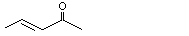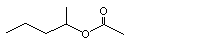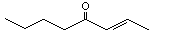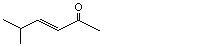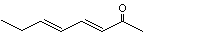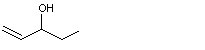WHO FOOD ADDITIVES SERIES: 50
Aliphatic Secondary Alcohols, Ketones and Related Esters
First draft prepared by
Professor I.G. Sipes
Department of Pharmacology, College of Medicine, University of Arizona, Tucson, Arizona, USA
and Professor A.G. Renwick
Clinical Pharmacology Group, University of Southampton, Southampton, England
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 39 flavouring agents that includes aliphatic acyclic secondary alcohols and ketones and esters derived from aliphatic secondary alcohols (see Table 1), using the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1). The Committee had not previously evaluated any of these agents.
Table 1. Summary of results of the safety evaluations of aliphatic secondary alcohols, ketones and related esters used as flavouring agentsa
|
Flavouring agent |
No. |
CAS No. and structure |
Step A3b
Does intake exceed the threshold for human intake? |
Comments |
Conclusion based on current intake |
|
Structural class 1 |
|
|
|
|
|
|
3-Penten-2-one |
1124 |
625-33-2
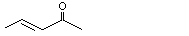 |
No
Europe: 0.3
USA: ND |
See notes 1,3 and 4. |
No safety concern |
|
4-Hexen-3-one |
1125 |
2497-21-4
 |
No
Europe: 15
USA: 1 |
See notes 1 and 3. |
No safety concern |
|
2-Hepten-4-one |
1126 |
4643-25-8
 |
No
Europe: 0.01
USA: ND |
See notes 1 and 3. |
No safety concern |
|
5-Methyl-2-hepten-4-one |
1133 |
81925-81-7
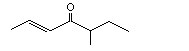 |
No
Europe: 7
USA: 1 |
See notes 1 and 3. |
No safety concern |
|
3-Octen-2-ol |
1140 |
76649-14-4
 |
No
Europe: 1
USA: ND |
See note 1. |
No safety concern |
|
(E)-2-Octen-4-ol |
1141 |
20125-81-9
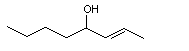 |
No
Europe: ND
USA: 1 |
See note 1. |
No safety concern |
|
2-Pentyl butyrate |
1142 |
60415-61-4
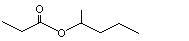 |
No
Europe: ND
USA: 1 |
See note 2. |
No safety concern |
|
(±) Heptan-3-yl acetate |
1143 |
5921-83-5
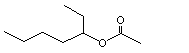 |
No
Europe: 4
USA: 3 |
See note 2. |
No safety concern |
|
(±) Heptan-2-yl butyrate |
1144 |
39026-94-3
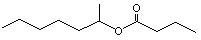 |
No
Europe: 4
USA: 3 |
See note 2. |
No safety concern |
|
(±) Nonan-3-yl acetate |
1145 |
60826-15-5
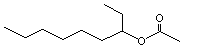 |
No
Europe: 4
USA: 3 |
See note 2. |
No safety concern |
|
Structural class II |
|
|
|
|
|
|
3-Decanone |
1118 |
928-80-3
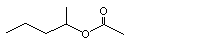 |
No
Europe: 4
USA: 3 |
See notes 1 and 3. |
No safety concern |
|
5-Methyl-5-hexen-2-one |
1119 |
3240-09-3
 |
No
Europe: ND
USA: 0.3 |
See notes 1, 3 and 4. |
No safety concern |
|
6-Methyl-5-hepten-2-one |
1120 |
110-93-0
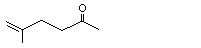 |
No
Europe: 120
USA: 40 |
See notes 1 and 4. |
No safety concern |
|
3,4,5,6-Tetrahydro-pseudo-ionone |
1121 |
4433-36-7
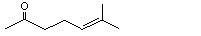 |
No
Europe: ND
USA: 0.01 |
See notes 1 and 4. |
No safety concern |
|
6,10-Dimethyl-5,9-undecadien-2-one |
1122 |
3796-70-1
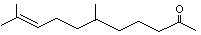 |
No
Europe: 50
USA: 2 |
See notes 1 and 4. |
No safety concern |
|
2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one |
1123 |
762-29-8
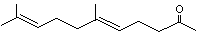 |
No
Europe: 0.1
USA: ND |
See notes 1 and 4. |
No safety concern |
|
3-Hepten-2-one |
1127 |
1119-44-4
 |
No
Europe: 0.2
USA: 0.07 |
See notes 1,3 and 4. |
No safety concern |
|
3-Octen-2-one |
1128 |
1669-44-9
 |
No
Europe: 1
USA: 1 |
See notes 1, 3 and 4. |
No safety concern |
|
2-Octen-4-one |
1129 |
4643-27-0
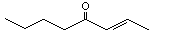 |
No
Europe: 1
USA: 3 |
See notes 1 and 3. |
No safety concern |
|
3-Decen-2-one |
1130 |
10519-33-2
 |
No
Europe: 0.01
USA: ND |
See notes 1, 3 and 4. |
No safety concern |
|
4-Methyl-3-penten-2-one |
1131 |
141-79-7
 |
No
Europe: 0.4
USA: ND |
See notes 1 and 4. |
No safety concern |
|
5-Methyl-3-hexen-2-one |
1132 |
5166-53-0
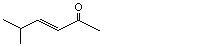 |
No
Europe: ND
USA: 0.1 |
See notes 1, 3 and 4. |
No safety concern |
|
6-Methyl-3,5-heptadien-2-one |
1134 |
1604-28-0
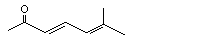 |
No
Europe: 15
USA: 5 |
See notes 1, 3 and 4. |
No safety concern |
|
(E)-7-Methyl-3-octen-2-one |
1135 |
33046-81-0
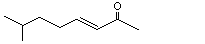 |
No
Europe: ND
USA: 2 |
See notes 1, 3 and 4. |
No safety concern |
|
3-Nonen-2-one |
1136 |
14309-57-0
 |
No
Europe: 14
USA: 13 |
See notes 1, 3 and 4. |
No safety concern |
|
(E) & (Z)-4,8-Dimethyl-3,7-nonadien-2-one |
1137 |
817-88-9
 |
No
Europe: 7
USA: 7 |
See notes 1, 3 and 4. |
No safety concern |
|
(E)-6-Methyl-3-hepten-2-one |
1138 |
20859-10-3
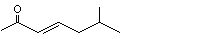 |
No
Europe: 4
USA: 3 |
See notes 1, 3 and 4. |
No safety concern |
|
(E,E)-3,5-Octadien-2-one |
1139 |
30086-02-3
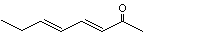 |
No
Europe: 4
USA: 3 |
See notes 1, 3 and 4. |
No safety concern |
|
1-Penten-3-one |
1147 |
1629-58-9
 |
No
Europe: 0.3
USA: 0.1 |
See notes 1 and 3. |
No safety concern |
|
1-Octen-3-one |
1148 |
4312-99-6
 |
No
Europe: 1
USA: 0.1 |
See notes 1 and 3. |
No safety concern |
|
2-Pentyl-1-buten-3-one |
1149 |
63759-55-7
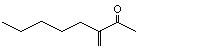 |
No
Europe: 0.2
USA: ND |
See notes 1, 3 and 4. |
No safety concern |
|
1-Penten-3-ol |
1150 |
616-25-1
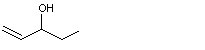 |
No
Europe: 2
USA: 1 |
See note 1. |
No safety concern |
|
1-Hexen-3-ol |
1151 |
4798-44-1
 |
No
Europe: 1
USA: 2 |
See note 1. |
No safety concern |
|
1-Octen-3-ol |
1152 |
3391-86-4
 |
No
Europe: 290
USA: 23 |
See note 1. |
No safety concern |
|
1-Decen-3-ol |
1153 |
51100-54-0
 |
No
Europe: ND
USA: 0.1 |
See note 1. |
No safety concern |
|
(E,R)-3,7-Dimethyl-1,5,7-octatrien-3-ol |
1154 |
20053-88-7
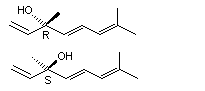 |
No
Europe: ND
USA: 6 |
See note 1. |
No safety concern |
|
6-Undecanone |
1155 |
927-49-1
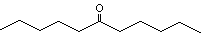 |
No
Europe: 4
USA: 3 |
See note 1. |
No safety concern |
|
2-Methylheptan-3-one |
1156 |
13019-20-0
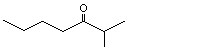 |
No
Europe: 4
USA: 3 |
See note 1. |
No safety concern |
|
CAS: Chemical Abstracts Service; ND: No intake data reported |
|
a |
Step 2: All of the flavouring agents in this group were predicted to be metabolized to innocuous products. |
|
b |
The threshold for human intake is 1800 µg/person per day for class I and 540 µg/person per day for class II. All intake levels expressed in µg/person per day. The combined intake of flavouring agents in class I is 58 and 16 µg per person per day in Europe and the USA, respectively. The combined intake of flavouring agents in class II is 520 and 120 µg per person per day in Europe and the USA, respectively. |
|
Notes: |
|
1. |
Detoxicated by reduction of the ketone followed by glucuronic acid conjugation of the corresponding alcohol or direct glucuronic acid conjugation of the secondary alcohol |
|
2. |
Detoxicated by hydrolysis of the ester and glucuronic acid conjugation of the resulting alicyclic alcohol and complete oxidation of the carboxylic acid |
|
3. |
Detoxicated by reduction of the ketone functional group followed by glucuronic acid conjugation of the resulting alcohol and glutathione conjugation of the parent ketone |
|
4. |
Detoxicated by reduction of the ketone and alkyl side-chain oxidation and excretion |
Twenty-six of the 39 substances in this group of flavouring agents (Nos 1118, 1120, 1122–1132, 1134–1136, 1139, 1142, 1144, 1147, 1148, 1150–1152, 1154 and 1156) have been reported to occur naturally in foods. They have been found in fruits, juices, spices, vegetables, cocoa, coffee and tea.
