
FLUBENDAZOLE
First draft prepared by
Dr Radovan Fuchs
Institute for Medical Research and Occupational Health
University of Zagreb, Croatia
1. EXPLANATION
Flubendazole([5-(4-fluorobenzoyl)-1H-benzimidazole-2-y1]-carbamic
acid methyl ester) belongs to the group of benzimidazole carbamates.
Flubendazole is an anthelminthic agent active against a range of
gastrointestinal parasites in pigs and poultry.
Flubendazole had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Groups of 18 Wistar rats and 12 multi-mammate rats (Mastomys
natalensis) were given an oral dose of 40 mg/kg bw of flubendazole
as a microsuspension. The same amount of test substance was given
subcutaneously to 24 Wistar rats and 12 multimammate rats. Groups of
six rats of both strains were sacrificed by decapitation at 4 and 8
h and at 24 and 48 h after administration to Wistar rats. Plasma
levels of flubendazole after oral administration were found to be 81
and 17 ng/ml at 4 h in the Wistar rats and multimammate rats,
respectively. The estimated half-life was 6-7 h. At 24 h the
flubendazole plasma level in the Wistar rats was 5.6 ng/ml. Plasma
concentrations after subcutaneous injection was very low. Peak
plasma levels of 7-9 ng/ml were observed after 4-8 h in both
strains. After 48 h, plasma levels represented 32% of the peak
level, indicating very slow absorption of the drug from the
injection site (Michiels et al., 1980a).
Groups of male Wistar rats were given oral doses of 10 mg/kg bw
microcrystalline suspension of 14C-labelled flubendazole. Animals
were sacrificed at different intervals (0.5, 1, 2, 4, 6, 16 and 24 h
after administration). Peak plasma levels of unchanged flubendazole
were observed after 0.5 h and the drug was eliminated with a
half-life of 6 h. Generally, the concentration of flubendazole in
blood and plasma was very low, and was almost unchanged at 0.5 h
(0.27 µg/ml) and 24 h (0.18 µg/ml) after exposure. After 24 h,
nearly 50% of the dose was excreted via faeces while 4% of the dose
was excreted in urine as metabolites. The amount of total
radioactivity present in the liver, lung, kidney, muscle and fat was
very low and did not exceed 3.1 µg/g of tissue (Michiels et al.,
1977a).
Five male Wistar rats were each given an oral dose of 10 mg/kg
bw of 14C-labelled flubendazole. Within 4 days, 7% of the dose was
excreted in urine, and 89% in faeces. After 48 h, 91% of
administered flubendazole was excreted. Radioactivity in urine was
due to metabolites while in faeces the radioactivity was mainly due
to the unchanged drug. The main metabolites identified in urine were
present mainly in the form of glucuronides and resulted from
carbamate hydrolysis and reduction of ketone (Meuldermans et al.,
1977).
14C-Labelled flubendazole was given orally to 3 female beagle
dogs at a dose of 10 mg/kg bw. A total of 88% of the radioactivity
was excreted within 4 days after administration. The majority of the
radioactivity (81.5%) was measured in faeces, while only 6.3% was
found in urine. Radioactivity found in urine was related to
metabolites of flubendazole. In samples of faeces collected during
48 h, the radioactivity was almost exclusively due to unchanged
flubendazone. There are indications that flubendazole may undergo
enterohepatic circulation (Meuldermans et al., 1978).
A group of 2 male and 4 female beagle dogs was treated orally
with flubendazole at a dose of 22 mg/kg bw. Plasma levels peaked at
2 to 8 h after dosing (4-5 ng/ml) (Michiels et al., 1987).
Eight male beagle dogs were divided into groups of two and were
given micro-suspensions of flubendazole intramuscularly. Doses of
2.5 and 25 mg/kg bw were given as single injections or as repeated
injections for five consecutive days. Blood tests were made during
42 days after administration. At both doses, three phases in plasma
concentration-time curves were observed. First there was a fast
phase, with more rapid release of the drug from the injection site
than its elimination from the body. A second phase showed faster
elimination than release from the depot and a third phase showed
very slow terminal resorption. After a single injection of 2.5 mg/kg
bw, peak plasma levels appeared after 3-5 days at concentrations up
to 0.6 ng/ml. With a single injection of 25 mg/kg bw, maximal plasma
levels up to 2.1 ng/ml were observed 5-7 days after administration.
Repeated injections for 5 consecutive days gave maximal plasma
levels at 3-4 days after the last dose; concentrations found were
2.4 and 13.2 ng/ml for the low and high dose, respectively. The
elimination half-life for both doses and regimes was estimated to be
24 h (Michiels et al., 1980b).
Pigs were given 14C-flubendazole, labelled in the 2-position
of the benzimidazole ring at a dose of 1.5 mg/kg bw for five
consecutive days. A total of 79% of the administered dose was
excreted within 30 days after the medication period (23% in urine
and 56% in faeces). The main metabolic pathways were carbamate
hydrolysis and ketone reduction (Meuldermans et al., 1982).
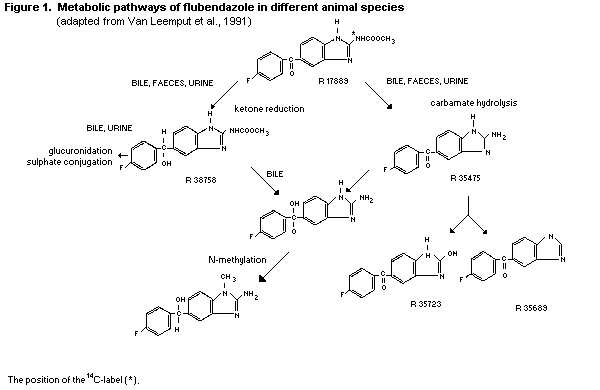
2.1.2 Biotransformation
Reduction of the ketone functional group and hydrolysis of the
carbamate moiety are the main biotransformation pathways of
flubendazole. To some extent, methylation has also been found as a
relatively minor pathway. In rats and dogs, the urinary metabolites
are formed exclusively by ketone reduction, carbamate hydrolysis and
glucuronide and sulfate conjugation (Meuldermans et al., 1977,
1978).
The metabolic pathways of flubendazole in different animal
species, adapted from Van Leemput, et al., 1991, is presented in
Figure 1.
 2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of flubendazole is summarized in Table 1. In
some cases the animals exhibited exophthalmus, hypotonia, slight
sedation, general depression, ataxia, convulsions and pilo-erection.
Deaths were recorded within the first 24 h after intraperitoneal
administration of test substance.
Table 1. Acute toxicity of flubendazole
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983a
M&F s.c.* >5000 Niemegeers, 1986a
M&F s.c.# >10 000 Nakaoka et al., 1983a
M i.p.# 528 Nakaoka et al., 1983a
F i.p.# 434 Nakaoka et al., 1983a
Rat M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983b
M i.p.# 435 Nakaoka et al., 1983b
F i.p.# 252 Nakaoka et al., 1983b
M&F s.c.# >5000 Nakaoka et al., 1983b
M&F s.c.* >10 000 Niemegeers, 1986b
Guinea-pig M&F oral* >5000 Niemegeers, 1974
M s.c.* 4679 Niemegeers, 1986c
F s.c.* 4834 Niemegeers, 1986c
Laying-hen F oral >640 Vanparijs &
Desplenter, 1982
Guinea fowl oral >1200 Le Brun, 1983
* vehicle: aqueous suspension with 1% polysorbate 80
# vehicle: 0.5% methylcellulose solution
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
In a 3-month study flubendazole admixed with the diet at levels
of 0, 10, 40 or 160 mg/100 g food was given to Wistar rats (10
rats/sex/dose level). The approximate intakes of flubendazole over
the period of the study were equal to 0, 8, 30 or 130 mg/kg bw/day
for males and 0, 9, 40 or 150 mg/kg bw/day for females. No
treatment-related effects were observed on mortality, behaviour,
appearance, food consumption, body weight, haematology, serum
analysis, urinalysis, microscopy, organ weight or histopathology
(Marsboom, 1975a).
2.2.2.2 Dogs
Groups of beagle dogs (3 dogs/sex/group) were given
flubendazole orally as a powder in gelatin capsules at doses of 0,
2.5, 10 or 40 mg/kg bw/day, six days per week, over 3 months.
Control animals received 250 mg lactose only. Observations included
behavioural changes, food consumption, body weight, ECG, blood
pressure, haematology, serum analysis, urinalysis, necropsy, organ
weight and histopathology. Small-sized prostate and congestion of
cauda of the epididymis were observed in all males at doses of 10
and 40 mg/kg bw/day.
Histopathologically some atrophic changes of the prostate were
noticed at the higher dose groups, but the changes were not
dose-related. Atresic changes in the ovaries were noted in 2 females
in the 2.5 mg/kg bw/day group, 1 in the 10 mg/kg bw/day group and 3
in the 40 mg/kg bw/day group. Atrophic changes of the uterine wall
and vagina in some dosed females were also observed. According to
historical control data the changes in the female genital tract were
considered to be within the normal range for the age of the dogs
(Marsboom et al., 1975).
Subsequent to the above report the histopathological slides
were evaluated by two independent pathologists. Both experts agreed
that the atrophic changes were not indicative of a treatment-related
toxic effect and should be considered as incomplete development in
sexually immature dogs (Thoonen, 1987; Powell, 1991). Because of the
lack of conclusive evidence as to the etiology of these changes, the
Committee concluded that the NOEL in this study was 2.5 mg/kg
bw/day.
2.2.2.3 Chickens
Flubendazole was given to 4 groups of broiler breeder pullets
(50 F + 5 M/group) over a period of 7 days. The drug was admixed in
the feed at concentrations of 0, 60, 120 or 180 mg/kg. Four days
after the end of treatment 10 hens and a cock were removed from the
groups for blood sampling and necropsy.
The only significant difference in haematological parameters
was a lower haematocrit value and red blood cell count in the
highest-dose group. Clinical biochemistry tests showed significant
increases in triglycerides and phospholypides at 120 mg/kg,
decreased aspartic aminotransaminase at 180 mg/kg and decreased
cholinesterase at 120 and 180 mg/kg. Histopathologically,
treatment-related effects were observed in the spleen of the
high-dose group, in reduction of the white pulp area and reduction
of the number of red blood cells in the red pulp. The NOEL in this
study was 120 mg/kg, equivalent to 15 mg/kg bw/day (Desplenter &
Coussement, 1985).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of 50 male and female Swiss albino mice were fed
diets containing 0, 5, 10 or 20 mg/100 g flubendazole for a period
of 18 months. The intake of the drug over the study period was
equivalent to 7.5, 15 or 30 mg/kg bw/day (data on food consumption
and body weight were not provided). The mortality rate, clinical
observations and presence of subcutaneous masses were recorded
daily. A complete necropsy and histopathological examination were
performed at termination of the study.
No treatment-related effects were noted for clinical condition
or survival. The percent survival for treated groups was comparable
to the controls, as shown in Table 2.
Table 2. Survival at 18 months in the long-term study in mice
Sex Dose mg/kg bw/day
0 7.5 15 30
M 54% 44% 38% 38%
F 40% 34% 40% 32%
The total number of benign and malignant tumours was similar
for treated and control groups. The most common types of tumours
were hepato-cellular tumours and alveologenic lung carcinoma. No
effect of flubendazole was evident histopathologically. The NOEL in
this study was 30 mg/kg bw/day (Verstraeten, et al., 1983a).
2.2.3.2 Rats
Groups of 50 Wistar rats of each sex were fed diets containing
0, 10, 20 or 40 mg/100 g flubandezole for 24 months. Drug intake was
equivalent to 5, 10, or 20 mg/kg bw/day.
All animals were observed once daily for signs of abnormal
behaviour and clinical effects. At termination of the study a full
necropsy was performed and the organs were examined
histopathologically.
The mortality rate was very high in all groups of animals
including the controls at the end of the study. There was no
statistically-significant difference in mortality rates between the
groups during the entire period of the study. There were no
treatment-related effects among the treated and control animals.
During the study subcutaneous masses in females were noted in
approximately 20% of the control and 40% of the high-dose animals.
On necropsy a significantly increased incidence of pale kidneys was
observed in females at 5 and 20 mg/kg bw/day. However, there was no
histopathological evidence for dose-related changes in the kidney.
Flubendazole at concentrations of up to 40 mg/100 g feed for 2 years
did not show biological or statistical evidence of incidence of
neo-plasms. The NOEL in this study was 20 mg/kg bw/day (Verstraeten
et al., 1983b).
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 30 female Swiss albino mice were given flubendazole
in a single dose of 0, 20, 80 or 320 mg/kg bw. The drug was
administered orally by gavage in microsuspension vehicle. A control
group of females received vehicle suspension only. All the females
were mated with untreated males. The animals were controlled daily
over a period of 360 days. During the experiment the mortality rate,
incidence of pregnancy, time interval between dosing and birth, and
number of pups were not affected by treatment.
By comparing the data from control and treated animals, no
differences were noted regarding mortality of females, the average
time interval between dosing and birth of first litters or litter
size per female. In the high-dose group (320 mg/kg bw) a reduction
of mean number of pups per female was observed. The NOEL in this
study was 80 mg/kg bw (Marsboom, 1977a).
2.2.4.2 Rats
In a fertility study, Wistar rats (20 animals/sex/dose) were
given flubendazole admixed in feed. The concentration of the test
substance in the diet was 0, 2.5, 10 or 40 mg/100 g, equivalent to
2.5, 10 or 40 mg/kg bw/day. Females were treated with flubendazole
for 14 days prior to cohabitation with males and during the
pregnancy period. Males received flubendazole over a period of 60
days before mating. Untreated animals were mated with treated ones.
No drug effects were noted regarding food consumption or
average weight gain of females. All the females were sacrificed on
the 22nd day post-mating. No drug effects were noted on incidence of
pregnancy and the percentage of pregnancies was 100% in almost all
groups. In all females the average number of implantations and
percentages of live, dead and resorbed fetuses were similar and not
affected by the treatment. Treatment-related fetal skeletal
abnormalities were not observed. The NOEL in this study was 40 mg/kg
bw/day (Marsboom, 1976a).
Four groups of 20 Wistar rats were given flubendazole admixed
in feed at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day from day 16 of gestation
up to the 3-week-lactation period.
The average body weight and food consumption were not
significantly different among the groups. Only one female from the
40 mg/kg bw/day group died during the time of treatment. The
pregnancy rate, average duration of gestation and fetal parameters
were comparable among the treated and control groups. No
abnormalities were found among offspring in any of the groups in the
study. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1976b).
2.2.4.3 Pigs
A study was performed on 5 different pig farms. On each farm 16
sows and 1 boar received flubendazole admixed in the feed, providing
a dose of 3 mg/kg bw/day. On each farm the same number of
non-treated animals served as controls. Each boar was mated with 8
treated and 8 control sows. The treated sows received flubendazole
diet from estrus till parturition, while boars were treated two
months prior to the first mating and until all sows were
successfully bred.
During the study no differences were observed on conception
rate of the boars, estrus behaviour or gestation length. Post-partum
conditions were normal except in 7 sows, which were found to be
related to dystocia. The number of live and dead piglets among the
different groups was not statistically different. There were no
significant differences in abnormalities observed among the piglets
from any groups except in slightly increased number of
mummifications. The investigators associated mummifications to the
endemic occurrence of pseudorabies and parvovirus infections in the
area where the study was performed. The time interval betwen weaning
to the next estrus were equal among the groups (De Keyser et al.,
1984)
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 young female Wistar rats were fed diet containing
flubendazole at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day, from day 6 to day 15 of
pregnancy. Parameters measuring body weight, food consumption,
mortality and pregnancy were recorded. The animals were sacrificed
on the 22nd day of pregnancy and the fetuses were delivered by
caesarean section. The number of live, dead and resorbed fetuses was
recorded, average body weight of pups was measured and the fetuses
examined for abnormalities.
