
ALITAME
First draft prepared by
Dr Peter J. Abbott
National Food Authority
Canberra, Australia
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on liver enzymes
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Long-term toxicity/carcinogenicity studies
Reproductive toxicity studies
Special studies on neonatal survival
Special studies on embryotoxicity/teratogenicity
Special studies on genotoxicity
Special studies on pharmacological effects
Special studies on neurotoxicity and neurobehavioural effects
Special studies on melanin binding
Special studies on ß-isomer of alitame
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
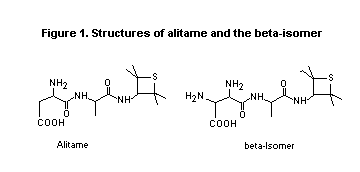
Alitame is a dipeptide amide, composed of three chemical
moieties: L-aspartic acid, D-alanine and 2,2,4,4- tetramethyl-
3-thietanyl amine. The major impurities are L-ß-aspartyl-N-
(2,2,4,4-tetramethyl-3-thietanyl)-D-alaninamide hydrate (2:5)
(referred to a ß-isomer), formed after re-arrangement of the aspartyl
unit; and N-(2,2,4,4-tetramethyl-3-thietanyl)-D-alaninamide (referred
to as alanine amide), formed after hydrolysis of the L-aspartic/
D-alanine bond. The chemical structures of alitame (CP-54,802) and the
6-isomer (CP-63,884) are shown in Figure 1.
Alitame has not been previously evaluated by the Committee.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
A group of 5 male CD-1 mice was administered by gavage a single
dose of 50 mg/kg bw 14C-alitame in distilled water. A second group
of 5 male CD-1 mice was fed a diet containing 14C-alitame at a
concentration of 0.3%, equal to 350 mg/kg bw. Following the gavage
dose, 77% of the radioactivity was recovered in urine in a 24-hour
period. About 50% of the administered dose was excreted between 4 and
20 h. Faecal radioactivity was not measured. Following dietary
administration, 60% of the radioactivity was found in urine and 32% in
faeces (Pfizer, 1986).
2.1.1.2 Rats
A male Long-Evans rat with a bile duct cannula was administered a
single oral dose of 50 mg/kg bw 14C-alitame. Bile and urine were
collected for 48 h. In a second study, 4 male and 4 female Long-Evans
rats were administered a single oral dose of 5 mg/kg bw 14C-alitame.
Urine and faeces were collected for 7 days and analyzed for
radioactivity. In the single animal study, 83% of the radioactivity
was recovered, with 14% in bile and 69% in urine over 48 h, indicating
extensive absorption. In the group study, most of the radioactivity
was recovered in urine (83% in males, and 95% in females) in a 24-hour
period. Faecal radioactivity was 20% in males and 4% in females during
24 h with little detected after that time (Pfizer, 1986).
In a study to investigate tissue distribution in rats, a group of
24 male and 24 female Long-Evans rats received by gavage a single dose
of 5 mg/kg bw 14C-alitame. Animals in groups of 3 males and 3
females were killed at 2, 6 or 24 h, and at 2, 3, 7, 14 or 29 days and
tissue levels determined. In most tissues, the half-life of clearance
was 3 to 6 h and the levels were below the level of detection by 7
days. Residue levels of radioactivity in the eyes decreased much more
slowly than in other tissues and levels of 0.08 mg/kg were detected at
day 29 (Pfizer, 1986).
In a study to examine the potential for transplacental transfer
of alitame, pregnant Long-Evans rats received a single oral dose of
1000 mg/kg bw 14C-alitame on day 13 of gestation. Four animals were
sacrificed 6 or 24 h after treatment and maternal plasma and fetuses
were examined for radioactivity. Radioactivity was high in the plasma
of dams at 6 and 24 h, and high levels were also found in the fetuses
at these times, indicating transplacental transfer of alitame
(Pfizer, 1986).
In a study to examine potential accumulation of alitame or its
metabolites in the milk of lactating rats, pregnant Long-Evans rats
were fed diet containing 1% alitame (equal to 750 mg/kg bw/day)
beginning on the last day before birth, and were continued on this
diet for 13 days of lactation. At the end of the lactation period,
rats were given a single oral dose of 750 mg/kg bw 14C-alitame,
and plasma and milk were collected 6 h later. Concentration in milk
and plasma were the same for each animal with no indication of
accumulation in milk. Intake for each pup, based on an average
consumption of 3.7 g milk/day on lactation day 10. was calculated to
be between 6 and 13 mg/kg bw/day, which is less than 2% of the
maternal dose level (Pfizer, 1986).
In a study to examine the toxicokinetics of alitame after i.v.
treatment, a group of 30 male and 4 female Long-Evans rats received a
single i.v. injection of 25 mg/kg bw. Males received non-radioactive
alitame and were used to determine the serum concentration over a
6-hour period. The females received 14C-alitame and were used to
determine the excretion rate and the identity of the urinary
metabolites. Radioactivity in serum declined with an estimated
half-life of 13 minutes. Most of the radioactivity (87%) was found in
urine in the 48-hour period of collection. Approximately 50% of
urinary radioactivity was unchanged alitame, in contrast to only 1%
after oral administration. This result was consistent with a high rate
of hydrolysis in either the intestine or during first-pass hydrolysis
in the liver (Pfizer, 1986).
2.1.1.3 Dogs
Two male beagle dogs with bile cannulas received a single dose of
10 mg/kg bw 14C-alitame. In one dog, the alitame was injected
directly into the stomach during cannulation surgery. This dog was
maintained under anaesthesia during the 24-h collection of urine,
faeces and bile. In a separate study, 4 males and 4 females received a
single oral dose of 5 or 25 mg/kg bw 14C-alitame. Urine and faeces
were collected at 24-hour intervals over a 7-day period and analyzed
for radioactivity. In the first experiment, biliary excretion
comprised only 0.2-0.3% of the total radioactivity. Urinary excretion
was approximately 36% of the administered radioactivity in the
anaesthetized dog, and 81% in the conscious dog. Faecal radioactivity
was 10% of the administered radioactivity in the conscious dog. In the
second experiment, the administered radioactivity was recovered in
urine (94-98%) and faeces (3-4%) in the first two days (Pfizer, 1986).
In a study to examine the toxicokinetics after i.v. treatment, a
male beagle dog received a single i.v. injection of 10 mg/kg bw
14C-alitame. Blood samples were taken at times up to 72 h, and urine
and faeces were collected in three 24- hour intervals. Radioactivity
in serum gave a biphasic decline, with half-lives of 34 minutes and
4 h. The vast majority of the radioactivity was excreted in the first
24 h, mainly in the urine (80%). Unchanged alitame was the major
urinary excretion product (60%) (Pfizer, 1986).
In a study to examine whether the site of hydrolysis of alitame
was the intestine or the liver, a female beagle dog under anaesthesia
had a 7-inch segment of the jejunum ligated and the mesenteric vein
cannulated. A 3 ml solution containing 14C-alitame (4 mg/kg bw) was
injected into the ligated jejunal segment and the mesenteric blood
collected at 2-minute intervals and peripheral blood at 15-minute
intervals over a 2-hour period. The mesenteric blood accounted for 4%
of the total radioactivity over the 2-hour period. The level of
unchanged alitame remained relatively constant and accounted for
approximately 50% of radioactivity while the percentage of the
hydrolysis product alanine tetramethylthietane amide (CP-57,207; see
Figure 2) increased with time from 12% to 38% over 2 h. Unchanged
alitame was the major substance present in the jejunum, while the
concentration of alanine tetramethylthietane amide increased with
time. No radioactivity was found in peripheral blood or urine. The
results suggested that the lumen of the jejunum is the site for
hydrolysis of alitame, and that further hydrolysis occurred during
passage through the intestinal wall (Pfizer, 1986).
2.1.1.4 Humans
Four male subjects were administered an oral dose of 14C-alitame
(50 mCi; 1 mg/kg bw). Blood samples were taken at various times up to
48 h. Exhaled 14CO2 was measured at various times up to 8 h.
Urine and faeces were collected over 5 days.
Plasma radioactivity levels peaked between 8 and 18 h and
decreased rapidly within the first 48 h, indicating slow but extensive
absorption followed by rapid excretion. There was no significant
increase in exhaled 14CO2 above zero. Urinary radioactivity was
maximal between 12 and 24 h with approximately 50% of radioactivity
excreted in the first 24 h and 90% within 5 days Faecal elimination
accounted for 7-10% of the total dose in 3 subjects and 2% in the 4th
subject.
The major urinary metabolites were alanine tetramethylthietane
amide sulfoxide (CP-58,290; see Figure 2) and alanine tetramethyl-
thietane amide glucuronide. Minor metabolites were alanine
tetramethylthietane amide (CP-57,207) and alanine tetramethylthietane
amide sulfone (CP-57,254). The glucuronide was the major urinary
metabolite in the first 24 h whereas the sulfoxide was the major
metabolite in the 24-48 h period. Unchanged alitame constituted less
than 1% of the total urinary radioactivity. In faeces, all of the
radioactivity consisted of unchanged alitame (CP-54,802) and alanine
tetramethylthietane amide (CP-57,207). In plasma, the major part of
the radioactivity (80%) consisted of alanine tetramethylthietane amide
(CP-57,207) and its glucuronide. In contrast to rats and dogs, the
major urinary metabolite in humans was found to be the glucuronide of
alanine tetramethylthietane amide (Caldwell et al., 1985).
2.1.2 Biotransformation
The metabolic pathways of alitame in different animal species and
in humans are shown in Figure 2.
2.1.2.1 Mice
Following a single oral dose of 50 mg/kg bw 14C-alitame or a
diet containing 3000 mg/kg 14C-alitame, metabolites identified were
D-alanine tetramethylthietane amide sulfoxide (CP-58,290; 64.3%),
D-alanine tetramethylthietane amide (CP-57,207; 24.7%), and unchanged
alitame (0.9%). The remainder metabolites (10.1%) were not identified.
Faecal radioactivity was identified as 39.5% unchanged alitame
(CP-54,802), 51.5% D-alanine tetramethyltbietane amide (CP-57,207) and
4.6% was not identified (Pfizer, 1986).
2.1.2.2 Rats
In a study to examine metabolites in plasma, a group of
Long-Evans rats received a single oral dose of 50 mg/kg bw
14C-alitame. Two rats were bled serially to determine the time
course of radioactivity and a further 3 male and 3 female rats were
killed at 6 h and the plasma radioactivity was assayed by HPLC.
Maximum plasma levels occurred at 4 to 6 h after administration. The
major metabolite was alanine tetramethylthietane amide sulfoxide
(CP-58,290; 70% of the total radioactivity), in both the free and
acetylated forms with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in urine, 4 male and 4 female
Long-Evans rats were administered a single oral dose of 5 mg/kg bw
14C-alitame. Urine and faeces were collected over a 24-hour period
and analyzed by HPLC. The major urinary metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 75-80%), in both free
and acetylated forms, with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in faeces, two Long-Evans
rats received a single oral dose of 50 mg/kg bw or 1000 mg/kg bw
14C-alitame. Urine and faeces were collected over 24 h and analyzed
by HPLC. The major product in the faeces was unchanged alitame
(CP-54,802; 70% of the radioactivity). A second metabolite was
identified as alanine tetramethylthietane amide (CP-57,207; 23%)
(Pfizer, 1986).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
A group of 5 male CD-1 mice was administered by gavage a single
dose of 50 mg/kg bw 14C-alitame in distilled water. A second group
of 5 male CD-1 mice was fed a diet containing 14C-alitame at a
concentration of 0.3%, equal to 350 mg/kg bw. Following the gavage
dose, 77% of the radioactivity was recovered in urine in a 24-hour
period. About 50% of the administered dose was excreted between 4 and
20 h. Faecal radioactivity was not measured. Following dietary
administration, 60% of the radioactivity was found in urine and 32% in
faeces (Pfizer, 1986).
2.1.1.2 Rats
A male Long-Evans rat with a bile duct cannula was administered a
single oral dose of 50 mg/kg bw 14C-alitame. Bile and urine were
collected for 48 h. In a second study, 4 male and 4 female Long-Evans
rats were administered a single oral dose of 5 mg/kg bw 14C-alitame.
Urine and faeces were collected for 7 days and analyzed for
radioactivity. In the single animal study, 83% of the radioactivity
was recovered, with 14% in bile and 69% in urine over 48 h, indicating
extensive absorption. In the group study, most of the radioactivity
was recovered in urine (83% in males, and 95% in females) in a 24-hour
period. Faecal radioactivity was 20% in males and 4% in females during
24 h with little detected after that time (Pfizer, 1986).
In a study to investigate tissue distribution in rats, a group of
24 male and 24 female Long-Evans rats received by gavage a single dose
of 5 mg/kg bw 14C-alitame. Animals in groups of 3 males and 3
females were killed at 2, 6 or 24 h, and at 2, 3, 7, 14 or 29 days and
tissue levels determined. In most tissues, the half-life of clearance
was 3 to 6 h and the levels were below the level of detection by 7
days. Residue levels of radioactivity in the eyes decreased much more
slowly than in other tissues and levels of 0.08 mg/kg were detected at
day 29 (Pfizer, 1986).
In a study to examine the potential for transplacental transfer
of alitame, pregnant Long-Evans rats received a single oral dose of
1000 mg/kg bw 14C-alitame on day 13 of gestation. Four animals were
sacrificed 6 or 24 h after treatment and maternal plasma and fetuses
were examined for radioactivity. Radioactivity was high in the plasma
of dams at 6 and 24 h, and high levels were also found in the fetuses
at these times, indicating transplacental transfer of alitame
(Pfizer, 1986).
In a study to examine potential accumulation of alitame or its
metabolites in the milk of lactating rats, pregnant Long-Evans rats
were fed diet containing 1% alitame (equal to 750 mg/kg bw/day)
beginning on the last day before birth, and were continued on this
diet for 13 days of lactation. At the end of the lactation period,
rats were given a single oral dose of 750 mg/kg bw 14C-alitame,
and plasma and milk were collected 6 h later. Concentration in milk
and plasma were the same for each animal with no indication of
accumulation in milk. Intake for each pup, based on an average
consumption of 3.7 g milk/day on lactation day 10. was calculated to
be between 6 and 13 mg/kg bw/day, which is less than 2% of the
maternal dose level (Pfizer, 1986).
In a study to examine the toxicokinetics of alitame after i.v.
treatment, a group of 30 male and 4 female Long-Evans rats received a
single i.v. injection of 25 mg/kg bw. Males received non-radioactive
alitame and were used to determine the serum concentration over a
6-hour period. The females received 14C-alitame and were used to
determine the excretion rate and the identity of the urinary
metabolites. Radioactivity in serum declined with an estimated
half-life of 13 minutes. Most of the radioactivity (87%) was found in
urine in the 48-hour period of collection. Approximately 50% of
urinary radioactivity was unchanged alitame, in contrast to only 1%
after oral administration. This result was consistent with a high rate
of hydrolysis in either the intestine or during first-pass hydrolysis
in the liver (Pfizer, 1986).
2.1.1.3 Dogs
Two male beagle dogs with bile cannulas received a single dose of
10 mg/kg bw 14C-alitame. In one dog, the alitame was injected
directly into the stomach during cannulation surgery. This dog was
maintained under anaesthesia during the 24-h collection of urine,
faeces and bile. In a separate study, 4 males and 4 females received a
single oral dose of 5 or 25 mg/kg bw 14C-alitame. Urine and faeces
were collected at 24-hour intervals over a 7-day period and analyzed
for radioactivity. In the first experiment, biliary excretion
comprised only 0.2-0.3% of the total radioactivity. Urinary excretion
was approximately 36% of the administered radioactivity in the
anaesthetized dog, and 81% in the conscious dog. Faecal radioactivity
was 10% of the administered radioactivity in the conscious dog. In the
second experiment, the administered radioactivity was recovered in
urine (94-98%) and faeces (3-4%) in the first two days (Pfizer, 1986).
In a study to examine the toxicokinetics after i.v. treatment, a
male beagle dog received a single i.v. injection of 10 mg/kg bw
14C-alitame. Blood samples were taken at times up to 72 h, and urine
and faeces were collected in three 24- hour intervals. Radioactivity
in serum gave a biphasic decline, with half-lives of 34 minutes and
4 h. The vast majority of the radioactivity was excreted in the first
24 h, mainly in the urine (80%). Unchanged alitame was the major
urinary excretion product (60%) (Pfizer, 1986).
In a study to examine whether the site of hydrolysis of alitame
was the intestine or the liver, a female beagle dog under anaesthesia
had a 7-inch segment of the jejunum ligated and the mesenteric vein
cannulated. A 3 ml solution containing 14C-alitame (4 mg/kg bw) was
injected into the ligated jejunal segment and the mesenteric blood
collected at 2-minute intervals and peripheral blood at 15-minute
intervals over a 2-hour period. The mesenteric blood accounted for 4%
of the total radioactivity over the 2-hour period. The level of
unchanged alitame remained relatively constant and accounted for
approximately 50% of radioactivity while the percentage of the
hydrolysis product alanine tetramethylthietane amide (CP-57,207; see
Figure 2) increased with time from 12% to 38% over 2 h. Unchanged
alitame was the major substance present in the jejunum, while the
concentration of alanine tetramethylthietane amide increased with
time. No radioactivity was found in peripheral blood or urine. The
results suggested that the lumen of the jejunum is the site for
hydrolysis of alitame, and that further hydrolysis occurred during
passage through the intestinal wall (Pfizer, 1986).
2.1.1.4 Humans
Four male subjects were administered an oral dose of 14C-alitame
(50 mCi; 1 mg/kg bw). Blood samples were taken at various times up to
48 h. Exhaled 14CO2 was measured at various times up to 8 h.
Urine and faeces were collected over 5 days.
Plasma radioactivity levels peaked between 8 and 18 h and
decreased rapidly within the first 48 h, indicating slow but extensive
absorption followed by rapid excretion. There was no significant
increase in exhaled 14CO2 above zero. Urinary radioactivity was
maximal between 12 and 24 h with approximately 50% of radioactivity
excreted in the first 24 h and 90% within 5 days Faecal elimination
accounted for 7-10% of the total dose in 3 subjects and 2% in the 4th
subject.
The major urinary metabolites were alanine tetramethylthietane
amide sulfoxide (CP-58,290; see Figure 2) and alanine tetramethyl-
thietane amide glucuronide. Minor metabolites were alanine
tetramethylthietane amide (CP-57,207) and alanine tetramethylthietane
amide sulfone (CP-57,254). The glucuronide was the major urinary
metabolite in the first 24 h whereas the sulfoxide was the major
metabolite in the 24-48 h period. Unchanged alitame constituted less
than 1% of the total urinary radioactivity. In faeces, all of the
radioactivity consisted of unchanged alitame (CP-54,802) and alanine
tetramethylthietane amide (CP-57,207). In plasma, the major part of
the radioactivity (80%) consisted of alanine tetramethylthietane amide
(CP-57,207) and its glucuronide. In contrast to rats and dogs, the
major urinary metabolite in humans was found to be the glucuronide of
alanine tetramethylthietane amide (Caldwell et al., 1985).
2.1.2 Biotransformation
The metabolic pathways of alitame in different animal species and
in humans are shown in Figure 2.
2.1.2.1 Mice
Following a single oral dose of 50 mg/kg bw 14C-alitame or a
diet containing 3000 mg/kg 14C-alitame, metabolites identified were
D-alanine tetramethylthietane amide sulfoxide (CP-58,290; 64.3%),
D-alanine tetramethylthietane amide (CP-57,207; 24.7%), and unchanged
alitame (0.9%). The remainder metabolites (10.1%) were not identified.
Faecal radioactivity was identified as 39.5% unchanged alitame
(CP-54,802), 51.5% D-alanine tetramethyltbietane amide (CP-57,207) and
4.6% was not identified (Pfizer, 1986).
2.1.2.2 Rats
In a study to examine metabolites in plasma, a group of
Long-Evans rats received a single oral dose of 50 mg/kg bw
14C-alitame. Two rats were bled serially to determine the time
course of radioactivity and a further 3 male and 3 female rats were
killed at 6 h and the plasma radioactivity was assayed by HPLC.
Maximum plasma levels occurred at 4 to 6 h after administration. The
major metabolite was alanine tetramethylthietane amide sulfoxide
(CP-58,290; 70% of the total radioactivity), in both the free and
acetylated forms with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in urine, 4 male and 4 female
Long-Evans rats were administered a single oral dose of 5 mg/kg bw
14C-alitame. Urine and faeces were collected over a 24-hour period
and analyzed by HPLC. The major urinary metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 75-80%), in both free
and acetylated forms, with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in faeces, two Long-Evans
rats received a single oral dose of 50 mg/kg bw or 1000 mg/kg bw
14C-alitame. Urine and faeces were collected over 24 h and analyzed
by HPLC. The major product in the faeces was unchanged alitame
(CP-54,802; 70% of the radioactivity). A second metabolite was
identified as alanine tetramethylthietane amide (CP-57,207; 23%)
(Pfizer, 1986).
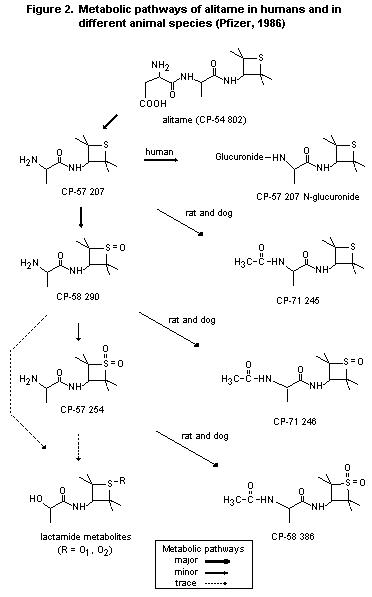 In a study in lactating dams, metabolites in milk were alanine
tetramethylthietane amide sulfoxide (CP-58,290) and its acetylated
form (54.4%), alanine tetramethylthietane amide (CP-57,207; 31.4%),
and unchanged alitame (1%) (Pfizer, 1986).
In a study to examine the effect of dose on metabolic pathways,
Long-Evans rats received a single oral dose of 10, 100 or 1000 mg/kg
bw 14C-alitame and urine and faeces were collected at 24-hour
intervals over 3 days. Urinary metabolites were analyzed by HPLC.
There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment, but the
metabolic profile was essentially unchanged (Pfizer, 1986).
In a study to compare the metabolic fate of D-alanine as either
free alanine or when incorporated into alitame, Long-Evans rats were
administered 14C-D-alanine or 14C-alitame (1-14C-D-alanine
label) by oral gavage and exhaled 14CO2 was measured. While
14CO2 was extensively exhaled following administration of
D-alanine, no 14CO2 was expired after administration of
14C-alitame, demonstrating the stability of the D-alanine
tetramethylthietane amide bond to metabolic hydrolysis (Pfizer, 1986).
2.1.2.3 Dogs
In a study to examine metabolites in plasma, groups of 3 male and
3 female beagle dogs received a single oral dose of 5 or 25 mg/kg bw
14C-alitame. Plasma samples were taken at various times and the
metabolites in plasma identified by HPLC. In both male and female
dogs, plasma radioactivity peaked at 2-3 h. The major metabolite was
alanine tetra-methylthietane amide sulfoxide (CP-58,290; 80%), with
10% in the acetylated form. Approximately 5% was unchanged alitame.
The remainder was alanine tetramethylthietane amide (CP-57,207), its
sulfone derivative (CP-57,254) and the acetylated forms of these
compounds. The metabolite profile was very similar to that seen in the
rat (Pfizer, 1986).
In a study to examine the metabolites in urine, a group of 3 male
and 3 female beagle dogs received a single oral dose of 14C-alitame,
and 24-hour urine and faeces collected. Urinary metabolites were
analyzed by HPLC. In males, the major metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 80%), including 9.5%
as the acetylated form. Approximately 5-15% of the urinary
radioactivity was unchanged alitame (Pfizer, 1986).
In a study to examine metabolites in faeces, a male beagle dog
received a single oral dose of 10 mg/kg bw 14C-alitame followed
several weeks later by a dose of 500 mg/kg bw. A female beagle dog
received a single oral dose of 500 mg/kg bw 14C-alitame Faecal
samples were collected over 24 h and analyzed by HPLC. Faeces
contained 18-40% of the administered radioactive dose over the 24 h
period. Faecal radioactivity was a mixture of unchanged alitame
(CP-54,802) and alanine tetramethylthietane amide (CP-57,207).
Together these two products accounted for approximately 90% of the
total radioactivity (Pfizer, 1986)
In a study to examine the effect of dose on metabolic pathways,
2 male and 2 female beagle dogs received a single oral dose of 10 or
500 mg/kg bw 14C-alitame and urine and faeces were collected in
24-hour intervals over 3 days. Urinary metabolites were analyzed by
HPLC. There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment than after the
low-dose treatment, but the metabolite profile was essentially
unchanged (Pfizer, 1986).
2.1.2.4 In vitro studies
The stability of alitame in gastric juices was examined to
clarify whether the cleavage of aspartic acid is initiated in the acid
environment of the stomach, and whether conversion of alitame to the
ß-isomer might occur under these conditions. Thus, alitame was
incubated in simulated gastric juices at 38°C to determine the degree
of hydrolysis. Controls were water and simulated gastric juices
(boiled). Samples were taken over 24 h and analyzed by HPLC. Alitame
was relatively stable, with only slight evidence of degradation after
prolonged incubation for 24 h, which was not considered to be due to
enzyme activity. Similarly, the small increase in ß-isomer observed in
both treated and control samples was not considered to be due to
enzyme activity (Pfizer, 1986).
The stability of alitame in rat intestinal homogenate was
examined in order to test the capacity of the rat small intestine to
metabolize alitame. The small intestine from male rats was flushed
with saline and homogenized in phosphate buffer at pH 7.4. The
homogenate was incubated with 500 mg alitame/ml at 37°C and the
reaction stopped by the addition of 0.25 tool/litre NaOH. Cleavage of
the peptidic bond of alitame was measured by the formation of alanine
tetramethylthietane amide (CP-57,207) and was dependent on both the
substrate concentration and time of incubation. The percentage
conversion to the amide ranged from 13-21% after 10 minutes of
incubation. This demonstrated the ability of intestinal homogenate
to cleave the peptidic bond of alitame. The reaction was also
demonstrated to be enzyme-mediated in a second experiment in which
it was shown that the extent of cleavage was dependent on the
concentration of homogenate. Little evidence of further metabolic
steps, such as sulfur oxidation or acetylation, was noted (Pfizer,
1986).
2.1.3 Effects on liver enzymes
2.1.3.1 Rats
In a study to compare the rat liver enzyme inducing properties of
alitame with those of butylated hydroxytoluene (BHT), groups of 4 male
rats received oral doses of either BHT (100 or 500 mg/kg bw), alitame
(100 or 500 mg/kg bw) or phenobarbital (50 mg/kg bw) daily for 5 days.
At 15 h following the last dose, animals were killed and livers
removed. Liver homogenates were assayed for O-demethylase, cytochrome
c reductase, and P-450 activity. Significant enzyme induction was
noted with BHT, but alitame showed no effect at the same dose levels
(Pfizer, 1986).
In a further study to examine the liver enzyme inducing potential
of alitame and its metabolites, groups of Sprague-Dawley rats received
single oral doses of either alitame (up to 2000 mg/kg bw), alanine
tetramethylthietane amide (CP-57,207) or its sulfoxide (CP-58,290; 100
or 500 mg/kg bw), or phenobarbital (50 mg/kg bw/day) for 5 days. At
15 h after the last dose, animals were killed and livers removed.
Liver homogenates were prepared and assayed for O-demethylase. Slight
induction of demethylase activity was noted with both alitame and its
metabolites. At 500 mg/kg bw, enzyme activity was approximately twice
the low baseline activity observed in controls with either alitame or
its metabolites (Pfizer. 1986).
In a recent detailed study, the hepatic enzyme-inducing activity
of alitame was examined in rats following gavage administration (pilot
study) and dietary administration (main study). In the pilot study,
groups of 5 male and 5 female Sprague-Dawley rats were administered
alitame daily by gavage for 7 days at dose levels of 0, 500 or
1000 mg/kg bw/day. Animals were killed on day 8, the livers excised,
sections taken for histology and electron microscopy, and the
remainder of the liver homogenized. Positive control animals were
given ß-naphthoflavone (80 mg/kg bw, i.p., 3 days), phenobarbitone
(80 mg/kg bw, i.p., 3 days), or dexamethasone (100 mg/kg bw, i.p., 3
days). In the main study, groups of 20 male and 20 female Sprague-
Dawley rats were fed a diet containing alitame at concentrations of
0, 0.3% or 1% for 4 weeks. An additional 20 males and 20 females acted
as pair-fed controls and received the basal diet at the level of
intake of the 1% alitame group. After 28 days, 10 males and 10 females
from each group were sacrificed, sections of liver taken for histology
and electron microscopy and the remainder of the liver homogenized.
Kidney and brain weights were also recorded. The remaining 10 males
and 10 females were withdrawn from the alitame diet and kept for a
further 14 days before being sacrificed. In each of the above studies,
the liver homogenate was used to determine protein content, cytochrome
P-450 content, NADPH-cytochrome P-450-dependent reductase activity,
ethoxyresorufin O-deethylase (EROD) activity, pentoxyresorufin
O-depentylase (PROD) activity, erythromycin N-demethylase (ERD)
activity, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
In the pilot study, the positive controls exhibited significant
increases with respect to hepatic microsomal enzymes and all caused an
increase in relative liver weight. Brain and kidney weights were
unaltered. Alitame at 500 or 1000 mg/kg bw/day for 7 days had only
minor effects by comparison. At 1000 mg/kg bw/day, cytochrome P-450
levels were 140% of controls, and EROD, PROD and ERD activities
increased 3.6-fold, 6.3-fold, and 4.7-fold, respectively. Examination
of the microsomes by SDS-PAGE provided similar evidence of low levels
of protein induction corresponding to the above enzymes. Light
microscopic examination revealed hepatocellular hypertrophy at both
dose levels, and some evidence of periportal inflammation and necrosis
in a few sections. Electron microscopy revealed an increase in rough
and smooth endoplasmic reticulum at both dose levels.
In the main study, growth curves indicated a reduced body-weight
gain in alitame-treated animals compared to controls. This effect
was more apparent in females than in males and appeared to be
dose-related. While the effects may not have been statistically
significant, the curves indicated a clear biologically significant
decrease in body-weight gain in females at both the 0.3 and 1% alitame
dose levels. There was also an increase in relative liver weight in
females at both dose levels which was dose-related. Relative brain and
kidney weights were unaltered.
The parameters used to measure hepatic enzyme induction were also
increased in a dose-related manner compared to controls and pair-fed
animals. At the 0.3 and 1% dose levels, cytochrome P-450 levels
increased by 12 and 20% in females, and by 46 and 101% in males,
respectively. The activity of EROD was increased slightly in males and
females while there was a significant increase in the activity of PROD
in males and females at 0.3% and 1% alitame. The activity of ERD was
only slightly increased in males at 1% alitame. Light microscopic
examination of the liver revealed centrilobular hypertrophy at both
dose levels, but more marked at the higher dose. Electron microscopy
revealed a dose-related increase in both rough and smooth endoplasmic
reticulum. Following the 14-day withdrawal period, the liver weight
changes were reversed, and none of the hepatic parameters was
significantly different from controls.
The results indicated that alitame, at both the 0.3% and 1% dose
levels, was a weak inducer of hepatic enzymes, which was accompanied
in females by a decrease in body-weight gain and an increase in
relative liver weight. The pattern of enzyme changes was essentially
similar to that caused by phenobarbitone, although quantitatively
smaller (Caldwell et al., 1994).
In a supplement to the above study, the cytochrome P-450
isoenzyme from the liver microsomes of alitame-treated rats were
separated by SDS-polyacrylamide gel electrophoresis and further
characterized by Western blot immunodetection. Following separation by
electrophoresis, the proteins were transferred to nitrocellulose
membranes and the blots either stained with antibodies to specific
cytochrome P-450 isoenzyme or, to allow visualization of total
protein, by staining with Amido Black solution.
The microsomes from alitame-treated rats showed an increase in
the band attributed to CYP2B (catalytic activity) at 52 kD which is a
protein also shown to be induced by phenobarbitone. The band at 56 kD
attributed to CYP1A1, shown to be induced by ß-naphthoflavone, was
conspicuous by its absence. The data further confirmed that the
hepatic changes produced by alitame were similar to those produced by
phenobarbitone (Caldwell & Reed, 1994).
2.1.3.2 Humans
In a 14-day study, 8 male subjects received alitame in gelatine
capsules as 4 daily oral doses totalling 15 mg/kg bw/day. This dose
was calculated to be 15 times the projected human exposure level,
assuming total sucrose replacement. Induction of microsomal enzyme
levels was measured by the rate of elimination of aminopyrine.
Measurements were taken before treatment, after 2 weeks of treatment,
and at 18 days post-treatment. Both plasma and saliva were collected.
Blood samples were taken at pre-test, after 1 and 2 weeks of
treatment, and at 7 and 18 days post-treatment for the analysis of
clinical chemistry and haematological parameters. On the 10th day of
treatment, a 24-hour urine sample was collected for the analysis of
metabolites. There was no adverse effects parameters were normal
during the course of the study.
The analysis of the aminopyrine elimination results indicated
that the saliva was a good indicator of plasma levels. When the
elimination rate constants and half-lives were compared, there was no
significant difference in aminopyrine kinetics from the pre-dose,
post-dose and post-withdrawal samples. Urine samples indicated the
presence of the major metabolites, alanine tetramethylthietane amide
(CP-57,207) and it sulfoxide (CP-57,290). Alitame was present only in
trace amounts (Caldwell et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of alitame was examined in both mice and rats
and the results are summarized in Table 1.
Table 1. Summary of acute toxicity studies with alitame.
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral > 5000 Levinsky et al. (1983)
Rat M&F oral > 5000 Levinsky et al. (1983)
Groups of 10 male and 10 female mice (Crl:COBS CD-1 (ICR) BR) and
10 male and 10 female Sprague-Dawley (Crl:COBS CD (SD) BR) rats were
administered alitame (purity 97.43%) by gavage at a single dose level
of 5000 mg/kg bw. Animals were observed for clinical signs over a
14-day period after treatment, during which food consumption and body
weights were recorded. Animals were examined for gross pathological
changes at day 14 after treatment.
In mice, there were no deaths during the study and no clinical
signs apart from an increase in soft faecal material at 2.5 h
post-treatment. Faeces were normal by day 2 post-treatment. During the
observation period, there was normal food consumption and steady
body-weight gain.
In rats, there were no deaths during the study and no clinical
signs during the first hour post-treatment, but from then onwards all
animals displayed an increased level of activity for 1-2 days. All
rats were jittery and exhibited a tendency for exophthalmia, with
females noticeably more affected than males. Females exhibited
incoordination in their movements but were not ataxic. By 24 h, 7/10
males were essentially asymptomatic, and by day 2, all males and
females were asymptomatic. During the observation period, food
consumption was normal, and there was a steady body-weight gain. There
were no gross pathological changes on day 14 in 20/20 mice and in
19/20 rats, with one rat having an enlarged liver (Levinsky et al.,
1983).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 2-month study, groups of 10 male and 10 female Crl:COBS
(CD-1) (ICR) BR mice were fed a diet containing alitame (purity
98-101%) at concentrations of 0, 1, 2, or 5%, equivalent to 0, 1500,
3000 or 7500 mg/kg bw/day.
Animals were observed for clinical signs, and food consumption
and body weights were recorded throughout the study period. At the end
of the study period, liver and kidney weights were recorded and
tissues from the major body organs were taken for histopathology.
There were no deaths and no clinical signs of toxicity throughout
the study period. Body weights of the intermediate- and high-dose
animals decreased slightly on days 1 and 2, but recovered quickly.
There was no treatment-related effect on body weight for the remainder
of the study. There was no change in food consumption. Both absolute
and relative liver weights increased in a dose-related manner compared
to controls. The increase in absolute liver weight was 20, 40 and 67%
in males and 20, 35 and 66% in females in low-, intermediate- and
high-dose animals, respectively. Histopathological examination of the
liver revealed a dose-related increase in centrilobular hypertrophy.
Electron microscopy of liver tissue indicated a dose-related increase
in proliferation of smooth endoplasmic reticulum. No degenerative
hepatocellular changes were noted. Effects were noted at all dose
levels and a NOEL was not established in this study (Holmes et al.,
1983a).
2.2.2.2 Rats
In a range-finding study, groups of 5 male and 5 female Crl:COBS
CD-1 (SD) BR rats (5/sex/dose) were fed a diet containing alitame
(purity 98-101%) at concentrations of 0, 1, 2 or 5%, equivalent to
500, 1000, or 2500 mg/kg bw/day for 15 days. Animals were examined for
clinical signs of toxicity, and food and water consumption was
monitored throughout the study period. At the end of the study period,
blood samples were taken for haematological and clinical chemistry
examination; tissues from the major body organs were taken for
histopathology; tissues from liver and kidney were taken for electron
microscopy; and tissue from liver for histochemistry. There were no
deaths throughout the study, and no clinical signs of toxicity. A
slight body-weight loss was noted at the 2% and 5% dose levels on day
3 but body-weight gain was normal thereafter. There was a dose-related
decrease in food consumption in all treated groups on days 1-2
(especially females at the 2% dose level and in both sexes at the 5%
dose level) possibly due to unpalatability of the diet. Water
consumption was similarly decreased. There was a slight, but not
significant, increase in plasma cholesterol and protein levels in all
treated groups. Haematological parameters were normal. There was a
dose-related increase in absolute and relative liver weights in
males and females but no microscopic abnormalities were noted.
Histochemistry indicated an increase in glucose-6-phosphate
dehydrogenase and NADPH2 diaphorase in liver tissue slices. Electron
microscopy indicated a dose-related increase in smooth endoplasmic
reticulum in liver tissue and, in some cases, an increase in lysosomes
and lipid vacuoles. Effects were observed at all dose levels and a
NOEL was not established in this study (Chvedoff et al., 1981a).
In a 5-week study, groups of 10 male and 10 female Long-Evans
rats were fed a diet containing alitame (purity 98.2%) at dose levels
of 0, 10, 50 or 100 mg/kg bw/day. Animals were observed daily and body
weights and food consumption recorded. Blood samples were taken from
the orbital sinus prior to treatment and at the end of the study
period, and clinical chemistry and haematological parameters examined.
After 5 weeks, the induction of liver microsomal enzymes was measured
in vitro and animals were examined for gross pathology. Tissues from
the major organs were prepared for histopathology.
There was no treatment-related effect on body-weight gain or food
consumption. The clinical chemistry, haematological data and
histopathology were unremarkable. It was not possible to determine
whether there was proliferation of smooth endoplasmic reticulum
because of the presence of excess glycogen clue to lack of fasting
before sacrifice. Induction of 0-demethylase was determined by the
release of p-chlorophenol. A 30% increase in enzyme induction
occurred at the high-dose level which was not statistically
significant. Nevertheless, on the basis of the biological significance
of this increase, the NOEL in this study was 50 mg/kg bw/day
(Holmes et al., 1983b).
In a 3-month study, groups of CrI:COBS-CD(SD)BR strain rats
(15/sex/dose) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0.5, 1.0 or 2.0%, equal to 380, 830 or 1500 mg/kg
bw/day. Animals were observed throughout the study period and body
weights, food and water consumption recorded. Blood samples were taken
for examination of clinical chemistry and haematology parameters at
the end of the study. Urinary parameters were also examined. At the
end of the study, gross pathological examinations were performed and
tissues from the major organs were taken for histopathology. A group
of 5 males and 5 females was observed for a further 1 month following
the treatment period.
There were no deaths throughout the study and no clinical signs
of toxicity. There was a dose-related decrease in body-weight gain in
both males and females in all treated groups. The decreases at 3
months in males/females were 4.5%/1.7%, 4.5%/3.3% and 5.3%/8.5% at
low, intermediate and high dose. Food consumption was slightly lower
in high-dose males, but not during the withdrawal period. Water
consumption was normal. The clinical chemistry analysis revealed a
significant increase in plasma cholesterol at all dose levels in males
and females. The increase was 23% at low dose and 42% at high dose.
Levels returned to normal during the 1-month withdrawal period. There
was also a dose-related decrease in plasma triglyceride levels in
males, which returned to normal levels during the withdrawal period.
The levels of cytochrome P-450 and b5 were increased in a
dose-related manner at the end of the treatment period. The activity
of microsomal N-demethylase was also increased in a close-related
manner. The changes were reversed during the withdrawal period except
for the cytochrome P-450 levels in males. Haematological parameters
were within the normal range.
Relative and absolute liver weights were increased in males at
the high-dose level, while relative liver weight was increased at all
doses in females. The liver weights returned to normal during the
withdrawal period.
Histopathology of the liver revealed centrilobular hypertrophy in
all high-dose females and in 5/10 high-dose males. Histochemical
analysis of the livers indicated increased activity of G-6-P
dehydrogenase, gamma-glutamyl transpeptidase and NADPH-diaphorase in
the high-dose animals. NADPH-diaphorase activity was also increased in
intermediate-dose females. All changes were reversible during the
withdrawal period. Electron microscopy revealed an increased
proliferation of smooth endoplasmic reticulum at all dose levels.
After the withdrawal period, the livers of female rats were normal
while those of the males still showed mild proliferation of smooth
endoplasmic reticulum at the high-dose level. The histopathological
and enzymatic changes were indicative of a reversible adaptive
response. There was no evidence of focal hyperplasia or hepatocellular
toxicity. Effects were noted at all dose levels and a NOEL was not
established in this study (Monro et al., 1982a).
In a one-year study, groups of 40 male and 40 female Long-Evans
rats were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.1, 0.3, or 1.0% (equivalent to 50, 150 or
500 mg/kg bw/day). Two other groups were fed a diet containing alitame
adjusted to achieve a nominal intake of 10 or 30 mg/kg bw/day. At 6
months, 20 male and 20 female animals at each of the dose levels were
removed from treatment. Of these, half were sacrificed while the other
half were returned to normal untreated diet. Animals were observed
throughout the study period and body weights, food and water
consumption recorded. Ophthalmoscopic examinations were performed in
all animals before necropsy. Blood samples were taken at 6, 9 and 12
months for clinical chemistry and haematology. Urinalysis parameters
were also examined. At the end of the study period, all animals were
examined for gross pathology, and tissues from major organs were taken
for histopathology.
There were no deaths throughout the study and no clinical signs
of toxicity. Ophthalmoscopic examination at 6 months and 1 year was
unremarkable. There were no treatment-related effects on body-weight
gain or on clinical pathology parameters. Cytochrome P-450 levels were
measured at 6 months and were significantly increased at the high-dose
level only (21% in males and 33% in females). The only organ weight
change noted was an increase in relative liver weight at the high-dose
level at 6 months, with a 12.1% increase in males and 16.0% increase
in females. At 12 months, liver weight increases at the high-dose
level were 8.5% and 11.7% in males and females respectively, compared
to controls. In animals withdrawn from treatment at 6 months, liver
weights had returned to normal levels at 12 months. The only
microscopic finding was a retention of glycogen in both control and
treated groups. This change was not considered to be treatment-
related. The NOEL in this study was 0.3% in the diet, equal to
145 mg/kg bw/day in males and 185 mg/kg bw/day in females
(Holmes et al., 1985a).
A study was conducted in Sprague-Dawley rats which included
dietary administration during mating, in utero exposure, exposure
during lactation, and dietary exposure for one year post-weaning.
Groups of 20 male and 20 female Sprague-Dawley rats were selected from
the F1 offspring of rats treated 4 weeks prior to mating and during
gestation and lactation (Levinsky et al., 1992). The groups were fed
a diet containing alitame (purity 99.3%) at concentrations of 0 (two
control groups), 0.1, 0.3 or 1% for one year. The animals were
observed daily for clinical signs and body weights and food
consumption were recorded weekly. Other measurements and observations
included ophthalmological examinations (pre-test and 6 and 12 months),
serum chemistry, haematology and urinalysis measurements at 6 and 12
months. Tissues were collected from all animals which died or became
moribund during the study. At the end of the study, the animals were
sacrificed and necropsied and tissues prepared for light microscopy.
Organ weights for liver, kidney, adrenal glands, testes and ovaries
were recorded.
Twenty rats died or were sacrificed during the study. The deaths
were distributed evenly between the groups although deaths in males
predominated over those in females by four to one. Of the 20 early
necropsies, 4 were due to neoplasms (1 control, 2 low-dose and 1
intermediate-dose rats). The most common non-neoplastic cause of death
(7/16) was described as 'unexplained death/heavy rats' and was not
accompanied by any remarkable clinical signs.
Body-weight measurements began at birth for this study, and at
weaning (day 21) the mean body weights for the intermediate- and
high-dose animals compared to the control groups were -9% and -21%,
respectively. Following selection of animals in groups for the 1-year
study, the body weights expressed as percent of control for the
intermediate-dose level were -9.5% (M) and -8.8% (F), and for the
high-dose level -16.3% (M) and -13.5% (F). Over the course of the
study, the initial body weight differentials for males at the
intermediate- and high-dose levels diminished by about 50%. For the
first 80 days, all groups of males appeared to gain weight at similar
rates apart from the control group 1. For the remaining period of the
study, the weight gains were similar for all groups. Over the 1-year
period, the body-weight difference for the intermediate-dose males
disappeared and the difference for the high-dose group was 6.6% and
11.1% compared with control groups 1 and 2, respectively.
For females, over the course of the study, the -8.8% difference
at the intermediate-dose level disappeared and the low-dose level
gained weight relative to the control group 1. The females in the
control group 1, like the males, showed a slower growth rate. The
high-dose females, unlike the males, did not recover their initial
body weight difference (-13.5%) and after 200 days, the body weight
difference began to increase, reaching -20% relative to control
group 1.
Ophthalmoscopic examination at 6 and 12 months was unremarkable.
There were no treatment-related changes in clinical chemistry,
haematology or urinary parameters apart from a 40-60% decrease in
serum triglyceride levels in the high-dose females, which appeared to
be related to the decreased weight gain in the high-dose females. The
high-dose males showed decreased serum triglyceride values at 6
months, but not at 12 months.
There were no gross pathological findings in any group. The organ
weight changes observed were a decrease in absolute liver weight in
the high-dose females, and an increase in relative liver, kidney and
ovary weight as a result of the decrease in mean body weights in the
high-dose females.
Histopathology revealed hepatocellular hypertrophy in the
high-dose males (13/20) and females (4/20). These changes were not
accompanied by an increase in liver weight. Other liver changes
observed could not be related to treatment. The NOEL in this study,
based on body and organ weight changes, was 0.3% in the diet, equal to
173 mg/kg bw/day in males, and 205 mg/kg bw/day in females (Fisher
et al., 1992).
2.2.2.3 Dogs
In a two-week range-finding study in dogs, groups of 2 male adult
beagle dogs were fed alitame (purity 98-101%) in the diet at
concentrations of 1, 2 or 5%, equivalent to 250, 500 or 1250 mg/kg
bw/day. There were no control animals. Animals were observed
throughout the study for clinical signs of toxicity and histopatho-
logical examinations were performed on lungs, kidney, liver and heart
after 14 days. A section of liver was examined by electron microscopy.
There were no deaths during the study and no clinical signs of
toxicity. There was no treatment-related effect on body weight.
Systolic blood pressure and heart rate were normal at all dose levels.
Clinical chemistry parameters were normal apart from a slight increase
in alkaline phosphatase activity at all dose levels which increased in
the second week. Urinalysis parameters were normal. Haematological
parameters indicated a slight decrease in haemoglobin concentration,
RBC count and packed cell volume in treated animals, but the changes
were within normal limits and not definitely related to treatment.
Relative liver weights were within normal limits. Microscopic
examination revealed an increase in cytoplasmic vacuolation in the
liver of 5/6 animals. Electron microscopic examination revealed a
dose-related increase in proliferation of smooth endoplasmic
reticulum. Lipid vacuoles and lysosomes were present in the liver of
all animals but, in the absence of control animals, it could not be
determined whether the observed microsomal changes were treatment-
related (Chvedoff et al., 1981b).
In a 3-month study, groups of beagle dogs (4/sex in the low- and
intermediate-dose groups, and 6/sex in the control and high-dose
group) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.5, 1, or 2%, equal to 0, 120, 220 or 490 mg/kg
bw/day. In the control and high-dose groups, 2 animals/sex were
transferred to normal diet for an additional month after the study
period. Animals were examined throughout the study period for clinical
signs of toxicity and body weight was recorded weekly. At various
times, clinical chemistry, urinary and haematology parameters were
measured. Tests of aminopyrine clearance were measured on days 1 and
63. Ophthalmological examinations were performed on high-dose animals.
Tissues were examined histopathologically at the end of 3 months.
Liver histochemistry, electron microscopy and content of microsomal
cytochrome P-450 and b5 were also performed.
There were no deaths throughout the study and no clinical signs
of toxicity. Food intake was normal although there was a slightly
decreased weight gain in high-dose animals, particularly males.
Cardiovascular parameters were normal. Ophthalmological examination of
high-dose animals was unremarkable. Haematological parameters were
within the normal range. Clinical chemistry revealed a dose-related
increase in plasma alkaline phosphatase when measured at 1, 2 and 3
months. The increase at the high-dose level was 400% at 3 months.
After the recovery period, levels in the high-dose group were reduced
but still higher than control levels.
At the end of the 3-month treatment, the levels of cytochrome
P-450 and b5 were increased in a dose-related manner. After the
1-month withdrawal period, cytochrome P-450 and b5 in the high-dose
group were reduced to near control levels. Induction of the liver
microsomal enzyme system was also demonstrated in the dogs of the
reversibility groups (control and high dose) following administration
of a single i.v. dose of aminopyrine (15 mg/kg bw) on days 1 and 63 of
alitame treatment, with blood levels measured at 0, 1, 1.5, 2, 3 and
5 h. The plasma levels reflected the induction of a liver microsomal
demethylase. On day 1, there was little difference between treated and
control animals. On day 63, there was a marked difference between
control and treated animals.
Relative and absolute liver weights were increased in all
treatment groups. Absolute liver weight was increased by 36% and 11%
at high and low dose, respectively. Histopathology of the liver and
other organs was unremarkable. Histochemical examination revealed an
increase in G-6-P dehydrogenase activity in all treated groups except
the low-dose females. NADPH-diaphorase activity was increased in
high-dose animals. All levels returned to normal during the recovery
period, except for an increased G-6-P phosphatase activity in
high-dose males. Electron microscopy revealed an increase in
proliferation of smooth endoplasmic reticulum in all groups. This
increase was reversed in females but persisted in males during the
recovery period. The liver changes appeared to be indicative of an
adaptive response due to induction of microsome oxidative metabolism.
Effects were observed at all dose levels and a NOEL was not
established in this study (Monro et al. 1982b).
In an 18-month study, groups of 4 male and 4 female beagle dogs
were fed a diet containing alitame (purity 98-101%) at dose levels of
0, 10, 30, 100 or 500 mg/kg bw/day. Animals were examined throughout
the study period for clinical signs of toxicity, and body weights were
recorded at regular intervals. Ophthalmoscopy was performed on animals
at 6-month intervals. Electrocardiographic tracings were performed at
regular intervals. Clinical chemistry, urinary and haematological
parameters were measured at regular intervals. Aminopyrine clearance
was measured in control and intermediate dose animals at pre-test and
during weeks 3, 5, 7, 9, 40 and 74, and in the high-dose group at 39
and 73 weeks. Gross pathological examination was performed at 18
months together with histopathological examination of a wide range of
tissues. There were no treatment-related effects on survival and no
clinical signs of toxicity. Ophthalmoscopic examinations and
cardiovascular parameters were normal. Body weights were comparable
between groups although body-weight gain in high-dose females was
slightly less than control animals between weeks 40 and 70. Food
consumption was normal in all groups. All of the clinical pathology
parameters were normal except for an increase in serum alkaline
phosphatase activity in high-dose males and females. The alkaline
phosphatase rise was not uniform at high dose, with some dogs showing
little or no change.
Analysis of the urinary metabolites over a 2-day period at week
10 showed that the excretion rate was proportional to the dose
administered, indicating a dose-related systemic exposure. Measurement
of aminopyrine clearance, as an indicator of the induction of the
liver microsomal enzyme system, revealed a slight decrease in t1/2
in males and females at 100 mg/kg bw/day at the 2-week period. The
significance of this result is unclear, and may be due to the high
t1/2 in the animals pre-dosing. In males, there appeared to be a
gradual increase in t1/2 over the study period but again the result
is difficult to interpret. Overall, the results at 100 mg/kg bw/day do
not appear to indicate any enzyme induction. At 500 mg/kg bw/day,
after 39 or 73 weeks, significant microsomal enzyme induction was
apparent.
The only gross pathological change observed was a moderate
increase in absolute liver weight of 31% and 20% in high-dose males
and females, respectively. This increase was statistically significant
in males only. Histopathology was unremarkable in the treated animals.
A slight increase in smooth endoplasmic reticulum was found in the
livers of high-dose animals. The NOEL in this study was 100 mg/kg
bw/day (Holmes et al., 1985b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 2-year carcinogenicity study, groups of 50 male and 50
female Crl: CD-1 (ICR) BR mice were fed a diet containing alitame
(purity, 98-101%) at concentrations of 0 (2 groups), 0.1. 0.3 or 0.7%.
Animals were observed throughout the study period. Body weight and
food consumption were recorded weekly for 13 weeks and then at
N-weekly intervals for the remainder of the study. At the end of the
study period, animals were sacrificed and subjected to gross
pathological examination and organs weights recorded. Tissues from all
of the major organs were prepared for histopathological examination.
Tumour incidence was recorded.
There was no treatment-related effect on survival. The survival
rate for controls, low-, intermediate-, and high-dose groups in
males/females was 52/24%, 22/26%, 22/38%, 32/36% and 38/26%,
respectively. There were no clinical signs of toxicity in the treated
groups. Body-weight gain and food consumption were similar for all
groups. Gross pathological examination revealed a significant increase
in absolute and relative liver weight at high dose in males only.
Absolute liver weight increased by 59% and 46% compared to the two
control groups, while relative liver weight increased by 58% and 38%
compared to the two control groups. Slight to mild centrilobular
hepatocellular hypertrophy was found in 12 male animals in the
high-dose group. At the intermediate-dose level, absolute and relative
liver weights of males were also increased. Absolute liver weight was
increased by 41% and 30% compared to the two control groups, while
relative liver weight was increased by 39% and 21% compared to the two
control groups. While the increases were not statistically
significant, nevertheless the Committee considered them to be of
biological significance at this dose level. No hepatocellular
hypertrophy was apparent at the intermediate- and low- dose levels.
There were no other histopathological changes, or any
treatment-related increase in tumour incidence. Under the conditions
of this assay, there was no evidence of carcinogenicity in mice
induced by alitame in the diet. The NOEL, based on the biologically
significant liver weight changes in males, was 0.1% in the diet, equal
to 125 mg/kg bw/day for males and 155 mg/kg bw/day for females (Holmes
et al., 1985c).
2.2.3.2 Rats
A carcinogenicity study was conducted with dietary administration
and in utero exposure to alitame in Long-Evans rats. Groups of 50
male and 50 female Long-Evans rats (strain Blu:(LE)BR) were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for 2 years.
Animals used in this study were taken from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Holmes et al., 1986b). Animals were observed throughout the study
period for clinical signs of toxicity. Body weights and food
consumption were recorded weekly. Ophthalmoscopic examinations were
carried out at 12, 18 and 24 months. Clinical chemistry and
haematological parameters were measured in 10 animals/sex/dose at 6,
12, 18 and 24 months. At the end of the study period, animals were
sacrificed and subjected to gross pathological examination. Organ
weights were recorded. Tissues from all the major organs were prepared
for histopathological examination.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examinations
revealed no treatment-related effects. The survival rate at 104 weeks
for the control, low, intermediate, and high-dose groups was for
males/females 40/46%, 34/40%, 30/42% and 28/34%, respectively. There
was a slight decrease in survival in treated animals compared to
controls but this was not necessarily treatment-related.
