
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
Jens-Jørgen Larsen
Institute of Food Safety and Toxicology
Ministry of Food, Agriculture and Fisheries, Søborg, Denmark
|
Mechanisms of inhibition of cholinesterase and neuropathy target esterase |
Methamidophos (O,S-dimethyl hydrogen phosphoramidothioate), an organophosphorus insecticide which acts by inhibiting cholinesterase activity, is a racemate and a major metabolite of acephate. It was last evaluated toxicologically by the 1990 JMPR (Annex 1, reference 59), which established an ADI of 0–0.004 mg/kg bw on the basis of inhibition of erythrocyte cholinesterase activity in a short-term study in humans. Methamidophos was considered by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
Rats
Studies on the absorption, distribution and excretion of methamidophos in rats were conducted with [S-methyl-14C]- and [32P]-labelled material. In one study, [S-methyl-14C]-methamidophos (purity, > 99.5%) was administered orally to female Sprague-Dawley rats at 0.18 mg per rat (0.5 mCi), and in the other study [32P]-labelled methamidophos (purity, > 99.5%) was administered orally at 0.21 mg per animal (2.7 mCi) to male and female Sprague-Dawley rats. Both [S-methyl-14C]- and [32P]-labelled methamidophos were rapidly absorbed, distributed, metabolized and excreted. The radioactivity was evenly distributed throughout the body. Two phases could be distinguished in the rate of excretion: first, a period of rapid excretion, lasting 1–3 days, during which > 50% of the administered dose was excreted, and a second period during which 1–2% of the dose was excreted daily. The dose remaining in the animals after the first phase of rapid metabolism and excretion consisted of traces of methamidophos, its metabolites and natural constituents of the body that contained the radiolabel. In the latter case, the radiolabel had been derived from complete breakdown of methamidophos. Elimination of 14CO2 within the first 6 h accounted for 32% of the recovered radioactivity, with 6% of the dose in the urine. Over a 5-day period, only 50% of the administered dose was recovered (39% as 14CO2 and the rest predominantly in urine). About 70% of the recovered 32P was observed in urine within 24 h. Faeces contained small quantities that were excreted over 28 days (Crossley & Tutass, 1969).
The tissue distribution and excretion of [14CH3S]methamidophos was followed in female Sprague-Dawley rats after intravenous injection of a toxic but not lethal dose (8 mg/kg bw). The radiolabel was distributed rapidly to all tissues at approximately equal concentrations. Peak tissue levels were achieved within 1–10 min, except in the central and peripheral nervous system, where the peak concentration (40 nmol/g) was found after 20–60 min, corresponding to peak signs of toxicity. Within 24 h of dosing, 47% of the radioactivity was recovered in urine and 34% as 14CO2, with < 5% in faeces over 7 days (Gray et al., 1982).
Male Wistar rats were given atropine sulfate at 100 mg/kg intraperitoneally 10–15 min before intravenous dosing with methamidophos (purity, 74%) in propylene glycol at a dose of 10 mg/kg bw. At various intervals after dosing (10–600 min), blood was taken and the concentrations of methamidophos in plasma were analysed. Pharmacokinetics was evaluated for a one-compartment model: The elimination half-life was 1.5 h, the volume of distribution was 0.81 l/kg bw; the clearance was 5.8 ml/min per kg bw, and the rate of elimination was 0.45 per h. The volume of distribution indicates that methamidophos is not strongly bound to tissues (Eigenberg et al., 1983).
Methanesulfonic acid was identified as a degradation product of methamidphos in plants but not in rats. This substance is an oxidation product of methyl mercaptan, which is probably also a degradation product of methamidophos in animals. Studies of the metabolism of this compound in rats in vivo or whole blood in vitro with 14C- and/or 35S-labelled methyl mercaptan showed that methyl mercaptan was rapidly oxidized to CO2 and sulfate. Methanesulfonic acid was detected as an oxidation product of methyl mercaptan in whole blood (Derr & Draves, 1983; Blom & Tangerman, 1988).
When [S-methyl-14C]methamidophos (specific radioactivity, 182 µCi/mg) was given orally at a single dose of 8 mg/kg bw on day 18 of gestation to Sprague-Dawley rats, it was rapidly absorbed and distributed (within 1 h) to various tissues, including the placenta and the fetus, and was eliminated within 48 h by both dams and fetuses. In a similar study, suckling rats were dosed immediately after delivery and killed 10 min and 0.5, 1, 3, 6, 12, 24 and 48 h later, and urine, faeces, expired air, blood, selected tissues and fetuses were analysed for radioactivity. Generally, the distribution of radioactivity was rapid, the concentrations in most tissues peaking within 1 h. Less radioactivity was found in the nervous system than in most other tissues. The concentrations of methamidophos in the pups indicated that it was present in milk (Salama, 1990).
In a study of dermal absorption, [S-methyl-14C]methamidophos (radiochemical purity, > 99.8%; specific radioactivity, 26 mCi/mmol per l) was dissolved in deionized water and applied topically at a single dose of 0.05, 0.5 or 5 mg onto an unabraded 10-cm2 application site on the shaved dorsal trunk of each of 12 male Sprague-Dawley rats. After each exposure, the animals were killed, and skin from the application site, blood, CO2 trap, volatile trap, carcass and excreta were measured for radioactivity. Up to 40–44% of the applied methamidophos was absorbed through the skin in a time-dependent manner. The total recovery of radioactivity was greatest at 2 h and had decreased by 10–24 h. Most of the absorbed material was recovered in urine and carcass at all doses. The average concentration of radioactivity in blood was almost constant at 2–24 h, showing that it was at or near the steady-state level in all groups during the test period. Methamidophos was effectively removed from the skin by scrubbing with soap and water (Bagos & Beatty, 1991).
The dermal absorption of [14C]methamidophos from a formulation was determined in rhesus monkeys after an 8-h exposure. The amount absorbed was calculated as the ratio of urinary and faecal excretion of 14C after dermal application and after intravenous administration (representing 100% absorption) of radiolabelled material at similar doses. The mean dermal absorption of radioactivity was calculated as the sum of total radioactivity excreted after dermal dosing divided by the sum after intravenous dosing. On the basis of these calculations, the per cent dermal absorption was 11% (Fuller, 2000).
The volatility of [14C]methamidophos from rat skin was determined with two solutions, containing 8.2 and 81 mg/g bw, with radioactivity of 81 ± 0.80 and 81 ± 0.91 µCi/g, respectively. The radiochemical purity of the two solutions was analysed by high-performance liquid chromatography after application to skin and determined to be 97% and 100%, respectively. [14C]Methamidophos was not appreciably volatile when applied to excised rat skin maintained at 37 ± 1 °C and collected for determination of radioactivity 0, 2, 8 and 24 h after application (Testman, 2000).
[S-methyl-14C]Methamidophos (radiochemical purity, > 99%; specific radioactivity, 26 mCi/mmol per l) was dissolved in physiological saline and given orally at a dose of 1 mg/kg bw to eight male Wistar rats. Urine, faeces and CO2 were collected, and radioactivity was determined in the excreta and in organs at sacrifice 24 h after dosing. In additional tests, metabolism cages were placed in a gas-tight box to prevent leakage of volatile radioactivity, and volatile metabolites were trapped on tubes inserted into a ‘cold trap injector’ mounted on the injection port of a gas chromatograph. After thermal desorption and concentration in dichlorobenzene, the volatiles were injected and chromatographed, with a flame-ionization detector and a radioactivity flow-through detector arranged in series. The renal and pulmonary excretion was rapid, as almost 50% of the administered radioactivity was eliminated in urine and expired air within 24 h. About one-third of the administered radioactivity was excreted with urine, while about 15% was expired; negligible amounts were excreted with faeces. The remaining radioactivity in the body, excluding the gastrointestinal tract, at sacrifice amounted to about 17% of the administered dose. The highest equivalent concentrations of radioactivity at sacrifice were measured in liver, the main metabolizing organ, while kidney and lung reflected the main excretory routes (Klein, 1997).
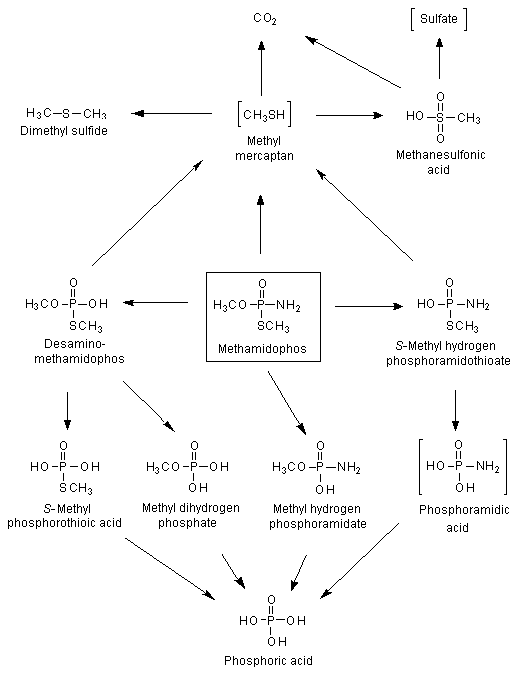
The metabolism of methamidophos in rats was studied with [S-methyl-14C]- and 32P-labelled material. In one study, [S-methyl-14C]methamidophos (purity, > 99.5%) was administered orally to female Sprague-Dawley rats at a dose of 0.18 mg per rat (0.5 mCi), and in the other study, [32P]methamidophos (purity, > 99.5%) was administered orally at 0.21 mg per animal (2.7 mCi) to male and female Sprague-Dawley rats. The metabolism was shown to be hydrolytic on the basis of the identity of the radioactive metabolites isolated from urine, faeces and tissues. Degradation appeared to involve rupture of the P–N bond to form O,S-dimethyl phosphorothioate and ammonia (Figure 1). Demethylation then occurred, first at the P–S bond and then at the P–O bond, to form methyl dihydrogen phosphate and then phosphoric acid (Crossley & Tutass, 1969).

Figure 1. Proposed metabolic pathway for methamidophos in rats
Postulated metabolites in square brackets
Male and female albino rats were each given [32P]methamidophos orally at a single dose of 15 mg/kg bw. After 24 h 77% of the administered dose was recovered in urine. The urinary metabolites identified were: O,S-dimethyl phosphorothioic acid, S-methyl phosphorothioic acid, O-methyl phosphoric acid amide, S-methyl phosphoramidothioic acid and phosphoric acid. Unchanged methamidophos and a highly non-polar, unidentified metabolite were also detected in urine (Fakhr et al., 1982).
The degradation of the proposed methamidophos metabolite methyl mercaptan was studied in groups of two to five male Sprague-Dawley rats, weighing 300 g, given [35S]methyl mercaptan intraperitoneally at a concentration of 1.1 µmol/l. Each rats was housed in a glass metabolism cage that allowed for separate collection of urine, faeces and expired gases. Air scrubbed for CO2 and water was pulled through the cage at a rate of 180–380 ml/min and then through three consecutive traps: the first trap contained isatin in concentrated sulfuric acid to trap methyl mercaptan, the second trap contained sodium hydroxide to trap hydrogen sulfide and other acidic compounds, and the third trap contained mercuric chloride to trap heavy metal-precipitable sulfur compounds. The terminal oxidation products of methyl mercaptan were CO2 and sulfate. The rate of urinary excretion of [35S]sulfate was compared with the excretion rate of 14CO2 reported by Canellakis & Tarver (1953) in one male rat weighing 180 g that was given [14C]methyl mercaptan orally at a concentration of 6.2 µmol/l. During the first 2 h after administration, the oxidation products (CO2 and sulfate) were excreted at similar rates. After another 2 h, oxidation of the methyl group to CO2 slowed, and the methyl group was then metabolized via the one-carbon fragment pool. [35S]Sulfate continued to be excreted, such that 80% of the sulfur of methyl mercaptan was removed from the body within 7 h and 94% within 21 h. The isatin trap for methyl mercaptan contained approximately 0.5% of the administered radioactivity in all cases. The sodium hydroxide trap for hydrogen sulfide and other acidic compounds collected only 0.02% of the dose, and the mercuric chloride trap for heavy metal-precipitable sulfur compounds contained no radioactivity (Derr & Draves, 1983).
The degradation of methyl mercaptan was also studied in vitro in fresh whole blood taken by venipuncture and collected in 15-ml glass vials closed by a septum and containing heparin. [14C]- or [35S]Methyl mercaptan was added to 2 ml of blood as a gas at room temperature at a concentration of 4–40 nmol/ml of blood and incubated for 30 min at 37 °C. After incubation, the headspace of the vial was analysed, and the amount of radioactivity extracted was estimated in plasma. Addition of methyl mercaptan to whole blood resulted in complete trapping of methyl mercaptan within 30 min; none was detectable fter that time After incubation, the 14C and 35S labels were almost equally distributed in plasma and erythrocytes. In an investigation of the chemical properties of the 14C and 35S labels, 80–90% of methyl mercaptan incubated with whole blood reacted with erythrocytes, resulting in products in which the C–S bond of methyl mercaptan had been cleaved. These results indicate that the sulfur atom of methyl mercaptan was oxidized to sulfite or sulfate and the carbon atom to formic acid. Thus, after incubation of methyl mercaptan with whole blood, 1% was recovered as methanesulfonic acid, an intermediate in this degradation process. Incubation of methyl mercaptan with whole blood also resulted in the formation of small amounts (0.5–1%) of dimethyl sulfide (Blom &Tangerman,1988).