1.2 Estimated daily intake
The total annual volume of production of the 39 aliphatic secondary alcohols and ketones in this group is approximately 3900 kg in Europe (International Organization of the Flavor Industry, 1995) and 1100 kg in the USA (Lucas et al., 1999). Approximately 73% and 47% of the total annual production volume in Europe and the USA, respectively, is accounted for by 6-methyl-5-hepten-2-one (No. 1120) and 1-octen-3-ol (No. 1152). The daily per capita intake of 6-methyl-5-hepten-2-one (No. 1120) and 1-octen-3-ol (No. 1152) was 120 µg and 290 µg in Europe and 44 µg and 23 µg, respectively, in the USA, respectively.
1.3 Absorption, distribution, metabolism and elimination
In general, the aliphatic esters in this group hydrolyse to the corresponding secondary alcohols. The secondary alcohols and their corresponding ketones are interconvertible under physiological conditions. In the principal excretion pathway, the ketones are reduced to the corresponding secondary alcohols, which are subsequently conjugated with glucuronic acid and excreted.
When the ketone carbonyl function is located at the 2-position (i.e. a methyl ketone), the methyl group may undergo alpha,beta-hydroxylation and subsequent oxidation, to yield a corresponding ketocarboxylic acid. The ketoacids are intermediary metabolites (e.g. alpha,beta-ketoacids), which can undergo oxidative decarboxylation to yield carbon dioxide and simple aliphatic carboxylic acids. The acid may be completely metabolized in the fatty acid pathway and citric acid cycle. When the substance is an alpha,beta-unsaturated ketone (Nos 1124–1139 and 1147–1149) or secondary alcohol (Nos 1140–1141 and 1150–1154) which is oxidized to an alpha,beta-unsaturated ketone, it can conjugate with glutathione. The glutathione conjugates are converted to the corresponding mercapturic acid derivatives (N-acetylcysteine derivative) and excreted.
As the alpha,beta-unsaturated carbonyl group is a structural alert for toxicity, the Committee, at previous meetings, has devoted considerable attention to the safety of flavouring agents containing this reactive moiety. The Committee concluded at its fifty-seventh meeting (Annex 1, reference 154) that the presence of protective processes in cells provides adequate detoxication capacity at the low doses associated with use of such compounds as flavouring agents. With respect to alpha,beta-unsaturated ketones, these protective processes include reduction of the ketone to the corresponding alcohol (followed by conjugation of the alcohol with glucuronic acid) and conjugation with glutathione. These processes operate for the aliphatic ketones used as flavouring agents.
1.4 Application of Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to these chemicals, the Committee assigned 11 of the 39 agents to structural class I (Cramer et al., 1978) on the basis that they are acyclic saturated and unsaturated aliphatic ketones with no other functional groups and three or fewer carbons on either side of the keto group (Nos 1124–1126 and 1133) and secondary alcohols (Nos 1140–1141) or esters of secondary alcohols (Nos 1142–1146). The remaining 28 flavouring agents, which are acyclic aliphatic ketones with four or more carbons on either side of the keto group (Nos 1118–1123, 1127– 1132, 1134–1139, 1148, 1155 and 1156) and either a ketone (Nos 1147 and 1149) or a secondary alcohol (Nos 1150–1154) attached to a terminal vinyl group, were assigned to structural class II. |
|
Step 2. |
All the flavouring agents in this group were predicted to be metabolized to innocuous products. The evaluation of these substances therefore proceeded via the A (left-hand) side of the decision tree. |
|
Step A3. |
The estimated daily per capita intakes of the 11 flavouring agents in structural class I and the 28 flavouring agents in structural class II are below the threshold for human intake for each class (i.e., 1800 µg per person for structural class I and 540 µg per person for structural class II). According to the Procedure, the safety of these 39 flavouring agents raises no concern when they are used at their current estimated levels of intake. |
Table 1 summarizes the evaluation of aliphatic secondary alcohols, ketones and related esters used as flavouring agents.
1.5 Consideration of combined intake from use as flavouring agents
In the unlikely event that all the flavouring agents in this group were to be consumed concurrently on a daily basis, the estimated combined intake would not exceed the human intake threshold for structural class I or II.
1.6 Conclusions
The Committee concluded that none of the flavouring agents in this group of 39 aliphatic secondary alcohols, ketones and related esters would raise a safety concern at the current estimated levels of intake. Other data on the toxicity of aliphatic secondary alcohols, ketones and related esters are consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This section summarizes the data relevant to the safety evaluation of 39 aliphatic ketones, secondary alcohols and related esters (see Table 1). The group of flavouring agents includes three saturated ketones (Nos 1118, 1155 and 1156), 24 unsaturated ketones (Nos 1119–1139 and 1147–1149), seven secondary alcohols (Nos 1140, 1141 and 1150–1154) and five esters formed from saturated secondary alcohols (Nos 1142–1146). The esters in this group are readily hydrolysed to the corresponding secondary alcohols. After hydrolysis, the resulting secondary alcohols, ketones and other secondary alcohols in the group are readily absorbed. Secondary alcohols are interconvertible with the corresponding ketones in vivo (McMahon, 1982). Therefore, all the substances in this group are expected to participate in common routes of absorption, metabolism, distribution and excretion and have similar toxicological profiles.
2.2 Additional considerations on intake
The total annual volume of use of the 39 secondary alcohols, ketones and related esters is approximately 3900 kg in Europe (International Organization of the Flavor Industry, 1995) and 1100 kg in the USA (Lucas et al., 1999) (see Table 2). In Europe and the USA, approximately 73% and 47% of the total annual production volume are accounted for by 6-methyl-5-hepten-2-one (No. 1120) and 1-octen-3-ol (No. 1152), respectively. Production volumes and intake values for each flavouring agent are reported in Table 2.
