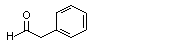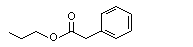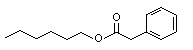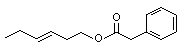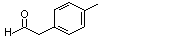WHO FOOD ADDITIVES SERIES: 50
Phenylethyl Alcohol, Aldehyde, Acid and Related Acetals and Esters and Related Substances
First draft prepared by
Dr A. Mattia
Division of Petition Review, Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, Maryland, USA
and Professor A.G. Renwick
Clinical Pharmacology Group, University of Southampton, Southampton, England
1. EVALUATION
1.1 Introduction
The Committee evaluated 43 flavouring agents that are derivatives of phenethyl alcohol (No. 987) and phenoxyethyl alcohol (see Table 1). The group includes 39 phenethyl derivatives, comprising phenylacetaldehyde (No. 1002), phenylacetic acid (No. 1007) and structurally related esters and acetals. The group also includes four phenoxyethyl alcohol derivatives: phenoxyacetic acid (No. 1026), the sodium salt of a structurally related phenoxyacetic acid (No. 1029), a phenoxyethyl ester (No. 1027) and a phenoxyacetic acid ester (No. 1028). The evaluations were conducted with the Procedure for the Safety Evaluation of Flavouring Agents (See Figure1). None of these agents has previously been evaluated by the Committee.
Table 1. Summary of results of safety evaluations of phenethyl alcohol, aldehyde, acid, and related acetals and estersa
|
Flavouring agent |
No. |
CAS No. and structure |
Steps A3 b
Does intake exceed the threshold for human intake? |
Step A4
Is the flavouring agent or are its metabolites endogenous? |
Step A5
Adequate margin of safety for the flavouring agent or related substance? |
Comments |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
|
Phenethyl alcohol |
987 |
60-12-8
 |
No
Europe: 1400
USA: 330 |
N/R |
N/R |
See note 1. |
No safety concern |
|
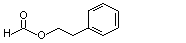
Phenethyl formate |
988 |
104-62-1
 |
No
Europe: 2
USA: 30 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl acetate |
989 |
103-45-7
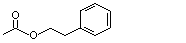 |
No
Europe: 100
USA: 60 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl propionate |
990 |
122-70-3
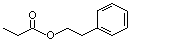 |
No
Europe: 1
USA: 3 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl butyrate |
991 |
103-52-6
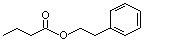 |
No
Europe: 30
USA: 30 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl isobutyrate |
992 |
103-48-0
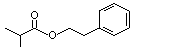 |
No
Europe: 20
USA: 60 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl-2-methylbutyrate |
993 |
24817-51-4
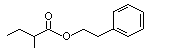 |
No
Europe: 0.4
USA: ND |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl isovalerate |
994 |
140-26-1
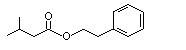 |
No
Europe: 100
USA: 30 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl hexanoate |
995 |
6290-37-5
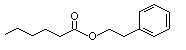 |
No
Europe: 10
USA: 2 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl octanoate |
996 |
5457-70-5
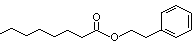 |
No
Europe: 30
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl tiglate |
997 |
55719-85-2
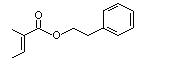 |
No
Europe: 0.3
USA: 1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl senecioate |
998 |
42078-65-9
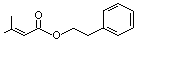 |
No
Europe: 2
USA: ND |
N/R |
N/R |
See note 2. |
No safety concern |
|
Phenethyl phenylacetate |
999 |
102-20-5
 |
No
Europe: 40
USA: 80 |
N/R |
N/R |
See note 3. |
No safety concern |
|
Acetaldehyde phenethyl propyl acetal |
1000 |
7493-57-4
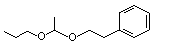 |
No
Europe: 0.1
USA: 6 |
N/R |
N/R |
See note 4. |
No safety concern |
|
Acetaldehyde butyl phenethyl acetal |
1001 |
64577-91-9
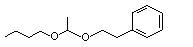 |
No
Europe: 0.01
USA: ND |
N/R |
N/R |
See note 4. |
No safety concern |
|
Phenylacetaldehyde |
1002 |
122-78-1
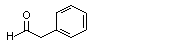 |
No
Europe: 40
USA: 60 |
N/R |
N/R |
See note 5. |
No safety concern |
|
Phenylacetaldehyde dimethyl acetal |
1003 |
101-48-4
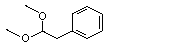 |
No
Europe: 20
USA: 40 |
N/R |
N/R |
See note 6. |
No safety concern |
|
Phenylacetaldehyde glyceryl acetal |
1004 |
29895-73-6
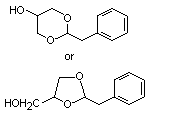 |
No
Europe: 0.1
USA: 1 |
N/R |
N/R |
See note 6. |
No safety concern |
|
Phenylacetaldehyde 2,3-butylene glycol acetal |
1005 |
5468-06-4
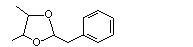 |
No
Europe: ND
USA: 1 |
N/R |
N/R |
See note 6. |
No safety concern |
|
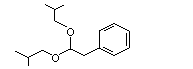
Phenylacetaldehyde diisobutylacetal |
1006 |
68345-22-2
 |
No
Europe: 30
USA: 0.4 |
N/R |
N/R |
See note 6. |
No safety concern |
|
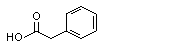
Phenylacetic acid |
1007 |
103-82-2
 |
No
Europe: 290
USA: 60 |
N/R |
N/R |
See note 7. |
No safety concern |
|
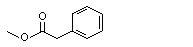
Methyl phenylacetate |
1008 |
101-41-7
 |
No
Europe: ND
USA: 20 |
N/R |
N/R |
See note 8. |
No safety concern |
|
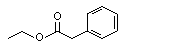
Ethyl phenylacetate |
1009 |
101-97-3
 |
No
Europe: 130
USA: 20 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Propyl phenylacetate |
1010 |
4606-15-9
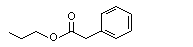 |
No
Europe: ND
USA: 0.3 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Isopropyl phenylacetate |
1011 |
4861-85-2
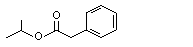 |
No
Europe: 0.07
USA: ND |
N/R |
N/R |
See note 8. |
No safety concern |
|
Butyl phenylacetate |
1012 |
122-43-0
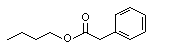 |
No
Europe: 3
USA: 3 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Isobutyl phenylacetate |
1013 |
102-13-6
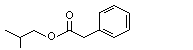 |
No
Europe: 20
USA: 20 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Isoamyl phenylacetate |
1014 |
102-19-2
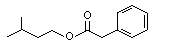 |
No
Europe: 30
USA: 30 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Hexyl phenylacetate |
1015 |
5421-17-0
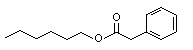 |
No
Europe: 8
USA: ND |
N/R |
N/R |
See note 8. |
No safety concern |
|
3-Hexenyl phenylacetate |
1016 |
42436-07-7
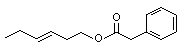 |
No
Europe: 1
USA: 0.05 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Octyl phenylacetate |
1017 |
122-45-2
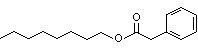 |
No
Europe: 0.004
USA: 0.006 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Rhodinyl phenylacetate |
1018 |
10486-14-3
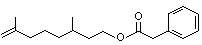 |
No
Europe: 0.001
USA: ND |
N/R |
N/R |
See note 9. |
No safety concern |
|
Linalyl phenylacetate |
1019 |
7143-69-3
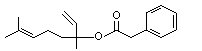 |
No
Europe: 0.09
USA: ND |
N/R |
N/R |
See note 9. |
No safety concern |
|
Geranyl phenylacetate |
1020 |
102-22-7
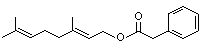 |
No
Europe: 2
USA: 2 |
N/R |
N/R |
See note 9. |
No safety concern |
|
Citronellyl phenylacetate |
1021 |
139-70-8
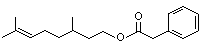 |
No
Europe: 1
USA: 2 |
N/R |
N/R |
See note 9. |
No safety concern |
|
Santalyl phenylacetate |
1022 |
1323-75-7
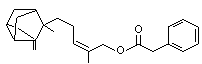
alpha |
No
Europe: ND
USA: 1 |
N/R |
N/R |
See note 9. |
No safety concern |
|
|
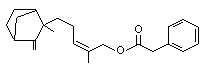
beta
|
|
|
|
|
|
|
para-Tolyacetaldehyde |
1023 |
104-09-6
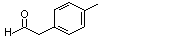 |
No
Europe: 6
USA: 3 |
N/R |
N/R |
See note 5. |
No safety concern |
|
para-Isopropylphenyl-acetaldehyde |
1024 |
4395-92-0
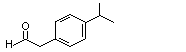 |
No
Europe: 0.1
USA: 0.01 |
N/R |
N/R |
See note 5. |
No safety concern |
|
Methyl para-tert-butylphenylacetate |
1025 |
3549-23-3
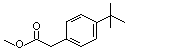 |
No
Europe: 20
USA: 20 |
N/R |
N/R |
See note 8. |
No safety concern |
|
Structural class III |
|
|
|
|
|
|
|
|
Phenoxyacetic acid |
1026 |
122-59-8
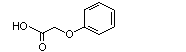 |
No
Europe: 40
USA: 0.1 |
N/R |
N/R |
See note 10. |
No safety concern |
|
Ethyl (para-tolyloxy)acetate |
1027 |
67028-40-4
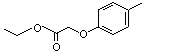 |
No
Europe: 0.1
USA: ND |
N/R |
N/R |
See note 11. |
No safety concern |
|
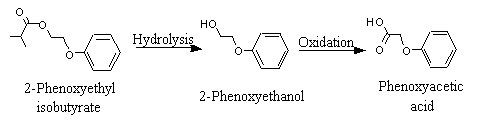
2-Phenoxyethyl isobutyrate |
1028 |
103-60-6
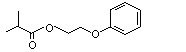 |
No
Europe: 2
USA: 110 |
N/R |
Yes. The NOEL of 15 mg/kg bw per day for the related chemical No. 1027 and the NOEL of 250 mg/kg bw per day for the realted chemical No. 1029 are > 1000 times the estimated intake of 2-phenoxy-ethyl isobutyrate when used as a flavouring agent. |
See note 12. |
No safety concern |
|
Sodium 2-(4-methoxy-phenoxy)propanoate |
1029 |
13794-15-5
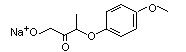 |
No
Europe: ND
USA: 6 |
N/R |
N/R |
See note 13. |
No safety concern |
|
CAS: Chemical Abstracts Service; ND: no intake data reported; N/R: not required for evaluation |
|
a |
Step 2: All the flavouring agents in this group were predicted to be metabolized to innocuous products. |
|
b |
The thresholds for human intake for classes I and III are 1800 µg/day and 90 µg/day, respectively. All intake values are expressed in µg/person per day. |
| |
The combined intakes of flavouring agents in class I are 2300 and 920 µg/person per day in Europe and the USA, respectively. The combined intakes of flavouring agents in class III are 42 and 120 µg/person per day in Europe and the USA, respectively. |
|
Notes: |
|
1. |
Oxidized to phenylacetic acid and either completely oxidized or conjugated and excreted primarily in the urine |
|
2. |
Hydrolysed to phenethyl alcohol (see note 1) and the corresponding acid, which is further oxidized to carbon dioxide and water |
|
3. |
Hydrolysed to phenethyl alcohol (see note 1) and phenylacetic acid (see note 7) |
|
4. |
Hydrolysed to phenethyl alcohol (see note 1) and the corresponding aldehyde, which is further oxidized to carbon dioxide and water |
|
5. |
Phenylacetaldehyde derivative is oxidized to phenylacetic acid derivative (see note 7). |
|
6. |
Hydrolysed to phenyacetaldehyde (see note 5) and the corresponding alcohol, which is further oxidized to carbon dioxide and water |
|
7. |
Phenylacetic acid is excreted as the glutamine acid conjugate. |
|
8. |
Hydrolysed to phenylacetic acid derivative (see note 7) and the corresponding alcohol, which is completely oxidized. |
|
9. |
Hydrolysed to phenylacetic acid (see note 7) and terpene alcohol, which is further oxidized to a polar, excretable metabolite. |
|
10. |
Excreted unchanged in the urine |
|
11. |
Hydrolysed to para-tolyloxy acetic acid and ethanol; the acid is excreted unchanged. |
|
12. |
Hydrolysed to isobutyric acid and 2-phenoxyethanol, which is oxidized to phenoxyacetic acid and excreted unchanged in the urine. |
|
13. |
Excreted mainly unchanged and as the O-demethylated metabolite, (±)2-(4-hydroxyphenoxy)propionic acid. |
Twenty of the 39 flavouring agents in this group are natural components of foods. Phenethyl alcohol has a mild rose aroma. The agents have been detected in a wide range of products; for example, the parent alcohol and its derivatives have been detected in beans, fruits, vegetables, cheeses, milk, oils and alcoholic and non-alcoholic beverages (Stofberg & Grundschober, 1987). Only one phenoxyacetic acid derivative is a natural component of food: sodium 2-(4-methoxyphenoxy)propanoate (No. 1029) has been detected in coffee (Maarse et al., 1999).
1.2 Estimated daily intake
The total annual volume of production of the 43 phenethyl alcohol and phenoxyethyl alcohol derivatives in this group is approximately 17 000 kg in Europe (International Organization of the Flavor Industry, 1995) and 7800 kg in the USA (Lucas et al., 1999) (Table 2). About 75% and 45% of the total annual volume in Europe and the USA, respectively, is accounted for by use of phenethyl alcohol (No. 987), its corresponding acetate ester (No. 989) and phenylacetic acid (No. 1007). The total annual volume of production of the four derivatives of phenoxyethyl alcohol (Nos 1026–1029) is approximately 260 kg in Europe (International Organization of the Flavor Industry, 1995) and 870 kg in the USA (Lucas et al., 1999), accounting for about 2% and 11% of the total annual volumes in Europe and the USA, respectively.
