
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
AZINPHOS-METHYL
Chemical name
O,O-dimethyl-S-(4-oxo-1,2,3,-benzotriazin-3,
(4H)-yl-methyl)-phosphoro-dithioate; [S-(3,4-dihydro-4-oxobenzo-[-d]
(1,2,3) triazin-3-yl-methyl]-dimethyl phosphoro-thiolothionate.
Synonyms
Guthion, Gusathion
Empirical formula
C10H12O3N3PS2
Structural formula
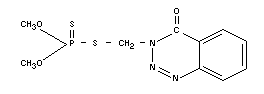 BIOLOGICAL DATA
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor. The molar I50 in rat brain is 2.99 × 10-8
(Schrader, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 11-25 DuBois et al., 1957;
Gaines, 1960
Rat Intraperitoneal 5-11.6 DuBois et al., 1957
Mouse Oral 8.0* Sato, 1959
Guinea-pig Oral 80.0 Dubois et al., 1957
Guinea-pig Intraperitoneal 40.0 DuBois et al., 1957
* Given as azinphos-methyl emulsion.
Short-term studies
Rat. The addition of azinphos-methyl to the diet of groups,
each 10 young male and 10 young female rats, at levels of 2, 5 and 20
ppm did not markedly alter the growth-rate over 60 days. The
body-weight of the male rats fed 20 ppm was about 4% less than that of
the controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10%) and in serum and erythrocytes (about 30%). After 120 days
no appreciable changes in the gross and microscopic appearance of
brain, heart, liver, spleen, adrenals, stomach, intestines, skeletal
muscle and bone marrow were found. There was no evidence of
demyelination in the nervous system (DuBois, 1956).
When weanling male rats were fed diets containing 50 and 100 ppm
of azinphos-methyl for 16 weeks, approximately half of each group
died. All animals receiving azinphos-methyl in the diet showed marked
cholinergic effects, including diarrhoea, salivation, lacrimation,
muscular tremors and fasciculations. These symptoms were most marked
during the first month on the diets. The rats fed 50 ppm of
azinphos-methyl weighed about 10% less and the rats fed 10 ppm, 18%
less than rats fed a normal diet. The cholinesterase activity of the
serum, brain, erythrocytes and submaxillary glands of rats fed 50 and
100 ppm was markedly inhibited. The inhibition was most marked in the
erythrocytes and brain and the animals did not fully recover from
these effects during a 3-week period on a normal diet.
Both gross and microscopic examination failed to indicate any
evidence of testicular atrophy due to the presence of the high levels
of azinphos-methyl in the diet (Doull et al., 1951).
Dog. Groups of 2 dogs, 1 male and 1 female, given 5, 10, 20 and
50 ppm of azinphos-methyl did not show any loss of weight nor any
symptoms of azinphos-methyl poisoning during a 12-week period. At
dietary levels of 5, 10 and 20 ppm there was no significant decrease
but at 50 ppm there was a 25% decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the 1 male and 1 female dog began to be inhibited at
10 ppm in the diet (DuBois, 1957).
Long-term studies
No data available.
Comments on experimental studies reported
Satisfactory studies have been carried out in rats but in the
studies in dogs only a very small number of animals was used.
EVALUATION
Level causing no significant toxicological effect in rat
5 ppm in the diet, equivalent to 0.25 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Doull, J., Amido, P. & DuBois, K. P. (1951) Unpublished report, 5 June
DuBois, K. P. (1956) University of Chicago. Unpublished final report,
3 May
DuBois, K. P. (1957) University of Chicago. Unpublished final report,
1 June
DuBois, K. P., Thursh, D. R. & Murphy, S. D. (1957) J. Pharmacol.
exp. Ther., 119, 208
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Sato, J. (1959) Kumamoto med. J., 12, 312 (from Chemical
Abstracts, 1960, 54, 21473)
Schrader, G. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
BIOLOGICAL DATA
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor. The molar I50 in rat brain is 2.99 × 10-8
(Schrader, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 11-25 DuBois et al., 1957;
Gaines, 1960
Rat Intraperitoneal 5-11.6 DuBois et al., 1957
Mouse Oral 8.0* Sato, 1959
Guinea-pig Oral 80.0 Dubois et al., 1957
Guinea-pig Intraperitoneal 40.0 DuBois et al., 1957
* Given as azinphos-methyl emulsion.
