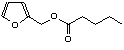
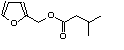
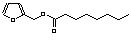
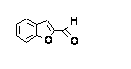
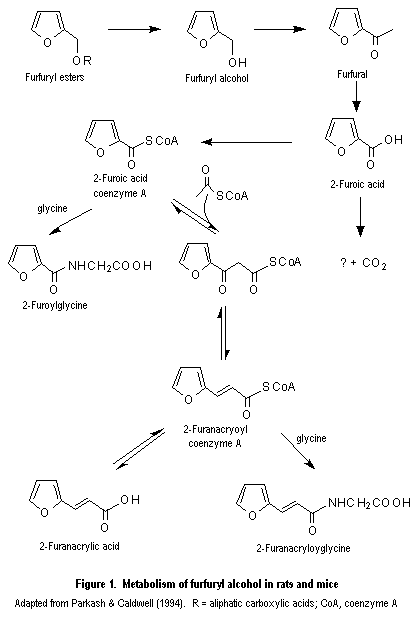
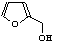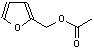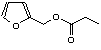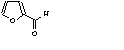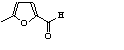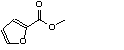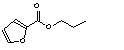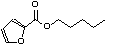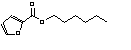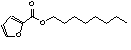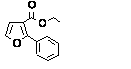c Concentration added to culture
3. REFERENCES
Adams, T.B., Lake, B.G., Beamad, J.A., Price, R.J., Ford, R.A. & Goodman, J.I. (1998) An investigation of the effect of furfural on the unscheduled DNA synthesis in cultured human liver slices. Toxicologist, 42, 79.
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon, R. (1989) Contribution of coffee aroma constituents to the mutagenicity of coffee. Food Chem. Toxicol., 27, 227–232.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York: Taylor & Francis, pp. 81–97.
Barciszewski, J., Siboska, G.E., Pedersen, B.O., Clark, B.F.C. & Rattan, S. (1996) Evidence for the presence of kinetin in DNA and cell extracts. FEBS Lett., 393, 197–200.
Barciszewski, J., Siboska, G.E., Pedersen, B.O., Clark, B.F.C. & Rattan, S. (1997a) Furfural; a presursor of the cyokinin hormone kinatin, and base propenals are formed by hydroxyl radical damage to DNA. Biochem. Biophys. Res. Commun., 238, 317–319.
Barciszewski, J., Siboska, G.E., Pedersen, B.O., Clark, B.F.C. & Rattan, S. (1997b) A mechanism of the in vivo formation of N6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett., 414, 457–460.
Boopathy, R., Bokang, H. & Daniels, L. (1993) Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J. Ind. Microbiol., 11, 147–150.
Boyd, M.R., Burka, L.T. & Wison, B.J. (1975) Metabolism studies with alkyl 14-C-ipomeanol in rats. Toxicol. Appl. Pharmacol., 32, 147–157.
Boyland, F. (1940) Experiments on the chemotherapy of cancer. 4. Further experiments with aldehydes and their derivatives. Biochem. J., 34, 1196–1201.
Buck, N.R. (2000) The hydrolysis of cinnamyl and furfuryl esters. Unpublished report from the University of Southampton, United Kingdom. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Bueld, J.E. & Netter, K.J. (1993) Factors affecting the distribution of ingested propionic acid in the rat forestomach. Food Chem. Toxicol., 31, 169–176.
Butterworth, K.R., Gaunt, I.F., Heading, C.E., Grasso, P. & Gangolli, S.D. (1978) Short-term toxicity of n-amyl alcohol in rats. Food Cosmet. Toxicol., 16, 203–207.
Caldwell, J. (1982) Conjugation reactions in foreign-compound metabolism: Definition, consequences, and species variations. J. Drug Metab. Rev., 13, 745–777.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Dillon, D., Combes, R. & Zeiger, E. (1998) The effectiveness of Salmonella strains TA100, TA102 and TA104 for detecting mutagenicity of some aldehydes and peroxides. Mutagenesis, 13, 19–26.
Dix, T.A., Hess, K.M., Medina, M.A., Sullivan, W., Tilly, S.L. & Webb, T.L. (1996) Mechanism of site-selected DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry, 35, 4578–4583.
