
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
ETHION
IDENTITY
Chemical name
OOO'O'-tetraethyl SS'-methylene di(phosphorothiolothionate) (IUPAC)
Formula
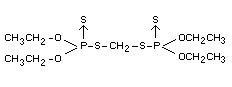 Other information on identity and properties
Liquid, practically non-volatile at ordinary temperatures, solidifying
at -12 to -15°C. Insoluble in water, somewhat soluble in kerosene and
petroleum oils, soluble in most organic solvents. Slowly oxidized in
air to a more reactive material.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Studies in vitro showed that the rate of reaction of ethion with
beef erythrocyte cholinesterase is relatively slow, the velocity
constant being about 3.5 × 103 litres per mole per minute. Liver
fortified with diphosphopyridine nucleotide (DPN) converts ethion into
a more potent cholinesterase inhibitor. Non-fortified liver did not
enhance the activity (Hazleton Laboratories, Inc., 1958a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 69 May and Baker. Ltd., 1960
s.c. 630 May and Baker, Ltd., 1960
Rat (M) oral 65 May and Baker, Ltd., 1960
(F) oral 63 May and Baker, Ltd., 1960
(continued)
LD50 mg/kg
Animal Route body-weight References
(M) s.c. 380 May and Baker, Ltd., 1960
(F) s.c. 360 May and Baker, Ltd., 1960
Rat (M) oral 97 Hazleton Laboratories, Inc., 1961
Rat oral 161 Industrial Bio-Test Laboratories, 1965
Short-term studies
In two separate studies, groups comprising equal numbers of male and
female rats were fed dietary levels of 0, 3, 10, 30 and 100 ppm of
ethion for periods up to 13 weeks. Five male and five female animals
from each group were sacrificed after 30, 63 and 93 days for
cholinesterase determination. All animals exhibited normal physical
appearance, behaviour, growth and food consumption during the studies.
There appeared to be little difference in the response of male and
female rats with respect to cholinesterase activity at these levels.
In the 100 ppm group significant inhibition of plasm, red blood cell
and brain cholinesterase activity was evident after the 30, 63 and 93
day test periods. Similar findings were noted in the 30 ppm group but
to a lesser degree. In the 10 ppm group slight depression in plasma
and red blood cell cholinesterase was noted after 30 days but not
after 63 and 93 days, and there was never any effect observed in brain
cholinesterase. At 3 ppm cholinesterase was normal. At the 100 ppm
level plasma cholinesterase activity returned to normal 14 days after
withdrawal of ethion from the diet, but red blood cell and brain
cholinesterase remained slightly depressed. No histological changes
were noted at any level (Hazleton Laboratories, Inc., 1958b).
Five groups, each containing 10 male and 10 female rats, were fed
ethion at dietary levels of 0, 300, 600, 1000 and 1500 ppm for 13
weeks. Growth suppression was observed in all the test groups, except
for the male rats at the 300 and 600 ppm levels. Signs of
intoxication, with increasing frequency and severity in proportion to
the dietary levels, were observed in all test animals, except for the
males fed 300 ppm. All the animals in the 1500 ppm group died during
the first two weeks. Six male and nine female rats died in the 1000
ppm group. Survival in the other groups was comparable to controls.
Complete inhibition of plasma and red cell cholinesterase activity
occurred in all test groups, except in the males fed 300 ppm, where
inhibition was marked but not complete. Almost total inhibition of
brain cholinesterase activity was observed in the female test animals.
The inhibition noted in the males was less marked, but in proportion
to increasing dietary levels. No gross or histological changes, which
could be attributed to ethion, were observed in any of the surviving
rats (Hazleton Laboratories Inc., 1959).
Dog
Groups, each containing two male and two female dogs, received ethion
orally in daily doses of 0.0125, 0.025, 0.075 and 0.25 mg/kg body
weight for 90 days. A control value relative to cholinesterase
activity was established for each dog during a two- week pre-dosage
period.Physical appearance, behaviour, appetite, body weight and
survival were unaffected. Autopsy at the conclusion of the experiment
revealed no pathological changes. Plasma cholinesterase activity was
significantly inhibited in one male dog and one female dog fed 0.25
mg/kg (Hazleton Laboratories Inc., 1958c).
Ethion was administered to groups, each containing three male and
three female dogs, for six days a week during a 90-day period. Dosage
levels were 0, 0.05, 0.075, 0.125, 0.25, 1.25 and 2.5 mg/kg body
weight. No significant cholinesterase depression was observed at
levels below 0.25 mg/kg. Depression of erythrocyte cholinesterase
occurred only in the 2.5 mg/kg group. This depression was observed
early in the test and persisted throughout its duration, but recovery
was complete 24 days after withdrawal. Plasma cholinesterase was
depressed more readily, occurring early in the test at the 2.5 mg/kg
level but was not evident until the eleventh week of the test at the
0.25 mg/kg level. Recovery was achieved 24 days after withdrawal in
the case of the higher dose level and within nine days in the case of
the 0.25 mg/kg level (Industrial Bio-Test Laboratories Inc., 1961a,
1961b).
Special studies
(a) Reproduction
Groups of male and female rats were fed diets containing 0, 2 and 30
ppm of ethion through three generations. The second litter from each
generation (F1b and F2b) was selected as parental animals to
establish the next generation. No adverse effects were noted among
either the parental animals or their progeny as regards growth,
mortality, reactions, pathology, reproduction performance and survival
indices of the young. Cholinesterase determinations were not conducted
in this study (Industrial Bio-Test Laboratories Inc., 1965b).
(b) Potentiation
When ethion was administered to rats in combination with nine other
organo-phosphorus insecticides, potentiation was observed only with
malathion, where the ratio of observed to theoretical acute LD50 was
2.9:1 (Hazleton Laboratories Inc., 1958d).
Comments
Ninety-day toxicity studies have been made in both rats and dogs
together with a three-generation reproduction study in rats. The
levels causing no toxic effects have been determined mainly on the
results of the extensive cholinesterase inhibition studies, and were
based on the absence of red cell cholinesterase depression. However,
it would have been desirable to have data on cholinesterase depression
at more frequent intervals.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day;
Dog: 0.125 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.00125 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Ethion is used as a pre-harvest topical application to a variety of
food crops especially citrus and deciduous fruit for the control of
many species of aphids, scale insects, mites, leaf miners and leaf
hoppers. It is also used in a cattle dip for the control of ticks and
as a "back-line" treatment for the control of buffalo fly.
Post-harvest treatments
No known post-harvest uses.
Residues resulting from supervised trials
The typical data presented below have been extracted from Internal
Reports of Niagara Chemical Division, FMC, except for that on grapes
(Taschenberg et al., 1963) and strawberries (Fakey et al., 1962).
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Almonds 1.9 0-28 < 0.1
Apples 1 45 1
(continued)
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Beans 1.9 1 7 0.27-0.43
Beef 0.075% emulsion 3 3 0.01
Beef, fat " 3 3 0.86
Citrus (juice) 1.25 2 15 0
" (peel) 1.25 1 30 1
Cucumbers 2.5 6 11 0.04
Grapes 2.5 1 28 2
Melons 1.25 1 7 0.07
Raisins 9 3 9 1.25-10.4
Strawberries 0.6 3 8 0.1
Tea, leaves 0.9 1 7 1.71-7.06
Tomatoes 0.6 2 7 0.17-0.34
Fate of residues
General comments
Ethion shows some systemic activity. It is taken into the plant and
translocated to other parts where it kills mites and certain insects.
This effect diminishes rather rapidly, and correlates with the
relative instability of the oxidized material by comparison with
ethion itself. The residues are reduced by oxidation and subsequent
hydrolysis on the surface or in the plant.
In soils. It is usually used as a spray and no data on its
degradation in soil have been made available.
In plants. A surface residue on cotton foliage of 86 ppm was reduced
to less than 1 ppm After eight days. Simultaneous analysis of
cottonseed showed no detectable residue. Onions planted in 20-feet
rows treated with 10 lb of three per cent dust per 20-feet rows
showed insignificant residues at harvest, 123 days later (Niagara,
1958).
Tea, at harvest, sometimes shows residues as high as 7 ppm, as the
result of "spot" treatment of particular bushes but the brewed product
of these particular leaves shows residues of 0.25 ppm or less. Since
tea is blended prior to sale to the ultimate consumer, a tolerance on
blended tea of not more than 1 ppm and a tolerance of 7 ppm on tea
from a particular estate appear justified (Niagara, 1966a).
