
CARTAP JMPR 1976
IDENTITY
Chemical name
1,3-di(carbamoylthio)-2-dimethylaminopropane
Synonyms
S,S'-[2-(Dimethylamino)trimethylene] bis(thiocarbamate)
CaldranR PadanR, PatapR, SanvexR, ThiobelR,
Vegetox, NTD-2, TA-7, TI-1258.
Structural formula
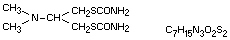 Other information on identity and properties
Cartap is invariably used as the hydrochloride, to which the
following information refers.
Molecular weight: 273.8
State: Colourless crystals
Melting point: 179-181°C(decomp.)
Solubility(g/100 ml): Soluble in water(13.2/10°C,
17.8/20°C, 25.3/30°C slightly soluble
in methanol(2.08/5°C), almost insoluble
in acetone, benzene, chloroform,
diethyl ether, ethyl acetate, and
n-hexane.
Purity of technical material: 97%. containing 3% of water and
ammonium chloride as impurities.
Hydroscopicity: Slightly hygroscopic
Volatility: Negligible
Formulation: 25% and 50% water-soluble powder, 2%
dust, 4% and 10% granules and 2% fine
granules are available. A bait
formulation has recently been
introduced.
Cartap hydrochloride is an insecticide recently developed by
Takeda Chemical Industries. It is a derivative of nereistoxin which
is a naturally occurring insecticidal substance isolated from the
marine segmented worms Lumbrinereis heteropoda and L.
brevicirra.
Nereistoxin was isolated by Nitta in 1934, the structure was
elucidated by Okaichi and Hashimoto (1962) and synthesized in 1965
by Hagiwara et al. A variety of nereistoxin derivatives were
prepared and their insecticidal activities tested. Cartap
hydrochloride is one of the most potent of the many compounds
prepared.
The chemistry and synthesis of nereistoxin and related
compounds are dealt with by a number of authors including Hashimoto
et al (1960); Okaichi and Hashimoto (1962); Hagiwara et al (1965);
Konishi (1968a, 1968b); Hagiwara and Numata (1968); Konishi (1970a,
1971); Sakai (1971); Nishi et al (1973).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Groups of 10 male and 20 female rats were administered cartap
hydrochloride in the diet at levels of 0, 100 and 1000 ppm for 9
weeks prior to mating and subsequently for the remainder of the
study which was a 2 litter, 2 generation reproduction study. One
half of the F2b litters were delivered by caesarean section on day
19 of gestation, the other half were delivered naturally and held
to weaning. Parental animals showed reduced body weight gain and
food consumption and an apparent impairment of fertility for the
F1a generation at 1000 ppm. Gestation and live birth indices of
the treated animals were comparable to those of the controls.
Weanling survival index was lower in the F1b generation and pup
weights in all generations at 7, 14 and 21 days after weaning were
reduced at the upper dose level. There were no meaningful
differences observed between groups with respect to number of
implantation sites, resorptions or live and dead fetuses. No
compound-related microscopic alterations were noted in selected
tissues from weanling F2b progeny of the 1000 ppm group. However,
a greater incidence of incomplete ossification was revealed on
skeletal examination in this group, (Olson and Busey, 1972).
Special studies on teratogenicity
Mouse
Three groups comprising 20, 12 and 14 pregnant mice received
cartap hydrochloride by stomach tube at dose levels of 0, 50 and
100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Twenty-one pregnant mice were employed as an untreated
control group. Animals were killed on day 19 of gestation and
fetuses removed by caesarean section. Weight gains were not
affected. No significant variations were observed in the number of
implantation sites, fetal mortality and mean body weight of fetuses
between control and treated groups. No visceral abnormalities were
observed. Skeletal examination revealed abnormalities (fusion of
cervical vertebral arches and fusion of sternebrae) in 2/110
fetuses (1.8%) at 50 mg/kg and 2/120 fetuses (1.7%) at 100 mg/kg.
In respect to other skeletal variations, there were no significant
differences between control and treated animals (Mizutani et al,
1971).
Rat
Cartap hydrochloride was administered orally to three groups
of 21, 12 and 17 pregnant rats at dose levels of 0, 50 and 100
mg/kg body weight respectively from gestation day 9 to day 15.
Twenty-three pregnant rats were used as an untreated control group.
Animals were sacrificed on day 21 and fetuses were delivered by
laparotomy. An increase in mortality and retarded weight gain were
observed in the maternal animals at 100 mg/kg during the dosing
period. There was no statistical difference between treated and
control groups with respect to number of implantation sites, fetal
mortality, sex ratio, external and visceral malformations. Skeletal
examination revealed no major abnormalities except for an increase
25/174 (14.4%) in bilateral twin thoracic vertebral centrae in the
100 mg/kg group (Mizutani et al, 1971).
Hamster
Five groups comprising 9, 7, 8, 9 and 7 pregnant hamsters
received cartap, hydrochloride orally at dose levels of 0, 2, 10,
50 and 100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Fifteen pregnant hamsters were used as an untreated
control group. Animals were killed on day 16 of gestation and
fetuses removed by caesarean section. Treatment with 100 mg/kg
resulted in the death of 2/7 animals and was associated with
reduced weight gain. No compound-related effects were observed in
the number of implantation sites, fetal mortality, fetal weights,
external and visceral malformations. In hamsters receiving 100
mg/kg, the frequency of skeletal anomalies increased slightly, 6/52
(11.5%) showing abnormalities in the thoracic vertebrae and/or
ribs. An increase in the incidence of fetuses with extra lumbar
ribs was noted at dose levels of 50 and 100 mg/kg (Mizutani et al,
1971).
Special studies on pharmacological properties
Cartap hydrochloride was shown to have neuromuscular blocking
activity in vitro and in vivo in several animal species
(rat, dog, cat). The effects were characterized as follows: 1)
inhibition of acetylcholine induced contraction of the muscle, 2)
antagonism by cholinesterase inhibitor, calcium ion,
tetraethylammonium and posttetanic stimulation against the partial
neuromuscular block caused by cartap hydrochloride, 3) complete
antagonism by several sulphhydryl compounds (BAL, L-cysteine,
cystine, D-penicillamine) against the neuromuscular block, 5)
respiratory failure due to neuromuscular block, 6) stimulant effect
on the superior cervical ganglion followed by postsynaptic
depression (Nagawa et al, 1971).
Special studies on antidotes
Male mice pre-treated with an intraperitoneal injection of
diazepam, phenobarbital, atropine sulphate, C-penicillamin cystine
or other sulphhydryl compounds delayed the onset of symptoms from
a toxic dose (500 mg/kg) of cartap hydrochloride. The intravenous
administration of cystine (ED50 40 mg/kg) was shown to be effective
in protecting rabbits from the toxic symptoms of an acute oral dose
(50 mg/kg) of cartap hydrochloride. In dogs, a minimal effective
dose of 12.5 mg/kg cystine was required in order to protect against
intoxication from the dermal application of 200 mg/kg of cartap
hydrochloride (Nagawa et al, 1970).
Special studies on dermal irritation
Cartap hydrochloride was applied as a 5, 10 and 20% paste to
the intact skin of two male rabbits daily for 5 days. No evidence
of irritation was observed (Aramaki, 1972).
Special studies on eye irritation
Cartap hydrochloride was instilled into the right eye of 3
rabbits as a 1.5 or 10% solution (0.2 ml) or 15 mg of the powder.
No evidence of irritation to the bulbar or palpebral conjunctiva
was observed when examined at 24 and 48 hours (Aramaki, 1972).
Special studies on respiratory effects
Groups of 5 male and 5 female rats were exposed continuously
for 6 hours to the dust of a 50% water-soluble cartap hydrochloride
powder at estimated concentrations of 0.086 and 0.54 mg/litre of
air. Animals were observed for a period of 7 days following
exposure. Severe eye and skin irritation, increased nasal and oral
secretions, respiratory irritation and lethargy were observed at
0.54 mg/litre. Exposure to 0.086 mg/litre caused slight dyspnoea,
temporary eye irritation and polyuria. The signs of irritation of
this group disappeared rapidly after termination of exposure.
Post-mortem examination revealed no dose-related abnormalities in
either test group (Berczy and Cobb, 1973)
Four groups of 5 male and 5 female rats were exposed
continuously for 6 hours/day, 5 days a week for 3 weeks to the dust
of a 50% water-soluble cartap hydrochloride powder at estimated
nominal concentrations of 0, 0.01, 0.1 and 1.0 mg/litre of air.
Severe respiratory irritation, loss in body weight, injury to the
eyes and skin were observed at the exposure level of 1.0 mg/litre.
Certain clinical parameters (mean cell volume, neutrophil and
lymphocyte cells, glucose, BUN, SGPT and SGOT) were found to be
elevated in the high dose group. The changes in relative organ
weights observed in this group were considered to be related to
loss in body weight. Gross and histopathological examination
revealed no abnormalities in the two lower dose groups. Extensive
chronic respiratory effects in both sexes and acute ulceration in
the urinary bladder in male rats were noted at the high dose level
(Berczy et al, 1973).
Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Mouse M oral 225 Aramaki, 1972
M oral 150 Toyoshima et al,
1972
F oral 154 ibid
M s.c. 34.5 ibid
F s.c. 35 ibid
M i.v. 51 ibid
F i.v. 52 ibid
Rat M oral 380 Aramaki, 1972
F oral 390 ibid
M oral 345 Toyoshima et al,
1972
F oral 325 ibid
M s.c. 40 ibid
F s.c. 42 ibid
M i.v. 44 ibid
F i.v. 36 ibid
Monkey oral 100-200** Yokotani et al,.
1968
Rabbit M&F dermal 819* Davies & Collins,
1973
* Padan water soluble powder (Cartap HCl 50%).
** exact estimate not possible owing to vomiting.
Short-term studies
Mouse (dermal)
Cartap hydrochloride (0.2 ml of a 5 or 10% aqueous solution)
was applied to the intact skin on the backs of 6 male mice. During
the 7-day observation period no symptoms of intoxication developed and
necropsy examination of subcutaneous tissues showed no adverse
effects (Aramaki, 1972).
Rabbit (dermal)
Cartap hydrochloride was applied to intact or abraded skin of
groups of 5 male and 5 female rabbits at dose levels of 0, 15, 60
and 240 mg/kg body weight/day. Rabbits treated with 240 mg/kg died
or were sacrificed after 1 week. Treatment of rabbits with 15 and
60 mg/kg was continued 7 days a week, for 3 consecutive weeks.
Pupillary dilatation and lack of hind limb coordination were
observed in some animals of each group. Body weight gain and food
consumption were reduced at 60 mg/kg especially during the 3rd
week. PCV, hemoglobin concentration and RBC counts were also
decreased in males (abraded) when compared to intact control
animals at this dose level. Increased BUN and reduced plasm
cholinesterase activity were observed at 240 mg/kg when compared to
control values at week 3. Slight dermal irritation was noted during
the 2nd and 3rd week in rabbits receiving 15 and 60 mg/kg.
Ophthalmoscopy and macroscopic and microscopic pathology were
comparable for all groups (Davies et al, 1974).
Mouse (oral)
Groups of 8 or 9 mice of each sex were fed cartap
hydrochloride at dietary levels of 0, 100, 300 and 900 ppm for
three months. Compound consumption was approximately 0, 15, 45 and
135 mg/kg body weight/day. Body weight gain and food efficiency
were slightly-reduced at the highest, dose level. No
compound-related effects were observed in hematology (RBC, total
WBC, hemoglobin and hematocrit) blood chemistry (plasma protein,
SGOT, SGPT, alkaline phosphatase, BUN and cholesterol),urinalysis
(pH, sugar & protein), organ weights or gross or histopathology
(Tsubura et al, 1975).
Rat (oral)
Groups of 15 male and 15 female rats were fed 0, 10, 20 and 40
mg/kg body weight/day of cartap hydrochloride in the diet for 13
weeks. Body weight gain and food consumption in male rats was
reduced at 40 mg/kg. Hematological values were within the normal
range for the rat although the hemoglobin concentration was
decreased and the erythrocyte count increased in male rats at the
upper dose level. No compound-related effects were observed in food
conversion ratio, urinalysis, ophthalmoscopy, plasma, erythrocyte
and brain cholinesterase, relative organ weights or gross and
microscopic pathological examination (Rivett et al, 1972).
Hen (oral)
Groups of 20 hens were fed cartap hydrochloride in the diet at
levels of 0, 10, 30, 90 and 270 ppm for 4 weeks followed by a
2-week post-treatment observation period. Body weight gain and food
consumption were decreased and egg shell weight/egg weight ratio
was lower at dietary levels of 90 and 270 ppm. Egg shell thickness
increased in each group except 90 and 270 ppm groups. No effect on
shell strength, egg yolk weight, egg yolk height or egg white
height were observed (Shiomi et al, 1973).
Long-term studies
Mouse
Groups of 40 male and 40 female mice were fed cartap
hydrochloride in the diet at levels of 0, 10, 20 and 40 mg/kg body
weight/day for a period of 80 weeks. No overt signs of toxicity
were observed and survival rate was not affected. Body weight gains
in the males during the first 52 weeks at 20 mg/kg and after 80
weeks at 40 mg/kg were slightly reduced. This effect was associated
with an impairment of food utilization. No compound-related effects
were noted on hematology and urinalysis parameters. Erythrocyte
cholinesterase activity was slightly reduced in the male and plasma
cholinesterase activity was lower in the female at 40 mg/kg. Other
blood chemistry parameters were within normal limits. No effect was
observed on brain cholinesterase activity. Increases in heart
weight (absolute and relative) in the female at 20 and 40 mg/kg and
thyroid weight in the male at 40 mg/kg were noted. The changes
noted on gross and histopathologic examination were common to both
treated and control animals. There was no evidence to suggest an
effect on the spontaneous tumour profile of this strain of mouse
(Hunter et al, 1974).
Rat
Groups of 45 male and 45 female rats were fed cartap
hydrochloride in the diet at 0, 10, 20 and 40 mg/kg body weight/day
for 104 weeks. A lower incidence in mortality in the treated males
was recorded. Body weight gain and food consumption were depressed
in both sexes, at 40 mg/kg in the male and at 20 mg/kg in the
female throughout the study. No evidence of a compound-related
effect was noted with respect to hematology, urinalysis, blood
chemistry including plasma and erythrocyte cholinesterase, brain
cholinesterase or ophthalmoscopy. There appeared to be a treatment-
related decrease in the relative liver weights of the male. This
effect was not dose-related. The increase in relative weights of
certain other organs receiving 40 mg/kg would appear to reflect the
body weight depression of this group. Gross and histopathological
changes observed in the treated groups were comparable to those in
the control group. There was no marked inter-group differences in
tumor incidence (Hunter et al, 1975).
OBSERVATIONS IN MAN
Cartap hydrochloride has been manufactured in Japan since
1966. Routine examinations of 98 workers employed in the
manufacture were considered essentially normal. A more detailed
medical examination which included urinalysis, blood chemistry,
hematology including routine examination was conducted yearly on
10 persons who were involved in the production of this chemical for
more than 5 years. No adverse effects relating to cartap
hydrochloride were observed in these workers (Hiraoka, 1975).
COMMENTS
Cartap hydrochloride is rapidly absorbed and metabolised.
Metabolites are excreted primarily in the urine and are not stored
to any great extent in the body.
No adverse effects at dietary levels up to 100 ppm were
observed in a two generation rat reproduction study. Teratogenic
studies in the mouse, rat and hamster revealed no compound-related
effect. However, these studies were considered inadequate because
the animals were not exposed to cartap hydrochloride for the full
period of organogenesis. Mutagenic studies were not preformed.
Cartap hydrochloride was shown to be moderately toxic to
mammals on acute exposure by several routes of administration. No
evidence of irritation was observed when cartap hydrochloride was
applied as a 20% paste to intact skin or was instilled as a 10%
solution into the eye of rabbits. However, inhalation exposure of
rats to relatively high concentrates of a 50% water-soluble dust
caused severe eye, dermal and respiratory irritation.
The reduced weight gain observed in the female rat at 20 and
40 mg/kg bw which appeared to be related to a lower food
consumption, was questioned. In various short and long term studies
in the mouse and rat a very weak anticholinesterase activity was
noted. In long term studies on the mouse and rat a compound-related
increase in tumour incidence was not observed. Since data were
submitted only on rodent species, concern was expressed as to the
effect of this compound on a non-rodent species. A detailed medical
examination of persons involved in the production of cartap
hydrochloride for more than 5 years revealed no compound-related
abnormalities.
No-effect levels in the rat and mouse have been established
and the data are sufficient to recommend a temporary acceptable
daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects:
Mouse 20 mg/kg bw/day
Rat 10 mg/kg bw/day
ESTIMATE FOR TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.05 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cartap hydrochloride insecticides have been registered in
Japan since 1967, and are widely used in Asia, Europe and South
America.
Cartap hydrochloride is used against a relatively broad
spectrum of insects, e.g., Lepidoptera, Coleoptera, Diptera and
Hemiptera. It is especially effective against Lepidopter such as
the rice stem borer, diamond-back moth and common cabbage worm, and
Coleoptera such as the Colorado potato beetle, Mexican bean beetle
etc.
Cartap is always applied as the hydrochloride, formulated into
25% and 50% water-soluble powder, 2% dust, 4% and 10% granules and
2% fine granules. A bait formulation has recently been introduced.
Approximately 70% of the world production is applied to rice and
30% to other crops, vegetables, potatoes, fruit, tea, etc.
Products based on cartap hydrochloride are registered in a
total of sixteen countries: Brazil, Bulgaria, Czechoslovakia, El
Salvador, France, German Democratic Republic, Hong Kong, Hungary,
Italy, Japan, Korea, Pakistan, Poland, Rumania, Spain, and Taiwan
province of China and are officially recommended in Greece,
Indonesia, Malaysia, Philippines, Thailand and Vietnam.
Pre-harvest treatments
Cartap hydrochloride is generally used at the rate of 0.5 -1.5
kg a.i./ha. The officially registered and/or recommended uses of
cartap hydrochloride are summarised in Table 1 which sets out
application rates and pre-harvest intervals.
Other uses
Cartap hydrochloride is used for controlling the rice
white-tip nematode by soaking rice seed in an aqueous solution of
the insecticide.
TABLE 1. Uses of cartap officially recommended in various countries
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Brazil
Cabbage, cotton,
passion fruit,
potato, sunflower, 0.5-0.75 14
tomato, wheat
Czechoslovakia
Mustard 0.5-0.6 7
Potato 0.5-0.6 14
Hungary
Potato, tomato 0.75-1.0 10
Italy
Potato 0.25-0.5 14
Japan (temporary)
Cabbage 0.5-0.75 7
Chestnut 1.5 14
Chinese cabbage 0.5-0.75 7
Ginger 0.5 3
Grape (vine) 1.5 14
Hop 1.0 14
Japanese persimmon 1.5 21
Japanese radish 0.35-0.5 7
Maize 1.0 7
TABLE 1. (Cont'd.)
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Potato 0.75 7
Rice 0.5-0.75 21
Sweet potato 0.5-0.75 7
Tea 1.0 7
Spain
Potato 0.35-0.65 15
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were obtained in Japan from supervised trials on
a variety of vegetables, fruits, hops, forage and rice. A summary
of these data is presented in Table 2 together with details of
application rate and pre-harvest interval.
Cabbage
Two applications of cartap hydrochloride to cabbage seldom
appear to produce detectable residues even when the last
application is made seven days before harvest. When four
applications were made throughout the growing season the residue
found ranged up to 0.1 mg/kg in cabbage harvested fourteen days
after the last treatment. Even eight treatments made at seven day
intervals produced residues of only 0.13 mg/kg fourteen days after
the last application.
The half-life appears to be of the order of 10-14 days.
Chestnut
Detectable residues were not found in the nuts when chestnut
trees were sprayed three times and the nuts were harvested 14 days
after the last application.
Chinese cabbage
Chinese cabbage appears to retain cartap residues at higher
levels and for longer periods than does cabbage. 4 sprays give rise
to residues ranging up to 0.27 mg/kg when chinese cabbage was
harvested 14 days after the last application. Residues up to 1.05
mg/kg were found after a preharvest interval of 7 days. Fine
granules appear to produce lower residues than sprays prepared from
soluble powders.
Ginger
Ginger appears to retain small quantities of cartap, (up to
0.03 mg/kg after 14 days) although the level of residues does not
appear to be significantly influenced by the number of treatments.
Grapes
The residue studies in grapes were designed to show the level
of residues resulting from the application of 2, 4 and 6 sprays in
grapes harvested 14, 21 and 30 days after the last application.
There is some indication that the residue levels in the fruit might
increase owing to systemic transfer for some time after application.
Generally the results found 30 days after the last spray were just
slightly lower than those found 21 days after the same treatment.
The level of residues was higher in grapes treated 6 times than
in those treated 4 times which in turn was distinctly higher than in
grapes treated only twice.
Hops
There is a very noticeable decline in the level of cartap
residues found in dried hops as the interval between last application
and harvest increases from 7 through 14 to 21 days. The half-life of
the residues appears to be of the order of 7 days but whether the
drying process has an influence on this is not clear.
Persimmon
The data on cartap residues in persimmons gathered from 2 trials
indicate only the quantity found 30 to 75 days after the last
application. Whilst detectable residues still occur 75 days after the
last application there is no great difference between the residue
level in samples from trees treated 6 or 4 times.
Japanese radish
Systematic studies of both leaves and roots of Japanese radish
(data on roots only are shown in Table 2) treated 2 and 4 times and
harvested 3, 7 and 14 days after the last application showed,
sometimes, a distinct increase in the residue concentration in the
TABLE 2. Cartrap residues resulting from supervised trials - Japan
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Cabbage 10/69 SP 0.6 2 <0,0.004 <0.004
4 0.01 0.008
11/69 SP 0.75 2 <0.004 <0.004
4 0.09 0.08
7/72 SP 0.75 2 0.02 0.06
4 0.14 0.09
12/72 FG 1.0 2 <0.006
5 0.07 0.05
8 0.13 0.07
12/72 FG 1.0 2 <0.006
3 <0.006
5 <0.006 <0.006
Chestnut 9/71 SP 60g/tree 3 <0.001 <0.001
3 <0.008 <0.008
Chinese 11/74 SP 0.6 2 0.03 0.02 0.02
cabbage 4 0.03 0.03 0.03
12/74 SP 0.6 2 0.7 0.07 0.06
4 0.5 0.16 0.15
1/75 SP 0.6 2 1.05 0.15 0.1
4 0.4 0.27 0.2
9/72 FG 1.0 2 0.03 0.01 <0.005
3 0.05 0.02 <0.005
5 0.12 0.03 <0.005
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Ginger 10/72 SP 0.7 4 0.006 <0.005
6 0.01 <0.005
9 0.007 <0.005
10/72 SP 1.0 3 0.02 0.03
5 0.02 0.02
11/73 SP 1.0 3 0.02 0.02
5 0.04 0.03
Grape 7/73 SP 2.0 2 0.08 0.12
4 0.54 0.38
6 0.57 0.97
9/73 SP 1.0 2 0.01 0.01 0.008
4 0.1 0.09 0.08
6 0.24 0.20 0.13
7/73 SP 2.0 2 0.08 0.13
4 0.35 0.40
6 0.75 0.80
9/73 SP 2.0 2 0.05 0.04 0.03
4 0.13 0.10 0.07
6 0.24 0.28 0.20
Hops 8/73 SP 2.0 3 0.56 0.04
(dry) 4 0.50 0.15
3.0 3 2.2 0.32
4 8.0 3.0
8/73 SP 2.0 3 0.30 0.03
4 0.34 0.14
3.0 3 0.92 0.28
4 4.9 2.7
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Persimmon 11/70 SP 2.25 4 0.02
6 0.03
11/70 SP 7.5 4 0.08 0.07
6 0.19 0.05
Potato 6/71 SP 1.5 3 <0.008 <0.008
6 <0.008 <0.008 <0.008
12/72 SP 1.0 2 <0.005 <0.005
5 <0.005 <0.005
8 <0.005 <0.005
FG 1.0 5 <0.001 <0.001
8 <0.001 <0.001
Radish 10/73 SP 0.75 2 0.10 0.12 0.03
(Japanese) 4 0.08 0.14 0.04
11/73 SP 0.5 2 0.52 0.12 0.15
4 0.57 0.22 0.22
11/73 SP 0.75 2 0.25 0.15 0.10
4 0.02 0.02 0.01
Rice
Grain 10/74 SP 0.5 8 <0.01 <0.01 <0.01
8/74 1.0 8 <0.01 <0.01 <0.01
0.5 8 0.05 <0.01 0.02
Straw 10/74 SP 1.0 8 <0.01 0.02 <0.01
8/74
Grain 10/74 SP 0.5 8 <0.005 0.005 <0.005
8/74 SP 1.0 8 <0.005 0.006 <0.005
Straw 10/74 SP 0.5 8 0.05 <0.01 0.02
8/74 SP 1.0 8 0.02 <0.01 <0.01
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Rice
Grain 10/74 SP 1.6 8 <0.01 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Straw 10/74 SP 1.6 8 0.02 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Grain 10/74 FG 0.8 8 <0.005 <0.005 <0.005
8/74 FG 1.0 8 <0.005 <0.005 <0.005
Straw 10/74 FG 0.8 8 0.05 <0.01 0.03
8/74 FG 1.0 8 <0.01 <0.01 <0.01
Tea (dry) 7/73 SP 1.0 1 1.0 1.2 0.6
7/73 SP 1.0 2 1.4 1.8 0.6
7/73 SP 1.0 1 0.7 0.8 0.7
Sweet corn 9/71 FG 0.6 1 <0.008 <0.008 <0.005 <0.005
Forage
Orchard grass 9/71 SP 0.5 1 0.03 0.01
2 0.06 0.04
Red clover 8/71 SP 0.5 1 0.01 0.03
2 0.11 0.01
White clover 9/71 SP 0.5 1 0.22 0.05
2 0.52 0.45
leaves during the first 7 days. This trend was not noticeable in the
roots. In radish root the half-life appears to be of the order of 10
days.
