First draft prepared by
Timothy C. Marrs
Food Standards Agency, Skipton House, London, United Kingdom
Imazalil was evaluated by the Joint Meeting in 1977, when a temporary ADI of 0–0.01 mg/kg bw was allocated (Annex 1, reference 28). The compound was reviewed again in 1986, when an ADI of 0–0.01 mg/kg bw was allocated on the basis of the NOAEL in a 2-year study in dogs (Annex 1, reference 47). The compound was re-evaluated in 1991, when a new study in dogs was available: an ADI of 0–0.03 mg/kg bw was established on the basis of the NOAEL in the study in dogs and a 100-fold safety factor (Annex 1, reference 62).
Imazalil is used as a human and veterinary pharmaceutical, the INN name being enilconazole (Pesticide Manual, 1994); it has not been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Imazalil was reviewed by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
(a) Absorption, distribution, and excretion
[14C]Imazalil (purity, 99.9%) and unlabelled technical-grade imazalil (purity, 98.63%) were given to groups of five male and five female Wistar rats. A single dose of 1.25 mg/kg bw [14C]imazalil was administered intravenously to one group, while a second group received the same dose by gavage; a third group received unlabelled imazalil at 1.25 mg/kg bw per day for 14 days by gavage and then a single oral dose of [14C]imazalil at 1.25 mg/kg bw per day. The fourth group of rats received [14C]imazalil at a single dose of 20 mg/kg bw by gavage. The final specific activities of the labelled formulations were 1.1 MBq/mg at the low dose and 74 kBq/mg at the high dose. The animals were observed before dosing and at the end of each faecal collection. Urine was collected over the periods 0–4 h, 4–8 h, 8–24 h, 24–48 h, 48–72 h, and 72–96 h. Faeces were collected 0–24 h, 24–48 h, 48–72 h, and 72–96 h after dosing with radiolabelled imazalil. The animals were killed at 96 h, and selected organs were removed. After the single intravenous dose, 84% of the label had been excreted in males and females within 24 h and 89% within 96 h. When imazalil was given at a single oral dose of 1.25 mg/kg bw, 90% was excreted within 24 h by males and 93% by females. After imazalil was given for 14 days at a dose of 1.25 mg/kg bw per day by gavage and then a single oral dose of [14C]imazalil, males excreted 94% within 24 h and females excreted 84%. Nearly all the label had been excreted in the urine and faeces of animals of each sex by 96 h. When labelled imazalil was given at a single dose of 20 mg/kg bw by gavage, 90% and 89% was excreted within 24 h by males and females, respectively; more than 95% was excreted in the faeces and urine. Somewhat more imazalil appeared in the urine than in the faeces with all dosing regimens, while there was no significant sex difference. Nearly 50% of the radiolabel retained in the body was found in the liver. Comparison of the excretion patterns after oral and intravenous dosing suggests that the bioavailability, and therefore the absorption, of imazalil given orally is high (Mannens et al., 1993).
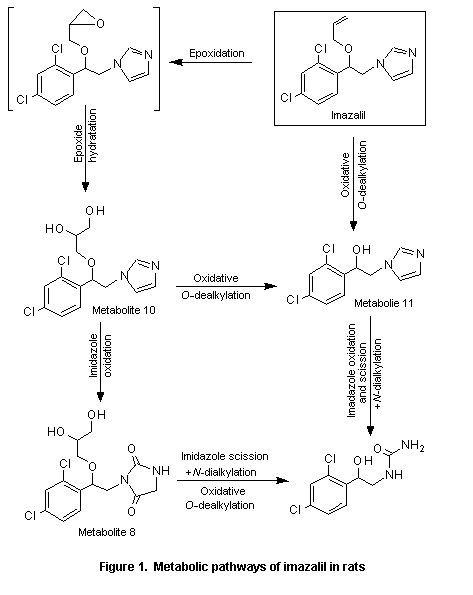
In the same study, little imazalil was excreted unchanged: less than 1% of the administered dose in the faeces and trace amounts in the urine. The compound was metabolized to at least 25 metabolites. Three major metabolites were identified, (±)-1-[2-(2,4-dichlorophenyl)-2-(2,3-dihydroxypropyloxy)ethyl]-imidaxolidine-2,5-dione (metabolite 8), (±)-1-[2-(2,4-dichlorophenyl)-2-(2,3-dihydroxypropyloxy)ethyl]-1H-imidazole (metabolite 10), and (±)-1-(2,4-dichlorophenyl)-2-imidazol-1-ylethanol (metabolite 11; see Figure 1). The main routes of metabolism were epoxidation, epoxide hydratation, oxidative O-dealkylation, oxidation, and scission and oxidative N-dealkylation. The metabolic pattern was similar after oral and intravenous administration and in animals of each sex (Mannens et al., 1993).

(c) Effects on enzymes and other biochemical parameters
(i) Hepatotoxicity of imazalil in mice
The effects of imazalil on liver morphology, enzymes, and metabolism were studied in albino Swiss mice. Imazalil (purity, 98.5%) was administered in the diet to groups of 40 male and 40 female mice at a concentration of 0, 50, 200, or 600 ppm for 1 or 3 months. These concentrations were equal to 0, 13, 53, and 160 mg/kg bw per day for males and 16, 64, and 200 mg/kg bw per day for females during the first month and 0, 12, 46, and 140 mg/kg bw per day for males and 0, 14, 55, and 170 mg/kg bw per day for females during 3 months. An interim sacrifice of 15 mice of each sex per group was carried out at 1 month, with 10 being assigned for toxicological and toxicokinetics evaluation and five for biochemistry. The remaining animals were killed at 3 months, 20 being used for toxicological and toxicokinetics evaluation and five for biochemistry. The mice were examined daily, and body weight and food consumption were measured weekly. Aminotranferase and alkaline phosphatase activities were measured in serum at both times of sacrifice, and selected organs were weighed and examined grossly. Only the livers were examined, but they were examined microscopically and the liver of one control and one mouse of each sex at the high dose at the interim kill was examined ultramicroscopically.
No deaths were observed during the study, and imazalil had no effects on appearance or behaviour. No difference in body weight was observed between groups. Food consumption was increased in males at 200 and 600 ppm over weeks 1–4 and in all treated females, probably due to food wastage. The activity of alanine aminotransferase was increased in animals of each sex at the highest dose at 1 month, while the activity of alkaline phosphatase was increased only in males at this dose. At 3 months, alanine aminotransferase activity was increased in males at 600 ppm, but the level was within the normal range seen in other studies. At 1 month, the absolute weight of the liver was increased in males at 200 ppm, and the absolute and relative weights were increased in males at 600 ppm; increased absolute and relative liver weights were seen in females at 600 ppm. At this time, males at the intermediate dose had increased absolute kidney weights, females at the highest dose had decreased absolute and relative pancreatic weights, and females at the intermediate dose had increased absolute and relative adrenal weights. At 3 months, males at the highest dose had increased absolute and relative liver weights, and males at 200 ppm had increased relative heart and kidney weights. A decrease in the absolute and relative thymus weights was seen at 200 ppm and a decrease in the absolute thymus weight at 600 ppm. In females at 3 months, the absolute and relative liver weights were increased and the relative pancreatic weight decreased at 600 ppm. In both males and females, the increase in liver weight appeared to be dose-related. The changes in liver weights were accompanied by an increased incidence of dark livers at the highest dose. Histological examination at 1 month showed an increase in the numbers of small and large vacuoles, especially in the periportal area, in males and females at the highest dose and in males at the intermediate dose. Similar findings were made at 3 months, with the addition of vacuolation in females at the intermediate dose. In mice at 600 ppm, ultramicroscopic examination of the liver showed an increased number of lipid droplets in hepatocytes, mainly in the periportal area. Moreover, the rough endoplasmic reticulum of the affected hepatocytes differed morphologically from that of hepatocytes from control animals: the rough endoplasmic reticulum in hepatocytes from treated animals was not arranged in parallel stacks of cysternae but diffused through the cytoplasm. The NOAEL was 50 ppm, equal to 12 mg/kg bw per day (Van Deun et al., 1994).
The hepatotoxic potential of imazalil (purity unstated), with and without L-buthionine-S,R-sulfoximine, a glutathione synthesis inhibitor, was examined by administering the inhibitor at 900 mg/kg bw to groups of four male Fischer 344/DuCrj rats by intraperitoneal injection, followed 1 h later by imazalil at a single dose by stomach tube of 0, 170, 255, or 340 mg/kg bw (the last dose being approximately equal to the LD50). Another group received imazalil without L-buthionine-S,R-sulfoximine, and two groups received the vehicle or L-buthionine-S,R-sulfoximine. The rats were killed 24 h after administration of imazalil. Serum was taken for measurements of aminotransferase activity and cholesterol, urea, phospholipid, and triglyceride concentrations. The kidneys and livers were removed and weighed; the livers were homogenized for measurement of malondialdehyde, and sections of the two organs were stained for histopathological examination.
Imazalil, with or without L-buthionine-S,R-sulfoximine, did not affect body, liver, or kidney weight. When given alone, it caused a dose-dependent decrease in aspartate but not alanine aminotransferase activity. Only the highest dose of imazalil alone decreased the serum concentration of triglycerides, while those of cholesterol and phospholipids were decreased at the two higher doses. The serum concentrations of triglycerides, cholesterol, and phospholipids were decreased at the two higher doses when L-buthionine-S,R-sulfoximine had been given, and there was some indication of a greater effect in these animals as compared with those given imazalil alone. L-Buthionine-S,R-sulfoximine and imazalil induced fatty infiltration of the liver, the distribution of which was predominantly midzonal or periportal, but there was no evidence of hepatocellular necrosis. Malondialdehyde, an index of lipid peroxidation, was found at increased concentrations in animals given imazalil at the highest dose, whether they also received L-buthionine-S,R-sulfoximine or not, but the concentration of malondialdehyde was higher when both compounds were given. No histopathological changes were observed in the kidneys, nor were urea concentrations in urine increased (Nakagawa & Tayama, 1997).