Methamidophos was administered to pregnant rats on day 18 of gestation, and the animals were killed 10 min and 0.5, 1, 3, 6, 12, 24 and 48 h after dosing. Urine, faeces, expired air, blood, selected tissues and fetuses were analysed. Methamidophos was the main compound present in urine, faeces and all tissues analysed. The degradation products detected were desamino-methamidophos, methyl dihydrogen phosphate, methyl hydrogen phosphoramidate, S-methyl hydrogen phosphoramidothioate and phosphoric acid. The metabolic degradation of methamidophos was proposed to involve desamination of methamidophos to desamino-methamidophos, followed by desmethylation to yield methyl dihydrogen phosphate and phosphoric acid. Methamidophos was also transformed to methyl hydrogen phosphoramidate, leading to phosphoric acid. Additionally, methamidophos was metabolized to S-methyl hydrogen phosphoramidothioate, which might be converted via phosphoramidic acid to phosphoric acid. Methamidophos, desamino-methamidophos and/or S-methyl hydrogen phosphoramidothioate were transformed to methyl mercaptan, which was converted in a final step to CO2.
Methamidophos was also administered to rats immediately after delivery, and groups of suckling pups were killed at intervals of 1, 3, 6, 12, 24, 36 and 48 h after administration to the dams. Females were killed at intervals of 12, 24 and 48 h. The compounds detected in the tissues of dams and suckling pups were methamidophos, desamino-methamidophos, methyl dihydrogen phosphate, methyl hydrogen phosphoramidate and S-methyl hydrogen phosphoramidothioate. In addition to these degradation products, phosphoric acid was found in liver, kidney, uterus, lung and plasma (Salama, 1990).
The ability of methamidophos, acephate and paraoxon (a known strong anticholinesterase) to inhibit cholinesterase activity was determined in human erythrocytes and in brain samples from rats, mice and rainbow trout in vitro. In all tissues except trout brain, acephate and methamidophos were six and three orders of magnitude weaker than paraoxon, respectively (Table 1) (Hussain et al., 1985).
Table 1. Concentrations of acephate, methamidophos and paraoxon required to inhibit 50% of the activity (IC50) of cholinesterase in human erythrocytes and rat, mouse and trout brain in vitro
|
Chemical |
Human erythrocytes |
Rat brain |
Mouse brain |
Trout brain |
|
Acephate |
1.9 ± 0.1 |
0.7 ± 0.08 |
6.0 ± 2.0 |
2.8 ± 0.3 |
|
Methamidophos |
2.3 ± 0.3 |
2.0 ± 0.2 |
2.0 ± 0.2 |
5.6 ± 0.9 |
|
Paraoxon |
3.3 ± 0.5 |
2.3 ± 0.3 |
5.0 ± 0.8 |
2200 ± 100 |
From Hussain et al. (1985)
Mean ± standard deviation of three determinations
Groups of five male and five female Sprague-Dawley rats received single topical applications of analytical-grade methamidophos (purity, 99.1%) or technical-grade methamidophos (purity, 74.7%) at a dose of 0, 1, 2.5, 6.2 or 16 mg of active ingredient per rat on a shaved dorsal area representing 6.6–8.7% of the total body surface area. The rats were killed 24 or 72 h after treatment and were observed daily for toxic signs and deaths. Body weight was measured before dosing and at sacrifice on day 1 or day 3. Gross and histopathological examinations were performed at sacrifice, and plasma, erythrocyte and brain cholinesterase activities were determined.
Three and two female rats given analytical-grade and technical-grade methamidophos, respectively, at 16 mg per rat died during the 24-h exposure, and one female at this dose of technical-grade material died after 72 h. No clinical signs of toxicity were observed at 1 mg per rat. No compound-related gross pathological changes were noted at necropsy, and no histopathological changes in the skin were found. Significant, dose-dependent inhibition of cholinesterase activity was observed in both males and females after treatment with both materials. In order to calculate the dose, the amount of active ingredient was determined per square centimetre of total body surface area. ID50 values were determined to compare the relative sensitivities of each tissue after 24 h. Erythrocyte and plasma cholinesterase activity were inhibited by both materials to a greater extent than brain cholinesterase activity (Table 2), and no consistent difference in cholinesterase inhibition was observed with the two materials (Easter & Rosenberg, 1986).
Table 2. ID50 (micrograms active ingredient per cm2 total body surface area) 24 h after administration of analytical-grade and technical-grade methamidophos
|
Sex |
Grade of methamidophos |
Cholinesterase activity |
||
|
Erythrocyte |
Brain |
Plasma |
||
|
Males |
Analytical |
7.0 |
23 |
9.5 |
|
Technical |
10 |
18 |
5.8 |
|
|
Females |
Analytical |
4.9 |
14 |
1.6 |
|
Technical |
5.4 |
8.3 |
0.57 |
|
From Easter & Rosenberg (1986)
Cholinesterase inhibition was measured in erythrocytes, plasma and various regions of the central nervous system at selected times after intravenous administration of a single dose of methamidophos at 8 mg/kg bw to rats. The degree of inhibition in three regions of the central nervous system was similar, reaching a minimum activity of 15–20% of control values at 30–60 min, when the toxicity was most severe. Erythrocyte cholinesterse was inhibited to a lesser degree than that of the central nervous system, although the time course was similar. Plasma cholinesterase inhibition was more rapid than that of the central nervous system or erythrocytes, and reactivation was slower. When concentrations of methamidophos similar to those found in vivo were incubated with central nervous system homogenates, plasma or erythrocytes in vitro (5 × 10–5 mol/l), the degree of inhibition was similar over the same time course. It was concluded that the cholinergic toxicity of methamidophos is a result of its stability in vivo, which permits its entry into the nervous system in sufficiently high concentrations to inhibit cholinesterase (Gray et al., 1982).
The methylthiophosphorus bond is cleaved during metabolism of methamidophos, leading to inhibition of acetylcholinesterase (Thompson & Fukuto, 1982).
The results of studies on the acute toxicity of methamidophos are summarized in Table 3. Acute oral or intraperitoneal administration or administration by inhalation of methamidophos induced significant toxicity in all laboratory animals tested. Dermal application resulted in moderate to high toxicity. In Sherman rats, no significant difference in toxicity was found in males, females and weanlings. In hens the (–) enantiomer of methamidophos was less toxic than the (+) enantiomer; however, in the surviving animals, the length of action and the recovery phase were longer than after treatment with the (+) enantiomer. In rats, the two enantiomers had the same acute toxic effect. The clinical signs of toxicity were indicative of cholinesterase inhibition, including salivation, lachrymation, rhinorrhoea, severe tremors, spasms, spastic gait, dyspnoea and apathy. The gross findings at necropsy included patchy distended lungs, congestion and oedema of lungs, dark liver, patchy spleen, hydronephrosis, cervical lymph node haemorrhage, gas-distended gastrointestinal tract and reddened intestinal mucosa.
Table 3. Acute toxicity of methamidophos
|
Species |
Strain |
Sex |
Route |
LD50 or LC50 |
Purity (%) |
Reference |
|
Mouse |
NR |
Male |
Oral |
23 |
Technical |
Mihail (1981) |
|
Swiss-Webster |
Female |
Oral |
16 |
95 |
Cavalli & Hallesy (1968a) |
|
|
Kunming |
Male |
Oral |
12 |
90.4 |
Guo et al. (1986a) |
|
|
Female |
11 |
|||||
|
NR |
NR |
Intraperitoneal |
5 |
NR |
Kao & Fukuto (1977) |
|
|
Rat |
Wistar |
M |
Oral |
16 |
95.3 (±) racemate |
Flucke (1990a) |
|
14 |
98.5 (+) enantiomer |
|||||
|
16 |
97.8 (–) enantiomer |
|||||
|
Sprague-Dawley |
Male |
Oral |
16 |
95 |
Cavalli & Hallesy (1968b) |
|
|
Female |
13 |
|||||
|
Wistar |
Male |
Oral |
23 |
90.4 |
Guo et al. (1986a) |
|
|
Female |
14 |
|||||
|
Wistar |
Male |
Dermal |
160 |
64.5 |
Heimann & Nash (1981) |
|
|
Female |
110 |
|||||
|
Sherman |
Male |
Dermal |
180 |
Technical |
Gaines & Linder (1986) |
|
|
Female |
150 |
|||||
|
Wistar |
Male |
Dermal |
360 |
90.4 |
Guo et al. (1986a) |
|
|
Female |
380 |
|||||
|
Sprague-Dawley |
Male |
Inhalation (1 h) |
380 |
75.1 |
Sangha (1983) |
|
|
Female |
240 |
|||||
|
Sprague-Dawley |
Male |
Inhalation (4 h) |
63 |
70.5 |
Sangha (1984) |
|
|
Female |
77 |
|||||
|
Wistar |
Male, female |
Inhalation (4 h) |
210 |
75.7 |
Pauluhn (1987) |
|
|
NR |
Male |
Intraperitoneal |
21 |
90 |
Kimmerle (1967) |
|
|
Female |
26 |
|||||
|
Sprague-Dawley |
Female |
Intraperitoneal |
8 |
Technical |
Crawford & Anderson (1973) |
|
|
Holtzman |
Male |
Intraperitoneal |
15 |
98 |
Robinson & Beiergrohslein (1980) |
|
|
Guinea-pig |
NR |
NR |
Oral |
40 |
90 |
Kimmerle (1967) |
|
Rabbit |
NR |
NR |
Oral |
20 |
90 |
Kimmerle (1967) |
|
New Zealand white |
Male |
Dermal |
120 |
75 |
Cavalli & Hallesy (1968c) |
|
|
New Zealand white |
Male |
Dermal |
120 |
73.2 |
Hixson (1980a) |
|
|
Female |
69 |
|||||
|
Cat |
NR |
NR |
Oral |
20 |
90 |
Kimmerle (1967) |
|
Dog |
NR |
NR |
Oral |
20 |
90 |
Kimmerle (1967) |
|
Hen |
White Leghorn |
Female |
Oral |
25 |
95.3 (±) racemate |
Flucke (1990b) |
|
43 |
98.5 (+) enantiomer |
|||||
|
82 |
96.5 (–) enantiomer |
|||||
|
White Leghorn |
Female |
Oral |
30 |
74 |
Kruckenberg et al. (1979) |
|
|
White Leghorn |
Female |
Oral |
48 |
72.5 |
Pauluhn & Kaliner (1984) |
|
|
White Leghorn |
Female |
Dermal |
50 |
74 |
Flucke (1985) |
|
|
White Leghorn |
Female |
Intraperitoneal |
10 |
90 |
Kimmerle (1967) |
NR, not reported
Mice
A group of 22 Kunming mice were given methamidophos (purity, 90.4%) orally at one-fifth the oral LD50 for 20 days. No deaths occurred, indicating no accumulation of the test compound (Guo et al. 1986a).
Four groups of 20 male and female Swiss mice were given diets containing methamidophos at a concentration of 0, 25, 50 or 100 ppm for 2 weeks and then allowed to recover for 2 weeks. All treated animals showed loss of body weight, and those at 100 ppm showed early tremors. Dose-dependent inhibition of plasma and erythrocyte cholinesterase activity was noted. Plasma cholinesterase was inhibited markedly (< 75%), but the level was comparable to the control value after the 2-week recovery period. Erythrocyte cholinesterase activity recovered more slowly than that in plasma and was not complete within 2 weeks (Zayed et al., 1984).
Rats
Groups of 25 male and 25 female Fischer 344 rats were fed diets containing technical-grade methamidophos (77.6% active ingredient) at a concentration of 0, 0.5, 1, 2 or 4 ppm, equal to 0, 0.03, 0.07, 0.13 and 0.24 mg/kg bw per day, for up to 56 days. The main parameters monitored during the study were body weight, food consumption, clinical signs and plasma, erythrocyte and brain cholinesterase activity. There were no effects on body weight or food consumption. At 4 ppm, plasma, erythrocyte and brain cholinesterase activities were statistically significantly decreased (by > 20%) in both sexes. The NOAEL was 2 ppm, equal to 0.13 mg/kg per day, on the basis of inhibition of erythrocyte and brain cholinesterase activity (Christenson, 1991).
Groups of 15 male and 15 female SPF Wistar rats (30 of each sex were used as controls), 28–32 days old, were fed diets containing methamidophos at a concentration of 0, 2, 6, 20 or 60 ppm, equivalent to 0, 0.2, 0.6, 2 and 6 mg/kg bw per day, for 3 months. Body weights were measured weekly. Laboratory examinations were performed on five male and five female rats at each dose after 4 weeks and 3 months of feeding.
No deaths or behavioural changes were observed, although growth was depressed significantly in both sexes. at 60 ppm. Blood chemistry, haematological and urine parameters were normal, except that the bilirubin concentration in males at 20 and 60 ppm and ornithine carbamoyl transferase activity in females at 60 ppm were depressed at 90 days. These unusual effects were reported to be within physiological limits. Both plasma and erythrocyte cholinesterase activity were depressed (by > 20 %) at doses > 6 ppm, the erythrocyte enzyme being more sensitive. No effects were noted at 2 ppm during the 3-month feeding period. Gross pathological examination of tissues and organs showed reduced size of thymus, liver, spleen, adrenals and gonads in both males and females at 60 ppm. No effects were seen at 20 ppm. Microscopic examination of tissues did not show any adverse effects related to the presence of methamidophos in the diet. The NOAEL was 2 ppm, equivalent to 0.2 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity (Löser 1970a; Gröning, 1976).
Dogs
Groups of three male and three female dogs were given methamidophos orally by capsule at a dose of 0, 0.025, 0.075 or 0.25 mg/kg bw per day, 7 days/week, for 90 days. There were no deaths, and growth and food consumption were unaffected. Erythrocyte and plasma cholinesterase activity, examined at several intervals before and during treatment, were normal. No inhibition of cholinesterase activity was seen in this study, and no NOAEL could be identified (Carlson et al., 1969).
The study was repeated with groups of three male and three female dogs, which were treated for 90 days. Body-weight gain was reduced in males at the highest dose and in females at all doses. Food consumption was reduced at the highest dose in both sexes. Depressed erythrocyte cholinesterase activity was seen at 0.25 mg/kg in both males and females. No effects were seen on plasma enzyme. The NOAEL was 0.075 mg/kg bw per day, on the basis of reduced body-weight gain and depression of erythrocyte cholinesterase activity (Lindberg et al., 1970).
