
WHO Pesticide Residues Series, No. 1
1971 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The evaluations contained in these monographs were prepared by the
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues that met
in Geneva from 22 to 29 November 1971.1
World Health Organization
Geneva
1972
1 Pesticide Residues in Food: Report of the 1971 Joint Meeting of
the FAO Working Party of Experts on Pesticide Residues and the WHO
Expert Committee on Pesticide Residues, Wld Hlth Org. techn. Rep.
Ser., No. 502; FAO Agricultural Studies, 1972, No. 88.
These monographs are also issued by the Food and Agriculture
Organization of the United Nations, Rome, as document AGP-1971/M/9/1.
FAO and WHO 1972
TRICHLORFON
IDENTITY
Chemical names
O,O-dimethyl-(2,2,2-trichloro-1-hydroxyethyl)-phosphonate
dimethyl 2,2,2-trichloro-1-hydroxyethyl-phosphonate
Synonyms
(R)Dipterex Crop protection
(R)Dylox Crop protection and veterinary medicine
(R)Neguvon Animal health
(R)Anthon Animal health
(R)Dyvon Animal health
(R)Tugon Control of pests of hygiene
(R)Metrifonate Medicine
(R)Bilarcil Medicine
(R)Masoten Medicine and animal health
Bayer 2349
Bay 15 922
L 13/59
Structural formula
O OH
CH3O " '
\ " '
P - CH - CCl3
/
CH3O
Other information on identity and properties
Trichlorfon is a white crystalline powder and has a melting point of
83-84°C. It has a vapour pressure of 7.8 × 10-6 mm Hg at 20°C and
its volatility is 0.11 mg/m3 at 20°C.
Its solubility in water is good (at 25°C 15.4 g in 100 ml) and
increases with rising temperature. It is also readily soluble in low
alcohols, ketones, aromatic chlorinated hydrocarbons, and dimethyl
sulfoxide. It is insoluble or only slightly soluble in carbon
tetrachloride, petroleum ether, ligroin, and cyclohexanone (Bayer,
1967).
Trichlorfon is very slowly hydrolyzed in acid media (at pH 1-5 50%
hydrolysis at 10°C 2400 days, at 20°C 526 days, and at 50°C 11 days).
In alkaline media it is hydrolyzed more readily (Mühlmann and
Schroder, 1957). In addition, in alkaline media trichlorfon is
transformed into dichlorvos (Metcalf et al., 1959).
Composition of the technical trichlorfon is reported to be (Bayer,
1971):
active ingredient >98%.
dichlorvos 0-0.2%
chloral 0-0.05%
dichloroacetaldehyde 0-0.03%
desmethyl trichlorfon
(by t.l.electrophoresis) 0-0.3%
H20 max. 0.3%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption and distribution
Trichlorfon is apparently absorbed, distributed, degraded and excreted
very rapidly in mammals. Robbins et al. (1956) administered
trichlorfon to a cow (25 mg/kg, oral application) and recovered
radioactive components in the blood in 0.5 hours after treatment. The
maximal content was obtained at 1-3 hours after treatment with almost
no material present at 24 hours. Radioactive components were secreted
into the milk 6-8 hours after treatment with a maximum occurring 18
hours after treatment. Traces of radioactive components were evident
at one week when administered intravenously to cattle (20 mg/kg). The
radioactive components obtained in blood at one hour were primarily
(95%) degradation products (Kühnert et al., 1963). Slower absorption
by intramuscular injection was evident when after six hours following
administration radioactive components were evident in blood.
Trichlorfon was present in the milk up to 10 hours after treatment by
intramuscular injection of 25 mg/kg. Administration to pigs by ip
injection (25 mg/kg) again showed rapid distribution. Radioactive
components were evident in blood within 15 minutes with a maxima
reached within one hour. In 5-7 hours 90% of the radioactivity was
removed from the blood. Within 30 minutes after treatment radioactive
components were present in the gut (Schwarz and Dedek, 1965b).
Schwarz and Dedek (1965a) administered 300 ml and 100 ml of
32P-labelled trichlorfon to two cows by dermal application. Maximum
blood concentration of active component was obtained 10-16 hours after
application, and the levels were 0.45-0.47 ppm with 300 ml and 0.1-0.2
ppm with 100 ml, respectively. Trace amount was still detected after
60 hours.
Secretion of active component into milk was observed, and maximum
concentration in milk was obtained after 14-18 hours with the level of
0.45-0.47 ppm in a high dose cow and 0.12-0.13 ppm in a low dose cow.
Trace amount (less than 0.01 ppm) was found even after seven days with
high dose.
Following subcutaneous administration to pigs (25 mg/kg) maximum
concentration of radioactive components was found in meat in two
hours. At six hours after treatment 90% of the radioactivity had been
removed. Dermal application to cattle resulted in milk residues within
eight hours which were absent at 24 hours following treatment (Leahy,
1964).
Trichlorfon solution was poured on the back of cows and the wash was
scrubbed into the animals coat by brush. Milk was collected at
two-hourly intervals and residues of trichlorfon were estimated by a
bioassay method. After treatment with 1 pt of an 8% wash the amount of
trichlorfon in milk increased to the level of 0.5-0.7 ppm within four
hours and this level was maintained up to 12 hours. After this time,
there was a rapid decline. After treatment with 1 or 2 pt of a 4 wash,
the level of trichlorfon in milk did not exceed 0.4 ppm at any time
after application, and not more than 0.1 ppm level was obtained after
six hours. Trace amounts were found after 48-69 hours (less than 0.06
ppm) (Wickman and Flanagan, 1962). Trichlorfon was administered to 10
dairy cows at a rate of 60 ml/kg by a "pour on" dressing method. The
determination of residue in milk, conducted by thin-layer and gas
chromatography, was found to reach the highest level (0.4 ppm) at six
hours, after which it declined to a mean of 0.05 ppm at 24 hours and
of 0.002 ppm at 48 hours (Juszkiewiez, 1970).
Oral administration to cattle and sheep resulted in meat residues
within one hour (Behrens, 1959). In 4-6 hours these residues were
dissipated by 99%. Trichlorfon is rapidly absorbed dermally and
although relative dermal absorption data are not available there are
distinct species differences. Sheep apparently absorb trichlorfon
dermally at a lower rate than cattle (Dedek and Schwarz, 1970),
Although signs of poisoning were not evident, cholinesterase
depression was noted when dogs were dipped once into a 1% solution of
trichlorfon (Bailey, 1956).
Biotransformation
Although studied extensively, the metabolism and mode of action of
trichlorfon remains uncertain. It has been established that
trichlorfon rearranges via dehydrochlorination to form dichlorvos
(DDVP, 2,2-dichlorovinyldimethyl phosphate) (Barthel et al., 1955;
Lorenz et al., 1955; Mattson et al., 1955; Miyamoto, 1961).
This conversion may occur spontaneously under physiological conditions
(Miyamoto, 1959) and small quantities of dichlorvos have been isolated
from biological tissues following trichlorfon treatment (Metcalf et
al., 1959; Dedek and Lohs, 1970; Schwarz and Dedek, 1965). Apparently
the conversion of trichlorfon to dichlorvos occurs, to a very minor
extent, in mammals although in several instances it has not been
demonstrated (Arthur and Casida, 1958; Hassan and Zayed, 1965; Bull
and Ridgeway, 1969; Kühnert et al., 1963), Dichlorvos does occur in
plants (Bull and Ridgeway, 1969) and insects (Metcalf et al., 1959;
Bull and Ridgeway, 1969). Degradation of trichlorfon apparently
follows several pathways. The two major reactions include: hydrolysis
of the methoxyl moiety (Robbins et al., 1956; Bull and Ridgeway, 1969;
Dedek and Lohs, 1970a) with the methyl group being incorporated by
alkylation or methyl transfer into proteins in liver and various
organs (Dedek and Lohs, 1970b) and hydrolysis of the phosphonate (P-C)
bond (Arthur and Casida, 1957, 1958; Miyamoto, 1961; Hassan et al.,
1960; Zayed and Hassan, 1965; Hassan and Zayed, 1965; Bull and
Ridgeway, 1969) yielding trichloroethanol which is subsequently
conjugated. Miyamoto (1961) has suggested that conjugated metabolites
from rabbits contain a molecule that has an altered trichloroethyl
moiety and an intact phosphorus atom. This alteration product has not
been further defined. In most instances the metabolism in plants and
animals appears to follow the same route.
 Excretion
Following acute administration trichlorfon is rapidly eliminated
primarily via the urine. Following intraperitoneal administration of
trichlorfon to rats, 71% of the total dose was eliminated in the urine
in 16 hours (Bull and Ridgeway, 1969). Following oral administration
to cows, 66% of the dose was eliminated within 12 hours (Robbins et
al., 1956). Arthur and Casida (1958) demonstrated that the major
quantity of radioactive components in urine of rats was hydrolysis
products with less than 1% of the products being extractable by
organic solvents. Secretion of trichlorfon into milk appears to occur
rapidly following application (Leahy, 1964; Robbins et al., 1956)
although this means of elimination is minor and residues are
eliminated rapidly. It seems apparent that elimination of the toxicant
is rapid although in one instance Arthur and Casida (1958) treated
rats with 2000 mg/kg and four hours later found 45% of the
administered dose in fat. This fat storage was not followed further.
The delayed recovery of cholinesterase systems in mammals suggests
that elimination of the toxicant is not complete and small quantities
of antiesterase agents may remain in the body for prolonged periods
(possibly in the fat).
Effect on enzymes and other biochemical parameters
Trichlorfon is a rapid irreversible inhibitor of cholinesterase.
Several investigators have reported in vitro values for inhibition
of this enzyme.
Enzyme source I50 (molar) References
Commercially purified 3.2 × 10-6 Arthur and Casida, 1957
Red blood cell (bovine) 3.2 × 10-6 Arthur and Casida, 1957
6.3 × 10-6 Bull and Ridgeway, 1959
Whole blood (human) 7.9 × 10-5 Arthur and Casida, 1957
Plasma (human) 1.5 × 10-5 Samir et al., 1966
Red blood cell (human) 3.4 × 10-6 Rosival et al., 1959
Serum (human) 1.3 × 10-7 Rosival et al., 1959
Brain (rat) 2.6 × 10-6 Dubois and Cotter, 1955
6.3 × 10-6 Hassan et al., 1965
8.7 × 10-4 Schulemann, 1957
In vivo, rat cholinesterase activity of brain serum and submaxillary
gland was maximally inhibited within 15 minutes of Rx by ip injection
(Dubois and Cotter, 1955). Recovery rates were dependent on the dose
administered (25, 50 or 75 mg/kg) with 75% of the enzyme recovered
within four hours at the highest treatment level. At the lowest level,
activity of cholinesterase was normal within an hour. Cholinesterase
inhibition in humans apparently recovers at a slower rate than
demonstrated with rats. Erythrocyte cholinesterase was not recovered
from two daily doses of 7.5 mg/kg (orally administered) for 38 days
after treatment. Maximum inhibition was only 50% of pretreatment
levels (Lebrun and Cerf, 1960). Cholinesterase levels in children
treated orally for 10 days with 5 or 10 mg/kg returned to normal
within four weeks.
Following instramuscular (25 mg/kg) or intravenous (20 mg/kg)
administration to cattle, calcium levels in the serum were reduced.
The calcium level returned to normal in three days (Kühnert et al.,
1963).
TOXICOLOGICAL STUDIES
Special studies
(a) Carcinogenicity
Following weekly subcutaneous administration of trichlorfon to rats,
two of 24 developed local sarcomas after a period of 800 days
(Pruessmann, 1968). Gibel et al. (1971) observed several incidents of
forestomach papilloma, liver carcinoma and abdominal sarcoma in rats
and mice administered trichlorfon (see "short-term studies").
(b) Reproduction
A three generation (two litter per generation) rat reproduction study
at levels of 0, 100, 300, 1000 and 3000 ppm in the diet resulted in
adverse effects on reproduction at 1000 ppm and above. At 1000 ppm
there was evidence of reduced fertility, smaller litters and reduced
body-weight of pups. At 3000 ppm the pregnancy rate was markedly
decreased and the pups were smaller and lighter in weight with none
surviving to weaning. No effects were noted at 300 ppm or below.
Microscopic examination of the F3b generation indicated no adverse
effects (Loser, 1969; Spicer and Urwin, 1971).
(c) Teratogenesis, mutagenesis
Daily oral administration of trichlorfon (100 mg/kg for 17 days) to
lactating rats resulted in no effect on the pups (Rahn, 1963).
Pregnant rats were administered trichlorfon by continuous inhalation
for 20 days at concentrations of 0.005, 0.02 and 9 mg/m3. At all
levels there were external and internal abnormalities in the
development of embryos, weight differences in organs of the rats,
weight differences in the embryos, shifts in the level of ascorbic
acid and nucleic acids in tissues of mothers and foetuses and
histopathological and histochemical changes in the placenta (Gofmekler
and Tabakova, 1970). No foetal abnormality or embryotoxic effects were
observed when trichlorfon was administered orally at 100 mg/kg/day to
pregnant rats from day six to 15 of gestation (Lorke, 1971).
Trichlorfon injected into chicken embryo egg sac at seven days after
fertilization at a dose of 0.0008 of the rat LD50 and examined at 21
days showed only a slight decrease in embryo viability (Dinerman et
al., 1970). Dominant lethal tests run with male mice injected with a
single dose of 0, 50 or 100 mg/kg and mated to untreated females
resulted in no adverse effects on reproduction or on the young (Arnold
et al., 1971).
Except for the one study by inhalation (Gofmekler and Tabakova, 1970),
trichlorfon does not appear to be a terata-inducing compound nor does
it induce mutations in rodents.
(d) Neurotoxicity
Subcutaneous administration of trichlorfon to chickens at a single
dose of 90 mg/kg did not result in ataxic neuropathy (Witter and
Gaines, 1963). When trichlorfon was fed to hens for 29 weeks at 130
ppm in the diet, no neurotoxic signs were observed (Ross and Sherman,
1960). Oral administration of a single dose of 100 mg/kg (with
atropine and PAM) or dietary levels of up to 5000 ppm for 30 days did
not result in delayed neurotoxicity. Trichlorfon does not induce
pathological demyelination or clinical signs of ataxia (Kimmerle and
Lorke, 1966; Hobik, 1967).
(e) Potentiation
Trichlorfon potentiates the toxicity of azinphos-methyl, EPN and
malathion but not several other organophosphates and carbamate
insecticides (Dubois, 1958; Doull et al., 1958; Kimmerle and Lorke,
1968).
Intraperitoneal administration of 10 mg/kg to rats resulted in a
decrease of the malathion detoxifying enzymes in liver and serum
(Murphy and Dubois, 1958). The effects on the detoxification system
were transient and were reversed in 24 hours. When rats were fed 100
ppm trichlorfon in combination with malathion (100 ppm) for two weeks,
no effects on the detoxifying enzyme were observed (Murphy and Dubois,
1958). However, when 100 ppm trichlorfon was fed to rats and dogs in
combination with malathion (1000 ppm), EPN (20 ppm) or azinphos-methyl
(5 ppm) for six weeks, effects on cholinesterase were noted with EPN
and malathion but not with azinphos-methyl (Doull et al., 1958). The
potentiated effects were greatest with EPN, less with malathion and
absent with azinphos-methyl.
(f) Antidotes
Trichlorfon intoxication in rodents, as with many antiesterase
organophosphate esters, responds to therapy with atropine and 2 - PAM
(Wills, 1959; Dubois and Cotter, 1955; Lorke and Kimmerle, 1968).
2 - PAM was effective in reactivating rabbit cholinesterase and
reducing mortality in mice (Wills, 1959) and rats (Lorke and Kimmerle,
1968).
A recent report on the action of thiamine and pyridoxime as
therapeutic agents indicates that the antivitamins (oxythiamine and
desoxypyridoxime) synergize the effects of poisoning. The two vitamins
when given prior to treatment have shown some beneficial effects
(Zhdanovich and Vdalov, 1970). There was little effect when given
after treatment.
Acute toxicity
Animal Sex Route LD50 References
(mg/kg)
Mouse M oral 660 Vbrovsky et al., 1959
M 950 Schulemann, 1955
M&F ip 500 Dubois and Cotter, 1955
M 650 Vbrovsky et al., 1959
F 575 Vbrovsky et al., 1959
M sc 267 Borgmann and Hunold, 1955
F 320 Borgmann and Hunold, 1955
Rat M&F oral 316-650 Dubois and Cotter, 1955
Deichman and Lampe, 1955
Edson and Noakes, 1960
Schulemann, 1955
Gaines, 1969
Hagan, 1958
Borgmann and Hunold, 1955
Animal Sex Route LD50 References
(mg/kg)
se 400 Arthur and Casida, 1958
ip 400 Arthur and Casida, 1957
160 Dubois, 1958
225 Dubois and Cotter, 1955
M 160 Murphy and Dubois, 1958
M
(adult) 250 Brodeur and Dubois, 1963
M
(weanling) 190 Brodeur and Dubois, 1963
M&F dermal 2 800 Edson and Noakes, 1960
2 000 Gaines, 1969
Guinea-pig M&F ip 300 Dubois and Cotter, 1955
200 Schulemann, 1955
Rabbit oral 160 Arant et al., 1971
dermal 5 000 Deichman and Lampe, 1955
Chicken oral 75-110 Dubois and Doull, 1955
Kimmerle and Lorke, 1966
sc 125 Witter and Gaines, 1963
65 Sherman and Ross, 1959
ip ca. 75 Kimmerle and Lorke, 1966
Duck
(1 week old) oral 105 Dubois and Doull, 1955
Dog oral 420 Deicbman and Lampe, 1955
Horse oral 100 Jackson et al., 1960
When trichlorfon was administered as a 10% solution to the
conjunctival sac of rabbits for six days, reversible effects,
including miosis and vasodilation of the blood vessels of the upper
lid, were observed (Deichman and Lampe, 1955). Dogs dipped once into a
1% solution showed no sign of toxicity although cholinesterase was
depressed (Bailey, 1956). Toxic signs of poisoning are typical of the
cholinergic response of organophosphate esters. Symptoms include
twitching, salivation, lacrymation, defaecation, urination, tonic and
clonic convulsions, prostration, cardiac arrest and respiratory
failure. The onset of symptoms is rapid as is the recovery following
sublethal poisoning. Intraperitoneal administration of toxic doses of
trichlorfon caused the appearance of symptoms in rats and mice in
about 10 minutes and death or recovery occurred within a few hours.
Symptoms included scattered muscular fibrillations and body twitches
which were followed by salivation, lacrymation, defaecation, and
urination, The severity of the symptoms increased with time and
included tonic and clonic convulsions, prostration and respiratory
failure preceded by cardiac arrest.
Skin irritation was absent when 1:1 mixture of trichlorfon and
emulsifier was applied to rat and guinea-pig backs daily for four
weeks (Borgmann and Hunold, 1955). Inhalation studies in a static
chamber with concentrations of 22 mg/l air caused symptoms of
cholinergic stimulation but no death. At 8 mg/l no signs of poisoning
were observed (Borgmann and Hunold, 1955).
Short-term studies
Rat
Rats (five rats per group) were administered trichlorfon
intraperitoneally at 50, 100 and 150 mg/kg/day for 60 days. Mortality
was observed at 100 mg/kg/day while at 50 mg/kg/day all animals
survived (Dubois and Cotter, 1955).
Rats (13 male and 13 female per group) were fed trichlorfon at dietary
levels of 0, 20, 100 and 300 ppm for 16 weeks. Significant
cholinesterase depression was noted at 300 ppm. No effects were
observed at 100 ppm on growth, behaviour, food consumption or gross
and microscopic examination of tissues (Doull and Dubois, 1956).
Rats (10 male rats per group) were fed trichlorfon at levels of 0. 1,
5, 25 and 125 ppm for 16 weeks. Cholinesterase depression was minor
(approximately 30%) at the initial phases of the test (4-8 weeks) and
slowly rose to near normal values. No effects were noted on food
consumption, growth or on gross examination of the tissues (Edson and
Noakes, 1960).
Cutaneous administration of trichlorfon to mice either alone or mixed
with "Krotonal" three times per week (alone) for five months or once
per week ("Krotonal") for six months resulted in a higher incidence of
pathological abnormalities than the controls. Cutaneous administration
alone resulted in an incidence of liver necrosis, liver cirrhosis,
liver carcinoma and forestomach papilloma. With "Krotonal" one case of
abdominal sarcoma was observed in addition to the other effects
(Gibel, 1971).
Dog
Dogs (two males and two females per group) were fed trichlorfon at
levels of O, 50, 250, 500 and 1000 ppm in the diet for one year.
Cholinesterase activity was depressed at 500 and 1000 ppm. At 500 and
1000 ppm an increased spleen weight with congestion and apparent
lymphoid atrophy was noted. Males at 1000 ppm exhibited a decrease in
spermatogenesis and the occurrence of hyperplastic nodules in the
adrenals. No effects were noted on mortality, growth, food
consumption, behaviour or gross and microscopic examination of tissues
other than those mentioned above (Doull et al., 1962a).
Two dogs were administered 45 mg trichlorfon per kg orally six days
per week for three months with no cumulative effects noted. The serum
cholinesterase level was 60% of normal at the conclusion. No mortality
was observed (Deichman and Lampe, 1955).
Dogs (one male and one female per group) were fed trichlorfon in the
diet for 12 weeks at levels of 20, 100, 300 and 500 ppm. The animals
were maintained on control diets for a further four weeks. Depression
of erythrocyte and serum cholinesterase activity was noted at 300 ppm
and above. Trichlorfon at 100 ppm did not affect cholinesterase. No
effects were noted at any level on the growth, food consumption or
behaviour of the dogs. Cholinesterase activity returned to normal
within two weeks of cessation of feeding (Doull and Vaughn, 1958).
Dogs (one male and one female per group, two males and two females
served as controls) were fed trichlorfon at levels of 0, 50, 200 and
500 ppm for 12 weeks in the diet. Plasma and erythrocyte
cholinesterase activity was depressed at 500 ppm and unaffected at 200
ppm. Recovery of enzyme activity was complete six weeks after
trichlorfon feeding stopped (Williams et al., 1959).
Long-term studies
Rat
Rats were administered trichlorfon three times weekly by oral or
subcutaneous administration for the life of the rats. Higher incidence
of liver necrosis, liver cirrhosis and forestomach papilloma were
evident than were described for the control (Gibel et al., 1971).
Five groups of rats (25 male and 25 female per group; controls
contained 50 males and 50 females) were fed diets of trichlorfon
containing 0, 50, 250, 500 and 1000 ppm for a period of two years,
Male rats at 1000 ppm gained less weight than the control after the
first two months and failed to regain the weight loss during the
remaining feeding period. Rats fed 1000 ppm weighed about 15% less
than the control animals. In male and female rats fed 1000 ppm the
onset of mortality occurred earlier than in rats from the other groups
and there was distinct shortening of the survival time in rats fed
this diet. Apparently 1000 ppm trichlorfon in the diet caused a
decrease in life span. Rats fed 500 ppm showed a moderate (25%)
inhibition of serum cholinesterase. No effect was noted on the
cholinesterase from brain submaxillary gland and erythrocyte at this
and lower levels. At 1000 ppm all cholinesterase determinations were
below normal, except for the brain. Gross pathological effects
resulting from the presence of trichlorfon in the diet included
mammary gland tumours which occurred in female rats fed 250 ppm (one
rat), 500 ppm (three rats) and 1000 ppm (two rats). Microscopic
examinations of the tissues indicated major adverse histological
findings observed in the mammary glands, gonads and blood vessels of
the trichlorfon fed animals. Three mammary tumours were observed at
1000 ppm (an adenocarcinoma, a sarcoma and a fibroma); three were
observed at 500 ppm (two adenocarcinomas and a fibroadenoma); and one
was found at 250 ppm (a fibroadenoma). Female rats, fed 500 and 1000
ppm, exhibited an absence of primary follicules and primative ova; one
rat fed 250 ppm also lacked follicules and ova. Two of the five rats
examined at 1000 ppm had tubular androblastomas which were composed of
epithelial components and stroma. Three of five male rats examined
from 1000 ppm feeding levels exhibited focal aspermogenesis not
observed at any other dietary levels. Lesions were observed in the
middle and small sized arteries of the lung, thymus, pancreas,
submucosa and adventitia of the gastrointestinal tract and in the
adrenal glands of several of the animals. The lesions were more common
in males than in females. The muscular and elastic tissue of the blood
vessel walls was frequently replaced by fibrous tissues and this was
associated with a necrotizing inflammation. The gross and microscopic
examination of the tissues from animals fed the trichlorfon-containing
diets revealed three pathological effects which appeared to be related
to the presence of the chemical in the diet. These consisted of an
increase in the incidence of mammary tumours in the female rats,
vascular changes and injury to the reproductive systems of both male
and female animals. The incidence of mammary tumour formation in the
entire group of rats used in the study (rather than the relatively
small number of animals used for histological examinations) shows that
the incidence in the rats fed control diets was 14%, 8% of the rats
fed 50 ppm, 20% of the rats fed 250 ppm, 21% at 500 ppm and 25% at
1000 ppm. The comparison of the time for the first tumours to appear
showed that in control diets 1.7 years, 50 ppm diet 1.6 years, 250 ppm
1.8 years, 500 ppm 1.5 years, and 1000 ppm 1.1 years. Thus, the total
frequency and onset of tumour formation appeared to be dose dependent
(Doull et al., 1962b).
Rats (25 male and 50 female per group) were fed dietary levels of
trichlorfon at 0, 100, 200 and 400 ppm for 1.5 years (Doull et al.,
1965). Serum cholinesterase depression was observed in both males and
females at 400 ppm. Erythrocyte cholinesterase was depressed in males
and females at 400 ppm; slightly in males at 200 ppm and very slightly
depressed in females at 100 ppm. There was a significant mortality in
the study in the control and experimental groups. The study was
prematurely concluded at approximately 70 weeks of feeding. At 400 ppm
the male spleen and liver weights were lighter than the control
values. At autopsy the major findings occurred in the ovaries and
mammary glands with slight effects on the lungs. Cystic granular
ovaries were seen in 40% of the female rats fed 400 ppm; 33% of the
rats fed 200 ppm; 14% of the rats fed 100 ppm; and 8% of the female
control rats. Fifteen per cent. of the female rats fed 400 ppm had one
or more mammary tumours; 11% of the rats fed 200 ppm; and 8% of the
control had mammary tumours. The induction time for mammary tumours
was not significantly decreased by increasing dietary concentrations
of trichlorfon. There were pulmonary abscesses in the lungs of two
female rats fed 400 ppm and two other female rats from this group
exhibited exudative pleuritis. Microscopic examination of the tissues
indicated an absence of primary follicules and primitive ova in four
of the five rats examined at 400 ppm. This was a significant increase
from those found at lower levels of feeding. The ovaries from the rats
fed 400 ppm were atrophic, small in size with nests of luteal or
granulosa cells, and an increased number of androblasts. Similar but
less marked changes were found in other groups. A greater frequency of
simple cysts of the ovaries was observed at 400 ppm than in the
control group. None of the cysts appeared to be neoplastic.
Examinations of the mammary tumours indicated that they were benign
fibro-epithelial tumours. Microscopic appearance of the tumours in the
trichlorfon fed animals was similar to the tumours seen in the control
animals. These studies suggest that the addition of trichlorfon to the
diet may have enhanced some of the aging changes, especially in the
reproductive tissue.
Rats (groups of 50 male and 50 female per group, 100 male and female
were used as controls) were fed trichlorfon at dietary levels of 0,
50, 250, 500 and 1000 ppm for two years. No effects were observed on
behaviour, food consumption, weight gain, survival, blood count,
urinalysis and liver protein content. Slight non-dose dependent
effects were noted in male and female liver on SDH activity and in
females on SG-OT activity. Cholinesterase was depressed in both males
and females at 1000 ppm but not at 500 ppm. The male liver weight was
significantly increased at 250 and 1000 ppm. An increase in weight was
observed although it was not statistically significant at 500 ppm.
There were no other dose-dependent, significant effects on tissue
weights. Clinical and histological examination of tissues or tumours
indicated that, of the total of 35 malignant tumours, 15 occurred in
the control group and only five were found at 1000 ppm dose levels.
There was no indication of an increased incidence of mammary tumours
although the total number of mammary tumours observed in the
trichlorfon fed animals was greater than that found in the controls
(the percentage of mammary tumour incidence in female rats was:
control, 9%; 1000 ppm, 8%; 500 ppm, 10%; 250 ppm, 12%; 50 ppm, 18%).
In the ovaries, cysts were found in 14 of the 33 control animals, and
at 1000 PPM cysts were found in eight of 20 animals examined. There
was no evidence of unusual interstitial fibrosis in the trichlorfon
fed animals. There appeared to be no evidence of acceleration of the
aging process of the gonads in this experiment. The frequency of
cystic atrophic ovaries or reduction in spermatogenesis was no
different in the controls than in the animals treated with 1000 ppm
trichlorfon in the diet (Lorke and Loser, 1966; Grundmann and Hobik,
1966).
Dog
Dogs (four male and four female per group) were maintained for four
years on diets containing 0, 50, 200, 800 and 3200 ppm trichlorfon
(Loser, 1970). Cholinesterase activity in plasma and erythrocytes was
depressed at 200 ppm. Trichlorfon, at 800 ppm and above, led to an
increased mortality rate. Increased uric acid and creatinine levels in
the male dogs at 800 ppm was indicative of kidney damage. Physical
appearance of the dogs was affected by 800 ppm in the diet. The
animals had a dull, shaggy coat and appeared weaker and sick.
Cholinergic symptoms occurred at the highest dose. Mortality was
evident in dogs fed 3200 ppm and 800 ppm (one male at 800 ppm and two
females at 800 ppm survived the test). Haematological values showed no
pathological changes in any group at two years. The activity of the
serum transaminases (G-OT and G-PT) of the female dogs at 3200 ppm at
two years was significantly high and regarded as pathological. No
other effects were noted on liver function activity. The transaminase
activity in the female dogs surviving the test to four years was
normal. There were no dose related changes in liver function or blood
values at four years. Urine examinations on all dogs at two and four
years showed uric acid and creatinine levels at the 800 ppm dose in
males and the 3200 ppm dose in the female were increased. There were
no differences in the clearance tests. Blood sugar and cholesterol
levels were not affected at 200 ppm. Cholinesterase activity was
depressed at 200 ppm and the depression was dose dependent. In
general, the depression of cholinesterase activity was noticeable
during the earliest parts of the experiment and tended to decrease as
the experiment progressed until at four years the plasma and
erythrocyte cholinesterase level were slightly depressed at 200 ppm in
both male and female with no depression noted at 50 ppm. Comparison of
the organs weights showed that male dogs at 800 ppm had enlarged
spleen, smaller adrenals and testis, and the female at 3200 ppm had an
increased liver weight, enlarged spleen and adrenals and reduced ovary
size. Based on histological examination of tissues there were no
changes in morphology which were considered to be significant or
related to trichlorfon in the diet (Spicer and Payne, 1971).
Observations in man
Over 6000 people, most in South Africa and South America, have been
treated over the past few years for various intestinal and body
parasites (reviewed by Wegner, 1970). The dosages varied up to 70
mg/kg/day for periods up to 12 days. The dose of 7.5 mg/kg given 2-4
times at two week intervals was believed to be the best level as the
symptoms observed were less severe. Symptoms include cholinesterase
depression, weakness, nausea, diarrhoea and abdominal pain. Higher
doses (24 mg/kg) gave more severe symptoms including tachycardia,
salivation, cholic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative and spontaneous recovery in
all cases was rapid. In a few cases, an indication was given that
spermatogenesis (size and shape of sperm) might be affected in humans.
In all cases for treatment of parasites cholinesterase depression was
evident.
Namba (1971) reviewing the human data alluded to the observation that
three persons exposed to trichlorfon showed signs of delayed
neurotoxicity.
Various studies on humans have shown two effects: (1) cholinesterase
depression in all cases which was usually recovered within 30 days of
the cessation of treatment, and (2) a possible effect on
spermatogenesis (Wegner, 1970; Hanna et al., 1966; Lebrun and Cerf,
1960) which included reduced sperm count, seminal fluid volume and a
decreased motility and viability of the cells in a very limited number
of cases. Other studies showing no adverse effects include: Davis and
Bailey, 1969; Abdalla et al., 1965; Abdel-Aal et al., 1970; Beheyet,
1961. The dose of 7.5 mg/kg given once every two weeks appears to be
the best available. There is no human no-effect level recorded.
Comments
Trichlorfon appears to be rapidly absorbed, distributed, metabolized
and excreted in animals. The conversion of trichlorfon to dichlorvos
occurs both in plants and mammals but to only a very minor extent.
Several long-term studies in rats and dogs are available. In two
long-term studies in the rat evidence of an increased frequency and/or
onset of tumour formation (particularly mammary tumours) appeared to
be dose dependent. A third study in rats did not confirm these
observations. An incidence of cystic atrophic ovaries and reduced
spermatogenesis was also observed in two studies and not confirmed in
the third study, in a three generation rat reproduction study or in a
dominant lethal test. None of the experiments individually considered
was by itself indicative of a carcinogenic effect, however the
cumulative evidence derived from all the experiments considered
suggests that further investigations of the potential carcinogenicity
of trichlorfon are required.
In view of the inconclusive nature of the findings in long-term rat
studies, only a temporary acceptable daily intake was established for
trichlorfon.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet equivalent to 2.5 mg/kg body-weight per day
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichlorfon is an insecticide with a broad spectrum of activity. It is
chiefly effective against Lepidoptera (moths), Diptera (flies),
and Heteroptera (bugs). In crop protection, trichlorfon is used
mainly against insect pests in field crops and fruit crops.
Trichlorfon is also of great importance as a public health pesticide
and as an animal health product, and is used in medicine as an
anthelmintic.
The amount of trichlorfon used in the different sectors of
application, expressed in percentages, is in the Western world as
follows:
45% in field crops (e,g. cereals, rice, maize, cotton, grassland,
tobacco)
35% in vegetable crops
10% in fruit crops (e.g. pome fruit, stone fruit, bush and cane
fruit, grapes, citrus fruit, olives)
10% for other uses (e.g. ornamental crops, hygiene, animal
health)
Approximately 25 formulations of trichlorfon are sold and registered
in 83 countries for the control of insect pests of crops and pests of
hygiene.
The following formulations are used in agriculture (crop protection):
50% emulsifiable concentrate, 50%. soluble powder, 80% soluble powder,
50% wettable powder, 2.5% granular, 5.0% granular, 5.0% dust.
Pre-harvest treatments
The recommended application rates and concentrations (in terms of
active ingredient) for pre-harvest treatments of the major crops are
as follows:
Bananas 400-600 g/ha
Beets 150-1000 g/ha, or
0.075-0.12% spray
Cereals (excl. corn and rice) 800-1200 g/ha
Citrus fruits 0.12% spray
Coffee 450-550 g/ha, or
0.075-0.12% spray
Corn 400-1500 g/ha
Cotton 400-1500 g/ha
Currants, gooseberries 0.075-0.12% spray
Deciduous fruits (apples, pears,
cherries, peaches, etc.) 0.075-0.12% spray
Grapes 0.075-0.12% spray
Grassland and forage crops 500-2000 g/ha
Oil palms 450-1600 g/ha,
or 0.08-0.2% spray
Olives 0.075-0.12% spray
Potatoes 600-1000 g/ha
Rice 800-1200 g/ha
Sugar cane 1200-1600 g/ha, or
0.075-0.2% spray
Tea 600-1200 g/ha
Tobacco 600-1200 g/ha
Vegetables 150-1200 g/ha, or
0.075-0.12% spray
For controlling soil-inhabiting cutworms use is made of baits which
are prepared by mixing 100 g active ingredient (soluble powder), 10 kg
bran, 500 g sugar in 10 litres of water. The crumbly mess is used to
treat 1/4-1/2 ha.
Bait sprays for controlling fruit flies consist of 0.08 or 0.32%
active ingredient and 0.25 or 0.5 protein hydrolyzate, respectively.
Post-harvest treatments
No recommendations.
Animal treatments
Trichlorfon is used as an animal health product for the control of
endoparasites and ectoparasites in/on cattle, sheep, goats, pigs,
horses, poultry, dogs, cats and fish. Following formulations are used:
97% soluble powder, 90% soluble powder, 80% soluble powder, 50%
soluble powder, 6% suspension, 11% solution, 5% injectable solution
tablets.
Methods of application are as follows: wash or spray treatment, dip
treatment, spot-on/pour-on treatment, injection, drench treatment,
feed mix, pond or immersion treatment.
Animal health products based on trichlorfon are registered and sold in
77 countries.
Spectrum of activity of trichlorfon in animal treatments is given in
Table 1.
Trichlorfon is normally applied to the animals at the rate of 25-75
mg/kg body-weight by various methods.
The most common method of application is as a wash or spray treatment
for the control of sucking lice, flies and biting lice. For this
purpose, the whole of the animal body is washed or sprayed with a
0.13% solution of Neguvon powder (repeat treatment after five days).
For the control of warble fly maggots on cattle and Sarcoptes mites
on pigs, a 2% solution is recommended (repeat treatment after 5-7
days).
For the external control of Stephanofilaria in cattle and Habronema
spp. in horses, use must be made of a 10% solution applied to the
affected areas.
The 11% ready-for-use Neguvon solution is used for the control of
warble fly maggots in cattle, a single application being made by the
spot-on method. An applicator is supplied with the solution.
Some of the trichlorfon enters through the skin into the blood system.
As a result, the effect is enhanced from inside the animal body. The
systemic action of trichlorfon against warble maggots which wander
through the body of the host animal until they eventually become
located beneath the skin of the animal's back, is also produced as a
result of the compound penetrating through the skin and entering the
blood system.
The success of an external treatment can be further increased by a
simultaneous internal application.
Internal treatment for the control of endoparasites is made as
follows:
TABLE 1. SPECTRUM OF ACTIVITY OF TRICHLORFON IN ANIMAL TREATMENTS
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
External application for control of
ectoparasites:
Biting lice X X X X X
Sucking lice/fleas X X X X X
Sarcoptes mites X X X X
Red avian mite/scaly leg mite X
Flies/sheep keds X X X X X
Warble fly maggots/larvae of
Dermatobia hominis X
Internal application for control of
ectoparasites:
Sarcoptes mites X
External application for control of
endoparasites on domestic animals:
Habronema spp. (summer sores) X
Stephanofilaria in cattle X
Internal application for control of
endoparasites:
Haemonchus spp., Mecistocirrus X X
Oesophagostomum spp. X X
Neoascaris vitulorum X
Bunostomum spp./Ostertagia spp./Cooperia
spp./Trichostrongylus spp. X X
TABLE 1. (Cont'd.)
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
Oxyguris equi X
Stephanofilaria X
Larvae of sheep keds (Oestrus ovis) X
Ascarids X X
Trichuris/Hyostrongylus X
Gastrophilus spp./Habronema spp. X
External application for control of
ectoparasites in fish:
Argulus/Ergasilus/Lernea/Dactylogyrus/
Gyrodactylus/Trichodina/different
fish leeches X
By means of a bottle or drenching gun. For this purpose, it is
recommended to use a 10% solution, the dose depending upon the
body-weight of the animal to be treated. The treatment should be
repeated after 2-3 months, or after an interval of three weeks if
infestation is severe.
In the feed (dry or liquid), mixture with Neguvon powder.
After oral application, trichlorfon is rapidly resorbed and is
translocated in the blood stream to the site at which it is required
to act. Degradation and excretion take place within a few hours.
The 50% Neguvon injectable formulation is a ready-for-use solution for
i.m. or s.c. injection, and is used for the control of Haemonchus
spp., Oesophagostomum spp., and Dermatobia hominis in cattle in
many countries of the Middle and Far East, Africa and Latin America.
For some time past, trichlorfon has also been in use as a pond
treatment and brief dip treatment for the control of ectoparasites in
fish, and has proved to be most successful. The required dose of the
80% powder formulation is 2.5 kg per ha of carp and eel ponds which
have a depth of 50 cm, the dose being raised to 5 kg per ha in 100 cm
deep ponds; the dosage rates needed for the treatment of trout ponds
are only half as large.
The dosage rate for the brief dip treatment for the control of
Argulus, Dactylogyrus and Gyrodactylus in carps is 2.5 kg per
100 litres of water, the duration of the dip treatment being five to
10 minutes.
In South Africa, trichlorfon is marketed also as a dog shampoo for the
control of fleas and ticks in dogs and cats, and as a powder
formulation for the control of fleas in dogs and cats.
Other uses
In hygiene trichlorfon is used for the control of flies in stables as
well as against many other pest species. Baits are used in most
instances.
Further, trichlorfon is used on ornamentals, tree nurseries; and
forestry.
Residues resulting from supervised trials
Trials on crops
The residue data obtained following application of trichlorfon to
fruit, vegetables and field crops are presented, in extracts, in Table
2. These data are from papers published in the literature as well as
from unpublished reports of Chemagro Corporation and Farbenfabriken
Bayer AG which have been compiled and submitted to the Meeting by
Bayer (1971). The residue values were determined initially by
enzymatic procedure (Delta pH method). and in recent years by gas
chromatography (electron-capture detector, phosphorus detector,
microcoulometer). Comparative analyses showed good agreement of these
methods.
Following application to green plant material, a half-life of about
1-2 days was obtained for trichlorfon, as shown by residue studies on
cotton leaves, grass, cabbage, clover, alfalfa and lettuce. Crops
treated 2-4 weeks before harvest are practically free of residues at
harvest time (maize, soybeans, rape, flax),
Following application to bananas, oranges and ground nuts, the bulk of
the trichlorfon residue is contained in the peel and shells,
respectively. Within a few days after the application only slight
residues are to be found in the pulp and nuts, respectively.
Trials on animals
On cattle
The studies of organs showed that following peroral application of
100 mg of active ingredient per kg, up to 10 ppm of active ingredient
is present in steak two hours after the application, and that this
amount decreases to a level of less than 0.1 ppm after 4-6 hours
(Behrenz, 1959). Following back-line and spray applications, organs
(liver, kidneys, brain, heart) and steak are practically free of
residues; in omental fat, on the other hand, maximum active ingredient
concentrations of 9.2 and 1.9 ppm were found one and seven days,
respectively, after the application (Adkins, 1966).
Following administration of trichlorfon to cattle by different routes,
residue studies were carried out by Chemagro Corporation, Kansas City,
United States of America, on practically all organs used for human
diet (meat, fat, liver, heart, kidneys and brain); the determinations
were made by sensitive gas chromatographic methods (limit of
determination 0.01-0.1 ppm; Chemagro Report 14.393; 24.808).
TABLE 2. RESIDUES RESULTING FROM SUPERVISED TRIALS
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Alfalfa 0.6 - 1.1 7 0.04 - 2.0
Alfalfa, seed hulls 1.7 8 0.2 - 1.6
" chaff 1.7 8 0.2 - 0.6
Apples (0.1 - 0.2%) 8 - 30 n.d. - 0.1
Artichokes, spray 1.1 0 - 12 n.d.
" dust 2.8 7 - 14 n.d.
Bananas, pulp 0.4 - 0.8 0 n.d. - 0.3
" peel 0.4 - 0.8 0 n.d. - 2.0
Barley 1.7 14 - 25 n.d.
Blackeyed, beans 1.7 14 n.d.
" vines 1.7 14 n.d. - 0.9
Brussel sprouts, spray 1.7 14 - 19 n.d.
" " dust 2.8 14 0.2
Cabbage 0.6 - 1.7 15 n.d. - 0.05
Cauliflower 1.7 19 - 21 n.d. - 0.15
Celery, spray 0.6 - 1.1 5 - 10 0.05 - 0.19
" dust 0.6 - 1.1 5 - 10 0.05 - 0.16
Cherries 0.3 - 0.5 3 - 8 0.001 - 0.12
Clover 1.1 7 - 8;14 <0.1 - 9.6;
<0.1 - 1.4
Clover, seed head 1.7 14 - 15 <0.1 - 0.7
" chaff 1.7 14 - 15 <0.1 - 0.6
Corn, kernel 0.8 - 1.7 30 - 144 n.d.
" cobs 0.8 - 1.7 30 - 144 n.d.
" husk 1.0 - 1.7 30 - 144 n.d.
" fodder, forage 1.0 - 1.7 30 - 144 n.d.
