDY, C.A. (1990). A comparison of
male rat and human urinary proteins: Implications for human
resistance to hyaline droplet nephropathy. Toxicol. Appl.
Pharmacol., 102: 524-536.
OLSON, M.J., MANCINI, M.A., GARG, B.D. & ROY, A.K. (1988).
Leupeptin-mediated alteration of renal phagolysosomes: Similarity to
hyaline droplet nephropathy of male rats exposed to unleaded
gasoline. Toxicol. Lett. 41: 245-254.
OPDYKE, D.L.J. (1975). d-Limonene. Monograph on fragrance raw
materials. Food and Cosmetics Toxicology, 13(suppl: 683-923):
825-826.
PAGE, N.P. & MEHLMAN, M. (1989). Health effects of gasoline
refueling vapors and measured exposures at service stations.
Toxicology and Industrial Health, 5: 869-890.
PARKER, G.A., BOGO, V. & YOUNG, R.W. (1981). Acute toxicity of
conventional versus shale-derived JP5 jet fuel: Light microscopic,
hematologic, and serum chemistry studies. Toxicology and Applied
Pharmacology, 57: 302-317.
PHILLIPS, R.D. & COCKRELL, B.Y. (1984). Chapter VII: Effect of
certain light hydrocarbons on kidney function and structure in male
rats. In: Advances in Modern Environmental Toxicology, Renal
Effects of Petroleum Hydrocarbons, M.A. Mehlman, editor, pp.
89-105, Princeton Scientific, Princeton, NJ.
PHILLIPS, R.D. & EGAN, G.F. (1984). Effect of C10-11 isoparaffinic
solvent on kidney function in Fischer 344 rats during eight weeks of
inhalation. Toxicology and Applied Pharmacology, 73: 500-510.
PIRILA, V., SILTANEN, E. & PIRILA, L. (1964). On the chemical nature
of the eczematogenic agent in oil of turpentine. Dermatologica,
128: 16-21.
RAABE, G.K. (1984). Chapter XVIII: Kidney cancer epidemiology in
petroleum related studies. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 259-271, Princeton Scientific, Princeton, NJ.
READ, N.G., ASTBURY, P.J., MORGAN, R.J.I., PARSONS, D.N. & PORT,
C.J. (1988). Induction and exacerbation of hyaline droplet formation
in the proximal tubular cells of the kidneys from male rats
receiving a variety of pharmacological agents. Toxicology, 52:
81-101.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1985). Acute
dermal irritation study; RIFM 1-32.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1990). The
absorption, distribution, and excretion of radioactivity after
topical application of 14C-limonene to rats.
RESEARCH INSTITUTE FOR THE DEVELOPMENT OF FRAGRANCE MATERIALS, INC.
(1984). Acute dermal irritation study; Ref. Nos. 307-338/8403.
RIDDER, G.M., VON BARGEN, E.C., ALDEN, C.L. & PARKER, R.D. (1990).
Increased hyaline droplet formation in male rats exposed to decalin
is dependent on the presence of alpha2u-globulin. Fundamental and
Applied Toxicology, 15: 732-743.
ROE, F.J.C. (1959). Oil of sweet orange: A possible role in
carcinogenesis. British Journal of Cancer, 13: 92-93.
ROE, F.J.C. & Peirce, W.E.H. (1960). Tumour promotion by citrus
oils: Tumours of the skin and urethral orifice in mice. Journal of
the National Cancer Institute, 24: 1389-1403.
ROY, A.K. & NEUHAUS, A.W. (1967) Androgenic control of a
sex-dependent protein in the rat. Nature, 214: 618-20.
SCHENK, J. DOBRONTE, Z., KOCH, H. & STOLTE, M. (1980).
Untersuchungen zur gewebevertraglichkeit von d-limonen als einem
losungsmittel cholesterineicher gallenstein. Gastroenterologie,
18: 389-394.
SCHOTTENFELD, D., WARSHAUER, M.E., ZAUBER, A.G., MEIKLE, J.G. &
HART, B.T. (1981). A prospective study of morbidity and mortality in
petroleum industry employees in the United States - a preliminary
report. In: Banbury Report 9: Quantification of Occupational
Cancer, R. Peto & M. Schneiderman, editors, pp. 247-265. Cold
Spring Harbor Laboratory, Cold Spring Harbor, NY.
SHORT, G.B., BURNETT, V.L., COX, M.G., BUS, J.S. & SWENBERG, J.A.
(1987). Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Laboratory
Investigation, 57: 564-577.
SHORT, G.B., BURNETT, V.L. & SWENBERG, J.A. (1989a). Elevated
proliferation of proximal tubule cells and localized accumulated
alpha2u-globulin in F344 rats during chronic exposure to unleaded
gasoline or 2,2,4-trimethylpentane. Toxicology and Applied
Pharmacology, 101: 414-431.
SHORT, G.B., STEINHAGEN, W.H. & SWENBERG, J.A. (1989b). Promoting
effects of unleaded gasoline and 2,2,4-trimethylpentane on the
development of atypical cell foci and renal tubular cell tumours in
rats exposed to n-ethyl-n-hydroxyethylnitrosamine. Cancer Research,
49: 6369-6378.
SHORT, G.B. & SWENBERG, J.A. (1988). Pathologic Investigations of
the mechanism of unleaded gasoline-induced renal tumours in rats.
CIIT Activities, 8: 1-6.
STONARD, M.C., PHILLIPS, P.G.N., FOSTER, J.R., SIMPSON, M.G. & LOCK,
E.A. (1986). Alpha2u-globulin: Measurement in rat kidney following
administration of 2,2,4-trimethylpentane, Toxicology, 41: 161-168.
STONE, L.C., KANERVA, R.L., BURNS, J.L. & ALDEN, C.L. (1987a).
Decalin-induced nephrotoxicity: Light and electron microscopic
examination of the effects of oral dosing on the development of
kidney lesions in the rat. Food and Chemical Toxicology, 25:
43-52.
STONE, L.C., McCRACKEN, M.S., KANERVA, R.L. & ALDEN, C.L. (1987b).
Development of a short-term model of decalin inhalation
nephrotoxicity in the male rat. Food and Chemical Toxicology, 25:
35-41.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumour
response in strain A mice. Cancer Research, 33: 3069-3085.
SWENBERG, J.A. (1991). Editorial. Risk assessment of chemicals
causing alpha2u-globulin nephropathy. Reg. Toxicol. Pharmacol.,
13: 1-2.
SWENBERG, J.A., SHORT, B., BORGHOFF, S., STRASSER, J. & CHARBONNEAU,
M. (1989). The comparative pathobiology of alpha2u-globulin
nephropathy. Toxicology and Applied Pharmacology, 97: 35-46.
THELESTAM, M., CURVALL, M. & ENZELL, C. (1980). Effect of tobacco
smoke compounds on the plasma membrane of cultured human lung
fibroblasts. Toxicology, 15: 203-217.
THOMAS, T.L., WAXWEILER, R.J., MOURE-ERASO, R., ITAYA, S. &
FRAUMENI, Jr., J.F. (1982). Mortality patterns among workers in
three Texas oil refineries. Journal of Occupational Medicine, 24:
135-141.
TRUMP, B.F., BEREZESKY, I.K., SATO, K. & SAIKO, K.U. (1984). Cell
calcium, cell injury, and cell death. Environ.Hlth. Revs., 57:
281-287.
TSUJI, M., FUJISAKI, Y., YAMACHIKA, K. NAKAGAMI, K., FUJISAKI, F.,
MITO, M., AOKI, T., KINOSHITA, S., OKUBO, A. & WATANABE, I. (1974).
Studies on d-limonene as gallstone solubilizer; I. General
pharmacological studies. Oyo Yakuri (Pharmacometrics), 8:
1439-1459.
TSUJI, M., FUJISAKI, Y., ARIKAWA, Y., MASUDA, S., KINOSHITA, S.,
OKUBO, A., NODA, K., IDE, H. & IWANAGA, Y. (1975a). Studies on
d-limonene, as gallstone solubilizer (II): Acute and subacute
toxicities. Oyo Yakuri (Pharmacometrics), 9: 387-401.
US EPA (1991). Alpha2u-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Risk
Assessment Forum, US Environmental Protection Agency, Washington,
DC, USA, EPA/625/3-91/019F.
VAN DUUREN, B.L. & GOLDSCHMIDT, B.M. (1976). Co-carcinogenic and
tumour-promoting agents in tobacco carcinogenesis. Journal of the
National Cancer Institute, 56: 1237-1242.
WADE, A.P., WILKINSON, G.S., DEAN, F.M. & PRICE, A.W. (1966). The
isolation, characterization and structure of uroterpenol, a
monoterpene from human urine. Biochem. J., 101: 727-734.
WATABE, T., HIRATSUKA, A., ISOBE, M. & OZAWA, N. (1980). Metabolism
of d-limonene by hepatic microsomes to non-mutagenic epoxides
toward Salmonella typhimurium. Biochemical Pharmacology, 29:
1068-1071.
WEBB, D.R., KANERVA, R.L., HYSELL, D.K., ALDEN, C.L. &
LEHMAN-MCKEEMAN, L.D. (1990). Assessment of the subchronic oral
toxicity of d-limonene in dogs. Fd. Chem. Toxic., 28: 669-675.
WEBB, D.R., RIDDER, G.M. & ALDEN, C.L. (1989). Acute and subacute
nephrotoxicity of d-limonene in Fischer 344 rats. Fd. Chem.
Toxicol., 27: 639-649.
WEN, C.P., ISAI, S.P., MOFFITT, K.B., BONDY, M. & GIBSON, R.L.
(1984). Chapter XVII: Epidemiologic studies of the role of gasoline
(hydrocarbon) exposure in kidney cancer risk. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 245-257, Princeton
Scientific, Princeton, NJ.
WONG, O. & RAABE, G. (1989). Critical review of cancer epidemiology
in petroleum industry employees, with a quantitative meta-analysis
by cancer site. American Journal of Industrial Medicine, 15:
283-310.
Wade et al. (1966) reported that dietary limonene gives rise
to uroterpenol (M-II: p-menth-1-ene-8,9-diol) in the urine of
humans.
2.1.3 Effects on enzymes and other biochemical parameters
Groups of control (6 animals) and experimental (4 animals) male
Wistar rats were administered single gavage doses of 0, 200, 400,
600, 800, or 1200 mg/kg bw d-limonene in 2% tragacanth solution in
a total volume of 4 ml/kg. Authors reported that no effects were
observed on liver triglycerides, microsomal proteins, cytochrome b5
and drug metabolizing enzymes.
In the same experiment, male Wistar rats were treated with
gavage doses of 0 or 400 mg/kg bw d-limonene in 2% tragacanth
solution in a total volume of 4 ml/kg for 2, 3, 15 or 30 days;
animals were killed 24 h following the last dose. Authors reported
that, following repeated treatment for 30 days, relative liver
weight and hepatic phospholipid content were slightly increased, and
liver and serum cholesterol were decreased 49% and 8%, respectively.