In the second year of the study, there was a dose-related
difference in body weights for males between control animals and those
in the high- and intermediate-dose groups. In females, the body weight
of high-dose animals was lower than controls at the start of the study
and continued to be significantly lower throughout the study.
Intermediate-dose females also had a slightly lower body weight than
controls which became more pronounced in the 2nd year of the study.
There was no treatment-related effect on food consumption. These
differences in body weights were significant only at high dose.
There were no treatment-related changes in clinical chemistry or
haematological parameters. Gross pathological findings were generally
unremarkable. There was a slight non-significant increase in relative
liver weight at the high and intermediate dose levels in both males
and females. Microscopic examination of the tissues did not reveal any
increase in the incidence of neoplastic lesions. In the high- dose
group, 5 males and one female showed mild centrilobular hepatocellular
hypertrophy. The incidence of proliferative liver lesions in females
is shown in Table 2. The first appearance of hyperplastic nodules in
the liver of females was at week 96. Survival at this time in control,
low-, intermediate- and high-dose females was 64%, 56%, 50% and 50%,
respectively. Alitame, under the conditions of this assay, showed no
evidence of carcinogenicity in rats. The NOEL, based on body-weight
changes in both males and females, was 0.3% in the diet, equal to
130 mg/kg bw/day for males and 160 mg/kg bw/day for females
(Holmes et al., 1986a).
Table 2. Incidence of proliferative liver lesions in female rats
(Holmes et al., 1986a).
Control 0.1% 0.3% 1%
No. of livers examined 50 46 48 49
Focal nodular hyperplasia 1 0 3 9
Eosinophilic foci 4 0 3 11
A re-examination of the pathology slides of the 2-year
carcinogenicity study in Long-Evans rats (Holmes et al., 1986a) was
conducted on two subsequent occasions. At the first re-examination,
all available liver slides from all groups of female rats were
examined. The reported incidence of proliferative lesions is shown in
Table 3. There was no evidence of hepatocellular carcinomas in females
(Farrell, 1987).
Table 3. Incidence of proliferative liver lesions in female rats
(first re-examination; Farrell, 1987)
Control 0.1% 0.3% 1%
No. of livers examined 50 49 50 50
Hepatocellular adenoma 0 0 1 1
Eosinophilic foci 3 3 6 12
Hyperplasia 0 4 3 6
(focal and multifocal)
In the second re-examination of the pathology slides, slides
showing proliferative changes in female rats in either the original
study or in the first re-examination were examined using a revised set
of diagnostic criteria for hepatic proliferative lesions. The
incidence of these lesions is shown in Table 4. There was no evidence
of hepatocellular carcinomas in females (Hardisty et al., 1994).
Table 4. Incidence of proliferative liver lesions in female rats
(second re-examination; Hardisty et al., 1994).
Control 0.1% 0.3% 1%
No. of livers examined1 8 7 16 25
Hepatocellular adenoma 0 0 2 12
Hepatocellular hyperplasia 0 0 0 3
Eosinophilic cell foci 2 3 11 18
1 In this re-examination, only slides identified in the original
report and in the first re-examination as showing proliferative
lesions were examined.
Because of the low survival rate at 104 weeks in the Long Evans
rats (Holmes et al., 1986a), a second carcinogenicity study was
conducted with dietary administration and in utero exposure to
alitame in Sprague-Dawley rats. Groups of 70 male and 70 female
Sprague-Dawley rats were selected from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Levinsky et al., 1992). The groups were fed a diet containing
alitame (purity 99.3%) at concentrations of 0 (two control groups),
0.1, 0.3 or 1% for two years.
The animals were observed daily for clinical signs, and body
weights and food consumption were recorded weekly for the first
6 months and then every 4 weeks thereafter. Ophthalmological
examinations were performed pre-test and at 12 and 21 months; serum
chemistry, haematology and urinalysis measurements were taken at 6,
12, 18, 21 and 24 months. Tissues were collected from all animals
which died or became moribund during the study. At the end of the
study the animals were sacrificed and necropsied and tissues prepared
for light microscopy. Organ weights (liver, kidney, adrenal glands,
testes and ovaries) were recorded.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examination
revealed no treatment-related effects. Survival rates were high for
the first 12 months (>93%) but by 18 months average survival was 65%
for males and 75% for females. Groups reached 50% survival between 18
and 19 months for males and between 20 and 21 months for females.
Survival rates at 24 months in the 2 controls, low-, intermediate- and
high-dose groups were for males/females 9/37%, 4/23%, 7/31%, 9/24% and
26/17%, respectively.
Body-weight measurement began at birth for this study, and at
weaning (day 21). The mean body weights for the intermediate- and
high-dose animals were -9% and -21%, respectively, compared to the
control groups. Following selection of animals into groups for the
2-year study, the body weights expressed as percent of controls at the
low dose were -4% (M) and -2% (F), at the intermediate dose -7% (M)
and -10% (F), and at the high dose -18% (M) and -16% (F). For males,
over tile course of the study, the initial body weight differential
decreased as the treatment group showed increased weight gain compared
to the controls. Body weights peaked at approximately 15 months
followed by a gradual decline in all groups, with the high-dose group
showing the greatest decline. In females, over the course of the first
few months of the study, the initial body weight differentials
decreased particularly in the low-and intermediate-dose groups, which
showed increased weight gain compared to controls. After 6 months,
weight gain in the low- and intermediate-dose groups was similar to
controls. In the high-dose group, however, there was a decrease in
weight gain compared to the controls, and by the end of the study,
body weight differential had increased to -32%. Food consumption may
have been slightly lower in high-dose males, but was similar in all
groups of females.
There were no treatment-related changes in haematology or
urinalysis parameters. Changes observed in clinical chemistry data
included a decrease in serum triglyceride values (50-60%) in females
at the intermediate-dose in the first 18 months, and in high-dose
females throughout the study. Cholesterol was also increased in female
rats at 6 months at the intermediate-dose level (28%), and at 6 and 12
months at the high-dose level (67%).
Significant organ weight changes were seen in both liver and
kidney. In males, there was a relative increase in both kidney and
liver weight at high dose. In females, there was an absolute decrease
in kidney and liver weights at both intermediate and high doses, and
a relative increase in kidney weight at high dose. These changes
accompanied the decrease in body weight in high-dose females.
There was also an overall trend for decreased body weight in
intermediate-dose females which was not statistically significant.
The relative testes weight was increased in high-dose males.
Neoplastic changes were observed in the following organs: kidney,
liver, pancreas, lung, heart, thymus, mesenteric node, adrenal cortex,
thymus, parathyroid, uterus, cervix, brain, spinal cord, Zymbal's
gland, skin, bone, skeletal muscle, abdominal cavity, and soft
tissues. There was no evidence that the incidence of these neoplasms
was treatment-related. In the liver, hepatocellular carcinomas were
reported in one control male and one high-dose male. There was no
reported incidence of hepatocellular adenomas. There were also no
treatment-related non-neoplastic changes. Alitame, under the
conditions of this assay, showed no evidence of carcinogenicity in
Sprague-Dawley rats. The NOEL, based on body-weight changes, was 0.3%
in the diet, equal to 230 mg/kg bw/day for males, and 250 mg/kg bw/day
for females (Fisher et al., 1993).
2.2.4 Reproductive toxicity studies
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (75/sex) were administered a diet containing alitame
(purity 98-101%) at concentrations of 0, 0.1, 0.3 or 1% for either 2
weeks (females) or 12 weeks (males) prior to mating. Alitame-
containing diet was continued throughout pregnancy and lactation. Of
the F1 pups, 50 pups/sex/dose level were selected for a 24-month
carcinogenicity study, a further 25/sex/dose level were raised to
maturity and mated to produce the F2a litters. Approximately 3 weeks
after weaning of the F2a litters, the F1 animals were again mated
to produce the F2b litters. Both F2a and F2b pups were exposed to
alitame via the milk and in the food.
In the F0 parental group, the body weight of males was similar
for all groups during the 12 weeks of treatment, while body weight and
food consumption of females were slightly depressed during the 2-week
treatment period at the high-dose level. There was no treatment-
related effect on fertility or gestation indices, and no abortions
were noted. There was no difference in the number of pups born alive
between treated and control groups in the F1 litters. There was no
treatment-related effect on day 4 pup survival rates or lactation
indices. F1 pup weights of treated animals were similar to those of
control animals on days I and 4 of lactation, but high-dose pups had
significantly lower body weights on days 7, 14 and 21. However, these
decreased body weights were not significantly different from the
historical control data.
In the F1 parental group, body weights were similar to control
except at the high-dose level where there was a slight decrease in
body-weight gain. There were no treatment-related changes in pregnancy
rates or gestation length for either F2a or F2b litters. There was
no difference in the number of pups born alive between treated and
control groups in the F2a or F2b litters. Survival indices for F2a
and F2b were normal for all groups on days 1 and 4 of lactation.
F2a pup weight for all treated groups was similar on days 1,
4 and 7 of lactation but a significant decrease was noted in the
high-dose animals on day 14 and in all treated groups on day 21
compared to controls. The body weights were still within the
historical control data range. The F2b pups weight at the high-dose
level was significantly lower than controls throughout lactation,
while at the intermediate-dose level body weights were lower at days
14 and 21, and at the low-dose level, body weights were significantly
lower than controls on days 4 to 21. No historical control data were
available for F2b litters.
Gross pathological examination of the F0 males and females, the
F1 pups, and the F2a and F2b pups revealed no treatment-related
abnormalities. Postnatal development in F2b pups was assessed with
regard to locomotor activity, auditory function and ophthalmoscopic
examination. At the F2b weaning, there was a significant decrease in
both horizontal and vertical locomotor activity in F2a male and
female pups at the high-dose level, and a significant increase in
horizontal (but not vertical) locomotor activity in the F1 dams at
the high-dose level group. Pups tested for auditory function and for
ophthalmoscopic changes revealed no treatment-related changes
(Holmes et al., 1986b).
In a second 2-generation study, groups of 30 male and 30 female
Long-Evans rats (strain Blu:(LE)BR) were administered alitame (purity
98-101%) at concentrations of 0, 0.1, 0.3, or 1% for either 2 weeks
(females) or 12 weeks (males) prior to mating. Alitame-containing diet
was continued through pregnancy and lactation. Of the F1 pups,
25/sex/dose were raised to maturity and mated to produce the F2a
litters. Approximately 3 weeks after weaning of the F2a litters, the
F1 animals were again mated to produce the F2b litters. Both the
F2a and F2b pups were exposed to alitame in utero, during lactation,
and in the food following weaning.
At the high-dose level, 2 additional F1 groups of 25/sex were
exposed to alitame differently. These groups were introduced to
address the lactation effect observed in the first study. In one of
these groups, the F1 females were removed from treated diet at the
birth of F2a pups. In this group, the F2a pups received no alitame
during lactation. In the other group, the F1 females were removed
from the treated diet 3 weeks after weaning until the birth of the
F2a pups. In this group, the F2b pups received alitame only during
lactation. Animals were then placed on treated diet again. Post-natal
development of F2b pups was tested with an ophthalmoscopic
examination, an auditory function test, and a locomotor activity test.
Locomotor activity tests were also conducted on F0 and F1 parents,
and on F2a and F2b pups.
In the F0 parental animals, the body weights of males were
similar for all groups during the 12 weeks of treatment, while there
were decreases in body-weight gain and food consumption in females at
the high-dose level during the 2-week treatment period. Fertility
rates for all groups were normal. The number of live F1 pups was
similar for all groups at day 1, but was slightly reduced at the
high-dose level on day 4. This was explained by several large litters
at the high-dose level which declined in the first few days. F1 pup
body weight was similar for all groups on day 1 of lactation but was
significantly less at the high-dose level from day 4 onward.
Body-weight gain at high dose was similar to that of control groups
from days 4 to 21. The mean number of pups alive at day 21 compared to
the number of pups alive at day 4 (the lactation index) was similar
for all groups. Gross pathological examination of the F0 males and
females and the F1 pups revealed no treatment-related changes.
In the F1 parental group, the body weights of all female groups
were similar. Pregnancy rates and gestation times were normal. The
F1 males in the treated groups had slightly lower body weights than
controls although body-weight gain was similar for all groups. The
numbers of F2a litter live pups were similar for control, low and
intermediate groups. For the high-dose group, the mean number of pups
born alive (11.54) was slightly lower than controls (12.96). However,
the mean number of pups born alive in the other two additional
high-dose groups were similar to controls (12.75 and 12.13). The
4-day survival indices for the F2a pups in the control, low-,
intermediate-and high-dose groups were 0.98, 0.98, 0.93 and 0.89,
respectively. In the two additional high-dose groups, the 4-day
survival indices were 0.97 and 0.95. Lactation indices were for the
control, low-, intermediate- and high-dose groups 0.98, 1.0, 0.97 and
0.96, respectively, and for the two additional high-dose groups 0.99
and 0.88, respectively.
The mean body weight of high-dose F2a pups were slightly but
significantly lower than controls at birth and throughout lactation.
The additional F2a pups which were exposed only during lactation had
slightly lower body weight after the first week of lactation. The
additional group of F2a pups which had no alitame exposure during
lactation had body weights similar to controls at all times after day
1 of lactation. The results indicated a slight lactation effect of
alitame at the high-dose level, although the high-dose body weights
were still within the historical control ranges. The F2a pups at the
intermediate-dose level also had slightly but significantly lower body
weights at days 14 and 21 of lactation. External examination and gross
internal examination of the F2a pups revealed no treatment-related
changes.
The fertility rates and gestation times associated with the
production of the F2b litters were normal for all groups. The mean
number of pups born alive was comparable at the control, low- and
intermediate-dose levels, being 12.1, 11.7 and 13.0, respectively. At
the high-dose level, the number of live pups was slightly lower (9.8).
Survival during lactation was similar for all groups. The mean body
weight for the F2b high-dose level pups was significantly lower than
controls on day 14 of lactation. External examination and gross
internal examination revealed no treatment-related changes.
Ophthalmoscopic examination and auditory function tests were
normal in all groups. Locomotor activity for both parental groups and
pups showed considerable variation and the changes could not be
correlated with treatment.
This second study provided supporting evidence that alitame at
high-dose levels caused some suppression of body-weight gain during
lactation. This was further investigated in a study to examine the
effect of alitame in the cage bedding material on the pups body weight
(see section 2.2.5). The NOEL, based on body-weight changes in the
F2 pups, was 0.1% in the diet, equivalent to 100 mg/kg bw/day
(Holmes et al., 1986c).
In a 1-generation study in Sprague-Dawley rats, groups of 105
male and 105 female rats (strain Crl:CDBR VAF/Plus) were administered
alitame (purity 99.3%) at concentrations of 0, 0.1, 0.3 or 1% for 4
weeks prior to mating, through the cohabitation period, through the
gestation period, and up to day 21 of lactation. The aim of this study
was to produce an F1 generation of rats, continuously exposed to
alitame in utero, via the milk during lactation, and through the
feed for use in 1-year and 2-year toxicity studies. The animals were
observed daily for clinical signs of toxicity and body weights and
food consumption were recorded. During cohabitation, only the body
weights of the males were recorded. During gestation, body weights of
females were recorded on days 0, 4, 7, 14 and 20. During lactation,
dams and viable young were weighed on days 1, 4, 7, 14 and 21
post partum. On day 4, all litters were culled to 8 pups, 4
animals/sex. At day 21 (weaning), litters from the control groups, 0.1
and 0.3% treatment groups were culled to 2 animals/sex, and the
respective dams sacrificed. High-dose litters were not culled due to
their lower body weights, and were allowed to remain with the
high-dose dams until it was deemed prudent to separate them. When the
F1 animals were 3 to 6 weeks of age, 70 pups/sex/dose were selected
for a 2-year oncogenicity study (Fisher et al., 1993), and 20
pups/sex/dose level were selected for a 1-year toxicity study
(Fisher et al., 1992). All F0 males were terminated after the
cohabitation period. F0 females which did not deliver by day 24 of
gestation were terminated and their uteri and ovaries were examined.
F0 pups not used for either study were terminated.
Reductions in body weights and food consumption were noted in the
high-dose F0 animals, particularly during the first 2 weeks of the
study. This may be associated with palatability. There was no
treatment-related effect on mating, gestation length, or body weight
during gestation and lactation. The number of implantation sites was
slightly lower in the high-dose group and this was reflected in a
slight reduction in the total number of pups born alive per litter.
There was no difference between control and treated groups with
respect to post-implantation losses. There was no treatment-related
effect on pup survival during the lactation period. The mean
body-weight gains at the intermediate- and high-dose levels were
depressed compared to controls, and at weaning the body weights of the
intermediate- and high-dose groups were 9% and 21% lower than
controls, respectively. There were no internal or external
abnormalities in the pups not selected for the long-term studies
(Levinsky et al., 1992).
2.2.5 Special studies on neonatal survival
A study was conducted in Long-Evans pups and lactating dams in
order to further investigate the apparent decrease in pup body weight
during lactation at the high-dose level in the reproductive toxicity
studies. In this study, dams and pups were exposed to alitame which
was added to the bedding material rather than to the feed. It was
argued that dams and pups are normally exposed to treated feed in this
way because of tipping over of the feed by the dam, or the tendency to
scratch the feed into the bedding material. In this study, 2 groups of
8 litters were formed and all dams received normal diet. On lactation
days 1 and 5 and every 2-4 days thereafter, one of the groups had
125 g of feed containing 1% alitame sprinkled on the bedding. The
control group had feed without alitame sprinkled on to the bedding.
Body weights of dams and pups were measured throughout the lactation
period and at one week after lactation.
Body-weight gain in 'treated' dams was slightly lower than in
control dams on days 1 to 4 but there was no difference in body-weight
gain on days 4 to 21 of lactation. Body-weight gain in 'treated' pups
was decreased from day 2 and continued to be decreased throughout
lactation and one week after lactation. For example, the mean
body-weight difference on days 2, 7, 14 and 21 were 0.5 g (-8.4%),
1.6 g (-13.7%), 2.7 g (-11.3%), and 3.1 g (-7.4%), respectively. After
weaning, the weight difference continued to increase to 5.2 g (-7.2%)
at one week. The differences were statistically different from
controls on days 2, 3 and 5, but not thereafter. Locomotor activity
was measured in dam and pups, and no treatment-related effect was
noted.
The results suggested that bedding containing 1% alitame had a
treatment-related effect on pup body-weight gain, although no
mechanism for this effect was apparent. Food consumption was not
measured in this study although it was claimed to be normal. The
consumption of food by the dams from the bedding or from their fur was
considered to be negligible (Holmes et al., 1986d).
2.2.6 Special studies on embryotoxicity/teratogenicity
2.2.6.1 Rats
A group of 80 Crl:COBS-CD(SD)BR strain female rats were
inseminated and divided into 4 groups of 20 animals each. The groups
of presumed pregnant rats were administered alitame in 0.1% methyl
cellulose by gavage at dose levels of 0, 100, 300 or 1000 mg/kg bw/day
on days 6 to 15 post-insemination. The dams were examined throughout
the study period and body weights recorded regularly. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half were stained for skeletal anomalies and half
were examined for soft tissue defects. The ovaries and uteri of dams
were examined on day 20.
The pregnancy rate was 20/20 in control and intermediate-dose
groups and 18/20 in the low- and high-dose groups. There were no
deaths throughout the study and no clinical signs of toxicity. A
slight decrease in food consumption occurred on day 14 at the
high-dose level. Body weight was depressed significantly during and
after the treatment period at the high-dose level only. The number
of viable litters was similar for all groups, as was the number
of corpora lutea. There were slight decreases in the number of
implantation sites in the intermediate- and high-dose groups (10.8 and
10.6 respectively) compared to the control (11.8) and low-dose group
(12.2). There were also slight decreases in the number of fetuses in
the intermediate- (9.9) and high-dose groups (9.7) compared to the
control (11.0) and low-dose group 111.3). The implantation rate
decreased with increasing close (91.1, 89.8, 86.7 and 84.9%,
respectively), and the embryotoxicity rate increased with increasing
dose (6.4, 6.8, 7.4, and 8.4%, respectively). The sex ratio (M/F) was
decreased at the high-dose level (74/100) compared to controls
(110/110).
With regard to fetal development, there was no difference between
the fetal weight of control and treated animals. Examination of the
fetuses did not reveal any treatment-related increase in gross,
visceral or skeletal malformations. Among all the fetuses examined,
only one external anomaly (cleft palate) was observed. There was no
treatment-related change in the rate of ossification. Under the
conditions of this study, alitame showed no evidence of teratogenicity
in the rat. The decrease in the number of fetuses at the higher dose
levels appeared to be a direct result of the lower level of
implantation in these groups rather than to embryotoxicity (Perraud
et al., 1983a).
2.2.6.2 Rabbits
A group of 77 New Zealand white rabbits was inseminated and
divided into 4 groups of 19, 18, 20 and 20 animals. The groups were
administered alitame by gavage at dose levels of 0, 100, 300 or
1000 mg/kg bw/day on days 7 to 18 post-insemination. The does were
examined throughout the study period and body weights recorded
regularly. Animals were killed on day 28 post-insemination. Fetuses
were examined for external malformations then half were stained for
skeletal anomalies and half were examined for soft tissue defects.
Ovaries and uteri of does were examined on day 28.
Four animals died during the study, one at each dose level and
one in the control group. The pregnancy rate was 16/19, 15/18, 18/20
and 16/20 in each of the groups, respectively. Food restrictions were
introduced on days 12 to 19 to reduce the incidence of diarrhoea.
Body-weight loss was observed in all groups during this 8-day period.
A significant treatment-related decrease in body-weight gain was also
noted at the high-dose level.
The number of viable litters was similar for all groups as was
the number of corpora lutea. There was one abortion on day 24 in the
high-dose group. The number of implantation sites was slightly
decreased at the low-dose level compared to other groups.
Embryo-mortality rate was slightly higher at the intermediate- and
high-dose levels but was not dose-related, and not considered to be
due to treatment.
With regard to fetal development, fetal body weights were
slightly but not significantly decreased (7%) at the intermediate- and
high-dose level compared to the control group. Examination of the
fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral abnormalities. Among all the fetuses, there was
one with cleft palate and one with club foot in the intermediate-dose
group, and one with edema in the control group. The only visceral
abnormality seen was agenesis of the left kidney in one control group
fetus. There were no major malformations observed, but there was a
significant increase in the incidence of fetuses with supernumerary
13th ribs at the intermediate- and high-dose levels. Under the
conditions of this assay, alitame showed no evidence of teratogenicity
in the rabbit (Perraud et al., 1983b).
2.2.7 Special studies on genotoxicity
The results of the genotoxicity tests with alitame are summarized
in Table 5.
2.2.8 Special studies on pharmacological effects
Alitame was tested for its pharmacological activity in a battery
of systems designed to assess autonomic, gastrointestinal, renal and
metabolic functions.
A solution of 10-4 mol/litre alitame had no contractile or
relaxant activity in guinea-pig ileum, rat fundus, or rat vas
deferens, and did not interact with any of the six neurotransmitters
used in these systems. Alitame at an oral dose level of 25 mg/kg bw
caused no change in intestinal transit time nor in renal excretion of
fluids or electrolytes. Alitame at an oral dose level of 25 mg/kg bw
had no effect on fasting blood glucose levels or on the deposition of
an oral glucose load (Pearson et al., 1982).
Table 5. Summary of genotoxicity tests with alitame
Test system Test organism Concentration1 Result Reference
Reverse mutation in S. typhimurium 20-10000 µg/plate - Holden et al.,
bacteria TA1535, TA 1537, TA98, (-S9) (1981)
TA100 10-10 000 µg/plate
(+S9)
Urinary metabolites CD-1 mice/ 50-1000 mg alitame/kg - Holden et al,.
in vivo/Reverse S. typhimurium in mice & 0.05-0.75 ml (1981)
mutation in bacteria2 TA1535, TA 1537, TA98, urine/plate
TA100
Reverse mutation in Mouse lymphoma cells 220-1655 µg/ml (+S9) - Holden et al.,
mammalian cells L5178Y (1981)
Chromosome aberration in CD-1 mice bone 0-1000 mg/kg - Holden et al.,
vivo3 marrow cells (1981)
Table 5. Summary of genotoxicity tests with alitame (cont'd).
Test system Test organism Concentration1 Result Reference
Chromosome aberrations Human lymphocytes 0-1000 µg/ml - Holden et al.,
in vitro4 (1981)
Human lymphocytes 0-3000 µg/ml (+/-S9) - Amacher et al.,
(1991)
Micronucleus assay5 CD-1 mice 0-2000 mg/kg/bw/day - Amacher et al.,
(1991)
1 The purity of alitame tested was not provided.
2 Urine from treated CD-1 mice (14/group) was pooled over 18 h and treated with beta-glucuronidase.
3 Groups of 5 CD-1 mice were administered alitame orally and bone marrow cells harvested at 6, 12
and 24 h.
4 In the first study, there was minimal provision of methodology and reporting. The second study
was conducted under more recent GLP regulations.
5 Groups of 5 male and 5 female mice were treated over three consecutive days. Bone marrow was
prepared 24 h after file last dose.
2.2.9 Special studies on neurotoxicity and neurobehavioural
effects
In a neurotoxicity study, alitame was tested for its ability to
inhibit in vitro receptor binding in rat brain homogenate and to
produce in vivo neurochemical effects in rats. In the in vitro
binding assays performed with rat brain homogenate, alitame was tested
for its ability to inhibit the binding of a variety of receptor
radioligands to brain membranes. Neither alitame nor the B-isomer, at
concentrations of 10 µM, had any effect on the level of binding of
these ligands. In the in vivo study, groups of Sprague-Dawley rats
(8 males) were administered an oral dose of vehicle, alitame (25 mg/kg
bw), ß-isomer of alitame (6.25 mg/kg bw), or alitame (12.5 mg/kg bw)
plus ß-isomer of alitame (6.25 mg/kg bw). At 2 or 4 hrs, animals were
killed and the levels of dopamine, serotonin and their metabolites in
the brain tissue were measured. There was no significant change in
neurotransmitter levels after treatment (Bacopoulos, undated).
In a study to examine the effect of alitame on the behaviour of
rats, groups of male CD rats were administered a single oral dose of
vehicle, alitame (25 mg/kg bw), ß-isomer of alitame (6.25 mg/kg bw),
or alitame (12.5 mg/kg bw) plus ß-isomer of alitame (6.25 mg/kg). In a
separate experiment, animals were administered single oral doses as
above for 13 consecutive days. No treatment-related effect on
horizontal or vertical locomotor activity was noted in either the
single or multiple treatment regimes (Bacopoulos, undated).
In a study to examine the potential neurotoxicity of alitame in
rats following acute administration, groups of 12 male and 12 female
Sprague-Dawley rats were given alitame at a single oral dose of
0, 2000, 3000 or 5000 mg/kg bw. The animals were examined using a
functional observational battery (FOB) (both qualitative and
quantitative), a motor activity test, and an auditory startle
habituation test prior to treatment, at 5 and 7 h post-dosing, and
on days 1 and 7 post-dosing. At the end of the study, half the
animals were randomly selected for perfusion for possible future
neuropathological examination while the remaining animals were given
a full necropsy.
Significant decreases in body-weight gains were noted in all
treated groups on the day following treatment (days 0 to 1), followed
by compensatory increases on the following days (days 1 to 7). A
decrease in food consumption on day 1 was noted in all female treated
groups and in males of the intermediate- and high-dose groups. For the
high-dose female group, clinical symptoms included yellow abdominal/
urogenital fur staining, dehydration, reduced activity and pallor
between days 1 and 4 post-treatment. Effects were noted in all
behavioural tests for both sexes in the high-dose group and in some
tests in the intermediate- and low-dose groups on the first day. By
day 7, results of all parameters in all treatment groups were similar
to the control group.
Qualitative FOB assessments on day 1 post-treatment revealed
significant differences between the control and high-dose group for
both sexes. At the high-dose level, there was a significant decrease
in pinna reflex and a decrease in rearing in the arena. There was also
an increased incidence in ataxic gait and diarrhoea in the high-dose
animals. Animals at the lower dose levels also showed some increase in
minor symptoms. By day 7, there were no significant differences
between the groups. Quantitative FOB assessments found changes in
temperature and hind limb splay in treated groups on day 1 but by day
7 no significant differences were detected. Changes were also noted in
treated groups with respect to the motor activity test and the
auditory startle habituation test on day 1, but no changes were
evident by day 7. There were no treatment-related gross pathological
changes found on day 7. Transitory behavioural changes were observed
immediately after treatment and in the 24 h following treatment. By
day 7, all animals appeared normal and there was no evidence of
neurotoxicity (Robinson et al., 1993a).
In a 13-week study of the potential effects of alitame on
behaviour and neuromorphology in rats, 12 male and 12 female
Sprague-Dawley rats were fed a diet containing alitame at
concentrations of 0, 0.1, 0.3 or 1%. Animals were examined throughout
the study period for clinical signs of toxicity and body weights and
food consumption were recorded. The animals were examined using a
functional observational battery (FOB) (qualitative and qualitative),
a motor activity test and an auditory startle habituation at
pre-treatment, after 7 and 14 days of treatment and in the 5th, 9th
and 13th weeks. Passive avoidance testing was performed in weeks 4 and
13. At the end of the study, half the animals (randomly selected) were
perfused for neuropathological examination. Tissues from brain, spinal
cord, and skeletal muscle were prepared as paraffin sections and
haematoxylin and examination. Tissues from the peripheral nervous
system and the central nervous system were prepared in epoxy
embedding.
There were no mortality or adverse clinical findings related to
the treatment. The body weights of females in the 1% treated group
were significantly lower after 3 weeks and throughout the second and
third months of the study. The food consumption for this group was
also significantly decreased during the first week of the study. The
body weights and food consumption of all the other treated groups were
normal.
Both the qualitative (observations and handling) and the
quantitative FOB evaluations (grip strength, hind limb splay, and body
temperature) were unaffected by treatment for both sexes. In the
locomotor activity tests, significant differences at single time
points were noted for high-dose males and females. However, these
differences were not sustained over the course of the study and could
not be clearly related to treatment. For auditory startle habituation
tests, occasional significant differences in the 0.1% group were noted
but these were not attributed to treatment as the effects were not
sustained and there was no dose-relationship. In passive avoidance
performance at 4 weeks there were no significant differences between
groups. At week 13, more females in the 1% group completed the
training trial than in the control group. However, it could not be
concluded that this was related to treatment and was of doubtful
neurotoxicological significance.
There were no treatment-related gross pathological findings in
animals not selected for perfusion or in those which underwent
perfusion. A few animals, primarily males in all groups, had pale
areas on the livers. For rats undergoing perfusion, gross pathological
findings in the nervous system were limited to a few animals with dark
areas in the meninges. Histopathological examination was only
performed in control and high-dose animals. There were no significant
differences in the finding in the two groups. Under the conditions of
this study, there were no behavioural or neuromorphological changes
observed which could be attributed to treatment with alitame (Robinson
et al., 1993b).
In a study to examine the potential developmental neurotoxicity
of alitame, groups of female Sprague-Dawley rats were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for one week
prior to mating, then throughout the mating and gestation periods and
up to 10 days post-partum. Animals were observed throughout the
study period and body weights and food consumption recorded. All
females were given a complete necropsy. Dams were killed on days 21-23
post-partum.
The F1 pups were examined for malformations, sexed and the
number alive and dead recorded. The pups in each litter were weighed
individually on days 1, 4, 7, 11, 14, 17 and 21. On day 4, the litters
were culled to 8 pups, 4 of each sex. Pups were examined for pinna
unfolding (day 2), eye opening (day 12), and air righting reflex
(day 14). A motor activity test was conducted on day 17. Pups were
weaned on day 21.
The F1 adult generation was formed from at least 20 different
litters and consisted of 80 rats/sex/group. Animals were examined for
clinical condition and physical development and body weights and food
consumption recorded. From each litter, 3 males and 3 females were
randomly selected for behavioural testing (motor activity test,
auditory startle habituation test, passive avoidance test and
water-maze test). Young adults not selected for the adult F1
generation behavioural testing were sacrificed and underwent gross
pathological examination. Six animals/sex/group were perfused for
histo-pathological examination. Tissues from brain, spinal cord, and
skeletal muscle were prepared as paraffin sections and H and E
stained. Tissues from the peripheral nervous system and the central
nervous system were prepared in epoxy embedding.
In the F0 animals, there were no deaths and there were no
treatment-related clinical findings. The body weights of the 1%
treated animals were significantly decreased throughout the study.
The food consumption values for the 1% treated group were also
significantly lower than control values during the study. Gross
pathological examinations revealed no treatment-related effects. There
was no treatment-related effect on oestrus cycles, pregnancy rate,
gestation index, number of live and dead pups, sex ratio or post
implantation loss.
In the F0 pups, viability, survival, lactation indices,
clinical findings and gross pathology were unaffected by treatment. In
the 1% group, pup weight was significantly decreased on days 11, 14
and 17 and slightly decreased on day 21. There were no treatment-
related effects on physical or reflexological development, or on
locomotor activity.
In the F1 adults, there were no treatment-related effects on
mortality or clinical findings. The body weights of males and females
in the 1% group were significantly decreased. Food consumption was
normal. Physical development was not affected by treatment. In the
behavioural testing, there was no treatment-related effect on
locomotor activity, auditory startle habituation, passive avoidance or
water maze tests. Gross pathological and neuropathological
examinations revealed no effects which could be attributed to alitame
treatment. Under the conditions of this study, there were no
behavioural or neuromorphological changes in adults or pups which
could be attributed to treatment with alitame (Robinson et al.,
1993c).
2.2.10 Special studies on melanin binding
The potential for radiolabelled alitame or its metabolites to
bind to melanin in the eye of rats was tested by whole body
autoradiography and by microhistoautoradiography. Two male rats from
the albino strain, Sprague-Dawley (Crl:CD(SD)BR) and two male rats
from the pigmented strain, Lister Hooded (Crl:(LIS)BR) were
administered a single oral dose of 65 mg/kg bw 14C-alitame (94%
purity). At 2 and 24 h, one animal from each strain was killed and
the right eye of each excised and stored at -80°C for micro-
histoautoradiography. The remaining carcass was rapidly frozen at
-75°C for whole-body autoradiography.
Microscopic examination of the micrographs of the eyes revealed a
large number of silver grains distributed widely throughout the layer
of the retina, choroid and sclera in animals of both pigmented and
non-pigmented strains at both sacrifice times. The results of the
microscopy could not be interpreted and the data were considered to be
artifactual.
The results of the autoradiography revealed much higher levels of
radioactivity in the uveal tract (choroid, ciliary body and iris) of
the pigmented rats. Quantification of the autoradiographies revealed
concentrations of 2.99 µ equivalent alitame/g tissue in the uveal
tract of albino rats at 2 h and no detectable radioactivity at 24 h.
In the pigmented rats, the radioactivity in the uveal tract was
28.24/µg equivalent alitame/g tissue at 2 h and slightly higher levels
at 24 h (30.55 µg/g). There was no difference in radioactivity in the
surrounding tissues in either the albino or pigmented rat.
The study provided evidence of binding of alitame or its
metabolites to the melanin contained in the uveal tract of the
pigmented rats.
In an addendum to this report, alitame and two metabolites were
tested in vitro for their binding affinity to a melanin preparation
from bovine eyeballs. Alanine tetramethylthietane amide sulfoxide,
which was found in the urine of both rats and humans, did not bind to
melanin under the conditions of this assay, whereas its acetylareal
form, which was found only in the urine of rats, did bind to the
melanin. The positive control, doxepin, produced a higher level of
binding than found with the acetylated sulfoxide metabolite. This
study provided some evidence that the melanin binding observed in rats
may be associated with a metabolite formed only in the rat (Whitby
et al., 1994).
2.2.11 Special studies on the ß-isomer of alitame
2.2.11.1 Absorption, distribution, excretion, and biotransformation
In a study to examine the toxicokinetics of the ß-isomer of
alitame, Sprague-Dawley rats (4/sex) were administered a single oral
dose of the 14C-ß-isomer of alitame (10 mg/kg bw in water).
Excretion of radioactivity in urine and faeces was measured over 72 h.
A similar study was conducted with 6 male and 3 female Long-Evans rats
following a single oral dose of 14C-ß-isomer of alitame (25 mg/kg
bw). A separate group of 7 male Long-Evans rats were fitted with a
bile cannula. These animals received an oral dose of 25 mg/kg bw
14C-ß-isomer, and bile and urine were collected over 48 h.
In Sprague-Dawley rats, the bulk of the radioactivity was
excreted within 48 h, with 24% and 65% excreted in urine and faeces,
respectively. In Long-Evans male rats, total excretion was similar,
with 25 and 64% excreted in urine and faeces, respectively. Excretion
in the female rats was slightly slower. In bile-duct cannulated
animals, 13% of the total radioactivity was in bile over 48 h
(Pfizer, 1986).
In a study to identify the metabolites of the ß-isomer of
alitame, 2 male and 2 female Long-Evans rats were administered a
single oral dose of 10 mg/kg bw 14C-ß-isomer of alitame. In a second
experiment, female Long-Evans rats received 14C-ß-isomer of alitame
at an oral dose of 25 mg/kg bw. In a third experiment, 3 females and 6
males received the same dose of 14C-ß-isomer of alitame. Urinary
metabolites were examined by HPLC. Urinary radioactivity contained
approximately 1% of the radioactivity as unchanged ß-isomer in the
males.
Unchanged ß-isomer was found in the urine of female rats and
represented approximately 13% of the total radioactivity. Other
metabolites which represented approximately 50% of the radioactivity
were alanine tetramethylthietane amide sulfoxide (CP-58,290), alanine
tetramethylthietane amide sulfone (CP-57,254) and their acetylated
derivatives (CP-71,246 and CP-58,386). The only different metabolite
identified was the N-acetyl derivative of the ß-isomer which was
formed by acetylation of the free amine group of the aspartic acid
moiety. The N-acetyl derivative of the ß-isomer comprised 9% and 31%
of the radioactivity in the urine of male and female rats,
respectively. In faeces, the ß-isomer accounted for the majority
(70-81%) of the radioactivity (Pfizer, 1986).
In a study to examine systemic exposure to B-alitame and N-acetyl
beta-isomer by examination of urinary metabolites in male Long-Evans
rats, 14C-ß-isomer of alitame or 14C-N-acetyl -ß-isomer was
administered i.v. initially, then orally, at a dose level of 25 mg/kg
bw. Urine and faeces were collected at 24-hour intervals for a 48-hour
period. Systemic exposure was calculated from a comparison of urinary
excretion following the oral and i.v. routes. The systemic exposure to
ß-isomer following oral administration was 9% in males and 10% in
females. The systemic exposure to ß-isomer plus N-acetyl-ß-isomer was
8% in males and 18% in females (Pfizer, 1986).
In a study to examine systemic exposure to ß-isomer by
examination of serum concentrations in Long-Evans male rats,
14C-ß-isomer was administered i.v. initially (25 mg/kg bw), then
orally at a dose level of 29 mg/kg bw. Serum samples were collected
from 3 animals at various time-points and analyzed by UV absorption
for ß-isomer. Serum concentration-time AUC determinations were made
for each route of administration. The systemic exposure to ß-isomer
following oral administration was 9.6%. The elimination half-life was
29 minutes following the i.v. dose (Pfizer, 1986).
In a study to examine the toxicokinetics and metabolism in dogs,
2 male and 2 female beagle dogs were administered an oral dose of
14C-ß-isomer of alitame followed by an unlabelled dose of 5 or
50 mg/kg bw. Urine and faeces were collected for 96 h. The two male
dogs excreted 35 and 39%, respectively, of the administered
radioactivity in the urine, and the females 14 and 16%, respectively.
Unchanged 13-isomer in urine accounted for 10-16% of the dose in all 4
dogs. All of the urinary metabolites were identical to the ones found
after administration of alitame, but the males had higher levels of
metabolites than the females (Pfizer, 1986).
2.2.11.2 Short-term toxicity studies
In a 3-month study, groups of 15 male and 15 female mice (Strain
Crl: COBS CD-1 (ICR) BR) were fed a diet containing the ß-isomer of
alitame (CP-63,884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption were recorded weekly. Urinary metabolites were
analyzed at weeks 11 and 13. Gross pathological examination was
performed on all animals and organ weights recorded. Tissues were
taken from all of the major organs for histopathological examination.
There was no treatment-related effect on body-weight gain
although the average body weight of treated male animals was lower
than in control groups. Food consumption was slightly decreased at
high dose in males. One of the urinary metabolites identified was the
N-acetyl derivative of the/%isomer. The amount of the isomer increased
proportionally with the dose. There were no treatment-related effects
on organ weights. Gross pathology was unremarkable and histopathology
did not reveal any treatment-related changes. The NOEL in this study
was >25 mg/kg bw/day (Stadnicki et al., 1986a).
In a 3-month study, groups of 15 male and 15 female Long-Evans
rats (Strain Crl: (LE) BR) were fed a diet containing ß-isomer of
alitame (CP-63884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption data were recorded weekly. Ophthalmoscopic
examinations were performed at pre-test and prior to necropsy. Blood
samples were taken for examination of clinical chemistry and
haematological parameters at pre-test and at 3, 9 and 13 weeks.
Urinary analyses were performed at 3, 9 and 13 weeks. At the end of
the study, gross pathological examinations were performed and tissues
from the major organs taken for histopathology.
There were no deaths through the study and the body-weight gains
and food consumption were similar for treated and untreated groups.
There were no treatment-related effects on clinical chemistry,
haematology or urinary parameters. There were no differences between
control and treated groups with respect to organs weights. Gross
pathology and histopathology were unremarkable The NOEL in this study
was > 25 mg/kg bw/day (Stadnicki et al., 1986b).
2.2.11.3 Special studies on embryotoxicity/teratogenicity
A group of 88 albino female rats (strain Crl:COBS-CD (SD)BR) was
inseminated and divided into 4 groups of 22 animals. The groups of
presumed pregnant rats were administered the 3-isomer of alitame
(CP-63,884) in distilled water by gavage at dose levels of 0, 25, 50
or 100 mg/kg bw/day on days 6 to 15 post-insemination. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half stained for skeletal anomalies and half
examined for soft tissue defects.
The pregnancy rate was 20/22 in the control and low-dose groups,
and 19/22 in the intermediate- and high-dose groups. There were no
deaths throughout the study, nor any clinical signs of toxicity. There
were no treatment-related effects on body weight or food consumption
of dams during or after the treatment period. The number of corpora
lutea, implantation sites and viable fetuses was similar for all
groups. With regard to fetal development, there was no difference
between fetal weight of control and treated animals. Examination of
the fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral malformations, nor any treatment-related effects
on the degree of ossification. Under the conditions of this study, the
ß-isomer of alitame showed no evidence of teratogenicity in rats
(Kessedjian et al., 1986).
2.2.11.4 Special studies on genotoxicity
The ß-isomer of alitame was tested for the induction of point
mutations, both base-pair and frameshift, at the his locus of
Salmonella typhimurium strains TA 1535, TA 1537, TA 98 and TA 100,
with or without S9 microsomal activation. The purity of the alitame
was not provided. Doses ranged from 20 to 10 000 µg/plate, with or
without activation. There was no increase in revertants above the
control (water) in any of the treated plates at any dose level. The
positive controls, 9-aminoacridine, nitrofurantoin, sodium nitrite,
2-nitrofluorene and 2-anthramine gave appropriate responses (Ellis
et al., 1991).
2.3 Observations in humans
In a 90-day double-blind tolerance study, groups of male and
female subjects (77 treated, 80 controls) were administered either
alitame in capsules divided over three meals at a dose level of
10 mg/kg bw/day for 90 days, or placebo in the same manner. This dose
was chosen because it is 10 times greater than the estimated daily
intake if all sucrose in the diet were replaced with alitame. Subjects
were assessed throughout the study period for side-effects (by
interview and observation). Other tests included blood pressure,
temperature, body weight, electrocardiogram, and ophthalmoscopic
examination. Various clinical pathology parameters were also measured.
Microsomal activation was estimated from the elimination kinetics of
aminopyrine at pre-dosing and at weeks 6 and 13 of treatment. Plasma
and urine samples were collected at various time points throughout the
study.
Four treated subjects and 2 control subjects discontinued from
the study due to side effects (angioedema of the lips, maculopapular
rash, urticaria (wheals) with erythema in treated subjects, and
tinnitus, dry eyes and abdominal cramps in the control subjects). A
rechallenge with alitame after 6 months produced none of the above
symptoms, making the possibility of allergic reaction very unlikely.
There were no treatment-related changes in blood pressure, pulse,
temperature or body weight. Electrocardiograms and ophthalmological
examinations were unremarkable. Haematological parameters were similar
in treated and control subjects. Measurement of aminopyrine
elimination rates at 6 and 13 weeks did not reveal any increase in
hepatic microsomal enzyme activity due to alitame treatment (Gerber
et al., 1987).
In a 90-day double-blind tolerance study in diabetic subjects,
groups of male and female subjects having either type I or type II
diabetes (75 treated, 80 control) were administered either alitame in
capsules divided over three meals at dose level of 10 mg/kg bw/day for
90 days, or placebo in the same manner. This dose was chosen because
it is ten times greater than the estimated daily intake if all sucrose
in the diet were replaced with alitame. Subjects were included on the
following criteria: diabetics of type I and type II, either sex and
aged between 15 and 70 years with stable diabetic therapy; fasting
blood glucose <250 mg/dL; free from investigational drugs; had not
suffered a myocardial infarction within the previous 6 months; and had
either no hypertension or it was controlled. Subjects were excluded on
the following criteria: females which were pregnant or nursing; drug
or alcohol dependence; systemic disease other than diabetes; clinical
chemistry and haematological parameters above the upper limit of
normal; hepatic disease; and donation of blood within 4 weeks prior to
the study. The groups were well matched with respect to such
parameters as age, sex distribution, weight, smoking, drinking,
intercurrent illnesses and medication as well as diabetic status.
Subjects were assessed at weeks 0, 1, 2, 3, 4, 6, 8, 10 and 13 of
the study period for side effects (by interview and observation).
Effects on diabetic control were assessed by measuring fasting
haemoglobin A1c and 2-hour postprandial blood glucose levels at
baseline and weeks 0, 6, 8 and 13. Physical examinations were
conducted at screening and at week 13 of the study and included blood
pressure, temperature, body weight and electrocardiogram. Clinical
pathology parameters (haematology, clinical chemistry and urinary)
were measured pre-treatment and at 13 weeks.
At the end of the study period, 16 treated and 9 control subjects
had discontinued from the programme and, of these, 5 treated and 2
control subjects withdrew due to illness. No subjects discontinued due
to side effects or abnormal laboratory parameters related to alitame
treatment. Side-effects were observed in 29% of treated subjects and
25% of control subjects. Gastrointestinal disturbances were the most
common side-effect. Incidence and severity of particular side-effects
were relatively evenly distributed between the groups. No significant
changes were noted in either the control or treated groups with
respect to diabetic control, clinical chemistry, haematological or
urinary parameters. No treatment-related effects were apparent from
the physical examinations, blood pressure and pulse rate, temperature,
body weight or EKG recordings.
Alitame at a dose of 10 mg/kg/bw/day for 90 days was well
tolerated by the type I and type II diabetic patients who continued in
this study. There was no effect of alitame at this dose level on the
ability to control serum glucose levels by these diabetic patients
(McMahon et al., 1989).
In a 1- and 2-year follow-up of the subjects in the 90-day study
in diabetics, all but one of the treated subjects received an
interview and complete physical examination. Subjects were interviewed
again within 24 months post-study for all but 6 treated and 4 control
subjects.
A variety of intercurrent illnesses were reported during the
follow-up period. Most of the reported illnesses related to diabetes
and its complications. The incidence of intercurrent illnesses during
the follow-up period was 40 treated and 36 control subjects. Two
subjects died during the follow-up period, both treated subjects. One
subject (type I diabetic) died during the month following the study,
cause unknown. At completion of the study no adverse reactions had
been noted in this subject. The second subject (type II diabetic)
suffered a fatal heart attack resulting from hypertensive
atherosclerotic cardiovascular disease.
At the first follow-up (12 months), 5 treated subjects and no
control subjects were found to have suffered myocardial infarction.
The possibility that the results were due to chance was examined using
available epidemiological data on the incidence of myocardial
infarction. Data from the US National Hospital Discharge Survey (NHDS)
were used to estimate the rate of myocardial infarctions among
diabetics in various age groups for both sexes. These data were
adjusted to take into account the number of myocardial infarction
casualties which are not admitted to hospital and the actual patient
years of observation. Based on the hospitalization data for diabetics
who suffered myocardial infarction in 1986, the numbers of subjects
expected to suffer a myocardial infarction in a single year were 2.9
and 3.2 in the treated and control groups, respectively. The observed
rates of 5% and 0%, respectively, were within the 95% tolerance
intervals of incidence expected based on the NHDS analysis. Two years
after the study, myocardial infarctions had been encountered by 6
treated and 3 control subjects. The statistical analysis of these data
at 2 years supports the null hypothesis that the incidence observed
were due to chance. The data indicate that in the two years after the
90-day study of alitame in diabetics, no evidence was provided that
alitame adversely affected the health of the study groups (McMahon
et al., 1989).
3. COMMENTS
A number of toxicity studies on alitame were available, as well
as studies on the ß-isomer of alitame, which is formed at low levels
in some foods.
In metabolism studies conducted in three animal species and in
humans, alitame was readily absorbed from the GI tract and then
rapidly metabolized and excreted. In mice, rats and dogs, the route of
metabolism involved cleavage of the peptide bond yielding aspartic
acid and D-alanine tetramethylthietane amide, the latter being further
biotransformed by oxidation at the thietane sulfur to form the
sulfoxide and sulfone. Acetylated derivatives of these metabolites
were commonly found in the urine of rats and dogs whereas, in humans,
the glucuronide derivative of D-alanine tetramethylthietane amide was
the major urinary metabolite. In rats, initial cleavage of the peptide
bond of alitame took place in the lumen of the jejunum. In pregnant
rats, alitame was readily transferred transplacentally, but there was
no evidence of active secretion in the milk. In a study in rats,
levels of alitame residues decreased rapidly in all tissues except the
eyes. Further studies indicated that it was bound to melanin contained
in the uveal tract (choroid, ciliary body and iris) and suggested that
such binding was associated with a metabolite formed only in rats. The
Committee considered that an in vivo study in a mammalian species
other than the rat is desirable to clarify the significance of the
retention of alitame in the eyes.
The ß-isomer of alitame was rapidly metabolized in rats, the only
urinary metabolite not also formed with alitame being the N-acetyl
derivative of this isomer. There was no evidence of the formation of
significant amounts of the ß-isomer of alitame in the stomach or small
intestine of the rat.
Acute and short-term toxicity studies indicated that the toxicity
of alitame is low. In studies conducted in mice, rats and dogs, the
major toxicological changes observed were associated with the liver. A
dose-related increase in liver weight and evidence of centrilobular
hypertrophy were seen in all of the studies in these three species,
and these changes were accompanied by an increase in the level of
hepatic microsomal enzymes. Short-term studies on hepatic enzyme
induction indicated that alitame was a weak inducer of microsomal
enzymes; the pattern of induction was qualitatively similar to that
caused by phenobarbitone but at a lower level. The liver changes were
indicative of an adaptive response and, in rats and dogs, withdrawal
of treatment after a 3-month period resulted in partial or complete
reversal of the observed changes within a month.
Two-year carcinogenicity studies have been conducted in CD-1
mice, Long-Evans rats and Sprague-Dawley rats. The two studies
conducted in rats included an in utero phase. There was no evidence
of carcinogenicity in the study in mice.
In rats, the first carcinogenicity study was conducted in
Long-Evans rats at dietary levels of 0.1, 0.3 or 1% and, while the
survival rate was greater than 50% in all groups at 22 months, poor
survival was noted at 24 months. Pathological examination of the liver
indicated a higher incidence of focal nodular hyperplasia and
eosinophilic foci in females at the 1% dose level than in controls,
according to the diagnostic criteria of Squire & Levitt (1975). Two
subsequent independent re-examinations of these data have been
performed using the diagnostic criteria of Maronpot et al. (1986).
In the first re-examination (Farrell, 1987), the higher incidence of
eosinophilic foci in females in the high-dose group was confirmed, but
the incidence of focal hyperplasia or hepatocellular adenomas was
similar to that in controls. In the second re-examination (Hardisty
et al., 1994), the higher incidence of eosinophilic foci in females
in the high-dose group was again confirmed. A higher incidence of
these foci was also found at the mid-dose level. Also in this
re-examination, the nodular hyperplastic lesions noted in the original
report were largely reclassified as hepatocellular adenomas, and an
increased incidence of these lesions was seen at the high-dose level.
No evidence of hepatocellular carcinomas was found in female rats in
the original examination or in the subsequent re-examinations. The
main difference between the first and second re-examinations was in
the nomenclature used, rather than in the recognition that lesions
existed. The Committee expressed concern at the change in the way that
these liver lesions were classified, possibly as a result of the use
of different diagnostic criteria. It considered that there was
evidence of a higher incidence of adenomas in females in this study,
at least at the high dose level. The adenomas were found late in the
study and, while considered unlikely to progress to hepatocellular
carcinomas, the data were inconclusive in this regard.
A second carcinogenicity study, this time in Sprague-Dawley rats,
was conducted because of the low survival rate at 24 months in the
Long-Evans rats. In this second study, the survival rate was lower at
both 22 and 24 months than that found in the Long-Evans rats, and
hence the study was considered inadequate to provide further
information regarding the potential carcinogenicity of alitame.
The Committee considered that the available studies did not
indicate that alitame was carcinogenic, but that they were deficient
in certain respects and did not fully address this question. It also
considered that further research to clarify the mechanism of the
formation of adenomas in female Long-Evans rats was desirable.
Genotoxicity studies both in vitro and in vivo did not
provide any evidence that alitame has a mutagenic potential.
Similarly, there was no evidence from studies conducted in rats and
rabbits that alitame has a teratogenic potential.
Two reproductive toxicity studies (2-generation) were conducted
in Long-Evans rats and one reproductive toxicity study (1-generation)
in Sprague-Dawley rats. The first reproductive toxicity study with
Long-Evans rats produced some evidence for slightly decreased
body-weight gain in the pups during lactation at 1% in the diet, the
highest dose administered. A similar effect was noted in the second
reproductive toxicity study at the same dose level, but not in the
1-generation reproductive toxicity study with Sprague-Dawley rats. A
study of the effects of alitame-containing diet in the bedding
material, which could have been a source of alitame for pups due to
spilled feed, while providing a possible explanation for the decrease
in pup body-weight gain, did not fully address this issue. A slight
effect of alitame on locomotor activity seen in the first reproductive
toxicity study was not substantiated in subsequent studies. The
Committee did not regard the changes seen in the reproductive toxicity
studies to be of toxicological significance.
Neurotoxicity and neurobehavioural toxicity studies using an
observational battery of tests indicated behavioural changes only when
very high doses were administered to rats as a single dose by gavage
(5000 mg/kg bw). These behavioural changes were of short duration and
animals appeared to be normal by day 7. There was no evidence of
neurotoxicity in rats fed diets containing 1% alitame (equivalent to
1000 mg/kg bw/day) over a 3-month period. Similarly, no changes
indicative of neurotoxicity were observed in dams or pups when the
same dose level was given over the reproductive period.
In a 14-day study conducted in humans there was no evidence of
hepatic enzyme induction at a dose level of 15 mg/kg bw/day.
Ninety-day tolerance studies were conducted in normal and
diabetic subjects. There was no evidence of adverse effects in
subjects of both types during the period of the study at a dose level
of 10 mg/kg bw/day. However, in the 1- and 2-year follow-up of the
diabetic subjects, there was a higher incidence of myocardial
infarction in the treatment group than in the controls, namely 5/58 in
the treatment group and 0/71 in the controls after 1 year, and 6/53 in
the treatment group and 3/67 in the controls after 2 years. While the
differences at 2 years were not statistically significant, and there
was no indication of cardiovascular effects in the animal studies, the
Committee considered that the increased incidence of myocardial
infarction in the treatment group has not been fully explained. It was
advised that a further study is to be performed in diabetic subjects.
