
WHO FOOD ADDITIVES SERIES: 52
First draft prepared by
Dr Gary Williams
Environmental Pathology and Toxicology,
New York Medical College, Valhalla, New York, USA
|
Influence of ferrous glycinate on the chemistry of nutrients |
The call for data for the sixty-first meeting referred to this substance as ferrous bisglycinate. The Committee decided that this name did not accurately describe the substance being evaluated and therefore agreed that it should be referred to as "ferrous glycinate (processed with citric acid)". Ferrous glycinate (processed with citric acid) is an iron [II] chelate with the amino acid glycine, and also contains citric acid. It is manufactured by the reaction of reduced iron with glycine, in the presence of citric acid. At chemical equilibrium, > 97% of the ferrous ions are chelated. The resulting product is spray-dried without prior removal of the citric acid. The substance is highly hygroscopic and may contain water in variable amounts.
At its twenty-seventh meeting (Annex 1, reference 62), the Committee allocated a provisional maximum tolerable daily intake of 0.8 mg/kg bw for iron from all sources, except for iron oxides used as food colouring agents, supplemental iron taken during pregnancy or lactation, and supplemental iron for specific clinical requirements. At its present meeting, the Committee was asked to comment on the safety of ferrous glycinate as a source of iron for dietary supplementation and as a fortificant for general use in food products.
The State of São Paulo, Brazil, has mandated the use of milk fortified with Ferrochel® ferrous glycinate in State-supported assistance programmes for the prevention and treatment of iron-deficiency and iron-deficiency anaemia in young children (Pineda, 2001). Other countries that currently use Ferrochel® ferrous glycinate for the fortification of the iron content of foods include seven in Latin America (Argentina, Chile, Colombia, Ecuador, Mexico, Paraguay, Venezuela), two in Europe (Italy, Spain), and one in Asia (Thailand), as well as Saudi Arabia and South Africa (Pineda, 2001; Allen, 2002).
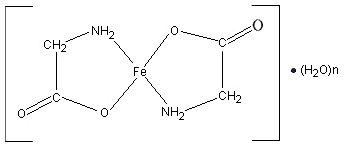
Ferrous glycinate consists of one molecule of ferrous iron covalently bound to two molecules of glycine (see Figure 1) (Ashmead, 2001; Allen, 2002). The iron is bound to the carboxyl group and the alpha-amino group of glycine by coordinate covalent bonds, to form two heterocyclic rings (Atkins & Beran, 1992; McMurry & Fay, 1995). This 1 : 2 metal to ligand ratio restricts reaction with dietary inhibitors of iron absorption, neutralizes the valence of the ferrous iron, and protects gastrointestinal surfaces from irritation by the iron (Jeppsen, 2001; Allen, 2002). This structure makes ferrous glycinate an ideal fortificant for foods with a high content of absorption inhibitors such as phytate (Jeppsen, 2001).

Figure 1. Structural formula of ferrous glycinate
The commercial food-grade formulation of ferrous glycinate is called Ferrochel® ferrous glycinate, and consists of 77% ferrous glycinate chelate, 17% citric acid, 4% moisture, 2% maltodextrin, and < 0.1% silicon dioxide by weight. The chelate itself consists of 27% iron and 73% glycine by weight, while 20% iron is present in the hydrated form of the compound. All components of Ferrochel® ferrous glycinate are acceptable food additives that meet appropriate food-grade specifications.
The solubility of iron from Ferrochel® ferrous glycinate is not affected by changes in pH (e.g. from pH 2 to pH 6) (García-Casal et al., 1997; García-Casal & Layrisse, 2001). Ferrochel® ferrous glycinate has weak pro-oxidant properties and is stable when exposed to air and ambient temperatures (Olivares et al., 1997).
In a study by Hendricks & Ashmead (1995), Ferrochel® ferrous glycinate was added to milk and yoghurt at concentrations providing iron at 3 mg/l, and to corn flour at a concentration providing approximately 9.1 mg/kg. The milk, yoghurt and corn flour fortified with Ferrochel® ferrous glycinate were packaged in sterile containers and stored in a freezer, in a refrigerator, or at room temperature (22 °C). Before packaging, Ferrochel® ferrous glycinate-fortified milk was also subjected to homogenization and pasteurization. Lipid peroxidation was measured by the presence of thiobarbituric acid-reactive substances (TBARS) in Ferrochel® ferrous glycinate-fortified milk and yoghurt after 3, 10, and 18 days of storage, and in the fortified corn flour after 4 weeks of storage. There were no significant differences in bacterial growth or in the quantity of TBARS produced in control and Ferrochel® ferrous glycinate-fortified milk and yoghurt after 3, 10 or 18 days of storage, at any of the temperatures examined; however, after 18 days of storage, the control milk had spoiled, while the Ferrochel® ferrous glycinate-fortified milk had not. Similarly, no significant differences in lipid peroxidation were noted between control and Ferrochel® ferrous glycinate-fortified corn flour after 4 weeks of storage at any of the temperatures examined.
Similar results were reported by Umbelino et al. (2001) after examination of the organoleptic characteristics (fermentation time, pH, titratable acidity, viscosity, consistency, iron concentration and sensory properties) of soya bean yoghurt fortified with iron at 12 mg/l as Ferrochel® ferrous glycinate, with no alterations in fermentation time, titratable acidity or the sensory and rheologic properties of the soya bean yoghurt.
The organoleptic characteristics of margarine fortified at a concentration of 20 or 60 mg/kg with Ferrochel® ferrous glycinate were evaluated every 30 days for 120 days (Name, 1996). Control margarine received a rating of "good" upon evaluation on days 30, 60, and 90 of the study, and a rating of "bad" on study day 120. Margarine fortified with Ferrochel® ferrous glycinate at 20 mg/kg was rated "acceptable" up to day 90, and "bad" on day 120 of the study. Conversely, margarine fortified at a level of 60 mg/kg received ratings of "acceptable" on days 30 and 60, and ratings of "bad" on days 90 and 120 of the study.
A panel of 15 judges trained in descriptive analyses who evaluated the sensory quality of maize meals fortified with ferrous glycinate providing iron at 30 or 60 mg/kg meal, reported no difference in colour between unfortified meals and meals fortified with ferrous glycinate. The degree of lipid oxidation in the meals was evaluated by measuring of hexanal production after storage. Maize meals fortified with ferrous glycinate and stored for 20 days at temperatures of 30, 40, or 50 °C were more rancid (as reflected by high hexanal production) than unfortified maize meals, or those fortified with iron sulfate (FeSO4), ferric trisglycinate, or NaFe–EDTA. The addition as antioxidants of butylated hydroxyanisole at 100 ppm or butylated hydroxytoluene at 200 ppm, singly or in combination with citric acid, decreased hexanal production in maize meals fortified with ferrous glycinate (Bovell-Benjamin et al., 1999).
Despite the results of Bovell-Benjamin et al. (1999), 40 infants aged 6–24 months and their mothers found unfortified maize meals and meals that had been fortified with ferrous glycinate, providing iron at 30 mg/kg and stored at 30°C for 20 days (Bovell-Benjamin et al., 1998) equally acceptable. Additionally, the acceptability rating of ferrous glycinate-fortified maize meal was not affected by the addition of butylated hydroxyanisole at 50 ppm.
Iron is a normal constituent of the diet and of the body, and is absorbed from the gastrointestinal tract by one of two pathways, depending on whether the iron is in the organic (haem) or inorganic (non-haem) form. Dietary haem iron, which is derived primarily from haemoglobin and myoglobin in meat, is absorbed into the intestinal cells as the intact porphyrin complex (INACG, 1993; IOM, 2001). Iron is released from this complex by the haem oxygenase enzyme and transferred to the bloodstream for transport, together with other iron taken up by the cells (INACG, 1993). Haem iron is highly bioavailable (20–25% absorption) and is relatively unaffected by dietary factors (INACG, 1993; IOM, 2001). Non-haem iron, which is derived from various food sources (e.g. vegetables, dairy products, meat and dietary iron fortificants), is solubilized and transferred into a common pool of non-haem iron located in the lumen of the upper gastrointestinal tract (Cook et al., 1972; INACG, 1993; IOM, 2001; MacPhail, 2001). In contrast to haem iron, the amount of non-haem iron absorbed from this pool is greatly affected by the presence of ligands in undigested or partially digested foods, which either enhance (e.g. ascorbic acid) or inhibit (e.g. polyphenols, phytate) absorption (Cook et al.,1972; INACG, 1993; IOM, 2001; MacPhail, 2001). Additionally, some insoluble forms of iron may be ingested; they therefore do not enter the common non-haem iron pool and are not absorbed (INACG, 1993).
Ferrous glycinate (processed with citric acid) enters the intestinal intraluminal pool of inorganic, non-haem iron. The absorption of ferrous glycinate in the intestine would be expected to follow the general mechanism of absorption for amino acid-metal chelates proposed by Ashmead et al., (1985), whereby ferrous glycinate is absorbed intact via the dipeptide pathway and subsequently hydrolysed into its respective iron and glycine components in the intestinal mucosa. In studies in which 59FeSO4 and 55Fe-glycinate (55Fe-Ferrochel® ferrous glycinate) were administered together in a whole-maize or wheat-flour meal (Allen et al., 1998; Bovell-Benjamin et al., 2000; Layrisse et al., 2000), significantly more iron was absorbed from ferrous glycinate (or Ferrochel® ferrous glycinate) than from FeSO4. There was no exchange of radiolabelled iron (i.e. 59Fe and 55Fe) between ferrous glycinate (or Ferrochel® ferrous glycinate) and FeSO4 in the non-haem iron pool prior to intestinal absorption, indicating that ferrous glycinate (or Ferrochel® ferrous glycinate) was absorbed intact into the mucosal cells of the intestine (Allen et al., 1998; Bovell-Benjamin et al., 2000; Layrisse et al., 2000).
