
ETHIOFENCARB JMPR 1977
IDENTITY
Chemical name
2-ethylthiomethylphenyl methylcarbamate
Synonyms
CronetonR 1 HOX 1901
Structural formula
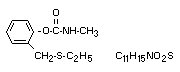 Other information on identity and properties
Molecular weight: 225
Specific gravity: 1.147 at 20° C
4°
Appearance: colourless crystals (pure a.i.)
Melting point: 33.4° C (pure a.i.)
Boiling point: decomposes
Vapour pressure: 5 × 10-6 mbar at 20° C
Solubility (pure): in water (20° C): approx. 0.19 g/100 g
in methylene chloride,)
propan-2-ol ) >60 g/100 g
and toluene: )
in ligroin (80-110° C): 1-5 g/100 g
Formulations: 10% Granular, 10% and 50% emulsifiable
concentrates
Minimum purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical ethiofencarb was
reported to the Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Studies on these points have been conducted with samples of
ethiofencarb labelled with 14C either in the ring or in the carbonyl
carbon. These studies have been described in several reports, all
issued during 1976-77. These experiments have demonstrated that
regardless of the position labelled the compound is readily absorbed
by the oral route, and is rapidly eliminated thereafter.
When administered as a single oral dose to rats, 41% of the
carbonyl-labelled compound was eliminated in the urine and 7% in the
faeces (within 72 hr), while 47% was eliminated as 14CO2. When
similarly administered but in ring-labelled form, 96% was eliminated
in the urine and 2% in the faeces within 72 hr. The compound,
therefore, undergoes hydrolysis, producing a phenol and a carbamic
acid derivative which breaks down further to CO2.
Maintenance of rats on a diet containing 6.6 ppm of ethiofencarb gave
similar results with the total excretion (urine plus faeces)
decreasing to 1% of one day's intake by the third day after return of
the animals to the normal diet. The major metabolites identified were
the sulphoxide and sulphone of ethiofencarb and the sulphoxide and
sulphone of the phenol; the latter two compounds were excreted mainly
as conjugates, and the parent compound was detected in the urine only
in trace amounts. Tissue residues detected after either a single oral
dose of 0.5 mg/kg b w or administration of 6.6 ppm in the diet for one
week were below 1 mg/kg, and these declined more rapidly after
administration of the ring-labelled than the carbonyl-labelled
compound. This suggests that the carbonyl-labelled residues were
incorporated into normal tissues components, as would be expected (Nye
et al., 1976).
Experiments bearing on the metabolism of ethiofencarb by livestock and
poultry and of some of its plant metabolites by rats, are described in
the section "Fate of residues", "In animals". Metabolic pathways are
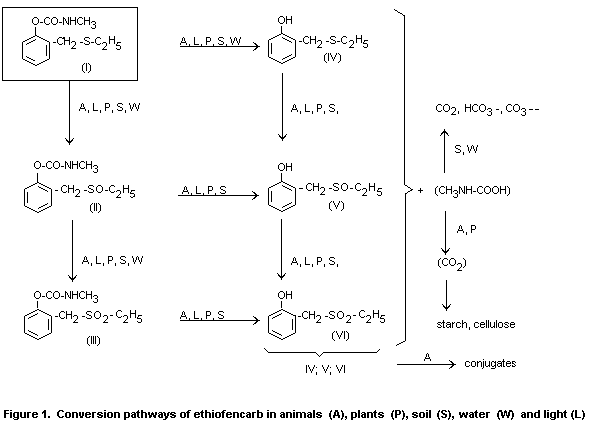
illustrated in Figure 1.
Effects on enzymes and other biochemical parameters
The depression of acetylcholinesterase activity in plasma,
erythrocytes and brain was investigated in rats and dogs (Kimmerle and
Eben, 1974).
Rats were treated with single oral doses of 0, 2.5, 10 and 50 mg/kg
and 0, 2.5, 10, 40, 125, 210 and 330 mg/kg for females and males
respectively. The maximum dose-related level of plasma cholinesterase
activity depression of 60% in females treated with 50 mg/kg and of
80-100% in males treated with 330 mg/kg was reached about 0.5 to 5 hours
after administration. Enzyme activity increased again thereafter. Two
males of the 330 mg/kg group died one hour after treatment.
Cholinesterase activity depression was lower in erythrocytes and brain
than in plasma. Maximum depression levels were reached after about 2
hours in erythrocytes and after about 1 hour in the brain.
In a 4-week oral study male and female rats received the test compound
daily at a rate of 0, 5, 10, 20 and 40 mg/kg. Dose-related depression
of plasma- and erythrocyte acetylcholinesterase activity was found in
the animals treated with 20 and 40 mg/kg. Male rats showed within 2
hours after administration of 10 mg/kg bw a depression of
plasma-cholinesterase of 25% and of erythrocyte-cholinesterase of 15%.
In male and female rats which were exposed for a period of 3 weeks to
concentrations of 0, 5.0, 29.7 and 148.4 mg/m3 of air for 6 hours on
5 consecutive days per week the concentrations of 29.7 mg/m3 and
above caused depression of the plasma cholinesterase, whereas
erythrocyte cholinesterase activity was depressed only at the highest
concentration level.
Following single oral administration of 5, 10, 25 and 50 mg/kg,
dose-dependent depression of the cholinesterase activity was found in
plasma at dose levels of 10 and above. Maximum depression was found
1 - 2 hours after administration. 3 hours post-administration the
activity increased again (Kimmerle and Eden, 1974).
TOXICOLOGICAL STUDIES
Special study on mutagenicity
In a dominant-lethal test, groups of 20 NMRI male mice were treated
with a single oral dose of 0 and 25 mg/kg. The male mice were mated
with untreated females for 8 consecutive weeks. The acute oral dose of
25 mg/kg was a sublethal dose causing transient toxicity symptoms such
as slight somnolence and piloerection. The application did not
influence the fertility of the male rats. This experiment gave no
indication of treatment-related pre- and post-implantation losses and
therefore gave no indication of a mutagenic effect of the test
substance (Machemer, 1973a).
Special study on neurotoxicity
A group of 30 atropinized hens was treated with a single oral LD50
dose of 870 mg/kg. During the 3-week observation period only one hen
died. After 3 weeks the survivors were atropinized again and re-dosed
with 870 mg/kg. Three other animals died 3 weeks after treatment. No
neurotoxic symptoms wore observed. The histopathological investigation
of the brain, spinal marrow and Nn. ischiadici gave no indication of
nerve damage, whereas the positive control animals which had been
treated with a single oral dose of 375 mg/kg tri-o-cresyl phosphate
(TOCP) showed evidence of delayed neurotoxicity with respect to
clinical symptoms as well as histopathology.
The test compound did not show any neurotoxic effect (Thyseen, 1975a).
Special study on teratogenicity
Rats
Groups of 20 female rats were treated with daily oral doses of 0, 5,
15 and 40 mg/kg from gestation day 6 to 15. None of the studied dose
levels had any adverse effect on the dams with respect to their health
condition and fertility. The embryonic and foetal development was not
affected by the treatment up to and including the dose level of 15
mg/kg. Treatment of the dams with 40 mg/kg caused reduction of the
average foetus weight. Two foetuses of dams treated with 40 mg/kg
showed multiple malformations, which are considered to be spontaneous
malformations of the strain. In a repeat experiment these
malformations could not be verified. Signs of immature skeletal
development in some foetuses and a higher frequency of slight bone
alterations of the 40 mg/kg group in comparison with controls were
found in the repeat experiment.
Based on these data the test compound was considered not to be
embryo-toxic or teratogenic (Machemer, 1973b).
Groups of 10 pregnant rabbits received daily oral doses of 0, 5, 15
and 40 mg/kg from gestation day 6 through 18. The administration did
not have any adverse effects on the dose. No significant differences
between the control group and treated groups with respect to embryonic
and foetal development could be found. The only malformed foetus
occurring in the 40 mg/kg group was considered to be a spontaneous
malformation. Therefore the test-compound at doses up to and including
40 mg/kg was considered not to have teratogenic effects (Machemer,
1975).
Special study on potentiation
The simultaneous administration of ethiofencarb with either malathion
or EPN to rats caused no potentiation effects on the acute oral
toxicity (Thysson, 1975b).
Simultaneous oral administration of ethiofencarb in combination with
trichlorfon produced likewise only a less than additive acute toxicity
(Thyssen and Kimmerle, 1975).
Other information on identity and properties
Molecular weight: 225
Specific gravity: 1.147 at 20° C
4°
Appearance: colourless crystals (pure a.i.)
Melting point: 33.4° C (pure a.i.)
Boiling point: decomposes
Vapour pressure: 5 × 10-6 mbar at 20° C
Solubility (pure): in water (20° C): approx. 0.19 g/100 g
in methylene chloride,)
propan-2-ol ) >60 g/100 g
and toluene: )
in ligroin (80-110° C): 1-5 g/100 g
Formulations: 10% Granular, 10% and 50% emulsifiable
concentrates
Minimum purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical ethiofencarb was
reported to the Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Studies on these points have been conducted with samples of
ethiofencarb labelled with 14C either in the ring or in the carbonyl
carbon. These studies have been described in several reports, all
issued during 1976-77. These experiments have demonstrated that
regardless of the position labelled the compound is readily absorbed
by the oral route, and is rapidly eliminated thereafter.
When administered as a single oral dose to rats, 41% of the
carbonyl-labelled compound was eliminated in the urine and 7% in the
faeces (within 72 hr), while 47% was eliminated as 14CO2. When
similarly administered but in ring-labelled form, 96% was eliminated
in the urine and 2% in the faeces within 72 hr. The compound,
therefore, undergoes hydrolysis, producing a phenol and a carbamic
acid derivative which breaks down further to CO2.
Maintenance of rats on a diet containing 6.6 ppm of ethiofencarb gave
similar results with the total excretion (urine plus faeces)
decreasing to 1% of one day's intake by the third day after return of
the animals to the normal diet. The major metabolites identified were
the sulphoxide and sulphone of ethiofencarb and the sulphoxide and
sulphone of the phenol; the latter two compounds were excreted mainly
as conjugates, and the parent compound was detected in the urine only
in trace amounts. Tissue residues detected after either a single oral
dose of 0.5 mg/kg b w or administration of 6.6 ppm in the diet for one
week were below 1 mg/kg, and these declined more rapidly after
administration of the ring-labelled than the carbonyl-labelled
compound. This suggests that the carbonyl-labelled residues were
incorporated into normal tissues components, as would be expected (Nye
et al., 1976).
Experiments bearing on the metabolism of ethiofencarb by livestock and
poultry and of some of its plant metabolites by rats, are described in
the section "Fate of residues", "In animals". Metabolic pathways are
illustrated in Figure 1.
Effects on enzymes and other biochemical parameters
The depression of acetylcholinesterase activity in plasma,
erythrocytes and brain was investigated in rats and dogs (Kimmerle and
Eben, 1974).
Rats were treated with single oral doses of 0, 2.5, 10 and 50 mg/kg
and 0, 2.5, 10, 40, 125, 210 and 330 mg/kg for females and males
respectively. The maximum dose-related level of plasma cholinesterase
activity depression of 60% in females treated with 50 mg/kg and of
80-100% in males treated with 330 mg/kg was reached about 0.5 to 5 hours
after administration. Enzyme activity increased again thereafter. Two
males of the 330 mg/kg group died one hour after treatment.
Cholinesterase activity depression was lower in erythrocytes and brain
than in plasma. Maximum depression levels were reached after about 2
hours in erythrocytes and after about 1 hour in the brain.
In a 4-week oral study male and female rats received the test compound
daily at a rate of 0, 5, 10, 20 and 40 mg/kg. Dose-related depression
of plasma- and erythrocyte acetylcholinesterase activity was found in
the animals treated with 20 and 40 mg/kg. Male rats showed within 2
hours after administration of 10 mg/kg bw a depression of
plasma-cholinesterase of 25% and of erythrocyte-cholinesterase of 15%.
In male and female rats which were exposed for a period of 3 weeks to
concentrations of 0, 5.0, 29.7 and 148.4 mg/m3 of air for 6 hours on
5 consecutive days per week the concentrations of 29.7 mg/m3 and
above caused depression of the plasma cholinesterase, whereas
erythrocyte cholinesterase activity was depressed only at the highest
concentration level.
Following single oral administration of 5, 10, 25 and 50 mg/kg,
dose-dependent depression of the cholinesterase activity was found in
plasma at dose levels of 10 and above. Maximum depression was found
1 - 2 hours after administration. 3 hours post-administration the
activity increased again (Kimmerle and Eden, 1974).
TOXICOLOGICAL STUDIES
Special study on mutagenicity
In a dominant-lethal test, groups of 20 NMRI male mice were treated
with a single oral dose of 0 and 25 mg/kg. The male mice were mated
with untreated females for 8 consecutive weeks. The acute oral dose of
25 mg/kg was a sublethal dose causing transient toxicity symptoms such
as slight somnolence and piloerection. The application did not
influence the fertility of the male rats. This experiment gave no
indication of treatment-related pre- and post-implantation losses and
therefore gave no indication of a mutagenic effect of the test
substance (Machemer, 1973a).
Special study on neurotoxicity
A group of 30 atropinized hens was treated with a single oral LD50
dose of 870 mg/kg. During the 3-week observation period only one hen
died. After 3 weeks the survivors were atropinized again and re-dosed
with 870 mg/kg. Three other animals died 3 weeks after treatment. No
neurotoxic symptoms wore observed. The histopathological investigation
of the brain, spinal marrow and Nn. ischiadici gave no indication of
nerve damage, whereas the positive control animals which had been
treated with a single oral dose of 375 mg/kg tri-o-cresyl phosphate
(TOCP) showed evidence of delayed neurotoxicity with respect to
clinical symptoms as well as histopathology.
The test compound did not show any neurotoxic effect (Thyseen, 1975a).
Special study on teratogenicity
Rats
Groups of 20 female rats were treated with daily oral doses of 0, 5,
15 and 40 mg/kg from gestation day 6 to 15. None of the studied dose
levels had any adverse effect on the dams with respect to their health
condition and fertility. The embryonic and foetal development was not
affected by the treatment up to and including the dose level of 15
mg/kg. Treatment of the dams with 40 mg/kg caused reduction of the
average foetus weight. Two foetuses of dams treated with 40 mg/kg
showed multiple malformations, which are considered to be spontaneous
malformations of the strain. In a repeat experiment these
malformations could not be verified. Signs of immature skeletal
development in some foetuses and a higher frequency of slight bone
alterations of the 40 mg/kg group in comparison with controls were
found in the repeat experiment.
Based on these data the test compound was considered not to be
embryo-toxic or teratogenic (Machemer, 1973b).
Groups of 10 pregnant rabbits received daily oral doses of 0, 5, 15
and 40 mg/kg from gestation day 6 through 18. The administration did
not have any adverse effects on the dose. No significant differences
between the control group and treated groups with respect to embryonic
and foetal development could be found. The only malformed foetus
occurring in the 40 mg/kg group was considered to be a spontaneous
malformation. Therefore the test-compound at doses up to and including
40 mg/kg was considered not to have teratogenic effects (Machemer,
1975).
Special study on potentiation
The simultaneous administration of ethiofencarb with either malathion
or EPN to rats caused no potentiation effects on the acute oral
toxicity (Thysson, 1975b).
Simultaneous oral administration of ethiofencarb in combination with
trichlorfon produced likewise only a less than additive acute toxicity
(Thyssen and Kimmerle, 1975).
 Acute toxicity
TABLE 1a, Acute toxicity of ethiofencarb
Species Sex Route LD50 References
mg/kg
Rat m oral 308 Thyssen and Kimmerle, 1975
Rat m oral 411 Kimmerle 1972b
Rat f oral 499 ibid.
Rat m i.p. 41.8 ibid.
Rat f i.p. 41.9 ibid.
Rat m dermal >1000 ibid.
Mouse m oral 256 ibid.
Mouse f oral 224 ibid.
Rabbit m oral approx. 225 Kimmerle 1972c
Rabbit m dermal >8000 Lamb and Matzkanin 1976a
Rabbit f dermal >8000 ibid.
Dog f oral >50 Kimmerle 1972b
Hen oral approx. 1000 ibid.
The typical symptoms of acetylcholinocaterase inhibition such as
salivation, muscle tremors, spasms and convulsions preceded death.
A good antidotal effect was obtained when rats orally treated with
ethiofencarb received i.p. injection of atropine sulphate (Kimmerle
1972d).
Short term studies
Rat
Groups of 15 male and 15 female rats were treated orally for 30 days
at dose levels of 0, 5, 10, 20 and 40 mg/kg. The administration had no
effects with respect to physical appearance, behaviour, body weight,
haematological and clinical chemistry test values. No gross
pathological alterations of tissues were observed and organ weights
did not significantly differ from those of the control animals.
Tissues were not examined microscopically (Kimmerle, 1972e).
In another subacute study 10 male and 10 female rats per dosage group
were exposed for 6 hours daily on 5 consecutive days per week over a
3-week period to aerosol concentrations of 0, 29.7 and 148.5 mg/m3
of air. All these concentrations were tolerated without any adverse
effect with respect to behaviour, general health condition, body
weight, haematological and clinical chemistry values.
The macroscopic examination of the major tissues revealed no
pathological alteration and organ weights did not differ significantly
between the control group and the treated groups (Kimmerle, 1972e).
Groups of 20 male and 20 female rats were fed ethiofencarb at
concentrations of O, 250, 500 and 1000 ppm for 3 months. Physical
appearance, behaviour, mortality and food consumption were not
affected by the diet. The average body weights of male rats fed 500
and 1000 ppm were higher than the corresponding weights of the control
animals. Values of urinalyses, haematology and clinical chemistry were
within the physiological range. Macroscopic and microscopic
examination of the principal organs revealed no treatment-related
alterations. The relative liver weights in male rats treated with 1000
ppm were higher than those of the control animals (Klimmer, 1973).
Dog
Groups of 4 male and 4 female dogs were maintained for 3 months on a
diet containing ethiofencarb at concentrations of 0, 100, 300 and 1000
ppm. The treatment did not effect physical appearance, behaviour, food
consumption, body weight, mortality or ophthalmoscopic and
neurological findings. Parameters of haematology, clinical chemistry
and urinalyses showed no significant differences between control and
treated groups. At 1000 ppm an increase of the relative liver weights
of about 10% was noted in both sexes and an increase of about 40% in
relative kidney and spleen weights in male animals only. Gross and
histopathological examination showed no treatment-related alteration
(Mührmann, 1973).
Groups of 4 male and 4 female dogs received a diet for a period of 104
weeks containing ethiofencarb at concentrations of 0, 330, 1000 and
3000 ppm. The administration of up to and including 1000 ppm did not
affect the test animals with respect to their physical appearance,
behaviour, food consumption and ophthalmoscopic findings. The dietary
level of 3000 ppm caused vomiting immediately after feeding,
especially in the second half of the study; food consumption was
retarded and reduced in some female dogs at the 3000 ppm level,
whereas female dogs at 330 and 1000 ppm made bigger gains in
comparison with controls. Haematological and urinalyses values
revealed no pathological alterations. Results of chemistry tests (with
respect to blood sugar, urea, creatinine, total protein, GOT,
bilirubin) were within the physiological range. The ALP activities
showed a 3 -4 fold increase at 3000 ppm and in some dogs of the same
group an increased level of GPT activity and cholesterol were noted.
Relative liver weights of the animals of both sexes at 1000 and 3000
ppm showed a dose-related increase. One dog at the 3000 ppm feeding
level showed enlargement of the liver. At 3000 ppm the
histopathological examination of tissues disclosed signs of treatment-
related alterations in liver such as enlarged hepatocytes, hepatocytes
nuclei and granular cytoplasmic structures (Hoffmann and Weischer,
1976).
Long term studies
Rat
Groups of 50 male and 50 female (100 male and 100 female in control
group) were fed a diet containing ethiofencarb at concentrations of 0,
330, 1000 and 3000 ppm for 24 months. The treatment did not affect the
physical appearance, behaviour and mortality of the test animals. The
values of haematological and clinical chemistry tests (with respect to
ALP, GOT, GPT, GLDH, bilirubin and proteins in serum), urinalyses and
kidney function tests were within the physiological ranges. Blood
sugar values did not differ from the control, whereas a dose-related
increase of the cholesterol values in treated animals was found,
especially at 3000 ppm. No gross pathological alterations that could
be attributable to treatment were seen in rats which died during the
feeding study or which were sacrificed at the end of the study. Some
variations in the relative organ weights were found.
A dose-dependent increase of the relative liver-weight at 1000 and
3000 ppm was observed. The histopathological examination of tissues
revealed no treatment-related alterations. The incidence and
localization of tumours provided no indication for a carcinogenic
effect of ethiofencarb (Bombard and Löser, 1976).
TABLE 1b. Acute toxicity of ethiofencarb metabolites
Species Sex Route LD50 References
mg/kg
Ethiofencarb-sulfoxide:
Rat m oral 200-250 Thyssen 1976
Rat f oral 200-250 ibid.
Rat f oral 133 Lamb and Matzkanin 1977a
Ethiofencarb-sulfone:
Rat m oral 600-750 Thyssen 1976
Rat m oral 468 Lamb and Matzkanin 1977b
Rat f oral approx. 600 Thyssen 1976
Ethiofencarb-phenol:
Rat m oral approx. 200 Lamb and Matzkanin 1977c
Rat f oral approx. 200 ibid.
Ethiofencarb-phenol-sulfoxide:
Rat m oral >1000 Lamb and Matzkanin 1977d
Rat f oral >1000 ibid.
Ethiofencarb-phenol-sulfone:
Rat m oral >1000 <20 Lamb and Matzkanin 1977e
Rat f oral >1000 <20 Ibid.
TABLE 1b. (Continued)
Species Sex Route LD50 References
mg/kg
N-methyl-hydroxy metabolite of ethiofencarb:
Rat m oral >2000 Lamb and Matzkanin 1977f
Rat f oral approx 2000 ibid.
N-methyl-hydroxy sulfoxide metabolite:
Rat m oral >2000 Lamb and Matzkanin 1977g
Rat f oral >2000 ibid.
N-methyl-hydroxy-sulfone metabolite
Rat m oral >2000 Lamb and Matzkanin 1977h
Rat f oral >2000 Ibid.
Comments
Ethiofencarb is almost completely absorbed in mammals and it is
excreted rapidly in the form of metabolites mainly in the urine. The
major metabolic reactions are demethylation and subsequent hydrolysis
of the carbamate and oxidations to sulphoxides and sulphones.
Ethiofencarb inhibits the activity of acetylcholinesterase only
transiently.
In a 4-week study daily oral administration of 10 mg/kg to male rats
led to a maximal depression of 25% of the plasma-cholinesterase and of
15% of the erythrocyte-cholinesterase after 2 hours. Recovery occurred
almost completely within 24 hours. This dose level was taken as a
basis for establishing an acceptable daily intake for humans. The fact
that at 10 mg/kg marginal effects occurred was taken into account by
using a safety factor higher than is usual for
cholinesterase-inhibiting compounds.
The compound was not mutagenic in a dominant lethal test, nor was it
teratogenic. No three-generation study was available. However, such a
study was felt to be important since the depression of the
cholinesterase activity often manifests itself unfavourably in a
reduced viability of the pups.
In a 2-year feeding study in dogs no untoward effects were seen up to
1000 ppm. A long-term feeding study in rats revealed no more than a
moderate increase of the relative liver weights at 3000 ppm.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 (mg/kg bw)/day
Dog: 1000 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Ethiofencarb in a systemic insecticide which has a selective effect
against aphids. It gives control of numerous aphid species on
vegetables, fruit crops, field crops and ornamental plants. It
displays good activity against both susceptible and
organophosphorusresistant strains. Used as a soil treatment, e.g. by
the soil drench method or as a granular application, ethiofencarb
displays long residual activity; when applied to the aerial parts of
plants it is somewhat less persistent. In trials conducted to date,
ethiofencarb has shown no phytotoxicity.
Ethiofencarb is sold or registered in Bulgaria, Chile, Denmark,
Federal Republic of Germany, Israel, Italy, Portugal, Spain, Sweden,
Switzerland and Turkey.
Applications for registration have been filed in a number of other
countries.
Pre-harvest treatments
Ethiofencarb is formulated as an emulsifiable concentrate for spraying
and as a granular product. Used as a spray, it is applied broadcast at
the onset of aphid infestation. As a granular formulation, it is
applied at sowing or planting, mainly in-furrow.
Details of uses and recommendations for pre-harvest treatments with
ethiofencarb are given in Table 2.
Post-harvest treatments
No treatments recommended.
Other uses
Ethiofencarb is also used on ornamental plants.
RESIDUES RESULTING PROM SUPERVISED TRIALS
The residue data obtained after the application of ethiofencarb to
fruit, vegetables and field crops are summarized in Table 3. The data
originate from trials conducted in the U.S.A., Japan, Great Britain,
France, the Netherlands and the Federal Republic of Germany.
The residues were determined by gas-chromatographic methods which
determine residues of ethiofencarb and the metabolites with an intact
carbamate group.
After application by spraying, half-lives of 2 to 18 days were noted
for the total residue.
Detailed studies with different formulations using the same active
ingredient dose produced the following half-lives for the given crops:
Apple: 13 (5-18) days
Bush bean: 5 (4-7 ) days
Head lettuce: 5 (4-6 ) days
Savoy cabbage: 5 (2-9 ) days
With crops of which the foliage and edible parts were analyzed
separately, e.g. potatoes, sugar beet, broad beans and radish, it was
found that the residues were present mainly in the foliage owing to
the pronounced systemic mode of action of ethiofencarb, residues were
found in the analyzed plant samples also after application of the
granular formulation to the soil. Here, too, there was an accumulation
of the residues in the leaves, as proved by studies on broad beans and
sugar beet.
FATE OF RESIDUES
General
The metabolism of ethiofencarb (I) is illustrated in Figure 1. It is
found to be of the same pattern, with oxidation of the sulphur and
hydrolysis of the carbamate group, in soil, plants and animals, with
only quantitative differences. In soil and plants, ethiofencarb
sulphoxide (II, Figure 1) and ethiofencarb sulphone (III) were the
main products with an intact carbamate group. In animal metabolism
studies, phenolic degradation products formed by hydrolysis of the
carbamate group were also detected. CO2 released from the carbamate
group by hydrolysis was found to be incorporated into plants.
In animals
Studies on ethiofencarb metabolism in rats are described previously
under "Biochemical aspects". Marshall and Dorough (1976,1977)
investigated the fate of water-soluble plant metabolites of
ethiofencarb in rats. Bean plants were stem-injected with either
ring-14C- or carbonyl-14C-ethiofencarb. After 20 days almost all
the radiocarbon residue was in the epicotyl leaves. The distribution
in the unextractable, organic and aqueous fraction was 3.3%, 45.8% and
40.7% for the ring label and 1.3%, 13.8% and 2.6% for the carbonyl
label. 14C in the aqueous fractions when orally dosed to rats was
largely excreted within 36 hours, mainly by routes similar to those of
the parent compound, viz. in urine (92% of ring and 39% of CO-label)
and as expired CO2 (30% of CO-label).
Sorghum plants treated with ring-14 C-ethiofencarb (I) in an
emulsifiable spray were used as a source of unextractable metabolites
(Marshall and Dorough, 1977). When these bound residues of I were
administered to rats, 90.8% of the dose was voided in the faeces and
16.4% in the urine within 2 days; the bile contained 2.5% of the dose.
These data demonstrated that bound residues of I were very poorly
absorbed from the gastrointestinal tract of rats, while the conjugated
ones were readily absorbed.
Gronberg at al. (1976) continuously fed I to dairy cows for 28 days at
50, 150 and 500 PPM in the diet, equivalent to 1.5, 4.5 and 15 mg/kg
body weight/day. From the residue data obtained it was calculated that
tissue residues would be less than 0.1 mg/kg I + II + III equivalents
and milk residues would be less than 0.01 mg/kg for animals on a
ration containing 90 ppm and 14 ppm of ethiofencarb respectively.
Residues in milk averaged about 1 mg/kg at the highest feed level.
Tissue residues are shown in Table 4.
A lactating cow and a pig were treated with a single oral dose of 0.5
mg/kg 14C-ring-labelled ethiofencarb (Dorough, 1976h). 24 hours
after administration 97.8% of the dose had been excreted via the urine
from the cow and 90% from the pig. Elimination of the radiocarbon in
the faeces was <1% and 5.1% in cow and pig respectively.
Tissues of the pig contained no detectable radioactive residues 24
hours after treatment. Of the bovine tissues only kidney, liver and
skin contained detectable residues (Table 4) of 0.016, 0.017 and 0.05
mg/kg ethiofencarb equivalents respectively. Maximum concentrations of
14C in the blood of the cow, reached 3 hours following
administration, were 0.3 mg/kg. The milk the peak residue level was
0.15 mg/kg, reached 4 hours after dosing. About 67% of the milk
residue consisted of free metabolites, mainly ethiofencarb sulphoxide
and sulphone.
11.6% of the urinary metabolites in the cow were in the free form,
predominantly as phenol sulphoxide and phenol sulphone. Other
metabolites were eliminated as conjugates. In the pig 40.6% of the
dose was excreted in free form.
Eight laying hens were treated with a single oral dose of
14C-ring-labelled ethiofencarb at a rate of 0.5 mg/kg (Dorough,
1976). over 80% of the administered radiocarbon was eliminated in the
excreta after 24 hours and over 90% after 3 days. The highest
radiocarbon level 8 hours after application was found in kidney at
0.062 mg/kg. Liver, muscle and blood showed lower residue levels and
C, fat and heart no residues could be detected (see Table 4). 24 hours
post-administration no 14C could be detected in the tissues. Eggs
laid during a 3-day period following the single treatment were free
from detectable residues.
In a further study (Dorough,1976i) 10 hens were given
14C-ring-labelled ethiofencarb as daily treatment for 7 days at a
rate of 0.5 mg/kg twice a day. 2 days after the start of treatment
about 60% of the cumulative dose was excreted and after 7 days about
92%. Tissues of animals sacrificed 3-4 hours after the final dose
contained 14C-ethiofencarb equivalents ranging from about 0.02 mg/kg
in fat to 0.32 mg/kg in kidney. 4 days after the termination of
treatment muscle, skin, kidney and liver contained 0.001, 0.006, 0.007
and 0.009 mg/kg 14C-ethiofencarb equivalents respectively. In bloody
brains fat and heart no residues were detectable. Levels of
radiocarbon in the eggs reached a maximum of 0.06 - 0.07 mg/kg after 7
days of continuous treatment. In eggs (white and yolk) residues were
about 0.03 - 0.04 mg/kg after 2 days of treatment, reached a maximum
of 0.06 - 0.07 mg/kg after 7 days and were below detectable levels 3
days after treatment was stopped.
TABLE 2. Use pattern of ethiofencarb
Crop Concentration or Formulation Number of Pre-harvest
application rate (a.i.) applications interval(days)
Vegetables 0.0375-0.05% E.C. 2-3 4-7
(Cucurbitaceae, foliar spray/overall
eggplants, peas application
brassicas, peppers, 0.075 -0.1 g./linear GR 1
lettuce, tomatoes metre at sowing/
Vicia, etc.) planting
Cereals 100-500 g./ha. E.C. 1-2 14-21
(including millet) foliar spray/overall
application
Potatoes 500-600 g./ha E.C. 2-3 14
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at planting
Pome and stone fruit, 0.05% E.C. 2-3 7-10
small (soft) fruit, foliar spray/overall
citrus fruit application
Beets 400-500 g./ha E.C. 2-4 14
foliar spray/overall
application
Tobacco 0.05-0.075% E.C. 2-3 4-7
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at sowing/
planting
TABLE 3. Residues of ethiofencarb, its sulphoxide and sulphone from supervised trials
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Apple D 2-3 1 EC 0.9-2.2 0.4 -2.0 0.4 -1.3 0.4 -1.6 0.2 -1.4 0.3-1.0 1)
(28d:)
F 1 0.5 EC 0.6 0.3 -0.4 0.4 1)
J 3/5 3-3.5 EC 1.2 - 1.3 0.8-1.2 2)
(30d.)
Apricot J 2/4 7.5 gEC/tree 0.8 -1.2 1.8-2.1 3)
(28d.)
Artichoke F 1 0.5-1EC 1.0 0.4 -4.7 0.2 -3.0 0.2 -1.0 1)
Beans
broad beans D 2-3 0.3 EC 1)
beans 0.02-0.04 0.02-0.04 0.02-0.03 <0.02
pods 0.2 -0.43 0.08-0.26 0.05-0.22 0.04-0.11 0.02-0.05
greens 5.5 -10.1 0.8 -4.6 0.6 - 4.6 0.5 -3.1 0.2 -1.0
broad beans D 1 1.5 G (55d.) (89d.) (111d.)
beans 0.02-0.13 0.02-0.04
pods 0.19-0.42 0.19-0.57
greens 14-30 2.2 -6.1 0.17-0.84
bush} D 2-3 0.3 EC 0.14-0.68 0.25-0.43 0.03-0.32 0.02-0.19 0.02-0.11 <0.02 1)
(28d.)
beans} F 1 0.75 EC 1.46-2.47 0.77-1.84
F 1 2 G 0.05 0.05 0.03 1)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Cabbage
Brussels sprouts D 3 0.3 EC 0.5 -1.4 0.4 -0.94 0.2 - 0.9 1)
Cauliflower D 2 0.3 EC 0.3 -0.5 0.1 -0.43 0.05-0.2
F 1 0.5 EC 0.26 0.11 0.07 1)
Chinese cabbage J 3/5 0.75-2EC 0.04-2.7 0.04-1.8 0.06-2.4 4)
(5d.)
Red cabbage D 2 0.3 EC 0.2 -1.1 0.2 - 0.4 0.05-0.5 1)
Savoy cabbage D 2 0.3 EC 0.4 -3.1 0.1 - 1.3 0.05-1.3 0.2 - 0.6 0.06-0.4 0.05-0.3 1)
(46d.) (78d.) (88d.) (28d.)
Savoy cabbage D 1 1 G 0.5 -0.6 0.05 <0.05 1)
White cabbage D 2 0.3 EG 0.2 0.1 <0.05 1)
White cabbage GB 1 3 G 0 09 1)
Cherry (see end of table) (105d.)
Cucumber J 3/5 0.75- 0.7 -0.9 0.3 -0.8 0.2 -0.5 0.1 -0.32 4)
2.5 EC
Currant
Blackcurrant D 2 1 EC 1.1 - 2.8 0.5 - 1.4 0.6 - 1.4
Red currant D 2 1 EC 0.5 - 2.9 0.4 - 1.0 0.2 - 0.6 1)
Eggplant F 1 0.75 EC 0.03-0.5 0.02-1.0 1)
Lettuce
(outdoor) D 2 0.3 EC 4.3 - 10.1 1.3 - 4.4 0.6 - 2.2 0.4 - 0.8 0.2 - 0.6 0.1 - 0.2 1)
(28d. )
(under glass) D 2 0.3 EC 2.3 - 18.8 2.2 - 10.1 1.6 -6.4 1.5 0.5 - 0.8 1)
(under glass) NL 1 5 G (44-67d.) 1)
Peach D 1-2 0.75-1EC 1.2 -2.3 1.1 -1.7 0.5 -2.0 0.4 -1.4 0.3 -1.1
Peach F 1 0.5 EC 0.3 -0.8 0.2 -0.3 0.1 -0.2
Plum (see end of table)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Potatoes D 3 0.4-0.6 0.16-0.23 0.09-0.25 0.08-0.17 0.02-0.16 1)
EC (28d.)
Potatoes US 2-6 0.5-2 EC <0.02-0.06 <0.02-0.06 6)
Potatoes J 4-5 0.9-1.75 0.07-0.1 0.04-0.16 0.04 7)
EC
Potatoes leaf J 1 0.7-1.7 EC 38-57 2.2 -4.6 0.3 -1.3 0.2 -0.6 7)
Potatoes D, NL, 1 1.5 G <0.02-0.06 1)
(91-135d.)
Radish J 3/5 1-1.5 EC 0.10-0.12 0.03-0.14 0.02-0.10
Radish leaf J 3/5 1-1.5 EC 0.1-1.5 0.06-1.2 0.1-0.6 8)
Soybean
Soybean, 3 0.75-1.5 0.02-1.04 <0.02-0.18
green+shell EC
Soybean, bean 3/5 0.75 EC 0.04-0.06 0.02-0.04 9)
(44-67d.)
