
RACTOPAMINE
First draft prepared by
Dr L. Ritter
Bureau of Veterinary Drugs
Health Protection Branch
Health and Welfare Canada, Ottawa
1. EXPLANATION
Ractopamine is a phenolethanolamine ß-adrenoceptor agonist that
is used for the improvement of weight gain, carcass leanness and
feed efficiency in pigs. Ractopamine is marketed as the
hydrochloride with a minimal purity of 92% and exists in two
diastereomeric forms which are identified as RS, SR and RR, SS. The
RR-isomer, butopamine, is a potent cardiotonic in humans.
Ractopamine hydrochloride had not been previously evaluated by the
Joint FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Studies with 14C-ractopamine in several animal species
indicated a rapid absorption following oral administration. Peak
plasma and whole blood levels occurred in rats 0.5-2.0 h after
dosing. Peak plasma and whole blood levels were similar in males
when compared to females over the dose range of 0.5 to 2 mg/kg bw.
At a dose of 20 mg/kg bw the levels were, however, approximately 2.5
times higher in females than in males. The elimination half-life was
approximately 7 h for males and females. Males receiving 20 mg/kg bw
had an unexplained longer elimination half-life of approximately 15
h (Dalidowicz et al., 1986a; Williams et al., 1987).
Oral gavage doses of 0.05, 0.5, or 5 mg/kg bw resulted in peak
levels in dogs 1-2 h after dosing (except for females at 5 mg/kg bw,
when maximum concentration was reached 4-8 h after dosing). The
elimination half-life from plasma and whole blood was approximately
6 h (Dalidowicz et al., 1986b; Dalidowicz, 1987).
The excretion of ractopamine after oral dosing was measured in
dogs, monkeys and pigs. A total of 79% and 70% of the administered
single oral dose of 0.125 mg/kg bw 14C-ractopamine hydrochloride
was recovered from the dog and monkey, respectively, during the 72 h
collection period. In a balance-excretion study, unlabelled
ractopamine hydrochloride administered to pigs in the feed at 20 ppm
combined with a one-time dose of 14C-ractopamine hydrochloride of
40 mg incorporated into control feed, was excreted almost
quantitatively with approximately 88% in urine and 9% in faeces
during a 7-day collection period. The bulk of the radiolabelled
ractopamine was excreted in the first three days (95%), while 85%
was eliminated during the first day after dosing (Williams, 1987a;
Dalidowicz & Babbitt, 1986; Dalidowicz et al., 1986; Dalidowicz,
1987).
2.1.2 Biotransformation
A study in pigs fed 14C-ractopamine hydrochloride showed that
the livers and kidneys of dosed animals contained three major
metabolites of ractopamine (Metabolites A, B, and C in figure 1).
These metabolites were formed by conjugation of the hydroxyl group
in ring A or ring B and are chromatographically distinct (Figure 1):
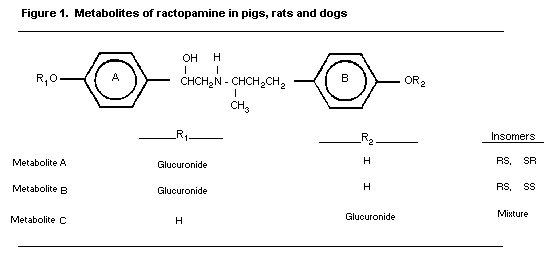 The same three metabolites were isolated from pig urine and
were all identified to be monoglucuronides of ractopamine. Of the
extractable liver residues, 30-50% was found to be ractopamine and
the rest conjugates of ractopamine. The kidneys contained a higher
amount of conjugates (64%) and correspondingly smaller amount of
ractopamine (20-30%). Studies in rats and dogs showed that urine
from animals dosed with 14C-ractopamine hydrochloride contained
the same three metabolites of ractopamine as in pigs (metabolites A,
B, and C). These three metabolites constituted a large portion of
14C-residues in rat and dog urine. The chromatographic profiles of
the 14C-residue extracts of rat, dog and pig liver were
quantitatively similar. The comparative quantitative data showing
the mean amounts in ppm of ractopamine hydrochloride and its major
metabolites (calculated as ractopamine hydrochloride) in kidney and
liver tissues of pigs, dogs, and rats are summarized in Table 1
(Dalidowicz & Babbitt, 1986; Dalidowicz, 1986a; Dalidowicz, 1986b).
Table 1. Metabolic profile in pigs, dogs and rats
Liver, ppm Kidney, ppm
Pig Dog Rat Pig Dog Rat
Ractopamine. HC1 0.12 0.59 0.40 0.10 0.50 0.33
Metabolite A 0.03 0.46 0.17 0.05 0.18 0.52
Metabolite B 0.04 0.77 0.15 0.06 0.27 0.57
Metabolite C 0.02 1.76 0.10 0.09 0.63 0.08
Dose: pigs (45 kg): 30 ppm in food for 4 days; pigs were killed
12 h after the last dose;
dogs: 0.5 mg/kg bw by gavage 3 times per day for 4 days and
once on the fifth day; dogs were killed 6 h after the
last dose;
rats: 2 mg/kg bw by gavage once daily for 7 days; rats were
killed 6 h after the last dose.
The concentrations of the metabolites in dog and rat tissues
were much higher than those in pig tissues. The nonextractable and
the uncharacterized residues in dog and rat tissues were also much
higher than in pig tissues (Dalidowicz, 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ractopamine are
summarized in Table 2.
2.2.2 Short-term studies
2.2.2.1 Mice
Diets containing 0, 0.02, 0.14 or 1.0% ractopamine
hydrochloride were fed to B6C3F1 mice (10/sex/dose) for 3
months, resulting in estimated time-weighted average daily doses of
0, 25, 175 or 1250 mg/kg bw/day, respectively. The mice were
observed daily for clinical signs of toxicity. Body-weights and food
consumption were measured weekly in all animals. Necropsies were
performed on all mice sacrificed at the end of the study. A set of
tissues was examined microscopically.
Table 2. Acute toxicity studies
Species Sex Route LD50 (mg/kg/bw Reference
Mouse M oral 3547 (2912-4321) Williams et al.,
1985a
F oral 2545 (2219-2919) Williams et al.,
1985a
Rat M oral 474 (398-564) Williams et al.,
1985b
F oral 367 Williams et al.,
(1985b)
Rat M intraperitoneal 132 (110-159) Williams et al.,
F intraperitoneal 122 (102-145) 1985c
Rabbit M dermal >2000 Williams et al.,
F 1984a
Rat M inhalation LC50 (4 h): 2.8 Williams et al.,
F mg/L 1985k
All of the treated animals survived the treatment period.
Alopaecia, a common finding in B61C3F1 mice, was observed in
22 animals of each sex. In this study the occurrence of alopaecia
was markedly decreased in the 1250 mg/kg bw/day group compared to
the other groups. No adverse signs of toxicity were attributed to
treatment with ractopamine hydrochloride. Male mice in the 25 and
175 mg/kg bw/day groups had a significant increase in cumulative
weight gain in the last eight weeks of the study. Differences in the
final mean body-weights of the treatment groups compared to the
controls were not statistically significant.
Treatment- and dose-related mild to moderate increases occurred
in erythrocyte counts, haemoglobin concentrations, and packed cell
volumes in both sexes of the 175 and 1250 mg/kg bw/day groups. Minor
changes occurred in the mean corpuscular volume and mean corpuscular
haemoglobin concentration in males of the 1250 mg/kg bw/day group.
The changes in thrombocyte counts were minimal in both sexes of the
1250 mg/kg bw/day group. All other haematologic parameters were
similar to control values. There were no treatment-related effects
on any haematologic parameters in mice of the 25 mg/kg bw/day group.
Treatment-related clinical chemistry changes consisted of mild
increases in urea nitrogen and cholesterol concentrations in males
of the 1250 mg/kg bw/day group. Female mice in both the 175 and 1250
mg/kg bw/day groups had mild increases in urea nitrogen and
cholesterol concentrations. The decrease in serum sodium
concentration in female mice of the 1250 mg/kg bw/day group,
although statistically significant, was minimal and the value fell
within the normal range. No alteration in the other electrocytes
concentration occurred. All other clinical chemistry parameters were
similar to control values. There were no treatment-related effects
on serum chemistry in mice of the 25 mg/kg bw/day group.
In males, a decrease in testicular weights, both absolute and
relative, occurred in a dose-dependent manner. Both males and
females in the 1250 mg/kg bw/day group had increased absolute and
relative heart weights. All the other organ weights were similar to
control values.
Microscopically, treatment-related changes involved the
periaortic and intercapsular brown fat in mice of both sexes in the
1250 mg/kg bw/day group. The plasma membranes of the affected brown
fat cells from the 1250 mg/kg bw/day group of mice were more
discrete and stained intensely with eosin. The cytoplasm of these
cells was filled with uniformly small spherical vacuoles, the
margins of which also stained intensely with eosin. These changes
were diagnosed as marked cytoplasmic changes. The cytoplasm of the
brown fat cells with minimal change was characterized by small
vacuoles, but the margins of the vacuoles did not stain intensely
with eosin. Brown fat in female mice and a few male mice of the
control and of the 25 and 175 mg/kg bw/day groups was characterized
as having a minimal cytoplasmic change. Due to the dose-dependent
decrease in testicular weights, a NOEL could not be established in
this study (Williams et al., 1985d).
2.2.2.2 Rats
Diets containing 0, 20, 200 or 2000 ppm ractopamine
hydochloride were fed to Fischer 344 rats (20/sex/dose) for three
months. The time-weighted average doses were 0, 1.3, 13, or 153
mg/kg bw/day for males and 0, 1.4, 15, or 157 mg/kg bw/day for
females. The rats were observed daily for clinical signs of
toxicity. Body-weights and food consumption were measured weekly for
all animals. Autopsies were done on animals at the end of the study.
Histological examination of tissue sections was conducted on all
tissues.
All of the treated animals survived the treatment period. The
clinical signs observed were decreased body-weight gain, increased
food consumption and decreased efficiency of food utilization. These
signs were observed in the 155 mg/kg bw/day group only, where they
were observed throughout the study in rats of both sexes. In
addition to these signs decreased serum triglyceride and cholesterol
levels and increased serum urea nitrogen concentrations were
observed.
An increase in serum potassium was observed in the 155 mg/kg
bw/day group in both sexes. Total erythrocyte count, haemoglobin
concentration, and packed cell volume all increased in rats of the
155 mg/kg bw/day group, as expected with a beta-agonist. There was a
decrease in uterine weight in rats of the 155 mg/kg bw/day group in
this study and a slight reduction in spleen weight in the 13-15 and
155 mg/kg bw/day groups. No change was observed in heart weight of
any treated animals and there was no microscopic evidence of
myocardial necrosis, which is a well-documented effect following
large doses of epinephrine and other sympathomimetic amines.
Histopathology examination of brown fat revealed slight to
moderate cytoplasmic change in the 13-15 and 155 mg/kg bw/day
groups. The NOEL for males was 1.3 mg/kg bw/day and 1.4 mg/kg bw/day
for females, based on the absence of biochemical or histological
changes (Williams et al., 1985e).
2.2.2.3 Dogs
Groups of 4 male and 4 female beagle dogs were given total
doses of 0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine
hydrochloride in gelatin capsules for 1 year. The doses were given
in capsules as three equal divided doses every 6 h. This study was
based on pilot studies in dogs, in which an oral dose of 0.05 mg/kg
bw/day was associated with skin and oral mucosal reddening and a
dose of 0.035 mg/kg bw/day was the NOEL. On the basis of this NOEL,
the frequency of dosing was increased to three times per day, 6 h
apart, in order to triple the daily exposure to 0.112 mg/kg bw/day
for the low-dose. The middle-dose, 0.224 mg/kg bw/day (0.075 mg/kg
bw, 3 times per day), was selected as a minimal cardiostimulatory
dose. The high-dose, 5.68 mg/kg bw/day (1.89 mg/kg bw, 3 times per
day) was selected because it produced near maximal cardiostimulation
at 2 h following a single oral dose of 1.5 mg/kg bw, in the pilot
study.
All dogs survived the one-year treatment period. Body-weight,
food consumption, ophthalmic examination, electrocardiogram wave
forms, bone marrow evaluation and urinalysis results were not
affected by treatment. The zero-time resting heart rate means for
the study (collected on test-days 29, 68, 83, 119, 155, 182, 211,
273 and 366) for low-, middle- and high-dose dogs (sex combined)
were 123, 116 and 115 beats per minute, respectively, and all were
significantly (p < 0.01) depressed compared to 141 beats per minute
for the controls.
Clinical signs of treatment-related effects were transient
cutaneous erythema and oily and unkept hair coat in the high-dose
dogs. Cutaneous erythema occurred in the mid-dose group in the first
five months of the study. Erythrocyte number, haemoglobin
concentration and packed cell volume were significantly decreased in
the high-dose group starting at 90 days, which and continuing up to
the end of the study. Several clinical chemistry parameters were
altered in the high-dose group by 90 days, which persisted until the
termination of the study. Serum potassium and urea nitrogen
concentration increased while serum glucose, cholesterol and
triglyceride concentration decreased.
Treatment-related gross and histopathological findings were
limited to the high-dose group. Both absolute and relative heart
weight and absolute thyroid and adrenal weights were significantly
decreased. Grossly, there was a slight decrease in abdominal and
thoracic fat. Microscopically, a decrease in hepatic centrilobular
glycogen and an increase in perithymic and periaortic brown fat was
noted in the high-dose dogs.
The appearance of bradycardia at all doses, most prominent
during the first 6 months, precluded a NOEL being established in
this study (Williams, 1987b).
2.2.2.4 Monkeys
Rhesus monkeys (2/sex/dose) were given ractopamine
hydrochloride dissolved in nanopure water at doses of 0.25, 0.5 or
4 mg/kg bw once daily by nasogastric gavage in a volume of 1 ml/kg
bw for 6 weeks. Control monkeys received vehicle (nanopure water)
only. This 6-week subchronic study was conducted in rhesus monkeys
to determine the doses at which toxic effects occurred so that
appropriate effect and no-effect doses can be selected for a 1-year
study in monkeys.
Treatment had no significant toxicological effect on
body-weight, food consumption, ophthalmic examination, daily
clinical examination, electro-cardiogram wave forms, haematology,
clinical chemistry, or urinalysis parameters. No treatment-related
abnormalities were observed at the final physical examination. There
was no induction of the hepatic enzyme p-nitroanisole o-demethylase,
and no compound-related gross lesions were observed at necropsy. No
organ weight changes were observed except for increased salivary
gland weight relative to body-weight (sexes combined) in the 4 mg/kg
bw/day group. The microscopic appearance of salivary gland and heart
from monkeys of the 4 mg/kg bw/day group was similar to that of
controls. Microscopic evaluation of the remaining tissues collected
by necropsy and determination of heart and lung ß-adrenergic
receptor numbers was in progress at the time of the review.
Monkeys given 4 mg/kg bw/day ractopamine hydrochloride
developed daily tachycardia, which was maximal by 0.5 h post-dose,
and remained elevated through 16 h after dosing. The monkeys did not
demonstrate the significant slowing of the night-time heart rates as
seen in the control, 0.25 and 0.5 mg/kg bw/day groups of monkeys. A
slight tachyphylaxis to ractopamine-induced heart rate stimulation
was apparent after the first day, which did not progress with time
of treatment. No effect on the electrocardiogram wave forms or
cardiac histopathology occurred in the presence of marked daily
tachycardia during the 6 weeks at 4 mg/kg bw/day. The NOEL was
0.5 mg/kg bw/day (Williams et al., 1985h).
Two groups, each composed of three male and three female rhesus
monkeys, were administered either vehicle or 0.125 m-g ractopamine
hydrochloride/kg bw once per day for 90 days by nasogastric gavage.
The test substance was prepared as a solution in nanopure water at a
concentration of 0.125 mg/ml.
One ml/kg bw of vehicle or the ractopamine solution was
administered and the monkeys were maintained in a conscious state
during the dosing and the cardiovascular monitoring procedures.
All of the monkeys were clinically normal throughout the study.
There were no effects on body-weight, food consumption, heart rate,
or on the electro-cardiographic wave forms in any control or treated
monkeys. The NOEL was determined to be 0.125 mg/kg bw/day
(Williams et al., 1985i). .