After absorption into the mucosal cells, the iron is hydrolysed and then distributed, reversibly bound to transferrin, for use in proteins, including storage proteins (e.g. ferritin and haemosiderin), transport proteins (e.g. transferrin and lactoferrin), haem-containing proteins (e.g. haemoglobin, myoglobin, and cytochromes), and enzymes (e.g. iron-containing and activated non-haem enzymes, iron-sulphur enzymes or flavoproteins) (IOM, 2001; MacPhail, 2001). The average total concentration of body iron is approximately 50 and 40 mg/kg bw in men and women, respectively (Bothwell et al., 1979). Iron in storage proteins makes up about 20–30% of the iron in the body, and primary iron storage sites have been identified in the cells of the liver, spleen and bone marrow. Approximately 60–70% of total body iron is present in the haemoglobin of circulating erythrocytes, while 10% has been identified in myoglobin, cytochromes, and other iron-containing enzymes (IOM, 2001). The amount of iron in the body is highly conserved, with daily basal losses of 0.60 mg in men, 0.64 mg in non-menstruating women, and 1.20 mg in menstruating women (Green et al., 1968). The majority of iron loss has been reported to be a consequence of faecal excretion. Iron losses also may occur in the urine, gastrointestinal tract and skin, which contribute approximately 0.08, 0.60, and 0.20–0.30 mg of the iron, respectively, that is lost from the body per day (Green et al., 1968; IOM, 2001).
After dissociation from ferrous glycinate, the free amino acid, glycine, is used in normal metabolic processes. Glycine, the smallest of the amino acids, is essential for the biosynthesis of nucleic acids and other amino acids, as well as proteins, such as collagen and elastin. In addition, glycine functions as a major inhibitory neurotransmitter in the spinal cord and brainstem of the central nervous system (Lodish et al., 1995; Silverthorn, 1998).
Ferrous fumarate and ferrous sulfate lose solubility as pH increases toward neutrality (García-Casal et al., 1997). Aided by its high solubility at physiological pH (García-Casal & Layrisse, 2001), ferrous glycinate (or Ferrochel® ferrous glycinate) maintains high iron bioavailability in foods, despite the presence of iron absorption inhibitory factors such as phytic acid, which form insoluble complexes with iron (Bovell-Benjamin et al., 2000; Layrisse et al., 2000). The absorption of iron from ferrous glycinate (or Ferrochel® ferrous glycinate) is regulated physiologically by the body’s iron status; like other iron compounds (Olivares et al., 1997; Allen et al., 1998; Iost et al., 1998; Bovell-Benjamin et al., 2000; Layrisse et al., 2000; Giorgini et al., 2001; Olivares & Pizarro, 2001). Therefore, fortification of foods with ferrous glycinate (or Ferrochel® ferrous glycinate) would not be expected to result in iron overload in otherwise normal populations.
Ferrous glycinate (or Ferrochel® ferrous glycinate) has been used in numerous studies of absorption conducted to assess iron absorption and/or iron status in healthy volunteers and in populations with low iron status. In several studies, ferrous ascorbate was administered as a reference dose under standardized conditions in order to minimize the effects of individual variations in absorptive capacity (i.e. absorption of iron from a given test meal/carrier food is expressed as a ratio to the reference dose) (Baynes et al., 1987). In each study, the carrier foods used to administer the iron, the level of use, and the resulting exposures to iron have been identified for comparison.
A comparative study of the absorption of iron from ferrous glycinate, ferric trisglycinate, and iron sulfate in a whole-maize meal was conducted by Bovell-Benjamin et al. (2000). Ten male healthy volunteers (aged 19–30 years) with normal iron status were provided with a porridge meal (consisting of 87.5 g whole-maize meal in 625 ml boiling water, 50 g dry non-fat milk, 125 g margarine, and 37 g Jamaican brown sugar) fortified with iron at 11 mg/kg maize as 59FeSO4 on day 1, and as 55Fe-glycinate on day 2. The total iron content of the meal was reported to be about 1.9 mg/serving. Fourteen days later (on day 16 of the study), fasting blood samples were obtained from each of the men for measurement of the amount of 59Fe and 55Fe incorporated into erythrocytes. Pre-study blood samples also were obtained for measurement of baseline serum ferritin, haemoglobin, and erythrocyte radioactivity. Assuming that 85% of absorbed radioactivity was incorporated into erythrocytes, iron absorption was calculated from the estimated blood volume of each subject. The geometric mean percentage of iron absorbed from ferrous glycinate (6%) was significantly greater than from FeSO4 (1.7%) in a whole-maize porridge. After fasting blood samples had been taken on day 16, the men ate a whole-maize porridge fortified with iron (as FeSO4) at 0.011 mg/g of maize and containing a mixture of 55.5 kBq 59FeSO4 and 111 kBq 55Fe-glycinate. Blood samples were again drawn from the men 14 days later (on day 31 of the study) for measurement of radioactivity incorporated into erythrocytes. The percentage of absorption from each source of iron was unaffected by the simultaneous presence of the respective sources. As reported after consecutive administration, a significantly greater geometric mean percentage of iron was absorbed from ferrous glycinate (6.8%) than from FeSO4 (1%). Additionally, when the results for the 31-day study period were combined, the average absorption of iron from ferrous glycinate was reported to be significantly higher (4.7-fold) than from FeSO4. Plasma ferritin values were reported to be negatively correlated with the percentage of iron absorbed from both sources of iron, which the authors indicated to be suggestive of down-regulation of iron absorption when iron reserves were sufficient. The men who absorbed the least or the most iron from FeSO4 were also reported to have absorbed the least or most iron from ferrous glycinate.
In a second study by Bovell-Benjamin et al. (2000), 23 healthy, non-pregnant female volunteers (aged 18– 48 years) with a wide range of iron statuses were given a dose of ferrous ascorbate reference solution (30 mg ascorbic acid and 3 mg iron as 59FeSO4) before or after drinking a solution containing 3 mg of iron as 55Fe-glycinate in 10 ml distilled water. Baseline blood radioactivity and serum ferritin, and haemoglobin concentrations were determined for each woman from blood samples obtained before treatment. Two weeks later (on day 16 of the study), fasting blood samples were obtained from each woman for measurement of the amounts of 59Fe and 55Fe incorporated into erythrocytes. Complete data were available for only 21 women as one woman withdrew for personal reasons and the data for another woman were discarded because of technical problems. Geometric mean iron absorption from the ferrous ascorbate reference dose (32.5%) was reported to be significantly higher than that from ferrous glycinate (9.1%). After fasting blood samples had been drawn on days 16 and 17, the women were randomly assigned to drink water containing 55.5 kBq 59Fe-trisglycinate or to eat whole-maize porridge fortified with iron (as 111 kBq 55Fe-trisglycinate at 11 mg)/kg of maize. The total iron content of the meal was reported to be 1.863 mg/serving. Blood samples were drawn from the women 14 days later (on day 31 of the study) for measurement of radioactivity incorporated into erythrocytes. Significantly more iron was absorbed from ferric trisglycinate administered in water (15.3%) than from ferric trisglycinate administered in whole-maize porridge (2.3%). The percentages of the geometric mean of iron absorption in water were significantly different for the three sources of iron: ferrous ascorbate (32.5%) > ferric trisglycinate (15.3%) > ferrous glycinate (9.1%). Although in the first study significantly less iron was observed after administration of FeSO4 in whole-maize porridge than after administration in water (as the reference dose, ferrous ascorbate), in this study no significant difference in iron absorption was reported from water or maize. On the basis of a highly significant negative correlation noted between iron absorption from any of the three sources of iron (ferrous ascorbate, ferrous glycinate, or ferric trisglycinate) and serum ferritin, the authors concluded that there is an effective down-regulation of iron absorption as iron reserves increase. They also concluded that the iron from ferrous glycinate does not exchange with the non-haem iron pool before intracellular incorporation; however, once inside the cell, its absorption is regulated by the same mechanisms as iron from FeSO4. Additionally, they concluded that ferrous glycinate is an effective and safe source of iron for use as a fortificant, particularly in foods rich in phytate.
The bioavailability of iron from an aqueous solution of ferrous glycinate (as Ferrochel® ferrous glycinate) was studied in a group of 14 healthy, non-pregnant, female volunteers aged 27–51 years (Olivares & Pizarro, 2001). The women were given 200 ml of an aqueous solution containing 111 kBq 55Fe-Ferrochel® ferrous glycinate on day 1 and a similar volume of a reference dose solution containing 37 kBq 59Fe-ascorbate on day 2, to correct for interindividual differences in iron status (both solutions providing 3 mg iron/day). Blood samples were obtained from each of the subjects 2 weeks later (on day 16 of the study) for measurement of erythrocyte radioactivity and iron status (i.e. haemoglobin, mean cell volume, free erythrocyte protoporphyrin, serum iron, total iron-binding capacity (TIBC) and serum ferritin). The bioavailability of iron from aqueous solutions of Ferrochel® ferrous glycinate and ferrous ascorbate was reported to be 34.6% and 29.9%, respectively. Upon standardization to 40% absorption of the reference dose of ferrous ascorbate, the corresponding absorption of iron from Ferrochel® ferrous glycinate was 46.3% (in a population with low iron stores). Nevertheless, these values were reported to be not significantly different for the two iron preparations. A significant inverse correlation was reported between the natural logarithm values of serum ferritin and iron absorption from Ferrochel® ferrous glycinate. According to the authors, this inverse relationship suggests regulation of iron absorption from Ferrochel® ferrous glycinate by iron stores (i.e. serum ferritin). This conclusion was further confirmed by a highly significant correlation between the natural logarithm values of iron absorption from aqueous solutions of ferrous ascorbate and Ferrochel® ferrous glycinate.
