First draft prepared by
Virginia A. Dobozy
Environmental Protection Agency, Washington DC, USA
Fipronil and some of its metabolites and degradation products were first evaluated by the 1997 Joint Meeting, when an ADI of 0–0.0002 mg/kg bw was established on the basis of a NOAEL of 0.019 mg/kg bw per day in a 2-year study of toxicity and carcinogenicity in rats and a safety factor of 100 (Annex 1, reference 80). The 1997 Meeting also considered whether a separate ADI should be established for fipronil-desulfinyl, a photodegradation product of fipronil, on the basis that it could be a significant residue, and its toxicity appeared to be greater than that of the parent molecule, fipronil. A temporary ADI of 0–0.00003 mg/kg bw was established for fipronil-desulfinyl on the basis of a NOAEL of 0.029 mg/kg bw per day in a 90-day study in rats and a safety factor of 1000, in view of the lack of a long-term study by oral administration in rats and a study of neurotoxicity in rats given repeated oral doses. The studies of fipronil-desulfinyl in rats that were required for the present Meeting included: a short-term study of neurotoxicity, a study of developmental neurotoxicity, and the results of an ongoing long-term study of toxicity.
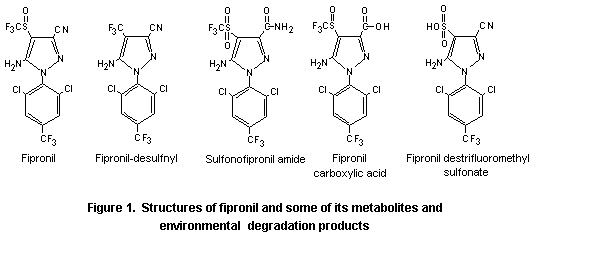
The following information was available on fipronil-desulfinyl for consideration by the present Meeting: metabolism in goats and hens, data on clinical signs of neurotoxicity (aggressivity, irritability) in control groups of mice and rats, and the results of a long-term study of toxicity and carcinogenicity in rats. Studies with the parent fipronil included a study of acute neurotoxicity in rats and a 6-week study of toxicity in mice treated orally. In addition, several studies were submitted on metabolites and photodegradation products, including the toxicity of single oral doses and induction of reverse mutation by sulfonofipronil amide, an environmental degradation product, and fipronil carboxylic acid, a metabolite and an environmental degradation product, and a 28-day study of toxicity with orally administered fipronil destrifluoromethyl sulfonate, an environmental degradation product. The structures of fipronil and the metabolites and environmental degradation products that were reviewed by the present Meeting are provided in Figure 1.

(a) Short-term studies of toxicity
Groups of 12 male and 12 female CD-1 mice were given diets containing fipronil (purity, 87.4–97.2%) at a concentration of 0, 15, 40, 110, 300, or 800 ppm for 6 weeks. The achieved doses were not calculated for animals at 300 and 800 ppm owing to extensive mortality in these groups. Those of the remaining groups were equal to 2.4, 6.5, and 20 mg/kg bw per day for males and 2.9, 8.2, and 22 mg/kg bw per day for females. The animals were monitored for deaths and clinical signs of toxicity; food consumption and body weight were measured, and food conversion efficiency was calculated. All animals that died before the end of the study underwent a complete necropsy. Complete macroscopic examinations were performed on all animals at terminal necropsy, and many organs were weighed. Tissues from all control animals, all those at 110 ppm, and males at 40 ppm were examined. In addition, the thyroids, parathyroids, and livers of animals at 15 ppm and females at 40 ppm and the brain and spinal cord of females at 40 ppm were examined. All animals at 300 and 800 ppm died or were killed in extremis during the first 2 weeks of the study, 11 males and four females at 110 ppm died during treatment, and two males at 40 ppm died during week 5. There were no deaths among animals at 15 ppm or females at 40 ppm. The clinical signs related to treatment included overactivity and irritability from week 2 in mice at 100, 300, and 800 ppm; one female at 40 ppm also displayed this sign during week 3. Convulsions were observed in three males and two females at 800 ppm and in one male at 300 ppm, and some of the animals died after these episodes. Other signs in animals treated at doses ł 300 ppm included thin build, hunched appearance, piloerection, respiratory abnormalities, pallor, body tremors, and abnormal gait and posture.
The food consumption of mice at 300 and 800 ppm was markedly depressed during the first week of treatment. Interpretation of the findings in males at 110 ppm was complicated by the high mortality rate in this group. The overall mean food consumption of males at 15 and 40 ppm and females at 110 ppm was decreased, but all were ł 90% of the control value. The overall mean body weights of males at 15 and 40 ppm were decreased by 19 and 37%, respectively, and a decrease was also found in females at 110 ppm (by 27%). The food conversion ratios of all treated animals were higher than those of controls. The weight of the liver relative to body weight was increased in animals that died or were killed during the study. At terminal sacrifice, significantly higher absolute and relative liver weights were observed in females at 15, 40, and 110 ppm. The absolute liver weights of males at 40 ppm were also increased, although the difference from controls was not significant; the relative weights in males at 15 and 40 ppm were significantly increased. Only one male at 300 ppm survived to terminal sacrifice; it also had significantly increased absolute and relative liver weights. The incidence and amount of fat in the liver were increased in all treated males, and the location of the fat was shifted from the centriacinar area in controls to the panacinar area in treated animals. Females showed a tendency towards increased fat only at 40 and 110 ppm. Significant toxicity was thus observed at dietary concentrations of 110 ppm and above, and the maximum tolerated dose was considered to be 15–40 ppm, equal to 2.4–6.5 mg/kg bw per day (Holmes, 1990).