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0 (air, control), 0.05, 0.17, or 0.44 mg
ractopamine hydrochloride/m3 of air for 4 h per day for 8 days
over a 10-day period (excluding week-ends). The activity median
equivalent aerodynamic diameters (AMEAD) of the aerosols were 5.6,
6.2 and 6.4 µm for groups exposed to 0.05, 0.17 or 0.44 mg
ractopamine hydrochloride/m3. There was a slight increase in
post-exposure (night-time) heart rates. No treatment-related changes
occurred in body-weights, organ weights, food consumption,
haematology, or clinical chemistry parameters. There were no
treatment-related gross or microscopic lesions. The
no-observed-effect exposure concentration was 0.17 mg ractopamine
hydrochloride/m3 (Williams et al., 1985f).
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0.38, 1.69, 6.42 or 23.8 mg ractopamine
hydrochloride/m3 for 4 h per day for 2 to 8 days over an 18-day
period (excluding weekends) following a 7-day period of control
heart rate data collection. The AMEAD of the aerosols were 6.2, 7.8,
8.3 and 11.4 µm for the four exposure groups, respectively.
Treatment was stopped after 2 exposures at 23.8 mg ractopamine
hydrochloride/m3 and after 7 exposures at 1.69 and 6.42 mg
ractopamine hydrochloride/m3. The 0.38 mg ractopamine
hydrochloride/m3 exposure group received no exposure until the
last 8 days of the study prior to necropsy. Two animals were
sacrificed prior to study termination due to rejection of the
implanted ECG transmitter. All other animals survived to termination
of the study. No clinical signs of toxicity were observed. Increased
heart rates were observed in all exposure groups during exposure
(daytime) and following exposure (night-time). The increased daytime
and night-time heart rates persisted after treatment was stopped and
required approximately two weeks to return to normal values. No
treatment-related changes occurred in body-weights, organ weights,
food consumption, haematology or clinical chemistry parameters. No
treatment-related gross or microscopic lesions were observed. A
no-observed-effect exposure concentration for inhalation of
ractopamine hydrochloride aerosol could not be determined in this
study (Williams et al., 1985g).
2.2.3 Long-term/carcinogenicity studies
No infomation available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 25 male and 25 female Crl:CD(SD)BR rats, about 35
days old at the beginning of the study were maintained on diets
containing 0, 2, 20, 200 or 2000 ppm ractopamine in two generations
of parental rats. Weanling F0 male rats were maintained on the
test diets for 70 days prior to mating and the female rats were on
the test diets for the 14 days prior to mating.
The F0 parental rats were mated once and the females were
allowed to deliver and rear their young through weaning. However,
F1 animals were mated for two breeding trials. In the first
breeding trial, the females were allowed to deliver and rear their
progeny through postpartum day 21. In the second breeding trial, the
females were killed on gestation day 20 for the assessment of
intrauterine reproduction parameters and the collection of fetuses
for external, visceral and skeletal examination. Significance was
defined as P <0.05 in this study.
Significant treatment-related depression in body-weight and
body-weight gain occurred in both F0 and F1 males of the 2000
ppm group. Significant depression in body-weight also occurred in
F1 females of the 2000 ppm group. Food consumption was
significantly depressed in F1 males of the 2000 ppm group only.
Mating performance and fertility were comparable with the
controls at each treatment level in each litter of the F0 (F1a)
and F1 (F2a and F2b) generations.
During the breeding trials for F1a and F2a litters,
gestation length was not affected; however, the mean litter size and
mean progeny survival indices were depressed significantly in the
2000 ppm group.
Significant reductions in the mean weanling weight of F1a and
F2a pups born to dams treated at 2000 ppm from days 1 through 21
postpartum were recorded. The percentage of male progeny was
significantly depressed only in the F1a litters.
Clinical signs such as pallor, apparent hypothermia, thinness,
dehydration and rough hair coat occurred with the highest frequency
in the neonatal and postnatal progeny of the 2000 ppm group.
Seventy-three pups of the F1a generation from the 2000 ppm group
were either born dead or died during the lactation period. Gross
external and internal examinations of the pups revealed that 13 of
these pups had abnormalities which included oedema (8), cleft
palate (7), limb abnormalities (5), brachygnathia (3), protruding
tongue (3), open eyelids (3), omphalocele (2), clotted blood in the
lateral ventricles of the brain (1), microphthalmia (2), and
enlarged heart (1).
Malformations were observed in 0, 1, 0, 2 and 63 F2a fetuses
from dams treated at 0, 2, 20, 200 and 2000 ppm, respectively.
However, significant increases in malformations were recorded in the
63 pups from the dams treated at 2000 ppm. These abnormalities
included oedema, hydraminos, cleft palate, protruding tongue, short
limbs, missing digits, open eyelids, brachygnathia, fused digits,
limb abnormalities (wavy humerus radius, ulna, femur, tibia and
fibula, mishapen scapula, micromelia, kinked tail, enlarged heart,
kyphosis adactyly (hindlimb), and omphalocele.
Percent early, late and total resorption were significantly
increased in dams treated at 2000 ppm. In dams of the 200 ppm and
2000 ppm groups, significant depression in the proportion of normal
fetuses was observed. In the 2000 ppm group, this depression was
accompanied by statistically-significant proportions of fetuses with
developmental variations and abnormalities.
In view of the fact that significant teratological effects were
observed only at the highest dose level tested, a dose level at
which maternal toxicity was also observed, it was concluded that the
NOEL was 200 ppm, equal to 15 mg/kg bw/day (Williams & Hoyt, 1986).
2.2.5 Special studies on genotoxicity
The results of a limited series of in vitro and in vivo
genotoxicity studies on ractopamine are summarized in Table 3. All
results were reported to be negative.
2.3 Observations in humans
A pilot clinical trial evaluating bronchodilator activity of
ractopamine in oral dosage form and as aerosol was conducted in
1960. Ractopamine was administered orally to four patients with
chronic bronchial asthma at doses of 30 and 45 mg. No clear-cut
evidence of bronchodilator activity, CNS stimulation, or increase in
pulse rate was seen in these patients. Two patients receiving 30 to
45 mg of ractopamine showed 15 to 20 mm Hg systolic elevation of
blood pressure lasting for about 1 h. A summary on the use of
50 mg/ml ractopamine with a DeVilbiss nebulizer in four asthmatic
patients indicated that on a 0 to 4 scale of effectiveness according
to patients, ractopamine scored 3, 3, 1 and 0 in these patients. It
was stated that ractopamine as an aerosol has failed to show any
activity at doses up to 6 mg (Shipley, 1960).
Table 3: Results of genotoxicity assays on ractopamine
Concentration of
Test System Test Object Ractopamine Results References
Unscheduled Fischer 344 rat 0.5-1000 µg/ml Negative Williams et al.,
DNA synthesis hepatocytes 1984b
in vitro
Ames test (1) S. typhimurium 50-5000 µg/ml Negative Williams et al.,
TA1535, TA1537, 1984c
TA1538, TA98,
TA100
Ames test (1) S. typhimurium 0.1-1000 µg/ml Negative Williams &
G46, TA1535, Thompson,
TA100, C3076, 1984
TA1537, D3052,
TA1338, TA98
E. coli
WP2, WP2uvrA-
Mouse lymphoma cell L5178Y mouse 10-350 µg/ml Negative Williams et al.,
thymidine kinase 1984d
100-700 µg/ml
Sister chromatid Chinese hamsters 200-500 mg/kg Negative Williams et al.,
exchange in bone 1985j
marrow of hamsters
in vivo
(1) With and without rat liver S-9 fraction.
3. COMMENTS
Results of various studies were reviewed by the Committee,
including pharmacokinetic, biotransformation, acute and short-term
toxicity, reproductive, teratogenicity, and genotoxicity studies and
a limited number of studies in humans.
Studies with 14C-ractopamine in several species have
indicated rapid absorption following oral administration. In pigs,
labelled ractopamine was excreted almost quantitatively;
approximately 88% was recovered in urine and 9% in faeces during a
7-day period. Studies in pigs, rats, and dogs fed 14C-ractopamine
showed three major metabolites, identified as monoglucuronides of
ractopamine.
In acute studies, ractopamine was substantially more toxic
orally to the rat (LD50 approximately 450 mg/kg bw) than the mouse
(LD50 approximately 3000 mg/kg bw).
The genotoxic potential of ractopamine was evaluated in a
limited series of in vitro and in vivo studies, all of which
were reported to be negative. However, in the absence of a
carcinogenicity study, and as human exposure is likely to be
extensive, the Committee concluded that additional genotoxicity
testing would be desirable.
The short-term toxicity of ractopamine has been evaluated in
mice, rats, dogs, and monkeys. Ractopamine was fed to B6C3F1
mice for 3 months at doses of 25, 175, or 1250 mg/kg bw/day. The
most significant effect noted was a dose-dependent decrease, both
absolute and relative, in testicular weights. In both males and
females in the highest-dose group, absolute and relative heart
weights were increased. However, no histopathological changes were
observed in either the heart or the testes. A clear NOEL could not
be established in this study.
Fischer 344 rats were fed doses up to approximately 155 mg/kg
bw/day for 3 months. The highest-dose group showed decreased
body-weight gain, increased food consumption, decreased efficiency
of food utilization, and an increase in serum potassium
concentration. There was a decrease in uterine weight in rats of
this group and a slight reduction in spleen weight in the top two
groups. The NOEL was 1.3 mg/kg bw/day in this study.
Beagle dogs were given three doses daily, 6 h apart, totalling
0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine in gelatin capsules
for 1 year. Treatment-related minor histopathological findings were
limited to the high-dose group and to the liver. The occurrence of
mild nocturnal bradycardia, most prominent during the first
6 months, meant that a clear no-effect level was not observed in
this study. The Committee noted that the quantity of ractopamine
residues consumed in 500 g of meat from animals slaughtered without
a withdrawal period would closely approach an ADI derived from the
lowest dose of 0.112 mg/kg bw/day and a safety factor of 100.
In a further study of cardiovascular effects, rhesus monkeys
were given either vehicle or ractopamine at 0.125 mg/kg bw/day for
90 days by gavage. This dose was 2.5 times the single dose known to
produce tachycardia and peripheral vasodilation in the dog. In
addition, the selected dose exceeded the total daily dose
(0.112 mg/kg bw/day) associated with nocturnal bradycardia in the
1-year dog study. The NOEL for this study was 0.125 mg/kg bw/day.
Rhesus monkeys were given ractopamine at doses of 0.25, 0.5, or
4 mg/kg bw/day once daily by gavage for 6 weeks to determine the
doses to be used in a 1-year study. Monkeys given 4 mg/kg bw/day
developed daily tachycardia which was maximal by 30 min agter
dosing, and continued for 16 h. Monkeys in the group did not
demonstrate the significant slowing of the nocturnal heart rates
seen in the other groups. The NOEL for this study was 0.5 mg/kg
bw/day.
The effect of ractopamine on reproductive and developmental
performance in Sprague-Dawley rats was evaluated at dosage levels of
up to 2000 ppm in the diet. Significant effects, which included a
reduction in mean litter size and an increase in the total number of
resorptions, were restricted to the high dose which was also
maternally toxic. The NOEL was 200 ppm, equal to 15 mg/kg bw/day, in
this study. The Committee considered the teratogenicity segment of
this study to be adequate to assess developmental toxicity. A minor
teratogenic response was observed only at the highest dose
(2000 ppm) tested, at which maternal toxicity was also noted.
The bronchodilator and inotropic effects of ractopamine were
evaluated in pilot clinical trials in humans. Four patients showed
little evidence of bronchodilator activity, central nervous system
stimulation, or an increase in pulse rate. Two patients showed a
mild elevation of blood pressure lasting for about 1 hour. An
infusion study showed inotropic and chronotropic enhancement in both
healthy volunteers and heart patients.
4. EVALUATION
The Committee concluded that, on the basis of the short-term
studies available, residues of ractopamine appeared to have little
toxic potential for the consumer. The effects recorded were in the
main those to be expected from a ß-adrenoceptor agonist. It might
therefore be appropriate to assess ractopamine on the basis of a
NOEL for pharmacological effects that are relevant to its ingestion
by humans as a residue in edible meats. However, because such a NOEL
could not be determined in the 1-year study in dogs, the Committee
was unable to establish an ADI.
The Committee noted that: (a) some ß-adrenoceptor agonists were
carcinogenic; (b) no long-term studies had been conducted in
rodents; and (c) there were no data relating to the long-term
exposure of humans to ractopamine. Therefore, before reviewing the
compound again, the Committee would wish to see evidence and
arguments in at least the following areas:
1. Genotoxicity
- A further in vivo study such as a micronucleus test.
2. Pharmacology
- investigations that fully explore the pharmacological
properties of ractopamine;
- the relative contributions of ß1- and ß2-adrenoceptor
activation to the spectrum of effects produced by
ractopamine;
- a sufficient basis from which to establish the most
sensitive indicator (test and species) of the
pharmacological effects of ractopamine;
- validation of the utility of this indicator in the setting
of a pharmacological NOEL for humans;
- a survey of the pharmacokinetic parameters of
ß-adrenoceptor agonists in humans and laboratory species,
including those relevant to oral administration;
- determination of appropriate timing for observations in
animal studies to reveal both the onset and the peak
values of all relevant effects.
3. Human data
- A survey of all non-therapeutic effects that follow
long-term ß-adrenoceptor agonist use in humans, to assist
in the prediction of the consequences of the long-term
intake of residues of ractopamine by consumers of animal
meat.
Depending on the results of the above investigations, it may be
necessary to perform other studies to explore further the potential
carcinogenicity of ractopamine.
5. REFERENCES
DALIDOWICZ, J.D. (1986a). Metabolism of 14C-ractopamine HCl in the
rat. Unpublished Report No. ABC-0285 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1986b). Metabolism of 14C-ractopamine HCl in the
dog. Unpublished Report No. ABC-0301 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. & BABBITT, G.E. (1986). Characterization of
14C residues in tissues and excreta from swine fed
14C-ractopamine HCl. Unpublished Report No. ABC-0355 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.E., THOMSON, T.D. & HERBERG, R.J. (1986).
14C-ractopamine HCl balance - excretion study in swine.
Unpublished Report No. ABC-0330 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1987). Comparative metabolism of 14C-ractopamine
HCl in swine, dogs, and rats. Unpublished Report No. ABC-0369 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
SHIPLEY, R.E. (1960). Summary for research project committee May 31,
1960. Unpublished memoranda from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & THOMPSON, C.Z. (1984). The effect of compound
31537, EL-737 on the induction of bacterial mutation using a
modification of the Ames test. Unpublished Report No. 840507GPA2000
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & MARKEY, T.F. (1984a). The acute
dermal, ocular, and inhalation toxicity of compound 31537 (EL-737).
Unpublished Reports Nos. B-D-82-84, B-E-108-84 and R-H-47-84 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., HILL, L.E. & PROBST, G.S. (1984b). The effect of
compound 31537, EL-737, on the induction of DNA repair synthesis in
primary cultures of adult rat hepatocytes. Unpublished Reports Nos.
840503UDS2000 and 840508UDS2000 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., REXROAT, M.A. & PROBST, G.S. (1984c). The effect of
EL-737 (compound 31537) the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished Report No.
840716AMS2000 from Lilly Research Laboratories, Division of Eli
Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., OBERLY, T.J., BEWSEY, B.J. & PROBST, G.S. (1984d).
The effect of 31537 (EL-737) on the induction of forward mutation at
the thymidine kinase locus of L517Y mouse lymphoma cells.
Unpublished Report No. 840627MLA2000 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., BRIDGE, T.L. (1985a). The acute
toxicity of compound 31537 (EL-737) administered orally to the ICR
mouse. Unpublished Reports Nos. M-0-178-84 and M-0-179-84 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985b). The acute
toxicity of compound 31537 (EL-737) administered orally to the
Fischer 344 rat. Unpublished Report No. R-0-132-84 and R-0-133-84
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985c). The acute
toxicity of compound 31537 (EL-737) administered intraperitoneally
to the Fischer 344 rat. Unpublished Reports Nos. R-P-08-84 and
R-P-09-84 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985d). A three-month
toxicity study of ractopamine hydrochloride fed in the diet to
B6C3F1 mice. Unpublished Report No. M01584 from Lilly Research
Laboratories, Division of Eli Lilly and Company Greenfield, Indiana,
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985e). A three-month
toxicity study of 031537 (EL-737) administered orally to Fischer 344
rats. Unpublished Report No. R06184 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield, Indiana
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985f). 8-Day
inhalation study in rhesus monkey. Unpublished Report No. P06288
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Lilly Corporate Center, Indianapolis, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985g). 18-Day
inhalation study in rhesus monkey. Unpublished Report No. P00388
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985h). An interim
summary of a 6-week subchronic toxicity study of ractopamine
hydrochloride administered by nasogastric gavage to rhesus monkeys.