Olivares et al. (1997) studied the bioavailability of iron supplied as Ferrochel® ferrous glycinate administered in water or milk to groups of 14 healthy, non-pregnant female volunteers aged 33–51 years. In the first study, one group of women received 200 ml of an aqueous solution containing 15 mg/l of iron as 111 kBq 55Fe-Ferrochel® ferrous glycinate on day 1, and 200 ml of a reference dose solution containing 15 mg/l of iron as 37 kBq 59Fe-ascorbate on day 2 (both solutions providing approximately 3 mg iron/day). Venous blood samples were obtained from each of the subjects 2 weeks later (on day 16 of the study) for measurement of erythrocyte radioactivity and iron status indicators (i.e. haemoglobin, mean cell volume, free erythrocyte protoporphyrin, serum iron, TIBC and serum ferritin). The women subsequently received 200 ml of whole cows’ milk fortified with 15 mg/l of iron as 111 kBq 55Fe-Ferrochel®ferrous glycinate (equivalent to 3 mg iron/day). Two weeks later (on day 31 of the study), venous blood samples were once again obtained for measurement of circulating erythrocyte radioactivity. The absorption of iron the two aqueous solutions of Ferrochel® ferrous glycinate (34.6%) and ferrous ascorbate (29.9%) was not significantly different between; however, a significantly lower iron bioavailability was reported from Ferrochel® ferrous glycinate administered in milk (8.3%) compared to that from Ferrochel® ferrous glycinate administered in water. Upon standardization of iron absorption to 40% of the reference dose of ferrous ascorbate, the corresponding percentages of iron absorption from Ferrochel® ferrous glycinate-fortified milk and water were 11.1% and 46.3%, respectively.
In a second study by Olivares et al. (1997), a group of 14 women were given 200 ml of an aqueous solution containing 15 mg/l of iron as 37 kBq 59Fe-ascorbate on day 1, and 200 ml of whole cows’ milk fortified with 15 mg/l of iron as 111 kBq 55Fe-Ferrochel® ferrous glycinate plus 100 mg/l of ascorbic acid on day 2 (both solutions providing approximately 3 mg iron/day). For measurement of circulating radioactivity and determination of iron status, blood samples were obtained from the women on day 16 of the study. Fortification of whole cows’ milk with Ferrochel® ferrous glycinate and ascorbic acid slightly increased iron absorption, from 8.3% to 10.7%, and from 11.1% to 15.4% when adjusted to 40% absorption of the reference dose, with the latter increase being statistically significant. To account for interindividual differences in iron status, the ratio of iron absorption from milk fortified with Ferrochel® ferrous glycinate to that from the reference dose solution (from study 1) was compared with the ratio of iron absorption from milk fortified with Ferrochel® ferrous glycinate plus ascorbic acid to that from the reference dose solution (from study 2). The ratio of iron absorption obtained from study 2 was significantly higher than that obtained from study 1. Significant inverse relationships were reported between serum ferritin and iron absorption from an aqueous solution of Ferrochel® ferrous glycinate or ferrous ascorbate. Additionally, the amount of iron absorbed from ferrous ascorbate was significantly and directly related to that absorbed from Ferrochel® ferrous glycinate in water or in milk (with ascorbic acid). According to the authors, these results suggested a controlled effect of the iron stores (i.e. serum ferritin) of the women on the absorption of iron from Ferrochel® ferrous glycinate. The amount of iron absorbed from milk fortified with Ferrochel® ferrous glycinate and ascorbic acid was not significantly correlated with serum ferritin or with the amount absorbed from the ferrous ascorbate reference dose solution. Therefore, the authors concluded that ascorbic acid had no significant effect on the absorption of iron from Ferrochel® ferrous glycinate-fortified milk.
A comparative study of the bioavailability of iron from Ferrochel® ferrous glycinate, Fe–EDTA, and FeSO4 was conducted by Layrisse et al. (2000). A total of 74 healthy volunteers aged 15–50 years (18 male and 56 female) of low socio-economic status in Valencia, Venezuela, participated in five studies of absorption. Each person was allowed to participate in only one study, and participation was determined by random selection. The number of participants and the meals administered in each study are presented in Table 1.
Table 1. Composition of meals fortified with Ferrochel ® ferrous glycinate, FeSO4, or Fe–EDTA administered to volunteers participating in studies of iron absorption1
|
Study No. |
No. of subjects |
Meal A |
Meal B |
Meal C |
Meal D |
|
1 |
13 (4 male |
Basal corn flour meal2 on the morning of day 1 |
Basal corn flour meal + 3 mg of iron as 55FeSO4 on afternoon of day 1 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate on the of day 15 |
Basal corn flour meal + 3 mg of iron as 55Fe–EDTA on the afternoon of day 15morning |
|
2 |
13 (1 male |
Basal wheat flour meal3 on the morning of day 1 |
Basal wheat flour meal+ 3 mg of iron as 55FeSO4 on the afternoon of day 1 |
Basal wheat flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate on the morning of day 15 |
Basal wheat flour meal + 3 mg of iron as 55Fe–EDTA on the afternoon of day 15 |
|
3 |
17 (all female) |
Basal wheat flour meal + 3 mg of iron as 59FeSO4 + 3 mg of iron as 55Fe-Ferrochel® ferrous glycinate on day 1 |
Basal wheat flour meal + 3 mg of iron as 59FeSO4 on day 15 |
Basal wheat flour meal + 3 mg of iron as 55Fe-Ferrochel® ferrous glycinate on day 15 |
— |
|
4 |
17 (8 male, |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate on day 1 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate + 2 g of a coffee beverage on day 1 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate + 4 g of a coffee beverage on day 15 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate + a tea beverage (containing 1.6 g of tea leaves) on day 15 |
|
5 |
14 (5 males, |
Basal corn flour meal + 3 mg of iron as 59FeSO4 on day 1 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate on day 1 |
Basal corn flour meal + 3 mg of iron as 59FeSO4 + 304 U of phytase on day 15 |
Basal corn flour meal + 3 mg of iron as 59Fe-Ferrochel® ferrous glycinate + 304 U of phytase on day 15 |
|
1 |
Table adapted from Layrisse et al. (2000) |
|
2 |
The basal corn flour meal consisted of 100 g pre-cooked corn flour + 50 g cheese + 10 g margarine, with a basal iron content of 1.5 mg |
|
3 |
The basal wheat flour meal consisted of 100 g white wheat flour + 50 g cheese + 10 g margarine, with a basal iron content of 1.6 mg |
Blood samples were obtained from each person 15 days after they had eaten the appropriate meals (i.e. on days 16 and 30 of the study) for measurement of erythrocyte radioactivity and haemoglobin and serum ferritin concentrations. In study 1, significantly more iron was absorbed from foods fortified with Ferrochel® ferrous glycinate (8.4%) or FeEDTA (10.5%) than from unfortified (3.2%) or FeSO4-fortified foods (4.7%). The mean iron absorption of 4/13 subjects who were iron-deficient in study 1 also was also reported to be significantly greater from foods fortified with Ferrochel® ferrous glycinate (13%) or FeEDTA (14%), than from foods fortified with FeSO4 (6%). In study 2, significantly more iron was absorbed from foods fortified with Ferrochel® ferrous glycinate (10.8%) or FeEDTA (14.9%) than from unfortified (3.0%) or FeSO4-fortified foods (5.3%). The mean iron absorption of 4/13 subjects who were iron-deficient in study 2 also was reported to be significantly higher from foods fortified with Ferrochel® ferrous glycinate (12%) or FeEDTA (15%), compared to that from foods fortified with FeSO4 (7%). In study 3, significantly more iron was absorbed from Ferrochel® ferrous glycinate, than from FeSO4 when they were administered together or in different meals. The percentages of iron absorbed from meals A (fortified with FeSO4 and Ferrochel® ferrous glycinate), B (fortified with FeSO4), and C (fortified with Ferrochel® ferrous glycinate) were reported to be 8.3%, 5.8%, and 9.7%, respectively; however, the difference in iron absorption between meals A and C was not statistically significant. Administration of 4 g of coffee or 1.6 g of tea with Ferrochel® ferrous glycinate-fortified corn meal (study 4) significantly decreased iron absorption from 7.5% to 3.9%; however, no significant change in iron absorption was observed when 2 g of coffee were taken with Ferrochel® ferrous glycinate-fortified corn meal (6.7%). In study 5, addition of phytase to corn flour meals fortified with FeSO4 and Ferrochel® ferrous glycinate significantly increased iron absorption, from 5.1% to 10.1% and from 7.9% to 13.2%, respectively.
