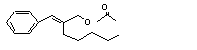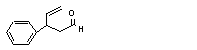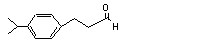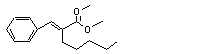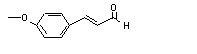WHO FOOD ADDITIVES SERIES 46:Cinnamyl Alcohol and Related Substances
First draft prepared by
Dr A. Mattia
Division of Product Policy, Office of Premarket Approval, Center for Food Safety and Applied Nutrition, US Food and Drug Administration, Washington DC, USA
and
Professor G.I. Sipes
Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, Tucson, Arizona, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of flavouring agents that includes cinnamyl alcohol (No. 647), cinnamaldehyde (No. 656), cinnamic acid (No. 657), and 52 structurally related substances (see Table 1). The evaluations were conducted according to the Procedure for the Safety Assessment of Flavouring Agents. One member of this group, allyl cinnamate (No. 19), had been evaluated previously by the Committee in a separate group of allyl ester flavouring agents examined by the Procedure (Annex 1, reference 122). Cinnamaldehyde (No. 656) was evaluated by the Committee at its eleventh meeting (Annex 1, reference 14), when it established a conditional ADI of 0–1.25 mg/kg bw. At its twenty-third meeting, the Committee converted the previous conditional ADI to a temporary ADI of 0–0.7 mg/kg bw (Annex 1, reference 50), which was extended at its twenty-fifth and twenty-eighth meetings (Annex 1, references 56 and 66). At its thirty-fifth meeting, the Committee did not extend the temporary ADI (Annex 1, reference 88) because the data that were required were not available. At its twenty-fifth meeting, the Committee concluded that cinnamyl anthranilate should not be used as a food additive (Annex 1, reference 56). This substance is structurally related to members of the group of cinnamyl alcohol and related flavouring agents but differs in that it is hydrolysed to cinnamyl alcohol and anthranilic acid only slowly, resulting in systemic intake of the intact ester. In contrast, the members of the group of flavouring agents undergo rapid hydrolysis.
Table 1. Summary of the results of safety evaluations of cinnamyl alcohol and related flavouring agentsa
|
Flavouring agent |
No. |
CAS no. and structure |
Step A3b
Does intake exceed the threshold for human intake?b |
Step A4
Is the substance or are its metabolites endogenous? |
Step A5
Adequate NOEL for substance or related substance? |
Comments |
Conclusion based on current intake |
|
Structural class I |
|
3-Phenyl-1-propanol |
636 |
122-97-4 |
No |
N/R |
N/R |
See note 1. |
No safety concern |
|

|
Europe: 60
USA: 31 |
|
|
|
|
|
3-Phenylpropyl formate |
637 |
104-64-3 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|

|
Europe: N/D
USA: 0.8 |
|
|
|
|
|
3-Phenylpropyl acetate |
638 |
122-72-5 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|

|
Europe: 41
USA: 9 |
|
|
|
|
|
3-Phenylpropyl propionate |
639 |
122-74-7 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
|
Europe: 0.2
USA: 0.3 |
|
|
|
|
|
3-Phenylpropyl isobutyrate |
640 |
103-58-2 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
|
Europe: 4
USA: 16 |
|
|
|
|
|
3-Phenylpropyl isovalerate concern |
641 |
5452-07-3 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
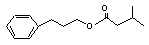
|
Europe: 0.01
USA: 0.1 |
|
|
|
|
|
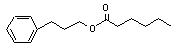
3-Phenylpropyl hexanoate concern |
642 |
6281-40-9 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
|
Europe: N/D
USA: 0.4 |
|
|
|
|
|
Methyl 3-phenylpropionate concern |
643 |
103-25-3 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
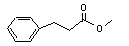
|
Europe: N/D
USA: 3 |
|
|
|
|
|
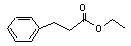
Ethyl 3-phenylpropionate concern |
644 |
2021-28-5 |
No |
N/R |
N/R |
See note 2. |
No safety concern |
|
|
Europe: 1
USA: 0.07 |
|
|
|
|
|
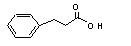
3-Phenylpropionic acid |
646 |
501-52-0 |
No |
N/R |
N/R |
See note 3. |
No safety concern |
|
|
Europe: 23
USA: 0.5 |
|
|
|
|
|
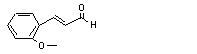
Cinnamyl alcohol |
647 |
104-54-1 |
Yes |
No |
Yes. |
See note 4. |
No safety concern |
|

|
Europe: 1800
USA: 1900 |
|
The NOEL of 54 mg/kg bw per day for cinnamyl alcohol (Zaitsev & Rakhmanina, 1974) is > 1000 times the daily intakes of 30 µg/kg bw per day in Europe and 32 µg/kg bw per day in the USA. |
|
|
|
Cinnamyl formate |
649 |
104-65-4 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
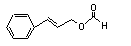
|
Europe: 2
USA: 17 |
|
|
|
|
|
Cinnamyl acetate |
650 |
103-54-8 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
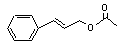
|
Europe: 210
USA: 300 |
|
|
|
|
|
Cinnamyl propionate |
651 |
103-56-0 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
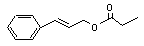
|
Europe: 4
USA: 25 |
|
|
|
|
|
Cinnamyl butyrate |
652 |
103-61-7 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
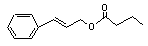
|
Europe: 3
USA: 2 |
|
|
|
|
|
Cinnamyl isobutyrate |
653 |
103-59-3 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
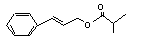
|
Europe: 13
USA: 22 |
|
|
|
|
|
Cinnamyl isovalerate |
654 |
140-27-2 |
No |
N/R |
N/R |
See note 5. |
No safety concern |
|
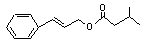
|
Europe: 5
USA: 8 |
|
|
|
|
|
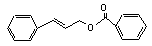
Cinnamyl benzoate |
760 |
5320-75-2 |
No |
N/R |
N/R |
See note 6. |
No safety concern |
|
|
Europe: N/D
USA: 1 |
|
|
|
|
|
Cinnamyl phenylacetate |
655 |
7492-65-1 |
No |
N/R |
N/R |
See note 7. |
No safety concern |
|
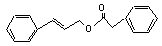
|
Europe: 0.003
USA: 1 |
|
|
|
|
|
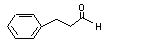
Cinnamaldehyde |
656 |
104-55-2 |
Yes |
No |
Yes. |
See note 4. |
No safety concern |
|
|
Europe: 2500
USA: 59 000 |
|
The NOEL of 620 mg/kg bw per day for cinnamaldehyde (National Toxicology Program, 1995) is > 10 000 and > 600 times the daily intakes of 42 µg/kg bw per day in Europe and 990 µg/kg bw per day in the USA. |
|
|
|
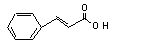
Cinnamic acid |
657 |
621-82-9 |
No |
N/R |
N/R |
See note 8. |
No safety concern |
|
|
Europe: 32
USA: 44 |
|
|
|
|
|
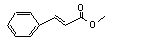
Methyl cinnamate |
658 |
103-26-4 |
Yes |
No |
Yes. |
See note 9. |
No safety concern |
|
|
Europe: 2800
USA: 830 |
|
The NOELs of 54 and 80 mg/kg bw per day for cinnamyl alcohol and ethyl connamate, respectively, are > 1000 times the daily per capita intake of methyl cinnamate in Europe |
|
|
|
Ethyl cinnamate |
659 |
103-36-6 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
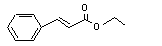
|
Europe: 100
USA: 70 |
|
|
|
|
|
Propyl cinnamate |
660 |
7778-83-8 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
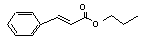
|
Europe: 0.4
USA: 4 |
|
|
|
|
|
Isopropyl cinnamate |
661 |
7780-06-5 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
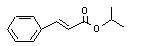
|
Europe: 19
USA: 3 |
|
|
|
|
|
Allyl cinnamate |
19 |
1866-31-5 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
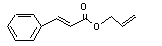
|
Europe: 5
USA: 0.3 |
|
|
|
|
|
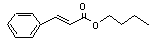
Butyl cinnamate |
663 |
538-65-8 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
|
Europe: 0.4
USA: 0.2 |
|
|
|
|
|
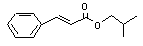
Isobutyl cinnamate |
664 |
122-67-8 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
|
Europe: 1
USA: 3 |
|
|
|
|
|
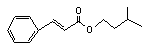
Isoamyl cinnamate |
665 |
7779-65-9 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
|
Europe: 8
USA: 6 |
|
|
|
|
|
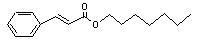
Heptyl cinnamate |
666 |
10032-08-3 |
No |
N/R |
N/R |
See note 9. |
No safety concern |
|
|
Europe: 2
USA: 52 |
|
|
|
|
|
Cyclohexyl cinnamate |
667 |
7779-17-1 |
No |
N/R |
N/R |
Hydrolysed to cinnamic acid |
No safety concern |
|
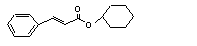
|
Europe: 0.4
USA: 0.04 |
|
|
(see note 8) and cyclo-hexanol. Cyclo-hexanol is mainly conjugated with glucuronic acid and excreted. |
|
|
Linalyl cinnamate |
668 |
78-37-5 |
No |
N/R |
N/R |
Hydrolysed to cinnamic acid. |
No safety concern |
|
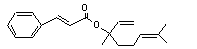
|
Europe: 7
USA: 3 |
|
|
(see note 8) and linalool. Linalool undergoes omega and omega-1 oxidation to yield polar, excretable metabolites. |
|
|
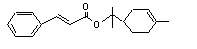
Terpinyl cinnamate |
669 |
10024-56-3 |
No |
N/R |
N/R |
Hydrolysed to cinnamic acid |
No safety concern |
|
|
Europe: 0.01
USA: 0.5 |
|
|
(see note 8) and terpineol. Terpineol undergoes omea and omea-1 oxidation to yield polar excretable metabolites |
|
|
Benzyl cinnamate |
670 |
103-41-3 |
No |
N/R |
N/R |
Hydrolysed to cinnamic acid |
No safety concern |
|
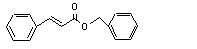
|
Europe: 44
USA: 69 |
|
|
(see note 8) and benzyl alcohol. Benzyl alcohol is oxidized to benzoic acid and excreted as hippuric acid. |
|
|
Phenethyl cinnamate |
671 |
103-53-7 |
No |
N/R |
N/R |
Hydrolysed to cinnamic acid |
No safety concern |
|
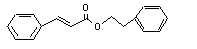
|
Europe: 6
USA: 50 |
|
|
(see note 8) and phenethyl alcohol. Phenethyl alcohol is oxidized to phenylacetic acid and excreted as the glucuronic acid conjugate. |
|
|
3-Phenylpropyl cinnamate |
672 |
122-68-9 |
No |
N/R |
N/R |
See notes 1 and 8. |
No safety concern |
|
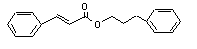
|
Europe: 0.6
USA: 37 |
|
|
|
|
|
Cinnamyl cinnamate |
673 |
122-69-0 |
No |
N/R |
N/R |
See notes 4 |
No safety concern |
|
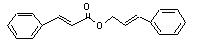
|
Europe: 2
USA: 36 |
|
|
|
and 8. |
|
alpha-Amylcinnamyl formate |
676 |
7493-79-0 |
No |
N/R |
N/R |
See note 10. |
No safety concern |
|
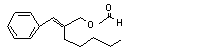
|
Europe: 1.4
USA: 0.5 |
|
|
|
|
|
alpha-Amylcinnamyl acetate |
677 |
7493-78-9 |
No |
N/R |
N/R |
See note 10. |
No safety concern |
|
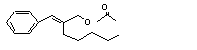
|
Europe: 3
USA: 260 |
|
|
|
|
|
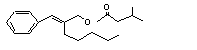
alpha-Amylcinnamyl isovalerate |
678 |
7493-80-3 |
No |
N/R |
N/R |
See note 10. |
No safety concern |
|
|
Europe: 0.01
USA: 0.5 |
|
|
|
|
|
3-Phenyl-4-pentenal |
679 |
939-21-9 |
No |
N/R |
N/R |
Oxidized to the corresponding acid and excreted |
No safety concern |
|
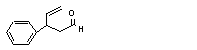
|
Europe: 1
USA: 2 |
|
|
|
|
|
3-(para-Isopropylphenyl)-propionaldehyde |
680 |
7775-00-0 |
No |
N/R |
N/R |
See note 1. |
No safety concern |
|
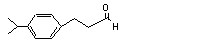
|
Europe: N/D
USA: 0.1 |
|
|
|
|
|
alpha-Amylcinnamaldehyde dimethyl acetal |
681 |
91-87-2 |
No |
N/R |
N/R |
See note 10. |
No safety concern |
|
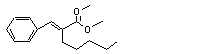
|
Europe: 0.01
USA: 0.007 |
|
|
|
|
|
para-Methylcinnam-aldehyde |
682 |
1504-75-2 |
No |
N/R |
N/R |
See note 4. |
No safety concern |
|
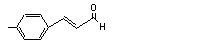
|
Europe: 0.01
USA: 0.9 |
|
|
|
|
|
alpha-Methylcinnamaldehyde |
683 |
101-39-3 |
No |
N/R |
N/R |
See note 4. |
No safety concern |
|
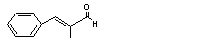
|
Europe: 3
USA: 390 |
|
|
|
|
|
para-Methoxycinnamaldehyde |
687 |
1963-36-6 |
No |
N/R |
N/R |
See note 4. |
No safety concern |
|
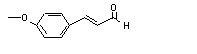
|
Europe: 0.04
USA: 0.01 |
|
|
|
|
|
ortho-Methoxycinnamal-dehyde |
688 |
1504-74-1 |
No |
N/R |
N/R |
Oxidized to the corres ponding acid, conju gated and excreted. |
No safety concern |
|
|
Europe: 0.6
USA: 71 |
|
|
Alternatively, the acid may undergo beta-oxida tion to yield the beta-hyd roxy carboxylic acid derivative, which is also excreted. |
|
|
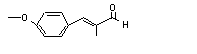
para-Methoxy-alpha-methyl cinnamaldehyde |
689 |
65405-67-6 |
No |
N/R |
N/R |
See note 4. |
No safety concern |
|
|
Europe: 0.3
USA: 0.05 |
|
|
|
|
|
Structural class II |
|
Cinnamaldehyde ethylene glycol acetal |
648 |
5600-60-6 |
Yes |
No |
Yes. |
|
No safety concern |
|
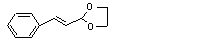
|
Europe: 690
USA: 0.007 |
|
The NOEL of 620 mg/kg bw per day for cinnamaldehyde is > 10 000 times the daily per capita intake of cinnamldehyde ethylene glycol acetal in Europe |
Hydrolysed to the corresponding alcohol and aldehyde |
|
|
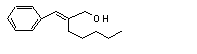
alpha-Amylcinnamyl alcohol |
674 |
101-85-9 |
No |
N/R |
N/R |
|
No safety concern |
|
|
Europe: 4
USA: 1 |
|
|
Oxidized to alpha-amylcin namaldehyde, which is further oxidized to alpha- amylcinnamic acid and excreted. |
|
|
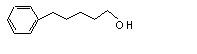
5-Phenylpentanol |
675 |
10521-91-2 |
No |
N/R |
N/R |
See note 1. |
No safety concern |
|
|
Europe: N/D
USA: 0.1 |
|
|
|
|
|
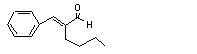
alpha-Butylcinnamal-dehyde |
684 |
7492-44-6 |
No |
N/R |
N/R |
See note 11. |
No safety concern |
|
|
Europe: 0.01
USA: 0.07 |
|
|
|
|
|
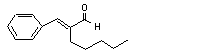
alpha-Amylcinnamal-dehyde |
685 |
122-40-7 |
No |
N/R |
N/R |
See note 11. |
No safety concern |
|
|
Europe: 25
USA: 23 |
|
|
|
|
|
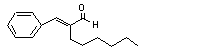
alpha-Hexylcinnamal-dehyde |
686 |
101-86-0 |
No |
N/R |
N/R |
See note 11 |
No safety concern |
|
|
Europe: 87
USA: 11 |
|
|
|
|
CAS: Chemical Abstracts Service; N/D: no intake data reported; N/R: not required for evaluation because consumption of the substance was determined to be of no safety concern at step A3 of the Procedure.
a Step 2: All of the substances in this group are expected to be metabolized to innocuous products.
b The thresholds for human intake for classes I and II are 1800 µg/day and 540 µg/day, respectively. All intake values are expressed in µg/day.
