
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
BUTYLATED HYDROXYTOLUENE
CHEMICAL NAMES 2:6-di-tert.-butyl-p-cresol;
4-methyl-2:6-ditertiary-butylphenol
SYNONYM BHT
EMPIRICAL FORMULA C15H24O
STRUCTURAL FORMULA
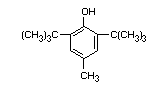 MOLECULAR WEIGHT 220.36
DEFINITION Butylated hydroxytoluene should contain not less
than 99.0% of C15H24O.
DESCRIPTION A white, crystalline, odourless solid. It is
insoluble in water but soluble in fats; 1 g is
soluble in 4 ml of ethanol.
USE As an antioxidant for fats and oils or in
packaging material for fat-containing foods. Its
activity is enhanced in combination with other
antioxidants and synergists.
Biological Data
Biochemical aspects
BHT is readily absorbed. Some deposition in adipose tissue bee been
described following high dosage in the rat, and this may cause
increased stability of the extracted perineal fat.1 The metabolism
of BHT is complicated by the presence of the butyl groups on each side
of the hydroxyl. Preliminary modification is necessary before
conjugation can occur. This takes the form of either oxidation of one
of the butyl groups to a 2-hydroxy-2:2-dimethyl-2-ethyl group, or
oxidation of the methyl group to a carboxy acid. The former yields
glucuronide conjugates, while the latter becomes conjugated with
glucuronic acid or glycine. Some of the oxidized material is also
excreted unconjugated.2,3
Rabbits were given single or repeated doses of BHT in the range
400-800 mg per kg body-weight. About 16% of the dose was excreted as
ester glucuronide and 19% as ether glucuronide. Unconjugated phenol
(8%), ethereal sulfate (8%) and a glycine conjugate (2%) were also
excreted. Excretion of all detectable metabolites was essentially
complete 3 to 4 days after administration of the compound and about
54% of the dose was accounted for as identified metabolites.4
Rats were given doses of 100 µg of BHT labelled with 3H
intraperitoneally and the urinary output of radioactivity was measured
for 4 consecutive days. Four days after the injection 34.5% of the
injected radioactivity was recovered in urine.5 The same dose of BHT
(100 µg) labelled with 14C was given to rats and 34% of the
radioactivity was excreted in the urine in the first 4 days, in close
agreement with the previous result using tritiated BHT.6
The liver and body fat of rats fed a diet containing 0.5% BHT for 35
days were analyzed. The concentration of BHT in the liver never rose
above 5 ppm in males or 1.5 ppm in females. In the body fat the level
fluctuated round 30 ppm in males and 45 ppm in females. Fat from rats
returned to normal diet showed a progressive fall in the concentration
of BHT, the half-life being about 7 to 10 days. The daily excretion
of radioactivity in urine and faeces was studied in rats given an oral
done of 14C-labelled BHT (12 mg/kg body-weight). Excretion became
negligible by the sixth day after administration when about 70% of the
injected dose had been recovered. Less than 1% was excreted as carbon
dioxide in the expired air. About 50% of the radioactivity was
excreted in the bile during the 24-hour period following the oral
dose.7
Acute toxicity
Animal Route LD50 Approximate lethal dose Reference
(mg/kg (mg/kg body-weight)
body-weight)
Rat oral 1700-1970 - 8
Cat oral - 940-2100 8
Rabbit oral - 2100-3200 8
Guinea-pig oral - 10 700 8
Short-term studies
Mouse. BHT was given to pregnant mice in daily doses of 750 mg per
kg body-weight for 18 days. Another group received the same dose for
a total of 50 to 64 days including 18 days of pregnancy. No foetal
abnormalities were observed.9
In a statistically planned experiment using 144 female mice no
blindness was observed in any of the 1162 litters representing 7765
babies born throughout the reproductive life span of the mothers.10
Rat. Feeding experiments were carried out on 45 pairs of weanling
male rats for 5 to 8 weeks with diets containing 0, 10 and 20% lard
supplements to which 0.001%, 0.1% or 0.5% BHT had been added. 0.001%
caused no changes in any of the serum constituents studied. 0.5%
produced increase in the serum cholesterol level within 5 weeks.
Female rate fed for 8 months on a diet containing a 10% lard
supplement with 0.1% BHT showed increased serum cholesterol levels,
but no other significant changes. 0.5% BHT in 10% and 20% lard
supplements fed to female rats for the same period increased serum
cholesterol, phospholipid and mucoprotein levels.11
0.3% BHT in the diet of pregnant rats that had been kept for 5 weeks
on a diet deficient in vitamin E produced no toxic symptoms. 1.55%
caused drastic loss of weight and foetal death.12
BHT fed to rats in groups of 12 for a period of 7 weeks at a dietary
level of 0.1% in conjunction with a 20% lard supplement significantly
reduced the initial growth rate and mature weight of male rats. No
effect was noted in female or male rats with a 10% lard supplement. A
paired feeding experiment showed that this inhibition of growth was a
direct toxic effect of BHT and could not be explained by a reduction
in the palatability of the diet. At this level BHT produced a
significant increase in the weight of the liver, both absolute and
relative to body-weight. Rats under increased stress showed
significant loss of hair from the top of the head. The toxic effect
of BHT was greater if the fat load in the diet was increased.
Anophthalmia occurred in 10% of the litter's.13
Groups of 6 weanling rats (3 male and 3 female) were fed BHT at
dietary levels of 0%, 0.1%. 0.2%, 0.3%, 0.4% and 0.5% in conjunction
with a 20% lard supplement for 6 weeks. BHT reduced the growth rate,
especially in the males, the effect appearing to become significant at
the 0.3% level. It also increased the absolute liver weight and the
ratio of liver weight to body-weight in both sexes, the latter effect
appearing to become significant at the 0.2% level. BHT increased the
ratio of left adrenal weight to body-weight in male rats but had no
consistent effect in females. There were no histological changes
attributable to the treatment in the adrenal. All dietary levels of
BHT increased the serum cholesterol and the concentration of the
cholesterol was directly proportional to the BHT level. There was
also a significant increase in the concentration of adrenal
cholesterol. BHT produced no significant changes in the concentration
of total or per cent. esterified liver cholesterol, total liver lipid
or concentration of total polyunsaturated fatty acids in the liver.14
BHT administered to rats at 250 mg per kg body-weight for 68 to 82
days caused reduction in rate of increase in weight and fatty
infiltration of the liver.15
Feeding experiments conducted for 20 and 90 days respectively
indicated that rats do not find food containing 0.5% or 1% BHT
palatable. However, the animals ingest foods so treated more readily
if these concentrations are attained gradually. Paired feeding
experiments with groups of 5 or 10 rats for 25 days demonstrated that
diets containing 0.8% and 1% BHT will reduce the daily intake of food
below control values. A level of 1% in the diet retarded weight
gain.8
(Work in progress) Rats were given single doses of 100 mg BHT per kg
body-weight daily for 7 weeks before mating and then throughout
pregnancy or were autopsied on the 20th day of pregnancy. No evidence
of foetal abnormality were found in any of these animals but
abnormalities did occur in the progeny of positive control groups
treated with vitamin A.9
(Work in progress) A three-generation reproduction study was started
by another group in May 1964. They also fed groups of 16 male and 16
female rats on levels of 0.03%, 0.1% and 0.3% BHT in a diet containing
20% fat for 10 weeks. There were two control groups each containing
16 male and 16 female animals. No definite effect on body-weight was
observed at any level in the females and there was only a slight
depression in the males at the 0.3% level. There was no significant
effect on blood cholesterol level in either sex after feeding BHT at
any of the levels for 10 weeks. Four of the males at the 0.3% and two
at the 0.1% level died during the experiment. Two deaths occurred
among the females at O.3%. Only one male rat died in both control
groups.16
(Work In progress) Groups of 20 male and 20 female rats fed 1% BHT in
the diet for 10 weeks showed recovery both in liver to body-weight
ratios and in morphological appearance of the liver cells within a few
weeks after restoring the animals to a normal diet.17
Rabbit. Acute effects on electrolyte excretion similar to those
described for large doses of BHA were also obtained following
administration of doses of BHT of 500-700 mg/kg body-weight (about 2%
in the diet). No such effects were observed at lower dosage
levels.18
Dog. A mild to moderately severe degree of diarrhoea was induced in
a group of 4 dogs fed doses of 1.4-4.7 g/kg body-weight every 2-4 days
over a period of 4 weeks. Post-mortem examination revealed no
significant gross pathological changes. No signs of intoxication and
no gross or histopathological changes were observed in dogs fed doses
of 0.17-0.94 g/kg body-weight 5 days a week for a period of 12
months.8
Fowl. When BHT was fed at a level of 0.125% for 34 weeks to a group
of 10 pullets, no differences in fertility, hatchability of eggs or
health of chicks in comparison with a similar control group were
found. The eggs of the antioxidant-treated birds contained more
carotenoids and vitamin A than those of the controls.19
Long-term studies
Rat. Groups of 15 male and 15 female rats given diets containing 1%
lard and 0.2%, 0.5% or 0.8% BHT for 24 months showed no specific signs
of intoxication, and micropathological studies were negative. For one
group given a diet containing .5% BHT, the BHT was dissolved in lard
and then heated for 30 minutes at 150°C before incorporation in the
diet. There were no effects on weight gain or blood constituents and
micropathological studies of the main organs were negative. The
feeding of 1% BHT was followed in both male and female rats by a
sub-normal weight gain and by an increase in the weight of the brain
and liver and some other organs in relation to body-weight.
