
PIRIMIPHOS-METHYL JMPR 1974
IDENTITY
Chemical Name
O-(2-diethylamino-6-methylpyrimidin-4-yl) O,O-dimethyl
phosphorothioate.
Synonyms
PP 511, 'Actellic'(R).
Structural Formula
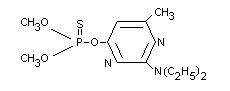 Other Information on Identity and Properties
Pirimiphos-methyl is a pale straw coloured liquid. It melts at
15-18°C and decomposes above 100°C. It has a vapour pressure of
approximately 1 × 10-4 Torr at 30°C and a density of 1.157 g/ml at
20°C.
Pirimiphos-methyl is stable for up to six months at room
temperature. It is hydrolysed by strong acid or alkali.
Pirimiphos-methyl is miscible with most organic solvents. Its
solubility in water is approximately 5 mg/l at 30°C.
Formulated products include emulsifiable concentrates (8%, 25%
and 50% a.i.), dusts (2% a.i.) and formulations for ultra-low volume
application (10% a.i. and 50% a.i.). A diluent-free formulation and a
smoke generator formulation are under development.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 0.6 mg/kg of 2-14C-ring-labelled pirimiphos-methyl was
given to adult male rats, either orally or by intraperitoneal
injection, 73 to 81% of the dose was excreted in the urine during the
first 24 hours. This indicates rapid absorption. At the end of 120
hours after oral dosing, the entire dose could be accounted for by
excretion of radioactive products in the urine (86% of dose) or faeces
(15.2% of dose). Similar results were obtained after intraperitoneal
injection although the excretion was more rapid after oral
administration.
Two male beagle dogs given 17-18 mg/kg orally also excreted most
of the dose in the urine during the first 24 to 48 hours.
Chromatographic and autoradiographic studies of the urine of dosed
dogs and rats revealed extensive metabolism by the presence of 9
radioactive chromatogram spots (Bratt and Dudley, 1970). The
concentration of label in the abdominal fat of female rats given four
daily doses of 5 mg/kg of 14C-pirimiphos-methyl revealed that the
metabolites were stored to some extent in fat: 0.46, 0.69, 1.87 and
1.00 ppm of pirimiphos-methyl equivalents in fat after 1, 2, 3 and 4
doses, respectively (Bratt and Jones, 1973).
In a lactating goat, 91% of the label of a single dose of 0.12
mg/kg of 2-14C ring-labelled pirimiphos-methyl was excreted (87% in
urine and 4% in faeces) within 8 days. The milk contained only 0.41%
of the label, primarily during the first 24 hours. The maximum residue
in milk was 0.026 ppm pirimiphos-methyl equivalents, and the parent
insecticide accounted for only 0.003 ppm (Bowker et al., 1973).
Bullock et al. (1974a) found that the pattern of excretion of
2-14C labelled pirimiphos-methyl in the cow was similar to that in
the goat. The label from a single dose of 0.5 mg/kg was quantitatively
recovered in the urine (85%) and faeces (14%) during the following 7
days. During the first 3 days, 0.35% of the label was secreted in the
milk. The milk contained 0.04 ppm of pirimiphos-methyl equivalents of
which less than 2% was unchanged pirimiphos-methyl and
phosphorus-containing metabolites. Hydroxypyrimidine hydrolysis
products or their conjugates constituted the major part of the residue
in milk.
Hens given a single oral dose of 14C-pirimiphos-methyl excreted
more than 70% of the label within 24 hours. When fed for 28 days in
the diet, at a level of 4 ppm, pirimiphos-methyl itself never exceeded
0.01 ppm in yolks and whites of eggs although the total concentration
of metabolites reached equilibrium levels of about 0.028 ppm, mostly
as water soluble metabolites. Increasing the dietary concentration to
32 ppm for a 7-day feeding period resulted in residues of 0.007 and
0.012 ppm of pirimiphos-methyl in the whites and yolks of eggs. In
muscle, 0.31 ppm and 0.16 ppm of the label was present in hens fed 32
and 4 ppm respectively; however, the parent insecticide or its oxygen
analogue was not detected (Green et al., 1973). See also the section
"Fate of residues in animals."
Biotransformation
The metabolism of pirimiphos-methyl in rats (100 mg/kg, p.o.) and
in one dog (20 mg/kg, p.o.) was studied by thin-layer chromatographic
separation of urinary metabolites. Twelve metabolites were detected.
None of these possessed anticholinesterase activity. No parent
pirimiphos-methyl was detected in the urine. Five of the twelve
metabolites were identified by comparison with authentic samples. In
both rats and does, 2-ethylamino-4-hydroxy-6-methylpyrimidine was the
major urinary metabolite (30% of dose). The next most predominant
metabolite in the dog was
4-O(2-diethylamino-6-methylpyrimidinyl)-ß-D-glucosiduronic acid (11%
of dose), and in the rat an unidentified phosphorus-containing
product thought to be a dealkylated derivative of either
pirimiphos-methyl or its oxygen analogue (12% of dose). Other
identified urinary metabolites included
2-amino-4-hydroxy-6-methylpyrimidine,
2-[N-ethyl-N-(2-hydroxyethyl) amino]-4-hydroxy-6-methylpyrimidine,
and 2-diethylamino-4-hydroxy-6-methylpyrimidine (Bratt and Jones,
1973).
These studies indicate that the P-O-C bond of pirimiphos-methyl
is extensively cleaved and that N-dealkylation and/or conjugation are
further steps in the metabolism of the pyrimidine leaving group.
Although the oxygen analogue of pirimiphos-methyl was not detected as
a urinary metabolite, the fact that cholinesterase inhibition occurs
in vivo suggests that the oxygen analogue is also formed and may be an
intermediate step leading to the identified urinary products.
Effect on enzymes and other biochemical parameters
The only biochemical effects consistently noted in acute or
chronic toxicity tests was inhibition of cholinesterase. A group of 36
male rats were given single oral doses of 1450 mg/kg of
pirimiphos-methyl. Symptoms were noted and they were sacrificed at
intervals up to 4 days after dosing for measurements of brain, plasma,
and red-cell cholinesterase activity. Few signs were noted at 6 hours
after dosing when cholinesterase inhibition was 0, 35 and 51%
respectively for brain, red cells and plasma. Clear signs of poisoning
only became apparent by 24 hours when brain was inhibited by 46% and
red cell and plasma by 70 and 80% respectively. Recovery of
cholinesterase activity began to be apparent by 72 hours. Plasma
cholinesterase activity had completely recovered by 96 hours, but red
cell and brain remained 47 and 30% inhibited, respectively, at this
time (Clark, 1970). From these studies it appears that inhibition of
brain cholinesterase by 40% or more results in obvious signs of
toxicity.
TOXICOLOGICAL STUDIES
Special studies on potentiation
Tests for potentiation of the acute oral toxicity of combinations
of pirimiphos-methyl and gamma-BHC were conducted by simultaneous
administration of one-half LD50 doses of the two compounds to ten
fasted female rats. Three of the ten animals died during a 14-day
observation period, suggesting no potentiation. Combinations of
one-half LD50 doses of pirimiphos-methyl and dichlorovos were tested
in the same manner and no mortality resulted in ten test rats. Under
these limited test conditions, there was no evidence of potentiation
of pirimiphos-methyl with 2 other insecticides that are likely to be
used in conjunction with it (Parkinson, 1972 a,b).
Special studies on reproduction
Rat
The effect of pirimiphos-methyl on the growth and reproductive
performance of the rat was assessed by feeding groups of 24 female and
12 male rats dietary concentrations of 0, 20 and 200 ppm of the
compound throughout three generations. However, dietary analysis of
the F0 generation nominally given 20 ppm revealed concentrations of
pirimiphos-methyl far below the intended levels and so animals treated
at 20 ppm were reared to produce a fourth (F4B) generation.
There were no effects among parent animals attributable to the
feeding of the compound. Mating performance and pregnancy rates were
reduced at 200 ppm for F1B and F2B generations. At 20 ppm there was an
effect at 2nd mating of the F1B generation and at both matings of the
F2B generation. When expressed as an average over all generations,
there was a dosage related decrease in pregnancy rate. Litter
parameters, as assessed by total litter loss, litter size, litter and
mean pup weights, pup mortality and the incidence of abnormalities
showed no consistent dosage-related trends. Examination of the
ultimate generation (F3B for 200 ppm dosage group, and F4B for 20 ppm)
by organ weight analysis and skeletal staining of 10 males and 10
females from all groups, and by microscopic examination of 10 male and
10 female pups from controls and from the 200 ppm group, showed no
difference that could be conclusively related to treatment (Palmer and
James, 1972).
The testes of the F1B and F2B male rats were examined
histopathologically in an attempt to find an explanation for their
reduced mating performance. However in the testes examined, from rats
of the control and both treatment groups, the degree of activity and
maturity of the process of spermatogenesis were comparable (Palmer and
Cherry, 1972).
Special studies on skin irritation, sensitization, and effects in
the eye
Application of a 50% solution of pirimiphos-methyl in olive oil
to ears of 6 guinea pigs did not produce erythema when challenge
applications of 10%, 5% and 1% were applied to shaved skin of their
flanks 4 days later. This indicated a lack of sensitizing potential.
Undiluted pirimiphos-methyl was applied to the clipped backs of 4
female rats and the site of application covered for 24 hours with an
impermeable dressing. No signs of primary irritation were noted after
a single such application nor after a second or third application at
alternate 24-hour intervals.
One drop of undiluted pirimiphos-methyl was placed in the left
conjunctival sac of the eyes of 4 albino rabbits. No signs of
irritation or myosis was noted on frequent observation over the next 7
days (Clark, 1970).
Special studies on teratogenicity
Rat
Three groups of 18 to 21 virgin female, Alderley Park specific
pathogen-free rats were mated and fed diets containing 0, 10 or 200
ppm of pirimiphos-methyl from day 1 to day 20 of pregnancy. On day 20,
the females were killed and foetuses immediately removed by Caesarean
section. The uterus was examined for resorptions, foetuses were
examined for viability and weighed. One third of the foetuses were
eviscerated and stained with alizarin red for examination of skeletal
defects. Viscera were fixed and examined for histopathology. Maternal
rats showed no toxic signs. It was presumed that 200 ppm produced
cholinesterase inhibition but this was not measured. The only
significant difference between pirimiphos-methyl and control litters
was a reduction in mean foetal weight; however, this may have been
because there were more foetuses per litter in the experimental
groups. No increased incidence of skeletal abnormalities were observed
in foetuses from the pirimiphos-methyl treated groups. The only
significant soft-tissue abnormality was increased hydronephrosis (7,
17 and 22 foetuses, representing incidence of 6, 17 and 16%) in the
foetuses examined from dams that received 0, 10 and 200 ppm in the
diet respectively. The investigators stated that these incidences of
hydronephrosis were within normal limits for Alderley Park rats and
they conclude that there was no evidence to suggest teratogenic action
for pirimiphos-methyl (Hodge and Moore, 1972).
Rabbit
Two groups of 16 to 17 pregnant rabbits were given gelatin
capsules containing pirimiphos-methyl dissolved in corn oil at doses
of 1 mg/kg and 16 mg/kg daily throughout pregnancy. A group of 16
controls was given capsules containing corn oil only. Twenty-nine days
after mating all the foetuses were delivered by Caesarean section. The
uterus was examined for resorptions and the foetuses for viability.
One half of the foetuses from each litter were stained with Alizarin
Red for skeletal examination and the remaining half were fixed in
Bouin's solution and dissected for soft-tissue examination. Does were
examined for gross and histological pathology and for blood
cholinesterase inhibition.
Three of the experimental does died during the gestational
period, two receiving 1 mg/kg/day and one receiving 16 mg/kg/day.
These deaths appeared to be due to causes unrelated to
pirimiphos-methyl. The does gained weight normally, but at the end of
4 weeks, the group average of RBC cholinesterase was 19% and 60% less
than controls in the 1 and 16 mg/kg dosage groups respectively. Data
on observations of foetuses were reported for 10, 10 and 11 litters
where implantations occurred in the 0, 1 and 16 mg/kg dosage groups
respectively. One grossly abnormal foetus was observed in a litter
from one doe receiving 16 mg/kg/day. No abnormalities were seen in the
remaining ten foetuses. There was no increase in resorptions in
experimental groups. Although the foetal weights of the 1 and 16 mg/kg
dosage groups were less than controls, there were more foetuses/litter
in these groups so that mean litter weights among the three groups did
not differ. The ratios of male/female foetuses were 1.0, 1.25 and 1.39
for the 0, 1 mg/kg and 16 mg/kg dosage groups. The authors state that
these are "within normal control limits." The only statistically
significant difference in the occurrence of abnormalities was a higher
incidence of 14 caudal vertebrae in the pirimiphos-methyl groups,
which the investigators state were isolated findings. This study
indicates no teratogenic action of pirimiphos-methyl in rabbits at
dosages which produce considerable inhibition of blood cholinesterase
in maternal animals (Gore et al., 1974a).
Acute toxicity
Pirimiphos-methyl, 90-94% purity, was administered to animals
undiluted or, for small doses, in propylene glycol. Single-dose LD50
values or estimates of LD50's by various routes are shown in Table
1. In the single-dose dermal test the skin of rats was washed with
soap and water 24 hours following application. All animals were
observed for 14 days or longer.
TABLE 1. Acute toxicity of pirimiphos-methyl
Animal Sex Route mg/kg bw References
Rat F Oral 2050 (1840-2260) Clark, 1970
Rat F ip approx. or equal to 800 Clark, 1970
Rat M ip approx. or equal to 800 Clark, 1970
Rat F dermal >2000 Clark, 1970
Mouse M Oral 1180 (1030-1360) Clark, 1970
Guinea Pig F Oral 1000-2000 Clark, 1970
Rabbit M Oral 1150-2300 Clark, 1970
Cat F Oral 575-1150 Clark, 1970
Dog M Oral >1500 Gage, 1972
Hen F Oral 30-60 Clark, 1970
Quail F Oral approx. or equal to 140 Gage, 1971a
Green Finch Oral 200-400 Gage, 1972
Figures in parentheses are 95% confidence limits.
The toxic signs exhibited after single doses were typical of
cholinesterase inhibition. In female rats given single oral doses,
only mild to moderate signs were noted at 12 hours after dosing, but
marked signs consisting of incontinence, salivation,
chromolacrymation, fibrillations, fasciculations and prostration were
present at 24 hours and persisted up to 11 days. No additional toxic
signs were noted during a 12 week observation period. Six surviving
hens of 8 dosed with 31 mg/kg were observed for 28 days after dosing
and no abnormal gait or other neurological signs were observed after
the initial acute toxic effects had subsided (within 7 days) (Clark,
1970).
2-Diethylamino-4-hydroxy-6-methylpyrimidine is a plant metabolite
of pirimiphos-methyl. It is also formed metabolically in rats and
dogs. Single-dose, range-finding acute toxicity tests (3 rats per
dose) indicated the acute oral LD50 in rats to be between 800 and
1600 mg/kg (Gage, 1971b). This is of the same order of acute toxicity
as the parent insecticide, but no description of toxic signs was
provided. Groups of 10 male and 10 female rats were given 100 and 400
mg/kg of the metabolite, orally, five days a week for two weeks. No
toxic signs, altered body weights or gross or histopathology were
noted. There was an approximate 25% increase in reticulocytes and a
reduction in total leucocytes which could be accounted for by a 30 to
40% reduction in lymphocytes, particularly in females, which the
investigators attribute to a "stress response" (Gage, 1971b).
Atropine sulphate and a mixture of atropine plus
pyridine-2-aldoxime methane-sulphonate partially counteracted the
acute oral toxicity of pirimiphos-ethyl in female rats when the
antidotes were given at intervals at which toxic signs appeared. The
combination of antidotes was more effective than atropine alone
(Clark, 1970).
Short-term studies
Rat (feeding)
Repeated daily oral dosing of 10 male and 10 female rats with 200
mg/kg of pirimiphos-methyl, 5 days a week for 2 weeks produced mild
signs of poisoning which were first noted after the 7th dose. The
signs did not progress in intensity with continued dosing; however,
body weight gain over this period was less than half that of controls.
An approximate 9% reduction of haemoglobin levels accompanied by
reticulocytosis was observed in the treated rats. Gross or
histopathology was not striking but slight to moderate haemopoesis of
the spleen was observed. When the experiment was repeated at a dose of
400 mg/kg, signs of poisoning appeared after 2 doses and increased in
severity as the experiment progressed. Males were more affected than
females and deaths occurred from the 4th day in males and from the 6th
in females. Cumulative mortality after 10 doses was 9 of 10 male rats
and 3 of 10 females (Clark, 1970).
Four groups of 25 male and 25 female Alderley Park specific
pathogen-free adult rats were maintained for 90 days on diets
containing 0, 8, 80 and 360 ppm of pirimiphos-methyl. Haematology and
plasma and erythrocyte cholinesterase activities were examined on
individual samples from five male and five female rats in each group
before, during and at the end of the feeding period. Brain
cholinesterase activities and organ:body weight ratios were calculated
for five males and five females in each group at the end of the
feeding period. Gross and histopathological examinations were
conducted. Body weight gain in the female rats fed diets containing 80
and 360 ppm pirimiphos-methyl averaged 18% and 21% less than controls.
This was apparently due to reduced food utilization as food
consumption did not differ significantly from controls. Plasma
cholinesterase was inhibited 65% in males and 80% in females fed 360
ppm and 40% in males and 60% in females fed 80 ppm, but no inhibition
was detected at 8 ppm. Erythrocyte cholinesterase was inhibited 20% in
males and 50% in females only at the highest dietary level. Both
plasma and erythrocyte cholinesterase returned to normal within one
week after cessation of dosage. Terminal measurements of brain
cholinesterase activity revealed inhibition in rats fed 80 and 360 ppm
with females affected most (about 40% inhibition in the 360 ppm
group). There was no inhibition in the 8 ppm group. Brain
cholinesterase was still inhibited at 4 weeks after cessation of
feeding pirimiphos-methyl. Haematological examinations revealed no
significant effects on haemoglobin concentration, packed cell volumes,
mean corpuscular diameter, white cell or platelet counts or clotting
function. There were no increases in gross or histopathological
lesions in the pirimiphos-methyl-fed rats over controls (Clapp and
Conning, 1970).
Rat (inhalation)
Four male and 4 female rats exposed by inhalation to a nominal
vapour-aerosol concentration of 3.5 ppm, 6 hours/day, 5 days/week for
3 weeks did not result in toxic signs nor reduction in body weight nor
decreased cholinesterase activities. An increase in alveolar
macrophages and slight lymphoid hyperplasia was noted (Clark, 1970).
Rabbit (dermal)
Daily application of 1000 mg/kg of pirimiphos-methyl to the
dorsal skin of 6 rabbits for 14 days (application site was washed 1
hour before each successive dose) resulted in fibrillations after 7 to
12 applications. Approximately 15% loss of body weight occurred and
one rabbit died after 14 applications (Clerk, 1970).
Avian species
Mallard ducklings and bobwhite quail chicks were fed
pirimiphos-methyl in their diets for five days and observed for an
additional 3 days. LC50 values were 633 and 207 ppm for ducklings and
quail chicks respectively (Fink, 1974a,b). Pirimiphos-methyl in the
diet of broiler chicks at levels from 4 to 48 ppm did not reduce feed
efficiencies or growth rate, except for a temporary period at the
beginning of the study (Graham and Jenkins, 1974). When 0, 4, 12 and
40 ppm were fed in the diet of laying hens for 28 days there was no
adverse effect on the hens. Egg production and egg quality were
considered normal although there was a small, dose-related increase in
the number of "small" eggs. There was some reduction in the number of
chicks hatched from eggs from the pirimiphos-methyl fed hens which was
not significant, although there were significantly greater numbers of
chicks dead in shell at the 12 and 40 ppm dietary levels (Ross et al.,
1974).
Dog
Groups of four male and four female beagle dogs were dosed orally
seven days a week for 13 weeks by administration of gelatin capsules
containing corn oil solutions of pirimiphos-methyl. The dosage rates
were 0, 2, 10 and 25 mg/kg given as single daily doses about 1 hour
after feeding the daily ration of complete dry diet. Clinical signs,
body weight, food and water consumption, electrocardiograms and
ophthalmic examinations were included among the observations.
Laboratory investigations included plasma and red cell cholinesterase
haematology, urinalysis, serum alkaline phosphate (SAP), serum
glutamic-pyruvic transaminase (SGPT) plasma urea and blood glucose. At
the end of the dosing period, two dogs of each sex in each dosage
group were sacrificed and the remaining dogs observed for an
additional four weeks before sacrifice. Gross and histopathological
examinations and brain cholinesterase assays were performed. There
were no deaths. Clinical symptoms were minimal with occasional
episodes of vomiting and watery stools. Body weight gain was
significantly reduced in both males and females given 25 mg/kg/day and
in females given 10 mg/kg/day. The rate of weight gain also tended to
be less for females given 2 mg/kg/day but this was of borderline
significance. At the highest dosage level (25 mg/kg) only, there was
reduced food consumption and a slower heart rate. Plasma and
erythrocyte cholinesterase activities were significantly depressed
(20% or more) at all three dosage levels. Plasma cholinesterase
rapidly returned to normal upon cessation of dosing but reversal of
red cell inhibition was delayed. At the lowest dosage level,
significant inhibition of red cell cholinesterase was only observed
during the latter weeks of the dosing period. Terminal brain
cholinesterase assays revealed no inhibition in any of the dosage
groups. Two dogs receiving 25 mg/kg showed very high SGPT and SAP
levels after three months dosing, while a third showed a less marked
SGPT increase. Post-mortem examination showed evidence of liver damage
in these animals. All other biochemical findings were within normal
limits. Histopathological examination post-mortem showed some bile
duct proliferation in one dog receiving 10 mg/kg and bile duct
proliferation together with portal cirrhosis in one dog receiving 25
mg/kg. After four weeks observation following the cessation of dosing,
two other dogs receiving 25 mg/kg showed minimal degrees of bile duct
proliferation. No other histopathological findings or other consistent
effects were noted (Noel et al., 1970).
Four groups each consisting of 4 male and 4 female beagle dogs
were orally dosed once daily, seven days/week, for two years with
gelatin capsules containing corn oil solutions of pirimiphos-methyl at
dosage rates of 0, 0.5, 2 or 10 mg/kg/day. Brain cholinesterase
activities at the end of the dosing period were 81, 78 and 44% of
control in the 0.5, 2 and 10 mg/kg groups respectively. The mean
activities in the 0.5 and 2 mg/kg groups were significantly less than
values for controls at the 1% level of probability, and in the 10
mg/kg group, brain cholinesterase depression was significant at the
0.1% level. Red cell cholinesterase was significantly inhibited with
respect to pre-dosing values, in both the 2 and 10 mg/kg groups from
the 8th week of dosing, but only occasionally inhibited in the 0.5
mg/kg group. Plasma cholinesterase was inhibited significantly
(30-50%) in all 3 groups from the 16th week of dosing. There was also
a decline in plasma cholinesterase activity in controls during the
experimental period. However, even with correction for this, 20-25%
depression was noted in the 0.5 mg/kg group, with only slightly
greater depressions with the higher dosages. It appears that plasma
cholinesterase inhibition did not discriminate well among the 3
experimental groups. One female dog in the 10 mg/kg group died after
the 400th day after showing few clinical signs. Clinical signs
consisting of loose stools were observed, in a dose-related frequency
in excess of controls, only in the 2 highest dosage groups. Vomiting
was infrequently noted. Dogs in the 10 mg/kg group showed some loss of
appetite and a reduced rate of weight gain. The 0.5 mg/kg/day dosage
rate appears to be a no effect level for clinical signs. No
abnormalities of the eyes, examined by ophthalmoscopy nor in
electrocardiogram records were observed. No appreciable or consistent
effects were noted in any of the dosage groups with respect to
haematology, standard urinalysis values or blood urea, blood glucose,
serum protein, SAP, SGPT, NA+ or K+ values. No consistent or
dose-related gross or histopathological changes were observed in post
mortem studies. However, a statistically significant increase in liver
weights and liver body weight ratios was seen in the group given 10
mg/kg/day (Rivett et al., 1973).
Long-term studies
Rat
Four groups of 48 male and 48 female rats were fed
pyrimiphos-methyl in their diet at levels of 0, 10, 50 and 300 ppm for
two years. Twenty-four additional animals of each sex were included in
each dosage group, and were killed at intervals up to one year to
provide interim data on brain cholinesterase activity and clotting
function. Eight animals of each sex in each of the dosage groups fed
for two years were continued on a control diet for 4 to 8 weeks beyond
the two year feeding period to allow for observation of recovery from
any treatment-induced effects.
Rats fed 300 ppm pirimiphos-methyl showed marked plasma
cholinesterase inhibition (50-80%) and some inhibition of erythrocyte
and brain cholinesterase activity (30-40%). The only other adverse
effect noted at this dosage rate was a slight anaemia in female rats.
Only female rat plasma cholinesterase was consistently inhibited
(50-65%) at the 50 ppm dietary level. Recovery from inhibited
cholinesterase, when it occurred, was usually complete by the end of
an 8-week period on the control diet. Slight (generally <25%)
transient depression of plasma cholinesterase activity in female rats
fed 10 ppm was observed. Erythrocyte and brain cholinesterases were
not depressed at this dietary level. Although cumulative mortality was
greater than for controls in male rats fed 300 ppm at 54, 60, 66 and
72 weeks of feeding, thereafter the cumulative mortalities of all
groups were approximately equal. Body weight gains, weights of major
organs and incidence of gross or microscopic pathology (including
tumors), in a comprehensive list of tissues, did not differ between
the control and experimental groups.
The results of this study indicate that 10 ppm in the diet of
rats is an experimental no-adverse-effect level (Gore et al., 1974b).
OBSERVATIONS IN MAN
A dose of 0.25 mg/kg/day of pirimiphos-methyl (97.8% purity) was
taken orally for 28 days by five healthy male volunteers (25-45 years
of age; 59.7-73 kg body-weight). Blood samples were taken before
dosing began, on days 1, 3, 7, 14, 21 and 28 during administration,
and 7 and 14 days after cessation of dosing. Only one subject had a
plasma cholinesterase depression (on day 28) which exceeded 20%
(21.5%). Otherwise variations, both above and below pre-dosing values
were within 12%. Four of the five subjects had red cell cholinesterase
values that were slightly below either of the pre-exposure values
during the last 2 weeks of the study. However, the group means for
each time interval did not differ significantly, and the variations
noted were within the range of variations found by others for normal
untreated subjects (Chart et al., 1974).
COMMENTS
Pirimiphos-methyl is rapidly absorbed from the gastrointestinal
tract, metabolised and quantitatively excreted in the urine and faeces
of several mammalian species.
The moderate acute toxicity of pirimiphos-methyl is due to its
inhibition of cholinesterase. After single toxic doses the onset of
inhibition of cholinesterase and appearance of toxic signs were
delayed for several hours and persisted for several days. Furthermore,
subacute and 2-year feeding studies in rats and dogs showed that
cholinesterase inhibition did not reach equilibrium for several weeks.
A two-year feeding study in rats provided a no-effect level based on
cholinesterase inhibition. Pirimiphos-methyl was not teratogenic in
rats and rabbits although hydronephrosis was noted. A decrease in
pregnancy rates was noted in a 3-generation reproduction study,
however cholinesterase activity was depressed at all dosages tested.
Other parameters of reproduction were not affected. No
compound-related histopathological effects were detected at dosage
rates considerably above those that inhibited cholinesterase, except
that in a 90-day study in dogs liver injury was observed in the
absence of cholinesterase inhibition.
In two-year studies in dogs, cholinesterase inhibition in brain
and plasma was noted at 0.5 mg/kg/day and above. No organ pathology
was observed in that study, however. Human volunteers who ingested
0.25 mg/kg/day of pirimiphos-methyl for 28 days had only a slight
reduction of plasma cholinesterase. Because of the slow decline in
cholinesterase activity in 2-year studies in the rat, and in the dog,
there is some question as to whether the experiments in volunteers
were of sufficient duration to detect maximum effect at the dosage
tested. Because of this, and because a no-effect level for brain
cholinesterase inhibition in the two-year dog studies was not clearly
established, the studies in rats provided the primary basis for
estimating an ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg bw.
Man: 0.25 mg/kg bw in 28 day period.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant action. It
shows activity against a wide spectrum of insect pests, including
ants, aphids, beetles, caterpillars, cockroaches, fleas, flies, mites,
mosquitoes, moths and thrips. It possesses only limited biological
persistence on leaf surfaces but gives long lasting control of insect
pests on inert surfaces such as wood, carpets, sacking and masonry. It
retains its biological activity when applied to stored agricultural
commodities including raw grain and nuts.
Commercial uses of pirimiphos-methyl are now developing in a wide
variety of outlets, including growing crops, public health and stored
products. The most important potential use appears to be as a grain
protectant and for use in the control of insect pests in stored
products. Such uses will be authorised as soon as appropriate maximum
residue limits have been recommended.
When used for the control of stored product pests,
pirimiphos-methyl is effective as a spray on structural surfaces and
on the outside of bagged produce and as an admixture treatment. The
recommended rates of application to bagged grain to control a complex
of beetles, weevils, moths and mites are normally in the range 250-500
mg/m2. For admixture with small grains the recommended rate of
application is 4 mg/kg except where Rhizopertha dominica is present
when a rate of 6 mg/kg is required.
Since the widespread development of strains of stored product
pests resistant to malathion (Pieterse et al., 1972; Waterhouse, 1973)
there has been considerable interest in pirimiphos-methyl which has
proved effective against all known strains of malathion-resistant
stored product insects.
Pirimiphos-methyl is regarded as more than a replacement for
malathion. At recommended rates it is effective against a wider
spectrum of insect pests, having an ability to destroy all forms other
than eggs and to confer long-term protection. It is effective at lower
rates of application and for much longer periods than is malathion.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest Treatments
The only information received by the Meeting on residues
resulting from pre-harvest application was provided by Hungarian
authorities and is summarised in Table 2.
TABLE 2. Residues resulting from pre-harvest applications of
pirimiphos-methyl1
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Tomato aphid cartridge 1/200 m3 1 day 0.15
2 days 0.06
5 days 0.01
Tomato 50% EC 2 l/ha 1 day 0.11
2 days 0.24
5 days 0.1
TABLE 2. (Cont'd.)
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Cucumber aphid 10% EC 7.5 l/ha 16 hours 0.11
24 hours 0.06
48 hours 0.04
Green
pepper 10% EC 7.5 l/ha 16 hours 0.12
24 hours 0.06
48 hours 0.04
1 Glasshouse experiments in 1974. The residue results are the average
of 3 samples. The limit of the determination was 0.01 mg/kg.
Post-harvest treatments
Surface sprays
Bullock (1973) has summarised the results of studies carried out
during 1970-1972 to determine the persistence of pirimiphos-methyl on
stored products. Trials were carried out in Colombia, Malaysia and the
United Kingdom involving the following bagged small grains: barley,
oats, rice in husk, milled rice, polished rice. In the period of 1-5
months after spraying the outside of the bags at rates up to 1000
mg/m2 the residues of pirimiphos-methyl in the whole grain taken from
the bag were found normally to be below 0.5 mg/kg and in no case to
have exceeded 1 mg/kg. The concentration was found to be highest in
grain sampled from within 2.5 cm of the inside of the bag. These
results are summarised in Table 3. No residues of the phosphorus
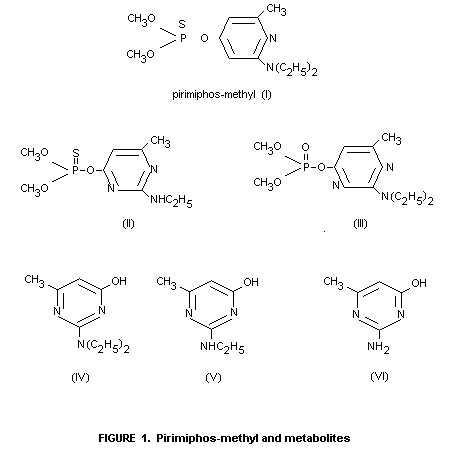
containing compounds (II) and (III) (Figure 1) were detected (Limit of
detection: 0.01 mg/kg in each case).
In most experiments the level of residues found in the grain in
the bags tended to rise during the first few weeks following
application and then to remain relatively constant over an interval of
three months. The level of residues in the grain was roughly
proportional to the rate of application to the surface of the bags.
Other Information on Identity and Properties
Pirimiphos-methyl is a pale straw coloured liquid. It melts at
15-18°C and decomposes above 100°C. It has a vapour pressure of
approximately 1 × 10-4 Torr at 30°C and a density of 1.157 g/ml at
20°C.
Pirimiphos-methyl is stable for up to six months at room
temperature. It is hydrolysed by strong acid or alkali.
Pirimiphos-methyl is miscible with most organic solvents. Its
solubility in water is approximately 5 mg/l at 30°C.
Formulated products include emulsifiable concentrates (8%, 25%
and 50% a.i.), dusts (2% a.i.) and formulations for ultra-low volume
application (10% a.i. and 50% a.i.). A diluent-free formulation and a
smoke generator formulation are under development.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 0.6 mg/kg of 2-14C-ring-labelled pirimiphos-methyl was
given to adult male rats, either orally or by intraperitoneal
injection, 73 to 81% of the dose was excreted in the urine during the
first 24 hours. This indicates rapid absorption. At the end of 120
hours after oral dosing, the entire dose could be accounted for by
excretion of radioactive products in the urine (86% of dose) or faeces
(15.2% of dose). Similar results were obtained after intraperitoneal
injection although the excretion was more rapid after oral
administration.
Two male beagle dogs given 17-18 mg/kg orally also excreted most
of the dose in the urine during the first 24 to 48 hours.
Chromatographic and autoradiographic studies of the urine of dosed
dogs and rats revealed extensive metabolism by the presence of 9
radioactive chromatogram spots (Bratt and Dudley, 1970). The
concentration of label in the abdominal fat of female rats given four
daily doses of 5 mg/kg of 14C-pirimiphos-methyl revealed that the
metabolites were stored to some extent in fat: 0.46, 0.69, 1.87 and
1.00 ppm of pirimiphos-methyl equivalents in fat after 1, 2, 3 and 4
doses, respectively (Bratt and Jones, 1973).
In a lactating goat, 91% of the label of a single dose of 0.12
mg/kg of 2-14C ring-labelled pirimiphos-methyl was excreted (87% in
urine and 4% in faeces) within 8 days. The milk contained only 0.41%
of the label, primarily during the first 24 hours. The maximum residue
in milk was 0.026 ppm pirimiphos-methyl equivalents, and the parent
insecticide accounted for only 0.003 ppm (Bowker et al., 1973).
Bullock et al. (1974a) found that the pattern of excretion of
2-14C labelled pirimiphos-methyl in the cow was similar to that in
the goat. The label from a single dose of 0.5 mg/kg was quantitatively
recovered in the urine (85%) and faeces (14%) during the following 7
days. During the first 3 days, 0.35% of the label was secreted in the
milk. The milk contained 0.04 ppm of pirimiphos-methyl equivalents of
which less than 2% was unchanged pirimiphos-methyl and
phosphorus-containing metabolites. Hydroxypyrimidine hydrolysis
products or their conjugates constituted the major part of the residue
in milk.
Hens given a single oral dose of 14C-pirimiphos-methyl excreted
more than 70% of the label within 24 hours. When fed for 28 days in
the diet, at a level of 4 ppm, pirimiphos-methyl itself never exceeded
0.01 ppm in yolks and whites of eggs although the total concentration
of metabolites reached equilibrium levels of about 0.028 ppm, mostly
as water soluble metabolites. Increasing the dietary concentration to
32 ppm for a 7-day feeding period resulted in residues of 0.007 and
0.012 ppm of pirimiphos-methyl in the whites and yolks of eggs. In
muscle, 0.31 ppm and 0.16 ppm of the label was present in hens fed 32
and 4 ppm respectively; however, the parent insecticide or its oxygen
analogue was not detected (Green et al., 1973). See also the section
"Fate of residues in animals."
Biotransformation
The metabolism of pirimiphos-methyl in rats (100 mg/kg, p.o.) and
in one dog (20 mg/kg, p.o.) was studied by thin-layer chromatographic
separation of urinary metabolites. Twelve metabolites were detected.
None of these possessed anticholinesterase activity. No parent
pirimiphos-methyl was detected in the urine. Five of the twelve
metabolites were identified by comparison with authentic samples. In
both rats and does, 2-ethylamino-4-hydroxy-6-methylpyrimidine was the
major urinary metabolite (30% of dose). The next most predominant
metabolite in the dog was
4-O(2-diethylamino-6-methylpyrimidinyl)-ß-D-glucosiduronic acid (11%
of dose), and in the rat an unidentified phosphorus-containing
product thought to be a dealkylated derivative of either
pirimiphos-methyl or its oxygen analogue (12% of dose). Other
identified urinary metabolites included
2-amino-4-hydroxy-6-methylpyrimidine,
2-[N-ethyl-N-(2-hydroxyethyl) amino]-4-hydroxy-6-methylpyrimidine,
and 2-diethylamino-4-hydroxy-6-methylpyrimidine (Bratt and Jones,
1973).
These studies indicate that the P-O-C bond of pirimiphos-methyl
is extensively cleaved and that N-dealkylation and/or conjugation are
further steps in the metabolism of the pyrimidine leaving group.
Although the oxygen analogue of pirimiphos-methyl was not detected as
a urinary metabolite, the fact that cholinesterase inhibition occurs
in vivo suggests that the oxygen analogue is also formed and may be an
intermediate step leading to the identified urinary products.
Effect on enzymes and other biochemical parameters
The only biochemical effects consistently noted in acute or
chronic toxicity tests was inhibition of cholinesterase. A group of 36
male rats were given single oral doses of 1450 mg/kg of
pirimiphos-methyl. Symptoms were noted and they were sacrificed at
intervals up to 4 days after dosing for measurements of brain, plasma,
and red-cell cholinesterase activity. Few signs were noted at 6 hours
after dosing when cholinesterase inhibition was 0, 35 and 51%
respectively for brain, red cells and plasma. Clear signs of poisoning
only became apparent by 24 hours when brain was inhibited by 46% and
red cell and plasma by 70 and 80% respectively. Recovery of
cholinesterase activity began to be apparent by 72 hours. Plasma
cholinesterase activity had completely recovered by 96 hours, but red
cell and brain remained 47 and 30% inhibited, respectively, at this
time (Clark, 1970). From these studies it appears that inhibition of
brain cholinesterase by 40% or more results in obvious signs of
toxicity.
