
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
PARATHION-METHYL
This pesticide was evaluated for acceptable daily intake under the
heading "Methyl parathion" by the Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues (FAO/WHO, 1965).
Since that time additional toxicological data have become available as
well as data on its residues in food and their evaluation. The earlier
monograph is now rendered obsolete and a completely revised monograph
is presented in its entirety below.
IDENTITY
Chemical name
OO-dimethyl O-(4-nitrophenyl) phosphorothioate (IUPAC)
Synonyms
Metaphos, Folidol M, E 605, Nitrox, Wofatox
Formula
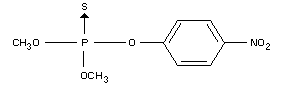 Other information on identity and properties
Typical analyses of technical parathion-methyl are not available. In
1966 the world production of parathion-methyl was 31 700 metric tons
(United States of America production, 14 800 metric tons).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When 32P-labelled parathion-methyl was administered orally to
guinea-pigs, the phosphorus was found to enter the organs almost
immediately, and the maximum tissue level was attained in one to two
hours. A high degree of absorption was found in the liver (Gar et al.,
1958).
Parathion-methyl is biologically similar to parathion and is
metabolized to its oxygen analogue, paraoxon-methyl (Augustinsson and
Jonsson, 1957).
Parathion-methyl is an in vivo cholinesterase inhibitor (Williams et
al., 1959). It also inhibits this enzyme in vitro, however it is
weaker in this respect than its ethyl analogue, parathion (DuBois and
Coon, 1952). The same is true of the respective oxygen metabolites;
paraoxon-methyl being a weaker cholinesterase inhibitor than paraoxon.
The in vitro molar I50 value for paraoxon-methyl, using rat brain
cholinesterase, is 4 × 10-8 (Davison, 1955).
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse oral 32.1 Ikeda, 1962
Mouse oral 150 Wills, 1968
Rat (M) oral 14 Wills, 1968
Rat (F) oral 24 Wills, 1968
Rat oral 17.2 Hagan, 1958
Rat (F) oral 9.7-14.8 Deichmann et al., 1952
Rat i.p. 3.5 DuBois and Coon, 1952
Rabbit oral 420 Wills, 1968
(in oil)
Rabbit oral 1 270 Wills, 1968
(undiluted)
Short-term studies
Dog. Pairs of dogs, comprising one male and one female, were fed
parathion-methyl for 12 weeks at dietary levels of 5, 20, and 50 ppm
along with four dogs used as controls. In the 20 and 50 ppm group,
erythrocyte cholinesterase began to be significantly depressed soon
after commencement of the test-diet. The same was true of plasma
cholinesterase in the 50 ppm group. Maximum extent of depression was
attained at the end of the 12-week period but recovery was complete
within four to eight weeks after withdrawal of parathion-methyl.
Depression of plasma cholinesterase was questionable at the 20 ppm
level and no significant anti-cholinesterase activity was found in the
5 pm group.
Long-term studies
No information available.
Special studies
(a) Reproduction
A three-generation reproduction study using 10 male and 20 female rats
per dose level for each generation, at 0, 10 and 30 ppm
parathion-methyl, and comprising two litters per generation, revealed
no consistent effect on the number of live or stillbirths, birth
weights, physical structure of newborn, litter size, weanling weights
or percentage survival to weaning.
Sporadic effects included lower weanling survival rate in F1a, F1b
and F2a generations at 30 ppm, and in F3a generation at 10 ppm,
increased stillbirth rate in F1b and F3a generations at 30 ppm,
and F3a at 10 ppm; reduced mean weanling rate in F2a generation at
30 ppm, and F1b generation at 10 ppm. Reduced reproductive
performance in F1a, F1b, F2aand F3b generations at 30 ppm, was
the only parameter consistently affected. No such activity was evident
at the 10 ppm level (Woodard Research Corp., 1966).
(b) Teratogenicity
Some cases of foetal deaths and malformations have been reported in
Japan and may possibly be related to the use of organo-phosphorus
insecticides in the field (Ogi and Hamada, 1965). Parathion was
identified in a human term foetus whose mother had used it to commit
suicide (Le Breton et al., 1963). No significant developmental defects
in rats whose mothers had been injected intraperitoneally with methyl
parathion was observed (Fish, 1966).
Because of these reports, the effect on organogenesis in the rat and
mouse was studied by injecting intraperitoneally parathion-methyl
suspended in a 0.5 per cent aqueous solution of sodium carboxymethyl
cellulose once on day 12 of gestation in rats and once on day 10 in
mice. The dosage was 5 to 15 mg/kg of body-weight in rats and 20 to 60
mg/kg in mice. The animals were killed near term on day 21 in rats and
on day 18 in mice. The foetuses were examined for intrauterine death,
external malformations, internal abnormalities and skeletal
abnormalities. All animals of both species showed signs of toxicity
about 30 minutes after administration of parathion-methyl. Ataxia and
paralysis of voluntary muscles were followed by hypersecretion of
saliva and tears, urinary incontinence, tremor and general
convulsions. Some died and the others recovered by the next day. Food
and water intake in rats was noted to be lower for three or four days
and there was a weight loss. No external or internal malformations
were found. In mice lethality, teratogenicity and suppression of
growth were noted in the group treated with the higher dosage. The
only malformation was cleft palate, the incidence of which was 0.71
per cent when used as control groups in other experiments.
Retardation of ossification of the caudal vertebrae and increased
incidence of cervical rib occurred in the group treated with the
higher dose (Tanimura et al., 1967).
Observations in man
Normal levels of red blood cell and plasma cholinesterase were
established in 12 human subjects, five of these subjects were given
parathion-methyl in dose levels of 3 mg/day for 28 days, 3.5 mg/day
for 28 days and 4.0 mg/day for 43 days. No anti-cholinesterase
activity in plasma on red blood cells, and no side effects, were
observed (Moeller and Rider, 1961)
In a second study, three groups of five subjects were given
parathion-methyl, The first group received 4.5 mg/day for 30 days
followed by 5.0 mg/day for 29 days; the second group, 5.5 mg/day for
28 days, then 6.0 mg/day for 29 days; and the third group, 6.5 mg/day
for 35 days, then 7.0 mg/day for 24 days. The maximum depression of
cholinesterase occurred in plasma and was approximately 15 per cent
of the control values established beforehand (Moeller and Rider,
1962).
In a continuing experiment, groups of five subjects were given
parathion-methyl for 30 days at daily doses of 7 mg, 7.5 mg, 8 mg and
9 mg. Plasma and red blood cell cholinesterase activities were within
20 per cent of the pre-established control values (Moeller and Rider,
1963).
Comments
Teratogenic effects were observed in mice only after parenteral
administration, on the other hand the reproduction studies in rats
showed some disturbance of the physiology of the reproductive process.
It was therefore found necessary to re-evaluate this compound by using
a higher safety factor and to change the acceptable daily intake to a
temporary acceptable daily intake.
TOXICOLOGICAL EVALUATION
Estimate of temporary acceptable intake
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Parathion-methyl is a broad spectrum insecticide and is mainly used on
cotton, but is also used on food crops including fruits and
vegetables.
For plant protection one, two or more applications are usually made,
the pre-harvest interval varying from 14 to 21 days and is usually
twice as long under greenhouse conditions.
Reported application rates are given in the following table:
Application rate*
Crop (g/ha)
Fruit 240-720
Vegetables 240-720
Grain crops (rice) 240-720
Hay, forage crops,
and cotton 100-1 000
* USDA, 1967.
Prescribed pre-harvest intervals in various countries are given below:
Pre-harvest
Country interval (days)
Austria 21
Belgium 14
Denmark 14
Finland 21
France 15
Germany (B.R.D.) 14
Germany (D.D.R.) 14/21
Italy 14
Netherlands, field conditions 21
Netherlands, greenhouses 28
Norway 14
Portugal 21
Switzerland 21
United States of America 5/21
Post-harvest treatments
Parathion-methyl is not used on stored food.
Other uses
Parathion-methyl has a very limited use for public health purposes.
Residues resulting from supervised trials
Many residue studies based on chemical methods, gas-liquid
chromatography and cholinesterase inhibition, are available. Residue
data include the oxon derivative. Parathion-methyl penetrates through
the cuticula but is usually not translocated within plants (Shipp,
1963; Stobwasser, 1967). Only in the case of carrots was it found that
parathion-methyl penetrates into the oil-cells and undergoes very slow
degradation (Engst, 1966). On the surfaces of plants parathion-methyl
residues decrease very rapidly as shown by Shipp (1963) on cotton. The
half-life is usually one to two days:
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cabbage- Germany 3 0.1-0.7 0.3 III 1-1.5
lettuce 7 <0.15 <0.15
(field)
Cabbage- Germany 4 0.76 II 2-3
lettuce 7 0.65
(greenhouse) 14 0.06
Tomatoes Germany 3 0.06 I
(field) 7 n.d.
Tomatoes Germany 3 n.d.-0.15 0.08 I 1.5
(greenhouse) 7 n.d. <0.03
3 0.07 II
7 0.04
Apples Italy 10 0.05 I
Apples Germany 3 0.38-0.54 II 2.5-5
7 0.11-0.12
14 0.05-0.15
Cabbage United 14 <1 <1 Hoelscher
States of 1968
America
Peas United 5 1.1 Hoelscher
States of 1968
America
Sorghum United 3 0.36 Dorough 1-1.5
grain States of 6 n.d. 1966
America
(continued)
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cotton United 3 2.5-18.0 8.7 Shipp 1
States of 7 1.0-11.8 5.3 1963
America 12 0.8-9.9 4.2
References: I Institut für Pflanzenschutz, Hohenheim, Germany
II Professor Maier-Bode, Bonn, Germany
III Biologisches Institut der Farbenfabriken Bayer, A.G. Leverkusen, Germany
Disappearance of residues is dependent on climatic factors, rain appreciably reducing
the residues (Hightower, 1958).
Fate of residues
General comments
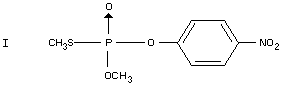
Under ultra-violet irradiation parathion-methyl is isomerized to the
S-methyl compound (I) (Metcalf, 1953). Ultra-violet degradation under
field conditions has not been reported. Upon heating to 140-160°C,
parathion-methyl is transformed to the S-methyl compound (I)
(McPherson, 1956; Metcalf, 1953). Thermal decomposition under field
conditions is not reported.
Other information on identity and properties
Typical analyses of technical parathion-methyl are not available. In
1966 the world production of parathion-methyl was 31 700 metric tons
(United States of America production, 14 800 metric tons).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When 32P-labelled parathion-methyl was administered orally to
guinea-pigs, the phosphorus was found to enter the organs almost
immediately, and the maximum tissue level was attained in one to two
hours. A high degree of absorption was found in the liver (Gar et al.,
1958).
Parathion-methyl is biologically similar to parathion and is
metabolized to its oxygen analogue, paraoxon-methyl (Augustinsson and
Jonsson, 1957).
