
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
METHYL SALICYLATE
Synonym Wintergreen oil
Chemical name Methyl salicylate
Empirical formula C8H8O3
Structural formula
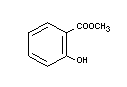 Molecular weight 152.15
Definition Methyl salicylate contains not less than
98 per cent. C8H8O3.
Description The volatile oil obtained by maceration
and subsequent steam distillation from
the leaves of Gaultheria procumbens
Linné (Fam. Ericaceae), or from the
bark of Betula lenta Linné (Fam.
Betulaceae). It is also produced
synthetically.
It is a colourless, yellowish or reddish
liquid, having the characteristic odour
and taste of wintergreen.
Biological Data
Biochemical aspects
The orally administered ester undergoes very rapid and nearly
complete hydrolysis in rats and dogs, salicylate being the only
significant finding in the blood of rats 20 minutes after an
intragastric dose, and in dogs within an hour of oral dosing. In man,
hydrolysis is somewhat slower after oral administration, some 21 per
cent. of salicylate as the ester being present in plasma after 90
minutes. The liver appears to be the main site of hydrolysis in rats,
rabbits, dogs and monkeys, the extent varying from 79-99 per cent.,
while plasma hydrolyzes some 15 per cent. (Davison et al., 1961). Dogs
excrete 0.2-0.5 per cent. of the ester in their urine after oral
administration and 14 per cent. after i.m. administration. Rabbits
excrete it mainly as glucuronate (12-55 per cent.); also as ethereal
sulfate (10 per cent.), conjugated before hydrolysis, and as other
metabolites, e.g. salicyluric acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 1110 Davison et al., 1961
Rat oral 887 Jenner et al., 1964
Rat oral 1250 Giroux et al., 1954
Guinea-pig oral 700 (MLD) Houghton, 1905
Guinea-pig oral 1060 Jenner et al., 1964
Guinea-pig s.c. 1500(MLD) Houghton, 1905
Rabbit oral 1300 Castagnou et al., 1952
Rabbit oral 2800 Leone, 1916
Dog oral 2100 Leone, 1916
The adult human oral LD50 is estimated at 0.5 g/kg body-weight
(Patty, 1963). The ester is potentially the most toxic salicylate for
infants and children, as 4 ml can be fatal (Davison et al., 1961;
Jacobziner, 1963).
Short-term studies
Rat. Groups of 10 males and 10 females were fed 0, 0.1 and 1.0
per cent. in their diet for 17 weeks. Both sexes showed a significant
reduction in growth rate at the 1.0 per cent. level, but histological
examination of the major organs revealed no abnormality. In another
experiment on 3 male and 3 female rats kept on a diet containing 2 per
cent. ester for up to 10 weeks, bone growth was specially studied by
X-ray and histology. Growth was reduced; there was excessive density
of bone with very reduced chondroclastic and osteoclastic activity
(Webb & Hansen, 1963). These together with decreased food intake, poor
weight gain and high mortality, were confirmed in other groups of rats
fed 1.12 per cent. and 2.0 per cent. ester in their diet for 10 weeks
(Harrison et al., 1963).
Dog. Groups of 1 male and 1 female were fed 50, 100, 250, 500,
800 and 1200 mg/kg body-weight/day of ester for up to 10 weeks. No
adverse effects were noted at levels including 250 mg/kg/day, but
there was increasing dose-dependent fatty metamorphosis of the liver
at higher test levels (Webb & Hansen, 1964).
In a 2-year study, groups of 2 males and 2 females were given 0,
50, 150, and 250 mg/kg body-weight/day of ester orally. Some growth
retardation. and liver enlargement was noted at the 150 and 250 mg/kg
level, and histology revealed enlarged hepatic parenchymal cells
(Webb & Hansen, 1963).
