
MANCOZEB JMPR 1974
Explanation
Mancozeb was considered by the Joint Meetings in 1967 and 1970
(FAO/WHO 1968, 1971). No tolerances were recommended in 1967, and a
single temporary tolerance of 1 mg/kg in potatoes was recommended in
1970. Since it was recognized that mancozeb is used in several
countries on a wide variety of crops the Meeting concluded that the
following information was required before further tolerances could be
recommended.
"Further study on the biotransformation of the compound in plants
to determine the chemical nature of the residues, followed by
appropriate toxicological studies. The extensive residue data already
submitted need to be validated by the diethylamine method and by
measuring the level of ethylenethiourea. (Some of these data are known
to be available but were not submitted in time for detailed scrutiny
by the Meeting)".
In response to these requirements the following information was
provided.
Ethylenethiourea (ETU) content in the technical and formulated
products
Bontoyan and Looker (1973a,b) studied the initial
ethylenethiourea content of various ethylenebisdithiocarbamate (EBDC)
products. The average ETU content in 76 different lots of mancozeb
manufactured at 6 different locations throughout the world was 0.07%.
Povlsen et al. (1974) analysed 9 samples of mancozeb, alone or as
a mixed product with dinocap. The ETU content (expressed as % w/w of
the declared EBDC content) varied between <0.01 and 0.04%.
Bontoyan and Looker (1973a) studied the effect of storage
conditions on the ETU content of formulated EBDC products. Samples of
mancozeb, maneb, and zineb were stored for 39 days at 48°C and a
relative humidity of 80%, thus simulating storage conditions which may
occur in normal practice during summer. The initial ETU content in
mancozeb formulations was about 0.02%, and in "maneb 80%" 0.05 -
1.26%. After 39 days of storage the ETU content in the formulated EBDC
products increased; in mancozeb to 0.13%. in "maneb 80%" preparation
to 0.58 - 14.04%, and in "zineb 80%" to 3.48 - 10.44%. The rate of ETU
formation was greatest in the maneb products; the zineb formulations
were degraded less rapidly and at a more uniform rate and the mancozeb
products were degraded slowly and formed little ETU.
TABLE 1 Residues of CS2 and ETU resulting from supervised trials with mancozeb
Application* Pre-harvest interval in days
Crop and No rate 0/1 0/1 7 7 14 14 21 21
Country kg a.i./ha CS2+ ETU CS2 ETU CS2 ETU CS2 ETU
Apples
USA (1972) 9 4.2 <0.01- <0.01- <0.01- <0.01
0.02 0.02 0.02
11 4.8 <0.01- <0.01
0.02
6 4.8 <0.01 <0.01
4 6.4 <0.01 <0.01
Banana (pulp)
Honduras (1972) 1 6.75 0.1 <0.01 0.1 <0.01 0.1 <0.01
1.1 <0.01 0.9 <0.01 0.9 <0.01
1 6.75 0.2 <0.01 0.2 <0.01 n.d. 0.01
0.1 <0.01 0.2 <0.01 n.d. <0.01
1 10.5 0.1 <0.01 0.1 <0.01 0.1 <0.01
1.6 <0.01 3.1 0.02- 3.1 0.03-
0.05 0.04
1 10.5 0.1 <0.01 0.2 <0.01 0.2 <0.01
0.2 <0.01 0.2 <0.01 0.1 <0.01
1 10.5 n.d. 0.01 n.d. <0.01 n.d. <0.01
0.3 <0.01 1.2 <0.01 1.4 <0.01
1 10.5 n.d. <0.01 0.1 <0.01 0.1 <0.01
n.d. <0.01 n.d. <0.01 n.d. <0.01
TABLE 1 (Cont'd.)
Application* Pre-harvest interval in days
Crop and No rate 0/1 0/1 7 7 14 14 21 21
Country kg a.i./ha CS2+ ETU CS2 ETU CS2 ETU CS2 ETU
1 1.4 n.d. <0.01 n.d. <0.01 n.d. 0.01
0.2 <0.01 0.8 <0.01 0.4 <0.01
Celery
USA (1972) 1 1.6 3.7- 0.02- 2.0- <0.01- 1.4- n.d. n.d. n.d.
5.7 0.06 4.4 <0.02 1.8 0.1
1 1.6 1.0- 0.01- 0.7- n.d. 0.1- n.d.
2.8 0.02 0.8 0.8
Spinach
USA (1972) 1 1.2 0.05- <0.01 n.d.
0.14 0.02
Tomatoes
USA (1972) 1 1.6 0.02- 0.03- <0.01
0.03 0.05 0.02
Maize
(sweetcorn)
USA (1972) 13 1.6 n.d. n.d. n.d.
13 2.4 n.d. n.d. n.d.
* Formulation used in all cases was 80% wettable powder.
+ All figures for CS2 and ETU expressed in mg/kg.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES ARISING FROM SUPERVISED TRIALS
Data on mancozeb residues in various crops (analysed as CS2) and
on potatoes (analysed as ethylenediamine) were considered by the 1967
and 1970 Joint Meetings respectively. More recently, residue data were
obtained from apples, bananas, celery, spinach, tomatoes and maize
(sweet corn) on levels of the parent compound, including some of the
intermediate degradation products, by the CS2 method, and on levels
of ETU. The figures are expressed in mg/kg CS2 and mg/kg ETU in Table
1.
FATE OF RESIDUES
General Comments
Lyman (1971), Lyman and Lacoste (1974) reviewed the fate of
mancozeb in plants, animals, water and soil. They demonstrated that
breakdown in plants and animals is similar, and proceeds through a
series of intermediates, the structures of most of which have been
elucidated. The final products are metallic cations (Mn2+ and Zn2+),
inorganic materials such as elemental sulphur, thiosulphate and
sulphate ions and, via a series of organic intermediates,
ethylenediamine. Earlier findings with respect to these intermediates
were confirmed.
In plants
Several recent studies illustrate the degradation pattern of
ethylenebisdithiocarbamates in plants, e.g. Czeglédi-Janke (1967);
Vekstejn and Klisenko (1970); Engst et al. (1968, 1969); Vonk et al.
(1970, 1974a, 1974b). A recent review of these studies by Engst and
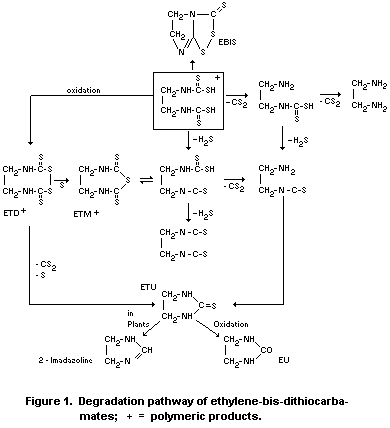
Schnaak (1974) gives the degradation pathway shown in Figure 1.
According to the few results available the degradation products
of EBDC compounds (mainly ETU and EBIS) vary in the extent of their
production in different plants and under different conditions.
According to Engst et al. (1968), EBIS and ETU residues increased
from about 0.05 mg/kg immediately after application to about 0.1 mg/kg
at the sixth day and then decreased rapidly up to the tenth day.
Klisenko and Vekstejn (1971) obtained similar degradation curves with
zineb in beets showing first an increase of EBIS and ETU then a rapid
degradation. Yip et al. (1971), however, found 0.45 and 0.15 mg/kg ETU
in maneb treated lettuce and cabbage respectively immediately after
treatment, which was completely degraded within 7 days.
 Vonk and Sijpesteijn (1970) studied the fate of the main
metabolites of EBDC's in the green parts of cucumber plants after
application of 14C-ethylene labelled nabam to the leaves. They found
the following distribution of radioactivity in water extracts from
cucumber leaves after 0.7 and 19 days. The compounds were separated by
TLC.
TABLE 2 Fate of nabam in cucumbers
% of total detected radioactivity
after interval (days)
Compound 0 7 19
ethylene thiourea (ETU) 31 11 3.5
2-imidazoline 12 14 19
ethyleneurea (EU) 4 17 21
polar material remaining
at origin 22 39.5 40
other unidentified compounds 31 18 16.5
From this and other experiments the conclusion could be drawn
that from solutions of EBDC's in which ETU and EBIS (DIDT) are formed
only ETU is taken up in the plants. This compound is converted almost
quantitatively into a mixture of ethylene urea (EU) and 2-imidazoline.
In a study with radio-labelled mancozeb (3H, 14C or 35S) on
leafy plants such as sugar beets, lettuce or turnips, applied at
exaggerated rates to facilitate identification of metabolites, the
following intermediates could be detected 13 days after application by
using reverse isotope dilution techniques (Lyman 1971).
Compound Percentage of 3H
activity
mancozeb 9
ethylene thiourea (ETU) 6
ethyleneurea (EU) 17
ethylenediamine 11
2-imidazoline 8
N-formylethylenediamine 8
5,6-dihydro-3H-imidazo-[2,1-C-1,2,4-dithiazolo-3-thione]
(DIDT or EBIS) 7
3-(2-imidazolin-2-yl)-2-imidazolidin-ethione
(Jaffe's base) 4
Total 62
The quantitative figures are an example and do not necessarily
indicate a typical distribution of percentages of metabolites present
in a residue.
Uptake and translocation of ETU in plants
It was shown by Sato and Tomiziwa (1960) and Vonk and Sijpesteijn
(1970, 1971) that ETU can be taken up by the roots and translocated
through the plant. In another study by Lyman and Lacoste (1974), 14C
ETU was applied either to the leaves or to the soil around young
potato or tomato plants. The tomato plants had 1 - 3 fruits at the
time of application. Only small amounts of radioactivity moved from
the site of application to the fruits or to other parts of the plant
(Table 3).
TABLE 3 Translocation of radioactivity from 14C ETU
14C activity expressed as ETU
(mg/kg) after indicated interval
in potatoes in tomatoes
Application tuber foliage roots fruit foliage
site
90 days 90 days 60 days 28 days 28 days
Leaves 0.14 24 0.05 0.8 64
Soil n.d.* 0.84 n.d. 0.4 6
* n.d. no detectable radioactivity = (<0.0001 mg/kg ETU)
In water
Exposure of mancozeb to spray tank conditions did not produce
significant ETU build-up during normal residence times. Analysis of a
spray tank slurry of "mancozeb 80%", containing 0.1% a.i. showed an
ETU content of 2.08 mg/kg after 1 minute increasing to 3.62 mg/kg
after 4 hours.
Lyman and Lacoste (1974) studied the hydrolysis of 14C labelled
mancozeb in water at PH 5, 7 and 9. Sterile water was used to
distinguish chemical from biological degradation. Suspensions of 20
ppm mancozeb were agitated for 28 days at 25°C in amber bottles (to
avoid photodecomposition). Samples taken at selected intervals were
analysed by TLC. Two different chromatographic systems were used to
provide confirmation of the identity of the spots. The radioactive
zones were detected by autoradiography and were subsequently
quantitated by liquid scintillation counting. The nonradioactive
standards were located on the TLC plates by UV light or with a spray
reagent.
It was shown that mancozeb is not stable in water and that the
half-life in the pH 5 - 9 range is less than 1 day (Table 4).
TABLE 4 Hydrolysis of 14C labelled mancozeb
% of total 14C found as parent mancozeb
after indicated interval
hours days
pH 2 24 48 3 6 10 14 21 28
5 62.3 27.2 11.5 15.2 13.6 10.6 4.0 6.4 6.1
7 25.6 13.6 14.8 16.9 2.3 10.0 8.2 7.0 4.2
9 0
The nature and quantity of the degradation products are
pH-dependent. ETU and ethyleneurea are found within the range studied.
Cruickshank and Jarrow (1973) showed that aqueous solutions of
ETU exposed to UV light (above 285 mm) undergo a very slow photolysis:
the process is markedly accelerated by photosensitisers. When kept in
the dark ETU is stable to hydrolysis over the pH range 5 - 9 at 90°C.
These results were confirmed by Bontoyan and Looker (1973b).
Ross and Crosby (1973) observed that photosensitisers present in
agricultural drainage water will catalyze the photodegradation of ETU
(Table 5). The degradation does not occur in the absence of UV light.
Laboratory experiments carried out by Lyman and Lacoste (1974) showed
similar results to those of Ross and Crosby.
TABLE 5 Effect of UV irradiation on degradation of ETU in
agricultural drainage water
Duration (hours) and % of initial 14C found as ETU*
type of exposure in sterile water in non-sterile water
5 UV 75 92
24 UV none none
48 UV none none
48 dark 104 101
* confirmed by reverse isotope dilution
In soil
A recent study of EBDC compounds in soil by Hylin (1973)
illustrates the degradation pattern discussed previously. Kaufman
(1973) studied the fate of 14C labelled ETU in two types of soil
(Hagerstown silty clay loam and lakeland sandy loam) both sterile and
non-sterile. Essentially all of the ETU was converted to
2-imidazolidone within two days in the Hagerstown silty clay loam
treated with 2 and 20 mg/kg ETU and within 8 days in this soil treated
with 200 mg/kg ETU. A slower but steady conversion of ETU to
2-imidazolidone occurred in sterile (autoclaved) soil. In the
non-sterile Hagerstown silty clay loam further degradation occurred
rapidly. Within 7 days after treatment with 2, 20 and 200 mg/kg ETU
43.4, 8.9 and 0.9% respectively of the initial 14C was evolved at
14CO2 was evolved from the sterile, autoclaved soil.
In the lakeland sandy loam a similar but somewhat slower
degradation of ETU was observed. Besides 14CO2, two products were
identified by co-chromatography as 2,4-imidazolidinedione and
1-(2'-imidazolin-2'-yl)-2-imidazolinethione (Jaffe's base). A third
was tentatively identified as a subsequent degradation product of the
latter compound, and a fourth was unidentified.
Lyman and Lacoste (1974) studied the fate of ETU applied at
exaggerated rates on a sandy soil under field conditions. The high
dosage rates applied provided considerably more ETU than could be
expected from the application of mancozeb at recommended dosages. The
experiment illustrates that ETU when exposed to field conditions
degrades rapidly in or on the top one cm of a relatively inert
substrate.