Notes to Table 1
1. Oxidized to yield the corresponding acid, which undergoes further side-chain beta-oxidation and cleavage to yield the benzoic acid derivative. It then conjugates with glycine and/or glucuronic acid, and is excreted in the urine.
2. Hydrolysed to the corresponding acid and alcohol. The acid is completely oxidized and the alcohol, 3-phenyl-1-propanol, is further metabolized and excreted (see note 1).
3. Undergoes side-chain beta-oxidation and cleavage to yield the corresponding benzoic acid derivative; it then conjugates with glycine and/or glucuronic acid and is excreted in the urine.
4. Oxidized to cinnamic acid or its corresponding derivative and further oxidized to benzoic acid or its corresponding derivative, which is excreted as hippuric acid or its corresponding derivative.
5. Hydrolysed to cinnamyl alcohol and the corresponding carboxylic acid. Cinnamyl alcohol is oxidized and excreted (see note 4); the carboxylic acid is either completely oxidized or conjugated and excreted primarily in the urine.
6. Hydrolysed to cinnamyl alcohol and benzoic acid. Cinnamyl alcohol is oxidized to cinnamic acid, which is further oxidized to benzoic acid (see note 4).
7. Hydrolysed to cinnamyl alcohol and phenylacetic acid. Cinnamyl alcohol is oxidized to cinnamic acid, which is further oxidized to benzoic acid (see note 4). Phenylacetic acid is excreted as the glucuronic acid conjugate.
8. Undergoes beta-oxidation and is excreted as hippuric acid
9. Rapidly hydrolysed to cinnamic acid (see note 8) and the corresponding alcohol. The corresponding alcohol is completely oxidized.
10. Hydrolysed to alpha-amylcinnamyl alcohol (No. 674) and the corresponding acid, which is excreted; alpha-amylcinnamyl alcohol is oxidized to alpha-amylcinnamaldehyde, which is further oxidized to alpha-amylcinnamic acid and excreted.
11. Oxidized to the corresponding acid and excreted.
Twenty-two of the 55 flavouring agents in this group are natural components of foods. Concentrations of cinnamaldehyde of up to 750 g/kg have been detected in oils from natural sources, such as cinnamon, cinnamomum, and cassia leaves; however, these agents are consumed predominantly as flavouring agents (Maarse et al., 1996).
1.2 Estimated daily intake
The total annual volume of production of the 55 cinnamyl compounds considered here is approximately 60 t in Europe (International Organization of the Flavor Industry, 1995) and 480 t in the USA (National Academy of Sciences, 1987; Lucas et al., 1999; see Table 2). Approximately 30% of the total annual volume in Europe and over 93% of that in the USA is accounted for by cinnamaldehyde (No. 656), while 54% of the total annual volume in Europe is accounted for by the use of cinnamyl alcohol (No. 647) and methyl cinnamate (No. 658). The estimated intakes in Europe are 2.5 mg/day of cinnamaldehyde, 1.8 mg/day of cinnamyl alcohol, and 2.8 mg/day of methyl cinnamate. The estimated intakes in the USA are 59 mg/day of cinnamaldehyde, 1.9 mg/day of cinnamyl alcohol, and 0.83 mg/day of methyl cinnamate. The intakes of all the other flavouring agents in the group are in the range 0.003–690 µg/day, most of the values being at the low end of this range. Production volumes and intake of each substance are reported in Table 2.
Table 2. Annual volumes of production of cinnamyl alcohol and related substances used as flavouring agents in Europe and the USA
|
Substance (No.) |
Most recent annual volume (kg)a |
Intakeb |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
3-Phenyl-1-propanol (636) |
|
Europe |
420 |
60 |
1 |
+ |
NA |
|
USA |
236 |
31 |
0.5 |
|
NA |
|
3-Phenylpropyl formate (637) |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
6 |
0.8 |
0.01 |
|
NA |
|
3-Phenylpropyl acetate (638) |
|
Europe |
289 |
41 |
0.7 |
140 |
0.5 |
|
USA |
68 |
9 |
0.1 |
|
2.1 |
|
3-Phenylpropyl propionate (639) |
|
Europe |
1 |
0.2 |
0.003 |
+ |
NA |
|
USA |
2 |
0.3 |
0.005 |
|
NA |
|
3-Phenylpropyl isobutyrate (640) |
|
Europe |
30 |
4 |
0.07 |
– |
NA |
|
USA |
123 |
16 |
0.3 |
|
NA |
|
3-Phenylpropyl isovalerate (641) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
0.5 |
0.1 |
0.001 |
|
NA |
|
3-Phenylpropyl hexanoate (642) |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
3 |
0.4 |
0.007 |
|
NA |
|
Methyl 3-phenylpropionate (643) |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
23 |
3 |
0.05 |
|
NA |
|
Ethyl 3-phenylpropionate (644) |
|
Europe |
10 |
1 |
0.02 |
47 |
4.7 |
|
USA |
0.5 |
0.07 |
0.001 |
|
94 |
|
3-Phenylpropionaldehyde (645) |
|
Europe |
134 |
19 |
0.3 |
+ |
NA |
|
USA |
146 |
19 |
0.3 |
|
NA |
|
3-Phenylpropionic acid (646) |
|
Europe |
161 |
23 |
0.4 |
+ |
NA |
|
USA |
4 |
0.5 |
0.008 |
|
NA |
|
Cinnamyl alcohol (647) |
|
Europe |
12 543 |
1 800 |
30 |
171 |
0.00 |
|
USA |
14 682 |
1 900 |
32 |
|
0.01 |
|
Cinnamaldehyde ethylene glycol (648) acetal |
|
Europe |
4 821 |
690 |
11 |
– |
NA |
|
USA |
0.05 |
0.007 |
0.0001 |
|
NA |
|
Cinnamyl formate (649) |
|
Europe |
15 |
2 |
0.04 |
– |
NA |
|
USA |
127 |
17 |
0.3 |
|
NA |
|
Cinnamyl acetate (650) |
|
Europe |
1 498 |
210 |
4 |
+ |
NA |
|
USA |
2 255 |
300 |
5 |
|
NA |
|
Cinnamyl propionate (651) |
|
Europe |
30 |
4 |
0.07 |
– |
NA |
|
USA |
191 |
25 |
0.4 |
|
NA |
|
Cinnamyl butyrate (652) |
|
Europe |
21 |
3 |
0.05 |
+ |
NA |
|
USA |
17 |
2 |
0.04 |
|
NA |
|
Cinnamyl isobutyrate (653) |
|
Europe |
90 |
13 |
0.2 |
– |
NA |
|
USA |
164 |
22 |
0.4 |
|
NA |
|
Cinnamyl isovalerate (654) |
|
Europe |
32 |
5 |
0.08 |
– |
NA |
|
USA |
64 |
8 |
0.14 |
|
NA |
|
Cinnamyl benzoate |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
5 |
1 |
0.01 |
|
NA |
|
Cinnamyl phenylacetate (655) |
|
Europe |
0.02 |
0.003 |
0.05 |
– |
NA |
|
USA |
11 |
1 |
0.02 |
|
NA |
|
Cinnamaldehyde (656) |
|
Europe |
17 623 |
2 500 |
42 |
38 642 |
2.2 |
|
USA |
451 364 |
59 000 |
991 |
|
0.09 |
|
Cinnamic acid (657) |
|
Europe |
227 |
32 |
0.5 |
183 |
0.8 |
|
USA |
332 |
44 |
0.7 |
|
0.6 |
|
Methyl cinnamate (658) |
|
Europe |
19 384 |
2 800 |
46 |
57 |
0.003 |
|
USA |
6 318 |
830 |
14 |
|
0.009 |
|
Ethyl cinnamate (659) |
|
Europe |
727 |
100 |
2 |
292 |
0.4 |
|
USA |
530 |
70 |
1 |
|
0.6 |
|
Propyl cinnamate (660) |
|
Europe |
2.6 |
0.4 |
0.006 |
– |
NA |
|
USA |
31 |
4 |
0.07 |
|
NA |
|
Isopropyl cinnamate (661) |
|
Europe |
135 |
19 |
0.3 |
– |
NA |
|
USA |
23 |
3 |
0.05 |
|
NA |
|
Allyl cinnamate (19) |
|
Europe |
38 |
5 |
0.09 |
– |
NA |
|
USA |
2 |
0.3 |
0.004 |
|
NA |
|
Butyl cinnamate (663) |
|
Europe |
3 |
0.4 |
0.006 |
– |
NA |
|
USA |
1 |
0.2 |
0.003 |
|
NA |
|
Isobutyl cinnamate (664) |
|
Europe |
10 |
1 |
0.02 |
+ |
NA |
|
USA |
21 |
3 |
0.05 |
|
NA |
|
Isoamyl cinnamate (665) |
|
Europe |
57 |
8 |
0.1 |
+ |
NA |
|
USA |
46 |
6 |
0.1 |
|
NA |
|
Heptyl cinnamate (666) |
|
Europe |
12 |
2 |
0.03 |
– |
NA |
|
USA |
391 |
52 |
0.9 |
|
NA |
|
Cyclohexyl cinnamate (667) |
|
Europe |
3 |
0.4 |
0.006 |
– |
NA |
|
USA |
0.3 |
0.04 |
0.001 |
|
NA |
|
Linalyl cinnamate (668) |
|
Europe |
49 |
7 |
0.1 |
– |
NA |
|
USA |
19 |
3 |
0.04 |
|
NA |
|
Terpinyl cinnamate (669) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
4 |
0.5 |
0.009 |
|
NA |
|
Benzyl cinnamate (670) |
|
Europe |
310 |
44 |
0.7 |
+ |
NA |
|
USA |
527 |
69 |
1 |
|
NA |
|
Phenethyl cinnamate (671) |
|
Europe |
40 |
6 |
0.1 |
– |
NA |
|
USA |
382 |
50 |
0.8 |
|
NA |
|
3-Phenylpropyl cinnamate (672) |
|
Europe |
4 |
0.6 |
0.01 |
– |
NA |
|
USA |
282 |
37 |
0.6 |
|
NA |
|
Cinnamyl cinnamate (673) |
|
Europe |
11 |
2 |
0.03 |
+ |
NA |
|
USA |
277 |
36 |
0.6 |
|
NA |
|
alpha-Amylcinnamyl alcohol (674) |
|
Europe |
27 |
4 |
0.06 |
– |
NA |
|
USA |
9 |
1 |
0.02 |
|
NA |
|
5-Phenylpentanol (675) |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
1 |
0.1 |
0.002 |
|
NA |
|
alpha-Amylcinnamyl formate (676) |
|
Europe |
10 |
1.4 |
0.02 |
– |
NA |
|
USA |
4 |
0.5 |
0.009 |
|
NA |
|
alpha-Amylcinnamyl acetate (677) |
|
Europe |
20 |
3 |
0.05 |
– |
NA |
|
USA |
1 996 |
263 |
4 |
|
NA |
|
alpha-Amylcinnamyl isovalerate (678) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
4 |
0.5 |
0.009 |
|
NA |
|
3-Phenyl-4-pentenal (679) |
|
Europe |
6 |
1 |
0.01 |
– |
NA |
|
USA |
16 |
2 |
0.04 |
|
NA |
|
3-(para-Isopropyl-phenyl)propionaldehyde (680) |
|
Europe |
N/D |
N/D |
N/D |
– |
NA |
|
USA |
1 |
0.1 |
0.002 |
|
NA |
|
alpha-Amylcinnamaldehyde dimethyl acetal (681) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
0.05 |
0.007 |
0.0001 |
|
NA |
|
para-Methylcinnamaldehyde (682) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
7 |
0.9 |
0.02 |
|
NA |
|
alpha-Methylcinnamaldehyde (683) |
|
Europe |
20 |
3 |
0.05 |
+ |
NA |
|
USA |
2 932 |
390 |
6 |
|
NA |
|
alpha-Butylcinnamaldehyde (684) |
|
Europe |
0.1 |
0.01 |
0.0002 |
– |
NA |
|
USA |
0.5 |
0.07 |
0.001 |
|
NA |
|
alpha-Amylcinnamaldehyde (685) |
|
Europe |
178 |
25 |
0.4 |
+ |
NA |
|
USA |
173 |
23 |
0.4 |
|
NA |
|
alpha-Hexylcinnamaldehyde (686) |
|
Europe |
611 |
87 |
1 |
+ |
NA |
|
USA |
82 |
11 |
0.2 |
|
NA |
|
para-Methoxycinnamaldehyde (687) |
|
Europe |
0.3 |
0.04 |
0.001 |
+ |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
NA |
|
ortho-Methoxycinnamaldehyde (688) |
|
Europe |
4 |
0.6 |
0.01 |
+ |
NA |
|
USA |
541 |
71 |
1 |
|
NA |
|
para-Methoxy-alpha-methyl-cinnamaldehyde (689) |
|
Europe |
2 |
0.3 |
0.005 |
– |
NA |
|
USA |
0.4 |
0.05 |
0.001 |
|
NA |
|
Total |
|
Europe |
60 000 |
|
|
|
|
|
USA |
480 000 |
|
|
|
|
NA, not available; N/D, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1996), but no quantitative data; –, not reported to occur naturally in foods
a From International Organization of the Flavor Industry (1995) and Lucas (1999)
b Intake (µg/person per day) calculated as follows: [(annual volume, kg) ´ (1 ´ 109 µg/kg)/ (population ´ survey correction factor ´ 365 days)], where population (10%, ‘eaters only’) = 32 x 106 for Europe and 26 x 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual volume of the flavour, respectively, was reported in the poundage surveys (International Organization of the Flavor Industry, 1995; Lucas et al., 1999). Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding.
c Quantitative data from Stofberg & Grundschober (1987)
d Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring agent, kg)
1.3 Metabolic considerations
Cinnamyl alcohol (No. 647), cinnamaldehyde (No. 656) and its para- and ortho-methoxy derivatives (Nos 687 and 688), cinnamic acid (No. 657) and its corres-ponding methyl ester (No. 658), and the saturated analogue (3-phenylpropionic acid, No. 646) have all been shown to be rapidly absorbed from the gut, metabolized, and excreted primarily in the urine and to a minor extent in the faeces.
Esters of cinnamic acid and structurally related aromatic esters have been shown to be hydrolysed rapidly to the component acid and alcohol. The aromatic primary alcohols and aldehydes in this group and those formed by the hydrolysis of esters and acetals are readily oxidized to cinnamic acid or one of its structurally related carboxylic acids. In animals, most carboxylic acids, such as cinnamic acid, are converted to acyl coenzyme A esters. Cinnamoyl coenzyme A undergoes either glycine conjugation catalysed by a glycine N-acyl transferase or beta-oxidation, eventually leading to the formation of benzoyl coenzyme A. This is in turn either conjugated with glycine, yielding hippuric acid, or hydrolysed to yield free benzoic acid, which is then excreted (Nutley et al., 1994).
Cinnamyl derivatives containing alpha-methyl substituents, such as alpha-methylcin-namaldehyde (No. 683), are extensively metabolized by beta-oxidation and cleavage to yield mainly the corresponding hippuric acid derivative. Because ortho-oxygenated ring substituents (e.g. ortho-methoxycinnamaldehyde, No. 688) selectively inhibit oxidation of coenzyme A esters of beta-hydroxyacids via the beta-oxidation pathway, these hydroxyacid derivatives are excreted as glycine conjugates. In contrast, para-oxygenated ring substituents (e.g. para-methoxycinnamaldehyde, No. 687) are oxidized via the beta-oxidation pathway, eventually yielding hippuric acid derivatives.