Table 2. Annual volumes of production and intake of aliphatic secondary alcohols, ketones and related esters used as flavouring agents in Europe and the USA
|
Substance (No.) |
Most recent annual volume (kg)a |
Intake (‘eaters only’)b |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
|
µg/day |
µg/kg bw
per day |
|
|
3-Decanone (1118)e |
|
Europe |
25 |
3.6 |
0.059 |
+ |
NA |
|
USA |
25 |
3.3 |
0.55 |
|
|
|
5-Methyl-5-hexen-2-one (1119)f |
|
Europe |
NR |
NA |
NA |
– |
NA |
|
USA |
2.0 |
0.26 |
0.0044 |
|
|
|
6-Methyl-5-hepten-2-one (1120) |
|
Europe |
820 |
120 |
1.9 |
4300 |
5 |
|
USA |
340 |
44 |
0.74 |
|
13 |
|
3,4,5,6-Tetrahydropseudoionone (1121) |
|
Europe |
NR |
NA |
NA |
– |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
|
|
6,10-Dimethyl-5,9-undecadien-2-one (1122) |
|
Europe |
340 |
49 |
0.82 |
3200 |
9 |
|
USA |
16 |
2.2 |
0.036 |
|
200 |
|
2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one (1123) |
|
Europe |
0.7 |
0.1 |
0.002 |
460 |
660 |
|
USA |
NR |
NA |
NA |
|
NA |
|
3-Penten-2-one (1124) |
|
Europe |
2.1 |
0.30 |
0.0050 |
660 |
320 |
|
USA |
NR |
NA |
NA |
|
NA |
|
4-Hexen-3-one (1125) |
|
Europe |
110 |
15 |
0.25 |
+ |
NA |
|
USA |
6.4 |
0.84 |
0.014 |
|
|
|
2-Hepten-4-one (1126) |
|
Europe |
0.1 |
0.01 |
0.0002 |
+ |
NA |
|
USA |
NR |
NA |
NA |
|
NA |
|
3-Hepten-2-one (1127) |
|
Europe |
1.3 |
0.19 |
0.0031 |
+ |
NA |
|
USA |
0.5 |
0.07 |
0.001 |
|
|
|
3-Octen-2-one (1128) |
|
Europe |
5.2 |
0.74 |
0.012 |
1 |
0.2 |
|
USA |
6.4 |
0.84 |
0.014 |
|
0.2 |
|
2-Octen-4-one (1129) |
|
Europe |
7.0 |
1.0 |
0.016 |
+ |
NA |
|
USA |
20 |
2.6 |
0.044 |
|
|
|
3-Decen-2-one (1130) |
|
Europe |
0.1 |
0.01 |
0.0002 |
+ |
NA |
|
USA |
NR |
NA |
NA |
|
|
|
4-Methyl-3-penten-2-one (1131) |
|
Europe |
2.8 |
0.40 |
0.0067 |
3 |
1 |
|
USA |
NR |
NA |
NA |
|
|
|
5-Methyl-3-hexen-2-one (1132) |
|
Europe |
NR |
NA |
NA |
+ |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
|
|
|
5-Methyl-2-hepten-4-one (1133) |
|
Europe |
48 |
6.8 |
0.11 |
– |
NA |
|
USA |
6.4 |
0.84 |
0.014 |
|
|
|
6-Methyl-3,5-heptadien-2-one (1134) |
|
Europe |
110 |
15 |
0.26 |
+ |
NA |
|
USA |
34 |
4.5 |
0.076 |
|
|
|
(E)-7-Methyl-3-octen-2-one (1135) |
|
Europe |
NR |
NA |
NA |
+ |
NA |
|
USA |
14 |
1.8 |
0.030 |
|
|
|
3-Nonen-2-one (1136)e |
|
Europe |
100 |
14 |
0.24 |
+ |
NA |
|
USA |
100 |
13 |
0.22 |
|
|
|
(E) and (Z)-4,8-Dimethyl-3,7-nonadien-2-one (1137)e |
|
Europe |
50 |
7.1 |
0.12 |
– |
NA |
|
USA |
50 |
6.6 |
0.11 |
|
|
|
(E)-6-Methyl-3-hepten-2-one (1138)e |
|
Europe |
25 |
3.6 |
0.059 |
– |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
(E,E)-3,5-Octadien-2-one (1139)e |
|
Europe |
25 |
3.6 |
0.059 |
+ |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
3-Octen-2-ol (1140) |
|
Europe |
10 |
1.4 |
0.024 |
– |
NA |
|
USA |
NR |
NA |
NA |
|
|
|
(E)-2-Octen-4-ol (1141) |
|
Europe |
NR |
NA |
NA |
– |
NA |
|
USA |
11 |
1.4 |
0.024 |
|
|
|
2-Pentyl butyrate (1142) |
|
Europe |
NR |
NA |
NA |
+ |
NA |
|
USA |
4.5 |
0.59 |
0.0099 |
|
|
|
(±) Heptan-3-yl acetate (1143)e |
|
Europe |
25 |
3.6 |
0.059 |
– |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
(±) Heptan-2-yl butyrate (1144)e |
|
Europe |
25 |
3.6 |
0.059 |
+ |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
(±) Nonan-3-yl acetate (1145)e |
|
Europe |
25 |
3.6 |
0.059 |
– |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
2-Pentyl acetate (1146)e |
|
Europe |
24 |
3.4 |
0.057 |
– |
NA |
|
USA |
24 |
3.2 |
0.053 |
|
|
|
1-Penten-3-one (1147) |
|
Europe |
2.4 |
0.34 |
0.0057 |
4 |
2 |
|
USA |
0.9 |
0.1 |
0.002 |
|
|
|
1-Octen-3-one (1148) |
|
Europe |
10 |
1.4 |
0.024 |
93 |
9 |
|
USA |
0.5 |
0.07 |
0.001 |
|
190 |
|
2-Pentyl-1-buten-3-one (1149) |
|
Europe |
1.2 |
0.17 |
0.0029 |
– |
NA |
|
USA |
NR |
NA |
NA |
|
|
|
1-Penten-3-ol (1150) |
|
Europe |
17 |
2.4 |
0.040 |
5300 |
310 |
|
USA |
9.1 |
1.2 |
0.020 |
|
580 |
|
1-Hexen-3-ol (1151) |
|
Europe |
4.5 |
0.64 |
0.011 |
+ |
NA |
|
USA |
18 |
2.3 |
0.039 |
|
|
|
1-Octen-3-ol (1152) |
|
Europe |
2000 |
290 |
4.8 |
15 000 |
7 |
|
USA |
180 |
23 |
0.39 |
|
83 |
|
1-Decen-3-ol (1153) |
|
Europe |
NR |
NA |
NA |
– |
NA |
|
USA |
1.0 |
0.13 |
0.0022 |
|
|
|
(E,R)-3,7-Dimethyl-1,5,7-octatrien-3-ol (1154) |
|
Europe |
NR |
NA |
NA |
+ |
NA |
|
USA |
45 |
5.9 |
0.099 |
|
|
|
6-Undecanone (1155)e |
|
Europe |
25 |
3.6 |
0.059 |
– |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
2-Methylheptan-3-one (1156)e |
|
Europe |
25 |
3.6 |
0.059 |
+ |
NA |
|
USA |
25 |
3.3 |
0.055 |
|
|
|
Total |
|
Europe |
3900 |
|
|
|
|
|
USA |
1100 |
|
|
|
|
|
NR, no data reported; NA, not applicable; +, reported to occur naturally in foods (Maarse et al., 1999) but no quantitative data available; –, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavor Industry (1995) and Lucas et al. (1999) |
|
b |
Intake (µg/person per day) calculated as follows: [(annual volume, kg) × (1 × 109 µg/kg)/(population × survey correction factor × 365 days)], where population (10%, ‘eaters only’) = 32 × 106 for Europe and 26 × 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual production volume of the flavour, resepctively, was reported in the poundage surveys. Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding. |
|
c |
Quantitative data from Stofberg & Grundschober (1987) |
|
d |
Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring agent, kg) |
|
e |
Anticipated annual volume in the USA as reported by the Flavor and Extract Manufacturers Association of the United States |
Twenty-six of the 39 flavouring substances in this group have been reported to occur naturally in foods. They have been detected in a variety of foods, including avocado, raspberry, mango, kiwi fruit, grapefruit juice, orange juice, cocoa, tea, tomato, asparagus, potato chips and rum (Maarse et al., 1999). Quantitative data on natural occurrence and consumption ratios have been reported for 10 of the substances: 6-methyl-5-hepten-2-one (No. 1120); 6,10-dimethyl-5,9-undecadien-2-one (No. 1122); 2,6,10-trimethyl-2,6,10-pentadecatrien-14-one (No. 1123); 3-penten-2-one (No. 1124); 3-octen-2-one (No. 1128); 4-methyl-3-penten-2-one (No. 1131); 1-penten-2-one (No. 1147); 1-octen-3-one (No. 1148); 1-penten-3-ol (No. 1150) and 1-octen-3-ol (No. 1152). The data demonstrate that their intake occurs predominantly from the consumption of traditional foods (i.e., consumption ratio > 1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987) (see Table 2).