Groups of two male and two female dogs were given methamidophos by capsule for periods of 21–28 days, at doses ranging from 0 to 0.6 mg/kg bw per day. Baseline cholinesterase activity was determined by examining each dog for 34 weeks before treatment. The animals were then given a dose of 0.025 mg/kg bw per day for 28 days, after which the dose was increased to 0.05 mg/kg bw per day. The total treatment sequence was 0.025, 0.05, 0.075 and 0.1 mg/kg bw per day each administered for 28 days, followed by 0.125, 0.2, 0.3, 0.4, 0.5 and 0.6 mg/kg bw per day each administered for 21 days, after which a control diet was administered for 28 days. Cholinesterase activity was measured generally at weekly intervals during treatment. In both males and females, a dose was reached at which plasma and erythrocyte cholinesterase activity was depressed. Depression was considered to have been achieved when the value for cholinesterase activity fell below the lowest range of the mean before treatment.
In males, plasma and erythrocyte cholinesterase activity was depressed when the dose reached 0.125 mg/kg bw per day. In females, plasma activity was depressed at 0.3 mg/kg bw per day, while erythrocyte activity was affected at approximately 0.2 mg/kg. On the basis of these findings, no effects would be expected on cholinesterase activity at doses around 0.1 mg/kg bw per day (Greco et al., 1971).
Groups of two male and two female beagle dogs (three of each sex were used as controls) were fed diets containing methamidophos at a concentration of 0, 1.5, 5 or15 ppm, equivalent to 0, 0.038, 0.13 and 0.38 mg/kg bw, for 90 days. There were no deaths or behavioural changes during treatment, and growth and food consumption were normal. There were no effects on blood chemistry or haematological or urine parameters. Inhibition (by > 20%) of plasma and erythrocyte cholinesterase activity was seen at 5 and 15 ppm in both sexes. Gross examination of tissues and calculation of relative organ weights did not indicate any effect at the highest dose. The NOAEL was 1.5 ppm, equivalent to 0.038 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity (Löser, 1970b; Gröning & Lorke, 1976).
Four groups of six male and six female beagle dogs were given diets containing methamidophos (70% pure active ingredient) at a concentration of 0, 2, 8 or 32 ppm, equal to 0, 0.06, 0.24 and 0.96 mg/kg bw per day, for 1 year. There were no deaths and no effects on body weight, food consumption, ophthalmic, haematological, clinical chemical or urine parameters, organ weights or gross or microscopic appearance in either sex at any concentration. Plasma, erythrocyte and brain cholinesterase activities were significantly decreased (by > 20%) in males at concentrations > 8 ppm. In females, erythrocyte and brain cholinesterase activities were significantly decreased (by > 20%) at these concentrations, while plasma cholinesterase activity was depressed (by > 20%) only at 32 ppm. At 32 ppm, erythrocyte cholinesterase activity was inhibited by 84–87% and brain cholinesterase by 66–71% in both sexes when compared with control values. Significant cholinesterase inhibition without deaths indicated that the maximum physiological limit had been reached in these animals at 32 ppm, with only 20–29% endogenous buffer capacity remaining to sustain life at the 12-month interval. The NOAEL was 2 ppm, equal to 0.06 mg/kg bw per day, on the basis of inhibition of erythrocyte and brain cholinesterase activity at higher doses (Hayes, 1984a).
Mice
Four groups of 50 male and 50 female CD1 mice were fed diets containing technical-grade methamidophos (purity, 70–72 %) at a concentration of 0, 1, 5 or 25 ppm, equal to 0, 0.14, 0.67 and 3.5 mg/kg bw per day for males and 0, 0.18, 0.78 and 4 mg/kg bw per day for females, for 106 weeks. Groups of 10 mice of each sex were included as satellite groups for haematological analysis at 6 and 12 months and at interim sacrifice at 53 weeks. The parameters monitored throughout the study were clinical signs, feed consumption and body weight. At termination, haematological parameters were measured in 10 randomly selected mice from each group. After sacrifice, all mice were subjected to a complete gross necropsy. The weights of several organs were recorded. All tissues from all animals were examined histologically. Cholinesterase activity was not measured in this study.
The mortality rate, clinical signs, haematological end-points and gross pathological appearance were not affected by treatment. Feed consumption and body-weight gain were significantly decreased for male and female mice at 25 ppm. The relative weights of the adrenals, heart, kidneys and lungs were increased in female rats at 25 ppm, and the relative weight of the brain was increased in both sexes at 25 ppm. Histopathologically, diffuse interstitial pneumonia was seen at increased incidence in males at 25 ppm. The neoplasms observed were similar in type, location, time of onset and incidence in control and treated mice. The NOAEL was 5 ppm, equal to 0.67 mg/kg bw per day, on the basis of reductions in mean body-weight gain and feed consumption at 25 ppm (Hayes, 1984b).
Rats
Five groups of 50 male and 50 female Fischer 344 rats were fed diets containing technical-grade methamidophos (purity, 70–72 %) at a concentration of 0, 2, 6, 18 or 54 ppm, equal to 0, 0.1, 0.29, 0.85 and 2.9 mg/kg bw per day for males and 0, 0.12, 0.35, 1.1 and 3.4 mg/kg bw per day for females, for 2 years. Groups of 10 rats of each sex were included for haematological and clinical chemical analysis at 6 and 12 months and at interim sacrifice at 1 year; and 10 rats of each sex per group were examined for cholinesterase activity at 1 month. The parameters monitored throughout the study were clinical signs, feed consumption, body weight and haematological and clinical chemical end-points. After sacrifice, all animals were subjected to a complete gross necropsy. The weights of several organs were recorded. Brain cholinesterase activity was measured in whole brain homogenate. No information was given about sample timing, sample handling or assay conditions. All tissues from all animals were examined histologically.
The mortality rate, feed consumption, haematological and clinical chemical end-points and gross and histopathological appearance were not affected by treatment. The frequency of clinical signs such as rough coat, urine staining, loose stools and skin lesions (on the tail) increased in male and female rats at 18 and 54 ppm after about 20 weeks of administration. Body-weight gain was significantly decreased for male rats at 18 and 54 ppm and for females at 54 ppm. The relative weight of the testes was decreased in rats at 18 and 54 ppm (although the values were within the range for other controls in the same laboratory), and the relative weight of the brain was increased in both sexes at 54 ppm. The neoplasms found were similar in type, location, time of onset and incidence in control and treated rats. Cholinesterase activity was inhibited by treatment in a dose-related manner in both sexes. Slight-to-moderate inhibition of brain (31–39%), erythrocyte (32–36%) and plasma (26–47%) cholinesterase activity was found at 6 ppm; moderate inhibition of brain (64%), erythrocyte (65–68%) and plasma (70–71%) cholinesterase activity was found at 18 ppm; and marked inhibition of brain (75–79%), erythrocyte (75–81%) and plasma (91%) cholinesterase activity was found at 54 ppm. The NOAEL for inhibition of erythrocyte and brain cholinesterase activity was 2 ppm, equal to 0.1 mg/kg bw per day (Hayes, 1984c).
An extensive range of studies for genotoxicity has been performed with methamidophos in vitro in bacteria and in mammalian cells, for point mutations, chromosomal aberrations and DNA damage, and several tests were conducted in mammals in vivo. The results are summarized in Table 4. Positive results were reported for chromosomal aberrations in mouse spleen cells in vitro and for micronucleus formation and sister chromatid exchange in the bone marrow of mice treated in vivo by one group (Amer & Sayed, 1987) and for sister chromatid exchange in red muntjac deer lung fibroblast cells invitro. The positive results were contradicted by the negative results obtained in eight other assays in vitro and in seven tests in vivo, some in the same assays as those in which positive findings were made. The Meeting concluded that methamidophos is unlikely to be genotoxic in vivo.
Table 4. Results of assays for genotoxicity with methamidophos
|
End-point |
Test system |
Concentration |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
DNA repair |
E. coli (K12)p 3478 and W 3110 |
0, 620, 1200, 2500, 5000, 10 000 µg/platea |
71.2 |
Negative |
Herbold (1983) |
|
Unscheduled DNA synthesis |
Male Sprague-Dawley rat primary hepatocytes |
0, 0.001, 0.003, 0.01, 0.03, 0.3, 1 µl/mlb |
71.2 |
Negative |
Curren (1988) |
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0, 100, 500, 1000, 5000, 10 000 µg/platea |
Technical |
Negative |
Machado (1982) |
|
S. typhimurium TA98, TA100, TA1535, TA1537 |
0, 16, 50, 160, 500, 1600, 5000 µg/platea |
73.4 |
Negative |
Herbold (1994) |
|
|
Point mutation |
Chinese hamster ovary cells (K1-BH4), Hprt locus |
0, 1000, 2000, 3000, 4000, 5000 µg/mla |
75.6 |
Negative |
Bigger & Sigler (1993) |
|
Chinese hamster ovary cells (K1-BH4), Hprt locus |
0, 0.2, 0.5, 1, 2, 3.5 µl/mla |
71.2 |
Negative |
Harbell & Jacobson-Kram (1990) |
|
|
Chromosomal aberration |
Chinese hamster ovary cells (WBL) |
0, 1900, 2500, 2600, 3200, 3800, 4200, 5100, 5200 µg/mlb |
74.5 |
Equivocal |
Murli (1990) |
|
0, 1200, 2500, 3800, 5000 µg/mlc |
Negative |
||||
|
Mouse spleen cells |
0, 0.25, 0.5, 1, 2 µg/mlb |
Pure |
Positive |
Amer & Sayed (1987) |
|
|
Sister chromatid exchange |
Chinese hamster ovary cells (V79) |
0, 10, 20, 40, 80 µg/mla |
99.8 |
Negative |
Chen et al. (1982a,b) |
|
Red muntjac deer lung fibroblast cells |
0, 4.2, 14, 42 µg/mlb |
97 |
Positive |
He et al. (1982); Guo et al. (1986b) |
|
|
In vivo |
|||||
|
Micronucleus formation |
Male, female NMRI/W 77 mice, bone-marrow cells |
0, 5, 10 mg/kg bw per day orally (twice) |
62.6 |
Negative |
Herbold (1981) |
|
Male and female Swiss mice, bone-marrow cells |
0, 4.5, 6 mg/kg bw per day intraperitoneally (once, twice or thrice) 0, 50, 100 ppm in diet, equivalent to 0, 7.5, 15 mg/kg bw, 2 weeks 0, 12, 24 mg/kg bw dermally (4 times) |
Pure |
Positive |
Amer & Sayed (1987) |
|
|
Male and female Hsd/Win: NMRI mice, bone-marrow cells |
8 mg/kg bw intraperitoneally (once) |
75.1 |
Negative |
Herbold (1996) |
|
|
Chromosomal aberration |
Male and female CD-1 mice, bone-marrow cells |
0, 0.6, 2, 6, 9, 12 mg/kg bw orally (once) |
74.4 |
Negative |
Esber (1983) |
|
Male Kunming mice, bone-marrow cells |
0, 1.5, 3 mg/kg bw per day orally (twice) |
90.4 |
Negative |
Guo et al. (1986b) |
|
|
0, 1.2, 2.5, 5 mg/kg bw subcutaneously (twice) |
|||||
|
Male and female Wistar rats, bone-marrow cells |
0, 10, 20 ppm in diet, equivalent to 0, 1, 2 mg/kg bw per day, 12 weeks |
Negative |
Guo et al. (1986b) |
||
|
Sister chromatid exchange |
Male and female Swiss mice, bone-marrow cells |
0, 4, 6, 8 mg/kg bw intraperitoneally (once) |
Pure |
Positive |
Amer & Sayed (1987) |
|
Dominant lethal mutation |
Male CD-1 mice, germ cells |
0, 5, 50, 150 ppm in diet, equivalent to 0, 0.75, 7.5, 22 mg/kg bw per day, 5 days |
74.3 |
Negative |
Eisenlord (1984) |
|
Male mice |
0, 0.2, 2 mg/kg bw per day orally (7 times) |
90.4 |
Negative |
Guo et al. (1986b) |
a With and without metabolic activation
b Without metabolic activation
c With metabolic activation
Rats
In a two-generation study of reproductive toxicity, four groups of 26 male and 26 female CD rats were fed diets containing technical-grade methamidophos (purity, 70.5 %) at a concentration of 0, 3, 10 or 33 ppm, equivalent to 0, 0.15, 0.5 and 1.6 mg/kg bw per day, for at least 100 days before mating and throughout gestation and lactation of F1 rats. Randomly selected groups of 26 male and 26 female F1 pups were given diets containing methamidophos for 120 days before mating and throughout gestation and lactation of F2 rats. After lactation of F2a pups and a 1-month rest period, the F1 rats were mated again to produce the F2b pups. Clinical signs, body weight, food consumption, deaths, fertility and the development of the progeny were evaluated. A complete gross necropsy was done on all rats., and the reproductive organs and gross lesions from F0 and F1 parental animals were examined histologically.
Methamidophos at 33 ppm increased the incidence of clinical signs in both parental rats and pups, generally reduced the body weight of parental rats (although the F0 females had increased body weight before gestation), reduced the litter and pup weights, decreased the viability of pups and reduced the proportion of fertilized females giving birth to F2b pups. The NOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day, for parental, reproductive and developmental toxicity (Hixson, 1984a).
In another two-generation study of reproductive toxicity, four groups of 30 male and 30 female CD Sprangue-Dawley rats were given diets containing technical-grade methamidophos (purity, 69–77%) at a concentration of 0, 1, 10 or 30 ppm, equal to 0, 0.1, 0.9 and 2.5 mg/kg bw per day for males and 0, 0.1, 0.9 and 2.4 mg/kg bw per day for females. Rats of the F0 and F1 generations received methamidophos in the diet throughout the study, beginning at 7 weeks of age for the F0 rats and at weaning for the F1 rats. The animals received treated feed for 10 weeks, and then F0 rats were mated to produce F1a and F1b litters; F1 rats (randomly selected F1b pups) were mated to produce the F2a and F2b litters. During the study, adult animals were evaluated for effects on body weight, food consumption, clinical signs, estrus cycling, insemination length, mating, fertility, gestation length and plasma, erythrocyte and brain cholinesterase activity. The offspring were evaluated for treatment-related effects on litter size, sex, viability, body-weight gain, clinical signs and cholinesterase activity. Gross necropsies were performed on all adults and pups, and the reproductive organs, pituitary and gross lesions from all F0 and F1 adults were examined histologically.