TOXICOLOGICAL STUDIES
Special studies on potentiation
Tests for potentiation of the acute oral toxicity of combinations
of pirimiphos-methyl and gamma-BHC were conducted by simultaneous
administration of one-half LD50 doses of the two compounds to ten
fasted female rats. Three of the ten animals died during a 14-day
observation period, suggesting no potentiation. Combinations of
one-half LD50 doses of pirimiphos-methyl and dichlorovos were tested
in the same manner and no mortality resulted in ten test rats. Under
these limited test conditions, there was no evidence of potentiation
of pirimiphos-methyl with 2 other insecticides that are likely to be
used in conjunction with it (Parkinson, 1972 a,b).
Special studies on reproduction
Rat
The effect of pirimiphos-methyl on the growth and reproductive
performance of the rat was assessed by feeding groups of 24 female and
12 male rats dietary concentrations of 0, 20 and 200 ppm of the
compound throughout three generations. However, dietary analysis of
the F0 generation nominally given 20 ppm revealed concentrations of
pirimiphos-methyl far below the intended levels and so animals treated
at 20 ppm were reared to produce a fourth (F4B) generation.
There were no effects among parent animals attributable to the
feeding of the compound. Mating performance and pregnancy rates were
reduced at 200 ppm for F1B and F2B generations. At 20 ppm there was an
effect at 2nd mating of the F1B generation and at both matings of the
F2B generation. When expressed as an average over all generations,
there was a dosage related decrease in pregnancy rate. Litter
parameters, as assessed by total litter loss, litter size, litter and
mean pup weights, pup mortality and the incidence of abnormalities
showed no consistent dosage-related trends. Examination of the
ultimate generation (F3B for 200 ppm dosage group, and F4B for 20 ppm)
by organ weight analysis and skeletal staining of 10 males and 10
females from all groups, and by microscopic examination of 10 male and
10 female pups from controls and from the 200 ppm group, showed no
difference that could be conclusively related to treatment (Palmer and
James, 1972).
The testes of the F1B and F2B male rats were examined
histopathologically in an attempt to find an explanation for their
reduced mating performance. However in the testes examined, from rats
of the control and both treatment groups, the degree of activity and
maturity of the process of spermatogenesis were comparable (Palmer and
Cherry, 1972).
Special studies on skin irritation, sensitization, and effects in
the eye
Application of a 50% solution of pirimiphos-methyl in olive oil
to ears of 6 guinea pigs did not produce erythema when challenge
applications of 10%, 5% and 1% were applied to shaved skin of their
flanks 4 days later. This indicated a lack of sensitizing potential.
Undiluted pirimiphos-methyl was applied to the clipped backs of 4
female rats and the site of application covered for 24 hours with an
impermeable dressing. No signs of primary irritation were noted after
a single such application nor after a second or third application at
alternate 24-hour intervals.
One drop of undiluted pirimiphos-methyl was placed in the left
conjunctival sac of the eyes of 4 albino rabbits. No signs of
irritation or myosis was noted on frequent observation over the next 7
days (Clark, 1970).
Special studies on teratogenicity
Rat
Three groups of 18 to 21 virgin female, Alderley Park specific
pathogen-free rats were mated and fed diets containing 0, 10 or 200
ppm of pirimiphos-methyl from day 1 to day 20 of pregnancy. On day 20,
the females were killed and foetuses immediately removed by Caesarean
section. The uterus was examined for resorptions, foetuses were
examined for viability and weighed. One third of the foetuses were
eviscerated and stained with alizarin red for examination of skeletal
defects. Viscera were fixed and examined for histopathology. Maternal
rats showed no toxic signs. It was presumed that 200 ppm produced
cholinesterase inhibition but this was not measured. The only
significant difference between pirimiphos-methyl and control litters
was a reduction in mean foetal weight; however, this may have been
because there were more foetuses per litter in the experimental
groups. No increased incidence of skeletal abnormalities were observed
in foetuses from the pirimiphos-methyl treated groups. The only
significant soft-tissue abnormality was increased hydronephrosis (7,
17 and 22 foetuses, representing incidence of 6, 17 and 16%) in the
foetuses examined from dams that received 0, 10 and 200 ppm in the
diet respectively. The investigators stated that these incidences of
hydronephrosis were within normal limits for Alderley Park rats and
they conclude that there was no evidence to suggest teratogenic action
for pirimiphos-methyl (Hodge and Moore, 1972).
Rabbit
Two groups of 16 to 17 pregnant rabbits were given gelatin
capsules containing pirimiphos-methyl dissolved in corn oil at doses
of 1 mg/kg and 16 mg/kg daily throughout pregnancy. A group of 16
controls was given capsules containing corn oil only. Twenty-nine days
after mating all the foetuses were delivered by Caesarean section. The
uterus was examined for resorptions and the foetuses for viability.
One half of the foetuses from each litter were stained with Alizarin
Red for skeletal examination and the remaining half were fixed in
Bouin's solution and dissected for soft-tissue examination. Does were
examined for gross and histological pathology and for blood
cholinesterase inhibition.
Three of the experimental does died during the gestational
period, two receiving 1 mg/kg/day and one receiving 16 mg/kg/day.
These deaths appeared to be due to causes unrelated to
pirimiphos-methyl. The does gained weight normally, but at the end of
4 weeks, the group average of RBC cholinesterase was 19% and 60% less
than controls in the 1 and 16 mg/kg dosage groups respectively. Data
on observations of foetuses were reported for 10, 10 and 11 litters
where implantations occurred in the 0, 1 and 16 mg/kg dosage groups
respectively. One grossly abnormal foetus was observed in a litter
from one doe receiving 16 mg/kg/day. No abnormalities were seen in the
remaining ten foetuses. There was no increase in resorptions in
experimental groups. Although the foetal weights of the 1 and 16 mg/kg
dosage groups were less than controls, there were more foetuses/litter
in these groups so that mean litter weights among the three groups did
not differ. The ratios of male/female foetuses were 1.0, 1.25 and 1.39
for the 0, 1 mg/kg and 16 mg/kg dosage groups. The authors state that
these are "within normal control limits." The only statistically
significant difference in the occurrence of abnormalities was a higher
incidence of 14 caudal vertebrae in the pirimiphos-methyl groups,
which the investigators state were isolated findings. This study
indicates no teratogenic action of pirimiphos-methyl in rabbits at
dosages which produce considerable inhibition of blood cholinesterase
in maternal animals (Gore et al., 1974a).
Acute toxicity
Pirimiphos-methyl, 90-94% purity, was administered to animals
undiluted or, for small doses, in propylene glycol. Single-dose LD50
values or estimates of LD50's by various routes are shown in Table
1. In the single-dose dermal test the skin of rats was washed with
soap and water 24 hours following application. All animals were
observed for 14 days or longer.
TABLE 1. Acute toxicity of pirimiphos-methyl
Animal Sex Route mg/kg bw References
Rat F Oral 2050 (1840-2260) Clark, 1970
Rat F ip approx. or equal to 800 Clark, 1970
Rat M ip approx. or equal to 800 Clark, 1970
Rat F dermal >2000 Clark, 1970
Mouse M Oral 1180 (1030-1360) Clark, 1970
Guinea Pig F Oral 1000-2000 Clark, 1970
Rabbit M Oral 1150-2300 Clark, 1970
Cat F Oral 575-1150 Clark, 1970
Dog M Oral >1500 Gage, 1972
Hen F Oral 30-60 Clark, 1970
Quail F Oral approx. or equal to 140 Gage, 1971a
Green Finch Oral 200-400 Gage, 1972
Figures in parentheses are 95% confidence limits.
The toxic signs exhibited after single doses were typical of
cholinesterase inhibition. In female rats given single oral doses,
only mild to moderate signs were noted at 12 hours after dosing, but
marked signs consisting of incontinence, salivation,
chromolacrymation, fibrillations, fasciculations and prostration were
present at 24 hours and persisted up to 11 days. No additional toxic
signs were noted during a 12 week observation period. Six surviving
hens of 8 dosed with 31 mg/kg were observed for 28 days after dosing
and no abnormal gait or other neurological signs were observed after
the initial acute toxic effects had subsided (within 7 days) (Clark,
1970).
2-Diethylamino-4-hydroxy-6-methylpyrimidine is a plant metabolite
of pirimiphos-methyl. It is also formed metabolically in rats and
dogs. Single-dose, range-finding acute toxicity tests (3 rats per
dose) indicated the acute oral LD50 in rats to be between 800 and
1600 mg/kg (Gage, 1971b). This is of the same order of acute toxicity
as the parent insecticide, but no description of toxic signs was
provided. Groups of 10 male and 10 female rats were given 100 and 400
mg/kg of the metabolite, orally, five days a week for two weeks. No
toxic signs, altered body weights or gross or histopathology were
noted. There was an approximate 25% increase in reticulocytes and a
reduction in total leucocytes which could be accounted for by a 30 to
40% reduction in lymphocytes, particularly in females, which the
investigators attribute to a "stress response" (Gage, 1971b).
Atropine sulphate and a mixture of atropine plus
pyridine-2-aldoxime methane-sulphonate partially counteracted the
acute oral toxicity of pirimiphos-ethyl in female rats when the
antidotes were given at intervals at which toxic signs appeared. The
combination of antidotes was more effective than atropine alone
(Clark, 1970).
Short-term studies
Rat (feeding)
Repeated daily oral dosing of 10 male and 10 female rats with 200
mg/kg of pirimiphos-methyl, 5 days a week for 2 weeks produced mild
signs of poisoning which were first noted after the 7th dose. The
signs did not progress in intensity with continued dosing; however,
body weight gain over this period was less than half that of controls.
An approximate 9% reduction of haemoglobin levels accompanied by
reticulocytosis was observed in the treated rats. Gross or
histopathology was not striking but slight to moderate haemopoesis of
the spleen was observed. When the experiment was repeated at a dose of
400 mg/kg, signs of poisoning appeared after 2 doses and increased in
severity as the experiment progressed. Males were more affected than
females and deaths occurred from the 4th day in males and from the 6th
in females. Cumulative mortality after 10 doses was 9 of 10 male rats
and 3 of 10 females (Clark, 1970).
Four groups of 25 male and 25 female Alderley Park specific
pathogen-free adult rats were maintained for 90 days on diets
containing 0, 8, 80 and 360 ppm of pirimiphos-methyl. Haematology and
plasma and erythrocyte cholinesterase activities were examined on
individual samples from five male and five female rats in each group
before, during and at the end of the feeding period. Brain
cholinesterase activities and organ:body weight ratios were calculated
for five males and five females in each group at the end of the
feeding period. Gross and histopathological examinations were
conducted. Body weight gain in the female rats fed diets containing 80
and 360 ppm pirimiphos-methyl averaged 18% and 21% less than controls.
This was apparently due to reduced food utilization as food
consumption did not differ significantly from controls. Plasma
cholinesterase was inhibited 65% in males and 80% in females fed 360
ppm and 40% in males and 60% in females fed 80 ppm, but no inhibition
was detected at 8 ppm. Erythrocyte cholinesterase was inhibited 20% in
males and 50% in females only at the highest dietary level. Both
plasma and erythrocyte cholinesterase returned to normal within one
week after cessation of dosage. Terminal measurements of brain
cholinesterase activity revealed inhibition in rats fed 80 and 360 ppm
with females affected most (about 40% inhibition in the 360 ppm
group). There was no inhibition in the 8 ppm group. Brain
cholinesterase was still inhibited at 4 weeks after cessation of
feeding pirimiphos-methyl. Haematological examinations revealed no
significant effects on haemoglobin concentration, packed cell volumes,
mean corpuscular diameter, white cell or platelet counts or clotting
function. There were no increases in gross or histopathological
lesions in the pirimiphos-methyl-fed rats over controls (Clapp and
Conning, 1970).
Rat (inhalation)
Four male and 4 female rats exposed by inhalation to a nominal
vapour-aerosol concentration of 3.5 ppm, 6 hours/day, 5 days/week for
3 weeks did not result in toxic signs nor reduction in body weight nor
decreased cholinesterase activities. An increase in alveolar
macrophages and slight lymphoid hyperplasia was noted (Clark, 1970).
Rabbit (dermal)
Daily application of 1000 mg/kg of pirimiphos-methyl to the
dorsal skin of 6 rabbits for 14 days (application site was washed 1
hour before each successive dose) resulted in fibrillations after 7 to
12 applications. Approximately 15% loss of body weight occurred and
one rabbit died after 14 applications (Clerk, 1970).
Avian species
Mallard ducklings and bobwhite quail chicks were fed
pirimiphos-methyl in their diets for five days and observed for an
additional 3 days. LC50 values were 633 and 207 ppm for ducklings and
quail chicks respectively (Fink, 1974a,b). Pirimiphos-methyl in the
diet of broiler chicks at levels from 4 to 48 ppm did not reduce feed
efficiencies or growth rate, except for a temporary period at the
beginning of the study (Graham and Jenkins, 1974). When 0, 4, 12 and
40 ppm were fed in the diet of laying hens for 28 days there was no
adverse effect on the hens. Egg production and egg quality were
considered normal although there was a small, dose-related increase in
the number of "small" eggs. There was some reduction in the number of
chicks hatched from eggs from the pirimiphos-methyl fed hens which was
not significant, although there were significantly greater numbers of
chicks dead in shell at the 12 and 40 ppm dietary levels (Ross et al.,
1974).
Dog
Groups of four male and four female beagle dogs were dosed orally
seven days a week for 13 weeks by administration of gelatin capsules
containing corn oil solutions of pirimiphos-methyl. The dosage rates
were 0, 2, 10 and 25 mg/kg given as single daily doses about 1 hour
after feeding the daily ration of complete dry diet. Clinical signs,
body weight, food and water consumption, electrocardiograms and
ophthalmic examinations were included among the observations.
Laboratory investigations included plasma and red cell cholinesterase
haematology, urinalysis, serum alkaline phosphate (SAP), serum
glutamic-pyruvic transaminase (SGPT) plasma urea and blood glucose. At
the end of the dosing period, two dogs of each sex in each dosage
group were sacrificed and the remaining dogs observed for an
additional four weeks before sacrifice. Gross and histopathological
examinations and brain cholinesterase assays were performed. There
were no deaths. Clinical symptoms were minimal with occasional
episodes of vomiting and watery stools. Body weight gain was
significantly reduced in both males and females given 25 mg/kg/day and
in females given 10 mg/kg/day. The rate of weight gain also tended to
be less for females given 2 mg/kg/day but this was of borderline
significance. At the highest dosage level (25 mg/kg) only, there was
reduced food consumption and a slower heart rate. Plasma and
erythrocyte cholinesterase activities were significantly depressed
(20% or more) at all three dosage levels. Plasma cholinesterase
rapidly returned to normal upon cessation of dosing but reversal of
red cell inhibition was delayed. At the lowest dosage level,
significant inhibition of red cell cholinesterase was only observed
during the latter weeks of the dosing period. Terminal brain
cholinesterase assays revealed no inhibition in any of the dosage
groups. Two dogs receiving 25 mg/kg showed very high SGPT and SAP
levels after three months dosing, while a third showed a less marked
SGPT increase. Post-mortem examination showed evidence of liver damage
in these animals. All other biochemical findings were within normal
limits. Histopathological examination post-mortem showed some bile
duct proliferation in one dog receiving 10 mg/kg and bile duct
proliferation together with portal cirrhosis in one dog receiving 25
mg/kg. After four weeks observation following the cessation of dosing,
two other dogs receiving 25 mg/kg showed minimal degrees of bile duct
proliferation. No other histopathological findings or other consistent
effects were noted (Noel et al., 1970).
Four groups each consisting of 4 male and 4 female beagle dogs
were orally dosed once daily, seven days/week, for two years with
gelatin capsules containing corn oil solutions of pirimiphos-methyl at
dosage rates of 0, 0.5, 2 or 10 mg/kg/day. Brain cholinesterase
activities at the end of the dosing period were 81, 78 and 44% of
control in the 0.5, 2 and 10 mg/kg groups respectively. The mean
activities in the 0.5 and 2 mg/kg groups were significantly less than
values for controls at the 1% level of probability, and in the 10
mg/kg group, brain cholinesterase depression was significant at the
0.1% level. Red cell cholinesterase was significantly inhibited with
respect to pre-dosing values, in both the 2 and 10 mg/kg groups from
the 8th week of dosing, but only occasionally inhibited in the 0.5
mg/kg group. Plasma cholinesterase was inhibited significantly
(30-50%) in all 3 groups from the 16th week of dosing. There was also
a decline in plasma cholinesterase activity in controls during the
experimental period. However, even with correction for this, 20-25%
depression was noted in the 0.5 mg/kg group, with only slightly
greater depressions with the higher dosages. It appears that plasma
cholinesterase inhibition did not discriminate well among the 3
experimental groups. One female dog in the 10 mg/kg group died after
the 400th day after showing few clinical signs. Clinical signs
consisting of loose stools were observed, in a dose-related frequency
in excess of controls, only in the 2 highest dosage groups. Vomiting
was infrequently noted. Dogs in the 10 mg/kg group showed some loss of
appetite and a reduced rate of weight gain. The 0.5 mg/kg/day dosage
rate appears to be a no effect level for clinical signs. No
abnormalities of the eyes, examined by ophthalmoscopy nor in
electrocardiogram records were observed. No appreciable or consistent
effects were noted in any of the dosage groups with respect to
haematology, standard urinalysis values or blood urea, blood glucose,
serum protein, SAP, SGPT, NA+ or K+ values. No consistent or
dose-related gross or histopathological changes were observed in post
mortem studies. However, a statistically significant increase in liver
weights and liver body weight ratios was seen in the group given 10
mg/kg/day (Rivett et al., 1973).
Long-term studies
Rat
Four groups of 48 male and 48 female rats were fed
pyrimiphos-methyl in their diet at levels of 0, 10, 50 and 300 ppm for
two years. Twenty-four additional animals of each sex were included in
each dosage group, and were killed at intervals up to one year to
provide interim data on brain cholinesterase activity and clotting
function. Eight animals of each sex in each of the dosage groups fed
for two years were continued on a control diet for 4 to 8 weeks beyond
the two year feeding period to allow for observation of recovery from
any treatment-induced effects.
Rats fed 300 ppm pirimiphos-methyl showed marked plasma
cholinesterase inhibition (50-80%) and some inhibition of erythrocyte
and brain cholinesterase activity (30-40%). The only other adverse
effect noted at this dosage rate was a slight anaemia in female rats.
Only female rat plasma cholinesterase was consistently inhibited
(50-65%) at the 50 ppm dietary level. Recovery from inhibited
cholinesterase, when it occurred, was usually complete by the end of
an 8-week period on the control diet. Slight (generally <25%)
transient depression of plasma cholinesterase activity in female rats
fed 10 ppm was observed. Erythrocyte and brain cholinesterases were
not depressed at this dietary level. Although cumulative mortality was
greater than for controls in male rats fed 300 ppm at 54, 60, 66 and
72 weeks of feeding, thereafter the cumulative mortalities of all
groups were approximately equal. Body weight gains, weights of major
organs and incidence of gross or microscopic pathology (including
tumors), in a comprehensive list of tissues, did not differ between
the control and experimental groups.
The results of this study indicate that 10 ppm in the diet of
rats is an experimental no-adverse-effect level (Gore et al., 1974b).
OBSERVATIONS IN MAN
A dose of 0.25 mg/kg/day of pirimiphos-methyl (97.8% purity) was
taken orally for 28 days by five healthy male volunteers (25-45 years
of age; 59.7-73 kg body-weight). Blood samples were taken before
dosing began, on days 1, 3, 7, 14, 21 and 28 during administration,
and 7 and 14 days after cessation of dosing. Only one subject had a
plasma cholinesterase depression (on day 28) which exceeded 20%
(21.5%). Otherwise variations, both above and below pre-dosing values
were within 12%. Four of the five subjects had red cell cholinesterase
values that were slightly below either of the pre-exposure values
during the last 2 weeks of the study. However, the group means for
each time interval did not differ significantly, and the variations
noted were within the range of variations found by others for normal
untreated subjects (Chart et al., 1974).
COMMENTS
Pirimiphos-methyl is rapidly absorbed from the gastrointestinal
tract, metabolised and quantitatively excreted in the urine and faeces
of several mammalian species.
The moderate acute toxicity of pirimiphos-methyl is due to its
inhibition of cholinesterase. After single toxic doses the onset of
inhibition of cholinesterase and appearance of toxic signs were
delayed for several hours and persisted for several days. Furthermore,
subacute and 2-year feeding studies in rats and dogs showed that
cholinesterase inhibition did not reach equilibrium for several weeks.
A two-year feeding study in rats provided a no-effect level based on
cholinesterase inhibition. Pirimiphos-methyl was not teratogenic in
rats and rabbits although hydronephrosis was noted. A decrease in
pregnancy rates was noted in a 3-generation reproduction study,
however cholinesterase activity was depressed at all dosages tested.
Other parameters of reproduction were not affected. No
compound-related histopathological effects were detected at dosage
rates considerably above those that inhibited cholinesterase, except
that in a 90-day study in dogs liver injury was observed in the
absence of cholinesterase inhibition.
In two-year studies in dogs, cholinesterase inhibition in brain
and plasma was noted at 0.5 mg/kg/day and above. No organ pathology
was observed in that study, however. Human volunteers who ingested
0.25 mg/kg/day of pirimiphos-methyl for 28 days had only a slight
reduction of plasma cholinesterase. Because of the slow decline in
cholinesterase activity in 2-year studies in the rat, and in the dog,
there is some question as to whether the experiments in volunteers
were of sufficient duration to detect maximum effect at the dosage
tested. Because of this, and because a no-effect level for brain
cholinesterase inhibition in the two-year dog studies was not clearly
established, the studies in rats provided the primary basis for
estimating an ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg bw.
Man: 0.25 mg/kg bw in 28 day period.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant action. It
shows activity against a wide spectrum of insect pests, including
ants, aphids, beetles, caterpillars, cockroaches, fleas, flies, mites,
mosquitoes, moths and thrips. It possesses only limited biological
persistence on leaf surfaces but gives long lasting control of insect
pests on inert surfaces such as wood, carpets, sacking and masonry. It
retains its biological activity when applied to stored agricultural
commodities including raw grain and nuts.
Commercial uses of pirimiphos-methyl are now developing in a wide
variety of outlets, including growing crops, public health and stored
products. The most important potential use appears to be as a grain
protectant and for use in the control of insect pests in stored
products. Such uses will be authorised as soon as appropriate maximum
residue limits have been recommended.
When used for the control of stored product pests,
pirimiphos-methyl is effective as a spray on structural surfaces and
on the outside of bagged produce and as an admixture treatment. The
recommended rates of application to bagged grain to control a complex
of beetles, weevils, moths and mites are normally in the range 250-500
mg/m2. For admixture with small grains the recommended rate of
application is 4 mg/kg except where Rhizopertha dominica is present
when a rate of 6 mg/kg is required.
Since the widespread development of strains of stored product
pests resistant to malathion (Pieterse et al., 1972; Waterhouse, 1973)
there has been considerable interest in pirimiphos-methyl which has
proved effective against all known strains of malathion-resistant
stored product insects.
Pirimiphos-methyl is regarded as more than a replacement for
malathion. At recommended rates it is effective against a wider
spectrum of insect pests, having an ability to destroy all forms other
than eggs and to confer long-term protection. It is effective at lower
rates of application and for much longer periods than is malathion.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest Treatments
The only information received by the Meeting on residues
resulting from pre-harvest application was provided by Hungarian
authorities and is summarised in Table 2.
TABLE 2. Residues resulting from pre-harvest applications of
pirimiphos-methyl1
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Tomato aphid cartridge 1/200 m3 1 day 0.15
2 days 0.06
5 days 0.01
Tomato 50% EC 2 l/ha 1 day 0.11
2 days 0.24
5 days 0.1
TABLE 2. (Cont'd.)
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Cucumber aphid 10% EC 7.5 l/ha 16 hours 0.11
24 hours 0.06
48 hours 0.04
Green
pepper 10% EC 7.5 l/ha 16 hours 0.12
24 hours 0.06
48 hours 0.04
1 Glasshouse experiments in 1974. The residue results are the average
of 3 samples. The limit of the determination was 0.01 mg/kg.
Post-harvest treatments
Surface sprays
Bullock (1973) has summarised the results of studies carried out
during 1970-1972 to determine the persistence of pirimiphos-methyl on
stored products. Trials were carried out in Colombia, Malaysia and the
United Kingdom involving the following bagged small grains: barley,
oats, rice in husk, milled rice, polished rice. In the period of 1-5
months after spraying the outside of the bags at rates up to 1000
mg/m2 the residues of pirimiphos-methyl in the whole grain taken from
the bag were found normally to be below 0.5 mg/kg and in no case to
have exceeded 1 mg/kg. The concentration was found to be highest in
grain sampled from within 2.5 cm of the inside of the bag. These
results are summarised in Table 3. No residues of the phosphorus
containing compounds (II) and (III) (Figure 1) were detected (Limit of
detection: 0.01 mg/kg in each case).
In most experiments the level of residues found in the grain in
the bags tended to rise during the first few weeks following
application and then to remain relatively constant over an interval of
three months. The level of residues in the grain was roughly
proportional to the rate of application to the surface of the bags.
 Admixture with small grain cereals
Bullock (1973 and 1974) reports numerous trials undertaken in
Australia, Argentina, Guyana, Malaysia, U.K. and the U.S.A. where
pirimiphos-methyl was admixed with wheat, barley, maize, and rice in
husk (residues were determined on the rice after milling). The results
are summarised in Table 4. Mean initial residues of pirimiphos-methyl
itself in the whole grains of wheat, barley and maize rarely exceeded
the nominal treatment rate and were frequently in the range of 40-60%
of the nominal dose. This appears to be a reflection of the method of
application and suggests that some at least of the nominal dose is
lost into the atmosphere during application. Higher percentage
retention of the applied chemical was obtained by treating 5-10% of
the grain with all the chemical rather than applying it to 50% or more
of the grain stream entering the storage. Again no residues of the
phosphorus-containing compounds (II) and (III) were detected.
TABLE 3. Residues of pirimiphos-methyl in whole grains
after spraying bagged grains. (Bullock, 1973)
Interval
between Range of
Application application residues
Rate and sampling found,
Crop mg/m2 months mg/kg Country
Barley 400 0-3 0.05-0.33 UK
Oats 400 2-3 0.07-0.19 UK
Rice in husk 300 0-3 <0.01-0.08 Colombia
Milled rice 500 0-5 0.02-0.28 Malaysia
Treated when
in husk 1000 0-5 0.01-0.70 Malaysia
TABLE 4. Residues of pirimiphos-methyl in whole grains
after admixture. (Bullock, 1973, 1974)
Interval
Nominal between Range of
admixture admixture residues
rate, and sampling found,
Crop mg/kg (months) mg/kg Country
Wheat 3 0-8 1.0-2.1 Australia
4 0-3 1.3-4.6 UK
5 1-3 1.7-4.7 USA
6 0-12 1.3-3.9 Argentina
0-8 3.0-4.9 Australia
10 1-3 4.7-8.4 USA
Barley 4 0-3 0.03-3.6 UK
8 0-3 0.26-4.4 UK
Maize 5 1-3 2.1-6.9 USA
10 1-3 4.7-8.0 USA
Milled rice 3 0-3 0.21-0.53 Guyana
treated when
in husk 4 0-5 0.12-0.26 Malaysia
5 0-4 0.16-0.59 Guyana
7.5 0-11 0.53-0.98 Guyana
8 0-5 0.21-0.85 Malaysia
Wheat
The stability of the deposit of pirimiphos-methyl on wheat grains
is quite remarkable and from the work of Bullock the half life has
been estimated to be of the order of 9 months. Degradation proceeds
uniformly and slowly as indicated in the results from a typical trial
carried out in Argentina on wheat with a moisture content ranging from
11 to 13% (Table 5).
TABLE 5. Pirimiphos-methyl residues in wheat at intervals after admixture-Argentina.
(Bullock, 1973)
Nominal
admixture
rate
mg/kg 1 Day 1 Month 2 Months 3 Months 4 Months 6 Months 9 Months 12 Months
2 1.60 1.16 1.47 2.06 2.00 0.90 0.80
3 1.61 1.53 1.30 1.34 1.13 1.96 1.03 0.80
4 2.04 2.40 1.89 1.79 1.53 1.80 1.66 0.92
5 2.26 2.79 2.06 2.00 1.93 2.00 1.73 1.31
6 3.91 3.65 2.62 2.42 2.39 2.14 2.02 1.29
7 3.90 3.71 3.12 3.06 2.43 2.30 2.20 1.35
Moisture content: low (11-13%)
Bengston et al. (1974) report the results of two extensive trials
in Australia with five grain protectant insecticides applied to wheat
having a mean moisture content of 10-12% and a mean temperature of
29-32°C. In these trials the target application rate was 6 mg/kg but
based on the analyst's determination of the concentrate strength and
the weight of grain treated (2000 tons per treatment) the calculated
application rate was 5.5 mg/kg. Analysis by five collaborating
laboratories showed the concentration of pirimiphos-methyl on samples
taken 1 week after application to range from 5.5-6.4 mg/kg.
Representative data summarised in Table 6 show relatively little
change over a 26 week period notwithstanding the high temperature
which prevailed throughout.
Based on this work calculations have been made of the half-life
of Pirimiphos-methyl deposits on wheat stored in concrete bins at
30°C. When using data from samples taken from within 10 cm of the
surface and using the rate of disappearance during weeks 1-11, a
half-life of 43 weeks is obtained. Based on data from weeks 11-26 the
half-life was 104 weeks. Using data from samples taken 6 metres
beneath the surface where the mean temperature exceeded 32°C a
half-life of 77 weeks is obtained whether the data from samples
collected during weeks 1-11 or weeks 11-26 are used. The mean
half-life from many data is 82 weeks. This compares with a half-life
for malathion of 3-9 weeks under comparable conditions.
Barley
Bullock (1973 and 1974) reports numerous trials where barley was
treated with pirimiphos-methyl in the United Kingdom by admixture of
2% Pirimiphos-methyl dust or by the application of dilute emulsion
designed to deposit from 2-8 mg/kg on the weight of grain.
Unfortunately moisture and temperature conditions are not stated and
extensive sampling and analysis has failed to recover more than 25-30%
of the amount that was supposed to have been applied. Residue analyses
however fail to show much decline in the level of residues over the
first three months following spray application. The deposit seems to
disappear more quickly when the application is made in a manner
designed to fully cover all grains than when only a proportion of the
grain receives the spray. Subject to some reservations about the
residue data, the half-life on barley from these trials appears to be
at least 30 weeks.
TABLE 6a. Pirimiphos-methyl residues in stored wheat from site M
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories.
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
1 week 0.1 5.5 6.6 - - 6.1
0.6 6.4 - - -
1.5 4.9 5.8 - -
6.0 5.6 5.6 - - 5.6
Mean 5.3 6.0 - -
6 weeks 0.1 5.2 5.2 5.5 5.3 5.3
0.6 - - - - -
1.5 4.6 5.1 4.9 4.5 4.8
6.0 4.8 5.1 5.3 3.8 4.8
Mean 4.9 5.1 5.2 4.5 -
11 weeks 0.1 5.6 5.1 6.0 4.8 5.4
0.6 - - - - -
1.5 5.1 4.5 5.2 3.8 4.7
6.0 5.4 4.9 5.8 3.8 5.0
Mean 5.3 4.8 5.7 4.1 -
16 weeks 0.1 5.4 - - 4.9 5.1
0.6 - - - - -
1.5 5.1 - - 4.3 4.7
6.0 5.4 - - 4.3 4.8
Mean 5.3 - - 4.6 -
22 weeks 0.1 5.6 4.3 4.8 4.1 4.7
0.6 - - - - -
1.5 5.5 4.1 4.1 3.7 4.4
6.0 5.2 4.2 4.4 3.6 4.4
Mean 5.4 4.2 4.4 3.8 -
26 weeks 0.1 - 4.2 5.2 - 4.7
0.6 - - - - -
1.5 - 3.6 4.6 - 4.1
6.0 3.6 3.6 4.4 - 3.9
Mean -
TABLE 6b. Pirimiphos-methyl residues in stored wheat from site W
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
2 weeks 0.1 - - - - -
0.6 5.6 5.4 4.5 4.5 5.0
1.5 6.0 4.9 4.7 4.0 5.0
6.0 5.8 4.9 4.4 4.0 4.8
Mean 5.1 4.5 4.2 -
8 weeks 0.1 - - - - -
0.6 4.4 3.9 4.5 4.0 4.2
1.5 4.8 4.5 5.0 4.5 4.7
6.0 4.5 3.9 4.7 4.2 4.2
Mean 4.6 4.1 4.7 4.2 -
13 weeks 0.1 - - - - -
0.6 4.2 4.0 - 2.3 3.5
1.5 4.8 4.2 - 3.3 4.1
6.0 4.2 3.9 - 2.7 3.6
Mean -
TRIAL TERMINATED BECAUSE OF INFESTATION WITH RHIZOPERTHA DOMINICA
Rice
Bullock (1973) reports trials in Guyana, Indonesia and Malaysia
where rice in husk and polished rice were treated by admixture of
varying amounts of pirimiphos-methyl. In all trials reported the husk
was removed after varying intervals and the residues on husk and grain
determined separately. There appears to be some migration of the
insecticide from the husk to the grain during parboiling prior to
dehusking. The level of the residue in the husk declines over a period
of one month to about 30% of its concentration shortly after
application. Table 7 shows results from Guyana where rice was treated
in husk. When the rate of application was 20 mg/kg, 55% of the deposit
was lost from the husk within three days, the level declining to 35%
of the initial value at the end of one month.
TABLE 7. Pirimiphos-methyl admixed with rice paddy and
analysed at intervals - Guyana. (Bullock, 1973)
Interval
Rate of between Before After
application, treatment Parboiling* Parboiling*
mg/kg and sampling Husk Grain Husk Grain
3 3 days 14 0.53 8.4 1.0
1 month 4.4 0.21 1.1 0.66
2 months 2.4 0.34 2.7 1.0
3 months 2.0 0.46 2.0 0.64
7.5 3 days 34 0.90 5.1 1.8
1 month 8.5 0.75 2.0 -
2 months 5.2 0.71 6.0 2.3
3 months 5.1 0.53 3.4 1.3
8 months 12 0.98 4.5 ND
11 months 0.59 0.58 1.4 0.38
19.2 0 days 80 1.2 17 5.6
3 days 36 2.1 - -
1 month 28 1.4 8.2 3.2
2 months 13 1.8 15 4.9
3 months 18 1.3 3.7 2.6
* Parboiling undertaken for dehusking purposes.
Moisture content: approximately 14%.
Bullock (1973) reports extensive trials carried out in Malaysia
where pirimiphos-methyl was applied to polished rice and to rice in
husk at various rates and by various methods of application. Polished
rice appears to retain the initial deposit over a period ranging up to
7 months. It is not possible to estimate the half-life from these data
but it can be assumed to be much more than 7 months. Other trials in
Indonesia confirm this long residual life. Malathion under comparable
conditions disappeared almost completely within two months. By
comparison malathion appears not to migrate in significant amounts
from the husk into the grain of treated paddy.
Maize
Bullock (1973) reports trials in the U.S.A. which revealed that
pirimiphos-methyl deposits on maize remained relatively stable over a
period of 3 months. When the rate of application was 5-10 mg/kg the
half-life appeared to be of the order of 3 months but when 20 mg/kg
was applied no significant change occurred over the three month
period.
Peanuts
Bullock (1973) reports two large scale trials in the U.S.A. where
pirimiphos-methyl was applied at 20 and 50 mg/kg to undecorticated
peanuts. Analytical results indicate that the target rate of treatment
was achieved. Very little change occurred in the level of residues
during the first month following application. Even at the end of three
months substantially all of the deposit remained. The data suggest
that the half-life is of the order of 5 months from the time of
application. Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied.
FATE OF RESIDUES
In stored products
a) Distribution and degradation
Residues of pirimiphos-methyl on wheat grains are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain, to give
principally the parent hydroxy-pyrimidine (IV), Figure 1, and also the
related compounds (V) and (VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grains.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residues of the
chemically-unstable oxygen analogue (III) were detected. The limit of
detection.was 0.01 mg/kg (Bowker, 1973: See Table 8).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products.
The general pattern of breakdown on stored rice is similar to
that found on wheat grain. The insecticide and its degradation
products were concentrated in the husk in which the rate of breakdown
appeared to be unaffected by the moisture content of the rice (Bowker,
1973; Bullock, 1973). Representative results are given in Table 9.
TABLE 8. Effect of moisture on degradation of pirimiphos-methyl residues in wheat
grain (Bowker, 1973)
Application Pirimiphos-methyl equivalents,
rate and mg/kg, after interval (weeks)
moisture
content Compound 0 2 4 8 16 32
4 mg/kg, I (PP511) 2.16 2.66 2.05 2.66 2.32 2.08
14% moisture II + unidentified* 0.04 0.04 0.04 0.06 0.04 -
IV, V, VI <0.01 0.07 0.08 0.17 0.21 0.29
TLC baseline <0.01 <0.01 0.04 0.04 0.08 0.26
4 mg/kg,
18% moisture I 2.36 2.73 1.12 0.80 0.79 0.38
II + unidentified* 0.05 0.03 0.03 0.03 0.03 0.03
IV, V, VI - 0.49 0.56 1.05 0.92 0.66
TLC baseline - - 0.21 0.03 0.16 0.14
8 mg/kg, I 4.03 5.46 3.80 4.64 5.53 5.37
14% moisture II + unidentified* 0.12 0.08 0.08 0.11 0.04 0.10
IV, V, VI - 0.15 0.11 0.16 0.32 0.59
TLC baseline - - 0.02 0.03 0.04 0.22
8 mg/kg, I 4.44 5.64 2.93 2.84 2.19 1.28
18% moisture II + unidentified* 0.69 0.27 0.08 0.09 0.05 0.04
IV, V, VI - 0.98 0.99 1.61 1.89 1.61
TLC baseline - - 0.41 0.06 0.24 0.27
* Unidentified compound (minor product).
TABLE 9. Effect of moisture on degradation of pirimiphos-methyl residues in brown rice
(Bowker, 1973)
Application Pirimiphos-methyl equivalents, mg/kg,
rate and after interval (weeks)
moisture
content Compound 0 2 4 8 16
4 mg/kg, I (PP511) 2.13 2.31 2.06 2.19 -
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.07 -
IV, (V, VI) 0.05 0.11 0.06 0.10 -
TLC baseline 0.01 0.07 0.23 0.07 0.02
4 mg/kg, I 1.89 1.99 1.5 1.17 0.76
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.12 <0.01
IV, (V, VI) 0.06 0.17 0.34 0.24 0.33
TLC baseline 0.01 0.12 0.26 0.27 0.17
8 mg/kg, I 3.7 4.37 4.1 3.73 2.99
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.11 0.09
IV, (V, VI) 0.1 0.18 0.11 0.34 0.17
TLC baseline 0.03 0.13 0.36 0.23 0.06
8 mg/kg, I 3.11 3.68 1.55 2.3 1.58
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.21 0.11
IV, (V, VI) 0.06 0.32 0.10 0.86 0.45
TLC baseline 0.02 0.19 1.48 0.43 0.06
* Unidentified compound (minor product)
Pirimiphos-methyl applied as a dust formulation to wheat and
brown rice is degraded in a similar manner to other organophosphorus
insecticides used to protect stored grain from infestation (Rowlands,
1966).