2.3 Biological data
2.3.1 Biochemical data
(a) Hydrolysis
The aliphatic esters, such as 2-pentyl butyrate (No. 1142), are rapidly hydrolysed to the parent secondary alcohol and the component aliphatic carboxylic acids by classes of enzymes recognized as carboxylesterases (Ford & Moran, 1978; Heymann, 1980; Anders, 1989; White, 1990), the most important of which are the beta-esterases. Esters of aliphatic secondary alcohols were hydrolysed to their corresponding alcohols and carboxylic acids when incubated with liver homogenates obtained from male Wistar albino rats (Longland et al., 1977) or intestinal mucosal homogenates obtained from pigs (Leegwater & van Straten, 1974b). The ester of the secondary alcohol, 3-methyl-2-pentanol, and acetic acid were completely hydrolysed in intestinal mucosal homogenate within 2 h (Leegwater & van Straten, 1974b). Esters exposed to artificial intestinal fluid and simulated gastric juice were also hydrolysed, but more slowly. Partial hydrolysis (15–40%) was reported 2 h after incubation of 2-propyl butyrate or 4-methyl-2-pentyl acetate with simulated intestinal fluid (Leegwater & van Straten, 1974a). Rat liver homogenates were found to be between 8 and > 25 000 times more efficient than artificial pancreatic juice in hydrolysing a wide variety of aliphatic esters, and hydrolysis in simulated intestinal fluid with pancreatin was much faster than in simulated gastric juice (Longland et al., 1977). The authors concluded that tissue homogenates provide a good model for the situation in vivo. Thus, it is anticipated that the esters (Nos 1142–1146) in this group rapidly hydrolyse to secondary alcohols and carboxylic acids, which are then readily absorbed.
(b) Absorption, distribution, metabolism and excretion
After oral administration, aliphatic secondary alcohols and ketones are absorbed through the gastrointestinal tract and subsequently rapidly eliminated from the blood. Peak blood concentrations normally occur within 1–2 h after dosing (Lehman et al., 1945; Nordmann et al., 1973; Bonte et al., 1981). Although ketones and secondary alcohols are readily interconverted, reduction of the ketones by cytosolic carbonyl reductases (Felsted & Bachur, 1980) is favoured in vivo, yielding the corresponding secondary alcohols, which are excreted in the urine mainly as glucuronic acid conjugates (Kasper & Henton, 1982; Annex 1, reference 138).
In the case of a methyl ketone, the terminal methyl group may undergo oxidation, eventually yielding alpha-ketoacid, which is the substrate for further oxidation in the fatty acid pathway and citric acid cycle (Annex 1, reference 138). In the case of alpha,beta-unsaturated ketones, glutathione may conjugate at the beta-position, yielding cysteine and mercapturic acid derivatives, which are also excreted primarily in the urine as the conjugated secondary alcohol (Williams, 1959; Portoghese et al., 1989).
(i) Reduction of aliphatic secondary alcohols and acyclic ketones
Single high doses of a homologous series of aliphatic secondary alcohols and ketones were administered individually by gavage to rabbits, principally to identify the extent of urinary glucuronide metabolite formation. The level of urinary excretion of glucuronic acid conjugates was determined over 24 h. The results showed that secondary alcohols, either administered directly or formed via ketone reduction, are largely excreted as glucuronic acid conjugates (Kamil et al., 1953). The substances, doses, and average urinary output of glucuronide as a percentage of the dose administered are listed in Table 3.
Table 3. Urinary glucuronic acid conjugate after administration of aliphatic
secondary alcohols and ketones to rabbits by gavage
|
Substance |
Dose
(mg/kg bw)a |
Urinary glucuronic acid conjugate (%) |
|
2-Pentanol |
740 |
45 |
|
2-Heptanone |
950 |
41 |
|
2-Heptanol |
960 |
55 |
|
3-Heptanol |
960 |
62 |
|
2-Octanol |
1100 |
16 |
a Calculated on the basis of a dose of 25 mmol/3 kg bw
alpha,beta-Unsaturated ketones are also reduced to the corresponding secondary alcohols. Cytoplasmic NADPH-dependent alpha,beta-unsaturated ketone reductase isolated from human liver catalysed the reduction of aliphatic alpha,beta-unsaturated ketones to the corresponding secondary alcohols (Fraser et al., 1967). The resulting alcohols can then be excreted as glucuronic acid conjugates (Williams, 1959). Glucuronic acid conjugates of substances like secondary alcohols are frequently the end-products of metabolism and are excreted in the urine or the bile (Kasper & Henton, 1982).
(ii) alpha-Oxidation of methylketones
Twenty aliphatic secondary ketones and related esters are methyl ketones (Nos 1119–1124, 1127, 1128, 1130–1132, 1134–1139 and 1149) or are secondary alcohols or related esters that are readily converted to methyl ketones in vivo (Nos 1140 and 1142) (see Table 1). Additional metabolic options are available for these substances. Methyl ketones undergo alpha-hydroxylation and subsequent oxidation of the terminal methyl group to eventually yield corresponding ketocarboxylic acids (Gabriel et al., 1972). The ketoacids are intermediary metabolites (e.g. alpha-ketoacids) that undergo oxidative decarboxylation to yield carbon dioxide and simple aliphatic carboxylic acids. The acids may be completely metabolized in the fatty acid pathway and citric acid cycle. The metabolism of alpha-hydroxyacids and alpha-ketoacids was recently reviewed (Annex 1, reference 144).
(iii) Glutathione conjugation of alpha,beta-unsaturated ketones
Twenty-six of the substances in this group are alpha,beta-unsaturated ketones (Nos 1124–1139, 1147–1149) or alcohols (Nos 1140, 1141, 1150–1154), which can be oxidized to alpha,beta-unsaturated ketones. In addition to reduction of the carbonyl function and, in some cases, alpha-oxidation of the methyl group, these substances may also react with free glutathione (GSH) in a nucleophilic Michael-type addition (Portoghese et al., 1989). GSH conjugates can be excreted in the bile or they can be converted enzymically to the mercapturic acid conjugate. It has been shown that alpha,beta-unsaturated carbonyl compounds have an affinity for sulfhydryl groups (Boyland & Chasseaud, 1967, 1968; Portoghese et al., 1989). These substances [e.g., ethyl vinyl ketone (No. 1147)] react with GSH through addition of a nucleophile (Nu–) to the beta-carbon atom of the double-bond. This reaction is catalysed by glutathione-S-transferase but can also occur non-enzymatically (see Figure 1) (Esterbauer et al., 1975; Chasseaud, 1979; Portoghese et al., 1989).
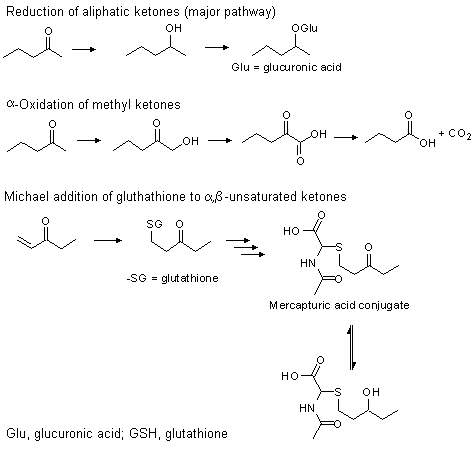
Figure 1. Metabolic fate of aliphatic ketones and secondary alcohols
The conjugation of glutathione with alpha,beta-unsaturated ketones in vivo is catalysed by glutathione-S-tranferases. Up to 13 different forms of this enzyme exist in the human liver. These isoenzymes have many similarities including subunit size, immunochemical properties, catalytic activities, other binding activities and resistance to sulfhydryl reagents (van der Jagt et al., 1985). They also are identifiable by isoelectric point and haematin binding properties. The conjugation reaction may occur, however, even in the absence of glutathione-S-transferase, since many alpha,beta-unsaturated ketones are sufficiently electrophilic to react with glutathione. The reactivities of several alpha,beta-unsaturated ketones for glutathione addition were studied in the absence of glutathione-S-transferase (Portoghese et al., 1989). Included in this group were 2-octene-4-one (No. 1129), 4-methyl-3-penten-2-one (No. 1131) and 6-methyl-3,5-heptadien-2-one (No. 1134). It was shown that the rate of reaction was extremely rapid for compounds such as 2-butenone, but that any substitution at the beta-position of the double-bond greatly reduced this reactivity. Thus, a methyl substitution at the beta-position in 4-methyl-3-penten-2-one (No. 1131) reduced the reactivity towards glutathione addition by 120 000 times as compared with 2-butenone. A similar reduction of relative reactivity was observed with 6-methyl-3,5-heptadien-2-one (No. 1134) (Portoghese et al., 1989).