TABLE 6. Decline of ETU in sand
Dosage rate ETU (mg/kg) at interval (days) after spraying
kg/ha 0 1 3 7
0.77 8.2 0.35 0.02 0.02
0.77 9.8 1.53 0.01 n.d.*
0.036 0.03 n.d. n.d. n.d.
* n.d.: No detectable residues (<0.01 mg/kg).
In a study in which 10 and 20 mg/kg 14C labelled mancozeb and
10 mg/kg 14C labelled ETU were applied to Hagerstown salt loam soil
it was demonstrated that both mancozeb and ETU are readily degraded by
soil micro-organisms to the point of releasing their ethylene carbons
as CO2. The 14CO2 released was trapped in sodium hydroxide and
measured by liquid scintillation to determine the loss of the best
compounds.
The experiments with each compound were done in both sterile and
non-sterile soils. No evolution of 14CO2 was observed from sterile
soil, but in non-sterile soil both mancozeb and ETU are rapidly
degraded to 14CO2. The half-life for ETU at the 10 mg/kg level is
about 22 days and that for 20 mg/kg mancozeb about 50 days. The
experiment with 10 mg/kg mancozeb was continued for 170 days; the
half-life was about 90 days.
Leaching experiments
Lyman and Lacoste (1974) studied the leaching of 14C mancozeb
and its degradation products in 5 different types of soil. The organic
matter in these soils ranged from 0.4 to 15% and the pH from 4.7 to
7.4.
An aqueous slurry of 14C labelled mancozeb was mixed with soil
which was then applied to the top of a 45 cm high column with a
diameter of 12.3 cm. The dosage applied was equivalent to a field
application of 8 kg mancozeb a.i./ha i.e. about 15.6 mg of mancozeb
80% to each column. Once a week for 9 weeks 2.5 cm of water was
applied to the top of each column. Radioactivity in the water emerging
from the column was determined by liquid scintillation counting. After
9 weeks the columns were cut into 2.5 cm sections and a sample from
each section was combusted to 14CO2, which was trapped and measured
by liquid scintillation counting. No radioactivity leached through
four of the 5 soils, and leaching beyond the top 2.5 cm was slight. In
cecil clay, however, 2 - 5% of the activity was leached from the
column and 37 - 47% was found below the top 2.5 cm. This soil was a
kaolinite clay containing 32% sand, 54% clay, 14% silt and 0.49%
organic matter. The pH was 4.7. Losses of radioactivity by
volatilization or by complete metabolism to CO2 were significant in
all soils except the cecil clay soil.
Effect of processing and cooking
Haines and Adler (1973) studied the effect of normal cooking (20
minute boiling) ETU residues in spinach. Various samples were selected
from field studies with mancozeb at different intervals (0-14 days)
after the application of 7 x 1.5 kg "mancozeb 80%"/ha. In cases where
ETU residues were found in the uncooked samples (0.03 - 0.11 mg/kg) no
residues were found after cooking (Table 7).
TABLE 7 Residues of ETU in spinach, before and after cooking
Interval after Residues, mg/kg
application (days) uncooked spinach cooked spinach
mancozeb ETU ETU
0 6.8-8.3 0.9-0.11 n.d.*
3 2.6-3.8 0.03-0.03 n.d.
7 0.6-1.2 n.d.-n.d. n.d.
14 0.6-0.8 n.d.-n.d. n.d.
* n.d.: <0.01 mg/kg
These results differ from those obtained by Watts et al. (1974)
after cooking spinach fortified with mancozeb. They found that ETU was
formed by cooking, the weight produced being about 20% of the weight
of mancozeb originally added. The effect on other EBDC compounds was
similar (Table 8). Newsome and Laver (1973) reported similar results
after cooking spinach, potatoes and carrots containing residues of
mancozeb and metiram.
TABLE 8 ETU produced by cooking vegetables fortified with 10 mg/kg
of EBDC compounds +
ETU (mg/kg)
EBDC Fortified Fortified Percentage of
Crop compound after before ETU formed by
cooking cooking cooking*
Spinach maneb 0.16 1.82 16.6
mancozeb
(Dithane M-45) 0.15 2.17 20.2
mancozeb
(Manzate 200) 0.11 2.42 23.1
metiram 0.07 2.72 26.5
Potato metiram 0.08 1.43 13.5
maneb 0.08 1.20 11.2
Carrot metiram 0.09 1.42 13.3
maneb 0.08 1.42 13.4
+ After Watts et al. (1974).
* Weight ETU formed as percentage of weight of mancozeb added.
METHODS OF RESIDUE ANALYSIS
Mancozeb and ethylenediamine-yielding metabolites
A method for the determination of ethylenediamine (EDA) which is
liberated from known components of residues [mancozeb, free
ethlylenediamine (EDA), ethyleneurea (EU), ETU, EBIS (DIDT)] was
presented at the 1970 JMPR by Rohm and Haas. A modified method was
developed by Newsome (1974). The EDA is isolated, after hydrolysis of
the residues with acid containing stannous chloride, by ion exchange
chromatography and quantitated by gas liquid chromatography of its
bis(triflouroacetate). Overall recoveries at levels of 0.16 - 1.3
mg/kg parent compound mancozeb were greater than 80% and generally
more than 95%. The limit of detection in terms of mancozeb is about
0.1 mg/kg.
ETU
Several methods are available for the determination of ETU
residues in food, soil and water. The gas-chromatographic methods are
suitable, or may be adapted, for regulatory purposes (Haines and Adler
1973, Newsome 1974, Onley and Yip 1971, Nash 1974).
Blazques (1973) developed a TLC method for the determination of
ETU in plants, soil and water with a limit of detection of 0.1 mg/kg.
Merek-Luenyo and Barragan-Allcaide (1974) developed a new TLC method,
in which ETU is detected by nitrosation and subsequent colour reaction
with N-(1-naphtyl)-ethylenediamine dihydrochloride. ETU, EBIS and
ethylene thiuram disulfide appear as purple spots.
Engst and Schnaak developed a polarographic procedure for the
determination of ETU in vegetable and animal products. After
extraction with methanol the interfering substances are separated by
column and paper chromatography. ETU is converted to the
nitroso-compound determined by its reduction wave using a cathode ray
polarograph. The limit of detection is about 0.05 mg/kg ETU.
NATIONAL TOLERANCES
a) Mancozeb
Tolerances are in effect in a number of countries around the
world. The tolerances are expressed and residues calculated as parent
compound, as zineb, or as the CS2 moiety.
b) ETU
No national tolerances have been established.
APPRAISAL
Mancozeb was considered by the 1967 and 1970 Joint Meetings. At
the 1970 Meeting further data on the biotransformation of the compound
in plants and animals was requested.
Several new studies have been carried out which clarify to a
large extent the metabolic pathway of mancozeb. It has been shown that
the breakdown of the compound in plants and animals is qualitatively
similar, and proceeds through a series of intermediates; the
structures of most of them have been clarified. Several other
ethylenebisdithiocarbamates (EBDCs) in common use show a similar
breakdown pattern on plants, leading to several identical
intermediates.
Information was obtained on the variation in the content of ETU
in technical mancozeb from several different manufacturers and in
various formulated products.
It has been shown that cooking of commodities of plant origin
containing mancozeb residues produce ETU to an extent corresponding to
about 25% of the weight of the original residue. Other EBDC compounds
gave similar results.
Additional data on the residues of mancozeb and ETU arising from
treatments according to good agricultural practice were obtained and
compared with those on zineb and maneb.
Several new sensitive methods are now available for the
determination of ethylenethiourea (ETU), the residue intermediate
which gives most concern. Some of these methods, which allow the
determination of ETU in plants and in products of animal origin at
levels of about 0.005 - 0.01 mg/kg, are suitable for regulatory
purposes. On the other hand, it is recognised that no specific methods
for determining mancozeb or for distinguishing its residues from those
of other EBDC compounds are available, and that development of such
methods in the near future is unlikely. It may therefore be of value,
in order to detect exaggerated uses of these compounds, to establish
maximum residue limits on the combined basis of either the
ethylenediamine or the CS2 moiety of the EBDC molecule, and the ETU
content. For regulatory purposes the determination of CS2 is more
convenient but suffers from the disadvantage of not distinguishing
EBDC residues from those of dimethyldithiocarbamates or thiram, and
toxicological considerations may make these distinctions important.
The ethylenediamine method of analysis is more time consuming
determination but more sensitive and not subject to interference by
the dimethyldithiocarbamates or thiram. The Meeting therefore
recommends (1) that residue limits should be based on the
determination of the ethylenediamine moiety; (2) that the specified
limit should be for ethylenediamine; (3) that limits for ETU should
also be observed. Specific limits for ethylenediamine and ETU are
recommended.
RECOMMENDATIONS
The following temporary tolerances for ethylenediamine and ETU
replace the single recommendation for "parent compound or sum of all
dithiocarbamates present" in potatoes, made in 1970. Neither limit
should be exceeded in any sample.
TEMPORARY TOLERANCES
Pre-harvest
intervals on which
ethylenediamine ETU recommendations
mg/kg mg/kg are based (days)
Potatoes 0.05 0.01* 14
Apples, pears 2 0.02 14
Banana pulp 0.05 0.01* 0
Celery 2 0.01* 14
Lettuce 2 0.01* 21
Tomatoes 1 0.05 7
Carrots 0.2 0.01* 14
Maize: sweetcorn
cob and kernel
(husks removed) 0.2 0.01* 7
Beans (with pod) 3 0.1 7 - 10
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED
1. Residue studies in which both the ethylenediamine moiety and
ethylenethiourea (ETU) are separately determined.
2. Further studies on the fate of residues during the preparation
and processing of foods with Particular reference to their conversion
to ETU.
REFERENCES
Benson, W.R., Rose, R.D., Chen, J.-Y.T., Barron, R.P. and Mastbrook,
D. (1972) Structure of ethylene thiurem monosulfide. J. Ass. Off.
Analyt. Chem., 55(1):44-46.
Blazques, C.H., (1973) Residue determination of ethylene thiourea
(2-imidazolidinethione) from tomato foliage, soil, and water. J. Agr.
Food. Chem., 21(3):330-332.
Bontoyan, W.R. and Looker, J.B. (1973a) Degradation of commercial
ethylene bisdithiocarbamate formulations to ethylenethiourea under
elevated temperature and humidity. J. Agr. Food Chem., 21(3):338-341.
Bontoyan, W.R., Looker, J.B. (1973b) Report to the 1973 AOAC Meeting.
Washington, D.C.
Cruickshank, P.A. and Jarrow, H.C. (1973) Ethylenethiourea
degradation. J. Agr. Food Chem., 21(3):333-335.
Czeglédi-Jankó, G. (1967) Determination of the degradation products of
ethylenebis-dithiocarbamates by thin layer chromatography and some
investigations of their decomposition in vitro. J. Chromat., 31:89-95.
Dekhuijzen, H.M., Vonk, J.W. and Sijpesteijn, A. Kaars. (1971)
Terminal residues of dithiocarbamate fungicides. Pesticide Terminal
Residues Butterworth, London (Suppl. Pure Appl. Chem.) p. 233-242.
Engst, R. and Schnaak, W. (1967) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, I. Mitteilung:
Identifizierung und fungitoxische Wirkung im Modellversuch erhaltener
Metabolite. Z. Lebensm. - Unters. - Forsch., 134:216-221.
Engst, R. and Schnaak, W. (1968/1969) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, II.
Mitteilung: Polarographische Bestimmung des unzersetzten
Wirkstoffanterls. Z. Lebensm. - Unters. - Forsch., 139:149-152.
Engst, R. and Schnaak, W. (1970) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, III.
Mitterlung: Zum Reaktionsverlauf des Abbaus. Z. Lebensm. - Unters. -
Forsch., 143:99-103.
Engst R. and Schnaak, W. (1970/1971) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, IV.
Mitterlung: Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid-Rückständen auf pflanzlichen
Erntegütern. Z. Lebensm. - Unters. - Forsch., 144:81-85.
Engst, R., Schnaak, W. and Lewerenz, H.-J. (1971) Untersuchungen zum
Metabolismus der fungiciden Äthylen-bis-dithiocarbamate Maneb, Zineb
und Nabam, V. Mitterlung: Zur Toxikologie der Abbauprodukte. Z.
Lebensm. - Unters. - Forsch., 146:91-97.
Engst, R., and Schnaak, W. (1969) Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid. Z. analyt. Chem., 248:321.
Engst, R., and Schnaak, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue reviews,
52:45-67.
Engst, R., Schnaak, W., and Rattba, H. (1968) Fungizide Wirkung und
Rückstandsbildung von Abbauprodukten des Maneb und Zineb an
freilandbehandelten Tomaten. NachrBl. dt. PflSchutzdienst, Berl.,
22:26-29.
FAO/WHO. (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL/1967/M/11/1; WHO/Food Add./68:30.
FAO/WHO. (1971) 1970 Evaluations of some pesticide residues in food.
AGP/1970/M/12/1; WHO/Food Add./71.42.
Haines, L.D., and Adler, I.L. (1974) Recommendation for using the ETU
method, of J. Ass. off. analyt. Chem., 56(2):333-337. Unpublished
report from Rohm and Haas.
Haines, L.D. and Adler, I.L. (1973) Gas chromatographic determination
of ethylene thiourea residues. J. Ass. off. analyt. Chem.,
56(2):333-337.
Hellwig, A., Liebmann, R. and Hempel, D. (1971) Zur
Rüchstandsbestimmung metallhaltiger Fungizide mit Hilfe einer
Enzymtestmethode. Archiv. Pflanzenschutz, 7:73-78.
Hylin, J.W. (1966) Thin layer chromatography of dithiocarbamate
fungicides. Bull. Environ. Contam. Toxicol., 1:76-77.
Hylin, J.W. (1973) Oxidative decomposition of ethylene-bis-
dithiocarbamates. Bull. Environ. Contam. Toxicol., 10(4):227-233.