Table 2. Annual volumes of usage of phenethyl alcohol, phenylacetaldehyde, phenylacetic acid and related substances used as flavouring agents in Europe and the USA
|
Substance (No.) |
Most recent annual volume (kg)a |
Intake (‘eaters only’)b |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
|
µg/day |
µg/kg bw |
| |
per day |
|
Phenethyl alcohol (987) |
|
Europe |
10 000 |
1400 |
23 |
|
|
|
USA |
2 500 |
330 |
6 |
700 000 |
280 |
|
Phenethyl formate (988) |
|
Europe |
17 |
2 |
0.04 |
|
|
|
USA |
200 |
27 |
0.4 |
14 |
0.1 |
|
Phenethyl acetate (989) |
|
Europe |
730 |
100 |
2 |
|
|
|
USA |
490 |
65 |
1 |
30 000 |
61 |
|
Phenethyl propionate (990) |
|
Europe |
8 |
1 |
0.02 |
|
|
|
USA |
24 |
3 |
0.1 |
220 |
9 |
|
Phenethyl butyrate (991) |
|
Europe |
230 |
33 |
1 |
|
|
|
USA |
210 |
28 |
0.5 |
1 |
000 |
|
Phenethyl isobutyrate (992) |
|
Europe |
160 |
22 |
0.4 |
|
|
|
USA |
440 |
57 |
1 |
+ |
NA |
|
Phenethyl 2-methylbutyrate (993) |
|
|
Europe |
3 |
0.4 |
0.007 |
|
|
USA |
N/D |
NA |
NA |
+ |
NA |
|
Phenethyl isovalerate (994) |
|
Europe |
670 |
95 |
2 |
|
|
|
USA |
200 |
27 |
0.4 |
430 |
2 |
|
Phenethyl hexanoate (995) |
|
Europe |
96 |
14 |
0.2 |
|
|
|
USA |
14 |
2 |
0.03 |
5700 |
410 |
|
Phenethyl octanoate (996) |
|
Europe |
189 |
27 |
0.4 |
|
|
|
USA |
1 |
0.1 |
0.002 |
3200 |
3200 |
|
Phenethyl tiglate (997) |
|
Europe |
2 |
0.3 |
0.005 |
|
|
|
USA |
9 |
1 |
0.02 |
– |
NA |
|
Phenethyl senecioate (998) |
|
Europe |
11 |
2 |
0.03 |
|
|
|
USA |
N/D |
NA |
NA |
– |
NA |
|
Phenethyl phenylacetate (999) |
|
Europe |
270 |
39 |
1 |
|
|
|
USA |
590 |
78 |
1 |
– |
NA |
|
Acetaldehyde phenethyl propyl acetal (1000) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USA |
45 |
6 |
0.1 |
– |
NA |
|
Acetaldehyde butyl phenethyl acetal (1001) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
|
|
USA |
N/D |
NA |
NA |
– |
NA |
|
Phenylacetaldehyde (1002) |
|
Europe |
300 |
43 |
1 |
|
|
|
USA |
430 |
56 |
1 |
10688 |
25 |
|
Phenylacetaldehyde dimethyl acetal (1003) |
|
Europe |
143 |
20 |
0.3 |
|
|
|
USA |
345 |
45 |
1 |
+ |
NA |
|
Phenylacetaldehyde glyceryl acetal (1004) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USA |
10 |
1 |
0.02 |
– |
NA |
|
Phenylacetaldehyde 2,3-butylene glycol acetal (1005) |
|
Europe |
N/D |
NA |
NA |
|
|
|
USA |
11 |
1 |
0.02 |
– |
NA |
|
Phenylacetaldehyde disobutyl acetal (1006) |
|
Europe |
220 |
31 |
1 |
|
|
|
USA |
3 |
0.4 |
0.01 |
– |
NA |
|
Phenylacetic acid (1007) |
|
Europe |
2000 |
290 |
5 |
|
|
|
USA |
490 |
65 |
1 |
280 |
1 |
|
Methyl phenylacetate (1008) |
|
Europe |
N/D |
NA |
NA |
|
|
|
USA |
170 |
23 |
0.4 |
55 |
0.3 |
|
Ethyl phenylacetate (1009) |
|
Europe |
900 |
130 |
2 |
|
|
|
USA |
130 |
17 |
0.3 |
41 |
0.3 |
|
Propyl phenylacetate (1010) |
|
Europe |
N/D |
NA |
NA |
|
|
|
USA |
2 |
0.3 |
0.004 |
– |
NA |
|
Isopropyl phenylacetate (1011) |
|
Europe |
0.5 |
0.07 |
0.001 |
|
|
|
USA |
N/D |
NA |
NA |
– |
NA |
|
Butyl phenylacetate (1012) |
|
Europe |
20 |
3 |
0.05 |
|
|
|
USA |
25 |
3 |
0.1 |
+ |
NA |
|
Isobutyl phenylacetate (1013) |
|
Europe |
150 |
21 |
0.4 |
|
|
|
USA |
170 |
22 |
0.4 |
+ |
NA |
|
Isoamyl phenylacetate (1014) |
|
Europe |
230 |
33 |
1 |
|
|
|
USA |
220 |
29 |
0.5 |
+ |
NA |
|
Hexyl phenylacetate (1015) |
|
Europe |
57 |
8 |
0.1 |
|
|
|
USA |
N/D |
NA |
NA |
32 |
0 |
|
3-Hexenyl phenylacetate (1016) |
|
Europe |
6 |
1 |
0.01 |
|
|
|
USA |
0.4 |
0.05 |
0.001 |
+ |
NA |
|
Octyl phenylacetate (1017) |
|
Europe |
0.03 |
0.004 |
0.0001 |
|
|
|
USA |
0.045 |
0.006 |
0.0001 |
– |
NA |
|
Rhodinyl phenylacetate (1018) |
|
Europe |
0.01 |
0.001 |
0.00002 |
|
|
|
USA |
N/D |
NA |
NA |
– |
NA |
|
Linalyl phenylacetate (1019) |
|
Europe |
0.6 |
0.09 |
0.001 |
|
|
|
USA |
N/D |
NA |
NA |
– |
NA |
|
Geranyl phenylacetate (1020) |
|
Europe |
14 |
2 |
0.03 |
|
|
|
USA |
14 |
2 |
0.03 |
– |
NA |
|
Citronellyl phenylacetate (1021) |
|
Europe |
10 |
1 |
0.02 |
|
|
|
USA |
14 |
2 |
0.03 |
– |
NA |
|
Santalyl phenylacetate (1022) |
|
Europe |
N/D |
NA |
NA |
|
|
|
USA |
4 |
1 |
0.01 |
– |
NA |
|
para-Tolyacetaldehyde (1023) |
|
Europe |
45 |
6 |
0.1 |
|
|
|
USA |
23 |
3 |
0.05 |
– |
NA |
|
para-Isopropylphenylacetaldehyde (1024) |
|
Europe |
0.5 |
0.1 |
0.001 |
|
|
|
USA |
0.045 |
000 |
0.0001 |
– |
NA |
|
Methyl para-tert-butylphenylacetate (1025) |
|
Europe |
140 |
19 |
0.3 |
|
|
|
USA |
150 |
20 |
0.3 |
– |
NA |
|
Phenoxyacetic acid (1026) |
|
Europe |
250 |
35 |
1 |
– |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
|
|
|
Ethyl (para-tolyloxy)acetate (1027) |
|
Europe |
1 |
0.1 |
0.002 |
– |
NA |
|
USA |
N/D |
NA |
NA |
|
|
|
2-Phenoxyethyl isobutyrate (1028) |
|
Europe |
14 |
2 |
0.03 |
– |
NA |
|
USA |
830 |
110 |
2 |
|
|
|
Sodium 2-(4-methoxyphenoxy) propanoate (1029) |
|
Europe |
N/D |
NA |
NA |
+ |
NA |
|
USA |
43 |
6 |
0.1 |
|
|
|
Total |
|
Europe |
17 000 |
|
|
|
|
|
USA |
7 800 |
|
|
|
|
|
NA, not available; N/D, no intake data reported; +, reported to occur naturally in foods |
|
(Maarse et al., 1996), but no quantitative data available; –, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavor Industry (1995) and Lucas et al. (1999) |
|
b |
Intake (µg/person per day) was calculated as follows: [(annual volume, kg) × (1 × 109 µg/kg)/(population × survey correction factor × 365 days)], where population (10%, ‘eaters only’) = 32 × 106 for Europe and 26 × 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual production volume of the flavour, resepctively, was reported in the poundage surveys. Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding. |
|
c |
Quantitative data from Stofberg & Grundschober (1987) |
|
d |
Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring agent, kg) |
Five flavouring agents in the group have the highest estimated intakes. These are 1400 µg/day for phenethyl alcohol (No. 987), 290 µg/day for phenylacetic acid (No. 1007), 130 µg/day for ethyl phenylacetate (No. 1009) and 100 µg/day for phenethyl acetate (No. 989) and for phenethyl isovalerate (No. 994) in Europe and 330 µg/day for phenethyl alcohol (No. 987) and 110 µg/day for phenoxyethyl isobutyrate (No. 1028) in the USA. The intakes of all the other flavouring agents in the group ranged from 0.001 to 80 µg/day, the intake of about 70% of them being less than 25 µg/day.
1.3 Hydrolysis, absorption, distribution, metabolism and elimination
Phenethyl and phenylacetate esters and phenyacetaldehyde acetals are rapidly hydrolysed in vivo to yield phenethyl alcohol (No. 987), phenylacetic acid (No. 1007) and phenylacetaldehyde (No. 1002), respectively (Williams, 1959). Phenethyl alcohol and phenylacetaldehyde are both oxidized to phenylacetic acid, which is conjugated and excreted primarily in the urine (Williams, 1959). Similarly, the phenoxyethyl and phenoxyacetate esters, 2-phenoxyethyl isobutyrate (No. 1028) and ethyl(para-tolyloxy)acetate (No. 1027), respectively, are anticipated to be hydrolysed to their component acids and alcohols. Phenoxyethyl alcohol is rapidly oxidized to phenoxyacetic acid, which in turn is rapidly absorbed and excreted primarily unchanged in the urine (Howes, 1988). Sodium 2-(4-methoxyphenoxy)propanoate (No. 1029) is also rapidly absorbed and excreted primarily unchanged, with a small amount of the O-demethylated metabolite, (±)2-(4-hydroxyphenoxy) propionic acid (Brown et al., 1986; Hawkins & Mayo, 1986; Sangster & Lindley, 1986; Caldwell, 1987). Therefore, all the flavouring agents in this group were predicted to hydrolyse and/or oxidize to yield phenylacetic acid or a phenoxyacetic acid derivative that is excreted either free or in conjugated form.
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure, the Committee assigned phenylacetic acid (No. 1007) to structural class I (Cramer et al., 1978) because it is a normal component of human urine. The other 38 phenethyl alcohol derivatives were also assigned to structural class I because they are simple aromatic compounds with a primary oxygenated functional group. |
| |
The Committee assigned all four phenoxyacetic acid derivatives in this group to structural class III (Cramer et al., 1978). These are aromatic substances with an ether linkage between the benzene ring and an unsaturated side-chain with a primary oxygenated functional group. |
|
Step 2. |
All the flavouring agents in this group were predicted to be metabolized to innocuous products. Their evaluation therefore proceeded via the A (left-hand) side of the decision tree. |
|
Step A3. |
The estimated daily intakes of each of the 39 flavouring agents in structural class I are below the threshold for daily human intake for that class (1800 µg per person). According to the Procedure, the safety of these 39 flavouring agents raises no concern when they are consumed at currently estimated levels. |
| |
The estimated daily intakes of three of the four flavouring agents in structural class III are below the threshold for daily human intake for that class (90 µg per person). According to the Procedure, the safety of these three flavouring agents (Nos 1026, 1027 and 1029) raises no concern when they are consumed at their currently estimated levels. The daily intake of 2-phenoxyethyl isobutyrate (No. 1028) in the USA (110 µg/person) exceeds the threshold for daily human intake for compounds in structural class III (90 µg per person). Accordingly, the evaluation of this substance proceeded to step A4. |
|
Step A4. |
2-Phenoxyethyl isobutyrate (No. 1028) is not endogenous. Therefore, its evaluation proceeded to step A5. |
|
Step A5. |
Although no NOEL was available for 2-phenoxyethyl isobutyrate (No. 1028), data were available on the toxicity of two structurally related flavouring agents in this group (Nos 1027 and 1029). The NOEL of 15 mg/kg bw per day for ethyl(para-tolyloxy)acetate (No. 1027) in a 90-day dietary study in rats (Posternak et al., 1969) and the NOEL of 250 mg/kg bw per day for sodium 2-(4-methoxyphenoxy)propanoate (No. 1029) in another 90-day dietary study in rats (Hill & Wood, 1986) provide adequate margins of safety (> 1000 times) in relation to the estimated daily per capita intake of 2-phenoxyethyl isobutyrate (No. 1028) in Europe (2 µg/kg bw) and the USA (110 µg/kg bw). The Committee therefore concluded that the safety of this agent would not be a concern. |
Table 1 summarizes the evaluations of the 43 flavouring agents in this group.
1.5 Consideration of combined intake from use as flavouring agents
In the unlikely event that all 39 of the phenethyl alcohol derivatives (Nos 987–1025) were consumed concurrently on a daily basis, the estimated combined intake would exceed the threshold for daily human intake of compounds in structural class I (1800 µg per person). At the levels of intake associated with their use as flavouring agents, all 39 agents are expected to be metabolized efficiently, without saturating metabolic pathways. The same holds true for two of the four flavouring agents in structural class III: the estimated combined intakes of phenoxyacetic acid (No. 1026) and 2-phenoxyethyl isobutyrate (No. 1028) would exceed the threshold for daily human intake of compounds in structural class III (90 µg per person). At the levels of intake associated with their use as flavouring agents, both agents are expected to be metabolized efficiently, without saturating metabolic pathways. The other two agents, ethyl (para-tolyloxy)acetate (No. 1027) and sodium 2-(4-methoxyphenoxy) propanoate (No. 1029), are not converted to a common metabolite, and there is no need to consider their combined intake from use as flavouring agents. On the basis of the evaluation of all the data, combined intake would raise no safety concern.
1.6 Conclusions
The Committee concluded that none of the 43 flavouring agents in this group would present a safety concern at current estimated levels of intake. No data on toxicity were required for application of the Procedure to 42 of the 43 flavouring agents, as they were predicted to be metabolized to innocuous agents and the estimated intakes were below the human intake threshold associated with the relevant structural class. Data on the toxicity of related agents were used to evaluate 2-phenoxyethyl isobutyrate (No. 1028). Data on the toxicity and metabolism of phenethyl alcohol, phenylacetaldehyde, phenylacetic acid, phenoxyethanol and phenoxyacetic acid and related agents were consistent with the results of the safety evaluation.
2. Background information
2.1 Explanation
The relevant background information summarizes the key data for the safety evaluation of 39 phenethyl alcohol derivatives and four phenoxyethyl alcohol derivatives. The group of flavouring substances includes the following: phenethyl alcohol (No. 987), 28 esters formed from phenethyl alcohol or phenylacetic acid (Nos 988–999, 1008–1022 and 1025), six acetals of phenethyl alcohol or phenylacetaldehyde (Nos 1000–1001 and 1003–1006), three related phenylacetaldehydes (Nos 1002 and 1023–1024) and phenylacetic acid (No. 1007). The four phenoxyacetic acid derivatives comprise phenoxyacetic acid (No. 1026), an ester of phenoxyacetic acid (No. 1027), an ester of phenoxyethyl alcohol (No. 1028) and a homologue of phenoxyacetic acid (No. 1029) (see Table 1).
The substances in this group are structurally related because they each have a 2-phenethyl or 2-phenoxyethyl-carbon skeleton containing a primary oxygenated functional group. Studies described in more detail below indicate that the phenethyl acetals and esters in this group are readily hydrolysed to yield phenethyl alcohol, phenylacetaldehyde or phenylacetic acid. Also. phenethyl alcohol and phenylacetaldehyde are readily oxidized to phenylacetic acid. Therefore, all the substances in this group are eventually hydrolysed and oxidized to yield phenylacetic acid, which is excreted primarily in the urine in conjugated form. Similarly, the esters of phenoxyethyl alcohol or phenoxyacetic acid are hydrolysed to the parent alcohol or acid. Phenoxyethyl alcohol is oxidized to phenoxyacetic acid, which is excreted primarily unchanged in the urine (Williams, 1959). Given their similar pharmacokinetics and their participation in a common metabolic pathway, the substances in this group are expected to have similar toxicological profiles (see Figure 1).