Potatoes
A number of trials confirm that detectable cartap residues do not
occur in the tubers of potatoes even when the crop is treated 8 times
during the season and the tubers are harvested 7, 14 or 21 days after
the last application.
Rice
Numerous trials have demonstrated that rice grain harvested 30 or
more days after 8 applications of cartap hydrochloride sprays almost
invariably contains no residues above the limit of determination. A
few of the many samples of rice straw from these trials were found to
contain residues at or just above the limit of determination.
Tea
Green tea produced from leaves harvested 7, 14 and 21 days after
the application of cartap hydrochloride contained residues ranging up
to almost 2 mg/kg. In 2 of the 3 trials the concentration of residues
increased slightly between the 7th and 14th day following treatment
but declined by the 21st day.
FATE OF RESIDUES
General comments
Cartap hydrochloride is stable to acid, but is hydrolysed in
neutral or alkaline solutions to give dihydronereistoxin (NTXH) which
is quite easily oxidised to nereistoxin (NTX) by air. NTXH and NTX may
be biologically convertible to one another, an shown in the following
reaction:
Other information on identity and properties
Cartap is invariably used as the hydrochloride, to which the
following information refers.
Molecular weight: 273.8
State: Colourless crystals
Melting point: 179-181°C(decomp.)
Solubility(g/100 ml): Soluble in water(13.2/10°C,
17.8/20°C, 25.3/30°C slightly soluble
in methanol(2.08/5°C), almost insoluble
in acetone, benzene, chloroform,
diethyl ether, ethyl acetate, and
n-hexane.
Purity of technical material: 97%. containing 3% of water and
ammonium chloride as impurities.
Hydroscopicity: Slightly hygroscopic
Volatility: Negligible
Formulation: 25% and 50% water-soluble powder, 2%
dust, 4% and 10% granules and 2% fine
granules are available. A bait
formulation has recently been
introduced.
Cartap hydrochloride is an insecticide recently developed by
Takeda Chemical Industries. It is a derivative of nereistoxin which
is a naturally occurring insecticidal substance isolated from the
marine segmented worms Lumbrinereis heteropoda and L.
brevicirra.
Nereistoxin was isolated by Nitta in 1934, the structure was
elucidated by Okaichi and Hashimoto (1962) and synthesized in 1965
by Hagiwara et al. A variety of nereistoxin derivatives were
prepared and their insecticidal activities tested. Cartap
hydrochloride is one of the most potent of the many compounds
prepared.
The chemistry and synthesis of nereistoxin and related
compounds are dealt with by a number of authors including Hashimoto
et al (1960); Okaichi and Hashimoto (1962); Hagiwara et al (1965);
Konishi (1968a, 1968b); Hagiwara and Numata (1968); Konishi (1970a,
1971); Sakai (1971); Nishi et al (1973).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Groups of 10 male and 20 female rats were administered cartap
hydrochloride in the diet at levels of 0, 100 and 1000 ppm for 9
weeks prior to mating and subsequently for the remainder of the
study which was a 2 litter, 2 generation reproduction study. One
half of the F2b litters were delivered by caesarean section on day
19 of gestation, the other half were delivered naturally and held
to weaning. Parental animals showed reduced body weight gain and
food consumption and an apparent impairment of fertility for the
F1a generation at 1000 ppm. Gestation and live birth indices of
the treated animals were comparable to those of the controls.
Weanling survival index was lower in the F1b generation and pup
weights in all generations at 7, 14 and 21 days after weaning were
reduced at the upper dose level. There were no meaningful
differences observed between groups with respect to number of
implantation sites, resorptions or live and dead fetuses. No
compound-related microscopic alterations were noted in selected
tissues from weanling F2b progeny of the 1000 ppm group. However,
a greater incidence of incomplete ossification was revealed on
skeletal examination in this group, (Olson and Busey, 1972).
Special studies on teratogenicity
Mouse
Three groups comprising 20, 12 and 14 pregnant mice received
cartap hydrochloride by stomach tube at dose levels of 0, 50 and
100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Twenty-one pregnant mice were employed as an untreated
control group. Animals were killed on day 19 of gestation and
fetuses removed by caesarean section. Weight gains were not
affected. No significant variations were observed in the number of
implantation sites, fetal mortality and mean body weight of fetuses
between control and treated groups. No visceral abnormalities were
observed. Skeletal examination revealed abnormalities (fusion of
cervical vertebral arches and fusion of sternebrae) in 2/110
fetuses (1.8%) at 50 mg/kg and 2/120 fetuses (1.7%) at 100 mg/kg.
In respect to other skeletal variations, there were no significant
differences between control and treated animals (Mizutani et al,
1971).
Rat
Cartap hydrochloride was administered orally to three groups
of 21, 12 and 17 pregnant rats at dose levels of 0, 50 and 100
mg/kg body weight respectively from gestation day 9 to day 15.
Twenty-three pregnant rats were used as an untreated control group.
Animals were sacrificed on day 21 and fetuses were delivered by
laparotomy. An increase in mortality and retarded weight gain were
observed in the maternal animals at 100 mg/kg during the dosing
period. There was no statistical difference between treated and
control groups with respect to number of implantation sites, fetal
mortality, sex ratio, external and visceral malformations. Skeletal
examination revealed no major abnormalities except for an increase
25/174 (14.4%) in bilateral twin thoracic vertebral centrae in the
100 mg/kg group (Mizutani et al, 1971).
Hamster
Five groups comprising 9, 7, 8, 9 and 7 pregnant hamsters
received cartap, hydrochloride orally at dose levels of 0, 2, 10,
50 and 100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Fifteen pregnant hamsters were used as an untreated
control group. Animals were killed on day 16 of gestation and
fetuses removed by caesarean section. Treatment with 100 mg/kg
resulted in the death of 2/7 animals and was associated with
reduced weight gain. No compound-related effects were observed in
the number of implantation sites, fetal mortality, fetal weights,
external and visceral malformations. In hamsters receiving 100
mg/kg, the frequency of skeletal anomalies increased slightly, 6/52
(11.5%) showing abnormalities in the thoracic vertebrae and/or
ribs. An increase in the incidence of fetuses with extra lumbar
ribs was noted at dose levels of 50 and 100 mg/kg (Mizutani et al,
1971).
Special studies on pharmacological properties
Cartap hydrochloride was shown to have neuromuscular blocking
activity in vitro and in vivo in several animal species
(rat, dog, cat). The effects were characterized as follows: 1)
inhibition of acetylcholine induced contraction of the muscle, 2)
antagonism by cholinesterase inhibitor, calcium ion,
tetraethylammonium and posttetanic stimulation against the partial
neuromuscular block caused by cartap hydrochloride, 3) complete
antagonism by several sulphhydryl compounds (BAL, L-cysteine,
cystine, D-penicillamine) against the neuromuscular block, 5)
respiratory failure due to neuromuscular block, 6) stimulant effect
on the superior cervical ganglion followed by postsynaptic
depression (Nagawa et al, 1971).
Special studies on antidotes
Male mice pre-treated with an intraperitoneal injection of
diazepam, phenobarbital, atropine sulphate, C-penicillamin cystine
or other sulphhydryl compounds delayed the onset of symptoms from
a toxic dose (500 mg/kg) of cartap hydrochloride. The intravenous
administration of cystine (ED50 40 mg/kg) was shown to be effective
in protecting rabbits from the toxic symptoms of an acute oral dose
(50 mg/kg) of cartap hydrochloride. In dogs, a minimal effective
dose of 12.5 mg/kg cystine was required in order to protect against
intoxication from the dermal application of 200 mg/kg of cartap
hydrochloride (Nagawa et al, 1970).
Special studies on dermal irritation
Cartap hydrochloride was applied as a 5, 10 and 20% paste to
the intact skin of two male rabbits daily for 5 days. No evidence
of irritation was observed (Aramaki, 1972).
Special studies on eye irritation
Cartap hydrochloride was instilled into the right eye of 3
rabbits as a 1.5 or 10% solution (0.2 ml) or 15 mg of the powder.
No evidence of irritation to the bulbar or palpebral conjunctiva
was observed when examined at 24 and 48 hours (Aramaki, 1972).
Special studies on respiratory effects
Groups of 5 male and 5 female rats were exposed continuously
for 6 hours to the dust of a 50% water-soluble cartap hydrochloride
powder at estimated concentrations of 0.086 and 0.54 mg/litre of
air. Animals were observed for a period of 7 days following
exposure. Severe eye and skin irritation, increased nasal and oral
secretions, respiratory irritation and lethargy were observed at
0.54 mg/litre. Exposure to 0.086 mg/litre caused slight dyspnoea,
temporary eye irritation and polyuria. The signs of irritation of
this group disappeared rapidly after termination of exposure.
Post-mortem examination revealed no dose-related abnormalities in
either test group (Berczy and Cobb, 1973)
Four groups of 5 male and 5 female rats were exposed
continuously for 6 hours/day, 5 days a week for 3 weeks to the dust
of a 50% water-soluble cartap hydrochloride powder at estimated
nominal concentrations of 0, 0.01, 0.1 and 1.0 mg/litre of air.
Severe respiratory irritation, loss in body weight, injury to the
eyes and skin were observed at the exposure level of 1.0 mg/litre.
Certain clinical parameters (mean cell volume, neutrophil and
lymphocyte cells, glucose, BUN, SGPT and SGOT) were found to be
elevated in the high dose group. The changes in relative organ
weights observed in this group were considered to be related to
loss in body weight. Gross and histopathological examination
revealed no abnormalities in the two lower dose groups. Extensive
chronic respiratory effects in both sexes and acute ulceration in
the urinary bladder in male rats were noted at the high dose level
(Berczy et al, 1973).
Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Mouse M oral 225 Aramaki, 1972
M oral 150 Toyoshima et al,
1972
F oral 154 ibid
M s.c. 34.5 ibid
F s.c. 35 ibid
M i.v. 51 ibid
F i.v. 52 ibid
Rat M oral 380 Aramaki, 1972
F oral 390 ibid
M oral 345 Toyoshima et al,
1972
F oral 325 ibid
M s.c. 40 ibid
F s.c. 42 ibid
M i.v. 44 ibid
F i.v. 36 ibid
Monkey oral 100-200** Yokotani et al,.
1968
Rabbit M&F dermal 819* Davies & Collins,
1973
* Padan water soluble powder (Cartap HCl 50%).
** exact estimate not possible owing to vomiting.
Short-term studies
Mouse (dermal)
Cartap hydrochloride (0.2 ml of a 5 or 10% aqueous solution)
was applied to the intact skin on the backs of 6 male mice. During
the 7-day observation period no symptoms of intoxication developed and
necropsy examination of subcutaneous tissues showed no adverse
effects (Aramaki, 1972).
Rabbit (dermal)
Cartap hydrochloride was applied to intact or abraded skin of
groups of 5 male and 5 female rabbits at dose levels of 0, 15, 60
and 240 mg/kg body weight/day. Rabbits treated with 240 mg/kg died
or were sacrificed after 1 week. Treatment of rabbits with 15 and
60 mg/kg was continued 7 days a week, for 3 consecutive weeks.
Pupillary dilatation and lack of hind limb coordination were
observed in some animals of each group. Body weight gain and food
consumption were reduced at 60 mg/kg especially during the 3rd
week. PCV, hemoglobin concentration and RBC counts were also
decreased in males (abraded) when compared to intact control
animals at this dose level. Increased BUN and reduced plasm
cholinesterase activity were observed at 240 mg/kg when compared to
control values at week 3. Slight dermal irritation was noted during
the 2nd and 3rd week in rabbits receiving 15 and 60 mg/kg.
Ophthalmoscopy and macroscopic and microscopic pathology were
comparable for all groups (Davies et al, 1974).
Mouse (oral)
Groups of 8 or 9 mice of each sex were fed cartap
hydrochloride at dietary levels of 0, 100, 300 and 900 ppm for
three months. Compound consumption was approximately 0, 15, 45 and
135 mg/kg body weight/day. Body weight gain and food efficiency
were slightly-reduced at the highest, dose level. No
compound-related effects were observed in hematology (RBC, total
WBC, hemoglobin and hematocrit) blood chemistry (plasma protein,
SGOT, SGPT, alkaline phosphatase, BUN and cholesterol),urinalysis
(pH, sugar & protein), organ weights or gross or histopathology
(Tsubura et al, 1975).
Rat (oral)
Groups of 15 male and 15 female rats were fed 0, 10, 20 and 40
mg/kg body weight/day of cartap hydrochloride in the diet for 13
weeks. Body weight gain and food consumption in male rats was
reduced at 40 mg/kg. Hematological values were within the normal
range for the rat although the hemoglobin concentration was
decreased and the erythrocyte count increased in male rats at the
upper dose level. No compound-related effects were observed in food
conversion ratio, urinalysis, ophthalmoscopy, plasma, erythrocyte
and brain cholinesterase, relative organ weights or gross and
microscopic pathological examination (Rivett et al, 1972).
Hen (oral)
Groups of 20 hens were fed cartap hydrochloride in the diet at
levels of 0, 10, 30, 90 and 270 ppm for 4 weeks followed by a
2-week post-treatment observation period. Body weight gain and food
consumption were decreased and egg shell weight/egg weight ratio
was lower at dietary levels of 90 and 270 ppm. Egg shell thickness
increased in each group except 90 and 270 ppm groups. No effect on
shell strength, egg yolk weight, egg yolk height or egg white
height were observed (Shiomi et al, 1973).
Long-term studies
Mouse
Groups of 40 male and 40 female mice were fed cartap
hydrochloride in the diet at levels of 0, 10, 20 and 40 mg/kg body
weight/day for a period of 80 weeks. No overt signs of toxicity
were observed and survival rate was not affected. Body weight gains
in the males during the first 52 weeks at 20 mg/kg and after 80
weeks at 40 mg/kg were slightly reduced. This effect was associated
with an impairment of food utilization. No compound-related effects
were noted on hematology and urinalysis parameters. Erythrocyte
cholinesterase activity was slightly reduced in the male and plasma
cholinesterase activity was lower in the female at 40 mg/kg. Other
blood chemistry parameters were within normal limits. No effect was
observed on brain cholinesterase activity. Increases in heart
weight (absolute and relative) in the female at 20 and 40 mg/kg and
thyroid weight in the male at 40 mg/kg were noted. The changes
noted on gross and histopathologic examination were common to both
treated and control animals. There was no evidence to suggest an
effect on the spontaneous tumour profile of this strain of mouse
(Hunter et al, 1974).
Rat
Groups of 45 male and 45 female rats were fed cartap
hydrochloride in the diet at 0, 10, 20 and 40 mg/kg body weight/day
for 104 weeks. A lower incidence in mortality in the treated males
was recorded. Body weight gain and food consumption were depressed
in both sexes, at 40 mg/kg in the male and at 20 mg/kg in the
female throughout the study. No evidence of a compound-related
effect was noted with respect to hematology, urinalysis, blood
chemistry including plasma and erythrocyte cholinesterase, brain
cholinesterase or ophthalmoscopy. There appeared to be a treatment-
related decrease in the relative liver weights of the male. This
effect was not dose-related. The increase in relative weights of
certain other organs receiving 40 mg/kg would appear to reflect the
body weight depression of this group. Gross and histopathological
changes observed in the treated groups were comparable to those in
the control group. There was no marked inter-group differences in
tumor incidence (Hunter et al, 1975).
OBSERVATIONS IN MAN
Cartap hydrochloride has been manufactured in Japan since
1966. Routine examinations of 98 workers employed in the
manufacture were considered essentially normal. A more detailed
medical examination which included urinalysis, blood chemistry,
hematology including routine examination was conducted yearly on
10 persons who were involved in the production of this chemical for
more than 5 years. No adverse effects relating to cartap
hydrochloride were observed in these workers (Hiraoka, 1975).
COMMENTS
Cartap hydrochloride is rapidly absorbed and metabolised.
Metabolites are excreted primarily in the urine and are not stored
to any great extent in the body.
No adverse effects at dietary levels up to 100 ppm were
observed in a two generation rat reproduction study. Teratogenic
studies in the mouse, rat and hamster revealed no compound-related
effect. However, these studies were considered inadequate because
the animals were not exposed to cartap hydrochloride for the full
period of organogenesis. Mutagenic studies were not preformed.
Cartap hydrochloride was shown to be moderately toxic to
mammals on acute exposure by several routes of administration. No
evidence of irritation was observed when cartap hydrochloride was
applied as a 20% paste to intact skin or was instilled as a 10%
solution into the eye of rabbits. However, inhalation exposure of
rats to relatively high concentrates of a 50% water-soluble dust
caused severe eye, dermal and respiratory irritation.
The reduced weight gain observed in the female rat at 20 and
40 mg/kg bw which appeared to be related to a lower food
consumption, was questioned. In various short and long term studies
in the mouse and rat a very weak anticholinesterase activity was
noted. In long term studies on the mouse and rat a compound-related
increase in tumour incidence was not observed. Since data were
submitted only on rodent species, concern was expressed as to the
effect of this compound on a non-rodent species. A detailed medical
examination of persons involved in the production of cartap
hydrochloride for more than 5 years revealed no compound-related
abnormalities.
No-effect levels in the rat and mouse have been established
and the data are sufficient to recommend a temporary acceptable
daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects:
Mouse 20 mg/kg bw/day
Rat 10 mg/kg bw/day
ESTIMATE FOR TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.05 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cartap hydrochloride insecticides have been registered in
Japan since 1967, and are widely used in Asia, Europe and South
America.
Cartap hydrochloride is used against a relatively broad
spectrum of insects, e.g., Lepidoptera, Coleoptera, Diptera and
Hemiptera. It is especially effective against Lepidopter such as
the rice stem borer, diamond-back moth and common cabbage worm, and
Coleoptera such as the Colorado potato beetle, Mexican bean beetle
etc.
Cartap is always applied as the hydrochloride, formulated into
25% and 50% water-soluble powder, 2% dust, 4% and 10% granules and
2% fine granules. A bait formulation has recently been introduced.
Approximately 70% of the world production is applied to rice and
30% to other crops, vegetables, potatoes, fruit, tea, etc.
Products based on cartap hydrochloride are registered in a
total of sixteen countries: Brazil, Bulgaria, Czechoslovakia, El
Salvador, France, German Democratic Republic, Hong Kong, Hungary,
Italy, Japan, Korea, Pakistan, Poland, Rumania, Spain, and Taiwan
province of China and are officially recommended in Greece,
Indonesia, Malaysia, Philippines, Thailand and Vietnam.
Pre-harvest treatments
Cartap hydrochloride is generally used at the rate of 0.5 -1.5
kg a.i./ha. The officially registered and/or recommended uses of
cartap hydrochloride are summarised in Table 1 which sets out
application rates and pre-harvest intervals.
Other uses
Cartap hydrochloride is used for controlling the rice
white-tip nematode by soaking rice seed in an aqueous solution of
the insecticide.
TABLE 1. Uses of cartap officially recommended in various countries
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Brazil
Cabbage, cotton,
passion fruit,
potato, sunflower, 0.5-0.75 14
tomato, wheat
Czechoslovakia
Mustard 0.5-0.6 7
Potato 0.5-0.6 14
Hungary
Potato, tomato 0.75-1.0 10
Italy
Potato 0.25-0.5 14
Japan (temporary)
Cabbage 0.5-0.75 7
Chestnut 1.5 14
Chinese cabbage 0.5-0.75 7
Ginger 0.5 3
Grape (vine) 1.5 14
Hop 1.0 14
Japanese persimmon 1.5 21
Japanese radish 0.35-0.5 7
Maize 1.0 7
TABLE 1. (Cont'd.)
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Potato 0.75 7
Rice 0.5-0.75 21
Sweet potato 0.5-0.75 7
Tea 1.0 7
Spain
Potato 0.35-0.65 15
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were obtained in Japan from supervised trials on
a variety of vegetables, fruits, hops, forage and rice. A summary
of these data is presented in Table 2 together with details of
application rate and pre-harvest interval.
Cabbage
Two applications of cartap hydrochloride to cabbage seldom
appear to produce detectable residues even when the last
application is made seven days before harvest. When four
applications were made throughout the growing season the residue
found ranged up to 0.1 mg/kg in cabbage harvested fourteen days
after the last treatment. Even eight treatments made at seven day
intervals produced residues of only 0.13 mg/kg fourteen days after
the last application.
The half-life appears to be of the order of 10-14 days.
Chestnut
Detectable residues were not found in the nuts when chestnut
trees were sprayed three times and the nuts were harvested 14 days
after the last application.
Chinese cabbage
Chinese cabbage appears to retain cartap residues at higher
levels and for longer periods than does cabbage. 4 sprays give rise
to residues ranging up to 0.27 mg/kg when chinese cabbage was
harvested 14 days after the last application. Residues up to 1.05
mg/kg were found after a preharvest interval of 7 days. Fine
granules appear to produce lower residues than sprays prepared from
soluble powders.
Ginger
Ginger appears to retain small quantities of cartap, (up to
0.03 mg/kg after 14 days) although the level of residues does not
appear to be significantly influenced by the number of treatments.
Grapes
The residue studies in grapes were designed to show the level
of residues resulting from the application of 2, 4 and 6 sprays in
grapes harvested 14, 21 and 30 days after the last application.
There is some indication that the residue levels in the fruit might
increase owing to systemic transfer for some time after application.
Generally the results found 30 days after the last spray were just
slightly lower than those found 21 days after the same treatment.
The level of residues was higher in grapes treated 6 times than
in those treated 4 times which in turn was distinctly higher than in
grapes treated only twice.
Hops
There is a very noticeable decline in the level of cartap
residues found in dried hops as the interval between last application
and harvest increases from 7 through 14 to 21 days. The half-life of
the residues appears to be of the order of 7 days but whether the
drying process has an influence on this is not clear.
Persimmon
The data on cartap residues in persimmons gathered from 2 trials
indicate only the quantity found 30 to 75 days after the last
application. Whilst detectable residues still occur 75 days after the
last application there is no great difference between the residue
level in samples from trees treated 6 or 4 times.
Japanese radish
Systematic studies of both leaves and roots of Japanese radish
(data on roots only are shown in Table 2) treated 2 and 4 times and
harvested 3, 7 and 14 days after the last application showed,
sometimes, a distinct increase in the residue concentration in the
TABLE 2. Cartrap residues resulting from supervised trials - Japan
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Cabbage 10/69 SP 0.6 2 <0,0.004 <0.004
4 0.01 0.008
11/69 SP 0.75 2 <0.004 <0.004
4 0.09 0.08
7/72 SP 0.75 2 0.02 0.06
4 0.14 0.09
12/72 FG 1.0 2 <0.006
5 0.07 0.05
8 0.13 0.07
12/72 FG 1.0 2 <0.006
3 <0.006
5 <0.006 <0.006
Chestnut 9/71 SP 60g/tree 3 <0.001 <0.001
3 <0.008 <0.008
Chinese 11/74 SP 0.6 2 0.03 0.02 0.02
cabbage 4 0.03 0.03 0.03
12/74 SP 0.6 2 0.7 0.07 0.06
4 0.5 0.16 0.15
1/75 SP 0.6 2 1.05 0.15 0.1
4 0.4 0.27 0.2
9/72 FG 1.0 2 0.03 0.01 <0.005
3 0.05 0.02 <0.005
5 0.12 0.03 <0.005
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Ginger 10/72 SP 0.7 4 0.006 <0.005
6 0.01 <0.005
9 0.007 <0.005
10/72 SP 1.0 3 0.02 0.03
5 0.02 0.02
11/73 SP 1.0 3 0.02 0.02
5 0.04 0.03
Grape 7/73 SP 2.0 2 0.08 0.12
4 0.54 0.38
6 0.57 0.97
9/73 SP 1.0 2 0.01 0.01 0.008
4 0.1 0.09 0.08
6 0.24 0.20 0.13
7/73 SP 2.0 2 0.08 0.13
4 0.35 0.40
6 0.75 0.80
9/73 SP 2.0 2 0.05 0.04 0.03
4 0.13 0.10 0.07
6 0.24 0.28 0.20
Hops 8/73 SP 2.0 3 0.56 0.04
(dry) 4 0.50 0.15
3.0 3 2.2 0.32
4 8.0 3.0
8/73 SP 2.0 3 0.30 0.03
4 0.34 0.14
3.0 3 0.92 0.28
4 4.9 2.7
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Persimmon 11/70 SP 2.25 4 0.02
6 0.03
11/70 SP 7.5 4 0.08 0.07
6 0.19 0.05
Potato 6/71 SP 1.5 3 <0.008 <0.008
6 <0.008 <0.008 <0.008
12/72 SP 1.0 2 <0.005 <0.005
5 <0.005 <0.005
8 <0.005 <0.005
FG 1.0 5 <0.001 <0.001
8 <0.001 <0.001
Radish 10/73 SP 0.75 2 0.10 0.12 0.03
(Japanese) 4 0.08 0.14 0.04
11/73 SP 0.5 2 0.52 0.12 0.15
4 0.57 0.22 0.22
11/73 SP 0.75 2 0.25 0.15 0.10
4 0.02 0.02 0.01
Rice
Grain 10/74 SP 0.5 8 <0.01 <0.01 <0.01
8/74 1.0 8 <0.01 <0.01 <0.01
0.5 8 0.05 <0.01 0.02
Straw 10/74 SP 1.0 8 <0.01 0.02 <0.01
8/74
Grain 10/74 SP 0.5 8 <0.005 0.005 <0.005
8/74 SP 1.0 8 <0.005 0.006 <0.005
Straw 10/74 SP 0.5 8 0.05 <0.01 0.02
8/74 SP 1.0 8 0.02 <0.01 <0.01
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Rice
Grain 10/74 SP 1.6 8 <0.01 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Straw 10/74 SP 1.6 8 0.02 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Grain 10/74 FG 0.8 8 <0.005 <0.005 <0.005
8/74 FG 1.0 8 <0.005 <0.005 <0.005
Straw 10/74 FG 0.8 8 0.05 <0.01 0.03
8/74 FG 1.0 8 <0.01 <0.01 <0.01
Tea (dry) 7/73 SP 1.0 1 1.0 1.2 0.6
7/73 SP 1.0 2 1.4 1.8 0.6
7/73 SP 1.0 1 0.7 0.8 0.7
Sweet corn 9/71 FG 0.6 1 <0.008 <0.008 <0.005 <0.005
Forage
Orchard grass 9/71 SP 0.5 1 0.03 0.01
2 0.06 0.04
Red clover 8/71 SP 0.5 1 0.01 0.03
2 0.11 0.01
White clover 9/71 SP 0.5 1 0.22 0.05
2 0.52 0.45
leaves during the first 7 days. This trend was not noticeable in the
roots. In radish root the half-life appears to be of the order of 10
days.