Unpublished Report No. P00691 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Lilly Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly,
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985i). The effect
of subchronic administration of ractopamine hydrochloride on heart
rate and electrocardiographic waveforms in conscious rhesus monkeys.
Unpublished Report No. P02186 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEAL, S.B. & PROBST, G.S. (1985j). The effect of
compound 31537 (EL-737) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters. Unpublished
Report No. 850121SCE2000 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & BRIDGE, T.L., MARKEY, T.F. (1985k).
The acute oral, dermal, ocular and inhalation toxicity of a granular
premix formulation (AFN-026) containing 10% compound 31537 (EL-737).
Unpublished Reports Nos. R-0-188-85, B-D-104-85, B-E-120-85, and
R-H-066-85 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., HOYT, J.A. (1986). An eleven-month two-generation
reproduction study, including a teratology segment, in Cd rats
maintained on diets containing ractopoamine hydrocholoride (EL-739,
Compound 31537). Unpublished report for study Nos. R11385 and R18985
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., (1987a). Comparative bioavailability of
14C-ractopamine hydrochloride (031537, EL-737) following a single
oral dose of 0.125 mg/kg in the dog and monkey. Unpublished Reports
Nos. D04686 and P03086 from Lilly Research Laboratories, Division of
Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., (1987b). A chronic toxicity study of ractopamine
hydrochloride administered orally to beagle dogs for one year.
Unpublished Report No. D05885 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & POHLAND, R.C. & BYRD, T.K. (1987). Bioavailability
of radiocarbon following the administration of single oral doses of
14C-EL-737 (31537) to F344/N Hsd BR rats. Unpublished Reports Nos.
R03985, RO4085 and RO4185 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
The same three metabolites were isolated from pig urine and
were all identified to be monoglucuronides of ractopamine. Of the
extractable liver residues, 30-50% was found to be ractopamine and
the rest conjugates of ractopamine. The kidneys contained a higher
amount of conjugates (64%) and correspondingly smaller amount of
ractopamine (20-30%). Studies in rats and dogs showed that urine
from animals dosed with 14C-ractopamine hydrochloride contained
the same three metabolites of ractopamine as in pigs (metabolites A,
B, and C). These three metabolites constituted a large portion of
14C-residues in rat and dog urine. The chromatographic profiles of
the 14C-residue extracts of rat, dog and pig liver were
quantitatively similar. The comparative quantitative data showing
the mean amounts in ppm of ractopamine hydrochloride and its major
metabolites (calculated as ractopamine hydrochloride) in kidney and
liver tissues of pigs, dogs, and rats are summarized in Table 1
(Dalidowicz & Babbitt, 1986; Dalidowicz, 1986a; Dalidowicz, 1986b).
Table 1. Metabolic profile in pigs, dogs and rats
Liver, ppm Kidney, ppm
Pig Dog Rat Pig Dog Rat
Ractopamine. HC1 0.12 0.59 0.40 0.10 0.50 0.33
Metabolite A 0.03 0.46 0.17 0.05 0.18 0.52
Metabolite B 0.04 0.77 0.15 0.06 0.27 0.57
Metabolite C 0.02 1.76 0.10 0.09 0.63 0.08
Dose: pigs (45 kg): 30 ppm in food for 4 days; pigs were killed
12 h after the last dose;
dogs: 0.5 mg/kg bw by gavage 3 times per day for 4 days and
once on the fifth day; dogs were killed 6 h after the
last dose;
rats: 2 mg/kg bw by gavage once daily for 7 days; rats were
killed 6 h after the last dose.
The concentrations of the metabolites in dog and rat tissues
were much higher than those in pig tissues. The nonextractable and
the uncharacterized residues in dog and rat tissues were also much
higher than in pig tissues (Dalidowicz, 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ractopamine are
summarized in Table 2.
2.2.2 Short-term studies
2.2.2.1 Mice
Diets containing 0, 0.02, 0.14 or 1.0% ractopamine
hydrochloride were fed to B6C3F1 mice (10/sex/dose) for 3
months, resulting in estimated time-weighted average daily doses of
0, 25, 175 or 1250 mg/kg bw/day, respectively. The mice were
observed daily for clinical signs of toxicity. Body-weights and food
consumption were measured weekly in all animals. Necropsies were
performed on all mice sacrificed at the end of the study. A set of
tissues was examined microscopically.
Table 2. Acute toxicity studies
Species Sex Route LD50 (mg/kg/bw Reference
Mouse M oral 3547 (2912-4321) Williams et al.,
1985a
F oral 2545 (2219-2919) Williams et al.,
1985a
Rat M oral 474 (398-564) Williams et al.,
1985b
F oral 367 Williams et al.,
(1985b)
Rat M intraperitoneal 132 (110-159) Williams et al.,
F intraperitoneal 122 (102-145) 1985c
Rabbit M dermal >2000 Williams et al.,
F 1984a
Rat M inhalation LC50 (4 h): 2.8 Williams et al.,
F mg/L 1985k
All of the treated animals survived the treatment period.
Alopaecia, a common finding in B61C3F1 mice, was observed in
22 animals of each sex. In this study the occurrence of alopaecia
was markedly decreased in the 1250 mg/kg bw/day group compared to
the other groups. No adverse signs of toxicity were attributed to
treatment with ractopamine hydrochloride. Male mice in the 25 and
175 mg/kg bw/day groups had a significant increase in cumulative
weight gain in the last eight weeks of the study. Differences in the
final mean body-weights of the treatment groups compared to the
controls were not statistically significant.
Treatment- and dose-related mild to moderate increases occurred
in erythrocyte counts, haemoglobin concentrations, and packed cell
volumes in both sexes of the 175 and 1250 mg/kg bw/day groups. Minor
changes occurred in the mean corpuscular volume and mean corpuscular
haemoglobin concentration in males of the 1250 mg/kg bw/day group.
The changes in thrombocyte counts were minimal in both sexes of the
1250 mg/kg bw/day group. All other haematologic parameters were
similar to control values. There were no treatment-related effects
on any haematologic parameters in mice of the 25 mg/kg bw/day group.
Treatment-related clinical chemistry changes consisted of mild
increases in urea nitrogen and cholesterol concentrations in males
of the 1250 mg/kg bw/day group. Female mice in both the 175 and 1250
mg/kg bw/day groups had mild increases in urea nitrogen and
cholesterol concentrations. The decrease in serum sodium
concentration in female mice of the 1250 mg/kg bw/day group,
although statistically significant, was minimal and the value fell
within the normal range. No alteration in the other electrocytes
concentration occurred. All other clinical chemistry parameters were
similar to control values. There were no treatment-related effects
on serum chemistry in mice of the 25 mg/kg bw/day group.
In males, a decrease in testicular weights, both absolute and
relative, occurred in a dose-dependent manner. Both males and
females in the 1250 mg/kg bw/day group had increased absolute and
relative heart weights. All the other organ weights were similar to
control values.
Microscopically, treatment-related changes involved the
periaortic and intercapsular brown fat in mice of both sexes in the
1250 mg/kg bw/day group. The plasma membranes of the affected brown
fat cells from the 1250 mg/kg bw/day group of mice were more
discrete and stained intensely with eosin. The cytoplasm of these
cells was filled with uniformly small spherical vacuoles, the
margins of which also stained intensely with eosin. These changes
were diagnosed as marked cytoplasmic changes. The cytoplasm of the
brown fat cells with minimal change was characterized by small
vacuoles, but the margins of the vacuoles did not stain intensely
with eosin. Brown fat in female mice and a few male mice of the
control and of the 25 and 175 mg/kg bw/day groups was characterized
as having a minimal cytoplasmic change. Due to the dose-dependent
decrease in testicular weights, a NOEL could not be established in
this study (Williams et al., 1985d).
2.2.2.2 Rats
Diets containing 0, 20, 200 or 2000 ppm ractopamine
hydochloride were fed to Fischer 344 rats (20/sex/dose) for three
months. The time-weighted average doses were 0, 1.3, 13, or 153
mg/kg bw/day for males and 0, 1.4, 15, or 157 mg/kg bw/day for
females. The rats were observed daily for clinical signs of
toxicity. Body-weights and food consumption were measured weekly for
all animals. Autopsies were done on animals at the end of the study.
Histological examination of tissue sections was conducted on all
tissues.
All of the treated animals survived the treatment period. The
clinical signs observed were decreased body-weight gain, increased
food consumption and decreased efficiency of food utilization. These
signs were observed in the 155 mg/kg bw/day group only, where they
were observed throughout the study in rats of both sexes. In
addition to these signs decreased serum triglyceride and cholesterol
levels and increased serum urea nitrogen concentrations were
observed.
An increase in serum potassium was observed in the 155 mg/kg
bw/day group in both sexes. Total erythrocyte count, haemoglobin
concentration, and packed cell volume all increased in rats of the
155 mg/kg bw/day group, as expected with a beta-agonist. There was a
decrease in uterine weight in rats of the 155 mg/kg bw/day group in
this study and a slight reduction in spleen weight in the 13-15 and
155 mg/kg bw/day groups. No change was observed in heart weight of
any treated animals and there was no microscopic evidence of
myocardial necrosis, which is a well-documented effect following
large doses of epinephrine and other sympathomimetic amines.
Histopathology examination of brown fat revealed slight to
moderate cytoplasmic change in the 13-15 and 155 mg/kg bw/day
groups. The NOEL for males was 1.3 mg/kg bw/day and 1.4 mg/kg bw/day
for females, based on the absence of biochemical or histological
changes (Williams et al., 1985e).
2.2.2.3 Dogs
Groups of 4 male and 4 female beagle dogs were given total
doses of 0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine
hydrochloride in gelatin capsules for 1 year. The doses were given
in capsules as three equal divided doses every 6 h. This study was
based on pilot studies in dogs, in which an oral dose of 0.05 mg/kg
bw/day was associated with skin and oral mucosal reddening and a
dose of 0.035 mg/kg bw/day was the NOEL. On the basis of this NOEL,
the frequency of dosing was increased to three times per day, 6 h
apart, in order to triple the daily exposure to 0.112 mg/kg bw/day
for the low-dose. The middle-dose, 0.224 mg/kg bw/day (0.075 mg/kg
bw, 3 times per day), was selected as a minimal cardiostimulatory
dose. The high-dose, 5.68 mg/kg bw/day (1.89 mg/kg bw, 3 times per
day) was selected because it produced near maximal cardiostimulation
at 2 h following a single oral dose of 1.5 mg/kg bw, in the pilot
study.
All dogs survived the one-year treatment period. Body-weight,
food consumption, ophthalmic examination, electrocardiogram wave
forms, bone marrow evaluation and urinalysis results were not
affected by treatment. The zero-time resting heart rate means for
the study (collected on test-days 29, 68, 83, 119, 155, 182, 211,
273 and 366) for low-, middle- and high-dose dogs (sex combined)
were 123, 116 and 115 beats per minute, respectively, and all were
significantly (p < 0.01) depressed compared to 141 beats per minute
for the controls.
Clinical signs of treatment-related effects were transient
cutaneous erythema and oily and unkept hair coat in the high-dose
dogs. Cutaneous erythema occurred in the mid-dose group in the first
five months of the study. Erythrocyte number, haemoglobin
concentration and packed cell volume were significantly decreased in
the high-dose group starting at 90 days, which and continuing up to
the end of the study. Several clinical chemistry parameters were
altered in the high-dose group by 90 days, which persisted until the
termination of the study. Serum potassium and urea nitrogen
concentration increased while serum glucose, cholesterol and
triglyceride concentration decreased.
Treatment-related gross and histopathological findings were
limited to the high-dose group. Both absolute and relative heart
weight and absolute thyroid and adrenal weights were significantly
decreased. Grossly, there was a slight decrease in abdominal and
thoracic fat. Microscopically, a decrease in hepatic centrilobular
glycogen and an increase in perithymic and periaortic brown fat was
noted in the high-dose dogs.
The appearance of bradycardia at all doses, most prominent
during the first 6 months, precluded a NOEL being established in
this study (Williams, 1987b).
2.2.2.4 Monkeys
Rhesus monkeys (2/sex/dose) were given ractopamine
hydrochloride dissolved in nanopure water at doses of 0.25, 0.5 or
4 mg/kg bw once daily by nasogastric gavage in a volume of 1 ml/kg
bw for 6 weeks. Control monkeys received vehicle (nanopure water)
only. This 6-week subchronic study was conducted in rhesus monkeys
to determine the doses at which toxic effects occurred so that
appropriate effect and no-effect doses can be selected for a 1-year
study in monkeys.
Treatment had no significant toxicological effect on
body-weight, food consumption, ophthalmic examination, daily
clinical examination, electro-cardiogram wave forms, haematology,
clinical chemistry, or urinalysis parameters. No treatment-related
abnormalities were observed at the final physical examination. There
was no induction of the hepatic enzyme p-nitroanisole o-demethylase,
and no compound-related gross lesions were observed at necropsy. No
organ weight changes were observed except for increased salivary
gland weight relative to body-weight (sexes combined) in the 4 mg/kg
bw/day group. The microscopic appearance of salivary gland and heart
from monkeys of the 4 mg/kg bw/day group was similar to that of
controls. Microscopic evaluation of the remaining tissues collected
by necropsy and determination of heart and lung ß-adrenergic
receptor numbers was in progress at the time of the review.
Monkeys given 4 mg/kg bw/day ractopamine hydrochloride
developed daily tachycardia, which was maximal by 0.5 h post-dose,
and remained elevated through 16 h after dosing. The monkeys did not
demonstrate the significant slowing of the night-time heart rates as
seen in the control, 0.25 and 0.5 mg/kg bw/day groups of monkeys. A
slight tachyphylaxis to ractopamine-induced heart rate stimulation
was apparent after the first day, which did not progress with time
of treatment. No effect on the electrocardiogram wave forms or
cardiac histopathology occurred in the presence of marked daily
tachycardia during the 6 weeks at 4 mg/kg bw/day. The NOEL was
0.5 mg/kg bw/day (Williams et al., 1985h).
Two groups, each composed of three male and three female rhesus
monkeys, were administered either vehicle or 0.125 m-g ractopamine
hydrochloride/kg bw once per day for 90 days by nasogastric gavage.
The test substance was prepared as a solution in nanopure water at a
concentration of 0.125 mg/ml.
One ml/kg bw of vehicle or the ractopamine solution was
administered and the monkeys were maintained in a conscious state
during the dosing and the cardiovascular monitoring procedures.
All of the monkeys were clinically normal throughout the study.
There were no effects on body-weight, food consumption, heart rate,
or on the electro-cardiographic wave forms in any control or treated
monkeys. The NOEL was determined to be 0.125 mg/kg bw/day
(Williams et al., 1985i). .
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0 (air, control), 0.05, 0.17, or 0.44 mg
ractopamine hydrochloride/m3 of air for 4 h per day for 8 days
over a 10-day period (excluding week-ends). The activity median
equivalent aerodynamic diameters (AMEAD) of the aerosols were 5.6,
6.2 and 6.4 µm for groups exposed to 0.05, 0.17 or 0.44 mg
ractopamine hydrochloride/m3. There was a slight increase in
post-exposure (night-time) heart rates. No treatment-related changes
occurred in body-weights, organ weights, food consumption,
haematology, or clinical chemistry parameters. There were no
treatment-related gross or microscopic lesions. The
no-observed-effect exposure concentration was 0.17 mg ractopamine
hydrochloride/m3 (Williams et al., 1985f).
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0.38, 1.69, 6.42 or 23.8 mg ractopamine
hydrochloride/m3 for 4 h per day for 2 to 8 days over an 18-day
period (excluding weekends) following a 7-day period of control
heart rate data collection. The AMEAD of the aerosols were 6.2, 7.8,
8.3 and 11.4 µm for the four exposure groups, respectively.