In animals. Lactating cows fed up to 20 ppm of radioactive ethion in
their diet showed no ethion residues in their milk, the bulk of the
radioactivity being associated with the protein fraction. In meat, the
highest radioactivity occurred in the liver which contained an average
of 3.15 ppm ethion-P32 equivalents at the end of the 28-day feeding
at 20 ppm. This dropped by 60 per cent within 12 days after
termination of the feeding period. However, chemical analysis showed
that the radioactive material was not ethion but products from its
metabolism (Hazelton Laboratories Inc., 1960; Niagara, 1960). Thus
ethion residues in citrus pulp fed to cattle would not contribute to
residues in milk.
When animals were dipped, residues arising from absorption through the
skin consisted largely of ethion and were mainly in the fat. The
active oxidation products, the monothiol and bisthiol derivatives,
were found at less than 0.01 ppm and 0.02 ppm respectively.
In storage and processing. No data have been made available.
Evidence of residues in food, in commerce or at consumption
Duggan and Weatherwax (1967), in their survey of dietary intake of
pesticide chemicals, found that the incidence and intake of ethion was
too low to be detected. Trace amounts contributing to less than 0.001
mg per day were found in fruit, samples being taken from June 1964 to
April 1966.
Martin and Duggan (1968) found residues of ethion at 0.024 to 0.054
ppm in three out of 30 composite samples of fruit collected from 30
markets in 29 different cities in the United States of America from
June 1966 to April 1967. In the same studies ethion was found at 0.025
ppm in one out of 30 composite samples of oils, fats and shortening.
Methods of residue analysis
A colorimetric procedure is based on the extraction of ethion, and
necessary clean-up, followed by hydrolysis to diethylphosphorodithioic
acid. The colour complex then formed with copper sulfate is measured
at 418 mµ. From the optical density the amount of ethion is determined
from a standard curve (Graham, 1964).
An enzymatic method is based on the oxidation of ethion with bromine
water to the active phosphorothiolate and subsequent measurement of
the extent of inhibition of the cholinesterase from human blood plasma
(Cook, 1954; Fallscheer and Cook, 1956).
The clean-up procedure associated with the colorimetric method is so
designed that determination of ethion in the presence of other organo-
phosphorus insecticides can be made. However, the method does not
measure oxidized or metabolized derivatives that contain phosphoryl
rather than phosphorothiono groups. The enzyme method on the other
hand measures the oxidized and unchanged ethion but does not
differentiate from other cholinesterase inhibitors. The two methods
are in substantial agreement however in the determination of ethion
residues, which implies that there is little of the residue in the
oxidized state (Niagara, 1958).
A microcoulometric gas chromatographic method (Cassil, 1962) has been
used to determine both ethion and its oxygen analogues independently
(Niagara, 1967). The methods are sensitive to 0.05 ppm in most foods.
For meat samples, following suitable extraction of macerated samples,
partitioning between solvents and further clean-up on Florisil
columns, the residue is determined by a gas chromatograph using a
sodium thermionic detector. Recoveries of 92 per cent are normal with
a sensitivity to 0.001 ppm. This precise method does not measure the
oxidation products which contribute an insignificant amount to the
residues (see above). With a different column the oxidation products
can be measured but at a lower recovery rate (Niagara, 1966b).
National tolerances
Tolerance
Country Crop (ppm)
Canada Raisins 4
Citrus, grapes, peaches, plums,
strawberries, nectarines, beans,
melons, tomatoes 2
Apples, pears, eggplant, onions,
peppers 1
Summer squash 0.5
Almonds 0.1
United States Citrus pulps, dehydrated for
of America cattle feed 10
Dried tea 7
Almond hulls 5
Raisins 4
(continued)
Tolerance
Country Crop (ppm)
Beef (fat basis) 2.5
Apples, citrus, grapes, plums,
strawberries, beans, melons,
tomatoes, sorghum grain 2
Pears, peaches, nectarines,
eggplant, onions, peppers 1
Beef or beef products 0.75
Cucumbers, summer squash 0.5
Almonds 0.1
Milk "0"
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Ethion is an insecticide and acaricide for use on both plants and
animals. The technical product contains a trace of an active oxidized
metabolite but it is not of significance in the final residue. Ethion
is used as a pre-harvest application to a variety of food crops
especially citrus and deciduous fruit for the control of aphids, scale
insects, mites, leaf miners and leaf hoppers. It shows minor systemic
activity and residues are reduced also by weathering. No residues of
ethion were found in milk or meat from lactating cows fed citrus pulp
containing ethion residues. However, where ethion was applied as a dip
to animals for the control of ticks, after a three-day interval
following treatment, the highest level was in the fat at 1.25 ppm with
less than 0.01 ppm in internal organs and muscle. The two active
oxidation metabolites were present in insignificant quantity.
Accordingly, animals dipped in ethion emulsion should be held three
days prior to slaughter to ensure that the recommended tolerances,
applied at slaughter, are not exceeded.
In market sample studies in 1966-67, residues were found at 0.024 to
0.054 ppm in three out of 30 composite samples of fruit in the United
States of America. The same survey on oils, fats and shortening showed
residues of 0.025 ppm in one out of 30 composite samples. Methods are
available for measuring residues for use in general regulatory
laboratories but further work is necessary to specify a method for
referee purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit-and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Temporary tolerances
Grapes 2 ppm
Other fruit 1 ppm
Vegetables 0.5 ppm
Tea (from a particular estate)
for blending only 7 ppm
Tea, blended 1 ppm
Beef (fat basis) (at slaughter) 1.5 ppm
Further work or information
ETHION
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre harvest intervals,
and the resultant residues.
2. Data on residue levels in raw agricultural commodities moving in
commerce.
3. Residue data in processed food, including meat, meat products and
wine.
Desirable:
1. Collaborative studies to establish a referee method.
2. Adequate observations of effects in man, including studies of the
metabolic fate.
3. Determination of the metabolic fate in animals.
4. Long-term studies in at least two species.
5. Cholinesterase depression studies at more frequent intervals in
animals.
REFERENCES
Cook, J. W. (1954) Report on determination of insecticides by
enzymatic methods. J. Assoc. Off. Agr. Chem., 37: 561-564
Cassil, C. C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Rev., 1: 37-65
Duggan, R. E. and Weatherwax, J. R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Fahey, J. E., Rodriquez, J. G., Rusk, H. W. and Chaplain, C. E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Entomol., 55: 179-184
Fallscheer, N. O. and Cook, J. W. (1956) Report on enzymatic methods
for insecticides. Studies on the conversion of some thionophosphates
and a dithiophosphate to in vitro cholinesterase inhibitors. J.
Assoc. Off. Agr. Chem., 39: 691-697
Graham, J. R. (1964) Vol. II, Anal. Methods for Pesticides, ed. G.
Zweig, Academic Press
Hazleton Laboratories, Inc. (1958a) Nialate Tech. (1240) Final report.
In vitro cholinesterase studies. Unpublished report
Hazleton Laboratories, Inc. (1958b) Nialate Tech. (1240) Final report.
Subacute feeding studies - rats. Unpublished report
Hazleton Laboratories, Inc. (1958c) Nialate Tech. (1240) Final report.
Subacute administration - dogs. Unpublished report
Hazleton Laboratories, Inc. (1958d) Nialate Techn. Potentiation study.
Acute oral administration - rats. Unpublished report
Hazleton Laboratories, Inc. (1959) Nialate Technical (ethion) Final
report. Ninety-day feeding study - rats. Unpublished report
Hazleton Laboratories, Inc., (1960) Palo Alto, Calif. Unpublished
report
Hazleton Laboratories, Inc. (1961) Ethion. Acute oral administration
- rats. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961a) Effects of ethion on
cholinesterase activity in the dog. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961b) Addendum report.
Effects of ethion on cholinesterase activity in the dog. Unpublished
report
Industrial Bio-Test Laboratories, Inc. (1965a) Acute oral toxicity of
ethion MR E423. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1965b) Three-generation
reproduction study in albino rats on ethion. First generation, second
generation and final report. Unpublished reports
Martin, R. J. and Duggan, R. E. (1968) Pesticide residues in total
diet samples (III). Pesticides Monitoring Journal, 1: 11-20
May and Baker, Ltd. (1960) Ethion. Unpublished report submitted to
the Ministry of Health, United Kingdom
Niagara. (1958) Unpublished report M-602. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1960) Unpublished report M-796. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1966a) Petition No. 351, Niagara Chemicals Division, F.M.C.
to U.S. Food and Drug Administration
Niagara Chemicals Division, F.M.C. Corpn., Middleport, N.Y. (1966b)
Ethion food additive petition. Report No. C.T.B. 23/2/20
Niagara. (1967) Unpublished report M-2131. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Taschenberg, E. F., Avens, A. W., Parsons, G. M. and Gibbs. S. D.