Wheat 1)
grain D 2 0.4 EC
straw 9.6 -11.4 1.4 -1.9 <0.02 <0.02 1)
grain F 1 0.5 EC 1.2 -1.7 0.06-0.8
straw <0.02
0.1 -0.32
(34.53d.)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Sugar beet
beet D,GB 2-5 0.25-0.5 0.04-0.06 0.03 0.02 0.01-0.03 1)
top (leaf) EC 3.0 -4.7 2.0 -2.2 2.7 -4.9 0.12-1.4
(31-76d.)
beet D, GB 1 1-5-3 G 0.01-0.08 1)
top (leaf) 0.05-1.1
(124-195d.)
Tobacco 3 0.3-0.4 12.5-52 5.4-37 4.5-21 1.3 - 9.3 2.9 - 5.7 1.1 -13
(25-28d.) 1)
air -
cured D 1 1.5 G 4.5 -12.3
(54-58d.+
132-137d.
cured)
Cherry
(morello) D 3 1 EC 1.8 -5.9 0.5 -5.9 1.2 -4.2 1)
Plum D 3 1 EC 2.4 -3.5 1.3 -3.4 1.3 -2.6 1)
Trials in a)
Fed. Rep. Germany = D
France = F
Great Britain = GB
Japan = J
Netherlands = NL
United States Amer. = US
TABLE 3. (Continued)
b)
EC = Emulsifiable concentrate
G = Granular
References c)
1) Bayer AG, 1972/77
2) Nitokuno Inst., 1976
3) Nitokuno Inst., 1975
4) Nitokuno Inst., 1975/76
5) Nitokuno Inst., 1975a
6) Chemagro Report, 1976
7) Nitokuno Inst., 1975/76a
8) Nitokuno Inst., 1976a
9) Nitokuno Inst., 1976b
TABLE 4. Ethiofencarb equivalents in various tissues of warm-blooded animals
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Rat 0.5 mg/kg Ring- 14C 2.5 h 0.021) 0.061) 0.041) 0.031) Dorough,
single oral dose 1976j
Rat 0.5 mg/kg N-Methyl-14C 8 h 0.24 0.88 Hurst and
single oral dose 24 h 0.19 0.46 Dorough,
1976
Rat 6.6 ppm/day in diet 0.24 0.18 0.06 0.29 1.18 0.10 Nye at al.,
for 7 days, carbonyl-14C 1976
Cow 0.5 mg/kg Ring-14C 3 h 0.32 0.016 0.017 0.05 Dorough,a
single oral dose 24 h 1976h
Cow 2xdaily 50 PPM in diet 0.03 0.042) 0.03 0.01 <0.01 0.083) Gronberg
for 28 days et al.,
2xdaily 150 PPm in diet 0.27 0.232) 0.29 0.10 0.01 0.853) 1976
for 28 days
2xdaily 500 PPM in diet 2.17 1.012) 2.53 0.89 0.13 4.313)
for 28 days
Hen 0.5 mg/kg Ring-14C 8 h 0.053 0.028 0.062 0.035 0.039 0.022 Dorough,
single oral dose 1976i
0.5 mg/kg Ring-14C 4 days 0.016 0.001 0.005 0.016 0.025 0.013
2xdaily for 7 days 7 days4) 0.099 0.039 0.019 0.072 0.324 0.180 0.069 0.075
TABLE 4. (Continued)
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Hen 45 ppm in diet for 28 days 0.03 0.04 0.05 0.05 Gronberg
150 ppm in diet for 28 days 0.07 0.13 0.08 0.10 and
450 ppm in diet for 28 days 0.16 0.41 0.29 0.24 Dorough,
1976
1) The values were derived from graphs
2) Mixed omental, renal and subcutaneous fat
3) Mixed loin, round, flank
4) Higher residues at 7 than at 4 days were due largely to the fact that the 7-day birds were sacrificed 3-4 h
after last treatment while 4-day birds were sacrificed 8 h after treatment
The major identified metabolites eliminated in the excreta were the
phenol sulphoxide and sulphone, existing as free metabolites or
conjugates, the proportion of free metabolites being higher in the
singly dosed birds. Small amounts of the sulphoxide and sulphone of
the carbamate were also found in the continuous feeding study only.
The same sulphoxidation products were found as the principal
metabolites in eggs, about 50% of the residue being the phenol
sulphone and about 20% the phenol sulphoxide.
Poultry were continuously fed with ethiofencarb for 28 days at a
ration level of 0, 15, 45, 150 and 450 ppm (Gronberg and Dorough,
1976). The average concentration of ethiofencarb equivalents (Table 4)
in fat, skins muscle and giblet ranged from 0.03 to 0.05 mg/kg at the
45 ppm level, from 0.07 to 0.13 mg/kg at the 150 ppm level and from
0.16 to 0.4 mg/kg at 450 ppm. Average residues in eggs collected at
days 26, 27 or 28 of treatment were approximately 0.01, 0.04 and 0.1
mg/kg at the 451 150 and 450 ppm feeding levels respectively. Prom
these residue data it me concluded that tissue residues would be less
then 0.1 mg/kg and egg residues less than 0.01 mg/kg for animals on a
ration of 100 ppm and 40 ppm respectively.
After continuous exposure of Channel catfish to 10 µg/1
14C-ring-labelled ethiofencarb for 28 days, 14C residues accumulated
to a plateau of about 50-75 mg/kg reached after 4 days. The edible
parts of the fish contained about 27% of the residues. During the
withdrawal period about 82% of the accumulated residues were excreted
wIthin 1 day and about 95% within 4 days (Lamb and Roney, 1976).
In plants
Ethiofencarb has good systemic properties and is absorbed in large
amounts by the plant roots and translocated to the aerial parts
(Homeyer, 1976).
Bean seedlings absorbed a total of 34.6% of the available
14C-ring-labelled ethiofencarb from a solution via the roots in 4
days, whilst 18.1% was found after application of
14C-carbonyl-labelled compound. The distribution of radiocarbon in
the plant after 4 days was: roots 5.1%, stems 9.0%, leaves 16.5%
(Dorough, 1976 g).
Dräger (1976a) applied 10% granules of ethiofencarb (I, Figure 1), its
sulphoxide (II) and its sulphone (III) separately to soil at the time
of sowing beans. Results are shown in Table 5. Generally, the total
residue in the green parts was highest after applying Ill and lowest
after the application of I. The highest residues ware in the green
parts (leaves and stems) followed by pods and beans; the highest
levels were found at the first sampling 4 weeks after sowing.
TABLE 5. Uptake of ethiofencarb (I), its sulphoxide (II) and sulphone (III) by beans
Interval Total residue (mg/kg) after application of
after
application, I Granular II Granular III Granular
weeks
a* b* c* a b c a b c
4 159 - - 210 - - 310 - -
6 61 - - 135 - - 204 - -
8 49 - - 74 - - 96 - -
12 78 0.95 0.27 25 0.18 <0.1 141 0.20 0.06
*a: green parts;
b: pods;
c: beans
Following foliar application of 14C-carbonyl-labelled and
14C-ring-labelled I to beans, potatoes and sorghum, it was found that
14 days after application only 60.7, 29.0 and 45.1% of the insecticide
had been absorbed from the leaf surfaces of the respective plants
(Dorough, 1976g).
Potato and sorghum plants were treated at 0.56 kg/ha with ring-14C-I
in an E.C. formulation. The plants were treated at 10-day intervals
5-7 times during the growing season. Radiocarbon residues were much
lower in potatoes than in sorghum. Total ethiofencarb equivalents
remained essentially constant in potato peels (0.2 mg/kg) and pulp
(about 0.15 mg/kg) for 31 days after treatment. In sorghum the highest
levels of ethiofencarb equivalents were located in the leaves (10-100
mg/kg) followed by heads (5-25 mg/kg), grain (1-10 mg/kg) and stalks
(0.05-0.5 mg/kg). Sorghum leaf levels showed an increase from 85 to
131 mi/kg during the first two weeks but declined to about 94 mg/kg at
31 days. Levels remained relatively constant in the stalks. Levels in
the grain appeared constant (6-9 mg/kg) throughout the entire study
except for the 1 week sample (19 mg/kg). Total radiocarbon in the
heads tripled from 50 mg/kg at time 0 to 167 mg/kg at 1 week and then
declined to 90 mg/kg at 31 days (Dorough, 1976f).
Dräger detected II and Ill in various plant parts after application of
10% granular 14C-carbonyl-labelled ethiofencarb at planting of beans
(0.05 g I/1.6 kg soil; 1976a) and potatoes (0.05 g /approx. 20 1 soil;
1976c). The extractable residues in the roots and green parts of both
potatoes and beans consisted mainly of II and Ill. 0.21-7.79 mg/kg II
and 0.14-0.31 mg/kg III were found in the roots of the potatoes
between days 34 and 96, with 1.39-16.2 mg/kg II and 3.08-8.79 mg/kg
Ill in the green parts. The bigger increase in the sulphone content of
the green parts of the potatoes is indicative of an increased
oxidation of the sulphoxide in the plant system. The two metabolites
were detected also in the potato tubers and beans but there was too
little material available for extensive quantitative studies on the
beans. The potato tubers contained 0.05-0.28 mg/kg II and 0.06-0.14
mg/kg Ill.
The unextractable radioactivity in the green parts of the beans was
less than 1% or the total applied, and accounted for a maximum of 7.7%
of the radioactivity measured at the sampling times (Drägert 1976a).
The major portion of the unextractable radioactivity in the potato
tuber was in the glucose moiety of the starch. Therefore, CO2 formed
by hydrolysis of the carbamate group was utilized in carbohydrate
metabolism. The unextractable but water soluble radioactivity in
rootsy green parts and potato tubers was also considered to be
incorporated in products of carbohydrate metabolism. For details of
the distribution of radioactivity in potatoes and beans, see Table 6.
TABLE 6. 14C-ethiofencarb and metabolites in plants (as % of radioactivity applied or recovered)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Bean 0 day 14CO+ 61.9 29.6 1.6 0.7 origin-0.1; 98.0 Dorough,
x2 = 4.1 1976g
3 days Ring- 10.8 55.9 4.4 6.7 1.9 origin = 11.3; x1=0.5; 94.0
x2 =2.5
7 days 14C 49.7 11.4 1.4 0.5 origin=20.2;x1=0.4; 84.6
x2=1.0
14 days EC 15.6 0.6 1.7 0.6 origin=49.8;x1=0.5 69.1
x2=0.3
Potato 0 day 41.3 50.0 1.0 origin=1.5; x1=1.8; 100.3
x2=4.7
3 days 23.1 48.6 3.3 2.0 origin=5.9; x1=1.8; 91.2
x2=7.9
7 days 7.0 42.2 6.5 2.6 0.4 origin=15.7; x1=1.9; 80.4
x2=4.1
14 days 1.8 38.0 9.6 3.5 0.8 origin=14.0; x1=6.2 77.1
X2=3.2
Sorghum 0 day 73.0 22.8 0.9 origin=0.9; x2=0.9 98.5
3 days 18.1 49.3 1.3 0.8 0.3 origin=5.9; x2=2.3 78.0
7 days 3.2 47.0 4.3 1.3 1.0 origin=10.3; x2=2.0 69.1
14 days 0.3 38.6 6.1 0.7 0.3 origin=23.6; x2=0.5 70.1
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Potato
Peel Ring-
org. 0 day 14C 5.2 10.0 2.7 3.4 2.6 origin=1.3; x1=3.8 29.0 Dorough, 1976f
-soluble
water- EC 1.7 3.3 origin=1.0; x1=2.1 8.1
soluble
unhydrolysed 18.1
by H2O
unextractable 44.8
100.0
(102.4)2)
Potato
Peel
org. 31 days Ring- 3.6 2.7 2.7 8.0 6.3 origin=0.9 24.2 Dorough, 1976f
soluble
water soluble 14C 20.9
unhydrolysed EC 31.0
by H2O
extractable 24.1
100.0
(90.9)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Pulp
org.-soluble 0 day 3.5 7.1 5.8 origin=0.3 16.7
water-soluble 4.2 5.3 origin=0.5; x1=3.9 14.0
unhydrolysed 20.7
by H2O
unextractable 48.6
100.0
(100.6)2)
org.-soluble 31 days 0.7 2.5 0.6 6.6 2.1 origin=0.2 12.7
water-soluble 16.2
unhydrolysed 38.8
by H2O
unextractable 32.3
100.0
(90.6)2)
Sorghum
Leaves
org.-soluble 0 day Ring- 1.4 50.5 11.6 0.9 2.1 5.2 origin=1.0; x3=0.5 73.2
water-soluble 14C 0.2 0.6 8.3 4.1 origin=0.2; x2=0.7 14.1
unhydrolysed EC 5.2
by H2O
unextractable 7.5
100.0
(98.8) 2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Leaves
org-soluble 31 days Ring- 0.4 11.3 15.7 3.1 17.8 origin=0.6; x2=0.4 50.4 Dorough, 1976f
14C x3=0.7; x5=0.4
water-soluble EC 0.6 0.4 4.2 4.9 origin=0.3; x2=0.7 11.1
unhydrolysed
by H2O 12.8
unextractable 25.7
100.0
(99.4)2)
Stalks
org.-soluble 0 day 56.2 15.6 2.5 origin=1.2 75.5
water-soluble 11.4 origin=0.5 11.9
unhydrolysed
by H2O 7.1
unextractable 5.4
99.9
(97.0)2)
Stalks
org.-soluble 31 days 14.6 25.5 1.8 3.0 origin=0.6 45.5
water-solube 27.8 origin=0.9 28.7
unhydrolysed
by H2O 15.3
unextractable 10.5
100.0
(105.4)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Heads
org.-soluble 0 day 1.7 45.2 14.4 0.9 1.8 5.2 origin=0.6; x2=0.7;
x5=2.1 72.6
water-soluble 0.4 2.2 3.9 0.4 origin=0.2; x1=0.3;
x2=0.5 7.9
unhydrolysed
by H2O 4.2
unextractable 15.3
100.0
(95.9)2)
Sorghum
Heads
org.-soluble 31 days Ring- 1.6 26.0 27.1 0.4 1.6 9.9 origin=1.0; x2=1.0; 70.6 Dorough, 1976f
14C x5=2.0
water-soluble EC 0.3 0.3 0.2 3.3 2.8 origin=0.2; x1=0.2; 7.8
x2=0.5
unhydrolysed 4.7
by H2O
unextractable 16.9
100.0
(98.7) 2)
Grain
org.-soluble 0 day 51.1 1.7 5.5 2.2 origin=0.8; x2=0.7;
x5=1.8 63.8
water-soluble 4.2 5.7 origin=0.9; x2=0.6 11.4
unhydrolyzed
by H2O 7.0
unextractable 17.8
100.0
(99.2)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Grain
org.-soluble 31 days 11.2 9.0 1.3 3.7 origin=0.5; x2=0.6;
x5=1.6 27.9
water-soluble 8.4 14.7 0.8 origin=0.5; x1=0.5;
x2=1.0 26.1
unhydrolysed
by H2O 12.5
unextractable 33.5
100.0
(99.3)2)
Potato unextractable by
organic solvents
Roots 34 days 14-CO 0.044 1.26 0.14 solid residue
0.10 in aqueous
layers 0.11 1.654 Dräger, 1976c
68 days Granular 0.055 0.42 0.10 solid residue
0.27 in aqueous
layers 0.15 0.945
96 days soil 0.04 0.05 solid residue
0.11; in aqueous
layers 0.07 0.27
TABLE 6 (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Green parts 34 days 7.15 3.89 solid residue
1.02 in aqueous
layers 0.94
68 days 10.28 9.03 solid residue
2.78 in aqueous
layers 3.04 25.12
96 days 4.38 10.18 solid residue
2.17 in aqueous
layers 3.42 20.15
Tubers 68 days 0.00 10.35 0.18 solid residue
0.973) in aqueous
layers 0.60 2.101
96 days 0.17 0.20 solid residue 2.393)
in aqueous layers 0.92 3.46
Bean
Roots 5 weeks 0.26 0.07 unextractable: 0.07 0.40 Dräger, 1976c
10 weeks 0.15 0.19 unextractable: 0.13 0.47
Green parts 5 weeks 3.53 0.95 unextractable: 0.27 4.75
10 weeks 3.02 4.27 unextractable: 0.61 7.90
TABLE 6 (Continued)
1) Compounds I-VI are identified in Figure 1.
2) The figures in Parentheses indicate percent of total recovery, based on combustion
3) Consisting of:
68days 96days
14C-CO2 (CO3-) 0.04 0.39
14C-Glucose in starch 0.85 1.66
Non-hydrolyzable 0.08 0.34
0.97 2.39
Following foliar and soil application, and stem injection of 14CO-and
ring-14C- labelled ethiofencarb to beans, potatoes and sorghum,
Dorough 1976f,g found that in addition to the major metabolites II and
III, minor amounts of IV, V and VI as well as unidentified metabolites
had formed. For quantitative data, see Table 6.
Bean plants were also treated with II and III (14C-ring-labelled),
After three days, 86.6% of II and 91.7% of III had been converted to
water-soluble metabolites which were easily degraded by acid
hydrolysis (Dorough, 1976g).
In soil
Studies with 14C-carbonyl-labelled ethiofencarb (granular
application; Dräger, 1976c 1977a) showed that ethiofencarb was
oxidized in soil to the corresponding sulphoxide (II) and sulphone
(III). Details are given in Table 7. After 5 weeks II was the main
residue but the proportion of III increased with time. Residues of
ethiofencarb in the first sample taken after 5 weeks were 2 mg/kg in
one experiment(1976c) and undetectable in another (1976a). The
unextractable residues amounted to 3-9% of the applied radioactivity.
Dräger (1977b) also found in laboratory studies with soil 34 clay
after the application of carbonyl-ethiofencarb (1) residues of 4.4% I,
68% II and 14% III. The carbamate group was hydrolyzed to CO2 with a
half-life of about 90 days. After 82 days, 43% of the radioactivity of
the carbonyl group was identified as gaseous CO2 Of the remaining
radioactivity left in the soil, II accounted for 1.7%, III for 38.3%,
and unextractrables for 17%; I could not be detected.
Ethiofencarb was degraded in the field in different soils with
half-lives of 20-30 days (Bayer, 1972/77), and in laboratory studies
with a maximum half-life of 32 days (Nitokuno, 1976d). In other
studies half-lives of approx. 90 and 130 days were obtained.
After the application of ethiofencarb 2 kg/ha as an emulsifiable
concentrate to different types of soil the following maximum residues
(I + II + III) were found: 8.8 mg/kg 0 days after application), 4.4
(7), 3.2 (14), 1.1 (30), 0.6 (60), 0.25 (92) and 0.1 (120) (Bayer
1972/77).
In greenhouse experiments with 14CO-labelled ethiofencarb granules,
Dräger calculated a half-life of about 6-7 weeks from the
radiochemical degradation rate of the total residue in soil planted
with potatoes (1976c) and a half-life of about 2 weeks in soil planted
with beans (1976a).
Half-lives for the degradation of ethiofencarb in soil are presented
in Table 8.
TABLE 7. Distribution of radioactivity in soil after application of
14C-carbonyl ethionfencarb
Compound % of initial 14C remaining on day
35 56 70 34 68 96
Ethiofencarb (I) n.d. n.d. n.d. 2.20 0.22 0.08
Ethiofencarb sulphoxide (II) 16.72 3.14 1.96 41.79 8.10 0.87
Ethiofencarb sulphone (III) 4.37 2.94 2.46 8.18 4.81 3.01
Unextractable 5.45 9.12 7.49 7.32 5.64 2.60
Total 26.54 15.20 11.91 59.49 18.77 6.56
References Dräger, 1976 a,b,c Dräger, 1976 a,b,c
The soils were planted either with beans (0.05 g I/1.6 kg soil;
Dräger, 1976a) or with potatoes T 0.05 g I/approx. 20 1 soil., Dräger,
1976 c) so that a portion of the radioactivity was absorbed by the
plants (see "Fate In Plants").
In laboratory studies to investigate the leaching behaviour of
ethiofencarb, normal dosages of formulations were applied to soil
which were eluted to simulate a rainfall of 200 mm in 2-21 days. The
leachate contained the following percentages of the applied dose as
total residues of I + II + III: low-humus sand 26.6-62.5%; high-humus
loamy sand 7.1-19.4%; sandy loam 18.2-74.5%. When II was applied to
low-humus sand and sandy loam, leachates contained 41% and 57% of the
dosage respectively. After application of Ill, the corresponding
figures were 50% and 40% (Bayer, 1972/77).
Rieck (1976a) found in laboratory studies with ring-labelled
14C-ethiofencarb (columns: 20 cm high and 10 cm diameter; application
of water equivalent to 500 m of rainfall) that the products of
ethiofencarb were not leached as rapidly from the Boils with higher
organic matter as from in the sandier, lean organic soils. In silty
clay, 88% of the radiocarbon was found in the upper 8 cm of the
column. Approximately 1-15% of the radiocarbon was found in the
leachates, of which at least 80% was II. The only other product found
in all the leachates was III. The products that remained in soil were
the same as those in the leachates and were in the same proportion.
Whereas carbamates were found in the leachate in these laboratory
experiments with small soil columns, no ethiofencarb and no products
with an intact carbamate structure were found in leachate samples
examined over a period of 16 months in leaching studies with an intact
soil core (10 cm diameter, 130 cm high) taken from the field after
rainfall typical of practical conditions (755 mm/year) (Dräger,
1976d).
Further studies of the leaching characteristics of aged ethiofencarb
residues under laboratory conditions were carried out by Dräger
(1977c) and Rieck (1976b) In the studies of Dräger, about 40-50% of
the applied 14Carbonyl activity was found in the leachate
irrespective of the soil type. Of this, only part was extractable with
chloroform. The unextractable portion was considered to be carbonate
or hydrogen carbonate formed during the experiment by hydrolysis of
the carbamate group of I (see also "Fate in Water"). The extractable
portions consisted of II and IlI, in a ratio similar to that of the
initial aged residues. I was no longer detectable. Rieck (1976b) also
did not detect I in four different soils after 30 days of aerobic
aging. The predominant residues were II and III, together with a
considerable amount of unextractable radioactive residue. In contrast
to the studies of Dräger (1977c) most of the radiocarbon was found in
the upper 8 cm of the soil with less than 2% in the leachate.
Thorton et al. (1976) compared the leaching behaviour of I with that
of 23 other pesticides. The pesticides were spotted on thin-layer
plates coated with six different types of soil ranging from
non-adsorptive sand to fine-textured clay. The plates were developed
and Rf values were compared. The compounds were grouped into five
categories ranging from immobile to highly mobile. I was placed in
class 49 as one of the mobile compounds.
TABLE 8. Half-lives of ethiofencarb (as total residues
of parent + sulphoxide + sulphone) in various soils
Soil Half-life
(days)
Laboratory trials
Carbon: 1.05%; fines: 30.4%; pH: 5.7 (Laacher Haf) 90
Carbon: 0.8% ; fines: 4.2%; pH: 7.0 (low-humus sand) 96
Carbon: 0.57%; fines: 19.5%; pH: 5.2 (sandy loam) 133
Clay: 14 %; org. matter: 5.85%; pH: 5.7 (sandy loam) 13
Clay: 13.6%; org. matter: 0.8%; pH: 7,0; Cation Exchange 32
Capacity:4-5 me/100g
(sandy loam)
Greenhouse trials
Carbon: 0.1%; fines: 22.1%; pH: 5.8; clay: 9.5%; fine silt: 12.6% ca.14
Carbon: 1.08%; fines: 23.2%; pH: 6.1; water cap.: 19-25% 42-49
Field trials
Clay 3.8%: silt: 18.3%; pH: 6.6; water cap. 36.1% 20
Clay: 13.7% silt: 29.4%; pH: 6.2; water cap. 61.3% 20
Carbon: 1.35%; fines: 9.1%; pH: 7.0 30
Carbon: 0.8 %; fines: 15.3% pH: 6.5 23
Clay: 14%; org. matter: 5.85%; pH: 5.7 (sandy loam) 22
Clay: 40%; org. matter: 5 %;
Cation Exchange Capacity 15-18 me/200 g (loam) 20
In water
Ring-labelled 14C-I was incubated under sterile conditions in aqueous
solutions buffered at pH 3, 6 and 9 at 25, 35 and 45°C. Ethiofencarb
was shown to be stable for 30 days at PH 3 and 25°C, whereas the
half-life at pH 9 and 45°C ranged from 0.3 to 0.7 days (Table 9). The
primary hydrolysis product was IV, Figure 1 (max. 92.6% of the
radioactivity of the sample) with only small amounts (<10%) of II
(Kurtz and Gronberg, 1976). The hydrolysis rates of
carbonyl-14C-labelled I, II and III were determined at pH 9.0, 9.5,
10.0, 10.5 and 11.0. I was 3-5 times as stable as II or Ill. Again,
the half-life of all compounds decreased with increasing pH (Table 9)
(Dorough, 1976 d).
Studies with 14CO-ethiofencarb showed that it decomposed in water (pH
6.8) with a half-life of about 8 days, largely by hydrolysis of the
carbamate group. In contrast to the metabolism in soil and plants, II
and Ill were very minor products: their sum represented about 10% of
the total carbamate residue throughout the experiment. After 31 days,
83% of the original carbonyl carbon could be determined as carbonate
or carbon dioxide gas, 3.4% was present as I, 0.3% as II and 0.2% as
III (Dräger, 1976 b, 1977a)
Photodecomposition
The photodegradation of ring-14C-or carbonyl-14C-I was studies under
artificial and natural sunlight on glass plates, silica gel plates,
soil plates, and in aqueous solution (distilled water or pond water).
5% or less of I was found after 6 hours irradiation. II was the major
product: Ill and VI were found as minor constituents (Dorough,1976e).
For quantitative data, see Table 10.
The effect of anthraquinone as a photosensitizer was profound when
applied as a mixture with I on silica gel plates. Although the extent
of degradation of 1 me essentially unaffected, there was an increase
in the number of minor photoproducts (those less than 1%) with
anthraquinone and an increase in the polar material remaining at the
origin after 7 days of exposure to a sunlamp. Almost no II was
produced and a compound with a similar Rf to IV was the major product
(48% after 7 days of exposure). There were also lesser amounts of Ill
and VI (Dorough, 1976e).
In storage and processing
In head lettuce that had been field-sprayed in accordance with good
agricultural practice, no decline of the total residue was noted after
one year of storage in a deep freezer at -20°C (Bayer, 1974).
TABLE 9. Half-lives of ethiofencarb in buffered solutions.
Half-life of compound
Conditions I II III Ref.
(ethiofencarb) (ethiofenoarb (ethiofencarb
sulphoxide) sulphone)
pH: 9.0 6.67 hours 2.03 hours 2.13 hours Dorough, 1976d
9.5 2.67 hours 0.47 hours 0.72 hours
10.0 1.17 hours 0.23 hours 0.40 hours
10.5 0.43 hours 0.08 hours 0.12 hours
11.0 0.09 hours 0.02 hours 0.03 hours
25 °C: 10 ppm; pH 3 stable Kurtz and
pH 6 324 days Gronberg, 1976
pH 9 3.5 days
25 °C; 100 ppm; pH 3 stable
pH 6 stable
pH 9 2.5 days
35 °C; 10 ppm; pH 3 stable
pH 6 68 days
pH 9 1.5 days
35 °C; 100 ppm; pH 3 stable
pH 6 68 days
pH 9 2.0 days
45 °C; 10 ppm; pH 3 stable
pH 6 22 days
pH 9 0.7 days
45 °C; 100 ppm; pH 3 stable
pH 6 20 days
pH 9 0.3 days
TABLE 10A. Photodegradation of 14c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound1) after indicated time
1 hour 3 hours
Conditions
O I II III IV V VI xn O I II III IV V VI xn
artifical light, 1.0 51.4 44.9 3.5 2.3 1.8 16.3 70.3 4.8 2.9
TLC plates
germicidal light, 14.2 6.6 22.7 24.1 0.6 15.6 9.5 20.1 1.3 10.8 23.9 1.0 14.6 10.0
TLC
plates
natural
light, 0.6 42.1 62.0 0.7 1.9 0.8 1.2 2.9 91.4 2.2 2.7 1.2
TLC plates
artifical light, 1.6 1.0 84.2 4.3 7.1 0.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
germicidal light, 1.6 1.8 80.1 44.4 10.4 1.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
natural light, 1.4 1.3 80.5 5.9 9.5 1.5 1.2 1.9 82.7 6.3 0.5 6.6 0.9
soil plates
natural light, 1.1 0.5 46.1 5.1 1.7 2.3 0.6 36.1 7.7 1.6
glass plates
natural light, 1.9 6.5 78.2 6.5 1.0 4.6 1.6 4.2 2.9 70.1 22.5 6.5 5.3
aquous
solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
TABLE 10B. Photodegradation of 14 c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound 1) after indicated time
6 hours
Conditions
O I II III IV V VI xn
artifical light, 2.4 5.2 78.6 6.3 3.5
TLC plates
germicidal light, 21.2 4.3 8.8 24.3 1.0 14.0 9.7
TLC plates
natural light, 1.4 0.8 90.5 2.7 2.9 1.2
TLC plates
artifical light, 2.1 1.0 83.6 5.9 5.1 1.6
soil plates
germicidal light, 1.9 2.6 76.3 6.5 1.0 9.1 1.3
soil plates
natural light, 1.0 1.1 87.8 5.7 0.8 2.6 1.1
soil plates
natural light, 4.2 0.3 26.0 13.1 0.9 2.3
glass plates
natural light, 5.8 0.4 65.6 11.7 1.8 8.4 3.2
aquous solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
Studies to investigate the stability of the sulphoxide and sulphone
metabolites in potatoes revealed that the content of both compounds
remained constant on storage for 11 months at -20°C (Chemagro, 1976b).
When bovine and poultry tissues, milk and eggs were fortified with
14C-labelled ethiofencarb and stored under frozen conditions for 7
months, 84-98% of the residue was found as intact carbamate (Chemagro,
1976c).
Poultry rations fortified with ethiofencarb for use in various animal
feeding studies were stored for 5 weeks at -10°C; no degradation of
the ethiofencarb was noted (Chemagro, 1975).
Processing of plant material by baking and boiling results in the
reduction of ethiofencarb residues. Broad beans which had a residue
content of 0.13 mg/kg before boiling were found to contain < 0.02 mg
I + II + III/kg after being boiled for 15 minutes. In the green parts
(stems and leaves), the residues were reduced by boiling to between 3
and 7% of the original content of 14-30 mg/kg; 0.4-3.4% of the
original residue (I + II + III) was found in the water used for
boiling (Bayer, 1975).
Raw potatoes fortified with 14C-labelled ethiofencarb (1 mg/kg) were
processed by pan frying, french frying and baking. Both pan and french
frying processes reduced the residue (I + II + III) with an average
loss of 52 and 58%, respectively. Leaching of the residue (2-20%) into
the frying oils and volatilization were observed. The baking process
showed a loss of about 2% of the 14C activity. Of the remaining
activity, ethiofencarb accounted for 66%, ethiofencarb sulphoxide for
14%, and ethiofencarb phenol for 20% (Chemagro Report, 1976d).
METHODS OF RESIDUE ANALYSIS
Residues of ethiofencarb can be determined by gas chromatography using
a sulphur-specific flame photometric detector. The methods available
for the analysis of plant samples# animal tissue samples and soil
samples simultaneously determine metabolites with an intact carbamate
structure, i.e. ethiofencarb sulphoxide and ethiofencarb sulphone.
Oxidation with potassium permanganate converts ethiofencarb and
ethiofencarb sulphoxide to the sulphone. For gas-chromatographic
determination, the total carbamate residue is derivatized by reaction
with bix-trimethylsilytrifluoroacetamide to the thermostable, volatile
2-ethylsulfonylmethylphenyl trimethylsilyl ether.
For the extraction step, acetone (Dräger, 1974) or methanol/water
(Morris and Gronberg, 1976; Dorough, 1976a) were used for plant and
soil samples. Soil samples can also be extracted with a mixture of
acetonitrile and 0.1 × hydrochloric acid (Morris and Gronberg, 1977)
or by reflux with a methanol/chloroform mixture (Morris, 1976a).
Water-containing animal tissues and eggs are extracted with
acetonitrile; prior to extraction, fat is dissolved in hexane and
acetone is added to milk to separate protein (Dorough, 1976b).
Isolation of the residues from the extracts is achieved by shaking
with chloroform, and separation of co-extractives is accomplished by
precipitating with a solution of ammonium chloride/phosphoric acid.
After oxidation of the residues with potassium permanganate to
ethiofencarb sulphone, silylation is carried out in acetone or
benzene.
With some types of sample, further chromatographic clean-up steps are
needed before silylation, in order to separate sulphur-containing
plant co-extractives which interfere with the gas-chromatographic
determination. With brassica extracts clean-up was by thinlayer
chromatography and with hop extracts by column chromatography (Dräger,
1974). In the analysis of animal tissues, eggs and milk, the residue
was hydrolyzed to the corresponding phenol sulphone before silylation
in order to increase reproducibility (Dorough, 1976b).
For the gas-chromatographic determination, columns packed with 5%
DC-200 on Gaschrom Q (Dräger, 1974) and with -3% OV-1 on Chromosorb W
(Morris and Gronberg, 1977) have proved suitable. For a confirmatory
procedure, a column packed with the polar phase Carbowax 20-M on
Chromosorb W (Morris,1976b) was used. The lower limit of determination
is generally 0.02-0.05 mg/kg for plant and soil samples. 0.01 mg/kg
for fat, and 0.005 mg/kg for eggs and milk.
The methods were shown to be specific in a study in which pesticides
registered in the U.S.A. for use on potatoes, sorghum, animal tissues,
milk and eggs were checked for possible interference: none of the 50
chemicals tested interfered at their maximum registered level
(Dorough, 1976c).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The only tolerances reported to the Meeting were on fruit and
vegetables in Switzerland, but pre-harvest intervals have been
specified in several other countries as shown below (Table 11).
APPRAISAL
Ethiofencarb is a systemic insecticide used especially against sacking
insects an vegetables, fruit, field crops and tobacco, It is effective
against some organophosphorasresistant strains. It is formulated an
granules and emulsifiable concentrates. The usual dose is 0.3-2 kg
a.i./ha in spray, or approximately 0.1 g a.i. per linear metre as
granules at sowing or planting. Ethiofencarb is used in various
European countries, Chile and Israel.
In plants and soil ethiofencarb (I) is oxidized to its sulphoxide (II)
and sulphone (III) which are also hydrolysed. In plants and soil the
main intact carbamates are II and III. Little or no ethiofencarb is
usually detectable at harvest. In animals, the corresponding phenols
and their conjugates are the main metabolites.
TABLE 11. National tolerances and pre-harvest intervals reported to the Meeting.
Country Crop Tolerances Pre-harvest
in mg/kg interval in
days
Germany Beets, sugar beets 28
(FRG) Head lettuce 4
Bush beans, pole beans,
broad beans 7
Potatoes 14
Israel Peppers 5
Potatoes 4
Cauliflower, drumhead
cabbage 7
Apples 7
Italy Vegetables 7
Fruit crops 14
Beets 30
Spain Fruit crops incl. citrus,
winter cereals, maize,
potatoes, beets, sugarcane,
cotton, oilseed crops 21
Switzerland Pome, stone and small
fruit 0.2
Vegetables excl. spinach 1.0 14
Fruit crops, peas for
harvesting dry (dried,
threshed) 21
Sugar beet, field beans 42
Half-lives of I + II + III in soil vary between 2 and 12 weeks in the
field.
On the basis of feeding a diet containing 50 ppm of I to cows, pigs
and hens, it can be estimated that residues of I + II + III are
unlikely to exceed 0.02 mg/kg in their meat or in eggs or milk.
Boiling broad beans reduced carbamate residues of 0.13 mg/kg to below
0.02 mg/kg. Cooking the green parts of beans reduced the residues to
3-7% of the original content of I + II + III (14-30 mg/kg). 0.4-3.4%
of these residues was found in the water used for boiling. Deep and
shallow frying of potatoes fortified with 1 mg ethiofencarb/kg
decreased the total carbamate residue by about 50%. Baking potatoes
caused no loss of carbamate residues.
Gas-chromatographic procedures for the determination of residues of I
+ II + III, suitable for regulatory purposes, in various crops, eggs,
milk and soil have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended. They
refer to the am of ethiofencarb, its sulphoxide and its sulphone,
expressed as ethiofencarb.
