
LEVAMISOLE
First draft prepared by
Dr G. Roberts
Environmental Health Branch
Department of Health, Housing and Community Services
Canberra, Australia
1. EXPLANATION
Levamisole has a long history of use as a broad spectrum
anthelmintic in animals. It is used in human medicine as an
anthelmintic and immunomodulator.
Levamisole had been previously evaluated at the thirty-sixth
Meeting of the Committee (Annex 1, reference 91). A temporary ADI
of 0-0.003 mg/kg bw was established based on a NOEL of 1.25 mg/kg
bw/day for hemolysis in dogs and a safety factor of 500.
The Committee requested information which addressed the
incidences of hematological effects in humans, the results of
studies that demonstrate the mechanism of production of hemolytic
anaemia in dogs and neutropenia in humans, and a comparison of the
metabolites of levamisole in humans, laboratory animals and
food-producing animals. The additional information is summarized
and discussed in the following addendum.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption and excretion
2.1.1.1 Dogs
Dogs were given single doses of 10 mg/kg bw levamisole by
either i.v. or oral (tablet) administration. The measurement of
pharmacokinetic parameters revealed that an oral dose was
moderately well absorbed, 64% in fasted animals and 44% in fed
animals. Excretion was relatively rapid with terminal half-lives of
elimination on the order of 1.3 to 1.8 hours (Watson et al.,
1988).
2.1.1.2 Humans
In an open three-way randomized cross-over trial, 3 male and
3 female volunteers were administered single oral doses of 1, 10,
or 50 mg levamisole as a 5 mg/ml solution. Peak plasma levels were
proportional to the dose and were achieved about 1 hour after
dosing; mean levels were 25.5 ± 8.8 ng/ml and 119 ± 42 ng/ml for
the 10 and 50 mg doses, respectively. With the 1 mg dose,
levamisole was detected at a concentration of 5.2 ng/ml, 1 hour
after drug administration, in one subject only. The detection limit
was 5 ng/ml. By extrapolation, it was estimated that, at the 1 mg
dose peak, drug levels would be approximately 2 ng/ml (Van Peer et
al., 1993).
A review was undertaken of the human pharmacokinetic data for
levamisole available up to January 1990. In summary, following
single oral doses of 3H-levamisole, peak plasma levels of
radioactivity and unchanged drug were reached approximately 2 hours
after dosing at which time 60% of the radioactivity was in the form
of metabolites. Over a range of 50 to 150 mg levamisole, systemic
exposure was proportional to dose. Levamisole was 51% protein-bound
in plasma. Levamisole was eliminated from plasma with a half-life
of 4 hours. For the radiolabel the half-life was 16 hours. About
70% of the administered radioactivity was excreted in urine in 3
days. About 4% of the dose was recovered in faeces. Biliary
excretion was not considered important (Heykants et al., 1990).
2.1.2 Biotransformation
2.1.2.1 In vitro
Microsomal fractions were extracted from the livers of dogs,
pigs, sheep, cattle, and humans. 14C-Levamisole was incubated
with microsomes at 37 °C for 2 hours and the metabolites were
analyzed by HPLC. Over the concentration range of 1 to 500 µM
levamisole, the rate of metabolism was slow with human, pig, and
cattle microsomes. At equivalent concentrations, the rate of
metabolism was at least 10-fold greater with dog and sheep
microsomes. Saturation of metabolism was not reached at the
concentrations used. The metabolite fractions were
co-chromatographed with mixtures of reference compounds, but no
attempt was made to further charact-erize the metabolites. The
results were suggestive of similar metabolic pathways in each
species, at least in a qualitative sense, apart from the presence
of an additional two polar metabolites with dog and sheep
microsomes. The post-microsomal supernatant did not produce
significant metabolism (Lavrijsen et al., 1993a).
Isolated hepatocytes from dogs, pigs, sheep, cattle, and
humans were incubated with 14C-levamisole; suspension cultures
were exposed for 2 hours at 37 °C and primary cell cultures were
exposed for up to 72 hours at 37 °C. Analysis for metabolites was
by HPLC with further characterization by co-chromatography with
reference compounds. Metabolism of levamisole was more extensive in
suspension cultures than in primary cell cultures; the rate of
metabolism was of the following order: dog >> sheep > pig >
cattle > human.
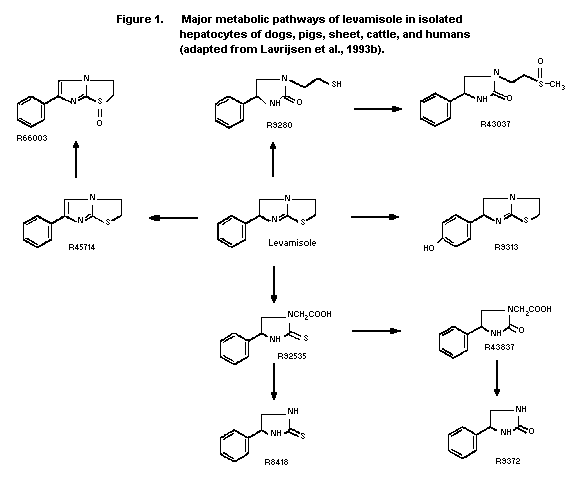
The major in vitro metabolites of levamisole in hepatocytes
of dogs, pigs, sheep, cattle, and humans are shown in Figure 1. The
major pathways seen in all species included scission of the
thiazolidine ring followed by aliphatic oxidation (R92535) and
hydrolysis of the thiazolidine ring followed by S-methylation and
sulphoxidation (R43037). The other major pathway in dogs, pigs,
sheep, and cattle was dehydrogenation in the imidazolidine ring
followed by sulphoxidation (R66003). With human hepatocytes, other
major metabolites were a result of dehydrogenation in the
imidazolidine ring (R45714) and aromatic hydroxylation (R9313).
Apart from the latter formation of p-hydroxy levamisole, the major
metabolic pathways in humans were also observed in other species
in vitro. Large numbers of minor metabolites were noted in each
species but remain unidentified. Approximately 10% to 30% of the
radioactivity in dog, pig, sheep, and cattle hepatocytes was
non-extractable (3.6 to 10% of the incubated dose). Human
hepatocytes were not tested in this study (Lavrijsen et al.,
1993b).
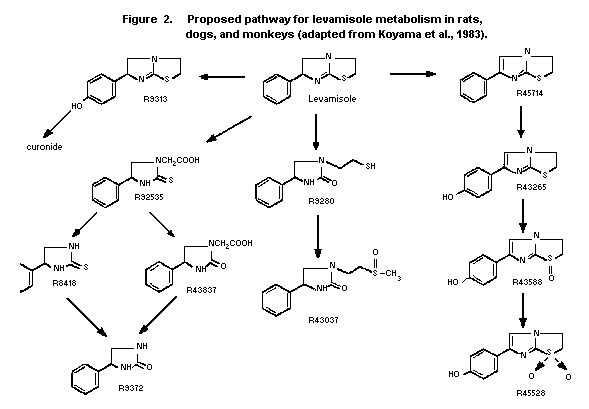
2.1.2.2 Rats
Wistar rats were given single oral doses of 20 mg/kg bw of
14C-levamisole and the urinary radiolabel was analyzed by HPLC.
The main metabolites were R92535 (20% of urinary radioactivity),
parent drug (16%) and R9313 and its glucuronide conjugate (13%).
There were lower levels of 10 other metabolites. The proposed
pathways of metabolism are outlined in Figure 2 (Koyama et al.,
1983).
2.1.2.3 Dogs
Beagle dogs were given single oral doses of 20 mg/kg bw of
14C-levamisole and the urinary radiolabel was analyzed by HPLC.
The major urinary product was parent drug (24% of urinary
radioactivity). Other major metabolites were R92535 (13%), R43037
(10%) and R9313 and its glucuronide conjugate (11%). Another 9
metabolites were identified at lower levels. The proposed metabolic
pathways are shown in Figure 2 (Koyama et al., 1983).
2.1.2.4 Monkeys
Crab-eating monkeys were administered single oral doses of 20
mg/kg bw of 14C-levamisole and the urinary radiolabel was
analyzed by HPLC. The greater part of the urinary radioactivity was
in the form of R92535 (62%). Other major metabolites were parent
drug (12%), R43837 (7%) and R9313 and its glucuronide conjugate
(7%). Lower levels of 9 other metabolites were detected. The
proposed metabolic pathways are shown in Figure 2 (Koyama et al.,
1983).
2.1.2.5 Humans
A review of the published literature on human pharmacokinetics
indicated that levamisole was extensively metabolized in humans,
with only 4.5% excreted unchanged. The p-hydroxy levamisole
metabolite (R9313) and its glucuronide conjugate accounted for up
to 17% of an administered dose. However, other metabolites had not
been identified (Heykants et al., 1990).
2.2 Toxicological studies
2.2.1 Special studies on haematological effects
2.2.1.1 Dogs
A group of 3 male and 5 female beagle dogs was given
levamisole in gelatin capsules for varying intervals during an
18-month study period. A complex dosing schedule was used; in
 general, dogs received initial doses of 20 mg/kg bw/day for 8 to 14
weeks followed by a treatment-free period of 2 to 7 weeks. The
animals were then challenged with doses of 2.5, 5, 10, or 20 mg/kg
bw/day. Overt signs of toxicity and haematological parameters were
reported.
Dosing with 20 mg/kg bw/day resulted in vomiting in all dogs.
Salivation was noted in most animals given 5, 10, or 20 mg/kg
bw/day. There was no obvious effect on body weight at any dose.
During the initial dosing period, 6 dogs exhibited haematological
changes, which necessitated cessation of dosing after 8 weeks.
There were decreases in white blood cells and thrombocytes in 3
animals and decreases in erythrocytes, haemoglobin, haematocrit and
thrombocytes in the other 3. Recovery of the haematological indices
was evident after 2 to 4 weeks of drug withdrawal.
In dogs showing leucopenia, challenge doses of 20, 10, 5, or
2.5 mg/kg bw/day for varying periods did not elicit further
haematological alterations in 2 of the 3 dogs. The other animal
died after 3 weeks of challenge with 20 mg/kg bw/day; gross
pathology of this dog was unremarkable and death was attributed to
leucopenia and thrombocytopenia.
In dogs showing haemolytic anaemia, challenge doses of 20, 10,
5, or 2.5 mg/kg bw resulted in the re-emergence of anaemia in all
3 animals. This sequence of anaemia during treatment and recovery
during drug-free periods was demonstrated for an overall duration
of approximately 18 months (Verstraeten et al., 1993a).
A group of 50 male and 50 female beagle dogs was given
levamisole in gelatin capsules for varying periods during a
14-month study. In many cases the presence of serious
haematological effects necessitated early cessation of dosing.
Overt signs of toxicity were recorded, and haematological and blood
biochemistry parameters and plasma drug levels were measured at
various times.