Degradation is marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
is caused by factors within the grain or by the associated microflora
is not known. At higher moisture contents (approximately 18%) less
pirimiphos-methyl is recovered on analysis but increased levels of the
hydrolysis product (IV) are obtained suggesting that a more rapid
degradation of the insecticide occurs. Bowker concludes that grain may
lack the enzymic activity believed to be present in plants and soil to
cleave the pyrimidine N-ethyl bonds. It is likely therefore that
following the treatment of wheat and brown rice with
pirimiphos-methyl, the major residues during storage will be the
insecticide itself and the simple hydrolysis product (IV). Under
optimum conditions, the maximum level of compound (IV) following
treatment at 4 mg/kg, was found to be 0.17 mg/kg; under poor storage
conditions with high moisture content grain, 0.62 mg/kg.
Solutions of pirimiphos-methyl-14C were applied in the
laboratory to wheat grains of 14 and 18 percent moisture, and also as
a 2 percept dust, to give levels of 4 mg/kg. Throughout a storage
period of 6 months, the residues of pirimiphos-methyl were found
almost entirely in the seed coat and aleurone layer, with only traces
present in the germ or endosperm. It was also clear that, as with
malathion but to a greater extent, there was transfer of insecticide
between grains, possibly in the vapour phase. About 10 percent of the
total residual radioactivity was bound to lipoprotein material in the
aleurone region of the grain and was unextractable except by digestion
of the aleurone protein. By contrast with other organophosphates
studied, the bound material appeared to be the unchanged pesticide,
rather than a metabolite. Certainly it was a P=S compound, but
degradation during the liberation of the bound material complicated
identification. During the storage period, the pirimiphos-methyl
available for decomposition decreased by 15 and 50 percent at the 14
and 18 percent moisture levels respectively. The main products found
were the free hydroxy-pyrimidine and the N-desethyl pyrimidinol
(Rowlands et al., 1974).
b) Toxicity of degradation products
The hydroxypyrimidines (IV), (V) and (VI) are members of a class
of compounds known to exhibit low mammalian toxicity and they are also
products of animal metabolism. It is therefore concluded that
pirimiphos-methyl itself represents the major toxic residue in stored
products. Traces of the phosphorus-containing degradation products
(II) and (III), not exceeding 0.05 mg/kg in total, may also occur.
They are determined by the preferred analytical method. The acute oral
LD50 of (II) to the female rat is 800-1600 mg/kg. The oxygen analogue
(III) is chemically unstable and it has not been possible to undertake
toxicity tests with it (Gage, 1971b).
In plants
In contrast to its fate in stored products pirimiphos-methyl is
rapidly lost from leaf surfaces during the first 2-3 days after
spraying, mainly by volatilisation.
Bowker and Hughes (1974a) identified by a combination of
thin-layer and gas-liquid chromatography of the compounds or their
derivatives, all the major extractable metabolites and degradation
products arising from the interaction of pirimiphos-methyl with
plants.
They showed that on plant surfaces, pirimiphos-methyl is lost
rapidly by vaporisation. Levels of the parent insecticide and the
major degradation product, compound (II), represent less than 10% of
the applied material after two to three days. In crops after foliar
treatment the hydroxypyrimidine (IV), formed by hydrolysis of the
insecticide, is unlikely to be a significant residue. They found that
the compound is usually present only in trace amounts and is degraded
photochemically and bound to plant material.
These same workers showed that photochemical degradation of
pirimiphos-methyl on leaf surfaces leads to the formation of compound
(II) which is not accumulated. When applied to water in which rice
seedlings were growing, pirimiphos-methyl is not translocated
significantly into the foliage; in these conditions the hydrolysis
product (IV) is likely to represent the predominant pyrimidine
residue. Compounds (V) and (VI) only occur in trace amounts.
In animals
Much of the information in this section is also to be found under
the heading "Absorption, distribution and excretion," but is repeated
here for convenience.
Goat
Bowker et al. (1973) report studies where a single dose of
radio-labelled pirimiphos-methyl was given orally to a lactating goat
(0.12 mg/kg bw, equivalent to approximately 6 ppm in the ration). 91%
of the radio-activity was excreted in the following 8 days: 87% in the
urine and 4% in the faeces. Only 0.41% of the radio-activity was
secreted in the milk, primarily during the first 24 hours. The maximum
residue in the milk was 0.026 mg/kg pirimiphos-methyl equivalents, of
which pirimiphos-methyl itself represented 0.003 mg/kg.
Cow
The pattern of excretion in the cow is very similar to that in
the goat (Bullock et al., 1974a). When a single dose of radio-labelled
pirimiphos-methyl was given orally to a cow at 0.5 mg/kg bw
(equivalent to approximately 17 ppm in the daily diet), the
radio-activity was quantitatively recovered during the following seven
days - 85% in the urine and 14% in the faeces. Only 0.37% of the
radio-activity was secreted in the milk (0.35% during the first three
days).
The highest levels of radioactivity in the milk, urine and faeces
were found during the first day after dosing. The milk contained 0.04
mg/kg pirimiphos-methyl equivalents, of which approximately 75% could
be extracted from the fat and protein fraction into organic solvents.
Pirimiphos-methyl accounted for approximately 12% (i.e. about 0.004
ppm), and compound (V) for about 50% of the total radioactivity in the
milk. The radioactivity was not extracted by organic solvents and
appeared to be incorporated into natural milk components.
Approximately 70% of the radio-label in "day 1" urine was
organo-soluble, the remainder dissolving only in water.
Four groups of three cows were maintained by Bullock et al.
(1974b) for 30 days on diets containing 0, 5, 15 and 50 ppm
pirimiphos-methyl. Residues of pirimiphos-methyl were determined in
milk samples taken every two days and in samples of kidney, liver,
heart, fat and muscle taken at the end of the study. None of these
residues exceeded 0.02 mg/kg. The authors prepared butter from the
milk of cows dosed at the 110 mg/kg bw rate but found this to contain
only 0.02-0.04 mg/kg pirimiphos-methyl. In all instances no residues
of the organophosphorus compounds (II) and (III) could be detected in
samples of meat, milk or butter. (Limit of detection: 0.01 mg/kg in
each case).
Hen
Green et al. (1973) report that when diets containing 4 ppm
pirimiphos-methyl were fed to laying hens for 28 days, or when a diet
containing 32 ppm was fed for 7 days, residues of pirimiphos-methyl
itself in eggs did not exceed 0.01 mg/kg and no residues of compound
(III) were detected (Limit of detection: 0.001 mg/kg). The same
authors using radio-labelled pirimiphos-methyl found that the total
amount of radio-activity in eggs reached a maximum of 0.03 mg/kg
pirimiphos-methyl equivalents after 15 days when fed at a
concentration of 4 ppm in the ration and a maximum of 0.15 mg/kg
pirimiphos-methyl equivalents at the end of the 32 ppm study. In
muscle samples taken at the end of the 32 ppm study no residues of
pirimiphos-methyl or of its P=0 analogue were detected, (Limit of
detection: 0.001 ppm). Since 86-90% of the radio-activity in eggs is
present as water-soluble unidentified metabolites and since at no time
does the concentration of pirimiphos-methyl itself in yolks or whites
exceed 0.001 mg/kg, Green et al. conclude that pirimiphos-methyl does
not accumulate in eggs during continuous feeding of the insecticide
and that at the proposed level of application the residues of the
pesticide in eggs are negligible.
Graham and Jenkins (1974), as part of a study of the effect of
pirimiphos-methyl on the growth rate of broiler chickens, analysed
muscle, skin and fat samples from birds receiving 4, 8, 16, 32 and 48
ppm pirimiphos-methyl continuously in rations for 9 weeks. Birds were
slaughtered within 1 hour of being removed from feed. The only
residues which appeared were detected in skin and fat. The highest
level, 0.017 mg/kg, was in the fat of a broiler receiving 48 ppm
pirimiphos-methyl in the ration. Another had 0.015 mg/kg. All other
samples contained less than 0.01 mg/kg or residues were not
detectable.
In soil
Pirimiphos-methyl shows very limited persistence and mobility in
soil.
Bowker, Riley and Gratton (1972) showed that in a range of soil
types the half-life of pirimiphos-methyl is less than one month. The
major soil metabolite is the parent hydroxypyrimidine (IV) together
with smaller amounts of the related compounds (V) and (VI). The routes
of metabolism in stored grain, plants and soil are therefore similar.
The same authors using a descending thick-layer chromatography
technique, showed pirimiphos-methyl to possess only limited mobility
in soil: considerably less than atrazine which was used as a standard
and which itself is recognised as being only moderately mobile.
In water
Pirimiphos-methyl in rapidly degraded in water, mainly by
hydrolysis with loss of the phosphorothionate ester side chain. This
process is accelerated in the presence of light. Bowker and Hughes
(1974b) confirmed this and showed that although some volatilisation
occurs from still water, this is less significant than hydrolysis in
accounting for loss of the insecticide. In their experiments 50%
degradation had occurred after one day in sunlight. The major
degradation product in water is compound (IV). Only trace quantities
of the P=0 analogue (III) were detected (less than 0.01 mg/l in water
treated initially with pirimiphos-methyl at 1 mg/l).
In processing
Wheat
Bullock (1973 and 1974) reported many separate experiments which
demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table
10 summarises the results of residue trials carried out in the U.K. on
wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 11 summarises results of a residue trial reported by
Bullock (1973) to have been carried out in the U.K. with wheat
nominally treated to contain 8 ppm pirimiphos-methyl. These data which
are substantially in agreement with those of Bengston et al. (1974)
show that there is relatively little penetration beyond the seed coat
even throughout a storage period of 9 weeks.
TABLE 10. Effect of milling and baking on residues in wheat admixed
with pirimiphos-methyl at 4 mg/kg-UK (Bullock, 1973, 1974)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
Whole 0 4.2 1.9 2.9 (9)
grain 3.0* -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Wholemeal 0 1.3 0.94 1.1 (3)
flour
1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White 0 0.88 0.30 0.52 (6)
flour 0.56* 0.53* 0.55* (3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27* (3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread
1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
TABLE 10. (Cont'd.)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
White 0 0.28 0.19 0.23 (6)
bread 0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 All results are from field trials except those marked*
which are from a small-scale trial. Figures in parentheses are
the numbers of results upon which the means are based.
TABLE 11. Residues of pirimiphos-methyl in whole grains and in milling
and baking fractions of whole grains treated in a laboratory
trial in UK at 8 mg/kg. (Bullock, 1973, 1974)
Interval between Residues, mg/kg.1
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52 - -
6.0 0.91 0.56 - -
6.0 1.0 0.57 - -
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 In all cases, no residues of the phosphorus-containing compounds (II)
or (III) (Figure 1) were detected. (Limit of detection: 0.01 ppm in
each case.)
Bengston et al. (1974) report an extensive series of trials in
which pirimiphos-methyl was applied to wheat at the rate of 6 mg/kg in
bulk silos in two areas of Australia. Wheat was submitted to two
separate laboratories, for analysis and for processing, milling and
baking. The results reported by the two independent laboratories are
summarised in Table 12. These studies revealed that though a
significant amount of the pirimiphos-methyl was removed during the
cleaning process prior to the milling of the wheat, by far the
greatest separation occurred in the bran. Although the bran
represented only 25% of the whole grain it contained just over two
thirds of the entire residue leading to a concentration in the bran of
250% of that in the whole grain from which the bran was derived. The
concentration in the flour after milling averaged only 22% of that in
the whole grain. When the grain was processed into bread and baked
there was a further 63% reduction in the level of residues according
to one co-operating laboratory and an almost complete destruction of
the insecticide reported by the other. Assuming that the amount of
residue found in the bread would always be as high as the highest
level reported (0.3 mg/kg) the processing, milling and baking of bread
from raw grain containing pirimiphos-methyl results in a 93%
destruction of the original residue. Under practical conditions this
could well be greater because there is normally a significant delay at
each stage of the process which would further promote degradation of
the residue.
Rice
Residues of pirimiphos-methyl and its degradation products in
rice in husk are concentrated in the husk. Bowker (1973) and Bullock
(1973, 1974) report that parboiling rice to remove the husk results in
a substantial loss of the insecticide and its degradation products
from the husk into the water used to soak the rice. Table 13, taken
from Bullock (1973), shows the results of experiments in Guyana
designed to determine the lose of residues during the processing of
rice which had been treated previously while in husk at a nominal rate
of 5 and 10 mg/kg with pirimiphos-methyl. These results clearly
demonstrate that the bulk of the residue is in the husk with the
remainder in the seed coat. Polishing removes virtually all of the
material deposited in the seed coat although the process of parboiling
appears to promote the transfer of a small amount of residue into the
grain itself.
TABLE 12. Fate of pirimiphos-methyl residues in wheat treated at 6 mg/kg and subjected to
milling and baking (Bengston et al., 1974).
Residues, mg/kg,
Residues, mg/kg, Wheat W Wheat M
Laboratory A Laboratory B Laboratory B
Stage of Processing 1 2 Mean 1 2 Mean 1 2 Mean
Whole grain 4.7 4.8 4.8 4.2 4.2 4.2 4.5 4.5 4.5
Cleaned grain 3.8 3.8 3.8 - - - - - -
Bran 10.0 9.9 10.0 8.9 9.3 9.1 11.9 12.7 12.4
Shorts (pollard) 7.4 7.7 7.6 8.2 8.5 8.3 10.1 10.6 10.4
Flour 0.85 0.88 0.9 0.6 0.8 0.7 1.3 1.3 1.3
Bread 0.31 0.32 0.3 <0.2 <0.2 <0.2 0.46 0.48 0.47
TABLE 13. Effect of milling and polishing on residues in rice admixed with
pirimiphos-methyl (5 or 10 mg/kg) while in husk - Guyana
(Bullock, 1973).
Residues, mg/kg.
Treated at 5 mg/kg Treated at 10 mg/kg
Not Not
Sample parboiled Parboiled parboiled Parboiled
Dehusked grain 0.55 0.58 0.72 1.46
Husks 8.7 2.1 9.4 4.95
Grain polished 1 min. 0.26 0.31 0.29 0.76
Polishings from 1st min. 5.9 6.3 9.0 14.0
Grain polished 2 min. 0.19 0.29 0.29 0.76
Polishings from 2nd min. 5.6 6.5 9.4 15.8
Grain polished 5 mins. <0.01 0.28 0.27 0.65
Polishings from 3rd to 5th mins. 5.3 7.1 9.3 14.9
Bullock (1973) reports experiments carried out in Indonesia to
determine the loss of pirimiphos-methyl residues from polished rice
during cooking. Rice which had been treated by admixture with
pirimiphos-methyl emulsifiable solution and which contained 1.96 mg/kg
pirimiphos-methyl was used in the trial. The rice was washed under the
cold water tap for two minutes before simmering for 20 minutes in 2.5
times its own weight of tap water. The rice by this time was a soft
mass. Cold tap water was poured over the grains to separate them and
the samples were then analysed in the usual manner. A second sample of
the same batch of rice was treated in the same way and duplicate
analyses were carried out on each sample. The results which are given
below show a 74% loss in cooking white rice.
Residue, mg/kg, before cooking: 1.96; 1.96
after cooking: 0.58, 0.50 (mean 0.54); 0.50, 0.47
(mean 0.49).
No residues of the phosphorus-containing compounds (II) or (III)
were detected (limit of detection: 0.01 ppm in both cases).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
Two methods of residue analysis are available. The more
sensitive, quicker and preferred method is that of Bullock (1972) in
which gas chromatography with a phosphorus detector is used. The
method is suitable for the determination of residues of
pirimiphos-methyl in crops, it also determines the
phosphorus-containing metabolites (II) and (III). The limit of
determination is 0.01 mg/kg in each case. Crop samples are extracted
with a 20% solution of acetone in n-hexane. A decanted portion of the
extract is washed with water, and then dried with anhydrous sodium
sulphate. A portion of the extract is then injected into a gas
chromatograph equipped with a flame photometric or thermionic
detector. The peaks obtained at the retention times of
pirimiphos-methyl and its metabolites (II) and (III) are compared with
standards. Grain samples are extracted with methanol instead of
acetone/hexane and oily or fatty materials are subjected to an
acetonitrile-n-hexane partition before analysis. The oxygen analogue
(III) has not been detected as a metabolite in any samples analysed in
the laboratory of the manufacturer, although (as stated above) its
limit of determination is 0.01 mg/kg.
Alternatively when GLC is not available, a colorimetric method
may be used. The colorimetric method reported by Bullock (1969) has
only been tried on field crop samples. An extract, obtained by
prolonged maceration or slurrying the sample with n-hexane, is dried
with anhydrous sodium sulphate, filtered, concentrated down to a small
volume in a rotary evaporator at low temperature and quantitatively
transferred to a preparative-layer plate together with a marker spot
of pirimiphos-methyl. The plate is developed in a mixture of n-hexane
and acetone and inspected under ultra-violet light. The band opposite
the marker spot is scraped off and refluxed for one hour with
concentrated hydrobromic acid to liberate hydrogen sulphide, which is
swept off in a stream of nitrogen, quantitatively absorbed in alkaline
zinc acetate and subsequently determined as methylene blue. The limit
of determination is normally about 0.1 mg/kg with recovery in excess
of 75%.
Bullock (1973) reports studies to determine the most effective
solvent for extracting pirimiphos-methyl and its metabolites from
"aged" residues in stored grain. Table 14 shows the results of these
studies indicating the residues of pirimiphos-methyl extractable from
aged samples by different extraction methods. The samples were ground
to a fine powder before analysis. The lowest recoveries are almost
invariably with methanol under reflux. Comparable results are obtained
with the other solvent systems when extraction is made immediately
after treatment, but on aged samples cold methanol generally gives
significantly higher results.
TABLE 14. Residues of pirimiphos-methyl extractable from "aged" samples by different
extraction methods. (Samples ground to fine powder before analysis.)
Residues determined (mg/kg) using various
Sample** Interval after extraction methods*
(Treatment rate) treatment 1 2 3 4 5
Wheat (4 mg/kg) 0 days 3.72 3.45 3.82 3.87 2.91
1 month 2.92 2.73 2.79 3.01 3.17
3 months 1.87 1.73 1.99 2.17 2.04
Barley (4 mg/kg) 0 days 3.07 3.12 3.49 3.09 1.90
1 month 2.51 2.36 2.17 2.69 2.54
3 months 1.53 1.71 1.47 1.84 1.70
Rice (4 mg/kg) 0 days 4.10 3.92 4.00 4.07 3.12
1 month 2.18 2.40 2.12 2.71 2.60
3 months 1.41 1.95 1.84 1.99 1.03
* Extraction methods: 1 Dichloromethane (cold); 2 Acetone (cold); 3 20% Acetone
in hexane (cold); 4 Methanol (cold); 5 Methanol (1 hour reflux).
** The samples had a moisture content of 13-14%.
Bullock also reports studies which show that it is preferable to
grind aged samples before attempting to extract with either methanol
or acetone/hexane. Grinding offers no advantage when dealing with
wheat or rice that has only just been treated but in view of the fact
that many regulatory analysts will receive samples which have been
treated three months to three years previously it is obvious that the
preparation of the sample and the selection of the solvent are of
critical importance. Table 15 shows results obtained by Bullock. The
samples were ground in a Moulinex coffee grinder. Later comparison
with Hobart laboratory grinder showed no significant difference in the
results and little temperature rise in the grain during grinding.
TABLE 15. Effect of grinding on extractability of aged residues
(Bullock, 1973).
Sample Residues, mg/kg, determined using
(Treated by Interval different extraction solvents
admixture after Original 20% acetone Methanol
at 4 mg/kg) treatment or ground in hexane
Wheat 0 days Original 3.82 3.91
Ground 3.82 3.87
1 month Original 2.70 2.81
Ground 3.10 3.04
3 months Original 1.43 1.55
Ground 1.87 1.99
Rice 0 days Original 4.01 4.05
Ground 4.10 4.07
1 month Original 2.00 1.91
Ground 2.18 2.34
3 months Original 1.67 1.71
Ground 1.84 1.99
As a check on the loss of residues during grinding Bullock took
wheat which had been freshly treated with pirimiphos-methyl and
measured the residue level before and after intense grinding for one
minute. The analytical results varied by less than 50% which is slight
by comparison with the loss which would occur if grain treated with
malathion or dichlorvos were ground in a similar manner. The same
author reports experiments designed to determine the loss of residue
from wheat, barley and rice when grain samples were stored at -14°C
while awaiting analysis. Both freshly treated grain and grain which
had been treated under semi-commercial conditions three months prior
to sampling were kept under such storage conditions but none of these
samples showed as much as 5% loss during two months at -14°C.
NATIONAL TOLERANCES
The information available to the meeting indicates that few
national tolerances have been established. It is understood that a
tolerance of 4 mg/kg has been proposed in France for pirimiphos-methyl
residues in cereals and 2 mg/kg in fruits and vegetables. In the
Netherlands a tolerance of 0.2 mg/kg has been established for
pirimiphos-methyl residues in cucumbers, melons, paprika and tomatoes.
APPRAISAL
Pirimiphos-methyl is a broad-spectrum organophosphorus
insecticide with both contact and fumigant action. It shows only
limited biological persistence on plant surfaces but gives long
lasting control of insect pests on inert surfaces and retains its
biological activity when applied to stored agricultural commodities
including raw grains and nuts.
The meeting had only limited Information concerning the
pre-harvest use and performance of this material on crops but
extensive data were available on its use, performance and fate on a
variety of small grains and nuts. The recommended rate of application
to raw cereals is 6 mg/kg.
Exceptional stability on grain results in an effective half-life
of up to 80 weeks. Although high temperatures and high moisture levels
in grain reduce the life of the deposit the effect of these influences
is much less than with other grain protectants approved or evaluated
to date.
Data were available to show that on both wheat and rice the
deposit resulting from admixture of pirimiphos-methyl with grain is
almost entirely in the seed coat and husk respectively. Cleaning,
processing and milling removes the bulk of the applied material. In
the case of wheat the milling process results in most of the residue
being separated into the bran where the concentration of
pirimiphos-methyl can range up to 20 mg/kg. In the case of rice
substantially all of the deposit is removed during the milling and
polishing operations. Depending upon the milling process, flour
prepared from treated wheat may contain significant amounts of
pirimiphos-methyl but a major proportion of this is destroyed in the
baking of bread. The small amount of residue remaining in polished
rice is substantially destroyed in cooking.
Pirimiphos-methyl and the corresponding hydroxypirimidine are the
only significant residues detected. Residues of the
phosphorus-containing metabolites when detected were always extremely
low (approximately 0.05 mg/kg).
Extensive data are available on the fate of pirimiphos-methyl in
plants, domestic animals, hens and their edible tissues, and foods of
animal origin. In living plants the major phosphorus-containing
degradation product is the N-desethyl metabolite (II) which is not
accumulated. The hydroxypyrimidine compound (IV) is the major
degradation product following root uptake. When fed to domestic
animals pirimiphos-methyl is rapidly metabolised and excreted, only
very small quantities being secreted into milk or eggs. Cattle
receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites could
be detected in meat, milk, butter or eggs.
A GLC method of analysis suitable for the determination of
residues of pirimiphos-methyl and the phosphorus-containing
metabolites in plant materials is available, the limit of
determination being reported to be 0.01 mg/kg. The method appears
suitable for regulatory purposes. Evidence of the importance of sample
preparation and method of extraction has been presented.
No national tolerances have yet been determined.
In proposing maximum residue limits for pirimiphos-methyl and its
metabolites on raw grain, milled cereal products and foods prepared
therefrom, careful consideration has been given to the fact that this
insecticide is to be used as a grain protectant, that a certain
concentration must be present on the grain to control infestations and
prevent damage to stored products and that the compound is
exceptionally stable under storage conditions. Under practical
conditions of grain handling and storage it is not possible to apply a
grain protectant with assurance that the deposit will be absolutely
uniform. There will always be a natural variation in the level of the
deposit resulting from fluctuations in the flow of grain and
insecticide. It is therefore not possible to fix the maximum limit on
the minimum necessary to control the insect pests. Furthermore
extensive work has demonstrated that the movement of bulk grain
results in the segregation of the various components of the bulk with
a resultant variation in the level of residues throughout the mass.
Due allowance must be made for the amplitude of these variations and
the problems of sampling and analysis.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl, expressed as pirimiphos-methyl.
TEMPORARY TOLERANCE
mg/kg
Bran (wheat, rice) 20
Wheat, rye, rice (in husk) 10
Barley, maize, oats 7
Wholemeal flour (wheat, rye) 5
Rice (dehusked), wheat flour (white) 2
Bread (wholemeal), rice (polished) 1
Bread (white) 0.5
Meat, milk, eggs 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 1976)
1. Additional studies to clearly establish no-effect level for
decreased pregnancy rates, and hydronephrosis in offspring.
2. Further studies to clarify the liver injury dogs observed in 90
day studies.
DESIRABLE
1. Longer duration observations in man.
2. Information from studies now in progress on other stored
commodities including nuts, peanuts and dried fruit. The
information is expected to be available during 1975.
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in food at
the point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
REFERENCES
Bengston, M., Connell, M., Desmarchelier, J., Phillips, M., Snelson,
J. and Sticka, R. (1974) Evaluation of four new grain protectants.
Report to Australian Wheat Board. (To be published)
Bowker, D.M. (1973) Pirimiphos-methyl (PP 511): fate of stored wheat
and rice grain in the laboratory. ICI Plant Protection Ltd. Report No.
AR 2457 AR. (Unpublished)
Bowker, D.M., Griggs, B.F. and Harper, P. (1973) Pirimiphos-methyl (PP
511): excretion by a goat. ICI Plant Protection Ltd. Report No. AR
2458 B. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in crops.
ICI Plant Protection Ltd. Report No. AR 2515 A. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in water.
ICI Plant Protection Ltd. Report No. AR 2516 A. (Unpublished)
Bowker, D.M., Riley, D. and Gratton, R.P. (1972) Pirimiphos-methyl:
fate in soil. ICI Plant Protection Ltd. Report No. TMJ 809 A.
(Unpublished)
Bratt, H. and Dudley, L.A. (1970) Pirimiphos-methyl (PP 511):
Excretion by rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bratt, H. and Jones, L.A. (1973) Pirimiphos-methyl (PP 511):
Metabolism in rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bullock, D.J.W. (1969) Provisional analytical method for the
determination of PP211 and PP511 residues in vegetables, grain and
fruit. ICI Plant Protection Ltd. Method No. PAM 310/A.
Bullock, D.J.W. (1972) Residue analytical method no. 11 for the
determination of residues of pirimiphos-methyl and its
phosphorus-containing metabolites in crops (gas chromatographic
method). ICI Plant Protection Ltd. Method PPRAM-11/A.
Bullock, D.J.W. (1973) Pirimiphos-methyl: residues in stored grain.
ICI Plant Protection Ltd. Report No. AR 2472 AR. (Unpublished)
Bullock, D.J.W. (1974) Pirimiphos-methyl: residues in stored grain
bread, flour and milled products. ICI Plant Protection Limited. Report
No. AR 2537 A. (Unpublished)
Bullock, D.J.W., Day, S., Hemingway, R.J. and Jegatheeswaran, T.
(1974) Pirimiphos-methyl: residue transfer study with cows. Report
from ICI Plant Protection Limited. (Unpublished)
Bullock, D.J.W., Day, S., and Griggs, B.F. (1974) Pirimiphos-methyl:
metabolism and residue transfer into the meat and milk of a lactating
cow. ICI Plant Protection Ltd. Report No. AR 2552 A. (Unpublished)
Chart, S., Foulkes, Caroline A., Gore, G.W. and Williamson, K.S.
(1974) Erythrocyte and plasma cholinesterase activity in human
volunteers administered pirimiphos-methyl. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Clapp, M.J. and Conning, D.M. (1970) Pirimiphos-methyl (PP511).
Ninety-day oral toxicity in rats. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Clark, D.G. (1970) The toxicity of PP511
[O-(2-diethylamino-6-methylpyrimidin-4-yl)
O,O-dimethylphosphorothioate] Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fink, R. (1974a) Eight-day dietary LC50 - mallard ducks: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Fink, R. (1974b) Eight-day dietary LC50 - bobwhite quail: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Gage, J.C. (1971a) Pirimiphos-methyl (PP511): Avian toxicity. Report
from ICI Industrial Hygiene Research Laboratories. (Unpublished)
Gage, J.C. (1971b) Pirimiphos-methyl (PP 211) and pirimiphos-methyl
(PP 511): acute and subacute oral toxicity in the rat of the plant
metabolite, 2-diethylamino-4-hydroxy-6-methylpyrimidine (R46382). ICI
Industrial Hygiene Research Laboratory. Report No. HO/IH/R/327.
(Unpublished)
Gage, J.C. (1972) Pirimiphos-methyl (PP511): Oral toxicity in the dog
and in a passerine bird species. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Gore, C.W. Palmer, S. and Pratt, I.S. (1974) Pirimiphos-methyl
(PP511): teratogenic studies in the rabbit. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Gore, C.W., Griffiths, D. and Phillips, C.E. (1974) Pirimiphos-methyl
(PP511): two year feeding study in the rat. Report from ICI Industrial
Hygiene Research Laboratories. (Unpublished)
Graham, C.A. and Jenkins, S.L. (1974) The effect of pirimiphos-methyl
on the growth rate of broiler chicks. ICI Australia Ltd. Merrindale
Research Station Report, July 1974.
Green, T., Monks, I.H. and Phillips, P.J. (1973) Pirimiphos-methyl (PP
511): sub-acute oral and residue studies in hens. ICI Industrial
Hygiene Research Laboratories. Report No. HO/IH/P/65B. (Unpublished)
Hodge, M.C.E. and Moore, S. (1972) Pirimiphos-methyl (PP 511):
Teratological studies in the rat. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Noel, P.R.B., Mawdesley-Thomas, L.E., Rivett, K.F., Squires, P.F. and
Street, E. (1970) PP 511: Oral toxicity studies in dogs - initial
studies and repeated dosage for thirteen weeks. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and Cherry, C.P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat: histopathological
examination on testes of F1B and F2B males. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and James, P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat. Report from Huntingdon
Research Centre. (Unpublished)
Parkinson, G.R. (1972a) Pirimiphos-methyl (PP 511): potentiation with
gamma-BHC. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Parkinson, G.R. (1972b) Pirimiphos-methyl (PP 511): potentiation with
dichlorvos. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Pieterse, A.H., Schulten, G.G.H. and Kuyken, W. (1972) A study of
insecticide resistance in Tribolium castaneum in Malawi. J. Stored
Prod. Res., 8(3):183.
Rivett, K.F., Edwards, B., Street, E. and Newman, A.J. (1973) PP 511:
Oral toxicity study in beagle dogs - repeated daily dosage for two
years. Report from Huntingdon Research Centre. (Unpublished)
Ross, D.B., Burroughs, Sheila J. and Roberts, Nicholas L. (1974) Egg
production and hatch-ability in the laying hen following dietary
inclusion of pirimiphos-methyl at various levels. Report from
Huntingdon Research Centre. (Unpublished)
Rowlands, D.G. (1966) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. Stored Prod. Res., 2:105-116.
Rowlands, D.G. et al. (1974) Preliminary report of studies with
pirimiphos-methyl as a grain protectant. Submission by United Kingdom
to FAO. (To be published)
Waterhouse, D. (1973) Background paper presented at the Ninth Session
of the FAO Working Party of Experts on Pest Resistance to Pesticides
(Rome, 20 June 1973). (Unpublished)
Admixture with small grain cereals
Bullock (1973 and 1974) reports numerous trials undertaken in
Australia, Argentina, Guyana, Malaysia, U.K. and the U.S.A. where
pirimiphos-methyl was admixed with wheat, barley, maize, and rice in
husk (residues were determined on the rice after milling). The results
are summarised in Table 4. Mean initial residues of pirimiphos-methyl
itself in the whole grains of wheat, barley and maize rarely exceeded
the nominal treatment rate and were frequently in the range of 40-60%
of the nominal dose. This appears to be a reflection of the method of
application and suggests that some at least of the nominal dose is
lost into the atmosphere during application. Higher percentage
retention of the applied chemical was obtained by treating 5-10% of
the grain with all the chemical rather than applying it to 50% or more
of the grain stream entering the storage. Again no residues of the
phosphorus-containing compounds (II) and (III) were detected.
TABLE 3. Residues of pirimiphos-methyl in whole grains
after spraying bagged grains. (Bullock, 1973)
Interval
between Range of
Application application residues
Rate and sampling found,
Crop mg/m2 months mg/kg Country
Barley 400 0-3 0.05-0.33 UK
Oats 400 2-3 0.07-0.19 UK
Rice in husk 300 0-3 <0.01-0.08 Colombia
Milled rice 500 0-5 0.02-0.28 Malaysia
Treated when
in husk 1000 0-5 0.01-0.70 Malaysia
TABLE 4. Residues of pirimiphos-methyl in whole grains
after admixture. (Bullock, 1973, 1974)
Interval
Nominal between Range of
admixture admixture residues
rate, and sampling found,
Crop mg/kg (months) mg/kg Country
Wheat 3 0-8 1.0-2.1 Australia
4 0-3 1.3-4.6 UK
5 1-3 1.7-4.7 USA
6 0-12 1.3-3.9 Argentina
0-8 3.0-4.9 Australia
10 1-3 4.7-8.4 USA
Barley 4 0-3 0.03-3.6 UK
8 0-3 0.26-4.4 UK
Maize 5 1-3 2.1-6.9 USA
10 1-3 4.7-8.0 USA
Milled rice 3 0-3 0.21-0.53 Guyana
treated when
in husk 4 0-5 0.12-0.26 Malaysia
5 0-4 0.16-0.59 Guyana
7.5 0-11 0.53-0.98 Guyana
8 0-5 0.21-0.85 Malaysia
Wheat
The stability of the deposit of pirimiphos-methyl on wheat grains
is quite remarkable and from the work of Bullock the half life has
been estimated to be of the order of 9 months. Degradation proceeds
uniformly and slowly as indicated in the results from a typical trial
carried out in Argentina on wheat with a moisture content ranging from
11 to 13% (Table 5).
TABLE 5. Pirimiphos-methyl residues in wheat at intervals after admixture-Argentina.
(Bullock, 1973)
Nominal
admixture
rate
mg/kg 1 Day 1 Month 2 Months 3 Months 4 Months 6 Months 9 Months 12 Months
2 1.60 1.16 1.47 2.06 2.00 0.90 0.80
3 1.61 1.53 1.30 1.34 1.13 1.96 1.03 0.80
4 2.04 2.40 1.89 1.79 1.53 1.80 1.66 0.92
5 2.26 2.79 2.06 2.00 1.93 2.00 1.73 1.31
6 3.91 3.65 2.62 2.42 2.39 2.14 2.02 1.29
7 3.90 3.71 3.12 3.06 2.43 2.30 2.20 1.35
Moisture content: low (11-13%)
Bengston et al. (1974) report the results of two extensive trials
in Australia with five grain protectant insecticides applied to wheat
having a mean moisture content of 10-12% and a mean temperature of
29-32°C. In these trials the target application rate was 6 mg/kg but
based on the analyst's determination of the concentrate strength and
the weight of grain treated (2000 tons per treatment) the calculated
application rate was 5.5 mg/kg. Analysis by five collaborating
laboratories showed the concentration of pirimiphos-methyl on samples
taken 1 week after application to range from 5.5-6.4 mg/kg.
Representative data summarised in Table 6 show relatively little
change over a 26 week period notwithstanding the high temperature
which prevailed throughout.
Based on this work calculations have been made of the half-life
of Pirimiphos-methyl deposits on wheat stored in concrete bins at
30°C. When using data from samples taken from within 10 cm of the
surface and using the rate of disappearance during weeks 1-11, a
half-life of 43 weeks is obtained. Based on data from weeks 11-26 the
half-life was 104 weeks. Using data from samples taken 6 metres
beneath the surface where the mean temperature exceeded 32°C a
half-life of 77 weeks is obtained whether the data from samples
collected during weeks 1-11 or weeks 11-26 are used. The mean
half-life from many data is 82 weeks. This compares with a half-life
for malathion of 3-9 weeks under comparable conditions.
Barley
Bullock (1973 and 1974) reports numerous trials where barley was
treated with pirimiphos-methyl in the United Kingdom by admixture of
2% Pirimiphos-methyl dust or by the application of dilute emulsion
designed to deposit from 2-8 mg/kg on the weight of grain.
Unfortunately moisture and temperature conditions are not stated and
extensive sampling and analysis has failed to recover more than 25-30%
of the amount that was supposed to have been applied. Residue analyses
however fail to show much decline in the level of residues over the
first three months following spray application. The deposit seems to
disappear more quickly when the application is made in a manner
designed to fully cover all grains than when only a proportion of the
grain receives the spray. Subject to some reservations about the
residue data, the half-life on barley from these trials appears to be
at least 30 weeks.
TABLE 6a. Pirimiphos-methyl residues in stored wheat from site M
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories.
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
1 week 0.1 5.5 6.6 - - 6.1
0.6 6.4 - - -
1.5 4.9 5.8 - -
6.0 5.6 5.6 - - 5.6
Mean 5.3 6.0 - -
6 weeks 0.1 5.2 5.2 5.5 5.3 5.3
0.6 - - - - -
1.5 4.6 5.1 4.9 4.5 4.8
6.0 4.8 5.1 5.3 3.8 4.8
Mean 4.9 5.1 5.2 4.5 -
11 weeks 0.1 5.6 5.1 6.0 4.8 5.4
0.6 - - - - -
1.5 5.1 4.5 5.2 3.8 4.7
6.0 5.4 4.9 5.8 3.8 5.0
Mean 5.3 4.8 5.7 4.1 -
16 weeks 0.1 5.4 - - 4.9 5.1
0.6 - - - - -
1.5 5.1 - - 4.3 4.7
6.0 5.4 - - 4.3 4.8
Mean 5.3 - - 4.6 -
22 weeks 0.1 5.6 4.3 4.8 4.1 4.7
0.6 - - - - -
1.5 5.5 4.1 4.1 3.7 4.4
6.0 5.2 4.2 4.4 3.6 4.4
Mean 5.4 4.2 4.4 3.8 -
26 weeks 0.1 - 4.2 5.2 - 4.7
0.6 - - - - -
1.5 - 3.6 4.6 - 4.1
6.0 3.6 3.6 4.4 - 3.9
Mean -
TABLE 6b. Pirimiphos-methyl residues in stored wheat from site W
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
2 weeks 0.1 - - - - -
0.6 5.6 5.4 4.5 4.5 5.0
1.5 6.0 4.9 4.7 4.0 5.0
6.0 5.8 4.9 4.4 4.0 4.8
Mean 5.1 4.5 4.2 -
8 weeks 0.1 - - - - -
0.6 4.4 3.9 4.5 4.0 4.2
1.5 4.8 4.5 5.0 4.5 4.7
6.0 4.5 3.9 4.7 4.2 4.2
Mean 4.6 4.1 4.7 4.2 -
13 weeks 0.1 - - - - -
0.6 4.2 4.0 - 2.3 3.5
1.5 4.8 4.2 - 3.3 4.1
6.0 4.2 3.9 - 2.7 3.6
Mean -
TRIAL TERMINATED BECAUSE OF INFESTATION WITH RHIZOPERTHA DOMINICA
Rice
Bullock (1973) reports trials in Guyana, Indonesia and Malaysia
where rice in husk and polished rice were treated by admixture of
varying amounts of pirimiphos-methyl. In all trials reported the husk
was removed after varying intervals and the residues on husk and grain
determined separately. There appears to be some migration of the
insecticide from the husk to the grain during parboiling prior to
dehusking. The level of the residue in the husk declines over a period
of one month to about 30% of its concentration shortly after
application. Table 7 shows results from Guyana where rice was treated
in husk. When the rate of application was 20 mg/kg, 55% of the deposit
was lost from the husk within three days, the level declining to 35%
of the initial value at the end of one month.