(1963) Disappearance of spray deposits of DDT, methoxychlor,
perthane, ethion and diazinon from Concord grapes. J. Econ. Entomol.,
56: 431-438
Other information on identity and properties
Liquid, practically non-volatile at ordinary temperatures, solidifying
at -12 to -15°C. Insoluble in water, somewhat soluble in kerosene and
petroleum oils, soluble in most organic solvents. Slowly oxidized in
air to a more reactive material.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Studies in vitro showed that the rate of reaction of ethion with
beef erythrocyte cholinesterase is relatively slow, the velocity
constant being about 3.5 × 103 litres per mole per minute. Liver
fortified with diphosphopyridine nucleotide (DPN) converts ethion into
a more potent cholinesterase inhibitor. Non-fortified liver did not
enhance the activity (Hazleton Laboratories, Inc., 1958a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 69 May and Baker. Ltd., 1960
s.c. 630 May and Baker, Ltd., 1960
Rat (M) oral 65 May and Baker, Ltd., 1960
(F) oral 63 May and Baker, Ltd., 1960
(continued)
LD50 mg/kg
Animal Route body-weight References
(M) s.c. 380 May and Baker, Ltd., 1960
(F) s.c. 360 May and Baker, Ltd., 1960
Rat (M) oral 97 Hazleton Laboratories, Inc., 1961
Rat oral 161 Industrial Bio-Test Laboratories, 1965
Short-term studies
In two separate studies, groups comprising equal numbers of male and
female rats were fed dietary levels of 0, 3, 10, 30 and 100 ppm of
ethion for periods up to 13 weeks. Five male and five female animals
from each group were sacrificed after 30, 63 and 93 days for
cholinesterase determination. All animals exhibited normal physical
appearance, behaviour, growth and food consumption during the studies.
There appeared to be little difference in the response of male and
female rats with respect to cholinesterase activity at these levels.
In the 100 ppm group significant inhibition of plasm, red blood cell
and brain cholinesterase activity was evident after the 30, 63 and 93
day test periods. Similar findings were noted in the 30 ppm group but
to a lesser degree. In the 10 ppm group slight depression in plasma
and red blood cell cholinesterase was noted after 30 days but not
after 63 and 93 days, and there was never any effect observed in brain
cholinesterase. At 3 ppm cholinesterase was normal. At the 100 ppm
level plasma cholinesterase activity returned to normal 14 days after
withdrawal of ethion from the diet, but red blood cell and brain
cholinesterase remained slightly depressed. No histological changes
were noted at any level (Hazleton Laboratories, Inc., 1958b).
Five groups, each containing 10 male and 10 female rats, were fed
ethion at dietary levels of 0, 300, 600, 1000 and 1500 ppm for 13
weeks. Growth suppression was observed in all the test groups, except
for the male rats at the 300 and 600 ppm levels. Signs of
intoxication, with increasing frequency and severity in proportion to
the dietary levels, were observed in all test animals, except for the
males fed 300 ppm. All the animals in the 1500 ppm group died during
the first two weeks. Six male and nine female rats died in the 1000
ppm group. Survival in the other groups was comparable to controls.
Complete inhibition of plasma and red cell cholinesterase activity
occurred in all test groups, except in the males fed 300 ppm, where
inhibition was marked but not complete. Almost total inhibition of
brain cholinesterase activity was observed in the female test animals.
The inhibition noted in the males was less marked, but in proportion
to increasing dietary levels. No gross or histological changes, which
could be attributed to ethion, were observed in any of the surviving
rats (Hazleton Laboratories Inc., 1959).
Dog
Groups, each containing two male and two female dogs, received ethion
orally in daily doses of 0.0125, 0.025, 0.075 and 0.25 mg/kg body
weight for 90 days. A control value relative to cholinesterase
activity was established for each dog during a two- week pre-dosage
period.Physical appearance, behaviour, appetite, body weight and
survival were unaffected. Autopsy at the conclusion of the experiment
revealed no pathological changes. Plasma cholinesterase activity was
significantly inhibited in one male dog and one female dog fed 0.25
mg/kg (Hazleton Laboratories Inc., 1958c).
Ethion was administered to groups, each containing three male and
three female dogs, for six days a week during a 90-day period. Dosage
levels were 0, 0.05, 0.075, 0.125, 0.25, 1.25 and 2.5 mg/kg body
weight. No significant cholinesterase depression was observed at
levels below 0.25 mg/kg. Depression of erythrocyte cholinesterase
occurred only in the 2.5 mg/kg group. This depression was observed
early in the test and persisted throughout its duration, but recovery
was complete 24 days after withdrawal. Plasma cholinesterase was
depressed more readily, occurring early in the test at the 2.5 mg/kg
level but was not evident until the eleventh week of the test at the
0.25 mg/kg level. Recovery was achieved 24 days after withdrawal in
the case of the higher dose level and within nine days in the case of
the 0.25 mg/kg level (Industrial Bio-Test Laboratories Inc., 1961a,
1961b).
Special studies
(a) Reproduction
Groups of male and female rats were fed diets containing 0, 2 and 30
ppm of ethion through three generations. The second litter from each
generation (F1b and F2b) was selected as parental animals to
establish the next generation. No adverse effects were noted among
either the parental animals or their progeny as regards growth,
mortality, reactions, pathology, reproduction performance and survival
indices of the young. Cholinesterase determinations were not conducted
in this study (Industrial Bio-Test Laboratories Inc., 1965b).
(b) Potentiation
When ethion was administered to rats in combination with nine other
organo-phosphorus insecticides, potentiation was observed only with
malathion, where the ratio of observed to theoretical acute LD50 was
2.9:1 (Hazleton Laboratories Inc., 1958d).
Comments
Ninety-day toxicity studies have been made in both rats and dogs
together with a three-generation reproduction study in rats. The
levels causing no toxic effects have been determined mainly on the
results of the extensive cholinesterase inhibition studies, and were
based on the absence of red cell cholinesterase depression. However,
it would have been desirable to have data on cholinesterase depression
at more frequent intervals.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day;
Dog: 0.125 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.00125 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Ethion is used as a pre-harvest topical application to a variety of
food crops especially citrus and deciduous fruit for the control of
many species of aphids, scale insects, mites, leaf miners and leaf
hoppers. It is also used in a cattle dip for the control of ticks and
as a "back-line" treatment for the control of buffalo fly.
Post-harvest treatments
No known post-harvest uses.
Residues resulting from supervised trials
The typical data presented below have been extracted from Internal
Reports of Niagara Chemical Division, FMC, except for that on grapes
(Taschenberg et al., 1963) and strawberries (Fakey et al., 1962).
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Almonds 1.9 0-28 < 0.1
Apples 1 45 1
(continued)
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Beans 1.9 1 7 0.27-0.43
Beef 0.075% emulsion 3 3 0.01
Beef, fat " 3 3 0.86
Citrus (juice) 1.25 2 15 0
" (peel) 1.25 1 30 1
Cucumbers 2.5 6 11 0.04
Grapes 2.5 1 28 2
Melons 1.25 1 7 0.07
Raisins 9 3 9 1.25-10.4
Strawberries 0.6 3 8 0.1
Tea, leaves 0.9 1 7 1.71-7.06
Tomatoes 0.6 2 7 0.17-0.34
Fate of residues
General comments
Ethion shows some systemic activity. It is taken into the plant and
translocated to other parts where it kills mites and certain insects.
This effect diminishes rather rapidly, and correlates with the
relative instability of the oxidized material by comparison with
ethion itself. The residues are reduced by oxidation and subsequent
hydrolysis on the surface or in the plant.
In soils. It is usually used as a spray and no data on its
degradation in soil have been made available.
In plants. A surface residue on cotton foliage of 86 ppm was reduced
to less than 1 ppm After eight days. Simultaneous analysis of
cottonseed showed no detectable residue. Onions planted in 20-feet
rows treated with 10 lb of three per cent dust per 20-feet rows
showed insignificant residues at harvest, 123 days later (Niagara,
1958).
Tea, at harvest, sometimes shows residues as high as 7 ppm, as the
result of "spot" treatment of particular bushes but the brewed product
of these particular leaves shows residues of 0.25 ppm or less. Since
tea is blended prior to sale to the ultimate consumer, a tolerance on
blended tea of not more than 1 ppm and a tolerance of 7 ppm on tea
from a particular estate appear justified (Niagara, 1966a).
In animals. Lactating cows fed up to 20 ppm of radioactive ethion in
their diet showed no ethion residues in their milk, the bulk of the
radioactivity being associated with the protein fraction. In meat, the
highest radioactivity occurred in the liver which contained an average
of 3.15 ppm ethion-P32 equivalents at the end of the 28-day feeding
at 20 ppm. This dropped by 60 per cent within 12 days after
termination of the feeding period. However, chemical analysis showed
that the radioactive material was not ethion but products from its
metabolism (Hazelton Laboratories Inc., 1960; Niagara, 1960). Thus
ethion residues in citrus pulp fed to cattle would not contribute to
residues in milk.