In addition, palmitic, linoleic and arachidonic acids were
increased, and stearic acid was decreased in the liver; aminopyrine
demethylase and aniline hydroxylase were increased 26% and 22%,
respectively, and cytochrome P-450 and b5 were increased by 31% and
30%, respectively (Ariyoshi et al., 1975).
d-Limonene was reported to enhance bile flow in Wistar rats
and mongrel dogs in a dose-related manner, and to decrease the ratio
of biliary bile salts and phospholipids to cholesterol (Kodama
et al., 1976). d-Limonene was reported to be an effective
gallstone solubilizer in animals and humans (Igimi et al., 1976;
Schenk et al., 1980), and to have no effect on liver weights nor
levels of serum lipids when fed to male Wistar rats at 0.5 and 1% in
the diet (Imaizumi et al., 1985).
d-Limonene was reported to significantly damage and increase
permeability of membranes of human lung fibroblasts (Thelestam
et al., 1980; Curvall et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Table 1: Summary of acute toxicity studies with d-limonene
Species Sex Route LD50 Reference
Mouse M&F oral, in 6.3 ml/kg (M) Tsuji et
juice, 7d 8.1 ml/kg (F) al., 1974
Mouse M&F ip, in 3.7 ml/kg (M) Tsuji et
juice, 3d 3.6 ml/kg (F) al., 1974
Mouse M&F ip, in 0.7 ml/kg (M) Tsuji et
juice, 10d 0.6 ml/kg (F) al., 1974
Mouse M&F sc, in >25.6 ml/kg (M) Tsuji et
juice, 7d >25.6 ml/kg (F) al., 1974
Mouse M&F oral 5600 mg/kg (M) Tsuji et
6600 mg/kg (F) al., 1975a
Mouse M&F ip 1300 mg/kg (M) Tsuji et
1300 mg/kg (F) al., 1975a
Mouse M&F sc >41500 mg/kg (M) Tsuji et
>41500 mg/kg (F) al., 1975a
Rat M&F oral 4400 mg/kg (M) Tsuji et
5100 mg/kg (F) al., 1975a
Rat M&F ip 3600 mg/kg (M) Tsuji et
4500 mg/kg (F) al., 1975a
Rat M&F sc >20200 mg/kg (M) Tsuji et
>20200 mg/kg (F) al., 1975a
An oral dose of 3 ml d-limonene (in juice)/kg decreased
spontaneous motor activities in rats and mice and potentiated
hexobarbital-induced sleeping and hypothermia in mice. Authors also
reported that nicotine-induced convulsion and death (but not maximum
electroshock-, pentetrazol-, strychnine-, nor picrotoxin-induced
convulsions) were inhibited by d-limonene in mice. Intravenous
administration of > 0.005 mg/kg d-limonene lowered the blood
pressure of rabbits and dogs, and > 0.1-0.3 mg/kg killed those
animals. Oral administration of 3 ml/kg d-limonene did not
decrease the blood pressure of rats, however. Authors also reported
that isolated smooth muscles of the intestine, vas deferens, uterus,
and peripheral vessel were constricted by d-limonene (Tsuji
et al., 1974). Single high s.c. doses of d-limonene produced
scratch behaviour and single high i.v. doses of d-limonene
produced stretch behaviour in mice and rats (Tsuji et al., 1975a).
2.2.2 Short-term studies
2.2.2.1 Mice
Groups of 5 B6C3F1 male and female mice were given daily
gavage doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg
bw in 10 ml/kg bw corn oil 5 days/week over a 16 day period (12
total dosings). All mice (5/5) exposed to 6600 mg/kg bw/day, 4/5
males and 5/5 females exposed to 3300 mg/kg bw/day, and 1/5 males
and 1/5 females exposed to 1650 mg/kg bw/day died before the end of
the study. The one female that died at the latter dose level was
reported to have been killed by a gavage error. Authors concluded
that no compound-related clinical signs were observed in mice that
received 1650 mg d-limonene/kg bw and lived to the end of the
study, and that no compound-related histopathologic effects were
seen (NTP, 1990).
Groups of 10 B6C3F1 mice of each sex were given daily
gavage exposures of 0, 125, 250, 500, 1000 or 2000 mg
d-limonene/kg bw in 10 ml/kg bw corn oil 5 days/week for 13 weeks.
One male exposed to 2000 mg/kg bw/day, two females exposed to
2000 mg/kg bw/day, and one female exposed to 500 mg/kg bw/day, died
during the course of the study. In addition, one female exposed to
125 mg/kg bw/day and five males (one each from the groups exposed to
250 and 1000 mg/kg bw/day, and three from the group exposed to
500 mg/kg bw/day) died prior to the end of the study, but their
deaths were attributed by the authors to accidents related to the
gavage procedure used. At the two highest dose levels, mice were
seen with rough hair coats and decreased activity. An alveolar cell
adenoma was observed in the lung of one female mouse that had
received 2000 mg d-limonene/kg bw/day (NTP, 1990).
2.2.2.2 Rats
Groups of 5 F344/N rats of each sex were given daily gavage
doses of 0, 413, 825, 1650, 3300 or 6600 mg d-limonene/kg bw in
10 ml/kg bw corn oil 5 days/week over a 16 day period (12 total
doses). All rats receiving 6600 mg d-limonene/kg bw/day as well as
5/5 male and 3/5 female rats that were exposed to 3300 mg/bw/day
died. The authors reported that no clinical signs were seen in rats
receiving 1650 mg d-limonene/kg bw/day or lower, and that no
compound-related histopathologic effects were seen in any rats (NTP,
1990).
Groups of 5 male Fischer-344 rats were given single daily
gavage doses containing 75, 150 or 300 mg d-limonene/kg bw in corn
oil (5 ml/kg bw) 5 days/week for 5 or 20 doses, primarily to follow
the development of renal alterations. The authors reported that no
signs of gross toxicity were observed nor was there any effect on
weight gain or feed consumption over the course of this experiment.
However, after only 5 days of exposure, liver and kidney weights
showed a dose-related increase and there was a dose-related increase
in the number and size of hyaline droplets and accumulation of
alpha2u-globulin in the proximal convoluted tubule epithelial
cells of the kidneys. In addition, after 20 doses, granular casts in
the outer zone of the medulla and multiple cortical changes
(classified as chronic nephrosis) were also seen in dose-related
severity (Kanerva et al., 1987a).
In a subacute study, groups of 5 male and female rats were
exposed daily to 0, 277, 554, 1385 or 2770 mg d-limonene/kg bw
orally for 30 days. A general decrease in food intake and a
dose-related decrease in body weight were seen in groups of exposed
males, but little or no effect on organ weights nor relative organ
weights was observed. No significant changes were seen in
urinalysis, haematology or biochemical values. The following tissues
were examined histopathologically: adrenals, duodenum, heart,
kidneys, liver, lungs, lymph nodes, pancreas, pituitary, spleen,
stomach, testes/ovaries, thymus and thyroids. Authors reported that
no significant changes were noted except that granular casts were
seen in the kidneys of most exposed male rats (0/5, 3/5, 5/5, 5/5 or
4/5 animals exposed to 0, 277, 554, 1385 or 2770 mg/kg bw/day
respectively) (Tsuji et al., 1975a).
Groups of 10 F334/N rats of each sex were given daily gavage
doses of 0, 150, 300, 600, 1200 or 2400 mg d-limonene/kg bw in
5 ml/kg bw corn oil 5 days/week for 13 weeks. Five male and nine
female rats exposed to 2400 mg d-limonene/kg bw/day died during
the course of the study. In all rats receiving 1200 or 2400 mg/kg
bw/day, rough hair coats, lethargy and excessive lacrimation were
observed. Authors reported that nephropathy was seen in all groups
of male rats, with a dose-related increase in severity. The
nephropathy was reported to be characterized by degeneration of
epithelium in the convoluted tubules, granular casts within tubular
lumens (primarily in the outer stripe of the outer medulla), and
regeneration of the tubular epithelium. In all groups of male rats
(including control animals), hyaline droplets were observed in the
epithelium of proximal convoluted tubules in at least some animals.
An attempt to determine by "blind" evaluation if the incidence of
these droplets was dose-related was reported to have resulted in an
equivocal conclusion.
The slides containing the renal sections made from male rats
exposed to d-limonene in this 13 week study were reviewed. They
reported that hyaline droplet accumulation within the cytoplasm of
proximal convoluted tubule (PCT) epithelial cells was uniform among
all groups, including controls. However, chronic nephrosis, although
seen in all animals (including control animals), appeared with a
dose-related increase in severity. The authors attributed the
uniformity of hyaline droplet accumulation to the fact that the
animals were not exposed to d-limonene for 4-5 days before
necropsy and examination, giving the animals time to rid themselves
of some of the droplets, although more extensive damage to the
kidneys (chronic nephrosis) was not repaired (Kanerva & Alden 1987).
Chronic nephrosis consisted of cytoplasmic basophilia of the
PCT epithelial cells, tubular hyperplasia or atrophy, fibrosis of
Bowman's capsule and an interstitial fibrolymphocytic response.
Atrophic tubules were characterized by thickened basement membranes
and a decrease in tubular cross-sectional diameter due to a decrease
in cell size, whereas tubules considered to be hyperplastic showed
increases in cell size and number and an increase in cross-sectional
diameter compared with the surrounding unaffected tubules. In
addition, occasional foci of PCT epithelial cell
necrosis/degeneration were observed in kidneys from all
d-limonene-treated animals but not in control animals. All treated
animals, but not controls, also showed a dose-related increase in
the number of granular casts found in the outer stripe of the
medulla. Granular casts were markedly dilated and had attenuated or
non-existent epithelium. In addition, animals exposed to the highest
dose level (2400 mg/kg bw/day) were reported to show multifocal
hyaline casts and tubular dilation in the cortex and medulla (NTP,
1990).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
To assess the pulmonary tumour carcinogenicity of d-limonene,
groups of 15 male or female A/He mice were injected i.p. with
d-limonene 3 times weekly for 8 weeks (4.8 or 24 g/kg bw total
dose) and then necropsied 24 weeks after the first injection. No
significant induction of lung tumours was seen (Stoner et al.,
1973).
Groups of 50 8-9 week old male B6C3F1 mice were given
daily gavage doses of 0, 250, or 500 mg d-limonene/kg bw and
groups of 50 8-9 week old female B6C3F1 mice were given 0,
500, or 1000 mg d-limonene/kg bw in 10 ml/kg bw corn oil
5 days/week for 103 weeks. Mice were observed twice a day and
weighed weekly for the first 12 weeks, after which they were weighed
monthly. Clinical signs were recorded at least monthly. Moribund
animals were killed and a necropsy was performed on all available
animals. The tissues of all mice that died during the study and
animals with grossly visible lesions, as well as all animals in the
control and high dose groups, were examined for histopathology.
The authors reported that no clinical signs were associated
with administration of the test chemical. The survival of male mice
exposed to 250 mg d-limonene/kg bw/day was lower than that of
controls at the end of the study (controls: 33/50; 250 mg/kg bw/day:
24/50; 500 mg/kg bw/day: 38/50). No other differences in survival
were seen among exposed groups of either sex. Mean body weights of
exposed and control male mice were generally comparable. Weights of
female mice in the 1000 mg d-limonene/kg bw/day group, however,
were 5-15% lower than control animals after week 28.
Multinucleated liver hepatocytes and cells with cytomegaly
occurred at increased incidence in male mice exposed to 500 mg
d-limonene/kg bw/day (multinucleated hepatocytes: controls - 8/49;
250 mg/kg bw/day - 4/36 [incomplete sampling]; 500 mg/kg bw/day -
32/50) (hepatocytes with cytomegaly: controls - 23/49; 250 mg/kg
bw/day - 11/36 [incomplete sampling]; 500 mg/kg bw/day - 38/50). The
combined incidence of hepatocellular adenomas and carcinomas,
however, did not differ significantly from their incidence in
control mice (controls: 22/49; 250 mg/kg bw/day: 14/36 [incomplete
sampling]; 500 mg/kg bw/day: 15/50). No significant differences were
observed in female mice for any of these endpoints.
The incidence of adenomas or the combined incidence of adenomas
and carcinomas in the anterior pituitary gland of female mice
exposed to 1000 mg d-limonene/kg bw/day were significantly lower
than controls. The incidence of hyperplasia did not differ
significantly from controls, however (hyperplasia: controls - 16/49;
500 mg/kg bw/day - 0/8 [incomplete sampling]; 1000 mg/kg bw/day -
17/48) (adenomas: controls - 12/49; 500 mg/kg bw/day - 5/8
[incomplete sampling]; 1000 mg/kg bw/day - 1/48) (combined adenoma
and carcinoma: controls - 12/49; 500 mg/kg bw/day - 5/8 [incomplete
sampling]; 500 mg/kg - 2/48) (NTP, 1990).