Potatoes
A number of trials confirm that detectable cartap residues do not
occur in the tubers of potatoes even when the crop is treated 8 times
during the season and the tubers are harvested 7, 14 or 21 days after
the last application.
Rice
Numerous trials have demonstrated that rice grain harvested 30 or
more days after 8 applications of cartap hydrochloride sprays almost
invariably contains no residues above the limit of determination. A
few of the many samples of rice straw from these trials were found to
contain residues at or just above the limit of determination.
Tea
Green tea produced from leaves harvested 7, 14 and 21 days after
the application of cartap hydrochloride contained residues ranging up
to almost 2 mg/kg. In 2 of the 3 trials the concentration of residues
increased slightly between the 7th and 14th day following treatment
but declined by the 21st day.
FATE OF RESIDUES
General comments
Cartap hydrochloride is stable to acid, but is hydrolysed in
neutral or alkaline solutions to give dihydronereistoxin (NTXH) which
is quite easily oxidised to nereistoxin (NTX) by air. NTXH and NTX may
be biologically convertible to one another, an shown in the following
reaction:
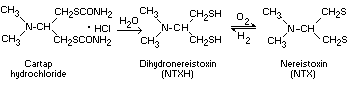 Sakai (1970) in discussing nereistoxin and its relatives
indicates that the contact toxicity to Azuki bean weevil, rice stem
borer and mouse, and the activity in blocking the synaptic response of
the 6th abdominal ganglion of the American cockroach are remarkably
similar for cartap hydrochloride and nereistoxin. It seems possible
that the activity of cartap hydrochloride is due to its conversion to
nereistoxin in plants and animals.
In animals
The absorption, distribution and excretion of cartap
hydrochloride in rats and mice is described above ("Bio-chemical
aspects") and further studies are in progress. Chromatographic studies
have revealed that there are about nine main metabolites in the urine
from animals receiving cartap, hydrochloride orally. There appears to
be no significant difference in the pattern between rats and mice.
Unchanged cartap hydrochloride could not be detected. One of the
identified metabolites was the mono-sulphoxide of
1,3-di(methylthio)-2-dimethylaminopropane and it comprised 18% and 8%
of the urinary metabolites in the rat and mouse respectively
(Shirakawa et al, 1971).
In plants
Tomizawa and Endo (1972) studied the uptake of 35S-labelled
cartap hydrochloride in rice plants. The basic fraction which was
assumed to retain biological activity reached a maximum concentration
in the leaf sheath and leaf blade on the 7th day after application and
thereafter declined rapidly until the 15th day. A small but consistent
amount of radio-activity remained in both leaf sheath and leaf blade
for 30 days. Radio-activity in the ear remained consistently low
throughout the test period.
Among the metabolites, nereistoxin and sulphuric acid were
identified. The metabolic degradation of cartap hydrochloride in
plants was judged to proceed in the following sequence (Tomizawa and
Endo, 1972; Tomizawa et al., 1974).
Sakai (1970) in discussing nereistoxin and its relatives
indicates that the contact toxicity to Azuki bean weevil, rice stem
borer and mouse, and the activity in blocking the synaptic response of
the 6th abdominal ganglion of the American cockroach are remarkably
similar for cartap hydrochloride and nereistoxin. It seems possible
that the activity of cartap hydrochloride is due to its conversion to
nereistoxin in plants and animals.
In animals
The absorption, distribution and excretion of cartap
hydrochloride in rats and mice is described above ("Bio-chemical
aspects") and further studies are in progress. Chromatographic studies
have revealed that there are about nine main metabolites in the urine
from animals receiving cartap, hydrochloride orally. There appears to
be no significant difference in the pattern between rats and mice.
Unchanged cartap hydrochloride could not be detected. One of the
identified metabolites was the mono-sulphoxide of
1,3-di(methylthio)-2-dimethylaminopropane and it comprised 18% and 8%
of the urinary metabolites in the rat and mouse respectively
(Shirakawa et al, 1971).
In plants
Tomizawa and Endo (1972) studied the uptake of 35S-labelled
cartap hydrochloride in rice plants. The basic fraction which was
assumed to retain biological activity reached a maximum concentration
in the leaf sheath and leaf blade on the 7th day after application and
thereafter declined rapidly until the 15th day. A small but consistent
amount of radio-activity remained in both leaf sheath and leaf blade
for 30 days. Radio-activity in the ear remained consistently low
throughout the test period.
Among the metabolites, nereistoxin and sulphuric acid were
identified. The metabolic degradation of cartap hydrochloride in
plants was judged to proceed in the following sequence (Tomizawa and
Endo, 1972; Tomizawa et al., 1974).
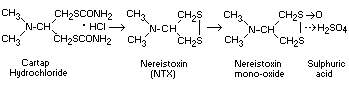 Dust, fine granule and granule formulations of cartap
hydrochloride were applied to rice plants in a paddy field, and the
cartap residue in the plants was determined by analysing the leaf
blades and leaf sheathes separately. Further measurements were also
made on water and soil from the treated plots. The concentration of
cartap in the rice plants immediately after application was found to
be of the order of 1 to 2 mg/kg for dust and fine granule treatments
but only 0.1 mg/kg in the case of granules. 3 days later the
concentration on the whole plants had fallen to approximately 0.05
mg/kg except on those treated with dust which were found to contain
0.2 mg/kg. After 5 days the concentration had fallen slightly in the
case of plants treated with dust and fine granules but plants treated
with granules were found to have a noticeable increase in cartap
residues, apparently owing to uptake from the water into which the
granules had fallen. This trend continued until the 7th day after
which residues began to decline slowly. The leaf blade was found to
have significantly higher residues than the leaf sheath (Koyama et al,
1975). It is difficult to tell how much of the residue determined in
these trials was a surface deposit on the outside of the leaves and
how much was taken into the plant.
Koyama et al (1975), quote work by Sakai which indicates that
when relatively high concentrations of cartap hydrochloride are
applied to rice plants in nursery boxes, the plants are capable of
taking up concentrations of the order of 20 mg/kg which, even after 42
days, remain at about 0.5 mg/kg. Due allowance must be made for the
extensive dilution which would occur owing to the growth of the plant
over this period.
Tomizawa et al (1974), using 35S-labelled cartap hydrochloride
showed that the rate and degree of absorption of cartap hydrochloride
by rice plants was much greater when the insecticide was applied in
the paddy water than when it was applied to the plants in the form of
a spray, and an effective concentration remained in the rice plant for
a longer period. By using ion-exchange chromatography it was possible
to show that the conversion of cartap hydrochloride to nereistoxin
occurred rapidly after application.
In soil
Tomizawa and Endo (1972) studied the movement of cartap
hydrochloride in soil under laboratory conditions. They took alluvial
clay from a paddy field and from an unirrigated field, volcanic ash
and sandy loam and applied radio-active cartap hydrochloride at a
dosage of 1 mg/50 g of soil. The authors do not mention the
temperature at which the treated soil was held but indicate that the
soil from the paddy field had the water content adjusted to 150% of
field capacity while for the other three soils the water content was
adjusted to 75% of field capacity. Samples of soil were removed at 6
intervals over the next 30 days and were extracted in order to
determine the cartap content. The concentration was measured in the
basic fraction extracted with aqueous ammonia. It was found that the
concentration in the soil one day after treatment was of the order of
1 to 2 mg/kg in all four samples. The concentration in the soil from
the paddy field remained virtually constant for 10 days and by the
30th day had declined to one-sixth. The volcanic ash yielded only one
thirtieth and the other two soils about one tenth of the original
concentration by the 30th day.
It is not clear whether the difference in concentration found
after 30 days is due to a difference in the speed of decomposition of
cartap hydrochloride or whether it results from the higher capacity of
volcanic ash to absorb cartap hydrochloride and its decomposition
products. There is a strong indication that cartap hydrochloride acts
as a chelating agent in soil (Takei, 1976). This may explain the low
recoveries reported by Tomizawa and Endo (1972).
Koyama et al (1975) demonstrated that when various cartap
hydrochloride formulations were applied to rice plants growing in
paddy fields there was a distinct transfer of cartap in the case of
granules. There appeared to be an increase in the concentration in the
soil of plots treated with granules over the first 14 days. In the
case of other formulations detectable residues were found in the soil
of all plots at the end of 14 days but at the time of harvest, 41
months after initial treatment, the concentration was below the limit
of determination.
In water
In the experiments with soil and rice plants described above,
Koyama et al (1975) also studied the concentration of cartap
hydrochloride in the water of paddy fields treated with various cartap
hydrochloride formulations. It was found that immediately after
application the concentration of cartap hydrochloride in the water of
paddy fields ranged between 1 and 2 mg/l but by the third day after
treatment it had generally fallen below the limit of determination,
except in the plots treated with granules where it was marginally
above this limit. The apparent difference in the stability of cartap
hydrochloride in water and soil of the same paddy fields seems
attributable to the fact that cartap hydrochloride dissolved in water
is easily degraded by direct sunlight while the residue held in soils
is relatively stable. Cartap hydrochloride is stable in aqueous
solution at pH3-4. In the water of paddy fields which would have a pH
ranging from 5 to 7, it may be decomposed gradually to nereistoxin as
described at the beginning of this section.
Tomizawa and Endo (1972) applied radio-active cartap
hydrochloride to the surface of water in which rice seedlings were
transplanted, at a rate corresponding to 1.5 kg/ha. The radio-activity
of samples of water was measured after various intervals. Although the
concentration in the water sampled immediately after application
indicated the presence of approximately 10 mg of cartap hydrochloride
per litre of water, within the first 24 hours the concentration had
fallen by more than 90% with a further slow decline over the
succeeding days. The loss of the compound from the aqueous phase may
probably be attributable to rapid absorption onto soil particles.
METHODS OF RESIDUE ANALYSIS
Several colorimetric, iodometric, oscillopolarographic and
gaschromatographic methods have been developed for the determination
of cartap. The oscillopolarographic and gaschromatographic methods are
suitable for residue analysis. Both are based on the measurement of
4.N,N-dimethylamino-1,2-dithiolane (nereistoxin, NTXH) which is
quantitatively derived from cartap. Cartap hydrochloride and its
metabolite dihydronereistoxin (NTXH) and nereistoxin are extracted
from samples with diluted hydrochloric acid and the unchanged cartap
hydrochloride is hydrolysed and oxidized in basic solution to NTX.
Total NTX is extracted from the reaction mixture with ethyl ether and
is used for analysis.
Recoveries by both the oscillopolarographic and the
gaschromatographic methods were generally between 75 and 95% (Nishi et
al, 1973). Soil, rice, tea and straw present difficulties in analysis
whereas substrates such as cabbage are easily extracted with good
recovery. Apparently there is a high degree of disulphide binding with
some commodities, because the addition of cystine results in a release
of residues and significantly better recovery (Takei, 1976).
In the oscillopolarographic method, liquid/liquid partition is
required during the clean-up in order to minimise the carry-over of
possible interfering compounds. A single sweep oscillopolarograph
(Randles-Sevcik type) is used. The polarogram of NTX appears at -0.78
±0.05 V vs. Hg pool, and the concentration is quantitatively
determined by the peak height. The limit of determination of this
method is 0.004 mg/kg. Some reducible pesticides, such as
organophosphorus pesticides containing a nitro group, did not
interfere with the determination (Nishi et al, 1973).
Residues of cartap can be determined easily by gaschromatography
with a high sensitivity and specificity. A flame photometric detector
(FPD) equipped with a sulphur mode filter and a glass column
containing 3% OV-1 on chromosorb W or 5% PEG 20 on Gaschrom Q at a
temperature of 130-165°C are used. The limit of determination by this
method is 0.005 mg/kg. Investigation by Nishi et al, (1973) of the
gas-chromatographic method indicates that it should be applicable to
all types of residue analysis. Since the treatment is simple and less
rigorous clean-up is required, the gas-chromatographic method is
superior to the polarographic method. It appears suitable for
regulatory analysis.
Most of the residue data from supervised trials have been
obtained by the gas-chromatographic method, and in the course of this
work almost 100 samples of 12 different commodities have been analyzed
independently by analysis from two of three separate laboratories. In
some cases the results presented by the two analysts differ by
relatively large factors. The percentage difference between the means
obtained by the two analysts does not give a true picture of the
variability. In Table 3 the significance of the difference between
laboratory means has been assessed in relation to the variation
between replicates (a) of pooled over values of <0.050 mg/kg, where
within-pair variability tends to be lowest and (b) pooled over values
between 0.050 and 0.500 mg/kg where this variability is greater. Where
levels above 0.5 mg/kg are found the differences within and between
pairs of duplicates are inconsistent and there are insufficient data
to make a valid comparison of laboratory means.
TABLE 3. Mean differences between samples analysed by 2 laboratories
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Chinese cabbage 0.0275 0.1195 0.092 P<.001
0.0335 0.1085 0.075 P<.001
0.0315 0.1625 0.131 P<.001
0.7060 0.9750 0.269 -
0.0700 0.1445 0.0745 P<.001
Ginger 0.0245 0.0220 0.0025 NS
0.0205 0.0330 0.0125 P<.001
0.0380 0.0220 0.016 P<.001
Grape 0.0765 0.0800 0.0035 NS
0.5345 0.3500 0.1845 P<.001
0.5645 0.7400 0.1755 P<.001
0.1245 0.1800 0.0555 P<.001
Hops 0.9150 2.0900 1.175 -
0.2700 0.3200 0.05 P<.001
4.8200 7.7150 2.895 -
0.2700 0.4950 0.225 P<.001
Japanese 0.0610 0.0935 0.0325 P<.01
radish 0.0520 0.1150 0.063 P<.001
leaf 0.0580 0.1270 0.069 P<.001
root 0.0290 0.0245 0.0045 NS
0.0115 0.0085 0.003 NS
0.0135 0.0160 0.0025 NS
Potato 0.0010 0.0050 0.004 NS
0.0070 0.0050 0.002 NS
Rice grain 0.0050 0.0100 0.005 NS
0.0060 0.0100 0.004 NS
Rice straw 0.0100 0.0200 0.01 P<.01
0.0450 0.0450 0.00 NS
Orchard grass 0.0260 0.0200 0.006 NS
0.0590 0.1500 0.091 P<.001
TABLE 3. (Cont'd.)
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Red clover 0.0095 0.1050 0.0955 P<.001
0.0255 0.1800 0.1545 P<.001
0.1080 0.0200 0.088 P<.001
White clover 0.2150 0.0800 0.135 P<.001
0.4430 0.0450 0.398 P<.001
Least difference in means necessary for significance:
P <0.05 P <0.01 P <0.00
Single estimates <0.050 = 0.0059 0.0080 0.0106
" " >0.050 = 0.0231 0.0311 0.0413
- = insufficient high values to assess significance.
NS = not significant
The results show that in most cases very highly significant
differences (P<0.001) occur between laboratories analysing samples of
the same commodity, while in some cases, notably commodities having
residues below 0.05 mg/kg, the differences are not significant. The
low residues occur mainly in root crops and grains, which would be
expected to be less subject than leafy plants to sampling variations
and therefore to give more consistent analytical results.
It is rarely possible to make such a comparison as this, since
investigators rarely submit samples to two different laboratories for
check analysis. The opportunity to see practical evidence of the
difficulties confronting residue analysts seeking to determine small
quantities of complex substances in a variety of raw agricultural
commodities is valuable. Such data are needed to provide an adequate
basis for the establishment of maximum residue limits appropriate to
the problems facing residue analysts and food control officials.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Brazil
Cotton, sunflower 0.03 mg/kg
Potato, passion fruit 0.01 mg/kg
Cabbage, tomato, wheat 0.01 mg/kg
Hungary
Potato, tomato 0.1 mg/kg
APPRAISAL
The insecticide cartap, invariably used as the hydrochloride, is
a derivative of nereistoxin, a naturally occurring insecticidal
substance isolated from the marine segmented worms, Lumbrineris spp.
Extensive information is available on the chemistry and synthesis of
nereistoxin and its derivatives, of which cartap hydrochloride is one
of the most potent.
Cartap hydrochloride insecticides have been registered in Japan
since 1967, and are widely used in Asia, Europe and South America.
Cartap hydrochloride is used against a relatively broad spectrum of
insects but particularly against the rice stem borer, Lepidopterous
pests of vegetables and Colorado potato beetle. Commercial
formulations include water soluble powders, dusts and granules.
Approximately 70% of the world production is applied to rice. Cartap
hydrochloride is generally used at the rate of 0.5 - 1.5 kg/ha with a
pre-harvest interval of 7 to 21 days.
Extensive data obtained from supervised trials in Japan on a
variety of vegetables, fruit, hops, forage and rice were available to
the Meeting. The compound shows some systemic action and some of the
data indicate an increase in the residue concentration in fruits and
vegetables during the first 7 days. Although the residues resulting
from approved applications are generally well below 1 mg/kg soon after
application, detectable residues remain for up to 45 days.
It appears that the insecticidal activity of cartap hydrochloride
is due to its conversion to nereistoxin in plants and animals. The
metabolism of cartap hydrochloride in mammals has been studied in rats
and mice where it is apparently rapidly converted to nereistoxin which
in turn is further metabolised. No information is available on the
fate in livestock.
Metabolism studies in plants indicate quantitative conversion to
nereistoxin which in turn is oxidised, through the mono-oxide,
eventually to sulphuric acid.
Cartap hydrochloride and nereistoxin are readily degraded in
water by direct sunlight but residues held in soils appear relatively
stable except in highly active soils such as volcanic ash.
Several methods have been developed for the determination of
cartap residues and a gas-chromatographic method appears suitable for
regulatory analysis. The method is based on the conversion of the
parent compound and metabolites to nereistoxin which can be measured
by means of a flame photometric detector equipped with a sulphur mode
filter. The limit of determination is 0.005 mg/kg.
The meeting welcomed the fact that most of the extensive residue
data generated from these supervised trials had been obtained by
independent analysis in two separate laboratories. Although there is
excellent agreement in the results presented for some samples by the
two laboratories there are many instances where the difference between
the means obtained by the two analysts is very highly significant.
This is not altogether remarkable in view of sampling difficulties and
the low levels of the residues analysed. The meeting welcomed this
practical expression of the difficulties confronting residue analysts
seeking to determine small quantities of complex substances in a
variety of raw agricultural commodities.
A number of countries have established maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits are for cartap, expressed as
the free base. (The usual methods of residue analysis convert cartap
to its metabolite nereistoxin, and will therefore record cartap,
nereistoxin and dihydronereistoxin as cartap.)
Pre-harvest Interval
Limit on which Recommendations
Commodity (mg/kg) are based
Hops (dried) 5 14
Chinese cabbage 2 7
Tea (green, dried) 2 7
Grapes 1 14
Persimmons 1 21
Radishes 1 7
Cabbage 0.02 7
Chestnut (seed including 0.01 14
pericarp)
Ginger 0.01 3
Potatoes 0.01 7
Rice (hulled) 0.01 21
Sweet corn 0.01 7
FURTHER WORK OR INFORMATION
Required (by 1978)
1. Submission of the details of the studies on metabolism and the
identification of metabolites.
2. Teratogenic studies covering full period of organogenesis.
3. A feeding study in a non-rodent species.
4. Information on the fate of residues in foods of animal origin
when treated fodder or plant parts are fed to livestock including
poultry.
5. Effect of cooking on the level and fate of cartap residues.
6. Information on the fate of residues in manufactured tea.
Desirable
1. A paired feeding study in the rat.
REFERENCES
Aramaki, Y. Acute oral, dermal and eye toxicity and irritation
1972 studies on cartap hydrochloride and its
formulation, padan water soluble powder. Submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Berczy, Z.S., and Cobb, L. M., Acute inhalation toxicity to the
1973 rat of Padan 50 SP. Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Berczy, Z.S., Cobb, L.M., Street, A.E. and Cherry, C.P. Subacute
1973 inhalation toxicity to the rat of Padan 50 SP.
Report from Huntingdon Research Centre, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Davies, R.E. and Collins, C.J. Acute percutaneous toxicity to
1973 rabbits of TA-7(50 s.p.). Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Davies, R.E., Elliott, P.H., Street, A.E., Heywood, R. and Cherry,
1974 C.P. The effect of repeated applications of TA-7
to the skin of rabbits for three weeks. Report
from Huntingdon Research Centre, submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Hagiwara, H., Numata, M., Konishi, K. and Oka, Y. Synthesis of
1965 nereistoxin and related compounds. I. Chem. Pharm.
Bull., 13, 253-260.
Hagiwara, H. and Numata, M. Synthesis of nereistoxin and related
1968 compounds. II. Chem. Pharm. Bull., 16, 311-317.
Hashimoto, Y. and Okaichi, T. Some chemical properties of
1960 nereistoxin. Ann. N.Y. Acad. Sco., 90, 667-673.
Hiraoka, T. The survey of the health condition in workers engaged
1975 in manufacturing cartap hydrochloride. Report from
Hikari Plant Hospital, submitted by Takeda
Chemical Industries, Ltd. (Unpublished).
Hunter, B., Graham, C., Street, A.E. and Gallagher, P.J. Long
1974 term feeding of TA-7 in mice. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Prentice,
1975 D.E. TA-7, Toxicity following dietary
administration to rats for two years. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Kato, M., Sato, Y. and Sakai, M. Foliage spray treatment of cartap
1967 hydrochloride mixed with DCPA for simultaneous
control of rice stem borer and barnyardgrass.
Japan J. appl. Ent. Zool., 32, 678-679.
Konishi, K. New insecticidally active derivatives of nereistoxin.
1968a Agr. Biol. Chem., 32, 678-679.
Konishi, K. Studies on organic insecticides. Part X. Synthesis
1968b of nereistoxin and related compounds. III. Agr.
Biol. Chem., 1199-1204.
Konishi, K. Studies on organic insecticides. Part XI. Synthesis
1970a of nereistoxin and related compounds. IV. Agr.
Biol. Chem., 34, 926-934.
Konishi, K. Studies on organic insecticides. Part XII. Synthesis
1970b of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 935-940.
Konishi, K. Studies on organic insecticides. Part XIII. Synthesis
1970c of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 1549-1560.
Konishi, K. Nereistoxin and its relatives. Proc. 2nd IUPAC
1971 International Congress of Pesticide Chemistry, 1,
179-189.
Kono, Y., Nagaarashi, C. and Sakai, M. Effects of cartap,
1975 chlorodimeform and diazinon on the probing
frequency of the green rice leafhopper (Hemiptera
deltocephalidae). Appl. Ent. Zool., 10, 58-60.
Koyama, S., Emura, K. and Kojima, A. Residue fate of cartap
1975 hydrochloride applied in paddy field and its
effect against the rice leaf beetle and the rice
stem borer of the first generation. Proc. Assoc.
Plant Prot. Hokuriku (Japan), No. 22, 72-76.
Translated in English.
Mizutani, M., Ihara, T., Kanamori, H., Takatani, O., Matsukawa,
1971 J., Amano, T. and Kaziwara, K. Teratogenesis
studies with cartap hydrochloride in the mouse,
rat and hamster. J. Takeda Res. Lab., 30, 776-785.
Nagawa, Y., Saji, Y., Chiba, S. and Yui, T. Neuromuscular blocking
1971 actions of nereistoxin and its derivatives and
antagonism by sulfhydryl compounds. Japan J.
Pharmacol., 21, 185-197.
Nagawa, Y., Saji, Y. and Kuriki, H. Antidotal effects of sulfhydryl
1970 compounds against intoxication with
1,3-bis(carbamoylthio)-2-N,N-dimethylaminopropane
hydrochloride(NTD-2) in experimental animals. J.
Takeda Res. Lab., 29, 624-629.
Nishi, K., Owaki, R. and Tan, N. Method for determination of
1968 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride. II. Polarography. Ann.
Rept. Takeda Res. Lab., 27, 47-53.
Nishi, K., Konishi, K. and Tan, N. PadanR. in Analytical Methods
1973 for Pesticides and Plant Growth Regulators, G.
Zweig. Ed. - Academic Press N.Y., 7, 371-384.
Nitta, S. Yakugaku Zasshi, 54, 648. Über Nereistoxin, einen
1934 giftigen Bestandteil von Lumbriconereis
heteropoda Marenz (Eunicidae)
Okaichi, T. and Hashimoto, Y. The structure of nereistoxin.
1962 Agr. Biol. Chem., 26, 224-227.
Olson, W.A. and Busey, W.M. Two-generation reproduction study
1972 - rats, cartap. Report from Hazleton Laboratories,
Inc., submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Rivett, K.F., Batham, P., Heywood, R., Street, A.E. and Newman, A.J.
1972 TA-7, oral toxicity to rats dietary administration
for 13 weeks. Report from Huntingdon Research
Centre, submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Sakai, M. Studies on the insecticidal action of nereistoxin.
1964 4-N,N-dimethylamino-1,2-dithiolane. I.
Insecticidal properties. Japan J. appl. Ent.
