
WHO FOOD ADDITIVES SERIES: 52
First draft prepared by
Dr P.J. Abbott
Food Standards Australia New Zealand, Canberra, Australia
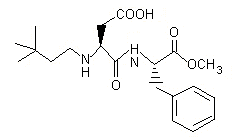
Neotame is a dipeptide methyl ester that is intended for use in food as a sweetener and flavour enhancer in a variety of applications. Neotame, the common name for N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine 1-methyl ester, is chemically related to aspartame. It has a sweetness potency of 7000–13 000 times greater than that of sucrose and 30–60 times greater than that of aspartame, depending on the food matrix in which it is used. Neotame, a white/off-white powder, is manufactured from aspartame and 3,3-dimethylbutyraldehyde in a one-step chemical synthesis, which includes a reductive alkylation, followed by purification, drying, and milling. Neotame has not been evaluated previously by the Committee.

Figure 1. Chemical structure of neotame
The absorption, distribution, metabolism and excretion of neotame have been studied in mice, rats, dogs, rabbits and humans. After oral administration, approximately 20–30% of the administered dose is absorbed and rapidly converted to the major metabolite, N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine (de-esterified neotame) and a number of minor metabolites. Neotame and its metabolites are rapidly eliminated in the urine and faeces. The metabolism of neotame, including pathways for minor metabolites comprising >1% of the dose, is described in Figure 2.
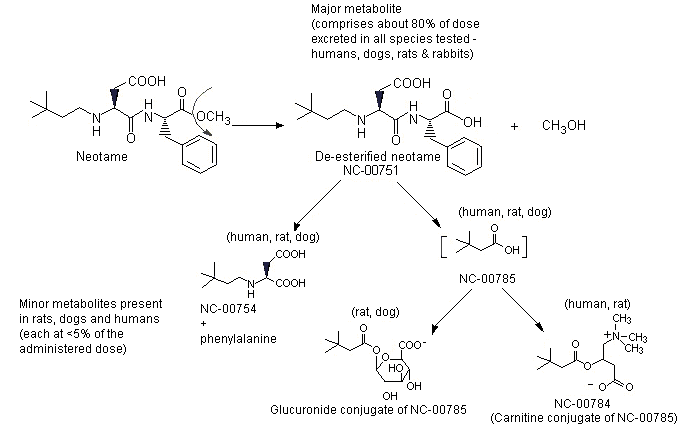
Figure 2. Metabolism of neotame
The major metabolic pathway is de-esterification of neotame to N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine (NC-00751) and methanol. Minor metabolites are N-(3,3-dimethylbutyl)-L-aspartic acid (NC-00754), a metabolite formed via peptide or amide hydrolysis of neotame; 3,3-dimethylbutyric acid (NC-00785), also referred to as 3,3-dimethylbutanoic acid; the carnitine conjugate of 3,3-dimethylbutyric acid (NC-00784); and the glucuronide conjugate of 3,3-dimethylbutyric acid (NC-00785).
Mice
The plasma concentrations of neotame and de-esterified neotame were examined during a 2-year study of carcinogenicity in CD-1 mice at doses of up to 4000 mg/kg bw per day. In this study, the effects of dose, duration of dosing, and sex were evaluated. Details of the study are provided in section 2.2.3.
Neotame and de-esterified neotame were detected in the plasma at all doses. There were no sex-specific differences in plasma concentrations of neotame or de-esterified neotame. Plasma concentrations of neotame and de-esterified neotame generally increased with increasing dose. There was no evidence for accumulation of either neotame or de-esterified neotame with continued treatment (Thomford & Carter, 1997a).
Rats
In a study examining the distribution of neotame in rat tissues, 21 male Lister Hooded rats were given [14C]neotame by gavage in a single oral dose of 15 mg/kg bw. Animals were killed in groups of three at various times up to 48 h. Samples of blood and specified tissues were taken from all animals at termination, and the amount of radiolabel in each specimen was determined.
Plasma concentrations of radiolabel peaked at 1 h after dosing, and decreased to approximately 10% of this value by 6 h. Most of the radiolabel was excreted within 24 h. The largest amounts of radiolabel were found in the contents of the gastrointestinal tract, with moderate amounts in the liver and kidney. Radiolabel was not retained in any organ, and the amounts detected in several tissues (e.g. bone marrow, brain, fat and muscle) were much lower than in plasma. There was no evidence for accumulation of radiolabel in any tissue at any time after treatment (Hawkins et al., 1995a).
In a further study to examine the distribution of neotame in rat tissues, 21 male Lister Hooded rats were given [14C]neotame in a single oral dose of 15 mg/kg bw by gavage. Pairs of rats (one of each sex) were killed after 0.5, 2, 6, 12 and 24 h, pinned out, frozen rapidly, and sagittal sections taken through the carcass at six levels were examined by autoradiography.
Qualitative assessment of radiolabel present in male and female rats indicated that the highest levels were present in rats killed at the earliest time-points after dosing. Levels decreased rapidly with time. At 0.5 h and 2 h after dosing, most radiolabel was found in the stomach, the gastrointestinal tract, liver, kidneys and bladder, with smaller amounts being distributed throughout the rest of the body.
Very small amounts were found in the central nervous system, and no binding to pigmented skin or the eye was observed. Levels were consistent with the circulation of radiolabel in the bloodstream. At subsequent time-points (6, 12 and 24 h), the passage of radiolabel through the excretory organs was seen. By 24 h after dosing, only very small amounts remained in the animal and there was no evidence of accumulation in any tissue (Hawkins et al., 1995b).
In a study designed to examine the distribution and elimination of radioactivity derived from neotame by whole-body autoradiography, eight pregnant and eight non-pregnant Sprague-Dawley rats were each given a single dose of 15 mg/kg bw of [14C]neotame by gavage. The rats were sacrificed at various times up to 24 h after dosing and the carcasses treated as in the previous study.
The tissue distribution of radiolabel was similar in pregnant and non-pregnant rats. Placental concentrations of radiolabel were low at 0.5 and 2 h after dosing, similar to those seen in other peripheral tissues and in circulating blood. No radiolabel was detected in the fetus at any time. The highest concentrations of radiolabel were seen shortly after dosing, initially in the stomach contents, gastrointestinal tract, liver, kidneys and bladder, with lower concentrations in the rest of the body. At subsequent time-points, the passage of radiolabel through the excretory organs was seen. No accumulation was seen in tissues, and concentrations were very low after 24 h. There was no significant difference between pregnant and non-pregnant rats in the time profile with which radiolabel was distributed in the tissues (Hawkins et al., 1996a).
In a pilot study to examine the metabolism of neotame, Sprague-Dawley Crl : CD BR VAF Plus rats were each given a single oral dose of 15 mg/kg bw of 14C]neotame by gavage and divided among four groups. In rats in group 1 (three rats of each sex), blood was taken at intervals up to 24 h after treatment, separated into cell and plasma fractions, and analysed for radiolabel. Rats in group 2 (two rats of each sex) were housed in glass metabolism cages for 72 h after treatment for collection of urine, faeces and expired air. Carcasses were solubilized for analysis of retained radiolabel, and urine and faeces were pooled for analysis of metabolites as well as total radiolabel. In group 3 (two rats of each sex), rats were anaesthetized 0.5 h or 2 h after dosing, and blood was collected and analysed. Rats in group 4 (two males) were anaesthetized and the bile ducts and stomach cannulated. Radiolabelled neotame was administered via the stomach cannula, and bile was collected at intervals up to 48 h after treatment. Urine and faeces were collected for 0–24 h and 24–48 h and radiolabel was measured.
Plasma concentrations of radiolabel after oral dosing with [14C]neotame peaked at 30 min after dosing in females and 1 h after dosing in males, followed by a rapid decline. The major metabolite identified in plasma, urine, faeces and bile was de-esterified neotame. The excretion of [14C]neotame was examined over 72 h; 8–10% of the radiolabel was recovered in urine, 90–92% in faeces, and 0.01–0.03% in expired air. After 72 h, 0.11–0.13% of the radiolabel remained in the carcass. In males, urinary excretion was virtually complete within 12 h, while in females, urinary excretion continued over 24 h. Most faecal excretion occurred between 6 h and 24 h after dosing in both sexes. In males in group 4, urinary excretion was similar to that seen in other groups, with around 5–9% of the administered dose being excreted in the urine. Biliary excretion accounted for approximately 5.7% of the administered dose, while faecal excretion accounted for around 85% of the administered dose. Little radiolabel was retained in the carcass.
According to the results of these studies, approximately 14% of the administered dose of [14C]neotame was absorbed. Most of the material recovered in the faeces was unabsorbed material. In all studies, around 100% of the administered dose was recovered; with very low retention of test material or metabolites in the carcass. The test material behaved similarly in males and females. The major metabolite found was de-esterifed neotame. No neotame was found in plasma, urine or faeces (Hawkins et al., 1995b).
In a more extensive study to examine the metabolism of neotame in rats, 14C]neotame was administered to groups of six male and six female Sprague-Dawley Crl : CD BR rats by gavage or by intravenous injection as a single dose of 15 mg/kg bw. Rats were individually housed in metabolism cages and urine and faeces were collected at intervals for 72 h after dosing. A additional group of three rats received a single oral dose of 120 mg/kg bw. All rats were killed after 72 h and the carcasses were retained for analysis. Radiolabel was measured in all samples and the metabolites present in the urine and faeces were determined.
After oral administration, >90% of the radiolabel was recovered in urine and faeces within 48 h. Within 72 h after oral administration of [14C]neotame at a dose of 15 or 120 mg/kg bw, 8.5–10.8% and 84.5–87.2% of the radiolabel was excreted in the urine and faeces, respectively. After intravenous administration of 14C]neotame at dose of 15 mg/kg bw, approximately 35% and 59% of the radiolabel was recovered in urine and faeces, respectively. Less than 0.3% of the radiolabel was recovered in the carcasses within 72 h after either oral or intravenous administration. Unchanged neotame was only detected in urine collected from female rats 0–6 h after intravenous administration and accounted for 3.7% of the administered dose. Unchanged neotame was not detected in the faeces of any animal regardless of the dose or route of administration.
The major metabolite found in urine after 48 h was de-esterified neotame, independent of the route of administration or the dose. N-(3,3-dimethylbutyl)-L-aspartic acid (NC-00754) was detected at lower concentrations (around 10% of the levels of de-esterified neotame after oral dosing). Parent compound was found only in the urine of female rats after intravenous dosing (3.7% of the dose); none was detected in the urine of any other groups. A glucuronide metabolite was also detected at low levels (0.4–0.5% of the administered dose) in the urine, independent of dose or route of administration. Two minor metabolites, each representing <1.6% of the administered dose, were identified.
In the faeces, de-esterified neotame was the major metabolite (approximately 70–80% of the dose after oral administration). N(3,3dimethylbutyl)Laspartic acid (NC-00754) was detected at lower levels, 0.8–2.5% of the dose. Low concentrations of an unidentified metabolite were also found, representing 0.7–1.2% of the administered dose (Kirkpatrick et al., 1997a).
In a further study to examine the pharmacokinetic profile of neotame, groups of Sprague-Dawley rats were given single oral doses of [14C]neotame in deionized water at 15 or 120 mg/kg bw (30 animals of each sex per group) or single intravenous doses of [14C]neotame in isotonic saline at 15 mg/kg bw (33 animals of each sex per group). An additional three rats of each sex per group were not treated and served as controls. Blood was taken from three rats of each sex per dose at intervals up to 24 h after treatment. Radioassay of the blood samples was performed and the radioactive constituents of plasma were analysed.
Radiolabel was detected in the plasma shortly after oral administration of 14C]neotame at a dose of 15 or 120 mg/kg bw, peaking at 0.5 h. No neotame was detected in plasma by liquid chromatograph-tandem mass spectroscopy (LC/MS/MS) methodology after oral or intravenous administration of a dose of 15 mg/kg bw; however, unchanged neotame was detected at concentrations slightly above the limit of detection (0.011 µg/ml) at the earliest sampling time (0.25 h) after oral administration of a dose of 120 mg/kg bw. The predominant metabolite of neotame was de-esterified neotame, which accounted for 80–90% of the total plasma radiolabel at early time-points after both oral and intravenous dosing. Peak mean concentrations of de-esterified neotame in plasma were detected by 0.1 h (first sampling time) after intravenous dosing and by 0.5 h after oral dosing. Plasma concentrations of de-esterified neotame declined rapidly with a t1/2 of approximately 1 h for oral administration. The clearance of de-esterified neotame was rapid and greater in females than males. Peak concentration (Cmax) and area under the curve of concentration–time at 24 h (AUC0–24) for total radiolabel were lower in females than in males, but increased proportionally in both sexes with increasing oral dose. The oral bioavailability of total radiolabel was approximately 20% in both males and females (Hawkins et al., 1997).
The plasma concentrations of neotame and de-esterified neotame were examined in a 1-year study of toxicity and 2-year study of carcinogenicity conducted in Sprague-Dawley rats fed doses of up to 1000 mg/kg bw per day. The effect of dose, duration of dosing, and sex were evaluated. Details of these studies are provided in Section 2.2.3.
There was no evidence for sex-specific differences in dose-adjusted plasma concentrations of neotame. The plasma concentration of neotame was generally below the quantifiable limit, 10 ng/ml, in rats receiving a dose of 300 mg/kg bw and above. Plasma concentrations of de-esterified neotame were quantifiable in both males and females at all doses and at all sampling times. Plasma concentrations of neotame and de-esterified neotame increased with increasing dose and there was no evidence for accumulation of neotame or de-esterified neotame (Burnett & Bartekian, 1998; Turk & Bartekian, 1998a).
Studies of metabolism and pharmacokinetics were also conducted with [14C]de-esterified neotame. Rats were given a single oral dose of [14C]de-esterified neotame at 15 mg/kg bw by gavage. Rats were then divided among four groups. Rats in group 1 (three of each sex in group housing) were dosed and blood was taken from the tail vein at intervals up to 24 h after treatment. In group 2, rats (two of each sex, housed in glass metabolism cages) were dosed and urine, faeces and expired air were collected for 72 h. Rats in group 3 (in group housing) were anaesthetized at 0.5 and 2 h (two of each sex per time period) after dosing, and blood samples collected. In group 4, rats (two males, housed individually in restraining cages) were anaesthetized and the bile ducts and stomach cannulated. [14C]De-esterified neotame was administered via the stomach cannula. Bile, urine and faeces were collected until 48 h after treatment. Rats were killed 48 h after dosing, the carcasses solubilized and radiolabel determined.
The recovery of radiolabel from intact rats ranged from 100% to 103%, virtually all of which was recovered within the first 48 h. Very little of the administered dose was excreted in expired air (<0.02%) or retained in the carcass (<0.01%). Only 1–2% of the radiolabel was excreted in the urine, while faecal excretion accounted for 99–102% of the dose. In females, urinary excretion was virtually complete within 12 h, while in males it continued over the first 24 h. Most faecal excretion occurred in both sexes between 12 h and 24 h after dosing, although there was considerable excretion in males between 6 h and 12 h after dosing. Biliary excretion accounted for 2% of the radiolabel.
Plasma concentrations of [14C]de-esterified neotame in rats in group 1 peaked at 30 min after dosing in females and at 1 h after dosing in males. There was a rapid decline over the first 4 h, with plasma concentrations below the level of detection from 4 h after dosing. Maximum concentrations of de-esterified neotame equivalents were 0.066 µg/ml plasma in males and 0.051 µg/ml plasma in females.
The predominant radiolabelled component in plasma, urine, faeces, and bile was unchanged de-esterified neotame. Several minor uncharacterized metabolites were also detected, none of which accounted for more than 0.4% of the radiolabel in urine, 2% in faeces, and approximately 6 ng de-esterified neotame equivalents/ml in plasma. The total combined amount (i.e. approximately 4%) of radiolabel excreted in the urine and bile after an oral bolus dose of [14C]de-esterified neotame was much less than that recovered after an identical dose of [14C]neotame. These data suggest that a proportion of the neotame dose was absorbed intact and subsequently hydrolysed to de-esterified neotame (Hawkins et al., 1996b).