Methamidophos at 10 and 30 ppm reduced the body weight of F1 male rats, reduced the body weights of F0 and F1 females during lactation but not gestation, decreased the weights of pups during lactation and reduced plasma, erythrocyte and brain cholinesterase activity in adults and pups. In addition, methamidophos at 30 ppm reduced the body weight of F0 male rats (despite increased food consumption), reduced the percentage of live pups per litter on day 21 from that at day 4 and increased the number of dams that cannibalized their pups. Methamidophos at 1 ppm reduced cholinesterase activity in adult males. No further effects of treatment were found. The NOAEL for parental and developmental toxicity was 1 ppm, equal to 0.1 mg/kg bw per day, on the basis of inhibition (> 20 %) of plasma, erythrocyte and brain cholinesterase activity and a statistically significant treatment-related reduction in body weight (8%). The NOAEL for repro-ductive toxicity was 30 ppm, equal to 2.4 mg/kg bw per day (the highest dose tested), on the basis of a statistically significant treatment-related reduction in body weight (Eigenberg et al., 1998).
Mice
In a study of developmental toxicity, four groups of female Kunming mice (number not specified) received methamidophos (purity not specified) orally at a dose of 0, 0.4, 2.2 or 4 mg/kg bw per day from day 16 of gestation until the pups were weaned on day 21 of lactation. The numbers of litters evaluated were 19–21 from treated groups and 34 from the control group. Maternal body weights and measures of physical and reflex development, locomotor activity and learning ability in the offspring were assessed. The cerebral cortexes of selected offspring were evaluated for neuron density and thickness and for protein.
Auditory startle, swimming ability, reflex suspension and T-water maze learning ability were statistically significantly affected at the lowest dose. The two higher doses also affected gestation length, eye opening and surface righting. The neuron density in the cerebral cortex of pups was statistically significantly increased at the two higher doses, and neuron thickness was statistically significantly decreased at the highest dose. A NOAEL for developmental delay could not be identified (Wang & Huang, 1987).
Rats
In a study of developmental toxicity, four groups of 22–26 female CD rats received methamidophos (purity, 70.5%) orally at a dose of 0, 0.3, 1 or 3 mg/kg bw per day on days 6–15 of gestation. Maternal clinical signs, body weight and feed consumption were reported. On day 21, the females were killed and inspected grossly, and their uterine contents were examined for sex of fetuses, fetal weights and numbers of implantations, resorptions, corpora lutea and viable fetuses. Fetuses were inspected grossly and preserved for visceral and skeletal examination.
The dams given 3 mg/kg bw per day showed typical signs of cholinergic toxicity and also decreased food consumption and body-weight gain relative to controls. The average fetal weights and total litter weights in this group were reduced. There were no treatment-related effects on the percentages of viable and malformed fetuses or on the incidences of external, visceral or skeletal fetal anomalies. No signs of maternal toxicity and no adverse effects on the fetuses were observed after administration of 0.3 or 1 mg/kg bw per day. The NOAEL was 1 mg/kg bw per day for both maternal toxicity, on the basis of clinical signs, decreased food consumption and decreased body-weight gain, and developmental toxicity, on the basis of decreased fetal and litter weights at 3 mg/kg bw per day (Hixson, 1984b).
In another study of developmental toxicity, three groups of 15–20 female albino rats received methamidophos (purity not specified) orally at a dose of 0, 1 or 2 mg/kg bw per day on days 6–15 of gestation. Maternal toxicity was not evaluated. On day 19, the females were killed and their uterine contents were examined for fetal weights and numbers of implantations and fetuses. Fetuses were inspected grossly and preserved for visceral and skeletal examination.
The average fetal weights and the numbers of implantations and viable fetuses were decreased at both doses. Embryo lethality and the incidence of malformations (anencephaly, anotia) were increased, particularly at 2 mg/kg bw per day. A NOAEL for developmental toxicity could not be identified (Hanafy et al., 1986).
Rabbits
In a study of developmental toxicity, four groups of 15 Himalayan rabbits received methamidophos (purity, 62%) orally at a dose of 0, 0.1, 0.5 or 2.5 mg/kg bw per day on days 6–18 of gestation. Maternal clinical signs and body weights were reported, but maternal food consumption and gross pathology were not. On day 29, the females were killed, and their uterine contents were examined for sex of fetuses, litter and average fetal weights and numbers of implantations, live and dead fetuses and stunted fetuses (weight, < 25 g). Fetuses were inspected grossly and preserved for visceral and skeletal examination.
The mean body-weight gain of the dams during treatment and throughout gestation was reduced in all treated groups, although statistical significance (p < 0.01) was reached only at the highest dose. The decrease was not dose-dependent, and no treatment-related deaths or clinical signs were seen. There were no treatment-related effects on litter size, fetal weight, other embryonic development parameters evaluated, percentages of viable and malformed fetuses or the incidences of external, visceral and skeletal fetal anomalies. The NOAEL for maternal toxicity was 0.5 mg/kg bw per day, on the basis of decreased body-weight gain at 2.5 mg/kg bw per day; and the NOAEL for developmental toxicity was 2.5 mg/kg bw per day (the highest dose tested) (Machemer, 1979).
Chickens
Groups of 5–23 Lohmann selected white Leghorn hens were given a single dose of technical-grade methamidophos (purity, 95.5 %) or its enantiomers dissolved in water by gavage with an antidote (atropine and/or 2-pyridine aldoxim hydrochloride) for protection. The doses given were 0, 200 or 400 mg/kg bw of racemic methamidophos, 100, 200 or 400 mg/kg bw of the R(+) enantiomer, or 400 mg/kg bw of the S(–) enantiomer. Six control hens and six at the highest dose of each of the enantiomers were examined for neuropathy target esterase (NTE) activity. In addition, groups of hens were dosed with 0 or 50 mg/kg bw of racemic methamidophos, 50, 100 or 400 mg/kg bw of the R(+) enantiomer, or 50 or 200 mg/kg bw of the S(–) enantiomer and examined for NTE activity. Tri-ortho-cresyl phosphate was given as a positive control at a dose of 100 or 300 mg/kg bw. Inhibition of NTE was assessed in three hens from each group at 24 and 48 h and, in some cases, 7 days after dosing, in lymphocytes, brain, spinal cord and sciatic nerve; reactivation of inhibited NTE was assessed in brain only. The hens that were not used for NTE determination were observed for 3–4 weeks after treatment. Throughout the study, birds were examined for clinical signs, motor activity and body weights. After death or sacrifice, all birds were subjected to gross necropsy.
Despite administration of antidote, 1/10 hens given 200 mg/kg bw of racemate, 7/10 given 400 mg/kg bw of racemate, 3/13 given 400 mg/kg bw of R(+) enantiomer and 15/23 given 400 mg/kg bw of S(–) enantiomer died within the first 6 days of dosing. Birds in all treated groups showed signs of acute cholinergic toxicity. Weak-to-moderate delayed neurotoxicity was observed in hens at 400 mg/kg bw of the racemate. Those at 400 mg/kg bw of R(+) enantiomer progressively developed marked signs of delayed neurotoxicity starting on day 8, whereas hens given the S(–) enantiomer showed no signs of delayed neuropathy. At 200 mg/kg bw of the R(+) enantiomer, four of five hens developed a slightly abnormal gait before day 25; this change was reversible and was no longer observed on day 28. Weight loss was observed in all treated hens at the beginning of the observation period, but most had regained their initial weight towards the end of the study.
Gross examination revealed pale, patchy livers in some sacrificed hens. Observations in dead hens included pale, dark, patchy livers, spleens and kidneys; distended, fluid-containing lungs; reddened duodenal mucosa; fluid-filled pericardium; and distended crop. Treatment at 50 mg/kg bw of racemic methamidophos led to 58–85% NTE inhibition in nervous tissues; however, nearly 90% of the inhibited NTE could be reactivated. Treatment with increasing doses of the enantiomers resulted in a dose-related increase in NTE inhibition during the first 2 days. There was less NTE inhibition after treatment with the S(–) enantiomer than with the R(+) enantiomer: R(+) enantiomer, 54–79% inhibition in brain at 50 mg/kg bw, 75–95% inhibition in nervous tissues at 100 mg/kg bw, and nearly 100% inhibition in brain at 400 mg/kg bw; S(–) enantiomer, 18–24% inhibition in brain at 50 mg/kg bw, 6–55% inhibition in nervous tissues at 100 mg/kg bw, and 58–84% inhibition in brain at 400 mg/kg bw. More than 80% of the NTE could be reactivated, even at the highest dose of the R(+) enantiomer; however, a relatively high proportion (73%) of the NTE inhibited by the high dose of the S(–) enantiomer could not be reactivated. NTE inhibition in lymphocytes was similar to that in nervous tissue on the first day, but the activity in lymphocytes recovered more quickly than in nervous tissue, normal levels being achieved within 7 days in all cases. Tri-ortho-cresyl phosphate caused irreversible NTE inhibition of 90–100%, confirming the sensitivity of the test system. The NOAEL for delayed neurotoxicity was 200 mg/kg bw for racemic methamidophos; 100 mg/kg bw for the R(+) enantiomer; and > 400 mg/kg bw for the S(–) enantiomer. Although methamidophos can cause delayed neurotoxicity after acute oral administration, its potential is low in view of the lethality of the doses that caused delayed neurotoxicity in this study (Flucke, 1990c; Flucke & Eben, 1990).
Six further studies of delayed neurotoxicity have been conducted (Kimmerle, 1967; Kruckenberg et al., 1979; Thyssen & Eben, 1982; Pauluhn & Kaliner, 1984; Guo et al., 1986a; El-Sebae et al., 1987), in which methamidophos was given as a single oral dose of < 75 mg/kg bw or at 30 mg/kg bw per day twice a day for 5 days. Nervous tissues were examined histologically, and NTE inhibition was measured in some studies. No clinical or histopathological signs of delayed neurotoxicity were found. The degree of NTE inhibition was similar to that measured in the study of Flucke & Eben, except in one study (El-Sebae et al., 1987) in which no inhibition was measured after a dose of 55 mg/kg bw.
Four groups of 16 white Leghorn hens were given technical-grade methamidophos (purity, 76%) dissolved in water at a dose of 0, 0.3, 1 or 3 mg/kg bw per day by gavage on 5 days/week for 90 days. Birds were monitored throughout the study for clinical signs, motor activity and body weight. Plasma cholinesterase activity was measured before treatment and monthly in 10 hens from each group. NTE inhibition was measured in brain and spinal cord at the end of the study in six hens from each group. All birds were subjected to gross necropsy, and nervous tissues from 10 hens in each group were evaluated histopathologically.
No treatment-related deaths occurred, and no treatment-related clinical signs or body-weight changes were seen in birds at the two lower doses. Somnolence and slight emaciation were seen in most hens at the highest dose, with treatment-related decreases in body weight. No clinical or histopathological signs of delayed neurotoxicity were found in any treated group. Plasma cholinesterase activity was inhibited by about 22% at the intermediate dose and by about 45% at the highest dose at all measurement times during the study. NTE activity was inhibited by 17% in brain and 41% in spinal cord at the highest dose and by 22% in spinal cord at the intermediate dose. The NOAEL for delayed neurotoxicity was 3 mg/kg bw per day, the highest dose tested (Sachsse et al., 1987).
Two doses of 200 mg/kg bw of technical-grade methamidophos (purity, 74%) were applied 3 weeks apart to the combs of 30 white Leghorn hens given antidote (atropine) protection. Five hens were given a single oral dose of 375 mg/kg bw tri-ortho-cresyl phosphate as a positive control. An untreated control group of six hens was also evaluated. All birds were observed for 42 days, except for the positive controls, which were killed on day 20 because of marked progressive ataxia and paresis. Throughout the study, the birds were monitored for clinical signs, motor activity and body weight. After death or sacrifice, all hens were subjected to a gross necropsy. Nervous tissue from six randomly selected treated hens and all positive and negative control birds was examined histologically.
Despite the use of antidote, 10 birds died, mainly within the first 3 days after dosing. Treated birds showed signs of acute cholinergic toxicity and weight loss for up to 1 week after each dose. No clinical or histopathological signs of delayed neurotoxicity were seen. Those given tri-ortho-cresyl phosphate showed degenerated or missing myelin and pronounced fibrodegeneration in the sciatic nerve and spinal cord. Gross examination revealed no treatment-related findings in hens that were killed. Dead hens were found to have distended, fluid-containing lungs; green mucus in the gastrointestinal tract and distended crop. Some had enlarged gall-bladders. The NOAEL for delayed neurotoxicity was 200 mg/kg bw (the only dose tested) (Flucke & Kaliner, 1985).
Groups of nine Lohmann selected white Leghorn hens under antidote (atropine) protection received a single application to the comb of technical-grade methamidophos (purity, 73%) at a dose of 0 or 200 mg/kg bw. Nine hens were given a single oral dose of 100 mg/kg bw tri-ortho-cresyl phosphate as a positive control. Brain and spinal cord NTE activity was evaluated in samples from three birds from each group taken 24 and 48 h and 7 days after dosing. The birds were weighed before treatment and on days 1, 2 and 7. Distinct weight losses were observed in all treated hens. The dose of 200 mg/kg bw led to maximum NTE inhibition of about 60% in brain 24 and 48 h after treatment. Tri-ortho-cresyl phosphate inhibited NTE activity in brain and spinal cord by > 80% during the first 2 days, confirming the sensitivity of the test system (Flucke & Eben, 1988).