Micropathological studies were negative in this group also. BHT in
these concentrations had no specific effect on the number of
erythrocytes and leucocytes, or on the concentration of haemoglobin in
the peripheral blood. A number of rats of both sexes died during this
experiment, but as the fatalities were in no relation to the
concentration of BHT fed, it was believed that the cause of death was
unrelated to the feeding of this substance. Micropathological studies
support this observation.8
When fed at the 0.5% level in the diet, BHT had no effect in rats on
the reproductive cycle, the histology of the spleen, kidney, liver and
skin, or on the weight of the heart, spleen or kidney. There was no
significant increase in mortality of rats fed on a diet containing
0.1% BHT and 10% hydrogenated coconut oil for a period of 2 years. The
effects on weight gain have already been described.13
Comment on experimental studies reported
Experimental studies have been carried out in several species. There
were, however, some important discrepancies between the results
obtained by earlier and by some more recent observers. Short-term and
long-term studies in rats and metabolic studies will be discussed
under evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
One extensive series of studies8 indicates that the level of BHT that
would cause no significant deleterious effect in the rat would be 0.8%
in a diet containing 1% lard. However, in two short-term studies11,13
it has been reported that 0.1% BHT in a 20% lard diet resulted in
increase of various lipid components of the blood and a significant
reduction in weight gain. More recently a similar trend of results
was obtained but the effects only became definite at the 0.3%
level.14 In another study 0.1% BHT in a 20% fat diet was found to
have no effect on the growth rate of weanling rats of either sex and
there was no effect on the blood cholesterol concentration at the 0.1%
or the 0.3% levels.16 In long-term studies with rats receiving 0.5%
BHT in a diet containing 10% lard no significant difference from the
controls was observed.13 The addition of 10% or 20% of lard to the
diet was found to enhance the deleterious action of BHT in some
studies. With a 20% lard supplement, fat provides about 33% of the
calorie intake and human diets frequently contain this level of fat.
The effect of such levels of dietary fat on BHT toxicity is therefore
relevant to its use as a food additive. Several investigators have
shown that BHT will cause an increase in liver weight relative to
body-weight. However, even in rats fed at 1% BHT in the diet for 10
weeks, there was recovery in the liver to body-weight ratio and
morphological appearance of the liver cells within a few weeks of
restoration of the animals to a normal diet.17
The chemical structure of BHT suggests the possibility of some delay
in metabolism. The results of studies on the excretion of labelled
BHT5,6,7 are difficult to compare because of the different dose levels
and routes of administration used and therefore the importance of
possible delayed metabolism is difficult to assess.
A possible teratogenic action of BHT was suggested by the occurrence
of anophthalmia in some litters of mothers fed the compound.13
Extensive reproduction studies now completed or in progress make this
unlikely.9,10,16
For the 0.1% level to be accepted as that causing no significant
toxicological effect in the rat it would be necessary to disregard
both the effect on weight gain and also the effect on blood lipids
reported by some investigators.
It would be inadvisable to dismiss these observations pending the
evaluation of results of work now in progress. The next lower dose
studied was 0.01% (= 100 ppm) in the diet in a group of 26 rats, which
is equivalent to 5 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-0.5
Further Work Considered Desirable
1. Further long-term studies, with particular reference to the effect
of BHT on lipid metabolism and the relationship between the dietary
fat load and toxicity.
2. Metabolic studies in human subjects.
References
1. Johnson, A. R., O'Halloran, M. W. & Hewgill, F. R. (1956) J. Amer.
Oil Chem. Soc., 35, 496
2. Dacre, J. C., Denz, F. A. & Kennedy. T. H. (1956) Biochem. J., 64,
777
3. Dacre, J. C. (1960) J. N.Z. Inst. Chem. , 24, 161
4. Dacre, J. C. (1961) Biochem. J., 78, 758
5. Golder, W. S., Ryan, A. J. & Wright, S. E. (1962) J. Pharm.
Pharmacol., 14, 268
6. Ladomery. L. F., Ryan, A. J. & Wright, S. E. (1963) J. Pharm.
Pharmacol., 15, 771
7. Gage. J. C. (1964) Unpublished report, Imperial Chemical Industries
Limited
8. Deichmann, W. B., Clemmer, J. J., Rakoczy, R. & Bianchine, J.
(1955) A.M.A. Arch. industr. Hlth, 11, 93
9. British Industrial Biological Research Association (Unpublished
report submitted to WHO in 1964)
10. Johnson, A. R. (Unpublished report submitted to WHO in 1964)
11. Day, A. J., Johnson, A. R., O'Halloran, M. W. & Schwartz, C. J.
(1959) Aust. J. exp. Biol. med. Sci., 37, 295
12. Ames, S.R., Ludwig, H. I., Swanson, W. J. & Harris, P. L. (1956)
Proc. Soc. exp. Biol. (N.Y.), 93, 39
13. Brown, W. D., Johnson, A. R. & O'Halloran, M. W. (1959) Aust. J.
exp. Biol. med. Sci., 37, 533
14. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
15. Karpliuk, I. A. (1959) Vop. Pitan., 18, 24
16. Industrial Bio-Test Laboratories (1964) Progress report to
Hercules Powder Company
17. Imperial Chemical Industries Limited (1964) Unpublished report
No. IHR/158
18. Denz, F. A. & Llaurado, J. G. (1957) Brit. J. exp. Path., 38, 515
19. Shellenberger. T. E., Parrish, D. B. & Sanford, P. E. (1957)
Poultry Sci., 36, 1313
MOLECULAR WEIGHT 220.36
DEFINITION Butylated hydroxytoluene should contain not less
than 99.0% of C15H24O.
