
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
MALATHION
Chemical name
O,O-dimethyl S-[1,2-di-(ethoxycarbonyl)ethyl]
phosphorodithioate; S-[1,2-di(ethoxycarbonyl)ethyl]
dimethyl-phosphorothiolothionate; S-[1,2-di(ethoxycarbonyl)ethyl]
dimethyl-phosphorothiolothionate; S[1, 2-bis
(ethoxycarbonyl)ethyl]-O,O-dimethyl phosphorodithioate.
Synonyms
Carbofos, malathon
Empirical formula
C10H19O6S2P
Structural formula
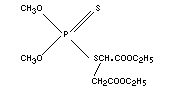 BIOLOGICAL DATA
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolysed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. In eggs from treated hens and milk from
cows treated with malathion, malathion or its metabolites were
recovered (O'Brien, 1960; Heath, 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro
(I50 7 × 10-7) (Heath, 1961).
The half time for the conversion in vivo of the reversibly
inhibited form of the dimethylphosphorilated cholinesterase to the
irreversibly inhibited form of this enzyme in the brain of chicken
given malathion has been found to be 2 hours. The same half time was
observed in vitro with the brain homogenate inhibited with paraoxon
(Witter & Gaines, 1964).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957; O'Brien, 1960).
In a colony of rats showing an oral LD50 of 925 mg/kg for
adults, the intragastric LD50 for a day-old rat was approximately 124
mg/kg (Lu et al., 1965).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm
for 6 weeks or after the administration of 5 oral doses of 500 mg/kg
the production of antibodies against B. Pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given Malathion at 100 or 500 ppm
in the diet or dipterex at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around
100% of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerna, 1965).
In another experiment 95% technical malathion was fed to 3 groups
of male rats, 10 animals per group, for 33 days at the levels of 100,
1000 and 5000 ppm. No sign of toxicity was observed, nor any deaths.
Food intake and weight gain in the groups fed 100 and 1000 ppm were
higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68% of normal in the
1000 ppm group, and in the 5000 ppm group plasm cholinesterase
activity was 78% and erythrocyte activity 22% of normal. At all levels
no depression of brain cholinesterase activity was found (American
Cyanamid Co., 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazelton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Intraperitoneal 420.474 Hazleton & Holland, 1953
Chicken Oral >850 (95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560 (95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
Ninety-eight per cent. technical malathion was fed to groups of 5
rats for 8 weeks at levels of 100 and 500 ppm without any inhibition
of whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent. technical malathion was fed to 40 male and
40 female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten wake after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. 95% technical malathion was fed to day-old chicks for
2 weeks at a level of 10 ppm. For the following 10 weeks they were
divided into groups of 10 and fed 100, 1000 and 5000 ppm in their
diets. The groups on 100 and 1000 ppm behaved normally and showed a
similar growth rate and food consumption to the controls. Four animals
died in the 5000 ppm group, and signs of intoxication and growth
retardation were observed. At autopsy, no pathological lesions were
found. Plasm and brain cholinesterase activity were significantly
lowered in the 5000 ppm group (American Cyanamid Co., 1955).