2.2.3.2 Rats
Groups of fifty 7-8 week old male F344/N rats were given daily
gavage doses of 0, 75, or 150 mg d-limonene/kg bw and groups of 50
7-8 week old female F344/N rats were given 0, 300, or 600 mg
d-limonene/kg bw in 5 ml/kg bw corn oil 5 days/week for 103 weeks.
Rats were observed twice a day; they were weighed weekly for the
first 12 weeks, and monthly thereafter. Clinical signs were recorded
at least monthly. Moribund animals were killed; necropsies were
performed on all available animals. The tissues of all rats that
died during the study, animals with grossly visible lesions, and all
animals in the control and high dose groups were examined for
histopathology.
No compound-related clinical signs were reported. The survival
of the female animals exposed to 600 mg/kg bw was lower than that of
control animals after week 39, while the survival of male rats
exposed. Mean body weights of male rats exposed to 150 mg/kg bw were
generally 4%-7% lower than controls from week 28.
Cataracts were observed at increased incidence in male rats
exposed to 150 and female rats exposed to 300 or 600 mg
d-limonene/kg bw/day, and retinal degeneration was seen in all
dosed animals. The authors pointed out, however, that the rats of
each sex that were exposed to their respective highest-dose levels
were housed in the top rows of the cage racks, the animals exposed
to the low-dose levels were below them, and the controls were housed
in the lowest rows for all but the last 10 weeks of the study.
Authors conclude that these effects were due to variations in the
proximity of animals in different dose groups to the light source.
Subcutaneous tissue fibromas in male rats occurred with a
negative dose-response trend (controls: 8/50; 75 mg/kg bw/day: 2/50;
150 mg/kg bw/day: 3/50). However, the combined incidence of fibromas
and fibrosarcomas was not significantly different when dosed males
were compared to controls (controls: 8/50; 75 mg/kg bw/day: 4/50;
150 mg/kg bw/day: 3/50).
Combined squamous cell papillomas or carcinomas in the skin of
male rats showed a positive dose-response trend (controls: 0/50;
75 mg/kg bw/day: 0/50; 150 mg/kg bw/day: 3/50). However, the
incidence of these neoplasms in dosed male rats was within the range
of incidences for NTP historical control rats, and did not differ
significantly from the incidence in control animals. The authors
concluded that the positive dose-response trend was not related to
d-limonene exposure.
Mononuclear cell leukaemia occurred in male rats with a
positive dose-response trend by the incidental tumour test
(controls: 10/50; 75 mg/kg bw/day: 10/50; 150 mg/kg bw/day: 19/50).
The incidences in dosed male rats were not significantly different
from the incidences in controls, however. The authors concluded that
the positive dose-response trend was not related to d-limonene
administration.
There was a significant positive dose-response trend in the
incidence of interstitial cell tumours in the testes of male rats,
and the incidences of these tumours in dosed groups were
significantly higher than the incidences in control animals
(control: 37/50; 75 mg/kg bw/day: 47/49; 150 mg/kg bw/day: 48/50).
The authors argued, however, that since historically this type of
tumour occurs in almost all aged F344 rats, this result should not
be considered to be related to d-limonene exposure, but rather was
an artifact caused by the low survival of the control animals in
this experiment (see above).
The incidence of uterine endometrial stromal polyps in females
exposed to 300 mg/kg bw/day was increased when compared to controls.
However, the incidence in the control animals was well below the NTP
historical incidence of this tumour. For this reason, and because
there was no positive dose-response trend, the authors argued that
this was not a compound-related effect.
d-Limonene exposure of male rats was associated with
dose-related increases in the incidences of mineralization and
epithelial hyperplasia in the kidneys (mineralization: controls -
7/50; 75 mg/kg bw/day - 43/50; 150 mg/kg bw/day - 48/50) (epithelial
hyperplasia: controls - 0/50; 75 mg/kg bw/day - 35/50; 150 mg/kg
bw/day - 43/50). The lesions consisted of linear deposits of mineral
in the medulla (renal papilla) and focal hyperplasia in the
transitional epithelium overlying the papilla. Hyperplasia was
frequently located near the fornices of the renal pelvis and was
occasionally bilateral. The severity of nephropathy, which occurs
spontaneously in aged male rats, was also positively dose-related in
this experiment (severity of nephropathy, rated on a scale from 0
[not present] to 4 [marked]: control mean - 1.5; 75 mg/kg bw/day
mean - 1.8; 150 mg/kg bw/day mean - 2.2). This nephropathy was
characterized by degeneration and atrophy of the tubular epithelium,
dilation of tubules with formation of hyaline droplets and granular
casts, regeneration of tubular epithelium, glomerulosclerosis, and
interstitial inflammation and fibrosis.
Kidney tubular cell hyperplasia and neoplasms were also
increased in dosed male rats (hyperplasia: controls - 0/50; 75 mg/kg
bw/day - 4/50; 150 mg/kg bw/day - 7/50) (adenomas: controls - 0/50;
75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 8/50) (adenocarcinomas:
controls - 0/50; 75 mg/kg bw/day - 4/50; 150 mg/kg bw/day - 3/50)
(combined adenomas and carcinomas: controls - 0/50; 75 mg/kg bw/day
- 8/50; 150 mg/kg bw/day - 11/50). Incidences of tubular cell
adenomas and the combined incidences of adenomas and
adeno-carcinomas in male rats showed significant positive
dose-response trends. High-dose males had a significantly higher
incidence than control males of both categories of tumours, while
low-dose males had a significantly higher incidence of the combined
category than control males. These neoplasms are historically rare
and none were seen in control males nor in any group of females.
The authors stated that kidney tubular cell hyperplasia,
adenomas, and adenocarcinomas were part of a morphologic spectrum.
Proliferative lesions diagnosed as hyperplasia generally consisted
of one to three adjacent cross-sections of enlarged tubules with
stratification of the epithelium. In some lesions, the epithelial
cells appeared to completely fill the lumen. The tubular cell
neoplasms varied in diameter from less than 1 mm to 15 mm. They
exhibited varied patterns of growth and were either solid, cystic,
or papillary. The solid and cystic neoplasms showed little evidence
of tubular structures and the cells were arranged in solid sheets or
small solid nests separated by a delicate vascular stroma. Some
neoplasms were reported to consist of layers of epithelium lining a
fibrous connective tissue stroma and were arranged in complex
branching papillary formations. The neoplasms were classified as
adenocarcinomas primarily if they exhibited cellular pleomorphism
and anaplasia, or if they were larger than 10 mm (NTP, 1990).
2.2.3.3 Dogs
Groups of 3 male or female Japanese beagle dogs were exposed
orally to 0, 0.4, 1.2 or 3.6 ml d-limonene/kg bw daily for 6
months. Dose-related occurrences of frequent vomiting and nausea
were seen in some animals. At doses of at least 1.2 ml/kg bw/day for
females and 3.6 ml/kg bw/day for males, a decrease in body weight
gain was noted compared to controls, but little or no effect on food
intake, organ weights or relative organ weights was reported. No
significant changes were seen in urinalysis, haematology nor
biochemical values, except that in animals at the 3.6 ml/kg bw/day
level, total cholesterol and blood sugar were lowered. The following
tissues were examined histopathologically: adrenals, bile duct,
duodenum, heart, kidneys, liver, lungs, pancreas, pituitary, rectum,
spleen, stomach, testes/ovaries, thymus and thyroids. No significant
changes were noted except that granular casts were seen in the
kidneys of all male dogs exposed to 3.6 ml/kg bw/day and all females
exposed to 0.4 ml/kg bw/day or more (1/3, 1/3, 2/3 or 3/3 for males;
1/3, 2/2, 3/3 or 3/3 for females exposed to 0, 0.4, 1.2 or 3.6 ml/kg
bw, respectively) (Tsuji et al., 1975b).
In a more recent study, groups of five male and female beagle
dogs were gavaged twice daily for six months with 0, 0.12, or
1.2 ml/kg bw/day (0, 10, or 1000 mg/kg bw/day) d-limonene. The
highest dose was reported to be near the maximum tolerated dose for
emesis. The authors stated that food consumption and body weight
were not affected by treatment. Although there was a positive
dose-related trend for absolute and relative female kidney weights
and for relative male kidney weight, authors reported that there
were no histo-pathological findings in the kidneys that could be
associated with the organ weight changes. In addition, there was no
hyaline droplet accumulation nor other indication of nephropathy
such as those associated with d-limonene consumption in male rats
(Webb et al., 1990)
2.2.3.4 Cocarcinogenicity/tumour promotion studies
Two publications have reported the results of experiments in
which 0.25 ml oil of sweet orange, composed of > 90% d-limonene,
was applied to the skin of 10 male and 10 female "101" mice weekly
for 42 weeks, starting 3 weeks after an application of 300 µg
7,12-dimethyl-1,2-benzanthracene (DMBA) in 0.2 ml acetone. While no
effects in the area of the treated skin were seen in mice exposed to
d-limonene or to DMBA alone, mice exposed to DMBA followed by
exposure to d-limonene began to develop papillomas during the
twelfth week of d-limonene exposure. After 33 weeks of treatment,
13/18 treated mice showed a total of 39 papillomas. No malignant
tumours were seen in the exposed area, however.
In a second study, mice were exposed to d-limonene following
exposure to: 1) 4 skin applications of 60 mg urethane in 0.3 ml
acetone at 3 day intervals, or 2) 4 i.p. injections of 16 mg
urethane in 0.1 ml distilled water at 3 day intervals. Papillomas
appeared on 3/13 mice in group 1 and 2/13 mice in group 2 by the
fourteenth week of exposure.
In a final experiment, the terpene fraction of oil of sweet
orange (containing d-limonene) was isolated and tested with DMBA
as described for the first experiment. Papillomas in mice exposed to
both DMBA and the terpene fraction of oil of sweet orange began to
appear in the eleventh week of treatment and were visible in 8/15
mice by the thirty-third week of treatment. Although pure
d-limonene was not tested in these experiments, the authors
concluded that the promoting effects seen were probably caused by
this chemical since it was the primary constituent of the test agent
(Roe, 1959, Roe & Peirce, 1960).
A group of 23 male and female albino mice were given single
doses of 50 µg benzo(a)pyrene (BAP) by stomach tube followed by 40
weekly doses of 0.05 ml d-limonene; seventeen male and female mice
received BAP only, 15 male and female mice received d-limonene
only and 18 male and female mice served as untreated controls. While
no tumours of the forestomach epithelium were seen in control
animals, 2 animals treated with BAP alone (12%), 2 animals treated
with d-limonene alone (13%), and 5 animals treated with both BAP
and d-limonene (22%), developed 0, 2, 3, and 8 tumours,
respectively, although none was judged to be a carcinoma. The
authors concluded that although d-limonene was weakly carcinogenic
for mouse forestomach epithelium it did not increase the tumour
yield caused by pretreatment with BAP and, therefore, was not a
promoter (Field & Roe, 1965).
Cells of embryos taken from F344 rats were infected with
Rauscher murine leukaemia virus and then treated with 0.05 µg/ml
3-methylcholanthrene (3-MCA) followed by 15 µg/ml d-limonene.
Following treatment with 3-MCA and d-limonene, colony formation in
semisolid medium was observed and was considered to denote cell
transformation. Authors reported that d-limonene alone failed to
transform cells and 3-MCA alone was only effective at concentrations
exceeding 0.2 µg/ml (Roe, 1959; Roe & Peirce, 1960; Traul et al.,
1981)).
Elegbede and co-workers attempted to repeat the 1960 study of
Roe and Peirce (see above) using purified d-limonene as well as
oil of sweet orange. The skin of groups of 24 female CD-1 mice were
treated once with 51.2 µg DMBA in 0.2 ml acetone followed 14 days
later by twice weekly applications of 0.1 ml d-limonene or oil of
sweet orange mixed with 0.1 ml acetone. Other groups of mice were
similarly initiated with DMBA, but beginning 7 days later were given
a diet containing 1% d-limonene or oil of sweet orange. In all
cases, administration of d-limonene or oil of sweet orange
continued for 40 weeks after DMBA treatment. Topical (but not
ingested) d-limonene was found to slightly increase the number of
tumours induced by DMBA alone, the increase becoming significant
only after 34 weeks of treatment. When oil of sweet orange (95%
d-limonene content) was applied to the skin, authors reported that
a promoting effect was seen after 7 weeks of treatment; promotion
was not reported, however, when oil of sweet orange was incorporated
into the diet. The authors concluded that a component of orange oil
other than d-limonene was responsible for the promoting activity
observed and that the very small promoting activity seen with
d-limonene may be due to its contamination by this unidentified
component (Elegbede et al., 1986b).
