
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
HEXACHLOROBENZENE
IDENTITY
Chemical name
hexachlorobenzene
Synonyms
HCB
perchlorobenzene
Structural formula
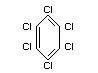 Other relevant chemical properties
Colourless white powder or needles MP 229°C - BP. 326°C. V.P. 1.089 ×
10-5 mm Hg at 20°C - sublimable. Insoluble in water and alcohol.
Soluble in hot benzene. The technical grade used in agriculture
contains 98 percent hexachlorobenzene, 1.8 percent pentachlorobenzene
together with 0.2 percent 1,2,4,5-tetrachlorobenzene.
Commercial formulations (dusts) contain 10-40 percent HCB alone or
together with small quantities of lindane (0.5-1.0 percent) added to
prevent insect attack on stored seed.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Residues of hexachlorobenzene are stored in the liver and fat of
birds. The biological half-life of hexachlorobenzene in the quail is
about three weeks (Vos et al., 1968) (see also "Short-term studies.
Quail").
When hexachlorobenzene, 400 mg/kg, was administered orally to rabbits,
the compound did not appear to be metabolized, because the main
portion was found in the gut contents five days after dosing, with
only 6 percent appearing in the faeces. There was no significant
urinary or pulmonary excretion of metabolites. Hexachlorobenzene did
not form conjugated glucuronic acids, ethereal sulphates or
mercapturic acids. Most of a dose (100 mg/kg) given subcutaneously was
found at the site of injection after five days, with no chlorinated
benzenes being found in the faeces over this period (Parke and
Williams, 1960).
There is evidence that hexachlorobenzene (or a toxic metabolite of it)
is excreted in milk. Two pregnant female rats were given
hexachlorobenzene in their food (the dose was not stated, it was
presumed to be 0.5 percent). One died, but the other was delivered
normally and reared the young until they died with convulsions after
seven to eight days. The mother was then given three normal week-old
rats to foster, which died three to four days later with convulsions.
The mother also died four weeks later with the usual symptoms of
hexachlorobenzene intoxication. This study indicated that a toxic
compound was excreted in the milk (De Matteis et al., 1961).
TOXICOLOGICAL STUDIES
Acute toxicity (oral)
Animal LD50 References
mg/kg body-weight
Mouse 4000 Savitskii, 1964
Rat 3500 Savitskii, 1964
Guinea pig > 1000 Melis, 1955
Rabbit 2600 Savitskii, 1964
Cat 1700 Savitskii, 1964
Short-term studies
Chicken
Hexachlorobenzene given at levels of 120-480 ppm in the diet for three
months, caused no toxic effects in chickens (Melis, 1955).
Guinea-pig
A diet containing 0.5 percent of hexachlorobenzene was given to
guinea-pig and to mice. These two species proved to be remarkably
susceptible to hexachlorobenzene and developed very marked
neurological symptoms within eight to 10 days (De Matteis et al.,
1961).
Mouse
See under "Guinea-pig".
Quail
Five groups of adult Japanese quail (10 females and two males per
group) were fed a mixed diet containing 0, 20, 100, 500 or 2500 ppm of
hexachlorobenzene for three months. Eggs from the 100 ppm, 20 ppm and
control groups were collected during three periods and incubated to
determine reproduction results. At the 2500 ppm level four birds died
within a week, the remainder within a month. Pre-mortem effects noted
were loss of weight, drooping wings, ruffled feathers, trembling,
ataxia and paralysis. On examination, bones, liver and kidney showed a
red fluorescence typical of porphyria. Degeneration and necrosis of
liver cells was seen. At 500 ppm all birds died within a month,
showing the same symptoms and lesions seen at the higher level. At 100
ppm, the first bird died on day 20, and 10 were dead within seven
weeks. An accumulation of porphyrins in liver and kidney was observed
in all birds in the group. Microscopic examination showed
degeneration, necrosis and local regeneration of liver cells. At 20
ppm all birds survived the three months test-period and did not
develop any visible symptoms. At autopsy one hen showed fluorescence
of bones and liver in gross ultraviolet examination. Fluorescence of
liver and kidney cells and swelling and regeneration of liver cells
was observed in this bird. In the other hens fluorescence of kidney
cells was seen under ultraviolet microscopy. Significant differences
were not found in body-weight or in relative weights of liver, spleen
and brain, nor was there any effect on egg production at 20 ppm. There
was a significant reduction in the number of chicks hatched, however,
at this level of hexachlorobenzene. It was tentatively concluded,
considering the disturbance in porphyrin metabolism and the diminished
reproduction results, that the no-effect level in this species is
lower than 20 ppm. (A comparative test in rats indicated the quail to
be much more sensitive). Residues in the liver of the birds fed
hexachlorobenzene were as follows:
TABLE I
Levels of hexachlorobenzene
Level of Number in the liver (ppm)
hexachlorobenzene History of birds Average Range
fed (ppm)
500 died 9 450 180-850
100 died 6 235 85-720
20 killed 6 36 14-94
20 killed 33 days 6 13 5-29
after termination
of hexachlorobenzene-
feeding
Residues were also found in eggs and in the chicks after hatching.
Several predatory birds, most of them found dead, had liver residues
of hexachlorobenzene in the same range as those in the 20 ppm group of
the quail study. It was concluded that hexachlorobenzene used as a
seed-dressing, may have toxic effects on seed-eating and predatory
birds (Vos et al., 1968)
Rabbit
Female rabbits were fed a diet containing 0.5 percent of
hexachlorobenzene. After about six weeks an increase in urinary
porphyrins was noted. Unless sacrificed earlier, the animals died in
eight to 12 weeks after having demonstrated neurological symptoms. (De
Matteis et al., 1961).
Rat
Groups of 10 male and 10 female rats were fed diets containing
hexachlorobenzene at levels of 0, 5, 25, 125, and 625 ppm for 13
weeks. After 13 weeks five males and five females from each group were
sacrificed for gross and microscopic examination. The rats left in the
groups given 125 and 625 ppm were transferred to the control diet for
a further two weeks, then sacrificed and examined. The rats in the 625
ppm group displayed signs of marked respiratory involvement; slight
tremors were also observed in several animals and the females
exhibited small sores in the skin of the head or neck. None of the
other groups showed these effects. Growth of male rats at the highest
level fed was reduced. Survival was not affected at any dose-level.
Total leukocyte counts were elevated during the test in the group fed
625 ppm. Organ-weight data showed an increase at 625 ppm, in thyroid,
liver, spleen and adrenals, and in male rats at 125 ppm, in liver.
Microscopic examination showed consistent effects in the liver
(lobular distortion, nuclear and cytoplasmic variations, focal loss of
cell outline, mitotic activity, and focal cellular necrosis) of the
rats at 125 and 625 ppm. Changes of a less distinct and less
consistent nature were observed in thyroid, kidneys, adrenals and
bone-marrow of animals in these two groups. No significant effects
were observed in organs and tissues of the rats fed at 5 or 25 ppm of
hexachlorobenzene (Weir, 1962).
Female rats were fed a standard died containing 0.2 percent
hexachlorobenzene. After one week, weight-loss and general debility
was noted in some rats. Within three weeks a few rats showed increased
urinary excretion of porphyrins, but this manifestation did not become
general until the seventh or eighth week. Rats with well established
porphyria were exposed continuously to ultraviolet light. If an area
of about 25 cm2 was plucked or shaved before exposure, most of the
animals died within three to 14 days, but a few rats survived three to
four months of this treatment. Rats on the hexachlorobenzene diet that
were not shaved or plucked tolerated the ultraviolet light exposure
well. After receiving hexachlorobenzene in the diet for six to eight
months, the rats were returned to a normal diet. Urinary porphyrin
excretion decreased considerably over the first four months but was
still above normal levels nine months after hexachlorobenzene has been
removed from the diet (Pearson and Malkinson, 1965).
A diet containing 0.2 percent of hexachlorobenzene was fed to 26 male
rats. Groups of two or three animals were killed at weekly intervals
over a period of one to 12 weeks. Retardation of weight-gain occurred
in the second and third week of the experiment. Evidence of a
localized toxic effect was confined to the liver. An increase
in liver-weight reached a maximum from the fifth to the ninth
week and then the weight of the organ declined. Degenerative changes
in the liver similar to those reported in earlier studies occurred.
Porphyrinuria and pathological amounts of porphyrin in liver, bones
and marrow were observed (Campbell, 1963).
Rats were given hexachlorobenzene at a level of 2 percent in the diet.
After appearing normal for the first 10-12 year, clinical and
biochemical signs of porphyria then appeared rapidly. There was a
considerable loss in body-weight, the mean for 13 rats being 25
percent of the initial weight. Cutaneous eruptions on the head, back
and feet occurred. Generalized tremor was observed. Porphyrinuria was
seen after two weeks of feeding hexachlorobenzene. Many rats died
after three to four weeks. Adenosine-5-monophosphoric acid, 20 mg
daily, was given by intramuscular injection to some of the rats,
starting on the 13th day of the experiment. By the 28th day, there was
a striking difference between most of the adenosine-5-monophosphoric
acid-treated and the other rats. Loss of weight was diminished, no new
cutaneous eruptions appeared and those that had existed healed
completely. There was a reduction in the urinary excretion of
porphyrins. Despite these improvements, however, some of the rats
treated with adenosine-5-monophosphoric acid died towards the end of
the fourth week of feeding hexachlorobenzene (Gajdos and Gajdos-Torök,
1961a, 1961b, 1961c).
A group of 33 male rats were fed a diet containing 0.2 percent of
hexachlorobenzene. Within the first month 13 rats died, exhibiting
terminal tremor, ataxia, weakness and paralysis. In the remaining
rats, a significant increase in urinary excretion of porphyrins and
porphyrin precursors was first noted after two to eight weeks of
hexachlorobenzene administration. In rats in which hexachlorobenzene
was discontinued shortly after peak excretory values were reached,
there was a return to normal excretion values within a week. However,
maintenance of high levels of porphyrin excretion by continued feeding
of hexachlorobenzene for two to three weeks resulted in an apparently
irreversible porphyric state, in which marked porphyrinuria continued
in spite of removal of hexachlorobenzene from the diet. Hepatomegaly
was common in the porphyric rats. Histological studies showed
liver-cell degeneration (Ockner and Schmid, 1961).
Long-term studies
No information available.
OBSERVATIONS IN MAN
Several published reports have described an outbreak of a cutaneous
type of porphyria that began in southeastern Turkey in 1955. The total
number of cases over a five-year period has been estimated at
3000-5000. Most of these were in children. The clinical manifestations
consisted of blistering and epidermolysis of the skin in areas exposed
to sunlight, particularly the face and hands. The lesions healed
poorly. Hyperpigmentation was invariable, usually accompanied by
marked hypertrichosis which was not limited to the exposed skin areas.
The urine contained large quantities of porphyrins. Weight loss and
hepatomegaly were frequently present. Neurological symptoms did not
appear to be evident but abdominal pain was reported in some cases and
liver enlargement was present in 35 percent of the cases surveyed. In
many cases bone and joint changes occurred, in some patients there was
osteoporosis of the bones of the extremities and interphalangeal
arthritis. The outbreak was traced to the consumption of wheat,
intended for planting, that had been treated with hexachlorobenzene.
It was estimated that the amount of hexachlorobenzene ingested by the
persons affected was from 50 to 200 mg/day for a relatively long
period before the disease became apparent. After stopping the
consumption of bread made from hexachlorobenzene-treated wheat, the
acute skin manifestations disappeared in about 20 to 30 days. Urinary
findings reverted to normal in most patients. Relapses during the
summer months were often seen. However one to two years after the
termination of other symptoms of porphyria the joint lesions were
found to be still present. It was concluded that the disease was the
result of a metabolic disorder caused by interference with porphyrin
metabolism in the liver by hexachlorobenzene. Upon recognition of the
situation, the use of hexachlorobenzene as a fungicide was
discontinued in 1959. Subsequently the disease gradually disappeared
(Cam and Nigogosyan, 1963; Cetingil and Ozen, 1960; Dogramaci, 1961,
1962, 1964; Dogramaci et al., 1962a, 1962b, Schmid, 1960, Wray et al.,
1962).
COMMENT
Evidence is presented that hexachlorobenzene is a highly toxic
compound. There is insufficient information on metabolism especially
in relation to the toxic compound excreted in milk. Use effect on the
bone-marrow gives rise to serious concern. In addition, no long-term
studies or studies on reproduction are available and there is little
information on the effect in mammalian species other than the rat. For
those reasons no acceptable daily intake can be established. However,
unintentional residues have been found in a variety of food
commodities in many countries. The Meeting therefore agreed to
consider the short-term study in rats as a basis for establishing a
tentative negligible daily intake using an extremely high safety
factor. It was stressed, however, that the use of this compound is
highly undesirable and a search for a more suitable substitute is
strongly recommended. In addition, extreme precautions should be taken
to prevent treated seeds and seed-grains from being consumed by humans
or farm animals.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TENTATIVE NEGLIGIBLE DAILY INTAKE
0 - 0.0006 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Seed wheat is treated with HCB to destroy seed-borne and soil-borne
spores of Bunt fungi (Tilletia spp.) and to ensure freedom from Bunt
in the subsequent crop. HCB has proved outstanding for this purpose
and has been used widely since being first introduced in 1945. (Costa
1952, Holton and Purdy 1957, Meagher 1953, Purdy 1961). Wheat infected
with "Bunt" or "Stinking Smut" has a bad appearance, an unpleasant
smell and is unsuitable for milling. "Bunt" can be regarded as a most
important and common disease of wheat.
Wheat crops become infected after sowing of seed, which has live Bunt
spores on its surface. These spores germinate and enter the young
plant, growing inside it throughout the season. At harvest a black
mass of spores replaces the starch of the grain whilst the skin of the
grain remains unaffected. During harvest these "Bunt Balls" are
broken, thus releasing myriads of spores over the clean grain, which
if sown without treatment, will lead to heavily infected crops.
HCB is usually applied in the form of dust containing 10-40 percent
a.i. but in Canada liquid preparations are also used (Houghton 1969).
Formulations are coloured to assist in application and to distinguish
treated seed from other grain. Usually, a blue pigment is used but
sometimes carbon black or a red dyestuff is added to reduce the risk
of treated seed being used for food. In Turkey where treated seed was
used for food with disastrous consequences it was proposed that a
denaturant with a strong taste or smell be added as well as colour.
In Australia and various other countries, wheat is selected for seed
at harvest time for the next season and after grading to remove small
or broken grains, fungicide is added automatically by machinery at the
rate of 1-2 ozs 30 percent HCB dust per bushel (330 ppm). The treated
seed is stored in labelled jute bags until required for planting some
months later. There is no evidence of significant contamination of
commercial grain or animal feeds during the process of applying the
HCB dust. The use of untreated seed wheat invariably leads to heavy
losses from fungus diseases (Bunt or Stinking Smut) and it is
therefore standard practice to treat sufficient seed for the
anticipated needs of the next season. It is exceptional for farmers to
buy seed: they normally produce and save for their own requirements.
Farmers are directed not to use treated seed wheat for animal feed or
to allow it to become mixed with commercial grain. Although such
directions are normally observed, the presence of 1 bushel of seed
wheat in 10,000 bushels of commercial grain is sufficient to lead to
significant residues in eggs from hens receiving such grain in their
ration. Contaminated grain sacks, machinery, storage premises,
transport and dust from commercial grain all contribute to the
residues found in animal products.
In Canada, seed wheat is usually treated just before planting.
Although some dusts are used, aqueous suspensions suitable for
application as a coarse spray are favoured. As an alternative,
"drill-box" treatments have been developed whereby the fungicide dust
is added to the seed wheat in the feed box of the seed drill where it
is distributed by simple agitation. Such drill-box treatments are less
effective but appear capable of reducing cross contamination of other
grain, (Wallace 1966).
Statistics on the production or use of HCB are not available for
countries other than Australia. In Australia 12 million bushels of
seed wheat are treated annually with HCB dust, requiring 200 tons of
technical HCB. A smaller proportion of the total crop is probably
treated in U.S.A., Canada, U.K. and some European countries but there
is apparently an extensive use in Turkey, Italy, Spain, Netherlands,
Germany, France and some Eastern European countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the time of the original introduction of HCB as a seed dressing and
for many years afterwards, the analytical methods were not capable of
detecting the level of residues found in commercial grain or animal
products. The only trial results available are those from Australia.
In animals
Craig (1959) fed HCB treated wheat to poultry at the rate of 1´ oz per
bird per day for six months. At the end of this period the fat of the
birds contained more than 300 ppm HCB. Craig and Dwyer (1961) fed seed
wheat containing 660 ppm HCB to aged sheep at the rate of 4 oz and 12
oz per head per day. After four months those receiving 4 oz per day
(80 mg HCB/day) had 330 ppm in the fat but none in liver or kidney.
Those on 12 oz per day (240 mg HCB/day) had 880 ppm in fat and 200 ppm
in liver and kidney.
Gardiner and Armstrong (1960) fed two pigs on HCB treated wheat
containing 660 ppm HCB for 71 days during which each consumed 329 lbs
of wheat. Animals gained 50-98 lbs in weight and at slaughter fat
contained 1000 ppm of HCB.
Watts (1968) reports trials where HCB residues were determined in eggs
and body fat of poultry receiving HCB treated wheat. Three groups of
chickens were fed wheat containing 340 ppm HCB for seven, fourteen and
twenty-eight days before being given untreated grain for twenty-eight
days. The maximum amount of HCB found in egg yolk was 76, 167 and 146
ppm respectively. The maximum amount in body fat of the same chickens
was 178, 349 and 528 ppm respectively. Controls fed on grain believed
to be free of residues showed 0.84 ppm in egg yolk and 3.3 ppm in fat.
It was found that the untreated controls received 0.02 ppm HCB in
their rations.
Later trials reported by Watts (1968a) showed that four groups of
chickens fed rations containing 0.02 ppm, 0.08 ppm, 0.7 ppm and 7 ppm
HCB for two months showed maximum residues of HCB in egg yolk of 0.2
ppm, 0.3 ppm, 2 ppm and 15 ppm respectively. Body fat from the same
chickens contained up to 0.7, 0.7, 5 and 29 ppm of HCB. Half of the
birds receiving the above rations were given treated grain after one
month. At the end of the second month the HCB residues in egg yolk
ranged from 0.18 to 0.2, 0.2 to 0.25, 0.7 to 1.2 and 6 to 12 ppm
respectively. The body fat of these birds contained HCB ranging from
0.6 to 0.7, 0.7 to 0.9, 1.5 to 2.6, 16 to 24 ppm respectively. It was
concluded that residues decline only slowly when birds which have
previously ingested HCB receive untreated feed for one month.
De Vos et al. (1968) as a result of identifying HCB in tissues of wild
birds in the Netherlands carried out semichronic toxicity tests on
Japanese quail and measured the HCB residues in liver, blood and fat.
Whilst exceptionally high levels were found in birds receiving toxic
concentrations in their diet, birds fed on diets containing 20 ppm of
HCB showed HCB residues in the fat of 350 to 520 ppm after three
months. The author concluded that the half-life of HCB in the quail is
about three weeks.
Wit (1969) reports no HCB in an extensive survey of animal fats
examined in the Netherlands but he notes that HCB would be obscured by
and reported as alpha BHC.
In plants
Johns (1969) reports results from six samples of wheat grown from HCB
treated seed in different soils. Residues range from 0.003 ppm to
0.062 ppm. Two samples of wheat grown from seed that had not been
treated with HCB showed 0.001 ppm and 0.003 ppm but it is not known
whether HCB treated wheat was grown in the name soil in previous
years.
Smith (1969) reported trials where wheat treated with 416 ppm HCB was
planted and the resulting grain analysed. Residues of 0.0033 ppm were
found. Untreated seed yielded grains containing 0.0022 ppm HCB. Soils
from twelve wheat farms were analysed and all showed traces of HCB
ranging from 0.001 to 0.02 ppm though nine were below 0.003 ppm.
Old (1969) reports investigations with wheat containing 0.05 ppm HCB,
to determine the effect of milling on the distribution of HCB residues
in the various milled fractions, and the HCB residues in bread made
from the flour fraction. Using wheat containing 0.05 ppm HCB it was
found that residues were distributed in all main milling fractions.
The total residues recovered were equivalent to 0.049 ppm. Of this 42
percent was recovered in the bran, 36 percent in the pollard and 22
percent in the flour. The flour which was found to contain 0.015 ppm
HCB produced bread containing 0.0024 ppm HCB. Residues in bread were
thus only 16 percent of the residues in the flour used for the making
of bread. Further confirmation of the loss during baking was obtained
from flour containing 0.006 ppm HCB which produced broad containing
0.0014 ppm HCB or 23 percent of the amount in the flour used.