Zool., 8, 324-333.
Sakai, M., Sato, Y. and Kato, M. Insecticidal activity of
1967 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride, cartap; with special
references to the effectiveness for controlling
the rice stem borer. Japan J. appl. Ent. Zool.,
11, 125-134.
Sakai, M. Nereistoxin and its derivatives; their ganglionic
1970 blocking and insecticidal activity. Biochemical
toxicology of insecticides, 13-20. (Academic Press
Inc.)
Sakai, M. The chemistry and action of cartap. Japan Pestic.
1971 Inform., (6) 15-19.
Sakai, M. and Sato, Y. Metabolic conversion of the nereistoxin
1971 related compounds into nereistoxin as a factor of
their insecticidal action. Insecticides Proc. 2nd
Intern. IUPAC Congr. Pesticide Chemistry, 1,
455-470. (Godon and Breach Sci. Pub. Ltd.)
Shiomi, Y., Sasa, Y. and Takeda, K. Effects of feeding cartap
1973 hydrochloride on laying performance and egg
quality in hens. Submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Shirakawa, Y., Fujita, T., Maki, K. and Suzauki, Z. Metabolic
1971 fate of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane(cartap) in rat and mouse. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Takei, N. Personal communication to FAO Panel of Experts 1976
on Pesticide Residues.
Tan, N., Nishi, K., Owaki, R. and Asahi, Y. Simultaneous analysis
1967 of 2-N,N-dimethylaminopropane-1,3-dithiocyanate
and 4,4-dimethylaminodithiolane by polargraphic
method. Noyaku Seisan Gizyutu (Japan), No. 16,
20-22.
Tomizawa, C. and Endo, T. Movement of cartap hydrochloride in soil,
1972 paddy water and rice plant. Report from National
Institute of Agricultural Sciences (Japan),
submitted by Takeda Chemical Industries, Ltd.
(Unpublished).
Tomizawa, C., Endo, T. and Naka, H. Fate and significance of
1974 labelled insecticide residues in rice. Isotope
Tracer Studies of Chemical Residues in Food and
the Agricultural Environment, 59-64. International
Atomic Energy Agency (Vienna).
Toyoshima, S., Sato, R. and Satoh, H. Acute toxicity of cartap
1972 hydrochloride in rats and mice. Report from Nippon
Experimental Medical Research Institute, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Tsubura, Y., Shimomura, T., Watanabe, T., Tsuji, H., Takahashi,
1976 A. and Fukuyama, T. Toxicity test of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride (cartap) on mice by oral
administration for three months. J. Nara Medical
Association (Japan), 26, 368-378.
Yokotani, H. Approximate estimation of acute oral toxicity of
1968 cartap hydrochloride in monkeys. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Dust, fine granule and granule formulations of cartap
hydrochloride were applied to rice plants in a paddy field, and the
cartap residue in the plants was determined by analysing the leaf
blades and leaf sheathes separately. Further measurements were also
made on water and soil from the treated plots. The concentration of
cartap in the rice plants immediately after application was found to
be of the order of 1 to 2 mg/kg for dust and fine granule treatments
but only 0.1 mg/kg in the case of granules. 3 days later the
concentration on the whole plants had fallen to approximately 0.05
mg/kg except on those treated with dust which were found to contain
0.2 mg/kg. After 5 days the concentration had fallen slightly in the
case of plants treated with dust and fine granules but plants treated
with granules were found to have a noticeable increase in cartap
residues, apparently owing to uptake from the water into which the
granules had fallen. This trend continued until the 7th day after
which residues began to decline slowly. The leaf blade was found to
have significantly higher residues than the leaf sheath (Koyama et al,
1975). It is difficult to tell how much of the residue determined in
these trials was a surface deposit on the outside of the leaves and
how much was taken into the plant.
Koyama et al (1975), quote work by Sakai which indicates that
when relatively high concentrations of cartap hydrochloride are
applied to rice plants in nursery boxes, the plants are capable of
taking up concentrations of the order of 20 mg/kg which, even after 42
days, remain at about 0.5 mg/kg. Due allowance must be made for the
extensive dilution which would occur owing to the growth of the plant
over this period.
Tomizawa et al (1974), using 35S-labelled cartap hydrochloride
showed that the rate and degree of absorption of cartap hydrochloride
by rice plants was much greater when the insecticide was applied in
the paddy water than when it was applied to the plants in the form of
a spray, and an effective concentration remained in the rice plant for
a longer period. By using ion-exchange chromatography it was possible
to show that the conversion of cartap hydrochloride to nereistoxin
occurred rapidly after application.
In soil
Tomizawa and Endo (1972) studied the movement of cartap
hydrochloride in soil under laboratory conditions. They took alluvial
clay from a paddy field and from an unirrigated field, volcanic ash
and sandy loam and applied radio-active cartap hydrochloride at a
dosage of 1 mg/50 g of soil. The authors do not mention the
temperature at which the treated soil was held but indicate that the
soil from the paddy field had the water content adjusted to 150% of
field capacity while for the other three soils the water content was
adjusted to 75% of field capacity. Samples of soil were removed at 6
intervals over the next 30 days and were extracted in order to
determine the cartap content. The concentration was measured in the
basic fraction extracted with aqueous ammonia. It was found that the
concentration in the soil one day after treatment was of the order of
1 to 2 mg/kg in all four samples. The concentration in the soil from
the paddy field remained virtually constant for 10 days and by the
30th day had declined to one-sixth. The volcanic ash yielded only one
thirtieth and the other two soils about one tenth of the original
concentration by the 30th day.
It is not clear whether the difference in concentration found
after 30 days is due to a difference in the speed of decomposition of
cartap hydrochloride or whether it results from the higher capacity of
volcanic ash to absorb cartap hydrochloride and its decomposition
products. There is a strong indication that cartap hydrochloride acts
as a chelating agent in soil (Takei, 1976). This may explain the low
recoveries reported by Tomizawa and Endo (1972).
Koyama et al (1975) demonstrated that when various cartap
hydrochloride formulations were applied to rice plants growing in
paddy fields there was a distinct transfer of cartap in the case of
granules. There appeared to be an increase in the concentration in the
soil of plots treated with granules over the first 14 days. In the
case of other formulations detectable residues were found in the soil
of all plots at the end of 14 days but at the time of harvest, 41
months after initial treatment, the concentration was below the limit
of determination.
In water
In the experiments with soil and rice plants described above,
Koyama et al (1975) also studied the concentration of cartap
hydrochloride in the water of paddy fields treated with various cartap
hydrochloride formulations. It was found that immediately after
application the concentration of cartap hydrochloride in the water of
paddy fields ranged between 1 and 2 mg/l but by the third day after
treatment it had generally fallen below the limit of determination,
except in the plots treated with granules where it was marginally
above this limit. The apparent difference in the stability of cartap
hydrochloride in water and soil of the same paddy fields seems
attributable to the fact that cartap hydrochloride dissolved in water
is easily degraded by direct sunlight while the residue held in soils
is relatively stable. Cartap hydrochloride is stable in aqueous
solution at pH3-4. In the water of paddy fields which would have a pH
ranging from 5 to 7, it may be decomposed gradually to nereistoxin as
described at the beginning of this section.
Tomizawa and Endo (1972) applied radio-active cartap
hydrochloride to the surface of water in which rice seedlings were
transplanted, at a rate corresponding to 1.5 kg/ha. The radio-activity
of samples of water was measured after various intervals. Although the
concentration in the water sampled immediately after application
indicated the presence of approximately 10 mg of cartap hydrochloride
per litre of water, within the first 24 hours the concentration had
fallen by more than 90% with a further slow decline over the
succeeding days. The loss of the compound from the aqueous phase may
probably be attributable to rapid absorption onto soil particles.
METHODS OF RESIDUE ANALYSIS
Several colorimetric, iodometric, oscillopolarographic and
gaschromatographic methods have been developed for the determination
of cartap. The oscillopolarographic and gaschromatographic methods are
suitable for residue analysis. Both are based on the measurement of
4.N,N-dimethylamino-1,2-dithiolane (nereistoxin, NTXH) which is
quantitatively derived from cartap. Cartap hydrochloride and its
metabolite dihydronereistoxin (NTXH) and nereistoxin are extracted
from samples with diluted hydrochloric acid and the unchanged cartap
hydrochloride is hydrolysed and oxidized in basic solution to NTX.
Total NTX is extracted from the reaction mixture with ethyl ether and
is used for analysis.
Recoveries by both the oscillopolarographic and the
gaschromatographic methods were generally between 75 and 95% (Nishi et
al, 1973). Soil, rice, tea and straw present difficulties in analysis
whereas substrates such as cabbage are easily extracted with good
recovery. Apparently there is a high degree of disulphide binding with
some commodities, because the addition of cystine results in a release
of residues and significantly better recovery (Takei, 1976).
In the oscillopolarographic method, liquid/liquid partition is
required during the clean-up in order to minimise the carry-over of
possible interfering compounds. A single sweep oscillopolarograph
(Randles-Sevcik type) is used. The polarogram of NTX appears at -0.78
±0.05 V vs. Hg pool, and the concentration is quantitatively
determined by the peak height. The limit of determination of this
method is 0.004 mg/kg. Some reducible pesticides, such as
organophosphorus pesticides containing a nitro group, did not
interfere with the determination (Nishi et al, 1973).
Residues of cartap can be determined easily by gaschromatography
with a high sensitivity and specificity. A flame photometric detector
(FPD) equipped with a sulphur mode filter and a glass column
containing 3% OV-1 on chromosorb W or 5% PEG 20 on Gaschrom Q at a
temperature of 130-165°C are used. The limit of determination by this
method is 0.005 mg/kg. Investigation by Nishi et al, (1973) of the
gas-chromatographic method indicates that it should be applicable to
all types of residue analysis. Since the treatment is simple and less
rigorous clean-up is required, the gas-chromatographic method is
superior to the polarographic method. It appears suitable for
regulatory analysis.
Most of the residue data from supervised trials have been
obtained by the gas-chromatographic method, and in the course of this
work almost 100 samples of 12 different commodities have been analyzed
independently by analysis from two of three separate laboratories. In
some cases the results presented by the two analysts differ by
relatively large factors. The percentage difference between the means
obtained by the two analysts does not give a true picture of the
variability. In Table 3 the significance of the difference between
laboratory means has been assessed in relation to the variation
between replicates (a) of pooled over values of <0.050 mg/kg, where
within-pair variability tends to be lowest and (b) pooled over values
between 0.050 and 0.500 mg/kg where this variability is greater. Where
levels above 0.5 mg/kg are found the differences within and between
pairs of duplicates are inconsistent and there are insufficient data
to make a valid comparison of laboratory means.
TABLE 3. Mean differences between samples analysed by 2 laboratories
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Chinese cabbage 0.0275 0.1195 0.092 P<.001
0.0335 0.1085 0.075 P<.001
0.0315 0.1625 0.131 P<.001
0.7060 0.9750 0.269 -
0.0700 0.1445 0.0745 P<.001
Ginger 0.0245 0.0220 0.0025 NS
0.0205 0.0330 0.0125 P<.001
0.0380 0.0220 0.016 P<.001
Grape 0.0765 0.0800 0.0035 NS
0.5345 0.3500 0.1845 P<.001
0.5645 0.7400 0.1755 P<.001
0.1245 0.1800 0.0555 P<.001
Hops 0.9150 2.0900 1.175 -
0.2700 0.3200 0.05 P<.001
4.8200 7.7150 2.895 -
0.2700 0.4950 0.225 P<.001
Japanese 0.0610 0.0935 0.0325 P<.01
radish 0.0520 0.1150 0.063 P<.001
leaf 0.0580 0.1270 0.069 P<.001
root 0.0290 0.0245 0.0045 NS
0.0115 0.0085 0.003 NS
0.0135 0.0160 0.0025 NS
Potato 0.0010 0.0050 0.004 NS
0.0070 0.0050 0.002 NS
Rice grain 0.0050 0.0100 0.005 NS
0.0060 0.0100 0.004 NS
Rice straw 0.0100 0.0200 0.01 P<.01
0.0450 0.0450 0.00 NS
Orchard grass 0.0260 0.0200 0.006 NS
0.0590 0.1500 0.091 P<.001
TABLE 3. (Cont'd.)
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Red clover 0.0095 0.1050 0.0955 P<.001
0.0255 0.1800 0.1545 P<.001
0.1080 0.0200 0.088 P<.001
White clover 0.2150 0.0800 0.135 P<.001
0.4430 0.0450 0.398 P<.001
Least difference in means necessary for significance:
P <0.05 P <0.01 P <0.00
Single estimates <0.050 = 0.0059 0.0080 0.0106
" " >0.050 = 0.0231 0.0311 0.0413
- = insufficient high values to assess significance.
NS = not significant
The results show that in most cases very highly significant
differences (P<0.001) occur between laboratories analysing samples of
the same commodity, while in some cases, notably commodities having
residues below 0.05 mg/kg, the differences are not significant. The
low residues occur mainly in root crops and grains, which would be
expected to be less subject than leafy plants to sampling variations
and therefore to give more consistent analytical results.
It is rarely possible to make such a comparison as this, since
investigators rarely submit samples to two different laboratories for
check analysis. The opportunity to see practical evidence of the
difficulties confronting residue analysts seeking to determine small
quantities of complex substances in a variety of raw agricultural
commodities is valuable. Such data are needed to provide an adequate
basis for the establishment of maximum residue limits appropriate to
the problems facing residue analysts and food control officials.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Brazil
Cotton, sunflower 0.03 mg/kg
Potato, passion fruit 0.01 mg/kg
Cabbage, tomato, wheat 0.01 mg/kg
Hungary
Potato, tomato 0.1 mg/kg
APPRAISAL
The insecticide cartap, invariably used as the hydrochloride, is
a derivative of nereistoxin, a naturally occurring insecticidal
substance isolated from the marine segmented worms, Lumbrineris spp.
Extensive information is available on the chemistry and synthesis of
nereistoxin and its derivatives, of which cartap hydrochloride is one
of the most potent.
Cartap hydrochloride insecticides have been registered in Japan
since 1967, and are widely used in Asia, Europe and South America.
Cartap hydrochloride is used against a relatively broad spectrum of
insects but particularly against the rice stem borer, Lepidopterous
pests of vegetables and Colorado potato beetle. Commercial
formulations include water soluble powders, dusts and granules.
Approximately 70% of the world production is applied to rice. Cartap
hydrochloride is generally used at the rate of 0.5 - 1.5 kg/ha with a
pre-harvest interval of 7 to 21 days.
Extensive data obtained from supervised trials in Japan on a
variety of vegetables, fruit, hops, forage and rice were available to
the Meeting. The compound shows some systemic action and some of the
data indicate an increase in the residue concentration in fruits and
vegetables during the first 7 days. Although the residues resulting
from approved applications are generally well below 1 mg/kg soon after
application, detectable residues remain for up to 45 days.
It appears that the insecticidal activity of cartap hydrochloride
is due to its conversion to nereistoxin in plants and animals. The
metabolism of cartap hydrochloride in mammals has been studied in rats
and mice where it is apparently rapidly converted to nereistoxin which
in turn is further metabolised. No information is available on the
fate in livestock.
Metabolism studies in plants indicate quantitative conversion to
nereistoxin which in turn is oxidised, through the mono-oxide,
eventually to sulphuric acid.
Cartap hydrochloride and nereistoxin are readily degraded in
water by direct sunlight but residues held in soils appear relatively
stable except in highly active soils such as volcanic ash.
Several methods have been developed for the determination of
cartap residues and a gas-chromatographic method appears suitable for
regulatory analysis. The method is based on the conversion of the
parent compound and metabolites to nereistoxin which can be measured
by means of a flame photometric detector equipped with a sulphur mode
filter. The limit of determination is 0.005 mg/kg.
The meeting welcomed the fact that most of the extensive residue
data generated from these supervised trials had been obtained by
independent analysis in two separate laboratories. Although there is
excellent agreement in the results presented for some samples by the
two laboratories there are many instances where the difference between
the means obtained by the two analysts is very highly significant.
This is not altogether remarkable in view of sampling difficulties and
the low levels of the residues analysed. The meeting welcomed this
practical expression of the difficulties confronting residue analysts
seeking to determine small quantities of complex substances in a
variety of raw agricultural commodities.
A number of countries have established maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits are for cartap, expressed as
the free base. (The usual methods of residue analysis convert cartap
to its metabolite nereistoxin, and will therefore record cartap,
nereistoxin and dihydronereistoxin as cartap.)
Pre-harvest Interval
Limit on which Recommendations
Commodity (mg/kg) are based
Hops (dried) 5 14
Chinese cabbage 2 7
Tea (green, dried) 2 7
Grapes 1 14
Persimmons 1 21
Radishes 1 7
Cabbage 0.02 7
Chestnut (seed including 0.01 14
pericarp)
Ginger 0.01 3
Potatoes 0.01 7
Rice (hulled) 0.01 21
Sweet corn 0.01 7
FURTHER WORK OR INFORMATION
Required (by 1978)
1. Submission of the details of the studies on metabolism and the
identification of metabolites.
2. Teratogenic studies covering full period of organogenesis.
3. A feeding study in a non-rodent species.
4. Information on the fate of residues in foods of animal origin
when treated fodder or plant parts are fed to livestock including
poultry.
5. Effect of cooking on the level and fate of cartap residues.
6. Information on the fate of residues in manufactured tea.
Desirable
1. A paired feeding study in the rat.
REFERENCES
Aramaki, Y. Acute oral, dermal and eye toxicity and irritation
1972 studies on cartap hydrochloride and its
formulation, padan water soluble powder. Submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Berczy, Z.S., and Cobb, L. M., Acute inhalation toxicity to the
1973 rat of Padan 50 SP. Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Berczy, Z.S., Cobb, L.M., Street, A.E. and Cherry, C.P. Subacute
1973 inhalation toxicity to the rat of Padan 50 SP.
Report from Huntingdon Research Centre, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Davies, R.E. and Collins, C.J. Acute percutaneous toxicity to
1973 rabbits of TA-7(50 s.p.). Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Davies, R.E., Elliott, P.H., Street, A.E., Heywood, R. and Cherry,
1974 C.P. The effect of repeated applications of TA-7
to the skin of rabbits for three weeks. Report
from Huntingdon Research Centre, submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Hagiwara, H., Numata, M., Konishi, K. and Oka, Y. Synthesis of
1965 nereistoxin and related compounds. I. Chem. Pharm.
Bull., 13, 253-260.
Hagiwara, H. and Numata, M. Synthesis of nereistoxin and related
1968 compounds. II. Chem. Pharm. Bull., 16, 311-317.
Hashimoto, Y. and Okaichi, T. Some chemical properties of
1960 nereistoxin. Ann. N.Y. Acad. Sco., 90, 667-673.
Hiraoka, T. The survey of the health condition in workers engaged
1975 in manufacturing cartap hydrochloride. Report from
Hikari Plant Hospital, submitted by Takeda
Chemical Industries, Ltd. (Unpublished).
Hunter, B., Graham, C., Street, A.E. and Gallagher, P.J. Long
1974 term feeding of TA-7 in mice. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Prentice,
1975 D.E. TA-7, Toxicity following dietary
administration to rats for two years. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Kato, M., Sato, Y. and Sakai, M. Foliage spray treatment of cartap
1967 hydrochloride mixed with DCPA for simultaneous
control of rice stem borer and barnyardgrass.
Japan J. appl. Ent. Zool., 32, 678-679.
Konishi, K. New insecticidally active derivatives of nereistoxin.
1968a Agr. Biol. Chem., 32, 678-679.
Konishi, K. Studies on organic insecticides. Part X. Synthesis
1968b of nereistoxin and related compounds. III. Agr.
Biol. Chem., 1199-1204.
Konishi, K. Studies on organic insecticides. Part XI. Synthesis
1970a of nereistoxin and related compounds. IV. Agr.
Biol. Chem., 34, 926-934.
Konishi, K. Studies on organic insecticides. Part XII. Synthesis
1970b of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 935-940.
Konishi, K. Studies on organic insecticides. Part XIII. Synthesis
1970c of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 1549-1560.
Konishi, K. Nereistoxin and its relatives. Proc. 2nd IUPAC
1971 International Congress of Pesticide Chemistry, 1,
179-189.
Kono, Y., Nagaarashi, C. and Sakai, M. Effects of cartap,
1975 chlorodimeform and diazinon on the probing
frequency of the green rice leafhopper (Hemiptera
deltocephalidae). Appl. Ent. Zool., 10, 58-60.
Koyama, S., Emura, K. and Kojima, A. Residue fate of cartap
1975 hydrochloride applied in paddy field and its
effect against the rice leaf beetle and the rice
stem borer of the first generation. Proc. Assoc.
Plant Prot. Hokuriku (Japan), No. 22, 72-76.
Translated in English.
Mizutani, M., Ihara, T., Kanamori, H., Takatani, O., Matsukawa,
1971 J., Amano, T. and Kaziwara, K. Teratogenesis
studies with cartap hydrochloride in the mouse,
rat and hamster. J. Takeda Res. Lab., 30, 776-785.
Nagawa, Y., Saji, Y., Chiba, S. and Yui, T. Neuromuscular blocking
1971 actions of nereistoxin and its derivatives and
antagonism by sulfhydryl compounds. Japan J.
Pharmacol., 21, 185-197.
Nagawa, Y., Saji, Y. and Kuriki, H. Antidotal effects of sulfhydryl
1970 compounds against intoxication with
1,3-bis(carbamoylthio)-2-N,N-dimethylaminopropane
hydrochloride(NTD-2) in experimental animals. J.
Takeda Res. Lab., 29, 624-629.
Nishi, K., Owaki, R. and Tan, N. Method for determination of
1968 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride. II. Polarography. Ann.
Rept. Takeda Res. Lab., 27, 47-53.
Nishi, K., Konishi, K. and Tan, N. PadanR. in Analytical Methods
1973 for Pesticides and Plant Growth Regulators, G.
Zweig. Ed. - Academic Press N.Y., 7, 371-384.
Nitta, S. Yakugaku Zasshi, 54, 648. Über Nereistoxin, einen
1934 giftigen Bestandteil von Lumbriconereis
heteropoda Marenz (Eunicidae)
Okaichi, T. and Hashimoto, Y. The structure of nereistoxin.
1962 Agr. Biol. Chem., 26, 224-227.
Olson, W.A. and Busey, W.M. Two-generation reproduction study
1972 - rats, cartap. Report from Hazleton Laboratories,
Inc., submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Rivett, K.F., Batham, P., Heywood, R., Street, A.E. and Newman, A.J.
1972 TA-7, oral toxicity to rats dietary administration
for 13 weeks. Report from Huntingdon Research
Centre, submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Sakai, M. Studies on the insecticidal action of nereistoxin.
1964 4-N,N-dimethylamino-1,2-dithiolane. I.
Insecticidal properties. Japan J. appl. Ent.
Zool., 8, 324-333.
Sakai, M., Sato, Y. and Kato, M. Insecticidal activity of
1967 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride, cartap; with special
references to the effectiveness for controlling
the rice stem borer. Japan J. appl. Ent. Zool.,
11, 125-134.
Sakai, M. Nereistoxin and its derivatives; their ganglionic
1970 blocking and insecticidal activity. Biochemical
toxicology of insecticides, 13-20. (Academic Press
Inc.)
Sakai, M. The chemistry and action of cartap. Japan Pestic.
1971 Inform., (6) 15-19.
Sakai, M. and Sato, Y. Metabolic conversion of the nereistoxin
1971 related compounds into nereistoxin as a factor of
their insecticidal action. Insecticides Proc. 2nd
Intern. IUPAC Congr. Pesticide Chemistry, 1,
455-470. (Godon and Breach Sci. Pub. Ltd.)
Shiomi, Y., Sasa, Y. and Takeda, K. Effects of feeding cartap
1973 hydrochloride on laying performance and egg
quality in hens. Submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Shirakawa, Y., Fujita, T., Maki, K. and Suzauki, Z. Metabolic
1971 fate of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane(cartap) in rat and mouse. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Takei, N. Personal communication to FAO Panel of Experts 1976
on Pesticide Residues.
Tan, N., Nishi, K., Owaki, R. and Asahi, Y. Simultaneous analysis
1967 of 2-N,N-dimethylaminopropane-1,3-dithiocyanate
and 4,4-dimethylaminodithiolane by polargraphic
method. Noyaku Seisan Gizyutu (Japan), No. 16,
20-22.
Tomizawa, C. and Endo, T. Movement of cartap hydrochloride in soil,
1972 paddy water and rice plant. Report from National
Institute of Agricultural Sciences (Japan),
submitted by Takeda Chemical Industries, Ltd.
(Unpublished).
Tomizawa, C., Endo, T. and Naka, H. Fate and significance of
1974 labelled insecticide residues in rice. Isotope
Tracer Studies of Chemical Residues in Food and
the Agricultural Environment, 59-64. International
Atomic Energy Agency (Vienna).
Toyoshima, S., Sato, R. and Satoh, H. Acute toxicity of cartap
1972 hydrochloride in rats and mice. Report from Nippon
Experimental Medical Research Institute, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Tsubura, Y., Shimomura, T., Watanabe, T., Tsuji, H., Takahashi,
1976 A. and Fukuyama, T. Toxicity test of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride (cartap) on mice by oral
administration for three months. J. Nara Medical
Association (Japan), 26, 368-378.
Yokotani, H. Approximate estimation of acute oral toxicity of
1968 cartap hydrochloride in monkeys. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
See Also:
Toxicological Abbreviations
Cartap (Pesticide residues in food: 1978 evaluations)
 Other information on identity and properties
Cartap is invariably used as the hydrochloride, to which the
following information refers.
Molecular weight: 273.8
State: Colourless crystals
Melting point: 179-181°C(decomp.)
