
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES: 43
Prepared by the Fifty-second meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
IPCS - International Programme on Chemical Safety
INSECTICIDE
PHOXIM
First draft prepared by M.E.J. Pronk and G.J. Schefferlie
Centre for Substances and Risk Assessment
National Institute of Public Health and the Environment
Bilthoven, The Netherlands
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Special studies on delayed neurotoxicity
Studies of metabolites
Comments
Evaluation
References
1. EXPLANATION
Phoxim is an organophosphorus insecticide used for topical
treatment of cattle, sheep, goats, and pigs. It has not been evaluated
previously by this Committee. Phoxim was evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues (JMPR) in 1982 and 1984
(FAO/WHO, 1983, 1985). The 1984 Joint Meeting established an ADI of
0-1 µg/kg bw on the basis of inhibition of plasma cholinesterase
activity. Phoxim was re-evaluated at the present meeting at the
request of the Codex Committee on Residues of Veterinary Drugs in
Foods. It is no longer supported for use as a plant protection
product.
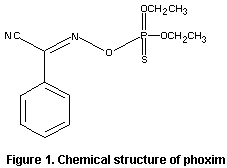
The chemical name of phoxim is
diethyl- O-(alpha-cyanobenzylideneamino)-thiophosphate. The structure
is shown in Figure 1. For reasons of stability, the active ingredient
phoxim is present as a pre-solution containing phoxim and 1-butanol in
which the content of phoxim varies from 82 to 91%. The preparation
used in the studies of toxicity is the pre-solution.
Organophosphorus insecticides exert their acute effects in both
insects and mammals by inhibiting acetylcholinesterase in the nervous
system, with subsequent accumulation of toxic levels of the
neurotransmitter acetylcholine. This results in over-stimulation of
central cholinergic neurons and of the parasympathetic nervous and
neuromuscular systems. Some organophosphorus insecticides also cause
delayed neuropathic effects by inhibiting neuropathy target esterase
in the nervous system and by ageing of the inhibited enzyme.
Phoxim is an insecticide with selective properties: it is toxic
to insects but virtually non-toxic to mammals. Although differences in
sensitivity to cholinesterase inhibition contribute to this
selectivity, metabolism plays a more important role. In both insects
and mammals, phoxim is oxidatively desulfurated to the oxo-analogue,
PO-phoxim, which is a more potent inhibitor of cholinesterases than
phoxim itself. In mammals, however, PO-phoxim is an extremely
short-lived intermediate and, together with phoxim, is rapidly
hydrolysed to non-toxic products (Kimmerle, 1968; Vinopal & Fukuto,
1971).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Male Swiss mice (number not given) were given 32P-phoxim
(specific activity unknown) in olive oil at doses of 10, 110, or 960
mg/kg bw. Urine and faeces were collected for up to 140 h after
treatment, and radioactivity in these samples was determined by
gas-flow counting. At all three doses, the total recovery of
administered radiolabel in urine and faeces was 73-82%, the majority
of which was in urine. The excretion rate in urine was, however, slow:
at 10 and 110 mg/kg bw, only 43 and 22%, respectively, of the
administered radiolabel was excreted in urine within 24 h, while at
960 mg/kg bw only 17% was excreted within 30 h. Autopsy of a mouse
given 110 mg/kg bw at 48 h, at which time approximately 43% of the
dose had been excreted in urine, revealed that virtually all internal
radiolabel was in the urinary bladder (88%), gut (8.8%), and liver
(1.7%). Nearly all of this radiolabel was water-soluble, indicating
that the P-containing moiety of phoxim was converted almost completely
to water-soluble derivatives. Apparently, the slow excretion of
P-derived metabolites in mice is due to their retention or storage in
the urinary bladder (Vinopal & Fukuto, 1971), although the reason for
this is unknown.
Phoxim uniformly radiolabelled with 14C in the phenyl moiety
(initial specific activity, 32 µCi/mg) was administered orally in
polyethylene glycol 200 by gavage to Charles River CD rats at 1 or 10
mg/kg bw (4 µCi at both doses). In the first experiment, groups of
three male rats were killed 0.5, 1, 4, 7.5, 24, 48, 72, or 144 h after
treatment with 10 mg/kg bw, and samples of blood, tissues, and organs
(liver, kidney, muscle, fat, skin, lung, brain, heart, spleen, gonads,
adrenals, and small and large intestine plus contents) were collected.
In a second experiment, groups of three male rats were killed 0.5, 1,
4, and 7.5 h after treatment with 1 or 10 mg/kg bw, and the stomach,
 small and large intestines, and their contents were collected. In a
third experiment, urine and faeces were collected at intervals for up
to 10 days after treatment of five male and five female rats with 10
mg/kg bw, and of five male rats with 1 mg/kg bw. After 10 days, the
animals were killed, and samples of the same tissues and organs as in
the first experiment were collected. In addition, bile was collected
from three male rats with cannulated bile ducts for up to 24 h after
treatment with 10 mg/kg bw. All samples were analysed for radiolabel
by liquid scintillation spectrometry.
Phoxim at 1 or 10 mg/kg bw was rapidly absorbed. In plasma, the
total residue concentrations reached a maximum of 0.35 and 2.44 µg/ml
phoxim equivalents, respectively, within 0.5 h of administration, but
the concentrations decreased to approximately 0.1 µg/ml within 1 h of
administration of 1 mg/kg bw and remained at that level until at least
7.5 h. After 10 mg/kg bw, a second peak of 1.9 µg/ml was detected
after 4 h in the animals in the first experiment but not in those in
the second. Thereafter, the plasma concentrations decreased to
approximately 0.8 µg/ml at 7.5 h and to 0.04 µg/ml at 24 h. The
radiolabel was rapidly taken up into the major organs and tissues,
with kinetics similar to that in plasma. Over the first 7.5 h after
administration of 10 mg/kg bw, the total residue concentrations in
tissues remained fairly constant, with the highest levels in the small
intestine and its contents and slightly higher concentrations in
kidney (2.1-6.1 mg/kg phoxim equivalents) and liver (1.2-3.1 mg/kg)
than in other tissues or organs (0.04-1.2 mg/kg). Thereafter, all
tissue concentrations declined, to below 0.4 mg/kg at 24 h and to
below 0.14 mg/kg at 144 h.
Most of the radiolabel was eliminated in urine within 24 h, and
elimination was virtually complete within two days, independently of
sex and dose. The total recovery in urine over 10 days was 93% for
males and 86% for females at 10 mg/kg bw and 82% for males given 1
mg/kg bw. In faeces, 4.9, 6.9, and 7.9%, respectively, of the
administered dose was recovered in the same period. Biliary excretion
amounted to approximately 4% of the administered dose over 24 h
(Daniel et al., 1978b).
[Phenyl-U-14C]phoxim (final specific activity, 20 µCi/mg) was
administered by oral intubation in gelatin capsules to two female
German Landrace piglets at a single dose of 5 mg/kg bw (2 mCi per
animal). Blood, urine, and faeces were collected at intervals for up
to 24 and 72 h after administration. After the animals were
slaughtered, samples of liver, kidney, muscle, loin, and subcutaneous
fat were collected. All samples were analysed for radiolabel by liquid
scintillation spectrometry. The study was (partly) certified for
compliance with GLP and quality assurance.
Phoxim was rapidly absorbed: the total residue concentrations in
plasma reached a maximum of 2.3 µg/ml phoxim equivalents within 1-2 h
after administration and declined biphasically with half-lives of 0.6
h in the initial phase and 25 h in the terminal phase (from 6 h
onwards). The radiolabel was rapidly eliminated, as 82% of the
administered dose was excreted via the urine within 24 h (72% within
the first 8 h) and an additional 1.5% in the following 48 h. Faecal
excretion amounted to 1.6% of the administered dose after 24 h in one
animal and to 12% after 72 h in the other. At 24 h, the highest total
residue concentrations in edible tissues were found in fat (1.3 mg/kg
phoxim equivalents), followed by liver (0.60 mg/kg), kidney (0.35
mg/kg), and loin and muscle (0.05 mg/kg). At 72 h, the tissue
concentrations were approximately half these values (Klein & Weber,
1988).
In order to study the dermal bioavailability of phoxim after
pour-on application, six piglets received 10 mg/kg bw phoxim in
glycerol-formal by intravenous injection into an ear vein. Seven days
later, groups of two piglets received a dermal application along the
mid-line of the back of 100 mg/kg bw phoxim in either dimethyl
sulfoxide, 1-octanol, or Carbowax. Blood samples were collected at
regular intervals up to 72 h after intravenous treatment and up to 10
days after dermal treatment, and the plasma was assayed for phoxim by
gas chromatography. Only 1.2-2.9% of the dermal dose was absorbed,
with little difference in the rate or extent of absorption among the
three vehicles (Gyrd-Hansen et al., 1993).
2.1.2 Biotransformation
In the study of Vinopal & Fukuto (1971) described above,
metabolites were identified in two to three pooled 24-h and 30-h urine
samples from animals treated with 110 and 960 mg/kg bw, respectively,
by ion-exchange chromatography and thin-layer chromatography. The
parent drug accounted for only 1 and 2% of the administered dose in
the samples from the two groups, respectively. Four metabolites were
detected: diethyl phosphoric acid (59 and 43%, respectively), diethyl
phosphorothioic acid (20 and 18%, respectively), phoxim carboxylic
acid (tentatively identified by infrared spectroscopy; 3 and 24%,
respectively), and either desethyl phoxim or desethyl PO-phoxim
(identification was tentative; 6 and 5%, respectively). It was
demonstrated by administration of diethyl phosphorothioic acid itself
that little, if any, diethyl phosphoric acid is formed by
desulfuration of this metabolite. Hence, metabolism of phoxim in mice
proceeds mainly by hydrolysis of the phosphor ester bond, via
oxidative desulfuration to PO-phoxim with subsequent extremely rapid
hydrolysis of the phosphor ester bond, and (at high doses only) via
hydrolysis of the cyano group. De-ethylation is not an important
pathway.
The biotransformation of phoxim was studied in groups of five
fasted male Charles River CD rats after administration of 10 mg/kg bw
[phenyl-U-14C]phoxim (4-12 µCi per animal) in polyethylene glycol 200
by oral gavage. Blood was sampled by cardiac puncture 1 h after
dosing, and urine was collected for periods of 6 and 24 h after
treatment. Metabolites in plasma and urine were characterized by
thin-layer chromatography, gas chromatography, and mass spectrometry.
Samples were pooled for analysis of metabolites. Four radiolabelled
components were detected in plasma. Most of the radiolabel was in the
form of the metabolite desethyl phoxim (80%), with smaller amounts in
desethyl PO-phoxim (12%); no PO-phoxim could be detected. The major
metabolites in urine (52-94%) were the glucuronic and sulfuric acid
conjugates of cyanobenzaldoxime (both syn and anti forms). Small
amounts (12%) of hippuric acid were also detected. The metabolism of
phoxim in rats involves de-ethylation hydrolysis of the phosphor ester
bond, followed by conjugation of cyanobenzaldoxime or its further
metabolism to hippuric acid (Daniel et al., 1978a).
In the study of Klein & Weber (1988) described above, metabolites
were identified in urine and tissue samples from pigs by thin-layer
chromatography, high-performance liquid chromatography, gas
chromatography, and mass spectrometry. In urine, two metabolites were
identified: cyanobenzaldoxime-glucuronide, which was the major
product, and hippuric acid, together accounting for approximately 90%
of the urinary radioactivity. In tissues, unchanged phoxim was present
in fat (90% of the radioactivity present), loin, and muscle.
Cyanobenzaldoxime was found in muscle, loin, and liver. In kidney, no
metabolites could be identified, although co-chromatography pointed to
hippuric acid. The main metabolic pathway for phoxim in pigs is
hydrolysis of the phosphor ester bond, followed by conjugation of the
resulting cyanobenzaldoxime. A small amount of cyanobenzaldoxime is
further oxidized to benzoic acid, which is subsequently metabolized to
hippuric acid.
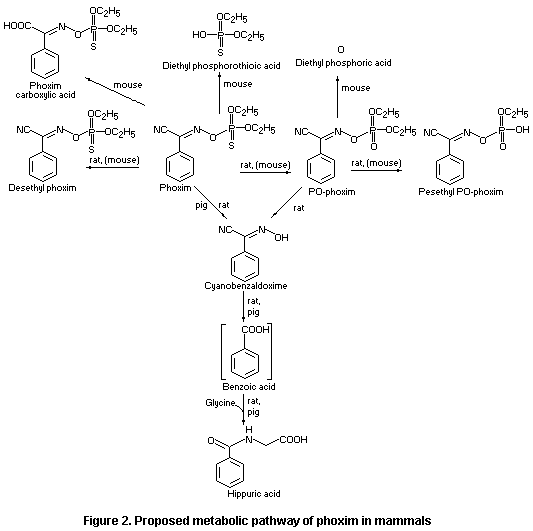
The metabolic pathway of phoxim in various species is shown in
Figure 2.
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of phoxim are shown
in Table 1. The toxic signs observed at lower oral doses were
deterioration of general condition and sedative effects. Only at the
highest, lethal doses were the signs indicative of acute
cholinesterase inhibition (spasms, trembling, diarrhoea, and red tears
in rats). Hens were considerably more sensitive to phoxim than mammals
and showed acute cholinergic signs but no indications of delayed
paralysis. After inhalation, no signs of cholinesterase inhibition
small and large intestines, and their contents were collected. In a
third experiment, urine and faeces were collected at intervals for up
to 10 days after treatment of five male and five female rats with 10
mg/kg bw, and of five male rats with 1 mg/kg bw. After 10 days, the
animals were killed, and samples of the same tissues and organs as in
the first experiment were collected. In addition, bile was collected
from three male rats with cannulated bile ducts for up to 24 h after
treatment with 10 mg/kg bw. All samples were analysed for radiolabel
by liquid scintillation spectrometry.
Phoxim at 1 or 10 mg/kg bw was rapidly absorbed. In plasma, the
total residue concentrations reached a maximum of 0.35 and 2.44 µg/ml
phoxim equivalents, respectively, within 0.5 h of administration, but
the concentrations decreased to approximately 0.1 µg/ml within 1 h of
administration of 1 mg/kg bw and remained at that level until at least
7.5 h. After 10 mg/kg bw, a second peak of 1.9 µg/ml was detected
after 4 h in the animals in the first experiment but not in those in
the second. Thereafter, the plasma concentrations decreased to
approximately 0.8 µg/ml at 7.5 h and to 0.04 µg/ml at 24 h. The
radiolabel was rapidly taken up into the major organs and tissues,
with kinetics similar to that in plasma. Over the first 7.5 h after
administration of 10 mg/kg bw, the total residue concentrations in
tissues remained fairly constant, with the highest levels in the small
intestine and its contents and slightly higher concentrations in
kidney (2.1-6.1 mg/kg phoxim equivalents) and liver (1.2-3.1 mg/kg)
than in other tissues or organs (0.04-1.2 mg/kg). Thereafter, all
tissue concentrations declined, to below 0.4 mg/kg at 24 h and to
below 0.14 mg/kg at 144 h.
Most of the radiolabel was eliminated in urine within 24 h, and
elimination was virtually complete within two days, independently of
sex and dose. The total recovery in urine over 10 days was 93% for
males and 86% for females at 10 mg/kg bw and 82% for males given 1
mg/kg bw. In faeces, 4.9, 6.9, and 7.9%, respectively, of the
administered dose was recovered in the same period. Biliary excretion
amounted to approximately 4% of the administered dose over 24 h
(Daniel et al., 1978b).
[Phenyl-U-14C]phoxim (final specific activity, 20 µCi/mg) was
administered by oral intubation in gelatin capsules to two female
German Landrace piglets at a single dose of 5 mg/kg bw (2 mCi per
animal). Blood, urine, and faeces were collected at intervals for up
to 24 and 72 h after administration. After the animals were
slaughtered, samples of liver, kidney, muscle, loin, and subcutaneous
fat were collected. All samples were analysed for radiolabel by liquid
scintillation spectrometry. The study was (partly) certified for
compliance with GLP and quality assurance.
Phoxim was rapidly absorbed: the total residue concentrations in
plasma reached a maximum of 2.3 µg/ml phoxim equivalents within 1-2 h
after administration and declined biphasically with half-lives of 0.6
h in the initial phase and 25 h in the terminal phase (from 6 h
onwards). The radiolabel was rapidly eliminated, as 82% of the
administered dose was excreted via the urine within 24 h (72% within
the first 8 h) and an additional 1.5% in the following 48 h. Faecal
excretion amounted to 1.6% of the administered dose after 24 h in one
animal and to 12% after 72 h in the other. At 24 h, the highest total
residue concentrations in edible tissues were found in fat (1.3 mg/kg
phoxim equivalents), followed by liver (0.60 mg/kg), kidney (0.35
mg/kg), and loin and muscle (0.05 mg/kg). At 72 h, the tissue
concentrations were approximately half these values (Klein & Weber,
1988).
In order to study the dermal bioavailability of phoxim after
pour-on application, six piglets received 10 mg/kg bw phoxim in
glycerol-formal by intravenous injection into an ear vein. Seven days
later, groups of two piglets received a dermal application along the
mid-line of the back of 100 mg/kg bw phoxim in either dimethyl
sulfoxide, 1-octanol, or Carbowax. Blood samples were collected at
regular intervals up to 72 h after intravenous treatment and up to 10
days after dermal treatment, and the plasma was assayed for phoxim by
gas chromatography. Only 1.2-2.9% of the dermal dose was absorbed,
with little difference in the rate or extent of absorption among the
three vehicles (Gyrd-Hansen et al., 1993).
2.1.2 Biotransformation
In the study of Vinopal & Fukuto (1971) described above,
metabolites were identified in two to three pooled 24-h and 30-h urine
samples from animals treated with 110 and 960 mg/kg bw, respectively,
by ion-exchange chromatography and thin-layer chromatography. The
parent drug accounted for only 1 and 2% of the administered dose in
the samples from the two groups, respectively. Four metabolites were
detected: diethyl phosphoric acid (59 and 43%, respectively), diethyl
phosphorothioic acid (20 and 18%, respectively), phoxim carboxylic
acid (tentatively identified by infrared spectroscopy; 3 and 24%,
respectively), and either desethyl phoxim or desethyl PO-phoxim
(identification was tentative; 6 and 5%, respectively). It was
demonstrated by administration of diethyl phosphorothioic acid itself
that little, if any, diethyl phosphoric acid is formed by
desulfuration of this metabolite. Hence, metabolism of phoxim in mice
proceeds mainly by hydrolysis of the phosphor ester bond, via
oxidative desulfuration to PO-phoxim with subsequent extremely rapid
hydrolysis of the phosphor ester bond, and (at high doses only) via
hydrolysis of the cyano group. De-ethylation is not an important
pathway.
The biotransformation of phoxim was studied in groups of five
fasted male Charles River CD rats after administration of 10 mg/kg bw
[phenyl-U-14C]phoxim (4-12 µCi per animal) in polyethylene glycol 200
by oral gavage. Blood was sampled by cardiac puncture 1 h after
dosing, and urine was collected for periods of 6 and 24 h after
treatment. Metabolites in plasma and urine were characterized by
thin-layer chromatography, gas chromatography, and mass spectrometry.
Samples were pooled for analysis of metabolites. Four radiolabelled
components were detected in plasma. Most of the radiolabel was in the
form of the metabolite desethyl phoxim (80%), with smaller amounts in
desethyl PO-phoxim (12%); no PO-phoxim could be detected. The major
metabolites in urine (52-94%) were the glucuronic and sulfuric acid
conjugates of cyanobenzaldoxime (both syn and anti forms). Small
amounts (12%) of hippuric acid were also detected. The metabolism of
phoxim in rats involves de-ethylation hydrolysis of the phosphor ester
bond, followed by conjugation of cyanobenzaldoxime or its further
metabolism to hippuric acid (Daniel et al., 1978a).
In the study of Klein & Weber (1988) described above, metabolites
were identified in urine and tissue samples from pigs by thin-layer
chromatography, high-performance liquid chromatography, gas
chromatography, and mass spectrometry. In urine, two metabolites were
identified: cyanobenzaldoxime-glucuronide, which was the major
product, and hippuric acid, together accounting for approximately 90%
of the urinary radioactivity. In tissues, unchanged phoxim was present
in fat (90% of the radioactivity present), loin, and muscle.
Cyanobenzaldoxime was found in muscle, loin, and liver. In kidney, no
metabolites could be identified, although co-chromatography pointed to
hippuric acid. The main metabolic pathway for phoxim in pigs is
hydrolysis of the phosphor ester bond, followed by conjugation of the
resulting cyanobenzaldoxime. A small amount of cyanobenzaldoxime is
further oxidized to benzoic acid, which is subsequently metabolized to
hippuric acid.
The metabolic pathway of phoxim in various species is shown in
Figure 2.
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of phoxim are shown
in Table 1. The toxic signs observed at lower oral doses were
deterioration of general condition and sedative effects. Only at the
highest, lethal doses were the signs indicative of acute
cholinesterase inhibition (spasms, trembling, diarrhoea, and red tears
in rats). Hens were considerably more sensitive to phoxim than mammals
and showed acute cholinergic signs but no indications of delayed
paralysis. After inhalation, no signs of cholinesterase inhibition
 were observed, even at the highest doses, when the only effects were
non-specific ones on the nervous system. No signs of poisoning were
observed after dermal application. Phoxim was only slightly to
moderately toxic in all of the mammalian species studied.
Poorly reported studies of skin irritation indicated that phoxim
had only a very slight irritating effect on rabbit's skin. The
calculated overall primary irritation index was < 0.5. Limited
reports of eye irritation in rabbits showed that phoxim can cause mild
to moderate irritation of the conjuctivae, without effects on the iris
or cornea (Lorke & Kimmerle, 1965; Kimmerle & Solmecke, 1970; Flucke &
Thyssen, 1979).
In a Magnussen-Kligman maximization test in 40 male DHPW
guinea-pigs, phoxim induced a sensitization response in 60% of the
animals, which was shown not to be due to 1-butanol (Flucke, 1984a).
In an open epicutaneous test (according to Klecak) in 64 male DHPW
guinea-pigs, administration of phoxim showed no sensitization
potential (Flucke, 1984b).
2.2.2 Short-term studies of toxicity
Mice
In a study to establish the doses for a study of carcinogenicity,
groups of 10 male and 10 female SPF B6C3F1 mice were fed diets
containing phoxim at 0, 5, 30, 150, or 750 mg/kg feed for six weeks,
equal to 0, 3, 18, 85, and 440 mg/kg bw per day for males and 0, 3,
20, 100, and 500 mg/kg bw per day for females. The study was certified
for compliance with GLP. No differences in mortality rate, clinical
signs, body weight, or food or water intake were observed that could
be associated with treatment. Plasma alkaline phosphatase activity and
total protein content were significantly increased in males at the
high dose. In females, the plasma cholesterol content increased with
dose, the increase becoming statistically significant from 30 mg/kg
feed onwards; however, the control concentrations of cholesterol and
total protein were low in comparison with historical data, and the
increased levels were still within the historical control range, as
were the alkaline phosphatase activities in males at the high dose.
Cholinesterase activity was increasingly reduced with dose in plasma,
with > 20% inhibition in all treated animals, and in erythrocytes,
with > 20% inhibition only in females at the high dose. Brain
acetylcholinesterase activity, which was determined in five animalsof
each sex in each group, was reduced in all treated groups, with
reductions of more than 50% in both males and females at the high
dose; however, the activities in concurrent controls were in the upper
range of that of historical controls, and the observed reduction
showed no dose-response relationship, was not always statistically
significant, and was still within historical control levels, except in
males at the high dose. Therefore, only the reduction in males at the
high dose is considered relevant.
Table 1. Acute toxicity of phoxim
Route Species Sex LD50a or LC50 Reference
Oral Mouse Male 2700 Kimmerle (1968)
(mg/kg bw) 1800 Kimmerle & Solmecke (1970)
1200b Flucke & Thyssen (1979)
Female 3600 Kimmerle (1968)
2200 Kimmerle & Solmecke (1970)
2800-4000 Flucke (1978a,b; 1979a,b; 1980);
Heimann (1982a,b); Mihail
(1981, 1982)
1800b Flucke & Thyssen (1979)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 2300 Kimmerle (1969)
> 2000d Vinopal & Fukuto (1971)
Rat Male 10 000 Lorke & Kimmerle (1965)
8000 Kimmerle (1968))
2100 Kimmerle & Solmecke (1970)
4900b Thyssen (1976)
2000b Flucke & Thyssen (1979)
Female 10 000 Lorke & Kimmerle (1965)
6500 Kimmerle (1968)
1900 Kimmerle & Solmecke (1970)
2900b Thyssen (1976)
1400b Flucke & Thyssen (1979)
Guinea-pig Female 390-560 Kimmerle (1968)
approx. 660 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
Rabbit Female 250-380 Kimmerle & Solmecke (1970)
Unspecified approx. 380c Lorke & Kimmerle (1965)
280-560 Kimmerle (1968)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Cat Female 250-500 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Dog Female 250-500 Kimmerle & Solmecke (1970)
> 500b Flucke & Thyssen (1979)
Unspecified > 1200 Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
20e Thyssen & Kimmerle (1973)
40b Pauluhn (1983)
Intraperitoneal Rat Male 2200c Lorke & Kimmerle (1965)
(mg/kg bw) 2000 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1100b Flucke & Thyssen (1979)
Female 2000c Lorke & Kimmerle (1965)
1900 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1500b Flucke & Thyssen (1979)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
Intravenous Mouse Female 1100 Kimmerle (1968)
(mg/kg bw) 480b Kimmerle & Solmecke (1970)
Dermal Rat Male > 1200 Lorke & Kimmerle (1965)
(mg/kg bw) > 1100 Kimmerle (1968)
> 1100 Kimmerle & Solmecke (1970)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Male/female > 5600 Flucke & Thyssen (1979)
> 5000f Bomann (1992)g
Inhalation Mouse Male > 2.1h Kimmerle (1968)
(mg/L, 4 h) > 3.1h Kimmerle & Solmecke (1970)
Unspecified > 1.3 Kimmerle (1968)
>1.7h Kimmerle & Solmecke (1970)
Rat Male > 2.0h Kimmerle (1968)
> 2.6h Kimmerle & Solmecke (1970)
Female > 2.2h Kimmerle (1968)
> 2.8h Kimmerle & Solmecke (1970)
Male/female > 3.0h Flucke & Thyssen (1979)
> 4.6 Märtins (1992)g
Unspecified > 1.3 Kimmerle (1968)
> 1.7h Kimmerle & Solmecke (1970)
Guinea-pig Unspecified > 1.3 Kimmerle (1968)
Rabbit Unspecified > 1.3 Kimmerle (1968)
1.2-1.7h Kimmerle & Solmecke (1970)
Subcutaneous Mouse Male/female > 5000b Flucke & Thyssen (1979)
(mg/kg bw ) Unspecified > 1000c Lorke & Kimmerle (1965)
a In the reports of Kimmerle (1968), Flucke (1978a,b; 1979a,b; 1980), Heimann (1982a,b), Mihail
(1981, 1982), Kimmerle & Solmecke (1970), and Flucke & Thyssen (1979), some or all of the
LD50 values for phoxim (as pre-solution) were cited in microlitres or millilitres per kilogram
of body weight. These values were converted to milligrams per kilogram of body weight by
Table 1. (continued)
correcting for the density of the pre-solution (1.126 mg/µl at 20 °C). In the report of Lorke
& Kimmerle (1965), some of the LD50 values for pure phoxim were cited in millilitres per
kilogram of body weight. These values were converted to milligrams per kilogram of body weight
by correcting for the density of the pure substance (1.176 mg/µl at 20 °C).
b Vehicle, water:Cremophor EL
c Vehicle, lutrol
d Vehicle, olive oil
e Vehicle, water
f Vehicle, 2% Cremophor EL:physiological saline
g The studies of Bomann (1992) and Märtins (1992) were of conventional design, with GLP
and quality assurance certification.
h Vehicle, ethanol:lutrol 1:1
The only gross change seen on post-mortem examination was
enlarged spleens in a number of control and treated females. As this
finding was unrelated to dose and occurred in more control than
treated animals, it is not considered to be related to treatment. The
relative kidney weights of females at 150 and 750 mg/kg feed were
statistically significantly increased with dose. The absolute and
relative liver weights were increased with dose in all treated
animals, the increase in relative weight becoming statistically
significant at 150 mg/kg feed and the increase in absolute weight in
males only; both the relative and absolute weights were increased
significantly at 750 mg/kg feed. Histopathological examination only of
the livers of controls and animals at the high dose revealed
enlargement of the centrilobular hepatocytes in males and females at
the high dose, indicating adaptive hypertrophy and possible enzyme
induction. No evidence of irreversible changes was found. One female
at the high dose had slight cytoplasmic vacuolation in periportal
cells, which may not have been related to treatment (Ivens-Kohl &
Hahnemann, 1989a).
In a follow-up study, groups of 10 male and 10 female SPF B6C3F1
mice were fed diets containing phoxim at 0, 0.5, 1, 5, or 30 mg/kg
feed, equal to 0, 0.28, 0.55, 2.8, and 18 mg/kg bw per day for males
and 0, 0.35, 0.66, 3.1, and 23 mg/kg bw per day for females, for eight
weeks. The study was certified for compliance with GLP. No differences
in mortality rate, clinical signs, body weight, food or water intake,
or macroscopic or histopathological appearance were observed that
could be related to treatment. The plasma cholesterol content was
statistically significantly increased in females at 30 mg/kg feed. As
in the previous study, however, the control cholesterol content was
lower than that of historical controls, and the increased level was
still within the range of historical controls. Cholinesterase activity
in plasma was reduced by > 40% at the two highest doses, and a small
inhibition (8-14%) of erythrocyte acetylcholinesterase activity was
observed in males and females at the high dose. There was no evidence
of a change in brain acetylcholinesterase activity in any treated
groups. Although the absolute and relative weights of the liver were
slightly but statistically significantly increased in females at the
high dose, no effects on organ weights were found. The NOEL was 5
mg/kg feed, equal to 3.1 mg/kg bw per day, on the basis of slightly
increased liver weights in female mice (Ivens-Kohl & Hahnemann,
1989b).
Rats
In a 21-day study of toxicity with special attention to the
reversibility of cholinesterase inhibition, groups of 15 SPF Wistar II
rats of each sex received phoxim emulsified in distilled water and
Cremophor EL by oral gavage at 0, 5, or 50 mg/kg bw per day. Five rats
of each sex in each group were killed at the end of treatment and two
and four weeks thereafter. The animals were examined regularly for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. Brain
cholinesterase activity was measured at termination of treatment and
two weeks thereafter.
There were no deaths and no treatment-related effects on
appearance, behaviour, or body weight. Plasma cholinesterase activity
was reduced by > 20% during treatment in females at the low dose
(when compared with concurrent controls but not when compared with
pre-treatment values in the same animals) and in males and females at
the high dose, but the activity was restored within one week after
cessation of treatment. Acetylcholines-terase activity in erythrocytes
was reduced by > 20% in males at the low dose during the first week
of treatment (when compared with concurrent controls but not when
compared with pre-treatment values in the same animals) and in females
at this dose throughout treatment but was restored within one week.
Erythrocyte cholinesterase activity was decreased by > 20% in males
and females at the high dose throughout the treatment period but was
restored within two weeks in males and within four weeks in females.
Brain acetylcholinesterase activity was within the physiological range
and very similar to control values in all treated males and in females
at the low dose, both at termination and two weeks thereafter. Females
at the high dose showed 30% inhibition of brain acetylcholinesterase
activity at termination, but this was restored within two weeks. Thus,
inhibition of cholinesterase activity was more pronounced in female
than in male rats and was reversible in animals of each sex (Thyssen,
1976).
Groups of 15 SPF Wistar II rats of each sex received phoxim
dissolved in polyethylene glycol 400 (lutrol) by oral gavage at 0, 2,
5, or 15 mg/kg bw per day for 30 days. The animals were examined for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. No
treatment-related effects were observed on appearance, behaviour or
body weight. The cholinesterase activity in plasma decreased with dose
in all treated females and in males at 5 and 15 mg/kg bw per day. The
acetylcholinesterase activity in erythrocytes was reduced by > 20%
in males and females at two highest doses (Kimmerle, 1973).
In a three-month study, groups of 15 young SPF Wistar rats of
each sex received diets containing phoxim at 0, 4, 12, 40, or 120
mg/kg feed, equivalent to 0, 0.4, 1.2, 4, or 12 mg/kg bw per day. The
animals were observed for physical appearance, behaviour, deaths, body
weight, food consumption, haematological and clincal chemical
parameters (liver function tests), plasma and erythrocyte
cholinesterase activity, urinary parameters, organ weights, and
macroscopic appearance. Brain acetylcholinesterase activity was not
investigated. Treatment-related effects were found on cholinesterase
activity in both plasma and erythrocytes, which were decreased by
> 20% at 40 mg/kg feed (within one month) and 120 mg/kg feed
(within one week) in animals of each sex. Statistically significantly
increased weights of the thyroid glands (both absolute and relative)
and adrenal glands (relative only) were observed in males at 120 mg/kg
feed. In the absence of data on brain acetylcholinesterase activity,
the NOEL was 12 mg/kg feed, equivalent to 1.2 mg/kg bw per day, on the
basis of inhibition of erythrocyte acetyl-cholinesterase activity
(Löser, 1969a,b).
In a second three-month study with groups of 15 young SPF Wistar
rats of each sex, phoxim was administered in the diet at
concentrations of 0, 5, 15, 50, 150, or 500 mg/kg feed, equivalent to
0, 0.5, 1.5, 5, 15, or 50 mg/kg bw per day. The animals were
observed for the same end-points as in the previous study, with the
addition of histopathological examination. Cholinergic signs (muscle
twitching, cramps) were observed occasionally in animals at the high
dose, particularly in the earlier part of the study. Although
body-weight gain was significantly retarded in males at the high dose,
animals at 50 mg/kg feed showed a very similar growth curve. Hence,
the effect on body-weight gain was not consistent or dose-dependent.
In male rats, cholinesterase activity in plasma and erythrocytes
decreased by > 20% with doses from 50 mg/kg feed. In female rats,
cholinesterase activity decreased by > 20% in plasma with doses
from 15 mg/kg feed (at 1 and 13 weeks only) and in erythrocytes with
doses from 150 mg/kg feed. Liver weights were statistically
significantly increased in animals at 150 mg/kg feed (relative) and
500 mg/kg feed (relative in males and females, absolute in females),
but there were no significant changes in liver function tests. At the
high dose, the absolute and relative thyroid weights were increased in
females, and the relative adrenal weights were increased in males.
Relative kidney weights were increased at 150 mg/kg feed in females
and 500 mg/kg feed in males and females. On the basis of inhibition of
erythrocyte acetylcholinesterase activity in males (in the absence of
data on brain acetylcholinesterase activity), the NOEL was 15 mg/kg
feed, equivalent to 1.5 mg/kg bw per day (Löser, 1970a; Vince &
Spicer, 1971).
Rabbits
Groups of three male and three female New Zealand white rabbits
received dermal applications of phoxim emulsified with Cremophor EL in
water on 5 × 5 cm areas of shorn intact skin of the flank or back or
of skin abraded with sandpaper at doses of 0, 0.5, or 15 mg/kg bw per
day, 7 h/day, five days per week, for three consecutive weeks. The
treated sites were not occluded. The observations included deaths,
physical appearance, behaviour, body weight, skin irritation,
haematological parameters, clinical chemical parameters (liver
function tests), urinary parameters, gross pathological appearance,
organ weights, histopathological appearance, and determination of
plasma, erythrocyte, and brain cholinesterase activities.