Sijpestijn, A. Kaars and Kaslander, J. (1965) Metabolism of fungicides
by plants and microorganisms. Outlook on Agriculture, 4(4):119-125.
Sijpestijn, A. Kaars and Vonk, J.W. (1970) Microbial conversion of
Med. Fac. Landbouwwetensch. Gent, 35(2):799-804.
Sijpesteijn A. Kaars and Vonk, J.W. Decomposition of
bis-dithiocarbamates and metabolism by plant and microorganisms. Env.
Quality and Safety. (In press).
Kuslander, J. (1966) Metabolic fate of dithiocarbamates. Thesis.
Utrecht. 95p.
Kaufman, D.D. (1973) Ethylenethiourea degradation in soil. Paper
presented at 2nd International Congress on Plant Pathology.
Minneapolis. September 1973.
Keppel, G.E. (1969) Modification of the carbon disulfide evolution
method for dithiocarbamate residues. J. Ass. off. Analyt. Chem.,
52(1):162-167.
Klisenko, M.A. and Vekstejn, M.S. (1971) O skorosti razlozenija i
produktach prevrascenija etilen-bis-dithiocarbamatov v razlicnych sel
skochozjaistvennych produktach. Vopr-Pitaniya, 30(5):79.
Lyman, W.R. (1971) The metabolic fate of Dithane M-75 (coordination
product of zinc iron and Manganous ethylenebisdithiocarbamate) in
Pestic. Terminal Residues (Suppl. Pure Appl. Chem.). p. 243-256.
Lyman, W.R. and Lacoste, R.J. New developments in the chemistry and
fate of ethylenebisdithiocarbamate fungicides. Paper presented at
Third International Congress of Pesticide Chemistry. Helsinki, July
1974. (In press).
Merek-Luenyo and Barragan-Allcaide. (1974) (Unpublished).
Nash, R.G. (1974) Improved gas-liquid chromatographic method for
determining ethylenethiourea in plants. J. Ass. off. Analyt. Chem.,
57(5):1015-1021.
Newsome, W.H. (1972) Determination of ethylene thiourea residues in
apples. J. Agr. Food Chem., 20(5):967-969.
Newsome, W.H. (1974) A method for determining ethylenebis
(dithiocarbamate) residues on food crops as bis (triflouro-acetamido)
ethane. J. Agr. Food Chem., 22(5):886-889.
Newsome, W.H. A method for the determination of ethylenethiuram
monosulfide on food crops. (In press).
Newsome, W.H., Brian, J.B. and Villeneuve, D.C. Residues of mancozeb,
ethylene thiuram monosulfide, ethylene thiourea, and ethylenediamine
on beans and tomatoes field-treated with mancozeb. (In press).
Newsome, W.H. and Laver, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb treated foods. Bull. Environ.
Contam. Toxicol., 10(3):151-154.
Onley, J.H. and Yip, G. (1971) Determination of ethylene thiourea
residues in food using thin layer and gas chromatography. J. Ass. off.
analyt. Chem., 54(1):165-169.
Pluijgers, C.W. and Vonk, J.W. Re-examination of the structure of
ethylene-thiuram monosulphide. Tetrahedron Letters, 18:1317.
Povlsen, H.H., Dahl, E. and Christensen, B.S. (1974) Report on
determination of ETU (ethylene thiourea) and water in dithiocarbamate
formulations. Paper provided to 1974 JMPR. (Unpublished).
Ross, R.D. and Crosby, D.G. (1973) Photolysis of ethylene thiourea. J.
Agr. Food. Chem., 21(3):335-337.
Sato, T., and Tomiziwa, C. (1960) Decomposition of zinc
ethylenebisdithiocarbamates (zineb) applied to plants. Bull. natn,
Inst. agric. Sci., Tokyo, Ser. C12, 181.
Thorn, G.D. and Ludwig, R.A. (1962) The dithiocarbamates and related
compounds. Elservier monographs. Amsterdam 298p.
Vekstejn, M.S. and Klisenko, M.A. (1970) Identifikacija i
kolicestvennoe opredelenie mekotorych dialkilditiokarbamatov i
produktov ich metabolisma v razlienych sredach rastitel nogo i
zivotnogo proischozdenija Vopr. Pitaniya, 29(1):56.
Vonk, J.W. (1971) Ethylene thiourea, a systemic decomposition product
of nabam. Med. Fak. Landbouw. Wetenschappen, 36(1):110-112.
Vonk, J.W. (1974) Transformation of dithiocarbamate fungicides and the
chemical nature of their residues in plants. Progress report to
FAO/IAEA Research Coord. Meeting on isotopic tracer aided studies of
foreign chemical residues in food. Vienna, June 1974.
Vonk, J.W. and Sijpestijn, A. Kaars. (1970) Studies on the fate in
plants of ethylenedithiocarbamate fungicides and their decomposition
products. Ann. appl. Biol., 65:489-496.
Vonk, J.W. and Sijpestijn, A. Kaars. (1971) Tentative identification
of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicide. Pest. Biochem. Physiol.,
1:163-165.
Watts, R.R., Storherr, R.W. and Onley, J.H. (1974) Effects of cooking
on ethylenebisdithiocarbamate degradation to ethylene thiourea. Bull.
Environ. Contam. Toxicol., 12(2):224-226.
Yip, G., Onley, J.H. and Howard, S.F. (1971) Residues of maneb and
ethylenethiourea on field-sprayed lettuce and kale. J. Ass. off.
analyt. Chem., 54(6):1373-1375
Vonk and Sijpesteijn (1970) studied the fate of the main
metabolites of EBDC's in the green parts of cucumber plants after
application of 14C-ethylene labelled nabam to the leaves. They found
the following distribution of radioactivity in water extracts from
cucumber leaves after 0.7 and 19 days. The compounds were separated by
TLC.
TABLE 2 Fate of nabam in cucumbers
% of total detected radioactivity
after interval (days)
Compound 0 7 19
ethylene thiourea (ETU) 31 11 3.5
2-imidazoline 12 14 19
ethyleneurea (EU) 4 17 21
polar material remaining
at origin 22 39.5 40
other unidentified compounds 31 18 16.5
From this and other experiments the conclusion could be drawn
that from solutions of EBDC's in which ETU and EBIS (DIDT) are formed
only ETU is taken up in the plants. This compound is converted almost
quantitatively into a mixture of ethylene urea (EU) and 2-imidazoline.
In a study with radio-labelled mancozeb (3H, 14C or 35S) on
leafy plants such as sugar beets, lettuce or turnips, applied at
exaggerated rates to facilitate identification of metabolites, the
following intermediates could be detected 13 days after application by
using reverse isotope dilution techniques (Lyman 1971).
Compound Percentage of 3H
activity
mancozeb 9
ethylene thiourea (ETU) 6
ethyleneurea (EU) 17
ethylenediamine 11
2-imidazoline 8
N-formylethylenediamine 8
5,6-dihydro-3H-imidazo-[2,1-C-1,2,4-dithiazolo-3-thione]
(DIDT or EBIS) 7
3-(2-imidazolin-2-yl)-2-imidazolidin-ethione
(Jaffe's base) 4
Total 62
The quantitative figures are an example and do not necessarily
indicate a typical distribution of percentages of metabolites present
in a residue.
Uptake and translocation of ETU in plants
It was shown by Sato and Tomiziwa (1960) and Vonk and Sijpesteijn
(1970, 1971) that ETU can be taken up by the roots and translocated
through the plant. In another study by Lyman and Lacoste (1974), 14C
ETU was applied either to the leaves or to the soil around young
potato or tomato plants. The tomato plants had 1 - 3 fruits at the
time of application. Only small amounts of radioactivity moved from
the site of application to the fruits or to other parts of the plant
(Table 3).
TABLE 3 Translocation of radioactivity from 14C ETU
14C activity expressed as ETU
(mg/kg) after indicated interval
in potatoes in tomatoes
Application tuber foliage roots fruit foliage
site
90 days 90 days 60 days 28 days 28 days
Leaves 0.14 24 0.05 0.8 64
Soil n.d.* 0.84 n.d. 0.4 6
* n.d. no detectable radioactivity = (<0.0001 mg/kg ETU)
In water
Exposure of mancozeb to spray tank conditions did not produce
significant ETU build-up during normal residence times. Analysis of a
spray tank slurry of "mancozeb 80%", containing 0.1% a.i. showed an
ETU content of 2.08 mg/kg after 1 minute increasing to 3.62 mg/kg
after 4 hours.
Lyman and Lacoste (1974) studied the hydrolysis of 14C labelled
mancozeb in water at PH 5, 7 and 9. Sterile water was used to
distinguish chemical from biological degradation. Suspensions of 20
ppm mancozeb were agitated for 28 days at 25°C in amber bottles (to
avoid photodecomposition). Samples taken at selected intervals were
analysed by TLC. Two different chromatographic systems were used to
provide confirmation of the identity of the spots. The radioactive
zones were detected by autoradiography and were subsequently
quantitated by liquid scintillation counting. The nonradioactive
standards were located on the TLC plates by UV light or with a spray
reagent.
It was shown that mancozeb is not stable in water and that the
half-life in the pH 5 - 9 range is less than 1 day (Table 4).
TABLE 4 Hydrolysis of 14C labelled mancozeb
% of total 14C found as parent mancozeb
after indicated interval
hours days
pH 2 24 48 3 6 10 14 21 28
5 62.3 27.2 11.5 15.2 13.6 10.6 4.0 6.4 6.1
7 25.6 13.6 14.8 16.9 2.3 10.0 8.2 7.0 4.2
9 0
The nature and quantity of the degradation products are
pH-dependent. ETU and ethyleneurea are found within the range studied.
Cruickshank and Jarrow (1973) showed that aqueous solutions of
ETU exposed to UV light (above 285 mm) undergo a very slow photolysis:
the process is markedly accelerated by photosensitisers. When kept in
the dark ETU is stable to hydrolysis over the pH range 5 - 9 at 90°C.
These results were confirmed by Bontoyan and Looker (1973b).
Ross and Crosby (1973) observed that photosensitisers present in
agricultural drainage water will catalyze the photodegradation of ETU
(Table 5). The degradation does not occur in the absence of UV light.
Laboratory experiments carried out by Lyman and Lacoste (1974) showed
similar results to those of Ross and Crosby.
TABLE 5 Effect of UV irradiation on degradation of ETU in
agricultural drainage water
Duration (hours) and % of initial 14C found as ETU*
type of exposure in sterile water in non-sterile water
5 UV 75 92
24 UV none none
48 UV none none
48 dark 104 101
* confirmed by reverse isotope dilution
In soil
A recent study of EBDC compounds in soil by Hylin (1973)
illustrates the degradation pattern discussed previously. Kaufman
(1973) studied the fate of 14C labelled ETU in two types of soil
(Hagerstown silty clay loam and lakeland sandy loam) both sterile and
non-sterile. Essentially all of the ETU was converted to
2-imidazolidone within two days in the Hagerstown silty clay loam
treated with 2 and 20 mg/kg ETU and within 8 days in this soil treated
with 200 mg/kg ETU. A slower but steady conversion of ETU to
2-imidazolidone occurred in sterile (autoclaved) soil. In the
non-sterile Hagerstown silty clay loam further degradation occurred
rapidly. Within 7 days after treatment with 2, 20 and 200 mg/kg ETU
43.4, 8.9 and 0.9% respectively of the initial 14C was evolved at
14CO2 was evolved from the sterile, autoclaved soil.
In the lakeland sandy loam a similar but somewhat slower
degradation of ETU was observed. Besides 14CO2, two products were
identified by co-chromatography as 2,4-imidazolidinedione and
1-(2'-imidazolin-2'-yl)-2-imidazolinethione (Jaffe's base). A third
was tentatively identified as a subsequent degradation product of the
latter compound, and a fourth was unidentified.
Lyman and Lacoste (1974) studied the fate of ETU applied at
exaggerated rates on a sandy soil under field conditions. The high
dosage rates applied provided considerably more ETU than could be
expected from the application of mancozeb at recommended dosages. The
experiment illustrates that ETU when exposed to field conditions
degrades rapidly in or on the top one cm of a relatively inert
substrate.
TABLE 6. Decline of ETU in sand
Dosage rate ETU (mg/kg) at interval (days) after spraying
kg/ha 0 1 3 7
0.77 8.2 0.35 0.02 0.02
0.77 9.8 1.53 0.01 n.d.*
0.036 0.03 n.d. n.d. n.d.
* n.d.: No detectable residues (<0.01 mg/kg).
In a study in which 10 and 20 mg/kg 14C labelled mancozeb and
10 mg/kg 14C labelled ETU were applied to Hagerstown salt loam soil
it was demonstrated that both mancozeb and ETU are readily degraded by
soil micro-organisms to the point of releasing their ethylene carbons
as CO2. The 14CO2 released was trapped in sodium hydroxide and
measured by liquid scintillation to determine the loss of the best
compounds.
The experiments with each compound were done in both sterile and
non-sterile soils. No evolution of 14CO2 was observed from sterile
soil, but in non-sterile soil both mancozeb and ETU are rapidly
degraded to 14CO2. The half-life for ETU at the 10 mg/kg level is
about 22 days and that for 20 mg/kg mancozeb about 50 days. The
experiment with 10 mg/kg mancozeb was continued for 170 days; the
half-life was about 90 days.
Leaching experiments
Lyman and Lacoste (1974) studied the leaching of 14C mancozeb
and its degradation products in 5 different types of soil. The organic
matter in these soils ranged from 0.4 to 15% and the pH from 4.7 to
7.4.