During the initial 14-week treatment period, 25 dogs showed
marked falls in erythrocytes, haemoglobin and haematocrit and 3
others showed slight falls. Of these animals, 18 also developed
reduced numbers of platelets and 7 showed reduced white blood
cells. The 25 dogs which demonstrated a marked response were
challenged according to the scheme in Table 1. The 75
"non-responding" dogs were no longer dosed.
There were a total of 7 deaths - 4 during interval I, 2 during
interval III and 1 during interval VI. The cause of death was
assumed to be related to abrupt decreases in haematology
parameters. Following the administration of 20 mg/kg bw/day,
levamisole produced vomiting in all dogs and salivation and
decubitus in some. Red urine was observed in a proportion of
animals when challenged with doses of 2.5 or 20 mg/kg bw/day
levamisole. Body weight fluctuations did not show any relationship
to treatment. There were no treatment-related trends in biochemical
parameters during periods of challenge with 1.25 or 2.5 mg/kg
bw/day levamisole.
A total of 20 of the 25 dogs responded with haemolytic anaemia
when challenged with 20 mg/kg bw/day levamisole, and of these, 16
also showed thrombocytopenia and 4 showed leucopenia. Haemolytic
anaemia was seen in 9 and 5 dogs challenged with 2.5 and 1.25 mg/kg
bw/day levamisole, respectively. Thrombocytopenia was observed in
some of the animals given 2.5 mg/kg bw/day levamisole, while
leucopenia was not produced at these lower challenge doses
(Verstraeten et al., 1993b).
Plasma levels of levamisole were measured in randomly chosen
dogs at various stages in the toxicity study. The results are given
in Table 1. The peak plasma levels increased proportionally with
increasing dose but there was no clear correlation of haemotoxicity
with plasma levels. Only parent drug was analyzed (Monbaliu et al.,
1993).
2.2.2 Special studies on immunological effects
2.2.2.1 Dogs
Three animals from the 18-month study in dogs by Verstraeten
et al., 1993a, reported in section 2.2.1.1 were studied further
to investigate immunological effects. Each dog was given oral doses
of levamisole in capsules at 20 mg/kg bw/day for 2 weeks, no drug
for 2 weeks, then 10 mg/kg bw/day for 2 weeks. Two dogs were
sensitized against levamisole while the other dog did not display
a sensitization response.
Blood samples were collected 1 day and 1 week after the last
dose. Serum was extracted for use in agglutination tests with
normal erythrocytes. The maximum dilutions of serum resulting in
agglutination were 40% (v/v) serum from the unsentisized dog and
2.5% (v/v) sera from the sentisized dogs.
The influence of levamisole and 9 of its metabolites was
tested by combining each separately with normal erythrocytes and
dilutions of serum which did not result in agglutination in earlier
experiments. Agglutination responses were elicited only with the
serum from one of the sensitized dogs. The response was moderate
with levamisole, with lesser responses with metabolites R8418 and
R9280 and a strong response with metabolite R45714. Agglutination
was not significantly influenced using serum from the other two
dogs. (See Figure 1 for metabolite structures).
Table 1. Design of 14-month study in dogs and results of plasma
levamisole analyses
Date Dose Levamisole peak plasma
(date.month.year) (mg/kg/bw/day) level (µg/ml)
03.02.92 -> 14.05.92 20 interval I 2.98-5.90: mean 4.76
in 6 dogs prior to
observation of
haematological effects.
0.019-7.23: mean
3.46 in 17 dogs
showing
haematological toxicity
15.05.92 -> 09.06.92 -
10.06.92 -> 10.07.92 20 interval II not measured
11.07.92 -> 03.08.92 -
04.08.92 -> 28.08.92 20 interval III not measured
29.08.92 -> 16.11.92 -
17.11.92 -> 08.12.92 1.25 interval IV 0.116-0.32: mean
0.174 in 6 dogs,
haemolytic anaemia
in 1 of 6 animals
09.12.92 -> 03.01.93 -
04.01.93 -> 25.01.93 2.5 interval V 0.278-0.638: mean
0.432 in 8 dogs
showing
haemolytic anaemia
16.01.93 -> 08.02.93 -
09.02.93 -> 02.03.93 20 interval VI not measured
Serum from the dog that exhibited an agglutination response
was chromatographed on bovine serum albumin/sepharose gels and an
IgM antibody fraction was purified. The immunoglobulin was
demonstrated to produce a strong agglutination reaction with
metabolite R45714 and a weaker reaction with levamisole (Moeremans
& Bols, 1993a).
Twenty-three sensitized animals from the 14-month study in
dogs by Verstraeten et al., 1993b reported in section 2.2.1.1
were used to investigate immunological parameters. During challenge
with 1.25, 2.5, and 20 mg/kg bw/day levamisole (Intervals IV, V and
VI), blood was collected weekly and 24 hours after the last dose at
each treatment level. Erythrocytes from treated dogs were incubated
with specific antisera, anti-dog IgG, anti-dog IgM, anti-dog C3 and
anti-dog C3c, for the determination of antibodies or complement on
cell surfaces.
During treatment with 1.25 mg/kg bw/day levamisole, anaemia
was observed in 5 of the 23 dogs but none of the serological tests
was positive. In 3 other dogs, IgG was consistently detected on
erythrocytes while IgM and complement were not apparent.
In dogs administered 2.5 mg/kg bw/day levamisole, anaemia was
seen in 9 of the 23 dogs and the presence of IgM and complement on
erythrocytes was demonstrated for each animal. Additionally, a
number of animals not exhibiting anaemia were shown to have IgM or
complement on their red blood cells. A total of 6 dogs showed IgG,
but correlation with anaemia was poor.
Of the 7 animals challenged with 20 mg/kg bw/day, anaemia was
produced in 5 dogs and 4 of these dogs revealed the presence of
complement on erythrocytes. IgM was not clearly detected in any
animals, while IgG was present on the cells of 2 dogs, one of which
developed anaemia.
In a further investigation, serum was extracted from blood
collected 24 hours after the last dose. Using dilutions of serum
which did not produce agglutination of normal erythrocytes, sera
from 2 of the 23 dogs, incubated in the presence of levamisole, led
to distinct agglutination of red blood cells (Moeremans & Bols,
1993b).
2.3 Observations in humans
2.3.1 Studies on immunological effects
Of 48 patients with rheumatic diseases receiving treatment
with levamisole, 2 developed severe leucopenia. The serum of both
subjects showed the presence of levamisole-dependent
leucocyte-agglutinating antibodies. Leucocytes did not agglutinate
when incubated with either patient's serum in the absence of
levamisole or normal serum in the presence of levamisole. The
results were interpreted as supportive of an immunological basis
for leucocyte-agglutination possibly mediated through the
production of anti-drug antibodies (Rosenthal et al., 1976).
Sera were obtained from 10 severely neutropenic patients who
had been treated with levamisole for breast cancer or rheumatoid
arthritis. When incubated with normal cells, the serum from each
patient showed complement-dependent toxicity to granulocytes, but
only 2 samples were toxic to lymphocytes and none caused leucocyte
agglutination. On withdrawal of drug treatment, neutrophil counts
increased rapidly in concert with reductions in serum
granulo-cytotoxicity titres. Sera from 10 levamisole-treated
patients who did not develop neutropenia tested negative for
granulocytotoxicity. Analysis of the sera from 3 patients, with
specific antisera, revealed the presence of IgM but not IgG
antibodies. However, there was no evidence for the presence of
anti-levamisole antibodies in the serum of neutropenic patients
(Thompson et al., 1980).
A group of 98 rheumatoid arthritis patients who had been
treated with levamisole for between 3 months and 7.2 years were
used to investigate the possible mechanism of haematological
toxicity. Agranulocytosis developed in 7 patients, and in each case
complement-dependent granulocytotoxic antibodies could be
demonstrated in their serum. It was stated that such antibodies
were absent in the serum of individuals who did not exhibit
agranulocytosis (Rosenthal, 1982).
2.3.2 Incidences of haematological effects.
Levamisole was registered for human use in 1966, initially as
an anthelmintic agent (150 mg single dose). It has also been found
useful in the treatment of rheumatoid disorders (150 mg/day for 3
consecutive days per fortnight) and in the treatment of Dukes C
colon cancer (150 mg/day for 3 consecutive days per fortnight).
Haemolytic anaemia has not been documented in humans as a
consequence of these uses.
Thrombocytopenia has been reported in 18 patients, 14 during
cancer therapy. The incidence of thrombocytopenia in 36 643 Dukes
C colon cancer patients in the USA, treated with levamisole, was
estimated to be 0.027%.
Leucopenia and agranulocytosis have not been reported
following administration of single doses of levamisole as an
anthelmintic. In US trials on Dukes C colon cancer patients,
incidences of leucopenia and agranulocytosis were 6% and 0.3%,
respectively. In another 46 controlled trials involving 2635
patients with various cancers, the incidence of agranulocytosis was
0.1%. Recent post-marketing data for levamisole therapy in 36 643
Dukes C colon cancer patients revealed an incidence of 0.08% for
agranulocytosis or granulocytopenia (Van Cauteren et al., 1993;
Vervaet, 1993).
3. COMMENTS
Information from a limited number of studies were available
for assessment, including data from pharmacokinetic and metabolism
studies, special studies on haematological and immunological
effects in dogs and results following administration in humans.
The in vitro biotransformation of levamisole was
investigated using hepatocytes and liver microsomes from dogs,
pigs, sheep, cattle and humans. The results indicated qualitatively
similar degradation pathways in each species. Following oral dosing
in animals, similar metabolic pathways were identified in rats,
dogs, monkeys and cattle, confirming previously reviewed studies in
rats. Characterization of human metabolites was limited, but the
available evidence indicates similar pathways to those in other
species. All metabolites identified in cattle were also observed in
dogs and rats, and therefore the toxicological potential of beef
residues may be considered to have been evaluated in laboratory
animal studies.
Pharmacokinetic studies in humans revealed that peak plasma
levels were achieved in 1 to 2 hours after an oral dose, with the
levels being proportional to the given dose. Metabolism of
levamisole was extensive and rapid and metabolites were eliminated
more slowly than the parent drug. Excretion was primarily in the
urine.
Two repeat dose toxicity studies in dogs, which utilized an
induction-challenge dosing regime, confirmed the susceptibility of
this species to the induction of haemolytic anaemia in some of the
levamisole-treated animals. Red cell parameters returned to normal
on cessation of dosing but anaemia quickly returned in most dogs
when treatment was recommenced. Additionally, thrombocytopenia and
leucopenia were induced at incidences lower than for haemolytic
anaemia but with a similar relationship to the treatment schedule.
The levamisole-induced incidence of granulocytopenia in both humans
and dogs was low.