Edwards A. (1999) An in vivo unscheduled DNA synthesis assay in the mouse with furfural. Report No. 3389/1/1/99. Unpublished report from BIBRA International, Carshalton, United Kingdom. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
Fassett, D.W. (1963) Aldehydes and acetals. J. Ind. Hyg. Toxicol., 2, 1959–1989.
Feron, V.J. (1972) Respiratory tract tumors in hamsters after intratracheal instillations of benzo(a)pyrene alone and with furfural. Cancer Res., 32, 28–36.
Feron, V.J. & Kruysse, A. (1978) Effects of exposure to furfural vapour in hamsters simultaneously treated with benzo(a)pyrene or diethylnitrosamine. Toxicology, 11, 127–144.
Flek, J. & Sedivec, V. (1978) The absorption, metabolism and excretion of furfural in man. Arch. Occup. Environ. Health, 41, 159–168.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 15, 219–232.
Friedmann, E. (1911) [Behaviour of furfuracrylic acid and furoylacetic acid in the animal body.] J. Biochem. Z., 35, 40–48 (in German).
Gajewski, J.R. & Alsdorf, W.R. (1949) Studies on furan compounds: Toxicity and pharmacological action of furfuryl alcohols. Fed. Proc., 8, 294.
Germond, J., Philippossian, G., Richli, U., Bracco, I. & Arnaud, M. (1987) Rapid and complete urinary elimination of [14C]-5-hydroxymethyl-2-furaldehyde administered orally or intravenously to rats. J. Toxicol. Environ. Health, 22, 79–89.
Godfrey, V.B., Chen, L., Griffin, R.J., Lebetkin, E.H. & Burka, L.T. (1999) Distribution and metabolism of (5-hydroxymethyl)furfural in male F344 rats and B6C3F1 mice after oral administration. J. Toxicol. Environ. Health Part A, 57, 199–210.
Gomez-Arroyo, S. & Souza, V. (1985) In vitro and occupational induction of sister-chromatid exchanges in human lymphocytes with furfuryl alcohol and furfural. Mutat. Res., 156, 233–238.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence of glycine conjugation on availability of glycine: Role of the glycine cleavage system. Xenobiotica, 23, 141–153
Grundschober, F. (1977) Toxicological assessment of flavoring esters. Toxicology, 8, 387–390.
Gupta, G.D., Misra, A. & Agarwal, D.K. (1991) Inhalation toxicity of furfural vapours: An assessment of biochemical response in rat lungs. J. Appl. Toxicol., 11, 343–347.
Haag, H.B., Finnegan, J.K., Larson, P.S. & Smith, R.B., Jr (1959) Studies on the acute toxicity and irritating properties of the congeners in whisky. Toxicol. Appl. Pharmacol., 1, 618–627.
Heymann, E. (1980) Carboxylesterases and amidases. In: Enzymatic Basis of Detoxication, New York: Academic Press, Vol. 2, pp. 291–323. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
International Organization of the Flavour Industry (1995) European Inquiry on Volume of Use. Unpublished report to the Flavor and Extract Manufacturers’ Association of the United States, Washington DC, USA.
Jansson, T., Curvall, M., Hedin, A. & Enzell, C.R. (1986) In vitro studies of biological effects of cigarette smoke condensate. II. Induction of sister-chromatid exchanges in human lymphocytes by weakly acidic, semivolatile constituents. Mutat. Res., 169, 129–139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure. I. Acute oral toxicity. Food Cosmet. Toxicol., 2, 327–343.
Jonek, J.J., Konecki, J. & Kaminski, M. (1975) Histoenzymatic changes in liver in acute poisoning with furfural. J. Morphol. Embryol., 21, 47–51.
Jonker, D. (1998) 14-day range-finding oral toxicity study in rats with micro-encapsulated furfural. Unpublished report from TNO Nutrition and Food Research Institute, Zeist, Netherlands. Submittted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
Jonker, D. (2000) Sub-chronic (13-week) oral toxicity study in rats with micro-encapsulated furfural. TNO Report V99.520, Vols 1–3. Unpublished report from TNO Nutrition and Food Research Institute, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
Kaminska, O. (1977) Morphological and histochemical investigations of liver and kidneys in different stage of furfural poisoning. Fol. Histochem. Cytochem., 15, 150–151.