When animals were dipped, residues arising from absorption through the
skin consisted largely of ethion and were mainly in the fat. The
active oxidation products, the monothiol and bisthiol derivatives,
were found at less than 0.01 ppm and 0.02 ppm respectively.
In storage and processing. No data have been made available.
Evidence of residues in food, in commerce or at consumption
Duggan and Weatherwax (1967), in their survey of dietary intake of
pesticide chemicals, found that the incidence and intake of ethion was
too low to be detected. Trace amounts contributing to less than 0.001
mg per day were found in fruit, samples being taken from June 1964 to
April 1966.
Martin and Duggan (1968) found residues of ethion at 0.024 to 0.054
ppm in three out of 30 composite samples of fruit collected from 30
markets in 29 different cities in the United States of America from
June 1966 to April 1967. In the same studies ethion was found at 0.025
ppm in one out of 30 composite samples of oils, fats and shortening.
Methods of residue analysis
A colorimetric procedure is based on the extraction of ethion, and
necessary clean-up, followed by hydrolysis to diethylphosphorodithioic
acid. The colour complex then formed with copper sulfate is measured
at 418 mµ. From the optical density the amount of ethion is determined
from a standard curve (Graham, 1964).
An enzymatic method is based on the oxidation of ethion with bromine
water to the active phosphorothiolate and subsequent measurement of
the extent of inhibition of the cholinesterase from human blood plasma
(Cook, 1954; Fallscheer and Cook, 1956).
The clean-up procedure associated with the colorimetric method is so
designed that determination of ethion in the presence of other organo-
phosphorus insecticides can be made. However, the method does not
measure oxidized or metabolized derivatives that contain phosphoryl
rather than phosphorothiono groups. The enzyme method on the other
hand measures the oxidized and unchanged ethion but does not
differentiate from other cholinesterase inhibitors. The two methods
are in substantial agreement however in the determination of ethion
residues, which implies that there is little of the residue in the
oxidized state (Niagara, 1958).
A microcoulometric gas chromatographic method (Cassil, 1962) has been
used to determine both ethion and its oxygen analogues independently
(Niagara, 1967). The methods are sensitive to 0.05 ppm in most foods.
For meat samples, following suitable extraction of macerated samples,
partitioning between solvents and further clean-up on Florisil
columns, the residue is determined by a gas chromatograph using a
sodium thermionic detector. Recoveries of 92 per cent are normal with
a sensitivity to 0.001 ppm. This precise method does not measure the
oxidation products which contribute an insignificant amount to the
residues (see above). With a different column the oxidation products
can be measured but at a lower recovery rate (Niagara, 1966b).
National tolerances
Tolerance
Country Crop (ppm)
Canada Raisins 4
Citrus, grapes, peaches, plums,
strawberries, nectarines, beans,
melons, tomatoes 2
Apples, pears, eggplant, onions,
peppers 1
Summer squash 0.5
Almonds 0.1
United States Citrus pulps, dehydrated for
of America cattle feed 10
Dried tea 7
Almond hulls 5
Raisins 4
(continued)
Tolerance
Country Crop (ppm)
Beef (fat basis) 2.5
Apples, citrus, grapes, plums,
strawberries, beans, melons,
tomatoes, sorghum grain 2
Pears, peaches, nectarines,
eggplant, onions, peppers 1
Beef or beef products 0.75
Cucumbers, summer squash 0.5
Almonds 0.1
Milk "0"
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Ethion is an insecticide and acaricide for use on both plants and
animals. The technical product contains a trace of an active oxidized
metabolite but it is not of significance in the final residue. Ethion
is used as a pre-harvest application to a variety of food crops
especially citrus and deciduous fruit for the control of aphids, scale
insects, mites, leaf miners and leaf hoppers. It shows minor systemic
activity and residues are reduced also by weathering. No residues of
ethion were found in milk or meat from lactating cows fed citrus pulp
containing ethion residues. However, where ethion was applied as a dip
to animals for the control of ticks, after a three-day interval
following treatment, the highest level was in the fat at 1.25 ppm with
less than 0.01 ppm in internal organs and muscle. The two active
oxidation metabolites were present in insignificant quantity.
Accordingly, animals dipped in ethion emulsion should be held three
days prior to slaughter to ensure that the recommended tolerances,
applied at slaughter, are not exceeded.
In market sample studies in 1966-67, residues were found at 0.024 to
0.054 ppm in three out of 30 composite samples of fruit in the United
States of America. The same survey on oils, fats and shortening showed
residues of 0.025 ppm in one out of 30 composite samples. Methods are
available for measuring residues for use in general regulatory
laboratories but further work is necessary to specify a method for
referee purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit-and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Temporary tolerances
Grapes 2 ppm
Other fruit 1 ppm
Vegetables 0.5 ppm
Tea (from a particular estate)
for blending only 7 ppm
Tea, blended 1 ppm
Beef (fat basis) (at slaughter) 1.5 ppm
Further work or information
ETHION
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre harvest intervals,
and the resultant residues.
2. Data on residue levels in raw agricultural commodities moving in
commerce.
3. Residue data in processed food, including meat, meat products and
wine.
Desirable:
1. Collaborative studies to establish a referee method.
2. Adequate observations of effects in man, including studies of the
metabolic fate.
3. Determination of the metabolic fate in animals.
4. Long-term studies in at least two species.
5. Cholinesterase depression studies at more frequent intervals in
animals.
REFERENCES
Cook, J. W. (1954) Report on determination of insecticides by
enzymatic methods. J. Assoc. Off. Agr. Chem., 37: 561-564
Cassil, C. C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Rev., 1: 37-65
Duggan, R. E. and Weatherwax, J. R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Fahey, J. E., Rodriquez, J. G., Rusk, H. W. and Chaplain, C. E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Entomol., 55: 179-184
Fallscheer, N. O. and Cook, J. W. (1956) Report on enzymatic methods
for insecticides. Studies on the conversion of some thionophosphates
and a dithiophosphate to in vitro cholinesterase inhibitors. J.
Assoc. Off. Agr. Chem., 39: 691-697
Graham, J. R. (1964) Vol. II, Anal. Methods for Pesticides, ed. G.
Zweig, Academic Press
Hazleton Laboratories, Inc. (1958a) Nialate Tech. (1240) Final report.
In vitro cholinesterase studies. Unpublished report
Hazleton Laboratories, Inc. (1958b) Nialate Tech. (1240) Final report.
Subacute feeding studies - rats. Unpublished report
Hazleton Laboratories, Inc. (1958c) Nialate Tech. (1240) Final report.
Subacute administration - dogs. Unpublished report
Hazleton Laboratories, Inc. (1958d) Nialate Techn. Potentiation study.
Acute oral administration - rats. Unpublished report
Hazleton Laboratories, Inc. (1959) Nialate Technical (ethion) Final
report. Ninety-day feeding study - rats. Unpublished report
Hazleton Laboratories, Inc., (1960) Palo Alto, Calif. Unpublished
report
Hazleton Laboratories, Inc. (1961) Ethion. Acute oral administration
- rats. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961a) Effects of ethion on
cholinesterase activity in the dog. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961b) Addendum report.
Effects of ethion on cholinesterase activity in the dog. Unpublished
report
Industrial Bio-Test Laboratories, Inc. (1965a) Acute oral toxicity of
ethion MR E423. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1965b) Three-generation
reproduction study in albino rats on ethion. First generation, second
generation and final report. Unpublished reports
Martin, R. J. and Duggan, R. E. (1968) Pesticide residues in total
diet samples (III). Pesticides Monitoring Journal, 1: 11-20
May and Baker, Ltd. (1960) Ethion. Unpublished report submitted to
the Ministry of Health, United Kingdom
Niagara. (1958) Unpublished report M-602. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1960) Unpublished report M-796. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1966a) Petition No. 351, Niagara Chemicals Division, F.M.C.
to U.S. Food and Drug Administration
Niagara Chemicals Division, F.M.C. Corpn., Middleport, N.Y. (1966b)
Ethion food additive petition. Report No. C.T.B. 23/2/20
Niagara. (1967) Unpublished report M-2131. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Taschenberg, E. F., Avens, A. W., Parsons, G. M. and Gibbs. S. D.