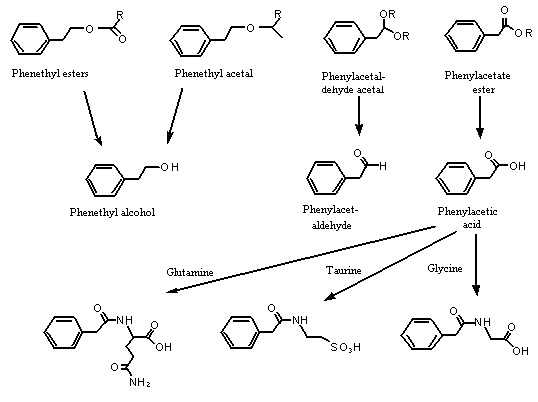
Figure 1. Metabolism of phenethyl alcohol and related substances in mammals
2.2 Additional considerations on intake
Production volumes and intake values for each flavouring agent in this group are reported in Table 2, which also gives the available information on natural occurrence.
Quantitative data on natural occurrence and consumption ratios have been reported for 13 phenethyl alcohol derivatives. The consumption ratios for phenethyl alcohol (No. 987), phenethyl acetate (No. 989), phenethyl propionate (No. 990), phenethyl hexanoate (No. 995), phenethyl octanoate (No. 996) and phenylacetaldehyde (No. 1002) are all > 1, indicating that they are consumed predominantly from traditional foods (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987). The six flavouring agents that have consumption ratios > 1 represent 67% and 44% of the total annual volume of usage in Europe and the USA, respectively.
Only one phenoxyacetic acid derivative is a natural component of food. Sodium 2-(4-methoxyphenoxy) propanoate (No. 1029) has been detected in coffee (Maarse et al., 1999), but no quantitative data were available on its natural occurrence.
2.3 Biological data
2.3.1 Biochemical data
(a) Hydrolysis
If evidence can be supplied to show that an ester is readily hydrolysed in the body to constituents whose metabolic fate and biological actions are fully understood, further toxicological studies may not be necessary. Consequently, artificial gastro-intestinal juices have been used to study the hydrolysis of esters (Longland et al., 1977). Before absorption in vivo, the esters and acetals in this group can be reasonably predicted to undergo hydrolysis (Williams, 1959) to yield phenethyl alcohol, phenoxyethyl alcohol, phenylacetaldehyde, phenylacetic acid and phenoxyacetic acid. Phenethyl acetate (No. 989), methyl phenylacetate (No. 1008), ethyl phenylacetate (No. 1009), isopropyl phenylacetate (No. 1011), isoamyl phenylacetate (No. 1014) and citronellyl phenylacetate (No. 1021) were rapidly hydrolysed in vitro in simulated gastric juice and pancreatic juice (Nos 989, 1008 and 1009) (Longland et al., 1977) or in a buffered solution of pancreatin (Nos 1008, 1011, 1014 and 1021) (Grundschober, 1977) to the corresponding phenethyl derivatives. Phenethyl acetate (No. 989), ethyl phenylacetate (No. 1009) and isoamyl phenylacetate (No. 1014) were not hydrolysed by partially purified human plasma arylesterase (Augustinsson & Ekedahl, 1962).
The finding of hydrolysis of aromatic acetals in simulated gastric juice and intestinal fluid leads indicates that the acetal functional group is hydrolysed before absorption in vivo. Incubation of 2-phenylpropanal dimethyl acetal (1 mmol/l) with simulated gastric juice at 37 °C resulted in 97% hydrolysis within 1 h. Under the same experimental conditions, benzaldehyde propylene glycol acetal (1 mmol/l) was 53% hydrolysed within 5 h, whereas the acetal in 0.1 N HCl under reflux was 99% hydrolysed (Morgareidge, 1962). The data on hydrolysis of acetals in vitro and in vivo indicate that aliphatic acetals undergo hydrolysis in humans before absorption.
(b) Absorption, distribution and excretion
When ingested in traditional foods, in foods to which they have been intentionally added or as hydrolysis products resulting from either condition, phenethyl and phenoxyethyl alcohols, phenylacetaldehyde and phenylacetic and phenoxyacetic acids are rapidly absorbed from the gastrointestinal tract. Once absorbed, the alcohols and aldehydes are rapidly oxidized to yield phenylacetic or phenoxyacetic acid derivatives, which are subsequently excreted in the urine, either free as in the case of phenoxyacetic acid or conjugated as in the case of phenylacetic acid (Williams, 1959; James et al., 1972; Sangster & Lindley, 1986; Hawkins & Mayo, 1986; Caldwell, 1987).
(i) Phenethyl alcohol (No. 987) and phenylacetic acid (No. 1007)
Phenylacetic acid is a normal component of human urine (250–500 mg/24 h), forming mainly from the breakdown of phenylalanine by intestinal bacteria (Seakins, 1971) or by oxidative deamination of endogenous phenethylamine (Richter, 1938; Seakins, 1971). Orally administered phenethylamine is rapidly metabolized to phenylacetylglutamine. Two volunteers, each fed a 300-mg dose of S-phenethyl-amine, excreted 60–62% of the administered dose as conjugated phenylacetic acid in the urine within 2–4.5 h (Richter, 1938; Seakins, 1971). Furthermore, > 80% of [14C]-S-phenethylamine fed to mice was rapidly excreted from urine as conjugated [14C]phenylacetic acid (Block, 1953).
One male volunteer excreted 26% of a 4000-mg oral dose of phenethyl alcohol (No. 987) in his urine as phenylacetylglutamine within 24 h (Thierfelder & Schempp, 1917). In rabbits, 42% and 5% of a single 300 mg/kg bw oral dose of phenethyl alcohol was excreted in the urine as glycine and glucuronic acid conjugates, respectively, of phenylacetic acid within 24 h. The ether-soluble acid extracted from the 24-h urine accounted for 61% of the dose (Bray et al., 1958). In an earlier study, 53% of a dose of 1300 mg bw of phenylacetic acid administered to rabbits by gavage was isolated from the 24-h urine as an ether-soluble acid. No appreciable quantity (< 0.5%) of free phenylacetic acid was recovered (Bray et al., 1946). In another study, only 0.4–3.1% of an oral dose of 740 mg/kg bw phenylacetic acid was excreted unconjugated in the urine of rabbits within 6 h (Tulane & Lewis, 1933).
More than 90% of a single oral dose of 80 mg of [carboxy-14C]phenylacetic acid administered to each of three healthy volunteers and two patients with phenylketouria was excreted in the urine within 24 h as the glutamine conjugate (In a third patient with phenylketouria in this study, 12% of the dose was excreted as the glutamine conjugate, but this was attributed to incomplete collection of urine.) (James et al., 1973). Two male volunteers excreted 98% of an oral dose of 1 mg/kg bw of [carboxy-14C]phenylacetic acid in their urine within 24 h (James et al., 1972), with > 90% of the dose recovered as the glutamine conjugate. The results of studies with radiolabelled phenylacetic acid indicate that it is rapidly absorbed and quantitatively excreted within 24 h.
(ii) Phenoxyethyl alcohol and phenoxyacetic acid (No. 1026) derivatives
Once hydrolysed, 2-phenoxyethanol is rapidly absorbed and oxidized to phenoxyacetic acid (No. 1026), which is then excreted almost exclusively in the urine in unconjugated form (Figure 2).
Figure 2. Metabolism of 2-phenoxyethanol and related substances in animals
The fate of 2-phenoxyethanol in rats and humans has been investigated. More than 90% of an oral dose of 16, 27 or 160 mg/kg bw of [2-14C]phenoxyethanol given to male Colworth rats by gavage was excreted in the urine within 24 h. A female rat also excreted about 90% of a dose of 27 mg/kg bw in the urine within 24 h. Approximately 2 and 1.3% of the ingested dose was recovered from expired air of female and male rats, respectively. The rate of intestinal absorption was rapid, with 60–70% of the excreted 14C detected at 3 h and > 95% of the total 4-day urinary 14C detected within the first 24 h. Trace amounts of radioactivity were detected in faeces. Four days after dosing, only trace amounts of radioactivity remained in the carcass, primarily in the liver (< 0.2% of the dose), fat and muscle. At 4 days, the 14C concentration in blood was only 0.001% (Howes, 1988).
After ingestion of a dose of 11 mg of unlabelled 2-phenoxyethanol by a volunteer, urine samples were pooled daily for 4 days. Quantitative urinary elimination (12 mg, 104% of the dose) of phenoxyacetic acid, mainly in the unconjugated form, was reported within 24 h. Minor amounts (0.34 mg) of phenoxyacetic acid were detected at day 2, but none was detectable at day 3 or 4 (Howes, 1988).
Phenoxyacetic acid was fed to male rabbits at a dose of 100–200 mg/kg bw, and some animals also received glycine in amounts corresponding to three equivalents of the acid. In this test, 44–72% of the phenoxyacetic acid was recovered unchanged in the urine within 6 h and 82–105% within 24 h. There was no evidence of conjugation with either glucuronic acid or glycine, even when the diet was supplemented with glycine. A rabbit that received an oral dose of 500 mg of the glycine conjugate of phenoxyacetic acid excreted 30% of the dose unconjugated in the urine after 18 h (Levey & Lewis, 1947). In another study, 55% of an oral dose of an unspecified amount of phenoxyacetic acid was recovered in the urine of dogs and 61% in the urine of humans. No evidence for glycine or glucuronic acid conjugation was found (Thierfelder & Schempp, 1917).
A series of studies performed with 2-(4-methoxyphenoxy) propanoate (No. 1029) indicated that this phenoxyacetic acid derivative is also rapidly absorbed and excreted unchanged in the urine. Groups of three male and three female rats were dosed by oral gavage with [14C]sodium 2-(4-methoxyphenoxy) propanoate at 50 mg/kg bw or 500 mg/kg bw (5 µCi), and urine was collected at 6, 12, 24, 48, 72, 96 and 120 h. Regardless of dose, most of the radioactivity (group mean activity, 81–95%) was eliminated in the urine within the first 24 h, most of the elimination (group mean activity, 59–76% of dose) occurring within the first 6 h. Only 0.8–4.6% was detected in the faeces at 120 h. The authors noted that contamination of faeces by urine may have been responsible for the radioactivity detected. At 120 h, the concentratuion of radioactivity in the urine of females was approximately twice that of males, suggesting that males eliminate the substance faster than females (Brown et al., 1986).
In the same study, 30 male and 27 female rats were dosed by oral gavage with [14C]sodium 2-(4-methoxyphenoxy) propanoate at 50 mg/kg bw (5 µCi/ml) 18 days after mating. Groups of three male and three female animals were killed 0.5, 1, 3, 5, 7, 12, 24, 48, 72 and 96 h after dosing, and blood was sampled and tissues and organs were removed. Sodium 2-(4-methoxyphenoxy) propanoate was rapidly absorbed and eliminated by both male and female rats. Only 12% (range, 6.3–23%) and 2.6% remained in the stomach of males and females after 0.5 h, indicating that absorption was slightly faster in females than in males. The total radioactivity was greatest in the liver (2.5% for males and 3.1% for females) and kidney (2.1% for males and 2.5% for females), the concentration of radioactivity being 3.5 times greater in the kidney than in the liver. Loss of radioactivity from tissues and organs was rapid, elimination being essentially complete with 24 h (Brown et al., 1986).
In pregnant females CD rats given [14C]sodium 2-(4-methoxyphenoxy) propanoate by gavage at a dose of 50 mg/kg bw (5 µCi/ml), examination of fetuses and amniotic fluid revealed that the radioactivity had crossed the placenta, although its elimination from the fetus and placenta was complete within 3 h. Elimination of radioactivity from amniotic fluid was complete within 12 h. Radioactivity was detected in uterine muscle for up to 72 h, although the activity represented < 0.05% of the dose at 7 h. The authors suggested that the acid might bind to uterine muscle protein. In a subsequent test with bovine serum albumin in vitro, sodium 2-(4-methoxy-phenoxy) propanoate bound to protein, an equilibrium (83–85% in bound fraction) being reached within 2 h. The authors concluded that, although the acid binds to plasma protein, it is still rapidly eliminated from the blood (Brown et al., 1986).
Measurements in plasma confirmed that [14C]sodium 2-(4-methoxyphenoxy) propanoate is rapidly absorbed and eliminated from male and pregnant female rats. The highest plasma concentration was found at the first sampling time (0.5 h); at 12 h, the concentration was only 0.3% of that achieved at 0.5 h. Repeated daily dosing resulted in 90% of the steady-state plasma concentration after 10 days. However, the steady state concentration was still < 1% of the plasma concentration at 0.5 h. Thus, repeated dosing had little effect on the plasma concentration–time profile (Brown et al., 1986).
In a subsequent study, three male and three female rats each received a single intravenous dose of [14C]sodium 2-(4-methoxyphenoxy) propanoate at a level of 50 mg/kg (10 µCi). Blood samples were collected at 5, 15, 30 and 60 min and 2, 4, 6, 12 and 24 h after dosing until the concentrations of radioactivity were near the limit of accurate measurement. The highest plasma concentration, achieved at 5 min, declined rapidly over the next hour. The mean concentrations of radioactivity decreased from 190 and 210 µg/ml of plasma equivalent at 5 min to 11 and 16 µg/ml at 1 h in male and female rats, respectively. The mean elimination half-lives of radioactivity during this time were estimated to be 13 min for males and 14 min for females. The concentrations of radioactivity declined to 0.25 and 0.32 µg/ml of plasma in male and female rats, respectively, at 6 h. No radioactivity was detected in male rats after 6 h or in female rats after 24 h. The mean area under the curve of plasma concentration–time was 77 µg.h per ml for male rats and 100 µg.h per ml for females, indicating slower elimination of radioactivity by females. The authors concluded that sodium 2-(4-methoxyphenoxy) propanoate was rapidly eliminated from the vascular system of male and female rats (Hawkins & Mayo, 1986).
In a subsequent investigation of the effect of repeated intake on metabolism and excretion, five male and five female rats were fed a diet containing 5000 ppm of uniformly ring-labelled [14C]sodium 2-(4-methoxyphenoxy) propanoate for 17 weeks. The study was conducted in three phases. Two unlabelled doses and one 14C-labelled dose of the sodium salt were given on separate occasions with 2 weeks between each dose. In the study with the labelled dose, 99% and 104% of the radioactivity was excreted in the urine of males and females, respectively, < 1% of the dose being detected in faeces. The 0–6-h urine samples accounted for 83% of the dose and the 6–12-h samples for 17%. No radioactivity was detected in whole blood, plasma or washed red blood cells 7 days after administration. More urinary metabolites were recovered than had been administered in the studies with unlabelled substance, indicating that the test material was not completely eliminated before oral administration. However, in both studies, 90% of the recovered material was excreted within the first 6 h after dosing and most of the remainder in the 6–12-h urine sample. The author concluded that prolonged intake of sodium 2-(4-methoxyphenoxy) propanoate does not affect the metabolism or disposition of this compound in rats (Hill, 1986).
The disposition of sodium 2-(4-methoxyphenoxy) propanoate was also investigated in three healthy male volunteers given capsules containing 114.7 mg, 112.9 mg and 114.3 mg of the test compound. About 75% of the dose was eliminated in urine within 72 h, of which 85% was recovered within 24 h (Sangster & Lindley, 1986). In another study, five healthy male volunteers were given an oral dose of 100 mg of [2-14C]sodium 2-(4-methoxyphenoxy) propanoate (50 µCi). About 97% of the dose was recovered within 5 days of administration, with approximately 93% recovered within the first 24 h (Caldwell, 1987). The author considered that the data show that sodium 2-(4-methoxyphenoxy) propanoate is rapidly absorbed and rapidly eliminated from the body via the urine.