Parathion-methyl is an in vivo cholinesterase inhibitor (Williams et
al., 1959). It also inhibits this enzyme in vitro, however it is
weaker in this respect than its ethyl analogue, parathion (DuBois and
Coon, 1952). The same is true of the respective oxygen metabolites;
paraoxon-methyl being a weaker cholinesterase inhibitor than paraoxon.
The in vitro molar I50 value for paraoxon-methyl, using rat brain
cholinesterase, is 4 × 10-8 (Davison, 1955).
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse oral 32.1 Ikeda, 1962
Mouse oral 150 Wills, 1968
Rat (M) oral 14 Wills, 1968
Rat (F) oral 24 Wills, 1968
Rat oral 17.2 Hagan, 1958
Rat (F) oral 9.7-14.8 Deichmann et al., 1952
Rat i.p. 3.5 DuBois and Coon, 1952
Rabbit oral 420 Wills, 1968
(in oil)
Rabbit oral 1 270 Wills, 1968
(undiluted)
Short-term studies
Dog. Pairs of dogs, comprising one male and one female, were fed
parathion-methyl for 12 weeks at dietary levels of 5, 20, and 50 ppm
along with four dogs used as controls. In the 20 and 50 ppm group,
erythrocyte cholinesterase began to be significantly depressed soon
after commencement of the test-diet. The same was true of plasma
cholinesterase in the 50 ppm group. Maximum extent of depression was
attained at the end of the 12-week period but recovery was complete
within four to eight weeks after withdrawal of parathion-methyl.
Depression of plasma cholinesterase was questionable at the 20 ppm
level and no significant anti-cholinesterase activity was found in the
5 pm group.
Long-term studies
No information available.
Special studies
(a) Reproduction
A three-generation reproduction study using 10 male and 20 female rats
per dose level for each generation, at 0, 10 and 30 ppm
parathion-methyl, and comprising two litters per generation, revealed
no consistent effect on the number of live or stillbirths, birth
weights, physical structure of newborn, litter size, weanling weights
or percentage survival to weaning.
Sporadic effects included lower weanling survival rate in F1a, F1b
and F2a generations at 30 ppm, and in F3a generation at 10 ppm,
increased stillbirth rate in F1b and F3a generations at 30 ppm,
and F3a at 10 ppm; reduced mean weanling rate in F2a generation at
30 ppm, and F1b generation at 10 ppm. Reduced reproductive
performance in F1a, F1b, F2aand F3b generations at 30 ppm, was
the only parameter consistently affected. No such activity was evident
at the 10 ppm level (Woodard Research Corp., 1966).
(b) Teratogenicity
Some cases of foetal deaths and malformations have been reported in
Japan and may possibly be related to the use of organo-phosphorus
insecticides in the field (Ogi and Hamada, 1965). Parathion was
identified in a human term foetus whose mother had used it to commit
suicide (Le Breton et al., 1963). No significant developmental defects
in rats whose mothers had been injected intraperitoneally with methyl
parathion was observed (Fish, 1966).
Because of these reports, the effect on organogenesis in the rat and
mouse was studied by injecting intraperitoneally parathion-methyl
suspended in a 0.5 per cent aqueous solution of sodium carboxymethyl
cellulose once on day 12 of gestation in rats and once on day 10 in
mice. The dosage was 5 to 15 mg/kg of body-weight in rats and 20 to 60
mg/kg in mice. The animals were killed near term on day 21 in rats and
on day 18 in mice. The foetuses were examined for intrauterine death,
external malformations, internal abnormalities and skeletal
abnormalities. All animals of both species showed signs of toxicity
about 30 minutes after administration of parathion-methyl. Ataxia and
paralysis of voluntary muscles were followed by hypersecretion of
saliva and tears, urinary incontinence, tremor and general
convulsions. Some died and the others recovered by the next day. Food
and water intake in rats was noted to be lower for three or four days
and there was a weight loss. No external or internal malformations
were found. In mice lethality, teratogenicity and suppression of
growth were noted in the group treated with the higher dosage. The
only malformation was cleft palate, the incidence of which was 0.71
per cent when used as control groups in other experiments.
Retardation of ossification of the caudal vertebrae and increased
incidence of cervical rib occurred in the group treated with the
higher dose (Tanimura et al., 1967).
Observations in man
Normal levels of red blood cell and plasma cholinesterase were
established in 12 human subjects, five of these subjects were given
parathion-methyl in dose levels of 3 mg/day for 28 days, 3.5 mg/day
for 28 days and 4.0 mg/day for 43 days. No anti-cholinesterase
activity in plasma on red blood cells, and no side effects, were
observed (Moeller and Rider, 1961)
In a second study, three groups of five subjects were given
parathion-methyl, The first group received 4.5 mg/day for 30 days
followed by 5.0 mg/day for 29 days; the second group, 5.5 mg/day for
28 days, then 6.0 mg/day for 29 days; and the third group, 6.5 mg/day
for 35 days, then 7.0 mg/day for 24 days. The maximum depression of
cholinesterase occurred in plasma and was approximately 15 per cent
of the control values established beforehand (Moeller and Rider,
1962).
In a continuing experiment, groups of five subjects were given
parathion-methyl for 30 days at daily doses of 7 mg, 7.5 mg, 8 mg and
9 mg. Plasma and red blood cell cholinesterase activities were within
20 per cent of the pre-established control values (Moeller and Rider,
1963).
Comments
Teratogenic effects were observed in mice only after parenteral
administration, on the other hand the reproduction studies in rats
showed some disturbance of the physiology of the reproductive process.
It was therefore found necessary to re-evaluate this compound by using
a higher safety factor and to change the acceptable daily intake to a
temporary acceptable daily intake.
TOXICOLOGICAL EVALUATION
Estimate of temporary acceptable intake
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Parathion-methyl is a broad spectrum insecticide and is mainly used on
cotton, but is also used on food crops including fruits and
vegetables.
For plant protection one, two or more applications are usually made,
the pre-harvest interval varying from 14 to 21 days and is usually
twice as long under greenhouse conditions.
Reported application rates are given in the following table:
Application rate*
Crop (g/ha)
Fruit 240-720
Vegetables 240-720
Grain crops (rice) 240-720
Hay, forage crops,
and cotton 100-1 000
* USDA, 1967.
Prescribed pre-harvest intervals in various countries are given below:
Pre-harvest
Country interval (days)
Austria 21
Belgium 14
Denmark 14
Finland 21
France 15
Germany (B.R.D.) 14
Germany (D.D.R.) 14/21
Italy 14
Netherlands, field conditions 21
Netherlands, greenhouses 28
Norway 14
Portugal 21
Switzerland 21
United States of America 5/21
Post-harvest treatments
Parathion-methyl is not used on stored food.
Other uses
Parathion-methyl has a very limited use for public health purposes.
Residues resulting from supervised trials
Many residue studies based on chemical methods, gas-liquid
chromatography and cholinesterase inhibition, are available. Residue
data include the oxon derivative. Parathion-methyl penetrates through
the cuticula but is usually not translocated within plants (Shipp,
1963; Stobwasser, 1967). Only in the case of carrots was it found that
parathion-methyl penetrates into the oil-cells and undergoes very slow
degradation (Engst, 1966). On the surfaces of plants parathion-methyl
residues decrease very rapidly as shown by Shipp (1963) on cotton. The
half-life is usually one to two days:
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cabbage- Germany 3 0.1-0.7 0.3 III 1-1.5
lettuce 7 <0.15 <0.15
(field)
Cabbage- Germany 4 0.76 II 2-3
lettuce 7 0.65
(greenhouse) 14 0.06
Tomatoes Germany 3 0.06 I
(field) 7 n.d.
Tomatoes Germany 3 n.d.-0.15 0.08 I 1.5
(greenhouse) 7 n.d. <0.03
3 0.07 II
7 0.04
Apples Italy 10 0.05 I
Apples Germany 3 0.38-0.54 II 2.5-5
7 0.11-0.12
14 0.05-0.15
Cabbage United 14 <1 <1 Hoelscher
States of 1968
America
Peas United 5 1.1 Hoelscher
States of 1968
America
Sorghum United 3 0.36 Dorough 1-1.5
grain States of 6 n.d. 1966
America
(continued)
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cotton United 3 2.5-18.0 8.7 Shipp 1
States of 7 1.0-11.8 5.3 1963
America 12 0.8-9.9 4.2
References: I Institut für Pflanzenschutz, Hohenheim, Germany
II Professor Maier-Bode, Bonn, Germany
III Biologisches Institut der Farbenfabriken Bayer, A.G. Leverkusen, Germany
Disappearance of residues is dependent on climatic factors, rain appreciably reducing
the residues (Hightower, 1958).
Fate of residues
General comments
Under ultra-violet irradiation parathion-methyl is isomerized to the
S-methyl compound (I) (Metcalf, 1953). Ultra-violet degradation under
field conditions has not been reported. Upon heating to 140-160°C,
parathion-methyl is transformed to the S-methyl compound (I)
(McPherson, 1956; Metcalf, 1953). Thermal decomposition under field
conditions is not reported.
 Parathion-methyl is quickly hydrolyzed by 1 N NaOH (K/min = 0.69), but
no information of hydrolysis under field conditions is available
(Metcalf, 1953).
No data on metabolism in humans has been reported. Dietary intake for
all organo-phosphates in the United States of America in 1966/1967 was
0.00025 mg/kg body-weight/day, the three-year average being 0.00013
mg/kg body-weight/day (Duggan, 1968).
In soils
Parathion-methyl is quickly broken down in soil. In silty loam, 30
days after application of 0.1 ppm active ingredient, only three per
cent was recovered. Disappearance of residues is accelerated by
moisture and micro-organisms (Lichtenstein and Schulz, 1964). Trials
on leaching in soil showed that in soil-water, parathion-methyl has a
persistence of two weeks and in lake-water of three months, but no
conclusions can be drawn concerning leaching of parathion-methyl from
soil to rivers (Lichtenstein et al., 1966).
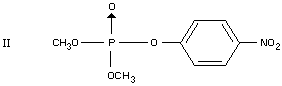
In plants
Three metabolites of parathion-methyl were found in cottonleaves, one
of which was identified as paraoxon-methyl (II) (Shipp, 1963; Coffin,
1964). After application of parathion-methyl to lettuce only small
amounts of residues of paraoxon-methyl were found (one to four per
cent of the total active residue) (Möllhoff, 1968). Under normal
field conditions, paraoxon-methyl constitutes only a small fraction (a
few per cent) of the residue of the parent compound (Möllhoff, 1968).
Under elevated temperatures (72-112°F) and high humidity (40-62 per
cent) the total residue on cotton after an interval of 72 hours
consisted of 14.5 per cent parathion-methyl and 38.5 per cent
paraoxon-methyl (Shipp, 1963). However, the oxon, when formed, is
subject to rapid hydrolysis in and on the plant (Möllhoff, 1968). The
classical colorimetric method by Averell et al. (1948) is able to
determine the parent compound plus the oxon. When applying GLC methods
the parent compound and the oxon can be determined separately.