(b) Special studies: Acute neurotoxicity
A single dose of fipronil (purity, 97.9%) was administered by gavage to groups of 10 male and 10 female Crl:CD BR rats at a dose of 0 (corn oil, 10 ml/kg bw), 2.5, 7.5, or 25 mg/kg bw. A battery of observational tests for function (FOB) and motor activity were evaluated before treatment, at the time of peak effect 7 h after dosing, and at 7 and 14 days after dosing. Clinical signs, body weight, and food consumption were measured at selected intervals during treatment. The animals were killed on day 15, and five males and five females from the control and high-dose groups were examined for neuropathological effects. The time to peak effect was established in a preliminary study in which four male and four female rats were given a single dose of fipronil (purity, 97.9%) by gavage at 25 mg/kg bw. An FOB was administered before treatment and 2, 4, and 7 h after dosing. Body weights were recorded daily after treatment. The animals were killed 7 days after treatment. No macroscopic examination was performed. The neurobehavioural signs seen included convulsions in one male at 4 and 7 h and in one female at 7 h; chewing in one male at 4 and 7 h; licking of lips in two females at 7 h; and wet anogenital region in animals of each sex at 7 h. Slight weight loss was seen on the first day after treatment and decreased body-weight gain up to day 3. The time to peak effect was established as 7 h after dosing.
In the definitive study, no treatment-related clinical signs of toxicity were seen on the day of treatment. Animals at 25 mg/kg bw showed unusual behaviour at 7 h: one male and one female were stationary with their chins on the floor; and other changes included decreased hindlimb splay, increased forelimb grip strength, and decreased body temperature. Only females had decreased counts for activity and rearing. The changes in motor activity included decreased large movements during the first 10 min of the 7-h evaluation in both males and females. On day 2 after treatment, the signs of toxicity in males and females included stained fur and soiled anogenital region, which lasted several days. The treatment-related effects at 7 days were limited to decreased body weight, food consumption, and food efficiency in males and females and decreased grooming in females. By 14 days, all of the previous effects had disappeared except for decreased grooming in females. At 7.5 mg/kg bw, males showed decreased hindlimb splay 7 h after dosing but no effects on other parameters. Females had decreased body weight gain, food consumption, and food efficiency during the first week and decreased grooming during tests at 14 days. The NOAEL was 2.5 mg/kg bw on the basis of decreased body-weight gain, food consumption, and feed efficiency in females, decreased hindlimb splay in males, and decreased grooming in females (Hughes, 1997)
Absorption, distribution, and excretion: [14C-phenyl]Fipronil-desulfinyl (radiochemical purity, 97.3%; specific activity, 58 µCi/mg) was administered orally in gelatin capsules to three lactating goats twice daily in the morning and afternoon before feeding and after milk and excreta collection for 7 consecutive days at a concentration of 0.05, 2, or 10 ppm. An additional goat served as a control and received empty gelatin capsules. The animals were killed about 23 h after the last dose, and samples of liver, kidney, muscle (hind- and forequarter), and fat (omental and renal) were collected for measurements of radioactivity.
The concentrations of radiolabel in milk approached a steady state by approximately 104 h after the initial dose. The maximum concentrations of fipronil-desulfinyl equivalents at the three doses were 0.0078, 0.056, and 0.36 mg/kg. The main route of excretion at each dose was via the faeces, but the proportion of the dose excreted declined as the dose decreased. At 0.05 ppm, 72% of the total dose was recovered; 20% was detected in the faeces and 7% in the urine. At 2 ppm, 52% of the radiolabel was recovered, with 26% in the faeces and 5% in the urine. At 10 ppm, 69% of the radiolabel was recovered, with 50% in the faeces and 3% in the urine. The highest concentrations of residues in tissues were found in the liver, omental fat, and renal fat, and the concentrations were higher than in circulating plasma. The lowest concentrations were found in muscle. The radiolabel in faeces, urine, milk, cage wash, and whole organs (liver and kidney) accounted for 37%, 35%, and 58% of the administered doses of 0.05, 2, and 10 ppm, respectively.
The main metabolite in urine was the 5-aminosulfate conjugate of fipronil-desulfinyl; others were the 5-aminoglucuronide conjugate, the 5-(N)-cysteine conjugate, and the N-oxide. Only fipronil-desulfinyl was detected in the faeces, and this was the main component in all edible tissues. In the liver, it accounted for 58% of the total radiolabelled residues. Three minor components representing Ł 3% of the total radiolabelled residues were identified as ring-opened derivatives; another metabolite representing 2% could not be identified. The core fipronil-desulfinyl ring structure was likely to be intact. In the kidney, fipronil-desulfinyl accounted for 49% of the total radiolabelled residues; other metabolites accounted for 1–5%. In muscle, fipronil-desulfinyl accounted for 70% of the total radiolabelled residues and a single unidentified compound for 1%. In renal and omental fat, only fipronil-desulfinyl was detected, representing 86% and 82% of the total radiolabelled residues. The only component detected in milk was fipronil-desulfinyl, which represented 94% of the total radiolabelled residues after 32 and 152 h (Johnson et al., 1996a).
[14C-phenyl]Fipronil-desulfinyl (radiochemical purity, 98.6%; specific activity, 58 µCi/mg) was administered orally in gelatin capsules to each of three groups of five laying hens once daily for 14 consecutive days at a concentration of 0.05, 2, or 10 ppm. Three hens served as controls and received empty gelatin capsules. Eggs were collected throughout the day after dosing and separated into whites and yolks. The hens were killed about 23 h after the last dose, and samples of partially formed eggs, liver, muscle (breast and thigh), omental fat, and skin with fat were collected.
The main route of elimination was the excreta, but the proportion of the administered dose declined as the dose decreased, from 71% at 10 ppm to 69% at 2 ppm and 53% at 0.05 ppm. The mean total radioactivity recovered was 81% at 10 ppm, 79% at 2 ppm, and 68% at 0.05 ppm. The proportion of the administered dose in egg whites and yolks and tissues increased as the dose decreased. The concentrations in egg whites reached a steady state or were declining by the end of the study; the maximum concentrations of [14C]fipronil-desulfinyl equivalents were 0.005, 0.18, and 0.85 mg/kg at the three doses, respectively. The concentrations in egg yolks were near steady state by the end of the study, with maxima of 0.05, 1.5, and 7.3 mg/kg. The highest concentrations in tissue were found in omental fat, partially formed eggs, liver, and skin with fat, and all were gretaer than that in circulating plasma. The concentrations in breast and thigh muscle were lower than those in plasma.