Studies conducted with the ß-isomer of alitame provided no
evidence of genotoxicity, teratogenicity or systemic toxicity in mice
or rats at dose levels of up to 25 mg/kg bw/day.
4. EVALUATION
The Committee recognized that the basis on which to consider
establishing an ADI for a chemical such as alitame is difficult to
specify, since the most sensitive treatment-related effects observed
were liver enzyme changes and liver weight changes, which may be
adaptive in nature. For alitame, these changes appeared to be closely
linked. While the prolonged hepatomegaly observed was not accompanied
in the long-term studies by any pathological changes indicative of
toxicity, it is not considered desirable. On the other hand, moderate
induction of hepatic enzymes is considered a normal adaptive process.
In the case of alitame, significant changes in body-weight gain or
liver weight were observed at doses above the NOELs. In the 18-month
study in dogs, the NOEL was 100 mg/kg bw/day. In the 2-year study in
mice, the NOEL was 0.1% in the diet, equal to 125 mg/kg bw/day. In the
lifetime studies in rats, the NOEL was 0.3% in the diet, equal to
130 mg/kg bw/day in Long-Evans rats and 230 mg/kg bw/day in
Sprague-Dawley rats.
The Committee concluded that the concerns raised by the
deficiencies in the carcinogenicity studies in rats were of such
significance that an ADI could not be allocated.
5. REFERENCES
AMACHER, D.E., MUEHBAUER, P.A., ZELLJADT, I. (1991) Genetic Toxicology
Report. Unpublished Report No. 91-444-21 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
BACOPOULOS, N.G. (undated) Investigation of alitame and its b-isomer
in neurochemical and behavioural animal models. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1984) Alitame: A Fourteen Day
Human Enzyme Induction Study. Unpublished Report No. 32 from
Bio-Organic Chemicals Research & Development, Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1985) Human Disposition of
14C-Alitame. Unpublished Report No. SC-2 from Department of
Pharmacology, University of London. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M., THATCHER N.J., MARSHALL, A.D. (1994)
Assessment of the Enzyme Inducing Characteristics of Alitame.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School., London, UK. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M. /1994) Assessment of the Enzyme Inducing
Characteristics of Alitame Suppl. Report on Ummunodetection of
Cytochrome P450 Isoenzymes in the Liver of Alitame-Treated Rats.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School, London, UK Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981a)
Alitame: Two-Week Range-Finding Feeding Study in Rats. Unpublished
Report No. 81-055 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981b)
Alitame: Two-Week Oral Range-Finding Study in Dogs. Unpublished Report
No. 81-070 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ELLIS, J.H., AMACHER, D.E., KLUWE, W.M. (1991) b-Isomer of Alitame:
Microbial Gene Mutation Assays. Unpublished Report from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
FARRELL, J.F. (1987) Histopathologic evaluation of selected
microslides from chronic rat and mouse feeding studies with alitame.
Unpublished Report No. PR-116 from Experimental Pathology Laboratories
Inc. USA. Submitted to WHO by Pfizer Inc., Groton CT, USA.
FISHER, D.O., MARSH, P.M., SOLER, G.N., COLEMAN, D.V., LEVINSKY, H.V.,
ESTES, P.C., DOUGHERTY, W.J. (1992) One-Year Toxicity Study with
Dietary Administration and In Utero Exposure in Sprague-Dawley Rats.
Unpublished Report No. 90-444-18 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
FISHER, D.O., LAITWAITE, B.S., BUTLER, B.T., COLEMAN, D.V., LEVINSKY,
H.V., OCHOA, R., DOUGHERTY, W.J. (1993) Two Year Oncogenicity Study
with Dietary Administration and In Utero Exposure in Sprague-Dawley
Rats. Unpublished Report No. 90-444-20 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
GERBER, N., McMAHON, M.D., WILLIAMS, R.L. (1987) Double-Blind Placebo
Controlled Ninety-Day Toleration Study of Alitame. Unpublished Report
No. 101 from Ohio State University, Columbus, Ohio. USA. Submitted to
WHO by Pfizer Inc. Groton, CT, USA.
HARDISTY, J.E., ABBOTT, D., GOPINATH, C., WARD, J. (1994) Pathology
Working Group Review of Two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report from Experimental Pathology
Laboratory Inc., Research Triangle Park, NC, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLDEN, H.E., ELLIS, J.H., AMACHER, D., RAY, V.A. (1981) Alitame:
Genetic Toxicology Report. Unpublished Report No. 91-444-21 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., TOOLAN, L.A, BURGAN, J.J., REYNOLDS, J.A., LEVINSKY,
H.V., ESTES, P.C. (1983a) Alitame: Two-Month Range-Finding Feeding
Study in Mice. Unpublished Report No. 82-444-03 from Pfizer Central
Research, Groton, CT, USA Laboratoires. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., ANGUS, T.A., FISHER, D.O., FIGDOR, S.K., REYNOLDS, J.A.,
ESTES, P.C., LEVINSKY, H.V. (1983b) Alitame: Five-Week Feeding Study
in Rats. Unpublished Report No. 82-444-01 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton,
CT, USA.
HOLMES, C.L., MARTZ, F., BOWDEN, P.R., FIGDOR, S.K., ROSS, P.E.,
LEVINSKY, H.V., ESTES, P.C.(1985a) Alitame: One-Year Feeding Study in
Long-Evans Rats. Unpublished Report No.s. 82-444-06 and 83-444-12 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., MARTZ, F., PUNZALAN, E.R., FIGDOR, S.K., ROESLER, A.R.,
LEVINSKY, H.V,, ESTES, P.C. (1985b) Alitame: Eighteen-Month Feeding
Study in Beagle Dogs. Unpublished Report No. 82-444-07 from
Laboratoires Pfizer Centre de Recherche, 37400 Ambroise, France.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., CACCIATORE. A., BURGAN, J.J., COLEMAN, G.L., FIGDOR,
S.K., LEVINSKY, H.V., REYNOLDS, J.A., ESTES, P.C., (1985c) Alitame:
Two-Year Oncogenicity Feeding Study in Mice. Unpublished Report
No. 82-444-08 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L. (1986a) Alitame: A two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report No. 83-444-11. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986b) Alitame: Two-Generation Reproduction
Feeding Study in Rat. Unpublished Report No. 83-444-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986c) Alitame: Second Two-Generation
Reproduction Study in Rat. Unpublished Report No. 83-444-13/14/15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROSE, E.M., STRADNICKI, S.W., LEVINSKY, H.V.
(1986d) Exploratory bedding study in the Long-Evans Rat. Unpublished
Report No. 85-444-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
KESSEDJIAN, M.J., STADLER, J., POOL, W.R., (1986) b-Isomer of Alitame:
Fetotoxicity Study in Rats by the Oral Route. Unpublished report
86-086 from Pfizer Central Research, Groton, CT, USA. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., BEUTER, N.J., STADNICKI, S.W. (1983) Alitame: Acute
Oral Toxicity Studies in Mice and Rats. Unpublished Report
No. 83-444-09 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., STADNICKI, S.W., ESTES, P.C., DOUGHERTY, W.J.,
CACCIATORE, A., KOPCYK, N., LUNDEEN, G.R. (1992) In Utero Exposure
with Alitame in Sprague-Dawley rats. Unpublished Report No. 90-444-19
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MARONPOT, R.R., MONTGOMERY Jr C.A., BOORMAN, G.A., McCONNELL (1986)
National toxicology programme nomenclature for hepatoproliferative
lesions of rats. Toxicol. Pathol., 14: 263-273.
McMAHON, F.G., CYRUS, J., RASKIN, P., STARR, I. (1989) Double-Blind
Placebo Controlled Ninety Day Toleration Study of Alitame in
Diabetics. Unpublished Report No. 102 from Central Research Centre,
New Orleans, LA, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
MONRO, A.M. CHVEDOFF, M., GREAVES, P., NACHBAUR, J., PERRAUD, J.,
(1982a) Three-Month Oral Toxicity Study in Rats plus 1-Month
Withdrawal. Unpublished Report No. 81-086 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MONRO, A.M., CHVEDOFF, M., PERRAUD, J., GREAVES, P., NACHBAUR, J.,
(1982b) Alitame: Three-Month Oral Toxicity Study in Dogs plus 1-Month
Withdrawal. Unpublished Report No. 81-106 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
PEARSON, D.L., GAYNOR, B.J., SWINDELL, A.C. (1982) General
Pharmacology Report on Alitame. Autonomic, Gastro-intestinal, Renal
and Metabolic Studies. Unpublished Report. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J. (1983a) Alitame: Foetotoxicity Study in
Rats (FDA Segment II). Unpublished Report No. 82-154 from Laboratoires
Pfizer Centre de Recherche, 37400 Ambroise, France. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J., SENKOWSKI, S. (1983b) Alitame:
Foetotoxicity Study in Rabbits (FDA Segment II). Unpublished Report
No, 83-026 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
PFIZER CENTRAL RESEARCH. (1986) Alitame: Metabolism and Disposition.
Unpublished Report from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., BROXUP, B., NOVEROSKE, J.W. (1993a) An Acute Study of
the Potential Effects of Orally Administered Alitame on Behaviour in
Rats. Unpublished Report No. 97148 form Bio-Research Laboratories Ltd.
Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W. (1993b) A
13-Week Dietary Study of the Potential Effects of Alitame on Behaviour
and Neuromorphology in Rats. Unpublished Report No. 97149 form
Bio-Research Laboratories Ltd. Senneville, Quebec, Canada. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W., (1993c) A
Developmental Neurotoxicity Study of Alitame Administered in the Diet
of Rats. Unpublished Report No. 97150 from Bio-Research Laboratories
Ltd. Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT. USA.
SQUIRE, R.A., LEVITT, M.H. (1975). Report of a workshop on a
classification of specific hepatocellular lesions in rats. Cancer
Res., 35: 3214-3223.
STADNICKI, S.W., PUNZALAN. E.R., FIGDOR, S.K., ROESLER, A.R., ESTES,
P.C., LEVINSKY, H.V. (1986a) b-Isomer of Alitame: Three-Month
Feeding Study in Study CD-I Mice. Unpublished Report No. 86-580-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
STADNICKI, S.W., SANTACROCE, E.M., FIGDOR, S.K., ROSS, P.E.,
ESTES, P.C., LEVINSKY, H.V. (1986b) b-Isomer of Alitame: Three-Month
Feeding Study in Long-Evans Rats. Unpublished Report No. 86-580-01
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
WHITBY, B.R., POWLES, P., LEWSLEY, R., FERNYHOUGH, P., MARSLAND, R.
(1994) 14C-Alitame: Whole-body autoradiography and microhistoauto-
radiography in the rat. Unpublished Report No. 727/5-1011 from
Hazleton Europe, Harrogate, UK. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
In a study in lactating dams, metabolites in milk were alanine
tetramethylthietane amide sulfoxide (CP-58,290) and its acetylated
form (54.4%), alanine tetramethylthietane amide (CP-57,207; 31.4%),
and unchanged alitame (1%) (Pfizer, 1986).
In a study to examine the effect of dose on metabolic pathways,
Long-Evans rats received a single oral dose of 10, 100 or 1000 mg/kg
bw 14C-alitame and urine and faeces were collected at 24-hour
intervals over 3 days. Urinary metabolites were analyzed by HPLC.
There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment, but the
metabolic profile was essentially unchanged (Pfizer, 1986).
In a study to compare the metabolic fate of D-alanine as either
free alanine or when incorporated into alitame, Long-Evans rats were
administered 14C-D-alanine or 14C-alitame (1-14C-D-alanine
label) by oral gavage and exhaled 14CO2 was measured. While
14CO2 was extensively exhaled following administration of
D-alanine, no 14CO2 was expired after administration of
14C-alitame, demonstrating the stability of the D-alanine
tetramethylthietane amide bond to metabolic hydrolysis (Pfizer, 1986).
2.1.2.3 Dogs
In a study to examine metabolites in plasma, groups of 3 male and
3 female beagle dogs received a single oral dose of 5 or 25 mg/kg bw
14C-alitame. Plasma samples were taken at various times and the
metabolites in plasma identified by HPLC. In both male and female
dogs, plasma radioactivity peaked at 2-3 h. The major metabolite was
alanine tetra-methylthietane amide sulfoxide (CP-58,290; 80%), with
10% in the acetylated form. Approximately 5% was unchanged alitame.
The remainder was alanine tetramethylthietane amide (CP-57,207), its
sulfone derivative (CP-57,254) and the acetylated forms of these
compounds. The metabolite profile was very similar to that seen in the
rat (Pfizer, 1986).
In a study to examine the metabolites in urine, a group of 3 male
and 3 female beagle dogs received a single oral dose of 14C-alitame,
and 24-hour urine and faeces collected. Urinary metabolites were
analyzed by HPLC. In males, the major metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 80%), including 9.5%
as the acetylated form. Approximately 5-15% of the urinary
radioactivity was unchanged alitame (Pfizer, 1986).
In a study to examine metabolites in faeces, a male beagle dog
received a single oral dose of 10 mg/kg bw 14C-alitame followed
several weeks later by a dose of 500 mg/kg bw. A female beagle dog
received a single oral dose of 500 mg/kg bw 14C-alitame Faecal
samples were collected over 24 h and analyzed by HPLC. Faeces
contained 18-40% of the administered radioactive dose over the 24 h
period. Faecal radioactivity was a mixture of unchanged alitame
(CP-54,802) and alanine tetramethylthietane amide (CP-57,207).
Together these two products accounted for approximately 90% of the
total radioactivity (Pfizer, 1986)
In a study to examine the effect of dose on metabolic pathways,
2 male and 2 female beagle dogs received a single oral dose of 10 or
500 mg/kg bw 14C-alitame and urine and faeces were collected in
24-hour intervals over 3 days. Urinary metabolites were analyzed by
HPLC. There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment than after the
low-dose treatment, but the metabolite profile was essentially
unchanged (Pfizer, 1986).
2.1.2.4 In vitro studies
The stability of alitame in gastric juices was examined to
clarify whether the cleavage of aspartic acid is initiated in the acid
environment of the stomach, and whether conversion of alitame to the
ß-isomer might occur under these conditions. Thus, alitame was
incubated in simulated gastric juices at 38°C to determine the degree
of hydrolysis. Controls were water and simulated gastric juices
(boiled). Samples were taken over 24 h and analyzed by HPLC. Alitame
was relatively stable, with only slight evidence of degradation after
prolonged incubation for 24 h, which was not considered to be due to
enzyme activity. Similarly, the small increase in ß-isomer observed in
both treated and control samples was not considered to be due to
enzyme activity (Pfizer, 1986).
The stability of alitame in rat intestinal homogenate was
examined in order to test the capacity of the rat small intestine to
metabolize alitame. The small intestine from male rats was flushed
with saline and homogenized in phosphate buffer at pH 7.4. The
homogenate was incubated with 500 mg alitame/ml at 37°C and the
reaction stopped by the addition of 0.25 tool/litre NaOH. Cleavage of
the peptidic bond of alitame was measured by the formation of alanine
tetramethylthietane amide (CP-57,207) and was dependent on both the
substrate concentration and time of incubation. The percentage
conversion to the amide ranged from 13-21% after 10 minutes of
incubation. This demonstrated the ability of intestinal homogenate
to cleave the peptidic bond of alitame. The reaction was also
demonstrated to be enzyme-mediated in a second experiment in which
it was shown that the extent of cleavage was dependent on the
concentration of homogenate. Little evidence of further metabolic
steps, such as sulfur oxidation or acetylation, was noted (Pfizer,
1986).
2.1.3 Effects on liver enzymes
2.1.3.1 Rats
In a study to compare the rat liver enzyme inducing properties of
alitame with those of butylated hydroxytoluene (BHT), groups of 4 male
rats received oral doses of either BHT (100 or 500 mg/kg bw), alitame
(100 or 500 mg/kg bw) or phenobarbital (50 mg/kg bw) daily for 5 days.
At 15 h following the last dose, animals were killed and livers
removed. Liver homogenates were assayed for O-demethylase, cytochrome
c reductase, and P-450 activity. Significant enzyme induction was
noted with BHT, but alitame showed no effect at the same dose levels
(Pfizer, 1986).
In a further study to examine the liver enzyme inducing potential
of alitame and its metabolites, groups of Sprague-Dawley rats received
single oral doses of either alitame (up to 2000 mg/kg bw), alanine
tetramethylthietane amide (CP-57,207) or its sulfoxide (CP-58,290; 100
or 500 mg/kg bw), or phenobarbital (50 mg/kg bw/day) for 5 days. At
15 h after the last dose, animals were killed and livers removed.
Liver homogenates were prepared and assayed for O-demethylase. Slight
induction of demethylase activity was noted with both alitame and its
metabolites. At 500 mg/kg bw, enzyme activity was approximately twice
the low baseline activity observed in controls with either alitame or
its metabolites (Pfizer. 1986).
In a recent detailed study, the hepatic enzyme-inducing activity
of alitame was examined in rats following gavage administration (pilot
study) and dietary administration (main study). In the pilot study,
groups of 5 male and 5 female Sprague-Dawley rats were administered
alitame daily by gavage for 7 days at dose levels of 0, 500 or
1000 mg/kg bw/day. Animals were killed on day 8, the livers excised,
sections taken for histology and electron microscopy, and the
remainder of the liver homogenized. Positive control animals were
given ß-naphthoflavone (80 mg/kg bw, i.p., 3 days), phenobarbitone
(80 mg/kg bw, i.p., 3 days), or dexamethasone (100 mg/kg bw, i.p., 3
days). In the main study, groups of 20 male and 20 female Sprague-
Dawley rats were fed a diet containing alitame at concentrations of
0, 0.3% or 1% for 4 weeks. An additional 20 males and 20 females acted
as pair-fed controls and received the basal diet at the level of
intake of the 1% alitame group. After 28 days, 10 males and 10 females
from each group were sacrificed, sections of liver taken for histology
and electron microscopy and the remainder of the liver homogenized.
Kidney and brain weights were also recorded. The remaining 10 males
and 10 females were withdrawn from the alitame diet and kept for a
further 14 days before being sacrificed. In each of the above studies,
the liver homogenate was used to determine protein content, cytochrome
P-450 content, NADPH-cytochrome P-450-dependent reductase activity,
ethoxyresorufin O-deethylase (EROD) activity, pentoxyresorufin
O-depentylase (PROD) activity, erythromycin N-demethylase (ERD)
activity, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
In the pilot study, the positive controls exhibited significant
increases with respect to hepatic microsomal enzymes and all caused an
increase in relative liver weight. Brain and kidney weights were
unaltered. Alitame at 500 or 1000 mg/kg bw/day for 7 days had only
minor effects by comparison. At 1000 mg/kg bw/day, cytochrome P-450
levels were 140% of controls, and EROD, PROD and ERD activities
increased 3.6-fold, 6.3-fold, and 4.7-fold, respectively. Examination
of the microsomes by SDS-PAGE provided similar evidence of low levels
of protein induction corresponding to the above enzymes. Light
microscopic examination revealed hepatocellular hypertrophy at both
dose levels, and some evidence of periportal inflammation and necrosis
in a few sections. Electron microscopy revealed an increase in rough
and smooth endoplasmic reticulum at both dose levels.
In the main study, growth curves indicated a reduced body-weight
gain in alitame-treated animals compared to controls. This effect
was more apparent in females than in males and appeared to be
dose-related. While the effects may not have been statistically
significant, the curves indicated a clear biologically significant
decrease in body-weight gain in females at both the 0.3 and 1% alitame
dose levels. There was also an increase in relative liver weight in
females at both dose levels which was dose-related. Relative brain and
kidney weights were unaltered.
The parameters used to measure hepatic enzyme induction were also
increased in a dose-related manner compared to controls and pair-fed
animals. At the 0.3 and 1% dose levels, cytochrome P-450 levels
increased by 12 and 20% in females, and by 46 and 101% in males,
respectively. The activity of EROD was increased slightly in males and
females while there was a significant increase in the activity of PROD
in males and females at 0.3% and 1% alitame. The activity of ERD was
only slightly increased in males at 1% alitame. Light microscopic
examination of the liver revealed centrilobular hypertrophy at both
dose levels, but more marked at the higher dose. Electron microscopy
revealed a dose-related increase in both rough and smooth endoplasmic
reticulum. Following the 14-day withdrawal period, the liver weight
changes were reversed, and none of the hepatic parameters was
significantly different from controls.
The results indicated that alitame, at both the 0.3% and 1% dose
levels, was a weak inducer of hepatic enzymes, which was accompanied
in females by a decrease in body-weight gain and an increase in
relative liver weight. The pattern of enzyme changes was essentially
similar to that caused by phenobarbitone, although quantitatively
smaller (Caldwell et al., 1994).
In a supplement to the above study, the cytochrome P-450
isoenzyme from the liver microsomes of alitame-treated rats were
separated by SDS-polyacrylamide gel electrophoresis and further
characterized by Western blot immunodetection. Following separation by
electrophoresis, the proteins were transferred to nitrocellulose
membranes and the blots either stained with antibodies to specific
cytochrome P-450 isoenzyme or, to allow visualization of total
protein, by staining with Amido Black solution.
The microsomes from alitame-treated rats showed an increase in
the band attributed to CYP2B (catalytic activity) at 52 kD which is a
protein also shown to be induced by phenobarbitone. The band at 56 kD
attributed to CYP1A1, shown to be induced by ß-naphthoflavone, was
conspicuous by its absence. The data further confirmed that the
hepatic changes produced by alitame were similar to those produced by
phenobarbitone (Caldwell & Reed, 1994).
2.1.3.2 Humans
In a 14-day study, 8 male subjects received alitame in gelatine
capsules as 4 daily oral doses totalling 15 mg/kg bw/day. This dose
was calculated to be 15 times the projected human exposure level,
assuming total sucrose replacement. Induction of microsomal enzyme
levels was measured by the rate of elimination of aminopyrine.
Measurements were taken before treatment, after 2 weeks of treatment,
and at 18 days post-treatment. Both plasma and saliva were collected.
Blood samples were taken at pre-test, after 1 and 2 weeks of
treatment, and at 7 and 18 days post-treatment for the analysis of
clinical chemistry and haematological parameters. On the 10th day of
treatment, a 24-hour urine sample was collected for the analysis of
metabolites. There was no adverse effects parameters were normal
during the course of the study.
The analysis of the aminopyrine elimination results indicated
that the saliva was a good indicator of plasma levels. When the
elimination rate constants and half-lives were compared, there was no
significant difference in aminopyrine kinetics from the pre-dose,
post-dose and post-withdrawal samples. Urine samples indicated the
presence of the major metabolites, alanine tetramethylthietane amide
(CP-57,207) and it sulfoxide (CP-57,290). Alitame was present only in
trace amounts (Caldwell et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of alitame was examined in both mice and rats
and the results are summarized in Table 1.
Table 1. Summary of acute toxicity studies with alitame.
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral > 5000 Levinsky et al. (1983)
Rat M&F oral > 5000 Levinsky et al. (1983)
Groups of 10 male and 10 female mice (Crl:COBS CD-1 (ICR) BR) and
10 male and 10 female Sprague-Dawley (Crl:COBS CD (SD) BR) rats were
administered alitame (purity 97.43%) by gavage at a single dose level
of 5000 mg/kg bw. Animals were observed for clinical signs over a
14-day period after treatment, during which food consumption and body
weights were recorded. Animals were examined for gross pathological
changes at day 14 after treatment.
In mice, there were no deaths during the study and no clinical
signs apart from an increase in soft faecal material at 2.5 h
post-treatment. Faeces were normal by day 2 post-treatment. During the
observation period, there was normal food consumption and steady
body-weight gain.
In rats, there were no deaths during the study and no clinical
signs during the first hour post-treatment, but from then onwards all
animals displayed an increased level of activity for 1-2 days. All
rats were jittery and exhibited a tendency for exophthalmia, with
females noticeably more affected than males. Females exhibited
incoordination in their movements but were not ataxic. By 24 h, 7/10
males were essentially asymptomatic, and by day 2, all males and
females were asymptomatic. During the observation period, food
consumption was normal, and there was a steady body-weight gain. There
were no gross pathological changes on day 14 in 20/20 mice and in
19/20 rats, with one rat having an enlarged liver (Levinsky et al.,
1983).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 2-month study, groups of 10 male and 10 female Crl:COBS
(CD-1) (ICR) BR mice were fed a diet containing alitame (purity
98-101%) at concentrations of 0, 1, 2, or 5%, equivalent to 0, 1500,
3000 or 7500 mg/kg bw/day.
Animals were observed for clinical signs, and food consumption
and body weights were recorded throughout the study period. At the end
of the study period, liver and kidney weights were recorded and
tissues from the major body organs were taken for histopathology.
There were no deaths and no clinical signs of toxicity throughout
the study period. Body weights of the intermediate- and high-dose
animals decreased slightly on days 1 and 2, but recovered quickly.
There was no treatment-related effect on body weight for the remainder
of the study. There was no change in food consumption. Both absolute
and relative liver weights increased in a dose-related manner compared
to controls. The increase in absolute liver weight was 20, 40 and 67%
in males and 20, 35 and 66% in females in low-, intermediate- and
high-dose animals, respectively. Histopathological examination of the
liver revealed a dose-related increase in centrilobular hypertrophy.
Electron microscopy of liver tissue indicated a dose-related increase
in proliferation of smooth endoplasmic reticulum. No degenerative
hepatocellular changes were noted. Effects were noted at all dose
levels and a NOEL was not established in this study (Holmes et al.,
1983a).
2.2.2.2 Rats
In a range-finding study, groups of 5 male and 5 female Crl:COBS
CD-1 (SD) BR rats (5/sex/dose) were fed a diet containing alitame
(purity 98-101%) at concentrations of 0, 1, 2 or 5%, equivalent to
500, 1000, or 2500 mg/kg bw/day for 15 days. Animals were examined for
clinical signs of toxicity, and food and water consumption was
monitored throughout the study period. At the end of the study period,
blood samples were taken for haematological and clinical chemistry
examination; tissues from the major body organs were taken for
histopathology; tissues from liver and kidney were taken for electron
microscopy; and tissue from liver for histochemistry. There were no
deaths throughout the study, and no clinical signs of toxicity. A
slight body-weight loss was noted at the 2% and 5% dose levels on day
3 but body-weight gain was normal thereafter. There was a dose-related
decrease in food consumption in all treated groups on days 1-2
(especially females at the 2% dose level and in both sexes at the 5%
dose level) possibly due to unpalatability of the diet. Water
consumption was similarly decreased. There was a slight, but not
significant, increase in plasma cholesterol and protein levels in all
treated groups. Haematological parameters were normal. There was a
dose-related increase in absolute and relative liver weights in
males and females but no microscopic abnormalities were noted.
Histochemistry indicated an increase in glucose-6-phosphate
dehydrogenase and NADPH2 diaphorase in liver tissue slices. Electron
microscopy indicated a dose-related increase in smooth endoplasmic
reticulum in liver tissue and, in some cases, an increase in lysosomes
and lipid vacuoles. Effects were observed at all dose levels and a
NOEL was not established in this study (Chvedoff et al., 1981a).
In a 5-week study, groups of 10 male and 10 female Long-Evans
rats were fed a diet containing alitame (purity 98.2%) at dose levels
of 0, 10, 50 or 100 mg/kg bw/day. Animals were observed daily and body
weights and food consumption recorded. Blood samples were taken from
the orbital sinus prior to treatment and at the end of the study
period, and clinical chemistry and haematological parameters examined.
After 5 weeks, the induction of liver microsomal enzymes was measured
in vitro and animals were examined for gross pathology. Tissues from
the major organs were prepared for histopathology.
There was no treatment-related effect on body-weight gain or food
consumption. The clinical chemistry, haematological data and
histopathology were unremarkable. It was not possible to determine
whether there was proliferation of smooth endoplasmic reticulum
because of the presence of excess glycogen clue to lack of fasting
before sacrifice. Induction of 0-demethylase was determined by the
release of p-chlorophenol. A 30% increase in enzyme induction
occurred at the high-dose level which was not statistically
significant. Nevertheless, on the basis of the biological significance
of this increase, the NOEL in this study was 50 mg/kg bw/day
(Holmes et al., 1983b).
In a 3-month study, groups of CrI:COBS-CD(SD)BR strain rats
(15/sex/dose) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0.5, 1.0 or 2.0%, equal to 380, 830 or 1500 mg/kg
bw/day. Animals were observed throughout the study period and body
weights, food and water consumption recorded. Blood samples were taken
for examination of clinical chemistry and haematology parameters at
the end of the study. Urinary parameters were also examined. At the
end of the study, gross pathological examinations were performed and
tissues from the major organs were taken for histopathology. A group
of 5 males and 5 females was observed for a further 1 month following
the treatment period.
There were no deaths throughout the study and no clinical signs
of toxicity. There was a dose-related decrease in body-weight gain in
both males and females in all treated groups. The decreases at 3
months in males/females were 4.5%/1.7%, 4.5%/3.3% and 5.3%/8.5% at
low, intermediate and high dose. Food consumption was slightly lower
in high-dose males, but not during the withdrawal period. Water
consumption was normal. The clinical chemistry analysis revealed a
significant increase in plasma cholesterol at all dose levels in males
and females. The increase was 23% at low dose and 42% at high dose.
Levels returned to normal during the 1-month withdrawal period. There
was also a dose-related decrease in plasma triglyceride levels in
males, which returned to normal levels during the withdrawal period.
The levels of cytochrome P-450 and b5 were increased in a
dose-related manner at the end of the treatment period. The activity
of microsomal N-demethylase was also increased in a close-related
manner. The changes were reversed during the withdrawal period except
for the cytochrome P-450 levels in males. Haematological parameters
were within the normal range.
Relative and absolute liver weights were increased in males at
the high-dose level, while relative liver weight was increased at all
doses in females. The liver weights returned to normal during the
withdrawal period.
Histopathology of the liver revealed centrilobular hypertrophy in
all high-dose females and in 5/10 high-dose males. Histochemical
analysis of the livers indicated increased activity of G-6-P
dehydrogenase, gamma-glutamyl transpeptidase and NADPH-diaphorase in
the high-dose animals. NADPH-diaphorase activity was also increased in
intermediate-dose females. All changes were reversible during the
withdrawal period. Electron microscopy revealed an increased
proliferation of smooth endoplasmic reticulum at all dose levels.
After the withdrawal period, the livers of female rats were normal
while those of the males still showed mild proliferation of smooth
endoplasmic reticulum at the high-dose level. The histopathological
and enzymatic changes were indicative of a reversible adaptive
response. There was no evidence of focal hyperplasia or hepatocellular
toxicity. Effects were noted at all dose levels and a NOEL was not
established in this study (Monro et al., 1982a).
In a one-year study, groups of 40 male and 40 female Long-Evans
rats were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.1, 0.3, or 1.0% (equivalent to 50, 150 or
500 mg/kg bw/day). Two other groups were fed a diet containing alitame
adjusted to achieve a nominal intake of 10 or 30 mg/kg bw/day. At 6
months, 20 male and 20 female animals at each of the dose levels were
removed from treatment. Of these, half were sacrificed while the other
half were returned to normal untreated diet. Animals were observed
throughout the study period and body weights, food and water
consumption recorded. Ophthalmoscopic examinations were performed in
all animals before necropsy. Blood samples were taken at 6, 9 and 12
months for clinical chemistry and haematology. Urinalysis parameters
were also examined. At the end of the study period, all animals were
examined for gross pathology, and tissues from major organs were taken
for histopathology.
There were no deaths throughout the study and no clinical signs
of toxicity. Ophthalmoscopic examination at 6 months and 1 year was
unremarkable. There were no treatment-related effects on body-weight
gain or on clinical pathology parameters. Cytochrome P-450 levels were
measured at 6 months and were significantly increased at the high-dose
level only (21% in males and 33% in females). The only organ weight
change noted was an increase in relative liver weight at the high-dose
level at 6 months, with a 12.1% increase in males and 16.0% increase
in females. At 12 months, liver weight increases at the high-dose
level were 8.5% and 11.7% in males and females respectively, compared
to controls. In animals withdrawn from treatment at 6 months, liver
weights had returned to normal levels at 12 months. The only
microscopic finding was a retention of glycogen in both control and
treated groups. This change was not considered to be treatment-
related. The NOEL in this study was 0.3% in the diet, equal to
145 mg/kg bw/day in males and 185 mg/kg bw/day in females
(Holmes et al., 1985a).
A study was conducted in Sprague-Dawley rats which included
dietary administration during mating, in utero exposure, exposure
during lactation, and dietary exposure for one year post-weaning.
Groups of 20 male and 20 female Sprague-Dawley rats were selected from
the F1 offspring of rats treated 4 weeks prior to mating and during
gestation and lactation (Levinsky et al., 1992). The groups were fed
a diet containing alitame (purity 99.3%) at concentrations of 0 (two
control groups), 0.1, 0.3 or 1% for one year. The animals were
observed daily for clinical signs and body weights and food
consumption were recorded weekly. Other measurements and observations
included ophthalmological examinations (pre-test and 6 and 12 months),
serum chemistry, haematology and urinalysis measurements at 6 and 12
months. Tissues were collected from all animals which died or became
moribund during the study. At the end of the study, the animals were
sacrificed and necropsied and tissues prepared for light microscopy.
Organ weights for liver, kidney, adrenal glands, testes and ovaries
were recorded.
Twenty rats died or were sacrificed during the study. The deaths
were distributed evenly between the groups although deaths in males
predominated over those in females by four to one. Of the 20 early
necropsies, 4 were due to neoplasms (1 control, 2 low-dose and 1
intermediate-dose rats). The most common non-neoplastic cause of death
(7/16) was described as 'unexplained death/heavy rats' and was not
accompanied by any remarkable clinical signs.
Body-weight measurements began at birth for this study, and at
weaning (day 21) the mean body weights for the intermediate- and
high-dose animals compared to the control groups were -9% and -21%,
respectively. Following selection of animals in groups for the 1-year
study, the body weights expressed as percent of control for the
intermediate-dose level were -9.5% (M) and -8.8% (F), and for the
high-dose level -16.3% (M) and -13.5% (F). Over the course of the
study, the initial body weight differentials for males at the
intermediate- and high-dose levels diminished by about 50%. For the
first 80 days, all groups of males appeared to gain weight at similar
rates apart from the control group 1. For the remaining period of the
study, the weight gains were similar for all groups. Over the 1-year
period, the body-weight difference for the intermediate-dose males
disappeared and the difference for the high-dose group was 6.6% and
11.1% compared with control groups 1 and 2, respectively.
For females, over the course of the study, the -8.8% difference
at the intermediate-dose level disappeared and the low-dose level
gained weight relative to the control group 1. The females in the
control group 1, like the males, showed a slower growth rate. The
high-dose females, unlike the males, did not recover their initial
body weight difference (-13.5%) and after 200 days, the body weight
difference began to increase, reaching -20% relative to control
group 1.
Ophthalmoscopic examination at 6 and 12 months was unremarkable.
There were no treatment-related changes in clinical chemistry,
haematology or urinary parameters apart from a 40-60% decrease in
serum triglyceride levels in the high-dose females, which appeared to
be related to the decreased weight gain in the high-dose females. The
high-dose males showed decreased serum triglyceride values at 6
months, but not at 12 months.
There were no gross pathological findings in any group. The organ
weight changes observed were a decrease in absolute liver weight in
the high-dose females, and an increase in relative liver, kidney and
ovary weight as a result of the decrease in mean body weights in the
high-dose females.
Histopathology revealed hepatocellular hypertrophy in the
high-dose males (13/20) and females (4/20). These changes were not
accompanied by an increase in liver weight. Other liver changes
observed could not be related to treatment. The NOEL in this study,
based on body and organ weight changes, was 0.3% in the diet, equal to
173 mg/kg bw/day in males, and 205 mg/kg bw/day in females (Fisher
et al., 1992).
2.2.2.3 Dogs
In a two-week range-finding study in dogs, groups of 2 male adult
beagle dogs were fed alitame (purity 98-101%) in the diet at
concentrations of 1, 2 or 5%, equivalent to 250, 500 or 1250 mg/kg
bw/day. There were no control animals. Animals were observed
throughout the study for clinical signs of toxicity and histopatho-
logical examinations were performed on lungs, kidney, liver and heart
after 14 days. A section of liver was examined by electron microscopy.
There were no deaths during the study and no clinical signs of
toxicity. There was no treatment-related effect on body weight.
Systolic blood pressure and heart rate were normal at all dose levels.
Clinical chemistry parameters were normal apart from a slight increase
in alkaline phosphatase activity at all dose levels which increased in
the second week. Urinalysis parameters were normal. Haematological
parameters indicated a slight decrease in haemoglobin concentration,
RBC count and packed cell volume in treated animals, but the changes
were within normal limits and not definitely related to treatment.
Relative liver weights were within normal limits. Microscopic
examination revealed an increase in cytoplasmic vacuolation in the
liver of 5/6 animals. Electron microscopic examination revealed a
dose-related increase in proliferation of smooth endoplasmic
reticulum. Lipid vacuoles and lysosomes were present in the liver of
all animals but, in the absence of control animals, it could not be
determined whether the observed microsomal changes were treatment-
related (Chvedoff et al., 1981b).
In a 3-month study, groups of beagle dogs (4/sex in the low- and
intermediate-dose groups, and 6/sex in the control and high-dose
group) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.5, 1, or 2%, equal to 0, 120, 220 or 490 mg/kg
bw/day. In the control and high-dose groups, 2 animals/sex were
transferred to normal diet for an additional month after the study
period. Animals were examined throughout the study period for clinical
signs of toxicity and body weight was recorded weekly. At various
times, clinical chemistry, urinary and haematology parameters were
measured. Tests of aminopyrine clearance were measured on days 1 and
63. Ophthalmological examinations were performed on high-dose animals.
Tissues were examined histopathologically at the end of 3 months.
Liver histochemistry, electron microscopy and content of microsomal
cytochrome P-450 and b5 were also performed.
There were no deaths throughout the study and no clinical signs
of toxicity. Food intake was normal although there was a slightly
decreased weight gain in high-dose animals, particularly males.
Cardiovascular parameters were normal. Ophthalmological examination of
high-dose animals was unremarkable. Haematological parameters were
within the normal range. Clinical chemistry revealed a dose-related
increase in plasma alkaline phosphatase when measured at 1, 2 and 3
months. The increase at the high-dose level was 400% at 3 months.
After the recovery period, levels in the high-dose group were reduced
but still higher than control levels.
At the end of the 3-month treatment, the levels of cytochrome
P-450 and b5 were increased in a dose-related manner. After the
1-month withdrawal period, cytochrome P-450 and b5 in the high-dose
group were reduced to near control levels. Induction of the liver
microsomal enzyme system was also demonstrated in the dogs of the
reversibility groups (control and high dose) following administration
of a single i.v. dose of aminopyrine (15 mg/kg bw) on days 1 and 63 of
alitame treatment, with blood levels measured at 0, 1, 1.5, 2, 3 and
5 h. The plasma levels reflected the induction of a liver microsomal
demethylase. On day 1, there was little difference between treated and
control animals. On day 63, there was a marked difference between
control and treated animals.
Relative and absolute liver weights were increased in all
treatment groups. Absolute liver weight was increased by 36% and 11%
at high and low dose, respectively. Histopathology of the liver and
other organs was unremarkable. Histochemical examination revealed an
increase in G-6-P dehydrogenase activity in all treated groups except
the low-dose females. NADPH-diaphorase activity was increased in
high-dose animals. All levels returned to normal during the recovery
period, except for an increased G-6-P phosphatase activity in
high-dose males. Electron microscopy revealed an increase in
proliferation of smooth endoplasmic reticulum in all groups. This
increase was reversed in females but persisted in males during the
recovery period. The liver changes appeared to be indicative of an
adaptive response due to induction of microsome oxidative metabolism.
Effects were observed at all dose levels and a NOEL was not
established in this study (Monro et al. 1982b).
In an 18-month study, groups of 4 male and 4 female beagle dogs
were fed a diet containing alitame (purity 98-101%) at dose levels of
0, 10, 30, 100 or 500 mg/kg bw/day. Animals were examined throughout
the study period for clinical signs of toxicity, and body weights were
recorded at regular intervals. Ophthalmoscopy was performed on animals
at 6-month intervals. Electrocardiographic tracings were performed at
regular intervals. Clinical chemistry, urinary and haematological
parameters were measured at regular intervals. Aminopyrine clearance
was measured in control and intermediate dose animals at pre-test and
during weeks 3, 5, 7, 9, 40 and 74, and in the high-dose group at 39
and 73 weeks. Gross pathological examination was performed at 18
months together with histopathological examination of a wide range of
tissues. There were no treatment-related effects on survival and no
clinical signs of toxicity. Ophthalmoscopic examinations and
cardiovascular parameters were normal. Body weights were comparable
between groups although body-weight gain in high-dose females was
slightly less than control animals between weeks 40 and 70. Food
consumption was normal in all groups. All of the clinical pathology
parameters were normal except for an increase in serum alkaline
phosphatase activity in high-dose males and females. The alkaline
phosphatase rise was not uniform at high dose, with some dogs showing
little or no change.
Analysis of the urinary metabolites over a 2-day period at week
10 showed that the excretion rate was proportional to the dose
administered, indicating a dose-related systemic exposure. Measurement
of aminopyrine clearance, as an indicator of the induction of the
liver microsomal enzyme system, revealed a slight decrease in t1/2
in males and females at 100 mg/kg bw/day at the 2-week period. The
significance of this result is unclear, and may be due to the high
t1/2 in the animals pre-dosing. In males, there appeared to be a
gradual increase in t1/2 over the study period but again the result
is difficult to interpret. Overall, the results at 100 mg/kg bw/day do
not appear to indicate any enzyme induction. At 500 mg/kg bw/day,
after 39 or 73 weeks, significant microsomal enzyme induction was
apparent.
The only gross pathological change observed was a moderate
increase in absolute liver weight of 31% and 20% in high-dose males
and females, respectively. This increase was statistically significant
in males only. Histopathology was unremarkable in the treated animals.
A slight increase in smooth endoplasmic reticulum was found in the
livers of high-dose animals. The NOEL in this study was 100 mg/kg
bw/day (Holmes et al., 1985b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 2-year carcinogenicity study, groups of 50 male and 50
female Crl: CD-1 (ICR) BR mice were fed a diet containing alitame
(purity, 98-101%) at concentrations of 0 (2 groups), 0.1. 0.3 or 0.7%.
Animals were observed throughout the study period. Body weight and
food consumption were recorded weekly for 13 weeks and then at
N-weekly intervals for the remainder of the study. At the end of the
study period, animals were sacrificed and subjected to gross
pathological examination and organs weights recorded. Tissues from all
of the major organs were prepared for histopathological examination.
Tumour incidence was recorded.
There was no treatment-related effect on survival. The survival
rate for controls, low-, intermediate-, and high-dose groups in
males/females was 52/24%, 22/26%, 22/38%, 32/36% and 38/26%,
respectively. There were no clinical signs of toxicity in the treated
groups. Body-weight gain and food consumption were similar for all
groups. Gross pathological examination revealed a significant increase
in absolute and relative liver weight at high dose in males only.
Absolute liver weight increased by 59% and 46% compared to the two
control groups, while relative liver weight increased by 58% and 38%
compared to the two control groups. Slight to mild centrilobular
hepatocellular hypertrophy was found in 12 male animals in the
high-dose group. At the intermediate-dose level, absolute and relative
liver weights of males were also increased. Absolute liver weight was
increased by 41% and 30% compared to the two control groups, while
relative liver weight was increased by 39% and 21% compared to the two
control groups. While the increases were not statistically
significant, nevertheless the Committee considered them to be of
biological significance at this dose level. No hepatocellular
hypertrophy was apparent at the intermediate- and low- dose levels.
There were no other histopathological changes, or any
treatment-related increase in tumour incidence. Under the conditions
of this assay, there was no evidence of carcinogenicity in mice
induced by alitame in the diet. The NOEL, based on the biologically
significant liver weight changes in males, was 0.1% in the diet, equal
to 125 mg/kg bw/day for males and 155 mg/kg bw/day for females (Holmes
et al., 1985c).
2.2.3.2 Rats
A carcinogenicity study was conducted with dietary administration
and in utero exposure to alitame in Long-Evans rats. Groups of 50
male and 50 female Long-Evans rats (strain Blu:(LE)BR) were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for 2 years.
Animals used in this study were taken from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Holmes et al., 1986b). Animals were observed throughout the study
period for clinical signs of toxicity. Body weights and food
consumption were recorded weekly. Ophthalmoscopic examinations were
carried out at 12, 18 and 24 months. Clinical chemistry and
haematological parameters were measured in 10 animals/sex/dose at 6,
12, 18 and 24 months. At the end of the study period, animals were
sacrificed and subjected to gross pathological examination. Organ
weights were recorded. Tissues from all the major organs were prepared
for histopathological examination.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examinations
revealed no treatment-related effects. The survival rate at 104 weeks
for the control, low, intermediate, and high-dose groups was for
males/females 40/46%, 34/40%, 30/42% and 28/34%, respectively. There
was a slight decrease in survival in treated animals compared to
controls but this was not necessarily treatment-related.
In the second year of the study, there was a dose-related
difference in body weights for males between control animals and those
in the high- and intermediate-dose groups. In females, the body weight
of high-dose animals was lower than controls at the start of the study
and continued to be significantly lower throughout the study.
Intermediate-dose females also had a slightly lower body weight than
controls which became more pronounced in the 2nd year of the study.
There was no treatment-related effect on food consumption. These
differences in body weights were significant only at high dose.
There were no treatment-related changes in clinical chemistry or
haematological parameters. Gross pathological findings were generally
unremarkable. There was a slight non-significant increase in relative
liver weight at the high and intermediate dose levels in both males
and females. Microscopic examination of the tissues did not reveal any
increase in the incidence of neoplastic lesions. In the high- dose
group, 5 males and one female showed mild centrilobular hepatocellular
hypertrophy. The incidence of proliferative liver lesions in females
is shown in Table 2. The first appearance of hyperplastic nodules in
the liver of females was at week 96. Survival at this time in control,
low-, intermediate- and high-dose females was 64%, 56%, 50% and 50%,
respectively. Alitame, under the conditions of this assay, showed no
evidence of carcinogenicity in rats. The NOEL, based on body-weight
changes in both males and females, was 0.3% in the diet, equal to
130 mg/kg bw/day for males and 160 mg/kg bw/day for females
(Holmes et al., 1986a).
Table 2. Incidence of proliferative liver lesions in female rats
(Holmes et al., 1986a).
Control 0.1% 0.3% 1%
No. of livers examined 50 46 48 49
Focal nodular hyperplasia 1 0 3 9
Eosinophilic foci 4 0 3 11
A re-examination of the pathology slides of the 2-year
carcinogenicity study in Long-Evans rats (Holmes et al., 1986a) was
conducted on two subsequent occasions. At the first re-examination,
all available liver slides from all groups of female rats were
examined. The reported incidence of proliferative lesions is shown in
Table 3. There was no evidence of hepatocellular carcinomas in females
(Farrell, 1987).
Table 3. Incidence of proliferative liver lesions in female rats
(first re-examination; Farrell, 1987)
Control 0.1% 0.3% 1%
No. of livers examined 50 49 50 50
Hepatocellular adenoma 0 0 1 1
Eosinophilic foci 3 3 6 12
Hyperplasia 0 4 3 6
(focal and multifocal)
In the second re-examination of the pathology slides, slides
showing proliferative changes in female rats in either the original
study or in the first re-examination were examined using a revised set
of diagnostic criteria for hepatic proliferative lesions. The
incidence of these lesions is shown in Table 4. There was no evidence
of hepatocellular carcinomas in females (Hardisty et al., 1994).
Table 4. Incidence of proliferative liver lesions in female rats
(second re-examination; Hardisty et al., 1994).
Control 0.1% 0.3% 1%
No. of livers examined1 8 7 16 25
Hepatocellular adenoma 0 0 2 12
Hepatocellular hyperplasia 0 0 0 3
Eosinophilic cell foci 2 3 11 18
1 In this re-examination, only slides identified in the original
report and in the first re-examination as showing proliferative
lesions were examined.
Because of the low survival rate at 104 weeks in the Long Evans
rats (Holmes et al., 1986a), a second carcinogenicity study was
conducted with dietary administration and in utero exposure to
alitame in Sprague-Dawley rats. Groups of 70 male and 70 female
Sprague-Dawley rats were selected from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Levinsky et al., 1992). The groups were fed a diet containing
alitame (purity 99.3%) at concentrations of 0 (two control groups),
0.1, 0.3 or 1% for two years.
The animals were observed daily for clinical signs, and body
weights and food consumption were recorded weekly for the first
6 months and then every 4 weeks thereafter. Ophthalmological
examinations were performed pre-test and at 12 and 21 months; serum
chemistry, haematology and urinalysis measurements were taken at 6,
12, 18, 21 and 24 months. Tissues were collected from all animals
which died or became moribund during the study. At the end of the
study the animals were sacrificed and necropsied and tissues prepared
for light microscopy. Organ weights (liver, kidney, adrenal glands,
testes and ovaries) were recorded.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examination
revealed no treatment-related effects. Survival rates were high for
the first 12 months (>93%) but by 18 months average survival was 65%
for males and 75% for females. Groups reached 50% survival between 18
and 19 months for males and between 20 and 21 months for females.
Survival rates at 24 months in the 2 controls, low-, intermediate- and
high-dose groups were for males/females 9/37%, 4/23%, 7/31%, 9/24% and
26/17%, respectively.
Body-weight measurement began at birth for this study, and at
weaning (day 21). The mean body weights for the intermediate- and
high-dose animals were -9% and -21%, respectively, compared to the
control groups. Following selection of animals into groups for the
2-year study, the body weights expressed as percent of controls at the
low dose were -4% (M) and -2% (F), at the intermediate dose -7% (M)
and -10% (F), and at the high dose -18% (M) and -16% (F). For males,
over tile course of the study, the initial body weight differential
decreased as the treatment group showed increased weight gain compared
to the controls. Body weights peaked at approximately 15 months
followed by a gradual decline in all groups, with the high-dose group
showing the greatest decline. In females, over the course of the first
few months of the study, the initial body weight differentials
decreased particularly in the low-and intermediate-dose groups, which
showed increased weight gain compared to controls. After 6 months,
weight gain in the low- and intermediate-dose groups was similar to
controls. In the high-dose group, however, there was a decrease in
weight gain compared to the controls, and by the end of the study,
body weight differential had increased to -32%. Food consumption may
have been slightly lower in high-dose males, but was similar in all
groups of females.
There were no treatment-related changes in haematology or
urinalysis parameters. Changes observed in clinical chemistry data
included a decrease in serum triglyceride values (50-60%) in females
at the intermediate-dose in the first 18 months, and in high-dose
females throughout the study. Cholesterol was also increased in female
rats at 6 months at the intermediate-dose level (28%), and at 6 and 12
months at the high-dose level (67%).
Significant organ weight changes were seen in both liver and
kidney. In males, there was a relative increase in both kidney and
liver weight at high dose. In females, there was an absolute decrease
in kidney and liver weights at both intermediate and high doses, and
a relative increase in kidney weight at high dose. These changes
accompanied the decrease in body weight in high-dose females.
There was also an overall trend for decreased body weight in
intermediate-dose females which was not statistically significant.
The relative testes weight was increased in high-dose males.
Neoplastic changes were observed in the following organs: kidney,
liver, pancreas, lung, heart, thymus, mesenteric node, adrenal cortex,
thymus, parathyroid, uterus, cervix, brain, spinal cord, Zymbal's
gland, skin, bone, skeletal muscle, abdominal cavity, and soft
tissues. There was no evidence that the incidence of these neoplasms
was treatment-related. In the liver, hepatocellular carcinomas were
reported in one control male and one high-dose male. There was no
reported incidence of hepatocellular adenomas. There were also no
treatment-related non-neoplastic changes. Alitame, under the
conditions of this assay, showed no evidence of carcinogenicity in
Sprague-Dawley rats. The NOEL, based on body-weight changes, was 0.3%
in the diet, equal to 230 mg/kg bw/day for males, and 250 mg/kg bw/day
for females (Fisher et al., 1993).
2.2.4 Reproductive toxicity studies
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (75/sex) were administered a diet containing alitame
(purity 98-101%) at concentrations of 0, 0.1, 0.3 or 1% for either 2
weeks (females) or 12 weeks (males) prior to mating. Alitame-
containing diet was continued throughout pregnancy and lactation. Of
the F1 pups, 50 pups/sex/dose level were selected for a 24-month
carcinogenicity study, a further 25/sex/dose level were raised to
maturity and mated to produce the F2a litters. Approximately 3 weeks
after weaning of the F2a litters, the F1 animals were again mated
to produce the F2b litters. Both F2a and F2b pups were exposed to
alitame via the milk and in the food.
In the F0 parental group, the body weight of males was similar
for all groups during the 12 weeks of treatment, while body weight and
food consumption of females were slightly depressed during the 2-week
treatment period at the high-dose level. There was no treatment-
related effect on fertility or gestation indices, and no abortions
were noted. There was no difference in the number of pups born alive
between treated and control groups in the F1 litters. There was no
treatment-related effect on day 4 pup survival rates or lactation
indices. F1 pup weights of treated animals were similar to those of
control animals on days I and 4 of lactation, but high-dose pups had
significantly lower body weights on days 7, 14 and 21. However, these
decreased body weights were not significantly different from the
historical control data.
In the F1 parental group, body weights were similar to control
except at the high-dose level where there was a slight decrease in
body-weight gain. There were no treatment-related changes in pregnancy
rates or gestation length for either F2a or F2b litters. There was
no difference in the number of pups born alive between treated and
control groups in the F2a or F2b litters. Survival indices for F2a
and F2b were normal for all groups on days 1 and 4 of lactation.
F2a pup weight for all treated groups was similar on days 1,
4 and 7 of lactation but a significant decrease was noted in the
high-dose animals on day 14 and in all treated groups on day 21
compared to controls. The body weights were still within the
historical control data range. The F2b pups weight at the high-dose
level was significantly lower than controls throughout lactation,
while at the intermediate-dose level body weights were lower at days
14 and 21, and at the low-dose level, body weights were significantly
lower than controls on days 4 to 21. No historical control data were
available for F2b litters.
Gross pathological examination of the F0 males and females, the
F1 pups, and the F2a and F2b pups revealed no treatment-related
abnormalities. Postnatal development in F2b pups was assessed with
regard to locomotor activity, auditory function and ophthalmoscopic
examination. At the F2b weaning, there was a significant decrease in
both horizontal and vertical locomotor activity in F2a male and
female pups at the high-dose level, and a significant increase in
horizontal (but not vertical) locomotor activity in the F1 dams at
the high-dose level group. Pups tested for auditory function and for
ophthalmoscopic changes revealed no treatment-related changes
(Holmes et al., 1986b).
In a second 2-generation study, groups of 30 male and 30 female
Long-Evans rats (strain Blu:(LE)BR) were administered alitame (purity
98-101%) at concentrations of 0, 0.1, 0.3, or 1% for either 2 weeks
(females) or 12 weeks (males) prior to mating. Alitame-containing diet
was continued through pregnancy and lactation. Of the F1 pups,
25/sex/dose were raised to maturity and mated to produce the F2a
litters. Approximately 3 weeks after weaning of the F2a litters, the
F1 animals were again mated to produce the F2b litters. Both the
F2a and F2b pups were exposed to alitame in utero, during lactation,
and in the food following weaning.
At the high-dose level, 2 additional F1 groups of 25/sex were
exposed to alitame differently. These groups were introduced to
address the lactation effect observed in the first study. In one of
these groups, the F1 females were removed from treated diet at the
birth of F2a pups. In this group, the F2a pups received no alitame
during lactation. In the other group, the F1 females were removed
from the treated diet 3 weeks after weaning until the birth of the
F2a pups. In this group, the F2b pups received alitame only during
lactation. Animals were then placed on treated diet again. Post-natal
development of F2b pups was tested with an ophthalmoscopic
examination, an auditory function test, and a locomotor activity test.
