
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
MEVINPHOS
Chemical name
2-methoxycarbonyl-1-methylvinyl dimethyl phosphate,
1-carbomethoxy 1-propen-2-yl dimethyl phosphate;
dimethyl-1-carbomethoxy-1-propenyl-2-phosphate;
dimethyl-2-methoxycarbomyl-1-methylvinyl-phosphate,
2-carbomethoxy-1-methylvinyl dimethyl phosphate.
Synonym
Phosdrin
Empirical formula
C7H13O6P
Structural formula
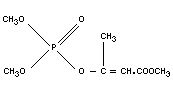 BIOLOGICAL DATA
Biochemical aspects
The half-line of the alpha-isomer in the plant is approximately
20 hours, while that of the ß-isomer is about 48 hours (Casida et al.,
1956). The principal metabolite is dimethyl phosphate (Casida et al.,
1956; O'Brien, 1960).
Mevinphos is hydrolysed to dimethylphosphoric acid by human and
bovine plasma (Casida et al., 1958). It is rapidly absorbed from the
intestinal tract and the skin (Casida et al., 1958; Gaines, 1960).
There is no tendency to accumulate in tissues and excretion takes
place rapidly as dimethylphosphoric acid in urine. Only hydrolysis
products were found in the milk from treated cows (Casida et al.,
1958).
Mevinphos is a direct cholinesterase-inhibitor. The molar I50
of the ß-isomer, in 60 minutes, is 1.2 × 10-6 and that of the
alpha-isomer 1.7 × 10-8 using human whole blood (Casida, 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral propylene glycol 4.3-6.8 Kodama et al., 1954
Rat, male Oral propylene glycol 6.0-6.8 Kodama et al., 1954
Rat, male Oral corn oil and 3.7-6.1 Gaines, 1960
peanut oil Kettering Lab., 1957b
Rat, female Intraperitoneal propylene glycol 1.5 Kodama et al., 1954
Rat Intraperitoneal 0.35 (alpha-) Casida, 1955
35 (beta-) Casida, 1955
Short-term studies
Rat. Groups, each of 6 female rats, were fed for 60 days on
diets containing 6.3, 12.5, 50 and 100 ppm of mevinphos. All the
animals in the 100-ppm group died during the third week. Slight
tremors were observed at all the lower levels. Weight gain was reduced
on 50 ppm. No significant histopathological changes were found (Kodama
et al., 1954). Cholinesterase determinations on plasma, erythrocytes
and brain all showed inhibition of activity proportional to the dose;
e.g., in the brain, 90% of the normal value was found at 6.3 ppm, 40%
at 12.5 ppm And 20% at 50 ppm.
Groups, each of 12 male and 12 female rats, were fed 25, 50, 100
and 200 ppm in their diets for 13 weeks. There was a slight increase
in mortality at 200 ppm and growth depression at the 100- and 200-ppm
levels. Clinical signs of intoxication were minimal at 25 ppm but
increased progressively with increasing concentration. Degeneration of
the epithelial cells lining ducts and acini were found in the
sub-maxillary, sublingual, parotid, Harderian and lacrimal glands, and
in the thymus and pancreas, most prominently in the male (Cleveland &
Treon, 1961).
Using groups of 30 male and 30 female rats, the same group of
investigators found inhibition of cholinesterase activity at low-level
feeding. The diets contained 0.32, 0.8, 2.0 and 5.0 ppm of mevinphos
and one male and one female were killed daily for determination of
brain cholinesterase activity during the second, fourth, eighth and
twelfth week of the experiment. On the basis of statistical analysis
it was concluded that the decrease in erythrocyte cholinesterase
activity became significant when the diets of males contained 1.1 ppm
and that of females 1.3 ppm mevinphos. A concentration in the diet
greater than 5 ppm was required to cause a significant reduction in
the cholinesterase activity of the plasm or brain of either sex
(Kettering Lab., 1957a).
Dog. Groups of 4 dogs were fed 0.3, 1,0, 2.5, 5.0, 75 and 200
ppm in their diets for 14 weeks. All the animals in the 200-ppm group
died. The dogs on 75 ppm level showed mild clinical signs of
intoxication and failed to gain weight. At autopsy, pathological
lesions of the exocrine glands were seen. The groups on the lower
levels behaved similarly to the control group as regards weight gain
and post-mortem examination. Determinations of cholinesterase
activities showed that 0.3 and 1.0 ppm had no effect on erythrocyte or
plasma cholinesterase activity. 2.5 ppm lowered erythrocyte
cholinesterase activity to 80%, 5 ppm to 70% of normal values. On 5
ppm plasm cholinesterase activity was 90% of normal and brain
cholinesterase activity was not affected by 0.3-5.0 ppm in the diet
(Cleveland & Treon, 1961; Kettering Lab., 1957c).