Allen et al. (1998) conducted a study involving the fortification of high-fat, un-de-germed maize with iron as 59FeSO4 or 55Fe-glycinate at 11 mg/g given to 10 fasted non-anaemic male volunteers (aged 19–30 years). The men ate a maize porridge meal containing 55.5 kBq 59FeSO4 at breakfast on day 1, and a similar meal containing 111 kBq 55Fe-glycinate at breakfast on day 2. Blood samples were collected before the start of the study and 2 weeks after the second maize porridge meal had been eaten (i.e. on day 15 of the study). On day 15, the subjects were given maize porridge meals containing both 55.5 kBq 59FeSO4 and 111 kBq 55Fe-glycinate. Two weeks later, blood samples were taken for measurement of erythrocyte radioactivity. Blood analysis indicated that four to seven times more iron was absorbed from ferrous glycinate than from FeSO4. When the data were analysed by analysis of variance, the differences in absorption from the two sources of iron were highly significant. The mean values for iron absorption from ferrous glycinate-fortified meals were reported to be 8.68% and 6.95% when administered with and without FeSO4, respectively, while the mean values for absorption from FeSO4-fortified meals were 1.3% and 1.59% when administered with and without ferrous glycinate, respectively. Additionally, a negative correlation between ferritin and iron absorption was noted with both compounds, which the authors reported to be suggestive of similar regulation of iron absorption by iron status for both iron sources.
Groups of infants aged 9 months participated in two studies of the bioavailability of iron from puréed vegetable meal and from whole-grain cereal fortified with 57Fe]- or [58Fe] ferrous glycinate or FeSO4 (Fox et al., 1998). Solutions of the two iron compounds were used to fortify the meals, and in all cases, 0.83 mg of ascorbic acid was added per mg of iron present in [57Fe]- or [58Fe]-FeSO4 solutions to reduce ferric iron to the ferrous form. In study 1, 22 infants (12 female and 10 male) with normal iron status (mean haemoglobin concentration, 114 g/l) were randomly assigned to two groups and given 150 g of commercial puréed vegetable weaning food (containing 1.62 mg of iron per serving) comprising parsnips, potatoes, cauliflower and milk, and fortified with FeSO4 or ferrous glycinate at 1.4 mg per serving as. One group received puréed vegetable meals fortified with [57Fe]-FeSO4 or [58Fe]-glycinate, while the other group received similar meals fortified with [58Fe]-FeSO4 or [57Fe]-glycinate, both on alternating days for 8 days. Fourteen days after the last test meal, all the infants were subjected to heel-prick blood sample testing, iron isotope ratio and body weight were measured, and blood volume was estimated. The mean per cent incorporation of iron from FeSO4 (9.1%) and ferrous glycinate (9.8%) into haemoglobin was reported to be not significantly different. In a second study, 24 infants (8 female and 16 male) with normal iron status (mean haemoglobin concentration, 118 g/l) were randomly divided into two groups and given 150 g of a puréed vegetable meal (as in study 1) or 20 g of a dry, commercial, whole-grain cereal food (containing 1.57 mg of iron per serving) made from wheat, rye, oats, rice, wheat bran, and 120 g milk, and fortified with FeSO4 or ferrous glycinate at 1.4 mg iron per serving as. Group 1 received [57Fe]-or [58Fe]-FeSO4, with the puréed vegetable meal or whole-grain cereal meal, administered alternately. Group 2 received [57Fe]- or [58Fe]-glycinate alternately with the same meals as group 1. Heel-prick blood samples were obtained from the infants 14 days after the last test meal, and body weights were measured and total circulating blood volume estimated. As in study 1, the mean incorporation of iron from FeSO4 (9.1%) and from ferrous glycinate (9.8%) into haemoglobin was not significantly different when these substances were administered to infants in a puréed vegetable meal. Lower iron bioavailability was, however, reported for FeSO4 (3.8%) and ferrous glycinate (5.2%) administered in a whole-grain cereal meal, although this difference was not statistically significant. The authors attributed the significantly lower iron bioavailability from the whole-grain cereal meal to its significantly higher phytic acid content (77.5%) compared to that of the puréed vegetable meal (22.1%). According to Fox et al. (1998), the inhibiting effect of phytic acid on the bioavailability of iron from ferrous glycinate suggested dissociation of iron from the iron-glycine complex and its subsequent incorporation into the common non-haem iron pool in the gastrointestinal tract.
Both the iron and glycine components of ferrous glycinate are metabolized via normal metabolic processes, without the generation of exogenous chemicals as a result of biotransformation.1
As previously noted, the chemical structure of ferrous glycinate confers protection from oxidation and other chemical reactions with compounds in the food matrix. The addition of ferrous glycinate to multivitamin preparations has been reported not to affect the stability of vitamins.
Iron Metalosate®, an animal feed supplement consisting of iron amino acid chelates containing ferrous glycinate, added to provide a concentration of iron of 5 mg/ml to a solution of vitamin A (20 000 UI/ml), had no effect on the level of vitamin A over 324 days (Marchetti et al., 2000). An accelerated degradation of vitamin A activity was, however, observed in the presence of 5 mg/ml of ferrous chloride.
The stability of vitamins and minerals was assayed in a 17-month study of a preparation in which several metal amino acid chelates and a multivitamin preparation containing vitamins A, C, D and E, and the vitamin B series were integrated into a powdered blend and subsequently encapsulated (Ashmead & Ashmead, 1995). Each capsule was formulated to contain Chelazome® metal amino acid chelates of copper, manganese, and zinc at concentrations of 0.45–0.55 mg, 0.86–1.05 mg, and 1.37–1.67 mg, respectively. Additionally, 36–44 µg of chromium (as the chelate product Chromium Chelavite®) and 1.8–2.2 mg of iron supplied as Ferrochel® ferrous glycinate were incorporated into each capsule. The stability of these chelated products was demonstrated by a lack of interaction between the metal amino acid chelates and any of the components of the capsules, including the vitamins, which were reported to retain levels within the specifications of the United States Pharmacopeia for the duration of the study.
The oral LD50 for ferrous glycinate (as Ferrochel® ferrous glycinate) was determined to be 2800 mg/kg bw in male and female rats, which corresponds to an iron concentration of approximately 560 mg/kg bw (95% CI, 399–786 mg/kg bw) (Kukulinski, 1993; Jeppsen & Borzelleca, 1999). Signs of toxicity observed in the rats included hunched posture, hypoactivity, hypothermia, prostration, poor coordination, and loose stools.
A range-finding study was conducted in CD Sprague-Dawley rats. Groups of three male and three female rats received 0, 300, or 500 mg/kg bw of ferrous glycinate (as Ferrochel® ferrous glycinate, containing 20.34% iron and 55% chelated glycine by weight) orally by gavage for 14 days. These treatments corresponded to doses of iron of approximately 0, 60, and 100 mg/kg bw per day. The rats were observed twice daily for signs of morbidity or mortality, and body weights and food consumption were measured weekly. The animals were killed at the end of the treatment period and a complete gross pathological examination was conducted. No deaths occurred during the study. With the exception of a red focus in the glandular portion of the stomach and thick black material in the caecum of female receiving the highest dose (500 mg/kg bw), no other clinical findings were reported in any of the rats treated with Ferrochel® ferrous glycinate (Jeppsen & Borzelleca, 1999).
On the basis of the results of the 14-day range-finding study, groups of CD Sprague-Dawley rats (20 of each sex per group) were fed ferrous glycinate (as Ferrochel® ferrous glycinate) in the diet at a concentration of 0, 100, 250, or 500 mg/kg bw per day for 13 weeks (Jeppsen & Borzelleca, 1999; Mandella, 2000). The actual daily intake of Ferrochel® ferrous glycinate was reported to be 0, 99.6, 249, and 497 mg/kg bw for males, and 0, 99.4, 247.3, and 499.4 mg/kg bw for females. This study was conducted in compliance with the United States Food and Drug Administration regulations for good laboratory practices. The animals were given access to water ad libitum. They were observed twice daily for signs of pharmacological or toxicological effects, morbidity and mortality, and a thorough physical examination was performed three times before initiation of the study, as well as weekly thereafter. Food consumption was measured twice and body weights were measured three times before the start of the study, and weekly thereafter. Ophthalmoscopic examinations were performed on each animal before initiation of the study and before necropsy at the end of 13 weeks of treatment. Blood samples were drawn for determination of haematological and clinical chemical parameters. The haematological parameters evaluated included haemoglobin concentration, erythrocyte volume fraction, erythrocyte count and morphology, reticulocyte count, platelet count, mean corpuscular volume, mean corpuscular haemoglobin concentration, prothrombin time, activated partial thromboplastin time, and total and differential leukocyte counts. The clinical chemistry parameters evaluated included the activities of aspartate aminotransferase, alanine amino-transferase, alkaline phosphatase and gamma-glutamyl transferase, the concentration of blood urea nitrogen, fasting glucose, total protein, albumin, globulin (calculated), albumin : globulin ratio (calculated), creatinine, total bilirubin, sodium, potassium, chloride, calcium and inorganic phosphorus. Upon gross necropsy of all animals at the end of 13 weeks, the adrenal glands, brain, kidneys, testes with epididymides, liver, ovaries, thymus, spleen and stomach were removed and weighed. A total of 60 samples of tissues and organs (including the lungs, liver, and kidney) from animals in the control groups and groups receiving the highest dose (500 mg/kg bw per day) were preserved for histopathological examination, and on the understanding that any tissue found to be abnormal in animals in the group receiving the high dose would also be examined in animals receiving the two lower doses. No deaths occurred during the study, and no compound-related, dose-dependent adverse effects on cageside observations, the results of physical and ophthalmoscopic examinations, body-weight gain, food consumption, haematological and clinical chemistry parameters, and absolute and relative organ weights were reported. Male rats at all doses and females at the highest dose had significantly increased food consumption at several intervals throughout the study, but this was not considered to be toxicologically significant. The mean hepatic non-haem iron concentration was increased by approximately 1.6-fold in males at the two higher doses, and by about 1.4-fold in females at the highest dose of Ferrochel® ferrous glycinate, compared with controls. These increases were not linearly related to dose, however, indicating the presence of a physiological control on the absorption and distribution of the iron from Ferrochel® ferrous glycinate. No significant differences in mean haemoglobin concentration or erythrocyte volume fraction were reported. Slight but statistically significant increases in mean corpuscular volume and haemoglobin were reported in males receiving the highest dose when compared to controls, but these values remained within the normal range, and were therefore not considered to be toxicologically significant. Males receiving the two lower doses had significantly lower concentrations of potassium than controls, and males receiving the intermediate dose also had decreased activity of aspartate aminotransferase; however, these effects were not related to dose and were considered not to be toxicologically significant. Two females receiving the intermediate dose had significantly increased absolute and relative spleen weights when compared with controls, but these effects were not considered to be toxicologically significant owing to the absence of a dose–response relationship. Histopathological examination revealed no biologically or statistically significant, dose-dependent, macroscopic or microscopic findings that could be attributed to treatment with Ferrochel® ferrous glycinate. The NOAEL for Ferrochel® ferrous glycinate was 500 mg/kg bw per day, corresponding to a dose of iron of approximately 100 mg/kg bw per day.