Skin irritation was observed only in animals with abraded skin,
in which there was a dose-related increase in the time necessary for
the erythema to disappear. Histopathological examination of the
treated skin of one control animal with intact skin, of one animal at
the high dose with intact skin, and of four animals at the high dose
with abraded skin showed, on average, moderate cellular infiltration
of the epidermis and corium by round cells, polymorphonuclear
leukocytes, and isolated inflammatory hair follicles, and a slight
increase in the thickness of the epithelium. These findings were
considered to be the result of repeated physical irritation,
exacerbated by the compound at the highest dose. Plasma and
erythrocyte cholinesterase activities were reduced in a dose-related
manner and were inhibited by > 20% at 0.5 mg/kg bw per day in males
with abraded skin and in females with intact skin only and in all
animals at 15 mg/kg bw per day. Brain acetylcholinesterase activity
was reduced by 14% in males with abraded skin at the low dose and by
23% in all males at the high dose but was not reduced in females
(Flucke & Schilde, 1978).
Chickens
Groups of 10 white Leghorn hens were fed diets containing phoxim
at a concentration of 0, 5, or 10 mg/kg feed for 28 days, equal to
average achieved intakes of 0, 0.32, or 0.68 mg/kg bw per day. Phoxim
had no effect on behaviour, and there were no signs of neurotoxicity.
Body-weight gain and food consumption were also unaffected. At the
high dose, a marked depression (> 20% inhibition) in whole blood
cholinesterase activity was observed (Thyssen & Kimmerle, 1973).
Dogs
In a three-month study, beagle dogs were fed phoxim in the diet
at doses of 0 (three of each sex), 2, 5, or 10 mg/kg feed (two of each
sex), equivalent to 0, 0.05, 0.13, or 0.25 mg/kg bw per day. Treatment
did not affect the mortality rate, physical appearance, behaviour,
food intake, ophthalmoscopic, haematological, clinical chemical (liver
function tests), or urinary parameters, macroscopic appearance, or
organ weights. Body-weight gain appeared to be reduced in males at 5
mg/kg feed and in animals of each sex at 10 mg/kg feed; however, the
smal number of animals per group and the high intra-group variation
did not allow statistical analysis. As this effect was not observed at
higher doses in a second three-month study (see below) or in the first
three months of a two-year study with larger groups (see below), it
was discounted. Inhibition of cholinesterase activity by > 20% was
observed in the plasma of dogs of each sex at 2 mg/kg feed (after one
month only) and at 5 and 10 mg/kg feed but not in erythrocytes. Brain
acetylcholinesterase activity was not determined, and
histopathological examination was not performed. The NOEL was 10 mg/kg
feed, equivalent to 0.25 mg/kg bw per day, the highest dose tested
(Löser, 1970b).
In a second three-month study, in which histopathological
examination was performed in addition to the observations in the first
study, beagle dogs were fed phoxim in the diet at doses of 0 (three
per sex), 50, 200, or 1000 mg/kg feed (two per sex), equivalent to
0, 1.3, 5, or 25 mg/kg bw per day. Animals at the high dose showed
signs of cholinergic poisoning (cramps, especially in the abdominal
region, muscle twitching, and salivation), but none died. Food
consumption was reduced in females at 1000 mg/kg feed, which resulted
in weight loss throughout the study, and these animals appeared
emaciated upon macroscopic examination, with little adipose tissue in
the subcutaneous connective tissue or in the mesentery. Plasma
alkaline phosphatase activity was increased in animals of each sex at
200 and 1000 mg/kg feed, and plasma lactate dehydrogenase activity was
increased in males at the high dose. Ophthalmoscopic, haematological,
and urinary determinations showed no treatment-related effects. The
absolute and relative liver weights were increased in males at the
high dose, and the relative liver weight was increased in females at
the high dose, probably because of the body-weight loss, although
changes were not seen in other tissues. The absolute and relative
weights of the male gonads were increased at the two highest doses,
but as the report did not include data on individual animals or
provide any measure of intra-group variation, the statistical
significance of these findings could not be determined. In both males
and females, cholinesterase activity was decreased by > 20% in a
dose-dependent fashion in plasma at all doses and erythrocytes at 200
and 1000 mg/kg feed, from within one week. Brain acetylcholinesterase
activity was not determined. Histopathological examination revealed no
difference between control and treated animals. The NOEL was 50 mg/kg
feed, equivalent to 1.3 mg/kg bw per day, on the basis of reduced
erythrocyte acetylcholinesterase activity (in the absence of data on
brain acetylcholinesterase activity) and increased plasma alkaline
phosphatase activity (Löser, 1971; Brooks & Cherry, 1974).
Groups of four male and four female beagles were fed diets
containing phoxim at 0, 0.3, 1, or 2 mg/kg feed for three months,
equivalent to 0, 0.0075, 0.025, or 0.05 mg/kg bw per day. The animals
were observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, plasma and
erythrocyte cholinesterase activity, gross and histopathological
appearance, and organ weights. Brain acetylcholinesterase activity was
not determined. The only effect observed was reduced plasma
cholinesterase activity (> 20% inhibition) in both males and
females at 1 and 2 mg/kg feed. The NOEL was 2 mg/kg feed, equivalent
to 0.05 mg/kg bw per day, the highest dose tested (Mürmann & Luckhaus,
1973).
Monkeys
Groups of five male and five female rhesus monkeys ( Macaca
mulatta) received phoxim dissolved in corn oil by oral gavage at
doses of 0, 0.2, 0.65, or 2 mg/kg bw per day on six days per week for
six months. The observations included clinical signs, physical
appearance, behaviour, body weight, haematological, clinical chemical,
and urinary parameters, and plasma and erythrocyte cholinesterase
activity. Liver was biopsied before and at the end of treatment for
histopathological assessment. Brain acetylcholinesterase activity was
not determined. Although plasma and erythrocyte cholinesterase
activity was inhibited, there were no signs of cholinergic poisoning
in animals at doses up to 2 mg/kg bw per day. There was no evidence
from either clinical chemistry or histopathology of an effect of
phoxim on the liver. Plasma cholinesterase activity was reduced by
> 20% in a dose-dependent manner in all treated animals.
Erythrocyte acetylcholinesterase activity was slightly reduced in
animals at the highest dose, reaching > 20% inhibition only after
treatment for four months, when compared with concurrent controls but
not when compared with pre-treatment values in the same animals. In
the absence of data on brain acetylcholinesterase activity, the NOEL
was 0.65 mg/kg bw per day on the basis of slight evidence of
inhibition of erythrocyte acetylcholinesterase activity (Coulston
et al., 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity, groups of 50 male and 50 female
SPF B6C3F1 mice were fed diets containing phoxim at doses of 0, 1, 5,
150, or 450 mg/kg feed, equal to average achieved intakes of 0, 0.47,
2.4, 67, or 200 mg/kg bw per day for males and 0, 0.55, 2.7, 79, or
210 mg/kg bw per day for females, for 24 months. Ten additional
animals of each sex at each dose (satellite groups) were killed at 12
months. The animals were observed for clinical signs, death, body
weight, food and water consumption, haematological and clinical
chemical parameters (in 10 animals per group during weeks 27, 53, 79,
and 105; including plasma and erythrocyte cholinesterase activities),
organ weights, detailed macroscopic and microscopic examinations, and
brain acetylcholinesterase activity (in 10 animals of each sex per
group at the terminal kill). The study was of conventional design,
with GLP and quality assurance certification.
Clinical signs, food and water consumption, and haematological
parameters were unaffected by treatment, as was survival (70-88% of
controls, 78-90% of treated animals). Statistically significantly
increased body weights were observed in males at 150 and 450 mg/kg
feed and in females at 450 mg/kg feed. Plasma cholesterol contents
were statistically significantly increased in animals of each sex at
doses of 150 mg/kg feed and higher, from 27 weeks onwards. The total
bilirubin content was decreased in males at the high dose, while
females at this dose showed slightly but statistically significantly
increased plasma alkaline phosphatase and alanine aminostransferase
activities from one year onwards. Plasma cholinesterase activity was
decreased in animals of each sex at 5 (23-45% inhibition), 150, and
450 (94-98% inhibition) mg/kg feed. Erythrocyte acetylcholinesterase
activity was inhibited by 14-26% in males and females at 150 and 450
mg/kg feed, and brain acetylcholinesterase activity was reduced in
animals of each sex at 150 mg/kg feed (19-28%) and 450 mg/kg feed
(45-54%).
The weights and macroscopic and microscopic appearance of organs
showed little treatment-related change, except in the liver. At 450
mg/kg feed, the absolute and relative liver weights were statistically
significantly increased in all animals, and histopathological
examination showed a significant increase in the incidence of
non-neoplastic hepatic foci, mainly eosinophilic and basophilic types,
in males (8/50 versus 1/50 in controls). The absolute weights of the
heart and kidneys were significantly increased in both males and
females at the high dose, and fermales at these doses had a
significant reduction in the number of ovarian cysts. The incidences
of hepatocellular carcinoma were comparable in treated and control
groups, but the incidences of hepatocellular adenoma were
statistically significantly increased in males at 150 mg/kg feed (9/50
versus 1/50 in concurrent controls and 1-7/50 in historical controls)
and in females at 450 mg/kg feed (8/50 versus 1/50 in concurrent
controls and 0-2/50 in historical controls). This finding is probably
not biologically significant in males, as no increase was seen at the
highest dose (5/50 at both the lowest and highest doses) and the
incidence was just beyond the range of historical controls. The
increased incidence of adenomas in females at the high dose is thought
to be due to the hepatoproliferative effect of the compound. The
incidence of lymphoreticular tumours was slightly but significantly
reduced in females receiving 150 and 450 mg/kg feed. The NOEL was 5
mg/kg feed, equal to 2.4 mg/kg bw per day, on the basis of increased
plasma cholesterol content and inhibition of brain
acetylcholinesterase activity (Jäger, 1992).
Rats
In a long-term study of toxicity, groups of 50 male and 50 female
SPF Wistar rats received diets containing phoxim at concentrations of
0, 15, 75, or 375 mg/kg feed, equal to average achieved intakes of 0,
0.8, 4, or 18 mg/kg bw per day for males and 0, 1.1, 5.4, or 27 mg/kg
bw per day for females, for 24 months. The control group comprisd 100
males and 100 females. Five additional animals of each sex per group
were used for clinical chemical examinations at 3, 6, and 12 months,
and 10 males and 10 females per group were used for these examinations
at termination. The observations included physical appearance,
behaviour, deaths, body weight, food consumption, haematological,
clinical chemical (liver function tests), and urinary parameters,
plasma and erythrocyte cholinesterase activities, absolute organ
weights, macroscopic and microscopic appearance, and brain
acetylcholinesterase activity (in five animals of each sex per group).
Treatment with phoxim did not affect the physical appearance,
behaviour, survival (76-79% of controls, 74-86% of treated animals),
or haematological, urinary, or macroscopic appearance of the rats.
Food consumption was reduced by 10% in males at the high dose, while
body-weight gain was slightly reduced in females at this dose. The
results of liver function tests were normal, other than a significant
reduction in plasma glutamate dehydrogenase activity in males at the
high dose and in bilirubin content in females at the high dose. Plasma
and erythrocyte cholinesterase activities were reduced by > 20% in
a dose-dependent manner throughout the study in rats treated with 75
and 375 mg/kg feed. Inhibition by > 20% of plasma and erythrocyte
cholinesterase activities was also observed in females at 15 mg/kg
feed (at 1, 2, and 52 weeks for plasma cholinesterase; at 2 weeks for
erythrocyte acetylcholinesterase). Brain acetylcholinesterase activity
was reduced by 18% in males and 23% in females at the highest dose.
Absolute liver weights were statistically significantly increased in
all treated males, but the effect is discounted as an inverse
dose-response relationship was seen. The absolute adrenal weights
were statistically significantly decreased in a dose-related manner
at doses > 75 mg/kg feed in animals of each sex, and the absolute
weights of the heart, lung, and spleen were statistically
significantly reduced in females at the high dose. None of the changes
in organ weights was accompanied by corresponding histopathological
changes. There was no difference in tumour incidence between control
and treated animals. The NOEL was 15 mg/kg feed, equal to 0.8 mg/kg bw
per day, on the basis of changes in adrenal weights (Bomhard & Löser,
1977; Finn, 1977).
Dogs
In a long-term study of toxicity, groups of four beagles of each
sex received phoxim in the diet at concentrations of 0, 0.3/0.1, 15,
or 750 mg/kg feed for 104 weeks, equivalent to 0, 0.0075/0.0025, 0.38,
or 19 mg/kg bw per day. Because plasma cholinesterase activity was
decreased by 26% in females at week 77, although erythrocyte
acetylcholinesterase activity was not affected, their dose was reduced
from 0.3 to 0.1 mg/kg feed from week 83 onwards. The animals were
observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, gross and
histopathological appearance, organ weights, and plasma, erythrocyte,
and brain cholinesterase activity.
No treatment-related effects were observed on mortality rates,
behaviour, food consumption, reflexes, or ophthalmoscopic,
haematological, or urinary parameters. All males at the high dose and
two of four at the intermediate dose showed a poor nutritional state.
In the second year, the coats of all males at the high dose were dull,
ruffled, and ungroomed, and, although the food consumption of these
animals was unaffected by treatment, they took much longer to eat
their food ration. The body-weight gain of all treated animals, but
especially the males, was reduced in a dose-dependent manner between 0
and 104 weeks due mainly to retarded growth in the second half of the
study; the effect reached statistical significance only in males at
the high dose. Plasma alanine aminotransferase activity was
statistically significantly increased in animals at the high dose from
week 52 onwards. Plasma alkaline phosphatase activity was
statistically significantly increased in these animals throughout the
treatment period. Although it was not increased at lower doses, the
natural decline was slowed, resulting in statistically significantly
higher values than in controls in females at the intermediate dose
from week 26 onwards and in males at the two lower doses from week 52
onwards. These effects on alkaline phosphatase activity are considered
to be adaptive, and were probably due to induction, as
organophosphorus esters are natural substrates for alkaline
phosphatase. The serum cholesterol concentration was reduced in
animals at the high dose throughout treatment, reaching statistical
significance at some times. Cholinesterase activity was reduced
throughout the experiment in males and females at 15 and 750 mg/kg
feed, in plasma (60 and 80% inhibition, respectively) and in
erythrocytes (20-30 and 60-80% inhibition, respectively). The
cholinesterase activity in plasma and erythrocytes was not reduced in
males at 0.3 mg/kg feed or in females at 0.1 mg/kg feed. Brain
acetylcholinesterase activity was significantly reduced, by
approximately 37%, in animals at the high dose.
Organ weights and gross and histopathological appearance showed
little treatment-related change other than in the liver. Histological
examination revealed hypolasia in both testes of one dog at the high
dose. As there was no cryptorchism, this effect might be related to
treatment. The livers of all females and one male at 750 mg/kg feed
were darker than those of the controls, and some had a markedly
exaggerated lobular pattern. The absolute and relative weights of the
liver were statistically significantly increased in all animals at the
high dose; although the relative weight of the liver was also
increased in males at the intermediate dose, the increase was not
statistically significant. Histopathological examination showed
hepatocytic alterations in three dogs and three bitches at the high
dose, involving dilated hepatocytes with a light, glassy,
structureless cytosol. On the basis of inhibition of brain
acetylcholinesterase activity and effects on the liver, the NOEL was
15 mg/kg feed, equivalent to 0.38 mg/kg bw per day (Hoffmann &
Gröning, 1977).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with phoxim are
summarized in Table 2. Phoxim was dissolved in dimethyl sulfoxide for
testing in vitro and in aqueous 0.5% Cremophor emulsion for testing
in vivo. The only effect seen in vitro or in vivo was induction of
chromosomal aberrations in vitro in lymphocytes; however, this
finding could not be interpreted owing to the cytotoxicity seen at
concentrations that affected the chromosomes. The summary tables in an
English translation of the Russian article show that phoxim induced a
very weak, dose-related increase in the number of cells with
aberrations, but no details were provided on the exposure time,
harvesting time, or absence or presence of S9. Furthermore, the
marginally increased frequencies reached statistical significance
because the frequency in concurrent controls both in vitro and in vivo
was very low, and these frequencies may have been within the range for
historical controls, for which data were lacking.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Phoxim was administered in the diet to four groups of 10 male and
20 female Long Evans FB 30 rats throughout mating, gestation, and
lactation in a three-generation study of reproductive toxicity at
doses of 0, 15, 75, or 375 mg/kg feed, equivalent to 0, 0.75, 3.8, and
19 mg/kg bw per day. The pups were suckled for up to four weeks,
weighed weekly, and examined grossly for malformations; the offspring
of each first mating (F1a, F2a, and F3a) were then killed. The
offspring of each second mating (F1b and F2b) were weaned and then
mated to produce two litters. After the dams of the F0, F1b, and F2b
generations had successfully nursed their offspring twice, they were
killed. Gross and histopathological examinations were performed on one
male and one female four-week-old pup of the F3b generation from each
of 10 mothers per group.
There were no differences between the control and treated parents
in any of the generations with respect to physical appearance,
behaviour, or body-weight gain, although some parents in the control
and treated groups died due to pneumonitis. No treatment-related
effects on fertility (pregnancy rate), litter size at birth or after
five days (viability index), or pup body weights at birth or during
four-week lactation were seen in any generation, and there were no
signs of malformation. The survival of pups in the F1a, F1b, F2a,
F2b, and F3a generations after four weeks' lactation was normal, but
the survival of pups in the F3b generation at 375 mg/kg feed was
slightly but statistically significantly reduced (84% versus 97% in
controls). Gross and histopathological examinations of the F3b pups
did not reveal any treatment-related alterations. The NOEL was 75
mg/kg feed, equivalent to 3.8 mg/kg bw per day, on the basis of
slightly reduced survival of pups in the second litter of the third
generation (Löser, 1979).
Table 2. Results of assays for genotoxicity with phoxim
End-point Test object Concentration Result GLP/ Reference
QA
In vitro
Reverse S. typhimurium TA98, 3.2-3200 nl/ Negativeb No Oesch
mutation TA100, TA1537 platea (+ S9) (1977)
32-3200 nl/
platea (- S9)
Reverse S. typhimurium TA98, 10-5000 µg/ Negativeb No Shirasu
mutation TA100 plate et al. (1978)
Reverse S. typhimurium TA98, < 5000 µg/ Negativeb No Moriya
mutation TA100, TA1535, plate et al. (1983)
TA1537, TA1538;
E. coli WP2 hcr
Reverse Saccharomyces 625-10 000 Negativeb Yes Herbold
mutation cerevisiae D7 µg/mlc (1985)
DNA damage Bacillus subtilis H-17, 1-100% v/v Negative No Shirasu
M-45 rec- (0.2-20 µl/disc) et al. (1978)
Chromosomal Human lymphocytes 30-300 µg/ml Positive Yes Herbold
aberration (- S9) at > 100 (1986)
50-500 µg/ml µg/ml - S9d
(+ S9)
Chromosomal Human lymphocytes 0.2 and 2 Equivocal No Kurinnyi
aberration µg/ml (1979)
Table 2. (continued)
End-point Test object Concentration Result GLP/ Reference
QA
In vivo
Micronucleus Mouse bone marrow 2 × 250 or Negativee No Herbold
formation (NMRI mice (SPF 500 mg/kg bw (1981)
Han) 5/sex per by gavage with
group) an interval of
24 h
Dominant Male NMRI mice Single dose of Negative No Machemer
lethal 500 mg/kg bw (1974)
mutation orally by gavage
Chromosomal Mouse bone marrow 1× 10 mg/kg bw Negative No Kurinnyi
aberration (noninbred white 1× 100 mg/kg bw, Negative (1979)
mice, 5 males/group) 5× 100 mg/kg bw, Equivocale
1× 250 mg/kg bw Equivocale
intragastrically
GLP, good laboratory practice; QA, quality assessment; S9, exogenous metabolic activation
system from rat liver microsomes
a At 1000 nl/plate, some test compound separated as droplets from the top agar.
b With and without S9
c No cytotoxicity up to 10 000 µg/ml
d The mitotic indices were 100, 37, and 1.9% at 30, 100, and 300 µg/ml,
respectively, in the absence of S9 and 84, 93, and 2.4% at 50, 100,
and 500 µg/ml, respectively, in the presence of S9. Significant
increases in chromosomal aberration frequency were found at 100 and
300 µg/ml in the absence of S9 and at 500 µg/ml in the presence of S9.
e The ratio of polychromatic to normochromatic erythrocytes at either
dose did not deviate from that in controls, and no clinical signs of
toxicity were noted. The doses were chosen on the basis of the results
of a preliminary test in which mice received 2 × 500 or 1000 mg/kg bw
orally and the lower dose was tolerated with induction of weak signs.
In view of this finding and the rapid and almost complete oral
absorption of phoxim by mice, it is likely that the bone marrow was
exposed.
2.2.5.2 Developmental toxicity
Rats
In a study of developmental toxicity, groups of 20-21 pregnant
Long Evans FB 30 rats received phoxim in 0.5% aqueous Cremophor
emulsion by oral gavage at daily doses of 0, 30, 100, or 300 mg/kg bw
on days 6-15 of gestation. On gestation day 20, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal malformations. None of the dams died
during the study, and no adverse effects were seen on their behaviour
or general appearance. The body-weight gain of dams at the high dose
was statistically significantly decreased during treatment but not
when considered over the entire gestation period. There were no
evident effects on embryonic or fetal development, and there was no
indication of teratogenicity up to the highest dose tested. The NOEL
for maternal toxicity was 100 mg/kg bw per day on the basis of the
decrease in body-weight gain. The NOEL for developmental toxicity was
300 mg/kg bw per day, the highest dose tested (Machemer, 1975).
Rabbits
In a study of developmental toxicity, groups of 20 pregnant
Chinchilla rabbits received phoxim in water with 2%
carboxymethylcellulose by oral gavage at daily doses of 0, 12, 36, or
72 mg/kg bw on days 6-18 of gestation. On gestation day 28, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external, visceral, and skeletal abnormalities. The study
was of conventional design, with quality assurance certification. No
treatment-related increase in mortality rate was observed. Clinical
signs of toxicity (diarrhoea, salivation, ventral body position,
dyspnoea, and abortion) were observed in dams at the high dose, and
phoxim caused reductions in food consumption and body-weight gain of
dams during treatment at 72 mg/kg bw. The only effects on fetuses were
an increased embryonic resorption rate and decreased fetal body
weights at the highest dose. There was no indication of teratogenicity
up to the highest dose tested. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of a decrease in food consumption and
body-weight gain. The NOEL for developmental toxicity was 36 mg/kg bw
per day on the basis of decreased fetal body weight and an increased
rate of embryonic resorptions (Becker, 1982).
2.2.6 Special studies on delayed neurotoxicity
Hens
In a study of delayed neurotoxicity, of which only a summary was
available, groups of one to five white Leghorn hens received a single
oral or intraperitoneal dose of phoxim dissolved in polyethylene
glycol, without an antidote. The oral doses used were 25, 38, 50, 250,
500, or 1000 mg/kg bw, and the intraperitoneal doses were 25, 38, or
50 mg/kg bw. In a second experiment, hens were given intraperitoneal
injections of 100 mg/kg bw pralidoxime iodide and 50 mg/kg bw atropine
sulfate before administration of phoxim at single oral doses of 38,
50, or 75 mg/kg bw (groups of 7-11 hens) or at single intraperitoneal
doses of 50, 75, 100, 150, or 200 mg/kg bw (groups of 1-14 hens). In
both studies, the birds were observed for 42 days; no
histopathological examinations were carried out, and a positive
control group was not included. No clinical signs of delayed
neurotoxicity were observed either with or without antidotal
protection. A LD50 of 38 mg/kg bw was derived for both oral and
intraperitoneal administration in the first experiment. The protective
effect of the antidote was more marked when phoxim was administered
intraperitoneally than when it was given orally (Kimmerle, 1972).
In another study of delayed neurotoxicity, 30 white Leghorn hens
received phoxim emulsified in water and 2% Cremophor EL by oral gavage
at a dose of 50 mg/kg bw, twice, at an interval of 21 days. Because
this dose was greater than the LD50, phoxim was administered with
atropine sulfate, administered intramuscularly at a dose of 50 mg/kg
bw. A negative control group of six hens received the vehicle and
atropine sulfate by the same schedule, and a positive control group of
five hens received a single dose of 375 mg/kg bw
tri- ortho-cresylphosphate in peanut oil. The hens treated with phoxim
and atropine and the negative controls were observed for 42 days and
the positive controls for 24 days. During the observation period, the
birds were inspected for clinical signs, body weight, and coordination
of movement. At the end of the observation period, they were killed
and nervous tissue (brain, spinal cord, and sciatic nerves) was
removed from six birds treated with phoxim and from all negative and
positive controls and examined histopathologically. The design of the
study closely resembled OECD guideline 418 in use in 1984, with GLP
certification.
After treatment with phoxim and atropine, the signs of toxicity
were staggering gait, prostration, apathy, salivation, drooping wings,
and fluffed plumage. From observation day 5 onwards, the only sign of
toxicity was staggering gait, while from observation day 26 onwards
the hens showed no abnormal signs or behaviour. The negative control
group showed only staggering gate. The positive control group showed
transient staggering gate in the acute phase, progressive disturbance
of coordination of movement from observation day 8 onwards, and severe
paralysis in the final stage which was of sufficient severity that the
birds were killed prematurely at observation day 24. Histopathological
examination showed no sign of peripheral neuropathy or demyelination
in phoxim-treated birds, whereas hens given tri- ortho-cresylphosphate
showed various degrees of degeneration of peripheral nerves including
demyelination, axonal lysis, ballooned fibre segments, and activated
Schwann cells. Thus, at a dose that would be lethal in the absence of
antidote, phoxim did not induce delayed neurotoxicity in hens (Pauluhn
& Kaliner, 1984).
2.2.7 Studies with metabolites
The oral LD50 of cyanobenzaldoxime (technically pure substance
formulated in lutrol) was 4500 mg/kg bw in male and 4100 mg/kg bw in
female rats. The signs of acute oral toxicity comprised slight to
moderate disturbance of behaviour, no grooming of coats, sedation, and
dyspnoea. Dermal application of 5000 mg/kg bw had no effect (Thyssen &
Kimmerle, 1976). The oral LD50 of the glucuronide of
cyanobenzaldoxime (formulated in aqueous Cremophor EL) in three female
rats was > 2500 mg/kg bw (Mihail, 1979). The oral LD50 value for
PO-phoxim in mice was approximately 800 mg/kg bw for the pure
substance (Kimmerle, 1969) and 1000 mg/kg bw for the pure substance
dissolved in olive oil (Vinopal & Fukuto, 1971) were determined.
In a short summary of a study on dermal irritation, 0.5 g of
cyanobenz-aldoxime applied under a plaster dressing for 24 h to the
ear did not irritate the skin of hairless rabbits. In a short summary
of a study on ocular irritation, cyanobenzaldoxime at a dose of 50 mg
placed in the conjuctival sac of the left eye of rabbits caused severe
redness and swelling of the conjunctivae, slight redness, swelling of
the iris, and diffuse opacity of the cornea (Thyssen & Kimmerle,
1976).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term, and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
delayed neurotoxicity of phoxim. Although most of the studies did not
meet current standards for study protocol and conduct, they did
provide satisfactory information for evaluation of the safety of the
compound.
After oral administration of radiolabelled phoxim to mice, rats,
and pigs, the radiolabel was rapidly and almost completely absorbed
and was rapidly taken up into the major organs and tissues. The main
route of elimination in mice, rats, and pigs was the urine, while
faecal excretion was of minor importance. In rats, there was also some
evidence of biliary excreation. In rats and pigs, elimination was
rapid (> 80% via the urine within 24 h) and was virtually complete
with three to four days. In mice, elimination of radiolabel was
somewhat slower, appraently because of retention of phosphate-derived
metabolites in the urinary blader. Although there were some
qualitative and quantitative differences in metabolism between mice,
rats, and pigs, the main routes of metabolism were the same, involving
de-ethylation, hydrolysis of the phosphorus ester bond (either before
or after oxidative desulfuration to yield PO-phoxim), and conjugation
of the resulting cyanobenzaldoxime. Despite the presence of products
that were assumed to have arisen from PO-phoxim, no PO-phoxim itself
was found in mice, rats, or pigs. Hence, if formed, it must be a very
short-lived intermediate in mammals.
Animal species differ in their sensitivity to single oral doses
of phoxim. The LD50 values ranged from 20 to 40 mg/kg bw in chickens,
from 250 to > 1200 mg/kg bw in guinea-pigs, rabbits, cats, and dogs,
and from 1200 to 10 000 mg/kg bw in mice and rats. Thus, phoxim is of
only low or moderate acute oral toxicity in mammalian species.
The short-term toxicity of phoxim was evaluated after oral
administration to mice, rats, dogs, and rhesus monkeys. In two dose
range-finding studies of six and eight weeks' duration, phoxim was
administered to mice in the feed at concentrations of 0.5-750 mg/kg
(equal to 0.28-510 mg/kg bw per day). In these studies, phoxim had
effects on the liver (increased weight and hepatocyte alterations
indicative of an adaptive response) at concentrations of 30 mg/kg of
feed and above and on the kidney (increased weight) at doses of 150
mg/kg of feed and above. Inhibition of plasma cholinesterase activity
was observed at and above concentrations of 5 m/kg of feed, while
inhibition of erythrocyte and brain acetylcholinesterase activity was
observed only at 750 mg/kg of feed. On the basis of a slight increase
in liver weight in female mice, the overall NOEL in these studies was
5 mg/kg of feed, equal to 3.1 mg/kg bw per day.
Studies were carried out in rats which received doses of 2-50
mg/kg bw per day by gavage for 21 or 30 days or in two studioes at
4-500 mg/kg of feed in the diet, equivalent to 0.4-50 mg/kg bw per
day, for three months. In the studies in which phoxim was given by
gavage, cholinesterase activity in plasma, erythrocytes, and brain was
inhibited by > 20% at doses of 2, 5, and 50 mg/kg bw per day,
respectively. In the two studies in which phoxim was administered in
the diet, signs of cholinergic poisoning were observed at 500 mg/kg of
feed, organ weight changes at doses > 120 mg/kg of feed, and
inhibition of cholinesterase activity in plasma at doses > 15 mg/kg
of feed and in erythrocytes at > 40 mg/kg of feed. The overall NOEL
in the studies involving dietary administration was 12 mg/kg of feed,
equivalent to 1.2 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholines-terase activity (in the absence of data on
brain acetylcholinesterase activity).
Three studies of three months' duration in dogs treated in the
diet were reported, in which phoxim was administered at doses ranging
from 0.3 to 1000 mg/kg of feed, equivalent to 0.0075-25 mg/kg bw per
day. Doses of 200 and 1000 mg/kg of feed resulted in changes in the
weights of gonads and liver and in the activities of alkaline
phosphatase and lactate dehydrogenase in plasma, inhibition of
erythrocyte acetylcholinesterase activity, and/or weight loss (females
only) and signs of cholinergic poisoning. Inhibition of plasma
cholinesterase activity was observed at doses from 1 to 1000 mg/kg of
feed. Brain acetylcholinesterase activity was not determined in these
studies. The overall NOEL was 50 mg/kg of feed, equivalent to 1.3
mg/kg bw per day, on the basis of inhibition of erythrocyte
acetyl-cholinesterase activity (in the absence of data on brain
acetylcholinesterase activity) and increased plasma alkalaine
phosphatase activity.
Rhesus monkeys received phoxim by gavage for six months at a dose
of 0.2, 0.65, or 3 mg/kg bw per day. Apart from marked inhibition of
plasma cholinesterase activity at doses > 0.2 mg/kg bw per day and
very slight inhibition of erythrocyte acetylcholinesterase activity at
2 mg/kg bw per day, phoxim had no effect. In the absence of data on
brain acetylcholinesterase activity, the NOEL was 0.65 mg/kg bw per
day on the basis of slight inhibition of erythrocyte
acetylcholinesterase activity.
In a 24-month study of carcinogenicity, phoxim was administered
in the diet at concentrations of 1-450 mg/kg of feed (equal to
0.47-210 mg/kg bw per day) to mice of a strain known to be highly
susceptible to the development of liver tumours. Plasma, erythrocyte,
and brain cholinesterase activities were decreased at doses > 150
mg/kg of feed. The body weight of males were increased at 150 mg/kg of
feed and the body weights of both males and females at 450 mg/kg of
feed. Signs of effects on the liver (including changes in weight, in
plasma cholesterol and total bilirubin levels, and in plasma alanine
aminotransferase and alkaline phosphatase activities) were evident at
450 mg/kg of feed. At this dose, there were also increased incidences
of non-neoplastic histological changes in the livers of males and of
hepatocellular adenomas in females. At 150 mg/kg of feed, the only
effect on the liver was increased plasma cholesterol concentrations in
animals of each sex. The NOEL was 5 mg/kg of feed, equal to 2.4 mg/kg
bw per day, on the basis of increased plasma cholesterol
concentrations and inhibition of brain acetylcholinesterase activity.
The increase in the incidence of adenomas observed in females at the
highest dose was thought to be a consequence of the
hepatoproliferative effect of the compound.
The long-term toxicity of phoxim was evaluated in rats and dogs
by oral administration for 24 months at 15-375 mg/kg of feed (equal to
0.8-27 mg/kg bw per day) to rats and 0.1-750 mg/kg of feed (equivalent
to 0.0025-19 mg/kg bw per day) to dogs. In rats dosed at 375 mg/kg of
feed, reductions were seen in food intake in males, in body weight in
females, and in plasma glutamate dehydrogenase activity, total
bilirubin level, the weights of the heart, lungs, spleen, and
adrenals, and plasma, erythrocyte, and brain cholinesterase activity
in animals of each sex. Decreased plasma and erythrocyte
cholinesterase activity and adrenal weights were also observed in rats
at 75 mg/kg of feed. There were no histopathological differences
between control and treated animals, nor was there any difference in
the incidence of tumours. The NOEL was 15 mg/kg of feed, equal to 0.8
mg/kg bw per day, on the basis of lowered adrenal weights at higher
doses.
Male and female dogs given phoxim at 750 mg/kg of feed had liver
damage, as shown by increased liver weights and plasma alanine
aminotransferase and alkaline phosphatase activities, decreased serum
cholesterol level, and histopathological alterations to hepatocytes.
Plasma, erythrocyte, and brain cholinesterase activities were also
reduced in animals of each sex at this dose. In addition, male dogs
showed clinical signs of toxicity and decreased body-weight gain.
Reductions in plasma and erythrocyte cholinesterase activities were
observed in male and female dogs at 15 mg/kg of feed. The NOEL was 15
mg/kg of feed, equivalent to 0.38 mg/kg bw per day, on the basis of
effects on the liver and inhibition of brain acetylcholinesterase
activity.
Phoxim has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium and Saccharomyces
cerevisiae, DNA damage in Bacillus subtilis, and chromosomal
aberrations in human lymphocytes. It has also been tested
in vivo for its ability to induce micronucleus formation,
chromosomal aberrations, and dominant lethal mutations in mice.
Cytogenetic alterations were found in human lymphocytes in vitro at
a cytotoxic dose in the absence of exogenous metabolic activation. The
results of all other tests were negative. On the basis of these data
and the results of the long-term assays in rodents, the Committee
concluded that phoxim is not genotoxici and is unlikely to have
carcinogenic potential in humans.
In a three-generation study of reproductive toxicity, rats were
given phoxim in the diet at a concentration of 0, 15, 75, or 375 mg/kg
of feed (equivalent to 0, 0.75, 3.8, and 19 mg/kg bw per day). The
only effect observed was a slight reduction in the number of pups in
the second litter of the third generation thaqt survived after four
weeks' lactation from dams receiving 375 mg/kg of feed. On the basis
of this effect, the NOEL was 75 mg/kg of feed, equivalent to 3.8 mg/kg
bw per day.