The presence of the neotame metabolite found in human urine, 3,3-dimethyl-butanoyl-L-carnitine, was investigated in rats. 3,3-Dimethyl-butanoyl-L-carnitine is the carnitine conjugate of the metabolite 3,3-dimethylbutyric acid (also referred to as 3,3-dimethylbutanoic acid) that has been identified in human studies. In order to investigate whether this metabolite occurs in rats, groups of 20 rats (10 male, 10 female) were given two doses of [14C]neotame of 15 mg/kg bw per dose, by oral gavage, separated by an interval of approximately 8 h. Recoveries of radiolabel in the urine of male and female rats were comparable to those reported in previous studies. The metabolite, 3,3-dimethyl-butanoyl-L-carnitine, was detected using thin-layer chromatography (TLC) in all urine specimens from females and was isolated from urine samples from females at 0–24 h. This metabolite was not detected in urine samples from males (Kirkpatrick et al., 1998a).
Rabbits
The plasma concentrations of neotame and de-esterified neotame were examined on days 6, 13 and 19 of gestation in a study of teratology in New Zealand white rabbits given doses of up to 500 mg/kg bw per day. Details of the study are described in section 2.2.5 (b).
The Cmax and AUC for neotame and the Cmax for de-esterified neotame increased with increasing dose; however, the increases were less than proportional, as indicated by statistically significant differences in the dose-adjusted Cmax and AUC values. The AUC for de-esterified neotame increased proportionally to dose. There was no evidence for accumulation of neotame or de-esterified neotame in plasma (Willoughby, 1996b).
Dogs
The plasma concentrations of neotame and de-esterified neotame were examined in a 13-week study in dogs fed neotame at doses of up to 1200 mg/kg bw per day, and in a 1-year study in dogs fed neotame at doses of up to 800 mg/kg bw per day. Details of these studies are described in section 2.2.2 and 2.2.3, respectively.
AUC0–24 and Cmax increased with dose for both neotame and de-esterified neotame at lower doses; however, the increases were more than proportional to the increase in dose at the higher doses, particularly at 1200 mg/kg bw per day in the 13-week study and 800 mg/kg bw per day in the 1-year study. There was no evidence for accumulation of neotame or de-esterified neotame in the plasma at any dose in either study (Thomford & Saunders, 1995; Thomford & Carter, 1997b).
In a study of metabolism in dogs, groups of beagles (three of each sex per dose) were given [14C]neotame (purity, >98%) as a single dose, either by gavage at a dose of 15 or 120 mg/kg bw, or intravenously at a dose of 15 mg/kg bw. Urine and faeces were collected and frozen 72 h after oral dosing. A cage wash was done at 72 h. A 10 ml blood sample was taken from the jugular vein before dosing and at intervals for 24 h after dosing. No postmortem was performed, and carcasses were discarded at the end of the trial. The intravenous dose was infused into the cephalic vein over 2–3 min. Sampling was carried out as for oral administration, with an additional blood sample being collected at the end of the infusion period. Radiolabel was measured and metabolites identified and quantified.
Radiolabel was detected in plasma shortly after oral administration and was rapidly excreted after oral or intravenous administration. Approximately 95% of the radiolabel was recovered in the urine and faeces. There were no differences between males and females in absorption or excretion. In general, >80% of the radiolabel was excreted in the urine and faeces within 48 h after dosing. After oral administration, approximately 13–20% of the radiolabel was excreted in the urine (approximately 90–97% of this being eliminated within 24 h), and the remainder was excreted in the faeces. After intravenous administration, approximately 40–43% of the radiolabel was excreted in the urine and the remainder in the faeces, suggesting excretion in the bile and/or gastrointestinal secretion. The mean plasma Cmax for radiolabel and for neotame occurred within 0.5 h after either oral or intravenous administration in both male and female dogs. Cmax and AUC0–24 appeared to increase more than proportionally compared with the increase in oral dose. Possible reasons for this observation include interindividual variability or increases in the volume and concentration of neotame administered in the gavage vehicle. The mean whole-body clearance of neotame after the intravenous dose was approximately 32 and 26 ml/min per kg bw in males and females, respectively. This suggests extra-hepatic clearance of neotame since these values are in excess of that for hepatic blood flow in the beagle dog. The mean volume of distribution of neotame was approximately 1 l/kg bw, a value that is in excess of the average total volume of body water in beagle dogs and indicates some distribution to tissues, without extensive tissue binding. Approximately 1–6% of orally administered neotame and 7–8% of intravenously injected neotame was excreted unchanged in the urine. Unchanged neotame was not detected in the faeces after any dose or route of administration.
The major metabolite in urine was de-esterified neotame, which represented approximately 6–9% of orally administered neotame and 19–20% of intravenously injected neotame. At least two other metabolites were identified in the urine at concentrations of >1% of the administered dose: one with similar chromatographic properties to those of a beta-glucuronide of 3,3-dimethylbutanoic acid (approximately 5% of the administered dose) and the other identified as N-(3,3-dimethylbutyl)-L-aspartic acid (approximately 0.4–2% of the administered dose). De-esterified neotame was the major component of faeces, representing approximately 62–74% of orally administered neotame and approximately 42–43% of intravenously administered neotame. In the faeces, N-(3,3-dimethylbutyl)-L-aspartic acid accounted for approximately 0.6–2% of the orally administered dose and approximately 2–4% of the intravenously injected dose (Kirkpatrick et al., 1997b).
Humans
In a study of tolerance, healthy men (mean age ± standard deviation (SD), 28 ±6 years) were each given a single dose of neotame in solution at 0.10, 0.25 or 0.50 mg/kg bw (n =7, 6, and 6 men per dose, respectively), after an overnight fast. Eighteen men completed the study. Clinical evaluations and laboratory tests were done immediately before dosing and approximately 48 h after dosing. Complete study details are provided in section 2.3.1.
The pharmacokinetic profiles of neotame were approximately linear across the three doses tested for Cmax and AUC. Absorption of neotame was rapid, with maximum plasma concentration attained approximately 0.5 h after administration. Neotame was rapidly eliminated with a half-life (t1/2) ranging from 0.61 h to 0.75 h. The short t1/2 was supported by the rapid disappearance of neotame from the urine (neotame was not detectable after 8 h). Approximately 1% of the administered dose was detected unchanged in the urine. Neotame was extensively metabolized to de-esterified neotame; approximately 14% of the administered dose was recovered in the urine as de-esterified neotame. The pharmacokinetics (AUC and Cmax) of de-esterified neotame were linear across the range of doses used in this study. The calculated t1/2 of de-esterified neotame in plasma was approximately 2 h. Small amounts of de-esterified neotame were measured in urine during the 24–48 h after treatment, representing about 1.5% of the total amount eliminated in the urine (Kisicki et al., 1997).
In a study of the pharmacokinetics of neotame, [14C]neotame in an aqueous solution was administered as a single oral dose of 18.75 mg (approximately 0.25 mg/kg bw) to seven healthy men (median age, 23 years). After an overnight fast, the men ingested [14C]neotame (purity, 98.6–99.8%) in 60 ml of water, followed immediately by 180 ml of water to rinse the dosing vessel, thus representing a total volume of 240 ml. Blood was taken before dosing and at predetermined times up to 168 h after dosing. All urine and faeces were collected for 12 h before dosing and at additional predetermined time-points up to 168 h after dosing. Blood, plasma, urine, and faeces were analysed for radiolabel by liquid scintillation counting, either directly or after combustion of the samples. Plasma and urine were analysed for neotame and de-esterified neotame by LC/MS/MS. Radioactive metabolites were separated from urine and faeces by extraction and chromatography.
The mean recovery of radiolabel in the urine and faeces of the seven men was 98.0%, most of which was recovered within 72 h. Means of 34.3% and 63.7% of the radiolabel were eliminated in the urine and faeces, respectively, and excretion was essentially complete by 96 h after dosing. Pharmacokinetic analysis of the data for plasma confirmed that neotame was rapidly absorbed (mean Tmax =0.4 h) and rapidly cleared from the body (mean t1/2 =0.6 h), mostly by conversion to de-esterified neotame. Mean concentrations of de-esterified neotame in plasma peaked at 1 h after dosing and were approximately 2.5-times greater than mean peak concentrations of neotame. Concentrations of de-esterified neotame declined with a mean t1/2 of 1.5 h.
Neotame and de-esterified neotame were detected in urine at 3.3% (0–8 h after treatment) and 23.8% (0–72 h after treatment) of the administered dose, respectively. Very small amounts of radioactive components other than neotame or de-esterified neotame were detected in urine. One minor component was detected in all men in urine only and accounted for a mean of 3.2% of the administered dose.
Unchanged neotame was not detected in faeces. The major radioactive component detected in faeces was de-esterified neotame (52.5% of the administered dose). N-(3,3-Dimethylbutyl)-L-aspartic acid was detected in faecal extracts and accounted for 4.9% of the administered dose (Holt & Kirkpatrick, 1997).
In a study to characterize and identify the unknown metabolite detected in the urine in the above pharmacokinetic study in humans, a non-blind study was carried out with neotame labelled with 14C in the C-1 position of the 3,3-dimethylbutyl moiety and with 13C in two of the methyl substituents of the tert-butyl function of the 3,3-dimethylbutyl moiety. [14C/13C]Neotame in solution was given to six healthy men (mean age, 31 years) in two oral doses of 37.5 mg (approximately 0.5 mg/kg bw per dose; a total dose of approximately 1 mg/kg bw), separated by an interval of 6 h. Urine and faeces were collected before dosing and at predetermined intervals for 120 h after the first dose.
The metabolite of interest was detected in urine from all men and represented 0.5–3.4% of the administered dose in the samples analysed. Mass spectral analysis showed that the isolate was enriched in 13C, which confirmed that the isolate was derived from neotame. Analysis of the fragmentation pattern suggested that the isolated metabolite was an acyl-ester of carnitine, 3,3-dimethyl-butanoyl-L-carnitine. Comparison of fragmentation patterns of the isolate with 3,3-dimethyl-butanoyl-L-carnitine synthesized as a reference confirmed the identity of this metabolite. Further confirmation was obtained by co-chromatography of the isolate with 3,3-dimethyl-butanoyl-L-carnitine, comparison of spectra and empirical formulae from fast atom bombardment mass spectrometry, and nuclear magnetic resonance spectrometry (Harry & Aikens, 1998).
In a study to assess the dose-related pharmacokinetics of neotame administered in solution, 12 healthy men (mean age ± SD, 41 ±8 years) were randomized in a non-blinded, three-way crossover study. Each man received a single dose of 0, 0.10, 0.25, or 0.50 mg/kg bw of neotame in a beverage solution after an overnight fast. The men were housed for the duration of the study (approximately 13 days), with a minimum washout period of 72 h between treatments.
Neotame was rapidly absorbed, metabolized to de-esterified neotame, and rapidly eliminated. Mean plasma half-lives were short, approximately 0.75 h for neotame and approximately 2.5 h for de-esterified neotame. The pharmacokinetics of neotame and de-esterified neotame were proportional to dose across the range of administered doses, as measured by AUC0–24 and Cmax. Neotame was rapidly converted to de-esterified neotame, with Cmax for de-esterified neotame occurring at approximately 1 h. De-esterified neotame was eliminated from the plasma of most men within 24 h (Weston et al., 1997).
The pharmacokinetics of both neotame and de-esterified neotame were examined in 12 healthy men (mean age ± SD, 34 ± 8.6 years) after repeated administration of neotame. Each man received neotame in solution in eight doses of approximately 0.25 mg/kg bw, administered hourly, representing a total dose of approximately 2 mg/kg bw. The men were housed at the clinical site for 7 days after dosing.
As in the studies of the administration of single doses, neotame was rapidly absorbed and de-esterified to N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine. Neotame was rapidly eliminated, with a plasma t1/2 of approximately 0.9 h. Steady-state concentration of neotame in plasma was achieved at 2 h after the first dose. Approximately 3% of the administered dose of neotame was excreted unchanged in the urine, and approximately 23% of the administered dose was recovered in the urine as de-esterified neotame. The elimination of de-esterified neotame from plasma was biphasic; approximately 93% was eliminated with a t1/2 of approximately 1.3 h, while the remaining 7% was eliminated with a terminal t1/2 of approximately 14 h (Kisicki et al., 1998a).
A comparison of the pharmacokinetics of neotame administered in solution and in capsules was conducted using 24 subjects (12 men and 12 women) randomized into two dosing regimens. The mean age ± SD for all 26 persons was 38.3 ± 10.7 years. All 26 persons were included in the safety evaluation while the 24 persons who completed the study were included in the pharmacokinetic evaluations.
There was no significant difference in relative bioavailability (AUC(0inf) and Cmax) between men and women after administration of neotame in solution. The relative bioavailability of neotame was greater in capsules than in solution in women only. The relative exposure to de-esterified neotame was greater from capsules than from solution for all subjects, however, there were no differences between the sexes. Exposure of subjects to neotame and de-esterified neotame was at least as great after administration in capsules as in solution. Thus, capsules were considered to be an appropriate means to administer neotame in clinical studies where it is desirable to blind subjects to treatment (Weston et al., 1998).
In a 2-week study of tolerance in humans, groups of 12 men and 12 women received placebo, or neotame at a dose of 0.5 or 1.5 mg neotame/kg bw per day in capsules as three divided doses over 2 weeks. Plasma concentrations of neotame and de-esterified neotame were analysed after overnight fasts at predetermined intervals. Details for the study are found in Section 2.3.2.
Plasma trough concentrations for neotame were below quantifiable limits for all subjects before morning dosing before treatment and 1, 2, 3, 6, 10, and 14 days after treatment. Plasma trough concentrations for de-esterified neotame were detectable after 1, 2, 3, 6, 10, and 14 days of treatment in most subjects receiving neotame. Steady-state concentrations of de-esterified neotame were achieved for both men and women within 72 h of the first dose of neotame (Kisicki et al., 1998b).
The stability of neotame and de-esterified neotame in simulated human gastric and intestinal fluids was investigated. Neotame or de-esterified neotame was mixed with simulated gastric or intestinal fluid at a single concentration (neotame, 50 mg/ml; de-esterified neotame, 25 mg/ml) and incubated at 37 °C for up to 120 min. Neotame was stable when incubated with simulated gastric fluid (with or without pepsin). Neotame was also stable in simulated intestinal fluid without pancreatin (approximately 2% degradation to de-esterified neotame). However, in simulated intestinal fluid containing pancreatin, neotame was almost completely hydrolysed to de-esterified neotame within 15 min at 37°C. De-esterified neotame was stable in simulated gastric and intestinal fluids (with or without enzymes) over the period of incubation (Kirkpatrick et al., 1998b).
The effect of neotame in the diet on the activity of rat hepatic enzymes was examined. Three groups of six male and six female Sprague-Dawley Crl : CD BR rats received diets containing neotame at a dose of 100, 300, or 1000 mg/kg bw per day for a period of 14 days. An additional two groups of six males and six females received untreated diet and acted as controls. One of these control groups, the positive control, was given sodium phenobarbital by gavage at a dose of 75 mg/kg bw per day on days 11–14 of the study. Animals were observed daily and body weights were measured weekly. At the end of the treatment period, the animals were killed and the liver fractionated into a microsomal and cytosolic fractions. The following enzyme activities and other parameters were measured to test the effect of neotame on specific cytochrome P450 isozymes: concentration of protein, concentration of cytochrome P450 and activities of 7-ethoxyresorufin O-de-ethylase (CYP1A), testosterone hydroxylase (CYP2B and CYP3A), lauric acid hydroxylase (CYP2E and CYP4A) and p-nitrophenol uridine diphosphate glucuronyl transferase (UDPGT, a phase II enzyme) in microsomal fractions, together with concentrations of protein and non-protein thiol in cytosolic fractions.
There was no difference in body weight between the treated groups. Body-weight gain was decreased at the highest dose. There was no neotame-related effect on liver weight (either absolute or relative), concentrations of non-protein thiol in the liver cytosol, hepatic microsomal or cytosolic protein, microsomal concentrations of cytochrome P450, or the activity of other cytochrome P450-related enzymes. In the positive control group of rats treated with phenobarbital, liver weight was increased, as were enzyme activities (Hall, 1997).
The binding of [14C]neotame or [14C]de-esterified neotame to human, dog and rat plasma proteins in vitro was evaluated. In addition, the binding of neotame and de-esterified neotame to human plasma albumin and alpha1-acid glycoprotein were evaluated.
In male Sprague-Dawley rats, the binding of de-esterified neotame to plasma proteins was 72–76%. Binding occurred very rapidly, with no evidence of an increase in the percentage bound with increasing time. There was a very slight decrease in the percentage of binding with increasing concentration. In beagle dogs, the binding of neotame and de-esterified neotame to plasma proteins was 74–89% and 50%, respectively. There was a notable decrease in the percentage of the neotame and de-esterified neotame bound with increasing concentration of neotame administered, and a slight increase in the percentage bound with increasing time of incubation.