Locomotor activity tests were also conducted on F0 and F1 parents,
and on F2a and F2b pups.
In the F0 parental animals, the body weights of males were
similar for all groups during the 12 weeks of treatment, while there
were decreases in body-weight gain and food consumption in females at
the high-dose level during the 2-week treatment period. Fertility
rates for all groups were normal. The number of live F1 pups was
similar for all groups at day 1, but was slightly reduced at the
high-dose level on day 4. This was explained by several large litters
at the high-dose level which declined in the first few days. F1 pup
body weight was similar for all groups on day 1 of lactation but was
significantly less at the high-dose level from day 4 onward.
Body-weight gain at high dose was similar to that of control groups
from days 4 to 21. The mean number of pups alive at day 21 compared to
the number of pups alive at day 4 (the lactation index) was similar
for all groups. Gross pathological examination of the F0 males and
females and the F1 pups revealed no treatment-related changes.
In the F1 parental group, the body weights of all female groups
were similar. Pregnancy rates and gestation times were normal. The
F1 males in the treated groups had slightly lower body weights than
controls although body-weight gain was similar for all groups. The
numbers of F2a litter live pups were similar for control, low and
intermediate groups. For the high-dose group, the mean number of pups
born alive (11.54) was slightly lower than controls (12.96). However,
the mean number of pups born alive in the other two additional
high-dose groups were similar to controls (12.75 and 12.13). The
4-day survival indices for the F2a pups in the control, low-,
intermediate-and high-dose groups were 0.98, 0.98, 0.93 and 0.89,
respectively. In the two additional high-dose groups, the 4-day
survival indices were 0.97 and 0.95. Lactation indices were for the
control, low-, intermediate- and high-dose groups 0.98, 1.0, 0.97 and
0.96, respectively, and for the two additional high-dose groups 0.99
and 0.88, respectively.
The mean body weight of high-dose F2a pups were slightly but
significantly lower than controls at birth and throughout lactation.
The additional F2a pups which were exposed only during lactation had
slightly lower body weight after the first week of lactation. The
additional group of F2a pups which had no alitame exposure during
lactation had body weights similar to controls at all times after day
1 of lactation. The results indicated a slight lactation effect of
alitame at the high-dose level, although the high-dose body weights
were still within the historical control ranges. The F2a pups at the
intermediate-dose level also had slightly but significantly lower body
weights at days 14 and 21 of lactation. External examination and gross
internal examination of the F2a pups revealed no treatment-related
changes.
The fertility rates and gestation times associated with the
production of the F2b litters were normal for all groups. The mean
number of pups born alive was comparable at the control, low- and
intermediate-dose levels, being 12.1, 11.7 and 13.0, respectively. At
the high-dose level, the number of live pups was slightly lower (9.8).
Survival during lactation was similar for all groups. The mean body
weight for the F2b high-dose level pups was significantly lower than
controls on day 14 of lactation. External examination and gross
internal examination revealed no treatment-related changes.
Ophthalmoscopic examination and auditory function tests were
normal in all groups. Locomotor activity for both parental groups and
pups showed considerable variation and the changes could not be
correlated with treatment.
This second study provided supporting evidence that alitame at
high-dose levels caused some suppression of body-weight gain during
lactation. This was further investigated in a study to examine the
effect of alitame in the cage bedding material on the pups body weight
(see section 2.2.5). The NOEL, based on body-weight changes in the
F2 pups, was 0.1% in the diet, equivalent to 100 mg/kg bw/day
(Holmes et al., 1986c).
In a 1-generation study in Sprague-Dawley rats, groups of 105
male and 105 female rats (strain Crl:CDBR VAF/Plus) were administered
alitame (purity 99.3%) at concentrations of 0, 0.1, 0.3 or 1% for 4
weeks prior to mating, through the cohabitation period, through the
gestation period, and up to day 21 of lactation. The aim of this study
was to produce an F1 generation of rats, continuously exposed to
alitame in utero, via the milk during lactation, and through the
feed for use in 1-year and 2-year toxicity studies. The animals were
observed daily for clinical signs of toxicity and body weights and
food consumption were recorded. During cohabitation, only the body
weights of the males were recorded. During gestation, body weights of
females were recorded on days 0, 4, 7, 14 and 20. During lactation,
dams and viable young were weighed on days 1, 4, 7, 14 and 21
post partum. On day 4, all litters were culled to 8 pups, 4
animals/sex. At day 21 (weaning), litters from the control groups, 0.1
and 0.3% treatment groups were culled to 2 animals/sex, and the
respective dams sacrificed. High-dose litters were not culled due to
their lower body weights, and were allowed to remain with the
high-dose dams until it was deemed prudent to separate them. When the
F1 animals were 3 to 6 weeks of age, 70 pups/sex/dose were selected
for a 2-year oncogenicity study (Fisher et al., 1993), and 20
pups/sex/dose level were selected for a 1-year toxicity study
(Fisher et al., 1992). All F0 males were terminated after the
cohabitation period. F0 females which did not deliver by day 24 of
gestation were terminated and their uteri and ovaries were examined.
F0 pups not used for either study were terminated.
Reductions in body weights and food consumption were noted in the
high-dose F0 animals, particularly during the first 2 weeks of the
study. This may be associated with palatability. There was no
treatment-related effect on mating, gestation length, or body weight
during gestation and lactation. The number of implantation sites was
slightly lower in the high-dose group and this was reflected in a
slight reduction in the total number of pups born alive per litter.
There was no difference between control and treated groups with
respect to post-implantation losses. There was no treatment-related
effect on pup survival during the lactation period. The mean
body-weight gains at the intermediate- and high-dose levels were
depressed compared to controls, and at weaning the body weights of the
intermediate- and high-dose groups were 9% and 21% lower than
controls, respectively. There were no internal or external
abnormalities in the pups not selected for the long-term studies
(Levinsky et al., 1992).
2.2.5 Special studies on neonatal survival
A study was conducted in Long-Evans pups and lactating dams in
order to further investigate the apparent decrease in pup body weight
during lactation at the high-dose level in the reproductive toxicity
studies. In this study, dams and pups were exposed to alitame which
was added to the bedding material rather than to the feed. It was
argued that dams and pups are normally exposed to treated feed in this
way because of tipping over of the feed by the dam, or the tendency to
scratch the feed into the bedding material. In this study, 2 groups of
8 litters were formed and all dams received normal diet. On lactation
days 1 and 5 and every 2-4 days thereafter, one of the groups had
125 g of feed containing 1% alitame sprinkled on the bedding. The
control group had feed without alitame sprinkled on to the bedding.
Body weights of dams and pups were measured throughout the lactation
period and at one week after lactation.
Body-weight gain in 'treated' dams was slightly lower than in
control dams on days 1 to 4 but there was no difference in body-weight
gain on days 4 to 21 of lactation. Body-weight gain in 'treated' pups
was decreased from day 2 and continued to be decreased throughout
lactation and one week after lactation. For example, the mean
body-weight difference on days 2, 7, 14 and 21 were 0.5 g (-8.4%),
1.6 g (-13.7%), 2.7 g (-11.3%), and 3.1 g (-7.4%), respectively. After
weaning, the weight difference continued to increase to 5.2 g (-7.2%)
at one week. The differences were statistically different from
controls on days 2, 3 and 5, but not thereafter. Locomotor activity
was measured in dam and pups, and no treatment-related effect was
noted.
The results suggested that bedding containing 1% alitame had a
treatment-related effect on pup body-weight gain, although no
mechanism for this effect was apparent. Food consumption was not
measured in this study although it was claimed to be normal. The
consumption of food by the dams from the bedding or from their fur was
considered to be negligible (Holmes et al., 1986d).
2.2.6 Special studies on embryotoxicity/teratogenicity
2.2.6.1 Rats
A group of 80 Crl:COBS-CD(SD)BR strain female rats were
inseminated and divided into 4 groups of 20 animals each. The groups
of presumed pregnant rats were administered alitame in 0.1% methyl
cellulose by gavage at dose levels of 0, 100, 300 or 1000 mg/kg bw/day
on days 6 to 15 post-insemination. The dams were examined throughout
the study period and body weights recorded regularly. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half were stained for skeletal anomalies and half
were examined for soft tissue defects. The ovaries and uteri of dams
were examined on day 20.
The pregnancy rate was 20/20 in control and intermediate-dose
groups and 18/20 in the low- and high-dose groups. There were no
deaths throughout the study and no clinical signs of toxicity. A
slight decrease in food consumption occurred on day 14 at the
high-dose level. Body weight was depressed significantly during and
after the treatment period at the high-dose level only. The number
of viable litters was similar for all groups, as was the number
of corpora lutea. There were slight decreases in the number of
implantation sites in the intermediate- and high-dose groups (10.8 and
10.6 respectively) compared to the control (11.8) and low-dose group
(12.2). There were also slight decreases in the number of fetuses in
the intermediate- (9.9) and high-dose groups (9.7) compared to the
control (11.0) and low-dose group 111.3). The implantation rate
decreased with increasing close (91.1, 89.8, 86.7 and 84.9%,
respectively), and the embryotoxicity rate increased with increasing
dose (6.4, 6.8, 7.4, and 8.4%, respectively). The sex ratio (M/F) was
decreased at the high-dose level (74/100) compared to controls
(110/110).
With regard to fetal development, there was no difference between
the fetal weight of control and treated animals. Examination of the
fetuses did not reveal any treatment-related increase in gross,
visceral or skeletal malformations. Among all the fetuses examined,
only one external anomaly (cleft palate) was observed. There was no
treatment-related change in the rate of ossification. Under the
conditions of this study, alitame showed no evidence of teratogenicity
in the rat. The decrease in the number of fetuses at the higher dose
levels appeared to be a direct result of the lower level of
implantation in these groups rather than to embryotoxicity (Perraud
et al., 1983a).
2.2.6.2 Rabbits
A group of 77 New Zealand white rabbits was inseminated and
divided into 4 groups of 19, 18, 20 and 20 animals. The groups were
administered alitame by gavage at dose levels of 0, 100, 300 or
1000 mg/kg bw/day on days 7 to 18 post-insemination. The does were
examined throughout the study period and body weights recorded
regularly. Animals were killed on day 28 post-insemination. Fetuses
were examined for external malformations then half were stained for
skeletal anomalies and half were examined for soft tissue defects.
Ovaries and uteri of does were examined on day 28.
Four animals died during the study, one at each dose level and
one in the control group. The pregnancy rate was 16/19, 15/18, 18/20
and 16/20 in each of the groups, respectively. Food restrictions were
introduced on days 12 to 19 to reduce the incidence of diarrhoea.
Body-weight loss was observed in all groups during this 8-day period.
A significant treatment-related decrease in body-weight gain was also
noted at the high-dose level.
The number of viable litters was similar for all groups as was
the number of corpora lutea. There was one abortion on day 24 in the
high-dose group. The number of implantation sites was slightly
decreased at the low-dose level compared to other groups.
Embryo-mortality rate was slightly higher at the intermediate- and
high-dose levels but was not dose-related, and not considered to be
due to treatment.
With regard to fetal development, fetal body weights were
slightly but not significantly decreased (7%) at the intermediate- and
high-dose level compared to the control group. Examination of the
fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral abnormalities. Among all the fetuses, there was
one with cleft palate and one with club foot in the intermediate-dose
group, and one with edema in the control group. The only visceral
abnormality seen was agenesis of the left kidney in one control group
fetus. There were no major malformations observed, but there was a
significant increase in the incidence of fetuses with supernumerary
13th ribs at the intermediate- and high-dose levels. Under the
conditions of this assay, alitame showed no evidence of teratogenicity
in the rabbit (Perraud et al., 1983b).
2.2.7 Special studies on genotoxicity
The results of the genotoxicity tests with alitame are summarized
in Table 5.
2.2.8 Special studies on pharmacological effects
Alitame was tested for its pharmacological activity in a battery
of systems designed to assess autonomic, gastrointestinal, renal and
metabolic functions.
A solution of 10-4 mol/litre alitame had no contractile or
relaxant activity in guinea-pig ileum, rat fundus, or rat vas
deferens, and did not interact with any of the six neurotransmitters
used in these systems. Alitame at an oral dose level of 25 mg/kg bw
caused no change in intestinal transit time nor in renal excretion of
fluids or electrolytes. Alitame at an oral dose level of 25 mg/kg bw
had no effect on fasting blood glucose levels or on the deposition of
an oral glucose load (Pearson et al., 1982).
Table 5. Summary of genotoxicity tests with alitame
Test system Test organism Concentration1 Result Reference
Reverse mutation in S. typhimurium 20-10000 µg/plate - Holden et al.,
bacteria TA1535, TA 1537, TA98, (-S9) (1981)
TA100 10-10 000 µg/plate
(+S9)
Urinary metabolites CD-1 mice/ 50-1000 mg alitame/kg - Holden et al,.
in vivo/Reverse S. typhimurium in mice & 0.05-0.75 ml (1981)
mutation in bacteria2 TA1535, TA 1537, TA98, urine/plate
TA100
Reverse mutation in Mouse lymphoma cells 220-1655 µg/ml (+S9) - Holden et al.,
mammalian cells L5178Y (1981)
Chromosome aberration in CD-1 mice bone 0-1000 mg/kg - Holden et al.,
vivo3 marrow cells (1981)
Table 5. Summary of genotoxicity tests with alitame (cont'd).
Test system Test organism Concentration1 Result Reference
Chromosome aberrations Human lymphocytes 0-1000 µg/ml - Holden et al.,
in vitro4 (1981)
Human lymphocytes 0-3000 µg/ml (+/-S9) - Amacher et al.,
(1991)
Micronucleus assay5 CD-1 mice 0-2000 mg/kg/bw/day - Amacher et al.,
(1991)
1 The purity of alitame tested was not provided.
2 Urine from treated CD-1 mice (14/group) was pooled over 18 h and treated with beta-glucuronidase.
3 Groups of 5 CD-1 mice were administered alitame orally and bone marrow cells harvested at 6, 12
and 24 h.
4 In the first study, there was minimal provision of methodology and reporting. The second study
was conducted under more recent GLP regulations.
5 Groups of 5 male and 5 female mice were treated over three consecutive days. Bone marrow was
prepared 24 h after file last dose.
2.2.9 Special studies on neurotoxicity and neurobehavioural
effects
In a neurotoxicity study, alitame was tested for its ability to
inhibit in vitro receptor binding in rat brain homogenate and to
produce in vivo neurochemical effects in rats. In the in vitro
binding assays performed with rat brain homogenate, alitame was tested
for its ability to inhibit the binding of a variety of receptor
radioligands to brain membranes. Neither alitame nor the B-isomer, at
concentrations of 10 µM, had any effect on the level of binding of
these ligands. In the in vivo study, groups of Sprague-Dawley rats
(8 males) were administered an oral dose of vehicle, alitame (25 mg/kg
bw), ß-isomer of alitame (6.25 mg/kg bw), or alitame (12.5 mg/kg bw)
plus ß-isomer of alitame (6.25 mg/kg bw). At 2 or 4 hrs, animals were
killed and the levels of dopamine, serotonin and their metabolites in
the brain tissue were measured. There was no significant change in
neurotransmitter levels after treatment (Bacopoulos, undated).
In a study to examine the effect of alitame on the behaviour of
rats, groups of male CD rats were administered a single oral dose of
vehicle, alitame (25 mg/kg bw), ß-isomer of alitame (6.25 mg/kg bw),
or alitame (12.5 mg/kg bw) plus ß-isomer of alitame (6.25 mg/kg). In a
separate experiment, animals were administered single oral doses as
above for 13 consecutive days. No treatment-related effect on
horizontal or vertical locomotor activity was noted in either the
single or multiple treatment regimes (Bacopoulos, undated).
In a study to examine the potential neurotoxicity of alitame in
rats following acute administration, groups of 12 male and 12 female
Sprague-Dawley rats were given alitame at a single oral dose of
0, 2000, 3000 or 5000 mg/kg bw. The animals were examined using a
functional observational battery (FOB) (both qualitative and
quantitative), a motor activity test, and an auditory startle
habituation test prior to treatment, at 5 and 7 h post-dosing, and
on days 1 and 7 post-dosing. At the end of the study, half the
animals were randomly selected for perfusion for possible future
neuropathological examination while the remaining animals were given
a full necropsy.
Significant decreases in body-weight gains were noted in all
treated groups on the day following treatment (days 0 to 1), followed
by compensatory increases on the following days (days 1 to 7). A
decrease in food consumption on day 1 was noted in all female treated
groups and in males of the intermediate- and high-dose groups. For the
high-dose female group, clinical symptoms included yellow abdominal/
urogenital fur staining, dehydration, reduced activity and pallor
between days 1 and 4 post-treatment. Effects were noted in all
behavioural tests for both sexes in the high-dose group and in some
tests in the intermediate- and low-dose groups on the first day. By
day 7, results of all parameters in all treatment groups were similar
to the control group.
Qualitative FOB assessments on day 1 post-treatment revealed
significant differences between the control and high-dose group for
both sexes. At the high-dose level, there was a significant decrease
in pinna reflex and a decrease in rearing in the arena. There was also
an increased incidence in ataxic gait and diarrhoea in the high-dose
animals. Animals at the lower dose levels also showed some increase in
minor symptoms. By day 7, there were no significant differences
between the groups. Quantitative FOB assessments found changes in
temperature and hind limb splay in treated groups on day 1 but by day
7 no significant differences were detected. Changes were also noted in
treated groups with respect to the motor activity test and the
auditory startle habituation test on day 1, but no changes were
evident by day 7. There were no treatment-related gross pathological
changes found on day 7. Transitory behavioural changes were observed
immediately after treatment and in the 24 h following treatment. By
day 7, all animals appeared normal and there was no evidence of
neurotoxicity (Robinson et al., 1993a).
In a 13-week study of the potential effects of alitame on
behaviour and neuromorphology in rats, 12 male and 12 female
Sprague-Dawley rats were fed a diet containing alitame at
concentrations of 0, 0.1, 0.3 or 1%. Animals were examined throughout
the study period for clinical signs of toxicity and body weights and
food consumption were recorded. The animals were examined using a
functional observational battery (FOB) (qualitative and qualitative),
a motor activity test and an auditory startle habituation at
pre-treatment, after 7 and 14 days of treatment and in the 5th, 9th
and 13th weeks. Passive avoidance testing was performed in weeks 4 and
13. At the end of the study, half the animals (randomly selected) were
perfused for neuropathological examination. Tissues from brain, spinal
cord, and skeletal muscle were prepared as paraffin sections and
haematoxylin and examination. Tissues from the peripheral nervous
system and the central nervous system were prepared in epoxy
embedding.
There were no mortality or adverse clinical findings related to
the treatment. The body weights of females in the 1% treated group
were significantly lower after 3 weeks and throughout the second and
third months of the study. The food consumption for this group was
also significantly decreased during the first week of the study. The
body weights and food consumption of all the other treated groups were
normal.
Both the qualitative (observations and handling) and the
quantitative FOB evaluations (grip strength, hind limb splay, and body
temperature) were unaffected by treatment for both sexes. In the
locomotor activity tests, significant differences at single time
points were noted for high-dose males and females. However, these
differences were not sustained over the course of the study and could
not be clearly related to treatment. For auditory startle habituation
tests, occasional significant differences in the 0.1% group were noted
but these were not attributed to treatment as the effects were not
sustained and there was no dose-relationship. In passive avoidance
performance at 4 weeks there were no significant differences between
groups. At week 13, more females in the 1% group completed the
training trial than in the control group. However, it could not be
concluded that this was related to treatment and was of doubtful
neurotoxicological significance.
There were no treatment-related gross pathological findings in
animals not selected for perfusion or in those which underwent
perfusion. A few animals, primarily males in all groups, had pale
areas on the livers. For rats undergoing perfusion, gross pathological
findings in the nervous system were limited to a few animals with dark
areas in the meninges. Histopathological examination was only
performed in control and high-dose animals. There were no significant
differences in the finding in the two groups. Under the conditions of
this study, there were no behavioural or neuromorphological changes
observed which could be attributed to treatment with alitame (Robinson
et al., 1993b).
In a study to examine the potential developmental neurotoxicity
of alitame, groups of female Sprague-Dawley rats were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for one week
prior to mating, then throughout the mating and gestation periods and
up to 10 days post-partum. Animals were observed throughout the
study period and body weights and food consumption recorded. All
females were given a complete necropsy. Dams were killed on days 21-23
post-partum.
The F1 pups were examined for malformations, sexed and the
number alive and dead recorded. The pups in each litter were weighed
individually on days 1, 4, 7, 11, 14, 17 and 21. On day 4, the litters
were culled to 8 pups, 4 of each sex. Pups were examined for pinna
unfolding (day 2), eye opening (day 12), and air righting reflex
(day 14). A motor activity test was conducted on day 17. Pups were
weaned on day 21.
The F1 adult generation was formed from at least 20 different
litters and consisted of 80 rats/sex/group. Animals were examined for
clinical condition and physical development and body weights and food
consumption recorded. From each litter, 3 males and 3 females were
randomly selected for behavioural testing (motor activity test,
auditory startle habituation test, passive avoidance test and
water-maze test). Young adults not selected for the adult F1
generation behavioural testing were sacrificed and underwent gross
pathological examination. Six animals/sex/group were perfused for
histo-pathological examination. Tissues from brain, spinal cord, and
skeletal muscle were prepared as paraffin sections and H and E
stained. Tissues from the peripheral nervous system and the central
nervous system were prepared in epoxy embedding.
In the F0 animals, there were no deaths and there were no
treatment-related clinical findings. The body weights of the 1%
treated animals were significantly decreased throughout the study.
The food consumption values for the 1% treated group were also
significantly lower than control values during the study. Gross
pathological examinations revealed no treatment-related effects. There
was no treatment-related effect on oestrus cycles, pregnancy rate,
gestation index, number of live and dead pups, sex ratio or post
implantation loss.
In the F0 pups, viability, survival, lactation indices,
clinical findings and gross pathology were unaffected by treatment. In
the 1% group, pup weight was significantly decreased on days 11, 14
and 17 and slightly decreased on day 21. There were no treatment-
related effects on physical or reflexological development, or on
locomotor activity.
In the F1 adults, there were no treatment-related effects on
mortality or clinical findings. The body weights of males and females
in the 1% group were significantly decreased. Food consumption was
normal. Physical development was not affected by treatment. In the
behavioural testing, there was no treatment-related effect on
locomotor activity, auditory startle habituation, passive avoidance or
water maze tests. Gross pathological and neuropathological
examinations revealed no effects which could be attributed to alitame
treatment. Under the conditions of this study, there were no
behavioural or neuromorphological changes in adults or pups which
could be attributed to treatment with alitame (Robinson et al.,
1993c).
2.2.10 Special studies on melanin binding
The potential for radiolabelled alitame or its metabolites to
bind to melanin in the eye of rats was tested by whole body
autoradiography and by microhistoautoradiography. Two male rats from
the albino strain, Sprague-Dawley (Crl:CD(SD)BR) and two male rats
from the pigmented strain, Lister Hooded (Crl:(LIS)BR) were
administered a single oral dose of 65 mg/kg bw 14C-alitame (94%
purity). At 2 and 24 h, one animal from each strain was killed and
the right eye of each excised and stored at -80°C for micro-
histoautoradiography. The remaining carcass was rapidly frozen at
-75°C for whole-body autoradiography.
Microscopic examination of the micrographs of the eyes revealed a
large number of silver grains distributed widely throughout the layer
of the retina, choroid and sclera in animals of both pigmented and
non-pigmented strains at both sacrifice times. The results of the
microscopy could not be interpreted and the data were considered to be
artifactual.
The results of the autoradiography revealed much higher levels of
radioactivity in the uveal tract (choroid, ciliary body and iris) of
the pigmented rats. Quantification of the autoradiographies revealed
concentrations of 2.99 µ equivalent alitame/g tissue in the uveal
tract of albino rats at 2 h and no detectable radioactivity at 24 h.
In the pigmented rats, the radioactivity in the uveal tract was
28.24/µg equivalent alitame/g tissue at 2 h and slightly higher levels
at 24 h (30.55 µg/g). There was no difference in radioactivity in the
surrounding tissues in either the albino or pigmented rat.
The study provided evidence of binding of alitame or its
metabolites to the melanin contained in the uveal tract of the
pigmented rats.
In an addendum to this report, alitame and two metabolites were
tested in vitro for their binding affinity to a melanin preparation
from bovine eyeballs. Alanine tetramethylthietane amide sulfoxide,
which was found in the urine of both rats and humans, did not bind to
melanin under the conditions of this assay, whereas its acetylareal
form, which was found only in the urine of rats, did bind to the
melanin. The positive control, doxepin, produced a higher level of
binding than found with the acetylated sulfoxide metabolite. This
study provided some evidence that the melanin binding observed in rats
may be associated with a metabolite formed only in the rat (Whitby
et al., 1994).
2.2.11 Special studies on the ß-isomer of alitame
2.2.11.1 Absorption, distribution, excretion, and biotransformation
In a study to examine the toxicokinetics of the ß-isomer of
alitame, Sprague-Dawley rats (4/sex) were administered a single oral
dose of the 14C-ß-isomer of alitame (10 mg/kg bw in water).
Excretion of radioactivity in urine and faeces was measured over 72 h.
A similar study was conducted with 6 male and 3 female Long-Evans rats
following a single oral dose of 14C-ß-isomer of alitame (25 mg/kg
bw). A separate group of 7 male Long-Evans rats were fitted with a
bile cannula. These animals received an oral dose of 25 mg/kg bw
14C-ß-isomer, and bile and urine were collected over 48 h.
In Sprague-Dawley rats, the bulk of the radioactivity was
excreted within 48 h, with 24% and 65% excreted in urine and faeces,
respectively. In Long-Evans male rats, total excretion was similar,
with 25 and 64% excreted in urine and faeces, respectively. Excretion
in the female rats was slightly slower. In bile-duct cannulated
animals, 13% of the total radioactivity was in bile over 48 h
(Pfizer, 1986).
In a study to identify the metabolites of the ß-isomer of
alitame, 2 male and 2 female Long-Evans rats were administered a
single oral dose of 10 mg/kg bw 14C-ß-isomer of alitame. In a second
experiment, female Long-Evans rats received 14C-ß-isomer of alitame
at an oral dose of 25 mg/kg bw. In a third experiment, 3 females and 6
males received the same dose of 14C-ß-isomer of alitame. Urinary
metabolites were examined by HPLC. Urinary radioactivity contained
approximately 1% of the radioactivity as unchanged ß-isomer in the
males.
Unchanged ß-isomer was found in the urine of female rats and
represented approximately 13% of the total radioactivity. Other
metabolites which represented approximately 50% of the radioactivity
were alanine tetramethylthietane amide sulfoxide (CP-58,290), alanine
tetramethylthietane amide sulfone (CP-57,254) and their acetylated
derivatives (CP-71,246 and CP-58,386). The only different metabolite
identified was the N-acetyl derivative of the ß-isomer which was
formed by acetylation of the free amine group of the aspartic acid
moiety. The N-acetyl derivative of the ß-isomer comprised 9% and 31%
of the radioactivity in the urine of male and female rats,
respectively. In faeces, the ß-isomer accounted for the majority
(70-81%) of the radioactivity (Pfizer, 1986).
In a study to examine systemic exposure to B-alitame and N-acetyl
beta-isomer by examination of urinary metabolites in male Long-Evans
rats, 14C-ß-isomer of alitame or 14C-N-acetyl -ß-isomer was
administered i.v. initially, then orally, at a dose level of 25 mg/kg
bw. Urine and faeces were collected at 24-hour intervals for a 48-hour
period. Systemic exposure was calculated from a comparison of urinary
excretion following the oral and i.v. routes. The systemic exposure to
ß-isomer following oral administration was 9% in males and 10% in
females. The systemic exposure to ß-isomer plus N-acetyl-ß-isomer was
8% in males and 18% in females (Pfizer, 1986).
In a study to examine systemic exposure to ß-isomer by
examination of serum concentrations in Long-Evans male rats,
14C-ß-isomer was administered i.v. initially (25 mg/kg bw), then
orally at a dose level of 29 mg/kg bw. Serum samples were collected
from 3 animals at various time-points and analyzed by UV absorption
for ß-isomer. Serum concentration-time AUC determinations were made
for each route of administration. The systemic exposure to ß-isomer
following oral administration was 9.6%. The elimination half-life was
29 minutes following the i.v. dose (Pfizer, 1986).
In a study to examine the toxicokinetics and metabolism in dogs,
2 male and 2 female beagle dogs were administered an oral dose of
14C-ß-isomer of alitame followed by an unlabelled dose of 5 or
50 mg/kg bw. Urine and faeces were collected for 96 h. The two male
dogs excreted 35 and 39%, respectively, of the administered
radioactivity in the urine, and the females 14 and 16%, respectively.
Unchanged 13-isomer in urine accounted for 10-16% of the dose in all 4
dogs. All of the urinary metabolites were identical to the ones found
after administration of alitame, but the males had higher levels of
metabolites than the females (Pfizer, 1986).
2.2.11.2 Short-term toxicity studies
In a 3-month study, groups of 15 male and 15 female mice (Strain
Crl: COBS CD-1 (ICR) BR) were fed a diet containing the ß-isomer of
alitame (CP-63,884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption were recorded weekly. Urinary metabolites were
analyzed at weeks 11 and 13. Gross pathological examination was
performed on all animals and organ weights recorded. Tissues were
taken from all of the major organs for histopathological examination.
There was no treatment-related effect on body-weight gain
although the average body weight of treated male animals was lower
than in control groups. Food consumption was slightly decreased at
high dose in males. One of the urinary metabolites identified was the
N-acetyl derivative of the/%isomer. The amount of the isomer increased
proportionally with the dose. There were no treatment-related effects
on organ weights. Gross pathology was unremarkable and histopathology
did not reveal any treatment-related changes. The NOEL in this study
was >25 mg/kg bw/day (Stadnicki et al., 1986a).
In a 3-month study, groups of 15 male and 15 female Long-Evans
rats (Strain Crl: (LE) BR) were fed a diet containing ß-isomer of
alitame (CP-63884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption data were recorded weekly. Ophthalmoscopic
examinations were performed at pre-test and prior to necropsy. Blood
samples were taken for examination of clinical chemistry and
haematological parameters at pre-test and at 3, 9 and 13 weeks.
Urinary analyses were performed at 3, 9 and 13 weeks. At the end of
the study, gross pathological examinations were performed and tissues
from the major organs taken for histopathology.
There were no deaths through the study and the body-weight gains
and food consumption were similar for treated and untreated groups.
There were no treatment-related effects on clinical chemistry,
haematology or urinary parameters. There were no differences between
control and treated groups with respect to organs weights. Gross
pathology and histopathology were unremarkable The NOEL in this study
was > 25 mg/kg bw/day (Stadnicki et al., 1986b).
2.2.11.3 Special studies on embryotoxicity/teratogenicity
A group of 88 albino female rats (strain Crl:COBS-CD (SD)BR) was
inseminated and divided into 4 groups of 22 animals. The groups of
presumed pregnant rats were administered the 3-isomer of alitame
(CP-63,884) in distilled water by gavage at dose levels of 0, 25, 50
or 100 mg/kg bw/day on days 6 to 15 post-insemination. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half stained for skeletal anomalies and half
examined for soft tissue defects.
The pregnancy rate was 20/22 in the control and low-dose groups,
and 19/22 in the intermediate- and high-dose groups. There were no
deaths throughout the study, nor any clinical signs of toxicity. There
were no treatment-related effects on body weight or food consumption
of dams during or after the treatment period. The number of corpora
lutea, implantation sites and viable fetuses was similar for all
groups. With regard to fetal development, there was no difference
between fetal weight of control and treated animals. Examination of
the fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral malformations, nor any treatment-related effects
on the degree of ossification. Under the conditions of this study, the
ß-isomer of alitame showed no evidence of teratogenicity in rats
(Kessedjian et al., 1986).
2.2.11.4 Special studies on genotoxicity
The ß-isomer of alitame was tested for the induction of point
mutations, both base-pair and frameshift, at the his locus of
Salmonella typhimurium strains TA 1535, TA 1537, TA 98 and TA 100,
with or without S9 microsomal activation. The purity of the alitame
was not provided. Doses ranged from 20 to 10 000 µg/plate, with or
without activation. There was no increase in revertants above the
control (water) in any of the treated plates at any dose level. The
positive controls, 9-aminoacridine, nitrofurantoin, sodium nitrite,
2-nitrofluorene and 2-anthramine gave appropriate responses (Ellis
et al., 1991).
2.3 Observations in humans
In a 90-day double-blind tolerance study, groups of male and
female subjects (77 treated, 80 controls) were administered either
alitame in capsules divided over three meals at a dose level of
10 mg/kg bw/day for 90 days, or placebo in the same manner. This dose
was chosen because it is 10 times greater than the estimated daily
intake if all sucrose in the diet were replaced with alitame. Subjects
were assessed throughout the study period for side-effects (by
interview and observation). Other tests included blood pressure,
temperature, body weight, electrocardiogram, and ophthalmoscopic
examination. Various clinical pathology parameters were also measured.
Microsomal activation was estimated from the elimination kinetics of
aminopyrine at pre-dosing and at weeks 6 and 13 of treatment. Plasma
and urine samples were collected at various time points throughout the
study.
Four treated subjects and 2 control subjects discontinued from
the study due to side effects (angioedema of the lips, maculopapular
rash, urticaria (wheals) with erythema in treated subjects, and
tinnitus, dry eyes and abdominal cramps in the control subjects). A
rechallenge with alitame after 6 months produced none of the above
symptoms, making the possibility of allergic reaction very unlikely.
There were no treatment-related changes in blood pressure, pulse,
temperature or body weight. Electrocardiograms and ophthalmological
examinations were unremarkable. Haematological parameters were similar
in treated and control subjects. Measurement of aminopyrine
elimination rates at 6 and 13 weeks did not reveal any increase in
hepatic microsomal enzyme activity due to alitame treatment (Gerber
et al., 1987).
In a 90-day double-blind tolerance study in diabetic subjects,
groups of male and female subjects having either type I or type II
diabetes (75 treated, 80 control) were administered either alitame in
capsules divided over three meals at dose level of 10 mg/kg bw/day for
90 days, or placebo in the same manner. This dose was chosen because
it is ten times greater than the estimated daily intake if all sucrose
in the diet were replaced with alitame. Subjects were included on the
following criteria: diabetics of type I and type II, either sex and
aged between 15 and 70 years with stable diabetic therapy; fasting
blood glucose <250 mg/dL; free from investigational drugs; had not
suffered a myocardial infarction within the previous 6 months; and had
either no hypertension or it was controlled. Subjects were excluded on
the following criteria: females which were pregnant or nursing; drug
or alcohol dependence; systemic disease other than diabetes; clinical
chemistry and haematological parameters above the upper limit of
normal; hepatic disease; and donation of blood within 4 weeks prior to
the study. The groups were well matched with respect to such
parameters as age, sex distribution, weight, smoking, drinking,
intercurrent illnesses and medication as well as diabetic status.
Subjects were assessed at weeks 0, 1, 2, 3, 4, 6, 8, 10 and 13 of
the study period for side effects (by interview and observation).
Effects on diabetic control were assessed by measuring fasting
haemoglobin A1c and 2-hour postprandial blood glucose levels at
baseline and weeks 0, 6, 8 and 13. Physical examinations were
conducted at screening and at week 13 of the study and included blood
pressure, temperature, body weight and electrocardiogram. Clinical
pathology parameters (haematology, clinical chemistry and urinary)
were measured pre-treatment and at 13 weeks.
At the end of the study period, 16 treated and 9 control subjects
had discontinued from the programme and, of these, 5 treated and 2
control subjects withdrew due to illness. No subjects discontinued due
to side effects or abnormal laboratory parameters related to alitame
treatment. Side-effects were observed in 29% of treated subjects and
25% of control subjects. Gastrointestinal disturbances were the most
common side-effect. Incidence and severity of particular side-effects
were relatively evenly distributed between the groups. No significant
changes were noted in either the control or treated groups with
respect to diabetic control, clinical chemistry, haematological or
urinary parameters. No treatment-related effects were apparent from
the physical examinations, blood pressure and pulse rate, temperature,
body weight or EKG recordings.
Alitame at a dose of 10 mg/kg/bw/day for 90 days was well
tolerated by the type I and type II diabetic patients who continued in
this study. There was no effect of alitame at this dose level on the
ability to control serum glucose levels by these diabetic patients
(McMahon et al., 1989).
In a 1- and 2-year follow-up of the subjects in the 90-day study
in diabetics, all but one of the treated subjects received an
interview and complete physical examination. Subjects were interviewed
again within 24 months post-study for all but 6 treated and 4 control
subjects.
A variety of intercurrent illnesses were reported during the
follow-up period. Most of the reported illnesses related to diabetes
and its complications. The incidence of intercurrent illnesses during
the follow-up period was 40 treated and 36 control subjects. Two
subjects died during the follow-up period, both treated subjects. One
subject (type I diabetic) died during the month following the study,
cause unknown. At completion of the study no adverse reactions had
been noted in this subject. The second subject (type II diabetic)
suffered a fatal heart attack resulting from hypertensive
atherosclerotic cardiovascular disease.
At the first follow-up (12 months), 5 treated subjects and no
control subjects were found to have suffered myocardial infarction.
The possibility that the results were due to chance was examined using
available epidemiological data on the incidence of myocardial
infarction. Data from the US National Hospital Discharge Survey (NHDS)
were used to estimate the rate of myocardial infarctions among
diabetics in various age groups for both sexes. These data were
adjusted to take into account the number of myocardial infarction
casualties which are not admitted to hospital and the actual patient
years of observation. Based on the hospitalization data for diabetics
who suffered myocardial infarction in 1986, the numbers of subjects
expected to suffer a myocardial infarction in a single year were 2.9
and 3.2 in the treated and control groups, respectively. The observed
rates of 5% and 0%, respectively, were within the 95% tolerance
intervals of incidence expected based on the NHDS analysis. Two years
after the study, myocardial infarctions had been encountered by 6
treated and 3 control subjects. The statistical analysis of these data
at 2 years supports the null hypothesis that the incidence observed
were due to chance. The data indicate that in the two years after the
90-day study of alitame in diabetics, no evidence was provided that
alitame adversely affected the health of the study groups (McMahon
et al., 1989).
3. COMMENTS
A number of toxicity studies on alitame were available, as well
as studies on the ß-isomer of alitame, which is formed at low levels
in some foods.
In metabolism studies conducted in three animal species and in
humans, alitame was readily absorbed from the GI tract and then
rapidly metabolized and excreted. In mice, rats and dogs, the route of
metabolism involved cleavage of the peptide bond yielding aspartic
acid and D-alanine tetramethylthietane amide, the latter being further
biotransformed by oxidation at the thietane sulfur to form the
sulfoxide and sulfone. Acetylated derivatives of these metabolites
were commonly found in the urine of rats and dogs whereas, in humans,
the glucuronide derivative of D-alanine tetramethylthietane amide was
the major urinary metabolite. In rats, initial cleavage of the peptide
bond of alitame took place in the lumen of the jejunum. In pregnant
rats, alitame was readily transferred transplacentally, but there was
no evidence of active secretion in the milk. In a study in rats,
levels of alitame residues decreased rapidly in all tissues except the
eyes. Further studies indicated that it was bound to melanin contained
in the uveal tract (choroid, ciliary body and iris) and suggested that
such binding was associated with a metabolite formed only in rats. The
Committee considered that an in vivo study in a mammalian species
other than the rat is desirable to clarify the significance of the
retention of alitame in the eyes.
The ß-isomer of alitame was rapidly metabolized in rats, the only
urinary metabolite not also formed with alitame being the N-acetyl
derivative of this isomer. There was no evidence of the formation of
significant amounts of the ß-isomer of alitame in the stomach or small
intestine of the rat.
Acute and short-term toxicity studies indicated that the toxicity
of alitame is low. In studies conducted in mice, rats and dogs, the
major toxicological changes observed were associated with the liver. A
dose-related increase in liver weight and evidence of centrilobular
hypertrophy were seen in all of the studies in these three species,
and these changes were accompanied by an increase in the level of
hepatic microsomal enzymes. Short-term studies on hepatic enzyme
induction indicated that alitame was a weak inducer of microsomal
enzymes; the pattern of induction was qualitatively similar to that
caused by phenobarbitone but at a lower level. The liver changes were
indicative of an adaptive response and, in rats and dogs, withdrawal
of treatment after a 3-month period resulted in partial or complete
reversal of the observed changes within a month.
Two-year carcinogenicity studies have been conducted in CD-1
mice, Long-Evans rats and Sprague-Dawley rats. The two studies
conducted in rats included an in utero phase. There was no evidence
of carcinogenicity in the study in mice.
In rats, the first carcinogenicity study was conducted in
Long-Evans rats at dietary levels of 0.1, 0.3 or 1% and, while the
survival rate was greater than 50% in all groups at 22 months, poor
survival was noted at 24 months. Pathological examination of the liver
indicated a higher incidence of focal nodular hyperplasia and
eosinophilic foci in females at the 1% dose level than in controls,
according to the diagnostic criteria of Squire & Levitt (1975). Two
subsequent independent re-examinations of these data have been
performed using the diagnostic criteria of Maronpot et al. (1986).
In the first re-examination (Farrell, 1987), the higher incidence of
eosinophilic foci in females in the high-dose group was confirmed, but
the incidence of focal hyperplasia or hepatocellular adenomas was
similar to that in controls. In the second re-examination (Hardisty
et al., 1994), the higher incidence of eosinophilic foci in females
in the high-dose group was again confirmed. A higher incidence of
these foci was also found at the mid-dose level. Also in this
re-examination, the nodular hyperplastic lesions noted in the original
report were largely reclassified as hepatocellular adenomas, and an
increased incidence of these lesions was seen at the high-dose level.
No evidence of hepatocellular carcinomas was found in female rats in
the original examination or in the subsequent re-examinations. The
main difference between the first and second re-examinations was in
the nomenclature used, rather than in the recognition that lesions
existed. The Committee expressed concern at the change in the way that
these liver lesions were classified, possibly as a result of the use
of different diagnostic criteria. It considered that there was
evidence of a higher incidence of adenomas in females in this study,
at least at the high dose level. The adenomas were found late in the
study and, while considered unlikely to progress to hepatocellular
carcinomas, the data were inconclusive in this regard.
A second carcinogenicity study, this time in Sprague-Dawley rats,
was conducted because of the low survival rate at 24 months in the
Long-Evans rats. In this second study, the survival rate was lower at
both 22 and 24 months than that found in the Long-Evans rats, and
hence the study was considered inadequate to provide further
information regarding the potential carcinogenicity of alitame.
The Committee considered that the available studies did not
indicate that alitame was carcinogenic, but that they were deficient
in certain respects and did not fully address this question. It also
considered that further research to clarify the mechanism of the
formation of adenomas in female Long-Evans rats was desirable.
Genotoxicity studies both in vitro and in vivo did not
provide any evidence that alitame has a mutagenic potential.
Similarly, there was no evidence from studies conducted in rats and
rabbits that alitame has a teratogenic potential.
Two reproductive toxicity studies (2-generation) were conducted
in Long-Evans rats and one reproductive toxicity study (1-generation)
in Sprague-Dawley rats. The first reproductive toxicity study with
Long-Evans rats produced some evidence for slightly decreased
body-weight gain in the pups during lactation at 1% in the diet, the
highest dose administered. A similar effect was noted in the second
reproductive toxicity study at the same dose level, but not in the
1-generation reproductive toxicity study with Sprague-Dawley rats. A
study of the effects of alitame-containing diet in the bedding
material, which could have been a source of alitame for pups due to
spilled feed, while providing a possible explanation for the decrease
in pup body-weight gain, did not fully address this issue. A slight
effect of alitame on locomotor activity seen in the first reproductive
toxicity study was not substantiated in subsequent studies. The
Committee did not regard the changes seen in the reproductive toxicity
studies to be of toxicological significance.
Neurotoxicity and neurobehavioural toxicity studies using an
observational battery of tests indicated behavioural changes only when
very high doses were administered to rats as a single dose by gavage
(5000 mg/kg bw). These behavioural changes were of short duration and
animals appeared to be normal by day 7. There was no evidence of
neurotoxicity in rats fed diets containing 1% alitame (equivalent to
1000 mg/kg bw/day) over a 3-month period. Similarly, no changes
indicative of neurotoxicity were observed in dams or pups when the
same dose level was given over the reproductive period.
In a 14-day study conducted in humans there was no evidence of
hepatic enzyme induction at a dose level of 15 mg/kg bw/day.
Ninety-day tolerance studies were conducted in normal and
diabetic subjects. There was no evidence of adverse effects in
subjects of both types during the period of the study at a dose level
of 10 mg/kg bw/day. However, in the 1- and 2-year follow-up of the
diabetic subjects, there was a higher incidence of myocardial
infarction in the treatment group than in the controls, namely 5/58 in
the treatment group and 0/71 in the controls after 1 year, and 6/53 in
the treatment group and 3/67 in the controls after 2 years. While the
differences at 2 years were not statistically significant, and there
was no indication of cardiovascular effects in the animal studies, the
Committee considered that the increased incidence of myocardial
infarction in the treatment group has not been fully explained. It was
advised that a further study is to be performed in diabetic subjects.
Studies conducted with the ß-isomer of alitame provided no
evidence of genotoxicity, teratogenicity or systemic toxicity in mice
or rats at dose levels of up to 25 mg/kg bw/day.
4. EVALUATION
The Committee recognized that the basis on which to consider
establishing an ADI for a chemical such as alitame is difficult to
specify, since the most sensitive treatment-related effects observed
were liver enzyme changes and liver weight changes, which may be
adaptive in nature. For alitame, these changes appeared to be closely
linked. While the prolonged hepatomegaly observed was not accompanied
in the long-term studies by any pathological changes indicative of
toxicity, it is not considered desirable. On the other hand, moderate
induction of hepatic enzymes is considered a normal adaptive process.
In the case of alitame, significant changes in body-weight gain or
liver weight were observed at doses above the NOELs. In the 18-month
study in dogs, the NOEL was 100 mg/kg bw/day. In the 2-year study in
mice, the NOEL was 0.1% in the diet, equal to 125 mg/kg bw/day. In the
lifetime studies in rats, the NOEL was 0.3% in the diet, equal to
130 mg/kg bw/day in Long-Evans rats and 230 mg/kg bw/day in
Sprague-Dawley rats.
The Committee concluded that the concerns raised by the
deficiencies in the carcinogenicity studies in rats were of such
significance that an ADI could not be allocated.
5. REFERENCES
AMACHER, D.E., MUEHBAUER, P.A., ZELLJADT, I. (1991) Genetic Toxicology
Report. Unpublished Report No. 91-444-21 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
BACOPOULOS, N.G. (undated) Investigation of alitame and its b-isomer
in neurochemical and behavioural animal models. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1984) Alitame: A Fourteen Day
Human Enzyme Induction Study. Unpublished Report No. 32 from
Bio-Organic Chemicals Research & Development, Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1985) Human Disposition of
14C-Alitame. Unpublished Report No. SC-2 from Department of
Pharmacology, University of London. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M., THATCHER N.J., MARSHALL, A.D. (1994)
Assessment of the Enzyme Inducing Characteristics of Alitame.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School., London, UK. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M. /1994) Assessment of the Enzyme Inducing
Characteristics of Alitame Suppl. Report on Ummunodetection of
Cytochrome P450 Isoenzymes in the Liver of Alitame-Treated Rats.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School, London, UK Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981a)
Alitame: Two-Week Range-Finding Feeding Study in Rats. Unpublished
Report No. 81-055 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981b)
Alitame: Two-Week Oral Range-Finding Study in Dogs. Unpublished Report
No. 81-070 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ELLIS, J.H., AMACHER, D.E., KLUWE, W.M. (1991) b-Isomer of Alitame:
Microbial Gene Mutation Assays. Unpublished Report from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
FARRELL, J.F. (1987) Histopathologic evaluation of selected
microslides from chronic rat and mouse feeding studies with alitame.
Unpublished Report No. PR-116 from Experimental Pathology Laboratories
Inc. USA. Submitted to WHO by Pfizer Inc., Groton CT, USA.
FISHER, D.O., MARSH, P.M., SOLER, G.N., COLEMAN, D.V., LEVINSKY, H.V.,
ESTES, P.C., DOUGHERTY, W.J. (1992) One-Year Toxicity Study with
Dietary Administration and In Utero Exposure in Sprague-Dawley Rats.
Unpublished Report No. 90-444-18 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
FISHER, D.O., LAITWAITE, B.S., BUTLER, B.T., COLEMAN, D.V., LEVINSKY,
H.V., OCHOA, R., DOUGHERTY, W.J. (1993) Two Year Oncogenicity Study
with Dietary Administration and In Utero Exposure in Sprague-Dawley
Rats. Unpublished Report No. 90-444-20 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
GERBER, N., McMAHON, M.D., WILLIAMS, R.L. (1987) Double-Blind Placebo
Controlled Ninety-Day Toleration Study of Alitame. Unpublished Report
No. 101 from Ohio State University, Columbus, Ohio. USA. Submitted to
WHO by Pfizer Inc. Groton, CT, USA.
HARDISTY, J.E., ABBOTT, D., GOPINATH, C., WARD, J. (1994) Pathology
Working Group Review of Two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report from Experimental Pathology
Laboratory Inc., Research Triangle Park, NC, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLDEN, H.E., ELLIS, J.H., AMACHER, D., RAY, V.A. (1981) Alitame:
Genetic Toxicology Report. Unpublished Report No. 91-444-21 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., TOOLAN, L.A, BURGAN, J.J., REYNOLDS, J.A., LEVINSKY,
H.V., ESTES, P.C. (1983a) Alitame: Two-Month Range-Finding Feeding
Study in Mice. Unpublished Report No. 82-444-03 from Pfizer Central
Research, Groton, CT, USA Laboratoires. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., ANGUS, T.A., FISHER, D.O., FIGDOR, S.K., REYNOLDS, J.A.,
ESTES, P.C., LEVINSKY, H.V. (1983b) Alitame: Five-Week Feeding Study
in Rats. Unpublished Report No. 82-444-01 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton,
CT, USA.
HOLMES, C.L., MARTZ, F., BOWDEN, P.R., FIGDOR, S.K., ROSS, P.E.,
LEVINSKY, H.V., ESTES, P.C.(1985a) Alitame: One-Year Feeding Study in
Long-Evans Rats. Unpublished Report No.s. 82-444-06 and 83-444-12 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., MARTZ, F., PUNZALAN, E.R., FIGDOR, S.K., ROESLER, A.R.,
LEVINSKY, H.V,, ESTES, P.C. (1985b) Alitame: Eighteen-Month Feeding
Study in Beagle Dogs. Unpublished Report No. 82-444-07 from
Laboratoires Pfizer Centre de Recherche, 37400 Ambroise, France.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., CACCIATORE. A., BURGAN, J.J., COLEMAN, G.L., FIGDOR,
S.K., LEVINSKY, H.V., REYNOLDS, J.A., ESTES, P.C., (1985c) Alitame:
Two-Year Oncogenicity Feeding Study in Mice. Unpublished Report
No. 82-444-08 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L. (1986a) Alitame: A two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report No. 83-444-11. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986b) Alitame: Two-Generation Reproduction
Feeding Study in Rat. Unpublished Report No. 83-444-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986c) Alitame: Second Two-Generation
Reproduction Study in Rat. Unpublished Report No. 83-444-13/14/15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROSE, E.M., STRADNICKI, S.W., LEVINSKY, H.V.
(1986d) Exploratory bedding study in the Long-Evans Rat. Unpublished
Report No. 85-444-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
KESSEDJIAN, M.J., STADLER, J., POOL, W.R., (1986) b-Isomer of Alitame:
Fetotoxicity Study in Rats by the Oral Route. Unpublished report
86-086 from Pfizer Central Research, Groton, CT, USA. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., BEUTER, N.J., STADNICKI, S.W. (1983) Alitame: Acute
Oral Toxicity Studies in Mice and Rats. Unpublished Report
No. 83-444-09 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., STADNICKI, S.W., ESTES, P.C., DOUGHERTY, W.J.,
CACCIATORE, A., KOPCYK, N., LUNDEEN, G.R. (1992) In Utero Exposure
with Alitame in Sprague-Dawley rats. Unpublished Report No. 90-444-19
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MARONPOT, R.R., MONTGOMERY Jr C.A., BOORMAN, G.A., McCONNELL (1986)
National toxicology programme nomenclature for hepatoproliferative
lesions of rats. Toxicol. Pathol., 14: 263-273.
McMAHON, F.G., CYRUS, J., RASKIN, P., STARR, I. (1989) Double-Blind
Placebo Controlled Ninety Day Toleration Study of Alitame in
Diabetics. Unpublished Report No. 102 from Central Research Centre,
New Orleans, LA, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
MONRO, A.M. CHVEDOFF, M., GREAVES, P., NACHBAUR, J., PERRAUD, J.,
(1982a) Three-Month Oral Toxicity Study in Rats plus 1-Month
Withdrawal. Unpublished Report No. 81-086 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MONRO, A.M., CHVEDOFF, M., PERRAUD, J., GREAVES, P., NACHBAUR, J.,
(1982b) Alitame: Three-Month Oral Toxicity Study in Dogs plus 1-Month
Withdrawal. Unpublished Report No. 81-106 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
PEARSON, D.L., GAYNOR, B.J., SWINDELL, A.C. (1982) General
Pharmacology Report on Alitame. Autonomic, Gastro-intestinal, Renal
and Metabolic Studies. Unpublished Report. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J. (1983a) Alitame: Foetotoxicity Study in
Rats (FDA Segment II). Unpublished Report No. 82-154 from Laboratoires
Pfizer Centre de Recherche, 37400 Ambroise, France. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J., SENKOWSKI, S. (1983b) Alitame:
Foetotoxicity Study in Rabbits (FDA Segment II). Unpublished Report
No, 83-026 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
PFIZER CENTRAL RESEARCH. (1986) Alitame: Metabolism and Disposition.
Unpublished Report from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., BROXUP, B., NOVEROSKE, J.W. (1993a) An Acute Study of
the Potential Effects of Orally Administered Alitame on Behaviour in
Rats. Unpublished Report No. 97148 form Bio-Research Laboratories Ltd.
Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W. (1993b) A
13-Week Dietary Study of the Potential Effects of Alitame on Behaviour
and Neuromorphology in Rats. Unpublished Report No. 97149 form
Bio-Research Laboratories Ltd. Senneville, Quebec, Canada. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W., (1993c) A
Developmental Neurotoxicity Study of Alitame Administered in the Diet
of Rats. Unpublished Report No. 97150 from Bio-Research Laboratories
Ltd. Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT. USA.
SQUIRE, R.A., LEVITT, M.H. (1975). Report of a workshop on a
classification of specific hepatocellular lesions in rats. Cancer
Res., 35: 3214-3223.
STADNICKI, S.W., PUNZALAN. E.R., FIGDOR, S.K., ROESLER, A.R., ESTES,
P.C., LEVINSKY, H.V. (1986a) b-Isomer of Alitame: Three-Month
Feeding Study in Study CD-I Mice. Unpublished Report No. 86-580-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
STADNICKI, S.W., SANTACROCE, E.M., FIGDOR, S.K., ROSS, P.E.,
ESTES, P.C., LEVINSKY, H.V. (1986b) b-Isomer of Alitame: Three-Month
Feeding Study in Long-Evans Rats. Unpublished Report No. 86-580-01
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
WHITBY, B.R., POWLES, P., LEWSLEY, R., FERNYHOUGH, P., MARSLAND, R.
