
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
FORMIC ACID
CHEMICAL NAMES Formic acid; methanoic acid
EMPIRICAL FORMULA CH2O2
STRUCTURAL FORMULA
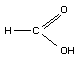 MOLECULAR WEIGHT 46.03
DEFINITION Formic acid contains not less than 88.0% of
CH2O2 and conforms to the following
specifications.
DESCRIPTION Formic acid is a clear, colourless, highly
corrosive liquid with a characteristic pungent
odour.
USE As a preservative and as a flavour adjunct.
IDENTIFICATION TESTS
A. Solubility: Miscible with water, ethanol, ether, glycerol.
B. Neutralize 1 ml of formic acid with sodium hydroxide TS and add 2
drops of the acid in excess; then add about 1 ml of ferric
chloride TS: a deep reddish orange colour results which turns to
yellowish orange on the addition of mineral acids.
C. Place 2 ml of formic acid in a test-tube, add 5 ml of sulfuric
acid and test the gas evolved with a lighted splinter: a blue
flame characteristic of carbon monoxide is produced.
PURITY TESTS
Acetic acid: Not more than 0.4%.
Dilute 1 ml (1.2 g) of formic acid to 100 ml. To 50 ml of this
solution contained in a 250-ml boiling flask add 5 g of yellow
mercuric oxide. Boil the solution under total reflux for 2 hours,
stirring the mixture continuously. Cool, filter and wash the residue
with about 25 ml of water. Add 0.10 ml of phenolphthalein indicator
solution to the combined filtrate and washings and titrate with 0.02
N sodium hydroxide. Not more than 2.0 ml of the 0.02 N sodium
hydroxide should be required to produce a pink colour.
Arsenic: Not more than 3 mg/kg.
Sulfite: Dilute 25 ml of formic acid with 25 ml of water and add
0.1 ml of 0.1 N iodine solution. The solution should retain a
distinct yellow colour.
Heavy metals: Not more than 5 mg/kg.
To 3.3 ml (4 g) add about 10 mg of sodium carbonate and evaporate to
dryness on a steam bath. Dissolve in 1 ml of 1 N acetic acid and
dilute to 25 ml.
ASSAY
Weigh accurately a 125-ml glass-stoppered flask containing 15 ml of
water. Quickly introduce 1.0 to 1.5 ml of formic acid and reweigh.
Dilute to about 50 ml, add 3 drops of phenolphthalein and titrate with
1 N sodium hydroxide. 1 ml of 1 N sodium hydroxide is equivalent
to 46.03 mg of CH2O2.
Biological Data
Biochemical aspects
Formate is an intermediate in normal metabolism. It takes part in the
metabolism of one-carbon compounds and its carbon may appear in methyl
groups undergoing transmethylation. It is eventually oxidized to
carbon dioxide.1 When formate is administered it could also be
expected to enter one-carbon metabolism. However, there is a species
difference in the extent of this metabolism, for in rabbits no
administered formate is excreted, whereas in dogs about half the
administered formate is excreted unchanged in the urine.2 Its
metabolism in human beings is probably somewhere between that in dogs
and that in rabbits, judging from the relative amounts of formate
excreted by man, dogs and rabbits receiving methanol.3,4 Formic acid
(or formate) is apparently more toxic than other fatty acids, possibly
owing to its enzyme-inhibiting activity.5 However, no cumulative
toxic effects are known.
Acute toxicity
Exact LD50 values are not available. In dogs, sodium formate in oral
doses of 4000 mg/kg and intravenous doses of 3000 mg/kg body-weight
produced toxic effects such as methaemoglobinaemia and heart
congestion.6 About 50 mg/kg in 10% aqueous solution given orally to
dogs or 6 mg/kg given subcutaneously to rabbits produced
methaemoglobinaemia which lasted about 10 days.2
This slow disappearance may be due to the inhibition of catalase by
formic acid.7 4.6 mg per kg intravenously given to 6 dogs produced
no ill effect and 13.8 mg per kg only slight hypertension.8
Short-term studies
Dog. 0.5 g of formic acid daily in the food has been tolerated by
dogs without effect.9
Man. 2-4 g of sodium formate daily did not produce toxic
manifestations in human subjects, even if they were suffering from
kidney disease. It has been stated that a daily intake of 2.4 g for
therapeutic purposes could be tolerated for months without untoward
effects.10
Comments on experimental studies reported
Since long-term toxicity studies are lacking, it is not possible to
give guidance on an unconditional acceptable daily intake level in
man.