Five dogs fed 1 ppm mevinphos for 6 weeks together with either
1 ppm parathion, 2 ppm demeton, 5 ppm methylparathion, 20 ppm EPN or
100 ppm malathion, showed no significant inhibition of cholinesterase
activity of the plasma or the erythrocytes. Feeding experiments on 32
female dogs with combinations of the above-mentioned organophosphates
in the stated concentrations did not show evidence of potentiation
when mevinphos was fed in combination with any of the compounds for 6
weeks (Kettering Lab., 1961).
Cattle. Twelve bull calves 1.5 to 4 weeks of age, divided into
groups of 3, were fed mevinphos in a milk diet at concentrations
equivalent to 0.02, 0.2 and 2.0 mg/kg body-weight per day for 3 weeks.
Cholinesterase determinations on whole blood revealed a progressive
decrease in enzyme activity. At 0.02 mg/kg body-weight activity
dropped to 75% of normal, at 0.2 mg/kg body-weight to 40% and at 2
mg/kg body-weight to below 5%. Only the group on 2 mg/kg body-weight
showed signs of intoxication. During an additional 4-week period the
cholinesterase activities returned almost to normal (Casida et al.,
1958).
Twelve lactating dairy cows in groups of 3 were given mevinphos
in capsules at the rate of 0.026, 0.13 and 0.52 mg/kg body-weight per
day for 12 weeks. The two highest doses depressed the rate of weight
gain but there was no evident effect on milk production or milk-fat
content. Signs of intoxication were not observed and no gross
pathological lesions were found. Erythrocyte cholinesterase activity
was not significantly lowered by 0.026 mg/kg body-weight per day. 0.13
mg/kg body-weight per day gradually decreased the activity to about
30% and 0.52 mg/kg body-weight per day to below 20% of the initial
value (Casida et al., 1958).
Long-term studies
No data available.
Comments on experimental studies reported and evaluation
From the present data a no-effect level in the dog would appear
to be 1 ppm, in the rat 0.8 ppm and in young calves <0.2 ppm. The
short duration of these studies, in view of the progressive decrease
in cholinesterase activity after minimal daily oral doses, makes it
impossible to determine an acceptable daily intake for man.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Casida, J. E. (1955) Science, 122, 597
Casida, J. E. et al. (1956) J. Agr. Food Chem. 4, 236
Casida, J. E., Gatterdam, P. E., Knaak, J. B., Lance, R. D. &
Niedermeier, R. P. (1958) J. Agr. Food Chem., 6, 658
Cleveland, F. P. & Treon, J. F. (1961) J. Agr. Food Chem., 9, 484
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Kettering Laboratory, Cincinnati, Ohio (1957a) Unpublished report,
15 March
Kettering Laboratory, Cincinnati, Ohio (1957b) Unpublished report,
22 March
Kettering Laboratory, Cincinnati, Ohio (1957c) Unpublished report,
19 June
Kettering Laboratory, Cincinnati, Ohio (1961) Unpublished report, 28
September
Kodama, J. K. et al. (1954) Arch. industr. Hyg., 9, 45
O'Brien, R. D. (1960) Toxic Phosphorus Esters, Academic Press, New
York
BIOLOGICAL DATA
Biochemical aspects
The half-line of the alpha-isomer in the plant is approximately
20 hours, while that of the ß-isomer is about 48 hours (Casida et al.,
1956). The principal metabolite is dimethyl phosphate (Casida et al.,
1956; O'Brien, 1960).
Mevinphos is hydrolysed to dimethylphosphoric acid by human and
bovine plasma (Casida et al., 1958). It is rapidly absorbed from the
intestinal tract and the skin (Casida et al., 1958; Gaines, 1960).
There is no tendency to accumulate in tissues and excretion takes
place rapidly as dimethylphosphoric acid in urine. Only hydrolysis
products were found in the milk from treated cows (Casida et al.,
1958).