The binding of neotame to human plasma proteins in vitro ranged from 94% to 98%. A slight increase in binding was observed with increased length of incubation, while a slight decrease in the percentage of the material bound was observed with increasing dose. The binding was predominantly to albumin and there was no evidence of saturation with increasing concentrations. The binding of neotame to alpha1-acid glycoprotein, 8–14%, was considered to be negligible. The binding of de-esterified neotame to human plasma proteins was 85–90%, with slight increases seen with increasing length of incubation. Approximately 30% of de-esterified neotame bound to albumin, while <10% bound to alpha1-acid glycoprotein. Overall, there was little evidence for saturation of plasma binding over the range of concentrations tested. Binding to plasma proteins was lower for de-esterified neotame than for neotame, in humans and dogs, in which neotame was largely bound to albumin (Kirkpatrick et al., 1997c).
No studies of the acute oral toxicity of neotame or de-esterified neotame (NC-00751) were conducted. The results of studies of the acute oral toxicity of the minor degradation products of neotame in rats are given in section 2.2.6 (c).
Mice
Two range-finding studies were conducted in mice. In the first study, groups of CD-1 mice (10 of each sex per group) were given diets containing neotame at a dose of 0, 10, 30, 100 or 300 mg/kg bw per day for 14 days. Animals were examined throughout the study period and body weight and food consumption monitored. Samples were taken for clinical pathology at day 15. A macroscopic examination of all animals was undertaken, as well as measurement of organ weights and microscopic examination of some tissues.
There were no abnormal clinical signs or changes in body weight or food consumption. There were no significant changes in clinical pathology parameters or in gross pathology or histopathology (Thomford, 1994a).
In the second range-finding study, groups of 10 male and 10 female mice were given diets containing neotame at a dose of 0, 500, 1000, 2000, 4000 or 8000 mg/kg bw per day for 14 days. Animals were examined in a similar way as in the first study, described above, however, no microscopic examination of tissues was undertaken.
There were no abnormal clinical signs or significant changes in body weight. Food consumption was decreased by 15–19% at 8000 mg/kg bw at certain times, but returned to control levels. In females, food consumption was decreased by 11% on days 8–15. Body-weight gain was significantly decreased in males at 8000 mg/kg bw, but not at 4000 mg/kg bw. Body-weight gain in females was significantly decreased at 500 and 2000 mg/kg, but no dose–response relationship was evident. There were no significant changes in clinical parameters or in gross pathology (Thomford, 1994b).
In a 13-week study, groups of 20 male and 20 female Crl: CD-1 (ICI) BR VAF Plus mice were fed neotame at a concentration of 0, 490–770 ppm, 4900–7400 ppm, 20 000–30 000 ppm, and 39 000–57 000 ppm in the diet, equivalent to a dose of 0, 100, 1000, 4000, or 8000 mg/kg bw per day, respectively. Animals in the control group received basal diet only. An additional satellite group of 20 rats of each sex per group for each dose was used for periodic sampling for pharmacokinetic analysis. Parameters studied included clinical signs, physical examinations, body weights, food consumption, test article intake, haematology, clinical chemistry, and organ weights. Complete gross and microscopic examinations were conducted on specified organs and tissues for each animal.
There were no deaths or treatment-related clinical signs of toxicity during the study. There were no significant changes in body weight at any dose in either males or females at the end of 13 weeks. However, body-weight gain was lower relative to controls for males at 4000 and 8000 mg/kg bw per day (88% and 85% of control values, respectively) and for females at 8000 mg/kg bw per day (93% of control values). This lower body-weight gain was associated with decreased food consumption in males on day 1, with food consumption at 4000 and 8000 mg/kg bw per day unable to be assessed owing to excessive food scattering by both sexes. It was not possible to measure food consumption accurately during week 1 at the higher doses because of the high incidence of spillage, which is indicative of reduced diet palatability. The incidence of excessive food spillage at later time-points was similar to that for controls.
There were no treatment-related effects on clinical chemistry parameters. In females, haematology revealed small significant decreases in mean corpuscular volume at 4000 and 8000 mg/kg bw per day, but these were within the historical reference range and not considered to be toxicologically significant. There were slight but significant increases in absolute liver weights at 8000 mg/kg bw per day in both sexes and slight but significant increases in relative liver weights in females at 4000 and 8000 mg/kg bw per day. These changes were generally within the ranges for historical controls and were not accompanied by microscopic changes. There were no treatment-related gross pathological changes. Histopathological examination revealed a slight increase in chronic inflammation of the kidney in both sexes, but no dose–response was evident. The NOEL was 1000 mg/kg bw per day on the basis of changes in relative liver weight (Thomford & Carter, 1995).
Rats
Two range-finding studies were conducted in rats. In the first study, groups of 10 male and 10 female Sprague-Dawley Crl : CD (SD) BR VAF Plus rats were fed diets containing neotame at a dose of 0, 10, 30, 100 or 300 mg/kg bw per day for 14 days. Animals were examined throughout the study period and body weight and food consumption were monitored. Samples were taken for clinical pathology at day 16. A macroscopic examination of all animals was undertaken, as well as measurement of organ weights and a microscopic examination of some tissues.
There were no abnormal clinical signs or changes in body weight or food consumption. Body-weight gain was reduced in males and females at >30 mg/kg bw. Food consumption was markedly decreased on days 1–3 in males and females but returned to normal during the study. There were no significant changes in clinical pathology parameters except for some sporadic changes in clinical chemistry parameters in females. Gross pathological changes were limited to dark foci in the stomach of males in several animals, which was confirmed as focal mucosal congestion upon histopathological examination. Pathology of females was normal (Thomford, 1994c).
In a second range-finding study, groups of 10 male and 10 female Crl : CD(SD)BR VAF Plus rats were given diets containing neotame at concentrations providing a dose of 0, 200, 600, 2000, 4000 or 6000 mg/kg bw per day for 14 days (males) or 15 days (females). Animals were examined in the same way as in the first study, however, no microscopic examination of tissues was undertaken.
There was an increased incidence of red nasal discharge in males at >4000 mg/kg bw and in females at >2000 mg/kg bw. Food consumption was reduced on days 1–3 in males at >2000 mg/kg bw and in females at >600 mg/kg bw. Body weight was reduced on day 3 in males at >4000 mg/kg bw and in females at 6000 mg/kg bw, but not at other times. Body-weight gain was reduced in males and females at 2000 mg/kg bw. Clinical chemical and haematology parameters were not significantly different to controls. There were no treatment-related changes in gross pathology in males or females (Thomford, 1994d).
In a 13-week study, groups of 20 male and 20 female Sprague-Dawley Crl: CD(SD)BR VAF Plus rats were given diets containing neotame at concentrations of approximately 0, 660–1800 ppm, 2000–5300 ppm, 6600–17 800 ppm, and 19 200–50 000 ppm throughout the study, equivalent to a dose of 0, 100, 300, 1000 or 3000 mg/kg bw per day, respectively, for 13 weeks. Control animals received basal diet only. An additional five rats of each sex were included in the control group and the groups receiving a dose of 1000 or 3000 mg/kg bw per day in order to examine reversibility in a 4-week period that commenced after completion of 13 weeks of dosing. Parameters studied included clinical signs, physical examinations, body weight, food/water consumption, test article intake, ophthalmology, haematology, clinical chemistry, urine analysis, and organ weights. Complete gross and microscopic examinations were performed on specified organs and tissues for each animal.
No treatment-related mortality occurred, and no changes were observed in appearance, behaviour, physical condition or clinical signs. No treatment-related ophthalmological changes were seen.
Food consumption was decreased on day 1 in males at 1000 mg/kg bw per day (33%, p <0.001) and during the first week at 3000 mg/kg bw per day (decreases up to 70%, p <0.001, with an average decrease for the first week of 11.2%, p <0.01). In males at 3000 mg/kg bw per day, food consumption was also decreased from week 7 until the end of the study, with decreases of 8–12%, p <0.01 or p <0.001. In females, food consumption was decreased on the first day in all treatment groups, by 20% at 100 mg/kg bw per day (p <0.05) to 80% at 3000 mg/kg bw per day (p <0.001). Food consumption on day 2 was decreased by 26% at 3000 mg/kg bw per day (p <0.001). Decreased food consumption was also seen in week 6 in females at 300 mg/kg bw per day, but was not otherwise during the study. No differences were seen between groups during the 4-week reversibility study. The presence of neotame in the diet had an immediate effect on food consumption at the start of the study in both males and females at all doses, except at the lowest dose in males. Mean food consumption in treated groups increased after day 1 as animals partially adjusted to diet containing neotame.
Body weights in males at 3000 mg/kg bw per day were 10–14% lower than those of controls throughout the study (p <0.001). In females, body weight at 3000 mg/kg bw per day was lower than that of controls until day 14, although the difference was <10% on days 7 and 14. The overall body-weight gain in males at 3000 mg/kg bw per day was 21% lower than that of controls for the 13-week study, while in females at 3000 mg/kg bw per day it was 8% lower than that of controls. During the reversibility phase, the difference between the control animals and males at 3000 mg/kg bw per day decreased, but was still 11% after 4 weeks of control diet.
Slight changes in some haematology and blood chemistry parameters were reported. At week 13, erythrocyte count was increased in females only at the highest dose; mean corpuscular volume was decreased in males only at 100, 1000 and 3000 mg/kg bw per day, with no dose-response relationship, and was unlikely to be of biological significance. Mean concentration of cholesterol was significantly decreased in females at 1000 and 3000 mg/kg bw per day at week 6, and in both males and females at 1000 and 3000 mg/kg bw per day at week 13, although individual values were generally within the ranges of the respective historical controls. These differences were not seen after the reversibility period, and may be related to body-weight changes. At week 13, mean activity of plasma alkaline phosphatase was slightly but statistically higher in males at the two higher doses (116 and 117 IU/l, respectively, versus 97 IU/l in controls) and in females at the highest dose (82 IU/l versus 60 IU/l in controls) than in rats in the control group. The mean activities of alkaline phosphatase were within the range of historical controls and there were no changes in clinical pathology parameters or microscopic findings that indicated target organ toxicity.
The absolute organ weights of adrenals, heart, kidneys, liver, prostate, spleen, and thymus were decreased in males at 3000 mg/kg bw per day compared with controls. There were no differences in organ : body weight ratios, with the exception of spleen weight being lower and ratios for brain and testes being higher in males at 3000 mg/kg bw per day. These results are consistent with the changes being secondary to lower body weights in the group of males receiving the highest dose. After the 4-week period of reversibility, the organ weights of all treated animals were not significantly different to those of controls, with the exception of the prostate.
The only microscopic finding was an increased incidence of slight corticomedullary mineralization in the kidneys of females at 1000 (not statistically significant) and 3000 mg/kg bw per day. There was no microscopic evidence of renal tubular damage or changes in clinical chemistry or urine analysis parameters in females that would have indicated toxicity or altered renal function.
The NOEL was 300 mg/kg bw per day on the basis of changes in clinical chemistry parameters (Mitchell & Brown, 1995).
Dogs
In a 13-week study, groups of male and female beagle dogs were given diets containing neotame at concentrations providing an initial dose of 0, 60, 200, 600, or 2000 mg/kg bw per day; These doses were equivalent to concentrations of approximately 0, 1400–1900 ppm, 4800–6900 ppm, 14 400–17 800 ppm, or 43 000–52 000 ppm in the diet. Control animals received basal diet only. There were six animals of each sex per group in the control group and in the groups receiving the two higher doses, and four animals of each sex per group in the other two groups. Two dogs of each sex from the control group and from the groups receiving the two higher doses were used for the 4-week reversibility phase of the study that commenced after the 13-week period of treatment. Parameters monitored included clinical signs, body weights, food consumption, and physical, neurological, electrocardiographic, and ophthalmologic examinations. Haematology, clinical chemistry, and urine analysis parameters and organ weights were measured. Complete gross and microscopic examinations were performed on specified organs and tissues in each animal.
There were no deaths at any dose and no test article-related clinical observations at 60 or 200 mg/kg bw per day. At the two highest doses, faeces were discoloured (grey and white). This observation was not associated with any effects on the gastrointestinal tract and was likely to be due to the presence of unabsorbed neotame.
There was an immediate and marked decrease in food consumption and body weight at 2000 mg/kg bw per day during the first 2 weeks of the study. It was necessary to decrease the concentration of neotame given to this group from approximately 5% in the diet (43 000–52 000 ppm) to approximately 3.5% (28 000–37 000 ppm), to provide an intake of 1200 mg/kg bw per day. At the highest dose, food intake decreased until week 10 by >10% in comparison with controls. At 600 mg/kg bw per day, food consumption in females was decreased during the first 2 weeks, although only at week 1 was the decrease significant (p <0.05, 20% and 11% for week 1 and 2 respectively). No other notable decreases in food consumption were seen throughout the study.
Body weight was statistically significantly decreased (p <0.05) in males at 1200/2000 mg/kg bw per day at week 2 (15% compared with controls) and from week 5 until the end of the study, with decreases of up to 19% compared with controls. During the reversibility phase, the males returned to the same body weight as the dogs in the control group by the end of week 2. In females at 1200/ 2000 mg/kg bw per day, body weight was statistically significantly (p <0.05) decreased by 17% at week 3. For the rest of the study, decreases in body weight were between 15% and 18%, compared with controls. No statistically significant decreases, or decreases of >10% were seen in any other groups. The cumulative body-weight gain at 1200/2000 mg/kg bw per day was statistically significantly lower than that of controls, with decreased body-weight gains of 57% in males and 75% in females, compared with controls. Statistically significant decreases were also seen in females at 600 mg/kg bw per day, with body-weight gains of less than half those seen in controls at up to week 5. The cumulative body-weight gain in females at 600 mg/kg bw per day was decreased by 24% compared with that of controls throughout the study, and in males was decreased by more than 15% compared with that of controls throughout the study. There were no effects on food consumption, body weight, or body-weight gain at 60 and 200 mg/kg bw per day.
Haematological examinations at week 2 revealed there was a decrease in activated partial thromboplastin time (p <0.01) in males at 600 mg/kg bw per day and an increase (p <0.05) in females at 1200/2000 mg/kg bw per day. At week 13, the erythrocyte count was decreased in both sexes at 1200/2000 mg/kg bw per day (p <0.01). Haemoglobin and erythrocyte volume fraction were also statistically significantly decreased, and mean corpuscular volume and mean corpuscular haemoglobin were increased in females. The changes seen at week 13 at the highest dose were considered to be related to treatment.
Examination of clinical chemistry parameters at week 2 revealed a significant increase in triglyceride concentrations (p <0.01) in males at 1200/2000 mg/kg bw per day. Glucose concentrations were decreased in a dose-related manner in week 2, which was probably related to decreased food consumption. In females at week 2, activities of aspartate aminotransferase and alanine aminotransferase were significantly decreased at 1200/2000 mg/kg bw per day, and alanine aminotransferase was also significantly decreased at 600 mg/kg bw per day. At week 13, but not at week 6, activity of alanine aminotransferase was significantly decreased in females at 600 mg/kg bw per day. Alkaline phosphatase activity was significantly increased at week 6 in males at 1200/2000 mg/kg bw per day (p <0.05). At week 13, alkaline phosphatase activity was significantly increased in males at 1200 mg/kg bw per day (p <0.01) and females at 600 and 1200 mg/kg bw per day (p <0.001). The small change in alkaline phosphatase activity in 3/4 animals at 200 mg/kg bw per day was not considered to be toxicologically significant. These changes in alkaline phosphatase activity were not accompanied by microscopic changes indicative of cholestasis or hepatotoxicity. The alkaline phosphatase activity returned to control levels during the 4-week reversibility phase of the study.
The absolute weight of the liver was increased by 18% in males in week 12 at 1200/2000 mg/kg bw per day, and by 12–23% in females at 600 mg/kg bw per day. There was a significant increase in relative liver weight (to body weight) at 1200/2000 mg/kg bw per day in males (49% more than controls) and in females at doses of >600 mg/kg bw per day (27–35%). Absolute spleen weight was decreased in males in all groups (15–43%), and spleen weights were increased in females at 200 and 1200/2000 mg/kg bw per day only, although none of these changes were statistically significant.
There were no significant abnormalities found on macroscopic examination. Histopathology revealed a higher incidence of minimal to moderate liver glycogen in both sexes at 600 and 1200 mg/kg bw per day.