Evaluation
Level causing no significant toxicological effect
Short-term studies in dogs and man indicate that formic acid has no
significant toxicological effect at a dosage of about 50 mg/kg
body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-5
Further Work Considered Desirable
Long-term toxicity studies in animals and metabolism studies in man.
References
1. Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall
2. Croner, P. & Selligmann, E. (1907) Z. Hyg. Infekt.-Kr., 56, 387
3. Lund, A. (1948) Acta pharmacol. (Kbh.), 4, 99
4. Lund, A. (1948) Acta pharmacol. (Kbh.) 4, 108
5. Bleyer, B., Diemair, W. & Leonhard, K. (1933) Arch. Pharm.
(Weinheim), 271, 539
6. Fleig, C. (1907) Arch. int. Pharmacodyn., 17, 147
7. Lück, H. (1957) Biochem. Z., 328, 411
8. Erra, U. (1958) Fol. med. (Napoli), 41. 366
9.Dick (1909) Hygienische Rundschan, 14, 313
10. Rost, E. (1917) Arb. Reichsgesundh.-Amte, 50, 405
MOLECULAR WEIGHT 46.03
DEFINITION Formic acid contains not less than 88.0% of
CH2O2 and conforms to the following
specifications.
DESCRIPTION Formic acid is a clear, colourless, highly
corrosive liquid with a characteristic pungent
odour.
USE As a preservative and as a flavour adjunct.
IDENTIFICATION TESTS
A. Solubility: Miscible with water, ethanol, ether, glycerol.
B. Neutralize 1 ml of formic acid with sodium hydroxide TS and add 2
drops of the acid in excess; then add about 1 ml of ferric
chloride TS: a deep reddish orange colour results which turns to
yellowish orange on the addition of mineral acids.
C. Place 2 ml of formic acid in a test-tube, add 5 ml of sulfuric
acid and test the gas evolved with a lighted splinter: a blue
flame characteristic of carbon monoxide is produced.
PURITY TESTS
Acetic acid: Not more than 0.4%.
Dilute 1 ml (1.2 g) of formic acid to 100 ml. To 50 ml of this
solution contained in a 250-ml boiling flask add 5 g of yellow
mercuric oxide. Boil the solution under total reflux for 2 hours,
stirring the mixture continuously. Cool, filter and wash the residue
with about 25 ml of water. Add 0.10 ml of phenolphthalein indicator
solution to the combined filtrate and washings and titrate with 0.02
N sodium hydroxide. Not more than 2.0 ml of the 0.02 N sodium
hydroxide should be required to produce a pink colour.
Arsenic: Not more than 3 mg/kg.
Sulfite: Dilute 25 ml of formic acid with 25 ml of water and add
0.1 ml of 0.1 N iodine solution. The solution should retain a
distinct yellow colour.
Heavy metals: Not more than 5 mg/kg.
To 3.3 ml (4 g) add about 10 mg of sodium carbonate and evaporate to
dryness on a steam bath. Dissolve in 1 ml of 1 N acetic acid and
dilute to 25 ml.
ASSAY
Weigh accurately a 125-ml glass-stoppered flask containing 15 ml of
water. Quickly introduce 1.0 to 1.5 ml of formic acid and reweigh.
Dilute to about 50 ml, add 3 drops of phenolphthalein and titrate with
1 N sodium hydroxide. 1 ml of 1 N sodium hydroxide is equivalent
to 46.03 mg of CH2O2.
Biological Data
Biochemical aspects
Formate is an intermediate in normal metabolism. It takes part in the
metabolism of one-carbon compounds and its carbon may appear in methyl
groups undergoing transmethylation. It is eventually oxidized to
carbon dioxide.1 When formate is administered it could also be
expected to enter one-carbon metabolism. However, there is a species
difference in the extent of this metabolism, for in rabbits no
administered formate is excreted, whereas in dogs about half the
administered formate is excreted unchanged in the urine.2 Its
metabolism in human beings is probably somewhere between that in dogs
and that in rabbits, judging from the relative amounts of formate
excreted by man, dogs and rabbits receiving methanol.3,4 Formic acid
(or formate) is apparently more toxic than other fatty acids, possibly
owing to its enzyme-inhibiting activity.5 However, no cumulative
toxic effects are known.