Mevinphos is a direct cholinesterase-inhibitor. The molar I50
of the ß-isomer, in 60 minutes, is 1.2 × 10-6 and that of the
alpha-isomer 1.7 × 10-8 using human whole blood (Casida, 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral propylene glycol 4.3-6.8 Kodama et al., 1954
Rat, male Oral propylene glycol 6.0-6.8 Kodama et al., 1954
Rat, male Oral corn oil and 3.7-6.1 Gaines, 1960
peanut oil Kettering Lab., 1957b
Rat, female Intraperitoneal propylene glycol 1.5 Kodama et al., 1954
Rat Intraperitoneal 0.35 (alpha-) Casida, 1955
35 (beta-) Casida, 1955
Short-term studies
Rat. Groups, each of 6 female rats, were fed for 60 days on
diets containing 6.3, 12.5, 50 and 100 ppm of mevinphos. All the
animals in the 100-ppm group died during the third week. Slight
tremors were observed at all the lower levels. Weight gain was reduced
on 50 ppm. No significant histopathological changes were found (Kodama
et al., 1954). Cholinesterase determinations on plasma, erythrocytes
and brain all showed inhibition of activity proportional to the dose;
e.g., in the brain, 90% of the normal value was found at 6.3 ppm, 40%
at 12.5 ppm And 20% at 50 ppm.
Groups, each of 12 male and 12 female rats, were fed 25, 50, 100
and 200 ppm in their diets for 13 weeks. There was a slight increase
in mortality at 200 ppm and growth depression at the 100- and 200-ppm
levels. Clinical signs of intoxication were minimal at 25 ppm but
increased progressively with increasing concentration. Degeneration of
the epithelial cells lining ducts and acini were found in the
sub-maxillary, sublingual, parotid, Harderian and lacrimal glands, and
in the thymus and pancreas, most prominently in the male (Cleveland &
Treon, 1961).
Using groups of 30 male and 30 female rats, the same group of
investigators found inhibition of cholinesterase activity at low-level
feeding. The diets contained 0.32, 0.8, 2.0 and 5.0 ppm of mevinphos
and one male and one female were killed daily for determination of
brain cholinesterase activity during the second, fourth, eighth and
twelfth week of the experiment. On the basis of statistical analysis
it was concluded that the decrease in erythrocyte cholinesterase
activity became significant when the diets of males contained 1.1 ppm
and that of females 1.3 ppm mevinphos. A concentration in the diet
greater than 5 ppm was required to cause a significant reduction in
the cholinesterase activity of the plasm or brain of either sex
(Kettering Lab., 1957a).
Dog. Groups of 4 dogs were fed 0.3, 1,0, 2.5, 5.0, 75 and 200
ppm in their diets for 14 weeks. All the animals in the 200-ppm group
died. The dogs on 75 ppm level showed mild clinical signs of
intoxication and failed to gain weight. At autopsy, pathological
lesions of the exocrine glands were seen. The groups on the lower
levels behaved similarly to the control group as regards weight gain
and post-mortem examination. Determinations of cholinesterase
activities showed that 0.3 and 1.0 ppm had no effect on erythrocyte or
plasma cholinesterase activity. 2.5 ppm lowered erythrocyte
cholinesterase activity to 80%, 5 ppm to 70% of normal values. On 5
ppm plasm cholinesterase activity was 90% of normal and brain
cholinesterase activity was not affected by 0.3-5.0 ppm in the diet
(Cleveland & Treon, 1961; Kettering Lab., 1957c).
Five dogs fed 1 ppm mevinphos for 6 weeks together with either
1 ppm parathion, 2 ppm demeton, 5 ppm methylparathion, 20 ppm EPN or
100 ppm malathion, showed no significant inhibition of cholinesterase
activity of the plasma or the erythrocytes. Feeding experiments on 32
female dogs with combinations of the above-mentioned organophosphates
in the stated concentrations did not show evidence of potentiation
when mevinphos was fed in combination with any of the compounds for 6
weeks (Kettering Lab., 1961).
Cattle. Twelve bull calves 1.5 to 4 weeks of age, divided into
groups of 3, were fed mevinphos in a milk diet at concentrations
equivalent to 0.02, 0.2 and 2.0 mg/kg body-weight per day for 3 weeks.