Solubility(g/100 ml): Soluble in water(13.2/10°C,
17.8/20°C, 25.3/30°C slightly soluble
in methanol(2.08/5°C), almost insoluble
in acetone, benzene, chloroform,
diethyl ether, ethyl acetate, and
n-hexane.
Purity of technical material: 97%. containing 3% of water and
ammonium chloride as impurities.
Hydroscopicity: Slightly hygroscopic
Volatility: Negligible
Formulation: 25% and 50% water-soluble powder, 2%
dust, 4% and 10% granules and 2% fine
granules are available. A bait
formulation has recently been
introduced.
Cartap hydrochloride is an insecticide recently developed by
Takeda Chemical Industries. It is a derivative of nereistoxin which
is a naturally occurring insecticidal substance isolated from the
marine segmented worms Lumbrinereis heteropoda and L.
brevicirra.
Nereistoxin was isolated by Nitta in 1934, the structure was
elucidated by Okaichi and Hashimoto (1962) and synthesized in 1965
by Hagiwara et al. A variety of nereistoxin derivatives were
prepared and their insecticidal activities tested. Cartap
hydrochloride is one of the most potent of the many compounds
prepared.
The chemistry and synthesis of nereistoxin and related
compounds are dealt with by a number of authors including Hashimoto
et al (1960); Okaichi and Hashimoto (1962); Hagiwara et al (1965);
Konishi (1968a, 1968b); Hagiwara and Numata (1968); Konishi (1970a,
1971); Sakai (1971); Nishi et al (1973).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Groups of 10 male and 20 female rats were administered cartap
hydrochloride in the diet at levels of 0, 100 and 1000 ppm for 9
weeks prior to mating and subsequently for the remainder of the
study which was a 2 litter, 2 generation reproduction study. One
half of the F2b litters were delivered by caesarean section on day
19 of gestation, the other half were delivered naturally and held
to weaning. Parental animals showed reduced body weight gain and
food consumption and an apparent impairment of fertility for the
F1a generation at 1000 ppm. Gestation and live birth indices of
the treated animals were comparable to those of the controls.
Weanling survival index was lower in the F1b generation and pup
weights in all generations at 7, 14 and 21 days after weaning were
reduced at the upper dose level. There were no meaningful
differences observed between groups with respect to number of
implantation sites, resorptions or live and dead fetuses. No
compound-related microscopic alterations were noted in selected
tissues from weanling F2b progeny of the 1000 ppm group. However,
a greater incidence of incomplete ossification was revealed on
skeletal examination in this group, (Olson and Busey, 1972).
Special studies on teratogenicity
Mouse
Three groups comprising 20, 12 and 14 pregnant mice received
cartap hydrochloride by stomach tube at dose levels of 0, 50 and
100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Twenty-one pregnant mice were employed as an untreated
control group. Animals were killed on day 19 of gestation and
fetuses removed by caesarean section. Weight gains were not
affected. No significant variations were observed in the number of
implantation sites, fetal mortality and mean body weight of fetuses
between control and treated groups. No visceral abnormalities were
observed. Skeletal examination revealed abnormalities (fusion of
cervical vertebral arches and fusion of sternebrae) in 2/110
fetuses (1.8%) at 50 mg/kg and 2/120 fetuses (1.7%) at 100 mg/kg.
In respect to other skeletal variations, there were no significant
differences between control and treated animals (Mizutani et al,
1971).
Rat
Cartap hydrochloride was administered orally to three groups
of 21, 12 and 17 pregnant rats at dose levels of 0, 50 and 100
mg/kg body weight respectively from gestation day 9 to day 15.
Twenty-three pregnant rats were used as an untreated control group.
Animals were sacrificed on day 21 and fetuses were delivered by
laparotomy. An increase in mortality and retarded weight gain were
observed in the maternal animals at 100 mg/kg during the dosing
period. There was no statistical difference between treated and
control groups with respect to number of implantation sites, fetal
mortality, sex ratio, external and visceral malformations. Skeletal
examination revealed no major abnormalities except for an increase
25/174 (14.4%) in bilateral twin thoracic vertebral centrae in the
100 mg/kg group (Mizutani et al, 1971).
Hamster
Five groups comprising 9, 7, 8, 9 and 7 pregnant hamsters
received cartap, hydrochloride orally at dose levels of 0, 2, 10,
50 and 100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Fifteen pregnant hamsters were used as an untreated
control group. Animals were killed on day 16 of gestation and
fetuses removed by caesarean section. Treatment with 100 mg/kg
resulted in the death of 2/7 animals and was associated with
reduced weight gain. No compound-related effects were observed in
the number of implantation sites, fetal mortality, fetal weights,
external and visceral malformations. In hamsters receiving 100
mg/kg, the frequency of skeletal anomalies increased slightly, 6/52
(11.5%) showing abnormalities in the thoracic vertebrae and/or
ribs. An increase in the incidence of fetuses with extra lumbar
ribs was noted at dose levels of 50 and 100 mg/kg (Mizutani et al,
1971).
Special studies on pharmacological properties
Cartap hydrochloride was shown to have neuromuscular blocking
activity in vitro and in vivo in several animal species
(rat, dog, cat). The effects were characterized as follows: 1)
inhibition of acetylcholine induced contraction of the muscle, 2)
antagonism by cholinesterase inhibitor, calcium ion,
tetraethylammonium and posttetanic stimulation against the partial
neuromuscular block caused by cartap hydrochloride, 3) complete
antagonism by several sulphhydryl compounds (BAL, L-cysteine,
cystine, D-penicillamine) against the neuromuscular block, 5)
respiratory failure due to neuromuscular block, 6) stimulant effect
on the superior cervical ganglion followed by postsynaptic
depression (Nagawa et al, 1971).
Special studies on antidotes
Male mice pre-treated with an intraperitoneal injection of
diazepam, phenobarbital, atropine sulphate, C-penicillamin cystine
or other sulphhydryl compounds delayed the onset of symptoms from
a toxic dose (500 mg/kg) of cartap hydrochloride. The intravenous
administration of cystine (ED50 40 mg/kg) was shown to be effective
in protecting rabbits from the toxic symptoms of an acute oral dose
(50 mg/kg) of cartap hydrochloride. In dogs, a minimal effective
dose of 12.5 mg/kg cystine was required in order to protect against
intoxication from the dermal application of 200 mg/kg of cartap
hydrochloride (Nagawa et al, 1970).
Special studies on dermal irritation
Cartap hydrochloride was applied as a 5, 10 and 20% paste to
the intact skin of two male rabbits daily for 5 days. No evidence
of irritation was observed (Aramaki, 1972).
Special studies on eye irritation
Cartap hydrochloride was instilled into the right eye of 3
rabbits as a 1.5 or 10% solution (0.2 ml) or 15 mg of the powder.
No evidence of irritation to the bulbar or palpebral conjunctiva
was observed when examined at 24 and 48 hours (Aramaki, 1972).
Special studies on respiratory effects
Groups of 5 male and 5 female rats were exposed continuously
for 6 hours to the dust of a 50% water-soluble cartap hydrochloride
powder at estimated concentrations of 0.086 and 0.54 mg/litre of
air. Animals were observed for a period of 7 days following
exposure. Severe eye and skin irritation, increased nasal and oral
secretions, respiratory irritation and lethargy were observed at
0.54 mg/litre. Exposure to 0.086 mg/litre caused slight dyspnoea,
temporary eye irritation and polyuria. The signs of irritation of
this group disappeared rapidly after termination of exposure.
Post-mortem examination revealed no dose-related abnormalities in
either test group (Berczy and Cobb, 1973)
Four groups of 5 male and 5 female rats were exposed
continuously for 6 hours/day, 5 days a week for 3 weeks to the dust
of a 50% water-soluble cartap hydrochloride powder at estimated
nominal concentrations of 0, 0.01, 0.1 and 1.0 mg/litre of air.
Severe respiratory irritation, loss in body weight, injury to the
eyes and skin were observed at the exposure level of 1.0 mg/litre.
Certain clinical parameters (mean cell volume, neutrophil and
lymphocyte cells, glucose, BUN, SGPT and SGOT) were found to be
elevated in the high dose group. The changes in relative organ
weights observed in this group were considered to be related to
loss in body weight. Gross and histopathological examination
revealed no abnormalities in the two lower dose groups. Extensive
chronic respiratory effects in both sexes and acute ulceration in
the urinary bladder in male rats were noted at the high dose level
(Berczy et al, 1973).
Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Mouse M oral 225 Aramaki, 1972
M oral 150 Toyoshima et al,
1972
F oral 154 ibid
M s.c. 34.5 ibid
F s.c. 35 ibid
M i.v. 51 ibid
F i.v. 52 ibid
Rat M oral 380 Aramaki, 1972
F oral 390 ibid
M oral 345 Toyoshima et al,
1972
F oral 325 ibid
M s.c. 40 ibid
F s.c. 42 ibid
M i.v. 44 ibid
F i.v. 36 ibid
Monkey oral 100-200** Yokotani et al,.
1968
Rabbit M&F dermal 819* Davies & Collins,
1973
* Padan water soluble powder (Cartap HCl 50%).
** exact estimate not possible owing to vomiting.
Short-term studies
Mouse (dermal)
Cartap hydrochloride (0.2 ml of a 5 or 10% aqueous solution)
was applied to the intact skin on the backs of 6 male mice. During
the 7-day observation period no symptoms of intoxication developed and
necropsy examination of subcutaneous tissues showed no adverse
effects (Aramaki, 1972).
Rabbit (dermal)
Cartap hydrochloride was applied to intact or abraded skin of
groups of 5 male and 5 female rabbits at dose levels of 0, 15, 60
and 240 mg/kg body weight/day. Rabbits treated with 240 mg/kg died
or were sacrificed after 1 week. Treatment of rabbits with 15 and
60 mg/kg was continued 7 days a week, for 3 consecutive weeks.
Pupillary dilatation and lack of hind limb coordination were
observed in some animals of each group. Body weight gain and food
consumption were reduced at 60 mg/kg especially during the 3rd
week. PCV, hemoglobin concentration and RBC counts were also
decreased in males (abraded) when compared to intact control
animals at this dose level. Increased BUN and reduced plasm
cholinesterase activity were observed at 240 mg/kg when compared to
control values at week 3. Slight dermal irritation was noted during
the 2nd and 3rd week in rabbits receiving 15 and 60 mg/kg.
Ophthalmoscopy and macroscopic and microscopic pathology were
comparable for all groups (Davies et al, 1974).
Mouse (oral)
Groups of 8 or 9 mice of each sex were fed cartap
hydrochloride at dietary levels of 0, 100, 300 and 900 ppm for
three months. Compound consumption was approximately 0, 15, 45 and
135 mg/kg body weight/day. Body weight gain and food efficiency
were slightly-reduced at the highest, dose level. No
compound-related effects were observed in hematology (RBC, total
WBC, hemoglobin and hematocrit) blood chemistry (plasma protein,
SGOT, SGPT, alkaline phosphatase, BUN and cholesterol),urinalysis
(pH, sugar & protein), organ weights or gross or histopathology
(Tsubura et al, 1975).
Rat (oral)
Groups of 15 male and 15 female rats were fed 0, 10, 20 and 40
mg/kg body weight/day of cartap hydrochloride in the diet for 13
weeks. Body weight gain and food consumption in male rats was
reduced at 40 mg/kg. Hematological values were within the normal
range for the rat although the hemoglobin concentration was
decreased and the erythrocyte count increased in male rats at the
upper dose level. No compound-related effects were observed in food
conversion ratio, urinalysis, ophthalmoscopy, plasma, erythrocyte
and brain cholinesterase, relative organ weights or gross and
microscopic pathological examination (Rivett et al, 1972).
Hen (oral)
Groups of 20 hens were fed cartap hydrochloride in the diet at
levels of 0, 10, 30, 90 and 270 ppm for 4 weeks followed by a
2-week post-treatment observation period. Body weight gain and food
consumption were decreased and egg shell weight/egg weight ratio
was lower at dietary levels of 90 and 270 ppm. Egg shell thickness
increased in each group except 90 and 270 ppm groups. No effect on
shell strength, egg yolk weight, egg yolk height or egg white
height were observed (Shiomi et al, 1973).
Long-term studies
Mouse
Groups of 40 male and 40 female mice were fed cartap
hydrochloride in the diet at levels of 0, 10, 20 and 40 mg/kg body
weight/day for a period of 80 weeks. No overt signs of toxicity
were observed and survival rate was not affected. Body weight gains
in the males during the first 52 weeks at 20 mg/kg and after 80
weeks at 40 mg/kg were slightly reduced. This effect was associated
with an impairment of food utilization. No compound-related effects
were noted on hematology and urinalysis parameters. Erythrocyte
cholinesterase activity was slightly reduced in the male and plasma
cholinesterase activity was lower in the female at 40 mg/kg. Other
blood chemistry parameters were within normal limits. No effect was
observed on brain cholinesterase activity. Increases in heart
weight (absolute and relative) in the female at 20 and 40 mg/kg and
thyroid weight in the male at 40 mg/kg were noted. The changes
noted on gross and histopathologic examination were common to both
treated and control animals. There was no evidence to suggest an
effect on the spontaneous tumour profile of this strain of mouse
(Hunter et al, 1974).
Rat
Groups of 45 male and 45 female rats were fed cartap
hydrochloride in the diet at 0, 10, 20 and 40 mg/kg body weight/day
for 104 weeks. A lower incidence in mortality in the treated males
was recorded. Body weight gain and food consumption were depressed
in both sexes, at 40 mg/kg in the male and at 20 mg/kg in the
female throughout the study. No evidence of a compound-related
effect was noted with respect to hematology, urinalysis, blood
chemistry including plasma and erythrocyte cholinesterase, brain
cholinesterase or ophthalmoscopy. There appeared to be a treatment-
related decrease in the relative liver weights of the male. This
effect was not dose-related. The increase in relative weights of
certain other organs receiving 40 mg/kg would appear to reflect the
body weight depression of this group. Gross and histopathological
changes observed in the treated groups were comparable to those in
the control group. There was no marked inter-group differences in
tumor incidence (Hunter et al, 1975).
OBSERVATIONS IN MAN
Cartap hydrochloride has been manufactured in Japan since
1966. Routine examinations of 98 workers employed in the
manufacture were considered essentially normal. A more detailed
medical examination which included urinalysis, blood chemistry,
hematology including routine examination was conducted yearly on
10 persons who were involved in the production of this chemical for
more than 5 years. No adverse effects relating to cartap
hydrochloride were observed in these workers (Hiraoka, 1975).
COMMENTS
Cartap hydrochloride is rapidly absorbed and metabolised.
Metabolites are excreted primarily in the urine and are not stored
to any great extent in the body.
No adverse effects at dietary levels up to 100 ppm were
observed in a two generation rat reproduction study. Teratogenic
studies in the mouse, rat and hamster revealed no compound-related
effect. However, these studies were considered inadequate because
the animals were not exposed to cartap hydrochloride for the full
period of organogenesis. Mutagenic studies were not preformed.
Cartap hydrochloride was shown to be moderately toxic to
mammals on acute exposure by several routes of administration. No
evidence of irritation was observed when cartap hydrochloride was
applied as a 20% paste to intact skin or was instilled as a 10%
solution into the eye of rabbits. However, inhalation exposure of
rats to relatively high concentrates of a 50% water-soluble dust
caused severe eye, dermal and respiratory irritation.
The reduced weight gain observed in the female rat at 20 and
40 mg/kg bw which appeared to be related to a lower food
consumption, was questioned. In various short and long term studies
in the mouse and rat a very weak anticholinesterase activity was
noted. In long term studies on the mouse and rat a compound-related
increase in tumour incidence was not observed. Since data were
submitted only on rodent species, concern was expressed as to the
effect of this compound on a non-rodent species. A detailed medical
examination of persons involved in the production of cartap
hydrochloride for more than 5 years revealed no compound-related
abnormalities.
No-effect levels in the rat and mouse have been established
and the data are sufficient to recommend a temporary acceptable
daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects:
Mouse 20 mg/kg bw/day
Rat 10 mg/kg bw/day
ESTIMATE FOR TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.05 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cartap hydrochloride insecticides have been registered in
Japan since 1967, and are widely used in Asia, Europe and South
America.
Cartap hydrochloride is used against a relatively broad
spectrum of insects, e.g., Lepidoptera, Coleoptera, Diptera and
Hemiptera. It is especially effective against Lepidopter such as
the rice stem borer, diamond-back moth and common cabbage worm, and
Coleoptera such as the Colorado potato beetle, Mexican bean beetle
etc.
Cartap is always applied as the hydrochloride, formulated into
25% and 50% water-soluble powder, 2% dust, 4% and 10% granules and
2% fine granules. A bait formulation has recently been introduced.
Approximately 70% of the world production is applied to rice and
30% to other crops, vegetables, potatoes, fruit, tea, etc.
Products based on cartap hydrochloride are registered in a
total of sixteen countries: Brazil, Bulgaria, Czechoslovakia, El
Salvador, France, German Democratic Republic, Hong Kong, Hungary,
Italy, Japan, Korea, Pakistan, Poland, Rumania, Spain, and Taiwan
province of China and are officially recommended in Greece,
Indonesia, Malaysia, Philippines, Thailand and Vietnam.
Pre-harvest treatments
Cartap hydrochloride is generally used at the rate of 0.5 -1.5
kg a.i./ha. The officially registered and/or recommended uses of
cartap hydrochloride are summarised in Table 1 which sets out
application rates and pre-harvest intervals.
Other uses
Cartap hydrochloride is used for controlling the rice
white-tip nematode by soaking rice seed in an aqueous solution of
the insecticide.
TABLE 1. Uses of cartap officially recommended in various countries
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Brazil
Cabbage, cotton,
passion fruit,
potato, sunflower, 0.5-0.75 14
tomato, wheat
Czechoslovakia
Mustard 0.5-0.6 7
Potato 0.5-0.6 14
Hungary
Potato, tomato 0.75-1.0 10
Italy
Potato 0.25-0.5 14
Japan (temporary)
Cabbage 0.5-0.75 7
Chestnut 1.5 14
Chinese cabbage 0.5-0.75 7
Ginger 0.5 3
Grape (vine) 1.5 14
Hop 1.0 14
Japanese persimmon 1.5 21
Japanese radish 0.35-0.5 7
Maize 1.0 7
TABLE 1. (Cont'd.)
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Potato 0.75 7
Rice 0.5-0.75 21
Sweet potato 0.5-0.75 7
Tea 1.0 7
Spain
Potato 0.35-0.65 15
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were obtained in Japan from supervised trials on
a variety of vegetables, fruits, hops, forage and rice. A summary
of these data is presented in Table 2 together with details of
application rate and pre-harvest interval.
Cabbage
Two applications of cartap hydrochloride to cabbage seldom
appear to produce detectable residues even when the last
application is made seven days before harvest. When four
applications were made throughout the growing season the residue
found ranged up to 0.1 mg/kg in cabbage harvested fourteen days
after the last treatment. Even eight treatments made at seven day
intervals produced residues of only 0.13 mg/kg fourteen days after
the last application.
The half-life appears to be of the order of 10-14 days.
Chestnut
Detectable residues were not found in the nuts when chestnut
trees were sprayed three times and the nuts were harvested 14 days
after the last application.
Chinese cabbage
Chinese cabbage appears to retain cartap residues at higher
levels and for longer periods than does cabbage. 4 sprays give rise
to residues ranging up to 0.27 mg/kg when chinese cabbage was
harvested 14 days after the last application. Residues up to 1.05
mg/kg were found after a preharvest interval of 7 days. Fine
granules appear to produce lower residues than sprays prepared from
soluble powders.
Ginger
Ginger appears to retain small quantities of cartap, (up to
0.03 mg/kg after 14 days) although the level of residues does not
appear to be significantly influenced by the number of treatments.
Grapes
The residue studies in grapes were designed to show the level
of residues resulting from the application of 2, 4 and 6 sprays in
grapes harvested 14, 21 and 30 days after the last application.
There is some indication that the residue levels in the fruit might
increase owing to systemic transfer for some time after application.
Generally the results found 30 days after the last spray were just
slightly lower than those found 21 days after the same treatment.
The level of residues was higher in grapes treated 6 times than
in those treated 4 times which in turn was distinctly higher than in
grapes treated only twice.
Hops
There is a very noticeable decline in the level of cartap
residues found in dried hops as the interval between last application
and harvest increases from 7 through 14 to 21 days. The half-life of
the residues appears to be of the order of 7 days but whether the
drying process has an influence on this is not clear.
Persimmon
The data on cartap residues in persimmons gathered from 2 trials
indicate only the quantity found 30 to 75 days after the last
application. Whilst detectable residues still occur 75 days after the
last application there is no great difference between the residue
level in samples from trees treated 6 or 4 times.
Japanese radish
Systematic studies of both leaves and roots of Japanese radish
(data on roots only are shown in Table 2) treated 2 and 4 times and
harvested 3, 7 and 14 days after the last application showed,
sometimes, a distinct increase in the residue concentration in the
TABLE 2. Cartrap residues resulting from supervised trials - Japan
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Cabbage 10/69 SP 0.6 2 <0,0.004 <0.004
4 0.01 0.008
11/69 SP 0.75 2 <0.004 <0.004
4 0.09 0.08
7/72 SP 0.75 2 0.02 0.06
4 0.14 0.09
12/72 FG 1.0 2 <0.006
5 0.07 0.05
8 0.13 0.07
12/72 FG 1.0 2 <0.006
3 <0.006
5 <0.006 <0.006
Chestnut 9/71 SP 60g/tree 3 <0.001 <0.001
3 <0.008 <0.008
Chinese 11/74 SP 0.6 2 0.03 0.02 0.02
cabbage 4 0.03 0.03 0.03
12/74 SP 0.6 2 0.7 0.07 0.06
4 0.5 0.16 0.15
1/75 SP 0.6 2 1.05 0.15 0.1
4 0.4 0.27 0.2
9/72 FG 1.0 2 0.03 0.01 <0.005
3 0.05 0.02 <0.005
5 0.12 0.03 <0.005
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Ginger 10/72 SP 0.7 4 0.006 <0.005
6 0.01 <0.005
9 0.007 <0.005
10/72 SP 1.0 3 0.02 0.03
5 0.02 0.02
11/73 SP 1.0 3 0.02 0.02
5 0.04 0.03
Grape 7/73 SP 2.0 2 0.08 0.12
4 0.54 0.38
6 0.57 0.97
9/73 SP 1.0 2 0.01 0.01 0.008
4 0.1 0.09 0.08
6 0.24 0.20 0.13
7/73 SP 2.0 2 0.08 0.13
4 0.35 0.40
6 0.75 0.80
9/73 SP 2.0 2 0.05 0.04 0.03
4 0.13 0.10 0.07
6 0.24 0.28 0.20
Hops 8/73 SP 2.0 3 0.56 0.04
(dry) 4 0.50 0.15
3.0 3 2.2 0.32
4 8.0 3.0
8/73 SP 2.0 3 0.30 0.03
4 0.34 0.14
3.0 3 0.92 0.28
4 4.9 2.7
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Persimmon 11/70 SP 2.25 4 0.02
6 0.03
11/70 SP 7.5 4 0.08 0.07
6 0.19 0.05
Potato 6/71 SP 1.5 3 <0.008 <0.008
6 <0.008 <0.008 <0.008
12/72 SP 1.0 2 <0.005 <0.005
5 <0.005 <0.005
8 <0.005 <0.005
FG 1.0 5 <0.001 <0.001
8 <0.001 <0.001
Radish 10/73 SP 0.75 2 0.10 0.12 0.03
(Japanese) 4 0.08 0.14 0.04
11/73 SP 0.5 2 0.52 0.12 0.15
4 0.57 0.22 0.22
11/73 SP 0.75 2 0.25 0.15 0.10
4 0.02 0.02 0.01
Rice
Grain 10/74 SP 0.5 8 <0.01 <0.01 <0.01
8/74 1.0 8 <0.01 <0.01 <0.01
0.5 8 0.05 <0.01 0.02
Straw 10/74 SP 1.0 8 <0.01 0.02 <0.01
8/74
Grain 10/74 SP 0.5 8 <0.005 0.005 <0.005
8/74 SP 1.0 8 <0.005 0.006 <0.005
Straw 10/74 SP 0.5 8 0.05 <0.01 0.02
8/74 SP 1.0 8 0.02 <0.01 <0.01
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Rice
Grain 10/74 SP 1.6 8 <0.01 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Straw 10/74 SP 1.6 8 0.02 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Grain 10/74 FG 0.8 8 <0.005 <0.005 <0.005
8/74 FG 1.0 8 <0.005 <0.005 <0.005
Straw 10/74 FG 0.8 8 0.05 <0.01 0.03
8/74 FG 1.0 8 <0.01 <0.01 <0.01
Tea (dry) 7/73 SP 1.0 1 1.0 1.2 0.6
7/73 SP 1.0 2 1.4 1.8 0.6
7/73 SP 1.0 1 0.7 0.8 0.7
Sweet corn 9/71 FG 0.6 1 <0.008 <0.008 <0.005 <0.005
Forage
Orchard grass 9/71 SP 0.5 1 0.03 0.01
2 0.06 0.04
Red clover 8/71 SP 0.5 1 0.01 0.03
2 0.11 0.01
White clover 9/71 SP 0.5 1 0.22 0.05
2 0.52 0.45
leaves during the first 7 days. This trend was not noticeable in the
roots. In radish root the half-life appears to be of the order of 10
days.
Potatoes
A number of trials confirm that detectable cartap residues do not
occur in the tubers of potatoes even when the crop is treated 8 times
during the season and the tubers are harvested 7, 14 or 21 days after
the last application.
Rice
Numerous trials have demonstrated that rice grain harvested 30 or
more days after 8 applications of cartap hydrochloride sprays almost
invariably contains no residues above the limit of determination. A
few of the many samples of rice straw from these trials were found to
contain residues at or just above the limit of determination.