(1994) 14C-Alitame: Whole-body autoradiography and microhistoauto-
radiography in the rat. Unpublished Report No. 727/5-1011 from
Hazleton Europe, Harrogate, UK. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
A group of 5 male CD-1 mice was administered by gavage a single
dose of 50 mg/kg bw 14C-alitame in distilled water. A second group
of 5 male CD-1 mice was fed a diet containing 14C-alitame at a
concentration of 0.3%, equal to 350 mg/kg bw. Following the gavage
dose, 77% of the radioactivity was recovered in urine in a 24-hour
period. About 50% of the administered dose was excreted between 4 and
20 h. Faecal radioactivity was not measured. Following dietary
administration, 60% of the radioactivity was found in urine and 32% in
faeces (Pfizer, 1986).
2.1.1.2 Rats
A male Long-Evans rat with a bile duct cannula was administered a
single oral dose of 50 mg/kg bw 14C-alitame. Bile and urine were
collected for 48 h. In a second study, 4 male and 4 female Long-Evans
rats were administered a single oral dose of 5 mg/kg bw 14C-alitame.
Urine and faeces were collected for 7 days and analyzed for
radioactivity. In the single animal study, 83% of the radioactivity
was recovered, with 14% in bile and 69% in urine over 48 h, indicating
extensive absorption. In the group study, most of the radioactivity
was recovered in urine (83% in males, and 95% in females) in a 24-hour
period. Faecal radioactivity was 20% in males and 4% in females during
24 h with little detected after that time (Pfizer, 1986).
In a study to investigate tissue distribution in rats, a group of
24 male and 24 female Long-Evans rats received by gavage a single dose
of 5 mg/kg bw 14C-alitame. Animals in groups of 3 males and 3
females were killed at 2, 6 or 24 h, and at 2, 3, 7, 14 or 29 days and
tissue levels determined. In most tissues, the half-life of clearance
was 3 to 6 h and the levels were below the level of detection by 7
days. Residue levels of radioactivity in the eyes decreased much more
slowly than in other tissues and levels of 0.08 mg/kg were detected at
day 29 (Pfizer, 1986).
In a study to examine the potential for transplacental transfer
of alitame, pregnant Long-Evans rats received a single oral dose of
1000 mg/kg bw 14C-alitame on day 13 of gestation. Four animals were
sacrificed 6 or 24 h after treatment and maternal plasma and fetuses
were examined for radioactivity. Radioactivity was high in the plasma
of dams at 6 and 24 h, and high levels were also found in the fetuses
at these times, indicating transplacental transfer of alitame
(Pfizer, 1986).
In a study to examine potential accumulation of alitame or its
metabolites in the milk of lactating rats, pregnant Long-Evans rats
were fed diet containing 1% alitame (equal to 750 mg/kg bw/day)
beginning on the last day before birth, and were continued on this
diet for 13 days of lactation. At the end of the lactation period,
rats were given a single oral dose of 750 mg/kg bw 14C-alitame,
and plasma and milk were collected 6 h later. Concentration in milk
and plasma were the same for each animal with no indication of
accumulation in milk. Intake for each pup, based on an average
consumption of 3.7 g milk/day on lactation day 10. was calculated to
be between 6 and 13 mg/kg bw/day, which is less than 2% of the
maternal dose level (Pfizer, 1986).
In a study to examine the toxicokinetics of alitame after i.v.
treatment, a group of 30 male and 4 female Long-Evans rats received a
single i.v. injection of 25 mg/kg bw. Males received non-radioactive
alitame and were used to determine the serum concentration over a
6-hour period. The females received 14C-alitame and were used to
determine the excretion rate and the identity of the urinary
metabolites. Radioactivity in serum declined with an estimated
half-life of 13 minutes. Most of the radioactivity (87%) was found in
urine in the 48-hour period of collection. Approximately 50% of
urinary radioactivity was unchanged alitame, in contrast to only 1%
after oral administration. This result was consistent with a high rate
of hydrolysis in either the intestine or during first-pass hydrolysis
in the liver (Pfizer, 1986).
2.1.1.3 Dogs
Two male beagle dogs with bile cannulas received a single dose of
10 mg/kg bw 14C-alitame. In one dog, the alitame was injected
directly into the stomach during cannulation surgery. This dog was
maintained under anaesthesia during the 24-h collection of urine,
faeces and bile. In a separate study, 4 males and 4 females received a
single oral dose of 5 or 25 mg/kg bw 14C-alitame. Urine and faeces
were collected at 24-hour intervals over a 7-day period and analyzed
for radioactivity. In the first experiment, biliary excretion
comprised only 0.2-0.3% of the total radioactivity. Urinary excretion
was approximately 36% of the administered radioactivity in the
anaesthetized dog, and 81% in the conscious dog. Faecal radioactivity
was 10% of the administered radioactivity in the conscious dog. In the
second experiment, the administered radioactivity was recovered in
urine (94-98%) and faeces (3-4%) in the first two days (Pfizer, 1986).
In a study to examine the toxicokinetics after i.v. treatment, a
male beagle dog received a single i.v. injection of 10 mg/kg bw
14C-alitame. Blood samples were taken at times up to 72 h, and urine
and faeces were collected in three 24- hour intervals. Radioactivity
in serum gave a biphasic decline, with half-lives of 34 minutes and
4 h. The vast majority of the radioactivity was excreted in the first
24 h, mainly in the urine (80%). Unchanged alitame was the major
urinary excretion product (60%) (Pfizer, 1986).
In a study to examine whether the site of hydrolysis of alitame
was the intestine or the liver, a female beagle dog under anaesthesia
had a 7-inch segment of the jejunum ligated and the mesenteric vein
cannulated. A 3 ml solution containing 14C-alitame (4 mg/kg bw) was
injected into the ligated jejunal segment and the mesenteric blood
collected at 2-minute intervals and peripheral blood at 15-minute
intervals over a 2-hour period. The mesenteric blood accounted for 4%
of the total radioactivity over the 2-hour period. The level of
unchanged alitame remained relatively constant and accounted for
approximately 50% of radioactivity while the percentage of the
hydrolysis product alanine tetramethylthietane amide (CP-57,207; see
Figure 2) increased with time from 12% to 38% over 2 h. Unchanged
alitame was the major substance present in the jejunum, while the
concentration of alanine tetramethylthietane amide increased with
time. No radioactivity was found in peripheral blood or urine. The
results suggested that the lumen of the jejunum is the site for
hydrolysis of alitame, and that further hydrolysis occurred during
passage through the intestinal wall (Pfizer, 1986).
2.1.1.4 Humans
Four male subjects were administered an oral dose of 14C-alitame
(50 mCi; 1 mg/kg bw). Blood samples were taken at various times up to
48 h. Exhaled 14CO2 was measured at various times up to 8 h.
Urine and faeces were collected over 5 days.
Plasma radioactivity levels peaked between 8 and 18 h and
decreased rapidly within the first 48 h, indicating slow but extensive
absorption followed by rapid excretion. There was no significant
increase in exhaled 14CO2 above zero. Urinary radioactivity was
maximal between 12 and 24 h with approximately 50% of radioactivity
excreted in the first 24 h and 90% within 5 days Faecal elimination
accounted for 7-10% of the total dose in 3 subjects and 2% in the 4th
subject.
The major urinary metabolites were alanine tetramethylthietane
amide sulfoxide (CP-58,290; see Figure 2) and alanine tetramethyl-
thietane amide glucuronide. Minor metabolites were alanine
tetramethylthietane amide (CP-57,207) and alanine tetramethylthietane
amide sulfone (CP-57,254). The glucuronide was the major urinary
metabolite in the first 24 h whereas the sulfoxide was the major
metabolite in the 24-48 h period. Unchanged alitame constituted less
than 1% of the total urinary radioactivity. In faeces, all of the
radioactivity consisted of unchanged alitame (CP-54,802) and alanine
tetramethylthietane amide (CP-57,207). In plasma, the major part of
the radioactivity (80%) consisted of alanine tetramethylthietane amide
(CP-57,207) and its glucuronide. In contrast to rats and dogs, the
major urinary metabolite in humans was found to be the glucuronide of
alanine tetramethylthietane amide (Caldwell et al., 1985).
2.1.2 Biotransformation
The metabolic pathways of alitame in different animal species and
in humans are shown in Figure 2.
2.1.2.1 Mice
Following a single oral dose of 50 mg/kg bw 14C-alitame or a
diet containing 3000 mg/kg 14C-alitame, metabolites identified were
D-alanine tetramethylthietane amide sulfoxide (CP-58,290; 64.3%),
D-alanine tetramethylthietane amide (CP-57,207; 24.7%), and unchanged
alitame (0.9%). The remainder metabolites (10.1%) were not identified.
Faecal radioactivity was identified as 39.5% unchanged alitame
(CP-54,802), 51.5% D-alanine tetramethyltbietane amide (CP-57,207) and
4.6% was not identified (Pfizer, 1986).
2.1.2.2 Rats
In a study to examine metabolites in plasma, a group of
Long-Evans rats received a single oral dose of 50 mg/kg bw
14C-alitame. Two rats were bled serially to determine the time
course of radioactivity and a further 3 male and 3 female rats were
killed at 6 h and the plasma radioactivity was assayed by HPLC.
Maximum plasma levels occurred at 4 to 6 h after administration. The
major metabolite was alanine tetramethylthietane amide sulfoxide
(CP-58,290; 70% of the total radioactivity), in both the free and
acetylated forms with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in urine, 4 male and 4 female
Long-Evans rats were administered a single oral dose of 5 mg/kg bw
14C-alitame. Urine and faeces were collected over a 24-hour period
and analyzed by HPLC. The major urinary metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 75-80%), in both free
and acetylated forms, with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in faeces, two Long-Evans
rats received a single oral dose of 50 mg/kg bw or 1000 mg/kg bw
14C-alitame. Urine and faeces were collected over 24 h and analyzed
by HPLC. The major product in the faeces was unchanged alitame
(CP-54,802; 70% of the radioactivity). A second metabolite was
identified as alanine tetramethylthietane amide (CP-57,207; 23%)
(Pfizer, 1986).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
A group of 5 male CD-1 mice was administered by gavage a single
dose of 50 mg/kg bw 14C-alitame in distilled water. A second group
of 5 male CD-1 mice was fed a diet containing 14C-alitame at a
concentration of 0.3%, equal to 350 mg/kg bw. Following the gavage
dose, 77% of the radioactivity was recovered in urine in a 24-hour
period. About 50% of the administered dose was excreted between 4 and
20 h. Faecal radioactivity was not measured. Following dietary
administration, 60% of the radioactivity was found in urine and 32% in
faeces (Pfizer, 1986).
2.1.1.2 Rats
A male Long-Evans rat with a bile duct cannula was administered a
single oral dose of 50 mg/kg bw 14C-alitame. Bile and urine were
collected for 48 h. In a second study, 4 male and 4 female Long-Evans
rats were administered a single oral dose of 5 mg/kg bw 14C-alitame.
Urine and faeces were collected for 7 days and analyzed for
radioactivity. In the single animal study, 83% of the radioactivity
was recovered, with 14% in bile and 69% in urine over 48 h, indicating
extensive absorption. In the group study, most of the radioactivity
was recovered in urine (83% in males, and 95% in females) in a 24-hour
period. Faecal radioactivity was 20% in males and 4% in females during
24 h with little detected after that time (Pfizer, 1986).
In a study to investigate tissue distribution in rats, a group of
24 male and 24 female Long-Evans rats received by gavage a single dose
of 5 mg/kg bw 14C-alitame. Animals in groups of 3 males and 3
females were killed at 2, 6 or 24 h, and at 2, 3, 7, 14 or 29 days and
tissue levels determined. In most tissues, the half-life of clearance
was 3 to 6 h and the levels were below the level of detection by 7
days. Residue levels of radioactivity in the eyes decreased much more
slowly than in other tissues and levels of 0.08 mg/kg were detected at
day 29 (Pfizer, 1986).
In a study to examine the potential for transplacental transfer
of alitame, pregnant Long-Evans rats received a single oral dose of
1000 mg/kg bw 14C-alitame on day 13 of gestation. Four animals were
sacrificed 6 or 24 h after treatment and maternal plasma and fetuses
were examined for radioactivity. Radioactivity was high in the plasma
of dams at 6 and 24 h, and high levels were also found in the fetuses
at these times, indicating transplacental transfer of alitame
(Pfizer, 1986).
In a study to examine potential accumulation of alitame or its
metabolites in the milk of lactating rats, pregnant Long-Evans rats
were fed diet containing 1% alitame (equal to 750 mg/kg bw/day)
beginning on the last day before birth, and were continued on this
diet for 13 days of lactation. At the end of the lactation period,
rats were given a single oral dose of 750 mg/kg bw 14C-alitame,
and plasma and milk were collected 6 h later. Concentration in milk
and plasma were the same for each animal with no indication of
accumulation in milk. Intake for each pup, based on an average
consumption of 3.7 g milk/day on lactation day 10. was calculated to
be between 6 and 13 mg/kg bw/day, which is less than 2% of the
maternal dose level (Pfizer, 1986).
In a study to examine the toxicokinetics of alitame after i.v.
treatment, a group of 30 male and 4 female Long-Evans rats received a
single i.v. injection of 25 mg/kg bw. Males received non-radioactive
alitame and were used to determine the serum concentration over a
6-hour period. The females received 14C-alitame and were used to
determine the excretion rate and the identity of the urinary
metabolites. Radioactivity in serum declined with an estimated
half-life of 13 minutes. Most of the radioactivity (87%) was found in
urine in the 48-hour period of collection. Approximately 50% of
urinary radioactivity was unchanged alitame, in contrast to only 1%
after oral administration. This result was consistent with a high rate
of hydrolysis in either the intestine or during first-pass hydrolysis
in the liver (Pfizer, 1986).
2.1.1.3 Dogs
Two male beagle dogs with bile cannulas received a single dose of
10 mg/kg bw 14C-alitame. In one dog, the alitame was injected
directly into the stomach during cannulation surgery. This dog was
maintained under anaesthesia during the 24-h collection of urine,
faeces and bile. In a separate study, 4 males and 4 females received a
single oral dose of 5 or 25 mg/kg bw 14C-alitame. Urine and faeces
were collected at 24-hour intervals over a 7-day period and analyzed
for radioactivity. In the first experiment, biliary excretion
comprised only 0.2-0.3% of the total radioactivity. Urinary excretion
was approximately 36% of the administered radioactivity in the
anaesthetized dog, and 81% in the conscious dog. Faecal radioactivity
was 10% of the administered radioactivity in the conscious dog. In the
second experiment, the administered radioactivity was recovered in
urine (94-98%) and faeces (3-4%) in the first two days (Pfizer, 1986).
In a study to examine the toxicokinetics after i.v. treatment, a
male beagle dog received a single i.v. injection of 10 mg/kg bw
14C-alitame. Blood samples were taken at times up to 72 h, and urine
and faeces were collected in three 24- hour intervals. Radioactivity
in serum gave a biphasic decline, with half-lives of 34 minutes and
4 h. The vast majority of the radioactivity was excreted in the first
24 h, mainly in the urine (80%). Unchanged alitame was the major
urinary excretion product (60%) (Pfizer, 1986).
In a study to examine whether the site of hydrolysis of alitame
was the intestine or the liver, a female beagle dog under anaesthesia
had a 7-inch segment of the jejunum ligated and the mesenteric vein
cannulated. A 3 ml solution containing 14C-alitame (4 mg/kg bw) was
injected into the ligated jejunal segment and the mesenteric blood
collected at 2-minute intervals and peripheral blood at 15-minute
intervals over a 2-hour period. The mesenteric blood accounted for 4%
of the total radioactivity over the 2-hour period. The level of
unchanged alitame remained relatively constant and accounted for
approximately 50% of radioactivity while the percentage of the
hydrolysis product alanine tetramethylthietane amide (CP-57,207; see
Figure 2) increased with time from 12% to 38% over 2 h. Unchanged
alitame was the major substance present in the jejunum, while the
concentration of alanine tetramethylthietane amide increased with
time. No radioactivity was found in peripheral blood or urine. The
results suggested that the lumen of the jejunum is the site for
hydrolysis of alitame, and that further hydrolysis occurred during
passage through the intestinal wall (Pfizer, 1986).
2.1.1.4 Humans
Four male subjects were administered an oral dose of 14C-alitame
(50 mCi; 1 mg/kg bw). Blood samples were taken at various times up to
48 h. Exhaled 14CO2 was measured at various times up to 8 h.
Urine and faeces were collected over 5 days.
Plasma radioactivity levels peaked between 8 and 18 h and
decreased rapidly within the first 48 h, indicating slow but extensive
absorption followed by rapid excretion. There was no significant
increase in exhaled 14CO2 above zero. Urinary radioactivity was
maximal between 12 and 24 h with approximately 50% of radioactivity
excreted in the first 24 h and 90% within 5 days Faecal elimination
accounted for 7-10% of the total dose in 3 subjects and 2% in the 4th
subject.
The major urinary metabolites were alanine tetramethylthietane
amide sulfoxide (CP-58,290; see Figure 2) and alanine tetramethyl-
thietane amide glucuronide. Minor metabolites were alanine
tetramethylthietane amide (CP-57,207) and alanine tetramethylthietane
amide sulfone (CP-57,254). The glucuronide was the major urinary
metabolite in the first 24 h whereas the sulfoxide was the major
metabolite in the 24-48 h period. Unchanged alitame constituted less
than 1% of the total urinary radioactivity. In faeces, all of the
radioactivity consisted of unchanged alitame (CP-54,802) and alanine
tetramethylthietane amide (CP-57,207). In plasma, the major part of
the radioactivity (80%) consisted of alanine tetramethylthietane amide
(CP-57,207) and its glucuronide. In contrast to rats and dogs, the
major urinary metabolite in humans was found to be the glucuronide of
alanine tetramethylthietane amide (Caldwell et al., 1985).
2.1.2 Biotransformation
The metabolic pathways of alitame in different animal species and
in humans are shown in Figure 2.
2.1.2.1 Mice
Following a single oral dose of 50 mg/kg bw 14C-alitame or a
diet containing 3000 mg/kg 14C-alitame, metabolites identified were
D-alanine tetramethylthietane amide sulfoxide (CP-58,290; 64.3%),
D-alanine tetramethylthietane amide (CP-57,207; 24.7%), and unchanged
alitame (0.9%). The remainder metabolites (10.1%) were not identified.
Faecal radioactivity was identified as 39.5% unchanged alitame
(CP-54,802), 51.5% D-alanine tetramethyltbietane amide (CP-57,207) and
4.6% was not identified (Pfizer, 1986).
2.1.2.2 Rats
In a study to examine metabolites in plasma, a group of
Long-Evans rats received a single oral dose of 50 mg/kg bw
14C-alitame. Two rats were bled serially to determine the time
course of radioactivity and a further 3 male and 3 female rats were
killed at 6 h and the plasma radioactivity was assayed by HPLC.
Maximum plasma levels occurred at 4 to 6 h after administration. The
major metabolite was alanine tetramethylthietane amide sulfoxide
(CP-58,290; 70% of the total radioactivity), in both the free and
acetylated forms with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in urine, 4 male and 4 female
Long-Evans rats were administered a single oral dose of 5 mg/kg bw
14C-alitame. Urine and faeces were collected over a 24-hour period
and analyzed by HPLC. The major urinary metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 75-80%), in both free
and acetylated forms, with unchanged alitame only 1% of the total
radioactivity (Pfizer, 1986).
In a study to examine metabolites in faeces, two Long-Evans
rats received a single oral dose of 50 mg/kg bw or 1000 mg/kg bw
14C-alitame. Urine and faeces were collected over 24 h and analyzed
by HPLC. The major product in the faeces was unchanged alitame
(CP-54,802; 70% of the radioactivity). A second metabolite was
identified as alanine tetramethylthietane amide (CP-57,207; 23%)
(Pfizer, 1986).
 In a study in lactating dams, metabolites in milk were alanine
tetramethylthietane amide sulfoxide (CP-58,290) and its acetylated
form (54.4%), alanine tetramethylthietane amide (CP-57,207; 31.4%),
and unchanged alitame (1%) (Pfizer, 1986).
In a study to examine the effect of dose on metabolic pathways,
Long-Evans rats received a single oral dose of 10, 100 or 1000 mg/kg
bw 14C-alitame and urine and faeces were collected at 24-hour
intervals over 3 days. Urinary metabolites were analyzed by HPLC.
There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment, but the
metabolic profile was essentially unchanged (Pfizer, 1986).
In a study to compare the metabolic fate of D-alanine as either
free alanine or when incorporated into alitame, Long-Evans rats were
administered 14C-D-alanine or 14C-alitame (1-14C-D-alanine
label) by oral gavage and exhaled 14CO2 was measured. While
14CO2 was extensively exhaled following administration of
D-alanine, no 14CO2 was expired after administration of
14C-alitame, demonstrating the stability of the D-alanine
tetramethylthietane amide bond to metabolic hydrolysis (Pfizer, 1986).
2.1.2.3 Dogs
In a study to examine metabolites in plasma, groups of 3 male and
3 female beagle dogs received a single oral dose of 5 or 25 mg/kg bw
14C-alitame. Plasma samples were taken at various times and the
metabolites in plasma identified by HPLC. In both male and female
dogs, plasma radioactivity peaked at 2-3 h. The major metabolite was
alanine tetra-methylthietane amide sulfoxide (CP-58,290; 80%), with
10% in the acetylated form. Approximately 5% was unchanged alitame.
The remainder was alanine tetramethylthietane amide (CP-57,207), its
sulfone derivative (CP-57,254) and the acetylated forms of these
compounds. The metabolite profile was very similar to that seen in the
rat (Pfizer, 1986).
In a study to examine the metabolites in urine, a group of 3 male
and 3 female beagle dogs received a single oral dose of 14C-alitame,
and 24-hour urine and faeces collected. Urinary metabolites were
analyzed by HPLC. In males, the major metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 80%), including 9.5%
as the acetylated form. Approximately 5-15% of the urinary
radioactivity was unchanged alitame (Pfizer, 1986).
In a study to examine metabolites in faeces, a male beagle dog
received a single oral dose of 10 mg/kg bw 14C-alitame followed
several weeks later by a dose of 500 mg/kg bw. A female beagle dog
received a single oral dose of 500 mg/kg bw 14C-alitame Faecal
samples were collected over 24 h and analyzed by HPLC. Faeces
contained 18-40% of the administered radioactive dose over the 24 h
period. Faecal radioactivity was a mixture of unchanged alitame
(CP-54,802) and alanine tetramethylthietane amide (CP-57,207).
Together these two products accounted for approximately 90% of the
total radioactivity (Pfizer, 1986)
In a study to examine the effect of dose on metabolic pathways,
2 male and 2 female beagle dogs received a single oral dose of 10 or
500 mg/kg bw 14C-alitame and urine and faeces were collected in
24-hour intervals over 3 days. Urinary metabolites were analyzed by
HPLC. There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment than after the
low-dose treatment, but the metabolite profile was essentially
unchanged (Pfizer, 1986).
2.1.2.4 In vitro studies
The stability of alitame in gastric juices was examined to
clarify whether the cleavage of aspartic acid is initiated in the acid
environment of the stomach, and whether conversion of alitame to the
ß-isomer might occur under these conditions. Thus, alitame was
incubated in simulated gastric juices at 38°C to determine the degree
of hydrolysis. Controls were water and simulated gastric juices
(boiled). Samples were taken over 24 h and analyzed by HPLC. Alitame
was relatively stable, with only slight evidence of degradation after
prolonged incubation for 24 h, which was not considered to be due to
enzyme activity. Similarly, the small increase in ß-isomer observed in
both treated and control samples was not considered to be due to
enzyme activity (Pfizer, 1986).
The stability of alitame in rat intestinal homogenate was
examined in order to test the capacity of the rat small intestine to
metabolize alitame. The small intestine from male rats was flushed
with saline and homogenized in phosphate buffer at pH 7.4. The
homogenate was incubated with 500 mg alitame/ml at 37°C and the
reaction stopped by the addition of 0.25 tool/litre NaOH. Cleavage of
the peptidic bond of alitame was measured by the formation of alanine
tetramethylthietane amide (CP-57,207) and was dependent on both the
substrate concentration and time of incubation. The percentage
conversion to the amide ranged from 13-21% after 10 minutes of
incubation. This demonstrated the ability of intestinal homogenate
to cleave the peptidic bond of alitame. The reaction was also
demonstrated to be enzyme-mediated in a second experiment in which
it was shown that the extent of cleavage was dependent on the
concentration of homogenate. Little evidence of further metabolic
steps, such as sulfur oxidation or acetylation, was noted (Pfizer,
1986).
2.1.3 Effects on liver enzymes
2.1.3.1 Rats
In a study to compare the rat liver enzyme inducing properties of
alitame with those of butylated hydroxytoluene (BHT), groups of 4 male
rats received oral doses of either BHT (100 or 500 mg/kg bw), alitame
(100 or 500 mg/kg bw) or phenobarbital (50 mg/kg bw) daily for 5 days.
At 15 h following the last dose, animals were killed and livers
removed. Liver homogenates were assayed for O-demethylase, cytochrome
c reductase, and P-450 activity. Significant enzyme induction was
noted with BHT, but alitame showed no effect at the same dose levels
(Pfizer, 1986).
In a further study to examine the liver enzyme inducing potential
of alitame and its metabolites, groups of Sprague-Dawley rats received
single oral doses of either alitame (up to 2000 mg/kg bw), alanine
tetramethylthietane amide (CP-57,207) or its sulfoxide (CP-58,290; 100
or 500 mg/kg bw), or phenobarbital (50 mg/kg bw/day) for 5 days. At
15 h after the last dose, animals were killed and livers removed.
Liver homogenates were prepared and assayed for O-demethylase. Slight
induction of demethylase activity was noted with both alitame and its
metabolites. At 500 mg/kg bw, enzyme activity was approximately twice
the low baseline activity observed in controls with either alitame or
its metabolites (Pfizer. 1986).
In a recent detailed study, the hepatic enzyme-inducing activity
of alitame was examined in rats following gavage administration (pilot
study) and dietary administration (main study). In the pilot study,
groups of 5 male and 5 female Sprague-Dawley rats were administered
alitame daily by gavage for 7 days at dose levels of 0, 500 or
1000 mg/kg bw/day. Animals were killed on day 8, the livers excised,
sections taken for histology and electron microscopy, and the
remainder of the liver homogenized. Positive control animals were
given ß-naphthoflavone (80 mg/kg bw, i.p., 3 days), phenobarbitone
(80 mg/kg bw, i.p., 3 days), or dexamethasone (100 mg/kg bw, i.p., 3
days). In the main study, groups of 20 male and 20 female Sprague-
Dawley rats were fed a diet containing alitame at concentrations of
0, 0.3% or 1% for 4 weeks. An additional 20 males and 20 females acted
as pair-fed controls and received the basal diet at the level of
intake of the 1% alitame group. After 28 days, 10 males and 10 females
from each group were sacrificed, sections of liver taken for histology
and electron microscopy and the remainder of the liver homogenized.
Kidney and brain weights were also recorded. The remaining 10 males
and 10 females were withdrawn from the alitame diet and kept for a
further 14 days before being sacrificed. In each of the above studies,
the liver homogenate was used to determine protein content, cytochrome
P-450 content, NADPH-cytochrome P-450-dependent reductase activity,
ethoxyresorufin O-deethylase (EROD) activity, pentoxyresorufin
O-depentylase (PROD) activity, erythromycin N-demethylase (ERD)
activity, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
In the pilot study, the positive controls exhibited significant
increases with respect to hepatic microsomal enzymes and all caused an
increase in relative liver weight. Brain and kidney weights were
unaltered. Alitame at 500 or 1000 mg/kg bw/day for 7 days had only
minor effects by comparison. At 1000 mg/kg bw/day, cytochrome P-450
levels were 140% of controls, and EROD, PROD and ERD activities
increased 3.6-fold, 6.3-fold, and 4.7-fold, respectively. Examination
of the microsomes by SDS-PAGE provided similar evidence of low levels
of protein induction corresponding to the above enzymes. Light
microscopic examination revealed hepatocellular hypertrophy at both
dose levels, and some evidence of periportal inflammation and necrosis
in a few sections. Electron microscopy revealed an increase in rough
and smooth endoplasmic reticulum at both dose levels.
In the main study, growth curves indicated a reduced body-weight
gain in alitame-treated animals compared to controls. This effect
was more apparent in females than in males and appeared to be
dose-related. While the effects may not have been statistically
significant, the curves indicated a clear biologically significant
decrease in body-weight gain in females at both the 0.3 and 1% alitame
dose levels. There was also an increase in relative liver weight in
females at both dose levels which was dose-related. Relative brain and
kidney weights were unaltered.
The parameters used to measure hepatic enzyme induction were also
increased in a dose-related manner compared to controls and pair-fed
animals. At the 0.3 and 1% dose levels, cytochrome P-450 levels
increased by 12 and 20% in females, and by 46 and 101% in males,
respectively. The activity of EROD was increased slightly in males and
females while there was a significant increase in the activity of PROD
in males and females at 0.3% and 1% alitame. The activity of ERD was
only slightly increased in males at 1% alitame. Light microscopic
examination of the liver revealed centrilobular hypertrophy at both
dose levels, but more marked at the higher dose. Electron microscopy
revealed a dose-related increase in both rough and smooth endoplasmic
reticulum. Following the 14-day withdrawal period, the liver weight
changes were reversed, and none of the hepatic parameters was
significantly different from controls.
The results indicated that alitame, at both the 0.3% and 1% dose
levels, was a weak inducer of hepatic enzymes, which was accompanied
in females by a decrease in body-weight gain and an increase in
relative liver weight. The pattern of enzyme changes was essentially
similar to that caused by phenobarbitone, although quantitatively
smaller (Caldwell et al., 1994).
In a supplement to the above study, the cytochrome P-450
isoenzyme from the liver microsomes of alitame-treated rats were
separated by SDS-polyacrylamide gel electrophoresis and further
characterized by Western blot immunodetection. Following separation by
electrophoresis, the proteins were transferred to nitrocellulose
membranes and the blots either stained with antibodies to specific
cytochrome P-450 isoenzyme or, to allow visualization of total
protein, by staining with Amido Black solution.
The microsomes from alitame-treated rats showed an increase in
the band attributed to CYP2B (catalytic activity) at 52 kD which is a
protein also shown to be induced by phenobarbitone. The band at 56 kD
attributed to CYP1A1, shown to be induced by ß-naphthoflavone, was
conspicuous by its absence. The data further confirmed that the
hepatic changes produced by alitame were similar to those produced by
phenobarbitone (Caldwell & Reed, 1994).
2.1.3.2 Humans
In a 14-day study, 8 male subjects received alitame in gelatine
capsules as 4 daily oral doses totalling 15 mg/kg bw/day. This dose
was calculated to be 15 times the projected human exposure level,
assuming total sucrose replacement. Induction of microsomal enzyme
levels was measured by the rate of elimination of aminopyrine.
Measurements were taken before treatment, after 2 weeks of treatment,
and at 18 days post-treatment. Both plasma and saliva were collected.
Blood samples were taken at pre-test, after 1 and 2 weeks of
treatment, and at 7 and 18 days post-treatment for the analysis of
clinical chemistry and haematological parameters. On the 10th day of
treatment, a 24-hour urine sample was collected for the analysis of
metabolites. There was no adverse effects parameters were normal
during the course of the study.
The analysis of the aminopyrine elimination results indicated
that the saliva was a good indicator of plasma levels. When the
elimination rate constants and half-lives were compared, there was no
significant difference in aminopyrine kinetics from the pre-dose,
post-dose and post-withdrawal samples. Urine samples indicated the
presence of the major metabolites, alanine tetramethylthietane amide
(CP-57,207) and it sulfoxide (CP-57,290). Alitame was present only in
trace amounts (Caldwell et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of alitame was examined in both mice and rats
and the results are summarized in Table 1.
Table 1. Summary of acute toxicity studies with alitame.
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral > 5000 Levinsky et al. (1983)
Rat M&F oral > 5000 Levinsky et al. (1983)
Groups of 10 male and 10 female mice (Crl:COBS CD-1 (ICR) BR) and
10 male and 10 female Sprague-Dawley (Crl:COBS CD (SD) BR) rats were
administered alitame (purity 97.43%) by gavage at a single dose level
of 5000 mg/kg bw. Animals were observed for clinical signs over a
14-day period after treatment, during which food consumption and body
weights were recorded. Animals were examined for gross pathological
changes at day 14 after treatment.
In mice, there were no deaths during the study and no clinical
signs apart from an increase in soft faecal material at 2.5 h
post-treatment. Faeces were normal by day 2 post-treatment. During the
observation period, there was normal food consumption and steady
body-weight gain.
In rats, there were no deaths during the study and no clinical
signs during the first hour post-treatment, but from then onwards all
animals displayed an increased level of activity for 1-2 days. All
rats were jittery and exhibited a tendency for exophthalmia, with
females noticeably more affected than males. Females exhibited
incoordination in their movements but were not ataxic. By 24 h, 7/10
males were essentially asymptomatic, and by day 2, all males and
females were asymptomatic. During the observation period, food
consumption was normal, and there was a steady body-weight gain. There
were no gross pathological changes on day 14 in 20/20 mice and in
19/20 rats, with one rat having an enlarged liver (Levinsky et al.,
1983).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 2-month study, groups of 10 male and 10 female Crl:COBS
(CD-1) (ICR) BR mice were fed a diet containing alitame (purity
98-101%) at concentrations of 0, 1, 2, or 5%, equivalent to 0, 1500,
3000 or 7500 mg/kg bw/day.
Animals were observed for clinical signs, and food consumption
and body weights were recorded throughout the study period. At the end
of the study period, liver and kidney weights were recorded and
tissues from the major body organs were taken for histopathology.
There were no deaths and no clinical signs of toxicity throughout
the study period. Body weights of the intermediate- and high-dose
animals decreased slightly on days 1 and 2, but recovered quickly.
There was no treatment-related effect on body weight for the remainder
of the study. There was no change in food consumption. Both absolute
and relative liver weights increased in a dose-related manner compared
to controls. The increase in absolute liver weight was 20, 40 and 67%
in males and 20, 35 and 66% in females in low-, intermediate- and
high-dose animals, respectively. Histopathological examination of the
liver revealed a dose-related increase in centrilobular hypertrophy.
Electron microscopy of liver tissue indicated a dose-related increase
in proliferation of smooth endoplasmic reticulum. No degenerative
hepatocellular changes were noted. Effects were noted at all dose
levels and a NOEL was not established in this study (Holmes et al.,
1983a).
2.2.2.2 Rats
In a range-finding study, groups of 5 male and 5 female Crl:COBS
CD-1 (SD) BR rats (5/sex/dose) were fed a diet containing alitame
(purity 98-101%) at concentrations of 0, 1, 2 or 5%, equivalent to
500, 1000, or 2500 mg/kg bw/day for 15 days. Animals were examined for
clinical signs of toxicity, and food and water consumption was
monitored throughout the study period. At the end of the study period,
blood samples were taken for haematological and clinical chemistry
examination; tissues from the major body organs were taken for
histopathology; tissues from liver and kidney were taken for electron
microscopy; and tissue from liver for histochemistry. There were no
deaths throughout the study, and no clinical signs of toxicity. A
slight body-weight loss was noted at the 2% and 5% dose levels on day
3 but body-weight gain was normal thereafter. There was a dose-related
decrease in food consumption in all treated groups on days 1-2
(especially females at the 2% dose level and in both sexes at the 5%
dose level) possibly due to unpalatability of the diet. Water
consumption was similarly decreased. There was a slight, but not
significant, increase in plasma cholesterol and protein levels in all
treated groups. Haematological parameters were normal. There was a
dose-related increase in absolute and relative liver weights in
males and females but no microscopic abnormalities were noted.
Histochemistry indicated an increase in glucose-6-phosphate
dehydrogenase and NADPH2 diaphorase in liver tissue slices. Electron
microscopy indicated a dose-related increase in smooth endoplasmic
reticulum in liver tissue and, in some cases, an increase in lysosomes
and lipid vacuoles. Effects were observed at all dose levels and a
NOEL was not established in this study (Chvedoff et al., 1981a).
In a 5-week study, groups of 10 male and 10 female Long-Evans
rats were fed a diet containing alitame (purity 98.2%) at dose levels
of 0, 10, 50 or 100 mg/kg bw/day. Animals were observed daily and body
weights and food consumption recorded. Blood samples were taken from
the orbital sinus prior to treatment and at the end of the study
period, and clinical chemistry and haematological parameters examined.
After 5 weeks, the induction of liver microsomal enzymes was measured
in vitro and animals were examined for gross pathology. Tissues from
the major organs were prepared for histopathology.
There was no treatment-related effect on body-weight gain or food
consumption. The clinical chemistry, haematological data and
histopathology were unremarkable. It was not possible to determine
whether there was proliferation of smooth endoplasmic reticulum
because of the presence of excess glycogen clue to lack of fasting
before sacrifice. Induction of 0-demethylase was determined by the
release of p-chlorophenol. A 30% increase in enzyme induction
occurred at the high-dose level which was not statistically
significant. Nevertheless, on the basis of the biological significance
of this increase, the NOEL in this study was 50 mg/kg bw/day
(Holmes et al., 1983b).
In a 3-month study, groups of CrI:COBS-CD(SD)BR strain rats
(15/sex/dose) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0.5, 1.0 or 2.0%, equal to 380, 830 or 1500 mg/kg
bw/day. Animals were observed throughout the study period and body
weights, food and water consumption recorded. Blood samples were taken
for examination of clinical chemistry and haematology parameters at
the end of the study. Urinary parameters were also examined. At the
end of the study, gross pathological examinations were performed and
tissues from the major organs were taken for histopathology. A group
of 5 males and 5 females was observed for a further 1 month following
the treatment period.
There were no deaths throughout the study and no clinical signs
of toxicity. There was a dose-related decrease in body-weight gain in
both males and females in all treated groups. The decreases at 3
months in males/females were 4.5%/1.7%, 4.5%/3.3% and 5.3%/8.5% at
low, intermediate and high dose. Food consumption was slightly lower
in high-dose males, but not during the withdrawal period. Water
consumption was normal. The clinical chemistry analysis revealed a
significant increase in plasma cholesterol at all dose levels in males
and females. The increase was 23% at low dose and 42% at high dose.
Levels returned to normal during the 1-month withdrawal period. There
was also a dose-related decrease in plasma triglyceride levels in
males, which returned to normal levels during the withdrawal period.
The levels of cytochrome P-450 and b5 were increased in a
dose-related manner at the end of the treatment period. The activity
of microsomal N-demethylase was also increased in a close-related
manner. The changes were reversed during the withdrawal period except
for the cytochrome P-450 levels in males. Haematological parameters
were within the normal range.
Relative and absolute liver weights were increased in males at
the high-dose level, while relative liver weight was increased at all
doses in females. The liver weights returned to normal during the
withdrawal period.
Histopathology of the liver revealed centrilobular hypertrophy in
all high-dose females and in 5/10 high-dose males. Histochemical
analysis of the livers indicated increased activity of G-6-P
dehydrogenase, gamma-glutamyl transpeptidase and NADPH-diaphorase in
the high-dose animals. NADPH-diaphorase activity was also increased in
intermediate-dose females. All changes were reversible during the
withdrawal period. Electron microscopy revealed an increased
proliferation of smooth endoplasmic reticulum at all dose levels.
After the withdrawal period, the livers of female rats were normal
while those of the males still showed mild proliferation of smooth
endoplasmic reticulum at the high-dose level. The histopathological
and enzymatic changes were indicative of a reversible adaptive
response. There was no evidence of focal hyperplasia or hepatocellular
toxicity. Effects were noted at all dose levels and a NOEL was not
established in this study (Monro et al., 1982a).
In a one-year study, groups of 40 male and 40 female Long-Evans
rats were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.1, 0.3, or 1.0% (equivalent to 50, 150 or
500 mg/kg bw/day). Two other groups were fed a diet containing alitame
adjusted to achieve a nominal intake of 10 or 30 mg/kg bw/day. At 6
months, 20 male and 20 female animals at each of the dose levels were
removed from treatment. Of these, half were sacrificed while the other
half were returned to normal untreated diet. Animals were observed
throughout the study period and body weights, food and water
consumption recorded. Ophthalmoscopic examinations were performed in
all animals before necropsy. Blood samples were taken at 6, 9 and 12
months for clinical chemistry and haematology. Urinalysis parameters
were also examined. At the end of the study period, all animals were
examined for gross pathology, and tissues from major organs were taken
for histopathology.
There were no deaths throughout the study and no clinical signs
of toxicity. Ophthalmoscopic examination at 6 months and 1 year was
unremarkable. There were no treatment-related effects on body-weight
gain or on clinical pathology parameters. Cytochrome P-450 levels were
measured at 6 months and were significantly increased at the high-dose
level only (21% in males and 33% in females). The only organ weight
change noted was an increase in relative liver weight at the high-dose
level at 6 months, with a 12.1% increase in males and 16.0% increase
in females. At 12 months, liver weight increases at the high-dose
level were 8.5% and 11.7% in males and females respectively, compared
to controls. In animals withdrawn from treatment at 6 months, liver
weights had returned to normal levels at 12 months. The only
microscopic finding was a retention of glycogen in both control and
treated groups. This change was not considered to be treatment-
related. The NOEL in this study was 0.3% in the diet, equal to
145 mg/kg bw/day in males and 185 mg/kg bw/day in females
(Holmes et al., 1985a).
A study was conducted in Sprague-Dawley rats which included
dietary administration during mating, in utero exposure, exposure
during lactation, and dietary exposure for one year post-weaning.
Groups of 20 male and 20 female Sprague-Dawley rats were selected from
the F1 offspring of rats treated 4 weeks prior to mating and during
gestation and lactation (Levinsky et al., 1992). The groups were fed
a diet containing alitame (purity 99.3%) at concentrations of 0 (two
control groups), 0.1, 0.3 or 1% for one year. The animals were
observed daily for clinical signs and body weights and food
consumption were recorded weekly. Other measurements and observations
included ophthalmological examinations (pre-test and 6 and 12 months),
serum chemistry, haematology and urinalysis measurements at 6 and 12
months. Tissues were collected from all animals which died or became
moribund during the study. At the end of the study, the animals were
sacrificed and necropsied and tissues prepared for light microscopy.
Organ weights for liver, kidney, adrenal glands, testes and ovaries
were recorded.
Twenty rats died or were sacrificed during the study. The deaths
were distributed evenly between the groups although deaths in males
predominated over those in females by four to one. Of the 20 early
necropsies, 4 were due to neoplasms (1 control, 2 low-dose and 1
intermediate-dose rats). The most common non-neoplastic cause of death
(7/16) was described as 'unexplained death/heavy rats' and was not
accompanied by any remarkable clinical signs.
Body-weight measurements began at birth for this study, and at
weaning (day 21) the mean body weights for the intermediate- and
high-dose animals compared to the control groups were -9% and -21%,
respectively. Following selection of animals in groups for the 1-year
study, the body weights expressed as percent of control for the
intermediate-dose level were -9.5% (M) and -8.8% (F), and for the
high-dose level -16.3% (M) and -13.5% (F). Over the course of the
study, the initial body weight differentials for males at the
intermediate- and high-dose levels diminished by about 50%. For the
first 80 days, all groups of males appeared to gain weight at similar
rates apart from the control group 1. For the remaining period of the
study, the weight gains were similar for all groups. Over the 1-year
period, the body-weight difference for the intermediate-dose males
disappeared and the difference for the high-dose group was 6.6% and
11.1% compared with control groups 1 and 2, respectively.
For females, over the course of the study, the -8.8% difference
at the intermediate-dose level disappeared and the low-dose level
gained weight relative to the control group 1. The females in the
control group 1, like the males, showed a slower growth rate. The
high-dose females, unlike the males, did not recover their initial
body weight difference (-13.5%) and after 200 days, the body weight
difference began to increase, reaching -20% relative to control
group 1.
Ophthalmoscopic examination at 6 and 12 months was unremarkable.
There were no treatment-related changes in clinical chemistry,
haematology or urinary parameters apart from a 40-60% decrease in
serum triglyceride levels in the high-dose females, which appeared to
be related to the decreased weight gain in the high-dose females. The
high-dose males showed decreased serum triglyceride values at 6
months, but not at 12 months.
There were no gross pathological findings in any group. The organ
weight changes observed were a decrease in absolute liver weight in
the high-dose females, and an increase in relative liver, kidney and
ovary weight as a result of the decrease in mean body weights in the
high-dose females.
Histopathology revealed hepatocellular hypertrophy in the
high-dose males (13/20) and females (4/20). These changes were not
accompanied by an increase in liver weight. Other liver changes
observed could not be related to treatment. The NOEL in this study,
based on body and organ weight changes, was 0.3% in the diet, equal to
173 mg/kg bw/day in males, and 205 mg/kg bw/day in females (Fisher
et al., 1992).
2.2.2.3 Dogs
In a two-week range-finding study in dogs, groups of 2 male adult
beagle dogs were fed alitame (purity 98-101%) in the diet at
concentrations of 1, 2 or 5%, equivalent to 250, 500 or 1250 mg/kg
bw/day. There were no control animals. Animals were observed
throughout the study for clinical signs of toxicity and histopatho-
logical examinations were performed on lungs, kidney, liver and heart
after 14 days. A section of liver was examined by electron microscopy.
There were no deaths during the study and no clinical signs of
toxicity. There was no treatment-related effect on body weight.
Systolic blood pressure and heart rate were normal at all dose levels.
Clinical chemistry parameters were normal apart from a slight increase
in alkaline phosphatase activity at all dose levels which increased in
the second week. Urinalysis parameters were normal. Haematological
parameters indicated a slight decrease in haemoglobin concentration,
RBC count and packed cell volume in treated animals, but the changes
were within normal limits and not definitely related to treatment.
Relative liver weights were within normal limits. Microscopic
examination revealed an increase in cytoplasmic vacuolation in the
liver of 5/6 animals. Electron microscopic examination revealed a
dose-related increase in proliferation of smooth endoplasmic
reticulum. Lipid vacuoles and lysosomes were present in the liver of
all animals but, in the absence of control animals, it could not be
determined whether the observed microsomal changes were treatment-
related (Chvedoff et al., 1981b).
In a 3-month study, groups of beagle dogs (4/sex in the low- and
intermediate-dose groups, and 6/sex in the control and high-dose
group) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.5, 1, or 2%, equal to 0, 120, 220 or 490 mg/kg
bw/day. In the control and high-dose groups, 2 animals/sex were
transferred to normal diet for an additional month after the study
period. Animals were examined throughout the study period for clinical
signs of toxicity and body weight was recorded weekly. At various
times, clinical chemistry, urinary and haematology parameters were
measured. Tests of aminopyrine clearance were measured on days 1 and
63. Ophthalmological examinations were performed on high-dose animals.
Tissues were examined histopathologically at the end of 3 months.
Liver histochemistry, electron microscopy and content of microsomal
cytochrome P-450 and b5 were also performed.
There were no deaths throughout the study and no clinical signs
of toxicity. Food intake was normal although there was a slightly
decreased weight gain in high-dose animals, particularly males.
Cardiovascular parameters were normal. Ophthalmological examination of
high-dose animals was unremarkable. Haematological parameters were
within the normal range. Clinical chemistry revealed a dose-related
increase in plasma alkaline phosphatase when measured at 1, 2 and 3
months. The increase at the high-dose level was 400% at 3 months.
After the recovery period, levels in the high-dose group were reduced
but still higher than control levels.
At the end of the 3-month treatment, the levels of cytochrome
P-450 and b5 were increased in a dose-related manner. After the
1-month withdrawal period, cytochrome P-450 and b5 in the high-dose
group were reduced to near control levels. Induction of the liver
microsomal enzyme system was also demonstrated in the dogs of the
reversibility groups (control and high dose) following administration
of a single i.v. dose of aminopyrine (15 mg/kg bw) on days 1 and 63 of
alitame treatment, with blood levels measured at 0, 1, 1.5, 2, 3 and
5 h. The plasma levels reflected the induction of a liver microsomal
demethylase. On day 1, there was little difference between treated and
control animals. On day 63, there was a marked difference between
control and treated animals.
Relative and absolute liver weights were increased in all
treatment groups. Absolute liver weight was increased by 36% and 11%
at high and low dose, respectively. Histopathology of the liver and
other organs was unremarkable. Histochemical examination revealed an
increase in G-6-P dehydrogenase activity in all treated groups except
the low-dose females. NADPH-diaphorase activity was increased in
high-dose animals. All levels returned to normal during the recovery
period, except for an increased G-6-P phosphatase activity in
high-dose males. Electron microscopy revealed an increase in
proliferation of smooth endoplasmic reticulum in all groups. This
increase was reversed in females but persisted in males during the
recovery period. The liver changes appeared to be indicative of an
adaptive response due to induction of microsome oxidative metabolism.
Effects were observed at all dose levels and a NOEL was not
established in this study (Monro et al. 1982b).
In an 18-month study, groups of 4 male and 4 female beagle dogs
were fed a diet containing alitame (purity 98-101%) at dose levels of
0, 10, 30, 100 or 500 mg/kg bw/day. Animals were examined throughout
the study period for clinical signs of toxicity, and body weights were
recorded at regular intervals. Ophthalmoscopy was performed on animals
at 6-month intervals. Electrocardiographic tracings were performed at
regular intervals. Clinical chemistry, urinary and haematological
parameters were measured at regular intervals. Aminopyrine clearance
was measured in control and intermediate dose animals at pre-test and
during weeks 3, 5, 7, 9, 40 and 74, and in the high-dose group at 39
and 73 weeks. Gross pathological examination was performed at 18
months together with histopathological examination of a wide range of
tissues. There were no treatment-related effects on survival and no
clinical signs of toxicity. Ophthalmoscopic examinations and
cardiovascular parameters were normal. Body weights were comparable
between groups although body-weight gain in high-dose females was
slightly less than control animals between weeks 40 and 70. Food
consumption was normal in all groups. All of the clinical pathology
parameters were normal except for an increase in serum alkaline
phosphatase activity in high-dose males and females. The alkaline
phosphatase rise was not uniform at high dose, with some dogs showing
little or no change.
Analysis of the urinary metabolites over a 2-day period at week
10 showed that the excretion rate was proportional to the dose
administered, indicating a dose-related systemic exposure. Measurement
of aminopyrine clearance, as an indicator of the induction of the
liver microsomal enzyme system, revealed a slight decrease in t1/2
in males and females at 100 mg/kg bw/day at the 2-week period. The
significance of this result is unclear, and may be due to the high
t1/2 in the animals pre-dosing. In males, there appeared to be a
gradual increase in t1/2 over the study period but again the result
is difficult to interpret. Overall, the results at 100 mg/kg bw/day do
not appear to indicate any enzyme induction. At 500 mg/kg bw/day,
after 39 or 73 weeks, significant microsomal enzyme induction was
apparent.
The only gross pathological change observed was a moderate
increase in absolute liver weight of 31% and 20% in high-dose males
and females, respectively. This increase was statistically significant
in males only. Histopathology was unremarkable in the treated animals.
A slight increase in smooth endoplasmic reticulum was found in the
livers of high-dose animals. The NOEL in this study was 100 mg/kg
bw/day (Holmes et al., 1985b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 2-year carcinogenicity study, groups of 50 male and 50
female Crl: CD-1 (ICR) BR mice were fed a diet containing alitame
(purity, 98-101%) at concentrations of 0 (2 groups), 0.1. 0.3 or 0.7%.
Animals were observed throughout the study period. Body weight and
food consumption were recorded weekly for 13 weeks and then at
N-weekly intervals for the remainder of the study. At the end of the
study period, animals were sacrificed and subjected to gross
pathological examination and organs weights recorded. Tissues from all
of the major organs were prepared for histopathological examination.
Tumour incidence was recorded.
There was no treatment-related effect on survival. The survival
rate for controls, low-, intermediate-, and high-dose groups in
males/females was 52/24%, 22/26%, 22/38%, 32/36% and 38/26%,
respectively. There were no clinical signs of toxicity in the treated
groups. Body-weight gain and food consumption were similar for all
groups. Gross pathological examination revealed a significant increase
in absolute and relative liver weight at high dose in males only.
Absolute liver weight increased by 59% and 46% compared to the two
control groups, while relative liver weight increased by 58% and 38%
compared to the two control groups. Slight to mild centrilobular
hepatocellular hypertrophy was found in 12 male animals in the
high-dose group. At the intermediate-dose level, absolute and relative
liver weights of males were also increased. Absolute liver weight was
increased by 41% and 30% compared to the two control groups, while
relative liver weight was increased by 39% and 21% compared to the two
control groups. While the increases were not statistically
significant, nevertheless the Committee considered them to be of
biological significance at this dose level. No hepatocellular
hypertrophy was apparent at the intermediate- and low- dose levels.
There were no other histopathological changes, or any
treatment-related increase in tumour incidence. Under the conditions
of this assay, there was no evidence of carcinogenicity in mice
induced by alitame in the diet. The NOEL, based on the biologically
significant liver weight changes in males, was 0.1% in the diet, equal
to 125 mg/kg bw/day for males and 155 mg/kg bw/day for females (Holmes
et al., 1985c).
2.2.3.2 Rats
A carcinogenicity study was conducted with dietary administration
and in utero exposure to alitame in Long-Evans rats. Groups of 50
male and 50 female Long-Evans rats (strain Blu:(LE)BR) were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for 2 years.
Animals used in this study were taken from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Holmes et al., 1986b). Animals were observed throughout the study
period for clinical signs of toxicity. Body weights and food
consumption were recorded weekly. Ophthalmoscopic examinations were
carried out at 12, 18 and 24 months. Clinical chemistry and
haematological parameters were measured in 10 animals/sex/dose at 6,
12, 18 and 24 months. At the end of the study period, animals were
sacrificed and subjected to gross pathological examination. Organ
weights were recorded. Tissues from all the major organs were prepared
for histopathological examination.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examinations
revealed no treatment-related effects. The survival rate at 104 weeks
for the control, low, intermediate, and high-dose groups was for
males/females 40/46%, 34/40%, 30/42% and 28/34%, respectively. There
was a slight decrease in survival in treated animals compared to
controls but this was not necessarily treatment-related.
In the second year of the study, there was a dose-related
difference in body weights for males between control animals and those
in the high- and intermediate-dose groups. In females, the body weight
of high-dose animals was lower than controls at the start of the study
and continued to be significantly lower throughout the study.
Intermediate-dose females also had a slightly lower body weight than
controls which became more pronounced in the 2nd year of the study.
There was no treatment-related effect on food consumption. These
differences in body weights were significant only at high dose.
There were no treatment-related changes in clinical chemistry or
haematological parameters. Gross pathological findings were generally
unremarkable. There was a slight non-significant increase in relative
liver weight at the high and intermediate dose levels in both males
and females. Microscopic examination of the tissues did not reveal any
increase in the incidence of neoplastic lesions. In the high- dose
group, 5 males and one female showed mild centrilobular hepatocellular
hypertrophy. The incidence of proliferative liver lesions in females
is shown in Table 2. The first appearance of hyperplastic nodules in
the liver of females was at week 96. Survival at this time in control,
low-, intermediate- and high-dose females was 64%, 56%, 50% and 50%,
respectively. Alitame, under the conditions of this assay, showed no
evidence of carcinogenicity in rats. The NOEL, based on body-weight
changes in both males and females, was 0.3% in the diet, equal to
130 mg/kg bw/day for males and 160 mg/kg bw/day for females
(Holmes et al., 1986a).
Table 2. Incidence of proliferative liver lesions in female rats
(Holmes et al., 1986a).
Control 0.1% 0.3% 1%
No. of livers examined 50 46 48 49
Focal nodular hyperplasia 1 0 3 9
Eosinophilic foci 4 0 3 11
A re-examination of the pathology slides of the 2-year
carcinogenicity study in Long-Evans rats (Holmes et al., 1986a) was
conducted on two subsequent occasions. At the first re-examination,
all available liver slides from all groups of female rats were
examined. The reported incidence of proliferative lesions is shown in
Table 3. There was no evidence of hepatocellular carcinomas in females
(Farrell, 1987).