The NOEL was 200 mg/kg bw per day on the basis of changes in alkaline phosphatase activity (Thomford & Saunders, 1995).
Mice
In a study of carcinogenicity in mice, groups of 70 male and 70 female Crl : CD (ICR) VAF Plus mice were fed diets containing neotame at concentrations providing a dose of 0, 50, 400, 2000, or 4000 mg/kg bw per day for 2 years. These doses were equivalent to concentrations of approximately 0, 230–420, 1800–3200, 9200–16 000, or 18 400–31 800 ppm in the diet, respectively. Control groups consisting of 140 mice of each sex received basal diet only. In addition, 35 mice of each sex per dose were designated "satellite" animals. Blood samples from satellite animals were used to determine plasma concentrations of neotame and de-esterified neotame at intervals up to week 52. All groups were observed twice daily for mortality or signs of moribundity. Parameters measured included clinical signs, physical examinations including palpation for masses, body weight, food consumption, test article intake, haematology, and organ weights. Complete gross and microscopic examinations were performed on specified organs and tissues in each animal.
There were no treatment-related effects on mortality, nor were there any clinical signs of toxicity during daily and periodic physical examinations.
Food consumption in males was significantly decreased, by up to 9% (p <0.01), at doses of >400 mg/kg bw per day intermittently throughout the study. In females, sporadic significant decreases in food consumption of up to 7% were seen in all treated groups. No decreases in food consumption were >10% and, although statistically significant, the decreases were not considered of biological significance and were probably related to decreased palatability of the diet.
Body weight in males was consistently decreased, by up to 7% (p <0.001), at doses of >400 mg/kg bw per day. In females, decreases of up to 10% were seen at 2000 mg/kg bw per day, and decreases of up to 8% (p <0.001) were seen at 400 mg/kg bw per day. Cumulative body-weight gain was decreased in males at doses of >400 mg/kg bw per day, with decreases of 18–20% at 400 mg/kg bw per day during weeks 1–77. In females, decreases in cumulative body-weight gain of 15% were seen at 50 mg/kg bw per day for weeks 1–77, with decreases of 20% seen at 400 mg/kg bw per day for the same period.
There were no treatment-related changes in haematology parameters. No clinical chemistry or urine analysis was conducted.
There were no macroscopic findings at necropsy that were considered to be related to treatment. There were no treatment-related effects on absolute organ weights or organ weight ratios, except for those that were secondary to lower body weights. There were no non-neoplastic findings that were considered to be related to treatment. The incidences of non-neoplastic lesions were within the expected range for ageing CD-1 mice.
There were no treatment-related effects on the incidence or onset of neoplasms in males or females. The types and incidences of neoplasms observed in all groups were typical of those expected in this strain and at this age. The incidence of hepatocellular adenomas in males at 4000 mg/kg bw per day was higher than that of controls, but the difference was not significant (p <0.01 for common tumours) by Peto trend analysis or pair-wise comparison. The incidence of hepatocellular carcinomas at 4000 mg/kg bw per day was no different than that in controls, and when the incidences of adenomas and carcinomas were combined, there was no statistical significance. There was no evidence of pre-neoplastic changes. In females, the incidence of hepatocellular adenomas and carcinomas was comparable between the groups of treated and control mice. A higher incidence of bronchiolar-alveolar carcinoma was observed in females at 4000 mg/kg bw per day when compared with controls, but this difference was not statistically significant. There was no positive trend with dose, and there also was no statistically significant difference when the incidences of adenomas and carcinomas were combined. Moreover, there were no non-neoplastic and pre-neoplastic changes in lung tissue. The NOEL was 4000 mg/kg bw per day, on the basis of the absence of target organ toxicity or carcinogenicity at the highest dose tested (Thomford & Carter, 1997a).
Rats
A 1-year study of toxicity in rats exposed in utero was conducted. In the in utero phase of the study, groups of F0 Sprague-Dawley rats strain Crl CBR VAF/Plus (25 of each sex per dose) were fed diets containing neotame at concentrations providing a dose of 0, 10, 30, 100, 300 or 1000 mg/kg bw per day. Control groups received basal diet only. F0 male and female groups were fed test diets for 4 weeks before and throughout mating. Females of the F0 generation were treated throughout gestation and lactation up to weaning of litters at day 21 after littering. The concentration of neotame in the diets of dams at the highest dose was reduced to provide a dose of 300 mg/kg bw per day from days 14–21 after littering to minimize differences in body weights of the offspring at the time that they began to consume solid food.
In the 1-year phase of the study, groups of rats (20 of each sex per dose) from the F1 generation were fed neotame in the diet at concentrations providing a dose of 0, 10, 30, 100, 300 or 1000 mg/kg bw per day for 52 weeks. These doses were equivalent to concentrations of approximately 0, 50–260 ppm, 150–730 ppm, 470–2300 ppm, 1500–7100 ppm, or 5100–23 000 ppm in the diet. Control groups received basal diet only. An additional 10 animals of each sex per group were assigned to the groups receiving a dose of 0, 100, 300, and 1000 mg/kg bw per day for a 4-week reversibility period after 52 weeks of dosing. Satellite groups consisted of six rats of each sex that were given a dose of 0, 10, or 30 mg/kg bw per day and 24 rats of each sex that were given a dose of 100, 300, or 1000 mg/kg bw per day. Blood was collected from satellite animals at specified intervals in order to quantitate plasma concentrations of neotame and de-esterified neotame. Parameters studied included clinical signs, physical examinations, body weight, food consumption, food conversion efficiency, test article intake, ophthalmology, haematology, clinical chemistry, urine analysis, and organ weights. Complete gross and histopathological examinations were performed on specified organs and tissues for each animal.
There was no treatment-related increase in mortality and no clinical signs of toxicity, with the exception of brown staining on the muzzle, possibly related to the adherence of brown diet in males at 1000 mg/kg bw per day.
There was no treatment-related effect on birth weight during the in utero phase of the study. At the start of the 1-year study of toxicity, the body weights of males and females at 100 mg/kg bw per day were 13% and 15% lower than those of controls, respectively. No differences were noted between the other groups. In females at 100 mg/kg bw per day, the difference in body weight compared with controls had decreased to 7% by the end of week 2. Other sporadic differences in body weight of males in treated groups and controls were noted at 13 weeks at 100 and 300 mg/kg bw per day and at 52 weeks at 100 mg/kg bw per day, but there was no dose-related effect. In females, body weights were decreased at the higher doses in the second half of the study (weeks 26–52). The decrease in body weight reached statistical significance in females at the two higher doses after week 48. Overall body-weight gain was significantly decreased compared with controls in females between weeks 0–52 at 300 and 1000 mg/kg bw per day. Body-weight gain was lower, but not significantly, at 100 mg/kg bw per day. In males, there was no treatment-related effect on body-weight gain.
There were no statistically significant sex-specific differences with regard to cumulative adjusted food consumption values in weeks 1–42, however, food consumption in males at 1000 mg/kg bw per day was decreased in weeks 10 and 11, and there were intermittent decreases in food consumption throughout the study in females at >100 mg/kg bw per day. At week 28, decreases were 11.7%, 11.1% and 10.6% at 100, 300 and 1000 mg/kg bw per day respectively. There were no significant decreases in food consumption between weeks 0–13 in females.
Although there was not a clear dose–response relationship, the decrease in food consumption in females at >100 mg/kg bw per day was considered to be related to treatment. This correlated with the observed decreases in body-weight gain in females at the higher doses in the latter part of the study. There was no treatment-related effect on food conversion efficiency during the active growth period (weeks 1–13) in either males or females.
Ophthalmoscopic examination revealed the presence of small, light grey, translucent spherical bodies in the centre of the lens. This finding, which was particularly apparent in male rats, was termed nuclear sclerosis. However, there was no real dose–response relationship, the incidence was comparable to that in controls in weeks 38 and 51, and the effect was reversible (in the 4-week reversibility phase). The reporting ophthalmologist did not consider this finding to be of any clinical significance or to be comparable with the clinical lesion of nuclear sclerosis reported in dogs or in humans. Sporadic changes in haematology parameters, clinical chemistry parameters and urine analysis parameters were observed but these were not considered to be related to treatment. There were no significant treatment-related changes in gross pathology or in histopathology.
The NOEL was 1000 mg/kg bw per day on the basis of the absence of target organ toxicity at the highest dose (Mitchell & Brown, 1997a).
A 2-year study of carcinogenicity was conducted in rats exposed in utero. In the in utero phase of the study, groups of F0 Sprague-Dawley rats of strain Crl CBR VAF/Plus (25 of each sex per dose) were fed diets containing neotame at concentrations providing a dose of 0, 50, 500, or 1000 mg/kg bw per day. Control groups, consisting of 170 rats of each sex, received basal diet only. F0 male and female groups were given test diets for 4 weeks before pairing and throughout pairing. Treatment of females of the F0 generation continued throughout gestation and lactation until weaning at day 21 after littering. Concentrations of neotame in the diet for dams in groups receiving the two highest doses were reduced to provide a dose of 300 mg/kg bw per day from days 14–21 after littering in order to minimize differences in body weights of the offspring at the time at which they begin to consume solid food.
In the carcinogenicity phase of the study, groups of approximately 75 males and 75 females of the F1 generation were fed diets containing neotame at concentrations providing a dose of 0, 50, 500, or 1000 mg/kg bw per day for 104 weeks. These doses were equivalent to concentrations of approximately 0, 240–1300 ppm, 2400–12 500 ppm, or 4800–25 000 ppm in the diet. Controls (147 rats of each sex) received basal diet only. An additional 12 animals of each sex per group were assigned to the groups receiving 0, 50, 500, or 1000 mg/kg bw per day to form satellite groups for collection of blood at specified intervals in order to quantitate plasma concentrations of neotame and de-esterified neotame and to assess water consumption and clinical pathology parameters (haematology, clinical chemistry, and urine analysis). Parameters studied included clinical signs, physical examinations including palpation for masses, body weight, food and water consumption, food conversion efficiency, test article intake, clinical pathology, and organ weights. Complete gross and microscopic examinations were performed on specified organs and tissues in each animal.
There were no treatment-related effects on moribundity and no changes in appearance, behaviour, palpable masses, food conversion efficiency or water consumption. The only test article-related effect observed during physical examinations was brown staining on the muzzle in males at 500 and 1000 mg/kg bw per day, probably the result of the brown diet adhering to the muzzle.
Treatment-related decreases in body weight of >10% were seen in all treated groups in both sexes, from week 11 in females and week 5 in males at >500 mg/kg bw per day, and from week 18 in females and week 22 in males at 50 mg/kg bw per day. Significant decreases in body-weight gain were seen in all treated groups at all doses. Food consumption was slightly lower in all treated groups.
There were sporadic changes in haematology parameters and in clinical chemistry parameters but these were not considered to be related to treatment.
In males at a dose of 1000 mg/kg bw per day, absolute adrenal weight was decreased by 27% compared with controls (p <0.01). The weight of the heart was decreased in all groups of treated males, from 12% (at 50 mg/kg bw per day, p <0.05) to 17% (at 1000 mg/kg bw per day, p <0.001). No other changes in absolute organ weight were seen in males. In females, the absolute weight of the uterus was higher in all treatment groups than that of controls, with increases of 14% at 50 and 500 mg/kg bw per day, and of 34% at 1000 mg/kg bw per day. None of these increases were statistically significant, and the relationship to treatment is not established. In males, adrenal weight relative to brain weight was significantly decreased (27%, p <0.01), and in females the weight of the uterus relative to body weight was increased at 500 and 1000 mg/kg bw per day (38–46%, p <0.05). These changes were considered secondary to lower body weights.
There were no treatment-related macroscopic effects seen at necropsy in either males or females. There were no treatment-related effects on the incidence or onset of neoplasms in either males or females. A range of tumours were seen, consistent with those expected in ageing Sprague-Dawley rats.
There were no treatment-related non-neoplastic findings. Other observations seen in a few animals at the highest dose included necrosis of the femoral marrow, thymic haemorrhage, papillary cysts in the kidney, erosion of the glandular stomach in males, and cystic follicular hyperplasia of the thyroid in females. These differences were considered to be normal variation and were not related to treatment with the test article.
There was no evidence for target organ toxicity or carcinogenicity in rats after exposure in utero and administration of diets containing neotame at a dose of up to 1000 mg/kg bw per day for 104 weeks. The NOEL was 1000 mg/kg bw per day on the basis of an absence of target organ toxicity or carcinogenicity at the highest dose (Mitchell & Brown, 1997b).
Dogs
In a 1-year study of toxicity, groups of beagle dogs (four to six of each sex per dose) were fed diets containing neotame at concentrations providing a dose of 0, 20, 60, 200, or 800 mg/kg bw per day for 52 weeks, followed by a 4-week recovery period, when basal diet only was given. The doses were equivalent to concentrations of approximately 0, 460–810 ppm, 1400–2500 ppm, 4600–8400 ppm, or 18 600–30 100 ppm in the diet. Animals in the control group received basal diet only. The control group and the groups receiving the two higher doses consisted of six dogs of each sex per group, while the other two groups consisted of four dogs of each sex per group. The additional two dogs of each sex used in the control group and the groups receiving the two higher doses were used for the reversibility phase of the study. Parameters studied included clinical signs, body weights, food consumption, physical, neurological, electrocardiographic, and ophthalmological examinations, haematology, clinical chemistry, and urine analysis parameters and organ weights. Complete gross and microscopic examinations were performed on specified organs and tissues in each animal. Alkaline phosphatase isozyme analysis was done on serum samples from the control group and the group receiving the higher doses.
There were no deaths during the study and no treatment-related clinical observations, apart from discoloured faeces at 800 mg/kg bw per day, which may be due to the presence of unabsorbed neotame and its major metabolite. This discoloration disappeared within 24 h after the last dosing in animals in the reversal phase of the study. There were no treatment related changes in heart rate, respiratory rate or body temperature. There were no abnormal findings on the neurological examination, ophthalmological examination or measurement of ECGs.
Body weights in males and females at 800 mg/kg bw per day were approximately 10% lower than those of controls from week 3 in males or week 6 in females until the end of the study. The body weight for both males and females at other doses were no more than 10% lower than those of controls at any point in the study, and none were statistically significantly different from those of controls.
Cumulative body-weight gain up to week 8 was significantly lower than that in controls for males at 800 mg/kg bw per day. At other doses, there was a decreased cumulative body-weight gain of more than 10% (not statistically significant) in males. No significant differences in cumulative body-weight gain were seen in females at any time during the study. Food consumption in males at 800 mg/kg bw per day was 19% lower than that for controls for weeks 1 and 2, respectively (p <0.05). In females at 800 mg/kg bw per day, food consumption was 12–23% lower than that of controls for weeks 1–3 (not significant). These changes were likely to be due to decreased palatability of the diet resulting from the addition of neotame.
There were no treatment-related changes in haematological parameters. The only change in clinical chemistry parameters was an increase in alkaline phosphatase activity throughout the study in both sexes at 800 mg/kg bw per day (p <0.001). These increases were not seen at lower doses, and returned to normal levels at the end of the reversal phase. Isoenzyme analysis indicated that the alkaline phosphatase was of hepatic origin, not from bone, and was not the form that is induced by glucocorticoids. There was no effect of neotame on other clinical chemistry parameters indicative of cholestasis or hepatotoxicity, such as liver enzymes. No changes were seen in urine analysis throughout the study.
No abnormalities were detected on macroscopic examination. There were no statistically significant treatment-related changes in absolute or relative organ weights, with the exception of increased liver weight relative to brain weight in females at 200 and 800 mg/kg bw per day (p <0.05). However, absolute liver weight and liver weight relative to body weight was unchanged at 200 and 800 mg/kg bw per day. In the absence of other clinical or histopathological changes, the increased liver weight relative to brain weight in female dogs is not considered to be toxicologically significant.
On histopathological examination, hepatocellular vacuolation was observed in both male and female controls and animals receiving doses of 800 mg/kg bw per day. This is an incidental finding of no clinical significance, as there were no significant differences in incidence between controls and animals receiving the highest dose, there was no dose–response relationship and at no other doses was this observed. There were a number of incidental observations but none of these were considered to be related to treatment.
There were no significant treatment-related changes in clinical pathology, organ weights or gross or histopathology. The NOEL was 200 mg/kg bw per day on the basis of the significant increase in alkaline phosphatase activity (Thomford & Carter, 1997b).