(ii) Cytotoxicity
Addition of imazalil to suspensions of isolated rat hepatocytes at a concentration of 0.75 mmol/L caused cell death accompanied by depletion of intracellular glutathione and protein thiols, with accumulation of intracellular malondialdehyde (Nakagawa & Moore, 1995).
(iii) Cytochrome P450 isoenzymes
Imazalil (purity, > 98%) administered orally to mice at 1 or 10 mg/kg bw daily for 3 days enhanced ethoxyresorufin O-deethylase and pentoxyresorufin O-depentylase activity in the microsomes of small intestinal mucosa and liver, indicating induction of CYP1A and CYP2B. Additionally, immunochemical analyses indicated induction of CYP2B, CYP2C, and CYP3A (Muto et al., 1997).
(iv) Endocrine disruption
Imazalil was tested for its ability to activate the estrogen receptor in vitro in a proliferation assay with MCF7 cells and a screening test for yeast estrogen. Statistically significant agonism was not observed (Vinggaard et al., 1999).
11beta-Hydroxylation and 19-hydroxylation in the mitochondria of gerbils (Meriones unguiculatus) in vitro were suppressed in parallel by about 75% by imazalil (purity unstated) at 1 µmol/L (Drummond et al., 1988). In a study of the effect of imazalil (purity, 97–99.9%) on CYP19 aromatase activity in human placental microsomes, the median inhibitory concentration was 0.34 µmol/L (Vinggaard et al., 2000).
Studies on the acute toxicity of imazilil in rats and rabbits are summarized in Table 1.
Table 1. Acute toxicity of imazalil
|
Species |
Strain |
Sex |
Route |
LD50 |
Purity |
Reference |
|
Rat |
Wistar |
Male |
Oral |
343 (262–448) |
Nitrate, > 95 |
Niemegeers (1979) |
|
|
|
Female |
|
288 (221–377) |
|
|
|
Rat |
Wistar |
Male |
Oral |
343 (262–448) |
Technical, 100.4 |
Niemegeers (1979) |
|
|
|
Female |
|
227 (174–297) |
|
|
|
Rat |
Wistar |
Male |
Oral |
355 (272–464) |
Sulfate, 102.7 |
Niemegeers (1979) |
|
|
|
Female |
|
309 (237–404) |
|
|
|
Rat |
Wistar |
Male |
Oral |
371 (284–485) |
Acetate, 99.1 |
Niemegeers (1979) |
|
|
|
Female |
|
309 (237–404) |
|
|
|
Rat |
Wistar Cpb; WU |
Male |
Inhalationa |
> 2 g/m3 |
99.5 |
Appelman & Woutersen |
|
|
Female |
(4 h) |
> 2g/m3 |
|
(1983) |
|
|
Rabbit |
New Zealand white |
Male |
Percutaneous |
> 2000 |
Technical, 97.6 |
Teuns et al. (1990a) |
|
|
|
|
|
> 2000 |
|
|
a
Deployed as smoke from a smoke generatorRabbits
The potential of imazalil to cause primary irritation of the skin was studied in three New Zealand white rabbits that received a single application of technical-grade imazalil (purity, 98.8%) to 6 cm2 of skin for 4 h at a dose of 0.5 g. The animals were observed for 1 h and then daily for 14 days, and irritation was scored according to the Draize method. Neither erythema nor oedema was observed at 24 or 72 h, nor was there any abnormality in body weight or weight gain during the 14-day observation period. It was concluded that, under the conditions of the study, imazalil was not irritating (Teuns & Marsboom, 1987).
The potential of imazalil to cause primary irritation of the eye was studied in three female New Zealand white rabbits that received a single instillation of technical-grade imazalil (purity, 97.6%) into the left conjunctival sac at a dose of 0.1 g. The animals were observed for 21 days, and the eyes were scored according to the Draize method. The material was moderately irritating (mean score, 29). Although some improvement was seen over the observation period, the irritation had not completely resolved by 21 days. Body weight was unaffected (Teuns et al., 1990b).
Guinea-pigs
The sensitization potential of technical-grade imazilil (purity, 98.5%) was examined in 20 Pirbight male guinea-pigs, which were induced by intradermal injection of 1% imazilil in sesame-seed oil followed by epicutaneous injection of 10% powder in petrolatum and epicutaneous challenge with 5% powder in petrolatum. Groups of 20 controls given the solvent and vehicle were included. The animals were scored for sensitization 48 and 72 h after challenge according to the Magnusson and Kligman scale. The sensitization rate was 5%. It was concluded that imazalil had weak sensitization potential (Teuns et al., 1990c).
(b) Short-term studies of toxicity
Rats
Technical-grade imazalil (purity, 98.1%) was administered in the diet to groups of 10 Cpb:WU Wistar rats of each sex as part of a lifetime study, summarized below (Lina et al., 1984; Til et al., 1985). Imazalil was admixed with the diet at a concentration of 0, 25, 100, or 400 ppm, equivalent to 0, 1.2, 5.0, and 20 mg/kg bw per day. General health and behaviour were checked daily, body weight was measured weekly for 12 weeks and then fortnightly, and food intake was measured weekly. Blood for haematological investigations was collected on day 174 from males and day 175 from females. Urine was collected on days 183 and 184 for analysis. At termination of the study, the animals were examined post mortem, blood samples for clinical chemistry were taken, and organs processed and examined histologically.
No treatment-related deaths or abnormal behaviour were noted. There were no treatment-related effects on weight gain or food intake. The leukocyte count was increased in males at 400 ppm in the absence of any significant change in the differential leukocyte count; the effect is thus of dubious significance. Lactate dehydrogenase activity was increased at the highest dose. Blood glucose concentration was increased in females at all doses. No significant differences between groups were seen in urinary parameters. Males at 400 ppm had increased relative kidney weights, and females had increased absolute and relative kidney and liver weights. Females also showed increased absolute thymus weight and relative lung weight. Gross and microscopic examination of the organs showed no effects that could be ascribed to treatment. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of changes in liver, kidney, and thymus weights in females at the highest dose (Lina et al., 1983).
Rabbits
Groups of five male and five female New Zealand white rabbits received imazalil (purity, 98.5%) under a porous gauze dressing at a dose of 0, 10, 40, or 160 mg/kg bw per day on the shaved back for 6 h/day, 5 days/week for 3 weeks. The animals were observed daily, body weights were determined initially and then weekly, and food consumption was determined weekly. Haematological, clinical chemical, and urinary parameters were measured at the end of the study. Irritancy was scored according to the Draize method. The animals were killed at the end of the study; the organs were weighed and examined grossly and microscopically. No adverse effects were observed on behaviour, and there was no irritation in the controls and little in the treated groups. Erythema was observed both with the vehicle (sesame oil) and the test compound; the severity was similar in all groups. Weight gain and food consumption were similar in all groups. No gross or microscopic adverse effects were seen on the skin, kidney, liver, or lung at any dose. Some urinary changes (reduced creatinine concentration, reduced specific gravity, and reduced urobilinogen concentration) were seen in males the highest dose. The NOAEL was 40 mg/kg bw per day (Teuns et al., 1991).
Dogs
Groups of four male and four female beagles were given technical-grade imazalil (purity, 98.8%) orally in gelatin capsules at a dose of 1.2, 2.5, or 20 mg/kg bw per day, while four controls of each sex received the capsules alone. The animals were observed twice daily and were examined for clinical signs weekly. An ophthalmic examination was carried out every 6 months and at the end of the study. An electrocardiogram and heart rate were recorded before the start of the study, at weeks 6, 14, 29, and 38, and at the end of the study. Body weights were recorded weekly. Haematological and clinical chemical parameters were measured 2 weeks after the start of dosing and monthly thereafter. Urine was analysed 1 month after the start of dosing and then every 3 months. At the end of the study, all the animals were killed, selected organs were weighed, and gross and microscopic examination was carried out. For statistical analysis of the histopathological findings, the two sexes were treated together.
All the animals survived to termination. Clinical effects consisting of soft faeces, vomiting, salivation, and decreased appetite were seen only at the highest dose. No effect on the electrocardiogram or the heart rate was seen at any dose. Decreased weight gain and food consumption were found only at the highest dose. No treatment-related effect was seen on haematological variables, and the only clinical chemical abnormalities observed were decreased calcium and substantially increased alkaline phosphatase activity at 20 mg/kg bw per day. No significant differences were seen between groups in urinary parameters. The organ weights were comparable, except that the weight of the liver was increased at the highest dose. No treatment-related change was seen on macroscopic or microscopic examination; in particular, the histological appearance of the liver did not differ between treated and control groups. The NOAEL was 2.5 mg/kg bw per day on the basis of clinical signs, decreased weight gain and food consumption, decreased serum calcium, increased serum alkaline phosphatase activity, and increased liver weight at the highest dose (Verstraeten et al., 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity, groups of 50 male and 50 female SPF albino Swiss mice received diets containing imazalil (purity, 96.9%) at a concentration of 0, 50, 200, or 600 ppm, equal to 0, 8.1, 33, and 100 mg/kg bw per day for males and 0, 9.9, 42, and 130 mg/kg bw per day for females, for approximately 23 calendar months. The mice were observed daily, and those in extremis were killed and examined grossly and post mortem. The animals were weighed at the start of the study, weekly for the first 12 months, and then monthly until immediately before sacrifice. Food consumption was recorded weekly for the first 12 months and then monthly. Haematological examinations were carried out at 12 months on samples from the tail vein and in those animals killed terminally by carotid exsanguination; additional haematological examinations were undertaken, when possible, on animals killed in extremis. When the leukocyte count was > 25 000/mm3, a differential leukocyte analysis was carried out. Clinical chemistry was undertaken on carotid blood at termination. At sacrifice, the surviving mice were examined grossly, and selected organs were weighed and examined grossly and microscopically.