Table 3. Incidence of proliferative liver lesions in female rats
(first re-examination; Farrell, 1987)
Control 0.1% 0.3% 1%
No. of livers examined 50 49 50 50
Hepatocellular adenoma 0 0 1 1
Eosinophilic foci 3 3 6 12
Hyperplasia 0 4 3 6
(focal and multifocal)
In the second re-examination of the pathology slides, slides
showing proliferative changes in female rats in either the original
study or in the first re-examination were examined using a revised set
of diagnostic criteria for hepatic proliferative lesions. The
incidence of these lesions is shown in Table 4. There was no evidence
of hepatocellular carcinomas in females (Hardisty et al., 1994).
Table 4. Incidence of proliferative liver lesions in female rats
(second re-examination; Hardisty et al., 1994).
Control 0.1% 0.3% 1%
No. of livers examined1 8 7 16 25
Hepatocellular adenoma 0 0 2 12
Hepatocellular hyperplasia 0 0 0 3
Eosinophilic cell foci 2 3 11 18
1 In this re-examination, only slides identified in the original
report and in the first re-examination as showing proliferative
lesions were examined.
Because of the low survival rate at 104 weeks in the Long Evans
rats (Holmes et al., 1986a), a second carcinogenicity study was
conducted with dietary administration and in utero exposure to
alitame in Sprague-Dawley rats. Groups of 70 male and 70 female
Sprague-Dawley rats were selected from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Levinsky et al., 1992). The groups were fed a diet containing
alitame (purity 99.3%) at concentrations of 0 (two control groups),
0.1, 0.3 or 1% for two years.
The animals were observed daily for clinical signs, and body
weights and food consumption were recorded weekly for the first
6 months and then every 4 weeks thereafter. Ophthalmological
examinations were performed pre-test and at 12 and 21 months; serum
chemistry, haematology and urinalysis measurements were taken at 6,
12, 18, 21 and 24 months. Tissues were collected from all animals
which died or became moribund during the study. At the end of the
study the animals were sacrificed and necropsied and tissues prepared
for light microscopy. Organ weights (liver, kidney, adrenal glands,
testes and ovaries) were recorded.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examination
revealed no treatment-related effects. Survival rates were high for
the first 12 months (>93%) but by 18 months average survival was 65%
for males and 75% for females. Groups reached 50% survival between 18
and 19 months for males and between 20 and 21 months for females.
Survival rates at 24 months in the 2 controls, low-, intermediate- and
high-dose groups were for males/females 9/37%, 4/23%, 7/31%, 9/24% and
26/17%, respectively.
Body-weight measurement began at birth for this study, and at
weaning (day 21). The mean body weights for the intermediate- and
high-dose animals were -9% and -21%, respectively, compared to the
control groups. Following selection of animals into groups for the
2-year study, the body weights expressed as percent of controls at the
low dose were -4% (M) and -2% (F), at the intermediate dose -7% (M)
and -10% (F), and at the high dose -18% (M) and -16% (F). For males,
over tile course of the study, the initial body weight differential
decreased as the treatment group showed increased weight gain compared
to the controls. Body weights peaked at approximately 15 months
followed by a gradual decline in all groups, with the high-dose group
showing the greatest decline. In females, over the course of the first
few months of the study, the initial body weight differentials
decreased particularly in the low-and intermediate-dose groups, which
showed increased weight gain compared to controls. After 6 months,
weight gain in the low- and intermediate-dose groups was similar to
controls. In the high-dose group, however, there was a decrease in
weight gain compared to the controls, and by the end of the study,
body weight differential had increased to -32%. Food consumption may
have been slightly lower in high-dose males, but was similar in all
groups of females.
There were no treatment-related changes in haematology or
urinalysis parameters. Changes observed in clinical chemistry data
included a decrease in serum triglyceride values (50-60%) in females
at the intermediate-dose in the first 18 months, and in high-dose
females throughout the study. Cholesterol was also increased in female
rats at 6 months at the intermediate-dose level (28%), and at 6 and 12
months at the high-dose level (67%).
Significant organ weight changes were seen in both liver and
kidney. In males, there was a relative increase in both kidney and
liver weight at high dose. In females, there was an absolute decrease
in kidney and liver weights at both intermediate and high doses, and
a relative increase in kidney weight at high dose. These changes
accompanied the decrease in body weight in high-dose females.
There was also an overall trend for decreased body weight in
intermediate-dose females which was not statistically significant.
The relative testes weight was increased in high-dose males.
Neoplastic changes were observed in the following organs: kidney,
liver, pancreas, lung, heart, thymus, mesenteric node, adrenal cortex,
thymus, parathyroid, uterus, cervix, brain, spinal cord, Zymbal's
gland, skin, bone, skeletal muscle, abdominal cavity, and soft
tissues. There was no evidence that the incidence of these neoplasms
was treatment-related. In the liver, hepatocellular carcinomas were
reported in one control male and one high-dose male. There was no
reported incidence of hepatocellular adenomas. There were also no
treatment-related non-neoplastic changes. Alitame, under the
conditions of this assay, showed no evidence of carcinogenicity in
Sprague-Dawley rats. The NOEL, based on body-weight changes, was 0.3%
in the diet, equal to 230 mg/kg bw/day for males, and 250 mg/kg bw/day
for females (Fisher et al., 1993).
2.2.4 Reproductive toxicity studies
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (75/sex) were administered a diet containing alitame
(purity 98-101%) at concentrations of 0, 0.1, 0.3 or 1% for either 2
weeks (females) or 12 weeks (males) prior to mating. Alitame-
containing diet was continued throughout pregnancy and lactation. Of
the F1 pups, 50 pups/sex/dose level were selected for a 24-month
carcinogenicity study, a further 25/sex/dose level were raised to
maturity and mated to produce the F2a litters. Approximately 3 weeks
after weaning of the F2a litters, the F1 animals were again mated
to produce the F2b litters. Both F2a and F2b pups were exposed to
alitame via the milk and in the food.
In the F0 parental group, the body weight of males was similar
for all groups during the 12 weeks of treatment, while body weight and
food consumption of females were slightly depressed during the 2-week
treatment period at the high-dose level. There was no treatment-
related effect on fertility or gestation indices, and no abortions
were noted. There was no difference in the number of pups born alive
between treated and control groups in the F1 litters. There was no
treatment-related effect on day 4 pup survival rates or lactation
indices. F1 pup weights of treated animals were similar to those of
control animals on days I and 4 of lactation, but high-dose pups had
significantly lower body weights on days 7, 14 and 21. However, these
decreased body weights were not significantly different from the
historical control data.
In the F1 parental group, body weights were similar to control
except at the high-dose level where there was a slight decrease in
body-weight gain. There were no treatment-related changes in pregnancy
rates or gestation length for either F2a or F2b litters. There was
no difference in the number of pups born alive between treated and
control groups in the F2a or F2b litters. Survival indices for F2a
and F2b were normal for all groups on days 1 and 4 of lactation.
F2a pup weight for all treated groups was similar on days 1,
4 and 7 of lactation but a significant decrease was noted in the
high-dose animals on day 14 and in all treated groups on day 21
compared to controls. The body weights were still within the
historical control data range. The F2b pups weight at the high-dose
level was significantly lower than controls throughout lactation,
while at the intermediate-dose level body weights were lower at days
14 and 21, and at the low-dose level, body weights were significantly
lower than controls on days 4 to 21. No historical control data were
available for F2b litters.
Gross pathological examination of the F0 males and females, the
F1 pups, and the F2a and F2b pups revealed no treatment-related
abnormalities. Postnatal development in F2b pups was assessed with
regard to locomotor activity, auditory function and ophthalmoscopic
examination. At the F2b weaning, there was a significant decrease in
both horizontal and vertical locomotor activity in F2a male and
female pups at the high-dose level, and a significant increase in
horizontal (but not vertical) locomotor activity in the F1 dams at
the high-dose level group. Pups tested for auditory function and for
ophthalmoscopic changes revealed no treatment-related changes
(Holmes et al., 1986b).
In a second 2-generation study, groups of 30 male and 30 female
Long-Evans rats (strain Blu:(LE)BR) were administered alitame (purity
98-101%) at concentrations of 0, 0.1, 0.3, or 1% for either 2 weeks
(females) or 12 weeks (males) prior to mating. Alitame-containing diet
was continued through pregnancy and lactation. Of the F1 pups,
25/sex/dose were raised to maturity and mated to produce the F2a
litters. Approximately 3 weeks after weaning of the F2a litters, the
F1 animals were again mated to produce the F2b litters. Both the
F2a and F2b pups were exposed to alitame in utero, during lactation,
and in the food following weaning.
At the high-dose level, 2 additional F1 groups of 25/sex were
exposed to alitame differently. These groups were introduced to
address the lactation effect observed in the first study. In one of
these groups, the F1 females were removed from treated diet at the
birth of F2a pups. In this group, the F2a pups received no alitame
during lactation. In the other group, the F1 females were removed
from the treated diet 3 weeks after weaning until the birth of the
F2a pups. In this group, the F2b pups received alitame only during
lactation. Animals were then placed on treated diet again. Post-natal
development of F2b pups was tested with an ophthalmoscopic
examination, an auditory function test, and a locomotor activity test.
Locomotor activity tests were also conducted on F0 and F1 parents,
and on F2a and F2b pups.
In the F0 parental animals, the body weights of males were
similar for all groups during the 12 weeks of treatment, while there
were decreases in body-weight gain and food consumption in females at
the high-dose level during the 2-week treatment period. Fertility
rates for all groups were normal. The number of live F1 pups was
similar for all groups at day 1, but was slightly reduced at the
high-dose level on day 4. This was explained by several large litters
at the high-dose level which declined in the first few days. F1 pup
body weight was similar for all groups on day 1 of lactation but was
significantly less at the high-dose level from day 4 onward.
Body-weight gain at high dose was similar to that of control groups
from days 4 to 21. The mean number of pups alive at day 21 compared to
the number of pups alive at day 4 (the lactation index) was similar
for all groups. Gross pathological examination of the F0 males and
females and the F1 pups revealed no treatment-related changes.
In the F1 parental group, the body weights of all female groups
were similar. Pregnancy rates and gestation times were normal. The
F1 males in the treated groups had slightly lower body weights than
controls although body-weight gain was similar for all groups. The
numbers of F2a litter live pups were similar for control, low and
intermediate groups. For the high-dose group, the mean number of pups
born alive (11.54) was slightly lower than controls (12.96). However,
the mean number of pups born alive in the other two additional
high-dose groups were similar to controls (12.75 and 12.13). The
4-day survival indices for the F2a pups in the control, low-,
intermediate-and high-dose groups were 0.98, 0.98, 0.93 and 0.89,
respectively. In the two additional high-dose groups, the 4-day
survival indices were 0.97 and 0.95. Lactation indices were for the
control, low-, intermediate- and high-dose groups 0.98, 1.0, 0.97 and
0.96, respectively, and for the two additional high-dose groups 0.99
and 0.88, respectively.
The mean body weight of high-dose F2a pups were slightly but
significantly lower than controls at birth and throughout lactation.
The additional F2a pups which were exposed only during lactation had
slightly lower body weight after the first week of lactation. The
additional group of F2a pups which had no alitame exposure during
lactation had body weights similar to controls at all times after day
1 of lactation. The results indicated a slight lactation effect of
alitame at the high-dose level, although the high-dose body weights
were still within the historical control ranges. The F2a pups at the
intermediate-dose level also had slightly but significantly lower body
weights at days 14 and 21 of lactation. External examination and gross
internal examination of the F2a pups revealed no treatment-related
changes.
The fertility rates and gestation times associated with the
production of the F2b litters were normal for all groups. The mean
number of pups born alive was comparable at the control, low- and
intermediate-dose levels, being 12.1, 11.7 and 13.0, respectively. At
the high-dose level, the number of live pups was slightly lower (9.8).
Survival during lactation was similar for all groups. The mean body
weight for the F2b high-dose level pups was significantly lower than
controls on day 14 of lactation. External examination and gross
internal examination revealed no treatment-related changes.
Ophthalmoscopic examination and auditory function tests were
normal in all groups. Locomotor activity for both parental groups and
pups showed considerable variation and the changes could not be
correlated with treatment.
This second study provided supporting evidence that alitame at
high-dose levels caused some suppression of body-weight gain during
lactation. This was further investigated in a study to examine the
effect of alitame in the cage bedding material on the pups body weight
(see section 2.2.5). The NOEL, based on body-weight changes in the
F2 pups, was 0.1% in the diet, equivalent to 100 mg/kg bw/day
(Holmes et al., 1986c).
In a 1-generation study in Sprague-Dawley rats, groups of 105
male and 105 female rats (strain Crl:CDBR VAF/Plus) were administered
alitame (purity 99.3%) at concentrations of 0, 0.1, 0.3 or 1% for 4
weeks prior to mating, through the cohabitation period, through the
gestation period, and up to day 21 of lactation. The aim of this study
was to produce an F1 generation of rats, continuously exposed to
alitame in utero, via the milk during lactation, and through the
feed for use in 1-year and 2-year toxicity studies. The animals were
observed daily for clinical signs of toxicity and body weights and
food consumption were recorded. During cohabitation, only the body
weights of the males were recorded. During gestation, body weights of
females were recorded on days 0, 4, 7, 14 and 20. During lactation,
dams and viable young were weighed on days 1, 4, 7, 14 and 21
post partum. On day 4, all litters were culled to 8 pups, 4
animals/sex. At day 21 (weaning), litters from the control groups, 0.1
and 0.3% treatment groups were culled to 2 animals/sex, and the
respective dams sacrificed. High-dose litters were not culled due to
their lower body weights, and were allowed to remain with the
high-dose dams until it was deemed prudent to separate them. When the
F1 animals were 3 to 6 weeks of age, 70 pups/sex/dose were selected
for a 2-year oncogenicity study (Fisher et al., 1993), and 20
pups/sex/dose level were selected for a 1-year toxicity study
(Fisher et al., 1992). All F0 males were terminated after the
cohabitation period. F0 females which did not deliver by day 24 of
gestation were terminated and their uteri and ovaries were examined.
F0 pups not used for either study were terminated.
Reductions in body weights and food consumption were noted in the
high-dose F0 animals, particularly during the first 2 weeks of the
study. This may be associated with palatability. There was no
treatment-related effect on mating, gestation length, or body weight
during gestation and lactation. The number of implantation sites was
slightly lower in the high-dose group and this was reflected in a
slight reduction in the total number of pups born alive per litter.
There was no difference between control and treated groups with
respect to post-implantation losses. There was no treatment-related
effect on pup survival during the lactation period. The mean
body-weight gains at the intermediate- and high-dose levels were
depressed compared to controls, and at weaning the body weights of the
intermediate- and high-dose groups were 9% and 21% lower than
controls, respectively. There were no internal or external
abnormalities in the pups not selected for the long-term studies
(Levinsky et al., 1992).
2.2.5 Special studies on neonatal survival
A study was conducted in Long-Evans pups and lactating dams in
order to further investigate the apparent decrease in pup body weight
during lactation at the high-dose level in the reproductive toxicity
studies. In this study, dams and pups were exposed to alitame which
was added to the bedding material rather than to the feed. It was
argued that dams and pups are normally exposed to treated feed in this
way because of tipping over of the feed by the dam, or the tendency to
scratch the feed into the bedding material. In this study, 2 groups of
8 litters were formed and all dams received normal diet. On lactation
days 1 and 5 and every 2-4 days thereafter, one of the groups had
125 g of feed containing 1% alitame sprinkled on the bedding. The
control group had feed without alitame sprinkled on to the bedding.
Body weights of dams and pups were measured throughout the lactation
period and at one week after lactation.
Body-weight gain in 'treated' dams was slightly lower than in
control dams on days 1 to 4 but there was no difference in body-weight
gain on days 4 to 21 of lactation. Body-weight gain in 'treated' pups
was decreased from day 2 and continued to be decreased throughout
lactation and one week after lactation. For example, the mean
body-weight difference on days 2, 7, 14 and 21 were 0.5 g (-8.4%),
1.6 g (-13.7%), 2.7 g (-11.3%), and 3.1 g (-7.4%), respectively. After
weaning, the weight difference continued to increase to 5.2 g (-7.2%)
at one week. The differences were statistically different from
controls on days 2, 3 and 5, but not thereafter. Locomotor activity
was measured in dam and pups, and no treatment-related effect was
noted.
The results suggested that bedding containing 1% alitame had a
treatment-related effect on pup body-weight gain, although no
mechanism for this effect was apparent. Food consumption was not
measured in this study although it was claimed to be normal. The
consumption of food by the dams from the bedding or from their fur was
considered to be negligible (Holmes et al., 1986d).
2.2.6 Special studies on embryotoxicity/teratogenicity
2.2.6.1 Rats
A group of 80 Crl:COBS-CD(SD)BR strain female rats were
inseminated and divided into 4 groups of 20 animals each. The groups
of presumed pregnant rats were administered alitame in 0.1% methyl
cellulose by gavage at dose levels of 0, 100, 300 or 1000 mg/kg bw/day
on days 6 to 15 post-insemination. The dams were examined throughout
the study period and body weights recorded regularly. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half were stained for skeletal anomalies and half
were examined for soft tissue defects. The ovaries and uteri of dams
were examined on day 20.
The pregnancy rate was 20/20 in control and intermediate-dose
groups and 18/20 in the low- and high-dose groups. There were no
deaths throughout the study and no clinical signs of toxicity. A
slight decrease in food consumption occurred on day 14 at the
high-dose level. Body weight was depressed significantly during and
after the treatment period at the high-dose level only. The number
of viable litters was similar for all groups, as was the number
of corpora lutea. There were slight decreases in the number of
implantation sites in the intermediate- and high-dose groups (10.8 and
10.6 respectively) compared to the control (11.8) and low-dose group
(12.2). There were also slight decreases in the number of fetuses in
the intermediate- (9.9) and high-dose groups (9.7) compared to the
control (11.0) and low-dose group 111.3). The implantation rate
decreased with increasing close (91.1, 89.8, 86.7 and 84.9%,
respectively), and the embryotoxicity rate increased with increasing
dose (6.4, 6.8, 7.4, and 8.4%, respectively). The sex ratio (M/F) was
decreased at the high-dose level (74/100) compared to controls
(110/110).
With regard to fetal development, there was no difference between
the fetal weight of control and treated animals. Examination of the
fetuses did not reveal any treatment-related increase in gross,
visceral or skeletal malformations. Among all the fetuses examined,
only one external anomaly (cleft palate) was observed. There was no
treatment-related change in the rate of ossification. Under the
conditions of this study, alitame showed no evidence of teratogenicity
in the rat. The decrease in the number of fetuses at the higher dose
levels appeared to be a direct result of the lower level of
implantation in these groups rather than to embryotoxicity (Perraud
et al., 1983a).
2.2.6.2 Rabbits
A group of 77 New Zealand white rabbits was inseminated and
divided into 4 groups of 19, 18, 20 and 20 animals. The groups were
administered alitame by gavage at dose levels of 0, 100, 300 or
1000 mg/kg bw/day on days 7 to 18 post-insemination. The does were
examined throughout the study period and body weights recorded
regularly. Animals were killed on day 28 post-insemination. Fetuses
were examined for external malformations then half were stained for
skeletal anomalies and half were examined for soft tissue defects.
Ovaries and uteri of does were examined on day 28.
Four animals died during the study, one at each dose level and
one in the control group. The pregnancy rate was 16/19, 15/18, 18/20
and 16/20 in each of the groups, respectively. Food restrictions were
introduced on days 12 to 19 to reduce the incidence of diarrhoea.
Body-weight loss was observed in all groups during this 8-day period.
A significant treatment-related decrease in body-weight gain was also
noted at the high-dose level.
The number of viable litters was similar for all groups as was
the number of corpora lutea. There was one abortion on day 24 in the
high-dose group. The number of implantation sites was slightly
decreased at the low-dose level compared to other groups.
Embryo-mortality rate was slightly higher at the intermediate- and
high-dose levels but was not dose-related, and not considered to be
due to treatment.
With regard to fetal development, fetal body weights were
slightly but not significantly decreased (7%) at the intermediate- and
high-dose level compared to the control group. Examination of the
fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral abnormalities. Among all the fetuses, there was
one with cleft palate and one with club foot in the intermediate-dose
group, and one with edema in the control group. The only visceral
abnormality seen was agenesis of the left kidney in one control group
fetus. There were no major malformations observed, but there was a
significant increase in the incidence of fetuses with supernumerary
13th ribs at the intermediate- and high-dose levels. Under the
conditions of this assay, alitame showed no evidence of teratogenicity
in the rabbit (Perraud et al., 1983b).
2.2.7 Special studies on genotoxicity
The results of the genotoxicity tests with alitame are summarized
in Table 5.
2.2.8 Special studies on pharmacological effects
Alitame was tested for its pharmacological activity in a battery
of systems designed to assess autonomic, gastrointestinal, renal and
metabolic functions.
A solution of 10-4 mol/litre alitame had no contractile or
relaxant activity in guinea-pig ileum, rat fundus, or rat vas
deferens, and did not interact with any of the six neurotransmitters
used in these systems. Alitame at an oral dose level of 25 mg/kg bw
caused no change in intestinal transit time nor in renal excretion of
fluids or electrolytes. Alitame at an oral dose level of 25 mg/kg bw
had no effect on fasting blood glucose levels or on the deposition of
an oral glucose load (Pearson et al., 1982).
Table 5. Summary of genotoxicity tests with alitame
Test system Test organism Concentration1 Result Reference
Reverse mutation in S. typhimurium 20-10000 µg/plate - Holden et al.,
bacteria TA1535, TA 1537, TA98, (-S9) (1981)
TA100 10-10 000 µg/plate
(+S9)
Urinary metabolites CD-1 mice/ 50-1000 mg alitame/kg - Holden et al,.
in vivo/Reverse S. typhimurium in mice & 0.05-0.75 ml (1981)
mutation in bacteria2 TA1535, TA 1537, TA98, urine/plate
TA100
Reverse mutation in Mouse lymphoma cells 220-1655 µg/ml (+S9) - Holden et al.,
mammalian cells L5178Y (1981)
Chromosome aberration in CD-1 mice bone 0-1000 mg/kg - Holden et al.,
vivo3 marrow cells (1981)
Table 5. Summary of genotoxicity tests with alitame (cont'd).
Test system Test organism Concentration1 Result Reference
Chromosome aberrations Human lymphocytes 0-1000 µg/ml - Holden et al.,
in vitro4 (1981)
Human lymphocytes 0-3000 µg/ml (+/-S9) - Amacher et al.,
(1991)
Micronucleus assay5 CD-1 mice 0-2000 mg/kg/bw/day - Amacher et al.,
(1991)
1 The purity of alitame tested was not provided.
2 Urine from treated CD-1 mice (14/group) was pooled over 18 h and treated with beta-glucuronidase.
3 Groups of 5 CD-1 mice were administered alitame orally and bone marrow cells harvested at 6, 12
and 24 h.
4 In the first study, there was minimal provision of methodology and reporting. The second study
was conducted under more recent GLP regulations.
5 Groups of 5 male and 5 female mice were treated over three consecutive days. Bone marrow was
prepared 24 h after file last dose.
2.2.9 Special studies on neurotoxicity and neurobehavioural
effects
In a neurotoxicity study, alitame was tested for its ability to
inhibit in vitro receptor binding in rat brain homogenate and to
produce in vivo neurochemical effects in rats. In the in vitro
binding assays performed with rat brain homogenate, alitame was tested
for its ability to inhibit the binding of a variety of receptor
radioligands to brain membranes. Neither alitame nor the B-isomer, at
concentrations of 10 µM, had any effect on the level of binding of
these ligands. In the in vivo study, groups of Sprague-Dawley rats
(8 males) were administered an oral dose of vehicle, alitame (25 mg/kg
bw), ß-isomer of alitame (6.25 mg/kg bw), or alitame (12.5 mg/kg bw)
plus ß-isomer of alitame (6.25 mg/kg bw). At 2 or 4 hrs, animals were
killed and the levels of dopamine, serotonin and their metabolites in
the brain tissue were measured. There was no significant change in
neurotransmitter levels after treatment (Bacopoulos, undated).
In a study to examine the effect of alitame on the behaviour of
rats, groups of male CD rats were administered a single oral dose of
vehicle, alitame (25 mg/kg bw), ß-isomer of alitame (6.25 mg/kg bw),
or alitame (12.5 mg/kg bw) plus ß-isomer of alitame (6.25 mg/kg). In a
separate experiment, animals were administered single oral doses as
above for 13 consecutive days. No treatment-related effect on
horizontal or vertical locomotor activity was noted in either the
single or multiple treatment regimes (Bacopoulos, undated).
In a study to examine the potential neurotoxicity of alitame in
rats following acute administration, groups of 12 male and 12 female
Sprague-Dawley rats were given alitame at a single oral dose of
0, 2000, 3000 or 5000 mg/kg bw. The animals were examined using a
functional observational battery (FOB) (both qualitative and
quantitative), a motor activity test, and an auditory startle
habituation test prior to treatment, at 5 and 7 h post-dosing, and
on days 1 and 7 post-dosing. At the end of the study, half the
animals were randomly selected for perfusion for possible future
neuropathological examination while the remaining animals were given
a full necropsy.
Significant decreases in body-weight gains were noted in all
treated groups on the day following treatment (days 0 to 1), followed
by compensatory increases on the following days (days 1 to 7). A
decrease in food consumption on day 1 was noted in all female treated
groups and in males of the intermediate- and high-dose groups. For the
high-dose female group, clinical symptoms included yellow abdominal/
urogenital fur staining, dehydration, reduced activity and pallor
between days 1 and 4 post-treatment. Effects were noted in all
behavioural tests for both sexes in the high-dose group and in some
tests in the intermediate- and low-dose groups on the first day. By
day 7, results of all parameters in all treatment groups were similar
to the control group.
Qualitative FOB assessments on day 1 post-treatment revealed
significant differences between the control and high-dose group for
both sexes. At the high-dose level, there was a significant decrease
in pinna reflex and a decrease in rearing in the arena. There was also
an increased incidence in ataxic gait and diarrhoea in the high-dose
animals. Animals at the lower dose levels also showed some increase in
minor symptoms. By day 7, there were no significant differences
between the groups. Quantitative FOB assessments found changes in
temperature and hind limb splay in treated groups on day 1 but by day
7 no significant differences were detected. Changes were also noted in
treated groups with respect to the motor activity test and the
auditory startle habituation test on day 1, but no changes were
evident by day 7. There were no treatment-related gross pathological
changes found on day 7. Transitory behavioural changes were observed
immediately after treatment and in the 24 h following treatment. By
day 7, all animals appeared normal and there was no evidence of
neurotoxicity (Robinson et al., 1993a).
In a 13-week study of the potential effects of alitame on
behaviour and neuromorphology in rats, 12 male and 12 female
Sprague-Dawley rats were fed a diet containing alitame at
concentrations of 0, 0.1, 0.3 or 1%. Animals were examined throughout
the study period for clinical signs of toxicity and body weights and
food consumption were recorded. The animals were examined using a
functional observational battery (FOB) (qualitative and qualitative),
a motor activity test and an auditory startle habituation at
pre-treatment, after 7 and 14 days of treatment and in the 5th, 9th
and 13th weeks. Passive avoidance testing was performed in weeks 4 and
13. At the end of the study, half the animals (randomly selected) were
perfused for neuropathological examination. Tissues from brain, spinal
cord, and skeletal muscle were prepared as paraffin sections and
haematoxylin and examination. Tissues from the peripheral nervous
system and the central nervous system were prepared in epoxy
embedding.
There were no mortality or adverse clinical findings related to
the treatment. The body weights of females in the 1% treated group
were significantly lower after 3 weeks and throughout the second and
third months of the study. The food consumption for this group was
also significantly decreased during the first week of the study. The
body weights and food consumption of all the other treated groups were
normal.
Both the qualitative (observations and handling) and the
quantitative FOB evaluations (grip strength, hind limb splay, and body
temperature) were unaffected by treatment for both sexes. In the
locomotor activity tests, significant differences at single time
points were noted for high-dose males and females. However, these
differences were not sustained over the course of the study and could
not be clearly related to treatment. For auditory startle habituation
tests, occasional significant differences in the 0.1% group were noted
but these were not attributed to treatment as the effects were not
sustained and there was no dose-relationship. In passive avoidance
performance at 4 weeks there were no significant differences between
groups. At week 13, more females in the 1% group completed the
training trial than in the control group. However, it could not be
concluded that this was related to treatment and was of doubtful
neurotoxicological significance.
There were no treatment-related gross pathological findings in
animals not selected for perfusion or in those which underwent
perfusion. A few animals, primarily males in all groups, had pale
areas on the livers. For rats undergoing perfusion, gross pathological
findings in the nervous system were limited to a few animals with dark
areas in the meninges. Histopathological examination was only
performed in control and high-dose animals. There were no significant
differences in the finding in the two groups. Under the conditions of
this study, there were no behavioural or neuromorphological changes
observed which could be attributed to treatment with alitame (Robinson
et al., 1993b).
In a study to examine the potential developmental neurotoxicity
of alitame, groups of female Sprague-Dawley rats were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for one week
prior to mating, then throughout the mating and gestation periods and
up to 10 days post-partum. Animals were observed throughout the
study period and body weights and food consumption recorded. All
females were given a complete necropsy. Dams were killed on days 21-23
post-partum.
The F1 pups were examined for malformations, sexed and the
number alive and dead recorded. The pups in each litter were weighed
individually on days 1, 4, 7, 11, 14, 17 and 21. On day 4, the litters
were culled to 8 pups, 4 of each sex. Pups were examined for pinna
unfolding (day 2), eye opening (day 12), and air righting reflex
(day 14). A motor activity test was conducted on day 17. Pups were
weaned on day 21.
The F1 adult generation was formed from at least 20 different
litters and consisted of 80 rats/sex/group. Animals were examined for
clinical condition and physical development and body weights and food
consumption recorded. From each litter, 3 males and 3 females were
randomly selected for behavioural testing (motor activity test,
auditory startle habituation test, passive avoidance test and
water-maze test). Young adults not selected for the adult F1
generation behavioural testing were sacrificed and underwent gross
pathological examination. Six animals/sex/group were perfused for
histo-pathological examination. Tissues from brain, spinal cord, and
skeletal muscle were prepared as paraffin sections and H and E
stained. Tissues from the peripheral nervous system and the central
nervous system were prepared in epoxy embedding.
In the F0 animals, there were no deaths and there were no
treatment-related clinical findings. The body weights of the 1%
treated animals were significantly decreased throughout the study.
The food consumption values for the 1% treated group were also
significantly lower than control values during the study. Gross
pathological examinations revealed no treatment-related effects. There
was no treatment-related effect on oestrus cycles, pregnancy rate,
gestation index, number of live and dead pups, sex ratio or post
implantation loss.
In the F0 pups, viability, survival, lactation indices,
clinical findings and gross pathology were unaffected by treatment. In
the 1% group, pup weight was significantly decreased on days 11, 14
and 17 and slightly decreased on day 21. There were no treatment-
related effects on physical or reflexological development, or on
locomotor activity.
In the F1 adults, there were no treatment-related effects on
mortality or clinical findings. The body weights of males and females
in the 1% group were significantly decreased. Food consumption was
normal. Physical development was not affected by treatment. In the
behavioural testing, there was no treatment-related effect on
locomotor activity, auditory startle habituation, passive avoidance or
water maze tests. Gross pathological and neuropathological
examinations revealed no effects which could be attributed to alitame
treatment. Under the conditions of this study, there were no
behavioural or neuromorphological changes in adults or pups which
could be attributed to treatment with alitame (Robinson et al.,
1993c).
2.2.10 Special studies on melanin binding
The potential for radiolabelled alitame or its metabolites to
bind to melanin in the eye of rats was tested by whole body
autoradiography and by microhistoautoradiography. Two male rats from
the albino strain, Sprague-Dawley (Crl:CD(SD)BR) and two male rats
from the pigmented strain, Lister Hooded (Crl:(LIS)BR) were
administered a single oral dose of 65 mg/kg bw 14C-alitame (94%
purity). At 2 and 24 h, one animal from each strain was killed and
the right eye of each excised and stored at -80°C for micro-
histoautoradiography. The remaining carcass was rapidly frozen at
-75°C for whole-body autoradiography.
Microscopic examination of the micrographs of the eyes revealed a
large number of silver grains distributed widely throughout the layer
of the retina, choroid and sclera in animals of both pigmented and
non-pigmented strains at both sacrifice times. The results of the
microscopy could not be interpreted and the data were considered to be
artifactual.
The results of the autoradiography revealed much higher levels of
radioactivity in the uveal tract (choroid, ciliary body and iris) of
the pigmented rats. Quantification of the autoradiographies revealed
concentrations of 2.99 µ equivalent alitame/g tissue in the uveal
tract of albino rats at 2 h and no detectable radioactivity at 24 h.
In the pigmented rats, the radioactivity in the uveal tract was
28.24/µg equivalent alitame/g tissue at 2 h and slightly higher levels
at 24 h (30.55 µg/g). There was no difference in radioactivity in the
surrounding tissues in either the albino or pigmented rat.
The study provided evidence of binding of alitame or its
metabolites to the melanin contained in the uveal tract of the
pigmented rats.
In an addendum to this report, alitame and two metabolites were
tested in vitro for their binding affinity to a melanin preparation
from bovine eyeballs. Alanine tetramethylthietane amide sulfoxide,
which was found in the urine of both rats and humans, did not bind to
melanin under the conditions of this assay, whereas its acetylareal
form, which was found only in the urine of rats, did bind to the
melanin. The positive control, doxepin, produced a higher level of
binding than found with the acetylated sulfoxide metabolite. This
study provided some evidence that the melanin binding observed in rats
may be associated with a metabolite formed only in the rat (Whitby
et al., 1994).
2.2.11 Special studies on the ß-isomer of alitame
2.2.11.1 Absorption, distribution, excretion, and biotransformation
In a study to examine the toxicokinetics of the ß-isomer of
alitame, Sprague-Dawley rats (4/sex) were administered a single oral
dose of the 14C-ß-isomer of alitame (10 mg/kg bw in water).
Excretion of radioactivity in urine and faeces was measured over 72 h.
A similar study was conducted with 6 male and 3 female Long-Evans rats
following a single oral dose of 14C-ß-isomer of alitame (25 mg/kg
bw). A separate group of 7 male Long-Evans rats were fitted with a
bile cannula. These animals received an oral dose of 25 mg/kg bw
14C-ß-isomer, and bile and urine were collected over 48 h.
In Sprague-Dawley rats, the bulk of the radioactivity was
excreted within 48 h, with 24% and 65% excreted in urine and faeces,
respectively. In Long-Evans male rats, total excretion was similar,
with 25 and 64% excreted in urine and faeces, respectively. Excretion
in the female rats was slightly slower. In bile-duct cannulated
animals, 13% of the total radioactivity was in bile over 48 h
(Pfizer, 1986).
In a study to identify the metabolites of the ß-isomer of
alitame, 2 male and 2 female Long-Evans rats were administered a
single oral dose of 10 mg/kg bw 14C-ß-isomer of alitame. In a second
experiment, female Long-Evans rats received 14C-ß-isomer of alitame
at an oral dose of 25 mg/kg bw. In a third experiment, 3 females and 6
males received the same dose of 14C-ß-isomer of alitame. Urinary
metabolites were examined by HPLC. Urinary radioactivity contained
approximately 1% of the radioactivity as unchanged ß-isomer in the
males.
Unchanged ß-isomer was found in the urine of female rats and
represented approximately 13% of the total radioactivity. Other
metabolites which represented approximately 50% of the radioactivity
were alanine tetramethylthietane amide sulfoxide (CP-58,290), alanine
tetramethylthietane amide sulfone (CP-57,254) and their acetylated
derivatives (CP-71,246 and CP-58,386). The only different metabolite
identified was the N-acetyl derivative of the ß-isomer which was
formed by acetylation of the free amine group of the aspartic acid
moiety. The N-acetyl derivative of the ß-isomer comprised 9% and 31%
of the radioactivity in the urine of male and female rats,
respectively. In faeces, the ß-isomer accounted for the majority
(70-81%) of the radioactivity (Pfizer, 1986).
In a study to examine systemic exposure to B-alitame and N-acetyl
beta-isomer by examination of urinary metabolites in male Long-Evans
rats, 14C-ß-isomer of alitame or 14C-N-acetyl -ß-isomer was
administered i.v. initially, then orally, at a dose level of 25 mg/kg
bw. Urine and faeces were collected at 24-hour intervals for a 48-hour
period. Systemic exposure was calculated from a comparison of urinary
excretion following the oral and i.v. routes. The systemic exposure to
ß-isomer following oral administration was 9% in males and 10% in
females. The systemic exposure to ß-isomer plus N-acetyl-ß-isomer was
8% in males and 18% in females (Pfizer, 1986).
In a study to examine systemic exposure to ß-isomer by
examination of serum concentrations in Long-Evans male rats,
14C-ß-isomer was administered i.v. initially (25 mg/kg bw), then
orally at a dose level of 29 mg/kg bw. Serum samples were collected
from 3 animals at various time-points and analyzed by UV absorption
for ß-isomer. Serum concentration-time AUC determinations were made
for each route of administration. The systemic exposure to ß-isomer
following oral administration was 9.6%. The elimination half-life was
29 minutes following the i.v. dose (Pfizer, 1986).
In a study to examine the toxicokinetics and metabolism in dogs,
2 male and 2 female beagle dogs were administered an oral dose of
14C-ß-isomer of alitame followed by an unlabelled dose of 5 or
50 mg/kg bw. Urine and faeces were collected for 96 h. The two male
dogs excreted 35 and 39%, respectively, of the administered
radioactivity in the urine, and the females 14 and 16%, respectively.
Unchanged 13-isomer in urine accounted for 10-16% of the dose in all 4
dogs. All of the urinary metabolites were identical to the ones found
after administration of alitame, but the males had higher levels of
metabolites than the females (Pfizer, 1986).
2.2.11.2 Short-term toxicity studies
In a 3-month study, groups of 15 male and 15 female mice (Strain
Crl: COBS CD-1 (ICR) BR) were fed a diet containing the ß-isomer of
alitame (CP-63,884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption were recorded weekly. Urinary metabolites were
analyzed at weeks 11 and 13. Gross pathological examination was
performed on all animals and organ weights recorded. Tissues were
taken from all of the major organs for histopathological examination.
There was no treatment-related effect on body-weight gain
although the average body weight of treated male animals was lower
than in control groups. Food consumption was slightly decreased at
high dose in males. One of the urinary metabolites identified was the
N-acetyl derivative of the/%isomer. The amount of the isomer increased
proportionally with the dose. There were no treatment-related effects
on organ weights. Gross pathology was unremarkable and histopathology
did not reveal any treatment-related changes. The NOEL in this study
was >25 mg/kg bw/day (Stadnicki et al., 1986a).
In a 3-month study, groups of 15 male and 15 female Long-Evans
rats (Strain Crl: (LE) BR) were fed a diet containing ß-isomer of
alitame (CP-63884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption data were recorded weekly. Ophthalmoscopic
examinations were performed at pre-test and prior to necropsy. Blood
samples were taken for examination of clinical chemistry and
haematological parameters at pre-test and at 3, 9 and 13 weeks.
Urinary analyses were performed at 3, 9 and 13 weeks. At the end of
the study, gross pathological examinations were performed and tissues
from the major organs taken for histopathology.
There were no deaths through the study and the body-weight gains
and food consumption were similar for treated and untreated groups.
There were no treatment-related effects on clinical chemistry,
haematology or urinary parameters. There were no differences between
control and treated groups with respect to organs weights. Gross
pathology and histopathology were unremarkable The NOEL in this study
was > 25 mg/kg bw/day (Stadnicki et al., 1986b).
2.2.11.3 Special studies on embryotoxicity/teratogenicity
A group of 88 albino female rats (strain Crl:COBS-CD (SD)BR) was
inseminated and divided into 4 groups of 22 animals. The groups of
presumed pregnant rats were administered the 3-isomer of alitame
(CP-63,884) in distilled water by gavage at dose levels of 0, 25, 50
or 100 mg/kg bw/day on days 6 to 15 post-insemination. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half stained for skeletal anomalies and half
examined for soft tissue defects.
The pregnancy rate was 20/22 in the control and low-dose groups,
and 19/22 in the intermediate- and high-dose groups. There were no
deaths throughout the study, nor any clinical signs of toxicity. There
were no treatment-related effects on body weight or food consumption
of dams during or after the treatment period. The number of corpora
lutea, implantation sites and viable fetuses was similar for all
groups. With regard to fetal development, there was no difference
between fetal weight of control and treated animals. Examination of
the fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral malformations, nor any treatment-related effects
on the degree of ossification. Under the conditions of this study, the
ß-isomer of alitame showed no evidence of teratogenicity in rats
(Kessedjian et al., 1986).
2.2.11.4 Special studies on genotoxicity
The ß-isomer of alitame was tested for the induction of point
mutations, both base-pair and frameshift, at the his locus of
Salmonella typhimurium strains TA 1535, TA 1537, TA 98 and TA 100,
with or without S9 microsomal activation. The purity of the alitame
was not provided. Doses ranged from 20 to 10 000 µg/plate, with or
without activation. There was no increase in revertants above the
control (water) in any of the treated plates at any dose level. The
positive controls, 9-aminoacridine, nitrofurantoin, sodium nitrite,
2-nitrofluorene and 2-anthramine gave appropriate responses (Ellis
et al., 1991).
2.3 Observations in humans
In a 90-day double-blind tolerance study, groups of male and
female subjects (77 treated, 80 controls) were administered either
alitame in capsules divided over three meals at a dose level of
10 mg/kg bw/day for 90 days, or placebo in the same manner. This dose
was chosen because it is 10 times greater than the estimated daily
intake if all sucrose in the diet were replaced with alitame. Subjects
were assessed throughout the study period for side-effects (by
interview and observation). Other tests included blood pressure,
temperature, body weight, electrocardiogram, and ophthalmoscopic
examination. Various clinical pathology parameters were also measured.
Microsomal activation was estimated from the elimination kinetics of
aminopyrine at pre-dosing and at weeks 6 and 13 of treatment. Plasma
and urine samples were collected at various time points throughout the
study.
Four treated subjects and 2 control subjects discontinued from
the study due to side effects (angioedema of the lips, maculopapular
rash, urticaria (wheals) with erythema in treated subjects, and
tinnitus, dry eyes and abdominal cramps in the control subjects). A
rechallenge with alitame after 6 months produced none of the above
symptoms, making the possibility of allergic reaction very unlikely.
There were no treatment-related changes in blood pressure, pulse,
temperature or body weight. Electrocardiograms and ophthalmological
examinations were unremarkable. Haematological parameters were similar
in treated and control subjects. Measurement of aminopyrine
elimination rates at 6 and 13 weeks did not reveal any increase in
hepatic microsomal enzyme activity due to alitame treatment (Gerber
et al., 1987).
In a 90-day double-blind tolerance study in diabetic subjects,
groups of male and female subjects having either type I or type II
diabetes (75 treated, 80 control) were administered either alitame in
capsules divided over three meals at dose level of 10 mg/kg bw/day for
90 days, or placebo in the same manner. This dose was chosen because
it is ten times greater than the estimated daily intake if all sucrose
in the diet were replaced with alitame. Subjects were included on the
following criteria: diabetics of type I and type II, either sex and
aged between 15 and 70 years with stable diabetic therapy; fasting
blood glucose <250 mg/dL; free from investigational drugs; had not
suffered a myocardial infarction within the previous 6 months; and had
either no hypertension or it was controlled. Subjects were excluded on
the following criteria: females which were pregnant or nursing; drug
or alcohol dependence; systemic disease other than diabetes; clinical
chemistry and haematological parameters above the upper limit of
normal; hepatic disease; and donation of blood within 4 weeks prior to
the study. The groups were well matched with respect to such
parameters as age, sex distribution, weight, smoking, drinking,
intercurrent illnesses and medication as well as diabetic status.
Subjects were assessed at weeks 0, 1, 2, 3, 4, 6, 8, 10 and 13 of
the study period for side effects (by interview and observation).
Effects on diabetic control were assessed by measuring fasting
haemoglobin A1c and 2-hour postprandial blood glucose levels at
baseline and weeks 0, 6, 8 and 13. Physical examinations were
conducted at screening and at week 13 of the study and included blood
pressure, temperature, body weight and electrocardiogram. Clinical
pathology parameters (haematology, clinical chemistry and urinary)
were measured pre-treatment and at 13 weeks.
At the end of the study period, 16 treated and 9 control subjects
had discontinued from the programme and, of these, 5 treated and 2
control subjects withdrew due to illness. No subjects discontinued due
to side effects or abnormal laboratory parameters related to alitame
treatment. Side-effects were observed in 29% of treated subjects and
25% of control subjects. Gastrointestinal disturbances were the most
common side-effect. Incidence and severity of particular side-effects
were relatively evenly distributed between the groups. No significant
changes were noted in either the control or treated groups with
respect to diabetic control, clinical chemistry, haematological or
urinary parameters. No treatment-related effects were apparent from
the physical examinations, blood pressure and pulse rate, temperature,
body weight or EKG recordings.
Alitame at a dose of 10 mg/kg/bw/day for 90 days was well
tolerated by the type I and type II diabetic patients who continued in
this study. There was no effect of alitame at this dose level on the
ability to control serum glucose levels by these diabetic patients
(McMahon et al., 1989).
In a 1- and 2-year follow-up of the subjects in the 90-day study
in diabetics, all but one of the treated subjects received an
interview and complete physical examination. Subjects were interviewed
again within 24 months post-study for all but 6 treated and 4 control
subjects.
A variety of intercurrent illnesses were reported during the
follow-up period. Most of the reported illnesses related to diabetes
and its complications. The incidence of intercurrent illnesses during
the follow-up period was 40 treated and 36 control subjects. Two
subjects died during the follow-up period, both treated subjects. One
subject (type I diabetic) died during the month following the study,
cause unknown. At completion of the study no adverse reactions had
been noted in this subject. The second subject (type II diabetic)
suffered a fatal heart attack resulting from hypertensive
atherosclerotic cardiovascular disease.
At the first follow-up (12 months), 5 treated subjects and no
control subjects were found to have suffered myocardial infarction.
The possibility that the results were due to chance was examined using
available epidemiological data on the incidence of myocardial
infarction. Data from the US National Hospital Discharge Survey (NHDS)
were used to estimate the rate of myocardial infarctions among
diabetics in various age groups for both sexes. These data were
adjusted to take into account the number of myocardial infarction
casualties which are not admitted to hospital and the actual patient
years of observation. Based on the hospitalization data for diabetics
who suffered myocardial infarction in 1986, the numbers of subjects
expected to suffer a myocardial infarction in a single year were 2.9
and 3.2 in the treated and control groups, respectively. The observed
rates of 5% and 0%, respectively, were within the 95% tolerance
intervals of incidence expected based on the NHDS analysis. Two years
after the study, myocardial infarctions had been encountered by 6
treated and 3 control subjects. The statistical analysis of these data
at 2 years supports the null hypothesis that the incidence observed
were due to chance. The data indicate that in the two years after the
90-day study of alitame in diabetics, no evidence was provided that
alitame adversely affected the health of the study groups (McMahon
et al., 1989).
3. COMMENTS
A number of toxicity studies on alitame were available, as well
as studies on the ß-isomer of alitame, which is formed at low levels
in some foods.
In metabolism studies conducted in three animal species and in
humans, alitame was readily absorbed from the GI tract and then
rapidly metabolized and excreted. In mice, rats and dogs, the route of
metabolism involved cleavage of the peptide bond yielding aspartic
acid and D-alanine tetramethylthietane amide, the latter being further
biotransformed by oxidation at the thietane sulfur to form the
sulfoxide and sulfone. Acetylated derivatives of these metabolites
were commonly found in the urine of rats and dogs whereas, in humans,
the glucuronide derivative of D-alanine tetramethylthietane amide was
the major urinary metabolite. In rats, initial cleavage of the peptide
bond of alitame took place in the lumen of the jejunum. In pregnant
rats, alitame was readily transferred transplacentally, but there was
no evidence of active secretion in the milk. In a study in rats,
levels of alitame residues decreased rapidly in all tissues except the
eyes. Further studies indicated that it was bound to melanin contained
in the uveal tract (choroid, ciliary body and iris) and suggested that
such binding was associated with a metabolite formed only in rats. The
Committee considered that an in vivo study in a mammalian species
other than the rat is desirable to clarify the significance of the
retention of alitame in the eyes.
The ß-isomer of alitame was rapidly metabolized in rats, the only
urinary metabolite not also formed with alitame being the N-acetyl
derivative of this isomer. There was no evidence of the formation of
significant amounts of the ß-isomer of alitame in the stomach or small
intestine of the rat.
Acute and short-term toxicity studies indicated that the toxicity
of alitame is low. In studies conducted in mice, rats and dogs, the
major toxicological changes observed were associated with the liver. A
dose-related increase in liver weight and evidence of centrilobular
hypertrophy were seen in all of the studies in these three species,
and these changes were accompanied by an increase in the level of
hepatic microsomal enzymes. Short-term studies on hepatic enzyme
induction indicated that alitame was a weak inducer of microsomal
enzymes; the pattern of induction was qualitatively similar to that
caused by phenobarbitone but at a lower level. The liver changes were
indicative of an adaptive response and, in rats and dogs, withdrawal
of treatment after a 3-month period resulted in partial or complete
reversal of the observed changes within a month.
Two-year carcinogenicity studies have been conducted in CD-1
mice, Long-Evans rats and Sprague-Dawley rats. The two studies
conducted in rats included an in utero phase. There was no evidence
of carcinogenicity in the study in mice.
In rats, the first carcinogenicity study was conducted in
Long-Evans rats at dietary levels of 0.1, 0.3 or 1% and, while the
survival rate was greater than 50% in all groups at 22 months, poor
survival was noted at 24 months. Pathological examination of the liver
indicated a higher incidence of focal nodular hyperplasia and
eosinophilic foci in females at the 1% dose level than in controls,
according to the diagnostic criteria of Squire & Levitt (1975). Two
subsequent independent re-examinations of these data have been
performed using the diagnostic criteria of Maronpot et al. (1986).
In the first re-examination (Farrell, 1987), the higher incidence of
eosinophilic foci in females in the high-dose group was confirmed, but
the incidence of focal hyperplasia or hepatocellular adenomas was
similar to that in controls. In the second re-examination (Hardisty
et al., 1994), the higher incidence of eosinophilic foci in females
in the high-dose group was again confirmed. A higher incidence of
these foci was also found at the mid-dose level. Also in this
re-examination, the nodular hyperplastic lesions noted in the original
report were largely reclassified as hepatocellular adenomas, and an
increased incidence of these lesions was seen at the high-dose level.
No evidence of hepatocellular carcinomas was found in female rats in
the original examination or in the subsequent re-examinations. The
main difference between the first and second re-examinations was in
the nomenclature used, rather than in the recognition that lesions
existed. The Committee expressed concern at the change in the way that
these liver lesions were classified, possibly as a result of the use
of different diagnostic criteria. It considered that there was
evidence of a higher incidence of adenomas in females in this study,
at least at the high dose level. The adenomas were found late in the
study and, while considered unlikely to progress to hepatocellular
carcinomas, the data were inconclusive in this regard.
A second carcinogenicity study, this time in Sprague-Dawley rats,
was conducted because of the low survival rate at 24 months in the
Long-Evans rats. In this second study, the survival rate was lower at
both 22 and 24 months than that found in the Long-Evans rats, and
hence the study was considered inadequate to provide further
information regarding the potential carcinogenicity of alitame.
The Committee considered that the available studies did not
indicate that alitame was carcinogenic, but that they were deficient
in certain respects and did not fully address this question. It also
considered that further research to clarify the mechanism of the
formation of adenomas in female Long-Evans rats was desirable.
Genotoxicity studies both in vitro and in vivo did not
provide any evidence that alitame has a mutagenic potential.
Similarly, there was no evidence from studies conducted in rats and
rabbits that alitame has a teratogenic potential.
Two reproductive toxicity studies (2-generation) were conducted
in Long-Evans rats and one reproductive toxicity study (1-generation)
in Sprague-Dawley rats. The first reproductive toxicity study with
Long-Evans rats produced some evidence for slightly decreased
body-weight gain in the pups during lactation at 1% in the diet, the
highest dose administered. A similar effect was noted in the second
reproductive toxicity study at the same dose level, but not in the
1-generation reproductive toxicity study with Sprague-Dawley rats. A
study of the effects of alitame-containing diet in the bedding
material, which could have been a source of alitame for pups due to
spilled feed, while providing a possible explanation for the decrease
in pup body-weight gain, did not fully address this issue. A slight
effect of alitame on locomotor activity seen in the first reproductive
toxicity study was not substantiated in subsequent studies. The
Committee did not regard the changes seen in the reproductive toxicity
studies to be of toxicological significance.
Neurotoxicity and neurobehavioural toxicity studies using an
observational battery of tests indicated behavioural changes only when
very high doses were administered to rats as a single dose by gavage
(5000 mg/kg bw). These behavioural changes were of short duration and
animals appeared to be normal by day 7. There was no evidence of
neurotoxicity in rats fed diets containing 1% alitame (equivalent to
1000 mg/kg bw/day) over a 3-month period. Similarly, no changes
indicative of neurotoxicity were observed in dams or pups when the
same dose level was given over the reproductive period.
In a 14-day study conducted in humans there was no evidence of
hepatic enzyme induction at a dose level of 15 mg/kg bw/day.
Ninety-day tolerance studies were conducted in normal and
diabetic subjects. There was no evidence of adverse effects in
subjects of both types during the period of the study at a dose level
of 10 mg/kg bw/day. However, in the 1- and 2-year follow-up of the
diabetic subjects, there was a higher incidence of myocardial
infarction in the treatment group than in the controls, namely 5/58 in
the treatment group and 0/71 in the controls after 1 year, and 6/53 in
the treatment group and 3/67 in the controls after 2 years. While the
differences at 2 years were not statistically significant, and there
was no indication of cardiovascular effects in the animal studies, the
Committee considered that the increased incidence of myocardial
infarction in the treatment group has not been fully explained. It was
advised that a further study is to be performed in diabetic subjects.
Studies conducted with the ß-isomer of alitame provided no
evidence of genotoxicity, teratogenicity or systemic toxicity in mice
or rats at dose levels of up to 25 mg/kg bw/day.
4. EVALUATION
The Committee recognized that the basis on which to consider
establishing an ADI for a chemical such as alitame is difficult to
specify, since the most sensitive treatment-related effects observed
were liver enzyme changes and liver weight changes, which may be
adaptive in nature. For alitame, these changes appeared to be closely
linked. While the prolonged hepatomegaly observed was not accompanied
in the long-term studies by any pathological changes indicative of
toxicity, it is not considered desirable. On the other hand, moderate
induction of hepatic enzymes is considered a normal adaptive process.
In the case of alitame, significant changes in body-weight gain or
liver weight were observed at doses above the NOELs. In the 18-month
study in dogs, the NOEL was 100 mg/kg bw/day. In the 2-year study in
mice, the NOEL was 0.1% in the diet, equal to 125 mg/kg bw/day. In the
lifetime studies in rats, the NOEL was 0.3% in the diet, equal to
130 mg/kg bw/day in Long-Evans rats and 230 mg/kg bw/day in
Sprague-Dawley rats.
The Committee concluded that the concerns raised by the
deficiencies in the carcinogenicity studies in rats were of such
significance that an ADI could not be allocated.