Acute toxicity
Exact LD50 values are not available. In dogs, sodium formate in oral
doses of 4000 mg/kg and intravenous doses of 3000 mg/kg body-weight
produced toxic effects such as methaemoglobinaemia and heart
congestion.6 About 50 mg/kg in 10% aqueous solution given orally to
dogs or 6 mg/kg given subcutaneously to rabbits produced
methaemoglobinaemia which lasted about 10 days.2
This slow disappearance may be due to the inhibition of catalase by
formic acid.7 4.6 mg per kg intravenously given to 6 dogs produced
no ill effect and 13.8 mg per kg only slight hypertension.8
Short-term studies
Dog. 0.5 g of formic acid daily in the food has been tolerated by
dogs without effect.9
Man. 2-4 g of sodium formate daily did not produce toxic
manifestations in human subjects, even if they were suffering from
kidney disease. It has been stated that a daily intake of 2.4 g for
therapeutic purposes could be tolerated for months without untoward
effects.10
Comments on experimental studies reported
Since long-term toxicity studies are lacking, it is not possible to
give guidance on an unconditional acceptable daily intake level in
man.
Evaluation
Level causing no significant toxicological effect
Short-term studies in dogs and man indicate that formic acid has no
significant toxicological effect at a dosage of about 50 mg/kg
body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-5
Further Work Considered Desirable
Long-term toxicity studies in animals and metabolism studies in man.
References
1. Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall
2. Croner, P. & Selligmann, E. (1907) Z. Hyg. Infekt.-Kr., 56, 387
3. Lund, A. (1948) Acta pharmacol. (Kbh.), 4, 99
4. Lund, A. (1948) Acta pharmacol. (Kbh.) 4, 108
5. Bleyer, B., Diemair, W. & Leonhard, K. (1933) Arch. Pharm.
(Weinheim), 271, 539
6. Fleig, C. (1907) Arch. int. Pharmacodyn., 17, 147
7. Lück, H. (1957) Biochem. Z., 328, 411
8. Erra, U. (1958) Fol. med. (Napoli), 41. 366
9.Dick (1909) Hygienische Rundschan, 14, 313
10. Rost, E. (1917) Arb. Reichsgesundh.-Amte, 50, 405
See Also:
Toxicological Abbreviations
Formic acid (ICSC)
Formic acid (WHO Food Additives Series 5)
FORMIC ACID (JECFA Evaluation)
 MOLECULAR WEIGHT 46.03
DEFINITION Formic acid contains not less than 88.0% of
CH2O2 and conforms to the following
specifications.
DESCRIPTION Formic acid is a clear, colourless, highly
corrosive liquid with a characteristic pungent
odour.
USE As a preservative and as a flavour adjunct.
IDENTIFICATION TESTS
A. Solubility: Miscible with water, ethanol, ether, glycerol.
B. Neutralize 1 ml of formic acid with sodium hydroxide TS and add 2
drops of the acid in excess; then add about 1 ml of ferric
chloride TS: a deep reddish orange colour results which turns to
yellowish orange on the addition of mineral acids.
C. Place 2 ml of formic acid in a test-tube, add 5 ml of sulfuric
acid and test the gas evolved with a lighted splinter: a blue
flame characteristic of carbon monoxide is produced.
PURITY TESTS
Acetic acid: Not more than 0.4%.
Dilute 1 ml (1.2 g) of formic acid to 100 ml. To 50 ml of this
solution contained in a 250-ml boiling flask add 5 g of yellow
mercuric oxide. Boil the solution under total reflux for 2 hours,
stirring the mixture continuously. Cool, filter and wash the residue
with about 25 ml of water. Add 0.10 ml of phenolphthalein indicator
solution to the combined filtrate and washings and titrate with 0.02
N sodium hydroxide. Not more than 2.0 ml of the 0.02 N sodium
hydroxide should be required to produce a pink colour.
Arsenic: Not more than 3 mg/kg.
Sulfite: Dilute 25 ml of formic acid with 25 ml of water and add
0.1 ml of 0.1 N iodine solution. The solution should retain a
distinct yellow colour.
Heavy metals: Not more than 5 mg/kg.
To 3.3 ml (4 g) add about 10 mg of sodium carbonate and evaporate to
dryness on a steam bath. Dissolve in 1 ml of 1 N acetic acid and
dilute to 25 ml.