2.2.3.5 Anticarcinogenicity studies
A group of 50 male C57Bl/6J mice were injected s.c. with 25 µg
dibenzpyrene (DBP) followed 24 h later by an injection of 0.15 ml
d-limonene. After 34 weeks of observation, the authors reported
that d-limonene significantly delayed the appearance of lung
adenomas induced by DBP and reduced the total number of tumours
seen. At 30 weeks post-DBP exposure without subsequent exposure to
d-limonene, lung adenomas were seen in approximately 70% of the
animals; with exposure to d-limonene, tumours were seen in
approximately 30% of the animals.
In a second experiment reported in the same paper, a group of
50 female A/J mice were injected s.c. with DBP followed by 16 weekly
injections into the tail vein of 1% v/v suspensions of 1 mg of
d-limonene. The incidence of lung adenomas was reduced from 75% of
animals exposed to DBP alone to 40% of animals exposed to DBP and
injected with d-limonene. Control incidence of lung adenomas in
this experiment was 27%. Treatment of mice with d-limonene alone
reduced this background incidence to approximately 7% (Homburger
et al., 1971).
Simultaneously, 5 µg of BAP and 10 mg d-limonene were applied
to the skin of groups of 50 female ICR/Ha Swiss mice three times a
week for 440 days. d-Limonene was found to partially inhibit BAP
carcinogenicity (Van Duuren & Goldschmidt, 1976).
Female Sprague-Dawley rats were fed diets containing 0, 1000,
or 10 000 ppm d-limonene for 1 wk, at which time they were given a
single gavage dose of 65 mg DMBA/kg bw. The d-limonene-containing
diet was then continued for 27 weeks. Authors reported that
d-limonene delayed the appearance of DMBA-induced mammary tumours
in a dose-related manner, reduced the incidence of tumours, and
caused increased regression of tumours that did appear (Elegbede
et al., 1984a,b).
Female (W/Fu x F344)F2 rats were fed a diet containing 10%
d-limonene for 80 days beginning after the first appearance of
tumours induced by intubation of rats with 130 mg DMBA/kg bw.
Significant tumour regressive action by d-limonene was observed,
and the development of subsequent tumours was also reduced
significantly (Elegbede et al., 1986a).
Following initiation with DMBA (single gavage dose of 65 mg/kg
in sesame oil), groups of female Sprague-Dawley rats were (1) fed a
basal cereal diet one week before and 24 weeks following initiation
with DMBA (controls), (2) fed a diet containing 5% d-limonene one
week before and one week after treatment with DMBA, then fed the
control diet for 24 weeks (initiation group), or (3) fed the control
diet for one week before and one week following DMBA initiation,
then fed the 5% d-limonene diet for 24 weeks
(promotion/progression group). d-Limonene was reported to be
effective in reducing the average number of rat mammary carcinomas
in DMBA-initiated rats when fed during the initiation phase or
during the initiation/progression phase of carcinogenesis. Time to
appearance of the first tumour was extended only when d-limonene
was fed during the initiation phase, however. Authors concluded that
these effects could not be attributed to changes in mammary-relevant
endocrine functions (serum levels of prolactin and duration of
estrus cycle) (Elson et al., 1988).
Female Wistar-Furth rats were (1) placed on diets containing 5%
d-limonene or 5% orange oil (source of d-limonene) for 2 weeks
prior to initiation with nitrosomethylurea (NMU; single i.v. dose of
50 mg/kg bw), then continued on these diets for 23 weeks; (2) fed
diets containing 5% d-limonene for two weeks before and one week
following initiation with NMU, then fed a basal diet for 22 weeks
(initiation group); or (3) fed basal diets prior to and for 1 week
following initiation with NMU, then fed diets containing 5%
d-limonene for 22 weeks (promotion/progression group). Both orange
oil and d-limonene decreased mammary tumour incidence (controls:
80%; d-limonene: 45%; orange oil: 47%) and the average number of
mammary tumours per rat (controls: 1.8; d-limonene: 0.4; orange
oil: 0.8) when present in the diet for two weeks before and 23 weeks
after NMU administration. d-Limonene decreased tumour incidence
and decreased the numbers of tumours per rat (by approximately 50%)
when fed during the promotion/progression experiment but not the
during the initiation experiment (Maltzman et al., 1989).
2.2.3.6 Studies on the mechanism of d-limonene carcinogenicity
This section discusses (1) spontaneously occurring nephropathy
in mature male rats and (2) the current proposed mechanism of
carcinogenicity of d-limonene and other chemicals/mixtures that
exacerbate this condition and lead to alpha2u-globulin-associated
male rat nephropathy.
(1) Mature male rat nephropathy
Normal male rats at least 30 days old exhibited marked
increased protein excretion in their urine not seen in younger males
nor in females of any age. This proteinuria reached a maximum level
of approximately 2.5-3 times that seen in females at 90 days of age.
Microscopically, the authors also observed intracellular droplets in
the epithelial cells of the upper two-thirds of the proximal
convoluted tubules of kidneys of male rats at least 60 days old. The
occurrence of these hyaline droplets consisted of a few groups of
small droplets in occasional cells in 60 day old animals. The number
of cells having droplets and the number and size of droplets per
cell increased with age; from 90 days of age most proximal tubule
cells contained them. In contrast, at 120 and 180 days of age, only
a small number of females showed occasional droplets in a few cells
of the proximal tubules.
These authors further demonstrated the sex-relatedness of this
phenomenon by castrating male rats at 30 days of age and injecting
female rats with 5 mg testosterone every other day from 50 days of
age. When examined at 120 days of age, the castrated male rats
showed proteinuria at a level equal to control females and hyaline
droplets were only seen in 4/20 animals. Testosterone-treated
females, on the other hand, had proteinuria at a level approximately
midway between control males and females. No hyaline droplets were
seen in the proximal tubule cells of these females, however, showing
that the addition of testosterone alone was insufficient to cause
them to appear (Logothetopoulos & Weinbren, 1955).
Numerous authors have since reported detailed descriptions of
spontaneously occurring nephropathy in mature male rats which is a
consequence of this hyaline droplet accumulation. The sequence of
events appears to be that: (1) protein accumulates in the lysosomes
in the cytoplasm of epithelial cells of the P2 segment of the
proximal convoluted tubules in the kidney cortex; (2) the
accumulated material becomes so abundant that it crystallizes,
forming the microscopically visible hyaline droplets; (3) the
continued build-up of material eventually leads to the death of
epithelial cells with a concomitant thinning of the epithelial layer
(although some regeneration is usually seen); and (4) the dead cell
debris becomes lodged in the outer strip of the outer medulla where
the tubules narrow, forming granular casts and causing tubule
dilation with pressure necrosis of the cells of the tubule walls.
There is also an increase in the relative weight of the kidneys
accompanying this phenomenon.
A steadily increasing number of agents have been identified
that appear to exacerbate this hyaline droplet formation and its
consequences in mature male rats. With some, noticeable changes
occur within days of exposure. These agents include complex mixtures
of hydrocarbons such as mineral spirits (Carpenter et al., 1975a;
Phillips & Cockrell, 1984), "60" solvent (Carpenter et al.,
1975b), "high naphthenic solvent" (Carpenter et al., 1977), the
petroleum-derived or shale-derived jet fuel JP-5 (MacEwen & Vernot,
1978a; Parker et al., 1981; Bruner, 1984; MacNaughton & Uddin,
1984), petroleum-derived and shale-derived diesel fuel marine (DFM),
the jet fuels JP-4, JP-TS and JP-7, and the missile propellants
JP-10 and RJ-5 (Bruner, 1984; MacNaughton & Uddin, 1984), C/10-C/11
isoparaffin (Phillips & Egan, 1984; Phillips & Cockrell, 1984), five
petroleum naphtha mixtures (Halder et al., 1984) and unleaded
gasoline (MacFarland et al., 1984; Busey & Cockrell, 1984; Halder
et al., 1984; Kitchen, 1984; Garg et al., 1988a,b, 1989; Short &
Swenberg, 1988; Short et al., 1989a). Also included on the list
are the individual chemicals decahydro-naphthalene (decalin, MacEwen
& Vernot, 1978b; Gaworski et al., 1980, 1981; Bruner, 1984; Alden
et al., 1984; Olson et al., 1986; Stone et al., 1987a,b;
Kanerva et al., 1987b,c; Read, 1988), pentachloroethane (NTP,
1983; Goldsworthy et al., 1988), 1,4-dichlorobenzene (NTP, 1987a;
Charbonneau et al., 1989), perchloroethylene (Goldsworthy et al.,
1988), dimethyl methylphosphonate (NTP, 1987b),
2,2,4-trimethylpentane (Stonard et al., 1986; Short et al.,
1987; Charbonneau et al., 1987; Lock et al., 1987; Loury
et al., 1987; Short et al., 1989a), the chemically unrelated
agents 3-methylamino-1-(3-trifluoromethyl-phenyl)-2-pyrazoline,
cis/trans-2-(4'-t-butylcyclohexyl)-3-hydroxy-1,4-naphthoquinone
and 2,3,5,6-tetrahycro-6-phenylimidazo-(2,1-b) thiazole (Read
et al., 1988) and d-limonene (Kanerva et al., 1987a; NTP,
1990).
There is no obvious common structure among the specifically
identified chemicals that cause an increased accumulation of hyaline
droplets in mature male rat kidneys. And there are examples of
agents that do have similar mixtures/structures to those listed
above that do not cause this phenomenon. These include "70" solvent
(Carpenter et al., 1975c), two other petroleum naphtha mixtures
(Halder et al., 1984), 1,2-dichlorobenzene (Charbonneau et al.,
1989) and an analog of d-limonene, 4-vinylcyclohexene (NTP, 1986).
Recently, however, Miller et al. (1989) reported that they have
identified correlations between structure and binding affinity to
alpha2u-globulin, but no specific details were given in their
published report.
While exposure to unleaded gasoline for 72 h has been shown to
cause effects that are reversible (Garg et al., 1988a), with
chronic exposure to several of these mixtures/chemicals the kidney
nephrosis has been reported to progress to hyperplasia, adenoma and
even adenocarcinoma (MacFarland et al., 1984; Kitchen, 1984;
MacNaughton & Uddin, 1984; NTP, 1987a,b,c; NTP, 1990).
(2) Mechanism for alpha2u-globulin-associated male rat
nephropathy
The mechanism proposed for alpha2u-globulin-associated male
rat nephrosis has recently been thoroughly reviewed by the US
Environmental Protection Agency (US EPA, 1991). Their review
incorporated the results of many studies, including Charbonneau &
Swenberg (1988), Swenberg et al. (1989), Flamm & Lehman-McKeeman
(1991), Lehman-McKeeman et al. (1989), Lehman-McKeeman et al.
(1990a,b), Short et al. (1987), Short et al. (1989a,b), Dietrich
& Swenberg (1991), Murty et al. (1988), Ridder et al. (1990),
and Webb et al. (1989). The review concluded that:
"Of the eight model substances tested in chronic animal
bioassays (decalin, dimethyl methyl phosphonate, JP-4 jet fuel,
JP-5 shale-derived jet fuel, d-limonene, methyl isobutyl
ketone, pentachloroethane, and unleaded gasoline), all invoked
a specific type of protein droplet nephropathy in male rats and
also produced renal tumours in male rats but not in other
species tested. It has been proposed that such renal tumours
are the end product in the following sequence of functional
changes in the epithelial cells of proximal tubules:
* Excessive accumulation of hyaline droplets in proximal
tubules, representing lysosomal overload, cell loss, and
regenerative cellular proliferation.