In a study of developmental toxicity in rats given a dose of 0,
30, 100, or 300 mg/kg bw per day orally on days 6-15 of gestation,
phoxim was toxic to the dams, retarding body-weight gain during
treatment at the highest dose. It was not embryotoxic, fetotoxic, or
teratogenic at any dose. The NOEL for maternal toxicity was 100 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 300 mg/kg bw per day, the highest dose
tested.
A study of developmental toxicity was conducted in rabbits given
phoxim at a dose of 0, 12, 36, or 72 mg/kg bw per day orally on days
6-18 of gestation. At the highest dose, phoxim increased the rate of
embryonic resorption, decreased fetal body weights, and was toxic to
the dams, which showed signs of toxicity a marked decreases in food
consumption and in body-weight gain. There was no indication of
teratogenicity at any dose. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of reduced food consumption and
body-weight gain. The NOEL for developmental toxicity was also 36
mg/kg bw per day, on the basis of decreased fetal body weight and an
increased rate of embryonic resorption.
In a study of delayed neurotoxicity, hens protected by the
antidote atropine received phoxim orally at a dose of 50 mg/kg bw,
which was repeated after 21 days. The birds were then observed for 42
days. They showed only transient signs of toxicity and no abnormal
signs or behaviour from day 26 after the second dose. At the end of
the study, no paralysis was present, and no histological evidence of
peripheral neuropathy or demyelination was observed. The Committee
concluded that phoxim does not induce delayed neurotoxicity in hens.
Although the effect of phoxim on the enzyme neuropathy target esterase
has not been investigated in hens, the Committee concluded that such a
study was not necessary in view of the negative results obtained in
adequately conducted assessments of the capacity of phoxim to induce
delayed neuropathy.
4. EVALUATION
The Committee established an ADI of 0-4 µg/kg bw for phoxim on
the basis of the NOEL of 0.38 mg/kg bw per day for effects on the
liver and inhibition of brain acetylcholinesterase activity in the
two-year study of toxicity in dogs and a safety factor of 100. This
ADI differs from that established by the Joint FAO/WHO Meeting on
Pesticide Residues, as the Expert Committee concluded that inhibition
of plasma cholinesterase activity is not a relevant end-point for risk
assessment. The Joint Meeting is now of a similar opinion (FAO/WHO,
1988).
5. REFERENCES
Becker, H. (1982) Embryotoxicity and teratogenicity study on SRA 7502
in rabbits. Unpublished report (project no. 004972, report no. R2354)
from Research & Consulting Company Ltd, Itingen, Switzerland, for
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomann, W. (1992) Volaton VL 80 (c.n.: phoxim). Studies on acute
dermal toxicity in rats. Unpublished report (study no. T 0040187,
report no. 21065) from Bayer AG, Fachbereich Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomhard, E. & Löser, E. (1977) SRA 7502. Chronic toxicology studies in
rats (feeding experiment over 2 years). Unpublished report (no. 7042)
from Bayer AG, Institute for Toxicology, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Brooks, P.N. & Cherry, C.P. (1974) Pathology report of Bay 77488.
Sub-chronic toxicological studies in dogs (3-month feeding
experiment). Addendum to report no. 2579. Unpublished report (no.
FBA130/73959) from Huntingdon Research Centre, Huntingdon, United
Kingdom, for Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F., Griffin, T. & Talley, W. (1978) A safety evaluation
study of phoxim in rhesus monkeys. Unpublished report from Institute
of Comparative and Human Toxicology, Albany, New York & International
Center of Environmental Safety, Holloman, New Mexico, United States.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Daniel, J.W., McLean, J. & Pringuer, M. (1978a)
Phoxim--Biotransformation in the rat. Unpublished report (no.
78/BAG6/317) from Life Science Research, Stock, Essex, United Kingdom
for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Daniel, J.W., Swanson, S. & McLean, J. (1978b) Phoxim:
Pharmacokinetics and biotransformation in the rat. Unpublished report
(no. 78/BAG5/194) from Life Science Research, Stock, Essex, United
Kingdom, for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
FAO/WHO (1983) Pesticide Residues in Food--1982. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 46).
FAO/WHO (1985) Pesticide Residues in Food--1984. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 62).
FAO/WHO (1988) Pesticide Residues in Food--1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and a WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 92).
Finn, J.P. (1977) Chronic toxicity study on rats with SRA 7502 (two
year feeding study). Unpublished report (no. 612/262/2) from Hazleton
Laboratories Europe Ltd, Harrogate, United Kingdom, for Bayer AG,
Institut für Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1978a) Determination of acute toxicity (LD50)--SRA 7502.
Unpublished letter report (dated 19 May 1978) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Flucke, W. (1978b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 26 June 1978) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979a) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 9 March 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 17 April 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity (LD50)--SRA 7502
Volaton VL. Unpublished letter report (dated 14 March 1980) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1984a) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in guinea pigs [in German]. Unpublished report
(study no. T 6016487, report no. 13071) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Flucke, W. (1984b) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in an open epicutaneous test with guinea pigs
[in German]. Unpublished report (study no. T 6016630, report no.
13086) from Bayer AG, Institut für Toxikiologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1978) Volaton VL. Subacute cutaneous
toxicity study with rabbits. Unpublished report (report no. 8034) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Thyssen, J. (1979) Volaton (SRA 7502) active ingredient
presolution. Acute toxicity studies. Unpublished report (no. 8081)
from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gyrd-Hansen, N., Brimer, L. & Rasmussen, F. (1993) Percutaneous
absorption of organophosphorus insecticides in pigs--The influence of
different vehicles. J. Vet. Pharmacol. Ther., 16, 174-180.
Heimann, K.-G. (1982a) Determination of acute toxicity (LD50)--
Volaton VL. Unpublished letter report (dated 16 February 1982) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.-G. (1982b) Determination of acute toxicity (LD50)--
Volaton VL 80. Unpublished letter report (dated 3 June 1982) from
Bayer, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) SRA 7502. Phoxim, Baythion(R)-active ingredient,
Volaton(R)-active ingredient. Micronucleus test on mouse to evaluate
SRA 7502 for mutagenic potential. Unpublished report (study no. T
5010077, report no. 9891) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985) SRA 7502 c.n. Phoxim. Test on S. cerevisiae D7
investigating for point mutagenic effect. Unpublished report (study
no. T 2018201, report no. 13708) from Bayer AG, Institute for
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1986) SRA 7502 (c.n. phoxim). Cytogenetic study with
human lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report (study no. T 9021736, report no.
15148) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1977) SRA 7502. Chronic toxicity study on
dogs (two-year feeding experiment). Unpublished report (no. 6865) from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989a) SRA 7502. Preliminary study on
mice (administration in feed to determine dosage for a long-term study
on carcinogenicity in mice). Unpublished report (study no. T1022043,
report no. 17610) from Bayer AG, Fachbereich Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989b) SRA 7502. Preliminary study on
mice (administration in feed for 8 weeks to determine dose for a
long-term study). Unpublished report (study no. T9022799, report no.
17611) from Bayer AG, Fachbereich Toxikologie, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jäger, R. (1992) SRA 7502 (common name: Phoxim). Study for
oncogenicity in B6C3F1 mice (twenty-four month feeding study).
Unpublished report (study no. T 2022297, report no. 21624) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1968) Bay 77488. Toxicological studies. Unpublished
report (no. 1067) from Farbenfabriken Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1969) Supplement to the toxicology of SRA 7502.
Unpublished letter report (dated 27 June 1969) from Bayer AG,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972) Phoxim: Tests for neurotoxicity in chickens.
Unpublished report (dated 21 June 1972) from Farbenfabriken Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1973) SRA 7502, SRA 7660, LOW 6599, SIR 5126, HOX 3082,
malathion. Comparative toxicological studies in rats with repeated
oral administration. Unpublished report (no. 3962) from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. (1970) Bay 77 488. Toxicological studies.
Unpublished report (no. 2235) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. & Weber, H. (1988) [Phenyl-U-14-C]-Phoxim: Biokinetic
behaviour and biotransformation in the edible tissues of the pig after
oral administration. Unpublished report (study no. M 183 0138-4,
PF-report no. 3077) from Bayer AG, Institute for Metabolism Research,
Leverkusen/Monheim, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kurinnyi, A.I. (1979) Cytogenetic activity of the pesticide valexon
and its influence on the mutability of mouse bone marrow cells.
Tsitol. Genet., 13, 370-374 (English translation). Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lorke, D. & Kimmerle G. (1965) Toxikologische Untersuchungen mit dem
Wirkstoff SRA 7502. Unpublished report (dated 14 December 1965) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969a) Bay 77 488. Subchronic toxicology studies in rats
(study over 3 months). Unpublished report (no. 1205) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969b) Bay 77 488. Subchronic toxicology studies with rats.
Animal and organ weights (Individual values for report no. 1205).
Unpublished report (no. 1215) from Farbenfabriken Bayer AG, Institut
für Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Löser, E. (1970a) Bay 77 488. Subchronic toxicological studies on rats
(three-month feeding experiment). Unpublished report (no. 2389) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1970b) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2418) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1971) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2579) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1979) SRA 7502. Multigeneration reproduction study on rats.
Unpublished report (no. 8447) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1974) SRA 7502 (phoxim). Dominant lethal test on male
mouse to evaluate SRA 7502 for mutagenic potential. Unpublished report
(no. 4943) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Machemer, L. (1975) SRA 7502 (phoxim). Studies for embryotoxic and
teratogenic effects on rats following oral administration. Unpublished
report (no. 5331) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Märtins, T. (1992) Volaton VL 80 (c.n.: phoxim). Acute inhalation
toxicity in rats in compliance with OECD guideline no. 403.
Unpublished report (study no. T 2040765, report no. 21429) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mihail, F. (1979) Cyanoxim acid glucoside (metabolite of phoxim).
Unpublished letter report (dated 4 July 1979) from Bayer AG, Institute
of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1981) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 4 May 1981) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1982) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 1 March 1982) from Bayer, Institute
of Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Moriya, M., Ohta, T., Watanabe K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Mürmann, P. & Luckhaus, G. (1973) SRA 7502. Subchronic toxicity study
on dogs (three-month feeding experiment). Unpublished report (no.
4136) from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Oesch, F. (1977) Ames test for Volaton (phoxim). Unpublished report
(dated 30 November 1977) from Pharmakologisches Institut, Universität
Mainz, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1983) SRA 7502 (active ingredient of Baythion(R) and
Volaton(R)) (common name: phoxim). Acute oral toxicity study on
hens. Unpublished report (study no. T 3006197, report no. 11978) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. & Kaliner, G. (1984) SRA 7502 (c.n.: phoxim). Study for
acute neurotoxicity in hens. Unpublished report (study no. T 3016385,
report no. 12632) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Watanabe, T. (1978) Phoxim. Mutagenicity
test on bacterial systems. Unpublished report (dated 18 July 1978)
from Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1976) SRA 7502. Subacute oral cumulation study on rats.
Unpublished report (no. 5954) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1973) SRA 7502. Toxicological studies on
hens. Unpublished report (no. 4236) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) Cyanoxim
(hydroxiiminophenylacetonitrile). Occupational toxicological study.
Unpublished report (no. 6392) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vince, A.A. & Spicer E.J.F. (1971) Pathology report on Bay 77 488.
Subchronic toxicological studies in rats (3 month feeding experiment).
Addendum to report no. 2389. Unpublished report (no. 4257/71/415) from
Huntingdon Research Centre, Huntingdon, United Kingdom, for
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vinopal, J.H. & Fukuto, T.R. (1971) Selective toxicity of phoxim
(phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate). Pestic.
Biochem. Physiol., 1, 44-60.
were observed, even at the highest doses, when the only effects were
non-specific ones on the nervous system. No signs of poisoning were
observed after dermal application. Phoxim was only slightly to
moderately toxic in all of the mammalian species studied.
Poorly reported studies of skin irritation indicated that phoxim
had only a very slight irritating effect on rabbit's skin. The
calculated overall primary irritation index was < 0.5. Limited
reports of eye irritation in rabbits showed that phoxim can cause mild
to moderate irritation of the conjuctivae, without effects on the iris
or cornea (Lorke & Kimmerle, 1965; Kimmerle & Solmecke, 1970; Flucke &
Thyssen, 1979).
In a Magnussen-Kligman maximization test in 40 male DHPW
guinea-pigs, phoxim induced a sensitization response in 60% of the
animals, which was shown not to be due to 1-butanol (Flucke, 1984a).
In an open epicutaneous test (according to Klecak) in 64 male DHPW
guinea-pigs, administration of phoxim showed no sensitization
potential (Flucke, 1984b).
2.2.2 Short-term studies of toxicity
Mice
In a study to establish the doses for a study of carcinogenicity,
groups of 10 male and 10 female SPF B6C3F1 mice were fed diets
containing phoxim at 0, 5, 30, 150, or 750 mg/kg feed for six weeks,
equal to 0, 3, 18, 85, and 440 mg/kg bw per day for males and 0, 3,
20, 100, and 500 mg/kg bw per day for females. The study was certified
for compliance with GLP. No differences in mortality rate, clinical
signs, body weight, or food or water intake were observed that could
be associated with treatment. Plasma alkaline phosphatase activity and
total protein content were significantly increased in males at the
high dose. In females, the plasma cholesterol content increased with
dose, the increase becoming statistically significant from 30 mg/kg
feed onwards; however, the control concentrations of cholesterol and
total protein were low in comparison with historical data, and the
increased levels were still within the historical control range, as
were the alkaline phosphatase activities in males at the high dose.
Cholinesterase activity was increasingly reduced with dose in plasma,
with > 20% inhibition in all treated animals, and in erythrocytes,
with > 20% inhibition only in females at the high dose. Brain
acetylcholinesterase activity, which was determined in five animalsof
each sex in each group, was reduced in all treated groups, with
reductions of more than 50% in both males and females at the high
dose; however, the activities in concurrent controls were in the upper
range of that of historical controls, and the observed reduction
showed no dose-response relationship, was not always statistically
significant, and was still within historical control levels, except in
males at the high dose. Therefore, only the reduction in males at the
high dose is considered relevant.
Table 1. Acute toxicity of phoxim
Route Species Sex LD50a or LC50 Reference
Oral Mouse Male 2700 Kimmerle (1968)
(mg/kg bw) 1800 Kimmerle & Solmecke (1970)
1200b Flucke & Thyssen (1979)
Female 3600 Kimmerle (1968)
2200 Kimmerle & Solmecke (1970)
2800-4000 Flucke (1978a,b; 1979a,b; 1980);
Heimann (1982a,b); Mihail
(1981, 1982)
1800b Flucke & Thyssen (1979)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 2300 Kimmerle (1969)
> 2000d Vinopal & Fukuto (1971)
Rat Male 10 000 Lorke & Kimmerle (1965)
8000 Kimmerle (1968))
2100 Kimmerle & Solmecke (1970)
4900b Thyssen (1976)
2000b Flucke & Thyssen (1979)
Female 10 000 Lorke & Kimmerle (1965)
6500 Kimmerle (1968)
1900 Kimmerle & Solmecke (1970)
2900b Thyssen (1976)
1400b Flucke & Thyssen (1979)
Guinea-pig Female 390-560 Kimmerle (1968)
approx. 660 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
Rabbit Female 250-380 Kimmerle & Solmecke (1970)
Unspecified approx. 380c Lorke & Kimmerle (1965)
280-560 Kimmerle (1968)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Cat Female 250-500 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Dog Female 250-500 Kimmerle & Solmecke (1970)
> 500b Flucke & Thyssen (1979)
Unspecified > 1200 Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
20e Thyssen & Kimmerle (1973)
40b Pauluhn (1983)
Intraperitoneal Rat Male 2200c Lorke & Kimmerle (1965)
(mg/kg bw) 2000 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1100b Flucke & Thyssen (1979)
Female 2000c Lorke & Kimmerle (1965)
1900 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1500b Flucke & Thyssen (1979)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
Intravenous Mouse Female 1100 Kimmerle (1968)
(mg/kg bw) 480b Kimmerle & Solmecke (1970)
Dermal Rat Male > 1200 Lorke & Kimmerle (1965)
(mg/kg bw) > 1100 Kimmerle (1968)
> 1100 Kimmerle & Solmecke (1970)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Male/female > 5600 Flucke & Thyssen (1979)
> 5000f Bomann (1992)g
Inhalation Mouse Male > 2.1h Kimmerle (1968)
(mg/L, 4 h) > 3.1h Kimmerle & Solmecke (1970)
Unspecified > 1.3 Kimmerle (1968)
>1.7h Kimmerle & Solmecke (1970)
Rat Male > 2.0h Kimmerle (1968)
> 2.6h Kimmerle & Solmecke (1970)
Female > 2.2h Kimmerle (1968)
> 2.8h Kimmerle & Solmecke (1970)
Male/female > 3.0h Flucke & Thyssen (1979)
> 4.6 Märtins (1992)g
Unspecified > 1.3 Kimmerle (1968)
> 1.7h Kimmerle & Solmecke (1970)
Guinea-pig Unspecified > 1.3 Kimmerle (1968)
Rabbit Unspecified > 1.3 Kimmerle (1968)
1.2-1.7h Kimmerle & Solmecke (1970)
Subcutaneous Mouse Male/female > 5000b Flucke & Thyssen (1979)
(mg/kg bw ) Unspecified > 1000c Lorke & Kimmerle (1965)
a In the reports of Kimmerle (1968), Flucke (1978a,b; 1979a,b; 1980), Heimann (1982a,b), Mihail
(1981, 1982), Kimmerle & Solmecke (1970), and Flucke & Thyssen (1979), some or all of the
LD50 values for phoxim (as pre-solution) were cited in microlitres or millilitres per kilogram
of body weight. These values were converted to milligrams per kilogram of body weight by
Table 1. (continued)
correcting for the density of the pre-solution (1.126 mg/µl at 20 °C). In the report of Lorke
& Kimmerle (1965), some of the LD50 values for pure phoxim were cited in millilitres per
kilogram of body weight. These values were converted to milligrams per kilogram of body weight
by correcting for the density of the pure substance (1.176 mg/µl at 20 °C).
b Vehicle, water:Cremophor EL
c Vehicle, lutrol
d Vehicle, olive oil
e Vehicle, water
f Vehicle, 2% Cremophor EL:physiological saline
g The studies of Bomann (1992) and Märtins (1992) were of conventional design, with GLP
and quality assurance certification.
h Vehicle, ethanol:lutrol 1:1
The only gross change seen on post-mortem examination was
enlarged spleens in a number of control and treated females. As this
finding was unrelated to dose and occurred in more control than
treated animals, it is not considered to be related to treatment. The
relative kidney weights of females at 150 and 750 mg/kg feed were
statistically significantly increased with dose. The absolute and
relative liver weights were increased with dose in all treated
animals, the increase in relative weight becoming statistically
significant at 150 mg/kg feed and the increase in absolute weight in
males only; both the relative and absolute weights were increased
significantly at 750 mg/kg feed. Histopathological examination only of
the livers of controls and animals at the high dose revealed
enlargement of the centrilobular hepatocytes in males and females at
the high dose, indicating adaptive hypertrophy and possible enzyme
induction. No evidence of irreversible changes was found. One female
at the high dose had slight cytoplasmic vacuolation in periportal
cells, which may not have been related to treatment (Ivens-Kohl &
Hahnemann, 1989a).
In a follow-up study, groups of 10 male and 10 female SPF B6C3F1
mice were fed diets containing phoxim at 0, 0.5, 1, 5, or 30 mg/kg
feed, equal to 0, 0.28, 0.55, 2.8, and 18 mg/kg bw per day for males
and 0, 0.35, 0.66, 3.1, and 23 mg/kg bw per day for females, for eight
weeks. The study was certified for compliance with GLP. No differences
in mortality rate, clinical signs, body weight, food or water intake,
or macroscopic or histopathological appearance were observed that
could be related to treatment. The plasma cholesterol content was
statistically significantly increased in females at 30 mg/kg feed. As
in the previous study, however, the control cholesterol content was
lower than that of historical controls, and the increased level was
still within the range of historical controls. Cholinesterase activity
in plasma was reduced by > 40% at the two highest doses, and a small
inhibition (8-14%) of erythrocyte acetylcholinesterase activity was
observed in males and females at the high dose. There was no evidence
of a change in brain acetylcholinesterase activity in any treated
groups. Although the absolute and relative weights of the liver were
slightly but statistically significantly increased in females at the
high dose, no effects on organ weights were found. The NOEL was 5
mg/kg feed, equal to 3.1 mg/kg bw per day, on the basis of slightly
increased liver weights in female mice (Ivens-Kohl & Hahnemann,
1989b).
Rats
In a 21-day study of toxicity with special attention to the
reversibility of cholinesterase inhibition, groups of 15 SPF Wistar II
rats of each sex received phoxim emulsified in distilled water and
Cremophor EL by oral gavage at 0, 5, or 50 mg/kg bw per day. Five rats
of each sex in each group were killed at the end of treatment and two
and four weeks thereafter. The animals were examined regularly for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. Brain
cholinesterase activity was measured at termination of treatment and
two weeks thereafter.
There were no deaths and no treatment-related effects on
appearance, behaviour, or body weight. Plasma cholinesterase activity
was reduced by > 20% during treatment in females at the low dose
(when compared with concurrent controls but not when compared with
pre-treatment values in the same animals) and in males and females at
the high dose, but the activity was restored within one week after
cessation of treatment. Acetylcholines-terase activity in erythrocytes
was reduced by > 20% in males at the low dose during the first week
of treatment (when compared with concurrent controls but not when
compared with pre-treatment values in the same animals) and in females
at this dose throughout treatment but was restored within one week.
Erythrocyte cholinesterase activity was decreased by > 20% in males
and females at the high dose throughout the treatment period but was
restored within two weeks in males and within four weeks in females.
Brain acetylcholinesterase activity was within the physiological range
and very similar to control values in all treated males and in females
at the low dose, both at termination and two weeks thereafter. Females
at the high dose showed 30% inhibition of brain acetylcholinesterase
activity at termination, but this was restored within two weeks. Thus,
inhibition of cholinesterase activity was more pronounced in female
than in male rats and was reversible in animals of each sex (Thyssen,
1976).
Groups of 15 SPF Wistar II rats of each sex received phoxim
dissolved in polyethylene glycol 400 (lutrol) by oral gavage at 0, 2,
5, or 15 mg/kg bw per day for 30 days. The animals were examined for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. No
treatment-related effects were observed on appearance, behaviour or
body weight. The cholinesterase activity in plasma decreased with dose
in all treated females and in males at 5 and 15 mg/kg bw per day. The
acetylcholinesterase activity in erythrocytes was reduced by > 20%
in males and females at two highest doses (Kimmerle, 1973).
In a three-month study, groups of 15 young SPF Wistar rats of
each sex received diets containing phoxim at 0, 4, 12, 40, or 120
mg/kg feed, equivalent to 0, 0.4, 1.2, 4, or 12 mg/kg bw per day. The
animals were observed for physical appearance, behaviour, deaths, body
weight, food consumption, haematological and clincal chemical
parameters (liver function tests), plasma and erythrocyte
cholinesterase activity, urinary parameters, organ weights, and
macroscopic appearance. Brain acetylcholinesterase activity was not
investigated. Treatment-related effects were found on cholinesterase
activity in both plasma and erythrocytes, which were decreased by
> 20% at 40 mg/kg feed (within one month) and 120 mg/kg feed
(within one week) in animals of each sex. Statistically significantly
increased weights of the thyroid glands (both absolute and relative)
and adrenal glands (relative only) were observed in males at 120 mg/kg
feed. In the absence of data on brain acetylcholinesterase activity,
the NOEL was 12 mg/kg feed, equivalent to 1.2 mg/kg bw per day, on the
basis of inhibition of erythrocyte acetyl-cholinesterase activity
(Löser, 1969a,b).
In a second three-month study with groups of 15 young SPF Wistar
rats of each sex, phoxim was administered in the diet at
concentrations of 0, 5, 15, 50, 150, or 500 mg/kg feed, equivalent to
0, 0.5, 1.5, 5, 15, or 50 mg/kg bw per day. The animals were
observed for the same end-points as in the previous study, with the
addition of histopathological examination. Cholinergic signs (muscle
twitching, cramps) were observed occasionally in animals at the high
dose, particularly in the earlier part of the study. Although
body-weight gain was significantly retarded in males at the high dose,
animals at 50 mg/kg feed showed a very similar growth curve. Hence,
the effect on body-weight gain was not consistent or dose-dependent.
In male rats, cholinesterase activity in plasma and erythrocytes
decreased by > 20% with doses from 50 mg/kg feed. In female rats,
cholinesterase activity decreased by > 20% in plasma with doses
from 15 mg/kg feed (at 1 and 13 weeks only) and in erythrocytes with
doses from 150 mg/kg feed. Liver weights were statistically
significantly increased in animals at 150 mg/kg feed (relative) and
500 mg/kg feed (relative in males and females, absolute in females),
but there were no significant changes in liver function tests. At the
high dose, the absolute and relative thyroid weights were increased in
females, and the relative adrenal weights were increased in males.
Relative kidney weights were increased at 150 mg/kg feed in females
and 500 mg/kg feed in males and females. On the basis of inhibition of
erythrocyte acetylcholinesterase activity in males (in the absence of
data on brain acetylcholinesterase activity), the NOEL was 15 mg/kg
feed, equivalent to 1.5 mg/kg bw per day (Löser, 1970a; Vince &
Spicer, 1971).
Rabbits
Groups of three male and three female New Zealand white rabbits
received dermal applications of phoxim emulsified with Cremophor EL in
water on 5 × 5 cm areas of shorn intact skin of the flank or back or
of skin abraded with sandpaper at doses of 0, 0.5, or 15 mg/kg bw per
day, 7 h/day, five days per week, for three consecutive weeks. The
treated sites were not occluded. The observations included deaths,
physical appearance, behaviour, body weight, skin irritation,
haematological parameters, clinical chemical parameters (liver
function tests), urinary parameters, gross pathological appearance,
organ weights, histopathological appearance, and determination of
plasma, erythrocyte, and brain cholinesterase activities.
Skin irritation was observed only in animals with abraded skin,
in which there was a dose-related increase in the time necessary for
the erythema to disappear. Histopathological examination of the
treated skin of one control animal with intact skin, of one animal at
the high dose with intact skin, and of four animals at the high dose
with abraded skin showed, on average, moderate cellular infiltration
of the epidermis and corium by round cells, polymorphonuclear
leukocytes, and isolated inflammatory hair follicles, and a slight
increase in the thickness of the epithelium. These findings were
considered to be the result of repeated physical irritation,
exacerbated by the compound at the highest dose. Plasma and
erythrocyte cholinesterase activities were reduced in a dose-related
manner and were inhibited by > 20% at 0.5 mg/kg bw per day in males
with abraded skin and in females with intact skin only and in all
animals at 15 mg/kg bw per day. Brain acetylcholinesterase activity
was reduced by 14% in males with abraded skin at the low dose and by
23% in all males at the high dose but was not reduced in females
(Flucke & Schilde, 1978).
Chickens
Groups of 10 white Leghorn hens were fed diets containing phoxim
at a concentration of 0, 5, or 10 mg/kg feed for 28 days, equal to
average achieved intakes of 0, 0.32, or 0.68 mg/kg bw per day. Phoxim
had no effect on behaviour, and there were no signs of neurotoxicity.
Body-weight gain and food consumption were also unaffected. At the
high dose, a marked depression (> 20% inhibition) in whole blood
cholinesterase activity was observed (Thyssen & Kimmerle, 1973).
Dogs
In a three-month study, beagle dogs were fed phoxim in the diet
at doses of 0 (three of each sex), 2, 5, or 10 mg/kg feed (two of each
sex), equivalent to 0, 0.05, 0.13, or 0.25 mg/kg bw per day. Treatment
did not affect the mortality rate, physical appearance, behaviour,
food intake, ophthalmoscopic, haematological, clinical chemical (liver
function tests), or urinary parameters, macroscopic appearance, or
organ weights. Body-weight gain appeared to be reduced in males at 5
mg/kg feed and in animals of each sex at 10 mg/kg feed; however, the
smal number of animals per group and the high intra-group variation
did not allow statistical analysis. As this effect was not observed at
higher doses in a second three-month study (see below) or in the first
three months of a two-year study with larger groups (see below), it
was discounted. Inhibition of cholinesterase activity by > 20% was
observed in the plasma of dogs of each sex at 2 mg/kg feed (after one
month only) and at 5 and 10 mg/kg feed but not in erythrocytes. Brain
acetylcholinesterase activity was not determined, and
histopathological examination was not performed. The NOEL was 10 mg/kg
feed, equivalent to 0.25 mg/kg bw per day, the highest dose tested
(Löser, 1970b).
In a second three-month study, in which histopathological
examination was performed in addition to the observations in the first
study, beagle dogs were fed phoxim in the diet at doses of 0 (three
per sex), 50, 200, or 1000 mg/kg feed (two per sex), equivalent to
0, 1.3, 5, or 25 mg/kg bw per day. Animals at the high dose showed
signs of cholinergic poisoning (cramps, especially in the abdominal
region, muscle twitching, and salivation), but none died. Food
consumption was reduced in females at 1000 mg/kg feed, which resulted
in weight loss throughout the study, and these animals appeared
emaciated upon macroscopic examination, with little adipose tissue in
the subcutaneous connective tissue or in the mesentery. Plasma
alkaline phosphatase activity was increased in animals of each sex at
200 and 1000 mg/kg feed, and plasma lactate dehydrogenase activity was
increased in males at the high dose. Ophthalmoscopic, haematological,
and urinary determinations showed no treatment-related effects. The
absolute and relative liver weights were increased in males at the
high dose, and the relative liver weight was increased in females at
the high dose, probably because of the body-weight loss, although
changes were not seen in other tissues. The absolute and relative
weights of the male gonads were increased at the two highest doses,
but as the report did not include data on individual animals or
provide any measure of intra-group variation, the statistical
significance of these findings could not be determined. In both males
and females, cholinesterase activity was decreased by > 20% in a
dose-dependent fashion in plasma at all doses and erythrocytes at 200
and 1000 mg/kg feed, from within one week. Brain acetylcholinesterase
activity was not determined. Histopathological examination revealed no
difference between control and treated animals. The NOEL was 50 mg/kg
feed, equivalent to 1.3 mg/kg bw per day, on the basis of reduced
erythrocyte acetylcholinesterase activity (in the absence of data on
brain acetylcholinesterase activity) and increased plasma alkaline
phosphatase activity (Löser, 1971; Brooks & Cherry, 1974).
Groups of four male and four female beagles were fed diets
containing phoxim at 0, 0.3, 1, or 2 mg/kg feed for three months,
equivalent to 0, 0.0075, 0.025, or 0.05 mg/kg bw per day. The animals
were observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, plasma and
erythrocyte cholinesterase activity, gross and histopathological
appearance, and organ weights. Brain acetylcholinesterase activity was
not determined. The only effect observed was reduced plasma
cholinesterase activity (> 20% inhibition) in both males and
females at 1 and 2 mg/kg feed. The NOEL was 2 mg/kg feed, equivalent
to 0.05 mg/kg bw per day, the highest dose tested (Mürmann & Luckhaus,
1973).
Monkeys
Groups of five male and five female rhesus monkeys ( Macaca
mulatta) received phoxim dissolved in corn oil by oral gavage at
doses of 0, 0.2, 0.65, or 2 mg/kg bw per day on six days per week for
six months. The observations included clinical signs, physical
appearance, behaviour, body weight, haematological, clinical chemical,
and urinary parameters, and plasma and erythrocyte cholinesterase
activity. Liver was biopsied before and at the end of treatment for
histopathological assessment. Brain acetylcholinesterase activity was
not determined. Although plasma and erythrocyte cholinesterase
activity was inhibited, there were no signs of cholinergic poisoning
in animals at doses up to 2 mg/kg bw per day. There was no evidence
from either clinical chemistry or histopathology of an effect of
phoxim on the liver. Plasma cholinesterase activity was reduced by
> 20% in a dose-dependent manner in all treated animals.
Erythrocyte acetylcholinesterase activity was slightly reduced in
animals at the highest dose, reaching > 20% inhibition only after
treatment for four months, when compared with concurrent controls but
not when compared with pre-treatment values in the same animals. In
the absence of data on brain acetylcholinesterase activity, the NOEL
was 0.65 mg/kg bw per day on the basis of slight evidence of
inhibition of erythrocyte acetylcholinesterase activity (Coulston
et al., 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity, groups of 50 male and 50 female
SPF B6C3F1 mice were fed diets containing phoxim at doses of 0, 1, 5,
150, or 450 mg/kg feed, equal to average achieved intakes of 0, 0.47,
2.4, 67, or 200 mg/kg bw per day for males and 0, 0.55, 2.7, 79, or
210 mg/kg bw per day for females, for 24 months. Ten additional
animals of each sex at each dose (satellite groups) were killed at 12
months. The animals were observed for clinical signs, death, body
weight, food and water consumption, haematological and clinical
chemical parameters (in 10 animals per group during weeks 27, 53, 79,
and 105; including plasma and erythrocyte cholinesterase activities),
organ weights, detailed macroscopic and microscopic examinations, and
brain acetylcholinesterase activity (in 10 animals of each sex per
group at the terminal kill). The study was of conventional design,
with GLP and quality assurance certification.
Clinical signs, food and water consumption, and haematological
parameters were unaffected by treatment, as was survival (70-88% of
controls, 78-90% of treated animals). Statistically significantly
increased body weights were observed in males at 150 and 450 mg/kg
feed and in females at 450 mg/kg feed. Plasma cholesterol contents
were statistically significantly increased in animals of each sex at
doses of 150 mg/kg feed and higher, from 27 weeks onwards. The total
bilirubin content was decreased in males at the high dose, while
females at this dose showed slightly but statistically significantly
increased plasma alkaline phosphatase and alanine aminostransferase
activities from one year onwards. Plasma cholinesterase activity was
decreased in animals of each sex at 5 (23-45% inhibition), 150, and
450 (94-98% inhibition) mg/kg feed. Erythrocyte acetylcholinesterase
activity was inhibited by 14-26% in males and females at 150 and 450
mg/kg feed, and brain acetylcholinesterase activity was reduced in
animals of each sex at 150 mg/kg feed (19-28%) and 450 mg/kg feed
(45-54%).
The weights and macroscopic and microscopic appearance of organs
showed little treatment-related change, except in the liver. At 450
mg/kg feed, the absolute and relative liver weights were statistically
significantly increased in all animals, and histopathological
examination showed a significant increase in the incidence of
non-neoplastic hepatic foci, mainly eosinophilic and basophilic types,
in males (8/50 versus 1/50 in controls). The absolute weights of the
heart and kidneys were significantly increased in both males and
females at the high dose, and fermales at these doses had a
significant reduction in the number of ovarian cysts. The incidences
of hepatocellular carcinoma were comparable in treated and control
groups, but the incidences of hepatocellular adenoma were
statistically significantly increased in males at 150 mg/kg feed (9/50
versus 1/50 in concurrent controls and 1-7/50 in historical controls)
and in females at 450 mg/kg feed (8/50 versus 1/50 in concurrent
controls and 0-2/50 in historical controls). This finding is probably
not biologically significant in males, as no increase was seen at the
highest dose (5/50 at both the lowest and highest doses) and the
incidence was just beyond the range of historical controls. The
increased incidence of adenomas in females at the high dose is thought
to be due to the hepatoproliferative effect of the compound. The
incidence of lymphoreticular tumours was slightly but significantly
reduced in females receiving 150 and 450 mg/kg feed. The NOEL was 5
mg/kg feed, equal to 2.4 mg/kg bw per day, on the basis of increased
plasma cholesterol content and inhibition of brain
acetylcholinesterase activity (Jäger, 1992).