Cholinesterase determinations on whole blood revealed a progressive
decrease in enzyme activity. At 0.02 mg/kg body-weight activity
dropped to 75% of normal, at 0.2 mg/kg body-weight to 40% and at 2
mg/kg body-weight to below 5%. Only the group on 2 mg/kg body-weight
showed signs of intoxication. During an additional 4-week period the
cholinesterase activities returned almost to normal (Casida et al.,
1958).
Twelve lactating dairy cows in groups of 3 were given mevinphos
in capsules at the rate of 0.026, 0.13 and 0.52 mg/kg body-weight per
day for 12 weeks. The two highest doses depressed the rate of weight
gain but there was no evident effect on milk production or milk-fat
content. Signs of intoxication were not observed and no gross
pathological lesions were found. Erythrocyte cholinesterase activity
was not significantly lowered by 0.026 mg/kg body-weight per day. 0.13
mg/kg body-weight per day gradually decreased the activity to about
30% and 0.52 mg/kg body-weight per day to below 20% of the initial
value (Casida et al., 1958).
Long-term studies
No data available.
Comments on experimental studies reported and evaluation
From the present data a no-effect level in the dog would appear
to be 1 ppm, in the rat 0.8 ppm and in young calves <0.2 ppm. The
short duration of these studies, in view of the progressive decrease
in cholinesterase activity after minimal daily oral doses, makes it
impossible to determine an acceptable daily intake for man.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Casida, J. E. (1955) Science, 122, 597
Casida, J. E. et al. (1956) J. Agr. Food Chem. 4, 236
Casida, J. E., Gatterdam, P. E., Knaak, J. B., Lance, R. D. &
Niedermeier, R. P. (1958) J. Agr. Food Chem., 6, 658
Cleveland, F. P. & Treon, J. F. (1961) J. Agr. Food Chem., 9, 484
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Kettering Laboratory, Cincinnati, Ohio (1957a) Unpublished report,
15 March
Kettering Laboratory, Cincinnati, Ohio (1957b) Unpublished report,
22 March
Kettering Laboratory, Cincinnati, Ohio (1957c) Unpublished report,
19 June
Kettering Laboratory, Cincinnati, Ohio (1961) Unpublished report, 28
September
Kodama, J. K. et al. (1954) Arch. industr. Hyg., 9, 45
O'Brien, R. D. (1960) Toxic Phosphorus Esters, Academic Press, New
York
See Also:
Toxicological Abbreviations
Mevinphos (WHO Pesticide Residues Series 2)
Mevinphos (Pesticide residues in food: 1996 evaluations Part II Toxicological)
Mevinphos (Pesticide residues in food: 1997 evaluations Part II Toxicological & Environmental)
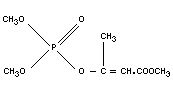 BIOLOGICAL DATA
Biochemical aspects
The half-line of the alpha-isomer in the plant is approximately
20 hours, while that of the ß-isomer is about 48 hours (Casida et al.,
1956). The principal metabolite is dimethyl phosphate (Casida et al.,
1956; O'Brien, 1960).
Mevinphos is hydrolysed to dimethylphosphoric acid by human and
bovine plasma (Casida et al., 1958). It is rapidly absorbed from the
intestinal tract and the skin (Casida et al., 1958; Gaines, 1960).
There is no tendency to accumulate in tissues and excretion takes
place rapidly as dimethylphosphoric acid in urine. Only hydrolysis
products were found in the milk from treated cows (Casida et al.,
1958).
Mevinphos is a direct cholinesterase-inhibitor. The molar I50
of the ß-isomer, in 60 minutes, is 1.2 × 10-6 and that of the
alpha-isomer 1.7 × 10-8 using human whole blood (Casida, 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral propylene glycol 4.3-6.8 Kodama et al., 1954
Rat, male Oral propylene glycol 6.0-6.8 Kodama et al., 1954
Rat, male Oral corn oil and 3.7-6.1 Gaines, 1960
peanut oil Kettering Lab., 1957b
Rat, female Intraperitoneal propylene glycol 1.5 Kodama et al., 1954
Rat Intraperitoneal 0.35 (alpha-) Casida, 1955
35 (beta-) Casida, 1955
Short-term studies
Rat. Groups, each of 6 female rats, were fed for 60 days on
diets containing 6.3, 12.5, 50 and 100 ppm of mevinphos. All the
animals in the 100-ppm group died during the third week. Slight
tremors were observed at all the lower levels. Weight gain was reduced
on 50 ppm. No significant histopathological changes were found (Kodama
et al., 1954). Cholinesterase determinations on plasma, erythrocytes
and brain all showed inhibition of activity proportional to the dose;
e.g., in the brain, 90% of the normal value was found at 6.3 ppm, 40%
at 12.5 ppm And 20% at 50 ppm.