Probably, breakdown of parathion-methyl in higher plants is the same
as the breakdown of parathion, but more rapid. The products of
hydrolysis - phosphorothioate and 4-nitro-phenol - are only present in
small amounts and seem to have no toxicological importance.
Parathion-methyl is quickly hydrolyzed by 1 N NaOH (K/min = 0.69), but
no information of hydrolysis under field conditions is available
(Metcalf, 1953).
No data on metabolism in humans has been reported. Dietary intake for
all organo-phosphates in the United States of America in 1966/1967 was
0.00025 mg/kg body-weight/day, the three-year average being 0.00013
mg/kg body-weight/day (Duggan, 1968).
In soils
Parathion-methyl is quickly broken down in soil. In silty loam, 30
days after application of 0.1 ppm active ingredient, only three per
cent was recovered. Disappearance of residues is accelerated by
moisture and micro-organisms (Lichtenstein and Schulz, 1964). Trials
on leaching in soil showed that in soil-water, parathion-methyl has a
persistence of two weeks and in lake-water of three months, but no
conclusions can be drawn concerning leaching of parathion-methyl from
soil to rivers (Lichtenstein et al., 1966).
In plants
Three metabolites of parathion-methyl were found in cottonleaves, one
of which was identified as paraoxon-methyl (II) (Shipp, 1963; Coffin,
1964). After application of parathion-methyl to lettuce only small
amounts of residues of paraoxon-methyl were found (one to four per
cent of the total active residue) (Möllhoff, 1968). Under normal
field conditions, paraoxon-methyl constitutes only a small fraction (a
few per cent) of the residue of the parent compound (Möllhoff, 1968).
Under elevated temperatures (72-112°F) and high humidity (40-62 per
cent) the total residue on cotton after an interval of 72 hours
consisted of 14.5 per cent parathion-methyl and 38.5 per cent
paraoxon-methyl (Shipp, 1963). However, the oxon, when formed, is
subject to rapid hydrolysis in and on the plant (Möllhoff, 1968). The
classical colorimetric method by Averell et al. (1948) is able to
determine the parent compound plus the oxon. When applying GLC methods
the parent compound and the oxon can be determined separately.
Probably, breakdown of parathion-methyl in higher plants is the same
as the breakdown of parathion, but more rapid. The products of
hydrolysis - phosphorothioate and 4-nitro-phenol - are only present in
small amounts and seem to have no toxicological importance.
 In animals
In warm-blooded animals parathion-methyl is transformed enzymatically
to paraoxon-methyl (II) (Augustinsson and Jonsson, 1957).
In storage and processing
Although residues of parathion-methyl in vegetables and fruit are
found near the peel, washing does not markedly reduce residues
(Stobwasser, 1967). During ensilage of pea plants the active
ingredient is quickly broken down because of the weak acids present.
One month after ensilage, residues up to 0.2 ppm were found; after
another month no residues could be detected (Heinisch, 1966).
Evidence of residues in food in commerce or at consumption
Samples of beef, butter and rice were examined for the presence of
organo-phosphorus compounds by the non-specific cholinesterase
inhibition method. Calculated as diasinon or parathion-methyl,
residues in all samples were 0.1 ppm or less (data from Woodstock
Agricultural Research Centre, Sittingbourne, 1967).
Methods of residue analysis
The residue analysis of parathion-methyl is usually carried out
colorimetrically, the results including metabolites and all other
substances with the nitrophenyl-group (Averell, 1948). Specific
detection of parathion-methyl is possible by paper and thin-layer
chromatography (Abbott, 1965; Getz, 1963). Gas-liquid chromatographic
analysis is carried out with microcoulometric (Nelson, 1966), electron
capture (Giuffrida, 1966; Egan, 1964; Möllhoff, 1967) and phosphorus
detectors (Möllhoff, 1967), The sensitivity of the colorimetric method
is 0.05 ppm, and of the gas-liquid chromatography methods, 0.01-0.02
ppm. The latter can be used as a referee method.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
National tolerances
Country Crop Tolerance*
(ppm)
Germany (Fed. Rep.) Fruit, vegetables 0.5
Netherlands General 0.5
Switzerland Fruit 0.75
United States of
America Fruit, vegetables 1.0
* Total of parathion and parathion-methyl.
Appraisal
Parathion-methyl is a broad spectrum insecticide which is widely and
heavily used in many countries for many purposes. It is especially
valuable in the control of cotton insects and is frequently used in
combination with DDT, endrin, toxaphene, and parathion. Although its
use involves an acute toxic hazard, the residues are relatively
non-persistent and rapidly disappear from treated crops. Residues
actually found after reasonable waiting periods rarely exceed 1 ppm
and are composed of the parent compound and paraoxon-methyl plus three
metabolites which have been identified and can be detected by
currently available analytical methods. However, additional
information on the occurrence of paraoxon-methyl and the three
metabolites is required. Further, the residue data which is available
comes mostly from experimental trials in the Federal Republic of
Germany with some data originating in the United States of America.
Residue data from other countries are required. Sensitive methods of
residue analysis are available for the parent compound and the oxygen
analogue. The meeting considered the possibility of combining
tolerances for parathion and parathion-methyl but rejected this
concept since modern analytical techniques allow for the
differentiation of the two compounds and their metabolites,
Accordingly, recommendations for tolerances of parathion-methyl
include paraoxon-methyl. If a tolerance is required for rice, residue
data should be furnished.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Vegetables 1 ppm
Cole crops, cucurbits, fruit 0.2 ppm
Cotton-seed oil (as processed) 0.05 ppm
The above figures are for parathion-methyl and its oxygen analogue.
Further work or information
Required before 30 June 1972
1. Quantitative data on the occurrence of paraoxon-methyl and the
other metabolites in plants and animal products.
2. Data from countries other than the Federal Republic of Germany and
the United States of America on the required rates and frequencies
of application, pre-harvest intervals, and the resultant residues.
3. If a tolerance is required on rice, data on the required rates and
frequencies of application, pre-harvest intervals, and the
resultant residues.
4. Further data on residue levels in raw agricultural products moving
in commerce.
5. Data on residue levels in total diet studies.
6. Comparative evaluation of available methods for regulatory
purposes.
7. Oral studies on teratogenicity and reproduction in species other
than rats and mice, preferably in subhuman primates.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Abbott, D. C., Crosby, N. T. and Thomson, J. (1965) Use of thin-layer
and semipreparative gas-liquid chromatography in the detection,
determination and identification of organophosphorous pesticide
residues. Proc. Soc. Anal. Chem. Conf., pp. 121-133
Augustinsson, K.-B. and Jonsson, G. (1957) Acta chem. scand.,
11:275. See Schrader: Die Entwicklung neuer insektizider
Phosphorsäureester, 444 pp. (1962). Verlag Chemie, Weinheim
Averell, P. R. and Norris, M. V. (1948) Estimation of small amounts of
O,O-di-ethyl O-(p-nitrophenyl) thiophosphate. Anal. Chem.,
20: 753-756
Coffin, D. E. (1964) Residues of parathion, methylparathion, EPN, and
their oxons in Canadian fruits and vegetables. Res. Rev., 7: 61-73
Davison, A. N. (1955) Return of cholinesterase activity in the rat
after inhibition by organophosphorus compounds. 2. A comparative study
of true and pseudo cholinesterase. Biochem. J., 60: 339-346
Deichmann, W. B., Pugliese, W. and Cassidy, J. (1952) Effects of
dimethyl and diethyl paranitrophenyl thiophosphate on experimental
animals. A.M.A. Arch. industr. Hyg., 5: 44-51
Dorough, H. W., Randolph, N. M. and Wimbish, G. H. (1966) Residual
nature of certain organophosphorus insecticides in grain sorghum and
coastal Bermuda grass. Bull. environm. Contam. Toxicol., 1: 46-58
DuBois, K. P. and Coon, J. M. (1952) Toxicology of
organicphosphorus-containing insecticides to mammals. A.M.A. Arch
industr. Hyg., 6: 9-13
Duggan, R. E. (1968) Residues in food and feed. Pesticides Monitoring
J., 2: 2-12
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorous pesticide residues by gas chromatography. Analyst,
89: 175-178
Engst, R., Seidler, H. and Härtig, M. (1966) Nahrung, 10: 413-425
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food, FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add/27.65
Fish, S. A. (1966) Organophosphorus cholinesterase inhibitors and
fetal development. Amer. J. Obstet. Gynec., 96: 1148-1154
Gar, K. A., Sazonova, N. A., Fadeev, Y. N., Vladimirova, I. L. and
Golubeva, Z. Z. (1958) Incorporation and excretion of dimethyl
4-nitrophenyl thiophosphate in guinea pigs. Org. Insektofungitsidy
i Gerbitsidy, pp. 93-105 [Chem. Abstr., 54: 15688e (1960)]
Getz, M. E. (1963) The determination of organophosphate pesticides and
their residues by paper chromatography. Res. Rev., 2: 9-25
Giuffrida, L., Ives, N. F. and Bostwick, D. C. (1966) J. Assoc. Off.
Anal. Chemists, 49: 8-21
Hagan, E. C. (1958) Personal communication cited in Williams at al.,
1959, q.v.
Heinisch, E. (1966) Nachrichtenblatt dt. Pflanzenschutzdienst,
20: 57-64
Hightower, B. G. and Martin, D. F. (1958) Effects of certain climatic
factors on the toxicities of several organic phosphorus insecticides.
J. Econ, Entomol., 51: 669-671
Hoelscher, C. E., Wolfenbarger, D. A. and Foster, N. E. (1968)
Parathion and methyl parathion residues on cabbage and southern peas.
J. Econ. Entomol., 61: 56-58
Ikeda, Y. (1962) "Report to the Japan Academy of Sciences"
Le Breton, R., Leyrie, J. and Garat, J. (1963) Intoxication
materno-foetale aigüe par dérivé organo-phosphoré. Ann. Méd. lég.,
43: 258-261
Lichtenstein, E. P. et al. (1966) Toxicity and fate of insecticide
residues in water. Insecticide residues in water after direct
applications or by leaching of agricultural soil. Arch. environm.
Hlth, 12: 199-212
Lichtenstein, E. P. and Schulz, K. R. (1964) Effect of moisture and
micro-organisms on the persistence of some organophosphorus
insecticides in soil, with special emphasis on parathion. J. Econ,
Entomol., 57: 618-627
McPherson, J. B. and Johnson, G. A. (1956) Thermal decomposition of
some phosphorothioate insecticides. J. Agr. Food Chem., 4: 42-49
Metcalf, R. L. and March, R. B. (1953) The isomerization of organic
thionophosphate insecticides. J. Econ. Entomol., 46: 228-294
Moeller, H. C. and Rider, J. A. (1961) Studies on the
anti-cholinesterase effect of parathion and methyl parathion in
humans. Fed. Proc., 20: 434
Moeller, H. C. and Rider, J. A. (1962) Threshold of incipient toxicity
to Systox and methyl parathion. Fed. Proc., 21: 451
Moeller, H. C. and Rider, J. A. (1963) Further studies on the toxicity
of Systox and methyl parathion. Fed. Proc., 22: 189
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605- und
Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21: 331-358
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E 605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten "Bayer", 20: 557-574
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by microcoulometric gas
chromatography. J. Assoc. Off. Anal. Chemists, 49: 763-766
Ogi, D. and Hamada, A. (1965) Case reports on fetal deaths and
malformations of extremities probably related to the insecticide
poisoning. Cited in Tanimura et al., 1967, q.v.