During the study none of the animals died and food consumption
and average body weight were comparable among the groups. The
percentage of pregnancies in all treated groups was 95% and in the
control group 90%. All the fetal parameters studied were comparable
among the treated and control groups. The absence of metacarpal and
metatarsal bones was noticed in only one fetus from the highest dose
group. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1974).
In a separate study, four groups of 20 female Wistar rats each
were treated with flubendazole in the diet at concentrations of 0,
2.5, 10 or 40 mg/100 g feed, equivalent to 0, 2.5, 10 or 40 mg/kg
bw/day. The study design and parameters studied were the same as
described in the previous study. There were no changes in any of the
parameters studied. The NOEL was 40 mg/kg bw/day (Marsboom, 1975b).
A third study (same experimental design) was carried out in
order to confirm the results of the previous studies. The test
substance in this experiment was given orally by gavage at doses of
0, 2.5, 10 or 40 mg/kg bw/day. All the maternal and fetal parameters
were comparable among the treated and control groups of animals. The
NOEL in this study was 40 mg/kg bw/day (Marsboom, 1977b).
Groups of female Wistar rats (20 animals/group) were given
flubendazole admixed in a powdered diet at concentrations of 0, 10,
40 or 160 mg/100 g feed, equivalent to 0, 10, 40 or 160 mg/kg bw/day
from days 6 to 15 of pregnancy. The same parameters as in the
previous experiments were studied. Food consumption and average body
weight were comparable among all groups. During the study no
mortalities occurred. The percentage of pregnancies was high and
comparable among the groups. No evidence of embryotoxicity or
teratogenicity was found. The NOEL in this study was 160 mg/kg
bw/day (Marsboom, 1978).
Flubendazole extracted from commercially formulated drug was
suspended in water and given by gavage to female Sprague-Dawley rats
(20 animals/group) at doses of 0, 2.5, 10, 40 or 160 mg/kg bw/day
from day 8 to day 15 of pregnancy. On day 21 of pregnancy the
animals were sacrificed and the number of implantation sites and
live fetuses were recorded. The fetuses were further examined for
malformations.
During the study period signs of maternal toxicity were not
observed. The highest dose (160 mg/kg bw/day) was found to be
embryocidal, resulting in a significant increase in the fetal
resorption rate. A dose-dependent decrease in fetal body weight
occurred, which was significant at 40 and 160 mg/kg bw/day. The two
highest doses induced significant gross, skeletal and internal
malformations. In the 160 mg/kg bw/day dose group, 16.8% of fetuses
were grossly malformed. The malformations were described as:
encephalocele, cranial meningocele, omphalocele, ectrodactyly, club
foot, anal atresia, spina bifida occulata and tail defects. Skeletal
malformations affected mainly the vertebrae and ribs, and
significant malformations were found in 24.6% and 32.6% of fetuses
in the two highest dose groups. Significant internal malformations
were observed in 19.8% and 47.7% of fetuses in the 40 and 160 mg/kg
bw/day group. respectively. The NOEL in this study was 10 mg/kg
bw/day (Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 female New Zeeland white rabbits were orally
administered 0, 10 or 40 mg flubendazole/kg bw/day from day 6 to day
18 of gestation. The females were sacrificed on gestation day 28 and
fetuses were delivered by caesarean section. The animals from all
doses were necropsied. The fetuses were weighed and examined for
external anomalies. After incubation of the pups the survival rate
was calculated. All the fetuses were radiographically examined.
One-third of the fetuses were examined for visceral anomalies and
the rest were preserved and used for additional analysis.
During the study one non-pregnant female in the highest dose
group died due to infection. The average body weight increase was
comparable among all groups. No differences were observed in
pregnancy rate among the groups and no teratogenic effects were
found among the offspring. The percentage of live, dead and resorbed
fetuses and survival rate of incubated pups from all groups were not
significantly different. The NOEL in this study was 40 mg/kg bw/day
(Marsboom, 1976c).
2.2.5.3 Pigs
Flubendazole (5% powder formulation) was admixed in the diet at
a concentration of 30 mg/kg and given to 6 crossed Landrace-Pietrain
sows from day 8 to day 50 of pregnancy. Altogether 62 piglets were
born. No piglets were stillborn and no abnormalities were recorded
(Marsboom, 1976d).
In a separate study flubendazole (5% powder formulation)
admixed in the feed at a concentration of 200 mg/kg, equivalent to 8
mg/kg bw/day, was given to 20 sows from the first day of mating
until the day of farrowing. Three sows were withdrawn from the
experiment after 3 weeks. In the group of 17 sows having successful
pregnancies 154 live and 8 stillborn piglets were recorded. There
were no detectable external abnormalities, except for a mild form of
sprayed legs observed in 2 piglets of one sow. Abnormalities were
not found in stillborn piglets (Rogiers, 1979).
A group of 8 sows was given 50 mg flubendazole/kg bw/day
admixed in the diet from the first day of mating until day 70 of
pregnancy. Sixty-three piglets of normal weight and 3 stillborn
piglets were born. No external abnormalities were recorded (Rogiers,
1980).
2.2.6 Special studies on eye and skin irritation
2.2.6.1 Rabbits
A group of 6 adult New Zeeland rabbits had 0.1 ml of a 50% w/w
suspension of a 5% premix formulation instilled into the
conjunctival sac of the left eye. There were no signs of eye
irritation during the observation time of 21 days (Teuns & Marsboom,
1987a).
A flubendazole formulation was applied to intact skin of New
Zeeland rabbits. The test sites were occluded for 24 h. After
removal of dressings, the test sites were scored for erythema and
oedema. A barely perceptible irritation (index 0.63) was noted
during the first 5 days in the treated animals according to the
Draize irritation index. This irritation was fully reversible after
5 days. This study was designed primarily to determine the dermal
LD50 value (Teuns & Marsboom, 1987b).
2.2.7 Special studies on genotoxicity
The genototoxicity of flubendazole is summarized in Table 3.
Table 3. Results of genotoxicity studies on flubendazole
Test system Test object Concentration Results Reference
In vitro
Repair test B. subtilis 1-5000 µg/disc Negative Yamashita &
Hattori, 1983
Ames test +/- S9 S. typhimurium 10-5000 µg/plate Negative Yamashita &
TA1535, TA1537, Hattori, 1983
TA1538, TA100,
TA98
E. coli
N/r WP2 trp hcr
S. typhimurium 0.5-1000 µg/plate Negative Jagannath &
TA535, TA1537, Brusick, 1978
TA1538, TA98,
TA100
S. cerevisiae D4
In vivo
Sex-linked Drosophila 500 and 2000 ppm Negative Vanparys &
melanogaster feeding/3d Marsboom, 1982
Micronucleus Male Swiss albino 2 oral doses: 40, Negative Vanparys &
test mice 80, 160, Marsboom, 1979
1280 mg/kg bw
female Wistar 2 oral doses: 80, Negative Vanparys &
rats 160, 640 mg/kg bw Marsboom, 1981
Dominant Male Swiss albino single oral dose: Negative Marsboom, 1977c
lethal mice 10, 40,
160 mg/kg bw
2.3 Observations in humans
Three male volunteers were given single doses of flubendazole
orally in 100 mg tablets. Flubendazole was mainly excreted in faeces
(77.3% of the administered dose) within 3 days after intake of the
drug. Less than 0.1% of the dose was excreted in the urine as
unchanged drug (Heykants et al., 1975).
Three male volunteers were given oral doses of 100 mg
flubendazole 2 h before meals, 2000 mg flubendazole immediately
after a heavy meal and 2000 mg flubendazole before a meal. Serum
concentrations of flubendazole were monitored. The plasma
concentration was very low, and peak levels after 100 and 2000 mg
doses taken before meals were 0.35 and 0.74 ng/ml, respectively. In
the case of flubendazole taken after a heavy meal, the peak plasma
concentration was markedly higher (4.06 ng/ml), which indicates that
the presence of food enhances the absorption of flubendazole from
the gastro-intestinal tract. By calculating the area under the curve
(AUC) values it was found that the absorption of the drug was not
dose-dependent. AUC values increased only 1.4-fold after a 20-fold
increase in dose (Michiels et al., 1977b).
3. COMMENTS
A substantial database was available for assessment, including
data on kinetics and metabolism, acute toxicity, short-term and
long-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The absorption, metabolism, and excretion of flubendazole have
been studied using radiolabelled drug. Flubendazole is poorly
absorbed and metabolized in a qualitatively similar way in all
species studied. More than 50% of the ingested drug is eliminated
unchanged in the faeces. The absorbed drug is rapidly metabolized,
so that levels of parent drug in the blood and urine are extremely
low. The main site of metabolism is the liver, and major metabolic
pathways are carbamate hydrolysis and ketone reduction. It seems
probable that flubendazole undergoes enterohepatic circulation.
Single oral doses of flubendazole were slightly toxic to
experimental animals, the median lethal dose (LD50) being greater
than 5000 mg/kg of body weight in mice, rats, and guinea-pigs.
Flubendazole was given orally in gelatin capsules to dogs at
doses of 2.5, 10 or 40 mg/kg bw/day, 6 days a week for 3 months.
Some atrophic changes and congestion of the epididymis were observed
in the male genital tract at doses of 10 and 40 mg/kg bw/day, and in
the female genital tract at all doses. The changes in the female
genital tract were considered to be within normal limits for dogs of
the age of those used in the study. On histological examination of
male sex organs, changes in the testes could not be clearly
associated with flubendazole treatment. The findings in male dogs
may not be compound-related, but because of the lack of conclusive
evidence as to the cause of these changes, the Committee concluded
that the no-observed-effect level (NOEL) was 2.5 mg/kg bw/day.
Carcinogenicity studies were performed in mice and rats at
doses up to 30 and 20 mg/kg bw/day respectively; no
treatment-related effects were observed. There was no
treatment-related increase in any type of neoplasm. The Committee
was of the opinion that flubendazole had no carcinogenic potential
at the highest doses administered in these studies.
The results from a range of in vitro and in vivo
genotoxicity tests were all negative.
The Committee considered data from reproduction,
embryotoxicity, and teratogenicity studies. Studies in mice,
rabbits, and pigs were negative. Flubendazole was extensively
studied in segmented reproduction studies in rats, performed as
required for human drug regulation purposes and accepted by the
Committee in lieu of a multigeneration reproduction study. In
several rat developmental
studies, doses of up to 40 and 160 mg/kg bw/day, given on gestation
days 6-15, did not produce any embryotoxic or teratogenic effects.
In a rat teratogenicity study published in 1987, using material
extracted from a commercial preparation, gross skeletal and internal
fetal malformations were recorded at doses of 40 and 160 mg/kg
bw/day. The NOEL in this study was 10 mg/kg bw/day.
4. EVALUATION
An ADI of 0-12 µg/kg of body weight was established for
flubendazole, based on the NOEL of 2.5 mg/kg bw/day in the 3-month
study in dogs, and a safety factor of 200. This safety factor was
used by the Committee to take account of the fact that the doses
were administered only 6 days per week in this study, the precise
consequences of which could not be assessed.
The Committee noted that the ADI also provided a safety margin
corresponding to a factor of about 1000 with respect to the NOEL of
10 mg/kg bw/day derived from the rat teratogenicity study. The
Committee considered that further carcinogenicity studies would not
be required, since the highest dose used in the negative studies
that it had evaluated exceeded the ADI by a factor of approximately
2000.
5. REFERENCES
DE KEYSER, H., DONY, J. & ROGIERS, M. (1984). Fertility study with
flubendazole (R 17889) in pigs. Unpublished results No. V4988 from
Janssen Pharmaceutica Veterinary Department. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
DESPLENTER, L. & COUSSEMENT, W. (1985). Safety evaluation of
flubendazole in broiler breeder chickens at higher than normal use
levels: haematological, biochemical and histopathological
evaluation. Unpublished results No.V5738 from Veterinary Clinical
Research and Department of Toxicology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
HEYKANTS, J., WYNANTS, J. & SCHEIJGROND, H. (1975). The absorption
and excretion of flubendazole in man. Unpublished results No. N10250
from Department of Drug Metabolism, Department of Separation
Techniques and Department of Clinical Pharmacology Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
JAGANNATH, D.R. & BRUSICK, D.J. (1978). Mutagenicity evaluation of
flubendazole in the Ames Salmonella/microsome plate test.
Unpublished results No. 20988 from Litton Bionetics, Inc., 5516
Nicholson Lane, Kensington, Maryland. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
LE BRUN, J. (1983). Acute toxicity of flubendazole in guinea fowls.
Unpublished results No. V4899 from Department of Toxicology and
Pharmacology JMG/MC. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1974). Potential of oral R 17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 556 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975a). Oral toxicity study in Wistar rats (repeated
dosage for 3 months). Unpublished results No. 567, Janssen
Pharmaceutica, Research Laboratories 2340 Beerse, Belgium. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975b). Potential of oral R17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 572 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R., HERIN, V., VANDESTEENE, R. & PARDOEL, L. (1975). Oral
toxicity study in beagle dogs (repeated dosage for 3 months).
Unpublished results No. 568, Janssen Pharmaceutica, Research
Laboratories 2340 Beerse, Belgium. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976a). Oral male and female fertility study in Wistar
rats. Unpublished results No. 695 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in Wistar rats. Unpublished results No. 692 from Department of
Toxicology, Janssen Pharmaceutica N.V. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976c). Oral embryotoxicity and teratogenicity study
in New Zealand white rabbits (segment II). Unpublished results No.
692 from Department of Toxicology, Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1976d). Potential of oral flubendazole (R 17889) for
teratogenic effects in pigs. Unpublished results No. 692 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977a). Total reproductive capacity test in female
mice. Unpublished results No. 773 from Department of Toxicology,
Janssen Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977b). Oral embryotoxicity and teratogenicity study
in Wistar rats (Segment II). Unpublished results No. 755 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977c). Dominant lethal test in male mice (single oral
dose). Unpublished results No. 688 from Janssen Pharmaceutica,
Research Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1978). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished results No. 820 from
Department of Toxicology, Janssen Pharmaceutica Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., HURKMANS, R., SWIJSEN, E. & HEYKANTS, J. (1977). A
comparative study on the excretion and metabolism of flubendazole
(R17889) and mebendazole (R17635) in the rat. Unpublished report No.
V2940 from Department of Drug Metabolism, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., SWIJSEN, E., HENDRICKX, J., LAUWERS, W., BRACKE,
J., LENAERTS, F., SNEYERS, R. & HEYKANTS, J. (1978). On the
absorption, excretion and biotransformation of flubendazole in the
dog. Unpublished report No. N15186 from Department of Drug
Metabolism and Pharmacology and Analytical Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., LEE, I.Y., HENDRICKX, J., SWYSEN, E., LAUWERS, W.,
PORTER, D. & HEYKANTS, J. (1982). Metabolism of flubendazole in
swine: Excretion pattern of [2-14C] flubendazole and its
metabolites and depletion kinetics of drug residues in edible
tissues. Preliminary report from Department of Drug Metabolism,
Department of Analytical Research, Janssen Pharmaceutica, and
Pitman-Moore, Inc., Washington Crossing, NJ., USA. Submitted to WHO
by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HEYKANTS, J. & HENDRICKX, J. (1977a). Distribution of
flubendazole (R17889) and its metabolites in the Wistar rat.
Unpublished report No. N12336 from Department of Drug Metabolism and
Analytical Department, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HENDRIKS, J. & HEYKANTS, J. (1977b). The
pharmacokinetics of mebendazole and flubendazole in animal and man.