On the basis of the extensive data available on the absorption, distribution and elimination of phenethyl and phenoxyethyl alcohols and phenylacetic and phenoxyacetic acids, the Committee concluded that, after hydrolysis, the parent alcohols are converted mainly to the corresponding carboxylic acids, which are then rapidly excreted in either free or conjugated form in urine. The pharmacokinetics of phenoxyethyl alcohol derivatives in rats and humans indicate that the rat is an acceptable model for human risk assessment.
(c) Biotransformation
(i) Phenethyl alcohol derivatives
Phenethyl alcohol is successively oxidized to phenylacetaldehyde and phenylacetic acid in vivo. Phenylacetic acid undergoes species-specific conjugation with a variety of amino acids, amines or glucuronic acid, followed by excretion almost exclusively in the urine (James et al., 1972; see Figure 2). Phenethyl alcohol is readily oxidized to phenylacetaldehyde by an assortment of NAD+-dependent alcohol and aldehyde dehydrogenases (Bosron & Li, 1980). The greatest activity of mammalian alcohol dehydrogenases (ALDH) occurs in the liver, where they show broad substrate specificity for the oxidation of primary aliphatic and aromatic alcohols. Human liver ALDH showed a decreased Michaelis-Menten constant (Km, the concentration of the specific substrate at which a given enzyme yields one-half its maximum velocity with increasing lipophilicity); however, the maximum rate or velocity of an enzymatic reaction which is indicative of all the enzyme active site(s) complexed with substrate, Vmax, remained essentially constant, suggesting that the rate-limiting step does not involve the binding or release of the alcohol or aldehyde intermediate (Pietruszko et al., 1973).
Once formed, phenylacetaldehyde is oxidized by inducible aldehyde dehydro-genases from rat liver cytosol. In rats, these isoenzymes can be induced by phenobarbital (Simpson et al., 1985). The Km and Vmax values of human mitochondrial ALDH (ALDH-2) and the cytosolic isoenzyme (ALDH-1) for oxidation of phenylacetal-dehyde (Table 3) indicate rapid conversion to phenylacetic acid (Klyosov, 1996).
Table 3. Pharmacokinetics of human mitochondrial aldehyde dehydrogenase (ALDH-2) and cytosolic isoenzyme (ALDH-1)
|
Pharmacokinetics |
ALDH1 |
ALDH2 |
|
Km (nmol/l) |
5500 ± 1200 |
29 ± 4 |
|
Vmax rel(%in relation to Vmax of acetaldehyde) |
380 ± 40 |
150 ± 20 |
|
kcata |
3000 ± 340 |
1800 ± 200 |
| |
From Klyosov (1996) |
|
a |
kcat values are based on the relative molecular masses of tetrameric enzymes of 230 000 (ALDH-1) and 240 000 (ALDH-2) The specific activities of purified ALDH-1 and ALDH-2 from five different batches were 3.4 ± 0.6 and 4.9 ± 0.8 µmol/min (per mg of protein), respectively, at pH 9.5. This corresponds to kcat values of 782 ± 138 per min for ALDH-1 and 1200 ± 190 per min for ALDH-2. |
Phenylacetaldehyde and 3- and 4-chlorophenylacetaldehyde are effectively oxidized to the corresponding phenylacetic acid derivatives when incubated with rat hepatic microsomal dehydrogenase containing NAD+ as a coenzyme. The rates of oxidation for the 3- and 4-chloro derivatives were markedly slower than that of the parent phenylacetaldehyde (Martini & Murray, 1996). In dogs, 32% of a dose of 1900 mg/kg bw phenylacetaldehyde (No. 1002) was rapidly oxidized and excreted as the glycine conjugate within 48 h (Kay & Raper, 1922).
(ii) Conjugation of phenylacetic acid (No. 1007)
Although phenylacetic acid has been studied extensively, investigations conducted before 1950 on human metabolism (Shiple & Sherwin, 1922; Power & Sherwin, 1927; Ambrose et al., 1933; Wagreich et al., 1940) failed to account for the endogenous level of 250–500 mg/kg per day of phenylacetic acid conjugated with glutamine (Stein et al., 1954) present in human urine and did not adequately characterize the array of urinary conjugates that were formed from phenylacetic acid. More recent work demonstrates that conjugation is both dose-dependent and species-specific. The major metabolic options available to phenyl acetic acid are conjugation with glucuronic acid, glycine, taurine or glutamine and elimination as the free acid.
Two men excreted an average of 91% and 7% of an oral dose of 1 mg/kg bw of [carboxy-14C]phenylacetic acid within 24 h as glutamine and taurine1 conjugates, respectively. In contrast to most animals, humans have only traces of the glycine conjugate (James et al., 1972). The distribution and type of conjugation are virtually unaffected by continued ingestion of phenylacetic acid. One person fed 34 doses of 1000–10 000 mg of the acid over 97 days excreted > 90% of the administered dose as the phenylacetylglutamine conjugate (Ambrose et al., 1933). Like humans, Old and New World monkeys conjugate phenylacetic acid with glutamine and, to a lesser extent, taurine; however, significant quantities of acid (1–44%) are excreted free. In carnivores (e.g., dogs, cats and ferrets), glycine conjugation predominates, with no detectable glutamine conjugation. Likewise, in rodents and lagomorphs (rabbits), phenylacetic acid is excreted primarily as the glycine conjugate. Unconjugated phenylacetic acid and minute amounts of taurine conjugates are also excreted. In rats, > 94% of a dose of 80 mg/kg bw of phenylacetic acid given by intraperitoneal injection was excreted as the glycine conjugate (James et al., 1972).
Clearly, the nature of the amino acid used for conjugation is a function of species. The sources and amounts of available amino acids alter the conjugating ability of different species. In humans, endogenous sources of glutamine include those from waste urea nitrogen. Ingestion of 5000 mg/day of phenylacetic acid for 3 consecutive days resulted in a 25–78% decrease in urinary urea nitrogen. Glutamine may be supplied by blood plasma glutathione (reduced tripeptide, Glu-Cys-Gly). An 18–23% reduction in the plasma tripeptide concentration was observed within a few hours of ingestion of a 4000-mg dose of phenylacetic acid in a volunteer(Shiple & Sherwin, 1922).
The capacity for glutamine conjugation was studied in three control subjects and three patients with phenylketouria, each of whom was given a single 80-mg dose of [carboxy-14C]phenylacetic acid. The average excretion of phenylacetylglutamine (measured as mmol/g creatinine) by the patients with phenylketouria was approximately five times that of two of the control subjects, indicating that the glutamine conjugation mechanism can cope with large amounts of phenylacetic acid (James & Smith, 1973). The mechanism for conjugation of glutamine with phenylacetic acid probably involves formation of a phenylacetic acid–coenzyme A intermediate. Perfusion of human kidney for 1 h or incubation of human liver homogenate with [14C]glutamine and phenylacetyl–coenzyme A resulted in yields of the 5 and 13% of the respective radioactive conjugates (Moldave & Meister, 1957).
In rodents, endogenous unconjugated phenylacetic acid can be found at concentrations at which glycine conjugation is capacity-limited, presumably by the supply of endogenous glycine (Gregus et al., 1993). Only small amounts of the glycine conjugate enter the bile: < 10% of a dose of phenylacetic acid of 100 mg/kg bw delivered to the small bowel via an indwelling catheter was collected from the bile of rats over 4 h (Koss & Lamprecht, 1968). Significant concentrations of free phenylacetic acid have been found after high doses (Teuchy et al., 1971; James et al., 1972). Prolonged high concentrations of free phenylacetic acid might be associated with toxic effects similar to those observed with high doses of other carboxylic acids with which conjugation is glycine-limited. Conversely, the high levels of glutamine available for conjugation in humans might allow metabolic pathways to cope with high levels of endogenously formed phenylacetic acid.
(iii) Phenoxyethyl alcohol derivatives
In rats, > 90% of a dose of of [2-14C]-2-phenoxyethanol of 16, 27 or 160 mg/kg bw in males and 27 mg/kg bw in females was excreted in the urine as phenoxyacetic acid within 24 h. An entire oral dose of 11 mg of unlabelled 2-phenoxyethanol was accounted for in the urine of one healthy male volunteer as 2-phenoxyacetic acid. Most of the acid was excreted unconjugated (Howes, 1988).
Urine of male rats collected for 6 h after an oral dose of 500 mg/kg (5 µCi) of [14C]sodium 2-(4-methoxyphenoxy)propanoate contained two excretion products, identified as 2-(4-methoxyphenoxy)propanoic acid (90%) and the O-demethylated metabolite (±)-2-(4-hydroxyphenoxy)propionic acid (7–10%) (Brown et al., 1986).
Groups of five rats of each sex were fed a diet cointaining [14C]sodium 2-(4-methoxyphenoxy)propanoate at a concentration of 5000 ppm, corresponding to 250 mg/kg bw per day, for 17 weeks. The majority (92–96%) of the dose was excreted as unchanged 2-(4-methoxyphenoxy)propanoic acid, while 6–7% of the dose in male rats and 8–9% of the dose in female rats was excreted as the O-demethylated metabolite (±)-2-(4-hydroxyphenoxy)propionic acid (Hill, 1986).
The metabolites in the urine of three men who received a single dose of 113–114 mg of 2-(4-methoxyphenoxy)propanoic acid included 2-(4-methoxyphenoxy) propanoic acid (65%) and 2-(4-hydroxyphenoxy)propanoic acid (10%) (Sangster & Lindley, 1986). In another study, approximately 97% an oral dose of 100 mg of [2-14C]sodium 2-(4-methoxyphenoxy)propanoate (50 µCi) given to five healthy male volunteers was excreted in the urine as 2-(4-methoxyphenoxy)propanoic acid; > 93% was recovered from the urine within the first 24 h (Caldwell, 1987). Thus, sodium 2-(4-methoxyphenoxy)propanoate is rapidly absorbed and rapidly eliminated in the urine, mainly as the unchanged acid. Minor amounts undergo O-demethylation to yield the corresponding metabolite 2-(4-hydroxyphenoxy)propanoic acid.
In summary, phenethyl and phenoxyethyl alcohol derivatives are rapidly oxidized to the corresponding carboxylic acids. In humans, phenylacetic acid is subsequently conjugated primarily with glutamine and excreted in the urine, while phenoxyacetic acid is excreted unconjugated almost exclusively in the urine.
(d) Other biochemical studies
Other studies have been conducted in rodents and humans on the effects of phenylacetic acid on various biochemical parameters, mostly associated with phenylketouria. Phenylacetic acid administered orally to fasted guinea-pigs at a dose of 380, 500 or 750 mg/kg bw resulted in a reduction in blood sugar to 13%, 44% and 60% of the initial value, respectively, within 2–4 h. Rabbits were as sensitive as guinea-pigs to the hypoglycaemic action of phenylacetic acid. Phenylacetic acid administered intravenously at a dose of 62 mg/kg bw also produced a marked reduction in blood sugar in a cat, which was sustained for at least 5 h (Stewart, 1962).
Phenylacetic acid given orally at a single dose of 20 mg/kg bw to each of five male volunteers resulted in a two- to fourfold increase in urinary indole-3-acetic acid concentration (Tashian, 1960). Presumably, the phenylacetic acid deactivated tryptophan decarboxylase, resulting in increased conversion of tryptophan to indole-3-acetic acid.
Incubation of a suspension of 0.1 ml or 0.1 g/100 ml of phenylacetic acid with human plasma caused prolonged clotting and increased thrombin activity (Nour-Eldin, 1968).
No changes in total nitrogen or ammonia content were seen in the urine of a rabbit fed a diet containing 0.5 g on the first day and 1 g on the next. An increase in amino acid and an insignificant decrease in urea output were noted in urine. In two other rabbits, one in nitrogen balance and one fasted, fed a dose of 1.2 g or 1.0 g, respectively, for 2 days by stomach tube, increased output of amino acids, total nitrogen, urea and ammonia was observed (Hijikata, 1922).
In a study of the effects of various phenyl-substituted acids on the activity of glycosyl transferase (used in cerebral glycoprotein synthesis) in human cerebrospinal fluid, a 1-h incubation with phenylacetic acid (2–10 mmol/l) produced up to 91% inhibition (Ko et al., 1973).
Incubation of 0.25% (2500 µg/ml) phenethyl alcohol with Escherichia coli strains DG75 and H500T– resulted in reversible inhibition of DNA synthesis and cell division, as measured by labelled thymine uptake and cell counts, respectively. The cells remained viable and RNA and protein synthesis capable. Increased sedimentation seen during treatment was interpreted as due to either increased formation of nucleoid bodies containing the replicating mechanism of the cell or increased cell lysis due to the detergent action of phenethyl alcohol (Brunner & Treick, 1982).
Numerous studies have been conducted to evaluate the effect of phenethyl alcohol on excision repair of DNA damage induced by ultraviolet radiation (UV) in non-standard E. coli strains. When solutions (0.2 and 0.4%) of phenethyl alcohol were incubated with UV-damaged E. coli strains H/r 30 and B (wild-type), excision repair of membrane-associated DNA was inhibited, as measured by a decrease in liquid holding recovery and a decrease in the removal of thymine dimers. The authors hypothesized that excision repair may require that DNA is bound to the cell membrane, and the test article may have inhibited that binding (Tachibana & Yonei, 1985). Similar results were obtained in an earlier study, in which it was shown that 0.05–0.5% solutions of phenethyl alcohol caused detachment of DNA from the DNA–membrane complex in E. coli B cells. The effect was concentration-dependent. Similar results were obtained with E. coli NG30 (rec A mutant) (Tachibana et al., 1982). In a related study, phenethyl alcohol inhibited the removal of thymine dimers from DNA in what was considered to be the incision step of excision repair in E. coli (Tomiyama et al., 1986). Phenethyl alcohol thus inhibited excision repair of UV-damaged DNA in E. coli (Yonei, 1980; Todo & Yonei, 1983; Tachibana & Yonei, 1985).
Wingard et al. (1955) classified phenylacetaldehyde (No. 1002) as a depressor on the basis of its effects on the blood pressure of dogs given doses of 3.4–40 mg/kg bw; they typically observed an initial large, rapid fall in blood pressure followed by a smaller, longer rise. Phenylacetaldehyde (No. 1002) was reported to have decreased blood pressure by 8 mm for 40 s in one dog (Romano et al., 1954).
Phenylacetaldehyde inhibited both Na+/K+-activated ATPase and Mg2+-ATPase. It also inhibited K+-dependent para-nitrophenylphosphatase activity. The Na+/K+ ATPase was much more sensitive than Mg2+ ATPase or K+-activated phosphatase to inhibition by aldehydes. The inhibition of Na+/K+ ATPase by aldehyde was reversible and was not competitive with ATP or K+ as the variable substrate or activator, respectively. Addition of cysteine or mercaptoethanol protected the enzymes from inhibition by aldehydes. All aldehydes, including acetaldehyde, were more potent inhibitors of Na+/K+ ATPase activity than was ethanol (Erwin et al., 1975). Glutathione peroxidase was inactivated by phenylacetaldehyde in vitro (Tabatabaie & Floyd, 1996).
2.3.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for 26 of the 39 phenethyl alcohol derivatives and are summarized in Table 4. The LD50 values in rats ranged from 1500 mg/kg bw for phenethyl alcohol to > 15 000 mg/kg bw for phenethyl phenylacetate, showing that their oral acute toxicity is low. In mice, the oral LD50 values ranged from 800 mg/kg for phenylacetic acid to > 15 000 mg/kg bw for linalyl phenylacetate. The oral LD50 values in guinea-pigs ranged from 400 mg/kg bw for phenylacetic acid to 3900 mg/kg for phenethylacetaldehyde.