Shipp, O. E., Lindquist, D. A. and Brazzel, J. R. (1963)
Characteristics of residues of methyl parathion applied to field
cotton. J. Econ. Entomol., 56: 793-798
Stobwasser, H., Rademacher, B. and Lange, E. (1967) Effect of post
harvest factors on occurrence of pesticide residues in fruit,
vegetables, etc. Res. Rev., 22: 45-112
Tanimura, T., Katsuya, T. and Nishimura, H. (1967) Embryotoxicity of
acute exposure to methyl parathion in rats and mice. Arch. environm.
Hlth., 15: 609-613
USDA. (1967) Summary of Registered Agricultural Pesticide Chemical
Uses.
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. appl.
Pharmacol., 1: 1-7
Wills, H. (1968) To Coulston, F., Division of Pharmacology, Albany
Medical College, Albany, N.Y. Unpublished report
Woodard Research Corp. (1966) Methyl parathion. Three-generation
reproduction study in the rat. Unpublished report
In animals
In warm-blooded animals parathion-methyl is transformed enzymatically
to paraoxon-methyl (II) (Augustinsson and Jonsson, 1957).
In storage and processing
Although residues of parathion-methyl in vegetables and fruit are
found near the peel, washing does not markedly reduce residues
(Stobwasser, 1967). During ensilage of pea plants the active
ingredient is quickly broken down because of the weak acids present.
One month after ensilage, residues up to 0.2 ppm were found; after
another month no residues could be detected (Heinisch, 1966).
Evidence of residues in food in commerce or at consumption
Samples of beef, butter and rice were examined for the presence of
organo-phosphorus compounds by the non-specific cholinesterase
inhibition method. Calculated as diasinon or parathion-methyl,
residues in all samples were 0.1 ppm or less (data from Woodstock
Agricultural Research Centre, Sittingbourne, 1967).
Methods of residue analysis
The residue analysis of parathion-methyl is usually carried out
colorimetrically, the results including metabolites and all other
substances with the nitrophenyl-group (Averell, 1948). Specific
detection of parathion-methyl is possible by paper and thin-layer
chromatography (Abbott, 1965; Getz, 1963). Gas-liquid chromatographic
analysis is carried out with microcoulometric (Nelson, 1966), electron
capture (Giuffrida, 1966; Egan, 1964; Möllhoff, 1967) and phosphorus
detectors (Möllhoff, 1967), The sensitivity of the colorimetric method
is 0.05 ppm, and of the gas-liquid chromatography methods, 0.01-0.02
ppm. The latter can be used as a referee method.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
National tolerances
Country Crop Tolerance*
(ppm)
Germany (Fed. Rep.) Fruit, vegetables 0.5
Netherlands General 0.5
Switzerland Fruit 0.75
United States of
America Fruit, vegetables 1.0
* Total of parathion and parathion-methyl.
Appraisal
Parathion-methyl is a broad spectrum insecticide which is widely and
heavily used in many countries for many purposes. It is especially
valuable in the control of cotton insects and is frequently used in
combination with DDT, endrin, toxaphene, and parathion. Although its
use involves an acute toxic hazard, the residues are relatively
non-persistent and rapidly disappear from treated crops. Residues
actually found after reasonable waiting periods rarely exceed 1 ppm
and are composed of the parent compound and paraoxon-methyl plus three
metabolites which have been identified and can be detected by
currently available analytical methods. However, additional
information on the occurrence of paraoxon-methyl and the three
metabolites is required. Further, the residue data which is available
comes mostly from experimental trials in the Federal Republic of
Germany with some data originating in the United States of America.
Residue data from other countries are required. Sensitive methods of
residue analysis are available for the parent compound and the oxygen
analogue. The meeting considered the possibility of combining
tolerances for parathion and parathion-methyl but rejected this
concept since modern analytical techniques allow for the
differentiation of the two compounds and their metabolites,
Accordingly, recommendations for tolerances of parathion-methyl
include paraoxon-methyl. If a tolerance is required for rice, residue
data should be furnished.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Vegetables 1 ppm
Cole crops, cucurbits, fruit 0.2 ppm
Cotton-seed oil (as processed) 0.05 ppm
The above figures are for parathion-methyl and its oxygen analogue.
Further work or information
Required before 30 June 1972
1. Quantitative data on the occurrence of paraoxon-methyl and the
other metabolites in plants and animal products.
2. Data from countries other than the Federal Republic of Germany and
the United States of America on the required rates and frequencies
of application, pre-harvest intervals, and the resultant residues.
3. If a tolerance is required on rice, data on the required rates and
frequencies of application, pre-harvest intervals, and the
resultant residues.
4. Further data on residue levels in raw agricultural products moving
in commerce.
5. Data on residue levels in total diet studies.
6. Comparative evaluation of available methods for regulatory
purposes.
7. Oral studies on teratogenicity and reproduction in species other
than rats and mice, preferably in subhuman primates.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Abbott, D. C., Crosby, N. T. and Thomson, J. (1965) Use of thin-layer
and semipreparative gas-liquid chromatography in the detection,
determination and identification of organophosphorous pesticide
residues. Proc. Soc. Anal. Chem. Conf., pp. 121-133
Augustinsson, K.-B. and Jonsson, G. (1957) Acta chem. scand.,
11:275. See Schrader: Die Entwicklung neuer insektizider
Phosphorsäureester, 444 pp. (1962). Verlag Chemie, Weinheim
Averell, P. R. and Norris, M. V. (1948) Estimation of small amounts of
O,O-di-ethyl O-(p-nitrophenyl) thiophosphate. Anal. Chem.,
20: 753-756
Coffin, D. E. (1964) Residues of parathion, methylparathion, EPN, and
their oxons in Canadian fruits and vegetables. Res. Rev., 7: 61-73
Davison, A. N. (1955) Return of cholinesterase activity in the rat
after inhibition by organophosphorus compounds. 2. A comparative study
of true and pseudo cholinesterase. Biochem. J., 60: 339-346
Deichmann, W. B., Pugliese, W. and Cassidy, J. (1952) Effects of
dimethyl and diethyl paranitrophenyl thiophosphate on experimental
animals. A.M.A. Arch. industr. Hyg., 5: 44-51
Dorough, H. W., Randolph, N. M. and Wimbish, G. H. (1966) Residual
nature of certain organophosphorus insecticides in grain sorghum and
coastal Bermuda grass. Bull. environm. Contam. Toxicol., 1: 46-58
DuBois, K. P. and Coon, J. M. (1952) Toxicology of
organicphosphorus-containing insecticides to mammals. A.M.A. Arch
industr. Hyg., 6: 9-13
Duggan, R. E. (1968) Residues in food and feed. Pesticides Monitoring
J., 2: 2-12
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorous pesticide residues by gas chromatography. Analyst,
89: 175-178
Engst, R., Seidler, H. and Härtig, M. (1966) Nahrung, 10: 413-425
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food, FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add/27.65
Fish, S. A. (1966) Organophosphorus cholinesterase inhibitors and
fetal development. Amer. J. Obstet. Gynec., 96: 1148-1154
Gar, K. A., Sazonova, N. A., Fadeev, Y. N., Vladimirova, I. L. and
Golubeva, Z. Z. (1958) Incorporation and excretion of dimethyl
4-nitrophenyl thiophosphate in guinea pigs. Org. Insektofungitsidy
i Gerbitsidy, pp. 93-105 [Chem. Abstr., 54: 15688e (1960)]
Getz, M. E. (1963) The determination of organophosphate pesticides and
their residues by paper chromatography. Res. Rev., 2: 9-25
Giuffrida, L., Ives, N. F. and Bostwick, D. C. (1966) J. Assoc. Off.
Anal. Chemists, 49: 8-21
Hagan, E. C. (1958) Personal communication cited in Williams at al.,
1959, q.v.
Heinisch, E. (1966) Nachrichtenblatt dt. Pflanzenschutzdienst,
20: 57-64
Hightower, B. G. and Martin, D. F. (1958) Effects of certain climatic
factors on the toxicities of several organic phosphorus insecticides.
J. Econ, Entomol., 51: 669-671
Hoelscher, C. E., Wolfenbarger, D. A. and Foster, N. E. (1968)
Parathion and methyl parathion residues on cabbage and southern peas.
J. Econ. Entomol., 61: 56-58
Ikeda, Y. (1962) "Report to the Japan Academy of Sciences"
Le Breton, R., Leyrie, J. and Garat, J. (1963) Intoxication
materno-foetale aigüe par dérivé organo-phosphoré. Ann. Méd. lég.,
43: 258-261
Lichtenstein, E. P. et al. (1966) Toxicity and fate of insecticide
residues in water. Insecticide residues in water after direct
applications or by leaching of agricultural soil. Arch. environm.
Hlth, 12: 199-212
Lichtenstein, E. P. and Schulz, K. R. (1964) Effect of moisture and
micro-organisms on the persistence of some organophosphorus
insecticides in soil, with special emphasis on parathion. J. Econ,
Entomol., 57: 618-627
McPherson, J. B. and Johnson, G. A. (1956) Thermal decomposition of
some phosphorothioate insecticides. J. Agr. Food Chem., 4: 42-49
Metcalf, R. L. and March, R. B. (1953) The isomerization of organic
thionophosphate insecticides. J. Econ. Entomol., 46: 228-294
Moeller, H. C. and Rider, J. A. (1961) Studies on the
anti-cholinesterase effect of parathion and methyl parathion in
humans. Fed. Proc., 20: 434
Moeller, H. C. and Rider, J. A. (1962) Threshold of incipient toxicity
to Systox and methyl parathion. Fed. Proc., 21: 451
Moeller, H. C. and Rider, J. A. (1963) Further studies on the toxicity
of Systox and methyl parathion. Fed. Proc., 22: 189
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605- und
Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21: 331-358
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E 605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten "Bayer", 20: 557-574
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by microcoulometric gas
chromatography. J. Assoc. Off. Anal. Chemists, 49: 763-766
Ogi, D. and Hamada, A. (1965) Case reports on fetal deaths and
malformations of extremities probably related to the insecticide
poisoning. Cited in Tanimura et al., 1967, q.v.
Shipp, O. E., Lindquist, D. A. and Brazzel, J. R. (1963)
Characteristics of residues of methyl parathion applied to field
cotton. J. Econ. Entomol., 56: 793-798
Stobwasser, H., Rademacher, B. and Lange, E. (1967) Effect of post
harvest factors on occurrence of pesticide residues in fruit,
vegetables, etc. Res. Rev., 22: 45-112
Tanimura, T., Katsuya, T. and Nishimura, H. (1967) Embryotoxicity of
acute exposure to methyl parathion in rats and mice. Arch. environm.