In a two-year study, 21 females were fed 250 ppm and 21 females
and 6 males 2500 ppm. The 250 ppm group did not differ significantly
from the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The hens came later into production and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At autopsy no macro- or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 8 mg of
malathion in gelatin capsules daily for 32 days. No effect on plasma
or erythrocyte cholinesterase activity could be detected. Five males
took 16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks after the first administration. Maximum depression amounting
to about 25% of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a light inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted, in 10
humans ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in
5 humans receiving 6 mg EPN for 88 days and 8 mg malathion for the
last 44 days, nor in 5 humans ingesting 16 mg malathion for 88 days
and 3 mg EPN for the last 41 days. However 10 humans ingesting 6 mg
EPN and 16 mg malathion daily for 42 days showed a slight depression
of both the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. 65% technical malathion as a 10% or 25% wettable powder
was mixed in the diets of groups of 20 male rats at the levels 100,
1000 and 5000 ppm, and fed for 2 years. The mortality rate was not
influenced, and at the 2 lower levels weight gain and food intake were
comparable to those of the controls. Five thousand ppm reduced food
intake and decreased weight gain. Cholinesterase determinations showed
no inhibition at the 100 ppm level; with a diet containing 1000 ppm,
36% inhibition of cholinesterase activity was found in the plasma, 73%
in the erythrocytes and 37% in the brain, while at the 5000 ppm level,
the plasma samples showed 80%, the erythrocytes 100% and the brain 77%
inhibition. At autopsy neither gross nor microscopic examination
revealed any pathological changes attributable to malathion (American
Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent. technical malathion was fed as 25% wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10.30% inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95% inhibition of erythrocyte cholinesterase
activity was observed. The 5000 ppm group showed total inhibition of
erythrocyte cholinesterase activity and 60-95% inhibition of
cholinesterase activity in plasma and brain (American Cyanamid Co.,
1955; Hazleton & Holland, 1953).
Ninety-nine per cent. technical malathion was fed for 2 years to
groups of 3-4 rats and produced, at 1000 and 5000 ppm levels,
inhibition of erythrocyte cholinesterase activity of the same order as
did the 90% compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90% technical
malathion (American Cyanamid Co., 1955, Hazleton & Holland, 1953).
Comments on experimental studies reported
The studies are extensive and have been carried out in several
species including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
EVALUATION
Level causing no significant toxicological effect in the rat and man
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per
day.
Man. 16 mg a day, equivalent to 0.2 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight.
Further work considered desirable
Reproduction studies in rats.
REFERENCES
American Cyanamid Company, New York (1955) Report on Malathion
American Cyanamid Company, New York (1960) Malathion pharmacology
and toxicology (Unpublished data)
Benes, V. & Cerna, V. (1965) Czech. Hyg., 10 (In press)
Benes, V., Pekarek, J. & Cerna, V. (1963) Czech. Hyg., 8, 3
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol, exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. G. (1953) Arch. industr. Hyg., 8,
399
Heath, D. F. (1961) Organophosphorus poisons, Pergamon Press
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3 (In press)
Moeller, H. C. & Rider, J. A. (1962) Toxicol. Appl. Pharmacol., 4,
123
O'Brien, R. D. (1960) Toxic phosphorus esters, Academic Press
Rider, J. A., Moeller, H. C., Swader, T. & Devereaux, R. J. (1959)
Chemical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1964) Biochem. Pharmacol., 12, 1421
BIOLOGICAL DATA
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolysed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. In eggs from treated hens and milk from
cows treated with malathion, malathion or its metabolites were
recovered (O'Brien, 1960; Heath, 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro
(I50 7 × 10-7) (Heath, 1961).
The half time for the conversion in vivo of the reversibly
inhibited form of the dimethylphosphorilated cholinesterase to the
irreversibly inhibited form of this enzyme in the brain of chicken
given malathion has been found to be 2 hours. The same half time was
observed in vitro with the brain homogenate inhibited with paraoxon
(Witter & Gaines, 1964).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957; O'Brien, 1960).
In a colony of rats showing an oral LD50 of 925 mg/kg for
adults, the intragastric LD50 for a day-old rat was approximately 124
mg/kg (Lu et al., 1965).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm
for 6 weeks or after the administration of 5 oral doses of 500 mg/kg
the production of antibodies against B. Pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given Malathion at 100 or 500 ppm
in the diet or dipterex at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around
100% of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerna, 1965).
In another experiment 95% technical malathion was fed to 3 groups
of male rats, 10 animals per group, for 33 days at the levels of 100,
1000 and 5000 ppm. No sign of toxicity was observed, nor any deaths.