There is extensive evidence from the literature that HCB is volatile
in water vapour even at low temperatures. It is assumed that the loss
during baking in due to steam volatilisation.
FATE OF RESIDUES
As far as can be gauged the HCB applied to seeds in not broken down by
physical, chemical or biological agencies but is simply dissipated and
diluted in the soil and atmosphere.
Because of the extreme stability of the molecule, solubility in fatty
tissue and the ease with which animals extract and concentrate
residues of HCB from their feed into their fatty tissues residues are
readily detectable in animal products.
The inadvertent contamination of commercial grain and animal feeds
with traces of HCB, though undesirable, appears hard to eliminate.
Evidence of residues in food in commerce or at consumption
A considerable amount of the date available results from surveys
conducted to determine the level and source of HCB residues in eggs,
meat fat and dairy produce from individual farms. Except in isolated
instances where farmers have not observed instructions not to feed
treated seed grain to domestic animals, the contamination invariably
results from the inadvertent feeding of grain or milling fractions
containing low levels of HCB residues. It is not possible for farmers
to know that such animal rations are contaminated and in view of the
slow rate of excretion the retention of residues in fatty tissues of
such animals presents serious economic and administrative problems.
The following table given results of an extensive survey of HCB
residues in animal products calculated on a fat basis.
TABLE II
HCB residues in Animal Products
ppm.
Commodity No. of No. Less 0.1 0.25 0.5 Over
Samples with than to to to
HCB 0.1 ppm 0.25 0.5 1.0 1.0
Beef Fat 2,029 14 12 1 1 0 0
Mutton Fat 833 20 2 8 9 1 0
Butter 514 15 8 5 0 2 0
Cheese 977 4 3 0 1 0 0
Frozen liquid 393 227 81 106 25 11 4
whole egg
Frozen liquid 154 90 8 42 17 19 4
egg yolk
A survey of commercial wheat samples carried out in Australia revealed
that HCB residues can be found in a significant proportion of the
samples in amounts ranging from traces (less than 0.005 ppm) to a
maximum of 0.05 ppm.
In the United Kingdom positive evidence of the presence of HCB has
been obtained in several shipments of imported wheat. Three samples
from one shipment contained 0.67, 1.3 and 0.48 ppm HCB. In two other
shipments the combined HCB and alpha BHC was less than 1 ppm.
Surveys of cheese imported into Australia reveal HCB residues in a
significant proportion of samples from many countries. More than 10
percent of such imports showed residues in excess of 0.1 ppm, with
many samples ranging from 0.3 to 0.9 ppm.
Examinations of food products imported into U.S.A. (Duggan, 1969)
showed a very low incidence of HCB residues for 1967 and 1968. One
and cheese in 1968). However, in 1969 the incidence increased, HCB
being reported in 170 samples of the 1,866 examined. The increase
probably reflects improvements in analytical technique more than an
increase in the incidence and level of contamination.
The majority of these findings were in manufactured dairy produce with
HCB being reported in 149 of 608 samples mainly of cheese. The range
of values for HCB in the positive sample was from a trace to 0.81 ppm
on a fat basis. The average of 608 samples was 0.01 ppm. Processed
foods, other than dairy produce, showed 12 positive findings in 345
samples examined. The range of positive findings in these commodities
was from a trace to 0.23 ppm.
One sample of butter was found to contain the following residues (on a
fat basis):-
BHC - 0.24 ppm
lindane - 0.32 ppm
HCB - 0.37 ppm
It is obvious that rather more than average care must be taken in
resolving such mixtures of residues which can easily become confused
by certain GLC analytical procedures.
HCB residues have been found in small proportion of the samples of
domestic food production. In 1967, HCB was reported in 12 samples out
of approximately 20,000 examined. In 1968 it was reported in only one
sample out of approximately 10,000 examined and in 1969 in 10 samples
out of approximately the name number tested. The level in positive
findings varied from a trace (less than 0.01 ppm) to 0.5 ppm.
METHODS OF RESIDUE ANALYSIS
On foods
Most chlorinated pesticides can be successfully isolated from fatty
substrates by methods based on the acetonitrile-hexane partition of
Mills (1959). However, HCB 10 exceptionally non-polar, and is
distributed preferentially into the hydrocarbon phase. Its partition
ratio by experiment using equal volumes of the two solvents in
approximately 0.75 to hexane at 20°C. In three counter-current
separations with equal volumes, recovery would be about 10 percent.
Increasing the volume of acetonitrile to increase extraction of the
pesticide would also increase extraction of unwanted fatty matter.
Methods using direct extraction with adsorption cleanup, but without
partition, give acceptable recoveries of HCB from eggs, Onley and
Mills (1962), butterfat, Moats (1963), Langlois et al. (1963) and meat
fat. A method using elution through florisil with methylene
chloride-hexane is now in use in the Australian Commonwealth
Laboratories (Taylor 1969, direct communication).
In wheat
The analytical problem here is somewhat different, as the significance
of the presence of HCB in grain for consumption is related to the
potential for concentration of residues in animals from low levels in
the wheat, as well as to that used for direct human consumption. Thus
the levels of interest are much lower than those in animal products.
Extraction of HCB from wheat was achieved by cracking in a blendor,
refluxing with "hexane" (Shell X4) and filtration through anhydrous
sodium sulphate. Beside the chlorinated residue, this extract contains
fat-soluble plant extractives. Passage through a column of activated
florisil cleans the solution sufficiently for determination by GLC and
confirmation by, TLC, if the levels are comparatively gross, (over 0.1
ppm). However, when the concentration is 0.01 ppm or less, the ratio
of interfering substances to pesticide becomes large enough to require
further cleanup if TLC is to be used. Alternatively, other means of
confirmation are required.
Investigation has therefore followed two lines: to Improve cleanup and
to devise other confirmatory tests.
Cleanup
Hexachlorobenzene is stable to concentrated sulphuric acid. A hexane
solution of residual substrate and HCB, after florisil treatment, was
washed with the acid several times until no further darkening
occurred. The hexane layer was washed with water until neutral, and
dried with anhydrous sodium sulphate. The infra-red spectrum of the
residue after removal of solvent appeared to be that of a hydrocarbon.
Gas chromatography produced meaningful peaks at retention times
corresponding to HCB, but resolution by TLC was prevented by the
streaking effect of the oily residue. This cleanup in inadequate for
low levels.
Attempts at separation of HCB from hydrocarbon material on alumina and
on activated carbon columns with a variety of eluting solvents did not
result in sufficiently clean products.
Refluxing of wheat extracts containing HCB with alcoholic potash
resulted in varying degrees of breakdown of the pesticide as well as
substrate. Although this precluded alkaline cleanup, it suggested
possibilities for confirmation of identity of HCB (vide infra).
Steam distillation of standard solutions of HCB was found to give high
recovery. Wheat extract previously partly cleaned by treatment with
sulphuric acid was therefore distilled from a large (twenty-fold)
excess of water, the vapour rising through a Vigreux column and
descending through a water-cooled condenser before collection under
hexane. The hexane extract from 20 ml of aqueous distillate produced
clear spots on TLC plates for both hexachlorobenzene and "benzene
hexachloride" the former at a level of 0.02 ppm (referred to the
original wheat). Recovery tests by adding known amounts of HCB
produced similar results, with recoveries close to 100 percent.
In spite of the high sensitivity of this method it is recommended that
the sensitivity be developed still further to provide for the need to
determine residues in cereal products at levels below 0.01 ppm.
Confirmation
Ideally, confirmation of identity should rest upon two or more methods
using properties as widely different as possible. For chlorinated
pesticides, chromatography by GLC and TIC are usually considered
suitable. However cleanup difficulty, together with small residues,
may preclude the use of TLC. It is acceptable, then, to use GLC on
columns of such different stationary phases that the substance has
substantially different retention characteristics, use being made of
different combinations of stationary phase-solute interactions.
Experimentally, the combination of retention values on a silicone
(low-polar) and polyester (polar) columns were found to provide useful
confirmations of the presence of HCB.
A number of experiments were carried out to make use of the
degradation of HCB by alkali. Both alcoholic potash and sodium
methylate were used, the latter being more dependably effective. When
small traces of the substance, of the order of 0.1 ppm or less, are
present, the pattern of degradation is consistent, in that two major
products appear in both TLC and GLC, one corresponding in retention
values with HCB, the other being lower. However, when greater amounts
of HCB are treated, the result is often obscured by the appearance of
several other products. The reaction is not, therefore, a reliable
confirmatory test for all samples.
A gas-chromatographic column commonly used in estimation of multiple
chlorinated pesticide residues contains the mixture of DC200 silicone
and QFI fluosilicone proposed by Burke and Holswade (1966). This
column does not separate HCB and the alpha isomer of "BHC", the latter
being the major constituent used in estimation of "BHC". In addition,
alpha-BHC does not produce a strong reaction on the silver nitrate
impregnated Thin layer used. Thus BHC may not be detected if in low
concentrations (at 0.1 ppm levels).
To distinguish and confirm these compounds, a stationary phase of a
more polar nature may be used. Separate peaks are produced on Reoplex
(poly propylene glycol adipate), XE60 (nitrile silicone) and Zonyl E7
(fluoroalkyl pyromellitic ester). Therefore an extract showing a peak
of HCB/BHC on the Burke column may be reanalysed on one of these
columns to distinguish between the two compounds.
The polar phases mentioned are, however, not suitable for all the
chlorinated hydrocarbons under investigation. On some, DDT is degraded
or in indistinguishable from DDD. On others, DDE and Dieldrin are not
separated. Work is currently on hand to select a column suitable for
separating all the chlorinated hydrocarbons for which goods are at
present analysed.
NATIONAL TOLERANCES
No national tolerances are known.
Australia, Canada and the U.S.A. approve the use of HCB seed dressings
on the basis that the seeds are not used for food or feed for animals.
APPRAISAL
HCB has been used extensively as a fungicide to control Bunt
(Tilletia caries, T. tritici and T. foetida) in wheat
since 1945. There are many reports of its action on other seed borne
and soil borne fungi of cereals and as seed treatment for onions but
these uses do not appear to be of great importance.
HCB formulations for seed treatment are sold in most wheat growing
countries. To date the only alternative materials are organomercurial
compounds. The rate of application is usually within the range of 1-3
ozs. of 30 percent dust per bushel of wheat seed equivalent to 330-990
ppm on the seed.
HCB is a comparatively pure material containing 98 percent
hexachlorobenzene. The impurities are tetrachlorobenzene and
pentachlorobenzene.
The data available to the meeting were obtained from the published
literature and by correspondence with more than 100 authors,
administrators and manufacturers throughout the world. In spite of the
wide search there is only limited information on residues in food
commodities in international trade or following good agricultural
practices, other than from Australia. One reason for this is that when
examining food commodities by several GLC analytical procedures HCB
has been obscured or confused with BHC.
All available data indicates that HCB is not metabolized to any
significant extent by plants or animals and that it persists in soil
and in animal tissues for long periods. There is an indication that
HCB might be translocated from treated seed into plant material and
subsequent grain but the level of contamination from this source is
insignificant.
Contamination of commercial grain from soil, seed, machinery and bags
may be minimized by strict attention to agricultural practices but it
is difficult to avoid all traces of HCB in commercial grain.
Animals feeding on grain or milling fractions containing traces of
HCB, concentrate and store the residues in body fat, and excrete
significant quantities in milk and eggs. The residue level in such
animal products ranges from ten to one hundred times the concentration
in feed.
The literature includes several methods of residue analysis which
measure HCB in grain and animal products by GLC but the results can be
confused as and by isomers of BHC. The sensitivity for the
determination of HCB is 0.005 ppm but special extraction and cleanup
procedures are necessary to ensure reasonable recovery from plant
products and especially from animal fats. However, no referee method
has been evaluated.
In view of the importance of HCB seed dressings in countries where
Bunt is endemic, and the lack of suitable alternatives, the Joint
Meeting recognized that recommendations for administrative action
levels were required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY PRACTICAL RESIDUE LIMITS (effective to 1973)
The following temporary practical residue limits are to apply to raw
agricultural commodities moving in commerce unless otherwise
indicated:
Raw cereal (wheat) 0.05 ppm
Cereal products from wheat 0.01 ppm
Fat of cattle, sheep, goats,
pigs and poultry 1.0 ppm
Milk 0.012 ppm
Milk products 0.3 ppm
Eggs (shellfree basis) 1.0 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Metabolic studies in animals including identification of the toxic
product or products excreted in milk.
2. Short-term studies in non-rodent mammalian species and long-term
studies especially in relation to the effect on bone-marrow.
3. Reproduction studies in rats.
REQUIRED before 30 June 1973 (for 1973 review of
Practical Residue Limits)
1. Data from countries other than Australia on residues in raw
agricultural commodities.
2. Data from surveys of residues in commodities moving in
international trade.
DESIRABLE
1. Further information on analytical procedures capable of
distinguishing HCB from BHC isomers suitable for use in
laboratories engaged in general regulatory work.
2. Collaborative studies on analytical methods capable of recovering
and identifying HCB residues in the above food commodities.
REFERENCES
Burke, Jerry A. and Holswade, Wendell. (1966) Gas chromatographic
column for pesticide residue analysis: retention times and response
data. J. Assoc. Offic. Anal. Chemists 49 (2) 374-85
Cam, C. and Nigogosyan, G. (1963) Acquired toxin porphyria cutanea
tarda due to hexachlorobenzene. J. Amer. med. Ass., 183:90-3
Campbell, J.A.H. (1963) Pathological aspects of hexachlorobenzene
feeding in rats. S. Afr. J. Lab. Clin. Med. 9(1):203-6
Cetingil, A.I. and Ozen, M.A. (1960) Toxic porphyria. Blood,
16:1002-11
Costa, J.J. (1952) A new material for eliminating bunt of cereals:
HCB. Rev. Argent, Agron. 19:44-51
Craig, J. (1959) Report of trials - feeding animals with grain
treated with fungicides. Reports - Department of Agriculture, Western
Australia, 1959-60
Craig, J. and Dwyer, H.P. (1961) "Pickled Wheat is Safe for Sheep".
HCB treated wheat is safe for sheep but should be fed with caution
for short periods only. J. of Agric. of W.A., Nov. 1961 p.909
De Matteis, F., Prior, B.E. and Rimington, C. (1961) Nervous and
biochemical disturbances following hexachlorobenzene intoxication.
Nature, 191:363-6
Dogramaci, I. (1961) An outbreak of toxic porphyria in southeastern
Turkey (editorial) Turk. J. Pediat., 3:57-60
Dogramaci, I. (1962) Porphyria turcica (Cutaneous porphyria seen
in southeastern Turkey). General consideration. Turk. J. Pediat.,
4:129-31
Dogramaci, I., Kenanoglu, A., Müftü, Y., Ergene, T. and Wray, J.D.
(1962a) Bone and joint changes in patients with porphyria turcica.
Turk. J. Pediat., 4:149-56
Dogramaci, I., Wray, J.D., Ergene, T., Sezar, V. and Müftü, Y.
(1962b) Porphyria turcica A Survey of 592 cases of cutaneous
porphyria seen in southeastern Turkey. Turk. J. Pediat., 4:138-48
Dogramaci, I. (1964) Porphyrias and porphyrin metabolism, with
special reference to porphyria in childhood in Advances in
Pediatrics, Year Book Medical Publishers, Inc., Vol. 13, pp.11-63
Duggan, R.E. (1969) Direct communication
Gajdos, A. and Gajdos-Torök, M. (1961a) The therapeutic effect of
adenosine-5-monophosphoric acid in porphyria. Lancet, 2:175-7
Gajdos, A. and Gajdos-Torök, M. (1961b) Porphyrie expérimental
observée chez le rat blanc à la suite d'intoxication par
l'hexachlorobènzene. Rev. franç. Etud. clin. biol. 6:549-52
Gajdos, A. and Gajdos-Torök, M. (1961c) Action thérapeutic de l'acide
adénosine-5-monophosphorique sur la porphyrie expérimental du
rat blanc due à l'intoxication par l'hexachlorobenzène. Rev.
franç. Etud. clin. biol., 6:553-9
Gardiner, M.R. and Armstrong, J. (1960) Feeding pickled wheat to
pigs. J. Dept. Agr. W. Australia 1, 237-8 C.A. 54.21368d.
Holton, C.S. and Purdy, L.H. (1957) Comparative effectiveness of HCB
and mercury preparations in controlling soil-borne common bunt in
commercial field trials. Pl. Dis. Reptr. Reprint 39:842-3
Houghton, E.R. (1969) Canada Department of Agriculture. Personal
communication
Johns, T.H. (1969) Translocation of HCB in Wheat. Report to
Australian Pesticides Sub-Committee from N.S.W. Dept. of
Agriculture, June 1969
Langlois, B.E., Stemp, A.R. and Leske, B.J. (1963) Milk fat from the
Babcock Test for Insecticide Residue Analysis in Dairy Produce. J.
Dairy Sc. 46 (1963) 854
Meagher, J.W. (1953) Bunt or stinking smut of wheat. Progress report
on seed dusting trials HCB. J. Dep. Agric. Vict. 51, 11:502-4
Melis, R. (1955) Toxic action of hexachlorobenzene for warm-blooded
animals. Nuovi Ann. Ig., 6:361-7 [Chem. Abstr. 50:5170 g, (1956)].
Mills, P.A. (1959) Detection and Semiquantitative Estimation of
Chlorinated Organic Pesticide Residues in Food by Paper
Chromatography. J.A.O.A.C. 42 (1959) 734
Middleton, John T. (1965) The presence, persistence and removal of
pesticides in air. Res. Pestic., Proc. Conf., Davis, Calif. 1964
191-7 (Pub. 1965)
Moats, W.A. (1963) One-step Cleanup Procedure Using Florisil
J.A.O.A.C. 46 (1963) 172
Ockner, R.K. and Schmid, R. (1961) Acquired porphyria in man and rat
due to hexachlorobenzene intoxication. Nature, 189:499
Old, A.N. (1969) HCB Residues in Milled Wheat Fractions and Baked
Bread. Report of Dept. of Agriculture of N.S.W. CB/66/825 (To be
published)
Onley, J.H. and Mills P.A. (1962) Detection and Estimation of
Chlorinated Pesticides in Eggs J.A.O.A.C. 45(4) 983
Parke, D.V. and Williams, R.T. (1960) Studies in Detoxication 81. The
metabolism of halogenobenzenes: (a) Penta- and hexa-chlorobenzenes.
Biochem. J., 74:5-9
Pearson, R.W. and Malkinson, F.D. (1965) Some observations on
hexachlorobenzene induced experimental porphyria. J. invest.
Derm., 44: 420-32
Purdy, L.H. (1961) Control of common bunt of wheat by seed treatment
in the Pacific North West. U.S.D.A. ARS 34-21:1-8
Savitskii, I.V. (1964) The basis for determining safe permissible
concentrations of hexachlorobenzene and pentachloronitrobenzene
in the air. (Russian) Vopr. Prom. i Sel' skokhoz. Toksikol.,
Kievsk. Med. Inst. 158-173 [Chem. Abstr. 63:8952 d, (1965)]
Schmid, R. (1960) Cutaneous porphyria in Turkey. New Engl. J. Med.,
263:397-8
Smith, W.S. (1969) Translocation of HCB in wheat. Report to Australian
Pesticides Sub-Committee from S.A. Dept. of Agriculture, June
1969
Vos de, J.G., Breeman, H.A. and Benschop, H. (1968) The occurrence of
the fungicide hexachlorobenzene in wild birds and its toxicological
importance. A preliminary communication. Mededelingen Rijksfakulteit
Landbouwwetenschappen Gent. 33:1263-9
Wallace, H.A.H. (1966) A comparison of standard and drillbox seed
treatment chemicals for covered smut of oats and barley. Canadian
Plant Disease. 46 (4) 134-6 Dec. 1966
Watts, R.M. (1968) HCB residues in eggs and body fat of poultry
receiving HCB treated wheat. Reports to Australian Pesticides
Sub-Committee by N.S.W. Department of Agriculture (June 1968)
Watts, R.M. (1968a) HCB residues in Poultry - Low-level feeding
trial. Report to Australian Pesticides Sub-Committee by N.S.W.
Department of Agriculture (June 1968)
Weir, R.J. (1962) Experimental pre-emergence herbicide HCB pure
(new), 90-day dietary administration - rats. Unpub. Rept. of Hazleton
Laboratories, Inc. sponsored by Diamond Alkali Co.
Wit, S.L. (1969) Residues of insecticides belonging to the Group of
chlorinated cyclic hydrocarbons in vegetable and animal fats.