Table 4. Studies of the acute toxicity of phenethyl alcohol, aldehyde, acid, and related acetals and esters used as flavouring agents
|
No. |
Agent |
Species |
Sex1 |
Route |
LD50
(mg/kg bw) |
Reference |
|
987 |
Phenethyl alcohol |
Mouse |
NR |
Oral |
800 |
Fassett (1963) |
|
987 |
Phenethyl alcohol |
Guinea-pig |
NR |
Oral |
400 |
Fassett (1963) |
|
987 |
Phenethyl alcohol |
Rat |
NR |
Oral |
1800 |
Rumyantsev et al. (1987) |
|
987 |
Phenethyl alcohol |
Rat |
NR |
Oral |
1500 |
Moreno (1982) |
|
987 |
Phenethyl alcohol |
Rat |
M,F |
Gavage |
1800 |
Jenner et al. (1964) |
|
987 |
Phenethyl alcohol |
Rat |
M,F |
Gavage |
2500 |
Zaitsev & Rakhamanina (1974) |
|
987 |
Phenethyl alcohol |
Mouse |
M,F |
Gavage |
2500 |
Zaitsev & Rakhamanina (1974) |
|
987 |
Phenethyl alcohol |
Guinea-pig |
M,F |
Gavage |
2500 |
Zaitsev & Rakhamanina (1974) |
|
987 |
Phenethyl alcohol |
Rat |
M |
Gavage |
1700 |
Mallory et al. (1982) |
|
988 |
Phenethyl formate |
Rat |
M,F |
Gavage |
3.2 ml/kg |
Levenstein (1973) |
|
989 |
Phenethyl acetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1973) |
|
989 |
Phenethyl acetate |
Rat |
NR |
Oral |
5200 |
Rumyantsev et al. (1987) |
|
989 |
Phenethyl acetate |
Rat |
M,F |
Gavage |
3700 |
Zaitsev & Rakhamanina (1974) |
|
989 |
Phenethyl acetate |
Mouse |
M,F |
Gavage |
3700 |
Zaitsev & Rakhamanina (1974) |
|
989 |
Phenethyl acetate |
Guinea-pig |
M,F |
Gavage |
3700 |
Zaitsev & Rakhamanina (1974) |
|
990 |
Phenethyl propionate |
Rat |
M,F |
Gavage |
4000 |
Beroza et al. (1975) |
|
990 |
Phenethyl propionate |
Rat |
NR |
Oral |
4000 |
Moreno (1973) |
|
991 |
Phenethyl butyrate |
Rat |
NR |
Oral |
4.6 ml/kg |
Levenstein (1974) |
|
992 |
Phenethyl isobutyrate |
Rat |
M,F |
Gavage |
> 5000 |
Shelanski & Moldovan (1971) |
|
993 |
Phenethyl 2-methylbutyrate |
Rat |
M |
Oral |
> 5000 |
Moreno (1982) |
|
997 |
Phenethyl tiglate |
Rat |
NR |
Oral |
> 5000 |
Levenstein (1974) |
|
999 |
Phenethyl phenylacetate |
Rat |
M.F |
Gavage |
15 000 |
Jenner et al. (1964) |
|
999 |
Phenethyl phenylacetate |
Rat |
M,F |
Gavage |
3200 |
Zaitsev & Rakhamanina (1974) |
|
999 |
Phenethyl phenylacetate |
Mouse |
M,F |
Gavage |
3200 |
Zaitsev & Rakhamanina (1974) |
|
999 |
Phenethyl phenylacetate |
Guinea-pig |
NR |
Gavage |
3200 |
Zaitsev & Rakhamanina (1974) |
|
1001 |
Acetaldehyde phenethyl propyl acetal |
Rat |
NR |
Oral |
> 5000 |
Moreno (1979) |
|
1002 |
Phenylacetaldehyde |
Rat |
NR |
Oral |
1600 |
Moreno (1977) |
|
1002 |
Phenylacetaldehyde |
Rat |
M,F |
Gavage |
3900 |
Zaitsev & Rakhamanina (1974) |
|
1002 |
Phenylacetaldehyde |
Mouse |
M,F |
Gavage |
3900 |
Zaitsev & Rakhamanina (1974) |
|
1002 |
Phenylacetaldehyde |
Guinea-pig |
NR |
Gavage |
3900 |
Zaitsev & Rakhamanina (1974) |
|
1003 |
Phenylacetaldehyde dimethyl acetal |
Rat |
M,F |
Gavage |
< 5000 |
Shelanski & Moldovan (1971) |
|
1004 |
Phenylacetaldehyde glyceryl acetal |
Rat |
M |
Oral |
1.7 ml/kg |
Moreno (1972) |
|
1004 |
Phenylacetaldehyde glyceryl acetal |
Rat |
M |
Oral |
< 5000 |
Moreno (1972) |
|
1007 |
Phenylacetic acid |
Rat |
NR |
Oral |
> 5000 |
Keating (1972) |
|
1007 |
Phenylacetic acid |
Rat |
M,F |
Gavage |
2200 |
Zaitsev & Rakhamanina (1974) |
|
1007 |
Phenylacetic acid |
Mouse |
M,F |
Gavage |
2200 |
Zaitsev & Rakhamanina (1974) |
|
1007 |
Phenylacetic acid |
Guinea-pig |
NR |
Gavage |
2250 |
Zaitsev & Rakhamanina (1974) |
|
1008 |
Methyl phenylacetate |
Rat |
NR |
Oral |
2600 |
Moreno (1974) |
|
1009 |
Ethyl phenylacetate |
Rat |
NR |
Oral |
3300 |
Moreno (1973) |
|
1012 |
Butyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1980) |
|
1013 |
Isobutyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1973) |
|
1014 |
Isoamyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1976) |
|
1016 |
3-Hexenyl phenyl acetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1976) |
|
1018 |
Rhodinyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1977) |
|
1019 |
Linalyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1974) |
|
1019 |
Linalyl phenylacetate |
Mouse |
M,F |
Oral |
15 000 |
Colaianni (1967) |
|
1020 |
Geranyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Russell (1973) |
|
1021 |
Citronellyl phenylacetate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1977) |
|
1023 |
para-Tolylacetaldehyde |
Mouse |
NR |
Oral |
> 5000 |
Levenstein (1975) |
|
1024 |
para-Isopropylphenyl acetaldehyde |
Rat |
NR |
Oral |
4100 |
Moreno (1977) |
|
1026 |
Phenoxyacetic acid |
Rat |
M,F |
Gavage |
1800 |
Burdock & Ford (1990) |
|
1026 |
Phenoxyacetic acid |
Rat |
M,F |
Gavage |
1800 |
Piccirillo et al. (1983) |
|
1026 |
Phenoxyacetic acid |
Rat |
NR |
Oral |
> 5000 |
Moreno (1976) |
|
1028 |
Phenoxyethyl iso butyrate |
Rat |
NR |
Oral |
> 5000 |
Moreno (1973) |
|
1029 |
Sodium 2-(4-methoxyphenoxy) propanoate |
Rat |
M,F |
Oral |
> 5000 |
Cummins (1985) |
M, male; F, female; NR, not reported
Oral LD50 values have been reported for three of the four phenoxyethyl alcohol derivatives (Table 4). In rats, the values ranged from 1500 mg/kg bw for phenoxyacetic acid to > 5000 mg/kg bw for phenoxyacetic acid, 2-phenoxyethyl isobutyrate and sodium 2-(4-methoxyphenoxy)propanoate. These results demonstrate that the acute toxicity of phenoxyacetic acid and related substances is low in rats.
(b) Short-term studies of toxicity
The results of short-term studies are summarized in Table 5 and described below. These studies show that phenethyl and phenoxyethyl alcohol derivatives have little toxic potential.
Table 5. Results of short-term and long-term studies of toxicity and carcinogenicity on phenethyl alcohol, aldehyde, acid and related acetals and estersused as flavouring agents
|
No. |
Substance |
Species; sex |
No. test groupsa/ no. per groupb |
Route |
Duration |
NOEL
(mg/kg bw per day) |
Reference |
|
987 |
Phenethyl alcohol |
Rat; M,F |
1/40 |
Oral |
56 weeks |
120d |
Johannsen & Purchase (1969) |
|
998 |
Phenethyl senecioate |
Rat; M,F |
1/24–28 |
Oral |
90 days |
< 1.5 (M) |
Rabinowitz (1969) |
|
< 1.8 (F) |
|
998 |
Phenethyl senecioate |
Rat; M,F |
1/20–32 |
Oral |
90 days |
1.5 (M)c |
Posternak et al. (1969) |
|
1.8 (F)c |
|
999 |
Phenethyl phenylacetate |
Rat; M,F |
3/20 |
Oral |
17 weeks |
500c |
Hagan et al. (1967) |
|
1024 |
para-Isopropyl-phenylacetaldehyde |
Rat; M,F |
1/20–32 |
Oral |
90 days |
17 (M)c |
Posternak et al. (1969) |
|
19 (F)c |
|
1027 |
Ethyl (para-tolyloxy) acetate |
Rat; M,F |
2/20–32 |
Oral |
90 days |
15 (M) |
Posternak et al. (1969) |
|
16 (F) |
|
1029 |
Sodium 2-(4-methoxyphenoxy) propanoate |
Rat; M,F |
5/20 |
Oral |
14 days |
200 |
Hill & Wood (1985) |
|
1029 |
Sodium 2-(4- methoxyphenoxy) propanoate |
Rat; M,F |
4/20 |
Oral |
90 days |
250 |
Hill & Wood (1986) |
|
M, male; F, female; NR, not reported |
|
a |
Total number of test groups does not include control animals. |
|
b |
Total number per test group includes both male and female animals. |
|
c |
Study performed at either a single or multiple doses that had no adverse effect. Therefore, this dose is not a true NOEL but is the highest dose tested that had no adverse effect. The actual NOEL would be higher. |
(i) Phenethyl alcohol (No. 987)
Groups of 20 male and 20 female Wistar albino rats were given a mixture of compounds dissolved in tap water as their only drinking-water for 56 weeks. The mixture comprised ethyl alcohol at 6000 mg/kg bw (6%), ethyl acetate at 4 mg/kg bw (0.004%), isoamyl alcohol at 120 mg/kg bw (0.12%), phenethyl alcohol at 120 mg/kg bw (0.12%), isobutyl alcohol at 200 mg/kg bw (0.2%) and acetic acid at 200 mg/kg bw (0.2%). A control group of 20 rats of each sex was maintained on tap water only. All groups of rats were fed a stock ration ad libitum, and their weights were recorded weekly. The activities of alcohol dehydrogenase and alanine and aspartate aminotransferases and the protein content of the liver were determined at 2–4-week intervals. At study termination, the liver, kidneys, heart, spleen and lungs were examined histologically. There was a slight, statistically nonsignificant decrease in the mean body weight of both groups at 28–29 weeks compared with that at 53–56 weeks. The absolute and relative liver weights were similar in test and control groups. A slight increase in aspartate aminotransferase activity was observed between 28 and 56 weeks in both test groups. No significant abnormalities were observed in any of the organs examined. Six animals contracted pneumonia and were discarded, and pneumonia was common in rats in all groups at termination. The authors concluded that the mixture of chemicals tested had no effects on the parameters tested (Johannsen & Purchase, 1969).
(ii) Phenethyl seneciote (No. 998), isopropylphenylacetaldehyde (No. 1024) and ethyl (para-tolyloxy)acetate (No. 1027)
Groups of 10–16 male and female Charles River CD rats were given diets adjusted during the study to provide constant concentrations of 1.5 mg/kg bw per day for males and 1.8 mg/kg bw per day for females of phenethyl senecioate, 15 mg/kg bw per day for males and 16 mg/kg bw per day for females of ethyl (para-tolyloxy) acetate or 17 mg/kg bw per day for males and 19 mg/kg bw per day for females of isopropylphenylacetaldehyde. The animals were housed in pairs of the same sex and given access to water and food ad libitum. The doses were chosen by methods described by Oser & Hall (1977). Clinical observations were recorded daily, and food consumption and body weights were determined weekly. During weeks 7 and 13, haematological and clinical chemical (blood urea) parameters were measured. After 90 days, all the animals were killed and subjected to detailed necropsy, and the liver and kidneys were weighed. A wide range of tissues and organs from each animal was preserved, and major organs and tissues were examined histologically.
No significant difference in body-weight gain was found between test and control animals. The kidneys of male rats given phenethyl senecioate (No. 998) were slightly enlarged, but the slight increase in relative weight became less marked when the values were compared with those for control groups in the same laboratory. The authors concluded that the changes in kidney weight were not toxicologically significant. No effects were observed in either male or female rats given isopropylphenylacetaldehyde (No. 1024) or ethyl (para-tolyloxy) acetate (No. 1027) (Posternak et al., 1969).
(iii) Phenethyl seneciote (No. 998)
Groups of 24 male and 28 female albino Charles River rats were fed a diet containing phenethyl seneociate in a gum arabic solution, at a concentration of 13 ppm for weeks 1–4, 22 ppm for weeks 5–10 and 26 ppm for weeks 11–13, corresponding to average concentrations ingested during the 13 weeks of the study of 1.5 (range, 1.1–1.9) mg/kg bw per day for males and 1.8 (range, 1.3–2.2) mg/kg bw per day for females. Losses in both body weight and food efficiency were noted in treated groups (p < 0.1), and the weight of the kidneys was significantly increased. At week 7 only, the per cent haemoglobin was significantly increased, accompanied by a significant increase in mean corpuscular haemoglobin concentration. Histological examination revealed the presence of renal lesions in both males and females, which were less severe in females (Rabinowitz, 1969).
In a study reported only as a memo, groups of 10 male and 10 female Osborne-Mendel rats were maintained on diets containing 0, 20, 1000, 2500 or 10 000 ppm of phenethyl senecioate, calculated to provide an average daily intake of 0, 1, 50, 125 or 500 mg/kg bw, for 4 months. Slight degenerative changes were seen in the kidneys of saome animals, which were not unexpected, as Osborne-Mendel rats of this age commonly show all gradations of chronic interstitial nephritis. Gross and histological examination of a wide variety of tissues revealed no alterations that could be related to administration of the test material (Bierbower, 1970).
(iv) Phenethyl phenylacetate (No. 999)
Groups of 10 male and 10 female Osborne-Mendel rats were given diets containing phenethyl phenylacetate at a concentration of 0, 1000, 2500 or 10 000 ppm, corresponding to an average daily intake of 0, 50, 250 or 500 mg/kg bw, for 17 weeks. Weekly measurement of body weight and food intake showed no significant difference between test and control animals. At termination, haematological examination revealed no effects due to treatment. At necropsy, no difference between test and control animals in the weights of major organs was reported. Gross examination of tissues from all animals revealed no remarkable changes, and histological examination of three to four animals of each sex at the high dose and from the control group revealed no treatment-related lesions (Hagan et al., 1967).