Long-term studies
Rat. Groups of 25 males and 25 females were kept for 2 years on
diets containing 0, 0.1, 0,5, 1.0 and 2.0 per cent. ester. Animals did
not survive 49 weeks at the highest level. At the 1.0 per cent. level,
growth rates were considerably reduced and enlargement of male testes
and female hearts and kidneys were noted. Excess cancellous bone
formation was seen at the 2.0, 1.0 and 0.5 per cent. levels (Webb &
Hansen, 1963). Another 2-year feeding study revealed no adverse
effects, including bone changes, up to a level of 0.21 per cent. ester
in the diet (Packman et al., 1961).
Comments
Man appears to handle methyl salicylate metabolically similarly
to other animals although hydrolysis is comparatively slow. Long-term
studies on rats and the 2-year study on dogs are adequate for the
establishment of an acceptable daily intake. However, in view of some
inconsistencies in the reported studies, the following additional work
is desirable: short-term studies in dogs, including, levels of 100 and
150 mg/kg body-weight/day, and reproduction studies in rats.
EVALUATION
Level causing no toxicological effect
Rat. 0.2 per cent. (= 2000 ppm) of the diet, equivalent to 100
mg/kg/day.
Dog. 50 mg/kg/day
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.5
REFERENCES
Castagnou, R., Larceban, S. & Queynent, A. (1952) Bull. Soc. Pharm.
Marseille 3, 30
Davison, C., Zimmerman, E. F. & Smith, P. K. (1961) J. Pharm. Exp.
Therap., 132, 207
Ciroux, J., Granger, R. & Monnier, P. (1954) Trav. Soc. Pharm.
Montpellier, 14, 383
Harrison, J. W. E., Abbott, D. D. & Packman, E. W. (1963) Fed.
Proc., 22, 554
Houghton, E. M. (1905) Amer. J. Physiol., 13, 331
Jacobziner, H. (1963) New York State J. Med., 63, 295
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Leone, G. (1916), Arch. farm. sper., 22, 327
Packman, E. W., Abbott, D. D., Wagner, B. M. & Harrison, J. W. E.
(1961) Pharmacologist 3, 62
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Webb, W. K. & Hansen, W. H. (1963) Toxicol. appl. Pharmacol., 5,
576
Williams, R. T. (1959) Detoxication Mechanism, Second Edition, Chapman
& Hall, London
Molecular weight 152.15
Definition Methyl salicylate contains not less than
98 per cent. C8H8O3.
Description The volatile oil obtained by maceration
and subsequent steam distillation from
the leaves of Gaultheria procumbens
Linné (Fam. Ericaceae), or from the
bark of Betula lenta Linné (Fam.
Betulaceae). It is also produced
synthetically.
It is a colourless, yellowish or reddish
liquid, having the characteristic odour
and taste of wintergreen.
Biological Data
Biochemical aspects
The orally administered ester undergoes very rapid and nearly
complete hydrolysis in rats and dogs, salicylate being the only
significant finding in the blood of rats 20 minutes after an
intragastric dose, and in dogs within an hour of oral dosing. In man,
hydrolysis is somewhat slower after oral administration, some 21 per
cent. of salicylate as the ester being present in plasma after 90
minutes. The liver appears to be the main site of hydrolysis in rats,
rabbits, dogs and monkeys, the extent varying from 79-99 per cent.,
while plasma hydrolyzes some 15 per cent. (Davison et al., 1961). Dogs
excrete 0.2-0.5 per cent. of the ester in their urine after oral
administration and 14 per cent. after i.m. administration. Rabbits
excrete it mainly as glucuronate (12-55 per cent.); also as ethereal
sulfate (10 per cent.), conjugated before hydrolysis, and as other
metabolites, e.g. salicyluric acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 1110 Davison et al., 1961
Rat oral 887 Jenner et al., 1964
Rat oral 1250 Giroux et al., 1954
Guinea-pig oral 700 (MLD) Houghton, 1905
Guinea-pig oral 1060 Jenner et al., 1964
Guinea-pig s.c. 1500(MLD) Houghton, 1905
Rabbit oral 1300 Castagnou et al., 1952
Rabbit oral 2800 Leone, 1916
Dog oral 2100 Leone, 1916
The adult human oral LD50 is estimated at 0.5 g/kg body-weight
(Patty, 1963). The ester is potentially the most toxic salicylate for
infants and children, as 4 ml can be fatal (Davison et al., 1961;
Jacobziner, 1963).