Commodity Limit, Pre-harvest interval (days)
mg/kg on which recommendations
are based
Cherries, lettuce 10 4-7
Apples, pears, apricots 4-7
Artichokes, beans with pod, 4-7
beet tops,
sugar beet tops (leaves), 14
brassicas peaches plums 5 4-7
Currants (black, red),
eggplants, 4-7
wheat straw 2 14
Cucumbers 1 4
Potatoes, 14
Radishes 0.5 4
Beans without pods
soybeans without pod 0.2 4-7
Fodder beets,
sugar beets (roots) 0.1 14
Raw grain
(barley, oats, rye, wheat) 0.05 30
Meat of cattle, pigs
and poultry, eggs, milk 0.02*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by July 1978)
1. Submission of the results of the reproduction study now in
progress.
2. Further residue data from supervised trials on apricots,
artichokes, various brassicas, cherries, cucumbers (including those
grown under glass), eggplants, plums, radishes, soybeans.
DESIRABLE
1. Further residue data from supervised trials on other crops on which
ethiofencarb is known to be used.
REFERENCES
Bayer, A.G. (1972/77) Ethiofencarb; Pflanzensohutzmittel-Rückstände,
Dräger, Bayer-Leverkusen, unpublished.
Bayer, A.G. (1974) Report RA 301; (unpublished).
Bayer, A.G. (1975) Report RA 655; 20 Nov. 1975.
Bothard, E. and Löser, E. (1976) HOX 1901 Chronic Toxicity Study on
Rats. Nov. 30, Rep. No. 6493 from Bayer AG, Inst. f. Tox., unpublished
report submitted by Bayer AG. Addendum., Histopathological Examination
by J.P. Finn, Hazleton Laboratories, Inc.
Chemagro Report (1975) 45 176; 12 Sept, 1975, R & D Dep. of Chemagro,
Agricult. Div., Mobay Chemical Corp., Kansas City, No. 64 1209 USA.
Chemagro Report (1976a) Chemagro Agricult. Div.; Groneton; Potatoes
(Kats; Russet Burbank), Rep. No. 49 735/49 751; 17 Nov. 1976
(unpublished).
Chemagro Report (1976b) No. 51 062; 30 Dec. 1976 (unpublished).
Chemagro Report (1976c) No. 49 896; 27 Dec. 1976.
Chemagro Report (1976d) No. 46995; 11 Feb. 1976.
Dorough H.W. (1976a) A Gas Chromatographic Method for the
Determination of Croneton Residues in Various Animal Tissues, Crops,
and Soil. CR 49 879, 1.12.1976.
Dorough H.W. (1976b) A Gas Chromatographic Method for the
Determination of Croneton Residues in Bovine Tissues and Milk and
Poultry Tissues and Eggs. CR 49 880; 18.11.1976.
Dorough H.W. (1976c) An Interference Study for the Residue Method for
Croneton and Metabolites in Various Crops and Animal Tissuee, Milk and
Eggs. CR 49 893, 17.12.1976.
Dorough H.W. (1976d) Rate of Hydrolysis of CRONETON TM GRONETON
Sulphoxide, and CRONSTON Sulphone in Aqueous Solutions. Chemagro
Report No. 49 890 (1976).
Dorough H.W. (1976e) Photodcoomposition of CRONETON TM on Various
Substrates. Chemagro Report No. 49 891 (1976).
Dorough H.W. (1976f) The Metabolism and Residue in Potatoes and
Sorghum Treated Under Field Conditions Using CRONETON
TM-ring-UL-14C. Chemagro Report No. 49 894 (1976).
Dorough H.W. (1976g) The Metabolism of CRONETON TM in Plants.
Chemagro Report No. 49 895 (1976).
Dorough H.W. (1976h) Metabolism of CRONETON TM in a Lactating
Holstein Cow and a Male Yorkshire Swine. Chemagro Report No. 49 897
(unpublished).
Dorough H.W. (1976i) The Metabolism of CRONETON TM Poultry. Chemagro
Report No. 49 898 (unpublished).
Dorough, H.W. (1976j) Correlation of Blood and Tissue Radiocarbon
Levels Following a Single Oral Dose of CRONETON TM-ring-14C to Rats.
Chemagro Report No. 49 887 (1976).
Dräger, G. (1974) Method for gas-chromatographic determination of
Croneton residues in plants and soil. Pflanzenschutz-Nachrichten Bayer
91, 144-155 (1974).
Dräger, G. (1976a) Study to investigate uptake and metabolism of
Croneton in beans (Vioia faba) after application in granular form.
Bayer AG, Pflanzensohutz-Anwendungstechnik, unpublished Report RA-79,
January 19, 1976.
Dräger, G. (1976b) Studying the Metabolism of Croneton in Water. Bayer
AG Pflanzenschutz-Anwenclungstcohnik, unpublished Report RA-438v June
9, 1976.
Dräger, G. (1976c) Studies on the Metabolism of CRONETON in Potatoes
and Soil and Identification of Bound Residues in the Potato Tuber.
Bayer AG, Pflanzenschut Anwendungstechnik, unpublished Report RA-7989
October 89 1976.
Dräger, G. (1976d) Untersuchunger zum Versickerungsverhalten von
CronetonR (Makro-Säulen-Versuch). Bayer AG,
Pflanzenschutz-Anwenclungeteohnik, unpublished Report RA-961.
Dräger, G. (1977a) Untersuchung des Metabolismus von Groneton in Boden
und Wasser. Pflanzenschutz-Nachrichten Bayer 30, 18-27 (1977).
Dräger, G. (1977b) Weiterer Abbau, von gealterten Croneton-Rückständen
im Boden unter Laborbedingungen Bayer AG Pflanzenschutz-
Anwendungsteohnik, unpublished report RA-133, February 16, 1977
Dräger, G. (1977c) Versickerungsverhplten von gealterten
Groneton-Rlickstgnden in Boden unter Laborbedingunger, Bayer AG,
Pflanzenschutz-Anwendungsteohnik, unpublished Report RA-346.
Gronberg, R.R., Dorough, H.W., (1976) Effect of Feeding CRONTEONTM
to Poultry. Chemagro Report No. 49 899 (unpublished).
Gronberg, R.R., Dorough, H.W., Hemken, R.W. (1976) Effect of Feeding
CRONETONTM to Dairy Cattle. Chemagro Report No. 50 500 (1976)
(unpublished).
Hoffmann, K. and Weischer, C.H. (1976) 1901/Chronio Study on Dogs. May
26, Rep. No. 6120 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Homeyer, B. (1976) Croneton als wurzelaystemieches Aphizid.
Ptlanzenschutz-Nachrichten Bayer 29 255-266 (1976).
Hurst, H., Dorough, H.W. (1976) The Metabolism of CRONETON TM-
N-methyl-14C in Rats-A Progress Report. Chemagro Report No. 49 886
(1976).
Kimmerle, G. (1972b) HOX 1901/Acute Toxicity Studies. July 10, Rep.
No. 3557 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. (1972c) HOX 1901/Acute orale Toxizität an männlichen
Kaninchen. Nov. 13, report from from Bayer AG, Inst. f. Tox.,
unpublished report submitted by Bayer AG.
Kimmerle, G. (1972d) Antidotal Effect of Atropine. July 10, report
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Kimmerle, G. (1972e) HOX 1901/Subacute Toxicity Studies. July 10, Rep.
No. 3558 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. and Eben, A. (1974) HOX 1901/Effect of Acute and Subacute
Oral Administration and Subacute Inhalation on Plasma, Erythrocyte and
Brain Acetylcholinesterase Activity in Rats and Dogs. Aug. 8, Rep. No.
4843 from Bayer AG, Inst. f. Tox.p unpublished report submitted by
Bayer AG.
Klimmer, O.R. (1973) Study Report / Bayer HOX 1901/Subchronic
Toxicological Studies on Rats. Febr. 27, Rep. No. R 739 from
Pharmakologisches Instit der Rhein. Friedr.-Wilh.-Univ., Bonn,
unpublished report submitted by Bayer, AG.
Kurtz, S.K., Gronberg, R.R. (1976) The Stability of CRONETON TM in
Aqueous Systems. Chemagro Report No. 50 798 (1976).
Lamb, D.W., Roncy, D.J. (1976) Accumulation and Persistence of
Residues in Channel Catfish Exposed to CRONETON-14C. Chemagro Report
No. 49 128 (unpublished).
Lamb, D.W. and Matzkanin, G.S. (1977a) The Acute Oral Toxicity of
CRONETON Sulphoxide. Febr. 18, Rep. No. 51 716 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lambt D.W. and Matzkanin, G.S. (1977b) The Acute Oral Toxicity of
CRONETON Sulphone. Febr. 21, Rep. No. 51 717 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, G.S. (1977c) The Acute Oral Toxicity of
CRONETON Phenol. Febr. 17, Rep. 51 719 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkaning G.S. (1977d) The Acute Oral Toxicity of
CRONETON Phenol Sulphoxide. Febr. 17, Rep. No. 51 718 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977e) The Acute Oral Toxicity of
CRONETON Phenol Sulphone. Febr. 17, Rep. No. 57 715 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977f) The Acute Oral Toxicity of
CRONETON (Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroxy). April
26, Rep. No. 52 878 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977g) Acute Oral Toxicity of CRONETON
(Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroyy) Sulphoxide.
April 29, Rep. No. 52 879 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977h) The Acute Oral Toxicity of
CRONETON Metabolite N-Methyl (Hydroxy) Sulphone. April 29, Rep. No. 52
880 from Mobay Chem. Corp., unpublished report submitted by Bayer AG.
Machemer, L. (1973a) HOX 1901/Dominant Lethal Test on the Male Mouse
to Test for Mutagenic Effect. Dec. 14, Rep. No. 4339 from Bayer AG,
Inst. f. Tox., unpublished report submitted by Bayer AG.
Machemer, L. (1973b) HOX 1901/Studies for Embryotoxic and Teratogenic
Effects on Rats Following Oral Administration. July 25, Rep. No. 4174
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Machemer, L. (1975) HOX 1901/Studies of Embryotoxic and Teratogenic
Effect on Rabbits Following Oral Administration. July 24, Rep. No.
5545 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Marshall, T., Dorough, H.W. (1976) Fate of Water Soluble CRONETON TM
Plant Metabolites in Rats (Progress Report). Chemagro Report No. 49
892 (1976).
Marshall, T.C., Dorough, H.W. (1977) Bioavailability in Rate of Bound
and Conjugated Plant Carbamate Insecticide Residues. Chemagro Report
No. 52 737 (1977).
Morris, R.A. (1976a) Determination of Baygon, Baytex, Bolster,
Croneton, Dasanit, Di-Syston, Dylox, Guthion, Hinosan, Mesurol,
Meyasystox-R, Monitor, Morestan, Nemacur, Systox Residues in Soil. CR
49 675, 15.9.1976.
Morris, R.A. (1976b) A Confirmatory Procedure for the Analytical
Residue Method for Croneton in Potatoes, Plant Forage and Cereal
Grains. CR 51 0639 30.12.1976.
Morris, R.A. Gronberg, R.R. (1976) A Gas Chromatographic Method for
the Determination of Residues of Croneton (Bay BOX 1901) and
Metabolites in Potatoes, Plant Forage, and Cereal Grains. CR 49 666#
23.8.1976.
Morris R.A., Gronberg, R.R. (1977) A Gas Chromatographic Procedure for
the Determination of Croneton (Bay HOX 1901) in Soil. CR 49 6659
10.2.1977.
Mürmann, P. (1973) HOX 1901/Subohronic Toxicity Study on Dogs. June
28, Rep. No. 4143 from Bayer AG, Inst. f. Tox., (unpublished).
Nitokuno Inst., (1975a) Report No. 3599 unpublished.
Nitokuno Inst., (1975b) Reports No. 335 and 336, unpublished.
Nitokuno Inst., (1975/76a) Reports No. 333, 334 and 390, unpublished.
Nitokuno Inst., (1975/76b) Reports No. 360-362 and 395-396,
unpublished.
Nitokuno Inst., (1976a) Report No. 379, unpublished.
Nitokuno Inst. (1976b) Reports No. 391-1949 unpublished.
Nitokuno Inst. (1976c) Reports No. 397-4009 unpublished.
Nitokuno Inst. (1976d) NIHON TOKUSHU NOYAKU SEIZO K.K. Residual
Analysis Results of HOX 1901 (ethiofencarb) in Upland Soil. Report No.
1042 (RA), January, 28, 1976.
Nye, D.E., Hurst, H.E., Dorough, H.W. (1976) Fate of Croneton
(2-Ethylthiomethylphenyl N-Methyl-carbamate) in Rats. J. Agric. Food
Chem. 24, 371-377.
Rieck, C.E. (1976a) Leaching characteristics of CRONETON TM in
various soils. Chemagro Report No. 50 783.
Rieck, C.E. (1976b) Leaching characteristics of CRONETON TM on aged
soil. Chemagro Report No. 51 028.
Thorton, J.S., Hurley, J.B. and Obrist, J.J. (1976) Soil thin-layer
mobility of twenty four pesticides chemicals. Chernagro Report No. 51
016.
Thyssen, J. and Kimmerle G. (1975) Präparat 6757/Untersuchungen zur
Anwendungstoxikologie. Nov. 27, Rep. No. 5751 from Bayer AG, Inst. f.
Tox., unpublished report submitted by Bayer AG.
Thyseen, J. (1975a) HOX 1901/Neurotoxicity Studies on Hens. Nov. 18,
Rep. No. 5735 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG. Appendix: ROX 1901 Neurotoxicity Studies on
Hens/Histopathological Finding by Prof. Dr. U. Mohr, Med. Hoohsohule
Hannover.
Thyssen, J. (1975b) Studies to Investigate Acute Oral Toxicity of HOX
1901 when Administered Simultaneously with Malathion or EPN, Nov. 18,
Rep. No. 5732 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Thyssen, J. (1976) HOX 1901-Metaboliten. Sept. 1, report from Bayer
AG, Inst. f. Tox., unpublished report submitted by Bayer AG.
Acute toxicity
TABLE 1a, Acute toxicity of ethiofencarb
Species Sex Route LD50 References
mg/kg
Rat m oral 308 Thyssen and Kimmerle, 1975
Rat m oral 411 Kimmerle 1972b
Rat f oral 499 ibid.
Rat m i.p. 41.8 ibid.
Rat f i.p. 41.9 ibid.
Rat m dermal >1000 ibid.
Mouse m oral 256 ibid.
Mouse f oral 224 ibid.
Rabbit m oral approx. 225 Kimmerle 1972c
Rabbit m dermal >8000 Lamb and Matzkanin 1976a
Rabbit f dermal >8000 ibid.
Dog f oral >50 Kimmerle 1972b
Hen oral approx. 1000 ibid.
The typical symptoms of acetylcholinocaterase inhibition such as
salivation, muscle tremors, spasms and convulsions preceded death.
A good antidotal effect was obtained when rats orally treated with
ethiofencarb received i.p. injection of atropine sulphate (Kimmerle
1972d).
Short term studies
Rat
Groups of 15 male and 15 female rats were treated orally for 30 days
at dose levels of 0, 5, 10, 20 and 40 mg/kg. The administration had no
effects with respect to physical appearance, behaviour, body weight,
haematological and clinical chemistry test values. No gross
pathological alterations of tissues were observed and organ weights
did not significantly differ from those of the control animals.
Tissues were not examined microscopically (Kimmerle, 1972e).
In another subacute study 10 male and 10 female rats per dosage group
were exposed for 6 hours daily on 5 consecutive days per week over a
3-week period to aerosol concentrations of 0, 29.7 and 148.5 mg/m3
of air. All these concentrations were tolerated without any adverse
effect with respect to behaviour, general health condition, body
weight, haematological and clinical chemistry values.
The macroscopic examination of the major tissues revealed no
pathological alteration and organ weights did not differ significantly
between the control group and the treated groups (Kimmerle, 1972e).
Groups of 20 male and 20 female rats were fed ethiofencarb at
concentrations of O, 250, 500 and 1000 ppm for 3 months. Physical
appearance, behaviour, mortality and food consumption were not
affected by the diet. The average body weights of male rats fed 500
and 1000 ppm were higher than the corresponding weights of the control
animals. Values of urinalyses, haematology and clinical chemistry were
within the physiological range. Macroscopic and microscopic
examination of the principal organs revealed no treatment-related
alterations. The relative liver weights in male rats treated with 1000
ppm were higher than those of the control animals (Klimmer, 1973).
Dog
Groups of 4 male and 4 female dogs were maintained for 3 months on a
diet containing ethiofencarb at concentrations of 0, 100, 300 and 1000
ppm. The treatment did not effect physical appearance, behaviour, food
consumption, body weight, mortality or ophthalmoscopic and
neurological findings. Parameters of haematology, clinical chemistry
and urinalyses showed no significant differences between control and
treated groups. At 1000 ppm an increase of the relative liver weights
of about 10% was noted in both sexes and an increase of about 40% in
relative kidney and spleen weights in male animals only. Gross and
histopathological examination showed no treatment-related alteration
(Mührmann, 1973).
Groups of 4 male and 4 female dogs received a diet for a period of 104
weeks containing ethiofencarb at concentrations of 0, 330, 1000 and
3000 ppm. The administration of up to and including 1000 ppm did not
affect the test animals with respect to their physical appearance,
behaviour, food consumption and ophthalmoscopic findings. The dietary
level of 3000 ppm caused vomiting immediately after feeding,
especially in the second half of the study; food consumption was
retarded and reduced in some female dogs at the 3000 ppm level,
whereas female dogs at 330 and 1000 ppm made bigger gains in
comparison with controls. Haematological and urinalyses values
revealed no pathological alterations. Results of chemistry tests (with
respect to blood sugar, urea, creatinine, total protein, GOT,
bilirubin) were within the physiological range. The ALP activities
showed a 3 -4 fold increase at 3000 ppm and in some dogs of the same
group an increased level of GPT activity and cholesterol were noted.
Relative liver weights of the animals of both sexes at 1000 and 3000
ppm showed a dose-related increase. One dog at the 3000 ppm feeding
level showed enlargement of the liver. At 3000 ppm the
histopathological examination of tissues disclosed signs of treatment-
related alterations in liver such as enlarged hepatocytes, hepatocytes
nuclei and granular cytoplasmic structures (Hoffmann and Weischer,
1976).
Long term studies
Rat
Groups of 50 male and 50 female (100 male and 100 female in control
group) were fed a diet containing ethiofencarb at concentrations of 0,
330, 1000 and 3000 ppm for 24 months. The treatment did not affect the
physical appearance, behaviour and mortality of the test animals. The
values of haematological and clinical chemistry tests (with respect to
ALP, GOT, GPT, GLDH, bilirubin and proteins in serum), urinalyses and
kidney function tests were within the physiological ranges. Blood
sugar values did not differ from the control, whereas a dose-related
increase of the cholesterol values in treated animals was found,
especially at 3000 ppm. No gross pathological alterations that could
be attributable to treatment were seen in rats which died during the
feeding study or which were sacrificed at the end of the study. Some
variations in the relative organ weights were found.
A dose-dependent increase of the relative liver-weight at 1000 and
3000 ppm was observed. The histopathological examination of tissues
revealed no treatment-related alterations. The incidence and
localization of tumours provided no indication for a carcinogenic
effect of ethiofencarb (Bombard and Löser, 1976).
TABLE 1b. Acute toxicity of ethiofencarb metabolites
Species Sex Route LD50 References
mg/kg
Ethiofencarb-sulfoxide:
Rat m oral 200-250 Thyssen 1976
Rat f oral 200-250 ibid.
Rat f oral 133 Lamb and Matzkanin 1977a
Ethiofencarb-sulfone:
Rat m oral 600-750 Thyssen 1976
Rat m oral 468 Lamb and Matzkanin 1977b
Rat f oral approx. 600 Thyssen 1976
Ethiofencarb-phenol:
Rat m oral approx. 200 Lamb and Matzkanin 1977c
Rat f oral approx. 200 ibid.
Ethiofencarb-phenol-sulfoxide:
Rat m oral >1000 Lamb and Matzkanin 1977d
Rat f oral >1000 ibid.
Ethiofencarb-phenol-sulfone:
Rat m oral >1000 <20 Lamb and Matzkanin 1977e
Rat f oral >1000 <20 Ibid.
TABLE 1b. (Continued)
Species Sex Route LD50 References
mg/kg
N-methyl-hydroxy metabolite of ethiofencarb:
Rat m oral >2000 Lamb and Matzkanin 1977f
Rat f oral approx 2000 ibid.
N-methyl-hydroxy sulfoxide metabolite:
Rat m oral >2000 Lamb and Matzkanin 1977g
Rat f oral >2000 ibid.
N-methyl-hydroxy-sulfone metabolite
Rat m oral >2000 Lamb and Matzkanin 1977h
Rat f oral >2000 Ibid.
Comments
Ethiofencarb is almost completely absorbed in mammals and it is
excreted rapidly in the form of metabolites mainly in the urine. The
major metabolic reactions are demethylation and subsequent hydrolysis
of the carbamate and oxidations to sulphoxides and sulphones.
Ethiofencarb inhibits the activity of acetylcholinesterase only
transiently.
In a 4-week study daily oral administration of 10 mg/kg to male rats
led to a maximal depression of 25% of the plasma-cholinesterase and of
15% of the erythrocyte-cholinesterase after 2 hours. Recovery occurred
almost completely within 24 hours. This dose level was taken as a
basis for establishing an acceptable daily intake for humans. The fact
that at 10 mg/kg marginal effects occurred was taken into account by
using a safety factor higher than is usual for
cholinesterase-inhibiting compounds.
The compound was not mutagenic in a dominant lethal test, nor was it
teratogenic. No three-generation study was available. However, such a
study was felt to be important since the depression of the
cholinesterase activity often manifests itself unfavourably in a
reduced viability of the pups.
In a 2-year feeding study in dogs no untoward effects were seen up to
1000 ppm. A long-term feeding study in rats revealed no more than a
moderate increase of the relative liver weights at 3000 ppm.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 (mg/kg bw)/day
Dog: 1000 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Ethiofencarb in a systemic insecticide which has a selective effect
against aphids. It gives control of numerous aphid species on
vegetables, fruit crops, field crops and ornamental plants. It
displays good activity against both susceptible and
organophosphorusresistant strains. Used as a soil treatment, e.g. by
the soil drench method or as a granular application, ethiofencarb
displays long residual activity; when applied to the aerial parts of
plants it is somewhat less persistent. In trials conducted to date,
ethiofencarb has shown no phytotoxicity.
Ethiofencarb is sold or registered in Bulgaria, Chile, Denmark,
Federal Republic of Germany, Israel, Italy, Portugal, Spain, Sweden,
Switzerland and Turkey.
Applications for registration have been filed in a number of other
countries.
Pre-harvest treatments
Ethiofencarb is formulated as an emulsifiable concentrate for spraying
and as a granular product. Used as a spray, it is applied broadcast at
the onset of aphid infestation. As a granular formulation, it is
applied at sowing or planting, mainly in-furrow.
Details of uses and recommendations for pre-harvest treatments with
ethiofencarb are given in Table 2.
Post-harvest treatments
No treatments recommended.
Other uses
Ethiofencarb is also used on ornamental plants.
RESIDUES RESULTING PROM SUPERVISED TRIALS
The residue data obtained after the application of ethiofencarb to
fruit, vegetables and field crops are summarized in Table 3. The data
originate from trials conducted in the U.S.A., Japan, Great Britain,
France, the Netherlands and the Federal Republic of Germany.
The residues were determined by gas-chromatographic methods which
determine residues of ethiofencarb and the metabolites with an intact
carbamate group.
After application by spraying, half-lives of 2 to 18 days were noted
for the total residue.
Detailed studies with different formulations using the same active
ingredient dose produced the following half-lives for the given crops:
Apple: 13 (5-18) days
Bush bean: 5 (4-7 ) days
Head lettuce: 5 (4-6 ) days
Savoy cabbage: 5 (2-9 ) days
With crops of which the foliage and edible parts were analyzed
separately, e.g. potatoes, sugar beet, broad beans and radish, it was
found that the residues were present mainly in the foliage owing to
the pronounced systemic mode of action of ethiofencarb, residues were
found in the analyzed plant samples also after application of the
granular formulation to the soil. Here, too, there was an accumulation
of the residues in the leaves, as proved by studies on broad beans and
sugar beet.
FATE OF RESIDUES
General
The metabolism of ethiofencarb (I) is illustrated in Figure 1. It is
found to be of the same pattern, with oxidation of the sulphur and
hydrolysis of the carbamate group, in soil, plants and animals, with
only quantitative differences. In soil and plants, ethiofencarb
sulphoxide (II, Figure 1) and ethiofencarb sulphone (III) were the
main products with an intact carbamate group. In animal metabolism
studies, phenolic degradation products formed by hydrolysis of the
carbamate group were also detected. CO2 released from the carbamate
group by hydrolysis was found to be incorporated into plants.
In animals
Studies on ethiofencarb metabolism in rats are described previously
under "Biochemical aspects". Marshall and Dorough (1976,1977)
investigated the fate of water-soluble plant metabolites of
ethiofencarb in rats. Bean plants were stem-injected with either
ring-14C- or carbonyl-14C-ethiofencarb. After 20 days almost all
the radiocarbon residue was in the epicotyl leaves. The distribution
in the unextractable, organic and aqueous fraction was 3.3%, 45.8% and
40.7% for the ring label and 1.3%, 13.8% and 2.6% for the carbonyl
label. 14C in the aqueous fractions when orally dosed to rats was
largely excreted within 36 hours, mainly by routes similar to those of
the parent compound, viz. in urine (92% of ring and 39% of CO-label)
and as expired CO2 (30% of CO-label).
Sorghum plants treated with ring-14 C-ethiofencarb (I) in an
emulsifiable spray were used as a source of unextractable metabolites
(Marshall and Dorough, 1977). When these bound residues of I were
administered to rats, 90.8% of the dose was voided in the faeces and
16.4% in the urine within 2 days; the bile contained 2.5% of the dose.
These data demonstrated that bound residues of I were very poorly
absorbed from the gastrointestinal tract of rats, while the conjugated
ones were readily absorbed.
Gronberg at al. (1976) continuously fed I to dairy cows for 28 days at
50, 150 and 500 PPM in the diet, equivalent to 1.5, 4.5 and 15 mg/kg
body weight/day. From the residue data obtained it was calculated that
tissue residues would be less than 0.1 mg/kg I + II + III equivalents
and milk residues would be less than 0.01 mg/kg for animals on a
ration containing 90 ppm and 14 ppm of ethiofencarb respectively.
Residues in milk averaged about 1 mg/kg at the highest feed level.
Tissue residues are shown in Table 4.
A lactating cow and a pig were treated with a single oral dose of 0.5
mg/kg 14C-ring-labelled ethiofencarb (Dorough, 1976h). 24 hours
after administration 97.8% of the dose had been excreted via the urine
from the cow and 90% from the pig. Elimination of the radiocarbon in
the faeces was <1% and 5.1% in cow and pig respectively.
Tissues of the pig contained no detectable radioactive residues 24
hours after treatment. Of the bovine tissues only kidney, liver and
skin contained detectable residues (Table 4) of 0.016, 0.017 and 0.05
mg/kg ethiofencarb equivalents respectively. Maximum concentrations of
14C in the blood of the cow, reached 3 hours following
administration, were 0.3 mg/kg. The milk the peak residue level was
0.15 mg/kg, reached 4 hours after dosing. About 67% of the milk
residue consisted of free metabolites, mainly ethiofencarb sulphoxide
and sulphone.
11.6% of the urinary metabolites in the cow were in the free form,
predominantly as phenol sulphoxide and phenol sulphone. Other
metabolites were eliminated as conjugates. In the pig 40.6% of the
dose was excreted in free form.
Eight laying hens were treated with a single oral dose of
14C-ring-labelled ethiofencarb at a rate of 0.5 mg/kg (Dorough,
1976). over 80% of the administered radiocarbon was eliminated in the
excreta after 24 hours and over 90% after 3 days. The highest
radiocarbon level 8 hours after application was found in kidney at
0.062 mg/kg. Liver, muscle and blood showed lower residue levels and
C, fat and heart no residues could be detected (see Table 4). 24 hours
post-administration no 14C could be detected in the tissues. Eggs
laid during a 3-day period following the single treatment were free
from detectable residues.
In a further study (Dorough,1976i) 10 hens were given
14C-ring-labelled ethiofencarb as daily treatment for 7 days at a
rate of 0.5 mg/kg twice a day. 2 days after the start of treatment
about 60% of the cumulative dose was excreted and after 7 days about
92%. Tissues of animals sacrificed 3-4 hours after the final dose
contained 14C-ethiofencarb equivalents ranging from about 0.02 mg/kg
in fat to 0.32 mg/kg in kidney. 4 days after the termination of
treatment muscle, skin, kidney and liver contained 0.001, 0.006, 0.007
and 0.009 mg/kg 14C-ethiofencarb equivalents respectively. In bloody
brains fat and heart no residues were detectable. Levels of
radiocarbon in the eggs reached a maximum of 0.06 - 0.07 mg/kg after 7
days of continuous treatment. In eggs (white and yolk) residues were
about 0.03 - 0.04 mg/kg after 2 days of treatment, reached a maximum
of 0.06 - 0.07 mg/kg after 7 days and were below detectable levels 3
days after treatment was stopped.
TABLE 2. Use pattern of ethiofencarb
Crop Concentration or Formulation Number of Pre-harvest
application rate (a.i.) applications interval(days)
Vegetables 0.0375-0.05% E.C. 2-3 4-7
(Cucurbitaceae, foliar spray/overall
eggplants, peas application
brassicas, peppers, 0.075 -0.1 g./linear GR 1
lettuce, tomatoes metre at sowing/
Vicia, etc.) planting
Cereals 100-500 g./ha. E.C. 1-2 14-21
(including millet) foliar spray/overall
application
Potatoes 500-600 g./ha E.C. 2-3 14
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at planting
Pome and stone fruit, 0.05% E.C. 2-3 7-10
small (soft) fruit, foliar spray/overall
citrus fruit application
Beets 400-500 g./ha E.C. 2-4 14
foliar spray/overall
application
Tobacco 0.05-0.075% E.C. 2-3 4-7
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at sowing/
planting
TABLE 3. Residues of ethiofencarb, its sulphoxide and sulphone from supervised trials
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Apple D 2-3 1 EC 0.9-2.2 0.4 -2.0 0.4 -1.3 0.4 -1.6 0.2 -1.4 0.3-1.0 1)
(28d:)
F 1 0.5 EC 0.6 0.3 -0.4 0.4 1)
J 3/5 3-3.5 EC 1.2 - 1.3 0.8-1.2 2)
(30d.)
Apricot J 2/4 7.5 gEC/tree 0.8 -1.2 1.8-2.1 3)
(28d.)
Artichoke F 1 0.5-1EC 1.0 0.4 -4.7 0.2 -3.0 0.2 -1.0 1)
Beans
broad beans D 2-3 0.3 EC 1)
beans 0.02-0.04 0.02-0.04 0.02-0.03 <0.02
pods 0.2 -0.43 0.08-0.26 0.05-0.22 0.04-0.11 0.02-0.05
greens 5.5 -10.1 0.8 -4.6 0.6 - 4.6 0.5 -3.1 0.2 -1.0
broad beans D 1 1.5 G (55d.) (89d.) (111d.)
beans 0.02-0.13 0.02-0.04
pods 0.19-0.42 0.19-0.57
greens 14-30 2.2 -6.1 0.17-0.84
bush} D 2-3 0.3 EC 0.14-0.68 0.25-0.43 0.03-0.32 0.02-0.19 0.02-0.11 <0.02 1)
(28d.)
beans} F 1 0.75 EC 1.46-2.47 0.77-1.84
F 1 2 G 0.05 0.05 0.03 1)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Cabbage
Brussels sprouts D 3 0.3 EC 0.5 -1.4 0.4 -0.94 0.2 - 0.9 1)
Cauliflower D 2 0.3 EC 0.3 -0.5 0.1 -0.43 0.05-0.2
F 1 0.5 EC 0.26 0.11 0.07 1)
Chinese cabbage J 3/5 0.75-2EC 0.04-2.7 0.04-1.8 0.06-2.4 4)
(5d.)
Red cabbage D 2 0.3 EC 0.2 -1.1 0.2 - 0.4 0.05-0.5 1)
Savoy cabbage D 2 0.3 EC 0.4 -3.1 0.1 - 1.3 0.05-1.3 0.2 - 0.6 0.06-0.4 0.05-0.3 1)
(46d.) (78d.) (88d.) (28d.)
Savoy cabbage D 1 1 G 0.5 -0.6 0.05 <0.05 1)
White cabbage D 2 0.3 EG 0.2 0.1 <0.05 1)
White cabbage GB 1 3 G 0 09 1)
Cherry (see end of table) (105d.)
Cucumber J 3/5 0.75- 0.7 -0.9 0.3 -0.8 0.2 -0.5 0.1 -0.32 4)
2.5 EC
Currant
Blackcurrant D 2 1 EC 1.1 - 2.8 0.5 - 1.4 0.6 - 1.4
Red currant D 2 1 EC 0.5 - 2.9 0.4 - 1.0 0.2 - 0.6 1)
Eggplant F 1 0.75 EC 0.03-0.5 0.02-1.0 1)
Lettuce
(outdoor) D 2 0.3 EC 4.3 - 10.1 1.3 - 4.4 0.6 - 2.2 0.4 - 0.8 0.2 - 0.6 0.1 - 0.2 1)
(28d. )
(under glass) D 2 0.3 EC 2.3 - 18.8 2.2 - 10.1 1.6 -6.4 1.5 0.5 - 0.8 1)
(under glass) NL 1 5 G (44-67d.) 1)
Peach D 1-2 0.75-1EC 1.2 -2.3 1.1 -1.7 0.5 -2.0 0.4 -1.4 0.3 -1.1
Peach F 1 0.5 EC 0.3 -0.8 0.2 -0.3 0.1 -0.2
Plum (see end of table)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Potatoes D 3 0.4-0.6 0.16-0.23 0.09-0.25 0.08-0.17 0.02-0.16 1)
EC (28d.)
Potatoes US 2-6 0.5-2 EC <0.02-0.06 <0.02-0.06 6)
Potatoes J 4-5 0.9-1.75 0.07-0.1 0.04-0.16 0.04 7)
EC
Potatoes leaf J 1 0.7-1.7 EC 38-57 2.2 -4.6 0.3 -1.3 0.2 -0.6 7)
Potatoes D, NL, 1 1.5 G <0.02-0.06 1)
(91-135d.)
Radish J 3/5 1-1.5 EC 0.10-0.12 0.03-0.14 0.02-0.10
Radish leaf J 3/5 1-1.5 EC 0.1-1.5 0.06-1.2 0.1-0.6 8)
Soybean
Soybean, 3 0.75-1.5 0.02-1.04 <0.02-0.18
green+shell EC
Soybean, bean 3/5 0.75 EC 0.04-0.06 0.02-0.04 9)
(44-67d.)
Wheat 1)
grain D 2 0.4 EC
straw 9.6 -11.4 1.4 -1.9 <0.02 <0.02 1)
grain F 1 0.5 EC 1.2 -1.7 0.06-0.8
straw <0.02
0.1 -0.32
(34.53d.)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Sugar beet
beet D,GB 2-5 0.25-0.5 0.04-0.06 0.03 0.02 0.01-0.03 1)
top (leaf) EC 3.0 -4.7 2.0 -2.2 2.7 -4.9 0.12-1.4
(31-76d.)
beet D, GB 1 1-5-3 G 0.01-0.08 1)
top (leaf) 0.05-1.1
(124-195d.)