In both repeat toxicity studies in dogs, the haemolytic
anaemia and leucopenia were severe enough to necessitate cessation
of dosing in many animals and resulted in the death of a number of
dogs. The induction dose was 20 mg/kg bw/day but in sensitized
animals challenged with doses of 1.25 mg/kg bw/day and above a
dose-related re-emergence of haemolytic anaemia occurred.
Nonetheless, a previous study in dogs showed no haematological
toxicity at a dose of 1.25 mg/kg bw/day given continuously for a
period of one year.
In one of the repeat toxicity studies, plasma levels of
levamisole were measured in randomly chosen dogs. Results showed an
increase in levamisole in proportion to the dose, but there was no
clear correlation of haematological toxicity with the plasma drug
level. However, the metabolites which may play a role in the
induction of anaemia were not measured.
Various immunological parameters were analyzed in the two
studies in dogs, with a view to investigating the mechanism
underlying the haematological effects. Sera obtained from dogs
which were sensitized to levamisole caused the agglutination of
erythrocytes from an untreated dog. The agglutination response was
enhanced in the presence of levamisole or some of its metabolites,
but only in 3 of 24 animals studied. Erythrocytes isolated from
some sensitized animals that were challenged with levamisole had
IgM antibodies, IgG antibodies, and/or complement on cell surfaces
during periods of levamisole-induced haemolytic anaemia. However,
IgG antibodies did not correlate well with anaemia in dogs.
Sera from humans treated with levamisole and showing severe
leucopenia or agranulocytosis caused leucocyte-agglutination or
complement-dependent granulocytotoxicity in vitro. The factors
responsible for these effects showed a high correlation with
haematological toxicity, while sera from patients not developing
agranulocytosis were not toxic to normal white blood cells.
Analysis of sera from a limited number of individuals revealed the
presence of IgM, but not IgG, antibodies. Leucocyte agglutination
was dependent on the presence of levamisole, but
granulocytotoxicity showed no such reliance.
Although the primary target cells in humans and dogs are
generally different, there is now evidence supporting an
immunological basis for the haematological toxicity observed in
both species. The available evidence implicates the involvement of
IgM antibodies and a dependence on complement in the mechanism of
cellular destruction. There is also limited evidence that
agglutination responses in humans and dogs are mediated through
anti-drug antibodies, possibly induced by immunogenic complexes
between levamisole and protein, to which the drug is known to bind.
The reasons for the differential cell sensitivity in humans and
dogs are not known; however, the similarities in aetiology and the
recent demonstration of leucopenia in dogs suggest that dogs are a
suitable model for the haematological toxicity of levamisole in
humans.
4. EVALUATION
The Committee noted that the further studies reviewed at the
present meeting provided information on the incidence and
mechanisms of the haematological effects in humans and dogs. The
Committee recognized that while continuous dosing of dogs with 1.25
mg/kg bw/day levamisole did not result in haemolytic anaemia, this
dose did cause the re-emergence of haemolytic anaemia in a number
of dogs previously sensitized with 20 mg/kg bw/day of levamisole.
Since there is a very small population of humans who are sensitized
to levamisole following therapeutic exposure, the Committee
concluded that the use of a safety factor of 200 would be
appropriate. On this basis, an ADI of 0-6 µg/kg bw was established.
5. REFERENCES
HEYKANTS, J., MEULDERMANS, W. & VAN PEER, A. (1990). Levamisole
pharmacokinetics in healthy volunteers and in cancer patients. A
review of the data available until January 1990. Unpublished Report
Serial No. R12564/117 from Janssen Research Foundation. Submitted
to WHO by Janssen Pharmaceutica, Beerse, Belgium.
KOYAMA, K., OISHI, T., ISHII, A. & DEGUCHI, T. (1983). Metabolic
fate of levamisole in rats, dogs and monkeys. Oyo Yakuri, 26:
869-876.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993a). The in
vitro metabolism of levamisole in subcellular liver fractions of
dogs, pigs, sheep, cattle and humans. Unpublished Report No.
R12564/FK1266 from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993b). The in
vitro metabolism of levamisole in hepatocytes of dogs, pigs,
sheep, cattle and humans. Unpublished Report No. R12564/FK1122 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993a). Evidence for the formation of
hapten antibodies in dogs after prolonged treatment with
levamisole. Unpublished Preclinical Research Report from Janssen
Research Foundation. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993b). In vitro study of
levamisole-induced hemolytic anaemia in dog. Unpublished
Preclinical Research Report from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
MONBALIU, J., VAN LEEMPUT, L. & HEYKANTS, J. (1993). Toxicokinetics
of levamisole in beagle dogs at the occasion of a fifteen-month
mechanistic toxicity study (Exp no. 2692) of levamisole
hydrochloride (R12564) at 1.25, 2.5 and 20 mg/kg/day. Unpublished
Report No. R12564/FK1317 from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M. (1982). Granulocytotoxic antibodies in
levamisole-induced agranulocytosis. Klinische Wochenschrift
60:1297-1302. English translation submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M., TRABERT, U. & MULLER, W. (1976). Leucocytotoxic
effect of levamisole. Lancet, I: 369.
THOMPSON, J.S., HERBICK, J.M., KLASSEN, L.W., SEVERSON, C.D.,
OVERLIN, V.L., BLASCHKE, J.W., SILVERMAN, M.A. & VOGEL, C.L.
(1980). Studies on levamisole induced agranulocytosis. Blood,
56: 114-122.
VAN CAUTEREN, H., LAMPO, A., COUSSEMENT, W., VAN LEEMPUT, L. &
MAES, L. (1993). Levamisole: Comprehensive answer to the questions
raised by the Codex Alimentarius Committee (JECFA). Unpublished
Report from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
VAN PEER, A., NICHOLLS, A., VAN DE POEL, S., WOESTENBORGHS, R., VAN
LEEMPUT, L. & HEYKANTS, J. (1993). Phase-I trial: Systemic
absorption of levamisole after single oral doses of 1, 10 and 50 mg
to healthy volunteers. Unpublished Report Trial No. LVM-GBR-2 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
VERSTRAETEN, A., LAMPO, A., COUSSEMENT, W. & VAN CAUTEREN, H.
(1993a). Mechanistic toxicity study in beagle dogs with levamisole.
Unpublished Report Experiment No. 2255 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., LAMPO, A., COUSSEMENT, W. & VAN
CAUTEREN, H. (1993b). Mechanistic toxicity study in beagle dogs.
Unpublished Report Experiment No. 2692 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERVAET, P. (1993). Statement on the observation of hemolytic
anaemia, thrombocytopenia and leucopenia/agranulocytosis during
treatment with levamisole. Unpublished Report from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
WATSON, A.D.J., SANGSTER, N.C., CHURCH, D.B. & VAN GOGH, H. (1988).
Levamisole pharmacokinetics and bioavailability in dogs. Res. Vet.
Sci., 45: 411-413.
general, dogs received initial doses of 20 mg/kg bw/day for 8 to 14
weeks followed by a treatment-free period of 2 to 7 weeks. The
animals were then challenged with doses of 2.5, 5, 10, or 20 mg/kg
bw/day. Overt signs of toxicity and haematological parameters were
reported.
Dosing with 20 mg/kg bw/day resulted in vomiting in all dogs.
Salivation was noted in most animals given 5, 10, or 20 mg/kg
bw/day. There was no obvious effect on body weight at any dose.
During the initial dosing period, 6 dogs exhibited haematological
changes, which necessitated cessation of dosing after 8 weeks.
There were decreases in white blood cells and thrombocytes in 3
animals and decreases in erythrocytes, haemoglobin, haematocrit and
thrombocytes in the other 3. Recovery of the haematological indices
was evident after 2 to 4 weeks of drug withdrawal.
In dogs showing leucopenia, challenge doses of 20, 10, 5, or
2.5 mg/kg bw/day for varying periods did not elicit further
haematological alterations in 2 of the 3 dogs. The other animal
died after 3 weeks of challenge with 20 mg/kg bw/day; gross
pathology of this dog was unremarkable and death was attributed to
leucopenia and thrombocytopenia.
In dogs showing haemolytic anaemia, challenge doses of 20, 10,
5, or 2.5 mg/kg bw resulted in the re-emergence of anaemia in all
3 animals. This sequence of anaemia during treatment and recovery
during drug-free periods was demonstrated for an overall duration
of approximately 18 months (Verstraeten et al., 1993a).
A group of 50 male and 50 female beagle dogs was given
levamisole in gelatin capsules for varying periods during a
14-month study. In many cases the presence of serious
haematological effects necessitated early cessation of dosing.
Overt signs of toxicity were recorded, and haematological and blood
biochemistry parameters and plasma drug levels were measured at
various times.
During the initial 14-week treatment period, 25 dogs showed
marked falls in erythrocytes, haemoglobin and haematocrit and 3
others showed slight falls. Of these animals, 18 also developed
reduced numbers of platelets and 7 showed reduced white blood
cells. The 25 dogs which demonstrated a marked response were
challenged according to the scheme in Table 1. The 75
"non-responding" dogs were no longer dosed.
There were a total of 7 deaths - 4 during interval I, 2 during
interval III and 1 during interval VI. The cause of death was
assumed to be related to abrupt decreases in haematology
parameters. Following the administration of 20 mg/kg bw/day,
levamisole produced vomiting in all dogs and salivation and
decubitus in some. Red urine was observed in a proportion of
animals when challenged with doses of 2.5 or 20 mg/kg bw/day
levamisole. Body weight fluctuations did not show any relationship
to treatment. There were no treatment-related trends in biochemical
parameters during periods of challenge with 1.25 or 2.5 mg/kg
bw/day levamisole.
A total of 20 of the 25 dogs responded with haemolytic anaemia
when challenged with 20 mg/kg bw/day levamisole, and of these, 16
also showed thrombocytopenia and 4 showed leucopenia. Haemolytic
anaemia was seen in 9 and 5 dogs challenged with 2.5 and 1.25 mg/kg
bw/day levamisole, respectively. Thrombocytopenia was observed in
some of the animals given 2.5 mg/kg bw/day levamisole, while
leucopenia was not produced at these lower challenge doses
(Verstraeten et al., 1993b).
Plasma levels of levamisole were measured in randomly chosen
dogs at various stages in the toxicity study. The results are given
in Table 1. The peak plasma levels increased proportionally with
increasing dose but there was no clear correlation of haemotoxicity
with plasma levels. Only parent drug was analyzed (Monbaliu et al.,
1993).
2.2.2 Special studies on immunological effects
2.2.2.1 Dogs
Three animals from the 18-month study in dogs by Verstraeten
et al., 1993a, reported in section 2.2.1.1 were studied further
to investigate immunological effects. Each dog was given oral doses
of levamisole in capsules at 20 mg/kg bw/day for 2 weeks, no drug
for 2 weeks, then 10 mg/kg bw/day for 2 weeks. Two dogs were
sensitized against levamisole while the other dog did not display
a sensitization response.