Treatment was stopped after 2 exposures at 23.8 mg ractopamine
hydrochloride/m3 and after 7 exposures at 1.69 and 6.42 mg
ractopamine hydrochloride/m3. The 0.38 mg ractopamine
hydrochloride/m3 exposure group received no exposure until the
last 8 days of the study prior to necropsy. Two animals were
sacrificed prior to study termination due to rejection of the
implanted ECG transmitter. All other animals survived to termination
of the study. No clinical signs of toxicity were observed. Increased
heart rates were observed in all exposure groups during exposure
(daytime) and following exposure (night-time). The increased daytime
and night-time heart rates persisted after treatment was stopped and
required approximately two weeks to return to normal values. No
treatment-related changes occurred in body-weights, organ weights,
food consumption, haematology or clinical chemistry parameters. No
treatment-related gross or microscopic lesions were observed. A
no-observed-effect exposure concentration for inhalation of
ractopamine hydrochloride aerosol could not be determined in this
study (Williams et al., 1985g).
2.2.3 Long-term/carcinogenicity studies
No infomation available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 25 male and 25 female Crl:CD(SD)BR rats, about 35
days old at the beginning of the study were maintained on diets
containing 0, 2, 20, 200 or 2000 ppm ractopamine in two generations
of parental rats. Weanling F0 male rats were maintained on the
test diets for 70 days prior to mating and the female rats were on
the test diets for the 14 days prior to mating.
The F0 parental rats were mated once and the females were
allowed to deliver and rear their young through weaning. However,
F1 animals were mated for two breeding trials. In the first
breeding trial, the females were allowed to deliver and rear their
progeny through postpartum day 21. In the second breeding trial, the
females were killed on gestation day 20 for the assessment of
intrauterine reproduction parameters and the collection of fetuses
for external, visceral and skeletal examination. Significance was
defined as P <0.05 in this study.
Significant treatment-related depression in body-weight and
body-weight gain occurred in both F0 and F1 males of the 2000
ppm group. Significant depression in body-weight also occurred in
F1 females of the 2000 ppm group. Food consumption was
significantly depressed in F1 males of the 2000 ppm group only.
Mating performance and fertility were comparable with the
controls at each treatment level in each litter of the F0 (F1a)
and F1 (F2a and F2b) generations.
During the breeding trials for F1a and F2a litters,
gestation length was not affected; however, the mean litter size and
mean progeny survival indices were depressed significantly in the
2000 ppm group.
Significant reductions in the mean weanling weight of F1a and
F2a pups born to dams treated at 2000 ppm from days 1 through 21
postpartum were recorded. The percentage of male progeny was
significantly depressed only in the F1a litters.
Clinical signs such as pallor, apparent hypothermia, thinness,
dehydration and rough hair coat occurred with the highest frequency
in the neonatal and postnatal progeny of the 2000 ppm group.
Seventy-three pups of the F1a generation from the 2000 ppm group
were either born dead or died during the lactation period. Gross
external and internal examinations of the pups revealed that 13 of
these pups had abnormalities which included oedema (8), cleft
palate (7), limb abnormalities (5), brachygnathia (3), protruding
tongue (3), open eyelids (3), omphalocele (2), clotted blood in the
lateral ventricles of the brain (1), microphthalmia (2), and
enlarged heart (1).
Malformations were observed in 0, 1, 0, 2 and 63 F2a fetuses
from dams treated at 0, 2, 20, 200 and 2000 ppm, respectively.
However, significant increases in malformations were recorded in the
63 pups from the dams treated at 2000 ppm. These abnormalities
included oedema, hydraminos, cleft palate, protruding tongue, short
limbs, missing digits, open eyelids, brachygnathia, fused digits,
limb abnormalities (wavy humerus radius, ulna, femur, tibia and
fibula, mishapen scapula, micromelia, kinked tail, enlarged heart,
kyphosis adactyly (hindlimb), and omphalocele.
Percent early, late and total resorption were significantly
increased in dams treated at 2000 ppm. In dams of the 200 ppm and
2000 ppm groups, significant depression in the proportion of normal
fetuses was observed. In the 2000 ppm group, this depression was
accompanied by statistically-significant proportions of fetuses with
developmental variations and abnormalities.
In view of the fact that significant teratological effects were
observed only at the highest dose level tested, a dose level at
which maternal toxicity was also observed, it was concluded that the
NOEL was 200 ppm, equal to 15 mg/kg bw/day (Williams & Hoyt, 1986).
2.2.5 Special studies on genotoxicity
The results of a limited series of in vitro and in vivo
genotoxicity studies on ractopamine are summarized in Table 3. All
results were reported to be negative.
2.3 Observations in humans
A pilot clinical trial evaluating bronchodilator activity of
ractopamine in oral dosage form and as aerosol was conducted in
1960. Ractopamine was administered orally to four patients with
chronic bronchial asthma at doses of 30 and 45 mg. No clear-cut
evidence of bronchodilator activity, CNS stimulation, or increase in
pulse rate was seen in these patients. Two patients receiving 30 to
45 mg of ractopamine showed 15 to 20 mm Hg systolic elevation of
blood pressure lasting for about 1 h. A summary on the use of
50 mg/ml ractopamine with a DeVilbiss nebulizer in four asthmatic
patients indicated that on a 0 to 4 scale of effectiveness according
to patients, ractopamine scored 3, 3, 1 and 0 in these patients. It
was stated that ractopamine as an aerosol has failed to show any
activity at doses up to 6 mg (Shipley, 1960).
Table 3: Results of genotoxicity assays on ractopamine
Concentration of
Test System Test Object Ractopamine Results References
Unscheduled Fischer 344 rat 0.5-1000 µg/ml Negative Williams et al.,
DNA synthesis hepatocytes 1984b
in vitro
Ames test (1) S. typhimurium 50-5000 µg/ml Negative Williams et al.,
TA1535, TA1537, 1984c
TA1538, TA98,
TA100
Ames test (1) S. typhimurium 0.1-1000 µg/ml Negative Williams &
G46, TA1535, Thompson,
TA100, C3076, 1984
TA1537, D3052,
TA1338, TA98
E. coli
WP2, WP2uvrA-
Mouse lymphoma cell L5178Y mouse 10-350 µg/ml Negative Williams et al.,
thymidine kinase 1984d
100-700 µg/ml
Sister chromatid Chinese hamsters 200-500 mg/kg Negative Williams et al.,
exchange in bone 1985j
marrow of hamsters
in vivo
(1) With and without rat liver S-9 fraction.
3. COMMENTS
Results of various studies were reviewed by the Committee,
including pharmacokinetic, biotransformation, acute and short-term
toxicity, reproductive, teratogenicity, and genotoxicity studies and
a limited number of studies in humans.
Studies with 14C-ractopamine in several species have
indicated rapid absorption following oral administration. In pigs,
labelled ractopamine was excreted almost quantitatively;
approximately 88% was recovered in urine and 9% in faeces during a
7-day period. Studies in pigs, rats, and dogs fed 14C-ractopamine
showed three major metabolites, identified as monoglucuronides of
ractopamine.
In acute studies, ractopamine was substantially more toxic
orally to the rat (LD50 approximately 450 mg/kg bw) than the mouse
(LD50 approximately 3000 mg/kg bw).
The genotoxic potential of ractopamine was evaluated in a
limited series of in vitro and in vivo studies, all of which
were reported to be negative. However, in the absence of a
carcinogenicity study, and as human exposure is likely to be
extensive, the Committee concluded that additional genotoxicity
testing would be desirable.
The short-term toxicity of ractopamine has been evaluated in
mice, rats, dogs, and monkeys. Ractopamine was fed to B6C3F1
mice for 3 months at doses of 25, 175, or 1250 mg/kg bw/day. The
most significant effect noted was a dose-dependent decrease, both
absolute and relative, in testicular weights. In both males and
females in the highest-dose group, absolute and relative heart
weights were increased. However, no histopathological changes were
observed in either the heart or the testes. A clear NOEL could not
be established in this study.
Fischer 344 rats were fed doses up to approximately 155 mg/kg
bw/day for 3 months. The highest-dose group showed decreased
body-weight gain, increased food consumption, decreased efficiency
of food utilization, and an increase in serum potassium
concentration. There was a decrease in uterine weight in rats of
this group and a slight reduction in spleen weight in the top two
groups. The NOEL was 1.3 mg/kg bw/day in this study.
Beagle dogs were given three doses daily, 6 h apart, totalling
0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine in gelatin capsules
for 1 year. Treatment-related minor histopathological findings were
limited to the high-dose group and to the liver. The occurrence of
mild nocturnal bradycardia, most prominent during the first
6 months, meant that a clear no-effect level was not observed in
this study. The Committee noted that the quantity of ractopamine
residues consumed in 500 g of meat from animals slaughtered without
a withdrawal period would closely approach an ADI derived from the
lowest dose of 0.112 mg/kg bw/day and a safety factor of 100.
In a further study of cardiovascular effects, rhesus monkeys
were given either vehicle or ractopamine at 0.125 mg/kg bw/day for
90 days by gavage. This dose was 2.5 times the single dose known to
produce tachycardia and peripheral vasodilation in the dog. In
addition, the selected dose exceeded the total daily dose
(0.112 mg/kg bw/day) associated with nocturnal bradycardia in the
1-year dog study. The NOEL for this study was 0.125 mg/kg bw/day.
Rhesus monkeys were given ractopamine at doses of 0.25, 0.5, or
4 mg/kg bw/day once daily by gavage for 6 weeks to determine the
doses to be used in a 1-year study. Monkeys given 4 mg/kg bw/day
developed daily tachycardia which was maximal by 30 min agter
dosing, and continued for 16 h. Monkeys in the group did not
demonstrate the significant slowing of the nocturnal heart rates
seen in the other groups. The NOEL for this study was 0.5 mg/kg
bw/day.
The effect of ractopamine on reproductive and developmental
performance in Sprague-Dawley rats was evaluated at dosage levels of
up to 2000 ppm in the diet. Significant effects, which included a
reduction in mean litter size and an increase in the total number of
resorptions, were restricted to the high dose which was also
maternally toxic. The NOEL was 200 ppm, equal to 15 mg/kg bw/day, in
this study. The Committee considered the teratogenicity segment of
this study to be adequate to assess developmental toxicity. A minor
teratogenic response was observed only at the highest dose
(2000 ppm) tested, at which maternal toxicity was also noted.
The bronchodilator and inotropic effects of ractopamine were
evaluated in pilot clinical trials in humans. Four patients showed
little evidence of bronchodilator activity, central nervous system
stimulation, or an increase in pulse rate. Two patients showed a
mild elevation of blood pressure lasting for about 1 hour. An
infusion study showed inotropic and chronotropic enhancement in both
healthy volunteers and heart patients.
4. EVALUATION
The Committee concluded that, on the basis of the short-term
studies available, residues of ractopamine appeared to have little
toxic potential for the consumer. The effects recorded were in the
main those to be expected from a ß-adrenoceptor agonist. It might
therefore be appropriate to assess ractopamine on the basis of a
NOEL for pharmacological effects that are relevant to its ingestion
by humans as a residue in edible meats. However, because such a NOEL
could not be determined in the 1-year study in dogs, the Committee
was unable to establish an ADI.
The Committee noted that: (a) some ß-adrenoceptor agonists were
carcinogenic; (b) no long-term studies had been conducted in
rodents; and (c) there were no data relating to the long-term
exposure of humans to ractopamine. Therefore, before reviewing the
compound again, the Committee would wish to see evidence and
arguments in at least the following areas:
1. Genotoxicity
- A further in vivo study such as a micronucleus test.
2. Pharmacology
- investigations that fully explore the pharmacological
properties of ractopamine;
- the relative contributions of ß1- and ß2-adrenoceptor
activation to the spectrum of effects produced by
ractopamine;
- a sufficient basis from which to establish the most
sensitive indicator (test and species) of the
pharmacological effects of ractopamine;
- validation of the utility of this indicator in the setting
of a pharmacological NOEL for humans;
- a survey of the pharmacokinetic parameters of
ß-adrenoceptor agonists in humans and laboratory species,
including those relevant to oral administration;
- determination of appropriate timing for observations in
animal studies to reveal both the onset and the peak
values of all relevant effects.
3. Human data
- A survey of all non-therapeutic effects that follow
long-term ß-adrenoceptor agonist use in humans, to assist
in the prediction of the consequences of the long-term
intake of residues of ractopamine by consumers of animal
meat.
Depending on the results of the above investigations, it may be
necessary to perform other studies to explore further the potential
carcinogenicity of ractopamine.
5. REFERENCES
DALIDOWICZ, J.D. (1986a). Metabolism of 14C-ractopamine HCl in the
rat. Unpublished Report No. ABC-0285 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1986b). Metabolism of 14C-ractopamine HCl in the
dog. Unpublished Report No. ABC-0301 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. & BABBITT, G.E. (1986). Characterization of
14C residues in tissues and excreta from swine fed
14C-ractopamine HCl. Unpublished Report No. ABC-0355 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.E., THOMSON, T.D. & HERBERG, R.J. (1986).
14C-ractopamine HCl balance - excretion study in swine.
Unpublished Report No. ABC-0330 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1987). Comparative metabolism of 14C-ractopamine
HCl in swine, dogs, and rats. Unpublished Report No. ABC-0369 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
SHIPLEY, R.E. (1960). Summary for research project committee May 31,
1960. Unpublished memoranda from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & THOMPSON, C.Z. (1984). The effect of compound
31537, EL-737 on the induction of bacterial mutation using a
modification of the Ames test. Unpublished Report No. 840507GPA2000
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & MARKEY, T.F. (1984a). The acute
dermal, ocular, and inhalation toxicity of compound 31537 (EL-737).
Unpublished Reports Nos. B-D-82-84, B-E-108-84 and R-H-47-84 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., HILL, L.E. & PROBST, G.S. (1984b). The effect of
compound 31537, EL-737, on the induction of DNA repair synthesis in
primary cultures of adult rat hepatocytes. Unpublished Reports Nos.
840503UDS2000 and 840508UDS2000 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., REXROAT, M.A. & PROBST, G.S. (1984c). The effect of
EL-737 (compound 31537) the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished Report No.
840716AMS2000 from Lilly Research Laboratories, Division of Eli
Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., OBERLY, T.J., BEWSEY, B.J. & PROBST, G.S. (1984d).
The effect of 31537 (EL-737) on the induction of forward mutation at
the thymidine kinase locus of L517Y mouse lymphoma cells.
Unpublished Report No. 840627MLA2000 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., BRIDGE, T.L. (1985a). The acute
toxicity of compound 31537 (EL-737) administered orally to the ICR
mouse. Unpublished Reports Nos. M-0-178-84 and M-0-179-84 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985b). The acute
toxicity of compound 31537 (EL-737) administered orally to the
Fischer 344 rat. Unpublished Report No. R-0-132-84 and R-0-133-84
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985c). The acute
toxicity of compound 31537 (EL-737) administered intraperitoneally
to the Fischer 344 rat. Unpublished Reports Nos. R-P-08-84 and
R-P-09-84 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985d). A three-month
toxicity study of ractopamine hydrochloride fed in the diet to
B6C3F1 mice. Unpublished Report No. M01584 from Lilly Research
Laboratories, Division of Eli Lilly and Company Greenfield, Indiana,
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985e). A three-month
toxicity study of 031537 (EL-737) administered orally to Fischer 344
rats. Unpublished Report No. R06184 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield, Indiana
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985f). 8-Day
inhalation study in rhesus monkey. Unpublished Report No. P06288
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Lilly Corporate Center, Indianapolis, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985g). 18-Day
inhalation study in rhesus monkey. Unpublished Report No. P00388
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985h). An interim
summary of a 6-week subchronic toxicity study of ractopamine
hydrochloride administered by nasogastric gavage to rhesus monkeys.
Unpublished Report No. P00691 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Lilly Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly,
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985i). The effect
of subchronic administration of ractopamine hydrochloride on heart
rate and electrocardiographic waveforms in conscious rhesus monkeys.
Unpublished Report No. P02186 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEAL, S.B. & PROBST, G.S. (1985j). The effect of
compound 31537 (EL-737) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters. Unpublished
Report No. 850121SCE2000 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & BRIDGE, T.L., MARKEY, T.F. (1985k).
The acute oral, dermal, ocular and inhalation toxicity of a granular
premix formulation (AFN-026) containing 10% compound 31537 (EL-737).