Tobacco 3 0.3-0.4 12.5-52 5.4-37 4.5-21 1.3 - 9.3 2.9 - 5.7 1.1 -13
(25-28d.) 1)
air -
cured D 1 1.5 G 4.5 -12.3
(54-58d.+
132-137d.
cured)
Cherry
(morello) D 3 1 EC 1.8 -5.9 0.5 -5.9 1.2 -4.2 1)
Plum D 3 1 EC 2.4 -3.5 1.3 -3.4 1.3 -2.6 1)
Trials in a)
Fed. Rep. Germany = D
France = F
Great Britain = GB
Japan = J
Netherlands = NL
United States Amer. = US
TABLE 3. (Continued)
b)
EC = Emulsifiable concentrate
G = Granular
References c)
1) Bayer AG, 1972/77
2) Nitokuno Inst., 1976
3) Nitokuno Inst., 1975
4) Nitokuno Inst., 1975/76
5) Nitokuno Inst., 1975a
6) Chemagro Report, 1976
7) Nitokuno Inst., 1975/76a
8) Nitokuno Inst., 1976a
9) Nitokuno Inst., 1976b
TABLE 4. Ethiofencarb equivalents in various tissues of warm-blooded animals
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Rat 0.5 mg/kg Ring- 14C 2.5 h 0.021) 0.061) 0.041) 0.031) Dorough,
single oral dose 1976j
Rat 0.5 mg/kg N-Methyl-14C 8 h 0.24 0.88 Hurst and
single oral dose 24 h 0.19 0.46 Dorough,
1976
Rat 6.6 ppm/day in diet 0.24 0.18 0.06 0.29 1.18 0.10 Nye at al.,
for 7 days, carbonyl-14C 1976
Cow 0.5 mg/kg Ring-14C 3 h 0.32 0.016 0.017 0.05 Dorough,a
single oral dose 24 h 1976h
Cow 2xdaily 50 PPM in diet 0.03 0.042) 0.03 0.01 <0.01 0.083) Gronberg
for 28 days et al.,
2xdaily 150 PPm in diet 0.27 0.232) 0.29 0.10 0.01 0.853) 1976
for 28 days
2xdaily 500 PPM in diet 2.17 1.012) 2.53 0.89 0.13 4.313)
for 28 days
Hen 0.5 mg/kg Ring-14C 8 h 0.053 0.028 0.062 0.035 0.039 0.022 Dorough,
single oral dose 1976i
0.5 mg/kg Ring-14C 4 days 0.016 0.001 0.005 0.016 0.025 0.013
2xdaily for 7 days 7 days4) 0.099 0.039 0.019 0.072 0.324 0.180 0.069 0.075
TABLE 4. (Continued)
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Hen 45 ppm in diet for 28 days 0.03 0.04 0.05 0.05 Gronberg
150 ppm in diet for 28 days 0.07 0.13 0.08 0.10 and
450 ppm in diet for 28 days 0.16 0.41 0.29 0.24 Dorough,
1976
1) The values were derived from graphs
2) Mixed omental, renal and subcutaneous fat
3) Mixed loin, round, flank
4) Higher residues at 7 than at 4 days were due largely to the fact that the 7-day birds were sacrificed 3-4 h
after last treatment while 4-day birds were sacrificed 8 h after treatment
The major identified metabolites eliminated in the excreta were the
phenol sulphoxide and sulphone, existing as free metabolites or
conjugates, the proportion of free metabolites being higher in the
singly dosed birds. Small amounts of the sulphoxide and sulphone of
the carbamate were also found in the continuous feeding study only.
The same sulphoxidation products were found as the principal
metabolites in eggs, about 50% of the residue being the phenol
sulphone and about 20% the phenol sulphoxide.
Poultry were continuously fed with ethiofencarb for 28 days at a
ration level of 0, 15, 45, 150 and 450 ppm (Gronberg and Dorough,
1976). The average concentration of ethiofencarb equivalents (Table 4)
in fat, skins muscle and giblet ranged from 0.03 to 0.05 mg/kg at the
45 ppm level, from 0.07 to 0.13 mg/kg at the 150 ppm level and from
0.16 to 0.4 mg/kg at 450 ppm. Average residues in eggs collected at
days 26, 27 or 28 of treatment were approximately 0.01, 0.04 and 0.1
mg/kg at the 451 150 and 450 ppm feeding levels respectively. Prom
these residue data it me concluded that tissue residues would be less
then 0.1 mg/kg and egg residues less than 0.01 mg/kg for animals on a
ration of 100 ppm and 40 ppm respectively.
After continuous exposure of Channel catfish to 10 µg/1
14C-ring-labelled ethiofencarb for 28 days, 14C residues accumulated
to a plateau of about 50-75 mg/kg reached after 4 days. The edible
parts of the fish contained about 27% of the residues. During the
withdrawal period about 82% of the accumulated residues were excreted
wIthin 1 day and about 95% within 4 days (Lamb and Roney, 1976).
In plants
Ethiofencarb has good systemic properties and is absorbed in large
amounts by the plant roots and translocated to the aerial parts
(Homeyer, 1976).
Bean seedlings absorbed a total of 34.6% of the available
14C-ring-labelled ethiofencarb from a solution via the roots in 4
days, whilst 18.1% was found after application of
14C-carbonyl-labelled compound. The distribution of radiocarbon in
the plant after 4 days was: roots 5.1%, stems 9.0%, leaves 16.5%
(Dorough, 1976 g).
Dräger (1976a) applied 10% granules of ethiofencarb (I, Figure 1), its
sulphoxide (II) and its sulphone (III) separately to soil at the time
of sowing beans. Results are shown in Table 5. Generally, the total
residue in the green parts was highest after applying Ill and lowest
after the application of I. The highest residues ware in the green
parts (leaves and stems) followed by pods and beans; the highest
levels were found at the first sampling 4 weeks after sowing.
TABLE 5. Uptake of ethiofencarb (I), its sulphoxide (II) and sulphone (III) by beans
Interval Total residue (mg/kg) after application of
after
application, I Granular II Granular III Granular
weeks
a* b* c* a b c a b c
4 159 - - 210 - - 310 - -
6 61 - - 135 - - 204 - -
8 49 - - 74 - - 96 - -
12 78 0.95 0.27 25 0.18 <0.1 141 0.20 0.06
*a: green parts;
b: pods;
c: beans
Following foliar application of 14C-carbonyl-labelled and
14C-ring-labelled I to beans, potatoes and sorghum, it was found that
14 days after application only 60.7, 29.0 and 45.1% of the insecticide
had been absorbed from the leaf surfaces of the respective plants
(Dorough, 1976g).
Potato and sorghum plants were treated at 0.56 kg/ha with ring-14C-I
in an E.C. formulation. The plants were treated at 10-day intervals
5-7 times during the growing season. Radiocarbon residues were much
lower in potatoes than in sorghum. Total ethiofencarb equivalents
remained essentially constant in potato peels (0.2 mg/kg) and pulp
(about 0.15 mg/kg) for 31 days after treatment. In sorghum the highest
levels of ethiofencarb equivalents were located in the leaves (10-100
mg/kg) followed by heads (5-25 mg/kg), grain (1-10 mg/kg) and stalks
(0.05-0.5 mg/kg). Sorghum leaf levels showed an increase from 85 to
131 mi/kg during the first two weeks but declined to about 94 mg/kg at
31 days. Levels remained relatively constant in the stalks. Levels in
the grain appeared constant (6-9 mg/kg) throughout the entire study
except for the 1 week sample (19 mg/kg). Total radiocarbon in the
heads tripled from 50 mg/kg at time 0 to 167 mg/kg at 1 week and then
declined to 90 mg/kg at 31 days (Dorough, 1976f).
Dräger detected II and Ill in various plant parts after application of
10% granular 14C-carbonyl-labelled ethiofencarb at planting of beans
(0.05 g I/1.6 kg soil; 1976a) and potatoes (0.05 g /approx. 20 1 soil;
1976c). The extractable residues in the roots and green parts of both
potatoes and beans consisted mainly of II and Ill. 0.21-7.79 mg/kg II
and 0.14-0.31 mg/kg III were found in the roots of the potatoes
between days 34 and 96, with 1.39-16.2 mg/kg II and 3.08-8.79 mg/kg
Ill in the green parts. The bigger increase in the sulphone content of
the green parts of the potatoes is indicative of an increased
oxidation of the sulphoxide in the plant system. The two metabolites
were detected also in the potato tubers and beans but there was too
little material available for extensive quantitative studies on the
beans. The potato tubers contained 0.05-0.28 mg/kg II and 0.06-0.14
mg/kg Ill.
The unextractable radioactivity in the green parts of the beans was
less than 1% or the total applied, and accounted for a maximum of 7.7%
of the radioactivity measured at the sampling times (Drägert 1976a).
The major portion of the unextractable radioactivity in the potato
tuber was in the glucose moiety of the starch. Therefore, CO2 formed
by hydrolysis of the carbamate group was utilized in carbohydrate
metabolism. The unextractable but water soluble radioactivity in
rootsy green parts and potato tubers was also considered to be
incorporated in products of carbohydrate metabolism. For details of
the distribution of radioactivity in potatoes and beans, see Table 6.
TABLE 6. 14C-ethiofencarb and metabolites in plants (as % of radioactivity applied or recovered)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Bean 0 day 14CO+ 61.9 29.6 1.6 0.7 origin-0.1; 98.0 Dorough,
x2 = 4.1 1976g
3 days Ring- 10.8 55.9 4.4 6.7 1.9 origin = 11.3; x1=0.5; 94.0
x2 =2.5
7 days 14C 49.7 11.4 1.4 0.5 origin=20.2;x1=0.4; 84.6
x2=1.0
14 days EC 15.6 0.6 1.7 0.6 origin=49.8;x1=0.5 69.1
x2=0.3
Potato 0 day 41.3 50.0 1.0 origin=1.5; x1=1.8; 100.3
x2=4.7
3 days 23.1 48.6 3.3 2.0 origin=5.9; x1=1.8; 91.2
x2=7.9
7 days 7.0 42.2 6.5 2.6 0.4 origin=15.7; x1=1.9; 80.4
x2=4.1
14 days 1.8 38.0 9.6 3.5 0.8 origin=14.0; x1=6.2 77.1
X2=3.2
Sorghum 0 day 73.0 22.8 0.9 origin=0.9; x2=0.9 98.5
3 days 18.1 49.3 1.3 0.8 0.3 origin=5.9; x2=2.3 78.0
7 days 3.2 47.0 4.3 1.3 1.0 origin=10.3; x2=2.0 69.1
14 days 0.3 38.6 6.1 0.7 0.3 origin=23.6; x2=0.5 70.1
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Potato
Peel Ring-
org. 0 day 14C 5.2 10.0 2.7 3.4 2.6 origin=1.3; x1=3.8 29.0 Dorough, 1976f
-soluble
water- EC 1.7 3.3 origin=1.0; x1=2.1 8.1
soluble
unhydrolysed 18.1
by H2O
unextractable 44.8
100.0
(102.4)2)
Potato
Peel
org. 31 days Ring- 3.6 2.7 2.7 8.0 6.3 origin=0.9 24.2 Dorough, 1976f
soluble
water soluble 14C 20.9
unhydrolysed EC 31.0
by H2O
extractable 24.1
100.0
(90.9)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Pulp
org.-soluble 0 day 3.5 7.1 5.8 origin=0.3 16.7
water-soluble 4.2 5.3 origin=0.5; x1=3.9 14.0
unhydrolysed 20.7
by H2O
unextractable 48.6
100.0
(100.6)2)
org.-soluble 31 days 0.7 2.5 0.6 6.6 2.1 origin=0.2 12.7
water-soluble 16.2
unhydrolysed 38.8
by H2O
unextractable 32.3
100.0
(90.6)2)
Sorghum
Leaves
org.-soluble 0 day Ring- 1.4 50.5 11.6 0.9 2.1 5.2 origin=1.0; x3=0.5 73.2
water-soluble 14C 0.2 0.6 8.3 4.1 origin=0.2; x2=0.7 14.1
unhydrolysed EC 5.2
by H2O
unextractable 7.5
100.0
(98.8) 2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Leaves
org-soluble 31 days Ring- 0.4 11.3 15.7 3.1 17.8 origin=0.6; x2=0.4 50.4 Dorough, 1976f
14C x3=0.7; x5=0.4
water-soluble EC 0.6 0.4 4.2 4.9 origin=0.3; x2=0.7 11.1
unhydrolysed
by H2O 12.8
unextractable 25.7
100.0
(99.4)2)
Stalks
org.-soluble 0 day 56.2 15.6 2.5 origin=1.2 75.5
water-soluble 11.4 origin=0.5 11.9
unhydrolysed
by H2O 7.1
unextractable 5.4
99.9
(97.0)2)
Stalks
org.-soluble 31 days 14.6 25.5 1.8 3.0 origin=0.6 45.5
water-solube 27.8 origin=0.9 28.7
unhydrolysed
by H2O 15.3
unextractable 10.5
100.0
(105.4)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Heads
org.-soluble 0 day 1.7 45.2 14.4 0.9 1.8 5.2 origin=0.6; x2=0.7;
x5=2.1 72.6
water-soluble 0.4 2.2 3.9 0.4 origin=0.2; x1=0.3;
x2=0.5 7.9
unhydrolysed
by H2O 4.2
unextractable 15.3
100.0
(95.9)2)
Sorghum
Heads
org.-soluble 31 days Ring- 1.6 26.0 27.1 0.4 1.6 9.9 origin=1.0; x2=1.0; 70.6 Dorough, 1976f
14C x5=2.0
water-soluble EC 0.3 0.3 0.2 3.3 2.8 origin=0.2; x1=0.2; 7.8
x2=0.5
unhydrolysed 4.7
by H2O
unextractable 16.9
100.0
(98.7) 2)
Grain
org.-soluble 0 day 51.1 1.7 5.5 2.2 origin=0.8; x2=0.7;
x5=1.8 63.8
water-soluble 4.2 5.7 origin=0.9; x2=0.6 11.4
unhydrolyzed
by H2O 7.0
unextractable 17.8
100.0
(99.2)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Grain
org.-soluble 31 days 11.2 9.0 1.3 3.7 origin=0.5; x2=0.6;
x5=1.6 27.9
water-soluble 8.4 14.7 0.8 origin=0.5; x1=0.5;
x2=1.0 26.1
unhydrolysed
by H2O 12.5
unextractable 33.5
100.0
(99.3)2)
Potato unextractable by
organic solvents
Roots 34 days 14-CO 0.044 1.26 0.14 solid residue
0.10 in aqueous
layers 0.11 1.654 Dräger, 1976c
68 days Granular 0.055 0.42 0.10 solid residue
0.27 in aqueous
layers 0.15 0.945
96 days soil 0.04 0.05 solid residue
0.11; in aqueous
layers 0.07 0.27
TABLE 6 (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Green parts 34 days 7.15 3.89 solid residue
1.02 in aqueous
layers 0.94
68 days 10.28 9.03 solid residue
2.78 in aqueous
layers 3.04 25.12
96 days 4.38 10.18 solid residue
2.17 in aqueous
layers 3.42 20.15
Tubers 68 days 0.00 10.35 0.18 solid residue
0.973) in aqueous
layers 0.60 2.101
96 days 0.17 0.20 solid residue 2.393)
in aqueous layers 0.92 3.46
Bean
Roots 5 weeks 0.26 0.07 unextractable: 0.07 0.40 Dräger, 1976c
10 weeks 0.15 0.19 unextractable: 0.13 0.47
Green parts 5 weeks 3.53 0.95 unextractable: 0.27 4.75
10 weeks 3.02 4.27 unextractable: 0.61 7.90
TABLE 6 (Continued)
1) Compounds I-VI are identified in Figure 1.
2) The figures in Parentheses indicate percent of total recovery, based on combustion
3) Consisting of:
68days 96days
14C-CO2 (CO3-) 0.04 0.39
14C-Glucose in starch 0.85 1.66
Non-hydrolyzable 0.08 0.34
0.97 2.39
Following foliar and soil application, and stem injection of 14CO-and
ring-14C- labelled ethiofencarb to beans, potatoes and sorghum,
Dorough 1976f,g found that in addition to the major metabolites II and
III, minor amounts of IV, V and VI as well as unidentified metabolites
had formed. For quantitative data, see Table 6.
Bean plants were also treated with II and III (14C-ring-labelled),
After three days, 86.6% of II and 91.7% of III had been converted to
water-soluble metabolites which were easily degraded by acid
hydrolysis (Dorough, 1976g).
In soil
Studies with 14C-carbonyl-labelled ethiofencarb (granular
application; Dräger, 1976c 1977a) showed that ethiofencarb was
oxidized in soil to the corresponding sulphoxide (II) and sulphone
(III). Details are given in Table 7. After 5 weeks II was the main
residue but the proportion of III increased with time. Residues of
ethiofencarb in the first sample taken after 5 weeks were 2 mg/kg in
one experiment(1976c) and undetectable in another (1976a). The
unextractable residues amounted to 3-9% of the applied radioactivity.
Dräger (1977b) also found in laboratory studies with soil 34 clay
after the application of carbonyl-ethiofencarb (1) residues of 4.4% I,
68% II and 14% III. The carbamate group was hydrolyzed to CO2 with a
half-life of about 90 days. After 82 days, 43% of the radioactivity of
the carbonyl group was identified as gaseous CO2 Of the remaining
radioactivity left in the soil, II accounted for 1.7%, III for 38.3%,
and unextractrables for 17%; I could not be detected.
Ethiofencarb was degraded in the field in different soils with
half-lives of 20-30 days (Bayer, 1972/77), and in laboratory studies
with a maximum half-life of 32 days (Nitokuno, 1976d). In other
studies half-lives of approx. 90 and 130 days were obtained.
After the application of ethiofencarb 2 kg/ha as an emulsifiable
concentrate to different types of soil the following maximum residues
(I + II + III) were found: 8.8 mg/kg 0 days after application), 4.4
(7), 3.2 (14), 1.1 (30), 0.6 (60), 0.25 (92) and 0.1 (120) (Bayer
1972/77).
In greenhouse experiments with 14CO-labelled ethiofencarb granules,
Dräger calculated a half-life of about 6-7 weeks from the
radiochemical degradation rate of the total residue in soil planted
with potatoes (1976c) and a half-life of about 2 weeks in soil planted
with beans (1976a).
Half-lives for the degradation of ethiofencarb in soil are presented
in Table 8.
TABLE 7. Distribution of radioactivity in soil after application of
14C-carbonyl ethionfencarb
Compound % of initial 14C remaining on day
35 56 70 34 68 96
Ethiofencarb (I) n.d. n.d. n.d. 2.20 0.22 0.08
Ethiofencarb sulphoxide (II) 16.72 3.14 1.96 41.79 8.10 0.87
Ethiofencarb sulphone (III) 4.37 2.94 2.46 8.18 4.81 3.01
Unextractable 5.45 9.12 7.49 7.32 5.64 2.60
Total 26.54 15.20 11.91 59.49 18.77 6.56
References Dräger, 1976 a,b,c Dräger, 1976 a,b,c
The soils were planted either with beans (0.05 g I/1.6 kg soil;
Dräger, 1976a) or with potatoes T 0.05 g I/approx. 20 1 soil., Dräger,
1976 c) so that a portion of the radioactivity was absorbed by the
plants (see "Fate In Plants").
In laboratory studies to investigate the leaching behaviour of
ethiofencarb, normal dosages of formulations were applied to soil
which were eluted to simulate a rainfall of 200 mm in 2-21 days. The
leachate contained the following percentages of the applied dose as
total residues of I + II + III: low-humus sand 26.6-62.5%; high-humus
loamy sand 7.1-19.4%; sandy loam 18.2-74.5%. When II was applied to
low-humus sand and sandy loam, leachates contained 41% and 57% of the
dosage respectively. After application of Ill, the corresponding
figures were 50% and 40% (Bayer, 1972/77).
Rieck (1976a) found in laboratory studies with ring-labelled
14C-ethiofencarb (columns: 20 cm high and 10 cm diameter; application
of water equivalent to 500 m of rainfall) that the products of
ethiofencarb were not leached as rapidly from the Boils with higher
organic matter as from in the sandier, lean organic soils. In silty
clay, 88% of the radiocarbon was found in the upper 8 cm of the
column. Approximately 1-15% of the radiocarbon was found in the
leachates, of which at least 80% was II. The only other product found
in all the leachates was III. The products that remained in soil were
the same as those in the leachates and were in the same proportion.
Whereas carbamates were found in the leachate in these laboratory
experiments with small soil columns, no ethiofencarb and no products
with an intact carbamate structure were found in leachate samples
examined over a period of 16 months in leaching studies with an intact
soil core (10 cm diameter, 130 cm high) taken from the field after
rainfall typical of practical conditions (755 mm/year) (Dräger,
1976d).
Further studies of the leaching characteristics of aged ethiofencarb
residues under laboratory conditions were carried out by Dräger
(1977c) and Rieck (1976b) In the studies of Dräger, about 40-50% of
the applied 14Carbonyl activity was found in the leachate
irrespective of the soil type. Of this, only part was extractable with
chloroform. The unextractable portion was considered to be carbonate
or hydrogen carbonate formed during the experiment by hydrolysis of
the carbamate group of I (see also "Fate in Water"). The extractable
portions consisted of II and IlI, in a ratio similar to that of the
initial aged residues. I was no longer detectable. Rieck (1976b) also
did not detect I in four different soils after 30 days of aerobic
aging. The predominant residues were II and III, together with a
considerable amount of unextractable radioactive residue. In contrast
to the studies of Dräger (1977c) most of the radiocarbon was found in
the upper 8 cm of the soil with less than 2% in the leachate.
Thorton et al. (1976) compared the leaching behaviour of I with that
of 23 other pesticides. The pesticides were spotted on thin-layer
plates coated with six different types of soil ranging from
non-adsorptive sand to fine-textured clay. The plates were developed
and Rf values were compared. The compounds were grouped into five
categories ranging from immobile to highly mobile. I was placed in
class 49 as one of the mobile compounds.
TABLE 8. Half-lives of ethiofencarb (as total residues
of parent + sulphoxide + sulphone) in various soils
Soil Half-life
(days)
Laboratory trials
Carbon: 1.05%; fines: 30.4%; pH: 5.7 (Laacher Haf) 90
Carbon: 0.8% ; fines: 4.2%; pH: 7.0 (low-humus sand) 96
Carbon: 0.57%; fines: 19.5%; pH: 5.2 (sandy loam) 133
Clay: 14 %; org. matter: 5.85%; pH: 5.7 (sandy loam) 13
Clay: 13.6%; org. matter: 0.8%; pH: 7,0; Cation Exchange 32
Capacity:4-5 me/100g
(sandy loam)
Greenhouse trials
Carbon: 0.1%; fines: 22.1%; pH: 5.8; clay: 9.5%; fine silt: 12.6% ca.14
Carbon: 1.08%; fines: 23.2%; pH: 6.1; water cap.: 19-25% 42-49
Field trials
Clay 3.8%: silt: 18.3%; pH: 6.6; water cap. 36.1% 20
Clay: 13.7% silt: 29.4%; pH: 6.2; water cap. 61.3% 20
Carbon: 1.35%; fines: 9.1%; pH: 7.0 30
Carbon: 0.8 %; fines: 15.3% pH: 6.5 23
Clay: 14%; org. matter: 5.85%; pH: 5.7 (sandy loam) 22
Clay: 40%; org. matter: 5 %;
Cation Exchange Capacity 15-18 me/200 g (loam) 20
In water
Ring-labelled 14C-I was incubated under sterile conditions in aqueous
solutions buffered at pH 3, 6 and 9 at 25, 35 and 45°C. Ethiofencarb
was shown to be stable for 30 days at PH 3 and 25°C, whereas the
half-life at pH 9 and 45°C ranged from 0.3 to 0.7 days (Table 9). The
primary hydrolysis product was IV, Figure 1 (max. 92.6% of the
radioactivity of the sample) with only small amounts (<10%) of II
(Kurtz and Gronberg, 1976). The hydrolysis rates of
carbonyl-14C-labelled I, II and III were determined at pH 9.0, 9.5,
10.0, 10.5 and 11.0. I was 3-5 times as stable as II or Ill. Again,
the half-life of all compounds decreased with increasing pH (Table 9)
(Dorough, 1976 d).
Studies with 14CO-ethiofencarb showed that it decomposed in water (pH
6.8) with a half-life of about 8 days, largely by hydrolysis of the
carbamate group. In contrast to the metabolism in soil and plants, II
and Ill were very minor products: their sum represented about 10% of
the total carbamate residue throughout the experiment. After 31 days,
83% of the original carbonyl carbon could be determined as carbonate
or carbon dioxide gas, 3.4% was present as I, 0.3% as II and 0.2% as
III (Dräger, 1976 b, 1977a)
Photodecomposition
The photodegradation of ring-14C-or carbonyl-14C-I was studies under
artificial and natural sunlight on glass plates, silica gel plates,
soil plates, and in aqueous solution (distilled water or pond water).
5% or less of I was found after 6 hours irradiation. II was the major
product: Ill and VI were found as minor constituents (Dorough,1976e).
For quantitative data, see Table 10.
The effect of anthraquinone as a photosensitizer was profound when
applied as a mixture with I on silica gel plates. Although the extent
of degradation of 1 me essentially unaffected, there was an increase
in the number of minor photoproducts (those less than 1%) with
anthraquinone and an increase in the polar material remaining at the
origin after 7 days of exposure to a sunlamp. Almost no II was
produced and a compound with a similar Rf to IV was the major product
(48% after 7 days of exposure). There were also lesser amounts of Ill
and VI (Dorough, 1976e).
In storage and processing
In head lettuce that had been field-sprayed in accordance with good
agricultural practice, no decline of the total residue was noted after
one year of storage in a deep freezer at -20°C (Bayer, 1974).
TABLE 9. Half-lives of ethiofencarb in buffered solutions.
Half-life of compound
Conditions I II III Ref.
(ethiofencarb) (ethiofenoarb (ethiofencarb
sulphoxide) sulphone)
pH: 9.0 6.67 hours 2.03 hours 2.13 hours Dorough, 1976d
9.5 2.67 hours 0.47 hours 0.72 hours
10.0 1.17 hours 0.23 hours 0.40 hours
10.5 0.43 hours 0.08 hours 0.12 hours
11.0 0.09 hours 0.02 hours 0.03 hours
25 °C: 10 ppm; pH 3 stable Kurtz and
pH 6 324 days Gronberg, 1976
pH 9 3.5 days
25 °C; 100 ppm; pH 3 stable
pH 6 stable
pH 9 2.5 days
35 °C; 10 ppm; pH 3 stable
pH 6 68 days
pH 9 1.5 days
35 °C; 100 ppm; pH 3 stable
pH 6 68 days
pH 9 2.0 days
45 °C; 10 ppm; pH 3 stable
pH 6 22 days
pH 9 0.7 days
45 °C; 100 ppm; pH 3 stable
pH 6 20 days
pH 9 0.3 days
TABLE 10A. Photodegradation of 14c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound1) after indicated time
1 hour 3 hours
Conditions
O I II III IV V VI xn O I II III IV V VI xn
artifical light, 1.0 51.4 44.9 3.5 2.3 1.8 16.3 70.3 4.8 2.9
TLC plates
germicidal light, 14.2 6.6 22.7 24.1 0.6 15.6 9.5 20.1 1.3 10.8 23.9 1.0 14.6 10.0
TLC
plates
natural
light, 0.6 42.1 62.0 0.7 1.9 0.8 1.2 2.9 91.4 2.2 2.7 1.2
TLC plates
artifical light, 1.6 1.0 84.2 4.3 7.1 0.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
germicidal light, 1.6 1.8 80.1 44.4 10.4 1.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
natural light, 1.4 1.3 80.5 5.9 9.5 1.5 1.2 1.9 82.7 6.3 0.5 6.6 0.9
soil plates
natural light, 1.1 0.5 46.1 5.1 1.7 2.3 0.6 36.1 7.7 1.6
glass plates
natural light, 1.9 6.5 78.2 6.5 1.0 4.6 1.6 4.2 2.9 70.1 22.5 6.5 5.3
aquous
solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
TABLE 10B. Photodegradation of 14 c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound 1) after indicated time
6 hours
Conditions
O I II III IV V VI xn
artifical light, 2.4 5.2 78.6 6.3 3.5
TLC plates
germicidal light, 21.2 4.3 8.8 24.3 1.0 14.0 9.7
TLC plates
natural light, 1.4 0.8 90.5 2.7 2.9 1.2
TLC plates
artifical light, 2.1 1.0 83.6 5.9 5.1 1.6
soil plates
germicidal light, 1.9 2.6 76.3 6.5 1.0 9.1 1.3
soil plates
natural light, 1.0 1.1 87.8 5.7 0.8 2.6 1.1
soil plates
natural light, 4.2 0.3 26.0 13.1 0.9 2.3
glass plates
natural light, 5.8 0.4 65.6 11.7 1.8 8.4 3.2
aquous solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
Studies to investigate the stability of the sulphoxide and sulphone
metabolites in potatoes revealed that the content of both compounds
remained constant on storage for 11 months at -20°C (Chemagro, 1976b).
When bovine and poultry tissues, milk and eggs were fortified with
14C-labelled ethiofencarb and stored under frozen conditions for 7
months, 84-98% of the residue was found as intact carbamate (Chemagro,
1976c).
Poultry rations fortified with ethiofencarb for use in various animal
feeding studies were stored for 5 weeks at -10°C; no degradation of
the ethiofencarb was noted (Chemagro, 1975).
Processing of plant material by baking and boiling results in the
reduction of ethiofencarb residues. Broad beans which had a residue
content of 0.13 mg/kg before boiling were found to contain < 0.02 mg
I + II + III/kg after being boiled for 15 minutes. In the green parts
(stems and leaves), the residues were reduced by boiling to between 3
and 7% of the original content of 14-30 mg/kg; 0.4-3.4% of the
original residue (I + II + III) was found in the water used for
boiling (Bayer, 1975).
Raw potatoes fortified with 14C-labelled ethiofencarb (1 mg/kg) were
processed by pan frying, french frying and baking. Both pan and french
frying processes reduced the residue (I + II + III) with an average
loss of 52 and 58%, respectively. Leaching of the residue (2-20%) into
the frying oils and volatilization were observed. The baking process
showed a loss of about 2% of the 14C activity. Of the remaining
activity, ethiofencarb accounted for 66%, ethiofencarb sulphoxide for
14%, and ethiofencarb phenol for 20% (Chemagro Report, 1976d).
METHODS OF RESIDUE ANALYSIS
Residues of ethiofencarb can be determined by gas chromatography using
a sulphur-specific flame photometric detector. The methods available
for the analysis of plant samples# animal tissue samples and soil
samples simultaneously determine metabolites with an intact carbamate
structure, i.e. ethiofencarb sulphoxide and ethiofencarb sulphone.
Oxidation with potassium permanganate converts ethiofencarb and
ethiofencarb sulphoxide to the sulphone. For gas-chromatographic
determination, the total carbamate residue is derivatized by reaction
with bix-trimethylsilytrifluoroacetamide to the thermostable, volatile
2-ethylsulfonylmethylphenyl trimethylsilyl ether.
For the extraction step, acetone (Dräger, 1974) or methanol/water
(Morris and Gronberg, 1976; Dorough, 1976a) were used for plant and
soil samples. Soil samples can also be extracted with a mixture of
acetonitrile and 0.1 × hydrochloric acid (Morris and Gronberg, 1977)
or by reflux with a methanol/chloroform mixture (Morris, 1976a).
Water-containing animal tissues and eggs are extracted with
acetonitrile; prior to extraction, fat is dissolved in hexane and
acetone is added to milk to separate protein (Dorough, 1976b).
Isolation of the residues from the extracts is achieved by shaking
with chloroform, and separation of co-extractives is accomplished by
precipitating with a solution of ammonium chloride/phosphoric acid.
After oxidation of the residues with potassium permanganate to
ethiofencarb sulphone, silylation is carried out in acetone or
benzene.
With some types of sample, further chromatographic clean-up steps are
needed before silylation, in order to separate sulphur-containing
plant co-extractives which interfere with the gas-chromatographic
determination. With brassica extracts clean-up was by thinlayer
chromatography and with hop extracts by column chromatography (Dräger,
1974). In the analysis of animal tissues, eggs and milk, the residue
was hydrolyzed to the corresponding phenol sulphone before silylation
in order to increase reproducibility (Dorough, 1976b).
For the gas-chromatographic determination, columns packed with 5%
DC-200 on Gaschrom Q (Dräger, 1974) and with -3% OV-1 on Chromosorb W
(Morris and Gronberg, 1977) have proved suitable. For a confirmatory
procedure, a column packed with the polar phase Carbowax 20-M on
Chromosorb W (Morris,1976b) was used. The lower limit of determination
is generally 0.02-0.05 mg/kg for plant and soil samples. 0.01 mg/kg
for fat, and 0.005 mg/kg for eggs and milk.
The methods were shown to be specific in a study in which pesticides
registered in the U.S.A. for use on potatoes, sorghum, animal tissues,
milk and eggs were checked for possible interference: none of the 50
chemicals tested interfered at their maximum registered level
(Dorough, 1976c).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The only tolerances reported to the Meeting were on fruit and
vegetables in Switzerland, but pre-harvest intervals have been
specified in several other countries as shown below (Table 11).
APPRAISAL
Ethiofencarb is a systemic insecticide used especially against sacking
insects an vegetables, fruit, field crops and tobacco, It is effective
against some organophosphorasresistant strains. It is formulated an
granules and emulsifiable concentrates. The usual dose is 0.3-2 kg
a.i./ha in spray, or approximately 0.1 g a.i. per linear metre as
granules at sowing or planting. Ethiofencarb is used in various
European countries, Chile and Israel.
In plants and soil ethiofencarb (I) is oxidized to its sulphoxide (II)
and sulphone (III) which are also hydrolysed. In plants and soil the
main intact carbamates are II and III. Little or no ethiofencarb is
usually detectable at harvest. In animals, the corresponding phenols
and their conjugates are the main metabolites.
TABLE 11. National tolerances and pre-harvest intervals reported to the Meeting.
Country Crop Tolerances Pre-harvest
in mg/kg interval in
days
Germany Beets, sugar beets 28
(FRG) Head lettuce 4
Bush beans, pole beans,
broad beans 7
Potatoes 14
Israel Peppers 5
Potatoes 4
Cauliflower, drumhead
cabbage 7
Apples 7
Italy Vegetables 7
Fruit crops 14
Beets 30
Spain Fruit crops incl. citrus,
winter cereals, maize,
potatoes, beets, sugarcane,
cotton, oilseed crops 21
Switzerland Pome, stone and small
fruit 0.2
Vegetables excl. spinach 1.0 14
Fruit crops, peas for
harvesting dry (dried,
threshed) 21
Sugar beet, field beans 42
Half-lives of I + II + III in soil vary between 2 and 12 weeks in the
field.
On the basis of feeding a diet containing 50 ppm of I to cows, pigs
and hens, it can be estimated that residues of I + II + III are
unlikely to exceed 0.02 mg/kg in their meat or in eggs or milk.
Boiling broad beans reduced carbamate residues of 0.13 mg/kg to below
0.02 mg/kg. Cooking the green parts of beans reduced the residues to
3-7% of the original content of I + II + III (14-30 mg/kg). 0.4-3.4%
of these residues was found in the water used for boiling. Deep and
shallow frying of potatoes fortified with 1 mg ethiofencarb/kg
decreased the total carbamate residue by about 50%. Baking potatoes
caused no loss of carbamate residues.
Gas-chromatographic procedures for the determination of residues of I
+ II + III, suitable for regulatory purposes, in various crops, eggs,
milk and soil have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended. They
refer to the am of ethiofencarb, its sulphoxide and its sulphone,
expressed as ethiofencarb.
Commodity Limit, Pre-harvest interval (days)
mg/kg on which recommendations
are based
Cherries, lettuce 10 4-7
Apples, pears, apricots 4-7
Artichokes, beans with pod, 4-7
beet tops,
sugar beet tops (leaves), 14
brassicas peaches plums 5 4-7
Currants (black, red),
eggplants, 4-7
wheat straw 2 14
Cucumbers 1 4
Potatoes, 14
Radishes 0.5 4
Beans without pods
soybeans without pod 0.2 4-7
Fodder beets,
sugar beets (roots) 0.1 14
Raw grain
(barley, oats, rye, wheat) 0.05 30
Meat of cattle, pigs
and poultry, eggs, milk 0.02*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by July 1978)
1. Submission of the results of the reproduction study now in
progress.
2. Further residue data from supervised trials on apricots,
artichokes, various brassicas, cherries, cucumbers (including those
grown under glass), eggplants, plums, radishes, soybeans.
DESIRABLE
1. Further residue data from supervised trials on other crops on which
ethiofencarb is known to be used.
REFERENCES
Bayer, A.G. (1972/77) Ethiofencarb; Pflanzensohutzmittel-Rückstände,
Dräger, Bayer-Leverkusen, unpublished.
Bayer, A.G. (1974) Report RA 301; (unpublished).
Bayer, A.G. (1975) Report RA 655; 20 Nov. 1975.
Bothard, E. and Löser, E. (1976) HOX 1901 Chronic Toxicity Study on
Rats. Nov. 30, Rep. No. 6493 from Bayer AG, Inst. f. Tox., unpublished
report submitted by Bayer AG. Addendum., Histopathological Examination
by J.P. Finn, Hazleton Laboratories, Inc.
Chemagro Report (1975) 45 176; 12 Sept, 1975, R & D Dep. of Chemagro,
Agricult. Div., Mobay Chemical Corp., Kansas City, No. 64 1209 USA.