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to the above-mentioned substances, the Committee assigned 49 of the 55 substances to structural class I (Cramer et al., 1978). These are simple aromatic compounds with a saturated propyl or unsaturated propenyl side-chain containing a primary oxygenated functional group, which have little toxic potential. The remaining six substances, which are those containing a heterocyclic ring (No. 648) or rings bearing substituents other than 1–5 carbon aliphatic groups (Nos 674, 675, 684–686), were assigned to structural class II. |
|
Step 2. |
All the substances in this group are predicted to be metabolized to innocuous products (see section 2.3). The evaluation of these substances therefore proceeded via the left-hand side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intakes of 46 of the 49 substances in structural class I and of five of six substances in structural class II are below the threshold for human intake for each structural class (i.e. 1800 µg/person per day for class I and 540 µg/person per day for class II). According to the Procedure, the safety of these 51 flavouring agents raises no concern when they are used at their currently estimated levels of intake. The intake of cinnamaldehyde (No. 656) is 2.5 mg/person per day in Europe and 59 mg/person per day in the USA. The intake of methyl cinnamate (No. 658) is 2800 µg/day in Europe and 830 µg/day in the USA. The intake of cinnamaldehyde ethylene glycol acetal (No. 648; structural class II) is 690 µg/day in Europe and 0.007 µg/day in the USA. The intake of cinnamyl alcohol (No. 647) is 1800 µg/person per day in Europe and 1900 µg/person per day in the USA. The intakes of these four flavouring agents therefore exceed the threshold for human intake for class I and class II (1800 and 540 µg/person per day, respectively). Accordingly, the evaluation of these substances proceeded to step A4. |
|
Step A4. |
None of these four flavouring agents is endogenous. Therefore, their evaluation proceeded to step A5. |
|
Step A5. |
The NOEL of 54 mg/kg bw per day for cinnamyl alcohol (No. 647) (Zaitsev & Rakhmanina, 1974) is about 1000 times greater than the estimated intake of cinnamyl alcohol from its use as a flavouring agent in Europe (29 µg/kg bw per day) and the USA (32 µg/kg bw per day). The NOEL of 620 mg/kg bw per day for cinnamaldehyde (No. 656) (National Toxicology Program, 1995) is 10 000 times greater than the estimated daily intake of this substance from use as a flavouring agent in Europe (42 µg/kg bw) and about 600 times greater than that in the USA (990 µg/kg bw). The NOEL of 54 mg/kg bw per day for cinnamyl alcohol is appropriate for evaluating the safety of methyl cinnamate (No. 658) because cinnamyl alcohol is oxidized to cinnamic acid, which is a product of hydrolysis of methyl cinnamate. In addition, a NOEL of 80 mg/kg bw per day has been identified for a closely related ester, ethyl cinnamate. Both of these NOELs are > 1000 times the daily per capita intake of methyl cinnamate. Cinnamaldehyde ethylene glycol acetate (No. 648) is rapidly hydrolysed to cinnamaldehyde; the NOEL of 620 Mg/kg bw per day for cinnamaldehyde is > 10 000 times the daily per capita intake of cinnamaldehyde ethylene glycol acetal. The Committee therefore concluded that the safety of these substances would not be expected to be of a concern. |
Table 1 summarizes the evaluation of cinnamyl alcohol and 54 related substances used as flavouring agents.
1.5 Consideration of combined intakes
In the unlikely event that all foods containing the 50 substances in structural class I were consumed concurrently on a daily basis, the estimated combined intake would exceed the threshold for human intake for class I. In the unlikely event that all foods containing the five substances in structural class II were consumed concurrently on a daily basis, the estimated combined intake would exceed the threshold for human intake for class II. However, all 55 substances in the group are expected to be efficiently metabolized and would not saturate the metabolic pathways. Overall evaluation of the data indicates that combined intake would not raise concern about safety.
1.6 Conclusions
The Committee concluded that the safety of the flavouring agents in this group would not raise concern at the current estimated levels of intake. Other data on the toxicity of cinnamyl derivatives were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes data relevant to an evaluation of the safety of cinnamyl alcohol (No. 647), cinnamaldehyde (No. 656), cinnamic acid (i.e., trans-3-phenylpropenoic acid, No. 657), and 52 structurally related substances. All members of this group are primary alcohols, aldehydes, or carboxylic acids or their corresponding esters and acetals. The primary oxygenated functional group is located on a three-carbon chain containing an aromatic ring at position 3 (i.e. a 3-phenylpropyl group). Structural variations among these substances include the presence of unsaturation in the propyl side-chain and/or alkyl, alkoxy, or hydroxy substituents on the aromatic ring. The parent saturated alcohol is 3-phenyl-1-propanol, and the parent unsaturated alcohol is cinnamyl alcohol (3-phenyl-2-propen-1-ol, No. 647).
The group includes 3-phenyl-1-propanol, (No. 636), eight related esters (Nos. 637-644), and the corresponding aldehyde (No. 645) and carboxylic acid (No. 646). It also includes cinnamyl alcohol (No. 647), eight related cinnamyl esters (Nos 649–655 and cinnamyl benzoate), cinnamaldehyde (No. 656), an acetal of cinnamaldehyde (No. 648), cinnamic acid (No. 657), and 16 cinnamic acid esters (Nos 658–673). Thirteen cinnamyl derivatives contain additional ring side-chain alkyl substituents (Nos 674–686), and three contain alkoxy-ring substituents (Nos 687–689).
The available data on cinnamyl anthranilate, which is no longer used as a flavouring agent (voluntarily discontinued in 1986), are not presented in this review. A review of the metabolism and the proposed mechanism of toxicity of this agent has been published (Newberne et al., 2000).
2.2 Additional considerations on intake
Cinnamyl compounds are fundamental to plant biochemistry. trans-Cinnamic acid is ubiquitous in the plant kingdom and is required for lignin formation in plants. It is derived from the action of L-phenylalanine ammonia lyase on L-phenylalanine, with the formation of ammonia and cinnamic acid (No. 657). Cinnamic acid is also converted to para-hydroxy cinnamic acid (para-coumaric acid) by plants. para-Coumaric acid is one of the more important precursors of lignins as it can be converted to polyphenolic alcohols, which readily polymerize to form lignin (Goodwin & Mercer, 1972).
Twenty-two of the 55 flavouring substances in this group have been detected as natural components of traditional foods (Maarse et al., 1996; see Table 2). A considerable amount (38 642 kg) of cinnamaldehyde (No. 656) occurs naturally in foods. This agent has been detected in oils derived from natural sources such as cinnamon, cinnamomum, and cassia leaf, at concentrations up to 750 000 mg/kg (Maarse et al., 1996). Quantitative data on natural occurrence have also been reported for 3-phenylpropyl acetate (No. 638), ethyl 3-phenylpropionate (No. 644), cinnamyl alcohol (No. 647), cinnamic acid (No. 657), methyl cinnamate (No. 658), and ethyl cinnamate (No. 659). Generally, the consumption ratios indicate that intake occurs predominately from use of these substances as flavours (i.e. the consumption ratio is < 1) (Stofberg & Kirschman, 1985; Stofberg & Grunschober, 1987).
2.3 Biological data
The cinnamyl derivatives used as flavouring substances are simple aromatic compounds with a propyl side-chain containing a primary oxygenated functional group, and they participate in common routes of absorption, distribution, and metabolism. The members of this group may be hydrolysed to yield the component alcohol, aldehyde, or acid. If the product is an alcohol or aldehyde, it is oxidized to yield the corresponding 3-phenylpropenoic acid or a 3-phenylpropanoic acid derivative which undergoes further side-chain beta-oxidation and cleavage to yield mainly the corresponding benzoic acid derivatives (Figure 2; Williams, 1959). The benzoic acid derivatives are conjugated with glycine and, to a lessor extent, glucuronic acid and excreted primarily in the urine (Snapper et al., 1940). ortho-Alkyl- and ortho-alkoxy-substituted cinnamaldehyde derivatives undergo beta-oxidation to a minor extent, to yield beta-hydroxy-3-phenylpropanoic acid metabolites that are excreted as the glucuronic acid conjugates (Solheim & Scheline, 1973, 1976; Samuelsen et al., 1986).
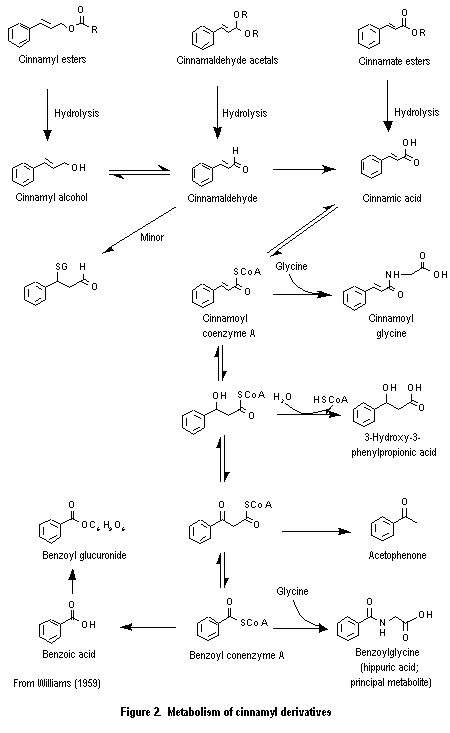
2.3.1 Absorption, distribution, and excretion
Cinnamyl alcohol (No. 647), cinnamaldehyde (No. 656) and its ortho- and para-methoxy derivatives (Nos 687 and 688), cinnamic acid (No. 657) and its corres-ponding methyl ester (No. 658), and the saturated analogue (3-phenylpropanoic acid, No. 646) have all been shown to be rapidly absorbed from the gut, metabolized, and excreted primarily in the urine and, to a minor extent, in the faeces. Results of studies conducted as long ago as 1909 indicate that cinnamyl derivatives are absorbed, metabolized, and excreted as polar metabolites within 24 h (see Table 3).
Table 3. Metabolism of cinnamyl alcohol and related substances used as flavouring agents
|
Agent |
Species |
Route |
Dose |
% dose in 24-h urine/ faeces |
Urinary metabolites (%) |
Other
|
Reference |
|
Benzoic acid (glycine conjugate / free) |
Cinnamic acid (glycine conjugate / free) |
|
Cinnamyl alcohol |
Rats |
Oral |
2.5 mmol/kg bw |
71/6 |
52/3 |
|
1% benzoyl glucuronide |
Nutley (1990) |
| |
Mice |
Intraperitoneal |
2.5 mmol/kg bw |
71/5 |
32/NR |
2/NR |
2.4% 3-hydroxy-3-phenyl-propionic acid; 4% benzoyl glucuronide |
Nutley (1990) |
| |
Rabbits |
Oral |
31.3 g |
|
22/NR |
NR/43 |
|
Fischer & Bielig (1940) |
|
Cinnamal-dehyde |
Mice |
Intraperitoneal |
2.5 mmol/kg bw |
54/15 |
11/0.6 |
1.4/2 |
|
Nutley (1990) |
| |
Rats |
Oral |
2.5 mmol/kg bw |
62/16 |
36/NR |
|
10% mercapturic acids; 2.2% 3-hydroxy-3-phenylpropionic acid |
Nutley (1990) |
|
|
Rats (M) |
Intraperitoneal |
2 mg/kg bw |
81/7.5 |
85/0.4 |
2.2/NR |
0.8% benzoyl glucuro-nide |
Peters & Caldwell (1994) |
|
|
Rats (M) |
Intraperitoneal |
250 mg/kg bw |
85/0.8 |
73/1 |
1/ 0.3 |
7% benzoyl glucuronide |
Peters & Caldwell (1994) |
|
|
Rats (M) |
Oral |
250 mg/kg bw |
91/7 |
87/0.8 |
0.3/NR |
|
Peters & Caldwell (1994) |
|
|
Rats (F) |
Intraperitoneal |
2 mg/kg bw |
81/8 |
88/NR |
1/NR |
|
Peters & Caldwell (1994) |
|
|
Rats (F) |
Intraperitoneal |
250 mg/kg bw |
70/9 |
84/NR |
0.4/NR |
2.3% benzoyl glucuro-nide |
Peters & Caldwell (1994) |
|
|
Mice (M) |
Intraperitoneal |
2 mg/kg bw |
86/9 |
72/3 |
11.3 |
1% benzoyl glucuronide |
Peters & Caldwell (1994) |
|
|
Mice (M) |
Intraperitoneal |
250 mg/kg bw |
81/6 |
72/NR |
8/NR |
2% benzoyl glucuronide |
Peters & Caldwell (1994) |
|
|
Mice (F) |
Intraperitoneal |
2 mg/kg bw |
71/16 |
72 |
13/NR |
|
Peters & Caldwell (1994) |
|
|
Mice (F) |
Intraperitoneal |
250 mg/kg bw |
79/10 |
71/NR |
4 /NR |
5% benzoyl glucuronide |
Peters & Caldwell (1994) |
|
Cinnamic acid |
Rats (M) |
Oral |
0.5 µmol to 2.5 mmol/kg bw |
85/5 |
50/2 |
|
5% benzoyl glucuronide |
Caldwell & Nutley (1986) |
|
|
Rats |
Intraperitoneal |
18–22 mg/kg bw |
48/25 |
38/9 |
9/NR |
|
Teuchy & Van Sumere (1971) |
|
|
Rats |
Oral |
50 mg/kg bw |
|
67/NR |
|
|
Fahelbum & James (1977) |
|
|
Mice |
Intraperitoneal |
2.5 mmol/kg bw |
90/4 |
67/6 |
3/1 |
5% 3-hydroxy-3-phenyl-propionic acid |
Nutley (1990) |
|
|
Rats |
Oral |
2.5 mmol/kg bw |
82/.9 |
73/2 |
NR/1 |
|
Nutley (1990) |
|
|
Mice |
Intraperitoneal |
0.5 µmol to 2.5 mmol/kg bw |
85/5a |
30/NR |
20/NR |
|
Caldwell & Nutley (1986) |
|
|
Rats (M) |
Oral |
0.5 µmol to 2.5 mmol/kg bw |
85/5a |
> 50/2 |
2/NR |
|
Caldwell & Nutley (1986) |
|
|
Rats |
Oral |
0.0005 mmol/kg bw |
74/0.5 |
71/0.4 |
NR/0.1 |
0.2% 3-hydroxy-3-phenyl-propionic acid; 0.4% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Rats |
Oral |
0.005 mmol/kg bw |
73/1 |
69/0.6 |
NR/0.1 |
0.2% 3-hydroxy-3-phen yl- propionic acid; 0.2% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Rats |
Oral |
0.05 mmol/kg bw |
80/0.9 |
77/0.4 |
NR/0.1 |
0.2% 3-hydroxy-3-phen yl- propionic acid; 0.3% benzoyl glucuronide |
Nutely et al. (1994) |
|
|
Rats |
Oral |
0.5 mmol/kg bw |
73/0.5 |
69/0.4 |
NR/0.1 |
0.2% 3-hydroxy-3-phenyl- propionic acid; 0.5% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Rats |
Oral |
2.5 mmol/kg bw |
88/1 |
76/2.3 |
NR/.3 |
5% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Mice |
Intraperitoneal |
0.0005 mmol/kg bw |
78/2.3 |
44/0.8 |
29 |
0.6% 3-hydroxy-3-phen yl- propionic acid; 0.1% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Mice |
Intraperitoneal |
0.005 mmol/kg bw |
88/1.5 |
54/0.7 |
27 |
0.3% 3-hydroxy-3-phenyl- propionic acid; 0.2% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Mice |
Intraperitoneal |
0.05 mmol/kg bw |
84/2.4 |
64/1 |
0.2/14 |
0.5% 3-hydroxy-3-phenyl- propionic acid; 0.3% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Mice |
Intraperitoneal |
0.5 mmol/kg bw |
85/3.1 |
66/.5 |
0.3/8.2 |
2.0% 3-hydroxy-3-phenyl- propionic acid; 0.2% benzoyl glucuronide |
Nutley et al. (1994) |
|
|
Mice |
Intraperitoneal |
2.5 mmol/kg bw |
93/3.2 |
57/8.6 |
2.3/2.4 |
9.8% 3-hydroxy-3-phenyl- propionic acid; 1.7% benzoyl glucuronide |
Nutley et al. (1994) |
|
Methyl cinnamate |
Rats |
Oral |
50 mg/kg bw |
|
66 |
|
5% benzoyl glucuronic acid |
Fahelbum & James (1977) |
|
|
Rabbits |
Oral |
50 mg/kg bw |
|
66 |
|
5% benzoyl glucuronic acid |
Fahelbum & James (1977) |
|
|
Rabbits (F) |
Oral |
500 mg/kg bw |
|
56 |
|
8% benzoyl glucuronic acid |
Fahelbum & James (1977) |
|
Ethyl cinnamate |
Cats |
Intraperitoneal |
1.19 g/kg bw |
|
55 |
|
|
Dakin (1909) |
|
3-Phenyl propionic acid |
Rabbits |
Oral |
500 mg/kg bw |
|
56 |
8 |
para-hydroxyhippuric acid (trace) |
Fahelbum & James (1977) |
|
3-Phenyl propionic acid, sodium salt |
Cats |
Intraperitoneal |
1190 mg/kg bw |
|
55 |
|
1% acetophenone |
Dakin (1909) |
|
|
Dogs |
Subcutaneous |
120 mg/kg bw |
|
77/NR |
|
|
Raper & Wayne (1928) |
|
|
Dogs |
Subcutaneous |
500 and 700 mg/kg bw |
|
|
50–67/NR |
|
Dakin (1909) |
|
|
Dogs |
Oral |
5, 6, or 7.4 g |
|
14–21/NR |
28–35 |
|
Quick (1928) |
|
|
Foxes |
Subcutaneous |
1280 mg |
|
77 |
|
|
Raper & Wayne (1928) |
|
ortho-Methoxy-cinnamaldehyde |
Rats |
Oral |
239 mg/kg bw |
91 |
trace/trace |
24/3.2 |
22% 3-hydroxy-3-(ortho- methoxy-phenyl) propio nic acid; 37% 2-(ortho- methoxy-phenyl) propionyl glycine |
Samuelson et al. (1986) |
NR, not reported
a Collected over 3 days
In groups of male Fischer 344 rats, 83% of an oral dose of 2.5 mmol/kg bw of [3-14C-d5]-cinnamyl alcohol (335 mg/kg bw), 77% of a dose of [3-14C-d5]-cinnamaldehyde (330 mg/kg bw), and 79% of a dose of [3-14C-d5]-cinnamic acid (370 mg/kg bw) were excreted mainly in the urine within 24 h. Excretion in the faeces accounted for only minor amounts of the administered alcohol (6.1%), aldehyde (16%), and acid (0.9%). More than 90% of the administered dose of any of the three substances was recovered in the urine and faeces within 72 h. Administration of the same doses of the parent alcohol, aldehyde, or acid to groups of CD-1 mice by intraperitoneal injection resulted in a similar pattern of excretion in the urine and faeces at 24 h (75%, 80%, and 93%, respectively) and 72 h (> 93%) (Nutley, 1990).