Unpublished Reports Nos. R-0-188-85, B-D-104-85, B-E-120-85, and
R-H-066-85 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., HOYT, J.A. (1986). An eleven-month two-generation
reproduction study, including a teratology segment, in Cd rats
maintained on diets containing ractopoamine hydrocholoride (EL-739,
Compound 31537). Unpublished report for study Nos. R11385 and R18985
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., (1987a). Comparative bioavailability of
14C-ractopamine hydrochloride (031537, EL-737) following a single
oral dose of 0.125 mg/kg in the dog and monkey. Unpublished Reports
Nos. D04686 and P03086 from Lilly Research Laboratories, Division of
Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., (1987b). A chronic toxicity study of ractopamine
hydrochloride administered orally to beagle dogs for one year.
Unpublished Report No. D05885 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & POHLAND, R.C. & BYRD, T.K. (1987). Bioavailability
of radiocarbon following the administration of single oral doses of
14C-EL-737 (31537) to F344/N Hsd BR rats. Unpublished Reports Nos.
R03985, RO4085 and RO4185 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
See Also:
Toxicological Abbreviations
Ractopamine (WHO Food Additives Series 53)
RACTOPAMINE (JECFA Evaluation)
 The same three metabolites were isolated from pig urine and
were all identified to be monoglucuronides of ractopamine. Of the
extractable liver residues, 30-50% was found to be ractopamine and
the rest conjugates of ractopamine. The kidneys contained a higher
amount of conjugates (64%) and correspondingly smaller amount of
ractopamine (20-30%). Studies in rats and dogs showed that urine
from animals dosed with 14C-ractopamine hydrochloride contained
the same three metabolites of ractopamine as in pigs (metabolites A,
B, and C). These three metabolites constituted a large portion of
14C-residues in rat and dog urine. The chromatographic profiles of
the 14C-residue extracts of rat, dog and pig liver were
quantitatively similar. The comparative quantitative data showing
the mean amounts in ppm of ractopamine hydrochloride and its major
metabolites (calculated as ractopamine hydrochloride) in kidney and
liver tissues of pigs, dogs, and rats are summarized in Table 1
(Dalidowicz & Babbitt, 1986; Dalidowicz, 1986a; Dalidowicz, 1986b).
Table 1. Metabolic profile in pigs, dogs and rats
Liver, ppm Kidney, ppm
Pig Dog Rat Pig Dog Rat
Ractopamine. HC1 0.12 0.59 0.40 0.10 0.50 0.33
Metabolite A 0.03 0.46 0.17 0.05 0.18 0.52
Metabolite B 0.04 0.77 0.15 0.06 0.27 0.57
Metabolite C 0.02 1.76 0.10 0.09 0.63 0.08
Dose: pigs (45 kg): 30 ppm in food for 4 days; pigs were killed
12 h after the last dose;
dogs: 0.5 mg/kg bw by gavage 3 times per day for 4 days and
once on the fifth day; dogs were killed 6 h after the
last dose;
rats: 2 mg/kg bw by gavage once daily for 7 days; rats were
killed 6 h after the last dose.
The concentrations of the metabolites in dog and rat tissues
were much higher than those in pig tissues. The nonextractable and
the uncharacterized residues in dog and rat tissues were also much
higher than in pig tissues (Dalidowicz, 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ractopamine are
summarized in Table 2.
2.2.2 Short-term studies
2.2.2.1 Mice
Diets containing 0, 0.02, 0.14 or 1.0% ractopamine
hydrochloride were fed to B6C3F1 mice (10/sex/dose) for 3
months, resulting in estimated time-weighted average daily doses of
0, 25, 175 or 1250 mg/kg bw/day, respectively. The mice were
observed daily for clinical signs of toxicity. Body-weights and food
consumption were measured weekly in all animals. Necropsies were
performed on all mice sacrificed at the end of the study. A set of
tissues was examined microscopically.
Table 2. Acute toxicity studies
Species Sex Route LD50 (mg/kg/bw Reference
Mouse M oral 3547 (2912-4321) Williams et al.,
1985a
F oral 2545 (2219-2919) Williams et al.,
1985a
Rat M oral 474 (398-564) Williams et al.,
1985b
F oral 367 Williams et al.,
(1985b)
Rat M intraperitoneal 132 (110-159) Williams et al.,
F intraperitoneal 122 (102-145) 1985c
Rabbit M dermal >2000 Williams et al.,
F 1984a
Rat M inhalation LC50 (4 h): 2.8 Williams et al.,
F mg/L 1985k
All of the treated animals survived the treatment period.
Alopaecia, a common finding in B61C3F1 mice, was observed in
22 animals of each sex. In this study the occurrence of alopaecia
was markedly decreased in the 1250 mg/kg bw/day group compared to
the other groups. No adverse signs of toxicity were attributed to
treatment with ractopamine hydrochloride. Male mice in the 25 and
175 mg/kg bw/day groups had a significant increase in cumulative
weight gain in the last eight weeks of the study. Differences in the
final mean body-weights of the treatment groups compared to the
controls were not statistically significant.
Treatment- and dose-related mild to moderate increases occurred
in erythrocyte counts, haemoglobin concentrations, and packed cell
volumes in both sexes of the 175 and 1250 mg/kg bw/day groups. Minor
changes occurred in the mean corpuscular volume and mean corpuscular
haemoglobin concentration in males of the 1250 mg/kg bw/day group.
The changes in thrombocyte counts were minimal in both sexes of the
1250 mg/kg bw/day group. All other haematologic parameters were
similar to control values. There were no treatment-related effects
on any haematologic parameters in mice of the 25 mg/kg bw/day group.
Treatment-related clinical chemistry changes consisted of mild
increases in urea nitrogen and cholesterol concentrations in males
of the 1250 mg/kg bw/day group. Female mice in both the 175 and 1250
mg/kg bw/day groups had mild increases in urea nitrogen and
cholesterol concentrations. The decrease in serum sodium
concentration in female mice of the 1250 mg/kg bw/day group,
although statistically significant, was minimal and the value fell
within the normal range. No alteration in the other electrocytes
concentration occurred. All other clinical chemistry parameters were
similar to control values. There were no treatment-related effects
on serum chemistry in mice of the 25 mg/kg bw/day group.
In males, a decrease in testicular weights, both absolute and
relative, occurred in a dose-dependent manner. Both males and
females in the 1250 mg/kg bw/day group had increased absolute and
relative heart weights. All the other organ weights were similar to
control values.
Microscopically, treatment-related changes involved the
periaortic and intercapsular brown fat in mice of both sexes in the
1250 mg/kg bw/day group. The plasma membranes of the affected brown
fat cells from the 1250 mg/kg bw/day group of mice were more
discrete and stained intensely with eosin. The cytoplasm of these
cells was filled with uniformly small spherical vacuoles, the
margins of which also stained intensely with eosin. These changes
were diagnosed as marked cytoplasmic changes. The cytoplasm of the
brown fat cells with minimal change was characterized by small
vacuoles, but the margins of the vacuoles did not stain intensely
with eosin. Brown fat in female mice and a few male mice of the
control and of the 25 and 175 mg/kg bw/day groups was characterized
as having a minimal cytoplasmic change. Due to the dose-dependent
decrease in testicular weights, a NOEL could not be established in
this study (Williams et al., 1985d).
2.2.2.2 Rats
Diets containing 0, 20, 200 or 2000 ppm ractopamine
hydochloride were fed to Fischer 344 rats (20/sex/dose) for three
months. The time-weighted average doses were 0, 1.3, 13, or 153
mg/kg bw/day for males and 0, 1.4, 15, or 157 mg/kg bw/day for
females. The rats were observed daily for clinical signs of
toxicity. Body-weights and food consumption were measured weekly for
all animals. Autopsies were done on animals at the end of the study.
Histological examination of tissue sections was conducted on all
tissues.
All of the treated animals survived the treatment period. The
clinical signs observed were decreased body-weight gain, increased
food consumption and decreased efficiency of food utilization. These
signs were observed in the 155 mg/kg bw/day group only, where they
were observed throughout the study in rats of both sexes. In
addition to these signs decreased serum triglyceride and cholesterol
levels and increased serum urea nitrogen concentrations were
observed.
An increase in serum potassium was observed in the 155 mg/kg
bw/day group in both sexes. Total erythrocyte count, haemoglobin
concentration, and packed cell volume all increased in rats of the
155 mg/kg bw/day group, as expected with a beta-agonist. There was a
decrease in uterine weight in rats of the 155 mg/kg bw/day group in
this study and a slight reduction in spleen weight in the 13-15 and
155 mg/kg bw/day groups. No change was observed in heart weight of
any treated animals and there was no microscopic evidence of
myocardial necrosis, which is a well-documented effect following
large doses of epinephrine and other sympathomimetic amines.
Histopathology examination of brown fat revealed slight to
moderate cytoplasmic change in the 13-15 and 155 mg/kg bw/day
groups. The NOEL for males was 1.3 mg/kg bw/day and 1.4 mg/kg bw/day
for females, based on the absence of biochemical or histological
changes (Williams et al., 1985e).
2.2.2.3 Dogs
Groups of 4 male and 4 female beagle dogs were given total
doses of 0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine
hydrochloride in gelatin capsules for 1 year. The doses were given
in capsules as three equal divided doses every 6 h. This study was
based on pilot studies in dogs, in which an oral dose of 0.05 mg/kg
bw/day was associated with skin and oral mucosal reddening and a
dose of 0.035 mg/kg bw/day was the NOEL. On the basis of this NOEL,
the frequency of dosing was increased to three times per day, 6 h
apart, in order to triple the daily exposure to 0.112 mg/kg bw/day
for the low-dose. The middle-dose, 0.224 mg/kg bw/day (0.075 mg/kg
bw, 3 times per day), was selected as a minimal cardiostimulatory
dose. The high-dose, 5.68 mg/kg bw/day (1.89 mg/kg bw, 3 times per
day) was selected because it produced near maximal cardiostimulation
at 2 h following a single oral dose of 1.5 mg/kg bw, in the pilot
study.
All dogs survived the one-year treatment period. Body-weight,
food consumption, ophthalmic examination, electrocardiogram wave
forms, bone marrow evaluation and urinalysis results were not
affected by treatment. The zero-time resting heart rate means for
the study (collected on test-days 29, 68, 83, 119, 155, 182, 211,
273 and 366) for low-, middle- and high-dose dogs (sex combined)
were 123, 116 and 115 beats per minute, respectively, and all were
significantly (p < 0.01) depressed compared to 141 beats per minute
for the controls.
Clinical signs of treatment-related effects were transient
cutaneous erythema and oily and unkept hair coat in the high-dose
dogs. Cutaneous erythema occurred in the mid-dose group in the first
five months of the study. Erythrocyte number, haemoglobin
concentration and packed cell volume were significantly decreased in
the high-dose group starting at 90 days, which and continuing up to
the end of the study. Several clinical chemistry parameters were
altered in the high-dose group by 90 days, which persisted until the
termination of the study. Serum potassium and urea nitrogen
concentration increased while serum glucose, cholesterol and
triglyceride concentration decreased.
Treatment-related gross and histopathological findings were
limited to the high-dose group. Both absolute and relative heart
weight and absolute thyroid and adrenal weights were significantly
decreased. Grossly, there was a slight decrease in abdominal and
thoracic fat. Microscopically, a decrease in hepatic centrilobular
glycogen and an increase in perithymic and periaortic brown fat was
noted in the high-dose dogs.
The appearance of bradycardia at all doses, most prominent
during the first 6 months, precluded a NOEL being established in
this study (Williams, 1987b).
2.2.2.4 Monkeys
Rhesus monkeys (2/sex/dose) were given ractopamine
hydrochloride dissolved in nanopure water at doses of 0.25, 0.5 or
4 mg/kg bw once daily by nasogastric gavage in a volume of 1 ml/kg
bw for 6 weeks. Control monkeys received vehicle (nanopure water)
only. This 6-week subchronic study was conducted in rhesus monkeys
to determine the doses at which toxic effects occurred so that
appropriate effect and no-effect doses can be selected for a 1-year
study in monkeys.
Treatment had no significant toxicological effect on
body-weight, food consumption, ophthalmic examination, daily
clinical examination, electro-cardiogram wave forms, haematology,
clinical chemistry, or urinalysis parameters. No treatment-related
abnormalities were observed at the final physical examination. There
was no induction of the hepatic enzyme p-nitroanisole o-demethylase,
and no compound-related gross lesions were observed at necropsy. No
organ weight changes were observed except for increased salivary
gland weight relative to body-weight (sexes combined) in the 4 mg/kg
bw/day group. The microscopic appearance of salivary gland and heart
from monkeys of the 4 mg/kg bw/day group was similar to that of
controls. Microscopic evaluation of the remaining tissues collected
by necropsy and determination of heart and lung ß-adrenergic
receptor numbers was in progress at the time of the review.
Monkeys given 4 mg/kg bw/day ractopamine hydrochloride
developed daily tachycardia, which was maximal by 0.5 h post-dose,
and remained elevated through 16 h after dosing. The monkeys did not
demonstrate the significant slowing of the night-time heart rates as
seen in the control, 0.25 and 0.5 mg/kg bw/day groups of monkeys. A
slight tachyphylaxis to ractopamine-induced heart rate stimulation
was apparent after the first day, which did not progress with time
of treatment. No effect on the electrocardiogram wave forms or
cardiac histopathology occurred in the presence of marked daily
tachycardia during the 6 weeks at 4 mg/kg bw/day. The NOEL was
0.5 mg/kg bw/day (Williams et al., 1985h).
Two groups, each composed of three male and three female rhesus
monkeys, were administered either vehicle or 0.125 m-g ractopamine
hydrochloride/kg bw once per day for 90 days by nasogastric gavage.
The test substance was prepared as a solution in nanopure water at a
concentration of 0.125 mg/ml.
One ml/kg bw of vehicle or the ractopamine solution was
administered and the monkeys were maintained in a conscious state
during the dosing and the cardiovascular monitoring procedures.
All of the monkeys were clinically normal throughout the study.
There were no effects on body-weight, food consumption, heart rate,
or on the electro-cardiographic wave forms in any control or treated
monkeys. The NOEL was determined to be 0.125 mg/kg bw/day
(Williams et al., 1985i). .
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0 (air, control), 0.05, 0.17, or 0.44 mg
ractopamine hydrochloride/m3 of air for 4 h per day for 8 days
over a 10-day period (excluding week-ends). The activity median
equivalent aerodynamic diameters (AMEAD) of the aerosols were 5.6,
6.2 and 6.4 µm for groups exposed to 0.05, 0.17 or 0.44 mg
ractopamine hydrochloride/m3. There was a slight increase in
post-exposure (night-time) heart rates. No treatment-related changes
occurred in body-weights, organ weights, food consumption,
haematology, or clinical chemistry parameters. There were no
treatment-related gross or microscopic lesions. The
no-observed-effect exposure concentration was 0.17 mg ractopamine
hydrochloride/m3 (Williams et al., 1985f).
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0.38, 1.69, 6.42 or 23.8 mg ractopamine
hydrochloride/m3 for 4 h per day for 2 to 8 days over an 18-day
period (excluding weekends) following a 7-day period of control
heart rate data collection. The AMEAD of the aerosols were 6.2, 7.8,
8.3 and 11.4 µm for the four exposure groups, respectively.
Treatment was stopped after 2 exposures at 23.8 mg ractopamine
hydrochloride/m3 and after 7 exposures at 1.69 and 6.42 mg
ractopamine hydrochloride/m3. The 0.38 mg ractopamine
hydrochloride/m3 exposure group received no exposure until the
last 8 days of the study prior to necropsy. Two animals were
sacrificed prior to study termination due to rejection of the
implanted ECG transmitter. All other animals survived to termination
of the study. No clinical signs of toxicity were observed. Increased
heart rates were observed in all exposure groups during exposure
(daytime) and following exposure (night-time). The increased daytime
and night-time heart rates persisted after treatment was stopped and
required approximately two weeks to return to normal values. No
treatment-related changes occurred in body-weights, organ weights,
food consumption, haematology or clinical chemistry parameters. No
treatment-related gross or microscopic lesions were observed. A
no-observed-effect exposure concentration for inhalation of
ractopamine hydrochloride aerosol could not be determined in this
study (Williams et al., 1985g).
2.2.3 Long-term/carcinogenicity studies
No infomation available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 25 male and 25 female Crl:CD(SD)BR rats, about 35
days old at the beginning of the study were maintained on diets
containing 0, 2, 20, 200 or 2000 ppm ractopamine in two generations
of parental rats. Weanling F0 male rats were maintained on the
test diets for 70 days prior to mating and the female rats were on
the test diets for the 14 days prior to mating.