Report No.15/69 Tox. (Feb. 1969) Rijks Institut Voor de
Volksgezondheid - Utrecht - Netherlands
Wray, J.D., Müftü, Y. and Dogramaci, I. (1962) Hexachlorobenzene as
a cause of porphyria turcica Turk. J. Pediat., 4:132-7
Other relevant chemical properties
Colourless white powder or needles MP 229°C - BP. 326°C. V.P. 1.089 ×
10-5 mm Hg at 20°C - sublimable. Insoluble in water and alcohol.
Soluble in hot benzene. The technical grade used in agriculture
contains 98 percent hexachlorobenzene, 1.8 percent pentachlorobenzene
together with 0.2 percent 1,2,4,5-tetrachlorobenzene.
Commercial formulations (dusts) contain 10-40 percent HCB alone or
together with small quantities of lindane (0.5-1.0 percent) added to
prevent insect attack on stored seed.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Residues of hexachlorobenzene are stored in the liver and fat of
birds. The biological half-life of hexachlorobenzene in the quail is
about three weeks (Vos et al., 1968) (see also "Short-term studies.
Quail").
When hexachlorobenzene, 400 mg/kg, was administered orally to rabbits,
the compound did not appear to be metabolized, because the main
portion was found in the gut contents five days after dosing, with
only 6 percent appearing in the faeces. There was no significant
urinary or pulmonary excretion of metabolites. Hexachlorobenzene did
not form conjugated glucuronic acids, ethereal sulphates or
mercapturic acids. Most of a dose (100 mg/kg) given subcutaneously was
found at the site of injection after five days, with no chlorinated
benzenes being found in the faeces over this period (Parke and
Williams, 1960).
There is evidence that hexachlorobenzene (or a toxic metabolite of it)
is excreted in milk. Two pregnant female rats were given
hexachlorobenzene in their food (the dose was not stated, it was
presumed to be 0.5 percent). One died, but the other was delivered
normally and reared the young until they died with convulsions after
seven to eight days. The mother was then given three normal week-old
rats to foster, which died three to four days later with convulsions.
The mother also died four weeks later with the usual symptoms of
hexachlorobenzene intoxication. This study indicated that a toxic
compound was excreted in the milk (De Matteis et al., 1961).
TOXICOLOGICAL STUDIES
Acute toxicity (oral)
Animal LD50 References
mg/kg body-weight
Mouse 4000 Savitskii, 1964
Rat 3500 Savitskii, 1964
Guinea pig > 1000 Melis, 1955
Rabbit 2600 Savitskii, 1964
Cat 1700 Savitskii, 1964
Short-term studies
Chicken
Hexachlorobenzene given at levels of 120-480 ppm in the diet for three
months, caused no toxic effects in chickens (Melis, 1955).
Guinea-pig
A diet containing 0.5 percent of hexachlorobenzene was given to
guinea-pig and to mice. These two species proved to be remarkably
susceptible to hexachlorobenzene and developed very marked
neurological symptoms within eight to 10 days (De Matteis et al.,
1961).
Mouse
See under "Guinea-pig".
Quail
Five groups of adult Japanese quail (10 females and two males per
group) were fed a mixed diet containing 0, 20, 100, 500 or 2500 ppm of
hexachlorobenzene for three months. Eggs from the 100 ppm, 20 ppm and
control groups were collected during three periods and incubated to
determine reproduction results. At the 2500 ppm level four birds died
within a week, the remainder within a month. Pre-mortem effects noted
were loss of weight, drooping wings, ruffled feathers, trembling,
ataxia and paralysis. On examination, bones, liver and kidney showed a
red fluorescence typical of porphyria. Degeneration and necrosis of
liver cells was seen. At 500 ppm all birds died within a month,
showing the same symptoms and lesions seen at the higher level. At 100
ppm, the first bird died on day 20, and 10 were dead within seven
weeks. An accumulation of porphyrins in liver and kidney was observed
in all birds in the group. Microscopic examination showed
degeneration, necrosis and local regeneration of liver cells. At 20
ppm all birds survived the three months test-period and did not
develop any visible symptoms. At autopsy one hen showed fluorescence
of bones and liver in gross ultraviolet examination. Fluorescence of
liver and kidney cells and swelling and regeneration of liver cells
was observed in this bird. In the other hens fluorescence of kidney
cells was seen under ultraviolet microscopy. Significant differences
were not found in body-weight or in relative weights of liver, spleen
and brain, nor was there any effect on egg production at 20 ppm. There
was a significant reduction in the number of chicks hatched, however,
at this level of hexachlorobenzene. It was tentatively concluded,
considering the disturbance in porphyrin metabolism and the diminished
reproduction results, that the no-effect level in this species is
lower than 20 ppm. (A comparative test in rats indicated the quail to
be much more sensitive). Residues in the liver of the birds fed
hexachlorobenzene were as follows:
TABLE I
Levels of hexachlorobenzene
Level of Number in the liver (ppm)
hexachlorobenzene History of birds Average Range
fed (ppm)
500 died 9 450 180-850
100 died 6 235 85-720
20 killed 6 36 14-94
20 killed 33 days 6 13 5-29
after termination
of hexachlorobenzene-
feeding
Residues were also found in eggs and in the chicks after hatching.
Several predatory birds, most of them found dead, had liver residues
of hexachlorobenzene in the same range as those in the 20 ppm group of
the quail study. It was concluded that hexachlorobenzene used as a
seed-dressing, may have toxic effects on seed-eating and predatory
birds (Vos et al., 1968)
Rabbit
Female rabbits were fed a diet containing 0.5 percent of
hexachlorobenzene. After about six weeks an increase in urinary
porphyrins was noted. Unless sacrificed earlier, the animals died in
eight to 12 weeks after having demonstrated neurological symptoms. (De
Matteis et al., 1961).
Rat
Groups of 10 male and 10 female rats were fed diets containing
hexachlorobenzene at levels of 0, 5, 25, 125, and 625 ppm for 13
weeks. After 13 weeks five males and five females from each group were
sacrificed for gross and microscopic examination. The rats left in the
groups given 125 and 625 ppm were transferred to the control diet for
a further two weeks, then sacrificed and examined. The rats in the 625
ppm group displayed signs of marked respiratory involvement; slight
tremors were also observed in several animals and the females
exhibited small sores in the skin of the head or neck. None of the
other groups showed these effects. Growth of male rats at the highest
level fed was reduced. Survival was not affected at any dose-level.
Total leukocyte counts were elevated during the test in the group fed
625 ppm. Organ-weight data showed an increase at 625 ppm, in thyroid,
liver, spleen and adrenals, and in male rats at 125 ppm, in liver.
Microscopic examination showed consistent effects in the liver
(lobular distortion, nuclear and cytoplasmic variations, focal loss of
cell outline, mitotic activity, and focal cellular necrosis) of the
rats at 125 and 625 ppm. Changes of a less distinct and less
consistent nature were observed in thyroid, kidneys, adrenals and
bone-marrow of animals in these two groups. No significant effects
were observed in organs and tissues of the rats fed at 5 or 25 ppm of
hexachlorobenzene (Weir, 1962).
Female rats were fed a standard died containing 0.2 percent
hexachlorobenzene. After one week, weight-loss and general debility
was noted in some rats. Within three weeks a few rats showed increased
urinary excretion of porphyrins, but this manifestation did not become
general until the seventh or eighth week. Rats with well established
porphyria were exposed continuously to ultraviolet light. If an area
of about 25 cm2 was plucked or shaved before exposure, most of the
animals died within three to 14 days, but a few rats survived three to
four months of this treatment. Rats on the hexachlorobenzene diet that
were not shaved or plucked tolerated the ultraviolet light exposure
well. After receiving hexachlorobenzene in the diet for six to eight
months, the rats were returned to a normal diet. Urinary porphyrin
excretion decreased considerably over the first four months but was
still above normal levels nine months after hexachlorobenzene has been
removed from the diet (Pearson and Malkinson, 1965).
A diet containing 0.2 percent of hexachlorobenzene was fed to 26 male
rats. Groups of two or three animals were killed at weekly intervals
over a period of one to 12 weeks. Retardation of weight-gain occurred
in the second and third week of the experiment. Evidence of a
localized toxic effect was confined to the liver. An increase
in liver-weight reached a maximum from the fifth to the ninth
week and then the weight of the organ declined. Degenerative changes
in the liver similar to those reported in earlier studies occurred.
Porphyrinuria and pathological amounts of porphyrin in liver, bones
and marrow were observed (Campbell, 1963).
Rats were given hexachlorobenzene at a level of 2 percent in the diet.
After appearing normal for the first 10-12 year, clinical and
biochemical signs of porphyria then appeared rapidly. There was a
considerable loss in body-weight, the mean for 13 rats being 25
percent of the initial weight. Cutaneous eruptions on the head, back
and feet occurred. Generalized tremor was observed. Porphyrinuria was
seen after two weeks of feeding hexachlorobenzene. Many rats died
after three to four weeks. Adenosine-5-monophosphoric acid, 20 mg
daily, was given by intramuscular injection to some of the rats,
starting on the 13th day of the experiment. By the 28th day, there was
a striking difference between most of the adenosine-5-monophosphoric
acid-treated and the other rats. Loss of weight was diminished, no new
cutaneous eruptions appeared and those that had existed healed
completely. There was a reduction in the urinary excretion of
porphyrins. Despite these improvements, however, some of the rats
treated with adenosine-5-monophosphoric acid died towards the end of
the fourth week of feeding hexachlorobenzene (Gajdos and Gajdos-Torök,
1961a, 1961b, 1961c).
A group of 33 male rats were fed a diet containing 0.2 percent of
hexachlorobenzene. Within the first month 13 rats died, exhibiting
terminal tremor, ataxia, weakness and paralysis. In the remaining
rats, a significant increase in urinary excretion of porphyrins and
porphyrin precursors was first noted after two to eight weeks of
hexachlorobenzene administration. In rats in which hexachlorobenzene
was discontinued shortly after peak excretory values were reached,
there was a return to normal excretion values within a week. However,
maintenance of high levels of porphyrin excretion by continued feeding
of hexachlorobenzene for two to three weeks resulted in an apparently
irreversible porphyric state, in which marked porphyrinuria continued
in spite of removal of hexachlorobenzene from the diet. Hepatomegaly
was common in the porphyric rats. Histological studies showed
liver-cell degeneration (Ockner and Schmid, 1961).
Long-term studies
No information available.
OBSERVATIONS IN MAN
Several published reports have described an outbreak of a cutaneous
type of porphyria that began in southeastern Turkey in 1955. The total
number of cases over a five-year period has been estimated at
3000-5000. Most of these were in children. The clinical manifestations
consisted of blistering and epidermolysis of the skin in areas exposed
to sunlight, particularly the face and hands. The lesions healed
poorly. Hyperpigmentation was invariable, usually accompanied by
marked hypertrichosis which was not limited to the exposed skin areas.
The urine contained large quantities of porphyrins. Weight loss and
hepatomegaly were frequently present. Neurological symptoms did not
appear to be evident but abdominal pain was reported in some cases and
liver enlargement was present in 35 percent of the cases surveyed. In
many cases bone and joint changes occurred, in some patients there was
osteoporosis of the bones of the extremities and interphalangeal
arthritis. The outbreak was traced to the consumption of wheat,
intended for planting, that had been treated with hexachlorobenzene.
It was estimated that the amount of hexachlorobenzene ingested by the
persons affected was from 50 to 200 mg/day for a relatively long
period before the disease became apparent. After stopping the
consumption of bread made from hexachlorobenzene-treated wheat, the
acute skin manifestations disappeared in about 20 to 30 days. Urinary
findings reverted to normal in most patients. Relapses during the
summer months were often seen. However one to two years after the
termination of other symptoms of porphyria the joint lesions were
found to be still present. It was concluded that the disease was the
result of a metabolic disorder caused by interference with porphyrin
metabolism in the liver by hexachlorobenzene. Upon recognition of the
situation, the use of hexachlorobenzene as a fungicide was
discontinued in 1959. Subsequently the disease gradually disappeared
(Cam and Nigogosyan, 1963; Cetingil and Ozen, 1960; Dogramaci, 1961,
1962, 1964; Dogramaci et al., 1962a, 1962b, Schmid, 1960, Wray et al.,
1962).
COMMENT
Evidence is presented that hexachlorobenzene is a highly toxic
compound. There is insufficient information on metabolism especially
in relation to the toxic compound excreted in milk. Use effect on the
bone-marrow gives rise to serious concern. In addition, no long-term
studies or studies on reproduction are available and there is little
information on the effect in mammalian species other than the rat. For
those reasons no acceptable daily intake can be established. However,
unintentional residues have been found in a variety of food
commodities in many countries. The Meeting therefore agreed to
consider the short-term study in rats as a basis for establishing a
tentative negligible daily intake using an extremely high safety
factor. It was stressed, however, that the use of this compound is
highly undesirable and a search for a more suitable substitute is
strongly recommended. In addition, extreme precautions should be taken
to prevent treated seeds and seed-grains from being consumed by humans
or farm animals.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TENTATIVE NEGLIGIBLE DAILY INTAKE
0 - 0.0006 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Seed wheat is treated with HCB to destroy seed-borne and soil-borne
spores of Bunt fungi (Tilletia spp.) and to ensure freedom from Bunt
in the subsequent crop. HCB has proved outstanding for this purpose
and has been used widely since being first introduced in 1945. (Costa
1952, Holton and Purdy 1957, Meagher 1953, Purdy 1961). Wheat infected
with "Bunt" or "Stinking Smut" has a bad appearance, an unpleasant
smell and is unsuitable for milling. "Bunt" can be regarded as a most
important and common disease of wheat.
Wheat crops become infected after sowing of seed, which has live Bunt
spores on its surface. These spores germinate and enter the young
plant, growing inside it throughout the season. At harvest a black
mass of spores replaces the starch of the grain whilst the skin of the
grain remains unaffected. During harvest these "Bunt Balls" are
broken, thus releasing myriads of spores over the clean grain, which
if sown without treatment, will lead to heavily infected crops.
HCB is usually applied in the form of dust containing 10-40 percent
a.i. but in Canada liquid preparations are also used (Houghton 1969).
Formulations are coloured to assist in application and to distinguish
treated seed from other grain. Usually, a blue pigment is used but
sometimes carbon black or a red dyestuff is added to reduce the risk
of treated seed being used for food. In Turkey where treated seed was
used for food with disastrous consequences it was proposed that a
denaturant with a strong taste or smell be added as well as colour.
In Australia and various other countries, wheat is selected for seed
at harvest time for the next season and after grading to remove small
or broken grains, fungicide is added automatically by machinery at the
rate of 1-2 ozs 30 percent HCB dust per bushel (330 ppm). The treated
seed is stored in labelled jute bags until required for planting some
months later. There is no evidence of significant contamination of
commercial grain or animal feeds during the process of applying the
HCB dust. The use of untreated seed wheat invariably leads to heavy
losses from fungus diseases (Bunt or Stinking Smut) and it is
therefore standard practice to treat sufficient seed for the
anticipated needs of the next season. It is exceptional for farmers to
buy seed: they normally produce and save for their own requirements.
Farmers are directed not to use treated seed wheat for animal feed or
to allow it to become mixed with commercial grain. Although such
directions are normally observed, the presence of 1 bushel of seed
wheat in 10,000 bushels of commercial grain is sufficient to lead to
significant residues in eggs from hens receiving such grain in their
ration. Contaminated grain sacks, machinery, storage premises,
transport and dust from commercial grain all contribute to the
residues found in animal products.
In Canada, seed wheat is usually treated just before planting.
Although some dusts are used, aqueous suspensions suitable for
application as a coarse spray are favoured. As an alternative,
"drill-box" treatments have been developed whereby the fungicide dust
is added to the seed wheat in the feed box of the seed drill where it
is distributed by simple agitation. Such drill-box treatments are less
effective but appear capable of reducing cross contamination of other
grain, (Wallace 1966).
Statistics on the production or use of HCB are not available for
countries other than Australia. In Australia 12 million bushels of
seed wheat are treated annually with HCB dust, requiring 200 tons of
technical HCB. A smaller proportion of the total crop is probably
treated in U.S.A., Canada, U.K. and some European countries but there
is apparently an extensive use in Turkey, Italy, Spain, Netherlands,
Germany, France and some Eastern European countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the time of the original introduction of HCB as a seed dressing and
for many years afterwards, the analytical methods were not capable of
detecting the level of residues found in commercial grain or animal
products. The only trial results available are those from Australia.
In animals
Craig (1959) fed HCB treated wheat to poultry at the rate of 1´ oz per
bird per day for six months. At the end of this period the fat of the
birds contained more than 300 ppm HCB. Craig and Dwyer (1961) fed seed
wheat containing 660 ppm HCB to aged sheep at the rate of 4 oz and 12
oz per head per day. After four months those receiving 4 oz per day
(80 mg HCB/day) had 330 ppm in the fat but none in liver or kidney.
Those on 12 oz per day (240 mg HCB/day) had 880 ppm in fat and 200 ppm
in liver and kidney.
Gardiner and Armstrong (1960) fed two pigs on HCB treated wheat
containing 660 ppm HCB for 71 days during which each consumed 329 lbs
of wheat. Animals gained 50-98 lbs in weight and at slaughter fat
contained 1000 ppm of HCB.
Watts (1968) reports trials where HCB residues were determined in eggs
and body fat of poultry receiving HCB treated wheat. Three groups of
chickens were fed wheat containing 340 ppm HCB for seven, fourteen and
twenty-eight days before being given untreated grain for twenty-eight
days. The maximum amount of HCB found in egg yolk was 76, 167 and 146
ppm respectively. The maximum amount in body fat of the same chickens
was 178, 349 and 528 ppm respectively. Controls fed on grain believed
to be free of residues showed 0.84 ppm in egg yolk and 3.3 ppm in fat.
It was found that the untreated controls received 0.02 ppm HCB in
their rations.
Later trials reported by Watts (1968a) showed that four groups of
chickens fed rations containing 0.02 ppm, 0.08 ppm, 0.7 ppm and 7 ppm
HCB for two months showed maximum residues of HCB in egg yolk of 0.2
ppm, 0.3 ppm, 2 ppm and 15 ppm respectively. Body fat from the same
chickens contained up to 0.7, 0.7, 5 and 29 ppm of HCB. Half of the
birds receiving the above rations were given treated grain after one
month. At the end of the second month the HCB residues in egg yolk
ranged from 0.18 to 0.2, 0.2 to 0.25, 0.7 to 1.2 and 6 to 12 ppm
respectively. The body fat of these birds contained HCB ranging from
0.6 to 0.7, 0.7 to 0.9, 1.5 to 2.6, 16 to 24 ppm respectively. It was
concluded that residues decline only slowly when birds which have
previously ingested HCB receive untreated feed for one month.
De Vos et al. (1968) as a result of identifying HCB in tissues of wild
birds in the Netherlands carried out semichronic toxicity tests on
Japanese quail and measured the HCB residues in liver, blood and fat.
Whilst exceptionally high levels were found in birds receiving toxic
concentrations in their diet, birds fed on diets containing 20 ppm of
HCB showed HCB residues in the fat of 350 to 520 ppm after three
months. The author concluded that the half-life of HCB in the quail is
about three weeks.
Wit (1969) reports no HCB in an extensive survey of animal fats
examined in the Netherlands but he notes that HCB would be obscured by
and reported as alpha BHC.
In plants
Johns (1969) reports results from six samples of wheat grown from HCB
treated seed in different soils. Residues range from 0.003 ppm to
0.062 ppm. Two samples of wheat grown from seed that had not been
treated with HCB showed 0.001 ppm and 0.003 ppm but it is not known
whether HCB treated wheat was grown in the name soil in previous
years.
Smith (1969) reported trials where wheat treated with 416 ppm HCB was
planted and the resulting grain analysed. Residues of 0.0033 ppm were
found. Untreated seed yielded grains containing 0.0022 ppm HCB. Soils
from twelve wheat farms were analysed and all showed traces of HCB
ranging from 0.001 to 0.02 ppm though nine were below 0.003 ppm.
Old (1969) reports investigations with wheat containing 0.05 ppm HCB,
to determine the effect of milling on the distribution of HCB residues
in the various milled fractions, and the HCB residues in bread made
from the flour fraction. Using wheat containing 0.05 ppm HCB it was
found that residues were distributed in all main milling fractions.
The total residues recovered were equivalent to 0.049 ppm. Of this 42
percent was recovered in the bran, 36 percent in the pollard and 22
percent in the flour. The flour which was found to contain 0.015 ppm
HCB produced bread containing 0.0024 ppm HCB. Residues in bread were
thus only 16 percent of the residues in the flour used for the making
of bread. Further confirmation of the loss during baking was obtained
from flour containing 0.006 ppm HCB which produced broad containing
0.0014 ppm HCB or 23 percent of the amount in the flour used.