ASSAY
Weigh accurately a 125-ml glass-stoppered flask containing 15 ml of
water. Quickly introduce 1.0 to 1.5 ml of formic acid and reweigh.
Dilute to about 50 ml, add 3 drops of phenolphthalein and titrate with
1 N sodium hydroxide. 1 ml of 1 N sodium hydroxide is equivalent
to 46.03 mg of CH2O2.
Biological Data
Biochemical aspects
Formate is an intermediate in normal metabolism. It takes part in the
metabolism of one-carbon compounds and its carbon may appear in methyl
groups undergoing transmethylation. It is eventually oxidized to
carbon dioxide.1 When formate is administered it could also be
expected to enter one-carbon metabolism. However, there is a species
difference in the extent of this metabolism, for in rabbits no
administered formate is excreted, whereas in dogs about half the
administered formate is excreted unchanged in the urine.2 Its
metabolism in human beings is probably somewhere between that in dogs
and that in rabbits, judging from the relative amounts of formate
excreted by man, dogs and rabbits receiving methanol.3,4 Formic acid
(or formate) is apparently more toxic than other fatty acids, possibly
owing to its enzyme-inhibiting activity.5 However, no cumulative
toxic effects are known.
Acute toxicity
Exact LD50 values are not available. In dogs, sodium formate in oral
doses of 4000 mg/kg and intravenous doses of 3000 mg/kg body-weight
produced toxic effects such as methaemoglobinaemia and heart
congestion.6 About 50 mg/kg in 10% aqueous solution given orally to
dogs or 6 mg/kg given subcutaneously to rabbits produced
methaemoglobinaemia which lasted about 10 days.2
This slow disappearance may be due to the inhibition of catalase by
formic acid.7 4.6 mg per kg intravenously given to 6 dogs produced
no ill effect and 13.8 mg per kg only slight hypertension.8
Short-term studies
Dog. 0.5 g of formic acid daily in the food has been tolerated by
dogs without effect.9
Man. 2-4 g of sodium formate daily did not produce toxic
manifestations in human subjects, even if they were suffering from
kidney disease. It has been stated that a daily intake of 2.4 g for
therapeutic purposes could be tolerated for months without untoward
effects.10
Comments on experimental studies reported
Since long-term toxicity studies are lacking, it is not possible to
give guidance on an unconditional acceptable daily intake level in
man.
Evaluation
Level causing no significant toxicological effect
Short-term studies in dogs and man indicate that formic acid has no
significant toxicological effect at a dosage of about 50 mg/kg
body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-5
Further Work Considered Desirable
Long-term toxicity studies in animals and metabolism studies in man.
References
1. Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall
2. Croner, P. & Selligmann, E. (1907) Z. Hyg. Infekt.-Kr., 56, 387
3. Lund, A. (1948) Acta pharmacol. (Kbh.), 4, 99
4. Lund, A. (1948) Acta pharmacol. (Kbh.) 4, 108
5. Bleyer, B., Diemair, W. & Leonhard, K. (1933) Arch. Pharm.
(Weinheim), 271, 539
6. Fleig, C. (1907) Arch. int. Pharmacodyn., 17, 147
7. Lück, H. (1957) Biochem. Z., 328, 411
8. Erra, U. (1958) Fol. med. (Napoli), 41. 366
9.Dick (1909) Hygienische Rundschan, 14, 313
10. Rost, E. (1917) Arb. Reichsgesundh.-Amte, 50, 405
MOLECULAR WEIGHT 46.03
DEFINITION Formic acid contains not less than 88.0% of
CH2O2 and conforms to the following
specifications.
DESCRIPTION Formic acid is a clear, colourless, highly
corrosive liquid with a characteristic pungent
odour.
USE As a preservative and as a flavour adjunct.
IDENTIFICATION TESTS
A. Solubility: Miscible with water, ethanol, ether, glycerol.
B. Neutralize 1 ml of formic acid with sodium hydroxide TS and add 2
drops of the acid in excess; then add about 1 ml of ferric
chloride TS: a deep reddish orange colour results which turns to
yellowish orange on the addition of mineral acids.
C. Place 2 ml of formic acid in a test-tube, add 5 ml of sulfuric
acid and test the gas evolved with a lighted splinter: a blue
flame characteristic of carbon monoxide is produced.
PURITY TESTS
Acetic acid: Not more than 0.4%.