Blood samples were collected 1 day and 1 week after the last
dose. Serum was extracted for use in agglutination tests with
normal erythrocytes. The maximum dilutions of serum resulting in
agglutination were 40% (v/v) serum from the unsentisized dog and
2.5% (v/v) sera from the sentisized dogs.
The influence of levamisole and 9 of its metabolites was
tested by combining each separately with normal erythrocytes and
dilutions of serum which did not result in agglutination in earlier
experiments. Agglutination responses were elicited only with the
serum from one of the sensitized dogs. The response was moderate
with levamisole, with lesser responses with metabolites R8418 and
R9280 and a strong response with metabolite R45714. Agglutination
was not significantly influenced using serum from the other two
dogs. (See Figure 1 for metabolite structures).
Table 1. Design of 14-month study in dogs and results of plasma
levamisole analyses
Date Dose Levamisole peak plasma
(date.month.year) (mg/kg/bw/day) level (µg/ml)
03.02.92 -> 14.05.92 20 interval I 2.98-5.90: mean 4.76
in 6 dogs prior to
observation of
haematological effects.
0.019-7.23: mean
3.46 in 17 dogs
showing
haematological toxicity
15.05.92 -> 09.06.92 -
10.06.92 -> 10.07.92 20 interval II not measured
11.07.92 -> 03.08.92 -
04.08.92 -> 28.08.92 20 interval III not measured
29.08.92 -> 16.11.92 -
17.11.92 -> 08.12.92 1.25 interval IV 0.116-0.32: mean
0.174 in 6 dogs,
haemolytic anaemia
in 1 of 6 animals
09.12.92 -> 03.01.93 -
04.01.93 -> 25.01.93 2.5 interval V 0.278-0.638: mean
0.432 in 8 dogs
showing
haemolytic anaemia
16.01.93 -> 08.02.93 -
09.02.93 -> 02.03.93 20 interval VI not measured
Serum from the dog that exhibited an agglutination response
was chromatographed on bovine serum albumin/sepharose gels and an
IgM antibody fraction was purified. The immunoglobulin was
demonstrated to produce a strong agglutination reaction with
metabolite R45714 and a weaker reaction with levamisole (Moeremans
& Bols, 1993a).
Twenty-three sensitized animals from the 14-month study in
dogs by Verstraeten et al., 1993b reported in section 2.2.1.1
were used to investigate immunological parameters. During challenge
with 1.25, 2.5, and 20 mg/kg bw/day levamisole (Intervals IV, V and
VI), blood was collected weekly and 24 hours after the last dose at
each treatment level. Erythrocytes from treated dogs were incubated
with specific antisera, anti-dog IgG, anti-dog IgM, anti-dog C3 and
anti-dog C3c, for the determination of antibodies or complement on
cell surfaces.
During treatment with 1.25 mg/kg bw/day levamisole, anaemia
was observed in 5 of the 23 dogs but none of the serological tests
was positive. In 3 other dogs, IgG was consistently detected on
erythrocytes while IgM and complement were not apparent.
In dogs administered 2.5 mg/kg bw/day levamisole, anaemia was
seen in 9 of the 23 dogs and the presence of IgM and complement on
erythrocytes was demonstrated for each animal. Additionally, a
number of animals not exhibiting anaemia were shown to have IgM or
complement on their red blood cells. A total of 6 dogs showed IgG,
but correlation with anaemia was poor.
Of the 7 animals challenged with 20 mg/kg bw/day, anaemia was
produced in 5 dogs and 4 of these dogs revealed the presence of
complement on erythrocytes. IgM was not clearly detected in any
animals, while IgG was present on the cells of 2 dogs, one of which
developed anaemia.
In a further investigation, serum was extracted from blood
collected 24 hours after the last dose. Using dilutions of serum
which did not produce agglutination of normal erythrocytes, sera
from 2 of the 23 dogs, incubated in the presence of levamisole, led
to distinct agglutination of red blood cells (Moeremans & Bols,
1993b).
2.3 Observations in humans
2.3.1 Studies on immunological effects
Of 48 patients with rheumatic diseases receiving treatment
with levamisole, 2 developed severe leucopenia. The serum of both
subjects showed the presence of levamisole-dependent
leucocyte-agglutinating antibodies. Leucocytes did not agglutinate
when incubated with either patient's serum in the absence of
levamisole or normal serum in the presence of levamisole. The
results were interpreted as supportive of an immunological basis
for leucocyte-agglutination possibly mediated through the
production of anti-drug antibodies (Rosenthal et al., 1976).
Sera were obtained from 10 severely neutropenic patients who
had been treated with levamisole for breast cancer or rheumatoid
arthritis. When incubated with normal cells, the serum from each
patient showed complement-dependent toxicity to granulocytes, but
only 2 samples were toxic to lymphocytes and none caused leucocyte
agglutination. On withdrawal of drug treatment, neutrophil counts
increased rapidly in concert with reductions in serum
granulo-cytotoxicity titres. Sera from 10 levamisole-treated
patients who did not develop neutropenia tested negative for
granulocytotoxicity. Analysis of the sera from 3 patients, with
specific antisera, revealed the presence of IgM but not IgG
antibodies. However, there was no evidence for the presence of
anti-levamisole antibodies in the serum of neutropenic patients
(Thompson et al., 1980).
A group of 98 rheumatoid arthritis patients who had been
treated with levamisole for between 3 months and 7.2 years were
used to investigate the possible mechanism of haematological
toxicity. Agranulocytosis developed in 7 patients, and in each case
complement-dependent granulocytotoxic antibodies could be
demonstrated in their serum. It was stated that such antibodies
were absent in the serum of individuals who did not exhibit
agranulocytosis (Rosenthal, 1982).
2.3.2 Incidences of haematological effects.
Levamisole was registered for human use in 1966, initially as
an anthelmintic agent (150 mg single dose). It has also been found
useful in the treatment of rheumatoid disorders (150 mg/day for 3
consecutive days per fortnight) and in the treatment of Dukes C
colon cancer (150 mg/day for 3 consecutive days per fortnight).
Haemolytic anaemia has not been documented in humans as a
consequence of these uses.
Thrombocytopenia has been reported in 18 patients, 14 during
cancer therapy. The incidence of thrombocytopenia in 36 643 Dukes
C colon cancer patients in the USA, treated with levamisole, was
estimated to be 0.027%.
Leucopenia and agranulocytosis have not been reported
following administration of single doses of levamisole as an
anthelmintic. In US trials on Dukes C colon cancer patients,
incidences of leucopenia and agranulocytosis were 6% and 0.3%,
respectively. In another 46 controlled trials involving 2635
patients with various cancers, the incidence of agranulocytosis was
0.1%. Recent post-marketing data for levamisole therapy in 36 643
Dukes C colon cancer patients revealed an incidence of 0.08% for
agranulocytosis or granulocytopenia (Van Cauteren et al., 1993;
Vervaet, 1993).
3. COMMENTS
Information from a limited number of studies were available
for assessment, including data from pharmacokinetic and metabolism
studies, special studies on haematological and immunological
effects in dogs and results following administration in humans.
The in vitro biotransformation of levamisole was
investigated using hepatocytes and liver microsomes from dogs,
pigs, sheep, cattle and humans. The results indicated qualitatively
similar degradation pathways in each species. Following oral dosing
in animals, similar metabolic pathways were identified in rats,
dogs, monkeys and cattle, confirming previously reviewed studies in
rats. Characterization of human metabolites was limited, but the
available evidence indicates similar pathways to those in other
species. All metabolites identified in cattle were also observed in
dogs and rats, and therefore the toxicological potential of beef
residues may be considered to have been evaluated in laboratory
animal studies.
Pharmacokinetic studies in humans revealed that peak plasma
levels were achieved in 1 to 2 hours after an oral dose, with the
levels being proportional to the given dose. Metabolism of
levamisole was extensive and rapid and metabolites were eliminated
more slowly than the parent drug. Excretion was primarily in the
urine.
Two repeat dose toxicity studies in dogs, which utilized an
induction-challenge dosing regime, confirmed the susceptibility of
this species to the induction of haemolytic anaemia in some of the
levamisole-treated animals. Red cell parameters returned to normal
on cessation of dosing but anaemia quickly returned in most dogs
when treatment was recommenced. Additionally, thrombocytopenia and
leucopenia were induced at incidences lower than for haemolytic
anaemia but with a similar relationship to the treatment schedule.
The levamisole-induced incidence of granulocytopenia in both humans
and dogs was low.
In both repeat toxicity studies in dogs, the haemolytic
anaemia and leucopenia were severe enough to necessitate cessation
of dosing in many animals and resulted in the death of a number of
dogs. The induction dose was 20 mg/kg bw/day but in sensitized
animals challenged with doses of 1.25 mg/kg bw/day and above a
dose-related re-emergence of haemolytic anaemia occurred.
Nonetheless, a previous study in dogs showed no haematological
toxicity at a dose of 1.25 mg/kg bw/day given continuously for a
period of one year.
In one of the repeat toxicity studies, plasma levels of
levamisole were measured in randomly chosen dogs. Results showed an
increase in levamisole in proportion to the dose, but there was no
clear correlation of haematological toxicity with the plasma drug
level. However, the metabolites which may play a role in the
induction of anaemia were not measured.
Various immunological parameters were analyzed in the two
studies in dogs, with a view to investigating the mechanism
underlying the haematological effects. Sera obtained from dogs
which were sensitized to levamisole caused the agglutination of
erythrocytes from an untreated dog. The agglutination response was
enhanced in the presence of levamisole or some of its metabolites,
but only in 3 of 24 animals studied. Erythrocytes isolated from
some sensitized animals that were challenged with levamisole had
IgM antibodies, IgG antibodies, and/or complement on cell surfaces
during periods of levamisole-induced haemolytic anaemia. However,
IgG antibodies did not correlate well with anaemia in dogs.
Sera from humans treated with levamisole and showing severe
leucopenia or agranulocytosis caused leucocyte-agglutination or
complement-dependent granulocytotoxicity in vitro. The factors
responsible for these effects showed a high correlation with
haematological toxicity, while sera from patients not developing
agranulocytosis were not toxic to normal white blood cells.
Analysis of sera from a limited number of individuals revealed the
presence of IgM, but not IgG, antibodies. Leucocyte agglutination
was dependent on the presence of levamisole, but
granulocytotoxicity showed no such reliance.
Although the primary target cells in humans and dogs are
generally different, there is now evidence supporting an
immunological basis for the haematological toxicity observed in
both species. The available evidence implicates the involvement of
IgM antibodies and a dependence on complement in the mechanism of
cellular destruction. There is also limited evidence that
agglutination responses in humans and dogs are mediated through
anti-drug antibodies, possibly induced by immunogenic complexes
between levamisole and protein, to which the drug is known to bind.
The reasons for the differential cell sensitivity in humans and
dogs are not known; however, the similarities in aetiology and the
recent demonstration of leucopenia in dogs suggest that dogs are a
suitable model for the haematological toxicity of levamisole in
humans.