There is extensive evidence from the literature that HCB is volatile
in water vapour even at low temperatures. It is assumed that the loss
during baking in due to steam volatilisation.
FATE OF RESIDUES
As far as can be gauged the HCB applied to seeds in not broken down by
physical, chemical or biological agencies but is simply dissipated and
diluted in the soil and atmosphere.
Because of the extreme stability of the molecule, solubility in fatty
tissue and the ease with which animals extract and concentrate
residues of HCB from their feed into their fatty tissues residues are
readily detectable in animal products.
The inadvertent contamination of commercial grain and animal feeds
with traces of HCB, though undesirable, appears hard to eliminate.
Evidence of residues in food in commerce or at consumption
A considerable amount of the date available results from surveys
conducted to determine the level and source of HCB residues in eggs,
meat fat and dairy produce from individual farms. Except in isolated
instances where farmers have not observed instructions not to feed
treated seed grain to domestic animals, the contamination invariably
results from the inadvertent feeding of grain or milling fractions
containing low levels of HCB residues. It is not possible for farmers
to know that such animal rations are contaminated and in view of the
slow rate of excretion the retention of residues in fatty tissues of
such animals presents serious economic and administrative problems.
The following table given results of an extensive survey of HCB
residues in animal products calculated on a fat basis.
TABLE II
HCB residues in Animal Products
ppm.
Commodity No. of No. Less 0.1 0.25 0.5 Over
Samples with than to to to
HCB 0.1 ppm 0.25 0.5 1.0 1.0
Beef Fat 2,029 14 12 1 1 0 0
Mutton Fat 833 20 2 8 9 1 0
Butter 514 15 8 5 0 2 0
Cheese 977 4 3 0 1 0 0
Frozen liquid 393 227 81 106 25 11 4
whole egg
Frozen liquid 154 90 8 42 17 19 4
egg yolk
A survey of commercial wheat samples carried out in Australia revealed
that HCB residues can be found in a significant proportion of the
samples in amounts ranging from traces (less than 0.005 ppm) to a
maximum of 0.05 ppm.
In the United Kingdom positive evidence of the presence of HCB has
been obtained in several shipments of imported wheat. Three samples
from one shipment contained 0.67, 1.3 and 0.48 ppm HCB. In two other
shipments the combined HCB and alpha BHC was less than 1 ppm.
Surveys of cheese imported into Australia reveal HCB residues in a
significant proportion of samples from many countries. More than 10
percent of such imports showed residues in excess of 0.1 ppm, with
many samples ranging from 0.3 to 0.9 ppm.
Examinations of food products imported into U.S.A. (Duggan, 1969)
showed a very low incidence of HCB residues for 1967 and 1968. One
and cheese in 1968). However, in 1969 the incidence increased, HCB
being reported in 170 samples of the 1,866 examined. The increase
probably reflects improvements in analytical technique more than an
increase in the incidence and level of contamination.
The majority of these findings were in manufactured dairy produce with
HCB being reported in 149 of 608 samples mainly of cheese. The range
of values for HCB in the positive sample was from a trace to 0.81 ppm
on a fat basis. The average of 608 samples was 0.01 ppm. Processed
foods, other than dairy produce, showed 12 positive findings in 345
samples examined. The range of positive findings in these commodities
was from a trace to 0.23 ppm.
One sample of butter was found to contain the following residues (on a
fat basis):-
BHC - 0.24 ppm
lindane - 0.32 ppm
HCB - 0.37 ppm
It is obvious that rather more than average care must be taken in
resolving such mixtures of residues which can easily become confused
by certain GLC analytical procedures.
HCB residues have been found in small proportion of the samples of
domestic food production. In 1967, HCB was reported in 12 samples out
of approximately 20,000 examined. In 1968 it was reported in only one
sample out of approximately 10,000 examined and in 1969 in 10 samples
out of approximately the name number tested. The level in positive
findings varied from a trace (less than 0.01 ppm) to 0.5 ppm.
METHODS OF RESIDUE ANALYSIS
On foods
Most chlorinated pesticides can be successfully isolated from fatty
substrates by methods based on the acetonitrile-hexane partition of
Mills (1959). However, HCB 10 exceptionally non-polar, and is
distributed preferentially into the hydrocarbon phase. Its partition
ratio by experiment using equal volumes of the two solvents in
approximately 0.75 to hexane at 20°C. In three counter-current
separations with equal volumes, recovery would be about 10 percent.
Increasing the volume of acetonitrile to increase extraction of the
pesticide would also increase extraction of unwanted fatty matter.
Methods using direct extraction with adsorption cleanup, but without
partition, give acceptable recoveries of HCB from eggs, Onley and
Mills (1962), butterfat, Moats (1963), Langlois et al. (1963) and meat
fat. A method using elution through florisil with methylene
chloride-hexane is now in use in the Australian Commonwealth
Laboratories (Taylor 1969, direct communication).
In wheat
The analytical problem here is somewhat different, as the significance
of the presence of HCB in grain for consumption is related to the
potential for concentration of residues in animals from low levels in
the wheat, as well as to that used for direct human consumption. Thus
the levels of interest are much lower than those in animal products.
Extraction of HCB from wheat was achieved by cracking in a blendor,
refluxing with "hexane" (Shell X4) and filtration through anhydrous
sodium sulphate. Beside the chlorinated residue, this extract contains
fat-soluble plant extractives. Passage through a column of activated
florisil cleans the solution sufficiently for determination by GLC and
confirmation by, TLC, if the levels are comparatively gross, (over 0.1
ppm). However, when the concentration is 0.01 ppm or less, the ratio
of interfering substances to pesticide becomes large enough to require
further cleanup if TLC is to be used. Alternatively, other means of
confirmation are required.
Investigation has therefore followed two lines: to Improve cleanup and
to devise other confirmatory tests.
Cleanup
Hexachlorobenzene is stable to concentrated sulphuric acid. A hexane
solution of residual substrate and HCB, after florisil treatment, was
washed with the acid several times until no further darkening
occurred. The hexane layer was washed with water until neutral, and
dried with anhydrous sodium sulphate. The infra-red spectrum of the
residue after removal of solvent appeared to be that of a hydrocarbon.
Gas chromatography produced meaningful peaks at retention times
corresponding to HCB, but resolution by TLC was prevented by the
streaking effect of the oily residue. This cleanup in inadequate for
low levels.
Attempts at separation of HCB from hydrocarbon material on alumina and
on activated carbon columns with a variety of eluting solvents did not
result in sufficiently clean products.
Refluxing of wheat extracts containing HCB with alcoholic potash
resulted in varying degrees of breakdown of the pesticide as well as
substrate. Although this precluded alkaline cleanup, it suggested
possibilities for confirmation of identity of HCB (vide infra).
Steam distillation of standard solutions of HCB was found to give high
recovery. Wheat extract previously partly cleaned by treatment with
sulphuric acid was therefore distilled from a large (twenty-fold)
excess of water, the vapour rising through a Vigreux column and
descending through a water-cooled condenser before collection under
hexane. The hexane extract from 20 ml of aqueous distillate produced
clear spots on TLC plates for both hexachlorobenzene and "benzene
hexachloride" the former at a level of 0.02 ppm (referred to the
original wheat). Recovery tests by adding known amounts of HCB
produced similar results, with recoveries close to 100 percent.
In spite of the high sensitivity of this method it is recommended that
the sensitivity be developed still further to provide for the need to
determine residues in cereal products at levels below 0.01 ppm.
Confirmation
Ideally, confirmation of identity should rest upon two or more methods
using properties as widely different as possible. For chlorinated
pesticides, chromatography by GLC and TIC are usually considered
suitable. However cleanup difficulty, together with small residues,
may preclude the use of TLC. It is acceptable, then, to use GLC on
columns of such different stationary phases that the substance has
substantially different retention characteristics, use being made of
different combinations of stationary phase-solute interactions.
Experimentally, the combination of retention values on a silicone
(low-polar) and polyester (polar) columns were found to provide useful
confirmations of the presence of HCB.
A number of experiments were carried out to make use of the
degradation of HCB by alkali. Both alcoholic potash and sodium
methylate were used, the latter being more dependably effective. When
small traces of the substance, of the order of 0.1 ppm or less, are
present, the pattern of degradation is consistent, in that two major
products appear in both TLC and GLC, one corresponding in retention
values with HCB, the other being lower. However, when greater amounts
of HCB are treated, the result is often obscured by the appearance of
several other products. The reaction is not, therefore, a reliable
confirmatory test for all samples.
A gas-chromatographic column commonly used in estimation of multiple
chlorinated pesticide residues contains the mixture of DC200 silicone
and QFI fluosilicone proposed by Burke and Holswade (1966). This
column does not separate HCB and the alpha isomer of "BHC", the latter
being the major constituent used in estimation of "BHC". In addition,
alpha-BHC does not produce a strong reaction on the silver nitrate
impregnated Thin layer used. Thus BHC may not be detected if in low
concentrations (at 0.1 ppm levels).
To distinguish and confirm these compounds, a stationary phase of a
more polar nature may be used. Separate peaks are produced on Reoplex
(poly propylene glycol adipate), XE60 (nitrile silicone) and Zonyl E7
(fluoroalkyl pyromellitic ester). Therefore an extract showing a peak
of HCB/BHC on the Burke column may be reanalysed on one of these
columns to distinguish between the two compounds.
The polar phases mentioned are, however, not suitable for all the
chlorinated hydrocarbons under investigation. On some, DDT is degraded
or in indistinguishable from DDD. On others, DDE and Dieldrin are not
separated. Work is currently on hand to select a column suitable for
separating all the chlorinated hydrocarbons for which goods are at
present analysed.
NATIONAL TOLERANCES
No national tolerances are known.
Australia, Canada and the U.S.A. approve the use of HCB seed dressings
on the basis that the seeds are not used for food or feed for animals.
APPRAISAL
HCB has been used extensively as a fungicide to control Bunt
(Tilletia caries, T. tritici and T. foetida) in wheat
since 1945. There are many reports of its action on other seed borne
and soil borne fungi of cereals and as seed treatment for onions but
these uses do not appear to be of great importance.
HCB formulations for seed treatment are sold in most wheat growing
countries. To date the only alternative materials are organomercurial
compounds. The rate of application is usually within the range of 1-3
ozs. of 30 percent dust per bushel of wheat seed equivalent to 330-990
ppm on the seed.
HCB is a comparatively pure material containing 98 percent
hexachlorobenzene. The impurities are tetrachlorobenzene and
pentachlorobenzene.
The data available to the meeting were obtained from the published
literature and by correspondence with more than 100 authors,
administrators and manufacturers throughout the world. In spite of the
wide search there is only limited information on residues in food
commodities in international trade or following good agricultural
practices, other than from Australia. One reason for this is that when
examining food commodities by several GLC analytical procedures HCB
has been obscured or confused with BHC.
All available data indicates that HCB is not metabolized to any
significant extent by plants or animals and that it persists in soil
and in animal tissues for long periods. There is an indication that
HCB might be translocated from treated seed into plant material and
subsequent grain but the level of contamination from this source is
insignificant.
Contamination of commercial grain from soil, seed, machinery and bags
may be minimized by strict attention to agricultural practices but it
is difficult to avoid all traces of HCB in commercial grain.
Animals feeding on grain or milling fractions containing traces of
HCB, concentrate and store the residues in body fat, and excrete
significant quantities in milk and eggs. The residue level in such
animal products ranges from ten to one hundred times the concentration
in feed.
The literature includes several methods of residue analysis which
measure HCB in grain and animal products by GLC but the results can be
confused as and by isomers of BHC. The sensitivity for the
determination of HCB is 0.005 ppm but special extraction and cleanup
procedures are necessary to ensure reasonable recovery from plant
products and especially from animal fats. However, no referee method
has been evaluated.
In view of the importance of HCB seed dressings in countries where
Bunt is endemic, and the lack of suitable alternatives, the Joint
Meeting recognized that recommendations for administrative action
levels were required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY PRACTICAL RESIDUE LIMITS (effective to 1973)
The following temporary practical residue limits are to apply to raw
agricultural commodities moving in commerce unless otherwise
indicated:
Raw cereal (wheat) 0.05 ppm
Cereal products from wheat 0.01 ppm
Fat of cattle, sheep, goats,
pigs and poultry 1.0 ppm
Milk 0.012 ppm
Milk products 0.3 ppm
Eggs (shellfree basis) 1.0 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Metabolic studies in animals including identification of the toxic
product or products excreted in milk.
2. Short-term studies in non-rodent mammalian species and long-term
studies especially in relation to the effect on bone-marrow.
3. Reproduction studies in rats.
REQUIRED before 30 June 1973 (for 1973 review of
Practical Residue Limits)
1. Data from countries other than Australia on residues in raw
agricultural commodities.
2. Data from surveys of residues in commodities moving in
international trade.
DESIRABLE
1. Further information on analytical procedures capable of
distinguishing HCB from BHC isomers suitable for use in
laboratories engaged in general regulatory work.
2. Collaborative studies on analytical methods capable of recovering
and identifying HCB residues in the above food commodities.
REFERENCES
Burke, Jerry A. and Holswade, Wendell. (1966) Gas chromatographic
column for pesticide residue analysis: retention times and response
data. J. Assoc. Offic. Anal. Chemists 49 (2) 374-85
Cam, C. and Nigogosyan, G. (1963) Acquired toxin porphyria cutanea
tarda due to hexachlorobenzene. J. Amer. med. Ass., 183:90-3
Campbell, J.A.H. (1963) Pathological aspects of hexachlorobenzene
feeding in rats. S. Afr. J. Lab. Clin. Med. 9(1):203-6
Cetingil, A.I. and Ozen, M.A. (1960) Toxic porphyria. Blood,
16:1002-11
Costa, J.J. (1952) A new material for eliminating bunt of cereals:
HCB. Rev. Argent, Agron. 19:44-51
Craig, J. (1959) Report of trials - feeding animals with grain
treated with fungicides. Reports - Department of Agriculture, Western
Australia, 1959-60
Craig, J. and Dwyer, H.P. (1961) "Pickled Wheat is Safe for Sheep".
HCB treated wheat is safe for sheep but should be fed with caution
for short periods only. J. of Agric. of W.A., Nov. 1961 p.909
De Matteis, F., Prior, B.E. and Rimington, C. (1961) Nervous and
biochemical disturbances following hexachlorobenzene intoxication.
Nature, 191:363-6
Dogramaci, I. (1961) An outbreak of toxic porphyria in southeastern
Turkey (editorial) Turk. J. Pediat., 3:57-60
Dogramaci, I. (1962) Porphyria turcica (Cutaneous porphyria seen
in southeastern Turkey). General consideration. Turk. J. Pediat.,
4:129-31
Dogramaci, I., Kenanoglu, A., Müftü, Y., Ergene, T. and Wray, J.D.
(1962a) Bone and joint changes in patients with porphyria turcica.
Turk. J. Pediat., 4:149-56
Dogramaci, I., Wray, J.D., Ergene, T., Sezar, V. and Müftü, Y.
(1962b) Porphyria turcica A Survey of 592 cases of cutaneous
porphyria seen in southeastern Turkey. Turk. J. Pediat., 4:138-48
Dogramaci, I. (1964) Porphyrias and porphyrin metabolism, with
special reference to porphyria in childhood in Advances in
Pediatrics, Year Book Medical Publishers, Inc., Vol. 13, pp.11-63
Duggan, R.E. (1969) Direct communication
Gajdos, A. and Gajdos-Torök, M. (1961a) The therapeutic effect of
adenosine-5-monophosphoric acid in porphyria. Lancet, 2:175-7
Gajdos, A. and Gajdos-Torök, M. (1961b) Porphyrie expérimental
observée chez le rat blanc à la suite d'intoxication par
l'hexachlorobènzene. Rev. franç. Etud. clin. biol. 6:549-52
Gajdos, A. and Gajdos-Torök, M. (1961c) Action thérapeutic de l'acide
adénosine-5-monophosphorique sur la porphyrie expérimental du
rat blanc due à l'intoxication par l'hexachlorobenzène. Rev.
franç. Etud. clin. biol., 6:553-9
Gardiner, M.R. and Armstrong, J. (1960) Feeding pickled wheat to
pigs. J. Dept. Agr. W. Australia 1, 237-8 C.A. 54.21368d.
Holton, C.S. and Purdy, L.H. (1957) Comparative effectiveness of HCB
and mercury preparations in controlling soil-borne common bunt in
commercial field trials. Pl. Dis. Reptr. Reprint 39:842-3
Houghton, E.R. (1969) Canada Department of Agriculture. Personal
communication
Johns, T.H. (1969) Translocation of HCB in Wheat. Report to
Australian Pesticides Sub-Committee from N.S.W. Dept. of
Agriculture, June 1969
Langlois, B.E., Stemp, A.R. and Leske, B.J. (1963) Milk fat from the
Babcock Test for Insecticide Residue Analysis in Dairy Produce. J.
Dairy Sc. 46 (1963) 854
Meagher, J.W. (1953) Bunt or stinking smut of wheat. Progress report
on seed dusting trials HCB. J. Dep. Agric. Vict. 51, 11:502-4
Melis, R. (1955) Toxic action of hexachlorobenzene for warm-blooded
animals. Nuovi Ann. Ig., 6:361-7 [Chem. Abstr. 50:5170 g, (1956)].
Mills, P.A. (1959) Detection and Semiquantitative Estimation of
Chlorinated Organic Pesticide Residues in Food by Paper
Chromatography. J.A.O.A.C. 42 (1959) 734
Middleton, John T. (1965) The presence, persistence and removal of
pesticides in air. Res. Pestic., Proc. Conf., Davis, Calif. 1964
191-7 (Pub. 1965)
Moats, W.A. (1963) One-step Cleanup Procedure Using Florisil
J.A.O.A.C. 46 (1963) 172
Ockner, R.K. and Schmid, R. (1961) Acquired porphyria in man and rat
due to hexachlorobenzene intoxication. Nature, 189:499
Old, A.N. (1969) HCB Residues in Milled Wheat Fractions and Baked
Bread. Report of Dept. of Agriculture of N.S.W. CB/66/825 (To be
published)
Onley, J.H. and Mills P.A. (1962) Detection and Estimation of
Chlorinated Pesticides in Eggs J.A.O.A.C. 45(4) 983
Parke, D.V. and Williams, R.T. (1960) Studies in Detoxication 81. The
metabolism of halogenobenzenes: (a) Penta- and hexa-chlorobenzenes.
Biochem. J., 74:5-9
Pearson, R.W. and Malkinson, F.D. (1965) Some observations on
hexachlorobenzene induced experimental porphyria. J. invest.
Derm., 44: 420-32
Purdy, L.H. (1961) Control of common bunt of wheat by seed treatment
in the Pacific North West. U.S.D.A. ARS 34-21:1-8
Savitskii, I.V. (1964) The basis for determining safe permissible
concentrations of hexachlorobenzene and pentachloronitrobenzene
in the air. (Russian) Vopr. Prom. i Sel' skokhoz. Toksikol.,
Kievsk. Med. Inst. 158-173 [Chem. Abstr. 63:8952 d, (1965)]
Schmid, R. (1960) Cutaneous porphyria in Turkey. New Engl. J. Med.,
263:397-8
Smith, W.S. (1969) Translocation of HCB in wheat. Report to Australian
Pesticides Sub-Committee from S.A. Dept. of Agriculture, June
1969
Vos de, J.G., Breeman, H.A. and Benschop, H. (1968) The occurrence of
the fungicide hexachlorobenzene in wild birds and its toxicological
importance. A preliminary communication. Mededelingen Rijksfakulteit
Landbouwwetenschappen Gent. 33:1263-9
Wallace, H.A.H. (1966) A comparison of standard and drillbox seed
treatment chemicals for covered smut of oats and barley. Canadian
Plant Disease. 46 (4) 134-6 Dec. 1966
Watts, R.M. (1968) HCB residues in eggs and body fat of poultry
receiving HCB treated wheat. Reports to Australian Pesticides
Sub-Committee by N.S.W. Department of Agriculture (June 1968)
Watts, R.M. (1968a) HCB residues in Poultry - Low-level feeding
trial. Report to Australian Pesticides Sub-Committee by N.S.W.
Department of Agriculture (June 1968)
Weir, R.J. (1962) Experimental pre-emergence herbicide HCB pure
(new), 90-day dietary administration - rats. Unpub. Rept. of Hazleton
Laboratories, Inc. sponsored by Diamond Alkali Co.
Wit, S.L. (1969) Residues of insecticides belonging to the Group of
chlorinated cyclic hydrocarbons in vegetable and animal fats.