(v) Sodium 2-(4-methoxyphenoxy) propanoate (No.1029)
Groups of 10 male and 10 female Sprague-Dawley-derived CD rats were maintained on diets containing sodium 2-(4-methoxyphenoxy)propanoate at a concentration of 0, 4000, 12 000, 25 000 or 50 000 ppm, calculated to provide an estimated daily intake of 0, 200, 600, 1250 or 2500 mg/kg bw, for 14 days. Reduced body-weight gain associated with reduced food consumption was found at the two higher doses, and water consumption was increased in animals of each sex at these doses. Increased activity of plasma alkaline phosphatase and aspartate aminotransferase was reported in half the animals at the highest dose, but the increases in females at the two higher doses were within the normal range. Male and female rats at 2500 mg/kg bw per day had lowered blood reticulocyte counts but no other haematological changes associated with treatment. No histopathological changes were found that were associated with treatment. The authors concluded that a dietary dose of 1000–1250 mg/kg bw per day would be suitable for a subsequent 90-day study (Hill & Wood, 1985).
In the 90-day study, groups of 20 male and 20 female CD rats received sodium 2-(4-methoxyphenoxy)propanoate in the diet at a concentration of 0, 5000, 10 000 or 20 000 ppm, corresponding to 0, 250, 500 or 1000 mg/kg bw per day. Body-weight gain was reduced in males (17%) and females (20%) at the highest dose, and males at 500 mg/kg bw per day showed a 7% reduction in body-weight gain. The authors did not consider these figures to be statistically significant. Haematological examination and blood chemistry revealed normal values. No treatment-related changes were found in urine cellularity, volume, specific gravity, pH or protein, bilirubin or ketone content or in blood glucose or sodium concentration, however, a reduction in urine potassium concentration was seen in males at the highest dose during weeks 6 and 13 and in females at 500 and 1000 mg/kg bw per day during week 13 of the treatment period. No microscopic changes were found that could be related to administration of the test substance (Hill & Wood, 1986).
(c) Genotoxicity
Tests for genotoxicity have been performed on seven representative phenethyl alcohol derivatives and three phenoxyethyl alcohol derivatives. The results are summarized in Table 6 and described below.
Table 6. Studies of genotoxicity with phenethyl alcohol, aldehyde, acid and related acetals and esters used as flavouring agents
|
No. |
Agent |
End-point |
Test object |
Maximum concentration |
Result |
Reference |
|
In vitro |
|
987 |
Phenethyl alcohol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 mmol/plate |
Negativea |
Florin et al. (1980) |
|
987 |
Phenethyl alcohol exchange |
Sister chromatid |
Human lymphocytes |
Not specified |
Negative |
Norppa & Vaino (1983) |
|
1002 |
Phenylacetaldehyde |
Reverse mutation |
S. typhimurium TA98, TA100, TA104 |
Not specified |
Negativea |
Kato et al. (1989) |
|
1002 |
Phenylacetaldehyde |
Mutation |
E. coli WP2uvrA/pkM101 |
Not specified |
Negativea |
Kato et al. (1989) |
|
1007 |
Phenylacetic acid |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
1000 mg |
Negativea |
Heck et al. (1989) |
|
1007 |
Phenylacetic acid synthesis |
Unscheduled DNA |
Rat hepatocytes |
500 mg |
Negative |
Heck et al. (1989) |
|
1007 |
Phenylacetic acid |
Mutation |
Mouse lymphoma L5178Y Tk+/– cells |
1500 mg |
Negativea |
Heck et al. (1989) |
|
1009 |
Ethyl phenylacetate |
Mutation |
B. subtilis H17 (rec+) and M45 (rec–) |
21 mg/disc |
Negative |
Oda et al. (1979) |
|
1009 |
Ethyl phenylacetate |
Mutation |
B. subtilis H17 (rec+) and M45 (rec–) |
20 ml/disc |
Positiveb |
Yoo (1986) |
|
1009 |
Ethyl phenylacetate |
Reverse mutation |
S. typhimurium TA92, TA94, TA98, TA100, TA1535, TA1537 |
5 mg |
Negativea |
Ishidate et al. (1984) |
|
1009 |
Ethyl phenylacetate |
Chromosomal abberation |
Chinese hamster fibroblast cells |
1 mg/ml |
Negativea |
Ishidate et al. (1984) |
|
1009 |
Ethyl phenylacetate |
Mutation |
E. coli WP2uvrA (trp–) |
200–1600 µg/plate |
Negativeb |
Yoo (1986) |
|
1013 |
Isobutyl phenylacetate |
Reverse mutation |
S. typhimurium TA97, TA102 |
0–0.1 mg/plate |
Negativea |
Fujita et al. (1994) |
|
1014 |
Isoamyl phenylacetate |
Mutation |
B. subtilis H17 (rec+) and M45 (rec–) |
20 mg/disc |
Positiveb |
Oda et al. (1979) |
|
1014 |
Isoamyl phenylacetate |
Mutation |
B. subtilis H17 (rec+) and M45 (rec–) |
20 ml/disc |
Negativeb |
Yoo (1986) |
|
1014 |
Isoamyl phenylacetate |
Reverse mutation |
S. typhimurium TA98, TA100 |
10 mg/plate |
Negativea |
Oda et al. (1979) |
|
50 mg/plate |
Lethala,b |
|
1023 |
para-Tolylacetaldehyde |
Reverse mutation |
S. typhimurium TA100 |
0.1–1000 µg/plate |
Negative |
Rapson et al. (1980) |
|
1023 |
para-Tolylacetaldehyde |
Mutation |
E. coli PQ37 |
Not specified |
Negative |
Ohshima et al. (1989) |
|
1027 |
Ethyl (para-tolyloxy) acetate |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA100, TA98 |
3600 µg/plate |
Negativea |
Wild et al. (1983) |
|
1028 |
2-Phenoxyethyl isobutyrate |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA100, TA98 |
3600 µg/plate |
Negativea |
Wild et al. (1983) |
|
1029 |
Sodium 2-(4-methoxyphenoxy) propanoate |
Reverse mutation |
S. typhimurium TA1535, TA98, TA100, TA1537 |
5000 µg/plate |
Negativea |
Varley & Trenchard-Morgan (1985) |
|
In vivo |
|
1028 |
2-Phenoxyethyl propanoate |
Micronucleus formation |
Mouse bone marrow cells |
1900 mg/kg/bw |
Negativeb |
Wild et al. (1983) |
|
1029 |
Sodium 2-(4-methoxyphenoxy) isobutyrate |
Micronucleus formation |
Mouse bone marrow cells |
2000 mg/kg/bw |
Negative |
Asquith & Pickering (1985) |
| |
Isoeugenol phenylacetaldehyde |
Micronucleus formation |
Mouse bone marrow cells |
2800 mg/kg bw |
Negativeb |
Wild et al. (1983) |
| |
Isoeugenol phenylacetaldehyde |
Sex-linked recessive lethal mutation |
D. melanogaster |
25 mmol/l |
Negative |
Wild et al. (1983) |
a With and without metabolic activation
b Administered intraperitoneally
(i) In vitro
Phenethyl alcohol (No. 987), phenylacetaldehyde (No. 1002), phenylacetic acid (No. 1007), ethyl phenylacetate (No. 1009), isobutyl phenylacetate (No. 1013), isoamyl phenylacetate (No. 1014) and para-tolylacetaldehyde (No. 1023) have been tested for their ability to induce reverse mutation in various strains of Salmonella typhimurium (e.g., TA98, TA100, TA1535, TA1537 and TA1538) in the presence or absence of an exogenous metabolic activation system. None of the compounds was mutagenic when tested at concentrations up to 5000 µg/ml or 50 mg/plate (Oda et al., 1979; Florin et al., 1980; Rapson et al., 1980; Ishidate et al., 1984; Heck et al., 1989; Kato et al., 1989; Fujita et al., 1994). No reverse mutation was seen when various strains of S. typhimurium (TA98, TA100, TA1535, TA1537 and TA1538) were incubated with ethyl (para-tolyloxy)acetate (No. 1027) at up to 3600 µg per plate (Wild et al., 1983), 2-phenoxyethyl isobutyrate (No. 1028) at 3600 µg per plate (Wild et al., 1983) or sodium 2-(4-methoxyphenoxy)propanoate (No. 1029) at up to 5000 µg per plate (Varley & Trenchard-Morgan, 1985), with or without metabolic activation.
Tests of the ability of ethyl phenylacetate (No. 1009) and isoamyl phenylacetate (No. 1014) to induce mutation in Bacillus subtilis H17 and M45 were inconclusive. In a study in which ethyl phenylacetate was incubated with B. subtilis H17 and M45 at 21 µg per disc, the difference in the zone of inhibition (0.8 mm) between the two strains indicated that it was not active (Oda et al., 1979). In a study with a lower concentration, ethyl phenylacetate was incubated at a concentration of 20 µl per disc with B. subtilis H17 and M45 in the same assay. The difference in the zone of inhibition (> 8 mm) between the two strains was considered to provide evidence of mutagenicity (Yoo, 1986). Contradictory data have also been reported with isoamyl phenylacetate (No. 1014). When 20 µg per disc were incubated with B. subtilis H17 and M45, a weak (2–5 mm difference) positive response was reported by Oda et al. (1979), while Yoo (1986) reported a negative response with 20 µl per disc.
Phenylacetaldehyde (No. 1002) was tested in E. coli strain WP2uvrA/pKM101 with preincubation (Kato et al., 1989), and para-tolylacetaldehyde (No. 1023) was studied in E. coli strain PQ37 (Ohshima et al., 1989), both at unspecified concentrations. There was no evidence of mutagenicity in either assay. In another assay, 200–1600 µg per plate of ethyl phenylacetate showed no evidence of mutagenicity when incubated with E. coli WP2uvrA (Yoo, 1986). The contradictory results reported by Yoo (1986) and Oda et al. (1979), the negative result in the WP2 uvrA strain and the fact that phenethyl alcohol is bactericidal in E. coli (Treick & Konetzka, 1964; Brunner & Treick, 1982) support the conclusion that the results with B. subtilis H17 and M45 should not be used in the overall assessment of the genotoxic potential of these substances.
No increase in sister chromatid exchange frequency was observed when human whole blood lymphocyte cultures were exposed to 2-phenethyl alcohol (No. 987) for 72 h (Norppa & Vainio, 1983). Also, no increase in unscheduled DNA synthesis was noted when rat hepatocytes were incubated with phenylacetic acid (Heck et al., 1989). Incubation of ethyl phenylacetate at 1000 µg/ml with Chinese hamster fibroblasts for 48 h caused chromosomal aberrations in 3% of cells. On the basis of a threshold of positivity of > 10%, ethyl phenylacetate gave negative results in this assay (Ishidate et al., 1984).
(ii) In vivo
The results of tests for genotoxicity in vivo with phenylacetate ester and 2-phenoxyethyl isobutyrate (No. 1028) and sodium 2-(4-methoxyphenoxy)propanoate (No. 1029) were negative. No significant increase in the number of micronucleated polychromatic erythrocytes was seen in mice given intraperitoneal injections of 2-phenoxyethyl isobutyrate at 620–1900 mg/kg bw (Wild et al., 1983). In another test, sodium 2-(4-methoxyphenoxy) propanoate was given to mice by gavage at doses of 500–2000 mg/kg bw. An increased frequency of micronucleated polychromatic erythrocytes was found in males at 500 mg/kg bw at 24 h (Asquith & Pickering, 1985).
A phenyl acetate ester that was not included in this group of substances, isoeugenol phenylacetate, was also tested for its ability to induce micronucleus formation. Groups of male and female NMRI mice were given the compound at doses of 1100–2800 mg/kg bw by intraperitoneal injection. After 30 h, they were killed, and the mean number of micronucleated polychromatic erythrocytes per 1000 normochromatic erythrocytes was calculated. No effect was seen at any dose. Furthermore, the frequency of sex-linked lethal mutations was not increased when Drosophila melanogaster were fed a solution of isoeugenol phenylacetate at 25 mmol/l for 3 days (Wild et al., 1983).
(d) Reproductive toxicity
(i) Multigeneration study
Male and female CD-1 mice received diets containing a hydrolysis product of the phenoxyethyl alcohol derivatives in the group, phenoxyethyl alcohol, at a concentration of 0.25, 1.2 or 2.5% for 18 weeks, corresponding to doses of 380, 1900 and 3700 mg/kg bw per day. No effect was seen on the number of pairs capable of producing at least one litter or on the sex of pups born alive. Reductions in litter size and the number of live pups per litter were reported at the highest dose when compared with the control group and the two lower doses, and there was a dose-related decrease in live pup weight at the two higher doses. The weights of live male pups (F1) were decreased, corresponding to a decrease in food consumption through week 18 among F0 pairs at the lower dose. Necropsy of control and high-dose F0 mice revealed decreased body weights (6% less) and increased absolute liver weights (14% more) in treated males and increased absolute liver weights among treated females. No effects were reported on the weights of other organs or on sperm indices.
In the second part of this study, phenoxyethyl alcohol was administered after weaning to the last F1 litter at the same dose provided to their F0 parents. A dose-related decrease in body weight was seen in the F1 generation at the two higher doses, from birth to post natal day 74. Increased mortality was reported at the two higher doses from weaning until postnatal day 74, with marked effects at the highest dose, only 6/56 pups surviving until mating. Consequently, this group was terminated. After mating of the F1 generation, no treatment-related effects were seen on F2 litter size or sex ratio. The weights of live F2 pups at the intermediate dose were decreased by 7%. Necropsy of these mice and of the controls revealed decreased body weight (13% less), decreased absolute testis weight (16% less), decreased relative seminal vesicle weight (14% less) in the male mice and decreased body weight (7%) in the female mice in comparison with the controls. No effects were reported on epididymal sperm concentration, motility or morphology. The decrease in body weight reported for live male F1 pups born to the pairs given the low dose (F0) was barely statistically significant, and its biological relevance is questionable as it was observed in male pups only. The NOEL for reproductive effects was 0.25%, corresponding to 380 mg/kg bw per day (National Toxicology Program, 1984).
(ii) Developmental toxicity
In screening studies, low doses of phenethyl alcohol (No. 987) and phenylacetic acid (No. 1007) were reported to have teratogenic effects resembling fetal alcohol syndrome (Mankes et al., 1983, 1984, 1985). These results were contradicted by those of a study in which phenethyl alcohol given to pregnant rats at high doses at critical periods of embryogenesis did not cause any visible anomalies in embryonal development (Burdock et al., 1987). More recent, comprehensive studies with high doses of phenethyl alcohol given orally (Bottomley et al., 1987) or dermally (Palmer et al., 1986) indicated that this group of substances has little reproductive or developmental toxicity.
Phenethyl alcohol (No. 987) and phenylacetic acid (No. 1007)
Long-Evans rats were given phenethyl alcohol by gavage at a dose of 4.3, 43 or 430 mg/kg bw per day on days 6–15 of gestation. The average birth weight and pup size in all treated groups were significantly lower than those of the control group, but the changes were not dose-related, and in fact the birth weights were greater in the group at the intermediate dose than in controls. The mean litter size was greater in the high-dose group (13) than at the two lower doses (9) or in controls (12). No embryolethality was seen at the high dose, while the rates were 18% at 43 mg/kg bw per day and 10% at the lowest dose. The authors reported a clear dose-related increase in the percentage of malformations in live offspring (100% at the highest dose, 93% at the intermediate and 50% at the lowest dose). The malformations were mainly ocular changes, neural tube defects, hydronephrosis and limb defects (Mankes et al., 1983). In abstracts of subsequent studies reported by the same authors (Mankes et al., 1984, 1985), doses of phenethyl alcohol equivalent to 0.02% and 24% of the oral LD50 were administered to pregnant Long-Evans rats. Intrauterine growth retardation (birth weight reduction) and embryolethality were reported at all doses. These observations are inconsistent with those of the original study.