Typically, two metabolic processes follow conjugation of the alpha,beta-unsaturated ketone. In one, the GSH conjugate undergoes enzyme-catalysed hydrolysis and N-acetylation, yielding the corresponding N-acetyl-L-cysteine derivative (i.e., mercapturic acid derivative) (Chasseaud, 1976). In the other, the ketone function may be reduced to the corresponding alcohol. The principal metabolite obtained from the urine of rats given 2-propenal by subcutaneous injection was N-acetyl-S-(3-hydroxypropyl)-L-cysteine (Kaye, 1973). Formation of the mercapturic acid derivative of the corresponding secondary alcohol has been reported with mesityl oxide [4-methyl-3-penten-2-one (No. 1131)] and with ethyl vinyl ketone [1-penten-3-one (No. 1147)] in vitro with rat liver homogenate (Boyland & Chasseaud, 1967; Chasseaud, 1976).
At high cellular concentrations, alpha,beta-unsaturated carbonyl compounds have the potential to react with DNA. A cyclic deoxyguanosine adduct is formed in vitro via Michael addition of the N-1 or exocyclic NH2 of guanine to the beta position of 2-butenone, followed by condensation of the ketone carbonyl with the remaining exocyclic NH2 or N-1 moiety (Chung et al., 1988). However, these adducts have been found to be formed at a considerably slower pace (24 h at 100 oC) than the glutathione reaction, suggesting that glutathione conjugation takes place in vivo before it can react with DNA (Portoghese et al., 1989).
In addition, the terminal double-bonds may undergo epoxidation, catalysed by microsomal cytochrome P450 isoenzymes (Belluci et al., 1996). In rat liver microsomes, substances such as 1-hexen-3-ol can be epoxidized to yield erythro- and threo-1,2-epoxyhexan-3-ol. These are intermediary metabolites, in that the epoxide function is rapidly hydrolysed to a diol in the presence of microsomal epoxide hydrolase or conjugated with glutathione (Chiappe et al., 1998). This results in formation of erythro- and threo-hexane-1,2,3-triol, a polar metabolite that can be rapidly excreted in the urine (Chiappe et al., 1998).
Analysis of 150 human urine samples revealed the presence of two substances used as flavouring agents. The samples were collected for 2 months on different days from the same volunteers and were analysed by gas chromatography. The urinary profiles varied among individuals but remained constant for the same person over 2 months. Dietary changes during the testing period did not change the urinary profiles. Constituents of normal urine samples were identified by mass spectrometry. Among the numerous compounds from this group of aliphatic secondary alcohols and ketones, 3-penten-2-one (No. 1124) and 4-methyl-3-penten-2-one (No. 1131) were identified as normal constituents of human urine. In a study under the same conditions for compounds present in the urine of 40 patients with diabetes mellitus, one of the several compounds identified as a normal urine constituent was again 3-penten-2-one (No. 1124) (Zlatkis et al., 1973).
A similar type of study was undertaken, in which urinary organic acid metabolites were studied in normal and diabetic C57BL/Ks male mice. The profiles of the diabetic animals showed significantly lower concentrations of certain ketones, including 6-methyl-6-hepten-2-one. These ketones were excreted in low amounts in diabetic animals with depleted fat stores, indicating that their production is related to a deficiency in fat breakdown. However, the concentrations of several other similar ketones, such as 6-methyl-5-hepten-2-one (No. 1120), 3-hepten-2-one (No. 1127) and 3-octen-2-one (No. 1128), showed no difference between normal and diabetic mice (Holland et al., 1984).
(iv) Summary
The studies demonstrate that these 39 aliphatic ketones, secondary alcohols and related esters are metabolized primarily by reduction of the ketone to yield the corresponding secondary alcohol, followed by conjugation with glucuronic acid and excretion in the urine. Other metabolic options include alpha-oxidation of methyl ketones and glutathione conjugation of the alpha,beta-unsaturated ketones, followed by elimination of the mercapturic acid derivative of the corresponding secondary alcohol. To a minor extent, the double-bond may undergo epoxidation and then hydrolysis or conjugation with glutathione.
Because the alpha,beta-unsaturated carbonyl group is a structural alert for toxicity, the Committee, at previous meetings, devoted considerable attention to the safety of flavouring agents containing this reactive moiety. The Committee concluded at its fifty-seventh meeting (Annex 1, reference 154) that the presence of cytoprotective processes provides adequate detoxication capacity at the low doses associated with use of such compounds as flavouring agents. With respect to alpha,beta-unsaturated ketones, these cytoprotective processes include reduction of the ketone to the corresponding alcohol (followed by conjugation of the alcohol with glucuronic acid) and conjugation of the glutathione. As reported above, these processes are operative for the aliphatic ketones used as flavouring agents.
2.3.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for 15 of the 39 substances in this group. In rats, the values ranged from 320 to > 6800 mg/kg bw (Table 4).
Table 4. Studies of acute toxicity of orally administered aliphatic secondary alcohols, ketones and related esters used as flavouring agents
|
No. |
Agent |
Species |
Sex |
LD50
(mg/kg bw) |
Reference |
|
1120 |
6-Methyl-5-hepten-2-one |
Rat |
M,F |
4100 |
Keating (1972) |
|
1120 |
6-Methyl-5-hepten-2-one |
Mouse |
M,F |
3600 |
Colaianni (1967) |
|
1121 |
3,4,5,6-Tetrahydropseudoionone |
Mouse |
M,F |
5200 |
Moreno (1982) |
|
1121 |
3,4,5,6-Tetrahydropseudoionone |
Rat |
M,F |
> 5000 |
Moreno (1977) |
|
1122 |
6,10-Dimethyl-5,9-undecadien-2-one |
Mouse |
M,F |
> 8600 |
Colaianni (1967) |
|
1122 |
6,10-Dimethyl-5,9-undecadien-2-one |
Rat |
M,F |
> 6800 |
Hoffman (1978) |
|
1122 |
6,10-Dimethyl-5,9-undecadien-2-one |
Rat |
M,F |
> 5000 |
Moreno (1975) |
|
1123 |
2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one |
Rat |
M,F |
> 5000 |
de Groot et al. (1974) |
|
1124 |
3-Penten-2-one |
Rat |
M,F |
320 |
Smyth & Carpenter (1948) |
|
1125 |
4-Hexen-3-one |
Mouse |
M,F |
780 |
Oser (1970) |
|
1132 |
5-Methyl-3-hexen-2-one |
Rat |
NR |
1400 |
Carpenter et al. (1974) |
|
1133 |
5-Methyl-2-hepten-4-one |
Rat |
M |
1400 |
Reagan & Becci |
|
F |
1400 |
(1984) |
|
1134 |
6-Methyl-3,5-heptadien-2-one |
Mouse |
M,F |
3200 |
Colaianni (1967) |
|
1135 |
(E)-7-Methyl-3-octen-2-one |
Rat |
M,F |
> 2000 |
Sanders (1996) |
|
1148 |
1-Octen-3-one |
Rat |
M |
982 |
Reagan & Becci |
|
F |
890 |
(1983a) |
|
1149 |
2-Pentyl-1-buten-3-one |
Rat |
M |
3700 |
Reagan & Becci |
|
F |
1800 |
(1983b) |
|
1151 |
1-Hexen-3-ol |
Rat |
M,F |
440 |
Til (1977) |
|
1152 |
1-Octen-3-ol |
Rat |
M,F |
340 |
Wohl (1974) |
|
1153 |
1-Decen-3-ol |
Rat |
M,F |
> 1500 |
Chubb (1995) |
M, male; F, female; NR, not reported
(b) Short-term and long-term studies of toxicity
The Committee previously evaluated the structurally related aliphatic ketones 2-heptanone (No. 283), 3-heptanone (No. 285), 4-heptanone (No. 287) and 2,6-dimethyl-4-heptanone (No. 302). The NOEL for 2-heptanone and was 20 mg/kg bw per day (Gaunt et al., 1972), while those of the remaining three aliphatic ketones were > 1000 mg/kg bw per day (O’Donoghue & Krasavage, 1980; O’Donoghue et al., 1984). The Committee concluded that all four agents were of no safety concern at current levels of intake when used as flavouring agents (Annex 1, reference 138). The results of short-term studies with repeated dose of two representative aliphatic secondary alcohols and five ketones are summarized in Table 5 and described below.