Report No.15/69 Tox. (Feb. 1969) Rijks Institut Voor de
Volksgezondheid - Utrecht - Netherlands
Wray, J.D., Müftü, Y. and Dogramaci, I. (1962) Hexachlorobenzene as
a cause of porphyria turcica Turk. J. Pediat., 4:132-7
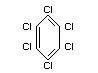 Other relevant chemical properties
Colourless white powder or needles MP 229°C - BP. 326°C. V.P. 1.089 ×
10-5 mm Hg at 20°C - sublimable. Insoluble in water and alcohol.
Soluble in hot benzene. The technical grade used in agriculture
contains 98 percent hexachlorobenzene, 1.8 percent pentachlorobenzene
together with 0.2 percent 1,2,4,5-tetrachlorobenzene.
Commercial formulations (dusts) contain 10-40 percent HCB alone or
together with small quantities of lindane (0.5-1.0 percent) added to
prevent insect attack on stored seed.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Residues of hexachlorobenzene are stored in the liver and fat of
birds. The biological half-life of hexachlorobenzene in the quail is
about three weeks (Vos et al., 1968) (see also "Short-term studies.
Quail").
When hexachlorobenzene, 400 mg/kg, was administered orally to rabbits,
the compound did not appear to be metabolized, because the main
portion was found in the gut contents five days after dosing, with
only 6 percent appearing in the faeces. There was no significant
urinary or pulmonary excretion of metabolites. Hexachlorobenzene did
not form conjugated glucuronic acids, ethereal sulphates or
mercapturic acids. Most of a dose (100 mg/kg) given subcutaneously was
found at the site of injection after five days, with no chlorinated
benzenes being found in the faeces over this period (Parke and
Williams, 1960).
There is evidence that hexachlorobenzene (or a toxic metabolite of it)
is excreted in milk. Two pregnant female rats were given
hexachlorobenzene in their food (the dose was not stated, it was
presumed to be 0.5 percent). One died, but the other was delivered
normally and reared the young until they died with convulsions after
seven to eight days. The mother was then given three normal week-old
rats to foster, which died three to four days later with convulsions.
The mother also died four weeks later with the usual symptoms of
hexachlorobenzene intoxication. This study indicated that a toxic
compound was excreted in the milk (De Matteis et al., 1961).
TOXICOLOGICAL STUDIES
Acute toxicity (oral)
Animal LD50 References
mg/kg body-weight
Mouse 4000 Savitskii, 1964
Rat 3500 Savitskii, 1964
Guinea pig > 1000 Melis, 1955
Rabbit 2600 Savitskii, 1964
Cat 1700 Savitskii, 1964
Short-term studies
Chicken
Hexachlorobenzene given at levels of 120-480 ppm in the diet for three
months, caused no toxic effects in chickens (Melis, 1955).
Guinea-pig
A diet containing 0.5 percent of hexachlorobenzene was given to
guinea-pig and to mice. These two species proved to be remarkably
susceptible to hexachlorobenzene and developed very marked
neurological symptoms within eight to 10 days (De Matteis et al.,
1961).
Mouse
See under "Guinea-pig".
Quail
Five groups of adult Japanese quail (10 females and two males per
group) were fed a mixed diet containing 0, 20, 100, 500 or 2500 ppm of
hexachlorobenzene for three months. Eggs from the 100 ppm, 20 ppm and
control groups were collected during three periods and incubated to
determine reproduction results. At the 2500 ppm level four birds died
within a week, the remainder within a month. Pre-mortem effects noted
were loss of weight, drooping wings, ruffled feathers, trembling,
ataxia and paralysis. On examination, bones, liver and kidney showed a
red fluorescence typical of porphyria. Degeneration and necrosis of
liver cells was seen. At 500 ppm all birds died within a month,
showing the same symptoms and lesions seen at the higher level. At 100
ppm, the first bird died on day 20, and 10 were dead within seven
weeks. An accumulation of porphyrins in liver and kidney was observed
in all birds in the group. Microscopic examination showed
degeneration, necrosis and local regeneration of liver cells. At 20
ppm all birds survived the three months test-period and did not
develop any visible symptoms. At autopsy one hen showed fluorescence
of bones and liver in gross ultraviolet examination. Fluorescence of
liver and kidney cells and swelling and regeneration of liver cells
was observed in this bird. In the other hens fluorescence of kidney
cells was seen under ultraviolet microscopy. Significant differences
were not found in body-weight or in relative weights of liver, spleen
and brain, nor was there any effect on egg production at 20 ppm. There
was a significant reduction in the number of chicks hatched, however,
at this level of hexachlorobenzene. It was tentatively concluded,
considering the disturbance in porphyrin metabolism and the diminished
reproduction results, that the no-effect level in this species is
lower than 20 ppm. (A comparative test in rats indicated the quail to
be much more sensitive). Residues in the liver of the birds fed
hexachlorobenzene were as follows:
TABLE I
Levels of hexachlorobenzene
Level of Number in the liver (ppm)
hexachlorobenzene History of birds Average Range
fed (ppm)
500 died 9 450 180-850
100 died 6 235 85-720
20 killed 6 36 14-94
20 killed 33 days 6 13 5-29
after termination
of hexachlorobenzene-
feeding
Residues were also found in eggs and in the chicks after hatching.
Several predatory birds, most of them found dead, had liver residues
of hexachlorobenzene in the same range as those in the 20 ppm group of
the quail study. It was concluded that hexachlorobenzene used as a
seed-dressing, may have toxic effects on seed-eating and predatory
birds (Vos et al., 1968)
Rabbit
Female rabbits were fed a diet containing 0.5 percent of
hexachlorobenzene. After about six weeks an increase in urinary
porphyrins was noted. Unless sacrificed earlier, the animals died in
eight to 12 weeks after having demonstrated neurological symptoms. (De
Matteis et al., 1961).
Rat
Groups of 10 male and 10 female rats were fed diets containing
hexachlorobenzene at levels of 0, 5, 25, 125, and 625 ppm for 13
weeks. After 13 weeks five males and five females from each group were
sacrificed for gross and microscopic examination. The rats left in the
groups given 125 and 625 ppm were transferred to the control diet for
a further two weeks, then sacrificed and examined. The rats in the 625
ppm group displayed signs of marked respiratory involvement; slight
tremors were also observed in several animals and the females
exhibited small sores in the skin of the head or neck. None of the
other groups showed these effects. Growth of male rats at the highest
level fed was reduced. Survival was not affected at any dose-level.
Total leukocyte counts were elevated during the test in the group fed
625 ppm. Organ-weight data showed an increase at 625 ppm, in thyroid,
liver, spleen and adrenals, and in male rats at 125 ppm, in liver.
Microscopic examination showed consistent effects in the liver
(lobular distortion, nuclear and cytoplasmic variations, focal loss of
cell outline, mitotic activity, and focal cellular necrosis) of the
rats at 125 and 625 ppm. Changes of a less distinct and less
consistent nature were observed in thyroid, kidneys, adrenals and
bone-marrow of animals in these two groups. No significant effects
were observed in organs and tissues of the rats fed at 5 or 25 ppm of
hexachlorobenzene (Weir, 1962).
Female rats were fed a standard died containing 0.2 percent
hexachlorobenzene. After one week, weight-loss and general debility
was noted in some rats. Within three weeks a few rats showed increased
urinary excretion of porphyrins, but this manifestation did not become
general until the seventh or eighth week. Rats with well established
porphyria were exposed continuously to ultraviolet light. If an area
of about 25 cm2 was plucked or shaved before exposure, most of the
animals died within three to 14 days, but a few rats survived three to
four months of this treatment. Rats on the hexachlorobenzene diet that
were not shaved or plucked tolerated the ultraviolet light exposure
well. After receiving hexachlorobenzene in the diet for six to eight
months, the rats were returned to a normal diet. Urinary porphyrin
excretion decreased considerably over the first four months but was
still above normal levels nine months after hexachlorobenzene has been
removed from the diet (Pearson and Malkinson, 1965).
A diet containing 0.2 percent of hexachlorobenzene was fed to 26 male
rats. Groups of two or three animals were killed at weekly intervals
over a period of one to 12 weeks. Retardation of weight-gain occurred
in the second and third week of the experiment. Evidence of a
localized toxic effect was confined to the liver. An increase
in liver-weight reached a maximum from the fifth to the ninth
week and then the weight of the organ declined. Degenerative changes
in the liver similar to those reported in earlier studies occurred.
Porphyrinuria and pathological amounts of porphyrin in liver, bones
and marrow were observed (Campbell, 1963).
Rats were given hexachlorobenzene at a level of 2 percent in the diet.
After appearing normal for the first 10-12 year, clinical and
biochemical signs of porphyria then appeared rapidly. There was a
considerable loss in body-weight, the mean for 13 rats being 25
percent of the initial weight. Cutaneous eruptions on the head, back
and feet occurred. Generalized tremor was observed. Porphyrinuria was
seen after two weeks of feeding hexachlorobenzene. Many rats died
after three to four weeks. Adenosine-5-monophosphoric acid, 20 mg
daily, was given by intramuscular injection to some of the rats,
starting on the 13th day of the experiment. By the 28th day, there was
a striking difference between most of the adenosine-5-monophosphoric
acid-treated and the other rats. Loss of weight was diminished, no new
cutaneous eruptions appeared and those that had existed healed
completely. There was a reduction in the urinary excretion of
porphyrins. Despite these improvements, however, some of the rats
treated with adenosine-5-monophosphoric acid died towards the end of
the fourth week of feeding hexachlorobenzene (Gajdos and Gajdos-Torök,
1961a, 1961b, 1961c).
A group of 33 male rats were fed a diet containing 0.2 percent of
hexachlorobenzene. Within the first month 13 rats died, exhibiting
terminal tremor, ataxia, weakness and paralysis. In the remaining
rats, a significant increase in urinary excretion of porphyrins and
porphyrin precursors was first noted after two to eight weeks of
hexachlorobenzene administration. In rats in which hexachlorobenzene
was discontinued shortly after peak excretory values were reached,
there was a return to normal excretion values within a week. However,
maintenance of high levels of porphyrin excretion by continued feeding
of hexachlorobenzene for two to three weeks resulted in an apparently
irreversible porphyric state, in which marked porphyrinuria continued
in spite of removal of hexachlorobenzene from the diet. Hepatomegaly
was common in the porphyric rats. Histological studies showed
liver-cell degeneration (Ockner and Schmid, 1961).
Long-term studies
No information available.
OBSERVATIONS IN MAN
Several published reports have described an outbreak of a cutaneous
type of porphyria that began in southeastern Turkey in 1955. The total
number of cases over a five-year period has been estimated at
3000-5000. Most of these were in children. The clinical manifestations
consisted of blistering and epidermolysis of the skin in areas exposed
to sunlight, particularly the face and hands. The lesions healed
poorly. Hyperpigmentation was invariable, usually accompanied by
marked hypertrichosis which was not limited to the exposed skin areas.
The urine contained large quantities of porphyrins. Weight loss and
hepatomegaly were frequently present. Neurological symptoms did not
appear to be evident but abdominal pain was reported in some cases and
liver enlargement was present in 35 percent of the cases surveyed. In
many cases bone and joint changes occurred, in some patients there was
osteoporosis of the bones of the extremities and interphalangeal
arthritis. The outbreak was traced to the consumption of wheat,
intended for planting, that had been treated with hexachlorobenzene.
It was estimated that the amount of hexachlorobenzene ingested by the
persons affected was from 50 to 200 mg/day for a relatively long
period before the disease became apparent. After stopping the
consumption of bread made from hexachlorobenzene-treated wheat, the
acute skin manifestations disappeared in about 20 to 30 days. Urinary
findings reverted to normal in most patients. Relapses during the
summer months were often seen. However one to two years after the
termination of other symptoms of porphyria the joint lesions were
found to be still present. It was concluded that the disease was the
result of a metabolic disorder caused by interference with porphyrin
metabolism in the liver by hexachlorobenzene. Upon recognition of the
situation, the use of hexachlorobenzene as a fungicide was
discontinued in 1959. Subsequently the disease gradually disappeared
(Cam and Nigogosyan, 1963; Cetingil and Ozen, 1960; Dogramaci, 1961,
1962, 1964; Dogramaci et al., 1962a, 1962b, Schmid, 1960, Wray et al.,
1962).
COMMENT
Evidence is presented that hexachlorobenzene is a highly toxic
compound. There is insufficient information on metabolism especially
in relation to the toxic compound excreted in milk. Use effect on the
bone-marrow gives rise to serious concern. In addition, no long-term
studies or studies on reproduction are available and there is little
information on the effect in mammalian species other than the rat. For
those reasons no acceptable daily intake can be established. However,
unintentional residues have been found in a variety of food
commodities in many countries. The Meeting therefore agreed to
consider the short-term study in rats as a basis for establishing a
tentative negligible daily intake using an extremely high safety
factor. It was stressed, however, that the use of this compound is
highly undesirable and a search for a more suitable substitute is
strongly recommended. In addition, extreme precautions should be taken
to prevent treated seeds and seed-grains from being consumed by humans
or farm animals.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TENTATIVE NEGLIGIBLE DAILY INTAKE
0 - 0.0006 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Seed wheat is treated with HCB to destroy seed-borne and soil-borne
spores of Bunt fungi (Tilletia spp.) and to ensure freedom from Bunt
in the subsequent crop. HCB has proved outstanding for this purpose
and has been used widely since being first introduced in 1945. (Costa
1952, Holton and Purdy 1957, Meagher 1953, Purdy 1961). Wheat infected
with "Bunt" or "Stinking Smut" has a bad appearance, an unpleasant
smell and is unsuitable for milling. "Bunt" can be regarded as a most
important and common disease of wheat.
Wheat crops become infected after sowing of seed, which has live Bunt
spores on its surface. These spores germinate and enter the young
plant, growing inside it throughout the season. At harvest a black
mass of spores replaces the starch of the grain whilst the skin of the
grain remains unaffected. During harvest these "Bunt Balls" are
broken, thus releasing myriads of spores over the clean grain, which
if sown without treatment, will lead to heavily infected crops.
HCB is usually applied in the form of dust containing 10-40 percent
a.i. but in Canada liquid preparations are also used (Houghton 1969).
Formulations are coloured to assist in application and to distinguish
treated seed from other grain. Usually, a blue pigment is used but
sometimes carbon black or a red dyestuff is added to reduce the risk
of treated seed being used for food. In Turkey where treated seed was
used for food with disastrous consequences it was proposed that a
denaturant with a strong taste or smell be added as well as colour.
In Australia and various other countries, wheat is selected for seed
at harvest time for the next season and after grading to remove small
or broken grains, fungicide is added automatically by machinery at the
rate of 1-2 ozs 30 percent HCB dust per bushel (330 ppm). The treated
seed is stored in labelled jute bags until required for planting some
months later. There is no evidence of significant contamination of
commercial grain or animal feeds during the process of applying the
HCB dust. The use of untreated seed wheat invariably leads to heavy
losses from fungus diseases (Bunt or Stinking Smut) and it is
therefore standard practice to treat sufficient seed for the
anticipated needs of the next season. It is exceptional for farmers to
buy seed: they normally produce and save for their own requirements.
Farmers are directed not to use treated seed wheat for animal feed or
to allow it to become mixed with commercial grain. Although such
directions are normally observed, the presence of 1 bushel of seed
wheat in 10,000 bushels of commercial grain is sufficient to lead to
significant residues in eggs from hens receiving such grain in their
ration. Contaminated grain sacks, machinery, storage premises,
transport and dust from commercial grain all contribute to the
residues found in animal products.
In Canada, seed wheat is usually treated just before planting.
Although some dusts are used, aqueous suspensions suitable for
application as a coarse spray are favoured. As an alternative,
"drill-box" treatments have been developed whereby the fungicide dust
is added to the seed wheat in the feed box of the seed drill where it
is distributed by simple agitation. Such drill-box treatments are less
effective but appear capable of reducing cross contamination of other
grain, (Wallace 1966).
Statistics on the production or use of HCB are not available for
countries other than Australia. In Australia 12 million bushels of
seed wheat are treated annually with HCB dust, requiring 200 tons of
technical HCB. A smaller proportion of the total crop is probably
treated in U.S.A., Canada, U.K. and some European countries but there
is apparently an extensive use in Turkey, Italy, Spain, Netherlands,
Germany, France and some Eastern European countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the time of the original introduction of HCB as a seed dressing and
for many years afterwards, the analytical methods were not capable of
detecting the level of residues found in commercial grain or animal
products. The only trial results available are those from Australia.
In animals
Craig (1959) fed HCB treated wheat to poultry at the rate of 1´ oz per
bird per day for six months. At the end of this period the fat of the
birds contained more than 300 ppm HCB. Craig and Dwyer (1961) fed seed
wheat containing 660 ppm HCB to aged sheep at the rate of 4 oz and 12
oz per head per day. After four months those receiving 4 oz per day
(80 mg HCB/day) had 330 ppm in the fat but none in liver or kidney.
Those on 12 oz per day (240 mg HCB/day) had 880 ppm in fat and 200 ppm
in liver and kidney.
Gardiner and Armstrong (1960) fed two pigs on HCB treated wheat
containing 660 ppm HCB for 71 days during which each consumed 329 lbs
of wheat. Animals gained 50-98 lbs in weight and at slaughter fat
contained 1000 ppm of HCB.
Watts (1968) reports trials where HCB residues were determined in eggs
and body fat of poultry receiving HCB treated wheat. Three groups of
chickens were fed wheat containing 340 ppm HCB for seven, fourteen and
twenty-eight days before being given untreated grain for twenty-eight
days. The maximum amount of HCB found in egg yolk was 76, 167 and 146
ppm respectively. The maximum amount in body fat of the same chickens
was 178, 349 and 528 ppm respectively. Controls fed on grain believed
to be free of residues showed 0.84 ppm in egg yolk and 3.3 ppm in fat.
It was found that the untreated controls received 0.02 ppm HCB in
their rations.
Later trials reported by Watts (1968a) showed that four groups of
chickens fed rations containing 0.02 ppm, 0.08 ppm, 0.7 ppm and 7 ppm
HCB for two months showed maximum residues of HCB in egg yolk of 0.2
ppm, 0.3 ppm, 2 ppm and 15 ppm respectively. Body fat from the same
chickens contained up to 0.7, 0.7, 5 and 29 ppm of HCB. Half of the
birds receiving the above rations were given treated grain after one
month. At the end of the second month the HCB residues in egg yolk
ranged from 0.18 to 0.2, 0.2 to 0.25, 0.7 to 1.2 and 6 to 12 ppm
respectively. The body fat of these birds contained HCB ranging from
0.6 to 0.7, 0.7 to 0.9, 1.5 to 2.6, 16 to 24 ppm respectively. It was
concluded that residues decline only slowly when birds which have
previously ingested HCB receive untreated feed for one month.
De Vos et al. (1968) as a result of identifying HCB in tissues of wild
birds in the Netherlands carried out semichronic toxicity tests on
Japanese quail and measured the HCB residues in liver, blood and fat.
Whilst exceptionally high levels were found in birds receiving toxic
concentrations in their diet, birds fed on diets containing 20 ppm of
HCB showed HCB residues in the fat of 350 to 520 ppm after three
months. The author concluded that the half-life of HCB in the quail is
about three weeks.
Wit (1969) reports no HCB in an extensive survey of animal fats
examined in the Netherlands but he notes that HCB would be obscured by
and reported as alpha BHC.
In plants
Johns (1969) reports results from six samples of wheat grown from HCB
treated seed in different soils. Residues range from 0.003 ppm to
0.062 ppm. Two samples of wheat grown from seed that had not been
treated with HCB showed 0.001 ppm and 0.003 ppm but it is not known
whether HCB treated wheat was grown in the name soil in previous
years.
Smith (1969) reported trials where wheat treated with 416 ppm HCB was
planted and the resulting grain analysed. Residues of 0.0033 ppm were
found. Untreated seed yielded grains containing 0.0022 ppm HCB. Soils
from twelve wheat farms were analysed and all showed traces of HCB
ranging from 0.001 to 0.02 ppm though nine were below 0.003 ppm.
Old (1969) reports investigations with wheat containing 0.05 ppm HCB,
to determine the effect of milling on the distribution of HCB residues
in the various milled fractions, and the HCB residues in bread made
from the flour fraction. Using wheat containing 0.05 ppm HCB it was
found that residues were distributed in all main milling fractions.
The total residues recovered were equivalent to 0.049 ppm. Of this 42
percent was recovered in the bran, 36 percent in the pollard and 22
percent in the flour. The flour which was found to contain 0.015 ppm
HCB produced bread containing 0.0024 ppm HCB. Residues in bread were
thus only 16 percent of the residues in the flour used for the making
of bread. Further confirmation of the loss during baking was obtained
from flour containing 0.006 ppm HCB which produced broad containing
0.0014 ppm HCB or 23 percent of the amount in the flour used.
There is extensive evidence from the literature that HCB is volatile
in water vapour even at low temperatures. It is assumed that the loss
during baking in due to steam volatilisation.