Rats
In a long-term study of toxicity, groups of 50 male and 50 female
SPF Wistar rats received diets containing phoxim at concentrations of
0, 15, 75, or 375 mg/kg feed, equal to average achieved intakes of 0,
0.8, 4, or 18 mg/kg bw per day for males and 0, 1.1, 5.4, or 27 mg/kg
bw per day for females, for 24 months. The control group comprisd 100
males and 100 females. Five additional animals of each sex per group
were used for clinical chemical examinations at 3, 6, and 12 months,
and 10 males and 10 females per group were used for these examinations
at termination. The observations included physical appearance,
behaviour, deaths, body weight, food consumption, haematological,
clinical chemical (liver function tests), and urinary parameters,
plasma and erythrocyte cholinesterase activities, absolute organ
weights, macroscopic and microscopic appearance, and brain
acetylcholinesterase activity (in five animals of each sex per group).
Treatment with phoxim did not affect the physical appearance,
behaviour, survival (76-79% of controls, 74-86% of treated animals),
or haematological, urinary, or macroscopic appearance of the rats.
Food consumption was reduced by 10% in males at the high dose, while
body-weight gain was slightly reduced in females at this dose. The
results of liver function tests were normal, other than a significant
reduction in plasma glutamate dehydrogenase activity in males at the
high dose and in bilirubin content in females at the high dose. Plasma
and erythrocyte cholinesterase activities were reduced by > 20% in
a dose-dependent manner throughout the study in rats treated with 75
and 375 mg/kg feed. Inhibition by > 20% of plasma and erythrocyte
cholinesterase activities was also observed in females at 15 mg/kg
feed (at 1, 2, and 52 weeks for plasma cholinesterase; at 2 weeks for
erythrocyte acetylcholinesterase). Brain acetylcholinesterase activity
was reduced by 18% in males and 23% in females at the highest dose.
Absolute liver weights were statistically significantly increased in
all treated males, but the effect is discounted as an inverse
dose-response relationship was seen. The absolute adrenal weights
were statistically significantly decreased in a dose-related manner
at doses > 75 mg/kg feed in animals of each sex, and the absolute
weights of the heart, lung, and spleen were statistically
significantly reduced in females at the high dose. None of the changes
in organ weights was accompanied by corresponding histopathological
changes. There was no difference in tumour incidence between control
and treated animals. The NOEL was 15 mg/kg feed, equal to 0.8 mg/kg bw
per day, on the basis of changes in adrenal weights (Bomhard & Löser,
1977; Finn, 1977).
Dogs
In a long-term study of toxicity, groups of four beagles of each
sex received phoxim in the diet at concentrations of 0, 0.3/0.1, 15,
or 750 mg/kg feed for 104 weeks, equivalent to 0, 0.0075/0.0025, 0.38,
or 19 mg/kg bw per day. Because plasma cholinesterase activity was
decreased by 26% in females at week 77, although erythrocyte
acetylcholinesterase activity was not affected, their dose was reduced
from 0.3 to 0.1 mg/kg feed from week 83 onwards. The animals were
observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, gross and
histopathological appearance, organ weights, and plasma, erythrocyte,
and brain cholinesterase activity.
No treatment-related effects were observed on mortality rates,
behaviour, food consumption, reflexes, or ophthalmoscopic,
haematological, or urinary parameters. All males at the high dose and
two of four at the intermediate dose showed a poor nutritional state.
In the second year, the coats of all males at the high dose were dull,
ruffled, and ungroomed, and, although the food consumption of these
animals was unaffected by treatment, they took much longer to eat
their food ration. The body-weight gain of all treated animals, but
especially the males, was reduced in a dose-dependent manner between 0
and 104 weeks due mainly to retarded growth in the second half of the
study; the effect reached statistical significance only in males at
the high dose. Plasma alanine aminotransferase activity was
statistically significantly increased in animals at the high dose from
week 52 onwards. Plasma alkaline phosphatase activity was
statistically significantly increased in these animals throughout the
treatment period. Although it was not increased at lower doses, the
natural decline was slowed, resulting in statistically significantly
higher values than in controls in females at the intermediate dose
from week 26 onwards and in males at the two lower doses from week 52
onwards. These effects on alkaline phosphatase activity are considered
to be adaptive, and were probably due to induction, as
organophosphorus esters are natural substrates for alkaline
phosphatase. The serum cholesterol concentration was reduced in
animals at the high dose throughout treatment, reaching statistical
significance at some times. Cholinesterase activity was reduced
throughout the experiment in males and females at 15 and 750 mg/kg
feed, in plasma (60 and 80% inhibition, respectively) and in
erythrocytes (20-30 and 60-80% inhibition, respectively). The
cholinesterase activity in plasma and erythrocytes was not reduced in
males at 0.3 mg/kg feed or in females at 0.1 mg/kg feed. Brain
acetylcholinesterase activity was significantly reduced, by
approximately 37%, in animals at the high dose.
Organ weights and gross and histopathological appearance showed
little treatment-related change other than in the liver. Histological
examination revealed hypolasia in both testes of one dog at the high
dose. As there was no cryptorchism, this effect might be related to
treatment. The livers of all females and one male at 750 mg/kg feed
were darker than those of the controls, and some had a markedly
exaggerated lobular pattern. The absolute and relative weights of the
liver were statistically significantly increased in all animals at the
high dose; although the relative weight of the liver was also
increased in males at the intermediate dose, the increase was not
statistically significant. Histopathological examination showed
hepatocytic alterations in three dogs and three bitches at the high
dose, involving dilated hepatocytes with a light, glassy,
structureless cytosol. On the basis of inhibition of brain
acetylcholinesterase activity and effects on the liver, the NOEL was
15 mg/kg feed, equivalent to 0.38 mg/kg bw per day (Hoffmann &
Gröning, 1977).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with phoxim are
summarized in Table 2. Phoxim was dissolved in dimethyl sulfoxide for
testing in vitro and in aqueous 0.5% Cremophor emulsion for testing
in vivo. The only effect seen in vitro or in vivo was induction of
chromosomal aberrations in vitro in lymphocytes; however, this
finding could not be interpreted owing to the cytotoxicity seen at
concentrations that affected the chromosomes. The summary tables in an
English translation of the Russian article show that phoxim induced a
very weak, dose-related increase in the number of cells with
aberrations, but no details were provided on the exposure time,
harvesting time, or absence or presence of S9. Furthermore, the
marginally increased frequencies reached statistical significance
because the frequency in concurrent controls both in vitro and in vivo
was very low, and these frequencies may have been within the range for
historical controls, for which data were lacking.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Phoxim was administered in the diet to four groups of 10 male and
20 female Long Evans FB 30 rats throughout mating, gestation, and
lactation in a three-generation study of reproductive toxicity at
doses of 0, 15, 75, or 375 mg/kg feed, equivalent to 0, 0.75, 3.8, and
19 mg/kg bw per day. The pups were suckled for up to four weeks,
weighed weekly, and examined grossly for malformations; the offspring
of each first mating (F1a, F2a, and F3a) were then killed. The
offspring of each second mating (F1b and F2b) were weaned and then
mated to produce two litters. After the dams of the F0, F1b, and F2b
generations had successfully nursed their offspring twice, they were
killed. Gross and histopathological examinations were performed on one
male and one female four-week-old pup of the F3b generation from each
of 10 mothers per group.
There were no differences between the control and treated parents
in any of the generations with respect to physical appearance,
behaviour, or body-weight gain, although some parents in the control
and treated groups died due to pneumonitis. No treatment-related
effects on fertility (pregnancy rate), litter size at birth or after
five days (viability index), or pup body weights at birth or during
four-week lactation were seen in any generation, and there were no
signs of malformation. The survival of pups in the F1a, F1b, F2a,
F2b, and F3a generations after four weeks' lactation was normal, but
the survival of pups in the F3b generation at 375 mg/kg feed was
slightly but statistically significantly reduced (84% versus 97% in
controls). Gross and histopathological examinations of the F3b pups
did not reveal any treatment-related alterations. The NOEL was 75
mg/kg feed, equivalent to 3.8 mg/kg bw per day, on the basis of
slightly reduced survival of pups in the second litter of the third
generation (Löser, 1979).
Table 2. Results of assays for genotoxicity with phoxim
End-point Test object Concentration Result GLP/ Reference
QA
In vitro
Reverse S. typhimurium TA98, 3.2-3200 nl/ Negativeb No Oesch
mutation TA100, TA1537 platea (+ S9) (1977)
32-3200 nl/
platea (- S9)
Reverse S. typhimurium TA98, 10-5000 µg/ Negativeb No Shirasu
mutation TA100 plate et al. (1978)
Reverse S. typhimurium TA98, < 5000 µg/ Negativeb No Moriya
mutation TA100, TA1535, plate et al. (1983)
TA1537, TA1538;
E. coli WP2 hcr
Reverse Saccharomyces 625-10 000 Negativeb Yes Herbold
mutation cerevisiae D7 µg/mlc (1985)
DNA damage Bacillus subtilis H-17, 1-100% v/v Negative No Shirasu
M-45 rec- (0.2-20 µl/disc) et al. (1978)
Chromosomal Human lymphocytes 30-300 µg/ml Positive Yes Herbold
aberration (- S9) at > 100 (1986)
50-500 µg/ml µg/ml - S9d
(+ S9)
Chromosomal Human lymphocytes 0.2 and 2 Equivocal No Kurinnyi
aberration µg/ml (1979)
Table 2. (continued)
End-point Test object Concentration Result GLP/ Reference
QA
In vivo
Micronucleus Mouse bone marrow 2 × 250 or Negativee No Herbold
formation (NMRI mice (SPF 500 mg/kg bw (1981)
Han) 5/sex per by gavage with
group) an interval of
24 h
Dominant Male NMRI mice Single dose of Negative No Machemer
lethal 500 mg/kg bw (1974)
mutation orally by gavage
Chromosomal Mouse bone marrow 1× 10 mg/kg bw Negative No Kurinnyi
aberration (noninbred white 1× 100 mg/kg bw, Negative (1979)
mice, 5 males/group) 5× 100 mg/kg bw, Equivocale
1× 250 mg/kg bw Equivocale
intragastrically
GLP, good laboratory practice; QA, quality assessment; S9, exogenous metabolic activation
system from rat liver microsomes
a At 1000 nl/plate, some test compound separated as droplets from the top agar.
b With and without S9
c No cytotoxicity up to 10 000 µg/ml
d The mitotic indices were 100, 37, and 1.9% at 30, 100, and 300 µg/ml,
respectively, in the absence of S9 and 84, 93, and 2.4% at 50, 100,
and 500 µg/ml, respectively, in the presence of S9. Significant
increases in chromosomal aberration frequency were found at 100 and
300 µg/ml in the absence of S9 and at 500 µg/ml in the presence of S9.
e The ratio of polychromatic to normochromatic erythrocytes at either
dose did not deviate from that in controls, and no clinical signs of
toxicity were noted. The doses were chosen on the basis of the results
of a preliminary test in which mice received 2 × 500 or 1000 mg/kg bw
orally and the lower dose was tolerated with induction of weak signs.
In view of this finding and the rapid and almost complete oral
absorption of phoxim by mice, it is likely that the bone marrow was
exposed.
2.2.5.2 Developmental toxicity
Rats
In a study of developmental toxicity, groups of 20-21 pregnant
Long Evans FB 30 rats received phoxim in 0.5% aqueous Cremophor
emulsion by oral gavage at daily doses of 0, 30, 100, or 300 mg/kg bw
on days 6-15 of gestation. On gestation day 20, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal malformations. None of the dams died
during the study, and no adverse effects were seen on their behaviour
or general appearance. The body-weight gain of dams at the high dose
was statistically significantly decreased during treatment but not
when considered over the entire gestation period. There were no
evident effects on embryonic or fetal development, and there was no
indication of teratogenicity up to the highest dose tested. The NOEL
for maternal toxicity was 100 mg/kg bw per day on the basis of the
decrease in body-weight gain. The NOEL for developmental toxicity was
300 mg/kg bw per day, the highest dose tested (Machemer, 1975).
Rabbits
In a study of developmental toxicity, groups of 20 pregnant
Chinchilla rabbits received phoxim in water with 2%
carboxymethylcellulose by oral gavage at daily doses of 0, 12, 36, or
72 mg/kg bw on days 6-18 of gestation. On gestation day 28, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external, visceral, and skeletal abnormalities. The study
was of conventional design, with quality assurance certification. No
treatment-related increase in mortality rate was observed. Clinical
signs of toxicity (diarrhoea, salivation, ventral body position,
dyspnoea, and abortion) were observed in dams at the high dose, and
phoxim caused reductions in food consumption and body-weight gain of
dams during treatment at 72 mg/kg bw. The only effects on fetuses were
an increased embryonic resorption rate and decreased fetal body
weights at the highest dose. There was no indication of teratogenicity
up to the highest dose tested. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of a decrease in food consumption and
body-weight gain. The NOEL for developmental toxicity was 36 mg/kg bw
per day on the basis of decreased fetal body weight and an increased
rate of embryonic resorptions (Becker, 1982).
2.2.6 Special studies on delayed neurotoxicity
Hens
In a study of delayed neurotoxicity, of which only a summary was
available, groups of one to five white Leghorn hens received a single
oral or intraperitoneal dose of phoxim dissolved in polyethylene
glycol, without an antidote. The oral doses used were 25, 38, 50, 250,
500, or 1000 mg/kg bw, and the intraperitoneal doses were 25, 38, or
50 mg/kg bw. In a second experiment, hens were given intraperitoneal
injections of 100 mg/kg bw pralidoxime iodide and 50 mg/kg bw atropine
sulfate before administration of phoxim at single oral doses of 38,
50, or 75 mg/kg bw (groups of 7-11 hens) or at single intraperitoneal
doses of 50, 75, 100, 150, or 200 mg/kg bw (groups of 1-14 hens). In
both studies, the birds were observed for 42 days; no
histopathological examinations were carried out, and a positive
control group was not included. No clinical signs of delayed
neurotoxicity were observed either with or without antidotal
protection. A LD50 of 38 mg/kg bw was derived for both oral and
intraperitoneal administration in the first experiment. The protective
effect of the antidote was more marked when phoxim was administered
intraperitoneally than when it was given orally (Kimmerle, 1972).
In another study of delayed neurotoxicity, 30 white Leghorn hens
received phoxim emulsified in water and 2% Cremophor EL by oral gavage
at a dose of 50 mg/kg bw, twice, at an interval of 21 days. Because
this dose was greater than the LD50, phoxim was administered with
atropine sulfate, administered intramuscularly at a dose of 50 mg/kg
bw. A negative control group of six hens received the vehicle and
atropine sulfate by the same schedule, and a positive control group of
five hens received a single dose of 375 mg/kg bw
tri- ortho-cresylphosphate in peanut oil. The hens treated with phoxim
and atropine and the negative controls were observed for 42 days and
the positive controls for 24 days. During the observation period, the
birds were inspected for clinical signs, body weight, and coordination
of movement. At the end of the observation period, they were killed
and nervous tissue (brain, spinal cord, and sciatic nerves) was
removed from six birds treated with phoxim and from all negative and
positive controls and examined histopathologically. The design of the
study closely resembled OECD guideline 418 in use in 1984, with GLP
certification.
After treatment with phoxim and atropine, the signs of toxicity
were staggering gait, prostration, apathy, salivation, drooping wings,
and fluffed plumage. From observation day 5 onwards, the only sign of
toxicity was staggering gait, while from observation day 26 onwards
the hens showed no abnormal signs or behaviour. The negative control
group showed only staggering gate. The positive control group showed
transient staggering gate in the acute phase, progressive disturbance
of coordination of movement from observation day 8 onwards, and severe
paralysis in the final stage which was of sufficient severity that the
birds were killed prematurely at observation day 24. Histopathological
examination showed no sign of peripheral neuropathy or demyelination
in phoxim-treated birds, whereas hens given tri- ortho-cresylphosphate
showed various degrees of degeneration of peripheral nerves including
demyelination, axonal lysis, ballooned fibre segments, and activated
Schwann cells. Thus, at a dose that would be lethal in the absence of
antidote, phoxim did not induce delayed neurotoxicity in hens (Pauluhn
& Kaliner, 1984).
2.2.7 Studies with metabolites
The oral LD50 of cyanobenzaldoxime (technically pure substance
formulated in lutrol) was 4500 mg/kg bw in male and 4100 mg/kg bw in
female rats. The signs of acute oral toxicity comprised slight to
moderate disturbance of behaviour, no grooming of coats, sedation, and
dyspnoea. Dermal application of 5000 mg/kg bw had no effect (Thyssen &
Kimmerle, 1976). The oral LD50 of the glucuronide of
cyanobenzaldoxime (formulated in aqueous Cremophor EL) in three female
rats was > 2500 mg/kg bw (Mihail, 1979). The oral LD50 value for
PO-phoxim in mice was approximately 800 mg/kg bw for the pure
substance (Kimmerle, 1969) and 1000 mg/kg bw for the pure substance
dissolved in olive oil (Vinopal & Fukuto, 1971) were determined.
In a short summary of a study on dermal irritation, 0.5 g of
cyanobenz-aldoxime applied under a plaster dressing for 24 h to the
ear did not irritate the skin of hairless rabbits. In a short summary
of a study on ocular irritation, cyanobenzaldoxime at a dose of 50 mg
placed in the conjuctival sac of the left eye of rabbits caused severe
redness and swelling of the conjunctivae, slight redness, swelling of
the iris, and diffuse opacity of the cornea (Thyssen & Kimmerle,
1976).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term, and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
delayed neurotoxicity of phoxim. Although most of the studies did not
meet current standards for study protocol and conduct, they did
provide satisfactory information for evaluation of the safety of the
compound.
After oral administration of radiolabelled phoxim to mice, rats,
and pigs, the radiolabel was rapidly and almost completely absorbed
and was rapidly taken up into the major organs and tissues. The main
route of elimination in mice, rats, and pigs was the urine, while
faecal excretion was of minor importance. In rats, there was also some
evidence of biliary excreation. In rats and pigs, elimination was
rapid (> 80% via the urine within 24 h) and was virtually complete
with three to four days. In mice, elimination of radiolabel was
somewhat slower, appraently because of retention of phosphate-derived
metabolites in the urinary blader. Although there were some
qualitative and quantitative differences in metabolism between mice,
rats, and pigs, the main routes of metabolism were the same, involving
de-ethylation, hydrolysis of the phosphorus ester bond (either before
or after oxidative desulfuration to yield PO-phoxim), and conjugation
of the resulting cyanobenzaldoxime. Despite the presence of products
that were assumed to have arisen from PO-phoxim, no PO-phoxim itself
was found in mice, rats, or pigs. Hence, if formed, it must be a very
short-lived intermediate in mammals.
Animal species differ in their sensitivity to single oral doses
of phoxim. The LD50 values ranged from 20 to 40 mg/kg bw in chickens,
from 250 to > 1200 mg/kg bw in guinea-pigs, rabbits, cats, and dogs,
and from 1200 to 10 000 mg/kg bw in mice and rats. Thus, phoxim is of
only low or moderate acute oral toxicity in mammalian species.
The short-term toxicity of phoxim was evaluated after oral
administration to mice, rats, dogs, and rhesus monkeys. In two dose
range-finding studies of six and eight weeks' duration, phoxim was
administered to mice in the feed at concentrations of 0.5-750 mg/kg
(equal to 0.28-510 mg/kg bw per day). In these studies, phoxim had
effects on the liver (increased weight and hepatocyte alterations
indicative of an adaptive response) at concentrations of 30 mg/kg of
feed and above and on the kidney (increased weight) at doses of 150
mg/kg of feed and above. Inhibition of plasma cholinesterase activity
was observed at and above concentrations of 5 m/kg of feed, while
inhibition of erythrocyte and brain acetylcholinesterase activity was
observed only at 750 mg/kg of feed. On the basis of a slight increase
in liver weight in female mice, the overall NOEL in these studies was
5 mg/kg of feed, equal to 3.1 mg/kg bw per day.
Studies were carried out in rats which received doses of 2-50
mg/kg bw per day by gavage for 21 or 30 days or in two studioes at
4-500 mg/kg of feed in the diet, equivalent to 0.4-50 mg/kg bw per
day, for three months. In the studies in which phoxim was given by
gavage, cholinesterase activity in plasma, erythrocytes, and brain was
inhibited by > 20% at doses of 2, 5, and 50 mg/kg bw per day,
respectively. In the two studies in which phoxim was administered in
the diet, signs of cholinergic poisoning were observed at 500 mg/kg of
feed, organ weight changes at doses > 120 mg/kg of feed, and
inhibition of cholinesterase activity in plasma at doses > 15 mg/kg
of feed and in erythrocytes at > 40 mg/kg of feed. The overall NOEL
in the studies involving dietary administration was 12 mg/kg of feed,
equivalent to 1.2 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholines-terase activity (in the absence of data on
brain acetylcholinesterase activity).
Three studies of three months' duration in dogs treated in the
diet were reported, in which phoxim was administered at doses ranging
from 0.3 to 1000 mg/kg of feed, equivalent to 0.0075-25 mg/kg bw per
day. Doses of 200 and 1000 mg/kg of feed resulted in changes in the
weights of gonads and liver and in the activities of alkaline
phosphatase and lactate dehydrogenase in plasma, inhibition of
erythrocyte acetylcholinesterase activity, and/or weight loss (females
only) and signs of cholinergic poisoning. Inhibition of plasma
cholinesterase activity was observed at doses from 1 to 1000 mg/kg of
feed. Brain acetylcholinesterase activity was not determined in these
studies. The overall NOEL was 50 mg/kg of feed, equivalent to 1.3
mg/kg bw per day, on the basis of inhibition of erythrocyte
acetyl-cholinesterase activity (in the absence of data on brain
acetylcholinesterase activity) and increased plasma alkalaine
phosphatase activity.
Rhesus monkeys received phoxim by gavage for six months at a dose
of 0.2, 0.65, or 3 mg/kg bw per day. Apart from marked inhibition of
plasma cholinesterase activity at doses > 0.2 mg/kg bw per day and
very slight inhibition of erythrocyte acetylcholinesterase activity at
2 mg/kg bw per day, phoxim had no effect. In the absence of data on
brain acetylcholinesterase activity, the NOEL was 0.65 mg/kg bw per
day on the basis of slight inhibition of erythrocyte
acetylcholinesterase activity.
In a 24-month study of carcinogenicity, phoxim was administered
in the diet at concentrations of 1-450 mg/kg of feed (equal to
0.47-210 mg/kg bw per day) to mice of a strain known to be highly
susceptible to the development of liver tumours. Plasma, erythrocyte,
and brain cholinesterase activities were decreased at doses > 150
mg/kg of feed. The body weight of males were increased at 150 mg/kg of
feed and the body weights of both males and females at 450 mg/kg of
feed. Signs of effects on the liver (including changes in weight, in
plasma cholesterol and total bilirubin levels, and in plasma alanine
aminotransferase and alkaline phosphatase activities) were evident at
450 mg/kg of feed. At this dose, there were also increased incidences
of non-neoplastic histological changes in the livers of males and of
hepatocellular adenomas in females. At 150 mg/kg of feed, the only
effect on the liver was increased plasma cholesterol concentrations in
animals of each sex. The NOEL was 5 mg/kg of feed, equal to 2.4 mg/kg
bw per day, on the basis of increased plasma cholesterol
concentrations and inhibition of brain acetylcholinesterase activity.
The increase in the incidence of adenomas observed in females at the
highest dose was thought to be a consequence of the
hepatoproliferative effect of the compound.
The long-term toxicity of phoxim was evaluated in rats and dogs
by oral administration for 24 months at 15-375 mg/kg of feed (equal to
0.8-27 mg/kg bw per day) to rats and 0.1-750 mg/kg of feed (equivalent
to 0.0025-19 mg/kg bw per day) to dogs. In rats dosed at 375 mg/kg of
feed, reductions were seen in food intake in males, in body weight in
females, and in plasma glutamate dehydrogenase activity, total
bilirubin level, the weights of the heart, lungs, spleen, and
adrenals, and plasma, erythrocyte, and brain cholinesterase activity
in animals of each sex. Decreased plasma and erythrocyte
cholinesterase activity and adrenal weights were also observed in rats
at 75 mg/kg of feed. There were no histopathological differences
between control and treated animals, nor was there any difference in
the incidence of tumours. The NOEL was 15 mg/kg of feed, equal to 0.8
mg/kg bw per day, on the basis of lowered adrenal weights at higher
doses.
Male and female dogs given phoxim at 750 mg/kg of feed had liver
damage, as shown by increased liver weights and plasma alanine
aminotransferase and alkaline phosphatase activities, decreased serum
cholesterol level, and histopathological alterations to hepatocytes.
Plasma, erythrocyte, and brain cholinesterase activities were also
reduced in animals of each sex at this dose. In addition, male dogs
showed clinical signs of toxicity and decreased body-weight gain.
Reductions in plasma and erythrocyte cholinesterase activities were
observed in male and female dogs at 15 mg/kg of feed. The NOEL was 15
mg/kg of feed, equivalent to 0.38 mg/kg bw per day, on the basis of
effects on the liver and inhibition of brain acetylcholinesterase
activity.
Phoxim has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium and Saccharomyces
cerevisiae, DNA damage in Bacillus subtilis, and chromosomal
aberrations in human lymphocytes. It has also been tested
in vivo for its ability to induce micronucleus formation,
chromosomal aberrations, and dominant lethal mutations in mice.
Cytogenetic alterations were found in human lymphocytes in vitro at
a cytotoxic dose in the absence of exogenous metabolic activation. The
results of all other tests were negative. On the basis of these data
and the results of the long-term assays in rodents, the Committee
concluded that phoxim is not genotoxici and is unlikely to have
carcinogenic potential in humans.
In a three-generation study of reproductive toxicity, rats were
given phoxim in the diet at a concentration of 0, 15, 75, or 375 mg/kg
of feed (equivalent to 0, 0.75, 3.8, and 19 mg/kg bw per day). The
only effect observed was a slight reduction in the number of pups in
the second litter of the third generation thaqt survived after four
weeks' lactation from dams receiving 375 mg/kg of feed. On the basis
of this effect, the NOEL was 75 mg/kg of feed, equivalent to 3.8 mg/kg
bw per day.
In a study of developmental toxicity in rats given a dose of 0,
30, 100, or 300 mg/kg bw per day orally on days 6-15 of gestation,
phoxim was toxic to the dams, retarding body-weight gain during
treatment at the highest dose. It was not embryotoxic, fetotoxic, or
teratogenic at any dose. The NOEL for maternal toxicity was 100 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 300 mg/kg bw per day, the highest dose
tested.
A study of developmental toxicity was conducted in rabbits given
phoxim at a dose of 0, 12, 36, or 72 mg/kg bw per day orally on days
6-18 of gestation. At the highest dose, phoxim increased the rate of
embryonic resorption, decreased fetal body weights, and was toxic to
the dams, which showed signs of toxicity a marked decreases in food
consumption and in body-weight gain. There was no indication of
teratogenicity at any dose. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of reduced food consumption and
body-weight gain. The NOEL for developmental toxicity was also 36
mg/kg bw per day, on the basis of decreased fetal body weight and an
increased rate of embryonic resorption.
In a study of delayed neurotoxicity, hens protected by the
antidote atropine received phoxim orally at a dose of 50 mg/kg bw,
which was repeated after 21 days. The birds were then observed for 42
days. They showed only transient signs of toxicity and no abnormal
signs or behaviour from day 26 after the second dose. At the end of
the study, no paralysis was present, and no histological evidence of
peripheral neuropathy or demyelination was observed. The Committee
concluded that phoxim does not induce delayed neurotoxicity in hens.
Although the effect of phoxim on the enzyme neuropathy target esterase
has not been investigated in hens, the Committee concluded that such a
study was not necessary in view of the negative results obtained in
adequately conducted assessments of the capacity of phoxim to induce
delayed neuropathy.
4. EVALUATION
The Committee established an ADI of 0-4 µg/kg bw for phoxim on
the basis of the NOEL of 0.38 mg/kg bw per day for effects on the
liver and inhibition of brain acetylcholinesterase activity in the
two-year study of toxicity in dogs and a safety factor of 100. This
ADI differs from that established by the Joint FAO/WHO Meeting on
Pesticide Residues, as the Expert Committee concluded that inhibition
of plasma cholinesterase activity is not a relevant end-point for risk
assessment. The Joint Meeting is now of a similar opinion (FAO/WHO,
1988).
5. REFERENCES
Becker, H. (1982) Embryotoxicity and teratogenicity study on SRA 7502
in rabbits. Unpublished report (project no. 004972, report no. R2354)
from Research & Consulting Company Ltd, Itingen, Switzerland, for
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomann, W. (1992) Volaton VL 80 (c.n.: phoxim). Studies on acute
dermal toxicity in rats. Unpublished report (study no. T 0040187,
report no. 21065) from Bayer AG, Fachbereich Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomhard, E. & Löser, E. (1977) SRA 7502. Chronic toxicology studies in
rats (feeding experiment over 2 years). Unpublished report (no. 7042)
from Bayer AG, Institute for Toxicology, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Brooks, P.N. & Cherry, C.P. (1974) Pathology report of Bay 77488.
Sub-chronic toxicological studies in dogs (3-month feeding
experiment). Addendum to report no. 2579. Unpublished report (no.
FBA130/73959) from Huntingdon Research Centre, Huntingdon, United
Kingdom, for Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F., Griffin, T. & Talley, W. (1978) A safety evaluation
study of phoxim in rhesus monkeys. Unpublished report from Institute
of Comparative and Human Toxicology, Albany, New York & International
Center of Environmental Safety, Holloman, New Mexico, United States.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Daniel, J.W., McLean, J. & Pringuer, M. (1978a)
Phoxim--Biotransformation in the rat. Unpublished report (no.
78/BAG6/317) from Life Science Research, Stock, Essex, United Kingdom
for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Daniel, J.W., Swanson, S. & McLean, J. (1978b) Phoxim:
Pharmacokinetics and biotransformation in the rat. Unpublished report
(no. 78/BAG5/194) from Life Science Research, Stock, Essex, United
Kingdom, for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
FAO/WHO (1983) Pesticide Residues in Food--1982. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 46).
FAO/WHO (1985) Pesticide Residues in Food--1984. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 62).
FAO/WHO (1988) Pesticide Residues in Food--1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and a WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 92).
Finn, J.P. (1977) Chronic toxicity study on rats with SRA 7502 (two
year feeding study). Unpublished report (no. 612/262/2) from Hazleton
Laboratories Europe Ltd, Harrogate, United Kingdom, for Bayer AG,
Institut für Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1978a) Determination of acute toxicity (LD50)--SRA 7502.
Unpublished letter report (dated 19 May 1978) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Flucke, W. (1978b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 26 June 1978) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979a) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 9 March 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 17 April 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity (LD50)--SRA 7502
Volaton VL. Unpublished letter report (dated 14 March 1980) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1984a) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in guinea pigs [in German]. Unpublished report
(study no. T 6016487, report no. 13071) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Flucke, W. (1984b) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in an open epicutaneous test with guinea pigs
[in German]. Unpublished report (study no. T 6016630, report no.
13086) from Bayer AG, Institut für Toxikiologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1978) Volaton VL. Subacute cutaneous
toxicity study with rabbits. Unpublished report (report no. 8034) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Thyssen, J. (1979) Volaton (SRA 7502) active ingredient
presolution. Acute toxicity studies. Unpublished report (no. 8081)
from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gyrd-Hansen, N., Brimer, L. & Rasmussen, F. (1993) Percutaneous
absorption of organophosphorus insecticides in pigs--The influence of
different vehicles. J. Vet. Pharmacol. Ther., 16, 174-180.
Heimann, K.-G. (1982a) Determination of acute toxicity (LD50)--
Volaton VL. Unpublished letter report (dated 16 February 1982) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.-G. (1982b) Determination of acute toxicity (LD50)--
Volaton VL 80. Unpublished letter report (dated 3 June 1982) from
Bayer, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) SRA 7502. Phoxim, Baythion(R)-active ingredient,
Volaton(R)-active ingredient. Micronucleus test on mouse to evaluate
SRA 7502 for mutagenic potential. Unpublished report (study no. T
5010077, report no. 9891) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985) SRA 7502 c.n. Phoxim. Test on S. cerevisiae D7
investigating for point mutagenic effect. Unpublished report (study
no. T 2018201, report no. 13708) from Bayer AG, Institute for
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1986) SRA 7502 (c.n. phoxim). Cytogenetic study with
human lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report (study no. T 9021736, report no.
15148) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1977) SRA 7502. Chronic toxicity study on
dogs (two-year feeding experiment). Unpublished report (no. 6865) from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989a) SRA 7502. Preliminary study on
mice (administration in feed to determine dosage for a long-term study
on carcinogenicity in mice). Unpublished report (study no. T1022043,
report no. 17610) from Bayer AG, Fachbereich Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989b) SRA 7502. Preliminary study on
mice (administration in feed for 8 weeks to determine dose for a
long-term study). Unpublished report (study no. T9022799, report no.
17611) from Bayer AG, Fachbereich Toxikologie, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jäger, R. (1992) SRA 7502 (common name: Phoxim). Study for
oncogenicity in B6C3F1 mice (twenty-four month feeding study).
Unpublished report (study no. T 2022297, report no. 21624) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1968) Bay 77488. Toxicological studies. Unpublished
report (no. 1067) from Farbenfabriken Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1969) Supplement to the toxicology of SRA 7502.
Unpublished letter report (dated 27 June 1969) from Bayer AG,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972) Phoxim: Tests for neurotoxicity in chickens.
Unpublished report (dated 21 June 1972) from Farbenfabriken Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1973) SRA 7502, SRA 7660, LOW 6599, SIR 5126, HOX 3082,
malathion. Comparative toxicological studies in rats with repeated
oral administration. Unpublished report (no. 3962) from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. (1970) Bay 77 488. Toxicological studies.
Unpublished report (no. 2235) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. & Weber, H. (1988) [Phenyl-U-14-C]-Phoxim: Biokinetic
behaviour and biotransformation in the edible tissues of the pig after
oral administration. Unpublished report (study no. M 183 0138-4,
PF-report no. 3077) from Bayer AG, Institute for Metabolism Research,
Leverkusen/Monheim, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kurinnyi, A.I. (1979) Cytogenetic activity of the pesticide valexon
and its influence on the mutability of mouse bone marrow cells.
Tsitol. Genet., 13, 370-374 (English translation). Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lorke, D. & Kimmerle G. (1965) Toxikologische Untersuchungen mit dem
Wirkstoff SRA 7502. Unpublished report (dated 14 December 1965) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969a) Bay 77 488. Subchronic toxicology studies in rats
(study over 3 months). Unpublished report (no. 1205) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969b) Bay 77 488. Subchronic toxicology studies with rats.
Animal and organ weights (Individual values for report no. 1205).
Unpublished report (no. 1215) from Farbenfabriken Bayer AG, Institut
für Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Löser, E. (1970a) Bay 77 488. Subchronic toxicological studies on rats
(three-month feeding experiment). Unpublished report (no. 2389) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1970b) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2418) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1971) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2579) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1979) SRA 7502. Multigeneration reproduction study on rats.
Unpublished report (no. 8447) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1974) SRA 7502 (phoxim). Dominant lethal test on male
mouse to evaluate SRA 7502 for mutagenic potential. Unpublished report
(no. 4943) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Machemer, L. (1975) SRA 7502 (phoxim). Studies for embryotoxic and
teratogenic effects on rats following oral administration. Unpublished
report (no. 5331) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Märtins, T. (1992) Volaton VL 80 (c.n.: phoxim). Acute inhalation
toxicity in rats in compliance with OECD guideline no. 403.