Chemagro Report (1976a) Chemagro Agricult. Div.; Groneton; Potatoes
(Kats; Russet Burbank), Rep. No. 49 735/49 751; 17 Nov. 1976
(unpublished).
Chemagro Report (1976b) No. 51 062; 30 Dec. 1976 (unpublished).
Chemagro Report (1976c) No. 49 896; 27 Dec. 1976.
Chemagro Report (1976d) No. 46995; 11 Feb. 1976.
Dorough H.W. (1976a) A Gas Chromatographic Method for the
Determination of Croneton Residues in Various Animal Tissues, Crops,
and Soil. CR 49 879, 1.12.1976.
Dorough H.W. (1976b) A Gas Chromatographic Method for the
Determination of Croneton Residues in Bovine Tissues and Milk and
Poultry Tissues and Eggs. CR 49 880; 18.11.1976.
Dorough H.W. (1976c) An Interference Study for the Residue Method for
Croneton and Metabolites in Various Crops and Animal Tissuee, Milk and
Eggs. CR 49 893, 17.12.1976.
Dorough H.W. (1976d) Rate of Hydrolysis of CRONETON TM GRONETON
Sulphoxide, and CRONSTON Sulphone in Aqueous Solutions. Chemagro
Report No. 49 890 (1976).
Dorough H.W. (1976e) Photodcoomposition of CRONETON TM on Various
Substrates. Chemagro Report No. 49 891 (1976).
Dorough H.W. (1976f) The Metabolism and Residue in Potatoes and
Sorghum Treated Under Field Conditions Using CRONETON
TM-ring-UL-14C. Chemagro Report No. 49 894 (1976).
Dorough H.W. (1976g) The Metabolism of CRONETON TM in Plants.
Chemagro Report No. 49 895 (1976).
Dorough H.W. (1976h) Metabolism of CRONETON TM in a Lactating
Holstein Cow and a Male Yorkshire Swine. Chemagro Report No. 49 897
(unpublished).
Dorough H.W. (1976i) The Metabolism of CRONETON TM Poultry. Chemagro
Report No. 49 898 (unpublished).
Dorough, H.W. (1976j) Correlation of Blood and Tissue Radiocarbon
Levels Following a Single Oral Dose of CRONETON TM-ring-14C to Rats.
Chemagro Report No. 49 887 (1976).
Dräger, G. (1974) Method for gas-chromatographic determination of
Croneton residues in plants and soil. Pflanzenschutz-Nachrichten Bayer
91, 144-155 (1974).
Dräger, G. (1976a) Study to investigate uptake and metabolism of
Croneton in beans (Vioia faba) after application in granular form.
Bayer AG, Pflanzensohutz-Anwendungstechnik, unpublished Report RA-79,
January 19, 1976.
Dräger, G. (1976b) Studying the Metabolism of Croneton in Water. Bayer
AG Pflanzenschutz-Anwenclungstcohnik, unpublished Report RA-438v June
9, 1976.
Dräger, G. (1976c) Studies on the Metabolism of CRONETON in Potatoes
and Soil and Identification of Bound Residues in the Potato Tuber.
Bayer AG, Pflanzenschut Anwendungstechnik, unpublished Report RA-7989
October 89 1976.
Dräger, G. (1976d) Untersuchunger zum Versickerungsverhalten von
CronetonR (Makro-Säulen-Versuch). Bayer AG,
Pflanzenschutz-Anwenclungeteohnik, unpublished Report RA-961.
Dräger, G. (1977a) Untersuchung des Metabolismus von Groneton in Boden
und Wasser. Pflanzenschutz-Nachrichten Bayer 30, 18-27 (1977).
Dräger, G. (1977b) Weiterer Abbau, von gealterten Croneton-Rückständen
im Boden unter Laborbedingungen Bayer AG Pflanzenschutz-
Anwendungsteohnik, unpublished report RA-133, February 16, 1977
Dräger, G. (1977c) Versickerungsverhplten von gealterten
Groneton-Rlickstgnden in Boden unter Laborbedingunger, Bayer AG,
Pflanzenschutz-Anwendungsteohnik, unpublished Report RA-346.
Gronberg, R.R., Dorough, H.W., (1976) Effect of Feeding CRONTEONTM
to Poultry. Chemagro Report No. 49 899 (unpublished).
Gronberg, R.R., Dorough, H.W., Hemken, R.W. (1976) Effect of Feeding
CRONETONTM to Dairy Cattle. Chemagro Report No. 50 500 (1976)
(unpublished).
Hoffmann, K. and Weischer, C.H. (1976) 1901/Chronio Study on Dogs. May
26, Rep. No. 6120 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Homeyer, B. (1976) Croneton als wurzelaystemieches Aphizid.
Ptlanzenschutz-Nachrichten Bayer 29 255-266 (1976).
Hurst, H., Dorough, H.W. (1976) The Metabolism of CRONETON TM-
N-methyl-14C in Rats-A Progress Report. Chemagro Report No. 49 886
(1976).
Kimmerle, G. (1972b) HOX 1901/Acute Toxicity Studies. July 10, Rep.
No. 3557 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. (1972c) HOX 1901/Acute orale Toxizität an männlichen
Kaninchen. Nov. 13, report from from Bayer AG, Inst. f. Tox.,
unpublished report submitted by Bayer AG.
Kimmerle, G. (1972d) Antidotal Effect of Atropine. July 10, report
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Kimmerle, G. (1972e) HOX 1901/Subacute Toxicity Studies. July 10, Rep.
No. 3558 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. and Eben, A. (1974) HOX 1901/Effect of Acute and Subacute
Oral Administration and Subacute Inhalation on Plasma, Erythrocyte and
Brain Acetylcholinesterase Activity in Rats and Dogs. Aug. 8, Rep. No.
4843 from Bayer AG, Inst. f. Tox.p unpublished report submitted by
Bayer AG.
Klimmer, O.R. (1973) Study Report / Bayer HOX 1901/Subchronic
Toxicological Studies on Rats. Febr. 27, Rep. No. R 739 from
Pharmakologisches Instit der Rhein. Friedr.-Wilh.-Univ., Bonn,
unpublished report submitted by Bayer, AG.
Kurtz, S.K., Gronberg, R.R. (1976) The Stability of CRONETON TM in
Aqueous Systems. Chemagro Report No. 50 798 (1976).
Lamb, D.W., Roncy, D.J. (1976) Accumulation and Persistence of
Residues in Channel Catfish Exposed to CRONETON-14C. Chemagro Report
No. 49 128 (unpublished).
Lamb, D.W. and Matzkanin, G.S. (1977a) The Acute Oral Toxicity of
CRONETON Sulphoxide. Febr. 18, Rep. No. 51 716 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lambt D.W. and Matzkanin, G.S. (1977b) The Acute Oral Toxicity of
CRONETON Sulphone. Febr. 21, Rep. No. 51 717 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, G.S. (1977c) The Acute Oral Toxicity of
CRONETON Phenol. Febr. 17, Rep. 51 719 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkaning G.S. (1977d) The Acute Oral Toxicity of
CRONETON Phenol Sulphoxide. Febr. 17, Rep. No. 51 718 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977e) The Acute Oral Toxicity of
CRONETON Phenol Sulphone. Febr. 17, Rep. No. 57 715 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977f) The Acute Oral Toxicity of
CRONETON (Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroxy). April
26, Rep. No. 52 878 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977g) Acute Oral Toxicity of CRONETON
(Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroyy) Sulphoxide.
April 29, Rep. No. 52 879 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977h) The Acute Oral Toxicity of
CRONETON Metabolite N-Methyl (Hydroxy) Sulphone. April 29, Rep. No. 52
880 from Mobay Chem. Corp., unpublished report submitted by Bayer AG.
Machemer, L. (1973a) HOX 1901/Dominant Lethal Test on the Male Mouse
to Test for Mutagenic Effect. Dec. 14, Rep. No. 4339 from Bayer AG,
Inst. f. Tox., unpublished report submitted by Bayer AG.
Machemer, L. (1973b) HOX 1901/Studies for Embryotoxic and Teratogenic
Effects on Rats Following Oral Administration. July 25, Rep. No. 4174
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Machemer, L. (1975) HOX 1901/Studies of Embryotoxic and Teratogenic
Effect on Rabbits Following Oral Administration. July 24, Rep. No.
5545 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Marshall, T., Dorough, H.W. (1976) Fate of Water Soluble CRONETON TM
Plant Metabolites in Rats (Progress Report). Chemagro Report No. 49
892 (1976).
Marshall, T.C., Dorough, H.W. (1977) Bioavailability in Rate of Bound
and Conjugated Plant Carbamate Insecticide Residues. Chemagro Report
No. 52 737 (1977).
Morris, R.A. (1976a) Determination of Baygon, Baytex, Bolster,
Croneton, Dasanit, Di-Syston, Dylox, Guthion, Hinosan, Mesurol,
Meyasystox-R, Monitor, Morestan, Nemacur, Systox Residues in Soil. CR
49 675, 15.9.1976.
Morris, R.A. (1976b) A Confirmatory Procedure for the Analytical
Residue Method for Croneton in Potatoes, Plant Forage and Cereal
Grains. CR 51 0639 30.12.1976.
Morris, R.A. Gronberg, R.R. (1976) A Gas Chromatographic Method for
the Determination of Residues of Croneton (Bay BOX 1901) and
Metabolites in Potatoes, Plant Forage, and Cereal Grains. CR 49 666#
23.8.1976.
Morris R.A., Gronberg, R.R. (1977) A Gas Chromatographic Procedure for
the Determination of Croneton (Bay HOX 1901) in Soil. CR 49 6659
10.2.1977.
Mürmann, P. (1973) HOX 1901/Subohronic Toxicity Study on Dogs. June
28, Rep. No. 4143 from Bayer AG, Inst. f. Tox., (unpublished).
Nitokuno Inst., (1975a) Report No. 3599 unpublished.
Nitokuno Inst., (1975b) Reports No. 335 and 336, unpublished.
Nitokuno Inst., (1975/76a) Reports No. 333, 334 and 390, unpublished.
Nitokuno Inst., (1975/76b) Reports No. 360-362 and 395-396,
unpublished.
Nitokuno Inst., (1976a) Report No. 379, unpublished.
Nitokuno Inst. (1976b) Reports No. 391-1949 unpublished.
Nitokuno Inst. (1976c) Reports No. 397-4009 unpublished.
Nitokuno Inst. (1976d) NIHON TOKUSHU NOYAKU SEIZO K.K. Residual
Analysis Results of HOX 1901 (ethiofencarb) in Upland Soil. Report No.
1042 (RA), January, 28, 1976.
Nye, D.E., Hurst, H.E., Dorough, H.W. (1976) Fate of Croneton
(2-Ethylthiomethylphenyl N-Methyl-carbamate) in Rats. J. Agric. Food
Chem. 24, 371-377.
Rieck, C.E. (1976a) Leaching characteristics of CRONETON TM in
various soils. Chemagro Report No. 50 783.
Rieck, C.E. (1976b) Leaching characteristics of CRONETON TM on aged
soil. Chemagro Report No. 51 028.
Thorton, J.S., Hurley, J.B. and Obrist, J.J. (1976) Soil thin-layer
mobility of twenty four pesticides chemicals. Chernagro Report No. 51
016.
Thyssen, J. and Kimmerle G. (1975) Präparat 6757/Untersuchungen zur
Anwendungstoxikologie. Nov. 27, Rep. No. 5751 from Bayer AG, Inst. f.
Tox., unpublished report submitted by Bayer AG.
Thyseen, J. (1975a) HOX 1901/Neurotoxicity Studies on Hens. Nov. 18,
Rep. No. 5735 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG. Appendix: ROX 1901 Neurotoxicity Studies on
Hens/Histopathological Finding by Prof. Dr. U. Mohr, Med. Hoohsohule
Hannover.
Thyssen, J. (1975b) Studies to Investigate Acute Oral Toxicity of HOX
1901 when Administered Simultaneously with Malathion or EPN, Nov. 18,
Rep. No. 5732 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Thyssen, J. (1976) HOX 1901-Metaboliten. Sept. 1, report from Bayer
AG, Inst. f. Tox., unpublished report submitted by Bayer AG.
See Also:
Toxicological Abbreviations
Ethiofencarb (ICSC)
Ethiofencarb (Pesticide residues in food: 1978 evaluations)
Ethiofencarb (Pesticide residues in food: 1980 evaluations)
Ethiofencarb (Pesticide residues in food: 1981 evaluations)
Ethiofencarb (Pesticide residues in food: 1982 evaluations)
Ethiofencarb (Pesticide residues in food: 1983 evaluations)
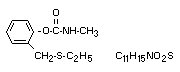 Other information on identity and properties
Molecular weight: 225
Specific gravity: 1.147 at 20° C
4°
Appearance: colourless crystals (pure a.i.)
Melting point: 33.4° C (pure a.i.)
Boiling point: decomposes
Vapour pressure: 5 × 10-6 mbar at 20° C
Solubility (pure): in water (20° C): approx. 0.19 g/100 g
in methylene chloride,)
propan-2-ol ) >60 g/100 g
and toluene: )
in ligroin (80-110° C): 1-5 g/100 g
Formulations: 10% Granular, 10% and 50% emulsifiable
concentrates
Minimum purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical ethiofencarb was
reported to the Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Studies on these points have been conducted with samples of
ethiofencarb labelled with 14C either in the ring or in the carbonyl
carbon. These studies have been described in several reports, all
issued during 1976-77. These experiments have demonstrated that
regardless of the position labelled the compound is readily absorbed
by the oral route, and is rapidly eliminated thereafter.
When administered as a single oral dose to rats, 41% of the
carbonyl-labelled compound was eliminated in the urine and 7% in the
faeces (within 72 hr), while 47% was eliminated as 14CO2. When
similarly administered but in ring-labelled form, 96% was eliminated
in the urine and 2% in the faeces within 72 hr. The compound,
therefore, undergoes hydrolysis, producing a phenol and a carbamic
acid derivative which breaks down further to CO2.
Maintenance of rats on a diet containing 6.6 ppm of ethiofencarb gave
similar results with the total excretion (urine plus faeces)
decreasing to 1% of one day's intake by the third day after return of
the animals to the normal diet. The major metabolites identified were
the sulphoxide and sulphone of ethiofencarb and the sulphoxide and
sulphone of the phenol; the latter two compounds were excreted mainly
as conjugates, and the parent compound was detected in the urine only
in trace amounts. Tissue residues detected after either a single oral
dose of 0.5 mg/kg b w or administration of 6.6 ppm in the diet for one
week were below 1 mg/kg, and these declined more rapidly after
administration of the ring-labelled than the carbonyl-labelled
compound. This suggests that the carbonyl-labelled residues were
incorporated into normal tissues components, as would be expected (Nye
et al., 1976).
Experiments bearing on the metabolism of ethiofencarb by livestock and
poultry and of some of its plant metabolites by rats, are described in
the section "Fate of residues", "In animals". Metabolic pathways are
illustrated in Figure 1.
Effects on enzymes and other biochemical parameters
The depression of acetylcholinesterase activity in plasma,
erythrocytes and brain was investigated in rats and dogs (Kimmerle and
Eben, 1974).
Rats were treated with single oral doses of 0, 2.5, 10 and 50 mg/kg
and 0, 2.5, 10, 40, 125, 210 and 330 mg/kg for females and males
respectively. The maximum dose-related level of plasma cholinesterase
activity depression of 60% in females treated with 50 mg/kg and of
80-100% in males treated with 330 mg/kg was reached about 0.5 to 5 hours
after administration. Enzyme activity increased again thereafter. Two
males of the 330 mg/kg group died one hour after treatment.
Cholinesterase activity depression was lower in erythrocytes and brain
than in plasma. Maximum depression levels were reached after about 2
hours in erythrocytes and after about 1 hour in the brain.
In a 4-week oral study male and female rats received the test compound
daily at a rate of 0, 5, 10, 20 and 40 mg/kg. Dose-related depression
of plasma- and erythrocyte acetylcholinesterase activity was found in
the animals treated with 20 and 40 mg/kg. Male rats showed within 2
hours after administration of 10 mg/kg bw a depression of
plasma-cholinesterase of 25% and of erythrocyte-cholinesterase of 15%.
In male and female rats which were exposed for a period of 3 weeks to
concentrations of 0, 5.0, 29.7 and 148.4 mg/m3 of air for 6 hours on
5 consecutive days per week the concentrations of 29.7 mg/m3 and
above caused depression of the plasma cholinesterase, whereas
erythrocyte cholinesterase activity was depressed only at the highest
concentration level.
Following single oral administration of 5, 10, 25 and 50 mg/kg,
dose-dependent depression of the cholinesterase activity was found in
plasma at dose levels of 10 and above. Maximum depression was found
1 - 2 hours after administration. 3 hours post-administration the
activity increased again (Kimmerle and Eden, 1974).
TOXICOLOGICAL STUDIES
Special study on mutagenicity
In a dominant-lethal test, groups of 20 NMRI male mice were treated
with a single oral dose of 0 and 25 mg/kg. The male mice were mated
with untreated females for 8 consecutive weeks. The acute oral dose of
25 mg/kg was a sublethal dose causing transient toxicity symptoms such
as slight somnolence and piloerection. The application did not
influence the fertility of the male rats. This experiment gave no
indication of treatment-related pre- and post-implantation losses and
therefore gave no indication of a mutagenic effect of the test
substance (Machemer, 1973a).
Special study on neurotoxicity
A group of 30 atropinized hens was treated with a single oral LD50
dose of 870 mg/kg. During the 3-week observation period only one hen
died. After 3 weeks the survivors were atropinized again and re-dosed
with 870 mg/kg. Three other animals died 3 weeks after treatment. No
neurotoxic symptoms wore observed. The histopathological investigation
of the brain, spinal marrow and Nn. ischiadici gave no indication of
nerve damage, whereas the positive control animals which had been
treated with a single oral dose of 375 mg/kg tri-o-cresyl phosphate
(TOCP) showed evidence of delayed neurotoxicity with respect to
clinical symptoms as well as histopathology.
The test compound did not show any neurotoxic effect (Thyseen, 1975a).
Special study on teratogenicity
Rats
Groups of 20 female rats were treated with daily oral doses of 0, 5,
15 and 40 mg/kg from gestation day 6 to 15. None of the studied dose
levels had any adverse effect on the dams with respect to their health
condition and fertility. The embryonic and foetal development was not
affected by the treatment up to and including the dose level of 15
mg/kg. Treatment of the dams with 40 mg/kg caused reduction of the
average foetus weight. Two foetuses of dams treated with 40 mg/kg
showed multiple malformations, which are considered to be spontaneous
malformations of the strain. In a repeat experiment these
malformations could not be verified. Signs of immature skeletal
development in some foetuses and a higher frequency of slight bone
alterations of the 40 mg/kg group in comparison with controls were
found in the repeat experiment.
Based on these data the test compound was considered not to be
embryo-toxic or teratogenic (Machemer, 1973b).
Groups of 10 pregnant rabbits received daily oral doses of 0, 5, 15
and 40 mg/kg from gestation day 6 through 18. The administration did
not have any adverse effects on the dose. No significant differences
between the control group and treated groups with respect to embryonic
and foetal development could be found. The only malformed foetus
occurring in the 40 mg/kg group was considered to be a spontaneous
malformation. Therefore the test-compound at doses up to and including
40 mg/kg was considered not to have teratogenic effects (Machemer,
1975).
Special study on potentiation
The simultaneous administration of ethiofencarb with either malathion
or EPN to rats caused no potentiation effects on the acute oral
toxicity (Thysson, 1975b).
Simultaneous oral administration of ethiofencarb in combination with
trichlorfon produced likewise only a less than additive acute toxicity
(Thyssen and Kimmerle, 1975).
Other information on identity and properties
Molecular weight: 225
Specific gravity: 1.147 at 20° C
4°
Appearance: colourless crystals (pure a.i.)
Melting point: 33.4° C (pure a.i.)
Boiling point: decomposes
Vapour pressure: 5 × 10-6 mbar at 20° C
Solubility (pure): in water (20° C): approx. 0.19 g/100 g
in methylene chloride,)
propan-2-ol ) >60 g/100 g
and toluene: )
in ligroin (80-110° C): 1-5 g/100 g
Formulations: 10% Granular, 10% and 50% emulsifiable
concentrates
Minimum purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical ethiofencarb was
reported to the Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Studies on these points have been conducted with samples of
ethiofencarb labelled with 14C either in the ring or in the carbonyl
carbon. These studies have been described in several reports, all
issued during 1976-77. These experiments have demonstrated that
regardless of the position labelled the compound is readily absorbed
by the oral route, and is rapidly eliminated thereafter.
When administered as a single oral dose to rats, 41% of the
carbonyl-labelled compound was eliminated in the urine and 7% in the
faeces (within 72 hr), while 47% was eliminated as 14CO2. When
similarly administered but in ring-labelled form, 96% was eliminated
in the urine and 2% in the faeces within 72 hr. The compound,
therefore, undergoes hydrolysis, producing a phenol and a carbamic
acid derivative which breaks down further to CO2.
Maintenance of rats on a diet containing 6.6 ppm of ethiofencarb gave
similar results with the total excretion (urine plus faeces)
decreasing to 1% of one day's intake by the third day after return of
the animals to the normal diet. The major metabolites identified were
the sulphoxide and sulphone of ethiofencarb and the sulphoxide and
sulphone of the phenol; the latter two compounds were excreted mainly
as conjugates, and the parent compound was detected in the urine only
in trace amounts. Tissue residues detected after either a single oral
dose of 0.5 mg/kg b w or administration of 6.6 ppm in the diet for one
week were below 1 mg/kg, and these declined more rapidly after
administration of the ring-labelled than the carbonyl-labelled
compound. This suggests that the carbonyl-labelled residues were
incorporated into normal tissues components, as would be expected (Nye
et al., 1976).
Experiments bearing on the metabolism of ethiofencarb by livestock and
poultry and of some of its plant metabolites by rats, are described in
the section "Fate of residues", "In animals". Metabolic pathways are
illustrated in Figure 1.
Effects on enzymes and other biochemical parameters
The depression of acetylcholinesterase activity in plasma,
erythrocytes and brain was investigated in rats and dogs (Kimmerle and
Eben, 1974).
Rats were treated with single oral doses of 0, 2.5, 10 and 50 mg/kg
and 0, 2.5, 10, 40, 125, 210 and 330 mg/kg for females and males
respectively. The maximum dose-related level of plasma cholinesterase
activity depression of 60% in females treated with 50 mg/kg and of
80-100% in males treated with 330 mg/kg was reached about 0.5 to 5 hours
after administration. Enzyme activity increased again thereafter. Two
males of the 330 mg/kg group died one hour after treatment.
Cholinesterase activity depression was lower in erythrocytes and brain
than in plasma. Maximum depression levels were reached after about 2
hours in erythrocytes and after about 1 hour in the brain.
In a 4-week oral study male and female rats received the test compound
daily at a rate of 0, 5, 10, 20 and 40 mg/kg. Dose-related depression
of plasma- and erythrocyte acetylcholinesterase activity was found in
the animals treated with 20 and 40 mg/kg. Male rats showed within 2
hours after administration of 10 mg/kg bw a depression of
plasma-cholinesterase of 25% and of erythrocyte-cholinesterase of 15%.
In male and female rats which were exposed for a period of 3 weeks to
concentrations of 0, 5.0, 29.7 and 148.4 mg/m3 of air for 6 hours on
5 consecutive days per week the concentrations of 29.7 mg/m3 and
above caused depression of the plasma cholinesterase, whereas
erythrocyte cholinesterase activity was depressed only at the highest
concentration level.
Following single oral administration of 5, 10, 25 and 50 mg/kg,
dose-dependent depression of the cholinesterase activity was found in
plasma at dose levels of 10 and above. Maximum depression was found
1 - 2 hours after administration. 3 hours post-administration the
activity increased again (Kimmerle and Eden, 1974).
TOXICOLOGICAL STUDIES
Special study on mutagenicity
In a dominant-lethal test, groups of 20 NMRI male mice were treated
with a single oral dose of 0 and 25 mg/kg. The male mice were mated
with untreated females for 8 consecutive weeks. The acute oral dose of
25 mg/kg was a sublethal dose causing transient toxicity symptoms such
as slight somnolence and piloerection. The application did not
influence the fertility of the male rats. This experiment gave no
indication of treatment-related pre- and post-implantation losses and
therefore gave no indication of a mutagenic effect of the test
substance (Machemer, 1973a).
Special study on neurotoxicity
A group of 30 atropinized hens was treated with a single oral LD50
dose of 870 mg/kg. During the 3-week observation period only one hen
died. After 3 weeks the survivors were atropinized again and re-dosed
with 870 mg/kg. Three other animals died 3 weeks after treatment. No
neurotoxic symptoms wore observed. The histopathological investigation
of the brain, spinal marrow and Nn. ischiadici gave no indication of
nerve damage, whereas the positive control animals which had been
treated with a single oral dose of 375 mg/kg tri-o-cresyl phosphate
(TOCP) showed evidence of delayed neurotoxicity with respect to
clinical symptoms as well as histopathology.
The test compound did not show any neurotoxic effect (Thyseen, 1975a).
Special study on teratogenicity
Rats
Groups of 20 female rats were treated with daily oral doses of 0, 5,
15 and 40 mg/kg from gestation day 6 to 15. None of the studied dose
levels had any adverse effect on the dams with respect to their health
condition and fertility. The embryonic and foetal development was not
affected by the treatment up to and including the dose level of 15
mg/kg. Treatment of the dams with 40 mg/kg caused reduction of the
average foetus weight. Two foetuses of dams treated with 40 mg/kg
showed multiple malformations, which are considered to be spontaneous
malformations of the strain. In a repeat experiment these
malformations could not be verified. Signs of immature skeletal
development in some foetuses and a higher frequency of slight bone
alterations of the 40 mg/kg group in comparison with controls were
found in the repeat experiment.
Based on these data the test compound was considered not to be
embryo-toxic or teratogenic (Machemer, 1973b).
Groups of 10 pregnant rabbits received daily oral doses of 0, 5, 15
and 40 mg/kg from gestation day 6 through 18. The administration did
not have any adverse effects on the dose. No significant differences
between the control group and treated groups with respect to embryonic
and foetal development could be found. The only malformed foetus
occurring in the 40 mg/kg group was considered to be a spontaneous
malformation. Therefore the test-compound at doses up to and including
40 mg/kg was considered not to have teratogenic effects (Machemer,
1975).
Special study on potentiation
The simultaneous administration of ethiofencarb with either malathion
or EPN to rats caused no potentiation effects on the acute oral
toxicity (Thysson, 1975b).
Simultaneous oral administration of ethiofencarb in combination with
trichlorfon produced likewise only a less than additive acute toxicity
(Thyssen and Kimmerle, 1975).
 Acute toxicity
TABLE 1a, Acute toxicity of ethiofencarb
Species Sex Route LD50 References
mg/kg
Rat m oral 308 Thyssen and Kimmerle, 1975
Rat m oral 411 Kimmerle 1972b
Rat f oral 499 ibid.
Rat m i.p. 41.8 ibid.
Rat f i.p. 41.9 ibid.
Rat m dermal >1000 ibid.
Mouse m oral 256 ibid.
Mouse f oral 224 ibid.
Rabbit m oral approx. 225 Kimmerle 1972c
Rabbit m dermal >8000 Lamb and Matzkanin 1976a
Rabbit f dermal >8000 ibid.
Dog f oral >50 Kimmerle 1972b
Hen oral approx. 1000 ibid.
The typical symptoms of acetylcholinocaterase inhibition such as
salivation, muscle tremors, spasms and convulsions preceded death.
A good antidotal effect was obtained when rats orally treated with
ethiofencarb received i.p. injection of atropine sulphate (Kimmerle
1972d).
Short term studies
Rat
Groups of 15 male and 15 female rats were treated orally for 30 days
at dose levels of 0, 5, 10, 20 and 40 mg/kg. The administration had no
effects with respect to physical appearance, behaviour, body weight,
haematological and clinical chemistry test values. No gross
pathological alterations of tissues were observed and organ weights
did not significantly differ from those of the control animals.
Tissues were not examined microscopically (Kimmerle, 1972e).
In another subacute study 10 male and 10 female rats per dosage group
were exposed for 6 hours daily on 5 consecutive days per week over a
3-week period to aerosol concentrations of 0, 29.7 and 148.5 mg/m3
of air. All these concentrations were tolerated without any adverse
effect with respect to behaviour, general health condition, body
weight, haematological and clinical chemistry values.
The macroscopic examination of the major tissues revealed no
pathological alteration and organ weights did not differ significantly
between the control group and the treated groups (Kimmerle, 1972e).
Groups of 20 male and 20 female rats were fed ethiofencarb at
concentrations of O, 250, 500 and 1000 ppm for 3 months. Physical
appearance, behaviour, mortality and food consumption were not
affected by the diet. The average body weights of male rats fed 500
and 1000 ppm were higher than the corresponding weights of the control
animals. Values of urinalyses, haematology and clinical chemistry were
within the physiological range. Macroscopic and microscopic
examination of the principal organs revealed no treatment-related
alterations. The relative liver weights in male rats treated with 1000
ppm were higher than those of the control animals (Klimmer, 1973).
Dog
Groups of 4 male and 4 female dogs were maintained for 3 months on a
diet containing ethiofencarb at concentrations of 0, 100, 300 and 1000
ppm. The treatment did not effect physical appearance, behaviour, food
consumption, body weight, mortality or ophthalmoscopic and
neurological findings. Parameters of haematology, clinical chemistry
and urinalyses showed no significant differences between control and
treated groups. At 1000 ppm an increase of the relative liver weights
of about 10% was noted in both sexes and an increase of about 40% in
relative kidney and spleen weights in male animals only. Gross and
histopathological examination showed no treatment-related alteration
(Mührmann, 1973).
Groups of 4 male and 4 female dogs received a diet for a period of 104
weeks containing ethiofencarb at concentrations of 0, 330, 1000 and
3000 ppm. The administration of up to and including 1000 ppm did not
affect the test animals with respect to their physical appearance,
behaviour, food consumption and ophthalmoscopic findings. The dietary
level of 3000 ppm caused vomiting immediately after feeding,
especially in the second half of the study; food consumption was
retarded and reduced in some female dogs at the 3000 ppm level,
whereas female dogs at 330 and 1000 ppm made bigger gains in
comparison with controls. Haematological and urinalyses values
revealed no pathological alterations. Results of chemistry tests (with
respect to blood sugar, urea, creatinine, total protein, GOT,
bilirubin) were within the physiological range. The ALP activities
showed a 3 -4 fold increase at 3000 ppm and in some dogs of the same
group an increased level of GPT activity and cholesterol were noted.
Relative liver weights of the animals of both sexes at 1000 and 3000
ppm showed a dose-related increase. One dog at the 3000 ppm feeding
level showed enlargement of the liver. At 3000 ppm the
histopathological examination of tissues disclosed signs of treatment-
related alterations in liver such as enlarged hepatocytes, hepatocytes
nuclei and granular cytoplasmic structures (Hoffmann and Weischer,
1976).
Long term studies
Rat
Groups of 50 male and 50 female (100 male and 100 female in control
group) were fed a diet containing ethiofencarb at concentrations of 0,
330, 1000 and 3000 ppm for 24 months. The treatment did not affect the
physical appearance, behaviour and mortality of the test animals. The
values of haematological and clinical chemistry tests (with respect to
ALP, GOT, GPT, GLDH, bilirubin and proteins in serum), urinalyses and
kidney function tests were within the physiological ranges. Blood
sugar values did not differ from the control, whereas a dose-related
increase of the cholesterol values in treated animals was found,
especially at 3000 ppm. No gross pathological alterations that could
be attributable to treatment were seen in rats which died during the
feeding study or which were sacrificed at the end of the study. Some
variations in the relative organ weights were found.
A dose-dependent increase of the relative liver-weight at 1000 and
3000 ppm was observed. The histopathological examination of tissues
revealed no treatment-related alterations. The incidence and
localization of tumours provided no indication for a carcinogenic
effect of ethiofencarb (Bombard and Löser, 1976).
TABLE 1b. Acute toxicity of ethiofencarb metabolites
Species Sex Route LD50 References
mg/kg
Ethiofencarb-sulfoxide:
Rat m oral 200-250 Thyssen 1976
Rat f oral 200-250 ibid.
Rat f oral 133 Lamb and Matzkanin 1977a
Ethiofencarb-sulfone:
Rat m oral 600-750 Thyssen 1976
Rat m oral 468 Lamb and Matzkanin 1977b
Rat f oral approx. 600 Thyssen 1976
Ethiofencarb-phenol:
Rat m oral approx. 200 Lamb and Matzkanin 1977c
Rat f oral approx. 200 ibid.
Ethiofencarb-phenol-sulfoxide:
Rat m oral >1000 Lamb and Matzkanin 1977d
Rat f oral >1000 ibid.
Ethiofencarb-phenol-sulfone:
Rat m oral >1000 <20 Lamb and Matzkanin 1977e
Rat f oral >1000 <20 Ibid.
TABLE 1b. (Continued)
Species Sex Route LD50 References
mg/kg
N-methyl-hydroxy metabolite of ethiofencarb:
Rat m oral >2000 Lamb and Matzkanin 1977f
Rat f oral approx 2000 ibid.
N-methyl-hydroxy sulfoxide metabolite:
Rat m oral >2000 Lamb and Matzkanin 1977g
Rat f oral >2000 ibid.
N-methyl-hydroxy-sulfone metabolite
Rat m oral >2000 Lamb and Matzkanin 1977h
Rat f oral >2000 Ibid.
Comments
Ethiofencarb is almost completely absorbed in mammals and it is
excreted rapidly in the form of metabolites mainly in the urine. The
major metabolic reactions are demethylation and subsequent hydrolysis
of the carbamate and oxidations to sulphoxides and sulphones.
Ethiofencarb inhibits the activity of acetylcholinesterase only
transiently.
In a 4-week study daily oral administration of 10 mg/kg to male rats
led to a maximal depression of 25% of the plasma-cholinesterase and of
15% of the erythrocyte-cholinesterase after 2 hours. Recovery occurred
almost completely within 24 hours. This dose level was taken as a
basis for establishing an acceptable daily intake for humans. The fact
that at 10 mg/kg marginal effects occurred was taken into account by
using a safety factor higher than is usual for
cholinesterase-inhibiting compounds.
The compound was not mutagenic in a dominant lethal test, nor was it
teratogenic. No three-generation study was available. However, such a
study was felt to be important since the depression of the
cholinesterase activity often manifests itself unfavourably in a
reduced viability of the pups.
In a 2-year feeding study in dogs no untoward effects were seen up to
1000 ppm. A long-term feeding study in rats revealed no more than a
moderate increase of the relative liver weights at 3000 ppm.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 (mg/kg bw)/day
Dog: 1000 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Ethiofencarb in a systemic insecticide which has a selective effect
against aphids. It gives control of numerous aphid species on
vegetables, fruit crops, field crops and ornamental plants. It
displays good activity against both susceptible and
organophosphorusresistant strains. Used as a soil treatment, e.g. by
the soil drench method or as a granular application, ethiofencarb
displays long residual activity; when applied to the aerial parts of
plants it is somewhat less persistent. In trials conducted to date,
ethiofencarb has shown no phytotoxicity.
Ethiofencarb is sold or registered in Bulgaria, Chile, Denmark,
Federal Republic of Germany, Israel, Italy, Portugal, Spain, Sweden,
Switzerland and Turkey.
Applications for registration have been filed in a number of other
countries.
Pre-harvest treatments
Ethiofencarb is formulated as an emulsifiable concentrate for spraying
and as a granular product. Used as a spray, it is applied broadcast at
the onset of aphid infestation. As a granular formulation, it is
applied at sowing or planting, mainly in-furrow.
Details of uses and recommendations for pre-harvest treatments with
ethiofencarb are given in Table 2.
Post-harvest treatments
No treatments recommended.
Other uses
Ethiofencarb is also used on ornamental plants.
RESIDUES RESULTING PROM SUPERVISED TRIALS
The residue data obtained after the application of ethiofencarb to
fruit, vegetables and field crops are summarized in Table 3. The data
originate from trials conducted in the U.S.A., Japan, Great Britain,
France, the Netherlands and the Federal Republic of Germany.
The residues were determined by gas-chromatographic methods which
determine residues of ethiofencarb and the metabolites with an intact
carbamate group.
After application by spraying, half-lives of 2 to 18 days were noted
for the total residue.