Appearance and behaviour were not affected by treatment. At the end of the study, the survival rates among controls were 30% of males and 54% of females, and no significant differences were seen among groups. Decreased body weight and body-weight gain were seen in males at 600 ppm from week 2 to the end of the study and in males at 200 ppm from time to time early in the study. Females at all doses showed decreased body weight and weight gain from time to time, but the effects were slight, infrequent, and probably biologically insignificant. Weekly or monthly food consumption was increased in all groups of treated males at many time intervals, and total food consumption (throughout the study) was increased among those at 50 and 600 ppm. Females in all treated groups showed occasional increases in weekly or monthly food consumption. There was considerable food wastage and no clear dose–response relationship; the significance of the findings on food intake are thus dubious. At termination, males at the highest dose had a significant decrease in body weight, which was accompanied by absolute and relative increases in liver weight. An increase in the relative brain weight at 600 ppm was probably a reflection of the decrease in body weight. Other changes in organ weights observed in males (increased relative pancreatic weight at 50 ppm) and those observed in females (decreased absolute and relative lung weights at the highest dose, relative lung weight at 50 ppm, and absolute and relative splenic weights at 200 ppm) did not appear to be related to treatment. Increases in relative heart weight at 200 ppm, decreases in relative kidney weight at 600 ppm, and decreases in absolute and relative brain weights at 50 ppm, all in females, likewise appeared to be without biological significance.
Some differences were found between groups in haematological parameters at 12 months. In males, the erythrocyte volume fraction was increased at 200 ppm as was the hemoglobin at 200 and 600 ppm. In females at 200 and 600 ppm, the erythrocyte volume fraction, haemoglobin concentration, and erythrocyte count were increased and the platelet count was decreased. By 23 months, no treatment-related differences were found between groups in haematological end-points. No difference in leukocyte count was seen at any time. No differences in clinical chemical variables were seen that could be ascribed to the test material. Statistically nonsignificant gross morphological changes, including foci and nodules, were seen in the livers of males at the two higher doses. No treatment-related non-neoplastic changes were observed at 50 ppm in mice of either sex or in females at 200 ppm. The livers of males at 200 and 600 ppm and those of females at 600 ppm showed focal cellular changes consisting of large and small vacuoles and pigmented, swollen sinusoidal cells. Increased incidences of animals with hepatic neoplasms were found among males at 200 and 600 ppm, with increased incidences of neoplastic hepatic nodules; a similar increase in the incidence of animals with hepatic neoplasms was found among females at the highest dose (Verstraeten et al., 1993).
The slides from this study were subjected to three evaluations: by the study pathologist, by Dr Stephen Sparrow, and by a working group. The results of the first evaluation were reported by Verstraeten et al. (1993), those of all three evaluations by Hess (1996), and those of the last two by Butler (1995).
In the first evaluation, the incidence of hepatocytic carcinoma was not found to be increased, but the incidences of hepatic neoplastic nodules and total hepatic neoplasms were increased in males at 200 and 600 ppm, with a dose-related trend. In females, the incidence of hepatic neoplasms was increased at the highest dose, with a trend for neoplastic nodules and all hepatic neoplasms. The main differences between the first and second readings of the slides were in nomenclature, in particular for hepatic neoplastic nodules. Sparrow considered that the study pathologist had used the term ‘hepatic neoplastic nodule’ to describe changes that he would have described as adenoma and some other non-neoplastic lesions. Sparrow found evidence for a trend in the incidence of hepatocellular carcinoma in males. A report on the relevance of the liver tumours prepared by Hess (1996) included a tabulation of Sparrow’s findings. In a further report on the relevance of the liver tumours by Butler (1995), based on the findings of the working group, it was concluded that there was evidence of an increased incidence of adenomas but not carcinomas in males at the two higher doses and that there was no statistically significant trend for carcinomas (see Table 2). In the absence of a clear dose–response relationship and of evidence for genotoxicity, the NOAEL for liver tumours was 50 ppm. A positive trend for vaginal polyps with an increased incidence at 600 ppm was seen by the Peto test. When the incidences of uterine and vaginal polyps were analysed together, there was no trend with dose and no pairwise difference. The overall NOAEL was 50 ppm, equal to 8.1 mg/kg bw per day, for morphological changes in the livers (adenomas, foci, and nodules) of males at 200 ppm, equal to 33 mg/kg bw per day.
Table 2. Classification of liver neoplasms reported by Verstraeten et al. (1993) according to the findings of a working group
|
Dose (ppm) |
Males |
Females |
||||
|
No, animals examined |
Adenoma |
Carcinoma |
No, animals examined |
Adenoma |
Carcinoma |
|
|
0 |
50 |
5 |
5 |
50 |
4 |
0 |
|
50 |
49 |
3 |
5 |
50 |
5 |
1 |
|
200 |
50 |
14* |
3 |
50 |
0 |
2 |
|
600 |
50 |
14*a |
8 |
50 |
9 |
1 |
* p < 0.05, pairwise comparison with controls; one-tailed chi2
a p < 0.05 for trend, Peto test
Rats
In a long-term study of toxicity, groups of 20 male and 20 female Wistar Cbp:WU rats were given diets containing imazalil (purity, 98.1%) at a concentration of 0, 25, 100, or 400 ppm, equivalent to 0, 1.2, 5, and 20 mg/kg bw per day, for 18 months. The animals were observed daily and more thoroughly every 2 weeks. Body weight was recorded at the start of the study, weekly for 12 weeks, and fortnightly for the remainder of the study. Food consumption was measured weekly. Blood was collected for haematological examination on days 538–539 from the tail vein. Urine samples from all animals were analysed on day 541. At autopsy, blood was collected from 10 animals per group for clinical chemistry. At the end of the study, the animals were killed and examined post mortem. Selected organs were removed, weighed, and examined grossly and microscopically.
General health, behaviour, and survival were not affected by treatment. Females at the highest dose had decreased weight, particularly in the second part of the study, and these animals showed reduced food intake at a few times. One minor change was seen in haematological variables, namely an increased thrombocyte count in females at the highest dose. The plasma albumin concentration was decreased in males at the highest dose, but no other treatment-related change in clinical chemical end-points was seen. Urinary parameters were unaffected. The absolute weight of the adrenals was increased in females at the two higher doses, and the relative weights of the adrenals, kidneys, heart, and brain were increased in females at the highest dose. The relative adrenal weights were also increased in the other treated groups of females. Treatment-related pathological effects were seen in the livers of males at the highest dose, involving an increase in the lobular pattern and periportal cytoplasmic vacuolation of hepatocytes. Multivacuolar hepatocytes were seen more frequently in groups at the high dose than at other doses. Intracytoplasmic inclusion bodies were seen. There was no evidence of treatment-related neoplasia. Staining with periodic acid–Schiff and oil red O showed that the vacuoles contained neither starch nor fat. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of decreased weight gain in females, decreased plasma albumin concentration in males, and pathological changes in the livers of males at the highest dose. Although there was no evidence that imazalil was carcinogenic, the duration of the study and the number of animals used were insufficient to exclude that possibility (Lina et al., 1984).
In another long-term study of toxicity, groups of 50 male and 50 female Wistar Cbp:WU rats were given diets containing imazalil (purity, 98.1%) at a concentration of 0, 25, 100, or 400 ppm, equal to 0, 1.0, 3.6, and 15 mg/kg bw per day for males and 0, 1.2, 4.7, and 20 mg/kg bw per day for females, for 30 months. The animals were observed daily, more thoroughly every 4 weeks during the first year, and thence fortnightly. Body weight was recorded at the start of the study, weekly for 12 weeks, and fortnightly for the remainder of the study. Food consumption per cage (i.e. per five animals) was measured weekly. Ophthalmic examinations were performed on animals in the control and highest-dose groups at 1 and 2 years. At the end of the study, the animals were killed and examined post mortem, and blood was collected from 10 animals per group for clinical chemistry. Selected organs were removed, weighed, and examined grossly and microscopically.
There were no differences in appearance, behaviour, or mortality rate that could be attributed to treatment. The mortality rate was 66–80% at the end of the study. No differences in ophthalmic end-points that could be related to treatment were seen. The body weights of males at the highest dose were lower than those of controls during the first 16 weeks of the study; thereafter, they recovered and were not significantly different from those of concurrent controls. The food intake was reduced of males at the highest dose, occasionally of males at the intermediate dose, and of females at all doses. No differences in plasma albumin concentration were found between groups. The absolute brain weight was slightly decreased in males at the two higher doses. No gross or microscopic treatment-related effects were found; notably there were no effects in the liver that could be attributed to treatment. No instance of neoplasia related to treatment was evident. The NOAEL was 100 ppm, equal to 3.6 mg/kg bw per day, on the basis of decreased weight gain of males at the highest dose (Til et al., 1985).