4. EVALUATION
The Committee noted that the further studies reviewed at the
present meeting provided information on the incidence and
mechanisms of the haematological effects in humans and dogs. The
Committee recognized that while continuous dosing of dogs with 1.25
mg/kg bw/day levamisole did not result in haemolytic anaemia, this
dose did cause the re-emergence of haemolytic anaemia in a number
of dogs previously sensitized with 20 mg/kg bw/day of levamisole.
Since there is a very small population of humans who are sensitized
to levamisole following therapeutic exposure, the Committee
concluded that the use of a safety factor of 200 would be
appropriate. On this basis, an ADI of 0-6 µg/kg bw was established.
5. REFERENCES
HEYKANTS, J., MEULDERMANS, W. & VAN PEER, A. (1990). Levamisole
pharmacokinetics in healthy volunteers and in cancer patients. A
review of the data available until January 1990. Unpublished Report
Serial No. R12564/117 from Janssen Research Foundation. Submitted
to WHO by Janssen Pharmaceutica, Beerse, Belgium.
KOYAMA, K., OISHI, T., ISHII, A. & DEGUCHI, T. (1983). Metabolic
fate of levamisole in rats, dogs and monkeys. Oyo Yakuri, 26:
869-876.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993a). The in
vitro metabolism of levamisole in subcellular liver fractions of
dogs, pigs, sheep, cattle and humans. Unpublished Report No.
R12564/FK1266 from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993b). The in
vitro metabolism of levamisole in hepatocytes of dogs, pigs,
sheep, cattle and humans. Unpublished Report No. R12564/FK1122 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993a). Evidence for the formation of
hapten antibodies in dogs after prolonged treatment with
levamisole. Unpublished Preclinical Research Report from Janssen
Research Foundation. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993b). In vitro study of
levamisole-induced hemolytic anaemia in dog. Unpublished
Preclinical Research Report from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
MONBALIU, J., VAN LEEMPUT, L. & HEYKANTS, J. (1993). Toxicokinetics
of levamisole in beagle dogs at the occasion of a fifteen-month
mechanistic toxicity study (Exp no. 2692) of levamisole
hydrochloride (R12564) at 1.25, 2.5 and 20 mg/kg/day. Unpublished
Report No. R12564/FK1317 from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M. (1982). Granulocytotoxic antibodies in
levamisole-induced agranulocytosis. Klinische Wochenschrift
60:1297-1302. English translation submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M., TRABERT, U. & MULLER, W. (1976). Leucocytotoxic
effect of levamisole. Lancet, I: 369.
THOMPSON, J.S., HERBICK, J.M., KLASSEN, L.W., SEVERSON, C.D.,
OVERLIN, V.L., BLASCHKE, J.W., SILVERMAN, M.A. & VOGEL, C.L.
(1980). Studies on levamisole induced agranulocytosis. Blood,
56: 114-122.
VAN CAUTEREN, H., LAMPO, A., COUSSEMENT, W., VAN LEEMPUT, L. &
MAES, L. (1993). Levamisole: Comprehensive answer to the questions
raised by the Codex Alimentarius Committee (JECFA). Unpublished
Report from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
VAN PEER, A., NICHOLLS, A., VAN DE POEL, S., WOESTENBORGHS, R., VAN
LEEMPUT, L. & HEYKANTS, J. (1993). Phase-I trial: Systemic
absorption of levamisole after single oral doses of 1, 10 and 50 mg
to healthy volunteers. Unpublished Report Trial No. LVM-GBR-2 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
VERSTRAETEN, A., LAMPO, A., COUSSEMENT, W. & VAN CAUTEREN, H.
(1993a). Mechanistic toxicity study in beagle dogs with levamisole.
Unpublished Report Experiment No. 2255 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., LAMPO, A., COUSSEMENT, W. & VAN
CAUTEREN, H. (1993b). Mechanistic toxicity study in beagle dogs.
Unpublished Report Experiment No. 2692 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERVAET, P. (1993). Statement on the observation of hemolytic
anaemia, thrombocytopenia and leucopenia/agranulocytosis during
treatment with levamisole. Unpublished Report from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
WATSON, A.D.J., SANGSTER, N.C., CHURCH, D.B. & VAN GOGH, H. (1988).
Levamisole pharmacokinetics and bioavailability in dogs. Res. Vet.
Sci., 45: 411-413.

 general, dogs received initial doses of 20 mg/kg bw/day for 8 to 14
weeks followed by a treatment-free period of 2 to 7 weeks. The
animals were then challenged with doses of 2.5, 5, 10, or 20 mg/kg
bw/day. Overt signs of toxicity and haematological parameters were
reported.
Dosing with 20 mg/kg bw/day resulted in vomiting in all dogs.
Salivation was noted in most animals given 5, 10, or 20 mg/kg
bw/day. There was no obvious effect on body weight at any dose.
During the initial dosing period, 6 dogs exhibited haematological
changes, which necessitated cessation of dosing after 8 weeks.
There were decreases in white blood cells and thrombocytes in 3
animals and decreases in erythrocytes, haemoglobin, haematocrit and
thrombocytes in the other 3. Recovery of the haematological indices
was evident after 2 to 4 weeks of drug withdrawal.
In dogs showing leucopenia, challenge doses of 20, 10, 5, or
2.5 mg/kg bw/day for varying periods did not elicit further
haematological alterations in 2 of the 3 dogs. The other animal
died after 3 weeks of challenge with 20 mg/kg bw/day; gross
pathology of this dog was unremarkable and death was attributed to
leucopenia and thrombocytopenia.
In dogs showing haemolytic anaemia, challenge doses of 20, 10,
5, or 2.5 mg/kg bw resulted in the re-emergence of anaemia in all
3 animals. This sequence of anaemia during treatment and recovery
during drug-free periods was demonstrated for an overall duration
of approximately 18 months (Verstraeten et al., 1993a).
A group of 50 male and 50 female beagle dogs was given
levamisole in gelatin capsules for varying periods during a
14-month study. In many cases the presence of serious
haematological effects necessitated early cessation of dosing.
Overt signs of toxicity were recorded, and haematological and blood
biochemistry parameters and plasma drug levels were measured at
various times.
During the initial 14-week treatment period, 25 dogs showed
marked falls in erythrocytes, haemoglobin and haematocrit and 3
others showed slight falls. Of these animals, 18 also developed
reduced numbers of platelets and 7 showed reduced white blood
cells. The 25 dogs which demonstrated a marked response were
challenged according to the scheme in Table 1. The 75
"non-responding" dogs were no longer dosed.
There were a total of 7 deaths - 4 during interval I, 2 during
interval III and 1 during interval VI. The cause of death was
assumed to be related to abrupt decreases in haematology
parameters. Following the administration of 20 mg/kg bw/day,
levamisole produced vomiting in all dogs and salivation and
decubitus in some. Red urine was observed in a proportion of
animals when challenged with doses of 2.5 or 20 mg/kg bw/day
levamisole. Body weight fluctuations did not show any relationship
to treatment. There were no treatment-related trends in biochemical
parameters during periods of challenge with 1.25 or 2.5 mg/kg
bw/day levamisole.
A total of 20 of the 25 dogs responded with haemolytic anaemia
when challenged with 20 mg/kg bw/day levamisole, and of these, 16
also showed thrombocytopenia and 4 showed leucopenia. Haemolytic
anaemia was seen in 9 and 5 dogs challenged with 2.5 and 1.25 mg/kg
bw/day levamisole, respectively. Thrombocytopenia was observed in
some of the animals given 2.5 mg/kg bw/day levamisole, while
leucopenia was not produced at these lower challenge doses
(Verstraeten et al., 1993b).
Plasma levels of levamisole were measured in randomly chosen
dogs at various stages in the toxicity study. The results are given
in Table 1. The peak plasma levels increased proportionally with
increasing dose but there was no clear correlation of haemotoxicity
with plasma levels. Only parent drug was analyzed (Monbaliu et al.,
1993).
2.2.2 Special studies on immunological effects
2.2.2.1 Dogs
Three animals from the 18-month study in dogs by Verstraeten
et al., 1993a, reported in section 2.2.1.1 were studied further
to investigate immunological effects. Each dog was given oral doses
of levamisole in capsules at 20 mg/kg bw/day for 2 weeks, no drug
for 2 weeks, then 10 mg/kg bw/day for 2 weeks. Two dogs were
sensitized against levamisole while the other dog did not display
a sensitization response.
Blood samples were collected 1 day and 1 week after the last
dose. Serum was extracted for use in agglutination tests with
normal erythrocytes. The maximum dilutions of serum resulting in
agglutination were 40% (v/v) serum from the unsentisized dog and
2.5% (v/v) sera from the sentisized dogs.
The influence of levamisole and 9 of its metabolites was
tested by combining each separately with normal erythrocytes and
dilutions of serum which did not result in agglutination in earlier
experiments. Agglutination responses were elicited only with the
serum from one of the sensitized dogs. The response was moderate
with levamisole, with lesser responses with metabolites R8418 and
R9280 and a strong response with metabolite R45714. Agglutination
was not significantly influenced using serum from the other two
dogs. (See Figure 1 for metabolite structures).
Table 1. Design of 14-month study in dogs and results of plasma
levamisole analyses
Date Dose Levamisole peak plasma
(date.month.year) (mg/kg/bw/day) level (µg/ml)
03.02.92 -> 14.05.92 20 interval I 2.98-5.90: mean 4.76
in 6 dogs prior to
observation of
haematological effects.
0.019-7.23: mean
3.46 in 17 dogs
showing
haematological toxicity
15.05.92 -> 09.06.92 -
10.06.92 -> 10.07.92 20 interval II not measured
11.07.92 -> 03.08.92 -
04.08.92 -> 28.08.92 20 interval III not measured
29.08.92 -> 16.11.92 -
17.11.92 -> 08.12.92 1.25 interval IV 0.116-0.32: mean
0.174 in 6 dogs,
haemolytic anaemia
in 1 of 6 animals
09.12.92 -> 03.01.93 -
04.01.93 -> 25.01.93 2.5 interval V 0.278-0.638: mean
0.432 in 8 dogs
showing
haemolytic anaemia
16.01.93 -> 08.02.93 -
09.02.93 -> 02.03.93 20 interval VI not measured
Serum from the dog that exhibited an agglutination response
was chromatographed on bovine serum albumin/sepharose gels and an
IgM antibody fraction was purified. The immunoglobulin was
demonstrated to produce a strong agglutination reaction with
metabolite R45714 and a weaker reaction with levamisole (Moeremans
& Bols, 1993a).
Twenty-three sensitized animals from the 14-month study in
dogs by Verstraeten et al., 1993b reported in section 2.2.1.1
were used to investigate immunological parameters. During challenge
with 1.25, 2.5, and 20 mg/kg bw/day levamisole (Intervals IV, V and
VI), blood was collected weekly and 24 hours after the last dose at
each treatment level. Erythrocytes from treated dogs were incubated
with specific antisera, anti-dog IgG, anti-dog IgM, anti-dog C3 and
anti-dog C3c, for the determination of antibodies or complement on
cell surfaces.