Four groups of 16–26 Lohmann selected Leghorn hens received an application to the comb of technical-grade methamidophos (purity, 76 %) formulated in 2-propanol at a dose of 0, 0.5, 1.5 or 4.5 mg/kg bw per day on 5 days/week for 13 weeks. Sixteen hens were treated in a similar manner with 15 mg/kg bw per day of tri-ortho-cresyl phosphate as a positive control. Ten birds each given 0 and 4.5 mg/kg bw per day were observed for an additional 4 weeks after the end of treatment to evaluate recovery. Throughout the study, the birds were monitored for clinical signs, motor activity, body weight and plasma cholinesterase activity. NTE inhibition was measured in brain and spinal cord from one to three hens in each group in weeks 4 and 13. All birds were subjected to gross necropsy. Nervous tissues from hens given 0 and 4.5 mg/kg bw per day were evaluated histopathologically.
In each group, one to two birds died or were killed in moribund condition. Only one of the deaths, at 4.5 mg/kg bw per day, was considered to be related to treatment. Acute cholinergic symptoms were seen in all hens at the highest dose, which persisted in some cases until the end of treatment. Changes were observed in a few clinical parameters in some groups but were considered not to be related to treatment as no dose–response relationship was found. All hens at the highest dose also showed an initial decrease in body weight, which disappeared during the recovery period. Detachment of the uppermost layer of skin at the application site was observed in all birds at the highest dose, in some at the intermediate dose and in one at the lowest dose. No clinical or histopathological signs of delayed neurotoxicity were seen, but the tri-ortho-cresyl phosphate-treated birds showed clear signs of delayed neurotoxicity, both clinically and histopathologically. Plasma cholinesterase activity was inhibited by 11–13% at the intermediate dose and 40–43% at the highest dose at all measurement times during the first 13 weeks. No inhibition was measured at week 17 in the birds allowed to recover. Slight inhibition of NTE activity (8–21%) was observed only in birds at the highest dose. In contrast, the activity of NTE in brain and spinal cord of the positive control birds was markedly inhibited (51–76%). The NOAEL for delayed neurotoxicity was 4.5 mg/kg bw per day (the highest dose tested) (Bomann et al., 1992).
Thymus and spleen cells from mice and peripheral blood lymphocytes from healthy male donors were treated with methamidophos at concentrations ranging from 10–7 mol/l to 10–3 mol/l. After incubation, viability, cell proliferation and antibody formation were measured. The viability of the cells was not affected, but the proliferation of T lymphocytes in thymus and the ability of B lymphocytes from mouse spleen and human blood to form antibodies was decreased in a dose-related manner. The IC50 values (concentration of substance causing 50% inhibition) estimated from dose–response curves were in the range 10–5 to 10–3 mol/l (Tiefenbach et al., 1990).
Male CBA mice were given a single intraperitoneal dose of methamidophos at 0.6, 1.2 or 6 mg/kg bw, and Wistar rats subjected to adrenalectomy were given a single intraperitoneal dose of 1.2 mg/kg bw. Within 24 h of treatment, blood parameters; the weight, proliferation (mice only) and number of cells in the thymus, spleen, mesenteric lymph nodes and bone marrow (mice only); the concentrations of glucose (mice only) and cortisol (mice only) in blood and the activity of tyrosine transaminase (mice only) in liver were measured. In addition, antibody formation was determined in mice in vivo and in vitro.
In mice, the doses of 1.2 and 6 mg/kg bw strongly inhibited cholinesterase activity in plasma and brain and induced a number of immunological effects, including decreased numbers of lymphocytes and monocytes, increased number of neutrophil granulocytes, decreased weights and numbers of lymphocytes in thymus and spleen, decreased cell proliferation in the thymus, increased cell proliferation in bone marrow and decreased antibody formation. In addition, the concentrations of cortisol and glucose and the activity of tyrosine transaminase were increased. The adrenalectomized rats showed no immunosuppressive effects after dosing with methamidophos. The authors suggested that the immunosuppression is a nonspecific effect due to stimulation of the adrenal gland, which releases glucocorticoids after the stress induced by inhibition of cholinesterase (Tiefenbach & Wichner, 1985).
In a study of dermal irritation, methamidophos was applied to the intact abraded skin or to the inside of rabbits’ ears for 24 h. Reactions ranging from no irritation to well-defined erythema were found in skin and moderate reddening and swelling were found on the inside of the ear. All the dermal irritation disappeared within 96 h (Kimmerle, 1967; Levy, 1979; Hixson, 1980b). No irritation was seen when methamidophos was applied dermally for 4 h (Märtins, 1990).
Methamidophos caused ocular irritation in rabbits. Slight-to-moderate corneal opacity and conjunctival irritation were observed in most rabbits up to 72 h. Iritis was observed in some animals up to 24 h. The eyes of all rabbits except one were normal by day 14 (Rittenhouse, 1977; Hixson, 1980b; Märtins, 1990).
No evidence of skin sensitizing potential was observed in a modified Buehler test in which methamidophos was administered at a concentration of 25% to Hartley guinea-pigs (Cushman, 1984).
Methamidophos inhibits cholinesterase by phosphorylating a serine hydroxyl group on the active site of the enzyme. The mechanism of phosphorylation involves cleavage of the P–S bond in methamidophos, after which the amido(methoxy)phosphinyl moiety of methamidophos binds to cholinesterase (de Jong et al., 1982; Thompson & Fukuto, 1982; Fukuto & Thompson, 1985). The general mechanism of inhibition of NTE by organophosphorus compounds involves phosphorylation of NTE, followed by ‘ageing’, in which an R–O–P bond is cleaved, resulting in an ionized phosphoryl residue which is believed to bind to the neural membrane and thus cause delayed neuropathy. During ageing, the inhibited enzyme becomes resistant to reactivation after treatment with nucleophilic agents, such as oximes and potassium fluoride (Johnson & Lotti, 1989). As for cholinesterase, the phosphorylation of NTE probably involves cleavage of the S-methyl group of methamidophos (Vilanova et al., 1987).
Methamidophos was shown to be a weak-to-moderate inhibitor of cholinesterase in vitro in tissues from humans, hens, rats, eels, houseflies and honeybees (Tucker, 1972; Khasawinah et al., 1978; Robinson & Beiergrohslein, 1980, 1982; Langenberg et al., 1987; Vilanova et al., 1987). The S(–) isomer was a more potent cholinesterase inhibitor than the R(+) isomer in tissues from humans and hens in vitro (Bertolazzi et al., 1991). Methamidophos was a more potent inhibitor of cholinesterase than of NTE in both human and hen tissue in vitro (Vilanova et al., 1987; Bertolazzi et al., 1991). The R(+) isomer appeared to be a more potent inhibitor of NTE than the S(–) isomer in tissue from humans and hens in vitro and in hens in vivo (Flucke & Eben, 1990; Bertolazzi et al., 1991). Inhibition of human and hen NTE followed first-order kinetics, whereas cholinesterase underwent spontaneous reactivation (Bertolazzi et al., 1991).
Significant, rapid, spontaneous reactivation of inhibited cholinesterase was found within 24 h in assays with rat, hen and eel tissues in vitro (Robinson & Beiergrohslein, 1980; de Jong et al., 1982; Langenberg et al., 1987; Vilanova et al., 1987). Addition of oximes (cholinesterase reactivators) initially increased the rate of reactivation, but this enhancement declined over time (Robinson & Beiergrohslein, 1980). Cholinesterase activity inhibited by the S(–) isomer appeared to be reactivated to a greater extent than cholinesterase inhibited by the R(+) isomer. About two times more reactivation was found for human cholinesterase than for hen cholinesterase inhibited by the S(–) isomer (Bertolazzi et al., 1991). Ageing of NTE by methamidophos was not clearly demonstrated in hen tissue in vitro (Vilanova et al., 1987), but no spontaneous reactivation was observed (Vilanova et al., 1987; Bertolazzi et al., 1991). Inhibition of NTE in hens in vivo by the R(+) isomer was reported to be reversible, while inhibition by high doses of the S(–) isomer was mainly irreversible (Flucke & Eben, 1990).
Although methamidophos is considered to be only a weakly-to-moderately potent cholinesterase inhibitor in vitro, high toxicity was observed in vivo. Its accumulation in the nervous system due to its relative stability and low rate of degradation, was suggested to contribute to its toxicity (Khasawinah et al., 1978; Fukuto & Thompson, 1985).
Brain homogenates and erythrocytes from rats and monkeys were incubated in vitro with various concentrations of methamidophos (purity, 99.6%), acephate, technical-grade acephate and mixtures of acephate and technical-grade acephate with 1–3% methamidophos for 60 min at 37 ×C. After incubation, the samples were analysed for cholinesterase activity by the method of Ellman et al. (1961), and IC50 concentrations were estimated for each material; the relative potencies of the subtances were then calculated. Technical-grade acephate containing 1% methamidophos as an impurity was a more potent inhibitor than acephate, and methamidophos was about 1000 times more potent than acephate in inhibiting erythrocyte and brain cholinesterase activity in both rats and monkeys. It was concluded that methamidophos present as an impurity in technical-grade acephate may account for essentially all the latter’s ability to inhibit cholinesterase (Wong & Kodama, 1979).
Rats
Four groups of 24 Sprague-Dawley rats of each sex were given a single dose of technical-grade methamidophos (purity, 76%) dissolved in 0.5% (w/v) methylcellulose with 0.4% (w/v) Tween 80 in deionized water by gavage at a dose of 0, 1, 3 or 9 mg/kg bw. In an additional study, doses of 0, 0.3 and 0.7 mg/kg bw were administered to groups of 18 rats of each sex. The animals were observed for 15 days after treatment. Throughout both studies, all rats were monitored for clinical signs and body weight. Twelve rats of each sex per group in the main study underwent behavioural testing (functional observational battery, motor and locomotor activity tests and habituation testing) and gross necropsy, and six rats of each sex per group were perfused at necropsy and examined for neuropathological changes. Neurological tissue from perfused controls and rats at the highest dose was examined histologically. Haematological and clinical chemical end-points were evaluated in six rats of each sex per group. Cholinesterase activity in plasma, erythrocytes and brain was measured in six rats of each sex per group. In the supplemental study, behavioural testing was performed on 12 rats, and plasma, erythrocyte and brain cholinesterase activity was measured in six rats.
No treatment-related deaths or changes in body weight were observed. Clinical signs of cholinergic poisoning occurred on the day of treatment but disappeared within 5 days in animals at 3 and 9 mg/kg bw. Dose-dependent, statistically significant decreases in cholinesterase activity (by > 20%) were found in all tissues from treated rats 2 h after dosing. In the supplemental study, inhibition ofcholinesterase activity by > 20% was observed at 0.7 mg/kg bw but not at 0.3 mg/kg bw. The behavioural test on the day of dosing revealed a dose-related reduction in activity and other signs of cholinergic poisoning in all treated rats, but all animals had recovered by 7 or 14 days after dosing. In the supplemental study, no treatment-related effects on behaviour were detected at any dose, and no treatment-related findings were observed at gross necropsy or histopathological evaluation of nervous tissues. Haematological end-points were not affected. Clinical chemical analysis showed increased serum aspartate aminotransferase activity in both sexes, decreased serum triglyceride concentrations in females at 3 and 9 mg/kg bw, increased serum alanine aminotransferase activity in both sexes, and increased cholesterol concentrations in males at 9 mg/kg bw. The NOAEL for inhibition of erythrocyte and brain cholinesterase activity was 0.3 mg/kg bw. The NOAEL for neurotoxicity (excluding cholinergic responses) was 9 mg/kg bw, the highest dose tested (Sheets, 1993).
Four groups of 18 Fischer 344 rats of each sex were given diets containing methamidophos (purity, 76%) at a concentration of 0, 1, 12 or 59 ppm, equal to 0, 0.067, 0.79 and 4.3 mg/kg bw per day for males and 0, 0.074, 0.9 and 4.9 mg/kg bw per day for females, for 90 days. Clinical signs, food consumption and body weights were monitored throughout the experiment in 12 rats of each sex per group. Behavioural testing (functional observational battery, motor and locomotor activity and habituation testing) and ophthalmic examinations were performed on the same rats. Cholinesterase activity in plasma, erythrocytes and brain was measured in six other rats of each sex per group. Six rats of each sex per group were grossly necropsied and perfused and used for neuropathology. Neurological tissues from controls and rats at the highest dietary concentration were examined histologically.
No treatment-related deaths were observed. Food consumption was increased in females at 59 ppm, and body-weight gain was reduced in males at that dietary concentration. Behavioural testing and clinical observations revealed a dose-related reduction in activity and other signs of cholinergic poisoning in rats at 12 and 59 ppm. The signs of toxicity persisted with continued administration, but there was no evidence of cumulative toxicity beyond weeks 4–8. Dose-dependent, statistically and biologically significant decreases (> 20%) in cholinesterase activity were seen in all tissues throughout the study in rats at the two higher dietary concentrations. No treatment-related findings were observed at necropsy, ophthalmic examination or histopathological evaluation of nervous tissues. The NOAEL for inhibition of erythrocyte and brain cholinesterase activity was 1 ppm, equal to 0.067 mg/kg bw per day. The NOAEL for other neurotoxicity was 59 ppm, the highest dose tested, equal to 4.3 mg/kg bw per day (Sheets, 1994, 1996).
Monitoring of production plant personnel following normal safety precautions has indicated no adverse effects, except for slight, transient inhibition of cholinesterase activity (Köllert, 1981; Sitt, 1990).