No information was available.
No information was available.
No information was available.
Ferrous glycinate (or Ferrochel® ferrous glycinate) has been used in field trials as a source of iron for food enrichment and fortification purposes in various countries, including Brazil (Queiroz & Torres, 1995; Fisberg et al., 1995; Gualandro & Name, 1996; Ashmead et al., 1997; Iost et al., 1998; Giorgini et al., 2001; Szarfarc et al., 2001), Guatemala (Pineda et al., 1994; Pineda & Ashmead, 2001), New Zealand (Heath et al., 2001), Saudi Arabia (Osman & al-Othaimeen, 2002) and the United Republic of Tanzania (Latham et al., 2001). These studies have shown that the use of ferrous glycinate (or Ferrochel® ferrous glycinate) as a dietary supplement or iron fortificant in foods is efficacious in reducing the prevalence of iron deficiency and iron deficiency anaemia in humans.
(a) Ferrous glycinate supplementation
Coplin et al. (1991) assessed the tolerability of daily doses of 50 mg of iron supplied as Ferrochel® ferrous glycinate or FeSO4 in a randomized, double-blind, crossover study of 40 pre-menopausal, non-pregnant women (aged 18–40 years) with normal iron status. The women were given a single capsule containing 50 mg of iron each morning before breakfast for 14 days. The severity and prevalence of eight symptoms (i.e. abdominal pain, bloating, constipation, diarrhoea, nausea, vomiting, headache, and fatigue), as well as any other self-reported symptoms, were evaluated by the women daily, on a scale of 0 (none present) to 3 (severe). A total of 38 women finished the 28-day study period, two women having with-drawn for reasons other than intolerance to the iron preparations. No severe scores were reported, and all the side-effects were evaluated as being mild to moderate. The incidence of side-effects did not increase from week 1 to week 2 of the treatment period, indicating that the effects were not cumulative. Supplementation with Ferrochel® ferrous glycinate or FeSO4 produced no significant difference in the fre-quency of side-effects, with weekly averages of 9.4 and 9.3 occurrences, respectively. Adverse effects while receiving Ferrochel® ferrous glycinate and FeSO4 supplementation were reported by a total of 25/38 (66%) and 26/38 (68%) participants, respectively. Of these, gastrointestinal side-effects were reported in 24%, 34%, and 32% of those receiving supplements of Ferrochel® ferrous glycinate, FeSO4, and both sources of iron, respectively. Of the 28 women with total scores for gastrointestinal side-effects of >5, 29%, 50%, and 21% of women had received supplements of Ferrochel® ferrous glycinate, FeSO4, and both sources of iron, respectively. These differences were not statistically significant. A rank sum analysis of symptom scores was performed for each subject, whereby the symptom score for the FeSO4 preparation was subtracted from the score for the Ferrochel® ferrous glycinate preparation, and the differences were subsequently ranked and summed. On the basis of the results of this analysis, the authors concluded that there was no significant difference in tolerance to the two iron preparations. In terms of total scores for gastrointestinal side-effects (especially bloating, constipation and nausea), however, a better tolerance for the Ferrochel® ferrous glycinate than to the FeSO4 preparation was noted. Significantly more women preferred the Ferrochel® ferrous glycinate preparation (61%) to the FeSO4 preparation (29%). The authors predicted that a significant difference in the frequency of side-effects between the two preparations would have been observed with a larger sample size of 120 subjects (Coplin et al., 1991).
Forty-two males and 58 females (aged 10–19 years) from the lowlands of Guatemala with a mean haemoglobin concentration of 11.23 g/dl (indicative of anaemia) were assigned to one of four groups (21–27 individuals per group). The participants received tablets containing 30 mg of iron supplied as Ferrochel® ferrous glycinate once (group 1), twice (group 2), or four times (group 3) daily for a period of 4 weeks, thus receiving a total dose of 30, 60, or 120 mg iron/day, respectively. Another group (group 4) received a total daily dose of 120 mg of iron supplied as FeSO4, administered as four tablets containing 30 mg iron per tablet for a period of 4 weeks. All of the subjects were also provided with 250 µg of folic acid per total daily dose of iron. The tablets were taken at breakfast or, in the case of persons receiving 120 mg iron per day (groups 3 and 4), two of the tablets were taken at breakfast while the other two tablets were taken at supper. Blood samples were obtained from each person before the start of the study, and at the end of treatment, to measure basal and post-treatment plasma concentrations of haemoglobin and ferritin. No significant differences among the groups in age, sex, or pre-treatment haemoglobin concentrations were reported. At the end of the 4-week treatment period, a significant increase in mean haemoglobin concentration (2.6 g/dl) over the basal value was reported in all four treatment groups; however, this increase was reported not to be significantly different between groups. The plasma ferritin concentrations of ndividuals in groups 2, 3, and 4 (dosed with 60 or 120 mg iron/day as Ferrochel® or FeSO4) were significantly increased over pre-treatment values, but no significant difference was found between groups. The plasma ferritin concentrations of individuals in group 1 were lower than basal values at the end of the 4 weeks of treatment. Gastrointestinal "upsets" were reported on days 15 and 30 of the study in 2/21 (9.5%), 4/26 (15.4%), and 9/27 (33.3%) of the persons in groups 2, 3 and 4, respectively. No such effects were reported by individuals in group 1. The authors concluded that daily dosing with 60 mg of iron, supplied as Ferrochel® ferrous glycinate, is effective in the treatment of iron deficiency anaemia, and has almost no gastric side-effects (Pineda et al., 1994).
Forty infants (aged 6–36 months) with iron deficiency anaemia (haemoglobin concentration of <11 g/dl), in the paediatric department of the San Juan de Dios General Hospital in Guatemala City, Guatemala, were paired for age, sex, weight and haemoglobin concentration, and randomly assigned to one of two groups (20 infants per group) in a double-blind controlled study. The children received a single daily dose of 250 µg folic acid and a dose of iron of 5 mg/kg bw in the form of a syrup containing 30 mg/ml as FeSO4 or Ferrochel® ferrous glycinate for 28 days. Blood samples were obtained from each child before and after the treatment for determination of haemoglobin and ferritin concentrations. Haemoglobin concentrations were significantly increased by the end of the treatment period in groups receiving supplements of FeSO4 or Ferrochel® ferrous glycinateover baseline values, but did not differ between groups. The change in haemoglobin concentrations was reported to be inversely proportional to basal levels, regardless of the iron compound administered. Mean plasma ferritin concentrations were increased over baseline values in both treatment groups, but the increase was statistically significant only in the group given Ferrochel® ferrous glycinate (Pineda & Ashmead, 2001).