During treatment with 1.25 mg/kg bw/day levamisole, anaemia
was observed in 5 of the 23 dogs but none of the serological tests
was positive. In 3 other dogs, IgG was consistently detected on
erythrocytes while IgM and complement were not apparent.
In dogs administered 2.5 mg/kg bw/day levamisole, anaemia was
seen in 9 of the 23 dogs and the presence of IgM and complement on
erythrocytes was demonstrated for each animal. Additionally, a
number of animals not exhibiting anaemia were shown to have IgM or
complement on their red blood cells. A total of 6 dogs showed IgG,
but correlation with anaemia was poor.
Of the 7 animals challenged with 20 mg/kg bw/day, anaemia was
produced in 5 dogs and 4 of these dogs revealed the presence of
complement on erythrocytes. IgM was not clearly detected in any
animals, while IgG was present on the cells of 2 dogs, one of which
developed anaemia.
In a further investigation, serum was extracted from blood
collected 24 hours after the last dose. Using dilutions of serum
which did not produce agglutination of normal erythrocytes, sera
from 2 of the 23 dogs, incubated in the presence of levamisole, led
to distinct agglutination of red blood cells (Moeremans & Bols,
1993b).
2.3 Observations in humans
2.3.1 Studies on immunological effects
Of 48 patients with rheumatic diseases receiving treatment
with levamisole, 2 developed severe leucopenia. The serum of both
subjects showed the presence of levamisole-dependent
leucocyte-agglutinating antibodies. Leucocytes did not agglutinate
when incubated with either patient's serum in the absence of
levamisole or normal serum in the presence of levamisole. The
results were interpreted as supportive of an immunological basis
for leucocyte-agglutination possibly mediated through the
production of anti-drug antibodies (Rosenthal et al., 1976).
Sera were obtained from 10 severely neutropenic patients who
had been treated with levamisole for breast cancer or rheumatoid
arthritis. When incubated with normal cells, the serum from each
patient showed complement-dependent toxicity to granulocytes, but
only 2 samples were toxic to lymphocytes and none caused leucocyte
agglutination. On withdrawal of drug treatment, neutrophil counts
increased rapidly in concert with reductions in serum
granulo-cytotoxicity titres. Sera from 10 levamisole-treated
patients who did not develop neutropenia tested negative for
granulocytotoxicity. Analysis of the sera from 3 patients, with
specific antisera, revealed the presence of IgM but not IgG
antibodies. However, there was no evidence for the presence of
anti-levamisole antibodies in the serum of neutropenic patients
(Thompson et al., 1980).
A group of 98 rheumatoid arthritis patients who had been
treated with levamisole for between 3 months and 7.2 years were
used to investigate the possible mechanism of haematological
toxicity. Agranulocytosis developed in 7 patients, and in each case
complement-dependent granulocytotoxic antibodies could be
demonstrated in their serum. It was stated that such antibodies
were absent in the serum of individuals who did not exhibit
agranulocytosis (Rosenthal, 1982).
2.3.2 Incidences of haematological effects.
Levamisole was registered for human use in 1966, initially as
an anthelmintic agent (150 mg single dose). It has also been found
useful in the treatment of rheumatoid disorders (150 mg/day for 3
consecutive days per fortnight) and in the treatment of Dukes C
colon cancer (150 mg/day for 3 consecutive days per fortnight).
Haemolytic anaemia has not been documented in humans as a
consequence of these uses.
Thrombocytopenia has been reported in 18 patients, 14 during
cancer therapy. The incidence of thrombocytopenia in 36 643 Dukes
C colon cancer patients in the USA, treated with levamisole, was
estimated to be 0.027%.
Leucopenia and agranulocytosis have not been reported
following administration of single doses of levamisole as an
anthelmintic. In US trials on Dukes C colon cancer patients,
incidences of leucopenia and agranulocytosis were 6% and 0.3%,
respectively. In another 46 controlled trials involving 2635
patients with various cancers, the incidence of agranulocytosis was
0.1%. Recent post-marketing data for levamisole therapy in 36 643
Dukes C colon cancer patients revealed an incidence of 0.08% for
agranulocytosis or granulocytopenia (Van Cauteren et al., 1993;
Vervaet, 1993).
3. COMMENTS
Information from a limited number of studies were available
for assessment, including data from pharmacokinetic and metabolism
studies, special studies on haematological and immunological
effects in dogs and results following administration in humans.
The in vitro biotransformation of levamisole was
investigated using hepatocytes and liver microsomes from dogs,
pigs, sheep, cattle and humans. The results indicated qualitatively
similar degradation pathways in each species. Following oral dosing
in animals, similar metabolic pathways were identified in rats,
dogs, monkeys and cattle, confirming previously reviewed studies in
rats. Characterization of human metabolites was limited, but the
available evidence indicates similar pathways to those in other
species. All metabolites identified in cattle were also observed in
dogs and rats, and therefore the toxicological potential of beef
residues may be considered to have been evaluated in laboratory
animal studies.
Pharmacokinetic studies in humans revealed that peak plasma
levels were achieved in 1 to 2 hours after an oral dose, with the
levels being proportional to the given dose. Metabolism of
levamisole was extensive and rapid and metabolites were eliminated
more slowly than the parent drug. Excretion was primarily in the
urine.
Two repeat dose toxicity studies in dogs, which utilized an
induction-challenge dosing regime, confirmed the susceptibility of
this species to the induction of haemolytic anaemia in some of the
levamisole-treated animals. Red cell parameters returned to normal
on cessation of dosing but anaemia quickly returned in most dogs
when treatment was recommenced. Additionally, thrombocytopenia and
leucopenia were induced at incidences lower than for haemolytic
anaemia but with a similar relationship to the treatment schedule.
The levamisole-induced incidence of granulocytopenia in both humans
and dogs was low.
In both repeat toxicity studies in dogs, the haemolytic
anaemia and leucopenia were severe enough to necessitate cessation
of dosing in many animals and resulted in the death of a number of
dogs. The induction dose was 20 mg/kg bw/day but in sensitized
animals challenged with doses of 1.25 mg/kg bw/day and above a
dose-related re-emergence of haemolytic anaemia occurred.
Nonetheless, a previous study in dogs showed no haematological
toxicity at a dose of 1.25 mg/kg bw/day given continuously for a
period of one year.
In one of the repeat toxicity studies, plasma levels of
levamisole were measured in randomly chosen dogs. Results showed an
increase in levamisole in proportion to the dose, but there was no
clear correlation of haematological toxicity with the plasma drug
level. However, the metabolites which may play a role in the
induction of anaemia were not measured.
Various immunological parameters were analyzed in the two
studies in dogs, with a view to investigating the mechanism
underlying the haematological effects. Sera obtained from dogs
which were sensitized to levamisole caused the agglutination of
erythrocytes from an untreated dog. The agglutination response was
enhanced in the presence of levamisole or some of its metabolites,
but only in 3 of 24 animals studied. Erythrocytes isolated from
some sensitized animals that were challenged with levamisole had
IgM antibodies, IgG antibodies, and/or complement on cell surfaces
during periods of levamisole-induced haemolytic anaemia. However,
IgG antibodies did not correlate well with anaemia in dogs.
Sera from humans treated with levamisole and showing severe
leucopenia or agranulocytosis caused leucocyte-agglutination or
complement-dependent granulocytotoxicity in vitro. The factors
responsible for these effects showed a high correlation with
haematological toxicity, while sera from patients not developing
agranulocytosis were not toxic to normal white blood cells.
Analysis of sera from a limited number of individuals revealed the
presence of IgM, but not IgG, antibodies. Leucocyte agglutination
was dependent on the presence of levamisole, but
granulocytotoxicity showed no such reliance.
Although the primary target cells in humans and dogs are
generally different, there is now evidence supporting an
immunological basis for the haematological toxicity observed in
both species. The available evidence implicates the involvement of
IgM antibodies and a dependence on complement in the mechanism of
cellular destruction. There is also limited evidence that
agglutination responses in humans and dogs are mediated through
anti-drug antibodies, possibly induced by immunogenic complexes
between levamisole and protein, to which the drug is known to bind.
The reasons for the differential cell sensitivity in humans and
dogs are not known; however, the similarities in aetiology and the
recent demonstration of leucopenia in dogs suggest that dogs are a
suitable model for the haematological toxicity of levamisole in
humans.
4. EVALUATION
The Committee noted that the further studies reviewed at the
present meeting provided information on the incidence and
mechanisms of the haematological effects in humans and dogs. The
Committee recognized that while continuous dosing of dogs with 1.25
mg/kg bw/day levamisole did not result in haemolytic anaemia, this
dose did cause the re-emergence of haemolytic anaemia in a number
of dogs previously sensitized with 20 mg/kg bw/day of levamisole.
Since there is a very small population of humans who are sensitized
to levamisole following therapeutic exposure, the Committee
concluded that the use of a safety factor of 200 would be
appropriate. On this basis, an ADI of 0-6 µg/kg bw was established.
5. REFERENCES
HEYKANTS, J., MEULDERMANS, W. & VAN PEER, A. (1990). Levamisole
pharmacokinetics in healthy volunteers and in cancer patients. A
review of the data available until January 1990. Unpublished Report
Serial No. R12564/117 from Janssen Research Foundation. Submitted
to WHO by Janssen Pharmaceutica, Beerse, Belgium.
KOYAMA, K., OISHI, T., ISHII, A. & DEGUCHI, T. (1983). Metabolic
fate of levamisole in rats, dogs and monkeys. Oyo Yakuri, 26:
869-876.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993a). The in
vitro metabolism of levamisole in subcellular liver fractions of
dogs, pigs, sheep, cattle and humans. Unpublished Report No.
R12564/FK1266 from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993b). The in
vitro metabolism of levamisole in hepatocytes of dogs, pigs,
sheep, cattle and humans. Unpublished Report No. R12564/FK1122 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993a). Evidence for the formation of
hapten antibodies in dogs after prolonged treatment with
levamisole. Unpublished Preclinical Research Report from Janssen
Research Foundation. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993b). In vitro study of
levamisole-induced hemolytic anaemia in dog. Unpublished
Preclinical Research Report from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
MONBALIU, J., VAN LEEMPUT, L. & HEYKANTS, J. (1993). Toxicokinetics
of levamisole in beagle dogs at the occasion of a fifteen-month
mechanistic toxicity study (Exp no. 2692) of levamisole
hydrochloride (R12564) at 1.25, 2.5 and 20 mg/kg/day. Unpublished
Report No. R12564/FK1317 from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M. (1982). Granulocytotoxic antibodies in
levamisole-induced agranulocytosis. Klinische Wochenschrift
60:1297-1302. English translation submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M., TRABERT, U. & MULLER, W. (1976). Leucocytotoxic
effect of levamisole. Lancet, I: 369.