An aqueous slurry of 14C labelled mancozeb was mixed with soil
which was then applied to the top of a 45 cm high column with a
diameter of 12.3 cm. The dosage applied was equivalent to a field
application of 8 kg mancozeb a.i./ha i.e. about 15.6 mg of mancozeb
80% to each column. Once a week for 9 weeks 2.5 cm of water was
applied to the top of each column. Radioactivity in the water emerging
from the column was determined by liquid scintillation counting. After
9 weeks the columns were cut into 2.5 cm sections and a sample from
each section was combusted to 14CO2, which was trapped and measured
by liquid scintillation counting. No radioactivity leached through
four of the 5 soils, and leaching beyond the top 2.5 cm was slight. In
cecil clay, however, 2 - 5% of the activity was leached from the
column and 37 - 47% was found below the top 2.5 cm. This soil was a
kaolinite clay containing 32% sand, 54% clay, 14% silt and 0.49%
organic matter. The pH was 4.7. Losses of radioactivity by
volatilization or by complete metabolism to CO2 were significant in
all soils except the cecil clay soil.
Effect of processing and cooking
Haines and Adler (1973) studied the effect of normal cooking (20
minute boiling) ETU residues in spinach. Various samples were selected
from field studies with mancozeb at different intervals (0-14 days)
after the application of 7 x 1.5 kg "mancozeb 80%"/ha. In cases where
ETU residues were found in the uncooked samples (0.03 - 0.11 mg/kg) no
residues were found after cooking (Table 7).
TABLE 7 Residues of ETU in spinach, before and after cooking
Interval after Residues, mg/kg
application (days) uncooked spinach cooked spinach
mancozeb ETU ETU
0 6.8-8.3 0.9-0.11 n.d.*
3 2.6-3.8 0.03-0.03 n.d.
7 0.6-1.2 n.d.-n.d. n.d.
14 0.6-0.8 n.d.-n.d. n.d.
* n.d.: <0.01 mg/kg
These results differ from those obtained by Watts et al. (1974)
after cooking spinach fortified with mancozeb. They found that ETU was
formed by cooking, the weight produced being about 20% of the weight
of mancozeb originally added. The effect on other EBDC compounds was
similar (Table 8). Newsome and Laver (1973) reported similar results
after cooking spinach, potatoes and carrots containing residues of
mancozeb and metiram.
TABLE 8 ETU produced by cooking vegetables fortified with 10 mg/kg
of EBDC compounds +
ETU (mg/kg)
EBDC Fortified Fortified Percentage of
Crop compound after before ETU formed by
cooking cooking cooking*
Spinach maneb 0.16 1.82 16.6
mancozeb
(Dithane M-45) 0.15 2.17 20.2
mancozeb
(Manzate 200) 0.11 2.42 23.1
metiram 0.07 2.72 26.5
Potato metiram 0.08 1.43 13.5
maneb 0.08 1.20 11.2
Carrot metiram 0.09 1.42 13.3
maneb 0.08 1.42 13.4
+ After Watts et al. (1974).
* Weight ETU formed as percentage of weight of mancozeb added.
METHODS OF RESIDUE ANALYSIS
Mancozeb and ethylenediamine-yielding metabolites
A method for the determination of ethylenediamine (EDA) which is
liberated from known components of residues [mancozeb, free
ethlylenediamine (EDA), ethyleneurea (EU), ETU, EBIS (DIDT)] was
presented at the 1970 JMPR by Rohm and Haas. A modified method was
developed by Newsome (1974). The EDA is isolated, after hydrolysis of
the residues with acid containing stannous chloride, by ion exchange
chromatography and quantitated by gas liquid chromatography of its
bis(triflouroacetate). Overall recoveries at levels of 0.16 - 1.3
mg/kg parent compound mancozeb were greater than 80% and generally
more than 95%. The limit of detection in terms of mancozeb is about
0.1 mg/kg.
ETU
Several methods are available for the determination of ETU
residues in food, soil and water. The gas-chromatographic methods are
suitable, or may be adapted, for regulatory purposes (Haines and Adler
1973, Newsome 1974, Onley and Yip 1971, Nash 1974).
Blazques (1973) developed a TLC method for the determination of
ETU in plants, soil and water with a limit of detection of 0.1 mg/kg.
Merek-Luenyo and Barragan-Allcaide (1974) developed a new TLC method,
in which ETU is detected by nitrosation and subsequent colour reaction
with N-(1-naphtyl)-ethylenediamine dihydrochloride. ETU, EBIS and
ethylene thiuram disulfide appear as purple spots.
Engst and Schnaak developed a polarographic procedure for the
determination of ETU in vegetable and animal products. After
extraction with methanol the interfering substances are separated by
column and paper chromatography. ETU is converted to the
nitroso-compound determined by its reduction wave using a cathode ray
polarograph. The limit of detection is about 0.05 mg/kg ETU.
NATIONAL TOLERANCES
a) Mancozeb
Tolerances are in effect in a number of countries around the
world. The tolerances are expressed and residues calculated as parent
compound, as zineb, or as the CS2 moiety.
b) ETU
No national tolerances have been established.
APPRAISAL
Mancozeb was considered by the 1967 and 1970 Joint Meetings. At
the 1970 Meeting further data on the biotransformation of the compound
in plants and animals was requested.
Several new studies have been carried out which clarify to a
large extent the metabolic pathway of mancozeb. It has been shown that
the breakdown of the compound in plants and animals is qualitatively
similar, and proceeds through a series of intermediates; the
structures of most of them have been clarified. Several other
ethylenebisdithiocarbamates (EBDCs) in common use show a similar
breakdown pattern on plants, leading to several identical
intermediates.
Information was obtained on the variation in the content of ETU
in technical mancozeb from several different manufacturers and in
various formulated products.
It has been shown that cooking of commodities of plant origin
containing mancozeb residues produce ETU to an extent corresponding to
about 25% of the weight of the original residue. Other EBDC compounds
gave similar results.
Additional data on the residues of mancozeb and ETU arising from
treatments according to good agricultural practice were obtained and
compared with those on zineb and maneb.
Several new sensitive methods are now available for the
determination of ethylenethiourea (ETU), the residue intermediate
which gives most concern. Some of these methods, which allow the
determination of ETU in plants and in products of animal origin at
levels of about 0.005 - 0.01 mg/kg, are suitable for regulatory
purposes. On the other hand, it is recognised that no specific methods
for determining mancozeb or for distinguishing its residues from those
of other EBDC compounds are available, and that development of such
methods in the near future is unlikely. It may therefore be of value,
in order to detect exaggerated uses of these compounds, to establish
maximum residue limits on the combined basis of either the
ethylenediamine or the CS2 moiety of the EBDC molecule, and the ETU
content. For regulatory purposes the determination of CS2 is more
convenient but suffers from the disadvantage of not distinguishing
EBDC residues from those of dimethyldithiocarbamates or thiram, and
toxicological considerations may make these distinctions important.
The ethylenediamine method of analysis is more time consuming
determination but more sensitive and not subject to interference by
the dimethyldithiocarbamates or thiram. The Meeting therefore
recommends (1) that residue limits should be based on the
determination of the ethylenediamine moiety; (2) that the specified
limit should be for ethylenediamine; (3) that limits for ETU should
also be observed. Specific limits for ethylenediamine and ETU are
recommended.
RECOMMENDATIONS
The following temporary tolerances for ethylenediamine and ETU
replace the single recommendation for "parent compound or sum of all
dithiocarbamates present" in potatoes, made in 1970. Neither limit
should be exceeded in any sample.
TEMPORARY TOLERANCES
Pre-harvest
intervals on which
ethylenediamine ETU recommendations
mg/kg mg/kg are based (days)
Potatoes 0.05 0.01* 14
Apples, pears 2 0.02 14
Banana pulp 0.05 0.01* 0
Celery 2 0.01* 14
Lettuce 2 0.01* 21
Tomatoes 1 0.05 7
Carrots 0.2 0.01* 14
Maize: sweetcorn
cob and kernel
(husks removed) 0.2 0.01* 7
Beans (with pod) 3 0.1 7 - 10
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED
1. Residue studies in which both the ethylenediamine moiety and
ethylenethiourea (ETU) are separately determined.
2. Further studies on the fate of residues during the preparation
and processing of foods with Particular reference to their conversion
to ETU.
REFERENCES
Benson, W.R., Rose, R.D., Chen, J.-Y.T., Barron, R.P. and Mastbrook,
D. (1972) Structure of ethylene thiurem monosulfide. J. Ass. Off.
Analyt. Chem., 55(1):44-46.
Blazques, C.H., (1973) Residue determination of ethylene thiourea
(2-imidazolidinethione) from tomato foliage, soil, and water. J. Agr.
Food. Chem., 21(3):330-332.
Bontoyan, W.R. and Looker, J.B. (1973a) Degradation of commercial
ethylene bisdithiocarbamate formulations to ethylenethiourea under
elevated temperature and humidity. J. Agr. Food Chem., 21(3):338-341.
Bontoyan, W.R., Looker, J.B. (1973b) Report to the 1973 AOAC Meeting.
Washington, D.C.
Cruickshank, P.A. and Jarrow, H.C. (1973) Ethylenethiourea
degradation. J. Agr. Food Chem., 21(3):333-335.
Czeglédi-Jankó, G. (1967) Determination of the degradation products of
ethylenebis-dithiocarbamates by thin layer chromatography and some
investigations of their decomposition in vitro. J. Chromat., 31:89-95.
Dekhuijzen, H.M., Vonk, J.W. and Sijpesteijn, A. Kaars. (1971)
Terminal residues of dithiocarbamate fungicides. Pesticide Terminal
Residues Butterworth, London (Suppl. Pure Appl. Chem.) p. 233-242.
Engst, R. and Schnaak, W. (1967) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, I. Mitteilung:
Identifizierung und fungitoxische Wirkung im Modellversuch erhaltener
Metabolite. Z. Lebensm. - Unters. - Forsch., 134:216-221.
Engst, R. and Schnaak, W. (1968/1969) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, II.
Mitteilung: Polarographische Bestimmung des unzersetzten
Wirkstoffanterls. Z. Lebensm. - Unters. - Forsch., 139:149-152.
Engst, R. and Schnaak, W. (1970) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, III.
Mitterlung: Zum Reaktionsverlauf des Abbaus. Z. Lebensm. - Unters. -
Forsch., 143:99-103.
Engst R. and Schnaak, W. (1970/1971) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, IV.
Mitterlung: Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid-Rückständen auf pflanzlichen
Erntegütern. Z. Lebensm. - Unters. - Forsch., 144:81-85.
Engst, R., Schnaak, W. and Lewerenz, H.-J. (1971) Untersuchungen zum
Metabolismus der fungiciden Äthylen-bis-dithiocarbamate Maneb, Zineb
und Nabam, V. Mitterlung: Zur Toxikologie der Abbauprodukte. Z.
Lebensm. - Unters. - Forsch., 146:91-97.
Engst, R., and Schnaak, W. (1969) Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid. Z. analyt. Chem., 248:321.
Engst, R., and Schnaak, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue reviews,
52:45-67.
Engst, R., Schnaak, W., and Rattba, H. (1968) Fungizide Wirkung und
Rückstandsbildung von Abbauprodukten des Maneb und Zineb an
freilandbehandelten Tomaten. NachrBl. dt. PflSchutzdienst, Berl.,
22:26-29.
FAO/WHO. (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL/1967/M/11/1; WHO/Food Add./68:30.
FAO/WHO. (1971) 1970 Evaluations of some pesticide residues in food.
AGP/1970/M/12/1; WHO/Food Add./71.42.
Haines, L.D., and Adler, I.L. (1974) Recommendation for using the ETU
method, of J. Ass. off. analyt. Chem., 56(2):333-337. Unpublished
report from Rohm and Haas.
Haines, L.D. and Adler, I.L. (1973) Gas chromatographic determination
of ethylene thiourea residues. J. Ass. off. analyt. Chem.,
56(2):333-337.
Hellwig, A., Liebmann, R. and Hempel, D. (1971) Zur
Rüchstandsbestimmung metallhaltiger Fungizide mit Hilfe einer
Enzymtestmethode. Archiv. Pflanzenschutz, 7:73-78.
Hylin, J.W. (1966) Thin layer chromatography of dithiocarbamate
fungicides. Bull. Environ. Contam. Toxicol., 1:76-77.
Hylin, J.W. (1973) Oxidative decomposition of ethylene-bis-
dithiocarbamates. Bull. Environ. Contam. Toxicol., 10(4):227-233.
Sijpestijn, A. Kaars and Kaslander, J. (1965) Metabolism of fungicides
by plants and microorganisms. Outlook on Agriculture, 4(4):119-125.
Sijpestijn, A. Kaars and Vonk, J.W. (1970) Microbial conversion of
Med. Fac. Landbouwwetensch. Gent, 35(2):799-804.
Sijpesteijn A. Kaars and Vonk, J.W. Decomposition of
bis-dithiocarbamates and metabolism by plant and microorganisms. Env.
Quality and Safety. (In press).
Kuslander, J. (1966) Metabolic fate of dithiocarbamates. Thesis.
Utrecht. 95p.
Kaufman, D.D. (1973) Ethylenethiourea degradation in soil. Paper
presented at 2nd International Congress on Plant Pathology.
Minneapolis. September 1973.
Keppel, G.E. (1969) Modification of the carbon disulfide evolution
method for dithiocarbamate residues. J. Ass. off. Analyt. Chem.,
52(1):162-167.
Klisenko, M.A. and Vekstejn, M.S. (1971) O skorosti razlozenija i
produktach prevrascenija etilen-bis-dithiocarbamatov v razlicnych sel
skochozjaistvennych produktach. Vopr-Pitaniya, 30(5):79.
Lyman, W.R. (1971) The metabolic fate of Dithane M-75 (coordination
product of zinc iron and Manganous ethylenebisdithiocarbamate) in
Pestic. Terminal Residues (Suppl. Pure Appl. Chem.). p. 243-256.
Lyman, W.R. and Lacoste, R.J. New developments in the chemistry and
fate of ethylenebisdithiocarbamate fungicides. Paper presented at
Third International Congress of Pesticide Chemistry. Helsinki, July
1974. (In press).
Merek-Luenyo and Barragan-Allcaide. (1974) (Unpublished).