Cotton, seed 1.1 - 1.7 7 - 10 <0.01 - 0.05
" foliage 2.2 - 2.5 7 n.d. - 0.4
Cowpeas 1.7 14 n.d.
Cowpeas, vines 1.7 14 n.d. - 0.9
Grass, spray 1.1 - 2.2 12 2 - 5
" granular 1.1 - 2.8 12 <1 - 2
Garden beets 1.7 14 - 28 n.d.
Green beans 1.7 14 n.d.
Kale 0.6 - 1.7 6 - 10 n.d. - 0.3
Lettuce, leaf
summer appl. 1.1 - 1.7 7 0.1 - 0.2
winter appl. 1.1 - 1.7 7;14 6.0;3.9
Lettuce, head
summer appl. 1.1 - 1.7 7 <0.1 - 0.24
winter appl. 1.1 - 1.7 7;14 11.1;3.7
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Lima beans 1.7 9 - 15 n.d.
" vines 1.7 9 - 15 n.d. - 0.6
Lin seed 1.7 14 - 27 n.d.
Mustard 1.7 14/15 n.d.
Oats, grain 1.1 - 1.7 13/14 n.d. - 1.4
Oranges, peel, washed 1.1 - 1.7 7 0.1 - 0.21
" pulp 1.1 - 1.7 7 <0.01
Peaches (0.1) 7 - 14 <0.85
Peanuts, spray
" nuts 1.7 - 2.5 0 - 8 n.d. - 0.1
" shells 1.7 - 2.5 0 - 8 0.1 - 2.65
" vines 1.7 - 2.5 3 - 8 0.2 - 0.4
Peanuts, bait
" nuts 1.7 - 2.5 0 - 3 <0.01 - 0.16
" shells 1.7 - 2.5 0 - 3 <0.03 - 0.56
" vines 1.7 - 2.5 0 - 3 <0.07 - 2.9
Peppers, spray 1.1 - 1.7 14/15 n.d. - 0.3
" dust 1.2 14 0.4 - 1.1
Pumpkins 1.1 - 1.7 13/15 n.d.
Rape, seed 0.8 - 1.7 23 - 32 n.d. - 0.1
" meal 1.1 - 1.7 23 n.d.
" oil 1.1 - 1.7 23 n.d.
Safflower 2.2 27;33;43 0.6;<0.1;
<0.1
Soybeans, green beans, pods 0.6 0 0.1 - 6.0
" vines 0.6 0 0.5 - 8.8
" dry beans 0.6 35 - 53 <0.01
" dry vines 0.6 35 - 53 <0.08
(1 x 0.45)
Spinach 0.8 - 1.1 14/15 <0.1 - 1.6
Strawberries 0.3 - 1.1 3/4 <0.07
Sugar beets, roots 0.3 - 1.1 29 - 34 <0.01
" " tops 0.3 - 1.1 29 - 34 <0.05
Sweet corn, spray
" " husk 1.7 - 2.2 7 - 14 n.d. - 0.6
" " kernel 1.7 - 2.2 7 - 14 n.d.
" " cob 1.7 - 2.2 7 - 14 n.d. - 0.1
" " fodder 1.7 - 2.2 7 - 14 0.2 - 1.1
Sweet corn, dust
" " husk 2.0 15 2.7 - 9.1
" " kernel, cob 2.0 15 n.d.
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Sweet corn, granular
" " husk 1.1 - 1.7 14 - 21 n.d. - 8.4
" " kernel 1.1 - 1.7 14 - 21 n.d. - 0.1
" " cob 1.1 - 1.7 14 - 21 n.d. - 0.2
" " fodder 1.1 - 1.7 14 - 21 n.d. - 3.0
Table beets, roots 1.7 7 - 13 n.d. - 0.2
" " tops 1.7 7 - 13 0.2 - 2.5
Tea 1.4 - 1.8 21 <1
Tobacco, green
" spray 1.1 - 1.7 14/15 n.d. - 1.3
" dust 1.0 - 2.5 10/14 n.d.
Tobacco, cured
" spray 1.1 - 1.7 0 - 21 n.d
" dust 1.0 - 2.5 0 - 21 n.d
Tomatoes 0.8 - 1.7 7 n.d. - 0.5
Turnips, roots 10 - 15 n.d. - 0.09
Wheat, grain 1.3 - 1.7 14 - 39 n.d. - 0.25
" straw 1 25 - 39 n.d. - 4.2
" flour 1 25 - 39 <0.05
" bread 1 25 - 39 <0.05
Following peroral uptake of the active ingredient (12.5 and 20 ppm in
feed), no trichlorfon residues were detected (<0.1 ppm) in any of the
examined tissues and organs (brain, heart, kidney, steak, fat) after a
four week feeding period (Chemagro Report 17.991; 18.884). Following
external application of the 9 and 16% formulation - single back-line
application of 25, 50 or 100 mg of active ingredient per kg
(examination of tissues 21 days after application) and four week mist
spray application of 1.1 g of active ingredient per animal and day
(examination of tissues after the final application) - no residues
were detected in any of the tissues or organs (Chemagro Report 14.412;
18.495; 26.117).
Only slight proportions of the parent compound or its metabolites are
to be found in milk, as shown by a number of studies using
P32-labelled and unlabelled trichlorfon.
Following peroral application of 25 mg of active ingredient per kg,
less than 0.2% of the radioactivity representing the total dose
administered was secreted in the milk at the end of 144 hours; of
this, about 10% was unchanged active ingredient and about 23% behaved
like inorganic phosphorus (Robbins et al., 1956). Following similar
application of 80 mg of active ingredient per kg, the maximum residue
at eight hours was 2.5 ppm in the milk samples and at 22 hours
0.1 ppm; in the following milk samples, the residue levels were below
the limit of determination of 0.05 ppm (Behrenz, 1961). Following
spray application of five litres of a 2% active ingredient solution
and back-line was application of one litre of a 2% solution, the milk
samples at eight hours were found to contain 0.05-0.25 ppm of active
ingredient and in the later milk samples no residues were detected
(Behrenz, 1961).
Studies by Ackermann et al. (1968) showed that following backline wash
application of one litre of a 2% trichlorfon solution, the maximum
residue in the first milk sample at eight hours was 0.2 ppm; the
residues in the third milk sample at 32 hours were below 0.01 ppm.
Analyses of these milk samples for dichlorvos showed a maximum content
of 0.03 ppm in the first sample and 0.01 ppm in the third sample.
Following a single dermal treatment with 6% suspension, equivalent to
36 mg of active ingredient per kg, 0.1-0.2 ppm of trichlorfon was
found to be present within the first eight hours (Leahy, 1964).
Following application of 1.15 litres of a 2% trichlorfon solution (or
0.57 litres of a 4% solution) as a dermal wash per animal, the level
of active ingredient present in the milk did not at any time exceed
0.4 ppm; milk samples taken later than six hours after the application
contained about 0.1 ppm of active ingredient (Wickham and Flanagan,
1962).
Following pour-on application (of 100 or 300 ml of a 5.7% solution
(P32-labelled)), the active ingredient concentration in the milk
reached a peak of 0.13 or 0.47 ppm after 14-18 hours; after four days,
the content was less than 0.05 ppm, and after seven days it was less
than 0.01 ppm (Schwarz and Dedek, 1965a).
Following intravenous and intramuscular injection of 20 and 25 mg of
P32-labelled active ingredient, radioactive substances were found in
the milk only at six and 10 hours after the application; dichlorvos
was not formed in these experiments (Kühnert et al., 1963).
Chemagro Corporation, Kansas City, United States of America, carried
out residue studies by gas chromatographic methods following
application of the active ingredient to dairy cows by different
routes. The lower limit of determination was 0.003-0.01 ppm (Chemagro
Report 16.460; 24.808).
Following peroral uptake of the compound in the feed (12.5, 20, 50,
150 and 325 ppm) for a period of one or four weeks, no trichlorfon
residues were found to be present in the milk at the end of the
feeding period (Chemagro Report 17.440; 18.321; 29.234).
Following external application of the active ingredient as a single
pour-on treatment (at 3.7 g/100 kg) or as a single spray treatment
(at 28 g/animal) or as a four week mist spray treatment (1.1 g per
animal per day), active ingredient residues of no more than 0.04 and
0.02 ppm were found in the milk samples taken on the first and second
day after the application only for those animals which received the
pour-on treatment (Chemagro Report 17.970; 18.322; 18.521; 21.289).
The majority of these milk samples were analysed for residues of
trichloroethanol, a possible metabolite of trichlorfon; however, this
compound could not be found in any of the examined samples.
Following a spray treatment of stable walls at a rate of 1 g of active
ingredient per square metre, no trichlorfon residues were found in the
milk of the cows kept in the treated stable (Chemagro Report 16.538).
On pigs
For the use of trichlorfon for the control of ectoparasites and
endoparasites in pigs, the degradation and excretion of the active
ingredient was studied following subcutaneous injection of 25 mg of
P32-labelled trichlorfon per kg body-weight. The maximum active
ingredient concentration in blood was reached after 15-60 minutes
(10-11 ppm) and in the intestinal contents after 20-150 minutes
(4-5 ppm). After 5-7 hours, the blood and intestinal contents still
contained about 1 ppm of active ingredient. Dichlorvos was no longer
detectable in blood 3.5 hours after the application. The trichlorfon
concentration in meat was always somewhat lower than in the blood; a
concentration of 5 ppm was found in the meat two hours after the
injection. The trichlorfon concentration in the meat decreased by a
power of 10 in each 6-7 hours (Schwarz and Dedek, 1965b, 1966).
Chemagro Corporation carried out studies using gas chromatographic
methods (limit of determination 0.01-0.1 ppm residue; Chemagro Report
14.393; 24.808). After addition of 1500 ppm of active ingredient to
the drinking water of pigs (the ingested amount was equivalent to a
single dose of 125 mg of active ingredient per kg body-weight),
0.02-0.07 ppm of active ingredient was found in the liver and
0.02-0.05 ppm was found in loin steak four days after the application;
brain, heart, kidneys and fat samples were free of residues. None of
the organs contained any trichlorfon residues after seven days
(Chemagro Report 27.533). Dichlorvos was not detectable in any of the
examined samples four days after the trichlorfon application (Chemagro
Report 27.561).
No trichlorfon residues were detectable in any of the examined organs
and tissues 14 days after application of the compound in the diet at a
dose level equivalent to 100 mg/kg body-weight (Chemagro Report
18.678).
On sheep
Following peroral application of 120 mg of trichlorfon per kg
body-weight, exploratory residue determinations were carried out in
organs by means of a fly larva test. The residues dropped below the
limit of determination of 0.1 ppm within 4-6 hours after the
application (Behrenz, 1959). Following percutaneous application of
50 mg of the compound per kg body-weight, the concentration in the
blood reached a level of 1 ppm at which it remained for only a brief
period. Dichlorvos was detectable only in slight amounts (Dedek and
Schwarz, 1970).
Fate of residues
General comments
The distribution of trichlorfon residues in organisms is characterized
by its hydrophilic properties.
The decomposition of the molecule is brought about both by splitting
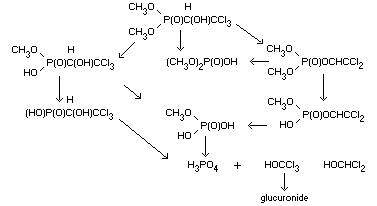
the P-C bond and by hydrolysis of the P-OCH3 bonds (Hassan and
Zayed, 1965). Furthermore, trichlorfon can be converted to the more
toxic dichlorvos, O,O-dimethyl-(2,2-dichlorovinyl)-phosphonate.
In animals
On account of its hydrophilic properties, trichlorfon is rapidly
absorbed by the organism, broken down and excreted in the urine.
Studies with P32-labelled compound on cattle showed that following
peroral application of dosages ranging from 25-100 mg of active
ingredient per kg body-weight, 66% of the P32 activity is excreted
in the urine within 12 hours; of this amount of excreted
radioactivity, 17% is dimethyl phosphate, 76% is accounted for by a
metabolite of unknown structure, and 0.26% by unchanged active
ingredient. After 45 hours, only 0.28% of the P32 activity
representing the total dose administered is found to be present in the
urine (Bolle, 1956; Robbins et al., 1956).
The degradation of the compound in blood proceeds at a very fast rate.
In cattle, 12 hours after peroral application of 40 mg of active
ingredient per kg, 0.03%, of the applied P32 activity is present in
the blood, and 0.003% after 45 hours (Bolle, 1956). The peak of
radioactivity is attained after two hours (Robbins et al., 1956).
Following intravenous application, the parent compound is broken down
by more than 95% after one hour; dichlorvos is found to be present in
a very low concentration only within the first four minutes (Kühnert
et al., 1963).
For further details, see for Biochemical aspects and residues
resulting from supervised trials: Trials on animals.
On poultry and eggs
Gas chromatographic determinations showed that no residues were
present in giblets, muscle and fat (limit of determination of 0.1 ppm)
or in the eggs (limit of determination of 0.003 ppm) of poultry which
had been maintained for four weeks on a diet containing 2.5 and 5 ppm
of the compound (Chemagro Report 20.945; 21.502).
In plants
Studies of the metabolism of trichlorfon in plants were carried out on
cotton plants.
The identified metabolites included dimethyl phosphate which accounted
for up to 70% for the applied trichlorfon dose; only small amounts of
monomethyl phosphate, O-demethyl trichlorfon, O-demethyl dichlorvos
and dichlorvos were formed (Hassan et al., 1966; Bull and Ridgway,
1969). Besides these metabolites, Bull and Ridgway found an
unidentified metabolite which accounted for a large percentage of the
applied dose of parent compound; this phosphorus-containing unknown is
split by ß-glucosidase but not by ß-glucuronidase.
Chloral and trichloroethanol are stated to be possible degradation
products (Arthur and Casida, 1957). However, these products could not
be detected in routine residue studies (Chemagro Corporation,
unpublished). Earlier studies also revealed that chloral and
trichloroethanol are metabolized in plants to form
B-2,2,2-trichloroethanol-gentiobioside (Miller, 1941).
In micro-organism cultures
Studies on Penicillium notatum, Fusarium sp. and Aspergillus niger
showed that approximately half of the applied parent compound is
transformed within 10 days. The principal metabolite found was
O-demethyl trichlorfon. A second degradation product formed is
probably 2,2,2-trichloro-1-hydroxyethyl-phosphonate (Zayed et al.,
1965).
In soil
Trichlorfon is not used for the control of soil pests. Studies
conducted by Chemagro Corporation have shown, however, that after
addition of 10 ppm of trichlorfon to soil, the residue level drops
below the limit of determination within 15-112 days depending upon the
soil type. Trichlorfon has a very low stability in soil; residues of
trichlorfon in runoff water from sandy loam, silt loam and high
organic silt loam soils showed a relatively low tendency to move
(Chemagro Report 28.937). After addition of 0.25 ppm of trichlorfon to
pond water, no residues are to be found in the mud (Chemagro Report
21.313).
In food processing
The published data on trichlorfon residues have generally been
obtained in studies of non-processed plant and animal products. When
these products are processed for the human diet, the trichlorfon
residue level is considerably reduced by such processing procedures as
boiling and sterilization.
In spinach, the residues which have an original level of 2.0 ppm in
the treated plants are reduced by bleaching (five minutes at 100°C) to
a concentration of 0.03 ppm and by further sterilization (100 minutes
at 115°C) to a level of 0.01 ppm. In dwarf beans, the residue level is
reduced from 1.22 ppm to 0.06 ppm and 0.05 ppm, respectively. In peas,
which had an original residue level of 0.21 ppm, no more residues were
detectable after bleaching and sterilization (Dormale et al., 1959).
Tomatoes were treated with 10 ppm of the compound and then canned
according to standard commercial procedure. The heat treatment
destroyed 80% of the compound (Chemagro Report 9012). Safflower seed
containing 0.9 ppm of the compound was processed into meal, crude oil
and refined oil. None of the three processed products contained
detectable residues (Chemagro Report 11.255).
Meat of sheep which ware slaughtered one hour after receiving a
peroral application of 120 mg/kg contained 1-10 ppm of the compound;
after the meat had been boiled for one hour, it contained no
detectable residue (Behrenz, 1959).
In beef which was immersed in a trichlorfon solution to prevent fly
maggot infestation, the residue level of the compound was reduced by
thorough washing from 0.77 ppm to 0.58 ppm. After one hour's boiling,
the residue level in the meat and in the broth was less than 0.1 ppm
(Börger and Maier-Bode, 1967).
Evidence of residues in food in commerce or at consumption
No data were submitted for consideration.
Methods of residue analysis
Many colorimetric, thin-layer chromatographic and gas chromatographic
methods for the determination of trichlorfon residues are described in
the literature. Some of the methods permit simultaneous determination
of trichlorfon and its possible metabolites, e.g. trichloroethanol and
dichlorvos. However, formation of trichloroethanol was not established
in any of the residue studies undertaken.
Trichlorfon can be extracted from plant material with chloroform
(Zadrozinska, 1966), acetone/hexane (Anderson et al., 1966), ethyl
acetate (Cernà, 1963; Watts et al., 1969) or diluted acetic acid
(Sissons and Telling, 1970). For clean-up, the extract residue is
transferred to water; after separating the plant constituents with
petroleum ether or heptane, the parent compound is extracted with
chloroform or diethyl ether (Zadrozinska, 1966; Anderson et al.,
1966). For determination of the compound by the cholinesterase
inhibition technique, extraction with water will suffice (Reynolds
et al., 1960; Chemagro Report 2412; 3581). Column chromatographic
clean-up of the plant extract is possible on charcoal (Watts et al.,
1969) or on aluminium oxide (Sissons and Telling, 1970). If plant
material with a high water content is involved, the compound can be
separated from the plant constituents by dialysis of the macerate in
diluted sulfuric acid, and extracted from the diffusate with diethyl
ether (Anderson et al., 1966; Chemagro Report 8839; 21811).
Trichlorfon can be extracted from animal tissues with acetonitrile
(Beck and Sherman, 1968; Anderson et al., 1966; Chemagro Report
14393), chloroform (Ackermann et al., 1969; Chemagro Report 24808) or
acetone (Chemagro Report 24808). For further clean-up, the extract
residue is transferred to water, fat portions are separated with
heptane, and the parent compound is extracted from the aqueous phase
with diethyl ether (Chemagro Report 14393; Anderson et al., 1966),
In milk, trichlorfon (after removal of fat by centrifuging and
separation of protein by precipitation) is determined either directly
by bioassay (Wickham and Flanagan, 1962) or (after extraction with
chloroform) by thin-layer chromatography, together with possibly
formed dichlorvos (Ackermann et al., 1968). For gas chromatographic
determination of trichlorfon, the milk is extracted with acetone and
benzene. In this method, trichloroethanol is co-determined as a
possible metabolite (Chemagro Report 16460).
Enzymatic determination
Giang and Hall (1951) developed a method for the enzymatic
determination of organic phosphorus insecticides (Delta pH method).
For the determination of trichlorfon residues, the parent compound is
converted to dichlorvos which is a strong cholinesterase inhibitor
(Reynolds et al., 1960; Chemagro Report 2412; 3581), The lower limit
of determination is approximately 0.01 ppm trichlorfon. The method is
unspecific.
Bioassays
Bioassays with mosquito larvae (Aedes aegypti) have been employed
for determining residues of trichlorfon in milk (Behrenz, 1961;
Wickham and Flanagan, 1962). Börger and Maier-Bode (1967) used
Daphnia magna as the test species for determining residues of
trichlorfon in meat. The limit of determination is approximately 0.05
ppm. The method is unspecific.
Thin-layer chromatography
Trichlorfon residues in plants and in animal tissues can be determined
by thin-layer chromatography on silica gel or aluminium oxide plates
sprayed with silver nitrate. The lower limit of determination is
0.25-0.5 ppm of trichlorfon in apples (Zadrozinska, 1966) and
0.2-0.5 µg in animal tissue extracts (Beck and Sherman, 1968). Smaller
amounts of trichlorfon and dichlorvos can be determined by the
cholinesterase inhibition technique (Ackermann et al., 1968, 1969).
Agar-diffusion method
The agar-diffusion method described by Sandi (1962) was modified for
routine determination of trichlorfon. A residue level of 0.1 ppm can
be determined in milk; however, it is not possible to separate
trichlorfon from dichlorvos (Ackermann et al., 1968).
Colorimetry
A colorimetric micro method for determining trichlorfon residues is
based on the determination of chloroform (which is separated from the
trichlorfon by pyrolysis at 550°C) with pyridine and sodium hydroxide
(Fujiwara reaction). This method was used for determining trichlorfon
residues in olive oil (Allessandrini and Lanforti, 1957) and in fruit
and lettuce (Cernà, 1963). The limit of determination is approximately
1 ppm in olive oil and 10 µg in plant material.
Trichlorfon residues can be determined colorimetrically also by a
total phosphorus procedure in plant material (Sissons and Telling,
1970) and in milk (Leahy, 1964); the lower limit of determination is
0.1-0.2 ppm.
Gas chromatography
Trichlorfon residues can be determined with a high degree of
sensitivity and specificity by gas chromatography. The intact molecule
is not determined by this method but instead the cleavage products
chloral or dimethyl phosphite which form upon pyrolytic cleavage of
trichlorfon in the injection port of the gas chromatograph. Chloral is
detected with the electron-capture detector (Anderson et al., 1966);
trichloroethanol can be simultaneously determined as a theoretically
possible metabolite. Chloral can also be determined with the Dohmann
microcoulometric gas chromatograph (Chemagro Report 8839). The
clean-up of extracts is simpler when trichlorfon is detected with a
phosphorus-specific detector, e.g. a modified flame ionization
detector (thermionic detector) (Chemagro Report 21811, 24808). The
limits of determination of these gas chromatographic methods depend
upon the material being analysed and the clean-up procedure used.
However, they are usually very low, e.g. 0.01-0.1 ppm with an
electron-capture detector and 0.003-0.06 ppm with the phosphorus
detector.
Studies on 30 different phosphorus-containing pesticides registered
for use in different crops show that only phosphamidon interferes with
the gas chromatographic determination of trichlorfon with the
phosphorus detector (Chemagro Report 21811, 27471, 29411). By using a
different column, this interference can be avoided (Chemagro Report
26335).
Parallel residue analyses of treated plant material showed agreement
of residue values obtained by the cholinesterase inhibition technique
and by gas chromatography. Determination by the cholinesterase
inhibition technique produces slightly higher residue values, which is
probably due to the presence of slight traces of dichlorvos (Chemagro
Report 9733).
For routine determination of trichlorfon residues, gas chromatography
is the most suitable method. The compound can be detected with a
microcoulometer, an electron-capture detector or a phosphorus-specific
detector, e.g. a thermionic or flame photometric detector.
Examples of national tolerances and safety intervals
Country Crop Tolerance Safety
ppm interval
days
Argentina Bananas 0.2 (provisional)
Bananas (peeled) 0
Australia General 2
Fruits, grains, vegetables 2.0
Austria General 14
Cucumbers, tomatoes,
peppers 4
Belgium General 10
Fruits, vegetables,
excl. potatoes 0.5
Brazil Vegetables 0.5 7
Fruits and field crops 0.5 10
Meat and milk 0.001
Bulgaria General 14
Canada Alfalfa 14
Rape 21
Corn 40
Sugar beets 14
Sugar beets, tops 28
Tobacco 3
Beans 14
Cabbage, cauliflower,
Brussels sprouts 21
Country Crop Tolerance Safety
ppm interval
days
Carrots, rutabagas,
salsify, turnips 28
Collards, kale, lettuce,
spinach 28
Peppers 21
Table beets 28
Tomatoes 21
Artichokes, bananas, NR
beans, beef cattle, NR
beets, Brussels NR
sprouts, cabbage, NR
carrots, cauliflower, NR
collards, corn, kale, NR
lettuce, peppers, rape NR
seed, rutabagas, NR
salsify, spinach, NR
sugar beets, tomatoes, NR
turnips NR
Finland General 14
France General 7
German Democratic Fruits, root vegetables,
Republic leafy vegetables,
cabbages, legumes,
fruit-producing
vegetables (tomatoes,
cucumbers) 1.0
Meat, fish, animal and
vegetable fats, eggs,
milk, baby foods 0
Field crops, fruits 10
Cherries 5
Vegetables 7
Special crops 14
Crops used for the
production of baby foods,
drugs and health foods 30
German Federal Fruits, field crops,
Republic incl. fodder crops 10
Vegetables 7
Application under glass:
general 10
Leafy vegetables,
fruit-producing vegetables,
root vegetables,
Country Crop Tolerance Safety
ppm interval
days
legumes, fruits incl.
grapes 0.5
Great Britain General 2
Hungary Animal products 0
Israel Fruits incl. grapes,
clover, alfalfa,
sub-tropical trees, sugar
beets, fodder crops 7
Maize 30
Tomatoes, cucumbers,
cucurbits, cabbage,
radish, lettuce,
spinach, beans, other
vegetables 10
Peppers, eggplants,
strawberries, cauliflower,
artichokes 14
Morocco General 7
Olives 30
Netherlands General 0.5
General, field-grown 10
Spinach, field-grown 4
General (under glass
from 1 March - 1 Nov.) 17
New Zealand Tomatoes (canned) 1
Other crops 14
Poland Fruits, legumes (not
vegetables), root
crops and other field
crops 14
Vegetables 10
Portugal General 7
Industrial tomatoes 4
South Africa Maize, wheat, citrus
fruits, apples, pears,
apricots, peaches,
sub-tropical crops 10
Cucurbits 7
Alfalfa 2
Tomatoes 3
General 2.0
Soviet Union General 1.0
Spain General 10
Country Crop Tolerance Safety
ppm interval
days
Sweden General 14
Switzerland General 21
Vegetables 14
Beets 42
Vegetables, legumes,
fruits incl. grapes 0.5
United States of See USDA Summary of
America Registered Agricultural
Pesticide Chemical Uses
Yugoslavia General 0.5
Fruits, vegetables,
field crops 14
Appraisal
Trichlorfon is an organophosphorus insecticide which is especially
used on crops against a variety of insects (moths, flies, bugs, etc.).
It is also widely used against ecto- and endoparasites of animals, in
public health and as an anthelmintic in medicine.
It is used on a wide variety of field and pasture crops with
application rates of 150-2000 g/ha, normally 500-1200 g/ha, or as
0.075-0.2% sprays. As an animal health product, it is normally applied
at a rate of 25-75 mg/kg body-weight externally as wash or spray and
spot-on treatment and internally by mouth and by injection. In public
health it is used against flies and other pests commonly in the form
of baits. Other uses are on ornamentals, in tree nurseries, and in
forestry.
Residue data for evaluation are satisfactory.
The behaviour of trichlorfon is characterized by its hydrophilic
properties. Its decomposition is brought about by splitting the P-C
bond and by hydrolysis of the P-OCH3 bonds. In addition, in tissues
it can be converted in trace amounts to dichlorvos. Products of more
advanced degradation have also been found and characterized. In food
processing, trichlorfon residues are substantially disappearing.
As a result of use of trichlorfon, residues may occur in animal feed.
When the applications are made in accordance with good agricultural
practice the residues are considered to be no hazard to the animal
health and no detectable contamination of the foods derived from these
animals is to be expected.
There are a number of methods of residue analysis available. For
regulatory purposes highly specific and sensitive gas chromatographic
methods with detection limits of less than 0.01-0.1 ppm have been
developed.
Due to the insignificant magnitude of dichlorvos and to the low
toxicity of other degradation products, recommendations for tolerances
of trichlorfon residues are made in terms of the parent compound.
Since the direct application of trichlorfon to the domestic animals
may result, although for a short limited period of time, in residues
of varying magnitude in milk, meat, and fat, it is necessary, in
accordance with the local needs of animal health, to set limitations
of use, e.g. type of formulation, route of application, dosage,
condition of the animals, and safety interval from treatment to use of
animal products for human food. The recommendations for trichlorfon
residues in animal products are made, because the use of insecticides
is necessary in animal health, application of trichlorfon according to
good agricultural practice will cause no health hazards to animals,
and, by adjusting properly the conditions of use, milk, meat, organs,
and fat can be obtained from the treated animals practically free from
the trichlorfon residues. Additional safeguard of the consumer against
the residues is the degradation of trichlorfon in storage, processing
and cooking of the animal products before they are consumed.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The following recommendations are made for temporary tolerances:
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Cereals:
Barley 21 0.1
Maize 30 0.1
Wheat 30 0.2
Fruits:
Apples 14 0.1
Bananas, pulp 0 0.2
Cherries 7 0.1
Oranges, pulp 7 0.1
Peaches 14 0.2
Strawberries 4 0.1
(continued)
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Vegetables:
Artichokes 7 0.1
Blackeyed, beans 14 0.1
Brussels sprouts 14 0.2
Cabbage 14 0.1
Cauliflower 21 0.2
Cowpeas 14 0.1
Green beans 14 0.1
Kale 14 0.2
Lima beans 14 0.1
Mustard, leaf 14 0.1
Peppers 14 1
Pumpkins 14 0.1
Sweet corn, kernels and cobs 14 0.2
Tomatoes 14 0.1
Celery 14 0.2
Sugar beets 30 0.05
Beets 14 0.2
Turnips 14 0.1
Oil seeds:
Cotton seed 14 0.1
Flax seed 14 0.1
Lin seed 30 0.1
Safflower seed 45 0.1
Soybeans, dry 45 0.1
Nuts:
Peanuts, shelled 7 0.1
Animal products:
Meat, fat and by-products
of cattle and pigs 14 0.1
Whole milk 2 0.05
Further work or information
Required before 30 June 1975
1. A two-generation carcinogenicity study to elucidate the possible
increase in the incidence of tumours including those of the
mammary gland.
2. More information on residues on oats.
3. More information on residues on lettuce and spinach under various
conditions (including greenhouses).
Desirable
1. Elucidation of the effect on spermatogenesis.
2. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abdalla, A., Saif, M., Taha, A., Askmawy, H., Tawfik, J., Abdel
Fattah, F., Sabet, S. and Abdel-Mequid, M. (1965) Evaluation of an
organophosphorus compound Dipterex on the treatment of Bilharziasis.
J. Egypt Med. Assoc., 48: 262-273
Abdel-Aal, A. M. A., El Hawary, M. F. S., Kamel, H., Abdel-Kalek,
M. K. and El-Diwany, K. M. J. (1970) Egypt Med. Assoc., 53: 265-271
Ackermann, H., Engst, R. and Fechner, G. (1968) Methode zur getrennten
Bestimmung von Trichlorphon und Dichlorphos-Rückständen in Milch.
Ztschr. Lebensmittelunters. Forsch. 137: 303-308
Ackermann, H., Lexow, B. and Plewka, E. (1969) Nachweis und
Identifizierung von insecticiden Phosphor-,
Thiophosphor- Phosphon- und Carbaminsäureestern im Biologischen
Material. Arch. f. Toxicol., 24: 316-324
Adkins, T. R., jr. (1966) Residues in cattle tissues following
back-line and spray applications of Trichlorfon. J. Econ. Entomol.,
59: 1423-1425
Alessandrini, M. E. and Lanforti, G. F. (1957) Determinazione di
residui di Dipterex (O,O-dimetil-2,2,2,trichloro-1-idrossietil
fosfato) nell' olio di oliva. Rend. Ist. super. Sanita, 20: 093-1003
Anderson, R. J., Anderson, C. A. and Olson, T. J. (1966) A Gas-liquid
chromatographic method for the determination of Trichlorfon in plant
and animal tissues. J. Agr. Food Chem., 14: 508-512
Arant, F. S., Atkins, T. R. and Sowell, W. L. Toxicity of Bayer L13/59
to rabbits. Unpublished report
Arnold, D., Keplinger, M. L., Fancher, O. E. and Calandra, J. C.
(1971) Mutagenic study with Dylox in Albino mice. Unpublished report
by Industrial Biotest Laboratories submitted by Farbenfabriken Bayer
A.G.,
Arthur, B. W. and Casida, J. E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2,-trichloro-1-hydroxethyl phosphonate and its acetyl
and vinyl derivatives. J. Agr. Food Chem., 5: 186-192
Arthur, B. W. and Casida, J. E. (1958) Biological activity of several
O,O-dialkyl alpha-acyloxyethyl phosphonates. J. Econ. Entomol.,
6: 360-365
Bailey, C. C., jr. (1956) Evaluation of the dermal toxicity of
malathion chlorthion and Dipterex to dogs. Thesis, Clemson College,
Clemson, S.C.
Bayer, A.G., (1967) Farbenfabriken, Pflanzenschutz. (R)Dipterex
(Bayer 15922, L 13/59) Leverkusen E. 10-6117/22 155
Bayer, A.G., Farbenfabriken, Pflanzenschutz. Documentation on 1971
trichlorfon for FAO
Beck, J. and Sherman, M. (1968) Detection by thin-layer chromatography
of organophosphorus insecticides in acutely poisoned rats and
chickens. Acta pharmacol. et toxicol., 26: 35-40
Beheyet, P., Lebrun. A., Cerf, J., Dierickx, J. and DeGroote. V.
(1961) Etude do la toxicite pour homme d'un insecticide
organophosphore. Bull. Wld Hlth Org., 24: 465-473
Behrenz, W. (1959) Biologische Bestimmung des Wirkstoffgehaltes im
Fleisch von Schafen und Rindern zu verschiedenen Zeiten nach peroraler
Behandlung mit Neguvon. Arch. Lebensmittelhyg., 10: 64
Behrenz, W. (1961) Ueber die Auascheidung von Neguvon(R) in der
Milch nach einmaliger oraler und percutaner Anwendung des Präparates
bei Milchkühen. Vet. mod. Nachr., 133-145
Bolle, W. R. (1956) Neguvon, ein äusserlich und innerlich anwendbares
Insektizid, Larvizid und Acarizid. Vet. med. Nachr., 155-172
Borgmann, W. and Hunnold, G. A. (1955) Report on the results of a
toxicological examination of Dipterex (L13/59). Unpublished report
submitted by Farbenfabriken Bayer A.G.
Brodeur, J. and Dubois, K. P. (1963) Comparison of acute toxicity of
anticholinesterase insecticides to weanling and adult male rats. Proc.
Soc. Exp. Biol., 114: 509-11
Bull, D. L. and Ridgway, R. L. (1969) Metabolism of trichlorfon in
animal and plants. J. Agr. Food Chem., 17: 837-841
Börger, K. and Maier-Bode, H. (1967) Versuche zur Verhinderung des
Fliegenmadon-Befalles von Fleisch. Arch. f. Lebens-mittelhyg., 18:
38-42
Cerná, V. (1963) Kolorimetrische Bestimmung von Dipterex-Rückstünden
in Lebensmittein. Die Nahrung, 7: 60-66
Chemagro-Report (Chemagro Corporation, Kansas City, USA)
2412 Tentative method for microestimation of Dipterex residues by
the Cholinesterase inhibition technique
3581 The determination of Dylox residues by the Cholinesterase
inhibition technique
8839 The microcoulometric determination of Dylox residues in
plant material
9012 The effect of canning on Dylox residues in tomatoes
9733 A comparison of Dylox residue results obtained by the
Cholinesterase inhibition procedure (Report 3581) and the
vapor phase chromatographic procedure (Report 8839)
11 255 The effect of processing on Dylox residues in Safflower
14 393 Determination of Neguvon, Chloral hydrate and
trichlorethanol residues in animal tissues by electron
capture gas chromatography
14 412 Trichlorfon residues in cattle tissues (Backline application
with 8 and 16% pour-on)
16 460 Determination of Trichlorfon, Chloral hydrate and
trichlorethanol residues in milk by electron capture gas
chromatography
16 538 Trichlorfon residues in milk (barn spray prepared with S.P.)
17 440 Trichlorfon residues in milk (in feed prepared)
17 970 Trichlorfon residues in cattle milk (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
17 991 Trichlorfon residues in cattle tissues (in feed prepared)
18 321 Trichlorfon residues in cattle milk (in feed prepared with
technical standard)
18 322 Trichlorfon residues in milk (spray prepared with 80%
soluble powder)
18 495 Trichlorfon residues in cattle tissues (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
18 521 Trichlorfon residues in milk (pour-on with 8% formulation)
18 678 Trichlorfon residues in swine tissues (in feed with 90%
soluble powder)
18 884 Trichlorfon residues in cattle tissues (in feed prepared
with technical standard)
20 945 Trichlorfon residues in poultry tissues (in feed prepared
with Dylox 80% S.P.)
21 289 Trichlorfon residues in milk (mist spray prepared with 1%
Co-Ral - 2% Trichlorfon combination)
21 313 Trichlorfon residues in mud (spray on surface with 50% S.P.)
21 502 Trichlorfon residues in eggs (in feed prepared with 80%
S.P.)
21 811 Determination of residues of Trichlorfon in Alfalfa by
thermionic emission gas chromatography
24 808 Determination of residues of Trichlorfon in bovine animal
tissues by thermionic emission gas chromatography
26 117 Trichlorfon residues in cattle tissues (Backline application
using 8% pour-on)
26 334 A confirmatory gas chromatographic procedure for Trichlorfon
residue analysis
27 471 An interference study for Trichlorfon residue determination
on Alfalfa and Clover
27 533 Trichlorfon residues in swine tissues (drinking water
treated with Neguvon)
27 561 DDVP-residues in swine tissues (drinking water treated with
Neguvon)
28 937 Trichlorfon residues in runoff water from soils
29 234 Trichlorfon residues in milk (Bolus-capsules fortified with
Dylox 80% S.P.)
29 411 An interference study for the residue method for Trichlorfon
on various crops
Davis, A. and Bailey, D. R. (1969) Metrifonate in urinary
schistosomiasis. Bull. Wld Hlth Org., 41: 209-224
Dedek, W. and Lohs, K. (1970a) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern I. Untersuchungen in vitro in Humanserum
mit 14C-Trichlorphon. Z. Naturforsch., 25b: 94-96
Dedek, W. and Lohs, K. (1970b) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern II. Verteilung von 14C in Organen und
Leberproteinen bei Ratten nach Applikation von 14C-Trichlorphon. Z.
Naturforsch., 25b: 1110-1113
Dedek, W. and Schwarz, H. (1970) Studien zur percutanen Resorption von
32P-Dimethoat am Schaf. Z. Naturforsch., 25b: 1193-1194
Deichmann, W. B. and Lampe, K. (1955) Dipterex, its pharmacological
action. Bull. Univ. Miami Sch. Med., 9: 7-12
Dinerman, A. A., Lavrent'eva, N. A. and Il'inskaia. N. A. (1970) The
embryotoxic action of some pesticides. Gigiena i. Sanit., 35: 39-42
Dormale, S., Martens, P. H., Decleire, M. and de Faetraets, L. (1959)
Etude do la persistance de résidus d'insecticides dans divers légumes
frais, blanchis et stérilisés. Bull. Inst. agron. et Stat. Rech.
Gembloux, 27: 137-147
Doull, J. and Dubois, K. P. (1956) The effects of diets containing
Dipterex on rats. Unpublished report by Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J. and Fitch, F.
(1962a) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Doull, J. and Dubois, K. P. (1958) The effects of diets containing
Dipterex for dogs. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Vaughn, G. and Dubois, K. P. (1958) Effect of diets
containing Dipterex in combination with organic phosphates on dogs and
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Fitch, F., Meskauskas, J., Root, M. and
Cowan, J. (1965) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Root; M., Cowan, J., Meskauskas, J. and
Fitch, F. (1962b) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Dubois, K. P. (1958) Potentiation of the toxicity of insecticidal
organic phosphates. AMA Arch. Indust. Health, 18: 488-496
Dubois, K. P. and Cotter, G. J. (1955) Studies on the toxicity and
mechanism of action of Dipterex. AMA Arch. Indust Health, 11: 53-60
Dubois, K. P. and Doull, J. (1955) Acute toxicity of Dipterex to
chickens and ducks. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol, appl.
Pharmacol., 14: 515-534
Giang, P. A. and Hall, S. A. Enzymatic determination of organic
phosphorus insecticides. Anal. Chem., 23: 1830-1834
Gibel, Von W., Lohs, Kh., Wildner. G-P, and Ziebarth, D. (1971)
Tierexperimentelle untersuchungen über die hepatotoxische und
Kanzerogene wirkung phosphoroganischer verbindungen. Arch. für
Geschwulstforschung, 37: 303-312
Gofmekler, V. A. add Tabakova, S. A. (1970) The effect of chorphos on
rat embrogenesis. Farmikol. i. Toksikol., 33: 735-737
Grundmann, E. and Hobik, H. P. (1966) Bay 15922/2 year feeding
experiment in rats/histology. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Hanna, S., Basmy, K., Osaima, S., Shoeb, S. M. and Awny, A. Y. (1966)
Effects of administration of an organophosphorus compound as an
antibilharzial agent with special reference to plasma cholinesterase.
Brit. Med. J., 1: 1390-1392
Hassan, A. and Zayed, S. M. A. D. (1965) Metabolism of
organophosphorus insecticides III. Fate of the methyl groups of
Dipterex in vivo. Can. J. Biochem., 43: 1271-1275
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides II. Metabolism of
O,O-Dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate (Dipterex) in
mammalian nervous tissue and kinetics involved in its reaction with
acetylcholine esterase. Can. J. Biochem., 43: 1263-1269
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides V. Mechanism of
detoxyfication of Dipterex in Prodenia Litura F. Biochem. Pharmacol.,
14: 1577-1584
Hassan, A., Zayed, S. M. A. D. and Hashish, S. (1965) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxyfication in the
rat. Biochem. Pharmacol., 14: 1692-1694
Hassan, A., Zayed, S. M. A. D. and Mostafa, I. Y. (1966) Metabolism of
organophosphorus insecticides VIII. Demethylation of Dipterex.
Z. Naturforsch., 21b: 498-500
Hobik, H. P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitat-sversuchen an huhnern mit
Dipterex. Unpublished report submitted by Farbenfabriken Bayer A.G.
Jackson, J. B., Drummond, R. O. Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Juszkiewicz, T. (1970) Insecticides residues in the tissues and milk
of cow following the dermal application of fenchlorvos and
trichlorphon: a preliminary report. Med. Weterynar (Poland),
26: 85-89
Kimmerle, G. and Lorke, D. (1966) Neurotoxische Untersuchungen an
Huhern mit Dipterex-Wirkstoff. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kühnert, M., Dedek, W. and Schwarz, H. (1963) Untersuchungen über die
Stoffwechselbeeinflussung und den Ausseheidungs-mechanismus des
Phosphonsäure-esters Trichlorphon im Handelspräparat "Bubulin" mit
Hilfe 32P-markierten Phosphors bei der intravenösen und
intramuskulären Injektion an Rindern. Arch. Exp. Vet. Med.,
17: 403-417
Leahy, J. S. (1964) Die Bestimmung von Rückstünden des Neguvon(R)
Milch nach dermaler Anwendung beim Rind. Vet. Med. Nachr., 37-48
Lebrun, A. and Cerf, C. (1960) Note preliminaire sur la Toxicite pour
l'homme d'un insecticide organophosphore (Dipterex). Bull. Wld Hlth
Org., 22: 579-582
Lindgren, P. D. and Ridgway, R. L. (1967) Toxicity of five
insecticides to several insect predators. J. Econ. Entomol.,
60: 1639-1641
Lorke, D. (1971) Trichlorfon. Untersuchungen auf embryotoxische und
teratogene wirkungen an der Ratte. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Lorke, D. and Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Lorke, D. and Kimmerle, G. (1968) Die wirkung von reactivatoren bei
der vergiftung mit phosphorsaureestern. Naunyn-Schmeidebergs Arch.
Pharmakol. Exp. Pathol., 263: S237
Löser, E. (1970) Bay 15922/Chronic toxicological studies on dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Löser, E. (1969) Generationsversuche an Ratten, Unpublished report
submitted by Farbenfabriken Bayer A.G.
Metcalf, R. L., Fukuto, T. R. and March, R. B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52: 44-49
Miller, L. P. (1941) Formation of 2,2,2-Trichloroethylgentiobioside in
tomato plants grown in media containing Chloral hydrate,
Trichloroethyl alcohol, or chloral cyanohydrin. Contribution from
Boyce Thompson Institute Plant Research, 12: 15-23
Miyamoto, J. (1959) Non-enzymic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24: 130-137
Miyamoto, J. (1961) Studies on the mode of action of Dipterex Part II.