5. REFERENCES
AMACHER, D.E., MUEHBAUER, P.A., ZELLJADT, I. (1991) Genetic Toxicology
Report. Unpublished Report No. 91-444-21 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
BACOPOULOS, N.G. (undated) Investigation of alitame and its b-isomer
in neurochemical and behavioural animal models. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1984) Alitame: A Fourteen Day
Human Enzyme Induction Study. Unpublished Report No. 32 from
Bio-Organic Chemicals Research & Development, Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1985) Human Disposition of
14C-Alitame. Unpublished Report No. SC-2 from Department of
Pharmacology, University of London. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M., THATCHER N.J., MARSHALL, A.D. (1994)
Assessment of the Enzyme Inducing Characteristics of Alitame.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School., London, UK. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M. /1994) Assessment of the Enzyme Inducing
Characteristics of Alitame Suppl. Report on Ummunodetection of
Cytochrome P450 Isoenzymes in the Liver of Alitame-Treated Rats.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School, London, UK Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981a)
Alitame: Two-Week Range-Finding Feeding Study in Rats. Unpublished
Report No. 81-055 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981b)
Alitame: Two-Week Oral Range-Finding Study in Dogs. Unpublished Report
No. 81-070 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ELLIS, J.H., AMACHER, D.E., KLUWE, W.M. (1991) b-Isomer of Alitame:
Microbial Gene Mutation Assays. Unpublished Report from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
FARRELL, J.F. (1987) Histopathologic evaluation of selected
microslides from chronic rat and mouse feeding studies with alitame.
Unpublished Report No. PR-116 from Experimental Pathology Laboratories
Inc. USA. Submitted to WHO by Pfizer Inc., Groton CT, USA.
FISHER, D.O., MARSH, P.M., SOLER, G.N., COLEMAN, D.V., LEVINSKY, H.V.,
ESTES, P.C., DOUGHERTY, W.J. (1992) One-Year Toxicity Study with
Dietary Administration and In Utero Exposure in Sprague-Dawley Rats.
Unpublished Report No. 90-444-18 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
FISHER, D.O., LAITWAITE, B.S., BUTLER, B.T., COLEMAN, D.V., LEVINSKY,
H.V., OCHOA, R., DOUGHERTY, W.J. (1993) Two Year Oncogenicity Study
with Dietary Administration and In Utero Exposure in Sprague-Dawley
Rats. Unpublished Report No. 90-444-20 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
GERBER, N., McMAHON, M.D., WILLIAMS, R.L. (1987) Double-Blind Placebo
Controlled Ninety-Day Toleration Study of Alitame. Unpublished Report
No. 101 from Ohio State University, Columbus, Ohio. USA. Submitted to
WHO by Pfizer Inc. Groton, CT, USA.
HARDISTY, J.E., ABBOTT, D., GOPINATH, C., WARD, J. (1994) Pathology
Working Group Review of Two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report from Experimental Pathology
Laboratory Inc., Research Triangle Park, NC, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLDEN, H.E., ELLIS, J.H., AMACHER, D., RAY, V.A. (1981) Alitame:
Genetic Toxicology Report. Unpublished Report No. 91-444-21 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., TOOLAN, L.A, BURGAN, J.J., REYNOLDS, J.A., LEVINSKY,
H.V., ESTES, P.C. (1983a) Alitame: Two-Month Range-Finding Feeding
Study in Mice. Unpublished Report No. 82-444-03 from Pfizer Central
Research, Groton, CT, USA Laboratoires. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., ANGUS, T.A., FISHER, D.O., FIGDOR, S.K., REYNOLDS, J.A.,
ESTES, P.C., LEVINSKY, H.V. (1983b) Alitame: Five-Week Feeding Study
in Rats. Unpublished Report No. 82-444-01 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton,
CT, USA.
HOLMES, C.L., MARTZ, F., BOWDEN, P.R., FIGDOR, S.K., ROSS, P.E.,
LEVINSKY, H.V., ESTES, P.C.(1985a) Alitame: One-Year Feeding Study in
Long-Evans Rats. Unpublished Report No.s. 82-444-06 and 83-444-12 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., MARTZ, F., PUNZALAN, E.R., FIGDOR, S.K., ROESLER, A.R.,
LEVINSKY, H.V,, ESTES, P.C. (1985b) Alitame: Eighteen-Month Feeding
Study in Beagle Dogs. Unpublished Report No. 82-444-07 from
Laboratoires Pfizer Centre de Recherche, 37400 Ambroise, France.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., CACCIATORE. A., BURGAN, J.J., COLEMAN, G.L., FIGDOR,
S.K., LEVINSKY, H.V., REYNOLDS, J.A., ESTES, P.C., (1985c) Alitame:
Two-Year Oncogenicity Feeding Study in Mice. Unpublished Report
No. 82-444-08 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L. (1986a) Alitame: A two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report No. 83-444-11. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986b) Alitame: Two-Generation Reproduction
Feeding Study in Rat. Unpublished Report No. 83-444-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986c) Alitame: Second Two-Generation
Reproduction Study in Rat. Unpublished Report No. 83-444-13/14/15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROSE, E.M., STRADNICKI, S.W., LEVINSKY, H.V.
(1986d) Exploratory bedding study in the Long-Evans Rat. Unpublished
Report No. 85-444-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
KESSEDJIAN, M.J., STADLER, J., POOL, W.R., (1986) b-Isomer of Alitame:
Fetotoxicity Study in Rats by the Oral Route. Unpublished report
86-086 from Pfizer Central Research, Groton, CT, USA. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., BEUTER, N.J., STADNICKI, S.W. (1983) Alitame: Acute
Oral Toxicity Studies in Mice and Rats. Unpublished Report
No. 83-444-09 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., STADNICKI, S.W., ESTES, P.C., DOUGHERTY, W.J.,
CACCIATORE, A., KOPCYK, N., LUNDEEN, G.R. (1992) In Utero Exposure
with Alitame in Sprague-Dawley rats. Unpublished Report No. 90-444-19
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MARONPOT, R.R., MONTGOMERY Jr C.A., BOORMAN, G.A., McCONNELL (1986)
National toxicology programme nomenclature for hepatoproliferative
lesions of rats. Toxicol. Pathol., 14: 263-273.
McMAHON, F.G., CYRUS, J., RASKIN, P., STARR, I. (1989) Double-Blind
Placebo Controlled Ninety Day Toleration Study of Alitame in
Diabetics. Unpublished Report No. 102 from Central Research Centre,
New Orleans, LA, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
MONRO, A.M. CHVEDOFF, M., GREAVES, P., NACHBAUR, J., PERRAUD, J.,
(1982a) Three-Month Oral Toxicity Study in Rats plus 1-Month
Withdrawal. Unpublished Report No. 81-086 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MONRO, A.M., CHVEDOFF, M., PERRAUD, J., GREAVES, P., NACHBAUR, J.,
(1982b) Alitame: Three-Month Oral Toxicity Study in Dogs plus 1-Month
Withdrawal. Unpublished Report No. 81-106 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
PEARSON, D.L., GAYNOR, B.J., SWINDELL, A.C. (1982) General
Pharmacology Report on Alitame. Autonomic, Gastro-intestinal, Renal
and Metabolic Studies. Unpublished Report. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J. (1983a) Alitame: Foetotoxicity Study in
Rats (FDA Segment II). Unpublished Report No. 82-154 from Laboratoires
Pfizer Centre de Recherche, 37400 Ambroise, France. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J., SENKOWSKI, S. (1983b) Alitame:
Foetotoxicity Study in Rabbits (FDA Segment II). Unpublished Report
No, 83-026 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
PFIZER CENTRAL RESEARCH. (1986) Alitame: Metabolism and Disposition.
Unpublished Report from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., BROXUP, B., NOVEROSKE, J.W. (1993a) An Acute Study of
the Potential Effects of Orally Administered Alitame on Behaviour in
Rats. Unpublished Report No. 97148 form Bio-Research Laboratories Ltd.
Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W. (1993b) A
13-Week Dietary Study of the Potential Effects of Alitame on Behaviour
and Neuromorphology in Rats. Unpublished Report No. 97149 form
Bio-Research Laboratories Ltd. Senneville, Quebec, Canada. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W., (1993c) A
Developmental Neurotoxicity Study of Alitame Administered in the Diet
of Rats. Unpublished Report No. 97150 from Bio-Research Laboratories
Ltd. Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT. USA.
SQUIRE, R.A., LEVITT, M.H. (1975). Report of a workshop on a
classification of specific hepatocellular lesions in rats. Cancer
Res., 35: 3214-3223.
STADNICKI, S.W., PUNZALAN. E.R., FIGDOR, S.K., ROESLER, A.R., ESTES,
P.C., LEVINSKY, H.V. (1986a) b-Isomer of Alitame: Three-Month
Feeding Study in Study CD-I Mice. Unpublished Report No. 86-580-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
STADNICKI, S.W., SANTACROCE, E.M., FIGDOR, S.K., ROSS, P.E.,
ESTES, P.C., LEVINSKY, H.V. (1986b) b-Isomer of Alitame: Three-Month
Feeding Study in Long-Evans Rats. Unpublished Report No. 86-580-01
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
WHITBY, B.R., POWLES, P., LEWSLEY, R., FERNYHOUGH, P., MARSLAND, R.
(1994) 14C-Alitame: Whole-body autoradiography and microhistoauto-
radiography in the rat. Unpublished Report No. 727/5-1011 from
Hazleton Europe, Harrogate, UK. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
In a study in lactating dams, metabolites in milk were alanine
tetramethylthietane amide sulfoxide (CP-58,290) and its acetylated
form (54.4%), alanine tetramethylthietane amide (CP-57,207; 31.4%),
and unchanged alitame (1%) (Pfizer, 1986).
In a study to examine the effect of dose on metabolic pathways,
Long-Evans rats received a single oral dose of 10, 100 or 1000 mg/kg
bw 14C-alitame and urine and faeces were collected at 24-hour
intervals over 3 days. Urinary metabolites were analyzed by HPLC.
There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment, but the
metabolic profile was essentially unchanged (Pfizer, 1986).
In a study to compare the metabolic fate of D-alanine as either
free alanine or when incorporated into alitame, Long-Evans rats were
administered 14C-D-alanine or 14C-alitame (1-14C-D-alanine
label) by oral gavage and exhaled 14CO2 was measured. While
14CO2 was extensively exhaled following administration of
D-alanine, no 14CO2 was expired after administration of
14C-alitame, demonstrating the stability of the D-alanine
tetramethylthietane amide bond to metabolic hydrolysis (Pfizer, 1986).
2.1.2.3 Dogs
In a study to examine metabolites in plasma, groups of 3 male and
3 female beagle dogs received a single oral dose of 5 or 25 mg/kg bw
14C-alitame. Plasma samples were taken at various times and the
metabolites in plasma identified by HPLC. In both male and female
dogs, plasma radioactivity peaked at 2-3 h. The major metabolite was
alanine tetra-methylthietane amide sulfoxide (CP-58,290; 80%), with
10% in the acetylated form. Approximately 5% was unchanged alitame.
The remainder was alanine tetramethylthietane amide (CP-57,207), its
sulfone derivative (CP-57,254) and the acetylated forms of these
compounds. The metabolite profile was very similar to that seen in the
rat (Pfizer, 1986).
In a study to examine the metabolites in urine, a group of 3 male
and 3 female beagle dogs received a single oral dose of 14C-alitame,
and 24-hour urine and faeces collected. Urinary metabolites were
analyzed by HPLC. In males, the major metabolite was alanine
tetramethylthietane amide sulfoxide (CP-58,290; 80%), including 9.5%
as the acetylated form. Approximately 5-15% of the urinary
radioactivity was unchanged alitame (Pfizer, 1986).
In a study to examine metabolites in faeces, a male beagle dog
received a single oral dose of 10 mg/kg bw 14C-alitame followed
several weeks later by a dose of 500 mg/kg bw. A female beagle dog
received a single oral dose of 500 mg/kg bw 14C-alitame Faecal
samples were collected over 24 h and analyzed by HPLC. Faeces
contained 18-40% of the administered radioactive dose over the 24 h
period. Faecal radioactivity was a mixture of unchanged alitame
(CP-54,802) and alanine tetramethylthietane amide (CP-57,207).
Together these two products accounted for approximately 90% of the
total radioactivity (Pfizer, 1986)
In a study to examine the effect of dose on metabolic pathways,
2 male and 2 female beagle dogs received a single oral dose of 10 or
500 mg/kg bw 14C-alitame and urine and faeces were collected in
24-hour intervals over 3 days. Urinary metabolites were analyzed by
HPLC. There was a slight decrease in the percentage of administered
radioactivity excreted after the high-dose treatment than after the
low-dose treatment, but the metabolite profile was essentially
unchanged (Pfizer, 1986).
2.1.2.4 In vitro studies
The stability of alitame in gastric juices was examined to
clarify whether the cleavage of aspartic acid is initiated in the acid
environment of the stomach, and whether conversion of alitame to the
ß-isomer might occur under these conditions. Thus, alitame was
incubated in simulated gastric juices at 38°C to determine the degree
of hydrolysis. Controls were water and simulated gastric juices
(boiled). Samples were taken over 24 h and analyzed by HPLC. Alitame
was relatively stable, with only slight evidence of degradation after
prolonged incubation for 24 h, which was not considered to be due to
enzyme activity. Similarly, the small increase in ß-isomer observed in
both treated and control samples was not considered to be due to
enzyme activity (Pfizer, 1986).
The stability of alitame in rat intestinal homogenate was
examined in order to test the capacity of the rat small intestine to
metabolize alitame. The small intestine from male rats was flushed
with saline and homogenized in phosphate buffer at pH 7.4. The
homogenate was incubated with 500 mg alitame/ml at 37°C and the
reaction stopped by the addition of 0.25 tool/litre NaOH. Cleavage of
the peptidic bond of alitame was measured by the formation of alanine
tetramethylthietane amide (CP-57,207) and was dependent on both the
substrate concentration and time of incubation. The percentage
conversion to the amide ranged from 13-21% after 10 minutes of
incubation. This demonstrated the ability of intestinal homogenate
to cleave the peptidic bond of alitame. The reaction was also
demonstrated to be enzyme-mediated in a second experiment in which
it was shown that the extent of cleavage was dependent on the
concentration of homogenate. Little evidence of further metabolic
steps, such as sulfur oxidation or acetylation, was noted (Pfizer,
1986).
2.1.3 Effects on liver enzymes
2.1.3.1 Rats
In a study to compare the rat liver enzyme inducing properties of
alitame with those of butylated hydroxytoluene (BHT), groups of 4 male
rats received oral doses of either BHT (100 or 500 mg/kg bw), alitame
(100 or 500 mg/kg bw) or phenobarbital (50 mg/kg bw) daily for 5 days.
At 15 h following the last dose, animals were killed and livers
removed. Liver homogenates were assayed for O-demethylase, cytochrome
c reductase, and P-450 activity. Significant enzyme induction was
noted with BHT, but alitame showed no effect at the same dose levels
(Pfizer, 1986).
In a further study to examine the liver enzyme inducing potential
of alitame and its metabolites, groups of Sprague-Dawley rats received
single oral doses of either alitame (up to 2000 mg/kg bw), alanine
tetramethylthietane amide (CP-57,207) or its sulfoxide (CP-58,290; 100
or 500 mg/kg bw), or phenobarbital (50 mg/kg bw/day) for 5 days. At
15 h after the last dose, animals were killed and livers removed.
Liver homogenates were prepared and assayed for O-demethylase. Slight
induction of demethylase activity was noted with both alitame and its
metabolites. At 500 mg/kg bw, enzyme activity was approximately twice
the low baseline activity observed in controls with either alitame or
its metabolites (Pfizer. 1986).
In a recent detailed study, the hepatic enzyme-inducing activity
of alitame was examined in rats following gavage administration (pilot
study) and dietary administration (main study). In the pilot study,
groups of 5 male and 5 female Sprague-Dawley rats were administered
alitame daily by gavage for 7 days at dose levels of 0, 500 or
1000 mg/kg bw/day. Animals were killed on day 8, the livers excised,
sections taken for histology and electron microscopy, and the
remainder of the liver homogenized. Positive control animals were
given ß-naphthoflavone (80 mg/kg bw, i.p., 3 days), phenobarbitone
(80 mg/kg bw, i.p., 3 days), or dexamethasone (100 mg/kg bw, i.p., 3
days). In the main study, groups of 20 male and 20 female Sprague-
Dawley rats were fed a diet containing alitame at concentrations of
0, 0.3% or 1% for 4 weeks. An additional 20 males and 20 females acted
as pair-fed controls and received the basal diet at the level of
intake of the 1% alitame group. After 28 days, 10 males and 10 females
from each group were sacrificed, sections of liver taken for histology
and electron microscopy and the remainder of the liver homogenized.
Kidney and brain weights were also recorded. The remaining 10 males
and 10 females were withdrawn from the alitame diet and kept for a
further 14 days before being sacrificed. In each of the above studies,
the liver homogenate was used to determine protein content, cytochrome
P-450 content, NADPH-cytochrome P-450-dependent reductase activity,
ethoxyresorufin O-deethylase (EROD) activity, pentoxyresorufin
O-depentylase (PROD) activity, erythromycin N-demethylase (ERD)
activity, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
In the pilot study, the positive controls exhibited significant
increases with respect to hepatic microsomal enzymes and all caused an
increase in relative liver weight. Brain and kidney weights were
unaltered. Alitame at 500 or 1000 mg/kg bw/day for 7 days had only
minor effects by comparison. At 1000 mg/kg bw/day, cytochrome P-450
levels were 140% of controls, and EROD, PROD and ERD activities
increased 3.6-fold, 6.3-fold, and 4.7-fold, respectively. Examination
of the microsomes by SDS-PAGE provided similar evidence of low levels
of protein induction corresponding to the above enzymes. Light
microscopic examination revealed hepatocellular hypertrophy at both
dose levels, and some evidence of periportal inflammation and necrosis
in a few sections. Electron microscopy revealed an increase in rough
and smooth endoplasmic reticulum at both dose levels.
In the main study, growth curves indicated a reduced body-weight
gain in alitame-treated animals compared to controls. This effect
was more apparent in females than in males and appeared to be
dose-related. While the effects may not have been statistically
significant, the curves indicated a clear biologically significant
decrease in body-weight gain in females at both the 0.3 and 1% alitame
dose levels. There was also an increase in relative liver weight in
females at both dose levels which was dose-related. Relative brain and
kidney weights were unaltered.
The parameters used to measure hepatic enzyme induction were also
increased in a dose-related manner compared to controls and pair-fed
animals. At the 0.3 and 1% dose levels, cytochrome P-450 levels
increased by 12 and 20% in females, and by 46 and 101% in males,
respectively. The activity of EROD was increased slightly in males and
females while there was a significant increase in the activity of PROD
in males and females at 0.3% and 1% alitame. The activity of ERD was
only slightly increased in males at 1% alitame. Light microscopic
examination of the liver revealed centrilobular hypertrophy at both
dose levels, but more marked at the higher dose. Electron microscopy
revealed a dose-related increase in both rough and smooth endoplasmic
reticulum. Following the 14-day withdrawal period, the liver weight
changes were reversed, and none of the hepatic parameters was
significantly different from controls.
The results indicated that alitame, at both the 0.3% and 1% dose
levels, was a weak inducer of hepatic enzymes, which was accompanied
in females by a decrease in body-weight gain and an increase in
relative liver weight. The pattern of enzyme changes was essentially
similar to that caused by phenobarbitone, although quantitatively
smaller (Caldwell et al., 1994).
In a supplement to the above study, the cytochrome P-450
isoenzyme from the liver microsomes of alitame-treated rats were
separated by SDS-polyacrylamide gel electrophoresis and further
characterized by Western blot immunodetection. Following separation by
electrophoresis, the proteins were transferred to nitrocellulose
membranes and the blots either stained with antibodies to specific
cytochrome P-450 isoenzyme or, to allow visualization of total
protein, by staining with Amido Black solution.
The microsomes from alitame-treated rats showed an increase in
the band attributed to CYP2B (catalytic activity) at 52 kD which is a
protein also shown to be induced by phenobarbitone. The band at 56 kD
attributed to CYP1A1, shown to be induced by ß-naphthoflavone, was
conspicuous by its absence. The data further confirmed that the
hepatic changes produced by alitame were similar to those produced by
phenobarbitone (Caldwell & Reed, 1994).
2.1.3.2 Humans
In a 14-day study, 8 male subjects received alitame in gelatine
capsules as 4 daily oral doses totalling 15 mg/kg bw/day. This dose
was calculated to be 15 times the projected human exposure level,
assuming total sucrose replacement. Induction of microsomal enzyme
levels was measured by the rate of elimination of aminopyrine.
Measurements were taken before treatment, after 2 weeks of treatment,
and at 18 days post-treatment. Both plasma and saliva were collected.
Blood samples were taken at pre-test, after 1 and 2 weeks of
treatment, and at 7 and 18 days post-treatment for the analysis of
clinical chemistry and haematological parameters. On the 10th day of
treatment, a 24-hour urine sample was collected for the analysis of
metabolites. There was no adverse effects parameters were normal
during the course of the study.
The analysis of the aminopyrine elimination results indicated
that the saliva was a good indicator of plasma levels. When the
elimination rate constants and half-lives were compared, there was no
significant difference in aminopyrine kinetics from the pre-dose,
post-dose and post-withdrawal samples. Urine samples indicated the
presence of the major metabolites, alanine tetramethylthietane amide
(CP-57,207) and it sulfoxide (CP-57,290). Alitame was present only in
trace amounts (Caldwell et al., 1984).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of alitame was examined in both mice and rats
and the results are summarized in Table 1.
Table 1. Summary of acute toxicity studies with alitame.
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral > 5000 Levinsky et al. (1983)
Rat M&F oral > 5000 Levinsky et al. (1983)
Groups of 10 male and 10 female mice (Crl:COBS CD-1 (ICR) BR) and
10 male and 10 female Sprague-Dawley (Crl:COBS CD (SD) BR) rats were
administered alitame (purity 97.43%) by gavage at a single dose level
of 5000 mg/kg bw. Animals were observed for clinical signs over a
14-day period after treatment, during which food consumption and body
weights were recorded. Animals were examined for gross pathological
changes at day 14 after treatment.
In mice, there were no deaths during the study and no clinical
signs apart from an increase in soft faecal material at 2.5 h
post-treatment. Faeces were normal by day 2 post-treatment. During the
observation period, there was normal food consumption and steady
body-weight gain.
In rats, there were no deaths during the study and no clinical
signs during the first hour post-treatment, but from then onwards all
animals displayed an increased level of activity for 1-2 days. All
rats were jittery and exhibited a tendency for exophthalmia, with
females noticeably more affected than males. Females exhibited
incoordination in their movements but were not ataxic. By 24 h, 7/10
males were essentially asymptomatic, and by day 2, all males and
females were asymptomatic. During the observation period, food
consumption was normal, and there was a steady body-weight gain. There
were no gross pathological changes on day 14 in 20/20 mice and in
19/20 rats, with one rat having an enlarged liver (Levinsky et al.,
1983).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 2-month study, groups of 10 male and 10 female Crl:COBS
(CD-1) (ICR) BR mice were fed a diet containing alitame (purity
98-101%) at concentrations of 0, 1, 2, or 5%, equivalent to 0, 1500,
3000 or 7500 mg/kg bw/day.
Animals were observed for clinical signs, and food consumption
and body weights were recorded throughout the study period. At the end
of the study period, liver and kidney weights were recorded and
tissues from the major body organs were taken for histopathology.
There were no deaths and no clinical signs of toxicity throughout
the study period. Body weights of the intermediate- and high-dose
animals decreased slightly on days 1 and 2, but recovered quickly.
There was no treatment-related effect on body weight for the remainder
of the study. There was no change in food consumption. Both absolute
and relative liver weights increased in a dose-related manner compared
to controls. The increase in absolute liver weight was 20, 40 and 67%
in males and 20, 35 and 66% in females in low-, intermediate- and
high-dose animals, respectively. Histopathological examination of the
liver revealed a dose-related increase in centrilobular hypertrophy.
Electron microscopy of liver tissue indicated a dose-related increase
in proliferation of smooth endoplasmic reticulum. No degenerative
hepatocellular changes were noted. Effects were noted at all dose
levels and a NOEL was not established in this study (Holmes et al.,
1983a).
2.2.2.2 Rats
In a range-finding study, groups of 5 male and 5 female Crl:COBS
CD-1 (SD) BR rats (5/sex/dose) were fed a diet containing alitame
(purity 98-101%) at concentrations of 0, 1, 2 or 5%, equivalent to
500, 1000, or 2500 mg/kg bw/day for 15 days. Animals were examined for
clinical signs of toxicity, and food and water consumption was
monitored throughout the study period. At the end of the study period,
blood samples were taken for haematological and clinical chemistry
examination; tissues from the major body organs were taken for
histopathology; tissues from liver and kidney were taken for electron
microscopy; and tissue from liver for histochemistry. There were no
deaths throughout the study, and no clinical signs of toxicity. A
slight body-weight loss was noted at the 2% and 5% dose levels on day
3 but body-weight gain was normal thereafter. There was a dose-related
decrease in food consumption in all treated groups on days 1-2
(especially females at the 2% dose level and in both sexes at the 5%
dose level) possibly due to unpalatability of the diet. Water
consumption was similarly decreased. There was a slight, but not
significant, increase in plasma cholesterol and protein levels in all
treated groups. Haematological parameters were normal. There was a
dose-related increase in absolute and relative liver weights in
males and females but no microscopic abnormalities were noted.
Histochemistry indicated an increase in glucose-6-phosphate
dehydrogenase and NADPH2 diaphorase in liver tissue slices. Electron
microscopy indicated a dose-related increase in smooth endoplasmic
reticulum in liver tissue and, in some cases, an increase in lysosomes
and lipid vacuoles. Effects were observed at all dose levels and a
NOEL was not established in this study (Chvedoff et al., 1981a).
In a 5-week study, groups of 10 male and 10 female Long-Evans
rats were fed a diet containing alitame (purity 98.2%) at dose levels
of 0, 10, 50 or 100 mg/kg bw/day. Animals were observed daily and body
weights and food consumption recorded. Blood samples were taken from
the orbital sinus prior to treatment and at the end of the study
period, and clinical chemistry and haematological parameters examined.
After 5 weeks, the induction of liver microsomal enzymes was measured
in vitro and animals were examined for gross pathology. Tissues from
the major organs were prepared for histopathology.
There was no treatment-related effect on body-weight gain or food
consumption. The clinical chemistry, haematological data and
histopathology were unremarkable. It was not possible to determine
whether there was proliferation of smooth endoplasmic reticulum
because of the presence of excess glycogen clue to lack of fasting
before sacrifice. Induction of 0-demethylase was determined by the
release of p-chlorophenol. A 30% increase in enzyme induction
occurred at the high-dose level which was not statistically
significant. Nevertheless, on the basis of the biological significance
of this increase, the NOEL in this study was 50 mg/kg bw/day
(Holmes et al., 1983b).
In a 3-month study, groups of CrI:COBS-CD(SD)BR strain rats
(15/sex/dose) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0.5, 1.0 or 2.0%, equal to 380, 830 or 1500 mg/kg
bw/day. Animals were observed throughout the study period and body
weights, food and water consumption recorded. Blood samples were taken
for examination of clinical chemistry and haematology parameters at
the end of the study. Urinary parameters were also examined. At the
end of the study, gross pathological examinations were performed and
tissues from the major organs were taken for histopathology. A group
of 5 males and 5 females was observed for a further 1 month following
the treatment period.
There were no deaths throughout the study and no clinical signs
of toxicity. There was a dose-related decrease in body-weight gain in
both males and females in all treated groups. The decreases at 3
months in males/females were 4.5%/1.7%, 4.5%/3.3% and 5.3%/8.5% at
low, intermediate and high dose. Food consumption was slightly lower
in high-dose males, but not during the withdrawal period. Water
consumption was normal. The clinical chemistry analysis revealed a
significant increase in plasma cholesterol at all dose levels in males
and females. The increase was 23% at low dose and 42% at high dose.
Levels returned to normal during the 1-month withdrawal period. There
was also a dose-related decrease in plasma triglyceride levels in
males, which returned to normal levels during the withdrawal period.
The levels of cytochrome P-450 and b5 were increased in a
dose-related manner at the end of the treatment period. The activity
of microsomal N-demethylase was also increased in a close-related
manner. The changes were reversed during the withdrawal period except
for the cytochrome P-450 levels in males. Haematological parameters
were within the normal range.
Relative and absolute liver weights were increased in males at
the high-dose level, while relative liver weight was increased at all
doses in females. The liver weights returned to normal during the
withdrawal period.
Histopathology of the liver revealed centrilobular hypertrophy in
all high-dose females and in 5/10 high-dose males. Histochemical
analysis of the livers indicated increased activity of G-6-P
dehydrogenase, gamma-glutamyl transpeptidase and NADPH-diaphorase in
the high-dose animals. NADPH-diaphorase activity was also increased in
intermediate-dose females. All changes were reversible during the
withdrawal period. Electron microscopy revealed an increased
proliferation of smooth endoplasmic reticulum at all dose levels.
After the withdrawal period, the livers of female rats were normal
while those of the males still showed mild proliferation of smooth
endoplasmic reticulum at the high-dose level. The histopathological
and enzymatic changes were indicative of a reversible adaptive
response. There was no evidence of focal hyperplasia or hepatocellular
toxicity. Effects were noted at all dose levels and a NOEL was not
established in this study (Monro et al., 1982a).
In a one-year study, groups of 40 male and 40 female Long-Evans
rats were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.1, 0.3, or 1.0% (equivalent to 50, 150 or
500 mg/kg bw/day). Two other groups were fed a diet containing alitame
adjusted to achieve a nominal intake of 10 or 30 mg/kg bw/day. At 6
months, 20 male and 20 female animals at each of the dose levels were
removed from treatment. Of these, half were sacrificed while the other
half were returned to normal untreated diet. Animals were observed
throughout the study period and body weights, food and water
consumption recorded. Ophthalmoscopic examinations were performed in
all animals before necropsy. Blood samples were taken at 6, 9 and 12
months for clinical chemistry and haematology. Urinalysis parameters
were also examined. At the end of the study period, all animals were
examined for gross pathology, and tissues from major organs were taken
for histopathology.
There were no deaths throughout the study and no clinical signs
of toxicity. Ophthalmoscopic examination at 6 months and 1 year was
unremarkable. There were no treatment-related effects on body-weight
gain or on clinical pathology parameters. Cytochrome P-450 levels were
measured at 6 months and were significantly increased at the high-dose
level only (21% in males and 33% in females). The only organ weight
change noted was an increase in relative liver weight at the high-dose
level at 6 months, with a 12.1% increase in males and 16.0% increase
in females. At 12 months, liver weight increases at the high-dose
level were 8.5% and 11.7% in males and females respectively, compared
to controls. In animals withdrawn from treatment at 6 months, liver
weights had returned to normal levels at 12 months. The only
microscopic finding was a retention of glycogen in both control and
treated groups. This change was not considered to be treatment-
related. The NOEL in this study was 0.3% in the diet, equal to
145 mg/kg bw/day in males and 185 mg/kg bw/day in females
(Holmes et al., 1985a).
A study was conducted in Sprague-Dawley rats which included
dietary administration during mating, in utero exposure, exposure
during lactation, and dietary exposure for one year post-weaning.
Groups of 20 male and 20 female Sprague-Dawley rats were selected from
the F1 offspring of rats treated 4 weeks prior to mating and during
gestation and lactation (Levinsky et al., 1992). The groups were fed
a diet containing alitame (purity 99.3%) at concentrations of 0 (two
control groups), 0.1, 0.3 or 1% for one year. The animals were
observed daily for clinical signs and body weights and food
consumption were recorded weekly. Other measurements and observations
included ophthalmological examinations (pre-test and 6 and 12 months),
serum chemistry, haematology and urinalysis measurements at 6 and 12
months. Tissues were collected from all animals which died or became
moribund during the study. At the end of the study, the animals were
sacrificed and necropsied and tissues prepared for light microscopy.
Organ weights for liver, kidney, adrenal glands, testes and ovaries
were recorded.
Twenty rats died or were sacrificed during the study. The deaths
were distributed evenly between the groups although deaths in males
predominated over those in females by four to one. Of the 20 early
necropsies, 4 were due to neoplasms (1 control, 2 low-dose and 1
intermediate-dose rats). The most common non-neoplastic cause of death
(7/16) was described as 'unexplained death/heavy rats' and was not
accompanied by any remarkable clinical signs.
Body-weight measurements began at birth for this study, and at
weaning (day 21) the mean body weights for the intermediate- and
high-dose animals compared to the control groups were -9% and -21%,
respectively. Following selection of animals in groups for the 1-year
study, the body weights expressed as percent of control for the
intermediate-dose level were -9.5% (M) and -8.8% (F), and for the
high-dose level -16.3% (M) and -13.5% (F). Over the course of the
study, the initial body weight differentials for males at the
intermediate- and high-dose levels diminished by about 50%. For the
first 80 days, all groups of males appeared to gain weight at similar
rates apart from the control group 1. For the remaining period of the
study, the weight gains were similar for all groups. Over the 1-year
period, the body-weight difference for the intermediate-dose males
disappeared and the difference for the high-dose group was 6.6% and
11.1% compared with control groups 1 and 2, respectively.
For females, over the course of the study, the -8.8% difference
at the intermediate-dose level disappeared and the low-dose level
gained weight relative to the control group 1. The females in the
control group 1, like the males, showed a slower growth rate. The
high-dose females, unlike the males, did not recover their initial
body weight difference (-13.5%) and after 200 days, the body weight
difference began to increase, reaching -20% relative to control
group 1.
Ophthalmoscopic examination at 6 and 12 months was unremarkable.
There were no treatment-related changes in clinical chemistry,
haematology or urinary parameters apart from a 40-60% decrease in
serum triglyceride levels in the high-dose females, which appeared to
be related to the decreased weight gain in the high-dose females. The
high-dose males showed decreased serum triglyceride values at 6
months, but not at 12 months.
There were no gross pathological findings in any group. The organ
weight changes observed were a decrease in absolute liver weight in
the high-dose females, and an increase in relative liver, kidney and
ovary weight as a result of the decrease in mean body weights in the
high-dose females.
Histopathology revealed hepatocellular hypertrophy in the
high-dose males (13/20) and females (4/20). These changes were not
accompanied by an increase in liver weight. Other liver changes
observed could not be related to treatment. The NOEL in this study,
based on body and organ weight changes, was 0.3% in the diet, equal to
173 mg/kg bw/day in males, and 205 mg/kg bw/day in females (Fisher
et al., 1992).
2.2.2.3 Dogs
In a two-week range-finding study in dogs, groups of 2 male adult
beagle dogs were fed alitame (purity 98-101%) in the diet at
concentrations of 1, 2 or 5%, equivalent to 250, 500 or 1250 mg/kg
bw/day. There were no control animals. Animals were observed
throughout the study for clinical signs of toxicity and histopatho-
logical examinations were performed on lungs, kidney, liver and heart
after 14 days. A section of liver was examined by electron microscopy.
There were no deaths during the study and no clinical signs of
toxicity. There was no treatment-related effect on body weight.
Systolic blood pressure and heart rate were normal at all dose levels.
Clinical chemistry parameters were normal apart from a slight increase
in alkaline phosphatase activity at all dose levels which increased in
the second week. Urinalysis parameters were normal. Haematological
parameters indicated a slight decrease in haemoglobin concentration,
RBC count and packed cell volume in treated animals, but the changes
were within normal limits and not definitely related to treatment.
Relative liver weights were within normal limits. Microscopic
examination revealed an increase in cytoplasmic vacuolation in the
liver of 5/6 animals. Electron microscopic examination revealed a
dose-related increase in proliferation of smooth endoplasmic
reticulum. Lipid vacuoles and lysosomes were present in the liver of
all animals but, in the absence of control animals, it could not be
determined whether the observed microsomal changes were treatment-
related (Chvedoff et al., 1981b).
In a 3-month study, groups of beagle dogs (4/sex in the low- and
intermediate-dose groups, and 6/sex in the control and high-dose
group) were fed a diet containing alitame (purity 98-101%) at
concentrations of 0, 0.5, 1, or 2%, equal to 0, 120, 220 or 490 mg/kg
bw/day. In the control and high-dose groups, 2 animals/sex were
transferred to normal diet for an additional month after the study
period. Animals were examined throughout the study period for clinical
signs of toxicity and body weight was recorded weekly. At various
times, clinical chemistry, urinary and haematology parameters were
measured. Tests of aminopyrine clearance were measured on days 1 and
63. Ophthalmological examinations were performed on high-dose animals.
Tissues were examined histopathologically at the end of 3 months.
Liver histochemistry, electron microscopy and content of microsomal
cytochrome P-450 and b5 were also performed.
There were no deaths throughout the study and no clinical signs
of toxicity. Food intake was normal although there was a slightly
decreased weight gain in high-dose animals, particularly males.
Cardiovascular parameters were normal. Ophthalmological examination of
high-dose animals was unremarkable. Haematological parameters were
within the normal range. Clinical chemistry revealed a dose-related
increase in plasma alkaline phosphatase when measured at 1, 2 and 3
months. The increase at the high-dose level was 400% at 3 months.
After the recovery period, levels in the high-dose group were reduced
but still higher than control levels.
At the end of the 3-month treatment, the levels of cytochrome
P-450 and b5 were increased in a dose-related manner. After the
1-month withdrawal period, cytochrome P-450 and b5 in the high-dose
group were reduced to near control levels. Induction of the liver
microsomal enzyme system was also demonstrated in the dogs of the
reversibility groups (control and high dose) following administration
of a single i.v. dose of aminopyrine (15 mg/kg bw) on days 1 and 63 of
alitame treatment, with blood levels measured at 0, 1, 1.5, 2, 3 and
5 h. The plasma levels reflected the induction of a liver microsomal
demethylase. On day 1, there was little difference between treated and
control animals. On day 63, there was a marked difference between
control and treated animals.
Relative and absolute liver weights were increased in all
treatment groups. Absolute liver weight was increased by 36% and 11%
at high and low dose, respectively. Histopathology of the liver and
other organs was unremarkable. Histochemical examination revealed an
increase in G-6-P dehydrogenase activity in all treated groups except
the low-dose females. NADPH-diaphorase activity was increased in
high-dose animals. All levels returned to normal during the recovery
period, except for an increased G-6-P phosphatase activity in
high-dose males. Electron microscopy revealed an increase in
proliferation of smooth endoplasmic reticulum in all groups. This
increase was reversed in females but persisted in males during the
recovery period. The liver changes appeared to be indicative of an
adaptive response due to induction of microsome oxidative metabolism.
Effects were observed at all dose levels and a NOEL was not
established in this study (Monro et al. 1982b).
In an 18-month study, groups of 4 male and 4 female beagle dogs
were fed a diet containing alitame (purity 98-101%) at dose levels of
0, 10, 30, 100 or 500 mg/kg bw/day. Animals were examined throughout
the study period for clinical signs of toxicity, and body weights were
recorded at regular intervals. Ophthalmoscopy was performed on animals
at 6-month intervals. Electrocardiographic tracings were performed at
regular intervals. Clinical chemistry, urinary and haematological
parameters were measured at regular intervals. Aminopyrine clearance
was measured in control and intermediate dose animals at pre-test and
during weeks 3, 5, 7, 9, 40 and 74, and in the high-dose group at 39
and 73 weeks. Gross pathological examination was performed at 18
months together with histopathological examination of a wide range of
tissues. There were no treatment-related effects on survival and no
clinical signs of toxicity. Ophthalmoscopic examinations and
cardiovascular parameters were normal. Body weights were comparable
between groups although body-weight gain in high-dose females was
slightly less than control animals between weeks 40 and 70. Food
consumption was normal in all groups. All of the clinical pathology
parameters were normal except for an increase in serum alkaline
phosphatase activity in high-dose males and females. The alkaline
phosphatase rise was not uniform at high dose, with some dogs showing
little or no change.
Analysis of the urinary metabolites over a 2-day period at week
10 showed that the excretion rate was proportional to the dose
administered, indicating a dose-related systemic exposure. Measurement
of aminopyrine clearance, as an indicator of the induction of the
liver microsomal enzyme system, revealed a slight decrease in t1/2
in males and females at 100 mg/kg bw/day at the 2-week period. The
significance of this result is unclear, and may be due to the high
t1/2 in the animals pre-dosing. In males, there appeared to be a
gradual increase in t1/2 over the study period but again the result
is difficult to interpret. Overall, the results at 100 mg/kg bw/day do
not appear to indicate any enzyme induction. At 500 mg/kg bw/day,
after 39 or 73 weeks, significant microsomal enzyme induction was
apparent.
The only gross pathological change observed was a moderate
increase in absolute liver weight of 31% and 20% in high-dose males
and females, respectively. This increase was statistically significant
in males only. Histopathology was unremarkable in the treated animals.
A slight increase in smooth endoplasmic reticulum was found in the
livers of high-dose animals. The NOEL in this study was 100 mg/kg
bw/day (Holmes et al., 1985b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 2-year carcinogenicity study, groups of 50 male and 50
female Crl: CD-1 (ICR) BR mice were fed a diet containing alitame
(purity, 98-101%) at concentrations of 0 (2 groups), 0.1. 0.3 or 0.7%.
Animals were observed throughout the study period. Body weight and
food consumption were recorded weekly for 13 weeks and then at
N-weekly intervals for the remainder of the study. At the end of the
study period, animals were sacrificed and subjected to gross
pathological examination and organs weights recorded. Tissues from all
of the major organs were prepared for histopathological examination.
Tumour incidence was recorded.
There was no treatment-related effect on survival. The survival
rate for controls, low-, intermediate-, and high-dose groups in
males/females was 52/24%, 22/26%, 22/38%, 32/36% and 38/26%,
respectively. There were no clinical signs of toxicity in the treated
groups. Body-weight gain and food consumption were similar for all
groups. Gross pathological examination revealed a significant increase
in absolute and relative liver weight at high dose in males only.
Absolute liver weight increased by 59% and 46% compared to the two
control groups, while relative liver weight increased by 58% and 38%
compared to the two control groups. Slight to mild centrilobular
hepatocellular hypertrophy was found in 12 male animals in the
high-dose group. At the intermediate-dose level, absolute and relative
liver weights of males were also increased. Absolute liver weight was
increased by 41% and 30% compared to the two control groups, while
relative liver weight was increased by 39% and 21% compared to the two
control groups. While the increases were not statistically
significant, nevertheless the Committee considered them to be of
biological significance at this dose level. No hepatocellular
hypertrophy was apparent at the intermediate- and low- dose levels.
There were no other histopathological changes, or any
treatment-related increase in tumour incidence. Under the conditions
of this assay, there was no evidence of carcinogenicity in mice
induced by alitame in the diet. The NOEL, based on the biologically
significant liver weight changes in males, was 0.1% in the diet, equal
to 125 mg/kg bw/day for males and 155 mg/kg bw/day for females (Holmes
et al., 1985c).
2.2.3.2 Rats
A carcinogenicity study was conducted with dietary administration
and in utero exposure to alitame in Long-Evans rats. Groups of 50
male and 50 female Long-Evans rats (strain Blu:(LE)BR) were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for 2 years.
Animals used in this study were taken from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Holmes et al., 1986b). Animals were observed throughout the study
period for clinical signs of toxicity. Body weights and food
consumption were recorded weekly. Ophthalmoscopic examinations were
carried out at 12, 18 and 24 months. Clinical chemistry and
haematological parameters were measured in 10 animals/sex/dose at 6,
12, 18 and 24 months. At the end of the study period, animals were
sacrificed and subjected to gross pathological examination. Organ
weights were recorded. Tissues from all the major organs were prepared
for histopathological examination.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examinations
revealed no treatment-related effects. The survival rate at 104 weeks
for the control, low, intermediate, and high-dose groups was for
males/females 40/46%, 34/40%, 30/42% and 28/34%, respectively. There
was a slight decrease in survival in treated animals compared to
controls but this was not necessarily treatment-related.
In the second year of the study, there was a dose-related
difference in body weights for males between control animals and those
in the high- and intermediate-dose groups. In females, the body weight
of high-dose animals was lower than controls at the start of the study
and continued to be significantly lower throughout the study.
Intermediate-dose females also had a slightly lower body weight than
controls which became more pronounced in the 2nd year of the study.
There was no treatment-related effect on food consumption. These
differences in body weights were significant only at high dose.
There were no treatment-related changes in clinical chemistry or
haematological parameters. Gross pathological findings were generally
unremarkable. There was a slight non-significant increase in relative
liver weight at the high and intermediate dose levels in both males
and females. Microscopic examination of the tissues did not reveal any
increase in the incidence of neoplastic lesions. In the high- dose
group, 5 males and one female showed mild centrilobular hepatocellular
hypertrophy. The incidence of proliferative liver lesions in females
is shown in Table 2. The first appearance of hyperplastic nodules in
the liver of females was at week 96. Survival at this time in control,
low-, intermediate- and high-dose females was 64%, 56%, 50% and 50%,
respectively. Alitame, under the conditions of this assay, showed no
evidence of carcinogenicity in rats. The NOEL, based on body-weight
changes in both males and females, was 0.3% in the diet, equal to
130 mg/kg bw/day for males and 160 mg/kg bw/day for females
(Holmes et al., 1986a).
Table 2. Incidence of proliferative liver lesions in female rats
(Holmes et al., 1986a).
Control 0.1% 0.3% 1%
No. of livers examined 50 46 48 49
Focal nodular hyperplasia 1 0 3 9
Eosinophilic foci 4 0 3 11
A re-examination of the pathology slides of the 2-year
carcinogenicity study in Long-Evans rats (Holmes et al., 1986a) was
conducted on two subsequent occasions. At the first re-examination,
all available liver slides from all groups of female rats were
examined. The reported incidence of proliferative lesions is shown in
Table 3. There was no evidence of hepatocellular carcinomas in females
(Farrell, 1987).
Table 3. Incidence of proliferative liver lesions in female rats
(first re-examination; Farrell, 1987)
Control 0.1% 0.3% 1%
No. of livers examined 50 49 50 50
Hepatocellular adenoma 0 0 1 1
Eosinophilic foci 3 3 6 12
Hyperplasia 0 4 3 6
(focal and multifocal)
In the second re-examination of the pathology slides, slides
showing proliferative changes in female rats in either the original
study or in the first re-examination were examined using a revised set
of diagnostic criteria for hepatic proliferative lesions. The
incidence of these lesions is shown in Table 4. There was no evidence
of hepatocellular carcinomas in females (Hardisty et al., 1994).
Table 4. Incidence of proliferative liver lesions in female rats
(second re-examination; Hardisty et al., 1994).
Control 0.1% 0.3% 1%
No. of livers examined1 8 7 16 25
Hepatocellular adenoma 0 0 2 12
Hepatocellular hyperplasia 0 0 0 3
Eosinophilic cell foci 2 3 11 18
1 In this re-examination, only slides identified in the original
report and in the first re-examination as showing proliferative
lesions were examined.
Because of the low survival rate at 104 weeks in the Long Evans
rats (Holmes et al., 1986a), a second carcinogenicity study was
conducted with dietary administration and in utero exposure to
alitame in Sprague-Dawley rats. Groups of 70 male and 70 female
Sprague-Dawley rats were selected from the F1 offspring of rats
treated 4 weeks prior to mating and during gestation and lactation
(Levinsky et al., 1992). The groups were fed a diet containing
alitame (purity 99.3%) at concentrations of 0 (two control groups),
0.1, 0.3 or 1% for two years.
The animals were observed daily for clinical signs, and body
weights and food consumption were recorded weekly for the first
6 months and then every 4 weeks thereafter. Ophthalmological
examinations were performed pre-test and at 12 and 21 months; serum
chemistry, haematology and urinalysis measurements were taken at 6,
12, 18, 21 and 24 months. Tissues were collected from all animals
which died or became moribund during the study. At the end of the
study the animals were sacrificed and necropsied and tissues prepared
for light microscopy. Organ weights (liver, kidney, adrenal glands,
testes and ovaries) were recorded.
There were no clinical signs of toxicity or behavioural changes
which could be associated with treatment. Ophthalmoscopic examination
revealed no treatment-related effects. Survival rates were high for
the first 12 months (>93%) but by 18 months average survival was 65%
for males and 75% for females. Groups reached 50% survival between 18
and 19 months for males and between 20 and 21 months for females.
Survival rates at 24 months in the 2 controls, low-, intermediate- and
high-dose groups were for males/females 9/37%, 4/23%, 7/31%, 9/24% and
26/17%, respectively.
Body-weight measurement began at birth for this study, and at
weaning (day 21). The mean body weights for the intermediate- and
high-dose animals were -9% and -21%, respectively, compared to the
control groups. Following selection of animals into groups for the
2-year study, the body weights expressed as percent of controls at the
low dose were -4% (M) and -2% (F), at the intermediate dose -7% (M)
and -10% (F), and at the high dose -18% (M) and -16% (F). For males,
over tile course of the study, the initial body weight differential
decreased as the treatment group showed increased weight gain compared
to the controls. Body weights peaked at approximately 15 months
followed by a gradual decline in all groups, with the high-dose group
showing the greatest decline. In females, over the course of the first
few months of the study, the initial body weight differentials
decreased particularly in the low-and intermediate-dose groups, which
showed increased weight gain compared to controls. After 6 months,
weight gain in the low- and intermediate-dose groups was similar to
controls. In the high-dose group, however, there was a decrease in
weight gain compared to the controls, and by the end of the study,
body weight differential had increased to -32%. Food consumption may
have been slightly lower in high-dose males, but was similar in all
groups of females.
There were no treatment-related changes in haematology or
urinalysis parameters. Changes observed in clinical chemistry data
included a decrease in serum triglyceride values (50-60%) in females
at the intermediate-dose in the first 18 months, and in high-dose
females throughout the study. Cholesterol was also increased in female
rats at 6 months at the intermediate-dose level (28%), and at 6 and 12
months at the high-dose level (67%).
Significant organ weight changes were seen in both liver and
kidney. In males, there was a relative increase in both kidney and
liver weight at high dose. In females, there was an absolute decrease
in kidney and liver weights at both intermediate and high doses, and
a relative increase in kidney weight at high dose. These changes
accompanied the decrease in body weight in high-dose females.
There was also an overall trend for decreased body weight in
intermediate-dose females which was not statistically significant.
The relative testes weight was increased in high-dose males.
Neoplastic changes were observed in the following organs: kidney,
liver, pancreas, lung, heart, thymus, mesenteric node, adrenal cortex,
thymus, parathyroid, uterus, cervix, brain, spinal cord, Zymbal's
gland, skin, bone, skeletal muscle, abdominal cavity, and soft
tissues. There was no evidence that the incidence of these neoplasms
was treatment-related. In the liver, hepatocellular carcinomas were
reported in one control male and one high-dose male. There was no
reported incidence of hepatocellular adenomas. There were also no
treatment-related non-neoplastic changes. Alitame, under the
conditions of this assay, showed no evidence of carcinogenicity in
Sprague-Dawley rats. The NOEL, based on body-weight changes, was 0.3%
in the diet, equal to 230 mg/kg bw/day for males, and 250 mg/kg bw/day
for females (Fisher et al., 1993).
2.2.4 Reproductive toxicity studies
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (75/sex) were administered a diet containing alitame
(purity 98-101%) at concentrations of 0, 0.1, 0.3 or 1% for either 2
weeks (females) or 12 weeks (males) prior to mating. Alitame-
containing diet was continued throughout pregnancy and lactation. Of
the F1 pups, 50 pups/sex/dose level were selected for a 24-month
carcinogenicity study, a further 25/sex/dose level were raised to
maturity and mated to produce the F2a litters. Approximately 3 weeks
after weaning of the F2a litters, the F1 animals were again mated
to produce the F2b litters. Both F2a and F2b pups were exposed to
alitame via the milk and in the food.
In the F0 parental group, the body weight of males was similar
for all groups during the 12 weeks of treatment, while body weight and
food consumption of females were slightly depressed during the 2-week
treatment period at the high-dose level. There was no treatment-
related effect on fertility or gestation indices, and no abortions
were noted. There was no difference in the number of pups born alive
between treated and control groups in the F1 litters. There was no
treatment-related effect on day 4 pup survival rates or lactation
indices. F1 pup weights of treated animals were similar to those of
control animals on days I and 4 of lactation, but high-dose pups had
significantly lower body weights on days 7, 14 and 21. However, these
decreased body weights were not significantly different from the
historical control data.
In the F1 parental group, body weights were similar to control
except at the high-dose level where there was a slight decrease in
body-weight gain. There were no treatment-related changes in pregnancy
rates or gestation length for either F2a or F2b litters. There was
no difference in the number of pups born alive between treated and
control groups in the F2a or F2b litters. Survival indices for F2a
and F2b were normal for all groups on days 1 and 4 of lactation.
F2a pup weight for all treated groups was similar on days 1,
4 and 7 of lactation but a significant decrease was noted in the
high-dose animals on day 14 and in all treated groups on day 21
compared to controls. The body weights were still within the
historical control data range. The F2b pups weight at the high-dose
level was significantly lower than controls throughout lactation,
while at the intermediate-dose level body weights were lower at days
14 and 21, and at the low-dose level, body weights were significantly
lower than controls on days 4 to 21. No historical control data were
available for F2b litters.
Gross pathological examination of the F0 males and females, the
F1 pups, and the F2a and F2b pups revealed no treatment-related
abnormalities. Postnatal development in F2b pups was assessed with
regard to locomotor activity, auditory function and ophthalmoscopic
examination. At the F2b weaning, there was a significant decrease in
both horizontal and vertical locomotor activity in F2a male and
female pups at the high-dose level, and a significant increase in
horizontal (but not vertical) locomotor activity in the F1 dams at
the high-dose level group. Pups tested for auditory function and for
ophthalmoscopic changes revealed no treatment-related changes
(Holmes et al., 1986b).
In a second 2-generation study, groups of 30 male and 30 female
Long-Evans rats (strain Blu:(LE)BR) were administered alitame (purity
98-101%) at concentrations of 0, 0.1, 0.3, or 1% for either 2 weeks
(females) or 12 weeks (males) prior to mating. Alitame-containing diet
was continued through pregnancy and lactation. Of the F1 pups,
25/sex/dose were raised to maturity and mated to produce the F2a
litters. Approximately 3 weeks after weaning of the F2a litters, the
F1 animals were again mated to produce the F2b litters. Both the
F2a and F2b pups were exposed to alitame in utero, during lactation,
and in the food following weaning.
At the high-dose level, 2 additional F1 groups of 25/sex were
exposed to alitame differently. These groups were introduced to
address the lactation effect observed in the first study. In one of
these groups, the F1 females were removed from treated diet at the
birth of F2a pups. In this group, the F2a pups received no alitame
during lactation. In the other group, the F1 females were removed
from the treated diet 3 weeks after weaning until the birth of the
F2a pups. In this group, the F2b pups received alitame only during
lactation. Animals were then placed on treated diet again. Post-natal
development of F2b pups was tested with an ophthalmoscopic
examination, an auditory function test, and a locomotor activity test.
Locomotor activity tests were also conducted on F0 and F1 parents,
and on F2a and F2b pups.
In the F0 parental animals, the body weights of males were
similar for all groups during the 12 weeks of treatment, while there
were decreases in body-weight gain and food consumption in females at
the high-dose level during the 2-week treatment period. Fertility
rates for all groups were normal. The number of live F1 pups was
similar for all groups at day 1, but was slightly reduced at the
high-dose level on day 4. This was explained by several large litters
at the high-dose level which declined in the first few days. F1 pup
body weight was similar for all groups on day 1 of lactation but was
significantly less at the high-dose level from day 4 onward.
Body-weight gain at high dose was similar to that of control groups
from days 4 to 21. The mean number of pups alive at day 21 compared to
the number of pups alive at day 4 (the lactation index) was similar
for all groups. Gross pathological examination of the F0 males and
females and the F1 pups revealed no treatment-related changes.
In the F1 parental group, the body weights of all female groups
were similar. Pregnancy rates and gestation times were normal. The
F1 males in the treated groups had slightly lower body weights than
controls although body-weight gain was similar for all groups. The
numbers of F2a litter live pups were similar for control, low and
intermediate groups. For the high-dose group, the mean number of pups
born alive (11.54) was slightly lower than controls (12.96). However,
the mean number of pups born alive in the other two additional
high-dose groups were similar to controls (12.75 and 12.13). The
4-day survival indices for the F2a pups in the control, low-,
intermediate-and high-dose groups were 0.98, 0.98, 0.93 and 0.89,
respectively. In the two additional high-dose groups, the 4-day
survival indices were 0.97 and 0.95. Lactation indices were for the
control, low-, intermediate- and high-dose groups 0.98, 1.0, 0.97 and
0.96, respectively, and for the two additional high-dose groups 0.99
and 0.88, respectively.