The results of studies on the genotoxicity of imazalil are shown in Table 3.
Table 3. Results of assays for the genotoxicity of imazalil
|
End-point |
Test system |
Concentration |
Purity |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
S. typhimurium TA1535, TA1538, TA97, TA98 |
5–500 mg/plate |
98.7 |
Negativea |
Vanparys & Marsboom (1988a) |
|
Chromosomal aberration |
Human lymphocytes |
9–145 mg/ml |
99.7 |
Negativea; not clastogenic |
Vanparys et al. (1990) |
|
Unscheduled DNA synthesis |
Male Wistar (WU) rat primary hepatocytes |
0.9–30 mg/ml |
97.6 |
Negative |
Miltenberger et al. (1990) |
|
Forward mutation |
Chinese hamster lung V79 cells; Hprt locus |
10–100 mg/ml |
98.3 |
Negativea |
Van Gompel et al. (1995) |
|
In vivo |
|||||
|
Micronucleus formation |
Mouse bone-marrow erythrocytes |
0, 20, 80, 320 mg/kg bw orally |
98.7 |
Negative |
Vanparys & Marsboom (1988b) |
|
Unscheduled DNA synthesis |
CD-1 mouse hepatocytes ex vivo |
125 or 250 mg/kg bw by gavage |
97–98.1 |
Negative; no cell proliferation at a cytotoxic dose (250 mg/kg bw) |
Clare (1996) |
a
In the presence and absence of an exogenous metabolic activation systemIn a two-generation study, groups of 24 male and 24 female Wistar rats were given diets containing imazalil (purity, 98.0%) at a nominal dose of 0, 5, 20, or 80 mg/kg bw per day for 60 days before mating (both sexes), during a 3-week mating period (both sexes), during gestation, and until weaning of the first generation. The F0 males were killed after mating. The first generation (F1) received imazalil during growth, mating, gestation, and weaning of the second generation (F2). The F1 males were killed after mating. The doses during the premating period were equal to 0, 4.2, 18, and 70 mg/kg bw per day for males and 0, 5.0, 22, and 100 mg/kg bw per day for females. During the F0 gestation, the intakes were 0, 4.0, 16, and 87 mg/kg bw per day, and those during the F1 gestation were 0, 4.3, 19, and 88 mg/kg bw per day. Thus, the intakes tended to be slightly lower than the nominal doses. The animals were observed daily for general health, appearance, and behaviour. Body weights were recorded weekly before mating and, for females, on days 1 and 22 after copulation. Body weights were also recorded during the 3-week lactation period. Food consumption was recorded weekly before mating for both sexes and during gestation and lactation for females. The body weights of both F1 and F2 pups in each litter were recorded 8–12 h after parturition and on days 4, 14, and 21. The kidneys, liver, and reproductive organs of the parental and F1 generations were examined histologically.
Two F1 females at the high dose were killed in extremis, and one F2 female at the high dose and one control died soon after parturition or during lactation. F0 males at the highest dose showed decreased weight gain before mating; a similar but less pronounced effect was seen in females before mating, but an effect was also seen in F1 and F2 dams during gestation, at parturition, and at days 4, 14, and 21 of lactation. No effects on weight gain were seen in other treated groups, and there was no clear effect on food consumption in any group. Histopathological changes (liver vacuoles) were seen in F0 males at the highest dose, but no lesions were seen in the male reproductive system at any dose. Decreased numbers of corpora lutea were seen in F0 females. At the highest dose, both F1 and F2 generations had considerably reduced numbers of live pups and larger numbers of stillborn pups, and the F1 generation had fewer implantations; the duration of gestation was increased in both generations, by approximately 1 day, with an associated increase in the incidence of dystocia. The survival rates of pups during lactation were decreased in all F1 treated groups on days 4, 14, and 21; in the F2 generation, survival was decreased at all times at the highest dose and on days 14 and 21 at the lower dose; at the intermediate dose, the survival was comparable to that of controls, so that there was no dose–response relationship. No differences in body weight were seen between F1 and F2 pups. No teratogenic effect was observed.
The NOAEL for maternal toxicity was 20 mg/kg bw per day on the basis of reduced maternal weight gain. The NOAEL for fetotoxicity was 20 mg/kg bw per day on the basis of decreased numbers of live pups and increased numbers of stillbirths at 80 mg/kg bw per day. As summarized above, the survival rates of F1 pups during lactation were decreased in all treated groups on days 4, 14, and 21 of lactation and in the F2 generation at 5 mg/kg bw per day (days 14 and 21) and 80 mg/kg bw per day (days 4, 14, and 21); moreover, there was no dose–response relationship for the effect in the F1 generation at day 4, although there was at days 14 and 21. It is arguable that the data, especially for days 14 and 21, indicate a dose-related effect, but there was clearly no dose-related effect in F2 pups. There is no biologically plausible explanation for the difference between the two generations, and a statistical re-examination on a litter basis showed that the mortality rate was significantly increased only in the group at the highest dose. As hepatotoxicity was observed in F0 males at the highest dose, the NOAEL for paternal toxicity was 20 mg/kg bw per day (Dirkx et al., 1992b; see also Janssen Pharmaceutica NV, 1994).
Mice
The developmental toxicity of imazalil (purity not stated) was studied in groups of 30 inseminated Cobs CD1 mice given a dose of 0, 40, 80, or 120 mg/kg bw per day by gavage on days 6–16 of gestation. Treatment did not affect the maternal mortality rate, but maternal body-weight gain and food consumption were decreased at the two higher doses. Litter size and the number of live pups were reduced in all treated groups. The number of resorptions was increased at the highest dose. No teratogenic effects were seen, and there were no differences between groups in mean pup weights. The NOAEL for maternal toxicity was 40 mg/kg bw per day; an NOAEL for fetotoxicity could not be established in view of the decreases in litter size and number of live pups at all doses (Gillardin et al., 1987).
A further study of the developmental toxicity of imazalil (purity, 99.3–99.5%) was carried out in groups of 30 inseminated Cobs CD1 mice given a dose of 0, 10, 40, 80, or 120 mg/kg bw per day by gavage on days 6–16 of gestation. Deaths were recorded during the study, and the animals were examined daily for abnormal clinical signs. Body weights were determined on day 1, daily on days 6–17, and on day 19 after copulation. Food consumption was recorded over days 1–5, days 6–16, and days 17–18. On day 19 after copulation and before killing, all survivors underwent a complete physical examination. The surviving dams were then killed, and macroscopic changes were recorded; the weights of the uteruses were recorded after removal of that organ in toto. The dams were examined for numbers of live and dead fetuses, implantation sites, and embryos undergoing resorption. All live fetuses were weighed, and live and dead fetuses were examined for external abnormalities. The pups were sexed and the fetuses examined radiographically. The fetuses were then dissected, and bones were stained with alizarin.
Fourteen mice died during the study: one control, four at 80 mg/kg bw per day, and nine at 120 mg/kg bw per day. At the highest dose, piloerection, excitability, convulsions, hypothermia, and prostration were observed. Body weights were decreased on days 17 and 19 in mice at the two higher doses in comparison with concurrent controls; moreover, the body-weight gain of these animals was decreased during days 6–17 after copulation, and that of animals at 40 and 120 mg/kg bw per day was decreased during days 17–19 after copulation. The total body-weight gain (uterine weight subtracted) over the whole period 1–19 days was decreased at doses of 40 mg/kg bw per day and more. Food consumption was decreased during dosing on days 6–16 in mice at 80 and 120 mg/kg bw per day and after dosing in those at 40 mg/kg bw per day and more. The pregnancy rates and number of implantations were comparable among groups, whereas fewer live fetuses were found in animals at the highest dose, and the number of resorptions was increased. The body weight of pups was decreased at the highest dose, but the sex ratio was similar in all groups. The frequency of minor changes in ribs was increased at 40 and 80 mg/kg bw per day but not at the highest dose; there was thus no clear dose–response relationship. The number of major abnormalities was comparable in all groups. The NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of decreased body-weight gain at 40 mg/kg bw per day and reduced food consumption after treatment. In addition, deaths occurred at doses of 80 mg/kg bw per day and more. The NOAEL for fetal toxicity was 80 mg/kg bw per day, as the number of live fetuses was reduced, the number of resorptions was increased, and the body weights of the pups were decreased at the highest dose. There was no evidence of teratogenicity (Levron et al., 1991).
If the results of the two studies of developmental toxicity in mice are taken together, the overall NOAEL for maternal toxicity was 10 mg/kg bw per day, but no NOAEL was identified for fetotoxicity.
Rats
The developmental toxicity of imazalil (sulfate; purity, 99.9%) was studied in groups of 24 pregnant Sprague-Dawley OFA.SD (IOPS Caw) rats treated by gavage at a dose of 0, 40, 80, or 120 mg/kg bw per day on days 6–16 of gestation. The dams were examined daily and weighed 1, 6, 17, and 22 days after copulation. Food consumption was recorded during days 1–5 (before dosing), 6–16 (during dosing), and 17–21 (after dosing). At the end of the study, on day 22 of gestation, the dams were killed and examined for live and dead fetuses, implantation sites, and resorptions. Live fetuses were weighed, and live and dead ones were examined for anomalies radiographically and by alizarin staining to visualize skeletal anomalies. One animal at 40 mg/kg bw per day died, but this death was considered to be unrelated to treatment as no others occurred during the study. Maternal body weight was reduced at the end of dosing at all three doses on day 17 and at the highest dose on day 22, in comparison with concurrent controls. Decreased body-weight gain was seen at the highest dose. Decreased food consumption was seen at all three doses throughout treatment. The rates of pregnancy and numbers of corpora lutea and implantations were similar in all groups. The number of live fetuses was decreased and the number of resorptions increased at 120 mg/kg bw per day. At the two higher doses, the birth weights of the pups were decreased. No teratogenic effects were seen. The NOAEL for fetal toxicity was 40 mg/kg bw per day, on the basis of reduced pup weight at the next higher dose. No NOAEL for maternal toxicity could be identified because of decreased maternal body weight at all doses (Gillardin et al., 1988).