* Cell debris in the form of granular casts accumulates at
the 'corticomedullary' junction with associated dilation
of the affected tubule segment and more distally,
mineralization of tubules within the renal medulla.
* Single-cell necrosis accompanied by compensatory cell
proliferation and exacerbation of the chronic progressive
nephropathy characteristically found in aging rats occurs.
* Renal tubule hyperplasia and neoplasia develop
subsequently.
According to this hypothesis, the increased proliferative
response caused by the chemically induced cytotoxicity results
in clonal expansion of spontaneously initiated renal tubule
cells and increased incidence of renal tumour formation (Trump
et al., 1984a; Alden, 1989; Swenberg et al., 1989). This
line of reasoning leads supporters of the hypothesis to
conclude that the acute and chronic renal effects induced in
male rats by such chemicals will be unlikely to occur in any
species not producing alpha2u-globulin, or a very closely
related protein, in the large quantities typically seen in the
male rat (Alden, 1989; Borghoff et al., 1990; Green et al.,
1990; Olson et al., 1990; Flamm & Lehman-McKeeman, 1991;
Swenberg, 1991)."
The following paragraphs summarize some of the data that
support this hypothesis:
Contents and catabolism of hyaline droplets/
phagolysosomes
The composition of the hyaline droplet inclusions has been
identified by Kanerva et al. (1987c) using two-dimensional gel
electrophoresis and immunological binding as being four species of
the low molecular weight protein alpha2u-globulin. As expected,
alpha2u-globulin was found to be absent in immature male rats and
female rats of all ages. It was present, however, in female rats
that had been ovariectomized and repeatedly injected with
testosterone. More recently, groups have confirmed that
alpha2u-globulin is the major constituent of the renal
phagolysosomal inclusions in male rats exposed to unleaded gasoline
(Garg et al., 1988b), and perchloroethylene and penta-chloroethane
(Goldsworthy et al., 1988). Finally, Dietrich and Swenberg (1991)
reported that male NCI-Black-Reiter rats, which do not produce
alpha2u-globulin, are not susceptible to nephropathy produced by
2,2,4-trimethylpentane, 1,4-dichloro-benzene, isophorone, PS-6
unleaded gasoline, or d-limonene.
A sex-specific protein has been identified in human urine
(Bernard et al., 1989), but the concentration (up to 108 µg/l) is
about 10 000 times less than that of alpha2u-globulin in male rats
(about 40 mg/24 h) (Roy & Neuhaus, 1967). Therefore assuming that
the protein in human urine shows the same binding characteristics as
alpha2u-globulin, it is reasonable to conclude that humans would
be comparably less sensitive than the male rat.
Alpha2u-globulin is synthesized in the liver of rats of both
sexes. This protein is filtered by the kidney glomeruli and
reabsorbed by the epithelial cells of the proximal convoluted
tubules by the formation of phagosomes at the lumenal surface.
Normally, the phagosomes then combine with cellular lysosomes and
the protein is degraded. In mature male rats, however, this
catabolism is slowed in some way under hormonal influence leading to
the intracellular accumulation of the protein which becomes
manifested as hyaline droplets. The degradation is slowed even more
by exposure to at least some of the agents listed in the previous
section (Stonard et al., 1986; Short et al., 1987; Kanerva
et al., 1987c; Charbonneau et al., 1987). A time- and
dose-dependent progressive increase in the number and size, and
alterations in the morphology of phagolysosomes in proximal
convoluted tubule epithelial cells have been demonstrated with
exposure to unleaded gasoline (Garg et al,. 1989). The ability of
unleaded gasoline to induce these effects declines with the age of
the male rat, in proportion to the declining production of
alpha2u-globulin (Murty et al., 1988).
Binding of chemicals to alpha2u-globulin
1,4-Dichlorobenzene as well as its major metabolite,
2,5-dichlorophenol, were both found to be reversibly bound (and
1,4-dichlorobenzene also covalently bound) to alpha2u-globulin in
the kidneys of treated male rats (Charbonneau et al., 1989).
Similarly, two groups have attempted to identify the specific
binding between one of these agents (2,2,4-tri-methylpentane) and
alpha2u-globulin. Loury et al. (1987) reported that no covalent
binding could be found, nor did there appear to be formation of a
Schiff base. Lock et al. (1987), however, detected reversible
binding of the metabolite 2,4,4-trimethyl-2-pentanol to
alpha2u-globulin. This specific metabolite was found in liver
cells of male rats only.
Likewise with decalin, an alcohol metabolite has been
identified that is unique to male rats (Olson et al., 1986). While
both male and female rats synthesize cis,cis-2-decalol and
trans,cis-2-decalol from cis-decalin in their livers,
cis,cis-1-decalol was found only in males and
cis,trans-1-decalol was produced in much higher concentrations
than in females. In similar fashion, with trans-decalin as the
substrate trans,trans-1-decalol was seen only in livers of male
rats. When kidneys were examined, 2-decalone (cis or trans,
depending on the original substrate) was found in the males while no
decalin metabolites whatever were seen in female kidneys. Whether
these unique male decalin metabolites react with alpha2u-globulin
has not yet been tested.
More recently, Lehman-McKeeman et al. (1989) have reported
that d-limonene and d-limonene-1,2-oxide, a major metabolite of
d-limonene, were found reversibly bound to alpha2u-globulin
(identified by amino acid sequencing) in the kidneys of treated
male, but not female, rats.
Inhibition of alpha2u-globulin catabolism
Charbonneau et al. (1988) reported that alpha2u-globulin
bound in kidney cytosol was not digested by purified proteases or
proteinase.
Treating rats with leupeptin, an inhibitor of cathepsin B, a
major lysosomal peptidase, mimics the ability of unleaded gasoline
to cause the accumulation of alpha2u-globulin in kidney
phagolysosomes. Authors concluded that the mechanism of
nephrotoxicity involves inhibition of renal phagolysosomal
proteolysis (Olsen et al., 1988).
Induction of cell proliferation
Short et al. (1989a) have shown that proliferation of
epithelial cells in the P2 proximal tubule segment in male rats is
associated with exposure of the animals to either
2,2,4-trimethylpentane or unleaded gasoline. They noted that this
proliferation closely paralleled the extent and severity of
detectable alpha2u-globulin in the same cells. The authors
speculated that this proliferation leads to the nephrocarcinogenic
effects caused by these agents.
Proliferation was also noted by Charbonneau et al. (1989) in
male rats treated with 1,4-dichlorobenzene. Short et al. (1989b)
observed increased numbers of atypical cell foci and renal cell
tumours in male, but not female, rats initiated with
N-ethyl-N-hydroxyethylnitrosamine and subsequently exposed to
unleaded gasoline or 2,2,4-trimethylpentane. The extent of the
promotion was reported to be dose-related and to parallel the
effects of these compounds on chronic cell proliferation.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity
d-Limonene was reported to increase the number of abnormal
chick embryos to approximately 50% when a single dose (25 µM/embryo)
in olive oil was injected suprablastodermically (Abramovici &
Rachmuth-Roizman, 1983).
Pregnant mice were given oral doses of 0, 591, or 2363 mg/kg bw
d-limonene from days 7 through 12 of gestation. Authors reported
significant decreases in body weight gain of dams in the high-dose
group; fetuses of dams exposed to the high-dose showed increased
incidences of lumbar rib, fused rib, delayed ossification, and
decreased body weight gain relative to fetuses of control dams
(Kodama et al., 1977a).
Pregnant Japanese white rabbits were given oral doses of 0,
250, 500, or 1000 mg/kg bw d-limonene from day 6 to day 18 of
gestation. Significant decreases in body weight gain in dams given
250 or 500 mg/kg bw/day d-limonene were observed, and survival in
dams given 1000 mg/kg bw/day was significantly reduced (40%
survival). From examination of the fetuses, authors concluded that
d-limonene was not teratogenic in rabbits (Kodama et al.,
1977b).
2.2.6 Special studies on genotoxicity
Table 2: Genotoxicity data on d-limonene
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium .03-3 µmol/plate neg Florin et
(+&-activ) TA98, TA100, al., 1980
TA1535, TA1537
Yeast S. cerevisiae .1-100 mM/5x107 (1) Fahrig,
(-activ) MP1 strain cells 1984
Mammalian Mouse embryo 0-215 mg/kg (2) Fahrig,
(-activ) system 1984
Ames test S. typhimurium 0-20 µM/plate neg Watabe
(+&-activ) TA1535, TA100, et al.,
TA1537, TA1538, 1980
TA98
Ames test S. typhimurium 0-3333 µg/plate neg Haworth
(+&-activ) TA98, TA100, et al.,
TA1535, TA1537 1983
Ames test S. typhimurium 0-3333 µg/plate neg NTP,
(+&-activ) TA100, TA1535, 1990
TA1537, TA98
Mammalian Mouse L5178Y neg NTP,
(+&-activ) cells in vitro 1990
Sister Chinese hamster 0-162 µg/ml neg NTP,
chromatid ovary cells 1990
exchange in vitro
(+&-activ)
Chromosomal Chinese hamster 0-500 µg/ml (3) neg NTP,
aberrations ovary cells 0-100 µg/ml (4) neg 1990
(+&-activ) in vitro
Table 2 cont'd
Test Test Concentration
System Object of d-limonene Results Reference
Ames test S. typhimurium 150 mg/plate neg Heck et
(+&-activ) TA1535, TA1537, al., 1989
TA1538, TA98,
TA100
Mammalian Mouse L5178Y 100 µg/ml neg Heck et
(+&-activ) cells in vitro al., 1989
(1) Author reported that results indicated that d-limonene was a
co-recombinant and an anti-mutagen.
(2) Author reported that results indicated that d-limonene is inactive
when given alone, but reduces the mutagenic effect of ethylnitrosourea
when the two are co-administered.
(3) Activated: addition of S9 from the livers of Aroclor 1254-induced
male Sprague Dawley rats.
(4) Not activated
2.2.7 Special studies on immune responses
Limonene has been identified as a primary skin irritant in
humans (Pirila et al., 1964; Klecak et al., 1977), rabbits
(research Institute for the Development of Fragrance Materials,
Inc., 1984; Research Institute for Fragrance Materials Inc., 1985),
guinea-pigs (Klecak et al., 1977), and mice (Gad et al., 1986).
d-Limonene was reported both to elicit (De Groot et al., 1985)
and not to elicit (Greif, 1967) allergic reactions in human skin and
to elicit allergic reactions in mouse skin (Gad et al., 1986).
d-Limonene was reported to eliminate human skin sensitization to
several aldehydes when they were co-administered with d-limonene
(Opdyke, 1975), and d-limonene was reported to reduce the
sensitivity and delayed hypersensitivity of guinea-pig skin to
citral (Hanau et al., 1983; Barbier & Benezra, 1983). Gozsy and
Kato (1957) reported that local endothelial phagocytosis is induced
in rat skin by d-limonene, and that d-limonene increases
phagocytosis in guinea-pig skin in vivo and in mouse skin
in vitro (Gozsy & Kato, 1958) and increases capillary permeability
in the guinea-pig (Kato & Gozsy, 1958).
Male BALB/c mice were treated intragastrically with 0, 0.002,
0.008, 0.033, 0.133, 0.531 or 2.125 mg/ml d-limonene daily. After
8 weeks, but not after 4 weeks, the mitogenic responses of spleen
cells taken from the treated animals and exposed in vitro to
concanavalin A and phytohaemagglutinin, which stimulate T-cells, and
lipopolysaccharide, which affects B-cells, were found to be
suppressed by the d-limonene treatment.
Other animals in groups of 10 were given these same doses of
d-limonene daily for 7 days prior to, or 8 days following,
immunization with keyhole limpet haemocyanin (KLH). Primary effects
on T- and B-cell responses were determined 10 days later.