Short-term studies
Rat. The addition of azinphos-methyl to the diet of groups,
each 10 young male and 10 young female rats, at levels of 2, 5 and 20
ppm did not markedly alter the growth-rate over 60 days. The
body-weight of the male rats fed 20 ppm was about 4% less than that of
the controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10%) and in serum and erythrocytes (about 30%). After 120 days
no appreciable changes in the gross and microscopic appearance of
brain, heart, liver, spleen, adrenals, stomach, intestines, skeletal
muscle and bone marrow were found. There was no evidence of
demyelination in the nervous system (DuBois, 1956).
When weanling male rats were fed diets containing 50 and 100 ppm
of azinphos-methyl for 16 weeks, approximately half of each group
died. All animals receiving azinphos-methyl in the diet showed marked
cholinergic effects, including diarrhoea, salivation, lacrimation,
muscular tremors and fasciculations. These symptoms were most marked
during the first month on the diets. The rats fed 50 ppm of
azinphos-methyl weighed about 10% less and the rats fed 10 ppm, 18%
less than rats fed a normal diet. The cholinesterase activity of the
serum, brain, erythrocytes and submaxillary glands of rats fed 50 and
100 ppm was markedly inhibited. The inhibition was most marked in the
erythrocytes and brain and the animals did not fully recover from
these effects during a 3-week period on a normal diet.
Both gross and microscopic examination failed to indicate any
evidence of testicular atrophy due to the presence of the high levels
of azinphos-methyl in the diet (Doull et al., 1951).
Dog. Groups of 2 dogs, 1 male and 1 female, given 5, 10, 20 and
50 ppm of azinphos-methyl did not show any loss of weight nor any
symptoms of azinphos-methyl poisoning during a 12-week period. At
dietary levels of 5, 10 and 20 ppm there was no significant decrease
but at 50 ppm there was a 25% decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the 1 male and 1 female dog began to be inhibited at
10 ppm in the diet (DuBois, 1957).
Long-term studies
No data available.
Comments on experimental studies reported
Satisfactory studies have been carried out in rats but in the
studies in dogs only a very small number of animals was used.
EVALUATION
Level causing no significant toxicological effect in rat
5 ppm in the diet, equivalent to 0.25 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Doull, J., Amido, P. & DuBois, K. P. (1951) Unpublished report, 5 June
DuBois, K. P. (1956) University of Chicago. Unpublished final report,
3 May
DuBois, K. P. (1957) University of Chicago. Unpublished final report,
1 June
DuBois, K. P., Thursh, D. R. & Murphy, S. D. (1957) J. Pharmacol.
exp. Ther., 119, 208
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Sato, J. (1959) Kumamoto med. J., 12, 312 (from Chemical
Abstracts, 1960, 54, 21473)
Schrader, G. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
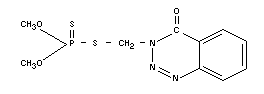 BIOLOGICAL DATA
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor. The molar I50 in rat brain is 2.99 × 10-8
(Schrader, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 11-25 DuBois et al., 1957;
Gaines, 1960
Rat Intraperitoneal 5-11.6 DuBois et al., 1957
Mouse Oral 8.0* Sato, 1959
Guinea-pig Oral 80.0 Dubois et al., 1957
Guinea-pig Intraperitoneal 40.0 DuBois et al., 1957
* Given as azinphos-methyl emulsion.
Short-term studies
Rat. The addition of azinphos-methyl to the diet of groups,
each 10 young male and 10 young female rats, at levels of 2, 5 and 20
ppm did not markedly alter the growth-rate over 60 days. The
body-weight of the male rats fed 20 ppm was about 4% less than that of
the controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10%) and in serum and erythrocytes (about 30%). After 120 days
no appreciable changes in the gross and microscopic appearance of
brain, heart, liver, spleen, adrenals, stomach, intestines, skeletal
muscle and bone marrow were found. There was no evidence of
demyelination in the nervous system (DuBois, 1956).
When weanling male rats were fed diets containing 50 and 100 ppm
of azinphos-methyl for 16 weeks, approximately half of each group
died. All animals receiving azinphos-methyl in the diet showed marked
cholinergic effects, including diarrhoea, salivation, lacrimation,
muscular tremors and fasciculations. These symptoms were most marked
during the first month on the diets. The rats fed 50 ppm of
azinphos-methyl weighed about 10% less and the rats fed 10 ppm, 18%
less than rats fed a normal diet. The cholinesterase activity of the
serum, brain, erythrocytes and submaxillary glands of rats fed 50 and
100 ppm was markedly inhibited. The inhibition was most marked in the
erythrocytes and brain and the animals did not fully recover from
these effects during a 3-week period on a normal diet.
Both gross and microscopic examination failed to indicate any
evidence of testicular atrophy due to the presence of the high levels
of azinphos-methyl in the diet (Doull et al., 1951).