New glucorunides obtained from the urine of rabbit following
administration of Dipterex. Agr. Biol. Chem., 25: 566-572
Miyamoto, J. (1968) Private communication to Bull, D. L. and Ridgway,
R. L.
Miyata, T., Iyatomi, K., Saito, T. and Morikawa, O. (1968) Metabolism
and cholinesterase inhibition of NS 2662,
O,O-dimethyl-2,2-dichloro-1-hydroxyethyl phosphonate, in the American
cockroach. Jap. Appl. Ent. Zool., 12: 211-219
Mühlmann, R. and Schrader, G. (1957) Hydrolyse der Insektiziden
Phosphorsäureester. Z. Naturforsch., 12b: 196-209
Murphy, S. D. and Dubois, K. P. (1958) Inhibitory effect of Dipterex
on the detoxification of malathion. Unpublished report by Department
of Pharmacology, University of Chicago
Namba, T. (1971) Cholinesterase inhibition by organophosphorus
compounds and its chemical effects. Bull. Wld Hlth Org., 44: 289-307
Pruessmann, R. (1968) Direct Alkylating Agents as Carcinogens. Food
cos. Toxicol., 6: 576-577
Rahn, H. W. (1963) Über die Moglichkeit der Trichlorphon Schadigung
bei Jung Tieren Laktierender Rattan. Arch. Exp. Veterinarmed.,
18: 713-717
Reynolds, H. T., Stern, V. M., Fukuto, T. R. and Peterson, G. D.
(1960) Potential use of Dylox and other insecticides in a control
program for field crop pests in California. J. Econ. Entomol.,
53: 72-78
Robbins, W. E., Hopkins, T. L. and Eddy, G. W. (1956) The metabolism
of 32P-labelled Bayer L 13/59 in a cow. J. Econ. Entomol.,
49: 801-806
Ross, E. and Sherman, M. (1960) The effect of selected insecticides on
growth and egg production when administered continuously in the feed.
Poultry Sci., 39: 1203-1211
Saito, T. (1969) Selective toxity of systemic insecticides. Res.
Reviews, 25: 175-186
Sándi. E. (1962) Kolorimetrische Bestimmung von Dipterex-Rückständen
in Lebensmitteln. Die Nahrung, 7: 60-66
Sato, K. and Saito, T. (1968) Selective Toxicity of NS 2662,
O,O-dimethyl dichloro-hydroxyethyl phosphonate, and Trichlorfon. Jap.
appl. Ent. Zool., 12: 148-155
Schulemann, W. (1955) Final opinion on the insecticide Bayer L13/59.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Schwarz, H. and Dedek, W. (1965a) Das Verhalten von radioaktiv
markiertem Trichlorphon nach Pour-on-Applikation (Aufgiessverfabren)
zur Dassellarvenbekämpfung beim Rind. Monatsh. Vet. Med.,
20: 958-968
Schwarz, H. and Dedek, W. (1965b) Untersuchungen über den Abbau und
die Ausseheidung von 32P-markiertem Trichlorphon beim Schwein. Zbl.
Vet. Med. (Reihe B), 12: 653-660
Schwarz, H, and Dedek, W. (1966) Untersuchungen zum Nachweis von
32P-markiertem Trichlorphon im Fleisch beim Schwein. Zbl. Vet. Med.
(Reihe B), 13: 489-494
Sherman, M. and Ross, E. (1959) Toxicity of housefly larvae to
insecticides administered as single oral doses to chicks. J. Econ.
Entomol., 52: 719-723
Sissons, D. J. and Telling, G. M. (1970) Rapid procedure for the
determination of organophosphorus insecticide residues in vegetables.
II. A screening procedure for watersoluble insecticides.
J. Chromatog., 48: 468-477
Spicer, E. J. F. and Payne, S. (1971) Pathology report of Bay 15922-4
year dog study (Loser, 1970). Unpublished report submitted by
Farbenfabriken Bayer A.G.
Spicer, E. J. F. and Urwin, C. (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Vbrovsky, L., Selecky, V. and Rosival, L. (1959) Toxikologische und
Pharmakologische Studien der Phosphorsaureester Insecticiden. Arch.
Exp. Pathol. Pharmol., 236: 202-205
Watts, R. R., Storherr, R. W. and Pardue, J. R. (1969) Charcoal column
clean up for many organophosphorus pesticide residues in crop
extracts. A.O.A.C., 52: 522-526
Wegner, D. (1970) Bilarcil(R) Bay 2349 - Klinische Erfahrungen
1960-1969. Unpublished report submitted by Farbenfabriken Bayer A.G.
Wickham, J. C. and Flanagan, P. (1962) Residues of Trichlorphon
(Neguvon) in milk after dermal application to cattle. J. Sci. Food
Agric., 13: 449-455
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Tox. appl. Pharmacol.,
1: 1-7
Wills, J. H. (1959) Recent studies of organic phosphate poisoning.
Fed. Proc., 18; 1020-1025
Witter, R. F. and Gaines, T. B. (1963) Relationship between depression
of brain or plasma cholinesterase and paralysis in chickens caused by
certain organic phosphorus compounds. Biochem. Pharmacol.,
12: 1377-1386
Zadrozinska, J. (1966) Oznaczanie Sladowych pozostalosci Diptereksu
w jablach metoda chromatografii cienkowwarstwowej (Determination of
trace residues of trichlorphon in apples by thin-layer
chromatography). Roczn. panst. Zakl. Hig., 17: 523-528
Zayed, S. M. A. D, and Hassan, A. (1965) Metabolism of
organophosphorus insecticides I. Distribution and metabolism of
Dipterex in adult larva of the cotton leaf worm (Prodenia Litura F.),
Can. J. Biochem., 43: 1257-1262
Zayed, S. M. A. D., Mostafa, I. Y. and Hassan, A. (1965) Organische
P-hattige Insectizide im Staffwichsel. VII. Umwandlung von
32-P-markiertem Dipterex durch Mikroorganismen. Arch. f. Mikrobiol.,
51: 118-121
Zhdanovich, N. V. and Udalov, I. U. F. (1970) The role of thiamine and
pyridoxime on acute and subacute intoxication with the
organophosphorus insecticide Dipterex. Vopr. Pitaniya, 29: 28-34
Excretion
Following acute administration trichlorfon is rapidly eliminated
primarily via the urine. Following intraperitoneal administration of
trichlorfon to rats, 71% of the total dose was eliminated in the urine
in 16 hours (Bull and Ridgeway, 1969). Following oral administration
to cows, 66% of the dose was eliminated within 12 hours (Robbins et
al., 1956). Arthur and Casida (1958) demonstrated that the major
quantity of radioactive components in urine of rats was hydrolysis
products with less than 1% of the products being extractable by
organic solvents. Secretion of trichlorfon into milk appears to occur
rapidly following application (Leahy, 1964; Robbins et al., 1956)
although this means of elimination is minor and residues are
eliminated rapidly. It seems apparent that elimination of the toxicant
is rapid although in one instance Arthur and Casida (1958) treated
rats with 2000 mg/kg and four hours later found 45% of the
administered dose in fat. This fat storage was not followed further.
The delayed recovery of cholinesterase systems in mammals suggests
that elimination of the toxicant is not complete and small quantities
of antiesterase agents may remain in the body for prolonged periods
(possibly in the fat).
Effect on enzymes and other biochemical parameters
Trichlorfon is a rapid irreversible inhibitor of cholinesterase.
Several investigators have reported in vitro values for inhibition
of this enzyme.
Enzyme source I50 (molar) References
Commercially purified 3.2 × 10-6 Arthur and Casida, 1957
Red blood cell (bovine) 3.2 × 10-6 Arthur and Casida, 1957
6.3 × 10-6 Bull and Ridgeway, 1959
Whole blood (human) 7.9 × 10-5 Arthur and Casida, 1957
Plasma (human) 1.5 × 10-5 Samir et al., 1966
Red blood cell (human) 3.4 × 10-6 Rosival et al., 1959
Serum (human) 1.3 × 10-7 Rosival et al., 1959
Brain (rat) 2.6 × 10-6 Dubois and Cotter, 1955
6.3 × 10-6 Hassan et al., 1965
8.7 × 10-4 Schulemann, 1957
In vivo, rat cholinesterase activity of brain serum and submaxillary
gland was maximally inhibited within 15 minutes of Rx by ip injection
(Dubois and Cotter, 1955). Recovery rates were dependent on the dose
administered (25, 50 or 75 mg/kg) with 75% of the enzyme recovered
within four hours at the highest treatment level. At the lowest level,
activity of cholinesterase was normal within an hour. Cholinesterase
inhibition in humans apparently recovers at a slower rate than
demonstrated with rats. Erythrocyte cholinesterase was not recovered
from two daily doses of 7.5 mg/kg (orally administered) for 38 days
after treatment. Maximum inhibition was only 50% of pretreatment
levels (Lebrun and Cerf, 1960). Cholinesterase levels in children
treated orally for 10 days with 5 or 10 mg/kg returned to normal
within four weeks.
Following instramuscular (25 mg/kg) or intravenous (20 mg/kg)
administration to cattle, calcium levels in the serum were reduced.
The calcium level returned to normal in three days (Kühnert et al.,
1963).
TOXICOLOGICAL STUDIES
Special studies
(a) Carcinogenicity
Following weekly subcutaneous administration of trichlorfon to rats,
two of 24 developed local sarcomas after a period of 800 days
(Pruessmann, 1968). Gibel et al. (1971) observed several incidents of
forestomach papilloma, liver carcinoma and abdominal sarcoma in rats
and mice administered trichlorfon (see "short-term studies").
(b) Reproduction
A three generation (two litter per generation) rat reproduction study
at levels of 0, 100, 300, 1000 and 3000 ppm in the diet resulted in
adverse effects on reproduction at 1000 ppm and above. At 1000 ppm
there was evidence of reduced fertility, smaller litters and reduced
body-weight of pups. At 3000 ppm the pregnancy rate was markedly
decreased and the pups were smaller and lighter in weight with none
surviving to weaning. No effects were noted at 300 ppm or below.
Microscopic examination of the F3b generation indicated no adverse
effects (Loser, 1969; Spicer and Urwin, 1971).
(c) Teratogenesis, mutagenesis
Daily oral administration of trichlorfon (100 mg/kg for 17 days) to
lactating rats resulted in no effect on the pups (Rahn, 1963).
Pregnant rats were administered trichlorfon by continuous inhalation
for 20 days at concentrations of 0.005, 0.02 and 9 mg/m3. At all
levels there were external and internal abnormalities in the
development of embryos, weight differences in organs of the rats,
weight differences in the embryos, shifts in the level of ascorbic
acid and nucleic acids in tissues of mothers and foetuses and
histopathological and histochemical changes in the placenta (Gofmekler
and Tabakova, 1970). No foetal abnormality or embryotoxic effects were
observed when trichlorfon was administered orally at 100 mg/kg/day to
pregnant rats from day six to 15 of gestation (Lorke, 1971).
Trichlorfon injected into chicken embryo egg sac at seven days after
fertilization at a dose of 0.0008 of the rat LD50 and examined at 21
days showed only a slight decrease in embryo viability (Dinerman et
al., 1970). Dominant lethal tests run with male mice injected with a
single dose of 0, 50 or 100 mg/kg and mated to untreated females
resulted in no adverse effects on reproduction or on the young (Arnold
et al., 1971).
Except for the one study by inhalation (Gofmekler and Tabakova, 1970),
trichlorfon does not appear to be a terata-inducing compound nor does
it induce mutations in rodents.
(d) Neurotoxicity
Subcutaneous administration of trichlorfon to chickens at a single
dose of 90 mg/kg did not result in ataxic neuropathy (Witter and
Gaines, 1963). When trichlorfon was fed to hens for 29 weeks at 130
ppm in the diet, no neurotoxic signs were observed (Ross and Sherman,
1960). Oral administration of a single dose of 100 mg/kg (with
atropine and PAM) or dietary levels of up to 5000 ppm for 30 days did
not result in delayed neurotoxicity. Trichlorfon does not induce
pathological demyelination or clinical signs of ataxia (Kimmerle and
Lorke, 1966; Hobik, 1967).
(e) Potentiation
Trichlorfon potentiates the toxicity of azinphos-methyl, EPN and
malathion but not several other organophosphates and carbamate
insecticides (Dubois, 1958; Doull et al., 1958; Kimmerle and Lorke,
1968).
Intraperitoneal administration of 10 mg/kg to rats resulted in a
decrease of the malathion detoxifying enzymes in liver and serum
(Murphy and Dubois, 1958). The effects on the detoxification system
were transient and were reversed in 24 hours. When rats were fed 100
ppm trichlorfon in combination with malathion (100 ppm) for two weeks,
no effects on the detoxifying enzyme were observed (Murphy and Dubois,
1958). However, when 100 ppm trichlorfon was fed to rats and dogs in
combination with malathion (1000 ppm), EPN (20 ppm) or azinphos-methyl
(5 ppm) for six weeks, effects on cholinesterase were noted with EPN
and malathion but not with azinphos-methyl (Doull et al., 1958). The
potentiated effects were greatest with EPN, less with malathion and
absent with azinphos-methyl.
(f) Antidotes
Trichlorfon intoxication in rodents, as with many antiesterase
organophosphate esters, responds to therapy with atropine and 2 - PAM
(Wills, 1959; Dubois and Cotter, 1955; Lorke and Kimmerle, 1968).
2 - PAM was effective in reactivating rabbit cholinesterase and
reducing mortality in mice (Wills, 1959) and rats (Lorke and Kimmerle,
1968).
A recent report on the action of thiamine and pyridoxime as
therapeutic agents indicates that the antivitamins (oxythiamine and
desoxypyridoxime) synergize the effects of poisoning. The two vitamins
when given prior to treatment have shown some beneficial effects
(Zhdanovich and Vdalov, 1970). There was little effect when given
after treatment.
Acute toxicity
Animal Sex Route LD50 References
(mg/kg)
Mouse M oral 660 Vbrovsky et al., 1959
M 950 Schulemann, 1955
M&F ip 500 Dubois and Cotter, 1955
M 650 Vbrovsky et al., 1959
F 575 Vbrovsky et al., 1959
M sc 267 Borgmann and Hunold, 1955
F 320 Borgmann and Hunold, 1955
Rat M&F oral 316-650 Dubois and Cotter, 1955
Deichman and Lampe, 1955
Edson and Noakes, 1960
Schulemann, 1955
Gaines, 1969
Hagan, 1958
Borgmann and Hunold, 1955
Animal Sex Route LD50 References
(mg/kg)
se 400 Arthur and Casida, 1958
ip 400 Arthur and Casida, 1957
160 Dubois, 1958
225 Dubois and Cotter, 1955
M 160 Murphy and Dubois, 1958
M
(adult) 250 Brodeur and Dubois, 1963
M
(weanling) 190 Brodeur and Dubois, 1963
M&F dermal 2 800 Edson and Noakes, 1960
2 000 Gaines, 1969
Guinea-pig M&F ip 300 Dubois and Cotter, 1955
200 Schulemann, 1955
Rabbit oral 160 Arant et al., 1971
dermal 5 000 Deichman and Lampe, 1955
Chicken oral 75-110 Dubois and Doull, 1955
Kimmerle and Lorke, 1966
sc 125 Witter and Gaines, 1963
65 Sherman and Ross, 1959
ip ca. 75 Kimmerle and Lorke, 1966
Duck
(1 week old) oral 105 Dubois and Doull, 1955
Dog oral 420 Deicbman and Lampe, 1955
Horse oral 100 Jackson et al., 1960
When trichlorfon was administered as a 10% solution to the
conjunctival sac of rabbits for six days, reversible effects,
including miosis and vasodilation of the blood vessels of the upper
lid, were observed (Deichman and Lampe, 1955). Dogs dipped once into a
1% solution showed no sign of toxicity although cholinesterase was
depressed (Bailey, 1956). Toxic signs of poisoning are typical of the
cholinergic response of organophosphate esters. Symptoms include
twitching, salivation, lacrymation, defaecation, urination, tonic and
clonic convulsions, prostration, cardiac arrest and respiratory
failure. The onset of symptoms is rapid as is the recovery following
sublethal poisoning. Intraperitoneal administration of toxic doses of
trichlorfon caused the appearance of symptoms in rats and mice in
about 10 minutes and death or recovery occurred within a few hours.
Symptoms included scattered muscular fibrillations and body twitches
which were followed by salivation, lacrymation, defaecation, and
urination, The severity of the symptoms increased with time and
included tonic and clonic convulsions, prostration and respiratory
failure preceded by cardiac arrest.
Skin irritation was absent when 1:1 mixture of trichlorfon and
emulsifier was applied to rat and guinea-pig backs daily for four
weeks (Borgmann and Hunold, 1955). Inhalation studies in a static
chamber with concentrations of 22 mg/l air caused symptoms of
cholinergic stimulation but no death. At 8 mg/l no signs of poisoning
were observed (Borgmann and Hunold, 1955).
Short-term studies
Rat
Rats (five rats per group) were administered trichlorfon
intraperitoneally at 50, 100 and 150 mg/kg/day for 60 days. Mortality
was observed at 100 mg/kg/day while at 50 mg/kg/day all animals
survived (Dubois and Cotter, 1955).
Rats (13 male and 13 female per group) were fed trichlorfon at dietary
levels of 0, 20, 100 and 300 ppm for 16 weeks. Significant
cholinesterase depression was noted at 300 ppm. No effects were
observed at 100 ppm on growth, behaviour, food consumption or gross
and microscopic examination of tissues (Doull and Dubois, 1956).
Rats (10 male rats per group) were fed trichlorfon at levels of 0. 1,
5, 25 and 125 ppm for 16 weeks. Cholinesterase depression was minor
(approximately 30%) at the initial phases of the test (4-8 weeks) and
slowly rose to near normal values. No effects were noted on food
consumption, growth or on gross examination of the tissues (Edson and
Noakes, 1960).
Cutaneous administration of trichlorfon to mice either alone or mixed
with "Krotonal" three times per week (alone) for five months or once
per week ("Krotonal") for six months resulted in a higher incidence of
pathological abnormalities than the controls. Cutaneous administration
alone resulted in an incidence of liver necrosis, liver cirrhosis,
liver carcinoma and forestomach papilloma. With "Krotonal" one case of
abdominal sarcoma was observed in addition to the other effects
(Gibel, 1971).
Dog
Dogs (two males and two females per group) were fed trichlorfon at
levels of O, 50, 250, 500 and 1000 ppm in the diet for one year.
Cholinesterase activity was depressed at 500 and 1000 ppm. At 500 and
1000 ppm an increased spleen weight with congestion and apparent
lymphoid atrophy was noted. Males at 1000 ppm exhibited a decrease in
spermatogenesis and the occurrence of hyperplastic nodules in the
adrenals. No effects were noted on mortality, growth, food
consumption, behaviour or gross and microscopic examination of tissues
other than those mentioned above (Doull et al., 1962a).
Two dogs were administered 45 mg trichlorfon per kg orally six days
per week for three months with no cumulative effects noted. The serum
cholinesterase level was 60% of normal at the conclusion. No mortality
was observed (Deichman and Lampe, 1955).
Dogs (one male and one female per group) were fed trichlorfon in the
diet for 12 weeks at levels of 20, 100, 300 and 500 ppm. The animals
were maintained on control diets for a further four weeks. Depression
of erythrocyte and serum cholinesterase activity was noted at 300 ppm
and above. Trichlorfon at 100 ppm did not affect cholinesterase. No
effects were noted at any level on the growth, food consumption or
behaviour of the dogs. Cholinesterase activity returned to normal
within two weeks of cessation of feeding (Doull and Vaughn, 1958).
Dogs (one male and one female per group, two males and two females
served as controls) were fed trichlorfon at levels of 0, 50, 200 and
500 ppm for 12 weeks in the diet. Plasma and erythrocyte
cholinesterase activity was depressed at 500 ppm and unaffected at 200
ppm. Recovery of enzyme activity was complete six weeks after
trichlorfon feeding stopped (Williams et al., 1959).
Long-term studies
Rat
Rats were administered trichlorfon three times weekly by oral or
subcutaneous administration for the life of the rats. Higher incidence
of liver necrosis, liver cirrhosis and forestomach papilloma were
evident than were described for the control (Gibel et al., 1971).
Five groups of rats (25 male and 25 female per group; controls
contained 50 males and 50 females) were fed diets of trichlorfon
containing 0, 50, 250, 500 and 1000 ppm for a period of two years,
Male rats at 1000 ppm gained less weight than the control after the
first two months and failed to regain the weight loss during the
remaining feeding period. Rats fed 1000 ppm weighed about 15% less
than the control animals. In male and female rats fed 1000 ppm the
onset of mortality occurred earlier than in rats from the other groups
and there was distinct shortening of the survival time in rats fed
this diet. Apparently 1000 ppm trichlorfon in the diet caused a
decrease in life span. Rats fed 500 ppm showed a moderate (25%)
inhibition of serum cholinesterase. No effect was noted on the
cholinesterase from brain submaxillary gland and erythrocyte at this
and lower levels. At 1000 ppm all cholinesterase determinations were
below normal, except for the brain. Gross pathological effects
resulting from the presence of trichlorfon in the diet included
mammary gland tumours which occurred in female rats fed 250 ppm (one
rat), 500 ppm (three rats) and 1000 ppm (two rats). Microscopic
examinations of the tissues indicated major adverse histological
findings observed in the mammary glands, gonads and blood vessels of
the trichlorfon fed animals. Three mammary tumours were observed at
1000 ppm (an adenocarcinoma, a sarcoma and a fibroma); three were
observed at 500 ppm (two adenocarcinomas and a fibroadenoma); and one
was found at 250 ppm (a fibroadenoma). Female rats, fed 500 and 1000
ppm, exhibited an absence of primary follicules and primative ova; one
rat fed 250 ppm also lacked follicules and ova. Two of the five rats
examined at 1000 ppm had tubular androblastomas which were composed of
epithelial components and stroma. Three of five male rats examined
from 1000 ppm feeding levels exhibited focal aspermogenesis not
observed at any other dietary levels. Lesions were observed in the
middle and small sized arteries of the lung, thymus, pancreas,
submucosa and adventitia of the gastrointestinal tract and in the
adrenal glands of several of the animals. The lesions were more common
in males than in females. The muscular and elastic tissue of the blood
vessel walls was frequently replaced by fibrous tissues and this was
associated with a necrotizing inflammation. The gross and microscopic
examination of the tissues from animals fed the trichlorfon-containing
diets revealed three pathological effects which appeared to be related
to the presence of the chemical in the diet. These consisted of an
increase in the incidence of mammary tumours in the female rats,
vascular changes and injury to the reproductive systems of both male
and female animals. The incidence of mammary tumour formation in the
entire group of rats used in the study (rather than the relatively
small number of animals used for histological examinations) shows that
the incidence in the rats fed control diets was 14%, 8% of the rats
fed 50 ppm, 20% of the rats fed 250 ppm, 21% at 500 ppm and 25% at
1000 ppm. The comparison of the time for the first tumours to appear
showed that in control diets 1.7 years, 50 ppm diet 1.6 years, 250 ppm
1.8 years, 500 ppm 1.5 years, and 1000 ppm 1.1 years. Thus, the total
frequency and onset of tumour formation appeared to be dose dependent
(Doull et al., 1962b).
Rats (25 male and 50 female per group) were fed dietary levels of
trichlorfon at 0, 100, 200 and 400 ppm for 1.5 years (Doull et al.,
1965). Serum cholinesterase depression was observed in both males and
females at 400 ppm. Erythrocyte cholinesterase was depressed in males
and females at 400 ppm; slightly in males at 200 ppm and very slightly
depressed in females at 100 ppm. There was a significant mortality in
the study in the control and experimental groups. The study was
prematurely concluded at approximately 70 weeks of feeding. At 400 ppm
the male spleen and liver weights were lighter than the control
values. At autopsy the major findings occurred in the ovaries and
mammary glands with slight effects on the lungs. Cystic granular
ovaries were seen in 40% of the female rats fed 400 ppm; 33% of the
rats fed 200 ppm; 14% of the rats fed 100 ppm; and 8% of the female
control rats. Fifteen per cent. of the female rats fed 400 ppm had one
or more mammary tumours; 11% of the rats fed 200 ppm; and 8% of the
control had mammary tumours. The induction time for mammary tumours
was not significantly decreased by increasing dietary concentrations
of trichlorfon. There were pulmonary abscesses in the lungs of two
female rats fed 400 ppm and two other female rats from this group
exhibited exudative pleuritis. Microscopic examination of the tissues
indicated an absence of primary follicules and primitive ova in four
of the five rats examined at 400 ppm. This was a significant increase
from those found at lower levels of feeding. The ovaries from the rats
fed 400 ppm were atrophic, small in size with nests of luteal or
granulosa cells, and an increased number of androblasts. Similar but
less marked changes were found in other groups. A greater frequency of
simple cysts of the ovaries was observed at 400 ppm than in the
control group. None of the cysts appeared to be neoplastic.
Examinations of the mammary tumours indicated that they were benign
fibro-epithelial tumours. Microscopic appearance of the tumours in the
trichlorfon fed animals was similar to the tumours seen in the control
animals. These studies suggest that the addition of trichlorfon to the
diet may have enhanced some of the aging changes, especially in the
reproductive tissue.
Rats (groups of 50 male and 50 female per group, 100 male and female
were used as controls) were fed trichlorfon at dietary levels of 0,
50, 250, 500 and 1000 ppm for two years. No effects were observed on
behaviour, food consumption, weight gain, survival, blood count,
urinalysis and liver protein content. Slight non-dose dependent
effects were noted in male and female liver on SDH activity and in
females on SG-OT activity. Cholinesterase was depressed in both males
and females at 1000 ppm but not at 500 ppm. The male liver weight was
significantly increased at 250 and 1000 ppm. An increase in weight was
observed although it was not statistically significant at 500 ppm.
There were no other dose-dependent, significant effects on tissue
weights. Clinical and histological examination of tissues or tumours
indicated that, of the total of 35 malignant tumours, 15 occurred in
the control group and only five were found at 1000 ppm dose levels.
There was no indication of an increased incidence of mammary tumours
although the total number of mammary tumours observed in the
trichlorfon fed animals was greater than that found in the controls
(the percentage of mammary tumour incidence in female rats was:
control, 9%; 1000 ppm, 8%; 500 ppm, 10%; 250 ppm, 12%; 50 ppm, 18%).
In the ovaries, cysts were found in 14 of the 33 control animals, and
at 1000 PPM cysts were found in eight of 20 animals examined. There
was no evidence of unusual interstitial fibrosis in the trichlorfon
fed animals. There appeared to be no evidence of acceleration of the
aging process of the gonads in this experiment. The frequency of
cystic atrophic ovaries or reduction in spermatogenesis was no
different in the controls than in the animals treated with 1000 ppm
trichlorfon in the diet (Lorke and Loser, 1966; Grundmann and Hobik,
1966).
Dog
Dogs (four male and four female per group) were maintained for four
years on diets containing 0, 50, 200, 800 and 3200 ppm trichlorfon
(Loser, 1970). Cholinesterase activity in plasma and erythrocytes was
depressed at 200 ppm. Trichlorfon, at 800 ppm and above, led to an
increased mortality rate. Increased uric acid and creatinine levels in
the male dogs at 800 ppm was indicative of kidney damage. Physical
appearance of the dogs was affected by 800 ppm in the diet. The
animals had a dull, shaggy coat and appeared weaker and sick.
Cholinergic symptoms occurred at the highest dose. Mortality was
evident in dogs fed 3200 ppm and 800 ppm (one male at 800 ppm and two
females at 800 ppm survived the test). Haematological values showed no
pathological changes in any group at two years. The activity of the
serum transaminases (G-OT and G-PT) of the female dogs at 3200 ppm at
two years was significantly high and regarded as pathological. No
other effects were noted on liver function activity. The transaminase
activity in the female dogs surviving the test to four years was
normal. There were no dose related changes in liver function or blood
values at four years. Urine examinations on all dogs at two and four
years showed uric acid and creatinine levels at the 800 ppm dose in
males and the 3200 ppm dose in the female were increased. There were
no differences in the clearance tests. Blood sugar and cholesterol
levels were not affected at 200 ppm. Cholinesterase activity was
depressed at 200 ppm and the depression was dose dependent. In
general, the depression of cholinesterase activity was noticeable
during the earliest parts of the experiment and tended to decrease as
the experiment progressed until at four years the plasma and
erythrocyte cholinesterase level were slightly depressed at 200 ppm in
both male and female with no depression noted at 50 ppm. Comparison of
the organs weights showed that male dogs at 800 ppm had enlarged
spleen, smaller adrenals and testis, and the female at 3200 ppm had an
increased liver weight, enlarged spleen and adrenals and reduced ovary
size. Based on histological examination of tissues there were no
changes in morphology which were considered to be significant or
related to trichlorfon in the diet (Spicer and Payne, 1971).
Observations in man
Over 6000 people, most in South Africa and South America, have been
treated over the past few years for various intestinal and body
parasites (reviewed by Wegner, 1970). The dosages varied up to 70
mg/kg/day for periods up to 12 days. The dose of 7.5 mg/kg given 2-4
times at two week intervals was believed to be the best level as the
symptoms observed were less severe. Symptoms include cholinesterase
depression, weakness, nausea, diarrhoea and abdominal pain. Higher
doses (24 mg/kg) gave more severe symptoms including tachycardia,
salivation, cholic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative and spontaneous recovery in
all cases was rapid. In a few cases, an indication was given that
spermatogenesis (size and shape of sperm) might be affected in humans.
In all cases for treatment of parasites cholinesterase depression was
evident.
Namba (1971) reviewing the human data alluded to the observation that
three persons exposed to trichlorfon showed signs of delayed
neurotoxicity.
Various studies on humans have shown two effects: (1) cholinesterase
depression in all cases which was usually recovered within 30 days of
the cessation of treatment, and (2) a possible effect on
spermatogenesis (Wegner, 1970; Hanna et al., 1966; Lebrun and Cerf,
1960) which included reduced sperm count, seminal fluid volume and a
decreased motility and viability of the cells in a very limited number
of cases. Other studies showing no adverse effects include: Davis and
Bailey, 1969; Abdalla et al., 1965; Abdel-Aal et al., 1970; Beheyet,
1961. The dose of 7.5 mg/kg given once every two weeks appears to be
the best available. There is no human no-effect level recorded.
Comments
Trichlorfon appears to be rapidly absorbed, distributed, metabolized
and excreted in animals. The conversion of trichlorfon to dichlorvos
occurs both in plants and mammals but to only a very minor extent.
Several long-term studies in rats and dogs are available. In two
long-term studies in the rat evidence of an increased frequency and/or
onset of tumour formation (particularly mammary tumours) appeared to
be dose dependent. A third study in rats did not confirm these
observations. An incidence of cystic atrophic ovaries and reduced
spermatogenesis was also observed in two studies and not confirmed in
the third study, in a three generation rat reproduction study or in a
dominant lethal test. None of the experiments individually considered
was by itself indicative of a carcinogenic effect, however the
cumulative evidence derived from all the experiments considered
suggests that further investigations of the potential carcinogenicity
of trichlorfon are required.
In view of the inconclusive nature of the findings in long-term rat
studies, only a temporary acceptable daily intake was established for
trichlorfon.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet equivalent to 2.5 mg/kg body-weight per day
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichlorfon is an insecticide with a broad spectrum of activity. It is
chiefly effective against Lepidoptera (moths), Diptera (flies),
and Heteroptera (bugs). In crop protection, trichlorfon is used
mainly against insect pests in field crops and fruit crops.
Trichlorfon is also of great importance as a public health pesticide
and as an animal health product, and is used in medicine as an
anthelmintic.
The amount of trichlorfon used in the different sectors of
application, expressed in percentages, is in the Western world as
follows:
45% in field crops (e,g. cereals, rice, maize, cotton, grassland,
tobacco)
35% in vegetable crops
10% in fruit crops (e.g. pome fruit, stone fruit, bush and cane
fruit, grapes, citrus fruit, olives)
10% for other uses (e.g. ornamental crops, hygiene, animal
health)
Approximately 25 formulations of trichlorfon are sold and registered
in 83 countries for the control of insect pests of crops and pests of
hygiene.
The following formulations are used in agriculture (crop protection):
50% emulsifiable concentrate, 50%. soluble powder, 80% soluble powder,
50% wettable powder, 2.5% granular, 5.0% granular, 5.0% dust.
Pre-harvest treatments
The recommended application rates and concentrations (in terms of
active ingredient) for pre-harvest treatments of the major crops are
as follows:
Bananas 400-600 g/ha
Beets 150-1000 g/ha, or
0.075-0.12% spray
Cereals (excl. corn and rice) 800-1200 g/ha
Citrus fruits 0.12% spray
Coffee 450-550 g/ha, or
0.075-0.12% spray
Corn 400-1500 g/ha
Cotton 400-1500 g/ha
Currants, gooseberries 0.075-0.12% spray
Deciduous fruits (apples, pears,
cherries, peaches, etc.) 0.075-0.12% spray
Grapes 0.075-0.12% spray
Grassland and forage crops 500-2000 g/ha
Oil palms 450-1600 g/ha,
or 0.08-0.2% spray
Olives 0.075-0.12% spray
Potatoes 600-1000 g/ha
Rice 800-1200 g/ha
Sugar cane 1200-1600 g/ha, or
0.075-0.2% spray
Tea 600-1200 g/ha
Tobacco 600-1200 g/ha
Vegetables 150-1200 g/ha, or
0.075-0.12% spray
For controlling soil-inhabiting cutworms use is made of baits which
are prepared by mixing 100 g active ingredient (soluble powder), 10 kg
bran, 500 g sugar in 10 litres of water. The crumbly mess is used to
treat 1/4-1/2 ha.
Bait sprays for controlling fruit flies consist of 0.08 or 0.32%
active ingredient and 0.25 or 0.5 protein hydrolyzate, respectively.
Post-harvest treatments
No recommendations.
Animal treatments
Trichlorfon is used as an animal health product for the control of
endoparasites and ectoparasites in/on cattle, sheep, goats, pigs,
horses, poultry, dogs, cats and fish. Following formulations are used:
97% soluble powder, 90% soluble powder, 80% soluble powder, 50%
soluble powder, 6% suspension, 11% solution, 5% injectable solution
tablets.
Methods of application are as follows: wash or spray treatment, dip
treatment, spot-on/pour-on treatment, injection, drench treatment,
feed mix, pond or immersion treatment.
Animal health products based on trichlorfon are registered and sold in
77 countries.
Spectrum of activity of trichlorfon in animal treatments is given in
Table 1.
Trichlorfon is normally applied to the animals at the rate of 25-75
mg/kg body-weight by various methods.
The most common method of application is as a wash or spray treatment
for the control of sucking lice, flies and biting lice. For this
purpose, the whole of the animal body is washed or sprayed with a
0.13% solution of Neguvon powder (repeat treatment after five days).
For the control of warble fly maggots on cattle and Sarcoptes mites
on pigs, a 2% solution is recommended (repeat treatment after 5-7
days).
For the external control of Stephanofilaria in cattle and Habronema
spp. in horses, use must be made of a 10% solution applied to the
affected areas.
The 11% ready-for-use Neguvon solution is used for the control of
warble fly maggots in cattle, a single application being made by the
spot-on method. An applicator is supplied with the solution.
Some of the trichlorfon enters through the skin into the blood system.
As a result, the effect is enhanced from inside the animal body. The
systemic action of trichlorfon against warble maggots which wander
through the body of the host animal until they eventually become
located beneath the skin of the animal's back, is also produced as a
result of the compound penetrating through the skin and entering the
blood system.
The success of an external treatment can be further increased by a
simultaneous internal application.
Internal treatment for the control of endoparasites is made as
follows:
TABLE 1. SPECTRUM OF ACTIVITY OF TRICHLORFON IN ANIMAL TREATMENTS
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
External application for control of
ectoparasites:
Biting lice X X X X X
Sucking lice/fleas X X X X X
Sarcoptes mites X X X X
Red avian mite/scaly leg mite X
Flies/sheep keds X X X X X
Warble fly maggots/larvae of
Dermatobia hominis X
Internal application for control of
ectoparasites:
Sarcoptes mites X
External application for control of
endoparasites on domestic animals:
Habronema spp. (summer sores) X
Stephanofilaria in cattle X
Internal application for control of
endoparasites:
Haemonchus spp., Mecistocirrus X X
Oesophagostomum spp. X X
Neoascaris vitulorum X
Bunostomum spp./Ostertagia spp./Cooperia
spp./Trichostrongylus spp. X X
TABLE 1. (Cont'd.)
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
Oxyguris equi X
Stephanofilaria X
Larvae of sheep keds (Oestrus ovis) X
Ascarids X X
Trichuris/Hyostrongylus X
Gastrophilus spp./Habronema spp. X
External application for control of
ectoparasites in fish:
Argulus/Ergasilus/Lernea/Dactylogyrus/
Gyrodactylus/Trichodina/different
fish leeches X
By means of a bottle or drenching gun. For this purpose, it is
recommended to use a 10% solution, the dose depending upon the
body-weight of the animal to be treated. The treatment should be
repeated after 2-3 months, or after an interval of three weeks if
infestation is severe.
In the feed (dry or liquid), mixture with Neguvon powder.
After oral application, trichlorfon is rapidly resorbed and is
translocated in the blood stream to the site at which it is required
to act. Degradation and excretion take place within a few hours.
The 50% Neguvon injectable formulation is a ready-for-use solution for
i.m. or s.c. injection, and is used for the control of Haemonchus
spp., Oesophagostomum spp., and Dermatobia hominis in cattle in
many countries of the Middle and Far East, Africa and Latin America.
For some time past, trichlorfon has also been in use as a pond
treatment and brief dip treatment for the control of ectoparasites in
fish, and has proved to be most successful. The required dose of the
80% powder formulation is 2.5 kg per ha of carp and eel ponds which
have a depth of 50 cm, the dose being raised to 5 kg per ha in 100 cm
deep ponds; the dosage rates needed for the treatment of trout ponds
are only half as large.
The dosage rate for the brief dip treatment for the control of
Argulus, Dactylogyrus and Gyrodactylus in carps is 2.5 kg per
100 litres of water, the duration of the dip treatment being five to
10 minutes.
In South Africa, trichlorfon is marketed also as a dog shampoo for the
control of fleas and ticks in dogs and cats, and as a powder
formulation for the control of fleas in dogs and cats.
Other uses
In hygiene trichlorfon is used for the control of flies in stables as
well as against many other pest species. Baits are used in most
instances.
Further, trichlorfon is used on ornamentals, tree nurseries; and
forestry.
Residues resulting from supervised trials
Trials on crops
The residue data obtained following application of trichlorfon to
fruit, vegetables and field crops are presented, in extracts, in Table
2. These data are from papers published in the literature as well as
from unpublished reports of Chemagro Corporation and Farbenfabriken
Bayer AG which have been compiled and submitted to the Meeting by
Bayer (1971). The residue values were determined initially by
enzymatic procedure (Delta pH method). and in recent years by gas
chromatography (electron-capture detector, phosphorus detector,
microcoulometer). Comparative analyses showed good agreement of these
methods.
Following application to green plant material, a half-life of about
1-2 days was obtained for trichlorfon, as shown by residue studies on
cotton leaves, grass, cabbage, clover, alfalfa and lettuce. Crops
treated 2-4 weeks before harvest are practically free of residues at
harvest time (maize, soybeans, rape, flax),
Following application to bananas, oranges and ground nuts, the bulk of
the trichlorfon residue is contained in the peel and shells,
respectively. Within a few days after the application only slight
residues are to be found in the pulp and nuts, respectively.
Trials on animals
On cattle
The studies of organs showed that following peroral application of
100 mg of active ingredient per kg, up to 10 ppm of active ingredient
is present in steak two hours after the application, and that this
amount decreases to a level of less than 0.1 ppm after 4-6 hours
(Behrenz, 1959). Following back-line and spray applications, organs
(liver, kidneys, brain, heart) and steak are practically free of
residues; in omental fat, on the other hand, maximum active ingredient
concentrations of 9.2 and 1.9 ppm were found one and seven days,
respectively, after the application (Adkins, 1966).
Following administration of trichlorfon to cattle by different routes,
residue studies were carried out by Chemagro Corporation, Kansas City,
United States of America, on practically all organs used for human
diet (meat, fat, liver, heart, kidneys and brain); the determinations
were made by sensitive gas chromatographic methods (limit of
determination 0.01-0.1 ppm; Chemagro Report 14.393; 24.808).
TABLE 2. RESIDUES RESULTING FROM SUPERVISED TRIALS
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Alfalfa 0.6 - 1.1 7 0.04 - 2.0
Alfalfa, seed hulls 1.7 8 0.2 - 1.6
" chaff 1.7 8 0.2 - 0.6
Apples (0.1 - 0.2%) 8 - 30 n.d. - 0.1
Artichokes, spray 1.1 0 - 12 n.d.
" dust 2.8 7 - 14 n.d.
Bananas, pulp 0.4 - 0.8 0 n.d. - 0.3
" peel 0.4 - 0.8 0 n.d. - 2.0
Barley 1.7 14 - 25 n.d.
Blackeyed, beans 1.7 14 n.d.
" vines 1.7 14 n.d. - 0.9
Brussel sprouts, spray 1.7 14 - 19 n.d.
" " dust 2.8 14 0.2
Cabbage 0.6 - 1.7 15 n.d. - 0.05
Cauliflower 1.7 19 - 21 n.d. - 0.15
Celery, spray 0.6 - 1.1 5 - 10 0.05 - 0.19
" dust 0.6 - 1.1 5 - 10 0.05 - 0.16
Cherries 0.3 - 0.5 3 - 8 0.001 - 0.12
Clover 1.1 7 - 8;14 <0.1 - 9.6;
<0.1 - 1.4
Clover, seed head 1.7 14 - 15 <0.1 - 0.7
" chaff 1.7 14 - 15 <0.1 - 0.6
Corn, kernel 0.8 - 1.7 30 - 144 n.d.
" cobs 0.8 - 1.7 30 - 144 n.d.
" husk 1.0 - 1.7 30 - 144 n.d.
" fodder, forage 1.0 - 1.7 30 - 144 n.d.
Cotton, seed 1.1 - 1.7 7 - 10 <0.01 - 0.05
" foliage 2.2 - 2.5 7 n.d. - 0.4
Cowpeas 1.7 14 n.d.
Cowpeas, vines 1.7 14 n.d. - 0.9
Grass, spray 1.1 - 2.2 12 2 - 5
" granular 1.1 - 2.8 12 <1 - 2
Garden beets 1.7 14 - 28 n.d.
Green beans 1.7 14 n.d.
Kale 0.6 - 1.7 6 - 10 n.d. - 0.3
Lettuce, leaf
summer appl. 1.1 - 1.7 7 0.1 - 0.2
winter appl. 1.1 - 1.7 7;14 6.0;3.9
Lettuce, head
summer appl. 1.1 - 1.7 7 <0.1 - 0.24
winter appl. 1.1 - 1.7 7;14 11.1;3.7
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Lima beans 1.7 9 - 15 n.d.
" vines 1.7 9 - 15 n.d. - 0.6
Lin seed 1.7 14 - 27 n.d.
Mustard 1.7 14/15 n.d.
Oats, grain 1.1 - 1.7 13/14 n.d. - 1.4
Oranges, peel, washed 1.1 - 1.7 7 0.1 - 0.21
" pulp 1.1 - 1.7 7 <0.01
Peaches (0.1) 7 - 14 <0.85
Peanuts, spray
" nuts 1.7 - 2.5 0 - 8 n.d. - 0.1
" shells 1.7 - 2.5 0 - 8 0.1 - 2.65
" vines 1.7 - 2.5 3 - 8 0.2 - 0.4
Peanuts, bait
" nuts 1.7 - 2.5 0 - 3 <0.01 - 0.16
" shells 1.7 - 2.5 0 - 3 <0.03 - 0.56
" vines 1.7 - 2.5 0 - 3 <0.07 - 2.9
Peppers, spray 1.1 - 1.7 14/15 n.d. - 0.3
" dust 1.2 14 0.4 - 1.1
Pumpkins 1.1 - 1.7 13/15 n.d.
Rape, seed 0.8 - 1.7 23 - 32 n.d. - 0.1
" meal 1.1 - 1.7 23 n.d.
" oil 1.1 - 1.7 23 n.d.