TABLE 7. Pirimiphos-methyl admixed with rice paddy and
analysed at intervals - Guyana. (Bullock, 1973)
Interval
Rate of between Before After
application, treatment Parboiling* Parboiling*
mg/kg and sampling Husk Grain Husk Grain
3 3 days 14 0.53 8.4 1.0
1 month 4.4 0.21 1.1 0.66
2 months 2.4 0.34 2.7 1.0
3 months 2.0 0.46 2.0 0.64
7.5 3 days 34 0.90 5.1 1.8
1 month 8.5 0.75 2.0 -
2 months 5.2 0.71 6.0 2.3
3 months 5.1 0.53 3.4 1.3
8 months 12 0.98 4.5 ND
11 months 0.59 0.58 1.4 0.38
19.2 0 days 80 1.2 17 5.6
3 days 36 2.1 - -
1 month 28 1.4 8.2 3.2
2 months 13 1.8 15 4.9
3 months 18 1.3 3.7 2.6
* Parboiling undertaken for dehusking purposes.
Moisture content: approximately 14%.
Bullock (1973) reports extensive trials carried out in Malaysia
where pirimiphos-methyl was applied to polished rice and to rice in
husk at various rates and by various methods of application. Polished
rice appears to retain the initial deposit over a period ranging up to
7 months. It is not possible to estimate the half-life from these data
but it can be assumed to be much more than 7 months. Other trials in
Indonesia confirm this long residual life. Malathion under comparable
conditions disappeared almost completely within two months. By
comparison malathion appears not to migrate in significant amounts
from the husk into the grain of treated paddy.
Maize
Bullock (1973) reports trials in the U.S.A. which revealed that
pirimiphos-methyl deposits on maize remained relatively stable over a
period of 3 months. When the rate of application was 5-10 mg/kg the
half-life appeared to be of the order of 3 months but when 20 mg/kg
was applied no significant change occurred over the three month
period.
Peanuts
Bullock (1973) reports two large scale trials in the U.S.A. where
pirimiphos-methyl was applied at 20 and 50 mg/kg to undecorticated
peanuts. Analytical results indicate that the target rate of treatment
was achieved. Very little change occurred in the level of residues
during the first month following application. Even at the end of three
months substantially all of the deposit remained. The data suggest
that the half-life is of the order of 5 months from the time of
application. Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied.
FATE OF RESIDUES
In stored products
a) Distribution and degradation
Residues of pirimiphos-methyl on wheat grains are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain, to give
principally the parent hydroxy-pyrimidine (IV), Figure 1, and also the
related compounds (V) and (VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grains.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residues of the
chemically-unstable oxygen analogue (III) were detected. The limit of
detection.was 0.01 mg/kg (Bowker, 1973: See Table 8).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products.
The general pattern of breakdown on stored rice is similar to
that found on wheat grain. The insecticide and its degradation
products were concentrated in the husk in which the rate of breakdown
appeared to be unaffected by the moisture content of the rice (Bowker,
1973; Bullock, 1973). Representative results are given in Table 9.
TABLE 8. Effect of moisture on degradation of pirimiphos-methyl residues in wheat
grain (Bowker, 1973)
Application Pirimiphos-methyl equivalents,
rate and mg/kg, after interval (weeks)
moisture
content Compound 0 2 4 8 16 32
4 mg/kg, I (PP511) 2.16 2.66 2.05 2.66 2.32 2.08
14% moisture II + unidentified* 0.04 0.04 0.04 0.06 0.04 -
IV, V, VI <0.01 0.07 0.08 0.17 0.21 0.29
TLC baseline <0.01 <0.01 0.04 0.04 0.08 0.26
4 mg/kg,
18% moisture I 2.36 2.73 1.12 0.80 0.79 0.38
II + unidentified* 0.05 0.03 0.03 0.03 0.03 0.03
IV, V, VI - 0.49 0.56 1.05 0.92 0.66
TLC baseline - - 0.21 0.03 0.16 0.14
8 mg/kg, I 4.03 5.46 3.80 4.64 5.53 5.37
14% moisture II + unidentified* 0.12 0.08 0.08 0.11 0.04 0.10
IV, V, VI - 0.15 0.11 0.16 0.32 0.59
TLC baseline - - 0.02 0.03 0.04 0.22
8 mg/kg, I 4.44 5.64 2.93 2.84 2.19 1.28
18% moisture II + unidentified* 0.69 0.27 0.08 0.09 0.05 0.04
IV, V, VI - 0.98 0.99 1.61 1.89 1.61
TLC baseline - - 0.41 0.06 0.24 0.27
* Unidentified compound (minor product).
TABLE 9. Effect of moisture on degradation of pirimiphos-methyl residues in brown rice
(Bowker, 1973)
Application Pirimiphos-methyl equivalents, mg/kg,
rate and after interval (weeks)
moisture
content Compound 0 2 4 8 16
4 mg/kg, I (PP511) 2.13 2.31 2.06 2.19 -
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.07 -
IV, (V, VI) 0.05 0.11 0.06 0.10 -
TLC baseline 0.01 0.07 0.23 0.07 0.02
4 mg/kg, I 1.89 1.99 1.5 1.17 0.76
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.12 <0.01
IV, (V, VI) 0.06 0.17 0.34 0.24 0.33
TLC baseline 0.01 0.12 0.26 0.27 0.17
8 mg/kg, I 3.7 4.37 4.1 3.73 2.99
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.11 0.09
IV, (V, VI) 0.1 0.18 0.11 0.34 0.17
TLC baseline 0.03 0.13 0.36 0.23 0.06
8 mg/kg, I 3.11 3.68 1.55 2.3 1.58
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.21 0.11
IV, (V, VI) 0.06 0.32 0.10 0.86 0.45
TLC baseline 0.02 0.19 1.48 0.43 0.06
* Unidentified compound (minor product)
Pirimiphos-methyl applied as a dust formulation to wheat and
brown rice is degraded in a similar manner to other organophosphorus
insecticides used to protect stored grain from infestation (Rowlands,
1966).
Degradation is marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
is caused by factors within the grain or by the associated microflora
is not known. At higher moisture contents (approximately 18%) less
pirimiphos-methyl is recovered on analysis but increased levels of the
hydrolysis product (IV) are obtained suggesting that a more rapid
degradation of the insecticide occurs. Bowker concludes that grain may
lack the enzymic activity believed to be present in plants and soil to
cleave the pyrimidine N-ethyl bonds. It is likely therefore that
following the treatment of wheat and brown rice with
pirimiphos-methyl, the major residues during storage will be the
insecticide itself and the simple hydrolysis product (IV). Under
optimum conditions, the maximum level of compound (IV) following
treatment at 4 mg/kg, was found to be 0.17 mg/kg; under poor storage
conditions with high moisture content grain, 0.62 mg/kg.
Solutions of pirimiphos-methyl-14C were applied in the
laboratory to wheat grains of 14 and 18 percent moisture, and also as
a 2 percept dust, to give levels of 4 mg/kg. Throughout a storage
period of 6 months, the residues of pirimiphos-methyl were found
almost entirely in the seed coat and aleurone layer, with only traces
present in the germ or endosperm. It was also clear that, as with
malathion but to a greater extent, there was transfer of insecticide
between grains, possibly in the vapour phase. About 10 percent of the
total residual radioactivity was bound to lipoprotein material in the
aleurone region of the grain and was unextractable except by digestion
of the aleurone protein. By contrast with other organophosphates
studied, the bound material appeared to be the unchanged pesticide,
rather than a metabolite. Certainly it was a P=S compound, but
degradation during the liberation of the bound material complicated
identification. During the storage period, the pirimiphos-methyl
available for decomposition decreased by 15 and 50 percent at the 14
and 18 percent moisture levels respectively. The main products found
were the free hydroxy-pyrimidine and the N-desethyl pyrimidinol
(Rowlands et al., 1974).
b) Toxicity of degradation products
The hydroxypyrimidines (IV), (V) and (VI) are members of a class
of compounds known to exhibit low mammalian toxicity and they are also
products of animal metabolism. It is therefore concluded that
pirimiphos-methyl itself represents the major toxic residue in stored
products. Traces of the phosphorus-containing degradation products
(II) and (III), not exceeding 0.05 mg/kg in total, may also occur.
They are determined by the preferred analytical method. The acute oral
LD50 of (II) to the female rat is 800-1600 mg/kg. The oxygen analogue
(III) is chemically unstable and it has not been possible to undertake
toxicity tests with it (Gage, 1971b).
In plants
In contrast to its fate in stored products pirimiphos-methyl is
rapidly lost from leaf surfaces during the first 2-3 days after
spraying, mainly by volatilisation.
Bowker and Hughes (1974a) identified by a combination of
thin-layer and gas-liquid chromatography of the compounds or their
derivatives, all the major extractable metabolites and degradation
products arising from the interaction of pirimiphos-methyl with
plants.
They showed that on plant surfaces, pirimiphos-methyl is lost
rapidly by vaporisation. Levels of the parent insecticide and the
major degradation product, compound (II), represent less than 10% of
the applied material after two to three days. In crops after foliar
treatment the hydroxypyrimidine (IV), formed by hydrolysis of the
insecticide, is unlikely to be a significant residue. They found that
the compound is usually present only in trace amounts and is degraded
photochemically and bound to plant material.
These same workers showed that photochemical degradation of
pirimiphos-methyl on leaf surfaces leads to the formation of compound
(II) which is not accumulated. When applied to water in which rice
seedlings were growing, pirimiphos-methyl is not translocated
significantly into the foliage; in these conditions the hydrolysis
product (IV) is likely to represent the predominant pyrimidine
residue. Compounds (V) and (VI) only occur in trace amounts.
In animals
Much of the information in this section is also to be found under
the heading "Absorption, distribution and excretion," but is repeated
here for convenience.
Goat
Bowker et al. (1973) report studies where a single dose of
radio-labelled pirimiphos-methyl was given orally to a lactating goat
(0.12 mg/kg bw, equivalent to approximately 6 ppm in the ration). 91%
of the radio-activity was excreted in the following 8 days: 87% in the
urine and 4% in the faeces. Only 0.41% of the radio-activity was
secreted in the milk, primarily during the first 24 hours. The maximum
residue in the milk was 0.026 mg/kg pirimiphos-methyl equivalents, of
which pirimiphos-methyl itself represented 0.003 mg/kg.
Cow
The pattern of excretion in the cow is very similar to that in
the goat (Bullock et al., 1974a). When a single dose of radio-labelled
pirimiphos-methyl was given orally to a cow at 0.5 mg/kg bw
(equivalent to approximately 17 ppm in the daily diet), the
radio-activity was quantitatively recovered during the following seven
days - 85% in the urine and 14% in the faeces. Only 0.37% of the
radio-activity was secreted in the milk (0.35% during the first three
days).
The highest levels of radioactivity in the milk, urine and faeces
were found during the first day after dosing. The milk contained 0.04
mg/kg pirimiphos-methyl equivalents, of which approximately 75% could
be extracted from the fat and protein fraction into organic solvents.
Pirimiphos-methyl accounted for approximately 12% (i.e. about 0.004
ppm), and compound (V) for about 50% of the total radioactivity in the
milk. The radioactivity was not extracted by organic solvents and
appeared to be incorporated into natural milk components.
Approximately 70% of the radio-label in "day 1" urine was
organo-soluble, the remainder dissolving only in water.
Four groups of three cows were maintained by Bullock et al.
(1974b) for 30 days on diets containing 0, 5, 15 and 50 ppm
pirimiphos-methyl. Residues of pirimiphos-methyl were determined in
milk samples taken every two days and in samples of kidney, liver,
heart, fat and muscle taken at the end of the study. None of these
residues exceeded 0.02 mg/kg. The authors prepared butter from the
milk of cows dosed at the 110 mg/kg bw rate but found this to contain
only 0.02-0.04 mg/kg pirimiphos-methyl. In all instances no residues
of the organophosphorus compounds (II) and (III) could be detected in
samples of meat, milk or butter. (Limit of detection: 0.01 mg/kg in
each case).
Hen
Green et al. (1973) report that when diets containing 4 ppm
pirimiphos-methyl were fed to laying hens for 28 days, or when a diet
containing 32 ppm was fed for 7 days, residues of pirimiphos-methyl
itself in eggs did not exceed 0.01 mg/kg and no residues of compound
(III) were detected (Limit of detection: 0.001 mg/kg). The same
authors using radio-labelled pirimiphos-methyl found that the total
amount of radio-activity in eggs reached a maximum of 0.03 mg/kg
pirimiphos-methyl equivalents after 15 days when fed at a
concentration of 4 ppm in the ration and a maximum of 0.15 mg/kg
pirimiphos-methyl equivalents at the end of the 32 ppm study. In
muscle samples taken at the end of the 32 ppm study no residues of
pirimiphos-methyl or of its P=0 analogue were detected, (Limit of
detection: 0.001 ppm). Since 86-90% of the radio-activity in eggs is
present as water-soluble unidentified metabolites and since at no time
does the concentration of pirimiphos-methyl itself in yolks or whites
exceed 0.001 mg/kg, Green et al. conclude that pirimiphos-methyl does
not accumulate in eggs during continuous feeding of the insecticide
and that at the proposed level of application the residues of the
pesticide in eggs are negligible.
Graham and Jenkins (1974), as part of a study of the effect of
pirimiphos-methyl on the growth rate of broiler chickens, analysed
muscle, skin and fat samples from birds receiving 4, 8, 16, 32 and 48
ppm pirimiphos-methyl continuously in rations for 9 weeks. Birds were
slaughtered within 1 hour of being removed from feed. The only
residues which appeared were detected in skin and fat. The highest
level, 0.017 mg/kg, was in the fat of a broiler receiving 48 ppm
pirimiphos-methyl in the ration. Another had 0.015 mg/kg. All other
samples contained less than 0.01 mg/kg or residues were not
detectable.
In soil
Pirimiphos-methyl shows very limited persistence and mobility in
soil.
Bowker, Riley and Gratton (1972) showed that in a range of soil
types the half-life of pirimiphos-methyl is less than one month. The
major soil metabolite is the parent hydroxypyrimidine (IV) together
with smaller amounts of the related compounds (V) and (VI). The routes
of metabolism in stored grain, plants and soil are therefore similar.
The same authors using a descending thick-layer chromatography
technique, showed pirimiphos-methyl to possess only limited mobility
in soil: considerably less than atrazine which was used as a standard
and which itself is recognised as being only moderately mobile.
In water
Pirimiphos-methyl in rapidly degraded in water, mainly by
hydrolysis with loss of the phosphorothionate ester side chain. This
process is accelerated in the presence of light. Bowker and Hughes
(1974b) confirmed this and showed that although some volatilisation
occurs from still water, this is less significant than hydrolysis in
accounting for loss of the insecticide. In their experiments 50%
degradation had occurred after one day in sunlight. The major
degradation product in water is compound (IV). Only trace quantities
of the P=0 analogue (III) were detected (less than 0.01 mg/l in water
treated initially with pirimiphos-methyl at 1 mg/l).
In processing
Wheat
Bullock (1973 and 1974) reported many separate experiments which
demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table
10 summarises the results of residue trials carried out in the U.K. on
wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 11 summarises results of a residue trial reported by
Bullock (1973) to have been carried out in the U.K. with wheat
nominally treated to contain 8 ppm pirimiphos-methyl. These data which
are substantially in agreement with those of Bengston et al. (1974)
show that there is relatively little penetration beyond the seed coat
even throughout a storage period of 9 weeks.
TABLE 10. Effect of milling and baking on residues in wheat admixed
with pirimiphos-methyl at 4 mg/kg-UK (Bullock, 1973, 1974)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
Whole 0 4.2 1.9 2.9 (9)
grain 3.0* -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Wholemeal 0 1.3 0.94 1.1 (3)
flour
1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White 0 0.88 0.30 0.52 (6)
flour 0.56* 0.53* 0.55* (3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27* (3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread
1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
TABLE 10. (Cont'd.)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
White 0 0.28 0.19 0.23 (6)
bread 0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 All results are from field trials except those marked*
which are from a small-scale trial. Figures in parentheses are
the numbers of results upon which the means are based.
TABLE 11. Residues of pirimiphos-methyl in whole grains and in milling
and baking fractions of whole grains treated in a laboratory
trial in UK at 8 mg/kg. (Bullock, 1973, 1974)
Interval between Residues, mg/kg.1
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52 - -
6.0 0.91 0.56 - -
6.0 1.0 0.57 - -
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 In all cases, no residues of the phosphorus-containing compounds (II)
or (III) (Figure 1) were detected. (Limit of detection: 0.01 ppm in
each case.)
Bengston et al. (1974) report an extensive series of trials in
which pirimiphos-methyl was applied to wheat at the rate of 6 mg/kg in
bulk silos in two areas of Australia. Wheat was submitted to two
separate laboratories, for analysis and for processing, milling and
baking. The results reported by the two independent laboratories are
summarised in Table 12. These studies revealed that though a
significant amount of the pirimiphos-methyl was removed during the
cleaning process prior to the milling of the wheat, by far the
greatest separation occurred in the bran. Although the bran
represented only 25% of the whole grain it contained just over two
thirds of the entire residue leading to a concentration in the bran of
250% of that in the whole grain from which the bran was derived. The
concentration in the flour after milling averaged only 22% of that in
the whole grain. When the grain was processed into bread and baked
there was a further 63% reduction in the level of residues according
to one co-operating laboratory and an almost complete destruction of
the insecticide reported by the other. Assuming that the amount of
residue found in the bread would always be as high as the highest
level reported (0.3 mg/kg) the processing, milling and baking of bread
from raw grain containing pirimiphos-methyl results in a 93%
destruction of the original residue. Under practical conditions this
could well be greater because there is normally a significant delay at
each stage of the process which would further promote degradation of
the residue.
Rice
Residues of pirimiphos-methyl and its degradation products in
rice in husk are concentrated in the husk. Bowker (1973) and Bullock
(1973, 1974) report that parboiling rice to remove the husk results in
a substantial loss of the insecticide and its degradation products
from the husk into the water used to soak the rice. Table 13, taken
from Bullock (1973), shows the results of experiments in Guyana
designed to determine the lose of residues during the processing of
rice which had been treated previously while in husk at a nominal rate
of 5 and 10 mg/kg with pirimiphos-methyl. These results clearly
demonstrate that the bulk of the residue is in the husk with the
remainder in the seed coat. Polishing removes virtually all of the
material deposited in the seed coat although the process of parboiling
appears to promote the transfer of a small amount of residue into the
grain itself.
TABLE 12. Fate of pirimiphos-methyl residues in wheat treated at 6 mg/kg and subjected to
milling and baking (Bengston et al., 1974).
Residues, mg/kg,
Residues, mg/kg, Wheat W Wheat M
Laboratory A Laboratory B Laboratory B
Stage of Processing 1 2 Mean 1 2 Mean 1 2 Mean
Whole grain 4.7 4.8 4.8 4.2 4.2 4.2 4.5 4.5 4.5
Cleaned grain 3.8 3.8 3.8 - - - - - -
Bran 10.0 9.9 10.0 8.9 9.3 9.1 11.9 12.7 12.4
Shorts (pollard) 7.4 7.7 7.6 8.2 8.5 8.3 10.1 10.6 10.4
Flour 0.85 0.88 0.9 0.6 0.8 0.7 1.3 1.3 1.3
Bread 0.31 0.32 0.3 <0.2 <0.2 <0.2 0.46 0.48 0.47
TABLE 13. Effect of milling and polishing on residues in rice admixed with
pirimiphos-methyl (5 or 10 mg/kg) while in husk - Guyana
(Bullock, 1973).
Residues, mg/kg.
Treated at 5 mg/kg Treated at 10 mg/kg
Not Not
Sample parboiled Parboiled parboiled Parboiled
Dehusked grain 0.55 0.58 0.72 1.46
Husks 8.7 2.1 9.4 4.95
Grain polished 1 min. 0.26 0.31 0.29 0.76
Polishings from 1st min. 5.9 6.3 9.0 14.0
Grain polished 2 min. 0.19 0.29 0.29 0.76
Polishings from 2nd min. 5.6 6.5 9.4 15.8
Grain polished 5 mins. <0.01 0.28 0.27 0.65
Polishings from 3rd to 5th mins. 5.3 7.1 9.3 14.9
Bullock (1973) reports experiments carried out in Indonesia to
determine the loss of pirimiphos-methyl residues from polished rice
during cooking. Rice which had been treated by admixture with
pirimiphos-methyl emulsifiable solution and which contained 1.96 mg/kg
pirimiphos-methyl was used in the trial. The rice was washed under the
cold water tap for two minutes before simmering for 20 minutes in 2.5
times its own weight of tap water. The rice by this time was a soft
mass. Cold tap water was poured over the grains to separate them and
the samples were then analysed in the usual manner. A second sample of
the same batch of rice was treated in the same way and duplicate
analyses were carried out on each sample. The results which are given
below show a 74% loss in cooking white rice.
Residue, mg/kg, before cooking: 1.96; 1.96
after cooking: 0.58, 0.50 (mean 0.54); 0.50, 0.47
(mean 0.49).
No residues of the phosphorus-containing compounds (II) or (III)
were detected (limit of detection: 0.01 ppm in both cases).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
Two methods of residue analysis are available. The more
sensitive, quicker and preferred method is that of Bullock (1972) in
which gas chromatography with a phosphorus detector is used. The
method is suitable for the determination of residues of
pirimiphos-methyl in crops, it also determines the
phosphorus-containing metabolites (II) and (III). The limit of
determination is 0.01 mg/kg in each case. Crop samples are extracted
with a 20% solution of acetone in n-hexane. A decanted portion of the
extract is washed with water, and then dried with anhydrous sodium
sulphate. A portion of the extract is then injected into a gas
chromatograph equipped with a flame photometric or thermionic
detector. The peaks obtained at the retention times of
pirimiphos-methyl and its metabolites (II) and (III) are compared with
standards. Grain samples are extracted with methanol instead of
acetone/hexane and oily or fatty materials are subjected to an
acetonitrile-n-hexane partition before analysis. The oxygen analogue
(III) has not been detected as a metabolite in any samples analysed in
the laboratory of the manufacturer, although (as stated above) its
limit of determination is 0.01 mg/kg.
Alternatively when GLC is not available, a colorimetric method
may be used. The colorimetric method reported by Bullock (1969) has
only been tried on field crop samples. An extract, obtained by
prolonged maceration or slurrying the sample with n-hexane, is dried
with anhydrous sodium sulphate, filtered, concentrated down to a small
volume in a rotary evaporator at low temperature and quantitatively
transferred to a preparative-layer plate together with a marker spot
of pirimiphos-methyl. The plate is developed in a mixture of n-hexane
and acetone and inspected under ultra-violet light. The band opposite
the marker spot is scraped off and refluxed for one hour with
concentrated hydrobromic acid to liberate hydrogen sulphide, which is
swept off in a stream of nitrogen, quantitatively absorbed in alkaline
zinc acetate and subsequently determined as methylene blue. The limit
of determination is normally about 0.1 mg/kg with recovery in excess
of 75%.
Bullock (1973) reports studies to determine the most effective
solvent for extracting pirimiphos-methyl and its metabolites from
"aged" residues in stored grain. Table 14 shows the results of these
studies indicating the residues of pirimiphos-methyl extractable from
aged samples by different extraction methods. The samples were ground
to a fine powder before analysis. The lowest recoveries are almost
invariably with methanol under reflux. Comparable results are obtained
with the other solvent systems when extraction is made immediately
after treatment, but on aged samples cold methanol generally gives
significantly higher results.
TABLE 14. Residues of pirimiphos-methyl extractable from "aged" samples by different
extraction methods. (Samples ground to fine powder before analysis.)
Residues determined (mg/kg) using various
Sample** Interval after extraction methods*
(Treatment rate) treatment 1 2 3 4 5
Wheat (4 mg/kg) 0 days 3.72 3.45 3.82 3.87 2.91
1 month 2.92 2.73 2.79 3.01 3.17
3 months 1.87 1.73 1.99 2.17 2.04
Barley (4 mg/kg) 0 days 3.07 3.12 3.49 3.09 1.90
1 month 2.51 2.36 2.17 2.69 2.54
3 months 1.53 1.71 1.47 1.84 1.70
Rice (4 mg/kg) 0 days 4.10 3.92 4.00 4.07 3.12
1 month 2.18 2.40 2.12 2.71 2.60
3 months 1.41 1.95 1.84 1.99 1.03
* Extraction methods: 1 Dichloromethane (cold); 2 Acetone (cold); 3 20% Acetone
in hexane (cold); 4 Methanol (cold); 5 Methanol (1 hour reflux).
** The samples had a moisture content of 13-14%.
Bullock also reports studies which show that it is preferable to
grind aged samples before attempting to extract with either methanol
or acetone/hexane. Grinding offers no advantage when dealing with
wheat or rice that has only just been treated but in view of the fact
that many regulatory analysts will receive samples which have been
treated three months to three years previously it is obvious that the
preparation of the sample and the selection of the solvent are of
critical importance. Table 15 shows results obtained by Bullock. The
samples were ground in a Moulinex coffee grinder. Later comparison
with Hobart laboratory grinder showed no significant difference in the
results and little temperature rise in the grain during grinding.
TABLE 15. Effect of grinding on extractability of aged residues
(Bullock, 1973).
Sample Residues, mg/kg, determined using
(Treated by Interval different extraction solvents
admixture after Original 20% acetone Methanol
at 4 mg/kg) treatment or ground in hexane
Wheat 0 days Original 3.82 3.91
Ground 3.82 3.87
1 month Original 2.70 2.81
Ground 3.10 3.04
3 months Original 1.43 1.55
Ground 1.87 1.99
Rice 0 days Original 4.01 4.05
Ground 4.10 4.07
1 month Original 2.00 1.91
Ground 2.18 2.34
3 months Original 1.67 1.71
Ground 1.84 1.99
As a check on the loss of residues during grinding Bullock took
wheat which had been freshly treated with pirimiphos-methyl and
measured the residue level before and after intense grinding for one
minute. The analytical results varied by less than 50% which is slight
by comparison with the loss which would occur if grain treated with
malathion or dichlorvos were ground in a similar manner. The same
author reports experiments designed to determine the loss of residue
from wheat, barley and rice when grain samples were stored at -14°C
while awaiting analysis. Both freshly treated grain and grain which
had been treated under semi-commercial conditions three months prior
to sampling were kept under such storage conditions but none of these
samples showed as much as 5% loss during two months at -14°C.
NATIONAL TOLERANCES
The information available to the meeting indicates that few
national tolerances have been established. It is understood that a
tolerance of 4 mg/kg has been proposed in France for pirimiphos-methyl
residues in cereals and 2 mg/kg in fruits and vegetables. In the
Netherlands a tolerance of 0.2 mg/kg has been established for
pirimiphos-methyl residues in cucumbers, melons, paprika and tomatoes.
APPRAISAL
Pirimiphos-methyl is a broad-spectrum organophosphorus
insecticide with both contact and fumigant action. It shows only
limited biological persistence on plant surfaces but gives long
lasting control of insect pests on inert surfaces and retains its
biological activity when applied to stored agricultural commodities
including raw grains and nuts.
The meeting had only limited Information concerning the
pre-harvest use and performance of this material on crops but
extensive data were available on its use, performance and fate on a
variety of small grains and nuts. The recommended rate of application
to raw cereals is 6 mg/kg.
Exceptional stability on grain results in an effective half-life
of up to 80 weeks. Although high temperatures and high moisture levels
in grain reduce the life of the deposit the effect of these influences
is much less than with other grain protectants approved or evaluated
to date.
Data were available to show that on both wheat and rice the
deposit resulting from admixture of pirimiphos-methyl with grain is
almost entirely in the seed coat and husk respectively. Cleaning,
processing and milling removes the bulk of the applied material. In
the case of wheat the milling process results in most of the residue
being separated into the bran where the concentration of
pirimiphos-methyl can range up to 20 mg/kg. In the case of rice
substantially all of the deposit is removed during the milling and
polishing operations. Depending upon the milling process, flour
prepared from treated wheat may contain significant amounts of
pirimiphos-methyl but a major proportion of this is destroyed in the
baking of bread. The small amount of residue remaining in polished
rice is substantially destroyed in cooking.
Pirimiphos-methyl and the corresponding hydroxypirimidine are the
only significant residues detected. Residues of the
phosphorus-containing metabolites when detected were always extremely
low (approximately 0.05 mg/kg).
Extensive data are available on the fate of pirimiphos-methyl in
plants, domestic animals, hens and their edible tissues, and foods of
animal origin. In living plants the major phosphorus-containing
degradation product is the N-desethyl metabolite (II) which is not
accumulated. The hydroxypyrimidine compound (IV) is the major
degradation product following root uptake. When fed to domestic
animals pirimiphos-methyl is rapidly metabolised and excreted, only
very small quantities being secreted into milk or eggs. Cattle
receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites could
be detected in meat, milk, butter or eggs.
A GLC method of analysis suitable for the determination of
residues of pirimiphos-methyl and the phosphorus-containing
metabolites in plant materials is available, the limit of
determination being reported to be 0.01 mg/kg. The method appears
suitable for regulatory purposes. Evidence of the importance of sample
preparation and method of extraction has been presented.
No national tolerances have yet been determined.
In proposing maximum residue limits for pirimiphos-methyl and its
metabolites on raw grain, milled cereal products and foods prepared
therefrom, careful consideration has been given to the fact that this
insecticide is to be used as a grain protectant, that a certain
concentration must be present on the grain to control infestations and
prevent damage to stored products and that the compound is
exceptionally stable under storage conditions. Under practical
conditions of grain handling and storage it is not possible to apply a
grain protectant with assurance that the deposit will be absolutely
uniform. There will always be a natural variation in the level of the
deposit resulting from fluctuations in the flow of grain and
insecticide. It is therefore not possible to fix the maximum limit on
the minimum necessary to control the insect pests. Furthermore
extensive work has demonstrated that the movement of bulk grain
results in the segregation of the various components of the bulk with
a resultant variation in the level of residues throughout the mass.
Due allowance must be made for the amplitude of these variations and
the problems of sampling and analysis.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl, expressed as pirimiphos-methyl.
TEMPORARY TOLERANCE
mg/kg
Bran (wheat, rice) 20
Wheat, rye, rice (in husk) 10
Barley, maize, oats 7
Wholemeal flour (wheat, rye) 5
Rice (dehusked), wheat flour (white) 2
Bread (wholemeal), rice (polished) 1
Bread (white) 0.5
Meat, milk, eggs 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 1976)
1. Additional studies to clearly establish no-effect level for
decreased pregnancy rates, and hydronephrosis in offspring.
2. Further studies to clarify the liver injury dogs observed in 90
day studies.
DESIRABLE
1. Longer duration observations in man.
2. Information from studies now in progress on other stored
commodities including nuts, peanuts and dried fruit. The
information is expected to be available during 1975.
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in food at
the point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
REFERENCES
Bengston, M., Connell, M., Desmarchelier, J., Phillips, M., Snelson,
J. and Sticka, R. (1974) Evaluation of four new grain protectants.
Report to Australian Wheat Board. (To be published)
Bowker, D.M. (1973) Pirimiphos-methyl (PP 511): fate of stored wheat
and rice grain in the laboratory. ICI Plant Protection Ltd. Report No.
AR 2457 AR. (Unpublished)
Bowker, D.M., Griggs, B.F. and Harper, P. (1973) Pirimiphos-methyl (PP
511): excretion by a goat. ICI Plant Protection Ltd. Report No. AR
2458 B. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in crops.
ICI Plant Protection Ltd. Report No. AR 2515 A. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in water.
ICI Plant Protection Ltd. Report No. AR 2516 A. (Unpublished)
Bowker, D.M., Riley, D. and Gratton, R.P. (1972) Pirimiphos-methyl:
fate in soil. ICI Plant Protection Ltd. Report No. TMJ 809 A.
(Unpublished)
Bratt, H. and Dudley, L.A. (1970) Pirimiphos-methyl (PP 511):
Excretion by rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bratt, H. and Jones, L.A. (1973) Pirimiphos-methyl (PP 511):
Metabolism in rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bullock, D.J.W. (1969) Provisional analytical method for the
determination of PP211 and PP511 residues in vegetables, grain and
fruit. ICI Plant Protection Ltd. Method No. PAM 310/A.
Bullock, D.J.W. (1972) Residue analytical method no. 11 for the
determination of residues of pirimiphos-methyl and its
phosphorus-containing metabolites in crops (gas chromatographic
method). ICI Plant Protection Ltd. Method PPRAM-11/A.
Bullock, D.J.W. (1973) Pirimiphos-methyl: residues in stored grain.
ICI Plant Protection Ltd. Report No. AR 2472 AR. (Unpublished)
Bullock, D.J.W. (1974) Pirimiphos-methyl: residues in stored grain
bread, flour and milled products. ICI Plant Protection Limited. Report
No. AR 2537 A. (Unpublished)
Bullock, D.J.W., Day, S., Hemingway, R.J. and Jegatheeswaran, T.
(1974) Pirimiphos-methyl: residue transfer study with cows. Report
from ICI Plant Protection Limited. (Unpublished)
Bullock, D.J.W., Day, S., and Griggs, B.F. (1974) Pirimiphos-methyl:
metabolism and residue transfer into the meat and milk of a lactating
cow. ICI Plant Protection Ltd. Report No. AR 2552 A. (Unpublished)
Chart, S., Foulkes, Caroline A., Gore, G.W. and Williamson, K.S.
(1974) Erythrocyte and plasma cholinesterase activity in human
volunteers administered pirimiphos-methyl. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Clapp, M.J. and Conning, D.M. (1970) Pirimiphos-methyl (PP511).
Ninety-day oral toxicity in rats. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Clark, D.G. (1970) The toxicity of PP511
[O-(2-diethylamino-6-methylpyrimidin-4-yl)
O,O-dimethylphosphorothioate] Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fink, R. (1974a) Eight-day dietary LC50 - mallard ducks: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Fink, R. (1974b) Eight-day dietary LC50 - bobwhite quail: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Gage, J.C. (1971a) Pirimiphos-methyl (PP511): Avian toxicity. Report
from ICI Industrial Hygiene Research Laboratories. (Unpublished)
Gage, J.C. (1971b) Pirimiphos-methyl (PP 211) and pirimiphos-methyl
(PP 511): acute and subacute oral toxicity in the rat of the plant
metabolite, 2-diethylamino-4-hydroxy-6-methylpyrimidine (R46382). ICI
Industrial Hygiene Research Laboratory. Report No. HO/IH/R/327.
(Unpublished)
Gage, J.C. (1972) Pirimiphos-methyl (PP511): Oral toxicity in the dog
and in a passerine bird species. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Gore, C.W. Palmer, S. and Pratt, I.S. (1974) Pirimiphos-methyl
(PP511): teratogenic studies in the rabbit. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Gore, C.W., Griffiths, D. and Phillips, C.E. (1974) Pirimiphos-methyl
(PP511): two year feeding study in the rat. Report from ICI Industrial
Hygiene Research Laboratories. (Unpublished)
Graham, C.A. and Jenkins, S.L. (1974) The effect of pirimiphos-methyl
on the growth rate of broiler chicks. ICI Australia Ltd. Merrindale
Research Station Report, July 1974.
Green, T., Monks, I.H. and Phillips, P.J. (1973) Pirimiphos-methyl (PP
511): sub-acute oral and residue studies in hens. ICI Industrial
Hygiene Research Laboratories. Report No. HO/IH/P/65B. (Unpublished)
Hodge, M.C.E. and Moore, S. (1972) Pirimiphos-methyl (PP 511):
Teratological studies in the rat. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Noel, P.R.B., Mawdesley-Thomas, L.E., Rivett, K.F., Squires, P.F. and
Street, E. (1970) PP 511: Oral toxicity studies in dogs - initial
studies and repeated dosage for thirteen weeks. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and Cherry, C.P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat: histopathological
examination on testes of F1B and F2B males. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and James, P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat. Report from Huntingdon
Research Centre. (Unpublished)
Parkinson, G.R. (1972a) Pirimiphos-methyl (PP 511): potentiation with
gamma-BHC. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Parkinson, G.R. (1972b) Pirimiphos-methyl (PP 511): potentiation with
dichlorvos. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Pieterse, A.H., Schulten, G.G.H. and Kuyken, W. (1972) A study of
insecticide resistance in Tribolium castaneum in Malawi. J. Stored
Prod. Res., 8(3):183.
Rivett, K.F., Edwards, B., Street, E. and Newman, A.J. (1973) PP 511:
Oral toxicity study in beagle dogs - repeated daily dosage for two
years. Report from Huntingdon Research Centre. (Unpublished)
Ross, D.B., Burroughs, Sheila J. and Roberts, Nicholas L. (1974) Egg
production and hatch-ability in the laying hen following dietary
inclusion of pirimiphos-methyl at various levels. Report from
Huntingdon Research Centre. (Unpublished)
Rowlands, D.G. (1966) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. Stored Prod. Res., 2:105-116.