Dog. Groups of 2 dogs, 1 male and 1 female, given 5, 10, 20 and
50 ppm of azinphos-methyl did not show any loss of weight nor any
symptoms of azinphos-methyl poisoning during a 12-week period. At
dietary levels of 5, 10 and 20 ppm there was no significant decrease
but at 50 ppm there was a 25% decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the 1 male and 1 female dog began to be inhibited at
10 ppm in the diet (DuBois, 1957).
Long-term studies
No data available.
Comments on experimental studies reported
Satisfactory studies have been carried out in rats but in the
studies in dogs only a very small number of animals was used.
EVALUATION
Level causing no significant toxicological effect in rat
5 ppm in the diet, equivalent to 0.25 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Doull, J., Amido, P. & DuBois, K. P. (1951) Unpublished report, 5 June
DuBois, K. P. (1956) University of Chicago. Unpublished final report,
3 May
DuBois, K. P. (1957) University of Chicago. Unpublished final report,
1 June
DuBois, K. P., Thursh, D. R. & Murphy, S. D. (1957) J. Pharmacol.
exp. Ther., 119, 208
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Sato, J. (1959) Kumamoto med. J., 12, 312 (from Chemical
Abstracts, 1960, 54, 21473)
Schrader, G. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
BIOLOGICAL DATA
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor. The molar I50 in rat brain is 2.99 × 10-8
(Schrader, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 11-25 DuBois et al., 1957;
Gaines, 1960
Rat Intraperitoneal 5-11.6 DuBois et al., 1957
Mouse Oral 8.0* Sato, 1959
Guinea-pig Oral 80.0 Dubois et al., 1957
Guinea-pig Intraperitoneal 40.0 DuBois et al., 1957
* Given as azinphos-methyl emulsion.
Short-term studies
Rat. The addition of azinphos-methyl to the diet of groups,
each 10 young male and 10 young female rats, at levels of 2, 5 and 20
ppm did not markedly alter the growth-rate over 60 days. The
body-weight of the male rats fed 20 ppm was about 4% less than that of
the controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10%) and in serum and erythrocytes (about 30%). After 120 days
no appreciable changes in the gross and microscopic appearance of
brain, heart, liver, spleen, adrenals, stomach, intestines, skeletal
muscle and bone marrow were found. There was no evidence of
demyelination in the nervous system (DuBois, 1956).
When weanling male rats were fed diets containing 50 and 100 ppm
of azinphos-methyl for 16 weeks, approximately half of each group
died. All animals receiving azinphos-methyl in the diet showed marked
cholinergic effects, including diarrhoea, salivation, lacrimation,
muscular tremors and fasciculations. These symptoms were most marked
during the first month on the diets. The rats fed 50 ppm of
azinphos-methyl weighed about 10% less and the rats fed 10 ppm, 18%
less than rats fed a normal diet. The cholinesterase activity of the
serum, brain, erythrocytes and submaxillary glands of rats fed 50 and
100 ppm was markedly inhibited. The inhibition was most marked in the
erythrocytes and brain and the animals did not fully recover from
these effects during a 3-week period on a normal diet.
Both gross and microscopic examination failed to indicate any
evidence of testicular atrophy due to the presence of the high levels
of azinphos-methyl in the diet (Doull et al., 1951).
Dog. Groups of 2 dogs, 1 male and 1 female, given 5, 10, 20 and
50 ppm of azinphos-methyl did not show any loss of weight nor any
symptoms of azinphos-methyl poisoning during a 12-week period. At
dietary levels of 5, 10 and 20 ppm there was no significant decrease
but at 50 ppm there was a 25% decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the 1 male and 1 female dog began to be inhibited at
10 ppm in the diet (DuBois, 1957).
Long-term studies
No data available.
Comments on experimental studies reported
Satisfactory studies have been carried out in rats but in the
studies in dogs only a very small number of animals was used.
EVALUATION
Level causing no significant toxicological effect in rat
5 ppm in the diet, equivalent to 0.25 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Doull, J., Amido, P. & DuBois, K. P. (1951) Unpublished report, 5 June
DuBois, K. P. (1956) University of Chicago. Unpublished final report,
3 May
DuBois, K. P. (1957) University of Chicago. Unpublished final report,
1 June
DuBois, K. P., Thursh, D. R. & Murphy, S. D. (1957) J. Pharmacol.
exp. Ther., 119, 208
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Sato, J. (1959) Kumamoto med. J., 12, 312 (from Chemical
Abstracts, 1960, 54, 21473)
Schrader, G. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