Kim, S.B., Hayase, F. & Kato, H. (1987) Demutagenic effects of alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic heterocyclic amines. Mutat. Res., 177, 9–15.
Koenig, K. & Andreesen, J.R. (1990) Xanthine dehydrogenase and 2-furoyl-coenzyme A dehydrogenase from Pseudomonas p. Fu1; two molybenum-containing dehydrogenases of novel structural composition. J. Bacteriol., 172, 5999–6009.
Liebert, J.J. (1988) Metabolism of 5-methyl-2-furfural in rats. III. Identification and determination of 5-methyl-2-furyl methyl ketone. Xenobiotica, 18, 887–892.
Loquet, C., Toussaint, G. & LeTalaer, J.Y. (1981) Studies of mutagenic constituents of apple brandy and various alcoholic beverages collected in western France. Mutat. Res., 88, 155–164.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B (1999) 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers’ Association of the United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens, M.H., eds (1994) Volatile Components in Food—Qualitative and Quantitative Data, 7th Ed., Centraal Instituut Voor Voedingsonderzioek TNO, Zeist, Netherlands.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens, M.H., eds (1996) Volatile Components in Food—Qualitative and Quantitative Data, Centraal Instituut Voor Voedingsonderzioek TNO, Zeist, Netherlands.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer, H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25–34.
Matsui, S., Yamamoto, R.. & Yamada, H. (1989) The Bacillus subtilis/microsome rec-assay for the detection of DNA damaging substances which may occur in chlorinated and ozonated waters. Water Sci. Technol., 21, 875–887.
McGregor, D.B., McConville, M.L., Prentice, R.D.M. & Riach, C.G. (1981) Mutagenic activity of 123 compounds with known carcinogenic potential. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk– mouse lymphoma cell forward mutation assay II: 18 coded chemicals. Environ. Mol. Mutag., 11, 91–118.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test chemicals in ten tester strains using a new modification of the Ames test for bacterial mutagens. Cancer Res., 39, 682–693.
Mellick, P.W., Leach, C.L., Chou, B.J., Irwin, R.D. & Roycroft, J.H. (1991) Nasal toxicity in F344 rats inhaling furfuryl alcohol for 2 or 13 weeks. Toxicologist, 11, 183.
Mishra, A., Dwivedi, P.D., Verma, A.S., Sinha, M., Mishra, J., Lal, K., Pandya, K.P. & Dutta, K.K. (1991) Pathological and biochemical alterations induced by inhalation of furfural vapor in rat lung. J. Bull. Environ. Contam. Toxicol., 47, 668–674.
Moreno, O. (1978) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories Inc. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. & Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutag., 8, 1–119.
Nakamura, S.-I., Oda, Y., Shimada, T., Oki, I. & Sugimoto, K. (1987) SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: Examination with 151 chemicals. Mutat. Res., 192, 239–246.
National Academy of Sciences (1989) 1987 Poundage and Technical Effects Update of Substances Added to Food, Washington DC.
National Toxicology Program (1990) Toxicology and Carcinogenesis Studies of Furfural (CAS No. 98-01-1) in F344/N Rats and B6C3F1 Mice (Gavage Studies), NTP Technical Report Series No. 382; NIH Publication No. 90-2837, Research Triangle Park, North Carolina, USA.
National Toxicology Program (1999) Toxicology and Carcinogenesis Studies of Furfuryl Alcohol (CAS No. 98-00-0) in F344/N Rats and B6C3F1 Mice (Inhalation Studies), NTP Technical Report Series 482; NIH Publication No. 99-3972, Research Triangle Park, North Carolina, USA.
Nishi, Y., Miyakawa, Y. & Kato, K. (1989) Chromosome aberrations induced by pyrolysates of carbohydrates in Chinese hamster V79 cells. Mutat. Res., 227, 117–123.
Nomeir, A.A., Silveria, D.M., McComish, M.F. & Chadwick, M. (1992) Comparative metabolism and disposition of furfural and furfuryl alcohol in rats. Drug Metab. Disposition, 20, 198–204.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of trans-cinnamic acid in the rat and the mouse and its variation with dose. J. Food Chem. Toxicol., 32, 877–886.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of nitrite with the food components analogous to sorbic acid. Agric. Biol. Chem., 45, 2299–2304.