Detailed studies with different formulations using the same active
ingredient dose produced the following half-lives for the given crops:
Apple: 13 (5-18) days
Bush bean: 5 (4-7 ) days
Head lettuce: 5 (4-6 ) days
Savoy cabbage: 5 (2-9 ) days
With crops of which the foliage and edible parts were analyzed
separately, e.g. potatoes, sugar beet, broad beans and radish, it was
found that the residues were present mainly in the foliage owing to
the pronounced systemic mode of action of ethiofencarb, residues were
found in the analyzed plant samples also after application of the
granular formulation to the soil. Here, too, there was an accumulation
of the residues in the leaves, as proved by studies on broad beans and
sugar beet.
FATE OF RESIDUES
General
The metabolism of ethiofencarb (I) is illustrated in Figure 1. It is
found to be of the same pattern, with oxidation of the sulphur and
hydrolysis of the carbamate group, in soil, plants and animals, with
only quantitative differences. In soil and plants, ethiofencarb
sulphoxide (II, Figure 1) and ethiofencarb sulphone (III) were the
main products with an intact carbamate group. In animal metabolism
studies, phenolic degradation products formed by hydrolysis of the
carbamate group were also detected. CO2 released from the carbamate
group by hydrolysis was found to be incorporated into plants.
In animals
Studies on ethiofencarb metabolism in rats are described previously
under "Biochemical aspects". Marshall and Dorough (1976,1977)
investigated the fate of water-soluble plant metabolites of
ethiofencarb in rats. Bean plants were stem-injected with either
ring-14C- or carbonyl-14C-ethiofencarb. After 20 days almost all
the radiocarbon residue was in the epicotyl leaves. The distribution
in the unextractable, organic and aqueous fraction was 3.3%, 45.8% and
40.7% for the ring label and 1.3%, 13.8% and 2.6% for the carbonyl
label. 14C in the aqueous fractions when orally dosed to rats was
largely excreted within 36 hours, mainly by routes similar to those of
the parent compound, viz. in urine (92% of ring and 39% of CO-label)
and as expired CO2 (30% of CO-label).
Sorghum plants treated with ring-14 C-ethiofencarb (I) in an
emulsifiable spray were used as a source of unextractable metabolites
(Marshall and Dorough, 1977). When these bound residues of I were
administered to rats, 90.8% of the dose was voided in the faeces and
16.4% in the urine within 2 days; the bile contained 2.5% of the dose.
These data demonstrated that bound residues of I were very poorly
absorbed from the gastrointestinal tract of rats, while the conjugated
ones were readily absorbed.
Gronberg at al. (1976) continuously fed I to dairy cows for 28 days at
50, 150 and 500 PPM in the diet, equivalent to 1.5, 4.5 and 15 mg/kg
body weight/day. From the residue data obtained it was calculated that
tissue residues would be less than 0.1 mg/kg I + II + III equivalents
and milk residues would be less than 0.01 mg/kg for animals on a
ration containing 90 ppm and 14 ppm of ethiofencarb respectively.
Residues in milk averaged about 1 mg/kg at the highest feed level.
Tissue residues are shown in Table 4.
A lactating cow and a pig were treated with a single oral dose of 0.5
mg/kg 14C-ring-labelled ethiofencarb (Dorough, 1976h). 24 hours
after administration 97.8% of the dose had been excreted via the urine
from the cow and 90% from the pig. Elimination of the radiocarbon in
the faeces was <1% and 5.1% in cow and pig respectively.
Tissues of the pig contained no detectable radioactive residues 24
hours after treatment. Of the bovine tissues only kidney, liver and
skin contained detectable residues (Table 4) of 0.016, 0.017 and 0.05
mg/kg ethiofencarb equivalents respectively. Maximum concentrations of
14C in the blood of the cow, reached 3 hours following
administration, were 0.3 mg/kg. The milk the peak residue level was
0.15 mg/kg, reached 4 hours after dosing. About 67% of the milk
residue consisted of free metabolites, mainly ethiofencarb sulphoxide
and sulphone.
11.6% of the urinary metabolites in the cow were in the free form,
predominantly as phenol sulphoxide and phenol sulphone. Other
metabolites were eliminated as conjugates. In the pig 40.6% of the
dose was excreted in free form.
Eight laying hens were treated with a single oral dose of
14C-ring-labelled ethiofencarb at a rate of 0.5 mg/kg (Dorough,
1976). over 80% of the administered radiocarbon was eliminated in the
excreta after 24 hours and over 90% after 3 days. The highest
radiocarbon level 8 hours after application was found in kidney at
0.062 mg/kg. Liver, muscle and blood showed lower residue levels and
C, fat and heart no residues could be detected (see Table 4). 24 hours
post-administration no 14C could be detected in the tissues. Eggs
laid during a 3-day period following the single treatment were free
from detectable residues.
In a further study (Dorough,1976i) 10 hens were given
14C-ring-labelled ethiofencarb as daily treatment for 7 days at a
rate of 0.5 mg/kg twice a day. 2 days after the start of treatment
about 60% of the cumulative dose was excreted and after 7 days about
92%. Tissues of animals sacrificed 3-4 hours after the final dose
contained 14C-ethiofencarb equivalents ranging from about 0.02 mg/kg
in fat to 0.32 mg/kg in kidney. 4 days after the termination of
treatment muscle, skin, kidney and liver contained 0.001, 0.006, 0.007
and 0.009 mg/kg 14C-ethiofencarb equivalents respectively. In bloody
brains fat and heart no residues were detectable. Levels of
radiocarbon in the eggs reached a maximum of 0.06 - 0.07 mg/kg after 7
days of continuous treatment. In eggs (white and yolk) residues were
about 0.03 - 0.04 mg/kg after 2 days of treatment, reached a maximum
of 0.06 - 0.07 mg/kg after 7 days and were below detectable levels 3
days after treatment was stopped.
TABLE 2. Use pattern of ethiofencarb
Crop Concentration or Formulation Number of Pre-harvest
application rate (a.i.) applications interval(days)
Vegetables 0.0375-0.05% E.C. 2-3 4-7
(Cucurbitaceae, foliar spray/overall
eggplants, peas application
brassicas, peppers, 0.075 -0.1 g./linear GR 1
lettuce, tomatoes metre at sowing/
Vicia, etc.) planting
Cereals 100-500 g./ha. E.C. 1-2 14-21
(including millet) foliar spray/overall
application
Potatoes 500-600 g./ha E.C. 2-3 14
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at planting
Pome and stone fruit, 0.05% E.C. 2-3 7-10
small (soft) fruit, foliar spray/overall
citrus fruit application
Beets 400-500 g./ha E.C. 2-4 14
foliar spray/overall
application
Tobacco 0.05-0.075% E.C. 2-3 4-7
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at sowing/
planting
TABLE 3. Residues of ethiofencarb, its sulphoxide and sulphone from supervised trials
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Apple D 2-3 1 EC 0.9-2.2 0.4 -2.0 0.4 -1.3 0.4 -1.6 0.2 -1.4 0.3-1.0 1)
(28d:)
F 1 0.5 EC 0.6 0.3 -0.4 0.4 1)
J 3/5 3-3.5 EC 1.2 - 1.3 0.8-1.2 2)
(30d.)
Apricot J 2/4 7.5 gEC/tree 0.8 -1.2 1.8-2.1 3)
(28d.)
Artichoke F 1 0.5-1EC 1.0 0.4 -4.7 0.2 -3.0 0.2 -1.0 1)
Beans
broad beans D 2-3 0.3 EC 1)
beans 0.02-0.04 0.02-0.04 0.02-0.03 <0.02
pods 0.2 -0.43 0.08-0.26 0.05-0.22 0.04-0.11 0.02-0.05
greens 5.5 -10.1 0.8 -4.6 0.6 - 4.6 0.5 -3.1 0.2 -1.0
broad beans D 1 1.5 G (55d.) (89d.) (111d.)
beans 0.02-0.13 0.02-0.04
pods 0.19-0.42 0.19-0.57
greens 14-30 2.2 -6.1 0.17-0.84
bush} D 2-3 0.3 EC 0.14-0.68 0.25-0.43 0.03-0.32 0.02-0.19 0.02-0.11 <0.02 1)
(28d.)
beans} F 1 0.75 EC 1.46-2.47 0.77-1.84
F 1 2 G 0.05 0.05 0.03 1)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Cabbage
Brussels sprouts D 3 0.3 EC 0.5 -1.4 0.4 -0.94 0.2 - 0.9 1)
Cauliflower D 2 0.3 EC 0.3 -0.5 0.1 -0.43 0.05-0.2
F 1 0.5 EC 0.26 0.11 0.07 1)
Chinese cabbage J 3/5 0.75-2EC 0.04-2.7 0.04-1.8 0.06-2.4 4)
(5d.)
Red cabbage D 2 0.3 EC 0.2 -1.1 0.2 - 0.4 0.05-0.5 1)
Savoy cabbage D 2 0.3 EC 0.4 -3.1 0.1 - 1.3 0.05-1.3 0.2 - 0.6 0.06-0.4 0.05-0.3 1)
(46d.) (78d.) (88d.) (28d.)
Savoy cabbage D 1 1 G 0.5 -0.6 0.05 <0.05 1)
White cabbage D 2 0.3 EG 0.2 0.1 <0.05 1)
White cabbage GB 1 3 G 0 09 1)
Cherry (see end of table) (105d.)
Cucumber J 3/5 0.75- 0.7 -0.9 0.3 -0.8 0.2 -0.5 0.1 -0.32 4)
2.5 EC
Currant
Blackcurrant D 2 1 EC 1.1 - 2.8 0.5 - 1.4 0.6 - 1.4
Red currant D 2 1 EC 0.5 - 2.9 0.4 - 1.0 0.2 - 0.6 1)
Eggplant F 1 0.75 EC 0.03-0.5 0.02-1.0 1)
Lettuce
(outdoor) D 2 0.3 EC 4.3 - 10.1 1.3 - 4.4 0.6 - 2.2 0.4 - 0.8 0.2 - 0.6 0.1 - 0.2 1)
(28d. )
(under glass) D 2 0.3 EC 2.3 - 18.8 2.2 - 10.1 1.6 -6.4 1.5 0.5 - 0.8 1)
(under glass) NL 1 5 G (44-67d.) 1)
Peach D 1-2 0.75-1EC 1.2 -2.3 1.1 -1.7 0.5 -2.0 0.4 -1.4 0.3 -1.1
Peach F 1 0.5 EC 0.3 -0.8 0.2 -0.3 0.1 -0.2
Plum (see end of table)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Potatoes D 3 0.4-0.6 0.16-0.23 0.09-0.25 0.08-0.17 0.02-0.16 1)
EC (28d.)
Potatoes US 2-6 0.5-2 EC <0.02-0.06 <0.02-0.06 6)
Potatoes J 4-5 0.9-1.75 0.07-0.1 0.04-0.16 0.04 7)
EC
Potatoes leaf J 1 0.7-1.7 EC 38-57 2.2 -4.6 0.3 -1.3 0.2 -0.6 7)
Potatoes D, NL, 1 1.5 G <0.02-0.06 1)
(91-135d.)
Radish J 3/5 1-1.5 EC 0.10-0.12 0.03-0.14 0.02-0.10
Radish leaf J 3/5 1-1.5 EC 0.1-1.5 0.06-1.2 0.1-0.6 8)
Soybean
Soybean, 3 0.75-1.5 0.02-1.04 <0.02-0.18
green+shell EC
Soybean, bean 3/5 0.75 EC 0.04-0.06 0.02-0.04 9)
(44-67d.)
Wheat 1)
grain D 2 0.4 EC
straw 9.6 -11.4 1.4 -1.9 <0.02 <0.02 1)
grain F 1 0.5 EC 1.2 -1.7 0.06-0.8
straw <0.02
0.1 -0.32
(34.53d.)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Sugar beet
beet D,GB 2-5 0.25-0.5 0.04-0.06 0.03 0.02 0.01-0.03 1)
top (leaf) EC 3.0 -4.7 2.0 -2.2 2.7 -4.9 0.12-1.4
(31-76d.)
beet D, GB 1 1-5-3 G 0.01-0.08 1)
top (leaf) 0.05-1.1
(124-195d.)
Tobacco 3 0.3-0.4 12.5-52 5.4-37 4.5-21 1.3 - 9.3 2.9 - 5.7 1.1 -13
(25-28d.) 1)
air -
cured D 1 1.5 G 4.5 -12.3
(54-58d.+
132-137d.
cured)
Cherry
(morello) D 3 1 EC 1.8 -5.9 0.5 -5.9 1.2 -4.2 1)
Plum D 3 1 EC 2.4 -3.5 1.3 -3.4 1.3 -2.6 1)
Trials in a)
Fed. Rep. Germany = D
France = F
Great Britain = GB
Japan = J
Netherlands = NL
United States Amer. = US
TABLE 3. (Continued)
b)
EC = Emulsifiable concentrate
G = Granular
References c)
1) Bayer AG, 1972/77
2) Nitokuno Inst., 1976
3) Nitokuno Inst., 1975
4) Nitokuno Inst., 1975/76
5) Nitokuno Inst., 1975a
6) Chemagro Report, 1976
7) Nitokuno Inst., 1975/76a
8) Nitokuno Inst., 1976a
9) Nitokuno Inst., 1976b
TABLE 4. Ethiofencarb equivalents in various tissues of warm-blooded animals
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Rat 0.5 mg/kg Ring- 14C 2.5 h 0.021) 0.061) 0.041) 0.031) Dorough,
single oral dose 1976j
Rat 0.5 mg/kg N-Methyl-14C 8 h 0.24 0.88 Hurst and
single oral dose 24 h 0.19 0.46 Dorough,
1976
Rat 6.6 ppm/day in diet 0.24 0.18 0.06 0.29 1.18 0.10 Nye at al.,
for 7 days, carbonyl-14C 1976
Cow 0.5 mg/kg Ring-14C 3 h 0.32 0.016 0.017 0.05 Dorough,a
single oral dose 24 h 1976h
Cow 2xdaily 50 PPM in diet 0.03 0.042) 0.03 0.01 <0.01 0.083) Gronberg
for 28 days et al.,
2xdaily 150 PPm in diet 0.27 0.232) 0.29 0.10 0.01 0.853) 1976
for 28 days
2xdaily 500 PPM in diet 2.17 1.012) 2.53 0.89 0.13 4.313)
for 28 days
Hen 0.5 mg/kg Ring-14C 8 h 0.053 0.028 0.062 0.035 0.039 0.022 Dorough,
single oral dose 1976i
0.5 mg/kg Ring-14C 4 days 0.016 0.001 0.005 0.016 0.025 0.013
2xdaily for 7 days 7 days4) 0.099 0.039 0.019 0.072 0.324 0.180 0.069 0.075
TABLE 4. (Continued)
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Hen 45 ppm in diet for 28 days 0.03 0.04 0.05 0.05 Gronberg
150 ppm in diet for 28 days 0.07 0.13 0.08 0.10 and
450 ppm in diet for 28 days 0.16 0.41 0.29 0.24 Dorough,
1976
1) The values were derived from graphs
2) Mixed omental, renal and subcutaneous fat
3) Mixed loin, round, flank
4) Higher residues at 7 than at 4 days were due largely to the fact that the 7-day birds were sacrificed 3-4 h
after last treatment while 4-day birds were sacrificed 8 h after treatment
The major identified metabolites eliminated in the excreta were the
phenol sulphoxide and sulphone, existing as free metabolites or
conjugates, the proportion of free metabolites being higher in the
singly dosed birds. Small amounts of the sulphoxide and sulphone of
the carbamate were also found in the continuous feeding study only.
The same sulphoxidation products were found as the principal
metabolites in eggs, about 50% of the residue being the phenol
sulphone and about 20% the phenol sulphoxide.
Poultry were continuously fed with ethiofencarb for 28 days at a
ration level of 0, 15, 45, 150 and 450 ppm (Gronberg and Dorough,
1976). The average concentration of ethiofencarb equivalents (Table 4)
in fat, skins muscle and giblet ranged from 0.03 to 0.05 mg/kg at the
45 ppm level, from 0.07 to 0.13 mg/kg at the 150 ppm level and from
0.16 to 0.4 mg/kg at 450 ppm. Average residues in eggs collected at
days 26, 27 or 28 of treatment were approximately 0.01, 0.04 and 0.1
mg/kg at the 451 150 and 450 ppm feeding levels respectively. Prom
these residue data it me concluded that tissue residues would be less
then 0.1 mg/kg and egg residues less than 0.01 mg/kg for animals on a
ration of 100 ppm and 40 ppm respectively.
After continuous exposure of Channel catfish to 10 µg/1
14C-ring-labelled ethiofencarb for 28 days, 14C residues accumulated
to a plateau of about 50-75 mg/kg reached after 4 days. The edible
parts of the fish contained about 27% of the residues. During the
withdrawal period about 82% of the accumulated residues were excreted
wIthin 1 day and about 95% within 4 days (Lamb and Roney, 1976).
In plants
Ethiofencarb has good systemic properties and is absorbed in large
amounts by the plant roots and translocated to the aerial parts
(Homeyer, 1976).
Bean seedlings absorbed a total of 34.6% of the available
14C-ring-labelled ethiofencarb from a solution via the roots in 4
days, whilst 18.1% was found after application of
14C-carbonyl-labelled compound. The distribution of radiocarbon in
the plant after 4 days was: roots 5.1%, stems 9.0%, leaves 16.5%
(Dorough, 1976 g).
Dräger (1976a) applied 10% granules of ethiofencarb (I, Figure 1), its
sulphoxide (II) and its sulphone (III) separately to soil at the time
of sowing beans. Results are shown in Table 5. Generally, the total
residue in the green parts was highest after applying Ill and lowest
after the application of I. The highest residues ware in the green
parts (leaves and stems) followed by pods and beans; the highest
levels were found at the first sampling 4 weeks after sowing.
TABLE 5. Uptake of ethiofencarb (I), its sulphoxide (II) and sulphone (III) by beans
Interval Total residue (mg/kg) after application of
after
application, I Granular II Granular III Granular
weeks
a* b* c* a b c a b c
4 159 - - 210 - - 310 - -
6 61 - - 135 - - 204 - -
8 49 - - 74 - - 96 - -
12 78 0.95 0.27 25 0.18 <0.1 141 0.20 0.06
*a: green parts;
b: pods;
c: beans
Following foliar application of 14C-carbonyl-labelled and
14C-ring-labelled I to beans, potatoes and sorghum, it was found that
14 days after application only 60.7, 29.0 and 45.1% of the insecticide
had been absorbed from the leaf surfaces of the respective plants
(Dorough, 1976g).
Potato and sorghum plants were treated at 0.56 kg/ha with ring-14C-I
in an E.C. formulation. The plants were treated at 10-day intervals
5-7 times during the growing season. Radiocarbon residues were much
lower in potatoes than in sorghum. Total ethiofencarb equivalents
remained essentially constant in potato peels (0.2 mg/kg) and pulp
(about 0.15 mg/kg) for 31 days after treatment. In sorghum the highest
levels of ethiofencarb equivalents were located in the leaves (10-100
mg/kg) followed by heads (5-25 mg/kg), grain (1-10 mg/kg) and stalks
(0.05-0.5 mg/kg). Sorghum leaf levels showed an increase from 85 to
131 mi/kg during the first two weeks but declined to about 94 mg/kg at
31 days. Levels remained relatively constant in the stalks. Levels in
the grain appeared constant (6-9 mg/kg) throughout the entire study
except for the 1 week sample (19 mg/kg). Total radiocarbon in the
heads tripled from 50 mg/kg at time 0 to 167 mg/kg at 1 week and then
declined to 90 mg/kg at 31 days (Dorough, 1976f).
Dräger detected II and Ill in various plant parts after application of
10% granular 14C-carbonyl-labelled ethiofencarb at planting of beans
(0.05 g I/1.6 kg soil; 1976a) and potatoes (0.05 g /approx. 20 1 soil;
1976c). The extractable residues in the roots and green parts of both
potatoes and beans consisted mainly of II and Ill. 0.21-7.79 mg/kg II
and 0.14-0.31 mg/kg III were found in the roots of the potatoes
between days 34 and 96, with 1.39-16.2 mg/kg II and 3.08-8.79 mg/kg
Ill in the green parts. The bigger increase in the sulphone content of
the green parts of the potatoes is indicative of an increased
oxidation of the sulphoxide in the plant system. The two metabolites
were detected also in the potato tubers and beans but there was too
little material available for extensive quantitative studies on the
beans. The potato tubers contained 0.05-0.28 mg/kg II and 0.06-0.14
mg/kg Ill.
The unextractable radioactivity in the green parts of the beans was
less than 1% or the total applied, and accounted for a maximum of 7.7%
of the radioactivity measured at the sampling times (Drägert 1976a).
The major portion of the unextractable radioactivity in the potato
tuber was in the glucose moiety of the starch. Therefore, CO2 formed
by hydrolysis of the carbamate group was utilized in carbohydrate
metabolism. The unextractable but water soluble radioactivity in
rootsy green parts and potato tubers was also considered to be
incorporated in products of carbohydrate metabolism. For details of
the distribution of radioactivity in potatoes and beans, see Table 6.
TABLE 6. 14C-ethiofencarb and metabolites in plants (as % of radioactivity applied or recovered)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Bean 0 day 14CO+ 61.9 29.6 1.6 0.7 origin-0.1; 98.0 Dorough,
x2 = 4.1 1976g
3 days Ring- 10.8 55.9 4.4 6.7 1.9 origin = 11.3; x1=0.5; 94.0
x2 =2.5
7 days 14C 49.7 11.4 1.4 0.5 origin=20.2;x1=0.4; 84.6
x2=1.0
14 days EC 15.6 0.6 1.7 0.6 origin=49.8;x1=0.5 69.1
x2=0.3
Potato 0 day 41.3 50.0 1.0 origin=1.5; x1=1.8; 100.3
x2=4.7
3 days 23.1 48.6 3.3 2.0 origin=5.9; x1=1.8; 91.2
x2=7.9
7 days 7.0 42.2 6.5 2.6 0.4 origin=15.7; x1=1.9; 80.4
x2=4.1
14 days 1.8 38.0 9.6 3.5 0.8 origin=14.0; x1=6.2 77.1
X2=3.2
Sorghum 0 day 73.0 22.8 0.9 origin=0.9; x2=0.9 98.5
3 days 18.1 49.3 1.3 0.8 0.3 origin=5.9; x2=2.3 78.0
7 days 3.2 47.0 4.3 1.3 1.0 origin=10.3; x2=2.0 69.1
14 days 0.3 38.6 6.1 0.7 0.3 origin=23.6; x2=0.5 70.1
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Potato
Peel Ring-
org. 0 day 14C 5.2 10.0 2.7 3.4 2.6 origin=1.3; x1=3.8 29.0 Dorough, 1976f
-soluble
water- EC 1.7 3.3 origin=1.0; x1=2.1 8.1
soluble
unhydrolysed 18.1
by H2O
unextractable 44.8
100.0
(102.4)2)
Potato
Peel
org. 31 days Ring- 3.6 2.7 2.7 8.0 6.3 origin=0.9 24.2 Dorough, 1976f
soluble
water soluble 14C 20.9
unhydrolysed EC 31.0
by H2O
extractable 24.1
100.0
(90.9)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Pulp
org.-soluble 0 day 3.5 7.1 5.8 origin=0.3 16.7
water-soluble 4.2 5.3 origin=0.5; x1=3.9 14.0
unhydrolysed 20.7
by H2O
unextractable 48.6
100.0
(100.6)2)
org.-soluble 31 days 0.7 2.5 0.6 6.6 2.1 origin=0.2 12.7
water-soluble 16.2
unhydrolysed 38.8
by H2O
unextractable 32.3
100.0
(90.6)2)
Sorghum
Leaves
org.-soluble 0 day Ring- 1.4 50.5 11.6 0.9 2.1 5.2 origin=1.0; x3=0.5 73.2
water-soluble 14C 0.2 0.6 8.3 4.1 origin=0.2; x2=0.7 14.1
unhydrolysed EC 5.2
by H2O
unextractable 7.5
100.0
(98.8) 2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Leaves
org-soluble 31 days Ring- 0.4 11.3 15.7 3.1 17.8 origin=0.6; x2=0.4 50.4 Dorough, 1976f
14C x3=0.7; x5=0.4
water-soluble EC 0.6 0.4 4.2 4.9 origin=0.3; x2=0.7 11.1
unhydrolysed
by H2O 12.8
unextractable 25.7
100.0
(99.4)2)
Stalks
org.-soluble 0 day 56.2 15.6 2.5 origin=1.2 75.5
water-soluble 11.4 origin=0.5 11.9
unhydrolysed
by H2O 7.1
unextractable 5.4
99.9
(97.0)2)
Stalks
org.-soluble 31 days 14.6 25.5 1.8 3.0 origin=0.6 45.5
water-solube 27.8 origin=0.9 28.7
unhydrolysed
by H2O 15.3
unextractable 10.5
100.0
(105.4)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Heads
org.-soluble 0 day 1.7 45.2 14.4 0.9 1.8 5.2 origin=0.6; x2=0.7;
x5=2.1 72.6
water-soluble 0.4 2.2 3.9 0.4 origin=0.2; x1=0.3;
x2=0.5 7.9
unhydrolysed
by H2O 4.2
unextractable 15.3
100.0
(95.9)2)
Sorghum
Heads
org.-soluble 31 days Ring- 1.6 26.0 27.1 0.4 1.6 9.9 origin=1.0; x2=1.0; 70.6 Dorough, 1976f
14C x5=2.0
water-soluble EC 0.3 0.3 0.2 3.3 2.8 origin=0.2; x1=0.2; 7.8
x2=0.5
unhydrolysed 4.7
by H2O
unextractable 16.9
100.0
(98.7) 2)
Grain
org.-soluble 0 day 51.1 1.7 5.5 2.2 origin=0.8; x2=0.7;
x5=1.8 63.8
water-soluble 4.2 5.7 origin=0.9; x2=0.6 11.4
unhydrolyzed
by H2O 7.0
unextractable 17.8
100.0
(99.2)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Grain
org.-soluble 31 days 11.2 9.0 1.3 3.7 origin=0.5; x2=0.6;
x5=1.6 27.9
water-soluble 8.4 14.7 0.8 origin=0.5; x1=0.5;
x2=1.0 26.1
unhydrolysed
by H2O 12.5
unextractable 33.5
100.0
(99.3)2)
Potato unextractable by
organic solvents
Roots 34 days 14-CO 0.044 1.26 0.14 solid residue
0.10 in aqueous
layers 0.11 1.654 Dräger, 1976c
68 days Granular 0.055 0.42 0.10 solid residue
0.27 in aqueous
layers 0.15 0.945
96 days soil 0.04 0.05 solid residue
0.11; in aqueous
layers 0.07 0.27
TABLE 6 (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Green parts 34 days 7.15 3.89 solid residue
1.02 in aqueous
layers 0.94
68 days 10.28 9.03 solid residue
2.78 in aqueous
layers 3.04 25.12
96 days 4.38 10.18 solid residue
2.17 in aqueous
layers 3.42 20.15
Tubers 68 days 0.00 10.35 0.18 solid residue
0.973) in aqueous
layers 0.60 2.101
96 days 0.17 0.20 solid residue 2.393)
in aqueous layers 0.92 3.46
Bean
Roots 5 weeks 0.26 0.07 unextractable: 0.07 0.40 Dräger, 1976c
10 weeks 0.15 0.19 unextractable: 0.13 0.47
Green parts 5 weeks 3.53 0.95 unextractable: 0.27 4.75
10 weeks 3.02 4.27 unextractable: 0.61 7.90
TABLE 6 (Continued)
1) Compounds I-VI are identified in Figure 1.
2) The figures in Parentheses indicate percent of total recovery, based on combustion
3) Consisting of:
68days 96days
14C-CO2 (CO3-) 0.04 0.39
14C-Glucose in starch 0.85 1.66
Non-hydrolyzable 0.08 0.34
0.97 2.39
Following foliar and soil application, and stem injection of 14CO-and
ring-14C- labelled ethiofencarb to beans, potatoes and sorghum,
Dorough 1976f,g found that in addition to the major metabolites II and
III, minor amounts of IV, V and VI as well as unidentified metabolites
had formed. For quantitative data, see Table 6.
Bean plants were also treated with II and III (14C-ring-labelled),
After three days, 86.6% of II and 91.7% of III had been converted to
water-soluble metabolites which were easily degraded by acid
hydrolysis (Dorough, 1976g).
In soil
Studies with 14C-carbonyl-labelled ethiofencarb (granular
application; Dräger, 1976c 1977a) showed that ethiofencarb was
oxidized in soil to the corresponding sulphoxide (II) and sulphone
(III). Details are given in Table 7. After 5 weeks II was the main
residue but the proportion of III increased with time. Residues of
ethiofencarb in the first sample taken after 5 weeks were 2 mg/kg in
one experiment(1976c) and undetectable in another (1976a). The
unextractable residues amounted to 3-9% of the applied radioactivity.
Dräger (1977b) also found in laboratory studies with soil 34 clay
after the application of carbonyl-ethiofencarb (1) residues of 4.4% I,
68% II and 14% III. The carbamate group was hydrolyzed to CO2 with a
half-life of about 90 days. After 82 days, 43% of the radioactivity of
the carbonyl group was identified as gaseous CO2 Of the remaining
radioactivity left in the soil, II accounted for 1.7%, III for 38.3%,
and unextractrables for 17%; I could not be detected.
Ethiofencarb was degraded in the field in different soils with
half-lives of 20-30 days (Bayer, 1972/77), and in laboratory studies
with a maximum half-life of 32 days (Nitokuno, 1976d). In other
studies half-lives of approx. 90 and 130 days were obtained.
After the application of ethiofencarb 2 kg/ha as an emulsifiable
concentrate to different types of soil the following maximum residues
(I + II + III) were found: 8.8 mg/kg 0 days after application), 4.4
(7), 3.2 (14), 1.1 (30), 0.6 (60), 0.25 (92) and 0.1 (120) (Bayer
1972/77).
In greenhouse experiments with 14CO-labelled ethiofencarb granules,
Dräger calculated a half-life of about 6-7 weeks from the
radiochemical degradation rate of the total residue in soil planted
with potatoes (1976c) and a half-life of about 2 weeks in soil planted
with beans (1976a).
Half-lives for the degradation of ethiofencarb in soil are presented
in Table 8.
TABLE 7. Distribution of radioactivity in soil after application of
14C-carbonyl ethionfencarb
Compound % of initial 14C remaining on day
35 56 70 34 68 96
Ethiofencarb (I) n.d. n.d. n.d. 2.20 0.22 0.08
Ethiofencarb sulphoxide (II) 16.72 3.14 1.96 41.79 8.10 0.87
Ethiofencarb sulphone (III) 4.37 2.94 2.46 8.18 4.81 3.01
Unextractable 5.45 9.12 7.49 7.32 5.64 2.60
Total 26.54 15.20 11.91 59.49 18.77 6.56
References Dräger, 1976 a,b,c Dräger, 1976 a,b,c
The soils were planted either with beans (0.05 g I/1.6 kg soil;
Dräger, 1976a) or with potatoes T 0.05 g I/approx. 20 1 soil., Dräger,
1976 c) so that a portion of the radioactivity was absorbed by the
plants (see "Fate In Plants").
In laboratory studies to investigate the leaching behaviour of
ethiofencarb, normal dosages of formulations were applied to soil
which were eluted to simulate a rainfall of 200 mm in 2-21 days. The
leachate contained the following percentages of the applied dose as
total residues of I + II + III: low-humus sand 26.6-62.5%; high-humus
loamy sand 7.1-19.4%; sandy loam 18.2-74.5%. When II was applied to
low-humus sand and sandy loam, leachates contained 41% and 57% of the
dosage respectively. After application of Ill, the corresponding
figures were 50% and 40% (Bayer, 1972/77).
Rieck (1976a) found in laboratory studies with ring-labelled
14C-ethiofencarb (columns: 20 cm high and 10 cm diameter; application
of water equivalent to 500 m of rainfall) that the products of
ethiofencarb were not leached as rapidly from the Boils with higher
organic matter as from in the sandier, lean organic soils. In silty
clay, 88% of the radiocarbon was found in the upper 8 cm of the
column. Approximately 1-15% of the radiocarbon was found in the
leachates, of which at least 80% was II. The only other product found
in all the leachates was III. The products that remained in soil were
the same as those in the leachates and were in the same proportion.
Whereas carbamates were found in the leachate in these laboratory
experiments with small soil columns, no ethiofencarb and no products
with an intact carbamate structure were found in leachate samples
examined over a period of 16 months in leaching studies with an intact
soil core (10 cm diameter, 130 cm high) taken from the field after
rainfall typical of practical conditions (755 mm/year) (Dräger,
1976d).
Further studies of the leaching characteristics of aged ethiofencarb
residues under laboratory conditions were carried out by Dräger
(1977c) and Rieck (1976b) In the studies of Dräger, about 40-50% of
the applied 14Carbonyl activity was found in the leachate
irrespective of the soil type. Of this, only part was extractable with
chloroform. The unextractable portion was considered to be carbonate
or hydrogen carbonate formed during the experiment by hydrolysis of
the carbamate group of I (see also "Fate in Water"). The extractable
portions consisted of II and IlI, in a ratio similar to that of the
initial aged residues. I was no longer detectable. Rieck (1976b) also
did not detect I in four different soils after 30 days of aerobic
aging. The predominant residues were II and III, together with a
considerable amount of unextractable radioactive residue. In contrast
to the studies of Dräger (1977c) most of the radiocarbon was found in
the upper 8 cm of the soil with less than 2% in the leachate.
Thorton et al. (1976) compared the leaching behaviour of I with that
of 23 other pesticides. The pesticides were spotted on thin-layer
plates coated with six different types of soil ranging from
non-adsorptive sand to fine-textured clay. The plates were developed
and Rf values were compared. The compounds were grouped into five
categories ranging from immobile to highly mobile. I was placed in
class 49 as one of the mobile compounds.
TABLE 8. Half-lives of ethiofencarb (as total residues
of parent + sulphoxide + sulphone) in various soils
Soil Half-life
(days)
Laboratory trials
Carbon: 1.05%; fines: 30.4%; pH: 5.7 (Laacher Haf) 90
Carbon: 0.8% ; fines: 4.2%; pH: 7.0 (low-humus sand) 96
Carbon: 0.57%; fines: 19.5%; pH: 5.2 (sandy loam) 133
Clay: 14 %; org. matter: 5.85%; pH: 5.7 (sandy loam) 13
Clay: 13.6%; org. matter: 0.8%; pH: 7,0; Cation Exchange 32
Capacity:4-5 me/100g
(sandy loam)
Greenhouse trials
Carbon: 0.1%; fines: 22.1%; pH: 5.8; clay: 9.5%; fine silt: 12.6% ca.14
Carbon: 1.08%; fines: 23.2%; pH: 6.1; water cap.: 19-25% 42-49
Field trials
Clay 3.8%: silt: 18.3%; pH: 6.6; water cap. 36.1% 20
Clay: 13.7% silt: 29.4%; pH: 6.2; water cap. 61.3% 20
Carbon: 1.35%; fines: 9.1%; pH: 7.0 30
Carbon: 0.8 %; fines: 15.3% pH: 6.5 23
Clay: 14%; org. matter: 5.85%; pH: 5.7 (sandy loam) 22
Clay: 40%; org. matter: 5 %;
Cation Exchange Capacity 15-18 me/200 g (loam) 20
In water
Ring-labelled 14C-I was incubated under sterile conditions in aqueous
solutions buffered at pH 3, 6 and 9 at 25, 35 and 45°C. Ethiofencarb
was shown to be stable for 30 days at PH 3 and 25°C, whereas the
half-life at pH 9 and 45°C ranged from 0.3 to 0.7 days (Table 9). The
primary hydrolysis product was IV, Figure 1 (max. 92.6% of the
radioactivity of the sample) with only small amounts (<10%) of II
(Kurtz and Gronberg, 1976). The hydrolysis rates of
carbonyl-14C-labelled I, II and III were determined at pH 9.0, 9.5,
10.0, 10.5 and 11.0. I was 3-5 times as stable as II or Ill. Again,
the half-life of all compounds decreased with increasing pH (Table 9)
(Dorough, 1976 d).
Studies with 14CO-ethiofencarb showed that it decomposed in water (pH
6.8) with a half-life of about 8 days, largely by hydrolysis of the
carbamate group. In contrast to the metabolism in soil and plants, II
and Ill were very minor products: their sum represented about 10% of
the total carbamate residue throughout the experiment. After 31 days,
83% of the original carbonyl carbon could be determined as carbonate
or carbon dioxide gas, 3.4% was present as I, 0.3% as II and 0.2% as
III (Dräger, 1976 b, 1977a)
Photodecomposition
The photodegradation of ring-14C-or carbonyl-14C-I was studies under
artificial and natural sunlight on glass plates, silica gel plates,
soil plates, and in aqueous solution (distilled water or pond water).