In edible tissues, the main component detected was fipronil-desulfinyl. It was the only component found in omental fat, skin with fat, and egg whites (on days 5 and 14) and represented 83% and 85% of the total radiolabelled residues in egg whites on days 5 and 14, 56% and 59% in egg yolks on those days, 14% in liver, 70% in muscle, 91% in omental fat, and 87% in skin with fat. A ring-opened derivative was tentatively identified in egg yolks on day 5 (3% of total radiolabelled residues) and day 14 (1%) and in liver (6%); and MB 46400 (see Figure 4 of the 1997 monograph; Annex 1, reference 82) was tentatively identified in egg yolks on day 5 (1%) and day 14 (5%) and in liver (3%). The glucuronide conjugate of fipronil-desulfinyl was tentatively identified in egg yolks on days 5 and 14 (1% and 6%, respectively), and the sulfate conjugate of fipronil-desulfinyl was tentatively identified in liver (2%). Three additional metabolites were tentatively identified in liver: two ring-opened derivatives representing 2 and 4% of the total and the monodechloro, monohydroxy fipronil-desulfinyl (2%) (Johnson et al., 1996b).
Short-term studies of toxicity
Mice: Fipronil-desulfinyl (purity, 96%) was administered to 10 male and 10 female OF1 mice in the diet for 90 days at a concentration of 0, 0.5, 2, or 10 ppm, equal to 0, 0.08, 0.32, and 1.7 mg/kg bw per day for males and 0, 0.11, 0.43, and 2.2 mg/kg bw per day for females. Standard evaluations of toxicity ante and post mortem were conducted. Histological examinations were conducted on all tissues from controls and animals at the two higher doses and on the liver, lung, and kidney and on tissues in which there were significant macroscopic findings from mice at the lower dose.
Nine males and one female at 10 ppm were found dead during the study. The significant clinical signs included excessive jumping in two males at 10 ppm and irritability to touch, aggressivity, and/or increased motor activity in one male at 10 ppm and in two at 2 ppm on two or more occasions. At 0.5 ppm, aggressivity was reported in one male on one occasion and in one on three occasions. None of these findings was reported in control animals. There were no significant changes in body weight, food consumption, or haematological or clinical chemical parameters. Gross examination of the dead animals showed liver enlargement in three males and a small thymus in four males at 10 ppm. Microscopy revealed centrilobular hypertrophy of the liver in six males at this dose. The only female at 10 ppm that died was severely autolysed. At scheduled sacrifice, gross and microscopic examinations revealed no treatment-related effects. The incidence of aggressivity or irritability to touch was low at 2 ppm, and there was no dose–response relationship; however, one male at this dose was irritable to touch and aggressive on days 70–72, and the other male at this dose that had behavioural changes was irritable to touch, had increased motor activity on day 29, and showed aggressivity on day 33. Therefore, two animals of the same sex showed multiple signs on two or three occasions of effects that have been recognized as clinical signs of toxicity in other studies with fipronil-desulfinyl and fipronil. In addition, signs of neurotoxicity were seen in males at the next highest dose and no signs of toxicity in concurrent controls. Therefore, the behavioural findings in males at 2 ppm should not be discounted, despite the lack of other treatment-related effects. It is easier to discount the behavioural effects at 0.5 ppm because they occcurred at low incidence and there was no dose–response relationship. The NOAEL was 0.5 ppm, equal to 0.08 mg/kg bw per day (WHO, 1997).
The author of the study concluded that the NOAEL was 2 ppm on the basis of the low incidence of aggressivity or irritability to touch and the lack of a dose–response relationship. Data on irritability and aggressivity in controls in five 90-day dietary studies conducted at Rhône-Poulenc Agro, Centre de Recherche, Sophia Antipolis, France, were submitted to JMPR. The company concluded that similar clinical signs had been observed in control animals in two of the five studies conducted in the same testing facility. In a study conducted in 1995, one control male was reported to show irritability on days 50 and 51; and in a study conducted in 1997, aggressivity was reported in two of 10 control males on days 71 and 56 (Blacker, 1998a). Two of the studies were conducted with CD-1 mice and three with OF-1 mice. Since the latter strain was used in the 90-day study with fipronil-desulfinyl, the data from these three studies are summarized separately from those on male CD-1 mice in Table 1. None of the females in the five studies had either clinical sign. The overall incidence of aggressivity and irritability in the controls in the previous studies might be construed as comparable to the findings in the 90-day study with fipronil-desulfinyl. However, a comparison of the temporal patterns and severity of the responses reveals some differences. As indicated above, one control male mouse was irritable on days 50 and 51, and two mice each showed aggressivity on one occasion on days 56 and 71. None of the control male mice had both clinical signs on the same occasion, whereas males at 2 ppm in the 90-day study with fipronil-desulfinyl did. In one animal, irritability and aggressivity were reported together on three occasions. In the other male at 2 ppm, irritability, increased motor activity, and aggressivity were reported together on one occasion. Data on motor activity in previous controls were not submitted to the present Meeting. The findings show that the clinical signs in the males at 2 ppm were more severe than those in the previous controls because they occurred together in the same animals. In addition, the incidence of 2/10 (20%) in the animals at 2 ppm is higher than 3/30 (10%), the combined incidence of both signs, in the previous male OF-1 controls. Consequently, the present Meeting concluded that the NOAEL was 0.5 ppm, equal to 0.08 mg/kg bw per day.