Unpublished report (study no. T 2040765, report no. 21429) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mihail, F. (1979) Cyanoxim acid glucoside (metabolite of phoxim).
Unpublished letter report (dated 4 July 1979) from Bayer AG, Institute
of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1981) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 4 May 1981) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1982) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 1 March 1982) from Bayer, Institute
of Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Moriya, M., Ohta, T., Watanabe K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Mürmann, P. & Luckhaus, G. (1973) SRA 7502. Subchronic toxicity study
on dogs (three-month feeding experiment). Unpublished report (no.
4136) from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Oesch, F. (1977) Ames test for Volaton (phoxim). Unpublished report
(dated 30 November 1977) from Pharmakologisches Institut, Universität
Mainz, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1983) SRA 7502 (active ingredient of Baythion(R) and
Volaton(R)) (common name: phoxim). Acute oral toxicity study on
hens. Unpublished report (study no. T 3006197, report no. 11978) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. & Kaliner, G. (1984) SRA 7502 (c.n.: phoxim). Study for
acute neurotoxicity in hens. Unpublished report (study no. T 3016385,
report no. 12632) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Watanabe, T. (1978) Phoxim. Mutagenicity
test on bacterial systems. Unpublished report (dated 18 July 1978)
from Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1976) SRA 7502. Subacute oral cumulation study on rats.
Unpublished report (no. 5954) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1973) SRA 7502. Toxicological studies on
hens. Unpublished report (no. 4236) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) Cyanoxim
(hydroxiiminophenylacetonitrile). Occupational toxicological study.
Unpublished report (no. 6392) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vince, A.A. & Spicer E.J.F. (1971) Pathology report on Bay 77 488.
Subchronic toxicological studies in rats (3 month feeding experiment).
Addendum to report no. 2389. Unpublished report (no. 4257/71/415) from
Huntingdon Research Centre, Huntingdon, United Kingdom, for
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vinopal, J.H. & Fukuto, T.R. (1971) Selective toxicity of phoxim
(phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate). Pestic.
Biochem. Physiol., 1, 44-60.
See Also:
Toxicological Abbreviations
 small and large intestines, and their contents were collected. In a
third experiment, urine and faeces were collected at intervals for up
to 10 days after treatment of five male and five female rats with 10
mg/kg bw, and of five male rats with 1 mg/kg bw. After 10 days, the
animals were killed, and samples of the same tissues and organs as in
the first experiment were collected. In addition, bile was collected
from three male rats with cannulated bile ducts for up to 24 h after
treatment with 10 mg/kg bw. All samples were analysed for radiolabel
by liquid scintillation spectrometry.
Phoxim at 1 or 10 mg/kg bw was rapidly absorbed. In plasma, the
total residue concentrations reached a maximum of 0.35 and 2.44 µg/ml
phoxim equivalents, respectively, within 0.5 h of administration, but
the concentrations decreased to approximately 0.1 µg/ml within 1 h of
administration of 1 mg/kg bw and remained at that level until at least
7.5 h. After 10 mg/kg bw, a second peak of 1.9 µg/ml was detected
after 4 h in the animals in the first experiment but not in those in
the second. Thereafter, the plasma concentrations decreased to
approximately 0.8 µg/ml at 7.5 h and to 0.04 µg/ml at 24 h. The
radiolabel was rapidly taken up into the major organs and tissues,
with kinetics similar to that in plasma. Over the first 7.5 h after
administration of 10 mg/kg bw, the total residue concentrations in
tissues remained fairly constant, with the highest levels in the small
intestine and its contents and slightly higher concentrations in
kidney (2.1-6.1 mg/kg phoxim equivalents) and liver (1.2-3.1 mg/kg)
than in other tissues or organs (0.04-1.2 mg/kg). Thereafter, all
tissue concentrations declined, to below 0.4 mg/kg at 24 h and to
below 0.14 mg/kg at 144 h.
Most of the radiolabel was eliminated in urine within 24 h, and
elimination was virtually complete within two days, independently of
sex and dose. The total recovery in urine over 10 days was 93% for
males and 86% for females at 10 mg/kg bw and 82% for males given 1
mg/kg bw. In faeces, 4.9, 6.9, and 7.9%, respectively, of the
administered dose was recovered in the same period. Biliary excretion
amounted to approximately 4% of the administered dose over 24 h
(Daniel et al., 1978b).
[Phenyl-U-14C]phoxim (final specific activity, 20 µCi/mg) was
administered by oral intubation in gelatin capsules to two female
German Landrace piglets at a single dose of 5 mg/kg bw (2 mCi per
animal). Blood, urine, and faeces were collected at intervals for up
to 24 and 72 h after administration. After the animals were
slaughtered, samples of liver, kidney, muscle, loin, and subcutaneous
fat were collected. All samples were analysed for radiolabel by liquid
scintillation spectrometry. The study was (partly) certified for
compliance with GLP and quality assurance.
Phoxim was rapidly absorbed: the total residue concentrations in
plasma reached a maximum of 2.3 µg/ml phoxim equivalents within 1-2 h
after administration and declined biphasically with half-lives of 0.6
h in the initial phase and 25 h in the terminal phase (from 6 h
onwards). The radiolabel was rapidly eliminated, as 82% of the
administered dose was excreted via the urine within 24 h (72% within
the first 8 h) and an additional 1.5% in the following 48 h. Faecal
excretion amounted to 1.6% of the administered dose after 24 h in one
animal and to 12% after 72 h in the other. At 24 h, the highest total
residue concentrations in edible tissues were found in fat (1.3 mg/kg
phoxim equivalents), followed by liver (0.60 mg/kg), kidney (0.35
mg/kg), and loin and muscle (0.05 mg/kg). At 72 h, the tissue
concentrations were approximately half these values (Klein & Weber,
1988).
In order to study the dermal bioavailability of phoxim after
pour-on application, six piglets received 10 mg/kg bw phoxim in
glycerol-formal by intravenous injection into an ear vein. Seven days
later, groups of two piglets received a dermal application along the
mid-line of the back of 100 mg/kg bw phoxim in either dimethyl
sulfoxide, 1-octanol, or Carbowax. Blood samples were collected at
regular intervals up to 72 h after intravenous treatment and up to 10
days after dermal treatment, and the plasma was assayed for phoxim by
gas chromatography. Only 1.2-2.9% of the dermal dose was absorbed,
with little difference in the rate or extent of absorption among the
three vehicles (Gyrd-Hansen et al., 1993).
2.1.2 Biotransformation
In the study of Vinopal & Fukuto (1971) described above,
metabolites were identified in two to three pooled 24-h and 30-h urine
samples from animals treated with 110 and 960 mg/kg bw, respectively,
by ion-exchange chromatography and thin-layer chromatography. The
parent drug accounted for only 1 and 2% of the administered dose in
the samples from the two groups, respectively. Four metabolites were
detected: diethyl phosphoric acid (59 and 43%, respectively), diethyl
phosphorothioic acid (20 and 18%, respectively), phoxim carboxylic
acid (tentatively identified by infrared spectroscopy; 3 and 24%,
respectively), and either desethyl phoxim or desethyl PO-phoxim
(identification was tentative; 6 and 5%, respectively). It was
demonstrated by administration of diethyl phosphorothioic acid itself
that little, if any, diethyl phosphoric acid is formed by
desulfuration of this metabolite. Hence, metabolism of phoxim in mice
proceeds mainly by hydrolysis of the phosphor ester bond, via
oxidative desulfuration to PO-phoxim with subsequent extremely rapid
hydrolysis of the phosphor ester bond, and (at high doses only) via
hydrolysis of the cyano group. De-ethylation is not an important
pathway.
The biotransformation of phoxim was studied in groups of five
fasted male Charles River CD rats after administration of 10 mg/kg bw
[phenyl-U-14C]phoxim (4-12 µCi per animal) in polyethylene glycol 200
by oral gavage. Blood was sampled by cardiac puncture 1 h after
dosing, and urine was collected for periods of 6 and 24 h after
treatment. Metabolites in plasma and urine were characterized by
thin-layer chromatography, gas chromatography, and mass spectrometry.
Samples were pooled for analysis of metabolites. Four radiolabelled
components were detected in plasma. Most of the radiolabel was in the
form of the metabolite desethyl phoxim (80%), with smaller amounts in
desethyl PO-phoxim (12%); no PO-phoxim could be detected. The major
metabolites in urine (52-94%) were the glucuronic and sulfuric acid
conjugates of cyanobenzaldoxime (both syn and anti forms). Small
amounts (12%) of hippuric acid were also detected. The metabolism of
phoxim in rats involves de-ethylation hydrolysis of the phosphor ester
bond, followed by conjugation of cyanobenzaldoxime or its further
metabolism to hippuric acid (Daniel et al., 1978a).
In the study of Klein & Weber (1988) described above, metabolites
were identified in urine and tissue samples from pigs by thin-layer
chromatography, high-performance liquid chromatography, gas
chromatography, and mass spectrometry. In urine, two metabolites were
identified: cyanobenzaldoxime-glucuronide, which was the major
product, and hippuric acid, together accounting for approximately 90%
of the urinary radioactivity. In tissues, unchanged phoxim was present
in fat (90% of the radioactivity present), loin, and muscle.
Cyanobenzaldoxime was found in muscle, loin, and liver. In kidney, no
metabolites could be identified, although co-chromatography pointed to
hippuric acid. The main metabolic pathway for phoxim in pigs is
hydrolysis of the phosphor ester bond, followed by conjugation of the
resulting cyanobenzaldoxime. A small amount of cyanobenzaldoxime is
further oxidized to benzoic acid, which is subsequently metabolized to
hippuric acid.
The metabolic pathway of phoxim in various species is shown in
Figure 2.
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of phoxim are shown
in Table 1. The toxic signs observed at lower oral doses were
deterioration of general condition and sedative effects. Only at the
highest, lethal doses were the signs indicative of acute
cholinesterase inhibition (spasms, trembling, diarrhoea, and red tears
in rats). Hens were considerably more sensitive to phoxim than mammals
and showed acute cholinergic signs but no indications of delayed
paralysis. After inhalation, no signs of cholinesterase inhibition
small and large intestines, and their contents were collected. In a
third experiment, urine and faeces were collected at intervals for up
to 10 days after treatment of five male and five female rats with 10
mg/kg bw, and of five male rats with 1 mg/kg bw. After 10 days, the
animals were killed, and samples of the same tissues and organs as in
the first experiment were collected. In addition, bile was collected
from three male rats with cannulated bile ducts for up to 24 h after
treatment with 10 mg/kg bw. All samples were analysed for radiolabel
by liquid scintillation spectrometry.
Phoxim at 1 or 10 mg/kg bw was rapidly absorbed. In plasma, the
total residue concentrations reached a maximum of 0.35 and 2.44 µg/ml
phoxim equivalents, respectively, within 0.5 h of administration, but
the concentrations decreased to approximately 0.1 µg/ml within 1 h of
administration of 1 mg/kg bw and remained at that level until at least
7.5 h. After 10 mg/kg bw, a second peak of 1.9 µg/ml was detected
after 4 h in the animals in the first experiment but not in those in
the second. Thereafter, the plasma concentrations decreased to
approximately 0.8 µg/ml at 7.5 h and to 0.04 µg/ml at 24 h. The
radiolabel was rapidly taken up into the major organs and tissues,
with kinetics similar to that in plasma. Over the first 7.5 h after
administration of 10 mg/kg bw, the total residue concentrations in
tissues remained fairly constant, with the highest levels in the small
intestine and its contents and slightly higher concentrations in
kidney (2.1-6.1 mg/kg phoxim equivalents) and liver (1.2-3.1 mg/kg)
than in other tissues or organs (0.04-1.2 mg/kg). Thereafter, all
tissue concentrations declined, to below 0.4 mg/kg at 24 h and to
below 0.14 mg/kg at 144 h.
Most of the radiolabel was eliminated in urine within 24 h, and
elimination was virtually complete within two days, independently of
sex and dose. The total recovery in urine over 10 days was 93% for
males and 86% for females at 10 mg/kg bw and 82% for males given 1
mg/kg bw. In faeces, 4.9, 6.9, and 7.9%, respectively, of the
administered dose was recovered in the same period. Biliary excretion
amounted to approximately 4% of the administered dose over 24 h
(Daniel et al., 1978b).
[Phenyl-U-14C]phoxim (final specific activity, 20 µCi/mg) was
administered by oral intubation in gelatin capsules to two female
German Landrace piglets at a single dose of 5 mg/kg bw (2 mCi per
animal). Blood, urine, and faeces were collected at intervals for up
to 24 and 72 h after administration. After the animals were
slaughtered, samples of liver, kidney, muscle, loin, and subcutaneous
fat were collected. All samples were analysed for radiolabel by liquid
scintillation spectrometry. The study was (partly) certified for
compliance with GLP and quality assurance.
Phoxim was rapidly absorbed: the total residue concentrations in
plasma reached a maximum of 2.3 µg/ml phoxim equivalents within 1-2 h
after administration and declined biphasically with half-lives of 0.6
h in the initial phase and 25 h in the terminal phase (from 6 h
onwards). The radiolabel was rapidly eliminated, as 82% of the
administered dose was excreted via the urine within 24 h (72% within
the first 8 h) and an additional 1.5% in the following 48 h. Faecal
excretion amounted to 1.6% of the administered dose after 24 h in one
animal and to 12% after 72 h in the other. At 24 h, the highest total
residue concentrations in edible tissues were found in fat (1.3 mg/kg
phoxim equivalents), followed by liver (0.60 mg/kg), kidney (0.35
mg/kg), and loin and muscle (0.05 mg/kg). At 72 h, the tissue
concentrations were approximately half these values (Klein & Weber,
1988).
In order to study the dermal bioavailability of phoxim after
pour-on application, six piglets received 10 mg/kg bw phoxim in
glycerol-formal by intravenous injection into an ear vein. Seven days
later, groups of two piglets received a dermal application along the
mid-line of the back of 100 mg/kg bw phoxim in either dimethyl
sulfoxide, 1-octanol, or Carbowax. Blood samples were collected at
regular intervals up to 72 h after intravenous treatment and up to 10
days after dermal treatment, and the plasma was assayed for phoxim by
gas chromatography. Only 1.2-2.9% of the dermal dose was absorbed,
with little difference in the rate or extent of absorption among the
three vehicles (Gyrd-Hansen et al., 1993).
2.1.2 Biotransformation
In the study of Vinopal & Fukuto (1971) described above,
metabolites were identified in two to three pooled 24-h and 30-h urine
samples from animals treated with 110 and 960 mg/kg bw, respectively,
by ion-exchange chromatography and thin-layer chromatography. The
parent drug accounted for only 1 and 2% of the administered dose in
the samples from the two groups, respectively. Four metabolites were
detected: diethyl phosphoric acid (59 and 43%, respectively), diethyl
phosphorothioic acid (20 and 18%, respectively), phoxim carboxylic
acid (tentatively identified by infrared spectroscopy; 3 and 24%,
respectively), and either desethyl phoxim or desethyl PO-phoxim
(identification was tentative; 6 and 5%, respectively). It was
demonstrated by administration of diethyl phosphorothioic acid itself
that little, if any, diethyl phosphoric acid is formed by
desulfuration of this metabolite. Hence, metabolism of phoxim in mice
proceeds mainly by hydrolysis of the phosphor ester bond, via
oxidative desulfuration to PO-phoxim with subsequent extremely rapid
hydrolysis of the phosphor ester bond, and (at high doses only) via
hydrolysis of the cyano group. De-ethylation is not an important
pathway.
The biotransformation of phoxim was studied in groups of five
fasted male Charles River CD rats after administration of 10 mg/kg bw
[phenyl-U-14C]phoxim (4-12 µCi per animal) in polyethylene glycol 200
by oral gavage. Blood was sampled by cardiac puncture 1 h after
dosing, and urine was collected for periods of 6 and 24 h after
treatment. Metabolites in plasma and urine were characterized by
thin-layer chromatography, gas chromatography, and mass spectrometry.
Samples were pooled for analysis of metabolites. Four radiolabelled
components were detected in plasma. Most of the radiolabel was in the
form of the metabolite desethyl phoxim (80%), with smaller amounts in
desethyl PO-phoxim (12%); no PO-phoxim could be detected. The major
metabolites in urine (52-94%) were the glucuronic and sulfuric acid
conjugates of cyanobenzaldoxime (both syn and anti forms). Small
amounts (12%) of hippuric acid were also detected. The metabolism of
phoxim in rats involves de-ethylation hydrolysis of the phosphor ester
bond, followed by conjugation of cyanobenzaldoxime or its further
metabolism to hippuric acid (Daniel et al., 1978a).
In the study of Klein & Weber (1988) described above, metabolites
were identified in urine and tissue samples from pigs by thin-layer
chromatography, high-performance liquid chromatography, gas
chromatography, and mass spectrometry. In urine, two metabolites were
identified: cyanobenzaldoxime-glucuronide, which was the major
product, and hippuric acid, together accounting for approximately 90%
of the urinary radioactivity. In tissues, unchanged phoxim was present
in fat (90% of the radioactivity present), loin, and muscle.
Cyanobenzaldoxime was found in muscle, loin, and liver. In kidney, no
metabolites could be identified, although co-chromatography pointed to
hippuric acid. The main metabolic pathway for phoxim in pigs is
hydrolysis of the phosphor ester bond, followed by conjugation of the
resulting cyanobenzaldoxime. A small amount of cyanobenzaldoxime is
further oxidized to benzoic acid, which is subsequently metabolized to
hippuric acid.
The metabolic pathway of phoxim in various species is shown in
Figure 2.
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of phoxim are shown
in Table 1. The toxic signs observed at lower oral doses were
deterioration of general condition and sedative effects. Only at the
highest, lethal doses were the signs indicative of acute
cholinesterase inhibition (spasms, trembling, diarrhoea, and red tears
in rats). Hens were considerably more sensitive to phoxim than mammals
and showed acute cholinergic signs but no indications of delayed
paralysis. After inhalation, no signs of cholinesterase inhibition
 were observed, even at the highest doses, when the only effects were
non-specific ones on the nervous system. No signs of poisoning were
observed after dermal application. Phoxim was only slightly to
moderately toxic in all of the mammalian species studied.
Poorly reported studies of skin irritation indicated that phoxim
had only a very slight irritating effect on rabbit's skin. The
calculated overall primary irritation index was < 0.5. Limited
reports of eye irritation in rabbits showed that phoxim can cause mild
to moderate irritation of the conjuctivae, without effects on the iris
or cornea (Lorke & Kimmerle, 1965; Kimmerle & Solmecke, 1970; Flucke &
Thyssen, 1979).
In a Magnussen-Kligman maximization test in 40 male DHPW
guinea-pigs, phoxim induced a sensitization response in 60% of the
animals, which was shown not to be due to 1-butanol (Flucke, 1984a).
In an open epicutaneous test (according to Klecak) in 64 male DHPW
guinea-pigs, administration of phoxim showed no sensitization
potential (Flucke, 1984b).
2.2.2 Short-term studies of toxicity
Mice
In a study to establish the doses for a study of carcinogenicity,
groups of 10 male and 10 female SPF B6C3F1 mice were fed diets
containing phoxim at 0, 5, 30, 150, or 750 mg/kg feed for six weeks,
equal to 0, 3, 18, 85, and 440 mg/kg bw per day for males and 0, 3,
20, 100, and 500 mg/kg bw per day for females. The study was certified
for compliance with GLP. No differences in mortality rate, clinical
signs, body weight, or food or water intake were observed that could
be associated with treatment. Plasma alkaline phosphatase activity and
total protein content were significantly increased in males at the
high dose. In females, the plasma cholesterol content increased with
dose, the increase becoming statistically significant from 30 mg/kg
feed onwards; however, the control concentrations of cholesterol and
total protein were low in comparison with historical data, and the
increased levels were still within the historical control range, as
were the alkaline phosphatase activities in males at the high dose.
Cholinesterase activity was increasingly reduced with dose in plasma,
with > 20% inhibition in all treated animals, and in erythrocytes,
with > 20% inhibition only in females at the high dose. Brain
acetylcholinesterase activity, which was determined in five animalsof
each sex in each group, was reduced in all treated groups, with
reductions of more than 50% in both males and females at the high
dose; however, the activities in concurrent controls were in the upper
range of that of historical controls, and the observed reduction
showed no dose-response relationship, was not always statistically
significant, and was still within historical control levels, except in
males at the high dose. Therefore, only the reduction in males at the
high dose is considered relevant.
Table 1. Acute toxicity of phoxim
Route Species Sex LD50a or LC50 Reference
Oral Mouse Male 2700 Kimmerle (1968)
(mg/kg bw) 1800 Kimmerle & Solmecke (1970)
1200b Flucke & Thyssen (1979)
Female 3600 Kimmerle (1968)
2200 Kimmerle & Solmecke (1970)
2800-4000 Flucke (1978a,b; 1979a,b; 1980);
Heimann (1982a,b); Mihail
(1981, 1982)
1800b Flucke & Thyssen (1979)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 2300 Kimmerle (1969)
> 2000d Vinopal & Fukuto (1971)
Rat Male 10 000 Lorke & Kimmerle (1965)
8000 Kimmerle (1968))
2100 Kimmerle & Solmecke (1970)
4900b Thyssen (1976)
2000b Flucke & Thyssen (1979)
Female 10 000 Lorke & Kimmerle (1965)
6500 Kimmerle (1968)
1900 Kimmerle & Solmecke (1970)
2900b Thyssen (1976)
1400b Flucke & Thyssen (1979)
Guinea-pig Female 390-560 Kimmerle (1968)
approx. 660 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
Rabbit Female 250-380 Kimmerle & Solmecke (1970)
Unspecified approx. 380c Lorke & Kimmerle (1965)
280-560 Kimmerle (1968)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Cat Female 250-500 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Dog Female 250-500 Kimmerle & Solmecke (1970)
> 500b Flucke & Thyssen (1979)
Unspecified > 1200 Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
20e Thyssen & Kimmerle (1973)
40b Pauluhn (1983)
Intraperitoneal Rat Male 2200c Lorke & Kimmerle (1965)
(mg/kg bw) 2000 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1100b Flucke & Thyssen (1979)
Female 2000c Lorke & Kimmerle (1965)
1900 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1500b Flucke & Thyssen (1979)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
Intravenous Mouse Female 1100 Kimmerle (1968)
(mg/kg bw) 480b Kimmerle & Solmecke (1970)
Dermal Rat Male > 1200 Lorke & Kimmerle (1965)
(mg/kg bw) > 1100 Kimmerle (1968)
> 1100 Kimmerle & Solmecke (1970)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Male/female > 5600 Flucke & Thyssen (1979)
> 5000f Bomann (1992)g
Inhalation Mouse Male > 2.1h Kimmerle (1968)
(mg/L, 4 h) > 3.1h Kimmerle & Solmecke (1970)
Unspecified > 1.3 Kimmerle (1968)
>1.7h Kimmerle & Solmecke (1970)
Rat Male > 2.0h Kimmerle (1968)
> 2.6h Kimmerle & Solmecke (1970)
Female > 2.2h Kimmerle (1968)
> 2.8h Kimmerle & Solmecke (1970)
Male/female > 3.0h Flucke & Thyssen (1979)
> 4.6 Märtins (1992)g
Unspecified > 1.3 Kimmerle (1968)
> 1.7h Kimmerle & Solmecke (1970)
Guinea-pig Unspecified > 1.3 Kimmerle (1968)
Rabbit Unspecified > 1.3 Kimmerle (1968)
1.2-1.7h Kimmerle & Solmecke (1970)
Subcutaneous Mouse Male/female > 5000b Flucke & Thyssen (1979)
(mg/kg bw ) Unspecified > 1000c Lorke & Kimmerle (1965)
a In the reports of Kimmerle (1968), Flucke (1978a,b; 1979a,b; 1980), Heimann (1982a,b), Mihail
(1981, 1982), Kimmerle & Solmecke (1970), and Flucke & Thyssen (1979), some or all of the
LD50 values for phoxim (as pre-solution) were cited in microlitres or millilitres per kilogram
of body weight. These values were converted to milligrams per kilogram of body weight by
Table 1. (continued)
correcting for the density of the pre-solution (1.126 mg/µl at 20 °C). In the report of Lorke
& Kimmerle (1965), some of the LD50 values for pure phoxim were cited in millilitres per
kilogram of body weight. These values were converted to milligrams per kilogram of body weight
by correcting for the density of the pure substance (1.176 mg/µl at 20 °C).
b Vehicle, water:Cremophor EL
c Vehicle, lutrol
d Vehicle, olive oil
e Vehicle, water
f Vehicle, 2% Cremophor EL:physiological saline
g The studies of Bomann (1992) and Märtins (1992) were of conventional design, with GLP
and quality assurance certification.
h Vehicle, ethanol:lutrol 1:1
The only gross change seen on post-mortem examination was
enlarged spleens in a number of control and treated females. As this
finding was unrelated to dose and occurred in more control than
treated animals, it is not considered to be related to treatment. The
relative kidney weights of females at 150 and 750 mg/kg feed were
statistically significantly increased with dose. The absolute and
relative liver weights were increased with dose in all treated
animals, the increase in relative weight becoming statistically
significant at 150 mg/kg feed and the increase in absolute weight in
males only; both the relative and absolute weights were increased
significantly at 750 mg/kg feed. Histopathological examination only of
the livers of controls and animals at the high dose revealed
enlargement of the centrilobular hepatocytes in males and females at
the high dose, indicating adaptive hypertrophy and possible enzyme
induction. No evidence of irreversible changes was found. One female
at the high dose had slight cytoplasmic vacuolation in periportal
cells, which may not have been related to treatment (Ivens-Kohl &
Hahnemann, 1989a).
In a follow-up study, groups of 10 male and 10 female SPF B6C3F1
mice were fed diets containing phoxim at 0, 0.5, 1, 5, or 30 mg/kg
feed, equal to 0, 0.28, 0.55, 2.8, and 18 mg/kg bw per day for males
and 0, 0.35, 0.66, 3.1, and 23 mg/kg bw per day for females, for eight
weeks. The study was certified for compliance with GLP. No differences
in mortality rate, clinical signs, body weight, food or water intake,
or macroscopic or histopathological appearance were observed that
could be related to treatment. The plasma cholesterol content was
statistically significantly increased in females at 30 mg/kg feed. As
in the previous study, however, the control cholesterol content was
lower than that of historical controls, and the increased level was
still within the range of historical controls. Cholinesterase activity
in plasma was reduced by > 40% at the two highest doses, and a small
inhibition (8-14%) of erythrocyte acetylcholinesterase activity was
observed in males and females at the high dose. There was no evidence
of a change in brain acetylcholinesterase activity in any treated
groups. Although the absolute and relative weights of the liver were
slightly but statistically significantly increased in females at the
high dose, no effects on organ weights were found. The NOEL was 5
mg/kg feed, equal to 3.1 mg/kg bw per day, on the basis of slightly
increased liver weights in female mice (Ivens-Kohl & Hahnemann,
1989b).
Rats
In a 21-day study of toxicity with special attention to the
reversibility of cholinesterase inhibition, groups of 15 SPF Wistar II
rats of each sex received phoxim emulsified in distilled water and
Cremophor EL by oral gavage at 0, 5, or 50 mg/kg bw per day. Five rats
of each sex in each group were killed at the end of treatment and two
and four weeks thereafter. The animals were examined regularly for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. Brain
cholinesterase activity was measured at termination of treatment and
two weeks thereafter.
There were no deaths and no treatment-related effects on
appearance, behaviour, or body weight. Plasma cholinesterase activity
was reduced by > 20% during treatment in females at the low dose
(when compared with concurrent controls but not when compared with
pre-treatment values in the same animals) and in males and females at
the high dose, but the activity was restored within one week after
cessation of treatment. Acetylcholines-terase activity in erythrocytes
was reduced by > 20% in males at the low dose during the first week
of treatment (when compared with concurrent controls but not when
compared with pre-treatment values in the same animals) and in females
at this dose throughout treatment but was restored within one week.
Erythrocyte cholinesterase activity was decreased by > 20% in males
and females at the high dose throughout the treatment period but was
restored within two weeks in males and within four weeks in females.
Brain acetylcholinesterase activity was within the physiological range
and very similar to control values in all treated males and in females
at the low dose, both at termination and two weeks thereafter. Females
at the high dose showed 30% inhibition of brain acetylcholinesterase
activity at termination, but this was restored within two weeks. Thus,
inhibition of cholinesterase activity was more pronounced in female
than in male rats and was reversible in animals of each sex (Thyssen,
1976).
Groups of 15 SPF Wistar II rats of each sex received phoxim
dissolved in polyethylene glycol 400 (lutrol) by oral gavage at 0, 2,
5, or 15 mg/kg bw per day for 30 days. The animals were examined for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. No
treatment-related effects were observed on appearance, behaviour or
body weight. The cholinesterase activity in plasma decreased with dose
in all treated females and in males at 5 and 15 mg/kg bw per day. The
acetylcholinesterase activity in erythrocytes was reduced by > 20%
in males and females at two highest doses (Kimmerle, 1973).
In a three-month study, groups of 15 young SPF Wistar rats of
each sex received diets containing phoxim at 0, 4, 12, 40, or 120
mg/kg feed, equivalent to 0, 0.4, 1.2, 4, or 12 mg/kg bw per day. The
animals were observed for physical appearance, behaviour, deaths, body
weight, food consumption, haematological and clincal chemical
parameters (liver function tests), plasma and erythrocyte
cholinesterase activity, urinary parameters, organ weights, and
macroscopic appearance. Brain acetylcholinesterase activity was not
investigated. Treatment-related effects were found on cholinesterase
activity in both plasma and erythrocytes, which were decreased by
> 20% at 40 mg/kg feed (within one month) and 120 mg/kg feed
(within one week) in animals of each sex. Statistically significantly
increased weights of the thyroid glands (both absolute and relative)
and adrenal glands (relative only) were observed in males at 120 mg/kg
feed. In the absence of data on brain acetylcholinesterase activity,
the NOEL was 12 mg/kg feed, equivalent to 1.2 mg/kg bw per day, on the
basis of inhibition of erythrocyte acetyl-cholinesterase activity
(Löser, 1969a,b).
In a second three-month study with groups of 15 young SPF Wistar
rats of each sex, phoxim was administered in the diet at
concentrations of 0, 5, 15, 50, 150, or 500 mg/kg feed, equivalent to
0, 0.5, 1.5, 5, 15, or 50 mg/kg bw per day. The animals were
observed for the same end-points as in the previous study, with the
addition of histopathological examination. Cholinergic signs (muscle
twitching, cramps) were observed occasionally in animals at the high
dose, particularly in the earlier part of the study. Although
body-weight gain was significantly retarded in males at the high dose,
animals at 50 mg/kg feed showed a very similar growth curve. Hence,
the effect on body-weight gain was not consistent or dose-dependent.
In male rats, cholinesterase activity in plasma and erythrocytes
decreased by > 20% with doses from 50 mg/kg feed. In female rats,
cholinesterase activity decreased by > 20% in plasma with doses
from 15 mg/kg feed (at 1 and 13 weeks only) and in erythrocytes with
doses from 150 mg/kg feed. Liver weights were statistically
significantly increased in animals at 150 mg/kg feed (relative) and
500 mg/kg feed (relative in males and females, absolute in females),
but there were no significant changes in liver function tests. At the
high dose, the absolute and relative thyroid weights were increased in
females, and the relative adrenal weights were increased in males.
Relative kidney weights were increased at 150 mg/kg feed in females
and 500 mg/kg feed in males and females. On the basis of inhibition of
erythrocyte acetylcholinesterase activity in males (in the absence of
data on brain acetylcholinesterase activity), the NOEL was 15 mg/kg
feed, equivalent to 1.5 mg/kg bw per day (Löser, 1970a; Vince &
Spicer, 1971).
Rabbits
Groups of three male and three female New Zealand white rabbits
received dermal applications of phoxim emulsified with Cremophor EL in
water on 5 × 5 cm areas of shorn intact skin of the flank or back or
of skin abraded with sandpaper at doses of 0, 0.5, or 15 mg/kg bw per
day, 7 h/day, five days per week, for three consecutive weeks. The
treated sites were not occluded. The observations included deaths,
physical appearance, behaviour, body weight, skin irritation,
haematological parameters, clinical chemical parameters (liver
function tests), urinary parameters, gross pathological appearance,
organ weights, histopathological appearance, and determination of
plasma, erythrocyte, and brain cholinesterase activities.
Skin irritation was observed only in animals with abraded skin,
in which there was a dose-related increase in the time necessary for
the erythema to disappear. Histopathological examination of the
treated skin of one control animal with intact skin, of one animal at
the high dose with intact skin, and of four animals at the high dose
with abraded skin showed, on average, moderate cellular infiltration
of the epidermis and corium by round cells, polymorphonuclear
leukocytes, and isolated inflammatory hair follicles, and a slight
increase in the thickness of the epithelium. These findings were
considered to be the result of repeated physical irritation,
exacerbated by the compound at the highest dose. Plasma and
erythrocyte cholinesterase activities were reduced in a dose-related
manner and were inhibited by > 20% at 0.5 mg/kg bw per day in males
with abraded skin and in females with intact skin only and in all
animals at 15 mg/kg bw per day. Brain acetylcholinesterase activity
was reduced by 14% in males with abraded skin at the low dose and by
23% in all males at the high dose but was not reduced in females
(Flucke & Schilde, 1978).
Chickens
Groups of 10 white Leghorn hens were fed diets containing phoxim
at a concentration of 0, 5, or 10 mg/kg feed for 28 days, equal to
average achieved intakes of 0, 0.32, or 0.68 mg/kg bw per day. Phoxim
had no effect on behaviour, and there were no signs of neurotoxicity.
Body-weight gain and food consumption were also unaffected. At the
high dose, a marked depression (> 20% inhibition) in whole blood
cholinesterase activity was observed (Thyssen & Kimmerle, 1973).
Dogs
In a three-month study, beagle dogs were fed phoxim in the diet
at doses of 0 (three of each sex), 2, 5, or 10 mg/kg feed (two of each
sex), equivalent to 0, 0.05, 0.13, or 0.25 mg/kg bw per day. Treatment
did not affect the mortality rate, physical appearance, behaviour,
food intake, ophthalmoscopic, haematological, clinical chemical (liver
function tests), or urinary parameters, macroscopic appearance, or
organ weights. Body-weight gain appeared to be reduced in males at 5
mg/kg feed and in animals of each sex at 10 mg/kg feed; however, the
smal number of animals per group and the high intra-group variation
did not allow statistical analysis. As this effect was not observed at
higher doses in a second three-month study (see below) or in the first
three months of a two-year study with larger groups (see below), it
was discounted. Inhibition of cholinesterase activity by > 20% was
observed in the plasma of dogs of each sex at 2 mg/kg feed (after one
month only) and at 5 and 10 mg/kg feed but not in erythrocytes. Brain
acetylcholinesterase activity was not determined, and
histopathological examination was not performed. The NOEL was 10 mg/kg
feed, equivalent to 0.25 mg/kg bw per day, the highest dose tested
(Löser, 1970b).