Rowlands, D.G. et al. (1974) Preliminary report of studies with
pirimiphos-methyl as a grain protectant. Submission by United Kingdom
to FAO. (To be published)
Waterhouse, D. (1973) Background paper presented at the Ninth Session
of the FAO Working Party of Experts on Pest Resistance to Pesticides
(Rome, 20 June 1973). (Unpublished)
See Also:
Toxicological Abbreviations
Pirimiphos-methyl (Pesticide residues in food: 1976 evaluations)
Pirimiphos-methyl (Pesticide residues in food: 1977 evaluations)
Pirimiphos-methyl (Pesticide residues in food: 1979 evaluations)
Pirimiphos-methyl (Pesticide residues in food: 1983 evaluations)
Pirimiphos-methyl (Pesticide residues in food: 1992 evaluations Part II Toxicology)
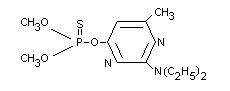 Other Information on Identity and Properties
Pirimiphos-methyl is a pale straw coloured liquid. It melts at
15-18°C and decomposes above 100°C. It has a vapour pressure of
approximately 1 × 10-4 Torr at 30°C and a density of 1.157 g/ml at
20°C.
Pirimiphos-methyl is stable for up to six months at room
temperature. It is hydrolysed by strong acid or alkali.
Pirimiphos-methyl is miscible with most organic solvents. Its
solubility in water is approximately 5 mg/l at 30°C.
Formulated products include emulsifiable concentrates (8%, 25%
and 50% a.i.), dusts (2% a.i.) and formulations for ultra-low volume
application (10% a.i. and 50% a.i.). A diluent-free formulation and a
smoke generator formulation are under development.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 0.6 mg/kg of 2-14C-ring-labelled pirimiphos-methyl was
given to adult male rats, either orally or by intraperitoneal
injection, 73 to 81% of the dose was excreted in the urine during the
first 24 hours. This indicates rapid absorption. At the end of 120
hours after oral dosing, the entire dose could be accounted for by
excretion of radioactive products in the urine (86% of dose) or faeces
(15.2% of dose). Similar results were obtained after intraperitoneal
injection although the excretion was more rapid after oral
administration.
Two male beagle dogs given 17-18 mg/kg orally also excreted most
of the dose in the urine during the first 24 to 48 hours.
Chromatographic and autoradiographic studies of the urine of dosed
dogs and rats revealed extensive metabolism by the presence of 9
radioactive chromatogram spots (Bratt and Dudley, 1970). The
concentration of label in the abdominal fat of female rats given four
daily doses of 5 mg/kg of 14C-pirimiphos-methyl revealed that the
metabolites were stored to some extent in fat: 0.46, 0.69, 1.87 and
1.00 ppm of pirimiphos-methyl equivalents in fat after 1, 2, 3 and 4
doses, respectively (Bratt and Jones, 1973).
In a lactating goat, 91% of the label of a single dose of 0.12
mg/kg of 2-14C ring-labelled pirimiphos-methyl was excreted (87% in
urine and 4% in faeces) within 8 days. The milk contained only 0.41%
of the label, primarily during the first 24 hours. The maximum residue
in milk was 0.026 ppm pirimiphos-methyl equivalents, and the parent
insecticide accounted for only 0.003 ppm (Bowker et al., 1973).
Bullock et al. (1974a) found that the pattern of excretion of
2-14C labelled pirimiphos-methyl in the cow was similar to that in
the goat. The label from a single dose of 0.5 mg/kg was quantitatively
recovered in the urine (85%) and faeces (14%) during the following 7
days. During the first 3 days, 0.35% of the label was secreted in the
milk. The milk contained 0.04 ppm of pirimiphos-methyl equivalents of
which less than 2% was unchanged pirimiphos-methyl and
phosphorus-containing metabolites. Hydroxypyrimidine hydrolysis
products or their conjugates constituted the major part of the residue
in milk.
Hens given a single oral dose of 14C-pirimiphos-methyl excreted
more than 70% of the label within 24 hours. When fed for 28 days in
the diet, at a level of 4 ppm, pirimiphos-methyl itself never exceeded
0.01 ppm in yolks and whites of eggs although the total concentration
of metabolites reached equilibrium levels of about 0.028 ppm, mostly
as water soluble metabolites. Increasing the dietary concentration to
32 ppm for a 7-day feeding period resulted in residues of 0.007 and
0.012 ppm of pirimiphos-methyl in the whites and yolks of eggs. In
muscle, 0.31 ppm and 0.16 ppm of the label was present in hens fed 32
and 4 ppm respectively; however, the parent insecticide or its oxygen
analogue was not detected (Green et al., 1973). See also the section
"Fate of residues in animals."
Biotransformation
The metabolism of pirimiphos-methyl in rats (100 mg/kg, p.o.) and
in one dog (20 mg/kg, p.o.) was studied by thin-layer chromatographic
separation of urinary metabolites. Twelve metabolites were detected.
None of these possessed anticholinesterase activity. No parent
pirimiphos-methyl was detected in the urine. Five of the twelve
metabolites were identified by comparison with authentic samples. In
both rats and does, 2-ethylamino-4-hydroxy-6-methylpyrimidine was the
major urinary metabolite (30% of dose). The next most predominant
metabolite in the dog was
4-O(2-diethylamino-6-methylpyrimidinyl)-ß-D-glucosiduronic acid (11%
of dose), and in the rat an unidentified phosphorus-containing
product thought to be a dealkylated derivative of either
pirimiphos-methyl or its oxygen analogue (12% of dose). Other
identified urinary metabolites included
2-amino-4-hydroxy-6-methylpyrimidine,
2-[N-ethyl-N-(2-hydroxyethyl) amino]-4-hydroxy-6-methylpyrimidine,
and 2-diethylamino-4-hydroxy-6-methylpyrimidine (Bratt and Jones,
1973).
These studies indicate that the P-O-C bond of pirimiphos-methyl
is extensively cleaved and that N-dealkylation and/or conjugation are
further steps in the metabolism of the pyrimidine leaving group.
Although the oxygen analogue of pirimiphos-methyl was not detected as
a urinary metabolite, the fact that cholinesterase inhibition occurs
in vivo suggests that the oxygen analogue is also formed and may be an
intermediate step leading to the identified urinary products.
Effect on enzymes and other biochemical parameters
The only biochemical effects consistently noted in acute or
chronic toxicity tests was inhibition of cholinesterase. A group of 36
male rats were given single oral doses of 1450 mg/kg of
pirimiphos-methyl. Symptoms were noted and they were sacrificed at
intervals up to 4 days after dosing for measurements of brain, plasma,
and red-cell cholinesterase activity. Few signs were noted at 6 hours
after dosing when cholinesterase inhibition was 0, 35 and 51%
respectively for brain, red cells and plasma. Clear signs of poisoning
only became apparent by 24 hours when brain was inhibited by 46% and
red cell and plasma by 70 and 80% respectively. Recovery of
cholinesterase activity began to be apparent by 72 hours. Plasma
cholinesterase activity had completely recovered by 96 hours, but red
cell and brain remained 47 and 30% inhibited, respectively, at this
time (Clark, 1970). From these studies it appears that inhibition of
brain cholinesterase by 40% or more results in obvious signs of
toxicity.
TOXICOLOGICAL STUDIES
Special studies on potentiation
Tests for potentiation of the acute oral toxicity of combinations
of pirimiphos-methyl and gamma-BHC were conducted by simultaneous
administration of one-half LD50 doses of the two compounds to ten
fasted female rats. Three of the ten animals died during a 14-day
observation period, suggesting no potentiation. Combinations of
one-half LD50 doses of pirimiphos-methyl and dichlorovos were tested
in the same manner and no mortality resulted in ten test rats. Under
these limited test conditions, there was no evidence of potentiation
of pirimiphos-methyl with 2 other insecticides that are likely to be
used in conjunction with it (Parkinson, 1972 a,b).
Special studies on reproduction
Rat
The effect of pirimiphos-methyl on the growth and reproductive
performance of the rat was assessed by feeding groups of 24 female and
12 male rats dietary concentrations of 0, 20 and 200 ppm of the
compound throughout three generations. However, dietary analysis of
the F0 generation nominally given 20 ppm revealed concentrations of
pirimiphos-methyl far below the intended levels and so animals treated
at 20 ppm were reared to produce a fourth (F4B) generation.
There were no effects among parent animals attributable to the
feeding of the compound. Mating performance and pregnancy rates were
reduced at 200 ppm for F1B and F2B generations. At 20 ppm there was an
effect at 2nd mating of the F1B generation and at both matings of the
F2B generation. When expressed as an average over all generations,
there was a dosage related decrease in pregnancy rate. Litter
parameters, as assessed by total litter loss, litter size, litter and
mean pup weights, pup mortality and the incidence of abnormalities
showed no consistent dosage-related trends. Examination of the
ultimate generation (F3B for 200 ppm dosage group, and F4B for 20 ppm)
by organ weight analysis and skeletal staining of 10 males and 10
females from all groups, and by microscopic examination of 10 male and
10 female pups from controls and from the 200 ppm group, showed no
difference that could be conclusively related to treatment (Palmer and
James, 1972).
The testes of the F1B and F2B male rats were examined
histopathologically in an attempt to find an explanation for their
reduced mating performance. However in the testes examined, from rats
of the control and both treatment groups, the degree of activity and
maturity of the process of spermatogenesis were comparable (Palmer and
Cherry, 1972).
Special studies on skin irritation, sensitization, and effects in
the eye
Application of a 50% solution of pirimiphos-methyl in olive oil
to ears of 6 guinea pigs did not produce erythema when challenge
applications of 10%, 5% and 1% were applied to shaved skin of their
flanks 4 days later. This indicated a lack of sensitizing potential.
Undiluted pirimiphos-methyl was applied to the clipped backs of 4
female rats and the site of application covered for 24 hours with an
impermeable dressing. No signs of primary irritation were noted after
a single such application nor after a second or third application at
alternate 24-hour intervals.
One drop of undiluted pirimiphos-methyl was placed in the left
conjunctival sac of the eyes of 4 albino rabbits. No signs of
irritation or myosis was noted on frequent observation over the next 7
days (Clark, 1970).
Special studies on teratogenicity
Rat
Three groups of 18 to 21 virgin female, Alderley Park specific
pathogen-free rats were mated and fed diets containing 0, 10 or 200
ppm of pirimiphos-methyl from day 1 to day 20 of pregnancy. On day 20,
the females were killed and foetuses immediately removed by Caesarean
section. The uterus was examined for resorptions, foetuses were
examined for viability and weighed. One third of the foetuses were
eviscerated and stained with alizarin red for examination of skeletal
defects. Viscera were fixed and examined for histopathology. Maternal
rats showed no toxic signs. It was presumed that 200 ppm produced
cholinesterase inhibition but this was not measured. The only
significant difference between pirimiphos-methyl and control litters
was a reduction in mean foetal weight; however, this may have been
because there were more foetuses per litter in the experimental
groups. No increased incidence of skeletal abnormalities were observed
in foetuses from the pirimiphos-methyl treated groups. The only
significant soft-tissue abnormality was increased hydronephrosis (7,
17 and 22 foetuses, representing incidence of 6, 17 and 16%) in the
foetuses examined from dams that received 0, 10 and 200 ppm in the
diet respectively. The investigators stated that these incidences of
hydronephrosis were within normal limits for Alderley Park rats and
they conclude that there was no evidence to suggest teratogenic action
for pirimiphos-methyl (Hodge and Moore, 1972).
Rabbit
Two groups of 16 to 17 pregnant rabbits were given gelatin
capsules containing pirimiphos-methyl dissolved in corn oil at doses
of 1 mg/kg and 16 mg/kg daily throughout pregnancy. A group of 16
controls was given capsules containing corn oil only. Twenty-nine days
after mating all the foetuses were delivered by Caesarean section. The
uterus was examined for resorptions and the foetuses for viability.
One half of the foetuses from each litter were stained with Alizarin
Red for skeletal examination and the remaining half were fixed in
Bouin's solution and dissected for soft-tissue examination. Does were
examined for gross and histological pathology and for blood
cholinesterase inhibition.
Three of the experimental does died during the gestational
period, two receiving 1 mg/kg/day and one receiving 16 mg/kg/day.
These deaths appeared to be due to causes unrelated to
pirimiphos-methyl. The does gained weight normally, but at the end of
4 weeks, the group average of RBC cholinesterase was 19% and 60% less
than controls in the 1 and 16 mg/kg dosage groups respectively. Data
on observations of foetuses were reported for 10, 10 and 11 litters
where implantations occurred in the 0, 1 and 16 mg/kg dosage groups
respectively. One grossly abnormal foetus was observed in a litter
from one doe receiving 16 mg/kg/day. No abnormalities were seen in the
remaining ten foetuses. There was no increase in resorptions in
experimental groups. Although the foetal weights of the 1 and 16 mg/kg
dosage groups were less than controls, there were more foetuses/litter
in these groups so that mean litter weights among the three groups did
not differ. The ratios of male/female foetuses were 1.0, 1.25 and 1.39
for the 0, 1 mg/kg and 16 mg/kg dosage groups. The authors state that
these are "within normal control limits." The only statistically
significant difference in the occurrence of abnormalities was a higher
incidence of 14 caudal vertebrae in the pirimiphos-methyl groups,
which the investigators state were isolated findings. This study
indicates no teratogenic action of pirimiphos-methyl in rabbits at
dosages which produce considerable inhibition of blood cholinesterase
in maternal animals (Gore et al., 1974a).
Acute toxicity
Pirimiphos-methyl, 90-94% purity, was administered to animals
undiluted or, for small doses, in propylene glycol. Single-dose LD50
values or estimates of LD50's by various routes are shown in Table
1. In the single-dose dermal test the skin of rats was washed with
soap and water 24 hours following application. All animals were
observed for 14 days or longer.
TABLE 1. Acute toxicity of pirimiphos-methyl
Animal Sex Route mg/kg bw References
Rat F Oral 2050 (1840-2260) Clark, 1970
Rat F ip approx. or equal to 800 Clark, 1970
Rat M ip approx. or equal to 800 Clark, 1970
Rat F dermal >2000 Clark, 1970
Mouse M Oral 1180 (1030-1360) Clark, 1970
Guinea Pig F Oral 1000-2000 Clark, 1970
Rabbit M Oral 1150-2300 Clark, 1970
Cat F Oral 575-1150 Clark, 1970
Dog M Oral >1500 Gage, 1972
Hen F Oral 30-60 Clark, 1970
Quail F Oral approx. or equal to 140 Gage, 1971a
Green Finch Oral 200-400 Gage, 1972
Figures in parentheses are 95% confidence limits.
The toxic signs exhibited after single doses were typical of
cholinesterase inhibition. In female rats given single oral doses,
only mild to moderate signs were noted at 12 hours after dosing, but
marked signs consisting of incontinence, salivation,
chromolacrymation, fibrillations, fasciculations and prostration were
present at 24 hours and persisted up to 11 days. No additional toxic
signs were noted during a 12 week observation period. Six surviving
hens of 8 dosed with 31 mg/kg were observed for 28 days after dosing
and no abnormal gait or other neurological signs were observed after
the initial acute toxic effects had subsided (within 7 days) (Clark,
1970).
2-Diethylamino-4-hydroxy-6-methylpyrimidine is a plant metabolite
of pirimiphos-methyl. It is also formed metabolically in rats and
dogs. Single-dose, range-finding acute toxicity tests (3 rats per
dose) indicated the acute oral LD50 in rats to be between 800 and
1600 mg/kg (Gage, 1971b). This is of the same order of acute toxicity
as the parent insecticide, but no description of toxic signs was
provided. Groups of 10 male and 10 female rats were given 100 and 400
mg/kg of the metabolite, orally, five days a week for two weeks. No
toxic signs, altered body weights or gross or histopathology were
noted. There was an approximate 25% increase in reticulocytes and a
reduction in total leucocytes which could be accounted for by a 30 to
40% reduction in lymphocytes, particularly in females, which the
investigators attribute to a "stress response" (Gage, 1971b).
Atropine sulphate and a mixture of atropine plus
pyridine-2-aldoxime methane-sulphonate partially counteracted the
acute oral toxicity of pirimiphos-ethyl in female rats when the
antidotes were given at intervals at which toxic signs appeared. The
combination of antidotes was more effective than atropine alone
(Clark, 1970).
Short-term studies
Rat (feeding)
Repeated daily oral dosing of 10 male and 10 female rats with 200
mg/kg of pirimiphos-methyl, 5 days a week for 2 weeks produced mild
signs of poisoning which were first noted after the 7th dose. The
signs did not progress in intensity with continued dosing; however,
body weight gain over this period was less than half that of controls.
An approximate 9% reduction of haemoglobin levels accompanied by
reticulocytosis was observed in the treated rats. Gross or
histopathology was not striking but slight to moderate haemopoesis of
the spleen was observed. When the experiment was repeated at a dose of
400 mg/kg, signs of poisoning appeared after 2 doses and increased in
severity as the experiment progressed. Males were more affected than
females and deaths occurred from the 4th day in males and from the 6th
in females. Cumulative mortality after 10 doses was 9 of 10 male rats
and 3 of 10 females (Clark, 1970).
Four groups of 25 male and 25 female Alderley Park specific
pathogen-free adult rats were maintained for 90 days on diets
containing 0, 8, 80 and 360 ppm of pirimiphos-methyl. Haematology and
plasma and erythrocyte cholinesterase activities were examined on
individual samples from five male and five female rats in each group
before, during and at the end of the feeding period. Brain
cholinesterase activities and organ:body weight ratios were calculated
for five males and five females in each group at the end of the
feeding period. Gross and histopathological examinations were
conducted. Body weight gain in the female rats fed diets containing 80
and 360 ppm pirimiphos-methyl averaged 18% and 21% less than controls.
This was apparently due to reduced food utilization as food
consumption did not differ significantly from controls. Plasma
cholinesterase was inhibited 65% in males and 80% in females fed 360
ppm and 40% in males and 60% in females fed 80 ppm, but no inhibition
was detected at 8 ppm. Erythrocyte cholinesterase was inhibited 20% in
males and 50% in females only at the highest dietary level. Both
plasma and erythrocyte cholinesterase returned to normal within one
week after cessation of dosage. Terminal measurements of brain
cholinesterase activity revealed inhibition in rats fed 80 and 360 ppm
with females affected most (about 40% inhibition in the 360 ppm
group). There was no inhibition in the 8 ppm group. Brain
cholinesterase was still inhibited at 4 weeks after cessation of
feeding pirimiphos-methyl. Haematological examinations revealed no
significant effects on haemoglobin concentration, packed cell volumes,
mean corpuscular diameter, white cell or platelet counts or clotting
function. There were no increases in gross or histopathological
lesions in the pirimiphos-methyl-fed rats over controls (Clapp and
Conning, 1970).
Rat (inhalation)
Four male and 4 female rats exposed by inhalation to a nominal
vapour-aerosol concentration of 3.5 ppm, 6 hours/day, 5 days/week for
3 weeks did not result in toxic signs nor reduction in body weight nor
decreased cholinesterase activities. An increase in alveolar
macrophages and slight lymphoid hyperplasia was noted (Clark, 1970).
Rabbit (dermal)
Daily application of 1000 mg/kg of pirimiphos-methyl to the
dorsal skin of 6 rabbits for 14 days (application site was washed 1
hour before each successive dose) resulted in fibrillations after 7 to
12 applications. Approximately 15% loss of body weight occurred and
one rabbit died after 14 applications (Clerk, 1970).
Avian species
Mallard ducklings and bobwhite quail chicks were fed
pirimiphos-methyl in their diets for five days and observed for an
additional 3 days. LC50 values were 633 and 207 ppm for ducklings and
quail chicks respectively (Fink, 1974a,b). Pirimiphos-methyl in the
diet of broiler chicks at levels from 4 to 48 ppm did not reduce feed
efficiencies or growth rate, except for a temporary period at the
beginning of the study (Graham and Jenkins, 1974). When 0, 4, 12 and
40 ppm were fed in the diet of laying hens for 28 days there was no
adverse effect on the hens. Egg production and egg quality were
considered normal although there was a small, dose-related increase in
the number of "small" eggs. There was some reduction in the number of
chicks hatched from eggs from the pirimiphos-methyl fed hens which was
not significant, although there were significantly greater numbers of
chicks dead in shell at the 12 and 40 ppm dietary levels (Ross et al.,
1974).
Dog
Groups of four male and four female beagle dogs were dosed orally
seven days a week for 13 weeks by administration of gelatin capsules
containing corn oil solutions of pirimiphos-methyl. The dosage rates
were 0, 2, 10 and 25 mg/kg given as single daily doses about 1 hour
after feeding the daily ration of complete dry diet. Clinical signs,
body weight, food and water consumption, electrocardiograms and
ophthalmic examinations were included among the observations.
Laboratory investigations included plasma and red cell cholinesterase
haematology, urinalysis, serum alkaline phosphate (SAP), serum
glutamic-pyruvic transaminase (SGPT) plasma urea and blood glucose. At
the end of the dosing period, two dogs of each sex in each dosage
group were sacrificed and the remaining dogs observed for an
additional four weeks before sacrifice. Gross and histopathological
examinations and brain cholinesterase assays were performed. There
were no deaths. Clinical symptoms were minimal with occasional
episodes of vomiting and watery stools. Body weight gain was
significantly reduced in both males and females given 25 mg/kg/day and
in females given 10 mg/kg/day. The rate of weight gain also tended to
be less for females given 2 mg/kg/day but this was of borderline
significance. At the highest dosage level (25 mg/kg) only, there was
reduced food consumption and a slower heart rate. Plasma and
erythrocyte cholinesterase activities were significantly depressed
(20% or more) at all three dosage levels. Plasma cholinesterase
rapidly returned to normal upon cessation of dosing but reversal of
red cell inhibition was delayed. At the lowest dosage level,
significant inhibition of red cell cholinesterase was only observed
during the latter weeks of the dosing period. Terminal brain
cholinesterase assays revealed no inhibition in any of the dosage
groups. Two dogs receiving 25 mg/kg showed very high SGPT and SAP
levels after three months dosing, while a third showed a less marked
SGPT increase. Post-mortem examination showed evidence of liver damage
in these animals. All other biochemical findings were within normal
limits. Histopathological examination post-mortem showed some bile
duct proliferation in one dog receiving 10 mg/kg and bile duct
proliferation together with portal cirrhosis in one dog receiving 25
mg/kg. After four weeks observation following the cessation of dosing,
two other dogs receiving 25 mg/kg showed minimal degrees of bile duct
proliferation. No other histopathological findings or other consistent
effects were noted (Noel et al., 1970).
Four groups each consisting of 4 male and 4 female beagle dogs
were orally dosed once daily, seven days/week, for two years with
gelatin capsules containing corn oil solutions of pirimiphos-methyl at
dosage rates of 0, 0.5, 2 or 10 mg/kg/day. Brain cholinesterase
activities at the end of the dosing period were 81, 78 and 44% of
control in the 0.5, 2 and 10 mg/kg groups respectively. The mean
activities in the 0.5 and 2 mg/kg groups were significantly less than
values for controls at the 1% level of probability, and in the 10
mg/kg group, brain cholinesterase depression was significant at the
0.1% level. Red cell cholinesterase was significantly inhibited with
respect to pre-dosing values, in both the 2 and 10 mg/kg groups from
the 8th week of dosing, but only occasionally inhibited in the 0.5
mg/kg group. Plasma cholinesterase was inhibited significantly
(30-50%) in all 3 groups from the 16th week of dosing. There was also
a decline in plasma cholinesterase activity in controls during the
experimental period. However, even with correction for this, 20-25%
depression was noted in the 0.5 mg/kg group, with only slightly
greater depressions with the higher dosages. It appears that plasma
cholinesterase inhibition did not discriminate well among the 3
experimental groups. One female dog in the 10 mg/kg group died after
the 400th day after showing few clinical signs. Clinical signs
consisting of loose stools were observed, in a dose-related frequency
in excess of controls, only in the 2 highest dosage groups. Vomiting
was infrequently noted. Dogs in the 10 mg/kg group showed some loss of
appetite and a reduced rate of weight gain. The 0.5 mg/kg/day dosage
rate appears to be a no effect level for clinical signs. No
abnormalities of the eyes, examined by ophthalmoscopy nor in
electrocardiogram records were observed. No appreciable or consistent
effects were noted in any of the dosage groups with respect to
haematology, standard urinalysis values or blood urea, blood glucose,
serum protein, SAP, SGPT, NA+ or K+ values. No consistent or
dose-related gross or histopathological changes were observed in post
mortem studies. However, a statistically significant increase in liver
weights and liver body weight ratios was seen in the group given 10
mg/kg/day (Rivett et al., 1973).
Long-term studies
Rat
Four groups of 48 male and 48 female rats were fed
pyrimiphos-methyl in their diet at levels of 0, 10, 50 and 300 ppm for
two years. Twenty-four additional animals of each sex were included in
each dosage group, and were killed at intervals up to one year to
provide interim data on brain cholinesterase activity and clotting
function. Eight animals of each sex in each of the dosage groups fed
for two years were continued on a control diet for 4 to 8 weeks beyond
the two year feeding period to allow for observation of recovery from
any treatment-induced effects.
Rats fed 300 ppm pirimiphos-methyl showed marked plasma
cholinesterase inhibition (50-80%) and some inhibition of erythrocyte
and brain cholinesterase activity (30-40%). The only other adverse
effect noted at this dosage rate was a slight anaemia in female rats.
Only female rat plasma cholinesterase was consistently inhibited
(50-65%) at the 50 ppm dietary level. Recovery from inhibited
cholinesterase, when it occurred, was usually complete by the end of
an 8-week period on the control diet. Slight (generally <25%)
transient depression of plasma cholinesterase activity in female rats
fed 10 ppm was observed. Erythrocyte and brain cholinesterases were
not depressed at this dietary level. Although cumulative mortality was
greater than for controls in male rats fed 300 ppm at 54, 60, 66 and
72 weeks of feeding, thereafter the cumulative mortalities of all
groups were approximately equal. Body weight gains, weights of major
organs and incidence of gross or microscopic pathology (including
tumors), in a comprehensive list of tissues, did not differ between
the control and experimental groups.
The results of this study indicate that 10 ppm in the diet of
rats is an experimental no-adverse-effect level (Gore et al., 1974b).
OBSERVATIONS IN MAN
A dose of 0.25 mg/kg/day of pirimiphos-methyl (97.8% purity) was
taken orally for 28 days by five healthy male volunteers (25-45 years
of age; 59.7-73 kg body-weight). Blood samples were taken before
dosing began, on days 1, 3, 7, 14, 21 and 28 during administration,
and 7 and 14 days after cessation of dosing. Only one subject had a
plasma cholinesterase depression (on day 28) which exceeded 20%
(21.5%). Otherwise variations, both above and below pre-dosing values
were within 12%. Four of the five subjects had red cell cholinesterase
values that were slightly below either of the pre-exposure values
during the last 2 weeks of the study. However, the group means for
each time interval did not differ significantly, and the variations
noted were within the range of variations found by others for normal
untreated subjects (Chart et al., 1974).
COMMENTS
Pirimiphos-methyl is rapidly absorbed from the gastrointestinal
tract, metabolised and quantitatively excreted in the urine and faeces
of several mammalian species.
The moderate acute toxicity of pirimiphos-methyl is due to its
inhibition of cholinesterase. After single toxic doses the onset of
inhibition of cholinesterase and appearance of toxic signs were
delayed for several hours and persisted for several days. Furthermore,
subacute and 2-year feeding studies in rats and dogs showed that
cholinesterase inhibition did not reach equilibrium for several weeks.
A two-year feeding study in rats provided a no-effect level based on
cholinesterase inhibition. Pirimiphos-methyl was not teratogenic in
rats and rabbits although hydronephrosis was noted. A decrease in
pregnancy rates was noted in a 3-generation reproduction study,
however cholinesterase activity was depressed at all dosages tested.
Other parameters of reproduction were not affected. No
compound-related histopathological effects were detected at dosage
rates considerably above those that inhibited cholinesterase, except
that in a 90-day study in dogs liver injury was observed in the
absence of cholinesterase inhibition.
In two-year studies in dogs, cholinesterase inhibition in brain
and plasma was noted at 0.5 mg/kg/day and above. No organ pathology
was observed in that study, however. Human volunteers who ingested
0.25 mg/kg/day of pirimiphos-methyl for 28 days had only a slight
reduction of plasma cholinesterase. Because of the slow decline in
cholinesterase activity in 2-year studies in the rat, and in the dog,
there is some question as to whether the experiments in volunteers
were of sufficient duration to detect maximum effect at the dosage
tested. Because of this, and because a no-effect level for brain
cholinesterase inhibition in the two-year dog studies was not clearly
established, the studies in rats provided the primary basis for
estimating an ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg bw.
Man: 0.25 mg/kg bw in 28 day period.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant action. It
shows activity against a wide spectrum of insect pests, including
ants, aphids, beetles, caterpillars, cockroaches, fleas, flies, mites,
mosquitoes, moths and thrips. It possesses only limited biological
persistence on leaf surfaces but gives long lasting control of insect
pests on inert surfaces such as wood, carpets, sacking and masonry. It
retains its biological activity when applied to stored agricultural
commodities including raw grain and nuts.
Commercial uses of pirimiphos-methyl are now developing in a wide
variety of outlets, including growing crops, public health and stored
products. The most important potential use appears to be as a grain
protectant and for use in the control of insect pests in stored
products. Such uses will be authorised as soon as appropriate maximum
residue limits have been recommended.
When used for the control of stored product pests,
pirimiphos-methyl is effective as a spray on structural surfaces and
on the outside of bagged produce and as an admixture treatment. The
recommended rates of application to bagged grain to control a complex
of beetles, weevils, moths and mites are normally in the range 250-500
mg/m2. For admixture with small grains the recommended rate of
application is 4 mg/kg except where Rhizopertha dominica is present
when a rate of 6 mg/kg is required.
Since the widespread development of strains of stored product
pests resistant to malathion (Pieterse et al., 1972; Waterhouse, 1973)
there has been considerable interest in pirimiphos-methyl which has
proved effective against all known strains of malathion-resistant
stored product insects.
Pirimiphos-methyl is regarded as more than a replacement for
malathion. At recommended rates it is effective against a wider
spectrum of insect pests, having an ability to destroy all forms other
than eggs and to confer long-term protection. It is effective at lower
rates of application and for much longer periods than is malathion.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest Treatments
The only information received by the Meeting on residues
resulting from pre-harvest application was provided by Hungarian
authorities and is summarised in Table 2.
TABLE 2. Residues resulting from pre-harvest applications of
pirimiphos-methyl1
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Tomato aphid cartridge 1/200 m3 1 day 0.15
2 days 0.06
5 days 0.01
Tomato 50% EC 2 l/ha 1 day 0.11
2 days 0.24
5 days 0.1
TABLE 2. (Cont'd.)
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Cucumber aphid 10% EC 7.5 l/ha 16 hours 0.11
24 hours 0.06
48 hours 0.04
Green
pepper 10% EC 7.5 l/ha 16 hours 0.12
24 hours 0.06
48 hours 0.04
1 Glasshouse experiments in 1974. The residue results are the average
of 3 samples. The limit of the determination was 0.01 mg/kg.
Post-harvest treatments
Surface sprays
Bullock (1973) has summarised the results of studies carried out
during 1970-1972 to determine the persistence of pirimiphos-methyl on
stored products. Trials were carried out in Colombia, Malaysia and the
United Kingdom involving the following bagged small grains: barley,
oats, rice in husk, milled rice, polished rice. In the period of 1-5
months after spraying the outside of the bags at rates up to 1000
mg/m2 the residues of pirimiphos-methyl in the whole grain taken from
the bag were found normally to be below 0.5 mg/kg and in no case to
have exceeded 1 mg/kg. The concentration was found to be highest in
grain sampled from within 2.5 cm of the inside of the bag. These
results are summarised in Table 3. No residues of the phosphorus
containing compounds (II) and (III) (Figure 1) were detected (Limit of
detection: 0.01 mg/kg in each case).
In most experiments the level of residues found in the grain in
the bags tended to rise during the first few weeks following
application and then to remain relatively constant over an interval of
three months. The level of residues in the grain was roughly
proportional to the rate of application to the surface of the bags.
Other Information on Identity and Properties
Pirimiphos-methyl is a pale straw coloured liquid. It melts at
15-18°C and decomposes above 100°C. It has a vapour pressure of
approximately 1 × 10-4 Torr at 30°C and a density of 1.157 g/ml at
20°C.
Pirimiphos-methyl is stable for up to six months at room
temperature. It is hydrolysed by strong acid or alkali.
Pirimiphos-methyl is miscible with most organic solvents. Its
solubility in water is approximately 5 mg/l at 30°C.
Formulated products include emulsifiable concentrates (8%, 25%
and 50% a.i.), dusts (2% a.i.) and formulations for ultra-low volume
application (10% a.i. and 50% a.i.). A diluent-free formulation and a
smoke generator formulation are under development.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 0.6 mg/kg of 2-14C-ring-labelled pirimiphos-methyl was
given to adult male rats, either orally or by intraperitoneal
injection, 73 to 81% of the dose was excreted in the urine during the
first 24 hours. This indicates rapid absorption. At the end of 120
hours after oral dosing, the entire dose could be accounted for by
excretion of radioactive products in the urine (86% of dose) or faeces
(15.2% of dose). Similar results were obtained after intraperitoneal
injection although the excretion was more rapid after oral
administration.
Two male beagle dogs given 17-18 mg/kg orally also excreted most
of the dose in the urine during the first 24 to 48 hours.
Chromatographic and autoradiographic studies of the urine of dosed
dogs and rats revealed extensive metabolism by the presence of 9
radioactive chromatogram spots (Bratt and Dudley, 1970). The
concentration of label in the abdominal fat of female rats given four
daily doses of 5 mg/kg of 14C-pirimiphos-methyl revealed that the
metabolites were stored to some extent in fat: 0.46, 0.69, 1.87 and
1.00 ppm of pirimiphos-methyl equivalents in fat after 1, 2, 3 and 4
doses, respectively (Bratt and Jones, 1973).
In a lactating goat, 91% of the label of a single dose of 0.12
mg/kg of 2-14C ring-labelled pirimiphos-methyl was excreted (87% in
urine and 4% in faeces) within 8 days. The milk contained only 0.41%
of the label, primarily during the first 24 hours. The maximum residue
in milk was 0.026 ppm pirimiphos-methyl equivalents, and the parent
insecticide accounted for only 0.003 ppm (Bowker et al., 1973).
Bullock et al. (1974a) found that the pattern of excretion of
2-14C labelled pirimiphos-methyl in the cow was similar to that in
the goat. The label from a single dose of 0.5 mg/kg was quantitatively
recovered in the urine (85%) and faeces (14%) during the following 7
days. During the first 3 days, 0.35% of the label was secreted in the
milk. The milk contained 0.04 ppm of pirimiphos-methyl equivalents of
which less than 2% was unchanged pirimiphos-methyl and
phosphorus-containing metabolites. Hydroxypyrimidine hydrolysis
products or their conjugates constituted the major part of the residue
in milk.
Hens given a single oral dose of 14C-pirimiphos-methyl excreted
more than 70% of the label within 24 hours. When fed for 28 days in
the diet, at a level of 4 ppm, pirimiphos-methyl itself never exceeded
0.01 ppm in yolks and whites of eggs although the total concentration
of metabolites reached equilibrium levels of about 0.028 ppm, mostly
as water soluble metabolites. Increasing the dietary concentration to
32 ppm for a 7-day feeding period resulted in residues of 0.007 and
0.012 ppm of pirimiphos-methyl in the whites and yolks of eggs. In
muscle, 0.31 ppm and 0.16 ppm of the label was present in hens fed 32
and 4 ppm respectively; however, the parent insecticide or its oxygen
analogue was not detected (Green et al., 1973). See also the section
"Fate of residues in animals."
Biotransformation
The metabolism of pirimiphos-methyl in rats (100 mg/kg, p.o.) and
in one dog (20 mg/kg, p.o.) was studied by thin-layer chromatographic
separation of urinary metabolites. Twelve metabolites were detected.
None of these possessed anticholinesterase activity. No parent
pirimiphos-methyl was detected in the urine. Five of the twelve
metabolites were identified by comparison with authentic samples. In
both rats and does, 2-ethylamino-4-hydroxy-6-methylpyrimidine was the
major urinary metabolite (30% of dose). The next most predominant
metabolite in the dog was
4-O(2-diethylamino-6-methylpyrimidinyl)-ß-D-glucosiduronic acid (11%
of dose), and in the rat an unidentified phosphorus-containing
product thought to be a dealkylated derivative of either
pirimiphos-methyl or its oxygen analogue (12% of dose). Other
identified urinary metabolites included
2-amino-4-hydroxy-6-methylpyrimidine,
2-[N-ethyl-N-(2-hydroxyethyl) amino]-4-hydroxy-6-methylpyrimidine,
and 2-diethylamino-4-hydroxy-6-methylpyrimidine (Bratt and Jones,
1973).
These studies indicate that the P-O-C bond of pirimiphos-methyl
is extensively cleaved and that N-dealkylation and/or conjugation are
further steps in the metabolism of the pyrimidine leaving group.
Although the oxygen analogue of pirimiphos-methyl was not detected as
a urinary metabolite, the fact that cholinesterase inhibition occurs
in vivo suggests that the oxygen analogue is also formed and may be an
intermediate step leading to the identified urinary products.
Effect on enzymes and other biochemical parameters
The only biochemical effects consistently noted in acute or
chronic toxicity tests was inhibition of cholinesterase. A group of 36
male rats were given single oral doses of 1450 mg/kg of
pirimiphos-methyl. Symptoms were noted and they were sacrificed at
intervals up to 4 days after dosing for measurements of brain, plasma,
and red-cell cholinesterase activity. Few signs were noted at 6 hours
after dosing when cholinesterase inhibition was 0, 35 and 51%
respectively for brain, red cells and plasma. Clear signs of poisoning
only became apparent by 24 hours when brain was inhibited by 46% and
red cell and plasma by 70 and 80% respectively. Recovery of
cholinesterase activity began to be apparent by 72 hours. Plasma
cholinesterase activity had completely recovered by 96 hours, but red
cell and brain remained 47 and 30% inhibited, respectively, at this
time (Clark, 1970). From these studies it appears that inhibition of
brain cholinesterase by 40% or more results in obvious signs of
toxicity.
TOXICOLOGICAL STUDIES
Special studies on potentiation
Tests for potentiation of the acute oral toxicity of combinations
of pirimiphos-methyl and gamma-BHC were conducted by simultaneous
administration of one-half LD50 doses of the two compounds to ten
fasted female rats. Three of the ten animals died during a 14-day
observation period, suggesting no potentiation. Combinations of
one-half LD50 doses of pirimiphos-methyl and dichlorovos were tested
in the same manner and no mortality resulted in ten test rats. Under
these limited test conditions, there was no evidence of potentiation
of pirimiphos-methyl with 2 other insecticides that are likely to be
used in conjunction with it (Parkinson, 1972 a,b).
Special studies on reproduction
Rat
The effect of pirimiphos-methyl on the growth and reproductive
performance of the rat was assessed by feeding groups of 24 female and
12 male rats dietary concentrations of 0, 20 and 200 ppm of the
compound throughout three generations. However, dietary analysis of
the F0 generation nominally given 20 ppm revealed concentrations of
pirimiphos-methyl far below the intended levels and so animals treated
at 20 ppm were reared to produce a fourth (F4B) generation.