A 16-week, double-blind, placebo-controlled intervention study was conducted in 75 women (aged 18–40 years) with mild iron deficiency (i.e. serum ferritin concentration, <20 µg/l; haemoglobin concentration, >120 g/l) and residents of Dunedin, New Zealand. The participants were randomly assigned to one of three groups receiving either a placebo tablet containing maltodextrin and regular diet (placebo group), a Ferrochel® ferrous glycinate tablet and regular diet (supplement group), or a placebo tablet and increased dietary iron intake (diet group). Laboratory analyses indicated that each tablet of Ferrochel® ferrous glycinate and placebo contained approximately 47 mg and 0.7 µg of iron, respectively, and participants took a single daily dose of the appropriate tablet with a meal for 16 weeks. Participants in the diet group were provided with dietary advice by New Zealand-registered dieticians in order to increase their dietary iron intake and the bioavailability of that iron (e.g. use of recipe books and cast-iron frying pans, and consumption of foods and beverages providing at least 50 mg of vitamin C per meal). Fasting blood samples were obtained from each woman once a month for determination of concentrations of haemoglobin, serum ferritin, serum transferrin receptor, and C-reactive protein. The average monthly dietary intake of haem, non-haem, and total iron was estimated from a food frequency questionnaire filled out by the women at study recruitment and during weeks 4, 8, and 16 of the study. Data on blood loss due to menstruation, blood donation and nosebleeds were collected from pre-tested questionnaires. The study was completed by 57 women (19, 16, and 22 women in the placebo, supplement, and diet groups, respectively). Eight of the women withdrew from the study because of personal reasons; while the data from another 10 women were excluded because they had anaemia, had donated blood, or were pregnant. The number of withdrawals and exclusions was not significantly different between groups. Additionally, no significant differences in the frequency and severity of adverse effects (i.e. abdominal discomfort, bloating, constipation, diarrhoea, nausea, vomiting, headache and fatigue) were reported between the placebo and supplement or diet groups (excluding symptoms occurring immediately before or during menses). There were no significant differences in the concentrations of haemoglobin, serum ferritin, or serum transferrin receptor, or in blood loss variations (e.g. menstruation and nosebleeds) reported between groups. A 97% compliance rate was reported for the group receiving the supplement. No significant differences in the mean dietary intake of haem, non-haem, and total iron were reported between the placebo and supplement groups; however, these parameters were significantly increased (haem iron, by 1.9 mg/day) in the diet group when compared with the placebo group. The total iron intakes of the three groups were reported not to be significantly different from each other. After 16 weeks, the serum ferritin concentrations in the diet and supplement groups were increased by 26% and 59%, respectively, over those in the placebo group; however, this increase was statistically significant only in the group receiving the supplement. The concentrations of haemoglobin at 16 weeks were not significantly different from baseline values in both the diet and supplement groups. The concentration of serum transferrin receptor and ratio of serum transferrin receptor : serum ferritin were significantly decreased in the supplement group compared to baseline values. According to the authors, the simultaneous decrease in serum transferrin receptor concentration and increase in serum ferritin concentration in the supplement group suggested concurrent replenishment of the functional tissue and storage iron compartments. No significant changes in serum transferrin receptor concentration or the ratio of serum transferrin receptor : serum ferritin compared with baseline values were reported in the diet group. The authors concluded that improvements in iron status could be achieved to a greater degree by iron supplementation than with dietary change, owing, in part, to the difficulties associated with embarking on and sustaining a dietary regimen requiring increased consumption of flesh foods (Heath et al., 2001).
A group of 145 pregnant female volunteers in Santo André, Brazil were given FeSO4 at 200 mg/day or Ferrochel® ferrous glycinate at 75 mg/day, providing iron at 40 and 15 mg/day, respectively, from week <20 of pregnancy until parturition. A total of 74 women received FeSO4 supplements, while 71 women received Ferrochel® ferrous glycinate supplements. Blood samples were obtained from each of the subjects at the time of enrollment in the study between weeks 20 and 29 of pregnancy, and at 30 or more weeks of pregnancy. Haematological parameters evaluated included the concentrations of haemoglobin and serum ferritin, and the percentage saturation of transferrin was calculated from the serum concentrations of iron, TIBC and ferritin. Compliance with supplement intake was reported to be significantly greater among women given Ferrochel® ferrous glycinate, with 52/71 (73%) and 26/74 (35%) of the women taking Ferrochel® ferrous glycinate and FeSO4 supplements, respectively, for >13 weeks. The taste of the iron supplement was the main reason for non-compliance by the women being given FeSO4. None of the women receiving Ferrochel® ferrous glycinate reported that it had a bad taste. Five women reported nausea, vomiting or diarrhoea as the reason for their non-compliance when taking iron as FeSO4, while two women reported these symptoms while taking iron as Ferrochel® ferrous glycinate. The authors attributed the greater compliance with Ferrochel® ferrous glycinate supplementation to the fact that the supplement could be ingested with meals, thus having fewer adverse gastric effects. There was a trend towards decreasing concentrations of haemoglobin and serum ferritin in the women as pregnancy progressed; however, the decreases in serum ferritin and percent transferrin saturation were lower in the group taking Ferrochel® ferrous glycinate than in the group taking FeSO4. The statistical significance of these decreases was not reported by the authors. Much information was missing, as only about 33% of the women who were originally enrolled in the study provided blood samples on all three sampling occasions. This problem was compounded by sample losses in the laboratory. When the authors analysed the results for women who had provided blood on all three occasions, significantly higher concentrations of haemoglobin and serum ferritin, and significantly higher percent transferrin saturation were reported for the women receiving Ferrochel® ferrous glycinate supplements as compared with women receiving FeSO4. Nevertheless, there was a large discrepancy in the sample sizes used in this analysis, with ranges of 7–14 and 17–40 in groups given Ferrochel® ferrous glycinate and FeSO4, respectively. Supplementation with Ferrochel® ferrous glycinate in compliant women at >30 weeks of pregnancy was reported to result in lower incidences of iron deficiency (31%) and iron deficiency anaemia (0%) than supplementation with FeSO4 (incidence of iron deficiency, 54.5%; iron deficiency anaemia, 11%) (Szarfarc et al., 2001).
(b) Ferrous glycinate-fortified foods
Ashmead et al. (1997) gave groups of seven persons (age and sex not reported; matched for age, sex and initial haemoglobin status) with iron at a dose of 22.5 mg/day as Ferrochel® ferrous glycinate or at 45 mg iron/day as FeSO4 for 28 days. Except for two infants who received iron in 100 ml of milk, the participants ate 20 g of a sweetened peanut butter bar (paçoca, a common snack in Brazil) fortified with iron from FeSO4 or Ferrochel® ferrous glycinate. Baseline and post-treatment concentrations of haemoglobin and serum ferritin were determined for each participant. At the end of 28 days of fortification, both groups had significantly higher haemoglobin and serum ferritin concentrations than at baseline, but these increases were not significantly different between groups. According to the authors, the bioavailability of iron from Ferrochel® ferrous glycinate was at least twice that from FeSO4, since only half as much iron was given to the group receiving Ferrochel® ferrous glycinate, with results equivalent to that of the group receiving FeSO4. Additionally, owing to the significant correlation between body iron status and the magnitude of iron absorption observed with both iron compounds, the authors concluded that there is little danger of iron overloading or subsequent toxicity in people with normal iron metabolism who eat foods fortified with either FeSO4 or Ferrochel® ferrous glycinate. Furthermore, the authors considered that the lower intake of iron from Ferrochel® ferrous glycinate provides a greater margin of safety for food fortification (Ashmead et al., 1997).
A similar study was conducted in 434 people (9% of whom were aged 1–3 years and 16% of whom were aged >15 years) given 20 g of sweetened peanut butter fortified withiron at 45 mg/day as FeSO4 or 23 mg/day as ferrous glycinate for an unspecified duration. Baseline and post-treatment concentrations of haemoglobin and serum ferritin were measured for each person. Both compounds were reported to be well tolerated. The mean haemoglobin values for the group receiving FeSO4 increased from 10.35 to 11.94 g/dl, while those of the ferrous glycinate group increased from 10.61 to 12.13 g/dl. Serum ferritin concentrations were also increased in the group receiving FeSO4, from 6.87 to 22.07 µg/l, while those in the group receiving ferrous glycinate increased from 5.38 to 14.35 µg/l; however, the authors cautioned that some of the serum ferritin values might have been artificially high, owing to infections or disease. The increases in haemoglobin and serum ferritin concentrations in both groups were reported to be statistically significant, but the differences in increases between groups were not. The authors concluded that 23 mg of iron as ferrous glycinate was as effective as 45 mg of iron as FeSO4 in correcting iron deficiency anaemia (Gualandro & Name, 1996).
Milk fortified with Ferrochel® ferrous glycinate at a concentration of 30 mg/l was given daily to 131 children aged 6–14 years in Riyadh, Saudi Arabia, for 3 months. Milk of three different flavours (plain, strawberry, and chocolate) was served and drunk three times daily with each meal. Social workers supervised the milk distribution and consumption. The total daily iron consumption was estimated to be 6 mg per child. Blood samples were obtained from each child at study initiation and termination. The milk fortified with Ferrochel® ferrous glycinate was reported to be well accepted by the children, the order of flavour preferences being: chocolate > strawberry > plain and was also well tolerated by the children, with no reports of gastrointestinal problems during the course of the study. The authors reported no clinical indications of iron overload in any of the children. The prevalence of haemoglobin concentrations of <12 g/dl dropped significantly, from 25.3% to 5.0% and from 23.0% to 8.6%, for boys and girls, respectively (p < 0.0001). The authors concluded that fortification of milk with Ferrochel® ferrous glycinate was an effective way of increasing the haemoglobin concentrations of children with low or close to normal haemoglobin levels (Osman & Al-Othaimeen, 2002).
Commercially prepared petit suisse cheese was fortified with ferrous glycinate to provide iron at 2 mg/90 g of cheese (approximately equivalent to 22.2 mg/kg of cheese) and given to 81 children (aged 2–6 years) of low socioeconomic status in São Paulo, Brazil, for 90 days. The concentrations of haemoglobin, ferritin, plasma zinc and copper, and anthropometric parameters were measured at the beginning and end of the study period. The children were reported to have an average daily consumption of 90 g of cheese, approximately equivalent to a daily iron intake of 2 mg. The cheese was adequately accepted and well tolerated by the children, as no product-related adverse effects were reported throughout the duration of the study. Mean pre-treatment values of 12.27 g/dl of haemoglobin and erythrocyte volume fraction of 0.38 were reported in 10.5% of the children, while 60% of children had a mean serum ferritin concentrations of 15.7 ng/ml. At the end of 90 days of treatment, 6.3% of the children had iron deficiency anaemia and 20.5% had levels of ferritin that were below normal. The improvements in the average values of haemoglobin and erythrocyte volume fraction were not significantly different from pre-treatment values; however, the increase in average serum ferritin concentration from 15.69 to 24.68 ng/ml was statistically significant. The average serum zinc concentration was reported to have decreased substantially throughout the study, but remained within the normal range. There were no changes in the overall consumption of food by the children, although the ratios of weight : age and weight : height increased as the study progressed; the authors attributed this to the reduction of iron deficiency parameters in the children. The authors concluded that ferrous glycinate is effective in increasing haemoglobin concentrations and replenishing iron reserves in children (Fisberg et al., 1995).