DESCRIPTION A white, crystalline, odourless solid. It is
insoluble in water but soluble in fats; 1 g is
soluble in 4 ml of ethanol.
USE As an antioxidant for fats and oils or in
packaging material for fat-containing foods. Its
activity is enhanced in combination with other
antioxidants and synergists.
Biological Data
Biochemical aspects
BHT is readily absorbed. Some deposition in adipose tissue bee been
described following high dosage in the rat, and this may cause
increased stability of the extracted perineal fat.1 The metabolism
of BHT is complicated by the presence of the butyl groups on each side
of the hydroxyl. Preliminary modification is necessary before
conjugation can occur. This takes the form of either oxidation of one
of the butyl groups to a 2-hydroxy-2:2-dimethyl-2-ethyl group, or
oxidation of the methyl group to a carboxy acid. The former yields
glucuronide conjugates, while the latter becomes conjugated with
glucuronic acid or glycine. Some of the oxidized material is also
excreted unconjugated.2,3
Rabbits were given single or repeated doses of BHT in the range
400-800 mg per kg body-weight. About 16% of the dose was excreted as
ester glucuronide and 19% as ether glucuronide. Unconjugated phenol
(8%), ethereal sulfate (8%) and a glycine conjugate (2%) were also
excreted. Excretion of all detectable metabolites was essentially
complete 3 to 4 days after administration of the compound and about
54% of the dose was accounted for as identified metabolites.4
Rats were given doses of 100 µg of BHT labelled with 3H
intraperitoneally and the urinary output of radioactivity was measured
for 4 consecutive days. Four days after the injection 34.5% of the
injected radioactivity was recovered in urine.5 The same dose of BHT
(100 µg) labelled with 14C was given to rats and 34% of the
radioactivity was excreted in the urine in the first 4 days, in close
agreement with the previous result using tritiated BHT.6
The liver and body fat of rats fed a diet containing 0.5% BHT for 35
days were analyzed. The concentration of BHT in the liver never rose
above 5 ppm in males or 1.5 ppm in females. In the body fat the level
fluctuated round 30 ppm in males and 45 ppm in females. Fat from rats
returned to normal diet showed a progressive fall in the concentration
of BHT, the half-life being about 7 to 10 days. The daily excretion
of radioactivity in urine and faeces was studied in rats given an oral
done of 14C-labelled BHT (12 mg/kg body-weight). Excretion became
negligible by the sixth day after administration when about 70% of the
injected dose had been recovered. Less than 1% was excreted as carbon
dioxide in the expired air. About 50% of the radioactivity was
excreted in the bile during the 24-hour period following the oral
dose.7
Acute toxicity
Animal Route LD50 Approximate lethal dose Reference
(mg/kg (mg/kg body-weight)
body-weight)
Rat oral 1700-1970 - 8
Cat oral - 940-2100 8
Rabbit oral - 2100-3200 8
Guinea-pig oral - 10 700 8
Short-term studies
Mouse. BHT was given to pregnant mice in daily doses of 750 mg per
kg body-weight for 18 days. Another group received the same dose for
a total of 50 to 64 days including 18 days of pregnancy. No foetal
abnormalities were observed.9
In a statistically planned experiment using 144 female mice no
blindness was observed in any of the 1162 litters representing 7765
babies born throughout the reproductive life span of the mothers.10
Rat. Feeding experiments were carried out on 45 pairs of weanling
male rats for 5 to 8 weeks with diets containing 0, 10 and 20% lard
supplements to which 0.001%, 0.1% or 0.5% BHT had been added. 0.001%
caused no changes in any of the serum constituents studied. 0.5%
produced increase in the serum cholesterol level within 5 weeks.
Female rate fed for 8 months on a diet containing a 10% lard
supplement with 0.1% BHT showed increased serum cholesterol levels,
but no other significant changes. 0.5% BHT in 10% and 20% lard
supplements fed to female rats for the same period increased serum
cholesterol, phospholipid and mucoprotein levels.11
0.3% BHT in the diet of pregnant rats that had been kept for 5 weeks
on a diet deficient in vitamin E produced no toxic symptoms. 1.55%
caused drastic loss of weight and foetal death.12
BHT fed to rats in groups of 12 for a period of 7 weeks at a dietary
level of 0.1% in conjunction with a 20% lard supplement significantly
reduced the initial growth rate and mature weight of male rats. No
effect was noted in female or male rats with a 10% lard supplement. A
paired feeding experiment showed that this inhibition of growth was a
direct toxic effect of BHT and could not be explained by a reduction
in the palatability of the diet. At this level BHT produced a
significant increase in the weight of the liver, both absolute and
relative to body-weight. Rats under increased stress showed
significant loss of hair from the top of the head. The toxic effect
of BHT was greater if the fat load in the diet was increased.
Anophthalmia occurred in 10% of the litter's.13
Groups of 6 weanling rats (3 male and 3 female) were fed BHT at
dietary levels of 0%, 0.1%. 0.2%, 0.3%, 0.4% and 0.5% in conjunction
with a 20% lard supplement for 6 weeks. BHT reduced the growth rate,
especially in the males, the effect appearing to become significant at
the 0.3% level. It also increased the absolute liver weight and the
ratio of liver weight to body-weight in both sexes, the latter effect
appearing to become significant at the 0.2% level. BHT increased the
ratio of left adrenal weight to body-weight in male rats but had no
consistent effect in females. There were no histological changes
attributable to the treatment in the adrenal. All dietary levels of
BHT increased the serum cholesterol and the concentration of the
cholesterol was directly proportional to the BHT level. There was
also a significant increase in the concentration of adrenal
cholesterol. BHT produced no significant changes in the concentration
of total or per cent. esterified liver cholesterol, total liver lipid
or concentration of total polyunsaturated fatty acids in the liver.14
BHT administered to rats at 250 mg per kg body-weight for 68 to 82
days caused reduction in rate of increase in weight and fatty
infiltration of the liver.15
Feeding experiments conducted for 20 and 90 days respectively
indicated that rats do not find food containing 0.5% or 1% BHT
palatable. However, the animals ingest foods so treated more readily
if these concentrations are attained gradually. Paired feeding
experiments with groups of 5 or 10 rats for 25 days demonstrated that
diets containing 0.8% and 1% BHT will reduce the daily intake of food
below control values. A level of 1% in the diet retarded weight
gain.8
(Work in progress) Rats were given single doses of 100 mg BHT per kg
body-weight daily for 7 weeks before mating and then throughout
pregnancy or were autopsied on the 20th day of pregnancy. No evidence
of foetal abnormality were found in any of these animals but
abnormalities did occur in the progeny of positive control groups
treated with vitamin A.9
(Work in progress) A three-generation reproduction study was started
by another group in May 1964. They also fed groups of 16 male and 16
female rats on levels of 0.03%, 0.1% and 0.3% BHT in a diet containing
20% fat for 10 weeks. There were two control groups each containing
16 male and 16 female animals. No definite effect on body-weight was
observed at any level in the females and there was only a slight
depression in the males at the 0.3% level. There was no significant
effect on blood cholesterol level in either sex after feeding BHT at
any of the levels for 10 weeks. Four of the males at the 0.3% and two
at the 0.1% level died during the experiment. Two deaths occurred
among the females at O.3%. Only one male rat died in both control
groups.16
(Work In progress) Groups of 20 male and 20 female rats fed 1% BHT in
the diet for 10 weeks showed recovery both in liver to body-weight
ratios and in morphological appearance of the liver cells within a few
weeks after restoring the animals to a normal diet.17
Rabbit. Acute effects on electrolyte excretion similar to those
described for large doses of BHA were also obtained following
administration of doses of BHT of 500-700 mg/kg body-weight (about 2%
in the diet). No such effects were observed at lower dosage
levels.18
Dog. A mild to moderately severe degree of diarrhoea was induced in
a group of 4 dogs fed doses of 1.4-4.7 g/kg body-weight every 2-4 days
over a period of 4 weeks. Post-mortem examination revealed no
significant gross pathological changes. No signs of intoxication and
no gross or histopathological changes were observed in dogs fed doses
of 0.17-0.94 g/kg body-weight 5 days a week for a period of 12
months.8
Fowl. When BHT was fed at a level of 0.125% for 34 weeks to a group
of 10 pullets, no differences in fertility, hatchability of eggs or
health of chicks in comparison with a similar control group were
found. The eggs of the antioxidant-treated birds contained more
carotenoids and vitamin A than those of the controls.19
Long-term studies
Rat. Groups of 15 male and 15 female rats given diets containing 1%
lard and 0.2%, 0.5% or 0.8% BHT for 24 months showed no specific signs
of intoxication, and micropathological studies were negative. For one
group given a diet containing .5% BHT, the BHT was dissolved in lard
and then heated for 30 minutes at 150°C before incorporation in the
diet. There were no effects on weight gain or blood constituents and
micropathological studies of the main organs were negative. The
feeding of 1% BHT was followed in both male and female rats by a
sub-normal weight gain and by an increase in the weight of the brain
and liver and some other organs in relation to body-weight.