Hlth., 15: 609-613
USDA. (1967) Summary of Registered Agricultural Pesticide Chemical
Uses.
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. appl.
Pharmacol., 1: 1-7
Wills, H. (1968) To Coulston, F., Division of Pharmacology, Albany
Medical College, Albany, N.Y. Unpublished report
Woodard Research Corp. (1966) Methyl parathion. Three-generation
reproduction study in the rat. Unpublished report
 Other information on identity and properties
Typical analyses of technical parathion-methyl are not available. In
1966 the world production of parathion-methyl was 31 700 metric tons
(United States of America production, 14 800 metric tons).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When 32P-labelled parathion-methyl was administered orally to
guinea-pigs, the phosphorus was found to enter the organs almost
immediately, and the maximum tissue level was attained in one to two
hours. A high degree of absorption was found in the liver (Gar et al.,
1958).
Parathion-methyl is biologically similar to parathion and is
metabolized to its oxygen analogue, paraoxon-methyl (Augustinsson and
Jonsson, 1957).
Parathion-methyl is an in vivo cholinesterase inhibitor (Williams et
al., 1959). It also inhibits this enzyme in vitro, however it is
weaker in this respect than its ethyl analogue, parathion (DuBois and
Coon, 1952). The same is true of the respective oxygen metabolites;
paraoxon-methyl being a weaker cholinesterase inhibitor than paraoxon.
The in vitro molar I50 value for paraoxon-methyl, using rat brain
cholinesterase, is 4 × 10-8 (Davison, 1955).
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse oral 32.1 Ikeda, 1962
Mouse oral 150 Wills, 1968
Rat (M) oral 14 Wills, 1968
Rat (F) oral 24 Wills, 1968
Rat oral 17.2 Hagan, 1958
Rat (F) oral 9.7-14.8 Deichmann et al., 1952
Rat i.p. 3.5 DuBois and Coon, 1952
Rabbit oral 420 Wills, 1968
(in oil)
Rabbit oral 1 270 Wills, 1968
(undiluted)
Short-term studies
Dog. Pairs of dogs, comprising one male and one female, were fed
parathion-methyl for 12 weeks at dietary levels of 5, 20, and 50 ppm
along with four dogs used as controls. In the 20 and 50 ppm group,
erythrocyte cholinesterase began to be significantly depressed soon
after commencement of the test-diet. The same was true of plasma
cholinesterase in the 50 ppm group. Maximum extent of depression was
attained at the end of the 12-week period but recovery was complete
within four to eight weeks after withdrawal of parathion-methyl.
Depression of plasma cholinesterase was questionable at the 20 ppm
level and no significant anti-cholinesterase activity was found in the
5 pm group.
Long-term studies
No information available.
Special studies
(a) Reproduction
A three-generation reproduction study using 10 male and 20 female rats
per dose level for each generation, at 0, 10 and 30 ppm
parathion-methyl, and comprising two litters per generation, revealed
no consistent effect on the number of live or stillbirths, birth
weights, physical structure of newborn, litter size, weanling weights
or percentage survival to weaning.
Sporadic effects included lower weanling survival rate in F1a, F1b
and F2a generations at 30 ppm, and in F3a generation at 10 ppm,
increased stillbirth rate in F1b and F3a generations at 30 ppm,
and F3a at 10 ppm; reduced mean weanling rate in F2a generation at
30 ppm, and F1b generation at 10 ppm. Reduced reproductive
performance in F1a, F1b, F2aand F3b generations at 30 ppm, was
the only parameter consistently affected. No such activity was evident
at the 10 ppm level (Woodard Research Corp., 1966).
(b) Teratogenicity
Some cases of foetal deaths and malformations have been reported in
Japan and may possibly be related to the use of organo-phosphorus
insecticides in the field (Ogi and Hamada, 1965). Parathion was
identified in a human term foetus whose mother had used it to commit
suicide (Le Breton et al., 1963). No significant developmental defects
in rats whose mothers had been injected intraperitoneally with methyl
parathion was observed (Fish, 1966).
Because of these reports, the effect on organogenesis in the rat and
mouse was studied by injecting intraperitoneally parathion-methyl
suspended in a 0.5 per cent aqueous solution of sodium carboxymethyl
cellulose once on day 12 of gestation in rats and once on day 10 in
mice. The dosage was 5 to 15 mg/kg of body-weight in rats and 20 to 60
mg/kg in mice. The animals were killed near term on day 21 in rats and
on day 18 in mice. The foetuses were examined for intrauterine death,
external malformations, internal abnormalities and skeletal
abnormalities. All animals of both species showed signs of toxicity
about 30 minutes after administration of parathion-methyl. Ataxia and
paralysis of voluntary muscles were followed by hypersecretion of
saliva and tears, urinary incontinence, tremor and general
convulsions. Some died and the others recovered by the next day. Food
and water intake in rats was noted to be lower for three or four days
and there was a weight loss. No external or internal malformations
were found. In mice lethality, teratogenicity and suppression of
growth were noted in the group treated with the higher dosage. The
only malformation was cleft palate, the incidence of which was 0.71
per cent when used as control groups in other experiments.
Retardation of ossification of the caudal vertebrae and increased
incidence of cervical rib occurred in the group treated with the
higher dose (Tanimura et al., 1967).
Observations in man
Normal levels of red blood cell and plasma cholinesterase were
established in 12 human subjects, five of these subjects were given
parathion-methyl in dose levels of 3 mg/day for 28 days, 3.5 mg/day
for 28 days and 4.0 mg/day for 43 days. No anti-cholinesterase
activity in plasma on red blood cells, and no side effects, were
observed (Moeller and Rider, 1961)
In a second study, three groups of five subjects were given
parathion-methyl, The first group received 4.5 mg/day for 30 days
followed by 5.0 mg/day for 29 days; the second group, 5.5 mg/day for
28 days, then 6.0 mg/day for 29 days; and the third group, 6.5 mg/day
for 35 days, then 7.0 mg/day for 24 days. The maximum depression of
cholinesterase occurred in plasma and was approximately 15 per cent
of the control values established beforehand (Moeller and Rider,
1962).
In a continuing experiment, groups of five subjects were given
parathion-methyl for 30 days at daily doses of 7 mg, 7.5 mg, 8 mg and
9 mg. Plasma and red blood cell cholinesterase activities were within
20 per cent of the pre-established control values (Moeller and Rider,
1963).
Comments
Teratogenic effects were observed in mice only after parenteral
administration, on the other hand the reproduction studies in rats
showed some disturbance of the physiology of the reproductive process.
It was therefore found necessary to re-evaluate this compound by using
a higher safety factor and to change the acceptable daily intake to a
temporary acceptable daily intake.
TOXICOLOGICAL EVALUATION
Estimate of temporary acceptable intake
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Parathion-methyl is a broad spectrum insecticide and is mainly used on
cotton, but is also used on food crops including fruits and
vegetables.
For plant protection one, two or more applications are usually made,
the pre-harvest interval varying from 14 to 21 days and is usually
twice as long under greenhouse conditions.
Reported application rates are given in the following table:
Application rate*
Crop (g/ha)
Fruit 240-720
Vegetables 240-720
Grain crops (rice) 240-720
Hay, forage crops,
and cotton 100-1 000
* USDA, 1967.
Prescribed pre-harvest intervals in various countries are given below:
Pre-harvest
Country interval (days)
Austria 21
Belgium 14
Denmark 14
Finland 21
France 15
Germany (B.R.D.) 14
Germany (D.D.R.) 14/21
Italy 14
Netherlands, field conditions 21
Netherlands, greenhouses 28
Norway 14
Portugal 21
Switzerland 21
United States of America 5/21
Post-harvest treatments
Parathion-methyl is not used on stored food.
Other uses
Parathion-methyl has a very limited use for public health purposes.
Residues resulting from supervised trials
Many residue studies based on chemical methods, gas-liquid
chromatography and cholinesterase inhibition, are available. Residue
data include the oxon derivative. Parathion-methyl penetrates through
the cuticula but is usually not translocated within plants (Shipp,
1963; Stobwasser, 1967). Only in the case of carrots was it found that
parathion-methyl penetrates into the oil-cells and undergoes very slow
degradation (Engst, 1966). On the surfaces of plants parathion-methyl
residues decrease very rapidly as shown by Shipp (1963) on cotton. The
half-life is usually one to two days:
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cabbage- Germany 3 0.1-0.7 0.3 III 1-1.5
lettuce 7 <0.15 <0.15
(field)
Cabbage- Germany 4 0.76 II 2-3
lettuce 7 0.65
(greenhouse) 14 0.06
Tomatoes Germany 3 0.06 I
(field) 7 n.d.
Tomatoes Germany 3 n.d.-0.15 0.08 I 1.5
(greenhouse) 7 n.d. <0.03
3 0.07 II
7 0.04
Apples Italy 10 0.05 I
Apples Germany 3 0.38-0.54 II 2.5-5
7 0.11-0.12
14 0.05-0.15
Cabbage United 14 <1 <1 Hoelscher
States of 1968
America
Peas United 5 1.1 Hoelscher
States of 1968
America
Sorghum United 3 0.36 Dorough 1-1.5
grain States of 6 n.d. 1966
America
(continued)
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cotton United 3 2.5-18.0 8.7 Shipp 1
States of 7 1.0-11.8 5.3 1963
America 12 0.8-9.9 4.2
References: I Institut für Pflanzenschutz, Hohenheim, Germany
II Professor Maier-Bode, Bonn, Germany
III Biologisches Institut der Farbenfabriken Bayer, A.G. Leverkusen, Germany
Disappearance of residues is dependent on climatic factors, rain appreciably reducing
the residues (Hightower, 1958).
Fate of residues
General comments
Under ultra-violet irradiation parathion-methyl is isomerized to the
S-methyl compound (I) (Metcalf, 1953). Ultra-violet degradation under
field conditions has not been reported. Upon heating to 140-160°C,
parathion-methyl is transformed to the S-methyl compound (I)
(McPherson, 1956; Metcalf, 1953). Thermal decomposition under field
conditions is not reported.
Other information on identity and properties
Typical analyses of technical parathion-methyl are not available. In
1966 the world production of parathion-methyl was 31 700 metric tons
(United States of America production, 14 800 metric tons).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When 32P-labelled parathion-methyl was administered orally to
guinea-pigs, the phosphorus was found to enter the organs almost
immediately, and the maximum tissue level was attained in one to two
hours. A high degree of absorption was found in the liver (Gar et al.,
1958).
Parathion-methyl is biologically similar to parathion and is
metabolized to its oxygen analogue, paraoxon-methyl (Augustinsson and
Jonsson, 1957).
Parathion-methyl is an in vivo cholinesterase inhibitor (Williams et
al., 1959). It also inhibits this enzyme in vitro, however it is
weaker in this respect than its ethyl analogue, parathion (DuBois and
Coon, 1952). The same is true of the respective oxygen metabolites;
paraoxon-methyl being a weaker cholinesterase inhibitor than paraoxon.
The in vitro molar I50 value for paraoxon-methyl, using rat brain
cholinesterase, is 4 × 10-8 (Davison, 1955).