Rabbits
The developmental toxicity of imazalil (purity, 99%) was studied in groups of 15 pregnant New Zealand white rabbits given a dose of 0, 1.2, 2.5, or 5 mg/kg bw per day by gavage on days 6–18 of gestation. The animals were weighed before the start of dosing, at day 19, and at termination. Food consumption was not recorded. On day 28 of gestation, the animals were killed and examined for the numbers of live and dead fetuses, implantation sites, and resorptions. Live fetuses were weighed, and dead and live fetuses were examined for anomalies radiographically and by alizarin staining. One rabbit at the low dose and one at the high dose died. There was no clear effect of treatment on body-weight gain or pregnancy rate. No differences were seen between groups in the numbers of live or dead fetuses or resorptions or in the birthweights of pups or 24-h survival rate. The NOAEL for both maternal and fetal toxicity was 5 mg/kg bw per day (Dirkx & Marsboom, 1985).
A further study of the developmental toxicity of imazalil was carried out in which groups of four pregnant albino rabbits were given imazalil sulfate (purity, 98.2–100%) by gavage at a dose of 0, 5, 10, or 20 mg/kg bw per day on days 6–18 of gestation. The animals were observed daily for ill health, abnormal behaviour, or other clinical effects. Body weights were recorded on the morning of day 0 and on days 6, 19, and 28 of gestation. Food consumption was recorded during days 0–5, 6–19, and 19–28. On day 28 of gestation, the surviving animals were killed and autopsied, the uterus was removed and the weight recorded, and the dams were examined for the numbers of dead and live fetuses and resorptions. The numbers of corpora lutea were determined, and the pups were sexed. Live fetuses were weighed, and both live and dead fetuses were examined externally by radiographic examination and alizarin staining. The fetuses were then dissected.
Eight animals at 20 mg/kg bw per day and one at 10 mg/kg bw per day died during the experiment. Five of those at the highest dose that died showed weight loss. No significant differences in clinical end-points were found in the survivors. On day 19 of gestation, the body weight of animals at 20 mg/kg bw per day was decreased in comparison with that of concurrent controls. Food consumption was reduced in animals at 10 mg/kg bw per day in comparison with concurrent controls during days 0–5, and in animals at 10 and 20 mg/kg bw per day during days 6–18. The pregnancy rates and numbers of corpora lutea were comparable between groups. Animals at 20 mg/kg bw per day had a decreased number of live pups, and those at 10 and 20 mg/kg bw per day had increased numbers of resorptions. Although these changes were not statistically significant, the numbers were small, and the decrease may be biologically significant. There was a significant decrease in the number of male fetuses at 5 mg/kg bw per day but not at higher doses. Fetuses of does at 20 mg/kg bw per day had an increased incidence of thirteenth rib and missing sternum in comparison with concurrent controls, but, especially in the latter case, there was no clear dose–response relationship, and the Meeting considered these findings to be coincidental. The NOAEL for maternal toxicity was 5 mg/kg bw per day on the basis of reduced food consumption at 10 mg/kg bw per day. The NOAEL for fetal toxicity was also 5 mg/kg bw per day on the basis of an increased number of resorptions and a decreased number of live pups at 10 mg/kg bw per day (Dirkx et al., 1992a).
(f) Special studies: Developmental neurotoxicity
A study of reproductive toxicity with measurement of neurobehavioural end-points was reported in which imazalil (purity, > 99%) was administered in the diet to groups of 10 Crj: CD-1 mice of each sex from 5 weeks of age in the F0 generation to 9 weeks of age in the F1 generation. The compound was given at a concentration of 0, 0.012, 0.024, or 0.048% (0, 120, 240, and 480 ppm, if % is assumed to equal w/w), resulting in intakes for the F0 generation of 0, 19, 31, and 79 mg/kg bw per day for males and 0, 26, 45, and 100 mg/kg bw per day for females before conception; 0, 18, 34, and 74 mg/kg bw per day during mating; 0, 21, 38, and 83 mg/kg bw per day during gestation; and 0, 65, 150, and 260 mg/kg bw per day during lactation. The intakes in the F1 generation were 0, 21, 41, and 87 mg/kg bw per day for males and 0, 24, 49, and 93 mg/kg bw per day for females.
In the F0 generation, there was no effect on the body weight of either sex before conception or on the body weights of dams during gestation or lactation. Food intake was increased in females at the low and high doses before conception but not in those at the intermediate dose. A number of indicators of exploratory behaviour were increased in males at the high dose. In the F1 generation, litter size and weight and sex ratio at birth were unaffected by treatment. The mean body weight of animals at the two higher doses was reduced in early lactation. Indicators of behavioural developmental were affected, as follows. In males, surface righting was affected at the two higher doses at postnatal day 4 and at the intermediate dose at postnatal day 7. Swimming head angle was affected in males at the highest dose at postnatal day 4. In females, surface righting was affected in all treated F1 offspring at postnatal day 7, without a clear dose–response relationship, and swimming head angle was affected in females at the highest dose at postnatal day 4. No convincing dose–response relationships were seen for other observations, such as on multiple water T-maze performance at week 7. By week 8, there were no effects on the exploratory behaviour of either sex. The doses used in this study were higher than those in other studies reviewed in this monograph. Furthermore, although the results suggest that neurobehavioural end-points can be adversely affected in mice exposed to imazalil in their diet during development, comparison with the study of Levron et al. (1991), described above, suggests that the lowest dose might have been almost maternally toxic. In view of the inconsistent results at the lowest dose, the many end-points measured, and lack of dose–reponse relationships in the adverse outcomes observed, the NOAEL was the lowest dose tested, about 20 mg/kg bw per day (Tanaka, 1995).
A 43-year-old female veterinary technician developed contact dermatitis to the veterinary antimycotic Imaverol, which contains imazalil (enilconazole). Patch testing showed sensitivity to several imidazoles, including imazalil (van Hecke & de Vos, 1983).
A case of palatal and nasal infection with Alternaria was treated with imazalil at oral doses up to 1200 mg/kg bw administered topically and orally. The drug was tolerated, but it was found to be bitter and unpleasant with an aftertaste lasting for hours, and nausea occurred at high doses (ł 800 mg). Very limited studies of pharmacokinetics were carried out: at a dose of 400 mg/day, the maximum serum concentration was about 2 µg/ml, while at a dose of 1200 mg, the maximum serum concentration was about 4 µg/ml. The half-time was about 2 h, and administration of 1200 mg/kg bw daily for 1 month did not result in accumulation. No changes in clinical chemical parameters were seen (Stiller & Stevens, 1986).
A study in volunteers was undertaken to examine the local effects of imazalil on the skin. Imazalil base 0.2%, imazalil base 0.2% ointment, imazalil sulfate (530 mg base/L), Fungaflor (68% w/w imazalil), and two control formulations not containing imazalil were applied under occlusive adhesive strips for 48 h. The skin was then examined for redness, oedema, irritation, or inflammation. There were no complaints of burning, itching, or pain, and the skin at the sites of application appeared normal in all cases (Desplenter & Verhamme, 1979).
After oral administration to rats, [14C]imazalil was rapidly and nearly completely absorbed. Most of the label was excreted within 96 h, predominantly in the urine but also in faeces. Nearly 50% of the radiolabel retained in the body was found in the liver. Very little imazalil was excreted unchanged, and the compound was metabolized to at least 25 metabolites. The main routes of metabolism were epoxidation, epoxide hydration, oxidative O-dealkylation, imidazole oxidation and scission, and oxidative N-dealkylation. No significant sex difference was seen in metabolism. The metabolic pattern was similar after oral and intravenous administration.
In a study of the hepatotoxicity of imazalil, the compound was found to affect liver morphology (vacuolation) when given in the diet to mice for up to 3 months. In rats, imazalil caused fatty infiltration of the liver and decreased serum concentrations of triglycerides, cholesterol, and phospholipids. Imazalil administered to mice by mouth induced the cytochrome P450 isoenzymes CYP1A, CYP2B, CYP2C, and CYP3A. Imazalil had no significant estrogenic effect when tested in vitro in an MCF7 cell proliferation assay or a yeast estrogen screen. Steroid 11beta-hydroxylation and 19-hydroxylation in the mitochondria of the gerbil were reported to be substantially suppressed in parallel by imazalil. Imazalil inhibited CYP19 aromatase activity in human placental microsomes.
The acute oral LD50 of imazalil in rats was 200–350 mg/kg bw. Imazalil was not irritating to the skin of rabbits and was moderately irritating to the eye. It had weak sensitizing potential when tested according to the Magnusson and Kligman method. A single case report of contact dermatitis in humans in response to imazalil was found. A further report was considered, in which imazalil had been used orally at high doses by an individual to treat a fungal infection; it was well tolerated, the only adverse effect noted being nausea. Imazalil has been classified by WHO (1999) as moderately hazardous.
Studies in mice, rats, and dogs showed that the target organ of toxicity was frequently the liver. In addition, imazalil, like other azole fungicides, affects steroid synthesis, but there was little indication that this was manifested in vivo in the studies examined by the Meeting.