Short-term studies
Rat. Groups of 10 males and 10 females were fed 0, 0.1 and 1.0
per cent. in their diet for 17 weeks. Both sexes showed a significant
reduction in growth rate at the 1.0 per cent. level, but histological
examination of the major organs revealed no abnormality. In another
experiment on 3 male and 3 female rats kept on a diet containing 2 per
cent. ester for up to 10 weeks, bone growth was specially studied by
X-ray and histology. Growth was reduced; there was excessive density
of bone with very reduced chondroclastic and osteoclastic activity
(Webb & Hansen, 1963). These together with decreased food intake, poor
weight gain and high mortality, were confirmed in other groups of rats
fed 1.12 per cent. and 2.0 per cent. ester in their diet for 10 weeks
(Harrison et al., 1963).
Dog. Groups of 1 male and 1 female were fed 50, 100, 250, 500,
800 and 1200 mg/kg body-weight/day of ester for up to 10 weeks. No
adverse effects were noted at levels including 250 mg/kg/day, but
there was increasing dose-dependent fatty metamorphosis of the liver
at higher test levels (Webb & Hansen, 1964).
In a 2-year study, groups of 2 males and 2 females were given 0,
50, 150, and 250 mg/kg body-weight/day of ester orally. Some growth
retardation. and liver enlargement was noted at the 150 and 250 mg/kg
level, and histology revealed enlarged hepatic parenchymal cells
(Webb & Hansen, 1963).
Long-term studies
Rat. Groups of 25 males and 25 females were kept for 2 years on
diets containing 0, 0.1, 0,5, 1.0 and 2.0 per cent. ester. Animals did
not survive 49 weeks at the highest level. At the 1.0 per cent. level,
growth rates were considerably reduced and enlargement of male testes
and female hearts and kidneys were noted. Excess cancellous bone
formation was seen at the 2.0, 1.0 and 0.5 per cent. levels (Webb &
Hansen, 1963). Another 2-year feeding study revealed no adverse
effects, including bone changes, up to a level of 0.21 per cent. ester
in the diet (Packman et al., 1961).
Comments
Man appears to handle methyl salicylate metabolically similarly
to other animals although hydrolysis is comparatively slow. Long-term
studies on rats and the 2-year study on dogs are adequate for the
establishment of an acceptable daily intake. However, in view of some
inconsistencies in the reported studies, the following additional work
is desirable: short-term studies in dogs, including, levels of 100 and
150 mg/kg body-weight/day, and reproduction studies in rats.
EVALUATION
Level causing no toxicological effect
Rat. 0.2 per cent. (= 2000 ppm) of the diet, equivalent to 100
mg/kg/day.
Dog. 50 mg/kg/day
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.5
REFERENCES
Castagnou, R., Larceban, S. & Queynent, A. (1952) Bull. Soc. Pharm.
Marseille 3, 30
Davison, C., Zimmerman, E. F. & Smith, P. K. (1961) J. Pharm. Exp.
Therap., 132, 207
Ciroux, J., Granger, R. & Monnier, P. (1954) Trav. Soc. Pharm.
Montpellier, 14, 383
Harrison, J. W. E., Abbott, D. D. & Packman, E. W. (1963) Fed.
Proc., 22, 554
Houghton, E. M. (1905) Amer. J. Physiol., 13, 331
Jacobziner, H. (1963) New York State J. Med., 63, 295
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Leone, G. (1916), Arch. farm. sper., 22, 327
Packman, E. W., Abbott, D. D., Wagner, B. M. & Harrison, J. W. E.