Food intake and weight gain in the groups fed 100 and 1000 ppm were
higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68% of normal in the
1000 ppm group, and in the 5000 ppm group plasm cholinesterase
activity was 78% and erythrocyte activity 22% of normal. At all levels
no depression of brain cholinesterase activity was found (American
Cyanamid Co., 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazelton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Intraperitoneal 420.474 Hazleton & Holland, 1953
Chicken Oral >850 (95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560 (95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
Ninety-eight per cent. technical malathion was fed to groups of 5
rats for 8 weeks at levels of 100 and 500 ppm without any inhibition
of whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent. technical malathion was fed to 40 male and
40 female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten wake after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. 95% technical malathion was fed to day-old chicks for
2 weeks at a level of 10 ppm. For the following 10 weeks they were
divided into groups of 10 and fed 100, 1000 and 5000 ppm in their
diets. The groups on 100 and 1000 ppm behaved normally and showed a
similar growth rate and food consumption to the controls. Four animals
died in the 5000 ppm group, and signs of intoxication and growth
retardation were observed. At autopsy, no pathological lesions were
found. Plasm and brain cholinesterase activity were significantly
lowered in the 5000 ppm group (American Cyanamid Co., 1955).
In a two-year study, 21 females were fed 250 ppm and 21 females
and 6 males 2500 ppm. The 250 ppm group did not differ significantly
from the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The hens came later into production and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At autopsy no macro- or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 8 mg of
malathion in gelatin capsules daily for 32 days. No effect on plasma
or erythrocyte cholinesterase activity could be detected. Five males
took 16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks after the first administration. Maximum depression amounting
to about 25% of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a light inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted, in 10
humans ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in
5 humans receiving 6 mg EPN for 88 days and 8 mg malathion for the
last 44 days, nor in 5 humans ingesting 16 mg malathion for 88 days
and 3 mg EPN for the last 41 days. However 10 humans ingesting 6 mg
EPN and 16 mg malathion daily for 42 days showed a slight depression
of both the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. 65% technical malathion as a 10% or 25% wettable powder
was mixed in the diets of groups of 20 male rats at the levels 100,
1000 and 5000 ppm, and fed for 2 years. The mortality rate was not
influenced, and at the 2 lower levels weight gain and food intake were
comparable to those of the controls. Five thousand ppm reduced food
intake and decreased weight gain. Cholinesterase determinations showed
no inhibition at the 100 ppm level; with a diet containing 1000 ppm,
36% inhibition of cholinesterase activity was found in the plasma, 73%
in the erythrocytes and 37% in the brain, while at the 5000 ppm level,
the plasma samples showed 80%, the erythrocytes 100% and the brain 77%
inhibition. At autopsy neither gross nor microscopic examination
revealed any pathological changes attributable to malathion (American
Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent. technical malathion was fed as 25% wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10.30% inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95% inhibition of erythrocyte cholinesterase
activity was observed. The 5000 ppm group showed total inhibition of
erythrocyte cholinesterase activity and 60-95% inhibition of
cholinesterase activity in plasma and brain (American Cyanamid Co.,
1955; Hazleton & Holland, 1953).
Ninety-nine per cent. technical malathion was fed for 2 years to
groups of 3-4 rats and produced, at 1000 and 5000 ppm levels,
inhibition of erythrocyte cholinesterase activity of the same order as
did the 90% compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90% technical
malathion (American Cyanamid Co., 1955, Hazleton & Holland, 1953).
Comments on experimental studies reported
The studies are extensive and have been carried out in several
species including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
EVALUATION
Level causing no significant toxicological effect in the rat and man
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per
day.
Man. 16 mg a day, equivalent to 0.2 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight.
Further work considered desirable
Reproduction studies in rats.
REFERENCES
American Cyanamid Company, New York (1955) Report on Malathion
American Cyanamid Company, New York (1960) Malathion pharmacology
and toxicology (Unpublished data)
Benes, V. & Cerna, V. (1965) Czech. Hyg., 10 (In press)
Benes, V., Pekarek, J. & Cerna, V. (1963) Czech. Hyg., 8, 3
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol, exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. G. (1953) Arch. industr. Hyg., 8,
399
Heath, D. F. (1961) Organophosphorus poisons, Pergamon Press
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3 (In press)
Moeller, H. C. & Rider, J. A. (1962) Toxicol. Appl. Pharmacol., 4,
123
O'Brien, R. D. (1960) Toxic phosphorus esters, Academic Press
Rider, J. A., Moeller, H. C., Swader, T. & Devereaux, R. J. (1959)
Chemical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1964) Biochem. Pharmacol., 12, 1421
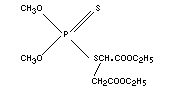 BIOLOGICAL DATA
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolysed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. In eggs from treated hens and milk from
cows treated with malathion, malathion or its metabolites were
recovered (O'Brien, 1960; Heath, 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro
(I50 7 × 10-7) (Heath, 1961).