Arch. Internat. Pharmacodinam. Therapie, 256: 180-191.
MICHIELS, M., HEYKANTS, J., VAN DEN BOSSCHE, H. & VERHOEVEN, H.
(1980a). A comparative study on the systemic absorption of oral and
subcutaneous mebendazole, flubendazole and R34803 in two different
rodents. Unpublished report No. N19702 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., PRINSEN, P., HEYKANTS, J. & VAN DEN BOSSCHE, H.
(1980b). Systemic absorption of intramuscular flubendazole in the
dog. Unpublished report No. N20709 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., MONBALIU, J., WOESTENBORGHS, R., HEYKANTS, J. & MAES,
L. (1987). Absorption and plasma concentrations of flubendazole in
the dog after single oral administration at the therapeutic dose
level of 22 mg/kg as the commercial flubendazole paste (Fflubenol KH
pasteR). Unpublished report No. V6282 from Department of Drug
Metabolism and Pharmacology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983a). Acute toxicity
study of flubendazole in mice. Unpublished report No. V5050 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983b). Acute toxicity
study of flubendazole in rats. Unpublished report No. V5049 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NIEMEGEERS, C.J.E. (1974) The acute oral toxicity of flubendazole in
Wistar rats, albino mice and guinea-pigs. Unpublished results No.
N9208 from Department of Pharmacology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986a). The acute subcutaneous toxicity of
R17889 in mice. Unpublished results No. N49012 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986b) The acute subcutaneous toxicity of R17889
in rats. Unpublished results No. N49023 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986c) The acute subcutaneous toxicity of R17889
in guinea-pigs. Unpublished results No. N49024 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
POWELL, C.J. (1991). Independent report on slides from the
flubendazole study No. 568. Unpublished results from Department of
Toxicology St. Bartholomew's Hospital Medical College, University of
London. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1979). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 200 ppm during the whole gestation
period. Unpublished results No. V3251 from Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1980). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 50 mg per kg of bodyweight,
administered during the first 10 weeks of the gestation period.
Unpublished results No. V3443 from Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
TEUNS, G. & MARSBOOM, R. (1987a). Primary eye irritation study in
rabbits. Unpublished results No. 1834 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
TEUNS, G. & MARSBOOM, R. (1987b). Acute dermal toxicity study in New
Zealand White rabbits (single dose). Unpublished results No. 1835
from Department of Toxicology, Janssen Pharmaceutica N.V. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
THOONEN, H. (1987). Independent report on slides from the
flubendazole study No 568. Unpublished results from Department of
Pathology, Faculty of Veterinary Medicine, State University Ghent,
Belgium. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VAN LEEMPUT, L., MAES, L., MEULDERMANS, W. & HAYKANTS, J. (1991).
Pharmacokinetic profile of flubendazole: critical review of
available data. Unpublished review report from Department of Drug
Metabolism and Pharmacokinetics, and Department of Parasitology,
Janssen Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1979). Micronucleus test in mice.
Unpublished results No. 912 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1981). Micronucleus test in rats.
Unpublished results No. 1032 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1982). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished results No. 1138 from
Department of Toxicology, Janssen Pharmaceutica, Research
Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VANPARIJS, O. & DESPLENTER, L. (1982). The acute toxicity of
flubendazole in poultry. Unpublished results No. V4501 from
Department of Parasitology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983a). Oral carcinogenicity study in Albino Swiss mice.
Unpublished results No. 685 from Department of Toxicology, Janssen
Pharmaceutica N.V. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983b). Carcinogenicity study in Wistar rats. Unpublished results
No. 684 from Department of Toxicology, Janssen Pharmaceutica N.V.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
YAMASHITA, T. & HATTORI, K. (1983). Mutagenic evaluation of
flubendazole in bacterial repair and reverse mutagenesis test.
Unpublished results No. V5051 from Research Laboratories Fujisawa
Pharmaceutical Co., Ltd., Osaka, Japan. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
YOSHIMURA, H. (1987). Teratogenicity of flubendazole in rats.
Toxicology, 43: 133-138.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of flubendazole is summarized in Table 1. In
some cases the animals exhibited exophthalmus, hypotonia, slight
sedation, general depression, ataxia, convulsions and pilo-erection.
Deaths were recorded within the first 24 h after intraperitoneal
administration of test substance.
Table 1. Acute toxicity of flubendazole
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983a
M&F s.c.* >5000 Niemegeers, 1986a
M&F s.c.# >10 000 Nakaoka et al., 1983a
M i.p.# 528 Nakaoka et al., 1983a
F i.p.# 434 Nakaoka et al., 1983a
Rat M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983b
M i.p.# 435 Nakaoka et al., 1983b
F i.p.# 252 Nakaoka et al., 1983b
M&F s.c.# >5000 Nakaoka et al., 1983b
M&F s.c.* >10 000 Niemegeers, 1986b
Guinea-pig M&F oral* >5000 Niemegeers, 1974
M s.c.* 4679 Niemegeers, 1986c
F s.c.* 4834 Niemegeers, 1986c
Laying-hen F oral >640 Vanparijs &
Desplenter, 1982
Guinea fowl oral >1200 Le Brun, 1983
* vehicle: aqueous suspension with 1% polysorbate 80
# vehicle: 0.5% methylcellulose solution
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
In a 3-month study flubendazole admixed with the diet at levels
of 0, 10, 40 or 160 mg/100 g food was given to Wistar rats (10
rats/sex/dose level). The approximate intakes of flubendazole over
the period of the study were equal to 0, 8, 30 or 130 mg/kg bw/day
for males and 0, 9, 40 or 150 mg/kg bw/day for females. No
treatment-related effects were observed on mortality, behaviour,
appearance, food consumption, body weight, haematology, serum
analysis, urinalysis, microscopy, organ weight or histopathology
(Marsboom, 1975a).
2.2.2.2 Dogs
Groups of beagle dogs (3 dogs/sex/group) were given
flubendazole orally as a powder in gelatin capsules at doses of 0,
2.5, 10 or 40 mg/kg bw/day, six days per week, over 3 months.
Control animals received 250 mg lactose only. Observations included
behavioural changes, food consumption, body weight, ECG, blood
pressure, haematology, serum analysis, urinalysis, necropsy, organ
weight and histopathology. Small-sized prostate and congestion of
cauda of the epididymis were observed in all males at doses of 10
and 40 mg/kg bw/day.
Histopathologically some atrophic changes of the prostate were
noticed at the higher dose groups, but the changes were not
dose-related. Atresic changes in the ovaries were noted in 2 females
in the 2.5 mg/kg bw/day group, 1 in the 10 mg/kg bw/day group and 3
in the 40 mg/kg bw/day group. Atrophic changes of the uterine wall
and vagina in some dosed females were also observed. According to
historical control data the changes in the female genital tract were
considered to be within the normal range for the age of the dogs
(Marsboom et al., 1975).
Subsequent to the above report the histopathological slides
were evaluated by two independent pathologists. Both experts agreed
that the atrophic changes were not indicative of a treatment-related
toxic effect and should be considered as incomplete development in
sexually immature dogs (Thoonen, 1987; Powell, 1991). Because of the
lack of conclusive evidence as to the etiology of these changes, the
Committee concluded that the NOEL in this study was 2.5 mg/kg
bw/day.
2.2.2.3 Chickens
Flubendazole was given to 4 groups of broiler breeder pullets
(50 F + 5 M/group) over a period of 7 days. The drug was admixed in
the feed at concentrations of 0, 60, 120 or 180 mg/kg. Four days
after the end of treatment 10 hens and a cock were removed from the
groups for blood sampling and necropsy.
The only significant difference in haematological parameters
was a lower haematocrit value and red blood cell count in the
highest-dose group. Clinical biochemistry tests showed significant
increases in triglycerides and phospholypides at 120 mg/kg,
decreased aspartic aminotransaminase at 180 mg/kg and decreased
cholinesterase at 120 and 180 mg/kg. Histopathologically,
treatment-related effects were observed in the spleen of the
high-dose group, in reduction of the white pulp area and reduction
of the number of red blood cells in the red pulp. The NOEL in this
study was 120 mg/kg, equivalent to 15 mg/kg bw/day (Desplenter &
Coussement, 1985).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of 50 male and female Swiss albino mice were fed
diets containing 0, 5, 10 or 20 mg/100 g flubendazole for a period
of 18 months. The intake of the drug over the study period was
equivalent to 7.5, 15 or 30 mg/kg bw/day (data on food consumption
and body weight were not provided). The mortality rate, clinical
observations and presence of subcutaneous masses were recorded
daily. A complete necropsy and histopathological examination were
performed at termination of the study.
No treatment-related effects were noted for clinical condition
or survival. The percent survival for treated groups was comparable
to the controls, as shown in Table 2.
Table 2. Survival at 18 months in the long-term study in mice
Sex Dose mg/kg bw/day
0 7.5 15 30
M 54% 44% 38% 38%
F 40% 34% 40% 32%
The total number of benign and malignant tumours was similar
for treated and control groups. The most common types of tumours
were hepato-cellular tumours and alveologenic lung carcinoma. No
effect of flubendazole was evident histopathologically. The NOEL in
this study was 30 mg/kg bw/day (Verstraeten, et al., 1983a).
2.2.3.2 Rats
Groups of 50 Wistar rats of each sex were fed diets containing
0, 10, 20 or 40 mg/100 g flubandezole for 24 months. Drug intake was
equivalent to 5, 10, or 20 mg/kg bw/day.
All animals were observed once daily for signs of abnormal
behaviour and clinical effects. At termination of the study a full
necropsy was performed and the organs were examined
histopathologically.
The mortality rate was very high in all groups of animals
including the controls at the end of the study. There was no
statistically-significant difference in mortality rates between the
groups during the entire period of the study. There were no
treatment-related effects among the treated and control animals.
During the study subcutaneous masses in females were noted in
approximately 20% of the control and 40% of the high-dose animals.
On necropsy a significantly increased incidence of pale kidneys was
observed in females at 5 and 20 mg/kg bw/day. However, there was no
histopathological evidence for dose-related changes in the kidney.
Flubendazole at concentrations of up to 40 mg/100 g feed for 2 years
did not show biological or statistical evidence of incidence of
neo-plasms. The NOEL in this study was 20 mg/kg bw/day (Verstraeten
et al., 1983b).
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 30 female Swiss albino mice were given flubendazole
in a single dose of 0, 20, 80 or 320 mg/kg bw. The drug was
administered orally by gavage in microsuspension vehicle. A control
group of females received vehicle suspension only. All the females
were mated with untreated males. The animals were controlled daily
over a period of 360 days. During the experiment the mortality rate,
incidence of pregnancy, time interval between dosing and birth, and
number of pups were not affected by treatment.
By comparing the data from control and treated animals, no
differences were noted regarding mortality of females, the average
time interval between dosing and birth of first litters or litter
size per female. In the high-dose group (320 mg/kg bw) a reduction
of mean number of pups per female was observed. The NOEL in this
study was 80 mg/kg bw (Marsboom, 1977a).
2.2.4.2 Rats
In a fertility study, Wistar rats (20 animals/sex/dose) were
given flubendazole admixed in feed. The concentration of the test
substance in the diet was 0, 2.5, 10 or 40 mg/100 g, equivalent to
2.5, 10 or 40 mg/kg bw/day. Females were treated with flubendazole
for 14 days prior to cohabitation with males and during the
pregnancy period. Males received flubendazole over a period of 60
days before mating. Untreated animals were mated with treated ones.
No drug effects were noted regarding food consumption or
average weight gain of females. All the females were sacrificed on
the 22nd day post-mating. No drug effects were noted on incidence of
pregnancy and the percentage of pregnancies was 100% in almost all
groups. In all females the average number of implantations and
percentages of live, dead and resorbed fetuses were similar and not
affected by the treatment. Treatment-related fetal skeletal
abnormalities were not observed. The NOEL in this study was 40 mg/kg
bw/day (Marsboom, 1976a).
Four groups of 20 Wistar rats were given flubendazole admixed
in feed at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day from day 16 of gestation
up to the 3-week-lactation period.
The average body weight and food consumption were not
significantly different among the groups. Only one female from the
40 mg/kg bw/day group died during the time of treatment. The
pregnancy rate, average duration of gestation and fetal parameters
were comparable among the treated and control groups. No
abnormalities were found among offspring in any of the groups in the
study. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1976b).
2.2.4.3 Pigs
A study was performed on 5 different pig farms. On each farm 16
sows and 1 boar received flubendazole admixed in the feed, providing
a dose of 3 mg/kg bw/day. On each farm the same number of
non-treated animals served as controls. Each boar was mated with 8
treated and 8 control sows. The treated sows received flubendazole
diet from estrus till parturition, while boars were treated two
months prior to the first mating and until all sows were
successfully bred.
During the study no differences were observed on conception
rate of the boars, estrus behaviour or gestation length. Post-partum
conditions were normal except in 7 sows, which were found to be
related to dystocia. The number of live and dead piglets among the
different groups was not statistically different. There were no
significant differences in abnormalities observed among the piglets
from any groups except in slightly increased number of
mummifications. The investigators associated mummifications to the
endemic occurrence of pseudorabies and parvovirus infections in the
area where the study was performed. The time interval betwen weaning
to the next estrus were equal among the groups (De Keyser et al.,
1984)
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 young female Wistar rats were fed diet containing
flubendazole at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day, from day 6 to day 15 of
pregnancy. Parameters measuring body weight, food consumption,
mortality and pregnancy were recorded. The animals were sacrificed
on the 22nd day of pregnancy and the fetuses were delivered by
caesarean section. The number of live, dead and resorbed fetuses was
recorded, average body weight of pups was measured and the fetuses
examined for abnormalities.
During the study none of the animals died and food consumption
and average body weight were comparable among the groups. The
percentage of pregnancies in all treated groups was 95% and in the
control group 90%. All the fetal parameters studied were comparable
among the treated and control groups. The absence of metacarpal and
metatarsal bones was noticed in only one fetus from the highest dose
group. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1974).
In a separate study, four groups of 20 female Wistar rats each
were treated with flubendazole in the diet at concentrations of 0,
2.5, 10 or 40 mg/100 g feed, equivalent to 0, 2.5, 10 or 40 mg/kg
bw/day. The study design and parameters studied were the same as
described in the previous study. There were no changes in any of the
parameters studied. The NOEL was 40 mg/kg bw/day (Marsboom, 1975b).
A third study (same experimental design) was carried out in
order to confirm the results of the previous studies. The test
substance in this experiment was given orally by gavage at doses of
0, 2.5, 10 or 40 mg/kg bw/day. All the maternal and fetal parameters
were comparable among the treated and control groups of animals. The
NOEL in this study was 40 mg/kg bw/day (Marsboom, 1977b).
Groups of female Wistar rats (20 animals/group) were given
flubendazole admixed in a powdered diet at concentrations of 0, 10,
40 or 160 mg/100 g feed, equivalent to 0, 10, 40 or 160 mg/kg bw/day
from days 6 to 15 of pregnancy. The same parameters as in the
previous experiments were studied. Food consumption and average body
weight were comparable among all groups. During the study no
mortalities occurred. The percentage of pregnancies was high and
comparable among the groups. No evidence of embryotoxicity or
teratogenicity was found. The NOEL in this study was 160 mg/kg
bw/day (Marsboom, 1978).
Flubendazole extracted from commercially formulated drug was
suspended in water and given by gavage to female Sprague-Dawley rats
(20 animals/group) at doses of 0, 2.5, 10, 40 or 160 mg/kg bw/day
from day 8 to day 15 of pregnancy. On day 21 of pregnancy the
animals were sacrificed and the number of implantation sites and
live fetuses were recorded. The fetuses were further examined for
malformations.