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse oral 32.1 Ikeda, 1962
Mouse oral 150 Wills, 1968
Rat (M) oral 14 Wills, 1968
Rat (F) oral 24 Wills, 1968
Rat oral 17.2 Hagan, 1958
Rat (F) oral 9.7-14.8 Deichmann et al., 1952
Rat i.p. 3.5 DuBois and Coon, 1952
Rabbit oral 420 Wills, 1968
(in oil)
Rabbit oral 1 270 Wills, 1968
(undiluted)
Short-term studies
Dog. Pairs of dogs, comprising one male and one female, were fed
parathion-methyl for 12 weeks at dietary levels of 5, 20, and 50 ppm
along with four dogs used as controls. In the 20 and 50 ppm group,
erythrocyte cholinesterase began to be significantly depressed soon
after commencement of the test-diet. The same was true of plasma
cholinesterase in the 50 ppm group. Maximum extent of depression was
attained at the end of the 12-week period but recovery was complete
within four to eight weeks after withdrawal of parathion-methyl.
Depression of plasma cholinesterase was questionable at the 20 ppm
level and no significant anti-cholinesterase activity was found in the
5 pm group.
Long-term studies
No information available.
Special studies
(a) Reproduction
A three-generation reproduction study using 10 male and 20 female rats
per dose level for each generation, at 0, 10 and 30 ppm
parathion-methyl, and comprising two litters per generation, revealed
no consistent effect on the number of live or stillbirths, birth
weights, physical structure of newborn, litter size, weanling weights
or percentage survival to weaning.
Sporadic effects included lower weanling survival rate in F1a, F1b
and F2a generations at 30 ppm, and in F3a generation at 10 ppm,
increased stillbirth rate in F1b and F3a generations at 30 ppm,
and F3a at 10 ppm; reduced mean weanling rate in F2a generation at
30 ppm, and F1b generation at 10 ppm. Reduced reproductive
performance in F1a, F1b, F2aand F3b generations at 30 ppm, was
the only parameter consistently affected. No such activity was evident
at the 10 ppm level (Woodard Research Corp., 1966).
(b) Teratogenicity
Some cases of foetal deaths and malformations have been reported in
Japan and may possibly be related to the use of organo-phosphorus
insecticides in the field (Ogi and Hamada, 1965). Parathion was
identified in a human term foetus whose mother had used it to commit
suicide (Le Breton et al., 1963). No significant developmental defects
in rats whose mothers had been injected intraperitoneally with methyl
parathion was observed (Fish, 1966).
Because of these reports, the effect on organogenesis in the rat and
mouse was studied by injecting intraperitoneally parathion-methyl
suspended in a 0.5 per cent aqueous solution of sodium carboxymethyl
cellulose once on day 12 of gestation in rats and once on day 10 in
mice. The dosage was 5 to 15 mg/kg of body-weight in rats and 20 to 60
mg/kg in mice. The animals were killed near term on day 21 in rats and
on day 18 in mice. The foetuses were examined for intrauterine death,
external malformations, internal abnormalities and skeletal
abnormalities. All animals of both species showed signs of toxicity
about 30 minutes after administration of parathion-methyl. Ataxia and
paralysis of voluntary muscles were followed by hypersecretion of
saliva and tears, urinary incontinence, tremor and general
convulsions. Some died and the others recovered by the next day. Food
and water intake in rats was noted to be lower for three or four days
and there was a weight loss. No external or internal malformations
were found. In mice lethality, teratogenicity and suppression of
growth were noted in the group treated with the higher dosage. The
only malformation was cleft palate, the incidence of which was 0.71
per cent when used as control groups in other experiments.
Retardation of ossification of the caudal vertebrae and increased
incidence of cervical rib occurred in the group treated with the
higher dose (Tanimura et al., 1967).
Observations in man
Normal levels of red blood cell and plasma cholinesterase were
established in 12 human subjects, five of these subjects were given
parathion-methyl in dose levels of 3 mg/day for 28 days, 3.5 mg/day
for 28 days and 4.0 mg/day for 43 days. No anti-cholinesterase
activity in plasma on red blood cells, and no side effects, were
observed (Moeller and Rider, 1961)
In a second study, three groups of five subjects were given
parathion-methyl, The first group received 4.5 mg/day for 30 days
followed by 5.0 mg/day for 29 days; the second group, 5.5 mg/day for
28 days, then 6.0 mg/day for 29 days; and the third group, 6.5 mg/day
for 35 days, then 7.0 mg/day for 24 days. The maximum depression of
cholinesterase occurred in plasma and was approximately 15 per cent
of the control values established beforehand (Moeller and Rider,
1962).
In a continuing experiment, groups of five subjects were given
parathion-methyl for 30 days at daily doses of 7 mg, 7.5 mg, 8 mg and
9 mg. Plasma and red blood cell cholinesterase activities were within
20 per cent of the pre-established control values (Moeller and Rider,
1963).
Comments
Teratogenic effects were observed in mice only after parenteral
administration, on the other hand the reproduction studies in rats
showed some disturbance of the physiology of the reproductive process.
It was therefore found necessary to re-evaluate this compound by using
a higher safety factor and to change the acceptable daily intake to a
temporary acceptable daily intake.
TOXICOLOGICAL EVALUATION
Estimate of temporary acceptable intake
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Parathion-methyl is a broad spectrum insecticide and is mainly used on
cotton, but is also used on food crops including fruits and
vegetables.
For plant protection one, two or more applications are usually made,
the pre-harvest interval varying from 14 to 21 days and is usually
twice as long under greenhouse conditions.
Reported application rates are given in the following table:
Application rate*
Crop (g/ha)
Fruit 240-720
Vegetables 240-720
Grain crops (rice) 240-720
Hay, forage crops,
and cotton 100-1 000
* USDA, 1967.
Prescribed pre-harvest intervals in various countries are given below:
Pre-harvest
Country interval (days)
Austria 21
Belgium 14
Denmark 14
Finland 21
France 15
Germany (B.R.D.) 14
Germany (D.D.R.) 14/21
Italy 14
Netherlands, field conditions 21
Netherlands, greenhouses 28
Norway 14
Portugal 21
Switzerland 21
United States of America 5/21
Post-harvest treatments
Parathion-methyl is not used on stored food.
Other uses
Parathion-methyl has a very limited use for public health purposes.
Residues resulting from supervised trials
Many residue studies based on chemical methods, gas-liquid
chromatography and cholinesterase inhibition, are available. Residue
data include the oxon derivative. Parathion-methyl penetrates through
the cuticula but is usually not translocated within plants (Shipp,
1963; Stobwasser, 1967). Only in the case of carrots was it found that
parathion-methyl penetrates into the oil-cells and undergoes very slow
degradation (Engst, 1966). On the surfaces of plants parathion-methyl
residues decrease very rapidly as shown by Shipp (1963) on cotton. The
half-life is usually one to two days:
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cabbage- Germany 3 0.1-0.7 0.3 III 1-1.5
lettuce 7 <0.15 <0.15
(field)
Cabbage- Germany 4 0.76 II 2-3
lettuce 7 0.65
(greenhouse) 14 0.06
Tomatoes Germany 3 0.06 I
(field) 7 n.d.
Tomatoes Germany 3 n.d.-0.15 0.08 I 1.5
(greenhouse) 7 n.d. <0.03
3 0.07 II
7 0.04
Apples Italy 10 0.05 I
Apples Germany 3 0.38-0.54 II 2.5-5
7 0.11-0.12
14 0.05-0.15
Cabbage United 14 <1 <1 Hoelscher
States of 1968
America
Peas United 5 1.1 Hoelscher
States of 1968
America
Sorghum United 3 0.36 Dorough 1-1.5
grain States of 6 n.d. 1966
America
(continued)
Pre-harvest Residue (ppm)
Crop Country interval Author Half-life
(days) range average (days)
Cotton United 3 2.5-18.0 8.7 Shipp 1
States of 7 1.0-11.8 5.3 1963
America 12 0.8-9.9 4.2
References: I Institut für Pflanzenschutz, Hohenheim, Germany
II Professor Maier-Bode, Bonn, Germany
III Biologisches Institut der Farbenfabriken Bayer, A.G. Leverkusen, Germany
Disappearance of residues is dependent on climatic factors, rain appreciably reducing
the residues (Hightower, 1958).
Fate of residues
General comments
Under ultra-violet irradiation parathion-methyl is isomerized to the
S-methyl compound (I) (Metcalf, 1953). Ultra-violet degradation under
field conditions has not been reported. Upon heating to 140-160°C,
parathion-methyl is transformed to the S-methyl compound (I)
(McPherson, 1956; Metcalf, 1953). Thermal decomposition under field
conditions is not reported.
 Parathion-methyl is quickly hydrolyzed by 1 N NaOH (K/min = 0.69), but
no information of hydrolysis under field conditions is available
(Metcalf, 1953).
No data on metabolism in humans has been reported. Dietary intake for
all organo-phosphates in the United States of America in 1966/1967 was
0.00025 mg/kg body-weight/day, the three-year average being 0.00013
mg/kg body-weight/day (Duggan, 1968).
In soils
Parathion-methyl is quickly broken down in soil. In silty loam, 30
days after application of 0.1 ppm active ingredient, only three per
cent was recovered. Disappearance of residues is accelerated by
moisture and micro-organisms (Lichtenstein and Schulz, 1964). Trials
on leaching in soil showed that in soil-water, parathion-methyl has a
persistence of two weeks and in lake-water of three months, but no
conclusions can be drawn concerning leaching of parathion-methyl from
soil to rivers (Lichtenstein et al., 1966).
In plants
Three metabolites of parathion-methyl were found in cottonleaves, one
of which was identified as paraoxon-methyl (II) (Shipp, 1963; Coffin,
1964). After application of parathion-methyl to lettuce only small
amounts of residues of paraoxon-methyl were found (one to four per
cent of the total active residue) (Möllhoff, 1968). Under normal
field conditions, paraoxon-methyl constitutes only a small fraction (a
few per cent) of the residue of the parent compound (Möllhoff, 1968).
Under elevated temperatures (72-112°F) and high humidity (40-62 per
cent) the total residue on cotton after an interval of 72 hours
consisted of 14.5 per cent parathion-methyl and 38.5 per cent
paraoxon-methyl (Shipp, 1963). However, the oxon, when formed, is
subject to rapid hydrolysis in and on the plant (Möllhoff, 1968). The
classical colorimetric method by Averell et al. (1948) is able to
determine the parent compound plus the oxon. When applying GLC methods
the parent compound and the oxon can be determined separately.
Probably, breakdown of parathion-methyl in higher plants is the same
as the breakdown of parathion, but more rapid. The products of
hydrolysis - phosphorothioate and 4-nitro-phenol - are only present in
small amounts and seem to have no toxicological importance.
Parathion-methyl is quickly hydrolyzed by 1 N NaOH (K/min = 0.69), but
no information of hydrolysis under field conditions is available
(Metcalf, 1953).