(1963) Disappearance of spray deposits of DDT, methoxychlor,
perthane, ethion and diazinon from Concord grapes. J. Econ. Entomol.,
56: 431-438
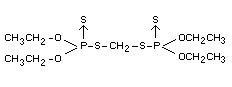 Other information on identity and properties
Liquid, practically non-volatile at ordinary temperatures, solidifying
at -12 to -15°C. Insoluble in water, somewhat soluble in kerosene and
petroleum oils, soluble in most organic solvents. Slowly oxidized in
air to a more reactive material.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Studies in vitro showed that the rate of reaction of ethion with
beef erythrocyte cholinesterase is relatively slow, the velocity
constant being about 3.5 × 103 litres per mole per minute. Liver
fortified with diphosphopyridine nucleotide (DPN) converts ethion into
a more potent cholinesterase inhibitor. Non-fortified liver did not
enhance the activity (Hazleton Laboratories, Inc., 1958a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 69 May and Baker. Ltd., 1960
s.c. 630 May and Baker, Ltd., 1960
Rat (M) oral 65 May and Baker, Ltd., 1960
(F) oral 63 May and Baker, Ltd., 1960
(continued)
LD50 mg/kg
Animal Route body-weight References
(M) s.c. 380 May and Baker, Ltd., 1960
(F) s.c. 360 May and Baker, Ltd., 1960
Rat (M) oral 97 Hazleton Laboratories, Inc., 1961
Rat oral 161 Industrial Bio-Test Laboratories, 1965
Short-term studies
In two separate studies, groups comprising equal numbers of male and
female rats were fed dietary levels of 0, 3, 10, 30 and 100 ppm of
ethion for periods up to 13 weeks. Five male and five female animals
from each group were sacrificed after 30, 63 and 93 days for
cholinesterase determination. All animals exhibited normal physical
appearance, behaviour, growth and food consumption during the studies.
There appeared to be little difference in the response of male and
female rats with respect to cholinesterase activity at these levels.
In the 100 ppm group significant inhibition of plasm, red blood cell
and brain cholinesterase activity was evident after the 30, 63 and 93
day test periods. Similar findings were noted in the 30 ppm group but
to a lesser degree. In the 10 ppm group slight depression in plasma
and red blood cell cholinesterase was noted after 30 days but not
after 63 and 93 days, and there was never any effect observed in brain
cholinesterase. At 3 ppm cholinesterase was normal. At the 100 ppm
level plasma cholinesterase activity returned to normal 14 days after
withdrawal of ethion from the diet, but red blood cell and brain
cholinesterase remained slightly depressed. No histological changes
were noted at any level (Hazleton Laboratories, Inc., 1958b).
Five groups, each containing 10 male and 10 female rats, were fed
ethion at dietary levels of 0, 300, 600, 1000 and 1500 ppm for 13
weeks. Growth suppression was observed in all the test groups, except
for the male rats at the 300 and 600 ppm levels. Signs of
intoxication, with increasing frequency and severity in proportion to
the dietary levels, were observed in all test animals, except for the
males fed 300 ppm. All the animals in the 1500 ppm group died during
the first two weeks. Six male and nine female rats died in the 1000
ppm group. Survival in the other groups was comparable to controls.
Complete inhibition of plasma and red cell cholinesterase activity
occurred in all test groups, except in the males fed 300 ppm, where
inhibition was marked but not complete. Almost total inhibition of
brain cholinesterase activity was observed in the female test animals.
The inhibition noted in the males was less marked, but in proportion
to increasing dietary levels. No gross or histological changes, which
could be attributed to ethion, were observed in any of the surviving
rats (Hazleton Laboratories Inc., 1959).
Dog
Groups, each containing two male and two female dogs, received ethion
orally in daily doses of 0.0125, 0.025, 0.075 and 0.25 mg/kg body
weight for 90 days. A control value relative to cholinesterase
activity was established for each dog during a two- week pre-dosage
period.Physical appearance, behaviour, appetite, body weight and
survival were unaffected. Autopsy at the conclusion of the experiment
revealed no pathological changes. Plasma cholinesterase activity was
significantly inhibited in one male dog and one female dog fed 0.25
mg/kg (Hazleton Laboratories Inc., 1958c).
Ethion was administered to groups, each containing three male and
three female dogs, for six days a week during a 90-day period. Dosage
levels were 0, 0.05, 0.075, 0.125, 0.25, 1.25 and 2.5 mg/kg body
weight. No significant cholinesterase depression was observed at
levels below 0.25 mg/kg. Depression of erythrocyte cholinesterase
occurred only in the 2.5 mg/kg group. This depression was observed
early in the test and persisted throughout its duration, but recovery
was complete 24 days after withdrawal. Plasma cholinesterase was
depressed more readily, occurring early in the test at the 2.5 mg/kg
level but was not evident until the eleventh week of the test at the
0.25 mg/kg level. Recovery was achieved 24 days after withdrawal in
the case of the higher dose level and within nine days in the case of
the 0.25 mg/kg level (Industrial Bio-Test Laboratories Inc., 1961a,
1961b).
Special studies
(a) Reproduction
Groups of male and female rats were fed diets containing 0, 2 and 30
ppm of ethion through three generations. The second litter from each
generation (F1b and F2b) was selected as parental animals to
establish the next generation. No adverse effects were noted among
either the parental animals or their progeny as regards growth,
mortality, reactions, pathology, reproduction performance and survival
indices of the young. Cholinesterase determinations were not conducted
in this study (Industrial Bio-Test Laboratories Inc., 1965b).
(b) Potentiation
When ethion was administered to rats in combination with nine other
organo-phosphorus insecticides, potentiation was observed only with
malathion, where the ratio of observed to theoretical acute LD50 was
2.9:1 (Hazleton Laboratories Inc., 1958d).
Comments
Ninety-day toxicity studies have been made in both rats and dogs
together with a three-generation reproduction study in rats. The
levels causing no toxic effects have been determined mainly on the
results of the extensive cholinesterase inhibition studies, and were
based on the absence of red cell cholinesterase depression. However,
it would have been desirable to have data on cholinesterase depression
at more frequent intervals.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day;
Dog: 0.125 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.00125 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Ethion is used as a pre-harvest topical application to a variety of
food crops especially citrus and deciduous fruit for the control of
many species of aphids, scale insects, mites, leaf miners and leaf
hoppers. It is also used in a cattle dip for the control of ticks and
as a "back-line" treatment for the control of buffalo fly.
Post-harvest treatments
No known post-harvest uses.
Residues resulting from supervised trials
The typical data presented below have been extracted from Internal
Reports of Niagara Chemical Division, FMC, except for that on grapes
(Taschenberg et al., 1963) and strawberries (Fakey et al., 1962).
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Almonds 1.9 0-28 < 0.1
Apples 1 45 1
(continued)
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Beans 1.9 1 7 0.27-0.43
Beef 0.075% emulsion 3 3 0.01
Beef, fat " 3 3 0.86
Citrus (juice) 1.25 2 15 0
" (peel) 1.25 1 30 1
Cucumbers 2.5 6 11 0.04
Grapes 2.5 1 28 2
Melons 1.25 1 7 0.07
Raisins 9 3 9 1.25-10.4
Strawberries 0.6 3 8 0.1
Tea, leaves 0.9 1 7 1.71-7.06
Tomatoes 0.6 2 7 0.17-0.34
Fate of residues
General comments
Ethion shows some systemic activity. It is taken into the plant and
translocated to other parts where it kills mites and certain insects.
This effect diminishes rather rapidly, and correlates with the
relative instability of the oxidized material by comparison with
ethion itself. The residues are reduced by oxidation and subsequent
hydrolysis on the surface or in the plant.
In soils. It is usually used as a spray and no data on its
degradation in soil have been made available.
In plants. A surface residue on cotton foliage of 86 ppm was reduced
to less than 1 ppm After eight days. Simultaneous analysis of
cottonseed showed no detectable residue. Onions planted in 20-feet
rows treated with 10 lb of three per cent dust per 20-feet rows
showed insignificant residues at harvest, 123 days later (Niagara,
1958).
Tea, at harvest, sometimes shows residues as high as 7 ppm, as the
result of "spot" treatment of particular bushes but the brewed product
of these particular leaves shows residues of 0.25 ppm or less. Since
tea is blended prior to sale to the ultimate consumer, a tolerance on
blended tea of not more than 1 ppm and a tolerance of 7 ppm on tea
from a particular estate appear justified (Niagara, 1966a).
In animals. Lactating cows fed up to 20 ppm of radioactive ethion in
their diet showed no ethion residues in their milk, the bulk of the
radioactivity being associated with the protein fraction. In meat, the
highest radioactivity occurred in the liver which contained an average
of 3.15 ppm ethion-P32 equivalents at the end of the 28-day feeding
at 20 ppm. This dropped by 60 per cent within 12 days after
termination of the feeding period. However, chemical analysis showed
that the radioactive material was not ethion but products from its
metabolism (Hazelton Laboratories Inc., 1960; Niagara, 1960). Thus
ethion residues in citrus pulp fed to cattle would not contribute to
residues in milk.