Table 5. Results of short- and long-term studies of toxicity in male and female rats of orally administered aliphatic secondary alcohols, ketones and related esters used as flavouring agents
|
No. |
Substance |
No. test groupsa/
no. per groupb |
Duration
(days) |
NOEL
(mg/kg bw per day) |
Reference |
|
1119 |
5-Methyl-5-hexen-2-one |
1/10 |
14 |
10d |
Gill & Van Miller (1987) |
|
1123 |
2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one |
2/10 |
14 |
3.5 |
de Groot et al. (1974) |
|
1125 |
4-Hexene-3-one |
1/46 |
90 |
6.6 (F) |
Shellenberger |
|
6.7 (M) |
(1970a,b) |
|
1129 |
2-Octen-4-one |
1/30 |
90 |
6.7 |
Cox et al. (1974a) |
|
1148 |
1-Octen-3-one |
1/30 |
90 |
6.7 |
Cox et al. (1974b) |
|
1152 |
1-Octen-3-ol |
1/28 |
90 |
12 (M) |
Posternak et al. |
|
14 (F) |
(1964) |
|
1154 |
(E,R)-3,7-Dimethyl-1,5,7-octatrien-3-ol |
1/14 |
14 |
10d |
Wnorowski (1997) |
|
a |
Does not include control animals |
|
b |
Includes both male and female animals |
|
c |
Performed at either a single or multiple doses that produced no adverse effects. Therefore, this dose level is not a true NOEL but the highest dose tested that produced no adverse effects. The actual NOEL would be higher. |
(i) 5-Methyl-5-hexen-2-one (No. 1119)
A 14-day study to screen for minimum toxicity was conducted, in which a group of five Fischer 344 rats of each sex was maintained on a diet containing 5-methyl-5-hexene-2-one (No. 1119) that provided a target intake of 10 mg/kg bw per day. Four other flavouring agents were tested, with a common control group. The animals were observed for deaths twice daily and underwent a detailed clinical examination daily. Food consumption was measured on days 7 and 14. No physical signs of toxicity, abnormal body-weight gain, abnormal food consumption or treatment-related effects were observed at necropsy. Statistically significant decreases in absolute and relative liver weights and absolute kidney weight were reported in treated males. As similar changes were found with the four other substances, the authors concluded that the decreases in organ weight were a reflection of higher than normal liver and kidney weights in the control group. This conclusion was supported by the fact that none of the five treated groups showed signs or histopathological changes in these organs (Gill & van Miller, 1987).
(ii) 2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one (No. 1123)
Groups of five weanling rats of each sex were given 2,6,10-trimethylpenta-decatrien-14-one in corn oil by gavage at a dose of 0, 0.35 or 3.5 mg/kg bw per day on 6 days per week for 2 weeks. Individual body weights and food intake were recorded on days 1, 7 and 14, and haemoglobin concentration was measured on day 14. All animals survived and were killed at the end of the study. Kidney and liver weights were recorded, and all organs were examined macro-scopically and microscopically. No differences in body-weight gain, food intake or food use efficiency were reported between treated and control groups. Histopathological examination revealed no evidence of alterations to the liver or kidneys. Liver weights were increased in male rats at the lower dose, while kidney weights were increased in male rats at the higher dose. In the absence of histopathological effects, these changes were considered to be of no toxicological significance (deGroot et al., 1974).
(iii) (E,R)-3,7-Dimethyl-1,5,7-octatrien-3-ol (No. 1154)
Groups of seven Sprague-Dawley rats of each sex were given (E,R)-3,7-dimethyl-1,5,7-octatrien-3-ol (No. 1154) in 1 ml of corn oil by gavage at a dose of 10 mg/kg bw per day for 14 consecutive days. Seven vehicle controls of each sex were given 1 ml of corn oil by gavage daily for 14 days. Individual doses were adjusted for body weight on days 1 and 8. All animals were observed daily for signs of gross toxicity and deaths. Weekly measurement of body weights and food consumption revealed no differences between test and control groups. All animals survived to completion of the study. At termination, gross and histopathological examination of the liver and kidneys from all treated animals revealed no lesions that could be associated with administration of the test substance (Wnorowski, 1997).
(iv) 4-Hexen-3-one (No. 1125)
Groups of 23 weanling Sprague-Dawley rats of each sex were maintained on a diet containing 4-hexene-3-one (No. 1125) at a concentration calculated to provide an average daily intake of 6.7 mg/kg bw for males and 6.6 mg/kg bw for females. Weekly measurements of body weight revealed no differences between treated and control groups. Biochemical, haematological and urinary analyses conducted after weeks 6 and 13 also showed no significant differences between treated and control rats. After 90 days, the animals were killed, and a complete necropsy was performed. A number of incidental lesions were reported, the incidences of which were low and randomly distributed among control and treated animals. The authors did not attribute the gross pathological lesions seen in the tissues to treatment with 4-hexen-3-one (Shellenberger, 1970a). In an addendum to the final report, which included the results of histopathological evaluation of tissues obtained from the rats, Shellenberger (1970b) concluded that administration of 4-hexen-3-one had no toxicologically significant effects.
(v) 2-Octen-4-one (No. 1129) and 1-octen-3-one (No. 1148)
In two studies with the same protocol, groups of 15 Wistar rats of each sex were maintained on a diet containing 2-octen-4-one (No. 1129) (Cox, 1974a) or 1-octen-3-one (No. 1148) (Cox, 1974b) at a concentration providing an average target intake of 7.5 mg/kg bw per day, for 90 days. Groups of 15 control rats of each sex were maintained on basal diet. The rats were housed individually and giventheir respective diet and tap water ad libitum. Measurements of food consumption throughout the study indicated that the treated groups received 6.7 mg/kg bw per day of 2-octen-4-one or 1-octen-3-one. All rats were monitored daily for survival, behaviour and physical appearance. Weekly measurement of body weight and food consumption revealed no significant differences between treated and control groups. Haematological, blood biochemical and urine analyses performed during weeks 6 and 12 on eight males and eight females from each group showed normal values. At 90 days, complete necropsies were performed. A detailed histopathological examination was undertaken on 23 major organs from eight male and eight female rats, and the livers and kidneys of the remaining seven animals in each group were examined for histopathological changes. No difference was found between treated and control animals in absolute or relative organ weights, and there was no evidence of gross or microscopic alterations with either substance (Cox, 1974a). A sporadic incidence of slight vacuolization of liver cells was observed in both control and treated animals (Cox, 1974b).
(vi) 1-Octen-3-ol (No. 1152)
A group of 14 male and 14 female Charles River rats was maintained on a diet containing 1-octen-3-ol at a concentration calculated to provide an average intake of 12 mg/kg bw per day for males and 14 mg/kg bw per day for females. The rats were given their respective diet and tap water ad libitum. All rats were monitored daily for survival, behaviour and physical appearance. Weekly measurements of body weight and food consumption revealed no significant differences between treated and control animals. In general, haematological, blood biochemical and urine analyses performed during weeks 7 and 13 revealed normal values, with the exception of increased haemoglobin and corpuscular values. At necropsy, gross and histopathological examination revealed no evidence of effects attributable to intake of the substance (Posternak, 1964).
(c) Genotoxicity
Seven representative substances in this group have been tested for genotoxicity in vitro. The results of these tests are summarized in Table 6 and described below.