Safflower 2.2 27;33;43 0.6;<0.1;
<0.1
Soybeans, green beans, pods 0.6 0 0.1 - 6.0
" vines 0.6 0 0.5 - 8.8
" dry beans 0.6 35 - 53 <0.01
" dry vines 0.6 35 - 53 <0.08
(1 x 0.45)
Spinach 0.8 - 1.1 14/15 <0.1 - 1.6
Strawberries 0.3 - 1.1 3/4 <0.07
Sugar beets, roots 0.3 - 1.1 29 - 34 <0.01
" " tops 0.3 - 1.1 29 - 34 <0.05
Sweet corn, spray
" " husk 1.7 - 2.2 7 - 14 n.d. - 0.6
" " kernel 1.7 - 2.2 7 - 14 n.d.
" " cob 1.7 - 2.2 7 - 14 n.d. - 0.1
" " fodder 1.7 - 2.2 7 - 14 0.2 - 1.1
Sweet corn, dust
" " husk 2.0 15 2.7 - 9.1
" " kernel, cob 2.0 15 n.d.
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Sweet corn, granular
" " husk 1.1 - 1.7 14 - 21 n.d. - 8.4
" " kernel 1.1 - 1.7 14 - 21 n.d. - 0.1
" " cob 1.1 - 1.7 14 - 21 n.d. - 0.2
" " fodder 1.1 - 1.7 14 - 21 n.d. - 3.0
Table beets, roots 1.7 7 - 13 n.d. - 0.2
" " tops 1.7 7 - 13 0.2 - 2.5
Tea 1.4 - 1.8 21 <1
Tobacco, green
" spray 1.1 - 1.7 14/15 n.d. - 1.3
" dust 1.0 - 2.5 10/14 n.d.
Tobacco, cured
" spray 1.1 - 1.7 0 - 21 n.d
" dust 1.0 - 2.5 0 - 21 n.d
Tomatoes 0.8 - 1.7 7 n.d. - 0.5
Turnips, roots 10 - 15 n.d. - 0.09
Wheat, grain 1.3 - 1.7 14 - 39 n.d. - 0.25
" straw 1 25 - 39 n.d. - 4.2
" flour 1 25 - 39 <0.05
" bread 1 25 - 39 <0.05
Following peroral uptake of the active ingredient (12.5 and 20 ppm in
feed), no trichlorfon residues were detected (<0.1 ppm) in any of the
examined tissues and organs (brain, heart, kidney, steak, fat) after a
four week feeding period (Chemagro Report 17.991; 18.884). Following
external application of the 9 and 16% formulation - single back-line
application of 25, 50 or 100 mg of active ingredient per kg
(examination of tissues 21 days after application) and four week mist
spray application of 1.1 g of active ingredient per animal and day
(examination of tissues after the final application) - no residues
were detected in any of the tissues or organs (Chemagro Report 14.412;
18.495; 26.117).
Only slight proportions of the parent compound or its metabolites are
to be found in milk, as shown by a number of studies using
P32-labelled and unlabelled trichlorfon.
Following peroral application of 25 mg of active ingredient per kg,
less than 0.2% of the radioactivity representing the total dose
administered was secreted in the milk at the end of 144 hours; of
this, about 10% was unchanged active ingredient and about 23% behaved
like inorganic phosphorus (Robbins et al., 1956). Following similar
application of 80 mg of active ingredient per kg, the maximum residue
at eight hours was 2.5 ppm in the milk samples and at 22 hours
0.1 ppm; in the following milk samples, the residue levels were below
the limit of determination of 0.05 ppm (Behrenz, 1961). Following
spray application of five litres of a 2% active ingredient solution
and back-line was application of one litre of a 2% solution, the milk
samples at eight hours were found to contain 0.05-0.25 ppm of active
ingredient and in the later milk samples no residues were detected
(Behrenz, 1961).
Studies by Ackermann et al. (1968) showed that following backline wash
application of one litre of a 2% trichlorfon solution, the maximum
residue in the first milk sample at eight hours was 0.2 ppm; the
residues in the third milk sample at 32 hours were below 0.01 ppm.
Analyses of these milk samples for dichlorvos showed a maximum content
of 0.03 ppm in the first sample and 0.01 ppm in the third sample.
Following a single dermal treatment with 6% suspension, equivalent to
36 mg of active ingredient per kg, 0.1-0.2 ppm of trichlorfon was
found to be present within the first eight hours (Leahy, 1964).
Following application of 1.15 litres of a 2% trichlorfon solution (or
0.57 litres of a 4% solution) as a dermal wash per animal, the level
of active ingredient present in the milk did not at any time exceed
0.4 ppm; milk samples taken later than six hours after the application
contained about 0.1 ppm of active ingredient (Wickham and Flanagan,
1962).
Following pour-on application (of 100 or 300 ml of a 5.7% solution
(P32-labelled)), the active ingredient concentration in the milk
reached a peak of 0.13 or 0.47 ppm after 14-18 hours; after four days,
the content was less than 0.05 ppm, and after seven days it was less
than 0.01 ppm (Schwarz and Dedek, 1965a).
Following intravenous and intramuscular injection of 20 and 25 mg of
P32-labelled active ingredient, radioactive substances were found in
the milk only at six and 10 hours after the application; dichlorvos
was not formed in these experiments (Kühnert et al., 1963).
Chemagro Corporation, Kansas City, United States of America, carried
out residue studies by gas chromatographic methods following
application of the active ingredient to dairy cows by different
routes. The lower limit of determination was 0.003-0.01 ppm (Chemagro
Report 16.460; 24.808).
Following peroral uptake of the compound in the feed (12.5, 20, 50,
150 and 325 ppm) for a period of one or four weeks, no trichlorfon
residues were found to be present in the milk at the end of the
feeding period (Chemagro Report 17.440; 18.321; 29.234).
Following external application of the active ingredient as a single
pour-on treatment (at 3.7 g/100 kg) or as a single spray treatment
(at 28 g/animal) or as a four week mist spray treatment (1.1 g per
animal per day), active ingredient residues of no more than 0.04 and
0.02 ppm were found in the milk samples taken on the first and second
day after the application only for those animals which received the
pour-on treatment (Chemagro Report 17.970; 18.322; 18.521; 21.289).
The majority of these milk samples were analysed for residues of
trichloroethanol, a possible metabolite of trichlorfon; however, this
compound could not be found in any of the examined samples.
Following a spray treatment of stable walls at a rate of 1 g of active
ingredient per square metre, no trichlorfon residues were found in the
milk of the cows kept in the treated stable (Chemagro Report 16.538).
On pigs
For the use of trichlorfon for the control of ectoparasites and
endoparasites in pigs, the degradation and excretion of the active
ingredient was studied following subcutaneous injection of 25 mg of
P32-labelled trichlorfon per kg body-weight. The maximum active
ingredient concentration in blood was reached after 15-60 minutes
(10-11 ppm) and in the intestinal contents after 20-150 minutes
(4-5 ppm). After 5-7 hours, the blood and intestinal contents still
contained about 1 ppm of active ingredient. Dichlorvos was no longer
detectable in blood 3.5 hours after the application. The trichlorfon
concentration in meat was always somewhat lower than in the blood; a
concentration of 5 ppm was found in the meat two hours after the
injection. The trichlorfon concentration in the meat decreased by a
power of 10 in each 6-7 hours (Schwarz and Dedek, 1965b, 1966).
Chemagro Corporation carried out studies using gas chromatographic
methods (limit of determination 0.01-0.1 ppm residue; Chemagro Report
14.393; 24.808). After addition of 1500 ppm of active ingredient to
the drinking water of pigs (the ingested amount was equivalent to a
single dose of 125 mg of active ingredient per kg body-weight),
0.02-0.07 ppm of active ingredient was found in the liver and
0.02-0.05 ppm was found in loin steak four days after the application;
brain, heart, kidneys and fat samples were free of residues. None of
the organs contained any trichlorfon residues after seven days
(Chemagro Report 27.533). Dichlorvos was not detectable in any of the
examined samples four days after the trichlorfon application (Chemagro
Report 27.561).
No trichlorfon residues were detectable in any of the examined organs
and tissues 14 days after application of the compound in the diet at a
dose level equivalent to 100 mg/kg body-weight (Chemagro Report
18.678).
On sheep
Following peroral application of 120 mg of trichlorfon per kg
body-weight, exploratory residue determinations were carried out in
organs by means of a fly larva test. The residues dropped below the
limit of determination of 0.1 ppm within 4-6 hours after the
application (Behrenz, 1959). Following percutaneous application of
50 mg of the compound per kg body-weight, the concentration in the
blood reached a level of 1 ppm at which it remained for only a brief
period. Dichlorvos was detectable only in slight amounts (Dedek and
Schwarz, 1970).
Fate of residues
General comments
The distribution of trichlorfon residues in organisms is characterized
by its hydrophilic properties.
The decomposition of the molecule is brought about both by splitting
the P-C bond and by hydrolysis of the P-OCH3 bonds (Hassan and
Zayed, 1965). Furthermore, trichlorfon can be converted to the more
toxic dichlorvos, O,O-dimethyl-(2,2-dichlorovinyl)-phosphonate.
In animals
On account of its hydrophilic properties, trichlorfon is rapidly
absorbed by the organism, broken down and excreted in the urine.
Studies with P32-labelled compound on cattle showed that following
peroral application of dosages ranging from 25-100 mg of active
ingredient per kg body-weight, 66% of the P32 activity is excreted
in the urine within 12 hours; of this amount of excreted
radioactivity, 17% is dimethyl phosphate, 76% is accounted for by a
metabolite of unknown structure, and 0.26% by unchanged active
ingredient. After 45 hours, only 0.28% of the P32 activity
representing the total dose administered is found to be present in the
urine (Bolle, 1956; Robbins et al., 1956).
The degradation of the compound in blood proceeds at a very fast rate.
In cattle, 12 hours after peroral application of 40 mg of active
ingredient per kg, 0.03%, of the applied P32 activity is present in
the blood, and 0.003% after 45 hours (Bolle, 1956). The peak of
radioactivity is attained after two hours (Robbins et al., 1956).
Following intravenous application, the parent compound is broken down
by more than 95% after one hour; dichlorvos is found to be present in
a very low concentration only within the first four minutes (Kühnert
et al., 1963).
For further details, see for Biochemical aspects and residues
resulting from supervised trials: Trials on animals.
On poultry and eggs
Gas chromatographic determinations showed that no residues were
present in giblets, muscle and fat (limit of determination of 0.1 ppm)
or in the eggs (limit of determination of 0.003 ppm) of poultry which
had been maintained for four weeks on a diet containing 2.5 and 5 ppm
of the compound (Chemagro Report 20.945; 21.502).
In plants
Studies of the metabolism of trichlorfon in plants were carried out on
cotton plants.
The identified metabolites included dimethyl phosphate which accounted
for up to 70% for the applied trichlorfon dose; only small amounts of
monomethyl phosphate, O-demethyl trichlorfon, O-demethyl dichlorvos
and dichlorvos were formed (Hassan et al., 1966; Bull and Ridgway,
1969). Besides these metabolites, Bull and Ridgway found an
unidentified metabolite which accounted for a large percentage of the
applied dose of parent compound; this phosphorus-containing unknown is
split by ß-glucosidase but not by ß-glucuronidase.
Chloral and trichloroethanol are stated to be possible degradation
products (Arthur and Casida, 1957). However, these products could not
be detected in routine residue studies (Chemagro Corporation,
unpublished). Earlier studies also revealed that chloral and
trichloroethanol are metabolized in plants to form
B-2,2,2-trichloroethanol-gentiobioside (Miller, 1941).
In micro-organism cultures
Studies on Penicillium notatum, Fusarium sp. and Aspergillus niger
showed that approximately half of the applied parent compound is
transformed within 10 days. The principal metabolite found was
O-demethyl trichlorfon. A second degradation product formed is
probably 2,2,2-trichloro-1-hydroxyethyl-phosphonate (Zayed et al.,
1965).
In soil
Trichlorfon is not used for the control of soil pests. Studies
conducted by Chemagro Corporation have shown, however, that after
addition of 10 ppm of trichlorfon to soil, the residue level drops
below the limit of determination within 15-112 days depending upon the
soil type. Trichlorfon has a very low stability in soil; residues of
trichlorfon in runoff water from sandy loam, silt loam and high
organic silt loam soils showed a relatively low tendency to move
(Chemagro Report 28.937). After addition of 0.25 ppm of trichlorfon to
pond water, no residues are to be found in the mud (Chemagro Report
21.313).
In food processing
The published data on trichlorfon residues have generally been
obtained in studies of non-processed plant and animal products. When
these products are processed for the human diet, the trichlorfon
residue level is considerably reduced by such processing procedures as
boiling and sterilization.
In spinach, the residues which have an original level of 2.0 ppm in
the treated plants are reduced by bleaching (five minutes at 100°C) to
a concentration of 0.03 ppm and by further sterilization (100 minutes
at 115°C) to a level of 0.01 ppm. In dwarf beans, the residue level is
reduced from 1.22 ppm to 0.06 ppm and 0.05 ppm, respectively. In peas,
which had an original residue level of 0.21 ppm, no more residues were
detectable after bleaching and sterilization (Dormale et al., 1959).
Tomatoes were treated with 10 ppm of the compound and then canned
according to standard commercial procedure. The heat treatment
destroyed 80% of the compound (Chemagro Report 9012). Safflower seed
containing 0.9 ppm of the compound was processed into meal, crude oil
and refined oil. None of the three processed products contained
detectable residues (Chemagro Report 11.255).
Meat of sheep which ware slaughtered one hour after receiving a
peroral application of 120 mg/kg contained 1-10 ppm of the compound;
after the meat had been boiled for one hour, it contained no
detectable residue (Behrenz, 1959).
In beef which was immersed in a trichlorfon solution to prevent fly
maggot infestation, the residue level of the compound was reduced by
thorough washing from 0.77 ppm to 0.58 ppm. After one hour's boiling,
the residue level in the meat and in the broth was less than 0.1 ppm
(Börger and Maier-Bode, 1967).
Evidence of residues in food in commerce or at consumption
No data were submitted for consideration.
Methods of residue analysis
Many colorimetric, thin-layer chromatographic and gas chromatographic
methods for the determination of trichlorfon residues are described in
the literature. Some of the methods permit simultaneous determination
of trichlorfon and its possible metabolites, e.g. trichloroethanol and
dichlorvos. However, formation of trichloroethanol was not established
in any of the residue studies undertaken.
Trichlorfon can be extracted from plant material with chloroform
(Zadrozinska, 1966), acetone/hexane (Anderson et al., 1966), ethyl
acetate (Cernà, 1963; Watts et al., 1969) or diluted acetic acid
(Sissons and Telling, 1970). For clean-up, the extract residue is
transferred to water; after separating the plant constituents with
petroleum ether or heptane, the parent compound is extracted with
chloroform or diethyl ether (Zadrozinska, 1966; Anderson et al.,
1966). For determination of the compound by the cholinesterase
inhibition technique, extraction with water will suffice (Reynolds
et al., 1960; Chemagro Report 2412; 3581). Column chromatographic
clean-up of the plant extract is possible on charcoal (Watts et al.,
1969) or on aluminium oxide (Sissons and Telling, 1970). If plant
material with a high water content is involved, the compound can be
separated from the plant constituents by dialysis of the macerate in
diluted sulfuric acid, and extracted from the diffusate with diethyl
ether (Anderson et al., 1966; Chemagro Report 8839; 21811).
Trichlorfon can be extracted from animal tissues with acetonitrile
(Beck and Sherman, 1968; Anderson et al., 1966; Chemagro Report
14393), chloroform (Ackermann et al., 1969; Chemagro Report 24808) or
acetone (Chemagro Report 24808). For further clean-up, the extract
residue is transferred to water, fat portions are separated with
heptane, and the parent compound is extracted from the aqueous phase
with diethyl ether (Chemagro Report 14393; Anderson et al., 1966),
In milk, trichlorfon (after removal of fat by centrifuging and
separation of protein by precipitation) is determined either directly
by bioassay (Wickham and Flanagan, 1962) or (after extraction with
chloroform) by thin-layer chromatography, together with possibly
formed dichlorvos (Ackermann et al., 1968). For gas chromatographic
determination of trichlorfon, the milk is extracted with acetone and
benzene. In this method, trichloroethanol is co-determined as a
possible metabolite (Chemagro Report 16460).
Enzymatic determination
Giang and Hall (1951) developed a method for the enzymatic
determination of organic phosphorus insecticides (Delta pH method).
For the determination of trichlorfon residues, the parent compound is
converted to dichlorvos which is a strong cholinesterase inhibitor
(Reynolds et al., 1960; Chemagro Report 2412; 3581), The lower limit
of determination is approximately 0.01 ppm trichlorfon. The method is
unspecific.
Bioassays
Bioassays with mosquito larvae (Aedes aegypti) have been employed
for determining residues of trichlorfon in milk (Behrenz, 1961;
Wickham and Flanagan, 1962). Börger and Maier-Bode (1967) used
Daphnia magna as the test species for determining residues of
trichlorfon in meat. The limit of determination is approximately 0.05
ppm. The method is unspecific.
Thin-layer chromatography
Trichlorfon residues in plants and in animal tissues can be determined
by thin-layer chromatography on silica gel or aluminium oxide plates
sprayed with silver nitrate. The lower limit of determination is
0.25-0.5 ppm of trichlorfon in apples (Zadrozinska, 1966) and
0.2-0.5 µg in animal tissue extracts (Beck and Sherman, 1968). Smaller
amounts of trichlorfon and dichlorvos can be determined by the
cholinesterase inhibition technique (Ackermann et al., 1968, 1969).
Agar-diffusion method
The agar-diffusion method described by Sandi (1962) was modified for
routine determination of trichlorfon. A residue level of 0.1 ppm can
be determined in milk; however, it is not possible to separate
trichlorfon from dichlorvos (Ackermann et al., 1968).
Colorimetry
A colorimetric micro method for determining trichlorfon residues is
based on the determination of chloroform (which is separated from the
trichlorfon by pyrolysis at 550°C) with pyridine and sodium hydroxide
(Fujiwara reaction). This method was used for determining trichlorfon
residues in olive oil (Allessandrini and Lanforti, 1957) and in fruit
and lettuce (Cernà, 1963). The limit of determination is approximately
1 ppm in olive oil and 10 µg in plant material.
Trichlorfon residues can be determined colorimetrically also by a
total phosphorus procedure in plant material (Sissons and Telling,
1970) and in milk (Leahy, 1964); the lower limit of determination is
0.1-0.2 ppm.
Gas chromatography
Trichlorfon residues can be determined with a high degree of
sensitivity and specificity by gas chromatography. The intact molecule
is not determined by this method but instead the cleavage products
chloral or dimethyl phosphite which form upon pyrolytic cleavage of
trichlorfon in the injection port of the gas chromatograph. Chloral is
detected with the electron-capture detector (Anderson et al., 1966);
trichloroethanol can be simultaneously determined as a theoretically
possible metabolite. Chloral can also be determined with the Dohmann
microcoulometric gas chromatograph (Chemagro Report 8839). The
clean-up of extracts is simpler when trichlorfon is detected with a
phosphorus-specific detector, e.g. a modified flame ionization
detector (thermionic detector) (Chemagro Report 21811, 24808). The
limits of determination of these gas chromatographic methods depend
upon the material being analysed and the clean-up procedure used.
However, they are usually very low, e.g. 0.01-0.1 ppm with an
electron-capture detector and 0.003-0.06 ppm with the phosphorus
detector.
Studies on 30 different phosphorus-containing pesticides registered
for use in different crops show that only phosphamidon interferes with
the gas chromatographic determination of trichlorfon with the
phosphorus detector (Chemagro Report 21811, 27471, 29411). By using a
different column, this interference can be avoided (Chemagro Report
26335).
Parallel residue analyses of treated plant material showed agreement
of residue values obtained by the cholinesterase inhibition technique
and by gas chromatography. Determination by the cholinesterase
inhibition technique produces slightly higher residue values, which is
probably due to the presence of slight traces of dichlorvos (Chemagro
Report 9733).
For routine determination of trichlorfon residues, gas chromatography
is the most suitable method. The compound can be detected with a
microcoulometer, an electron-capture detector or a phosphorus-specific
detector, e.g. a thermionic or flame photometric detector.
Examples of national tolerances and safety intervals
Country Crop Tolerance Safety
ppm interval
days
Argentina Bananas 0.2 (provisional)
Bananas (peeled) 0
Australia General 2
Fruits, grains, vegetables 2.0
Austria General 14
Cucumbers, tomatoes,
peppers 4
Belgium General 10
Fruits, vegetables,
excl. potatoes 0.5
Brazil Vegetables 0.5 7
Fruits and field crops 0.5 10
Meat and milk 0.001
Bulgaria General 14
Canada Alfalfa 14
Rape 21
Corn 40
Sugar beets 14
Sugar beets, tops 28
Tobacco 3
Beans 14
Cabbage, cauliflower,
Brussels sprouts 21
Country Crop Tolerance Safety
ppm interval
days
Carrots, rutabagas,
salsify, turnips 28
Collards, kale, lettuce,
spinach 28
Peppers 21
Table beets 28
Tomatoes 21
Artichokes, bananas, NR
beans, beef cattle, NR
beets, Brussels NR
sprouts, cabbage, NR
carrots, cauliflower, NR
collards, corn, kale, NR
lettuce, peppers, rape NR
seed, rutabagas, NR
salsify, spinach, NR
sugar beets, tomatoes, NR
turnips NR
Finland General 14
France General 7
German Democratic Fruits, root vegetables,
Republic leafy vegetables,
cabbages, legumes,
fruit-producing
vegetables (tomatoes,
cucumbers) 1.0
Meat, fish, animal and
vegetable fats, eggs,
milk, baby foods 0
Field crops, fruits 10
Cherries 5
Vegetables 7
Special crops 14
Crops used for the
production of baby foods,
drugs and health foods 30
German Federal Fruits, field crops,
Republic incl. fodder crops 10
Vegetables 7
Application under glass:
general 10
Leafy vegetables,
fruit-producing vegetables,
root vegetables,
Country Crop Tolerance Safety
ppm interval
days
legumes, fruits incl.
grapes 0.5
Great Britain General 2
Hungary Animal products 0
Israel Fruits incl. grapes,
clover, alfalfa,
sub-tropical trees, sugar
beets, fodder crops 7
Maize 30
Tomatoes, cucumbers,
cucurbits, cabbage,
radish, lettuce,
spinach, beans, other
vegetables 10
Peppers, eggplants,
strawberries, cauliflower,
artichokes 14
Morocco General 7
Olives 30
Netherlands General 0.5
General, field-grown 10
Spinach, field-grown 4
General (under glass
from 1 March - 1 Nov.) 17
New Zealand Tomatoes (canned) 1
Other crops 14
Poland Fruits, legumes (not
vegetables), root
crops and other field
crops 14
Vegetables 10
Portugal General 7
Industrial tomatoes 4
South Africa Maize, wheat, citrus
fruits, apples, pears,
apricots, peaches,
sub-tropical crops 10
Cucurbits 7
Alfalfa 2
Tomatoes 3
General 2.0
Soviet Union General 1.0
Spain General 10
Country Crop Tolerance Safety
ppm interval
days
Sweden General 14
Switzerland General 21
Vegetables 14
Beets 42
Vegetables, legumes,
fruits incl. grapes 0.5
United States of See USDA Summary of
America Registered Agricultural
Pesticide Chemical Uses
Yugoslavia General 0.5
Fruits, vegetables,
field crops 14
Appraisal
Trichlorfon is an organophosphorus insecticide which is especially
used on crops against a variety of insects (moths, flies, bugs, etc.).
It is also widely used against ecto- and endoparasites of animals, in
public health and as an anthelmintic in medicine.
It is used on a wide variety of field and pasture crops with
application rates of 150-2000 g/ha, normally 500-1200 g/ha, or as
0.075-0.2% sprays. As an animal health product, it is normally applied
at a rate of 25-75 mg/kg body-weight externally as wash or spray and
spot-on treatment and internally by mouth and by injection. In public
health it is used against flies and other pests commonly in the form
of baits. Other uses are on ornamentals, in tree nurseries, and in
forestry.
Residue data for evaluation are satisfactory.
The behaviour of trichlorfon is characterized by its hydrophilic
properties. Its decomposition is brought about by splitting the P-C
bond and by hydrolysis of the P-OCH3 bonds. In addition, in tissues
it can be converted in trace amounts to dichlorvos. Products of more
advanced degradation have also been found and characterized. In food
processing, trichlorfon residues are substantially disappearing.
As a result of use of trichlorfon, residues may occur in animal feed.
When the applications are made in accordance with good agricultural
practice the residues are considered to be no hazard to the animal
health and no detectable contamination of the foods derived from these
animals is to be expected.
There are a number of methods of residue analysis available. For
regulatory purposes highly specific and sensitive gas chromatographic
methods with detection limits of less than 0.01-0.1 ppm have been
developed.
Due to the insignificant magnitude of dichlorvos and to the low
toxicity of other degradation products, recommendations for tolerances
of trichlorfon residues are made in terms of the parent compound.
Since the direct application of trichlorfon to the domestic animals
may result, although for a short limited period of time, in residues
of varying magnitude in milk, meat, and fat, it is necessary, in
accordance with the local needs of animal health, to set limitations
of use, e.g. type of formulation, route of application, dosage,
condition of the animals, and safety interval from treatment to use of
animal products for human food. The recommendations for trichlorfon
residues in animal products are made, because the use of insecticides
is necessary in animal health, application of trichlorfon according to
good agricultural practice will cause no health hazards to animals,
and, by adjusting properly the conditions of use, milk, meat, organs,
and fat can be obtained from the treated animals practically free from
the trichlorfon residues. Additional safeguard of the consumer against
the residues is the degradation of trichlorfon in storage, processing
and cooking of the animal products before they are consumed.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The following recommendations are made for temporary tolerances:
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Cereals:
Barley 21 0.1
Maize 30 0.1
Wheat 30 0.2
Fruits:
Apples 14 0.1
Bananas, pulp 0 0.2
Cherries 7 0.1
Oranges, pulp 7 0.1
Peaches 14 0.2
Strawberries 4 0.1
(continued)
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Vegetables:
Artichokes 7 0.1
Blackeyed, beans 14 0.1
Brussels sprouts 14 0.2
Cabbage 14 0.1
Cauliflower 21 0.2
Cowpeas 14 0.1
Green beans 14 0.1
Kale 14 0.2
Lima beans 14 0.1
Mustard, leaf 14 0.1
Peppers 14 1
Pumpkins 14 0.1
Sweet corn, kernels and cobs 14 0.2
Tomatoes 14 0.1
Celery 14 0.2
Sugar beets 30 0.05
Beets 14 0.2
Turnips 14 0.1
Oil seeds:
Cotton seed 14 0.1
Flax seed 14 0.1
Lin seed 30 0.1
Safflower seed 45 0.1
Soybeans, dry 45 0.1
Nuts:
Peanuts, shelled 7 0.1
Animal products:
Meat, fat and by-products
of cattle and pigs 14 0.1
Whole milk 2 0.05
Further work or information
Required before 30 June 1975
1. A two-generation carcinogenicity study to elucidate the possible
increase in the incidence of tumours including those of the
mammary gland.
2. More information on residues on oats.
3. More information on residues on lettuce and spinach under various
conditions (including greenhouses).
Desirable
1. Elucidation of the effect on spermatogenesis.
2. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abdalla, A., Saif, M., Taha, A., Askmawy, H., Tawfik, J., Abdel
Fattah, F., Sabet, S. and Abdel-Mequid, M. (1965) Evaluation of an
organophosphorus compound Dipterex on the treatment of Bilharziasis.
J. Egypt Med. Assoc., 48: 262-273
Abdel-Aal, A. M. A., El Hawary, M. F. S., Kamel, H., Abdel-Kalek,
M. K. and El-Diwany, K. M. J. (1970) Egypt Med. Assoc., 53: 265-271
Ackermann, H., Engst, R. and Fechner, G. (1968) Methode zur getrennten
Bestimmung von Trichlorphon und Dichlorphos-Rückständen in Milch.
Ztschr. Lebensmittelunters. Forsch. 137: 303-308
Ackermann, H., Lexow, B. and Plewka, E. (1969) Nachweis und
Identifizierung von insecticiden Phosphor-,
Thiophosphor- Phosphon- und Carbaminsäureestern im Biologischen
Material. Arch. f. Toxicol., 24: 316-324
Adkins, T. R., jr. (1966) Residues in cattle tissues following
back-line and spray applications of Trichlorfon. J. Econ. Entomol.,
59: 1423-1425
Alessandrini, M. E. and Lanforti, G. F. (1957) Determinazione di
residui di Dipterex (O,O-dimetil-2,2,2,trichloro-1-idrossietil
fosfato) nell' olio di oliva. Rend. Ist. super. Sanita, 20: 093-1003
Anderson, R. J., Anderson, C. A. and Olson, T. J. (1966) A Gas-liquid
chromatographic method for the determination of Trichlorfon in plant
and animal tissues. J. Agr. Food Chem., 14: 508-512
Arant, F. S., Atkins, T. R. and Sowell, W. L. Toxicity of Bayer L13/59
to rabbits. Unpublished report
Arnold, D., Keplinger, M. L., Fancher, O. E. and Calandra, J. C.
(1971) Mutagenic study with Dylox in Albino mice. Unpublished report
by Industrial Biotest Laboratories submitted by Farbenfabriken Bayer
A.G.,
Arthur, B. W. and Casida, J. E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2,-trichloro-1-hydroxethyl phosphonate and its acetyl
and vinyl derivatives. J. Agr. Food Chem., 5: 186-192
Arthur, B. W. and Casida, J. E. (1958) Biological activity of several
O,O-dialkyl alpha-acyloxyethyl phosphonates. J. Econ. Entomol.,
6: 360-365
Bailey, C. C., jr. (1956) Evaluation of the dermal toxicity of
malathion chlorthion and Dipterex to dogs. Thesis, Clemson College,
Clemson, S.C.
Bayer, A.G., (1967) Farbenfabriken, Pflanzenschutz. (R)Dipterex
(Bayer 15922, L 13/59) Leverkusen E. 10-6117/22 155
Bayer, A.G., Farbenfabriken, Pflanzenschutz. Documentation on 1971
trichlorfon for FAO
Beck, J. and Sherman, M. (1968) Detection by thin-layer chromatography
of organophosphorus insecticides in acutely poisoned rats and
chickens. Acta pharmacol. et toxicol., 26: 35-40
Beheyet, P., Lebrun. A., Cerf, J., Dierickx, J. and DeGroote. V.
(1961) Etude do la toxicite pour homme d'un insecticide
organophosphore. Bull. Wld Hlth Org., 24: 465-473
Behrenz, W. (1959) Biologische Bestimmung des Wirkstoffgehaltes im
Fleisch von Schafen und Rindern zu verschiedenen Zeiten nach peroraler
Behandlung mit Neguvon. Arch. Lebensmittelhyg., 10: 64
Behrenz, W. (1961) Ueber die Auascheidung von Neguvon(R) in der
Milch nach einmaliger oraler und percutaner Anwendung des Präparates
bei Milchkühen. Vet. mod. Nachr., 133-145
Bolle, W. R. (1956) Neguvon, ein äusserlich und innerlich anwendbares
Insektizid, Larvizid und Acarizid. Vet. med. Nachr., 155-172
Borgmann, W. and Hunnold, G. A. (1955) Report on the results of a
toxicological examination of Dipterex (L13/59). Unpublished report
submitted by Farbenfabriken Bayer A.G.
Brodeur, J. and Dubois, K. P. (1963) Comparison of acute toxicity of
anticholinesterase insecticides to weanling and adult male rats. Proc.
Soc. Exp. Biol., 114: 509-11
Bull, D. L. and Ridgway, R. L. (1969) Metabolism of trichlorfon in
animal and plants. J. Agr. Food Chem., 17: 837-841
Börger, K. and Maier-Bode, H. (1967) Versuche zur Verhinderung des
Fliegenmadon-Befalles von Fleisch. Arch. f. Lebens-mittelhyg., 18:
38-42
Cerná, V. (1963) Kolorimetrische Bestimmung von Dipterex-Rückstünden
in Lebensmittein. Die Nahrung, 7: 60-66
Chemagro-Report (Chemagro Corporation, Kansas City, USA)
2412 Tentative method for microestimation of Dipterex residues by
the Cholinesterase inhibition technique
3581 The determination of Dylox residues by the Cholinesterase
inhibition technique
8839 The microcoulometric determination of Dylox residues in
plant material
9012 The effect of canning on Dylox residues in tomatoes
9733 A comparison of Dylox residue results obtained by the
Cholinesterase inhibition procedure (Report 3581) and the
vapor phase chromatographic procedure (Report 8839)
11 255 The effect of processing on Dylox residues in Safflower
14 393 Determination of Neguvon, Chloral hydrate and
trichlorethanol residues in animal tissues by electron
capture gas chromatography
14 412 Trichlorfon residues in cattle tissues (Backline application
with 8 and 16% pour-on)
16 460 Determination of Trichlorfon, Chloral hydrate and
trichlorethanol residues in milk by electron capture gas
chromatography
16 538 Trichlorfon residues in milk (barn spray prepared with S.P.)
17 440 Trichlorfon residues in milk (in feed prepared)
17 970 Trichlorfon residues in cattle milk (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
17 991 Trichlorfon residues in cattle tissues (in feed prepared)
18 321 Trichlorfon residues in cattle milk (in feed prepared with
technical standard)
18 322 Trichlorfon residues in milk (spray prepared with 80%
soluble powder)
18 495 Trichlorfon residues in cattle tissues (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
18 521 Trichlorfon residues in milk (pour-on with 8% formulation)
18 678 Trichlorfon residues in swine tissues (in feed with 90%
soluble powder)
18 884 Trichlorfon residues in cattle tissues (in feed prepared
with technical standard)
20 945 Trichlorfon residues in poultry tissues (in feed prepared
with Dylox 80% S.P.)
21 289 Trichlorfon residues in milk (mist spray prepared with 1%
Co-Ral - 2% Trichlorfon combination)
21 313 Trichlorfon residues in mud (spray on surface with 50% S.P.)
21 502 Trichlorfon residues in eggs (in feed prepared with 80%
S.P.)
21 811 Determination of residues of Trichlorfon in Alfalfa by
thermionic emission gas chromatography
24 808 Determination of residues of Trichlorfon in bovine animal
tissues by thermionic emission gas chromatography
26 117 Trichlorfon residues in cattle tissues (Backline application
using 8% pour-on)
26 334 A confirmatory gas chromatographic procedure for Trichlorfon
residue analysis
27 471 An interference study for Trichlorfon residue determination
on Alfalfa and Clover
27 533 Trichlorfon residues in swine tissues (drinking water
treated with Neguvon)
27 561 DDVP-residues in swine tissues (drinking water treated with
Neguvon)
28 937 Trichlorfon residues in runoff water from soils
29 234 Trichlorfon residues in milk (Bolus-capsules fortified with
Dylox 80% S.P.)
29 411 An interference study for the residue method for Trichlorfon
on various crops
Davis, A. and Bailey, D. R. (1969) Metrifonate in urinary
schistosomiasis. Bull. Wld Hlth Org., 41: 209-224
Dedek, W. and Lohs, K. (1970a) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern I. Untersuchungen in vitro in Humanserum
mit 14C-Trichlorphon. Z. Naturforsch., 25b: 94-96
Dedek, W. and Lohs, K. (1970b) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern II. Verteilung von 14C in Organen und
Leberproteinen bei Ratten nach Applikation von 14C-Trichlorphon. Z.
Naturforsch., 25b: 1110-1113
Dedek, W. and Schwarz, H. (1970) Studien zur percutanen Resorption von
32P-Dimethoat am Schaf. Z. Naturforsch., 25b: 1193-1194
Deichmann, W. B. and Lampe, K. (1955) Dipterex, its pharmacological
action. Bull. Univ. Miami Sch. Med., 9: 7-12
Dinerman, A. A., Lavrent'eva, N. A. and Il'inskaia. N. A. (1970) The
embryotoxic action of some pesticides. Gigiena i. Sanit., 35: 39-42
Dormale, S., Martens, P. H., Decleire, M. and de Faetraets, L. (1959)
Etude do la persistance de résidus d'insecticides dans divers légumes
frais, blanchis et stérilisés. Bull. Inst. agron. et Stat. Rech.
Gembloux, 27: 137-147
Doull, J. and Dubois, K. P. (1956) The effects of diets containing
Dipterex on rats. Unpublished report by Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J. and Fitch, F.
(1962a) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Doull, J. and Dubois, K. P. (1958) The effects of diets containing
Dipterex for dogs. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Vaughn, G. and Dubois, K. P. (1958) Effect of diets
containing Dipterex in combination with organic phosphates on dogs and
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Fitch, F., Meskauskas, J., Root, M. and
Cowan, J. (1965) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Root; M., Cowan, J., Meskauskas, J. and
Fitch, F. (1962b) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Dubois, K. P. (1958) Potentiation of the toxicity of insecticidal
organic phosphates. AMA Arch. Indust. Health, 18: 488-496
Dubois, K. P. and Cotter, G. J. (1955) Studies on the toxicity and
mechanism of action of Dipterex. AMA Arch. Indust Health, 11: 53-60
Dubois, K. P. and Doull, J. (1955) Acute toxicity of Dipterex to
chickens and ducks. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol, appl.
Pharmacol., 14: 515-534
Giang, P. A. and Hall, S. A. Enzymatic determination of organic
phosphorus insecticides. Anal. Chem., 23: 1830-1834
Gibel, Von W., Lohs, Kh., Wildner. G-P, and Ziebarth, D. (1971)
Tierexperimentelle untersuchungen über die hepatotoxische und
Kanzerogene wirkung phosphoroganischer verbindungen. Arch. für
Geschwulstforschung, 37: 303-312
Gofmekler, V. A. add Tabakova, S. A. (1970) The effect of chorphos on
rat embrogenesis. Farmikol. i. Toksikol., 33: 735-737
Grundmann, E. and Hobik, H. P. (1966) Bay 15922/2 year feeding
experiment in rats/histology. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Hanna, S., Basmy, K., Osaima, S., Shoeb, S. M. and Awny, A. Y. (1966)
Effects of administration of an organophosphorus compound as an
antibilharzial agent with special reference to plasma cholinesterase.
Brit. Med. J., 1: 1390-1392
Hassan, A. and Zayed, S. M. A. D. (1965) Metabolism of
organophosphorus insecticides III. Fate of the methyl groups of
Dipterex in vivo. Can. J. Biochem., 43: 1271-1275
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides II. Metabolism of
O,O-Dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate (Dipterex) in
mammalian nervous tissue and kinetics involved in its reaction with
acetylcholine esterase. Can. J. Biochem., 43: 1263-1269
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides V. Mechanism of
detoxyfication of Dipterex in Prodenia Litura F. Biochem. Pharmacol.,
14: 1577-1584
Hassan, A., Zayed, S. M. A. D. and Hashish, S. (1965) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxyfication in the
rat. Biochem. Pharmacol., 14: 1692-1694
Hassan, A., Zayed, S. M. A. D. and Mostafa, I. Y. (1966) Metabolism of
organophosphorus insecticides VIII. Demethylation of Dipterex.
Z. Naturforsch., 21b: 498-500
Hobik, H. P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitat-sversuchen an huhnern mit
Dipterex. Unpublished report submitted by Farbenfabriken Bayer A.G.
Jackson, J. B., Drummond, R. O. Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Juszkiewicz, T. (1970) Insecticides residues in the tissues and milk
of cow following the dermal application of fenchlorvos and
trichlorphon: a preliminary report. Med. Weterynar (Poland),
26: 85-89
Kimmerle, G. and Lorke, D. (1966) Neurotoxische Untersuchungen an
Huhern mit Dipterex-Wirkstoff. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kühnert, M., Dedek, W. and Schwarz, H. (1963) Untersuchungen über die
Stoffwechselbeeinflussung und den Ausseheidungs-mechanismus des
Phosphonsäure-esters Trichlorphon im Handelspräparat "Bubulin" mit
Hilfe 32P-markierten Phosphors bei der intravenösen und
intramuskulären Injektion an Rindern. Arch. Exp. Vet. Med.,
17: 403-417
Leahy, J. S. (1964) Die Bestimmung von Rückstünden des Neguvon(R)
Milch nach dermaler Anwendung beim Rind. Vet. Med. Nachr., 37-48
Lebrun, A. and Cerf, C. (1960) Note preliminaire sur la Toxicite pour
l'homme d'un insecticide organophosphore (Dipterex). Bull. Wld Hlth
Org., 22: 579-582
Lindgren, P. D. and Ridgway, R. L. (1967) Toxicity of five
insecticides to several insect predators. J. Econ. Entomol.,
60: 1639-1641
Lorke, D. (1971) Trichlorfon. Untersuchungen auf embryotoxische und
teratogene wirkungen an der Ratte. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Lorke, D. and Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Lorke, D. and Kimmerle, G. (1968) Die wirkung von reactivatoren bei
der vergiftung mit phosphorsaureestern. Naunyn-Schmeidebergs Arch.
Pharmakol. Exp. Pathol., 263: S237
Löser, E. (1970) Bay 15922/Chronic toxicological studies on dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Löser, E. (1969) Generationsversuche an Ratten, Unpublished report
submitted by Farbenfabriken Bayer A.G.
Metcalf, R. L., Fukuto, T. R. and March, R. B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52: 44-49
Miller, L. P. (1941) Formation of 2,2,2-Trichloroethylgentiobioside in
tomato plants grown in media containing Chloral hydrate,
Trichloroethyl alcohol, or chloral cyanohydrin. Contribution from
Boyce Thompson Institute Plant Research, 12: 15-23
Miyamoto, J. (1959) Non-enzymic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24: 130-137
Miyamoto, J. (1961) Studies on the mode of action of Dipterex Part II.
New glucorunides obtained from the urine of rabbit following
administration of Dipterex. Agr. Biol. Chem., 25: 566-572
Miyamoto, J. (1968) Private communication to Bull, D. L. and Ridgway,
R. L.
Miyata, T., Iyatomi, K., Saito, T. and Morikawa, O. (1968) Metabolism
and cholinesterase inhibition of NS 2662,
O,O-dimethyl-2,2-dichloro-1-hydroxyethyl phosphonate, in the American
cockroach. Jap. Appl. Ent. Zool., 12: 211-219
Mühlmann, R. and Schrader, G. (1957) Hydrolyse der Insektiziden
Phosphorsäureester. Z. Naturforsch., 12b: 196-209
Murphy, S. D. and Dubois, K. P. (1958) Inhibitory effect of Dipterex
on the detoxification of malathion. Unpublished report by Department
of Pharmacology, University of Chicago
Namba, T. (1971) Cholinesterase inhibition by organophosphorus
compounds and its chemical effects. Bull. Wld Hlth Org., 44: 289-307
Pruessmann, R. (1968) Direct Alkylating Agents as Carcinogens. Food
cos. Toxicol., 6: 576-577
Rahn, H. W. (1963) Über die Moglichkeit der Trichlorphon Schadigung
bei Jung Tieren Laktierender Rattan. Arch. Exp. Veterinarmed.,
18: 713-717
Reynolds, H. T., Stern, V. M., Fukuto, T. R. and Peterson, G. D.
(1960) Potential use of Dylox and other insecticides in a control
program for field crop pests in California. J. Econ. Entomol.,
53: 72-78
Robbins, W. E., Hopkins, T. L. and Eddy, G. W. (1956) The metabolism
of 32P-labelled Bayer L 13/59 in a cow. J. Econ. Entomol.,
49: 801-806
Ross, E. and Sherman, M. (1960) The effect of selected insecticides on
growth and egg production when administered continuously in the feed.
Poultry Sci., 39: 1203-1211
Saito, T. (1969) Selective toxity of systemic insecticides. Res.
Reviews, 25: 175-186
Sándi. E. (1962) Kolorimetrische Bestimmung von Dipterex-Rückständen
in Lebensmitteln. Die Nahrung, 7: 60-66
Sato, K. and Saito, T. (1968) Selective Toxicity of NS 2662,
O,O-dimethyl dichloro-hydroxyethyl phosphonate, and Trichlorfon. Jap.
appl. Ent. Zool., 12: 148-155
Schulemann, W. (1955) Final opinion on the insecticide Bayer L13/59.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Schwarz, H. and Dedek, W. (1965a) Das Verhalten von radioaktiv
markiertem Trichlorphon nach Pour-on-Applikation (Aufgiessverfabren)
zur Dassellarvenbekämpfung beim Rind. Monatsh. Vet. Med.,
20: 958-968
Schwarz, H. and Dedek, W. (1965b) Untersuchungen über den Abbau und
die Ausseheidung von 32P-markiertem Trichlorphon beim Schwein. Zbl.