Secondary effects were determined by reinoculating the animals
with KLH 21 days after the first inoculation and then assaying on
day 24. The primary and secondary responses were reported to be
suppressed by d-limonene if the animals were exposed to KLH first,
but stimulated in treated mice if exposed to KLH after d-limonene
exposure.
Histopathological examination of secondary lymphoreticular
tissue taken from animals exposed to d-limonene showed significant
secondary follicle development and prominent lymphoid nodules and
aggregates in the pancreas and intestinal mucosa, which were evident
in animals which had received the highest dose of d-limonene
(Evans et al., 1987).
2.3 Observations in humans
Numerous prospective and retrospective cohort epidemiological
studies have been done on populations of refinery, chemical plant,
and service station workers, exposed to mixtures of chemicals that
have been associated with male rat alpha2u-globulin nephropathy
(Hanis et al., 1979; Schottenfeld et al., 1981; Hanis et al.,
1982; Thomas et al., 1982; Austin & Schnatter, 1983; Higginson
et al., 1984; Raabe, 1984; Wen et al., 1984; Wong & Raabe, 1989;
Page & Mehlman, 1989). No statistically significant evidence was
found in any study to link incidence of cancer of the kidney to
work-place exposure to mixtures of complex hydrocarbons.
Igimi et al. (1976) reported that d-limonene was a safe and
effective gallstone solubilizer in animals and humans; side effects
in humans were reported to be pain and tenderness radiating from the
upper abdomen to the anterior chest, nausea and vomiting, and
diarrhoea.
3. COMMENTS
d-Limonene has been shown to reduce body weights
significantly in male and female mice and rats and in female
rabbits. The NOEL for this effect was 150 mg of d-limonene kg
bw/day (administered by gavage) in a 2-year study in male rats.
Oral administration of d-limonene (400 mg/kg bw/day) to rats
for 20 days slightly increased liver weight and phospholipid
content, decreased liver and serum cholesterol levels, increased the
concentration of cytochromes P450 and B5, and increased the
activities of aminopyrine demethylase and aniline hydroxylase.
Although liver lesions were not associated with the administration
of d-limonene in a 2-year study in rats (doses up to 150 mg/kg
bw/day for males; doses up to 600 mg/kg bw/day for females), a daily
gavage dose of 500 mg d-limonene/kg bw/day for 2 years was
associated with an increased incidence of multinucleated liver
hepatocytes and cytomegaly in male mice. The NOEL for these effects
was 250 mg/kg bw/day administered by gavage to male mice for 2
years.
Dermal application of d-limonene has been reported to cause
irritation and immune-mediated skin reactions in a number of
species, including humans. Oral administration of d-limonene to
mice was reported to have immunological effects, including
suppression of the in vitro mitogenic response by mouse T-cells
and B-cells and both suppression and stimulation of antigenic
responses. However, the biological significance of these in vitro
changes is not known.
Results of teratogenicity studies in mice suggest that maternal
consumption of 2400 mg of d-limonene/kg bw/day but not of
600 mg/kg bw/day, affected the development of fetuses (decreased
body weight gain, increased incidences of lumbar rib and fused rib,
and delayed ossification). However, d-limonene was reported to be
non-teratogenic in rabbits at doses ranging from 250 to 1000 mg/kg
bw/day (although the highest dose significantly reduced survival of
the dams).
d-Limonene consistently produced negative results in
genotoxicity studies, has been reported to inhibit the activity of
other mutagens and carcinogens, and gave mixed responses in
tumour-promotion studies.
Data clearly demonstrate that d-limonene exacerbates the
spontaneously occurring nephropathy in mature male rats which, after
prolonged exposure, is associated with an increase in the incidence
of renal tumours that are not commonly observed in control male
rats. However, d-limonene is not the only substance that has this
effect: various hydrocarbon mixtures and several other chemicals
have also been reported to exacerbate spontaneous nephropathy and
cause renal tumours in male rats. Although, in studies designed to
elucidate the mechanism of this carcinogenic process, chemicals
other than d-limonene were generally used as test substances, such
studies have shown that the following mechanism is likely to be
applicable to d-limonene-induced nephropathy and renal tumours in
male rats: (1) d-limonene and d-limonene-1,2-oxide (a metabolite
of d-limonene) bind reversibly to alpha2u-globulin in the
kidneys of d-limonene-treated male rats; (2) this binding further
slows down the normally slow processing of reabsorbed
alpha2u-globulin; and (3) as a result, alpha2u-globulin
accumulates in the phagolysosomes (hyaline droplets) of epithelial
cells of the proximal convoluted tubules of the kidney, leading to
necrosis, accumulation of cell debris, and regenerative hyperplasia.
The postulated mechanism of action for some non-genotoxic
carcinogens suggests that regenerative hyperplasia may increase
clonal expansion of spontaneously initiated cells and lead to the
development of the renal tumours observed.
A NOEL for the d-limonene-associated increase in
alpha2u-globulin levels in male rat kidney has not been
identified, but the lowest-observed-effect level from a 21-day
gavage study of d-limonene in male rats was 75 mg/kg bw/day. A
sex-specific protein with molecular features similar to
alpha2u-globulin has been identified in human urine, but the
concentration is at least four orders of magnitude less than that of
alpha2u-globulin in male rats.
The Committee concluded that the postulated mechanism for
d-limonene-induced nephropathy and renal tumours in the male rat
was probably not relevant to humans, and that toxic end points
associated with this effect were not appropriate bases for the
derivation of an ADI for d-limonene.
4. EVALUATION
Based on the significant decreases in body weight gain
associated with administration of d-limonene to male and female
mice and rats and female rabbits, an ADI of 0-1.5 mg/kg bw was
established for this substance. The Committee considered the known
natural occurrence and food additive uses of d-limonene, and
concluded that only a small proportion of total intake is likely to
be derived from direct additive use. The Committee therefore
recommended that food additive intake be restricted to 75 µg/kg
bw/day, which represents 5% of the maximum ADI for d-limonene.
5. REFERENCES
ABRAMOVICI, A. & RACHMUTH-ROIZMAN, P. (1983). Molecular
structure-teratogenicity relationships of some fragrance additives.
Toxicology, 29: 143-156.
ALDEN, C.L., KANERVA, R.L., RIDDER, G. & STONE, L.C. (1984). Chapter
VIII: The pathogenesis of the nephrotoxicity of volatile
hydrocarbons in the male rat. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 107-120, Princeton Scientific, Princeton, NJ.
ALDEN, C.L. (1989) Male rat specific alpha2u-globulin nephropathy
and renal tumorigenesis. In: Bach, P.H. & Lock, E.A. (eds.),
Handbook of Toxicologic Pathology. Academic Press, New York. In
press (cited in US EPA 1991).
ARIYOSHI, T., ARAKAKI, M., IDEGUCHI, K., ISHIZUKA, Y., NODA, K. &
IDE, H. (1975). Studies on the metabolism of d-limonene
(p-mentha-1,8-diene); III. Effects of d-limonene on the lipids and
drug metabolizing enzymes in rat livers. Xenobiotica, 5: 33-38.
AUSTIN, S.G. & SCHNATTER, A.R. (1983). A cohort mortality study of
petrochemical workers. Journal of Occupational Medicine, 25:
304-312.
BARBIER, P. & BENEZRA, C. (1983) The influence on induced
hypersensitivity to citral in guinea-pigs; II. Label distribution in
the skin of 14C-labelled citral. Acta Dermatovener (Stockholm),
63: 93-96.
BERNARD, A.M., LAUWERYS, R.R., NOEL, A., VANDELEENE, B. & LAMBERT,
A. (1989). Urine protein I: a sex-dependent marker of tubular or
glomerular dysfunction. Clin. Chem., 35: 2141-2.
BORGHOFF, S.J., SHORT, B.G., & SWENBERG, J.A. (1990) Biochemical
mechanisms and pathobiology of alpha2u-globulin nephropathy.
Ann. Rev. Pharmacol. Toxicol., 30: 349-367.
BRUNER, R.H. (1984). Chapter X: Pathologic findings in laboratory
animals exposed to hydrocarbon fuels of military interest. In:
Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 133-140,
Princeton Scientific, Princeton, NJ.
BUSEY, W.M. & COCKRELL, B.Y. (1984). Chapter IV: Non-neoplastic
exposure-related renal lesions in rats following inhalation of
unleaded gasoline vapors. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 57-64, Princeton Scientific, Princeton, NJ.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975a). Petroleum hydrocarbon toxicity studies: III.
Animal and human response to vapors of Stoddard solvent. Toxicology
and Applied Pharmacology, 32: 282-297.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975b). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "60 solvent." Toxicology
and Applied Pharmacology, 32: 374-394.
CARPENTER, C.P., KINKEAD, E.R., GEARY, Jr., D.L., SULLIVAN, L.J. &
KING, J.M. (1975c). Petroleum hydrocarbon toxicity studies: VI.
Animal and human responses to vapors of "70 solvent." Toxicology
and Applied Pharmacology, 34: 395-412.
CARPENTER, C.P., GEARY, JR., D.L., MYERS, R.C., NACHREINER, D.J.,
SULLIVAN, L.J. & KING, J.M. (1977). Petroleum hydrocarbon toxicity
studies: XV. Animal and human responses to vapors of "high
naphthenic solvent." Toxicology and Applied Pharmacology, 41:
251-260.
CHARBONNEAU, M., LOCK, E.A., STRASSER, J., COX, M.G., TURNER, M.J. &
BUS, J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: I.
Metabolic disposition of TMP in male and female Fischer 344 rats.
Toxicology and Applied Pharmacology, 91: 171-181.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J. & SWENBERG,
J.A. (1989). Involvement of reversible binding to alpha2u-globulin
in 1,4-dichlorobenzene-induced nephrotoxicity. Toxicology and
Applied Pharmacology, 99: 122-132.
CHARBONNEAU, M. & SWENBERG, J.A. (1988). Studies on the bio-chemical
mechanism of alpha2u-globulin nephropathy in rats. CIIT
Activities, 8: 1-5.
CURVALL, M., ENZELL, C. & PETTERSSON, B. (1984). An evaluation of
the utility of four in vitro short-term tests for predicting the
cytotoxicity of individual compounds derived from tobacco smoke.
Cell Biology and Toxicology, 1: 173-193.
DIETRICH, D.R. & SWENBERG, J.A. (1991). NCI-Black-Reiter (NBR) male
rats fail to develop renal disease following exposure to agents that
induce alpha2u-globulin nephropathy. Fundamentals of Applied
Toxicology, 16: 749-762.
DE GROOT, A.C., LIEM, D.H., NATER, J.P. & VAN KETEL, W.G. (1985).
Patch tests with fragrance materials and preservatives. Contact
Dermatitis, 12: 87-92.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984a). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Carcinogenesis, 5: 661-664.
ELEGBEDE, J.A., ELSON, C.E., QURESHI, A., TANNER, M.A. & GOULD, M.N.
(1984b). Inhibition of DMBA-induced mammary cancer by the
monoterpene d-limonene. Proceedings of the American Association
of Cancer Researchers, 25: 127A.
ELEGBEDE, J.A., ELSON, C.E., TANNER, M.A., QURESHI, A. & GOULD, M.N.
(1986a). Regression of rat primary mammary tumours following dietary
d-limonene. Journal of the National Cancer Institute, 76:
323-325.
ELEGBEDE, J.A., MALTZMAN, T.H., VERMA, A.K., TANNER, M.A., ELSON,
C.E. & GOULD, M.N. (1986b). Mouse skin tumour promoting activity of
orange peel oil and d-limonene: a re-evaluation. Carcinogenesis,
7: 2047-2049.
ELSON, C.E., MALTZMAN, T.H., BOSTON, J.L., TANNER, M.A. & GOULD,
M.N. (1988). Anti-carcinogenic activity of d-limonene during the
initiation and promotion/progression stages of DMBA-induced rat
mammary carcinogenesis. Carcinogenesis, 9: 331-332.
FAHRIG, R. (1984). Genetic mode of action of cocarcinogens and
promoters in yeast and mice. Molecular and General Genetics, 194:
7-14.