(1961) Pharmacologist 3, 62
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Webb, W. K. & Hansen, W. H. (1963) Toxicol. appl. Pharmacol., 5,
576
Williams, R. T. (1959) Detoxication Mechanism, Second Edition, Chapman
& Hall, London
See Also:
Toxicological Abbreviations
METHYL SALICYLATE (JECFA Evaluation)
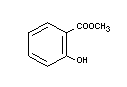 Molecular weight 152.15
Definition Methyl salicylate contains not less than
98 per cent. C8H8O3.
Description The volatile oil obtained by maceration
and subsequent steam distillation from
the leaves of Gaultheria procumbens
Linné (Fam. Ericaceae), or from the
bark of Betula lenta Linné (Fam.
Betulaceae). It is also produced
synthetically.
It is a colourless, yellowish or reddish
liquid, having the characteristic odour
and taste of wintergreen.
Biological Data
Biochemical aspects
The orally administered ester undergoes very rapid and nearly
complete hydrolysis in rats and dogs, salicylate being the only
significant finding in the blood of rats 20 minutes after an
intragastric dose, and in dogs within an hour of oral dosing. In man,
hydrolysis is somewhat slower after oral administration, some 21 per
cent. of salicylate as the ester being present in plasma after 90
minutes. The liver appears to be the main site of hydrolysis in rats,
rabbits, dogs and monkeys, the extent varying from 79-99 per cent.,
while plasma hydrolyzes some 15 per cent. (Davison et al., 1961). Dogs
excrete 0.2-0.5 per cent. of the ester in their urine after oral
administration and 14 per cent. after i.m. administration. Rabbits
excrete it mainly as glucuronate (12-55 per cent.); also as ethereal
sulfate (10 per cent.), conjugated before hydrolysis, and as other
metabolites, e.g. salicyluric acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 1110 Davison et al., 1961
Rat oral 887 Jenner et al., 1964
Rat oral 1250 Giroux et al., 1954
Guinea-pig oral 700 (MLD) Houghton, 1905
Guinea-pig oral 1060 Jenner et al., 1964
Guinea-pig s.c. 1500(MLD) Houghton, 1905
Rabbit oral 1300 Castagnou et al., 1952
Rabbit oral 2800 Leone, 1916
Dog oral 2100 Leone, 1916
The adult human oral LD50 is estimated at 0.5 g/kg body-weight
(Patty, 1963). The ester is potentially the most toxic salicylate for
infants and children, as 4 ml can be fatal (Davison et al., 1961;
Jacobziner, 1963).
Short-term studies
Rat. Groups of 10 males and 10 females were fed 0, 0.1 and 1.0
per cent. in their diet for 17 weeks. Both sexes showed a significant
reduction in growth rate at the 1.0 per cent. level, but histological
examination of the major organs revealed no abnormality. In another
experiment on 3 male and 3 female rats kept on a diet containing 2 per
cent. ester for up to 10 weeks, bone growth was specially studied by
X-ray and histology. Growth was reduced; there was excessive density
of bone with very reduced chondroclastic and osteoclastic activity
(Webb & Hansen, 1963). These together with decreased food intake, poor
weight gain and high mortality, were confirmed in other groups of rats
fed 1.12 per cent. and 2.0 per cent. ester in their diet for 10 weeks
(Harrison et al., 1963).
Dog. Groups of 1 male and 1 female were fed 50, 100, 250, 500,
800 and 1200 mg/kg body-weight/day of ester for up to 10 weeks. No
adverse effects were noted at levels including 250 mg/kg/day, but
there was increasing dose-dependent fatty metamorphosis of the liver
at higher test levels (Webb & Hansen, 1964).
In a 2-year study, groups of 2 males and 2 females were given 0,
50, 150, and 250 mg/kg body-weight/day of ester orally. Some growth
retardation. and liver enlargement was noted at the 150 and 250 mg/kg
level, and histology revealed enlarged hepatic parenchymal cells
(Webb & Hansen, 1963).