Table 1. Incidences of irritability and aggressivity in male control mice
|
Study No. |
Mouse strain |
Incidence of irritability |
Incidence of aggressivity |
|
SA 95242 |
OF-1 |
1/10 |
0/10 |
|
SA 95309 |
OF-1 |
0/10 |
0/10 |
|
SA 96528 |
OF-1 |
0/10 |
2/10 |
|
All OF-1 mice |
|
1/30 |
2/30 |
|
|
|
|
|
|
SA 92132 |
CD-1 |
0/10 |
0/10 |
|
SA 93109 |
CD-1 |
0/10 |
0/10 |
|
All CD-1 mice |
|
0/20 |
0/20 |
From Blacker (1998a)
Rats: Fipronil-desulfinyl (purity, at least 97.5%) was administered in the diet to groups of 10 male and 10 female Sprague-Dawley rats at a concentration of 0, 0.5, 3, 10, or 30 ppm, equal to 0, 0.029, 0.18, 0.59, and 1.8 mg/kg bw per day for males and 0, 0.035, 0.21, 0.71, and 2.1 mg/kg bw per day for females, for 90 days. In addition to standard ante and post mortem evaluations of toxicity, triiodothyronine, thyroxine, and thyroid-stimulating hormone were assayed in unfasted animals at weeks 2 and 10. During treatment, one male and three females at 30 ppm died with clinical signs of distress. Signs of neurotoxicity (aggressivity, irritability to touch, increased motor activity, and curling up on handling) were seen in animals at 10 and 30 ppm, and excessive vocalization was observed in some animals. None of these findings was seen in controls, in males at 0.5 ppm, or in females at doses 3 ppm. (The 1997 monograph states, "None of these effects was observed in controls, in males at 0.5 ppm, or in females at doses ł 3 ppm." Review of the original study report indicates that the correct statement should be as given here.) One male at 3 ppm showed aggressivity, irritability to touch on several occasions, and excessive vocalization, mainly on days 3–5. The mean body weights were significantly decreased in males and females at 30 ppm and in males at 10 ppm several times during the study. The overall mean body-weight gain of males was decreased by 15% for those at 10 ppm and 13% at 30 ppm. The mean weekly food consumption and food conversion efficiency of males and females at 30 ppm were lower than those of controls only during the first 2 weeks of the study. No treatment-related changes in haematological, urinary, or ophthalmic parameters were seen. Decreases in total bilirubin (–43%), total cholesterol (–25%), and triglyceride (–24%) concentrations seen in females at 30 ppm were considered to be related to treatment but not toxicologically significant since the individual values were within the normal range for these parameters for this age and strain of rat. At 30 ppm, treatment-related decreases in thyroxine concentration were seen in males (–48%, p < 0.01 at week 2 and –25% at week 10) and females (–29% at week 10), and the concentration of triiodothyronine was decreased (–29%, p < 0.05) in males at week 10. The altered hormone concentrations were reported to be within the normal range of values for this strain of rat, and, as no changes were found in the thyroid gland on macroscopic or microscopic examination, the toxicological significance of the hormonal alterations is questionable. The activity of thyroid-stimulating hormone was not affected. There were no treatment-related macroscopic changes at necropsy, no changes in organ weights, and no histopathological findings. The NOAEL was 0.5 ppm, equal to 0.029 mg/kg bw per day, on the basis of clinical signs of toxicity in one male (WHO, 1997).
Data on the incidences of irritability, aggressivity, and increased motor activity from 17 90-day studies conducted at Rhône-Poulenc Agro, Sophia Antipolis, France, between 5 June 1991 and 16 December 1997 were submitted to JMPR. The company concluded that, on the basis of the occurrence of these effects at similar or greater incidences in control animals in concurrent studies, the clinical signs observed in the single male at 3 ppm should be considered to be unrelated to treatment (Blacker, 1998b).
Of the 17 studies submitted, four were not conducted in complicance with GLP and one involved administration by gavage. The rats used in the remaining 12 studies involving 120 animals were either Sprague-Dawley Crl:CD(SD)BR from Charles River France (five studies) or Sprague-Dawley Ico:OFA.SD.(IOPS Caw) from Iffa-Credo (seven studies). Since the rats used in the 90-day study with fipronil-desulfinyl were of the former strain, data from those studies were considered separately. In the five studies in which rats from this supplier were used, irritability (in 4/10 males and 4/10 females) and aggressivity (in 0/10 males and 2/10 females) were reported in only one study, SA 96277. Aggressivity and/or irritability was reported in three of the seven studies in which the strain from Iffa-Credo was used, and increased motor activity was reported in another study. The data from studies in which either irritability or aggressivity was reported are presented in Table 2. Since increased motor activity was not observed in the male at 3 ppm in the 90-day study with fipronil-desulfinyl, the data are not included.
Table 2. Incidences of irritability and aggressivity in control Sprague-Dawley rats in 12 previous studies in France
|
Study No. |
Strain |
Animal supplier |
Incidence of irritability |
Incidence of aggressivity |
||
|
Males |
Females |
Males |
Females |
|||
|
SA 96097 |
Ico:OFA:SD(IOPS Caw) |
Iffa-Credo, L'Arbresle |
0/10 |
1/10 |
0/10 |
0/10 |
|
SA 96277 |
Crl:CD(SD)BR |
Charles River France, St Aubin-les-Elbeuf |
4/10 |
4/10 |
0/10 |
2/10 |
|
SA 97004 |
Ico:OFA:SD(IOPS Caw) |
Iffa-Credo, L'Arbresle |
3/10 |
1/10 |
1/10 |
0/10 |
|
SA 97078 |
Ico:OFA:SD(IOPS Caw) |
Iffa-Credo, L'Arbresle |
1/10 |
1/10 |
0/10 |
0/10 |
|
Total incidence |
|
Charles River France |
4/50 |
4/50 |
0/50 |
2/50 |
|
|
Iffa-Credo |
4/70 |
3/70 |
1/70 |
0/70 |
|
|
|
All |
8/120a |
7/120 |
1/120 |
2/120 |
|
From Blacker (1988b)
a
Denominator based on total number of animals in 12 studies considered acceptable for the database on previous controlsIn the 90-day study with fipronil-desulfinyl, aggressivity was observed on one occasion (day 41), irritability on three occasions (days 50–84), and excessive vocalization on one occasion (day 70). The presentation of the data on individual animals in the report did not permit determination that the single occasion of aggressivity occurred alone or whether irritability and excessive vocalization occurred on the same day. The data on previous controls showed that irritability and aggressivity were observed in control animals at incidences comparable to those reported at 3 ppm.
The results of a long-term study of toxicity and carcinogenicity were available from the same testing facility with the same strain of rat and supplier (Iffa-Credo) at nearly comparable doses. In this study, the concentration of 2 ppm was equal to a time-weighted average dose of 0.098 mg/kg bw per day for males in the 104-week study. Given that the dose would have been higher at the beginning of the study, the value of 0.098 mg/kg bw per day is comparable to the dose of 0.18 mg/kg bw per day for males in the 90-day study. Examination of the data on individual males at 2 ppm in the long-term study indicated that 8/70 animals showed signs of aggressivity or irritability to touch; however, the earliest time at which the signs were observed was day 209. The Meeting concluded that the clinical signs seen in the one male at 3 ppm in the 90-day study were not replicated in the long-term study. The NOAEL in the 90-day study was therefore 3 ppm, equal to 0.18 mg/kg bw per day.