Imazalil was applied to the shaved backs of New Zealand white rabbits at a dose of 0, 10, 40, or 160 mg/kg bw per day for 6 h/day, 5 days/week for 3 weeks, on a porous gauze dressing. The highest dose reduced the creatinine concentration, specific gravity, and urobilinogen concentration in urine. The NOAEL was 40 mg/kg bw per day.
Imazalil was administered to groups of 10 rats of each sex at a dietary concentration of 0, 25, 100, or 400 ppm. The NOAEL was 100 ppm (equivalent to 5 mg/kg bw per day), on the basis of increased relative kidney weights in males and increased absolute and relative liver and kidney weights and increased absolute thymus weight in females at the highest concentration.
In a 1-year study of toxicity in beagle dogs, groups of four animals of each sex received imazalil at a dose of 0, 1.2, 2.5, or 20 mg/kg bw per day orally in gelatine capsules. The NOAEL was 2.5 mg/kg bw per day on the basis of clinical signs, decreased body-weight gain and food consumption, decreased serum calcium concentration, increased alkaline phosphatase activity, and increased liver weight at the highest dose.
In a 23-month study of carcinogenicity in mice, groups of 50 males and 50 females received imazalil at a dietary concentration of 0, 50, 200, or 600 ppm. Males at the two higher doses and females at the highest dose showed focal cellular changes, large and small vacuoles, and pigmented and swollen sinusoidal cells in the liver. Increased incidences of hepatic neoplasms were found in males at 200 and 600 ppm, with increased incidences of hepatic neoplastic nodules; a similar increase in the incidence of hepatic neoplasms was found in females at the highest dietary concentration. The hepatic neoplasms were evaluated three times, the last time by a pathology working group which concluded that the incidence of adenomas, but not that of carcinomas, was increased in males at the two highest doses. Therefore, the NOAEL for carcinogenicity was 50 ppm. The overall NOAEL for the study was 50 ppm, equal to 8.1 mg/kg bw per day, on the basis of morphological changes (adenomas, foci and nodules) in the livers of males at 200 ppm.
Imazalil was administered to groups of 20 male and 20 female rats at a dietary concentration of 0, 25, 100, or 400 ppm for 18 months. Decreased body-weight gain was observed in females at the highest dose, and males at this dose showed treatment-related gross (increase in the lobular pattern) and microscopic (periportal cytoplasmic vacuolation of hepatocytes) effects in the liver. There was no evidence of treatment-related neoplasia. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of decreased body-weight gain in females, decreased plasma albumin concentration in males, and pathological changes in the livers of males at the highest dose.
Imazalil was administered to groups of 50 male and 50 female rats at a dietary concentration of 0, 25, 100, or 400 ppm for 30 months. The NOAEL was 100 ppm, equal to 3.6 mg/ kg bw per day, on the basis of decreased body-weight gain in males at the highest dose. No treatment-related histopathological effects were observed in the liver. There was no evidence that imazalil was carcinogenic.
Imazalil has been tested for genotoxicity in an adequate range of tests in vivo and in vitro. The Meeting concluded that imazalil is unlikely to have genotoxic potential. In view of the lack of genotoxicity and the finding of tumours only in mice, the Meeting concluded that imazalil is unlikely to pose a carcinogenic risk to humans. However, the Meeting was aware that the toxicological dossier supplied was incomplete.
A two-generation study of reproductive toxicity was conducted in rats, in which imazalil was administered in the diet at a nominal dose of 0, 5, 20, or 80 mg/kg bw per day. The NOAEL for maternal toxicity was 20 mg/kg bw per day on the basis of reduced maternal weight gain at 80 mg/kg bw per day. Decreased numbers of live pups and increased numbers of stillbirths were observed at this dose. The survival rate of pups during lactation was decreased in all test groups of the F1 generation at days 4, 14, and 21 of lactation and in the F2 generation, at 5 mg/kg bw per day (days 14 and 21) and 80 mg/kg bw per day (days 4, 14, and 21). However, when these data were evaluated on a per litter basis, the differences in survival were not significant. On this basis, the NOAEL for fetotoxicity was 20 mg/kg bw per day.
A study of reproductive toxicity in which neurobehavioural end-points were measured was conducted with dietary concentrations of 0, 120, 240, and 480 ppm. The lowest concentration used was high in comparison with the doses used in other studies that were reviewed. Nevertheless, the results suggest that neurobehavioural end-points in the offspring of mice exposed to imazalil in their diet, during pregnancy and perinatally, can be adversely affected. In view of the inconsistent results found at the lowest dose, the multiple end-points measured, and the lack of a dose–response relationship, the Meeting concluded that the NOAEL for developmental neurotoxicity was 120 ppm, equal to 20 mg/kg bw per day.
Two studies of the developmental toxicity of imazalil in mice were available for review. In the first, imazalil was administered by gavage at a dose of 0, 40, 80, or 120 mg/kg bw per day. The NOAEL for maternal toxicity was 40 mg/kg bw per day, on the basis of reduced body-weight gain and food consumption. No NOAEL was identified for fetal toxicity, as litter size and the number of live pups were decreased in all groups. In the second study, imazalil was administered by gavage at a dose of 0, 10, 40, 80, or 120 mg/kg bw per day. At the highest dose, the number of live fetuses was reduced, and the number of resorptions was increased. The body weights of pups at this dose were decreased, but the sex ratio was similar in all groups. The NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of decreased body-weight gain at 40 mg/kg bw per day and reduced food consumption after dosing. In addition, deaths occurred at doses of 80 mg/kg bw per day and above. The NOAEL for fetal toxicity was 80 mg/kg bw per day, as the highest dose reduced the number of live fetuses,increased the number of resorptions, and decreased the body weights of the pups. There was no evidence of teratogenicity. When the two studies were considered together, the Meeting concluded that the NOAEL for maternal toxicity was 10 mg/kg bw per day, but that a NOAEL for fetal toxicity could not be identified.
In a study of developmental toxicity in rats, imazalil was administered at a dose of 0, 40, 80, or 120 mg/kg bw per day by gavage. No teratogenic effects were seen, and the NOAEL for fetal toxicity was 40 mg/kg bw per day, on the basis of reduced pup weight at the higher dose. A NOAEL for maternal toxicity was not identified because of decreased maternal body weight in all the groups when compared with concurrent controls.
The developmental toxicity of imazalil in rabbits was studied at doses of 0, 1.2, 2.5, and 5 mg/kg bw per day. The NOAEL for both maternal and fetotoxicity was 5 mg/kg bw per day, the highest dose tested. In another study of developmental toxicity in rabbits, at doses of 0, 5, 10, and 20 mg/kg bw per day, the NOAEL for maternal toxicity was 5 mg/kg bw per day on the basis of reduced food consumption at 10 and 20 mg/kg bw per day. The NOAEL for fetal toxicity was also 5 mg/kg bw per day, on the basis of an increased incidence of resorptions and a decrease in the number of live pups at 10 and 20 mg/kg bw per day. In neither case was imazalil teratogenic.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of imazalil to fetuses, infants, and children
The ADI of 0–0.03 mg/kg bw established by the 1991 Joint Meeting was reaffirmed. The ADI is based on a NOAEL of 2.5 mg/kg bw per day in a 1-year study of toxicity in dogs and a 100-fold safety factor.
The establishment of an acute RfD was considered unnecessary as no relevant end-point was identified.