There were no effects among parent animals attributable to the
feeding of the compound. Mating performance and pregnancy rates were
reduced at 200 ppm for F1B and F2B generations. At 20 ppm there was an
effect at 2nd mating of the F1B generation and at both matings of the
F2B generation. When expressed as an average over all generations,
there was a dosage related decrease in pregnancy rate. Litter
parameters, as assessed by total litter loss, litter size, litter and
mean pup weights, pup mortality and the incidence of abnormalities
showed no consistent dosage-related trends. Examination of the
ultimate generation (F3B for 200 ppm dosage group, and F4B for 20 ppm)
by organ weight analysis and skeletal staining of 10 males and 10
females from all groups, and by microscopic examination of 10 male and
10 female pups from controls and from the 200 ppm group, showed no
difference that could be conclusively related to treatment (Palmer and
James, 1972).
The testes of the F1B and F2B male rats were examined
histopathologically in an attempt to find an explanation for their
reduced mating performance. However in the testes examined, from rats
of the control and both treatment groups, the degree of activity and
maturity of the process of spermatogenesis were comparable (Palmer and
Cherry, 1972).
Special studies on skin irritation, sensitization, and effects in
the eye
Application of a 50% solution of pirimiphos-methyl in olive oil
to ears of 6 guinea pigs did not produce erythema when challenge
applications of 10%, 5% and 1% were applied to shaved skin of their
flanks 4 days later. This indicated a lack of sensitizing potential.
Undiluted pirimiphos-methyl was applied to the clipped backs of 4
female rats and the site of application covered for 24 hours with an
impermeable dressing. No signs of primary irritation were noted after
a single such application nor after a second or third application at
alternate 24-hour intervals.
One drop of undiluted pirimiphos-methyl was placed in the left
conjunctival sac of the eyes of 4 albino rabbits. No signs of
irritation or myosis was noted on frequent observation over the next 7
days (Clark, 1970).
Special studies on teratogenicity
Rat
Three groups of 18 to 21 virgin female, Alderley Park specific
pathogen-free rats were mated and fed diets containing 0, 10 or 200
ppm of pirimiphos-methyl from day 1 to day 20 of pregnancy. On day 20,
the females were killed and foetuses immediately removed by Caesarean
section. The uterus was examined for resorptions, foetuses were
examined for viability and weighed. One third of the foetuses were
eviscerated and stained with alizarin red for examination of skeletal
defects. Viscera were fixed and examined for histopathology. Maternal
rats showed no toxic signs. It was presumed that 200 ppm produced
cholinesterase inhibition but this was not measured. The only
significant difference between pirimiphos-methyl and control litters
was a reduction in mean foetal weight; however, this may have been
because there were more foetuses per litter in the experimental
groups. No increased incidence of skeletal abnormalities were observed
in foetuses from the pirimiphos-methyl treated groups. The only
significant soft-tissue abnormality was increased hydronephrosis (7,
17 and 22 foetuses, representing incidence of 6, 17 and 16%) in the
foetuses examined from dams that received 0, 10 and 200 ppm in the
diet respectively. The investigators stated that these incidences of
hydronephrosis were within normal limits for Alderley Park rats and
they conclude that there was no evidence to suggest teratogenic action
for pirimiphos-methyl (Hodge and Moore, 1972).
Rabbit
Two groups of 16 to 17 pregnant rabbits were given gelatin
capsules containing pirimiphos-methyl dissolved in corn oil at doses
of 1 mg/kg and 16 mg/kg daily throughout pregnancy. A group of 16
controls was given capsules containing corn oil only. Twenty-nine days
after mating all the foetuses were delivered by Caesarean section. The
uterus was examined for resorptions and the foetuses for viability.
One half of the foetuses from each litter were stained with Alizarin
Red for skeletal examination and the remaining half were fixed in
Bouin's solution and dissected for soft-tissue examination. Does were
examined for gross and histological pathology and for blood
cholinesterase inhibition.
Three of the experimental does died during the gestational
period, two receiving 1 mg/kg/day and one receiving 16 mg/kg/day.
These deaths appeared to be due to causes unrelated to
pirimiphos-methyl. The does gained weight normally, but at the end of
4 weeks, the group average of RBC cholinesterase was 19% and 60% less
than controls in the 1 and 16 mg/kg dosage groups respectively. Data
on observations of foetuses were reported for 10, 10 and 11 litters
where implantations occurred in the 0, 1 and 16 mg/kg dosage groups
respectively. One grossly abnormal foetus was observed in a litter
from one doe receiving 16 mg/kg/day. No abnormalities were seen in the
remaining ten foetuses. There was no increase in resorptions in
experimental groups. Although the foetal weights of the 1 and 16 mg/kg
dosage groups were less than controls, there were more foetuses/litter
in these groups so that mean litter weights among the three groups did
not differ. The ratios of male/female foetuses were 1.0, 1.25 and 1.39
for the 0, 1 mg/kg and 16 mg/kg dosage groups. The authors state that
these are "within normal control limits." The only statistically
significant difference in the occurrence of abnormalities was a higher
incidence of 14 caudal vertebrae in the pirimiphos-methyl groups,
which the investigators state were isolated findings. This study
indicates no teratogenic action of pirimiphos-methyl in rabbits at
dosages which produce considerable inhibition of blood cholinesterase
in maternal animals (Gore et al., 1974a).
Acute toxicity
Pirimiphos-methyl, 90-94% purity, was administered to animals
undiluted or, for small doses, in propylene glycol. Single-dose LD50
values or estimates of LD50's by various routes are shown in Table
1. In the single-dose dermal test the skin of rats was washed with
soap and water 24 hours following application. All animals were
observed for 14 days or longer.
TABLE 1. Acute toxicity of pirimiphos-methyl
Animal Sex Route mg/kg bw References
Rat F Oral 2050 (1840-2260) Clark, 1970
Rat F ip approx. or equal to 800 Clark, 1970
Rat M ip approx. or equal to 800 Clark, 1970
Rat F dermal >2000 Clark, 1970
Mouse M Oral 1180 (1030-1360) Clark, 1970
Guinea Pig F Oral 1000-2000 Clark, 1970
Rabbit M Oral 1150-2300 Clark, 1970
Cat F Oral 575-1150 Clark, 1970
Dog M Oral >1500 Gage, 1972
Hen F Oral 30-60 Clark, 1970
Quail F Oral approx. or equal to 140 Gage, 1971a
Green Finch Oral 200-400 Gage, 1972
Figures in parentheses are 95% confidence limits.
The toxic signs exhibited after single doses were typical of
cholinesterase inhibition. In female rats given single oral doses,
only mild to moderate signs were noted at 12 hours after dosing, but
marked signs consisting of incontinence, salivation,
chromolacrymation, fibrillations, fasciculations and prostration were
present at 24 hours and persisted up to 11 days. No additional toxic
signs were noted during a 12 week observation period. Six surviving
hens of 8 dosed with 31 mg/kg were observed for 28 days after dosing
and no abnormal gait or other neurological signs were observed after
the initial acute toxic effects had subsided (within 7 days) (Clark,
1970).
2-Diethylamino-4-hydroxy-6-methylpyrimidine is a plant metabolite
of pirimiphos-methyl. It is also formed metabolically in rats and
dogs. Single-dose, range-finding acute toxicity tests (3 rats per
dose) indicated the acute oral LD50 in rats to be between 800 and
1600 mg/kg (Gage, 1971b). This is of the same order of acute toxicity
as the parent insecticide, but no description of toxic signs was
provided. Groups of 10 male and 10 female rats were given 100 and 400
mg/kg of the metabolite, orally, five days a week for two weeks. No
toxic signs, altered body weights or gross or histopathology were
noted. There was an approximate 25% increase in reticulocytes and a
reduction in total leucocytes which could be accounted for by a 30 to
40% reduction in lymphocytes, particularly in females, which the
investigators attribute to a "stress response" (Gage, 1971b).
Atropine sulphate and a mixture of atropine plus
pyridine-2-aldoxime methane-sulphonate partially counteracted the
acute oral toxicity of pirimiphos-ethyl in female rats when the
antidotes were given at intervals at which toxic signs appeared. The
combination of antidotes was more effective than atropine alone
(Clark, 1970).
Short-term studies
Rat (feeding)
Repeated daily oral dosing of 10 male and 10 female rats with 200
mg/kg of pirimiphos-methyl, 5 days a week for 2 weeks produced mild
signs of poisoning which were first noted after the 7th dose. The
signs did not progress in intensity with continued dosing; however,
body weight gain over this period was less than half that of controls.
An approximate 9% reduction of haemoglobin levels accompanied by
reticulocytosis was observed in the treated rats. Gross or
histopathology was not striking but slight to moderate haemopoesis of
the spleen was observed. When the experiment was repeated at a dose of
400 mg/kg, signs of poisoning appeared after 2 doses and increased in
severity as the experiment progressed. Males were more affected than
females and deaths occurred from the 4th day in males and from the 6th
in females. Cumulative mortality after 10 doses was 9 of 10 male rats
and 3 of 10 females (Clark, 1970).
Four groups of 25 male and 25 female Alderley Park specific
pathogen-free adult rats were maintained for 90 days on diets
containing 0, 8, 80 and 360 ppm of pirimiphos-methyl. Haematology and
plasma and erythrocyte cholinesterase activities were examined on
individual samples from five male and five female rats in each group
before, during and at the end of the feeding period. Brain
cholinesterase activities and organ:body weight ratios were calculated
for five males and five females in each group at the end of the
feeding period. Gross and histopathological examinations were
conducted. Body weight gain in the female rats fed diets containing 80
and 360 ppm pirimiphos-methyl averaged 18% and 21% less than controls.
This was apparently due to reduced food utilization as food
consumption did not differ significantly from controls. Plasma
cholinesterase was inhibited 65% in males and 80% in females fed 360
ppm and 40% in males and 60% in females fed 80 ppm, but no inhibition
was detected at 8 ppm. Erythrocyte cholinesterase was inhibited 20% in
males and 50% in females only at the highest dietary level. Both
plasma and erythrocyte cholinesterase returned to normal within one
week after cessation of dosage. Terminal measurements of brain
cholinesterase activity revealed inhibition in rats fed 80 and 360 ppm
with females affected most (about 40% inhibition in the 360 ppm
group). There was no inhibition in the 8 ppm group. Brain
cholinesterase was still inhibited at 4 weeks after cessation of
feeding pirimiphos-methyl. Haematological examinations revealed no
significant effects on haemoglobin concentration, packed cell volumes,
mean corpuscular diameter, white cell or platelet counts or clotting
function. There were no increases in gross or histopathological
lesions in the pirimiphos-methyl-fed rats over controls (Clapp and
Conning, 1970).
Rat (inhalation)
Four male and 4 female rats exposed by inhalation to a nominal
vapour-aerosol concentration of 3.5 ppm, 6 hours/day, 5 days/week for
3 weeks did not result in toxic signs nor reduction in body weight nor
decreased cholinesterase activities. An increase in alveolar
macrophages and slight lymphoid hyperplasia was noted (Clark, 1970).
Rabbit (dermal)
Daily application of 1000 mg/kg of pirimiphos-methyl to the
dorsal skin of 6 rabbits for 14 days (application site was washed 1
hour before each successive dose) resulted in fibrillations after 7 to
12 applications. Approximately 15% loss of body weight occurred and
one rabbit died after 14 applications (Clerk, 1970).
Avian species
Mallard ducklings and bobwhite quail chicks were fed
pirimiphos-methyl in their diets for five days and observed for an
additional 3 days. LC50 values were 633 and 207 ppm for ducklings and
quail chicks respectively (Fink, 1974a,b). Pirimiphos-methyl in the
diet of broiler chicks at levels from 4 to 48 ppm did not reduce feed
efficiencies or growth rate, except for a temporary period at the
beginning of the study (Graham and Jenkins, 1974). When 0, 4, 12 and
40 ppm were fed in the diet of laying hens for 28 days there was no
adverse effect on the hens. Egg production and egg quality were
considered normal although there was a small, dose-related increase in
the number of "small" eggs. There was some reduction in the number of
chicks hatched from eggs from the pirimiphos-methyl fed hens which was
not significant, although there were significantly greater numbers of
chicks dead in shell at the 12 and 40 ppm dietary levels (Ross et al.,
1974).
Dog
Groups of four male and four female beagle dogs were dosed orally
seven days a week for 13 weeks by administration of gelatin capsules
containing corn oil solutions of pirimiphos-methyl. The dosage rates
were 0, 2, 10 and 25 mg/kg given as single daily doses about 1 hour
after feeding the daily ration of complete dry diet. Clinical signs,
body weight, food and water consumption, electrocardiograms and
ophthalmic examinations were included among the observations.
Laboratory investigations included plasma and red cell cholinesterase
haematology, urinalysis, serum alkaline phosphate (SAP), serum
glutamic-pyruvic transaminase (SGPT) plasma urea and blood glucose. At
the end of the dosing period, two dogs of each sex in each dosage
group were sacrificed and the remaining dogs observed for an
additional four weeks before sacrifice. Gross and histopathological
examinations and brain cholinesterase assays were performed. There
were no deaths. Clinical symptoms were minimal with occasional
episodes of vomiting and watery stools. Body weight gain was
significantly reduced in both males and females given 25 mg/kg/day and
in females given 10 mg/kg/day. The rate of weight gain also tended to
be less for females given 2 mg/kg/day but this was of borderline
significance. At the highest dosage level (25 mg/kg) only, there was
reduced food consumption and a slower heart rate. Plasma and
erythrocyte cholinesterase activities were significantly depressed
(20% or more) at all three dosage levels. Plasma cholinesterase
rapidly returned to normal upon cessation of dosing but reversal of
red cell inhibition was delayed. At the lowest dosage level,
significant inhibition of red cell cholinesterase was only observed
during the latter weeks of the dosing period. Terminal brain
cholinesterase assays revealed no inhibition in any of the dosage
groups. Two dogs receiving 25 mg/kg showed very high SGPT and SAP
levels after three months dosing, while a third showed a less marked
SGPT increase. Post-mortem examination showed evidence of liver damage
in these animals. All other biochemical findings were within normal
limits. Histopathological examination post-mortem showed some bile
duct proliferation in one dog receiving 10 mg/kg and bile duct
proliferation together with portal cirrhosis in one dog receiving 25
mg/kg. After four weeks observation following the cessation of dosing,
two other dogs receiving 25 mg/kg showed minimal degrees of bile duct
proliferation. No other histopathological findings or other consistent
effects were noted (Noel et al., 1970).
Four groups each consisting of 4 male and 4 female beagle dogs
were orally dosed once daily, seven days/week, for two years with
gelatin capsules containing corn oil solutions of pirimiphos-methyl at
dosage rates of 0, 0.5, 2 or 10 mg/kg/day. Brain cholinesterase
activities at the end of the dosing period were 81, 78 and 44% of
control in the 0.5, 2 and 10 mg/kg groups respectively. The mean
activities in the 0.5 and 2 mg/kg groups were significantly less than
values for controls at the 1% level of probability, and in the 10
mg/kg group, brain cholinesterase depression was significant at the
0.1% level. Red cell cholinesterase was significantly inhibited with
respect to pre-dosing values, in both the 2 and 10 mg/kg groups from
the 8th week of dosing, but only occasionally inhibited in the 0.5
mg/kg group. Plasma cholinesterase was inhibited significantly
(30-50%) in all 3 groups from the 16th week of dosing. There was also
a decline in plasma cholinesterase activity in controls during the
experimental period. However, even with correction for this, 20-25%
depression was noted in the 0.5 mg/kg group, with only slightly
greater depressions with the higher dosages. It appears that plasma
cholinesterase inhibition did not discriminate well among the 3
experimental groups. One female dog in the 10 mg/kg group died after
the 400th day after showing few clinical signs. Clinical signs
consisting of loose stools were observed, in a dose-related frequency
in excess of controls, only in the 2 highest dosage groups. Vomiting
was infrequently noted. Dogs in the 10 mg/kg group showed some loss of
appetite and a reduced rate of weight gain. The 0.5 mg/kg/day dosage
rate appears to be a no effect level for clinical signs. No
abnormalities of the eyes, examined by ophthalmoscopy nor in
electrocardiogram records were observed. No appreciable or consistent
effects were noted in any of the dosage groups with respect to
haematology, standard urinalysis values or blood urea, blood glucose,
serum protein, SAP, SGPT, NA+ or K+ values. No consistent or
dose-related gross or histopathological changes were observed in post
mortem studies. However, a statistically significant increase in liver
weights and liver body weight ratios was seen in the group given 10
mg/kg/day (Rivett et al., 1973).
Long-term studies
Rat
Four groups of 48 male and 48 female rats were fed
pyrimiphos-methyl in their diet at levels of 0, 10, 50 and 300 ppm for
two years. Twenty-four additional animals of each sex were included in
each dosage group, and were killed at intervals up to one year to
provide interim data on brain cholinesterase activity and clotting
function. Eight animals of each sex in each of the dosage groups fed
for two years were continued on a control diet for 4 to 8 weeks beyond
the two year feeding period to allow for observation of recovery from
any treatment-induced effects.
Rats fed 300 ppm pirimiphos-methyl showed marked plasma
cholinesterase inhibition (50-80%) and some inhibition of erythrocyte
and brain cholinesterase activity (30-40%). The only other adverse
effect noted at this dosage rate was a slight anaemia in female rats.
Only female rat plasma cholinesterase was consistently inhibited
(50-65%) at the 50 ppm dietary level. Recovery from inhibited
cholinesterase, when it occurred, was usually complete by the end of
an 8-week period on the control diet. Slight (generally <25%)
transient depression of plasma cholinesterase activity in female rats
fed 10 ppm was observed. Erythrocyte and brain cholinesterases were
not depressed at this dietary level. Although cumulative mortality was
greater than for controls in male rats fed 300 ppm at 54, 60, 66 and
72 weeks of feeding, thereafter the cumulative mortalities of all
groups were approximately equal. Body weight gains, weights of major
organs and incidence of gross or microscopic pathology (including
tumors), in a comprehensive list of tissues, did not differ between
the control and experimental groups.
The results of this study indicate that 10 ppm in the diet of
rats is an experimental no-adverse-effect level (Gore et al., 1974b).
OBSERVATIONS IN MAN
A dose of 0.25 mg/kg/day of pirimiphos-methyl (97.8% purity) was
taken orally for 28 days by five healthy male volunteers (25-45 years
of age; 59.7-73 kg body-weight). Blood samples were taken before
dosing began, on days 1, 3, 7, 14, 21 and 28 during administration,
and 7 and 14 days after cessation of dosing. Only one subject had a
plasma cholinesterase depression (on day 28) which exceeded 20%
(21.5%). Otherwise variations, both above and below pre-dosing values
were within 12%. Four of the five subjects had red cell cholinesterase
values that were slightly below either of the pre-exposure values
during the last 2 weeks of the study. However, the group means for
each time interval did not differ significantly, and the variations
noted were within the range of variations found by others for normal
untreated subjects (Chart et al., 1974).
COMMENTS
Pirimiphos-methyl is rapidly absorbed from the gastrointestinal
tract, metabolised and quantitatively excreted in the urine and faeces
of several mammalian species.
The moderate acute toxicity of pirimiphos-methyl is due to its
inhibition of cholinesterase. After single toxic doses the onset of
inhibition of cholinesterase and appearance of toxic signs were
delayed for several hours and persisted for several days. Furthermore,
subacute and 2-year feeding studies in rats and dogs showed that
cholinesterase inhibition did not reach equilibrium for several weeks.
A two-year feeding study in rats provided a no-effect level based on
cholinesterase inhibition. Pirimiphos-methyl was not teratogenic in
rats and rabbits although hydronephrosis was noted. A decrease in
pregnancy rates was noted in a 3-generation reproduction study,
however cholinesterase activity was depressed at all dosages tested.
Other parameters of reproduction were not affected. No
compound-related histopathological effects were detected at dosage
rates considerably above those that inhibited cholinesterase, except
that in a 90-day study in dogs liver injury was observed in the
absence of cholinesterase inhibition.
In two-year studies in dogs, cholinesterase inhibition in brain
and plasma was noted at 0.5 mg/kg/day and above. No organ pathology
was observed in that study, however. Human volunteers who ingested
0.25 mg/kg/day of pirimiphos-methyl for 28 days had only a slight
reduction of plasma cholinesterase. Because of the slow decline in
cholinesterase activity in 2-year studies in the rat, and in the dog,
there is some question as to whether the experiments in volunteers
were of sufficient duration to detect maximum effect at the dosage
tested. Because of this, and because a no-effect level for brain
cholinesterase inhibition in the two-year dog studies was not clearly
established, the studies in rats provided the primary basis for
estimating an ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg bw.
Man: 0.25 mg/kg bw in 28 day period.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant action. It
shows activity against a wide spectrum of insect pests, including
ants, aphids, beetles, caterpillars, cockroaches, fleas, flies, mites,
mosquitoes, moths and thrips. It possesses only limited biological
persistence on leaf surfaces but gives long lasting control of insect
pests on inert surfaces such as wood, carpets, sacking and masonry. It
retains its biological activity when applied to stored agricultural
commodities including raw grain and nuts.
Commercial uses of pirimiphos-methyl are now developing in a wide
variety of outlets, including growing crops, public health and stored
products. The most important potential use appears to be as a grain
protectant and for use in the control of insect pests in stored
products. Such uses will be authorised as soon as appropriate maximum
residue limits have been recommended.
When used for the control of stored product pests,
pirimiphos-methyl is effective as a spray on structural surfaces and
on the outside of bagged produce and as an admixture treatment. The
recommended rates of application to bagged grain to control a complex
of beetles, weevils, moths and mites are normally in the range 250-500
mg/m2. For admixture with small grains the recommended rate of
application is 4 mg/kg except where Rhizopertha dominica is present
when a rate of 6 mg/kg is required.
Since the widespread development of strains of stored product
pests resistant to malathion (Pieterse et al., 1972; Waterhouse, 1973)
there has been considerable interest in pirimiphos-methyl which has
proved effective against all known strains of malathion-resistant
stored product insects.
Pirimiphos-methyl is regarded as more than a replacement for
malathion. At recommended rates it is effective against a wider
spectrum of insect pests, having an ability to destroy all forms other
than eggs and to confer long-term protection. It is effective at lower
rates of application and for much longer periods than is malathion.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest Treatments
The only information received by the Meeting on residues
resulting from pre-harvest application was provided by Hungarian
authorities and is summarised in Table 2.
TABLE 2. Residues resulting from pre-harvest applications of
pirimiphos-methyl1
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Tomato aphid cartridge 1/200 m3 1 day 0.15
2 days 0.06
5 days 0.01
Tomato 50% EC 2 l/ha 1 day 0.11
2 days 0.24
5 days 0.1
TABLE 2. (Cont'd.)
Interval
Application after Residue
Crop Pest Formulation rate treatment mg/kg
Cucumber aphid 10% EC 7.5 l/ha 16 hours 0.11
24 hours 0.06
48 hours 0.04
Green
pepper 10% EC 7.5 l/ha 16 hours 0.12
24 hours 0.06
48 hours 0.04
1 Glasshouse experiments in 1974. The residue results are the average
of 3 samples. The limit of the determination was 0.01 mg/kg.
Post-harvest treatments
Surface sprays
Bullock (1973) has summarised the results of studies carried out
during 1970-1972 to determine the persistence of pirimiphos-methyl on
stored products. Trials were carried out in Colombia, Malaysia and the
United Kingdom involving the following bagged small grains: barley,
oats, rice in husk, milled rice, polished rice. In the period of 1-5
months after spraying the outside of the bags at rates up to 1000
mg/m2 the residues of pirimiphos-methyl in the whole grain taken from
the bag were found normally to be below 0.5 mg/kg and in no case to
have exceeded 1 mg/kg. The concentration was found to be highest in
grain sampled from within 2.5 cm of the inside of the bag. These
results are summarised in Table 3. No residues of the phosphorus
containing compounds (II) and (III) (Figure 1) were detected (Limit of
detection: 0.01 mg/kg in each case).
In most experiments the level of residues found in the grain in
the bags tended to rise during the first few weeks following
application and then to remain relatively constant over an interval of
three months. The level of residues in the grain was roughly
proportional to the rate of application to the surface of the bags.
 Admixture with small grain cereals
Bullock (1973 and 1974) reports numerous trials undertaken in
Australia, Argentina, Guyana, Malaysia, U.K. and the U.S.A. where
pirimiphos-methyl was admixed with wheat, barley, maize, and rice in
husk (residues were determined on the rice after milling). The results
are summarised in Table 4. Mean initial residues of pirimiphos-methyl
itself in the whole grains of wheat, barley and maize rarely exceeded
the nominal treatment rate and were frequently in the range of 40-60%
of the nominal dose. This appears to be a reflection of the method of
application and suggests that some at least of the nominal dose is
lost into the atmosphere during application. Higher percentage
retention of the applied chemical was obtained by treating 5-10% of
the grain with all the chemical rather than applying it to 50% or more
of the grain stream entering the storage. Again no residues of the
phosphorus-containing compounds (II) and (III) were detected.
TABLE 3. Residues of pirimiphos-methyl in whole grains
after spraying bagged grains. (Bullock, 1973)
Interval
between Range of
Application application residues
Rate and sampling found,
Crop mg/m2 months mg/kg Country
Barley 400 0-3 0.05-0.33 UK
Oats 400 2-3 0.07-0.19 UK
Rice in husk 300 0-3 <0.01-0.08 Colombia
Milled rice 500 0-5 0.02-0.28 Malaysia
Treated when
in husk 1000 0-5 0.01-0.70 Malaysia
TABLE 4. Residues of pirimiphos-methyl in whole grains
after admixture. (Bullock, 1973, 1974)
Interval
Nominal between Range of
admixture admixture residues
rate, and sampling found,
Crop mg/kg (months) mg/kg Country
Wheat 3 0-8 1.0-2.1 Australia
4 0-3 1.3-4.6 UK
5 1-3 1.7-4.7 USA
6 0-12 1.3-3.9 Argentina
0-8 3.0-4.9 Australia
10 1-3 4.7-8.4 USA
Barley 4 0-3 0.03-3.6 UK
8 0-3 0.26-4.4 UK
Maize 5 1-3 2.1-6.9 USA
10 1-3 4.7-8.0 USA
Milled rice 3 0-3 0.21-0.53 Guyana
treated when
in husk 4 0-5 0.12-0.26 Malaysia
5 0-4 0.16-0.59 Guyana
7.5 0-11 0.53-0.98 Guyana
8 0-5 0.21-0.85 Malaysia
Wheat
The stability of the deposit of pirimiphos-methyl on wheat grains
is quite remarkable and from the work of Bullock the half life has
been estimated to be of the order of 9 months. Degradation proceeds
uniformly and slowly as indicated in the results from a typical trial
carried out in Argentina on wheat with a moisture content ranging from
11 to 13% (Table 5).
TABLE 5. Pirimiphos-methyl residues in wheat at intervals after admixture-Argentina.
(Bullock, 1973)
Nominal
admixture
rate
mg/kg 1 Day 1 Month 2 Months 3 Months 4 Months 6 Months 9 Months 12 Months
2 1.60 1.16 1.47 2.06 2.00 0.90 0.80
3 1.61 1.53 1.30 1.34 1.13 1.96 1.03 0.80
4 2.04 2.40 1.89 1.79 1.53 1.80 1.66 0.92
5 2.26 2.79 2.06 2.00 1.93 2.00 1.73 1.31
6 3.91 3.65 2.62 2.42 2.39 2.14 2.02 1.29
7 3.90 3.71 3.12 3.06 2.43 2.30 2.20 1.35
Moisture content: low (11-13%)
Bengston et al. (1974) report the results of two extensive trials
in Australia with five grain protectant insecticides applied to wheat
having a mean moisture content of 10-12% and a mean temperature of
29-32°C. In these trials the target application rate was 6 mg/kg but
based on the analyst's determination of the concentrate strength and
the weight of grain treated (2000 tons per treatment) the calculated
application rate was 5.5 mg/kg. Analysis by five collaborating
laboratories showed the concentration of pirimiphos-methyl on samples
taken 1 week after application to range from 5.5-6.4 mg/kg.
Representative data summarised in Table 6 show relatively little
change over a 26 week period notwithstanding the high temperature
which prevailed throughout.
Based on this work calculations have been made of the half-life
of Pirimiphos-methyl deposits on wheat stored in concrete bins at
30°C. When using data from samples taken from within 10 cm of the
surface and using the rate of disappearance during weeks 1-11, a
half-life of 43 weeks is obtained. Based on data from weeks 11-26 the
half-life was 104 weeks. Using data from samples taken 6 metres
beneath the surface where the mean temperature exceeded 32°C a
half-life of 77 weeks is obtained whether the data from samples
collected during weeks 1-11 or weeks 11-26 are used. The mean
half-life from many data is 82 weeks. This compares with a half-life
for malathion of 3-9 weeks under comparable conditions.
Barley
Bullock (1973 and 1974) reports numerous trials where barley was
treated with pirimiphos-methyl in the United Kingdom by admixture of
2% Pirimiphos-methyl dust or by the application of dilute emulsion
designed to deposit from 2-8 mg/kg on the weight of grain.
Unfortunately moisture and temperature conditions are not stated and
extensive sampling and analysis has failed to recover more than 25-30%
of the amount that was supposed to have been applied. Residue analyses
however fail to show much decline in the level of residues over the
first three months following spray application. The deposit seems to
disappear more quickly when the application is made in a manner
designed to fully cover all grains than when only a proportion of the
grain receives the spray. Subject to some reservations about the
residue data, the half-life on barley from these trials appears to be
at least 30 weeks.
TABLE 6a. Pirimiphos-methyl residues in stored wheat from site M
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories.
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
1 week 0.1 5.5 6.6 - - 6.1
0.6 6.4 - - -
1.5 4.9 5.8 - -
6.0 5.6 5.6 - - 5.6
Mean 5.3 6.0 - -
6 weeks 0.1 5.2 5.2 5.5 5.3 5.3
0.6 - - - - -
1.5 4.6 5.1 4.9 4.5 4.8
6.0 4.8 5.1 5.3 3.8 4.8
Mean 4.9 5.1 5.2 4.5 -
11 weeks 0.1 5.6 5.1 6.0 4.8 5.4
0.6 - - - - -
1.5 5.1 4.5 5.2 3.8 4.7
6.0 5.4 4.9 5.8 3.8 5.0
Mean 5.3 4.8 5.7 4.1 -
16 weeks 0.1 5.4 - - 4.9 5.1
0.6 - - - - -
1.5 5.1 - - 4.3 4.7
6.0 5.4 - - 4.3 4.8
Mean 5.3 - - 4.6 -
22 weeks 0.1 5.6 4.3 4.8 4.1 4.7
0.6 - - - - -
1.5 5.5 4.1 4.1 3.7 4.4
6.0 5.2 4.2 4.4 3.6 4.4
Mean 5.4 4.2 4.4 3.8 -
26 weeks 0.1 - 4.2 5.2 - 4.7
0.6 - - - - -
1.5 - 3.6 4.6 - 4.1
6.0 3.6 3.6 4.4 - 3.9
Mean -
TABLE 6b. Pirimiphos-methyl residues in stored wheat from site W
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
2 weeks 0.1 - - - - -
0.6 5.6 5.4 4.5 4.5 5.0
1.5 6.0 4.9 4.7 4.0 5.0
6.0 5.8 4.9 4.4 4.0 4.8
Mean 5.1 4.5 4.2 -
8 weeks 0.1 - - - - -
0.6 4.4 3.9 4.5 4.0 4.2
1.5 4.8 4.5 5.0 4.5 4.7
6.0 4.5 3.9 4.7 4.2 4.2
Mean 4.6 4.1 4.7 4.2 -
13 weeks 0.1 - - - - -
0.6 4.2 4.0 - 2.3 3.5
1.5 4.8 4.2 - 3.3 4.1
6.0 4.2 3.9 - 2.7 3.6
Mean -
TRIAL TERMINATED BECAUSE OF INFESTATION WITH RHIZOPERTHA DOMINICA
Rice
Bullock (1973) reports trials in Guyana, Indonesia and Malaysia
where rice in husk and polished rice were treated by admixture of
varying amounts of pirimiphos-methyl. In all trials reported the husk
was removed after varying intervals and the residues on husk and grain
determined separately. There appears to be some migration of the
insecticide from the husk to the grain during parboiling prior to
dehusking. The level of the residue in the husk declines over a period
of one month to about 30% of its concentration shortly after
application. Table 7 shows results from Guyana where rice was treated
in husk. When the rate of application was 20 mg/kg, 55% of the deposit
was lost from the husk within three days, the level declining to 35%
of the initial value at the end of one month.
TABLE 7. Pirimiphos-methyl admixed with rice paddy and
analysed at intervals - Guyana. (Bullock, 1973)
Interval
Rate of between Before After
application, treatment Parboiling* Parboiling*
mg/kg and sampling Husk Grain Husk Grain
3 3 days 14 0.53 8.4 1.0
1 month 4.4 0.21 1.1 0.66
2 months 2.4 0.34 2.7 1.0
3 months 2.0 0.46 2.0 0.64
7.5 3 days 34 0.90 5.1 1.8
1 month 8.5 0.75 2.0 -
2 months 5.2 0.71 6.0 2.3
3 months 5.1 0.53 3.4 1.3
8 months 12 0.98 4.5 ND
11 months 0.59 0.58 1.4 0.38
19.2 0 days 80 1.2 17 5.6
3 days 36 2.1 - -
1 month 28 1.4 8.2 3.2
2 months 13 1.8 15 4.9
3 months 18 1.3 3.7 2.6
* Parboiling undertaken for dehusking purposes.
Moisture content: approximately 14%.
Bullock (1973) reports extensive trials carried out in Malaysia
where pirimiphos-methyl was applied to polished rice and to rice in
husk at various rates and by various methods of application. Polished
rice appears to retain the initial deposit over a period ranging up to
7 months. It is not possible to estimate the half-life from these data
but it can be assumed to be much more than 7 months. Other trials in
Indonesia confirm this long residual life. Malathion under comparable
conditions disappeared almost completely within two months. By
comparison malathion appears not to migrate in significant amounts
from the husk into the grain of treated paddy.
Maize
Bullock (1973) reports trials in the U.S.A. which revealed that
pirimiphos-methyl deposits on maize remained relatively stable over a
period of 3 months. When the rate of application was 5-10 mg/kg the
half-life appeared to be of the order of 3 months but when 20 mg/kg
was applied no significant change occurred over the three month
period.
Peanuts
Bullock (1973) reports two large scale trials in the U.S.A. where
pirimiphos-methyl was applied at 20 and 50 mg/kg to undecorticated
peanuts. Analytical results indicate that the target rate of treatment
was achieved. Very little change occurred in the level of residues
during the first month following application. Even at the end of three
months substantially all of the deposit remained. The data suggest
that the half-life is of the order of 5 months from the time of
application. Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied.
FATE OF RESIDUES
In stored products
a) Distribution and degradation
Residues of pirimiphos-methyl on wheat grains are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain, to give
principally the parent hydroxy-pyrimidine (IV), Figure 1, and also the
related compounds (V) and (VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grains.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residues of the
chemically-unstable oxygen analogue (III) were detected. The limit of
detection.was 0.01 mg/kg (Bowker, 1973: See Table 8).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products.
The general pattern of breakdown on stored rice is similar to
that found on wheat grain. The insecticide and its degradation
products were concentrated in the husk in which the rate of breakdown
appeared to be unaffected by the moisture content of the rice (Bowker,
1973; Bullock, 1973). Representative results are given in Table 9.
TABLE 8. Effect of moisture on degradation of pirimiphos-methyl residues in wheat
grain (Bowker, 1973)
Application Pirimiphos-methyl equivalents,
rate and mg/kg, after interval (weeks)
moisture
content Compound 0 2 4 8 16 32
4 mg/kg, I (PP511) 2.16 2.66 2.05 2.66 2.32 2.08
14% moisture II + unidentified* 0.04 0.04 0.04 0.06 0.04 -
IV, V, VI <0.01 0.07 0.08 0.17 0.21 0.29
TLC baseline <0.01 <0.01 0.04 0.04 0.08 0.26
4 mg/kg,
18% moisture I 2.36 2.73 1.12 0.80 0.79 0.38
II + unidentified* 0.05 0.03 0.03 0.03 0.03 0.03
IV, V, VI - 0.49 0.56 1.05 0.92 0.66
TLC baseline - - 0.21 0.03 0.16 0.14
8 mg/kg, I 4.03 5.46 3.80 4.64 5.53 5.37
14% moisture II + unidentified* 0.12 0.08 0.08 0.11 0.04 0.10
IV, V, VI - 0.15 0.11 0.16 0.32 0.59
TLC baseline - - 0.02 0.03 0.04 0.22
8 mg/kg, I 4.44 5.64 2.93 2.84 2.19 1.28
18% moisture II + unidentified* 0.69 0.27 0.08 0.09 0.05 0.04
IV, V, VI - 0.98 0.99 1.61 1.89 1.61
TLC baseline - - 0.41 0.06 0.24 0.27
* Unidentified compound (minor product).
TABLE 9. Effect of moisture on degradation of pirimiphos-methyl residues in brown rice
(Bowker, 1973)
Application Pirimiphos-methyl equivalents, mg/kg,
rate and after interval (weeks)
moisture
content Compound 0 2 4 8 16
4 mg/kg, I (PP511) 2.13 2.31 2.06 2.19 -
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.07 -
IV, (V, VI) 0.05 0.11 0.06 0.10 -
TLC baseline 0.01 0.07 0.23 0.07 0.02
4 mg/kg, I 1.89 1.99 1.5 1.17 0.76
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.12 <0.01
IV, (V, VI) 0.06 0.17 0.34 0.24 0.33
TLC baseline 0.01 0.12 0.26 0.27 0.17
8 mg/kg, I 3.7 4.37 4.1 3.73 2.99
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.11 0.09
IV, (V, VI) 0.1 0.18 0.11 0.34 0.17
TLC baseline 0.03 0.13 0.36 0.23 0.06
8 mg/kg, I 3.11 3.68 1.55 2.3 1.58
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.21 0.11
IV, (V, VI) 0.06 0.32 0.10 0.86 0.45
TLC baseline 0.02 0.19 1.48 0.43 0.06
* Unidentified compound (minor product)
Pirimiphos-methyl applied as a dust formulation to wheat and
brown rice is degraded in a similar manner to other organophosphorus
insecticides used to protect stored grain from infestation (Rowlands,
1966).