The tissue distribution and excretion of cinnamaldehyde (No. 656) were studied in groups of eight male Fischer 344 rats pretreated with single daily oral doses of 5, 50, or 500 mg/kg bw of cinnamaldehyde by gavage for 7 days and the same single oral dose of [3-14C]cinnamaldehyde 24 h later. Further groups received no pretreatment but the same single doses. The radiolabel was distributed primarily to the gastrointestinal tract, kidneys, and liver in all groups. After 24 h, > 80% of the radiolabel was recovered in the urine and < 7% in the faeces from all rats, regardless of dose. In all groups, a small amount of the dose was distributed to fat. Radiolabel was still present in animals killed 3 days after receiving 50 or 500 mg/kg bw. In animals pretreated with the two lower doses, the main urinary metabolite was hippuric acid, with small amounts of cinnamic and benzoic acid. In those pretreated with the high dose, benzoic acid was the major metabolite, suggesting that saturation of the glycine conjugation pathway occurs with repeated high doses of cinnamaldehyde (Sapienza et al., 1993).
The effect of dose and sex on the disposition of [3-14C]cinnamaldehyde was studied in Fischer 344 rats and CD-1 mice. More than 85% of doses of 2.0 and 250 mg/kg bw administered to groups of four male and four female rats and six male and six female mice by intraperitoneal injection was recovered in the urine and faeces within 24 h, and > 90% was recovered by 72 h. Of a dose of 250 mg/kg bw of [3-14C]cinnamaldehyde administered orally to Fischer 344 rats, 98% was recovered from the urine (91%) and faeces (7%) within 24 h (Peters & Caldwell, 1994). The effect of dose on the disposition of [3-14C-d5]-cinnamic acid was also studied in Fischer 344 rats and CD-1 mice. Five doses of cinnamic acid in the range 0.0005–2.5 mmol/kg bw were given orally to groups of four rats or by intraperitoneal injection to groups of four mice. After 24 h, 73–88% of the radiolabel was recovered in the urine of rats and 78–93% in the urine of mice; after 72 h, 85–100% of the radiolabel was recovered from rats and 89–100% from mice, mainly in the urine (Caldwell & Nutley, 1986). Only trace amounts of radiolabel were present in the carcass, indicating that cinnamic acid was readily and quantitatively excreted at all doses. The parent alcohol, aldehyde, and acid therefore appear to undergo rapid absorption, metabolism, and excretion, independently of dose up to 250 mg/kg bw, species, sex, and mode of administration (Nutley et al., 1994).
Eleven persons each received a single intravenous dose of cinnamic acid (No. 657) equivalent to 5 mg/kg bw. Analysis of blood showed that 100% of the dose was present within 2.5 min and none after 20 min (Quarto di Palo & Bertolini, 1961).
An oral dose of 1.5 mmol/kg bw (240 mg/kg bw) methyl cinnamate (No. 658) was rapidly and almost completely (95%) absorbed from the gut in rats. The agent was partially hydrolysed to cinnamic acid in the stomach (9%) and gut (40%), and the rate of absorption of cinnamic acid and methyl cinnamate from the gut was similar. No ester was detected in the peripheral blood of dosed rabbits or rats. Only traces were detected in portal and heart blood taken from the rats, indicating that almost complete hydrolysis of methyl cinnamate had occurred during intestinal absorption (Fahelbum & James, 1977).
After administration of a single oral dose of 57 mg of ring-deuterated 3-phenyl-propionic acid to one person, deuterobenzoic acid corresponding to 110% of the dose was isolated from alkaline hydrolysed urine within 100 min (Pollitt, 1974).
These studies indicate that cinnamyl derivatives can be anticipated to be rapidly absorbed, metabolized, and excreted, mainly in the urine, within 24 h.
2.3.1.1 Hydrolysis of esters and acetals
In general, esters containing an aromatic ring system are expected to be hydrolysed in vivo. The hydrolysis is catalysed by classes of enzymes recognized as carboxylesterases or esterases (Heymann, 1980), the most important of which are the A-esterases. In mammals, A-esterases occur in most tissues of the body (Anders, 1989; Heymann, 1980) but predominate in hepatocytes (Heymann, 1980). Acetals are rapidly hydrolysed in acidic media (Morgareidge, 1962).
Esters of cinnamic acid (No. 657) and structurally related aromatic esters have been shown to hydrolyse rapidly to the component acid and alcohol. Oral administration of a single dose of 50 mg/kg bw of methyl cinnamate (No. 658) resulted in the urinary excretion, after 24 h, of hippuric acid (66%) and benzoylglucuronide (5%). This distribution of metabolites, which was nearly identical to that for cinnamic acid, indicates that rapid hydrolysis of the ester in vivo precedes metabolism of the acid (Fahelbum & James, 1977). Ethyl cinnamate (No. 659) administered subcutaneously to a cat also produced only cinnamic acid metabolites in the urine (Dakin, 1909). Incubation of benzyl cinnamate (No. 670) or benzyl acetate with simulated intestinal fluid (pH 7.5; pancreatin) at 37 °C for 2 h resulted in 80% and 50% hydrolysis, respectively (Grundschober, 1977). Incubation of the structurally related aromatic acetal, 2-phenylpropanal dimethyl acetal (1 mmol/L) with simulated gastric juice at 37 °C resulted in 97% hydrolysis within 1 h. Under the same experimental conditions, benzaldehyde propylene glycol acetal (1 mmol/L) was 97% hydrolysed within 5 h (Morgareidge, 1962).
2.3.1.2 Oxidation and conjugation reactions of cinnamyl alcohol (No. 647) and cinnamaldehyde derivatives
The aromatic primary alcohols and aldehydes used as flavouring substances or formed by the hydrolysis of esters and acetals are readily oxidized to a cinnamic acid derivative (see Figure 2). Human NAD+-dependent alcohol dehydrogenase catalyses oxidation of primary alcohols to aldehydes (Pietruszko et al., 1973), and isoenzyme mixtures of NAD+-dependent aldehyde dehydrogenase (Weiner, 1980) catalyse oxidation of aldehydes to carboxylic acids. Aromatic alcohols and aldehydes have been reported to be excellent substrates for alcohol dehydrogenase (Sund & Theorell, 1963) and aldehyde dehydrogenase (Feldman & Wiener, 1972), respec-tively. The urinary metabolites of cinnamyl alcohol (No. 647) and cinnamaldehyde (No. 656) are mainly those derived from metabolism of cinnamic acid (see Figure 2).
In four rats given cinnamyl alcohol (No. 647) orally at a dose of 335 mg/kg bw, 52% was recovered in the urine within 24 h as the glycine conjugate of benzoic acid (hippuric acid). Ten minor metabolites cumulatively accounted for about 10% of the dose. When cinnamyl alcohol (No. 647) was administered to mice by intraperitoneal injection, hippuric acid was the major urinary metabolite (Nutley, 1990).
trans-[3-14C]Cinnamaldehyde was given at doses of 2 and 250 mg/kg bw by intraperitoneal injection to male and female Fischer 344 rats and CD-1 mice, and doses of 250 mg/kg bw were administered by oral gavage to male rats and mice only. In both species, the major urinary metabolites were formed from oxidation of cinnamaldehyde (No. 656) to cinnamic acid (No. 657), which was subsequently oxidized in the -oxidation pathway. The major urinary metabolite was hippuric acid (71–75% in mice and 73–87% in rats), and this was accompanied by small amounts of metabolites, including 3-hydroxy-3-phenylpropionic acid (0.4–4%), benzoic acid (0.4–3%), and benzoyl glucuronide (0.8–7.0%). The glycine conjugate of cinnamic acid was formed to a considerable extent only in the mice (4–13%). Glutathione conjugation of cinnamaldehyde competes to a small extent with the oxidation pathway. Approximately 6–9% of either dose was excreted within 24 h as glutathione conjugates of cinnamaldehyde. The authors concluded that the excretion pattern and metabolic profile of cinnamaldehyde in rats and mice are not systematically affected by sex, dose size, or route of administration (Peters & Caldwell, 1994).
The toxicokinetics of cinnamaldehyde (No. 656) has been investigated in male Fischer 344 rats. Cinnamaldehyde (limit of detection, < 0.1 µg/ml) and cinnamic acid (No. 657; < 1 µg/ml) were not detectable in the plasma of groups of three to six rats given a single oral dose of 50 mg/kg bw by gavage in corn oil. At doses of 250 and 500 mg/kg bw, the plasma concentrations of cinnamaldehyde and cinnamic acid were approximately 1 and > 10 µg/ml, respectively. The bioavailability of cinnamaldehyde was calculated to be < 20% at both doses. A dose-dependent increase in the amount of hippuric acid, the major urinary metabolite, occurred 6 h after gavage and continued over the next 18 h. Only small amounts of cinnamic acid were excreted in the urine either free or as the glucuronic acid conjugate. The hippuric acid recovered in the urine over 50 h accounted for 72–81% over doses ranging from 50 to 500 mg/kg bw (Yuan et al., 1992).
Approximately 15% of an oral dose of 250 mg/kg bw of cinnamaldehyde (No. 656) administered to rats by gavage was excreted in the urine as two mercapturic acid derivatives, N-acetyl-S-(1-phenyl-3-hydroxypropyl)cysteine and N-acetyl-S-(1-phenyl-2-carboxyethyl)cysteine, in a ratio of 4:1. Approximately 9% of an oral dose of 125 mg/kg bw of cinnamyl alcohol (No. 647) was excreted in the urine as N-acetyl-S-(1-phenyl-3-hydroxypropyl)cysteine (Delbressine et al., 1981).
2.3.1.3 Metabolism of cinnamic acid (No. 657)
In animals, aromatic carboxylic acids such as cinnamic acid (No. 657) that enter the cell are converted to acyl coenzyme A (CoA) esters. Cinnamoyl CoA either conjugates with glycine, a reaction catalysed by N-acyl transferase, or undergoes -oxidation eventually leading to the formation of benzoyl CoA. The reactions which form benzoic acid from cinnamic acid are reversible but the equilibrium favours formation of the benzoic acid CoA ester. Benzoyl CoA is in turn conjugated with glycine, yielding hippuric acid, or the CoA thioester is hydrolysed to yield free benzoic acid, which is then excreted (Nutley et al., 1994). CoA thioesters of carboxylic acids are obligatory intermediates in amino acid conjugation reactions (Hutt & Caldwell, 1990). The reactions in this sequence are of historical significance in biochemistry, since it was studies on cinnamic acid and fatty acids that revealed the beta-oxidation pathway of fatty acid catabolism (Nutley et al., 1994). Regardless of dose or species, the beta-oxidation pathway is the predominant pathway of metabolic detoxication of cinnamic acid in animals (see Table 3).
Six doses in the range 0.0005–2.5 mmol/kg (0.08–400 mg/kg bw) of [14C]- or [14C/5H2]-cinnamic acid (No. 657) were administered orally to male Fischer 344 rats or by intraperitoneal injection to male CD-1 mice. In both species, 84–101% was recovered within 72 h, most (73–93%) being recovered from the urine within 24 h. The metabolites identified at all doses included hippuric acid, benzoyl glucuronide, 3-hydroxy-3-phenyl-propionic acid, benzoic acid, and unchanged cinnamic acid. The major metabolite at all doses was hippuric acid (44–77%). At the highest dose tested (2.5 mmol/kg bw), the percentage of hippuric acid decreased while the percentages of benzoyl glucuronide and benzoic acid increased. The formation of larger amounts of benzoyl glucuronide (0.5–5%) and free benzoic acid (0.4–2%) at doses > 0.5 mmol/kg bw provides evidence that saturation of the glycine conjugation pathway occurs at these doses. The fact that the excretion of 3-hydroxy-3-phenyl-propionic acid differed little over the dose range (0.2–0.9%) supports the conclusion that the capacity of the beta-oxidation pathway is not limited at doses of cinnamic acid up to 2.5 mmol/kg bw in male rats (Nutley et al., 1994). The increasing role of glucuronic acid conjugation relative to glycine conjugation as the dose increases is a general trend observed in the metabolism of carboxylic acids (Caldwell et al., 1980).
In mice, glycine conjugation of cinnamic acid (No. 657) competes with the beta-oxidation pathway, but only at low doses. As the dose was increased from 0.0005 to 2.5 mmol/kg bw, the urinary concentration of hippuric acid increased from 44 to 67%, while that of cinnamoylglycine decreased from 29 to 2.4%. These results suggest that gylcine N-acetyl transferase has a stronger affinity but a lower capacity for cinnamic acid than for benzoic acid. At the highest dose, 2.5 mmol/kg bw, the amount of free benzoic acid excreted was increased from 0.8 to 8.6%, suggesting that the capacity of glycine conjugation of benzoyl CoA is also limited in mice. At all doses, mice excreted only a small proportion of benzoyl glucuronide, indicating that this conjugation reaction is of minimal importance in this species (Nutley et al., 1994).
Eleven volunteers received a single intravenous dose of cinnamic acid (No. 657) equivalent to 5 mg/kg bw. In blood plasma, 100% of the dose was found within 2.5 min and 0% after 20 min. Ninety minutes after dosing, hippuric acid, cinnamoyl-glucuronide, and benzoylglucuronide were found in a ratio of 74:24:1.5 (Quarto di Palo & Bertolini, 1961).
2.3.1.4 Effects of ring and chain substituents on metabolism of cinnamyl derivatives
The position and size of the substituent play a role in the metabolism of cinnamyl derivatives. Those containing alpha-methyl substituents (e.g. alpha-methylcinnamaldehyde, No. 683) are extensively metabolized via beta-oxidation and cleavage to yield mainly the corresponding hippuric acid derivative. A benzoic acid metabolite was isolated from the urine of dogs given either alpha-methylcinnamic acid or alpha-methylphenylpropionic acid (Kay & Raper, 1924). Larger substituents located at the alpha- or beta-position inhibited beta-oxidation to some extent (Deuel, 1957; Kassahun et al., 1991), in which case there may be direct conjugation of the carboxylic acid with glucuronic acid, followed by excretion. While alpha-methylcinnamic acid undergoes oxidation to benzoic acid, alpha-ethyl- and alpha-propylcinnamic acids are excreted unchanged (Carter, 1941). alpha-Ethylcinnamic alcohol and alpha-ethylcinnamaldehyde administered orally to rabbits resulted in urinary excretion of alpha-ethylcinnamic acid and of small amounts of benzoic acid (Fischer & Bielig, 1940). These observations suggest that alpha-methylcinnam-aldehyde undergoes oxidation to benzoic acid, while higher homologues are excreted primarily unchanged or as the conjugated form of the cinnamic acid derivative.
ortho-ring substituents (e.g. ortho-methoxycinnamaldehyde, No. 688) selectively inhibit oxidation of CoA esters of beta-hydroxyacids within the beta-oxidation pathway, and the hydroxyacid derivative is excreted as a glycine conjugate. The metabolism of ortho-methoxycinnamaldehyde ceases after formation of the beta-hydroxy derivative (Samuelsen et al., 1986).