THOMPSON, J.S., HERBICK, J.M., KLASSEN, L.W., SEVERSON, C.D.,
OVERLIN, V.L., BLASCHKE, J.W., SILVERMAN, M.A. & VOGEL, C.L.
(1980). Studies on levamisole induced agranulocytosis. Blood,
56: 114-122.
VAN CAUTEREN, H., LAMPO, A., COUSSEMENT, W., VAN LEEMPUT, L. &
MAES, L. (1993). Levamisole: Comprehensive answer to the questions
raised by the Codex Alimentarius Committee (JECFA). Unpublished
Report from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
VAN PEER, A., NICHOLLS, A., VAN DE POEL, S., WOESTENBORGHS, R., VAN
LEEMPUT, L. & HEYKANTS, J. (1993). Phase-I trial: Systemic
absorption of levamisole after single oral doses of 1, 10 and 50 mg
to healthy volunteers. Unpublished Report Trial No. LVM-GBR-2 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
VERSTRAETEN, A., LAMPO, A., COUSSEMENT, W. & VAN CAUTEREN, H.
(1993a). Mechanistic toxicity study in beagle dogs with levamisole.
Unpublished Report Experiment No. 2255 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., LAMPO, A., COUSSEMENT, W. & VAN
CAUTEREN, H. (1993b). Mechanistic toxicity study in beagle dogs.
Unpublished Report Experiment No. 2692 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERVAET, P. (1993). Statement on the observation of hemolytic
anaemia, thrombocytopenia and leucopenia/agranulocytosis during
treatment with levamisole. Unpublished Report from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
WATSON, A.D.J., SANGSTER, N.C., CHURCH, D.B. & VAN GOGH, H. (1988).
Levamisole pharmacokinetics and bioavailability in dogs. Res. Vet.
Sci., 45: 411-413.
general, dogs received initial doses of 20 mg/kg bw/day for 8 to 14
weeks followed by a treatment-free period of 2 to 7 weeks. The
animals were then challenged with doses of 2.5, 5, 10, or 20 mg/kg
bw/day. Overt signs of toxicity and haematological parameters were
reported.
Dosing with 20 mg/kg bw/day resulted in vomiting in all dogs.
Salivation was noted in most animals given 5, 10, or 20 mg/kg
bw/day. There was no obvious effect on body weight at any dose.
During the initial dosing period, 6 dogs exhibited haematological
changes, which necessitated cessation of dosing after 8 weeks.
There were decreases in white blood cells and thrombocytes in 3
animals and decreases in erythrocytes, haemoglobin, haematocrit and
thrombocytes in the other 3. Recovery of the haematological indices
was evident after 2 to 4 weeks of drug withdrawal.
In dogs showing leucopenia, challenge doses of 20, 10, 5, or
2.5 mg/kg bw/day for varying periods did not elicit further
haematological alterations in 2 of the 3 dogs. The other animal
died after 3 weeks of challenge with 20 mg/kg bw/day; gross
pathology of this dog was unremarkable and death was attributed to
leucopenia and thrombocytopenia.
In dogs showing haemolytic anaemia, challenge doses of 20, 10,
5, or 2.5 mg/kg bw resulted in the re-emergence of anaemia in all
3 animals. This sequence of anaemia during treatment and recovery
during drug-free periods was demonstrated for an overall duration
of approximately 18 months (Verstraeten et al., 1993a).
A group of 50 male and 50 female beagle dogs was given
levamisole in gelatin capsules for varying periods during a
14-month study. In many cases the presence of serious
haematological effects necessitated early cessation of dosing.
Overt signs of toxicity were recorded, and haematological and blood
biochemistry parameters and plasma drug levels were measured at
various times.
During the initial 14-week treatment period, 25 dogs showed
marked falls in erythrocytes, haemoglobin and haematocrit and 3
others showed slight falls. Of these animals, 18 also developed
reduced numbers of platelets and 7 showed reduced white blood
cells. The 25 dogs which demonstrated a marked response were
challenged according to the scheme in Table 1. The 75
"non-responding" dogs were no longer dosed.
There were a total of 7 deaths - 4 during interval I, 2 during
interval III and 1 during interval VI. The cause of death was
assumed to be related to abrupt decreases in haematology
parameters. Following the administration of 20 mg/kg bw/day,
levamisole produced vomiting in all dogs and salivation and
decubitus in some. Red urine was observed in a proportion of
animals when challenged with doses of 2.5 or 20 mg/kg bw/day
levamisole. Body weight fluctuations did not show any relationship
to treatment. There were no treatment-related trends in biochemical
parameters during periods of challenge with 1.25 or 2.5 mg/kg
bw/day levamisole.
A total of 20 of the 25 dogs responded with haemolytic anaemia
when challenged with 20 mg/kg bw/day levamisole, and of these, 16
also showed thrombocytopenia and 4 showed leucopenia. Haemolytic
anaemia was seen in 9 and 5 dogs challenged with 2.5 and 1.25 mg/kg
bw/day levamisole, respectively. Thrombocytopenia was observed in
some of the animals given 2.5 mg/kg bw/day levamisole, while
leucopenia was not produced at these lower challenge doses
(Verstraeten et al., 1993b).
Plasma levels of levamisole were measured in randomly chosen
dogs at various stages in the toxicity study. The results are given
in Table 1. The peak plasma levels increased proportionally with
increasing dose but there was no clear correlation of haemotoxicity
with plasma levels. Only parent drug was analyzed (Monbaliu et al.,
1993).
2.2.2 Special studies on immunological effects
2.2.2.1 Dogs
Three animals from the 18-month study in dogs by Verstraeten
et al., 1993a, reported in section 2.2.1.1 were studied further
to investigate immunological effects. Each dog was given oral doses
of levamisole in capsules at 20 mg/kg bw/day for 2 weeks, no drug
for 2 weeks, then 10 mg/kg bw/day for 2 weeks. Two dogs were
sensitized against levamisole while the other dog did not display
a sensitization response.
Blood samples were collected 1 day and 1 week after the last
dose. Serum was extracted for use in agglutination tests with
normal erythrocytes. The maximum dilutions of serum resulting in
agglutination were 40% (v/v) serum from the unsentisized dog and
2.5% (v/v) sera from the sentisized dogs.
The influence of levamisole and 9 of its metabolites was
tested by combining each separately with normal erythrocytes and
dilutions of serum which did not result in agglutination in earlier
experiments. Agglutination responses were elicited only with the
serum from one of the sensitized dogs. The response was moderate
with levamisole, with lesser responses with metabolites R8418 and
R9280 and a strong response with metabolite R45714. Agglutination
was not significantly influenced using serum from the other two
dogs. (See Figure 1 for metabolite structures).
Table 1. Design of 14-month study in dogs and results of plasma
levamisole analyses
Date Dose Levamisole peak plasma
(date.month.year) (mg/kg/bw/day) level (µg/ml)
03.02.92 -> 14.05.92 20 interval I 2.98-5.90: mean 4.76
in 6 dogs prior to
observation of
haematological effects.
0.019-7.23: mean
3.46 in 17 dogs
showing
haematological toxicity
15.05.92 -> 09.06.92 -
10.06.92 -> 10.07.92 20 interval II not measured
11.07.92 -> 03.08.92 -
04.08.92 -> 28.08.92 20 interval III not measured
29.08.92 -> 16.11.92 -
17.11.92 -> 08.12.92 1.25 interval IV 0.116-0.32: mean
0.174 in 6 dogs,
haemolytic anaemia
in 1 of 6 animals
09.12.92 -> 03.01.93 -
04.01.93 -> 25.01.93 2.5 interval V 0.278-0.638: mean
0.432 in 8 dogs
showing
haemolytic anaemia
16.01.93 -> 08.02.93 -
09.02.93 -> 02.03.93 20 interval VI not measured
Serum from the dog that exhibited an agglutination response
was chromatographed on bovine serum albumin/sepharose gels and an
IgM antibody fraction was purified. The immunoglobulin was
demonstrated to produce a strong agglutination reaction with
metabolite R45714 and a weaker reaction with levamisole (Moeremans
& Bols, 1993a).
Twenty-three sensitized animals from the 14-month study in
dogs by Verstraeten et al., 1993b reported in section 2.2.1.1
were used to investigate immunological parameters. During challenge
with 1.25, 2.5, and 20 mg/kg bw/day levamisole (Intervals IV, V and
VI), blood was collected weekly and 24 hours after the last dose at
each treatment level. Erythrocytes from treated dogs were incubated
with specific antisera, anti-dog IgG, anti-dog IgM, anti-dog C3 and
anti-dog C3c, for the determination of antibodies or complement on
cell surfaces.
During treatment with 1.25 mg/kg bw/day levamisole, anaemia
was observed in 5 of the 23 dogs but none of the serological tests
was positive. In 3 other dogs, IgG was consistently detected on
erythrocytes while IgM and complement were not apparent.
In dogs administered 2.5 mg/kg bw/day levamisole, anaemia was
seen in 9 of the 23 dogs and the presence of IgM and complement on
erythrocytes was demonstrated for each animal. Additionally, a
number of animals not exhibiting anaemia were shown to have IgM or
complement on their red blood cells. A total of 6 dogs showed IgG,
but correlation with anaemia was poor.
Of the 7 animals challenged with 20 mg/kg bw/day, anaemia was
produced in 5 dogs and 4 of these dogs revealed the presence of
complement on erythrocytes. IgM was not clearly detected in any
animals, while IgG was present on the cells of 2 dogs, one of which
developed anaemia.
In a further investigation, serum was extracted from blood
collected 24 hours after the last dose. Using dilutions of serum
which did not produce agglutination of normal erythrocytes, sera
from 2 of the 23 dogs, incubated in the presence of levamisole, led
to distinct agglutination of red blood cells (Moeremans & Bols,
1993b).
2.3 Observations in humans
2.3.1 Studies on immunological effects
Of 48 patients with rheumatic diseases receiving treatment
with levamisole, 2 developed severe leucopenia. The serum of both
subjects showed the presence of levamisole-dependent
leucocyte-agglutinating antibodies. Leucocytes did not agglutinate
when incubated with either patient's serum in the absence of
levamisole or normal serum in the presence of levamisole. The
results were interpreted as supportive of an immunological basis
for leucocyte-agglutination possibly mediated through the
production of anti-drug antibodies (Rosenthal et al., 1976).