The F0 parental rats were mated once and the females were
allowed to deliver and rear their young through weaning. However,
F1 animals were mated for two breeding trials. In the first
breeding trial, the females were allowed to deliver and rear their
progeny through postpartum day 21. In the second breeding trial, the
females were killed on gestation day 20 for the assessment of
intrauterine reproduction parameters and the collection of fetuses
for external, visceral and skeletal examination. Significance was
defined as P <0.05 in this study.
Significant treatment-related depression in body-weight and
body-weight gain occurred in both F0 and F1 males of the 2000
ppm group. Significant depression in body-weight also occurred in
F1 females of the 2000 ppm group. Food consumption was
significantly depressed in F1 males of the 2000 ppm group only.
Mating performance and fertility were comparable with the
controls at each treatment level in each litter of the F0 (F1a)
and F1 (F2a and F2b) generations.
During the breeding trials for F1a and F2a litters,
gestation length was not affected; however, the mean litter size and
mean progeny survival indices were depressed significantly in the
2000 ppm group.
Significant reductions in the mean weanling weight of F1a and
F2a pups born to dams treated at 2000 ppm from days 1 through 21
postpartum were recorded. The percentage of male progeny was
significantly depressed only in the F1a litters.
Clinical signs such as pallor, apparent hypothermia, thinness,
dehydration and rough hair coat occurred with the highest frequency
in the neonatal and postnatal progeny of the 2000 ppm group.
Seventy-three pups of the F1a generation from the 2000 ppm group
were either born dead or died during the lactation period. Gross
external and internal examinations of the pups revealed that 13 of
these pups had abnormalities which included oedema (8), cleft
palate (7), limb abnormalities (5), brachygnathia (3), protruding
tongue (3), open eyelids (3), omphalocele (2), clotted blood in the
lateral ventricles of the brain (1), microphthalmia (2), and
enlarged heart (1).
Malformations were observed in 0, 1, 0, 2 and 63 F2a fetuses
from dams treated at 0, 2, 20, 200 and 2000 ppm, respectively.
However, significant increases in malformations were recorded in the
63 pups from the dams treated at 2000 ppm. These abnormalities
included oedema, hydraminos, cleft palate, protruding tongue, short
limbs, missing digits, open eyelids, brachygnathia, fused digits,
limb abnormalities (wavy humerus radius, ulna, femur, tibia and
fibula, mishapen scapula, micromelia, kinked tail, enlarged heart,
kyphosis adactyly (hindlimb), and omphalocele.
Percent early, late and total resorption were significantly
increased in dams treated at 2000 ppm. In dams of the 200 ppm and
2000 ppm groups, significant depression in the proportion of normal
fetuses was observed. In the 2000 ppm group, this depression was
accompanied by statistically-significant proportions of fetuses with
developmental variations and abnormalities.
In view of the fact that significant teratological effects were
observed only at the highest dose level tested, a dose level at
which maternal toxicity was also observed, it was concluded that the
NOEL was 200 ppm, equal to 15 mg/kg bw/day (Williams & Hoyt, 1986).
2.2.5 Special studies on genotoxicity
The results of a limited series of in vitro and in vivo
genotoxicity studies on ractopamine are summarized in Table 3. All
results were reported to be negative.
2.3 Observations in humans
A pilot clinical trial evaluating bronchodilator activity of
ractopamine in oral dosage form and as aerosol was conducted in
1960. Ractopamine was administered orally to four patients with
chronic bronchial asthma at doses of 30 and 45 mg. No clear-cut
evidence of bronchodilator activity, CNS stimulation, or increase in
pulse rate was seen in these patients. Two patients receiving 30 to
45 mg of ractopamine showed 15 to 20 mm Hg systolic elevation of
blood pressure lasting for about 1 h. A summary on the use of
50 mg/ml ractopamine with a DeVilbiss nebulizer in four asthmatic
patients indicated that on a 0 to 4 scale of effectiveness according
to patients, ractopamine scored 3, 3, 1 and 0 in these patients. It
was stated that ractopamine as an aerosol has failed to show any
activity at doses up to 6 mg (Shipley, 1960).
Table 3: Results of genotoxicity assays on ractopamine
Concentration of
Test System Test Object Ractopamine Results References
Unscheduled Fischer 344 rat 0.5-1000 µg/ml Negative Williams et al.,
DNA synthesis hepatocytes 1984b
in vitro
Ames test (1) S. typhimurium 50-5000 µg/ml Negative Williams et al.,
TA1535, TA1537, 1984c
TA1538, TA98,
TA100
Ames test (1) S. typhimurium 0.1-1000 µg/ml Negative Williams &
G46, TA1535, Thompson,
TA100, C3076, 1984
TA1537, D3052,
TA1338, TA98
E. coli
WP2, WP2uvrA-
Mouse lymphoma cell L5178Y mouse 10-350 µg/ml Negative Williams et al.,
thymidine kinase 1984d
100-700 µg/ml
Sister chromatid Chinese hamsters 200-500 mg/kg Negative Williams et al.,
exchange in bone 1985j
marrow of hamsters
in vivo
(1) With and without rat liver S-9 fraction.
3. COMMENTS
Results of various studies were reviewed by the Committee,
including pharmacokinetic, biotransformation, acute and short-term
toxicity, reproductive, teratogenicity, and genotoxicity studies and
a limited number of studies in humans.
Studies with 14C-ractopamine in several species have
indicated rapid absorption following oral administration. In pigs,
labelled ractopamine was excreted almost quantitatively;
approximately 88% was recovered in urine and 9% in faeces during a
7-day period. Studies in pigs, rats, and dogs fed 14C-ractopamine
showed three major metabolites, identified as monoglucuronides of
ractopamine.
In acute studies, ractopamine was substantially more toxic
orally to the rat (LD50 approximately 450 mg/kg bw) than the mouse
(LD50 approximately 3000 mg/kg bw).
The genotoxic potential of ractopamine was evaluated in a
limited series of in vitro and in vivo studies, all of which
were reported to be negative. However, in the absence of a
carcinogenicity study, and as human exposure is likely to be
extensive, the Committee concluded that additional genotoxicity
testing would be desirable.
The short-term toxicity of ractopamine has been evaluated in
mice, rats, dogs, and monkeys. Ractopamine was fed to B6C3F1
mice for 3 months at doses of 25, 175, or 1250 mg/kg bw/day. The
most significant effect noted was a dose-dependent decrease, both
absolute and relative, in testicular weights. In both males and
females in the highest-dose group, absolute and relative heart
weights were increased. However, no histopathological changes were
observed in either the heart or the testes. A clear NOEL could not
be established in this study.
Fischer 344 rats were fed doses up to approximately 155 mg/kg
bw/day for 3 months. The highest-dose group showed decreased
body-weight gain, increased food consumption, decreased efficiency
of food utilization, and an increase in serum potassium
concentration. There was a decrease in uterine weight in rats of
this group and a slight reduction in spleen weight in the top two
groups. The NOEL was 1.3 mg/kg bw/day in this study.
Beagle dogs were given three doses daily, 6 h apart, totalling
0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine in gelatin capsules
for 1 year. Treatment-related minor histopathological findings were
limited to the high-dose group and to the liver. The occurrence of
mild nocturnal bradycardia, most prominent during the first
6 months, meant that a clear no-effect level was not observed in
this study. The Committee noted that the quantity of ractopamine
residues consumed in 500 g of meat from animals slaughtered without
a withdrawal period would closely approach an ADI derived from the
lowest dose of 0.112 mg/kg bw/day and a safety factor of 100.
In a further study of cardiovascular effects, rhesus monkeys
were given either vehicle or ractopamine at 0.125 mg/kg bw/day for
90 days by gavage. This dose was 2.5 times the single dose known to
produce tachycardia and peripheral vasodilation in the dog. In
addition, the selected dose exceeded the total daily dose
(0.112 mg/kg bw/day) associated with nocturnal bradycardia in the
1-year dog study. The NOEL for this study was 0.125 mg/kg bw/day.
Rhesus monkeys were given ractopamine at doses of 0.25, 0.5, or
4 mg/kg bw/day once daily by gavage for 6 weeks to determine the
doses to be used in a 1-year study. Monkeys given 4 mg/kg bw/day
developed daily tachycardia which was maximal by 30 min agter
dosing, and continued for 16 h. Monkeys in the group did not
demonstrate the significant slowing of the nocturnal heart rates
seen in the other groups. The NOEL for this study was 0.5 mg/kg
bw/day.
The effect of ractopamine on reproductive and developmental
performance in Sprague-Dawley rats was evaluated at dosage levels of
up to 2000 ppm in the diet. Significant effects, which included a
reduction in mean litter size and an increase in the total number of
resorptions, were restricted to the high dose which was also
maternally toxic. The NOEL was 200 ppm, equal to 15 mg/kg bw/day, in
this study. The Committee considered the teratogenicity segment of
this study to be adequate to assess developmental toxicity. A minor
teratogenic response was observed only at the highest dose
(2000 ppm) tested, at which maternal toxicity was also noted.
The bronchodilator and inotropic effects of ractopamine were
evaluated in pilot clinical trials in humans. Four patients showed
little evidence of bronchodilator activity, central nervous system
stimulation, or an increase in pulse rate. Two patients showed a
mild elevation of blood pressure lasting for about 1 hour. An
infusion study showed inotropic and chronotropic enhancement in both
healthy volunteers and heart patients.
4. EVALUATION
The Committee concluded that, on the basis of the short-term
studies available, residues of ractopamine appeared to have little
toxic potential for the consumer. The effects recorded were in the
main those to be expected from a ß-adrenoceptor agonist. It might
therefore be appropriate to assess ractopamine on the basis of a
NOEL for pharmacological effects that are relevant to its ingestion
by humans as a residue in edible meats. However, because such a NOEL
could not be determined in the 1-year study in dogs, the Committee
was unable to establish an ADI.
The Committee noted that: (a) some ß-adrenoceptor agonists were
carcinogenic; (b) no long-term studies had been conducted in
rodents; and (c) there were no data relating to the long-term
exposure of humans to ractopamine. Therefore, before reviewing the
compound again, the Committee would wish to see evidence and
arguments in at least the following areas:
1. Genotoxicity
- A further in vivo study such as a micronucleus test.
2. Pharmacology
- investigations that fully explore the pharmacological
properties of ractopamine;
- the relative contributions of ß1- and ß2-adrenoceptor
activation to the spectrum of effects produced by
ractopamine;
- a sufficient basis from which to establish the most
sensitive indicator (test and species) of the
pharmacological effects of ractopamine;
- validation of the utility of this indicator in the setting
of a pharmacological NOEL for humans;
- a survey of the pharmacokinetic parameters of
ß-adrenoceptor agonists in humans and laboratory species,
including those relevant to oral administration;
- determination of appropriate timing for observations in
animal studies to reveal both the onset and the peak
values of all relevant effects.
3. Human data
- A survey of all non-therapeutic effects that follow
long-term ß-adrenoceptor agonist use in humans, to assist
in the prediction of the consequences of the long-term
intake of residues of ractopamine by consumers of animal
meat.
Depending on the results of the above investigations, it may be
necessary to perform other studies to explore further the potential
carcinogenicity of ractopamine.
5. REFERENCES
DALIDOWICZ, J.D. (1986a). Metabolism of 14C-ractopamine HCl in the
rat. Unpublished Report No. ABC-0285 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1986b). Metabolism of 14C-ractopamine HCl in the
dog. Unpublished Report No. ABC-0301 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. & BABBITT, G.E. (1986). Characterization of
14C residues in tissues and excreta from swine fed
14C-ractopamine HCl. Unpublished Report No. ABC-0355 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.E., THOMSON, T.D. & HERBERG, R.J. (1986).
14C-ractopamine HCl balance - excretion study in swine.
Unpublished Report No. ABC-0330 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1987). Comparative metabolism of 14C-ractopamine
HCl in swine, dogs, and rats. Unpublished Report No. ABC-0369 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
SHIPLEY, R.E. (1960). Summary for research project committee May 31,
1960. Unpublished memoranda from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & THOMPSON, C.Z. (1984). The effect of compound
31537, EL-737 on the induction of bacterial mutation using a
modification of the Ames test. Unpublished Report No. 840507GPA2000
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & MARKEY, T.F. (1984a). The acute
dermal, ocular, and inhalation toxicity of compound 31537 (EL-737).
Unpublished Reports Nos. B-D-82-84, B-E-108-84 and R-H-47-84 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., HILL, L.E. & PROBST, G.S. (1984b). The effect of
compound 31537, EL-737, on the induction of DNA repair synthesis in
primary cultures of adult rat hepatocytes. Unpublished Reports Nos.
840503UDS2000 and 840508UDS2000 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., REXROAT, M.A. & PROBST, G.S. (1984c). The effect of
EL-737 (compound 31537) the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished Report No.
840716AMS2000 from Lilly Research Laboratories, Division of Eli
Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., OBERLY, T.J., BEWSEY, B.J. & PROBST, G.S. (1984d).
The effect of 31537 (EL-737) on the induction of forward mutation at
the thymidine kinase locus of L517Y mouse lymphoma cells.
Unpublished Report No. 840627MLA2000 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., BRIDGE, T.L. (1985a). The acute
toxicity of compound 31537 (EL-737) administered orally to the ICR
mouse. Unpublished Reports Nos. M-0-178-84 and M-0-179-84 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985b). The acute
toxicity of compound 31537 (EL-737) administered orally to the
Fischer 344 rat. Unpublished Report No. R-0-132-84 and R-0-133-84
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985c). The acute
toxicity of compound 31537 (EL-737) administered intraperitoneally
to the Fischer 344 rat. Unpublished Reports Nos. R-P-08-84 and
R-P-09-84 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985d). A three-month
toxicity study of ractopamine hydrochloride fed in the diet to
B6C3F1 mice. Unpublished Report No. M01584 from Lilly Research
Laboratories, Division of Eli Lilly and Company Greenfield, Indiana,
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985e). A three-month
toxicity study of 031537 (EL-737) administered orally to Fischer 344
rats. Unpublished Report No. R06184 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield, Indiana
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985f). 8-Day
inhalation study in rhesus monkey. Unpublished Report No. P06288
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Lilly Corporate Center, Indianapolis, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985g). 18-Day
inhalation study in rhesus monkey. Unpublished Report No. P00388
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985h). An interim
summary of a 6-week subchronic toxicity study of ractopamine
hydrochloride administered by nasogastric gavage to rhesus monkeys.
Unpublished Report No. P00691 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Lilly Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly,
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985i). The effect
of subchronic administration of ractopamine hydrochloride on heart
rate and electrocardiographic waveforms in conscious rhesus monkeys.
Unpublished Report No. P02186 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEAL, S.B. & PROBST, G.S. (1985j). The effect of
compound 31537 (EL-737) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters. Unpublished
Report No. 850121SCE2000 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & BRIDGE, T.L., MARKEY, T.F. (1985k).
The acute oral, dermal, ocular and inhalation toxicity of a granular
premix formulation (AFN-026) containing 10% compound 31537 (EL-737).
Unpublished Reports Nos. R-0-188-85, B-D-104-85, B-E-120-85, and
R-H-066-85 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., HOYT, J.A. (1986). An eleven-month two-generation
reproduction study, including a teratology segment, in Cd rats
maintained on diets containing ractopoamine hydrocholoride (EL-739,
Compound 31537). Unpublished report for study Nos. R11385 and R18985
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., (1987a). Comparative bioavailability of
14C-ractopamine hydrochloride (031537, EL-737) following a single
oral dose of 0.125 mg/kg in the dog and monkey. Unpublished Reports
Nos. D04686 and P03086 from Lilly Research Laboratories, Division of
Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., (1987b). A chronic toxicity study of ractopamine
hydrochloride administered orally to beagle dogs for one year.
Unpublished Report No. D05885 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & POHLAND, R.C. & BYRD, T.K. (1987). Bioavailability
of radiocarbon following the administration of single oral doses of
14C-EL-737 (31537) to F344/N Hsd BR rats. Unpublished Reports Nos.