In a second three-month study, in which histopathological
examination was performed in addition to the observations in the first
study, beagle dogs were fed phoxim in the diet at doses of 0 (three
per sex), 50, 200, or 1000 mg/kg feed (two per sex), equivalent to
0, 1.3, 5, or 25 mg/kg bw per day. Animals at the high dose showed
signs of cholinergic poisoning (cramps, especially in the abdominal
region, muscle twitching, and salivation), but none died. Food
consumption was reduced in females at 1000 mg/kg feed, which resulted
in weight loss throughout the study, and these animals appeared
emaciated upon macroscopic examination, with little adipose tissue in
the subcutaneous connective tissue or in the mesentery. Plasma
alkaline phosphatase activity was increased in animals of each sex at
200 and 1000 mg/kg feed, and plasma lactate dehydrogenase activity was
increased in males at the high dose. Ophthalmoscopic, haematological,
and urinary determinations showed no treatment-related effects. The
absolute and relative liver weights were increased in males at the
high dose, and the relative liver weight was increased in females at
the high dose, probably because of the body-weight loss, although
changes were not seen in other tissues. The absolute and relative
weights of the male gonads were increased at the two highest doses,
but as the report did not include data on individual animals or
provide any measure of intra-group variation, the statistical
significance of these findings could not be determined. In both males
and females, cholinesterase activity was decreased by > 20% in a
dose-dependent fashion in plasma at all doses and erythrocytes at 200
and 1000 mg/kg feed, from within one week. Brain acetylcholinesterase
activity was not determined. Histopathological examination revealed no
difference between control and treated animals. The NOEL was 50 mg/kg
feed, equivalent to 1.3 mg/kg bw per day, on the basis of reduced
erythrocyte acetylcholinesterase activity (in the absence of data on
brain acetylcholinesterase activity) and increased plasma alkaline
phosphatase activity (Löser, 1971; Brooks & Cherry, 1974).
Groups of four male and four female beagles were fed diets
containing phoxim at 0, 0.3, 1, or 2 mg/kg feed for three months,
equivalent to 0, 0.0075, 0.025, or 0.05 mg/kg bw per day. The animals
were observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, plasma and
erythrocyte cholinesterase activity, gross and histopathological
appearance, and organ weights. Brain acetylcholinesterase activity was
not determined. The only effect observed was reduced plasma
cholinesterase activity (> 20% inhibition) in both males and
females at 1 and 2 mg/kg feed. The NOEL was 2 mg/kg feed, equivalent
to 0.05 mg/kg bw per day, the highest dose tested (Mürmann & Luckhaus,
1973).
Monkeys
Groups of five male and five female rhesus monkeys ( Macaca
mulatta) received phoxim dissolved in corn oil by oral gavage at
doses of 0, 0.2, 0.65, or 2 mg/kg bw per day on six days per week for
six months. The observations included clinical signs, physical
appearance, behaviour, body weight, haematological, clinical chemical,
and urinary parameters, and plasma and erythrocyte cholinesterase
activity. Liver was biopsied before and at the end of treatment for
histopathological assessment. Brain acetylcholinesterase activity was
not determined. Although plasma and erythrocyte cholinesterase
activity was inhibited, there were no signs of cholinergic poisoning
in animals at doses up to 2 mg/kg bw per day. There was no evidence
from either clinical chemistry or histopathology of an effect of
phoxim on the liver. Plasma cholinesterase activity was reduced by
> 20% in a dose-dependent manner in all treated animals.
Erythrocyte acetylcholinesterase activity was slightly reduced in
animals at the highest dose, reaching > 20% inhibition only after
treatment for four months, when compared with concurrent controls but
not when compared with pre-treatment values in the same animals. In
the absence of data on brain acetylcholinesterase activity, the NOEL
was 0.65 mg/kg bw per day on the basis of slight evidence of
inhibition of erythrocyte acetylcholinesterase activity (Coulston
et al., 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity, groups of 50 male and 50 female
SPF B6C3F1 mice were fed diets containing phoxim at doses of 0, 1, 5,
150, or 450 mg/kg feed, equal to average achieved intakes of 0, 0.47,
2.4, 67, or 200 mg/kg bw per day for males and 0, 0.55, 2.7, 79, or
210 mg/kg bw per day for females, for 24 months. Ten additional
animals of each sex at each dose (satellite groups) were killed at 12
months. The animals were observed for clinical signs, death, body
weight, food and water consumption, haematological and clinical
chemical parameters (in 10 animals per group during weeks 27, 53, 79,
and 105; including plasma and erythrocyte cholinesterase activities),
organ weights, detailed macroscopic and microscopic examinations, and
brain acetylcholinesterase activity (in 10 animals of each sex per
group at the terminal kill). The study was of conventional design,
with GLP and quality assurance certification.
Clinical signs, food and water consumption, and haematological
parameters were unaffected by treatment, as was survival (70-88% of
controls, 78-90% of treated animals). Statistically significantly
increased body weights were observed in males at 150 and 450 mg/kg
feed and in females at 450 mg/kg feed. Plasma cholesterol contents
were statistically significantly increased in animals of each sex at
doses of 150 mg/kg feed and higher, from 27 weeks onwards. The total
bilirubin content was decreased in males at the high dose, while
females at this dose showed slightly but statistically significantly
increased plasma alkaline phosphatase and alanine aminostransferase
activities from one year onwards. Plasma cholinesterase activity was
decreased in animals of each sex at 5 (23-45% inhibition), 150, and
450 (94-98% inhibition) mg/kg feed. Erythrocyte acetylcholinesterase
activity was inhibited by 14-26% in males and females at 150 and 450
mg/kg feed, and brain acetylcholinesterase activity was reduced in
animals of each sex at 150 mg/kg feed (19-28%) and 450 mg/kg feed
(45-54%).
The weights and macroscopic and microscopic appearance of organs
showed little treatment-related change, except in the liver. At 450
mg/kg feed, the absolute and relative liver weights were statistically
significantly increased in all animals, and histopathological
examination showed a significant increase in the incidence of
non-neoplastic hepatic foci, mainly eosinophilic and basophilic types,
in males (8/50 versus 1/50 in controls). The absolute weights of the
heart and kidneys were significantly increased in both males and
females at the high dose, and fermales at these doses had a
significant reduction in the number of ovarian cysts. The incidences
of hepatocellular carcinoma were comparable in treated and control
groups, but the incidences of hepatocellular adenoma were
statistically significantly increased in males at 150 mg/kg feed (9/50
versus 1/50 in concurrent controls and 1-7/50 in historical controls)
and in females at 450 mg/kg feed (8/50 versus 1/50 in concurrent
controls and 0-2/50 in historical controls). This finding is probably
not biologically significant in males, as no increase was seen at the
highest dose (5/50 at both the lowest and highest doses) and the
incidence was just beyond the range of historical controls. The
increased incidence of adenomas in females at the high dose is thought
to be due to the hepatoproliferative effect of the compound. The
incidence of lymphoreticular tumours was slightly but significantly
reduced in females receiving 150 and 450 mg/kg feed. The NOEL was 5
mg/kg feed, equal to 2.4 mg/kg bw per day, on the basis of increased
plasma cholesterol content and inhibition of brain
acetylcholinesterase activity (Jäger, 1992).
Rats
In a long-term study of toxicity, groups of 50 male and 50 female
SPF Wistar rats received diets containing phoxim at concentrations of
0, 15, 75, or 375 mg/kg feed, equal to average achieved intakes of 0,
0.8, 4, or 18 mg/kg bw per day for males and 0, 1.1, 5.4, or 27 mg/kg
bw per day for females, for 24 months. The control group comprisd 100
males and 100 females. Five additional animals of each sex per group
were used for clinical chemical examinations at 3, 6, and 12 months,
and 10 males and 10 females per group were used for these examinations
at termination. The observations included physical appearance,
behaviour, deaths, body weight, food consumption, haematological,
clinical chemical (liver function tests), and urinary parameters,
plasma and erythrocyte cholinesterase activities, absolute organ
weights, macroscopic and microscopic appearance, and brain
acetylcholinesterase activity (in five animals of each sex per group).
Treatment with phoxim did not affect the physical appearance,
behaviour, survival (76-79% of controls, 74-86% of treated animals),
or haematological, urinary, or macroscopic appearance of the rats.
Food consumption was reduced by 10% in males at the high dose, while
body-weight gain was slightly reduced in females at this dose. The
results of liver function tests were normal, other than a significant
reduction in plasma glutamate dehydrogenase activity in males at the
high dose and in bilirubin content in females at the high dose. Plasma
and erythrocyte cholinesterase activities were reduced by > 20% in
a dose-dependent manner throughout the study in rats treated with 75
and 375 mg/kg feed. Inhibition by > 20% of plasma and erythrocyte
cholinesterase activities was also observed in females at 15 mg/kg
feed (at 1, 2, and 52 weeks for plasma cholinesterase; at 2 weeks for
erythrocyte acetylcholinesterase). Brain acetylcholinesterase activity
was reduced by 18% in males and 23% in females at the highest dose.
Absolute liver weights were statistically significantly increased in
all treated males, but the effect is discounted as an inverse
dose-response relationship was seen. The absolute adrenal weights
were statistically significantly decreased in a dose-related manner
at doses > 75 mg/kg feed in animals of each sex, and the absolute
weights of the heart, lung, and spleen were statistically
significantly reduced in females at the high dose. None of the changes
in organ weights was accompanied by corresponding histopathological
changes. There was no difference in tumour incidence between control
and treated animals. The NOEL was 15 mg/kg feed, equal to 0.8 mg/kg bw
per day, on the basis of changes in adrenal weights (Bomhard & Löser,
1977; Finn, 1977).
Dogs
In a long-term study of toxicity, groups of four beagles of each
sex received phoxim in the diet at concentrations of 0, 0.3/0.1, 15,
or 750 mg/kg feed for 104 weeks, equivalent to 0, 0.0075/0.0025, 0.38,
or 19 mg/kg bw per day. Because plasma cholinesterase activity was
decreased by 26% in females at week 77, although erythrocyte
acetylcholinesterase activity was not affected, their dose was reduced
from 0.3 to 0.1 mg/kg feed from week 83 onwards. The animals were
observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, gross and
histopathological appearance, organ weights, and plasma, erythrocyte,
and brain cholinesterase activity.
No treatment-related effects were observed on mortality rates,
behaviour, food consumption, reflexes, or ophthalmoscopic,
haematological, or urinary parameters. All males at the high dose and
two of four at the intermediate dose showed a poor nutritional state.
In the second year, the coats of all males at the high dose were dull,
ruffled, and ungroomed, and, although the food consumption of these
animals was unaffected by treatment, they took much longer to eat
their food ration. The body-weight gain of all treated animals, but
especially the males, was reduced in a dose-dependent manner between 0
and 104 weeks due mainly to retarded growth in the second half of the
study; the effect reached statistical significance only in males at
the high dose. Plasma alanine aminotransferase activity was
statistically significantly increased in animals at the high dose from
week 52 onwards. Plasma alkaline phosphatase activity was
statistically significantly increased in these animals throughout the
treatment period. Although it was not increased at lower doses, the
natural decline was slowed, resulting in statistically significantly
higher values than in controls in females at the intermediate dose
from week 26 onwards and in males at the two lower doses from week 52
onwards. These effects on alkaline phosphatase activity are considered
to be adaptive, and were probably due to induction, as
organophosphorus esters are natural substrates for alkaline
phosphatase. The serum cholesterol concentration was reduced in
animals at the high dose throughout treatment, reaching statistical
significance at some times. Cholinesterase activity was reduced
throughout the experiment in males and females at 15 and 750 mg/kg
feed, in plasma (60 and 80% inhibition, respectively) and in
erythrocytes (20-30 and 60-80% inhibition, respectively). The
cholinesterase activity in plasma and erythrocytes was not reduced in
males at 0.3 mg/kg feed or in females at 0.1 mg/kg feed. Brain
acetylcholinesterase activity was significantly reduced, by
approximately 37%, in animals at the high dose.
Organ weights and gross and histopathological appearance showed
little treatment-related change other than in the liver. Histological
examination revealed hypolasia in both testes of one dog at the high
dose. As there was no cryptorchism, this effect might be related to
treatment. The livers of all females and one male at 750 mg/kg feed
were darker than those of the controls, and some had a markedly
exaggerated lobular pattern. The absolute and relative weights of the
liver were statistically significantly increased in all animals at the
high dose; although the relative weight of the liver was also
increased in males at the intermediate dose, the increase was not
statistically significant. Histopathological examination showed
hepatocytic alterations in three dogs and three bitches at the high
dose, involving dilated hepatocytes with a light, glassy,
structureless cytosol. On the basis of inhibition of brain
acetylcholinesterase activity and effects on the liver, the NOEL was
15 mg/kg feed, equivalent to 0.38 mg/kg bw per day (Hoffmann &
Gröning, 1977).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with phoxim are
summarized in Table 2. Phoxim was dissolved in dimethyl sulfoxide for
testing in vitro and in aqueous 0.5% Cremophor emulsion for testing
in vivo. The only effect seen in vitro or in vivo was induction of
chromosomal aberrations in vitro in lymphocytes; however, this
finding could not be interpreted owing to the cytotoxicity seen at
concentrations that affected the chromosomes. The summary tables in an
English translation of the Russian article show that phoxim induced a
very weak, dose-related increase in the number of cells with
aberrations, but no details were provided on the exposure time,
harvesting time, or absence or presence of S9. Furthermore, the
marginally increased frequencies reached statistical significance
because the frequency in concurrent controls both in vitro and in vivo
was very low, and these frequencies may have been within the range for
historical controls, for which data were lacking.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Phoxim was administered in the diet to four groups of 10 male and
20 female Long Evans FB 30 rats throughout mating, gestation, and
lactation in a three-generation study of reproductive toxicity at
doses of 0, 15, 75, or 375 mg/kg feed, equivalent to 0, 0.75, 3.8, and
19 mg/kg bw per day. The pups were suckled for up to four weeks,
weighed weekly, and examined grossly for malformations; the offspring
of each first mating (F1a, F2a, and F3a) were then killed. The
offspring of each second mating (F1b and F2b) were weaned and then
mated to produce two litters. After the dams of the F0, F1b, and F2b
generations had successfully nursed their offspring twice, they were
killed. Gross and histopathological examinations were performed on one
male and one female four-week-old pup of the F3b generation from each
of 10 mothers per group.
There were no differences between the control and treated parents
in any of the generations with respect to physical appearance,
behaviour, or body-weight gain, although some parents in the control
and treated groups died due to pneumonitis. No treatment-related
effects on fertility (pregnancy rate), litter size at birth or after
five days (viability index), or pup body weights at birth or during
four-week lactation were seen in any generation, and there were no
signs of malformation. The survival of pups in the F1a, F1b, F2a,
F2b, and F3a generations after four weeks' lactation was normal, but
the survival of pups in the F3b generation at 375 mg/kg feed was
slightly but statistically significantly reduced (84% versus 97% in
controls). Gross and histopathological examinations of the F3b pups
did not reveal any treatment-related alterations. The NOEL was 75
mg/kg feed, equivalent to 3.8 mg/kg bw per day, on the basis of
slightly reduced survival of pups in the second litter of the third
generation (Löser, 1979).
Table 2. Results of assays for genotoxicity with phoxim
End-point Test object Concentration Result GLP/ Reference
QA
In vitro
Reverse S. typhimurium TA98, 3.2-3200 nl/ Negativeb No Oesch
mutation TA100, TA1537 platea (+ S9) (1977)
32-3200 nl/
platea (- S9)
Reverse S. typhimurium TA98, 10-5000 µg/ Negativeb No Shirasu
mutation TA100 plate et al. (1978)
Reverse S. typhimurium TA98, < 5000 µg/ Negativeb No Moriya
mutation TA100, TA1535, plate et al. (1983)
TA1537, TA1538;
E. coli WP2 hcr
Reverse Saccharomyces 625-10 000 Negativeb Yes Herbold
mutation cerevisiae D7 µg/mlc (1985)
DNA damage Bacillus subtilis H-17, 1-100% v/v Negative No Shirasu
M-45 rec- (0.2-20 µl/disc) et al. (1978)
Chromosomal Human lymphocytes 30-300 µg/ml Positive Yes Herbold
aberration (- S9) at > 100 (1986)
50-500 µg/ml µg/ml - S9d
(+ S9)
Chromosomal Human lymphocytes 0.2 and 2 Equivocal No Kurinnyi
aberration µg/ml (1979)
Table 2. (continued)
End-point Test object Concentration Result GLP/ Reference
QA
In vivo
Micronucleus Mouse bone marrow 2 × 250 or Negativee No Herbold
formation (NMRI mice (SPF 500 mg/kg bw (1981)
Han) 5/sex per by gavage with
group) an interval of
24 h
Dominant Male NMRI mice Single dose of Negative No Machemer
lethal 500 mg/kg bw (1974)
mutation orally by gavage
Chromosomal Mouse bone marrow 1× 10 mg/kg bw Negative No Kurinnyi
aberration (noninbred white 1× 100 mg/kg bw, Negative (1979)
mice, 5 males/group) 5× 100 mg/kg bw, Equivocale
1× 250 mg/kg bw Equivocale
intragastrically
GLP, good laboratory practice; QA, quality assessment; S9, exogenous metabolic activation
system from rat liver microsomes
a At 1000 nl/plate, some test compound separated as droplets from the top agar.
b With and without S9
c No cytotoxicity up to 10 000 µg/ml
d The mitotic indices were 100, 37, and 1.9% at 30, 100, and 300 µg/ml,
respectively, in the absence of S9 and 84, 93, and 2.4% at 50, 100,
and 500 µg/ml, respectively, in the presence of S9. Significant
increases in chromosomal aberration frequency were found at 100 and
300 µg/ml in the absence of S9 and at 500 µg/ml in the presence of S9.
e The ratio of polychromatic to normochromatic erythrocytes at either
dose did not deviate from that in controls, and no clinical signs of
toxicity were noted. The doses were chosen on the basis of the results
of a preliminary test in which mice received 2 × 500 or 1000 mg/kg bw
orally and the lower dose was tolerated with induction of weak signs.
In view of this finding and the rapid and almost complete oral
absorption of phoxim by mice, it is likely that the bone marrow was
exposed.
2.2.5.2 Developmental toxicity
Rats
In a study of developmental toxicity, groups of 20-21 pregnant
Long Evans FB 30 rats received phoxim in 0.5% aqueous Cremophor
emulsion by oral gavage at daily doses of 0, 30, 100, or 300 mg/kg bw
on days 6-15 of gestation. On gestation day 20, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal malformations. None of the dams died
during the study, and no adverse effects were seen on their behaviour
or general appearance. The body-weight gain of dams at the high dose
was statistically significantly decreased during treatment but not
when considered over the entire gestation period. There were no
evident effects on embryonic or fetal development, and there was no
indication of teratogenicity up to the highest dose tested. The NOEL
for maternal toxicity was 100 mg/kg bw per day on the basis of the
decrease in body-weight gain. The NOEL for developmental toxicity was
300 mg/kg bw per day, the highest dose tested (Machemer, 1975).
Rabbits
In a study of developmental toxicity, groups of 20 pregnant
Chinchilla rabbits received phoxim in water with 2%
carboxymethylcellulose by oral gavage at daily doses of 0, 12, 36, or
72 mg/kg bw on days 6-18 of gestation. On gestation day 28, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external, visceral, and skeletal abnormalities. The study
was of conventional design, with quality assurance certification. No
treatment-related increase in mortality rate was observed. Clinical
signs of toxicity (diarrhoea, salivation, ventral body position,
dyspnoea, and abortion) were observed in dams at the high dose, and
phoxim caused reductions in food consumption and body-weight gain of
dams during treatment at 72 mg/kg bw. The only effects on fetuses were
an increased embryonic resorption rate and decreased fetal body
weights at the highest dose. There was no indication of teratogenicity
up to the highest dose tested. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of a decrease in food consumption and
body-weight gain. The NOEL for developmental toxicity was 36 mg/kg bw
per day on the basis of decreased fetal body weight and an increased
rate of embryonic resorptions (Becker, 1982).
2.2.6 Special studies on delayed neurotoxicity
Hens
In a study of delayed neurotoxicity, of which only a summary was
available, groups of one to five white Leghorn hens received a single
oral or intraperitoneal dose of phoxim dissolved in polyethylene
glycol, without an antidote. The oral doses used were 25, 38, 50, 250,
500, or 1000 mg/kg bw, and the intraperitoneal doses were 25, 38, or
50 mg/kg bw. In a second experiment, hens were given intraperitoneal
injections of 100 mg/kg bw pralidoxime iodide and 50 mg/kg bw atropine
sulfate before administration of phoxim at single oral doses of 38,
50, or 75 mg/kg bw (groups of 7-11 hens) or at single intraperitoneal
doses of 50, 75, 100, 150, or 200 mg/kg bw (groups of 1-14 hens). In
both studies, the birds were observed for 42 days; no
histopathological examinations were carried out, and a positive
control group was not included. No clinical signs of delayed
neurotoxicity were observed either with or without antidotal
protection. A LD50 of 38 mg/kg bw was derived for both oral and
intraperitoneal administration in the first experiment. The protective
effect of the antidote was more marked when phoxim was administered
intraperitoneally than when it was given orally (Kimmerle, 1972).
In another study of delayed neurotoxicity, 30 white Leghorn hens
received phoxim emulsified in water and 2% Cremophor EL by oral gavage
at a dose of 50 mg/kg bw, twice, at an interval of 21 days. Because
this dose was greater than the LD50, phoxim was administered with
atropine sulfate, administered intramuscularly at a dose of 50 mg/kg
bw. A negative control group of six hens received the vehicle and
atropine sulfate by the same schedule, and a positive control group of
five hens received a single dose of 375 mg/kg bw
tri- ortho-cresylphosphate in peanut oil. The hens treated with phoxim
and atropine and the negative controls were observed for 42 days and
the positive controls for 24 days. During the observation period, the
birds were inspected for clinical signs, body weight, and coordination
of movement. At the end of the observation period, they were killed
and nervous tissue (brain, spinal cord, and sciatic nerves) was
removed from six birds treated with phoxim and from all negative and
positive controls and examined histopathologically. The design of the
study closely resembled OECD guideline 418 in use in 1984, with GLP
certification.
After treatment with phoxim and atropine, the signs of toxicity
were staggering gait, prostration, apathy, salivation, drooping wings,
and fluffed plumage. From observation day 5 onwards, the only sign of
toxicity was staggering gait, while from observation day 26 onwards
the hens showed no abnormal signs or behaviour. The negative control
group showed only staggering gate. The positive control group showed
transient staggering gate in the acute phase, progressive disturbance
of coordination of movement from observation day 8 onwards, and severe
paralysis in the final stage which was of sufficient severity that the
birds were killed prematurely at observation day 24. Histopathological
examination showed no sign of peripheral neuropathy or demyelination
in phoxim-treated birds, whereas hens given tri- ortho-cresylphosphate
showed various degrees of degeneration of peripheral nerves including
demyelination, axonal lysis, ballooned fibre segments, and activated
Schwann cells. Thus, at a dose that would be lethal in the absence of
antidote, phoxim did not induce delayed neurotoxicity in hens (Pauluhn
& Kaliner, 1984).
2.2.7 Studies with metabolites
The oral LD50 of cyanobenzaldoxime (technically pure substance
formulated in lutrol) was 4500 mg/kg bw in male and 4100 mg/kg bw in
female rats. The signs of acute oral toxicity comprised slight to
moderate disturbance of behaviour, no grooming of coats, sedation, and
dyspnoea. Dermal application of 5000 mg/kg bw had no effect (Thyssen &
Kimmerle, 1976). The oral LD50 of the glucuronide of
cyanobenzaldoxime (formulated in aqueous Cremophor EL) in three female
rats was > 2500 mg/kg bw (Mihail, 1979). The oral LD50 value for
PO-phoxim in mice was approximately 800 mg/kg bw for the pure
substance (Kimmerle, 1969) and 1000 mg/kg bw for the pure substance
dissolved in olive oil (Vinopal & Fukuto, 1971) were determined.
In a short summary of a study on dermal irritation, 0.5 g of
cyanobenz-aldoxime applied under a plaster dressing for 24 h to the
ear did not irritate the skin of hairless rabbits. In a short summary
of a study on ocular irritation, cyanobenzaldoxime at a dose of 50 mg
placed in the conjuctival sac of the left eye of rabbits caused severe
redness and swelling of the conjunctivae, slight redness, swelling of
the iris, and diffuse opacity of the cornea (Thyssen & Kimmerle,
1976).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term, and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
delayed neurotoxicity of phoxim. Although most of the studies did not
meet current standards for study protocol and conduct, they did
provide satisfactory information for evaluation of the safety of the
compound.
After oral administration of radiolabelled phoxim to mice, rats,
and pigs, the radiolabel was rapidly and almost completely absorbed
and was rapidly taken up into the major organs and tissues. The main
route of elimination in mice, rats, and pigs was the urine, while
faecal excretion was of minor importance. In rats, there was also some
evidence of biliary excreation. In rats and pigs, elimination was
rapid (> 80% via the urine within 24 h) and was virtually complete
with three to four days. In mice, elimination of radiolabel was
somewhat slower, appraently because of retention of phosphate-derived
metabolites in the urinary blader. Although there were some
qualitative and quantitative differences in metabolism between mice,
rats, and pigs, the main routes of metabolism were the same, involving
de-ethylation, hydrolysis of the phosphorus ester bond (either before
or after oxidative desulfuration to yield PO-phoxim), and conjugation
of the resulting cyanobenzaldoxime. Despite the presence of products
that were assumed to have arisen from PO-phoxim, no PO-phoxim itself
was found in mice, rats, or pigs. Hence, if formed, it must be a very
short-lived intermediate in mammals.
Animal species differ in their sensitivity to single oral doses
of phoxim. The LD50 values ranged from 20 to 40 mg/kg bw in chickens,
from 250 to > 1200 mg/kg bw in guinea-pigs, rabbits, cats, and dogs,
and from 1200 to 10 000 mg/kg bw in mice and rats. Thus, phoxim is of
only low or moderate acute oral toxicity in mammalian species.
The short-term toxicity of phoxim was evaluated after oral
administration to mice, rats, dogs, and rhesus monkeys. In two dose
range-finding studies of six and eight weeks' duration, phoxim was
administered to mice in the feed at concentrations of 0.5-750 mg/kg
(equal to 0.28-510 mg/kg bw per day). In these studies, phoxim had
effects on the liver (increased weight and hepatocyte alterations
indicative of an adaptive response) at concentrations of 30 mg/kg of
feed and above and on the kidney (increased weight) at doses of 150
mg/kg of feed and above. Inhibition of plasma cholinesterase activity
was observed at and above concentrations of 5 m/kg of feed, while
inhibition of erythrocyte and brain acetylcholinesterase activity was
observed only at 750 mg/kg of feed. On the basis of a slight increase
in liver weight in female mice, the overall NOEL in these studies was
5 mg/kg of feed, equal to 3.1 mg/kg bw per day.
Studies were carried out in rats which received doses of 2-50
mg/kg bw per day by gavage for 21 or 30 days or in two studioes at
4-500 mg/kg of feed in the diet, equivalent to 0.4-50 mg/kg bw per
day, for three months. In the studies in which phoxim was given by
gavage, cholinesterase activity in plasma, erythrocytes, and brain was
inhibited by > 20% at doses of 2, 5, and 50 mg/kg bw per day,
respectively. In the two studies in which phoxim was administered in
the diet, signs of cholinergic poisoning were observed at 500 mg/kg of
feed, organ weight changes at doses > 120 mg/kg of feed, and
inhibition of cholinesterase activity in plasma at doses > 15 mg/kg
of feed and in erythrocytes at > 40 mg/kg of feed. The overall NOEL
in the studies involving dietary administration was 12 mg/kg of feed,
equivalent to 1.2 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholines-terase activity (in the absence of data on
brain acetylcholinesterase activity).
Three studies of three months' duration in dogs treated in the
diet were reported, in which phoxim was administered at doses ranging
from 0.3 to 1000 mg/kg of feed, equivalent to 0.0075-25 mg/kg bw per
day. Doses of 200 and 1000 mg/kg of feed resulted in changes in the
weights of gonads and liver and in the activities of alkaline
phosphatase and lactate dehydrogenase in plasma, inhibition of
erythrocyte acetylcholinesterase activity, and/or weight loss (females
only) and signs of cholinergic poisoning. Inhibition of plasma
cholinesterase activity was observed at doses from 1 to 1000 mg/kg of
feed. Brain acetylcholinesterase activity was not determined in these
studies. The overall NOEL was 50 mg/kg of feed, equivalent to 1.3
mg/kg bw per day, on the basis of inhibition of erythrocyte
acetyl-cholinesterase activity (in the absence of data on brain
acetylcholinesterase activity) and increased plasma alkalaine
phosphatase activity.
Rhesus monkeys received phoxim by gavage for six months at a dose
of 0.2, 0.65, or 3 mg/kg bw per day. Apart from marked inhibition of
plasma cholinesterase activity at doses > 0.2 mg/kg bw per day and
very slight inhibition of erythrocyte acetylcholinesterase activity at
2 mg/kg bw per day, phoxim had no effect. In the absence of data on
brain acetylcholinesterase activity, the NOEL was 0.65 mg/kg bw per
day on the basis of slight inhibition of erythrocyte
acetylcholinesterase activity.
In a 24-month study of carcinogenicity, phoxim was administered
in the diet at concentrations of 1-450 mg/kg of feed (equal to
0.47-210 mg/kg bw per day) to mice of a strain known to be highly
susceptible to the development of liver tumours. Plasma, erythrocyte,
and brain cholinesterase activities were decreased at doses > 150
mg/kg of feed. The body weight of males were increased at 150 mg/kg of
feed and the body weights of both males and females at 450 mg/kg of
feed. Signs of effects on the liver (including changes in weight, in
plasma cholesterol and total bilirubin levels, and in plasma alanine
aminotransferase and alkaline phosphatase activities) were evident at
450 mg/kg of feed. At this dose, there were also increased incidences
of non-neoplastic histological changes in the livers of males and of
hepatocellular adenomas in females. At 150 mg/kg of feed, the only
effect on the liver was increased plasma cholesterol concentrations in
animals of each sex. The NOEL was 5 mg/kg of feed, equal to 2.4 mg/kg
bw per day, on the basis of increased plasma cholesterol
concentrations and inhibition of brain acetylcholinesterase activity.
The increase in the incidence of adenomas observed in females at the
highest dose was thought to be a consequence of the
hepatoproliferative effect of the compound.
The long-term toxicity of phoxim was evaluated in rats and dogs
by oral administration for 24 months at 15-375 mg/kg of feed (equal to
0.8-27 mg/kg bw per day) to rats and 0.1-750 mg/kg of feed (equivalent
to 0.0025-19 mg/kg bw per day) to dogs. In rats dosed at 375 mg/kg of
feed, reductions were seen in food intake in males, in body weight in
females, and in plasma glutamate dehydrogenase activity, total
bilirubin level, the weights of the heart, lungs, spleen, and
adrenals, and plasma, erythrocyte, and brain cholinesterase activity
in animals of each sex. Decreased plasma and erythrocyte
cholinesterase activity and adrenal weights were also observed in rats
at 75 mg/kg of feed. There were no histopathological differences
between control and treated animals, nor was there any difference in
the incidence of tumours. The NOEL was 15 mg/kg of feed, equal to 0.8
mg/kg bw per day, on the basis of lowered adrenal weights at higher
doses.
Male and female dogs given phoxim at 750 mg/kg of feed had liver
damage, as shown by increased liver weights and plasma alanine
aminotransferase and alkaline phosphatase activities, decreased serum
cholesterol level, and histopathological alterations to hepatocytes.
Plasma, erythrocyte, and brain cholinesterase activities were also
reduced in animals of each sex at this dose. In addition, male dogs
showed clinical signs of toxicity and decreased body-weight gain.
Reductions in plasma and erythrocyte cholinesterase activities were
observed in male and female dogs at 15 mg/kg of feed. The NOEL was 15
mg/kg of feed, equivalent to 0.38 mg/kg bw per day, on the basis of
effects on the liver and inhibition of brain acetylcholinesterase
activity.
Phoxim has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium and Saccharomyces
cerevisiae, DNA damage in Bacillus subtilis, and chromosomal
aberrations in human lymphocytes. It has also been tested
in vivo for its ability to induce micronucleus formation,
chromosomal aberrations, and dominant lethal mutations in mice.
Cytogenetic alterations were found in human lymphocytes in vitro at
a cytotoxic dose in the absence of exogenous metabolic activation. The
results of all other tests were negative. On the basis of these data
and the results of the long-term assays in rodents, the Committee
concluded that phoxim is not genotoxici and is unlikely to have
carcinogenic potential in humans.
In a three-generation study of reproductive toxicity, rats were
given phoxim in the diet at a concentration of 0, 15, 75, or 375 mg/kg
of feed (equivalent to 0, 0.75, 3.8, and 19 mg/kg bw per day). The
only effect observed was a slight reduction in the number of pups in
the second litter of the third generation thaqt survived after four
weeks' lactation from dams receiving 375 mg/kg of feed. On the basis
of this effect, the NOEL was 75 mg/kg of feed, equivalent to 3.8 mg/kg
bw per day.
In a study of developmental toxicity in rats given a dose of 0,
30, 100, or 300 mg/kg bw per day orally on days 6-15 of gestation,
phoxim was toxic to the dams, retarding body-weight gain during
treatment at the highest dose. It was not embryotoxic, fetotoxic, or
teratogenic at any dose. The NOEL for maternal toxicity was 100 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 300 mg/kg bw per day, the highest dose
tested.
A study of developmental toxicity was conducted in rabbits given
phoxim at a dose of 0, 12, 36, or 72 mg/kg bw per day orally on days
6-18 of gestation. At the highest dose, phoxim increased the rate of
embryonic resorption, decreased fetal body weights, and was toxic to
the dams, which showed signs of toxicity a marked decreases in food
consumption and in body-weight gain. There was no indication of
teratogenicity at any dose. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of reduced food consumption and
body-weight gain. The NOEL for developmental toxicity was also 36
mg/kg bw per day, on the basis of decreased fetal body weight and an
increased rate of embryonic resorption.
In a study of delayed neurotoxicity, hens protected by the
antidote atropine received phoxim orally at a dose of 50 mg/kg bw,
which was repeated after 21 days. The birds were then observed for 42
days. They showed only transient signs of toxicity and no abnormal
signs or behaviour from day 26 after the second dose. At the end of
the study, no paralysis was present, and no histological evidence of
peripheral neuropathy or demyelination was observed. The Committee
concluded that phoxim does not induce delayed neurotoxicity in hens.
Although the effect of phoxim on the enzyme neuropathy target esterase
has not been investigated in hens, the Committee concluded that such a
study was not necessary in view of the negative results obtained in
adequately conducted assessments of the capacity of phoxim to induce
delayed neuropathy.
4. EVALUATION
The Committee established an ADI of 0-4 µg/kg bw for phoxim on
the basis of the NOEL of 0.38 mg/kg bw per day for effects on the
liver and inhibition of brain acetylcholinesterase activity in the
two-year study of toxicity in dogs and a safety factor of 100. This
ADI differs from that established by the Joint FAO/WHO Meeting on
Pesticide Residues, as the Expert Committee concluded that inhibition
of plasma cholinesterase activity is not a relevant end-point for risk
assessment. The Joint Meeting is now of a similar opinion (FAO/WHO,
1988).
5. REFERENCES
Becker, H. (1982) Embryotoxicity and teratogenicity study on SRA 7502
in rabbits. Unpublished report (project no. 004972, report no. R2354)
from Research & Consulting Company Ltd, Itingen, Switzerland, for
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomann, W. (1992) Volaton VL 80 (c.n.: phoxim). Studies on acute
dermal toxicity in rats. Unpublished report (study no. T 0040187,
report no. 21065) from Bayer AG, Fachbereich Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomhard, E. & Löser, E. (1977) SRA 7502. Chronic toxicology studies in
rats (feeding experiment over 2 years). Unpublished report (no. 7042)
from Bayer AG, Institute for Toxicology, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Brooks, P.N. & Cherry, C.P. (1974) Pathology report of Bay 77488.
Sub-chronic toxicological studies in dogs (3-month feeding
experiment). Addendum to report no. 2579. Unpublished report (no.