When animals were dipped, residues arising from absorption through the
skin consisted largely of ethion and were mainly in the fat. The
active oxidation products, the monothiol and bisthiol derivatives,
were found at less than 0.01 ppm and 0.02 ppm respectively.
In storage and processing. No data have been made available.
Evidence of residues in food, in commerce or at consumption
Duggan and Weatherwax (1967), in their survey of dietary intake of
pesticide chemicals, found that the incidence and intake of ethion was
too low to be detected. Trace amounts contributing to less than 0.001
mg per day were found in fruit, samples being taken from June 1964 to
April 1966.
Martin and Duggan (1968) found residues of ethion at 0.024 to 0.054
ppm in three out of 30 composite samples of fruit collected from 30
markets in 29 different cities in the United States of America from
June 1966 to April 1967. In the same studies ethion was found at 0.025
ppm in one out of 30 composite samples of oils, fats and shortening.
Methods of residue analysis
A colorimetric procedure is based on the extraction of ethion, and
necessary clean-up, followed by hydrolysis to diethylphosphorodithioic
acid. The colour complex then formed with copper sulfate is measured
at 418 mµ. From the optical density the amount of ethion is determined
from a standard curve (Graham, 1964).
An enzymatic method is based on the oxidation of ethion with bromine
water to the active phosphorothiolate and subsequent measurement of
the extent of inhibition of the cholinesterase from human blood plasma
(Cook, 1954; Fallscheer and Cook, 1956).
The clean-up procedure associated with the colorimetric method is so
designed that determination of ethion in the presence of other organo-
phosphorus insecticides can be made. However, the method does not
measure oxidized or metabolized derivatives that contain phosphoryl
rather than phosphorothiono groups. The enzyme method on the other
hand measures the oxidized and unchanged ethion but does not
differentiate from other cholinesterase inhibitors. The two methods
are in substantial agreement however in the determination of ethion
residues, which implies that there is little of the residue in the
oxidized state (Niagara, 1958).
A microcoulometric gas chromatographic method (Cassil, 1962) has been
used to determine both ethion and its oxygen analogues independently
(Niagara, 1967). The methods are sensitive to 0.05 ppm in most foods.
For meat samples, following suitable extraction of macerated samples,
partitioning between solvents and further clean-up on Florisil
columns, the residue is determined by a gas chromatograph using a
sodium thermionic detector. Recoveries of 92 per cent are normal with
a sensitivity to 0.001 ppm. This precise method does not measure the
oxidation products which contribute an insignificant amount to the
residues (see above). With a different column the oxidation products
can be measured but at a lower recovery rate (Niagara, 1966b).
National tolerances
Tolerance
Country Crop (ppm)
Canada Raisins 4
Citrus, grapes, peaches, plums,
strawberries, nectarines, beans,
melons, tomatoes 2
Apples, pears, eggplant, onions,
peppers 1
Summer squash 0.5
Almonds 0.1
United States Citrus pulps, dehydrated for
of America cattle feed 10
Dried tea 7
Almond hulls 5
Raisins 4
(continued)
Tolerance
Country Crop (ppm)
Beef (fat basis) 2.5
Apples, citrus, grapes, plums,
strawberries, beans, melons,
tomatoes, sorghum grain 2
Pears, peaches, nectarines,
eggplant, onions, peppers 1
Beef or beef products 0.75
Cucumbers, summer squash 0.5
Almonds 0.1
Milk "0"
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Ethion is an insecticide and acaricide for use on both plants and
animals. The technical product contains a trace of an active oxidized
metabolite but it is not of significance in the final residue. Ethion
is used as a pre-harvest application to a variety of food crops
especially citrus and deciduous fruit for the control of aphids, scale
insects, mites, leaf miners and leaf hoppers. It shows minor systemic
activity and residues are reduced also by weathering. No residues of
ethion were found in milk or meat from lactating cows fed citrus pulp
containing ethion residues. However, where ethion was applied as a dip
to animals for the control of ticks, after a three-day interval
following treatment, the highest level was in the fat at 1.25 ppm with
less than 0.01 ppm in internal organs and muscle. The two active
oxidation metabolites were present in insignificant quantity.
Accordingly, animals dipped in ethion emulsion should be held three
days prior to slaughter to ensure that the recommended tolerances,
applied at slaughter, are not exceeded.
In market sample studies in 1966-67, residues were found at 0.024 to
0.054 ppm in three out of 30 composite samples of fruit in the United
States of America. The same survey on oils, fats and shortening showed
residues of 0.025 ppm in one out of 30 composite samples. Methods are
available for measuring residues for use in general regulatory
laboratories but further work is necessary to specify a method for
referee purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit-and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Temporary tolerances
Grapes 2 ppm
Other fruit 1 ppm
Vegetables 0.5 ppm
Tea (from a particular estate)
for blending only 7 ppm
Tea, blended 1 ppm
Beef (fat basis) (at slaughter) 1.5 ppm
Further work or information
ETHION
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre harvest intervals,
and the resultant residues.
2. Data on residue levels in raw agricultural commodities moving in
commerce.
3. Residue data in processed food, including meat, meat products and
wine.
Desirable:
1. Collaborative studies to establish a referee method.
2. Adequate observations of effects in man, including studies of the
metabolic fate.
3. Determination of the metabolic fate in animals.
4. Long-term studies in at least two species.
5. Cholinesterase depression studies at more frequent intervals in
animals.
REFERENCES
Cook, J. W. (1954) Report on determination of insecticides by
enzymatic methods. J. Assoc. Off. Agr. Chem., 37: 561-564
Cassil, C. C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Rev., 1: 37-65
Duggan, R. E. and Weatherwax, J. R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Fahey, J. E., Rodriquez, J. G., Rusk, H. W. and Chaplain, C. E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Entomol., 55: 179-184
Fallscheer, N. O. and Cook, J. W. (1956) Report on enzymatic methods
for insecticides. Studies on the conversion of some thionophosphates
and a dithiophosphate to in vitro cholinesterase inhibitors. J.
Assoc. Off. Agr. Chem., 39: 691-697
Graham, J. R. (1964) Vol. II, Anal. Methods for Pesticides, ed. G.
Zweig, Academic Press
Hazleton Laboratories, Inc. (1958a) Nialate Tech. (1240) Final report.
In vitro cholinesterase studies. Unpublished report
Hazleton Laboratories, Inc. (1958b) Nialate Tech. (1240) Final report.
Subacute feeding studies - rats. Unpublished report
Hazleton Laboratories, Inc. (1958c) Nialate Tech. (1240) Final report.
Subacute administration - dogs. Unpublished report
Hazleton Laboratories, Inc. (1958d) Nialate Techn. Potentiation study.
Acute oral administration - rats. Unpublished report
Hazleton Laboratories, Inc. (1959) Nialate Technical (ethion) Final
report. Ninety-day feeding study - rats. Unpublished report
Hazleton Laboratories, Inc., (1960) Palo Alto, Calif. Unpublished
report
Hazleton Laboratories, Inc. (1961) Ethion. Acute oral administration
- rats. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961a) Effects of ethion on
cholinesterase activity in the dog. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961b) Addendum report.
Effects of ethion on cholinesterase activity in the dog. Unpublished
report
Industrial Bio-Test Laboratories, Inc. (1965a) Acute oral toxicity of
ethion MR E423. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1965b) Three-generation
reproduction study in albino rats on ethion. First generation, second
generation and final report. Unpublished reports
Martin, R. J. and Duggan, R. E. (1968) Pesticide residues in total
diet samples (III). Pesticides Monitoring Journal, 1: 11-20
May and Baker, Ltd. (1960) Ethion. Unpublished report submitted to
the Ministry of Health, United Kingdom
Niagara. (1958) Unpublished report M-602. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1960) Unpublished report M-796. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1966a) Petition No. 351, Niagara Chemicals Division, F.M.C.
to U.S. Food and Drug Administration
Niagara Chemicals Division, F.M.C. Corpn., Middleport, N.Y. (1966b)
Ethion food additive petition. Report No. C.T.B. 23/2/20
Niagara. (1967) Unpublished report M-2131. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Taschenberg, E. F., Avens, A. W., Parsons, G. M. and Gibbs. S. D.