No data on metabolism in humans has been reported. Dietary intake for
all organo-phosphates in the United States of America in 1966/1967 was
0.00025 mg/kg body-weight/day, the three-year average being 0.00013
mg/kg body-weight/day (Duggan, 1968).
In soils
Parathion-methyl is quickly broken down in soil. In silty loam, 30
days after application of 0.1 ppm active ingredient, only three per
cent was recovered. Disappearance of residues is accelerated by
moisture and micro-organisms (Lichtenstein and Schulz, 1964). Trials
on leaching in soil showed that in soil-water, parathion-methyl has a
persistence of two weeks and in lake-water of three months, but no
conclusions can be drawn concerning leaching of parathion-methyl from
soil to rivers (Lichtenstein et al., 1966).
In plants
Three metabolites of parathion-methyl were found in cottonleaves, one
of which was identified as paraoxon-methyl (II) (Shipp, 1963; Coffin,
1964). After application of parathion-methyl to lettuce only small
amounts of residues of paraoxon-methyl were found (one to four per
cent of the total active residue) (Möllhoff, 1968). Under normal
field conditions, paraoxon-methyl constitutes only a small fraction (a
few per cent) of the residue of the parent compound (Möllhoff, 1968).
Under elevated temperatures (72-112°F) and high humidity (40-62 per
cent) the total residue on cotton after an interval of 72 hours
consisted of 14.5 per cent parathion-methyl and 38.5 per cent
paraoxon-methyl (Shipp, 1963). However, the oxon, when formed, is
subject to rapid hydrolysis in and on the plant (Möllhoff, 1968). The
classical colorimetric method by Averell et al. (1948) is able to
determine the parent compound plus the oxon. When applying GLC methods
the parent compound and the oxon can be determined separately.
Probably, breakdown of parathion-methyl in higher plants is the same
as the breakdown of parathion, but more rapid. The products of
hydrolysis - phosphorothioate and 4-nitro-phenol - are only present in
small amounts and seem to have no toxicological importance.
 In animals
In warm-blooded animals parathion-methyl is transformed enzymatically
to paraoxon-methyl (II) (Augustinsson and Jonsson, 1957).
In storage and processing
Although residues of parathion-methyl in vegetables and fruit are
found near the peel, washing does not markedly reduce residues
(Stobwasser, 1967). During ensilage of pea plants the active
ingredient is quickly broken down because of the weak acids present.
One month after ensilage, residues up to 0.2 ppm were found; after
another month no residues could be detected (Heinisch, 1966).
Evidence of residues in food in commerce or at consumption
Samples of beef, butter and rice were examined for the presence of
organo-phosphorus compounds by the non-specific cholinesterase
inhibition method. Calculated as diasinon or parathion-methyl,
residues in all samples were 0.1 ppm or less (data from Woodstock
Agricultural Research Centre, Sittingbourne, 1967).
Methods of residue analysis
The residue analysis of parathion-methyl is usually carried out
colorimetrically, the results including metabolites and all other
substances with the nitrophenyl-group (Averell, 1948). Specific
detection of parathion-methyl is possible by paper and thin-layer
chromatography (Abbott, 1965; Getz, 1963). Gas-liquid chromatographic
analysis is carried out with microcoulometric (Nelson, 1966), electron
capture (Giuffrida, 1966; Egan, 1964; Möllhoff, 1967) and phosphorus
detectors (Möllhoff, 1967), The sensitivity of the colorimetric method
is 0.05 ppm, and of the gas-liquid chromatography methods, 0.01-0.02
ppm. The latter can be used as a referee method.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
National tolerances
Country Crop Tolerance*
(ppm)
Germany (Fed. Rep.) Fruit, vegetables 0.5
Netherlands General 0.5
Switzerland Fruit 0.75
United States of
America Fruit, vegetables 1.0
* Total of parathion and parathion-methyl.
Appraisal
Parathion-methyl is a broad spectrum insecticide which is widely and
heavily used in many countries for many purposes. It is especially
valuable in the control of cotton insects and is frequently used in
combination with DDT, endrin, toxaphene, and parathion. Although its
use involves an acute toxic hazard, the residues are relatively
non-persistent and rapidly disappear from treated crops. Residues
actually found after reasonable waiting periods rarely exceed 1 ppm
and are composed of the parent compound and paraoxon-methyl plus three
metabolites which have been identified and can be detected by
currently available analytical methods. However, additional
information on the occurrence of paraoxon-methyl and the three
metabolites is required. Further, the residue data which is available
comes mostly from experimental trials in the Federal Republic of
Germany with some data originating in the United States of America.
Residue data from other countries are required. Sensitive methods of
residue analysis are available for the parent compound and the oxygen
analogue. The meeting considered the possibility of combining
tolerances for parathion and parathion-methyl but rejected this
concept since modern analytical techniques allow for the
differentiation of the two compounds and their metabolites,
Accordingly, recommendations for tolerances of parathion-methyl
include paraoxon-methyl. If a tolerance is required for rice, residue
data should be furnished.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Vegetables 1 ppm
Cole crops, cucurbits, fruit 0.2 ppm
Cotton-seed oil (as processed) 0.05 ppm
The above figures are for parathion-methyl and its oxygen analogue.
Further work or information
Required before 30 June 1972
1. Quantitative data on the occurrence of paraoxon-methyl and the
other metabolites in plants and animal products.
2. Data from countries other than the Federal Republic of Germany and
the United States of America on the required rates and frequencies
of application, pre-harvest intervals, and the resultant residues.
3. If a tolerance is required on rice, data on the required rates and
frequencies of application, pre-harvest intervals, and the
resultant residues.
4. Further data on residue levels in raw agricultural products moving
in commerce.
5. Data on residue levels in total diet studies.
6. Comparative evaluation of available methods for regulatory
purposes.
7. Oral studies on teratogenicity and reproduction in species other
than rats and mice, preferably in subhuman primates.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Abbott, D. C., Crosby, N. T. and Thomson, J. (1965) Use of thin-layer
and semipreparative gas-liquid chromatography in the detection,
determination and identification of organophosphorous pesticide
residues. Proc. Soc. Anal. Chem. Conf., pp. 121-133
Augustinsson, K.-B. and Jonsson, G. (1957) Acta chem. scand.,
11:275. See Schrader: Die Entwicklung neuer insektizider
Phosphorsäureester, 444 pp. (1962). Verlag Chemie, Weinheim
Averell, P. R. and Norris, M. V. (1948) Estimation of small amounts of
O,O-di-ethyl O-(p-nitrophenyl) thiophosphate. Anal. Chem.,
20: 753-756
Coffin, D. E. (1964) Residues of parathion, methylparathion, EPN, and
their oxons in Canadian fruits and vegetables. Res. Rev., 7: 61-73
Davison, A. N. (1955) Return of cholinesterase activity in the rat
after inhibition by organophosphorus compounds. 2. A comparative study
of true and pseudo cholinesterase. Biochem. J., 60: 339-346
Deichmann, W. B., Pugliese, W. and Cassidy, J. (1952) Effects of
dimethyl and diethyl paranitrophenyl thiophosphate on experimental
animals. A.M.A. Arch. industr. Hyg., 5: 44-51
Dorough, H. W., Randolph, N. M. and Wimbish, G. H. (1966) Residual
nature of certain organophosphorus insecticides in grain sorghum and
coastal Bermuda grass. Bull. environm. Contam. Toxicol., 1: 46-58
DuBois, K. P. and Coon, J. M. (1952) Toxicology of
organicphosphorus-containing insecticides to mammals. A.M.A. Arch
industr. Hyg., 6: 9-13
Duggan, R. E. (1968) Residues in food and feed. Pesticides Monitoring
J., 2: 2-12
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorous pesticide residues by gas chromatography. Analyst,
89: 175-178
Engst, R., Seidler, H. and Härtig, M. (1966) Nahrung, 10: 413-425
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food, FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add/27.65
Fish, S. A. (1966) Organophosphorus cholinesterase inhibitors and
fetal development. Amer. J. Obstet. Gynec., 96: 1148-1154
Gar, K. A., Sazonova, N. A., Fadeev, Y. N., Vladimirova, I. L. and
Golubeva, Z. Z. (1958) Incorporation and excretion of dimethyl
4-nitrophenyl thiophosphate in guinea pigs. Org. Insektofungitsidy
i Gerbitsidy, pp. 93-105 [Chem. Abstr., 54: 15688e (1960)]
Getz, M. E. (1963) The determination of organophosphate pesticides and
their residues by paper chromatography. Res. Rev., 2: 9-25
Giuffrida, L., Ives, N. F. and Bostwick, D. C. (1966) J. Assoc. Off.
Anal. Chemists, 49: 8-21
Hagan, E. C. (1958) Personal communication cited in Williams at al.,
1959, q.v.
Heinisch, E. (1966) Nachrichtenblatt dt. Pflanzenschutzdienst,
20: 57-64
Hightower, B. G. and Martin, D. F. (1958) Effects of certain climatic
factors on the toxicities of several organic phosphorus insecticides.
J. Econ, Entomol., 51: 669-671
Hoelscher, C. E., Wolfenbarger, D. A. and Foster, N. E. (1968)
Parathion and methyl parathion residues on cabbage and southern peas.
J. Econ. Entomol., 61: 56-58
Ikeda, Y. (1962) "Report to the Japan Academy of Sciences"
Le Breton, R., Leyrie, J. and Garat, J. (1963) Intoxication
materno-foetale aigüe par dérivé organo-phosphoré. Ann. Méd. lég.,
43: 258-261
Lichtenstein, E. P. et al. (1966) Toxicity and fate of insecticide
residues in water. Insecticide residues in water after direct
applications or by leaching of agricultural soil. Arch. environm.
Hlth, 12: 199-212
Lichtenstein, E. P. and Schulz, K. R. (1964) Effect of moisture and
micro-organisms on the persistence of some organophosphorus
insecticides in soil, with special emphasis on parathion. J. Econ,
Entomol., 57: 618-627
McPherson, J. B. and Johnson, G. A. (1956) Thermal decomposition of
some phosphorothioate insecticides. J. Agr. Food Chem., 4: 42-49
Metcalf, R. L. and March, R. B. (1953) The isomerization of organic
thionophosphate insecticides. J. Econ. Entomol., 46: 228-294
Moeller, H. C. and Rider, J. A. (1961) Studies on the
anti-cholinesterase effect of parathion and methyl parathion in
humans. Fed. Proc., 20: 434
Moeller, H. C. and Rider, J. A. (1962) Threshold of incipient toxicity
to Systox and methyl parathion. Fed. Proc., 21: 451
Moeller, H. C. and Rider, J. A. (1963) Further studies on the toxicity
of Systox and methyl parathion. Fed. Proc., 22: 189
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605- und
Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21: 331-358
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E 605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten "Bayer", 20: 557-574
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by microcoulometric gas
chromatography. J. Assoc. Off. Anal. Chemists, 49: 763-766
Ogi, D. and Hamada, A. (1965) Case reports on fetal deaths and
malformations of extremities probably related to the insecticide
poisoning. Cited in Tanimura et al., 1967, q.v.