Tea
Green tea produced from leaves harvested 7, 14 and 21 days after
the application of cartap hydrochloride contained residues ranging up
to almost 2 mg/kg. In 2 of the 3 trials the concentration of residues
increased slightly between the 7th and 14th day following treatment
but declined by the 21st day.
FATE OF RESIDUES
General comments
Cartap hydrochloride is stable to acid, but is hydrolysed in
neutral or alkaline solutions to give dihydronereistoxin (NTXH) which
is quite easily oxidised to nereistoxin (NTX) by air. NTXH and NTX may
be biologically convertible to one another, an shown in the following
reaction:
Other information on identity and properties
Cartap is invariably used as the hydrochloride, to which the
following information refers.
Molecular weight: 273.8
State: Colourless crystals
Melting point: 179-181°C(decomp.)
Solubility(g/100 ml): Soluble in water(13.2/10°C,
17.8/20°C, 25.3/30°C slightly soluble
in methanol(2.08/5°C), almost insoluble
in acetone, benzene, chloroform,
diethyl ether, ethyl acetate, and
n-hexane.
Purity of technical material: 97%. containing 3% of water and
ammonium chloride as impurities.
Hydroscopicity: Slightly hygroscopic
Volatility: Negligible
Formulation: 25% and 50% water-soluble powder, 2%
dust, 4% and 10% granules and 2% fine
granules are available. A bait
formulation has recently been
introduced.
Cartap hydrochloride is an insecticide recently developed by
Takeda Chemical Industries. It is a derivative of nereistoxin which
is a naturally occurring insecticidal substance isolated from the
marine segmented worms Lumbrinereis heteropoda and L.
brevicirra.
Nereistoxin was isolated by Nitta in 1934, the structure was
elucidated by Okaichi and Hashimoto (1962) and synthesized in 1965
by Hagiwara et al. A variety of nereistoxin derivatives were
prepared and their insecticidal activities tested. Cartap
hydrochloride is one of the most potent of the many compounds
prepared.
The chemistry and synthesis of nereistoxin and related
compounds are dealt with by a number of authors including Hashimoto
et al (1960); Okaichi and Hashimoto (1962); Hagiwara et al (1965);
Konishi (1968a, 1968b); Hagiwara and Numata (1968); Konishi (1970a,
1971); Sakai (1971); Nishi et al (1973).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Groups of 10 male and 20 female rats were administered cartap
hydrochloride in the diet at levels of 0, 100 and 1000 ppm for 9
weeks prior to mating and subsequently for the remainder of the
study which was a 2 litter, 2 generation reproduction study. One
half of the F2b litters were delivered by caesarean section on day
19 of gestation, the other half were delivered naturally and held
to weaning. Parental animals showed reduced body weight gain and
food consumption and an apparent impairment of fertility for the
F1a generation at 1000 ppm. Gestation and live birth indices of
the treated animals were comparable to those of the controls.
Weanling survival index was lower in the F1b generation and pup
weights in all generations at 7, 14 and 21 days after weaning were
reduced at the upper dose level. There were no meaningful
differences observed between groups with respect to number of
implantation sites, resorptions or live and dead fetuses. No
compound-related microscopic alterations were noted in selected
tissues from weanling F2b progeny of the 1000 ppm group. However,
a greater incidence of incomplete ossification was revealed on
skeletal examination in this group, (Olson and Busey, 1972).
Special studies on teratogenicity
Mouse
Three groups comprising 20, 12 and 14 pregnant mice received
cartap hydrochloride by stomach tube at dose levels of 0, 50 and
100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Twenty-one pregnant mice were employed as an untreated
control group. Animals were killed on day 19 of gestation and
fetuses removed by caesarean section. Weight gains were not
affected. No significant variations were observed in the number of
implantation sites, fetal mortality and mean body weight of fetuses
between control and treated groups. No visceral abnormalities were
observed. Skeletal examination revealed abnormalities (fusion of
cervical vertebral arches and fusion of sternebrae) in 2/110
fetuses (1.8%) at 50 mg/kg and 2/120 fetuses (1.7%) at 100 mg/kg.
In respect to other skeletal variations, there were no significant
differences between control and treated animals (Mizutani et al,
1971).
Rat
Cartap hydrochloride was administered orally to three groups
of 21, 12 and 17 pregnant rats at dose levels of 0, 50 and 100
mg/kg body weight respectively from gestation day 9 to day 15.
Twenty-three pregnant rats were used as an untreated control group.
Animals were sacrificed on day 21 and fetuses were delivered by
laparotomy. An increase in mortality and retarded weight gain were
observed in the maternal animals at 100 mg/kg during the dosing
period. There was no statistical difference between treated and
control groups with respect to number of implantation sites, fetal
mortality, sex ratio, external and visceral malformations. Skeletal
examination revealed no major abnormalities except for an increase
25/174 (14.4%) in bilateral twin thoracic vertebral centrae in the
100 mg/kg group (Mizutani et al, 1971).
Hamster
Five groups comprising 9, 7, 8, 9 and 7 pregnant hamsters
received cartap, hydrochloride orally at dose levels of 0, 2, 10,
50 and 100 mg/kg body weight respectively from day 8 to day 13 of
gestation. Fifteen pregnant hamsters were used as an untreated
control group. Animals were killed on day 16 of gestation and
fetuses removed by caesarean section. Treatment with 100 mg/kg
resulted in the death of 2/7 animals and was associated with
reduced weight gain. No compound-related effects were observed in
the number of implantation sites, fetal mortality, fetal weights,
external and visceral malformations. In hamsters receiving 100
mg/kg, the frequency of skeletal anomalies increased slightly, 6/52
(11.5%) showing abnormalities in the thoracic vertebrae and/or
ribs. An increase in the incidence of fetuses with extra lumbar
ribs was noted at dose levels of 50 and 100 mg/kg (Mizutani et al,
1971).
Special studies on pharmacological properties
Cartap hydrochloride was shown to have neuromuscular blocking
activity in vitro and in vivo in several animal species
(rat, dog, cat). The effects were characterized as follows: 1)
inhibition of acetylcholine induced contraction of the muscle, 2)
antagonism by cholinesterase inhibitor, calcium ion,
tetraethylammonium and posttetanic stimulation against the partial
neuromuscular block caused by cartap hydrochloride, 3) complete
antagonism by several sulphhydryl compounds (BAL, L-cysteine,
cystine, D-penicillamine) against the neuromuscular block, 5)
respiratory failure due to neuromuscular block, 6) stimulant effect
on the superior cervical ganglion followed by postsynaptic
depression (Nagawa et al, 1971).
Special studies on antidotes
Male mice pre-treated with an intraperitoneal injection of
diazepam, phenobarbital, atropine sulphate, C-penicillamin cystine
or other sulphhydryl compounds delayed the onset of symptoms from
a toxic dose (500 mg/kg) of cartap hydrochloride. The intravenous
administration of cystine (ED50 40 mg/kg) was shown to be effective
in protecting rabbits from the toxic symptoms of an acute oral dose
(50 mg/kg) of cartap hydrochloride. In dogs, a minimal effective
dose of 12.5 mg/kg cystine was required in order to protect against
intoxication from the dermal application of 200 mg/kg of cartap
hydrochloride (Nagawa et al, 1970).
Special studies on dermal irritation
Cartap hydrochloride was applied as a 5, 10 and 20% paste to
the intact skin of two male rabbits daily for 5 days. No evidence
of irritation was observed (Aramaki, 1972).
Special studies on eye irritation
Cartap hydrochloride was instilled into the right eye of 3
rabbits as a 1.5 or 10% solution (0.2 ml) or 15 mg of the powder.
No evidence of irritation to the bulbar or palpebral conjunctiva
was observed when examined at 24 and 48 hours (Aramaki, 1972).
Special studies on respiratory effects
Groups of 5 male and 5 female rats were exposed continuously
for 6 hours to the dust of a 50% water-soluble cartap hydrochloride
powder at estimated concentrations of 0.086 and 0.54 mg/litre of
air. Animals were observed for a period of 7 days following
exposure. Severe eye and skin irritation, increased nasal and oral
secretions, respiratory irritation and lethargy were observed at
0.54 mg/litre. Exposure to 0.086 mg/litre caused slight dyspnoea,
temporary eye irritation and polyuria. The signs of irritation of
this group disappeared rapidly after termination of exposure.
Post-mortem examination revealed no dose-related abnormalities in
either test group (Berczy and Cobb, 1973)
Four groups of 5 male and 5 female rats were exposed
continuously for 6 hours/day, 5 days a week for 3 weeks to the dust
of a 50% water-soluble cartap hydrochloride powder at estimated
nominal concentrations of 0, 0.01, 0.1 and 1.0 mg/litre of air.
Severe respiratory irritation, loss in body weight, injury to the
eyes and skin were observed at the exposure level of 1.0 mg/litre.
Certain clinical parameters (mean cell volume, neutrophil and
lymphocyte cells, glucose, BUN, SGPT and SGOT) were found to be
elevated in the high dose group. The changes in relative organ
weights observed in this group were considered to be related to
loss in body weight. Gross and histopathological examination
revealed no abnormalities in the two lower dose groups. Extensive
chronic respiratory effects in both sexes and acute ulceration in
the urinary bladder in male rats were noted at the high dose level
(Berczy et al, 1973).
Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Mouse M oral 225 Aramaki, 1972
M oral 150 Toyoshima et al,
1972
F oral 154 ibid
M s.c. 34.5 ibid
F s.c. 35 ibid
M i.v. 51 ibid
F i.v. 52 ibid
Rat M oral 380 Aramaki, 1972
F oral 390 ibid
M oral 345 Toyoshima et al,
1972
F oral 325 ibid
M s.c. 40 ibid
F s.c. 42 ibid
M i.v. 44 ibid
F i.v. 36 ibid
Monkey oral 100-200** Yokotani et al,.
1968
Rabbit M&F dermal 819* Davies & Collins,
1973
* Padan water soluble powder (Cartap HCl 50%).
** exact estimate not possible owing to vomiting.
Short-term studies
Mouse (dermal)
Cartap hydrochloride (0.2 ml of a 5 or 10% aqueous solution)
was applied to the intact skin on the backs of 6 male mice. During
the 7-day observation period no symptoms of intoxication developed and
necropsy examination of subcutaneous tissues showed no adverse
effects (Aramaki, 1972).
Rabbit (dermal)
Cartap hydrochloride was applied to intact or abraded skin of
groups of 5 male and 5 female rabbits at dose levels of 0, 15, 60
and 240 mg/kg body weight/day. Rabbits treated with 240 mg/kg died
or were sacrificed after 1 week. Treatment of rabbits with 15 and
60 mg/kg was continued 7 days a week, for 3 consecutive weeks.
Pupillary dilatation and lack of hind limb coordination were
observed in some animals of each group. Body weight gain and food
consumption were reduced at 60 mg/kg especially during the 3rd
week. PCV, hemoglobin concentration and RBC counts were also
decreased in males (abraded) when compared to intact control
animals at this dose level. Increased BUN and reduced plasm
cholinesterase activity were observed at 240 mg/kg when compared to
control values at week 3. Slight dermal irritation was noted during
the 2nd and 3rd week in rabbits receiving 15 and 60 mg/kg.
Ophthalmoscopy and macroscopic and microscopic pathology were
comparable for all groups (Davies et al, 1974).
Mouse (oral)
Groups of 8 or 9 mice of each sex were fed cartap
hydrochloride at dietary levels of 0, 100, 300 and 900 ppm for
three months. Compound consumption was approximately 0, 15, 45 and
135 mg/kg body weight/day. Body weight gain and food efficiency
were slightly-reduced at the highest, dose level. No
compound-related effects were observed in hematology (RBC, total
WBC, hemoglobin and hematocrit) blood chemistry (plasma protein,
SGOT, SGPT, alkaline phosphatase, BUN and cholesterol),urinalysis
(pH, sugar & protein), organ weights or gross or histopathology
(Tsubura et al, 1975).
Rat (oral)
Groups of 15 male and 15 female rats were fed 0, 10, 20 and 40
mg/kg body weight/day of cartap hydrochloride in the diet for 13
weeks. Body weight gain and food consumption in male rats was
reduced at 40 mg/kg. Hematological values were within the normal
range for the rat although the hemoglobin concentration was
decreased and the erythrocyte count increased in male rats at the
upper dose level. No compound-related effects were observed in food
conversion ratio, urinalysis, ophthalmoscopy, plasma, erythrocyte
and brain cholinesterase, relative organ weights or gross and
microscopic pathological examination (Rivett et al, 1972).
Hen (oral)
Groups of 20 hens were fed cartap hydrochloride in the diet at
levels of 0, 10, 30, 90 and 270 ppm for 4 weeks followed by a
2-week post-treatment observation period. Body weight gain and food
consumption were decreased and egg shell weight/egg weight ratio
was lower at dietary levels of 90 and 270 ppm. Egg shell thickness
increased in each group except 90 and 270 ppm groups. No effect on
shell strength, egg yolk weight, egg yolk height or egg white
height were observed (Shiomi et al, 1973).
Long-term studies
Mouse
Groups of 40 male and 40 female mice were fed cartap
hydrochloride in the diet at levels of 0, 10, 20 and 40 mg/kg body
weight/day for a period of 80 weeks. No overt signs of toxicity
were observed and survival rate was not affected. Body weight gains
in the males during the first 52 weeks at 20 mg/kg and after 80
weeks at 40 mg/kg were slightly reduced. This effect was associated
with an impairment of food utilization. No compound-related effects
were noted on hematology and urinalysis parameters. Erythrocyte
cholinesterase activity was slightly reduced in the male and plasma
cholinesterase activity was lower in the female at 40 mg/kg. Other
blood chemistry parameters were within normal limits. No effect was
observed on brain cholinesterase activity. Increases in heart
weight (absolute and relative) in the female at 20 and 40 mg/kg and
thyroid weight in the male at 40 mg/kg were noted. The changes
noted on gross and histopathologic examination were common to both
treated and control animals. There was no evidence to suggest an
effect on the spontaneous tumour profile of this strain of mouse
(Hunter et al, 1974).
Rat
Groups of 45 male and 45 female rats were fed cartap
hydrochloride in the diet at 0, 10, 20 and 40 mg/kg body weight/day
for 104 weeks. A lower incidence in mortality in the treated males
was recorded. Body weight gain and food consumption were depressed
in both sexes, at 40 mg/kg in the male and at 20 mg/kg in the
female throughout the study. No evidence of a compound-related
effect was noted with respect to hematology, urinalysis, blood
chemistry including plasma and erythrocyte cholinesterase, brain
cholinesterase or ophthalmoscopy. There appeared to be a treatment-
related decrease in the relative liver weights of the male. This
effect was not dose-related. The increase in relative weights of
certain other organs receiving 40 mg/kg would appear to reflect the
body weight depression of this group. Gross and histopathological
changes observed in the treated groups were comparable to those in
the control group. There was no marked inter-group differences in
tumor incidence (Hunter et al, 1975).
OBSERVATIONS IN MAN
Cartap hydrochloride has been manufactured in Japan since
1966. Routine examinations of 98 workers employed in the
manufacture were considered essentially normal. A more detailed
medical examination which included urinalysis, blood chemistry,
hematology including routine examination was conducted yearly on
10 persons who were involved in the production of this chemical for
more than 5 years. No adverse effects relating to cartap
hydrochloride were observed in these workers (Hiraoka, 1975).
COMMENTS
Cartap hydrochloride is rapidly absorbed and metabolised.
Metabolites are excreted primarily in the urine and are not stored
to any great extent in the body.
No adverse effects at dietary levels up to 100 ppm were
observed in a two generation rat reproduction study. Teratogenic
studies in the mouse, rat and hamster revealed no compound-related
effect. However, these studies were considered inadequate because
the animals were not exposed to cartap hydrochloride for the full
period of organogenesis. Mutagenic studies were not preformed.
Cartap hydrochloride was shown to be moderately toxic to
mammals on acute exposure by several routes of administration. No
evidence of irritation was observed when cartap hydrochloride was
applied as a 20% paste to intact skin or was instilled as a 10%
solution into the eye of rabbits. However, inhalation exposure of
rats to relatively high concentrates of a 50% water-soluble dust
caused severe eye, dermal and respiratory irritation.
The reduced weight gain observed in the female rat at 20 and
40 mg/kg bw which appeared to be related to a lower food
consumption, was questioned. In various short and long term studies
in the mouse and rat a very weak anticholinesterase activity was
noted. In long term studies on the mouse and rat a compound-related
increase in tumour incidence was not observed. Since data were
submitted only on rodent species, concern was expressed as to the
effect of this compound on a non-rodent species. A detailed medical
examination of persons involved in the production of cartap
hydrochloride for more than 5 years revealed no compound-related
abnormalities.
No-effect levels in the rat and mouse have been established
and the data are sufficient to recommend a temporary acceptable
daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects:
Mouse 20 mg/kg bw/day
Rat 10 mg/kg bw/day
ESTIMATE FOR TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.05 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cartap hydrochloride insecticides have been registered in
Japan since 1967, and are widely used in Asia, Europe and South
America.
Cartap hydrochloride is used against a relatively broad
spectrum of insects, e.g., Lepidoptera, Coleoptera, Diptera and
Hemiptera. It is especially effective against Lepidopter such as
the rice stem borer, diamond-back moth and common cabbage worm, and
Coleoptera such as the Colorado potato beetle, Mexican bean beetle
etc.
Cartap is always applied as the hydrochloride, formulated into
25% and 50% water-soluble powder, 2% dust, 4% and 10% granules and
2% fine granules. A bait formulation has recently been introduced.
Approximately 70% of the world production is applied to rice and
30% to other crops, vegetables, potatoes, fruit, tea, etc.
Products based on cartap hydrochloride are registered in a
total of sixteen countries: Brazil, Bulgaria, Czechoslovakia, El
Salvador, France, German Democratic Republic, Hong Kong, Hungary,
Italy, Japan, Korea, Pakistan, Poland, Rumania, Spain, and Taiwan
province of China and are officially recommended in Greece,
Indonesia, Malaysia, Philippines, Thailand and Vietnam.
Pre-harvest treatments
Cartap hydrochloride is generally used at the rate of 0.5 -1.5
kg a.i./ha. The officially registered and/or recommended uses of
cartap hydrochloride are summarised in Table 1 which sets out
application rates and pre-harvest intervals.
Other uses
Cartap hydrochloride is used for controlling the rice
white-tip nematode by soaking rice seed in an aqueous solution of
the insecticide.
TABLE 1. Uses of cartap officially recommended in various countries
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Brazil
Cabbage, cotton,
passion fruit,
potato, sunflower, 0.5-0.75 14
tomato, wheat
Czechoslovakia
Mustard 0.5-0.6 7
Potato 0.5-0.6 14
Hungary
Potato, tomato 0.75-1.0 10
Italy
Potato 0.25-0.5 14
Japan (temporary)
Cabbage 0.5-0.75 7
Chestnut 1.5 14
Chinese cabbage 0.5-0.75 7
Ginger 0.5 3
Grape (vine) 1.5 14
Hop 1.0 14
Japanese persimmon 1.5 21
Japanese radish 0.35-0.5 7
Maize 1.0 7
TABLE 1. (Cont'd.)
Country and Crop Dosage rate Minimum
kg a.i./ha pre-harvest
(as hydrochloride) interval, days
Potato 0.75 7
Rice 0.5-0.75 21
Sweet potato 0.5-0.75 7
Tea 1.0 7
Spain
Potato 0.35-0.65 15
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were obtained in Japan from supervised trials on
a variety of vegetables, fruits, hops, forage and rice. A summary
of these data is presented in Table 2 together with details of
application rate and pre-harvest interval.
Cabbage
Two applications of cartap hydrochloride to cabbage seldom
appear to produce detectable residues even when the last
application is made seven days before harvest. When four
applications were made throughout the growing season the residue
found ranged up to 0.1 mg/kg in cabbage harvested fourteen days
after the last treatment. Even eight treatments made at seven day
intervals produced residues of only 0.13 mg/kg fourteen days after
the last application.
The half-life appears to be of the order of 10-14 days.
Chestnut
Detectable residues were not found in the nuts when chestnut
trees were sprayed three times and the nuts were harvested 14 days
after the last application.
Chinese cabbage
Chinese cabbage appears to retain cartap residues at higher
levels and for longer periods than does cabbage. 4 sprays give rise
to residues ranging up to 0.27 mg/kg when chinese cabbage was
harvested 14 days after the last application. Residues up to 1.05
mg/kg were found after a preharvest interval of 7 days. Fine
granules appear to produce lower residues than sprays prepared from
soluble powders.
Ginger
Ginger appears to retain small quantities of cartap, (up to
0.03 mg/kg after 14 days) although the level of residues does not
appear to be significantly influenced by the number of treatments.
Grapes
The residue studies in grapes were designed to show the level
of residues resulting from the application of 2, 4 and 6 sprays in
grapes harvested 14, 21 and 30 days after the last application.
There is some indication that the residue levels in the fruit might
increase owing to systemic transfer for some time after application.
Generally the results found 30 days after the last spray were just
slightly lower than those found 21 days after the same treatment.
The level of residues was higher in grapes treated 6 times than
in those treated 4 times which in turn was distinctly higher than in
grapes treated only twice.
Hops
There is a very noticeable decline in the level of cartap
residues found in dried hops as the interval between last application
and harvest increases from 7 through 14 to 21 days. The half-life of
the residues appears to be of the order of 7 days but whether the
drying process has an influence on this is not clear.
Persimmon
The data on cartap residues in persimmons gathered from 2 trials
indicate only the quantity found 30 to 75 days after the last
application. Whilst detectable residues still occur 75 days after the
last application there is no great difference between the residue
level in samples from trees treated 6 or 4 times.
Japanese radish
Systematic studies of both leaves and roots of Japanese radish
(data on roots only are shown in Table 2) treated 2 and 4 times and
harvested 3, 7 and 14 days after the last application showed,
sometimes, a distinct increase in the residue concentration in the
TABLE 2. Cartrap residues resulting from supervised trials - Japan
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Cabbage 10/69 SP 0.6 2 <0,0.004 <0.004
4 0.01 0.008
11/69 SP 0.75 2 <0.004 <0.004
4 0.09 0.08
7/72 SP 0.75 2 0.02 0.06
4 0.14 0.09
12/72 FG 1.0 2 <0.006
5 0.07 0.05
8 0.13 0.07
12/72 FG 1.0 2 <0.006
3 <0.006
5 <0.006 <0.006
Chestnut 9/71 SP 60g/tree 3 <0.001 <0.001
3 <0.008 <0.008
Chinese 11/74 SP 0.6 2 0.03 0.02 0.02
cabbage 4 0.03 0.03 0.03
12/74 SP 0.6 2 0.7 0.07 0.06
4 0.5 0.16 0.15
1/75 SP 0.6 2 1.05 0.15 0.1
4 0.4 0.27 0.2
9/72 FG 1.0 2 0.03 0.01 <0.005
3 0.05 0.02 <0.005
5 0.12 0.03 <0.005
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Ginger 10/72 SP 0.7 4 0.006 <0.005
6 0.01 <0.005
9 0.007 <0.005
10/72 SP 1.0 3 0.02 0.03
5 0.02 0.02
11/73 SP 1.0 3 0.02 0.02
5 0.04 0.03
Grape 7/73 SP 2.0 2 0.08 0.12
4 0.54 0.38
6 0.57 0.97
9/73 SP 1.0 2 0.01 0.01 0.008
4 0.1 0.09 0.08
6 0.24 0.20 0.13
7/73 SP 2.0 2 0.08 0.13
4 0.35 0.40
6 0.75 0.80
9/73 SP 2.0 2 0.05 0.04 0.03
4 0.13 0.10 0.07
6 0.24 0.28 0.20
Hops 8/73 SP 2.0 3 0.56 0.04
(dry) 4 0.50 0.15
3.0 3 2.2 0.32
4 8.0 3.0
8/73 SP 2.0 3 0.30 0.03
4 0.34 0.14
3.0 3 0.92 0.28
4 4.9 2.7
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Persimmon 11/70 SP 2.25 4 0.02
6 0.03
11/70 SP 7.5 4 0.08 0.07
6 0.19 0.05
Potato 6/71 SP 1.5 3 <0.008 <0.008
6 <0.008 <0.008 <0.008
12/72 SP 1.0 2 <0.005 <0.005
5 <0.005 <0.005
8 <0.005 <0.005
FG 1.0 5 <0.001 <0.001
8 <0.001 <0.001
Radish 10/73 SP 0.75 2 0.10 0.12 0.03
(Japanese) 4 0.08 0.14 0.04
11/73 SP 0.5 2 0.52 0.12 0.15
4 0.57 0.22 0.22
11/73 SP 0.75 2 0.25 0.15 0.10
4 0.02 0.02 0.01
Rice
Grain 10/74 SP 0.5 8 <0.01 <0.01 <0.01
8/74 1.0 8 <0.01 <0.01 <0.01
0.5 8 0.05 <0.01 0.02
Straw 10/74 SP 1.0 8 <0.01 0.02 <0.01
8/74
Grain 10/74 SP 0.5 8 <0.005 0.005 <0.005
8/74 SP 1.0 8 <0.005 0.006 <0.005
Straw 10/74 SP 0.5 8 0.05 <0.01 0.02
8/74 SP 1.0 8 0.02 <0.01 <0.01
TABLE 2. (Cont'd.)