Several poisoning incidents have been reported with methamidophos, including cases of intentional ingestion by people attempting suicide. The initial symptoms of poisoning were consistent with cholinesterase inhibition, including miosis, salivation, sweating, muscular fasciculations and, after severe poisoning, unconsciousness. When tested, reduced plasma and erythrocyte cholinesterase activity was observed. Most of the cases of acute cholinergic poisoning were successfully treated with atropine and cholinesterase reactivators such as oximes. An intermediate syndrome was observed in a few patients within 24–96 h after poisoning, which predominantly affected muscles innervated by cranial nerves (neck flexors, proximal muscles of the limbs and muscles of respiration), resulting in muscle weakness. After a latent period ranging from 10 to 30 days after exposure, delayed neuropathy occurred in some patients who had been severely (potentially fatally) poisoned with methamidophos. The symptoms included pain, weakness and paralysis of the distal muscles of the limbs. Electromyography confirmed lesions in the distal portions of peripheral nerves. Some patients recovered completely within 6 weeks to 2 years after the onset of symptoms (Senanayake & Johnson, 1982; Senanayake & Karalliede, 1987; Brown et al., 1989; Goh et al., 1990). A woman in the 36th week of pregnancy who attempted suicide by ingesting methamidophos was treated and delivered a healthy boy 44 days after the incident (Karalliedde et al., 1988).
In a study that was not conducted according to current standards, seven female and seven male healthy volunteers aged 21–48 years and weighing 55–122 kg were divided randomly into a control and two treatment groups. The control group and a group given a 1:4 mixture of methamidophos:acephate contained four subjects, and the second treated group consisted of six people given a 1:9 methamidophos:acephate mixture. The doses were given orally each day in capsules in three equally divided doses. After a 2-week pre-treatment period, the test period consisted of four 21-day segments in which the daily dose was increased from 0.1 mg/kg bw per day to 0.2, 0.3 and 0.4 mg/kg bw per day. Dosing was terminated when a significant effect on any monitored parameter was detected. The dosing period was followed by a 1-week recovery period. Plasma and erythrocyte cholinesterase activity and various blood parameters were measured throughout the experiment, and the subjects were observed for clinical signs of poisoning. No information was provided about sampling times, sample handling or the assay for cholinesterase activity.
Plasma cholinesterase activity was significantly depressed at 0.2 mg/kg bw per day in men (35%) and women (45%) receiving the 1:4 mixture, at 0.3 mg/kg bw per day in men (25%) receiving the 1:9 mixture and at 0.4 mg/kg bw per day in women (25%) receiving the 1:9 mixture. No effects on erythrocyte cholinesterase activity or on haematological parameters were detected at any time during the study, and no clinical signs were found. The NOAEL for erythrocyte cholinesterase inhibition was 0.3 mg/kg bw per day for the 1:9 mixture and 0.2 mg/kg bw per day for the 1:4 mixture, equivalent to 0.03 and 0.04 mg/kg bw per day, respectively, for methamidophos (Garofalo, 1973).
The dermal absorption and excretion of methamidophos were investigated in healthy male volunteers given [14C]methamidophos at a dose of 3 µg/cm2. Urine, faeces and serial venous blood samples were collected for analysis over 5 days. The dose was reported to represent that typically encountered by agricultural workers. Topically applied radioactivity bound to the skin, and the absorbed radioactivity was slowly eliminated from the body. The mean total recovery of administered radioactivity was 72%. A mean of 0.55% of the dermally administered radioactivity was excreted in urine, the highest per cent (0.11%) being excreted during the first 2–3 days. No significant amount of radioactivity was excreted in faeces. The mean of 0.55% was reported to be less than that excreted in the urine of monkeys treated dermally (1.2%), indicating that human dermal absorption is less extensive. In monkeys, dermal absorption was calculated as the ratio of the sum of radioactivity excreted after intravenous and dermal administration (ratio, 8.8). As the results of the studies in monkeys and humans showed that the compound behaved similarly, and intravenous administration of the compound to humans would be unacceptable, the ratio used for monkeys was used to determine the dermal absorption of methamidophos in humans. On the basis of these assumptions and using the ratio of 8.8 calculated from the study in monkeys, the dermal absorption of methamidophos by volunteers receiving a single dermal application of 3 µg/cm2 was estimated to be 4.8% (Selim, 2000).
While many of the studies that were reviewed by the present Meeting were conducted prior to the development of good laboratory practice, most of the pivotal studies were carried out according to appropriate standards for study protocol and conduct.
[S-methyl-14C]- and [32P]-Labelled methamidophos administered orally, intraperitoneally or intravenously to rats was rapidly absorbed and distributed, 50–77% of the administered dose being eliminated within the first 1–3 days after dosing. Urine and expired CO2 were the major media of elimination of the 14C-labelled material and urine and faeces the major repositories of 32P-labelled methamidophos. Some radioactivity was incorporated into the body in the form of 14C fragments and ultimately eliminated with the natural turnover of these compounds.
Oral administration of a single dose of [S-methyl-14C]methamidophos on day 18 to pregnant rats resulted in rapid absorption and distribution (within 1 h) to various tissues, including the placenta and the fetus, and elimination within 48 h by both dams and fetuses. In a corresponding study with suckling rats, methamidophos was also rapidly absorbed and subsequently distributed throughout the body. The concentrations in the pups indicated that it was present in milk.
The extent of percutaneous absorption of [S-methyl-14C]methamidophos over 24 h was about 40% in rats, 11% in monkeys and 5% in humans. The total recovery of administered radioactivity in humans was 72%, and 0.55% was excreted in urine.
Methamidophos was rapidly degraded through deamination and/or demethylation in rats. The first step was cleavage of P–O, P–N or P–S bonds, followed by demethylation. The major degradation products found, in addition to unchanged methamidophos, were desamino-methamidophos, methyl dihydrogen phosphate, S-methyl phosphorothioic acid, methyl hydrogen phosphoramidate, S-methyl hydrogen phosphoramidothioate, and phosphoric acid. The existence of volatile metabolites other than CO2 at concentrations above the detection limit of 5 µg per animal could not be established unequivocally; however, their formation was considered to be very likely.
Methamidophos is acutely toxicity when administered orally, with an LD50 of 13–23 mg/kg bw in rats. The clinical signs of toxicity are those typical of cholinergic effects. Its toxicity to hens, guinea-pigs, rabbits, cats and dogs was comparable to that in rodents. Adult male rats were more sensitive to methamidophos than were females and young rats. The acute toxicity after oral administration was greater in fasted than in non-fasted mice and rats. Methamidophos had moderate-to-high acute toxicity when administered dermally to experimental animals (LD50: 110–380 mg/kg bw in rats) and had high acute toxicity when administered by inhalation (LC50: 0.063–0.21 mg/l of air in rats). Methamidophos was a mild dermal irritant and was slightly irritating to the eyes of rabbits. It was not a skin sensitizer. WHO has classified methamidophos as ‘highly hazardous’ (WHO, 2000).
In a study of neurotoxicity with methamidophos in rats given a single oral dose of 0, 0.3, 0.7, 1, 3 or 9 mg/kg bw, a dose-dependent, statistically significant decrease in erythrocyte and brain cholinesterase activity (> 20%) was observed at 0.7 mg/kg bw but not at 0.3 mg/kg bw. A dose-related reduction in activity and signs of cholinergic intoxication were observed at 1–9 mg/kg bw. The NOAEL for inhibition of erythrocyte and brain cholinesterase activity was 0.3 mg/kg bw.
In a 90-day study of neurotoxicity in rats given methamidophos at 0, 1, 12 or 59 ppm of diet (equal to 0, 0.067, 0.79 and 4.3 mg/kg bw per day), dose-dependent, statistically significant decreases (> 20%) in erythrocyte and brain cholinesterase activity, a reduction in locomotor activity and other signs of cholinergic intoxication were seen at 12 and 59 ppm. The NOAEL for inhibition of erythrocyte and brain cholinesterase activity was 1 ppm, equal to 0.067 mg/kg bw per day.
In short-term studies of toxicity in mice (oral, dermal, intraperitoneal), rats (oral, dermal, inhalation, intraperitoneal), rabbits (dermal) and dogs (oral), methamidophos caused signs of toxicity related to inhibition of cholinesterase activity . Effects on organ weights were observed in only a few rats. The NOAEL was 2 ppm, equal to of 0.13 mg/kg per day, in rats, and 2 ppm, equal to 0.06 mg/kg bw per day, in dogs on the basis of inhibition (> 20%) of erythrocyte and brain cholinesterase activity.
In long-term studies of toxicity in mice and rats, there was no evidence of organ-specific toxicity, and no additional effects were seen over those observed in the short-term studies. Inhibition of cholinesterase activity was the most sensitive end-point. During a recovery period of 2–4 weeks after long-term administration, cholinesterase activity returned to control levels in rats. In mice, the NOAEL was 5 ppm, equal to 0.67 mg/kg bw per day, on the basis of reduced body-weight gain and feed consumption. In rats, the NOAEL was 2 ppm, equal to 0.1 mg/kg bw per day, on the basis of inhibition (> 20%) of erythrocyte and brain cholinesterase activity.
In the absence of carcinogenic effects observed in mice or rats, the Meeting concluded that there was no evidence that methamidophos has a carcinogenic potential in either species.
An extensive range of tests for genotoxicity has been performed with methamidophos both in vitro and in vivo. The positive results obtained in a few assays were contradicted by the negative results obtained in a range of adequate tests, including assays in which positive findings were also observed. The Meeting concluded that methamidophos is unlikely to be genotoxic in vivo.
In view of the lack of evidence for genotoxicity or for carcinogenic potential in mice or rats, the Meeting concluded that methamidophos is unlikely to pose a carcinogenic risk to humans.
In two studies of reproductive toxicity in rats given methamidophos at 0, 3, 10 or 33 ppm in the diet, equivalent to 0, 0.15, 0.5 and 1.6 mg/kg bw per day, or 0, 1, 10 or 30 ppm, equal to 0, 0.1, 0.9 and 2.4 mg/kg bw per day, reproductive performance and pup development were affected only at the highest doses. The toxicity of methamidophos to parental rats was expressed as depression of body-weight gain and a reduced fertility index. Reductions in the body-weight gain and viability of pups were attributed to the parental toxicity. The NOAEL was 1 ppm, equal to 0.1 mg/kg bw per day, for parental toxicity, on the basis of inhibition of erythrocyte and brain cholinesterase activity (> 20 %) at higher doses.
Methamidophos was tested for its ability to induce developmental toxicity in mice, rats and rabbits at doses up to 4, 3 and 2.5 mg/kg bw per day, respectively. In rats at the highest dose, delays observed in pup development were associated with maternal toxicity, characterized by a depression in body-weight gain. Anencephaly was reported at a dose of < 1 mg/kg bw per day in a published study of the developmental toxicity of methamidophos in rats. However, in adequately conducted studies of developmental toxicity in rats and rabbits, no evidence of malformations was found at doses higher than 1 mg/kg bw per day. The Meeting concluded that methamidophos is not teratogenic.
The Meeting concluded that the existing database was adequate to characterize the potential for hazard of methamidophos to fetuses, infants and children.
Studies of delayed polyneuropathy were conducted with methamidophos [racemate, R(+) enantiomer or S(–) enantiomer] in hens. When the racemate was given at a single oral dose of 400 mg/kg bw, it resulted in weak-to-moderate delayed polyneuropathy. Hens dosed with the R(+) enantiomer showed marked signs of delayed polyneuropathy and inhibition of neuropathy target esterase activity in the brain (by nearly 100%). Hens dosed with the S(-) enantiomer did not show signs of delayed polyneuropathy and had less inhibition of neuropathy target esterase activity in the brain (58–84%). Treatment with any of the compounds was associated with severe toxicity and death, despite administration of an antidote. The Meeting concluded that delayed polyneuropathy develops only at doses well above the LD50.
Monitoring of production plant personnel indicated no adverse effects, except for slight, transient inhibition of cholinesterase activity in workers complying with normal safety precautions.
Delayed polyneuropathy was reported in humans after exposure to large, but unknown, quantities of methamidophos, usually by ingestion. When tested, reduced plasma and erythrocyte cholinesterase activities were observed. Long-term follow-up indicated complete recovery in some patients within several months to 2 years after the onset of symptoms.
A mixture of methamidophos and acephate (in a ratio of 1:4 or 1:9) was administered to volunteers in repeated doses over 21 days, and plasma and erythrocyte cholinesterase activities were measured. Erythrocyte cholinesterase activity did not appear to be inhibited in either sex at doses of the 1:9 mixture up to 0.3 mg/kg bw per day, equivalent to a dose of methamidophos of 0.03 mg/kg bw per day. As this study was not conducted according to current standards, the Meeting considered that it could be used only to support a reference value.
The Meeting established an ADI of 0–0.004 mg/kg bw on the basis of the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte and brain cholinesterase activity in the 2-year study of toxicity and carcinogenicity in rats and a safety factor of 25. This value is supported by the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte cholinesterase activity in the multigeneration study of reproductive toxicity in rats and by the NOAEL of 0.06 mg/kg bw per day for inhibition of erythrocyte and brain cholinesterase activity in the 1-year study of toxicity in dogs. Use of a safety factor of 25 is supported by the information provided by the 21-day study in volunteers, by the finding of negligible species differences in inhibition of cholinesterase activity in rats, dogs and humans in vitro, by the absence of differences in inhibition of erythrocyte and brain cholinesterase activity in various animal species and because the effect was dependent on the Cmax (see section 2.2 of the report: Annex 1, reference 95).
The Meeting established an acute RfD of 0.01 mg/kg bw on the basis of the NOAEL of 0.3 mg/kg bw for inhibition of erythrocyte and brain cholinesterase activity in the study of neurotoxicity in rats given single doses and a safety factor of 25. This safety factor was used for the same reasons as those given above.