During the study period signs of maternal toxicity were not
observed. The highest dose (160 mg/kg bw/day) was found to be
embryocidal, resulting in a significant increase in the fetal
resorption rate. A dose-dependent decrease in fetal body weight
occurred, which was significant at 40 and 160 mg/kg bw/day. The two
highest doses induced significant gross, skeletal and internal
malformations. In the 160 mg/kg bw/day dose group, 16.8% of fetuses
were grossly malformed. The malformations were described as:
encephalocele, cranial meningocele, omphalocele, ectrodactyly, club
foot, anal atresia, spina bifida occulata and tail defects. Skeletal
malformations affected mainly the vertebrae and ribs, and
significant malformations were found in 24.6% and 32.6% of fetuses
in the two highest dose groups. Significant internal malformations
were observed in 19.8% and 47.7% of fetuses in the 40 and 160 mg/kg
bw/day group. respectively. The NOEL in this study was 10 mg/kg
bw/day (Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 female New Zeeland white rabbits were orally
administered 0, 10 or 40 mg flubendazole/kg bw/day from day 6 to day
18 of gestation. The females were sacrificed on gestation day 28 and
fetuses were delivered by caesarean section. The animals from all
doses were necropsied. The fetuses were weighed and examined for
external anomalies. After incubation of the pups the survival rate
was calculated. All the fetuses were radiographically examined.
One-third of the fetuses were examined for visceral anomalies and
the rest were preserved and used for additional analysis.
During the study one non-pregnant female in the highest dose
group died due to infection. The average body weight increase was
comparable among all groups. No differences were observed in
pregnancy rate among the groups and no teratogenic effects were
found among the offspring. The percentage of live, dead and resorbed
fetuses and survival rate of incubated pups from all groups were not
significantly different. The NOEL in this study was 40 mg/kg bw/day
(Marsboom, 1976c).
2.2.5.3 Pigs
Flubendazole (5% powder formulation) was admixed in the diet at
a concentration of 30 mg/kg and given to 6 crossed Landrace-Pietrain
sows from day 8 to day 50 of pregnancy. Altogether 62 piglets were
born. No piglets were stillborn and no abnormalities were recorded
(Marsboom, 1976d).
In a separate study flubendazole (5% powder formulation)
admixed in the feed at a concentration of 200 mg/kg, equivalent to 8
mg/kg bw/day, was given to 20 sows from the first day of mating
until the day of farrowing. Three sows were withdrawn from the
experiment after 3 weeks. In the group of 17 sows having successful
pregnancies 154 live and 8 stillborn piglets were recorded. There
were no detectable external abnormalities, except for a mild form of
sprayed legs observed in 2 piglets of one sow. Abnormalities were
not found in stillborn piglets (Rogiers, 1979).
A group of 8 sows was given 50 mg flubendazole/kg bw/day
admixed in the diet from the first day of mating until day 70 of
pregnancy. Sixty-three piglets of normal weight and 3 stillborn
piglets were born. No external abnormalities were recorded (Rogiers,
1980).
2.2.6 Special studies on eye and skin irritation
2.2.6.1 Rabbits
A group of 6 adult New Zeeland rabbits had 0.1 ml of a 50% w/w
suspension of a 5% premix formulation instilled into the
conjunctival sac of the left eye. There were no signs of eye
irritation during the observation time of 21 days (Teuns & Marsboom,
1987a).
A flubendazole formulation was applied to intact skin of New
Zeeland rabbits. The test sites were occluded for 24 h. After
removal of dressings, the test sites were scored for erythema and
oedema. A barely perceptible irritation (index 0.63) was noted
during the first 5 days in the treated animals according to the
Draize irritation index. This irritation was fully reversible after
5 days. This study was designed primarily to determine the dermal
LD50 value (Teuns & Marsboom, 1987b).
2.2.7 Special studies on genotoxicity
The genototoxicity of flubendazole is summarized in Table 3.
Table 3. Results of genotoxicity studies on flubendazole
Test system Test object Concentration Results Reference
In vitro
Repair test B. subtilis 1-5000 µg/disc Negative Yamashita &
Hattori, 1983
Ames test +/- S9 S. typhimurium 10-5000 µg/plate Negative Yamashita &
TA1535, TA1537, Hattori, 1983
TA1538, TA100,
TA98
E. coli
N/r WP2 trp hcr
S. typhimurium 0.5-1000 µg/plate Negative Jagannath &
TA535, TA1537, Brusick, 1978
TA1538, TA98,
TA100
S. cerevisiae D4
In vivo
Sex-linked Drosophila 500 and 2000 ppm Negative Vanparys &
melanogaster feeding/3d Marsboom, 1982
Micronucleus Male Swiss albino 2 oral doses: 40, Negative Vanparys &
test mice 80, 160, Marsboom, 1979
1280 mg/kg bw
female Wistar 2 oral doses: 80, Negative Vanparys &
rats 160, 640 mg/kg bw Marsboom, 1981
Dominant Male Swiss albino single oral dose: Negative Marsboom, 1977c
lethal mice 10, 40,
160 mg/kg bw
2.3 Observations in humans
Three male volunteers were given single doses of flubendazole
orally in 100 mg tablets. Flubendazole was mainly excreted in faeces
(77.3% of the administered dose) within 3 days after intake of the
drug. Less than 0.1% of the dose was excreted in the urine as
unchanged drug (Heykants et al., 1975).
Three male volunteers were given oral doses of 100 mg
flubendazole 2 h before meals, 2000 mg flubendazole immediately
after a heavy meal and 2000 mg flubendazole before a meal. Serum
concentrations of flubendazole were monitored. The plasma
concentration was very low, and peak levels after 100 and 2000 mg
doses taken before meals were 0.35 and 0.74 ng/ml, respectively. In
the case of flubendazole taken after a heavy meal, the peak plasma
concentration was markedly higher (4.06 ng/ml), which indicates that
the presence of food enhances the absorption of flubendazole from
the gastro-intestinal tract. By calculating the area under the curve
(AUC) values it was found that the absorption of the drug was not
dose-dependent. AUC values increased only 1.4-fold after a 20-fold
increase in dose (Michiels et al., 1977b).
3. COMMENTS
A substantial database was available for assessment, including
data on kinetics and metabolism, acute toxicity, short-term and
long-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The absorption, metabolism, and excretion of flubendazole have
been studied using radiolabelled drug. Flubendazole is poorly
absorbed and metabolized in a qualitatively similar way in all
species studied. More than 50% of the ingested drug is eliminated
unchanged in the faeces. The absorbed drug is rapidly metabolized,
so that levels of parent drug in the blood and urine are extremely
low. The main site of metabolism is the liver, and major metabolic
pathways are carbamate hydrolysis and ketone reduction. It seems
probable that flubendazole undergoes enterohepatic circulation.
Single oral doses of flubendazole were slightly toxic to
experimental animals, the median lethal dose (LD50) being greater
than 5000 mg/kg of body weight in mice, rats, and guinea-pigs.
Flubendazole was given orally in gelatin capsules to dogs at
doses of 2.5, 10 or 40 mg/kg bw/day, 6 days a week for 3 months.
Some atrophic changes and congestion of the epididymis were observed
in the male genital tract at doses of 10 and 40 mg/kg bw/day, and in
the female genital tract at all doses. The changes in the female
genital tract were considered to be within normal limits for dogs of
the age of those used in the study. On histological examination of
male sex organs, changes in the testes could not be clearly
associated with flubendazole treatment. The findings in male dogs
may not be compound-related, but because of the lack of conclusive
evidence as to the cause of these changes, the Committee concluded
that the no-observed-effect level (NOEL) was 2.5 mg/kg bw/day.
Carcinogenicity studies were performed in mice and rats at
doses up to 30 and 20 mg/kg bw/day respectively; no
treatment-related effects were observed. There was no
treatment-related increase in any type of neoplasm. The Committee
was of the opinion that flubendazole had no carcinogenic potential
at the highest doses administered in these studies.
The results from a range of in vitro and in vivo
genotoxicity tests were all negative.
The Committee considered data from reproduction,
embryotoxicity, and teratogenicity studies. Studies in mice,
rabbits, and pigs were negative. Flubendazole was extensively
studied in segmented reproduction studies in rats, performed as
required for human drug regulation purposes and accepted by the
Committee in lieu of a multigeneration reproduction study. In
several rat developmental
studies, doses of up to 40 and 160 mg/kg bw/day, given on gestation
days 6-15, did not produce any embryotoxic or teratogenic effects.
In a rat teratogenicity study published in 1987, using material
extracted from a commercial preparation, gross skeletal and internal
fetal malformations were recorded at doses of 40 and 160 mg/kg
bw/day. The NOEL in this study was 10 mg/kg bw/day.
4. EVALUATION
An ADI of 0-12 µg/kg of body weight was established for
flubendazole, based on the NOEL of 2.5 mg/kg bw/day in the 3-month
study in dogs, and a safety factor of 200. This safety factor was
used by the Committee to take account of the fact that the doses
were administered only 6 days per week in this study, the precise
consequences of which could not be assessed.
The Committee noted that the ADI also provided a safety margin
corresponding to a factor of about 1000 with respect to the NOEL of
10 mg/kg bw/day derived from the rat teratogenicity study. The
Committee considered that further carcinogenicity studies would not
be required, since the highest dose used in the negative studies
that it had evaluated exceeded the ADI by a factor of approximately
2000.
5. REFERENCES
DE KEYSER, H., DONY, J. & ROGIERS, M. (1984). Fertility study with
flubendazole (R 17889) in pigs. Unpublished results No. V4988 from
Janssen Pharmaceutica Veterinary Department. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
DESPLENTER, L. & COUSSEMENT, W. (1985). Safety evaluation of
flubendazole in broiler breeder chickens at higher than normal use
levels: haematological, biochemical and histopathological
evaluation. Unpublished results No.V5738 from Veterinary Clinical
Research and Department of Toxicology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
HEYKANTS, J., WYNANTS, J. & SCHEIJGROND, H. (1975). The absorption
and excretion of flubendazole in man. Unpublished results No. N10250
from Department of Drug Metabolism, Department of Separation
Techniques and Department of Clinical Pharmacology Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
JAGANNATH, D.R. & BRUSICK, D.J. (1978). Mutagenicity evaluation of
flubendazole in the Ames Salmonella/microsome plate test.
Unpublished results No. 20988 from Litton Bionetics, Inc., 5516
Nicholson Lane, Kensington, Maryland. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
LE BRUN, J. (1983). Acute toxicity of flubendazole in guinea fowls.
Unpublished results No. V4899 from Department of Toxicology and
Pharmacology JMG/MC. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1974). Potential of oral R 17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 556 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975a). Oral toxicity study in Wistar rats (repeated
dosage for 3 months). Unpublished results No. 567, Janssen
Pharmaceutica, Research Laboratories 2340 Beerse, Belgium. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975b). Potential of oral R17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 572 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R., HERIN, V., VANDESTEENE, R. & PARDOEL, L. (1975). Oral
toxicity study in beagle dogs (repeated dosage for 3 months).
Unpublished results No. 568, Janssen Pharmaceutica, Research
Laboratories 2340 Beerse, Belgium. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976a). Oral male and female fertility study in Wistar
rats. Unpublished results No. 695 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in Wistar rats. Unpublished results No. 692 from Department of
Toxicology, Janssen Pharmaceutica N.V. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976c). Oral embryotoxicity and teratogenicity study
in New Zealand white rabbits (segment II). Unpublished results No.
692 from Department of Toxicology, Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1976d). Potential of oral flubendazole (R 17889) for
teratogenic effects in pigs. Unpublished results No. 692 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977a). Total reproductive capacity test in female
mice. Unpublished results No. 773 from Department of Toxicology,
Janssen Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977b). Oral embryotoxicity and teratogenicity study
in Wistar rats (Segment II). Unpublished results No. 755 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977c). Dominant lethal test in male mice (single oral
dose). Unpublished results No. 688 from Janssen Pharmaceutica,
Research Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1978). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished results No. 820 from
Department of Toxicology, Janssen Pharmaceutica Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., HURKMANS, R., SWIJSEN, E. & HEYKANTS, J. (1977). A
comparative study on the excretion and metabolism of flubendazole
(R17889) and mebendazole (R17635) in the rat. Unpublished report No.
V2940 from Department of Drug Metabolism, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., SWIJSEN, E., HENDRICKX, J., LAUWERS, W., BRACKE,
J., LENAERTS, F., SNEYERS, R. & HEYKANTS, J. (1978). On the
absorption, excretion and biotransformation of flubendazole in the
dog. Unpublished report No. N15186 from Department of Drug
Metabolism and Pharmacology and Analytical Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., LEE, I.Y., HENDRICKX, J., SWYSEN, E., LAUWERS, W.,
PORTER, D. & HEYKANTS, J. (1982). Metabolism of flubendazole in
swine: Excretion pattern of [2-14C] flubendazole and its
metabolites and depletion kinetics of drug residues in edible
tissues. Preliminary report from Department of Drug Metabolism,
Department of Analytical Research, Janssen Pharmaceutica, and
Pitman-Moore, Inc., Washington Crossing, NJ., USA. Submitted to WHO
by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HEYKANTS, J. & HENDRICKX, J. (1977a). Distribution of
flubendazole (R17889) and its metabolites in the Wistar rat.
Unpublished report No. N12336 from Department of Drug Metabolism and
Analytical Department, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HENDRIKS, J. & HEYKANTS, J. (1977b). The
pharmacokinetics of mebendazole and flubendazole in animal and man.
Arch. Internat. Pharmacodinam. Therapie, 256: 180-191.
MICHIELS, M., HEYKANTS, J., VAN DEN BOSSCHE, H. & VERHOEVEN, H.
(1980a). A comparative study on the systemic absorption of oral and
subcutaneous mebendazole, flubendazole and R34803 in two different
rodents. Unpublished report No. N19702 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., PRINSEN, P., HEYKANTS, J. & VAN DEN BOSSCHE, H.
(1980b). Systemic absorption of intramuscular flubendazole in the
dog. Unpublished report No. N20709 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., MONBALIU, J., WOESTENBORGHS, R., HEYKANTS, J. & MAES,
L. (1987). Absorption and plasma concentrations of flubendazole in
the dog after single oral administration at the therapeutic dose
level of 22 mg/kg as the commercial flubendazole paste (Fflubenol KH
pasteR). Unpublished report No. V6282 from Department of Drug
Metabolism and Pharmacology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983a). Acute toxicity
study of flubendazole in mice. Unpublished report No. V5050 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983b). Acute toxicity
study of flubendazole in rats. Unpublished report No. V5049 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NIEMEGEERS, C.J.E. (1974) The acute oral toxicity of flubendazole in
Wistar rats, albino mice and guinea-pigs. Unpublished results No.
N9208 from Department of Pharmacology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986a). The acute subcutaneous toxicity of
R17889 in mice. Unpublished results No. N49012 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986b) The acute subcutaneous toxicity of R17889
in rats. Unpublished results No. N49023 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986c) The acute subcutaneous toxicity of R17889
in guinea-pigs. Unpublished results No. N49024 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
POWELL, C.J. (1991). Independent report on slides from the
flubendazole study No. 568. Unpublished results from Department of
Toxicology St. Bartholomew's Hospital Medical College, University of
London. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1979). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 200 ppm during the whole gestation
period. Unpublished results No. V3251 from Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1980). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 50 mg per kg of bodyweight,
administered during the first 10 weeks of the gestation period.