Long-term studies of toxicity and carcinogenicity
Rats: Fipronil-desulfinyl (purity, 96–99.2%) was administered in the diet to groups of 70 male and 70 female Sprague-Dawley rats at a concentration of 0, 0.5, 2, or 10 ppm for 104 weeks, equal to 0, 0.025, 0.098, and 0.50 mg/kg bw per day for males and 0, 0.032, 0.13, and 0.55 mg/kg bw per day for females. The 10-ppm dose was reduced to 6 ppm for females after week 26 because of an unacceptably high mortality rate. About 10 males and 10 females per group were killed at week 53 for interim evaluations. Body weights and ophthalmoscopic parameters were evaluated before treatment and at selected intervals during treatment. Animals were inspected once or twice daily for moribundity and deaths. Detailed physical examinations, palpation of masses, and measurements of food consumption were conducted periodically throughout the study. Haematological and clinical chemical parameters were evaluated at weeks 26, 52, 78, and 104; urine was analysed at weeks 26, 51, 77, and 103. At necropsy, the animals were examined macroscopically, and selected organs were weighed. Tissues from selected organs of control and high-dose groups were examined at the interim sacrifice. In addition, the liver, lungs, kidney, and spleen of animals at the low and intermediate doses were examined microscopically. At the final sacrifice, all tissues from all animals were examined microscopically.
By week 26, seven females at 10 ppm had died as compared to one in the control group and none at 0.5 or 2 ppm. The concentration of fipronil-desulfinyl was therefore reduced to 6 ppm to align the actual doses of males and females. The terminal mortality rates (adjusted for animals killed at week 53) for males at the low and high doses and females at the high dose were significantly (p < 0.05) greater than that of the corresponding controls, while that of males at the intermediate dose was just above the limit of significance (p = 0.053). The mortality rate in females showed a statistically significant (p < 0.05), dose-related trend. The primary causes of death were chronic progressive nephropathy, large pituitary gland tumours, and ulcerated mammary fibroadenomas, which are common lesions in aged rats.
Clinical signs of toxicity were observed in males at 10 ppm, females at 10 or 6 ppm, and females at 2 ppm. Males showed a statistically significant increase in the incidence of aggressivity and irritability to touch at 10 ppm. Females at 10 or 6 ppm had a statistically significantly increased incidence of chromodacryorrhoea, aggressivity, irritability to touch, increased salivation, and convulsions (tonic, clonic, and tonic and/or clonic), and females at 2 and 10 or 6 ppm had a statistically significant increase in the incidence of tonic and/or clonic convulsions. The incidences of tonic and/or clonic convulsions were 7.1% in controls, 11% at 0.5 ppm, 19% at 2 ppm, and 29% at 10 ppm. The incidence of convulsions in females at the low dose (11%) was within the range of controls in other studies (2.5–16%). The average day on which convulsions were first observed was 390 ± 61 in controls, 360 ± 61 for groups at the low dose, 340 ± 170 at the intermediate dose, and 300 ± 170 at the high dose; the early mean onset in treated females was marginally significant (p = 0.06) at the high dose. Data on the incidence of tonic and/or clonic convulsions among controls were provided from six studies finalized or being conducted at the Aventis CropScience (formerly Rhône-Poulenc) Toxicology Centre in Sophia Antipolis, France, with rats supplied by Iffa Credo, France. Although the incidence of convulsions was increased in females at 0.5 ppm, the sign was considered not to be toxicologically significant, because the difference from controls was not statistically significant, there was no statistically significant decrease in the average day that convulsions first occurred, the incidence was within the range for controls in other studies, and there was no dose–response relationship for the increase in the number of seizures observed in affected animals, although the number of animals with tonic/clonic convulsions, i.e. grand mal seizures, cannot be determined from the presentation of the data on individual animals in the report. The data on convulsions in the study on fipronil-desulfinyl are presented in Table 3; the data for controls in previous studies are presented in Table 4.
Table 3. Incidences (%) of convulsions in rats treated with fipronil-desulfinyl for 104 weeks
|
Convulsions |
Concentration (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
0.5 |
2 |
10 |
0 |
0.5 |
2 |
10 or 6 |
|
|
Tonic |
2.9 |
1.4 |
8.6 |
4.3 |
2.9 |
7.1 |
13* |
16** |
|
Clonic |
10 |
2.9 |
13 |
13 |
7.1 |
11 |
19* |
23** |
|
Tonic and/or clonic |
10 |
2.9 |
13 |
14 |
7.1 |
11 |
18* |
29** |
70 animals/group
* p 0.05; ** p 0.01 in comparison with controls
Table 4. Incidences of female rats with convulsions in control groups in previous studies
|
Study No. |
Starting date |
Incidence |
|
SA 94247 |
7 July 1994 |
3/80 (3.8%) |
|
SA 94296 |
14 September 1994 |
9/80 (11.3%) |
|
SA 94356 |
16 November 1994 |
2/80 (2.5%) |
|
SA 95083 |
14 March 1995 |
4/70 (5.7%) |
|
SA 96188 |
4 September 1996 |
11/70 (15.7%) |
|
SA 96426 |
10 October 1996 |
5/70 (7.1%) |
The mean body weight and body-weight gain of males were not adversely affected. Females at 10 or 6 ppm showed a statistically significant increase in mean body weight during the first year of the study; body-weight gain was also increased in this group, but the change was not analysed statistically. During the second year of the study, the mean body-weight gain decreased, and the overall body-weight gain was 9% lower than the control value. Females at 10 or 6 ppm consumed more food (up to 24%) than control rats during weeks 4–69 of the study, and statistical significance was achieved at some times. No treatment-related effect on haematological parameters was observed. A statistically significant increase in the per cent of neutrophils and a decrease in the per cent of lymphocytes in males at 10 ppm were considered not to be of toxicological significance, since the absolute counts of the cells were not affected. No treatment-related effects on chemical parameters were observed. Statistically significant decreases in serum bilirubin and triglyceride concentrations and increases in glucose and inorganic phosphate concentrations in females at 10 or 6 ppm were also considered not toxicologically significant since they were small and occurred at only one time during the study. No treatment-related effects on urinary parameters were observed. A statistically significant increase in urinary pH in females at 10 or 6 ppm at week 25 and in males at 0.5 ppm at the end of the study was not toxicologically significant because of the lack of a dose–response relationship for males and the sporadic occurrence in females.