Levels relevant for risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
23-month study of toxicity and carcinogenicitya |
Toxicity |
50 ppm, equal to 8.1 mg/kg bw per day |
200 ppm, equal to 33 mg/kg bw per day |
|
|
|
Carcinogenicity |
50 ppm, equal to 8.1 mg/kg bw per day |
200 ppm, equal to 33.4 mg/kg bw per day |
|
|
Developmental toxicityb |
Maternal toxicity |
10 mg/kg bw per day |
40 mg/kg bw per day |
|
Embryo- and feto-toxicity |
– |
10 mg/kg bw per dayc |
||
|
Rat |
30-month study of toxicity and carcinogenicitya |
Toxicity |
100 ppm, equal to 3.6 mg/kg bw per day |
400 ppm, equal to 15 mg/kg bw per day |
|
|
|
Carcinogenicity |
400 ppm, equal to 15 mg/kg bw per dayd |
– |
|
|
Two-generation study of reproductive toxicitya |
Maternal toxicity |
20 mg/kg bw per day |
80 mg/kg bw per day |
|
|
|
Pup toxicity |
20 mg/kg bw per day |
80 mg/kg bw per day |
|
|
Developmental toxicityb |
Maternal toxicity |
– |
40 mg/kg bw per dayc |
|
|
|
Embryo- and feto-toxicity |
40 mg/kg bw per day |
80 mg/kg bw per day |
|
|
Two-generation study of reproductive toxicitya |
Developmental neurotoxicity |
120 ppm, about 20 mg/kg bw per day |
240 ppm, about 30 mg/kg bw per day |
|
Rabbit |
Developmental toxicityb |
Maternal toxicity |
5 mg/kg bw per day |
10 mg/kg bw per day |
|
|
|
Embryo- and feto-toxicity |
5 mg/kg bw per day |
10 mg/kg bw per day |
|
Dog |
1-year study of toxicitye |
Toxicity |
2.5 mg/kg bw per day |
20 mg/kg bw per day |
a
Dietary administrationb
Gavagec
Lowest dose testedd
Highest dose testede
CapsuleEstimate of acceptable daily intake for humans
0–0.03 mg/kg bw
Acute reference dose
Unnecessary
Studies that would provide information valuable for continued evaluation of the compound
Summary of critical end-points
|
Absorption, distribution, excretion, and metabolism in mammals |
|
|
Rate and extent of oral absorption |
High bioavailability |
|
Distribution |
Extensive; highest concentration in liver |
|
Potential for accumulation |
Low |
|
Rate and extent of excretion |
Rapid: > 80% within 24 h |
|
Metabolism in animals |
Extensive metabolism by epoxidation, epoxide hydration, oxidative O-dealkylation, imidazole oxidation and scission, and oxidative N-dealkylation, rat |
|
Toxicologically significant compounds |
Parent compound |
|
Acute toxicity |
|
|
Rats, LD50, oral |
220–350 mg/kg bw |
|
Rats, LD50, intraperitoneal |
No data |
|
Mice, LD50, oral |
No data |
|
Dermal sensitization (test method used) |
Weak response in guinea-pigs (Magnusson and Kligman) |
|
Short-term toxicity |
|
|
Target/critical effect |
Effects on body weight and food consumption |
|
Lowest relevant oral NOAEL |
2.5 mg/kg bw per day |
|
Genotoxicity |
None |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Decreased weight gain; pathological changes in liver, mice and rats |
|
Lowest relevant NOAEL |
3.6 mg/kg bw per day |
|
Carcinogenicity |
Liver tumours in mice; clear NOAELs identified |
|
Reproduction target/critical effect |
Reduced pup viability |
|
Lowest relevant reproductive NOAEL |
20 mg/kg bw per day |
|
Developmental target/critical effect |
Not teratogenic; fetotoxicity usually seen with maternal toxicity |
|
Lowest relevant developmental NOAEL |
5 mg/kg bw per day for maternal and fetal toxicity |
|
Medical data |
Used as a human drug (enilconazole) and well tolerated as such |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.03 mg/kg bw |
1 year study in dogs |
100 |
|
Acute RfD |
None |
|
|
Appelman, L. & Woutersen, R. (1983) Acute inhalation toxicity study of an imazalil containing smoke, developed by a smoke-generator in rats. Unpublished report No V83.308/230831. Civo Institutes TNO, Zeist, Netherlands. Submitted to WHO by Janssen Pharmaceutica NV. In house protocol ‘complies to a great extent with EEC method B2’ 1984. GLP.
Butler, W.H. (1995).Pathology working group imazalil. Unpublished report prepared by WH Butler, BIBRA International, Carshalton, Surrey, United Kingdom. Submitted to WHO by Janssen Pharmaceutica NV.
Clare, C. (1996) Imazalil: measurement of unscheduled DNA synthesis and replicative DNA synthesis in mouse liver using an in vivo/in vitro procedure. Unpublished report 1073/3-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Janssen Pharmaceutica NV. Complies with United Kingdom Environmental Mutagen Society Guidelines, 1993. GLP: 21 CFR parts 58.31, 58.35 (b) (7); United Kingdom: Principles of Good Laboratory Practice; OECD Final report on Good Laboratory Practice in the Testing of Chemicals, 1982.
Desplenter, L. & Verhamme, G. (1979) Local effects of imazalil after application to the human skin. Unpublished preclinical research report, Department of Veterinary Clinical Research, Janssen Pharmaceutica Laboratories, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV.
Dirkx, P. & Marsboom, R. (1985) R 18531 oral embryotoxicity and teratogencity study in New Zealand white rabbits (segment II) administration: oral. Unpublished report 1482 (85.18.04). Department of Toxicology, Janssen Pharmaceutica NV, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals: teratogenicity no. 414, 12 May 1981. GLP: 21CFR part 85.35 (b) (7), 58.35 (c ).
Dirkx, P., Lampo, A., Coussement, W. & Van Cauteren, H. (1992a) R 23979—Imazalil sulphate. Embryotoxicity and teratogenicity study in albino rabbits. Administration: orally by gavage. Unpublished report No 2615. Department of Toxicology, Janssen Research Foundation, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. Stated to conform with OECD guideline 452, 1981; US Food and Drug Administration, Toxicological principles for the safety assessment of direct food additives and color additives in food and the rules governing medicinal products in the European Community, Vol. III, guidelines on the quality, safety and efficacy of medicinal products for human use. January 1989. GLP compliant—US Environmental Protection Agency 40 CFR part 160.12(a).
Dirkx, P., Lampo, A., Vandenberghe, J., Coussement, W. & Van Cauteren, H. (1992b) R 23979—Imazalil 2-generation reproduction study with 1 litter per generation in Wistar rats. Administration orally through the diet. Unpublished report No 2337. Department of Toxicology, Janssen Research Foundation, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals no. 452, 1981; US Food and Drug Administration, toxicological principles for the safety assessment of direct food additives and color additives used in food, 1982; EEC, rules governing medicinal products in the European Community, Vol. III: guidelines on the quality safety and efficacy of medicinal products for human use, revised and completed, January 1989. GLP: 40CFR parts 160.12 (a) 160.35(b) (7), 160.31.
Drummond, T.D., Mason, J.I. & McCarthy, J.L. (1988) Gerbil adrenal 11beta- and 19-hydroxylating activities respond similarly to inhibitory or stimulatory agents: two activities of a single enzyme. J. Steroid Biochem., 29, 641–648. No mention of GLP status.
Gillardin, J.M., Sanz, G. & Marsboom, R. (1987) R 27180 embryotoxicity and teratogenicity study in COBS CD1 mice (segment II): oral by gavage. Unpublished report no. 86-07 (86.09.18). Research Department, Laboratoires Janssen, Aubervilliers, France. Submitted to WHO by Janssen Pharmaceutica NV. Stated to be compliant with GLP.
Gillardin, J.M., van Cauteren, H., Sanz, G. & Marsboom, R. (1988) R 27180 embryotoxicity and teratogenicity study in Sprague-Dawley rats (segment II): oral by gavage. Unpublished report no 2003/88-05. Research Department, Laboratoires Janssen, Aubervilliers, France. Submitted to WHO by Janssen Pharmaceutica NV. Complies with OECD guideline no 414; Directive 87/302/EEC. GLP 21 CFR part 58.35 (b) (7), 58.35 (c) 58.185.
van Gompel, J., Vanparys, P. & van Cauteren, H. (1995) RO23979—imazalil in vitro mammalian gene mutation assay administration: incubation with or without a rat liver metabolic activation system. Unpublished report No. 3470. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals 476, 4 April 1984. Also stated to comply with EEC Directive 88/302/EEC, annex V of Directive 67/548/EEC, part B: Methods for the determination of toxicity, other effects—mutagenicity: in vitro mammalian cell mutation test; EEC publication L133, minor deviation on expression of results. GLP: 21CFR parts 58.35 (b) (7), 58.35 (c); US Environmental Protection Agency 40 CFR 160.
van Hecke, E. & de Vos, L. (1983) Contact sensitivity to enilconazole. Contact Derm., 9, 144–173. No mention of GLP status.
Hess, R. (1996) Imazalil (enilconazole) relevance of liver tumor formation in mice. Report to Janssen Research Foundation, Beerse, Belgium, by Professor Robert Hess, University of Basle, Switzerland. Submitted to WHO by Janssen Pharmaceutica. NV.
Janssen Pharmaceutica NV (1994) Amendment to report no 2337 (Dirkx, P., Lampo, A., Vandenberghe, J., Coussement, W. & Van Cauteren, H. (1992) 2-Generation reproduction study with imazalil technical grade with 1 litter per generation in Wistar rats, when orally administered through the diet. Department of Toxicology, Janssen Research Foundation, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV.
Levron, J.C., van Cauteren, H., Sanz, G. & Marsboom, R. (1991) Imazalil sulfate R 27180S embryotoxicity and teratogenicity study in COBS CD1 mice. Unpublished report 2374/90-06. Department de Recherche, Laboratoires Janssen, Aubervilliers, France. Submitted to WHO by Janssen Pharmaceutica NV. GLP: stated to be compliant; 21 CFR part 58.185; 40 CFR 160.
Lina, B., Til, H., van Nesselrooij, J., Kuper, C. & Falke, H. (1983) Six-month oral toxicity study with imazalil technical grade in rats. Unpublished report V 83.186/220555. Netherlands Organization for Applied Scientific Research, Division for Nutrition and Food Research, TNO, Zeist, Netherlands. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals: Subchronic oral toxicity—rodent: 90 day study, no 408, 12 May 1981. GLP: stated to be compliant.
Lina, B., Til, H.P., van Nesselrooij, J.H.J., Beems, R.B. & Falke, H.E. (1984) Eighteen-month oral toxicity study with imazalil base-R 23979 in rats. Unpublished report V 84.140/220555. Netherlands Organization for Applied Scientific Research, Division for Nutrition and Food Research, TNO, Zeist, Netherlands. Submitted to WHO by Janssen Pharmaceutica NV. Stated to be compliant with an approved protocol; no indication of GLP status.
Mannens, G., Van Leemput, L. & Heykants, J. (1993) General metabolism of imazalil. Unpublished report No. R 23979/FK1116. Department of Drug Metabolism and Pharmacokinetics, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD Guideline for testing chemicals ‘toxicokinetics’ 417, 4 April 1984; Directive 87/302/EEC Part B. GLP: OECD, US Food and Drug Administration (21CFR part 58), US Environmental Protection Agency (40CFR part 160).