Groups, each of 12 male and 12 female rats, were fed 25, 50, 100
and 200 ppm in their diets for 13 weeks. There was a slight increase
in mortality at 200 ppm and growth depression at the 100- and 200-ppm
levels. Clinical signs of intoxication were minimal at 25 ppm but
increased progressively with increasing concentration. Degeneration of
the epithelial cells lining ducts and acini were found in the
sub-maxillary, sublingual, parotid, Harderian and lacrimal glands, and
in the thymus and pancreas, most prominently in the male (Cleveland &
Treon, 1961).
Using groups of 30 male and 30 female rats, the same group of
investigators found inhibition of cholinesterase activity at low-level
feeding. The diets contained 0.32, 0.8, 2.0 and 5.0 ppm of mevinphos
and one male and one female were killed daily for determination of
brain cholinesterase activity during the second, fourth, eighth and
twelfth week of the experiment. On the basis of statistical analysis
it was concluded that the decrease in erythrocyte cholinesterase
activity became significant when the diets of males contained 1.1 ppm
and that of females 1.3 ppm mevinphos. A concentration in the diet
greater than 5 ppm was required to cause a significant reduction in
the cholinesterase activity of the plasm or brain of either sex
(Kettering Lab., 1957a).
Dog. Groups of 4 dogs were fed 0.3, 1,0, 2.5, 5.0, 75 and 200
ppm in their diets for 14 weeks. All the animals in the 200-ppm group
died. The dogs on 75 ppm level showed mild clinical signs of
intoxication and failed to gain weight. At autopsy, pathological
lesions of the exocrine glands were seen. The groups on the lower
levels behaved similarly to the control group as regards weight gain
and post-mortem examination. Determinations of cholinesterase
activities showed that 0.3 and 1.0 ppm had no effect on erythrocyte or
plasma cholinesterase activity. 2.5 ppm lowered erythrocyte
cholinesterase activity to 80%, 5 ppm to 70% of normal values. On 5
ppm plasm cholinesterase activity was 90% of normal and brain
cholinesterase activity was not affected by 0.3-5.0 ppm in the diet
(Cleveland & Treon, 1961; Kettering Lab., 1957c).
Five dogs fed 1 ppm mevinphos for 6 weeks together with either
1 ppm parathion, 2 ppm demeton, 5 ppm methylparathion, 20 ppm EPN or
100 ppm malathion, showed no significant inhibition of cholinesterase
activity of the plasma or the erythrocytes. Feeding experiments on 32
female dogs with combinations of the above-mentioned organophosphates
in the stated concentrations did not show evidence of potentiation
when mevinphos was fed in combination with any of the compounds for 6
weeks (Kettering Lab., 1961).
Cattle. Twelve bull calves 1.5 to 4 weeks of age, divided into
groups of 3, were fed mevinphos in a milk diet at concentrations
equivalent to 0.02, 0.2 and 2.0 mg/kg body-weight per day for 3 weeks.
Cholinesterase determinations on whole blood revealed a progressive
decrease in enzyme activity. At 0.02 mg/kg body-weight activity
dropped to 75% of normal, at 0.2 mg/kg body-weight to 40% and at 2
mg/kg body-weight to below 5%. Only the group on 2 mg/kg body-weight
showed signs of intoxication. During an additional 4-week period the
cholinesterase activities returned almost to normal (Casida et al.,
1958).
Twelve lactating dairy cows in groups of 3 were given mevinphos
in capsules at the rate of 0.026, 0.13 and 0.52 mg/kg body-weight per
day for 12 weeks. The two highest doses depressed the rate of weight
gain but there was no evident effect on milk production or milk-fat
content. Signs of intoxication were not observed and no gross
pathological lesions were found. Erythrocyte cholinesterase activity
was not significantly lowered by 0.026 mg/kg body-weight per day. 0.13
mg/kg body-weight per day gradually decreased the activity to about
30% and 0.52 mg/kg body-weight per day to below 20% of the initial
value (Casida et al., 1958).
Long-term studies
No data available.