Table 6. Studies of genotoxicity in vitro with aliphatic secondary alcohols, ketones and related esters used as flavouring agents
|
No. |
Agent |
End-point |
Test object |
Maximum concentrati.† |
Results |
Reference |
|
1120 |
6-Methyl-5-hepten-2-one |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
380 µg/plate |
Negativea |
Florin et al. (1980) |
|
1124 |
3-Penten-2-one |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
250 µg/plate |
Negativea |
Florin et al. (1980) |
|
1131 |
4-Methyl-3-penten-2-one |
Reverse mutation |
S. typhimurium TA100 |
5.8–200 mg/plate |
Negativeb |
Cheh (1986) |
|
1134 |
6-Methyl-3,5-heptadien-2-one |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
370 µg/plate |
Negativea |
Florin et al. (1980) |
|
1135 |
(E)-7-Methyl-3-octen-2-one |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
15–1500 µg/plate |
Negativea |
Thompson (19896) |
|
1147 |
1-Penten-3-one |
Reverse mutation |
S. typhimurium TA100 |
42–130 µg/plate |
Positivea,c |
Deininger et al. (1990) |
|
1147 |
1-Penten-3-one |
DNA damage |
E. coli pQ37 |
0–4.2 µg/plate |
Positivea |
Deininger et al. (1990) |
|
1153 |
1-Decen-3-ol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0.3–1000 µg/plate |
Negativea,d |
Durward (1995) |
a With and without metabolic activation
b Without metabolic activation
c Cytotoxic at 50 µg/plate in the absence of metabolic activation
d Toxic in some species at 300 µg/plate
(i) Reverse mutation
Assays for reverse mutation were performed with 6-methyl-5-hepten-2-one (No. 1120), 3-penten-2-one (No.1124), 4-methyl-3-penten-2-one (No. 1131), 6-methyl-3,5-heptadien-2-one (No. 1134), (E)-7-methyl-3-octen-2-one (No. 1135) and 1-decen-3-ol (No. 1153). There was no evidence of mutagenicity at concentrations up to 1500 µg/ml in TA98, TA100, TA1535, TA1537 or TA1538 strains of Salmonella typhimurium (Florin et al. 1980; Cheh, 1986; Durward, 1995; Thompson, 1996).
1-Penten-3-one (No. 1147) caused reverse mutation in an assay with preincubation in a single strain of S. typhimurium (TA100) in the presence and absence of metabolic activation (Deininger et al., 1990). No increase over spontaneous background reversion rates was reported with 1-penten-3-one at a concentration of 42 µg/plate in the presence of metabolic activation; however, at a concentration of 84 µg/plate, it induced a threefold increase in the rate of reversions. Bacterial toxicity was reduced in the presence of a metabolic activation system, probably due to detoxication by reaction with nucleophilic components in the system, such as glutathione and other SH-group-containing compounds. Similarly, the toxicity was reduced by addition of glutathione, with no effect on mutagenicity (Deininger et al., 1990; Eder et al., 1993).
In order to evaluate the effect of epoxidation of the double-bond on potential mutagenicity, 1-penten-3-one was further evaluated in the presence of SKF 525A, an inhibitor of microsomal monooxygenases, and 1,1,1-trichloropropene-2,3-oxide, an inhibitor of epoxide hydrolase. No mutagenic activity was observed in the presence of 100 µg/ml of SKF 525A, but addition of 1,1,1-trichloropropene-2,3-oxide resulted in an increase in the frequency of reverse mutations in a concentration-dependent manner (Deininger et al., 1990).
(ii) DNA damage
In a non-standardized assay for SOS DNA repair, Escherichia coli strain PQ37, with rfA and uvrA mutations, was incubated with 1-penten-3-one (No. 1147), with and without metabolic activation. sfiA gene-linked beta-galactosidase activity was used as a measure of the induction of SOS repair. In the absence of metabolic activation, 1-penten-3-one was a weak SOS-inducing agent, with a potential of 0.027 calculated from the linear part of the dose–response curve. Adding metabolic activation did not increase the genotoxicity. The authors concluded that the genotoxicity of 1-penten-3-one is probably a result of its toxicity (Deininger, 1990). As has been pointed out by other authors, it is difficult to detect genotoxic effects of alpha,beta-unsaturated substances in vitro because of their general toxicity to bacteria (Eder et al., 1991).
(iii) Conclusion
Positive results in vitro were obtained only with 1-penten-3-one, for which sufficient metabolic processes are available for detoxication at low levels of intake. These include known conjugation of alpha,beta-unsaturated ketones with glutathione and metabolic conversion of metabolically formed epoxides to dihydrodiols by epoxide hydrolase.
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson, G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York: Taylor & Francis, pp. 81–97.
Belluci, G., Chiappe, C., Pucci, L. & Gervasi, P.G. (1996) The mechanism of the oxidation of allylic alcohol to alpha,beta -unsaturated ketones by cytochrome P450. Chem. Res. Toxicol., 9, 871–874.
Bonte, W., Rudell, E., Sprung, R., Frauenrath, C., Blanke, E., Kupilas, G., Wochinik, J. & Zah, G. (1981) Experimental investigations concerning the blood-analytical detection of small doses of higher aliphatic alcohols in man. J. Blutalkohol, 18, 399–411.
Boyland, E. & Chasseaud, L.F. (1966) Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem. J., 104, 95–102.
Boyland, E. & Chasseaud, L.F. (1967) Enzyme catalyzing conjugations of glutathione with alpha, beta-unsaturated carbonyl compounds. Biochem. J., 109, 651–660.
Carpenter, C.P., Weil, C.S. & Smyth, H.F., Jr (1974) Range finding toxicity data: List VIII. Toxicol. Appl. Pharmacol., 28, 313–319.
Chasseaud, L.F. (1976) Conjugation with glutathione and mercapturic acid excretion. In: Arias, I.R. & Jakoby, W.B., eds,Glutathione Metabolism and Function, New York: Raven Press, pp. 77–114.
Chasseaud, L.F. (1979) The role of gluthathione and glutathione-S-transferase in the metabolism of chemical carcinogens and other electrophilic agents. Adv. Cancer Res., 29, 175–274.
Cheh, A.M. (1986) Mutagen production by chlorination of methylated alpha,beta-unsaturated ketones. Mutat. Res., 169, 1–9.
Chiappe, C., DeRubertis, A., Amato, G. & Gervasi, P.G. (1998) Sterochemistry and biotransformation of 1-hexene and 2-methylh-1-hexene with rat liver microsomes and purified P450s of rats and humans. Chem. Res. Toxicol., 11, 1487–1493.
Chubb, D.R. (1995) Acute oral toxicity study in the rat (fixed dose procedure). Study No. A/O/41323. Unpublished report from Toxicology Laboratories Ltd, Herefordshire, England. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Chung, F.L., Roy, K.R. & Hecht, S.S. (1988) A study of reactions of alpha,beta-unsaturated carbonyl compounds with deoxyguanosine. J. Org. Chem., 53, 14–17.
Colaianni, L.J. (1967) Acute toxicity, eye and skin irritation tests on aromatic compounds. Unpublished report from Roche Chemical Division. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974a) 90-day feeding study in rats with 2-octen-4-one. Unpublished report from FDRL, Inc., Waverly, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974b) 90-day feeding study in rats with 1-octen-3-one. Unpublished report from FDRL, Inc., Waverly, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Deininger, C., Eder, E., Neudecker, T. & Hoffman, C. (1990) Mutagenicity and genotoxicity of ethylvinyl ketone in bacterial tests. J. Appl. Toxicol., 10, 167–171.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. & Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its metabolites in the rat. J. Pharmacokinet. Biopharmacol., 9, 553–576.
Durward, R. (1995) Reverse mutation assay ‘Ames test’ using Salmonella typhimurium. Unpublished report from Safepharm Laboratories Ltd, Derby, England. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Eder, E., Hoffman, C. & Deininger, C. (1991) Identification and characterization of deoxyguanosine adducts of methyl vinyl ketone and ethyl vinyl ketone. Genotoxicity of the ketones in the SOS chromotest. Chem. Res. Toxicol.. 4, 50–57.
Eder, E., Scheckenbach, S., Deininger, C. & Hoffman, C. (1993) The possible role of alpha,beta-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol. Lett., 67, 87–103.