Unpublished results No. V3443 from Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
TEUNS, G. & MARSBOOM, R. (1987a). Primary eye irritation study in
rabbits. Unpublished results No. 1834 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
TEUNS, G. & MARSBOOM, R. (1987b). Acute dermal toxicity study in New
Zealand White rabbits (single dose). Unpublished results No. 1835
from Department of Toxicology, Janssen Pharmaceutica N.V. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
THOONEN, H. (1987). Independent report on slides from the
flubendazole study No 568. Unpublished results from Department of
Pathology, Faculty of Veterinary Medicine, State University Ghent,
Belgium. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VAN LEEMPUT, L., MAES, L., MEULDERMANS, W. & HAYKANTS, J. (1991).
Pharmacokinetic profile of flubendazole: critical review of
available data. Unpublished review report from Department of Drug
Metabolism and Pharmacokinetics, and Department of Parasitology,
Janssen Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1979). Micronucleus test in mice.
Unpublished results No. 912 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1981). Micronucleus test in rats.
Unpublished results No. 1032 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1982). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished results No. 1138 from
Department of Toxicology, Janssen Pharmaceutica, Research
Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VANPARIJS, O. & DESPLENTER, L. (1982). The acute toxicity of
flubendazole in poultry. Unpublished results No. V4501 from
Department of Parasitology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983a). Oral carcinogenicity study in Albino Swiss mice.
Unpublished results No. 685 from Department of Toxicology, Janssen
Pharmaceutica N.V. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983b). Carcinogenicity study in Wistar rats. Unpublished results
No. 684 from Department of Toxicology, Janssen Pharmaceutica N.V.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
YAMASHITA, T. & HATTORI, K. (1983). Mutagenic evaluation of
flubendazole in bacterial repair and reverse mutagenesis test.
Unpublished results No. V5051 from Research Laboratories Fujisawa
Pharmaceutical Co., Ltd., Osaka, Japan. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
YOSHIMURA, H. (1987). Teratogenicity of flubendazole in rats.
Toxicology, 43: 133-138.
See Also:
Toxicological Abbreviations
FLUBENDAZOLE (JECFA Evaluation)
 2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of flubendazole is summarized in Table 1. In
some cases the animals exhibited exophthalmus, hypotonia, slight
sedation, general depression, ataxia, convulsions and pilo-erection.
Deaths were recorded within the first 24 h after intraperitoneal
administration of test substance.
Table 1. Acute toxicity of flubendazole
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983a
M&F s.c.* >5000 Niemegeers, 1986a
M&F s.c.# >10 000 Nakaoka et al., 1983a
M i.p.# 528 Nakaoka et al., 1983a
F i.p.# 434 Nakaoka et al., 1983a
Rat M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983b
M i.p.# 435 Nakaoka et al., 1983b
F i.p.# 252 Nakaoka et al., 1983b
M&F s.c.# >5000 Nakaoka et al., 1983b
M&F s.c.* >10 000 Niemegeers, 1986b
Guinea-pig M&F oral* >5000 Niemegeers, 1974
M s.c.* 4679 Niemegeers, 1986c
F s.c.* 4834 Niemegeers, 1986c
Laying-hen F oral >640 Vanparijs &
Desplenter, 1982
Guinea fowl oral >1200 Le Brun, 1983
* vehicle: aqueous suspension with 1% polysorbate 80
# vehicle: 0.5% methylcellulose solution
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
In a 3-month study flubendazole admixed with the diet at levels
of 0, 10, 40 or 160 mg/100 g food was given to Wistar rats (10
rats/sex/dose level). The approximate intakes of flubendazole over
the period of the study were equal to 0, 8, 30 or 130 mg/kg bw/day
for males and 0, 9, 40 or 150 mg/kg bw/day for females. No
treatment-related effects were observed on mortality, behaviour,
appearance, food consumption, body weight, haematology, serum
analysis, urinalysis, microscopy, organ weight or histopathology
(Marsboom, 1975a).
2.2.2.2 Dogs
Groups of beagle dogs (3 dogs/sex/group) were given
flubendazole orally as a powder in gelatin capsules at doses of 0,
2.5, 10 or 40 mg/kg bw/day, six days per week, over 3 months.
Control animals received 250 mg lactose only. Observations included
behavioural changes, food consumption, body weight, ECG, blood
pressure, haematology, serum analysis, urinalysis, necropsy, organ
weight and histopathology. Small-sized prostate and congestion of
cauda of the epididymis were observed in all males at doses of 10
and 40 mg/kg bw/day.
Histopathologically some atrophic changes of the prostate were
noticed at the higher dose groups, but the changes were not
dose-related. Atresic changes in the ovaries were noted in 2 females
in the 2.5 mg/kg bw/day group, 1 in the 10 mg/kg bw/day group and 3
in the 40 mg/kg bw/day group. Atrophic changes of the uterine wall
and vagina in some dosed females were also observed. According to
historical control data the changes in the female genital tract were
considered to be within the normal range for the age of the dogs
(Marsboom et al., 1975).
Subsequent to the above report the histopathological slides
were evaluated by two independent pathologists. Both experts agreed
that the atrophic changes were not indicative of a treatment-related
toxic effect and should be considered as incomplete development in
sexually immature dogs (Thoonen, 1987; Powell, 1991). Because of the
lack of conclusive evidence as to the etiology of these changes, the
Committee concluded that the NOEL in this study was 2.5 mg/kg
bw/day.
2.2.2.3 Chickens
Flubendazole was given to 4 groups of broiler breeder pullets
(50 F + 5 M/group) over a period of 7 days. The drug was admixed in
the feed at concentrations of 0, 60, 120 or 180 mg/kg. Four days
after the end of treatment 10 hens and a cock were removed from the
groups for blood sampling and necropsy.
The only significant difference in haematological parameters
was a lower haematocrit value and red blood cell count in the
highest-dose group. Clinical biochemistry tests showed significant
increases in triglycerides and phospholypides at 120 mg/kg,
decreased aspartic aminotransaminase at 180 mg/kg and decreased
cholinesterase at 120 and 180 mg/kg. Histopathologically,
treatment-related effects were observed in the spleen of the
high-dose group, in reduction of the white pulp area and reduction
of the number of red blood cells in the red pulp. The NOEL in this
study was 120 mg/kg, equivalent to 15 mg/kg bw/day (Desplenter &
Coussement, 1985).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of 50 male and female Swiss albino mice were fed
diets containing 0, 5, 10 or 20 mg/100 g flubendazole for a period
of 18 months. The intake of the drug over the study period was
equivalent to 7.5, 15 or 30 mg/kg bw/day (data on food consumption
and body weight were not provided). The mortality rate, clinical
observations and presence of subcutaneous masses were recorded
daily. A complete necropsy and histopathological examination were
performed at termination of the study.
No treatment-related effects were noted for clinical condition
or survival. The percent survival for treated groups was comparable
to the controls, as shown in Table 2.
Table 2. Survival at 18 months in the long-term study in mice
Sex Dose mg/kg bw/day
0 7.5 15 30
M 54% 44% 38% 38%
F 40% 34% 40% 32%
The total number of benign and malignant tumours was similar
for treated and control groups. The most common types of tumours
were hepato-cellular tumours and alveologenic lung carcinoma. No
effect of flubendazole was evident histopathologically. The NOEL in
this study was 30 mg/kg bw/day (Verstraeten, et al., 1983a).
2.2.3.2 Rats
Groups of 50 Wistar rats of each sex were fed diets containing
0, 10, 20 or 40 mg/100 g flubandezole for 24 months. Drug intake was
equivalent to 5, 10, or 20 mg/kg bw/day.
All animals were observed once daily for signs of abnormal
behaviour and clinical effects. At termination of the study a full
necropsy was performed and the organs were examined
histopathologically.
The mortality rate was very high in all groups of animals
including the controls at the end of the study. There was no
statistically-significant difference in mortality rates between the
groups during the entire period of the study. There were no
treatment-related effects among the treated and control animals.
During the study subcutaneous masses in females were noted in
approximately 20% of the control and 40% of the high-dose animals.
On necropsy a significantly increased incidence of pale kidneys was
observed in females at 5 and 20 mg/kg bw/day. However, there was no
histopathological evidence for dose-related changes in the kidney.
Flubendazole at concentrations of up to 40 mg/100 g feed for 2 years
did not show biological or statistical evidence of incidence of
neo-plasms. The NOEL in this study was 20 mg/kg bw/day (Verstraeten
et al., 1983b).
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 30 female Swiss albino mice were given flubendazole
in a single dose of 0, 20, 80 or 320 mg/kg bw. The drug was
administered orally by gavage in microsuspension vehicle. A control
group of females received vehicle suspension only. All the females
were mated with untreated males. The animals were controlled daily
over a period of 360 days. During the experiment the mortality rate,
incidence of pregnancy, time interval between dosing and birth, and
number of pups were not affected by treatment.
By comparing the data from control and treated animals, no
differences were noted regarding mortality of females, the average
time interval between dosing and birth of first litters or litter
size per female. In the high-dose group (320 mg/kg bw) a reduction
of mean number of pups per female was observed. The NOEL in this
study was 80 mg/kg bw (Marsboom, 1977a).
2.2.4.2 Rats
In a fertility study, Wistar rats (20 animals/sex/dose) were
given flubendazole admixed in feed. The concentration of the test
substance in the diet was 0, 2.5, 10 or 40 mg/100 g, equivalent to
2.5, 10 or 40 mg/kg bw/day. Females were treated with flubendazole
for 14 days prior to cohabitation with males and during the
pregnancy period. Males received flubendazole over a period of 60
days before mating. Untreated animals were mated with treated ones.
No drug effects were noted regarding food consumption or
average weight gain of females. All the females were sacrificed on
the 22nd day post-mating. No drug effects were noted on incidence of
pregnancy and the percentage of pregnancies was 100% in almost all
groups. In all females the average number of implantations and
percentages of live, dead and resorbed fetuses were similar and not
affected by the treatment. Treatment-related fetal skeletal
abnormalities were not observed. The NOEL in this study was 40 mg/kg
bw/day (Marsboom, 1976a).
Four groups of 20 Wistar rats were given flubendazole admixed
in feed at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day from day 16 of gestation
up to the 3-week-lactation period.
The average body weight and food consumption were not
significantly different among the groups. Only one female from the
40 mg/kg bw/day group died during the time of treatment. The
pregnancy rate, average duration of gestation and fetal parameters
were comparable among the treated and control groups. No
abnormalities were found among offspring in any of the groups in the
study. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1976b).
2.2.4.3 Pigs
A study was performed on 5 different pig farms. On each farm 16
sows and 1 boar received flubendazole admixed in the feed, providing
a dose of 3 mg/kg bw/day. On each farm the same number of
non-treated animals served as controls. Each boar was mated with 8
treated and 8 control sows. The treated sows received flubendazole
diet from estrus till parturition, while boars were treated two
months prior to the first mating and until all sows were
successfully bred.
During the study no differences were observed on conception
rate of the boars, estrus behaviour or gestation length. Post-partum
conditions were normal except in 7 sows, which were found to be
related to dystocia. The number of live and dead piglets among the
different groups was not statistically different. There were no
significant differences in abnormalities observed among the piglets
from any groups except in slightly increased number of
mummifications. The investigators associated mummifications to the
endemic occurrence of pseudorabies and parvovirus infections in the
area where the study was performed. The time interval betwen weaning
to the next estrus were equal among the groups (De Keyser et al.,
1984)
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 young female Wistar rats were fed diet containing
flubendazole at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day, from day 6 to day 15 of
pregnancy. Parameters measuring body weight, food consumption,
mortality and pregnancy were recorded. The animals were sacrificed
on the 22nd day of pregnancy and the fetuses were delivered by
caesarean section. The number of live, dead and resorbed fetuses was
recorded, average body weight of pups was measured and the fetuses
examined for abnormalities.
During the study none of the animals died and food consumption
and average body weight were comparable among the groups. The
percentage of pregnancies in all treated groups was 95% and in the
control group 90%. All the fetal parameters studied were comparable
among the treated and control groups. The absence of metacarpal and
metatarsal bones was noticed in only one fetus from the highest dose
group. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1974).
In a separate study, four groups of 20 female Wistar rats each
were treated with flubendazole in the diet at concentrations of 0,
2.5, 10 or 40 mg/100 g feed, equivalent to 0, 2.5, 10 or 40 mg/kg
bw/day. The study design and parameters studied were the same as
described in the previous study. There were no changes in any of the
parameters studied. The NOEL was 40 mg/kg bw/day (Marsboom, 1975b).
A third study (same experimental design) was carried out in
order to confirm the results of the previous studies. The test
substance in this experiment was given orally by gavage at doses of
0, 2.5, 10 or 40 mg/kg bw/day. All the maternal and fetal parameters
were comparable among the treated and control groups of animals. The
NOEL in this study was 40 mg/kg bw/day (Marsboom, 1977b).
Groups of female Wistar rats (20 animals/group) were given
flubendazole admixed in a powdered diet at concentrations of 0, 10,
40 or 160 mg/100 g feed, equivalent to 0, 10, 40 or 160 mg/kg bw/day
from days 6 to 15 of pregnancy. The same parameters as in the
previous experiments were studied. Food consumption and average body
weight were comparable among all groups. During the study no
mortalities occurred. The percentage of pregnancies was high and
comparable among the groups. No evidence of embryotoxicity or
teratogenicity was found. The NOEL in this study was 160 mg/kg
bw/day (Marsboom, 1978).
Flubendazole extracted from commercially formulated drug was
suspended in water and given by gavage to female Sprague-Dawley rats
(20 animals/group) at doses of 0, 2.5, 10, 40 or 160 mg/kg bw/day
from day 8 to day 15 of pregnancy. On day 21 of pregnancy the
animals were sacrificed and the number of implantation sites and
live fetuses were recorded. The fetuses were further examined for
malformations.
During the study period signs of maternal toxicity were not
observed. The highest dose (160 mg/kg bw/day) was found to be
embryocidal, resulting in a significant increase in the fetal
resorption rate. A dose-dependent decrease in fetal body weight
occurred, which was significant at 40 and 160 mg/kg bw/day. The two
highest doses induced significant gross, skeletal and internal
malformations. In the 160 mg/kg bw/day dose group, 16.8% of fetuses
were grossly malformed. The malformations were described as:
encephalocele, cranial meningocele, omphalocele, ectrodactyly, club
foot, anal atresia, spina bifida occulata and tail defects. Skeletal
malformations affected mainly the vertebrae and ribs, and
significant malformations were found in 24.6% and 32.6% of fetuses
in the two highest dose groups. Significant internal malformations
were observed in 19.8% and 47.7% of fetuses in the 40 and 160 mg/kg
bw/day group. respectively. The NOEL in this study was 10 mg/kg
bw/day (Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 female New Zeeland white rabbits were orally
administered 0, 10 or 40 mg flubendazole/kg bw/day from day 6 to day
18 of gestation. The females were sacrificed on gestation day 28 and
fetuses were delivered by caesarean section. The animals from all
doses were necropsied. The fetuses were weighed and examined for
external anomalies. After incubation of the pups the survival rate
was calculated. All the fetuses were radiographically examined.
One-third of the fetuses were examined for visceral anomalies and
the rest were preserved and used for additional analysis.
During the study one non-pregnant female in the highest dose
group died due to infection. The average body weight increase was
comparable among all groups. No differences were observed in
pregnancy rate among the groups and no teratogenic effects were
found among the offspring. The percentage of live, dead and resorbed
fetuses and survival rate of incubated pups from all groups were not
significantly different. The NOEL in this study was 40 mg/kg bw/day
(Marsboom, 1976c).