Sweet rolls prepared from ferrous glycinate-fortified flour were provided twice daily, 5 days a week (providing iron at approximately 4 mg per day) for 6 months to 89 children aged 1-6 years of low socioeconomic status in São Paulo, Brazil. Haematological (i.e. concentrations of haemoglobin and serum ferritin) and anthropometric parameters (i.e. body weight and height) were measured before the start and at the end of the intervention. At the end of the 6-month period, the mean ratios of body weight : age and height : age were significantly greater than the pre-intervention values. The haemoglobin concentration increased significantly (from 11.5 to 12.6g/dl), the prevalence of iron deficiency anaemia (concentration of haemoglobin, <11 g/dl) decreased significantly (from 28% to 9%), and the prevalence of low iron stores decreased significantly (from 62% to 25%) in all children. The average increase in haemoglobin concentrations (1.10 g/dl) was reported to be significant for all children, with a greater mean increase (1.42 g/dl) in anaemic children than in those with a pre-intervention haemoglobin concentration of >11 g/dl (mean increase of 0.96 g/dl). Significant increases (mean increase, 8.90 µg/l) were also reported in the mean serum ferritin concentrations, particularly in children with depleted iron stores (mean increase, 13.03 µg/l). According to the authors, the greater increase in serum ferritin concentrations observed in children with lower basal concentrations of ferritin (as compared with the increases in subjects with concentrations closer to normal) is consistent with the regulation of iron absorption from ferrous glycinate by iron stores (Giorgini et al., 2001).
A randomized, double-blind, placebo-controlled study was conducted in 775 children (aged 6–12 years) in the United Republic of Tanzania. For 6 months, each child received a sachet containing 25 g of a placebo or fortified powdered orange drink, which provided iron as ferrous glycinate at 0 or 5.4 mg/day. Each sachet also contained 1750 IU vitamin A, 45 µg iodine, 5.25 mg zinc, 72 mg ascorbic acid, 0.6 mg riboflavin, 0.14 mg folic acid, 3 µg vitamin B12, 0.7 mg vitamin B6 and 10.5 mg vitamin E. Anthropometric parameters (weight, height, body mass index (BMI)), and vitamin A and iron status (i.e. haemoglobin, erythrocyte volume fraction, zinc protoporphyrin, and serum ferritin) were measured at the beginning and end of the study. The pre-treatment levels of serum retinol and measures of iron status and the anthropometric parameters were not significantly different between groups given the placebo and the fortified orange drink. The mean haemoglobin concentration for the group given the fortified orange drink significantly increased, from 9.3 to 10.6g/dl, during the 6-month intervention period when compared with that for the placebo group, in whom the mean haemoglobin concentration increased by only 0.02 g/dl. There also was a significant increase (16 µg/l) in mean serum ferritin concentrations in the group given the fortified drink when compared with that (2 µg/l) noted in the group receiving the placebo at the end of the intervention. The group given the fortified drink also had a significantly lower incidence of vitamin A deficiency after 6 months than the group treated with the placebo; the number of children with a serum retinol concentration of <20 µg/dl (indicating vitamin A deficiency) was decreased by 50% in the former group. Highly significant increases in body-weight gain, height gain, and BMI were also found in the group receiving the drink fortified with micronutrients when compared with the group receiving the placebo (Latham et al., 2001).
One litre of milk fortified with Ferrochel® ferrous glycinate at 15 mg/l (containing a concentration of iron of 3 mg/l) was given daily for 7.3 months to 185 infants and young children (aged 6–24 months) in Tupã, Brazil. According to the WHO guidelines for identification of iron deficiency anaemia (haemoglobin concentration: severe anaemia, <9.5 g/dl; less severe, 9.5–11.0 g/dl; normal, >11.0 g/dl)), severe iron deficiency anaemia was diagnosed in 54% of the children, 33% were classified as having less severe iron deficiency anaemia, and 13% were considered to have normal haemoglobin concentrations. At the conclusion of the study, the children who remained severely anaemic were given higher doses of iron. The average daily consumption of Ferrochel® ferrous glycinate-fortified milk was estimated to be 750 ml, providing iron at approximately 2.1 mg/day. Finger-prick blood samples were obtained from each child before the start of the study, 4.4 months into the study (i.e. at a mean of 133 days), and at the end (i.e. at a mean of 222 days) in order to monitor any changes in haemoglobin concentration. The mean haemoglobin concentrations for all children were 9.3, 10.5, and 11.2 g/dl at 0, mean 133 days, and mean 222 days of the study, respectively. At a mean of 222 days of the study, significantly increased haemoglobin concentrations were found in children who were initially diagnosed with severe or less severe anaemia, but no significant changes were reported in those who initially had normal haemoglobin concentrations. The authors concluded that fortification of commercial milk with Ferrochel® ferrous glycinate is an effective dietary intervention for the treatment of iron deficiency and iron deficiency anaemia in children (Iost et al., 1998).
In a study in Angaluba, Brazil, whole milk was fortified with iron as ferrous glycinate at a concentration of 3 mg/l, and 1 litre was given daily to 269 preschool children for 12 months. The prevalence of anaemia was significantly reduced, from 62.3% at the beginning of the study to 26.4% at the end. Specifically, the prevalence of children with severe anaemia (haemoglobin concentration, <9.5 g/dl) was reduced from 20.4% to 5.2%, while the prevalence of those with moderate anaemia (haemoglobin concentration, 9.5–10.9 g/dl) was decreased from 31.1% to 16.6%, and the prevalence of those with normal iron status (haemoglobin concentration, >11 g/dl) increased from 31.2% to 60.9%. The authors concluded that fortification of milk with ferrous glycinate was an effective way of reducing, and in some cases eliminating, iron deficiency anaemia in children (Queiroz & Torres, 1995).
The Committee noted that ferrous glycinate is absorbed by the mucosal cells of the intestine, and is subsequently dissociated into its iron and glycine components within the intestinal mucosa. The available studies indicate that the absorption of iron from ferrous glycinate is regulated physiologically according to the body’s iron status, in a manner similar to other non-haem iron compounds. The bioavailability of iron from ferrous glycinate is comparable to that of Fe–EDTA (evaluated by the Committee at its forty-first and fifty-third meetings; Annex 1, references 107 and 143) and is generally greater than that of FeSO4. As is the case with other non-haem iron compounds, the nature of the food matrix may affect the bioavailability of the iron from ferrous glycinate.
In consideration of the potential for overuse of this product, the Committee noted the results of studies of dietary supplementation and fortification at doses of up to 60 mg iron per day, which confirmed the efficacy of ferrous glycinate in correcting iron status in persons with iron deficiency, while showing no gastric side-effects. In iron-sufficient individuals, including children, iron absorption from ferrous glycinate is down-regulated according to iron status, and haemoglobin and serum ferritin concentrations are not significantly increased relative to pre-treatment or normal-range values at doses of up to 23 mg iron per day. The Committee therefore concluded that there was no evidence that the administration of iron in the form of ferrous glycinate would result in increased body stores of iron after the nutritional requirement for iron had been satisfied.
The Committee reviewed a 90-day study of toxicity in rats fed diets containing ferrous glycinate. Despite the fact that a slight increase in iron deposition in the liver of rats of each sex occurred at high doses, no compound-related toxicological effects were noted at doses of 100, 250 or 500 mg/kg bw per day. The NOEL of ferrous glycinate was 500 mg/kg bw per day, corresponding to a concentration of iron of 100 mg/kg bw per day. This NOEL is 125-fold the provisional maximum tolerable daily intake of 0.8 mg/kg bw of iron from all sources.
On the basis of the available data on bioavailability, metabolism, and toxicity, and the studies in humans, the Committee concluded that ferrous glycinate is suitable for use as a source of iron for supplementation and fortification, provided that the total intake of iron does not exceed the provisional maximum tolerable daily intake of 0.8 mg/kg bw.
Products, including ferrous glycinate, that are intended to provide a source of additional iron should not be consumed by individuals with any type of iron storage disease, except under medical supervision.
The Committee did not receive information on estimated intakes of ferrous glycinate, either from its use in food or any possible use as an iron supplement. Information on levels of fortification in food, provided by the sponsor, suggest that intakes approaching the provisional maximum tolerable daily intake could not be attained unless extremely large amounts of foodstuffs fortified at the suggested levels were eaten.
Allen, L.H. (2002) Advantages and limitations of iron amino acid chelates as iron fortificants. Nutr. Rev., 60, S18–S21.
Allen, L.H., Bovell-Benjamin, A.C. & Viteri, F. (1998) Ferrous bis- and ferric tris-glycinates as iron fortificants for whole maize: bioavailability and regulation by iron status. FASEB J., 12, A821 (Abstract No. 4759).
Ashmead, S.D. (2001) The chemistry of ferrous bis-glycinate chelate. Arch. Latinoam. Nutr., 51, 7–12.
Ashmead, H. & Ashmead, S. (1995) Albion research: Past, present and future. Proceedings of the Albion Laboratories, Inc. International Conference on Human Nutrition. January 21–22 1995, Salt Lake City, Utah, USA, pp. 1–8.
Ashmead, H.D., Graff, D.J. & Ashmead, H.H. (1985) Intestinal absorption of metal ions and chelates. Springfield: Charles C. Thomas, pp. 113–212.