The results of the study in volunteers were supportive of both the ADI and the acute RfD because methamidophos did not cause toxic effects other than those associated with, or secondary to, inhibition of cholinesterase activity.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya |
Toxicity |
5 ppm, equal to |
25 ppm, equal to |
|
Carcinogenicity |
25 ppm equal to |
– |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
2 ppm, equal to |
6 ppm, equal to |
|
Carcinogenicity |
54 ppm, equal to |
– |
||
|
Multigeneration study of reproductive toxicitya |
Parental toxicity |
1 ppm, equal to |
10 ppm, equal to |
|
|
Reproductive toxicity |
300 ppm, equal to |
– |
||
|
Developmental toxicityb |
Maternal toxicity |
1 mg/kg bw per day |
3 mg/kg bw per day |
|
|
Embryo and feto-toxicity |
1 mg/kg bw per day |
3 mg/kg bw per day |
||
|
Acute neurotoxicityc |
Inhibition of cholinesterase activity |
0.3 mg/kg bw |
0.7 mg/kg bw |
|
|
Neurotoxicity |
9 mg/kg bwb |
– |
||
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
0.5 mg/kg bw per day |
2.5 mg/kg bw per day |
|
Embryo- and feto-toxicity |
2.5 mg/kg bw per dayb |
– |
||
|
Dog |
1-year study of toxicitya |
Toxicity |
2 ppm, equal to |
8 ppm, equal to |
|
Human |
21-day study of toxicityd |
Inhibition of cholinesterase activity |
0.03 mg/kg bw per dayb |
– |
aDietary administration
bHighest dose tested
cGavage
dCapsule; only supportive for establishment of reference values
Estimate of acceptable daily intake for humans
0–0.004 mg/kg bw
Estimate of acute reference dose
0.01 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid and extensive |
|
Distribution |
Extensive |
|
Potential for accumulation |
None |
|
Rate and extent of excretion |
Rapid |
|
Metabolism in animals |
Very extensive through deamination or demethylation |
|
Toxicologically significant compounds (animals, plants and environment) |
Methamidophos |
|
Acute toxicity |
|
|
Rat, LD50, oral |
13–23 mg/kg bw |
|
Rat, LD50, dermal |
110–380 mg/kg bw |
|
Rat, LC50, inhalation |
0.063–0.21 mg/l (4 h, nose-only) |
|
Mouse LD50, oral |
10–30 mg/kg bw |
|
Skin irritation |
Mildly irritating |
|
Eye irritation |
Mildly irritating |
|
Skin sensitization |
Not sensitizing (modified Buehler test) |
|
Short-term studies of toxicity |
|
|
Target /critical effect |
Inhibition of cholinesterase activity |
|
Lowest relevant oral NOAEL |
2.1 ppm (equal to 0.1 mg/kg bw per day, 56-day study in rats) |
|
2 ppm (equal to 0.06 mg/kg bw per day, 1-year study in dogs) |
|
|
Genotoxicity |
Unlikely to be genotoxic in vivo |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Inhibition of cholinesterase activity |
|
Lowest relevant NOAEL |
2 ppm (equal to 0.1 mg/kg bw per day, 2-year study in rats) |
|
Carcinogenicity |
Unlikely to pose a carcinogenic risk to humans |
|
Reproductive toxicity |
|
|
Target/critical effect for reproductive toxicity |
Reduced parental and pup weight and viability |
|
Lowest relevant NOAEL for reproductivetoxicity |
1 ppm (equal to 0.1 mg/kg bw per day, rats) |
|
Target/critical effect for developmental toxicity |
Decreased fetal body weight only at maternally toxic doses |
|
Lowest relevant NOAEL for developmental toxicity |
1 mg/kg bw (rats) |
|
Neurotoxicity |
|
|
Acute |
NOAEL: 0.3 mg/kg bw for inhibition of cholinesterase activity (rats) |
|
90-day |
NOAEL: 1 ppm (equal to 0.067 mg/kg bw for inhibition of cholinesterase activity, rats) |
|
Delayed polyneuropathy |
Delayed polyneuropathy at doses well above the LD50 |
|
Human dataa |
In a 21-day study in volunteers treated orally, no inhibition of erythrocyte cholinesterase activity at 0.03 mg/kg bw per day |
|
Delayed polyneuropathy developed after severe poisoning |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.004 mg/kg bw |
Rat (long-term study of toxicity) supported by short-term study in dogs, study in volunteers and multigeneration study of reproductive toxicity in rats |
25 |
|
Acute RfD |
0.01 mg/kg bw |
Rat (single-dose study of neurotoxicity) |
25 |
aOnly supportive for establishment of reference values
Amer, S.M. & Sayed, M.A. (1987) Cytogenetic effects of the insecticide methamidophos in mouse bone marrow and cultured mouse spleen cells. Z. Naturforsch. 42C, 21-30.
Bagos, A.C. & Beatty, P.W. (1991) The percutaneous absorption of methamidophos (SX-1757) in male rats. Unpublished report from Chevron Chemical Co. Submitted to WHO by Bayer/Tomen.
Bertolazzi, M., Caroldi, S., Moretto, A. & Lotti, M. (1991) Interaction of methamidophos with hen and human acetylcholinesterase and neuropathy target esterase. Arch. Toxicol., 65, 580–585.
Bigger, C.A. & Sigler, C.I. (1993) CHO/HGPRT mutation assay—Monitor technical. Unpublished report from Microbiological Associates, Inc. Submitted to WHO by Bayer/Tomen.
Blom, H.J. & Tangerman, A. (1988) Methanethiol metabolism in whole blood. J. Lab. Clin. Med., 111, 606–610.
Bomann, W., Kaliner, G. & Mager, H. (1992) SRA 5172 (c.n. Methamidophos) subchronic dermal neurotoxicity study (ninety-day hen study). Unpublished report from Bayer AG, file No. 21428. Submitted to WHO by Bayer/Tomen.
Brown, R., Ames, R. & Mengle, D. (1989) Occupational illness from cholinesterase-inhibiting pesticides among agricultural applicators in California. Arch. Environ. Health, 44, 1.
Canellakis, E.S. & Tarver, H. (1953) The metabolism of methyl mercaptan in the intact animal. Arch. Biochem. Biophys., 42, 446–455.
Carlson, D., Vondruska, J.F. & Fancher, O.E. (1969) Effects of RE 9006-75%SX-171 on the cholinesterase activity in the beagle dog. Unpublished report from Industrial Bio-test Laboratories, Inc. Submitted to WHO by Bayer/Tomen.
Cavalli, R.D. & Hallesy, D.W. (1968a) Acute oral toxicity of RE 9006 (95%) in mice. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Cavalli, R.D. & Hallesy, D.W. (1968b) Acute oral toxicity of RE 9006 (95%) in rats. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Cavalli, R.D. & Hallesy, D.W. (1968c) Acute dermal toxicity of Monitor technical. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982a) Sister chromatid exchanges and cell-cycle delay in Chinese hamster V79 cells treated with 9 organophosphorus compounds (8 pesticides and 1 defoliant). Mutat. Res., 103, 307–313.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982b) Sister chromatid exchanges in Chinese hamster cells treated with seventeen organophosphorus compounds in the presence of a metabolic activation system. Environ. Mutag., 4, 621–624.
Christenson, W.R. (1991) Technical grade methamidophos (Monitor), an eight-week subchronic cholinesterase study in Fischer 344 rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Crawford, C.R. & Anderson, R.H. (1973) The acute toxicity of Monitor technical in combination with malathion technical to female rats. Unpublished report from ChemAgro Division of Baychem Corp. Submitted to WHO by Bayer/Tomen.
Crossley, J. & Tutass, H.O. (1969) Metabolism of Monitor insecticide by rats. Unpublished report from Chevron Chemical Co. Submitted to WHO by Bayer/Tomen.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary hepatocytes. Unpublished report from Microbiological Associates, Inc. Submitted to WHO by Bayer/Tomen.
Cushman, J.R. (1984) Modified Buehler test for the skin sensitization potential of methamidophos. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Derr, R.F. & Draves, K. (1983) Methanethiol metabolism in the rat. Res. Communications Chem. Pathol. Pharmacol., 39/3, 503–506.
Easter, M.D. & Rosenberg, D.W. (1986) The cholinesterase inhibition potential of analytical grade methamidophos (SX-1672) and methamidophos technical (SX-1490) following topical application of a single dose to adult male and female rats. Unpublished report from Chevron Environmental Health Center, Inc. Submitted to WHO by Bayer/Tomen.
Eigenberg, D.A., Pazdernik, T.L. & Doull, J. (1983) Haemoperfusion and pharmacokinetic studies with methamidophos in the rat. Fundam. Appl. Toxicol., 3, 490–501.
Eigenberg, D.A., Freshwater, K.J. & Lake, S.G. (1998) A two-generation dietary reproduction study in rats using technical methamidophos. Unpublished report from Bayer Corp., dated 5 January 1998, file No. 8398. Submitted to WHO by Bayer/Tomen.
Eisenlord, G.H. (1984) Dominant lethal study of methamidophos technical in mice. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Ellman, G.L., Courtney, K.D., Andres, V. & Featherstone, R.M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7, 88–95.
El-Sebae, A.H., Ahmed, N.S., EL-Gendy, K.S., EL-Bakary, A.S. & Soliman, S.A. (1987) Methamidophos (Tamaron)—A delayed neuropathic compound to man, but negative to chickens. In: Proceedings of the Second National Conference on Pests and Diseases in Vegetables and Fruits, Ismailia, Abstract No. 15, pp. 474–475.
Esber, H.J. (1983) In vivo cytogenetics study in mice, methamidophos technical (SX 1244). Unpublished report from EG&G Mason Research Institute. Submitted to WHO by Bayer/Tomen.
Fakhr, I.M.I., Abdel-Hamid, F.M. & Afifi, L.M. (1982) In vivo metabolism of 32P-‘Tamaron’ in the rat. Isotope Radiat. Res., 14, 49–55.
Flucke, W. (1985) SRA 5172 TA (Tamaron TA)—c.n. methamidophos—Study for acute dermal toxicity to the hen (Gallus domesticus). Unpublished report from Bayer AG, dated 17 August 1985, file No. 13780. Submitted to WHO by Bayer/Tomen.
Flucke, W. (1990a) Methamidophos (Tamaron) racemate and enantiomers—Study for acute oral toxicity to rats. Unpublished report from Bayer AG, dated 4 May 1990, file No. 19034. Submitted to WHO by Bayer/Tomen.
Flucke, W. (1990b) Methamidophos (Tamaron) racemate and enantiomers study for acute oral toxicity to the hen (Gallus domesticus). Unpublished report from Bayer AG, dated 29 May 1990, file No. 19091. Submitted to WHO by Bayer/Tomen.
Flucke, W. (1990c) Methamidophos (racemate and enantiomers) study for OPIDP (organophosphorous ester-induced delayed polyneuropathy) exploratory studies on hens. Unpublished report from Bayer AG, file No. 18987. Submitted to WHO by Bayer/Tomen.
Flucke, W. & Eben, A. (1988) SRA 5172 TA (Tamaron TA) (c.n. Methamidophos)—Study for the effect on the neurotoxic esterase (NTE) after dermal administration to chickens. Unpublished report from Bayer AG, file No. 17268. Submitted to WHO by Bayer/Tomen.
Flucke, W. & Eben, A. (1990) Methamidophos—Racemate and enantiomers—Study for the effect on NTE (neuropathy target esterase) in hens following oral administration. Unpublished report from Bayer AG, file No. 19092. Submitted to WHO by Bayer/Tomen.
Flucke, W. & Kaliner, G. (1985) SRA 5172 (Tamaron TA)—c.n. Methamidophos—Study for acute neurotoxicity to the chicken after dermal administration. Unpublished report from Bayer AG, file No. 13852. Submitted to WHO by Bayer/Tomen.
Fukuto, T.R. & Thompson, C.M. (1985) Utilization of 13C and 14C isotopes in toxicological studies with organophosphorus compounds. In: Proceedings of the 2nd International Symposium, Kansas City, Missouri, USA, September 3–6, 1985, Amsterdam: Elsevier Science Publishers.
Fuller, B. (2000) A dermal/intravenous crossover study to determine the dermal absoption of (14C)-methamidophos in male rhesus monkeys. Unpublished report No. 109812, dated 15 August 2000, from Bayer AG.
Gaines, T.B. & Linder, R.E. (1986) Acute toxicity of pesticides in adult and weanling rats. Fundam. Appl. Toxico., 7, 299–308.
Garofalo, M. (1973) A study of the effects of orthene and Monitor on plasma and erythrocyte cholinesterase activity in human subjects during subacute oral administration. Unpublished report from Industrial Bio-Test Laboratories, Inc., file No. 98473. Submitted to WHO by Bayer/Tomen.
Goh, K.T., Yew, F.S. & Ong, K.H. (1990) Acute organophosphorus food poisoning caused by contaminated green leafy vegetables. Arch. Environ. Health, 45, 180–184.
Gray, A.J., Thompson, C.M. & Fukuto, T.R. (1982) Distribution and excretion of [14CH3S] methamidophos after intravenous administration of a toxic dose and the relationship with anticholinesterase activity. Pestic. Biochem. Physiol., 18, 28–37.
Greco, R.A., Lindberg, D., Keplinger, M.L. & Fancher, O.E. (1971) Effects of RE 9006 on plasma and erythrocyte cholinesterase activity in the beagle dog. Unpublished report from Industrial Bio-test Laboratories, Inc. Submitted to WHO by Bayer/Tomen.
Gröning, P. (1976) Bay 71628 subchronic toxicity study on rats (three-month feeding experiment)—Histopathology report. Unpublished report from Bayer AG, dated 31 August 1976, file No. 6313. Submitted to WHO by Bayer/Tomen.
Gröning, P. & Lorke, D. (1976) Subchronic toxicity in dogs. Unpublished report from Industrial Bio-test Laboratories, Inc. Submitted to WHO by Bayer/Tomen (in German).
Guo, L.J., Chen, Y.G., Lu, R.Z., Liu, Y.L., Chen, J.P., Zhang, X.H. & Mao, J.Y. (1986a) Toxicity studies of methamidophos. (I) Acute, cumulative, and subchronic toxicity, delayed neurotoxicity and experimental treatment of intoxication. Zhonghua Laodong-Weisheng Zhiyebing Zazhi, 4, 257–260 (in Chinese, with English translation).