2.2.5.3 Pigs
Flubendazole (5% powder formulation) was admixed in the diet at
a concentration of 30 mg/kg and given to 6 crossed Landrace-Pietrain
sows from day 8 to day 50 of pregnancy. Altogether 62 piglets were
born. No piglets were stillborn and no abnormalities were recorded
(Marsboom, 1976d).
In a separate study flubendazole (5% powder formulation)
admixed in the feed at a concentration of 200 mg/kg, equivalent to 8
mg/kg bw/day, was given to 20 sows from the first day of mating
until the day of farrowing. Three sows were withdrawn from the
experiment after 3 weeks. In the group of 17 sows having successful
pregnancies 154 live and 8 stillborn piglets were recorded. There
were no detectable external abnormalities, except for a mild form of
sprayed legs observed in 2 piglets of one sow. Abnormalities were
not found in stillborn piglets (Rogiers, 1979).
A group of 8 sows was given 50 mg flubendazole/kg bw/day
admixed in the diet from the first day of mating until day 70 of
pregnancy. Sixty-three piglets of normal weight and 3 stillborn
piglets were born. No external abnormalities were recorded (Rogiers,
1980).
2.2.6 Special studies on eye and skin irritation
2.2.6.1 Rabbits
A group of 6 adult New Zeeland rabbits had 0.1 ml of a 50% w/w
suspension of a 5% premix formulation instilled into the
conjunctival sac of the left eye. There were no signs of eye
irritation during the observation time of 21 days (Teuns & Marsboom,
1987a).
A flubendazole formulation was applied to intact skin of New
Zeeland rabbits. The test sites were occluded for 24 h. After
removal of dressings, the test sites were scored for erythema and
oedema. A barely perceptible irritation (index 0.63) was noted
during the first 5 days in the treated animals according to the
Draize irritation index. This irritation was fully reversible after
5 days. This study was designed primarily to determine the dermal
LD50 value (Teuns & Marsboom, 1987b).
2.2.7 Special studies on genotoxicity
The genototoxicity of flubendazole is summarized in Table 3.
Table 3. Results of genotoxicity studies on flubendazole
Test system Test object Concentration Results Reference
In vitro
Repair test B. subtilis 1-5000 µg/disc Negative Yamashita &
Hattori, 1983
Ames test +/- S9 S. typhimurium 10-5000 µg/plate Negative Yamashita &
TA1535, TA1537, Hattori, 1983
TA1538, TA100,
TA98
E. coli
N/r WP2 trp hcr
S. typhimurium 0.5-1000 µg/plate Negative Jagannath &
TA535, TA1537, Brusick, 1978
TA1538, TA98,
TA100
S. cerevisiae D4
In vivo
Sex-linked Drosophila 500 and 2000 ppm Negative Vanparys &
melanogaster feeding/3d Marsboom, 1982
Micronucleus Male Swiss albino 2 oral doses: 40, Negative Vanparys &
test mice 80, 160, Marsboom, 1979
1280 mg/kg bw
female Wistar 2 oral doses: 80, Negative Vanparys &
rats 160, 640 mg/kg bw Marsboom, 1981
Dominant Male Swiss albino single oral dose: Negative Marsboom, 1977c
lethal mice 10, 40,
160 mg/kg bw
2.3 Observations in humans
Three male volunteers were given single doses of flubendazole
orally in 100 mg tablets. Flubendazole was mainly excreted in faeces
(77.3% of the administered dose) within 3 days after intake of the
drug. Less than 0.1% of the dose was excreted in the urine as
unchanged drug (Heykants et al., 1975).
Three male volunteers were given oral doses of 100 mg
flubendazole 2 h before meals, 2000 mg flubendazole immediately
after a heavy meal and 2000 mg flubendazole before a meal. Serum
concentrations of flubendazole were monitored. The plasma
concentration was very low, and peak levels after 100 and 2000 mg
doses taken before meals were 0.35 and 0.74 ng/ml, respectively. In
the case of flubendazole taken after a heavy meal, the peak plasma
concentration was markedly higher (4.06 ng/ml), which indicates that
the presence of food enhances the absorption of flubendazole from
the gastro-intestinal tract. By calculating the area under the curve
(AUC) values it was found that the absorption of the drug was not
dose-dependent. AUC values increased only 1.4-fold after a 20-fold
increase in dose (Michiels et al., 1977b).
3. COMMENTS
A substantial database was available for assessment, including
data on kinetics and metabolism, acute toxicity, short-term and
long-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The absorption, metabolism, and excretion of flubendazole have
been studied using radiolabelled drug. Flubendazole is poorly
absorbed and metabolized in a qualitatively similar way in all
species studied. More than 50% of the ingested drug is eliminated
unchanged in the faeces. The absorbed drug is rapidly metabolized,
so that levels of parent drug in the blood and urine are extremely
low. The main site of metabolism is the liver, and major metabolic
pathways are carbamate hydrolysis and ketone reduction. It seems
probable that flubendazole undergoes enterohepatic circulation.
Single oral doses of flubendazole were slightly toxic to
experimental animals, the median lethal dose (LD50) being greater
than 5000 mg/kg of body weight in mice, rats, and guinea-pigs.
Flubendazole was given orally in gelatin capsules to dogs at
doses of 2.5, 10 or 40 mg/kg bw/day, 6 days a week for 3 months.
Some atrophic changes and congestion of the epididymis were observed
in the male genital tract at doses of 10 and 40 mg/kg bw/day, and in
the female genital tract at all doses. The changes in the female
genital tract were considered to be within normal limits for dogs of
the age of those used in the study. On histological examination of
male sex organs, changes in the testes could not be clearly
associated with flubendazole treatment. The findings in male dogs
may not be compound-related, but because of the lack of conclusive
evidence as to the cause of these changes, the Committee concluded
that the no-observed-effect level (NOEL) was 2.5 mg/kg bw/day.
Carcinogenicity studies were performed in mice and rats at
doses up to 30 and 20 mg/kg bw/day respectively; no
treatment-related effects were observed. There was no
treatment-related increase in any type of neoplasm. The Committee
was of the opinion that flubendazole had no carcinogenic potential
at the highest doses administered in these studies.
The results from a range of in vitro and in vivo
genotoxicity tests were all negative.
The Committee considered data from reproduction,
embryotoxicity, and teratogenicity studies. Studies in mice,
rabbits, and pigs were negative. Flubendazole was extensively
studied in segmented reproduction studies in rats, performed as
required for human drug regulation purposes and accepted by the
Committee in lieu of a multigeneration reproduction study. In
several rat developmental
studies, doses of up to 40 and 160 mg/kg bw/day, given on gestation
days 6-15, did not produce any embryotoxic or teratogenic effects.
In a rat teratogenicity study published in 1987, using material
extracted from a commercial preparation, gross skeletal and internal
fetal malformations were recorded at doses of 40 and 160 mg/kg
bw/day. The NOEL in this study was 10 mg/kg bw/day.
4. EVALUATION
An ADI of 0-12 µg/kg of body weight was established for
flubendazole, based on the NOEL of 2.5 mg/kg bw/day in the 3-month
study in dogs, and a safety factor of 200. This safety factor was
used by the Committee to take account of the fact that the doses
were administered only 6 days per week in this study, the precise
consequences of which could not be assessed.
The Committee noted that the ADI also provided a safety margin
corresponding to a factor of about 1000 with respect to the NOEL of
10 mg/kg bw/day derived from the rat teratogenicity study. The
Committee considered that further carcinogenicity studies would not
be required, since the highest dose used in the negative studies
that it had evaluated exceeded the ADI by a factor of approximately
2000.
5. REFERENCES
DE KEYSER, H., DONY, J. & ROGIERS, M. (1984). Fertility study with
flubendazole (R 17889) in pigs. Unpublished results No. V4988 from
Janssen Pharmaceutica Veterinary Department. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
DESPLENTER, L. & COUSSEMENT, W. (1985). Safety evaluation of
flubendazole in broiler breeder chickens at higher than normal use
levels: haematological, biochemical and histopathological
evaluation. Unpublished results No.V5738 from Veterinary Clinical
Research and Department of Toxicology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
HEYKANTS, J., WYNANTS, J. & SCHEIJGROND, H. (1975). The absorption
and excretion of flubendazole in man. Unpublished results No. N10250
from Department of Drug Metabolism, Department of Separation
Techniques and Department of Clinical Pharmacology Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
JAGANNATH, D.R. & BRUSICK, D.J. (1978). Mutagenicity evaluation of
flubendazole in the Ames Salmonella/microsome plate test.
Unpublished results No. 20988 from Litton Bionetics, Inc., 5516
Nicholson Lane, Kensington, Maryland. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
LE BRUN, J. (1983). Acute toxicity of flubendazole in guinea fowls.
Unpublished results No. V4899 from Department of Toxicology and
Pharmacology JMG/MC. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1974). Potential of oral R 17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 556 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975a). Oral toxicity study in Wistar rats (repeated
dosage for 3 months). Unpublished results No. 567, Janssen
Pharmaceutica, Research Laboratories 2340 Beerse, Belgium. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975b). Potential of oral R17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 572 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R., HERIN, V., VANDESTEENE, R. & PARDOEL, L. (1975). Oral
toxicity study in beagle dogs (repeated dosage for 3 months).
Unpublished results No. 568, Janssen Pharmaceutica, Research
Laboratories 2340 Beerse, Belgium. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976a). Oral male and female fertility study in Wistar
rats. Unpublished results No. 695 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in Wistar rats. Unpublished results No. 692 from Department of
Toxicology, Janssen Pharmaceutica N.V. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976c). Oral embryotoxicity and teratogenicity study
in New Zealand white rabbits (segment II). Unpublished results No.
692 from Department of Toxicology, Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1976d). Potential of oral flubendazole (R 17889) for
teratogenic effects in pigs. Unpublished results No. 692 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977a). Total reproductive capacity test in female
mice. Unpublished results No. 773 from Department of Toxicology,
Janssen Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977b). Oral embryotoxicity and teratogenicity study
in Wistar rats (Segment II). Unpublished results No. 755 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977c). Dominant lethal test in male mice (single oral
dose). Unpublished results No. 688 from Janssen Pharmaceutica,
Research Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1978). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished results No. 820 from
Department of Toxicology, Janssen Pharmaceutica Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., HURKMANS, R., SWIJSEN, E. & HEYKANTS, J. (1977). A
comparative study on the excretion and metabolism of flubendazole
(R17889) and mebendazole (R17635) in the rat. Unpublished report No.
V2940 from Department of Drug Metabolism, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., SWIJSEN, E., HENDRICKX, J., LAUWERS, W., BRACKE,
J., LENAERTS, F., SNEYERS, R. & HEYKANTS, J. (1978). On the
absorption, excretion and biotransformation of flubendazole in the
dog. Unpublished report No. N15186 from Department of Drug
Metabolism and Pharmacology and Analytical Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., LEE, I.Y., HENDRICKX, J., SWYSEN, E., LAUWERS, W.,
PORTER, D. & HEYKANTS, J. (1982). Metabolism of flubendazole in
swine: Excretion pattern of [2-14C] flubendazole and its
metabolites and depletion kinetics of drug residues in edible
tissues. Preliminary report from Department of Drug Metabolism,
Department of Analytical Research, Janssen Pharmaceutica, and
Pitman-Moore, Inc., Washington Crossing, NJ., USA. Submitted to WHO
by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HEYKANTS, J. & HENDRICKX, J. (1977a). Distribution of
flubendazole (R17889) and its metabolites in the Wistar rat.
Unpublished report No. N12336 from Department of Drug Metabolism and
Analytical Department, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HENDRIKS, J. & HEYKANTS, J. (1977b). The
pharmacokinetics of mebendazole and flubendazole in animal and man.
Arch. Internat. Pharmacodinam. Therapie, 256: 180-191.
MICHIELS, M., HEYKANTS, J., VAN DEN BOSSCHE, H. & VERHOEVEN, H.
(1980a). A comparative study on the systemic absorption of oral and
subcutaneous mebendazole, flubendazole and R34803 in two different
rodents. Unpublished report No. N19702 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., PRINSEN, P., HEYKANTS, J. & VAN DEN BOSSCHE, H.
(1980b). Systemic absorption of intramuscular flubendazole in the
dog. Unpublished report No. N20709 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., MONBALIU, J., WOESTENBORGHS, R., HEYKANTS, J. & MAES,
L. (1987). Absorption and plasma concentrations of flubendazole in
the dog after single oral administration at the therapeutic dose
level of 22 mg/kg as the commercial flubendazole paste (Fflubenol KH
pasteR). Unpublished report No. V6282 from Department of Drug
Metabolism and Pharmacology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983a). Acute toxicity
study of flubendazole in mice. Unpublished report No. V5050 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983b). Acute toxicity
study of flubendazole in rats. Unpublished report No. V5049 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NIEMEGEERS, C.J.E. (1974) The acute oral toxicity of flubendazole in
Wistar rats, albino mice and guinea-pigs. Unpublished results No.
N9208 from Department of Pharmacology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986a). The acute subcutaneous toxicity of
R17889 in mice. Unpublished results No. N49012 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986b) The acute subcutaneous toxicity of R17889
in rats. Unpublished results No. N49023 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986c) The acute subcutaneous toxicity of R17889
in guinea-pigs. Unpublished results No. N49024 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
POWELL, C.J. (1991). Independent report on slides from the
flubendazole study No. 568. Unpublished results from Department of
Toxicology St. Bartholomew's Hospital Medical College, University of
London. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1979). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 200 ppm during the whole gestation
period. Unpublished results No. V3251 from Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1980). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 50 mg per kg of bodyweight,
administered during the first 10 weeks of the gestation period.
Unpublished results No. V3443 from Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
TEUNS, G. & MARSBOOM, R. (1987a). Primary eye irritation study in
rabbits. Unpublished results No. 1834 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
TEUNS, G. & MARSBOOM, R. (1987b). Acute dermal toxicity study in New
Zealand White rabbits (single dose). Unpublished results No. 1835
from Department of Toxicology, Janssen Pharmaceutica N.V. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
THOONEN, H. (1987). Independent report on slides from the
flubendazole study No 568. Unpublished results from Department of
Pathology, Faculty of Veterinary Medicine, State University Ghent,
Belgium. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VAN LEEMPUT, L., MAES, L., MEULDERMANS, W. & HAYKANTS, J. (1991).
Pharmacokinetic profile of flubendazole: critical review of
available data. Unpublished review report from Department of Drug
Metabolism and Pharmacokinetics, and Department of Parasitology,
Janssen Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1979). Micronucleus test in mice.
Unpublished results No. 912 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1981). Micronucleus test in rats.
Unpublished results No. 1032 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1982). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished results No. 1138 from
Department of Toxicology, Janssen Pharmaceutica, Research
Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VANPARIJS, O. & DESPLENTER, L. (1982). The acute toxicity of
flubendazole in poultry. Unpublished results No. V4501 from
Department of Parasitology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983a). Oral carcinogenicity study in Albino Swiss mice.
Unpublished results No. 685 from Department of Toxicology, Janssen
Pharmaceutica N.V. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983b). Carcinogenicity study in Wistar rats. Unpublished results
No. 684 from Department of Toxicology, Janssen Pharmaceutica N.V.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
YAMASHITA, T. & HATTORI, K. (1983). Mutagenic evaluation of
flubendazole in bacterial repair and reverse mutagenesis test.
Unpublished results No. V5051 from Research Laboratories Fujisawa
Pharmaceutical Co., Ltd., Osaka, Japan. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
YOSHIMURA, H. (1987). Teratogenicity of flubendazole in rats.
Toxicology, 43: 133-138.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of flubendazole is summarized in Table 1. In
some cases the animals exhibited exophthalmus, hypotonia, slight
sedation, general depression, ataxia, convulsions and pilo-erection.