Dilute 1 ml (1.2 g) of formic acid to 100 ml. To 50 ml of this
solution contained in a 250-ml boiling flask add 5 g of yellow
mercuric oxide. Boil the solution under total reflux for 2 hours,
stirring the mixture continuously. Cool, filter and wash the residue
with about 25 ml of water. Add 0.10 ml of phenolphthalein indicator
solution to the combined filtrate and washings and titrate with 0.02
N sodium hydroxide. Not more than 2.0 ml of the 0.02 N sodium
hydroxide should be required to produce a pink colour.
Arsenic: Not more than 3 mg/kg.
Sulfite: Dilute 25 ml of formic acid with 25 ml of water and add
0.1 ml of 0.1 N iodine solution. The solution should retain a
distinct yellow colour.
Heavy metals: Not more than 5 mg/kg.
To 3.3 ml (4 g) add about 10 mg of sodium carbonate and evaporate to
dryness on a steam bath. Dissolve in 1 ml of 1 N acetic acid and
dilute to 25 ml.
ASSAY
Weigh accurately a 125-ml glass-stoppered flask containing 15 ml of
water. Quickly introduce 1.0 to 1.5 ml of formic acid and reweigh.
Dilute to about 50 ml, add 3 drops of phenolphthalein and titrate with
1 N sodium hydroxide. 1 ml of 1 N sodium hydroxide is equivalent
to 46.03 mg of CH2O2.
Biological Data
Biochemical aspects
Formate is an intermediate in normal metabolism. It takes part in the
metabolism of one-carbon compounds and its carbon may appear in methyl
groups undergoing transmethylation. It is eventually oxidized to
carbon dioxide.1 When formate is administered it could also be
expected to enter one-carbon metabolism. However, there is a species
difference in the extent of this metabolism, for in rabbits no
administered formate is excreted, whereas in dogs about half the
administered formate is excreted unchanged in the urine.2 Its
metabolism in human beings is probably somewhere between that in dogs
and that in rabbits, judging from the relative amounts of formate
excreted by man, dogs and rabbits receiving methanol.3,4 Formic acid
(or formate) is apparently more toxic than other fatty acids, possibly
owing to its enzyme-inhibiting activity.5 However, no cumulative
toxic effects are known.
Acute toxicity
Exact LD50 values are not available. In dogs, sodium formate in oral
doses of 4000 mg/kg and intravenous doses of 3000 mg/kg body-weight
produced toxic effects such as methaemoglobinaemia and heart
congestion.6 About 50 mg/kg in 10% aqueous solution given orally to
dogs or 6 mg/kg given subcutaneously to rabbits produced
methaemoglobinaemia which lasted about 10 days.2
This slow disappearance may be due to the inhibition of catalase by
formic acid.7 4.6 mg per kg intravenously given to 6 dogs produced
no ill effect and 13.8 mg per kg only slight hypertension.8
Short-term studies
Dog. 0.5 g of formic acid daily in the food has been tolerated by
dogs without effect.9
Man. 2-4 g of sodium formate daily did not produce toxic
manifestations in human subjects, even if they were suffering from
kidney disease. It has been stated that a daily intake of 2.4 g for
therapeutic purposes could be tolerated for months without untoward
effects.10
Comments on experimental studies reported
Since long-term toxicity studies are lacking, it is not possible to
give guidance on an unconditional acceptable daily intake level in
man.
Evaluation
Level causing no significant toxicological effect
Short-term studies in dogs and man indicate that formic acid has no
significant toxicological effect at a dosage of about 50 mg/kg
body-weight per day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-5
Further Work Considered Desirable
Long-term toxicity studies in animals and metabolism studies in man.
References
1. Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall
2. Croner, P. & Selligmann, E. (1907) Z. Hyg. Infekt.-Kr., 56, 387
3. Lund, A. (1948) Acta pharmacol. (Kbh.), 4, 99
4. Lund, A. (1948) Acta pharmacol. (Kbh.) 4, 108
5. Bleyer, B., Diemair, W. & Leonhard, K. (1933) Arch. Pharm.
(Weinheim), 271, 539
6. Fleig, C. (1907) Arch. int. Pharmacodyn., 17, 147
7. Lück, H. (1957) Biochem. Z., 328, 411
8. Erra, U. (1958) Fol. med. (Napoli), 41. 366
9.Dick (1909) Hygienische Rundschan, 14, 313
10. Rost, E. (1917) Arb. Reichsgesundh.-Amte, 50, 405