The results of studies of genotoxicity with neotame are summarized in Table 1. No genotoxic effects were reported. The results of studies of genotoxicity with the degradation products of neotame are described in section 2.2.6 (c).
Table 1. Results of studies of the genotoxicity of neotame
|
Endpoint |
Test object |
Concentration/ dose |
Results |
Reference |
|
In vitro |
|
|
|
|
|
Reverse mutation |
S. typhimurium strains TA1535, TA1537, TA1538, TA98, TA100. E. coli strain WP2 uvrA |
312–10 000 mg/ plate ±S9a |
Negative |
Riccio (1994) |
|
Reverse mutation |
Mouse lymphoma L5178Y cells |
100–1 000 mg/ ml ±S9a |
Negative |
Rudd (1994) |
|
Chromosomal aberration |
Chinese hamster ovary cells |
62.5–500 mg/ ml S9; 250 to 1 000 mg/ml +S9a |
Negative |
Winegar (1994) |
|
In vivo |
|
|
|
|
|
Micronucleus formation |
CD-1 mice bone-marrow |
500, 1 000 or 2 000 mg/kg bw by oral gavage |
Negative |
Garrett et al. (1997) |
|
a |
S9, 9000 × g supernatant of liver homogenate from rats induced with Aroclor 1254 |
Rats
In a range-finding one-generation study in Sprague-Dawley rats, groups of eight male and eight female Crl : CD BR VAF Plus rats were fed diets containing neotame at concentrations providing a dose of 0, 10, 30, 100, 300 or 1000 mg/kg bw per day. Females were given the treated diet from 29 days before mating until the pups were aged 25 days. Males were given the treated diet until after successful littering of the females. Animals were observed daily and body weight and food consumption were monitored. Females were examined for reproductive parameters. Pups were examined daily and litter size culled to eight pups (four of each sex, if possible) on day 4. Females and litters were killed on day 25 and underwent internal and external macroscopic examination.
There were no treatment-related signs of toxicity. Food consumption was decreased in week 1 in males at >100 mg/kg bw per day, and in females at 1000 mg/kg bw per day. Body weight on days 1–4 in males at >30 mg/kg bw per day was significantly lower than that of controls. In females, the only significant decreases in body weights were seen on days 1 and 4 at 1000 mg/kg bw per day.
There were no treatment-related effects on the number of animals mating, the conception rate or the mean gestation rate. Litter size and survival were not affected by treatment, nor were there any treatment-related effects on sex ratio. The total body weight of the litter on days 21 and 25 of lactation was decreased by 12% (p <0.05) at 1000 mg/kg bw per day. Body-weight gain of the litter was also reduced on days 1–25 at 1000 mg/kg bw per day, and during days 18–21 of lactation at 300 mg/kg bw per day (26%; p <0.01).
On the basis of this study, there is no evidence that neotame affects reproductive capacity in rats at a dose of up to 1000 mg/kg bw per day (Willoughby, 1996a).
In a second range-finding one-generation study in Sprague-Dawley (Crl : CD BR VAF Plus) rats, groups of eight male and eight female rats were given diets containing neotame at concentrations providing a dose of 0, 500, 1000, 1500, 200, 2500 or 3000 mg/kg bw per day. The study was conducted in a similar way as the first range-finding study (described above).
At a dose of >2500 mg/kg bw per day, there was an increased incidence of brown staining of the coat, an ungroomed appearance, thin rats and some tremors on handling. Food consumption in males and females was decreased at all doses during the first week. At 500 mg/kg bw per day, food intake was decreased by 14% (p <0.05). Body weight in males and females was significantly lower on days 1 and 2 at a dose of >500 mg/kg bw per day.
There were no treatment-related effects on number of animals mating, the conception rate, or the mean duration of gestation. Litter size, survival and sex ratio were not affected by treatment. The litter body weight was decreased by 10% or more on day 21 of lactation at 1500 mg/kg bw per day. Body-weight gain was decreased on days 1–21 at >1500 mg/kg bw per day and on days 18–21 of lactation at >500 mg/kg bw per day.
On the basis of this study, there is no evidence that neotame affects reproductive capacity in rats at a dose of up to 3000 mg/kg bw per day (Willoughby, 1996b).
In a two-generation study of reproductive toxicity in Sprague-Dawley (CRL : CD BR VAF Plus) rats, groups of rats were given diets containing neotame at concentrations providing a dose of 0, 100, 300 or 1000 mg/kg bw per day. These doses were equivalent to concentrations throughout the study of approximately 0, 920–2000 ppm, 2800–6100 ppm or 9200–18 800 ppm in the diet. Control animals received basal diets only. Groups of 28 male and 28 female rats of the P0 generation were treated for 10 or 4 weeks before pairing, respectively. Dosing continued throughout pairing, gestation, and lactation until weaning on day 21 after littering and until selection for the next generation. Offspring (the F1 generation) were weaned on postnatal day 21, and developmental, behavioural, and performance parameters were evaluated. At age approximately 4 weeks, offspring were selected to form the breeding F1 generation and were treated for a minimum of 10 weeks before pairing to generate the F2 offspring that were raised to postnatal day 21 before termination. Growth and reproductive parameters were assessed for the F0 and F1 generations. Growth, physical maturation and behaviour parameters were assessed for the offspring. All adults were subjected to a detailed necropsy, and weights of the reproductive organ were measured.
There were no treatment-related deaths or clinical signs of toxicity in either generation. Food consumption in males of the F1 generation was less than that of controls throughout the study at all doses, decreasing by 4–9% (p <0.05) at 100 mg/kg bw per day, and by 5–9% (p <0.05) at 1000 mg/kg bw per day. In females of the F1 generation at 1000 mg/kg bw per day, food consumption during week 1 was 9% (p <0.001) less than that of controls. There were no other statistically significant decreases in food consumption in females in the F1 generation. In males of the F2 generation, food consumption was consistently decreased (7–10%, p <0.01) from week 4, at 1000 mg/kg bw per day. No statistically significant decreases in food consumption were observed in females.
Body weight and food consumption were lower in treated F0 males than in controls during the 10-week pre-mating period. The lower relative body weight and food consumption were seen at 100 and 1000 mg/kg bw per day. In females at 1000 mg/kg bw per day, body weight was lower during the 4-week pre-mating period and throughout gestation. On day 20 of gestation, body weight of females at the highest dose was 95% that of controls. In adults of the F1 generation, significantly lower body weights were seen at 1000 mg/kg bw per day, with decreases of 8–12% in males and 7–10% in females. Throughout gestation, body weights of 1 females at 1000 mg/kg bw per day remained lower than those of controls .
There were decreases in total body weight per litter for F1 male offspring on days 1 and 21 of lactation at 300 mg/kg bw per day, and for male and female offspring on days 1 and 21 at 1000 mg/kg bw per day. In the F2 generation, pup weight on day 21 at 1000 mg/kg bw per day was about 15% less than that of controls. This effect was probably because the treated groups consumed less food than the controls during days 14–21 (before weaning) when pups begin to consume solid food.
There were no treatment-related effects on reproductive parameters (i.e. estrus cycle, mating performance, fertility, duration of gestation, parturition, and gestation index) throughout two successive generations at doses of up to 1000 mg/kg bw per day, the highest dose. Litter size, sex ratio, and offspring viability indices were unchanged by treatment, and there were no effects on physical development or on auditory or visual performance.
There was a slight (not statistically significant) decrease in motor activity for F1 males at 1000 mg/kg bw per day. There was also a significant increase in the swim time in three out of six trials with F1 males in a water-filled Y-maze at 1000 mg/kg bw per day. The increased swim time was, however, within the range for historical controls observed in this laboratory. Both of these effects are likely to be related to reduced body weight in males at the highest dose, and not indicative of a reduced learning ability. This conclusion is supported by the subsequent statistical analyses performed by Tesh & Tesh (2002). No similar effect was seen in F1 females at any dose.
There were no treatment-related macroscopic changes observed at necropsy. There were no effects observed in this study that were related to reproductive performance and the NOEL, therefore, was the highest dose tested, 1000 mg/kg bw per day (Willoughby, 1997).
Rats
Groups of 24 female Sprague-Dawley Crl : CD BR VAF Plus rats were fed diets containing neotame at concentrations providing a dose of 0, 100, 300, or 1000 mg/kg bw per day for 4 weeks before pairing with untreated males. These doses were equivalent to dietary concentrations of approximately 0, 1100–1300 ppm, 3300–3900 ppm or 10 700–12 800 ppm in the diet. Neotame continued to be administered in the diet throughout gestation until necropsy on day 20 after mating, when the contents of the uterus were examined. Control animals were fed the basal diet. The pre-mating period of treatment was included to allow the animals to adapt to the presence of neotame in their diets. Animals were examined twice daily for mortality and morbidity and once daily for clinical signs. Body weights and food consumption was monitored and, after necropsy on day 20, reproductive parameters were examined. Fetuses were weighed and examined externally at necropsy. Approximately 50% of fetuses were examined internally at necropsy and were subsequently examined for skeletal development. The remaining fetuses were fixed and examined for visceral abnormalities after serial sectioning.
There was an immediate but transient decrease in food consumption on day 1 of the pre-mating phase at 300 and 1000 mg/kg bw per day, which resulted in lower relative food consumption and body-weight gain (57% that of controls) for the first week of pre-mating, at 1000 mg/kg bw per day. Overall body-weight gain at 1000 mg/kg bw per day improved to reach 87% that of controls by the end of the 4-week pre-mating period. There were no effects on food consumption, body weight, or body-weight gain at any interval during gestation, at any dose up to the highest dose administered in the diet. Body weights at day 20 of gestation at 1000 mg/kg bw per day were 98% those of controls.
There were no treatment-related changes in the number of corpora lutea, pre- and postimplantations, viable young, sex ratios, litter size, or fetal body weights at any dose. In the fetuses, there were no treatment-related increases in the incidence of either skeletal or soft tissue malformations or of pre- or postimplantation loss.
On the basis of this study, there is no evidence of embryotoxicity or teratogenicity in rats after administration of neotame at doses of up to 1000 mg/kg bw per day (Willoughby, 1996a).
Rabbits
In a range-finding study in rabbits, groups of six mated New Zealand white rabbits were given neotame in aqueous methylcellulose by gavage at a dose of 0, 30, 100, 300 or 1000 mg/kg bw per day on days 6–19 of gestation. Rabbits were examined throughout the study for abnormal clinical signs, and body weight and food consumption were monitored daily. On day 29 of gestation, dams underwent detailed necropsy and reproductive parameters were analysed. Fetuses were discarded.
At 1000 mg/kg bw per day, one rabbit was found dead and one had a total litter loss. The only treatment-related clinical signs of toxicity were pale faeces at the highest dose. There were no treatment-related effects on body weight during gestation and no treatment-related effects on reproductive parameters.
On the basis of this study, there were minimal signs of maternal toxicity at a dose of 1000 mg/kg bw (Willoughby, 1996b)
In another study in rabbits, groups of 20 mated New Zealand white rabbits were given neotame in aqueous methylcellulose at a dose of 0, 50, 150, or 500 mg/kg bw per day by gavage (10 ml/kg) between days 6 and 19 (inclusive) of gestation. Controls were given the vehicle only, 0.5% aqueous methylcellulose containing 0.1% polysorbate 80. Approximately 20% of the rabbits in each group, including controls, were not pregnant, and five animals were added to each of the control and 500 mg/kg bw per day groups to supplement the number of litters for fetal examination. Animals were examined twice daily for abnormal clinical signs, and body weight and food consumption were assessed daily. All dams were subjected to a detailed necropsy on day 29 after mating. Fetuses were weighed and examined externally and internally at necropsy. Fetuses were processed and examined for skeletal development. The heads of one-third of the fetuses were fixed and examined after serial sectioning.
Satellite groups of pregnant rabbits (five rabbits per group) were given neotame at a dose of 0, 50, 150, or 500 mg/kg bw per day by gavage. Blood samples were collected on days 1, 8 and 14 for measurement of plasma concentrations of neotame and de-esterified neotame. These animals were killed after sampling and were not necropsied.
One animal at 150 mg/kg bw per day died during the study as a result of a dosing injury. Another animal at 500 mg/kg bw per day died during the study, after a marked decrease in food consumption. Two animals at 500 mg/kg bw per day aborted late in pregnancy; this was also related to markedly reduced food consumption. Overall food consumption and body-weight gain at 500 mg/kg bw per day were not significantly different from those of controls.
Pharmacokinetic data in the satellite animals demonstrate that plasma concentrations of neotame and de-esterified neotame increased with dose, and that there was no evidence of increasing plasma concentrations with increased duration of dosing.
There were no treatment-related effects on corpora lutea, implantations, viable young, early or late resorptions or pre- or postimplantation losses. There were no macroscopic abnormalities in dams or fetuses. Skeletal and visceral examination of the fetuses did not reveal any treatment-related increase in abnormalities.
On the basis of this study, there is no evidence for embrotoxicity or teratogenicity in rabbits after administration of neotame at doses of up to 500 mg/kg bw per day (Willoughby, 1996c).
(a) Cardiovascular, respiratory and renal parameters
Cardiovascular, respiratory and renal parameters were evaluated in dogs after intraduodenal administration of neotame. Twelve male beagle dogs were anaesthetized with alpha-chloralose/sodium pentobarbitone, and baseline cardiovascular and respiratory parameters were recorded. Baseline urinary electrolyte and protein concentrations were also measured. After a stabilization period, each dog was given a single intraduodenal dose of vehicle (0.5% methylcellulose/0.1% (v/v) polysorbate 80), followed by neotame at a dose of 5 or 15 mg/kg bw. Blood pressure, heart rate, left ventricular systolic pressure, left ventricular cardiac output (dp/dt max), lead II electrocardiogram, respiratory rate, tidal volume and urinary concentrations of sodium, protein, potassium and chloride were recorded after doses of the vehicle and neotame.
There were no treatment-related effects on any of the cardiovascular or respiratory parameters, or on urinary excretion of protein or electrolytes, at either dose (Algate, 1997).
(b) Special studies on the pharmacology of neotame
(i) Effects on gastrointestinal motility
The possible effects of neotame on gastrointestinal motility were studied in male Sprague-Dawley rats using the charcoal propulsion test. Rats were assigned randomly to groups (10 rats per group) and given, by gavage, a single dose of (1) neotame, at 5 or 15 mg/kg bw per day; or (2) morphine sulfate, at 100 mg/kg bw per day as a positive control; or (3) vehicle alone (0.5% w/v methylcellulose and 0.1% v/v polysorbate 80). An oral suspension of 5% (w/v) charcoal in water was administered approximately 30 min after dosing. The rats were killed, their gastrointestinal tracts removed, and the distance travelled by the charcoal meal between pyloric sphincter and caecum was measured. The positive control significantly decreased gut, while neotame did not affect gastrointestinal motility at either dose (Atterson, 1997a).
(ii) Effects on the autonomic nervous system
The effects of neotame and de-esterified neotame on responses of the autonomic nervous system to acetylcholine, histamine, 5-hydroxytryptamine (5-HT) and barium chloride were examined. The ileum was removed from Dunkin-Hartley guinea-pigs and suspended in an organ bath. The concentrations tested in the organ bath were: neotame, 0 (vehicle only), 20, 60, and 200 mg/ml; and de-esterified neotame, 60, 200, and 600 ng/ml. The effect of neotame and de-esterified neotame on agonist responses was evaluated in separate tissues.
Neotame and de-esterified neotame did not affect autonomic responses to agonist-induced spasmogenic effects in the guinea-pig ileum. In addition, neotame and de-esterified did not affect the basal tone of the isolated ileum. Thus, neotame and de-esterified neotame had no agonistic, synergistic, or antagonistic effects on the receptor systems evaluated (Atterson, 1997c).
(iii) Effects on hexobarbital sleeping time
An assessment of the possible effects of orally administered neotame on hexobarbital-induced sleeping time was conducted. Groups of five Sprague-Dawley rats of each sex were given orally administered (1) neotame, at 5 or 15 mg /kg bw per day; or (2) chlorpromazine hydrochloride, at 15 mg /kg bw per day as a positive control; or (3) vehicle alone (0.5% (w/v) methylcellulose and 0.1% (v/v) polysorbate 80). These treatments were administered approximately 30 min before an intraperitoneal injection of sodium hexobarbital at 150 and 100 mg/kg bw per day, in males and females respectively. Sleep onset and duration of sleeping time were recorded, based on loss and reappearance of the righting reflex.