Guo, L.J., Chen, Y.G., Lu, R.Z., Zhang, W.Q., Liu, Y.L., Li, H.J., Chen, J.P. & Zhang, X.H. (1986b) Toxicity studies of methamidophos. (II) Teratogenicity and mutagenicity tests. Zhonghua Laodong-Weisheng Zhiyebing Zazhi, 4, 261 (in Chinese, with English translation).
Hanafy, M.S. Atta, A.H. & Hashim, M.M. (1986) Studies on the teratogenic effects of Tamaron (an organophosphate pesticide). Vet. Med. J., 34, 357–363.
Harbell, J.W. & Jacobson-Kram, D. (1990) CHO/HGPRT mutation assay Monitor technical. Unpublished report from Microbiological Associates, Inc. Submitted to WHO by Bayer/Tomen.
Hayes, R.H. (1984a) One-year feeding study of methamidophos (Monitor) in dogs. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hayes, R.H. (1984b) Oncogenicity study of methamidophos technical (Monitor) on mice. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hayes, R.H. (1984c) Chronic feeding/oncogenicity study of technical methamidophos (Monitor) to rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
He, W.S., Liu, A.H. & Bao, H.X. (1982) Effects of seven pesticides on chromosome aberration, sister-chromatid exchange and cell-cycle kinetics change in cultured red muntjac (Muntiacus muntjak) cells. Dongwuxue Vanjiu, 3, 129–136 (in Chinese, with English translation).
Heimann, K.G. & Nash, G. (1981) SRA 5172—Subacute dermal toxicity study on rabbits. Unpublished report from Bayer AG, dated 30 October 1981, file No. 10330. Submitted to WHO by Bayer/Tomen.
Herbold, B. (1981) SRA 5172—Methamidophos—Tamaron active ingredient—Micronucleus test on the mouse to evaluate for mutagenic effect. Unpublished report from Bayer AG, dated 22 January 1981, file No. 9707. Submitted to WHO by Bayer/Tomen.
Herbold, B. (1983) SRA 5172—Methamidophos—Pol-test on E. coli for DNA damage. Unpublished report from Bayer AG, dated 19 December 1983, file No. 12318. Submitted to WHO by Bayer/Tomen.
Herbold, B. (1994) SRA 5172 Salmonella/microsome test. Unpublished report from Bayer AG, dated 14 September 1994, file No. 23343. Submitted to WHO by Bayer/Tomen.
Herbold, B. (1996) SRA 5172—Micronucleus test on the mouse. Unpublished report from Bayer AG, dated 29 May 1996, file No. 25116. Submitted to WHO by Bayer/Tomen.
Hixson, E.J. (1980a) Acute dermal toxicity of methamidophos (Monitor) to rabbits. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hixson, E.J. (1980b) Eye and dermal irritation of methamidophos (Monitor). Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hixson, E.J. (1984a) Effect of methamidophos (Monitor) on reproduction in rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hixson, E.J. (1984b) Embryotoxic and teratogenic effects of methamidophos (Monitor) in rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Hussain, M.A., Muhamed, R.B. & Oloffs, P.C. (1985) Studies of the toxicity, metabolism, and anticholinesterase properties of acephate and methamidophos. J. Environ. Sci. Health, 20, 129–147.
Johnson, M. & Lotti, M. (1989) Can methamidophos cause delayed polyneuropathy in man or test animals. Unpublished report from Medical Research Council Toxicology Unit and Universita’ degli Studi di Padova. Submitted to WHO by Bayer/Tomen.
de Jong, L.P.A., Wolring, G.Z & Benschop, H.P. (1982) Reactivation of acetylcholinesterase inhibited by methamidophos and analogous (di)methylphosphoramidates. Arch. Toxicol., 49, 175–183.
Kao, T.S. & Fukuto, T.R. (1977) Metabolism of O,S-dimethyl propionyl- and hexanoylphosphoroamidothioate in the house fly and white mouse. Pestic. Biochem. Physiol., 9, 211–221.
Karalliedde, L., Senanayake, N. & Ariaratnam, A. (1988) Acute organophosphorus insecticide poisoning during pregnancy. Human Toxicol., 7, 363–364.
Khasawinah, A.M.A., March, R.B. & Fukuto, T.R. (1978) Insecticide properties, antiesterases activities and metabolism of methamidophos. Pestic. Biochem. Physiol., 9, 211–281.
Kimmerle, G. (1967) Toxicological studies on the active ingredient Bayer 71 628. Unpublished report from Bayer AG. Submitted to WHO by Bayer/Tomen.
Klein, O. (1997) Position paper on metabolism of methamidophos in rats. Unpublished report from Bayer AG, dated 19 February 1997, file No. M9942. Submitted to WHO by Bayer/Tomen.
Kruckenberg, S.M., Fenwick, B.W., Brown, S.M., Kassebaum, L.J. & Nilson, R.K. (1979) Acute delayed neurotoxicity study on Monitor technical. Unpublished report from Kansas State University. Submitted to WHO by Bayer/Tomen.
Köllert, W. (1981) Medical department letter report of 5 June 1981; BBA requirement / effect on humans. Unpublished report from Bayer AG. Submitted to WHO by Bayer/Tomen.
Langenberg, J.P., de Jong, L.P.A., Otto, M.F. & Benschop, H.P. (1987) Spontaneous and oxime-induced reactivation of acetylcholinesterase inhibited by phosphoroamidates. Arch. Toxicol., 62, 305–310.
Levy, J.E. (1979) The skin irritation potential of Monitor technical. Unpublished report from Chevron Chemical Co. Submitted to WHO by Bayer/Tomen.
Lindberg, D., Keplinger, M.L. & Fancher, O.E. (1970) Effects of RE 9006 (70%) SX-171 on the cholinesterase activity in the beagle dog. Unpublished report from Industrial Bio-test Laboratories, Inc. Submitted to WHO by Bayer/Tomen.
Löser, E. (1970a) Bay 71628 subchronic toxicological studies on rats (three-month feeding experiment). Unpublished report from Bayer AG, dated 29 June 1970, file No. 2165. Submitted to WHO by Bayer/Tomen.
Löser, E. (1970b) Bay 71628 subchronic toxicological studies on dogs (three month feeding experiment). Unpublished report from Bayer AG, dated 26 June 1970, file No. 2164. Submitted to WHO by Bayer/Tomen.
Machado, M.L. (1982) Salmonella/mammalian microsome mutagenicity test (Ames test) with Monitor technical. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Machemer, L. (1979) SRA 5172 (methamidophos)—Studies of embryotoxic and teratogenic effects on rabbits following oral administration. Unpublished report from Bayer AG, dated 31 May 1979, file No. 8410. Submitted to WHO by Bayer/Tomen.
Märtins, T. (1990) SRA 5172 (c.n.: Methamidophos), study for skin and eye irritation/corrosion in rabbits. Unpublished report from Bayer AG, dated 10 October 1990, file No. 19610. Submitted to WHO by Bayer/Tomen.
Mihail, F. (1981) Determination of acute toxicity (LD50) (letter report). Unpublished report from Bayer AG, dated 7 December 1981. Submitted to WHO by Bayer/Tomen.
Murli, H. (1990) Mutagenicity test on SRA 5172 in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells. Unpublished report from Hazleton Laboratories America, Inc. Submitted to WHO by Bayer/Tomen.
Pauluhn, J. (1987) SRA 5172 TA (c.n. Methamidophos)—Study for acute inhalation toxicity to the rat. Unpublished report from Bayer AG, dated 18 March 1987a, file No. 15661. Submitted to WHO by Bayer/Tomen.
Pauluhn, J. & Kaliner, G. (1984) SRA 5172 and trimethylphosphate (TMPO)—c.n. methamidophos—Study for the combination’s acute neurotoxicity to hens and turkey hens. Unpublished report from Bayer AG, dated 19 November 1984, file No. 13056. Submitted to WHO by Bayer/Tomen.
Rittenhouse, J.R. (1977) The eye irritation potential of Monitor technical. Unpublished report from Standard Oil Company of California. Submitted to WHO by Bayer/Tomen.
Robinson, C.P. & Beiergrohslein, D. (1980) Cholinesterase inhibition by methamidophos and its subsequent reactivation. Pestic. Biochem. Physio., 13, 267–273.
Robinson, C.P. & Beiergrohslein, D. (1982) Inhibition of human erythrocyte and plasma cholinesterase by methamidophos. J. Appl. Toxico., 2, 217–218.
Sachsse, K., Oharek, A., Zbinden, K., Madoerin, K., Luetkemeier, H., Wilson, J., Terrier, C. & Vogel, W. (1987) 3-month subchronic delayed neurotoxicity study with SRA 5172 (c.n. Methamidophos) in the hen. Unpublished report from Research & Consulting Co. AG, file No. R 4106. Submitted to WHO by Bayer/Tomen.
Salama, A.K.M. (1990) Toxicological studies on some insecticides. Pharmacokinetics, placental transfer, milk transfer, and metabolism studies of [14CH3S]-methamidophos and/or [14C]-acephate in rat. Dissertation, Alexandria University, Faculty of Agriculture. Submitted to WHO by Bayer/Tomen.
Sangha, G.K. (1983) Acute inhalation toxicity study with technical methamidophos (Monitor) in rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Sangha, G.K. (1984) Acute inhalation toxicity study with technical methamidophos (Monitor) in rats. Unpublished report from Mobay Chemical Corp. Submitted to WHO by Bayer/Tomen.
Selim, S. (2000) Absorption, excretion, balance and pharmacokinetics of 14C radioactivity after single dose dermal application of one dose level of 14C labeled methamidophos from a Tamaron 600 SL formulation administered to healthy volunteers. Unpublished report No. BC9267 from Bayer AG, dated 11 June 2000.
Senanayake, N. & Johnson, M.K. (1982) Acute polyneuropathy after poisoning by a new organophosphate insecticide. New Engl. J. Med., 306, 155–157.
Senanayake, N. & Karalliedde, L. (1987) Neurotoxic effects of organophosphorus insecticides. New Engl. J. Med., 316, 761–763.
Sheets, L.P. (1993) An acute oral neurotoxicity-screening study with technical grade methamidophos (Monitor) in rats. Unpublished report from Miles, Inc., file No. 7133, dated 5 November 1993, Supplement, 12 August 1994, Supplement, 14 October 1995. Submitted to WHO by Bayer/Tomen.
Sheets, L.P. (1994) A subchronic dietary neurotoxicity-screening study with technical grade methamidophos (Monitor) in Fischer 344 rats. Unpublished report from Miles, Inc., file No. 7252. Submitted to WHO by Bayer/Tomen.
Sheets, L.P. (1996) Supplemental submission to US EPA MRID No. 43197901, California EPA DPR Document #315-135; record #129816. A subchronic dietary neurotoxicity-screening study with technical grade methamidophos (Monitor) in Fischer 344 rats Unpublished report from Miles, Inc., file No. 7252 (supplemental submission). Submitted to WHO by Bayer/Tomen.
Sitt, R. (1990) Bayer, Medical Department, letter report of 6 March 1990—Methamidophos (SRA 5172), formulation: Tamaron. Unpublished report from Bayer AG Medical Department. Submitted to WHO by Bayer/Tomen.
Testman, R. (2000) Determination of the volatility of 14C-methamidophos from rat skin. Unpublished report No. BC9245 from Bayer AG, dated 8 August 2000.
Thompson, C.M. & Fukuto, T.R. (1982) Mechanism of cholinesterase inhibition by methamidophos. J. Agric. Food Chem., 30, 282–284.
Thyssen, J. & Eben, A. (1982) Methamidophos (Tamaron—active ingredient) and Tamaron, Sri Lanka formulation—Special study for neurotoxic effects on the chicken. Unpublished report from Bayer AG, file No. 1081. Submitted to WHO by Bayer/Tomen.
Tiefenbach, B., Henninghausen, G. & Wichner, S. (1990) Effects of some phosphororganic pesticides on functions and viability of lymphocytes in vitro. Wiss. Beitr. Martin Luther Univ. Halle-Wittenberg, 19, 43–50.
Tiefenbach, B. & Wichner, S. (1985) Dosage and mechanism of action of methamidophos in the mouse immune system. Z. Ges. Hyg., 31, 228–231 (in German).
Tucker, B.V. (1972) Acetylcholinesterase inhibition of Orthene and Ortho 9006. Unpublished report from Chevron Chemical Co., file No. 721.13. Submitted to WHO by Bayer/Tomen.
Vilanova, E., Johnson, M.K. & Vicedo, J.L. (1987) Interaction of some unsubstituted phosphoroamidate analogs of methamidophos (O,S-dimethyl phosphorothioamidate) with acetylcholinesterase and neuropathy target esterase of hen brain. Pestic. Biochem. Physiol., 28, 224–238.
Wang, S. & Huang, X. (1987) Behavioral toxicity in the offspring of mice following maternal exposure to methamidophos. Chin. J. Pharmacol. Toxicol., 2, 371–375 (in Chinese, with English abstract and tables).
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 2000–2202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
Wong, Z.A. & Kodama, J.K. (1979) Characterization of the in vitro inhibition of rat and monkey red blood cell and brain acetylcholinesterase by acephate, technical acephate and methamidophos and their mixtures. Unpublished report from Chevron Chemical Co., file No. S-1747. Submitted to WHO by Bayer/Tomen.
Zayed, S.M.A.D., Fakhr, I.M.I. & El-Magraby, S. (1984) Some toxicological aspects of methamidophos exposure in mice. J. Environ. Sci. Health B, 4, 467–478 and 5, 292–293.
See Also:
Toxicological Abbreviations
Methamidophos (HSG 79, 1993)
Methamidophos (ICSC)
Methamidophos (Pesticide residues in food: 1976 evaluations)
Methamidophos (Pesticide residues in food: 1979 evaluations)
Methamidophos (Pesticide residues in food: 1981 evaluations)
Methamidophos (Pesticide residues in food: 1982 evaluations)
Methamidophos (Pesticide residues in food: 1984 evaluations)
Methamidophos (Pesticide residues in food: 1985 evaluations Part II Toxicology)
Methamidophos (Pesticide residues in food: 1990 evaluations Toxicology)