The mean body weight of high-dose F2a pups were slightly but
significantly lower than controls at birth and throughout lactation.
The additional F2a pups which were exposed only during lactation had
slightly lower body weight after the first week of lactation. The
additional group of F2a pups which had no alitame exposure during
lactation had body weights similar to controls at all times after day
1 of lactation. The results indicated a slight lactation effect of
alitame at the high-dose level, although the high-dose body weights
were still within the historical control ranges. The F2a pups at the
intermediate-dose level also had slightly but significantly lower body
weights at days 14 and 21 of lactation. External examination and gross
internal examination of the F2a pups revealed no treatment-related
changes.
The fertility rates and gestation times associated with the
production of the F2b litters were normal for all groups. The mean
number of pups born alive was comparable at the control, low- and
intermediate-dose levels, being 12.1, 11.7 and 13.0, respectively. At
the high-dose level, the number of live pups was slightly lower (9.8).
Survival during lactation was similar for all groups. The mean body
weight for the F2b high-dose level pups was significantly lower than
controls on day 14 of lactation. External examination and gross
internal examination revealed no treatment-related changes.
Ophthalmoscopic examination and auditory function tests were
normal in all groups. Locomotor activity for both parental groups and
pups showed considerable variation and the changes could not be
correlated with treatment.
This second study provided supporting evidence that alitame at
high-dose levels caused some suppression of body-weight gain during
lactation. This was further investigated in a study to examine the
effect of alitame in the cage bedding material on the pups body weight
(see section 2.2.5). The NOEL, based on body-weight changes in the
F2 pups, was 0.1% in the diet, equivalent to 100 mg/kg bw/day
(Holmes et al., 1986c).
In a 1-generation study in Sprague-Dawley rats, groups of 105
male and 105 female rats (strain Crl:CDBR VAF/Plus) were administered
alitame (purity 99.3%) at concentrations of 0, 0.1, 0.3 or 1% for 4
weeks prior to mating, through the cohabitation period, through the
gestation period, and up to day 21 of lactation. The aim of this study
was to produce an F1 generation of rats, continuously exposed to
alitame in utero, via the milk during lactation, and through the
feed for use in 1-year and 2-year toxicity studies. The animals were
observed daily for clinical signs of toxicity and body weights and
food consumption were recorded. During cohabitation, only the body
weights of the males were recorded. During gestation, body weights of
females were recorded on days 0, 4, 7, 14 and 20. During lactation,
dams and viable young were weighed on days 1, 4, 7, 14 and 21
post partum. On day 4, all litters were culled to 8 pups, 4
animals/sex. At day 21 (weaning), litters from the control groups, 0.1
and 0.3% treatment groups were culled to 2 animals/sex, and the
respective dams sacrificed. High-dose litters were not culled due to
their lower body weights, and were allowed to remain with the
high-dose dams until it was deemed prudent to separate them. When the
F1 animals were 3 to 6 weeks of age, 70 pups/sex/dose were selected
for a 2-year oncogenicity study (Fisher et al., 1993), and 20
pups/sex/dose level were selected for a 1-year toxicity study
(Fisher et al., 1992). All F0 males were terminated after the
cohabitation period. F0 females which did not deliver by day 24 of
gestation were terminated and their uteri and ovaries were examined.
F0 pups not used for either study were terminated.
Reductions in body weights and food consumption were noted in the
high-dose F0 animals, particularly during the first 2 weeks of the
study. This may be associated with palatability. There was no
treatment-related effect on mating, gestation length, or body weight
during gestation and lactation. The number of implantation sites was
slightly lower in the high-dose group and this was reflected in a
slight reduction in the total number of pups born alive per litter.
There was no difference between control and treated groups with
respect to post-implantation losses. There was no treatment-related
effect on pup survival during the lactation period. The mean
body-weight gains at the intermediate- and high-dose levels were
depressed compared to controls, and at weaning the body weights of the
intermediate- and high-dose groups were 9% and 21% lower than
controls, respectively. There were no internal or external
abnormalities in the pups not selected for the long-term studies
(Levinsky et al., 1992).
2.2.5 Special studies on neonatal survival
A study was conducted in Long-Evans pups and lactating dams in
order to further investigate the apparent decrease in pup body weight
during lactation at the high-dose level in the reproductive toxicity
studies. In this study, dams and pups were exposed to alitame which
was added to the bedding material rather than to the feed. It was
argued that dams and pups are normally exposed to treated feed in this
way because of tipping over of the feed by the dam, or the tendency to
scratch the feed into the bedding material. In this study, 2 groups of
8 litters were formed and all dams received normal diet. On lactation
days 1 and 5 and every 2-4 days thereafter, one of the groups had
125 g of feed containing 1% alitame sprinkled on the bedding. The
control group had feed without alitame sprinkled on to the bedding.
Body weights of dams and pups were measured throughout the lactation
period and at one week after lactation.
Body-weight gain in 'treated' dams was slightly lower than in
control dams on days 1 to 4 but there was no difference in body-weight
gain on days 4 to 21 of lactation. Body-weight gain in 'treated' pups
was decreased from day 2 and continued to be decreased throughout
lactation and one week after lactation. For example, the mean
body-weight difference on days 2, 7, 14 and 21 were 0.5 g (-8.4%),
1.6 g (-13.7%), 2.7 g (-11.3%), and 3.1 g (-7.4%), respectively. After
weaning, the weight difference continued to increase to 5.2 g (-7.2%)
at one week. The differences were statistically different from
controls on days 2, 3 and 5, but not thereafter. Locomotor activity
was measured in dam and pups, and no treatment-related effect was
noted.
The results suggested that bedding containing 1% alitame had a
treatment-related effect on pup body-weight gain, although no
mechanism for this effect was apparent. Food consumption was not
measured in this study although it was claimed to be normal. The
consumption of food by the dams from the bedding or from their fur was
considered to be negligible (Holmes et al., 1986d).
2.2.6 Special studies on embryotoxicity/teratogenicity
2.2.6.1 Rats
A group of 80 Crl:COBS-CD(SD)BR strain female rats were
inseminated and divided into 4 groups of 20 animals each. The groups
of presumed pregnant rats were administered alitame in 0.1% methyl
cellulose by gavage at dose levels of 0, 100, 300 or 1000 mg/kg bw/day
on days 6 to 15 post-insemination. The dams were examined throughout
the study period and body weights recorded regularly. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half were stained for skeletal anomalies and half
were examined for soft tissue defects. The ovaries and uteri of dams
were examined on day 20.
The pregnancy rate was 20/20 in control and intermediate-dose
groups and 18/20 in the low- and high-dose groups. There were no
deaths throughout the study and no clinical signs of toxicity. A
slight decrease in food consumption occurred on day 14 at the
high-dose level. Body weight was depressed significantly during and
after the treatment period at the high-dose level only. The number
of viable litters was similar for all groups, as was the number
of corpora lutea. There were slight decreases in the number of
implantation sites in the intermediate- and high-dose groups (10.8 and
10.6 respectively) compared to the control (11.8) and low-dose group
(12.2). There were also slight decreases in the number of fetuses in
the intermediate- (9.9) and high-dose groups (9.7) compared to the
control (11.0) and low-dose group 111.3). The implantation rate
decreased with increasing close (91.1, 89.8, 86.7 and 84.9%,
respectively), and the embryotoxicity rate increased with increasing
dose (6.4, 6.8, 7.4, and 8.4%, respectively). The sex ratio (M/F) was
decreased at the high-dose level (74/100) compared to controls
(110/110).
With regard to fetal development, there was no difference between
the fetal weight of control and treated animals. Examination of the
fetuses did not reveal any treatment-related increase in gross,
visceral or skeletal malformations. Among all the fetuses examined,
only one external anomaly (cleft palate) was observed. There was no
treatment-related change in the rate of ossification. Under the
conditions of this study, alitame showed no evidence of teratogenicity
in the rat. The decrease in the number of fetuses at the higher dose
levels appeared to be a direct result of the lower level of
implantation in these groups rather than to embryotoxicity (Perraud
et al., 1983a).
2.2.6.2 Rabbits
A group of 77 New Zealand white rabbits was inseminated and
divided into 4 groups of 19, 18, 20 and 20 animals. The groups were
administered alitame by gavage at dose levels of 0, 100, 300 or
1000 mg/kg bw/day on days 7 to 18 post-insemination. The does were
examined throughout the study period and body weights recorded
regularly. Animals were killed on day 28 post-insemination. Fetuses
were examined for external malformations then half were stained for
skeletal anomalies and half were examined for soft tissue defects.
Ovaries and uteri of does were examined on day 28.
Four animals died during the study, one at each dose level and
one in the control group. The pregnancy rate was 16/19, 15/18, 18/20
and 16/20 in each of the groups, respectively. Food restrictions were
introduced on days 12 to 19 to reduce the incidence of diarrhoea.
Body-weight loss was observed in all groups during this 8-day period.
A significant treatment-related decrease in body-weight gain was also
noted at the high-dose level.
The number of viable litters was similar for all groups as was
the number of corpora lutea. There was one abortion on day 24 in the
high-dose group. The number of implantation sites was slightly
decreased at the low-dose level compared to other groups.
Embryo-mortality rate was slightly higher at the intermediate- and
high-dose levels but was not dose-related, and not considered to be
due to treatment.
With regard to fetal development, fetal body weights were
slightly but not significantly decreased (7%) at the intermediate- and
high-dose level compared to the control group. Examination of the
fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral abnormalities. Among all the fetuses, there was
one with cleft palate and one with club foot in the intermediate-dose
group, and one with edema in the control group. The only visceral
abnormality seen was agenesis of the left kidney in one control group
fetus. There were no major malformations observed, but there was a
significant increase in the incidence of fetuses with supernumerary
13th ribs at the intermediate- and high-dose levels. Under the
conditions of this assay, alitame showed no evidence of teratogenicity
in the rabbit (Perraud et al., 1983b).
2.2.7 Special studies on genotoxicity
The results of the genotoxicity tests with alitame are summarized
in Table 5.
2.2.8 Special studies on pharmacological effects
Alitame was tested for its pharmacological activity in a battery
of systems designed to assess autonomic, gastrointestinal, renal and
metabolic functions.
A solution of 10-4 mol/litre alitame had no contractile or
relaxant activity in guinea-pig ileum, rat fundus, or rat vas
deferens, and did not interact with any of the six neurotransmitters
used in these systems. Alitame at an oral dose level of 25 mg/kg bw
caused no change in intestinal transit time nor in renal excretion of
fluids or electrolytes. Alitame at an oral dose level of 25 mg/kg bw
had no effect on fasting blood glucose levels or on the deposition of
an oral glucose load (Pearson et al., 1982).
Table 5. Summary of genotoxicity tests with alitame
Test system Test organism Concentration1 Result Reference
Reverse mutation in S. typhimurium 20-10000 µg/plate - Holden et al.,
bacteria TA1535, TA 1537, TA98, (-S9) (1981)
TA100 10-10 000 µg/plate
(+S9)
Urinary metabolites CD-1 mice/ 50-1000 mg alitame/kg - Holden et al,.
in vivo/Reverse S. typhimurium in mice & 0.05-0.75 ml (1981)
mutation in bacteria2 TA1535, TA 1537, TA98, urine/plate
TA100
Reverse mutation in Mouse lymphoma cells 220-1655 µg/ml (+S9) - Holden et al.,
mammalian cells L5178Y (1981)
Chromosome aberration in CD-1 mice bone 0-1000 mg/kg - Holden et al.,
vivo3 marrow cells (1981)
Table 5. Summary of genotoxicity tests with alitame (cont'd).
Test system Test organism Concentration1 Result Reference
Chromosome aberrations Human lymphocytes 0-1000 µg/ml - Holden et al.,
in vitro4 (1981)
Human lymphocytes 0-3000 µg/ml (+/-S9) - Amacher et al.,
(1991)
Micronucleus assay5 CD-1 mice 0-2000 mg/kg/bw/day - Amacher et al.,
(1991)
1 The purity of alitame tested was not provided.
2 Urine from treated CD-1 mice (14/group) was pooled over 18 h and treated with beta-glucuronidase.
3 Groups of 5 CD-1 mice were administered alitame orally and bone marrow cells harvested at 6, 12
and 24 h.
4 In the first study, there was minimal provision of methodology and reporting. The second study
was conducted under more recent GLP regulations.
5 Groups of 5 male and 5 female mice were treated over three consecutive days. Bone marrow was
prepared 24 h after file last dose.
2.2.9 Special studies on neurotoxicity and neurobehavioural
effects
In a neurotoxicity study, alitame was tested for its ability to
inhibit in vitro receptor binding in rat brain homogenate and to
produce in vivo neurochemical effects in rats. In the in vitro
binding assays performed with rat brain homogenate, alitame was tested
for its ability to inhibit the binding of a variety of receptor
radioligands to brain membranes. Neither alitame nor the B-isomer, at
concentrations of 10 µM, had any effect on the level of binding of
these ligands. In the in vivo study, groups of Sprague-Dawley rats
(8 males) were administered an oral dose of vehicle, alitame (25 mg/kg
bw), ß-isomer of alitame (6.25 mg/kg bw), or alitame (12.5 mg/kg bw)
plus ß-isomer of alitame (6.25 mg/kg bw). At 2 or 4 hrs, animals were
killed and the levels of dopamine, serotonin and their metabolites in
the brain tissue were measured. There was no significant change in
neurotransmitter levels after treatment (Bacopoulos, undated).
In a study to examine the effect of alitame on the behaviour of
rats, groups of male CD rats were administered a single oral dose of
vehicle, alitame (25 mg/kg bw), ß-isomer of alitame (6.25 mg/kg bw),
or alitame (12.5 mg/kg bw) plus ß-isomer of alitame (6.25 mg/kg). In a
separate experiment, animals were administered single oral doses as
above for 13 consecutive days. No treatment-related effect on
horizontal or vertical locomotor activity was noted in either the
single or multiple treatment regimes (Bacopoulos, undated).
In a study to examine the potential neurotoxicity of alitame in
rats following acute administration, groups of 12 male and 12 female
Sprague-Dawley rats were given alitame at a single oral dose of
0, 2000, 3000 or 5000 mg/kg bw. The animals were examined using a
functional observational battery (FOB) (both qualitative and
quantitative), a motor activity test, and an auditory startle
habituation test prior to treatment, at 5 and 7 h post-dosing, and
on days 1 and 7 post-dosing. At the end of the study, half the
animals were randomly selected for perfusion for possible future
neuropathological examination while the remaining animals were given
a full necropsy.
Significant decreases in body-weight gains were noted in all
treated groups on the day following treatment (days 0 to 1), followed
by compensatory increases on the following days (days 1 to 7). A
decrease in food consumption on day 1 was noted in all female treated
groups and in males of the intermediate- and high-dose groups. For the
high-dose female group, clinical symptoms included yellow abdominal/
urogenital fur staining, dehydration, reduced activity and pallor
between days 1 and 4 post-treatment. Effects were noted in all
behavioural tests for both sexes in the high-dose group and in some
tests in the intermediate- and low-dose groups on the first day. By
day 7, results of all parameters in all treatment groups were similar
to the control group.
Qualitative FOB assessments on day 1 post-treatment revealed
significant differences between the control and high-dose group for
both sexes. At the high-dose level, there was a significant decrease
in pinna reflex and a decrease in rearing in the arena. There was also
an increased incidence in ataxic gait and diarrhoea in the high-dose
animals. Animals at the lower dose levels also showed some increase in
minor symptoms. By day 7, there were no significant differences
between the groups. Quantitative FOB assessments found changes in
temperature and hind limb splay in treated groups on day 1 but by day
7 no significant differences were detected. Changes were also noted in
treated groups with respect to the motor activity test and the
auditory startle habituation test on day 1, but no changes were
evident by day 7. There were no treatment-related gross pathological
changes found on day 7. Transitory behavioural changes were observed
immediately after treatment and in the 24 h following treatment. By
day 7, all animals appeared normal and there was no evidence of
neurotoxicity (Robinson et al., 1993a).
In a 13-week study of the potential effects of alitame on
behaviour and neuromorphology in rats, 12 male and 12 female
Sprague-Dawley rats were fed a diet containing alitame at
concentrations of 0, 0.1, 0.3 or 1%. Animals were examined throughout
the study period for clinical signs of toxicity and body weights and
food consumption were recorded. The animals were examined using a
functional observational battery (FOB) (qualitative and qualitative),
a motor activity test and an auditory startle habituation at
pre-treatment, after 7 and 14 days of treatment and in the 5th, 9th
and 13th weeks. Passive avoidance testing was performed in weeks 4 and
13. At the end of the study, half the animals (randomly selected) were
perfused for neuropathological examination. Tissues from brain, spinal
cord, and skeletal muscle were prepared as paraffin sections and
haematoxylin and examination. Tissues from the peripheral nervous
system and the central nervous system were prepared in epoxy
embedding.
There were no mortality or adverse clinical findings related to
the treatment. The body weights of females in the 1% treated group
were significantly lower after 3 weeks and throughout the second and
third months of the study. The food consumption for this group was
also significantly decreased during the first week of the study. The
body weights and food consumption of all the other treated groups were
normal.
Both the qualitative (observations and handling) and the
quantitative FOB evaluations (grip strength, hind limb splay, and body
temperature) were unaffected by treatment for both sexes. In the
locomotor activity tests, significant differences at single time
points were noted for high-dose males and females. However, these
differences were not sustained over the course of the study and could
not be clearly related to treatment. For auditory startle habituation
tests, occasional significant differences in the 0.1% group were noted
but these were not attributed to treatment as the effects were not
sustained and there was no dose-relationship. In passive avoidance
performance at 4 weeks there were no significant differences between
groups. At week 13, more females in the 1% group completed the
training trial than in the control group. However, it could not be
concluded that this was related to treatment and was of doubtful
neurotoxicological significance.
There were no treatment-related gross pathological findings in
animals not selected for perfusion or in those which underwent
perfusion. A few animals, primarily males in all groups, had pale
areas on the livers. For rats undergoing perfusion, gross pathological
findings in the nervous system were limited to a few animals with dark
areas in the meninges. Histopathological examination was only
performed in control and high-dose animals. There were no significant
differences in the finding in the two groups. Under the conditions of
this study, there were no behavioural or neuromorphological changes
observed which could be attributed to treatment with alitame (Robinson
et al., 1993b).
In a study to examine the potential developmental neurotoxicity
of alitame, groups of female Sprague-Dawley rats were fed a diet
containing alitame at concentrations of 0, 0.1, 0.3 or 1% for one week
prior to mating, then throughout the mating and gestation periods and
up to 10 days post-partum. Animals were observed throughout the
study period and body weights and food consumption recorded. All
females were given a complete necropsy. Dams were killed on days 21-23
post-partum.
The F1 pups were examined for malformations, sexed and the
number alive and dead recorded. The pups in each litter were weighed
individually on days 1, 4, 7, 11, 14, 17 and 21. On day 4, the litters
were culled to 8 pups, 4 of each sex. Pups were examined for pinna
unfolding (day 2), eye opening (day 12), and air righting reflex
(day 14). A motor activity test was conducted on day 17. Pups were
weaned on day 21.
The F1 adult generation was formed from at least 20 different
litters and consisted of 80 rats/sex/group. Animals were examined for
clinical condition and physical development and body weights and food
consumption recorded. From each litter, 3 males and 3 females were
randomly selected for behavioural testing (motor activity test,
auditory startle habituation test, passive avoidance test and
water-maze test). Young adults not selected for the adult F1
generation behavioural testing were sacrificed and underwent gross
pathological examination. Six animals/sex/group were perfused for
histo-pathological examination. Tissues from brain, spinal cord, and
skeletal muscle were prepared as paraffin sections and H and E
stained. Tissues from the peripheral nervous system and the central
nervous system were prepared in epoxy embedding.
In the F0 animals, there were no deaths and there were no
treatment-related clinical findings. The body weights of the 1%
treated animals were significantly decreased throughout the study.
The food consumption values for the 1% treated group were also
significantly lower than control values during the study. Gross
pathological examinations revealed no treatment-related effects. There
was no treatment-related effect on oestrus cycles, pregnancy rate,
gestation index, number of live and dead pups, sex ratio or post
implantation loss.
In the F0 pups, viability, survival, lactation indices,
clinical findings and gross pathology were unaffected by treatment. In
the 1% group, pup weight was significantly decreased on days 11, 14
and 17 and slightly decreased on day 21. There were no treatment-
related effects on physical or reflexological development, or on
locomotor activity.
In the F1 adults, there were no treatment-related effects on
mortality or clinical findings. The body weights of males and females
in the 1% group were significantly decreased. Food consumption was
normal. Physical development was not affected by treatment. In the
behavioural testing, there was no treatment-related effect on
locomotor activity, auditory startle habituation, passive avoidance or
water maze tests. Gross pathological and neuropathological
examinations revealed no effects which could be attributed to alitame
treatment. Under the conditions of this study, there were no
behavioural or neuromorphological changes in adults or pups which
could be attributed to treatment with alitame (Robinson et al.,
1993c).
2.2.10 Special studies on melanin binding
The potential for radiolabelled alitame or its metabolites to
bind to melanin in the eye of rats was tested by whole body
autoradiography and by microhistoautoradiography. Two male rats from
the albino strain, Sprague-Dawley (Crl:CD(SD)BR) and two male rats
from the pigmented strain, Lister Hooded (Crl:(LIS)BR) were
administered a single oral dose of 65 mg/kg bw 14C-alitame (94%
purity). At 2 and 24 h, one animal from each strain was killed and
the right eye of each excised and stored at -80°C for micro-
histoautoradiography. The remaining carcass was rapidly frozen at
-75°C for whole-body autoradiography.
Microscopic examination of the micrographs of the eyes revealed a
large number of silver grains distributed widely throughout the layer
of the retina, choroid and sclera in animals of both pigmented and
non-pigmented strains at both sacrifice times. The results of the
microscopy could not be interpreted and the data were considered to be
artifactual.
The results of the autoradiography revealed much higher levels of
radioactivity in the uveal tract (choroid, ciliary body and iris) of
the pigmented rats. Quantification of the autoradiographies revealed
concentrations of 2.99 µ equivalent alitame/g tissue in the uveal
tract of albino rats at 2 h and no detectable radioactivity at 24 h.
In the pigmented rats, the radioactivity in the uveal tract was
28.24/µg equivalent alitame/g tissue at 2 h and slightly higher levels
at 24 h (30.55 µg/g). There was no difference in radioactivity in the
surrounding tissues in either the albino or pigmented rat.
The study provided evidence of binding of alitame or its
metabolites to the melanin contained in the uveal tract of the
pigmented rats.
In an addendum to this report, alitame and two metabolites were
tested in vitro for their binding affinity to a melanin preparation
from bovine eyeballs. Alanine tetramethylthietane amide sulfoxide,
which was found in the urine of both rats and humans, did not bind to
melanin under the conditions of this assay, whereas its acetylareal
form, which was found only in the urine of rats, did bind to the
melanin. The positive control, doxepin, produced a higher level of
binding than found with the acetylated sulfoxide metabolite. This
study provided some evidence that the melanin binding observed in rats
may be associated with a metabolite formed only in the rat (Whitby
et al., 1994).
2.2.11 Special studies on the ß-isomer of alitame
2.2.11.1 Absorption, distribution, excretion, and biotransformation
In a study to examine the toxicokinetics of the ß-isomer of
alitame, Sprague-Dawley rats (4/sex) were administered a single oral
dose of the 14C-ß-isomer of alitame (10 mg/kg bw in water).
Excretion of radioactivity in urine and faeces was measured over 72 h.
A similar study was conducted with 6 male and 3 female Long-Evans rats
following a single oral dose of 14C-ß-isomer of alitame (25 mg/kg
bw). A separate group of 7 male Long-Evans rats were fitted with a
bile cannula. These animals received an oral dose of 25 mg/kg bw
14C-ß-isomer, and bile and urine were collected over 48 h.
In Sprague-Dawley rats, the bulk of the radioactivity was
excreted within 48 h, with 24% and 65% excreted in urine and faeces,
respectively. In Long-Evans male rats, total excretion was similar,
with 25 and 64% excreted in urine and faeces, respectively. Excretion
in the female rats was slightly slower. In bile-duct cannulated
animals, 13% of the total radioactivity was in bile over 48 h
(Pfizer, 1986).
In a study to identify the metabolites of the ß-isomer of
alitame, 2 male and 2 female Long-Evans rats were administered a
single oral dose of 10 mg/kg bw 14C-ß-isomer of alitame. In a second
experiment, female Long-Evans rats received 14C-ß-isomer of alitame
at an oral dose of 25 mg/kg bw. In a third experiment, 3 females and 6
males received the same dose of 14C-ß-isomer of alitame. Urinary
metabolites were examined by HPLC. Urinary radioactivity contained
approximately 1% of the radioactivity as unchanged ß-isomer in the
males.
Unchanged ß-isomer was found in the urine of female rats and
represented approximately 13% of the total radioactivity. Other
metabolites which represented approximately 50% of the radioactivity
were alanine tetramethylthietane amide sulfoxide (CP-58,290), alanine
tetramethylthietane amide sulfone (CP-57,254) and their acetylated
derivatives (CP-71,246 and CP-58,386). The only different metabolite
identified was the N-acetyl derivative of the ß-isomer which was
formed by acetylation of the free amine group of the aspartic acid
moiety. The N-acetyl derivative of the ß-isomer comprised 9% and 31%
of the radioactivity in the urine of male and female rats,
respectively. In faeces, the ß-isomer accounted for the majority
(70-81%) of the radioactivity (Pfizer, 1986).
In a study to examine systemic exposure to B-alitame and N-acetyl
beta-isomer by examination of urinary metabolites in male Long-Evans
rats, 14C-ß-isomer of alitame or 14C-N-acetyl -ß-isomer was
administered i.v. initially, then orally, at a dose level of 25 mg/kg
bw. Urine and faeces were collected at 24-hour intervals for a 48-hour
period. Systemic exposure was calculated from a comparison of urinary
excretion following the oral and i.v. routes. The systemic exposure to
ß-isomer following oral administration was 9% in males and 10% in
females. The systemic exposure to ß-isomer plus N-acetyl-ß-isomer was
8% in males and 18% in females (Pfizer, 1986).
In a study to examine systemic exposure to ß-isomer by
examination of serum concentrations in Long-Evans male rats,
14C-ß-isomer was administered i.v. initially (25 mg/kg bw), then
orally at a dose level of 29 mg/kg bw. Serum samples were collected
from 3 animals at various time-points and analyzed by UV absorption
for ß-isomer. Serum concentration-time AUC determinations were made
for each route of administration. The systemic exposure to ß-isomer
following oral administration was 9.6%. The elimination half-life was
29 minutes following the i.v. dose (Pfizer, 1986).
In a study to examine the toxicokinetics and metabolism in dogs,
2 male and 2 female beagle dogs were administered an oral dose of
14C-ß-isomer of alitame followed by an unlabelled dose of 5 or
50 mg/kg bw. Urine and faeces were collected for 96 h. The two male
dogs excreted 35 and 39%, respectively, of the administered
radioactivity in the urine, and the females 14 and 16%, respectively.
Unchanged 13-isomer in urine accounted for 10-16% of the dose in all 4
dogs. All of the urinary metabolites were identical to the ones found
after administration of alitame, but the males had higher levels of
metabolites than the females (Pfizer, 1986).
2.2.11.2 Short-term toxicity studies
In a 3-month study, groups of 15 male and 15 female mice (Strain
Crl: COBS CD-1 (ICR) BR) were fed a diet containing the ß-isomer of
alitame (CP-63,884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption were recorded weekly. Urinary metabolites were
analyzed at weeks 11 and 13. Gross pathological examination was
performed on all animals and organ weights recorded. Tissues were
taken from all of the major organs for histopathological examination.
There was no treatment-related effect on body-weight gain
although the average body weight of treated male animals was lower
than in control groups. Food consumption was slightly decreased at
high dose in males. One of the urinary metabolites identified was the
N-acetyl derivative of the/%isomer. The amount of the isomer increased
proportionally with the dose. There were no treatment-related effects
on organ weights. Gross pathology was unremarkable and histopathology
did not reveal any treatment-related changes. The NOEL in this study
was >25 mg/kg bw/day (Stadnicki et al., 1986a).
In a 3-month study, groups of 15 male and 15 female Long-Evans
rats (Strain Crl: (LE) BR) were fed a diet containing ß-isomer of
alitame (CP-63884) at dose levels of 0, 6.3, 12.5 or 25 mg/kg bw/day.
Animals were observed throughout the study period and body weights and
food consumption data were recorded weekly. Ophthalmoscopic
examinations were performed at pre-test and prior to necropsy. Blood
samples were taken for examination of clinical chemistry and
haematological parameters at pre-test and at 3, 9 and 13 weeks.
Urinary analyses were performed at 3, 9 and 13 weeks. At the end of
the study, gross pathological examinations were performed and tissues
from the major organs taken for histopathology.
There were no deaths through the study and the body-weight gains
and food consumption were similar for treated and untreated groups.
There were no treatment-related effects on clinical chemistry,
haematology or urinary parameters. There were no differences between
control and treated groups with respect to organs weights. Gross
pathology and histopathology were unremarkable The NOEL in this study
was > 25 mg/kg bw/day (Stadnicki et al., 1986b).
2.2.11.3 Special studies on embryotoxicity/teratogenicity
A group of 88 albino female rats (strain Crl:COBS-CD (SD)BR) was
inseminated and divided into 4 groups of 22 animals. The groups of
presumed pregnant rats were administered the 3-isomer of alitame
(CP-63,884) in distilled water by gavage at dose levels of 0, 25, 50
or 100 mg/kg bw/day on days 6 to 15 post-insemination. Animals were
killed on day 20 post-insemination. Fetuses were examined for external
malformations, then half stained for skeletal anomalies and half
examined for soft tissue defects.
The pregnancy rate was 20/22 in the control and low-dose groups,
and 19/22 in the intermediate- and high-dose groups. There were no
deaths throughout the study, nor any clinical signs of toxicity. There
were no treatment-related effects on body weight or food consumption
of dams during or after the treatment period. The number of corpora
lutea, implantation sites and viable fetuses was similar for all
groups. With regard to fetal development, there was no difference
between fetal weight of control and treated animals. Examination of
the fetuses did not reveal any treatment-related increase in gross,
skeletal or visceral malformations, nor any treatment-related effects
on the degree of ossification. Under the conditions of this study, the
ß-isomer of alitame showed no evidence of teratogenicity in rats
(Kessedjian et al., 1986).
2.2.11.4 Special studies on genotoxicity
The ß-isomer of alitame was tested for the induction of point
mutations, both base-pair and frameshift, at the his locus of
Salmonella typhimurium strains TA 1535, TA 1537, TA 98 and TA 100,
with or without S9 microsomal activation. The purity of the alitame
was not provided. Doses ranged from 20 to 10 000 µg/plate, with or
without activation. There was no increase in revertants above the
control (water) in any of the treated plates at any dose level. The
positive controls, 9-aminoacridine, nitrofurantoin, sodium nitrite,
2-nitrofluorene and 2-anthramine gave appropriate responses (Ellis
et al., 1991).
2.3 Observations in humans
In a 90-day double-blind tolerance study, groups of male and
female subjects (77 treated, 80 controls) were administered either
alitame in capsules divided over three meals at a dose level of
10 mg/kg bw/day for 90 days, or placebo in the same manner. This dose
was chosen because it is 10 times greater than the estimated daily
intake if all sucrose in the diet were replaced with alitame. Subjects
were assessed throughout the study period for side-effects (by
interview and observation). Other tests included blood pressure,
temperature, body weight, electrocardiogram, and ophthalmoscopic
examination. Various clinical pathology parameters were also measured.
Microsomal activation was estimated from the elimination kinetics of
aminopyrine at pre-dosing and at weeks 6 and 13 of treatment. Plasma
and urine samples were collected at various time points throughout the
study.
Four treated subjects and 2 control subjects discontinued from
the study due to side effects (angioedema of the lips, maculopapular
rash, urticaria (wheals) with erythema in treated subjects, and
tinnitus, dry eyes and abdominal cramps in the control subjects). A
rechallenge with alitame after 6 months produced none of the above
symptoms, making the possibility of allergic reaction very unlikely.
There were no treatment-related changes in blood pressure, pulse,
temperature or body weight. Electrocardiograms and ophthalmological
examinations were unremarkable. Haematological parameters were similar
in treated and control subjects. Measurement of aminopyrine
elimination rates at 6 and 13 weeks did not reveal any increase in
hepatic microsomal enzyme activity due to alitame treatment (Gerber
et al., 1987).
In a 90-day double-blind tolerance study in diabetic subjects,
groups of male and female subjects having either type I or type II
diabetes (75 treated, 80 control) were administered either alitame in
capsules divided over three meals at dose level of 10 mg/kg bw/day for
90 days, or placebo in the same manner. This dose was chosen because
it is ten times greater than the estimated daily intake if all sucrose
in the diet were replaced with alitame. Subjects were included on the
following criteria: diabetics of type I and type II, either sex and
aged between 15 and 70 years with stable diabetic therapy; fasting
blood glucose <250 mg/dL; free from investigational drugs; had not
suffered a myocardial infarction within the previous 6 months; and had
either no hypertension or it was controlled. Subjects were excluded on
the following criteria: females which were pregnant or nursing; drug
or alcohol dependence; systemic disease other than diabetes; clinical
chemistry and haematological parameters above the upper limit of
normal; hepatic disease; and donation of blood within 4 weeks prior to
the study. The groups were well matched with respect to such
parameters as age, sex distribution, weight, smoking, drinking,
intercurrent illnesses and medication as well as diabetic status.
Subjects were assessed at weeks 0, 1, 2, 3, 4, 6, 8, 10 and 13 of
the study period for side effects (by interview and observation).
Effects on diabetic control were assessed by measuring fasting
haemoglobin A1c and 2-hour postprandial blood glucose levels at
baseline and weeks 0, 6, 8 and 13. Physical examinations were
conducted at screening and at week 13 of the study and included blood
pressure, temperature, body weight and electrocardiogram. Clinical
pathology parameters (haematology, clinical chemistry and urinary)
were measured pre-treatment and at 13 weeks.
At the end of the study period, 16 treated and 9 control subjects
had discontinued from the programme and, of these, 5 treated and 2
control subjects withdrew due to illness. No subjects discontinued due
to side effects or abnormal laboratory parameters related to alitame
treatment. Side-effects were observed in 29% of treated subjects and
25% of control subjects. Gastrointestinal disturbances were the most
common side-effect. Incidence and severity of particular side-effects
were relatively evenly distributed between the groups. No significant
changes were noted in either the control or treated groups with
respect to diabetic control, clinical chemistry, haematological or
urinary parameters. No treatment-related effects were apparent from
the physical examinations, blood pressure and pulse rate, temperature,
body weight or EKG recordings.
Alitame at a dose of 10 mg/kg/bw/day for 90 days was well
tolerated by the type I and type II diabetic patients who continued in
this study. There was no effect of alitame at this dose level on the
ability to control serum glucose levels by these diabetic patients
(McMahon et al., 1989).
In a 1- and 2-year follow-up of the subjects in the 90-day study
in diabetics, all but one of the treated subjects received an
interview and complete physical examination. Subjects were interviewed
again within 24 months post-study for all but 6 treated and 4 control
subjects.
A variety of intercurrent illnesses were reported during the
follow-up period. Most of the reported illnesses related to diabetes
and its complications. The incidence of intercurrent illnesses during
the follow-up period was 40 treated and 36 control subjects. Two
subjects died during the follow-up period, both treated subjects. One
subject (type I diabetic) died during the month following the study,
cause unknown. At completion of the study no adverse reactions had
been noted in this subject. The second subject (type II diabetic)
suffered a fatal heart attack resulting from hypertensive
atherosclerotic cardiovascular disease.
At the first follow-up (12 months), 5 treated subjects and no
control subjects were found to have suffered myocardial infarction.
The possibility that the results were due to chance was examined using
available epidemiological data on the incidence of myocardial
infarction. Data from the US National Hospital Discharge Survey (NHDS)
were used to estimate the rate of myocardial infarctions among
diabetics in various age groups for both sexes. These data were
adjusted to take into account the number of myocardial infarction
casualties which are not admitted to hospital and the actual patient
years of observation. Based on the hospitalization data for diabetics
who suffered myocardial infarction in 1986, the numbers of subjects
expected to suffer a myocardial infarction in a single year were 2.9
and 3.2 in the treated and control groups, respectively. The observed
rates of 5% and 0%, respectively, were within the 95% tolerance
intervals of incidence expected based on the NHDS analysis. Two years
after the study, myocardial infarctions had been encountered by 6
treated and 3 control subjects. The statistical analysis of these data
at 2 years supports the null hypothesis that the incidence observed
were due to chance. The data indicate that in the two years after the
90-day study of alitame in diabetics, no evidence was provided that
alitame adversely affected the health of the study groups (McMahon
et al., 1989).
3. COMMENTS
A number of toxicity studies on alitame were available, as well
as studies on the ß-isomer of alitame, which is formed at low levels
in some foods.
In metabolism studies conducted in three animal species and in
humans, alitame was readily absorbed from the GI tract and then
rapidly metabolized and excreted. In mice, rats and dogs, the route of
metabolism involved cleavage of the peptide bond yielding aspartic
acid and D-alanine tetramethylthietane amide, the latter being further
biotransformed by oxidation at the thietane sulfur to form the
sulfoxide and sulfone. Acetylated derivatives of these metabolites
were commonly found in the urine of rats and dogs whereas, in humans,
the glucuronide derivative of D-alanine tetramethylthietane amide was
the major urinary metabolite. In rats, initial cleavage of the peptide
bond of alitame took place in the lumen of the jejunum. In pregnant
rats, alitame was readily transferred transplacentally, but there was
no evidence of active secretion in the milk. In a study in rats,
levels of alitame residues decreased rapidly in all tissues except the
eyes. Further studies indicated that it was bound to melanin contained
in the uveal tract (choroid, ciliary body and iris) and suggested that
such binding was associated with a metabolite formed only in rats. The
Committee considered that an in vivo study in a mammalian species
other than the rat is desirable to clarify the significance of the
retention of alitame in the eyes.
The ß-isomer of alitame was rapidly metabolized in rats, the only
urinary metabolite not also formed with alitame being the N-acetyl
derivative of this isomer. There was no evidence of the formation of
significant amounts of the ß-isomer of alitame in the stomach or small
intestine of the rat.
Acute and short-term toxicity studies indicated that the toxicity
of alitame is low. In studies conducted in mice, rats and dogs, the
major toxicological changes observed were associated with the liver. A
dose-related increase in liver weight and evidence of centrilobular
hypertrophy were seen in all of the studies in these three species,
and these changes were accompanied by an increase in the level of
hepatic microsomal enzymes. Short-term studies on hepatic enzyme
induction indicated that alitame was a weak inducer of microsomal
enzymes; the pattern of induction was qualitatively similar to that
caused by phenobarbitone but at a lower level. The liver changes were
indicative of an adaptive response and, in rats and dogs, withdrawal
of treatment after a 3-month period resulted in partial or complete
reversal of the observed changes within a month.
Two-year carcinogenicity studies have been conducted in CD-1
mice, Long-Evans rats and Sprague-Dawley rats. The two studies
conducted in rats included an in utero phase. There was no evidence
of carcinogenicity in the study in mice.
In rats, the first carcinogenicity study was conducted in
Long-Evans rats at dietary levels of 0.1, 0.3 or 1% and, while the
survival rate was greater than 50% in all groups at 22 months, poor
survival was noted at 24 months. Pathological examination of the liver
indicated a higher incidence of focal nodular hyperplasia and
eosinophilic foci in females at the 1% dose level than in controls,
according to the diagnostic criteria of Squire & Levitt (1975). Two
subsequent independent re-examinations of these data have been
performed using the diagnostic criteria of Maronpot et al. (1986).
In the first re-examination (Farrell, 1987), the higher incidence of
eosinophilic foci in females in the high-dose group was confirmed, but
the incidence of focal hyperplasia or hepatocellular adenomas was
similar to that in controls. In the second re-examination (Hardisty
et al., 1994), the higher incidence of eosinophilic foci in females
in the high-dose group was again confirmed. A higher incidence of
these foci was also found at the mid-dose level. Also in this
re-examination, the nodular hyperplastic lesions noted in the original
report were largely reclassified as hepatocellular adenomas, and an
increased incidence of these lesions was seen at the high-dose level.
No evidence of hepatocellular carcinomas was found in female rats in
the original examination or in the subsequent re-examinations. The
main difference between the first and second re-examinations was in
the nomenclature used, rather than in the recognition that lesions
existed. The Committee expressed concern at the change in the way that
these liver lesions were classified, possibly as a result of the use
of different diagnostic criteria. It considered that there was
evidence of a higher incidence of adenomas in females in this study,
at least at the high dose level. The adenomas were found late in the
study and, while considered unlikely to progress to hepatocellular
carcinomas, the data were inconclusive in this regard.
A second carcinogenicity study, this time in Sprague-Dawley rats,
was conducted because of the low survival rate at 24 months in the
Long-Evans rats. In this second study, the survival rate was lower at
both 22 and 24 months than that found in the Long-Evans rats, and
hence the study was considered inadequate to provide further
information regarding the potential carcinogenicity of alitame.
The Committee considered that the available studies did not
indicate that alitame was carcinogenic, but that they were deficient
in certain respects and did not fully address this question. It also
considered that further research to clarify the mechanism of the
formation of adenomas in female Long-Evans rats was desirable.
Genotoxicity studies both in vitro and in vivo did not
provide any evidence that alitame has a mutagenic potential.
Similarly, there was no evidence from studies conducted in rats and
rabbits that alitame has a teratogenic potential.
Two reproductive toxicity studies (2-generation) were conducted
in Long-Evans rats and one reproductive toxicity study (1-generation)
in Sprague-Dawley rats. The first reproductive toxicity study with
Long-Evans rats produced some evidence for slightly decreased
body-weight gain in the pups during lactation at 1% in the diet, the
highest dose administered. A similar effect was noted in the second
reproductive toxicity study at the same dose level, but not in the
1-generation reproductive toxicity study with Sprague-Dawley rats. A
study of the effects of alitame-containing diet in the bedding
material, which could have been a source of alitame for pups due to
spilled feed, while providing a possible explanation for the decrease
in pup body-weight gain, did not fully address this issue. A slight
effect of alitame on locomotor activity seen in the first reproductive
toxicity study was not substantiated in subsequent studies. The
Committee did not regard the changes seen in the reproductive toxicity
studies to be of toxicological significance.
Neurotoxicity and neurobehavioural toxicity studies using an
observational battery of tests indicated behavioural changes only when
very high doses were administered to rats as a single dose by gavage
(5000 mg/kg bw). These behavioural changes were of short duration and
animals appeared to be normal by day 7. There was no evidence of
neurotoxicity in rats fed diets containing 1% alitame (equivalent to
1000 mg/kg bw/day) over a 3-month period. Similarly, no changes
indicative of neurotoxicity were observed in dams or pups when the
same dose level was given over the reproductive period.
In a 14-day study conducted in humans there was no evidence of
hepatic enzyme induction at a dose level of 15 mg/kg bw/day.
Ninety-day tolerance studies were conducted in normal and
diabetic subjects. There was no evidence of adverse effects in
subjects of both types during the period of the study at a dose level
of 10 mg/kg bw/day. However, in the 1- and 2-year follow-up of the
diabetic subjects, there was a higher incidence of myocardial
infarction in the treatment group than in the controls, namely 5/58 in
the treatment group and 0/71 in the controls after 1 year, and 6/53 in
the treatment group and 3/67 in the controls after 2 years. While the
differences at 2 years were not statistically significant, and there
was no indication of cardiovascular effects in the animal studies, the
Committee considered that the increased incidence of myocardial
infarction in the treatment group has not been fully explained. It was
advised that a further study is to be performed in diabetic subjects.
Studies conducted with the ß-isomer of alitame provided no
evidence of genotoxicity, teratogenicity or systemic toxicity in mice
or rats at dose levels of up to 25 mg/kg bw/day.
4. EVALUATION
The Committee recognized that the basis on which to consider
establishing an ADI for a chemical such as alitame is difficult to
specify, since the most sensitive treatment-related effects observed
were liver enzyme changes and liver weight changes, which may be
adaptive in nature. For alitame, these changes appeared to be closely
linked. While the prolonged hepatomegaly observed was not accompanied
in the long-term studies by any pathological changes indicative of
toxicity, it is not considered desirable. On the other hand, moderate
induction of hepatic enzymes is considered a normal adaptive process.
In the case of alitame, significant changes in body-weight gain or
liver weight were observed at doses above the NOELs. In the 18-month
study in dogs, the NOEL was 100 mg/kg bw/day. In the 2-year study in
mice, the NOEL was 0.1% in the diet, equal to 125 mg/kg bw/day. In the
lifetime studies in rats, the NOEL was 0.3% in the diet, equal to
130 mg/kg bw/day in Long-Evans rats and 230 mg/kg bw/day in
Sprague-Dawley rats.
The Committee concluded that the concerns raised by the
deficiencies in the carcinogenicity studies in rats were of such
significance that an ADI could not be allocated.
5. REFERENCES
AMACHER, D.E., MUEHBAUER, P.A., ZELLJADT, I. (1991) Genetic Toxicology
Report. Unpublished Report No. 91-444-21 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
BACOPOULOS, N.G. (undated) Investigation of alitame and its b-isomer
in neurochemical and behavioural animal models. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1984) Alitame: A Fourteen Day
Human Enzyme Induction Study. Unpublished Report No. 32 from
Bio-Organic Chemicals Research & Development, Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
CALDWELL, J., SEVER, P.S., CZUBA, L.J. (1985) Human Disposition of
14C-Alitame. Unpublished Report No. SC-2 from Department of
Pharmacology, University of London. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M., THATCHER N.J., MARSHALL, A.D. (1994)
Assessment of the Enzyme Inducing Characteristics of Alitame.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School., London, UK. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CALDWELL, J., REED, P.M. /1994) Assessment of the Enzyme Inducing
Characteristics of Alitame Suppl. Report on Ummunodetection of
Cytochrome P450 Isoenzymes in the Liver of Alitame-Treated Rats.
Unpublished report from Dept. Pharmacol. & Toxicol. St. Mary's
Hospital Medical School, London, UK Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981a)
Alitame: Two-Week Range-Finding Feeding Study in Rats. Unpublished
Report No. 81-055 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
CHVEDOFF, M., GREAVES, P., DANCLA, J.L., FACCINI, J.M. (1981b)
Alitame: Two-Week Oral Range-Finding Study in Dogs. Unpublished Report
No. 81-070 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ELLIS, J.H., AMACHER, D.E., KLUWE, W.M. (1991) b-Isomer of Alitame:
Microbial Gene Mutation Assays. Unpublished Report from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
FARRELL, J.F. (1987) Histopathologic evaluation of selected
microslides from chronic rat and mouse feeding studies with alitame.
Unpublished Report No. PR-116 from Experimental Pathology Laboratories
Inc. USA. Submitted to WHO by Pfizer Inc., Groton CT, USA.
FISHER, D.O., MARSH, P.M., SOLER, G.N., COLEMAN, D.V., LEVINSKY, H.V.,
ESTES, P.C., DOUGHERTY, W.J. (1992) One-Year Toxicity Study with
Dietary Administration and In Utero Exposure in Sprague-Dawley Rats.
Unpublished Report No. 90-444-18 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
FISHER, D.O., LAITWAITE, B.S., BUTLER, B.T., COLEMAN, D.V., LEVINSKY,
H.V., OCHOA, R., DOUGHERTY, W.J. (1993) Two Year Oncogenicity Study
with Dietary Administration and In Utero Exposure in Sprague-Dawley
Rats. Unpublished Report No. 90-444-20 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
GERBER, N., McMAHON, M.D., WILLIAMS, R.L. (1987) Double-Blind Placebo
Controlled Ninety-Day Toleration Study of Alitame. Unpublished Report
No. 101 from Ohio State University, Columbus, Ohio. USA. Submitted to
WHO by Pfizer Inc. Groton, CT, USA.
HARDISTY, J.E., ABBOTT, D., GOPINATH, C., WARD, J. (1994) Pathology
Working Group Review of Two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report from Experimental Pathology
Laboratory Inc., Research Triangle Park, NC, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLDEN, H.E., ELLIS, J.H., AMACHER, D., RAY, V.A. (1981) Alitame:
Genetic Toxicology Report. Unpublished Report No. 91-444-21 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., TOOLAN, L.A, BURGAN, J.J., REYNOLDS, J.A., LEVINSKY,
H.V., ESTES, P.C. (1983a) Alitame: Two-Month Range-Finding Feeding
Study in Mice. Unpublished Report No. 82-444-03 from Pfizer Central
Research, Groton, CT, USA Laboratoires. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., ANGUS, T.A., FISHER, D.O., FIGDOR, S.K., REYNOLDS, J.A.,
ESTES, P.C., LEVINSKY, H.V. (1983b) Alitame: Five-Week Feeding Study
in Rats. Unpublished Report No. 82-444-01 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc. Groton,
CT, USA.
HOLMES, C.L., MARTZ, F., BOWDEN, P.R., FIGDOR, S.K., ROSS, P.E.,
LEVINSKY, H.V., ESTES, P.C.(1985a) Alitame: One-Year Feeding Study in
Long-Evans Rats. Unpublished Report No.s. 82-444-06 and 83-444-12 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., MARTZ, F., PUNZALAN, E.R., FIGDOR, S.K., ROESLER, A.R.,
LEVINSKY, H.V,, ESTES, P.C. (1985b) Alitame: Eighteen-Month Feeding
Study in Beagle Dogs. Unpublished Report No. 82-444-07 from
Laboratoires Pfizer Centre de Recherche, 37400 Ambroise, France.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., CACCIATORE. A., BURGAN, J.J., COLEMAN, G.L., FIGDOR,
S.K., LEVINSKY, H.V., REYNOLDS, J.A., ESTES, P.C., (1985c) Alitame:
Two-Year Oncogenicity Feeding Study in Mice. Unpublished Report
No. 82-444-08 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
HOLMES, C.L. (1986a) Alitame: A two-Year Oncogenicity Feeding Study in
Long-Evans Rats. Unpublished Report No. 83-444-11. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986b) Alitame: Two-Generation Reproduction
Feeding Study in Rat. Unpublished Report No. 83-444-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
HOLMES, C.L., SANTACROCE, E.M., DARROW, T.E., CALCAGNI, A., STADNICKI,
S.W., LEVINSKY, H.V. (1986c) Alitame: Second Two-Generation
Reproduction Study in Rat. Unpublished Report No. 83-444-13/14/15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
HOLMES, C.L., SANTACROSE, E.M., STRADNICKI, S.W., LEVINSKY, H.V.
(1986d) Exploratory bedding study in the Long-Evans Rat. Unpublished
Report No. 85-444-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
KESSEDJIAN, M.J., STADLER, J., POOL, W.R., (1986) b-Isomer of Alitame:
Fetotoxicity Study in Rats by the Oral Route. Unpublished report
86-086 from Pfizer Central Research, Groton, CT, USA. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., BEUTER, N.J., STADNICKI, S.W. (1983) Alitame: Acute
Oral Toxicity Studies in Mice and Rats. Unpublished Report
No. 83-444-09 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
LEVINSKY, H.V., STADNICKI, S.W., ESTES, P.C., DOUGHERTY, W.J.,
CACCIATORE, A., KOPCYK, N., LUNDEEN, G.R. (1992) In Utero Exposure
with Alitame in Sprague-Dawley rats. Unpublished Report No. 90-444-19
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MARONPOT, R.R., MONTGOMERY Jr C.A., BOORMAN, G.A., McCONNELL (1986)
National toxicology programme nomenclature for hepatoproliferative
lesions of rats. Toxicol. Pathol., 14: 263-273.
McMAHON, F.G., CYRUS, J., RASKIN, P., STARR, I. (1989) Double-Blind
Placebo Controlled Ninety Day Toleration Study of Alitame in
Diabetics. Unpublished Report No. 102 from Central Research Centre,
New Orleans, LA, USA. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
MONRO, A.M. CHVEDOFF, M., GREAVES, P., NACHBAUR, J., PERRAUD, J.,
(1982a) Three-Month Oral Toxicity Study in Rats plus 1-Month
Withdrawal. Unpublished Report No. 81-086 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
MONRO, A.M., CHVEDOFF, M., PERRAUD, J., GREAVES, P., NACHBAUR, J.,
(1982b) Alitame: Three-Month Oral Toxicity Study in Dogs plus 1-Month
Withdrawal. Unpublished Report No. 81-106 from Laboratoires Pfizer
Centre de Recherche, 37400 Ambroise, France. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
PEARSON, D.L., GAYNOR, B.J., SWINDELL, A.C. (1982) General
Pharmacology Report on Alitame. Autonomic, Gastro-intestinal, Renal
and Metabolic Studies. Unpublished Report. Submitted to WHO by Pfizer
Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J. (1983a) Alitame: Foetotoxicity Study in
Rats (FDA Segment II). Unpublished Report No. 82-154 from Laboratoires
Pfizer Centre de Recherche, 37400 Ambroise, France. Submitted to WHO
by Pfizer Inc. Groton, CT, USA.
PERRAUD, J., KESSEDJIAN, M.J., SENKOWSKI, S. (1983b) Alitame:
Foetotoxicity Study in Rabbits (FDA Segment II). Unpublished Report
No, 83-026 from Laboratoires Pfizer Centre de Recherche, 37400
Ambroise, France. Submitted to WHO by Pfizer Inc. Groton, CT, USA.
PFIZER CENTRAL RESEARCH. (1986) Alitame: Metabolism and Disposition.
Unpublished Report from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., BROXUP, B., NOVEROSKE, J.W. (1993a) An Acute Study of
the Potential Effects of Orally Administered Alitame on Behaviour in
Rats. Unpublished Report No. 97148 form Bio-Research Laboratories Ltd.
Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W. (1993b) A
13-Week Dietary Study of the Potential Effects of Alitame on Behaviour
and Neuromorphology in Rats. Unpublished Report No. 97149 form
Bio-Research Laboratories Ltd. Senneville, Quebec, Canada. Submitted
to WHO by Pfizer Inc. Groton, CT, USA.
ROBINSON, K., PINSONNEAULT, L., BROXUP, B., NOVEROSKE, J.W., (1993c) A
Developmental Neurotoxicity Study of Alitame Administered in the Diet
of Rats. Unpublished Report No. 97150 from Bio-Research Laboratories
Ltd. Senneville, Quebec, Canada. Submitted to WHO by Pfizer Inc.
Groton, CT. USA.
SQUIRE, R.A., LEVITT, M.H. (1975). Report of a workshop on a
classification of specific hepatocellular lesions in rats. Cancer
Res., 35: 3214-3223.
STADNICKI, S.W., PUNZALAN. E.R., FIGDOR, S.K., ROESLER, A.R., ESTES,
P.C., LEVINSKY, H.V. (1986a) b-Isomer of Alitame: Three-Month
Feeding Study in Study CD-I Mice. Unpublished Report No. 86-580-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
STADNICKI, S.W., SANTACROCE, E.M., FIGDOR, S.K., ROSS, P.E.,
ESTES, P.C., LEVINSKY, H.V. (1986b) b-Isomer of Alitame: Three-Month
Feeding Study in Long-Evans Rats. Unpublished Report No. 86-580-01
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc. Groton, CT, USA.
WHITBY, B.R., POWLES, P., LEWSLEY, R., FERNYHOUGH, P., MARSLAND, R.
(1994) 14C-Alitame: Whole-body autoradiography and microhistoauto-
radiography in the rat. Unpublished Report No. 727/5-1011 from
Hazleton Europe, Harrogate, UK. Submitted to WHO by Pfizer Inc.
Groton, CT, USA.