FATE OF RESIDUES
As far as can be gauged the HCB applied to seeds in not broken down by
physical, chemical or biological agencies but is simply dissipated and
diluted in the soil and atmosphere.
Because of the extreme stability of the molecule, solubility in fatty
tissue and the ease with which animals extract and concentrate
residues of HCB from their feed into their fatty tissues residues are
readily detectable in animal products.
The inadvertent contamination of commercial grain and animal feeds
with traces of HCB, though undesirable, appears hard to eliminate.
Evidence of residues in food in commerce or at consumption
A considerable amount of the date available results from surveys
conducted to determine the level and source of HCB residues in eggs,
meat fat and dairy produce from individual farms. Except in isolated
instances where farmers have not observed instructions not to feed
treated seed grain to domestic animals, the contamination invariably
results from the inadvertent feeding of grain or milling fractions
containing low levels of HCB residues. It is not possible for farmers
to know that such animal rations are contaminated and in view of the
slow rate of excretion the retention of residues in fatty tissues of
such animals presents serious economic and administrative problems.
The following table given results of an extensive survey of HCB
residues in animal products calculated on a fat basis.
TABLE II
HCB residues in Animal Products
ppm.
Commodity No. of No. Less 0.1 0.25 0.5 Over
Samples with than to to to
HCB 0.1 ppm 0.25 0.5 1.0 1.0
Beef Fat 2,029 14 12 1 1 0 0
Mutton Fat 833 20 2 8 9 1 0
Butter 514 15 8 5 0 2 0
Cheese 977 4 3 0 1 0 0
Frozen liquid 393 227 81 106 25 11 4
whole egg
Frozen liquid 154 90 8 42 17 19 4
egg yolk
A survey of commercial wheat samples carried out in Australia revealed
that HCB residues can be found in a significant proportion of the
samples in amounts ranging from traces (less than 0.005 ppm) to a
maximum of 0.05 ppm.
In the United Kingdom positive evidence of the presence of HCB has
been obtained in several shipments of imported wheat. Three samples
from one shipment contained 0.67, 1.3 and 0.48 ppm HCB. In two other
shipments the combined HCB and alpha BHC was less than 1 ppm.
Surveys of cheese imported into Australia reveal HCB residues in a
significant proportion of samples from many countries. More than 10
percent of such imports showed residues in excess of 0.1 ppm, with
many samples ranging from 0.3 to 0.9 ppm.
Examinations of food products imported into U.S.A. (Duggan, 1969)
showed a very low incidence of HCB residues for 1967 and 1968. One
and cheese in 1968). However, in 1969 the incidence increased, HCB
being reported in 170 samples of the 1,866 examined. The increase
probably reflects improvements in analytical technique more than an
increase in the incidence and level of contamination.
The majority of these findings were in manufactured dairy produce with
HCB being reported in 149 of 608 samples mainly of cheese. The range
of values for HCB in the positive sample was from a trace to 0.81 ppm
on a fat basis. The average of 608 samples was 0.01 ppm. Processed
foods, other than dairy produce, showed 12 positive findings in 345
samples examined. The range of positive findings in these commodities
was from a trace to 0.23 ppm.
One sample of butter was found to contain the following residues (on a
fat basis):-
BHC - 0.24 ppm
lindane - 0.32 ppm
HCB - 0.37 ppm
It is obvious that rather more than average care must be taken in
resolving such mixtures of residues which can easily become confused
by certain GLC analytical procedures.
HCB residues have been found in small proportion of the samples of
domestic food production. In 1967, HCB was reported in 12 samples out
of approximately 20,000 examined. In 1968 it was reported in only one
sample out of approximately 10,000 examined and in 1969 in 10 samples
out of approximately the name number tested. The level in positive
findings varied from a trace (less than 0.01 ppm) to 0.5 ppm.
METHODS OF RESIDUE ANALYSIS
On foods
Most chlorinated pesticides can be successfully isolated from fatty
substrates by methods based on the acetonitrile-hexane partition of
Mills (1959). However, HCB 10 exceptionally non-polar, and is
distributed preferentially into the hydrocarbon phase. Its partition
ratio by experiment using equal volumes of the two solvents in
approximately 0.75 to hexane at 20°C. In three counter-current
separations with equal volumes, recovery would be about 10 percent.
Increasing the volume of acetonitrile to increase extraction of the
pesticide would also increase extraction of unwanted fatty matter.
Methods using direct extraction with adsorption cleanup, but without
partition, give acceptable recoveries of HCB from eggs, Onley and
Mills (1962), butterfat, Moats (1963), Langlois et al. (1963) and meat
fat. A method using elution through florisil with methylene
chloride-hexane is now in use in the Australian Commonwealth
Laboratories (Taylor 1969, direct communication).
In wheat
The analytical problem here is somewhat different, as the significance
of the presence of HCB in grain for consumption is related to the
potential for concentration of residues in animals from low levels in
the wheat, as well as to that used for direct human consumption. Thus
the levels of interest are much lower than those in animal products.
Extraction of HCB from wheat was achieved by cracking in a blendor,
refluxing with "hexane" (Shell X4) and filtration through anhydrous
sodium sulphate. Beside the chlorinated residue, this extract contains
fat-soluble plant extractives. Passage through a column of activated
florisil cleans the solution sufficiently for determination by GLC and
confirmation by, TLC, if the levels are comparatively gross, (over 0.1
ppm). However, when the concentration is 0.01 ppm or less, the ratio
of interfering substances to pesticide becomes large enough to require
further cleanup if TLC is to be used. Alternatively, other means of
confirmation are required.
Investigation has therefore followed two lines: to Improve cleanup and
to devise other confirmatory tests.
Cleanup
Hexachlorobenzene is stable to concentrated sulphuric acid. A hexane
solution of residual substrate and HCB, after florisil treatment, was
washed with the acid several times until no further darkening
occurred. The hexane layer was washed with water until neutral, and
dried with anhydrous sodium sulphate. The infra-red spectrum of the
residue after removal of solvent appeared to be that of a hydrocarbon.
Gas chromatography produced meaningful peaks at retention times
corresponding to HCB, but resolution by TLC was prevented by the
streaking effect of the oily residue. This cleanup in inadequate for
low levels.
Attempts at separation of HCB from hydrocarbon material on alumina and
on activated carbon columns with a variety of eluting solvents did not
result in sufficiently clean products.
Refluxing of wheat extracts containing HCB with alcoholic potash
resulted in varying degrees of breakdown of the pesticide as well as
substrate. Although this precluded alkaline cleanup, it suggested
possibilities for confirmation of identity of HCB (vide infra).
Steam distillation of standard solutions of HCB was found to give high
recovery. Wheat extract previously partly cleaned by treatment with
sulphuric acid was therefore distilled from a large (twenty-fold)
excess of water, the vapour rising through a Vigreux column and
descending through a water-cooled condenser before collection under
hexane. The hexane extract from 20 ml of aqueous distillate produced
clear spots on TLC plates for both hexachlorobenzene and "benzene
hexachloride" the former at a level of 0.02 ppm (referred to the
original wheat). Recovery tests by adding known amounts of HCB
produced similar results, with recoveries close to 100 percent.
In spite of the high sensitivity of this method it is recommended that
the sensitivity be developed still further to provide for the need to
determine residues in cereal products at levels below 0.01 ppm.
Confirmation
Ideally, confirmation of identity should rest upon two or more methods
using properties as widely different as possible. For chlorinated
pesticides, chromatography by GLC and TIC are usually considered
suitable. However cleanup difficulty, together with small residues,
may preclude the use of TLC. It is acceptable, then, to use GLC on
columns of such different stationary phases that the substance has
substantially different retention characteristics, use being made of
different combinations of stationary phase-solute interactions.
Experimentally, the combination of retention values on a silicone
(low-polar) and polyester (polar) columns were found to provide useful
confirmations of the presence of HCB.
A number of experiments were carried out to make use of the
degradation of HCB by alkali. Both alcoholic potash and sodium
methylate were used, the latter being more dependably effective. When
small traces of the substance, of the order of 0.1 ppm or less, are
present, the pattern of degradation is consistent, in that two major
products appear in both TLC and GLC, one corresponding in retention
values with HCB, the other being lower. However, when greater amounts
of HCB are treated, the result is often obscured by the appearance of
several other products. The reaction is not, therefore, a reliable
confirmatory test for all samples.
A gas-chromatographic column commonly used in estimation of multiple
chlorinated pesticide residues contains the mixture of DC200 silicone
and QFI fluosilicone proposed by Burke and Holswade (1966). This
column does not separate HCB and the alpha isomer of "BHC", the latter
being the major constituent used in estimation of "BHC". In addition,
alpha-BHC does not produce a strong reaction on the silver nitrate
impregnated Thin layer used. Thus BHC may not be detected if in low
concentrations (at 0.1 ppm levels).
To distinguish and confirm these compounds, a stationary phase of a
more polar nature may be used. Separate peaks are produced on Reoplex
(poly propylene glycol adipate), XE60 (nitrile silicone) and Zonyl E7
(fluoroalkyl pyromellitic ester). Therefore an extract showing a peak
of HCB/BHC on the Burke column may be reanalysed on one of these
columns to distinguish between the two compounds.
The polar phases mentioned are, however, not suitable for all the
chlorinated hydrocarbons under investigation. On some, DDT is degraded
or in indistinguishable from DDD. On others, DDE and Dieldrin are not
separated. Work is currently on hand to select a column suitable for
separating all the chlorinated hydrocarbons for which goods are at
present analysed.
NATIONAL TOLERANCES
No national tolerances are known.
Australia, Canada and the U.S.A. approve the use of HCB seed dressings
on the basis that the seeds are not used for food or feed for animals.
APPRAISAL
HCB has been used extensively as a fungicide to control Bunt
(Tilletia caries, T. tritici and T. foetida) in wheat
since 1945. There are many reports of its action on other seed borne
and soil borne fungi of cereals and as seed treatment for onions but
these uses do not appear to be of great importance.
HCB formulations for seed treatment are sold in most wheat growing
countries. To date the only alternative materials are organomercurial
compounds. The rate of application is usually within the range of 1-3
ozs. of 30 percent dust per bushel of wheat seed equivalent to 330-990
ppm on the seed.
HCB is a comparatively pure material containing 98 percent
hexachlorobenzene. The impurities are tetrachlorobenzene and
pentachlorobenzene.
The data available to the meeting were obtained from the published
literature and by correspondence with more than 100 authors,
administrators and manufacturers throughout the world. In spite of the
wide search there is only limited information on residues in food
commodities in international trade or following good agricultural
practices, other than from Australia. One reason for this is that when
examining food commodities by several GLC analytical procedures HCB
has been obscured or confused with BHC.
All available data indicates that HCB is not metabolized to any
significant extent by plants or animals and that it persists in soil
and in animal tissues for long periods. There is an indication that
HCB might be translocated from treated seed into plant material and
subsequent grain but the level of contamination from this source is
insignificant.
Contamination of commercial grain from soil, seed, machinery and bags
may be minimized by strict attention to agricultural practices but it
is difficult to avoid all traces of HCB in commercial grain.
Animals feeding on grain or milling fractions containing traces of
HCB, concentrate and store the residues in body fat, and excrete
significant quantities in milk and eggs. The residue level in such
animal products ranges from ten to one hundred times the concentration
in feed.
The literature includes several methods of residue analysis which
measure HCB in grain and animal products by GLC but the results can be
confused as and by isomers of BHC. The sensitivity for the
determination of HCB is 0.005 ppm but special extraction and cleanup
procedures are necessary to ensure reasonable recovery from plant
products and especially from animal fats. However, no referee method
has been evaluated.
In view of the importance of HCB seed dressings in countries where
Bunt is endemic, and the lack of suitable alternatives, the Joint
Meeting recognized that recommendations for administrative action
levels were required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY PRACTICAL RESIDUE LIMITS (effective to 1973)
The following temporary practical residue limits are to apply to raw
agricultural commodities moving in commerce unless otherwise
indicated:
Raw cereal (wheat) 0.05 ppm
Cereal products from wheat 0.01 ppm
Fat of cattle, sheep, goats,
pigs and poultry 1.0 ppm
Milk 0.012 ppm
Milk products 0.3 ppm
Eggs (shellfree basis) 1.0 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Metabolic studies in animals including identification of the toxic
product or products excreted in milk.
2. Short-term studies in non-rodent mammalian species and long-term
studies especially in relation to the effect on bone-marrow.
3. Reproduction studies in rats.
REQUIRED before 30 June 1973 (for 1973 review of
Practical Residue Limits)
1. Data from countries other than Australia on residues in raw
agricultural commodities.
2. Data from surveys of residues in commodities moving in
international trade.
DESIRABLE
1. Further information on analytical procedures capable of
distinguishing HCB from BHC isomers suitable for use in
laboratories engaged in general regulatory work.
2. Collaborative studies on analytical methods capable of recovering
and identifying HCB residues in the above food commodities.
REFERENCES
Burke, Jerry A. and Holswade, Wendell. (1966) Gas chromatographic
column for pesticide residue analysis: retention times and response
data. J. Assoc. Offic. Anal. Chemists 49 (2) 374-85
Cam, C. and Nigogosyan, G. (1963) Acquired toxin porphyria cutanea
tarda due to hexachlorobenzene. J. Amer. med. Ass., 183:90-3
Campbell, J.A.H. (1963) Pathological aspects of hexachlorobenzene
feeding in rats. S. Afr. J. Lab. Clin. Med. 9(1):203-6
Cetingil, A.I. and Ozen, M.A. (1960) Toxic porphyria. Blood,
16:1002-11
Costa, J.J. (1952) A new material for eliminating bunt of cereals:
HCB. Rev. Argent, Agron. 19:44-51
Craig, J. (1959) Report of trials - feeding animals with grain
treated with fungicides. Reports - Department of Agriculture, Western
Australia, 1959-60
Craig, J. and Dwyer, H.P. (1961) "Pickled Wheat is Safe for Sheep".
HCB treated wheat is safe for sheep but should be fed with caution
for short periods only. J. of Agric. of W.A., Nov. 1961 p.909
De Matteis, F., Prior, B.E. and Rimington, C. (1961) Nervous and
biochemical disturbances following hexachlorobenzene intoxication.
Nature, 191:363-6
Dogramaci, I. (1961) An outbreak of toxic porphyria in southeastern
Turkey (editorial) Turk. J. Pediat., 3:57-60
Dogramaci, I. (1962) Porphyria turcica (Cutaneous porphyria seen
in southeastern Turkey). General consideration. Turk. J. Pediat.,
4:129-31
Dogramaci, I., Kenanoglu, A., Müftü, Y., Ergene, T. and Wray, J.D.
(1962a) Bone and joint changes in patients with porphyria turcica.
Turk. J. Pediat., 4:149-56
Dogramaci, I., Wray, J.D., Ergene, T., Sezar, V. and Müftü, Y.
(1962b) Porphyria turcica A Survey of 592 cases of cutaneous
porphyria seen in southeastern Turkey. Turk. J. Pediat., 4:138-48
Dogramaci, I. (1964) Porphyrias and porphyrin metabolism, with
special reference to porphyria in childhood in Advances in
Pediatrics, Year Book Medical Publishers, Inc., Vol. 13, pp.11-63
Duggan, R.E. (1969) Direct communication
Gajdos, A. and Gajdos-Torök, M. (1961a) The therapeutic effect of
adenosine-5-monophosphoric acid in porphyria. Lancet, 2:175-7
Gajdos, A. and Gajdos-Torök, M. (1961b) Porphyrie expérimental
observée chez le rat blanc à la suite d'intoxication par
l'hexachlorobènzene. Rev. franç. Etud. clin. biol. 6:549-52
Gajdos, A. and Gajdos-Torök, M. (1961c) Action thérapeutic de l'acide
adénosine-5-monophosphorique sur la porphyrie expérimental du
rat blanc due à l'intoxication par l'hexachlorobenzène. Rev.
franç. Etud. clin. biol., 6:553-9
Gardiner, M.R. and Armstrong, J. (1960) Feeding pickled wheat to
pigs. J. Dept. Agr. W. Australia 1, 237-8 C.A. 54.21368d.
Holton, C.S. and Purdy, L.H. (1957) Comparative effectiveness of HCB
and mercury preparations in controlling soil-borne common bunt in
commercial field trials. Pl. Dis. Reptr. Reprint 39:842-3
Houghton, E.R. (1969) Canada Department of Agriculture. Personal
communication
Johns, T.H. (1969) Translocation of HCB in Wheat. Report to
Australian Pesticides Sub-Committee from N.S.W. Dept. of
Agriculture, June 1969
Langlois, B.E., Stemp, A.R. and Leske, B.J. (1963) Milk fat from the
Babcock Test for Insecticide Residue Analysis in Dairy Produce. J.
Dairy Sc. 46 (1963) 854
Meagher, J.W. (1953) Bunt or stinking smut of wheat. Progress report
on seed dusting trials HCB. J. Dep. Agric. Vict. 51, 11:502-4
Melis, R. (1955) Toxic action of hexachlorobenzene for warm-blooded
animals. Nuovi Ann. Ig., 6:361-7 [Chem. Abstr. 50:5170 g, (1956)].
Mills, P.A. (1959) Detection and Semiquantitative Estimation of
Chlorinated Organic Pesticide Residues in Food by Paper
Chromatography. J.A.O.A.C. 42 (1959) 734
Middleton, John T. (1965) The presence, persistence and removal of
pesticides in air. Res. Pestic., Proc. Conf., Davis, Calif. 1964
191-7 (Pub. 1965)
Moats, W.A. (1963) One-step Cleanup Procedure Using Florisil
J.A.O.A.C. 46 (1963) 172
Ockner, R.K. and Schmid, R. (1961) Acquired porphyria in man and rat
due to hexachlorobenzene intoxication. Nature, 189:499
Old, A.N. (1969) HCB Residues in Milled Wheat Fractions and Baked
Bread. Report of Dept. of Agriculture of N.S.W. CB/66/825 (To be
published)
Onley, J.H. and Mills P.A. (1962) Detection and Estimation of
Chlorinated Pesticides in Eggs J.A.O.A.C. 45(4) 983
Parke, D.V. and Williams, R.T. (1960) Studies in Detoxication 81. The
metabolism of halogenobenzenes: (a) Penta- and hexa-chlorobenzenes.
Biochem. J., 74:5-9
Pearson, R.W. and Malkinson, F.D. (1965) Some observations on
hexachlorobenzene induced experimental porphyria. J. invest.
Derm., 44: 420-32
Purdy, L.H. (1961) Control of common bunt of wheat by seed treatment
in the Pacific North West. U.S.D.A. ARS 34-21:1-8
Savitskii, I.V. (1964) The basis for determining safe permissible
concentrations of hexachlorobenzene and pentachloronitrobenzene
in the air. (Russian) Vopr. Prom. i Sel' skokhoz. Toksikol.,
Kievsk. Med. Inst. 158-173 [Chem. Abstr. 63:8952 d, (1965)]
Schmid, R. (1960) Cutaneous porphyria in Turkey. New Engl. J. Med.,
263:397-8
Smith, W.S. (1969) Translocation of HCB in wheat. Report to Australian
Pesticides Sub-Committee from S.A. Dept. of Agriculture, June
1969
Vos de, J.G., Breeman, H.A. and Benschop, H. (1968) The occurrence of
the fungicide hexachlorobenzene in wild birds and its toxicological
importance. A preliminary communication. Mededelingen Rijksfakulteit
Landbouwwetenschappen Gent. 33:1263-9
Wallace, H.A.H. (1966) A comparison of standard and drillbox seed
treatment chemicals for covered smut of oats and barley. Canadian
Plant Disease. 46 (4) 134-6 Dec. 1966
Watts, R.M. (1968) HCB residues in eggs and body fat of poultry
receiving HCB treated wheat. Reports to Australian Pesticides
Sub-Committee by N.S.W. Department of Agriculture (June 1968)
Watts, R.M. (1968a) HCB residues in Poultry - Low-level feeding
trial. Report to Australian Pesticides Sub-Committee by N.S.W.
Department of Agriculture (June 1968)
Weir, R.J. (1962) Experimental pre-emergence herbicide HCB pure
(new), 90-day dietary administration - rats. Unpub. Rept. of Hazleton
Laboratories, Inc. sponsored by Diamond Alkali Co.
Wit, S.L. (1969) Residues of insecticides belonging to the Group of
chlorinated cyclic hydrocarbons in vegetable and animal fats.