The half time for the conversion in vivo of the reversibly
inhibited form of the dimethylphosphorilated cholinesterase to the
irreversibly inhibited form of this enzyme in the brain of chicken
given malathion has been found to be 2 hours. The same half time was
observed in vitro with the brain homogenate inhibited with paraoxon
(Witter & Gaines, 1964).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957; O'Brien, 1960).
In a colony of rats showing an oral LD50 of 925 mg/kg for
adults, the intragastric LD50 for a day-old rat was approximately 124
mg/kg (Lu et al., 1965).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm
for 6 weeks or after the administration of 5 oral doses of 500 mg/kg
the production of antibodies against B. Pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given Malathion at 100 or 500 ppm
in the diet or dipterex at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around
100% of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerna, 1965).
In another experiment 95% technical malathion was fed to 3 groups
of male rats, 10 animals per group, for 33 days at the levels of 100,
1000 and 5000 ppm. No sign of toxicity was observed, nor any deaths.
Food intake and weight gain in the groups fed 100 and 1000 ppm were
higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68% of normal in the
1000 ppm group, and in the 5000 ppm group plasm cholinesterase
activity was 78% and erythrocyte activity 22% of normal. At all levels
no depression of brain cholinesterase activity was found (American
Cyanamid Co., 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazelton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Intraperitoneal 420.474 Hazleton & Holland, 1953
Chicken Oral >850 (95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560 (95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
Ninety-eight per cent. technical malathion was fed to groups of 5
rats for 8 weeks at levels of 100 and 500 ppm without any inhibition
of whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent. technical malathion was fed to 40 male and
40 female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten wake after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. 95% technical malathion was fed to day-old chicks for
2 weeks at a level of 10 ppm. For the following 10 weeks they were
divided into groups of 10 and fed 100, 1000 and 5000 ppm in their
diets. The groups on 100 and 1000 ppm behaved normally and showed a
similar growth rate and food consumption to the controls. Four animals
died in the 5000 ppm group, and signs of intoxication and growth
retardation were observed. At autopsy, no pathological lesions were
found. Plasm and brain cholinesterase activity were significantly
lowered in the 5000 ppm group (American Cyanamid Co., 1955).
In a two-year study, 21 females were fed 250 ppm and 21 females
and 6 males 2500 ppm. The 250 ppm group did not differ significantly
from the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The hens came later into production and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At autopsy no macro- or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 8 mg of
malathion in gelatin capsules daily for 32 days. No effect on plasma
or erythrocyte cholinesterase activity could be detected. Five males
took 16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks after the first administration. Maximum depression amounting
to about 25% of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a light inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted, in 10
humans ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in
5 humans receiving 6 mg EPN for 88 days and 8 mg malathion for the
last 44 days, nor in 5 humans ingesting 16 mg malathion for 88 days
and 3 mg EPN for the last 41 days. However 10 humans ingesting 6 mg
EPN and 16 mg malathion daily for 42 days showed a slight depression
of both the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. 65% technical malathion as a 10% or 25% wettable powder
was mixed in the diets of groups of 20 male rats at the levels 100,
1000 and 5000 ppm, and fed for 2 years. The mortality rate was not
influenced, and at the 2 lower levels weight gain and food intake were
comparable to those of the controls. Five thousand ppm reduced food
intake and decreased weight gain. Cholinesterase determinations showed
no inhibition at the 100 ppm level; with a diet containing 1000 ppm,
36% inhibition of cholinesterase activity was found in the plasma, 73%
in the erythrocytes and 37% in the brain, while at the 5000 ppm level,
the plasma samples showed 80%, the erythrocytes 100% and the brain 77%
inhibition. At autopsy neither gross nor microscopic examination
revealed any pathological changes attributable to malathion (American
Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent. technical malathion was fed as 25% wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10.30% inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95% inhibition of erythrocyte cholinesterase
activity was observed. The 5000 ppm group showed total inhibition of
erythrocyte cholinesterase activity and 60-95% inhibition of
cholinesterase activity in plasma and brain (American Cyanamid Co.,
1955; Hazleton & Holland, 1953).