Degradation is marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
is caused by factors within the grain or by the associated microflora
is not known. At higher moisture contents (approximately 18%) less
pirimiphos-methyl is recovered on analysis but increased levels of the
hydrolysis product (IV) are obtained suggesting that a more rapid
degradation of the insecticide occurs. Bowker concludes that grain may
lack the enzymic activity believed to be present in plants and soil to
cleave the pyrimidine N-ethyl bonds. It is likely therefore that
following the treatment of wheat and brown rice with
pirimiphos-methyl, the major residues during storage will be the
insecticide itself and the simple hydrolysis product (IV). Under
optimum conditions, the maximum level of compound (IV) following
treatment at 4 mg/kg, was found to be 0.17 mg/kg; under poor storage
conditions with high moisture content grain, 0.62 mg/kg.
Solutions of pirimiphos-methyl-14C were applied in the
laboratory to wheat grains of 14 and 18 percent moisture, and also as
a 2 percept dust, to give levels of 4 mg/kg. Throughout a storage
period of 6 months, the residues of pirimiphos-methyl were found
almost entirely in the seed coat and aleurone layer, with only traces
present in the germ or endosperm. It was also clear that, as with
malathion but to a greater extent, there was transfer of insecticide
between grains, possibly in the vapour phase. About 10 percent of the
total residual radioactivity was bound to lipoprotein material in the
aleurone region of the grain and was unextractable except by digestion
of the aleurone protein. By contrast with other organophosphates
studied, the bound material appeared to be the unchanged pesticide,
rather than a metabolite. Certainly it was a P=S compound, but
degradation during the liberation of the bound material complicated
identification. During the storage period, the pirimiphos-methyl
available for decomposition decreased by 15 and 50 percent at the 14
and 18 percent moisture levels respectively. The main products found
were the free hydroxy-pyrimidine and the N-desethyl pyrimidinol
(Rowlands et al., 1974).
b) Toxicity of degradation products
The hydroxypyrimidines (IV), (V) and (VI) are members of a class
of compounds known to exhibit low mammalian toxicity and they are also
products of animal metabolism. It is therefore concluded that
pirimiphos-methyl itself represents the major toxic residue in stored
products. Traces of the phosphorus-containing degradation products
(II) and (III), not exceeding 0.05 mg/kg in total, may also occur.
They are determined by the preferred analytical method. The acute oral
LD50 of (II) to the female rat is 800-1600 mg/kg. The oxygen analogue
(III) is chemically unstable and it has not been possible to undertake
toxicity tests with it (Gage, 1971b).
In plants
In contrast to its fate in stored products pirimiphos-methyl is
rapidly lost from leaf surfaces during the first 2-3 days after
spraying, mainly by volatilisation.
Bowker and Hughes (1974a) identified by a combination of
thin-layer and gas-liquid chromatography of the compounds or their
derivatives, all the major extractable metabolites and degradation
products arising from the interaction of pirimiphos-methyl with
plants.
They showed that on plant surfaces, pirimiphos-methyl is lost
rapidly by vaporisation. Levels of the parent insecticide and the
major degradation product, compound (II), represent less than 10% of
the applied material after two to three days. In crops after foliar
treatment the hydroxypyrimidine (IV), formed by hydrolysis of the
insecticide, is unlikely to be a significant residue. They found that
the compound is usually present only in trace amounts and is degraded
photochemically and bound to plant material.
These same workers showed that photochemical degradation of
pirimiphos-methyl on leaf surfaces leads to the formation of compound
(II) which is not accumulated. When applied to water in which rice
seedlings were growing, pirimiphos-methyl is not translocated
significantly into the foliage; in these conditions the hydrolysis
product (IV) is likely to represent the predominant pyrimidine
residue. Compounds (V) and (VI) only occur in trace amounts.
In animals
Much of the information in this section is also to be found under
the heading "Absorption, distribution and excretion," but is repeated
here for convenience.
Goat
Bowker et al. (1973) report studies where a single dose of
radio-labelled pirimiphos-methyl was given orally to a lactating goat
(0.12 mg/kg bw, equivalent to approximately 6 ppm in the ration). 91%
of the radio-activity was excreted in the following 8 days: 87% in the
urine and 4% in the faeces. Only 0.41% of the radio-activity was
secreted in the milk, primarily during the first 24 hours. The maximum
residue in the milk was 0.026 mg/kg pirimiphos-methyl equivalents, of
which pirimiphos-methyl itself represented 0.003 mg/kg.
Cow
The pattern of excretion in the cow is very similar to that in
the goat (Bullock et al., 1974a). When a single dose of radio-labelled
pirimiphos-methyl was given orally to a cow at 0.5 mg/kg bw
(equivalent to approximately 17 ppm in the daily diet), the
radio-activity was quantitatively recovered during the following seven
days - 85% in the urine and 14% in the faeces. Only 0.37% of the
radio-activity was secreted in the milk (0.35% during the first three
days).
The highest levels of radioactivity in the milk, urine and faeces
were found during the first day after dosing. The milk contained 0.04
mg/kg pirimiphos-methyl equivalents, of which approximately 75% could
be extracted from the fat and protein fraction into organic solvents.
Pirimiphos-methyl accounted for approximately 12% (i.e. about 0.004
ppm), and compound (V) for about 50% of the total radioactivity in the
milk. The radioactivity was not extracted by organic solvents and
appeared to be incorporated into natural milk components.
Approximately 70% of the radio-label in "day 1" urine was
organo-soluble, the remainder dissolving only in water.
Four groups of three cows were maintained by Bullock et al.
(1974b) for 30 days on diets containing 0, 5, 15 and 50 ppm
pirimiphos-methyl. Residues of pirimiphos-methyl were determined in
milk samples taken every two days and in samples of kidney, liver,
heart, fat and muscle taken at the end of the study. None of these
residues exceeded 0.02 mg/kg. The authors prepared butter from the
milk of cows dosed at the 110 mg/kg bw rate but found this to contain
only 0.02-0.04 mg/kg pirimiphos-methyl. In all instances no residues
of the organophosphorus compounds (II) and (III) could be detected in
samples of meat, milk or butter. (Limit of detection: 0.01 mg/kg in
each case).
Hen
Green et al. (1973) report that when diets containing 4 ppm
pirimiphos-methyl were fed to laying hens for 28 days, or when a diet
containing 32 ppm was fed for 7 days, residues of pirimiphos-methyl
itself in eggs did not exceed 0.01 mg/kg and no residues of compound
(III) were detected (Limit of detection: 0.001 mg/kg). The same
authors using radio-labelled pirimiphos-methyl found that the total
amount of radio-activity in eggs reached a maximum of 0.03 mg/kg
pirimiphos-methyl equivalents after 15 days when fed at a
concentration of 4 ppm in the ration and a maximum of 0.15 mg/kg
pirimiphos-methyl equivalents at the end of the 32 ppm study. In
muscle samples taken at the end of the 32 ppm study no residues of
pirimiphos-methyl or of its P=0 analogue were detected, (Limit of
detection: 0.001 ppm). Since 86-90% of the radio-activity in eggs is
present as water-soluble unidentified metabolites and since at no time
does the concentration of pirimiphos-methyl itself in yolks or whites
exceed 0.001 mg/kg, Green et al. conclude that pirimiphos-methyl does
not accumulate in eggs during continuous feeding of the insecticide
and that at the proposed level of application the residues of the
pesticide in eggs are negligible.
Graham and Jenkins (1974), as part of a study of the effect of
pirimiphos-methyl on the growth rate of broiler chickens, analysed
muscle, skin and fat samples from birds receiving 4, 8, 16, 32 and 48
ppm pirimiphos-methyl continuously in rations for 9 weeks. Birds were
slaughtered within 1 hour of being removed from feed. The only
residues which appeared were detected in skin and fat. The highest
level, 0.017 mg/kg, was in the fat of a broiler receiving 48 ppm
pirimiphos-methyl in the ration. Another had 0.015 mg/kg. All other
samples contained less than 0.01 mg/kg or residues were not
detectable.
In soil
Pirimiphos-methyl shows very limited persistence and mobility in
soil.
Bowker, Riley and Gratton (1972) showed that in a range of soil
types the half-life of pirimiphos-methyl is less than one month. The
major soil metabolite is the parent hydroxypyrimidine (IV) together
with smaller amounts of the related compounds (V) and (VI). The routes
of metabolism in stored grain, plants and soil are therefore similar.
The same authors using a descending thick-layer chromatography
technique, showed pirimiphos-methyl to possess only limited mobility
in soil: considerably less than atrazine which was used as a standard
and which itself is recognised as being only moderately mobile.
In water
Pirimiphos-methyl in rapidly degraded in water, mainly by
hydrolysis with loss of the phosphorothionate ester side chain. This
process is accelerated in the presence of light. Bowker and Hughes
(1974b) confirmed this and showed that although some volatilisation
occurs from still water, this is less significant than hydrolysis in
accounting for loss of the insecticide. In their experiments 50%
degradation had occurred after one day in sunlight. The major
degradation product in water is compound (IV). Only trace quantities
of the P=0 analogue (III) were detected (less than 0.01 mg/l in water
treated initially with pirimiphos-methyl at 1 mg/l).
In processing
Wheat
Bullock (1973 and 1974) reported many separate experiments which
demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table
10 summarises the results of residue trials carried out in the U.K. on
wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 11 summarises results of a residue trial reported by
Bullock (1973) to have been carried out in the U.K. with wheat
nominally treated to contain 8 ppm pirimiphos-methyl. These data which
are substantially in agreement with those of Bengston et al. (1974)
show that there is relatively little penetration beyond the seed coat
even throughout a storage period of 9 weeks.
TABLE 10. Effect of milling and baking on residues in wheat admixed
with pirimiphos-methyl at 4 mg/kg-UK (Bullock, 1973, 1974)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
Whole 0 4.2 1.9 2.9 (9)
grain 3.0* -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Wholemeal 0 1.3 0.94 1.1 (3)
flour
1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White 0 0.88 0.30 0.52 (6)
flour 0.56* 0.53* 0.55* (3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27* (3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread
1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
TABLE 10. (Cont'd.)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
White 0 0.28 0.19 0.23 (6)
bread 0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 All results are from field trials except those marked*
which are from a small-scale trial. Figures in parentheses are
the numbers of results upon which the means are based.
TABLE 11. Residues of pirimiphos-methyl in whole grains and in milling
and baking fractions of whole grains treated in a laboratory
trial in UK at 8 mg/kg. (Bullock, 1973, 1974)
Interval between Residues, mg/kg.1
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52 - -
6.0 0.91 0.56 - -
6.0 1.0 0.57 - -
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 In all cases, no residues of the phosphorus-containing compounds (II)
or (III) (Figure 1) were detected. (Limit of detection: 0.01 ppm in
each case.)
Bengston et al. (1974) report an extensive series of trials in
which pirimiphos-methyl was applied to wheat at the rate of 6 mg/kg in
bulk silos in two areas of Australia. Wheat was submitted to two
separate laboratories, for analysis and for processing, milling and
baking. The results reported by the two independent laboratories are
summarised in Table 12. These studies revealed that though a
significant amount of the pirimiphos-methyl was removed during the
cleaning process prior to the milling of the wheat, by far the
greatest separation occurred in the bran. Although the bran
represented only 25% of the whole grain it contained just over two
thirds of the entire residue leading to a concentration in the bran of
250% of that in the whole grain from which the bran was derived. The
concentration in the flour after milling averaged only 22% of that in
the whole grain. When the grain was processed into bread and baked
there was a further 63% reduction in the level of residues according
to one co-operating laboratory and an almost complete destruction of
the insecticide reported by the other. Assuming that the amount of
residue found in the bread would always be as high as the highest
level reported (0.3 mg/kg) the processing, milling and baking of bread
from raw grain containing pirimiphos-methyl results in a 93%
destruction of the original residue. Under practical conditions this
could well be greater because there is normally a significant delay at
each stage of the process which would further promote degradation of
the residue.
Rice
Residues of pirimiphos-methyl and its degradation products in
rice in husk are concentrated in the husk. Bowker (1973) and Bullock
(1973, 1974) report that parboiling rice to remove the husk results in
a substantial loss of the insecticide and its degradation products
from the husk into the water used to soak the rice. Table 13, taken
from Bullock (1973), shows the results of experiments in Guyana
designed to determine the lose of residues during the processing of
rice which had been treated previously while in husk at a nominal rate
of 5 and 10 mg/kg with pirimiphos-methyl. These results clearly
demonstrate that the bulk of the residue is in the husk with the
remainder in the seed coat. Polishing removes virtually all of the
material deposited in the seed coat although the process of parboiling
appears to promote the transfer of a small amount of residue into the
grain itself.
TABLE 12. Fate of pirimiphos-methyl residues in wheat treated at 6 mg/kg and subjected to
milling and baking (Bengston et al., 1974).
Residues, mg/kg,
Residues, mg/kg, Wheat W Wheat M
Laboratory A Laboratory B Laboratory B
Stage of Processing 1 2 Mean 1 2 Mean 1 2 Mean
Whole grain 4.7 4.8 4.8 4.2 4.2 4.2 4.5 4.5 4.5
Cleaned grain 3.8 3.8 3.8 - - - - - -
Bran 10.0 9.9 10.0 8.9 9.3 9.1 11.9 12.7 12.4
Shorts (pollard) 7.4 7.7 7.6 8.2 8.5 8.3 10.1 10.6 10.4
Flour 0.85 0.88 0.9 0.6 0.8 0.7 1.3 1.3 1.3
Bread 0.31 0.32 0.3 <0.2 <0.2 <0.2 0.46 0.48 0.47
TABLE 13. Effect of milling and polishing on residues in rice admixed with
pirimiphos-methyl (5 or 10 mg/kg) while in husk - Guyana
(Bullock, 1973).
Residues, mg/kg.
Treated at 5 mg/kg Treated at 10 mg/kg
Not Not
Sample parboiled Parboiled parboiled Parboiled
Dehusked grain 0.55 0.58 0.72 1.46
Husks 8.7 2.1 9.4 4.95
Grain polished 1 min. 0.26 0.31 0.29 0.76
Polishings from 1st min. 5.9 6.3 9.0 14.0
Grain polished 2 min. 0.19 0.29 0.29 0.76
Polishings from 2nd min. 5.6 6.5 9.4 15.8
Grain polished 5 mins. <0.01 0.28 0.27 0.65
Polishings from 3rd to 5th mins. 5.3 7.1 9.3 14.9
Bullock (1973) reports experiments carried out in Indonesia to
determine the loss of pirimiphos-methyl residues from polished rice
during cooking. Rice which had been treated by admixture with
pirimiphos-methyl emulsifiable solution and which contained 1.96 mg/kg
pirimiphos-methyl was used in the trial. The rice was washed under the
cold water tap for two minutes before simmering for 20 minutes in 2.5
times its own weight of tap water. The rice by this time was a soft
mass. Cold tap water was poured over the grains to separate them and
the samples were then analysed in the usual manner. A second sample of
the same batch of rice was treated in the same way and duplicate
analyses were carried out on each sample. The results which are given
below show a 74% loss in cooking white rice.
Residue, mg/kg, before cooking: 1.96; 1.96
after cooking: 0.58, 0.50 (mean 0.54); 0.50, 0.47
(mean 0.49).
No residues of the phosphorus-containing compounds (II) or (III)
were detected (limit of detection: 0.01 ppm in both cases).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
Two methods of residue analysis are available. The more
sensitive, quicker and preferred method is that of Bullock (1972) in
which gas chromatography with a phosphorus detector is used. The
method is suitable for the determination of residues of
pirimiphos-methyl in crops, it also determines the
phosphorus-containing metabolites (II) and (III). The limit of
determination is 0.01 mg/kg in each case. Crop samples are extracted
with a 20% solution of acetone in n-hexane. A decanted portion of the
extract is washed with water, and then dried with anhydrous sodium
sulphate. A portion of the extract is then injected into a gas
chromatograph equipped with a flame photometric or thermionic
detector. The peaks obtained at the retention times of
pirimiphos-methyl and its metabolites (II) and (III) are compared with
standards. Grain samples are extracted with methanol instead of
acetone/hexane and oily or fatty materials are subjected to an
acetonitrile-n-hexane partition before analysis. The oxygen analogue
(III) has not been detected as a metabolite in any samples analysed in
the laboratory of the manufacturer, although (as stated above) its
limit of determination is 0.01 mg/kg.
Alternatively when GLC is not available, a colorimetric method
may be used. The colorimetric method reported by Bullock (1969) has
only been tried on field crop samples. An extract, obtained by
prolonged maceration or slurrying the sample with n-hexane, is dried
with anhydrous sodium sulphate, filtered, concentrated down to a small
volume in a rotary evaporator at low temperature and quantitatively
transferred to a preparative-layer plate together with a marker spot
of pirimiphos-methyl. The plate is developed in a mixture of n-hexane
and acetone and inspected under ultra-violet light. The band opposite
the marker spot is scraped off and refluxed for one hour with
concentrated hydrobromic acid to liberate hydrogen sulphide, which is
swept off in a stream of nitrogen, quantitatively absorbed in alkaline
zinc acetate and subsequently determined as methylene blue. The limit
of determination is normally about 0.1 mg/kg with recovery in excess
of 75%.
Bullock (1973) reports studies to determine the most effective
solvent for extracting pirimiphos-methyl and its metabolites from
"aged" residues in stored grain. Table 14 shows the results of these
studies indicating the residues of pirimiphos-methyl extractable from
aged samples by different extraction methods. The samples were ground
to a fine powder before analysis. The lowest recoveries are almost
invariably with methanol under reflux. Comparable results are obtained
with the other solvent systems when extraction is made immediately
after treatment, but on aged samples cold methanol generally gives
significantly higher results.
TABLE 14. Residues of pirimiphos-methyl extractable from "aged" samples by different
extraction methods. (Samples ground to fine powder before analysis.)
Residues determined (mg/kg) using various
Sample** Interval after extraction methods*
(Treatment rate) treatment 1 2 3 4 5
Wheat (4 mg/kg) 0 days 3.72 3.45 3.82 3.87 2.91
1 month 2.92 2.73 2.79 3.01 3.17
3 months 1.87 1.73 1.99 2.17 2.04
Barley (4 mg/kg) 0 days 3.07 3.12 3.49 3.09 1.90
1 month 2.51 2.36 2.17 2.69 2.54
3 months 1.53 1.71 1.47 1.84 1.70
Rice (4 mg/kg) 0 days 4.10 3.92 4.00 4.07 3.12
1 month 2.18 2.40 2.12 2.71 2.60
3 months 1.41 1.95 1.84 1.99 1.03
* Extraction methods: 1 Dichloromethane (cold); 2 Acetone (cold); 3 20% Acetone
in hexane (cold); 4 Methanol (cold); 5 Methanol (1 hour reflux).
** The samples had a moisture content of 13-14%.
Bullock also reports studies which show that it is preferable to
grind aged samples before attempting to extract with either methanol
or acetone/hexane. Grinding offers no advantage when dealing with
wheat or rice that has only just been treated but in view of the fact
that many regulatory analysts will receive samples which have been
treated three months to three years previously it is obvious that the
preparation of the sample and the selection of the solvent are of
critical importance. Table 15 shows results obtained by Bullock. The
samples were ground in a Moulinex coffee grinder. Later comparison
with Hobart laboratory grinder showed no significant difference in the
results and little temperature rise in the grain during grinding.
TABLE 15. Effect of grinding on extractability of aged residues
(Bullock, 1973).
Sample Residues, mg/kg, determined using
(Treated by Interval different extraction solvents
admixture after Original 20% acetone Methanol
at 4 mg/kg) treatment or ground in hexane
Wheat 0 days Original 3.82 3.91
Ground 3.82 3.87
1 month Original 2.70 2.81
Ground 3.10 3.04
3 months Original 1.43 1.55
Ground 1.87 1.99
Rice 0 days Original 4.01 4.05
Ground 4.10 4.07
1 month Original 2.00 1.91
Ground 2.18 2.34
3 months Original 1.67 1.71
Ground 1.84 1.99
As a check on the loss of residues during grinding Bullock took
wheat which had been freshly treated with pirimiphos-methyl and
measured the residue level before and after intense grinding for one
minute. The analytical results varied by less than 50% which is slight
by comparison with the loss which would occur if grain treated with
malathion or dichlorvos were ground in a similar manner. The same
author reports experiments designed to determine the loss of residue
from wheat, barley and rice when grain samples were stored at -14°C
while awaiting analysis. Both freshly treated grain and grain which
had been treated under semi-commercial conditions three months prior
to sampling were kept under such storage conditions but none of these
samples showed as much as 5% loss during two months at -14°C.
NATIONAL TOLERANCES
The information available to the meeting indicates that few
national tolerances have been established. It is understood that a
tolerance of 4 mg/kg has been proposed in France for pirimiphos-methyl
residues in cereals and 2 mg/kg in fruits and vegetables. In the
Netherlands a tolerance of 0.2 mg/kg has been established for
pirimiphos-methyl residues in cucumbers, melons, paprika and tomatoes.
APPRAISAL
Pirimiphos-methyl is a broad-spectrum organophosphorus
insecticide with both contact and fumigant action. It shows only
limited biological persistence on plant surfaces but gives long
lasting control of insect pests on inert surfaces and retains its
biological activity when applied to stored agricultural commodities
including raw grains and nuts.
The meeting had only limited Information concerning the
pre-harvest use and performance of this material on crops but
extensive data were available on its use, performance and fate on a
variety of small grains and nuts. The recommended rate of application
to raw cereals is 6 mg/kg.
Exceptional stability on grain results in an effective half-life
of up to 80 weeks. Although high temperatures and high moisture levels
in grain reduce the life of the deposit the effect of these influences
is much less than with other grain protectants approved or evaluated
to date.
Data were available to show that on both wheat and rice the
deposit resulting from admixture of pirimiphos-methyl with grain is
almost entirely in the seed coat and husk respectively. Cleaning,
processing and milling removes the bulk of the applied material. In
the case of wheat the milling process results in most of the residue
being separated into the bran where the concentration of
pirimiphos-methyl can range up to 20 mg/kg. In the case of rice
substantially all of the deposit is removed during the milling and
polishing operations. Depending upon the milling process, flour
prepared from treated wheat may contain significant amounts of
pirimiphos-methyl but a major proportion of this is destroyed in the
baking of bread. The small amount of residue remaining in polished
rice is substantially destroyed in cooking.
Pirimiphos-methyl and the corresponding hydroxypirimidine are the
only significant residues detected. Residues of the
phosphorus-containing metabolites when detected were always extremely
low (approximately 0.05 mg/kg).
Extensive data are available on the fate of pirimiphos-methyl in
plants, domestic animals, hens and their edible tissues, and foods of
animal origin. In living plants the major phosphorus-containing
degradation product is the N-desethyl metabolite (II) which is not
accumulated. The hydroxypyrimidine compound (IV) is the major
degradation product following root uptake. When fed to domestic
animals pirimiphos-methyl is rapidly metabolised and excreted, only
very small quantities being secreted into milk or eggs. Cattle
receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites could
be detected in meat, milk, butter or eggs.
A GLC method of analysis suitable for the determination of
residues of pirimiphos-methyl and the phosphorus-containing
metabolites in plant materials is available, the limit of
determination being reported to be 0.01 mg/kg. The method appears
suitable for regulatory purposes. Evidence of the importance of sample
preparation and method of extraction has been presented.
No national tolerances have yet been determined.
In proposing maximum residue limits for pirimiphos-methyl and its
metabolites on raw grain, milled cereal products and foods prepared
therefrom, careful consideration has been given to the fact that this
insecticide is to be used as a grain protectant, that a certain
concentration must be present on the grain to control infestations and
prevent damage to stored products and that the compound is
exceptionally stable under storage conditions. Under practical
conditions of grain handling and storage it is not possible to apply a
grain protectant with assurance that the deposit will be absolutely
uniform. There will always be a natural variation in the level of the
deposit resulting from fluctuations in the flow of grain and
insecticide. It is therefore not possible to fix the maximum limit on
the minimum necessary to control the insect pests. Furthermore
extensive work has demonstrated that the movement of bulk grain
results in the segregation of the various components of the bulk with
a resultant variation in the level of residues throughout the mass.
Due allowance must be made for the amplitude of these variations and
the problems of sampling and analysis.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl, expressed as pirimiphos-methyl.
TEMPORARY TOLERANCE
mg/kg
Bran (wheat, rice) 20
Wheat, rye, rice (in husk) 10
Barley, maize, oats 7
Wholemeal flour (wheat, rye) 5
Rice (dehusked), wheat flour (white) 2
Bread (wholemeal), rice (polished) 1
Bread (white) 0.5
Meat, milk, eggs 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 1976)
1. Additional studies to clearly establish no-effect level for
decreased pregnancy rates, and hydronephrosis in offspring.
2. Further studies to clarify the liver injury dogs observed in 90
day studies.
DESIRABLE
1. Longer duration observations in man.
2. Information from studies now in progress on other stored
commodities including nuts, peanuts and dried fruit. The
information is expected to be available during 1975.
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in food at
the point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
REFERENCES
Bengston, M., Connell, M., Desmarchelier, J., Phillips, M., Snelson,
J. and Sticka, R. (1974) Evaluation of four new grain protectants.
Report to Australian Wheat Board. (To be published)
Bowker, D.M. (1973) Pirimiphos-methyl (PP 511): fate of stored wheat
and rice grain in the laboratory. ICI Plant Protection Ltd. Report No.
AR 2457 AR. (Unpublished)
Bowker, D.M., Griggs, B.F. and Harper, P. (1973) Pirimiphos-methyl (PP
511): excretion by a goat. ICI Plant Protection Ltd. Report No. AR
2458 B. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in crops.
ICI Plant Protection Ltd. Report No. AR 2515 A. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in water.
ICI Plant Protection Ltd. Report No. AR 2516 A. (Unpublished)
Bowker, D.M., Riley, D. and Gratton, R.P. (1972) Pirimiphos-methyl:
fate in soil. ICI Plant Protection Ltd. Report No. TMJ 809 A.
(Unpublished)
Bratt, H. and Dudley, L.A. (1970) Pirimiphos-methyl (PP 511):
Excretion by rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bratt, H. and Jones, L.A. (1973) Pirimiphos-methyl (PP 511):
Metabolism in rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bullock, D.J.W. (1969) Provisional analytical method for the
determination of PP211 and PP511 residues in vegetables, grain and
fruit. ICI Plant Protection Ltd. Method No. PAM 310/A.
Bullock, D.J.W. (1972) Residue analytical method no. 11 for the
determination of residues of pirimiphos-methyl and its
phosphorus-containing metabolites in crops (gas chromatographic
method). ICI Plant Protection Ltd. Method PPRAM-11/A.
Bullock, D.J.W. (1973) Pirimiphos-methyl: residues in stored grain.
ICI Plant Protection Ltd. Report No. AR 2472 AR. (Unpublished)
Bullock, D.J.W. (1974) Pirimiphos-methyl: residues in stored grain
bread, flour and milled products. ICI Plant Protection Limited. Report
No. AR 2537 A. (Unpublished)
Bullock, D.J.W., Day, S., Hemingway, R.J. and Jegatheeswaran, T.
(1974) Pirimiphos-methyl: residue transfer study with cows. Report
from ICI Plant Protection Limited. (Unpublished)
Bullock, D.J.W., Day, S., and Griggs, B.F. (1974) Pirimiphos-methyl:
metabolism and residue transfer into the meat and milk of a lactating
cow. ICI Plant Protection Ltd. Report No. AR 2552 A. (Unpublished)
Chart, S., Foulkes, Caroline A., Gore, G.W. and Williamson, K.S.
(1974) Erythrocyte and plasma cholinesterase activity in human
volunteers administered pirimiphos-methyl. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Clapp, M.J. and Conning, D.M. (1970) Pirimiphos-methyl (PP511).
Ninety-day oral toxicity in rats. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Clark, D.G. (1970) The toxicity of PP511
[O-(2-diethylamino-6-methylpyrimidin-4-yl)
O,O-dimethylphosphorothioate] Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fink, R. (1974a) Eight-day dietary LC50 - mallard ducks: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Fink, R. (1974b) Eight-day dietary LC50 - bobwhite quail: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Gage, J.C. (1971a) Pirimiphos-methyl (PP511): Avian toxicity. Report
from ICI Industrial Hygiene Research Laboratories. (Unpublished)
Gage, J.C. (1971b) Pirimiphos-methyl (PP 211) and pirimiphos-methyl
(PP 511): acute and subacute oral toxicity in the rat of the plant
metabolite, 2-diethylamino-4-hydroxy-6-methylpyrimidine (R46382). ICI
Industrial Hygiene Research Laboratory. Report No. HO/IH/R/327.
(Unpublished)
Gage, J.C. (1972) Pirimiphos-methyl (PP511): Oral toxicity in the dog
and in a passerine bird species. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Gore, C.W. Palmer, S. and Pratt, I.S. (1974) Pirimiphos-methyl
(PP511): teratogenic studies in the rabbit. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Gore, C.W., Griffiths, D. and Phillips, C.E. (1974) Pirimiphos-methyl
(PP511): two year feeding study in the rat. Report from ICI Industrial
Hygiene Research Laboratories. (Unpublished)
Graham, C.A. and Jenkins, S.L. (1974) The effect of pirimiphos-methyl
on the growth rate of broiler chicks. ICI Australia Ltd. Merrindale
Research Station Report, July 1974.
Green, T., Monks, I.H. and Phillips, P.J. (1973) Pirimiphos-methyl (PP
511): sub-acute oral and residue studies in hens. ICI Industrial
Hygiene Research Laboratories. Report No. HO/IH/P/65B. (Unpublished)
Hodge, M.C.E. and Moore, S. (1972) Pirimiphos-methyl (PP 511):
Teratological studies in the rat. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Noel, P.R.B., Mawdesley-Thomas, L.E., Rivett, K.F., Squires, P.F. and
Street, E. (1970) PP 511: Oral toxicity studies in dogs - initial
studies and repeated dosage for thirteen weeks. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and Cherry, C.P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat: histopathological
examination on testes of F1B and F2B males. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and James, P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat. Report from Huntingdon
Research Centre. (Unpublished)
Parkinson, G.R. (1972a) Pirimiphos-methyl (PP 511): potentiation with
gamma-BHC. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Parkinson, G.R. (1972b) Pirimiphos-methyl (PP 511): potentiation with
dichlorvos. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Pieterse, A.H., Schulten, G.G.H. and Kuyken, W. (1972) A study of
insecticide resistance in Tribolium castaneum in Malawi. J. Stored
Prod. Res., 8(3):183.
Rivett, K.F., Edwards, B., Street, E. and Newman, A.J. (1973) PP 511:
Oral toxicity study in beagle dogs - repeated daily dosage for two
years. Report from Huntingdon Research Centre. (Unpublished)
Ross, D.B., Burroughs, Sheila J. and Roberts, Nicholas L. (1974) Egg
production and hatch-ability in the laying hen following dietary
inclusion of pirimiphos-methyl at various levels. Report from
Huntingdon Research Centre. (Unpublished)
Rowlands, D.G. (1966) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. Stored Prod. Res., 2:105-116.
Rowlands, D.G. et al. (1974) Preliminary report of studies with
pirimiphos-methyl as a grain protectant. Submission by United Kingdom
to FAO. (To be published)
Waterhouse, D. (1973) Background paper presented at the Ninth Session
of the FAO Working Party of Experts on Pest Resistance to Pesticides
(Rome, 20 June 1973). (Unpublished)
Admixture with small grain cereals
Bullock (1973 and 1974) reports numerous trials undertaken in
Australia, Argentina, Guyana, Malaysia, U.K. and the U.S.A. where
pirimiphos-methyl was admixed with wheat, barley, maize, and rice in
husk (residues were determined on the rice after milling). The results
are summarised in Table 4. Mean initial residues of pirimiphos-methyl
itself in the whole grains of wheat, barley and maize rarely exceeded
the nominal treatment rate and were frequently in the range of 40-60%
of the nominal dose. This appears to be a reflection of the method of
application and suggests that some at least of the nominal dose is
lost into the atmosphere during application. Higher percentage
retention of the applied chemical was obtained by treating 5-10% of
the grain with all the chemical rather than applying it to 50% or more
of the grain stream entering the storage. Again no residues of the
phosphorus-containing compounds (II) and (III) were detected.
TABLE 3. Residues of pirimiphos-methyl in whole grains
after spraying bagged grains. (Bullock, 1973)
Interval
between Range of
Application application residues
Rate and sampling found,
Crop mg/m2 months mg/kg Country
Barley 400 0-3 0.05-0.33 UK
Oats 400 2-3 0.07-0.19 UK
Rice in husk 300 0-3 <0.01-0.08 Colombia
Milled rice 500 0-5 0.02-0.28 Malaysia
Treated when
in husk 1000 0-5 0.01-0.70 Malaysia
TABLE 4. Residues of pirimiphos-methyl in whole grains
after admixture. (Bullock, 1973, 1974)
Interval
Nominal between Range of
admixture admixture residues
rate, and sampling found,
Crop mg/kg (months) mg/kg Country
Wheat 3 0-8 1.0-2.1 Australia
4 0-3 1.3-4.6 UK
5 1-3 1.7-4.7 USA
6 0-12 1.3-3.9 Argentina
0-8 3.0-4.9 Australia
10 1-3 4.7-8.4 USA
Barley 4 0-3 0.03-3.6 UK
8 0-3 0.26-4.4 UK
Maize 5 1-3 2.1-6.9 USA
10 1-3 4.7-8.0 USA
Milled rice 3 0-3 0.21-0.53 Guyana
treated when
in husk 4 0-5 0.12-0.26 Malaysia
5 0-4 0.16-0.59 Guyana
7.5 0-11 0.53-0.98 Guyana
8 0-5 0.21-0.85 Malaysia
Wheat
The stability of the deposit of pirimiphos-methyl on wheat grains
is quite remarkable and from the work of Bullock the half life has
been estimated to be of the order of 9 months. Degradation proceeds
uniformly and slowly as indicated in the results from a typical trial
carried out in Argentina on wheat with a moisture content ranging from
11 to 13% (Table 5).
TABLE 5. Pirimiphos-methyl residues in wheat at intervals after admixture-Argentina.
(Bullock, 1973)
Nominal
admixture
rate
mg/kg 1 Day 1 Month 2 Months 3 Months 4 Months 6 Months 9 Months 12 Months
2 1.60 1.16 1.47 2.06 2.00 0.90 0.80
3 1.61 1.53 1.30 1.34 1.13 1.96 1.03 0.80
4 2.04 2.40 1.89 1.79 1.53 1.80 1.66 0.92
5 2.26 2.79 2.06 2.00 1.93 2.00 1.73 1.31
6 3.91 3.65 2.62 2.42 2.39 2.14 2.02 1.29
7 3.90 3.71 3.12 3.06 2.43 2.30 2.20 1.35
Moisture content: low (11-13%)
Bengston et al. (1974) report the results of two extensive trials
in Australia with five grain protectant insecticides applied to wheat
having a mean moisture content of 10-12% and a mean temperature of
29-32°C. In these trials the target application rate was 6 mg/kg but
based on the analyst's determination of the concentrate strength and
the weight of grain treated (2000 tons per treatment) the calculated
application rate was 5.5 mg/kg. Analysis by five collaborating
laboratories showed the concentration of pirimiphos-methyl on samples
taken 1 week after application to range from 5.5-6.4 mg/kg.
Representative data summarised in Table 6 show relatively little
change over a 26 week period notwithstanding the high temperature
which prevailed throughout.
Based on this work calculations have been made of the half-life
of Pirimiphos-methyl deposits on wheat stored in concrete bins at
30°C. When using data from samples taken from within 10 cm of the
surface and using the rate of disappearance during weeks 1-11, a
half-life of 43 weeks is obtained. Based on data from weeks 11-26 the
half-life was 104 weeks. Using data from samples taken 6 metres
beneath the surface where the mean temperature exceeded 32°C a
half-life of 77 weeks is obtained whether the data from samples
collected during weeks 1-11 or weeks 11-26 are used. The mean
half-life from many data is 82 weeks. This compares with a half-life
for malathion of 3-9 weeks under comparable conditions.
Barley
Bullock (1973 and 1974) reports numerous trials where barley was
treated with pirimiphos-methyl in the United Kingdom by admixture of
2% Pirimiphos-methyl dust or by the application of dilute emulsion
designed to deposit from 2-8 mg/kg on the weight of grain.
Unfortunately moisture and temperature conditions are not stated and
extensive sampling and analysis has failed to recover more than 25-30%
of the amount that was supposed to have been applied. Residue analyses
however fail to show much decline in the level of residues over the
first three months following spray application. The deposit seems to
disappear more quickly when the application is made in a manner
designed to fully cover all grains than when only a proportion of the
grain receives the spray. Subject to some reservations about the
residue data, the half-life on barley from these trials appears to be
at least 30 weeks.
TABLE 6a. Pirimiphos-methyl residues in stored wheat from site M
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories.
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
1 week 0.1 5.5 6.6 - - 6.1
0.6 6.4 - - -
1.5 4.9 5.8 - -
6.0 5.6 5.6 - - 5.6
Mean 5.3 6.0 - -
6 weeks 0.1 5.2 5.2 5.5 5.3 5.3
0.6 - - - - -
1.5 4.6 5.1 4.9 4.5 4.8
6.0 4.8 5.1 5.3 3.8 4.8
Mean 4.9 5.1 5.2 4.5 -
11 weeks 0.1 5.6 5.1 6.0 4.8 5.4
0.6 - - - - -
1.5 5.1 4.5 5.2 3.8 4.7
6.0 5.4 4.9 5.8 3.8 5.0
Mean 5.3 4.8 5.7 4.1 -
16 weeks 0.1 5.4 - - 4.9 5.1
0.6 - - - - -
1.5 5.1 - - 4.3 4.7
6.0 5.4 - - 4.3 4.8
Mean 5.3 - - 4.6 -
22 weeks 0.1 5.6 4.3 4.8 4.1 4.7
0.6 - - - - -
1.5 5.5 4.1 4.1 3.7 4.4
6.0 5.2 4.2 4.4 3.6 4.4
Mean 5.4 4.2 4.4 3.8 -
26 weeks 0.1 - 4.2 5.2 - 4.7
0.6 - - - - -
1.5 - 3.6 4.6 - 4.1
6.0 3.6 3.6 4.4 - 3.9
Mean -
TABLE 6b. Pirimiphos-methyl residues in stored wheat from site W
of two trials in Australia. Samples taken at various
depths analysed by independent laboratories
(Bengston et al., 1974)
Interval since Sample Analyst
treatment depth, m. A B C D Mean
2 weeks 0.1 - - - - -
0.6 5.6 5.4 4.5 4.5 5.0
1.5 6.0 4.9 4.7 4.0 5.0
6.0 5.8 4.9 4.4 4.0 4.8
Mean 5.1 4.5 4.2 -
8 weeks 0.1 - - - - -
0.6 4.4 3.9 4.5 4.0 4.2
1.5 4.8 4.5 5.0 4.5 4.7
6.0 4.5 3.9 4.7 4.2 4.2
Mean 4.6 4.1 4.7 4.2 -
13 weeks 0.1 - - - - -
0.6 4.2 4.0 - 2.3 3.5
1.5 4.8 4.2 - 3.3 4.1
6.0 4.2 3.9 - 2.7 3.6
Mean -
TRIAL TERMINATED BECAUSE OF INFESTATION WITH RHIZOPERTHA DOMINICA
Rice
Bullock (1973) reports trials in Guyana, Indonesia and Malaysia
where rice in husk and polished rice were treated by admixture of
varying amounts of pirimiphos-methyl. In all trials reported the husk
was removed after varying intervals and the residues on husk and grain
determined separately. There appears to be some migration of the
insecticide from the husk to the grain during parboiling prior to
dehusking. The level of the residue in the husk declines over a period
of one month to about 30% of its concentration shortly after
application. Table 7 shows results from Guyana where rice was treated
in husk. When the rate of application was 20 mg/kg, 55% of the deposit
was lost from the husk within three days, the level declining to 35%
of the initial value at the end of one month.