Vet. Med. (Reihe B), 12: 653-660
Schwarz, H, and Dedek, W. (1966) Untersuchungen zum Nachweis von
32P-markiertem Trichlorphon im Fleisch beim Schwein. Zbl. Vet. Med.
(Reihe B), 13: 489-494
Sherman, M. and Ross, E. (1959) Toxicity of housefly larvae to
insecticides administered as single oral doses to chicks. J. Econ.
Entomol., 52: 719-723
Sissons, D. J. and Telling, G. M. (1970) Rapid procedure for the
determination of organophosphorus insecticide residues in vegetables.
II. A screening procedure for watersoluble insecticides.
J. Chromatog., 48: 468-477
Spicer, E. J. F. and Payne, S. (1971) Pathology report of Bay 15922-4
year dog study (Loser, 1970). Unpublished report submitted by
Farbenfabriken Bayer A.G.
Spicer, E. J. F. and Urwin, C. (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Vbrovsky, L., Selecky, V. and Rosival, L. (1959) Toxikologische und
Pharmakologische Studien der Phosphorsaureester Insecticiden. Arch.
Exp. Pathol. Pharmol., 236: 202-205
Watts, R. R., Storherr, R. W. and Pardue, J. R. (1969) Charcoal column
clean up for many organophosphorus pesticide residues in crop
extracts. A.O.A.C., 52: 522-526
Wegner, D. (1970) Bilarcil(R) Bay 2349 - Klinische Erfahrungen
1960-1969. Unpublished report submitted by Farbenfabriken Bayer A.G.
Wickham, J. C. and Flanagan, P. (1962) Residues of Trichlorphon
(Neguvon) in milk after dermal application to cattle. J. Sci. Food
Agric., 13: 449-455
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Tox. appl. Pharmacol.,
1: 1-7
Wills, J. H. (1959) Recent studies of organic phosphate poisoning.
Fed. Proc., 18; 1020-1025
Witter, R. F. and Gaines, T. B. (1963) Relationship between depression
of brain or plasma cholinesterase and paralysis in chickens caused by
certain organic phosphorus compounds. Biochem. Pharmacol.,
12: 1377-1386
Zadrozinska, J. (1966) Oznaczanie Sladowych pozostalosci Diptereksu
w jablach metoda chromatografii cienkowwarstwowej (Determination of
trace residues of trichlorphon in apples by thin-layer
chromatography). Roczn. panst. Zakl. Hig., 17: 523-528
Zayed, S. M. A. D, and Hassan, A. (1965) Metabolism of
organophosphorus insecticides I. Distribution and metabolism of
Dipterex in adult larva of the cotton leaf worm (Prodenia Litura F.),
Can. J. Biochem., 43: 1257-1262
Zayed, S. M. A. D., Mostafa, I. Y. and Hassan, A. (1965) Organische
P-hattige Insectizide im Staffwichsel. VII. Umwandlung von
32-P-markiertem Dipterex durch Mikroorganismen. Arch. f. Mikrobiol.,
51: 118-121
Zhdanovich, N. V. and Udalov, I. U. F. (1970) The role of thiamine and
pyridoxime on acute and subacute intoxication with the
organophosphorus insecticide Dipterex. Vopr. Pitaniya, 29: 28-34
 Excretion
Following acute administration trichlorfon is rapidly eliminated
primarily via the urine. Following intraperitoneal administration of
trichlorfon to rats, 71% of the total dose was eliminated in the urine
in 16 hours (Bull and Ridgeway, 1969). Following oral administration
to cows, 66% of the dose was eliminated within 12 hours (Robbins et
al., 1956). Arthur and Casida (1958) demonstrated that the major
quantity of radioactive components in urine of rats was hydrolysis
products with less than 1% of the products being extractable by
organic solvents. Secretion of trichlorfon into milk appears to occur
rapidly following application (Leahy, 1964; Robbins et al., 1956)
although this means of elimination is minor and residues are
eliminated rapidly. It seems apparent that elimination of the toxicant
is rapid although in one instance Arthur and Casida (1958) treated
rats with 2000 mg/kg and four hours later found 45% of the
administered dose in fat. This fat storage was not followed further.
The delayed recovery of cholinesterase systems in mammals suggests
that elimination of the toxicant is not complete and small quantities
of antiesterase agents may remain in the body for prolonged periods
(possibly in the fat).
Effect on enzymes and other biochemical parameters
Trichlorfon is a rapid irreversible inhibitor of cholinesterase.
Several investigators have reported in vitro values for inhibition
of this enzyme.
Enzyme source I50 (molar) References
Commercially purified 3.2 × 10-6 Arthur and Casida, 1957
Red blood cell (bovine) 3.2 × 10-6 Arthur and Casida, 1957
6.3 × 10-6 Bull and Ridgeway, 1959
Whole blood (human) 7.9 × 10-5 Arthur and Casida, 1957
Plasma (human) 1.5 × 10-5 Samir et al., 1966
Red blood cell (human) 3.4 × 10-6 Rosival et al., 1959
Serum (human) 1.3 × 10-7 Rosival et al., 1959
Brain (rat) 2.6 × 10-6 Dubois and Cotter, 1955
6.3 × 10-6 Hassan et al., 1965
8.7 × 10-4 Schulemann, 1957
In vivo, rat cholinesterase activity of brain serum and submaxillary
gland was maximally inhibited within 15 minutes of Rx by ip injection
(Dubois and Cotter, 1955). Recovery rates were dependent on the dose
administered (25, 50 or 75 mg/kg) with 75% of the enzyme recovered
within four hours at the highest treatment level. At the lowest level,
activity of cholinesterase was normal within an hour. Cholinesterase
inhibition in humans apparently recovers at a slower rate than
demonstrated with rats. Erythrocyte cholinesterase was not recovered
from two daily doses of 7.5 mg/kg (orally administered) for 38 days
after treatment. Maximum inhibition was only 50% of pretreatment
levels (Lebrun and Cerf, 1960). Cholinesterase levels in children
treated orally for 10 days with 5 or 10 mg/kg returned to normal
within four weeks.
Following instramuscular (25 mg/kg) or intravenous (20 mg/kg)
administration to cattle, calcium levels in the serum were reduced.
The calcium level returned to normal in three days (Kühnert et al.,
1963).
TOXICOLOGICAL STUDIES
Special studies
(a) Carcinogenicity
Following weekly subcutaneous administration of trichlorfon to rats,
two of 24 developed local sarcomas after a period of 800 days
(Pruessmann, 1968). Gibel et al. (1971) observed several incidents of
forestomach papilloma, liver carcinoma and abdominal sarcoma in rats
and mice administered trichlorfon (see "short-term studies").
(b) Reproduction
A three generation (two litter per generation) rat reproduction study
at levels of 0, 100, 300, 1000 and 3000 ppm in the diet resulted in
adverse effects on reproduction at 1000 ppm and above. At 1000 ppm
there was evidence of reduced fertility, smaller litters and reduced
body-weight of pups. At 3000 ppm the pregnancy rate was markedly
decreased and the pups were smaller and lighter in weight with none
surviving to weaning. No effects were noted at 300 ppm or below.
Microscopic examination of the F3b generation indicated no adverse
effects (Loser, 1969; Spicer and Urwin, 1971).
(c) Teratogenesis, mutagenesis
Daily oral administration of trichlorfon (100 mg/kg for 17 days) to
lactating rats resulted in no effect on the pups (Rahn, 1963).
Pregnant rats were administered trichlorfon by continuous inhalation
for 20 days at concentrations of 0.005, 0.02 and 9 mg/m3. At all
levels there were external and internal abnormalities in the
development of embryos, weight differences in organs of the rats,
weight differences in the embryos, shifts in the level of ascorbic
acid and nucleic acids in tissues of mothers and foetuses and
histopathological and histochemical changes in the placenta (Gofmekler
and Tabakova, 1970). No foetal abnormality or embryotoxic effects were
observed when trichlorfon was administered orally at 100 mg/kg/day to
pregnant rats from day six to 15 of gestation (Lorke, 1971).
Trichlorfon injected into chicken embryo egg sac at seven days after
fertilization at a dose of 0.0008 of the rat LD50 and examined at 21
days showed only a slight decrease in embryo viability (Dinerman et
al., 1970). Dominant lethal tests run with male mice injected with a
single dose of 0, 50 or 100 mg/kg and mated to untreated females
resulted in no adverse effects on reproduction or on the young (Arnold
et al., 1971).
Except for the one study by inhalation (Gofmekler and Tabakova, 1970),
trichlorfon does not appear to be a terata-inducing compound nor does
it induce mutations in rodents.
(d) Neurotoxicity
Subcutaneous administration of trichlorfon to chickens at a single
dose of 90 mg/kg did not result in ataxic neuropathy (Witter and
Gaines, 1963). When trichlorfon was fed to hens for 29 weeks at 130
ppm in the diet, no neurotoxic signs were observed (Ross and Sherman,
1960). Oral administration of a single dose of 100 mg/kg (with
atropine and PAM) or dietary levels of up to 5000 ppm for 30 days did
not result in delayed neurotoxicity. Trichlorfon does not induce
pathological demyelination or clinical signs of ataxia (Kimmerle and
Lorke, 1966; Hobik, 1967).
(e) Potentiation
Trichlorfon potentiates the toxicity of azinphos-methyl, EPN and
malathion but not several other organophosphates and carbamate
insecticides (Dubois, 1958; Doull et al., 1958; Kimmerle and Lorke,
1968).
Intraperitoneal administration of 10 mg/kg to rats resulted in a
decrease of the malathion detoxifying enzymes in liver and serum
(Murphy and Dubois, 1958). The effects on the detoxification system
were transient and were reversed in 24 hours. When rats were fed 100
ppm trichlorfon in combination with malathion (100 ppm) for two weeks,
no effects on the detoxifying enzyme were observed (Murphy and Dubois,
1958). However, when 100 ppm trichlorfon was fed to rats and dogs in
combination with malathion (1000 ppm), EPN (20 ppm) or azinphos-methyl
(5 ppm) for six weeks, effects on cholinesterase were noted with EPN
and malathion but not with azinphos-methyl (Doull et al., 1958). The
potentiated effects were greatest with EPN, less with malathion and
absent with azinphos-methyl.
(f) Antidotes
Trichlorfon intoxication in rodents, as with many antiesterase
organophosphate esters, responds to therapy with atropine and 2 - PAM
(Wills, 1959; Dubois and Cotter, 1955; Lorke and Kimmerle, 1968).
2 - PAM was effective in reactivating rabbit cholinesterase and
reducing mortality in mice (Wills, 1959) and rats (Lorke and Kimmerle,
1968).
A recent report on the action of thiamine and pyridoxime as
therapeutic agents indicates that the antivitamins (oxythiamine and
desoxypyridoxime) synergize the effects of poisoning. The two vitamins
when given prior to treatment have shown some beneficial effects
(Zhdanovich and Vdalov, 1970). There was little effect when given
after treatment.
Acute toxicity
Animal Sex Route LD50 References
(mg/kg)
Mouse M oral 660 Vbrovsky et al., 1959
M 950 Schulemann, 1955
M&F ip 500 Dubois and Cotter, 1955
M 650 Vbrovsky et al., 1959
F 575 Vbrovsky et al., 1959
M sc 267 Borgmann and Hunold, 1955
F 320 Borgmann and Hunold, 1955
Rat M&F oral 316-650 Dubois and Cotter, 1955
Deichman and Lampe, 1955
Edson and Noakes, 1960
Schulemann, 1955
Gaines, 1969
Hagan, 1958
Borgmann and Hunold, 1955
Animal Sex Route LD50 References
(mg/kg)
se 400 Arthur and Casida, 1958
ip 400 Arthur and Casida, 1957
160 Dubois, 1958
225 Dubois and Cotter, 1955
M 160 Murphy and Dubois, 1958
M
(adult) 250 Brodeur and Dubois, 1963
M
(weanling) 190 Brodeur and Dubois, 1963
M&F dermal 2 800 Edson and Noakes, 1960
2 000 Gaines, 1969
Guinea-pig M&F ip 300 Dubois and Cotter, 1955
200 Schulemann, 1955
Rabbit oral 160 Arant et al., 1971
dermal 5 000 Deichman and Lampe, 1955
Chicken oral 75-110 Dubois and Doull, 1955
Kimmerle and Lorke, 1966
sc 125 Witter and Gaines, 1963
65 Sherman and Ross, 1959
ip ca. 75 Kimmerle and Lorke, 1966
Duck
(1 week old) oral 105 Dubois and Doull, 1955
Dog oral 420 Deicbman and Lampe, 1955
Horse oral 100 Jackson et al., 1960
When trichlorfon was administered as a 10% solution to the
conjunctival sac of rabbits for six days, reversible effects,
including miosis and vasodilation of the blood vessels of the upper
lid, were observed (Deichman and Lampe, 1955). Dogs dipped once into a
1% solution showed no sign of toxicity although cholinesterase was
depressed (Bailey, 1956). Toxic signs of poisoning are typical of the
cholinergic response of organophosphate esters. Symptoms include
twitching, salivation, lacrymation, defaecation, urination, tonic and
clonic convulsions, prostration, cardiac arrest and respiratory
failure. The onset of symptoms is rapid as is the recovery following
sublethal poisoning. Intraperitoneal administration of toxic doses of
trichlorfon caused the appearance of symptoms in rats and mice in
about 10 minutes and death or recovery occurred within a few hours.
Symptoms included scattered muscular fibrillations and body twitches
which were followed by salivation, lacrymation, defaecation, and
urination, The severity of the symptoms increased with time and
included tonic and clonic convulsions, prostration and respiratory
failure preceded by cardiac arrest.
Skin irritation was absent when 1:1 mixture of trichlorfon and
emulsifier was applied to rat and guinea-pig backs daily for four
weeks (Borgmann and Hunold, 1955). Inhalation studies in a static
chamber with concentrations of 22 mg/l air caused symptoms of
cholinergic stimulation but no death. At 8 mg/l no signs of poisoning
were observed (Borgmann and Hunold, 1955).
Short-term studies
Rat
Rats (five rats per group) were administered trichlorfon
intraperitoneally at 50, 100 and 150 mg/kg/day for 60 days. Mortality
was observed at 100 mg/kg/day while at 50 mg/kg/day all animals
survived (Dubois and Cotter, 1955).
Rats (13 male and 13 female per group) were fed trichlorfon at dietary
levels of 0, 20, 100 and 300 ppm for 16 weeks. Significant
cholinesterase depression was noted at 300 ppm. No effects were
observed at 100 ppm on growth, behaviour, food consumption or gross
and microscopic examination of tissues (Doull and Dubois, 1956).
Rats (10 male rats per group) were fed trichlorfon at levels of 0. 1,
5, 25 and 125 ppm for 16 weeks. Cholinesterase depression was minor
(approximately 30%) at the initial phases of the test (4-8 weeks) and
slowly rose to near normal values. No effects were noted on food
consumption, growth or on gross examination of the tissues (Edson and
Noakes, 1960).
Cutaneous administration of trichlorfon to mice either alone or mixed
with "Krotonal" three times per week (alone) for five months or once
per week ("Krotonal") for six months resulted in a higher incidence of
pathological abnormalities than the controls. Cutaneous administration
alone resulted in an incidence of liver necrosis, liver cirrhosis,
liver carcinoma and forestomach papilloma. With "Krotonal" one case of
abdominal sarcoma was observed in addition to the other effects
(Gibel, 1971).
Dog
Dogs (two males and two females per group) were fed trichlorfon at
levels of O, 50, 250, 500 and 1000 ppm in the diet for one year.
Cholinesterase activity was depressed at 500 and 1000 ppm. At 500 and
1000 ppm an increased spleen weight with congestion and apparent
lymphoid atrophy was noted. Males at 1000 ppm exhibited a decrease in
spermatogenesis and the occurrence of hyperplastic nodules in the
adrenals. No effects were noted on mortality, growth, food
consumption, behaviour or gross and microscopic examination of tissues
other than those mentioned above (Doull et al., 1962a).
Two dogs were administered 45 mg trichlorfon per kg orally six days
per week for three months with no cumulative effects noted. The serum
cholinesterase level was 60% of normal at the conclusion. No mortality
was observed (Deichman and Lampe, 1955).
Dogs (one male and one female per group) were fed trichlorfon in the
diet for 12 weeks at levels of 20, 100, 300 and 500 ppm. The animals
were maintained on control diets for a further four weeks. Depression
of erythrocyte and serum cholinesterase activity was noted at 300 ppm
and above. Trichlorfon at 100 ppm did not affect cholinesterase. No
effects were noted at any level on the growth, food consumption or
behaviour of the dogs. Cholinesterase activity returned to normal
within two weeks of cessation of feeding (Doull and Vaughn, 1958).
Dogs (one male and one female per group, two males and two females
served as controls) were fed trichlorfon at levels of 0, 50, 200 and
500 ppm for 12 weeks in the diet. Plasma and erythrocyte
cholinesterase activity was depressed at 500 ppm and unaffected at 200
ppm. Recovery of enzyme activity was complete six weeks after
trichlorfon feeding stopped (Williams et al., 1959).
Long-term studies
Rat
Rats were administered trichlorfon three times weekly by oral or
subcutaneous administration for the life of the rats. Higher incidence
of liver necrosis, liver cirrhosis and forestomach papilloma were
evident than were described for the control (Gibel et al., 1971).
Five groups of rats (25 male and 25 female per group; controls
contained 50 males and 50 females) were fed diets of trichlorfon
containing 0, 50, 250, 500 and 1000 ppm for a period of two years,
Male rats at 1000 ppm gained less weight than the control after the
first two months and failed to regain the weight loss during the
remaining feeding period. Rats fed 1000 ppm weighed about 15% less
than the control animals. In male and female rats fed 1000 ppm the
onset of mortality occurred earlier than in rats from the other groups
and there was distinct shortening of the survival time in rats fed
this diet. Apparently 1000 ppm trichlorfon in the diet caused a
decrease in life span. Rats fed 500 ppm showed a moderate (25%)
inhibition of serum cholinesterase. No effect was noted on the
cholinesterase from brain submaxillary gland and erythrocyte at this
and lower levels. At 1000 ppm all cholinesterase determinations were
below normal, except for the brain. Gross pathological effects
resulting from the presence of trichlorfon in the diet included
mammary gland tumours which occurred in female rats fed 250 ppm (one
rat), 500 ppm (three rats) and 1000 ppm (two rats). Microscopic
examinations of the tissues indicated major adverse histological
findings observed in the mammary glands, gonads and blood vessels of
the trichlorfon fed animals. Three mammary tumours were observed at
1000 ppm (an adenocarcinoma, a sarcoma and a fibroma); three were
observed at 500 ppm (two adenocarcinomas and a fibroadenoma); and one
was found at 250 ppm (a fibroadenoma). Female rats, fed 500 and 1000
ppm, exhibited an absence of primary follicules and primative ova; one
rat fed 250 ppm also lacked follicules and ova. Two of the five rats
examined at 1000 ppm had tubular androblastomas which were composed of
epithelial components and stroma. Three of five male rats examined
from 1000 ppm feeding levels exhibited focal aspermogenesis not
observed at any other dietary levels. Lesions were observed in the
middle and small sized arteries of the lung, thymus, pancreas,
submucosa and adventitia of the gastrointestinal tract and in the
adrenal glands of several of the animals. The lesions were more common
in males than in females. The muscular and elastic tissue of the blood
vessel walls was frequently replaced by fibrous tissues and this was
associated with a necrotizing inflammation. The gross and microscopic
examination of the tissues from animals fed the trichlorfon-containing
diets revealed three pathological effects which appeared to be related
to the presence of the chemical in the diet. These consisted of an
increase in the incidence of mammary tumours in the female rats,
vascular changes and injury to the reproductive systems of both male
and female animals. The incidence of mammary tumour formation in the
entire group of rats used in the study (rather than the relatively
small number of animals used for histological examinations) shows that
the incidence in the rats fed control diets was 14%, 8% of the rats
fed 50 ppm, 20% of the rats fed 250 ppm, 21% at 500 ppm and 25% at
1000 ppm. The comparison of the time for the first tumours to appear
showed that in control diets 1.7 years, 50 ppm diet 1.6 years, 250 ppm
1.8 years, 500 ppm 1.5 years, and 1000 ppm 1.1 years. Thus, the total
frequency and onset of tumour formation appeared to be dose dependent
(Doull et al., 1962b).
Rats (25 male and 50 female per group) were fed dietary levels of
trichlorfon at 0, 100, 200 and 400 ppm for 1.5 years (Doull et al.,
1965). Serum cholinesterase depression was observed in both males and
females at 400 ppm. Erythrocyte cholinesterase was depressed in males
and females at 400 ppm; slightly in males at 200 ppm and very slightly
depressed in females at 100 ppm. There was a significant mortality in
the study in the control and experimental groups. The study was
prematurely concluded at approximately 70 weeks of feeding. At 400 ppm
the male spleen and liver weights were lighter than the control
values. At autopsy the major findings occurred in the ovaries and
mammary glands with slight effects on the lungs. Cystic granular
ovaries were seen in 40% of the female rats fed 400 ppm; 33% of the
rats fed 200 ppm; 14% of the rats fed 100 ppm; and 8% of the female
control rats. Fifteen per cent. of the female rats fed 400 ppm had one
or more mammary tumours; 11% of the rats fed 200 ppm; and 8% of the
control had mammary tumours. The induction time for mammary tumours
was not significantly decreased by increasing dietary concentrations
of trichlorfon. There were pulmonary abscesses in the lungs of two
female rats fed 400 ppm and two other female rats from this group
exhibited exudative pleuritis. Microscopic examination of the tissues
indicated an absence of primary follicules and primitive ova in four
of the five rats examined at 400 ppm. This was a significant increase
from those found at lower levels of feeding. The ovaries from the rats
fed 400 ppm were atrophic, small in size with nests of luteal or
granulosa cells, and an increased number of androblasts. Similar but
less marked changes were found in other groups. A greater frequency of
simple cysts of the ovaries was observed at 400 ppm than in the
control group. None of the cysts appeared to be neoplastic.
Examinations of the mammary tumours indicated that they were benign
fibro-epithelial tumours. Microscopic appearance of the tumours in the
trichlorfon fed animals was similar to the tumours seen in the control
animals. These studies suggest that the addition of trichlorfon to the
diet may have enhanced some of the aging changes, especially in the
reproductive tissue.
Rats (groups of 50 male and 50 female per group, 100 male and female
were used as controls) were fed trichlorfon at dietary levels of 0,
50, 250, 500 and 1000 ppm for two years. No effects were observed on
behaviour, food consumption, weight gain, survival, blood count,
urinalysis and liver protein content. Slight non-dose dependent
effects were noted in male and female liver on SDH activity and in
females on SG-OT activity. Cholinesterase was depressed in both males
and females at 1000 ppm but not at 500 ppm. The male liver weight was
significantly increased at 250 and 1000 ppm. An increase in weight was
observed although it was not statistically significant at 500 ppm.
There were no other dose-dependent, significant effects on tissue
weights. Clinical and histological examination of tissues or tumours
indicated that, of the total of 35 malignant tumours, 15 occurred in
the control group and only five were found at 1000 ppm dose levels.
There was no indication of an increased incidence of mammary tumours
although the total number of mammary tumours observed in the
trichlorfon fed animals was greater than that found in the controls
(the percentage of mammary tumour incidence in female rats was:
control, 9%; 1000 ppm, 8%; 500 ppm, 10%; 250 ppm, 12%; 50 ppm, 18%).
In the ovaries, cysts were found in 14 of the 33 control animals, and
at 1000 PPM cysts were found in eight of 20 animals examined. There
was no evidence of unusual interstitial fibrosis in the trichlorfon
fed animals. There appeared to be no evidence of acceleration of the
aging process of the gonads in this experiment. The frequency of
cystic atrophic ovaries or reduction in spermatogenesis was no
different in the controls than in the animals treated with 1000 ppm
trichlorfon in the diet (Lorke and Loser, 1966; Grundmann and Hobik,
1966).
Dog
Dogs (four male and four female per group) were maintained for four
years on diets containing 0, 50, 200, 800 and 3200 ppm trichlorfon
(Loser, 1970). Cholinesterase activity in plasma and erythrocytes was
depressed at 200 ppm. Trichlorfon, at 800 ppm and above, led to an
increased mortality rate. Increased uric acid and creatinine levels in
the male dogs at 800 ppm was indicative of kidney damage. Physical
appearance of the dogs was affected by 800 ppm in the diet. The
animals had a dull, shaggy coat and appeared weaker and sick.
Cholinergic symptoms occurred at the highest dose. Mortality was
evident in dogs fed 3200 ppm and 800 ppm (one male at 800 ppm and two
females at 800 ppm survived the test). Haematological values showed no
pathological changes in any group at two years. The activity of the
serum transaminases (G-OT and G-PT) of the female dogs at 3200 ppm at
two years was significantly high and regarded as pathological. No
other effects were noted on liver function activity. The transaminase
activity in the female dogs surviving the test to four years was
normal. There were no dose related changes in liver function or blood
values at four years. Urine examinations on all dogs at two and four
years showed uric acid and creatinine levels at the 800 ppm dose in
males and the 3200 ppm dose in the female were increased. There were
no differences in the clearance tests. Blood sugar and cholesterol
levels were not affected at 200 ppm. Cholinesterase activity was
depressed at 200 ppm and the depression was dose dependent. In
general, the depression of cholinesterase activity was noticeable
during the earliest parts of the experiment and tended to decrease as
the experiment progressed until at four years the plasma and
erythrocyte cholinesterase level were slightly depressed at 200 ppm in
both male and female with no depression noted at 50 ppm. Comparison of
the organs weights showed that male dogs at 800 ppm had enlarged
spleen, smaller adrenals and testis, and the female at 3200 ppm had an
increased liver weight, enlarged spleen and adrenals and reduced ovary
size. Based on histological examination of tissues there were no
changes in morphology which were considered to be significant or
related to trichlorfon in the diet (Spicer and Payne, 1971).
Observations in man
Over 6000 people, most in South Africa and South America, have been
treated over the past few years for various intestinal and body
parasites (reviewed by Wegner, 1970). The dosages varied up to 70
mg/kg/day for periods up to 12 days. The dose of 7.5 mg/kg given 2-4
times at two week intervals was believed to be the best level as the
symptoms observed were less severe. Symptoms include cholinesterase
depression, weakness, nausea, diarrhoea and abdominal pain. Higher
doses (24 mg/kg) gave more severe symptoms including tachycardia,
salivation, cholic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative and spontaneous recovery in
all cases was rapid. In a few cases, an indication was given that
spermatogenesis (size and shape of sperm) might be affected in humans.
In all cases for treatment of parasites cholinesterase depression was
evident.
Namba (1971) reviewing the human data alluded to the observation that
three persons exposed to trichlorfon showed signs of delayed
neurotoxicity.
Various studies on humans have shown two effects: (1) cholinesterase
depression in all cases which was usually recovered within 30 days of
the cessation of treatment, and (2) a possible effect on
spermatogenesis (Wegner, 1970; Hanna et al., 1966; Lebrun and Cerf,
1960) which included reduced sperm count, seminal fluid volume and a
decreased motility and viability of the cells in a very limited number
of cases. Other studies showing no adverse effects include: Davis and
Bailey, 1969; Abdalla et al., 1965; Abdel-Aal et al., 1970; Beheyet,
1961. The dose of 7.5 mg/kg given once every two weeks appears to be
the best available. There is no human no-effect level recorded.
Comments
Trichlorfon appears to be rapidly absorbed, distributed, metabolized
and excreted in animals. The conversion of trichlorfon to dichlorvos
occurs both in plants and mammals but to only a very minor extent.
Several long-term studies in rats and dogs are available. In two
long-term studies in the rat evidence of an increased frequency and/or
onset of tumour formation (particularly mammary tumours) appeared to
be dose dependent. A third study in rats did not confirm these
observations. An incidence of cystic atrophic ovaries and reduced
spermatogenesis was also observed in two studies and not confirmed in
the third study, in a three generation rat reproduction study or in a
dominant lethal test. None of the experiments individually considered
was by itself indicative of a carcinogenic effect, however the
cumulative evidence derived from all the experiments considered
suggests that further investigations of the potential carcinogenicity
of trichlorfon are required.
In view of the inconclusive nature of the findings in long-term rat
studies, only a temporary acceptable daily intake was established for
trichlorfon.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet equivalent to 2.5 mg/kg body-weight per day
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichlorfon is an insecticide with a broad spectrum of activity. It is
chiefly effective against Lepidoptera (moths), Diptera (flies),
and Heteroptera (bugs). In crop protection, trichlorfon is used
mainly against insect pests in field crops and fruit crops.
Trichlorfon is also of great importance as a public health pesticide
and as an animal health product, and is used in medicine as an
anthelmintic.
The amount of trichlorfon used in the different sectors of
application, expressed in percentages, is in the Western world as
follows:
45% in field crops (e,g. cereals, rice, maize, cotton, grassland,
tobacco)
35% in vegetable crops
10% in fruit crops (e.g. pome fruit, stone fruit, bush and cane
fruit, grapes, citrus fruit, olives)
10% for other uses (e.g. ornamental crops, hygiene, animal
health)
Approximately 25 formulations of trichlorfon are sold and registered
in 83 countries for the control of insect pests of crops and pests of
hygiene.
The following formulations are used in agriculture (crop protection):
50% emulsifiable concentrate, 50%. soluble powder, 80% soluble powder,
50% wettable powder, 2.5% granular, 5.0% granular, 5.0% dust.
Pre-harvest treatments
The recommended application rates and concentrations (in terms of
active ingredient) for pre-harvest treatments of the major crops are
as follows:
Bananas 400-600 g/ha
Beets 150-1000 g/ha, or
0.075-0.12% spray
Cereals (excl. corn and rice) 800-1200 g/ha
Citrus fruits 0.12% spray
Coffee 450-550 g/ha, or
0.075-0.12% spray
Corn 400-1500 g/ha
Cotton 400-1500 g/ha
Currants, gooseberries 0.075-0.12% spray
Deciduous fruits (apples, pears,
cherries, peaches, etc.) 0.075-0.12% spray
Grapes 0.075-0.12% spray
Grassland and forage crops 500-2000 g/ha
Oil palms 450-1600 g/ha,
or 0.08-0.2% spray
Olives 0.075-0.12% spray
Potatoes 600-1000 g/ha
Rice 800-1200 g/ha
Sugar cane 1200-1600 g/ha, or
0.075-0.2% spray
Tea 600-1200 g/ha
Tobacco 600-1200 g/ha
Vegetables 150-1200 g/ha, or
0.075-0.12% spray
For controlling soil-inhabiting cutworms use is made of baits which
are prepared by mixing 100 g active ingredient (soluble powder), 10 kg
bran, 500 g sugar in 10 litres of water. The crumbly mess is used to
treat 1/4-1/2 ha.
Bait sprays for controlling fruit flies consist of 0.08 or 0.32%
active ingredient and 0.25 or 0.5 protein hydrolyzate, respectively.
Post-harvest treatments
No recommendations.
Animal treatments
Trichlorfon is used as an animal health product for the control of
endoparasites and ectoparasites in/on cattle, sheep, goats, pigs,
horses, poultry, dogs, cats and fish. Following formulations are used:
97% soluble powder, 90% soluble powder, 80% soluble powder, 50%
soluble powder, 6% suspension, 11% solution, 5% injectable solution
tablets.
Methods of application are as follows: wash or spray treatment, dip
treatment, spot-on/pour-on treatment, injection, drench treatment,
feed mix, pond or immersion treatment.
Animal health products based on trichlorfon are registered and sold in
77 countries.
Spectrum of activity of trichlorfon in animal treatments is given in
Table 1.
Trichlorfon is normally applied to the animals at the rate of 25-75
mg/kg body-weight by various methods.
The most common method of application is as a wash or spray treatment
for the control of sucking lice, flies and biting lice. For this
purpose, the whole of the animal body is washed or sprayed with a
0.13% solution of Neguvon powder (repeat treatment after five days).
For the control of warble fly maggots on cattle and Sarcoptes mites
on pigs, a 2% solution is recommended (repeat treatment after 5-7
days).
For the external control of Stephanofilaria in cattle and Habronema
spp. in horses, use must be made of a 10% solution applied to the
affected areas.
The 11% ready-for-use Neguvon solution is used for the control of
warble fly maggots in cattle, a single application being made by the
spot-on method. An applicator is supplied with the solution.
Some of the trichlorfon enters through the skin into the blood system.
As a result, the effect is enhanced from inside the animal body. The
systemic action of trichlorfon against warble maggots which wander
through the body of the host animal until they eventually become
located beneath the skin of the animal's back, is also produced as a
result of the compound penetrating through the skin and entering the
blood system.
The success of an external treatment can be further increased by a
simultaneous internal application.
Internal treatment for the control of endoparasites is made as
follows:
TABLE 1. SPECTRUM OF ACTIVITY OF TRICHLORFON IN ANIMAL TREATMENTS
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
External application for control of
ectoparasites:
Biting lice X X X X X
Sucking lice/fleas X X X X X
Sarcoptes mites X X X X
Red avian mite/scaly leg mite X
Flies/sheep keds X X X X X
Warble fly maggots/larvae of
Dermatobia hominis X
Internal application for control of
ectoparasites:
Sarcoptes mites X
External application for control of
endoparasites on domestic animals:
Habronema spp. (summer sores) X
Stephanofilaria in cattle X
Internal application for control of
endoparasites:
Haemonchus spp., Mecistocirrus X X
Oesophagostomum spp. X X
Neoascaris vitulorum X
Bunostomum spp./Ostertagia spp./Cooperia
spp./Trichostrongylus spp. X X
TABLE 1. (Cont'd.)
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
Oxyguris equi X
Stephanofilaria X
Larvae of sheep keds (Oestrus ovis) X
Ascarids X X
Trichuris/Hyostrongylus X
Gastrophilus spp./Habronema spp. X
External application for control of
ectoparasites in fish:
Argulus/Ergasilus/Lernea/Dactylogyrus/
Gyrodactylus/Trichodina/different
fish leeches X
By means of a bottle or drenching gun. For this purpose, it is
recommended to use a 10% solution, the dose depending upon the
body-weight of the animal to be treated. The treatment should be
repeated after 2-3 months, or after an interval of three weeks if
infestation is severe.
In the feed (dry or liquid), mixture with Neguvon powder.
After oral application, trichlorfon is rapidly resorbed and is
translocated in the blood stream to the site at which it is required
to act. Degradation and excretion take place within a few hours.
The 50% Neguvon injectable formulation is a ready-for-use solution for
i.m. or s.c. injection, and is used for the control of Haemonchus
spp., Oesophagostomum spp., and Dermatobia hominis in cattle in
many countries of the Middle and Far East, Africa and Latin America.
For some time past, trichlorfon has also been in use as a pond
treatment and brief dip treatment for the control of ectoparasites in
fish, and has proved to be most successful. The required dose of the
80% powder formulation is 2.5 kg per ha of carp and eel ponds which
have a depth of 50 cm, the dose being raised to 5 kg per ha in 100 cm
deep ponds; the dosage rates needed for the treatment of trout ponds
are only half as large.
The dosage rate for the brief dip treatment for the control of
Argulus, Dactylogyrus and Gyrodactylus in carps is 2.5 kg per
100 litres of water, the duration of the dip treatment being five to
10 minutes.
In South Africa, trichlorfon is marketed also as a dog shampoo for the
control of fleas and ticks in dogs and cats, and as a powder
formulation for the control of fleas in dogs and cats.
Other uses
In hygiene trichlorfon is used for the control of flies in stables as
well as against many other pest species. Baits are used in most
instances.
Further, trichlorfon is used on ornamentals, tree nurseries; and
forestry.
Residues resulting from supervised trials
Trials on crops
The residue data obtained following application of trichlorfon to
fruit, vegetables and field crops are presented, in extracts, in Table
2. These data are from papers published in the literature as well as
from unpublished reports of Chemagro Corporation and Farbenfabriken
Bayer AG which have been compiled and submitted to the Meeting by
Bayer (1971). The residue values were determined initially by
enzymatic procedure (Delta pH method). and in recent years by gas
chromatography (electron-capture detector, phosphorus detector,
microcoulometer). Comparative analyses showed good agreement of these
methods.
Following application to green plant material, a half-life of about
1-2 days was obtained for trichlorfon, as shown by residue studies on
cotton leaves, grass, cabbage, clover, alfalfa and lettuce. Crops
treated 2-4 weeks before harvest are practically free of residues at
harvest time (maize, soybeans, rape, flax),
Following application to bananas, oranges and ground nuts, the bulk of
the trichlorfon residue is contained in the peel and shells,
respectively. Within a few days after the application only slight
residues are to be found in the pulp and nuts, respectively.
Trials on animals
On cattle
The studies of organs showed that following peroral application of
100 mg of active ingredient per kg, up to 10 ppm of active ingredient
is present in steak two hours after the application, and that this
amount decreases to a level of less than 0.1 ppm after 4-6 hours
(Behrenz, 1959). Following back-line and spray applications, organs
(liver, kidneys, brain, heart) and steak are practically free of
residues; in omental fat, on the other hand, maximum active ingredient
concentrations of 9.2 and 1.9 ppm were found one and seven days,
respectively, after the application (Adkins, 1966).
Following administration of trichlorfon to cattle by different routes,
residue studies were carried out by Chemagro Corporation, Kansas City,
United States of America, on practically all organs used for human
diet (meat, fat, liver, heart, kidneys and brain); the determinations
were made by sensitive gas chromatographic methods (limit of
determination 0.01-0.1 ppm; Chemagro Report 14.393; 24.808).
TABLE 2. RESIDUES RESULTING FROM SUPERVISED TRIALS
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Alfalfa 0.6 - 1.1 7 0.04 - 2.0
Alfalfa, seed hulls 1.7 8 0.2 - 1.6
" chaff 1.7 8 0.2 - 0.6
Apples (0.1 - 0.2%) 8 - 30 n.d. - 0.1
Artichokes, spray 1.1 0 - 12 n.d.
" dust 2.8 7 - 14 n.d.
Bananas, pulp 0.4 - 0.8 0 n.d. - 0.3
" peel 0.4 - 0.8 0 n.d. - 2.0
Barley 1.7 14 - 25 n.d.
Blackeyed, beans 1.7 14 n.d.
" vines 1.7 14 n.d. - 0.9
Brussel sprouts, spray 1.7 14 - 19 n.d.
" " dust 2.8 14 0.2
Cabbage 0.6 - 1.7 15 n.d. - 0.05
Cauliflower 1.7 19 - 21 n.d. - 0.15
Celery, spray 0.6 - 1.1 5 - 10 0.05 - 0.19
" dust 0.6 - 1.1 5 - 10 0.05 - 0.16
Cherries 0.3 - 0.5 3 - 8 0.001 - 0.12
Clover 1.1 7 - 8;14 <0.1 - 9.6;
<0.1 - 1.4
Clover, seed head 1.7 14 - 15 <0.1 - 0.7
" chaff 1.7 14 - 15 <0.1 - 0.6
Corn, kernel 0.8 - 1.7 30 - 144 n.d.
" cobs 0.8 - 1.7 30 - 144 n.d.
" husk 1.0 - 1.7 30 - 144 n.d.
" fodder, forage 1.0 - 1.7 30 - 144 n.d.
Cotton, seed 1.1 - 1.7 7 - 10 <0.01 - 0.05
" foliage 2.2 - 2.5 7 n.d. - 0.4
Cowpeas 1.7 14 n.d.
Cowpeas, vines 1.7 14 n.d. - 0.9
Grass, spray 1.1 - 2.2 12 2 - 5
" granular 1.1 - 2.8 12 <1 - 2
Garden beets 1.7 14 - 28 n.d.
Green beans 1.7 14 n.d.
Kale 0.6 - 1.7 6 - 10 n.d. - 0.3
Lettuce, leaf
summer appl. 1.1 - 1.7 7 0.1 - 0.2
winter appl. 1.1 - 1.7 7;14 6.0;3.9
Lettuce, head
summer appl. 1.1 - 1.7 7 <0.1 - 0.24
winter appl. 1.1 - 1.7 7;14 11.1;3.7
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Lima beans 1.7 9 - 15 n.d.
" vines 1.7 9 - 15 n.d. - 0.6
Lin seed 1.7 14 - 27 n.d.
Mustard 1.7 14/15 n.d.
Oats, grain 1.1 - 1.7 13/14 n.d. - 1.4
Oranges, peel, washed 1.1 - 1.7 7 0.1 - 0.21
" pulp 1.1 - 1.7 7 <0.01
Peaches (0.1) 7 - 14 <0.85
Peanuts, spray
" nuts 1.7 - 2.5 0 - 8 n.d. - 0.1
" shells 1.7 - 2.5 0 - 8 0.1 - 2.65
" vines 1.7 - 2.5 3 - 8 0.2 - 0.4
Peanuts, bait
" nuts 1.7 - 2.5 0 - 3 <0.01 - 0.16
" shells 1.7 - 2.5 0 - 3 <0.03 - 0.56
" vines 1.7 - 2.5 0 - 3 <0.07 - 2.9
Peppers, spray 1.1 - 1.7 14/15 n.d. - 0.3
" dust 1.2 14 0.4 - 1.1
Pumpkins 1.1 - 1.7 13/15 n.d.
Rape, seed 0.8 - 1.7 23 - 32 n.d. - 0.1
" meal 1.1 - 1.7 23 n.d.
" oil 1.1 - 1.7 23 n.d.
Safflower 2.2 27;33;43 0.6;<0.1;
<0.1
Soybeans, green beans, pods 0.6 0 0.1 - 6.0
" vines 0.6 0 0.5 - 8.8
" dry beans 0.6 35 - 53 <0.01
" dry vines 0.6 35 - 53 <0.08
(1 x 0.45)
Spinach 0.8 - 1.1 14/15 <0.1 - 1.6
Strawberries 0.3 - 1.1 3/4 <0.07
Sugar beets, roots 0.3 - 1.1 29 - 34 <0.01
" " tops 0.3 - 1.1 29 - 34 <0.05
Sweet corn, spray
" " husk 1.7 - 2.2 7 - 14 n.d. - 0.6
" " kernel 1.7 - 2.2 7 - 14 n.d.
" " cob 1.7 - 2.2 7 - 14 n.d. - 0.1
" " fodder 1.7 - 2.2 7 - 14 0.2 - 1.1
Sweet corn, dust
" " husk 2.0 15 2.7 - 9.1
" " kernel, cob 2.0 15 n.d.
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Sweet corn, granular
" " husk 1.1 - 1.7 14 - 21 n.d. - 8.4
" " kernel 1.1 - 1.7 14 - 21 n.d. - 0.1
" " cob 1.1 - 1.7 14 - 21 n.d. - 0.2
" " fodder 1.1 - 1.7 14 - 21 n.d. - 3.0
Table beets, roots 1.7 7 - 13 n.d. - 0.2
" " tops 1.7 7 - 13 0.2 - 2.5
Tea 1.4 - 1.8 21 <1
Tobacco, green
" spray 1.1 - 1.7 14/15 n.d. - 1.3
" dust 1.0 - 2.5 10/14 n.d.
Tobacco, cured
" spray 1.1 - 1.7 0 - 21 n.d
" dust 1.0 - 2.5 0 - 21 n.d
Tomatoes 0.8 - 1.7 7 n.d. - 0.5
Turnips, roots 10 - 15 n.d. - 0.09
Wheat, grain 1.3 - 1.7 14 - 39 n.d. - 0.25
" straw 1 25 - 39 n.d. - 4.2
" flour 1 25 - 39 <0.05
" bread 1 25 - 39 <0.05
Following peroral uptake of the active ingredient (12.5 and 20 ppm in
feed), no trichlorfon residues were detected (<0.1 ppm) in any of the
examined tissues and organs (brain, heart, kidney, steak, fat) after a
four week feeding period (Chemagro Report 17.991; 18.884). Following
external application of the 9 and 16% formulation - single back-line
application of 25, 50 or 100 mg of active ingredient per kg
(examination of tissues 21 days after application) and four week mist
spray application of 1.1 g of active ingredient per animal and day
(examination of tissues after the final application) - no residues
were detected in any of the tissues or organs (Chemagro Report 14.412;
18.495; 26.117).