The glycine conjugates of ortho-methoxycinnamic and ortho-methoxyphenyl-propionic acids are the principal urinary metabolites of ortho-methoxycinnamaldehyde in rats. Relatively large amounts of the beta-hydroxylated phenylpropionic acid derivatives were also detected, but only traces of benzoic and hippuric acid derivatives (products of further beta-oxidation) were excreted. The detection of relatively large amounts of a beta-hydroxylated derivative suggests that this metabolite is not readily oxidized, perhaps because of steric hindrance of the ortho substituent (Solheim & Scheline, 1973).
In contrast, para-ring substituents (e.g. 3-(para-isopropyl-phenyl)propional-dehyde, No. 680, and para-methylcinnamaldehyde, No. 682) may not affect metabolism via beta-oxidation significantly. In male albino rats, para-methoxycinnamic acid was metabolized primarily to para-methoxybenzoic acid and its corresponding glycine conjugate (Solheim & Scheline, 1973). Similar results were reported with 3,4-dimethoxycinnamic acid (which is meta and para substituted) (Solheim & Scheline, 1976). The structurally related substance para-tolualdehyde has been reported to be metabolized to para-methylbenzoic acid with no apparent oxidation of the methyl group (Williams, 1959). These observations indicate that the presence of side-chain alkyl substituents with more than one carbon atom and of ortho-ring substituents inhibits the beta-oxidation pathway. In these cases, the parent acid (cinnamic acid derivative) or an intermediary beta-oxidation metabolite (e.g. beta-hydroxy-3-phenylpropanoic acid derivative) is excreted as the glycine or glucuronic acid conjugate.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
LD50 values after oral administration have been reported for 39 of the 55 substances in this group. In rats, the values were in the range 1500 to > 5000 mg/kg bw (Jenner et al., 1964; Moreno, 1971; Weir & Wong, 1971; Keating, 1972; Levenstein & Wolven, 1972; Moreno, 1972; Denine & Palanker, 1973; Moreno, 1973; Russell, 1973; Moreno, 1974; Opdyke, 1974; Wohl, 1974; Zaitsev & Rakhmanina, 1974; Levenstein, 1975; Moreno, 1975; Levenstein, 1976; Moreno, 1976, 1977, 1981, 1982; Schafer et al., 1983), demonstrating that the acute toxicity of these substances after oral administration is low. In rats, the values were 900 to > 5000 mg/kg bw (Draize et al., 1948; Harada & Ozaki, 1972; Zaitsev & Rakhmanina, 1974; Levenstein, 1975; Schafer & Bowles, 1985), and in guinea-pigs they were 3100 to > 5000 mg/kg bw (Draize et al., 1948; Zaitsev & Rakhmanina, 1974).
2.3.2.2 Short-term and long-term studies of toxicity
Toxicological studies have been reported for 11 substances in the group. The results of those with the parent alcohol cinnamyl alcohol (No. 647), the corresponding aldehyde, two cinnamate esters, two alpha-alkyl substituted cinnamaldehyde derivatives, two alkoxy-substituted cinnamaldehyde derivatives, and a mixture of five cinnamyl derivatives are described below and summarized in Table 4.
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on cinnamyl alcohol and related substances used as flavouring agents administered orally
|
No. |
Substance |
Species; sex |
No. test groupsa/ no. per groupb |
Duration |
NOEL (mg/kg bw per day) |
Reference |
|
647 |
Cinnamyl alcohol |
Rat; M |
1/12 |
4 months |
54c |
Zaitsev & Rakhmanina (1974) |
|
656 |
Cinnamaldehyde |
Rat; M |
1/12 |
4 months |
68c |
Zaitsev & Rakhmanina (1974) |
|
656 |
Cinnamaldehyde |
Rat; M, F |
3/10 |
12 weeks |
230c |
Trubeck Laboratories (1958a) |
|
656 |
Cinnamaldehyde |
Rat; M, F |
2/24 |
12 weeks |
100c,d |
Trubek Laboratories (1958b) |
|
656 |
Cinnamaldehyde |
Rat; M, F |
4/20 |
13 weeks |
620 |
National Toxicology Program (1995) |
|
656 |
Cinnamaldehyde |
Rat; M, F |
3/20 |
16 weeks |
120c |
Hagan et al. (1967) |
|
658 |
Methyl cinnamate |
Rat; M, F |
2/24 |
12 weeks |
3c,d |
Trubeck Laboratories (1958b) |
|
659 |
Ethyl cinnamate |
Rat; M |
1/12 |
4 months |
80c |
Zaitsev & Rakhmanina (1974) |
|
659 |
Ethyl cinnamate |
Rat; M, F |
2/24 |
12 weeks |
3c,d |
Trubeck Laboratories (1958b) |
|
668 |
Linalyl cinnamate |
Rat; M, F |
3/20 |
17 weeks |
500c |
Hagan et al. (1967) |
|
670 |
Benzyl cinnamate |
Rat; M, F |
2/20 |
19 weeks |
500c |
Hagan et al. (1967) |
|
670 |
Cinnamyl cinnamate |
Rat; M, F |
2/24 |
12 weeks |
3c,d |
Trubeck Laboratories (1958b) |
|
683 |
alpha-Methylcinnamaldehyde |
Rat; M |
3/10 |
90 days |
220c |
Trubeck Laboratories (1958c) |
|
683 |
alpha-Methylcinnamaldehyde |
Rat; M, F |
2/24 |
12 weeks |
3c,d |
Trubeck Laboratories (1958b) |
|
685 |
alpha-Amylcinnamaldehyde |
Rat; M, F |
3/30 |
14 weeks |
290c (M)
320c (F) |
Carpanini et al. (1973) |
|
685 |
alpha-Amylcinnamaldehyde |
Rat; M, F |
1/30 |
90 days |
6.1c (M)
6.6c (F) |
Oser et al. (1965) |
|
688 |
ortho-Methoxycinnamaldehyde |
Rat; M, F |
1/20–32 |
90 days |
47c (M)
52c (F) |
Posternak et al. (1969) |
|
689 |
para-Methoxy-alpha-methyl-cinnamaldehyde |
Rat; M, F |
1/20–32 |
90 days |
2.4c (M)
2.7c (F) |
Posternak et al. (1969) |
M, male; F, female
a Total number of test groups does not include control animals.
b Total number per test group includes both male and female animals.
c Study performed with either a single dose or multiple doses that had no adverse effect; the value is therefore the highest dose tested.
d The substance was administered as a component of a mixture.
Cinnamyl alcohol (No. 647), cinnamaldehyde (No. 656), and ethyl cinnamate (No. 659)
Sunflower oil solutions containing cinnamyl alcohol (No. 647; 0.2 ml/100 g bw) providing a dose of 54 mg/kg bw per day, cinnamaldehyde (No. 656) providing a dose of 68 mg/kg bw per day, or ethyl cinnamate (No. 659) providing a dose of 80 mg/kg bw per day, equivalent to 0.02 of the LD50 for the respective substance, was administered to groups of 12 male white rats (strain not identified) by oral intubation once daily for 4 months. Liver function was tested at days 40 and 140. Increased (26%) blood serum fructose diphosphate aldolase activity was observed in the groups given cinnamyl alcohol (No. 647) and ethyl cinnamate (No. 659) on day 140, but the activities of serum cholinesterase and alanine aminotransferase and the concentration of SH groups in serum showed no change from control values. The authors concluded that none of the three cinnamyl derivatives caused pronounced pathological changes in rat liver; however, the study was described by the authors as preliminary (Zaitsev & Rakhmanina, 1974).
Cinnamaldehyde (No. 656)
Groups of 10 male and 10 female Osborne-Mendel rats were maintained on a diet containing cinnamaldehyde (No. 656) at a concentration of 0 (control), 1000, 2500, or 10 000 mg/kg, equivalent to 50, 120, and 500 mg/kg bw per day, for 16 weeks. Body weights and food intake, recorded weekly, did not differ significantly between treated and control animals. Haematological parameters at termination were normal. At necropsy, no differences in the weights of the major organ were found. Gross examination of the tissues showed no remarkable changes. Histological examination of three to four male and female animals at the high dose revealed slight hepatic cellular swelling and slight hyperkeratosis of the squamous epithelium of the stomach. The NOEL was 120 mg/kg bw per day (Hagan et al., 1967).
Groups of five male and five female rats were maintained on a diet containing cinnamaldehyde (No. 656) at concentrations calculated to result in a daily intake of 0 (control), 58, 110, or 230 mg/kg bw for 12 weeks. General condition, behaviour, body weight, food intake, and efficiency of food use were recorded regularly, and no statistically significant differences were seen between treated and control animals. Haematological examination after 12 weeks revealed normal blood haemoglobin concentration, and urine analysis revealed the absence of glucose and only traces of albumin in males (attributed to the possible presence of semen). At necropsy, no significant difference in liver or kidney weights was seen between treated and control groups. Gross examination revealed occasional respiratory infection in animals in all groups (Trubeck Laboratories, 1958a).
In a 13-week study, groups of 10 male and 10 female Fischer 344/N rats were given diets containing 0, 1.25, 2.5, 5.0, or 10% microencapsulated trans-cinnamaldehyde (No. 656), equal to 0, 620, 1250, 2500, or 5000 mg/kg bw per day. Necropsies were performed on all survivors, and tissues from animals at the two highest doses and the control group were examined histologically. There were no early deaths and no treatment-related clinical toxicity. The mean terminal body weights of untreated controls and vehicle controls were similar, but the mean body weights of animals at the three higher doses were decreased. The food consumption of treated animals was depressed during the first week, possible because of unpalata-bility. No overt haematological effects were seen. The clinical chemical parameters that were increased by treatment included bile salt concentration and alanine transaminase activity (in males and females at the highest dose), suggesting mild cholestasis. Microscopic examination showed no morphological alterations to the liver. Gross and microscopic examination of the stomach and forestomach indicated irritation at all doses of trans-cinnamaldehyde. The NOEL was 620 mg/kg bw per day (National Toxicology Program, 1995).
Mixture of cinnamaldehyde (No. 656), methyl cinnamate (No. 658), ethyl cinnamate (No. 659), cinnamyl cinnamate (No. 673), and alpha-methyl-cinnamaldehyde (No. 683)
A mixture of flavourings containing 900 mg/kg cinnamaldehyde (No. 22) and 25 mg/kg each of methyl cinnamate (No. 656), ethyl cinnamate (No. 659), cinnamyl cinnamate (No. 673), and alpha-methyl-cinnamaldehyde (No. 683) was added to the diet of groups of 12 rats of each sex for 12 weeks, resulting in daily intakes of 110 mg/kg bw for males and 120 mg/kg bw for females, which were equivalent to 100 mg/kg bw of cinnamaldehyde and 3 mg/kg bw of each of the other components. Weekly measurements of body weight and food intake revealed a statistically nonsignificant decrease in weight gain in treated males when compared with controls. The efficiency of food use was statistically significantly decreased in treated males (p < 0.01) and females (p < 0.05) when compared with their respective controls. At week 12, blood haemoglobin, urinary sugar, and urinary albumin concentrations, measured in three animals of each sex, were normal. At necropsy, the weights of the liver, kidney, and brain were within normal limits. Gross examination revealed no obvious differences between treated and control groups. The results of histological examinations were not reported (Trubeck Laboratories, 1958b).
Linalyl cinnamate (No. 668) and benzyl cinnamate (No. 670)
Groups of 10 male and 10 female Osborne-Mendel rats were fed a diet containing linalyl cinnamate (No. 668) at a concentration of 0 (control), 1000, 2500, or 10 000 mg/kg (equivalent to 0, 50, 120, or 500 mg/kg bw per day) for 17 weeks, and groups of five animals of each sex were given benzyl cinnamate (No. 670) at a concentration of 0 (control), 1000, or 10 000 mg/kg of diet (equivalent to 0, 50, or 500 mg/kg bw per day) for 19 weeks. The diets were prepared weekly; analysis of old diet preparations revealed a 4% weekly loss of linalyl cinnamate, but the dietary loss of benzyl cinnamate was not determined. No significant differences in body weight or food intake, recorded weekly, were seen between treated and control animals. Haematological examination at termination revealed no significant difference between treated and control animals, and no difference in the weights of the major organs was found at necropsy. Gross examination of the tissue of animals given either agent showed no remarkable change. Histological examination of three to four animals of each sex at the high dose of linalyl cinnamate and in the control group revealed no treatment-related lesions. The tissues of animals given benzyl cinnamate were not examined histologically (Hagan et al., 1967).
ortho-Methoxycinnamaldehyde (No. 688) and para-methoxy-alpha-methyl-cinnamaldehyde (No. 689)
Groups of 10–16 Charles River CD rats of each sex were maintained on diets containing ortho-methoxycinnamaldehyde (No. 688) at concentrations calculated to result in daily intakes of 0 (control) or 47 mg/kg bw for males and 52 mg/kg bw for females or para-methoxy-alpha-methycinnamaldehyde at concentrations calculated to result in daily intakes of 2.4 mg/kg bw for males and 2.7 mg/kg bw for females, for 90 days. The control groups received basal diets only. The animals were housed in pairs of the same sex and given access to water and food ad libitum. The concentration of the test material in the diet was adjusted during the study to maintain constant dietary intakes. Clinical observations were recorded daily, and food consumption and body weights were determined weekly. Haematological parameters and blood urea were determined in 50% of the animals at week 7 and on all animals at week 13. After 90 days, all animals were killed and necropsied, and the livers and kidneys were weighed. A wide range of tissues and organs from each animal were preserved, and major organs and tissues were examined histologically. No differences in growth, food intake, haematological or clinical chemical parameters, organ weights, or organ appearance were observed between treated and control animals (Posternak et al., 1969).
alpha-Methylcinnamaldehyde (No. 683)
Groups of five rats of each sex were maintained on a diet containing alpha-methylcinnamaldehyde (No. 683) at concentrations calculated to result in average daily intakes of 0, 58, 120, or 220 mg/kg bw for 90 days. Growth and food intake were recorded weekly, as were the results of regular examinations for physical appearance, behaviour, and efficiency of food use. At week 12, urine samples were collected and analysed for the presence of sugar and albumin, and blood samples were taken for determination of haemoglobin. No statistically significant differences were found between treated and control animals, and no differences in liver or kidney weights were seen (Trubeck Laboratories, 1958c).
alpha-Amylcinnamaldehyde (No. 685)
Groups of 15 male and 15 female CFE rats were maintained on a diet containing alpha-amylcinnamaldehyde (No. 685) at a concentration of 0 (control), 80, 400, or 4000 mg/kg for 14 weeks. Additional groups of five male and five female rats were maintained on diets containing 400 or 4000 mg/kg of the agent for 2 and 6 weeks. The mean dietary intakes over 14 weeks were reported to be 0, 6.1, 30, and 290 mg/kg bw per day for males and 0, 6.7, 35, and 320 mg/kg bw per day for females. Measurement of body weight and food and water consumption revealed no significant differences between treated and control groups. Evaluations of haemoglobin content, haematocrit, erythrocyte and leukocyte counts, and individual leukocyte counts and of blood chemistry at 2, 6, and 14 weeks revealed normal values. Reticulocyte counts, performed only on controls and animals at the high dose, showed no significant difference. Urine analysis performed during the final week of treatment revealed no difference in cell content or renal concentration. At autopsy, a statistically significant increase in the relative weight of the liver was seen in males (p < 0.01) and females (p < 0.05) at 4000 mg/kg of diet after 14 weeks, the stomach weights of males at 400 mg/kg of diet were decreased after 6 weeks, and the relative weight of the kidneys was increased in males (p < 0.01) at 4000 mg/kg after 14 weeks. The increases in relative organ weights were not associated with histological lesions. Microscopic examination of tissues from all major organs revealed no histopathological changes that could be associated with administration of the agent (Carpanini et al., 1973).
Groups of 15 male and 15 female FDRL rats were maintained on a diet containing alpha-amylcinnamaldehyde (No. 685) at concentrations calculated to result daily intakes of 6.1 mg/kg bw for males and 6.6 mg/kg bw for females, for 90 days. Body weight, food consumption, and general condition were recorded regularly. Haematological and clinical chemical parameters were measured in eight rats of each sex at week 6 and in all animals at week 12. The measurements of growth, haematology, and clinical chemistry and histopathology at necropsy gave no evidence of toxic effects (Oser et al., 1965).