5% or less of I was found after 6 hours irradiation. II was the major
product: Ill and VI were found as minor constituents (Dorough,1976e).
For quantitative data, see Table 10.
The effect of anthraquinone as a photosensitizer was profound when
applied as a mixture with I on silica gel plates. Although the extent
of degradation of 1 me essentially unaffected, there was an increase
in the number of minor photoproducts (those less than 1%) with
anthraquinone and an increase in the polar material remaining at the
origin after 7 days of exposure to a sunlamp. Almost no II was
produced and a compound with a similar Rf to IV was the major product
(48% after 7 days of exposure). There were also lesser amounts of Ill
and VI (Dorough, 1976e).
In storage and processing
In head lettuce that had been field-sprayed in accordance with good
agricultural practice, no decline of the total residue was noted after
one year of storage in a deep freezer at -20°C (Bayer, 1974).
TABLE 9. Half-lives of ethiofencarb in buffered solutions.
Half-life of compound
Conditions I II III Ref.
(ethiofencarb) (ethiofenoarb (ethiofencarb
sulphoxide) sulphone)
pH: 9.0 6.67 hours 2.03 hours 2.13 hours Dorough, 1976d
9.5 2.67 hours 0.47 hours 0.72 hours
10.0 1.17 hours 0.23 hours 0.40 hours
10.5 0.43 hours 0.08 hours 0.12 hours
11.0 0.09 hours 0.02 hours 0.03 hours
25 °C: 10 ppm; pH 3 stable Kurtz and
pH 6 324 days Gronberg, 1976
pH 9 3.5 days
25 °C; 100 ppm; pH 3 stable
pH 6 stable
pH 9 2.5 days
35 °C; 10 ppm; pH 3 stable
pH 6 68 days
pH 9 1.5 days
35 °C; 100 ppm; pH 3 stable
pH 6 68 days
pH 9 2.0 days
45 °C; 10 ppm; pH 3 stable
pH 6 22 days
pH 9 0.7 days
45 °C; 100 ppm; pH 3 stable
pH 6 20 days
pH 9 0.3 days
TABLE 10A. Photodegradation of 14c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound1) after indicated time
1 hour 3 hours
Conditions
O I II III IV V VI xn O I II III IV V VI xn
artifical light, 1.0 51.4 44.9 3.5 2.3 1.8 16.3 70.3 4.8 2.9
TLC plates
germicidal light, 14.2 6.6 22.7 24.1 0.6 15.6 9.5 20.1 1.3 10.8 23.9 1.0 14.6 10.0
TLC
plates
natural
light, 0.6 42.1 62.0 0.7 1.9 0.8 1.2 2.9 91.4 2.2 2.7 1.2
TLC plates
artifical light, 1.6 1.0 84.2 4.3 7.1 0.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
germicidal light, 1.6 1.8 80.1 44.4 10.4 1.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
natural light, 1.4 1.3 80.5 5.9 9.5 1.5 1.2 1.9 82.7 6.3 0.5 6.6 0.9
soil plates
natural light, 1.1 0.5 46.1 5.1 1.7 2.3 0.6 36.1 7.7 1.6
glass plates
natural light, 1.9 6.5 78.2 6.5 1.0 4.6 1.6 4.2 2.9 70.1 22.5 6.5 5.3
aquous
solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
TABLE 10B. Photodegradation of 14 c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound 1) after indicated time
6 hours
Conditions
O I II III IV V VI xn
artifical light, 2.4 5.2 78.6 6.3 3.5
TLC plates
germicidal light, 21.2 4.3 8.8 24.3 1.0 14.0 9.7
TLC plates
natural light, 1.4 0.8 90.5 2.7 2.9 1.2
TLC plates
artifical light, 2.1 1.0 83.6 5.9 5.1 1.6
soil plates
germicidal light, 1.9 2.6 76.3 6.5 1.0 9.1 1.3
soil plates
natural light, 1.0 1.1 87.8 5.7 0.8 2.6 1.1
soil plates
natural light, 4.2 0.3 26.0 13.1 0.9 2.3
glass plates
natural light, 5.8 0.4 65.6 11.7 1.8 8.4 3.2
aquous solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
Studies to investigate the stability of the sulphoxide and sulphone
metabolites in potatoes revealed that the content of both compounds
remained constant on storage for 11 months at -20°C (Chemagro, 1976b).
When bovine and poultry tissues, milk and eggs were fortified with
14C-labelled ethiofencarb and stored under frozen conditions for 7
months, 84-98% of the residue was found as intact carbamate (Chemagro,
1976c).
Poultry rations fortified with ethiofencarb for use in various animal
feeding studies were stored for 5 weeks at -10°C; no degradation of
the ethiofencarb was noted (Chemagro, 1975).
Processing of plant material by baking and boiling results in the
reduction of ethiofencarb residues. Broad beans which had a residue
content of 0.13 mg/kg before boiling were found to contain < 0.02 mg
I + II + III/kg after being boiled for 15 minutes. In the green parts
(stems and leaves), the residues were reduced by boiling to between 3
and 7% of the original content of 14-30 mg/kg; 0.4-3.4% of the
original residue (I + II + III) was found in the water used for
boiling (Bayer, 1975).
Raw potatoes fortified with 14C-labelled ethiofencarb (1 mg/kg) were
processed by pan frying, french frying and baking. Both pan and french
frying processes reduced the residue (I + II + III) with an average
loss of 52 and 58%, respectively. Leaching of the residue (2-20%) into
the frying oils and volatilization were observed. The baking process
showed a loss of about 2% of the 14C activity. Of the remaining
activity, ethiofencarb accounted for 66%, ethiofencarb sulphoxide for
14%, and ethiofencarb phenol for 20% (Chemagro Report, 1976d).
METHODS OF RESIDUE ANALYSIS
Residues of ethiofencarb can be determined by gas chromatography using
a sulphur-specific flame photometric detector. The methods available
for the analysis of plant samples# animal tissue samples and soil
samples simultaneously determine metabolites with an intact carbamate
structure, i.e. ethiofencarb sulphoxide and ethiofencarb sulphone.
Oxidation with potassium permanganate converts ethiofencarb and
ethiofencarb sulphoxide to the sulphone. For gas-chromatographic
determination, the total carbamate residue is derivatized by reaction
with bix-trimethylsilytrifluoroacetamide to the thermostable, volatile
2-ethylsulfonylmethylphenyl trimethylsilyl ether.
For the extraction step, acetone (Dräger, 1974) or methanol/water
(Morris and Gronberg, 1976; Dorough, 1976a) were used for plant and
soil samples. Soil samples can also be extracted with a mixture of
acetonitrile and 0.1 × hydrochloric acid (Morris and Gronberg, 1977)
or by reflux with a methanol/chloroform mixture (Morris, 1976a).
Water-containing animal tissues and eggs are extracted with
acetonitrile; prior to extraction, fat is dissolved in hexane and
acetone is added to milk to separate protein (Dorough, 1976b).
Isolation of the residues from the extracts is achieved by shaking
with chloroform, and separation of co-extractives is accomplished by
precipitating with a solution of ammonium chloride/phosphoric acid.
After oxidation of the residues with potassium permanganate to
ethiofencarb sulphone, silylation is carried out in acetone or
benzene.
With some types of sample, further chromatographic clean-up steps are
needed before silylation, in order to separate sulphur-containing
plant co-extractives which interfere with the gas-chromatographic
determination. With brassica extracts clean-up was by thinlayer
chromatography and with hop extracts by column chromatography (Dräger,
1974). In the analysis of animal tissues, eggs and milk, the residue
was hydrolyzed to the corresponding phenol sulphone before silylation
in order to increase reproducibility (Dorough, 1976b).
For the gas-chromatographic determination, columns packed with 5%
DC-200 on Gaschrom Q (Dräger, 1974) and with -3% OV-1 on Chromosorb W
(Morris and Gronberg, 1977) have proved suitable. For a confirmatory
procedure, a column packed with the polar phase Carbowax 20-M on
Chromosorb W (Morris,1976b) was used. The lower limit of determination
is generally 0.02-0.05 mg/kg for plant and soil samples. 0.01 mg/kg
for fat, and 0.005 mg/kg for eggs and milk.
The methods were shown to be specific in a study in which pesticides
registered in the U.S.A. for use on potatoes, sorghum, animal tissues,
milk and eggs were checked for possible interference: none of the 50
chemicals tested interfered at their maximum registered level
(Dorough, 1976c).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The only tolerances reported to the Meeting were on fruit and
vegetables in Switzerland, but pre-harvest intervals have been
specified in several other countries as shown below (Table 11).
APPRAISAL
Ethiofencarb is a systemic insecticide used especially against sacking
insects an vegetables, fruit, field crops and tobacco, It is effective
against some organophosphorasresistant strains. It is formulated an
granules and emulsifiable concentrates. The usual dose is 0.3-2 kg
a.i./ha in spray, or approximately 0.1 g a.i. per linear metre as
granules at sowing or planting. Ethiofencarb is used in various
European countries, Chile and Israel.
In plants and soil ethiofencarb (I) is oxidized to its sulphoxide (II)
and sulphone (III) which are also hydrolysed. In plants and soil the
main intact carbamates are II and III. Little or no ethiofencarb is
usually detectable at harvest. In animals, the corresponding phenols
and their conjugates are the main metabolites.
TABLE 11. National tolerances and pre-harvest intervals reported to the Meeting.
Country Crop Tolerances Pre-harvest
in mg/kg interval in
days
Germany Beets, sugar beets 28
(FRG) Head lettuce 4
Bush beans, pole beans,
broad beans 7
Potatoes 14
Israel Peppers 5
Potatoes 4
Cauliflower, drumhead
cabbage 7
Apples 7
Italy Vegetables 7
Fruit crops 14
Beets 30
Spain Fruit crops incl. citrus,
winter cereals, maize,
potatoes, beets, sugarcane,
cotton, oilseed crops 21
Switzerland Pome, stone and small
fruit 0.2
Vegetables excl. spinach 1.0 14
Fruit crops, peas for
harvesting dry (dried,
threshed) 21
Sugar beet, field beans 42
Half-lives of I + II + III in soil vary between 2 and 12 weeks in the
field.
On the basis of feeding a diet containing 50 ppm of I to cows, pigs
and hens, it can be estimated that residues of I + II + III are
unlikely to exceed 0.02 mg/kg in their meat or in eggs or milk.
Boiling broad beans reduced carbamate residues of 0.13 mg/kg to below
0.02 mg/kg. Cooking the green parts of beans reduced the residues to
3-7% of the original content of I + II + III (14-30 mg/kg). 0.4-3.4%
of these residues was found in the water used for boiling. Deep and
shallow frying of potatoes fortified with 1 mg ethiofencarb/kg
decreased the total carbamate residue by about 50%. Baking potatoes
caused no loss of carbamate residues.
Gas-chromatographic procedures for the determination of residues of I
+ II + III, suitable for regulatory purposes, in various crops, eggs,
milk and soil have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended. They
refer to the am of ethiofencarb, its sulphoxide and its sulphone,
expressed as ethiofencarb.
Commodity Limit, Pre-harvest interval (days)
mg/kg on which recommendations
are based
Cherries, lettuce 10 4-7
Apples, pears, apricots 4-7
Artichokes, beans with pod, 4-7
beet tops,
sugar beet tops (leaves), 14
brassicas peaches plums 5 4-7
Currants (black, red),
eggplants, 4-7
wheat straw 2 14
Cucumbers 1 4
Potatoes, 14
Radishes 0.5 4
Beans without pods
soybeans without pod 0.2 4-7
Fodder beets,
sugar beets (roots) 0.1 14
Raw grain
(barley, oats, rye, wheat) 0.05 30
Meat of cattle, pigs
and poultry, eggs, milk 0.02*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by July 1978)
1. Submission of the results of the reproduction study now in
progress.
2. Further residue data from supervised trials on apricots,
artichokes, various brassicas, cherries, cucumbers (including those
grown under glass), eggplants, plums, radishes, soybeans.
DESIRABLE
1. Further residue data from supervised trials on other crops on which
ethiofencarb is known to be used.
REFERENCES
Bayer, A.G. (1972/77) Ethiofencarb; Pflanzensohutzmittel-Rückstände,
Dräger, Bayer-Leverkusen, unpublished.
Bayer, A.G. (1974) Report RA 301; (unpublished).
Bayer, A.G. (1975) Report RA 655; 20 Nov. 1975.
Bothard, E. and Löser, E. (1976) HOX 1901 Chronic Toxicity Study on
Rats. Nov. 30, Rep. No. 6493 from Bayer AG, Inst. f. Tox., unpublished
report submitted by Bayer AG. Addendum., Histopathological Examination
by J.P. Finn, Hazleton Laboratories, Inc.
Chemagro Report (1975) 45 176; 12 Sept, 1975, R & D Dep. of Chemagro,
Agricult. Div., Mobay Chemical Corp., Kansas City, No. 64 1209 USA.
Chemagro Report (1976a) Chemagro Agricult. Div.; Groneton; Potatoes
(Kats; Russet Burbank), Rep. No. 49 735/49 751; 17 Nov. 1976
(unpublished).
Chemagro Report (1976b) No. 51 062; 30 Dec. 1976 (unpublished).
Chemagro Report (1976c) No. 49 896; 27 Dec. 1976.
Chemagro Report (1976d) No. 46995; 11 Feb. 1976.
Dorough H.W. (1976a) A Gas Chromatographic Method for the
Determination of Croneton Residues in Various Animal Tissues, Crops,
and Soil. CR 49 879, 1.12.1976.
Dorough H.W. (1976b) A Gas Chromatographic Method for the
Determination of Croneton Residues in Bovine Tissues and Milk and
Poultry Tissues and Eggs. CR 49 880; 18.11.1976.
Dorough H.W. (1976c) An Interference Study for the Residue Method for
Croneton and Metabolites in Various Crops and Animal Tissuee, Milk and
Eggs. CR 49 893, 17.12.1976.
Dorough H.W. (1976d) Rate of Hydrolysis of CRONETON TM GRONETON
Sulphoxide, and CRONSTON Sulphone in Aqueous Solutions. Chemagro
Report No. 49 890 (1976).
Dorough H.W. (1976e) Photodcoomposition of CRONETON TM on Various
Substrates. Chemagro Report No. 49 891 (1976).
Dorough H.W. (1976f) The Metabolism and Residue in Potatoes and
Sorghum Treated Under Field Conditions Using CRONETON
TM-ring-UL-14C. Chemagro Report No. 49 894 (1976).
Dorough H.W. (1976g) The Metabolism of CRONETON TM in Plants.
Chemagro Report No. 49 895 (1976).
Dorough H.W. (1976h) Metabolism of CRONETON TM in a Lactating
Holstein Cow and a Male Yorkshire Swine. Chemagro Report No. 49 897
(unpublished).
Dorough H.W. (1976i) The Metabolism of CRONETON TM Poultry. Chemagro
Report No. 49 898 (unpublished).
Dorough, H.W. (1976j) Correlation of Blood and Tissue Radiocarbon
Levels Following a Single Oral Dose of CRONETON TM-ring-14C to Rats.
Chemagro Report No. 49 887 (1976).
Dräger, G. (1974) Method for gas-chromatographic determination of
Croneton residues in plants and soil. Pflanzenschutz-Nachrichten Bayer
91, 144-155 (1974).
Dräger, G. (1976a) Study to investigate uptake and metabolism of
Croneton in beans (Vioia faba) after application in granular form.
Bayer AG, Pflanzensohutz-Anwendungstechnik, unpublished Report RA-79,
January 19, 1976.
Dräger, G. (1976b) Studying the Metabolism of Croneton in Water. Bayer
AG Pflanzenschutz-Anwenclungstcohnik, unpublished Report RA-438v June
9, 1976.
Dräger, G. (1976c) Studies on the Metabolism of CRONETON in Potatoes
and Soil and Identification of Bound Residues in the Potato Tuber.
Bayer AG, Pflanzenschut Anwendungstechnik, unpublished Report RA-7989
October 89 1976.
Dräger, G. (1976d) Untersuchunger zum Versickerungsverhalten von
CronetonR (Makro-Säulen-Versuch). Bayer AG,
Pflanzenschutz-Anwenclungeteohnik, unpublished Report RA-961.
Dräger, G. (1977a) Untersuchung des Metabolismus von Groneton in Boden
und Wasser. Pflanzenschutz-Nachrichten Bayer 30, 18-27 (1977).
Dräger, G. (1977b) Weiterer Abbau, von gealterten Croneton-Rückständen
im Boden unter Laborbedingungen Bayer AG Pflanzenschutz-
Anwendungsteohnik, unpublished report RA-133, February 16, 1977
Dräger, G. (1977c) Versickerungsverhplten von gealterten
Groneton-Rlickstgnden in Boden unter Laborbedingunger, Bayer AG,
Pflanzenschutz-Anwendungsteohnik, unpublished Report RA-346.
Gronberg, R.R., Dorough, H.W., (1976) Effect of Feeding CRONTEONTM
to Poultry. Chemagro Report No. 49 899 (unpublished).
Gronberg, R.R., Dorough, H.W., Hemken, R.W. (1976) Effect of Feeding
CRONETONTM to Dairy Cattle. Chemagro Report No. 50 500 (1976)
(unpublished).
Hoffmann, K. and Weischer, C.H. (1976) 1901/Chronio Study on Dogs. May
26, Rep. No. 6120 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Homeyer, B. (1976) Croneton als wurzelaystemieches Aphizid.
Ptlanzenschutz-Nachrichten Bayer 29 255-266 (1976).
Hurst, H., Dorough, H.W. (1976) The Metabolism of CRONETON TM-
N-methyl-14C in Rats-A Progress Report. Chemagro Report No. 49 886
(1976).
Kimmerle, G. (1972b) HOX 1901/Acute Toxicity Studies. July 10, Rep.
No. 3557 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. (1972c) HOX 1901/Acute orale Toxizität an männlichen
Kaninchen. Nov. 13, report from from Bayer AG, Inst. f. Tox.,
unpublished report submitted by Bayer AG.
Kimmerle, G. (1972d) Antidotal Effect of Atropine. July 10, report
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Kimmerle, G. (1972e) HOX 1901/Subacute Toxicity Studies. July 10, Rep.
No. 3558 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. and Eben, A. (1974) HOX 1901/Effect of Acute and Subacute
Oral Administration and Subacute Inhalation on Plasma, Erythrocyte and
Brain Acetylcholinesterase Activity in Rats and Dogs. Aug. 8, Rep. No.
4843 from Bayer AG, Inst. f. Tox.p unpublished report submitted by
Bayer AG.
Klimmer, O.R. (1973) Study Report / Bayer HOX 1901/Subchronic
Toxicological Studies on Rats. Febr. 27, Rep. No. R 739 from
Pharmakologisches Instit der Rhein. Friedr.-Wilh.-Univ., Bonn,
unpublished report submitted by Bayer, AG.
Kurtz, S.K., Gronberg, R.R. (1976) The Stability of CRONETON TM in
Aqueous Systems. Chemagro Report No. 50 798 (1976).
Lamb, D.W., Roncy, D.J. (1976) Accumulation and Persistence of
Residues in Channel Catfish Exposed to CRONETON-14C. Chemagro Report
No. 49 128 (unpublished).
Lamb, D.W. and Matzkanin, G.S. (1977a) The Acute Oral Toxicity of
CRONETON Sulphoxide. Febr. 18, Rep. No. 51 716 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lambt D.W. and Matzkanin, G.S. (1977b) The Acute Oral Toxicity of
CRONETON Sulphone. Febr. 21, Rep. No. 51 717 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, G.S. (1977c) The Acute Oral Toxicity of
CRONETON Phenol. Febr. 17, Rep. 51 719 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkaning G.S. (1977d) The Acute Oral Toxicity of
CRONETON Phenol Sulphoxide. Febr. 17, Rep. No. 51 718 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977e) The Acute Oral Toxicity of
CRONETON Phenol Sulphone. Febr. 17, Rep. No. 57 715 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977f) The Acute Oral Toxicity of
CRONETON (Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroxy). April
26, Rep. No. 52 878 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977g) Acute Oral Toxicity of CRONETON
(Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroyy) Sulphoxide.
April 29, Rep. No. 52 879 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977h) The Acute Oral Toxicity of
CRONETON Metabolite N-Methyl (Hydroxy) Sulphone. April 29, Rep. No. 52
880 from Mobay Chem. Corp., unpublished report submitted by Bayer AG.
Machemer, L. (1973a) HOX 1901/Dominant Lethal Test on the Male Mouse
to Test for Mutagenic Effect. Dec. 14, Rep. No. 4339 from Bayer AG,
Inst. f. Tox., unpublished report submitted by Bayer AG.
Machemer, L. (1973b) HOX 1901/Studies for Embryotoxic and Teratogenic
Effects on Rats Following Oral Administration. July 25, Rep. No. 4174
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Machemer, L. (1975) HOX 1901/Studies of Embryotoxic and Teratogenic
Effect on Rabbits Following Oral Administration. July 24, Rep. No.
5545 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Marshall, T., Dorough, H.W. (1976) Fate of Water Soluble CRONETON TM
Plant Metabolites in Rats (Progress Report). Chemagro Report No. 49
892 (1976).
Marshall, T.C., Dorough, H.W. (1977) Bioavailability in Rate of Bound
and Conjugated Plant Carbamate Insecticide Residues. Chemagro Report
No. 52 737 (1977).
Morris, R.A. (1976a) Determination of Baygon, Baytex, Bolster,
Croneton, Dasanit, Di-Syston, Dylox, Guthion, Hinosan, Mesurol,
Meyasystox-R, Monitor, Morestan, Nemacur, Systox Residues in Soil. CR
49 675, 15.9.1976.
Morris, R.A. (1976b) A Confirmatory Procedure for the Analytical
Residue Method for Croneton in Potatoes, Plant Forage and Cereal
Grains. CR 51 0639 30.12.1976.
Morris, R.A. Gronberg, R.R. (1976) A Gas Chromatographic Method for
the Determination of Residues of Croneton (Bay BOX 1901) and
Metabolites in Potatoes, Plant Forage, and Cereal Grains. CR 49 666#
23.8.1976.
Morris R.A., Gronberg, R.R. (1977) A Gas Chromatographic Procedure for
the Determination of Croneton (Bay HOX 1901) in Soil. CR 49 6659
10.2.1977.
Mürmann, P. (1973) HOX 1901/Subohronic Toxicity Study on Dogs. June
28, Rep. No. 4143 from Bayer AG, Inst. f. Tox., (unpublished).
Nitokuno Inst., (1975a) Report No. 3599 unpublished.
Nitokuno Inst., (1975b) Reports No. 335 and 336, unpublished.
Nitokuno Inst., (1975/76a) Reports No. 333, 334 and 390, unpublished.
Nitokuno Inst., (1975/76b) Reports No. 360-362 and 395-396,
unpublished.
Nitokuno Inst., (1976a) Report No. 379, unpublished.
Nitokuno Inst. (1976b) Reports No. 391-1949 unpublished.
Nitokuno Inst. (1976c) Reports No. 397-4009 unpublished.
Nitokuno Inst. (1976d) NIHON TOKUSHU NOYAKU SEIZO K.K. Residual
Analysis Results of HOX 1901 (ethiofencarb) in Upland Soil. Report No.
1042 (RA), January, 28, 1976.
Nye, D.E., Hurst, H.E., Dorough, H.W. (1976) Fate of Croneton
(2-Ethylthiomethylphenyl N-Methyl-carbamate) in Rats. J. Agric. Food
Chem. 24, 371-377.
Rieck, C.E. (1976a) Leaching characteristics of CRONETON TM in
various soils. Chemagro Report No. 50 783.
Rieck, C.E. (1976b) Leaching characteristics of CRONETON TM on aged
soil. Chemagro Report No. 51 028.
Thorton, J.S., Hurley, J.B. and Obrist, J.J. (1976) Soil thin-layer
mobility of twenty four pesticides chemicals. Chernagro Report No. 51
016.
Thyssen, J. and Kimmerle G. (1975) Präparat 6757/Untersuchungen zur
Anwendungstoxikologie. Nov. 27, Rep. No. 5751 from Bayer AG, Inst. f.
Tox., unpublished report submitted by Bayer AG.
Thyseen, J. (1975a) HOX 1901/Neurotoxicity Studies on Hens. Nov. 18,
Rep. No. 5735 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG. Appendix: ROX 1901 Neurotoxicity Studies on
Hens/Histopathological Finding by Prof. Dr. U. Mohr, Med. Hoohsohule
Hannover.
Thyssen, J. (1975b) Studies to Investigate Acute Oral Toxicity of HOX
1901 when Administered Simultaneously with Malathion or EPN, Nov. 18,
Rep. No. 5732 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Thyssen, J. (1976) HOX 1901-Metaboliten. Sept. 1, report from Bayer
AG, Inst. f. Tox., unpublished report submitted by Bayer AG.
Acute toxicity
TABLE 1a, Acute toxicity of ethiofencarb
Species Sex Route LD50 References
mg/kg
Rat m oral 308 Thyssen and Kimmerle, 1975
Rat m oral 411 Kimmerle 1972b
Rat f oral 499 ibid.
Rat m i.p. 41.8 ibid.
Rat f i.p. 41.9 ibid.
Rat m dermal >1000 ibid.
Mouse m oral 256 ibid.
Mouse f oral 224 ibid.
Rabbit m oral approx. 225 Kimmerle 1972c
Rabbit m dermal >8000 Lamb and Matzkanin 1976a
Rabbit f dermal >8000 ibid.
Dog f oral >50 Kimmerle 1972b
Hen oral approx. 1000 ibid.
The typical symptoms of acetylcholinocaterase inhibition such as
salivation, muscle tremors, spasms and convulsions preceded death.
A good antidotal effect was obtained when rats orally treated with
ethiofencarb received i.p. injection of atropine sulphate (Kimmerle
1972d).
Short term studies
Rat
Groups of 15 male and 15 female rats were treated orally for 30 days
at dose levels of 0, 5, 10, 20 and 40 mg/kg. The administration had no
effects with respect to physical appearance, behaviour, body weight,
haematological and clinical chemistry test values. No gross
pathological alterations of tissues were observed and organ weights
did not significantly differ from those of the control animals.
Tissues were not examined microscopically (Kimmerle, 1972e).
In another subacute study 10 male and 10 female rats per dosage group
were exposed for 6 hours daily on 5 consecutive days per week over a
3-week period to aerosol concentrations of 0, 29.7 and 148.5 mg/m3
of air. All these concentrations were tolerated without any adverse
effect with respect to behaviour, general health condition, body
weight, haematological and clinical chemistry values.
The macroscopic examination of the major tissues revealed no
pathological alteration and organ weights did not differ significantly
between the control group and the treated groups (Kimmerle, 1972e).
Groups of 20 male and 20 female rats were fed ethiofencarb at
concentrations of O, 250, 500 and 1000 ppm for 3 months. Physical
appearance, behaviour, mortality and food consumption were not
affected by the diet. The average body weights of male rats fed 500
and 1000 ppm were higher than the corresponding weights of the control
animals. Values of urinalyses, haematology and clinical chemistry were
within the physiological range. Macroscopic and microscopic
examination of the principal organs revealed no treatment-related
alterations. The relative liver weights in male rats treated with 1000
ppm were higher than those of the control animals (Klimmer, 1973).
Dog
Groups of 4 male and 4 female dogs were maintained for 3 months on a
diet containing ethiofencarb at concentrations of 0, 100, 300 and 1000
ppm. The treatment did not effect physical appearance, behaviour, food
consumption, body weight, mortality or ophthalmoscopic and
neurological findings. Parameters of haematology, clinical chemistry
and urinalyses showed no significant differences between control and
treated groups. At 1000 ppm an increase of the relative liver weights
of about 10% was noted in both sexes and an increase of about 40% in
relative kidney and spleen weights in male animals only. Gross and
histopathological examination showed no treatment-related alteration
(Mührmann, 1973).
Groups of 4 male and 4 female dogs received a diet for a period of 104
weeks containing ethiofencarb at concentrations of 0, 330, 1000 and
3000 ppm. The administration of up to and including 1000 ppm did not
affect the test animals with respect to their physical appearance,
behaviour, food consumption and ophthalmoscopic findings. The dietary
level of 3000 ppm caused vomiting immediately after feeding,
especially in the second half of the study; food consumption was
retarded and reduced in some female dogs at the 3000 ppm level,
whereas female dogs at 330 and 1000 ppm made bigger gains in
comparison with controls. Haematological and urinalyses values
revealed no pathological alterations. Results of chemistry tests (with
respect to blood sugar, urea, creatinine, total protein, GOT,
bilirubin) were within the physiological range. The ALP activities
showed a 3 -4 fold increase at 3000 ppm and in some dogs of the same
group an increased level of GPT activity and cholesterol were noted.
Relative liver weights of the animals of both sexes at 1000 and 3000
ppm showed a dose-related increase. One dog at the 3000 ppm feeding
level showed enlargement of the liver. At 3000 ppm the
histopathological examination of tissues disclosed signs of treatment-
related alterations in liver such as enlarged hepatocytes, hepatocytes
nuclei and granular cytoplasmic structures (Hoffmann and Weischer,
1976).
Long term studies
Rat
Groups of 50 male and 50 female (100 male and 100 female in control
group) were fed a diet containing ethiofencarb at concentrations of 0,
330, 1000 and 3000 ppm for 24 months. The treatment did not affect the
physical appearance, behaviour and mortality of the test animals. The
values of haematological and clinical chemistry tests (with respect to
ALP, GOT, GPT, GLDH, bilirubin and proteins in serum), urinalyses and
kidney function tests were within the physiological ranges. Blood
sugar values did not differ from the control, whereas a dose-related
increase of the cholesterol values in treated animals was found,
especially at 3000 ppm. No gross pathological alterations that could
be attributable to treatment were seen in rats which died during the
feeding study or which were sacrificed at the end of the study. Some
variations in the relative organ weights were found.
A dose-dependent increase of the relative liver-weight at 1000 and
3000 ppm was observed. The histopathological examination of tissues
revealed no treatment-related alterations. The incidence and
localization of tumours provided no indication for a carcinogenic
effect of ethiofencarb (Bombard and Löser, 1976).
TABLE 1b. Acute toxicity of ethiofencarb metabolites
Species Sex Route LD50 References
mg/kg
Ethiofencarb-sulfoxide:
Rat m oral 200-250 Thyssen 1976
Rat f oral 200-250 ibid.
Rat f oral 133 Lamb and Matzkanin 1977a
Ethiofencarb-sulfone:
Rat m oral 600-750 Thyssen 1976
Rat m oral 468 Lamb and Matzkanin 1977b
Rat f oral approx. 600 Thyssen 1976
Ethiofencarb-phenol:
Rat m oral approx. 200 Lamb and Matzkanin 1977c
Rat f oral approx. 200 ibid.
Ethiofencarb-phenol-sulfoxide:
Rat m oral >1000 Lamb and Matzkanin 1977d
Rat f oral >1000 ibid.
Ethiofencarb-phenol-sulfone:
Rat m oral >1000 <20 Lamb and Matzkanin 1977e
Rat f oral >1000 <20 Ibid.
TABLE 1b. (Continued)
Species Sex Route LD50 References
mg/kg
N-methyl-hydroxy metabolite of ethiofencarb:
Rat m oral >2000 Lamb and Matzkanin 1977f
Rat f oral approx 2000 ibid.
N-methyl-hydroxy sulfoxide metabolite:
Rat m oral >2000 Lamb and Matzkanin 1977g
Rat f oral >2000 ibid.
N-methyl-hydroxy-sulfone metabolite
Rat m oral >2000 Lamb and Matzkanin 1977h
Rat f oral >2000 Ibid.
Comments
Ethiofencarb is almost completely absorbed in mammals and it is
excreted rapidly in the form of metabolites mainly in the urine. The
major metabolic reactions are demethylation and subsequent hydrolysis
of the carbamate and oxidations to sulphoxides and sulphones.
Ethiofencarb inhibits the activity of acetylcholinesterase only
transiently.
In a 4-week study daily oral administration of 10 mg/kg to male rats
led to a maximal depression of 25% of the plasma-cholinesterase and of
15% of the erythrocyte-cholinesterase after 2 hours. Recovery occurred
almost completely within 24 hours. This dose level was taken as a
basis for establishing an acceptable daily intake for humans. The fact
that at 10 mg/kg marginal effects occurred was taken into account by
using a safety factor higher than is usual for
cholinesterase-inhibiting compounds.
The compound was not mutagenic in a dominant lethal test, nor was it
teratogenic. No three-generation study was available. However, such a
study was felt to be important since the depression of the
cholinesterase activity often manifests itself unfavourably in a
reduced viability of the pups.
In a 2-year feeding study in dogs no untoward effects were seen up to
1000 ppm. A long-term feeding study in rats revealed no more than a
moderate increase of the relative liver weights at 3000 ppm.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 (mg/kg bw)/day
Dog: 1000 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Ethiofencarb in a systemic insecticide which has a selective effect
against aphids. It gives control of numerous aphid species on
vegetables, fruit crops, field crops and ornamental plants. It
displays good activity against both susceptible and
organophosphorusresistant strains. Used as a soil treatment, e.g. by
the soil drench method or as a granular application, ethiofencarb
displays long residual activity; when applied to the aerial parts of
plants it is somewhat less persistent. In trials conducted to date,
ethiofencarb has shown no phytotoxicity.
Ethiofencarb is sold or registered in Bulgaria, Chile, Denmark,
Federal Republic of Germany, Israel, Italy, Portugal, Spain, Sweden,
Switzerland and Turkey.
Applications for registration have been filed in a number of other
countries.
Pre-harvest treatments
Ethiofencarb is formulated as an emulsifiable concentrate for spraying
and as a granular product. Used as a spray, it is applied broadcast at
the onset of aphid infestation. As a granular formulation, it is
applied at sowing or planting, mainly in-furrow.
Details of uses and recommendations for pre-harvest treatments with
ethiofencarb are given in Table 2.
Post-harvest treatments
No treatments recommended.
Other uses
Ethiofencarb is also used on ornamental plants.
RESIDUES RESULTING PROM SUPERVISED TRIALS
The residue data obtained after the application of ethiofencarb to
fruit, vegetables and field crops are summarized in Table 3. The data
originate from trials conducted in the U.S.A., Japan, Great Britain,
France, the Netherlands and the Federal Republic of Germany.
The residues were determined by gas-chromatographic methods which
determine residues of ethiofencarb and the metabolites with an intact
carbamate group.
After application by spraying, half-lives of 2 to 18 days were noted
for the total residue.
Detailed studies with different formulations using the same active
ingredient dose produced the following half-lives for the given crops:
Apple: 13 (5-18) days
Bush bean: 5 (4-7 ) days
Head lettuce: 5 (4-6 ) days
Savoy cabbage: 5 (2-9 ) days
With crops of which the foliage and edible parts were analyzed
separately, e.g. potatoes, sugar beet, broad beans and radish, it was
found that the residues were present mainly in the foliage owing to
the pronounced systemic mode of action of ethiofencarb, residues were
found in the analyzed plant samples also after application of the
granular formulation to the soil. Here, too, there was an accumulation
of the residues in the leaves, as proved by studies on broad beans and
sugar beet.
FATE OF RESIDUES
General
The metabolism of ethiofencarb (I) is illustrated in Figure 1. It is
found to be of the same pattern, with oxidation of the sulphur and
hydrolysis of the carbamate group, in soil, plants and animals, with
only quantitative differences. In soil and plants, ethiofencarb
sulphoxide (II, Figure 1) and ethiofencarb sulphone (III) were the
main products with an intact carbamate group. In animal metabolism
studies, phenolic degradation products formed by hydrolysis of the
carbamate group were also detected. CO2 released from the carbamate
group by hydrolysis was found to be incorporated into plants.
In animals
Studies on ethiofencarb metabolism in rats are described previously
under "Biochemical aspects". Marshall and Dorough (1976,1977)
investigated the fate of water-soluble plant metabolites of
ethiofencarb in rats. Bean plants were stem-injected with either
ring-14C- or carbonyl-14C-ethiofencarb. After 20 days almost all
the radiocarbon residue was in the epicotyl leaves. The distribution
in the unextractable, organic and aqueous fraction was 3.3%, 45.8% and
40.7% for the ring label and 1.3%, 13.8% and 2.6% for the carbonyl
label. 14C in the aqueous fractions when orally dosed to rats was
largely excreted within 36 hours, mainly by routes similar to those of
the parent compound, viz. in urine (92% of ring and 39% of CO-label)
and as expired CO2 (30% of CO-label).