Micropathological studies were negative in this group also. BHT in
these concentrations had no specific effect on the number of
erythrocytes and leucocytes, or on the concentration of haemoglobin in
the peripheral blood. A number of rats of both sexes died during this
experiment, but as the fatalities were in no relation to the
concentration of BHT fed, it was believed that the cause of death was
unrelated to the feeding of this substance. Micropathological studies
support this observation.8
When fed at the 0.5% level in the diet, BHT had no effect in rats on
the reproductive cycle, the histology of the spleen, kidney, liver and
skin, or on the weight of the heart, spleen or kidney. There was no
significant increase in mortality of rats fed on a diet containing
0.1% BHT and 10% hydrogenated coconut oil for a period of 2 years. The
effects on weight gain have already been described.13
Comment on experimental studies reported
Experimental studies have been carried out in several species. There
were, however, some important discrepancies between the results
obtained by earlier and by some more recent observers. Short-term and
long-term studies in rats and metabolic studies will be discussed
under evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
One extensive series of studies8 indicates that the level of BHT that
would cause no significant deleterious effect in the rat would be 0.8%
in a diet containing 1% lard. However, in two short-term studies11,13
it has been reported that 0.1% BHT in a 20% lard diet resulted in
increase of various lipid components of the blood and a significant
reduction in weight gain. More recently a similar trend of results
was obtained but the effects only became definite at the 0.3%
level.14 In another study 0.1% BHT in a 20% fat diet was found to
have no effect on the growth rate of weanling rats of either sex and
there was no effect on the blood cholesterol concentration at the 0.1%
or the 0.3% levels.16 In long-term studies with rats receiving 0.5%
BHT in a diet containing 10% lard no significant difference from the
controls was observed.13 The addition of 10% or 20% of lard to the
diet was found to enhance the deleterious action of BHT in some
studies. With a 20% lard supplement, fat provides about 33% of the
calorie intake and human diets frequently contain this level of fat.
The effect of such levels of dietary fat on BHT toxicity is therefore
relevant to its use as a food additive. Several investigators have
shown that BHT will cause an increase in liver weight relative to
body-weight. However, even in rats fed at 1% BHT in the diet for 10
weeks, there was recovery in the liver to body-weight ratio and
morphological appearance of the liver cells within a few weeks of
restoration of the animals to a normal diet.17
The chemical structure of BHT suggests the possibility of some delay
in metabolism. The results of studies on the excretion of labelled
BHT5,6,7 are difficult to compare because of the different dose levels
and routes of administration used and therefore the importance of
possible delayed metabolism is difficult to assess.
A possible teratogenic action of BHT was suggested by the occurrence
of anophthalmia in some litters of mothers fed the compound.13
Extensive reproduction studies now completed or in progress make this
unlikely.9,10,16
For the 0.1% level to be accepted as that causing no significant
toxicological effect in the rat it would be necessary to disregard
both the effect on weight gain and also the effect on blood lipids
reported by some investigators.
It would be inadvisable to dismiss these observations pending the
evaluation of results of work now in progress. The next lower dose
studied was 0.01% (= 100 ppm) in the diet in a group of 26 rats, which
is equivalent to 5 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-0.5
Further Work Considered Desirable
1. Further long-term studies, with particular reference to the effect
of BHT on lipid metabolism and the relationship between the dietary
fat load and toxicity.
2. Metabolic studies in human subjects.
References
1. Johnson, A. R., O'Halloran, M. W. & Hewgill, F. R. (1956) J. Amer.
Oil Chem. Soc., 35, 496
2. Dacre, J. C., Denz, F. A. & Kennedy. T. H. (1956) Biochem. J., 64,
777
3. Dacre, J. C. (1960) J. N.Z. Inst. Chem. , 24, 161
4. Dacre, J. C. (1961) Biochem. J., 78, 758
5. Golder, W. S., Ryan, A. J. & Wright, S. E. (1962) J. Pharm.
Pharmacol., 14, 268
6. Ladomery. L. F., Ryan, A. J. & Wright, S. E. (1963) J. Pharm.
Pharmacol., 15, 771
7. Gage. J. C. (1964) Unpublished report, Imperial Chemical Industries
Limited
8. Deichmann, W. B., Clemmer, J. J., Rakoczy, R. & Bianchine, J.
(1955) A.M.A. Arch. industr. Hlth, 11, 93
9. British Industrial Biological Research Association (Unpublished
report submitted to WHO in 1964)
10. Johnson, A. R. (Unpublished report submitted to WHO in 1964)
11. Day, A. J., Johnson, A. R., O'Halloran, M. W. & Schwartz, C. J.
(1959) Aust. J. exp. Biol. med. Sci., 37, 295
12. Ames, S.R., Ludwig, H. I., Swanson, W. J. & Harris, P. L. (1956)
Proc. Soc. exp. Biol. (N.Y.), 93, 39
13. Brown, W. D., Johnson, A. R. & O'Halloran, M. W. (1959) Aust. J.
exp. Biol. med. Sci., 37, 533
14. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
15. Karpliuk, I. A. (1959) Vop. Pitan., 18, 24
16. Industrial Bio-Test Laboratories (1964) Progress report to
Hercules Powder Company
17. Imperial Chemical Industries Limited (1964) Unpublished report
No. IHR/158
18. Denz, F. A. & Llaurado, J. G. (1957) Brit. J. exp. Path., 38, 515
19. Shellenberger. T. E., Parrish, D. B. & Sanford, P. E. (1957)
Poultry Sci., 36, 1313
See Also:
Toxicological Abbreviations
Butylated hydroxytoluene (ICSC)
Butylated hydroxytoluene (FAO Nutrition Meetings Report Series 40abc)
Butylated hydroxytoluene (WHO Food Additives Series 5)
Butylated hydroxytoluene (WHO Food Additives Series 10)
Butylated hydroxytoluene (WHO Food Additives Series 21)
Butylated hydroxytoluene (WHO Food Additives Series 35)
BUTYLATED HYDROXYTOLUENE (JECFA Evaluation)
 MOLECULAR WEIGHT 220.36
DEFINITION Butylated hydroxytoluene should contain not less
than 99.0% of C15H24O.