Sera were obtained from 10 severely neutropenic patients who
had been treated with levamisole for breast cancer or rheumatoid
arthritis. When incubated with normal cells, the serum from each
patient showed complement-dependent toxicity to granulocytes, but
only 2 samples were toxic to lymphocytes and none caused leucocyte
agglutination. On withdrawal of drug treatment, neutrophil counts
increased rapidly in concert with reductions in serum
granulo-cytotoxicity titres. Sera from 10 levamisole-treated
patients who did not develop neutropenia tested negative for
granulocytotoxicity. Analysis of the sera from 3 patients, with
specific antisera, revealed the presence of IgM but not IgG
antibodies. However, there was no evidence for the presence of
anti-levamisole antibodies in the serum of neutropenic patients
(Thompson et al., 1980).
A group of 98 rheumatoid arthritis patients who had been
treated with levamisole for between 3 months and 7.2 years were
used to investigate the possible mechanism of haematological
toxicity. Agranulocytosis developed in 7 patients, and in each case
complement-dependent granulocytotoxic antibodies could be
demonstrated in their serum. It was stated that such antibodies
were absent in the serum of individuals who did not exhibit
agranulocytosis (Rosenthal, 1982).
2.3.2 Incidences of haematological effects.
Levamisole was registered for human use in 1966, initially as
an anthelmintic agent (150 mg single dose). It has also been found
useful in the treatment of rheumatoid disorders (150 mg/day for 3
consecutive days per fortnight) and in the treatment of Dukes C
colon cancer (150 mg/day for 3 consecutive days per fortnight).
Haemolytic anaemia has not been documented in humans as a
consequence of these uses.
Thrombocytopenia has been reported in 18 patients, 14 during
cancer therapy. The incidence of thrombocytopenia in 36 643 Dukes
C colon cancer patients in the USA, treated with levamisole, was
estimated to be 0.027%.
Leucopenia and agranulocytosis have not been reported
following administration of single doses of levamisole as an
anthelmintic. In US trials on Dukes C colon cancer patients,
incidences of leucopenia and agranulocytosis were 6% and 0.3%,
respectively. In another 46 controlled trials involving 2635
patients with various cancers, the incidence of agranulocytosis was
0.1%. Recent post-marketing data for levamisole therapy in 36 643
Dukes C colon cancer patients revealed an incidence of 0.08% for
agranulocytosis or granulocytopenia (Van Cauteren et al., 1993;
Vervaet, 1993).
3. COMMENTS
Information from a limited number of studies were available
for assessment, including data from pharmacokinetic and metabolism
studies, special studies on haematological and immunological
effects in dogs and results following administration in humans.
The in vitro biotransformation of levamisole was
investigated using hepatocytes and liver microsomes from dogs,
pigs, sheep, cattle and humans. The results indicated qualitatively
similar degradation pathways in each species. Following oral dosing
in animals, similar metabolic pathways were identified in rats,
dogs, monkeys and cattle, confirming previously reviewed studies in
rats. Characterization of human metabolites was limited, but the
available evidence indicates similar pathways to those in other
species. All metabolites identified in cattle were also observed in
dogs and rats, and therefore the toxicological potential of beef
residues may be considered to have been evaluated in laboratory
animal studies.
Pharmacokinetic studies in humans revealed that peak plasma
levels were achieved in 1 to 2 hours after an oral dose, with the
levels being proportional to the given dose. Metabolism of
levamisole was extensive and rapid and metabolites were eliminated
more slowly than the parent drug. Excretion was primarily in the
urine.
Two repeat dose toxicity studies in dogs, which utilized an
induction-challenge dosing regime, confirmed the susceptibility of
this species to the induction of haemolytic anaemia in some of the
levamisole-treated animals. Red cell parameters returned to normal
on cessation of dosing but anaemia quickly returned in most dogs
when treatment was recommenced. Additionally, thrombocytopenia and
leucopenia were induced at incidences lower than for haemolytic
anaemia but with a similar relationship to the treatment schedule.
The levamisole-induced incidence of granulocytopenia in both humans
and dogs was low.
In both repeat toxicity studies in dogs, the haemolytic
anaemia and leucopenia were severe enough to necessitate cessation
of dosing in many animals and resulted in the death of a number of
dogs. The induction dose was 20 mg/kg bw/day but in sensitized
animals challenged with doses of 1.25 mg/kg bw/day and above a
dose-related re-emergence of haemolytic anaemia occurred.
Nonetheless, a previous study in dogs showed no haematological
toxicity at a dose of 1.25 mg/kg bw/day given continuously for a
period of one year.
In one of the repeat toxicity studies, plasma levels of
levamisole were measured in randomly chosen dogs. Results showed an
increase in levamisole in proportion to the dose, but there was no
clear correlation of haematological toxicity with the plasma drug
level. However, the metabolites which may play a role in the
induction of anaemia were not measured.
Various immunological parameters were analyzed in the two
studies in dogs, with a view to investigating the mechanism
underlying the haematological effects. Sera obtained from dogs
which were sensitized to levamisole caused the agglutination of
erythrocytes from an untreated dog. The agglutination response was
enhanced in the presence of levamisole or some of its metabolites,
but only in 3 of 24 animals studied. Erythrocytes isolated from
some sensitized animals that were challenged with levamisole had
IgM antibodies, IgG antibodies, and/or complement on cell surfaces
during periods of levamisole-induced haemolytic anaemia. However,
IgG antibodies did not correlate well with anaemia in dogs.
Sera from humans treated with levamisole and showing severe
leucopenia or agranulocytosis caused leucocyte-agglutination or
complement-dependent granulocytotoxicity in vitro. The factors
responsible for these effects showed a high correlation with
haematological toxicity, while sera from patients not developing
agranulocytosis were not toxic to normal white blood cells.
Analysis of sera from a limited number of individuals revealed the
presence of IgM, but not IgG, antibodies. Leucocyte agglutination
was dependent on the presence of levamisole, but
granulocytotoxicity showed no such reliance.
Although the primary target cells in humans and dogs are
generally different, there is now evidence supporting an
immunological basis for the haematological toxicity observed in
both species. The available evidence implicates the involvement of
IgM antibodies and a dependence on complement in the mechanism of
cellular destruction. There is also limited evidence that
agglutination responses in humans and dogs are mediated through
anti-drug antibodies, possibly induced by immunogenic complexes
between levamisole and protein, to which the drug is known to bind.
The reasons for the differential cell sensitivity in humans and
dogs are not known; however, the similarities in aetiology and the
recent demonstration of leucopenia in dogs suggest that dogs are a
suitable model for the haematological toxicity of levamisole in
humans.
4. EVALUATION
The Committee noted that the further studies reviewed at the
present meeting provided information on the incidence and
mechanisms of the haematological effects in humans and dogs. The
Committee recognized that while continuous dosing of dogs with 1.25
mg/kg bw/day levamisole did not result in haemolytic anaemia, this
dose did cause the re-emergence of haemolytic anaemia in a number
of dogs previously sensitized with 20 mg/kg bw/day of levamisole.
Since there is a very small population of humans who are sensitized
to levamisole following therapeutic exposure, the Committee
concluded that the use of a safety factor of 200 would be
appropriate. On this basis, an ADI of 0-6 µg/kg bw was established.
5. REFERENCES
HEYKANTS, J., MEULDERMANS, W. & VAN PEER, A. (1990). Levamisole
pharmacokinetics in healthy volunteers and in cancer patients. A
review of the data available until January 1990. Unpublished Report
Serial No. R12564/117 from Janssen Research Foundation. Submitted
to WHO by Janssen Pharmaceutica, Beerse, Belgium.
KOYAMA, K., OISHI, T., ISHII, A. & DEGUCHI, T. (1983). Metabolic
fate of levamisole in rats, dogs and monkeys. Oyo Yakuri, 26:
869-876.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993a). The in
vitro metabolism of levamisole in subcellular liver fractions of
dogs, pigs, sheep, cattle and humans. Unpublished Report No.
R12564/FK1266 from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
LAVRIJSEN, K., VAN LEEMPUT, L. & HEYKANTS, J. (1993b). The in
vitro metabolism of levamisole in hepatocytes of dogs, pigs,
sheep, cattle and humans. Unpublished Report No. R12564/FK1122 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993a). Evidence for the formation of
hapten antibodies in dogs after prolonged treatment with
levamisole. Unpublished Preclinical Research Report from Janssen
Research Foundation. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
MOEREMANS, M. & BOLS, L. (1993b). In vitro study of
levamisole-induced hemolytic anaemia in dog. Unpublished
Preclinical Research Report from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
MONBALIU, J., VAN LEEMPUT, L. & HEYKANTS, J. (1993). Toxicokinetics
of levamisole in beagle dogs at the occasion of a fifteen-month
mechanistic toxicity study (Exp no. 2692) of levamisole
hydrochloride (R12564) at 1.25, 2.5 and 20 mg/kg/day. Unpublished
Report No. R12564/FK1317 from Janssen Research Foundation.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M. (1982). Granulocytotoxic antibodies in
levamisole-induced agranulocytosis. Klinische Wochenschrift
60:1297-1302. English translation submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
ROSENTHAL, M., TRABERT, U. & MULLER, W. (1976). Leucocytotoxic
effect of levamisole. Lancet, I: 369.
THOMPSON, J.S., HERBICK, J.M., KLASSEN, L.W., SEVERSON, C.D.,
OVERLIN, V.L., BLASCHKE, J.W., SILVERMAN, M.A. & VOGEL, C.L.
(1980). Studies on levamisole induced agranulocytosis. Blood,
56: 114-122.
VAN CAUTEREN, H., LAMPO, A., COUSSEMENT, W., VAN LEEMPUT, L. &
MAES, L. (1993). Levamisole: Comprehensive answer to the questions
raised by the Codex Alimentarius Committee (JECFA). Unpublished
Report from Janssen Research Foundation. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
VAN PEER, A., NICHOLLS, A., VAN DE POEL, S., WOESTENBORGHS, R., VAN
LEEMPUT, L. & HEYKANTS, J. (1993). Phase-I trial: Systemic
absorption of levamisole after single oral doses of 1, 10 and 50 mg
to healthy volunteers. Unpublished Report Trial No. LVM-GBR-2 from
Janssen Research Foundation. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
VERSTRAETEN, A., LAMPO, A., COUSSEMENT, W. & VAN CAUTEREN, H.
(1993a). Mechanistic toxicity study in beagle dogs with levamisole.
Unpublished Report Experiment No. 2255 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERSTRAETEN, A., VANDENBERGHE, J., LAMPO, A., COUSSEMENT, W. & VAN
CAUTEREN, H. (1993b). Mechanistic toxicity study in beagle dogs.
Unpublished Report Experiment No. 2692 from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
VERVAET, P. (1993). Statement on the observation of hemolytic
anaemia, thrombocytopenia and leucopenia/agranulocytosis during
treatment with levamisole. Unpublished Report from Janssen Research
Foundation. Submitted to WHO by Janssen Pharmaceutica, Beerse,
Belgium.
WATSON, A.D.J., SANGSTER, N.C., CHURCH, D.B. & VAN GOGH, H. (1988).
Levamisole pharmacokinetics and bioavailability in dogs. Res. Vet.
Sci., 45: 411-413.