Deaths were recorded within the first 24 h after intraperitoneal
administration of test substance.
Table 1. Acute toxicity of flubendazole
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983a
M&F s.c.* >5000 Niemegeers, 1986a
M&F s.c.# >10 000 Nakaoka et al., 1983a
M i.p.# 528 Nakaoka et al., 1983a
F i.p.# 434 Nakaoka et al., 1983a
Rat M&F oral* >5000 Niemegeers, 1974
M&F oral# >10 000 Nakaoka et al., 1983b
M i.p.# 435 Nakaoka et al., 1983b
F i.p.# 252 Nakaoka et al., 1983b
M&F s.c.# >5000 Nakaoka et al., 1983b
M&F s.c.* >10 000 Niemegeers, 1986b
Guinea-pig M&F oral* >5000 Niemegeers, 1974
M s.c.* 4679 Niemegeers, 1986c
F s.c.* 4834 Niemegeers, 1986c
Laying-hen F oral >640 Vanparijs &
Desplenter, 1982
Guinea fowl oral >1200 Le Brun, 1983
* vehicle: aqueous suspension with 1% polysorbate 80
# vehicle: 0.5% methylcellulose solution
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
In a 3-month study flubendazole admixed with the diet at levels
of 0, 10, 40 or 160 mg/100 g food was given to Wistar rats (10
rats/sex/dose level). The approximate intakes of flubendazole over
the period of the study were equal to 0, 8, 30 or 130 mg/kg bw/day
for males and 0, 9, 40 or 150 mg/kg bw/day for females. No
treatment-related effects were observed on mortality, behaviour,
appearance, food consumption, body weight, haematology, serum
analysis, urinalysis, microscopy, organ weight or histopathology
(Marsboom, 1975a).
2.2.2.2 Dogs
Groups of beagle dogs (3 dogs/sex/group) were given
flubendazole orally as a powder in gelatin capsules at doses of 0,
2.5, 10 or 40 mg/kg bw/day, six days per week, over 3 months.
Control animals received 250 mg lactose only. Observations included
behavioural changes, food consumption, body weight, ECG, blood
pressure, haematology, serum analysis, urinalysis, necropsy, organ
weight and histopathology. Small-sized prostate and congestion of
cauda of the epididymis were observed in all males at doses of 10
and 40 mg/kg bw/day.
Histopathologically some atrophic changes of the prostate were
noticed at the higher dose groups, but the changes were not
dose-related. Atresic changes in the ovaries were noted in 2 females
in the 2.5 mg/kg bw/day group, 1 in the 10 mg/kg bw/day group and 3
in the 40 mg/kg bw/day group. Atrophic changes of the uterine wall
and vagina in some dosed females were also observed. According to
historical control data the changes in the female genital tract were
considered to be within the normal range for the age of the dogs
(Marsboom et al., 1975).
Subsequent to the above report the histopathological slides
were evaluated by two independent pathologists. Both experts agreed
that the atrophic changes were not indicative of a treatment-related
toxic effect and should be considered as incomplete development in
sexually immature dogs (Thoonen, 1987; Powell, 1991). Because of the
lack of conclusive evidence as to the etiology of these changes, the
Committee concluded that the NOEL in this study was 2.5 mg/kg
bw/day.
2.2.2.3 Chickens
Flubendazole was given to 4 groups of broiler breeder pullets
(50 F + 5 M/group) over a period of 7 days. The drug was admixed in
the feed at concentrations of 0, 60, 120 or 180 mg/kg. Four days
after the end of treatment 10 hens and a cock were removed from the
groups for blood sampling and necropsy.
The only significant difference in haematological parameters
was a lower haematocrit value and red blood cell count in the
highest-dose group. Clinical biochemistry tests showed significant
increases in triglycerides and phospholypides at 120 mg/kg,
decreased aspartic aminotransaminase at 180 mg/kg and decreased
cholinesterase at 120 and 180 mg/kg. Histopathologically,
treatment-related effects were observed in the spleen of the
high-dose group, in reduction of the white pulp area and reduction
of the number of red blood cells in the red pulp. The NOEL in this
study was 120 mg/kg, equivalent to 15 mg/kg bw/day (Desplenter &
Coussement, 1985).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of 50 male and female Swiss albino mice were fed
diets containing 0, 5, 10 or 20 mg/100 g flubendazole for a period
of 18 months. The intake of the drug over the study period was
equivalent to 7.5, 15 or 30 mg/kg bw/day (data on food consumption
and body weight were not provided). The mortality rate, clinical
observations and presence of subcutaneous masses were recorded
daily. A complete necropsy and histopathological examination were
performed at termination of the study.
No treatment-related effects were noted for clinical condition
or survival. The percent survival for treated groups was comparable
to the controls, as shown in Table 2.
Table 2. Survival at 18 months in the long-term study in mice
Sex Dose mg/kg bw/day
0 7.5 15 30
M 54% 44% 38% 38%
F 40% 34% 40% 32%
The total number of benign and malignant tumours was similar
for treated and control groups. The most common types of tumours
were hepato-cellular tumours and alveologenic lung carcinoma. No
effect of flubendazole was evident histopathologically. The NOEL in
this study was 30 mg/kg bw/day (Verstraeten, et al., 1983a).
2.2.3.2 Rats
Groups of 50 Wistar rats of each sex were fed diets containing
0, 10, 20 or 40 mg/100 g flubandezole for 24 months. Drug intake was
equivalent to 5, 10, or 20 mg/kg bw/day.
All animals were observed once daily for signs of abnormal
behaviour and clinical effects. At termination of the study a full
necropsy was performed and the organs were examined
histopathologically.
The mortality rate was very high in all groups of animals
including the controls at the end of the study. There was no
statistically-significant difference in mortality rates between the
groups during the entire period of the study. There were no
treatment-related effects among the treated and control animals.
During the study subcutaneous masses in females were noted in
approximately 20% of the control and 40% of the high-dose animals.
On necropsy a significantly increased incidence of pale kidneys was
observed in females at 5 and 20 mg/kg bw/day. However, there was no
histopathological evidence for dose-related changes in the kidney.
Flubendazole at concentrations of up to 40 mg/100 g feed for 2 years
did not show biological or statistical evidence of incidence of
neo-plasms. The NOEL in this study was 20 mg/kg bw/day (Verstraeten
et al., 1983b).
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 30 female Swiss albino mice were given flubendazole
in a single dose of 0, 20, 80 or 320 mg/kg bw. The drug was
administered orally by gavage in microsuspension vehicle. A control
group of females received vehicle suspension only. All the females
were mated with untreated males. The animals were controlled daily
over a period of 360 days. During the experiment the mortality rate,
incidence of pregnancy, time interval between dosing and birth, and
number of pups were not affected by treatment.
By comparing the data from control and treated animals, no
differences were noted regarding mortality of females, the average
time interval between dosing and birth of first litters or litter
size per female. In the high-dose group (320 mg/kg bw) a reduction
of mean number of pups per female was observed. The NOEL in this
study was 80 mg/kg bw (Marsboom, 1977a).
2.2.4.2 Rats
In a fertility study, Wistar rats (20 animals/sex/dose) were
given flubendazole admixed in feed. The concentration of the test
substance in the diet was 0, 2.5, 10 or 40 mg/100 g, equivalent to
2.5, 10 or 40 mg/kg bw/day. Females were treated with flubendazole
for 14 days prior to cohabitation with males and during the
pregnancy period. Males received flubendazole over a period of 60
days before mating. Untreated animals were mated with treated ones.
No drug effects were noted regarding food consumption or
average weight gain of females. All the females were sacrificed on
the 22nd day post-mating. No drug effects were noted on incidence of
pregnancy and the percentage of pregnancies was 100% in almost all
groups. In all females the average number of implantations and
percentages of live, dead and resorbed fetuses were similar and not
affected by the treatment. Treatment-related fetal skeletal
abnormalities were not observed. The NOEL in this study was 40 mg/kg
bw/day (Marsboom, 1976a).
Four groups of 20 Wistar rats were given flubendazole admixed
in feed at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day from day 16 of gestation
up to the 3-week-lactation period.
The average body weight and food consumption were not
significantly different among the groups. Only one female from the
40 mg/kg bw/day group died during the time of treatment. The
pregnancy rate, average duration of gestation and fetal parameters
were comparable among the treated and control groups. No
abnormalities were found among offspring in any of the groups in the
study. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1976b).
2.2.4.3 Pigs
A study was performed on 5 different pig farms. On each farm 16
sows and 1 boar received flubendazole admixed in the feed, providing
a dose of 3 mg/kg bw/day. On each farm the same number of
non-treated animals served as controls. Each boar was mated with 8
treated and 8 control sows. The treated sows received flubendazole
diet from estrus till parturition, while boars were treated two
months prior to the first mating and until all sows were
successfully bred.
During the study no differences were observed on conception
rate of the boars, estrus behaviour or gestation length. Post-partum
conditions were normal except in 7 sows, which were found to be
related to dystocia. The number of live and dead piglets among the
different groups was not statistically different. There were no
significant differences in abnormalities observed among the piglets
from any groups except in slightly increased number of
mummifications. The investigators associated mummifications to the
endemic occurrence of pseudorabies and parvovirus infections in the
area where the study was performed. The time interval betwen weaning
to the next estrus were equal among the groups (De Keyser et al.,
1984)
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 young female Wistar rats were fed diet containing
flubendazole at concentrations of 0, 2.5, 10 or 40 mg/100 g feed,
equivalent to 0, 2.5, 10 or 40 mg/kg bw/day, from day 6 to day 15 of
pregnancy. Parameters measuring body weight, food consumption,
mortality and pregnancy were recorded. The animals were sacrificed
on the 22nd day of pregnancy and the fetuses were delivered by
caesarean section. The number of live, dead and resorbed fetuses was
recorded, average body weight of pups was measured and the fetuses
examined for abnormalities.
During the study none of the animals died and food consumption
and average body weight were comparable among the groups. The
percentage of pregnancies in all treated groups was 95% and in the
control group 90%. All the fetal parameters studied were comparable
among the treated and control groups. The absence of metacarpal and
metatarsal bones was noticed in only one fetus from the highest dose
group. The NOEL in this study was 40 mg/kg bw/day (Marsboom, 1974).
In a separate study, four groups of 20 female Wistar rats each
were treated with flubendazole in the diet at concentrations of 0,
2.5, 10 or 40 mg/100 g feed, equivalent to 0, 2.5, 10 or 40 mg/kg
bw/day. The study design and parameters studied were the same as
described in the previous study. There were no changes in any of the
parameters studied. The NOEL was 40 mg/kg bw/day (Marsboom, 1975b).
A third study (same experimental design) was carried out in
order to confirm the results of the previous studies. The test
substance in this experiment was given orally by gavage at doses of
0, 2.5, 10 or 40 mg/kg bw/day. All the maternal and fetal parameters
were comparable among the treated and control groups of animals. The
NOEL in this study was 40 mg/kg bw/day (Marsboom, 1977b).
Groups of female Wistar rats (20 animals/group) were given
flubendazole admixed in a powdered diet at concentrations of 0, 10,
40 or 160 mg/100 g feed, equivalent to 0, 10, 40 or 160 mg/kg bw/day
from days 6 to 15 of pregnancy. The same parameters as in the
previous experiments were studied. Food consumption and average body
weight were comparable among all groups. During the study no
mortalities occurred. The percentage of pregnancies was high and
comparable among the groups. No evidence of embryotoxicity or
teratogenicity was found. The NOEL in this study was 160 mg/kg
bw/day (Marsboom, 1978).
Flubendazole extracted from commercially formulated drug was
suspended in water and given by gavage to female Sprague-Dawley rats
(20 animals/group) at doses of 0, 2.5, 10, 40 or 160 mg/kg bw/day
from day 8 to day 15 of pregnancy. On day 21 of pregnancy the
animals were sacrificed and the number of implantation sites and
live fetuses were recorded. The fetuses were further examined for
malformations.
During the study period signs of maternal toxicity were not
observed. The highest dose (160 mg/kg bw/day) was found to be
embryocidal, resulting in a significant increase in the fetal
resorption rate. A dose-dependent decrease in fetal body weight
occurred, which was significant at 40 and 160 mg/kg bw/day. The two
highest doses induced significant gross, skeletal and internal
malformations. In the 160 mg/kg bw/day dose group, 16.8% of fetuses
were grossly malformed. The malformations were described as:
encephalocele, cranial meningocele, omphalocele, ectrodactyly, club
foot, anal atresia, spina bifida occulata and tail defects. Skeletal
malformations affected mainly the vertebrae and ribs, and
significant malformations were found in 24.6% and 32.6% of fetuses
in the two highest dose groups. Significant internal malformations
were observed in 19.8% and 47.7% of fetuses in the 40 and 160 mg/kg
bw/day group. respectively. The NOEL in this study was 10 mg/kg
bw/day (Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 female New Zeeland white rabbits were orally
administered 0, 10 or 40 mg flubendazole/kg bw/day from day 6 to day
18 of gestation. The females were sacrificed on gestation day 28 and
fetuses were delivered by caesarean section. The animals from all
doses were necropsied. The fetuses were weighed and examined for
external anomalies. After incubation of the pups the survival rate
was calculated. All the fetuses were radiographically examined.
One-third of the fetuses were examined for visceral anomalies and
the rest were preserved and used for additional analysis.
During the study one non-pregnant female in the highest dose
group died due to infection. The average body weight increase was
comparable among all groups. No differences were observed in
pregnancy rate among the groups and no teratogenic effects were
found among the offspring. The percentage of live, dead and resorbed
fetuses and survival rate of incubated pups from all groups were not
significantly different. The NOEL in this study was 40 mg/kg bw/day
(Marsboom, 1976c).
2.2.5.3 Pigs
Flubendazole (5% powder formulation) was admixed in the diet at
a concentration of 30 mg/kg and given to 6 crossed Landrace-Pietrain
sows from day 8 to day 50 of pregnancy. Altogether 62 piglets were
born. No piglets were stillborn and no abnormalities were recorded
(Marsboom, 1976d).
In a separate study flubendazole (5% powder formulation)
admixed in the feed at a concentration of 200 mg/kg, equivalent to 8
mg/kg bw/day, was given to 20 sows from the first day of mating
until the day of farrowing. Three sows were withdrawn from the
experiment after 3 weeks. In the group of 17 sows having successful
pregnancies 154 live and 8 stillborn piglets were recorded. There
were no detectable external abnormalities, except for a mild form of
sprayed legs observed in 2 piglets of one sow. Abnormalities were
not found in stillborn piglets (Rogiers, 1979).
A group of 8 sows was given 50 mg flubendazole/kg bw/day
admixed in the diet from the first day of mating until day 70 of
pregnancy. Sixty-three piglets of normal weight and 3 stillborn
piglets were born. No external abnormalities were recorded (Rogiers,
1980).
2.2.6 Special studies on eye and skin irritation
2.2.6.1 Rabbits
A group of 6 adult New Zeeland rabbits had 0.1 ml of a 50% w/w
suspension of a 5% premix formulation instilled into the
conjunctival sac of the left eye. There were no signs of eye
irritation during the observation time of 21 days (Teuns & Marsboom,
1987a).
A flubendazole formulation was applied to intact skin of New
Zeeland rabbits. The test sites were occluded for 24 h. After
removal of dressings, the test sites were scored for erythema and
oedema. A barely perceptible irritation (index 0.63) was noted
during the first 5 days in the treated animals according to the
Draize irritation index. This irritation was fully reversible after
5 days. This study was designed primarily to determine the dermal
LD50 value (Teuns & Marsboom, 1987b).
2.2.7 Special studies on genotoxicity
The genototoxicity of flubendazole is summarized in Table 3.