2.3.2.3 Genotoxicity
Numerous studies of the genotoxicity of the substances in this group of flavouring substances have been reported and are summarized in Table 5 and described below.
Table 5. Studies of genotoxicity with cinnamyl alcohol and related substances used as flavouring agents
|
No. |
Agent |
End-point |
Test object |
Maximum concentration |
Result |
Reference |
|
In vitro |
|
645 |
3-Phenylpropion-aldehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate
(402 µg/plate) |
Negativea |
Florin et al. (1980) |
|
645 |
3-phenylpropion-aldehyde |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(4468 µg) |
Negativeb |
Sasaki et al. (1989) |
|
647 |
cinnamyl alcohol |
Reverse mutationc |
S. typhimurium TA1537, TA1535 |
3000 µg/plate |
Negativea |
Sekizawa & Shibimoto (1982) |
|
647 |
cinnamyl alcohol |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
21 µg/disc |
Negativeb |
Oda et al. (1979) |
|
647 |
cinnamyl alcohol |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
1.0 mg/disc
(1000 µg/disc) |
Positivea |
Sekizawa & Shibimoto (1982) |
|
647 |
cinnamyl alcohol |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
10 µl/disc
(10 400 µg/disc) |
Positiveb |
Yoo (1986 |
|
647 |
cinnamyl alcohol |
Mutation |
E. coli WP2 uvrA |
3000 µg/plate |
Negativeb |
Sekizawa & Shibimoto (1982) |
|
647 |
cinnamyl alcohol |
Mutation |
E. coli WP2 uvrA |
4.0 mg/plate
(4000 µg/plate) |
Negativeb |
Yoo (1986) |
|
647 |
cinnamyl alcohol |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(4468 µg) |
Negativeb |
Sasaki et al. (1989) |
|
650 |
cinnamyl acetate |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(5868 µg) |
Negativeb |
Sasaki et al. (1989) |
|
656 |
Cinnamaldehyde |
Reverse mutationc |
S. typhimurium TA1537, TA1538, TA98, TA100, TA1535 |
600 µg/plate |
Negativea |
Sekizawa & Shibamoto (1982) |
|
656 |
trans-cinnamaldehyde |
Reverse mutation |
S. typhimurium TA1537, TA98, TA100, TA1535 |
10 mg/plate
(10,000 µg/plate) |
Negativea |
Prival et al. (1982) |
|
656 |
cinnamaldehyde |
Reverse mutation |
S. typhimurium TA104 (with preincubation) |
0.8 µmol
(105 µg) |
Negativea |
Marnett et al. (1985) |
|
656 |
Cinnamaldehyde |
Reverse mutation |
S. typhimurium TA1537, TA92, TA94, TA98, TA100, TA1535 (with preincubation) |
0.5 mg/plate
(500 µg/plate) |
Positivea,d |
Ishidate et al. (1984) |
|
656 |
trans-Cinnamaldehyde |
Reverse mutation |
S. typhimurium TA1537, TA92, TA94, TA98, TA100, TA1535 (with plate incorporation and preincubation) |
500 µg/plate |
Negativea |
Lijinsky & Andrews (1980) |
|
656 |
trans-Cinnamaldehyde |
Reverse mutation |
S. typhimurium TA1537, TA1538, TA98, TA100, TA1535 (with plate incorporation and preincubation) |
500 µg/plate |
Negativea |
Kasamaki et al. (1982) |
|
656 |
cinnamaldehyde |
Reverse mutation |
S. typhimurium TA97, TA98, TA100 (with preincubation) |
1 mg/ml
(1000 µg/ml) |
Negativea |
Azizan & Blevins (1995) |
|
656 |
trans-cinnamaldehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA104 (with preincubation) |
Not reported |
Negativea |
Kato et al. (1989) |
|
656 |
trans-cinnamaldehyde |
Reverse mutation |
S. typhimurium TA1537, TA98, TA100, TA1535 (with preincubation) |
100 µg/plate |
Negativea |
Mortelmans et al. (1986) |
|
656 |
trans-cinnamaldehyde |
Reverse mutation |
S. typhimurium TA100 (with preincubation) |
5 µmol/plate
(661 µg/plate) |
Negativea |
Neudecker et al. (1983) |
|
656 |
cinnamaldehyde |
Mutation |
E. coli WP2 uvrA |
600 µg/plate |
Negativeb |
Sekizawa & Shibimoto (1982) |
|
656 |
cinnamaldehyde |
Mutation |
E. coli WP2 uvrA |
0.8 mg/plate
(800 µg/plate) |
Negativeb |
Yoo (1986) |
|
656 |
cinnamaldehyde |
DNA repair |
B. subtilis M45 (rec-) |
0.2 mg/disk
(200 µg/disc) |
Positiveb |
Sekizawa & Shibimoto (1982) |
|
656 |
cinnamaldehyde |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
10 µl/disc
(10,500 µg/disc) |
Positiveb |
Yoo (1986) |
|
656 |
cinnamaldehyde |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
10 µl/disc
(10,500 µg/disc) |
Positivea |
Kuroda et al. (1984) |
|
656 |
cinnamaldehyde |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
21 µg/disc |
Negativeb |
Oda et al. (1979) |
|
656 |
cinnamaldehyde |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(4401 µg) |
Negativeb |
Sasaki et al. (1987) |
|
656 |
cinnamaldehyde |
Chromosomal aberration |
Chinese hamster fibroblasts |
0.015 mg/ml
(15 µg/ml) |
Positiveb |
Ishidate et al. (1984) |
|
656 |
cinnamaldehyde |
Chromosomal aberration |
Chinese hamster B241 cells |
20 nmol/L
(2.6 µg) |
Positiveb |
Kasamaki & Urasawa (1985) |
|
656 |
cinnamaldehyde |
Chromosomal aberration |
Chinese hamster B241 cells |
10 nmol/L
(1.3 µg) |
Positivea |
Kasamaki et al. (1982) |
|
656 |
trans-cinnamaldehyde |
Chromosomal aberration |
Chinese hamster ovary cells |
18.3 µg/ml
100 µg/ml |
Negativeb
Negativee |
Galloway et al. (1987) |
|
656 |
trans-cinnamaldehyde |
Sister chromatid exchange |
Chinese hamster ovary cells |
6.8 µg/ml |
Weakly positiveb |
Galloway et al. (1987) |
|
|
|
|
|
91.8 µg/ml |
Weakly positivee |
|
|
656 |
Cinnamaldehyde |
DNA strand breaks |
Mouse L1210 lymphoma cells |
500 µmol
(66 080 µg) |
Positiveb |
Eder et al. (1993) |
|
656 |
Cinnamaldehyde |
Cytotoxicity |
Mouse L1210 lymphoma cells |
10 µg/ml |
Positiveb |
Moon & Pack (1983) |
|
656 |
Cinnamaldehyde |
Mutation |
Chinese hamster V79 cells |
100 µmol/L
(13 216 µg) |
Negativeb |
Fiorio & Bronzetti (1994) |
|
656 |
Cinnamaldehyde |
Micronucleus formation |
Hep-G2 cells |
500 µg/ml |
Weakly positiveb |
Sanyal et al. (1997) |
|
657 |
Cinnamic acid |
Reverse mutation |
S. typhimurium TA1537, TA1538, TA98, TA100, TA1535 (with plate incorporation and preincubation) |
1000 µg |
Negativea |
Lijinsky & Andrews (1980) |
|
657 |
Cinnamic acid |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
25 µg/disc |
Negativeb |
Oda et al. (1979) |
|
657 |
Cinnamic acid |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
2.0 mg/disc
(2000 µg/disc) |
Negativeb |
Yoo (1986) |
|
657 |
Cinnamic acid |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(4934 µg) |
Positiveb |
Sasaki et al. (1989) |
|
658 |
Methyl cinnamate |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
20 µg/disc |
Negativeb |
Oda et al. (1979) |
|
658 |
Methyl cinnamate |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(5401 µg) |
Positiveb |
Sasaki et al. (1989) |
|
659 |
Ethyl cinnamate |
Reverse mutation |
S. typhimurium TA1537, TA92, TA94, TA98, TA100,TA1535 (with preincubation) |
5.0 mg/plate
(5000 µg/plate) |
Negativea |
Ishidate et al. (1984) |
|
659 |
Ethyl cinnamate |
Chromosomal aberration |
Chinese hamster fibroblasts |
0.063 mg/ml
(63 µg/ml) |
Equivocalb |
Ishidate et al. (1984) |
|
659 |
Ethyl cinnamate |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
20 µg/disc |
Negativeb |
Oda et al. (1979) |
|
659 |
Ethyl cinnamate |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3 µmol/L
(5868 µg) |
Positiveb |
Sasaki et al. (1989) |
|
19 |
Allyl cinnamate |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
667 |
Cyclohexyl cinnamate |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
670 |
Benzyl cinnamate |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate
(715 µg/plate) |
Negativea |
Florin et al. (1980) |
|
670 |
Benzyl cinnamate |
DNA repair |
B. subtilis M45 (rec-) and H17 (rec+) |
1.0 mg/disc
(1000 µg/disc) |
Negativeb |
Yoo (1986) |
|
674 |
alpha-Amylcinnamyl alcohol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
683 |
alpha-Methylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA100(with preincubation) |
4 µmol/plate
(585 µg/plate) |
Negativea |
Neudecker et al. (1983) |
|
683 |
alpha-Methylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 (with preincubation) |
500 µg/plate |
Negativea |
Mortelmans et al. (1986) |
|
683 |
alpha-Methylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
683 |
alpha-Methylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
685 |
alpha-Amylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA97, TA102 (with preincubation) |
1.0 mg/plate
(1000 µg/plate) |
Negativea |
Fujita & Sasaki (1987) |
|
686 |
alpha-Hexylcinnamal-dehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
688 |
ortho-Methoxycinnamal-dehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 (with preincubation) |
666 µg/plate |
Positivea |
Mortelmans et al. (1986) |
|
689 |
para-Methoxy-alpha-methyl- cinnamaldehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3.6 mg/plate
(3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
In vivo |
|
656 |
trans-Cinnamaldehyde |
Sex-linked recessive lethal mutation |
D. melanogaster |
800 mg/kg od diet
(800 µg/g) |
Negative |
Woodruff et al. (1985) |
|
19 |
Allyl cinnamate |
Sex-linked recessive lethal mutation |
D. melanogaster |
1 mmol/L
(188 000 µg) |
Negative |
Wild et al. (1983) |
|
674 |
alpha-Amylcinnamyl alcohol |
Sex-linked recessive lethal mutation |
D. melanogaster |
45 mmol/L
(9 194 000 µg) |
Negative |
Wild et al. (1983) |
|
683 |
alpha-Methylcinnamal-dehyde |
Sex-linked recessive lethal mutation |
D. melanogaster |
5 mmol/L
(731 000 µg) |
Negative |
Wild et al. (1983) |
|
685 |
alpha-Amylcinnamal-dehyde |
Sex-linked recessive lethal mutation |
D. melanogaster |
10 mmol/L
(2 023 000 µg) |
Negative |
Wild et al. (1983) |
|
686 |
alpha-Hexylcinnamal-dehyde |
Sex-linked recessive lethal mutation |
D. melanogaster |
10 mmol/L
(2 163 000 µg) |
Negative |
Wild et al. (1983) |
|
656 |
Cinnamaldehyde |
Unscheduled DNA synthesis |
Rat and mouse hepatocytes |
1 000 000 µg/kg bw |
Negative |
Mirsalis et al. (1989) |
|
656 |
Cinnamaldehyde |
Micronucleus formation |
Mouse bone-marrow cells |
500 000 µg/kg bw |
Negative |
Hayashi et al. (1984, 1988) |
|
656 |
trans-Cinnamaldehyde |
Micronucleus formation |
Rat and mouse hepatocytes |
1 700 000 µg/kg bw (mice)
1 100 000 µg/kg bw (rats) |
Positive |
Mereto et al. (1994) |
|
656 |
trans-Cinnamaldehyde |
Micronucleus formation |
Rat and mouse bone marrow |
1 700 000 µg/kg bw (mice)
1 100 000 µg/kg bw (rats) |
Negative |
Mereto et al. (1994) |
|
656 |
Cinnamaldehyde |
Nuclear anomaliesf |
Rat and mouse fore- stomach mucosal cells |
1 700 000 µg/kg bw (mice) |
Negative |
Mereto et al. (1994) |
|
|
|
|
|
1 100 000 µg/kg bw (rats) |
Positive |
|
|
656 |
trans-Cinnamaldehyde |
DNA fragmentation |
Rat hepatocytes and gastric mucosal cells |
1 100 000 µg/kg bw |
Negative |
Mereto et al. (1994) |
|
656 |
Cinnamaldehyde |
Hyperplastic foci |
Rat hepatocytes |
500 000 µg/kg bw per dayg |
Positive |
Mereto et al. (1994) |
|
19 |
Allyl cinnamate |
Micronucleus formation |
Mouse bone-marrow cells |
282 000 µg/kg bw |
Negative |
Wild et al. (1983) |
|
674 |
alpha-Amylcinnamyl alcohol |
Micronucleus formation |
Mouse bone-marrow cells |
510 000 µg/kg bw |
Negative |
Wild et al. (1983) |
|
683 |
alpha-Methylcinna-maldehyde |
Micronucleus formation |
Mouse bone-marrow cells |
438 000 µg/kg bw |
Negative |
Wild et al. (1983) |
|
685 |
alpha-Amylcinnamal-dehyde |
Micronucleus formation |
Mouse bone-marrow cells |
1 213 000 µg/kg bw |
Negative |
Wild et al. (1983) |
|
686 |
alpha-Hexylcinna-maldehyde |
Micronucleus formation |
Mouse bone-marrow cells |
657 000 µg/kg bw |
Negative |
Wild et al. (1983) |
a With and without metabolic activation
b Without metabolic activation
c Method included both plate incorporation (without metabolic activation) and preincubation method (with metabolic activation)
d Positive results in strain TA100 only
e With metabolic activation
f Includes % micronuclei, pyknosis, and karyorrhexis
g Rats were initiated with N-nitrosodiethylamine then given cinnamaldehyde by oral gavage for 14 consecutive days.
In vitro
Cinnamaldehyde (trans- and unspecified stereochemistry), cinnamyl alcohol (No. 647) (trans- and unspecified stereochemistry), cinnamic acid (No. 657), alpha-methylcinnamaldehyde (No. 683), cinnamyl acetate (No. 650), benzyl cinnamate (No. 670), cyclohexyl cinnamate (No. 667), alpha-amylcinnamaldehyde (No. 685), alpha-hexylcinnamaldehyde (No. 686), para-methoxy-alpha-methylcinnamaldehyde (No. 689), and 3-phenylpropionaldehyde (No. 645) generally did not cause reverse mutation in Salmonella typhimurium strains TA92, TA94, TA97, TA98, TA100, TA102, TA104, TA1535, TA1537, TA1538, and TA2637. The assays were performed at concentrations up to the level of cytotoxicity, both in the absence and presence of metabolic activation obtained from the livers of Aroclor 1254- or methylcholanthrene-induced Sprague-Dawley rats or Syrian hamsters (Dunkel & Simon, 1980; Eder et al., 1980; Florin et al., 1980; Lijinsky & Andrews, 1980; Lutz et al., 1980; Eder et al., 1982a,b; Kasamaki et al., 1982; Lutz et al., 1982; Prival et al., 1982; Sekizawa & Shibamoto, 1982; Neudecker et al., 1983; Wild et al., 1983; Ishidate et al., 1984; Huang et al., 1985; Marnett et al., 1985; Mortelmans et al., 1986; Fujita & Sasaki, 1987; Tennant et al., 1987; Kato et al., 1989; Eder et al., 1991; Dillon et al., 1992; Azizan & Blevins, 1995).
Weakly positive or positive results were reported for cinnamaldehyde (No. 656) in S. typhimurium strain TA100 with the pre-incubation method (Dillon et al., 1992; Ishidate et al., 1984), but most other studies in this strain, including a recent study with a prolonged pre-incubation time (120 min) and others in which the standard plate incorporation method was used gave no evidence of mutagenicity (Sasaki & Endo, 1978; Lijinsky & Andrews, 1980; Eder et al., 1982a,b; Kasamaki et al., 1982; Lutz et al., 1982; Prival et al., 1982; Sekizawa & Shibamoto, 1982; Neudecker et al., 1983; Marnett et al., 1985; Mortelmans et al., 1986; Kato et al., 1989; Eder et al., 1991; Azizan & Blevins, 1995).