R03985, RO4085 and RO4185 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
The same three metabolites were isolated from pig urine and
were all identified to be monoglucuronides of ractopamine. Of the
extractable liver residues, 30-50% was found to be ractopamine and
the rest conjugates of ractopamine. The kidneys contained a higher
amount of conjugates (64%) and correspondingly smaller amount of
ractopamine (20-30%). Studies in rats and dogs showed that urine
from animals dosed with 14C-ractopamine hydrochloride contained
the same three metabolites of ractopamine as in pigs (metabolites A,
B, and C). These three metabolites constituted a large portion of
14C-residues in rat and dog urine. The chromatographic profiles of
the 14C-residue extracts of rat, dog and pig liver were
quantitatively similar. The comparative quantitative data showing
the mean amounts in ppm of ractopamine hydrochloride and its major
metabolites (calculated as ractopamine hydrochloride) in kidney and
liver tissues of pigs, dogs, and rats are summarized in Table 1
(Dalidowicz & Babbitt, 1986; Dalidowicz, 1986a; Dalidowicz, 1986b).
Table 1. Metabolic profile in pigs, dogs and rats
Liver, ppm Kidney, ppm
Pig Dog Rat Pig Dog Rat
Ractopamine. HC1 0.12 0.59 0.40 0.10 0.50 0.33
Metabolite A 0.03 0.46 0.17 0.05 0.18 0.52
Metabolite B 0.04 0.77 0.15 0.06 0.27 0.57
Metabolite C 0.02 1.76 0.10 0.09 0.63 0.08
Dose: pigs (45 kg): 30 ppm in food for 4 days; pigs were killed
12 h after the last dose;
dogs: 0.5 mg/kg bw by gavage 3 times per day for 4 days and
once on the fifth day; dogs were killed 6 h after the
last dose;
rats: 2 mg/kg bw by gavage once daily for 7 days; rats were
killed 6 h after the last dose.
The concentrations of the metabolites in dog and rat tissues
were much higher than those in pig tissues. The nonextractable and
the uncharacterized residues in dog and rat tissues were also much
higher than in pig tissues (Dalidowicz, 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ractopamine are
summarized in Table 2.
2.2.2 Short-term studies
2.2.2.1 Mice
Diets containing 0, 0.02, 0.14 or 1.0% ractopamine
hydrochloride were fed to B6C3F1 mice (10/sex/dose) for 3
months, resulting in estimated time-weighted average daily doses of
0, 25, 175 or 1250 mg/kg bw/day, respectively. The mice were
observed daily for clinical signs of toxicity. Body-weights and food
consumption were measured weekly in all animals. Necropsies were
performed on all mice sacrificed at the end of the study. A set of
tissues was examined microscopically.
Table 2. Acute toxicity studies
Species Sex Route LD50 (mg/kg/bw Reference
Mouse M oral 3547 (2912-4321) Williams et al.,
1985a
F oral 2545 (2219-2919) Williams et al.,
1985a
Rat M oral 474 (398-564) Williams et al.,
1985b
F oral 367 Williams et al.,
(1985b)
Rat M intraperitoneal 132 (110-159) Williams et al.,
F intraperitoneal 122 (102-145) 1985c
Rabbit M dermal >2000 Williams et al.,
F 1984a
Rat M inhalation LC50 (4 h): 2.8 Williams et al.,
F mg/L 1985k
All of the treated animals survived the treatment period.
Alopaecia, a common finding in B61C3F1 mice, was observed in
22 animals of each sex. In this study the occurrence of alopaecia
was markedly decreased in the 1250 mg/kg bw/day group compared to
the other groups. No adverse signs of toxicity were attributed to
treatment with ractopamine hydrochloride. Male mice in the 25 and
175 mg/kg bw/day groups had a significant increase in cumulative
weight gain in the last eight weeks of the study. Differences in the
final mean body-weights of the treatment groups compared to the
controls were not statistically significant.
Treatment- and dose-related mild to moderate increases occurred
in erythrocyte counts, haemoglobin concentrations, and packed cell
volumes in both sexes of the 175 and 1250 mg/kg bw/day groups. Minor
changes occurred in the mean corpuscular volume and mean corpuscular
haemoglobin concentration in males of the 1250 mg/kg bw/day group.
The changes in thrombocyte counts were minimal in both sexes of the
1250 mg/kg bw/day group. All other haematologic parameters were
similar to control values. There were no treatment-related effects
on any haematologic parameters in mice of the 25 mg/kg bw/day group.
Treatment-related clinical chemistry changes consisted of mild
increases in urea nitrogen and cholesterol concentrations in males
of the 1250 mg/kg bw/day group. Female mice in both the 175 and 1250
mg/kg bw/day groups had mild increases in urea nitrogen and
cholesterol concentrations. The decrease in serum sodium
concentration in female mice of the 1250 mg/kg bw/day group,
although statistically significant, was minimal and the value fell
within the normal range. No alteration in the other electrocytes
concentration occurred. All other clinical chemistry parameters were
similar to control values. There were no treatment-related effects
on serum chemistry in mice of the 25 mg/kg bw/day group.
In males, a decrease in testicular weights, both absolute and
relative, occurred in a dose-dependent manner. Both males and
females in the 1250 mg/kg bw/day group had increased absolute and
relative heart weights. All the other organ weights were similar to
control values.
Microscopically, treatment-related changes involved the
periaortic and intercapsular brown fat in mice of both sexes in the
1250 mg/kg bw/day group. The plasma membranes of the affected brown
fat cells from the 1250 mg/kg bw/day group of mice were more
discrete and stained intensely with eosin. The cytoplasm of these
cells was filled with uniformly small spherical vacuoles, the
margins of which also stained intensely with eosin. These changes
were diagnosed as marked cytoplasmic changes. The cytoplasm of the
brown fat cells with minimal change was characterized by small
vacuoles, but the margins of the vacuoles did not stain intensely
with eosin. Brown fat in female mice and a few male mice of the
control and of the 25 and 175 mg/kg bw/day groups was characterized
as having a minimal cytoplasmic change. Due to the dose-dependent
decrease in testicular weights, a NOEL could not be established in
this study (Williams et al., 1985d).
2.2.2.2 Rats
Diets containing 0, 20, 200 or 2000 ppm ractopamine
hydochloride were fed to Fischer 344 rats (20/sex/dose) for three
months. The time-weighted average doses were 0, 1.3, 13, or 153
mg/kg bw/day for males and 0, 1.4, 15, or 157 mg/kg bw/day for
females. The rats were observed daily for clinical signs of
toxicity. Body-weights and food consumption were measured weekly for
all animals. Autopsies were done on animals at the end of the study.
Histological examination of tissue sections was conducted on all
tissues.
All of the treated animals survived the treatment period. The
clinical signs observed were decreased body-weight gain, increased
food consumption and decreased efficiency of food utilization. These
signs were observed in the 155 mg/kg bw/day group only, where they
were observed throughout the study in rats of both sexes. In
addition to these signs decreased serum triglyceride and cholesterol
levels and increased serum urea nitrogen concentrations were
observed.
An increase in serum potassium was observed in the 155 mg/kg
bw/day group in both sexes. Total erythrocyte count, haemoglobin
concentration, and packed cell volume all increased in rats of the
155 mg/kg bw/day group, as expected with a beta-agonist. There was a
decrease in uterine weight in rats of the 155 mg/kg bw/day group in
this study and a slight reduction in spleen weight in the 13-15 and
155 mg/kg bw/day groups. No change was observed in heart weight of
any treated animals and there was no microscopic evidence of
myocardial necrosis, which is a well-documented effect following
large doses of epinephrine and other sympathomimetic amines.
Histopathology examination of brown fat revealed slight to
moderate cytoplasmic change in the 13-15 and 155 mg/kg bw/day
groups. The NOEL for males was 1.3 mg/kg bw/day and 1.4 mg/kg bw/day
for females, based on the absence of biochemical or histological
changes (Williams et al., 1985e).
2.2.2.3 Dogs
Groups of 4 male and 4 female beagle dogs were given total
doses of 0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine
hydrochloride in gelatin capsules for 1 year. The doses were given
in capsules as three equal divided doses every 6 h. This study was
based on pilot studies in dogs, in which an oral dose of 0.05 mg/kg
bw/day was associated with skin and oral mucosal reddening and a
dose of 0.035 mg/kg bw/day was the NOEL. On the basis of this NOEL,
the frequency of dosing was increased to three times per day, 6 h
apart, in order to triple the daily exposure to 0.112 mg/kg bw/day
for the low-dose. The middle-dose, 0.224 mg/kg bw/day (0.075 mg/kg
bw, 3 times per day), was selected as a minimal cardiostimulatory
dose. The high-dose, 5.68 mg/kg bw/day (1.89 mg/kg bw, 3 times per
day) was selected because it produced near maximal cardiostimulation
at 2 h following a single oral dose of 1.5 mg/kg bw, in the pilot
study.
All dogs survived the one-year treatment period. Body-weight,
food consumption, ophthalmic examination, electrocardiogram wave
forms, bone marrow evaluation and urinalysis results were not
affected by treatment. The zero-time resting heart rate means for
the study (collected on test-days 29, 68, 83, 119, 155, 182, 211,
273 and 366) for low-, middle- and high-dose dogs (sex combined)
were 123, 116 and 115 beats per minute, respectively, and all were
significantly (p < 0.01) depressed compared to 141 beats per minute
for the controls.
Clinical signs of treatment-related effects were transient
cutaneous erythema and oily and unkept hair coat in the high-dose
dogs. Cutaneous erythema occurred in the mid-dose group in the first
five months of the study. Erythrocyte number, haemoglobin
concentration and packed cell volume were significantly decreased in
the high-dose group starting at 90 days, which and continuing up to
the end of the study. Several clinical chemistry parameters were
altered in the high-dose group by 90 days, which persisted until the
termination of the study. Serum potassium and urea nitrogen
concentration increased while serum glucose, cholesterol and
triglyceride concentration decreased.
Treatment-related gross and histopathological findings were
limited to the high-dose group. Both absolute and relative heart
weight and absolute thyroid and adrenal weights were significantly
decreased. Grossly, there was a slight decrease in abdominal and
thoracic fat. Microscopically, a decrease in hepatic centrilobular
glycogen and an increase in perithymic and periaortic brown fat was
noted in the high-dose dogs.
The appearance of bradycardia at all doses, most prominent
during the first 6 months, precluded a NOEL being established in
this study (Williams, 1987b).
2.2.2.4 Monkeys
Rhesus monkeys (2/sex/dose) were given ractopamine
hydrochloride dissolved in nanopure water at doses of 0.25, 0.5 or
4 mg/kg bw once daily by nasogastric gavage in a volume of 1 ml/kg
bw for 6 weeks. Control monkeys received vehicle (nanopure water)
only. This 6-week subchronic study was conducted in rhesus monkeys
to determine the doses at which toxic effects occurred so that
appropriate effect and no-effect doses can be selected for a 1-year
study in monkeys.
Treatment had no significant toxicological effect on
body-weight, food consumption, ophthalmic examination, daily
clinical examination, electro-cardiogram wave forms, haematology,
clinical chemistry, or urinalysis parameters. No treatment-related
abnormalities were observed at the final physical examination. There
was no induction of the hepatic enzyme p-nitroanisole o-demethylase,
and no compound-related gross lesions were observed at necropsy. No
organ weight changes were observed except for increased salivary
gland weight relative to body-weight (sexes combined) in the 4 mg/kg
bw/day group. The microscopic appearance of salivary gland and heart
from monkeys of the 4 mg/kg bw/day group was similar to that of
controls. Microscopic evaluation of the remaining tissues collected
by necropsy and determination of heart and lung ß-adrenergic
receptor numbers was in progress at the time of the review.
Monkeys given 4 mg/kg bw/day ractopamine hydrochloride
developed daily tachycardia, which was maximal by 0.5 h post-dose,
and remained elevated through 16 h after dosing. The monkeys did not
demonstrate the significant slowing of the night-time heart rates as
seen in the control, 0.25 and 0.5 mg/kg bw/day groups of monkeys. A
slight tachyphylaxis to ractopamine-induced heart rate stimulation
was apparent after the first day, which did not progress with time
of treatment. No effect on the electrocardiogram wave forms or
cardiac histopathology occurred in the presence of marked daily
tachycardia during the 6 weeks at 4 mg/kg bw/day. The NOEL was
0.5 mg/kg bw/day (Williams et al., 1985h).
Two groups, each composed of three male and three female rhesus
monkeys, were administered either vehicle or 0.125 m-g ractopamine
hydrochloride/kg bw once per day for 90 days by nasogastric gavage.
The test substance was prepared as a solution in nanopure water at a
concentration of 0.125 mg/ml.
One ml/kg bw of vehicle or the ractopamine solution was
administered and the monkeys were maintained in a conscious state
during the dosing and the cardiovascular monitoring procedures.
All of the monkeys were clinically normal throughout the study.
There were no effects on body-weight, food consumption, heart rate,
or on the electro-cardiographic wave forms in any control or treated
monkeys. The NOEL was determined to be 0.125 mg/kg bw/day
(Williams et al., 1985i). .
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0 (air, control), 0.05, 0.17, or 0.44 mg
ractopamine hydrochloride/m3 of air for 4 h per day for 8 days
over a 10-day period (excluding week-ends). The activity median
equivalent aerodynamic diameters (AMEAD) of the aerosols were 5.6,
6.2 and 6.4 µm for groups exposed to 0.05, 0.17 or 0.44 mg
ractopamine hydrochloride/m3. There was a slight increase in
post-exposure (night-time) heart rates. No treatment-related changes
occurred in body-weights, organ weights, food consumption,
haematology, or clinical chemistry parameters. There were no
treatment-related gross or microscopic lesions. The
no-observed-effect exposure concentration was 0.17 mg ractopamine
hydrochloride/m3 (Williams et al., 1985f).
Rhesus monkeys, 2/sex/group, were exposed to mean aerosol
concentrations of 0.38, 1.69, 6.42 or 23.8 mg ractopamine
hydrochloride/m3 for 4 h per day for 2 to 8 days over an 18-day
period (excluding weekends) following a 7-day period of control
heart rate data collection. The AMEAD of the aerosols were 6.2, 7.8,
8.3 and 11.4 µm for the four exposure groups, respectively.
Treatment was stopped after 2 exposures at 23.8 mg ractopamine
hydrochloride/m3 and after 7 exposures at 1.69 and 6.42 mg
ractopamine hydrochloride/m3. The 0.38 mg ractopamine
hydrochloride/m3 exposure group received no exposure until the
last 8 days of the study prior to necropsy. Two animals were
sacrificed prior to study termination due to rejection of the
implanted ECG transmitter. All other animals survived to termination
of the study. No clinical signs of toxicity were observed. Increased
heart rates were observed in all exposure groups during exposure
(daytime) and following exposure (night-time). The increased daytime
and night-time heart rates persisted after treatment was stopped and
required approximately two weeks to return to normal values. No
treatment-related changes occurred in body-weights, organ weights,
food consumption, haematology or clinical chemistry parameters. No
treatment-related gross or microscopic lesions were observed. A
no-observed-effect exposure concentration for inhalation of
ractopamine hydrochloride aerosol could not be determined in this
study (Williams et al., 1985g).
2.2.3 Long-term/carcinogenicity studies
No infomation available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 25 male and 25 female Crl:CD(SD)BR rats, about 35
days old at the beginning of the study were maintained on diets
containing 0, 2, 20, 200 or 2000 ppm ractopamine in two generations
of parental rats. Weanling F0 male rats were maintained on the
test diets for 70 days prior to mating and the female rats were on
the test diets for the 14 days prior to mating.
The F0 parental rats were mated once and the females were
allowed to deliver and rear their young through weaning. However,
F1 animals were mated for two breeding trials. In the first
breeding trial, the females were allowed to deliver and rear their
progeny through postpartum day 21. In the second breeding trial, the
females were killed on gestation day 20 for the assessment of
intrauterine reproduction parameters and the collection of fetuses
for external, visceral and skeletal examination. Significance was
defined as P <0.05 in this study.
Significant treatment-related depression in body-weight and
body-weight gain occurred in both F0 and F1 males of the 2000
ppm group. Significant depression in body-weight also occurred in
F1 females of the 2000 ppm group. Food consumption was
significantly depressed in F1 males of the 2000 ppm group only.
Mating performance and fertility were comparable with the
controls at each treatment level in each litter of the F0 (F1a)
and F1 (F2a and F2b) generations.
During the breeding trials for F1a and F2a litters,
gestation length was not affected; however, the mean litter size and
mean progeny survival indices were depressed significantly in the
2000 ppm group.
Significant reductions in the mean weanling weight of F1a and
F2a pups born to dams treated at 2000 ppm from days 1 through 21
postpartum were recorded. The percentage of male progeny was
significantly depressed only in the F1a litters.