Table 3. Results of genotoxicity studies on flubendazole
Test system Test object Concentration Results Reference
In vitro
Repair test B. subtilis 1-5000 µg/disc Negative Yamashita &
Hattori, 1983
Ames test +/- S9 S. typhimurium 10-5000 µg/plate Negative Yamashita &
TA1535, TA1537, Hattori, 1983
TA1538, TA100,
TA98
E. coli
N/r WP2 trp hcr
S. typhimurium 0.5-1000 µg/plate Negative Jagannath &
TA535, TA1537, Brusick, 1978
TA1538, TA98,
TA100
S. cerevisiae D4
In vivo
Sex-linked Drosophila 500 and 2000 ppm Negative Vanparys &
melanogaster feeding/3d Marsboom, 1982
Micronucleus Male Swiss albino 2 oral doses: 40, Negative Vanparys &
test mice 80, 160, Marsboom, 1979
1280 mg/kg bw
female Wistar 2 oral doses: 80, Negative Vanparys &
rats 160, 640 mg/kg bw Marsboom, 1981
Dominant Male Swiss albino single oral dose: Negative Marsboom, 1977c
lethal mice 10, 40,
160 mg/kg bw
2.3 Observations in humans
Three male volunteers were given single doses of flubendazole
orally in 100 mg tablets. Flubendazole was mainly excreted in faeces
(77.3% of the administered dose) within 3 days after intake of the
drug. Less than 0.1% of the dose was excreted in the urine as
unchanged drug (Heykants et al., 1975).
Three male volunteers were given oral doses of 100 mg
flubendazole 2 h before meals, 2000 mg flubendazole immediately
after a heavy meal and 2000 mg flubendazole before a meal. Serum
concentrations of flubendazole were monitored. The plasma
concentration was very low, and peak levels after 100 and 2000 mg
doses taken before meals were 0.35 and 0.74 ng/ml, respectively. In
the case of flubendazole taken after a heavy meal, the peak plasma
concentration was markedly higher (4.06 ng/ml), which indicates that
the presence of food enhances the absorption of flubendazole from
the gastro-intestinal tract. By calculating the area under the curve
(AUC) values it was found that the absorption of the drug was not
dose-dependent. AUC values increased only 1.4-fold after a 20-fold
increase in dose (Michiels et al., 1977b).
3. COMMENTS
A substantial database was available for assessment, including
data on kinetics and metabolism, acute toxicity, short-term and
long-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The absorption, metabolism, and excretion of flubendazole have
been studied using radiolabelled drug. Flubendazole is poorly
absorbed and metabolized in a qualitatively similar way in all
species studied. More than 50% of the ingested drug is eliminated
unchanged in the faeces. The absorbed drug is rapidly metabolized,
so that levels of parent drug in the blood and urine are extremely
low. The main site of metabolism is the liver, and major metabolic
pathways are carbamate hydrolysis and ketone reduction. It seems
probable that flubendazole undergoes enterohepatic circulation.
Single oral doses of flubendazole were slightly toxic to
experimental animals, the median lethal dose (LD50) being greater
than 5000 mg/kg of body weight in mice, rats, and guinea-pigs.
Flubendazole was given orally in gelatin capsules to dogs at
doses of 2.5, 10 or 40 mg/kg bw/day, 6 days a week for 3 months.
Some atrophic changes and congestion of the epididymis were observed
in the male genital tract at doses of 10 and 40 mg/kg bw/day, and in
the female genital tract at all doses. The changes in the female
genital tract were considered to be within normal limits for dogs of
the age of those used in the study. On histological examination of
male sex organs, changes in the testes could not be clearly
associated with flubendazole treatment. The findings in male dogs
may not be compound-related, but because of the lack of conclusive
evidence as to the cause of these changes, the Committee concluded
that the no-observed-effect level (NOEL) was 2.5 mg/kg bw/day.
Carcinogenicity studies were performed in mice and rats at
doses up to 30 and 20 mg/kg bw/day respectively; no
treatment-related effects were observed. There was no
treatment-related increase in any type of neoplasm. The Committee
was of the opinion that flubendazole had no carcinogenic potential
at the highest doses administered in these studies.
The results from a range of in vitro and in vivo
genotoxicity tests were all negative.
The Committee considered data from reproduction,
embryotoxicity, and teratogenicity studies. Studies in mice,
rabbits, and pigs were negative. Flubendazole was extensively
studied in segmented reproduction studies in rats, performed as
required for human drug regulation purposes and accepted by the
Committee in lieu of a multigeneration reproduction study. In
several rat developmental
studies, doses of up to 40 and 160 mg/kg bw/day, given on gestation
days 6-15, did not produce any embryotoxic or teratogenic effects.
In a rat teratogenicity study published in 1987, using material
extracted from a commercial preparation, gross skeletal and internal
fetal malformations were recorded at doses of 40 and 160 mg/kg
bw/day. The NOEL in this study was 10 mg/kg bw/day.
4. EVALUATION
An ADI of 0-12 µg/kg of body weight was established for
flubendazole, based on the NOEL of 2.5 mg/kg bw/day in the 3-month
study in dogs, and a safety factor of 200. This safety factor was
used by the Committee to take account of the fact that the doses
were administered only 6 days per week in this study, the precise
consequences of which could not be assessed.
The Committee noted that the ADI also provided a safety margin
corresponding to a factor of about 1000 with respect to the NOEL of
10 mg/kg bw/day derived from the rat teratogenicity study. The
Committee considered that further carcinogenicity studies would not
be required, since the highest dose used in the negative studies
that it had evaluated exceeded the ADI by a factor of approximately
2000.
5. REFERENCES
DE KEYSER, H., DONY, J. & ROGIERS, M. (1984). Fertility study with
flubendazole (R 17889) in pigs. Unpublished results No. V4988 from
Janssen Pharmaceutica Veterinary Department. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
DESPLENTER, L. & COUSSEMENT, W. (1985). Safety evaluation of
flubendazole in broiler breeder chickens at higher than normal use
levels: haematological, biochemical and histopathological
evaluation. Unpublished results No.V5738 from Veterinary Clinical
Research and Department of Toxicology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
HEYKANTS, J., WYNANTS, J. & SCHEIJGROND, H. (1975). The absorption
and excretion of flubendazole in man. Unpublished results No. N10250
from Department of Drug Metabolism, Department of Separation
Techniques and Department of Clinical Pharmacology Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
JAGANNATH, D.R. & BRUSICK, D.J. (1978). Mutagenicity evaluation of
flubendazole in the Ames Salmonella/microsome plate test.
Unpublished results No. 20988 from Litton Bionetics, Inc., 5516
Nicholson Lane, Kensington, Maryland. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
LE BRUN, J. (1983). Acute toxicity of flubendazole in guinea fowls.
Unpublished results No. V4899 from Department of Toxicology and
Pharmacology JMG/MC. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1974). Potential of oral R 17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 556 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975a). Oral toxicity study in Wistar rats (repeated
dosage for 3 months). Unpublished results No. 567, Janssen
Pharmaceutica, Research Laboratories 2340 Beerse, Belgium. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1975b). Potential of oral R17889 for embryotoxicity
and teratogenic effects in rats. Unpublished results No. 572 from
Department of Toxicology, Janssen Pharmaceutica N.V. Submitted to
WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R., HERIN, V., VANDESTEENE, R. & PARDOEL, L. (1975). Oral
toxicity study in beagle dogs (repeated dosage for 3 months).
Unpublished results No. 568, Janssen Pharmaceutica, Research
Laboratories 2340 Beerse, Belgium. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976a). Oral male and female fertility study in Wistar
rats. Unpublished results No. 695 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in Wistar rats. Unpublished results No. 692 from Department of
Toxicology, Janssen Pharmaceutica N.V. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
MARSBOOM, R. (1976c). Oral embryotoxicity and teratogenicity study
in New Zealand white rabbits (segment II). Unpublished results No.
692 from Department of Toxicology, Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1976d). Potential of oral flubendazole (R 17889) for
teratogenic effects in pigs. Unpublished results No. 692 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977a). Total reproductive capacity test in female
mice. Unpublished results No. 773 from Department of Toxicology,
Janssen Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977b). Oral embryotoxicity and teratogenicity study
in Wistar rats (Segment II). Unpublished results No. 755 from
Department of Toxicology, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1977c). Dominant lethal test in male mice (single oral
dose). Unpublished results No. 688 from Janssen Pharmaceutica,
Research Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MARSBOOM, R. (1978). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished results No. 820 from
Department of Toxicology, Janssen Pharmaceutica Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., HURKMANS, R., SWIJSEN, E. & HEYKANTS, J. (1977). A
comparative study on the excretion and metabolism of flubendazole
(R17889) and mebendazole (R17635) in the rat. Unpublished report No.
V2940 from Department of Drug Metabolism, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., SWIJSEN, E., HENDRICKX, J., LAUWERS, W., BRACKE,
J., LENAERTS, F., SNEYERS, R. & HEYKANTS, J. (1978). On the
absorption, excretion and biotransformation of flubendazole in the
dog. Unpublished report No. N15186 from Department of Drug
Metabolism and Pharmacology and Analytical Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
MEULDERMANS, W., LEE, I.Y., HENDRICKX, J., SWYSEN, E., LAUWERS, W.,
PORTER, D. & HEYKANTS, J. (1982). Metabolism of flubendazole in
swine: Excretion pattern of [2-14C] flubendazole and its
metabolites and depletion kinetics of drug residues in edible
tissues. Preliminary report from Department of Drug Metabolism,
Department of Analytical Research, Janssen Pharmaceutica, and
Pitman-Moore, Inc., Washington Crossing, NJ., USA. Submitted to WHO
by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HEYKANTS, J. & HENDRICKX, J. (1977a). Distribution of
flubendazole (R17889) and its metabolites in the Wistar rat.
Unpublished report No. N12336 from Department of Drug Metabolism and
Analytical Department, Janssen Pharmaceutica. Submitted to WHO by
Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
MICHIELS, M., HENDRIKS, J. & HEYKANTS, J. (1977b). The
pharmacokinetics of mebendazole and flubendazole in animal and man.
Arch. Internat. Pharmacodinam. Therapie, 256: 180-191.
MICHIELS, M., HEYKANTS, J., VAN DEN BOSSCHE, H. & VERHOEVEN, H.
(1980a). A comparative study on the systemic absorption of oral and
subcutaneous mebendazole, flubendazole and R34803 in two different
rodents. Unpublished report No. N19702 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., PRINSEN, P., HEYKANTS, J. & VAN DEN BOSSCHE, H.
(1980b). Systemic absorption of intramuscular flubendazole in the
dog. Unpublished report No. N20709 from Department of Drug
Metabolism and Department of Comparative Biochemistry, Janssen
Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
MICHIELS, M., MONBALIU, J., WOESTENBORGHS, R., HEYKANTS, J. & MAES,
L. (1987). Absorption and plasma concentrations of flubendazole in
the dog after single oral administration at the therapeutic dose
level of 22 mg/kg as the commercial flubendazole paste (Fflubenol KH
pasteR). Unpublished report No. V6282 from Department of Drug
Metabolism and Pharmacology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983a). Acute toxicity
study of flubendazole in mice. Unpublished report No. V5050 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NAKAOKA, A., FURUKAWA, T. & FUKUHARA, K. (1983b). Acute toxicity
study of flubendazole in rats. Unpublished report No. V5049 from
Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka,
Japan. Submitted to WHO by Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
NIEMEGEERS, C.J.E. (1974) The acute oral toxicity of flubendazole in
Wistar rats, albino mice and guinea-pigs. Unpublished results No.
N9208 from Department of Pharmacology, Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986a). The acute subcutaneous toxicity of
R17889 in mice. Unpublished results No. N49012 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986b) The acute subcutaneous toxicity of R17889
in rats. Unpublished results No. N49023 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
NIEMEGEERS, C.J.E. (1986c) The acute subcutaneous toxicity of R17889
in guinea-pigs. Unpublished results No. N49024 from Department of
Pharmacology, Janssen Pharmaceutica. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
POWELL, C.J. (1991). Independent report on slides from the
flubendazole study No. 568. Unpublished results from Department of
Toxicology St. Bartholomew's Hospital Medical College, University of
London. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1979). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 200 ppm during the whole gestation
period. Unpublished results No. V3251 from Janssen Pharmaceutica.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
ROGIERS, M. (1980). Potential of oral flubendazole (R17889) for
teratogenic effects in pigs at 50 mg per kg of bodyweight,
administered during the first 10 weeks of the gestation period.
Unpublished results No. V3443 from Janssen Pharmaceutica. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
TEUNS, G. & MARSBOOM, R. (1987a). Primary eye irritation study in
rabbits. Unpublished results No. 1834 from Department of Toxicology,
Janssen Pharmaceutica N.V. Submitted to WHO by Janssen Research
Products Information Service, Janssen Pharmaceutica, B-2340 Beerse,
Belgium.
TEUNS, G. & MARSBOOM, R. (1987b). Acute dermal toxicity study in New
Zealand White rabbits (single dose). Unpublished results No. 1835
from Department of Toxicology, Janssen Pharmaceutica N.V. Submitted
to WHO by Janssen Research Products Information Service, Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
THOONEN, H. (1987). Independent report on slides from the
flubendazole study No 568. Unpublished results from Department of
Pathology, Faculty of Veterinary Medicine, State University Ghent,
Belgium. Submitted to WHO by Janssen Research Products Information
Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VAN LEEMPUT, L., MAES, L., MEULDERMANS, W. & HAYKANTS, J. (1991).
Pharmacokinetic profile of flubendazole: critical review of
available data. Unpublished review report from Department of Drug
Metabolism and Pharmacokinetics, and Department of Parasitology,
Janssen Pharmaceutic. Submitted to WHO by Jansen Research Products
Information Service, Janssen Pharmaceutical, B-2340, Beerse,
Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1979). Micronucleus test in mice.
Unpublished results No. 912 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1981). Micronucleus test in rats.
Unpublished results No. 1032 from Department of Toxicology, Janssen
Pharmaceutica, Research Laboratories. Submitted to WHO by Janssen
Research Products Information Service, Janssen Pharmaceutica, B-2340
Beerse, Belgium.
VANPARYS, Ph. & MARSBOOM, R. (1982). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished results No. 1138 from
Department of Toxicology, Janssen Pharmaceutica, Research
Laboratories. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VANPARIJS, O. & DESPLENTER, L. (1982). The acute toxicity of
flubendazole in poultry. Unpublished results No. V4501 from
Department of Parasitology and Animal Health Department, Janssen
Pharmaceutica. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983a). Oral carcinogenicity study in Albino Swiss mice.
Unpublished results No. 685 from Department of Toxicology, Janssen
Pharmaceutica N.V. Submitted to WHO by Janssen Research Products
Information Service, Janssen Pharmaceutica, B-2340 Beerse, Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., VAN CAUTEREN, H. & MARSBOOM, R.
(1983b). Carcinogenicity study in Wistar rats. Unpublished results
No. 684 from Department of Toxicology, Janssen Pharmaceutica N.V.
Submitted to WHO by Janssen Research Products Information Service,
Janssen Pharmaceutica, B-2340 Beerse, Belgium.
YAMASHITA, T. & HATTORI, K. (1983). Mutagenic evaluation of
flubendazole in bacterial repair and reverse mutagenesis test.
Unpublished results No. V5051 from Research Laboratories Fujisawa
Pharmaceutical Co., Ltd., Osaka, Japan. Submitted to WHO by Janssen
Pharmaceutica, B-2340 Beerse, Belgium.
YOSHIMURA, H. (1987). Teratogenicity of flubendazole in rats.
Toxicology, 43: 133-138.