Crop Date Formulation Rate No of Residues (mg/kg) at interval (days) after last treatment
kg/ha treatments
(hydrochloride) 3 7 14 21 30 45 60-75
Rice
Grain 10/74 SP 1.6 8 <0.01 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Straw 10/74 SP 1.6 8 0.02 <0.01 <0.01
8/74 SP 2.0 8 <0.01 <0.01 <0.01
Grain 10/74 FG 0.8 8 <0.005 <0.005 <0.005
8/74 FG 1.0 8 <0.005 <0.005 <0.005
Straw 10/74 FG 0.8 8 0.05 <0.01 0.03
8/74 FG 1.0 8 <0.01 <0.01 <0.01
Tea (dry) 7/73 SP 1.0 1 1.0 1.2 0.6
7/73 SP 1.0 2 1.4 1.8 0.6
7/73 SP 1.0 1 0.7 0.8 0.7
Sweet corn 9/71 FG 0.6 1 <0.008 <0.008 <0.005 <0.005
Forage
Orchard grass 9/71 SP 0.5 1 0.03 0.01
2 0.06 0.04
Red clover 8/71 SP 0.5 1 0.01 0.03
2 0.11 0.01
White clover 9/71 SP 0.5 1 0.22 0.05
2 0.52 0.45
leaves during the first 7 days. This trend was not noticeable in the
roots. In radish root the half-life appears to be of the order of 10
days.
Potatoes
A number of trials confirm that detectable cartap residues do not
occur in the tubers of potatoes even when the crop is treated 8 times
during the season and the tubers are harvested 7, 14 or 21 days after
the last application.
Rice
Numerous trials have demonstrated that rice grain harvested 30 or
more days after 8 applications of cartap hydrochloride sprays almost
invariably contains no residues above the limit of determination. A
few of the many samples of rice straw from these trials were found to
contain residues at or just above the limit of determination.
Tea
Green tea produced from leaves harvested 7, 14 and 21 days after
the application of cartap hydrochloride contained residues ranging up
to almost 2 mg/kg. In 2 of the 3 trials the concentration of residues
increased slightly between the 7th and 14th day following treatment
but declined by the 21st day.
FATE OF RESIDUES
General comments
Cartap hydrochloride is stable to acid, but is hydrolysed in
neutral or alkaline solutions to give dihydronereistoxin (NTXH) which
is quite easily oxidised to nereistoxin (NTX) by air. NTXH and NTX may
be biologically convertible to one another, an shown in the following
reaction:
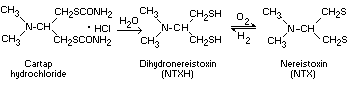 Sakai (1970) in discussing nereistoxin and its relatives
indicates that the contact toxicity to Azuki bean weevil, rice stem
borer and mouse, and the activity in blocking the synaptic response of
the 6th abdominal ganglion of the American cockroach are remarkably
similar for cartap hydrochloride and nereistoxin. It seems possible
that the activity of cartap hydrochloride is due to its conversion to
nereistoxin in plants and animals.
In animals
The absorption, distribution and excretion of cartap
hydrochloride in rats and mice is described above ("Bio-chemical
aspects") and further studies are in progress. Chromatographic studies
have revealed that there are about nine main metabolites in the urine
from animals receiving cartap, hydrochloride orally. There appears to
be no significant difference in the pattern between rats and mice.
Unchanged cartap hydrochloride could not be detected. One of the
identified metabolites was the mono-sulphoxide of
1,3-di(methylthio)-2-dimethylaminopropane and it comprised 18% and 8%
of the urinary metabolites in the rat and mouse respectively
(Shirakawa et al, 1971).
In plants
Tomizawa and Endo (1972) studied the uptake of 35S-labelled
cartap hydrochloride in rice plants. The basic fraction which was
assumed to retain biological activity reached a maximum concentration
in the leaf sheath and leaf blade on the 7th day after application and
thereafter declined rapidly until the 15th day. A small but consistent
amount of radio-activity remained in both leaf sheath and leaf blade
for 30 days. Radio-activity in the ear remained consistently low
throughout the test period.
Among the metabolites, nereistoxin and sulphuric acid were
identified. The metabolic degradation of cartap hydrochloride in
plants was judged to proceed in the following sequence (Tomizawa and
Endo, 1972; Tomizawa et al., 1974).
Sakai (1970) in discussing nereistoxin and its relatives
indicates that the contact toxicity to Azuki bean weevil, rice stem
borer and mouse, and the activity in blocking the synaptic response of
the 6th abdominal ganglion of the American cockroach are remarkably
similar for cartap hydrochloride and nereistoxin. It seems possible
that the activity of cartap hydrochloride is due to its conversion to
nereistoxin in plants and animals.
In animals
The absorption, distribution and excretion of cartap
hydrochloride in rats and mice is described above ("Bio-chemical
aspects") and further studies are in progress. Chromatographic studies
have revealed that there are about nine main metabolites in the urine
from animals receiving cartap, hydrochloride orally. There appears to
be no significant difference in the pattern between rats and mice.
Unchanged cartap hydrochloride could not be detected. One of the
identified metabolites was the mono-sulphoxide of
1,3-di(methylthio)-2-dimethylaminopropane and it comprised 18% and 8%
of the urinary metabolites in the rat and mouse respectively
(Shirakawa et al, 1971).
In plants
Tomizawa and Endo (1972) studied the uptake of 35S-labelled
cartap hydrochloride in rice plants. The basic fraction which was
assumed to retain biological activity reached a maximum concentration
in the leaf sheath and leaf blade on the 7th day after application and
thereafter declined rapidly until the 15th day. A small but consistent
amount of radio-activity remained in both leaf sheath and leaf blade
for 30 days. Radio-activity in the ear remained consistently low
throughout the test period.
Among the metabolites, nereistoxin and sulphuric acid were
identified. The metabolic degradation of cartap hydrochloride in
plants was judged to proceed in the following sequence (Tomizawa and
Endo, 1972; Tomizawa et al., 1974).
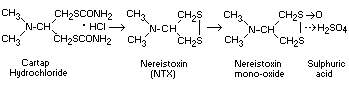 Dust, fine granule and granule formulations of cartap
hydrochloride were applied to rice plants in a paddy field, and the
cartap residue in the plants was determined by analysing the leaf
blades and leaf sheathes separately. Further measurements were also
made on water and soil from the treated plots. The concentration of
cartap in the rice plants immediately after application was found to
be of the order of 1 to 2 mg/kg for dust and fine granule treatments
but only 0.1 mg/kg in the case of granules. 3 days later the
concentration on the whole plants had fallen to approximately 0.05
mg/kg except on those treated with dust which were found to contain
0.2 mg/kg. After 5 days the concentration had fallen slightly in the
case of plants treated with dust and fine granules but plants treated
with granules were found to have a noticeable increase in cartap
residues, apparently owing to uptake from the water into which the
granules had fallen. This trend continued until the 7th day after
which residues began to decline slowly. The leaf blade was found to
have significantly higher residues than the leaf sheath (Koyama et al,
1975). It is difficult to tell how much of the residue determined in
these trials was a surface deposit on the outside of the leaves and
how much was taken into the plant.
Koyama et al (1975), quote work by Sakai which indicates that
when relatively high concentrations of cartap hydrochloride are
applied to rice plants in nursery boxes, the plants are capable of
taking up concentrations of the order of 20 mg/kg which, even after 42
days, remain at about 0.5 mg/kg. Due allowance must be made for the
extensive dilution which would occur owing to the growth of the plant
over this period.
Tomizawa et al (1974), using 35S-labelled cartap hydrochloride
showed that the rate and degree of absorption of cartap hydrochloride
by rice plants was much greater when the insecticide was applied in
the paddy water than when it was applied to the plants in the form of
a spray, and an effective concentration remained in the rice plant for
a longer period. By using ion-exchange chromatography it was possible
to show that the conversion of cartap hydrochloride to nereistoxin
occurred rapidly after application.
In soil
Tomizawa and Endo (1972) studied the movement of cartap
hydrochloride in soil under laboratory conditions. They took alluvial
clay from a paddy field and from an unirrigated field, volcanic ash
and sandy loam and applied radio-active cartap hydrochloride at a
dosage of 1 mg/50 g of soil. The authors do not mention the
temperature at which the treated soil was held but indicate that the
soil from the paddy field had the water content adjusted to 150% of
field capacity while for the other three soils the water content was
adjusted to 75% of field capacity. Samples of soil were removed at 6
intervals over the next 30 days and were extracted in order to
determine the cartap content. The concentration was measured in the
basic fraction extracted with aqueous ammonia. It was found that the
concentration in the soil one day after treatment was of the order of
1 to 2 mg/kg in all four samples. The concentration in the soil from
the paddy field remained virtually constant for 10 days and by the
30th day had declined to one-sixth. The volcanic ash yielded only one
thirtieth and the other two soils about one tenth of the original
concentration by the 30th day.
It is not clear whether the difference in concentration found
after 30 days is due to a difference in the speed of decomposition of
cartap hydrochloride or whether it results from the higher capacity of
volcanic ash to absorb cartap hydrochloride and its decomposition
products. There is a strong indication that cartap hydrochloride acts
as a chelating agent in soil (Takei, 1976). This may explain the low
recoveries reported by Tomizawa and Endo (1972).
Koyama et al (1975) demonstrated that when various cartap
hydrochloride formulations were applied to rice plants growing in
paddy fields there was a distinct transfer of cartap in the case of
granules. There appeared to be an increase in the concentration in the
soil of plots treated with granules over the first 14 days. In the
case of other formulations detectable residues were found in the soil
of all plots at the end of 14 days but at the time of harvest, 41
months after initial treatment, the concentration was below the limit
of determination.
In water
In the experiments with soil and rice plants described above,
Koyama et al (1975) also studied the concentration of cartap
hydrochloride in the water of paddy fields treated with various cartap
hydrochloride formulations. It was found that immediately after
application the concentration of cartap hydrochloride in the water of
paddy fields ranged between 1 and 2 mg/l but by the third day after
treatment it had generally fallen below the limit of determination,
except in the plots treated with granules where it was marginally
above this limit. The apparent difference in the stability of cartap
hydrochloride in water and soil of the same paddy fields seems
attributable to the fact that cartap hydrochloride dissolved in water
is easily degraded by direct sunlight while the residue held in soils
is relatively stable. Cartap hydrochloride is stable in aqueous
solution at pH3-4. In the water of paddy fields which would have a pH
ranging from 5 to 7, it may be decomposed gradually to nereistoxin as
described at the beginning of this section.
Tomizawa and Endo (1972) applied radio-active cartap
hydrochloride to the surface of water in which rice seedlings were
transplanted, at a rate corresponding to 1.5 kg/ha. The radio-activity
of samples of water was measured after various intervals. Although the
concentration in the water sampled immediately after application
indicated the presence of approximately 10 mg of cartap hydrochloride
per litre of water, within the first 24 hours the concentration had
fallen by more than 90% with a further slow decline over the
succeeding days. The loss of the compound from the aqueous phase may
probably be attributable to rapid absorption onto soil particles.
METHODS OF RESIDUE ANALYSIS
Several colorimetric, iodometric, oscillopolarographic and
gaschromatographic methods have been developed for the determination
of cartap. The oscillopolarographic and gaschromatographic methods are
suitable for residue analysis. Both are based on the measurement of
4.N,N-dimethylamino-1,2-dithiolane (nereistoxin, NTXH) which is
quantitatively derived from cartap. Cartap hydrochloride and its
metabolite dihydronereistoxin (NTXH) and nereistoxin are extracted
from samples with diluted hydrochloric acid and the unchanged cartap
hydrochloride is hydrolysed and oxidized in basic solution to NTX.
Total NTX is extracted from the reaction mixture with ethyl ether and
is used for analysis.
Recoveries by both the oscillopolarographic and the
gaschromatographic methods were generally between 75 and 95% (Nishi et
al, 1973). Soil, rice, tea and straw present difficulties in analysis
whereas substrates such as cabbage are easily extracted with good
recovery. Apparently there is a high degree of disulphide binding with
some commodities, because the addition of cystine results in a release
of residues and significantly better recovery (Takei, 1976).
In the oscillopolarographic method, liquid/liquid partition is
required during the clean-up in order to minimise the carry-over of
possible interfering compounds. A single sweep oscillopolarograph
(Randles-Sevcik type) is used. The polarogram of NTX appears at -0.78
±0.05 V vs. Hg pool, and the concentration is quantitatively
determined by the peak height. The limit of determination of this
method is 0.004 mg/kg. Some reducible pesticides, such as
organophosphorus pesticides containing a nitro group, did not
interfere with the determination (Nishi et al, 1973).
Residues of cartap can be determined easily by gaschromatography
with a high sensitivity and specificity. A flame photometric detector
(FPD) equipped with a sulphur mode filter and a glass column
containing 3% OV-1 on chromosorb W or 5% PEG 20 on Gaschrom Q at a
temperature of 130-165°C are used. The limit of determination by this
method is 0.005 mg/kg. Investigation by Nishi et al, (1973) of the
gas-chromatographic method indicates that it should be applicable to
all types of residue analysis. Since the treatment is simple and less
rigorous clean-up is required, the gas-chromatographic method is
superior to the polarographic method. It appears suitable for
regulatory analysis.
Most of the residue data from supervised trials have been
obtained by the gas-chromatographic method, and in the course of this
work almost 100 samples of 12 different commodities have been analyzed
independently by analysis from two of three separate laboratories. In
some cases the results presented by the two analysts differ by
relatively large factors. The percentage difference between the means
obtained by the two analysts does not give a true picture of the
variability. In Table 3 the significance of the difference between
laboratory means has been assessed in relation to the variation
between replicates (a) of pooled over values of <0.050 mg/kg, where
within-pair variability tends to be lowest and (b) pooled over values
between 0.050 and 0.500 mg/kg where this variability is greater. Where
levels above 0.5 mg/kg are found the differences within and between
pairs of duplicates are inconsistent and there are insufficient data
to make a valid comparison of laboratory means.
TABLE 3. Mean differences between samples analysed by 2 laboratories
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Chinese cabbage 0.0275 0.1195 0.092 P<.001
0.0335 0.1085 0.075 P<.001
0.0315 0.1625 0.131 P<.001
0.7060 0.9750 0.269 -
0.0700 0.1445 0.0745 P<.001
Ginger 0.0245 0.0220 0.0025 NS
0.0205 0.0330 0.0125 P<.001
0.0380 0.0220 0.016 P<.001
Grape 0.0765 0.0800 0.0035 NS
0.5345 0.3500 0.1845 P<.001
0.5645 0.7400 0.1755 P<.001
0.1245 0.1800 0.0555 P<.001
Hops 0.9150 2.0900 1.175 -
0.2700 0.3200 0.05 P<.001
4.8200 7.7150 2.895 -
0.2700 0.4950 0.225 P<.001
Japanese 0.0610 0.0935 0.0325 P<.01
radish 0.0520 0.1150 0.063 P<.001
leaf 0.0580 0.1270 0.069 P<.001
root 0.0290 0.0245 0.0045 NS
0.0115 0.0085 0.003 NS
0.0135 0.0160 0.0025 NS
Potato 0.0010 0.0050 0.004 NS
0.0070 0.0050 0.002 NS
Rice grain 0.0050 0.0100 0.005 NS
0.0060 0.0100 0.004 NS
Rice straw 0.0100 0.0200 0.01 P<.01
0.0450 0.0450 0.00 NS
Orchard grass 0.0260 0.0200 0.006 NS
0.0590 0.1500 0.091 P<.001
TABLE 3. (Cont'd.)
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Red clover 0.0095 0.1050 0.0955 P<.001
0.0255 0.1800 0.1545 P<.001
0.1080 0.0200 0.088 P<.001
White clover 0.2150 0.0800 0.135 P<.001
0.4430 0.0450 0.398 P<.001
Least difference in means necessary for significance:
P <0.05 P <0.01 P <0.00
Single estimates <0.050 = 0.0059 0.0080 0.0106
" " >0.050 = 0.0231 0.0311 0.0413
- = insufficient high values to assess significance.
NS = not significant
The results show that in most cases very highly significant
differences (P<0.001) occur between laboratories analysing samples of
the same commodity, while in some cases, notably commodities having
residues below 0.05 mg/kg, the differences are not significant. The
low residues occur mainly in root crops and grains, which would be
expected to be less subject than leafy plants to sampling variations
and therefore to give more consistent analytical results.
It is rarely possible to make such a comparison as this, since
investigators rarely submit samples to two different laboratories for
check analysis. The opportunity to see practical evidence of the
difficulties confronting residue analysts seeking to determine small
quantities of complex substances in a variety of raw agricultural
commodities is valuable. Such data are needed to provide an adequate
basis for the establishment of maximum residue limits appropriate to
the problems facing residue analysts and food control officials.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Brazil
Cotton, sunflower 0.03 mg/kg
Potato, passion fruit 0.01 mg/kg
Cabbage, tomato, wheat 0.01 mg/kg
Hungary
Potato, tomato 0.1 mg/kg
APPRAISAL
The insecticide cartap, invariably used as the hydrochloride, is
a derivative of nereistoxin, a naturally occurring insecticidal
substance isolated from the marine segmented worms, Lumbrineris spp.
Extensive information is available on the chemistry and synthesis of
nereistoxin and its derivatives, of which cartap hydrochloride is one
of the most potent.
Cartap hydrochloride insecticides have been registered in Japan
since 1967, and are widely used in Asia, Europe and South America.
Cartap hydrochloride is used against a relatively broad spectrum of
insects but particularly against the rice stem borer, Lepidopterous
pests of vegetables and Colorado potato beetle. Commercial
formulations include water soluble powders, dusts and granules.
Approximately 70% of the world production is applied to rice. Cartap
hydrochloride is generally used at the rate of 0.5 - 1.5 kg/ha with a
pre-harvest interval of 7 to 21 days.
Extensive data obtained from supervised trials in Japan on a
variety of vegetables, fruit, hops, forage and rice were available to
the Meeting. The compound shows some systemic action and some of the
data indicate an increase in the residue concentration in fruits and
vegetables during the first 7 days. Although the residues resulting
from approved applications are generally well below 1 mg/kg soon after
application, detectable residues remain for up to 45 days.
It appears that the insecticidal activity of cartap hydrochloride
is due to its conversion to nereistoxin in plants and animals. The
metabolism of cartap hydrochloride in mammals has been studied in rats
and mice where it is apparently rapidly converted to nereistoxin which
in turn is further metabolised. No information is available on the
fate in livestock.
Metabolism studies in plants indicate quantitative conversion to
nereistoxin which in turn is oxidised, through the mono-oxide,
eventually to sulphuric acid.
Cartap hydrochloride and nereistoxin are readily degraded in
water by direct sunlight but residues held in soils appear relatively
stable except in highly active soils such as volcanic ash.
Several methods have been developed for the determination of
cartap residues and a gas-chromatographic method appears suitable for
regulatory analysis. The method is based on the conversion of the
parent compound and metabolites to nereistoxin which can be measured
by means of a flame photometric detector equipped with a sulphur mode
filter. The limit of determination is 0.005 mg/kg.
The meeting welcomed the fact that most of the extensive residue
data generated from these supervised trials had been obtained by
independent analysis in two separate laboratories. Although there is
excellent agreement in the results presented for some samples by the
two laboratories there are many instances where the difference between
the means obtained by the two analysts is very highly significant.
This is not altogether remarkable in view of sampling difficulties and
the low levels of the residues analysed. The meeting welcomed this
practical expression of the difficulties confronting residue analysts
seeking to determine small quantities of complex substances in a
variety of raw agricultural commodities.
A number of countries have established maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits are for cartap, expressed as
the free base. (The usual methods of residue analysis convert cartap
to its metabolite nereistoxin, and will therefore record cartap,
nereistoxin and dihydronereistoxin as cartap.)
Pre-harvest Interval
Limit on which Recommendations
Commodity (mg/kg) are based
Hops (dried) 5 14
Chinese cabbage 2 7
Tea (green, dried) 2 7
Grapes 1 14
Persimmons 1 21
Radishes 1 7
Cabbage 0.02 7
Chestnut (seed including 0.01 14
pericarp)
Ginger 0.01 3
Potatoes 0.01 7
Rice (hulled) 0.01 21
Sweet corn 0.01 7
FURTHER WORK OR INFORMATION
Required (by 1978)
1. Submission of the details of the studies on metabolism and the
identification of metabolites.
2. Teratogenic studies covering full period of organogenesis.
3. A feeding study in a non-rodent species.
4. Information on the fate of residues in foods of animal origin
when treated fodder or plant parts are fed to livestock including
poultry.
5. Effect of cooking on the level and fate of cartap residues.
6. Information on the fate of residues in manufactured tea.
Desirable
1. A paired feeding study in the rat.
REFERENCES
Aramaki, Y. Acute oral, dermal and eye toxicity and irritation
1972 studies on cartap hydrochloride and its
formulation, padan water soluble powder. Submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Berczy, Z.S., and Cobb, L. M., Acute inhalation toxicity to the
1973 rat of Padan 50 SP. Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Berczy, Z.S., Cobb, L.M., Street, A.E. and Cherry, C.P. Subacute
1973 inhalation toxicity to the rat of Padan 50 SP.
Report from Huntingdon Research Centre, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Davies, R.E. and Collins, C.J. Acute percutaneous toxicity to
1973 rabbits of TA-7(50 s.p.). Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Davies, R.E., Elliott, P.H., Street, A.E., Heywood, R. and Cherry,
1974 C.P. The effect of repeated applications of TA-7
to the skin of rabbits for three weeks. Report
from Huntingdon Research Centre, submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Hagiwara, H., Numata, M., Konishi, K. and Oka, Y. Synthesis of
1965 nereistoxin and related compounds. I. Chem. Pharm.
Bull., 13, 253-260.
Hagiwara, H. and Numata, M. Synthesis of nereistoxin and related
1968 compounds. II. Chem. Pharm. Bull., 16, 311-317.
Hashimoto, Y. and Okaichi, T. Some chemical properties of
1960 nereistoxin. Ann. N.Y. Acad. Sco., 90, 667-673.
Hiraoka, T. The survey of the health condition in workers engaged
1975 in manufacturing cartap hydrochloride. Report from
Hikari Plant Hospital, submitted by Takeda
Chemical Industries, Ltd. (Unpublished).
Hunter, B., Graham, C., Street, A.E. and Gallagher, P.J. Long
1974 term feeding of TA-7 in mice. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Prentice,
1975 D.E. TA-7, Toxicity following dietary
administration to rats for two years. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Kato, M., Sato, Y. and Sakai, M. Foliage spray treatment of cartap
1967 hydrochloride mixed with DCPA for simultaneous
control of rice stem borer and barnyardgrass.
Japan J. appl. Ent. Zool., 32, 678-679.
Konishi, K. New insecticidally active derivatives of nereistoxin.
1968a Agr. Biol. Chem., 32, 678-679.
Konishi, K. Studies on organic insecticides. Part X. Synthesis
1968b of nereistoxin and related compounds. III. Agr.
Biol. Chem., 1199-1204.
Konishi, K. Studies on organic insecticides. Part XI. Synthesis
1970a of nereistoxin and related compounds. IV. Agr.
Biol. Chem., 34, 926-934.
Konishi, K. Studies on organic insecticides. Part XII. Synthesis
1970b of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 935-940.
Konishi, K. Studies on organic insecticides. Part XIII. Synthesis
1970c of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 1549-1560.
Konishi, K. Nereistoxin and its relatives. Proc. 2nd IUPAC
1971 International Congress of Pesticide Chemistry, 1,
179-189.
Kono, Y., Nagaarashi, C. and Sakai, M. Effects of cartap,
1975 chlorodimeform and diazinon on the probing
frequency of the green rice leafhopper (Hemiptera
deltocephalidae). Appl. Ent. Zool., 10, 58-60.
Koyama, S., Emura, K. and Kojima, A. Residue fate of cartap
1975 hydrochloride applied in paddy field and its
effect against the rice leaf beetle and the rice
stem borer of the first generation. Proc. Assoc.
Plant Prot. Hokuriku (Japan), No. 22, 72-76.
Translated in English.
Mizutani, M., Ihara, T., Kanamori, H., Takatani, O., Matsukawa,
1971 J., Amano, T. and Kaziwara, K. Teratogenesis
studies with cartap hydrochloride in the mouse,
rat and hamster. J. Takeda Res. Lab., 30, 776-785.
Nagawa, Y., Saji, Y., Chiba, S. and Yui, T. Neuromuscular blocking
1971 actions of nereistoxin and its derivatives and
antagonism by sulfhydryl compounds. Japan J.
Pharmacol., 21, 185-197.
Nagawa, Y., Saji, Y. and Kuriki, H. Antidotal effects of sulfhydryl
1970 compounds against intoxication with
1,3-bis(carbamoylthio)-2-N,N-dimethylaminopropane
hydrochloride(NTD-2) in experimental animals. J.
Takeda Res. Lab., 29, 624-629.
Nishi, K., Owaki, R. and Tan, N. Method for determination of
1968 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride. II. Polarography. Ann.
Rept. Takeda Res. Lab., 27, 47-53.
Nishi, K., Konishi, K. and Tan, N. PadanR. in Analytical Methods
1973 for Pesticides and Plant Growth Regulators, G.
Zweig. Ed. - Academic Press N.Y., 7, 371-384.
Nitta, S. Yakugaku Zasshi, 54, 648. Über Nereistoxin, einen
1934 giftigen Bestandteil von Lumbriconereis
heteropoda Marenz (Eunicidae)
Okaichi, T. and Hashimoto, Y. The structure of nereistoxin.
1962 Agr. Biol. Chem., 26, 224-227.
Olson, W.A. and Busey, W.M. Two-generation reproduction study
1972 - rats, cartap. Report from Hazleton Laboratories,
Inc., submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Rivett, K.F., Batham, P., Heywood, R., Street, A.E. and Newman, A.J.
1972 TA-7, oral toxicity to rats dietary administration
for 13 weeks. Report from Huntingdon Research
Centre, submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Sakai, M. Studies on the insecticidal action of nereistoxin.
1964 4-N,N-dimethylamino-1,2-dithiolane. I.
Insecticidal properties. Japan J. appl. Ent.
Zool., 8, 324-333.
Sakai, M., Sato, Y. and Kato, M. Insecticidal activity of
1967 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride, cartap; with special
references to the effectiveness for controlling
the rice stem borer. Japan J. appl. Ent. Zool.,
11, 125-134.