FBA130/73959) from Huntingdon Research Centre, Huntingdon, United
Kingdom, for Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F., Griffin, T. & Talley, W. (1978) A safety evaluation
study of phoxim in rhesus monkeys. Unpublished report from Institute
of Comparative and Human Toxicology, Albany, New York & International
Center of Environmental Safety, Holloman, New Mexico, United States.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Daniel, J.W., McLean, J. & Pringuer, M. (1978a)
Phoxim--Biotransformation in the rat. Unpublished report (no.
78/BAG6/317) from Life Science Research, Stock, Essex, United Kingdom
for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Daniel, J.W., Swanson, S. & McLean, J. (1978b) Phoxim:
Pharmacokinetics and biotransformation in the rat. Unpublished report
(no. 78/BAG5/194) from Life Science Research, Stock, Essex, United
Kingdom, for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
FAO/WHO (1983) Pesticide Residues in Food--1982. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 46).
FAO/WHO (1985) Pesticide Residues in Food--1984. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 62).
FAO/WHO (1988) Pesticide Residues in Food--1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and a WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 92).
Finn, J.P. (1977) Chronic toxicity study on rats with SRA 7502 (two
year feeding study). Unpublished report (no. 612/262/2) from Hazleton
Laboratories Europe Ltd, Harrogate, United Kingdom, for Bayer AG,
Institut für Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1978a) Determination of acute toxicity (LD50)--SRA 7502.
Unpublished letter report (dated 19 May 1978) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Flucke, W. (1978b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 26 June 1978) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979a) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 9 March 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 17 April 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity (LD50)--SRA 7502
Volaton VL. Unpublished letter report (dated 14 March 1980) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1984a) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in guinea pigs [in German]. Unpublished report
(study no. T 6016487, report no. 13071) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Flucke, W. (1984b) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in an open epicutaneous test with guinea pigs
[in German]. Unpublished report (study no. T 6016630, report no.
13086) from Bayer AG, Institut für Toxikiologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1978) Volaton VL. Subacute cutaneous
toxicity study with rabbits. Unpublished report (report no. 8034) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Thyssen, J. (1979) Volaton (SRA 7502) active ingredient
presolution. Acute toxicity studies. Unpublished report (no. 8081)
from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gyrd-Hansen, N., Brimer, L. & Rasmussen, F. (1993) Percutaneous
absorption of organophosphorus insecticides in pigs--The influence of
different vehicles. J. Vet. Pharmacol. Ther., 16, 174-180.
Heimann, K.-G. (1982a) Determination of acute toxicity (LD50)--
Volaton VL. Unpublished letter report (dated 16 February 1982) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.-G. (1982b) Determination of acute toxicity (LD50)--
Volaton VL 80. Unpublished letter report (dated 3 June 1982) from
Bayer, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) SRA 7502. Phoxim, Baythion(R)-active ingredient,
Volaton(R)-active ingredient. Micronucleus test on mouse to evaluate
SRA 7502 for mutagenic potential. Unpublished report (study no. T
5010077, report no. 9891) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985) SRA 7502 c.n. Phoxim. Test on S. cerevisiae D7
investigating for point mutagenic effect. Unpublished report (study
no. T 2018201, report no. 13708) from Bayer AG, Institute for
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1986) SRA 7502 (c.n. phoxim). Cytogenetic study with
human lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report (study no. T 9021736, report no.
15148) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1977) SRA 7502. Chronic toxicity study on
dogs (two-year feeding experiment). Unpublished report (no. 6865) from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989a) SRA 7502. Preliminary study on
mice (administration in feed to determine dosage for a long-term study
on carcinogenicity in mice). Unpublished report (study no. T1022043,
report no. 17610) from Bayer AG, Fachbereich Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989b) SRA 7502. Preliminary study on
mice (administration in feed for 8 weeks to determine dose for a
long-term study). Unpublished report (study no. T9022799, report no.
17611) from Bayer AG, Fachbereich Toxikologie, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jäger, R. (1992) SRA 7502 (common name: Phoxim). Study for
oncogenicity in B6C3F1 mice (twenty-four month feeding study).
Unpublished report (study no. T 2022297, report no. 21624) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1968) Bay 77488. Toxicological studies. Unpublished
report (no. 1067) from Farbenfabriken Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1969) Supplement to the toxicology of SRA 7502.
Unpublished letter report (dated 27 June 1969) from Bayer AG,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972) Phoxim: Tests for neurotoxicity in chickens.
Unpublished report (dated 21 June 1972) from Farbenfabriken Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1973) SRA 7502, SRA 7660, LOW 6599, SIR 5126, HOX 3082,
malathion. Comparative toxicological studies in rats with repeated
oral administration. Unpublished report (no. 3962) from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. (1970) Bay 77 488. Toxicological studies.
Unpublished report (no. 2235) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. & Weber, H. (1988) [Phenyl-U-14-C]-Phoxim: Biokinetic
behaviour and biotransformation in the edible tissues of the pig after
oral administration. Unpublished report (study no. M 183 0138-4,
PF-report no. 3077) from Bayer AG, Institute for Metabolism Research,
Leverkusen/Monheim, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kurinnyi, A.I. (1979) Cytogenetic activity of the pesticide valexon
and its influence on the mutability of mouse bone marrow cells.
Tsitol. Genet., 13, 370-374 (English translation). Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lorke, D. & Kimmerle G. (1965) Toxikologische Untersuchungen mit dem
Wirkstoff SRA 7502. Unpublished report (dated 14 December 1965) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969a) Bay 77 488. Subchronic toxicology studies in rats
(study over 3 months). Unpublished report (no. 1205) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969b) Bay 77 488. Subchronic toxicology studies with rats.
Animal and organ weights (Individual values for report no. 1205).
Unpublished report (no. 1215) from Farbenfabriken Bayer AG, Institut
für Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Löser, E. (1970a) Bay 77 488. Subchronic toxicological studies on rats
(three-month feeding experiment). Unpublished report (no. 2389) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1970b) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2418) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1971) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2579) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1979) SRA 7502. Multigeneration reproduction study on rats.
Unpublished report (no. 8447) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1974) SRA 7502 (phoxim). Dominant lethal test on male
mouse to evaluate SRA 7502 for mutagenic potential. Unpublished report
(no. 4943) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Machemer, L. (1975) SRA 7502 (phoxim). Studies for embryotoxic and
teratogenic effects on rats following oral administration. Unpublished
report (no. 5331) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Märtins, T. (1992) Volaton VL 80 (c.n.: phoxim). Acute inhalation
toxicity in rats in compliance with OECD guideline no. 403.
Unpublished report (study no. T 2040765, report no. 21429) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mihail, F. (1979) Cyanoxim acid glucoside (metabolite of phoxim).
Unpublished letter report (dated 4 July 1979) from Bayer AG, Institute
of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1981) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 4 May 1981) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1982) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 1 March 1982) from Bayer, Institute
of Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Moriya, M., Ohta, T., Watanabe K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Mürmann, P. & Luckhaus, G. (1973) SRA 7502. Subchronic toxicity study
on dogs (three-month feeding experiment). Unpublished report (no.
4136) from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Oesch, F. (1977) Ames test for Volaton (phoxim). Unpublished report
(dated 30 November 1977) from Pharmakologisches Institut, Universität
Mainz, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1983) SRA 7502 (active ingredient of Baythion(R) and
Volaton(R)) (common name: phoxim). Acute oral toxicity study on
hens. Unpublished report (study no. T 3006197, report no. 11978) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. & Kaliner, G. (1984) SRA 7502 (c.n.: phoxim). Study for
acute neurotoxicity in hens. Unpublished report (study no. T 3016385,
report no. 12632) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Watanabe, T. (1978) Phoxim. Mutagenicity
test on bacterial systems. Unpublished report (dated 18 July 1978)
from Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1976) SRA 7502. Subacute oral cumulation study on rats.
Unpublished report (no. 5954) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1973) SRA 7502. Toxicological studies on
hens. Unpublished report (no. 4236) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) Cyanoxim
(hydroxiiminophenylacetonitrile). Occupational toxicological study.
Unpublished report (no. 6392) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vince, A.A. & Spicer E.J.F. (1971) Pathology report on Bay 77 488.
Subchronic toxicological studies in rats (3 month feeding experiment).
Addendum to report no. 2389. Unpublished report (no. 4257/71/415) from
Huntingdon Research Centre, Huntingdon, United Kingdom, for
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vinopal, J.H. & Fukuto, T.R. (1971) Selective toxicity of phoxim
(phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate). Pestic.
Biochem. Physiol., 1, 44-60.
were observed, even at the highest doses, when the only effects were
non-specific ones on the nervous system. No signs of poisoning were
observed after dermal application. Phoxim was only slightly to
moderately toxic in all of the mammalian species studied.
Poorly reported studies of skin irritation indicated that phoxim
had only a very slight irritating effect on rabbit's skin. The
calculated overall primary irritation index was < 0.5. Limited
reports of eye irritation in rabbits showed that phoxim can cause mild
to moderate irritation of the conjuctivae, without effects on the iris
or cornea (Lorke & Kimmerle, 1965; Kimmerle & Solmecke, 1970; Flucke &
Thyssen, 1979).
In a Magnussen-Kligman maximization test in 40 male DHPW
guinea-pigs, phoxim induced a sensitization response in 60% of the
animals, which was shown not to be due to 1-butanol (Flucke, 1984a).
In an open epicutaneous test (according to Klecak) in 64 male DHPW
guinea-pigs, administration of phoxim showed no sensitization
potential (Flucke, 1984b).
2.2.2 Short-term studies of toxicity
Mice
In a study to establish the doses for a study of carcinogenicity,
groups of 10 male and 10 female SPF B6C3F1 mice were fed diets
containing phoxim at 0, 5, 30, 150, or 750 mg/kg feed for six weeks,
equal to 0, 3, 18, 85, and 440 mg/kg bw per day for males and 0, 3,
20, 100, and 500 mg/kg bw per day for females. The study was certified
for compliance with GLP. No differences in mortality rate, clinical
signs, body weight, or food or water intake were observed that could
be associated with treatment. Plasma alkaline phosphatase activity and
total protein content were significantly increased in males at the
high dose. In females, the plasma cholesterol content increased with
dose, the increase becoming statistically significant from 30 mg/kg
feed onwards; however, the control concentrations of cholesterol and
total protein were low in comparison with historical data, and the
increased levels were still within the historical control range, as
were the alkaline phosphatase activities in males at the high dose.
Cholinesterase activity was increasingly reduced with dose in plasma,
with > 20% inhibition in all treated animals, and in erythrocytes,
with > 20% inhibition only in females at the high dose. Brain
acetylcholinesterase activity, which was determined in five animalsof
each sex in each group, was reduced in all treated groups, with
reductions of more than 50% in both males and females at the high
dose; however, the activities in concurrent controls were in the upper
range of that of historical controls, and the observed reduction
showed no dose-response relationship, was not always statistically
significant, and was still within historical control levels, except in
males at the high dose. Therefore, only the reduction in males at the
high dose is considered relevant.
Table 1. Acute toxicity of phoxim
Route Species Sex LD50a or LC50 Reference
Oral Mouse Male 2700 Kimmerle (1968)
(mg/kg bw) 1800 Kimmerle & Solmecke (1970)
1200b Flucke & Thyssen (1979)
Female 3600 Kimmerle (1968)
2200 Kimmerle & Solmecke (1970)
2800-4000 Flucke (1978a,b; 1979a,b; 1980);
Heimann (1982a,b); Mihail
(1981, 1982)
1800b Flucke & Thyssen (1979)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 2300 Kimmerle (1969)
> 2000d Vinopal & Fukuto (1971)
Rat Male 10 000 Lorke & Kimmerle (1965)
8000 Kimmerle (1968))
2100 Kimmerle & Solmecke (1970)
4900b Thyssen (1976)
2000b Flucke & Thyssen (1979)
Female 10 000 Lorke & Kimmerle (1965)
6500 Kimmerle (1968)
1900 Kimmerle & Solmecke (1970)
2900b Thyssen (1976)
1400b Flucke & Thyssen (1979)
Guinea-pig Female 390-560 Kimmerle (1968)
approx. 660 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
Rabbit Female 250-380 Kimmerle & Solmecke (1970)
Unspecified approx. 380c Lorke & Kimmerle (1965)
280-560 Kimmerle (1968)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Cat Female 250-500 Kimmerle & Solmecke (1970)
Unspecified > 1000c Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Dog Female 250-500 Kimmerle & Solmecke (1970)
> 500b Flucke & Thyssen (1979)
Unspecified > 1200 Lorke & Kimmerle (1965)
> 1100 Kimmerle (1968)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
20e Thyssen & Kimmerle (1973)
40b Pauluhn (1983)
Intraperitoneal Rat Male 2200c Lorke & Kimmerle (1965)
(mg/kg bw) 2000 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1100b Flucke & Thyssen (1979)
Female 2000c Lorke & Kimmerle (1965)
1900 Kimmerle (1968)
1800 Kimmerle & Solmecke (1970)
1500b Flucke & Thyssen (1979)
Chicken Female approx. 38c Lorke & Kimmerle (1965);
Kimmerle (1972)
Intravenous Mouse Female 1100 Kimmerle (1968)
(mg/kg bw) 480b Kimmerle & Solmecke (1970)
Dermal Rat Male > 1200 Lorke & Kimmerle (1965)
(mg/kg bw) > 1100 Kimmerle (1968)
> 1100 Kimmerle & Solmecke (1970)
Table 1. (continued)
Route Species Sex LD50a or LC50 Reference
Male/female > 5600 Flucke & Thyssen (1979)
> 5000f Bomann (1992)g
Inhalation Mouse Male > 2.1h Kimmerle (1968)
(mg/L, 4 h) > 3.1h Kimmerle & Solmecke (1970)
Unspecified > 1.3 Kimmerle (1968)
>1.7h Kimmerle & Solmecke (1970)
Rat Male > 2.0h Kimmerle (1968)
> 2.6h Kimmerle & Solmecke (1970)
Female > 2.2h Kimmerle (1968)
> 2.8h Kimmerle & Solmecke (1970)
Male/female > 3.0h Flucke & Thyssen (1979)
> 4.6 Märtins (1992)g
Unspecified > 1.3 Kimmerle (1968)
> 1.7h Kimmerle & Solmecke (1970)
Guinea-pig Unspecified > 1.3 Kimmerle (1968)
Rabbit Unspecified > 1.3 Kimmerle (1968)
1.2-1.7h Kimmerle & Solmecke (1970)
Subcutaneous Mouse Male/female > 5000b Flucke & Thyssen (1979)
(mg/kg bw ) Unspecified > 1000c Lorke & Kimmerle (1965)
a In the reports of Kimmerle (1968), Flucke (1978a,b; 1979a,b; 1980), Heimann (1982a,b), Mihail
(1981, 1982), Kimmerle & Solmecke (1970), and Flucke & Thyssen (1979), some or all of the
LD50 values for phoxim (as pre-solution) were cited in microlitres or millilitres per kilogram
of body weight. These values were converted to milligrams per kilogram of body weight by
Table 1. (continued)
correcting for the density of the pre-solution (1.126 mg/µl at 20 °C). In the report of Lorke
& Kimmerle (1965), some of the LD50 values for pure phoxim were cited in millilitres per
kilogram of body weight. These values were converted to milligrams per kilogram of body weight
by correcting for the density of the pure substance (1.176 mg/µl at 20 °C).
b Vehicle, water:Cremophor EL
c Vehicle, lutrol
d Vehicle, olive oil
e Vehicle, water
f Vehicle, 2% Cremophor EL:physiological saline
g The studies of Bomann (1992) and Märtins (1992) were of conventional design, with GLP
and quality assurance certification.
h Vehicle, ethanol:lutrol 1:1
The only gross change seen on post-mortem examination was
enlarged spleens in a number of control and treated females. As this
finding was unrelated to dose and occurred in more control than
treated animals, it is not considered to be related to treatment. The
relative kidney weights of females at 150 and 750 mg/kg feed were
statistically significantly increased with dose. The absolute and
relative liver weights were increased with dose in all treated
animals, the increase in relative weight becoming statistically
significant at 150 mg/kg feed and the increase in absolute weight in
males only; both the relative and absolute weights were increased
significantly at 750 mg/kg feed. Histopathological examination only of
the livers of controls and animals at the high dose revealed
enlargement of the centrilobular hepatocytes in males and females at
the high dose, indicating adaptive hypertrophy and possible enzyme
induction. No evidence of irreversible changes was found. One female
at the high dose had slight cytoplasmic vacuolation in periportal
cells, which may not have been related to treatment (Ivens-Kohl &
Hahnemann, 1989a).
In a follow-up study, groups of 10 male and 10 female SPF B6C3F1
mice were fed diets containing phoxim at 0, 0.5, 1, 5, or 30 mg/kg
feed, equal to 0, 0.28, 0.55, 2.8, and 18 mg/kg bw per day for males
and 0, 0.35, 0.66, 3.1, and 23 mg/kg bw per day for females, for eight
weeks. The study was certified for compliance with GLP. No differences
in mortality rate, clinical signs, body weight, food or water intake,
or macroscopic or histopathological appearance were observed that
could be related to treatment. The plasma cholesterol content was
statistically significantly increased in females at 30 mg/kg feed. As
in the previous study, however, the control cholesterol content was
lower than that of historical controls, and the increased level was
still within the range of historical controls. Cholinesterase activity
in plasma was reduced by > 40% at the two highest doses, and a small
inhibition (8-14%) of erythrocyte acetylcholinesterase activity was
observed in males and females at the high dose. There was no evidence
of a change in brain acetylcholinesterase activity in any treated
groups. Although the absolute and relative weights of the liver were
slightly but statistically significantly increased in females at the
high dose, no effects on organ weights were found. The NOEL was 5
mg/kg feed, equal to 3.1 mg/kg bw per day, on the basis of slightly
increased liver weights in female mice (Ivens-Kohl & Hahnemann,
1989b).
Rats
In a 21-day study of toxicity with special attention to the
reversibility of cholinesterase inhibition, groups of 15 SPF Wistar II
rats of each sex received phoxim emulsified in distilled water and
Cremophor EL by oral gavage at 0, 5, or 50 mg/kg bw per day. Five rats
of each sex in each group were killed at the end of treatment and two
and four weeks thereafter. The animals were examined regularly for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. Brain
cholinesterase activity was measured at termination of treatment and
two weeks thereafter.
There were no deaths and no treatment-related effects on
appearance, behaviour, or body weight. Plasma cholinesterase activity
was reduced by > 20% during treatment in females at the low dose
(when compared with concurrent controls but not when compared with
pre-treatment values in the same animals) and in males and females at
the high dose, but the activity was restored within one week after
cessation of treatment. Acetylcholines-terase activity in erythrocytes
was reduced by > 20% in males at the low dose during the first week
of treatment (when compared with concurrent controls but not when
compared with pre-treatment values in the same animals) and in females
at this dose throughout treatment but was restored within one week.
Erythrocyte cholinesterase activity was decreased by > 20% in males
and females at the high dose throughout the treatment period but was
restored within two weeks in males and within four weeks in females.
Brain acetylcholinesterase activity was within the physiological range
and very similar to control values in all treated males and in females
at the low dose, both at termination and two weeks thereafter. Females
at the high dose showed 30% inhibition of brain acetylcholinesterase
activity at termination, but this was restored within two weeks. Thus,
inhibition of cholinesterase activity was more pronounced in female
than in male rats and was reversible in animals of each sex (Thyssen,
1976).
Groups of 15 SPF Wistar II rats of each sex received phoxim
dissolved in polyethylene glycol 400 (lutrol) by oral gavage at 0, 2,
5, or 15 mg/kg bw per day for 30 days. The animals were examined for
effects on physical appearance, behaviour, body weight, and
cholinesterase activity in plasma and erythrocytes. No
treatment-related effects were observed on appearance, behaviour or
body weight. The cholinesterase activity in plasma decreased with dose
in all treated females and in males at 5 and 15 mg/kg bw per day. The
acetylcholinesterase activity in erythrocytes was reduced by > 20%
in males and females at two highest doses (Kimmerle, 1973).
In a three-month study, groups of 15 young SPF Wistar rats of
each sex received diets containing phoxim at 0, 4, 12, 40, or 120
mg/kg feed, equivalent to 0, 0.4, 1.2, 4, or 12 mg/kg bw per day. The
animals were observed for physical appearance, behaviour, deaths, body
weight, food consumption, haematological and clincal chemical
parameters (liver function tests), plasma and erythrocyte
cholinesterase activity, urinary parameters, organ weights, and
macroscopic appearance. Brain acetylcholinesterase activity was not
investigated. Treatment-related effects were found on cholinesterase
activity in both plasma and erythrocytes, which were decreased by
> 20% at 40 mg/kg feed (within one month) and 120 mg/kg feed
(within one week) in animals of each sex. Statistically significantly
increased weights of the thyroid glands (both absolute and relative)
and adrenal glands (relative only) were observed in males at 120 mg/kg
feed. In the absence of data on brain acetylcholinesterase activity,
the NOEL was 12 mg/kg feed, equivalent to 1.2 mg/kg bw per day, on the
basis of inhibition of erythrocyte acetyl-cholinesterase activity
(Löser, 1969a,b).
In a second three-month study with groups of 15 young SPF Wistar
rats of each sex, phoxim was administered in the diet at
concentrations of 0, 5, 15, 50, 150, or 500 mg/kg feed, equivalent to
0, 0.5, 1.5, 5, 15, or 50 mg/kg bw per day. The animals were
observed for the same end-points as in the previous study, with the
addition of histopathological examination. Cholinergic signs (muscle
twitching, cramps) were observed occasionally in animals at the high
dose, particularly in the earlier part of the study. Although
body-weight gain was significantly retarded in males at the high dose,
animals at 50 mg/kg feed showed a very similar growth curve. Hence,
the effect on body-weight gain was not consistent or dose-dependent.
In male rats, cholinesterase activity in plasma and erythrocytes
decreased by > 20% with doses from 50 mg/kg feed. In female rats,
cholinesterase activity decreased by > 20% in plasma with doses
from 15 mg/kg feed (at 1 and 13 weeks only) and in erythrocytes with
doses from 150 mg/kg feed. Liver weights were statistically
significantly increased in animals at 150 mg/kg feed (relative) and
500 mg/kg feed (relative in males and females, absolute in females),
but there were no significant changes in liver function tests. At the
high dose, the absolute and relative thyroid weights were increased in
females, and the relative adrenal weights were increased in males.
Relative kidney weights were increased at 150 mg/kg feed in females
and 500 mg/kg feed in males and females. On the basis of inhibition of
erythrocyte acetylcholinesterase activity in males (in the absence of
data on brain acetylcholinesterase activity), the NOEL was 15 mg/kg
feed, equivalent to 1.5 mg/kg bw per day (Löser, 1970a; Vince &
Spicer, 1971).
Rabbits
Groups of three male and three female New Zealand white rabbits
received dermal applications of phoxim emulsified with Cremophor EL in
water on 5 × 5 cm areas of shorn intact skin of the flank or back or
of skin abraded with sandpaper at doses of 0, 0.5, or 15 mg/kg bw per
day, 7 h/day, five days per week, for three consecutive weeks. The
treated sites were not occluded. The observations included deaths,
physical appearance, behaviour, body weight, skin irritation,
haematological parameters, clinical chemical parameters (liver
function tests), urinary parameters, gross pathological appearance,
organ weights, histopathological appearance, and determination of
plasma, erythrocyte, and brain cholinesterase activities.
Skin irritation was observed only in animals with abraded skin,
in which there was a dose-related increase in the time necessary for
the erythema to disappear. Histopathological examination of the
treated skin of one control animal with intact skin, of one animal at
the high dose with intact skin, and of four animals at the high dose
with abraded skin showed, on average, moderate cellular infiltration
of the epidermis and corium by round cells, polymorphonuclear
leukocytes, and isolated inflammatory hair follicles, and a slight
increase in the thickness of the epithelium. These findings were
considered to be the result of repeated physical irritation,
exacerbated by the compound at the highest dose. Plasma and
erythrocyte cholinesterase activities were reduced in a dose-related
manner and were inhibited by > 20% at 0.5 mg/kg bw per day in males
with abraded skin and in females with intact skin only and in all
animals at 15 mg/kg bw per day. Brain acetylcholinesterase activity
was reduced by 14% in males with abraded skin at the low dose and by
23% in all males at the high dose but was not reduced in females
(Flucke & Schilde, 1978).
Chickens
Groups of 10 white Leghorn hens were fed diets containing phoxim
at a concentration of 0, 5, or 10 mg/kg feed for 28 days, equal to
average achieved intakes of 0, 0.32, or 0.68 mg/kg bw per day. Phoxim
had no effect on behaviour, and there were no signs of neurotoxicity.
Body-weight gain and food consumption were also unaffected. At the
high dose, a marked depression (> 20% inhibition) in whole blood
cholinesterase activity was observed (Thyssen & Kimmerle, 1973).
Dogs
In a three-month study, beagle dogs were fed phoxim in the diet
at doses of 0 (three of each sex), 2, 5, or 10 mg/kg feed (two of each
sex), equivalent to 0, 0.05, 0.13, or 0.25 mg/kg bw per day. Treatment
did not affect the mortality rate, physical appearance, behaviour,
food intake, ophthalmoscopic, haematological, clinical chemical (liver
function tests), or urinary parameters, macroscopic appearance, or
organ weights. Body-weight gain appeared to be reduced in males at 5
mg/kg feed and in animals of each sex at 10 mg/kg feed; however, the
smal number of animals per group and the high intra-group variation
did not allow statistical analysis. As this effect was not observed at
higher doses in a second three-month study (see below) or in the first
three months of a two-year study with larger groups (see below), it
was discounted. Inhibition of cholinesterase activity by > 20% was
observed in the plasma of dogs of each sex at 2 mg/kg feed (after one
month only) and at 5 and 10 mg/kg feed but not in erythrocytes. Brain
acetylcholinesterase activity was not determined, and
histopathological examination was not performed. The NOEL was 10 mg/kg
feed, equivalent to 0.25 mg/kg bw per day, the highest dose tested
(Löser, 1970b).
In a second three-month study, in which histopathological
examination was performed in addition to the observations in the first
study, beagle dogs were fed phoxim in the diet at doses of 0 (three
per sex), 50, 200, or 1000 mg/kg feed (two per sex), equivalent to
0, 1.3, 5, or 25 mg/kg bw per day. Animals at the high dose showed
signs of cholinergic poisoning (cramps, especially in the abdominal
region, muscle twitching, and salivation), but none died. Food
consumption was reduced in females at 1000 mg/kg feed, which resulted
in weight loss throughout the study, and these animals appeared
emaciated upon macroscopic examination, with little adipose tissue in
the subcutaneous connective tissue or in the mesentery. Plasma
alkaline phosphatase activity was increased in animals of each sex at
200 and 1000 mg/kg feed, and plasma lactate dehydrogenase activity was
increased in males at the high dose. Ophthalmoscopic, haematological,
and urinary determinations showed no treatment-related effects. The
absolute and relative liver weights were increased in males at the
high dose, and the relative liver weight was increased in females at
the high dose, probably because of the body-weight loss, although
changes were not seen in other tissues. The absolute and relative
weights of the male gonads were increased at the two highest doses,
but as the report did not include data on individual animals or
provide any measure of intra-group variation, the statistical
significance of these findings could not be determined. In both males
and females, cholinesterase activity was decreased by > 20% in a
dose-dependent fashion in plasma at all doses and erythrocytes at 200
and 1000 mg/kg feed, from within one week. Brain acetylcholinesterase
activity was not determined. Histopathological examination revealed no
difference between control and treated animals. The NOEL was 50 mg/kg
feed, equivalent to 1.3 mg/kg bw per day, on the basis of reduced
erythrocyte acetylcholinesterase activity (in the absence of data on
brain acetylcholinesterase activity) and increased plasma alkaline
phosphatase activity (Löser, 1971; Brooks & Cherry, 1974).
Groups of four male and four female beagles were fed diets
containing phoxim at 0, 0.3, 1, or 2 mg/kg feed for three months,
equivalent to 0, 0.0075, 0.025, or 0.05 mg/kg bw per day. The animals
were observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, plasma and
erythrocyte cholinesterase activity, gross and histopathological
appearance, and organ weights. Brain acetylcholinesterase activity was
not determined. The only effect observed was reduced plasma
cholinesterase activity (> 20% inhibition) in both males and
females at 1 and 2 mg/kg feed. The NOEL was 2 mg/kg feed, equivalent
to 0.05 mg/kg bw per day, the highest dose tested (Mürmann & Luckhaus,
1973).
Monkeys
Groups of five male and five female rhesus monkeys ( Macaca
mulatta) received phoxim dissolved in corn oil by oral gavage at
doses of 0, 0.2, 0.65, or 2 mg/kg bw per day on six days per week for
six months. The observations included clinical signs, physical
appearance, behaviour, body weight, haematological, clinical chemical,
and urinary parameters, and plasma and erythrocyte cholinesterase
activity. Liver was biopsied before and at the end of treatment for
histopathological assessment. Brain acetylcholinesterase activity was
not determined. Although plasma and erythrocyte cholinesterase
activity was inhibited, there were no signs of cholinergic poisoning
in animals at doses up to 2 mg/kg bw per day. There was no evidence
from either clinical chemistry or histopathology of an effect of
phoxim on the liver. Plasma cholinesterase activity was reduced by
> 20% in a dose-dependent manner in all treated animals.
Erythrocyte acetylcholinesterase activity was slightly reduced in
animals at the highest dose, reaching > 20% inhibition only after
treatment for four months, when compared with concurrent controls but
not when compared with pre-treatment values in the same animals. In
the absence of data on brain acetylcholinesterase activity, the NOEL
was 0.65 mg/kg bw per day on the basis of slight evidence of
inhibition of erythrocyte acetylcholinesterase activity (Coulston
et al., 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity, groups of 50 male and 50 female
SPF B6C3F1 mice were fed diets containing phoxim at doses of 0, 1, 5,
150, or 450 mg/kg feed, equal to average achieved intakes of 0, 0.47,
2.4, 67, or 200 mg/kg bw per day for males and 0, 0.55, 2.7, 79, or
210 mg/kg bw per day for females, for 24 months. Ten additional
animals of each sex at each dose (satellite groups) were killed at 12
months. The animals were observed for clinical signs, death, body
weight, food and water consumption, haematological and clinical
chemical parameters (in 10 animals per group during weeks 27, 53, 79,
and 105; including plasma and erythrocyte cholinesterase activities),
organ weights, detailed macroscopic and microscopic examinations, and
brain acetylcholinesterase activity (in 10 animals of each sex per
group at the terminal kill). The study was of conventional design,
with GLP and quality assurance certification.
Clinical signs, food and water consumption, and haematological
parameters were unaffected by treatment, as was survival (70-88% of
controls, 78-90% of treated animals). Statistically significantly
increased body weights were observed in males at 150 and 450 mg/kg
feed and in females at 450 mg/kg feed. Plasma cholesterol contents
were statistically significantly increased in animals of each sex at
doses of 150 mg/kg feed and higher, from 27 weeks onwards. The total
bilirubin content was decreased in males at the high dose, while
females at this dose showed slightly but statistically significantly
increased plasma alkaline phosphatase and alanine aminostransferase
activities from one year onwards. Plasma cholinesterase activity was
decreased in animals of each sex at 5 (23-45% inhibition), 150, and
450 (94-98% inhibition) mg/kg feed. Erythrocyte acetylcholinesterase
activity was inhibited by 14-26% in males and females at 150 and 450
mg/kg feed, and brain acetylcholinesterase activity was reduced in
animals of each sex at 150 mg/kg feed (19-28%) and 450 mg/kg feed
(45-54%).
The weights and macroscopic and microscopic appearance of organs
showed little treatment-related change, except in the liver. At 450
mg/kg feed, the absolute and relative liver weights were statistically
significantly increased in all animals, and histopathological
examination showed a significant increase in the incidence of
non-neoplastic hepatic foci, mainly eosinophilic and basophilic types,
in males (8/50 versus 1/50 in controls). The absolute weights of the
heart and kidneys were significantly increased in both males and
females at the high dose, and fermales at these doses had a
significant reduction in the number of ovarian cysts. The incidences
of hepatocellular carcinoma were comparable in treated and control
groups, but the incidences of hepatocellular adenoma were
statistically significantly increased in males at 150 mg/kg feed (9/50
versus 1/50 in concurrent controls and 1-7/50 in historical controls)
and in females at 450 mg/kg feed (8/50 versus 1/50 in concurrent
controls and 0-2/50 in historical controls). This finding is probably
not biologically significant in males, as no increase was seen at the
highest dose (5/50 at both the lowest and highest doses) and the
incidence was just beyond the range of historical controls. The
increased incidence of adenomas in females at the high dose is thought
to be due to the hepatoproliferative effect of the compound. The
incidence of lymphoreticular tumours was slightly but significantly
reduced in females receiving 150 and 450 mg/kg feed. The NOEL was 5
mg/kg feed, equal to 2.4 mg/kg bw per day, on the basis of increased
plasma cholesterol content and inhibition of brain
acetylcholinesterase activity (Jäger, 1992).
Rats
In a long-term study of toxicity, groups of 50 male and 50 female
SPF Wistar rats received diets containing phoxim at concentrations of
0, 15, 75, or 375 mg/kg feed, equal to average achieved intakes of 0,
0.8, 4, or 18 mg/kg bw per day for males and 0, 1.1, 5.4, or 27 mg/kg
bw per day for females, for 24 months. The control group comprisd 100
males and 100 females. Five additional animals of each sex per group
were used for clinical chemical examinations at 3, 6, and 12 months,
and 10 males and 10 females per group were used for these examinations
at termination. The observations included physical appearance,
behaviour, deaths, body weight, food consumption, haematological,
clinical chemical (liver function tests), and urinary parameters,
plasma and erythrocyte cholinesterase activities, absolute organ
weights, macroscopic and microscopic appearance, and brain
acetylcholinesterase activity (in five animals of each sex per group).
Treatment with phoxim did not affect the physical appearance,
behaviour, survival (76-79% of controls, 74-86% of treated animals),
or haematological, urinary, or macroscopic appearance of the rats.
Food consumption was reduced by 10% in males at the high dose, while
body-weight gain was slightly reduced in females at this dose. The
results of liver function tests were normal, other than a significant
reduction in plasma glutamate dehydrogenase activity in males at the
high dose and in bilirubin content in females at the high dose. Plasma
and erythrocyte cholinesterase activities were reduced by > 20% in
a dose-dependent manner throughout the study in rats treated with 75
and 375 mg/kg feed. Inhibition by > 20% of plasma and erythrocyte
cholinesterase activities was also observed in females at 15 mg/kg
feed (at 1, 2, and 52 weeks for plasma cholinesterase; at 2 weeks for
erythrocyte acetylcholinesterase). Brain acetylcholinesterase activity
was reduced by 18% in males and 23% in females at the highest dose.
Absolute liver weights were statistically significantly increased in
all treated males, but the effect is discounted as an inverse
dose-response relationship was seen. The absolute adrenal weights
were statistically significantly decreased in a dose-related manner
at doses > 75 mg/kg feed in animals of each sex, and the absolute
weights of the heart, lung, and spleen were statistically
significantly reduced in females at the high dose. None of the changes
in organ weights was accompanied by corresponding histopathological
changes. There was no difference in tumour incidence between control
and treated animals. The NOEL was 15 mg/kg feed, equal to 0.8 mg/kg bw
per day, on the basis of changes in adrenal weights (Bomhard & Löser,
1977; Finn, 1977).
Dogs
In a long-term study of toxicity, groups of four beagles of each
sex received phoxim in the diet at concentrations of 0, 0.3/0.1, 15,
or 750 mg/kg feed for 104 weeks, equivalent to 0, 0.0075/0.0025, 0.38,
or 19 mg/kg bw per day. Because plasma cholinesterase activity was
decreased by 26% in females at week 77, although erythrocyte
acetylcholinesterase activity was not affected, their dose was reduced
from 0.3 to 0.1 mg/kg feed from week 83 onwards. The animals were
observed for deaths, physical appearance, behaviour, neuronal
reflexes, food consumption, body weight, ophthalmoscopic,
haematological, clinical chemical, and urinary parameters, gross and
histopathological appearance, organ weights, and plasma, erythrocyte,
and brain cholinesterase activity.