Only slight proportions of the parent compound or its metabolites are
to be found in milk, as shown by a number of studies using
P32-labelled and unlabelled trichlorfon.
Following peroral application of 25 mg of active ingredient per kg,
less than 0.2% of the radioactivity representing the total dose
administered was secreted in the milk at the end of 144 hours; of
this, about 10% was unchanged active ingredient and about 23% behaved
like inorganic phosphorus (Robbins et al., 1956). Following similar
application of 80 mg of active ingredient per kg, the maximum residue
at eight hours was 2.5 ppm in the milk samples and at 22 hours
0.1 ppm; in the following milk samples, the residue levels were below
the limit of determination of 0.05 ppm (Behrenz, 1961). Following
spray application of five litres of a 2% active ingredient solution
and back-line was application of one litre of a 2% solution, the milk
samples at eight hours were found to contain 0.05-0.25 ppm of active
ingredient and in the later milk samples no residues were detected
(Behrenz, 1961).
Studies by Ackermann et al. (1968) showed that following backline wash
application of one litre of a 2% trichlorfon solution, the maximum
residue in the first milk sample at eight hours was 0.2 ppm; the
residues in the third milk sample at 32 hours were below 0.01 ppm.
Analyses of these milk samples for dichlorvos showed a maximum content
of 0.03 ppm in the first sample and 0.01 ppm in the third sample.
Following a single dermal treatment with 6% suspension, equivalent to
36 mg of active ingredient per kg, 0.1-0.2 ppm of trichlorfon was
found to be present within the first eight hours (Leahy, 1964).
Following application of 1.15 litres of a 2% trichlorfon solution (or
0.57 litres of a 4% solution) as a dermal wash per animal, the level
of active ingredient present in the milk did not at any time exceed
0.4 ppm; milk samples taken later than six hours after the application
contained about 0.1 ppm of active ingredient (Wickham and Flanagan,
1962).
Following pour-on application (of 100 or 300 ml of a 5.7% solution
(P32-labelled)), the active ingredient concentration in the milk
reached a peak of 0.13 or 0.47 ppm after 14-18 hours; after four days,
the content was less than 0.05 ppm, and after seven days it was less
than 0.01 ppm (Schwarz and Dedek, 1965a).
Following intravenous and intramuscular injection of 20 and 25 mg of
P32-labelled active ingredient, radioactive substances were found in
the milk only at six and 10 hours after the application; dichlorvos
was not formed in these experiments (Kühnert et al., 1963).
Chemagro Corporation, Kansas City, United States of America, carried
out residue studies by gas chromatographic methods following
application of the active ingredient to dairy cows by different
routes. The lower limit of determination was 0.003-0.01 ppm (Chemagro
Report 16.460; 24.808).
Following peroral uptake of the compound in the feed (12.5, 20, 50,
150 and 325 ppm) for a period of one or four weeks, no trichlorfon
residues were found to be present in the milk at the end of the
feeding period (Chemagro Report 17.440; 18.321; 29.234).
Following external application of the active ingredient as a single
pour-on treatment (at 3.7 g/100 kg) or as a single spray treatment
(at 28 g/animal) or as a four week mist spray treatment (1.1 g per
animal per day), active ingredient residues of no more than 0.04 and
0.02 ppm were found in the milk samples taken on the first and second
day after the application only for those animals which received the
pour-on treatment (Chemagro Report 17.970; 18.322; 18.521; 21.289).
The majority of these milk samples were analysed for residues of
trichloroethanol, a possible metabolite of trichlorfon; however, this
compound could not be found in any of the examined samples.
Following a spray treatment of stable walls at a rate of 1 g of active
ingredient per square metre, no trichlorfon residues were found in the
milk of the cows kept in the treated stable (Chemagro Report 16.538).
On pigs
For the use of trichlorfon for the control of ectoparasites and
endoparasites in pigs, the degradation and excretion of the active
ingredient was studied following subcutaneous injection of 25 mg of
P32-labelled trichlorfon per kg body-weight. The maximum active
ingredient concentration in blood was reached after 15-60 minutes
(10-11 ppm) and in the intestinal contents after 20-150 minutes
(4-5 ppm). After 5-7 hours, the blood and intestinal contents still
contained about 1 ppm of active ingredient. Dichlorvos was no longer
detectable in blood 3.5 hours after the application. The trichlorfon
concentration in meat was always somewhat lower than in the blood; a
concentration of 5 ppm was found in the meat two hours after the
injection. The trichlorfon concentration in the meat decreased by a
power of 10 in each 6-7 hours (Schwarz and Dedek, 1965b, 1966).
Chemagro Corporation carried out studies using gas chromatographic
methods (limit of determination 0.01-0.1 ppm residue; Chemagro Report
14.393; 24.808). After addition of 1500 ppm of active ingredient to
the drinking water of pigs (the ingested amount was equivalent to a
single dose of 125 mg of active ingredient per kg body-weight),
0.02-0.07 ppm of active ingredient was found in the liver and
0.02-0.05 ppm was found in loin steak four days after the application;
brain, heart, kidneys and fat samples were free of residues. None of
the organs contained any trichlorfon residues after seven days
(Chemagro Report 27.533). Dichlorvos was not detectable in any of the
examined samples four days after the trichlorfon application (Chemagro
Report 27.561).
No trichlorfon residues were detectable in any of the examined organs
and tissues 14 days after application of the compound in the diet at a
dose level equivalent to 100 mg/kg body-weight (Chemagro Report
18.678).
On sheep
Following peroral application of 120 mg of trichlorfon per kg
body-weight, exploratory residue determinations were carried out in
organs by means of a fly larva test. The residues dropped below the
limit of determination of 0.1 ppm within 4-6 hours after the
application (Behrenz, 1959). Following percutaneous application of
50 mg of the compound per kg body-weight, the concentration in the
blood reached a level of 1 ppm at which it remained for only a brief
period. Dichlorvos was detectable only in slight amounts (Dedek and
Schwarz, 1970).
Fate of residues
General comments
The distribution of trichlorfon residues in organisms is characterized
by its hydrophilic properties.
The decomposition of the molecule is brought about both by splitting
the P-C bond and by hydrolysis of the P-OCH3 bonds (Hassan and
Zayed, 1965). Furthermore, trichlorfon can be converted to the more
toxic dichlorvos, O,O-dimethyl-(2,2-dichlorovinyl)-phosphonate.
In animals
On account of its hydrophilic properties, trichlorfon is rapidly
absorbed by the organism, broken down and excreted in the urine.
Studies with P32-labelled compound on cattle showed that following
peroral application of dosages ranging from 25-100 mg of active
ingredient per kg body-weight, 66% of the P32 activity is excreted
in the urine within 12 hours; of this amount of excreted
radioactivity, 17% is dimethyl phosphate, 76% is accounted for by a
metabolite of unknown structure, and 0.26% by unchanged active
ingredient. After 45 hours, only 0.28% of the P32 activity
representing the total dose administered is found to be present in the
urine (Bolle, 1956; Robbins et al., 1956).
The degradation of the compound in blood proceeds at a very fast rate.
In cattle, 12 hours after peroral application of 40 mg of active
ingredient per kg, 0.03%, of the applied P32 activity is present in
the blood, and 0.003% after 45 hours (Bolle, 1956). The peak of
radioactivity is attained after two hours (Robbins et al., 1956).
Following intravenous application, the parent compound is broken down
by more than 95% after one hour; dichlorvos is found to be present in
a very low concentration only within the first four minutes (Kühnert
et al., 1963).
For further details, see for Biochemical aspects and residues
resulting from supervised trials: Trials on animals.
On poultry and eggs
Gas chromatographic determinations showed that no residues were
present in giblets, muscle and fat (limit of determination of 0.1 ppm)
or in the eggs (limit of determination of 0.003 ppm) of poultry which
had been maintained for four weeks on a diet containing 2.5 and 5 ppm
of the compound (Chemagro Report 20.945; 21.502).
In plants
Studies of the metabolism of trichlorfon in plants were carried out on
cotton plants.
The identified metabolites included dimethyl phosphate which accounted
for up to 70% for the applied trichlorfon dose; only small amounts of
monomethyl phosphate, O-demethyl trichlorfon, O-demethyl dichlorvos
and dichlorvos were formed (Hassan et al., 1966; Bull and Ridgway,
1969). Besides these metabolites, Bull and Ridgway found an
unidentified metabolite which accounted for a large percentage of the
applied dose of parent compound; this phosphorus-containing unknown is
split by ß-glucosidase but not by ß-glucuronidase.
Chloral and trichloroethanol are stated to be possible degradation
products (Arthur and Casida, 1957). However, these products could not
be detected in routine residue studies (Chemagro Corporation,
unpublished). Earlier studies also revealed that chloral and
trichloroethanol are metabolized in plants to form
B-2,2,2-trichloroethanol-gentiobioside (Miller, 1941).
In micro-organism cultures
Studies on Penicillium notatum, Fusarium sp. and Aspergillus niger
showed that approximately half of the applied parent compound is
transformed within 10 days. The principal metabolite found was
O-demethyl trichlorfon. A second degradation product formed is
probably 2,2,2-trichloro-1-hydroxyethyl-phosphonate (Zayed et al.,
1965).
In soil
Trichlorfon is not used for the control of soil pests. Studies
conducted by Chemagro Corporation have shown, however, that after
addition of 10 ppm of trichlorfon to soil, the residue level drops
below the limit of determination within 15-112 days depending upon the
soil type. Trichlorfon has a very low stability in soil; residues of
trichlorfon in runoff water from sandy loam, silt loam and high
organic silt loam soils showed a relatively low tendency to move
(Chemagro Report 28.937). After addition of 0.25 ppm of trichlorfon to
pond water, no residues are to be found in the mud (Chemagro Report
21.313).
In food processing
The published data on trichlorfon residues have generally been
obtained in studies of non-processed plant and animal products. When
these products are processed for the human diet, the trichlorfon
residue level is considerably reduced by such processing procedures as
boiling and sterilization.
In spinach, the residues which have an original level of 2.0 ppm in
the treated plants are reduced by bleaching (five minutes at 100°C) to
a concentration of 0.03 ppm and by further sterilization (100 minutes
at 115°C) to a level of 0.01 ppm. In dwarf beans, the residue level is
reduced from 1.22 ppm to 0.06 ppm and 0.05 ppm, respectively. In peas,
which had an original residue level of 0.21 ppm, no more residues were
detectable after bleaching and sterilization (Dormale et al., 1959).
Tomatoes were treated with 10 ppm of the compound and then canned
according to standard commercial procedure. The heat treatment
destroyed 80% of the compound (Chemagro Report 9012). Safflower seed
containing 0.9 ppm of the compound was processed into meal, crude oil
and refined oil. None of the three processed products contained
detectable residues (Chemagro Report 11.255).
Meat of sheep which ware slaughtered one hour after receiving a
peroral application of 120 mg/kg contained 1-10 ppm of the compound;
after the meat had been boiled for one hour, it contained no
detectable residue (Behrenz, 1959).
In beef which was immersed in a trichlorfon solution to prevent fly
maggot infestation, the residue level of the compound was reduced by
thorough washing from 0.77 ppm to 0.58 ppm. After one hour's boiling,
the residue level in the meat and in the broth was less than 0.1 ppm
(Börger and Maier-Bode, 1967).
Evidence of residues in food in commerce or at consumption
No data were submitted for consideration.
Methods of residue analysis
Many colorimetric, thin-layer chromatographic and gas chromatographic
methods for the determination of trichlorfon residues are described in
the literature. Some of the methods permit simultaneous determination
of trichlorfon and its possible metabolites, e.g. trichloroethanol and
dichlorvos. However, formation of trichloroethanol was not established
in any of the residue studies undertaken.
Trichlorfon can be extracted from plant material with chloroform
(Zadrozinska, 1966), acetone/hexane (Anderson et al., 1966), ethyl
acetate (Cernà, 1963; Watts et al., 1969) or diluted acetic acid
(Sissons and Telling, 1970). For clean-up, the extract residue is
transferred to water; after separating the plant constituents with
petroleum ether or heptane, the parent compound is extracted with
chloroform or diethyl ether (Zadrozinska, 1966; Anderson et al.,
1966). For determination of the compound by the cholinesterase
inhibition technique, extraction with water will suffice (Reynolds
et al., 1960; Chemagro Report 2412; 3581). Column chromatographic
clean-up of the plant extract is possible on charcoal (Watts et al.,
1969) or on aluminium oxide (Sissons and Telling, 1970). If plant
material with a high water content is involved, the compound can be
separated from the plant constituents by dialysis of the macerate in
diluted sulfuric acid, and extracted from the diffusate with diethyl
ether (Anderson et al., 1966; Chemagro Report 8839; 21811).
Trichlorfon can be extracted from animal tissues with acetonitrile
(Beck and Sherman, 1968; Anderson et al., 1966; Chemagro Report
14393), chloroform (Ackermann et al., 1969; Chemagro Report 24808) or
acetone (Chemagro Report 24808). For further clean-up, the extract
residue is transferred to water, fat portions are separated with
heptane, and the parent compound is extracted from the aqueous phase
with diethyl ether (Chemagro Report 14393; Anderson et al., 1966),
In milk, trichlorfon (after removal of fat by centrifuging and
separation of protein by precipitation) is determined either directly
by bioassay (Wickham and Flanagan, 1962) or (after extraction with
chloroform) by thin-layer chromatography, together with possibly
formed dichlorvos (Ackermann et al., 1968). For gas chromatographic
determination of trichlorfon, the milk is extracted with acetone and
benzene. In this method, trichloroethanol is co-determined as a
possible metabolite (Chemagro Report 16460).
Enzymatic determination
Giang and Hall (1951) developed a method for the enzymatic
determination of organic phosphorus insecticides (Delta pH method).
For the determination of trichlorfon residues, the parent compound is
converted to dichlorvos which is a strong cholinesterase inhibitor
(Reynolds et al., 1960; Chemagro Report 2412; 3581), The lower limit
of determination is approximately 0.01 ppm trichlorfon. The method is
unspecific.
Bioassays
Bioassays with mosquito larvae (Aedes aegypti) have been employed
for determining residues of trichlorfon in milk (Behrenz, 1961;
Wickham and Flanagan, 1962). Börger and Maier-Bode (1967) used
Daphnia magna as the test species for determining residues of
trichlorfon in meat. The limit of determination is approximately 0.05
ppm. The method is unspecific.
Thin-layer chromatography
Trichlorfon residues in plants and in animal tissues can be determined
by thin-layer chromatography on silica gel or aluminium oxide plates
sprayed with silver nitrate. The lower limit of determination is
0.25-0.5 ppm of trichlorfon in apples (Zadrozinska, 1966) and
0.2-0.5 µg in animal tissue extracts (Beck and Sherman, 1968). Smaller
amounts of trichlorfon and dichlorvos can be determined by the
cholinesterase inhibition technique (Ackermann et al., 1968, 1969).
Agar-diffusion method
The agar-diffusion method described by Sandi (1962) was modified for
routine determination of trichlorfon. A residue level of 0.1 ppm can
be determined in milk; however, it is not possible to separate
trichlorfon from dichlorvos (Ackermann et al., 1968).
Colorimetry
A colorimetric micro method for determining trichlorfon residues is
based on the determination of chloroform (which is separated from the
trichlorfon by pyrolysis at 550°C) with pyridine and sodium hydroxide
(Fujiwara reaction). This method was used for determining trichlorfon
residues in olive oil (Allessandrini and Lanforti, 1957) and in fruit
and lettuce (Cernà, 1963). The limit of determination is approximately
1 ppm in olive oil and 10 µg in plant material.
Trichlorfon residues can be determined colorimetrically also by a
total phosphorus procedure in plant material (Sissons and Telling,
1970) and in milk (Leahy, 1964); the lower limit of determination is
0.1-0.2 ppm.
Gas chromatography
Trichlorfon residues can be determined with a high degree of
sensitivity and specificity by gas chromatography. The intact molecule
is not determined by this method but instead the cleavage products
chloral or dimethyl phosphite which form upon pyrolytic cleavage of
trichlorfon in the injection port of the gas chromatograph. Chloral is
detected with the electron-capture detector (Anderson et al., 1966);
trichloroethanol can be simultaneously determined as a theoretically
possible metabolite. Chloral can also be determined with the Dohmann
microcoulometric gas chromatograph (Chemagro Report 8839). The
clean-up of extracts is simpler when trichlorfon is detected with a
phosphorus-specific detector, e.g. a modified flame ionization
detector (thermionic detector) (Chemagro Report 21811, 24808). The
limits of determination of these gas chromatographic methods depend
upon the material being analysed and the clean-up procedure used.
However, they are usually very low, e.g. 0.01-0.1 ppm with an
electron-capture detector and 0.003-0.06 ppm with the phosphorus
detector.
Studies on 30 different phosphorus-containing pesticides registered
for use in different crops show that only phosphamidon interferes with
the gas chromatographic determination of trichlorfon with the
phosphorus detector (Chemagro Report 21811, 27471, 29411). By using a
different column, this interference can be avoided (Chemagro Report
26335).
Parallel residue analyses of treated plant material showed agreement
of residue values obtained by the cholinesterase inhibition technique
and by gas chromatography. Determination by the cholinesterase
inhibition technique produces slightly higher residue values, which is
probably due to the presence of slight traces of dichlorvos (Chemagro
Report 9733).
For routine determination of trichlorfon residues, gas chromatography
is the most suitable method. The compound can be detected with a
microcoulometer, an electron-capture detector or a phosphorus-specific
detector, e.g. a thermionic or flame photometric detector.
Examples of national tolerances and safety intervals
Country Crop Tolerance Safety
ppm interval
days
Argentina Bananas 0.2 (provisional)
Bananas (peeled) 0
Australia General 2
Fruits, grains, vegetables 2.0
Austria General 14
Cucumbers, tomatoes,
peppers 4
Belgium General 10
Fruits, vegetables,
excl. potatoes 0.5
Brazil Vegetables 0.5 7
Fruits and field crops 0.5 10
Meat and milk 0.001
Bulgaria General 14
Canada Alfalfa 14
Rape 21
Corn 40
Sugar beets 14
Sugar beets, tops 28
Tobacco 3
Beans 14
Cabbage, cauliflower,
Brussels sprouts 21
Country Crop Tolerance Safety
ppm interval
days
Carrots, rutabagas,
salsify, turnips 28
Collards, kale, lettuce,
spinach 28
Peppers 21
Table beets 28
Tomatoes 21
Artichokes, bananas, NR
beans, beef cattle, NR
beets, Brussels NR
sprouts, cabbage, NR
carrots, cauliflower, NR
collards, corn, kale, NR
lettuce, peppers, rape NR
seed, rutabagas, NR
salsify, spinach, NR
sugar beets, tomatoes, NR
turnips NR
Finland General 14
France General 7
German Democratic Fruits, root vegetables,
Republic leafy vegetables,
cabbages, legumes,
fruit-producing
vegetables (tomatoes,
cucumbers) 1.0
Meat, fish, animal and
vegetable fats, eggs,
milk, baby foods 0
Field crops, fruits 10
Cherries 5
Vegetables 7
Special crops 14
Crops used for the
production of baby foods,
drugs and health foods 30
German Federal Fruits, field crops,
Republic incl. fodder crops 10
Vegetables 7
Application under glass:
general 10
Leafy vegetables,
fruit-producing vegetables,
root vegetables,
Country Crop Tolerance Safety
ppm interval
days
legumes, fruits incl.
grapes 0.5
Great Britain General 2
Hungary Animal products 0
Israel Fruits incl. grapes,
clover, alfalfa,
sub-tropical trees, sugar
beets, fodder crops 7
Maize 30
Tomatoes, cucumbers,
cucurbits, cabbage,
radish, lettuce,
spinach, beans, other
vegetables 10
Peppers, eggplants,
strawberries, cauliflower,
artichokes 14
Morocco General 7
Olives 30
Netherlands General 0.5
General, field-grown 10
Spinach, field-grown 4
General (under glass
from 1 March - 1 Nov.) 17
New Zealand Tomatoes (canned) 1
Other crops 14
Poland Fruits, legumes (not
vegetables), root
crops and other field
crops 14
Vegetables 10
Portugal General 7
Industrial tomatoes 4
South Africa Maize, wheat, citrus
fruits, apples, pears,
apricots, peaches,
sub-tropical crops 10
Cucurbits 7
Alfalfa 2
Tomatoes 3
General 2.0
Soviet Union General 1.0
Spain General 10
Country Crop Tolerance Safety
ppm interval
days
Sweden General 14
Switzerland General 21
Vegetables 14
Beets 42
Vegetables, legumes,
fruits incl. grapes 0.5
United States of See USDA Summary of
America Registered Agricultural
Pesticide Chemical Uses
Yugoslavia General 0.5
Fruits, vegetables,
field crops 14
Appraisal
Trichlorfon is an organophosphorus insecticide which is especially
used on crops against a variety of insects (moths, flies, bugs, etc.).
It is also widely used against ecto- and endoparasites of animals, in
public health and as an anthelmintic in medicine.
It is used on a wide variety of field and pasture crops with
application rates of 150-2000 g/ha, normally 500-1200 g/ha, or as
0.075-0.2% sprays. As an animal health product, it is normally applied
at a rate of 25-75 mg/kg body-weight externally as wash or spray and
spot-on treatment and internally by mouth and by injection. In public
health it is used against flies and other pests commonly in the form
of baits. Other uses are on ornamentals, in tree nurseries, and in
forestry.
Residue data for evaluation are satisfactory.
The behaviour of trichlorfon is characterized by its hydrophilic
properties. Its decomposition is brought about by splitting the P-C
bond and by hydrolysis of the P-OCH3 bonds. In addition, in tissues
it can be converted in trace amounts to dichlorvos. Products of more
advanced degradation have also been found and characterized. In food
processing, trichlorfon residues are substantially disappearing.
As a result of use of trichlorfon, residues may occur in animal feed.
When the applications are made in accordance with good agricultural
practice the residues are considered to be no hazard to the animal
health and no detectable contamination of the foods derived from these
animals is to be expected.
There are a number of methods of residue analysis available. For
regulatory purposes highly specific and sensitive gas chromatographic
methods with detection limits of less than 0.01-0.1 ppm have been
developed.
Due to the insignificant magnitude of dichlorvos and to the low
toxicity of other degradation products, recommendations for tolerances
of trichlorfon residues are made in terms of the parent compound.
Since the direct application of trichlorfon to the domestic animals
may result, although for a short limited period of time, in residues
of varying magnitude in milk, meat, and fat, it is necessary, in
accordance with the local needs of animal health, to set limitations
of use, e.g. type of formulation, route of application, dosage,
condition of the animals, and safety interval from treatment to use of
animal products for human food. The recommendations for trichlorfon
residues in animal products are made, because the use of insecticides
is necessary in animal health, application of trichlorfon according to
good agricultural practice will cause no health hazards to animals,
and, by adjusting properly the conditions of use, milk, meat, organs,
and fat can be obtained from the treated animals practically free from
the trichlorfon residues. Additional safeguard of the consumer against
the residues is the degradation of trichlorfon in storage, processing
and cooking of the animal products before they are consumed.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The following recommendations are made for temporary tolerances:
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Cereals:
Barley 21 0.1
Maize 30 0.1
Wheat 30 0.2
Fruits:
Apples 14 0.1
Bananas, pulp 0 0.2
Cherries 7 0.1
Oranges, pulp 7 0.1
Peaches 14 0.2
Strawberries 4 0.1
(continued)
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Vegetables:
Artichokes 7 0.1
Blackeyed, beans 14 0.1
Brussels sprouts 14 0.2
Cabbage 14 0.1
Cauliflower 21 0.2
Cowpeas 14 0.1
Green beans 14 0.1
Kale 14 0.2
Lima beans 14 0.1
Mustard, leaf 14 0.1
Peppers 14 1
Pumpkins 14 0.1
Sweet corn, kernels and cobs 14 0.2
Tomatoes 14 0.1
Celery 14 0.2
Sugar beets 30 0.05
Beets 14 0.2
Turnips 14 0.1
Oil seeds:
Cotton seed 14 0.1
Flax seed 14 0.1
Lin seed 30 0.1
Safflower seed 45 0.1
Soybeans, dry 45 0.1
Nuts:
Peanuts, shelled 7 0.1
Animal products:
Meat, fat and by-products
of cattle and pigs 14 0.1
Whole milk 2 0.05
Further work or information
Required before 30 June 1975
1. A two-generation carcinogenicity study to elucidate the possible
increase in the incidence of tumours including those of the
mammary gland.
2. More information on residues on oats.
3. More information on residues on lettuce and spinach under various
conditions (including greenhouses).
Desirable
1. Elucidation of the effect on spermatogenesis.
2. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abdalla, A., Saif, M., Taha, A., Askmawy, H., Tawfik, J., Abdel
Fattah, F., Sabet, S. and Abdel-Mequid, M. (1965) Evaluation of an
organophosphorus compound Dipterex on the treatment of Bilharziasis.
J. Egypt Med. Assoc., 48: 262-273
Abdel-Aal, A. M. A., El Hawary, M. F. S., Kamel, H., Abdel-Kalek,
M. K. and El-Diwany, K. M. J. (1970) Egypt Med. Assoc., 53: 265-271
Ackermann, H., Engst, R. and Fechner, G. (1968) Methode zur getrennten
Bestimmung von Trichlorphon und Dichlorphos-Rückständen in Milch.
Ztschr. Lebensmittelunters. Forsch. 137: 303-308
Ackermann, H., Lexow, B. and Plewka, E. (1969) Nachweis und
Identifizierung von insecticiden Phosphor-,
Thiophosphor- Phosphon- und Carbaminsäureestern im Biologischen
Material. Arch. f. Toxicol., 24: 316-324
Adkins, T. R., jr. (1966) Residues in cattle tissues following
back-line and spray applications of Trichlorfon. J. Econ. Entomol.,
59: 1423-1425
Alessandrini, M. E. and Lanforti, G. F. (1957) Determinazione di
residui di Dipterex (O,O-dimetil-2,2,2,trichloro-1-idrossietil
fosfato) nell' olio di oliva. Rend. Ist. super. Sanita, 20: 093-1003
Anderson, R. J., Anderson, C. A. and Olson, T. J. (1966) A Gas-liquid
chromatographic method for the determination of Trichlorfon in plant
and animal tissues. J. Agr. Food Chem., 14: 508-512
Arant, F. S., Atkins, T. R. and Sowell, W. L. Toxicity of Bayer L13/59
to rabbits. Unpublished report
Arnold, D., Keplinger, M. L., Fancher, O. E. and Calandra, J. C.
(1971) Mutagenic study with Dylox in Albino mice. Unpublished report
by Industrial Biotest Laboratories submitted by Farbenfabriken Bayer
A.G.,
Arthur, B. W. and Casida, J. E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2,-trichloro-1-hydroxethyl phosphonate and its acetyl
and vinyl derivatives. J. Agr. Food Chem., 5: 186-192
Arthur, B. W. and Casida, J. E. (1958) Biological activity of several
O,O-dialkyl alpha-acyloxyethyl phosphonates. J. Econ. Entomol.,
6: 360-365
Bailey, C. C., jr. (1956) Evaluation of the dermal toxicity of
malathion chlorthion and Dipterex to dogs. Thesis, Clemson College,
Clemson, S.C.
Bayer, A.G., (1967) Farbenfabriken, Pflanzenschutz. (R)Dipterex
(Bayer 15922, L 13/59) Leverkusen E. 10-6117/22 155
Bayer, A.G., Farbenfabriken, Pflanzenschutz. Documentation on 1971
trichlorfon for FAO
Beck, J. and Sherman, M. (1968) Detection by thin-layer chromatography
of organophosphorus insecticides in acutely poisoned rats and
chickens. Acta pharmacol. et toxicol., 26: 35-40
Beheyet, P., Lebrun. A., Cerf, J., Dierickx, J. and DeGroote. V.
(1961) Etude do la toxicite pour homme d'un insecticide
organophosphore. Bull. Wld Hlth Org., 24: 465-473
Behrenz, W. (1959) Biologische Bestimmung des Wirkstoffgehaltes im
Fleisch von Schafen und Rindern zu verschiedenen Zeiten nach peroraler
Behandlung mit Neguvon. Arch. Lebensmittelhyg., 10: 64
Behrenz, W. (1961) Ueber die Auascheidung von Neguvon(R) in der
Milch nach einmaliger oraler und percutaner Anwendung des Präparates
bei Milchkühen. Vet. mod. Nachr., 133-145
Bolle, W. R. (1956) Neguvon, ein äusserlich und innerlich anwendbares
Insektizid, Larvizid und Acarizid. Vet. med. Nachr., 155-172
Borgmann, W. and Hunnold, G. A. (1955) Report on the results of a
toxicological examination of Dipterex (L13/59). Unpublished report
submitted by Farbenfabriken Bayer A.G.
Brodeur, J. and Dubois, K. P. (1963) Comparison of acute toxicity of
anticholinesterase insecticides to weanling and adult male rats. Proc.
Soc. Exp. Biol., 114: 509-11
Bull, D. L. and Ridgway, R. L. (1969) Metabolism of trichlorfon in
animal and plants. J. Agr. Food Chem., 17: 837-841
Börger, K. and Maier-Bode, H. (1967) Versuche zur Verhinderung des
Fliegenmadon-Befalles von Fleisch. Arch. f. Lebens-mittelhyg., 18:
38-42
Cerná, V. (1963) Kolorimetrische Bestimmung von Dipterex-Rückstünden
in Lebensmittein. Die Nahrung, 7: 60-66
Chemagro-Report (Chemagro Corporation, Kansas City, USA)
2412 Tentative method for microestimation of Dipterex residues by
the Cholinesterase inhibition technique
3581 The determination of Dylox residues by the Cholinesterase
inhibition technique
8839 The microcoulometric determination of Dylox residues in
plant material
9012 The effect of canning on Dylox residues in tomatoes
9733 A comparison of Dylox residue results obtained by the
Cholinesterase inhibition procedure (Report 3581) and the
vapor phase chromatographic procedure (Report 8839)
11 255 The effect of processing on Dylox residues in Safflower
14 393 Determination of Neguvon, Chloral hydrate and
trichlorethanol residues in animal tissues by electron
capture gas chromatography
14 412 Trichlorfon residues in cattle tissues (Backline application
with 8 and 16% pour-on)
16 460 Determination of Trichlorfon, Chloral hydrate and
trichlorethanol residues in milk by electron capture gas
chromatography
16 538 Trichlorfon residues in milk (barn spray prepared with S.P.)
17 440 Trichlorfon residues in milk (in feed prepared)
17 970 Trichlorfon residues in cattle milk (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
17 991 Trichlorfon residues in cattle tissues (in feed prepared)
18 321 Trichlorfon residues in cattle milk (in feed prepared with
technical standard)
18 322 Trichlorfon residues in milk (spray prepared with 80%
soluble powder)
18 495 Trichlorfon residues in cattle tissues (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
18 521 Trichlorfon residues in milk (pour-on with 8% formulation)
18 678 Trichlorfon residues in swine tissues (in feed with 90%
soluble powder)
18 884 Trichlorfon residues in cattle tissues (in feed prepared
with technical standard)
20 945 Trichlorfon residues in poultry tissues (in feed prepared
with Dylox 80% S.P.)
21 289 Trichlorfon residues in milk (mist spray prepared with 1%
Co-Ral - 2% Trichlorfon combination)
21 313 Trichlorfon residues in mud (spray on surface with 50% S.P.)
21 502 Trichlorfon residues in eggs (in feed prepared with 80%
S.P.)
21 811 Determination of residues of Trichlorfon in Alfalfa by
thermionic emission gas chromatography
24 808 Determination of residues of Trichlorfon in bovine animal
tissues by thermionic emission gas chromatography
26 117 Trichlorfon residues in cattle tissues (Backline application
using 8% pour-on)
26 334 A confirmatory gas chromatographic procedure for Trichlorfon
residue analysis
27 471 An interference study for Trichlorfon residue determination
on Alfalfa and Clover
27 533 Trichlorfon residues in swine tissues (drinking water
treated with Neguvon)
27 561 DDVP-residues in swine tissues (drinking water treated with
Neguvon)
28 937 Trichlorfon residues in runoff water from soils
29 234 Trichlorfon residues in milk (Bolus-capsules fortified with
Dylox 80% S.P.)
29 411 An interference study for the residue method for Trichlorfon
on various crops
Davis, A. and Bailey, D. R. (1969) Metrifonate in urinary
schistosomiasis. Bull. Wld Hlth Org., 41: 209-224
Dedek, W. and Lohs, K. (1970a) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern I. Untersuchungen in vitro in Humanserum
mit 14C-Trichlorphon. Z. Naturforsch., 25b: 94-96
Dedek, W. and Lohs, K. (1970b) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern II. Verteilung von 14C in Organen und
Leberproteinen bei Ratten nach Applikation von 14C-Trichlorphon. Z.
Naturforsch., 25b: 1110-1113
Dedek, W. and Schwarz, H. (1970) Studien zur percutanen Resorption von
32P-Dimethoat am Schaf. Z. Naturforsch., 25b: 1193-1194
Deichmann, W. B. and Lampe, K. (1955) Dipterex, its pharmacological
action. Bull. Univ. Miami Sch. Med., 9: 7-12
Dinerman, A. A., Lavrent'eva, N. A. and Il'inskaia. N. A. (1970) The
embryotoxic action of some pesticides. Gigiena i. Sanit., 35: 39-42
Dormale, S., Martens, P. H., Decleire, M. and de Faetraets, L. (1959)
Etude do la persistance de résidus d'insecticides dans divers légumes
frais, blanchis et stérilisés. Bull. Inst. agron. et Stat. Rech.
Gembloux, 27: 137-147
Doull, J. and Dubois, K. P. (1956) The effects of diets containing
Dipterex on rats. Unpublished report by Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J. and Fitch, F.
(1962a) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Doull, J. and Dubois, K. P. (1958) The effects of diets containing
Dipterex for dogs. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Vaughn, G. and Dubois, K. P. (1958) Effect of diets
containing Dipterex in combination with organic phosphates on dogs and
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Fitch, F., Meskauskas, J., Root, M. and
Cowan, J. (1965) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Root; M., Cowan, J., Meskauskas, J. and
Fitch, F. (1962b) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Dubois, K. P. (1958) Potentiation of the toxicity of insecticidal
organic phosphates. AMA Arch. Indust. Health, 18: 488-496
Dubois, K. P. and Cotter, G. J. (1955) Studies on the toxicity and
mechanism of action of Dipterex. AMA Arch. Indust Health, 11: 53-60
Dubois, K. P. and Doull, J. (1955) Acute toxicity of Dipterex to
chickens and ducks. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol, appl.
Pharmacol., 14: 515-534
Giang, P. A. and Hall, S. A. Enzymatic determination of organic
phosphorus insecticides. Anal. Chem., 23: 1830-1834
Gibel, Von W., Lohs, Kh., Wildner. G-P, and Ziebarth, D. (1971)
Tierexperimentelle untersuchungen über die hepatotoxische und
Kanzerogene wirkung phosphoroganischer verbindungen. Arch. für
Geschwulstforschung, 37: 303-312
Gofmekler, V. A. add Tabakova, S. A. (1970) The effect of chorphos on
rat embrogenesis. Farmikol. i. Toksikol., 33: 735-737
Grundmann, E. and Hobik, H. P. (1966) Bay 15922/2 year feeding
experiment in rats/histology. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Hanna, S., Basmy, K., Osaima, S., Shoeb, S. M. and Awny, A. Y. (1966)
Effects of administration of an organophosphorus compound as an
antibilharzial agent with special reference to plasma cholinesterase.
Brit. Med. J., 1: 1390-1392
Hassan, A. and Zayed, S. M. A. D. (1965) Metabolism of
organophosphorus insecticides III. Fate of the methyl groups of
Dipterex in vivo. Can. J. Biochem., 43: 1271-1275
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides II. Metabolism of
O,O-Dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate (Dipterex) in
mammalian nervous tissue and kinetics involved in its reaction with
acetylcholine esterase. Can. J. Biochem., 43: 1263-1269
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides V. Mechanism of
detoxyfication of Dipterex in Prodenia Litura F. Biochem. Pharmacol.,
14: 1577-1584
Hassan, A., Zayed, S. M. A. D. and Hashish, S. (1965) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxyfication in the
rat. Biochem. Pharmacol., 14: 1692-1694
Hassan, A., Zayed, S. M. A. D. and Mostafa, I. Y. (1966) Metabolism of
organophosphorus insecticides VIII. Demethylation of Dipterex.
Z. Naturforsch., 21b: 498-500
Hobik, H. P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitat-sversuchen an huhnern mit
Dipterex. Unpublished report submitted by Farbenfabriken Bayer A.G.
Jackson, J. B., Drummond, R. O. Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Juszkiewicz, T. (1970) Insecticides residues in the tissues and milk
of cow following the dermal application of fenchlorvos and
trichlorphon: a preliminary report. Med. Weterynar (Poland),
26: 85-89
Kimmerle, G. and Lorke, D. (1966) Neurotoxische Untersuchungen an
Huhern mit Dipterex-Wirkstoff. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kühnert, M., Dedek, W. and Schwarz, H. (1963) Untersuchungen über die
Stoffwechselbeeinflussung und den Ausseheidungs-mechanismus des
Phosphonsäure-esters Trichlorphon im Handelspräparat "Bubulin" mit
Hilfe 32P-markierten Phosphors bei der intravenösen und
intramuskulären Injektion an Rindern. Arch. Exp. Vet. Med.,
17: 403-417
Leahy, J. S. (1964) Die Bestimmung von Rückstünden des Neguvon(R)
Milch nach dermaler Anwendung beim Rind. Vet. Med. Nachr., 37-48
Lebrun, A. and Cerf, C. (1960) Note preliminaire sur la Toxicite pour
l'homme d'un insecticide organophosphore (Dipterex). Bull. Wld Hlth
Org., 22: 579-582
Lindgren, P. D. and Ridgway, R. L. (1967) Toxicity of five
insecticides to several insect predators. J. Econ. Entomol.,
60: 1639-1641
Lorke, D. (1971) Trichlorfon. Untersuchungen auf embryotoxische und
teratogene wirkungen an der Ratte. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Lorke, D. and Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Lorke, D. and Kimmerle, G. (1968) Die wirkung von reactivatoren bei
der vergiftung mit phosphorsaureestern. Naunyn-Schmeidebergs Arch.
Pharmakol. Exp. Pathol., 263: S237
Löser, E. (1970) Bay 15922/Chronic toxicological studies on dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Löser, E. (1969) Generationsversuche an Ratten, Unpublished report
submitted by Farbenfabriken Bayer A.G.
Metcalf, R. L., Fukuto, T. R. and March, R. B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52: 44-49
Miller, L. P. (1941) Formation of 2,2,2-Trichloroethylgentiobioside in
tomato plants grown in media containing Chloral hydrate,
Trichloroethyl alcohol, or chloral cyanohydrin. Contribution from
Boyce Thompson Institute Plant Research, 12: 15-23
Miyamoto, J. (1959) Non-enzymic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24: 130-137
Miyamoto, J. (1961) Studies on the mode of action of Dipterex Part II.
New glucorunides obtained from the urine of rabbit following
administration of Dipterex. Agr. Biol. Chem., 25: 566-572
Miyamoto, J. (1968) Private communication to Bull, D. L. and Ridgway,
R. L.
Miyata, T., Iyatomi, K., Saito, T. and Morikawa, O. (1968) Metabolism
and cholinesterase inhibition of NS 2662,
O,O-dimethyl-2,2-dichloro-1-hydroxyethyl phosphonate, in the American
cockroach. Jap. Appl. Ent. Zool., 12: 211-219
Mühlmann, R. and Schrader, G. (1957) Hydrolyse der Insektiziden
Phosphorsäureester. Z. Naturforsch., 12b: 196-209
Murphy, S. D. and Dubois, K. P. (1958) Inhibitory effect of Dipterex
on the detoxification of malathion. Unpublished report by Department
of Pharmacology, University of Chicago
Namba, T. (1971) Cholinesterase inhibition by organophosphorus
compounds and its chemical effects. Bull. Wld Hlth Org., 44: 289-307
Pruessmann, R. (1968) Direct Alkylating Agents as Carcinogens. Food
cos. Toxicol., 6: 576-577
Rahn, H. W. (1963) Über die Moglichkeit der Trichlorphon Schadigung
bei Jung Tieren Laktierender Rattan. Arch. Exp. Veterinarmed.,
18: 713-717
Reynolds, H. T., Stern, V. M., Fukuto, T. R. and Peterson, G. D.
(1960) Potential use of Dylox and other insecticides in a control
program for field crop pests in California. J. Econ. Entomol.,
53: 72-78
Robbins, W. E., Hopkins, T. L. and Eddy, G. W. (1956) The metabolism
of 32P-labelled Bayer L 13/59 in a cow. J. Econ. Entomol.,
49: 801-806
Ross, E. and Sherman, M. (1960) The effect of selected insecticides on
growth and egg production when administered continuously in the feed.
Poultry Sci., 39: 1203-1211
Saito, T. (1969) Selective toxity of systemic insecticides. Res.
Reviews, 25: 175-186
Sándi. E. (1962) Kolorimetrische Bestimmung von Dipterex-Rückständen
in Lebensmitteln. Die Nahrung, 7: 60-66
Sato, K. and Saito, T. (1968) Selective Toxicity of NS 2662,
O,O-dimethyl dichloro-hydroxyethyl phosphonate, and Trichlorfon. Jap.
appl. Ent. Zool., 12: 148-155
Schulemann, W. (1955) Final opinion on the insecticide Bayer L13/59.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Schwarz, H. and Dedek, W. (1965a) Das Verhalten von radioaktiv
markiertem Trichlorphon nach Pour-on-Applikation (Aufgiessverfabren)
zur Dassellarvenbekämpfung beim Rind. Monatsh. Vet. Med.,
20: 958-968
Schwarz, H. and Dedek, W. (1965b) Untersuchungen über den Abbau und
die Ausseheidung von 32P-markiertem Trichlorphon beim Schwein. Zbl.
Vet. Med. (Reihe B), 12: 653-660
Schwarz, H, and Dedek, W. (1966) Untersuchungen zum Nachweis von
32P-markiertem Trichlorphon im Fleisch beim Schwein. Zbl. Vet. Med.
(Reihe B), 13: 489-494
Sherman, M. and Ross, E. (1959) Toxicity of housefly larvae to
insecticides administered as single oral doses to chicks. J. Econ.
Entomol., 52: 719-723
Sissons, D. J. and Telling, G. M. (1970) Rapid procedure for the
determination of organophosphorus insecticide residues in vegetables.
II. A screening procedure for watersoluble insecticides.
J. Chromatog., 48: 468-477
Spicer, E. J. F. and Payne, S. (1971) Pathology report of Bay 15922-4
year dog study (Loser, 1970). Unpublished report submitted by
Farbenfabriken Bayer A.G.
Spicer, E. J. F. and Urwin, C. (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Vbrovsky, L., Selecky, V. and Rosival, L. (1959) Toxikologische und
Pharmakologische Studien der Phosphorsaureester Insecticiden. Arch.
Exp. Pathol. Pharmol., 236: 202-205
Watts, R. R., Storherr, R. W. and Pardue, J. R. (1969) Charcoal column
clean up for many organophosphorus pesticide residues in crop
extracts. A.O.A.C., 52: 522-526
Wegner, D. (1970) Bilarcil(R) Bay 2349 - Klinische Erfahrungen
1960-1969. Unpublished report submitted by Farbenfabriken Bayer A.G.
Wickham, J. C. and Flanagan, P. (1962) Residues of Trichlorphon
(Neguvon) in milk after dermal application to cattle. J. Sci. Food
Agric., 13: 449-455
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Tox. appl. Pharmacol.,
1: 1-7
Wills, J. H. (1959) Recent studies of organic phosphate poisoning.