Shipp, O. E., Lindquist, D. A. and Brazzel, J. R. (1963)
Characteristics of residues of methyl parathion applied to field
cotton. J. Econ. Entomol., 56: 793-798
Stobwasser, H., Rademacher, B. and Lange, E. (1967) Effect of post
harvest factors on occurrence of pesticide residues in fruit,
vegetables, etc. Res. Rev., 22: 45-112
Tanimura, T., Katsuya, T. and Nishimura, H. (1967) Embryotoxicity of
acute exposure to methyl parathion in rats and mice. Arch. environm.
Hlth., 15: 609-613
USDA. (1967) Summary of Registered Agricultural Pesticide Chemical
Uses.
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. appl.
Pharmacol., 1: 1-7
Wills, H. (1968) To Coulston, F., Division of Pharmacology, Albany
Medical College, Albany, N.Y. Unpublished report
Woodard Research Corp. (1966) Methyl parathion. Three-generation
reproduction study in the rat. Unpublished report
In animals
In warm-blooded animals parathion-methyl is transformed enzymatically
to paraoxon-methyl (II) (Augustinsson and Jonsson, 1957).
In storage and processing
Although residues of parathion-methyl in vegetables and fruit are
found near the peel, washing does not markedly reduce residues
(Stobwasser, 1967). During ensilage of pea plants the active
ingredient is quickly broken down because of the weak acids present.
One month after ensilage, residues up to 0.2 ppm were found; after
another month no residues could be detected (Heinisch, 1966).
Evidence of residues in food in commerce or at consumption
Samples of beef, butter and rice were examined for the presence of
organo-phosphorus compounds by the non-specific cholinesterase
inhibition method. Calculated as diasinon or parathion-methyl,
residues in all samples were 0.1 ppm or less (data from Woodstock
Agricultural Research Centre, Sittingbourne, 1967).
Methods of residue analysis
The residue analysis of parathion-methyl is usually carried out
colorimetrically, the results including metabolites and all other
substances with the nitrophenyl-group (Averell, 1948). Specific
detection of parathion-methyl is possible by paper and thin-layer
chromatography (Abbott, 1965; Getz, 1963). Gas-liquid chromatographic
analysis is carried out with microcoulometric (Nelson, 1966), electron
capture (Giuffrida, 1966; Egan, 1964; Möllhoff, 1967) and phosphorus
detectors (Möllhoff, 1967), The sensitivity of the colorimetric method
is 0.05 ppm, and of the gas-liquid chromatography methods, 0.01-0.02
ppm. The latter can be used as a referee method.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
National tolerances
Country Crop Tolerance*
(ppm)
Germany (Fed. Rep.) Fruit, vegetables 0.5
Netherlands General 0.5
Switzerland Fruit 0.75
United States of
America Fruit, vegetables 1.0
* Total of parathion and parathion-methyl.
Appraisal
Parathion-methyl is a broad spectrum insecticide which is widely and
heavily used in many countries for many purposes. It is especially
valuable in the control of cotton insects and is frequently used in
combination with DDT, endrin, toxaphene, and parathion. Although its
use involves an acute toxic hazard, the residues are relatively
non-persistent and rapidly disappear from treated crops. Residues
actually found after reasonable waiting periods rarely exceed 1 ppm
and are composed of the parent compound and paraoxon-methyl plus three
metabolites which have been identified and can be detected by
currently available analytical methods. However, additional
information on the occurrence of paraoxon-methyl and the three
metabolites is required. Further, the residue data which is available
comes mostly from experimental trials in the Federal Republic of
Germany with some data originating in the United States of America.
Residue data from other countries are required. Sensitive methods of
residue analysis are available for the parent compound and the oxygen
analogue. The meeting considered the possibility of combining
tolerances for parathion and parathion-methyl but rejected this
concept since modern analytical techniques allow for the
differentiation of the two compounds and their metabolites,
Accordingly, recommendations for tolerances of parathion-methyl
include paraoxon-methyl. If a tolerance is required for rice, residue
data should be furnished.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Vegetables 1 ppm
Cole crops, cucurbits, fruit 0.2 ppm
Cotton-seed oil (as processed) 0.05 ppm
The above figures are for parathion-methyl and its oxygen analogue.
Further work or information
Required before 30 June 1972
1. Quantitative data on the occurrence of paraoxon-methyl and the
other metabolites in plants and animal products.
2. Data from countries other than the Federal Republic of Germany and
the United States of America on the required rates and frequencies
of application, pre-harvest intervals, and the resultant residues.
3. If a tolerance is required on rice, data on the required rates and
frequencies of application, pre-harvest intervals, and the
resultant residues.
4. Further data on residue levels in raw agricultural products moving
in commerce.
5. Data on residue levels in total diet studies.
6. Comparative evaluation of available methods for regulatory
purposes.
7. Oral studies on teratogenicity and reproduction in species other
than rats and mice, preferably in subhuman primates.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Abbott, D. C., Crosby, N. T. and Thomson, J. (1965) Use of thin-layer
and semipreparative gas-liquid chromatography in the detection,
determination and identification of organophosphorous pesticide
residues. Proc. Soc. Anal. Chem. Conf., pp. 121-133
Augustinsson, K.-B. and Jonsson, G. (1957) Acta chem. scand.,
11:275. See Schrader: Die Entwicklung neuer insektizider
Phosphorsäureester, 444 pp. (1962). Verlag Chemie, Weinheim
Averell, P. R. and Norris, M. V. (1948) Estimation of small amounts of
O,O-di-ethyl O-(p-nitrophenyl) thiophosphate. Anal. Chem.,
20: 753-756
Coffin, D. E. (1964) Residues of parathion, methylparathion, EPN, and
their oxons in Canadian fruits and vegetables. Res. Rev., 7: 61-73
Davison, A. N. (1955) Return of cholinesterase activity in the rat
after inhibition by organophosphorus compounds. 2. A comparative study
of true and pseudo cholinesterase. Biochem. J., 60: 339-346
Deichmann, W. B., Pugliese, W. and Cassidy, J. (1952) Effects of
dimethyl and diethyl paranitrophenyl thiophosphate on experimental
animals. A.M.A. Arch. industr. Hyg., 5: 44-51
Dorough, H. W., Randolph, N. M. and Wimbish, G. H. (1966) Residual
nature of certain organophosphorus insecticides in grain sorghum and
coastal Bermuda grass. Bull. environm. Contam. Toxicol., 1: 46-58
DuBois, K. P. and Coon, J. M. (1952) Toxicology of
organicphosphorus-containing insecticides to mammals. A.M.A. Arch
industr. Hyg., 6: 9-13
Duggan, R. E. (1968) Residues in food and feed. Pesticides Monitoring
J., 2: 2-12
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorous pesticide residues by gas chromatography. Analyst,
89: 175-178
Engst, R., Seidler, H. and Härtig, M. (1966) Nahrung, 10: 413-425
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food, FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add/27.65
Fish, S. A. (1966) Organophosphorus cholinesterase inhibitors and
fetal development. Amer. J. Obstet. Gynec., 96: 1148-1154
Gar, K. A., Sazonova, N. A., Fadeev, Y. N., Vladimirova, I. L. and
Golubeva, Z. Z. (1958) Incorporation and excretion of dimethyl
4-nitrophenyl thiophosphate in guinea pigs. Org. Insektofungitsidy
i Gerbitsidy, pp. 93-105 [Chem. Abstr., 54: 15688e (1960)]
Getz, M. E. (1963) The determination of organophosphate pesticides and
their residues by paper chromatography. Res. Rev., 2: 9-25
Giuffrida, L., Ives, N. F. and Bostwick, D. C. (1966) J. Assoc. Off.
Anal. Chemists, 49: 8-21
Hagan, E. C. (1958) Personal communication cited in Williams at al.,
1959, q.v.
Heinisch, E. (1966) Nachrichtenblatt dt. Pflanzenschutzdienst,
20: 57-64
Hightower, B. G. and Martin, D. F. (1958) Effects of certain climatic
factors on the toxicities of several organic phosphorus insecticides.
J. Econ, Entomol., 51: 669-671
Hoelscher, C. E., Wolfenbarger, D. A. and Foster, N. E. (1968)
Parathion and methyl parathion residues on cabbage and southern peas.
J. Econ. Entomol., 61: 56-58
Ikeda, Y. (1962) "Report to the Japan Academy of Sciences"
Le Breton, R., Leyrie, J. and Garat, J. (1963) Intoxication
materno-foetale aigüe par dérivé organo-phosphoré. Ann. Méd. lég.,
43: 258-261
Lichtenstein, E. P. et al. (1966) Toxicity and fate of insecticide
residues in water. Insecticide residues in water after direct
applications or by leaching of agricultural soil. Arch. environm.
Hlth, 12: 199-212
Lichtenstein, E. P. and Schulz, K. R. (1964) Effect of moisture and
micro-organisms on the persistence of some organophosphorus
insecticides in soil, with special emphasis on parathion. J. Econ,
Entomol., 57: 618-627
McPherson, J. B. and Johnson, G. A. (1956) Thermal decomposition of
some phosphorothioate insecticides. J. Agr. Food Chem., 4: 42-49
Metcalf, R. L. and March, R. B. (1953) The isomerization of organic
thionophosphate insecticides. J. Econ. Entomol., 46: 228-294
Moeller, H. C. and Rider, J. A. (1961) Studies on the
anti-cholinesterase effect of parathion and methyl parathion in
humans. Fed. Proc., 20: 434
Moeller, H. C. and Rider, J. A. (1962) Threshold of incipient toxicity
to Systox and methyl parathion. Fed. Proc., 21: 451
Moeller, H. C. and Rider, J. A. (1963) Further studies on the toxicity
of Systox and methyl parathion. Fed. Proc., 22: 189
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605- und
Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21: 331-358
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E 605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten "Bayer", 20: 557-574
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by microcoulometric gas
chromatography. J. Assoc. Off. Anal. Chemists, 49: 763-766
Ogi, D. and Hamada, A. (1965) Case reports on fetal deaths and
malformations of extremities probably related to the insecticide
poisoning. Cited in Tanimura et al., 1967, q.v.
Shipp, O. E., Lindquist, D. A. and Brazzel, J. R. (1963)
Characteristics of residues of methyl parathion applied to field
cotton. J. Econ. Entomol., 56: 793-798
Stobwasser, H., Rademacher, B. and Lange, E. (1967) Effect of post
harvest factors on occurrence of pesticide residues in fruit,
vegetables, etc. Res. Rev., 22: 45-112
Tanimura, T., Katsuya, T. and Nishimura, H. (1967) Embryotoxicity of
acute exposure to methyl parathion in rats and mice. Arch. environm.
Hlth., 15: 609-613
USDA. (1967) Summary of Registered Agricultural Pesticide Chemical
Uses.
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Toxicol. appl.
Pharmacol., 1: 1-7
Wills, H. (1968) To Coulston, F., Division of Pharmacology, Albany
Medical College, Albany, N.Y. Unpublished report
Woodard Research Corp. (1966) Methyl parathion. Three-generation
reproduction study in the rat. Unpublished report