Administration of the positive control significantly increased the sleeping times in males and females, while neotame had no effect on hexobarbital-induced sleeping time in rats. Thus, neotame does not interfere with the metabolism of hexobarbital or its pharmacodynamic effects on the central nervous system (Atterson, 1997b).
(c) Degradation products of neotame
(i) Source, structure and chemistry
The degradation of neotame was assessed at an artificially high concentration of 200 ppm in mock beverages containing phosphate- and citrate-buffered solutions simulating formulations used in commercial cola soft drinks (pH 2.8 and 3.2) lemon-lime soft drink (pH 3.8) and root beer soft drink (pH 4.5), and covered a range of temperatures (5, 20, 30, and 35°C) and storage for up to 8 weeks. These conditions simulated typical commercial, as well as extreme, storage conditions for beverages, with respect to temperature and time. Dependency on pH, time, and temperature was assessed for all degradation products of neotame. Higher concentrations of neotame were used to allow detection of low concentrations of degradation products. The use of higher concentrations of neotame was justified on the basis of similar kinetic profiles for neotame at 200 ppm and 15 ppm, a concentration relevant to intended use.
The principle degradation product of neotame at concentrations of intended use or at the much higher concentrations used in mock beverage formulations was de-esterified neotame. It comprised approximately 7% of the initial amount of neotame after storage for 8 weeks at 20 °C, pH 3.2. Hydrolysis of neotame to de-esterified neotame occurs slowly and is dependent upon pH and temperature.
In addition to de-esterified neotame, three minor degradation products were detected, specifically N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester formed by cyclization of neotame, N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester formed by beta-rearrangement of neotame, and N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine formed by methyl ester hydrolysis of N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1methyl ester. These minor degradation products represented <1% of the initial concentration of neotame of 200 ppm after 8 weeks of storage at 20 °C. When the initial concentration of neotame was 15 ppm, these products could not be detected.
The degradation pathways for neotame in mock beverages at a concentration of 200 ppm are shown in Figure 3 (Lui & Cleary, 1998).
Figure 3. Degradation of neotame (200 ppm) in mock beverage stored at 20 °C, pH 3.2, for 8 weeks
Two other minor products of degradation are methanol and phenylalanine, both of which are normal components of the diet. The amounts of these components contributed by consumption of neotame are insignificant compared to the amounts found normally in food.
At extreme conditions of temperature and time, two additional minor products of degradation were detected in mock beverages containing neotame at a concentration of 200 ppm. These conditions are not likely to be encountered during typical storage and use of neotame in soft drink beverages. The first was N-(3,3-dimethylbutyl)-L-aspartic acid, which is a known metabolite of neotame formed via peptide or amide hydrolysis; exposure to this minor product thus occurred in the studies of toxicity in laboratory animals and in humans. The other minor degradant was N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine formed by the beta-rearrangement of neotame to N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester followed by hydrolysis of the methyl ester group. Animal safety studies with N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester include exposure to N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine, since this substance is a metabolite of N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester based on studies with digestion enzymes in vitro (Kirkpatrick & Aiken, 1998).
(ii) Safety evaluation of the degradation products of neotame
Acute toxicity
The acute toxicity of the minor degradation products of neotame was examined in Sprague-Dawley rats. Groups of 10 male and 10 female Sprague-Dawley rats were given a single dose of N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester (NC-00764) or N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester (NC-00777) at 0, 0.6, 2.0 or 6.0 mg/kg bw per day by gavage and observed for clinical signs for 14 days. Similarly, N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine (NC-00779) was administered at a dose of 0, 0.3, 1.0, or 3.0 mg/kg bw per day. There were no deaths and no significant changes in body weight or food consumption in the treated animals. After administration of NC-00764, body-weight gain was decreased by 23% in females at the highest dose on day 14 after dosing. There were no treatment-related changes in gross pathology after treatment with any of the degradation products. The results are summarized in Table 2 (Bechtel, 1998a–c).
Table 2. Acute toxicity of the minor degradation products of neotame
|
Species |
Degradation product |
Route |
LD50 (mg/kg bw) |
Reference |
|
Rat |
N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester (NC-00777) |
Gavage |
>6000 mg/kg |
Bechtel (1998b) |
|
N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester (NC-00764) |
Gavage |
>6000 mg/kg |
Bechtel (1998a) |
|
N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine (NC-00779) |
Gavage |
>3000 mg/kg |
Bechtel (1998c) |
Genotoxicity
Table 3 summarizes the results of studies of genotoxicity in vitro and in vivo with the major degradation product of neotame, N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine (de-esterified neotame) and each of the minor degradation products.
Table 3. Summary of studies of genotoxicity with degradation products of neotame
|
Endpoint |
Test object |
Concentration or dose |
Result |
Reference |
|
N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine (de-esterified neotame) |
||||
|
In vitro |
||||
|
Bacterial gene mutation |
S. typhimurium strains TA1535, TA1537, TA98, TA100, TA102 |
500–5000 µg/ plate ±S9a |
Negative |
Curtiss et al. (1997) |
|
Mammalian gene mutation |
Chinese hamster ovary cell |
20–5000 µg/ml ±S9a |
Negative |
Cabonce et al. (1997) |
|
N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester |
||||
|
In vitro |
||||
|
Bacterial gene mutation |
S. typhimurium strains TA1535, TA97A, TA98, TA100, TA102 |
10–5000 µg/ plate ±S9a |
Negative |
Balwierz & Bunch (1998a) |
|
Mammalian gene mutation |
Chinese hamster ovary cell |
20–5000 µg/ml ±S9a |
Negative |
Cabonce et al. (1998b) |
|
In vivo |
||||
|
Micronucleus formation |
CD-1 mice |
500, 1000, 2000 mg/kg bw, by oral gavage |
Negative |
Soelter et al. (1998) |
|
N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester |
||||
|
In vitro |
||||
|
Bacterial gene mutation |
S. typhimurium strains TA1535, TA1537, TA98, TA100, TA102 |
500–5000 µg/ plate ±S9a |
Negative |
Curtiss et al. (1998) |
|
Mammalian gene mutation |
Chinese hamster ovary cell |
20–5000 µg/ml ±S9a |
Negative |
Cabonce et al. (1998a) |
|
Micronucleus formation |
CD-1 mice bone marrow |
500, 1000, 2000 mg/kg bw, by oral gavage |
Negative |
Garrett et al. (1998) |
|
N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine |
||||
|
In vitro |
||||
|
Bacterial gene mutation |
S. typhimurium strains TA1535, TA97A, TA98, TA100, TA102 |
10–5000 µg/ plate ±S9a |
Negative |
Balwierz & Bunch (1998b) |
|
Mammalian gene mutation |
Chinese hamster ovary cell |
20–5000 µg/ml ±S9a |
Negative |
Cabonce et al. (1998c) |
|
In vivo |
||||
|
Micronucleus formation |
CD-1 mice bone marrow |
500, 1000, 2000 mg/kg bw, by oral gavage |
Negative (1998) |
Nicollette & Bunch |
|
a |
S9, 9000 × g supernatant of liver homogenate obtained from Aroclor 1254induced rats |
Short-term studies of toxicity
In a 4-week study, groups of three male and three female Sprague-Dawley Crl : CD(SD)BR VAF Plus rats were fed diets containing a mixture of N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester, N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester, and N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine. The relative concentration of the components of the above mixture was formulated to simulate the ratio of such components that could be expected in degraded soft-drink beverages. The diets provided a dose of N-[N-(3,3-dimethylbutyl)-L-beta-aspartyl]-L-phenylalanine 1-methyl ester/N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine 1-methyl ester/N-[N-(3,3-dimethylbutyl)-L-aspartamidyl]-L-phenylalanine of 0 mg/kg bw per day, 0.2/0.2/0.1 mg/kg bw per day, 0.6/0.6/0.3 mg/kg bw per day, 2.0/2.0/1.0 mg/kg bw per day or 6.0/6.0/3.0 mg/kg bw per day.
Rats were observed for mortality and clinical signs of toxicity. Body weights and food consumption were determined periodically. Detailed clinical observations were performed weekly. Ophthalmological examinations were performed before treatment and during week 4. At termination, blood and urine samples were collected for analysis of clinical pathology parameters after an overnight fast. A gross necropsy was performed, including determination of organ weights and collection of tissues for microscopic examination.
During the study, there were no unscheduled deaths and no clinical signs attributed to dosing with any of the mixtures. No abnormal findings were detected on ophthalmoscopic examination. There were no treatment-related effects on body weights, body-weight gains or food consumption. There were no treatment-related changes to haematological, clinical chemistry or urine analysis parameters.
There were no treatment-related changes to organ weights or organ weight ratios and no treatment-related abnormalities on gross histopathological examination (Lemen, 1998).
(d) Palatability of neotame in rats
In a feasibility study on the dietary preferences of rats, groups of five male and five female Sprague-Dawley Crl CD(SD)BR VAF Plus rats were fed diets containing neotame at a concentration of 0, 50, 150, 500, 1500, 5000 or 15 000 ppm (equivalent to 0, 3, 10, 30, 100, 300 or 1000 mg/kg bw per day) in males. Rats were housed individually in cages provided with two food jars, each containing 50 g of food. In the pretreatment phase, the same diet was used in both jars, and the position of the jars was not altered. Any preference for one jar was noted. During the first preference phase of 3 days, treated diets were provided in one jar and basal diet in the other (controls received basal diet only). During this phase, the position of the jars in the cage was altered on a daily basis. For the next 7 days, the rats were given treated diet in both jars. This was followed by a second preference phase to determine whether acclimatization to the treated diet altered the initial preferences determined. Diet containing quinine sulfate at 5000 ppm was used as a positive control.
Rats were observed twice daily during the study for any reaction to treatment or signs of ill health. Food consumption was determined daily throughout the study, and was reported as food consumption from each jar in the cage. Body weight was measured 7 days before treatment, and on days 0, 3, 10 and 13, being the beginning and end of each study phase. At the end of the study, rats were killed and discarded without postmortem examination.
No preference for either food bowl was identified during the pretreatment phase. During the first preference phase (days 1–3), a preference for the basal diet was seen at doses of >150 ppm, with consumption of the treated diet comprising only 40% of the total intake in males and 45% in females. At the highest concentration of neotame, virtually none (1% of the total intake) of the treated diet was eaten. Consumption of neotame was thus similar to that of quinine sulfate in the first preference phase (1.2% of total intake in males, 0% in females).
On days 4–10, food consumption was decreased at 15 000 ppm by more than 10% compared with controls. Consumption of the positive control diet containing quinine sulfate was decreased to 63% that of controls in males and 44.9% in females. During the second preference phase (days 11–14), a similar consumption pattern to that of the first preference phase was observed, although the preference for basal diet at 150 ppm was more marked.
There were only limited effects on body weight in this study. On day 10, the body weight of males at 15 000 ppm was 11% lower than that of controls (not statistically significant). No significant differences were seen in females. On days 3–10, body-weight gain was decreased at 5000 and 15 000 ppm; the decrease in males was 63% and 44% of controls, respectively, and in females was 55% and 60% of controls, respectively (Nicholls, 1997a).
In a more comprehensive study on the dietary preferences of rats, six groups of 14 male and 14 female Crl : CD(SD)BR VAF Plus rats were fed diets containing neotame at a concentration of 0, 50, 150, 500, 1500, 5000, or 15 000 ppm. The study design was similar to that described in the feasibility study. In this case, however, the pre-treatment phase was 5 days and the first preference phase was 5 days. This was followed by a 5-day phase in which all animals were offered only treated diet, and then by a second preference phase of 5 days.
Rats were observed twice daily during the study for any reaction to treatment or signs of ill health. Food consumption was determined daily throughout the study, and was reported as food consumption from each jar in the cage. Body weight was measured 5 days before treatment, and on days 0, 5, 10 and 15, being the beginning and end of each study phase. At the end of the study, rats were killed and discarded without postmortem examination.
Total food consumption was decreased during the treatment phase in males and females at doses of >5000 ppm. In males, food consumption was decreased by 12% at 5000 ppm and by 25% at 15 000 ppm. In females, food consumption was decreased by 7% at 5000 ppm and by 16% at 15 000 ppm. In both preference phases, a preference for the basal diet was seen at doses of >150 ppm, with intakes of the treated diet representing 23–32% of the total food intake. At concentrations of neotame of >5000 ppm, there was almost complete preference for control diet over the treated diet. Although there was some accommodation to the treated diet when a choice was not offered, this did not affect the preference for the untreated diet when offered in the second preference study.
No significant differences in body weight were seen at any time during the study. Body-weight gain during the treatment phase was significantly decreased in males at 5000 ppm (42%) and 15000 ppm (95%). In females at 15000 ppm, body-weight gain was decreased by 45%, but this was not statistically significant.
On the basis of the results of this study, there was a decrease in the palatability of the diet containing neotame at dietary concentrations of >150 ppm (Nicholls, 1997b).
A study was conducted to evaluate tolerance of a single dose of neotame ingested in solution by healthy men. The safety of neotame was evaluated in a stepwise fashion, starting with the lowest dose, followed by the intermediate dose and then the high dose only after safety at lower doses had been confirmed. Nineteen healthy men (mean age ±SD, 28 ±6 years) were given single doses of 0.10, 0.25, or 0.50 mg/kg bw (seven, six, six men per dose, repectively) of neotame in solution. The study was randomized, single dose and not double-blinded. Each man received only one treatment regimen after an overnight fast. Eighteen men completed the study; one man was excluded due to poor venous access. Clinical evaluations and laboratory tests were done immediately before dosing and approximately 48 h after dosing.
There were no treatment-related changes in pulse rate or blood pressure, and no changes in haematology, clinical chemistry or urine analysis parameters. Two men experienced mild headaches, one before dosing and one after a dose of 0.1 mg/kg bw per day. At 0.5 mg/kg bw per day, another two men had mild headaches, one before dosing and one after, and one had lower back pain. These signs resolved without further treatment and were not attributed to dosing with neotame (Kisicki et al., 1997).
In a two-week study of tolerance in men and women, neotame was administered to 74 healthy adults (mean age ±SD, 32 ±10 years). The study was randomized, double-blind, and placebo-controlled, and inclusion and exclusion criteria were used to select appropriate subjects. Seventy-two healthy men and women were randomized into three groups of 24 subjects (12 male and 12 female). Two subjects were dropped for reasons unrelated to administration of the test article and were replaced by subjects of the same sex. The groups received neotame at a dose of 0, 0.5 mg/kg bw per day, or 1.5 mg/kg bw per day in capsules, as three divided doses (given at 07:00, 12:00 and 17:00). The men and women attended the clinic to receive each dose and to eat a standardized meal. The first morning dose followed an overnight fast of at least 8 h.
Body weight was determined before the first dose. The sitting blood pressure, temperature, pulse rate and respiratory rate were determined before the morning dose on days 1, 3, 5, 7, 9, 11 and 14. Blood for clinical pathology was taken before dosing on days 1, 3 and 7. Blood samples were taken to determine concentrations of neotame and de-esterified neotame on days 1, 2, 3, 4, 7, 11 and 15. Plasma was separated and stored frozen for later analysis. A screen for drugs in urine was done before dosing on days 1 and 7. An electrocardiogram (ECG) was performed before dosing on days 1 and 3, and a physical examination (including measurement of body weight) was carried out on day 7. Men and women were monitored for adverse experiences or unusual symptoms. A physical examination was done after treatment. Vital signs and body weight were measured, and an ophthalmological examination, ECG and clinical laboratory tests were performed after the last blood sample had been collected.
Of the 74 (70 Caucasian, 1 Hispanic, 1 Middle-Eastern and 2 Asian) persons starting the study, 72 successfully completed the study. The failure of two subjects to complete the study was unrelated to administration of neotame. The mean age was 33 (range, 20–53) years for women, and 31 (range, 20–53) years for men.
A range of clinical symptoms was documented throughout the study. The most common finding was headache, namely, eight headaches in five men in the control group, 16 headaches in seven men at 0.5 mg/kg bw per day and 10 headaches in four men at 1.5 mg/kg bw per day. Diarrhoea was reported in controls and at 0.5 mg/kg bw per day, but was not reported at 1.5 mg/kg bw per day. Abdominal pain was reported in one man at 0.5 mg/kg bw per day and two men at 1.5 mg/kg bw per day. None of these clinical symptoms required medical intervention, and most were considered mild to moderate, although four headaches were documented as severe. These clinical symptoms could not be linked to ingestion of neotame.
There were no treatment-related changes in clinical pathology parameters, heart rate, blood pressure, respiratory rate, temperature, body weight or ECG.