Treatment did not affect the eyes. At the interim necropsy, no treatment-related effects were seen. At the terminal necropsy, the incidence of focal spongiosis hepatis was increased in males at the high dose (32%; 6% in controls). The overall incidence of this lesion in males that died or were killed (2/69 at 0.5 ppm, 5/69 at 2.0 ppm, and 8/70 at 10 ppm) approximated the overall incidence in this laboratory (2/69 control males in this study and 7/70 control males in a concurrent study). The incidence of slight or mild diffuse hepatocyte hypertrophy was increased in treated females at terminal sacrifice, but the differences from the control were not dose-dependent and were therefore judged to be irrelevant. A statistically significant increase in the incidence of pituitary (pars distalis) adenomas in males at the high dose [31/70 (44.3%); 24/68 (35.3%) in controls] was seen, but the test for trend was negative and there were no statistically significant differences in other groups of treated males. The NOAEL for toxicity was 0.5 ppm, equal to 0.025 mg/kg bw per day, on the basis of clinical signs of toxicity. There was no evidence of carcinogenicity at doses considered adequate to measure carcinogenic potential (Bigot, 1998).
(iii) Other metabolites and degradation products
Acute toxicity: Studies of acute toxicity after oral administration were submitted for two metabolites or degradation products, sulfonofipronil amide and fipronil carboxylic acid. In studies in male and female Sprague-Dawley rats, the LD50 values for sulfonofipronil amide (purity, 98.7%) and fipronil carboxylic acid (purity, 97.5%) administered orally were > 2000 mg/kg bw. No deaths, clinical signs of toxicity, changes in body weight, or findings at necropsy were found in either study (Dange, 1994; Katchedourian, 1995).
Short-term studies of toxicity: Fipronil destrifluoromethyl sulfonate (purity, 93%) was administered in the diet to groups of 10 male and 10 female Sprague-Dawley rats at a concentration of 0, 50, 500, 5000, or 10 000 ppm for 28 days, equal to 0, 4.5, 46, 460, and 900 mg/kg bw per day for males and 0, 4.7, 50, 490, and 950 mg/kg bw per day for females. Physical condition, body weight, and ophthalmic parameters were evaluated before treatment and at selected intervals during treatment. Food consumption was measured periodically during treatment. Haematological and clinical chemical parameters (including triiodothyronine, thyroxine, and thyroid-stimulating hormone) were measured on day 22, 23, or 24. On day 29, 30, or 31, urine was collected before death for analysis. At necropsy, the animals were examined macroscopically, and selected organs were weighed. Tissues from selected organs of control and high-dose animals and the lungs, liver, thyroids, and kidneys of all animalswere examined histologically.
There were no deaths or clinical signs of toxicity during the study. Body weight, body-weight changes, and food consumption were not affected. Mean prothrombin times were increased by 8% in males at 5000 ppm (alpha = 0.05) and by 16% (alpha = 0.01) in males at 10 000 ppm. The mean alkaline phosphatase activity was increased in males (by 34–230%) and females (190–430%) at 5000 and 10 000 ppm. In females, the mean total cholesterol concentration was increased at 10 000 ppm by 35%; the mean triglyceride concentrations were increased at 5000 ppm (by 120%) and 10 000 ppm (110%), although not in a dose-dependent manner. In males, urinary pH was increased at the three higher doses, although not in a dose-dependent manner. The signifi-cance of this finding is questionable since no other toxic effect on the urinary tract was observed.
At 10 000 ppm, males had an increased absolute liver weight (by 15%), and males and females had increased relative liver weights (by 12%). The absolute and relative weights of the epididymides and testes of males at the three higher doses were increased, although the effect was not dose-related. The toxicological significance of this finding is questionable since no effects were observed microscopically in these organs. Macroscopically, there was a non-dose-related increase in the number of treated females with prominent liver lobulation. Microscopically, a slight increase in the incidence and severity of sinusoidal lymphoid aggregations was found, associated with occasional degenerative hepatocytes in both males and females at 10 000 ppm. All the foci were graded as minimal or slight. Females at this dose had a minimal or slight increase in the incidence and severity of fine vacuolation of hepatocytes, mainly periportal, usually diffused throughout the liver. The thyroid of males at 10 000 ppm and one female at this dose showed an increased incidence of follicular epithelial hypertrophy. The NOAEL was 500 ppm, equal to 46 mg/kg bw per day, on the basis of changes in alkaline phosphatase activity and triglyceride concentrations in females and changes in the relative liver weight (Dange, 1998).
In an amendment to the study, dated 21 April 2000, it was concluded that dietary administration of fipronil detrifluoromethyl sulfonate for 28 days provoked slight changes in thyroid hormone activity in males at 10 000 ppm. None of the changes in thyroid hormone values was statistically significantly different from those of controls, but there was a dose-related increase in thyroid-stimulating hormone concentration in treated males as compared with controls, by 11% at 50 ppm, 13% at 500 ppm, 19% at 5000 ppm, and 39% at 10 000 ppm. These changes were correlated with decreases in thyroxine only at 5000 and 10 000 ppm (7% and 15%, respectively). The concentration of triiodothyronine in treated males was increased over that of controls in a non-dose-related manner. In females, there were statistically significant decreases in thyroxine values at 5000 and 10 000 ppm (by 20% and 23%, respectively) as compared with the control mean; however, a correlation with an increase in thyroid-stimulating hormone activity (12%) was seen only at the high dose. In addition, the concentration of triiodothyronine was decreased (by 14%) in females at this dose. Although the data varied, it was concluded that males and females treated at 10 000 ppm and males at 5000 ppm of fipronil detrifluoromethyl sulfonate for 28 days showed effects on thyroid hormones. These findings do not alter the original NOAEL.