DESCRIPTION A white, crystalline, odourless solid. It is
insoluble in water but soluble in fats; 1 g is
soluble in 4 ml of ethanol.
USE As an antioxidant for fats and oils or in
packaging material for fat-containing foods. Its
activity is enhanced in combination with other
antioxidants and synergists.
Biological Data
Biochemical aspects
BHT is readily absorbed. Some deposition in adipose tissue bee been
described following high dosage in the rat, and this may cause
increased stability of the extracted perineal fat.1 The metabolism
of BHT is complicated by the presence of the butyl groups on each side
of the hydroxyl. Preliminary modification is necessary before
conjugation can occur. This takes the form of either oxidation of one
of the butyl groups to a 2-hydroxy-2:2-dimethyl-2-ethyl group, or
oxidation of the methyl group to a carboxy acid. The former yields
glucuronide conjugates, while the latter becomes conjugated with
glucuronic acid or glycine. Some of the oxidized material is also
excreted unconjugated.2,3
Rabbits were given single or repeated doses of BHT in the range
400-800 mg per kg body-weight. About 16% of the dose was excreted as
ester glucuronide and 19% as ether glucuronide. Unconjugated phenol
(8%), ethereal sulfate (8%) and a glycine conjugate (2%) were also
excreted. Excretion of all detectable metabolites was essentially
complete 3 to 4 days after administration of the compound and about
54% of the dose was accounted for as identified metabolites.4
Rats were given doses of 100 µg of BHT labelled with 3H
intraperitoneally and the urinary output of radioactivity was measured
for 4 consecutive days. Four days after the injection 34.5% of the
injected radioactivity was recovered in urine.5 The same dose of BHT
(100 µg) labelled with 14C was given to rats and 34% of the
radioactivity was excreted in the urine in the first 4 days, in close
agreement with the previous result using tritiated BHT.6
The liver and body fat of rats fed a diet containing 0.5% BHT for 35
days were analyzed. The concentration of BHT in the liver never rose
above 5 ppm in males or 1.5 ppm in females. In the body fat the level
fluctuated round 30 ppm in males and 45 ppm in females. Fat from rats
returned to normal diet showed a progressive fall in the concentration
of BHT, the half-life being about 7 to 10 days. The daily excretion
of radioactivity in urine and faeces was studied in rats given an oral
done of 14C-labelled BHT (12 mg/kg body-weight). Excretion became
negligible by the sixth day after administration when about 70% of the
injected dose had been recovered. Less than 1% was excreted as carbon
dioxide in the expired air. About 50% of the radioactivity was
excreted in the bile during the 24-hour period following the oral
dose.7
Acute toxicity
Animal Route LD50 Approximate lethal dose Reference
(mg/kg (mg/kg body-weight)
body-weight)
Rat oral 1700-1970 - 8
Cat oral - 940-2100 8
Rabbit oral - 2100-3200 8
Guinea-pig oral - 10 700 8
Short-term studies
Mouse. BHT was given to pregnant mice in daily doses of 750 mg per
kg body-weight for 18 days. Another group received the same dose for
a total of 50 to 64 days including 18 days of pregnancy. No foetal
abnormalities were observed.9
In a statistically planned experiment using 144 female mice no
blindness was observed in any of the 1162 litters representing 7765
babies born throughout the reproductive life span of the mothers.10
Rat. Feeding experiments were carried out on 45 pairs of weanling
male rats for 5 to 8 weeks with diets containing 0, 10 and 20% lard
supplements to which 0.001%, 0.1% or 0.5% BHT had been added. 0.001%
caused no changes in any of the serum constituents studied. 0.5%
produced increase in the serum cholesterol level within 5 weeks.
Female rate fed for 8 months on a diet containing a 10% lard
supplement with 0.1% BHT showed increased serum cholesterol levels,
but no other significant changes. 0.5% BHT in 10% and 20% lard
supplements fed to female rats for the same period increased serum
cholesterol, phospholipid and mucoprotein levels.11
0.3% BHT in the diet of pregnant rats that had been kept for 5 weeks
on a diet deficient in vitamin E produced no toxic symptoms. 1.55%
caused drastic loss of weight and foetal death.12
BHT fed to rats in groups of 12 for a period of 7 weeks at a dietary
level of 0.1% in conjunction with a 20% lard supplement significantly
reduced the initial growth rate and mature weight of male rats. No
effect was noted in female or male rats with a 10% lard supplement. A
paired feeding experiment showed that this inhibition of growth was a
direct toxic effect of BHT and could not be explained by a reduction
in the palatability of the diet. At this level BHT produced a
significant increase in the weight of the liver, both absolute and
relative to body-weight. Rats under increased stress showed
significant loss of hair from the top of the head. The toxic effect
of BHT was greater if the fat load in the diet was increased.