On analysis of blood samples, concentrations of neotame were below the level of quantification at all time periods. Plasma concentrations of de-esterified neotame were approximately proportional to administered dose. In men and women, plasma concentrations of de-esterified neotame reached steady state after 24 h and 72 h, respectively.
On the basis of this study, neotame was well tolerated in humans when administered at a dose of up to 1.5 mg/kg bw for a period of 2 weeks (Kisicki et al., 1998b).
In a 2-week study of tolerance in persons with diabetes, 18 men and 19 women with non-insulin dependent diabetes mellitus (NIDDM) were enrolled in a randomized, double-blind, placebo-controlled, multiple dose, crossover study. Inclusion and exclusion criteria were used to select appropriate subjects. Thirty-four subjects (mean age ±SD, 53 ±8 years) completed the study. Before treatment, subjects provided a medical history and underwent a physical examination, eye examination, ECG, and screening for drugs in urine, pregnancy and hepatitis B virus and human immunodeficiency virus (HIV). An evaluation of clinical chemistry parameters, which included plasma concentrations of glucose and insulin, was also carried out.
Neotame was provided in a blister pack of five gelatin capsules containing 10 mg of neotame or placebo per capsule. A mixture of placebo and treatment capsules was eaten in order to achieve the appropriate dose of neotame, 0, 0.5 or 1.5 mg/kg bw per day. As average body weight was 100 kg, intake was 0, 60 or 150 mg/day. The test material was self-administered in divided doses, three times daily (07:00, 12:00 and 17:00) for 43 doses (14 days). Each subject received each dose, with the order randomized between subjects, and a 72 h period allowed between study periods.
Neotame did not affect plasma concentrations of glucose and insulin in diabetic men and women. There were no statistically significant differences in maximum observed effect (Emax) or area under the effect–time curve (AUEC0–180) between treatments for either glucose or insulin.
Neotame was well tolerated by this population. There were no treatment-related differences in heart rate, respiratory rate, temperature, blood pressure, body weight, ECG, or haematological, clinical chemistry or urine analysis parameters. Ten subjects reported adverse experiences, with the most frequently reported being flu syndrome, headache, and sinusitis; these were not related to treatment.
On the basis of this study, neotame was well tolerated in persons with non-insulin dependent diabetes mellitus when administered at a dose of up to 1.5 mg/kg bw per day for a period of 2 weeks (Morrison et al., 1998).
In a 3-month study of tolerance, 151 healthy men and women (mean age ±SD, 35 ±11 years) were enrolled in a two site, randomized, double-blind, placebo-controlled, parallel study, consisting of three treatment groups. Inclusion and exclusion criteria were used to select appropriate subjects. A total of 144 men and women (24 of each sex per group) participated. Neotame was provided in a blister pack of four gelatine capsules containing 10 mg of neotame or placebo per capsule. A mixture of placebo and treatment capsules was eaten to yield the appropriate dose of neotame of 0, 0.5 or 1.5 mg/kg bw per day. Average body weight was 70 kg for women and 80 kg for men, therefore, the doses administered were 0, 30 or 110 mg/day for women and 0, 40 or 120 mg/day for men. The test material was self-administered in divided doses, three times daily (07:00, 12:00 and 17:00). The first morning dose followed an overnight fast of at least 8 h. The dates and times of administration were recorded in a daily logbook. Subjects attended the clinic on a weekly basis to receive test material for 7 days; test material for 3 additional days was also supplied to allow flexibility in attendance at the clinic.
On day 1, before the first dose, the sitting blood pressure, temperature, pulse rate and respiratory rate, ECG and body weight were measured, analysis of urine, haematological and clinical chemistry parameters was performed and screening for alcohol or drugs of abuse, and pregnancy was undertaken. On the mornings of study days 7, 14, 28, 42, 56, 84 and 92, subjects were instructed not to take the morning dose or to eat until after attendance at the clinic and blood sampling. Haematology, clinical chemistry and urine analysis, as well as a physical examination, were done at these times. Vital signs were determined before the morning dose weekly throughout the study (on attendance at the clinic to obtain additional test material). On the morning of day 92, blood was taken for clinical pathology tests, clotting times, thyroxine, pregnancy test and possible analysis of concentrations of compound in the plasma. Urine was collected for urine analysis and screening for alcohol and drugs. A physical examination, including vital signs, body weight, ophthalmology and ECG was also done.
The majority of men and women in the study were Caucasian, with a small number being American Indian, persons of African origin, European/Middle Eastern and Hispanic. The mean age was 35 (range, 19–65) years for women and 34 (range, 19–54) years for men.
With respect to clinical symptoms, 82 persons reported at least one adverse reaction during the study. Most of these were determined to be of mild or moderate severity, and were reported in all three treatment groups, with no dose–response relationship or statistically significant differences between groups. Headache was the most common adverse experience, occurring in 16, 15 and 13 persons at 0, 0.5 and 1.5 mg/kg bw per day, respectively. There were no serious adverse reactions during treatment.
There were no treatment-related changes throughout the study in pulse rate, blood pressure, respiratory rate, temperature, body weight, ophthalmological or haematological parameters. The sporadic changes observed in clinical chemistry parameters were not considered to be of biological significance or to be treatment-related.
On the basis of the results of this study, neotame was well tolerated at a dose of up to 1.5 mg/kg bw per day for 91 days, with no treatment-related adverse effects (Kisicki et al., 1998c).
Neotame is currently permitted for use in the United States, Australia, China, Costa Rica, the Czech Republic, Ecuador, Mexico, New Zealand, Peru, Poland, Romania, and Trinidad and Tobago. In the Czech Republic, Poland and Slovakia, maximum permitted levels have been established for various categories of foods and beverages. In other countries, neotame is allowed at levels necessary to achieve desired technical effects, according to good manufacturing practice (GMP). In Australia and New Zealand, an acceptable daily intake (ADI) for neotame of 0–2 mg/kg bw was established in 2001; neotame is permitted to be used at levels consistent with GMP in a large number of solid and liquid food categories (Australia New Zealand Food Standards Code, 2003). In the United States, an ADI of 0–0.3 mg/kg bw was established in 2002; neotame has general approval for use in foods and beverages and is authorized at levels consistent with GMP as a non-nutritive sweetener in all foods, except meat and poultry (Food and Drug Administration, 2002).
Typical levels of use as provided by the sponsor vary from 15 to 17 mg/kg in beverages and from 15 to 70 mg/kg in solid foods, with the exception of chewing gum, 250 mg/kg.
The budget method was used to estimate the theoretical maximum level of neotame in foods and beverages likely to contain neotame that would not result in the ADI being exceeded by the population (Table 4). The proportion of both solid foods and beverages that may contain neotame was set at 50%.
Table 4. Estimated theoretical maximum level of neotame in food, by the budget method
|
Distribution of use in the food supply |
% of solid foods or beverage supply containing neotame |
Theoretical maximum level (mg/kg) ADI = 0–2 mg/kg bwa |
Typical use levelsa (mg/kg) |
|
50% solid foods |
50 |
80 |
70 |
|
50% beverages |
50 |
20 |
17 |
|
a |
Highest level excluding chewing gum (for which the typical use level is 250 mg/kg) and tabletop sweeteners, for which use levels are not applicable |
The theoretical maximum levels calculated are higher than the typical use levels received from the sponsor for all products, with the exception of tabletop sweeteners and chewing gums. However, use of neotame in these two categories will not lead to intakes greater than the ADI since conservative calculations based on the lowest sweetness potency of neotame (7000 times that of sugar) suggest that substitution of neotame for dietary sugar at up to 14 g/kg bw (i.e. 840 g in a 60-kg adult) would be necessary to reach the ADI. Exceeding the ADI is therefore extremely unlikely and further intake estimates are not warranted.
Projections of intake were provided by Australia and New Zealand (Hambridge, 2003a, b) and by the sponsor (Stargel, 2002). Such projections are not reported here since refinements of intake were not necessary.
The metabolism and pharmacokinetics of neotame have been examined in mice, rats, dogs, rabbits and humans. Approximately 20–30% of orally administered neotame is absorbed in all species studied. The major metabolic pathway for both absorbed and non-absorbed neotame is de-esterification to N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine and methanol, a reaction that is mediated by non-specific esterases. More than 95% of orally administered neotame is metabolized. De-esterified neotame accounted for approximately 80% of the neotame administered, while other metabolites each accounted for <5%.
In studies in rats given radiolabelled neotame by gavage, radiolabel was found to be primarily confined to the stomach, gastrointestinal tract, liver, kidney, and bladder, with smaller amounts of radiolabel detected throughout the rest of the body. There was no evidence for accumulation of radiolabel in any tissue. In a study in pregnant rats, radiolabel was not detected in the fetus. After oral administration of radiolabelled neotame to rats and dogs, 90–95% of the radiolabel was recovered in the urine and faeces within 48 h. The major metabolite found in the urine and faeces of the rat and dog was de-esterified neotame. Unchanged neotame was not detected in rat urine but was present at 1–6% of the administered dose in the urine of dogs. Unchanged neotame was not detected in the faeces of rats or dogs. Pharmacokinetic analysis of plasma metabolites after oral administration of radiolabelled neotame to rats indicated that peak plasma concentrations occurred after 0.5 h, followed by a rapid decline with a t1/2of approximately 1 h.
Pharmacokinetic analysis of absorbed neotame in human plasma after oral administration of radiolabelled neotame indicated rapid absorption, with a maximum plasma concentration at 0.4 h followed by rapid clearance (t1/2, 0.6 h). Concentrations of de-esterified neotame in plasma peaked at 1 h and declined with a t1/2 of 1.5 h. After oral administration of radiolabelled neotame to humans, 98% of the radiolabel was recovered in the urine and faeces within 72 h. De-esterified neotame was the major metabolite in both urine and faeces. The unchanged neotame detected in the urine represented 3.3% of the administered dose, while no unchanged neotame was found in the faeces. A metabolite detected initially only in human urine (and subsequently also in female rat urine) was identified as 3,3-dimethyl-butanoyl-L-carnitine and represented 0.5–3.4% of the administered dose of neotame. On the basis of the data evaluated, the Committee considered that neotame is rapidly but only partially absorbed in all species studied, and that both absorbed and non-absorbed neotame are metabolized via well-characterized pathways to nontoxic metabolites. The major metabolite, de-esterified neotame, is itself eliminated rapidly in the urine and faeces, with no evidence of tissue accumulation.
Short-term and long-term studies with neotame have been conducted in mice, rats and dogs using a body-weight adjusted constant dose regime. In all of these studies, the major effect observed was a treatment-related decrease in body-weight gain, which was linked in most cases to a measurable decrease in food consumption, particularly at high doses. The Committee considered that this effect was due to reduced palatability of the diet containing neotame, rather than toxicity induced by neotame. This conclusion is supported by several observations. First, in the 13-week study in mice given neotame in the diet, the high incidence of food scattering is indicative of reduced palatability of the diet. Second, the body-weight changes observed in the 13-week studies in rats and dogs were partially reversible when the animals were returned to a basal diet for a 4-week period at the end of the study. Third, reduced food consumption often occurred at the start of treatment at all doses, followed by some degree of adaptation as the animals adjusted to the diet. Fourth, studies were conducted over a wide range of doses (50–1000 mg/kg bw per day) and body-weight changes were not closely related to dose, as would be expected if the observed changes were a manifestation of treatment-related toxicity. Fifth, in the 1-year study in rats, there were no changes in body-weight gain or food conversion efficiency in rapidly growing rats consuming diets diets containing neotame. Sixth, when the effect of neotame on palatability of the diet was specifically examined in a preference study comparing basal diets with and without neotame at a concentration of 50–15 000 mg/kg diet, the rats showed a clear preference for the diet without neotame when the concentration of neotame was >150 mg/kg diet. There was no significant decrease in body weight observed in this study, but body-weight gain was reduced in males at >5000 mg/kg diet, when the reduced palatability caused a significant decrease in food consumption. In the light of the above information, the Committee agreed that the NOELs for the various short-term and long-term studies of toxicity should not be assigned on the basis of decreases in body weight or body-weight gain.
Aside from the palatability-related decreases in body-weight gain, neotame was well tolerated in all species at high doses in the diet in both the short-term and long-term studies, with no clinical signs of toxicity. In the 13-week study in mice, the NOEL was 1000 mg neotame/kg bw per day in the diet, on the basis of an increase in liver weight relative to body weight. In the 13-week study in rats, there was a small but significant increase in serum alkaline phosphatase activity at 1000 and 3000 mg/kg bw per day at week 13. On the basis of these changes, the NOEL for neotame was 300 mg/kg bw per day. However, in the 1-year study in rats exposed in utero, no increases in alkaline phosphatase activity were observed and the NOEL for neotame was 1000 mg/kg bw per day, the highest dose tested.
In the 13-week study in dogs, there were changes in clinical chemistry parameters in dogs fed neotame at >600 mg/kg bw per day, including significant increases in the activity of serum alkaline phosphatase, which isoenzyme analysis confirmed to be of hepatic origin. The small increase in activity of alkaline phosphatase observed at 200 mg/kg bw per day in three out of four female dogs was not considered to be toxicologically significant, and a NOEL for neotame of 200 mg/kg bw per day was established. In the 1-year study in dogs, the only significant change was an increase in the activity of alkaline phosphatase (hepatic isoenzyme) that was observed only at the highest dose (800 mg/kg bw per day) in both males and females. The increase in activity of alkaline phosphatase was rapidly reversible and was not accompanied by changes in other parameters indicative of cholestasis or other hepatotoxicity. Nevertheless, the Committee considered this to be indicative of a treatment-related effect and it was therefore used as the basis of the NOEL for neotame of 200 mg/kg bw per day.
In the 2-year study of carcinogenicity in mice, there was no treatment-related increase in tumour incidence at doses of up to 4000 mg/kg bw per day. In the 2-year study of carcinogenicity in rats exposed in utero, there was no treatment-related increase in tumour incidence at doses of up to 1000 mg/kg bw per day.
In a study of reproductive toxicity in rats, there were no treatment-related effects on reproductive parameters (estrus cycle, mating performance, fertility, duration of gestation, parturition, and gestation index) or on litter size, sex ratio, offspring viability, physical development or learning at doses of up to 1000 mg/kg bw per day. The major effect observed was a reduction in body-weight gain in treated animals compared with controls. As in the short and long-term studies, the Committee considered this to be related to a decrease in food consumption caused by the reduced palatability of the diet containing neotame. Mean litter body weights in treated groups in both the F1 and F2 generations were also reduced at day 21 compared with controls, probably as a result of a decrease in food consumption during pre-weaning days 14–21 when pups begin to consume solid food. The significant increase in swim time for F1 males in a water-filled Y-maze at 1000 mg/kg bw per day was within the variability seen in studies of this type and was likely to be related to reduced body weight rather than reduced learning ability. The Committee considered that the NOEL for neotame in this study, on the basis of the highest dose tested, was 1000 mg/kg bw per day.
The developmental toxicity of neotame was examined in rats and rabbits at a dose of up to 1000 mg/kg bw per day and 500 mg/kg bw per day, respectively. In neither species was there any evidence of embryotoxicity or teratogenicity. In rats, there was an immediate but transitory decrease in food consumption resulting in lower body-weight gain in the treated animals; however, there was no significant effect on body weight or body-weight gain during gestation. In rabbits, there was no significant effect on overall food consumption, body weight or body-weight gain.
Studies of genotoxicity have examined the ability of neotame to induce gene mutations in both bacterial and mammalian cells, as well as chromosome aberrations in Chinese hamster ovary cells in vitro and in bone marrow cells from mice in vivo. There was no evidence of genotoxicity in any of the tests.
The potential pharmacological effects of neotame on the gastrointestinal system in rats, on the autonomic nervous system in guinea-pig ileum, on the parameters associated with the cardiovascular, respiratory or renal systems in dogs, and on the hexobarbital-induced sleeping time in rats were examined. There was no evidence of pharmacological activity associated with neotame.
Studies of tolerance in humans included a study of single doses in men, 2-week studies in both diabetic and nondiabetic men and women, and a 3-month study in men and women. Single doses of neotame at 0.5 mg/kg bw per day were tolerated without treatment-related signs or symptoms. In both the 2-week studies in diabetic and non-diabetic adults, there were no signs or symptoms associated with the administration of neotame. In the study in diabetic adults, treatment with neotame had no effect on plasma glucose or insulin concentrations. In both these 2-week studies, neotame was well tolerated at a dose of up to 1.5 mg/kg bw per day. In the 3-month study of tolerance in non-diabetic adults, there were no signs or symptoms associated with the administration of neotame at a dose of up to 1.5 mg/kg bw per day.