(1963) Disappearance of spray deposits of DDT, methoxychlor,
perthane, ethion and diazinon from Concord grapes. J. Econ. Entomol.,
56: 431-438
Other information on identity and properties
Liquid, practically non-volatile at ordinary temperatures, solidifying
at -12 to -15°C. Insoluble in water, somewhat soluble in kerosene and
petroleum oils, soluble in most organic solvents. Slowly oxidized in
air to a more reactive material.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Studies in vitro showed that the rate of reaction of ethion with
beef erythrocyte cholinesterase is relatively slow, the velocity
constant being about 3.5 × 103 litres per mole per minute. Liver
fortified with diphosphopyridine nucleotide (DPN) converts ethion into
a more potent cholinesterase inhibitor. Non-fortified liver did not
enhance the activity (Hazleton Laboratories, Inc., 1958a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 69 May and Baker. Ltd., 1960
s.c. 630 May and Baker, Ltd., 1960
Rat (M) oral 65 May and Baker, Ltd., 1960
(F) oral 63 May and Baker, Ltd., 1960
(continued)
LD50 mg/kg
Animal Route body-weight References
(M) s.c. 380 May and Baker, Ltd., 1960
(F) s.c. 360 May and Baker, Ltd., 1960
Rat (M) oral 97 Hazleton Laboratories, Inc., 1961
Rat oral 161 Industrial Bio-Test Laboratories, 1965
Short-term studies
In two separate studies, groups comprising equal numbers of male and
female rats were fed dietary levels of 0, 3, 10, 30 and 100 ppm of
ethion for periods up to 13 weeks. Five male and five female animals
from each group were sacrificed after 30, 63 and 93 days for
cholinesterase determination. All animals exhibited normal physical
appearance, behaviour, growth and food consumption during the studies.
There appeared to be little difference in the response of male and
female rats with respect to cholinesterase activity at these levels.
In the 100 ppm group significant inhibition of plasm, red blood cell
and brain cholinesterase activity was evident after the 30, 63 and 93
day test periods. Similar findings were noted in the 30 ppm group but
to a lesser degree. In the 10 ppm group slight depression in plasma
and red blood cell cholinesterase was noted after 30 days but not
after 63 and 93 days, and there was never any effect observed in brain
cholinesterase. At 3 ppm cholinesterase was normal. At the 100 ppm
level plasma cholinesterase activity returned to normal 14 days after
withdrawal of ethion from the diet, but red blood cell and brain
cholinesterase remained slightly depressed. No histological changes
were noted at any level (Hazleton Laboratories, Inc., 1958b).
Five groups, each containing 10 male and 10 female rats, were fed
ethion at dietary levels of 0, 300, 600, 1000 and 1500 ppm for 13
weeks. Growth suppression was observed in all the test groups, except
for the male rats at the 300 and 600 ppm levels. Signs of
intoxication, with increasing frequency and severity in proportion to
the dietary levels, were observed in all test animals, except for the
males fed 300 ppm. All the animals in the 1500 ppm group died during
the first two weeks. Six male and nine female rats died in the 1000
ppm group. Survival in the other groups was comparable to controls.
Complete inhibition of plasma and red cell cholinesterase activity
occurred in all test groups, except in the males fed 300 ppm, where
inhibition was marked but not complete. Almost total inhibition of
brain cholinesterase activity was observed in the female test animals.
The inhibition noted in the males was less marked, but in proportion
to increasing dietary levels. No gross or histological changes, which
could be attributed to ethion, were observed in any of the surviving
rats (Hazleton Laboratories Inc., 1959).
Dog
Groups, each containing two male and two female dogs, received ethion
orally in daily doses of 0.0125, 0.025, 0.075 and 0.25 mg/kg body
weight for 90 days. A control value relative to cholinesterase
activity was established for each dog during a two- week pre-dosage
period.Physical appearance, behaviour, appetite, body weight and
survival were unaffected. Autopsy at the conclusion of the experiment
revealed no pathological changes. Plasma cholinesterase activity was
significantly inhibited in one male dog and one female dog fed 0.25
mg/kg (Hazleton Laboratories Inc., 1958c).
Ethion was administered to groups, each containing three male and
three female dogs, for six days a week during a 90-day period. Dosage
levels were 0, 0.05, 0.075, 0.125, 0.25, 1.25 and 2.5 mg/kg body
weight. No significant cholinesterase depression was observed at
levels below 0.25 mg/kg. Depression of erythrocyte cholinesterase
occurred only in the 2.5 mg/kg group. This depression was observed
early in the test and persisted throughout its duration, but recovery
was complete 24 days after withdrawal. Plasma cholinesterase was
depressed more readily, occurring early in the test at the 2.5 mg/kg
level but was not evident until the eleventh week of the test at the
0.25 mg/kg level. Recovery was achieved 24 days after withdrawal in
the case of the higher dose level and within nine days in the case of
the 0.25 mg/kg level (Industrial Bio-Test Laboratories Inc., 1961a,
1961b).
Special studies
(a) Reproduction
Groups of male and female rats were fed diets containing 0, 2 and 30
ppm of ethion through three generations. The second litter from each
generation (F1b and F2b) was selected as parental animals to
establish the next generation. No adverse effects were noted among
either the parental animals or their progeny as regards growth,
mortality, reactions, pathology, reproduction performance and survival
indices of the young. Cholinesterase determinations were not conducted
in this study (Industrial Bio-Test Laboratories Inc., 1965b).
(b) Potentiation
When ethion was administered to rats in combination with nine other
organo-phosphorus insecticides, potentiation was observed only with
malathion, where the ratio of observed to theoretical acute LD50 was
2.9:1 (Hazleton Laboratories Inc., 1958d).
Comments
Ninety-day toxicity studies have been made in both rats and dogs
together with a three-generation reproduction study in rats. The
levels causing no toxic effects have been determined mainly on the
results of the extensive cholinesterase inhibition studies, and were
based on the absence of red cell cholinesterase depression. However,
it would have been desirable to have data on cholinesterase depression
at more frequent intervals.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day;
Dog: 0.125 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.00125 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Ethion is used as a pre-harvest topical application to a variety of
food crops especially citrus and deciduous fruit for the control of
many species of aphids, scale insects, mites, leaf miners and leaf
hoppers. It is also used in a cattle dip for the control of ticks and
as a "back-line" treatment for the control of buffalo fly.
Post-harvest treatments
No known post-harvest uses.
Residues resulting from supervised trials
The typical data presented below have been extracted from Internal
Reports of Niagara Chemical Division, FMC, except for that on grapes
(Taschenberg et al., 1963) and strawberries (Fakey et al., 1962).
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Almonds 1.9 0-28 < 0.1
Apples 1 45 1
(continued)
Rate of No. of Pre-harvest Residue
Crop application (kg/ha) treatments interval (days) (ppm)
Beans 1.9 1 7 0.27-0.43
Beef 0.075% emulsion 3 3 0.01
Beef, fat " 3 3 0.86
Citrus (juice) 1.25 2 15 0
" (peel) 1.25 1 30 1
Cucumbers 2.5 6 11 0.04
Grapes 2.5 1 28 2
Melons 1.25 1 7 0.07
Raisins 9 3 9 1.25-10.4
Strawberries 0.6 3 8 0.1
Tea, leaves 0.9 1 7 1.71-7.06
Tomatoes 0.6 2 7 0.17-0.34
Fate of residues
General comments
Ethion shows some systemic activity. It is taken into the plant and
translocated to other parts where it kills mites and certain insects.
This effect diminishes rather rapidly, and correlates with the
relative instability of the oxidized material by comparison with
ethion itself. The residues are reduced by oxidation and subsequent
hydrolysis on the surface or in the plant.
In soils. It is usually used as a spray and no data on its
degradation in soil have been made available.
In plants. A surface residue on cotton foliage of 86 ppm was reduced
to less than 1 ppm After eight days. Simultaneous analysis of
cottonseed showed no detectable residue. Onions planted in 20-feet
rows treated with 10 lb of three per cent dust per 20-feet rows
showed insignificant residues at harvest, 123 days later (Niagara,
1958).
Tea, at harvest, sometimes shows residues as high as 7 ppm, as the
result of "spot" treatment of particular bushes but the brewed product
of these particular leaves shows residues of 0.25 ppm or less. Since
tea is blended prior to sale to the ultimate consumer, a tolerance on
blended tea of not more than 1 ppm and a tolerance of 7 ppm on tea
from a particular estate appear justified (Niagara, 1966a).
In animals. Lactating cows fed up to 20 ppm of radioactive ethion in
their diet showed no ethion residues in their milk, the bulk of the
radioactivity being associated with the protein fraction. In meat, the
highest radioactivity occurred in the liver which contained an average
of 3.15 ppm ethion-P32 equivalents at the end of the 28-day feeding
at 20 ppm. This dropped by 60 per cent within 12 days after
termination of the feeding period. However, chemical analysis showed
that the radioactive material was not ethion but products from its
metabolism (Hazelton Laboratories Inc., 1960; Niagara, 1960). Thus
ethion residues in citrus pulp fed to cattle would not contribute to
residues in milk.