Parkash, M.K. & Caldwell, J. (1994) Metabolism and excretion of [14C]-furfural in the rat and the mouse. Food Chem. Toxicol., 32, 887–895.
Paul, H.E., Austin, F.L., Paul, M.F. & Ells, U.R. (1948) Metabolism of the nitrofurans. I. Ultraviolet absorption studies of the urinary products after oral administration. J. Biol. Chem., 180, 345–363.
Phillips, B.J., Jackson, L.I., Tate, B., Price, R.J., Adams, T.B., Ford, R.A., Goodman, J.I. & Lake B.J. (1997) Furfural does not induce unscheduled DNA synthesis (UDS) in the in vivo rat hepatocyte DNA repair assay. Submitted to WHO by the Flavor and Extract Manufacturers’ Association of the United States.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summary of toxicological data. Toxicological tests on flavor matters. Food Cosmet. Toxicol., 7, 405–407.
Ramsdell, H.S. & Eaton, D.L. (1990) Species susceptibility to aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal biotransformation. Cancer Res., 50, 615.
Ravindranath, A. & Boyd, M.R. (1985) Metabolic activiation of 2-methylfuran by rat microsomal systems. Toxicol. Appl. Pharmacol., 78, 370–376.
Rice, W.W. (1972) Furfural: Exogenous precursor of certain urinary furans and possible toxicologic agent in humans. Clin. Chem., 18, 1550–1551.
Rodriguez-Arnaiz, R., Morales, P.R., Moctezuma, R.V. & Salas, R.M.B. (1989) Evidence for the absence of mutagenic activity of furfuryl alcohol in tests of germ cells in Drosophila melanogaster. Mutat. Res., 223, 309–311.
Rodriquez-Arnaiz, R., Morales, P.R. & Zimmering, S. (1992) Evaluation in Drosophila melanogaster of the mutagenic potential of furfural in the mei-9(a) test for chromosome loss in germ-line cells. Mutat. Res., 280, 75–80.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988) Comparative mutagenicity of nine brands of coffee to Salmonella typhimurium TA100, TA102, and TA104. Environ. Mol. Mutag., 11, 195–206.
Shimizu, A. & Kanisawa, M. (1986) Experimental studies on hepatic cirrhosis and hepato-carcinogenesis. I. Production of hepatic cirrhosis by furfural administration. Acta Pathol. Jpn., 36, 1027–1038.
Shinohara, K., Kim, E. & Omura, H. (1986) Furans as the mutagens formed by amino-carbonyl reactions. In: Fujimaki, M., Namaiki, M. & Kato, H., eds, Developments in Food Science 13: Amino–Carbonyl Reactions in Food and Biological Systems, Tokyo: Kodansha Ltd.
Stich, H.F., Rosin, M.P., San, R.H.C., Wu, C.H. & Powrie, W.D. (1981a) Intake, formation, and release of mutagens by man. In: Gastrointestinal Cancer, Endogenous Factors (Banbury Report 7), Cold Spring Harbor, New York: CSH Press, pp. 247–266.
Stich, H.F., Rosin, M.P., Wu, C.H. & Powrie, W.D. (1981b) Clastogenicity of furans found in food. Cancer Lett., 13, 89–95.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857–860.
Subramanyam, S., Sailaja, D. & Rathnapra, D. (1989) Genotoxic assay of two dietary furans by some in vivo cytogenic parameters. Environ. Mol. Mutag., 14, 239.
Sujatha, P.S. & Subramanyam, S. (1994) Clastogenicity of furfuryl alcohol in a mouse bone marrow system. Med. Sci. Res., 22, 281–284.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York: John Wiley & Sons.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985) Chemical mutagenesis testing in Drosophila. V. Results of 53 coded compounds tested for the National Toxicology Program. Environ. Mutag., 7, 677–702.
Zdzienicka, M., Tudek, B., Zielenska, M. & Szymczyk, T. (1978) Mutagenic activity of furfural in Salmonella typhimurium TA100. Mutat. Res., 58, 205–209.
See Also:
Toxicological Abbreviations
.