Anophthalmia occurred in 10% of the litter's.13
Groups of 6 weanling rats (3 male and 3 female) were fed BHT at
dietary levels of 0%, 0.1%. 0.2%, 0.3%, 0.4% and 0.5% in conjunction
with a 20% lard supplement for 6 weeks. BHT reduced the growth rate,
especially in the males, the effect appearing to become significant at
the 0.3% level. It also increased the absolute liver weight and the
ratio of liver weight to body-weight in both sexes, the latter effect
appearing to become significant at the 0.2% level. BHT increased the
ratio of left adrenal weight to body-weight in male rats but had no
consistent effect in females. There were no histological changes
attributable to the treatment in the adrenal. All dietary levels of
BHT increased the serum cholesterol and the concentration of the
cholesterol was directly proportional to the BHT level. There was
also a significant increase in the concentration of adrenal
cholesterol. BHT produced no significant changes in the concentration
of total or per cent. esterified liver cholesterol, total liver lipid
or concentration of total polyunsaturated fatty acids in the liver.14
BHT administered to rats at 250 mg per kg body-weight for 68 to 82
days caused reduction in rate of increase in weight and fatty
infiltration of the liver.15
Feeding experiments conducted for 20 and 90 days respectively
indicated that rats do not find food containing 0.5% or 1% BHT
palatable. However, the animals ingest foods so treated more readily
if these concentrations are attained gradually. Paired feeding
experiments with groups of 5 or 10 rats for 25 days demonstrated that
diets containing 0.8% and 1% BHT will reduce the daily intake of food
below control values. A level of 1% in the diet retarded weight
gain.8
(Work in progress) Rats were given single doses of 100 mg BHT per kg
body-weight daily for 7 weeks before mating and then throughout
pregnancy or were autopsied on the 20th day of pregnancy. No evidence
of foetal abnormality were found in any of these animals but
abnormalities did occur in the progeny of positive control groups
treated with vitamin A.9
(Work in progress) A three-generation reproduction study was started
by another group in May 1964. They also fed groups of 16 male and 16
female rats on levels of 0.03%, 0.1% and 0.3% BHT in a diet containing
20% fat for 10 weeks. There were two control groups each containing
16 male and 16 female animals. No definite effect on body-weight was
observed at any level in the females and there was only a slight
depression in the males at the 0.3% level. There was no significant
effect on blood cholesterol level in either sex after feeding BHT at
any of the levels for 10 weeks. Four of the males at the 0.3% and two
at the 0.1% level died during the experiment. Two deaths occurred
among the females at O.3%. Only one male rat died in both control
groups.16
(Work In progress) Groups of 20 male and 20 female rats fed 1% BHT in
the diet for 10 weeks showed recovery both in liver to body-weight
ratios and in morphological appearance of the liver cells within a few
weeks after restoring the animals to a normal diet.17
Rabbit. Acute effects on electrolyte excretion similar to those
described for large doses of BHA were also obtained following
administration of doses of BHT of 500-700 mg/kg body-weight (about 2%
in the diet). No such effects were observed at lower dosage
levels.18
Dog. A mild to moderately severe degree of diarrhoea was induced in
a group of 4 dogs fed doses of 1.4-4.7 g/kg body-weight every 2-4 days
over a period of 4 weeks. Post-mortem examination revealed no
significant gross pathological changes. No signs of intoxication and
no gross or histopathological changes were observed in dogs fed doses
of 0.17-0.94 g/kg body-weight 5 days a week for a period of 12
months.8
Fowl. When BHT was fed at a level of 0.125% for 34 weeks to a group
of 10 pullets, no differences in fertility, hatchability of eggs or
health of chicks in comparison with a similar control group were
found. The eggs of the antioxidant-treated birds contained more
carotenoids and vitamin A than those of the controls.19
Long-term studies
Rat. Groups of 15 male and 15 female rats given diets containing 1%
lard and 0.2%, 0.5% or 0.8% BHT for 24 months showed no specific signs
of intoxication, and micropathological studies were negative. For one
group given a diet containing .5% BHT, the BHT was dissolved in lard
and then heated for 30 minutes at 150°C before incorporation in the
diet. There were no effects on weight gain or blood constituents and
micropathological studies of the main organs were negative. The
feeding of 1% BHT was followed in both male and female rats by a
sub-normal weight gain and by an increase in the weight of the brain
and liver and some other organs in relation to body-weight.
Micropathological studies were negative in this group also. BHT in
these concentrations had no specific effect on the number of
erythrocytes and leucocytes, or on the concentration of haemoglobin in
the peripheral blood. A number of rats of both sexes died during this
experiment, but as the fatalities were in no relation to the
concentration of BHT fed, it was believed that the cause of death was
unrelated to the feeding of this substance. Micropathological studies
support this observation.8
When fed at the 0.5% level in the diet, BHT had no effect in rats on
the reproductive cycle, the histology of the spleen, kidney, liver and
skin, or on the weight of the heart, spleen or kidney. There was no
significant increase in mortality of rats fed on a diet containing
0.1% BHT and 10% hydrogenated coconut oil for a period of 2 years. The
effects on weight gain have already been described.13
Comment on experimental studies reported
Experimental studies have been carried out in several species. There
were, however, some important discrepancies between the results
obtained by earlier and by some more recent observers. Short-term and
long-term studies in rats and metabolic studies will be discussed
under evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
One extensive series of studies8 indicates that the level of BHT that
would cause no significant deleterious effect in the rat would be 0.8%
in a diet containing 1% lard. However, in two short-term studies11,13
it has been reported that 0.1% BHT in a 20% lard diet resulted in
increase of various lipid components of the blood and a significant
reduction in weight gain. More recently a similar trend of results
was obtained but the effects only became definite at the 0.3%
level.14 In another study 0.1% BHT in a 20% fat diet was found to
have no effect on the growth rate of weanling rats of either sex and
there was no effect on the blood cholesterol concentration at the 0.1%
or the 0.3% levels.16 In long-term studies with rats receiving 0.5%
BHT in a diet containing 10% lard no significant difference from the
controls was observed.13 The addition of 10% or 20% of lard to the
diet was found to enhance the deleterious action of BHT in some
studies. With a 20% lard supplement, fat provides about 33% of the
calorie intake and human diets frequently contain this level of fat.
The effect of such levels of dietary fat on BHT toxicity is therefore
relevant to its use as a food additive. Several investigators have
shown that BHT will cause an increase in liver weight relative to
body-weight. However, even in rats fed at 1% BHT in the diet for 10
weeks, there was recovery in the liver to body-weight ratio and
morphological appearance of the liver cells within a few weeks of
restoration of the animals to a normal diet.17
The chemical structure of BHT suggests the possibility of some delay
in metabolism. The results of studies on the excretion of labelled
BHT5,6,7 are difficult to compare because of the different dose levels
and routes of administration used and therefore the importance of
possible delayed metabolism is difficult to assess.
A possible teratogenic action of BHT was suggested by the occurrence
of anophthalmia in some litters of mothers fed the compound.13
Extensive reproduction studies now completed or in progress make this
unlikely.9,10,16
For the 0.1% level to be accepted as that causing no significant
toxicological effect in the rat it would be necessary to disregard
both the effect on weight gain and also the effect on blood lipids
reported by some investigators.
It would be inadvisable to dismiss these observations pending the
evaluation of results of work now in progress. The next lower dose
studied was 0.01% (= 100 ppm) in the diet in a group of 26 rats, which
is equivalent to 5 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-0.5
Further Work Considered Desirable
1. Further long-term studies, with particular reference to the effect
of BHT on lipid metabolism and the relationship between the dietary
fat load and toxicity.
2. Metabolic studies in human subjects.
References
1. Johnson, A. R., O'Halloran, M. W. & Hewgill, F. R. (1956) J. Amer.
Oil Chem. Soc., 35, 496
2. Dacre, J. C., Denz, F. A. & Kennedy. T. H. (1956) Biochem. J., 64,
777
3. Dacre, J. C. (1960) J. N.Z. Inst. Chem. , 24, 161
4. Dacre, J. C. (1961) Biochem. J., 78, 758
5. Golder, W. S., Ryan, A. J. & Wright, S. E. (1962) J. Pharm.
Pharmacol., 14, 268
6. Ladomery. L. F., Ryan, A. J. & Wright, S. E. (1963) J. Pharm.
Pharmacol., 15, 771
7. Gage. J. C. (1964) Unpublished report, Imperial Chemical Industries
Limited
8. Deichmann, W. B., Clemmer, J. J., Rakoczy, R. & Bianchine, J.
(1955) A.M.A. Arch. industr. Hlth, 11, 93
9. British Industrial Biological Research Association (Unpublished
report submitted to WHO in 1964)
10. Johnson, A. R. (Unpublished report submitted to WHO in 1964)
11. Day, A. J., Johnson, A. R., O'Halloran, M. W. & Schwartz, C. J.
(1959) Aust. J. exp. Biol. med. Sci., 37, 295
12. Ames, S.R., Ludwig, H. I., Swanson, W. J. & Harris, P. L. (1956)
Proc. Soc. exp. Biol. (N.Y.), 93, 39
13. Brown, W. D., Johnson, A. R. & O'Halloran, M. W. (1959) Aust. J.
exp. Biol. med. Sci., 37, 533
14. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
15. Karpliuk, I. A. (1959) Vop. Pitan., 18, 24
16. Industrial Bio-Test Laboratories (1964) Progress report to
Hercules Powder Company
17. Imperial Chemical Industries Limited (1964) Unpublished report
No. IHR/158
18. Denz, F. A. & Llaurado, J. G. (1957) Brit. J. exp. Path., 38, 515
19. Shellenberger. T. E., Parrish, D. B. & Sanford, P. E. (1957)
Poultry Sci., 36, 1313
MOLECULAR WEIGHT 220.36
DEFINITION Butylated hydroxytoluene should contain not less
than 99.0% of C15H24O.