Comments on experimental studies reported and evaluation
From the present data a no-effect level in the dog would appear
to be 1 ppm, in the rat 0.8 ppm and in young calves <0.2 ppm. The
short duration of these studies, in view of the progressive decrease
in cholinesterase activity after minimal daily oral doses, makes it
impossible to determine an acceptable daily intake for man.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Casida, J. E. (1955) Science, 122, 597
Casida, J. E. et al. (1956) J. Agr. Food Chem. 4, 236
Casida, J. E., Gatterdam, P. E., Knaak, J. B., Lance, R. D. &
Niedermeier, R. P. (1958) J. Agr. Food Chem., 6, 658
Cleveland, F. P. & Treon, J. F. (1961) J. Agr. Food Chem., 9, 484
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Kettering Laboratory, Cincinnati, Ohio (1957a) Unpublished report,
15 March
Kettering Laboratory, Cincinnati, Ohio (1957b) Unpublished report,
22 March
Kettering Laboratory, Cincinnati, Ohio (1957c) Unpublished report,
19 June
Kettering Laboratory, Cincinnati, Ohio (1961) Unpublished report, 28
September
Kodama, J. K. et al. (1954) Arch. industr. Hyg., 9, 45
O'Brien, R. D. (1960) Toxic Phosphorus Esters, Academic Press, New
York
BIOLOGICAL DATA
Biochemical aspects
The half-line of the alpha-isomer in the plant is approximately
20 hours, while that of the ß-isomer is about 48 hours (Casida et al.,
1956). The principal metabolite is dimethyl phosphate (Casida et al.,
1956; O'Brien, 1960).
Mevinphos is hydrolysed to dimethylphosphoric acid by human and
bovine plasma (Casida et al., 1958). It is rapidly absorbed from the
intestinal tract and the skin (Casida et al., 1958; Gaines, 1960).
There is no tendency to accumulate in tissues and excretion takes
place rapidly as dimethylphosphoric acid in urine. Only hydrolysis
products were found in the milk from treated cows (Casida et al.,
1958).
Mevinphos is a direct cholinesterase-inhibitor. The molar I50
of the ß-isomer, in 60 minutes, is 1.2 × 10-6 and that of the
alpha-isomer 1.7 × 10-8 using human whole blood (Casida, 1955).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral propylene glycol 4.3-6.8 Kodama et al., 1954
Rat, male Oral propylene glycol 6.0-6.8 Kodama et al., 1954
Rat, male Oral corn oil and 3.7-6.1 Gaines, 1960
peanut oil Kettering Lab., 1957b
Rat, female Intraperitoneal propylene glycol 1.5 Kodama et al., 1954
Rat Intraperitoneal 0.35 (alpha-) Casida, 1955
35 (beta-) Casida, 1955
Short-term studies
Rat. Groups, each of 6 female rats, were fed for 60 days on
diets containing 6.3, 12.5, 50 and 100 ppm of mevinphos. All the
animals in the 100-ppm group died during the third week. Slight
tremors were observed at all the lower levels. Weight gain was reduced
on 50 ppm. No significant histopathological changes were found (Kodama
et al., 1954). Cholinesterase determinations on plasma, erythrocytes
and brain all showed inhibition of activity proportional to the dose;
e.g., in the brain, 90% of the normal value was found at 6.3 ppm, 40%
at 12.5 ppm And 20% at 50 ppm.
Groups, each of 12 male and 12 female rats, were fed 25, 50, 100
and 200 ppm in their diets for 13 weeks. There was a slight increase
in mortality at 200 ppm and growth depression at the 100- and 200-ppm
levels. Clinical signs of intoxication were minimal at 25 ppm but
increased progressively with increasing concentration. Degeneration of
the epithelial cells lining ducts and acini were found in the
sub-maxillary, sublingual, parotid, Harderian and lacrimal glands, and
in the thymus and pancreas, most prominently in the male (Cleveland &
Treon, 1961).
Using groups of 30 male and 30 female rats, the same group of
investigators found inhibition of cholinesterase activity at low-level
feeding. The diets contained 0.32, 0.8, 2.0 and 5.0 ppm of mevinphos
and one male and one female were killed daily for determination of
brain cholinesterase activity during the second, fourth, eighth and
twelfth week of the experiment. On the basis of statistical analysis
it was concluded that the decrease in erythrocyte cholinesterase
activity became significant when the diets of males contained 1.1 ppm
and that of females 1.3 ppm mevinphos. A concentration in the diet
greater than 5 ppm was required to cause a significant reduction in
the cholinesterase activity of the plasm or brain of either sex
(Kettering Lab., 1957a).