Fed. Proc., 18; 1020-1025
Witter, R. F. and Gaines, T. B. (1963) Relationship between depression
of brain or plasma cholinesterase and paralysis in chickens caused by
certain organic phosphorus compounds. Biochem. Pharmacol.,
12: 1377-1386
Zadrozinska, J. (1966) Oznaczanie Sladowych pozostalosci Diptereksu
w jablach metoda chromatografii cienkowwarstwowej (Determination of
trace residues of trichlorphon in apples by thin-layer
chromatography). Roczn. panst. Zakl. Hig., 17: 523-528
Zayed, S. M. A. D, and Hassan, A. (1965) Metabolism of
organophosphorus insecticides I. Distribution and metabolism of
Dipterex in adult larva of the cotton leaf worm (Prodenia Litura F.),
Can. J. Biochem., 43: 1257-1262
Zayed, S. M. A. D., Mostafa, I. Y. and Hassan, A. (1965) Organische
P-hattige Insectizide im Staffwichsel. VII. Umwandlung von
32-P-markiertem Dipterex durch Mikroorganismen. Arch. f. Mikrobiol.,
51: 118-121
Zhdanovich, N. V. and Udalov, I. U. F. (1970) The role of thiamine and
pyridoxime on acute and subacute intoxication with the
organophosphorus insecticide Dipterex. Vopr. Pitaniya, 29: 28-34
Excretion
Following acute administration trichlorfon is rapidly eliminated
primarily via the urine. Following intraperitoneal administration of
trichlorfon to rats, 71% of the total dose was eliminated in the urine
in 16 hours (Bull and Ridgeway, 1969). Following oral administration
to cows, 66% of the dose was eliminated within 12 hours (Robbins et
al., 1956). Arthur and Casida (1958) demonstrated that the major
quantity of radioactive components in urine of rats was hydrolysis
products with less than 1% of the products being extractable by
organic solvents. Secretion of trichlorfon into milk appears to occur
rapidly following application (Leahy, 1964; Robbins et al., 1956)
although this means of elimination is minor and residues are
eliminated rapidly. It seems apparent that elimination of the toxicant
is rapid although in one instance Arthur and Casida (1958) treated
rats with 2000 mg/kg and four hours later found 45% of the
administered dose in fat. This fat storage was not followed further.
The delayed recovery of cholinesterase systems in mammals suggests
that elimination of the toxicant is not complete and small quantities
of antiesterase agents may remain in the body for prolonged periods
(possibly in the fat).
Effect on enzymes and other biochemical parameters
Trichlorfon is a rapid irreversible inhibitor of cholinesterase.
Several investigators have reported in vitro values for inhibition
of this enzyme.
Enzyme source I50 (molar) References
Commercially purified 3.2 × 10-6 Arthur and Casida, 1957
Red blood cell (bovine) 3.2 × 10-6 Arthur and Casida, 1957
6.3 × 10-6 Bull and Ridgeway, 1959
Whole blood (human) 7.9 × 10-5 Arthur and Casida, 1957
Plasma (human) 1.5 × 10-5 Samir et al., 1966
Red blood cell (human) 3.4 × 10-6 Rosival et al., 1959
Serum (human) 1.3 × 10-7 Rosival et al., 1959
Brain (rat) 2.6 × 10-6 Dubois and Cotter, 1955
6.3 × 10-6 Hassan et al., 1965
8.7 × 10-4 Schulemann, 1957
In vivo, rat cholinesterase activity of brain serum and submaxillary
gland was maximally inhibited within 15 minutes of Rx by ip injection
(Dubois and Cotter, 1955). Recovery rates were dependent on the dose
administered (25, 50 or 75 mg/kg) with 75% of the enzyme recovered
within four hours at the highest treatment level. At the lowest level,
activity of cholinesterase was normal within an hour. Cholinesterase
inhibition in humans apparently recovers at a slower rate than
demonstrated with rats. Erythrocyte cholinesterase was not recovered
from two daily doses of 7.5 mg/kg (orally administered) for 38 days
after treatment. Maximum inhibition was only 50% of pretreatment
levels (Lebrun and Cerf, 1960). Cholinesterase levels in children
treated orally for 10 days with 5 or 10 mg/kg returned to normal
within four weeks.
Following instramuscular (25 mg/kg) or intravenous (20 mg/kg)
administration to cattle, calcium levels in the serum were reduced.
The calcium level returned to normal in three days (Kühnert et al.,
1963).
TOXICOLOGICAL STUDIES
Special studies
(a) Carcinogenicity
Following weekly subcutaneous administration of trichlorfon to rats,
two of 24 developed local sarcomas after a period of 800 days
(Pruessmann, 1968). Gibel et al. (1971) observed several incidents of
forestomach papilloma, liver carcinoma and abdominal sarcoma in rats
and mice administered trichlorfon (see "short-term studies").
(b) Reproduction
A three generation (two litter per generation) rat reproduction study
at levels of 0, 100, 300, 1000 and 3000 ppm in the diet resulted in
adverse effects on reproduction at 1000 ppm and above. At 1000 ppm
there was evidence of reduced fertility, smaller litters and reduced
body-weight of pups. At 3000 ppm the pregnancy rate was markedly
decreased and the pups were smaller and lighter in weight with none
surviving to weaning. No effects were noted at 300 ppm or below.
Microscopic examination of the F3b generation indicated no adverse
effects (Loser, 1969; Spicer and Urwin, 1971).
(c) Teratogenesis, mutagenesis
Daily oral administration of trichlorfon (100 mg/kg for 17 days) to
lactating rats resulted in no effect on the pups (Rahn, 1963).
Pregnant rats were administered trichlorfon by continuous inhalation
for 20 days at concentrations of 0.005, 0.02 and 9 mg/m3. At all
levels there were external and internal abnormalities in the
development of embryos, weight differences in organs of the rats,
weight differences in the embryos, shifts in the level of ascorbic
acid and nucleic acids in tissues of mothers and foetuses and
histopathological and histochemical changes in the placenta (Gofmekler
and Tabakova, 1970). No foetal abnormality or embryotoxic effects were
observed when trichlorfon was administered orally at 100 mg/kg/day to
pregnant rats from day six to 15 of gestation (Lorke, 1971).
Trichlorfon injected into chicken embryo egg sac at seven days after
fertilization at a dose of 0.0008 of the rat LD50 and examined at 21
days showed only a slight decrease in embryo viability (Dinerman et
al., 1970). Dominant lethal tests run with male mice injected with a
single dose of 0, 50 or 100 mg/kg and mated to untreated females
resulted in no adverse effects on reproduction or on the young (Arnold
et al., 1971).
Except for the one study by inhalation (Gofmekler and Tabakova, 1970),
trichlorfon does not appear to be a terata-inducing compound nor does
it induce mutations in rodents.
(d) Neurotoxicity
Subcutaneous administration of trichlorfon to chickens at a single
dose of 90 mg/kg did not result in ataxic neuropathy (Witter and
Gaines, 1963). When trichlorfon was fed to hens for 29 weeks at 130
ppm in the diet, no neurotoxic signs were observed (Ross and Sherman,
1960). Oral administration of a single dose of 100 mg/kg (with
atropine and PAM) or dietary levels of up to 5000 ppm for 30 days did
not result in delayed neurotoxicity. Trichlorfon does not induce
pathological demyelination or clinical signs of ataxia (Kimmerle and
Lorke, 1966; Hobik, 1967).
(e) Potentiation
Trichlorfon potentiates the toxicity of azinphos-methyl, EPN and
malathion but not several other organophosphates and carbamate
insecticides (Dubois, 1958; Doull et al., 1958; Kimmerle and Lorke,
1968).
Intraperitoneal administration of 10 mg/kg to rats resulted in a
decrease of the malathion detoxifying enzymes in liver and serum
(Murphy and Dubois, 1958). The effects on the detoxification system
were transient and were reversed in 24 hours. When rats were fed 100
ppm trichlorfon in combination with malathion (100 ppm) for two weeks,
no effects on the detoxifying enzyme were observed (Murphy and Dubois,
1958). However, when 100 ppm trichlorfon was fed to rats and dogs in
combination with malathion (1000 ppm), EPN (20 ppm) or azinphos-methyl
(5 ppm) for six weeks, effects on cholinesterase were noted with EPN
and malathion but not with azinphos-methyl (Doull et al., 1958). The
potentiated effects were greatest with EPN, less with malathion and
absent with azinphos-methyl.
(f) Antidotes
Trichlorfon intoxication in rodents, as with many antiesterase
organophosphate esters, responds to therapy with atropine and 2 - PAM
(Wills, 1959; Dubois and Cotter, 1955; Lorke and Kimmerle, 1968).
2 - PAM was effective in reactivating rabbit cholinesterase and
reducing mortality in mice (Wills, 1959) and rats (Lorke and Kimmerle,
1968).
A recent report on the action of thiamine and pyridoxime as
therapeutic agents indicates that the antivitamins (oxythiamine and
desoxypyridoxime) synergize the effects of poisoning. The two vitamins
when given prior to treatment have shown some beneficial effects
(Zhdanovich and Vdalov, 1970). There was little effect when given
after treatment.
Acute toxicity
Animal Sex Route LD50 References
(mg/kg)
Mouse M oral 660 Vbrovsky et al., 1959
M 950 Schulemann, 1955
M&F ip 500 Dubois and Cotter, 1955
M 650 Vbrovsky et al., 1959
F 575 Vbrovsky et al., 1959
M sc 267 Borgmann and Hunold, 1955
F 320 Borgmann and Hunold, 1955
Rat M&F oral 316-650 Dubois and Cotter, 1955
Deichman and Lampe, 1955
Edson and Noakes, 1960
Schulemann, 1955
Gaines, 1969
Hagan, 1958
Borgmann and Hunold, 1955
Animal Sex Route LD50 References
(mg/kg)
se 400 Arthur and Casida, 1958
ip 400 Arthur and Casida, 1957
160 Dubois, 1958
225 Dubois and Cotter, 1955
M 160 Murphy and Dubois, 1958
M
(adult) 250 Brodeur and Dubois, 1963
M
(weanling) 190 Brodeur and Dubois, 1963
M&F dermal 2 800 Edson and Noakes, 1960
2 000 Gaines, 1969
Guinea-pig M&F ip 300 Dubois and Cotter, 1955
200 Schulemann, 1955
Rabbit oral 160 Arant et al., 1971
dermal 5 000 Deichman and Lampe, 1955
Chicken oral 75-110 Dubois and Doull, 1955
Kimmerle and Lorke, 1966
sc 125 Witter and Gaines, 1963
65 Sherman and Ross, 1959
ip ca. 75 Kimmerle and Lorke, 1966
Duck
(1 week old) oral 105 Dubois and Doull, 1955
Dog oral 420 Deicbman and Lampe, 1955
Horse oral 100 Jackson et al., 1960
When trichlorfon was administered as a 10% solution to the
conjunctival sac of rabbits for six days, reversible effects,
including miosis and vasodilation of the blood vessels of the upper
lid, were observed (Deichman and Lampe, 1955). Dogs dipped once into a
1% solution showed no sign of toxicity although cholinesterase was
depressed (Bailey, 1956). Toxic signs of poisoning are typical of the
cholinergic response of organophosphate esters. Symptoms include
twitching, salivation, lacrymation, defaecation, urination, tonic and
clonic convulsions, prostration, cardiac arrest and respiratory
failure. The onset of symptoms is rapid as is the recovery following
sublethal poisoning. Intraperitoneal administration of toxic doses of
trichlorfon caused the appearance of symptoms in rats and mice in
about 10 minutes and death or recovery occurred within a few hours.
Symptoms included scattered muscular fibrillations and body twitches
which were followed by salivation, lacrymation, defaecation, and
urination, The severity of the symptoms increased with time and
included tonic and clonic convulsions, prostration and respiratory
failure preceded by cardiac arrest.
Skin irritation was absent when 1:1 mixture of trichlorfon and
emulsifier was applied to rat and guinea-pig backs daily for four
weeks (Borgmann and Hunold, 1955). Inhalation studies in a static
chamber with concentrations of 22 mg/l air caused symptoms of
cholinergic stimulation but no death. At 8 mg/l no signs of poisoning
were observed (Borgmann and Hunold, 1955).
Short-term studies
Rat
Rats (five rats per group) were administered trichlorfon
intraperitoneally at 50, 100 and 150 mg/kg/day for 60 days. Mortality
was observed at 100 mg/kg/day while at 50 mg/kg/day all animals
survived (Dubois and Cotter, 1955).
Rats (13 male and 13 female per group) were fed trichlorfon at dietary
levels of 0, 20, 100 and 300 ppm for 16 weeks. Significant
cholinesterase depression was noted at 300 ppm. No effects were
observed at 100 ppm on growth, behaviour, food consumption or gross
and microscopic examination of tissues (Doull and Dubois, 1956).
Rats (10 male rats per group) were fed trichlorfon at levels of 0. 1,
5, 25 and 125 ppm for 16 weeks. Cholinesterase depression was minor
(approximately 30%) at the initial phases of the test (4-8 weeks) and
slowly rose to near normal values. No effects were noted on food
consumption, growth or on gross examination of the tissues (Edson and
Noakes, 1960).
Cutaneous administration of trichlorfon to mice either alone or mixed
with "Krotonal" three times per week (alone) for five months or once
per week ("Krotonal") for six months resulted in a higher incidence of
pathological abnormalities than the controls. Cutaneous administration
alone resulted in an incidence of liver necrosis, liver cirrhosis,
liver carcinoma and forestomach papilloma. With "Krotonal" one case of
abdominal sarcoma was observed in addition to the other effects
(Gibel, 1971).
Dog
Dogs (two males and two females per group) were fed trichlorfon at
levels of O, 50, 250, 500 and 1000 ppm in the diet for one year.
Cholinesterase activity was depressed at 500 and 1000 ppm. At 500 and
1000 ppm an increased spleen weight with congestion and apparent
lymphoid atrophy was noted. Males at 1000 ppm exhibited a decrease in
spermatogenesis and the occurrence of hyperplastic nodules in the
adrenals. No effects were noted on mortality, growth, food
consumption, behaviour or gross and microscopic examination of tissues
other than those mentioned above (Doull et al., 1962a).
Two dogs were administered 45 mg trichlorfon per kg orally six days
per week for three months with no cumulative effects noted. The serum
cholinesterase level was 60% of normal at the conclusion. No mortality
was observed (Deichman and Lampe, 1955).
Dogs (one male and one female per group) were fed trichlorfon in the
diet for 12 weeks at levels of 20, 100, 300 and 500 ppm. The animals
were maintained on control diets for a further four weeks. Depression
of erythrocyte and serum cholinesterase activity was noted at 300 ppm
and above. Trichlorfon at 100 ppm did not affect cholinesterase. No
effects were noted at any level on the growth, food consumption or
behaviour of the dogs. Cholinesterase activity returned to normal
within two weeks of cessation of feeding (Doull and Vaughn, 1958).
Dogs (one male and one female per group, two males and two females
served as controls) were fed trichlorfon at levels of 0, 50, 200 and
500 ppm for 12 weeks in the diet. Plasma and erythrocyte
cholinesterase activity was depressed at 500 ppm and unaffected at 200
ppm. Recovery of enzyme activity was complete six weeks after
trichlorfon feeding stopped (Williams et al., 1959).
Long-term studies
Rat
Rats were administered trichlorfon three times weekly by oral or
subcutaneous administration for the life of the rats. Higher incidence
of liver necrosis, liver cirrhosis and forestomach papilloma were
evident than were described for the control (Gibel et al., 1971).
Five groups of rats (25 male and 25 female per group; controls
contained 50 males and 50 females) were fed diets of trichlorfon
containing 0, 50, 250, 500 and 1000 ppm for a period of two years,
Male rats at 1000 ppm gained less weight than the control after the
first two months and failed to regain the weight loss during the
remaining feeding period. Rats fed 1000 ppm weighed about 15% less
than the control animals. In male and female rats fed 1000 ppm the
onset of mortality occurred earlier than in rats from the other groups
and there was distinct shortening of the survival time in rats fed
this diet. Apparently 1000 ppm trichlorfon in the diet caused a
decrease in life span. Rats fed 500 ppm showed a moderate (25%)
inhibition of serum cholinesterase. No effect was noted on the
cholinesterase from brain submaxillary gland and erythrocyte at this
and lower levels. At 1000 ppm all cholinesterase determinations were
below normal, except for the brain. Gross pathological effects
resulting from the presence of trichlorfon in the diet included
mammary gland tumours which occurred in female rats fed 250 ppm (one
rat), 500 ppm (three rats) and 1000 ppm (two rats). Microscopic
examinations of the tissues indicated major adverse histological
findings observed in the mammary glands, gonads and blood vessels of
the trichlorfon fed animals. Three mammary tumours were observed at
1000 ppm (an adenocarcinoma, a sarcoma and a fibroma); three were
observed at 500 ppm (two adenocarcinomas and a fibroadenoma); and one
was found at 250 ppm (a fibroadenoma). Female rats, fed 500 and 1000
ppm, exhibited an absence of primary follicules and primative ova; one
rat fed 250 ppm also lacked follicules and ova. Two of the five rats
examined at 1000 ppm had tubular androblastomas which were composed of
epithelial components and stroma. Three of five male rats examined
from 1000 ppm feeding levels exhibited focal aspermogenesis not
observed at any other dietary levels. Lesions were observed in the
middle and small sized arteries of the lung, thymus, pancreas,
submucosa and adventitia of the gastrointestinal tract and in the
adrenal glands of several of the animals. The lesions were more common
in males than in females. The muscular and elastic tissue of the blood
vessel walls was frequently replaced by fibrous tissues and this was
associated with a necrotizing inflammation. The gross and microscopic
examination of the tissues from animals fed the trichlorfon-containing
diets revealed three pathological effects which appeared to be related
to the presence of the chemical in the diet. These consisted of an
increase in the incidence of mammary tumours in the female rats,
vascular changes and injury to the reproductive systems of both male
and female animals. The incidence of mammary tumour formation in the
entire group of rats used in the study (rather than the relatively
small number of animals used for histological examinations) shows that
the incidence in the rats fed control diets was 14%, 8% of the rats
fed 50 ppm, 20% of the rats fed 250 ppm, 21% at 500 ppm and 25% at
1000 ppm. The comparison of the time for the first tumours to appear
showed that in control diets 1.7 years, 50 ppm diet 1.6 years, 250 ppm
1.8 years, 500 ppm 1.5 years, and 1000 ppm 1.1 years. Thus, the total
frequency and onset of tumour formation appeared to be dose dependent
(Doull et al., 1962b).
Rats (25 male and 50 female per group) were fed dietary levels of
trichlorfon at 0, 100, 200 and 400 ppm for 1.5 years (Doull et al.,
1965). Serum cholinesterase depression was observed in both males and
females at 400 ppm. Erythrocyte cholinesterase was depressed in males
and females at 400 ppm; slightly in males at 200 ppm and very slightly
depressed in females at 100 ppm. There was a significant mortality in
the study in the control and experimental groups. The study was
prematurely concluded at approximately 70 weeks of feeding. At 400 ppm
the male spleen and liver weights were lighter than the control
values. At autopsy the major findings occurred in the ovaries and
mammary glands with slight effects on the lungs. Cystic granular
ovaries were seen in 40% of the female rats fed 400 ppm; 33% of the
rats fed 200 ppm; 14% of the rats fed 100 ppm; and 8% of the female
control rats. Fifteen per cent. of the female rats fed 400 ppm had one
or more mammary tumours; 11% of the rats fed 200 ppm; and 8% of the
control had mammary tumours. The induction time for mammary tumours
was not significantly decreased by increasing dietary concentrations
of trichlorfon. There were pulmonary abscesses in the lungs of two
female rats fed 400 ppm and two other female rats from this group
exhibited exudative pleuritis. Microscopic examination of the tissues
indicated an absence of primary follicules and primitive ova in four
of the five rats examined at 400 ppm. This was a significant increase
from those found at lower levels of feeding. The ovaries from the rats
fed 400 ppm were atrophic, small in size with nests of luteal or
granulosa cells, and an increased number of androblasts. Similar but
less marked changes were found in other groups. A greater frequency of
simple cysts of the ovaries was observed at 400 ppm than in the
control group. None of the cysts appeared to be neoplastic.
Examinations of the mammary tumours indicated that they were benign
fibro-epithelial tumours. Microscopic appearance of the tumours in the
trichlorfon fed animals was similar to the tumours seen in the control
animals. These studies suggest that the addition of trichlorfon to the
diet may have enhanced some of the aging changes, especially in the
reproductive tissue.
Rats (groups of 50 male and 50 female per group, 100 male and female
were used as controls) were fed trichlorfon at dietary levels of 0,
50, 250, 500 and 1000 ppm for two years. No effects were observed on
behaviour, food consumption, weight gain, survival, blood count,
urinalysis and liver protein content. Slight non-dose dependent
effects were noted in male and female liver on SDH activity and in
females on SG-OT activity. Cholinesterase was depressed in both males
and females at 1000 ppm but not at 500 ppm. The male liver weight was
significantly increased at 250 and 1000 ppm. An increase in weight was
observed although it was not statistically significant at 500 ppm.
There were no other dose-dependent, significant effects on tissue
weights. Clinical and histological examination of tissues or tumours
indicated that, of the total of 35 malignant tumours, 15 occurred in
the control group and only five were found at 1000 ppm dose levels.
There was no indication of an increased incidence of mammary tumours
although the total number of mammary tumours observed in the
trichlorfon fed animals was greater than that found in the controls
(the percentage of mammary tumour incidence in female rats was:
control, 9%; 1000 ppm, 8%; 500 ppm, 10%; 250 ppm, 12%; 50 ppm, 18%).
In the ovaries, cysts were found in 14 of the 33 control animals, and
at 1000 PPM cysts were found in eight of 20 animals examined. There
was no evidence of unusual interstitial fibrosis in the trichlorfon
fed animals. There appeared to be no evidence of acceleration of the
aging process of the gonads in this experiment. The frequency of
cystic atrophic ovaries or reduction in spermatogenesis was no
different in the controls than in the animals treated with 1000 ppm
trichlorfon in the diet (Lorke and Loser, 1966; Grundmann and Hobik,
1966).
Dog
Dogs (four male and four female per group) were maintained for four
years on diets containing 0, 50, 200, 800 and 3200 ppm trichlorfon
(Loser, 1970). Cholinesterase activity in plasma and erythrocytes was
depressed at 200 ppm. Trichlorfon, at 800 ppm and above, led to an
increased mortality rate. Increased uric acid and creatinine levels in
the male dogs at 800 ppm was indicative of kidney damage. Physical
appearance of the dogs was affected by 800 ppm in the diet. The
animals had a dull, shaggy coat and appeared weaker and sick.
Cholinergic symptoms occurred at the highest dose. Mortality was
evident in dogs fed 3200 ppm and 800 ppm (one male at 800 ppm and two
females at 800 ppm survived the test). Haematological values showed no
pathological changes in any group at two years. The activity of the
serum transaminases (G-OT and G-PT) of the female dogs at 3200 ppm at
two years was significantly high and regarded as pathological. No
other effects were noted on liver function activity. The transaminase
activity in the female dogs surviving the test to four years was
normal. There were no dose related changes in liver function or blood
values at four years. Urine examinations on all dogs at two and four
years showed uric acid and creatinine levels at the 800 ppm dose in
males and the 3200 ppm dose in the female were increased. There were
no differences in the clearance tests. Blood sugar and cholesterol
levels were not affected at 200 ppm. Cholinesterase activity was
depressed at 200 ppm and the depression was dose dependent. In
general, the depression of cholinesterase activity was noticeable
during the earliest parts of the experiment and tended to decrease as
the experiment progressed until at four years the plasma and
erythrocyte cholinesterase level were slightly depressed at 200 ppm in
both male and female with no depression noted at 50 ppm. Comparison of
the organs weights showed that male dogs at 800 ppm had enlarged
spleen, smaller adrenals and testis, and the female at 3200 ppm had an
increased liver weight, enlarged spleen and adrenals and reduced ovary
size. Based on histological examination of tissues there were no
changes in morphology which were considered to be significant or
related to trichlorfon in the diet (Spicer and Payne, 1971).
Observations in man
Over 6000 people, most in South Africa and South America, have been
treated over the past few years for various intestinal and body
parasites (reviewed by Wegner, 1970). The dosages varied up to 70
mg/kg/day for periods up to 12 days. The dose of 7.5 mg/kg given 2-4
times at two week intervals was believed to be the best level as the
symptoms observed were less severe. Symptoms include cholinesterase
depression, weakness, nausea, diarrhoea and abdominal pain. Higher
doses (24 mg/kg) gave more severe symptoms including tachycardia,
salivation, cholic pain, vomiting, nausea, fatigue, tremors, and
sweating. The effects were not cumulative and spontaneous recovery in
all cases was rapid. In a few cases, an indication was given that
spermatogenesis (size and shape of sperm) might be affected in humans.
In all cases for treatment of parasites cholinesterase depression was
evident.
Namba (1971) reviewing the human data alluded to the observation that
three persons exposed to trichlorfon showed signs of delayed
neurotoxicity.
Various studies on humans have shown two effects: (1) cholinesterase
depression in all cases which was usually recovered within 30 days of
the cessation of treatment, and (2) a possible effect on
spermatogenesis (Wegner, 1970; Hanna et al., 1966; Lebrun and Cerf,
1960) which included reduced sperm count, seminal fluid volume and a
decreased motility and viability of the cells in a very limited number
of cases. Other studies showing no adverse effects include: Davis and
Bailey, 1969; Abdalla et al., 1965; Abdel-Aal et al., 1970; Beheyet,
1961. The dose of 7.5 mg/kg given once every two weeks appears to be
the best available. There is no human no-effect level recorded.
Comments
Trichlorfon appears to be rapidly absorbed, distributed, metabolized
and excreted in animals. The conversion of trichlorfon to dichlorvos
occurs both in plants and mammals but to only a very minor extent.
Several long-term studies in rats and dogs are available. In two
long-term studies in the rat evidence of an increased frequency and/or
onset of tumour formation (particularly mammary tumours) appeared to
be dose dependent. A third study in rats did not confirm these
observations. An incidence of cystic atrophic ovaries and reduced
spermatogenesis was also observed in two studies and not confirmed in
the third study, in a three generation rat reproduction study or in a
dominant lethal test. None of the experiments individually considered
was by itself indicative of a carcinogenic effect, however the
cumulative evidence derived from all the experiments considered
suggests that further investigations of the potential carcinogenicity
of trichlorfon are required.
In view of the inconclusive nature of the findings in long-term rat
studies, only a temporary acceptable daily intake was established for
trichlorfon.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet equivalent to 2.5 mg/kg body-weight per day
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichlorfon is an insecticide with a broad spectrum of activity. It is
chiefly effective against Lepidoptera (moths), Diptera (flies),
and Heteroptera (bugs). In crop protection, trichlorfon is used
mainly against insect pests in field crops and fruit crops.
Trichlorfon is also of great importance as a public health pesticide
and as an animal health product, and is used in medicine as an
anthelmintic.
The amount of trichlorfon used in the different sectors of
application, expressed in percentages, is in the Western world as
follows:
45% in field crops (e,g. cereals, rice, maize, cotton, grassland,
tobacco)
35% in vegetable crops
10% in fruit crops (e.g. pome fruit, stone fruit, bush and cane
fruit, grapes, citrus fruit, olives)
10% for other uses (e.g. ornamental crops, hygiene, animal
health)
Approximately 25 formulations of trichlorfon are sold and registered
in 83 countries for the control of insect pests of crops and pests of
hygiene.
The following formulations are used in agriculture (crop protection):
50% emulsifiable concentrate, 50%. soluble powder, 80% soluble powder,
50% wettable powder, 2.5% granular, 5.0% granular, 5.0% dust.
Pre-harvest treatments
The recommended application rates and concentrations (in terms of
active ingredient) for pre-harvest treatments of the major crops are
as follows:
Bananas 400-600 g/ha
Beets 150-1000 g/ha, or
0.075-0.12% spray
Cereals (excl. corn and rice) 800-1200 g/ha
Citrus fruits 0.12% spray
Coffee 450-550 g/ha, or
0.075-0.12% spray
Corn 400-1500 g/ha
Cotton 400-1500 g/ha
Currants, gooseberries 0.075-0.12% spray
Deciduous fruits (apples, pears,
cherries, peaches, etc.) 0.075-0.12% spray
Grapes 0.075-0.12% spray
Grassland and forage crops 500-2000 g/ha
Oil palms 450-1600 g/ha,
or 0.08-0.2% spray
Olives 0.075-0.12% spray
Potatoes 600-1000 g/ha
Rice 800-1200 g/ha
Sugar cane 1200-1600 g/ha, or
0.075-0.2% spray
Tea 600-1200 g/ha
Tobacco 600-1200 g/ha
Vegetables 150-1200 g/ha, or
0.075-0.12% spray
For controlling soil-inhabiting cutworms use is made of baits which
are prepared by mixing 100 g active ingredient (soluble powder), 10 kg
bran, 500 g sugar in 10 litres of water. The crumbly mess is used to
treat 1/4-1/2 ha.
Bait sprays for controlling fruit flies consist of 0.08 or 0.32%
active ingredient and 0.25 or 0.5 protein hydrolyzate, respectively.
Post-harvest treatments
No recommendations.
Animal treatments
Trichlorfon is used as an animal health product for the control of
endoparasites and ectoparasites in/on cattle, sheep, goats, pigs,
horses, poultry, dogs, cats and fish. Following formulations are used:
97% soluble powder, 90% soluble powder, 80% soluble powder, 50%
soluble powder, 6% suspension, 11% solution, 5% injectable solution
tablets.
Methods of application are as follows: wash or spray treatment, dip
treatment, spot-on/pour-on treatment, injection, drench treatment,
feed mix, pond or immersion treatment.
Animal health products based on trichlorfon are registered and sold in
77 countries.
Spectrum of activity of trichlorfon in animal treatments is given in
Table 1.
Trichlorfon is normally applied to the animals at the rate of 25-75
mg/kg body-weight by various methods.
The most common method of application is as a wash or spray treatment
for the control of sucking lice, flies and biting lice. For this
purpose, the whole of the animal body is washed or sprayed with a
0.13% solution of Neguvon powder (repeat treatment after five days).
For the control of warble fly maggots on cattle and Sarcoptes mites
on pigs, a 2% solution is recommended (repeat treatment after 5-7
days).
For the external control of Stephanofilaria in cattle and Habronema
spp. in horses, use must be made of a 10% solution applied to the
affected areas.
The 11% ready-for-use Neguvon solution is used for the control of
warble fly maggots in cattle, a single application being made by the
spot-on method. An applicator is supplied with the solution.
Some of the trichlorfon enters through the skin into the blood system.
As a result, the effect is enhanced from inside the animal body. The
systemic action of trichlorfon against warble maggots which wander
through the body of the host animal until they eventually become
located beneath the skin of the animal's back, is also produced as a
result of the compound penetrating through the skin and entering the
blood system.
The success of an external treatment can be further increased by a
simultaneous internal application.
Internal treatment for the control of endoparasites is made as
follows:
TABLE 1. SPECTRUM OF ACTIVITY OF TRICHLORFON IN ANIMAL TREATMENTS
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
External application for control of
ectoparasites:
Biting lice X X X X X
Sucking lice/fleas X X X X X
Sarcoptes mites X X X X
Red avian mite/scaly leg mite X
Flies/sheep keds X X X X X
Warble fly maggots/larvae of
Dermatobia hominis X
Internal application for control of
ectoparasites:
Sarcoptes mites X
External application for control of
endoparasites on domestic animals:
Habronema spp. (summer sores) X
Stephanofilaria in cattle X
Internal application for control of
endoparasites:
Haemonchus spp., Mecistocirrus X X
Oesophagostomum spp. X X
Neoascaris vitulorum X
Bunostomum spp./Ostertagia spp./Cooperia
spp./Trichostrongylus spp. X X
TABLE 1. (Cont'd.)
Cattle Sheep/ Pigs Horses Poultry Dogs/ Fish
Goats Cats
Oxyguris equi X
Stephanofilaria X
Larvae of sheep keds (Oestrus ovis) X
Ascarids X X
Trichuris/Hyostrongylus X
Gastrophilus spp./Habronema spp. X
External application for control of
ectoparasites in fish:
Argulus/Ergasilus/Lernea/Dactylogyrus/
Gyrodactylus/Trichodina/different
fish leeches X
By means of a bottle or drenching gun. For this purpose, it is
recommended to use a 10% solution, the dose depending upon the
body-weight of the animal to be treated. The treatment should be
repeated after 2-3 months, or after an interval of three weeks if
infestation is severe.
In the feed (dry or liquid), mixture with Neguvon powder.
After oral application, trichlorfon is rapidly resorbed and is
translocated in the blood stream to the site at which it is required
to act. Degradation and excretion take place within a few hours.
The 50% Neguvon injectable formulation is a ready-for-use solution for
i.m. or s.c. injection, and is used for the control of Haemonchus
spp., Oesophagostomum spp., and Dermatobia hominis in cattle in
many countries of the Middle and Far East, Africa and Latin America.
For some time past, trichlorfon has also been in use as a pond
treatment and brief dip treatment for the control of ectoparasites in
fish, and has proved to be most successful. The required dose of the
80% powder formulation is 2.5 kg per ha of carp and eel ponds which
have a depth of 50 cm, the dose being raised to 5 kg per ha in 100 cm
deep ponds; the dosage rates needed for the treatment of trout ponds
are only half as large.
The dosage rate for the brief dip treatment for the control of
Argulus, Dactylogyrus and Gyrodactylus in carps is 2.5 kg per
100 litres of water, the duration of the dip treatment being five to
10 minutes.
In South Africa, trichlorfon is marketed also as a dog shampoo for the
control of fleas and ticks in dogs and cats, and as a powder
formulation for the control of fleas in dogs and cats.
Other uses
In hygiene trichlorfon is used for the control of flies in stables as
well as against many other pest species. Baits are used in most
instances.
Further, trichlorfon is used on ornamentals, tree nurseries; and
forestry.
Residues resulting from supervised trials
Trials on crops
The residue data obtained following application of trichlorfon to
fruit, vegetables and field crops are presented, in extracts, in Table
2. These data are from papers published in the literature as well as
from unpublished reports of Chemagro Corporation and Farbenfabriken
Bayer AG which have been compiled and submitted to the Meeting by
Bayer (1971). The residue values were determined initially by
enzymatic procedure (Delta pH method). and in recent years by gas
chromatography (electron-capture detector, phosphorus detector,
microcoulometer). Comparative analyses showed good agreement of these
methods.
Following application to green plant material, a half-life of about
1-2 days was obtained for trichlorfon, as shown by residue studies on
cotton leaves, grass, cabbage, clover, alfalfa and lettuce. Crops
treated 2-4 weeks before harvest are practically free of residues at
harvest time (maize, soybeans, rape, flax),
Following application to bananas, oranges and ground nuts, the bulk of
the trichlorfon residue is contained in the peel and shells,
respectively. Within a few days after the application only slight
residues are to be found in the pulp and nuts, respectively.
Trials on animals
On cattle
The studies of organs showed that following peroral application of
100 mg of active ingredient per kg, up to 10 ppm of active ingredient
is present in steak two hours after the application, and that this
amount decreases to a level of less than 0.1 ppm after 4-6 hours
(Behrenz, 1959). Following back-line and spray applications, organs
(liver, kidneys, brain, heart) and steak are practically free of
residues; in omental fat, on the other hand, maximum active ingredient
concentrations of 9.2 and 1.9 ppm were found one and seven days,
respectively, after the application (Adkins, 1966).
Following administration of trichlorfon to cattle by different routes,
residue studies were carried out by Chemagro Corporation, Kansas City,
United States of America, on practically all organs used for human
diet (meat, fat, liver, heart, kidneys and brain); the determinations
were made by sensitive gas chromatographic methods (limit of
determination 0.01-0.1 ppm; Chemagro Report 14.393; 24.808).
TABLE 2. RESIDUES RESULTING FROM SUPERVISED TRIALS
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Alfalfa 0.6 - 1.1 7 0.04 - 2.0
Alfalfa, seed hulls 1.7 8 0.2 - 1.6
" chaff 1.7 8 0.2 - 0.6
Apples (0.1 - 0.2%) 8 - 30 n.d. - 0.1
Artichokes, spray 1.1 0 - 12 n.d.
" dust 2.8 7 - 14 n.d.
Bananas, pulp 0.4 - 0.8 0 n.d. - 0.3
" peel 0.4 - 0.8 0 n.d. - 2.0
Barley 1.7 14 - 25 n.d.
Blackeyed, beans 1.7 14 n.d.
" vines 1.7 14 n.d. - 0.9
Brussel sprouts, spray 1.7 14 - 19 n.d.
" " dust 2.8 14 0.2
Cabbage 0.6 - 1.7 15 n.d. - 0.05
Cauliflower 1.7 19 - 21 n.d. - 0.15
Celery, spray 0.6 - 1.1 5 - 10 0.05 - 0.19
" dust 0.6 - 1.1 5 - 10 0.05 - 0.16
Cherries 0.3 - 0.5 3 - 8 0.001 - 0.12
Clover 1.1 7 - 8;14 <0.1 - 9.6;
<0.1 - 1.4
Clover, seed head 1.7 14 - 15 <0.1 - 0.7
" chaff 1.7 14 - 15 <0.1 - 0.6
Corn, kernel 0.8 - 1.7 30 - 144 n.d.
" cobs 0.8 - 1.7 30 - 144 n.d.
" husk 1.0 - 1.7 30 - 144 n.d.
" fodder, forage 1.0 - 1.7 30 - 144 n.d.
Cotton, seed 1.1 - 1.7 7 - 10 <0.01 - 0.05
" foliage 2.2 - 2.5 7 n.d. - 0.4
Cowpeas 1.7 14 n.d.
Cowpeas, vines 1.7 14 n.d. - 0.9
Grass, spray 1.1 - 2.2 12 2 - 5
" granular 1.1 - 2.8 12 <1 - 2
Garden beets 1.7 14 - 28 n.d.
Green beans 1.7 14 n.d.
Kale 0.6 - 1.7 6 - 10 n.d. - 0.3
Lettuce, leaf
summer appl. 1.1 - 1.7 7 0.1 - 0.2
winter appl. 1.1 - 1.7 7;14 6.0;3.9
Lettuce, head
summer appl. 1.1 - 1.7 7 <0.1 - 0.24
winter appl. 1.1 - 1.7 7;14 11.1;3.7
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Lima beans 1.7 9 - 15 n.d.
" vines 1.7 9 - 15 n.d. - 0.6
Lin seed 1.7 14 - 27 n.d.
Mustard 1.7 14/15 n.d.
Oats, grain 1.1 - 1.7 13/14 n.d. - 1.4
Oranges, peel, washed 1.1 - 1.7 7 0.1 - 0.21
" pulp 1.1 - 1.7 7 <0.01
Peaches (0.1) 7 - 14 <0.85
Peanuts, spray
" nuts 1.7 - 2.5 0 - 8 n.d. - 0.1
" shells 1.7 - 2.5 0 - 8 0.1 - 2.65
" vines 1.7 - 2.5 3 - 8 0.2 - 0.4
Peanuts, bait
" nuts 1.7 - 2.5 0 - 3 <0.01 - 0.16
" shells 1.7 - 2.5 0 - 3 <0.03 - 0.56
" vines 1.7 - 2.5 0 - 3 <0.07 - 2.9
Peppers, spray 1.1 - 1.7 14/15 n.d. - 0.3
" dust 1.2 14 0.4 - 1.1
Pumpkins 1.1 - 1.7 13/15 n.d.
Rape, seed 0.8 - 1.7 23 - 32 n.d. - 0.1
" meal 1.1 - 1.7 23 n.d.
" oil 1.1 - 1.7 23 n.d.
Safflower 2.2 27;33;43 0.6;<0.1;
<0.1
Soybeans, green beans, pods 0.6 0 0.1 - 6.0
" vines 0.6 0 0.5 - 8.8
" dry beans 0.6 35 - 53 <0.01
" dry vines 0.6 35 - 53 <0.08
(1 x 0.45)
Spinach 0.8 - 1.1 14/15 <0.1 - 1.6
Strawberries 0.3 - 1.1 3/4 <0.07
Sugar beets, roots 0.3 - 1.1 29 - 34 <0.01
" " tops 0.3 - 1.1 29 - 34 <0.05
Sweet corn, spray
" " husk 1.7 - 2.2 7 - 14 n.d. - 0.6
" " kernel 1.7 - 2.2 7 - 14 n.d.
" " cob 1.7 - 2.2 7 - 14 n.d. - 0.1
" " fodder 1.7 - 2.2 7 - 14 0.2 - 1.1
Sweet corn, dust
" " husk 2.0 15 2.7 - 9.1
" " kernel, cob 2.0 15 n.d.
TABLE 2. (Cont'd.)
Dosage active Pre-harvest Residue
Crop ingredient interval at harvest
kg/ha
(or % spray) days ppm
Sweet corn, granular
" " husk 1.1 - 1.7 14 - 21 n.d. - 8.4
" " kernel 1.1 - 1.7 14 - 21 n.d. - 0.1
" " cob 1.1 - 1.7 14 - 21 n.d. - 0.2
" " fodder 1.1 - 1.7 14 - 21 n.d. - 3.0
Table beets, roots 1.7 7 - 13 n.d. - 0.2
" " tops 1.7 7 - 13 0.2 - 2.5
Tea 1.4 - 1.8 21 <1
Tobacco, green
" spray 1.1 - 1.7 14/15 n.d. - 1.3
" dust 1.0 - 2.5 10/14 n.d.
Tobacco, cured
" spray 1.1 - 1.7 0 - 21 n.d
" dust 1.0 - 2.5 0 - 21 n.d
Tomatoes 0.8 - 1.7 7 n.d. - 0.5
Turnips, roots 10 - 15 n.d. - 0.09
Wheat, grain 1.3 - 1.7 14 - 39 n.d. - 0.25
" straw 1 25 - 39 n.d. - 4.2
" flour 1 25 - 39 <0.05
" bread 1 25 - 39 <0.05
Following peroral uptake of the active ingredient (12.5 and 20 ppm in
feed), no trichlorfon residues were detected (<0.1 ppm) in any of the
examined tissues and organs (brain, heart, kidney, steak, fat) after a
four week feeding period (Chemagro Report 17.991; 18.884). Following
external application of the 9 and 16% formulation - single back-line
application of 25, 50 or 100 mg of active ingredient per kg
(examination of tissues 21 days after application) and four week mist
spray application of 1.1 g of active ingredient per animal and day
(examination of tissues after the final application) - no residues
were detected in any of the tissues or organs (Chemagro Report 14.412;
18.495; 26.117).
Only slight proportions of the parent compound or its metabolites are
to be found in milk, as shown by a number of studies using
P32-labelled and unlabelled trichlorfon.
Following peroral application of 25 mg of active ingredient per kg,
less than 0.2% of the radioactivity representing the total dose
administered was secreted in the milk at the end of 144 hours; of
this, about 10% was unchanged active ingredient and about 23% behaved
like inorganic phosphorus (Robbins et al., 1956). Following similar
application of 80 mg of active ingredient per kg, the maximum residue
at eight hours was 2.5 ppm in the milk samples and at 22 hours
0.1 ppm; in the following milk samples, the residue levels were below
the limit of determination of 0.05 ppm (Behrenz, 1961). Following
spray application of five litres of a 2% active ingredient solution
and back-line was application of one litre of a 2% solution, the milk
samples at eight hours were found to contain 0.05-0.25 ppm of active
ingredient and in the later milk samples no residues were detected
(Behrenz, 1961).
Studies by Ackermann et al. (1968) showed that following backline wash
application of one litre of a 2% trichlorfon solution, the maximum
residue in the first milk sample at eight hours was 0.2 ppm; the
residues in the third milk sample at 32 hours were below 0.01 ppm.
Analyses of these milk samples for dichlorvos showed a maximum content
of 0.03 ppm in the first sample and 0.01 ppm in the third sample.
Following a single dermal treatment with 6% suspension, equivalent to
36 mg of active ingredient per kg, 0.1-0.2 ppm of trichlorfon was
found to be present within the first eight hours (Leahy, 1964).
Following application of 1.15 litres of a 2% trichlorfon solution (or
0.57 litres of a 4% solution) as a dermal wash per animal, the level
of active ingredient present in the milk did not at any time exceed
0.4 ppm; milk samples taken later than six hours after the application
contained about 0.1 ppm of active ingredient (Wickham and Flanagan,
1962).