Ashmead, H.D., Gualandro, S.F.M. & Name, J.J. (1997) Increases in haemoglobin and ferritin resulting from consumption of food containing ferrous amino acid (Ferrochel) versus ferrous sulphate. In: Fischer, P.W.F., L’Abbé, M.R., Cockell, K.A. & Gibson, R.S., eds, Proceedings of the 9th International Symposium on Trace Elements in Man and Animals. Ottawa: NRC Research Press, pp. 284–285.
Atkins, P.W. & Beran, J.A. (1992) General chemistry, 2nd Ed., Annotated instructor’s version. New York: WH Freeman, pp. 804–809.
Baynes, R.D., Bothwell, T.H., Bezwoda, W.R., Macphail, A.P. & Derman, D.P. (1987) Relationship between absorption of inorganic and food iron in field studies. Ann. Nutr. Metab., 31, 109–116.
Bothwell, T.H., Charlton, R.W., Cook, J.D. & Finch, C.A. (1979) Iron metabolism in man. Oxford: Blackwell Scientific Publications. Cited In: IOM, 2001.
Bovell-Benjamin, A.C., Allen, L.H. & Guinard, J.-X. (1998) Toddler’s acceptance of whole maize meal porridge fortified with ferrous bisglycinate. FASEB J., 12, A847 (Abstract No. 4904).
Bovell-Benjamin, A.C., Allen, L.H., Frankel, E.N. & Guinard, J.-X. (1999) Sensory quality and lipid oxidation of maize porridge as affected by iron-amino acid chelates and EDTA. J. Food. Sci., 64, 371–376.
Bovell-Benjamin, A.C., Viteri, F.E. & Allen, L.H. (2000) Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am. J. Clin. Nutr., 71, 1563–1569.
Cook, J.D., Layrisse, M., Martinez-Torres, C., Walker, R., Monsen, E.R. & Finch, C.A. (1972) Food iron absorption measured by an extrinsic tag. J. Clin. Invest., 51, 805– 815.
Coplin, M., Leichtmann, G. & Lashner, B. (1991) Tolerability of iron: Comparison of bis-glycino iron II and ferrous sulfate. Clin. Ther., 13, 606–612.
Fisberg, M., Braga, J.A.P., Kliamca, P.E., Ferreira, A.M.A. & Berezowski, M. (1995) [Use of "Petit Suisse" cheese enriched with iron in the prevention of anemia among preschool children.] Clin. Ped., 19, 14–24 (in Portuguese).
Fox, T.E., Eagles, J. & Fairweather-Tait, S.J. (1998) Bioavailability of iron glycine as a fortificant in infant foods. Am. J. Clin. Nutr., 67 (4), 664–668.
García-Casal, M.N. & Layrisse, M. (2001) The effect of change in pH on the solubility of iron bis-glycinate chelate and other iron compounds. Arch. Latinoam. Nutr., 51, 35–36.
García-Casal, M.N., Layrisse, M., Solano, L., Barón, M.A., Arguello, F., Llovera, D., Ramírez, J., Leets, I. & Tropper, E. (1997) Vitamin A and beta-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr., 128, 646–650.
Giorgini, E., Fisberg, M., de Paula, R.A., Ferreira, A.M., Valle, J. & Braga, J.A. (2001) The use of sweet rolls fortified with iron bis-glycinate chelate in the prevention of iron deficiency anemia in preschool children. Arch. Latinoam. Nutr., 51, 48–53.
Green, R., Charlton, R., Seftel, H., Bothwell, T., Mayet, F., Adams, B., Finch, C. & Layrisse, M. (1968) Body iron excretion in man. Am. J. Med., 45, 336–353.
Gualandro, S. & Name, J.J. (1996) Anaemia and iron deficiency in a population in Brazil. The effect of the consumption of food containing ferrous amino acid chelate or ferrous sulphate. Presented at the 26th Congress of The International Congress of Hematology in Singapore. Abstract No. 134. Submitted to WHO by Albion Laboratories, Inc., Clearfield, Utah, USA.
Heath, A-L.M., Skeaff, C.M., O’Brien, S.M., Williams, S.M. & Gibson, R.S. (2001) Can dietary treatment of non-anemic iron deficiency improve iron status? J. Am. Coll. Nutr., 20, 477–484.
Hendricks, D.G. & Ashmead, S. (1995) Stability of Ferrochel in food fortification as measured by TBARS and lipid peroxidation: preliminary report. Unpublished report. Submitted to WHO by Albion Laboratories, Inc., Clearfield, Utah, USA.
INACG (1993) Iron EDTA for food fortification: A report of the International Nutritional Anemia Consultative Group. International Nutritional Anemia Consultative Group (INACG). Washington: Nutrition Foundation Inc.
IOM (2001) Iron. In: IOM (Institute of Medicine), Food and Nutrition Board, Dietary Reference Intakes For Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. A report of the Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington: National Academy Press, pp. 290–393.
Iost, C., Name, J.J., Jeppsen, R.B. & Ashmead, H.D. (1998) Repleting hemoglobin in iron deficiency anemia in young children through liquid milk fortification with bioavailable iron amino acid chelate. J. Am. Coll. Nutr., 17, 187–194.
Jeppsen, R.B. (2001) Toxicology and safety of ferrous bisglycinate chelate and other iron amino acid chelates. Arch. Latinoam. Nutr., 51, 26–34.
Jeppsen, R.B. & Borzelleca, J.F. (1999) Safety evaluation of ferrous bisglycinate chelate. Food Chem. Toxicol., 37, 723–731.
Kukulinski, M. (1993) Acute oral toxicity study in the Sprague-Dawley rat (LD50). Test article: Ferrochel, Lot No. 31733. Unpublished report No. 043-002 from Biologic Safety Research, Inc., Muskegon, Michigan, USA. Submitted to WHO by Albion Laboratories, Inc., Clearfield, Utah, USA.
Latham, M.C., Ash, D., Ndossi, G., Mehansho, H. & Tatala, S. (2001) Micronutrient dietary supplements—A new fourth approach. Arch. Latinoam. Nutr., 51, 37–41.
Layrisse, M., García-Casal, M.N., Solano, L., Barón, M.A., Arguello, F., Llovera, D., Ramírez, J., Leets, I. & Tropper, E. (2000) Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr., 130 (9), 2195–2199 & Erratum, 12, 3106.
Lodish, H., Baltimore, D., Berk, A., Zipursky, S.L., Matsudaira, P. & Darnell, J. (1995) Molecular Cell Biology, 3rd Ed. New York: Scientific American Books, pp. 54, 966–967, 1128.
MacPhail, A.P. (2001) Iron deficiency and the developing world. Arch. Latinoam. Nutr., 51, 2–6.
Mandella, R.C. (2000) A subchronic (3-month) toxicity study of Ferrochel in the rat adminis-tered via diet: Final report. Unpublished report No. 96–2503 from Huntingdon Life Sciences Inc., East Millstone, New Jersey, USA. Submitted to WHO by Albion Laboratories, Inc., Clearfield, Utah, USA.
Marchetti, M., Ashmead, D., Tossani, N., Marchetti, S. & Ashmead, S.D. (2000) Comparison of the rates of vitamin degradation when mixed with metal sulphates or metal amino acid chelates. J. Food Composition Anal., 13, 845–884.
McMurry, J. & Fay, R.C. (1995) Chemistry. New Jersey: Prentice Hall, pp. 812–817.
Name, J.J. (1996) [Food fortification with amino acid chelated minerals.] Presented at the 2nd Seminar on Enriched Foods. Unpublished report. Submitted to WHO by Albion Laboratories, Inc., Clearfield, Utah, USA (in Spanish).
Olivares, M. & Pizarro, F. (2001) Bioavailability of iron bis-glycinate chelate in water. Arch. Latinoam. Nutr., 51, 22–25.
Olivares, M., Pizarro, F., Pineda, O., Name, J.J., Hertrampf, E. & Walter, T. (1997) Milk inhibits and ascorbic acid favors ferrous bis-glycine chelate bioavailability in humans. J. Nutr., 127, 1407–1411.
Osman, A.K. & Al-Othaimeen, A. (2002) Experience with ferrous bis-glycine chelate as an iron fortificant in milk. Int J Vitam Nutr Res, 72, 257–263.
Pineda, O. (2001) Final comment. Arch. Latinoam. Nutr., 51, 60–61.
Pineda, O. & Ashmead, H.D. (2001) Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition, 17, 381–384.
Pineda, O., Ashmead, H.D., Perez, J.M. & Lemus, C.P. (1994) Effectiveness of iron amino acid chelate on the treatment of iron deficiency anemia in adolescents. J. Appl. Nutr., 46, 2–13.
Queiroz, S. & Torres, M. (1995) [Iron-deficiency anemia: Physiopathological aspects and experiment with the use of iron-enriched milk.] Ped. Mod., 31 (in Spanish).
Silverthorn, D.U. (1998) Human Physiology: An Integrated Approach. New Jersey: Prentice Hall, Inc., pp. 226, 250.
Szarfarc, S.C., de Cassana, L.M.N., Fujimori, E., Guerra-Shinohara, E.M. & de Oliveira, I.M.V. (2001) Relative effectiveness of iron bis-glycinate chelate (Ferrochel) and ferrous sulfate in the control of iron deficiency in pregnant women. Arch. Latinoam. Nutr., 51, 42–47.
Umbelino, D.C., Cardello, H.M.A.B. & Rossi, E.A. (2001) [Effect of iron salts addition on the sensory characteristics of soy yogurt.] Arch. Latinoam. Nutr., 51, 199–203 (in Spanish).
See Also:
Toxicological Abbreviations