Nash, R.G. (1974) Improved gas-liquid chromatographic method for
determining ethylenethiourea in plants. J. Ass. off. Analyt. Chem.,
57(5):1015-1021.
Newsome, W.H. (1972) Determination of ethylene thiourea residues in
apples. J. Agr. Food Chem., 20(5):967-969.
Newsome, W.H. (1974) A method for determining ethylenebis
(dithiocarbamate) residues on food crops as bis (triflouro-acetamido)
ethane. J. Agr. Food Chem., 22(5):886-889.
Newsome, W.H. A method for the determination of ethylenethiuram
monosulfide on food crops. (In press).
Newsome, W.H., Brian, J.B. and Villeneuve, D.C. Residues of mancozeb,
ethylene thiuram monosulfide, ethylene thiourea, and ethylenediamine
on beans and tomatoes field-treated with mancozeb. (In press).
Newsome, W.H. and Laver, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb treated foods. Bull. Environ.
Contam. Toxicol., 10(3):151-154.
Onley, J.H. and Yip, G. (1971) Determination of ethylene thiourea
residues in food using thin layer and gas chromatography. J. Ass. off.
analyt. Chem., 54(1):165-169.
Pluijgers, C.W. and Vonk, J.W. Re-examination of the structure of
ethylene-thiuram monosulphide. Tetrahedron Letters, 18:1317.
Povlsen, H.H., Dahl, E. and Christensen, B.S. (1974) Report on
determination of ETU (ethylene thiourea) and water in dithiocarbamate
formulations. Paper provided to 1974 JMPR. (Unpublished).
Ross, R.D. and Crosby, D.G. (1973) Photolysis of ethylene thiourea. J.
Agr. Food. Chem., 21(3):335-337.
Sato, T., and Tomiziwa, C. (1960) Decomposition of zinc
ethylenebisdithiocarbamates (zineb) applied to plants. Bull. natn,
Inst. agric. Sci., Tokyo, Ser. C12, 181.
Thorn, G.D. and Ludwig, R.A. (1962) The dithiocarbamates and related
compounds. Elservier monographs. Amsterdam 298p.
Vekstejn, M.S. and Klisenko, M.A. (1970) Identifikacija i
kolicestvennoe opredelenie mekotorych dialkilditiokarbamatov i
produktov ich metabolisma v razlienych sredach rastitel nogo i
zivotnogo proischozdenija Vopr. Pitaniya, 29(1):56.
Vonk, J.W. (1971) Ethylene thiourea, a systemic decomposition product
of nabam. Med. Fak. Landbouw. Wetenschappen, 36(1):110-112.
Vonk, J.W. (1974) Transformation of dithiocarbamate fungicides and the
chemical nature of their residues in plants. Progress report to
FAO/IAEA Research Coord. Meeting on isotopic tracer aided studies of
foreign chemical residues in food. Vienna, June 1974.
Vonk, J.W. and Sijpestijn, A. Kaars. (1970) Studies on the fate in
plants of ethylenedithiocarbamate fungicides and their decomposition
products. Ann. appl. Biol., 65:489-496.
Vonk, J.W. and Sijpestijn, A. Kaars. (1971) Tentative identification
of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicide. Pest. Biochem. Physiol.,
1:163-165.
Watts, R.R., Storherr, R.W. and Onley, J.H. (1974) Effects of cooking
on ethylenebisdithiocarbamate degradation to ethylene thiourea. Bull.
Environ. Contam. Toxicol., 12(2):224-226.
Yip, G., Onley, J.H. and Howard, S.F. (1971) Residues of maneb and
ethylenethiourea on field-sprayed lettuce and kale. J. Ass. off.
analyt. Chem., 54(6):1373-1375
 Vonk and Sijpesteijn (1970) studied the fate of the main
metabolites of EBDC's in the green parts of cucumber plants after
application of 14C-ethylene labelled nabam to the leaves. They found
the following distribution of radioactivity in water extracts from
cucumber leaves after 0.7 and 19 days. The compounds were separated by
TLC.
TABLE 2 Fate of nabam in cucumbers
% of total detected radioactivity
after interval (days)
Compound 0 7 19
ethylene thiourea (ETU) 31 11 3.5
2-imidazoline 12 14 19
ethyleneurea (EU) 4 17 21
polar material remaining
at origin 22 39.5 40
other unidentified compounds 31 18 16.5
From this and other experiments the conclusion could be drawn
that from solutions of EBDC's in which ETU and EBIS (DIDT) are formed
only ETU is taken up in the plants. This compound is converted almost
quantitatively into a mixture of ethylene urea (EU) and 2-imidazoline.
In a study with radio-labelled mancozeb (3H, 14C or 35S) on
leafy plants such as sugar beets, lettuce or turnips, applied at
exaggerated rates to facilitate identification of metabolites, the
following intermediates could be detected 13 days after application by
using reverse isotope dilution techniques (Lyman 1971).
Compound Percentage of 3H
activity
mancozeb 9
ethylene thiourea (ETU) 6
ethyleneurea (EU) 17
ethylenediamine 11
2-imidazoline 8
N-formylethylenediamine 8
5,6-dihydro-3H-imidazo-[2,1-C-1,2,4-dithiazolo-3-thione]
(DIDT or EBIS) 7
3-(2-imidazolin-2-yl)-2-imidazolidin-ethione
(Jaffe's base) 4
Total 62
The quantitative figures are an example and do not necessarily
indicate a typical distribution of percentages of metabolites present
in a residue.
Uptake and translocation of ETU in plants
It was shown by Sato and Tomiziwa (1960) and Vonk and Sijpesteijn
(1970, 1971) that ETU can be taken up by the roots and translocated
through the plant. In another study by Lyman and Lacoste (1974), 14C
ETU was applied either to the leaves or to the soil around young
potato or tomato plants. The tomato plants had 1 - 3 fruits at the
time of application. Only small amounts of radioactivity moved from
the site of application to the fruits or to other parts of the plant
(Table 3).
TABLE 3 Translocation of radioactivity from 14C ETU
14C activity expressed as ETU
(mg/kg) after indicated interval
in potatoes in tomatoes
Application tuber foliage roots fruit foliage
site
90 days 90 days 60 days 28 days 28 days
Leaves 0.14 24 0.05 0.8 64
Soil n.d.* 0.84 n.d. 0.4 6
* n.d. no detectable radioactivity = (<0.0001 mg/kg ETU)
In water
Exposure of mancozeb to spray tank conditions did not produce
significant ETU build-up during normal residence times. Analysis of a
spray tank slurry of "mancozeb 80%", containing 0.1% a.i. showed an
ETU content of 2.08 mg/kg after 1 minute increasing to 3.62 mg/kg
after 4 hours.
Lyman and Lacoste (1974) studied the hydrolysis of 14C labelled
mancozeb in water at PH 5, 7 and 9. Sterile water was used to
distinguish chemical from biological degradation. Suspensions of 20
ppm mancozeb were agitated for 28 days at 25°C in amber bottles (to
avoid photodecomposition). Samples taken at selected intervals were
analysed by TLC. Two different chromatographic systems were used to
provide confirmation of the identity of the spots. The radioactive
zones were detected by autoradiography and were subsequently
quantitated by liquid scintillation counting. The nonradioactive
standards were located on the TLC plates by UV light or with a spray
reagent.
It was shown that mancozeb is not stable in water and that the
half-life in the pH 5 - 9 range is less than 1 day (Table 4).
TABLE 4 Hydrolysis of 14C labelled mancozeb
% of total 14C found as parent mancozeb
after indicated interval
hours days
pH 2 24 48 3 6 10 14 21 28
5 62.3 27.2 11.5 15.2 13.6 10.6 4.0 6.4 6.1
7 25.6 13.6 14.8 16.9 2.3 10.0 8.2 7.0 4.2
9 0
The nature and quantity of the degradation products are
pH-dependent. ETU and ethyleneurea are found within the range studied.
Cruickshank and Jarrow (1973) showed that aqueous solutions of
ETU exposed to UV light (above 285 mm) undergo a very slow photolysis:
the process is markedly accelerated by photosensitisers. When kept in
the dark ETU is stable to hydrolysis over the pH range 5 - 9 at 90°C.
These results were confirmed by Bontoyan and Looker (1973b).
Ross and Crosby (1973) observed that photosensitisers present in
agricultural drainage water will catalyze the photodegradation of ETU
(Table 5). The degradation does not occur in the absence of UV light.
Laboratory experiments carried out by Lyman and Lacoste (1974) showed
similar results to those of Ross and Crosby.
TABLE 5 Effect of UV irradiation on degradation of ETU in
agricultural drainage water
Duration (hours) and % of initial 14C found as ETU*
type of exposure in sterile water in non-sterile water
5 UV 75 92
24 UV none none
48 UV none none
48 dark 104 101
* confirmed by reverse isotope dilution
In soil
A recent study of EBDC compounds in soil by Hylin (1973)
illustrates the degradation pattern discussed previously. Kaufman
(1973) studied the fate of 14C labelled ETU in two types of soil
(Hagerstown silty clay loam and lakeland sandy loam) both sterile and
non-sterile. Essentially all of the ETU was converted to
2-imidazolidone within two days in the Hagerstown silty clay loam
treated with 2 and 20 mg/kg ETU and within 8 days in this soil treated
with 200 mg/kg ETU. A slower but steady conversion of ETU to
2-imidazolidone occurred in sterile (autoclaved) soil. In the
non-sterile Hagerstown silty clay loam further degradation occurred
rapidly. Within 7 days after treatment with 2, 20 and 200 mg/kg ETU
43.4, 8.9 and 0.9% respectively of the initial 14C was evolved at
14CO2 was evolved from the sterile, autoclaved soil.
In the lakeland sandy loam a similar but somewhat slower
degradation of ETU was observed. Besides 14CO2, two products were
identified by co-chromatography as 2,4-imidazolidinedione and
1-(2'-imidazolin-2'-yl)-2-imidazolinethione (Jaffe's base). A third
was tentatively identified as a subsequent degradation product of the
latter compound, and a fourth was unidentified.
Lyman and Lacoste (1974) studied the fate of ETU applied at
exaggerated rates on a sandy soil under field conditions. The high
dosage rates applied provided considerably more ETU than could be
expected from the application of mancozeb at recommended dosages. The
experiment illustrates that ETU when exposed to field conditions
degrades rapidly in or on the top one cm of a relatively inert
substrate.
TABLE 6. Decline of ETU in sand
Dosage rate ETU (mg/kg) at interval (days) after spraying
kg/ha 0 1 3 7
0.77 8.2 0.35 0.02 0.02
0.77 9.8 1.53 0.01 n.d.*
0.036 0.03 n.d. n.d. n.d.
* n.d.: No detectable residues (<0.01 mg/kg).
In a study in which 10 and 20 mg/kg 14C labelled mancozeb and
10 mg/kg 14C labelled ETU were applied to Hagerstown salt loam soil
it was demonstrated that both mancozeb and ETU are readily degraded by
soil micro-organisms to the point of releasing their ethylene carbons
as CO2. The 14CO2 released was trapped in sodium hydroxide and
measured by liquid scintillation to determine the loss of the best
compounds.
The experiments with each compound were done in both sterile and
non-sterile soils. No evolution of 14CO2 was observed from sterile
soil, but in non-sterile soil both mancozeb and ETU are rapidly
degraded to 14CO2. The half-life for ETU at the 10 mg/kg level is
about 22 days and that for 20 mg/kg mancozeb about 50 days. The
experiment with 10 mg/kg mancozeb was continued for 170 days; the
half-life was about 90 days.
Leaching experiments
Lyman and Lacoste (1974) studied the leaching of 14C mancozeb
and its degradation products in 5 different types of soil. The organic
matter in these soils ranged from 0.4 to 15% and the pH from 4.7 to
7.4.
An aqueous slurry of 14C labelled mancozeb was mixed with soil
which was then applied to the top of a 45 cm high column with a
diameter of 12.3 cm. The dosage applied was equivalent to a field
application of 8 kg mancozeb a.i./ha i.e. about 15.6 mg of mancozeb
80% to each column. Once a week for 9 weeks 2.5 cm of water was
applied to the top of each column. Radioactivity in the water emerging
from the column was determined by liquid scintillation counting. After
9 weeks the columns were cut into 2.5 cm sections and a sample from
each section was combusted to 14CO2, which was trapped and measured
by liquid scintillation counting. No radioactivity leached through
four of the 5 soils, and leaching beyond the top 2.5 cm was slight. In
cecil clay, however, 2 - 5% of the activity was leached from the
column and 37 - 47% was found below the top 2.5 cm. This soil was a
kaolinite clay containing 32% sand, 54% clay, 14% silt and 0.49%
organic matter. The pH was 4.7. Losses of radioactivity by
volatilization or by complete metabolism to CO2 were significant in
all soils except the cecil clay soil.
Effect of processing and cooking
Haines and Adler (1973) studied the effect of normal cooking (20
minute boiling) ETU residues in spinach. Various samples were selected
from field studies with mancozeb at different intervals (0-14 days)
after the application of 7 x 1.5 kg "mancozeb 80%"/ha. In cases where
ETU residues were found in the uncooked samples (0.03 - 0.11 mg/kg) no
residues were found after cooking (Table 7).
TABLE 7 Residues of ETU in spinach, before and after cooking
Interval after Residues, mg/kg
application (days) uncooked spinach cooked spinach
mancozeb ETU ETU
0 6.8-8.3 0.9-0.11 n.d.*
3 2.6-3.8 0.03-0.03 n.d.
7 0.6-1.2 n.d.-n.d. n.d.
14 0.6-0.8 n.d.-n.d. n.d.
* n.d.: <0.01 mg/kg
These results differ from those obtained by Watts et al. (1974)
after cooking spinach fortified with mancozeb. They found that ETU was
formed by cooking, the weight produced being about 20% of the weight
of mancozeb originally added. The effect on other EBDC compounds was
similar (Table 8). Newsome and Laver (1973) reported similar results
after cooking spinach, potatoes and carrots containing residues of
mancozeb and metiram.