Following pour-on application (of 100 or 300 ml of a 5.7% solution
(P32-labelled)), the active ingredient concentration in the milk
reached a peak of 0.13 or 0.47 ppm after 14-18 hours; after four days,
the content was less than 0.05 ppm, and after seven days it was less
than 0.01 ppm (Schwarz and Dedek, 1965a).
Following intravenous and intramuscular injection of 20 and 25 mg of
P32-labelled active ingredient, radioactive substances were found in
the milk only at six and 10 hours after the application; dichlorvos
was not formed in these experiments (Kühnert et al., 1963).
Chemagro Corporation, Kansas City, United States of America, carried
out residue studies by gas chromatographic methods following
application of the active ingredient to dairy cows by different
routes. The lower limit of determination was 0.003-0.01 ppm (Chemagro
Report 16.460; 24.808).
Following peroral uptake of the compound in the feed (12.5, 20, 50,
150 and 325 ppm) for a period of one or four weeks, no trichlorfon
residues were found to be present in the milk at the end of the
feeding period (Chemagro Report 17.440; 18.321; 29.234).
Following external application of the active ingredient as a single
pour-on treatment (at 3.7 g/100 kg) or as a single spray treatment
(at 28 g/animal) or as a four week mist spray treatment (1.1 g per
animal per day), active ingredient residues of no more than 0.04 and
0.02 ppm were found in the milk samples taken on the first and second
day after the application only for those animals which received the
pour-on treatment (Chemagro Report 17.970; 18.322; 18.521; 21.289).
The majority of these milk samples were analysed for residues of
trichloroethanol, a possible metabolite of trichlorfon; however, this
compound could not be found in any of the examined samples.
Following a spray treatment of stable walls at a rate of 1 g of active
ingredient per square metre, no trichlorfon residues were found in the
milk of the cows kept in the treated stable (Chemagro Report 16.538).
On pigs
For the use of trichlorfon for the control of ectoparasites and
endoparasites in pigs, the degradation and excretion of the active
ingredient was studied following subcutaneous injection of 25 mg of
P32-labelled trichlorfon per kg body-weight. The maximum active
ingredient concentration in blood was reached after 15-60 minutes
(10-11 ppm) and in the intestinal contents after 20-150 minutes
(4-5 ppm). After 5-7 hours, the blood and intestinal contents still
contained about 1 ppm of active ingredient. Dichlorvos was no longer
detectable in blood 3.5 hours after the application. The trichlorfon
concentration in meat was always somewhat lower than in the blood; a
concentration of 5 ppm was found in the meat two hours after the
injection. The trichlorfon concentration in the meat decreased by a
power of 10 in each 6-7 hours (Schwarz and Dedek, 1965b, 1966).
Chemagro Corporation carried out studies using gas chromatographic
methods (limit of determination 0.01-0.1 ppm residue; Chemagro Report
14.393; 24.808). After addition of 1500 ppm of active ingredient to
the drinking water of pigs (the ingested amount was equivalent to a
single dose of 125 mg of active ingredient per kg body-weight),
0.02-0.07 ppm of active ingredient was found in the liver and
0.02-0.05 ppm was found in loin steak four days after the application;
brain, heart, kidneys and fat samples were free of residues. None of
the organs contained any trichlorfon residues after seven days
(Chemagro Report 27.533). Dichlorvos was not detectable in any of the
examined samples four days after the trichlorfon application (Chemagro
Report 27.561).
No trichlorfon residues were detectable in any of the examined organs
and tissues 14 days after application of the compound in the diet at a
dose level equivalent to 100 mg/kg body-weight (Chemagro Report
18.678).
On sheep
Following peroral application of 120 mg of trichlorfon per kg
body-weight, exploratory residue determinations were carried out in
organs by means of a fly larva test. The residues dropped below the
limit of determination of 0.1 ppm within 4-6 hours after the
application (Behrenz, 1959). Following percutaneous application of
50 mg of the compound per kg body-weight, the concentration in the
blood reached a level of 1 ppm at which it remained for only a brief
period. Dichlorvos was detectable only in slight amounts (Dedek and
Schwarz, 1970).
Fate of residues
General comments
The distribution of trichlorfon residues in organisms is characterized
by its hydrophilic properties.
The decomposition of the molecule is brought about both by splitting
the P-C bond and by hydrolysis of the P-OCH3 bonds (Hassan and
Zayed, 1965). Furthermore, trichlorfon can be converted to the more
toxic dichlorvos, O,O-dimethyl-(2,2-dichlorovinyl)-phosphonate.
In animals
On account of its hydrophilic properties, trichlorfon is rapidly
absorbed by the organism, broken down and excreted in the urine.
Studies with P32-labelled compound on cattle showed that following
peroral application of dosages ranging from 25-100 mg of active
ingredient per kg body-weight, 66% of the P32 activity is excreted
in the urine within 12 hours; of this amount of excreted
radioactivity, 17% is dimethyl phosphate, 76% is accounted for by a
metabolite of unknown structure, and 0.26% by unchanged active
ingredient. After 45 hours, only 0.28% of the P32 activity
representing the total dose administered is found to be present in the
urine (Bolle, 1956; Robbins et al., 1956).
The degradation of the compound in blood proceeds at a very fast rate.
In cattle, 12 hours after peroral application of 40 mg of active
ingredient per kg, 0.03%, of the applied P32 activity is present in
the blood, and 0.003% after 45 hours (Bolle, 1956). The peak of
radioactivity is attained after two hours (Robbins et al., 1956).
Following intravenous application, the parent compound is broken down
by more than 95% after one hour; dichlorvos is found to be present in
a very low concentration only within the first four minutes (Kühnert
et al., 1963).
For further details, see for Biochemical aspects and residues
resulting from supervised trials: Trials on animals.
On poultry and eggs
Gas chromatographic determinations showed that no residues were
present in giblets, muscle and fat (limit of determination of 0.1 ppm)
or in the eggs (limit of determination of 0.003 ppm) of poultry which
had been maintained for four weeks on a diet containing 2.5 and 5 ppm
of the compound (Chemagro Report 20.945; 21.502).
In plants
Studies of the metabolism of trichlorfon in plants were carried out on
cotton plants.
The identified metabolites included dimethyl phosphate which accounted
for up to 70% for the applied trichlorfon dose; only small amounts of
monomethyl phosphate, O-demethyl trichlorfon, O-demethyl dichlorvos
and dichlorvos were formed (Hassan et al., 1966; Bull and Ridgway,
1969). Besides these metabolites, Bull and Ridgway found an
unidentified metabolite which accounted for a large percentage of the
applied dose of parent compound; this phosphorus-containing unknown is
split by ß-glucosidase but not by ß-glucuronidase.
Chloral and trichloroethanol are stated to be possible degradation
products (Arthur and Casida, 1957). However, these products could not
be detected in routine residue studies (Chemagro Corporation,
unpublished). Earlier studies also revealed that chloral and
trichloroethanol are metabolized in plants to form
B-2,2,2-trichloroethanol-gentiobioside (Miller, 1941).
In micro-organism cultures
Studies on Penicillium notatum, Fusarium sp. and Aspergillus niger
showed that approximately half of the applied parent compound is
transformed within 10 days. The principal metabolite found was
O-demethyl trichlorfon. A second degradation product formed is
probably 2,2,2-trichloro-1-hydroxyethyl-phosphonate (Zayed et al.,
1965).
In soil
Trichlorfon is not used for the control of soil pests. Studies
conducted by Chemagro Corporation have shown, however, that after
addition of 10 ppm of trichlorfon to soil, the residue level drops
below the limit of determination within 15-112 days depending upon the
soil type. Trichlorfon has a very low stability in soil; residues of
trichlorfon in runoff water from sandy loam, silt loam and high
organic silt loam soils showed a relatively low tendency to move
(Chemagro Report 28.937). After addition of 0.25 ppm of trichlorfon to
pond water, no residues are to be found in the mud (Chemagro Report
21.313).
In food processing
The published data on trichlorfon residues have generally been
obtained in studies of non-processed plant and animal products. When
these products are processed for the human diet, the trichlorfon
residue level is considerably reduced by such processing procedures as
boiling and sterilization.
In spinach, the residues which have an original level of 2.0 ppm in
the treated plants are reduced by bleaching (five minutes at 100°C) to
a concentration of 0.03 ppm and by further sterilization (100 minutes
at 115°C) to a level of 0.01 ppm. In dwarf beans, the residue level is
reduced from 1.22 ppm to 0.06 ppm and 0.05 ppm, respectively. In peas,
which had an original residue level of 0.21 ppm, no more residues were
detectable after bleaching and sterilization (Dormale et al., 1959).
Tomatoes were treated with 10 ppm of the compound and then canned
according to standard commercial procedure. The heat treatment
destroyed 80% of the compound (Chemagro Report 9012). Safflower seed
containing 0.9 ppm of the compound was processed into meal, crude oil
and refined oil. None of the three processed products contained
detectable residues (Chemagro Report 11.255).
Meat of sheep which ware slaughtered one hour after receiving a
peroral application of 120 mg/kg contained 1-10 ppm of the compound;
after the meat had been boiled for one hour, it contained no
detectable residue (Behrenz, 1959).
In beef which was immersed in a trichlorfon solution to prevent fly
maggot infestation, the residue level of the compound was reduced by
thorough washing from 0.77 ppm to 0.58 ppm. After one hour's boiling,
the residue level in the meat and in the broth was less than 0.1 ppm
(Börger and Maier-Bode, 1967).
Evidence of residues in food in commerce or at consumption
No data were submitted for consideration.
Methods of residue analysis
Many colorimetric, thin-layer chromatographic and gas chromatographic
methods for the determination of trichlorfon residues are described in
the literature. Some of the methods permit simultaneous determination
of trichlorfon and its possible metabolites, e.g. trichloroethanol and
dichlorvos. However, formation of trichloroethanol was not established
in any of the residue studies undertaken.
Trichlorfon can be extracted from plant material with chloroform
(Zadrozinska, 1966), acetone/hexane (Anderson et al., 1966), ethyl
acetate (Cernà, 1963; Watts et al., 1969) or diluted acetic acid
(Sissons and Telling, 1970). For clean-up, the extract residue is
transferred to water; after separating the plant constituents with
petroleum ether or heptane, the parent compound is extracted with
chloroform or diethyl ether (Zadrozinska, 1966; Anderson et al.,
1966). For determination of the compound by the cholinesterase
inhibition technique, extraction with water will suffice (Reynolds
et al., 1960; Chemagro Report 2412; 3581). Column chromatographic
clean-up of the plant extract is possible on charcoal (Watts et al.,
1969) or on aluminium oxide (Sissons and Telling, 1970). If plant
material with a high water content is involved, the compound can be
separated from the plant constituents by dialysis of the macerate in
diluted sulfuric acid, and extracted from the diffusate with diethyl
ether (Anderson et al., 1966; Chemagro Report 8839; 21811).
Trichlorfon can be extracted from animal tissues with acetonitrile
(Beck and Sherman, 1968; Anderson et al., 1966; Chemagro Report
14393), chloroform (Ackermann et al., 1969; Chemagro Report 24808) or
acetone (Chemagro Report 24808). For further clean-up, the extract
residue is transferred to water, fat portions are separated with
heptane, and the parent compound is extracted from the aqueous phase
with diethyl ether (Chemagro Report 14393; Anderson et al., 1966),
In milk, trichlorfon (after removal of fat by centrifuging and
separation of protein by precipitation) is determined either directly
by bioassay (Wickham and Flanagan, 1962) or (after extraction with
chloroform) by thin-layer chromatography, together with possibly
formed dichlorvos (Ackermann et al., 1968). For gas chromatographic
determination of trichlorfon, the milk is extracted with acetone and
benzene. In this method, trichloroethanol is co-determined as a
possible metabolite (Chemagro Report 16460).
Enzymatic determination
Giang and Hall (1951) developed a method for the enzymatic
determination of organic phosphorus insecticides (Delta pH method).
For the determination of trichlorfon residues, the parent compound is
converted to dichlorvos which is a strong cholinesterase inhibitor
(Reynolds et al., 1960; Chemagro Report 2412; 3581), The lower limit
of determination is approximately 0.01 ppm trichlorfon. The method is
unspecific.
Bioassays
Bioassays with mosquito larvae (Aedes aegypti) have been employed
for determining residues of trichlorfon in milk (Behrenz, 1961;
Wickham and Flanagan, 1962). Börger and Maier-Bode (1967) used
Daphnia magna as the test species for determining residues of
trichlorfon in meat. The limit of determination is approximately 0.05
ppm. The method is unspecific.
Thin-layer chromatography
Trichlorfon residues in plants and in animal tissues can be determined
by thin-layer chromatography on silica gel or aluminium oxide plates
sprayed with silver nitrate. The lower limit of determination is
0.25-0.5 ppm of trichlorfon in apples (Zadrozinska, 1966) and
0.2-0.5 µg in animal tissue extracts (Beck and Sherman, 1968). Smaller
amounts of trichlorfon and dichlorvos can be determined by the
cholinesterase inhibition technique (Ackermann et al., 1968, 1969).
Agar-diffusion method
The agar-diffusion method described by Sandi (1962) was modified for
routine determination of trichlorfon. A residue level of 0.1 ppm can
be determined in milk; however, it is not possible to separate
trichlorfon from dichlorvos (Ackermann et al., 1968).
Colorimetry
A colorimetric micro method for determining trichlorfon residues is
based on the determination of chloroform (which is separated from the
trichlorfon by pyrolysis at 550°C) with pyridine and sodium hydroxide
(Fujiwara reaction). This method was used for determining trichlorfon
residues in olive oil (Allessandrini and Lanforti, 1957) and in fruit
and lettuce (Cernà, 1963). The limit of determination is approximately
1 ppm in olive oil and 10 µg in plant material.
Trichlorfon residues can be determined colorimetrically also by a
total phosphorus procedure in plant material (Sissons and Telling,
1970) and in milk (Leahy, 1964); the lower limit of determination is
0.1-0.2 ppm.
Gas chromatography
Trichlorfon residues can be determined with a high degree of
sensitivity and specificity by gas chromatography. The intact molecule
is not determined by this method but instead the cleavage products
chloral or dimethyl phosphite which form upon pyrolytic cleavage of
trichlorfon in the injection port of the gas chromatograph. Chloral is
detected with the electron-capture detector (Anderson et al., 1966);
trichloroethanol can be simultaneously determined as a theoretically
possible metabolite. Chloral can also be determined with the Dohmann
microcoulometric gas chromatograph (Chemagro Report 8839). The
clean-up of extracts is simpler when trichlorfon is detected with a
phosphorus-specific detector, e.g. a modified flame ionization
detector (thermionic detector) (Chemagro Report 21811, 24808). The
limits of determination of these gas chromatographic methods depend
upon the material being analysed and the clean-up procedure used.
However, they are usually very low, e.g. 0.01-0.1 ppm with an
electron-capture detector and 0.003-0.06 ppm with the phosphorus
detector.
Studies on 30 different phosphorus-containing pesticides registered
for use in different crops show that only phosphamidon interferes with
the gas chromatographic determination of trichlorfon with the
phosphorus detector (Chemagro Report 21811, 27471, 29411). By using a
different column, this interference can be avoided (Chemagro Report
26335).
Parallel residue analyses of treated plant material showed agreement
of residue values obtained by the cholinesterase inhibition technique
and by gas chromatography. Determination by the cholinesterase
inhibition technique produces slightly higher residue values, which is
probably due to the presence of slight traces of dichlorvos (Chemagro
Report 9733).
For routine determination of trichlorfon residues, gas chromatography
is the most suitable method. The compound can be detected with a
microcoulometer, an electron-capture detector or a phosphorus-specific
detector, e.g. a thermionic or flame photometric detector.
Examples of national tolerances and safety intervals
Country Crop Tolerance Safety
ppm interval
days
Argentina Bananas 0.2 (provisional)
Bananas (peeled) 0
Australia General 2
Fruits, grains, vegetables 2.0
Austria General 14
Cucumbers, tomatoes,
peppers 4
Belgium General 10
Fruits, vegetables,
excl. potatoes 0.5
Brazil Vegetables 0.5 7
Fruits and field crops 0.5 10
Meat and milk 0.001
Bulgaria General 14
Canada Alfalfa 14
Rape 21
Corn 40
Sugar beets 14
Sugar beets, tops 28
Tobacco 3
Beans 14
Cabbage, cauliflower,
Brussels sprouts 21
Country Crop Tolerance Safety
ppm interval
days
Carrots, rutabagas,
salsify, turnips 28
Collards, kale, lettuce,
spinach 28
Peppers 21
Table beets 28
Tomatoes 21
Artichokes, bananas, NR
beans, beef cattle, NR
beets, Brussels NR
sprouts, cabbage, NR
carrots, cauliflower, NR
collards, corn, kale, NR
lettuce, peppers, rape NR
seed, rutabagas, NR
salsify, spinach, NR
sugar beets, tomatoes, NR
turnips NR
Finland General 14
France General 7
German Democratic Fruits, root vegetables,
Republic leafy vegetables,
cabbages, legumes,
fruit-producing
vegetables (tomatoes,
cucumbers) 1.0
Meat, fish, animal and
vegetable fats, eggs,
milk, baby foods 0
Field crops, fruits 10
Cherries 5
Vegetables 7
Special crops 14
Crops used for the
production of baby foods,
drugs and health foods 30
German Federal Fruits, field crops,
Republic incl. fodder crops 10
Vegetables 7
Application under glass:
general 10
Leafy vegetables,
fruit-producing vegetables,
root vegetables,
Country Crop Tolerance Safety
ppm interval
days
legumes, fruits incl.
grapes 0.5
Great Britain General 2
Hungary Animal products 0
Israel Fruits incl. grapes,
clover, alfalfa,
sub-tropical trees, sugar
beets, fodder crops 7
Maize 30
Tomatoes, cucumbers,
cucurbits, cabbage,
radish, lettuce,
spinach, beans, other
vegetables 10
Peppers, eggplants,
strawberries, cauliflower,
artichokes 14
Morocco General 7
Olives 30
Netherlands General 0.5
General, field-grown 10
Spinach, field-grown 4
General (under glass
from 1 March - 1 Nov.) 17
New Zealand Tomatoes (canned) 1
Other crops 14
Poland Fruits, legumes (not
vegetables), root
crops and other field
crops 14
Vegetables 10
Portugal General 7
Industrial tomatoes 4
South Africa Maize, wheat, citrus
fruits, apples, pears,
apricots, peaches,
sub-tropical crops 10
Cucurbits 7
Alfalfa 2
Tomatoes 3
General 2.0
Soviet Union General 1.0
Spain General 10
Country Crop Tolerance Safety
ppm interval
days
Sweden General 14
Switzerland General 21
Vegetables 14
Beets 42
Vegetables, legumes,
fruits incl. grapes 0.5
United States of See USDA Summary of
America Registered Agricultural
Pesticide Chemical Uses
Yugoslavia General 0.5
Fruits, vegetables,
field crops 14
Appraisal
Trichlorfon is an organophosphorus insecticide which is especially
used on crops against a variety of insects (moths, flies, bugs, etc.).
It is also widely used against ecto- and endoparasites of animals, in
public health and as an anthelmintic in medicine.
It is used on a wide variety of field and pasture crops with
application rates of 150-2000 g/ha, normally 500-1200 g/ha, or as
0.075-0.2% sprays. As an animal health product, it is normally applied
at a rate of 25-75 mg/kg body-weight externally as wash or spray and
spot-on treatment and internally by mouth and by injection. In public
health it is used against flies and other pests commonly in the form
of baits. Other uses are on ornamentals, in tree nurseries, and in
forestry.
Residue data for evaluation are satisfactory.
The behaviour of trichlorfon is characterized by its hydrophilic
properties. Its decomposition is brought about by splitting the P-C
bond and by hydrolysis of the P-OCH3 bonds. In addition, in tissues
it can be converted in trace amounts to dichlorvos. Products of more
advanced degradation have also been found and characterized. In food
processing, trichlorfon residues are substantially disappearing.
As a result of use of trichlorfon, residues may occur in animal feed.
When the applications are made in accordance with good agricultural
practice the residues are considered to be no hazard to the animal
health and no detectable contamination of the foods derived from these
animals is to be expected.
There are a number of methods of residue analysis available. For
regulatory purposes highly specific and sensitive gas chromatographic
methods with detection limits of less than 0.01-0.1 ppm have been
developed.
Due to the insignificant magnitude of dichlorvos and to the low
toxicity of other degradation products, recommendations for tolerances
of trichlorfon residues are made in terms of the parent compound.
Since the direct application of trichlorfon to the domestic animals
may result, although for a short limited period of time, in residues
of varying magnitude in milk, meat, and fat, it is necessary, in
accordance with the local needs of animal health, to set limitations
of use, e.g. type of formulation, route of application, dosage,
condition of the animals, and safety interval from treatment to use of
animal products for human food. The recommendations for trichlorfon
residues in animal products are made, because the use of insecticides
is necessary in animal health, application of trichlorfon according to
good agricultural practice will cause no health hazards to animals,
and, by adjusting properly the conditions of use, milk, meat, organs,
and fat can be obtained from the treated animals practically free from
the trichlorfon residues. Additional safeguard of the consumer against
the residues is the degradation of trichlorfon in storage, processing
and cooking of the animal products before they are consumed.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The following recommendations are made for temporary tolerances:
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Cereals:
Barley 21 0.1
Maize 30 0.1
Wheat 30 0.2
Fruits:
Apples 14 0.1
Bananas, pulp 0 0.2
Cherries 7 0.1
Oranges, pulp 7 0.1
Peaches 14 0.2
Strawberries 4 0.1
(continued)
Period from
Crop treatment Tolerance
to analysis (ppm)
(days)
Vegetables:
Artichokes 7 0.1
Blackeyed, beans 14 0.1
Brussels sprouts 14 0.2
Cabbage 14 0.1
Cauliflower 21 0.2
Cowpeas 14 0.1
Green beans 14 0.1
Kale 14 0.2
Lima beans 14 0.1
Mustard, leaf 14 0.1
Peppers 14 1
Pumpkins 14 0.1
Sweet corn, kernels and cobs 14 0.2
Tomatoes 14 0.1
Celery 14 0.2
Sugar beets 30 0.05
Beets 14 0.2
Turnips 14 0.1
Oil seeds:
Cotton seed 14 0.1
Flax seed 14 0.1
Lin seed 30 0.1
Safflower seed 45 0.1
Soybeans, dry 45 0.1
Nuts:
Peanuts, shelled 7 0.1
Animal products:
Meat, fat and by-products
of cattle and pigs 14 0.1
Whole milk 2 0.05
Further work or information
Required before 30 June 1975
1. A two-generation carcinogenicity study to elucidate the possible
increase in the incidence of tumours including those of the
mammary gland.
2. More information on residues on oats.
3. More information on residues on lettuce and spinach under various
conditions (including greenhouses).
Desirable
1. Elucidation of the effect on spermatogenesis.
2. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abdalla, A., Saif, M., Taha, A., Askmawy, H., Tawfik, J., Abdel
Fattah, F., Sabet, S. and Abdel-Mequid, M. (1965) Evaluation of an
organophosphorus compound Dipterex on the treatment of Bilharziasis.
J. Egypt Med. Assoc., 48: 262-273
Abdel-Aal, A. M. A., El Hawary, M. F. S., Kamel, H., Abdel-Kalek,
M. K. and El-Diwany, K. M. J. (1970) Egypt Med. Assoc., 53: 265-271
Ackermann, H., Engst, R. and Fechner, G. (1968) Methode zur getrennten
Bestimmung von Trichlorphon und Dichlorphos-Rückständen in Milch.
Ztschr. Lebensmittelunters. Forsch. 137: 303-308
Ackermann, H., Lexow, B. and Plewka, E. (1969) Nachweis und
Identifizierung von insecticiden Phosphor-,
Thiophosphor- Phosphon- und Carbaminsäureestern im Biologischen
Material. Arch. f. Toxicol., 24: 316-324
Adkins, T. R., jr. (1966) Residues in cattle tissues following
back-line and spray applications of Trichlorfon. J. Econ. Entomol.,
59: 1423-1425
Alessandrini, M. E. and Lanforti, G. F. (1957) Determinazione di
residui di Dipterex (O,O-dimetil-2,2,2,trichloro-1-idrossietil
fosfato) nell' olio di oliva. Rend. Ist. super. Sanita, 20: 093-1003
Anderson, R. J., Anderson, C. A. and Olson, T. J. (1966) A Gas-liquid
chromatographic method for the determination of Trichlorfon in plant
and animal tissues. J. Agr. Food Chem., 14: 508-512
Arant, F. S., Atkins, T. R. and Sowell, W. L. Toxicity of Bayer L13/59
to rabbits. Unpublished report
Arnold, D., Keplinger, M. L., Fancher, O. E. and Calandra, J. C.
(1971) Mutagenic study with Dylox in Albino mice. Unpublished report
by Industrial Biotest Laboratories submitted by Farbenfabriken Bayer
A.G.,
Arthur, B. W. and Casida, J. E. (1957) Metabolism and selectivity of
O,O-dimethyl 2,2,2,-trichloro-1-hydroxethyl phosphonate and its acetyl
and vinyl derivatives. J. Agr. Food Chem., 5: 186-192
Arthur, B. W. and Casida, J. E. (1958) Biological activity of several
O,O-dialkyl alpha-acyloxyethyl phosphonates. J. Econ. Entomol.,
6: 360-365
Bailey, C. C., jr. (1956) Evaluation of the dermal toxicity of
malathion chlorthion and Dipterex to dogs. Thesis, Clemson College,
Clemson, S.C.
Bayer, A.G., (1967) Farbenfabriken, Pflanzenschutz. (R)Dipterex
(Bayer 15922, L 13/59) Leverkusen E. 10-6117/22 155
Bayer, A.G., Farbenfabriken, Pflanzenschutz. Documentation on 1971
trichlorfon for FAO
Beck, J. and Sherman, M. (1968) Detection by thin-layer chromatography
of organophosphorus insecticides in acutely poisoned rats and
chickens. Acta pharmacol. et toxicol., 26: 35-40
Beheyet, P., Lebrun. A., Cerf, J., Dierickx, J. and DeGroote. V.
(1961) Etude do la toxicite pour homme d'un insecticide
organophosphore. Bull. Wld Hlth Org., 24: 465-473
Behrenz, W. (1959) Biologische Bestimmung des Wirkstoffgehaltes im
Fleisch von Schafen und Rindern zu verschiedenen Zeiten nach peroraler
Behandlung mit Neguvon. Arch. Lebensmittelhyg., 10: 64
Behrenz, W. (1961) Ueber die Auascheidung von Neguvon(R) in der
Milch nach einmaliger oraler und percutaner Anwendung des Präparates
bei Milchkühen. Vet. mod. Nachr., 133-145
Bolle, W. R. (1956) Neguvon, ein äusserlich und innerlich anwendbares
Insektizid, Larvizid und Acarizid. Vet. med. Nachr., 155-172
Borgmann, W. and Hunnold, G. A. (1955) Report on the results of a
toxicological examination of Dipterex (L13/59). Unpublished report
submitted by Farbenfabriken Bayer A.G.
Brodeur, J. and Dubois, K. P. (1963) Comparison of acute toxicity of
anticholinesterase insecticides to weanling and adult male rats. Proc.
Soc. Exp. Biol., 114: 509-11
Bull, D. L. and Ridgway, R. L. (1969) Metabolism of trichlorfon in
animal and plants. J. Agr. Food Chem., 17: 837-841
Börger, K. and Maier-Bode, H. (1967) Versuche zur Verhinderung des
Fliegenmadon-Befalles von Fleisch. Arch. f. Lebens-mittelhyg., 18:
38-42
Cerná, V. (1963) Kolorimetrische Bestimmung von Dipterex-Rückstünden
in Lebensmittein. Die Nahrung, 7: 60-66
Chemagro-Report (Chemagro Corporation, Kansas City, USA)
2412 Tentative method for microestimation of Dipterex residues by
the Cholinesterase inhibition technique
3581 The determination of Dylox residues by the Cholinesterase
inhibition technique
8839 The microcoulometric determination of Dylox residues in
plant material
9012 The effect of canning on Dylox residues in tomatoes
9733 A comparison of Dylox residue results obtained by the
Cholinesterase inhibition procedure (Report 3581) and the
vapor phase chromatographic procedure (Report 8839)
11 255 The effect of processing on Dylox residues in Safflower
14 393 Determination of Neguvon, Chloral hydrate and
trichlorethanol residues in animal tissues by electron
capture gas chromatography
14 412 Trichlorfon residues in cattle tissues (Backline application
with 8 and 16% pour-on)
16 460 Determination of Trichlorfon, Chloral hydrate and
trichlorethanol residues in milk by electron capture gas
chromatography
16 538 Trichlorfon residues in milk (barn spray prepared with S.P.)
17 440 Trichlorfon residues in milk (in feed prepared)
17 970 Trichlorfon residues in cattle milk (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
17 991 Trichlorfon residues in cattle tissues (in feed prepared)
18 321 Trichlorfon residues in cattle milk (in feed prepared with
technical standard)
18 322 Trichlorfon residues in milk (spray prepared with 80%
soluble powder)
18 495 Trichlorfon residues in cattle tissues (mist spray with 1%
Co-Ral - 2% Trichlorfon combination)
18 521 Trichlorfon residues in milk (pour-on with 8% formulation)
18 678 Trichlorfon residues in swine tissues (in feed with 90%
soluble powder)
18 884 Trichlorfon residues in cattle tissues (in feed prepared
with technical standard)
20 945 Trichlorfon residues in poultry tissues (in feed prepared
with Dylox 80% S.P.)
21 289 Trichlorfon residues in milk (mist spray prepared with 1%
Co-Ral - 2% Trichlorfon combination)
21 313 Trichlorfon residues in mud (spray on surface with 50% S.P.)
21 502 Trichlorfon residues in eggs (in feed prepared with 80%
S.P.)
21 811 Determination of residues of Trichlorfon in Alfalfa by
thermionic emission gas chromatography
24 808 Determination of residues of Trichlorfon in bovine animal
tissues by thermionic emission gas chromatography
26 117 Trichlorfon residues in cattle tissues (Backline application
using 8% pour-on)
26 334 A confirmatory gas chromatographic procedure for Trichlorfon
residue analysis
27 471 An interference study for Trichlorfon residue determination
on Alfalfa and Clover
27 533 Trichlorfon residues in swine tissues (drinking water
treated with Neguvon)
27 561 DDVP-residues in swine tissues (drinking water treated with
Neguvon)
28 937 Trichlorfon residues in runoff water from soils
29 234 Trichlorfon residues in milk (Bolus-capsules fortified with
Dylox 80% S.P.)
29 411 An interference study for the residue method for Trichlorfon
on various crops
Davis, A. and Bailey, D. R. (1969) Metrifonate in urinary
schistosomiasis. Bull. Wld Hlth Org., 41: 209-224
Dedek, W. and Lohs, K. (1970a) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern I. Untersuchungen in vitro in Humanserum
mit 14C-Trichlorphon. Z. Naturforsch., 25b: 94-96
Dedek, W. and Lohs, K. (1970b) Zur alkylierenden Wirkung von
Trichlorphon in Warmblütern II. Verteilung von 14C in Organen und
Leberproteinen bei Ratten nach Applikation von 14C-Trichlorphon. Z.
Naturforsch., 25b: 1110-1113
Dedek, W. and Schwarz, H. (1970) Studien zur percutanen Resorption von
32P-Dimethoat am Schaf. Z. Naturforsch., 25b: 1193-1194
Deichmann, W. B. and Lampe, K. (1955) Dipterex, its pharmacological
action. Bull. Univ. Miami Sch. Med., 9: 7-12
Dinerman, A. A., Lavrent'eva, N. A. and Il'inskaia. N. A. (1970) The
embryotoxic action of some pesticides. Gigiena i. Sanit., 35: 39-42
Dormale, S., Martens, P. H., Decleire, M. and de Faetraets, L. (1959)
Etude do la persistance de résidus d'insecticides dans divers légumes
frais, blanchis et stérilisés. Bull. Inst. agron. et Stat. Rech.
Gembloux, 27: 137-147
Doull, J. and Dubois, K. P. (1956) The effects of diets containing
Dipterex on rats. Unpublished report by Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Vesselinovitch, D., Meskauskas, J. and Fitch, F.
(1962a) Chronic oral toxicity of Dylox to male and female dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Doull, J. and Dubois, K. P. (1958) The effects of diets containing
Dipterex for dogs. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Vaughn, G. and Dubois, K. P. (1958) Effect of diets
containing Dipterex in combination with organic phosphates on dogs and
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Fitch, F., Meskauskas, J., Root, M. and
Cowan, J. (1965) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Doull, J., Vesselinovitch, D., Root; M., Cowan, J., Meskauskas, J. and
Fitch, F. (1962b) Chronic oral toxicity of Dylox to male and female
rats. Unpublished report by Department of Pharmacology, University of
Chicago
Dubois, K. P. (1958) Potentiation of the toxicity of insecticidal
organic phosphates. AMA Arch. Indust. Health, 18: 488-496
Dubois, K. P. and Cotter, G. J. (1955) Studies on the toxicity and
mechanism of action of Dipterex. AMA Arch. Indust Health, 11: 53-60
Dubois, K. P. and Doull, J. (1955) Acute toxicity of Dipterex to
chickens and ducks. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol, appl.
Pharmacol., 14: 515-534
Giang, P. A. and Hall, S. A. Enzymatic determination of organic
phosphorus insecticides. Anal. Chem., 23: 1830-1834
Gibel, Von W., Lohs, Kh., Wildner. G-P, and Ziebarth, D. (1971)
Tierexperimentelle untersuchungen über die hepatotoxische und
Kanzerogene wirkung phosphoroganischer verbindungen. Arch. für
Geschwulstforschung, 37: 303-312
Gofmekler, V. A. add Tabakova, S. A. (1970) The effect of chorphos on
rat embrogenesis. Farmikol. i. Toksikol., 33: 735-737
Grundmann, E. and Hobik, H. P. (1966) Bay 15922/2 year feeding
experiment in rats/histology. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Hanna, S., Basmy, K., Osaima, S., Shoeb, S. M. and Awny, A. Y. (1966)
Effects of administration of an organophosphorus compound as an
antibilharzial agent with special reference to plasma cholinesterase.
Brit. Med. J., 1: 1390-1392
Hassan, A. and Zayed, S. M. A. D. (1965) Metabolism of
organophosphorus insecticides III. Fate of the methyl groups of
Dipterex in vivo. Can. J. Biochem., 43: 1271-1275
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides II. Metabolism of
O,O-Dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate (Dipterex) in
mammalian nervous tissue and kinetics involved in its reaction with
acetylcholine esterase. Can. J. Biochem., 43: 1263-1269
Hassan, A., Zayed, S. M. A. D. and Abdel-Hamid, F. M. (1965)
Metabolism of organophosphorus insecticides V. Mechanism of
detoxyfication of Dipterex in Prodenia Litura F. Biochem. Pharmacol.,
14: 1577-1584
Hassan, A., Zayed, S. M. A. D. and Hashish, S. (1965) Metabolism of
organophosphorus insecticides. VI. Mechanism of detoxyfication in the
rat. Biochem. Pharmacol., 14: 1692-1694
Hassan, A., Zayed, S. M. A. D. and Mostafa, I. Y. (1966) Metabolism of
organophosphorus insecticides VIII. Demethylation of Dipterex.
Z. Naturforsch., 21b: 498-500
Hobik, H. P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitat-sversuchen an huhnern mit
Dipterex. Unpublished report submitted by Farbenfabriken Bayer A.G.
Jackson, J. B., Drummond, R. O. Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Juszkiewicz, T. (1970) Insecticides residues in the tissues and milk
of cow following the dermal application of fenchlorvos and
trichlorphon: a preliminary report. Med. Weterynar (Poland),
26: 85-89
Kimmerle, G. and Lorke, D. (1966) Neurotoxische Untersuchungen an
Huhern mit Dipterex-Wirkstoff. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kühnert, M., Dedek, W. and Schwarz, H. (1963) Untersuchungen über die
Stoffwechselbeeinflussung und den Ausseheidungs-mechanismus des
Phosphonsäure-esters Trichlorphon im Handelspräparat "Bubulin" mit
Hilfe 32P-markierten Phosphors bei der intravenösen und
intramuskulären Injektion an Rindern. Arch. Exp. Vet. Med.,
17: 403-417
Leahy, J. S. (1964) Die Bestimmung von Rückstünden des Neguvon(R)
Milch nach dermaler Anwendung beim Rind. Vet. Med. Nachr., 37-48
Lebrun, A. and Cerf, C. (1960) Note preliminaire sur la Toxicite pour
l'homme d'un insecticide organophosphore (Dipterex). Bull. Wld Hlth
Org., 22: 579-582
Lindgren, P. D. and Ridgway, R. L. (1967) Toxicity of five
insecticides to several insect predators. J. Econ. Entomol.,
60: 1639-1641
Lorke, D. (1971) Trichlorfon. Untersuchungen auf embryotoxische und
teratogene wirkungen an der Ratte. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Lorke, D. and Loser, E. (1966) Chronic toxicological studies on rats.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Lorke, D. and Kimmerle, G. (1968) Die wirkung von reactivatoren bei
der vergiftung mit phosphorsaureestern. Naunyn-Schmeidebergs Arch.
Pharmakol. Exp. Pathol., 263: S237
Löser, E. (1970) Bay 15922/Chronic toxicological studies on dogs.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Löser, E. (1969) Generationsversuche an Ratten, Unpublished report
submitted by Farbenfabriken Bayer A.G.
Metcalf, R. L., Fukuto, T. R. and March, R. B. (1959) Toxic action of
Dipterex and DDVP to the house fly. J. Econ. Entomol., 52: 44-49
Miller, L. P. (1941) Formation of 2,2,2-Trichloroethylgentiobioside in
tomato plants grown in media containing Chloral hydrate,
Trichloroethyl alcohol, or chloral cyanohydrin. Contribution from
Boyce Thompson Institute Plant Research, 12: 15-23
Miyamoto, J. (1959) Non-enzymic conversion of Dipterex into DDVP and
their inhibitory action on enzymes. Botyu-Kagaku, 24: 130-137
Miyamoto, J. (1961) Studies on the mode of action of Dipterex Part II.
New glucorunides obtained from the urine of rabbit following
administration of Dipterex. Agr. Biol. Chem., 25: 566-572
Miyamoto, J. (1968) Private communication to Bull, D. L. and Ridgway,
R. L.
Miyata, T., Iyatomi, K., Saito, T. and Morikawa, O. (1968) Metabolism
and cholinesterase inhibition of NS 2662,
O,O-dimethyl-2,2-dichloro-1-hydroxyethyl phosphonate, in the American
cockroach. Jap. Appl. Ent. Zool., 12: 211-219
Mühlmann, R. and Schrader, G. (1957) Hydrolyse der Insektiziden
Phosphorsäureester. Z. Naturforsch., 12b: 196-209
Murphy, S. D. and Dubois, K. P. (1958) Inhibitory effect of Dipterex
on the detoxification of malathion. Unpublished report by Department
of Pharmacology, University of Chicago
Namba, T. (1971) Cholinesterase inhibition by organophosphorus
compounds and its chemical effects. Bull. Wld Hlth Org., 44: 289-307
Pruessmann, R. (1968) Direct Alkylating Agents as Carcinogens. Food
cos. Toxicol., 6: 576-577
Rahn, H. W. (1963) Über die Moglichkeit der Trichlorphon Schadigung
bei Jung Tieren Laktierender Rattan. Arch. Exp. Veterinarmed.,
18: 713-717
Reynolds, H. T., Stern, V. M., Fukuto, T. R. and Peterson, G. D.
(1960) Potential use of Dylox and other insecticides in a control
program for field crop pests in California. J. Econ. Entomol.,
53: 72-78
Robbins, W. E., Hopkins, T. L. and Eddy, G. W. (1956) The metabolism
of 32P-labelled Bayer L 13/59 in a cow. J. Econ. Entomol.,
49: 801-806
Ross, E. and Sherman, M. (1960) The effect of selected insecticides on
growth and egg production when administered continuously in the feed.
Poultry Sci., 39: 1203-1211
Saito, T. (1969) Selective toxity of systemic insecticides. Res.
Reviews, 25: 175-186
Sándi. E. (1962) Kolorimetrische Bestimmung von Dipterex-Rückständen
in Lebensmitteln. Die Nahrung, 7: 60-66
Sato, K. and Saito, T. (1968) Selective Toxicity of NS 2662,
O,O-dimethyl dichloro-hydroxyethyl phosphonate, and Trichlorfon. Jap.
appl. Ent. Zool., 12: 148-155
Schulemann, W. (1955) Final opinion on the insecticide Bayer L13/59.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Schwarz, H. and Dedek, W. (1965a) Das Verhalten von radioaktiv
markiertem Trichlorphon nach Pour-on-Applikation (Aufgiessverfabren)
zur Dassellarvenbekämpfung beim Rind. Monatsh. Vet. Med.,
20: 958-968
Schwarz, H. and Dedek, W. (1965b) Untersuchungen über den Abbau und
die Ausseheidung von 32P-markiertem Trichlorphon beim Schwein. Zbl.
Vet. Med. (Reihe B), 12: 653-660
Schwarz, H, and Dedek, W. (1966) Untersuchungen zum Nachweis von
32P-markiertem Trichlorphon im Fleisch beim Schwein. Zbl. Vet. Med.
(Reihe B), 13: 489-494
Sherman, M. and Ross, E. (1959) Toxicity of housefly larvae to
insecticides administered as single oral doses to chicks. J. Econ.
Entomol., 52: 719-723
Sissons, D. J. and Telling, G. M. (1970) Rapid procedure for the
determination of organophosphorus insecticide residues in vegetables.
II. A screening procedure for watersoluble insecticides.
J. Chromatog., 48: 468-477
Spicer, E. J. F. and Payne, S. (1971) Pathology report of Bay 15922-4
year dog study (Loser, 1970). Unpublished report submitted by
Farbenfabriken Bayer A.G.
Spicer, E. J. F. and Urwin, C. (1971) Pathology report of Bay 15922
Generation experiment in rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Vbrovsky, L., Selecky, V. and Rosival, L. (1959) Toxikologische und
Pharmakologische Studien der Phosphorsaureester Insecticiden. Arch.
Exp. Pathol. Pharmol., 236: 202-205
Watts, R. R., Storherr, R. W. and Pardue, J. R. (1969) Charcoal column
clean up for many organophosphorus pesticide residues in crop
extracts. A.O.A.C., 52: 522-526
Wegner, D. (1970) Bilarcil(R) Bay 2349 - Klinische Erfahrungen
1960-1969. Unpublished report submitted by Farbenfabriken Bayer A.G.
Wickham, J. C. and Flanagan, P. (1962) Residues of Trichlorphon
(Neguvon) in milk after dermal application to cattle. J. Sci. Food
Agric., 13: 449-455
Williams, M. W., Fuyat, H. N. and Fitzhugh, O. G. (1959) The subacute
toxicity of four organic phosphates to dogs. Tox. appl. Pharmacol.,
1: 1-7
Wills, J. H. (1959) Recent studies of organic phosphate poisoning.
Fed. Proc., 18; 1020-1025
Witter, R. F. and Gaines, T. B. (1963) Relationship between depression
of brain or plasma cholinesterase and paralysis in chickens caused by
certain organic phosphorus compounds. Biochem. Pharmacol.,
12: 1377-1386
Zadrozinska, J. (1966) Oznaczanie Sladowych pozostalosci Diptereksu
w jablach metoda chromatografii cienkowwarstwowej (Determination of
trace residues of trichlorphon in apples by thin-layer
chromatography). Roczn. panst. Zakl. Hig., 17: 523-528
Zayed, S. M. A. D, and Hassan, A. (1965) Metabolism of
organophosphorus insecticides I. Distribution and metabolism of
Dipterex in adult larva of the cotton leaf worm (Prodenia Litura F.),
Can. J. Biochem., 43: 1257-1262
Zayed, S. M. A. D., Mostafa, I. Y. and Hassan, A. (1965) Organische
P-hattige Insectizide im Staffwichsel. VII. Umwandlung von
32-P-markiertem Dipterex durch Mikroorganismen. Arch. f. Mikrobiol.,
51: 118-121
Zhdanovich, N. V. and Udalov, I. U. F. (1970) The role of thiamine and
pyridoxime on acute and subacute intoxication with the
organophosphorus insecticide Dipterex. Vopr. Pitaniya, 29: 28-34