No treatment-related effects were observed on mortality rates,
behaviour, food consumption, reflexes, or ophthalmoscopic,
haematological, or urinary parameters. All males at the high dose and
two of four at the intermediate dose showed a poor nutritional state.
In the second year, the coats of all males at the high dose were dull,
ruffled, and ungroomed, and, although the food consumption of these
animals was unaffected by treatment, they took much longer to eat
their food ration. The body-weight gain of all treated animals, but
especially the males, was reduced in a dose-dependent manner between 0
and 104 weeks due mainly to retarded growth in the second half of the
study; the effect reached statistical significance only in males at
the high dose. Plasma alanine aminotransferase activity was
statistically significantly increased in animals at the high dose from
week 52 onwards. Plasma alkaline phosphatase activity was
statistically significantly increased in these animals throughout the
treatment period. Although it was not increased at lower doses, the
natural decline was slowed, resulting in statistically significantly
higher values than in controls in females at the intermediate dose
from week 26 onwards and in males at the two lower doses from week 52
onwards. These effects on alkaline phosphatase activity are considered
to be adaptive, and were probably due to induction, as
organophosphorus esters are natural substrates for alkaline
phosphatase. The serum cholesterol concentration was reduced in
animals at the high dose throughout treatment, reaching statistical
significance at some times. Cholinesterase activity was reduced
throughout the experiment in males and females at 15 and 750 mg/kg
feed, in plasma (60 and 80% inhibition, respectively) and in
erythrocytes (20-30 and 60-80% inhibition, respectively). The
cholinesterase activity in plasma and erythrocytes was not reduced in
males at 0.3 mg/kg feed or in females at 0.1 mg/kg feed. Brain
acetylcholinesterase activity was significantly reduced, by
approximately 37%, in animals at the high dose.
Organ weights and gross and histopathological appearance showed
little treatment-related change other than in the liver. Histological
examination revealed hypolasia in both testes of one dog at the high
dose. As there was no cryptorchism, this effect might be related to
treatment. The livers of all females and one male at 750 mg/kg feed
were darker than those of the controls, and some had a markedly
exaggerated lobular pattern. The absolute and relative weights of the
liver were statistically significantly increased in all animals at the
high dose; although the relative weight of the liver was also
increased in males at the intermediate dose, the increase was not
statistically significant. Histopathological examination showed
hepatocytic alterations in three dogs and three bitches at the high
dose, involving dilated hepatocytes with a light, glassy,
structureless cytosol. On the basis of inhibition of brain
acetylcholinesterase activity and effects on the liver, the NOEL was
15 mg/kg feed, equivalent to 0.38 mg/kg bw per day (Hoffmann &
Gröning, 1977).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with phoxim are
summarized in Table 2. Phoxim was dissolved in dimethyl sulfoxide for
testing in vitro and in aqueous 0.5% Cremophor emulsion for testing
in vivo. The only effect seen in vitro or in vivo was induction of
chromosomal aberrations in vitro in lymphocytes; however, this
finding could not be interpreted owing to the cytotoxicity seen at
concentrations that affected the chromosomes. The summary tables in an
English translation of the Russian article show that phoxim induced a
very weak, dose-related increase in the number of cells with
aberrations, but no details were provided on the exposure time,
harvesting time, or absence or presence of S9. Furthermore, the
marginally increased frequencies reached statistical significance
because the frequency in concurrent controls both in vitro and in vivo
was very low, and these frequencies may have been within the range for
historical controls, for which data were lacking.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
Phoxim was administered in the diet to four groups of 10 male and
20 female Long Evans FB 30 rats throughout mating, gestation, and
lactation in a three-generation study of reproductive toxicity at
doses of 0, 15, 75, or 375 mg/kg feed, equivalent to 0, 0.75, 3.8, and
19 mg/kg bw per day. The pups were suckled for up to four weeks,
weighed weekly, and examined grossly for malformations; the offspring
of each first mating (F1a, F2a, and F3a) were then killed. The
offspring of each second mating (F1b and F2b) were weaned and then
mated to produce two litters. After the dams of the F0, F1b, and F2b
generations had successfully nursed their offspring twice, they were
killed. Gross and histopathological examinations were performed on one
male and one female four-week-old pup of the F3b generation from each
of 10 mothers per group.
There were no differences between the control and treated parents
in any of the generations with respect to physical appearance,
behaviour, or body-weight gain, although some parents in the control
and treated groups died due to pneumonitis. No treatment-related
effects on fertility (pregnancy rate), litter size at birth or after
five days (viability index), or pup body weights at birth or during
four-week lactation were seen in any generation, and there were no
signs of malformation. The survival of pups in the F1a, F1b, F2a,
F2b, and F3a generations after four weeks' lactation was normal, but
the survival of pups in the F3b generation at 375 mg/kg feed was
slightly but statistically significantly reduced (84% versus 97% in
controls). Gross and histopathological examinations of the F3b pups
did not reveal any treatment-related alterations. The NOEL was 75
mg/kg feed, equivalent to 3.8 mg/kg bw per day, on the basis of
slightly reduced survival of pups in the second litter of the third
generation (Löser, 1979).
Table 2. Results of assays for genotoxicity with phoxim
End-point Test object Concentration Result GLP/ Reference
QA
In vitro
Reverse S. typhimurium TA98, 3.2-3200 nl/ Negativeb No Oesch
mutation TA100, TA1537 platea (+ S9) (1977)
32-3200 nl/
platea (- S9)
Reverse S. typhimurium TA98, 10-5000 µg/ Negativeb No Shirasu
mutation TA100 plate et al. (1978)
Reverse S. typhimurium TA98, < 5000 µg/ Negativeb No Moriya
mutation TA100, TA1535, plate et al. (1983)
TA1537, TA1538;
E. coli WP2 hcr
Reverse Saccharomyces 625-10 000 Negativeb Yes Herbold
mutation cerevisiae D7 µg/mlc (1985)
DNA damage Bacillus subtilis H-17, 1-100% v/v Negative No Shirasu
M-45 rec- (0.2-20 µl/disc) et al. (1978)
Chromosomal Human lymphocytes 30-300 µg/ml Positive Yes Herbold
aberration (- S9) at > 100 (1986)
50-500 µg/ml µg/ml - S9d
(+ S9)
Chromosomal Human lymphocytes 0.2 and 2 Equivocal No Kurinnyi
aberration µg/ml (1979)
Table 2. (continued)
End-point Test object Concentration Result GLP/ Reference
QA
In vivo
Micronucleus Mouse bone marrow 2 × 250 or Negativee No Herbold
formation (NMRI mice (SPF 500 mg/kg bw (1981)
Han) 5/sex per by gavage with
group) an interval of
24 h
Dominant Male NMRI mice Single dose of Negative No Machemer
lethal 500 mg/kg bw (1974)
mutation orally by gavage
Chromosomal Mouse bone marrow 1× 10 mg/kg bw Negative No Kurinnyi
aberration (noninbred white 1× 100 mg/kg bw, Negative (1979)
mice, 5 males/group) 5× 100 mg/kg bw, Equivocale
1× 250 mg/kg bw Equivocale
intragastrically
GLP, good laboratory practice; QA, quality assessment; S9, exogenous metabolic activation
system from rat liver microsomes
a At 1000 nl/plate, some test compound separated as droplets from the top agar.
b With and without S9
c No cytotoxicity up to 10 000 µg/ml
d The mitotic indices were 100, 37, and 1.9% at 30, 100, and 300 µg/ml,
respectively, in the absence of S9 and 84, 93, and 2.4% at 50, 100,
and 500 µg/ml, respectively, in the presence of S9. Significant
increases in chromosomal aberration frequency were found at 100 and
300 µg/ml in the absence of S9 and at 500 µg/ml in the presence of S9.
e The ratio of polychromatic to normochromatic erythrocytes at either
dose did not deviate from that in controls, and no clinical signs of
toxicity were noted. The doses were chosen on the basis of the results
of a preliminary test in which mice received 2 × 500 or 1000 mg/kg bw
orally and the lower dose was tolerated with induction of weak signs.
In view of this finding and the rapid and almost complete oral
absorption of phoxim by mice, it is likely that the bone marrow was
exposed.
2.2.5.2 Developmental toxicity
Rats
In a study of developmental toxicity, groups of 20-21 pregnant
Long Evans FB 30 rats received phoxim in 0.5% aqueous Cremophor
emulsion by oral gavage at daily doses of 0, 30, 100, or 300 mg/kg bw
on days 6-15 of gestation. On gestation day 20, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal malformations. None of the dams died
during the study, and no adverse effects were seen on their behaviour
or general appearance. The body-weight gain of dams at the high dose
was statistically significantly decreased during treatment but not
when considered over the entire gestation period. There were no
evident effects on embryonic or fetal development, and there was no
indication of teratogenicity up to the highest dose tested. The NOEL
for maternal toxicity was 100 mg/kg bw per day on the basis of the
decrease in body-weight gain. The NOEL for developmental toxicity was
300 mg/kg bw per day, the highest dose tested (Machemer, 1975).
Rabbits
In a study of developmental toxicity, groups of 20 pregnant
Chinchilla rabbits received phoxim in water with 2%
carboxymethylcellulose by oral gavage at daily doses of 0, 12, 36, or
72 mg/kg bw on days 6-18 of gestation. On gestation day 28, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external, visceral, and skeletal abnormalities. The study
was of conventional design, with quality assurance certification. No
treatment-related increase in mortality rate was observed. Clinical
signs of toxicity (diarrhoea, salivation, ventral body position,
dyspnoea, and abortion) were observed in dams at the high dose, and
phoxim caused reductions in food consumption and body-weight gain of
dams during treatment at 72 mg/kg bw. The only effects on fetuses were
an increased embryonic resorption rate and decreased fetal body
weights at the highest dose. There was no indication of teratogenicity
up to the highest dose tested. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of a decrease in food consumption and
body-weight gain. The NOEL for developmental toxicity was 36 mg/kg bw
per day on the basis of decreased fetal body weight and an increased
rate of embryonic resorptions (Becker, 1982).
2.2.6 Special studies on delayed neurotoxicity
Hens
In a study of delayed neurotoxicity, of which only a summary was
available, groups of one to five white Leghorn hens received a single
oral or intraperitoneal dose of phoxim dissolved in polyethylene
glycol, without an antidote. The oral doses used were 25, 38, 50, 250,
500, or 1000 mg/kg bw, and the intraperitoneal doses were 25, 38, or
50 mg/kg bw. In a second experiment, hens were given intraperitoneal
injections of 100 mg/kg bw pralidoxime iodide and 50 mg/kg bw atropine
sulfate before administration of phoxim at single oral doses of 38,
50, or 75 mg/kg bw (groups of 7-11 hens) or at single intraperitoneal
doses of 50, 75, 100, 150, or 200 mg/kg bw (groups of 1-14 hens). In
both studies, the birds were observed for 42 days; no
histopathological examinations were carried out, and a positive
control group was not included. No clinical signs of delayed
neurotoxicity were observed either with or without antidotal
protection. A LD50 of 38 mg/kg bw was derived for both oral and
intraperitoneal administration in the first experiment. The protective
effect of the antidote was more marked when phoxim was administered
intraperitoneally than when it was given orally (Kimmerle, 1972).
In another study of delayed neurotoxicity, 30 white Leghorn hens
received phoxim emulsified in water and 2% Cremophor EL by oral gavage
at a dose of 50 mg/kg bw, twice, at an interval of 21 days. Because
this dose was greater than the LD50, phoxim was administered with
atropine sulfate, administered intramuscularly at a dose of 50 mg/kg
bw. A negative control group of six hens received the vehicle and
atropine sulfate by the same schedule, and a positive control group of
five hens received a single dose of 375 mg/kg bw
tri- ortho-cresylphosphate in peanut oil. The hens treated with phoxim
and atropine and the negative controls were observed for 42 days and
the positive controls for 24 days. During the observation period, the
birds were inspected for clinical signs, body weight, and coordination
of movement. At the end of the observation period, they were killed
and nervous tissue (brain, spinal cord, and sciatic nerves) was
removed from six birds treated with phoxim and from all negative and
positive controls and examined histopathologically. The design of the
study closely resembled OECD guideline 418 in use in 1984, with GLP
certification.
After treatment with phoxim and atropine, the signs of toxicity
were staggering gait, prostration, apathy, salivation, drooping wings,
and fluffed plumage. From observation day 5 onwards, the only sign of
toxicity was staggering gait, while from observation day 26 onwards
the hens showed no abnormal signs or behaviour. The negative control
group showed only staggering gate. The positive control group showed
transient staggering gate in the acute phase, progressive disturbance
of coordination of movement from observation day 8 onwards, and severe
paralysis in the final stage which was of sufficient severity that the
birds were killed prematurely at observation day 24. Histopathological
examination showed no sign of peripheral neuropathy or demyelination
in phoxim-treated birds, whereas hens given tri- ortho-cresylphosphate
showed various degrees of degeneration of peripheral nerves including
demyelination, axonal lysis, ballooned fibre segments, and activated
Schwann cells. Thus, at a dose that would be lethal in the absence of
antidote, phoxim did not induce delayed neurotoxicity in hens (Pauluhn
& Kaliner, 1984).
2.2.7 Studies with metabolites
The oral LD50 of cyanobenzaldoxime (technically pure substance
formulated in lutrol) was 4500 mg/kg bw in male and 4100 mg/kg bw in
female rats. The signs of acute oral toxicity comprised slight to
moderate disturbance of behaviour, no grooming of coats, sedation, and
dyspnoea. Dermal application of 5000 mg/kg bw had no effect (Thyssen &
Kimmerle, 1976). The oral LD50 of the glucuronide of
cyanobenzaldoxime (formulated in aqueous Cremophor EL) in three female
rats was > 2500 mg/kg bw (Mihail, 1979). The oral LD50 value for
PO-phoxim in mice was approximately 800 mg/kg bw for the pure
substance (Kimmerle, 1969) and 1000 mg/kg bw for the pure substance
dissolved in olive oil (Vinopal & Fukuto, 1971) were determined.
In a short summary of a study on dermal irritation, 0.5 g of
cyanobenz-aldoxime applied under a plaster dressing for 24 h to the
ear did not irritate the skin of hairless rabbits. In a short summary
of a study on ocular irritation, cyanobenzaldoxime at a dose of 50 mg
placed in the conjuctival sac of the left eye of rabbits caused severe
redness and swelling of the conjunctivae, slight redness, swelling of
the iris, and diffuse opacity of the cornea (Thyssen & Kimmerle,
1976).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term, and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
delayed neurotoxicity of phoxim. Although most of the studies did not
meet current standards for study protocol and conduct, they did
provide satisfactory information for evaluation of the safety of the
compound.
After oral administration of radiolabelled phoxim to mice, rats,
and pigs, the radiolabel was rapidly and almost completely absorbed
and was rapidly taken up into the major organs and tissues. The main
route of elimination in mice, rats, and pigs was the urine, while
faecal excretion was of minor importance. In rats, there was also some
evidence of biliary excreation. In rats and pigs, elimination was
rapid (> 80% via the urine within 24 h) and was virtually complete
with three to four days. In mice, elimination of radiolabel was
somewhat slower, appraently because of retention of phosphate-derived
metabolites in the urinary blader. Although there were some
qualitative and quantitative differences in metabolism between mice,
rats, and pigs, the main routes of metabolism were the same, involving
de-ethylation, hydrolysis of the phosphorus ester bond (either before
or after oxidative desulfuration to yield PO-phoxim), and conjugation
of the resulting cyanobenzaldoxime. Despite the presence of products
that were assumed to have arisen from PO-phoxim, no PO-phoxim itself
was found in mice, rats, or pigs. Hence, if formed, it must be a very
short-lived intermediate in mammals.
Animal species differ in their sensitivity to single oral doses
of phoxim. The LD50 values ranged from 20 to 40 mg/kg bw in chickens,
from 250 to > 1200 mg/kg bw in guinea-pigs, rabbits, cats, and dogs,
and from 1200 to 10 000 mg/kg bw in mice and rats. Thus, phoxim is of
only low or moderate acute oral toxicity in mammalian species.
The short-term toxicity of phoxim was evaluated after oral
administration to mice, rats, dogs, and rhesus monkeys. In two dose
range-finding studies of six and eight weeks' duration, phoxim was
administered to mice in the feed at concentrations of 0.5-750 mg/kg
(equal to 0.28-510 mg/kg bw per day). In these studies, phoxim had
effects on the liver (increased weight and hepatocyte alterations
indicative of an adaptive response) at concentrations of 30 mg/kg of
feed and above and on the kidney (increased weight) at doses of 150
mg/kg of feed and above. Inhibition of plasma cholinesterase activity
was observed at and above concentrations of 5 m/kg of feed, while
inhibition of erythrocyte and brain acetylcholinesterase activity was
observed only at 750 mg/kg of feed. On the basis of a slight increase
in liver weight in female mice, the overall NOEL in these studies was
5 mg/kg of feed, equal to 3.1 mg/kg bw per day.
Studies were carried out in rats which received doses of 2-50
mg/kg bw per day by gavage for 21 or 30 days or in two studioes at
4-500 mg/kg of feed in the diet, equivalent to 0.4-50 mg/kg bw per
day, for three months. In the studies in which phoxim was given by
gavage, cholinesterase activity in plasma, erythrocytes, and brain was
inhibited by > 20% at doses of 2, 5, and 50 mg/kg bw per day,
respectively. In the two studies in which phoxim was administered in
the diet, signs of cholinergic poisoning were observed at 500 mg/kg of
feed, organ weight changes at doses > 120 mg/kg of feed, and
inhibition of cholinesterase activity in plasma at doses > 15 mg/kg
of feed and in erythrocytes at > 40 mg/kg of feed. The overall NOEL
in the studies involving dietary administration was 12 mg/kg of feed,
equivalent to 1.2 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholines-terase activity (in the absence of data on
brain acetylcholinesterase activity).
Three studies of three months' duration in dogs treated in the
diet were reported, in which phoxim was administered at doses ranging
from 0.3 to 1000 mg/kg of feed, equivalent to 0.0075-25 mg/kg bw per
day. Doses of 200 and 1000 mg/kg of feed resulted in changes in the
weights of gonads and liver and in the activities of alkaline
phosphatase and lactate dehydrogenase in plasma, inhibition of
erythrocyte acetylcholinesterase activity, and/or weight loss (females
only) and signs of cholinergic poisoning. Inhibition of plasma
cholinesterase activity was observed at doses from 1 to 1000 mg/kg of
feed. Brain acetylcholinesterase activity was not determined in these
studies. The overall NOEL was 50 mg/kg of feed, equivalent to 1.3
mg/kg bw per day, on the basis of inhibition of erythrocyte
acetyl-cholinesterase activity (in the absence of data on brain
acetylcholinesterase activity) and increased plasma alkalaine
phosphatase activity.
Rhesus monkeys received phoxim by gavage for six months at a dose
of 0.2, 0.65, or 3 mg/kg bw per day. Apart from marked inhibition of
plasma cholinesterase activity at doses > 0.2 mg/kg bw per day and
very slight inhibition of erythrocyte acetylcholinesterase activity at
2 mg/kg bw per day, phoxim had no effect. In the absence of data on
brain acetylcholinesterase activity, the NOEL was 0.65 mg/kg bw per
day on the basis of slight inhibition of erythrocyte
acetylcholinesterase activity.
In a 24-month study of carcinogenicity, phoxim was administered
in the diet at concentrations of 1-450 mg/kg of feed (equal to
0.47-210 mg/kg bw per day) to mice of a strain known to be highly
susceptible to the development of liver tumours. Plasma, erythrocyte,
and brain cholinesterase activities were decreased at doses > 150
mg/kg of feed. The body weight of males were increased at 150 mg/kg of
feed and the body weights of both males and females at 450 mg/kg of
feed. Signs of effects on the liver (including changes in weight, in
plasma cholesterol and total bilirubin levels, and in plasma alanine
aminotransferase and alkaline phosphatase activities) were evident at
450 mg/kg of feed. At this dose, there were also increased incidences
of non-neoplastic histological changes in the livers of males and of
hepatocellular adenomas in females. At 150 mg/kg of feed, the only
effect on the liver was increased plasma cholesterol concentrations in
animals of each sex. The NOEL was 5 mg/kg of feed, equal to 2.4 mg/kg
bw per day, on the basis of increased plasma cholesterol
concentrations and inhibition of brain acetylcholinesterase activity.
The increase in the incidence of adenomas observed in females at the
highest dose was thought to be a consequence of the
hepatoproliferative effect of the compound.
The long-term toxicity of phoxim was evaluated in rats and dogs
by oral administration for 24 months at 15-375 mg/kg of feed (equal to
0.8-27 mg/kg bw per day) to rats and 0.1-750 mg/kg of feed (equivalent
to 0.0025-19 mg/kg bw per day) to dogs. In rats dosed at 375 mg/kg of
feed, reductions were seen in food intake in males, in body weight in
females, and in plasma glutamate dehydrogenase activity, total
bilirubin level, the weights of the heart, lungs, spleen, and
adrenals, and plasma, erythrocyte, and brain cholinesterase activity
in animals of each sex. Decreased plasma and erythrocyte
cholinesterase activity and adrenal weights were also observed in rats
at 75 mg/kg of feed. There were no histopathological differences
between control and treated animals, nor was there any difference in
the incidence of tumours. The NOEL was 15 mg/kg of feed, equal to 0.8
mg/kg bw per day, on the basis of lowered adrenal weights at higher
doses.
Male and female dogs given phoxim at 750 mg/kg of feed had liver
damage, as shown by increased liver weights and plasma alanine
aminotransferase and alkaline phosphatase activities, decreased serum
cholesterol level, and histopathological alterations to hepatocytes.
Plasma, erythrocyte, and brain cholinesterase activities were also
reduced in animals of each sex at this dose. In addition, male dogs
showed clinical signs of toxicity and decreased body-weight gain.
Reductions in plasma and erythrocyte cholinesterase activities were
observed in male and female dogs at 15 mg/kg of feed. The NOEL was 15
mg/kg of feed, equivalent to 0.38 mg/kg bw per day, on the basis of
effects on the liver and inhibition of brain acetylcholinesterase
activity.
Phoxim has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium and Saccharomyces
cerevisiae, DNA damage in Bacillus subtilis, and chromosomal
aberrations in human lymphocytes. It has also been tested
in vivo for its ability to induce micronucleus formation,
chromosomal aberrations, and dominant lethal mutations in mice.
Cytogenetic alterations were found in human lymphocytes in vitro at
a cytotoxic dose in the absence of exogenous metabolic activation. The
results of all other tests were negative. On the basis of these data
and the results of the long-term assays in rodents, the Committee
concluded that phoxim is not genotoxici and is unlikely to have
carcinogenic potential in humans.
In a three-generation study of reproductive toxicity, rats were
given phoxim in the diet at a concentration of 0, 15, 75, or 375 mg/kg
of feed (equivalent to 0, 0.75, 3.8, and 19 mg/kg bw per day). The
only effect observed was a slight reduction in the number of pups in
the second litter of the third generation thaqt survived after four
weeks' lactation from dams receiving 375 mg/kg of feed. On the basis
of this effect, the NOEL was 75 mg/kg of feed, equivalent to 3.8 mg/kg
bw per day.
In a study of developmental toxicity in rats given a dose of 0,
30, 100, or 300 mg/kg bw per day orally on days 6-15 of gestation,
phoxim was toxic to the dams, retarding body-weight gain during
treatment at the highest dose. It was not embryotoxic, fetotoxic, or
teratogenic at any dose. The NOEL for maternal toxicity was 100 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 300 mg/kg bw per day, the highest dose
tested.
A study of developmental toxicity was conducted in rabbits given
phoxim at a dose of 0, 12, 36, or 72 mg/kg bw per day orally on days
6-18 of gestation. At the highest dose, phoxim increased the rate of
embryonic resorption, decreased fetal body weights, and was toxic to
the dams, which showed signs of toxicity a marked decreases in food
consumption and in body-weight gain. There was no indication of
teratogenicity at any dose. The NOEL for maternal toxicity was 36
mg/kg bw per day on the basis of reduced food consumption and
body-weight gain. The NOEL for developmental toxicity was also 36
mg/kg bw per day, on the basis of decreased fetal body weight and an
increased rate of embryonic resorption.
In a study of delayed neurotoxicity, hens protected by the
antidote atropine received phoxim orally at a dose of 50 mg/kg bw,
which was repeated after 21 days. The birds were then observed for 42
days. They showed only transient signs of toxicity and no abnormal
signs or behaviour from day 26 after the second dose. At the end of
the study, no paralysis was present, and no histological evidence of
peripheral neuropathy or demyelination was observed. The Committee
concluded that phoxim does not induce delayed neurotoxicity in hens.
Although the effect of phoxim on the enzyme neuropathy target esterase
has not been investigated in hens, the Committee concluded that such a
study was not necessary in view of the negative results obtained in
adequately conducted assessments of the capacity of phoxim to induce
delayed neuropathy.
4. EVALUATION
The Committee established an ADI of 0-4 µg/kg bw for phoxim on
the basis of the NOEL of 0.38 mg/kg bw per day for effects on the
liver and inhibition of brain acetylcholinesterase activity in the
two-year study of toxicity in dogs and a safety factor of 100. This
ADI differs from that established by the Joint FAO/WHO Meeting on
Pesticide Residues, as the Expert Committee concluded that inhibition
of plasma cholinesterase activity is not a relevant end-point for risk
assessment. The Joint Meeting is now of a similar opinion (FAO/WHO,
1988).
5. REFERENCES
Becker, H. (1982) Embryotoxicity and teratogenicity study on SRA 7502
in rabbits. Unpublished report (project no. 004972, report no. R2354)
from Research & Consulting Company Ltd, Itingen, Switzerland, for
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomann, W. (1992) Volaton VL 80 (c.n.: phoxim). Studies on acute
dermal toxicity in rats. Unpublished report (study no. T 0040187,
report no. 21065) from Bayer AG, Fachbereich Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Bomhard, E. & Löser, E. (1977) SRA 7502. Chronic toxicology studies in
rats (feeding experiment over 2 years). Unpublished report (no. 7042)
from Bayer AG, Institute for Toxicology, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Brooks, P.N. & Cherry, C.P. (1974) Pathology report of Bay 77488.
Sub-chronic toxicological studies in dogs (3-month feeding
experiment). Addendum to report no. 2579. Unpublished report (no.
FBA130/73959) from Huntingdon Research Centre, Huntingdon, United
Kingdom, for Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F., Griffin, T. & Talley, W. (1978) A safety evaluation
study of phoxim in rhesus monkeys. Unpublished report from Institute
of Comparative and Human Toxicology, Albany, New York & International
Center of Environmental Safety, Holloman, New Mexico, United States.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Daniel, J.W., McLean, J. & Pringuer, M. (1978a)
Phoxim--Biotransformation in the rat. Unpublished report (no.
78/BAG6/317) from Life Science Research, Stock, Essex, United Kingdom
for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Daniel, J.W., Swanson, S. & McLean, J. (1978b) Phoxim:
Pharmacokinetics and biotransformation in the rat. Unpublished report
(no. 78/BAG5/194) from Life Science Research, Stock, Essex, United
Kingdom, for Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
FAO/WHO (1983) Pesticide Residues in Food--1982. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 46).
FAO/WHO (1985) Pesticide Residues in Food--1984. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 62).
FAO/WHO (1988) Pesticide Residues in Food--1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and a WHO Expert Group on Pesticide Residues. Rome,
Food and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper, No. 92).
Finn, J.P. (1977) Chronic toxicity study on rats with SRA 7502 (two
year feeding study). Unpublished report (no. 612/262/2) from Hazleton
Laboratories Europe Ltd, Harrogate, United Kingdom, for Bayer AG,
Institut für Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1978a) Determination of acute toxicity (LD50)--SRA 7502.
Unpublished letter report (dated 19 May 1978) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Flucke, W. (1978b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 26 June 1978) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979a) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 9 March 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1979b) Determination of acute toxicity (LD50)--SRA 7502
VL. Unpublished letter report (dated 17 April 1979) from Bayer,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity (LD50)--SRA 7502
Volaton VL. Unpublished letter report (dated 14 March 1980) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1984a) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in guinea pigs [in German]. Unpublished report
(study no. T 6016487, report no. 13071) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Flucke, W. (1984b) SRA 7502 (c.n.: phoxim). Determination of skin
sensitising potential in an open epicutaneous test with guinea pigs
[in German]. Unpublished report (study no. T 6016630, report no.
13086) from Bayer AG, Institut für Toxikiologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1978) Volaton VL. Subacute cutaneous
toxicity study with rabbits. Unpublished report (report no. 8034) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Thyssen, J. (1979) Volaton (SRA 7502) active ingredient
presolution. Acute toxicity studies. Unpublished report (no. 8081)
from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gyrd-Hansen, N., Brimer, L. & Rasmussen, F. (1993) Percutaneous
absorption of organophosphorus insecticides in pigs--The influence of
different vehicles. J. Vet. Pharmacol. Ther., 16, 174-180.
Heimann, K.-G. (1982a) Determination of acute toxicity (LD50)--
Volaton VL. Unpublished letter report (dated 16 February 1982) from
Bayer, Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.-G. (1982b) Determination of acute toxicity (LD50)--
Volaton VL 80. Unpublished letter report (dated 3 June 1982) from
Bayer, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) SRA 7502. Phoxim, Baythion(R)-active ingredient,
Volaton(R)-active ingredient. Micronucleus test on mouse to evaluate
SRA 7502 for mutagenic potential. Unpublished report (study no. T
5010077, report no. 9891) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985) SRA 7502 c.n. Phoxim. Test on S. cerevisiae D7
investigating for point mutagenic effect. Unpublished report (study
no. T 2018201, report no. 13708) from Bayer AG, Institute for
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1986) SRA 7502 (c.n. phoxim). Cytogenetic study with
human lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report (study no. T 9021736, report no.
15148) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1977) SRA 7502. Chronic toxicity study on
dogs (two-year feeding experiment). Unpublished report (no. 6865) from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989a) SRA 7502. Preliminary study on
mice (administration in feed to determine dosage for a long-term study
on carcinogenicity in mice). Unpublished report (study no. T1022043,
report no. 17610) from Bayer AG, Fachbereich Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ivens-Kohl, I. & Hahnemann, S. (1989b) SRA 7502. Preliminary study on
mice (administration in feed for 8 weeks to determine dose for a
long-term study). Unpublished report (study no. T9022799, report no.
17611) from Bayer AG, Fachbereich Toxikologie, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jäger, R. (1992) SRA 7502 (common name: Phoxim). Study for
oncogenicity in B6C3F1 mice (twenty-four month feeding study).
Unpublished report (study no. T 2022297, report no. 21624) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1968) Bay 77488. Toxicological studies. Unpublished
report (no. 1067) from Farbenfabriken Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1969) Supplement to the toxicology of SRA 7502.
Unpublished letter report (dated 27 June 1969) from Bayer AG,
Institute of Toxicology, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972) Phoxim: Tests for neurotoxicity in chickens.
Unpublished report (dated 21 June 1972) from Farbenfabriken Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1973) SRA 7502, SRA 7660, LOW 6599, SIR 5126, HOX 3082,
malathion. Comparative toxicological studies in rats with repeated
oral administration. Unpublished report (no. 3962) from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. (1970) Bay 77 488. Toxicological studies.
Unpublished report (no. 2235) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. & Weber, H. (1988) [Phenyl-U-14-C]-Phoxim: Biokinetic
behaviour and biotransformation in the edible tissues of the pig after
oral administration. Unpublished report (study no. M 183 0138-4,
PF-report no. 3077) from Bayer AG, Institute for Metabolism Research,
Leverkusen/Monheim, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kurinnyi, A.I. (1979) Cytogenetic activity of the pesticide valexon
and its influence on the mutability of mouse bone marrow cells.
Tsitol. Genet., 13, 370-374 (English translation). Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lorke, D. & Kimmerle G. (1965) Toxikologische Untersuchungen mit dem
Wirkstoff SRA 7502. Unpublished report (dated 14 December 1965) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969a) Bay 77 488. Subchronic toxicology studies in rats
(study over 3 months). Unpublished report (no. 1205) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1969b) Bay 77 488. Subchronic toxicology studies with rats.
Animal and organ weights (Individual values for report no. 1205).
Unpublished report (no. 1215) from Farbenfabriken Bayer AG, Institut
für Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Löser, E. (1970a) Bay 77 488. Subchronic toxicological studies on rats
(three-month feeding experiment). Unpublished report (no. 2389) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1970b) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2418) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1971) Bay 77488. Subchronic toxicological studies on dogs
(three-month feeding experiment). Unpublished report (no. 2579) from
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1979) SRA 7502. Multigeneration reproduction study on rats.
Unpublished report (no. 8447) from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1974) SRA 7502 (phoxim). Dominant lethal test on male
mouse to evaluate SRA 7502 for mutagenic potential. Unpublished report
(no. 4943) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Machemer, L. (1975) SRA 7502 (phoxim). Studies for embryotoxic and
teratogenic effects on rats following oral administration. Unpublished
report (no. 5331) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Märtins, T. (1992) Volaton VL 80 (c.n.: phoxim). Acute inhalation
toxicity in rats in compliance with OECD guideline no. 403.
Unpublished report (study no. T 2040765, report no. 21429) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mihail, F. (1979) Cyanoxim acid glucoside (metabolite of phoxim).
Unpublished letter report (dated 4 July 1979) from Bayer AG, Institute
of Toxicology, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1981) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 4 May 1981) from Bayer, Institute of
Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1982) Determination of acute toxicity (LD50)--Volaton VL.
Unpublished letter report (dated 1 March 1982) from Bayer, Institute
of Toxicology, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Moriya, M., Ohta, T., Watanabe K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Mürmann, P. & Luckhaus, G. (1973) SRA 7502. Subchronic toxicity study
on dogs (three-month feeding experiment). Unpublished report (no.
4136) from Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Oesch, F. (1977) Ames test for Volaton (phoxim). Unpublished report
(dated 30 November 1977) from Pharmakologisches Institut, Universität
Mainz, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1983) SRA 7502 (active ingredient of Baythion(R) and
Volaton(R)) (common name: phoxim). Acute oral toxicity study on
hens. Unpublished report (study no. T 3006197, report no. 11978) from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. & Kaliner, G. (1984) SRA 7502 (c.n.: phoxim). Study for
acute neurotoxicity in hens. Unpublished report (study no. T 3016385,
report no. 12632) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Watanabe, T. (1978) Phoxim. Mutagenicity
test on bacterial systems. Unpublished report (dated 18 July 1978)
from Institute of Environmental Toxicology, Japan. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1976) SRA 7502. Subacute oral cumulation study on rats.
Unpublished report (no. 5954) from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1973) SRA 7502. Toxicological studies on
hens. Unpublished report (no. 4236) from Bayer AG, Institut für
Toxikologie, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) Cyanoxim
(hydroxiiminophenylacetonitrile). Occupational toxicological study.
Unpublished report (no. 6392) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vince, A.A. & Spicer E.J.F. (1971) Pathology report on Bay 77 488.
Subchronic toxicological studies in rats (3 month feeding experiment).
Addendum to report no. 2389. Unpublished report (no. 4257/71/415) from
Huntingdon Research Centre, Huntingdon, United Kingdom, for
Farbenfabriken Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Vinopal, J.H. & Fukuto, T.R. (1971) Selective toxicity of phoxim
(phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate). Pestic.
Biochem. Physiol., 1, 44-60.