TABLE 8 ETU produced by cooking vegetables fortified with 10 mg/kg
of EBDC compounds +
ETU (mg/kg)
EBDC Fortified Fortified Percentage of
Crop compound after before ETU formed by
cooking cooking cooking*
Spinach maneb 0.16 1.82 16.6
mancozeb
(Dithane M-45) 0.15 2.17 20.2
mancozeb
(Manzate 200) 0.11 2.42 23.1
metiram 0.07 2.72 26.5
Potato metiram 0.08 1.43 13.5
maneb 0.08 1.20 11.2
Carrot metiram 0.09 1.42 13.3
maneb 0.08 1.42 13.4
+ After Watts et al. (1974).
* Weight ETU formed as percentage of weight of mancozeb added.
METHODS OF RESIDUE ANALYSIS
Mancozeb and ethylenediamine-yielding metabolites
A method for the determination of ethylenediamine (EDA) which is
liberated from known components of residues [mancozeb, free
ethlylenediamine (EDA), ethyleneurea (EU), ETU, EBIS (DIDT)] was
presented at the 1970 JMPR by Rohm and Haas. A modified method was
developed by Newsome (1974). The EDA is isolated, after hydrolysis of
the residues with acid containing stannous chloride, by ion exchange
chromatography and quantitated by gas liquid chromatography of its
bis(triflouroacetate). Overall recoveries at levels of 0.16 - 1.3
mg/kg parent compound mancozeb were greater than 80% and generally
more than 95%. The limit of detection in terms of mancozeb is about
0.1 mg/kg.
ETU
Several methods are available for the determination of ETU
residues in food, soil and water. The gas-chromatographic methods are
suitable, or may be adapted, for regulatory purposes (Haines and Adler
1973, Newsome 1974, Onley and Yip 1971, Nash 1974).
Blazques (1973) developed a TLC method for the determination of
ETU in plants, soil and water with a limit of detection of 0.1 mg/kg.
Merek-Luenyo and Barragan-Allcaide (1974) developed a new TLC method,
in which ETU is detected by nitrosation and subsequent colour reaction
with N-(1-naphtyl)-ethylenediamine dihydrochloride. ETU, EBIS and
ethylene thiuram disulfide appear as purple spots.
Engst and Schnaak developed a polarographic procedure for the
determination of ETU in vegetable and animal products. After
extraction with methanol the interfering substances are separated by
column and paper chromatography. ETU is converted to the
nitroso-compound determined by its reduction wave using a cathode ray
polarograph. The limit of detection is about 0.05 mg/kg ETU.
NATIONAL TOLERANCES
a) Mancozeb
Tolerances are in effect in a number of countries around the
world. The tolerances are expressed and residues calculated as parent
compound, as zineb, or as the CS2 moiety.
b) ETU
No national tolerances have been established.
APPRAISAL
Mancozeb was considered by the 1967 and 1970 Joint Meetings. At
the 1970 Meeting further data on the biotransformation of the compound
in plants and animals was requested.
Several new studies have been carried out which clarify to a
large extent the metabolic pathway of mancozeb. It has been shown that
the breakdown of the compound in plants and animals is qualitatively
similar, and proceeds through a series of intermediates; the
structures of most of them have been clarified. Several other
ethylenebisdithiocarbamates (EBDCs) in common use show a similar
breakdown pattern on plants, leading to several identical
intermediates.
Information was obtained on the variation in the content of ETU
in technical mancozeb from several different manufacturers and in
various formulated products.
It has been shown that cooking of commodities of plant origin
containing mancozeb residues produce ETU to an extent corresponding to
about 25% of the weight of the original residue. Other EBDC compounds
gave similar results.
Additional data on the residues of mancozeb and ETU arising from
treatments according to good agricultural practice were obtained and
compared with those on zineb and maneb.
Several new sensitive methods are now available for the
determination of ethylenethiourea (ETU), the residue intermediate
which gives most concern. Some of these methods, which allow the
determination of ETU in plants and in products of animal origin at
levels of about 0.005 - 0.01 mg/kg, are suitable for regulatory
purposes. On the other hand, it is recognised that no specific methods
for determining mancozeb or for distinguishing its residues from those
of other EBDC compounds are available, and that development of such
methods in the near future is unlikely. It may therefore be of value,
in order to detect exaggerated uses of these compounds, to establish
maximum residue limits on the combined basis of either the
ethylenediamine or the CS2 moiety of the EBDC molecule, and the ETU
content. For regulatory purposes the determination of CS2 is more
convenient but suffers from the disadvantage of not distinguishing
EBDC residues from those of dimethyldithiocarbamates or thiram, and
toxicological considerations may make these distinctions important.
The ethylenediamine method of analysis is more time consuming
determination but more sensitive and not subject to interference by
the dimethyldithiocarbamates or thiram. The Meeting therefore
recommends (1) that residue limits should be based on the
determination of the ethylenediamine moiety; (2) that the specified
limit should be for ethylenediamine; (3) that limits for ETU should
also be observed. Specific limits for ethylenediamine and ETU are
recommended.
RECOMMENDATIONS
The following temporary tolerances for ethylenediamine and ETU
replace the single recommendation for "parent compound or sum of all
dithiocarbamates present" in potatoes, made in 1970. Neither limit
should be exceeded in any sample.
TEMPORARY TOLERANCES
Pre-harvest
intervals on which
ethylenediamine ETU recommendations
mg/kg mg/kg are based (days)
Potatoes 0.05 0.01* 14
Apples, pears 2 0.02 14
Banana pulp 0.05 0.01* 0
Celery 2 0.01* 14
Lettuce 2 0.01* 21
Tomatoes 1 0.05 7
Carrots 0.2 0.01* 14
Maize: sweetcorn
cob and kernel
(husks removed) 0.2 0.01* 7
Beans (with pod) 3 0.1 7 - 10
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED
1. Residue studies in which both the ethylenediamine moiety and
ethylenethiourea (ETU) are separately determined.
2. Further studies on the fate of residues during the preparation
and processing of foods with Particular reference to their conversion
to ETU.
REFERENCES
Benson, W.R., Rose, R.D., Chen, J.-Y.T., Barron, R.P. and Mastbrook,
D. (1972) Structure of ethylene thiurem monosulfide. J. Ass. Off.
Analyt. Chem., 55(1):44-46.
Blazques, C.H., (1973) Residue determination of ethylene thiourea
(2-imidazolidinethione) from tomato foliage, soil, and water. J. Agr.
Food. Chem., 21(3):330-332.
Bontoyan, W.R. and Looker, J.B. (1973a) Degradation of commercial
ethylene bisdithiocarbamate formulations to ethylenethiourea under
elevated temperature and humidity. J. Agr. Food Chem., 21(3):338-341.
Bontoyan, W.R., Looker, J.B. (1973b) Report to the 1973 AOAC Meeting.
Washington, D.C.
Cruickshank, P.A. and Jarrow, H.C. (1973) Ethylenethiourea
degradation. J. Agr. Food Chem., 21(3):333-335.
Czeglédi-Jankó, G. (1967) Determination of the degradation products of
ethylenebis-dithiocarbamates by thin layer chromatography and some
investigations of their decomposition in vitro. J. Chromat., 31:89-95.
Dekhuijzen, H.M., Vonk, J.W. and Sijpesteijn, A. Kaars. (1971)
Terminal residues of dithiocarbamate fungicides. Pesticide Terminal
Residues Butterworth, London (Suppl. Pure Appl. Chem.) p. 233-242.
Engst, R. and Schnaak, W. (1967) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, I. Mitteilung:
Identifizierung und fungitoxische Wirkung im Modellversuch erhaltener
Metabolite. Z. Lebensm. - Unters. - Forsch., 134:216-221.
Engst, R. and Schnaak, W. (1968/1969) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, II.
Mitteilung: Polarographische Bestimmung des unzersetzten
Wirkstoffanterls. Z. Lebensm. - Unters. - Forsch., 139:149-152.
Engst, R. and Schnaak, W. (1970) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, III.
Mitterlung: Zum Reaktionsverlauf des Abbaus. Z. Lebensm. - Unters. -
Forsch., 143:99-103.
Engst R. and Schnaak, W. (1970/1971) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, IV.
Mitterlung: Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid-Rückständen auf pflanzlichen
Erntegütern. Z. Lebensm. - Unters. - Forsch., 144:81-85.
Engst, R., Schnaak, W. and Lewerenz, H.-J. (1971) Untersuchungen zum
Metabolismus der fungiciden Äthylen-bis-dithiocarbamate Maneb, Zineb
und Nabam, V. Mitterlung: Zur Toxikologie der Abbauprodukte. Z.
Lebensm. - Unters. - Forsch., 146:91-97.
Engst, R., and Schnaak, W. (1969) Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid. Z. analyt. Chem., 248:321.
Engst, R., and Schnaak, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue reviews,
52:45-67.
Engst, R., Schnaak, W., and Rattba, H. (1968) Fungizide Wirkung und
Rückstandsbildung von Abbauprodukten des Maneb und Zineb an
freilandbehandelten Tomaten. NachrBl. dt. PflSchutzdienst, Berl.,
22:26-29.
FAO/WHO. (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL/1967/M/11/1; WHO/Food Add./68:30.
FAO/WHO. (1971) 1970 Evaluations of some pesticide residues in food.
AGP/1970/M/12/1; WHO/Food Add./71.42.
Haines, L.D., and Adler, I.L. (1974) Recommendation for using the ETU
method, of J. Ass. off. analyt. Chem., 56(2):333-337. Unpublished
report from Rohm and Haas.
Haines, L.D. and Adler, I.L. (1973) Gas chromatographic determination
of ethylene thiourea residues. J. Ass. off. analyt. Chem.,
56(2):333-337.
Hellwig, A., Liebmann, R. and Hempel, D. (1971) Zur
Rüchstandsbestimmung metallhaltiger Fungizide mit Hilfe einer
Enzymtestmethode. Archiv. Pflanzenschutz, 7:73-78.
Hylin, J.W. (1966) Thin layer chromatography of dithiocarbamate
fungicides. Bull. Environ. Contam. Toxicol., 1:76-77.
Hylin, J.W. (1973) Oxidative decomposition of ethylene-bis-
dithiocarbamates. Bull. Environ. Contam. Toxicol., 10(4):227-233.
Sijpestijn, A. Kaars and Kaslander, J. (1965) Metabolism of fungicides
by plants and microorganisms. Outlook on Agriculture, 4(4):119-125.
Sijpestijn, A. Kaars and Vonk, J.W. (1970) Microbial conversion of
Med. Fac. Landbouwwetensch. Gent, 35(2):799-804.
Sijpesteijn A. Kaars and Vonk, J.W. Decomposition of
bis-dithiocarbamates and metabolism by plant and microorganisms. Env.
Quality and Safety. (In press).
Kuslander, J. (1966) Metabolic fate of dithiocarbamates. Thesis.
Utrecht. 95p.
Kaufman, D.D. (1973) Ethylenethiourea degradation in soil. Paper
presented at 2nd International Congress on Plant Pathology.
Minneapolis. September 1973.
Keppel, G.E. (1969) Modification of the carbon disulfide evolution
method for dithiocarbamate residues. J. Ass. off. Analyt. Chem.,
52(1):162-167.
Klisenko, M.A. and Vekstejn, M.S. (1971) O skorosti razlozenija i
produktach prevrascenija etilen-bis-dithiocarbamatov v razlicnych sel
skochozjaistvennych produktach. Vopr-Pitaniya, 30(5):79.
Lyman, W.R. (1971) The metabolic fate of Dithane M-75 (coordination
product of zinc iron and Manganous ethylenebisdithiocarbamate) in
Pestic. Terminal Residues (Suppl. Pure Appl. Chem.). p. 243-256.
Lyman, W.R. and Lacoste, R.J. New developments in the chemistry and
fate of ethylenebisdithiocarbamate fungicides. Paper presented at
Third International Congress of Pesticide Chemistry. Helsinki, July
1974. (In press).
Merek-Luenyo and Barragan-Allcaide. (1974) (Unpublished).
Nash, R.G. (1974) Improved gas-liquid chromatographic method for
determining ethylenethiourea in plants. J. Ass. off. Analyt. Chem.,
57(5):1015-1021.
Newsome, W.H. (1972) Determination of ethylene thiourea residues in
apples. J. Agr. Food Chem., 20(5):967-969.
Newsome, W.H. (1974) A method for determining ethylenebis
(dithiocarbamate) residues on food crops as bis (triflouro-acetamido)
ethane. J. Agr. Food Chem., 22(5):886-889.
Newsome, W.H. A method for the determination of ethylenethiuram
monosulfide on food crops. (In press).
Newsome, W.H., Brian, J.B. and Villeneuve, D.C. Residues of mancozeb,
ethylene thiuram monosulfide, ethylene thiourea, and ethylenediamine
on beans and tomatoes field-treated with mancozeb. (In press).
Newsome, W.H. and Laver, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb treated foods. Bull. Environ.
Contam. Toxicol., 10(3):151-154.
Onley, J.H. and Yip, G. (1971) Determination of ethylene thiourea
residues in food using thin layer and gas chromatography. J. Ass. off.
analyt. Chem., 54(1):165-169.
Pluijgers, C.W. and Vonk, J.W. Re-examination of the structure of
ethylene-thiuram monosulphide. Tetrahedron Letters, 18:1317.
Povlsen, H.H., Dahl, E. and Christensen, B.S. (1974) Report on
determination of ETU (ethylene thiourea) and water in dithiocarbamate
formulations. Paper provided to 1974 JMPR. (Unpublished).
Ross, R.D. and Crosby, D.G. (1973) Photolysis of ethylene thiourea. J.
Agr. Food. Chem., 21(3):335-337.
Sato, T., and Tomiziwa, C. (1960) Decomposition of zinc
ethylenebisdithiocarbamates (zineb) applied to plants. Bull. natn,
Inst. agric. Sci., Tokyo, Ser. C12, 181.