Ninety-nine per cent. technical malathion was fed for 2 years to
groups of 3-4 rats and produced, at 1000 and 5000 ppm levels,
inhibition of erythrocyte cholinesterase activity of the same order as
did the 90% compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90% technical
malathion (American Cyanamid Co., 1955, Hazleton & Holland, 1953).
Comments on experimental studies reported
The studies are extensive and have been carried out in several
species including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
EVALUATION
Level causing no significant toxicological effect in the rat and man
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per
day.
Man. 16 mg a day, equivalent to 0.2 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight.
Further work considered desirable
Reproduction studies in rats.
REFERENCES
American Cyanamid Company, New York (1955) Report on Malathion
American Cyanamid Company, New York (1960) Malathion pharmacology
and toxicology (Unpublished data)
Benes, V. & Cerna, V. (1965) Czech. Hyg., 10 (In press)
Benes, V., Pekarek, J. & Cerna, V. (1963) Czech. Hyg., 8, 3
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol, exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. G. (1953) Arch. industr. Hyg., 8,
399
Heath, D. F. (1961) Organophosphorus poisons, Pergamon Press
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3 (In press)
Moeller, H. C. & Rider, J. A. (1962) Toxicol. Appl. Pharmacol., 4,
123
O'Brien, R. D. (1960) Toxic phosphorus esters, Academic Press
Rider, J. A., Moeller, H. C., Swader, T. & Devereaux, R. J. (1959)
Chemical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1964) Biochem. Pharmacol., 12, 1421
BIOLOGICAL DATA
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolysed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. In eggs from treated hens and milk from
cows treated with malathion, malathion or its metabolites were
recovered (O'Brien, 1960; Heath, 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro
(I50 7 × 10-7) (Heath, 1961).
The half time for the conversion in vivo of the reversibly
inhibited form of the dimethylphosphorilated cholinesterase to the
irreversibly inhibited form of this enzyme in the brain of chicken
given malathion has been found to be 2 hours. The same half time was
observed in vitro with the brain homogenate inhibited with paraoxon
(Witter & Gaines, 1964).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957; O'Brien, 1960).
In a colony of rats showing an oral LD50 of 925 mg/kg for
adults, the intragastric LD50 for a day-old rat was approximately 124
mg/kg (Lu et al., 1965).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm
for 6 weeks or after the administration of 5 oral doses of 500 mg/kg
the production of antibodies against B. Pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given Malathion at 100 or 500 ppm
in the diet or dipterex at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around
100% of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerna, 1965).
In another experiment 95% technical malathion was fed to 3 groups
of male rats, 10 animals per group, for 33 days at the levels of 100,
1000 and 5000 ppm. No sign of toxicity was observed, nor any deaths.
Food intake and weight gain in the groups fed 100 and 1000 ppm were
higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68% of normal in the
1000 ppm group, and in the 5000 ppm group plasm cholinesterase
activity was 78% and erythrocyte activity 22% of normal. At all levels
no depression of brain cholinesterase activity was found (American
Cyanamid Co., 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazelton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Intraperitoneal 420.474 Hazleton & Holland, 1953
Chicken Oral >850 (95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560 (95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
Ninety-eight per cent. technical malathion was fed to groups of 5
rats for 8 weeks at levels of 100 and 500 ppm without any inhibition
of whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent. technical malathion was fed to 40 male and
40 female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten wake after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. 95% technical malathion was fed to day-old chicks for
2 weeks at a level of 10 ppm. For the following 10 weeks they were
divided into groups of 10 and fed 100, 1000 and 5000 ppm in their
diets. The groups on 100 and 1000 ppm behaved normally and showed a
similar growth rate and food consumption to the controls. Four animals
died in the 5000 ppm group, and signs of intoxication and growth
retardation were observed. At autopsy, no pathological lesions were
found. Plasm and brain cholinesterase activity were significantly
lowered in the 5000 ppm group (American Cyanamid Co., 1955).