Dog. Groups of 4 dogs were fed 0.3, 1,0, 2.5, 5.0, 75 and 200
ppm in their diets for 14 weeks. All the animals in the 200-ppm group
died. The dogs on 75 ppm level showed mild clinical signs of
intoxication and failed to gain weight. At autopsy, pathological
lesions of the exocrine glands were seen. The groups on the lower
levels behaved similarly to the control group as regards weight gain
and post-mortem examination. Determinations of cholinesterase
activities showed that 0.3 and 1.0 ppm had no effect on erythrocyte or
plasma cholinesterase activity. 2.5 ppm lowered erythrocyte
cholinesterase activity to 80%, 5 ppm to 70% of normal values. On 5
ppm plasm cholinesterase activity was 90% of normal and brain
cholinesterase activity was not affected by 0.3-5.0 ppm in the diet
(Cleveland & Treon, 1961; Kettering Lab., 1957c).
Five dogs fed 1 ppm mevinphos for 6 weeks together with either
1 ppm parathion, 2 ppm demeton, 5 ppm methylparathion, 20 ppm EPN or
100 ppm malathion, showed no significant inhibition of cholinesterase
activity of the plasma or the erythrocytes. Feeding experiments on 32
female dogs with combinations of the above-mentioned organophosphates
in the stated concentrations did not show evidence of potentiation
when mevinphos was fed in combination with any of the compounds for 6
weeks (Kettering Lab., 1961).
Cattle. Twelve bull calves 1.5 to 4 weeks of age, divided into
groups of 3, were fed mevinphos in a milk diet at concentrations
equivalent to 0.02, 0.2 and 2.0 mg/kg body-weight per day for 3 weeks.
Cholinesterase determinations on whole blood revealed a progressive
decrease in enzyme activity. At 0.02 mg/kg body-weight activity
dropped to 75% of normal, at 0.2 mg/kg body-weight to 40% and at 2
mg/kg body-weight to below 5%. Only the group on 2 mg/kg body-weight
showed signs of intoxication. During an additional 4-week period the
cholinesterase activities returned almost to normal (Casida et al.,
1958).
Twelve lactating dairy cows in groups of 3 were given mevinphos
in capsules at the rate of 0.026, 0.13 and 0.52 mg/kg body-weight per
day for 12 weeks. The two highest doses depressed the rate of weight
gain but there was no evident effect on milk production or milk-fat
content. Signs of intoxication were not observed and no gross
pathological lesions were found. Erythrocyte cholinesterase activity
was not significantly lowered by 0.026 mg/kg body-weight per day. 0.13
mg/kg body-weight per day gradually decreased the activity to about
30% and 0.52 mg/kg body-weight per day to below 20% of the initial
value (Casida et al., 1958).
Long-term studies
No data available.
Comments on experimental studies reported and evaluation
From the present data a no-effect level in the dog would appear
to be 1 ppm, in the rat 0.8 ppm and in young calves <0.2 ppm. The
short duration of these studies, in view of the progressive decrease
in cholinesterase activity after minimal daily oral doses, makes it
impossible to determine an acceptable daily intake for man.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Casida, J. E. (1955) Science, 122, 597
Casida, J. E. et al. (1956) J. Agr. Food Chem. 4, 236
Casida, J. E., Gatterdam, P. E., Knaak, J. B., Lance, R. D. &
Niedermeier, R. P. (1958) J. Agr. Food Chem., 6, 658
Cleveland, F. P. & Treon, J. F. (1961) J. Agr. Food Chem., 9, 484
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Kettering Laboratory, Cincinnati, Ohio (1957a) Unpublished report,
15 March
Kettering Laboratory, Cincinnati, Ohio (1957b) Unpublished report,
22 March
Kettering Laboratory, Cincinnati, Ohio (1957c) Unpublished report,
19 June
Kettering Laboratory, Cincinnati, Ohio (1961) Unpublished report, 28
September
Kodama, J. K. et al. (1954) Arch. industr. Hyg., 9, 45
O'Brien, R. D. (1960) Toxic Phosphorus Esters, Academic Press, New
York