Miltenberger, H.G., Fautz, R. & Völkner, W. (1990) Unscheduled DNA synthesis in primary hepatocytes of male rats in vitro with imazalil. Unpublished report no. 192600, Cytotest cell research GMBH and Co. KG, Rossdorf, Germany, and Laboratory for Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Janssen Pharmaceutica NV. Complies with OECD guideline 482, 23 October 1986; US Environmental Protection Agency 40 CFR subpart F, revised 1 July 1986, unscheduled DNA synthesis in mammalian cells in culture. GLP: OECD guidelines 1981.
Muto, N., Hirai, H., Tanaka, T., Itoh, N. & Tanaka, K. (1997) Induction and inhibition of cytochrome P450 isoforms by imazalil, a food contaminant, in mouse small intestine and liver. Xenobiotica, 27, 1215–1223. No mention of GLP.
Nakagawa, Y. & Moore, G.A. (1995) Cytotoxic effects of postharvest fungicides, ortho-phenylphenol, thiabendazole and imazalil, on isolated rat hepatocytes. Life Sci., 57, 1433–1440. No mention of GLP status.
Nakagawa, Y. & Tayama, K. (1997) Acute hepatotoxic potential of imazalil fungicide in rats. Bull. Environ. Contam. Toxicol., 58, 402–407.
Niemegeers, C. (1979) Comparative acute oral toxicity studies of the different salts of imazalil in rats. Unpublished report No. N17417. Department of Pharmacology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. No GLP. Method: in house protocol.
Stiller, R.L. & Stevens, D.A. (1986) Studies with a plant fungicide, imazalil, with vapor phase activity, in the therapy of human alternariosis. Mycopathologia, 93, 169–172. No mention of GLP status.
Tanaka, T. (1995) Reproductive and neurobehavioral effects of imazalil administered to mice. Reprod. Toxicol., 9, 281–288. No mention of GLP status.
Teuns, G. & Marsboom, R. (1987) Imazalil (R 23979) primary dermal irritation study in rabbits. Unpublished report No 1864. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing chemicals, 404: 12 May 1981; EEC 79/831/EEC part V, paragraph B.4. GLP: US Food and Drug Administration 21CFR part 58.185.
Teuns, G., Ligtvoet, T., Coussement, W., Lampo, A., van Cauteren, H. & Marsboom, R. (1990a) Study of the acute dermal toxicity of imazalil: R 23979 (technical grade) in New Zealand white rabbits. Unpublished report No. 2344. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing chemicals, section 4 (health effects), subsection 402: acute dermal toxicity, 24 February 1987; EEC method B3 GLP: US Environmental Protection Agency 40CFR part 160.12, 160.31, 160.35 (b) (7).
Teuns, G., Peeters, V., Coussement, W., Lampo, A., van Cauteren, H. & Marsboom, R. (1990b) Imazalil: R 23979 technical grade primary eye irritation study in New Zealand white rabbits. Unpublished report No. 2253. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing chemicals, section 4 (health effects), subsection 405: acute eye irritation/corrosion, 24 February 1987; US Environmental Protection Agency, pesticide assessment guideline, subdivision F. Hazard evaluation, human and domestic animals, revised edition. § 81-4: primary eye irritation study, pp. 55a–55c; Directive 79/831/EEC Annex V, part B: methods for the determination of toxicity, subpart bB5: acute toxicity: eye irritation. GLP: US Food and Drug Administration 21 CFR part 58, subparts 58.31, 58.35.
Teuns, G., Coussement, W., van Cauteren, H. & Marsboom, R. (1990c) Imazalil: R 23979 (technical grade) dermal sensitization study according to the Magnusson guinea-pig maximization test. Unpublished report No. 2417. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing chemicals, section 4 (health effects), subsection 406: dermal sensitization, 12 May 1981; US Environmental Protection Agency pesticide assessment guidelines, subdivision S—hazard evaluation: human and domestic animals series 81-6 dermal sensitization addendum 4 on data reporting (January 1988); 50 CFR 188 subpart 798.4100; Directive 79/831/EEC Annex V, part B: methods for the determination of toxicity, subpart B.6G, skin sensitization; Agricultural chemicals law and regulations (Japan) II: testing guidelines for toxicology studies pp. 27–29: dermal sensitization toxicity study: 28 January 1985. GLP: US Environmental Protection Agency 40 CFR parts 160.12a, 160.31, 160.35.
Teuns, G., Vandenberghe, J., Lampo, A., Coussement, W. & van Cauteren, H. (1991) Imazalil: R 23979. Repeated dose dermal toxicity study in New Zealand white rabbits (21-days). Unpublished report No. 2418. Department of Toxicology, Janssen Pharmaceutica NV, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing chemicals no. 414; EEC 92/69/EEC method B.9. GLP: US Food and Drug Administration 40CFR parts 160.12 (a), 160.31, 160.35 (b) (7).
Til, H.P., Lina, B.A.R., van Nesselrooij, J.H.J., Beems, R.B. & Falke, H.E. (1985) Lifespan oral carcinogenicity study with imazalil base-R 23979 in rats. Unpublished report V 85.153/220555. Netherland Organization for Applied Scientific Research, Division for Nutrition and Food Research, TNO, Zeist, Netherlands. Submitted to WHO by Janssen Pharmaceutica NV. GLP: stated to be compliant.
Van Deun, K., Vandenbergh, J., Lammens, L., Lampo, A., Coussement, W. & Van Cauteren, H. (1994) Three-month oral mechanistic toxicity study with one month interim sacrifice in SPF albino Swiss mice. Unpublished report No. 3140. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. GLP: 40 CFR part 160.31; 160.35 (b) (7).
Vanparys, P. & Marsboom, R. (1988a) Ames reverse mutation test of imazalil technical. Unpublished report No. 1999. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline no. 471, 26 May 1983; complies ‘to a great extent’ with EEC B.14. GLP: 21CFR parts 58.35 (b) (7), 58.35 (c).
Vanparys, P. & Marsboom, R. (1988b) Micronucleus test in mice at a single oral dose of imazalil technical. Unpublished report No. 1911. Department of Toxicology, Janssen Research Foundation, Turnhoutseweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals 474, micronucleus test; also stated to comply with 84/449/EEC test method B.12 Other effects—mutagenicity (micronucleus test) EEC Directive 84/449. GLP: 21CFR part 58.35 (b) (7), part 58.35 (c).
Vanparys, P., Marsboom, R., Lenaerts, P. & Deknudt, G. (1990) R 23979 In vitro chromosome aberration study on human lymphocytes incubated with or without a metabolic activation system. Unpublished report No. SCK 86/O2D/R23979. Mammalian Genetics Laboratory, Department of Biology, SCK/CEN Mol, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. In accordance with OECD guideline 473 (1983); stated partly to comply with EEC method B14. GLP: 21CFR parts 58.35 (b) (7), 58.35 (c).
Verstraeten, A., Teuns, G., Vandenberghe, J., Coussement, W., van Cauteren, H. & Marsboom, R. (1989) Imazalil base: R 23979 technical. Chronic toxicity study in beagle dogs subjected to repeated dosage for 12 months by oral administration. Unpublished report No. 1899. Department of Toxicology, Janssen Research Foundation, Turnhoutsweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. OECD guideline for testing of chemicals 452, 12 May 1981; also stated to comply ‘to a great extent’ with part B Directive 87/302/EEC. GLP: US Environmental Protection Agency 40CFR part 160.12, 160.31, 160.35 (b) 7.
Verstraeten, A., Vandenberghe, J., Lampo, A., Van Deun, K., Coussement, W. & van Cauteren, H. (1993) Imazalil base: carcinogenicity study in Swiss mice administration through the diet for 2 years. Unpublished report No. 2194. Department of Toxicology, Janssen Research Foundation, Turnhoutsweg, Beerse, Belgium. Submitted to WHO by Janssen Pharmaceutica NV. Stated to be based upon Toxicological principles for the safety assessment oif direct food additives and food colors, US Food and Drug Administration Washington DC, 1982, US Environmental Protection Agency FIFRA guidelines, pesticide assessments guidelines, subdivision F, hazard evaluation, Washington DC, 1984, toxicity test guidelines, Ministry of Health and Welfare, Japan, 1989, rules governing medicinal products in the European Community, Vol. III. Guidelines on the quality, safety and efficacy of medicinal products for human use. Revised and completed edition, January 1989. Directive 87/302/EEC. GLP: US Environmental Protection Agency 40CFR parts 160.12 (a), 160.31.
Vinggaard, A.M., Breinholt, V. & Larsen, J.C. (1999) Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit. Comtam., 16, 533–542. No indication of GLP status.
Vinggaard, A.M., Hnida, C., Breinholt, V. & Larsen, J.C. (2000) Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. In Vitro, 14, 227–234. No indication of GLP status.
WHO (1999) Recommended classification of pesticides by hazard and guidelines to classification 1998–1999 (WHO/PCS/98.21/Rev.1), Geneva, International Programme on Chemical Safety (www.who.int/pcs/pcs-act.htm).
See Also:
Toxicological Abbreviations
Imazalil (ICSC)
Imazalil (Pesticide residues in food: 1977 evaluations)
Imazalil (Pesticide residues in food: 1980 evaluations)
Imazalil (Pesticide residues in food: 1984 evaluations)
Imazalil (Pesticide residues in food: 1984 evaluations)
Imazalil (Pesticide residues in food: 1985 evaluations Part II Toxicology)
Imazalil (Pesticide residues in food: 1986 evaluations Part II Toxicology)
Imazalil (Pesticide residues in food: 1991 evaluations Part II Toxicology)
Imazalil (JMPR Evaluations 2001 Part II Toxicological)
Imazalil (JMPR Evaluations 2005 Part II Toxicological)