No teratogenic effects were reported in rats fed diets containing phenethyl alcohol at concentrations exceeding the normal human intake by > 8000 times. Microencapsulated phenethyl alcohol was administered in the diet to nulliparous Sprague-Dawley rats during the period of major organogenesis (days 6–15 post coitum) at a concentration of 1000, 3000 or 10 000 ppm, calculated to provide an average daily intake of 50, 150 or 500 mg/kg bw per day. The effects on the dams were limited to transient suppression of maternal food consumption, resulting in slight weight loss during the first 2 days of treatment with the high dose. The effects on the offspring were minimal, malformations being found in only five pups (three controls and two at the intermediate dose). The number and type of visceral anomalies were comparable in control and treated groups. An increased incidence of incomplete calcification was seen only in fetuses of dams at 500 mg/kg bw per day, and this was considered to be a possible consequence of the earlier impairment of maternal weight gain. There were no obvious differences between control and treated groups in skeletal variants, number of live young, embryolethality, number of implants, litter weight, mean fetal weight or sex ratio (Burdock et al., 1987).
Sprague-Dawley-derived rats received diets containing micro-encapsulated phenethyl alcohol at a nominal concentration of 0, 1000, 3000 or 10 000 ppm, equivalent to 0, 50, 150 and 500 mg/kg bw per day, on days 6–15 of gestation. Spray-dried gum arabic, the microencapsulant, was used as a placebo control and was also added with the lower concentrations so that the total dose remained constant for all groups at 5%. The animals were killed on day 20 post coitum, and development was assessed by determination of litter values and examination of fetuses for structural malformations or anomalies. The achieved intake of phenethyl alcohol by dams during the treatment period was calculated, and the values were adjusted to take account of the assayed content of test material in the microcapsules. The actual intake was thus 83, 270 and 800 mg/kg bw per day for the three groups, respectively. Treatment at the highest dose had a negligible detrimental effect on uterine development. Although there was clear evidence of impaired weight gain in dams after initial treatment, fetal development was virtually unaffected, the only possible exception being a marginal delay in ossification, which is usually considered to be transient and self-correcting during postnatal maturation. At the two lower doses, phenethyl alcohol did not elicit any overt response in the dams, and embryofetal development and morphology were unaffected (Bottomley et al., 1987).
Sprague-Dawley-derived rats received phenethyl alcohol topically at a dose of 0, 0.14, 0.43 or 1.4 ml/kg bw, corresponding to 140, 440 or 1400 mg/kg bw per day, on days 6–15 of gestation. These doses were chosen so that the intermediate dose was roughly equivalent to the highest dose used in a previous study of oral administration (Mankes et al., 1983) and designed to extend the range in case of differential absorption by the dermal route. An occlusive bandage was used to minimize oral ingestion of the test material, and this was put on immediately after dosing and not removed until the following day. The animals were killed on day 20 of gestation, and uterine development was assessed by determining litter values and examining fetuses for soft tissue and skeletal changes. The highest dose clearly induced both maternal toxicity, including lethality, suppression of mean food intake and growth rate, and embryo-fetal toxicity, indicated by resorption, wastage, reduction in mean litter size, depression of fetal weight, a wide range of soft tissue and skeletal changes and incomplete ossification. The pattern of incomplete ossification and the comprehensive nature of the morphological changes were considered by the authors to be more severe than those that would occur merely as a secondary consequence of the maternal response. Thus, the intermediate dose was considered close to the threshold for maternal toxicity. While there was no evidence of an adverse effect on litter values, dose-dependent increases were found in some morphological changes in fetuses. The lowest dose did not elicit any adverse effects in litter values. The slight increases over control values in morphological changes (cervical ribs, thoracic vertebral irregularities) at the lowest dose indicated that 140 mg/kg bw per day was a threshold for developmental toxicity in rats (Palmer et al., 1986).
Phenoxyacetic acid (No. 1026)
Phenoxyacetic acid was administered by gavage to mice on one of days 8–15 of gestation at a dose of 800–900 mg/kg bw or on three consecutive days (7–9, 10–12 or 13–15) at a dose of 250–300 mg/kg bw per day. Treated females were killed on day 18 of gestation, and their uteri were examined to determine the numbes of live, dead and resorbed fetuses. Live fetuses were examined for gross external malformations and weighed. Fetuses from each litter were further examined for visceral abnormalities and skeletal malformations. The authors stated that phenoxyacetic acid was not strongly teratogenic or fetotoxic (Hood et al., 1979).
Sodium 2-(4-methoxyphenoxy)propanoate (No. 1029)
Groups of five time-mated Charles River female rats were given suspensions of sodium 2-(4-methoxyphenoxy) propanoate at a single daily dose of 0, 100, 500, 1000 or 5000 mg/kg bw by gavage on days 6–15 of gestation, in order to determine suitable doses for a second study. The surviving animals were killed on day 20 of gestation and grossly necropsied. No adverse effects were observed at 100 or 500 mg/kg bw per day, and at 1000 and 5000 mg/kg bw per day, there were no treatment-related clinical signs of toxicity, mortality or abnormal findings at necropsy among dams, and embryonic and fetal development was not affected. Maternal body-weight gain and food consumption were adversely affected from the onset of dosing at 1000 mg/kg bw per day, and food consumption was significantly lower (p < 0.01, Student’s t test) than that of controls throughout dosing. These differences were considered to be treatment-related even though statistical significance was not achieved (probably because of the small group size). However, there were no deaths or adverse findings at necropsy. There was a trend towards slightly lower fetal body weight (not statistically significant), which may have been related to the reduced food consumption of the dams. Two animals at 5000 mg/kg bw per day were found dead on day 8 of gestation, having received two doses of sodium 2-(4-methoxyphenoxy) propanoate; the remaining three animals were reported to be in poor condition on day 7, after having received one dose, and were killed. The authors also reported brown stains on the fur and piloerection in most animals. Additionally, there were lesions of the gastric mucosa, which included inflammation, haemorrhage or ulceration in four animals. The authors concluded that 1000 mg/kg per day was a suitable highest dose for the main study. Although slight toxicity was observed at this dose, there were no maternal deaths (Ridgway, 1985).
The next study was designed to investigate the effects of sodium 2-(4-methoxyphenoxy) propanoate on embryonic and fetal development when administered during the period of organogenesis. Groups of 24 time-mated female CD rats were dosed once daily by gavage with suspensions of sodium 2-(4-methoxyphenoxy) propanoate at 0, 100, 300 or 1000 mg/kg per day on days 6–15 of gestation and were killed and necropsied on day 20 of gestation. The fetuses were subjected to detailed external, visceral and skeletal examinations. No maternal deaths occurred. Treatment-related clinical signs were reported only at the highest dose. A slight, transient retardation in maternal body-weight gain was seen at all doses at the start of treatment, with statistically significant differences of on days 6–7 at 100 and 300 mg/kg bw per day (p < 0.05) and on days 6–7 and 6–9 at 1000 mg/kg bw per day (p < 0.001). Maternal food consumption was not affected. There were no abnormal findings at necropsy of dams and no evidence of developmental toxicity. Implantation, post-implantation loss, fetal weight, sex ratio and the incidence of fetal abnormalities were not affected by treatment (Ridgway, 1986).
(e) Other studies
(i) Phenethyl alcohol (No. 987) and phenethyl acetate (No. 989)
Male rats were given phenethyl alcohol at a dose of 51 mg/kg bw per day or phenethyl acetate at 73 mg/kg bw per day by gavage for 4 months. On days 40 and 140, cholinesterase activity, the activity of serum enzymes such as aldolases, amylase, sorbitol dehydrogenase and aspartate and alanine aminotransferases, and the content of thiol groups and total serum protein were measured in serum. Treatment with phenethyl alcohol increased cholinesterase and alanine aminotransferase activities and increased the content of thiol groups in the blood at day 40. The serum protein content decreased to 7.2 g/100 ml after 40 days of treatment. The effects on thiol group content and cholinesterase activity persisted at 140 days. The only change reported was an increase in cholinesterase activity 140 days after the start of the experiment (Zaitsev & Rakhmanina, 1974).
(ii) Phenylacetic acid (No. 1007)
A rapid screening protocol was used to evaluate the potential immunotoxicity of flavouring ingredients, including phenylacetic acid. The protocol incorporated key elements of the National Toxicology Program’s tier testing strategy, including measurement of body weight, lymphoid organ weight and cellularity, as well as functional tests of the humoral (antibody plaque-forming cells) response to sheep erythrocytes and cell-mediated immunity to Listeria monocytogenes bacterial challenge. Decreases in body weight, spleen and/or thymus weight or a decrease in spleen cellularity may be indicative of depressed immune competence. The number of antibody-producing plasma cells, the result of antigen-driven B-cell differentiation, after immunization with a T-cell-dependent antigen such as sheep red blood cells, provides information about the functional integrity of, and communication among, several cell populations important in antibody-mediated immunity, including T cells, B cells and macrophages. The Listeria model system was selected because the pathogenesis of this infection and the host’s immune response are similar in mice and humans. The model system is useful for assessing immunosuppression since both immunocompetent T cells and macrophages are required to control infection and supply protective immunity. Phenylacetic acid administered orally to groups of 10–20 female CD-1 or B6C3F1 mice, aged 6–8 weeks, at doses as high as 100 mg/kg per day had no effect on spleen weight, thymus weight, spleen cellularity, anti-sheep red blood cell response or Listeria mortality (Vollmuth et al., 1989).
Phenylacetic acid was shown to be devoid of immunomodulatory effects in a testing strategy to evaluate the effects of 35 commonly used flavouring ingredients on humoral and cell-mediated immune responses. Female CD-1 or B6C3F1 mice were given phenylacetic acid at a dose of 250, 500 or 1000 mg/kg bw per day intragastrically for 5 days. L. monocytogenes bacterial challenge was conducted to assess cell-mediated immunity and the antibody response to sheep erythrocytes was determined as a measure of humoral immunity. Body weights, lymphoid organ weights and spleen cellularity were also measured. Cyclophosphamide served as a positive control. Phenylacetic acid did not modulate the cell-mediated or humoral response at any dose tested (Gaworski et al., 1994).
In a study of the effects of excess L-phenylalanine, L-tyrosine, L-valine and phenylacetic acid on serotonin in brain and liver, 5% or 7% phenylacetic acid in the diet for 1–3 weeks increased brain serotonin and decreased liver serotonin in rats (Boggs et al., 1963).
(iii) Sodium 2-(4-methoxyphenoxy) propanoate (No. 1029)
The effects of sodium 2-(4-methoxyphenoxy) propanoate and ibuprofen on prostaglandin E2 levels and leukocyte counts were studied in rats in which carrageenan-soaked sponges had been implanted subcutaneously. Oral administration of sodium 2-(4-methoxyphenoxy) propanoate at 3, 10, 30 or 100 mg/kg bw to male and female rats did not alter leukocyte migration into the sponges, and, in general, the test substance had no significant or dose-related effect on the prostaglandin E2 content of the sponges. However, there was a marked increase in the levels in male rats treated at 30 mg/kg bw and a significant reduction in females at 100 mg/kg bw. The reference standard, ibuprofen, at 100 mg/kg bw did not alter the leukocyte counts but significantly reduced the prostaglandin E2 content of the sponges in both male and female rats. The authors concluded that this reduction is consistent with the anti-inflammatory action of ibuprofen and that sodium 2-(4-methoxyphenoxy) propanoate could have a slight inhibitory effect on prostaglandin biosynthesis (Algate et al., 1986a).
In a study by the same authors, sodium 2-(4-methoxyphenoxy) propanoate at doses of 10, 30 and 100 mg/kg bw produced statistically significant inhibition of carrageenan-induced oedema, at both the 3-h (30 and 100 mg/kg bw) and 6-h (10, 30 and 100 mg/kg bw) observation times. Ibuprofen caused statistically significant inhibition of oedema at all three intervals, with peak activity recorded at the 6-h observation time. The authors concluded that sodium 2-(4-methoxyphenoxy) propanoate and the reference standard ibuprofen suppressed the inflammatory response induced by the irritant carrageenan; however, the activity of 2-(4-methoxyphenoxy) propanoate was reported to be minor (Algate et al., 1986b).
The effects of sodium 2-(4-methoxyphenoxy) propanoate (lactisole) on glucose tolerance and insulin secretion were investigated in eight healthy male volunteers. The men received either a glucose solution (75 g) or a glucose solution containing 80 mg of the test substance on two separate occasions, at least 7 days apart. Blood samples were collected at 30-min intervals for 3 h and then hourly until 5 h after the glucose load. Glucose, insulin, C-peptide and glucagon levels were assayed. Lactisole had no effect on glucose metabolism or insulin, C-peptide or glucagon secretion under the conditions of the study (Marks, 1988).
3. REFERENCES
Algate, D.R., Lawrence, D.J. & Atterson, P.R. (1986a) An investigation of the anti-inflammatory activity of ORP 178 against carrageenan-induced oedema in the rat. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Algate, D.R., Munt, P.L. & Green, T.D. (1986b) Effects of ORP 178 and ibuprofen on PGE2 levels and leucocyte counts in subcutaneously implanted sponges in the rat. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Ambrose, A.M., Power, F.W. & Sherwin, C.P. (1933) Further studies on the detoxication of phenylacetic acid. J. Biol. Chem., 101, 669–675.
Asquith, J.C. & Pickering, K.J. (1985) Mouse micronucleus test on ORP 178. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Augustinsson, K.B. & Ekedahl, G. (1962) On the specificity of arylesterases. Acta Chem. Scand., 16, 240–241.
Beroza, M., Inscoe, M.N., Schwartz, P.H., Keplinger, M.L. & Mastri, C.W. (1975) Acute toxicity studies with insect attractants. Toxicol. Appl. Pharmacol., 31, 421–429.
Bierbower, G.W. (1970) Memo to J. Taylor regarding a pathology report on Osborne-Mendel rats fed phenethyl senocioate in rations for four months. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Block, W. (1953) On the physiology of C(14)-radioactive mescaline in animals. Part IV. Comparative studies with C(14)-mescaline and C(14)-beta-phenylethylamine. Z. Naturforsch., 8B, 440–444.
Boggs, D.E., Rosenberg, R. & Waiman, H.A. (1963) Effects of phenylalanine, phenylacetic acid, tyrosine and valine on brain and liver serotonin in rats. Proc. Soc. Biol. Med., 114, 356–358.
Bosron, W.F. & Li, T.K. (1980) Alcohol dehydrogenase. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxication, Orlando, Florida: Academic Press, Vol. 1, pp. 231–248.
Bottomley, A.M., Ratcliffe, H.E., John, D.M., Anderson, A. & Dawe, I.S. (1987) Effect of dietary administration of micro-encapsulated phenylethyl alcohol on pregnancy of the rat (embryotoxicity study). Unpublished report to RIFM. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Bray, H.G., Neale, F.C. & Thorpe, W.V. (1946) The fate of certain organic acids and amines in the rabbit. Biochem. J., 40, 134–139.
Bray, H.G., James, S.P. & Thorpe, W.V. (1958) Metabolism of some omega-halogenoalkylbenzenes and related alcohols in the rabbit. Biochem. J., 70, 570–579.
Brown, P.M., Rhenius, S.T. & Ryder, J.R. (1986) ORP/178: Absorption, distribution, and excretion in rat. Final report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Brunner, D.P. & Treick, R.W. (1982) Effects of phenethyl alcohol treatment upon the folded chromosome of Escherichia coli. J. Gen. Appl. Microbiol., 28, 491–498.