Report No.15/69 Tox. (Feb. 1969) Rijks Institut Voor de
Volksgezondheid - Utrecht - Netherlands
Wray, J.D., Müftü, Y. and Dogramaci, I. (1962) Hexachlorobenzene as
a cause of porphyria turcica Turk. J. Pediat., 4:132-7
Other relevant chemical properties
Colourless white powder or needles MP 229°C - BP. 326°C. V.P. 1.089 ×
10-5 mm Hg at 20°C - sublimable. Insoluble in water and alcohol.
Soluble in hot benzene. The technical grade used in agriculture
contains 98 percent hexachlorobenzene, 1.8 percent pentachlorobenzene
together with 0.2 percent 1,2,4,5-tetrachlorobenzene.
Commercial formulations (dusts) contain 10-40 percent HCB alone or
together with small quantities of lindane (0.5-1.0 percent) added to
prevent insect attack on stored seed.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Residues of hexachlorobenzene are stored in the liver and fat of
birds. The biological half-life of hexachlorobenzene in the quail is
about three weeks (Vos et al., 1968) (see also "Short-term studies.
Quail").
When hexachlorobenzene, 400 mg/kg, was administered orally to rabbits,
the compound did not appear to be metabolized, because the main
portion was found in the gut contents five days after dosing, with
only 6 percent appearing in the faeces. There was no significant
urinary or pulmonary excretion of metabolites. Hexachlorobenzene did
not form conjugated glucuronic acids, ethereal sulphates or
mercapturic acids. Most of a dose (100 mg/kg) given subcutaneously was
found at the site of injection after five days, with no chlorinated
benzenes being found in the faeces over this period (Parke and
Williams, 1960).
There is evidence that hexachlorobenzene (or a toxic metabolite of it)
is excreted in milk. Two pregnant female rats were given
hexachlorobenzene in their food (the dose was not stated, it was
presumed to be 0.5 percent). One died, but the other was delivered
normally and reared the young until they died with convulsions after
seven to eight days. The mother was then given three normal week-old
rats to foster, which died three to four days later with convulsions.
The mother also died four weeks later with the usual symptoms of
hexachlorobenzene intoxication. This study indicated that a toxic
compound was excreted in the milk (De Matteis et al., 1961).
TOXICOLOGICAL STUDIES
Acute toxicity (oral)
Animal LD50 References
mg/kg body-weight
Mouse 4000 Savitskii, 1964
Rat 3500 Savitskii, 1964
Guinea pig > 1000 Melis, 1955
Rabbit 2600 Savitskii, 1964
Cat 1700 Savitskii, 1964
Short-term studies
Chicken
Hexachlorobenzene given at levels of 120-480 ppm in the diet for three
months, caused no toxic effects in chickens (Melis, 1955).
Guinea-pig
A diet containing 0.5 percent of hexachlorobenzene was given to
guinea-pig and to mice. These two species proved to be remarkably
susceptible to hexachlorobenzene and developed very marked
neurological symptoms within eight to 10 days (De Matteis et al.,
1961).
Mouse
See under "Guinea-pig".
Quail
Five groups of adult Japanese quail (10 females and two males per
group) were fed a mixed diet containing 0, 20, 100, 500 or 2500 ppm of
hexachlorobenzene for three months. Eggs from the 100 ppm, 20 ppm and
control groups were collected during three periods and incubated to
determine reproduction results. At the 2500 ppm level four birds died
within a week, the remainder within a month. Pre-mortem effects noted
were loss of weight, drooping wings, ruffled feathers, trembling,
ataxia and paralysis. On examination, bones, liver and kidney showed a
red fluorescence typical of porphyria. Degeneration and necrosis of
liver cells was seen. At 500 ppm all birds died within a month,
showing the same symptoms and lesions seen at the higher level. At 100
ppm, the first bird died on day 20, and 10 were dead within seven
weeks. An accumulation of porphyrins in liver and kidney was observed
in all birds in the group. Microscopic examination showed
degeneration, necrosis and local regeneration of liver cells. At 20
ppm all birds survived the three months test-period and did not
develop any visible symptoms. At autopsy one hen showed fluorescence
of bones and liver in gross ultraviolet examination. Fluorescence of
liver and kidney cells and swelling and regeneration of liver cells
was observed in this bird. In the other hens fluorescence of kidney
cells was seen under ultraviolet microscopy. Significant differences
were not found in body-weight or in relative weights of liver, spleen
and brain, nor was there any effect on egg production at 20 ppm. There
was a significant reduction in the number of chicks hatched, however,
at this level of hexachlorobenzene. It was tentatively concluded,
considering the disturbance in porphyrin metabolism and the diminished
reproduction results, that the no-effect level in this species is
lower than 20 ppm. (A comparative test in rats indicated the quail to
be much more sensitive). Residues in the liver of the birds fed
hexachlorobenzene were as follows:
TABLE I
Levels of hexachlorobenzene
Level of Number in the liver (ppm)
hexachlorobenzene History of birds Average Range
fed (ppm)
500 died 9 450 180-850
100 died 6 235 85-720
20 killed 6 36 14-94
20 killed 33 days 6 13 5-29
after termination
of hexachlorobenzene-
feeding
Residues were also found in eggs and in the chicks after hatching.
Several predatory birds, most of them found dead, had liver residues
of hexachlorobenzene in the same range as those in the 20 ppm group of
the quail study. It was concluded that hexachlorobenzene used as a
seed-dressing, may have toxic effects on seed-eating and predatory
birds (Vos et al., 1968)
Rabbit
Female rabbits were fed a diet containing 0.5 percent of
hexachlorobenzene. After about six weeks an increase in urinary
porphyrins was noted. Unless sacrificed earlier, the animals died in
eight to 12 weeks after having demonstrated neurological symptoms. (De
Matteis et al., 1961).
Rat
Groups of 10 male and 10 female rats were fed diets containing
hexachlorobenzene at levels of 0, 5, 25, 125, and 625 ppm for 13
weeks. After 13 weeks five males and five females from each group were
sacrificed for gross and microscopic examination. The rats left in the
groups given 125 and 625 ppm were transferred to the control diet for
a further two weeks, then sacrificed and examined. The rats in the 625
ppm group displayed signs of marked respiratory involvement; slight
tremors were also observed in several animals and the females
exhibited small sores in the skin of the head or neck. None of the
other groups showed these effects. Growth of male rats at the highest
level fed was reduced. Survival was not affected at any dose-level.
Total leukocyte counts were elevated during the test in the group fed
625 ppm. Organ-weight data showed an increase at 625 ppm, in thyroid,
liver, spleen and adrenals, and in male rats at 125 ppm, in liver.
Microscopic examination showed consistent effects in the liver
(lobular distortion, nuclear and cytoplasmic variations, focal loss of
cell outline, mitotic activity, and focal cellular necrosis) of the
rats at 125 and 625 ppm. Changes of a less distinct and less
consistent nature were observed in thyroid, kidneys, adrenals and
bone-marrow of animals in these two groups. No significant effects
were observed in organs and tissues of the rats fed at 5 or 25 ppm of
hexachlorobenzene (Weir, 1962).
Female rats were fed a standard died containing 0.2 percent
hexachlorobenzene. After one week, weight-loss and general debility
was noted in some rats. Within three weeks a few rats showed increased
urinary excretion of porphyrins, but this manifestation did not become
general until the seventh or eighth week. Rats with well established
porphyria were exposed continuously to ultraviolet light. If an area
of about 25 cm2 was plucked or shaved before exposure, most of the
animals died within three to 14 days, but a few rats survived three to
four months of this treatment. Rats on the hexachlorobenzene diet that
were not shaved or plucked tolerated the ultraviolet light exposure
well. After receiving hexachlorobenzene in the diet for six to eight
months, the rats were returned to a normal diet. Urinary porphyrin
excretion decreased considerably over the first four months but was
still above normal levels nine months after hexachlorobenzene has been
removed from the diet (Pearson and Malkinson, 1965).
A diet containing 0.2 percent of hexachlorobenzene was fed to 26 male
rats. Groups of two or three animals were killed at weekly intervals
over a period of one to 12 weeks. Retardation of weight-gain occurred
in the second and third week of the experiment. Evidence of a
localized toxic effect was confined to the liver. An increase
in liver-weight reached a maximum from the fifth to the ninth
week and then the weight of the organ declined. Degenerative changes
in the liver similar to those reported in earlier studies occurred.
Porphyrinuria and pathological amounts of porphyrin in liver, bones
and marrow were observed (Campbell, 1963).
Rats were given hexachlorobenzene at a level of 2 percent in the diet.
After appearing normal for the first 10-12 year, clinical and
biochemical signs of porphyria then appeared rapidly. There was a
considerable loss in body-weight, the mean for 13 rats being 25
percent of the initial weight. Cutaneous eruptions on the head, back
and feet occurred. Generalized tremor was observed. Porphyrinuria was
seen after two weeks of feeding hexachlorobenzene. Many rats died
after three to four weeks. Adenosine-5-monophosphoric acid, 20 mg
daily, was given by intramuscular injection to some of the rats,
starting on the 13th day of the experiment. By the 28th day, there was
a striking difference between most of the adenosine-5-monophosphoric
acid-treated and the other rats. Loss of weight was diminished, no new
cutaneous eruptions appeared and those that had existed healed
completely. There was a reduction in the urinary excretion of
porphyrins. Despite these improvements, however, some of the rats
treated with adenosine-5-monophosphoric acid died towards the end of
the fourth week of feeding hexachlorobenzene (Gajdos and Gajdos-Torök,
1961a, 1961b, 1961c).
A group of 33 male rats were fed a diet containing 0.2 percent of
hexachlorobenzene. Within the first month 13 rats died, exhibiting
terminal tremor, ataxia, weakness and paralysis. In the remaining
rats, a significant increase in urinary excretion of porphyrins and
porphyrin precursors was first noted after two to eight weeks of
hexachlorobenzene administration. In rats in which hexachlorobenzene
was discontinued shortly after peak excretory values were reached,
there was a return to normal excretion values within a week. However,
maintenance of high levels of porphyrin excretion by continued feeding
of hexachlorobenzene for two to three weeks resulted in an apparently
irreversible porphyric state, in which marked porphyrinuria continued
in spite of removal of hexachlorobenzene from the diet. Hepatomegaly
was common in the porphyric rats. Histological studies showed
liver-cell degeneration (Ockner and Schmid, 1961).
Long-term studies
No information available.
OBSERVATIONS IN MAN
Several published reports have described an outbreak of a cutaneous
type of porphyria that began in southeastern Turkey in 1955. The total
number of cases over a five-year period has been estimated at
3000-5000. Most of these were in children. The clinical manifestations
consisted of blistering and epidermolysis of the skin in areas exposed
to sunlight, particularly the face and hands. The lesions healed
poorly. Hyperpigmentation was invariable, usually accompanied by
marked hypertrichosis which was not limited to the exposed skin areas.
The urine contained large quantities of porphyrins. Weight loss and
hepatomegaly were frequently present. Neurological symptoms did not
appear to be evident but abdominal pain was reported in some cases and
liver enlargement was present in 35 percent of the cases surveyed. In
many cases bone and joint changes occurred, in some patients there was
osteoporosis of the bones of the extremities and interphalangeal
arthritis. The outbreak was traced to the consumption of wheat,
intended for planting, that had been treated with hexachlorobenzene.
It was estimated that the amount of hexachlorobenzene ingested by the
persons affected was from 50 to 200 mg/day for a relatively long
period before the disease became apparent. After stopping the
consumption of bread made from hexachlorobenzene-treated wheat, the
acute skin manifestations disappeared in about 20 to 30 days. Urinary
findings reverted to normal in most patients. Relapses during the
summer months were often seen. However one to two years after the
termination of other symptoms of porphyria the joint lesions were
found to be still present. It was concluded that the disease was the
result of a metabolic disorder caused by interference with porphyrin
metabolism in the liver by hexachlorobenzene. Upon recognition of the
situation, the use of hexachlorobenzene as a fungicide was
discontinued in 1959. Subsequently the disease gradually disappeared
(Cam and Nigogosyan, 1963; Cetingil and Ozen, 1960; Dogramaci, 1961,
1962, 1964; Dogramaci et al., 1962a, 1962b, Schmid, 1960, Wray et al.,
1962).
COMMENT
Evidence is presented that hexachlorobenzene is a highly toxic
compound. There is insufficient information on metabolism especially
in relation to the toxic compound excreted in milk. Use effect on the
bone-marrow gives rise to serious concern. In addition, no long-term
studies or studies on reproduction are available and there is little
information on the effect in mammalian species other than the rat. For
those reasons no acceptable daily intake can be established. However,
unintentional residues have been found in a variety of food
commodities in many countries. The Meeting therefore agreed to
consider the short-term study in rats as a basis for establishing a
tentative negligible daily intake using an extremely high safety
factor. It was stressed, however, that the use of this compound is
highly undesirable and a search for a more suitable substitute is
strongly recommended. In addition, extreme precautions should be taken
to prevent treated seeds and seed-grains from being consumed by humans
or farm animals.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TENTATIVE NEGLIGIBLE DAILY INTAKE
0 - 0.0006 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Seed wheat is treated with HCB to destroy seed-borne and soil-borne
spores of Bunt fungi (Tilletia spp.) and to ensure freedom from Bunt
in the subsequent crop. HCB has proved outstanding for this purpose
and has been used widely since being first introduced in 1945. (Costa
1952, Holton and Purdy 1957, Meagher 1953, Purdy 1961). Wheat infected
with "Bunt" or "Stinking Smut" has a bad appearance, an unpleasant
smell and is unsuitable for milling. "Bunt" can be regarded as a most
important and common disease of wheat.
Wheat crops become infected after sowing of seed, which has live Bunt
spores on its surface. These spores germinate and enter the young
plant, growing inside it throughout the season. At harvest a black
mass of spores replaces the starch of the grain whilst the skin of the
grain remains unaffected. During harvest these "Bunt Balls" are
broken, thus releasing myriads of spores over the clean grain, which
if sown without treatment, will lead to heavily infected crops.
HCB is usually applied in the form of dust containing 10-40 percent
a.i. but in Canada liquid preparations are also used (Houghton 1969).
Formulations are coloured to assist in application and to distinguish
treated seed from other grain. Usually, a blue pigment is used but
sometimes carbon black or a red dyestuff is added to reduce the risk
of treated seed being used for food. In Turkey where treated seed was
used for food with disastrous consequences it was proposed that a
denaturant with a strong taste or smell be added as well as colour.
In Australia and various other countries, wheat is selected for seed
at harvest time for the next season and after grading to remove small
or broken grains, fungicide is added automatically by machinery at the
rate of 1-2 ozs 30 percent HCB dust per bushel (330 ppm). The treated
seed is stored in labelled jute bags until required for planting some
months later. There is no evidence of significant contamination of
commercial grain or animal feeds during the process of applying the
HCB dust. The use of untreated seed wheat invariably leads to heavy
losses from fungus diseases (Bunt or Stinking Smut) and it is
therefore standard practice to treat sufficient seed for the
anticipated needs of the next season. It is exceptional for farmers to
buy seed: they normally produce and save for their own requirements.
Farmers are directed not to use treated seed wheat for animal feed or
to allow it to become mixed with commercial grain. Although such
directions are normally observed, the presence of 1 bushel of seed
wheat in 10,000 bushels of commercial grain is sufficient to lead to
significant residues in eggs from hens receiving such grain in their
ration. Contaminated grain sacks, machinery, storage premises,
transport and dust from commercial grain all contribute to the
residues found in animal products.
In Canada, seed wheat is usually treated just before planting.
Although some dusts are used, aqueous suspensions suitable for
application as a coarse spray are favoured. As an alternative,
"drill-box" treatments have been developed whereby the fungicide dust
is added to the seed wheat in the feed box of the seed drill where it
is distributed by simple agitation. Such drill-box treatments are less
effective but appear capable of reducing cross contamination of other
grain, (Wallace 1966).
Statistics on the production or use of HCB are not available for
countries other than Australia. In Australia 12 million bushels of
seed wheat are treated annually with HCB dust, requiring 200 tons of
technical HCB. A smaller proportion of the total crop is probably
treated in U.S.A., Canada, U.K. and some European countries but there
is apparently an extensive use in Turkey, Italy, Spain, Netherlands,
Germany, France and some Eastern European countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the time of the original introduction of HCB as a seed dressing and
for many years afterwards, the analytical methods were not capable of
detecting the level of residues found in commercial grain or animal
products. The only trial results available are those from Australia.
In animals
Craig (1959) fed HCB treated wheat to poultry at the rate of 1´ oz per
bird per day for six months. At the end of this period the fat of the
birds contained more than 300 ppm HCB. Craig and Dwyer (1961) fed seed
wheat containing 660 ppm HCB to aged sheep at the rate of 4 oz and 12
oz per head per day. After four months those receiving 4 oz per day
(80 mg HCB/day) had 330 ppm in the fat but none in liver or kidney.
Those on 12 oz per day (240 mg HCB/day) had 880 ppm in fat and 200 ppm
in liver and kidney.
Gardiner and Armstrong (1960) fed two pigs on HCB treated wheat
containing 660 ppm HCB for 71 days during which each consumed 329 lbs
of wheat. Animals gained 50-98 lbs in weight and at slaughter fat
contained 1000 ppm of HCB.
Watts (1968) reports trials where HCB residues were determined in eggs
and body fat of poultry receiving HCB treated wheat. Three groups of
chickens were fed wheat containing 340 ppm HCB for seven, fourteen and
twenty-eight days before being given untreated grain for twenty-eight
days. The maximum amount of HCB found in egg yolk was 76, 167 and 146
ppm respectively. The maximum amount in body fat of the same chickens
was 178, 349 and 528 ppm respectively. Controls fed on grain believed
to be free of residues showed 0.84 ppm in egg yolk and 3.3 ppm in fat.
It was found that the untreated controls received 0.02 ppm HCB in
their rations.
Later trials reported by Watts (1968a) showed that four groups of
chickens fed rations containing 0.02 ppm, 0.08 ppm, 0.7 ppm and 7 ppm
HCB for two months showed maximum residues of HCB in egg yolk of 0.2
ppm, 0.3 ppm, 2 ppm and 15 ppm respectively. Body fat from the same
chickens contained up to 0.7, 0.7, 5 and 29 ppm of HCB. Half of the
birds receiving the above rations were given treated grain after one
month. At the end of the second month the HCB residues in egg yolk
ranged from 0.18 to 0.2, 0.2 to 0.25, 0.7 to 1.2 and 6 to 12 ppm
respectively. The body fat of these birds contained HCB ranging from
0.6 to 0.7, 0.7 to 0.9, 1.5 to 2.6, 16 to 24 ppm respectively. It was
concluded that residues decline only slowly when birds which have
previously ingested HCB receive untreated feed for one month.
De Vos et al. (1968) as a result of identifying HCB in tissues of wild
birds in the Netherlands carried out semichronic toxicity tests on
Japanese quail and measured the HCB residues in liver, blood and fat.
Whilst exceptionally high levels were found in birds receiving toxic
concentrations in their diet, birds fed on diets containing 20 ppm of
HCB showed HCB residues in the fat of 350 to 520 ppm after three
months. The author concluded that the half-life of HCB in the quail is
about three weeks.
Wit (1969) reports no HCB in an extensive survey of animal fats
examined in the Netherlands but he notes that HCB would be obscured by
and reported as alpha BHC.
In plants
Johns (1969) reports results from six samples of wheat grown from HCB
treated seed in different soils. Residues range from 0.003 ppm to
0.062 ppm. Two samples of wheat grown from seed that had not been
treated with HCB showed 0.001 ppm and 0.003 ppm but it is not known
whether HCB treated wheat was grown in the name soil in previous
years.
Smith (1969) reported trials where wheat treated with 416 ppm HCB was
planted and the resulting grain analysed. Residues of 0.0033 ppm were
found. Untreated seed yielded grains containing 0.0022 ppm HCB. Soils
from twelve wheat farms were analysed and all showed traces of HCB
ranging from 0.001 to 0.02 ppm though nine were below 0.003 ppm.
Old (1969) reports investigations with wheat containing 0.05 ppm HCB,
to determine the effect of milling on the distribution of HCB residues
in the various milled fractions, and the HCB residues in bread made
from the flour fraction. Using wheat containing 0.05 ppm HCB it was
found that residues were distributed in all main milling fractions.
The total residues recovered were equivalent to 0.049 ppm. Of this 42
percent was recovered in the bran, 36 percent in the pollard and 22
percent in the flour. The flour which was found to contain 0.015 ppm
HCB produced bread containing 0.0024 ppm HCB. Residues in bread were
thus only 16 percent of the residues in the flour used for the making
of bread. Further confirmation of the loss during baking was obtained
from flour containing 0.006 ppm HCB which produced broad containing
0.0014 ppm HCB or 23 percent of the amount in the flour used.
There is extensive evidence from the literature that HCB is volatile
in water vapour even at low temperatures. It is assumed that the loss
during baking in due to steam volatilisation.