Negative or weakly positive results were obtained in S. typhimurium with pre-incubation with ortho-methoxycinnamaldehyde (No. 688) (Eder et al., 1991; Mortelmans et al., 1986). The weakly positive results in strain TA100 with metabolic activation were obtained with two different activation systems (Mortelmans et al., 1986). Negative results were obtained in strains TA1535, TA1537, and TA98 both with and without metabolic activation. In the study with strain TA100, negative results were reported in the absence of metabolic activation (Eder et al., 1991). No standard plate incorporation assay was available for ortho-methoxycinnamaldehyde, which might be expected to behave similarly to the other cinnamyl compounds on the basis of structural and metabolic similarities.
The results of assays for mutation in Escherichia coli strains WP2uvrA, PQ37, and Sd-4-73, including several in which the pre-incubation method was used, were negative with cinnamaldehyde (No. 656), cinnamyl alcohol (No. 647), cinnamic acid (No. 657), alpha-methylcinnamaldehdye (No. 683), and alpha-amylcinnamaldehyde (No. 685) (Szybalski, 1958; Sekizawa & Shibamoto, 1982; Ohta et al., 1986; Yoo, 1986; Kato et al., 1989; Eder et al., 1991, 1993). In the rec assay in Bacillus subtilis, positive results were reported with cinnamaldehyde (No. 656) and cinnamyl alcohol (No. 647), whereas cinnamic acid (No. 657), ethyl cinnamate (No. 659), methyl cinnamate (No. 648), and benzyl cinnamate (No. 670) gave negative results in all such tests (Oda et al., 1979; Sekizawa & Shibamoto, 1982; Kuroda et al., 1984; Yoo, 1986).
Assays with isolated mammalian cells gave mixed but generally positive results for cinnamyl esters overall. Equivocal to positive results were obtained for cinnamaldehyde (No. 656) in the assay for forward mutation in L5178Y mouse lymphoma cells with and without metabolic activation, but the reports of these tests did not provide sufficient detail of the method, concentrations tested, or cytotoxic effects to allow adequate evaluation of the results (Rudd et al., 1983; Palmer, 1984). In L1210 mouse lymphoma cells, DNA strand breaks were observed only at cytotoxic concentrations of cinnamaldehyde (Eder et al., 1993).
The results of tests for the induction of sister chromatid exchange in Chinese hamster ovary cells exposed to cinnamaldehyde (No. 656) were negative at low concentrations and weakly positive at concentrations approaching cytotoxic levels, suggesting only weak activity (Galloway et al., 1987; Sasaki et al., 1987). A dose-dependent increase in the frequency of sister chromatid exchange was reported only when cultures were pre-treated with mitomycin C (Sasaki et al., 1987); however, the activity in conjunction with mitomycin contributes little to an evaluation of potential sister chromatid exchange activity. Cinnamaldehyde at concentrations < 15 µg/ml was reported to induce chromosomal aberrations in Chinese hamster fibroblasts and B241 cells tested with and without metabolic activation (Kasamaki et al., 1982; Ishidate et al., 1984; Kasamaki & Urasawa, 1985). However, higher concentrations did not induce chromosomal aberrations in Chinese hamster ovary cells in the presence or absence of metabolic activation in a well-conducted, repeated assay (Galloway et al., 1987).
The results of assays for cell transformation with cinnamaldehyde (No. 656) were positive at near-cytotoxic concentrations or after multiple generations of growth and negative in human HAIN-55 cells (Kasamaki et al., 1987; Matthews et al., 1993). Subcutaneous injection of the transformed cells into nude mice led to the formation of nodules at the site of injection and neoplastic growth in the spleen (Kasamaki et al., 1987). Negative results were obtained with cinnamaldehyde (No. 656) in Chinese hamster V79 cells (Fiorio & Bronzetti, 1994), while a weak increase in the incidence of micronucleated Hep-G2 cells was reported by Sanyal et al. (1997).
The results obtained with the other cinnamyl compounds in isolated mammalian cells were, in general, comparable to those obtained with cinnamaldehyde (No. 656). Sister chromatid exchange was not observed in Chinese hamster ovary cells exposed to cinnamyl alcohol (No. 647), cinnamic acid (No. 657), ethyl cinnamate (No. 659), methyl cinnamate (No. 658), cinnamyl acetate (No. 650), or 3-phenylpropionaldehyde (No. 645). Pretreatment with mitomycin C increased the incidence of sister chromatid exchange in assays with cinnamic acid (No. 657), methyl cinnamate (No. 658), and ethyl cinnamate (No. 659) but not cinnamyl alcohol (No. 647), cinnamyl acetate (No. 650), or 3-phenylpropionaldehyde (No. 645) (Sasaki et al., 1989). Palmer (1984) reported reproducible, dose-related increases in the incidence of reversions in L5178Y mouse lymphoma cells, with and without metabolic activation, after treatment with cinnamyl alcohol (No. 647), cinnamic acid (No. 657), cinnamyl cinnamate (No. 673), and ortho-methoxycinnamaldehyde (No. 688).
The results of assays in L5178Y mouse lymphoma cells at the Tk+/– locus have yielded equivocal results. The positive results were seen at near-lethal concentrations in studies in which this was reported. The results of assays with simple aliphatic and aromatic substances were not consistent with the results of other, standard assays for genotoxicity (Tennant et al., 1987; Heck et al., 1989). Culture conditions of low pH and high osmolality, which may pertain with substances that have a potentially acidifying effect on the culture medium (aldehydes, carboxylic acids, lactones, and hydrolysed esters), have been shown to produce false-positive results in this and other assays (Heck et al., 1989). Other reports of positive responses in the mouse lymphoma cell assay lacked information on the concentration tested and on cytolethality (Rudd et al., 1983; Palmer, 1984).
In vivo
Most of the results of tests of the administration of cinnamyl compounds in vivo pertains to cinnamaldehyde (No. 656). An increase in the frequency of sex-linked recessive lethal mutations was reported when Drosophila melanogaster were injected with cinnamaldehyde at 20 000 mg/kg of diet, but no increase in the frequency of mutations was seen when D. melanogaster were fed 800 mg/kg of diet for 3 days. Reciprocal translocations were not observed in either assay (Woodruff et al., 1985). No increase in the frequency of unscheduled DNA synthesis was found in the hepatocytes of rats or mice given cinnamaldehyde at 1000 mg/kg bw by oral gavage (Mirsalis et al., 1989). The frequency of micronuclei was not increased when rats or mice were given 1700 mg/kg bw or 1100 mg/kg bw, respectively, of cinnamaldehyde by oral gavage (Mereto et al., 1994) or when mice were given 500 mg/kg bw by intraperitoneal injection (Hayashi et al., 1984, 1988). The frequency of micronucleated bone-marrow cells in mice that had been exposed to X-rays decreased after injection of 500 mg of cinnamaldehyde (Sasaki et al., 1990).
An increase in the frequency of micronucleated cells was reported in rat and mouse hepatocytes and in rat (but not mouse) forestomach cells after the animals had received up to 1100 (rats) or 1700 (mice) mg/kg bw of cinnamaldehyde by oral gavage. No increase in the frequency of micronuclei in liver or forestomach was observed at doses ³ 850 mg/kg bw, and no DNA fragmentation was observed in rat hepatocytes or gastric mucosal cells. The incidence and size of gamma-glutamyl transferase-positive foci were increased in hepatocytes of rats pretreated with N-nitrosodiethylamine and then given cinnamaldehyde at 500 mg/kg bw per day by oral gavage for 14 days (Mereto et al., 1994).
The positive findings with cinnamaldehyde (No. 656) in rat forestomach and in the livers of both rats and mice treated in vivo are not consistent with the results of the standard assays in bone marrow and were observed at doses that far exceeded those resulting from intake of cinnamaldehyde in foods. Cinnamaldehyde given at oral doses ³ 500 mg/kg bw depleted hepatocellular glutathione concentrations (Swales & Caldwell, 1991, 1992, 1993), and the increases in micronucleus frequency were found at doses that appeared to affect cellular defence mechanisms, such as glutathione depletion. As the micronucleus formation was dose-dependent, induction of micronuclei may be a threshold phenomenon which occurs at intakes orders of magnitude greater than that of cinnamaldehyde as a flavouring agent. Furthermore, the bolus doses resulting from gavage probably resulted in much greater exposure of both the forestomach and the liver than administration in a dietary admixture. The author of the study in which these results were obtained (Mereto et al., 1994) acknowledged these facts and concluded that their data did not justify the conclusion that cinnamaldehyde is clastogenic. In view of the apparent threshold for micronucleus induction and the lack of activity in other studies in vivo, the effects induced by the bolus dose in the liver and forestomach are considered irrelevant to the evaluation of the safety of cinnamaldehyde when used as a flavouring agent.
Wild et al. (1983) reported negative results in tests for sex-linked recessive lethal mutation in D. melanogaster and in an assay for micronucleus formation in mouse bone-marrow cells after administration of alpha-methylcinnamaldehyde (No. 683), allyl cinnamate (No. 19), alpha-amylcinnamyl alcohol (No. 674), alpha-amylcinnamaldehyde (No. 685), or alpha-hexylcinnamaldehyde (No. 686).
2.3.2.4 Reproductive toxicity
Cinnamyl alcohol (No. 647)
Groups of 14 or 15 female rats were given cinnamyl alcohol (No. 647) orally at a dose of 53.5 mg/kg bw on day 4 (implantation) or days 10–12 (organogenesis) of gestation. On day 20 of gestation, all animals were killed, and their fetuses were removed for examination. Fetal body weight, length, and the number surviving did not differ significantly between treated and control groups. Histological examination revealed a slight reduction in skeletal ossification of the extremities. Examination of the saggital sections revealed no anomalies in relation to palatal structure, eyes, brain, or other internal organs (Maganova & Zaitsev, 1973).
In a second study, groups of 14 or 15 female rats were given cinnamyl alcohol (No. 647) orally at a dose of 53.5 mg/kg bw per day throughout gestation. On day 20 of gestation, 50% of the treated and control animals were killed, and their fetuses were removed for examination. Fetal body weight, liver nucleic acids, number of survivors, and bone development did not differ significantly between test and control groups. The remaining females from both groups were allowed to deliver normally. Again, the body weights, number surviving, and size and general development of offspring at birth or at 1 month did not differ significantly between treated and control groups (Zaitsev & Maganova, 1975).
Cinnamaldehyde (No. 656)
Groups of 14–16 rats were given cinnamaldehyde (No. 656) at a dose of 5, 25, or 250 mg/kg bw per day by gavage in olive oil on days 7–17 of gestation. A control group was included, but it was not stated whether they received the olive oil vehicle. The fetal abnormalities observed included poor cranial ossification at all doses; increased incidences of dilated pelvis, reduced renal papillae, and dilated ureters at the low and intermediate doses; and an increased number of fetuses with two or more abnormal sternebrae at the intermediate dose. These effects were not dose-related and might be attributable to the decrease in maternal weight gain at the two higher doses (Mantovani et al., 1989).
Cinnamic acid (No. 657)
Groups of 14 or 15 female rats were given cinnamic acid (No. 657) orally at a dose of 0, 5, or 50 mg/kg bw per day throughout gestation. On day 20 of gestation, 50% of the females in all groups were killed, and their fetuses were removed for examination. No significant differences in fetal body weight, number of survivors, bone development, or hepatic nucleic acids were found between treated and control groups. The remaining females in both treated and control groups were allowed to deliver normally on days 22–23 of gestation. No significant differences in the body weights, size, number surviving, or general development of offspring at birth or at 1 month were found between treated and control groups (Zaitsev & Maganova, 1975).
Conclusion
Cinnamyl alcohol (No. 647) and related compounds lack direct mutagenic or genotoxic activity, as indicated by the negative results obtained in bacterial test systems. The mixed results in the assay for DNA repair and in various studies of antimutagenicity were associated with cytotoxicity, as noted by Sekizawa & Shibamoto (1982). Evidence of genotoxic activity was found in isolated mammalian cells, the cinnamyl compounds inducing chromosomal aberrations and/or mutations in the presence or absence of metabolic activation; however, the reported activity in vitro was not seen as mutagenic, clastogenic, or genotoxic activity in vivo.
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D,, eds, Intermediary Xenobiotic Metabolism in Animals, New York: Taylor & Francis, pp. 81–97.
Azizan, A. & Blevins, R.D. (1995) Mutagenicity and antimutagenicity testing of six chemicals associated with the pungent properties of specific spices as revealed by the Ames Salmonella/microsomal assay. Arch. Environ. Contam. Toxicol., 28, 248–258.
Caldwell, J. & Nutley, B. (1986) Comparative metabolism of cinnamic acid in rats and mice and its variation with dose. Br. J. Pharmacol., 88 (Suppl.), 423.
Caldwell, J., Idle, J. & Smith, R.L. (1980) The amino acid conjugations. In: Gram, T.E., ed., The Extrahepatic Metabolism of Drugs and Other Foreign Compounds, New York: SP Medical and Scientific Books, pp. 453–492.
Carpanini, F.M.B., Gaunt, I.F., Wright, M.G., Grasso, P. & Gangolli, S.D. (1973) Short-term toxicity of amyl cinnamic aldehyde in rats. Food Cosmet. Toxicol., 11, 725–734.
Carter, H.E. (1941) The oxidation of branched-chain fatty acids. Biol. Sym., 5, 47–63.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard: A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Dakin, H.D. (1909) The mode of oxidation in the animal organism of phenyl derivatives of fatty acids. Part IV. J. Biol. Chem., 6, 203–209.
Delbressine, L., Klippert, P., Reuvers, J. & Seutter-Berlage, F. (1981) Isolation and identification of mercapturic acids of cinnamic aldehyde and cinnamyl alcohol from urine of female rats. Arch. Toxicol., 49, 57–64.
Denine, E.P. & Palanker, A. (1973) Acute oral and dermal toxicity studies. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Deuel, H.J. (1957) The oxidation and metabolism of triglycerides, fatty acids, and glycerol in the animal body. In: The Lipids, Their Chemistry and Biochemistry, New York: Wiley Interscience, Vol. III, pp. 71–99, 291–301.
Dillon, D.M., McGregor, D.B., Combes, R.D. & Zeiger, E. (1992) Detection of mutagenicity in Salmonella of some aldehydes and peroxides. Environ. Mol. Mutag., 19,15.
Draize, J.H., Alvarez, E., Whitesell, M.F., Woodward, G., Hagen, E.C. & Nelson, A.A. (1948) Toxicological investigations of compounds proposed for use as insect repellants. J. Pharmacol. Exp. Ther., 93, 26-39.
Dunkel, V. & Simon, V. (1980) Mutagenic activity of chemicals previously tested for carcinogenicity in the National Cancer Institute Bioassay program. In: Montesano, R., Bartsch, H. & Tomatis, L., eds, Molecular and Cellular Aspects of Carcinogen Screening Tests (IARC Scientific Publications 27), Lyon: IARCPress, pp. 283–302.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic potential of allyl and allylic compounds: Structure–activity relationship as determined by alkylating and direct in vitro mutagenic properties. Biochem. Pharmacol., 29, 993–998.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1982a) Correlation of alkylating and mutagenic activities of allyl and allylic compounds: Standard alkylation test vs. kinetic investigation. Chem. Biol. Interactions, 38, 303–315.
Eder, E., Henschler, D. & Neudecker, T. (1982b) Mutagenic properties of allylic and alpha, beta-unsaturated carbonylic compounds: Consideration of alkylating mechanisms. Xenobiotica, 12, 831–848.
Eder, E., Deininger, C. & Muth, D. (1991) Genotoxicity of p-nitrocinnamaldehyde and related alpha, beta-unsaturated carbonyl compounds in two bacterial assays. Mutagenesis, 6, 261–269.
Eder, E., Scheckenbach, S., Deininger, C. & Hoffman, C. (1993) The possible role of alpha, beta-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol. Lett., 67, 87–103.
Fahelbum, I. & James, S. (1977) The absorption and metabolism of methyl cinnamate. Toxicology, 7, 123–132.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase. I. Purification and characterization. J. Biol. Chem., 247, 260–266.
Fiorio, R. & Bronzetti, G. (1994) Effects of cinnamaldehyde on survival and formation of HGPRT— mutants in V79 cells treated with methyl methanesulfonate, N-nitroso-N-methylurea, ethyl methanesulfonate and UV light. Mutat. Res., 324, 51–57.