In a two-year study, 21 females were fed 250 ppm and 21 females
and 6 males 2500 ppm. The 250 ppm group did not differ significantly
from the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The hens came later into production and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At autopsy no macro- or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 8 mg of
malathion in gelatin capsules daily for 32 days. No effect on plasma
or erythrocyte cholinesterase activity could be detected. Five males
took 16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks after the first administration. Maximum depression amounting
to about 25% of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a light inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted, in 10
humans ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in
5 humans receiving 6 mg EPN for 88 days and 8 mg malathion for the
last 44 days, nor in 5 humans ingesting 16 mg malathion for 88 days
and 3 mg EPN for the last 41 days. However 10 humans ingesting 6 mg
EPN and 16 mg malathion daily for 42 days showed a slight depression
of both the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. 65% technical malathion as a 10% or 25% wettable powder
was mixed in the diets of groups of 20 male rats at the levels 100,
1000 and 5000 ppm, and fed for 2 years. The mortality rate was not
influenced, and at the 2 lower levels weight gain and food intake were
comparable to those of the controls. Five thousand ppm reduced food
intake and decreased weight gain. Cholinesterase determinations showed
no inhibition at the 100 ppm level; with a diet containing 1000 ppm,
36% inhibition of cholinesterase activity was found in the plasma, 73%
in the erythrocytes and 37% in the brain, while at the 5000 ppm level,
the plasma samples showed 80%, the erythrocytes 100% and the brain 77%
inhibition. At autopsy neither gross nor microscopic examination
revealed any pathological changes attributable to malathion (American
Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent. technical malathion was fed as 25% wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10.30% inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95% inhibition of erythrocyte cholinesterase
activity was observed. The 5000 ppm group showed total inhibition of
erythrocyte cholinesterase activity and 60-95% inhibition of
cholinesterase activity in plasma and brain (American Cyanamid Co.,
1955; Hazleton & Holland, 1953).
Ninety-nine per cent. technical malathion was fed for 2 years to
groups of 3-4 rats and produced, at 1000 and 5000 ppm levels,
inhibition of erythrocyte cholinesterase activity of the same order as
did the 90% compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90% technical
malathion (American Cyanamid Co., 1955, Hazleton & Holland, 1953).
Comments on experimental studies reported
The studies are extensive and have been carried out in several
species including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
EVALUATION
Level causing no significant toxicological effect in the rat and man
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per
day.
Man. 16 mg a day, equivalent to 0.2 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.02 mg/kg body-weight.
Further work considered desirable
Reproduction studies in rats.
REFERENCES
American Cyanamid Company, New York (1955) Report on Malathion
American Cyanamid Company, New York (1960) Malathion pharmacology
and toxicology (Unpublished data)
Benes, V. & Cerna, V. (1965) Czech. Hyg., 10 (In press)
Benes, V., Pekarek, J. & Cerna, V. (1963) Czech. Hyg., 8, 3
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol, exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. G. (1953) Arch. industr. Hyg., 8,
399
Heath, D. F. (1961) Organophosphorus poisons, Pergamon Press
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3 (In press)
Moeller, H. C. & Rider, J. A. (1962) Toxicol. Appl. Pharmacol., 4,
123
O'Brien, R. D. (1960) Toxic phosphorus esters, Academic Press
Rider, J. A., Moeller, H. C., Swader, T. & Devereaux, R. J. (1959)
Chemical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1964) Biochem. Pharmacol., 12, 1421