The major degradation product of neotame under normal storage conditions was de-esterified neotame, which accounted for 7% of the initial concentration of neotame after 8 weeks storage. Three minor degradation products were formed that represented <1% of the initial concentration of neotame. All the degradation products have low acute toxicity and gave negative results in tests for genotoxicity. Furthermore, no treatment-related adverse effects were observed in a 4-week study in rats fed with a mixture of the three minor degradation products. The Committee also noted that the safety studies with neotame in laboratory animals and humans would have included low levels of these degradation products.
On the basis of the available studies, the Committee considered neotame to be a substance of low toxicity in a range of species, including humans. Appropriate studies indicated that neotame is not carcinogenic, mutagenic, teratogenic or associated with any reproductive/developmental toxicity. The only consistent treatment-related effect observed was an increase in serum alkaline phosphatase activity in the 13-week and 1-year studies in dogs fed diets containing neotame . While the increase in alkaline phosphatase was moderate, reversible, and was not accompanied by other evidence of liver toxicity, the observed change was reproducible, of high statistical significance and treatment-related. The Committee agreed there were insufficient data to discount this effect and therefore accepted the dog as the most sensitive species with a NOEL for neotame of 200 mg/kg bw per day, on the basis of the 1-year study in dogs fed diets containing neotame. Studies of tolerance in humans confirmed the lack of any treatment-related signs or symptoms at a dose of up to 1.5 mg/kg bw per day in diabetic and non-diabetic subjects. Although a 1-year study is not considered to be a long-term study in dogs, an additional safety factor was not considered necessary, in light of the data for humans.
The Committee established an ADI of 0–2 mg/kg bw for neotame on the basis of a NOEL of 200 mg/kg bw per day in a 1-year study in dogs and a safety factor of 100.
Assessment of intake. Neotame is intended for use as a tabletop sweetener as well as in a large variety of solid and liquid foods. Conservative calculations based on its lowest sweetness potency (7000 times that of sugar) suggest that an intake of 2 mg/kg bw per day would correspond to the replacement of 840 g of sugar in the diet of a 60-kg adult. Therefore even a total replacement of sugar with neotame would not lead to the ADI being exceeded.
The Committee agreed that the ADI also applied to individuals with phenylketonuria, since the formation of phenylalanine from the normal use of neotame would not be significant in relation to this condition.
Algate, C. (1997) NC-00723: Cardiovascular, respiratory and renal evaluation in the anaesthetised dog following intraduodenal administration. Study No. PCR 1167. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England Submitted to WHO by The NutraSweet Company, Illinois, USA.
Atterson, P. (1997a) NC-00723: Charcoal propulsion test in rats (oral administration). Study No. PCR 1169. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Atterson, P. (1997b) NC-00723: Assessment of hexobarbital sleeping time in rats (oral administration). Study No. PCR 1168. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Atterson, P. (1997c) NC-00723 and NC-00751: Effects on the isolated guineapig ileum. Study No. PCR 1170. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Australia New Zealand Food Standards Code (2003) Standard 1.3.1—Food Additives. Canberra (www.foodstandards.gov.ai/foodstandardscode/).
Balwierz, P. & Bunch, R. (1998a) Evaluation of the mutagenic potential of NC-00777 in the Ames Salmonella/microsome assay. Study No. (PCR 1191). Unpublished report from G.D. Searle and Co., Skokie, Illinois, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Balwierz, P. & Bunch, R. (1998b) Evaluation of the mutagenic potential of NC-00779 in the Ames Salmonella/microsome assay. Study No. PCR 1201. Unpublished report from G.D. Searle & Co., Skokie, IL. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Bechtel, C. (1998a) Single gavage dose study in rats with NC-00764. Study No. PCR 1134. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Bechtel, C. (1998b) Single gavage dose study in rats with NC-00777. Study No. PCR 1189. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Bechtel, C. (1998c) Single gavage dose study in rats with NC-00779. Study No. PCR 1199. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Burnett, P. & Bartekian, V. (1998) Measurement of concentrations of NC-00723 and NC-00751 in plasma samples from an oncogenicity study in CD rats with exposure in utero. Study No. PCR 1049 (addendum to PCR 1000). Unpublished report from Phoenix International Life Sciences Inc., Saint-Laurent (Montreal), Quebec, Canada. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Cabonce, M., Asbury, K., McAdams, J., Wagner, C. & Kier, L. (1997) AS52/XPRT Gene mutation assay with NC-00751. Study No. PCR 1138. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Cabonce, M., Asbury, J., McAdams, J., Wagner, C. & Kier, L. (1998a) AS52/XPRT Gene mutation assay with NC-00764. Study No. PCR 1087. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Cabonce, M., Asbury, K., McAdams, J., Wagner, C. & Kier, L. (1998b) AS52/XPRT Gene mutation assay with NC-00777. Study No. PCR 1192. Unpublished report from Monsanto Company, Monsanto Safety Evaluation, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Cabonce, M., Asbury, K., McAdams, J. & Wagner, C. (1998c) AS52/XPRT Gene mutation assay with NC-00779. Study No. PCR 1202. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Curtiss, S., McAdams, J. & Kier, L. (1997) Ames/Salmonella assay of NC-00751. Study No. PCR 1137. Unpublished report from Ceregen, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Curtiss, S., McAdams, J. & Kier, L. (1998). Ames/Salmonella assay of NC-00764. Study No. PCR 1086. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Food and Drug Administration (2002) Federal Register 172.829. Food additives permitted for direct addition to food for human consumption. Washington, USA (www.cfsan.fda.gov/).
Garrett, S., Kier, L., Carbone, L. & McAdams, J. (1997) Mouse bone marrow micronucleus assay of NC-00723. Study No. PCR 1026. Unpublished report from Monsanto Company, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Garrett, S., Kier, L., Carbone, L. & McAdams, J. (1998) Mouse bone marrow micronucleus assay of NC-00764. Study No. PCR 1090. Unpublished report from Monsanto Company, Environmental Health Laboratory, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hall, M. (1997) NC-00723 Effect on hepatic xenobiotic metabolising enzyme activities in rats by dietary administration for 14 days. Study No. PCR 1032. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hambridge, T. (2003a) Information sheet for Australia on neotame. Unpublished report from Food Standards Australia New Zealand, Canberra, Australia. Submitted to WHO by Food Standards Australia, New Zealand.
Hambridge, T. (2003b) Information sheet for New Zealand on neotame. Unpublished report from Food Standards Australia New Zealand, Canberra, Australia. Submitted to WHO by Food Standards Australia, New Zealand.
Harry, J. & Aikens, P. (1998) An investigation of a urinary metabolite in healthy male subjects after administration of [14C/13C]NC-00723. Study No. (PCR 1215). Unpublished report from Leicester Clinical Research Centre, (clinical phase) Leicester, UK, and Huntingdon Life Sciences, Ltd. (analytical phase), Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hawkins, D., Kirkpatrick, D., Aikens, P. & Saxton, J. (1995a) 14CNC-00723 Tissue distribution in the rat. Study No. PCR 0959. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hawkins, D., Kirkpatrick, D., Aikens, P. & Beeby, T. (1995b) 14CNC-00723 Determination of the distribution in rats by whole-body autoradiography. Study No. PCR 0958. Unpublished report from Huntingdon Life Science Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hawkins, D., Aikens, P. & Beeby, T. (1996a) 14CNC-00723 Determination of the distribution in pregnant and non-pregnant rats by whole-body autoradiography. Study No. PCR 1031. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hawkins, D., Kirkpatrick, D., Shaw, D. & Bennett, S. (1996b) Metabolism of 14CNC-00751 in the rat. Study No. PCR 1119. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Hawkins, D., Kirkpatrick, D., Aikens, P. & Saxton, J. (1997). NC-00723: Pharmacokinetics of single doses in the rat after oral and intravenous administration. Study No. PCR 1028. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Holt, P. & Kirkpatrick, D. (1997) A pharmacokinetics study of 14CNC-00723 in healthy male subjects. Study No. PCR 1039. Unpublished report from Leicester Clinical Research Centre (Clinical Phase), Envington, Leicester, England., and Huntingdon Life Science Ltd. (analytical phase), Huntingdon, Cambridgeshire, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D. & Aikens, P. (1998) 14CNC-00764: Stability in simulated gastric fluid and intestinal fluid. Study No. (PCR 1229). Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D., Aikens, P., Nicholson, J. & Saxton, J. (1997a) 14CNC-00723: Metabolism in the rat. Study No. PCR 1027. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D., Aikens, P., Nicholson, J., Saxton, J. & Harris, K. (1997b) 14CNC-00723: Metabolism and pharmacokinetics in the dog. Study No. PCR 1029. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D., Aikens, P. & Saxton, J. (1997c) 14CNC-00723 and 14CNC-00751: Studies of plasma protein binding in vitro (rat, dog and human). Study No. PCR 1208. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D., Aikens, P. & Harris, K. (1998a) 14CNC-00723: Metabolite isolation from the rat. Study No. PCR 1214. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kirkpatrick, D., Aikens, P. & Hobbs, G. (1998b) Stability of NC-00723 and NC-00751 in simulated gastric and intestinal fluids. Study No. PCR 1218. Unpublished report from Huntingdon Life Sciences Ltd., Huntingdon, Cambridgeshire, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kisicki, J., Azzam, S. & Gao, X. (1997) Single dose tolerance of NC-00723 in healthy male subjects. Study No. PCR 1035. Unpublished report from Harris Laboratories, Inc., Lincoln, Nebraska, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kisicki, J., Combs, M. & Gao, X. (1998a) Effect of repeated ingestion of NC-00723 in solution administered in healthy male subjects. Study No. PCR 1145. Unpublished Report from MDS Harris, Lincoln, Nebraska, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kisicki, J., Combs, M. & Gao, X. (1998b) Twoweek tolerance study of NC-00723 administered to healthy male and female subjects. Study No. PCR 1113. Unpublished report from MDS Harris, Lincoln, Nebraska, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Kisicki, J., Weston, I. & Combs, M. (1998c) 13-week tolerance study of NC-00723 administered to healthy male and female subjects. Study No. PCR 1114. Unpublished report from MDS Harris, Phoenix Arizona/Lincoln, Nebraska, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Lemen, J. (1998) Four-week dietary study of NC-00764/NC-00777/NC-00779 mixture in rats. Study No. PCR 1186. Unpublished report from Monsanto Safety Evaluation, St. Louis, Missouri, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Lui, P. & Cleary, M. (1998) Twenty-six-week stability study of NC-00723 in mock beverages. Study No. NP96–001. Unpublished report from The NutraSweet Kelco Company, Mt. Prospect, Illinois, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Mitchell, D. & Brown, M. (1995) NC-00723: Toxicity study by dietary administration to CD rats for 13-weeks followed by a 4-week reversibility period. Study No. PCR 0988. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Mitchell, D. & Brown, M. (1997a) NC-00723: 52-week toxicity study by dietary administration to CD rats with exposure in utero and followed by a 4-week reversibility period. Study No. PCR 1011. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Mitchell, D. & Brown, M. (1997b) NC-00723: Oncogenicity study by dietary administration to CD Rats with exposure in utero. Study No. PCR 1000. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Morrison, D., Combs, M. & Lu, M. (1998) Effect of multiple doses of NC-00723 compared to placebo on plasma glucose and insulin concentrations in non-insulin dependent diabetes mellitus (NIDDM) subjects. Study No. PCR 1115. Unpublished report from Biokinetic Clinical Applications, Inc. (clinical phase), Springfield, Missouri, and MDS Harris, Phoenix Arizona/Lincoln, Nebraska, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Nicholls, I. (1997a) NC-00723: Dietary preference feasibility study. Study No. PCR 1132. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Nicholls, I. (1997b) NC-00723: Dietary preference study. Study No. PCR 1150. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, UK. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Nicolette, J. & Bunch, R. (1998) An evaluation of the potential of NC-00779 to induce micronucleated polychromatic erythrocytes in the bone marrow of mice (micronucleus test). Study No. PCR 1206. Unpublished Report from G.D. Searle & Co., Skokie, Illinois, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Riccio, E. (1994) Salmonella-Escherichia coli/microsome plate incorporation assay of NC-00723. Study No. PCR 096). Unpublished report from SRI International, Menlo Park, California, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Rudd, C. (1994) L5178Y mouse lymphoma (MOLY) cell tk+/tk/ gene mutation assay with NC-00723. Study No. PCR 0965. Unpublished report from SRI International, Menlo Park, California, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Soelter, S., Bunch, R. & Nicolette, J. (1998) An evaluation of the potential of NC-00777 to induce micronucleated polychromatic erythrocytes in the bone marrow of mice (micronucleus test). Study No. PCR 1196. Unpublished report from G.D. Searle & Co., Skokie, Illinois, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Stargel, W. (2002) Estimated dietary intake of neotame. Unpublished report from The Nutrasweet Company, Chicago, Illinois, USA. Submitted to WHO by The Nutrasweet Company, Illinois, USA.
Tesh, J. & Tesh, S. (2002) Expert Review—Neotame: Two-generation reproduction study in rats. Unpublished report. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. (1994a) Two-week dietary range-finding study of NC-00723 in mice. Study No. PCR-0936. Unpublished report from Hazleton Wisconson Inc., Madison, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. (1994b) Two-week dietary range-finding study of NC-00723 in mice. Study No. PCR-0992. Unpublished report from Hazleton Wisconson Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. (1994c) Two-week dietary range-finding study of NC-00723 in rats. Study No. PCR-0949. Unpublished report from Hazleton Wisconson Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. (1994d) Two-week dietary range-finding study of NC-00723 in rats. Study No. PCR-0994. Unpublished report from Hazleton Wisconson Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. & Carter, J. (1995) 13-week dietary range-finding study of NC-00723 in mice. Study No. PCR 0989. Unpublished report from Hazleton Wisconsin Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. & Carter, J. (1997a) 104-week dietary carcinogenicity study with NC-00723 in CD-1 mice. Study No. PCR 1014. Unpublished report from Covance Laboratories Inc., Madison, WIisconsin, USA Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. & Carter, J. (1997b) 52-week dietary toxicity study of NC-00723 in dogs followed by a 4-week reversibility period. Study No. PCR 1017. Unpublished report from Covance Laboratories Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Thomford, P. & Saunders, W. (1995) 13-week dietary toxicity study of NC-00723 in dogs followed by a 4-week reversibility period. Study No. PCR 0990. Unpublished report from Hazleton Wisconsin Inc., Madison, Wisconsin, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Turk, M. & Bartekian, V. (1998) Measurement of concentrations of NC-00723 and NC-00751 in plasma samples from a 52-week toxicology study in rats. Study No. PCR 1013 (addendum to PCR 1011). Unpublished report from Phoenix International Life Sciences Inc., Saint-Laurent (Montreal), Quebec, Canada. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Weston, I., Azzam, S. & Gao, X. (1997) Assessment of the dose-related pharmacokinetic profile of NC-00723 in solution administered to healthy male subjects. Study No. PCR 1111. Unpublished report from MDS Harris, Phoenix, Arizona, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Weston, I., Combs, M. & Gao, X. (1998) A comparison of the pharmacokinetic profile of NC-00723 in solution and capsules administered to healthy subjects. Study No. PCR 1112. Unpublished report from MDS Harris, Phoenix, Arizona, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Willoughby, C. (1996a) NC-00723: Dietary teratology study in the rat. Study No. PCR 0999. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Willoughby, C. (1996b) NC-00723: Maternal toxicity range finding study administered by gavage to the rabbit. Study No. PCR 1038. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Willoughby, C. (1996c) NC-00723: Teratology study in the rabbit by gavage. Study No. PCR 1023. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Willoughby, C. (1997) NC-00723: Two-generation reproductive study by dietary administration to CD Rats. Study No. PCR 1001. Unpublished report from Huntingdon Life Sciences Ltd., Eye, Suffolk, England. Submitted to WHO by The NutraSweet Company, Illinois, USA.
Winegar, R. (1994) Measurement of chromosomal damage in Chinese hamster ovary (CHO) Cells treated with NC-00723. Study No. PCR 0964. Unpublished report from SRI International, Menlo Park, California, USA. Submitted to WHO by The NutraSweet Company, Illinois, USA.
See Also:
Toxicological Abbreviations
NEOTAME (JECFA Evaluation)