TABLE 7. Pirimiphos-methyl admixed with rice paddy and
analysed at intervals - Guyana. (Bullock, 1973)
Interval
Rate of between Before After
application, treatment Parboiling* Parboiling*
mg/kg and sampling Husk Grain Husk Grain
3 3 days 14 0.53 8.4 1.0
1 month 4.4 0.21 1.1 0.66
2 months 2.4 0.34 2.7 1.0
3 months 2.0 0.46 2.0 0.64
7.5 3 days 34 0.90 5.1 1.8
1 month 8.5 0.75 2.0 -
2 months 5.2 0.71 6.0 2.3
3 months 5.1 0.53 3.4 1.3
8 months 12 0.98 4.5 ND
11 months 0.59 0.58 1.4 0.38
19.2 0 days 80 1.2 17 5.6
3 days 36 2.1 - -
1 month 28 1.4 8.2 3.2
2 months 13 1.8 15 4.9
3 months 18 1.3 3.7 2.6
* Parboiling undertaken for dehusking purposes.
Moisture content: approximately 14%.
Bullock (1973) reports extensive trials carried out in Malaysia
where pirimiphos-methyl was applied to polished rice and to rice in
husk at various rates and by various methods of application. Polished
rice appears to retain the initial deposit over a period ranging up to
7 months. It is not possible to estimate the half-life from these data
but it can be assumed to be much more than 7 months. Other trials in
Indonesia confirm this long residual life. Malathion under comparable
conditions disappeared almost completely within two months. By
comparison malathion appears not to migrate in significant amounts
from the husk into the grain of treated paddy.
Maize
Bullock (1973) reports trials in the U.S.A. which revealed that
pirimiphos-methyl deposits on maize remained relatively stable over a
period of 3 months. When the rate of application was 5-10 mg/kg the
half-life appeared to be of the order of 3 months but when 20 mg/kg
was applied no significant change occurred over the three month
period.
Peanuts
Bullock (1973) reports two large scale trials in the U.S.A. where
pirimiphos-methyl was applied at 20 and 50 mg/kg to undecorticated
peanuts. Analytical results indicate that the target rate of treatment
was achieved. Very little change occurred in the level of residues
during the first month following application. Even at the end of three
months substantially all of the deposit remained. The data suggest
that the half-life is of the order of 5 months from the time of
application. Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied.
FATE OF RESIDUES
In stored products
a) Distribution and degradation
Residues of pirimiphos-methyl on wheat grains are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain, to give
principally the parent hydroxy-pyrimidine (IV), Figure 1, and also the
related compounds (V) and (VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grains.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residues of the
chemically-unstable oxygen analogue (III) were detected. The limit of
detection.was 0.01 mg/kg (Bowker, 1973: See Table 8).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products.
The general pattern of breakdown on stored rice is similar to
that found on wheat grain. The insecticide and its degradation
products were concentrated in the husk in which the rate of breakdown
appeared to be unaffected by the moisture content of the rice (Bowker,
1973; Bullock, 1973). Representative results are given in Table 9.
TABLE 8. Effect of moisture on degradation of pirimiphos-methyl residues in wheat
grain (Bowker, 1973)
Application Pirimiphos-methyl equivalents,
rate and mg/kg, after interval (weeks)
moisture
content Compound 0 2 4 8 16 32
4 mg/kg, I (PP511) 2.16 2.66 2.05 2.66 2.32 2.08
14% moisture II + unidentified* 0.04 0.04 0.04 0.06 0.04 -
IV, V, VI <0.01 0.07 0.08 0.17 0.21 0.29
TLC baseline <0.01 <0.01 0.04 0.04 0.08 0.26
4 mg/kg,
18% moisture I 2.36 2.73 1.12 0.80 0.79 0.38
II + unidentified* 0.05 0.03 0.03 0.03 0.03 0.03
IV, V, VI - 0.49 0.56 1.05 0.92 0.66
TLC baseline - - 0.21 0.03 0.16 0.14
8 mg/kg, I 4.03 5.46 3.80 4.64 5.53 5.37
14% moisture II + unidentified* 0.12 0.08 0.08 0.11 0.04 0.10
IV, V, VI - 0.15 0.11 0.16 0.32 0.59
TLC baseline - - 0.02 0.03 0.04 0.22
8 mg/kg, I 4.44 5.64 2.93 2.84 2.19 1.28
18% moisture II + unidentified* 0.69 0.27 0.08 0.09 0.05 0.04
IV, V, VI - 0.98 0.99 1.61 1.89 1.61
TLC baseline - - 0.41 0.06 0.24 0.27
* Unidentified compound (minor product).
TABLE 9. Effect of moisture on degradation of pirimiphos-methyl residues in brown rice
(Bowker, 1973)
Application Pirimiphos-methyl equivalents, mg/kg,
rate and after interval (weeks)
moisture
content Compound 0 2 4 8 16
4 mg/kg, I (PP511) 2.13 2.31 2.06 2.19 -
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.07 -
IV, (V, VI) 0.05 0.11 0.06 0.10 -
TLC baseline 0.01 0.07 0.23 0.07 0.02
4 mg/kg, I 1.89 1.99 1.5 1.17 0.76
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.12 <0.01
IV, (V, VI) 0.06 0.17 0.34 0.24 0.33
TLC baseline 0.01 0.12 0.26 0.27 0.17
8 mg/kg, I 3.7 4.37 4.1 3.73 2.99
14% moisture II + unidentified* <0.01 <0.01 <0.01 0.11 0.09
IV, (V, VI) 0.1 0.18 0.11 0.34 0.17
TLC baseline 0.03 0.13 0.36 0.23 0.06
8 mg/kg, I 3.11 3.68 1.55 2.3 1.58
18% moisture II + unidentified* <0.01 <0.01 <0.01 0.21 0.11
IV, (V, VI) 0.06 0.32 0.10 0.86 0.45
TLC baseline 0.02 0.19 1.48 0.43 0.06
* Unidentified compound (minor product)
Pirimiphos-methyl applied as a dust formulation to wheat and
brown rice is degraded in a similar manner to other organophosphorus
insecticides used to protect stored grain from infestation (Rowlands,
1966).
Degradation is marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
is caused by factors within the grain or by the associated microflora
is not known. At higher moisture contents (approximately 18%) less
pirimiphos-methyl is recovered on analysis but increased levels of the
hydrolysis product (IV) are obtained suggesting that a more rapid
degradation of the insecticide occurs. Bowker concludes that grain may
lack the enzymic activity believed to be present in plants and soil to
cleave the pyrimidine N-ethyl bonds. It is likely therefore that
following the treatment of wheat and brown rice with
pirimiphos-methyl, the major residues during storage will be the
insecticide itself and the simple hydrolysis product (IV). Under
optimum conditions, the maximum level of compound (IV) following
treatment at 4 mg/kg, was found to be 0.17 mg/kg; under poor storage
conditions with high moisture content grain, 0.62 mg/kg.
Solutions of pirimiphos-methyl-14C were applied in the
laboratory to wheat grains of 14 and 18 percent moisture, and also as
a 2 percept dust, to give levels of 4 mg/kg. Throughout a storage
period of 6 months, the residues of pirimiphos-methyl were found
almost entirely in the seed coat and aleurone layer, with only traces
present in the germ or endosperm. It was also clear that, as with
malathion but to a greater extent, there was transfer of insecticide
between grains, possibly in the vapour phase. About 10 percent of the
total residual radioactivity was bound to lipoprotein material in the
aleurone region of the grain and was unextractable except by digestion
of the aleurone protein. By contrast with other organophosphates
studied, the bound material appeared to be the unchanged pesticide,
rather than a metabolite. Certainly it was a P=S compound, but
degradation during the liberation of the bound material complicated
identification. During the storage period, the pirimiphos-methyl
available for decomposition decreased by 15 and 50 percent at the 14
and 18 percent moisture levels respectively. The main products found
were the free hydroxy-pyrimidine and the N-desethyl pyrimidinol
(Rowlands et al., 1974).
b) Toxicity of degradation products
The hydroxypyrimidines (IV), (V) and (VI) are members of a class
of compounds known to exhibit low mammalian toxicity and they are also
products of animal metabolism. It is therefore concluded that
pirimiphos-methyl itself represents the major toxic residue in stored
products. Traces of the phosphorus-containing degradation products
(II) and (III), not exceeding 0.05 mg/kg in total, may also occur.
They are determined by the preferred analytical method. The acute oral
LD50 of (II) to the female rat is 800-1600 mg/kg. The oxygen analogue
(III) is chemically unstable and it has not been possible to undertake
toxicity tests with it (Gage, 1971b).
In plants
In contrast to its fate in stored products pirimiphos-methyl is
rapidly lost from leaf surfaces during the first 2-3 days after
spraying, mainly by volatilisation.
Bowker and Hughes (1974a) identified by a combination of
thin-layer and gas-liquid chromatography of the compounds or their
derivatives, all the major extractable metabolites and degradation
products arising from the interaction of pirimiphos-methyl with
plants.
They showed that on plant surfaces, pirimiphos-methyl is lost
rapidly by vaporisation. Levels of the parent insecticide and the
major degradation product, compound (II), represent less than 10% of
the applied material after two to three days. In crops after foliar
treatment the hydroxypyrimidine (IV), formed by hydrolysis of the
insecticide, is unlikely to be a significant residue. They found that
the compound is usually present only in trace amounts and is degraded
photochemically and bound to plant material.
These same workers showed that photochemical degradation of
pirimiphos-methyl on leaf surfaces leads to the formation of compound
(II) which is not accumulated. When applied to water in which rice
seedlings were growing, pirimiphos-methyl is not translocated
significantly into the foliage; in these conditions the hydrolysis
product (IV) is likely to represent the predominant pyrimidine
residue. Compounds (V) and (VI) only occur in trace amounts.
In animals
Much of the information in this section is also to be found under
the heading "Absorption, distribution and excretion," but is repeated
here for convenience.
Goat
Bowker et al. (1973) report studies where a single dose of
radio-labelled pirimiphos-methyl was given orally to a lactating goat
(0.12 mg/kg bw, equivalent to approximately 6 ppm in the ration). 91%
of the radio-activity was excreted in the following 8 days: 87% in the
urine and 4% in the faeces. Only 0.41% of the radio-activity was
secreted in the milk, primarily during the first 24 hours. The maximum
residue in the milk was 0.026 mg/kg pirimiphos-methyl equivalents, of
which pirimiphos-methyl itself represented 0.003 mg/kg.
Cow
The pattern of excretion in the cow is very similar to that in
the goat (Bullock et al., 1974a). When a single dose of radio-labelled
pirimiphos-methyl was given orally to a cow at 0.5 mg/kg bw
(equivalent to approximately 17 ppm in the daily diet), the
radio-activity was quantitatively recovered during the following seven
days - 85% in the urine and 14% in the faeces. Only 0.37% of the
radio-activity was secreted in the milk (0.35% during the first three
days).
The highest levels of radioactivity in the milk, urine and faeces
were found during the first day after dosing. The milk contained 0.04
mg/kg pirimiphos-methyl equivalents, of which approximately 75% could
be extracted from the fat and protein fraction into organic solvents.
Pirimiphos-methyl accounted for approximately 12% (i.e. about 0.004
ppm), and compound (V) for about 50% of the total radioactivity in the
milk. The radioactivity was not extracted by organic solvents and
appeared to be incorporated into natural milk components.
Approximately 70% of the radio-label in "day 1" urine was
organo-soluble, the remainder dissolving only in water.
Four groups of three cows were maintained by Bullock et al.
(1974b) for 30 days on diets containing 0, 5, 15 and 50 ppm
pirimiphos-methyl. Residues of pirimiphos-methyl were determined in
milk samples taken every two days and in samples of kidney, liver,
heart, fat and muscle taken at the end of the study. None of these
residues exceeded 0.02 mg/kg. The authors prepared butter from the
milk of cows dosed at the 110 mg/kg bw rate but found this to contain
only 0.02-0.04 mg/kg pirimiphos-methyl. In all instances no residues
of the organophosphorus compounds (II) and (III) could be detected in
samples of meat, milk or butter. (Limit of detection: 0.01 mg/kg in
each case).
Hen
Green et al. (1973) report that when diets containing 4 ppm
pirimiphos-methyl were fed to laying hens for 28 days, or when a diet
containing 32 ppm was fed for 7 days, residues of pirimiphos-methyl
itself in eggs did not exceed 0.01 mg/kg and no residues of compound
(III) were detected (Limit of detection: 0.001 mg/kg). The same
authors using radio-labelled pirimiphos-methyl found that the total
amount of radio-activity in eggs reached a maximum of 0.03 mg/kg
pirimiphos-methyl equivalents after 15 days when fed at a
concentration of 4 ppm in the ration and a maximum of 0.15 mg/kg
pirimiphos-methyl equivalents at the end of the 32 ppm study. In
muscle samples taken at the end of the 32 ppm study no residues of
pirimiphos-methyl or of its P=0 analogue were detected, (Limit of
detection: 0.001 ppm). Since 86-90% of the radio-activity in eggs is
present as water-soluble unidentified metabolites and since at no time
does the concentration of pirimiphos-methyl itself in yolks or whites
exceed 0.001 mg/kg, Green et al. conclude that pirimiphos-methyl does
not accumulate in eggs during continuous feeding of the insecticide
and that at the proposed level of application the residues of the
pesticide in eggs are negligible.
Graham and Jenkins (1974), as part of a study of the effect of
pirimiphos-methyl on the growth rate of broiler chickens, analysed
muscle, skin and fat samples from birds receiving 4, 8, 16, 32 and 48
ppm pirimiphos-methyl continuously in rations for 9 weeks. Birds were
slaughtered within 1 hour of being removed from feed. The only
residues which appeared were detected in skin and fat. The highest
level, 0.017 mg/kg, was in the fat of a broiler receiving 48 ppm
pirimiphos-methyl in the ration. Another had 0.015 mg/kg. All other
samples contained less than 0.01 mg/kg or residues were not
detectable.
In soil
Pirimiphos-methyl shows very limited persistence and mobility in
soil.
Bowker, Riley and Gratton (1972) showed that in a range of soil
types the half-life of pirimiphos-methyl is less than one month. The
major soil metabolite is the parent hydroxypyrimidine (IV) together
with smaller amounts of the related compounds (V) and (VI). The routes
of metabolism in stored grain, plants and soil are therefore similar.
The same authors using a descending thick-layer chromatography
technique, showed pirimiphos-methyl to possess only limited mobility
in soil: considerably less than atrazine which was used as a standard
and which itself is recognised as being only moderately mobile.
In water
Pirimiphos-methyl in rapidly degraded in water, mainly by
hydrolysis with loss of the phosphorothionate ester side chain. This
process is accelerated in the presence of light. Bowker and Hughes
(1974b) confirmed this and showed that although some volatilisation
occurs from still water, this is less significant than hydrolysis in
accounting for loss of the insecticide. In their experiments 50%
degradation had occurred after one day in sunlight. The major
degradation product in water is compound (IV). Only trace quantities
of the P=0 analogue (III) were detected (less than 0.01 mg/l in water
treated initially with pirimiphos-methyl at 1 mg/l).
In processing
Wheat
Bullock (1973 and 1974) reported many separate experiments which
demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table
10 summarises the results of residue trials carried out in the U.K. on
wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 11 summarises results of a residue trial reported by
Bullock (1973) to have been carried out in the U.K. with wheat
nominally treated to contain 8 ppm pirimiphos-methyl. These data which
are substantially in agreement with those of Bengston et al. (1974)
show that there is relatively little penetration beyond the seed coat
even throughout a storage period of 9 weeks.
TABLE 10. Effect of milling and baking on residues in wheat admixed
with pirimiphos-methyl at 4 mg/kg-UK (Bullock, 1973, 1974)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
Whole 0 4.2 1.9 2.9 (9)
grain 3.0* -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Wholemeal 0 1.3 0.94 1.1 (3)
flour
1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White 0 0.88 0.30 0.52 (6)
flour 0.56* 0.53* 0.55* (3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27* (3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread
1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
TABLE 10. (Cont'd.)
Interval between
Grain treatment and Residues, mg/kg1
fraction sampling (months) Highest Lowest Mean
White 0 0.28 0.19 0.23 (6)
bread 0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 All results are from field trials except those marked*
which are from a small-scale trial. Figures in parentheses are
the numbers of results upon which the means are based.
TABLE 11. Residues of pirimiphos-methyl in whole grains and in milling
and baking fractions of whole grains treated in a laboratory
trial in UK at 8 mg/kg. (Bullock, 1973, 1974)
Interval between Residues, mg/kg.1
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52 - -
6.0 0.91 0.56 - -
6.0 1.0 0.57 - -
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 In all cases, no residues of the phosphorus-containing compounds (II)
or (III) (Figure 1) were detected. (Limit of detection: 0.01 ppm in
each case.)
Bengston et al. (1974) report an extensive series of trials in
which pirimiphos-methyl was applied to wheat at the rate of 6 mg/kg in
bulk silos in two areas of Australia. Wheat was submitted to two
separate laboratories, for analysis and for processing, milling and
baking. The results reported by the two independent laboratories are
summarised in Table 12. These studies revealed that though a
significant amount of the pirimiphos-methyl was removed during the
cleaning process prior to the milling of the wheat, by far the
greatest separation occurred in the bran. Although the bran
represented only 25% of the whole grain it contained just over two
thirds of the entire residue leading to a concentration in the bran of
250% of that in the whole grain from which the bran was derived. The
concentration in the flour after milling averaged only 22% of that in
the whole grain. When the grain was processed into bread and baked
there was a further 63% reduction in the level of residues according
to one co-operating laboratory and an almost complete destruction of
the insecticide reported by the other. Assuming that the amount of
residue found in the bread would always be as high as the highest
level reported (0.3 mg/kg) the processing, milling and baking of bread
from raw grain containing pirimiphos-methyl results in a 93%
destruction of the original residue. Under practical conditions this
could well be greater because there is normally a significant delay at
each stage of the process which would further promote degradation of
the residue.
Rice
Residues of pirimiphos-methyl and its degradation products in
rice in husk are concentrated in the husk. Bowker (1973) and Bullock
(1973, 1974) report that parboiling rice to remove the husk results in
a substantial loss of the insecticide and its degradation products
from the husk into the water used to soak the rice. Table 13, taken
from Bullock (1973), shows the results of experiments in Guyana
designed to determine the lose of residues during the processing of
rice which had been treated previously while in husk at a nominal rate
of 5 and 10 mg/kg with pirimiphos-methyl. These results clearly
demonstrate that the bulk of the residue is in the husk with the
remainder in the seed coat. Polishing removes virtually all of the
material deposited in the seed coat although the process of parboiling
appears to promote the transfer of a small amount of residue into the
grain itself.
TABLE 12. Fate of pirimiphos-methyl residues in wheat treated at 6 mg/kg and subjected to
milling and baking (Bengston et al., 1974).
Residues, mg/kg,
Residues, mg/kg, Wheat W Wheat M
Laboratory A Laboratory B Laboratory B
Stage of Processing 1 2 Mean 1 2 Mean 1 2 Mean
Whole grain 4.7 4.8 4.8 4.2 4.2 4.2 4.5 4.5 4.5
Cleaned grain 3.8 3.8 3.8 - - - - - -
Bran 10.0 9.9 10.0 8.9 9.3 9.1 11.9 12.7 12.4
Shorts (pollard) 7.4 7.7 7.6 8.2 8.5 8.3 10.1 10.6 10.4
Flour 0.85 0.88 0.9 0.6 0.8 0.7 1.3 1.3 1.3
Bread 0.31 0.32 0.3 <0.2 <0.2 <0.2 0.46 0.48 0.47
TABLE 13. Effect of milling and polishing on residues in rice admixed with
pirimiphos-methyl (5 or 10 mg/kg) while in husk - Guyana
(Bullock, 1973).
Residues, mg/kg.
Treated at 5 mg/kg Treated at 10 mg/kg
Not Not
Sample parboiled Parboiled parboiled Parboiled
Dehusked grain 0.55 0.58 0.72 1.46
Husks 8.7 2.1 9.4 4.95
Grain polished 1 min. 0.26 0.31 0.29 0.76
Polishings from 1st min. 5.9 6.3 9.0 14.0
Grain polished 2 min. 0.19 0.29 0.29 0.76
Polishings from 2nd min. 5.6 6.5 9.4 15.8
Grain polished 5 mins. <0.01 0.28 0.27 0.65
Polishings from 3rd to 5th mins. 5.3 7.1 9.3 14.9
Bullock (1973) reports experiments carried out in Indonesia to
determine the loss of pirimiphos-methyl residues from polished rice
during cooking. Rice which had been treated by admixture with
pirimiphos-methyl emulsifiable solution and which contained 1.96 mg/kg
pirimiphos-methyl was used in the trial. The rice was washed under the
cold water tap for two minutes before simmering for 20 minutes in 2.5
times its own weight of tap water. The rice by this time was a soft
mass. Cold tap water was poured over the grains to separate them and
the samples were then analysed in the usual manner. A second sample of
the same batch of rice was treated in the same way and duplicate
analyses were carried out on each sample. The results which are given
below show a 74% loss in cooking white rice.
Residue, mg/kg, before cooking: 1.96; 1.96
after cooking: 0.58, 0.50 (mean 0.54); 0.50, 0.47
(mean 0.49).
No residues of the phosphorus-containing compounds (II) or (III)
were detected (limit of detection: 0.01 ppm in both cases).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
Two methods of residue analysis are available. The more
sensitive, quicker and preferred method is that of Bullock (1972) in
which gas chromatography with a phosphorus detector is used. The
method is suitable for the determination of residues of
pirimiphos-methyl in crops, it also determines the
phosphorus-containing metabolites (II) and (III). The limit of
determination is 0.01 mg/kg in each case. Crop samples are extracted
with a 20% solution of acetone in n-hexane. A decanted portion of the
extract is washed with water, and then dried with anhydrous sodium
sulphate. A portion of the extract is then injected into a gas
chromatograph equipped with a flame photometric or thermionic
detector. The peaks obtained at the retention times of
pirimiphos-methyl and its metabolites (II) and (III) are compared with
standards. Grain samples are extracted with methanol instead of
acetone/hexane and oily or fatty materials are subjected to an
acetonitrile-n-hexane partition before analysis. The oxygen analogue
(III) has not been detected as a metabolite in any samples analysed in
the laboratory of the manufacturer, although (as stated above) its
limit of determination is 0.01 mg/kg.
Alternatively when GLC is not available, a colorimetric method
may be used. The colorimetric method reported by Bullock (1969) has
only been tried on field crop samples. An extract, obtained by
prolonged maceration or slurrying the sample with n-hexane, is dried
with anhydrous sodium sulphate, filtered, concentrated down to a small
volume in a rotary evaporator at low temperature and quantitatively
transferred to a preparative-layer plate together with a marker spot
of pirimiphos-methyl. The plate is developed in a mixture of n-hexane
and acetone and inspected under ultra-violet light. The band opposite
the marker spot is scraped off and refluxed for one hour with
concentrated hydrobromic acid to liberate hydrogen sulphide, which is
swept off in a stream of nitrogen, quantitatively absorbed in alkaline
zinc acetate and subsequently determined as methylene blue. The limit
of determination is normally about 0.1 mg/kg with recovery in excess
of 75%.
Bullock (1973) reports studies to determine the most effective
solvent for extracting pirimiphos-methyl and its metabolites from
"aged" residues in stored grain. Table 14 shows the results of these
studies indicating the residues of pirimiphos-methyl extractable from
aged samples by different extraction methods. The samples were ground
to a fine powder before analysis. The lowest recoveries are almost
invariably with methanol under reflux. Comparable results are obtained
with the other solvent systems when extraction is made immediately
after treatment, but on aged samples cold methanol generally gives
significantly higher results.
TABLE 14. Residues of pirimiphos-methyl extractable from "aged" samples by different
extraction methods. (Samples ground to fine powder before analysis.)
Residues determined (mg/kg) using various
Sample** Interval after extraction methods*
(Treatment rate) treatment 1 2 3 4 5
Wheat (4 mg/kg) 0 days 3.72 3.45 3.82 3.87 2.91
1 month 2.92 2.73 2.79 3.01 3.17
3 months 1.87 1.73 1.99 2.17 2.04
Barley (4 mg/kg) 0 days 3.07 3.12 3.49 3.09 1.90
1 month 2.51 2.36 2.17 2.69 2.54
3 months 1.53 1.71 1.47 1.84 1.70
Rice (4 mg/kg) 0 days 4.10 3.92 4.00 4.07 3.12
1 month 2.18 2.40 2.12 2.71 2.60
3 months 1.41 1.95 1.84 1.99 1.03
* Extraction methods: 1 Dichloromethane (cold); 2 Acetone (cold); 3 20% Acetone
in hexane (cold); 4 Methanol (cold); 5 Methanol (1 hour reflux).
** The samples had a moisture content of 13-14%.
Bullock also reports studies which show that it is preferable to
grind aged samples before attempting to extract with either methanol
or acetone/hexane. Grinding offers no advantage when dealing with
wheat or rice that has only just been treated but in view of the fact
that many regulatory analysts will receive samples which have been
treated three months to three years previously it is obvious that the
preparation of the sample and the selection of the solvent are of
critical importance. Table 15 shows results obtained by Bullock. The
samples were ground in a Moulinex coffee grinder. Later comparison
with Hobart laboratory grinder showed no significant difference in the
results and little temperature rise in the grain during grinding.
TABLE 15. Effect of grinding on extractability of aged residues
(Bullock, 1973).
Sample Residues, mg/kg, determined using
(Treated by Interval different extraction solvents
admixture after Original 20% acetone Methanol
at 4 mg/kg) treatment or ground in hexane
Wheat 0 days Original 3.82 3.91
Ground 3.82 3.87
1 month Original 2.70 2.81
Ground 3.10 3.04
3 months Original 1.43 1.55
Ground 1.87 1.99
Rice 0 days Original 4.01 4.05
Ground 4.10 4.07
1 month Original 2.00 1.91
Ground 2.18 2.34
3 months Original 1.67 1.71
Ground 1.84 1.99
As a check on the loss of residues during grinding Bullock took
wheat which had been freshly treated with pirimiphos-methyl and
measured the residue level before and after intense grinding for one
minute. The analytical results varied by less than 50% which is slight
by comparison with the loss which would occur if grain treated with
malathion or dichlorvos were ground in a similar manner. The same
author reports experiments designed to determine the loss of residue
from wheat, barley and rice when grain samples were stored at -14°C
while awaiting analysis. Both freshly treated grain and grain which
had been treated under semi-commercial conditions three months prior
to sampling were kept under such storage conditions but none of these
samples showed as much as 5% loss during two months at -14°C.
NATIONAL TOLERANCES
The information available to the meeting indicates that few
national tolerances have been established. It is understood that a
tolerance of 4 mg/kg has been proposed in France for pirimiphos-methyl
residues in cereals and 2 mg/kg in fruits and vegetables. In the
Netherlands a tolerance of 0.2 mg/kg has been established for
pirimiphos-methyl residues in cucumbers, melons, paprika and tomatoes.
APPRAISAL
Pirimiphos-methyl is a broad-spectrum organophosphorus
insecticide with both contact and fumigant action. It shows only
limited biological persistence on plant surfaces but gives long
lasting control of insect pests on inert surfaces and retains its
biological activity when applied to stored agricultural commodities
including raw grains and nuts.
The meeting had only limited Information concerning the
pre-harvest use and performance of this material on crops but
extensive data were available on its use, performance and fate on a
variety of small grains and nuts. The recommended rate of application
to raw cereals is 6 mg/kg.
Exceptional stability on grain results in an effective half-life
of up to 80 weeks. Although high temperatures and high moisture levels
in grain reduce the life of the deposit the effect of these influences
is much less than with other grain protectants approved or evaluated
to date.
Data were available to show that on both wheat and rice the
deposit resulting from admixture of pirimiphos-methyl with grain is
almost entirely in the seed coat and husk respectively. Cleaning,
processing and milling removes the bulk of the applied material. In
the case of wheat the milling process results in most of the residue
being separated into the bran where the concentration of
pirimiphos-methyl can range up to 20 mg/kg. In the case of rice
substantially all of the deposit is removed during the milling and
polishing operations. Depending upon the milling process, flour
prepared from treated wheat may contain significant amounts of
pirimiphos-methyl but a major proportion of this is destroyed in the
baking of bread. The small amount of residue remaining in polished
rice is substantially destroyed in cooking.
Pirimiphos-methyl and the corresponding hydroxypirimidine are the
only significant residues detected. Residues of the
phosphorus-containing metabolites when detected were always extremely
low (approximately 0.05 mg/kg).
Extensive data are available on the fate of pirimiphos-methyl in
plants, domestic animals, hens and their edible tissues, and foods of
animal origin. In living plants the major phosphorus-containing
degradation product is the N-desethyl metabolite (II) which is not
accumulated. The hydroxypyrimidine compound (IV) is the major
degradation product following root uptake. When fed to domestic
animals pirimiphos-methyl is rapidly metabolised and excreted, only
very small quantities being secreted into milk or eggs. Cattle
receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites could
be detected in meat, milk, butter or eggs.
A GLC method of analysis suitable for the determination of
residues of pirimiphos-methyl and the phosphorus-containing
metabolites in plant materials is available, the limit of
determination being reported to be 0.01 mg/kg. The method appears
suitable for regulatory purposes. Evidence of the importance of sample
preparation and method of extraction has been presented.
No national tolerances have yet been determined.
In proposing maximum residue limits for pirimiphos-methyl and its
metabolites on raw grain, milled cereal products and foods prepared
therefrom, careful consideration has been given to the fact that this
insecticide is to be used as a grain protectant, that a certain
concentration must be present on the grain to control infestations and
prevent damage to stored products and that the compound is
exceptionally stable under storage conditions. Under practical
conditions of grain handling and storage it is not possible to apply a
grain protectant with assurance that the deposit will be absolutely
uniform. There will always be a natural variation in the level of the
deposit resulting from fluctuations in the flow of grain and
insecticide. It is therefore not possible to fix the maximum limit on
the minimum necessary to control the insect pests. Furthermore
extensive work has demonstrated that the movement of bulk grain
results in the segregation of the various components of the bulk with
a resultant variation in the level of residues throughout the mass.
Due allowance must be made for the amplitude of these variations and
the problems of sampling and analysis.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl, expressed as pirimiphos-methyl.
TEMPORARY TOLERANCE
mg/kg
Bran (wheat, rice) 20
Wheat, rye, rice (in husk) 10
Barley, maize, oats 7
Wholemeal flour (wheat, rye) 5
Rice (dehusked), wheat flour (white) 2
Bread (wholemeal), rice (polished) 1
Bread (white) 0.5
Meat, milk, eggs 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 1976)
1. Additional studies to clearly establish no-effect level for
decreased pregnancy rates, and hydronephrosis in offspring.
2. Further studies to clarify the liver injury dogs observed in 90
day studies.
DESIRABLE
1. Longer duration observations in man.
2. Information from studies now in progress on other stored
commodities including nuts, peanuts and dried fruit. The
information is expected to be available during 1975.
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in food at
the point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
REFERENCES
Bengston, M., Connell, M., Desmarchelier, J., Phillips, M., Snelson,
J. and Sticka, R. (1974) Evaluation of four new grain protectants.
Report to Australian Wheat Board. (To be published)
Bowker, D.M. (1973) Pirimiphos-methyl (PP 511): fate of stored wheat
and rice grain in the laboratory. ICI Plant Protection Ltd. Report No.
AR 2457 AR. (Unpublished)
Bowker, D.M., Griggs, B.F. and Harper, P. (1973) Pirimiphos-methyl (PP
511): excretion by a goat. ICI Plant Protection Ltd. Report No. AR
2458 B. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in crops.
ICI Plant Protection Ltd. Report No. AR 2515 A. (Unpublished)
Bowker, D.M. and Hughes, H.E. (1974) Pirimiphos-methyl: fate in water.
ICI Plant Protection Ltd. Report No. AR 2516 A. (Unpublished)
Bowker, D.M., Riley, D. and Gratton, R.P. (1972) Pirimiphos-methyl:
fate in soil. ICI Plant Protection Ltd. Report No. TMJ 809 A.
(Unpublished)
Bratt, H. and Dudley, L.A. (1970) Pirimiphos-methyl (PP 511):
Excretion by rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bratt, H. and Jones, L.A. (1973) Pirimiphos-methyl (PP 511):
Metabolism in rats and dogs. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Bullock, D.J.W. (1969) Provisional analytical method for the
determination of PP211 and PP511 residues in vegetables, grain and
fruit. ICI Plant Protection Ltd. Method No. PAM 310/A.
Bullock, D.J.W. (1972) Residue analytical method no. 11 for the
determination of residues of pirimiphos-methyl and its
phosphorus-containing metabolites in crops (gas chromatographic
method). ICI Plant Protection Ltd. Method PPRAM-11/A.
Bullock, D.J.W. (1973) Pirimiphos-methyl: residues in stored grain.
ICI Plant Protection Ltd. Report No. AR 2472 AR. (Unpublished)
Bullock, D.J.W. (1974) Pirimiphos-methyl: residues in stored grain
bread, flour and milled products. ICI Plant Protection Limited. Report
No. AR 2537 A. (Unpublished)
Bullock, D.J.W., Day, S., Hemingway, R.J. and Jegatheeswaran, T.
(1974) Pirimiphos-methyl: residue transfer study with cows. Report
from ICI Plant Protection Limited. (Unpublished)
Bullock, D.J.W., Day, S., and Griggs, B.F. (1974) Pirimiphos-methyl:
metabolism and residue transfer into the meat and milk of a lactating
cow. ICI Plant Protection Ltd. Report No. AR 2552 A. (Unpublished)
Chart, S., Foulkes, Caroline A., Gore, G.W. and Williamson, K.S.
(1974) Erythrocyte and plasma cholinesterase activity in human
volunteers administered pirimiphos-methyl. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Clapp, M.J. and Conning, D.M. (1970) Pirimiphos-methyl (PP511).
Ninety-day oral toxicity in rats. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Clark, D.G. (1970) The toxicity of PP511
[O-(2-diethylamino-6-methylpyrimidin-4-yl)
O,O-dimethylphosphorothioate] Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fink, R. (1974a) Eight-day dietary LC50 - mallard ducks: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Fink, R. (1974b) Eight-day dietary LC50 - bobwhite quail: technical
pirimiphos-methyl. Report from Truslow Farms Inc. (Unpublished)
Gage, J.C. (1971a) Pirimiphos-methyl (PP511): Avian toxicity. Report
from ICI Industrial Hygiene Research Laboratories. (Unpublished)
Gage, J.C. (1971b) Pirimiphos-methyl (PP 211) and pirimiphos-methyl
(PP 511): acute and subacute oral toxicity in the rat of the plant
metabolite, 2-diethylamino-4-hydroxy-6-methylpyrimidine (R46382). ICI
Industrial Hygiene Research Laboratory. Report No. HO/IH/R/327.
(Unpublished)
Gage, J.C. (1972) Pirimiphos-methyl (PP511): Oral toxicity in the dog
and in a passerine bird species. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Gore, C.W. Palmer, S. and Pratt, I.S. (1974) Pirimiphos-methyl
(PP511): teratogenic studies in the rabbit. Report from ICI Central
Toxicology Laboratory. (Unpublished)
Gore, C.W., Griffiths, D. and Phillips, C.E. (1974) Pirimiphos-methyl
(PP511): two year feeding study in the rat. Report from ICI Industrial
Hygiene Research Laboratories. (Unpublished)
Graham, C.A. and Jenkins, S.L. (1974) The effect of pirimiphos-methyl
on the growth rate of broiler chicks. ICI Australia Ltd. Merrindale
Research Station Report, July 1974.
Green, T., Monks, I.H. and Phillips, P.J. (1973) Pirimiphos-methyl (PP
511): sub-acute oral and residue studies in hens. ICI Industrial
Hygiene Research Laboratories. Report No. HO/IH/P/65B. (Unpublished)
Hodge, M.C.E. and Moore, S. (1972) Pirimiphos-methyl (PP 511):
Teratological studies in the rat. Report from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Noel, P.R.B., Mawdesley-Thomas, L.E., Rivett, K.F., Squires, P.F. and
Street, E. (1970) PP 511: Oral toxicity studies in dogs - initial
studies and repeated dosage for thirteen weeks. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and Cherry, C.P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat: histopathological
examination on testes of F1B and F2B males. Report from Huntingdon
Research Centre. (Unpublished)
Palmer, A.K. and James, P. (1972) Effect of PP 511 on reproductive
function of multiple generations in the rat. Report from Huntingdon
Research Centre. (Unpublished)
Parkinson, G.R. (1972a) Pirimiphos-methyl (PP 511): potentiation with
gamma-BHC. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Parkinson, G.R. (1972b) Pirimiphos-methyl (PP 511): potentiation with
dichlorvos. Report from ICI Industrial Hygiene Research Laboratories.
(Unpublished)
Pieterse, A.H., Schulten, G.G.H. and Kuyken, W. (1972) A study of
insecticide resistance in Tribolium castaneum in Malawi. J. Stored
Prod. Res., 8(3):183.
Rivett, K.F., Edwards, B., Street, E. and Newman, A.J. (1973) PP 511:
Oral toxicity study in beagle dogs - repeated daily dosage for two
years. Report from Huntingdon Research Centre. (Unpublished)
Ross, D.B., Burroughs, Sheila J. and Roberts, Nicholas L. (1974) Egg
production and hatch-ability in the laying hen following dietary
inclusion of pirimiphos-methyl at various levels. Report from
Huntingdon Research Centre. (Unpublished)
Rowlands, D.G. (1966) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. Stored Prod. Res., 2:105-116.
Rowlands, D.G. et al. (1974) Preliminary report of studies with
pirimiphos-methyl as a grain protectant. Submission by United Kingdom
to FAO. (To be published)
Waterhouse, D. (1973) Background paper presented at the Ninth Session
of the FAO Working Party of Experts on Pest Resistance to Pesticides
(Rome, 20 June 1973). (Unpublished)