When animals were dipped, residues arising from absorption through the
skin consisted largely of ethion and were mainly in the fat. The
active oxidation products, the monothiol and bisthiol derivatives,
were found at less than 0.01 ppm and 0.02 ppm respectively.
In storage and processing. No data have been made available.
Evidence of residues in food, in commerce or at consumption
Duggan and Weatherwax (1967), in their survey of dietary intake of
pesticide chemicals, found that the incidence and intake of ethion was
too low to be detected. Trace amounts contributing to less than 0.001
mg per day were found in fruit, samples being taken from June 1964 to
April 1966.
Martin and Duggan (1968) found residues of ethion at 0.024 to 0.054
ppm in three out of 30 composite samples of fruit collected from 30
markets in 29 different cities in the United States of America from
June 1966 to April 1967. In the same studies ethion was found at 0.025
ppm in one out of 30 composite samples of oils, fats and shortening.
Methods of residue analysis
A colorimetric procedure is based on the extraction of ethion, and
necessary clean-up, followed by hydrolysis to diethylphosphorodithioic
acid. The colour complex then formed with copper sulfate is measured
at 418 mµ. From the optical density the amount of ethion is determined
from a standard curve (Graham, 1964).
An enzymatic method is based on the oxidation of ethion with bromine
water to the active phosphorothiolate and subsequent measurement of
the extent of inhibition of the cholinesterase from human blood plasma
(Cook, 1954; Fallscheer and Cook, 1956).
The clean-up procedure associated with the colorimetric method is so
designed that determination of ethion in the presence of other organo-
phosphorus insecticides can be made. However, the method does not
measure oxidized or metabolized derivatives that contain phosphoryl
rather than phosphorothiono groups. The enzyme method on the other
hand measures the oxidized and unchanged ethion but does not
differentiate from other cholinesterase inhibitors. The two methods
are in substantial agreement however in the determination of ethion
residues, which implies that there is little of the residue in the
oxidized state (Niagara, 1958).
A microcoulometric gas chromatographic method (Cassil, 1962) has been
used to determine both ethion and its oxygen analogues independently
(Niagara, 1967). The methods are sensitive to 0.05 ppm in most foods.
For meat samples, following suitable extraction of macerated samples,
partitioning between solvents and further clean-up on Florisil
columns, the residue is determined by a gas chromatograph using a
sodium thermionic detector. Recoveries of 92 per cent are normal with
a sensitivity to 0.001 ppm. This precise method does not measure the
oxidation products which contribute an insignificant amount to the
residues (see above). With a different column the oxidation products
can be measured but at a lower recovery rate (Niagara, 1966b).
National tolerances
Tolerance
Country Crop (ppm)
Canada Raisins 4
Citrus, grapes, peaches, plums,
strawberries, nectarines, beans,
melons, tomatoes 2
Apples, pears, eggplant, onions,
peppers 1
Summer squash 0.5
Almonds 0.1
United States Citrus pulps, dehydrated for
of America cattle feed 10
Dried tea 7
Almond hulls 5
Raisins 4
(continued)
Tolerance
Country Crop (ppm)
Beef (fat basis) 2.5
Apples, citrus, grapes, plums,
strawberries, beans, melons,
tomatoes, sorghum grain 2
Pears, peaches, nectarines,
eggplant, onions, peppers 1
Beef or beef products 0.75
Cucumbers, summer squash 0.5
Almonds 0.1
Milk "0"
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Ethion is an insecticide and acaricide for use on both plants and
animals. The technical product contains a trace of an active oxidized
metabolite but it is not of significance in the final residue. Ethion
is used as a pre-harvest application to a variety of food crops
especially citrus and deciduous fruit for the control of aphids, scale
insects, mites, leaf miners and leaf hoppers. It shows minor systemic
activity and residues are reduced also by weathering. No residues of
ethion were found in milk or meat from lactating cows fed citrus pulp
containing ethion residues. However, where ethion was applied as a dip
to animals for the control of ticks, after a three-day interval
following treatment, the highest level was in the fat at 1.25 ppm with
less than 0.01 ppm in internal organs and muscle. The two active
oxidation metabolites were present in insignificant quantity.
Accordingly, animals dipped in ethion emulsion should be held three
days prior to slaughter to ensure that the recommended tolerances,
applied at slaughter, are not exceeded.
In market sample studies in 1966-67, residues were found at 0.024 to
0.054 ppm in three out of 30 composite samples of fruit in the United
States of America. The same survey on oils, fats and shortening showed
residues of 0.025 ppm in one out of 30 composite samples. Methods are
available for measuring residues for use in general regulatory
laboratories but further work is necessary to specify a method for
referee purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit-and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Temporary tolerances
Grapes 2 ppm
Other fruit 1 ppm
Vegetables 0.5 ppm
Tea (from a particular estate)
for blending only 7 ppm
Tea, blended 1 ppm
Beef (fat basis) (at slaughter) 1.5 ppm
Further work or information
ETHION
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre harvest intervals,
and the resultant residues.
2. Data on residue levels in raw agricultural commodities moving in
commerce.
3. Residue data in processed food, including meat, meat products and
wine.
Desirable:
1. Collaborative studies to establish a referee method.
2. Adequate observations of effects in man, including studies of the
metabolic fate.
3. Determination of the metabolic fate in animals.
4. Long-term studies in at least two species.
5. Cholinesterase depression studies at more frequent intervals in
animals.
REFERENCES
Cook, J. W. (1954) Report on determination of insecticides by
enzymatic methods. J. Assoc. Off. Agr. Chem., 37: 561-564
Cassil, C. C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Rev., 1: 37-65
Duggan, R. E. and Weatherwax, J. R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Fahey, J. E., Rodriquez, J. G., Rusk, H. W. and Chaplain, C. E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Entomol., 55: 179-184
Fallscheer, N. O. and Cook, J. W. (1956) Report on enzymatic methods
for insecticides. Studies on the conversion of some thionophosphates
and a dithiophosphate to in vitro cholinesterase inhibitors. J.
Assoc. Off. Agr. Chem., 39: 691-697
Graham, J. R. (1964) Vol. II, Anal. Methods for Pesticides, ed. G.
Zweig, Academic Press
Hazleton Laboratories, Inc. (1958a) Nialate Tech. (1240) Final report.
In vitro cholinesterase studies. Unpublished report
Hazleton Laboratories, Inc. (1958b) Nialate Tech. (1240) Final report.
Subacute feeding studies - rats. Unpublished report
Hazleton Laboratories, Inc. (1958c) Nialate Tech. (1240) Final report.
Subacute administration - dogs. Unpublished report
Hazleton Laboratories, Inc. (1958d) Nialate Techn. Potentiation study.
Acute oral administration - rats. Unpublished report
Hazleton Laboratories, Inc. (1959) Nialate Technical (ethion) Final
report. Ninety-day feeding study - rats. Unpublished report
Hazleton Laboratories, Inc., (1960) Palo Alto, Calif. Unpublished
report
Hazleton Laboratories, Inc. (1961) Ethion. Acute oral administration
- rats. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961a) Effects of ethion on
cholinesterase activity in the dog. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1961b) Addendum report.
Effects of ethion on cholinesterase activity in the dog. Unpublished
report
Industrial Bio-Test Laboratories, Inc. (1965a) Acute oral toxicity of
ethion MR E423. Unpublished report
Industrial Bio-Test Laboratories, Inc. (1965b) Three-generation
reproduction study in albino rats on ethion. First generation, second
generation and final report. Unpublished reports
Martin, R. J. and Duggan, R. E. (1968) Pesticide residues in total
diet samples (III). Pesticides Monitoring Journal, 1: 11-20
May and Baker, Ltd. (1960) Ethion. Unpublished report submitted to
the Ministry of Health, United Kingdom
Niagara. (1958) Unpublished report M-602. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1960) Unpublished report M-796. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Niagara. (1966a) Petition No. 351, Niagara Chemicals Division, F.M.C.
to U.S. Food and Drug Administration
Niagara Chemicals Division, F.M.C. Corpn., Middleport, N.Y. (1966b)
Ethion food additive petition. Report No. C.T.B. 23/2/20
Niagara. (1967) Unpublished report M-2131. Niagara Chemicals Division,
F.M.C. Corpn., Middleport, N.Y.
Taschenberg, E. F., Avens, A. W., Parsons, G. M. and Gibbs. S. D.
(1963) Disappearance of spray deposits of DDT, methoxychlor,
perthane, ethion and diazinon from Concord grapes. J. Econ. Entomol.,
56: 431-438