Genotoxicity: Sulfonofipronil amide (purity, 96.4%) and fipronil carboxylic acid (purity, 97.5%) were tested for their ability to induce reverse mutation in Salmonella typhimurium at doses of 0–5000 µg/plate in dimethyl sulfoxide. Negative results were obtained in the presence and absence of metabolic activation from a 9000 x g fraction of liver. The appropriate positive controls gave the expected positive responses (Percy, 1994, 1995).
Fipronil
In a study of neurotoxicity, fipronil was administered by gavage to rats at a single oral dose of 0, 2.5, 7.5, or 25 mg/kg bw. Decreased hind-foot splay was observed in males at 7.5 and 25 mg/kg bw 7 h after dosing, and females at these doses showed decreased grooming and decreased body-weight gain, food consumption, and food efficiency. The NOAEL was 2.5 mg/kg bw. In another study of neurotoxicity reviewed by the 1997 JMPR, the NOAEL was 0.5 mg/kg bw on the basis of decreased hindleg splay 7 h after treatment at 5 mg/kg bw.
In a range-finding study, fipronil was administered in the diet to mice at a concentration of 0, 15, 40, 110, 300, or 800 ppm for 6 weeks. One female at 40 ppm showed clinical signs of neurotoxicity (overactivity and irritability), which were observed consistently in males and females at higher doses. Body weight and food consumption were decreased in males at 15 ppm and above. The absolute and/or relative weights of the liver were increased in males and females at 15 ppm and above. As this was a range-finding study, the maximum tolerated dose for a long-term study was considered to be 15–40 ppm, equal to 2.4–6.5 mg/kg bw per day.
Fipronil-desulfinyl
In a study evaluated by the 1997 JMPR, fipronil-desulfinyl was administered in the diet of mice for 90 days at a concentration of 0, 0.5, 2, or 10 ppm. The study was re-evaluated by the present Meeting in the light of additional information on the incidence of key toxicological findings in historical controls. The Meeting concluded that the NOAEL was 0.5 ppm, equal to 0.08 mg/kg bw per day.
In a 90-day study of toxicity in rats, also evaluated by the 1997 JMPR, fipronil-desulfinyl was administered in the diet at a concentration of 0, 0.5, 3, 10, or 30 ppm. The NOAEL was considered to be 0.5 ppm, equal to 0.029 mg/kg bw per day. The study was re-evaluated by the present Meeting in the light of additional information on the incidence of key toxicological findings in historical controls. The present Meeting concluded that, in view of comparable incidences of clinical signs in historical controls and the lack of clinical signs at comparable doses after 90 days of treatment in the long-term study of toxicity and carcinogenicity, the NOAEL was 3 ppm, equal to 0.18 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity, fipronil-desulfinyl was administered in the diet to rats at a concentration of 0, 0.5, 2, or 10 ppm for 104 weeks. The 10 ppm concentration was reduced to 6 ppm in females after week 26 owing to an increased mortality rate. The incidences of clinical signs of neurotoxicity (tonic and/or clonic convulsions) in females were 7.1% in controls, 11% at 0.5 ppm, 19% at 2 ppm, and 29% at 10 ppm. These were statistically significant (p < 0.05) at the two higher doses but not at 0.5 ppm. The Meeting noted that the incidences in control females and those at 0.5 ppm fell within the range (2.5–16%) for historical control rats obtained from the same source at around the time of the study. The NOAEL for toxicity was 0.5 ppm, equal to 0.025 mg/kg bw per day, on the basis of clinical signs of toxicity. There was no evidence of carcinogenicity at doses considered adequate to measure the carcinogenic potential of fipronil-desulfinyl. The Meeting noted that there was no study of the carcinogenicity of fipronil-desulfinyl in mice; however, fipronil-desulfinyl did not produce thyroid tumours in rats as does the parent compound fipronil. Furthermore, the short-term studies provided no evidence of disruption of thyroid homeostasis, as was found with fipronil. There was no evidence of genotoxicity in three assays in vitro (for reverse mutation, gene mutation, and chromosomal aberration). The Meeting concluded that fipronil-desulfinyl is unlikely to pose a carcinogenic risk to humans.
Fipronil metabolites and degradation products
The LD50 values for both sulfonofipronil amide and fipronil carboxylic acid were > 2000 mg/kg after oral administration. Neither compound induced reverse mutation in bacteria, either with or without metabolic activation.
Fipronil destrifluromethyl sulfonate was administered in the diet to rats at a concentration of 0, 50, 500, 5000, or 10 000 ppm for 28 days. The triglyceride concentration was increased in females and alkaline phosphatase activity in animals of each sex at 5000 ppm, equal to 460 mg/kg bw per day. The NOAEL was 500 ppm, equal to 46 mg/kg bw per day.
Conclusion
The present Meeting concluded that the NOAELs in the long-term studies of toxicity and carcinogenicity with fipronil and fipronil-desulfinyl, both based on clinical signs of neurotoxicity, were comparable. The Meeting therefore established a group ADI of 0–0.0002 mg/kg bw for fipronil and fipronil-desulfinyl, in accordance with that established for fipronil in 1997. This value is supported by the NOAEL of 0.025 mg/kg bw per day for fipronil-desulfinyl in the long-term study of toxicity and carcinogenicity in rats, with a safety factor of 100.
In addition, comparison of the NOAEL for the developmental toxicity of fipronil-desulfinyl in rats (1 mg/kg bw per day) with the NOAEL in the long-term study of toxicity and carcinogenicity (0.025 mg/kg bw per day) shows a 40-fold difference. Use of the NOAEL in the long-term study of toxicity and carcinogenicity in establishing the ADI for fipronil-desulfinyl would therefore protect developing organisms. The Meeting concluded that the additional studies of neurotoxicity (short-term study of neurotoxicity and developmental neurotoxicity) required by the 1997 JMPR were no longer necessary.