DESCRIPTION A white, crystalline, odourless solid. It is
insoluble in water but soluble in fats; 1 g is
soluble in 4 ml of ethanol.
USE As an antioxidant for fats and oils or in
packaging material for fat-containing foods. Its
activity is enhanced in combination with other
antioxidants and synergists.
Biological Data
Biochemical aspects
BHT is readily absorbed. Some deposition in adipose tissue bee been
described following high dosage in the rat, and this may cause
increased stability of the extracted perineal fat.1 The metabolism
of BHT is complicated by the presence of the butyl groups on each side
of the hydroxyl. Preliminary modification is necessary before
conjugation can occur. This takes the form of either oxidation of one
of the butyl groups to a 2-hydroxy-2:2-dimethyl-2-ethyl group, or
oxidation of the methyl group to a carboxy acid. The former yields
glucuronide conjugates, while the latter becomes conjugated with
glucuronic acid or glycine. Some of the oxidized material is also
excreted unconjugated.2,3
Rabbits were given single or repeated doses of BHT in the range
400-800 mg per kg body-weight. About 16% of the dose was excreted as
ester glucuronide and 19% as ether glucuronide. Unconjugated phenol
(8%), ethereal sulfate (8%) and a glycine conjugate (2%) were also
excreted. Excretion of all detectable metabolites was essentially
complete 3 to 4 days after administration of the compound and about
54% of the dose was accounted for as identified metabolites.4
Rats were given doses of 100 µg of BHT labelled with 3H
intraperitoneally and the urinary output of radioactivity was measured
for 4 consecutive days. Four days after the injection 34.5% of the
injected radioactivity was recovered in urine.5 The same dose of BHT
(100 µg) labelled with 14C was given to rats and 34% of the
radioactivity was excreted in the urine in the first 4 days, in close
agreement with the previous result using tritiated BHT.6
The liver and body fat of rats fed a diet containing 0.5% BHT for 35
days were analyzed. The concentration of BHT in the liver never rose
above 5 ppm in males or 1.5 ppm in females. In the body fat the level
fluctuated round 30 ppm in males and 45 ppm in females. Fat from rats
returned to normal diet showed a progressive fall in the concentration
of BHT, the half-life being about 7 to 10 days. The daily excretion
of radioactivity in urine and faeces was studied in rats given an oral
done of 14C-labelled BHT (12 mg/kg body-weight). Excretion became
negligible by the sixth day after administration when about 70% of the
injected dose had been recovered. Less than 1% was excreted as carbon
dioxide in the expired air. About 50% of the radioactivity was
excreted in the bile during the 24-hour period following the oral
dose.7
Acute toxicity
Animal Route LD50 Approximate lethal dose Reference
(mg/kg (mg/kg body-weight)
body-weight)
Rat oral 1700-1970 - 8
Cat oral - 940-2100 8
Rabbit oral - 2100-3200 8
Guinea-pig oral - 10 700 8
Short-term studies
Mouse. BHT was given to pregnant mice in daily doses of 750 mg per
kg body-weight for 18 days. Another group received the same dose for
a total of 50 to 64 days including 18 days of pregnancy. No foetal
abnormalities were observed.9
In a statistically planned experiment using 144 female mice no
blindness was observed in any of the 1162 litters representing 7765
babies born throughout the reproductive life span of the mothers.10
Rat. Feeding experiments were carried out on 45 pairs of weanling
male rats for 5 to 8 weeks with diets containing 0, 10 and 20% lard
supplements to which 0.001%, 0.1% or 0.5% BHT had been added. 0.001%
caused no changes in any of the serum constituents studied. 0.5%
produced increase in the serum cholesterol level within 5 weeks.
Female rate fed for 8 months on a diet containing a 10% lard
supplement with 0.1% BHT showed increased serum cholesterol levels,
but no other significant changes. 0.5% BHT in 10% and 20% lard
supplements fed to female rats for the same period increased serum
cholesterol, phospholipid and mucoprotein levels.11
0.3% BHT in the diet of pregnant rats that had been kept for 5 weeks
on a diet deficient in vitamin E produced no toxic symptoms. 1.55%
caused drastic loss of weight and foetal death.12
BHT fed to rats in groups of 12 for a period of 7 weeks at a dietary
level of 0.1% in conjunction with a 20% lard supplement significantly
reduced the initial growth rate and mature weight of male rats. No
effect was noted in female or male rats with a 10% lard supplement. A
paired feeding experiment showed that this inhibition of growth was a
direct toxic effect of BHT and could not be explained by a reduction
in the palatability of the diet. At this level BHT produced a
significant increase in the weight of the liver, both absolute and
relative to body-weight. Rats under increased stress showed
significant loss of hair from the top of the head. The toxic effect
of BHT was greater if the fat load in the diet was increased.
Anophthalmia occurred in 10% of the litter's.13
Groups of 6 weanling rats (3 male and 3 female) were fed BHT at
dietary levels of 0%, 0.1%. 0.2%, 0.3%, 0.4% and 0.5% in conjunction
with a 20% lard supplement for 6 weeks. BHT reduced the growth rate,
especially in the males, the effect appearing to become significant at
the 0.3% level. It also increased the absolute liver weight and the
ratio of liver weight to body-weight in both sexes, the latter effect
appearing to become significant at the 0.2% level. BHT increased the
ratio of left adrenal weight to body-weight in male rats but had no
consistent effect in females. There were no histological changes
attributable to the treatment in the adrenal. All dietary levels of
BHT increased the serum cholesterol and the concentration of the
cholesterol was directly proportional to the BHT level. There was
also a significant increase in the concentration of adrenal
cholesterol. BHT produced no significant changes in the concentration
of total or per cent. esterified liver cholesterol, total liver lipid
or concentration of total polyunsaturated fatty acids in the liver.14
BHT administered to rats at 250 mg per kg body-weight for 68 to 82
days caused reduction in rate of increase in weight and fatty
infiltration of the liver.15
Feeding experiments conducted for 20 and 90 days respectively
indicated that rats do not find food containing 0.5% or 1% BHT
palatable. However, the animals ingest foods so treated more readily
if these concentrations are attained gradually. Paired feeding
experiments with groups of 5 or 10 rats for 25 days demonstrated that
diets containing 0.8% and 1% BHT will reduce the daily intake of food
below control values. A level of 1% in the diet retarded weight
gain.8
(Work in progress) Rats were given single doses of 100 mg BHT per kg
body-weight daily for 7 weeks before mating and then throughout
pregnancy or were autopsied on the 20th day of pregnancy. No evidence
of foetal abnormality were found in any of these animals but
abnormalities did occur in the progeny of positive control groups
treated with vitamin A.9
(Work in progress) A three-generation reproduction study was started
by another group in May 1964. They also fed groups of 16 male and 16
female rats on levels of 0.03%, 0.1% and 0.3% BHT in a diet containing
20% fat for 10 weeks. There were two control groups each containing
16 male and 16 female animals. No definite effect on body-weight was
observed at any level in the females and there was only a slight
depression in the males at the 0.3% level. There was no significant
effect on blood cholesterol level in either sex after feeding BHT at
any of the levels for 10 weeks. Four of the males at the 0.3% and two
at the 0.1% level died during the experiment. Two deaths occurred
among the females at O.3%. Only one male rat died in both control
groups.16
(Work In progress) Groups of 20 male and 20 female rats fed 1% BHT in
the diet for 10 weeks showed recovery both in liver to body-weight
ratios and in morphological appearance of the liver cells within a few
weeks after restoring the animals to a normal diet.17
Rabbit. Acute effects on electrolyte excretion similar to those
described for large doses of BHA were also obtained following
administration of doses of BHT of 500-700 mg/kg body-weight (about 2%
in the diet). No such effects were observed at lower dosage
levels.18
Dog. A mild to moderately severe degree of diarrhoea was induced in
a group of 4 dogs fed doses of 1.4-4.7 g/kg body-weight every 2-4 days
over a period of 4 weeks. Post-mortem examination revealed no
significant gross pathological changes. No signs of intoxication and
no gross or histopathological changes were observed in dogs fed doses
of 0.17-0.94 g/kg body-weight 5 days a week for a period of 12
months.8
Fowl. When BHT was fed at a level of 0.125% for 34 weeks to a group
of 10 pullets, no differences in fertility, hatchability of eggs or
health of chicks in comparison with a similar control group were
found. The eggs of the antioxidant-treated birds contained more
carotenoids and vitamin A than those of the controls.19
Long-term studies
Rat. Groups of 15 male and 15 female rats given diets containing 1%
lard and 0.2%, 0.5% or 0.8% BHT for 24 months showed no specific signs
of intoxication, and micropathological studies were negative. For one
group given a diet containing .5% BHT, the BHT was dissolved in lard
and then heated for 30 minutes at 150°C before incorporation in the
diet. There were no effects on weight gain or blood constituents and
micropathological studies of the main organs were negative. The
feeding of 1% BHT was followed in both male and female rats by a
sub-normal weight gain and by an increase in the weight of the brain
and liver and some other organs in relation to body-weight.