Thorn, G.D. and Ludwig, R.A. (1962) The dithiocarbamates and related
compounds. Elservier monographs. Amsterdam 298p.
Vekstejn, M.S. and Klisenko, M.A. (1970) Identifikacija i
kolicestvennoe opredelenie mekotorych dialkilditiokarbamatov i
produktov ich metabolisma v razlienych sredach rastitel nogo i
zivotnogo proischozdenija Vopr. Pitaniya, 29(1):56.
Vonk, J.W. (1971) Ethylene thiourea, a systemic decomposition product
of nabam. Med. Fak. Landbouw. Wetenschappen, 36(1):110-112.
Vonk, J.W. (1974) Transformation of dithiocarbamate fungicides and the
chemical nature of their residues in plants. Progress report to
FAO/IAEA Research Coord. Meeting on isotopic tracer aided studies of
foreign chemical residues in food. Vienna, June 1974.
Vonk, J.W. and Sijpestijn, A. Kaars. (1970) Studies on the fate in
plants of ethylenedithiocarbamate fungicides and their decomposition
products. Ann. appl. Biol., 65:489-496.
Vonk, J.W. and Sijpestijn, A. Kaars. (1971) Tentative identification
of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicide. Pest. Biochem. Physiol.,
1:163-165.
Watts, R.R., Storherr, R.W. and Onley, J.H. (1974) Effects of cooking
on ethylenebisdithiocarbamate degradation to ethylene thiourea. Bull.
Environ. Contam. Toxicol., 12(2):224-226.
Yip, G., Onley, J.H. and Howard, S.F. (1971) Residues of maneb and
ethylenethiourea on field-sprayed lettuce and kale. J. Ass. off.
analyt. Chem., 54(6):1373-1375
Vonk and Sijpesteijn (1970) studied the fate of the main
metabolites of EBDC's in the green parts of cucumber plants after
application of 14C-ethylene labelled nabam to the leaves. They found
the following distribution of radioactivity in water extracts from
cucumber leaves after 0.7 and 19 days. The compounds were separated by
TLC.
TABLE 2 Fate of nabam in cucumbers
% of total detected radioactivity
after interval (days)
Compound 0 7 19
ethylene thiourea (ETU) 31 11 3.5
2-imidazoline 12 14 19
ethyleneurea (EU) 4 17 21
polar material remaining
at origin 22 39.5 40
other unidentified compounds 31 18 16.5
From this and other experiments the conclusion could be drawn
that from solutions of EBDC's in which ETU and EBIS (DIDT) are formed
only ETU is taken up in the plants. This compound is converted almost
quantitatively into a mixture of ethylene urea (EU) and 2-imidazoline.
In a study with radio-labelled mancozeb (3H, 14C or 35S) on
leafy plants such as sugar beets, lettuce or turnips, applied at
exaggerated rates to facilitate identification of metabolites, the
following intermediates could be detected 13 days after application by
using reverse isotope dilution techniques (Lyman 1971).
Compound Percentage of 3H
activity
mancozeb 9
ethylene thiourea (ETU) 6
ethyleneurea (EU) 17
ethylenediamine 11
2-imidazoline 8
N-formylethylenediamine 8
5,6-dihydro-3H-imidazo-[2,1-C-1,2,4-dithiazolo-3-thione]
(DIDT or EBIS) 7
3-(2-imidazolin-2-yl)-2-imidazolidin-ethione
(Jaffe's base) 4
Total 62
The quantitative figures are an example and do not necessarily
indicate a typical distribution of percentages of metabolites present
in a residue.
Uptake and translocation of ETU in plants
It was shown by Sato and Tomiziwa (1960) and Vonk and Sijpesteijn
(1970, 1971) that ETU can be taken up by the roots and translocated
through the plant. In another study by Lyman and Lacoste (1974), 14C
ETU was applied either to the leaves or to the soil around young
potato or tomato plants. The tomato plants had 1 - 3 fruits at the
time of application. Only small amounts of radioactivity moved from
the site of application to the fruits or to other parts of the plant
(Table 3).
TABLE 3 Translocation of radioactivity from 14C ETU
14C activity expressed as ETU
(mg/kg) after indicated interval
in potatoes in tomatoes
Application tuber foliage roots fruit foliage
site
90 days 90 days 60 days 28 days 28 days
Leaves 0.14 24 0.05 0.8 64
Soil n.d.* 0.84 n.d. 0.4 6
* n.d. no detectable radioactivity = (<0.0001 mg/kg ETU)
In water
Exposure of mancozeb to spray tank conditions did not produce
significant ETU build-up during normal residence times. Analysis of a
spray tank slurry of "mancozeb 80%", containing 0.1% a.i. showed an
ETU content of 2.08 mg/kg after 1 minute increasing to 3.62 mg/kg
after 4 hours.
Lyman and Lacoste (1974) studied the hydrolysis of 14C labelled
mancozeb in water at PH 5, 7 and 9. Sterile water was used to
distinguish chemical from biological degradation. Suspensions of 20
ppm mancozeb were agitated for 28 days at 25°C in amber bottles (to
avoid photodecomposition). Samples taken at selected intervals were
analysed by TLC. Two different chromatographic systems were used to
provide confirmation of the identity of the spots. The radioactive
zones were detected by autoradiography and were subsequently
quantitated by liquid scintillation counting. The nonradioactive
standards were located on the TLC plates by UV light or with a spray
reagent.
It was shown that mancozeb is not stable in water and that the
half-life in the pH 5 - 9 range is less than 1 day (Table 4).
TABLE 4 Hydrolysis of 14C labelled mancozeb
% of total 14C found as parent mancozeb
after indicated interval
hours days
pH 2 24 48 3 6 10 14 21 28
5 62.3 27.2 11.5 15.2 13.6 10.6 4.0 6.4 6.1
7 25.6 13.6 14.8 16.9 2.3 10.0 8.2 7.0 4.2
9 0
The nature and quantity of the degradation products are
pH-dependent. ETU and ethyleneurea are found within the range studied.
Cruickshank and Jarrow (1973) showed that aqueous solutions of
ETU exposed to UV light (above 285 mm) undergo a very slow photolysis:
the process is markedly accelerated by photosensitisers. When kept in
the dark ETU is stable to hydrolysis over the pH range 5 - 9 at 90°C.
These results were confirmed by Bontoyan and Looker (1973b).
Ross and Crosby (1973) observed that photosensitisers present in
agricultural drainage water will catalyze the photodegradation of ETU
(Table 5). The degradation does not occur in the absence of UV light.
Laboratory experiments carried out by Lyman and Lacoste (1974) showed
similar results to those of Ross and Crosby.
TABLE 5 Effect of UV irradiation on degradation of ETU in
agricultural drainage water
Duration (hours) and % of initial 14C found as ETU*
type of exposure in sterile water in non-sterile water
5 UV 75 92
24 UV none none
48 UV none none
48 dark 104 101
* confirmed by reverse isotope dilution
In soil
A recent study of EBDC compounds in soil by Hylin (1973)
illustrates the degradation pattern discussed previously. Kaufman
(1973) studied the fate of 14C labelled ETU in two types of soil
(Hagerstown silty clay loam and lakeland sandy loam) both sterile and
non-sterile. Essentially all of the ETU was converted to
2-imidazolidone within two days in the Hagerstown silty clay loam
treated with 2 and 20 mg/kg ETU and within 8 days in this soil treated
with 200 mg/kg ETU. A slower but steady conversion of ETU to
2-imidazolidone occurred in sterile (autoclaved) soil. In the
non-sterile Hagerstown silty clay loam further degradation occurred
rapidly. Within 7 days after treatment with 2, 20 and 200 mg/kg ETU
43.4, 8.9 and 0.9% respectively of the initial 14C was evolved at
14CO2 was evolved from the sterile, autoclaved soil.
In the lakeland sandy loam a similar but somewhat slower
degradation of ETU was observed. Besides 14CO2, two products were
identified by co-chromatography as 2,4-imidazolidinedione and
1-(2'-imidazolin-2'-yl)-2-imidazolinethione (Jaffe's base). A third
was tentatively identified as a subsequent degradation product of the
latter compound, and a fourth was unidentified.
Lyman and Lacoste (1974) studied the fate of ETU applied at
exaggerated rates on a sandy soil under field conditions. The high
dosage rates applied provided considerably more ETU than could be
expected from the application of mancozeb at recommended dosages. The
experiment illustrates that ETU when exposed to field conditions
degrades rapidly in or on the top one cm of a relatively inert
substrate.
TABLE 6. Decline of ETU in sand
Dosage rate ETU (mg/kg) at interval (days) after spraying
kg/ha 0 1 3 7
0.77 8.2 0.35 0.02 0.02
0.77 9.8 1.53 0.01 n.d.*
0.036 0.03 n.d. n.d. n.d.
* n.d.: No detectable residues (<0.01 mg/kg).
In a study in which 10 and 20 mg/kg 14C labelled mancozeb and
10 mg/kg 14C labelled ETU were applied to Hagerstown salt loam soil
it was demonstrated that both mancozeb and ETU are readily degraded by
soil micro-organisms to the point of releasing their ethylene carbons
as CO2. The 14CO2 released was trapped in sodium hydroxide and
measured by liquid scintillation to determine the loss of the best
compounds.
The experiments with each compound were done in both sterile and
non-sterile soils. No evolution of 14CO2 was observed from sterile
soil, but in non-sterile soil both mancozeb and ETU are rapidly
degraded to 14CO2. The half-life for ETU at the 10 mg/kg level is
about 22 days and that for 20 mg/kg mancozeb about 50 days. The
experiment with 10 mg/kg mancozeb was continued for 170 days; the
half-life was about 90 days.
Leaching experiments
Lyman and Lacoste (1974) studied the leaching of 14C mancozeb
and its degradation products in 5 different types of soil. The organic
matter in these soils ranged from 0.4 to 15% and the pH from 4.7 to
7.4.
An aqueous slurry of 14C labelled mancozeb was mixed with soil
which was then applied to the top of a 45 cm high column with a
diameter of 12.3 cm. The dosage applied was equivalent to a field
application of 8 kg mancozeb a.i./ha i.e. about 15.6 mg of mancozeb
80% to each column. Once a week for 9 weeks 2.5 cm of water was
applied to the top of each column. Radioactivity in the water emerging
from the column was determined by liquid scintillation counting. After
9 weeks the columns were cut into 2.5 cm sections and a sample from
each section was combusted to 14CO2, which was trapped and measured
by liquid scintillation counting. No radioactivity leached through
four of the 5 soils, and leaching beyond the top 2.5 cm was slight. In
cecil clay, however, 2 - 5% of the activity was leached from the
column and 37 - 47% was found below the top 2.5 cm. This soil was a
kaolinite clay containing 32% sand, 54% clay, 14% silt and 0.49%
organic matter. The pH was 4.7. Losses of radioactivity by
volatilization or by complete metabolism to CO2 were significant in
all soils except the cecil clay soil.
Effect of processing and cooking
Haines and Adler (1973) studied the effect of normal cooking (20
minute boiling) ETU residues in spinach. Various samples were selected
from field studies with mancozeb at different intervals (0-14 days)
after the application of 7 x 1.5 kg "mancozeb 80%"/ha. In cases where
ETU residues were found in the uncooked samples (0.03 - 0.11 mg/kg) no
residues were found after cooking (Table 7).
TABLE 7 Residues of ETU in spinach, before and after cooking
Interval after Residues, mg/kg
application (days) uncooked spinach cooked spinach
mancozeb ETU ETU
0 6.8-8.3 0.9-0.11 n.d.*
3 2.6-3.8 0.03-0.03 n.d.
7 0.6-1.2 n.d.-n.d. n.d.
14 0.6-0.8 n.d.-n.d. n.d.
* n.d.: <0.01 mg/kg
These results differ from those obtained by Watts et al. (1974)
after cooking spinach fortified with mancozeb. They found that ETU was
formed by cooking, the weight produced being about 20% of the weight
of mancozeb originally added. The effect on other EBDC compounds was
similar (Table 8). Newsome and Laver (1973) reported similar results
after cooking spinach, potatoes and carrots containing residues of
mancozeb and metiram.
TABLE 8 ETU produced by cooking vegetables fortified with 10 mg/kg
of EBDC compounds +
ETU (mg/kg)
EBDC Fortified Fortified Percentage of
Crop compound after before ETU formed by
cooking cooking cooking*
Spinach maneb 0.16 1.82 16.6
mancozeb
(Dithane M-45) 0.15 2.17 20.2
mancozeb
(Manzate 200) 0.11 2.42 23.1
metiram 0.07 2.72 26.5
Potato metiram 0.08 1.43 13.5
maneb 0.08 1.20 11.2
Carrot metiram 0.09 1.42 13.3
maneb 0.08 1.42 13.4
+ After Watts et al. (1974).
* Weight ETU formed as percentage of weight of mancozeb added.
METHODS OF RESIDUE ANALYSIS
Mancozeb and ethylenediamine-yielding metabolites
A method for the determination of ethylenediamine (EDA) which is
liberated from known components of residues [mancozeb, free
ethlylenediamine (EDA), ethyleneurea (EU), ETU, EBIS (DIDT)] was
presented at the 1970 JMPR by Rohm and Haas. A modified method was
developed by Newsome (1974). The EDA is isolated, after hydrolysis of
the residues with acid containing stannous chloride, by ion exchange
chromatography and quantitated by gas liquid chromatography of its
bis(triflouroacetate). Overall recoveries at levels of 0.16 - 1.3
mg/kg parent compound mancozeb were greater than 80% and generally
more than 95%. The limit of detection in terms of mancozeb is about
0.1 mg/kg.
ETU
Several methods are available for the determination of ETU
residues in food, soil and water. The gas-chromatographic methods are
suitable, or may be adapted, for regulatory purposes (Haines and Adler
1973, Newsome 1974, Onley and Yip 1971, Nash 1974).
Blazques (1973) developed a TLC method for the determination of
ETU in plants, soil and water with a limit of detection of 0.1 mg/kg.