FLAMM, W.G. & LEHMAN-McKEEMAN, L. (1991). The human relevance of the
renal tumour-inducing potential of d-limonene in male rats:
Implications for risk assessment. Regulatory Toxicology and
Pharmacology, 13: 70-86.
FLORIN, I., RUTBERG, L., CURVALL, M. & ENZELL, C. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18: 219-232.
GAD, S.C., DUNN, B.J., DOBBS, D.W., REILLY, C. & WALSH R.D. (1986).
Development and validation of an alternative dermal sensitization
test: the mouse ear swelling test (MEST). Toxicology and Applied
Pharmacology, 84: 93-114.
GARG, B.D., OLSON, M.J., DEMYAN, W.F. & ROY, A.K. (1988a). Rapid
post-exposure decay of alpha2u-globulin and hyaline droplets in
the kidneys of gasoline-treated male rats. Journal of Toxicology
and Environmental Health, 24: 145-160.
GARG, B.D., OLSON, M.J., MANCINI, M.A. & ROY, A.K. (1988b).
Localization of alpha2u-globulin in hyaline droplets
(phagolysosomes) of the male rat renal cortex by immunohistochemical
techniques. Journal of Histochemistry and Cytochemistry, 36: 960.
GARG, B.D., OLSON, M.J., LI, L.C. & ROY, A.K. (1989). Phagolysosomal
alterations induced by unleaded gasoline in epithelial cells of the
proximal convoluted tubules of male rats: Effect of dose and
treatment duration. Journal of Toxicology and Environmental Health,
26: 101-118.
GAWORSKI, C.L., HAUN, C.C. & MacEWEN, J.D. (1981). A ninety-day
inhalation toxicity study of decalin. The Toxicologist, 1, 76.
GAWORSKI, C.L., LEAHY, H.F. & BRUNER, H.F. (1980). Subchronic
inhalation toxicity of decalin, Proceedings of the Tenth Conference
on Environmental Toxicology, AFAMRL-TR-79-121 (ADA086341) Aerospace
Medical Research Laboratory, Wright-Patterson Air Force Base, OH.
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L. & POPP, J.A. (1988).
Potential role of alpha2u-globulin, protein droplet accumulation,
and cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicology and Applied Pharmacology, 96: 367-379.
GOZSY, B. & KATO, L. (1957). Action of reticulo-endothelial-system
stimulators on the histamine depleted rats. Arch. Int. Pharmacodyn,
CXII: 235-246.
GOZSY, B. & KATO, L. (1958). Reticulo-endothelial system stimulating
compounds and their mechanism of action. Can. J. Biochem. Physiol.,
36: 319-326.
GREEN, T., ODUM, J., NASH, J.A. & FOSTER, J.R. (1990).
Perchloroethylene-induced rat kidney tumours: An investigation of
the mechanisms involved and their relevance to humans. Toxicol.
Appl. Pharmacol., 102: 77-89.
GREIF, N. (1967). Cutaneous safety of fragrance material as measured
by the Maximization Test. American Perfumer and Cosmetics, 82:
54-57.
HALDER, C.A., WARNE, T.M. & HATOUM, N.S. (1984). Chapter VI: Renal
toxicity of gasoline and related petroleum naphthas in male rats.
In: Advances in Modern Environmental Toxicology, Renal Effects of
Petroleum Hydrocarbons, M.A. Mehlman, editor, pp. 73-88, Princeton
Scientific, Princeton, NJ.
HANAU, D., GROSSHANS, E., BARBIER, P. & BENEZRA, C. (1983). The
influence of limonene on induced delayed hypersensitivity to citral
in guinea-pigs; I. Histology study. Acta Dermatovener (Stockholm)
63: 1-7.
HANIS, N.M., HOLMES, T.M., SHALLENBERGER, L.G. & JONES, K.E. (1982).
Epidemiologic study of refinery and chemical plant workers. Journal
of Occupational Medicine, 24: 203-212.
HANIS, N.M., STRAVRAKY, K.M. & FOWLER, J.L. (1979). Cancer mortality
in oil refinery workers. Journal of Occupational Medicine, 21:
167-174.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W. & ZEIGER, E.
(1983). Salmonella mutagenicity test results for 250 chemicals.
Environmental Mutagenesis Supplement, 1: 3-142.
HECK, J.D., VOLLMUTH, T.A., CIFONE, M.A., JAGANNATH, D.R., MYHR, B.
& CURREN, R.D. (1989). An evaluation of food flavoring ingredients
in a genetic toxicity screening battery. The Toxicologist, 1: 257.
HIGGINSON, J., MUIR, C. & BUFFLER, P.A. (1984). Chapter XV: The
epidemiology of renal carcinoma in humans with a note on the effect
of exposure to gasoline. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 203-226, Princeton Scientific, Princeton, NJ.
HOMBURGER, F., TREGER, A. & BOGER, E. (1971). Inhibition of murine
subcutaneous and intravenous benzo(rst)pentaphene carcinogenesis by
sweet orange oils and d-limonene. Oncology, 25: 1-10.
IGIMI, H., HISATSUGU, T. & NISHIMURA, M. (1976). The use of
d-limonene preparation as a dissolving agent of gallstones.
Digestive Diseases, 21: 926-939.
IGIMI, H., NISHIMURA, M., KODAMA, R. & IDE, H. (1974). Studies on
the metabolism of d-limonene (p-mentha-1,8-diene): I. The
absorption, distribution and excretion of d-limonene in rats.
Xenobiotica, 4: 77-84.
IMAIZUMI, K. HANADA, K., MAWATARI, K. & SUGANO, M. (1985). Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49: 2795-2796.
KANERVA, R.L. & ALDEN, C.L. (1987). Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and Chemical Toxicology, 25: 355-358.
KANERVA, R.L., RIDDER, G.M., LEFEVER, F.R. & ALDEN, C.L. (1987a).
Comparison of short-term renal effects due to oral administration of
decalin or d-limonene in young adult male Fischer-344 rats.
Food and Chemical Toxicology, 25: 345-353.
KANERVA, R.L., MCCRACKEN, M.S., ALDEN, C.L. & STONE, L.C. (1987b).
Morphogenesis of decalin-induced renal alterations in the male rat.
Food and Chemical Toxicology, 25: 53-61.
KANERVA, R.L., RIDDER, G.M., STONE, L.C. & ALDEN, C.L. (1987c).
Characterization of spontaneous and decalin-induced hyaline droplets
in kidneys of adult male rats. Food and Chemical Toxicology, 25:
63-82.
KATO, L. & GOZSY, B. (1958). Treatment of experimental tuberculosis
of the guinea-pig with dihydrostreptomycin and simultaneously with
substances acting on the host. Arch. Int. Pharmacodyn, CXVII:
52-62.
KITCHEN D.N. (1984). Chapter V: Neoplastic renal effects of unleaded
gasoline in Fischer 344 rats. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 65-88, Princeton Scientific, Princeton, NJ.
KLECAK, G., GELEICK, H. & FREY, R. (1977). Screening of fragrance
materials for allergenicity in the guinea-pig; comparison of four
testing methods. J. Soc. Cosmet. Chem., 28: 53-64.
KODAMA, R., NODA, K. & IDE, H. (1974). Studies on the metabolism of
d-limonene (p-mentha-1,8-diene): II. The metabolic fate of
d-limonene in rabbits. Xenobiotica, 4: 85-95.
KODAMA, R., INOUE, H., NODA, K., IDE, H., YAMAMOTO, H., YOSHIHARA,
S. & YOSHIMURA, H. (1976a). Effect of d-limonene and related
compounds on bile flow and biliary lipid composition in rats and
dogs. Life Sciences, 19: 1559-1568.
KODAMA, R., YANO, T., FURUKAWA, K., NODA, K. & IDE, H. (1976b).
Studies on the metabolism of d-limonene (p-mentha-1,8-diene): IV.
Isolation and characterization of new metabolites and species
differences in metabolism. Xenobiotica, 6: 377-389.
KODAMA, R., OKUBO, A., ARAKI, E., NODA, K., IDE, H. & IKEDA, T.
(1977a). Studies on d-limonene as a gallstone solubilizer; VII.
Effects on development of mouse fetuses and offspring. Oyo Yakuri
(Pharmacometrics) 13: 863-873.
KODAMA, R., OKUBO, A., SATO, K., ARAKI, E., NODA, K., IDE, H. &
IKEDA, T. (1977b). Studies on d-limonene as a gallstone
solubilizer; IX. Effects on development of rabbit fetuses and
offspring. Oyo Yakuri (Pharmacometrics), 13: 885-898.
LEHMAN-McKEEMAN, L.D., CAUDILL, D., TAKIJIKU, R., SCHNEIDER, R.E. &
YOUNG, J.A. (1990a). Comparative disposition of d-limonene in rats
and mice: Relevance to male-rat-specific nephrotoxicity. Toxicol.
Lett., 53: 193-195.
LEHMAN-McKEEMAN, L.D., RIVERA-TORRES, M.I. & CAUDILL, D. (1990b).
Lysosomal degradation of alpha2u-globulin and
alpha2u-globulin-xenobiotic conjugates. Toxicology and Applied
Pharmacology, 103: 539-548.
LEHMAN-McKEEMAN, L.D., RODRIGUEZ, P.A., TAKIGIKU, R., CAUDILL, D. &
FEY, M.L. (1989). d-Limonene-induced male rat-specific
nephrotoxicity: Evaluation of the association between d-limonene
and alpha2u-globulin. Toxicology and Applied Pharmacology, 99:
250-259.
LOCK, E.A., CHARBONNEAU, M., STRASSER, J., SWENBERG, J.A. & BUS,
J.S. (1987). 2,2,4-trimethylpentane-induced nephrotoxicity: II. The
reversible binding of a TMP metabolite to a renal protein fraction
containing alpha/2u-globulin. Toxicology and Applied
Pharmacology, 91: 182-192.
LOGOTHETOPOULOS, J. & WEINBREN, K. (1955). Naturally occurring
protein droplets in the proximal tubule of the rat's kidney.
British Journal of Experimental Pathology, 36: 402-406.
LOURY, D.J., SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987). Assessment
of the covalent binding potential of 2,2,4-trimethylpentane to rat
alpha2u-globulin. Toxicology and Applied Pharmacology, 88:
44-56.
MacEWEN, J.D. & VERNOT, E. (1978a). Evaluation of toxic effects of
90-day continuous exposure to conventional JP-5 jet fuel. In: Toxic
Hazards Research Unit Annual Report, AFAMRL-TR-78-55 (ADA062138)
Aerospace Medical Research Laboratory, Wright-Patterson Air Force
Base, OH.
MacEWEN, J.D. & VERNOT, E. (1978b). The effects of subchronic
exposure of rodents to inhaled decalin. In: Toxic Hazards Research
Unit Annual Report, AFAMRL-TR-78-55 (ADA062138) Aerospace Medical
Research Laboratory, Wright-Patterson Air Force Base, OH.
MacFARLAND, H.N., ULRICH, C.E., HOLDSWORTH, C.E., KITCHEN, D.N.,
HALLIWELL, W.H. & BLUM S.C. (1984). A chronic inhalation study with
unleaded gasoline vapor. Journal of the American College of
Toxicology, 3: 231-248.
MacNAUGHTON, M.G. & UDDIN, D.E. (1984). Chapter IX: Toxicology of
mixed distillate and high-energy synthetic fuels. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 121-132, Princeton
Scientific, Princeton, NJ.
MALTZMAN, T.H., HURT, L.M., ELSON, C.E., TANNER, M.A. & GOULD, M.N.
(1989). The prevention of nitrosomethylurea-induced mammary tumours
by d-limonene and orange oil. Carcinogenesis, 10: 781-783.
MEYER, F. & MEYER, E. (1959). Absorption of ethereal oils and
substances contained in them through the skin.
Arzneimittelforschung (Drug Research), 9: 516-519.
MILLER, A.B., BOWEN, J.P., BORGHOFF, S.J. & SWENBERG, J.A. (1989).