Long-term studies
Rat. Groups of 25 males and 25 females were kept for 2 years on
diets containing 0, 0.1, 0,5, 1.0 and 2.0 per cent. ester. Animals did
not survive 49 weeks at the highest level. At the 1.0 per cent. level,
growth rates were considerably reduced and enlargement of male testes
and female hearts and kidneys were noted. Excess cancellous bone
formation was seen at the 2.0, 1.0 and 0.5 per cent. levels (Webb &
Hansen, 1963). Another 2-year feeding study revealed no adverse
effects, including bone changes, up to a level of 0.21 per cent. ester
in the diet (Packman et al., 1961).
Comments
Man appears to handle methyl salicylate metabolically similarly
to other animals although hydrolysis is comparatively slow. Long-term
studies on rats and the 2-year study on dogs are adequate for the
establishment of an acceptable daily intake. However, in view of some
inconsistencies in the reported studies, the following additional work
is desirable: short-term studies in dogs, including, levels of 100 and
150 mg/kg body-weight/day, and reproduction studies in rats.
EVALUATION
Level causing no toxicological effect
Rat. 0.2 per cent. (= 2000 ppm) of the diet, equivalent to 100
mg/kg/day.
Dog. 50 mg/kg/day
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.5
REFERENCES
Castagnou, R., Larceban, S. & Queynent, A. (1952) Bull. Soc. Pharm.
Marseille 3, 30
Davison, C., Zimmerman, E. F. & Smith, P. K. (1961) J. Pharm. Exp.
Therap., 132, 207
Ciroux, J., Granger, R. & Monnier, P. (1954) Trav. Soc. Pharm.
Montpellier, 14, 383
Harrison, J. W. E., Abbott, D. D. & Packman, E. W. (1963) Fed.
Proc., 22, 554
Houghton, E. M. (1905) Amer. J. Physiol., 13, 331
Jacobziner, H. (1963) New York State J. Med., 63, 295
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Leone, G. (1916), Arch. farm. sper., 22, 327
Packman, E. W., Abbott, D. D., Wagner, B. M. & Harrison, J. W. E.
(1961) Pharmacologist 3, 62
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Webb, W. K. & Hansen, W. H. (1963) Toxicol. appl. Pharmacol., 5,
576
Williams, R. T. (1959) Detoxication Mechanism, Second Edition, Chapman
& Hall, London
Molecular weight 152.15
Definition Methyl salicylate contains not less than
98 per cent. C8H8O3.
Description The volatile oil obtained by maceration
and subsequent steam distillation from
the leaves of Gaultheria procumbens
Linné (Fam. Ericaceae), or from the
bark of Betula lenta Linné (Fam.
Betulaceae). It is also produced
synthetically.
It is a colourless, yellowish or reddish
liquid, having the characteristic odour
and taste of wintergreen.
Biological Data
Biochemical aspects
The orally administered ester undergoes very rapid and nearly
complete hydrolysis in rats and dogs, salicylate being the only
significant finding in the blood of rats 20 minutes after an
intragastric dose, and in dogs within an hour of oral dosing. In man,
hydrolysis is somewhat slower after oral administration, some 21 per
cent. of salicylate as the ester being present in plasma after 90
minutes. The liver appears to be the main site of hydrolysis in rats,
rabbits, dogs and monkeys, the extent varying from 79-99 per cent.,
while plasma hydrolyzes some 15 per cent. (Davison et al., 1961). Dogs
excrete 0.2-0.5 per cent. of the ester in their urine after oral
administration and 14 per cent. after i.m. administration. Rabbits
excrete it mainly as glucuronate (12-55 per cent.); also as ethereal
sulfate (10 per cent.), conjugated before hydrolysis, and as other
metabolites, e.g. salicyluric acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 1110 Davison et al., 1961
Rat oral 887 Jenner et al., 1964
Rat oral 1250 Giroux et al., 1954
Guinea-pig oral 700 (MLD) Houghton, 1905
Guinea-pig oral 1060 Jenner et al., 1964
Guinea-pig s.c. 1500(MLD) Houghton, 1905
Rabbit oral 1300 Castagnou et al., 1952
Rabbit oral 2800 Leone, 1916
Dog oral 2100 Leone, 1916
The adult human oral LD50 is estimated at 0.5 g/kg body-weight
(Patty, 1963). The ester is potentially the most toxic salicylate for
infants and children, as 4 ml can be fatal (Davison et al., 1961;
Jacobziner, 1963).