Fischer, F.G. & Bielig, H.J. (1940) On the hydrogenation of unsaturated materials in the animal body. Z. Physiol. Chem., 266, 73–98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102 (II). Ann. Rep. Tokyo Metr. Res. Lab. Public Health, 38, 423–430.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B., Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., Rimpo, J., Margolin, B.H., Resnick, M.A., Anderson, B. & Zeiger, E. (1987) Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol. Mutag., 10 (Suppl. 10), 1–175.
Goodwin, T.W. & Mercer, E.I. (1972) The plant cell wall. In: Introduction to Plant Biochemistry, Oxford: Pergamon Press, pp. 52–73.
Grundschober, F. (1977) Toxicological assessment of flavoring esters. Toxicology, 8, 387–390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food flavorings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol., 5, 141–157.
Harada, M. & Ozaki, Y. (1972) Pharmocological studies on Chinese cinnamon. I. Central effects of cinnamaldehyde. J. Pharm. Soc. Jpn, 92, 135–140.
Hayashi, M., Sofuni, T. & Ishidate, M., Jr (1984) A pilot experiment for the micronucleus test. The multi-sampling at multi-dose levels method. Mutat. Res., 141, 165–169.
Hayashi, M., Kishi, M., Sofuni, T. & Ishidate, M., Jr (1988) Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem. Toxicol., 26, 487–500.
Heck, J., Vollmuth, T., Cifone, M., Jagannath, D., Myrh, B. & Curren, R. (1989) An evaluation of food flavoring ingredients in a genetic toxicity screening battery. Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Enzymatic Basis of Detoxication, New York: Academic Press, pp. 291–323.
Huang, M., Chang, R.L., Wood, A.W., Newmark, H.L., Sayer, J.M., Yagi, H., Jerina, D.M. & Conney, A.H. (1985) Inhibition of the mutagenicity of bay-region diol-epoxides of polycyclic aromatic hydrocarbons by tannic acid, hydroxylated anthraquinones and hydroxylated cinnamic acid derivatives. Carcinogenesis, 6, 237–242.
Hutt, A.J. & Caldwell, J. (1990) Amino acid conjugations. In: Mulder, G.J., ed., Conjugation Reactions in Drug Metabolism, London: Taylor & Francis, Ltd, pp. 273–305.
International Organization of the Flavour Industry (1995) European inquiry on volume of use. Private communication to the Flavor and Extract Manufacturers’ Association of the United States.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T., Sawada M. & Matsuoka, A. (1984) Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol., 22, 623–636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure. I. Acute oral toxicity. Food Cosmet. Toxicol., 2, 327–343.
Kasamaki, A. & Urasawa, S. (1985) Transforming potency of flavoring agents in Chinese hamster cells. J. Toxicol. Sci., 10, 177–185.
Kasamaki, A., Yasuhara, T. & Urasawa, S. (1987) Neoplastic transformation of Chinese hamster cells in vitro after treatment with flavoring agents. J. Toxicol. Sci., 12, 383–396.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. & Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res., 105, 387–392.
Kassahun, K., Farrell, K. & Abbott, F. (1991) Identification and characterization of the glutathione and N-acetylcysteine conjugates of (E)-2-propyl-2,4-pentadienoic acid, a toxic metabolite of valproic acid, in rats and humans. Drug Metab. Disposition, 19, 525–535.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity of aldehydes and diketones. Mutat. Res., 216, 366–367.
Kay, H. & Raper, H. (1924) The mode of oxidation of fatty acids with branched chains. III. Biochemistry, 18, 153–160.
Keating, J.W. (1972) Acute toxicity study in rats and rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of food additives. Jpn. Soc. Public Health, 31, 277.
Levenstein, I. (1975) Acute toxicity studies in rats, mice, and rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Levenstein, I. (1976) Acute oral toxicity study in rats and acute dermal toxicity study in rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Levenstein, I. & Wolven, A.M. (1972) Acute toxicity studies in rats and in rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Lijinsky, W. & Andrews, A.W. (1980) Mutagenicity of vinyl compounds in Salmonella typhimurium. Teratog. Carcinog. Mutag., 1, 259–267.
Lucas, C., Putnam, J., Hallagan, J. & the Flavor Ingredients Committee (1999) 1995 Poundage and Technical Effects Update Survey, Washington DC, Flavor and Extract Manufacturers’ Association of the United States.
Lutz, D., Neudecker, T. & Eder, E. (1980) Mutagenic effects of allylic alcohols and their corresponding aldehydes. Naunyn-Schmiedebergs Arch. Pharmacol., 311 (Suppl.), R25 (Abstract No. 97).
Lutz, D., Eder, E., Neudecker, T. & Henschler, D. (1982) Structure–mutagenicity relationship in alpha, beta-unsaturated carbonylic compounds and their corresponding allylic alcohols. Mutat. Res., 93, 305–315.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens, M.H., eds (1996) Volatile Components in Food, Suppl. 5 to the 6th Edition, Zeist, TNO Nutrition and Food Research.
Maganova, N. & Zaitsev, A. (1973) Study of the embryotoxic action of some synthetic food flavorings. Vop. Pitan., 32, 50–54.
Mantovani, A., Stazi, A., Macri, C., Ricciardi, C., Piccioni, A. & Badellino, E. (1989) Pre-natal toxicity study of cinnamic aldehyde in the Sprague-Dawley rat. Food Chem. Toxicol., 27, 781–786.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer, H. & Ames, B.N. (1985) Naturally occuring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25–34.
Matthews, E.J., Spalding, J.W. & Tennant, R.W. (1993) Transformation of BALB/c-3T3 cells: IV. Rank-ordered potency of 24 chemical responses detected in a sensitive new assay procedure. Environ. Health Perspectives, 101 (Suppl. 2), 319–345.
Mereto, E., Brambilla-Campart, G., Ghia, M., Martelli, A. & Brambilla, G. (1994) Cinnamaldehyde-induced micronuclei in rodent liver. Mutat. Res., 322, 1–8.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L., Loh, E.K., Hamilton, C.M., Bakke, J.P. & Spalding, J.W. (1989) Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following in vivo treatment: Testing of 24 compounds. Environ. Mol. Mutag., 14, 155–164.
Moon, K.H. & Pack, M.Y. (1983) Cytotoxicity of cinnamic aldehyde on leukemia L1210 cells. Drug Chem. Toxicol., 6, 521–535.
Moreno, O.M. (1971) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1972) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1973) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1974) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1975) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1977) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1981) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Moreno, O.M. (1982) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Morgareidge, K. (1962) In vitro digestion of four acetals. Private communication to the Flavor and Extract Manufacturers Association of the United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. & Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mol. Mutag., 8, 1–119.
National Academy of Sciences (1987) Evaluating the Safety of Food Chemicals, Washington DC.
National Toxicology Program (1995) 13-Week Subchronic Study of trans-Cinnamaldehyde in Fischer F344 Rats. Unpublished report.
Neudecker, T., Öhrlein, K., Eder, E. & Henschler, D. (1983) Effect of methyl and halogen substitutions in the alphaC position on the mutagencity of cinnameldehyde. Mutat. Res., 110, 1–8.
Newberne, P., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adamas, T.B. & Hallagan, J.B. (2000) GRAS flavoring substances 19. Food Technol., 54, 66–84.
Nutley, B.P. (1990) Investigations into the Metabolism of Cinnamic Acid, Cinnamyl Alcohol, and Cinnamaldehyde in Relation to their Safety Evaluation, PhD Thesis, University of London, Department of Pharmacology.
Nutley, B., Farmer, P. & Caldwell, J. (1994) Metabolism of trans-cinnamic acid in the rat and mouse and its variation with dose. Food Chem. Toxicol., 32, 877–886.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N. (1979) Mutagenicity of food flavors in bacteria. Osaka-Furitsu Koshu Eisei Kenkyu Hokoku. Shokuhin Eisei Hen, 91, 325. (Chem. Abstr., 1979, Abstr. No. 207194r).
Ohta, T., Watanabe, M., Watanabe, K., Shirasu, Y. & Kada, T. (1986) Inhibitory effects of flavourings on mutagenesis induced by chemicals in bacteria. Food Chem. Toxicol., 24, 51–54.
Opdyke, D.L.J. (1974) Monographs on fragrance raw materials. Cinnamic alcohol. Food Cosmet. Toxicol., 12, 855–856.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on flavoring matters. Food Cosmet. Toxicol., 3, 563–569.
Palmer, K.A. (1984) L5178Y TK+/– assay of cinnamaldehyde and several structurally related compounds. Environ. Mutag., 6, 423–424.
Peters, M. & Caldwell, J. (1994) Studies on trans-cinnamaldehyde. The influence of dose size and sex on its disposition in the mouse and rat. Food Chem. Toxicol., 32, 869–876.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of substrate specificity of alcohol dehydrogenase from human liver, horse liver, and yeast towards saturated and 2-enoic alcohols and aldehydes. Arch. Biochem. Biophys., 159, 50–60.
Pollitt, R.J. (1974) Phenylpropionic acid in the urine of patients with phenylketonuria and normals. Clin. Chim. Acta, 55, 317–322.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of toxicological data. Toxicological tests on flavoring matters. Food Cosmet. Toxicol., 7, 405–407.
Prival, M.J., Sheldon, A.T., Jr & Popkin, D. (1982) Evaluation, using Salmonella typhimurium, of the mutagenicity of seven chemicals found in cosmetics. Food Chem. Toxicol., 20, 427–432.
Quarto di Palo, F.M. & Bertolini, A.M. (1961) Cinnamic acid administration to renal patients. Atti Accad. Med. Lombarada, 16, 180–183.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. I. The conjugation of benzoic acid and phenylacetic acid formed as the end-products from the oxidation of phenyl-substituted aliphatic acids. J. Biol. Chem., 77, 581–593.
Raper, H.S. & Wayne, E.J. (1928) A quantitative study of the oxidation of phenyl-fatty acids in the animal organism. Biochem. J., 22, 188–197.
Rudd, C.J., Mitchell, A.D. & Spalding, J. (1983) L5178Y mouse lymphoma cell mutagenesis assay of coded chemicals incorporating analyses of the colony size distributions. Environ. Mutag., 5, 419 (Abstract No. Cd-19).
Russell, T. (1973) Acute oral and dermal toxicity studies. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Samuelsen, O., Brenna, J., Solheim, E. & Scheline, R. (1986) Metabolism of the cinnamon constituent o-methoxycinnamaldehyde in the rat. Xenobiotica, 16, 845–852.
Sanyal, R., Darroudi, F., Parzefall, W., Nagao, M. & Knasmüller, S. (1997) Inhibition of the genotoxic effects of heterocyclic amines in human derived hepatoma cells by dietary bioantimutagens. Mutagenesis, 12, 297–303.
Sapienza, P., Ikeda, G.J., Warr, P.I., Plummer, S.L., Dailey, R.E. & Lin, C.S. (1993) Tissue distribution and excretion of 14C-labelled cinnamic aldehyde following single and multiple oral administration in male Fischer 344 rats. Food Chem. Toxicol., 31, 253–261.
Sasaki, Y. & Endo, R. (1978) Mutagencity of aldehydes in Salmonella. Mutat. Res., 54, 251–252.
Sasaki, Y.F., Imanishi, H., Ohta, T. & Shirasu, Y. (1987) Effects of antimutagenic flavourings of SCEs induced by chemical mutagens in cultured Chinese hamster cells. Mutat. Res., 189, 313–318.
Sasaki, Y.F., Imanishi, H., Ohta, T. & Shirasum Y. (1989) Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mutat. Res., 226, 103–110.
Sasaki, Y.F., Ohta, T., Imanishi, H., Watanabe, M., Matsumoto, K., Kato, T. & Shirasu, Y. (1990) Suppressing effects of vanillin, cinnamaldehyde, and anisaldehyde on chromosome aberrations induced by X-rays in mice. Mutat. Res., 243, 299–302.
Schafer, E.W. & Bowles, W.A. (1985) Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Arch. Environ. Contam. Toxicol., 14, 111–129.
Schafer, E.W., Bowles, W.A. & Hurlbut, J. (1983) The acute oral toxicity, repellency, and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environ. Contam. Toxicol., 12, 355–382.
Sekizawa, J. & Shibamoto, T. (1982) Genotoxicity of safrole-related chemicals in microbial test systems. Mutat. Res., 101, 127–140.
Snapper, I., Yu, T.F. & Chiang, Y.T. (1940) Cinnamic acid metabolism in man. Proc. Soc. Exp. Biol. Med., 44, 30–34.
Solheim, E. & Scheline, R. (1973) Metabolism of alkenebenzene derivatives in the rat. I. p-Methoxyallybenzene (estragole) and p-methoxypropenylbenzene (anethole). Xenobiotica, 3, 493–510.
Solheim, E. & Scheline, R. (1976) Metabolism of alkenylbenzene derivatives in the rat. II. Eugenol and Isoeugenol methyl esters. Xenobiotica, 6, 137–150.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfum. Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C.(1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857–860.
Sund, H. & Theorell, H. (1963) Alcohol dehydrogenases. In: Boyer, P.D., Lardy, H. & Myrback, K., eds, The Enzymes, 2nd Ed., New York: Academic Press, Vol. 7, pp. 25–83.
Swales, N.J. & Caldwell, J. (1991) Cytotoxicity and depletion of glutathione (GSH) by cinnamaldehyde in rat hepatocytes. Hum. Exp. Toxicol., 10, 488–489.
Swales, N.J. & Caldwell, J. (1992) Cytotoxicity and glutathione depletion in hepatocytes by esters of cinnamic acid. Hum. Exp. Toxicol., 6, 589–590.
Swales, N.J. & Caldwell, J. (1993) The depletion of hepatic reduced glutathione (Gsh), cysteine and protein sulphydryls by cinnamaldehyde in F344 rats. Proc. Int. Soc. Stud. Xenobiotics, 3.
Szybalski, W. (1958) Special microbiological systems. II. Observations on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76, 475–489.
Tennant, R., Margolin, B., Shelby, M., Zeiger, E., Haseman, J., Spalding, J., Caspary, W., Resnick, M., Stasiewicz, S., Anderson, B. & Minor, R. (1987) Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science, 236, 933–941.
Teuchy, H. & VanSumere, C. (1971) The metabolism of [1-14C]phenylalanine, [3-14C]cinnamic acid, and [2-14C]ferulic acid in the rat. Arch. Int. Physiol. Biochem., 79, 589–618.
Trubeck Laboratories (1958a) Toxicological examination of cinnamic aldehyde in rats (Class IV, Part 2). Unpublished report.
Trubeck Laboratories (1958b) Toxicological screening of components of food flavors. Class IV. Cinnamates. Unpublished report.
Trubeck Laboratories (1958c) Toxicological examination of methyl cinnamic aldehyde (Class IV, Part 3). Unpublished report.
Weiner, H. (1980) Aldehyde oxidizing enzymes. In: Jakoby, W., ed., Enzymatic Basis of Detoxication, New York: Academic Press, Vol. 1.
Weir, R.J. & Wong, L.C.K. (1971) Acute oral and dermal toxicity studies in rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, BASC and micronucleus tests. Food Chem. Toxicol., 21, 707–719.
Williams, R.T. (1959) Aliphatic alcohols, glycols and polyols; The metabolism of some aliphatic aldehydes, ketones and acids. In: Detoxication Mechanisms: The Metabolism and Detoxication of Drugs, Toxic Substances, and Other Organic Compounds, 2nd Ed., London: Chapman & Hall Ltd, pp. 46–97.
Yuan, J.H., Dieter, M.P., Bucher, J.R. & Jameson, C.W. (1992) Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem. Toxicol., 30, 997–1004.
Wohl, A. (1974) Acute oral dermal toxicity studies. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985) Chemical mutagenesis testing in Drosophila. V. Results of 53 coded compounds tested for the National Toxicology Program. Environ. Mutag., 7, 677–702.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring agents used in foodstuffs. J. Osaka City Med. Center, 4, 267–288 .
Yuan, J.H., Dieter, M.P., Bucher, J.R. & Jameson, C.W. (1992) Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem. Toxicol., 30, 997–1004.
Zaitsev, A.N. & Maganova, N.B. (1975) Embryotoxic effects of some aromatizers for food products. Vopr. Pitan., 3, 64–68.
Zaitsev, A.N. & Rakhmanina, N.L. (1974) Toxic properties of phenylethanol and cinnamic alcohol derivatives. Vopr. Pitan., 5, 48–53.
See Also:
Toxicological Abbreviations
.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()