Computational and molecular modeling studies of alpha2u-globulin.
Abstract papers of the American Chemical Society, 198: Medi-39.
MURTY, C.V.R., OLSON, M.J., GARG, B.D. & ROY, A.K. (1988).
Hydrocarbon-induced hyaline droplet nephropathy in male rats during
senescence. Toxicology and Applied Pharmacology, 96: 380-392.
NTP (1983). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of pentachloroethane (CAS No.
76-01-7) in F344/N rats and B6C3F1 mice (Gavage Studies), NTP
TR 232, NIH Publication No. 83-1788.
NTP (1986). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 4-vinyl-cyclohexene (CAS
No. 100-40-3) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 303, NIH Publication No. 86-2559.
NTP (1987a). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of 1,4-di-chlorobenzene (CAS
No. 106-46-7) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 319, NIH Publication No. 87-2570.
NTP (1987b). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of dimethylmethylphosphonate
(CAS No. 756-79-6) in F344/N rats and B6C3F1 mice (Gavage
Studies), NTP TR 323, NIH Publication No. 88-2579.
NTP (1990). National Toxicology Program technical report on the
toxicology and carcinogenesis studies of d-limonene (CAS No.
5989-27-5) in F344/N rats and B6C3F1 mice (Gavage Studies),
NTP TR 347, NIH Publication No. 90-2802.
OLSON, C.T., YU, K.O. & SERVE, M.P. (1986). Metabolism of
nephrotoxic cis- and trans-decalin in Fischer-344 rats. Journal
of Toxicology and Environmental Health, 18: 285-292.
OLSON, M.J., JOHNSON, J.T. & REIDY, C.A. (1990). A comparison of
male rat and human urinary proteins: Implications for human
resistance to hyaline droplet nephropathy. Toxicol. Appl.
Pharmacol., 102: 524-536.
OLSON, M.J., MANCINI, M.A., GARG, B.D. & ROY, A.K. (1988).
Leupeptin-mediated alteration of renal phagolysosomes: Similarity to
hyaline droplet nephropathy of male rats exposed to unleaded
gasoline. Toxicol. Lett. 41: 245-254.
OPDYKE, D.L.J. (1975). d-Limonene. Monograph on fragrance raw
materials. Food and Cosmetics Toxicology, 13(suppl: 683-923):
825-826.
PAGE, N.P. & MEHLMAN, M. (1989). Health effects of gasoline
refueling vapors and measured exposures at service stations.
Toxicology and Industrial Health, 5: 869-890.
PARKER, G.A., BOGO, V. & YOUNG, R.W. (1981). Acute toxicity of
conventional versus shale-derived JP5 jet fuel: Light microscopic,
hematologic, and serum chemistry studies. Toxicology and Applied
Pharmacology, 57: 302-317.
PHILLIPS, R.D. & COCKRELL, B.Y. (1984). Chapter VII: Effect of
certain light hydrocarbons on kidney function and structure in male
rats. In: Advances in Modern Environmental Toxicology, Renal
Effects of Petroleum Hydrocarbons, M.A. Mehlman, editor, pp.
89-105, Princeton Scientific, Princeton, NJ.
PHILLIPS, R.D. & EGAN, G.F. (1984). Effect of C10-11 isoparaffinic
solvent on kidney function in Fischer 344 rats during eight weeks of
inhalation. Toxicology and Applied Pharmacology, 73: 500-510.
PIRILA, V., SILTANEN, E. & PIRILA, L. (1964). On the chemical nature
of the eczematogenic agent in oil of turpentine. Dermatologica,
128: 16-21.
RAABE, G.K. (1984). Chapter XVIII: Kidney cancer epidemiology in
petroleum related studies. In: Advances in Modern Environmental
Toxicology, Renal Effects of Petroleum Hydrocarbons, M.A. Mehlman,
editor, pp. 259-271, Princeton Scientific, Princeton, NJ.
READ, N.G., ASTBURY, P.J., MORGAN, R.J.I., PARSONS, D.N. & PORT,
C.J. (1988). Induction and exacerbation of hyaline droplet formation
in the proximal tubular cells of the kidneys from male rats
receiving a variety of pharmacological agents. Toxicology, 52:
81-101.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1985). Acute
dermal irritation study; RIFM 1-32.
RESEARCH INSTITUTE FOR FRAGRANCE MATERIALS, INC. (1990). The
absorption, distribution, and excretion of radioactivity after
topical application of 14C-limonene to rats.
RESEARCH INSTITUTE FOR THE DEVELOPMENT OF FRAGRANCE MATERIALS, INC.
(1984). Acute dermal irritation study; Ref. Nos. 307-338/8403.
RIDDER, G.M., VON BARGEN, E.C., ALDEN, C.L. & PARKER, R.D. (1990).
Increased hyaline droplet formation in male rats exposed to decalin
is dependent on the presence of alpha2u-globulin. Fundamental and
Applied Toxicology, 15: 732-743.
ROE, F.J.C. (1959). Oil of sweet orange: A possible role in
carcinogenesis. British Journal of Cancer, 13: 92-93.
ROE, F.J.C. & Peirce, W.E.H. (1960). Tumour promotion by citrus
oils: Tumours of the skin and urethral orifice in mice. Journal of
the National Cancer Institute, 24: 1389-1403.
ROY, A.K. & NEUHAUS, A.W. (1967) Androgenic control of a
sex-dependent protein in the rat. Nature, 214: 618-20.
SCHENK, J. DOBRONTE, Z., KOCH, H. & STOLTE, M. (1980).
Untersuchungen zur gewebevertraglichkeit von d-limonen als einem
losungsmittel cholesterineicher gallenstein. Gastroenterologie,
18: 389-394.
SCHOTTENFELD, D., WARSHAUER, M.E., ZAUBER, A.G., MEIKLE, J.G. &
HART, B.T. (1981). A prospective study of morbidity and mortality in
petroleum industry employees in the United States - a preliminary
report. In: Banbury Report 9: Quantification of Occupational
Cancer, R. Peto & M. Schneiderman, editors, pp. 247-265. Cold
Spring Harbor Laboratory, Cold Spring Harbor, NY.
SHORT, G.B., BURNETT, V.L., COX, M.G., BUS, J.S. & SWENBERG, J.A.
(1987). Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Laboratory
Investigation, 57: 564-577.
SHORT, G.B., BURNETT, V.L. & SWENBERG, J.A. (1989a). Elevated
proliferation of proximal tubule cells and localized accumulated
alpha2u-globulin in F344 rats during chronic exposure to unleaded
gasoline or 2,2,4-trimethylpentane. Toxicology and Applied
Pharmacology, 101: 414-431.
SHORT, G.B., STEINHAGEN, W.H. & SWENBERG, J.A. (1989b). Promoting
effects of unleaded gasoline and 2,2,4-trimethylpentane on the
development of atypical cell foci and renal tubular cell tumours in
rats exposed to n-ethyl-n-hydroxyethylnitrosamine. Cancer Research,
49: 6369-6378.
SHORT, G.B. & SWENBERG, J.A. (1988). Pathologic Investigations of
the mechanism of unleaded gasoline-induced renal tumours in rats.
CIIT Activities, 8: 1-6.
STONARD, M.C., PHILLIPS, P.G.N., FOSTER, J.R., SIMPSON, M.G. & LOCK,
E.A. (1986). Alpha2u-globulin: Measurement in rat kidney following
administration of 2,2,4-trimethylpentane, Toxicology, 41: 161-168.
STONE, L.C., KANERVA, R.L., BURNS, J.L. & ALDEN, C.L. (1987a).
Decalin-induced nephrotoxicity: Light and electron microscopic
examination of the effects of oral dosing on the development of
kidney lesions in the rat. Food and Chemical Toxicology, 25:
43-52.
STONE, L.C., McCRACKEN, M.S., KANERVA, R.L. & ALDEN, C.L. (1987b).
Development of a short-term model of decalin inhalation
nephrotoxicity in the male rat. Food and Chemical Toxicology, 25:
35-41.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumour
response in strain A mice. Cancer Research, 33: 3069-3085.
SWENBERG, J.A. (1991). Editorial. Risk assessment of chemicals
causing alpha2u-globulin nephropathy. Reg. Toxicol. Pharmacol.,
13: 1-2.
SWENBERG, J.A., SHORT, B., BORGHOFF, S., STRASSER, J. & CHARBONNEAU,
M. (1989). The comparative pathobiology of alpha2u-globulin
nephropathy. Toxicology and Applied Pharmacology, 97: 35-46.
THELESTAM, M., CURVALL, M. & ENZELL, C. (1980). Effect of tobacco
smoke compounds on the plasma membrane of cultured human lung
fibroblasts. Toxicology, 15: 203-217.
THOMAS, T.L., WAXWEILER, R.J., MOURE-ERASO, R., ITAYA, S. &
FRAUMENI, Jr., J.F. (1982). Mortality patterns among workers in
three Texas oil refineries. Journal of Occupational Medicine, 24:
135-141.
TRUMP, B.F., BEREZESKY, I.K., SATO, K. & SAIKO, K.U. (1984). Cell
calcium, cell injury, and cell death. Environ.Hlth. Revs., 57:
281-287.
TSUJI, M., FUJISAKI, Y., YAMACHIKA, K. NAKAGAMI, K., FUJISAKI, F.,
MITO, M., AOKI, T., KINOSHITA, S., OKUBO, A. & WATANABE, I. (1974).
Studies on d-limonene as gallstone solubilizer; I. General
pharmacological studies. Oyo Yakuri (Pharmacometrics), 8:
1439-1459.
TSUJI, M., FUJISAKI, Y., ARIKAWA, Y., MASUDA, S., KINOSHITA, S.,
OKUBO, A., NODA, K., IDE, H. & IWANAGA, Y. (1975a). Studies on
d-limonene, as gallstone solubilizer (II): Acute and subacute
toxicities. Oyo Yakuri (Pharmacometrics), 9: 387-401.
US EPA (1991). Alpha2u-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Risk
Assessment Forum, US Environmental Protection Agency, Washington,
DC, USA, EPA/625/3-91/019F.
VAN DUUREN, B.L. & GOLDSCHMIDT, B.M. (1976). Co-carcinogenic and
tumour-promoting agents in tobacco carcinogenesis. Journal of the
National Cancer Institute, 56: 1237-1242.
WADE, A.P., WILKINSON, G.S., DEAN, F.M. & PRICE, A.W. (1966). The
isolation, characterization and structure of uroterpenol, a
monoterpene from human urine. Biochem. J., 101: 727-734.
WATABE, T., HIRATSUKA, A., ISOBE, M. & OZAWA, N. (1980). Metabolism
of d-limonene by hepatic microsomes to non-mutagenic epoxides
toward Salmonella typhimurium. Biochemical Pharmacology, 29:
1068-1071.
WEBB, D.R., KANERVA, R.L., HYSELL, D.K., ALDEN, C.L. &
LEHMAN-MCKEEMAN, L.D. (1990). Assessment of the subchronic oral
toxicity of d-limonene in dogs. Fd. Chem. Toxic., 28: 669-675.
WEBB, D.R., RIDDER, G.M. & ALDEN, C.L. (1989). Acute and subacute
nephrotoxicity of d-limonene in Fischer 344 rats. Fd. Chem.
Toxicol., 27: 639-649.
WEN, C.P., ISAI, S.P., MOFFITT, K.B., BONDY, M. & GIBSON, R.L.
(1984). Chapter XVII: Epidemiologic studies of the role of gasoline
(hydrocarbon) exposure in kidney cancer risk. In: Advances in
Modern Environmental Toxicology, Renal Effects of Petroleum
Hydrocarbons, M.A. Mehlman, editor, pp. 245-257, Princeton
Scientific, Princeton, NJ.
WONG, O. & RAABE, G. (1989). Critical review of cancer epidemiology
in petroleum industry employees, with a quantitative meta-analysis
by cancer site. American Journal of Industrial Medicine, 15:
283-310.