Clinical signs such as pallor, apparent hypothermia, thinness,
dehydration and rough hair coat occurred with the highest frequency
in the neonatal and postnatal progeny of the 2000 ppm group.
Seventy-three pups of the F1a generation from the 2000 ppm group
were either born dead or died during the lactation period. Gross
external and internal examinations of the pups revealed that 13 of
these pups had abnormalities which included oedema (8), cleft
palate (7), limb abnormalities (5), brachygnathia (3), protruding
tongue (3), open eyelids (3), omphalocele (2), clotted blood in the
lateral ventricles of the brain (1), microphthalmia (2), and
enlarged heart (1).
Malformations were observed in 0, 1, 0, 2 and 63 F2a fetuses
from dams treated at 0, 2, 20, 200 and 2000 ppm, respectively.
However, significant increases in malformations were recorded in the
63 pups from the dams treated at 2000 ppm. These abnormalities
included oedema, hydraminos, cleft palate, protruding tongue, short
limbs, missing digits, open eyelids, brachygnathia, fused digits,
limb abnormalities (wavy humerus radius, ulna, femur, tibia and
fibula, mishapen scapula, micromelia, kinked tail, enlarged heart,
kyphosis adactyly (hindlimb), and omphalocele.
Percent early, late and total resorption were significantly
increased in dams treated at 2000 ppm. In dams of the 200 ppm and
2000 ppm groups, significant depression in the proportion of normal
fetuses was observed. In the 2000 ppm group, this depression was
accompanied by statistically-significant proportions of fetuses with
developmental variations and abnormalities.
In view of the fact that significant teratological effects were
observed only at the highest dose level tested, a dose level at
which maternal toxicity was also observed, it was concluded that the
NOEL was 200 ppm, equal to 15 mg/kg bw/day (Williams & Hoyt, 1986).
2.2.5 Special studies on genotoxicity
The results of a limited series of in vitro and in vivo
genotoxicity studies on ractopamine are summarized in Table 3. All
results were reported to be negative.
2.3 Observations in humans
A pilot clinical trial evaluating bronchodilator activity of
ractopamine in oral dosage form and as aerosol was conducted in
1960. Ractopamine was administered orally to four patients with
chronic bronchial asthma at doses of 30 and 45 mg. No clear-cut
evidence of bronchodilator activity, CNS stimulation, or increase in
pulse rate was seen in these patients. Two patients receiving 30 to
45 mg of ractopamine showed 15 to 20 mm Hg systolic elevation of
blood pressure lasting for about 1 h. A summary on the use of
50 mg/ml ractopamine with a DeVilbiss nebulizer in four asthmatic
patients indicated that on a 0 to 4 scale of effectiveness according
to patients, ractopamine scored 3, 3, 1 and 0 in these patients. It
was stated that ractopamine as an aerosol has failed to show any
activity at doses up to 6 mg (Shipley, 1960).
Table 3: Results of genotoxicity assays on ractopamine
Concentration of
Test System Test Object Ractopamine Results References
Unscheduled Fischer 344 rat 0.5-1000 µg/ml Negative Williams et al.,
DNA synthesis hepatocytes 1984b
in vitro
Ames test (1) S. typhimurium 50-5000 µg/ml Negative Williams et al.,
TA1535, TA1537, 1984c
TA1538, TA98,
TA100
Ames test (1) S. typhimurium 0.1-1000 µg/ml Negative Williams &
G46, TA1535, Thompson,
TA100, C3076, 1984
TA1537, D3052,
TA1338, TA98
E. coli
WP2, WP2uvrA-
Mouse lymphoma cell L5178Y mouse 10-350 µg/ml Negative Williams et al.,
thymidine kinase 1984d
100-700 µg/ml
Sister chromatid Chinese hamsters 200-500 mg/kg Negative Williams et al.,
exchange in bone 1985j
marrow of hamsters
in vivo
(1) With and without rat liver S-9 fraction.
3. COMMENTS
Results of various studies were reviewed by the Committee,
including pharmacokinetic, biotransformation, acute and short-term
toxicity, reproductive, teratogenicity, and genotoxicity studies and
a limited number of studies in humans.
Studies with 14C-ractopamine in several species have
indicated rapid absorption following oral administration. In pigs,
labelled ractopamine was excreted almost quantitatively;
approximately 88% was recovered in urine and 9% in faeces during a
7-day period. Studies in pigs, rats, and dogs fed 14C-ractopamine
showed three major metabolites, identified as monoglucuronides of
ractopamine.
In acute studies, ractopamine was substantially more toxic
orally to the rat (LD50 approximately 450 mg/kg bw) than the mouse
(LD50 approximately 3000 mg/kg bw).
The genotoxic potential of ractopamine was evaluated in a
limited series of in vitro and in vivo studies, all of which
were reported to be negative. However, in the absence of a
carcinogenicity study, and as human exposure is likely to be
extensive, the Committee concluded that additional genotoxicity
testing would be desirable.
The short-term toxicity of ractopamine has been evaluated in
mice, rats, dogs, and monkeys. Ractopamine was fed to B6C3F1
mice for 3 months at doses of 25, 175, or 1250 mg/kg bw/day. The
most significant effect noted was a dose-dependent decrease, both
absolute and relative, in testicular weights. In both males and
females in the highest-dose group, absolute and relative heart
weights were increased. However, no histopathological changes were
observed in either the heart or the testes. A clear NOEL could not
be established in this study.
Fischer 344 rats were fed doses up to approximately 155 mg/kg
bw/day for 3 months. The highest-dose group showed decreased
body-weight gain, increased food consumption, decreased efficiency
of food utilization, and an increase in serum potassium
concentration. There was a decrease in uterine weight in rats of
this group and a slight reduction in spleen weight in the top two
groups. The NOEL was 1.3 mg/kg bw/day in this study.
Beagle dogs were given three doses daily, 6 h apart, totalling
0.112, 0.224 or 5.68 mg/kg bw/day of ractopamine in gelatin capsules
for 1 year. Treatment-related minor histopathological findings were
limited to the high-dose group and to the liver. The occurrence of
mild nocturnal bradycardia, most prominent during the first
6 months, meant that a clear no-effect level was not observed in
this study. The Committee noted that the quantity of ractopamine
residues consumed in 500 g of meat from animals slaughtered without
a withdrawal period would closely approach an ADI derived from the
lowest dose of 0.112 mg/kg bw/day and a safety factor of 100.
In a further study of cardiovascular effects, rhesus monkeys
were given either vehicle or ractopamine at 0.125 mg/kg bw/day for
90 days by gavage. This dose was 2.5 times the single dose known to
produce tachycardia and peripheral vasodilation in the dog. In
addition, the selected dose exceeded the total daily dose
(0.112 mg/kg bw/day) associated with nocturnal bradycardia in the
1-year dog study. The NOEL for this study was 0.125 mg/kg bw/day.
Rhesus monkeys were given ractopamine at doses of 0.25, 0.5, or
4 mg/kg bw/day once daily by gavage for 6 weeks to determine the
doses to be used in a 1-year study. Monkeys given 4 mg/kg bw/day
developed daily tachycardia which was maximal by 30 min agter
dosing, and continued for 16 h. Monkeys in the group did not
demonstrate the significant slowing of the nocturnal heart rates
seen in the other groups. The NOEL for this study was 0.5 mg/kg
bw/day.
The effect of ractopamine on reproductive and developmental
performance in Sprague-Dawley rats was evaluated at dosage levels of
up to 2000 ppm in the diet. Significant effects, which included a
reduction in mean litter size and an increase in the total number of
resorptions, were restricted to the high dose which was also
maternally toxic. The NOEL was 200 ppm, equal to 15 mg/kg bw/day, in
this study. The Committee considered the teratogenicity segment of
this study to be adequate to assess developmental toxicity. A minor
teratogenic response was observed only at the highest dose
(2000 ppm) tested, at which maternal toxicity was also noted.
The bronchodilator and inotropic effects of ractopamine were
evaluated in pilot clinical trials in humans. Four patients showed
little evidence of bronchodilator activity, central nervous system
stimulation, or an increase in pulse rate. Two patients showed a
mild elevation of blood pressure lasting for about 1 hour. An
infusion study showed inotropic and chronotropic enhancement in both
healthy volunteers and heart patients.
4. EVALUATION
The Committee concluded that, on the basis of the short-term
studies available, residues of ractopamine appeared to have little
toxic potential for the consumer. The effects recorded were in the
main those to be expected from a ß-adrenoceptor agonist. It might
therefore be appropriate to assess ractopamine on the basis of a
NOEL for pharmacological effects that are relevant to its ingestion
by humans as a residue in edible meats. However, because such a NOEL
could not be determined in the 1-year study in dogs, the Committee
was unable to establish an ADI.
The Committee noted that: (a) some ß-adrenoceptor agonists were
carcinogenic; (b) no long-term studies had been conducted in
rodents; and (c) there were no data relating to the long-term
exposure of humans to ractopamine. Therefore, before reviewing the
compound again, the Committee would wish to see evidence and
arguments in at least the following areas:
1. Genotoxicity
- A further in vivo study such as a micronucleus test.
2. Pharmacology
- investigations that fully explore the pharmacological
properties of ractopamine;
- the relative contributions of ß1- and ß2-adrenoceptor
activation to the spectrum of effects produced by
ractopamine;
- a sufficient basis from which to establish the most
sensitive indicator (test and species) of the
pharmacological effects of ractopamine;
- validation of the utility of this indicator in the setting
of a pharmacological NOEL for humans;
- a survey of the pharmacokinetic parameters of
ß-adrenoceptor agonists in humans and laboratory species,
including those relevant to oral administration;
- determination of appropriate timing for observations in
animal studies to reveal both the onset and the peak
values of all relevant effects.
3. Human data
- A survey of all non-therapeutic effects that follow
long-term ß-adrenoceptor agonist use in humans, to assist
in the prediction of the consequences of the long-term
intake of residues of ractopamine by consumers of animal
meat.
Depending on the results of the above investigations, it may be
necessary to perform other studies to explore further the potential
carcinogenicity of ractopamine.
5. REFERENCES
DALIDOWICZ, J.D. (1986a). Metabolism of 14C-ractopamine HCl in the
rat. Unpublished Report No. ABC-0285 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1986b). Metabolism of 14C-ractopamine HCl in the
dog. Unpublished Report No. ABC-0301 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. & BABBITT, G.E. (1986). Characterization of
14C residues in tissues and excreta from swine fed
14C-ractopamine HCl. Unpublished Report No. ABC-0355 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.E., THOMSON, T.D. & HERBERG, R.J. (1986).
14C-ractopamine HCl balance - excretion study in swine.
Unpublished Report No. ABC-0330 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
DALIDOWICZ, J.D. (1987). Comparative metabolism of 14C-ractopamine
HCl in swine, dogs, and rats. Unpublished Report No. ABC-0369 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
SHIPLEY, R.E. (1960). Summary for research project committee May 31,
1960. Unpublished memoranda from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & THOMPSON, C.Z. (1984). The effect of compound
31537, EL-737 on the induction of bacterial mutation using a
modification of the Ames test. Unpublished Report No. 840507GPA2000
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & MARKEY, T.F. (1984a). The acute
dermal, ocular, and inhalation toxicity of compound 31537 (EL-737).
Unpublished Reports Nos. B-D-82-84, B-E-108-84 and R-H-47-84 from
Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., HILL, L.E. & PROBST, G.S. (1984b). The effect of
compound 31537, EL-737, on the induction of DNA repair synthesis in
primary cultures of adult rat hepatocytes. Unpublished Reports Nos.
840503UDS2000 and 840508UDS2000 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., REXROAT, M.A. & PROBST, G.S. (1984c). The effect of
EL-737 (compound 31537) the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished Report No.
840716AMS2000 from Lilly Research Laboratories, Division of Eli
Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., OBERLY, T.J., BEWSEY, B.J. & PROBST, G.S. (1984d).
The effect of 31537 (EL-737) on the induction of forward mutation at
the thymidine kinase locus of L517Y mouse lymphoma cells.
Unpublished Report No. 840627MLA2000 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield,
Indiana, USA. Submitted to WHO by Elanco Products Company, Division
of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., BRIDGE, T.L. (1985a). The acute
toxicity of compound 31537 (EL-737) administered orally to the ICR
mouse. Unpublished Reports Nos. M-0-178-84 and M-0-179-84 from Lilly
Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985b). The acute
toxicity of compound 31537 (EL-737) administered orally to the
Fischer 344 rat. Unpublished Report No. R-0-132-84 and R-0-133-84
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985c). The acute
toxicity of compound 31537 (EL-737) administered intraperitoneally
to the Fischer 344 rat. Unpublished Reports Nos. R-P-08-84 and
R-P-09-84 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985d). A three-month
toxicity study of ractopamine hydrochloride fed in the diet to
B6C3F1 mice. Unpublished Report No. M01584 from Lilly Research
Laboratories, Division of Eli Lilly and Company Greenfield, Indiana,
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985e). A three-month
toxicity study of 031537 (EL-737) administered orally to Fischer 344
rats. Unpublished Report No. R06184 from Lilly Research
Laboratories, Division of Eli Lilly and Company, Greenfield, Indiana
USA. Submitted to WHO by Elanco Products Company, Division of Eli
Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985f). 8-Day
inhalation study in rhesus monkey. Unpublished Report No. P06288
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Lilly Corporate Center, Indianapolis, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R. & BRIDGE, T.L. (1985g). 18-Day
inhalation study in rhesus monkey. Unpublished Report No. P00388
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985h). An interim
summary of a 6-week subchronic toxicity study of ractopamine
hydrochloride administered by nasogastric gavage to rhesus monkeys.
Unpublished Report No. P00691 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Lilly Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly,
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., McKINLEY, E.R., & BRIDGE, T.L. (1985i). The effect
of subchronic administration of ractopamine hydrochloride on heart
rate and electrocardiographic waveforms in conscious rhesus monkeys.
Unpublished Report No. P02186 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., NEAL, S.B. & PROBST, G.S. (1985j). The effect of
compound 31537 (EL-737) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters. Unpublished
Report No. 850121SCE2000 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., NEGILSKI, D.S. & BRIDGE, T.L., MARKEY, T.F. (1985k).
The acute oral, dermal, ocular and inhalation toxicity of a granular
premix formulation (AFN-026) containing 10% compound 31537 (EL-737).
Unpublished Reports Nos. R-0-188-85, B-D-104-85, B-E-120-85, and
R-H-066-85 from Lilly Research Laboratories, Division of Eli Lilly
and Company, Greenfield, Indiana, USA. Submitted to WHO by Elanco
Products Company, Division of Eli Lilly and Company, Indianapolis,
IN, USA.
WILLIAMS, G.D., HOYT, J.A. (1986). An eleven-month two-generation
reproduction study, including a teratology segment, in Cd rats
maintained on diets containing ractopoamine hydrocholoride (EL-739,
Compound 31537). Unpublished report for study Nos. R11385 and R18985
from Lilly Research Laboratories, Division of Eli Lilly and Company,
Greenfield, Indiana, USA. Submitted to WHO by Elanco Products
Company, Division of Eli Lilly and Company, Indianapolis, IN, USA.
WILLIAMS, G.D., (1987a). Comparative bioavailability of
14C-ractopamine hydrochloride (031537, EL-737) following a single
oral dose of 0.125 mg/kg in the dog and monkey. Unpublished Reports
Nos. D04686 and P03086 from Lilly Research Laboratories, Division of
Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO by
Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
WILLIAMS, G.D., (1987b). A chronic toxicity study of ractopamine
hydrochloride administered orally to beagle dogs for one year.
Unpublished Report No. D05885 from Lilly Research Laboratories,
Division of Eli Lilly and Company, Greenfield, Indiana, USA.
Submitted to WHO by Elanco Products Company, Division of Eli Lilly
and Company, Indianapolis, IN, USA.
WILLIAMS, G.D. & POHLAND, R.C. & BYRD, T.K. (1987). Bioavailability
of radiocarbon following the administration of single oral doses of
14C-EL-737 (31537) to F344/N Hsd BR rats. Unpublished Reports Nos.
R03985, RO4085 and RO4185 from Lilly Research Laboratories, Division
of Eli Lilly and Company, Greenfield, Indiana, USA. Submitted to WHO
by Elanco Products Company, Division of Eli Lilly and Company,
Indianapolis, IN, USA.