Micropathological studies were negative in this group also. BHT in
these concentrations had no specific effect on the number of
erythrocytes and leucocytes, or on the concentration of haemoglobin in
the peripheral blood. A number of rats of both sexes died during this
experiment, but as the fatalities were in no relation to the
concentration of BHT fed, it was believed that the cause of death was
unrelated to the feeding of this substance. Micropathological studies
support this observation.8
When fed at the 0.5% level in the diet, BHT had no effect in rats on
the reproductive cycle, the histology of the spleen, kidney, liver and
skin, or on the weight of the heart, spleen or kidney. There was no
significant increase in mortality of rats fed on a diet containing
0.1% BHT and 10% hydrogenated coconut oil for a period of 2 years. The
effects on weight gain have already been described.13
Comment on experimental studies reported
Experimental studies have been carried out in several species. There
were, however, some important discrepancies between the results
obtained by earlier and by some more recent observers. Short-term and
long-term studies in rats and metabolic studies will be discussed
under evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
One extensive series of studies8 indicates that the level of BHT that
would cause no significant deleterious effect in the rat would be 0.8%
in a diet containing 1% lard. However, in two short-term studies11,13
it has been reported that 0.1% BHT in a 20% lard diet resulted in
increase of various lipid components of the blood and a significant
reduction in weight gain. More recently a similar trend of results
was obtained but the effects only became definite at the 0.3%
level.14 In another study 0.1% BHT in a 20% fat diet was found to
have no effect on the growth rate of weanling rats of either sex and
there was no effect on the blood cholesterol concentration at the 0.1%
or the 0.3% levels.16 In long-term studies with rats receiving 0.5%
BHT in a diet containing 10% lard no significant difference from the
controls was observed.13 The addition of 10% or 20% of lard to the
diet was found to enhance the deleterious action of BHT in some
studies. With a 20% lard supplement, fat provides about 33% of the
calorie intake and human diets frequently contain this level of fat.
The effect of such levels of dietary fat on BHT toxicity is therefore
relevant to its use as a food additive. Several investigators have
shown that BHT will cause an increase in liver weight relative to
body-weight. However, even in rats fed at 1% BHT in the diet for 10
weeks, there was recovery in the liver to body-weight ratio and
morphological appearance of the liver cells within a few weeks of
restoration of the animals to a normal diet.17
The chemical structure of BHT suggests the possibility of some delay
in metabolism. The results of studies on the excretion of labelled
BHT5,6,7 are difficult to compare because of the different dose levels
and routes of administration used and therefore the importance of
possible delayed metabolism is difficult to assess.
A possible teratogenic action of BHT was suggested by the occurrence
of anophthalmia in some litters of mothers fed the compound.13
Extensive reproduction studies now completed or in progress make this
unlikely.9,10,16
For the 0.1% level to be accepted as that causing no significant
toxicological effect in the rat it would be necessary to disregard
both the effect on weight gain and also the effect on blood lipids
reported by some investigators.
It would be inadvisable to dismiss these observations pending the
evaluation of results of work now in progress. The next lower dose
studied was 0.01% (= 100 ppm) in the diet in a group of 26 rats, which
is equivalent to 5 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-0.5
Further Work Considered Desirable
1. Further long-term studies, with particular reference to the effect
of BHT on lipid metabolism and the relationship between the dietary
fat load and toxicity.
2. Metabolic studies in human subjects.
References
1. Johnson, A. R., O'Halloran, M. W. & Hewgill, F. R. (1956) J. Amer.
Oil Chem. Soc., 35, 496
2. Dacre, J. C., Denz, F. A. & Kennedy. T. H. (1956) Biochem. J., 64,
777
3. Dacre, J. C. (1960) J. N.Z. Inst. Chem. , 24, 161
4. Dacre, J. C. (1961) Biochem. J., 78, 758
5. Golder, W. S., Ryan, A. J. & Wright, S. E. (1962) J. Pharm.
Pharmacol., 14, 268
6. Ladomery. L. F., Ryan, A. J. & Wright, S. E. (1963) J. Pharm.
Pharmacol., 15, 771
7. Gage. J. C. (1964) Unpublished report, Imperial Chemical Industries
Limited
8. Deichmann, W. B., Clemmer, J. J., Rakoczy, R. & Bianchine, J.
(1955) A.M.A. Arch. industr. Hlth, 11, 93
9. British Industrial Biological Research Association (Unpublished
report submitted to WHO in 1964)
10. Johnson, A. R. (Unpublished report submitted to WHO in 1964)
11. Day, A. J., Johnson, A. R., O'Halloran, M. W. & Schwartz, C. J.
(1959) Aust. J. exp. Biol. med. Sci., 37, 295
12. Ames, S.R., Ludwig, H. I., Swanson, W. J. & Harris, P. L. (1956)
Proc. Soc. exp. Biol. (N.Y.), 93, 39
13. Brown, W. D., Johnson, A. R. & O'Halloran, M. W. (1959) Aust. J.
exp. Biol. med. Sci., 37, 533
14. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
15. Karpliuk, I. A. (1959) Vop. Pitan., 18, 24
16. Industrial Bio-Test Laboratories (1964) Progress report to
Hercules Powder Company
17. Imperial Chemical Industries Limited (1964) Unpublished report
No. IHR/158
18. Denz, F. A. & Llaurado, J. G. (1957) Brit. J. exp. Path., 38, 515
19. Shellenberger. T. E., Parrish, D. B. & Sanford, P. E. (1957)
Poultry Sci., 36, 1313