Short-term studies
Rat. Groups of 10 males and 10 females were fed 0, 0.1 and 1.0
per cent. in their diet for 17 weeks. Both sexes showed a significant
reduction in growth rate at the 1.0 per cent. level, but histological
examination of the major organs revealed no abnormality. In another
experiment on 3 male and 3 female rats kept on a diet containing 2 per
cent. ester for up to 10 weeks, bone growth was specially studied by
X-ray and histology. Growth was reduced; there was excessive density
of bone with very reduced chondroclastic and osteoclastic activity
(Webb & Hansen, 1963). These together with decreased food intake, poor
weight gain and high mortality, were confirmed in other groups of rats
fed 1.12 per cent. and 2.0 per cent. ester in their diet for 10 weeks
(Harrison et al., 1963).
Dog. Groups of 1 male and 1 female were fed 50, 100, 250, 500,
800 and 1200 mg/kg body-weight/day of ester for up to 10 weeks. No
adverse effects were noted at levels including 250 mg/kg/day, but
there was increasing dose-dependent fatty metamorphosis of the liver
at higher test levels (Webb & Hansen, 1964).
In a 2-year study, groups of 2 males and 2 females were given 0,
50, 150, and 250 mg/kg body-weight/day of ester orally. Some growth
retardation. and liver enlargement was noted at the 150 and 250 mg/kg
level, and histology revealed enlarged hepatic parenchymal cells
(Webb & Hansen, 1963).
Long-term studies
Rat. Groups of 25 males and 25 females were kept for 2 years on
diets containing 0, 0.1, 0,5, 1.0 and 2.0 per cent. ester. Animals did
not survive 49 weeks at the highest level. At the 1.0 per cent. level,
growth rates were considerably reduced and enlargement of male testes
and female hearts and kidneys were noted. Excess cancellous bone
formation was seen at the 2.0, 1.0 and 0.5 per cent. levels (Webb &
Hansen, 1963). Another 2-year feeding study revealed no adverse
effects, including bone changes, up to a level of 0.21 per cent. ester
in the diet (Packman et al., 1961).
Comments
Man appears to handle methyl salicylate metabolically similarly
to other animals although hydrolysis is comparatively slow. Long-term
studies on rats and the 2-year study on dogs are adequate for the
establishment of an acceptable daily intake. However, in view of some
inconsistencies in the reported studies, the following additional work
is desirable: short-term studies in dogs, including, levels of 100 and
150 mg/kg body-weight/day, and reproduction studies in rats.
EVALUATION
Level causing no toxicological effect
Rat. 0.2 per cent. (= 2000 ppm) of the diet, equivalent to 100
mg/kg/day.
Dog. 50 mg/kg/day
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.5
REFERENCES
Castagnou, R., Larceban, S. & Queynent, A. (1952) Bull. Soc. Pharm.
Marseille 3, 30
Davison, C., Zimmerman, E. F. & Smith, P. K. (1961) J. Pharm. Exp.
Therap., 132, 207
Ciroux, J., Granger, R. & Monnier, P. (1954) Trav. Soc. Pharm.
Montpellier, 14, 383
Harrison, J. W. E., Abbott, D. D. & Packman, E. W. (1963) Fed.
Proc., 22, 554
Houghton, E. M. (1905) Amer. J. Physiol., 13, 331
Jacobziner, H. (1963) New York State J. Med., 63, 295
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Leone, G. (1916), Arch. farm. sper., 22, 327
Packman, E. W., Abbott, D. D., Wagner, B. M. & Harrison, J. W. E.
(1961) Pharmacologist 3, 62
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Webb, W. K. & Hansen, W. H. (1963) Toxicol. appl. Pharmacol., 5,
576
Williams, R. T. (1959) Detoxication Mechanism, Second Edition, Chapman
& Hall, London