Burdock, G.A. & Ford, R.A. (1990) Acute oral toxicity (LD50) study in the rat with phenoxyacetic acid. Acute toxicity data. J. Am. Coll. Toxicol. B, 1, 94–95.
Burdock, G.A., Ford, R.A., Bottomley, A.M. & John, D.M. (1987) An evaluation of the teratogenic potential of orally administered phenylethyl alcohol. Toxicologist, 7, 702.
Caldwell, J. (1987) Human disposition of [14C]-ORP/178. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Colaianni, L. (1967) Acute toxicity, eye and skin irritation test of fragrance materials. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol.,16, 255–276.
Cummins, H.A. (1985) Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Department of Agriculture (1965) Food intake and nutritive value of the diets of men, women, and children in the in the United States, Spring 1965, a preliminary report by the consumer and Food Economics Research Division, Agriculture Research Service, Washington DC.
Erwin, V.G., Kim, J. & Anderson, A.D. (1975) Effects of aldehydes on sodium plus potassium ion-stimulated adenosine triphosphatase of mouse brain. Biochem. Pharmacol., 24, 2089–2095.
Fassett, D.W. (1963) Toxicity of phenethyl alcohol. Ind. Hyg. Toxicol., Vol. ?, 1476–1477.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames test. Toxicology, 18, 219–232.
Fujita, H., Aoki, N. & Sasaki, M. (1994) Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102(IX). Tokyo-toritsu Eisei Kenkysho Kenkyu Nenpo, 45, 191–199.
Gaworski, C.L., Vollmuth, T.A., Dosier, M.M., Heck, J.D., Dunn, L.T., Ratajczak, H.V. & Thomas, P.T. (1994) An immunotoxicity assessment of food flavouring ingredients. Food Chem. Toxicol., 32, 409–415.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence of glycine conjugation on availability of glycine: Role of the glycine cleavage system. Xenobiotica, 23,141–153.
Grundschober, F. (1977) Toxicological assessment of flavouring esters. Toxicology, 8, 387–390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol., 5, 141–157.
Hawkins, D.R. & Mayo, B.C. (1986) Plasma kinetics of [14C]-ORP/178 in the rat. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. & Curren, R.D. (1989) An evaluation of food flavouring ingredients in a genetic toxicity screening battery. Toxicologist, 9, 257.
Hijikata, Y. (1922) The influence of putrefaction products on cellular metabolism. II. On the influence of phenylacetic and phenylproionic acids on the distribution of nitrogen in the urine. J. Biol. Chem., 51, 141–154.
Hill, R.E. (1986) Metabolism phase of 90 day toxicity study in the rat ORP-178. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Hill, R.E. & Wood, C.M. (1985) 14-day oral toxicity study in the rat ORP-178. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Hill, R.E. & Wood, C.M. (1986) 90-day oral toxicity study in the rat ORP-178. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Hood, R.D., Patterson, B.L., Thacker, G.T., Sloan, G.L & Szczech, G.M. (1979) Prenatal effect of 2,4,5-T,2,4,5-trichlorophenol and phenoxyacetic acid in mice. J. Environ. Sci. Health, C13, 189–204.
Howes, D. (1988) Absorption and metabolism of 2-phenoxyethanol in rat and man. Cosmet.Toiletries, 103, 75.
International Organization of the Flavour Industry (1995) European inquiry on volume use. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Ishidate, M., Sofuni, K., Yoshikawa, K., Hayashi, M., Nohmi, T., Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol., 22, 623–636.
James, M.O. & Smith, R.L. (1973) Conjugation of phenylacetic acid in phenylketonurics. Eur. J. Clin. Pharmacol., 5, 243–246.
James, M.O., Smith, R.L., Williams, R.T. & Reidenberg, M. (1972) The conjugation of phenylacetic acid in man, sub-human primates and some non-primate species. Proc. R. Soc. London B, 182, 25–35.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavourings and compounds of related structure. I. Acute oral toxicity. Food Cosmet. Toxicol., 2, 327–343.
Johannsen, E. & Purchase, I.F.H. (1969) Kaffircorn malting and brewing studies. XXI: The effect of the fusel oils of Bantu beer on rat liver. S.A. Med. J., 43, 326–328.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity of aldehydes and diketones. Mutat. Res., 216, 366.
Kay, H.D. & Raper, H.S. (1922) Mode of oxidation of fatty acids with branched chains. II. The fate in the body of hydratropic, tropic, atrolactic and atropic acids together with phenylacetaldehyde. Biochem. J., 16, 465–474.
Keating, J.W. (1972) Acute toxicity study in rats and rabbits. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Klyosov, A.A. (1996) Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic and fused polycyclic aldehydes. Biochemistry, 35, 4457–4467.
Ko, G.K.W., Raghupathy, E. & McKean, C.M. (1973) UDP-galactose: Glycoprotein galactosyl transferase and UDP-N-acetylgalactosamine: Protein N-acetylgalactosaminyl transferase activities of human cerebrospinal fluid. Can. J. Biochem., 51, 1460–1469.
Koss, F.W. & Lamprecht, W. (1968) Experimental investigations in choleresis. Eur. J. Pharmacol., 4, 215–223.
Levenstein, I. (1973) Acute oral toxicity reports on rats. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Levenstein, I. (1974) Acute toxicity study in rats and rabbits. Unpublished report to RIFM. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Levenstein, I. (1975) Acute toxicity studies in rats, mice and rabbits. Unpublished report to RIFM. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Levey, S. & Lewis, H.B. (1947) The metabolism of phenoxyacetic acid, its homologues, and some monochlorophenoxyacetic acids. New examples of oxidation. J. Biol. Chem., 168, 213–221.
Longland, R.C., Shilling, W.H. & Gangolli, S.D. (1977) The hydrolysis of flavouring esters by artificial gastrointestinal juices and rat tissue preparations. Toxicology, 8, 197–204.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavour and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavour and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999) Volatile Components in Food—Qualitative and Quantitative Data, Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982) Acute oral toxicity study in rats (14 days). Unpublished report No. PH 402-IFF-018-82. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Mankes, R.F., LeFevre, R., Bates, H. & Abraham, R. (1983) Effects of various exposure levels of 2-phenylethanol on fetal development and survival in Long-Evans rats. J. Toxicol. Environ. Health, 12, 235–244.
Mankes. R.F., LeFevre, R. & Abraham, R. (1984) Reproductive effects of some solvent alcohols: Evaluation of structure–activity relationships in 2-substituted ethanols. J. Am. Coll. Toxicol., 3, 166.
Mankes, R.F., LeFevre, R., Renak, R., Fiesher, J. & Abraham, R. (1985) Reproductive effects of some solvent alcohols with differing partition coefficients. Teratology, 31, 67A.
Marks, V. (1988) The effect of lactisole on glucose tolerance and insulin secretion in healthy subjects. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Martini, R. & Murray, M. (1996) Rat hepatic microsomal aldehyde dehydrogenase. Identification of 3- and 4-substituted aromatic aldehydes as substrates of the enzyme. Chem. Res. Toxicol., 9, 268–276.
Moldave, K. & Meister, A. (1957) Enzymic acylation of glutamine by phenylacetic acid. Biochim. Biophys. Acta, 24, 654–655.
Moreno, O.M. (1972) Single dose toxicity in rats. Unpublished report by Toxicological Resources, East Millstone, New Jersey, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1973) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1974) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1976) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1977) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1979) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1980) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moreno, O.M. (1982) Acute oral toxicity in rats. Unpublished report by MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Morgareidge, K. (1962) In vitro digestion of four acetals. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
National Toxicology Program (1984) Ethylene glycol monophenyl ether: Reproductive and fertility assessment in CD-1 mice when administered in the feed. Report No. NTP-84-410; NTP-83-FACB022, Washington DC.
Norppa, H. & Vainio, H. (1983) Induction of sister-chromatid exchanges by styrene analogues in cultured human lymphocytes. Mutat. Res., 116, 379–387.
Nour-Eldin, F. (1968) Phenols and blood coagulation. J. Biomed. Mater. Res., 2, 23–42.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N. (1979) Mutagenicity of food flavours in bacteria. Shokuhin Eisei Hen, 9, 177–181 (in Japanese, with English abstract and tables).
Ohshima, H., Friesen, M., Malaveille, C., Brouet, I., Hautefeuille, A. & Bartsch, H. (1989) Formation of direct-acting genotoxic substances in nitrosated smoked fish and meat products: Identification of simple phenolic precursors and phenyldiazonium ions as reactive products. Food Chem. Toxicol., 27, 193–203.
Oser, B.L. & Hall, R.L. (1977) Criteria employed by the expert panel of Flavor and Extract Manufacturers’ Association for the GRAS evaluation of flavouring substances. Food Cosmet. Toxicol.,15, 457–466.
Palmer, A.K., Bottomley, A.M., Ratcliffe, H.E., Clark, R. & John, D.M. (1986) Effect of phenylethyl alcohol (PEA) on pregnancy of the rat. Unpublished report from Huntingdon Research Center to RIFM. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Piccirillo, V.J., Hartman, W.C., Dauvin, E. & Swidersky, ?. (1983) Acute oral toxicity (LD50) study in the rat with phenoxyacetic acid. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of substrate specificity of alcohol dehydrogenases from human liver, horse liver, and yeast towards saturated and 2-enoic alcohols ands aldehydes. Arch. Biochem. Biophys., 159, 50–60.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of toxicological data. Toxicological tests on flavouring matters. Food Cosmet. Toxicol., 7, 405–407.
Power, F.W. & Sherwin, C.P. (1927) The detoxication of putrefactive products by the human body. Arch. Intern. Med., 39, 60–66.
Rabinowitz, T. (1969) Subacute toxicity (90 days) of senecioic acid, phenethyl ester. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced by aqueous chlorination of organic compounds. Bull. Environ. Contam. Toxicol., 24, 590–596.
Richter, D. (1938) Elimination of amines in man. Biochem. J., 32, 1763–1769.
Ridgway, P. (1985) Rat teratology dose ranging study. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Ridgway, P. (1986) Rat teratology test. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Romano, C., Meyers, F.H. & Anderson, H.H. (1954) Pharmacological relationship between aldehydes and arterenol. Arch. Int. Pharmacodyn., 99, 378–398.
Rumyantsev, G.I., Novikov, S.M., Fursova, T.N.M., Kochetkova, T.A. & Ivanov, Yu.V. (1987) Experimental study of the toxic properties of phenylethyl alcohol and phenylethyl acetate. Gig. Sanit., 10, 83–84.
Russell, T. (1973) Acute oral and dermal toxicity studies. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Sangster, S.A. & Lindley, M.G. (1986) Metabolism and excretion of ORP/178 in man. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Seakins, J.W.T. (1971) The determination of urinary phenylacetylglutamine as phenylacetic acid: Studies on its origin in normal subjects and children with cystic fibrosis. Clin. Chim. Acta, 35, 121–131.
Shelanski, M. & Moldovan, M. (1971) Acute oral toxicity in rats. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Shiple, G.J. & Sherwin, C.P. (1922) Fate of phenylacetyl derivatives of the amino acids in the animal organism. J. Biol. Chem., 53, 463.
Simpson, V.J., Baker, R. & Deitrich, R.A. (1985) Inducible aldehyde dehydrogenases from rat liver cytosol. Toxicol. Appl. Pharmacol., 79, 193–203.
Stein, W.H., Paladini, A.C., Hirs, C.H. & Moore, S. (1954) A phenylacetylglutamine as a constituent of normal human urine (Letter to the Editor). J. Chem. Soc. Perkin Trans., 76, 2848–2849.
Stewart, G.A. (1962) Pharmacological studies on oral hypoglycaemic agents. Anglo-Ger. Med. Rev., 1, 334–347.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavouring materials. Perfumer Flavourist, 12, 27–68.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavouring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857–860.
Tabatabaie, T. & Floyd, R.A. (1996) Inactivation of glutathione peroxidase by benzaldehyde. Toxicol. Appl. Pharmacol., 141, 389–393.
Tachibana, A. & Yonei, S. (1985) Inhibition of excision repair in DNA in UV-irradiated Escherichia coli by phenethyl alcohol. Int. J. Radiat. Biol., 47, 663–671.
Tachibana, A., Yonei, T., Todo, T. & Kato, M. (1982) Inhibitory effect of phenethyl alcohol on DNA repair in UV-radiated E. coli cells (Abstract No. 23910). J. Radiat. Res., 23.
Tashian, R.E. (1960) Effect of phenylalanine metabolites on urinary excretion of indole-3-acetic acid and 5-hydroxyindole-3-acetic acid in man. Proc. Soc. Exp. Biol. Med., 103, 407–410.
Teuchy, H., Quatacker, G. & Van Sumere, C.F. (1971) Quantitative investigation of the hippuric acid formation in the rat after administration of some possible aromatic and hydroaromatic precursors. Arch. Intern. Physiol. Biochem., 79, 573–587.
Thierfelder, H. & Schempp, E. (1917) Behaviour of benzoylpropionic acid, phenethyl alcohol and phenoxyacetic acid in the body of men and dogs. Arch. Ges. Physiol., 167, 280–288.
Todo, T. & Yonei, S. (1983) Inhibitory effect of membrane-binding drugs on excision repair of DNA damage in UV-irradiated Escherichia coli. Mutat. Res., 112, 97–107.
Tomiyama, H., Tachibana, A. & Yonei, S. (1986) Differential effects of procaine and phenethyl alcohol on excision repair in DNA in UV-irradiated Escherichia coli. Int. J. Radiat. Biol., 50, 973–981.
Treick, R.W. & Konetzka, W.A. (1964) Physiological state of Escherichia coli and the inhibition of deoxyribonucleic acid synthesis by phenethyl alcohol. J. Bacteriol., 88, 1580–1584.
Tulane, V.J. & Lewis, H.B. (1933) Studies in the synthesis of hippuric acid in the animal organism. IX. A comparative study of the rate of synthesis and excretion of hippuric and phenaceturic acids by the rabbit. J. Biol. Chem., 103, 151–160.
Varley, R. & Trenchard-Morgan, S. (1985) Completion of bacterial reverse mutation tests to on ORP 178 to OECD guideline 471 (Ames test). Tate and Lyle Group Research and Development, Ledbury, Herefordshire, England. Unpublished report. Private communication. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989) Immunotoxicity assessment of flavouring Ingredients using a rapid and economical screen. Toxicologist, 9, 206.
Wagreich, H., Kamin, H. & Harrow, B. (1940) On the detoxication of phenylacetic acid by glucuronic acid in humans. Proc. Soc. Exp. Biol. Med., 43, 468–470.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, Basc and micronucleus tests. Food Chem. Toxicol., 21, 707–719.
Williams, R.T. (1959) Detoxication Mechanisms—The Metabolism and Detoxication of Drugs, Toxic Substances and Other Organic Compounds, 2nd Ed., London, Chapman & Hall.
Wingard, C., Hitchcock, P. & Teague, R.S. (1955) A survey of aldehydes with respect to their action on the blood pressure. Arch. Int. Pharmacodyn. Ther., 102, 65–84.
Yonei, S. (1980) Inhibitory effect of membrane-specific drugs on liquid-holding recovery in UV-irradiated E. coli cells. Int. J. Radiat. Biol., 37, 685–689.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring agents used in foodstuffs. J. Osaka City Med. Center, 34, 267–288.
Zaitsev, A.N. & Rakhmanina, N.L. (1974) Some data on the toxic properties of phenylethyl and cinnamyl alcohols. Vop. Pitan., 6, 48–53.
ENDNOTES
1.
See Also:
Toxicological Abbreviations