FATE OF RESIDUES
As far as can be gauged the HCB applied to seeds in not broken down by
physical, chemical or biological agencies but is simply dissipated and
diluted in the soil and atmosphere.
Because of the extreme stability of the molecule, solubility in fatty
tissue and the ease with which animals extract and concentrate
residues of HCB from their feed into their fatty tissues residues are
readily detectable in animal products.
The inadvertent contamination of commercial grain and animal feeds
with traces of HCB, though undesirable, appears hard to eliminate.
Evidence of residues in food in commerce or at consumption
A considerable amount of the date available results from surveys
conducted to determine the level and source of HCB residues in eggs,
meat fat and dairy produce from individual farms. Except in isolated
instances where farmers have not observed instructions not to feed
treated seed grain to domestic animals, the contamination invariably
results from the inadvertent feeding of grain or milling fractions
containing low levels of HCB residues. It is not possible for farmers
to know that such animal rations are contaminated and in view of the
slow rate of excretion the retention of residues in fatty tissues of
such animals presents serious economic and administrative problems.
The following table given results of an extensive survey of HCB
residues in animal products calculated on a fat basis.
TABLE II
HCB residues in Animal Products
ppm.
Commodity No. of No. Less 0.1 0.25 0.5 Over
Samples with than to to to
HCB 0.1 ppm 0.25 0.5 1.0 1.0
Beef Fat 2,029 14 12 1 1 0 0
Mutton Fat 833 20 2 8 9 1 0
Butter 514 15 8 5 0 2 0
Cheese 977 4 3 0 1 0 0
Frozen liquid 393 227 81 106 25 11 4
whole egg
Frozen liquid 154 90 8 42 17 19 4
egg yolk
A survey of commercial wheat samples carried out in Australia revealed
that HCB residues can be found in a significant proportion of the
samples in amounts ranging from traces (less than 0.005 ppm) to a
maximum of 0.05 ppm.
In the United Kingdom positive evidence of the presence of HCB has
been obtained in several shipments of imported wheat. Three samples
from one shipment contained 0.67, 1.3 and 0.48 ppm HCB. In two other
shipments the combined HCB and alpha BHC was less than 1 ppm.
Surveys of cheese imported into Australia reveal HCB residues in a
significant proportion of samples from many countries. More than 10
percent of such imports showed residues in excess of 0.1 ppm, with
many samples ranging from 0.3 to 0.9 ppm.
Examinations of food products imported into U.S.A. (Duggan, 1969)
showed a very low incidence of HCB residues for 1967 and 1968. One
and cheese in 1968). However, in 1969 the incidence increased, HCB
being reported in 170 samples of the 1,866 examined. The increase
probably reflects improvements in analytical technique more than an
increase in the incidence and level of contamination.
The majority of these findings were in manufactured dairy produce with
HCB being reported in 149 of 608 samples mainly of cheese. The range
of values for HCB in the positive sample was from a trace to 0.81 ppm
on a fat basis. The average of 608 samples was 0.01 ppm. Processed
foods, other than dairy produce, showed 12 positive findings in 345
samples examined. The range of positive findings in these commodities
was from a trace to 0.23 ppm.
One sample of butter was found to contain the following residues (on a
fat basis):-
BHC - 0.24 ppm
lindane - 0.32 ppm
HCB - 0.37 ppm
It is obvious that rather more than average care must be taken in
resolving such mixtures of residues which can easily become confused
by certain GLC analytical procedures.
HCB residues have been found in small proportion of the samples of
domestic food production. In 1967, HCB was reported in 12 samples out
of approximately 20,000 examined. In 1968 it was reported in only one
sample out of approximately 10,000 examined and in 1969 in 10 samples
out of approximately the name number tested. The level in positive
findings varied from a trace (less than 0.01 ppm) to 0.5 ppm.
METHODS OF RESIDUE ANALYSIS
On foods
Most chlorinated pesticides can be successfully isolated from fatty
substrates by methods based on the acetonitrile-hexane partition of
Mills (1959). However, HCB 10 exceptionally non-polar, and is
distributed preferentially into the hydrocarbon phase. Its partition
ratio by experiment using equal volumes of the two solvents in
approximately 0.75 to hexane at 20°C. In three counter-current
separations with equal volumes, recovery would be about 10 percent.
Increasing the volume of acetonitrile to increase extraction of the
pesticide would also increase extraction of unwanted fatty matter.
Methods using direct extraction with adsorption cleanup, but without
partition, give acceptable recoveries of HCB from eggs, Onley and
Mills (1962), butterfat, Moats (1963), Langlois et al. (1963) and meat
fat. A method using elution through florisil with methylene
chloride-hexane is now in use in the Australian Commonwealth
Laboratories (Taylor 1969, direct communication).
In wheat
The analytical problem here is somewhat different, as the significance
of the presence of HCB in grain for consumption is related to the
potential for concentration of residues in animals from low levels in
the wheat, as well as to that used for direct human consumption. Thus
the levels of interest are much lower than those in animal products.
Extraction of HCB from wheat was achieved by cracking in a blendor,
refluxing with "hexane" (Shell X4) and filtration through anhydrous
sodium sulphate. Beside the chlorinated residue, this extract contains
fat-soluble plant extractives. Passage through a column of activated
florisil cleans the solution sufficiently for determination by GLC and
confirmation by, TLC, if the levels are comparatively gross, (over 0.1
ppm). However, when the concentration is 0.01 ppm or less, the ratio
of interfering substances to pesticide becomes large enough to require
further cleanup if TLC is to be used. Alternatively, other means of
confirmation are required.
Investigation has therefore followed two lines: to Improve cleanup and
to devise other confirmatory tests.
Cleanup
Hexachlorobenzene is stable to concentrated sulphuric acid. A hexane
solution of residual substrate and HCB, after florisil treatment, was
washed with the acid several times until no further darkening
occurred. The hexane layer was washed with water until neutral, and
dried with anhydrous sodium sulphate. The infra-red spectrum of the
residue after removal of solvent appeared to be that of a hydrocarbon.
Gas chromatography produced meaningful peaks at retention times
corresponding to HCB, but resolution by TLC was prevented by the
streaking effect of the oily residue. This cleanup in inadequate for
low levels.
Attempts at separation of HCB from hydrocarbon material on alumina and
on activated carbon columns with a variety of eluting solvents did not
result in sufficiently clean products.
Refluxing of wheat extracts containing HCB with alcoholic potash
resulted in varying degrees of breakdown of the pesticide as well as
substrate. Although this precluded alkaline cleanup, it suggested
possibilities for confirmation of identity of HCB (vide infra).
Steam distillation of standard solutions of HCB was found to give high
recovery. Wheat extract previously partly cleaned by treatment with
sulphuric acid was therefore distilled from a large (twenty-fold)
excess of water, the vapour rising through a Vigreux column and
descending through a water-cooled condenser before collection under
hexane. The hexane extract from 20 ml of aqueous distillate produced
clear spots on TLC plates for both hexachlorobenzene and "benzene
hexachloride" the former at a level of 0.02 ppm (referred to the
original wheat). Recovery tests by adding known amounts of HCB
produced similar results, with recoveries close to 100 percent.
In spite of the high sensitivity of this method it is recommended that
the sensitivity be developed still further to provide for the need to
determine residues in cereal products at levels below 0.01 ppm.
Confirmation
Ideally, confirmation of identity should rest upon two or more methods
using properties as widely different as possible. For chlorinated
pesticides, chromatography by GLC and TIC are usually considered
suitable. However cleanup difficulty, together with small residues,
may preclude the use of TLC. It is acceptable, then, to use GLC on
columns of such different stationary phases that the substance has
substantially different retention characteristics, use being made of
different combinations of stationary phase-solute interactions.
Experimentally, the combination of retention values on a silicone
(low-polar) and polyester (polar) columns were found to provide useful
confirmations of the presence of HCB.
A number of experiments were carried out to make use of the
degradation of HCB by alkali. Both alcoholic potash and sodium
methylate were used, the latter being more dependably effective. When
small traces of the substance, of the order of 0.1 ppm or less, are
present, the pattern of degradation is consistent, in that two major
products appear in both TLC and GLC, one corresponding in retention
values with HCB, the other being lower. However, when greater amounts
of HCB are treated, the result is often obscured by the appearance of
several other products. The reaction is not, therefore, a reliable
confirmatory test for all samples.
A gas-chromatographic column commonly used in estimation of multiple
chlorinated pesticide residues contains the mixture of DC200 silicone
and QFI fluosilicone proposed by Burke and Holswade (1966). This
column does not separate HCB and the alpha isomer of "BHC", the latter
being the major constituent used in estimation of "BHC". In addition,
alpha-BHC does not produce a strong reaction on the silver nitrate
impregnated Thin layer used. Thus BHC may not be detected if in low
concentrations (at 0.1 ppm levels).
To distinguish and confirm these compounds, a stationary phase of a
more polar nature may be used. Separate peaks are produced on Reoplex
(poly propylene glycol adipate), XE60 (nitrile silicone) and Zonyl E7
(fluoroalkyl pyromellitic ester). Therefore an extract showing a peak
of HCB/BHC on the Burke column may be reanalysed on one of these
columns to distinguish between the two compounds.
The polar phases mentioned are, however, not suitable for all the
chlorinated hydrocarbons under investigation. On some, DDT is degraded
or in indistinguishable from DDD. On others, DDE and Dieldrin are not
separated. Work is currently on hand to select a column suitable for
separating all the chlorinated hydrocarbons for which goods are at
present analysed.
NATIONAL TOLERANCES
No national tolerances are known.
Australia, Canada and the U.S.A. approve the use of HCB seed dressings
on the basis that the seeds are not used for food or feed for animals.
APPRAISAL
HCB has been used extensively as a fungicide to control Bunt
(Tilletia caries, T. tritici and T. foetida) in wheat
since 1945. There are many reports of its action on other seed borne
and soil borne fungi of cereals and as seed treatment for onions but
these uses do not appear to be of great importance.
HCB formulations for seed treatment are sold in most wheat growing
countries. To date the only alternative materials are organomercurial
compounds. The rate of application is usually within the range of 1-3
ozs. of 30 percent dust per bushel of wheat seed equivalent to 330-990
ppm on the seed.
HCB is a comparatively pure material containing 98 percent
hexachlorobenzene. The impurities are tetrachlorobenzene and
pentachlorobenzene.
The data available to the meeting were obtained from the published
literature and by correspondence with more than 100 authors,
administrators and manufacturers throughout the world. In spite of the
wide search there is only limited information on residues in food
commodities in international trade or following good agricultural
practices, other than from Australia. One reason for this is that when
examining food commodities by several GLC analytical procedures HCB
has been obscured or confused with BHC.
All available data indicates that HCB is not metabolized to any
significant extent by plants or animals and that it persists in soil
and in animal tissues for long periods. There is an indication that
HCB might be translocated from treated seed into plant material and
subsequent grain but the level of contamination from this source is
insignificant.
Contamination of commercial grain from soil, seed, machinery and bags
may be minimized by strict attention to agricultural practices but it
is difficult to avoid all traces of HCB in commercial grain.
Animals feeding on grain or milling fractions containing traces of
HCB, concentrate and store the residues in body fat, and excrete
significant quantities in milk and eggs. The residue level in such
animal products ranges from ten to one hundred times the concentration
in feed.
The literature includes several methods of residue analysis which
measure HCB in grain and animal products by GLC but the results can be
confused as and by isomers of BHC. The sensitivity for the
determination of HCB is 0.005 ppm but special extraction and cleanup
procedures are necessary to ensure reasonable recovery from plant
products and especially from animal fats. However, no referee method
has been evaluated.
In view of the importance of HCB seed dressings in countries where
Bunt is endemic, and the lack of suitable alternatives, the Joint
Meeting recognized that recommendations for administrative action
levels were required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY PRACTICAL RESIDUE LIMITS (effective to 1973)
The following temporary practical residue limits are to apply to raw
agricultural commodities moving in commerce unless otherwise
indicated:
Raw cereal (wheat) 0.05 ppm
Cereal products from wheat 0.01 ppm
Fat of cattle, sheep, goats,
pigs and poultry 1.0 ppm
Milk 0.012 ppm
Milk products 0.3 ppm
Eggs (shellfree basis) 1.0 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Metabolic studies in animals including identification of the toxic
product or products excreted in milk.
2. Short-term studies in non-rodent mammalian species and long-term
studies especially in relation to the effect on bone-marrow.
3. Reproduction studies in rats.
REQUIRED before 30 June 1973 (for 1973 review of
Practical Residue Limits)
1. Data from countries other than Australia on residues in raw
agricultural commodities.
2. Data from surveys of residues in commodities moving in
international trade.
DESIRABLE
1. Further information on analytical procedures capable of
distinguishing HCB from BHC isomers suitable for use in
laboratories engaged in general regulatory work.
2. Collaborative studies on analytical methods capable of recovering
and identifying HCB residues in the above food commodities.
REFERENCES
Burke, Jerry A. and Holswade, Wendell. (1966) Gas chromatographic
column for pesticide residue analysis: retention times and response
data. J. Assoc. Offic. Anal. Chemists 49 (2) 374-85
Cam, C. and Nigogosyan, G. (1963) Acquired toxin porphyria cutanea
tarda due to hexachlorobenzene. J. Amer. med. Ass., 183:90-3
Campbell, J.A.H. (1963) Pathological aspects of hexachlorobenzene
feeding in rats. S. Afr. J. Lab. Clin. Med. 9(1):203-6
Cetingil, A.I. and Ozen, M.A. (1960) Toxic porphyria. Blood,
16:1002-11
Costa, J.J. (1952) A new material for eliminating bunt of cereals:
HCB. Rev. Argent, Agron. 19:44-51
Craig, J. (1959) Report of trials - feeding animals with grain
treated with fungicides. Reports - Department of Agriculture, Western
Australia, 1959-60
Craig, J. and Dwyer, H.P. (1961) "Pickled Wheat is Safe for Sheep".
HCB treated wheat is safe for sheep but should be fed with caution
for short periods only. J. of Agric. of W.A., Nov. 1961 p.909
De Matteis, F., Prior, B.E. and Rimington, C. (1961) Nervous and
biochemical disturbances following hexachlorobenzene intoxication.
Nature, 191:363-6
Dogramaci, I. (1961) An outbreak of toxic porphyria in southeastern
Turkey (editorial) Turk. J. Pediat., 3:57-60
Dogramaci, I. (1962) Porphyria turcica (Cutaneous porphyria seen
in southeastern Turkey). General consideration. Turk. J. Pediat.,
4:129-31
Dogramaci, I., Kenanoglu, A., Müftü, Y., Ergene, T. and Wray, J.D.
(1962a) Bone and joint changes in patients with porphyria turcica.
Turk. J. Pediat., 4:149-56
Dogramaci, I., Wray, J.D., Ergene, T., Sezar, V. and Müftü, Y.
(1962b) Porphyria turcica A Survey of 592 cases of cutaneous
porphyria seen in southeastern Turkey. Turk. J. Pediat., 4:138-48
Dogramaci, I. (1964) Porphyrias and porphyrin metabolism, with
special reference to porphyria in childhood in Advances in
Pediatrics, Year Book Medical Publishers, Inc., Vol. 13, pp.11-63
Duggan, R.E. (1969) Direct communication
Gajdos, A. and Gajdos-Torök, M. (1961a) The therapeutic effect of
adenosine-5-monophosphoric acid in porphyria. Lancet, 2:175-7
Gajdos, A. and Gajdos-Torök, M. (1961b) Porphyrie expérimental
observée chez le rat blanc à la suite d'intoxication par
l'hexachlorobènzene. Rev. franç. Etud. clin. biol. 6:549-52
Gajdos, A. and Gajdos-Torök, M. (1961c) Action thérapeutic de l'acide
adénosine-5-monophosphorique sur la porphyrie expérimental du
rat blanc due à l'intoxication par l'hexachlorobenzène. Rev.
franç. Etud. clin. biol., 6:553-9
Gardiner, M.R. and Armstrong, J. (1960) Feeding pickled wheat to
pigs. J. Dept. Agr. W. Australia 1, 237-8 C.A. 54.21368d.
Holton, C.S. and Purdy, L.H. (1957) Comparative effectiveness of HCB
and mercury preparations in controlling soil-borne common bunt in
commercial field trials. Pl. Dis. Reptr. Reprint 39:842-3
Houghton, E.R. (1969) Canada Department of Agriculture. Personal
communication
Johns, T.H. (1969) Translocation of HCB in Wheat. Report to
Australian Pesticides Sub-Committee from N.S.W. Dept. of
Agriculture, June 1969
Langlois, B.E., Stemp, A.R. and Leske, B.J. (1963) Milk fat from the
Babcock Test for Insecticide Residue Analysis in Dairy Produce. J.
Dairy Sc. 46 (1963) 854
Meagher, J.W. (1953) Bunt or stinking smut of wheat. Progress report
on seed dusting trials HCB. J. Dep. Agric. Vict. 51, 11:502-4
Melis, R. (1955) Toxic action of hexachlorobenzene for warm-blooded
animals. Nuovi Ann. Ig., 6:361-7 [Chem. Abstr. 50:5170 g, (1956)].
Mills, P.A. (1959) Detection and Semiquantitative Estimation of
Chlorinated Organic Pesticide Residues in Food by Paper
Chromatography. J.A.O.A.C. 42 (1959) 734
Middleton, John T. (1965) The presence, persistence and removal of
pesticides in air. Res. Pestic., Proc. Conf., Davis, Calif. 1964
191-7 (Pub. 1965)
Moats, W.A. (1963) One-step Cleanup Procedure Using Florisil
J.A.O.A.C. 46 (1963) 172
Ockner, R.K. and Schmid, R. (1961) Acquired porphyria in man and rat
due to hexachlorobenzene intoxication. Nature, 189:499
Old, A.N. (1969) HCB Residues in Milled Wheat Fractions and Baked
Bread. Report of Dept. of Agriculture of N.S.W. CB/66/825 (To be
published)
Onley, J.H. and Mills P.A. (1962) Detection and Estimation of
Chlorinated Pesticides in Eggs J.A.O.A.C. 45(4) 983
Parke, D.V. and Williams, R.T. (1960) Studies in Detoxication 81. The
metabolism of halogenobenzenes: (a) Penta- and hexa-chlorobenzenes.
Biochem. J., 74:5-9
Pearson, R.W. and Malkinson, F.D. (1965) Some observations on
hexachlorobenzene induced experimental porphyria. J. invest.
Derm., 44: 420-32
Purdy, L.H. (1961) Control of common bunt of wheat by seed treatment
in the Pacific North West. U.S.D.A. ARS 34-21:1-8
Savitskii, I.V. (1964) The basis for determining safe permissible
concentrations of hexachlorobenzene and pentachloronitrobenzene
in the air. (Russian) Vopr. Prom. i Sel' skokhoz. Toksikol.,
Kievsk. Med. Inst. 158-173 [Chem. Abstr. 63:8952 d, (1965)]
Schmid, R. (1960) Cutaneous porphyria in Turkey. New Engl. J. Med.,
263:397-8
Smith, W.S. (1969) Translocation of HCB in wheat. Report to Australian
Pesticides Sub-Committee from S.A. Dept. of Agriculture, June
1969
Vos de, J.G., Breeman, H.A. and Benschop, H. (1968) The occurrence of
the fungicide hexachlorobenzene in wild birds and its toxicological
importance. A preliminary communication. Mededelingen Rijksfakulteit
Landbouwwetenschappen Gent. 33:1263-9
Wallace, H.A.H. (1966) A comparison of standard and drillbox seed
treatment chemicals for covered smut of oats and barley. Canadian
Plant Disease. 46 (4) 134-6 Dec. 1966
Watts, R.M. (1968) HCB residues in eggs and body fat of poultry
receiving HCB treated wheat. Reports to Australian Pesticides
Sub-Committee by N.S.W. Department of Agriculture (June 1968)
Watts, R.M. (1968a) HCB residues in Poultry - Low-level feeding
trial. Report to Australian Pesticides Sub-Committee by N.S.W.
Department of Agriculture (June 1968)
Weir, R.J. (1962) Experimental pre-emergence herbicide HCB pure
(new), 90-day dietary administration - rats. Unpub. Rept. of Hazleton
Laboratories, Inc. sponsored by Diamond Alkali Co.
Wit, S.L. (1969) Residues of insecticides belonging to the Group of
chlorinated cyclic hydrocarbons in vegetable and animal fats.
Report No.15/69 Tox. (Feb. 1969) Rijks Institut Voor de
Volksgezondheid - Utrecht - Netherlands
Wray, J.D., Müftü, Y. and Dogramaci, I. (1962) Hexachlorobenzene as
a cause of porphyria turcica Turk. J. Pediat., 4:132-7