Merek-Luenyo and Barragan-Allcaide (1974) developed a new TLC method,
in which ETU is detected by nitrosation and subsequent colour reaction
with N-(1-naphtyl)-ethylenediamine dihydrochloride. ETU, EBIS and
ethylene thiuram disulfide appear as purple spots.
Engst and Schnaak developed a polarographic procedure for the
determination of ETU in vegetable and animal products. After
extraction with methanol the interfering substances are separated by
column and paper chromatography. ETU is converted to the
nitroso-compound determined by its reduction wave using a cathode ray
polarograph. The limit of detection is about 0.05 mg/kg ETU.
NATIONAL TOLERANCES
a) Mancozeb
Tolerances are in effect in a number of countries around the
world. The tolerances are expressed and residues calculated as parent
compound, as zineb, or as the CS2 moiety.
b) ETU
No national tolerances have been established.
APPRAISAL
Mancozeb was considered by the 1967 and 1970 Joint Meetings. At
the 1970 Meeting further data on the biotransformation of the compound
in plants and animals was requested.
Several new studies have been carried out which clarify to a
large extent the metabolic pathway of mancozeb. It has been shown that
the breakdown of the compound in plants and animals is qualitatively
similar, and proceeds through a series of intermediates; the
structures of most of them have been clarified. Several other
ethylenebisdithiocarbamates (EBDCs) in common use show a similar
breakdown pattern on plants, leading to several identical
intermediates.
Information was obtained on the variation in the content of ETU
in technical mancozeb from several different manufacturers and in
various formulated products.
It has been shown that cooking of commodities of plant origin
containing mancozeb residues produce ETU to an extent corresponding to
about 25% of the weight of the original residue. Other EBDC compounds
gave similar results.
Additional data on the residues of mancozeb and ETU arising from
treatments according to good agricultural practice were obtained and
compared with those on zineb and maneb.
Several new sensitive methods are now available for the
determination of ethylenethiourea (ETU), the residue intermediate
which gives most concern. Some of these methods, which allow the
determination of ETU in plants and in products of animal origin at
levels of about 0.005 - 0.01 mg/kg, are suitable for regulatory
purposes. On the other hand, it is recognised that no specific methods
for determining mancozeb or for distinguishing its residues from those
of other EBDC compounds are available, and that development of such
methods in the near future is unlikely. It may therefore be of value,
in order to detect exaggerated uses of these compounds, to establish
maximum residue limits on the combined basis of either the
ethylenediamine or the CS2 moiety of the EBDC molecule, and the ETU
content. For regulatory purposes the determination of CS2 is more
convenient but suffers from the disadvantage of not distinguishing
EBDC residues from those of dimethyldithiocarbamates or thiram, and
toxicological considerations may make these distinctions important.
The ethylenediamine method of analysis is more time consuming
determination but more sensitive and not subject to interference by
the dimethyldithiocarbamates or thiram. The Meeting therefore
recommends (1) that residue limits should be based on the
determination of the ethylenediamine moiety; (2) that the specified
limit should be for ethylenediamine; (3) that limits for ETU should
also be observed. Specific limits for ethylenediamine and ETU are
recommended.
RECOMMENDATIONS
The following temporary tolerances for ethylenediamine and ETU
replace the single recommendation for "parent compound or sum of all
dithiocarbamates present" in potatoes, made in 1970. Neither limit
should be exceeded in any sample.
TEMPORARY TOLERANCES
Pre-harvest
intervals on which
ethylenediamine ETU recommendations
mg/kg mg/kg are based (days)
Potatoes 0.05 0.01* 14
Apples, pears 2 0.02 14
Banana pulp 0.05 0.01* 0
Celery 2 0.01* 14
Lettuce 2 0.01* 21
Tomatoes 1 0.05 7
Carrots 0.2 0.01* 14
Maize: sweetcorn
cob and kernel
(husks removed) 0.2 0.01* 7
Beans (with pod) 3 0.1 7 - 10
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED
1. Residue studies in which both the ethylenediamine moiety and
ethylenethiourea (ETU) are separately determined.
2. Further studies on the fate of residues during the preparation
and processing of foods with Particular reference to their conversion
to ETU.
REFERENCES
Benson, W.R., Rose, R.D., Chen, J.-Y.T., Barron, R.P. and Mastbrook,
D. (1972) Structure of ethylene thiurem monosulfide. J. Ass. Off.
Analyt. Chem., 55(1):44-46.
Blazques, C.H., (1973) Residue determination of ethylene thiourea
(2-imidazolidinethione) from tomato foliage, soil, and water. J. Agr.
Food. Chem., 21(3):330-332.
Bontoyan, W.R. and Looker, J.B. (1973a) Degradation of commercial
ethylene bisdithiocarbamate formulations to ethylenethiourea under
elevated temperature and humidity. J. Agr. Food Chem., 21(3):338-341.
Bontoyan, W.R., Looker, J.B. (1973b) Report to the 1973 AOAC Meeting.
Washington, D.C.
Cruickshank, P.A. and Jarrow, H.C. (1973) Ethylenethiourea
degradation. J. Agr. Food Chem., 21(3):333-335.
Czeglédi-Jankó, G. (1967) Determination of the degradation products of
ethylenebis-dithiocarbamates by thin layer chromatography and some
investigations of their decomposition in vitro. J. Chromat., 31:89-95.
Dekhuijzen, H.M., Vonk, J.W. and Sijpesteijn, A. Kaars. (1971)
Terminal residues of dithiocarbamate fungicides. Pesticide Terminal
Residues Butterworth, London (Suppl. Pure Appl. Chem.) p. 233-242.
Engst, R. and Schnaak, W. (1967) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, I. Mitteilung:
Identifizierung und fungitoxische Wirkung im Modellversuch erhaltener
Metabolite. Z. Lebensm. - Unters. - Forsch., 134:216-221.
Engst, R. and Schnaak, W. (1968/1969) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, II.
Mitteilung: Polarographische Bestimmung des unzersetzten
Wirkstoffanterls. Z. Lebensm. - Unters. - Forsch., 139:149-152.
Engst, R. and Schnaak, W. (1970) Untersuchungen zum Metabolismus der
fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, III.
Mitterlung: Zum Reaktionsverlauf des Abbaus. Z. Lebensm. - Unters. -
Forsch., 143:99-103.
Engst R. and Schnaak, W. (1970/1971) Untersuchungen zum Metabolismus
der fungiciden Äthylen-bis-dithiocarbamate Maneb und Zineb, IV.
Mitterlung: Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid-Rückständen auf pflanzlichen
Erntegütern. Z. Lebensm. - Unters. - Forsch., 144:81-85.
Engst, R., Schnaak, W. and Lewerenz, H.-J. (1971) Untersuchungen zum
Metabolismus der fungiciden Äthylen-bis-dithiocarbamate Maneb, Zineb
und Nabam, V. Mitterlung: Zur Toxikologie der Abbauprodukte. Z.
Lebensm. - Unters. - Forsch., 146:91-97.
Engst, R., and Schnaak, W. (1969) Polarographische Bestimmung von
Äthylen-bis-thiurammonosulfid. Z. analyt. Chem., 248:321.
Engst, R., and Schnaak, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue reviews,
52:45-67.
Engst, R., Schnaak, W., and Rattba, H. (1968) Fungizide Wirkung und
Rückstandsbildung von Abbauprodukten des Maneb und Zineb an
freilandbehandelten Tomaten. NachrBl. dt. PflSchutzdienst, Berl.,
22:26-29.
FAO/WHO. (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL/1967/M/11/1; WHO/Food Add./68:30.
FAO/WHO. (1971) 1970 Evaluations of some pesticide residues in food.
AGP/1970/M/12/1; WHO/Food Add./71.42.
Haines, L.D., and Adler, I.L. (1974) Recommendation for using the ETU
method, of J. Ass. off. analyt. Chem., 56(2):333-337. Unpublished
report from Rohm and Haas.
Haines, L.D. and Adler, I.L. (1973) Gas chromatographic determination
of ethylene thiourea residues. J. Ass. off. analyt. Chem.,
56(2):333-337.
Hellwig, A., Liebmann, R. and Hempel, D. (1971) Zur
Rüchstandsbestimmung metallhaltiger Fungizide mit Hilfe einer
Enzymtestmethode. Archiv. Pflanzenschutz, 7:73-78.
Hylin, J.W. (1966) Thin layer chromatography of dithiocarbamate
fungicides. Bull. Environ. Contam. Toxicol., 1:76-77.
Hylin, J.W. (1973) Oxidative decomposition of ethylene-bis-
dithiocarbamates. Bull. Environ. Contam. Toxicol., 10(4):227-233.
Sijpestijn, A. Kaars and Kaslander, J. (1965) Metabolism of fungicides
by plants and microorganisms. Outlook on Agriculture, 4(4):119-125.
Sijpestijn, A. Kaars and Vonk, J.W. (1970) Microbial conversion of
Med. Fac. Landbouwwetensch. Gent, 35(2):799-804.
Sijpesteijn A. Kaars and Vonk, J.W. Decomposition of
bis-dithiocarbamates and metabolism by plant and microorganisms. Env.
Quality and Safety. (In press).
Kuslander, J. (1966) Metabolic fate of dithiocarbamates. Thesis.
Utrecht. 95p.
Kaufman, D.D. (1973) Ethylenethiourea degradation in soil. Paper
presented at 2nd International Congress on Plant Pathology.
Minneapolis. September 1973.
Keppel, G.E. (1969) Modification of the carbon disulfide evolution
method for dithiocarbamate residues. J. Ass. off. Analyt. Chem.,
52(1):162-167.
Klisenko, M.A. and Vekstejn, M.S. (1971) O skorosti razlozenija i
produktach prevrascenija etilen-bis-dithiocarbamatov v razlicnych sel
skochozjaistvennych produktach. Vopr-Pitaniya, 30(5):79.
Lyman, W.R. (1971) The metabolic fate of Dithane M-75 (coordination
product of zinc iron and Manganous ethylenebisdithiocarbamate) in
Pestic. Terminal Residues (Suppl. Pure Appl. Chem.). p. 243-256.
Lyman, W.R. and Lacoste, R.J. New developments in the chemistry and
fate of ethylenebisdithiocarbamate fungicides. Paper presented at
Third International Congress of Pesticide Chemistry. Helsinki, July
1974. (In press).
Merek-Luenyo and Barragan-Allcaide. (1974) (Unpublished).
Nash, R.G. (1974) Improved gas-liquid chromatographic method for
determining ethylenethiourea in plants. J. Ass. off. Analyt. Chem.,
57(5):1015-1021.
Newsome, W.H. (1972) Determination of ethylene thiourea residues in
apples. J. Agr. Food Chem., 20(5):967-969.
Newsome, W.H. (1974) A method for determining ethylenebis
(dithiocarbamate) residues on food crops as bis (triflouro-acetamido)
ethane. J. Agr. Food Chem., 22(5):886-889.
Newsome, W.H. A method for the determination of ethylenethiuram
monosulfide on food crops. (In press).
Newsome, W.H., Brian, J.B. and Villeneuve, D.C. Residues of mancozeb,
ethylene thiuram monosulfide, ethylene thiourea, and ethylenediamine
on beans and tomatoes field-treated with mancozeb. (In press).
Newsome, W.H. and Laver, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb treated foods. Bull. Environ.
Contam. Toxicol., 10(3):151-154.
Onley, J.H. and Yip, G. (1971) Determination of ethylene thiourea
residues in food using thin layer and gas chromatography. J. Ass. off.
analyt. Chem., 54(1):165-169.
Pluijgers, C.W. and Vonk, J.W. Re-examination of the structure of
ethylene-thiuram monosulphide. Tetrahedron Letters, 18:1317.
Povlsen, H.H., Dahl, E. and Christensen, B.S. (1974) Report on
determination of ETU (ethylene thiourea) and water in dithiocarbamate
formulations. Paper provided to 1974 JMPR. (Unpublished).
Ross, R.D. and Crosby, D.G. (1973) Photolysis of ethylene thiourea. J.
Agr. Food. Chem., 21(3):335-337.
Sato, T., and Tomiziwa, C. (1960) Decomposition of zinc
ethylenebisdithiocarbamates (zineb) applied to plants. Bull. natn,
Inst. agric. Sci., Tokyo, Ser. C12, 181.
Thorn, G.D. and Ludwig, R.A. (1962) The dithiocarbamates and related
compounds. Elservier monographs. Amsterdam 298p.
Vekstejn, M.S. and Klisenko, M.A. (1970) Identifikacija i
kolicestvennoe opredelenie mekotorych dialkilditiokarbamatov i
produktov ich metabolisma v razlienych sredach rastitel nogo i
zivotnogo proischozdenija Vopr. Pitaniya, 29(1):56.
Vonk, J.W. (1971) Ethylene thiourea, a systemic decomposition product
of nabam. Med. Fak. Landbouw. Wetenschappen, 36(1):110-112.
Vonk, J.W. (1974) Transformation of dithiocarbamate fungicides and the
chemical nature of their residues in plants. Progress report to
FAO/IAEA Research Coord. Meeting on isotopic tracer aided studies of
foreign chemical residues in food. Vienna, June 1974.
Vonk, J.W. and Sijpestijn, A. Kaars. (1970) Studies on the fate in
plants of ethylenedithiocarbamate fungicides and their decomposition
products. Ann. appl. Biol., 65:489-496.
Vonk, J.W. and Sijpestijn, A. Kaars. (1971) Tentative identification
of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicide. Pest. Biochem. Physiol.,
1:163-165.
Watts, R.R., Storherr, R.W. and Onley, J.H. (1974) Effects of cooking
on ethylenebisdithiocarbamate degradation to ethylene thiourea. Bull.
Environ. Contam. Toxicol., 12(2):224-226.
Yip, G., Onley, J.H. and Howard, S.F. (1971) Residues of maneb and
ethylenethiourea on field-sprayed lettuce and kale. J. Ass. off.
analyt. Chem., 54(6):1373-1375