The acute RfD established by the 1997 JMPR was 0.003 mg/kg bw for both fipronil and fipronil-desulfinyl, on the basis of the NOAEL of 0.3 mg/kg bw per day in a study of neurotoxicity in rats given repeated doses of fipronil, and a safety factor of 100. The present Meeting confirmed this as a group acute RfD for fipronil and fipronil-desulfinyl, alone or in combination.
Levels relevant for risk assessment of fipronil-desulfinyl
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Rat |
104-week study of toxicity |
Toxicity and carcinogenicitya |
0.5 ppm, equal to 0.025 mg/kg bw per dayb |
2 ppm, equal to 0.098 mg/kg bw per day |
|
|
|
Carcinogenicity |
10 ppm, equal to 0.497 mg/kg bw per day |
– |
|
|
Developmental toxicityc |
Maternal toxicity |
1 mg/kg bw per day |
2.5 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
1 mg/kg bw per day |
2.5 mg/kg bw per day |
|
Dog |
90-day study of toxicitya |
Toxicity |
9.5 ppm, equal to 0.29 mg/kg bw per day |
35 ppm, equal to 0.95 mg/kg bw per day |
a
Dietary administrationb
Highest dose testedc
Gavage administrationEstimate of acceptable daily intake for humans
0–0.0002 mg/kg bw (for fipronil and fipronil-desulfinyl, alone or in combination)
Estimate of acute reference dose
0.003 mg/kg bw (for fipronil and fipronil-desulfinyl, alone or in combination)
Studies that would provide information useful for further evaluation of the compound
• Observations in humans
Summary of critical end-points
|
Absorption, distribution, excretion, and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Slowly absorbed: maximum blood concentration 46–73 h after dosing |
|
Dermal absorption |
0.2–7% of applied dose within 24 h |
|
Distribution |
Widely distributed in tissues |
|
Potential for accumulation |
Long half-time (183–195 h) and high fat:plasma ratios suggest potential bioaccumulation |
|
Rate and extent of excretion |
70% eliminated within 96–120 h after dosing |
|
Metabolism in animals |
Extensive metabolism; numerous metabolites in urine and faeces |
|
Toxicologically significant compounds |
Fipronil, fipronil-desulfinyl, fipronil sulfone, and fipronil thioether |
|
(animals, plants and environment) |
|
|
Acute toxicity |
|
|
Rat, LD50, oral |
Males: 18 mg/kg; females: 15 mg/kg |
|
Rat, LD50, dermal |
> 2000 mg/kg |
|
Rat, LC50, inhalation |
– |
|
Dermal irritation |
– |
|
Ocular irritation |
– |
|
Dermal sensitization |
– |
|
Short-term toxicity |
|
|
Target/critical effect |
Clinical signs of neurotoxicity |
|
Lowest relevant oral NOAEL |
0.08 mg/kg bw per day (90-day dietary study in mice) |
|
Lowest relevant dermal NOAEL |
– |
|
Lowest relevant inhalation NOAEL |
– |
|
Genotoxicity |
No evidence of genotoxicity |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Clinical signs of neurotoxicity |
|
Lowest relevant NOAEL |
0.025 mg/kg bw per day |
|
Carcinogenicity |
Not carcinogenic |
|
Reproductive toxicity |
|
|
Reproductive target/critical effect |
– |
|
Lowest relevant reproductive NOAEL |
– |
|
Developmental target/critical effect |
Increased incidence of incomplete or reduced ossification of several bones |
|
Lowest relevant developmental NOAEL |
1.0 mg/kg bw per day |
|
Neurotoxicity/Delayed neurotoxicity |
Evidence of neurotoxicity in several studies |
|
Other toxicological studies |
None |
|
Medical data |
None |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.0002 mg/kg bw (for fipronil and fipronil-desulfinyl, alone or in combination) |
2-year study of toxicity and carcinogenicity in rats with fipronil |
100 |
|
Acute RfD |
0.003 mg/kg bw (for fipronil and fipronil-desulfinyl, alone or in combination) |
Study of neurotoxicity in rats given repeated doses |
100 |
Bigot, D. (1998) Chronic toxicity and carcinogenicity study of MB 46513 in the Sprague Dawley rat by dietary administration. Unpublished report No. SA 95156 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Blacker, A. (1998a) Historical control data (1992 to 1997) for clinical; signs observed in mice from 90-day toxicity studies. Unpublished report from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Blacker, A. (1998b) Historical control data (1991 to 1997) for clinical signs observed in rats from 90-day toxicity studies. Unpublished report from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Dange, M. (1994) RPA 105320: Acute oral LD50 in the rat. Unpublished report No. SA 93436 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Dange, M. (1998) RPA 104615: 28-day toxicity study in the rat by dietary administration. Unpublished report No. SA 96368 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Holmes, P. (1990) M&B 46030: Preliminary toxicity study by dietary administration to CD-1 mice for six weeks. Unpublished report no. 90/RHA299/0325 from Life Science Research, Ltd. Previously submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Hughes, E.W. (1997) Fipronil: Neurotoxicity to rats by acute oral administration (including a time to peak effect study) Unpublished report No. RNP 536/973345 from Huntingdon Life Sciences. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Johnson, S., Johnston, A.M., McCorquodale, G.Y. & Phillips, M. (1996a) The distribution and metabolism of 14C-MB 46513 in the lactating goat. Unpublished report no. 157352 from Inveresk Research. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Johnson, S., Johnston, A.M., McCorquodale, G.Y., Phillips, M. (1996b) The distribution and metabolism of 14C-MB 46513 in the laying hen. Unpublished report no. 157347 from Inveresk Research. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Katchedourian, P. (1995) RPA 200761. Oral limit test in the rat. Unpublished report No. SA 95214 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Percy, A. (1994) RPA 105320 Salmonella typhimurium reverse mutation assay (Ames test). Unpublished report No. SA 94012 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
Percy, A. (1995) RPA 200761 Salmonella typhimurium reverse mutation assay (Ames test). Unpublished report No. SA 95160 from Rhône-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., RTP, North Carolina, USA.
WHO (1997) Pesticide residues in food–1997. Toxicological and environmental evaluations. Geneva.
See Also:
Toxicological Abbreviations
Fipronil (ICSC)
Fipronil (Pesticide residues in food: 1997 evaluations Part II Toxicological & Environmental)