Esterbauer, H., Zollner, H. & Scholz, N. (1975) Reaction of glutathione with conjugated carbonyls. Z. Naturforsch. C, 30, 466–473.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxification, New York: Academic Press, Vol. 1, pp. 281–293.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using Ames’ test. Toxicology, 18, 219–232.
Ford, D.M. & Moran, E.J. (1978) Preliminary indications of in vitro hydrolysis of two flavor chemical esters. Private communication. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Fraser, I.M., Peters, M.A. & Hardinge, G.M. (1967) Purification and some characteristics of an alpha,beta-unsaturated keone reductases from dog erythrocytes and human liver. Mol. Pharmacol., 3, 233–247.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of acetoin to CO2 in intact animals and in liver mince preparation. Comp. Biochem. Physiol., 41B, 493–502.
Gaunt, I.F., Carpanini, F.M.B., Wright, M.G., Grasso, P. & Gangolli, S.D. (1972) Short-term toxicity of methyl amyl ketones in rats. Food Cosmet. Toxicol., 10, 625–636.
Gill, M.W. & van Miller, J.P. (1987) Fourteen-day dietary minimum toxicity screen (MTS) in albino rats. Unpublished report from Union Carbide Export, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
deGroot, A.P., Spanjers, M.T. & van der Heijden, C.A. (1974) Acute and sub-acute oral toxicity studies in rats with five flavour compounds. Unpublished report from Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxication, 2nd Ed., New York: Academic Press, pp. 291-323.
Hoffman, W. (1974) Methylheptenon. Unpublished report from BASF, Ludwigshafen, Germany. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Hoffman, W. (1978) Acute toxicity study of 2,6-dimethylundecadien-2,6-on-10 (geranylaceton). Unpublished report from BASF, Ludwigshafen, Germany. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States (in German).
Holland, M.L., Rhodes, G.R., Wiesler, D. & Novotny, M. (1984) Chromatographic profiling of urinary volatile and organic acid metabolites of normal and diabetic C57BL/Ka mice. J. Chromatogr., 306, 23–37.
Honkanen, E., Karvonen, P. & Virtanen, A.I. (1964) Studies on the transfer of some flavour compounds to milk. Acta Chem. Scand., 18, 612–618.
International Organization of the Flavor Industry (1995) European inquiry on volume use. Private communication. Submitted to WHO by Flavor and Extract Manufacturers Association of the United States.
van der Jagt, D.L., Hunsaker, L.A., Garcia, K.B. & Royer, R.E. (1985) Isolation and characterization of the multiple gluthathione-S-transferases from human liver. J. Biol. Chem., 260, 11603–11610.
Kasper, C.B. & Henton, D. (1982) Glucuronidation. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxification, New York: Academic Press, Vol. 2, pp. 4–36.
Kamil, I.A., Smith, J.N. & Williams, R.T. (1953) Studies in detoxication. 46. The metabolism of aliphatic alcohols; the glucuronic acid conjugation of acyclic aliphatic alcohols. Biochem. J., 53, 129–136.
Kaye, C.M. (1973) Biosynthesis of mercapturic acids from allyl alcohol, allyl esters and acreolin. Biochem. J., 134, 1093–1101.
Keating, J.W. (1972) Acute toxicity study in rats and rabbits. Unpublished report from Foster D. Snell, Inc., Elizabeth, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Leegwater, D.C. & van Straten, S. (1974a) In vitro study on the hydrolysis of twenty-six organic esters by pancreatin. Unpublished report from Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Leegwater, D.C. & van Straten, S. (1974b) In vitro study on the hydrolysis of eight carboxylic esters by intestinal and liver enzymes. Unpublished report from Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States..
Lehman, A.J., Schwerma, H. & Rickards, E. (1945) Isopropyl alcohol: Acquired tolerance in dogs, rate of disappearance from the blood stream in various species, and effects on successive generation of rats. J. Pharmacol. Exp. Ther., 85, 61–69.
Longland, R.C., Shilling, W.H. & Gangolli, S.D. (1977) The hydrolysis of flavouring estersby artificial gastrointestinal juices and rat tissue preparations. Toxicology, 8, 197–204.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999) Volatile Components in Food—Qualitative and Quantitative Data, Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
McMahon, R.E. (1982) Alcohols, aldehydes and ketones. in: Jakoby , W.B., Bend, J.R. & Caldwell, J., eds, Metabolic Basis for Detoxification. Metabolism of Functional Groups, New York: Academic Press, p. 91.
Moreno, O.M. (1975) Acute toxicity studies in rats rabbits and guinea pigs. Private communication to Research Institute for Fragrance Materials, Hackensack, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Moreno, O.M.(1977) Acute toxicity studies in rats rabbits and guinea pigs. Private communication to Research Institute for Fragrance Materials, Hackensack, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States
Moreno, O.M.(1982) Acute toxicity studies in rats rabbits and guinea pigs. Private communication to Research Institute for Fragrance Materials, Hackensack, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Nordmann, R., Ribière, C., Rouach, H., Béauge, F., Giudicelli, Y. & Nordmann, J. (1973) Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sci., 13, 919–932.
O’Donohogue, J.L. & Krasavage, W.J. (1980) 90-day repeated oral administration of five ketones and n-heptane to rats. Private communication. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
O’Donoghue, J.L., Krasavage, W.J., DiVincenzo, G.D. & Katz, G.V. (1984) Further studies on ketone neurotoxicity and interactions. Toxicol. Appl. Pharmacol., 72, 201–209.
Oser, B.L. (1970) The acute oral toxicity of ten compounds. Unpublished report from FDRL, Inc., Maspeth, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall, R.L. (1989) Reactivity of glutathione with alpha,beta-unsaturated ketone flavouring substances. Food Chem. Toxicol., 27, 773–776.
Posternak, J. (1964) Subacute toxicity (90 days) report on 1-octen-3-ol (amyl vinyl carbinol). Private communication. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Reagan, E.L. & Becci, P.J. (1983a) Acute oral toxicity study in rats with 1-octene-3-one. Unpublished report from FDRL, Inc., Waverly, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Reagan, E.L. & Becci, P.J. (1983b) Acute oral toxicity study in rats with 3-methylene-2-octanone. Unpublished report from FDRL, Inc., Waverly, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of filbertone in Sprague-Dawley rats. Unpublished report from FDRL, Inc., Waverly, New York, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Sanders, A. (1996) Acute oral toxicity (limit test) in the rat. Unpublished report from Safepharm Laboratories Ltd, Derby, England. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Shellenberger, T.E. (1970a) Subacute toxicity evaluation of 2-hexene-4-one with rats. Private communication. Unpublished report from Gulf South Research Institute, New Iberia, Louisiana, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Shellenberger T.E. (1970b) Histology evaluations: Subacute toxicity evaluation of 2-hexene-4-one with rats. Addendum to the final report. Unpublished report from Gulf South Research Institute, New Iberia, Louisiana, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Smyth, H.F., Jr & Carpenter, C.P. (1948) Further experience with the range finding test in the industrial toxicology laboratory. J. Ind. Hyg. Toxicol., 30, 63–68.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chemical Toxicol., 23, 857–860.
Thompson, P.W. (1996) Reverse mutation assay ‘Ames test’ using Salmonella typhimurium. Unpublished report from Safepharm Laboratories Ltd, Derby, England. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Til, H.P. (1977) Determination of the acute oral toxicity of 1-vinyl butanol in rats. Unpublished report from Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, Netherlands. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. & Knights, D. (1990) Chemical synthesis of dual radiolabelled cyclandelate and its metabolism in rat hepatocytes and mouse J774 cells. Xenobiotica, 20, 71.
Wnorowski, G. (1997) 14-day repeat dose oral toxicity study: rats. Unpublished report from Product Safety Labs, East Brunswick, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Wohl, A.J. (1974) Acute oral toxicity of amyl vinyl carbinol. Unpublished report from Foster D. Snell, Inc., Elizabeth, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
Zlatkis, A., Bertsch, W., Lichtenstein, H.A., Tishbee, A., Shunbo, F., Liebich, H.M., Coscia, A.M. & Fleischer, N. (1973) Profile of volatile metabolites in urine by gas chromatography–mass spectrometry. Anal. Chem., 45, 783–787.
See Also:
Toxicological Abbreviations