Sakai, M. Nereistoxin and its derivatives; their ganglionic
1970 blocking and insecticidal activity. Biochemical
toxicology of insecticides, 13-20. (Academic Press
Inc.)
Sakai, M. The chemistry and action of cartap. Japan Pestic.
1971 Inform., (6) 15-19.
Sakai, M. and Sato, Y. Metabolic conversion of the nereistoxin
1971 related compounds into nereistoxin as a factor of
their insecticidal action. Insecticides Proc. 2nd
Intern. IUPAC Congr. Pesticide Chemistry, 1,
455-470. (Godon and Breach Sci. Pub. Ltd.)
Shiomi, Y., Sasa, Y. and Takeda, K. Effects of feeding cartap
1973 hydrochloride on laying performance and egg
quality in hens. Submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Shirakawa, Y., Fujita, T., Maki, K. and Suzauki, Z. Metabolic
1971 fate of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane(cartap) in rat and mouse. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Takei, N. Personal communication to FAO Panel of Experts 1976
on Pesticide Residues.
Tan, N., Nishi, K., Owaki, R. and Asahi, Y. Simultaneous analysis
1967 of 2-N,N-dimethylaminopropane-1,3-dithiocyanate
and 4,4-dimethylaminodithiolane by polargraphic
method. Noyaku Seisan Gizyutu (Japan), No. 16,
20-22.
Tomizawa, C. and Endo, T. Movement of cartap hydrochloride in soil,
1972 paddy water and rice plant. Report from National
Institute of Agricultural Sciences (Japan),
submitted by Takeda Chemical Industries, Ltd.
(Unpublished).
Tomizawa, C., Endo, T. and Naka, H. Fate and significance of
1974 labelled insecticide residues in rice. Isotope
Tracer Studies of Chemical Residues in Food and
the Agricultural Environment, 59-64. International
Atomic Energy Agency (Vienna).
Toyoshima, S., Sato, R. and Satoh, H. Acute toxicity of cartap
1972 hydrochloride in rats and mice. Report from Nippon
Experimental Medical Research Institute, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Tsubura, Y., Shimomura, T., Watanabe, T., Tsuji, H., Takahashi,
1976 A. and Fukuyama, T. Toxicity test of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride (cartap) on mice by oral
administration for three months. J. Nara Medical
Association (Japan), 26, 368-378.
Yokotani, H. Approximate estimation of acute oral toxicity of
1968 cartap hydrochloride in monkeys. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Dust, fine granule and granule formulations of cartap
hydrochloride were applied to rice plants in a paddy field, and the
cartap residue in the plants was determined by analysing the leaf
blades and leaf sheathes separately. Further measurements were also
made on water and soil from the treated plots. The concentration of
cartap in the rice plants immediately after application was found to
be of the order of 1 to 2 mg/kg for dust and fine granule treatments
but only 0.1 mg/kg in the case of granules. 3 days later the
concentration on the whole plants had fallen to approximately 0.05
mg/kg except on those treated with dust which were found to contain
0.2 mg/kg. After 5 days the concentration had fallen slightly in the
case of plants treated with dust and fine granules but plants treated
with granules were found to have a noticeable increase in cartap
residues, apparently owing to uptake from the water into which the
granules had fallen. This trend continued until the 7th day after
which residues began to decline slowly. The leaf blade was found to
have significantly higher residues than the leaf sheath (Koyama et al,
1975). It is difficult to tell how much of the residue determined in
these trials was a surface deposit on the outside of the leaves and
how much was taken into the plant.
Koyama et al (1975), quote work by Sakai which indicates that
when relatively high concentrations of cartap hydrochloride are
applied to rice plants in nursery boxes, the plants are capable of
taking up concentrations of the order of 20 mg/kg which, even after 42
days, remain at about 0.5 mg/kg. Due allowance must be made for the
extensive dilution which would occur owing to the growth of the plant
over this period.
Tomizawa et al (1974), using 35S-labelled cartap hydrochloride
showed that the rate and degree of absorption of cartap hydrochloride
by rice plants was much greater when the insecticide was applied in
the paddy water than when it was applied to the plants in the form of
a spray, and an effective concentration remained in the rice plant for
a longer period. By using ion-exchange chromatography it was possible
to show that the conversion of cartap hydrochloride to nereistoxin
occurred rapidly after application.
In soil
Tomizawa and Endo (1972) studied the movement of cartap
hydrochloride in soil under laboratory conditions. They took alluvial
clay from a paddy field and from an unirrigated field, volcanic ash
and sandy loam and applied radio-active cartap hydrochloride at a
dosage of 1 mg/50 g of soil. The authors do not mention the
temperature at which the treated soil was held but indicate that the
soil from the paddy field had the water content adjusted to 150% of
field capacity while for the other three soils the water content was
adjusted to 75% of field capacity. Samples of soil were removed at 6
intervals over the next 30 days and were extracted in order to
determine the cartap content. The concentration was measured in the
basic fraction extracted with aqueous ammonia. It was found that the
concentration in the soil one day after treatment was of the order of
1 to 2 mg/kg in all four samples. The concentration in the soil from
the paddy field remained virtually constant for 10 days and by the
30th day had declined to one-sixth. The volcanic ash yielded only one
thirtieth and the other two soils about one tenth of the original
concentration by the 30th day.
It is not clear whether the difference in concentration found
after 30 days is due to a difference in the speed of decomposition of
cartap hydrochloride or whether it results from the higher capacity of
volcanic ash to absorb cartap hydrochloride and its decomposition
products. There is a strong indication that cartap hydrochloride acts
as a chelating agent in soil (Takei, 1976). This may explain the low
recoveries reported by Tomizawa and Endo (1972).
Koyama et al (1975) demonstrated that when various cartap
hydrochloride formulations were applied to rice plants growing in
paddy fields there was a distinct transfer of cartap in the case of
granules. There appeared to be an increase in the concentration in the
soil of plots treated with granules over the first 14 days. In the
case of other formulations detectable residues were found in the soil
of all plots at the end of 14 days but at the time of harvest, 41
months after initial treatment, the concentration was below the limit
of determination.
In water
In the experiments with soil and rice plants described above,
Koyama et al (1975) also studied the concentration of cartap
hydrochloride in the water of paddy fields treated with various cartap
hydrochloride formulations. It was found that immediately after
application the concentration of cartap hydrochloride in the water of
paddy fields ranged between 1 and 2 mg/l but by the third day after
treatment it had generally fallen below the limit of determination,
except in the plots treated with granules where it was marginally
above this limit. The apparent difference in the stability of cartap
hydrochloride in water and soil of the same paddy fields seems
attributable to the fact that cartap hydrochloride dissolved in water
is easily degraded by direct sunlight while the residue held in soils
is relatively stable. Cartap hydrochloride is stable in aqueous
solution at pH3-4. In the water of paddy fields which would have a pH
ranging from 5 to 7, it may be decomposed gradually to nereistoxin as
described at the beginning of this section.
Tomizawa and Endo (1972) applied radio-active cartap
hydrochloride to the surface of water in which rice seedlings were
transplanted, at a rate corresponding to 1.5 kg/ha. The radio-activity
of samples of water was measured after various intervals. Although the
concentration in the water sampled immediately after application
indicated the presence of approximately 10 mg of cartap hydrochloride
per litre of water, within the first 24 hours the concentration had
fallen by more than 90% with a further slow decline over the
succeeding days. The loss of the compound from the aqueous phase may
probably be attributable to rapid absorption onto soil particles.
METHODS OF RESIDUE ANALYSIS
Several colorimetric, iodometric, oscillopolarographic and
gaschromatographic methods have been developed for the determination
of cartap. The oscillopolarographic and gaschromatographic methods are
suitable for residue analysis. Both are based on the measurement of
4.N,N-dimethylamino-1,2-dithiolane (nereistoxin, NTXH) which is
quantitatively derived from cartap. Cartap hydrochloride and its
metabolite dihydronereistoxin (NTXH) and nereistoxin are extracted
from samples with diluted hydrochloric acid and the unchanged cartap
hydrochloride is hydrolysed and oxidized in basic solution to NTX.
Total NTX is extracted from the reaction mixture with ethyl ether and
is used for analysis.
Recoveries by both the oscillopolarographic and the
gaschromatographic methods were generally between 75 and 95% (Nishi et
al, 1973). Soil, rice, tea and straw present difficulties in analysis
whereas substrates such as cabbage are easily extracted with good
recovery. Apparently there is a high degree of disulphide binding with
some commodities, because the addition of cystine results in a release
of residues and significantly better recovery (Takei, 1976).
In the oscillopolarographic method, liquid/liquid partition is
required during the clean-up in order to minimise the carry-over of
possible interfering compounds. A single sweep oscillopolarograph
(Randles-Sevcik type) is used. The polarogram of NTX appears at -0.78
±0.05 V vs. Hg pool, and the concentration is quantitatively
determined by the peak height. The limit of determination of this
method is 0.004 mg/kg. Some reducible pesticides, such as
organophosphorus pesticides containing a nitro group, did not
interfere with the determination (Nishi et al, 1973).
Residues of cartap can be determined easily by gaschromatography
with a high sensitivity and specificity. A flame photometric detector
(FPD) equipped with a sulphur mode filter and a glass column
containing 3% OV-1 on chromosorb W or 5% PEG 20 on Gaschrom Q at a
temperature of 130-165°C are used. The limit of determination by this
method is 0.005 mg/kg. Investigation by Nishi et al, (1973) of the
gas-chromatographic method indicates that it should be applicable to
all types of residue analysis. Since the treatment is simple and less
rigorous clean-up is required, the gas-chromatographic method is
superior to the polarographic method. It appears suitable for
regulatory analysis.
Most of the residue data from supervised trials have been
obtained by the gas-chromatographic method, and in the course of this
work almost 100 samples of 12 different commodities have been analyzed
independently by analysis from two of three separate laboratories. In
some cases the results presented by the two analysts differ by
relatively large factors. The percentage difference between the means
obtained by the two analysts does not give a true picture of the
variability. In Table 3 the significance of the difference between
laboratory means has been assessed in relation to the variation
between replicates (a) of pooled over values of <0.050 mg/kg, where
within-pair variability tends to be lowest and (b) pooled over values
between 0.050 and 0.500 mg/kg where this variability is greater. Where
levels above 0.5 mg/kg are found the differences within and between
pairs of duplicates are inconsistent and there are insufficient data
to make a valid comparison of laboratory means.
TABLE 3. Mean differences between samples analysed by 2 laboratories
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Chinese cabbage 0.0275 0.1195 0.092 P<.001
0.0335 0.1085 0.075 P<.001
0.0315 0.1625 0.131 P<.001
0.7060 0.9750 0.269 -
0.0700 0.1445 0.0745 P<.001
Ginger 0.0245 0.0220 0.0025 NS
0.0205 0.0330 0.0125 P<.001
0.0380 0.0220 0.016 P<.001
Grape 0.0765 0.0800 0.0035 NS
0.5345 0.3500 0.1845 P<.001
0.5645 0.7400 0.1755 P<.001
0.1245 0.1800 0.0555 P<.001
Hops 0.9150 2.0900 1.175 -
0.2700 0.3200 0.05 P<.001
4.8200 7.7150 2.895 -
0.2700 0.4950 0.225 P<.001
Japanese 0.0610 0.0935 0.0325 P<.01
radish 0.0520 0.1150 0.063 P<.001
leaf 0.0580 0.1270 0.069 P<.001
root 0.0290 0.0245 0.0045 NS
0.0115 0.0085 0.003 NS
0.0135 0.0160 0.0025 NS
Potato 0.0010 0.0050 0.004 NS
0.0070 0.0050 0.002 NS
Rice grain 0.0050 0.0100 0.005 NS
0.0060 0.0100 0.004 NS
Rice straw 0.0100 0.0200 0.01 P<.01
0.0450 0.0450 0.00 NS
Orchard grass 0.0260 0.0200 0.006 NS
0.0590 0.1500 0.091 P<.001
TABLE 3. (Cont'd.)
Laboratory 1 Lab. 2 Mean Significance
Mean Mean difference
Red clover 0.0095 0.1050 0.0955 P<.001
0.0255 0.1800 0.1545 P<.001
0.1080 0.0200 0.088 P<.001
White clover 0.2150 0.0800 0.135 P<.001
0.4430 0.0450 0.398 P<.001
Least difference in means necessary for significance:
P <0.05 P <0.01 P <0.00
Single estimates <0.050 = 0.0059 0.0080 0.0106
" " >0.050 = 0.0231 0.0311 0.0413
- = insufficient high values to assess significance.
NS = not significant
The results show that in most cases very highly significant
differences (P<0.001) occur between laboratories analysing samples of
the same commodity, while in some cases, notably commodities having
residues below 0.05 mg/kg, the differences are not significant. The
low residues occur mainly in root crops and grains, which would be
expected to be less subject than leafy plants to sampling variations
and therefore to give more consistent analytical results.
It is rarely possible to make such a comparison as this, since
investigators rarely submit samples to two different laboratories for
check analysis. The opportunity to see practical evidence of the
difficulties confronting residue analysts seeking to determine small
quantities of complex substances in a variety of raw agricultural
commodities is valuable. Such data are needed to provide an adequate
basis for the establishment of maximum residue limits appropriate to
the problems facing residue analysts and food control officials.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Brazil
Cotton, sunflower 0.03 mg/kg
Potato, passion fruit 0.01 mg/kg
Cabbage, tomato, wheat 0.01 mg/kg
Hungary
Potato, tomato 0.1 mg/kg
APPRAISAL
The insecticide cartap, invariably used as the hydrochloride, is
a derivative of nereistoxin, a naturally occurring insecticidal
substance isolated from the marine segmented worms, Lumbrineris spp.
Extensive information is available on the chemistry and synthesis of
nereistoxin and its derivatives, of which cartap hydrochloride is one
of the most potent.
Cartap hydrochloride insecticides have been registered in Japan
since 1967, and are widely used in Asia, Europe and South America.
Cartap hydrochloride is used against a relatively broad spectrum of
insects but particularly against the rice stem borer, Lepidopterous
pests of vegetables and Colorado potato beetle. Commercial
formulations include water soluble powders, dusts and granules.
Approximately 70% of the world production is applied to rice. Cartap
hydrochloride is generally used at the rate of 0.5 - 1.5 kg/ha with a
pre-harvest interval of 7 to 21 days.
Extensive data obtained from supervised trials in Japan on a
variety of vegetables, fruit, hops, forage and rice were available to
the Meeting. The compound shows some systemic action and some of the
data indicate an increase in the residue concentration in fruits and
vegetables during the first 7 days. Although the residues resulting
from approved applications are generally well below 1 mg/kg soon after
application, detectable residues remain for up to 45 days.
It appears that the insecticidal activity of cartap hydrochloride
is due to its conversion to nereistoxin in plants and animals. The
metabolism of cartap hydrochloride in mammals has been studied in rats
and mice where it is apparently rapidly converted to nereistoxin which
in turn is further metabolised. No information is available on the
fate in livestock.
Metabolism studies in plants indicate quantitative conversion to
nereistoxin which in turn is oxidised, through the mono-oxide,
eventually to sulphuric acid.
Cartap hydrochloride and nereistoxin are readily degraded in
water by direct sunlight but residues held in soils appear relatively
stable except in highly active soils such as volcanic ash.
Several methods have been developed for the determination of
cartap residues and a gas-chromatographic method appears suitable for
regulatory analysis. The method is based on the conversion of the
parent compound and metabolites to nereistoxin which can be measured
by means of a flame photometric detector equipped with a sulphur mode
filter. The limit of determination is 0.005 mg/kg.
The meeting welcomed the fact that most of the extensive residue
data generated from these supervised trials had been obtained by
independent analysis in two separate laboratories. Although there is
excellent agreement in the results presented for some samples by the
two laboratories there are many instances where the difference between
the means obtained by the two analysts is very highly significant.
This is not altogether remarkable in view of sampling difficulties and
the low levels of the residues analysed. The meeting welcomed this
practical expression of the difficulties confronting residue analysts
seeking to determine small quantities of complex substances in a
variety of raw agricultural commodities.
A number of countries have established maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits are for cartap, expressed as
the free base. (The usual methods of residue analysis convert cartap
to its metabolite nereistoxin, and will therefore record cartap,
nereistoxin and dihydronereistoxin as cartap.)
Pre-harvest Interval
Limit on which Recommendations
Commodity (mg/kg) are based
Hops (dried) 5 14
Chinese cabbage 2 7
Tea (green, dried) 2 7
Grapes 1 14
Persimmons 1 21
Radishes 1 7
Cabbage 0.02 7
Chestnut (seed including 0.01 14
pericarp)
Ginger 0.01 3
Potatoes 0.01 7
Rice (hulled) 0.01 21
Sweet corn 0.01 7
FURTHER WORK OR INFORMATION
Required (by 1978)
1. Submission of the details of the studies on metabolism and the
identification of metabolites.
2. Teratogenic studies covering full period of organogenesis.
3. A feeding study in a non-rodent species.
4. Information on the fate of residues in foods of animal origin
when treated fodder or plant parts are fed to livestock including
poultry.
5. Effect of cooking on the level and fate of cartap residues.
6. Information on the fate of residues in manufactured tea.
Desirable
1. A paired feeding study in the rat.
REFERENCES
Aramaki, Y. Acute oral, dermal and eye toxicity and irritation
1972 studies on cartap hydrochloride and its
formulation, padan water soluble powder. Submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Berczy, Z.S., and Cobb, L. M., Acute inhalation toxicity to the
1973 rat of Padan 50 SP. Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Berczy, Z.S., Cobb, L.M., Street, A.E. and Cherry, C.P. Subacute
1973 inhalation toxicity to the rat of Padan 50 SP.
Report from Huntingdon Research Centre, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Davies, R.E. and Collins, C.J. Acute percutaneous toxicity to
1973 rabbits of TA-7(50 s.p.). Report from Huntingdon
Research Centre, submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Davies, R.E., Elliott, P.H., Street, A.E., Heywood, R. and Cherry,
1974 C.P. The effect of repeated applications of TA-7
to the skin of rabbits for three weeks. Report
from Huntingdon Research Centre, submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Hagiwara, H., Numata, M., Konishi, K. and Oka, Y. Synthesis of
1965 nereistoxin and related compounds. I. Chem. Pharm.
Bull., 13, 253-260.
Hagiwara, H. and Numata, M. Synthesis of nereistoxin and related
1968 compounds. II. Chem. Pharm. Bull., 16, 311-317.
Hashimoto, Y. and Okaichi, T. Some chemical properties of
1960 nereistoxin. Ann. N.Y. Acad. Sco., 90, 667-673.
Hiraoka, T. The survey of the health condition in workers engaged
1975 in manufacturing cartap hydrochloride. Report from
Hikari Plant Hospital, submitted by Takeda
Chemical Industries, Ltd. (Unpublished).
Hunter, B., Graham, C., Street, A.E. and Gallagher, P.J. Long
1974 term feeding of TA-7 in mice. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Prentice,
1975 D.E. TA-7, Toxicity following dietary
administration to rats for two years. Report from
Huntingdon Research Centre, submitted by Takeda
Chemical Industries, Ltd., (Unpublished).
Kato, M., Sato, Y. and Sakai, M. Foliage spray treatment of cartap
1967 hydrochloride mixed with DCPA for simultaneous
control of rice stem borer and barnyardgrass.
Japan J. appl. Ent. Zool., 32, 678-679.
Konishi, K. New insecticidally active derivatives of nereistoxin.
1968a Agr. Biol. Chem., 32, 678-679.
Konishi, K. Studies on organic insecticides. Part X. Synthesis
1968b of nereistoxin and related compounds. III. Agr.
Biol. Chem., 1199-1204.
Konishi, K. Studies on organic insecticides. Part XI. Synthesis
1970a of nereistoxin and related compounds. IV. Agr.
Biol. Chem., 34, 926-934.
Konishi, K. Studies on organic insecticides. Part XII. Synthesis
1970b of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 935-940.
Konishi, K. Studies on organic insecticides. Part XIII. Synthesis
1970c of nereistoxin and related compounds. VI. Agr.
Biol. Chem., 34, 1549-1560.
Konishi, K. Nereistoxin and its relatives. Proc. 2nd IUPAC
1971 International Congress of Pesticide Chemistry, 1,
179-189.
Kono, Y., Nagaarashi, C. and Sakai, M. Effects of cartap,
1975 chlorodimeform and diazinon on the probing
frequency of the green rice leafhopper (Hemiptera
deltocephalidae). Appl. Ent. Zool., 10, 58-60.
Koyama, S., Emura, K. and Kojima, A. Residue fate of cartap
1975 hydrochloride applied in paddy field and its
effect against the rice leaf beetle and the rice
stem borer of the first generation. Proc. Assoc.
Plant Prot. Hokuriku (Japan), No. 22, 72-76.
Translated in English.
Mizutani, M., Ihara, T., Kanamori, H., Takatani, O., Matsukawa,
1971 J., Amano, T. and Kaziwara, K. Teratogenesis
studies with cartap hydrochloride in the mouse,
rat and hamster. J. Takeda Res. Lab., 30, 776-785.
Nagawa, Y., Saji, Y., Chiba, S. and Yui, T. Neuromuscular blocking
1971 actions of nereistoxin and its derivatives and
antagonism by sulfhydryl compounds. Japan J.
Pharmacol., 21, 185-197.
Nagawa, Y., Saji, Y. and Kuriki, H. Antidotal effects of sulfhydryl
1970 compounds against intoxication with
1,3-bis(carbamoylthio)-2-N,N-dimethylaminopropane
hydrochloride(NTD-2) in experimental animals. J.
Takeda Res. Lab., 29, 624-629.
Nishi, K., Owaki, R. and Tan, N. Method for determination of
1968 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride. II. Polarography. Ann.
Rept. Takeda Res. Lab., 27, 47-53.
Nishi, K., Konishi, K. and Tan, N. PadanR. in Analytical Methods
1973 for Pesticides and Plant Growth Regulators, G.
Zweig. Ed. - Academic Press N.Y., 7, 371-384.
Nitta, S. Yakugaku Zasshi, 54, 648. Über Nereistoxin, einen
1934 giftigen Bestandteil von Lumbriconereis
heteropoda Marenz (Eunicidae)
Okaichi, T. and Hashimoto, Y. The structure of nereistoxin.
1962 Agr. Biol. Chem., 26, 224-227.
Olson, W.A. and Busey, W.M. Two-generation reproduction study
1972 - rats, cartap. Report from Hazleton Laboratories,
Inc., submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Rivett, K.F., Batham, P., Heywood, R., Street, A.E. and Newman, A.J.
1972 TA-7, oral toxicity to rats dietary administration
for 13 weeks. Report from Huntingdon Research
Centre, submitted by Takeda Chemical Industries,
Ltd. (Unpublished).
Sakai, M. Studies on the insecticidal action of nereistoxin.
1964 4-N,N-dimethylamino-1,2-dithiolane. I.
Insecticidal properties. Japan J. appl. Ent.
Zool., 8, 324-333.
Sakai, M., Sato, Y. and Kato, M. Insecticidal activity of
1967 1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride, cartap; with special
references to the effectiveness for controlling
the rice stem borer. Japan J. appl. Ent. Zool.,
11, 125-134.
Sakai, M. Nereistoxin and its derivatives; their ganglionic
1970 blocking and insecticidal activity. Biochemical
toxicology of insecticides, 13-20. (Academic Press
Inc.)
Sakai, M. The chemistry and action of cartap. Japan Pestic.
1971 Inform., (6) 15-19.
Sakai, M. and Sato, Y. Metabolic conversion of the nereistoxin
1971 related compounds into nereistoxin as a factor of
their insecticidal action. Insecticides Proc. 2nd
Intern. IUPAC Congr. Pesticide Chemistry, 1,
455-470. (Godon and Breach Sci. Pub. Ltd.)
Shiomi, Y., Sasa, Y. and Takeda, K. Effects of feeding cartap
1973 hydrochloride on laying performance and egg
quality in hens. Submitted by Takeda Chemical
Industries, Ltd. (Unpublished).
Shirakawa, Y., Fujita, T., Maki, K. and Suzauki, Z. Metabolic
1971 fate of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane(cartap) in rat and mouse. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
Takei, N. Personal communication to FAO Panel of Experts 1976
on Pesticide Residues.
Tan, N., Nishi, K., Owaki, R. and Asahi, Y. Simultaneous analysis
1967 of 2-N,N-dimethylaminopropane-1,3-dithiocyanate
and 4,4-dimethylaminodithiolane by polargraphic
method. Noyaku Seisan Gizyutu (Japan), No. 16,
20-22.
Tomizawa, C. and Endo, T. Movement of cartap hydrochloride in soil,
1972 paddy water and rice plant. Report from National
Institute of Agricultural Sciences (Japan),
submitted by Takeda Chemical Industries, Ltd.
(Unpublished).
Tomizawa, C., Endo, T. and Naka, H. Fate and significance of
1974 labelled insecticide residues in rice. Isotope
Tracer Studies of Chemical Residues in Food and
the Agricultural Environment, 59-64. International
Atomic Energy Agency (Vienna).
Toyoshima, S., Sato, R. and Satoh, H. Acute toxicity of cartap
1972 hydrochloride in rats and mice. Report from Nippon
Experimental Medical Research Institute, submitted
by Takeda Chemical Industries, Ltd. (Unpublished).
Tsubura, Y., Shimomura, T., Watanabe, T., Tsuji, H., Takahashi,
1976 A. and Fukuyama, T. Toxicity test of
1,3-bis(carbamoylthio)-2-(N,N-dimethylamino)
propane hydrochloride (cartap) on mice by oral
administration for three months. J. Nara Medical
Association (Japan), 26, 368-378.
Yokotani, H. Approximate estimation of acute oral toxicity of
1968 cartap hydrochloride in monkeys. Submitted by
Takeda Chemical Industries, Ltd. (Unpublished).