Sorghum plants treated with ring-14 C-ethiofencarb (I) in an
emulsifiable spray were used as a source of unextractable metabolites
(Marshall and Dorough, 1977). When these bound residues of I were
administered to rats, 90.8% of the dose was voided in the faeces and
16.4% in the urine within 2 days; the bile contained 2.5% of the dose.
These data demonstrated that bound residues of I were very poorly
absorbed from the gastrointestinal tract of rats, while the conjugated
ones were readily absorbed.
Gronberg at al. (1976) continuously fed I to dairy cows for 28 days at
50, 150 and 500 PPM in the diet, equivalent to 1.5, 4.5 and 15 mg/kg
body weight/day. From the residue data obtained it was calculated that
tissue residues would be less than 0.1 mg/kg I + II + III equivalents
and milk residues would be less than 0.01 mg/kg for animals on a
ration containing 90 ppm and 14 ppm of ethiofencarb respectively.
Residues in milk averaged about 1 mg/kg at the highest feed level.
Tissue residues are shown in Table 4.
A lactating cow and a pig were treated with a single oral dose of 0.5
mg/kg 14C-ring-labelled ethiofencarb (Dorough, 1976h). 24 hours
after administration 97.8% of the dose had been excreted via the urine
from the cow and 90% from the pig. Elimination of the radiocarbon in
the faeces was <1% and 5.1% in cow and pig respectively.
Tissues of the pig contained no detectable radioactive residues 24
hours after treatment. Of the bovine tissues only kidney, liver and
skin contained detectable residues (Table 4) of 0.016, 0.017 and 0.05
mg/kg ethiofencarb equivalents respectively. Maximum concentrations of
14C in the blood of the cow, reached 3 hours following
administration, were 0.3 mg/kg. The milk the peak residue level was
0.15 mg/kg, reached 4 hours after dosing. About 67% of the milk
residue consisted of free metabolites, mainly ethiofencarb sulphoxide
and sulphone.
11.6% of the urinary metabolites in the cow were in the free form,
predominantly as phenol sulphoxide and phenol sulphone. Other
metabolites were eliminated as conjugates. In the pig 40.6% of the
dose was excreted in free form.
Eight laying hens were treated with a single oral dose of
14C-ring-labelled ethiofencarb at a rate of 0.5 mg/kg (Dorough,
1976). over 80% of the administered radiocarbon was eliminated in the
excreta after 24 hours and over 90% after 3 days. The highest
radiocarbon level 8 hours after application was found in kidney at
0.062 mg/kg. Liver, muscle and blood showed lower residue levels and
C, fat and heart no residues could be detected (see Table 4). 24 hours
post-administration no 14C could be detected in the tissues. Eggs
laid during a 3-day period following the single treatment were free
from detectable residues.
In a further study (Dorough,1976i) 10 hens were given
14C-ring-labelled ethiofencarb as daily treatment for 7 days at a
rate of 0.5 mg/kg twice a day. 2 days after the start of treatment
about 60% of the cumulative dose was excreted and after 7 days about
92%. Tissues of animals sacrificed 3-4 hours after the final dose
contained 14C-ethiofencarb equivalents ranging from about 0.02 mg/kg
in fat to 0.32 mg/kg in kidney. 4 days after the termination of
treatment muscle, skin, kidney and liver contained 0.001, 0.006, 0.007
and 0.009 mg/kg 14C-ethiofencarb equivalents respectively. In bloody
brains fat and heart no residues were detectable. Levels of
radiocarbon in the eggs reached a maximum of 0.06 - 0.07 mg/kg after 7
days of continuous treatment. In eggs (white and yolk) residues were
about 0.03 - 0.04 mg/kg after 2 days of treatment, reached a maximum
of 0.06 - 0.07 mg/kg after 7 days and were below detectable levels 3
days after treatment was stopped.
TABLE 2. Use pattern of ethiofencarb
Crop Concentration or Formulation Number of Pre-harvest
application rate (a.i.) applications interval(days)
Vegetables 0.0375-0.05% E.C. 2-3 4-7
(Cucurbitaceae, foliar spray/overall
eggplants, peas application
brassicas, peppers, 0.075 -0.1 g./linear GR 1
lettuce, tomatoes metre at sowing/
Vicia, etc.) planting
Cereals 100-500 g./ha. E.C. 1-2 14-21
(including millet) foliar spray/overall
application
Potatoes 500-600 g./ha E.C. 2-3 14
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at planting
Pome and stone fruit, 0.05% E.C. 2-3 7-10
small (soft) fruit, foliar spray/overall
citrus fruit application
Beets 400-500 g./ha E.C. 2-4 14
foliar spray/overall
application
Tobacco 0.05-0.075% E.C. 2-3 4-7
foliar spray/overall
application
0.075-0.1 g./linear GR 1
metre at sowing/
planting
TABLE 3. Residues of ethiofencarb, its sulphoxide and sulphone from supervised trials
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Apple D 2-3 1 EC 0.9-2.2 0.4 -2.0 0.4 -1.3 0.4 -1.6 0.2 -1.4 0.3-1.0 1)
(28d:)
F 1 0.5 EC 0.6 0.3 -0.4 0.4 1)
J 3/5 3-3.5 EC 1.2 - 1.3 0.8-1.2 2)
(30d.)
Apricot J 2/4 7.5 gEC/tree 0.8 -1.2 1.8-2.1 3)
(28d.)
Artichoke F 1 0.5-1EC 1.0 0.4 -4.7 0.2 -3.0 0.2 -1.0 1)
Beans
broad beans D 2-3 0.3 EC 1)
beans 0.02-0.04 0.02-0.04 0.02-0.03 <0.02
pods 0.2 -0.43 0.08-0.26 0.05-0.22 0.04-0.11 0.02-0.05
greens 5.5 -10.1 0.8 -4.6 0.6 - 4.6 0.5 -3.1 0.2 -1.0
broad beans D 1 1.5 G (55d.) (89d.) (111d.)
beans 0.02-0.13 0.02-0.04
pods 0.19-0.42 0.19-0.57
greens 14-30 2.2 -6.1 0.17-0.84
bush} D 2-3 0.3 EC 0.14-0.68 0.25-0.43 0.03-0.32 0.02-0.19 0.02-0.11 <0.02 1)
(28d.)
beans} F 1 0.75 EC 1.46-2.47 0.77-1.84
F 1 2 G 0.05 0.05 0.03 1)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Cabbage
Brussels sprouts D 3 0.3 EC 0.5 -1.4 0.4 -0.94 0.2 - 0.9 1)
Cauliflower D 2 0.3 EC 0.3 -0.5 0.1 -0.43 0.05-0.2
F 1 0.5 EC 0.26 0.11 0.07 1)
Chinese cabbage J 3/5 0.75-2EC 0.04-2.7 0.04-1.8 0.06-2.4 4)
(5d.)
Red cabbage D 2 0.3 EC 0.2 -1.1 0.2 - 0.4 0.05-0.5 1)
Savoy cabbage D 2 0.3 EC 0.4 -3.1 0.1 - 1.3 0.05-1.3 0.2 - 0.6 0.06-0.4 0.05-0.3 1)
(46d.) (78d.) (88d.) (28d.)
Savoy cabbage D 1 1 G 0.5 -0.6 0.05 <0.05 1)
White cabbage D 2 0.3 EG 0.2 0.1 <0.05 1)
White cabbage GB 1 3 G 0 09 1)
Cherry (see end of table) (105d.)
Cucumber J 3/5 0.75- 0.7 -0.9 0.3 -0.8 0.2 -0.5 0.1 -0.32 4)
2.5 EC
Currant
Blackcurrant D 2 1 EC 1.1 - 2.8 0.5 - 1.4 0.6 - 1.4
Red currant D 2 1 EC 0.5 - 2.9 0.4 - 1.0 0.2 - 0.6 1)
Eggplant F 1 0.75 EC 0.03-0.5 0.02-1.0 1)
Lettuce
(outdoor) D 2 0.3 EC 4.3 - 10.1 1.3 - 4.4 0.6 - 2.2 0.4 - 0.8 0.2 - 0.6 0.1 - 0.2 1)
(28d. )
(under glass) D 2 0.3 EC 2.3 - 18.8 2.2 - 10.1 1.6 -6.4 1.5 0.5 - 0.8 1)
(under glass) NL 1 5 G (44-67d.) 1)
Peach D 1-2 0.75-1EC 1.2 -2.3 1.1 -1.7 0.5 -2.0 0.4 -1.4 0.3 -1.1
Peach F 1 0.5 EC 0.3 -0.8 0.2 -0.3 0.1 -0.2
Plum (see end of table)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Potatoes D 3 0.4-0.6 0.16-0.23 0.09-0.25 0.08-0.17 0.02-0.16 1)
EC (28d.)
Potatoes US 2-6 0.5-2 EC <0.02-0.06 <0.02-0.06 6)
Potatoes J 4-5 0.9-1.75 0.07-0.1 0.04-0.16 0.04 7)
EC
Potatoes leaf J 1 0.7-1.7 EC 38-57 2.2 -4.6 0.3 -1.3 0.2 -0.6 7)
Potatoes D, NL, 1 1.5 G <0.02-0.06 1)
(91-135d.)
Radish J 3/5 1-1.5 EC 0.10-0.12 0.03-0.14 0.02-0.10
Radish leaf J 3/5 1-1.5 EC 0.1-1.5 0.06-1.2 0.1-0.6 8)
Soybean
Soybean, 3 0.75-1.5 0.02-1.04 <0.02-0.18
green+shell EC
Soybean, bean 3/5 0.75 EC 0.04-0.06 0.02-0.04 9)
(44-67d.)
Wheat 1)
grain D 2 0.4 EC
straw 9.6 -11.4 1.4 -1.9 <0.02 <0.02 1)
grain F 1 0.5 EC 1.2 -1.7 0.06-0.8
straw <0.02
0.1 -0.32
(34.53d.)
TABLE 3. (Continued)
Application Residues (mg/kg) at interval (days) after last application
Crop Country a) No. kg/hab) 0 3-4 7-10 14-16 18-21 >21 Ref. c)
(number in
brackets)
Sugar beet
beet D,GB 2-5 0.25-0.5 0.04-0.06 0.03 0.02 0.01-0.03 1)
top (leaf) EC 3.0 -4.7 2.0 -2.2 2.7 -4.9 0.12-1.4
(31-76d.)
beet D, GB 1 1-5-3 G 0.01-0.08 1)
top (leaf) 0.05-1.1
(124-195d.)
Tobacco 3 0.3-0.4 12.5-52 5.4-37 4.5-21 1.3 - 9.3 2.9 - 5.7 1.1 -13
(25-28d.) 1)
air -
cured D 1 1.5 G 4.5 -12.3
(54-58d.+
132-137d.
cured)
Cherry
(morello) D 3 1 EC 1.8 -5.9 0.5 -5.9 1.2 -4.2 1)
Plum D 3 1 EC 2.4 -3.5 1.3 -3.4 1.3 -2.6 1)
Trials in a)
Fed. Rep. Germany = D
France = F
Great Britain = GB
Japan = J
Netherlands = NL
United States Amer. = US
TABLE 3. (Continued)
b)
EC = Emulsifiable concentrate
G = Granular
References c)
1) Bayer AG, 1972/77
2) Nitokuno Inst., 1976
3) Nitokuno Inst., 1975
4) Nitokuno Inst., 1975/76
5) Nitokuno Inst., 1975a
6) Chemagro Report, 1976
7) Nitokuno Inst., 1975/76a
8) Nitokuno Inst., 1976a
9) Nitokuno Inst., 1976b
TABLE 4. Ethiofencarb equivalents in various tissues of warm-blooded animals
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Rat 0.5 mg/kg Ring- 14C 2.5 h 0.021) 0.061) 0.041) 0.031) Dorough,
single oral dose 1976j
Rat 0.5 mg/kg N-Methyl-14C 8 h 0.24 0.88 Hurst and
single oral dose 24 h 0.19 0.46 Dorough,
1976
Rat 6.6 ppm/day in diet 0.24 0.18 0.06 0.29 1.18 0.10 Nye at al.,
for 7 days, carbonyl-14C 1976
Cow 0.5 mg/kg Ring-14C 3 h 0.32 0.016 0.017 0.05 Dorough,a
single oral dose 24 h 1976h
Cow 2xdaily 50 PPM in diet 0.03 0.042) 0.03 0.01 <0.01 0.083) Gronberg
for 28 days et al.,
2xdaily 150 PPm in diet 0.27 0.232) 0.29 0.10 0.01 0.853) 1976
for 28 days
2xdaily 500 PPM in diet 2.17 1.012) 2.53 0.89 0.13 4.313)
for 28 days
Hen 0.5 mg/kg Ring-14C 8 h 0.053 0.028 0.062 0.035 0.039 0.022 Dorough,
single oral dose 1976i
0.5 mg/kg Ring-14C 4 days 0.016 0.001 0.005 0.016 0.025 0.013
2xdaily for 7 days 7 days4) 0.099 0.039 0.019 0.072 0.324 0.180 0.069 0.075
TABLE 4. (Continued)
Radioactivity expressed as ethiofencarb (mg/kg) in
Animal Application Time after Blood Brain Fat Giblet Heart Kidney Liver Muscle Skin Ref.
first
application
Hen 45 ppm in diet for 28 days 0.03 0.04 0.05 0.05 Gronberg
150 ppm in diet for 28 days 0.07 0.13 0.08 0.10 and
450 ppm in diet for 28 days 0.16 0.41 0.29 0.24 Dorough,
1976
1) The values were derived from graphs
2) Mixed omental, renal and subcutaneous fat
3) Mixed loin, round, flank
4) Higher residues at 7 than at 4 days were due largely to the fact that the 7-day birds were sacrificed 3-4 h
after last treatment while 4-day birds were sacrificed 8 h after treatment
The major identified metabolites eliminated in the excreta were the
phenol sulphoxide and sulphone, existing as free metabolites or
conjugates, the proportion of free metabolites being higher in the
singly dosed birds. Small amounts of the sulphoxide and sulphone of
the carbamate were also found in the continuous feeding study only.
The same sulphoxidation products were found as the principal
metabolites in eggs, about 50% of the residue being the phenol
sulphone and about 20% the phenol sulphoxide.
Poultry were continuously fed with ethiofencarb for 28 days at a
ration level of 0, 15, 45, 150 and 450 ppm (Gronberg and Dorough,
1976). The average concentration of ethiofencarb equivalents (Table 4)
in fat, skins muscle and giblet ranged from 0.03 to 0.05 mg/kg at the
45 ppm level, from 0.07 to 0.13 mg/kg at the 150 ppm level and from
0.16 to 0.4 mg/kg at 450 ppm. Average residues in eggs collected at
days 26, 27 or 28 of treatment were approximately 0.01, 0.04 and 0.1
mg/kg at the 451 150 and 450 ppm feeding levels respectively. Prom
these residue data it me concluded that tissue residues would be less
then 0.1 mg/kg and egg residues less than 0.01 mg/kg for animals on a
ration of 100 ppm and 40 ppm respectively.
After continuous exposure of Channel catfish to 10 µg/1
14C-ring-labelled ethiofencarb for 28 days, 14C residues accumulated
to a plateau of about 50-75 mg/kg reached after 4 days. The edible
parts of the fish contained about 27% of the residues. During the
withdrawal period about 82% of the accumulated residues were excreted
wIthin 1 day and about 95% within 4 days (Lamb and Roney, 1976).
In plants
Ethiofencarb has good systemic properties and is absorbed in large
amounts by the plant roots and translocated to the aerial parts
(Homeyer, 1976).
Bean seedlings absorbed a total of 34.6% of the available
14C-ring-labelled ethiofencarb from a solution via the roots in 4
days, whilst 18.1% was found after application of
14C-carbonyl-labelled compound. The distribution of radiocarbon in
the plant after 4 days was: roots 5.1%, stems 9.0%, leaves 16.5%
(Dorough, 1976 g).
Dräger (1976a) applied 10% granules of ethiofencarb (I, Figure 1), its
sulphoxide (II) and its sulphone (III) separately to soil at the time
of sowing beans. Results are shown in Table 5. Generally, the total
residue in the green parts was highest after applying Ill and lowest
after the application of I. The highest residues ware in the green
parts (leaves and stems) followed by pods and beans; the highest
levels were found at the first sampling 4 weeks after sowing.
TABLE 5. Uptake of ethiofencarb (I), its sulphoxide (II) and sulphone (III) by beans
Interval Total residue (mg/kg) after application of
after
application, I Granular II Granular III Granular
weeks
a* b* c* a b c a b c
4 159 - - 210 - - 310 - -
6 61 - - 135 - - 204 - -
8 49 - - 74 - - 96 - -
12 78 0.95 0.27 25 0.18 <0.1 141 0.20 0.06
*a: green parts;
b: pods;
c: beans
Following foliar application of 14C-carbonyl-labelled and
14C-ring-labelled I to beans, potatoes and sorghum, it was found that
14 days after application only 60.7, 29.0 and 45.1% of the insecticide
had been absorbed from the leaf surfaces of the respective plants
(Dorough, 1976g).
Potato and sorghum plants were treated at 0.56 kg/ha with ring-14C-I
in an E.C. formulation. The plants were treated at 10-day intervals
5-7 times during the growing season. Radiocarbon residues were much
lower in potatoes than in sorghum. Total ethiofencarb equivalents
remained essentially constant in potato peels (0.2 mg/kg) and pulp
(about 0.15 mg/kg) for 31 days after treatment. In sorghum the highest
levels of ethiofencarb equivalents were located in the leaves (10-100
mg/kg) followed by heads (5-25 mg/kg), grain (1-10 mg/kg) and stalks
(0.05-0.5 mg/kg). Sorghum leaf levels showed an increase from 85 to
131 mi/kg during the first two weeks but declined to about 94 mg/kg at
31 days. Levels remained relatively constant in the stalks. Levels in
the grain appeared constant (6-9 mg/kg) throughout the entire study
except for the 1 week sample (19 mg/kg). Total radiocarbon in the
heads tripled from 50 mg/kg at time 0 to 167 mg/kg at 1 week and then
declined to 90 mg/kg at 31 days (Dorough, 1976f).
Dräger detected II and Ill in various plant parts after application of
10% granular 14C-carbonyl-labelled ethiofencarb at planting of beans
(0.05 g I/1.6 kg soil; 1976a) and potatoes (0.05 g /approx. 20 1 soil;
1976c). The extractable residues in the roots and green parts of both
potatoes and beans consisted mainly of II and Ill. 0.21-7.79 mg/kg II
and 0.14-0.31 mg/kg III were found in the roots of the potatoes
between days 34 and 96, with 1.39-16.2 mg/kg II and 3.08-8.79 mg/kg
Ill in the green parts. The bigger increase in the sulphone content of
the green parts of the potatoes is indicative of an increased
oxidation of the sulphoxide in the plant system. The two metabolites
were detected also in the potato tubers and beans but there was too
little material available for extensive quantitative studies on the
beans. The potato tubers contained 0.05-0.28 mg/kg II and 0.06-0.14
mg/kg Ill.
The unextractable radioactivity in the green parts of the beans was
less than 1% or the total applied, and accounted for a maximum of 7.7%
of the radioactivity measured at the sampling times (Drägert 1976a).
The major portion of the unextractable radioactivity in the potato
tuber was in the glucose moiety of the starch. Therefore, CO2 formed
by hydrolysis of the carbamate group was utilized in carbohydrate
metabolism. The unextractable but water soluble radioactivity in
rootsy green parts and potato tubers was also considered to be
incorporated in products of carbohydrate metabolism. For details of
the distribution of radioactivity in potatoes and beans, see Table 6.
TABLE 6. 14C-ethiofencarb and metabolites in plants (as % of radioactivity applied or recovered)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Bean 0 day 14CO+ 61.9 29.6 1.6 0.7 origin-0.1; 98.0 Dorough,
x2 = 4.1 1976g
3 days Ring- 10.8 55.9 4.4 6.7 1.9 origin = 11.3; x1=0.5; 94.0
x2 =2.5
7 days 14C 49.7 11.4 1.4 0.5 origin=20.2;x1=0.4; 84.6
x2=1.0
14 days EC 15.6 0.6 1.7 0.6 origin=49.8;x1=0.5 69.1
x2=0.3
Potato 0 day 41.3 50.0 1.0 origin=1.5; x1=1.8; 100.3
x2=4.7
3 days 23.1 48.6 3.3 2.0 origin=5.9; x1=1.8; 91.2
x2=7.9
7 days 7.0 42.2 6.5 2.6 0.4 origin=15.7; x1=1.9; 80.4
x2=4.1
14 days 1.8 38.0 9.6 3.5 0.8 origin=14.0; x1=6.2 77.1
X2=3.2
Sorghum 0 day 73.0 22.8 0.9 origin=0.9; x2=0.9 98.5
3 days 18.1 49.3 1.3 0.8 0.3 origin=5.9; x2=2.3 78.0
7 days 3.2 47.0 4.3 1.3 1.0 origin=10.3; x2=2.0 69.1
14 days 0.3 38.6 6.1 0.7 0.3 origin=23.6; x2=0.5 70.1
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Potato
Peel Ring-
org. 0 day 14C 5.2 10.0 2.7 3.4 2.6 origin=1.3; x1=3.8 29.0 Dorough, 1976f
-soluble
water- EC 1.7 3.3 origin=1.0; x1=2.1 8.1
soluble
unhydrolysed 18.1
by H2O
unextractable 44.8
100.0
(102.4)2)
Potato
Peel
org. 31 days Ring- 3.6 2.7 2.7 8.0 6.3 origin=0.9 24.2 Dorough, 1976f
soluble
water soluble 14C 20.9
unhydrolysed EC 31.0
by H2O
extractable 24.1
100.0
(90.9)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Pulp
org.-soluble 0 day 3.5 7.1 5.8 origin=0.3 16.7
water-soluble 4.2 5.3 origin=0.5; x1=3.9 14.0
unhydrolysed 20.7
by H2O
unextractable 48.6
100.0
(100.6)2)
org.-soluble 31 days 0.7 2.5 0.6 6.6 2.1 origin=0.2 12.7
water-soluble 16.2
unhydrolysed 38.8
by H2O
unextractable 32.3
100.0
(90.6)2)
Sorghum
Leaves
org.-soluble 0 day Ring- 1.4 50.5 11.6 0.9 2.1 5.2 origin=1.0; x3=0.5 73.2
water-soluble 14C 0.2 0.6 8.3 4.1 origin=0.2; x2=0.7 14.1
unhydrolysed EC 5.2
by H2O
unextractable 7.5
100.0
(98.8) 2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Leaves
org-soluble 31 days Ring- 0.4 11.3 15.7 3.1 17.8 origin=0.6; x2=0.4 50.4 Dorough, 1976f
14C x3=0.7; x5=0.4
water-soluble EC 0.6 0.4 4.2 4.9 origin=0.3; x2=0.7 11.1
unhydrolysed
by H2O 12.8
unextractable 25.7
100.0
(99.4)2)
Stalks
org.-soluble 0 day 56.2 15.6 2.5 origin=1.2 75.5
water-soluble 11.4 origin=0.5 11.9
unhydrolysed
by H2O 7.1
unextractable 5.4
99.9
(97.0)2)
Stalks
org.-soluble 31 days 14.6 25.5 1.8 3.0 origin=0.6 45.5
water-solube 27.8 origin=0.9 28.7
unhydrolysed
by H2O 15.3
unextractable 10.5
100.0
(105.4)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Heads
org.-soluble 0 day 1.7 45.2 14.4 0.9 1.8 5.2 origin=0.6; x2=0.7;
x5=2.1 72.6
water-soluble 0.4 2.2 3.9 0.4 origin=0.2; x1=0.3;
x2=0.5 7.9
unhydrolysed
by H2O 4.2
unextractable 15.3
100.0
(95.9)2)
Sorghum
Heads
org.-soluble 31 days Ring- 1.6 26.0 27.1 0.4 1.6 9.9 origin=1.0; x2=1.0; 70.6 Dorough, 1976f
14C x5=2.0
water-soluble EC 0.3 0.3 0.2 3.3 2.8 origin=0.2; x1=0.2; 7.8
x2=0.5
unhydrolysed 4.7
by H2O
unextractable 16.9
100.0
(98.7) 2)
Grain
org.-soluble 0 day 51.1 1.7 5.5 2.2 origin=0.8; x2=0.7;
x5=1.8 63.8
water-soluble 4.2 5.7 origin=0.9; x2=0.6 11.4
unhydrolyzed
by H2O 7.0
unextractable 17.8
100.0
(99.2)2)
TABLE 6. (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Grain
org.-soluble 31 days 11.2 9.0 1.3 3.7 origin=0.5; x2=0.6;
x5=1.6 27.9
water-soluble 8.4 14.7 0.8 origin=0.5; x1=0.5;
x2=1.0 26.1
unhydrolysed
by H2O 12.5
unextractable 33.5
100.0
(99.3)2)
Potato unextractable by
organic solvents
Roots 34 days 14-CO 0.044 1.26 0.14 solid residue
0.10 in aqueous
layers 0.11 1.654 Dräger, 1976c
68 days Granular 0.055 0.42 0.10 solid residue
0.27 in aqueous
layers 0.15 0.945
96 days soil 0.04 0.05 solid residue
0.11; in aqueous
layers 0.07 0.27
TABLE 6 (Continued)
% of activity recovered as 1)
Plant Time after Label and
application Formulation I II III IV V VI unidentified activity As% of As% of Ref.
% found at indicated applied recovered
TLC positions activity activity
Green parts 34 days 7.15 3.89 solid residue
1.02 in aqueous
layers 0.94
68 days 10.28 9.03 solid residue
2.78 in aqueous
layers 3.04 25.12
96 days 4.38 10.18 solid residue
2.17 in aqueous
layers 3.42 20.15
Tubers 68 days 0.00 10.35 0.18 solid residue
0.973) in aqueous
layers 0.60 2.101
96 days 0.17 0.20 solid residue 2.393)
in aqueous layers 0.92 3.46
Bean
Roots 5 weeks 0.26 0.07 unextractable: 0.07 0.40 Dräger, 1976c
10 weeks 0.15 0.19 unextractable: 0.13 0.47
Green parts 5 weeks 3.53 0.95 unextractable: 0.27 4.75
10 weeks 3.02 4.27 unextractable: 0.61 7.90
TABLE 6 (Continued)
1) Compounds I-VI are identified in Figure 1.
2) The figures in Parentheses indicate percent of total recovery, based on combustion
3) Consisting of:
68days 96days
14C-CO2 (CO3-) 0.04 0.39
14C-Glucose in starch 0.85 1.66
Non-hydrolyzable 0.08 0.34
0.97 2.39
Following foliar and soil application, and stem injection of 14CO-and
ring-14C- labelled ethiofencarb to beans, potatoes and sorghum,
Dorough 1976f,g found that in addition to the major metabolites II and
III, minor amounts of IV, V and VI as well as unidentified metabolites
had formed. For quantitative data, see Table 6.
Bean plants were also treated with II and III (14C-ring-labelled),
After three days, 86.6% of II and 91.7% of III had been converted to
water-soluble metabolites which were easily degraded by acid
hydrolysis (Dorough, 1976g).
In soil
Studies with 14C-carbonyl-labelled ethiofencarb (granular
application; Dräger, 1976c 1977a) showed that ethiofencarb was
oxidized in soil to the corresponding sulphoxide (II) and sulphone
(III). Details are given in Table 7. After 5 weeks II was the main
residue but the proportion of III increased with time. Residues of
ethiofencarb in the first sample taken after 5 weeks were 2 mg/kg in
one experiment(1976c) and undetectable in another (1976a). The
unextractable residues amounted to 3-9% of the applied radioactivity.
Dräger (1977b) also found in laboratory studies with soil 34 clay
after the application of carbonyl-ethiofencarb (1) residues of 4.4% I,
68% II and 14% III. The carbamate group was hydrolyzed to CO2 with a
half-life of about 90 days. After 82 days, 43% of the radioactivity of
the carbonyl group was identified as gaseous CO2 Of the remaining
radioactivity left in the soil, II accounted for 1.7%, III for 38.3%,
and unextractrables for 17%; I could not be detected.
Ethiofencarb was degraded in the field in different soils with
half-lives of 20-30 days (Bayer, 1972/77), and in laboratory studies
with a maximum half-life of 32 days (Nitokuno, 1976d). In other
studies half-lives of approx. 90 and 130 days were obtained.
After the application of ethiofencarb 2 kg/ha as an emulsifiable
concentrate to different types of soil the following maximum residues
(I + II + III) were found: 8.8 mg/kg 0 days after application), 4.4
(7), 3.2 (14), 1.1 (30), 0.6 (60), 0.25 (92) and 0.1 (120) (Bayer
1972/77).
In greenhouse experiments with 14CO-labelled ethiofencarb granules,
Dräger calculated a half-life of about 6-7 weeks from the
radiochemical degradation rate of the total residue in soil planted
with potatoes (1976c) and a half-life of about 2 weeks in soil planted
with beans (1976a).
Half-lives for the degradation of ethiofencarb in soil are presented
in Table 8.
TABLE 7. Distribution of radioactivity in soil after application of
14C-carbonyl ethionfencarb
Compound % of initial 14C remaining on day
35 56 70 34 68 96
Ethiofencarb (I) n.d. n.d. n.d. 2.20 0.22 0.08
Ethiofencarb sulphoxide (II) 16.72 3.14 1.96 41.79 8.10 0.87
Ethiofencarb sulphone (III) 4.37 2.94 2.46 8.18 4.81 3.01
Unextractable 5.45 9.12 7.49 7.32 5.64 2.60
Total 26.54 15.20 11.91 59.49 18.77 6.56
References Dräger, 1976 a,b,c Dräger, 1976 a,b,c
The soils were planted either with beans (0.05 g I/1.6 kg soil;
Dräger, 1976a) or with potatoes T 0.05 g I/approx. 20 1 soil., Dräger,
1976 c) so that a portion of the radioactivity was absorbed by the
plants (see "Fate In Plants").
In laboratory studies to investigate the leaching behaviour of
ethiofencarb, normal dosages of formulations were applied to soil
which were eluted to simulate a rainfall of 200 mm in 2-21 days. The
leachate contained the following percentages of the applied dose as
total residues of I + II + III: low-humus sand 26.6-62.5%; high-humus
loamy sand 7.1-19.4%; sandy loam 18.2-74.5%. When II was applied to
low-humus sand and sandy loam, leachates contained 41% and 57% of the
dosage respectively. After application of Ill, the corresponding
figures were 50% and 40% (Bayer, 1972/77).
Rieck (1976a) found in laboratory studies with ring-labelled
14C-ethiofencarb (columns: 20 cm high and 10 cm diameter; application
of water equivalent to 500 m of rainfall) that the products of
ethiofencarb were not leached as rapidly from the Boils with higher
organic matter as from in the sandier, lean organic soils. In silty
clay, 88% of the radiocarbon was found in the upper 8 cm of the
column. Approximately 1-15% of the radiocarbon was found in the
leachates, of which at least 80% was II. The only other product found
in all the leachates was III. The products that remained in soil were
the same as those in the leachates and were in the same proportion.
Whereas carbamates were found in the leachate in these laboratory
experiments with small soil columns, no ethiofencarb and no products
with an intact carbamate structure were found in leachate samples
examined over a period of 16 months in leaching studies with an intact
soil core (10 cm diameter, 130 cm high) taken from the field after
rainfall typical of practical conditions (755 mm/year) (Dräger,
1976d).
Further studies of the leaching characteristics of aged ethiofencarb
residues under laboratory conditions were carried out by Dräger
(1977c) and Rieck (1976b) In the studies of Dräger, about 40-50% of
the applied 14Carbonyl activity was found in the leachate
irrespective of the soil type. Of this, only part was extractable with
chloroform. The unextractable portion was considered to be carbonate
or hydrogen carbonate formed during the experiment by hydrolysis of
the carbamate group of I (see also "Fate in Water"). The extractable
portions consisted of II and IlI, in a ratio similar to that of the
initial aged residues. I was no longer detectable. Rieck (1976b) also
did not detect I in four different soils after 30 days of aerobic
aging. The predominant residues were II and III, together with a
considerable amount of unextractable radioactive residue. In contrast
to the studies of Dräger (1977c) most of the radiocarbon was found in
the upper 8 cm of the soil with less than 2% in the leachate.
Thorton et al. (1976) compared the leaching behaviour of I with that
of 23 other pesticides. The pesticides were spotted on thin-layer
plates coated with six different types of soil ranging from
non-adsorptive sand to fine-textured clay. The plates were developed
and Rf values were compared. The compounds were grouped into five
categories ranging from immobile to highly mobile. I was placed in
class 49 as one of the mobile compounds.
TABLE 8. Half-lives of ethiofencarb (as total residues
of parent + sulphoxide + sulphone) in various soils
Soil Half-life
(days)
Laboratory trials
Carbon: 1.05%; fines: 30.4%; pH: 5.7 (Laacher Haf) 90
Carbon: 0.8% ; fines: 4.2%; pH: 7.0 (low-humus sand) 96
Carbon: 0.57%; fines: 19.5%; pH: 5.2 (sandy loam) 133
Clay: 14 %; org. matter: 5.85%; pH: 5.7 (sandy loam) 13
Clay: 13.6%; org. matter: 0.8%; pH: 7,0; Cation Exchange 32
Capacity:4-5 me/100g
(sandy loam)
Greenhouse trials
Carbon: 0.1%; fines: 22.1%; pH: 5.8; clay: 9.5%; fine silt: 12.6% ca.14
Carbon: 1.08%; fines: 23.2%; pH: 6.1; water cap.: 19-25% 42-49
Field trials
Clay 3.8%: silt: 18.3%; pH: 6.6; water cap. 36.1% 20
Clay: 13.7% silt: 29.4%; pH: 6.2; water cap. 61.3% 20
Carbon: 1.35%; fines: 9.1%; pH: 7.0 30
Carbon: 0.8 %; fines: 15.3% pH: 6.5 23
Clay: 14%; org. matter: 5.85%; pH: 5.7 (sandy loam) 22
Clay: 40%; org. matter: 5 %;
Cation Exchange Capacity 15-18 me/200 g (loam) 20
In water
Ring-labelled 14C-I was incubated under sterile conditions in aqueous
solutions buffered at pH 3, 6 and 9 at 25, 35 and 45°C. Ethiofencarb
was shown to be stable for 30 days at PH 3 and 25°C, whereas the
half-life at pH 9 and 45°C ranged from 0.3 to 0.7 days (Table 9). The
primary hydrolysis product was IV, Figure 1 (max. 92.6% of the
radioactivity of the sample) with only small amounts (<10%) of II
(Kurtz and Gronberg, 1976). The hydrolysis rates of
carbonyl-14C-labelled I, II and III were determined at pH 9.0, 9.5,
10.0, 10.5 and 11.0. I was 3-5 times as stable as II or Ill. Again,
the half-life of all compounds decreased with increasing pH (Table 9)
(Dorough, 1976 d).
Studies with 14CO-ethiofencarb showed that it decomposed in water (pH
6.8) with a half-life of about 8 days, largely by hydrolysis of the
carbamate group. In contrast to the metabolism in soil and plants, II
and Ill were very minor products: their sum represented about 10% of
the total carbamate residue throughout the experiment. After 31 days,
83% of the original carbonyl carbon could be determined as carbonate
or carbon dioxide gas, 3.4% was present as I, 0.3% as II and 0.2% as
III (Dräger, 1976 b, 1977a)
Photodecomposition
The photodegradation of ring-14C-or carbonyl-14C-I was studies under
artificial and natural sunlight on glass plates, silica gel plates,
soil plates, and in aqueous solution (distilled water or pond water).
5% or less of I was found after 6 hours irradiation. II was the major
product: Ill and VI were found as minor constituents (Dorough,1976e).
For quantitative data, see Table 10.
The effect of anthraquinone as a photosensitizer was profound when
applied as a mixture with I on silica gel plates. Although the extent
of degradation of 1 me essentially unaffected, there was an increase
in the number of minor photoproducts (those less than 1%) with
anthraquinone and an increase in the polar material remaining at the
origin after 7 days of exposure to a sunlamp. Almost no II was
produced and a compound with a similar Rf to IV was the major product
(48% after 7 days of exposure). There were also lesser amounts of Ill
and VI (Dorough, 1976e).
In storage and processing
In head lettuce that had been field-sprayed in accordance with good
agricultural practice, no decline of the total residue was noted after
one year of storage in a deep freezer at -20°C (Bayer, 1974).
TABLE 9. Half-lives of ethiofencarb in buffered solutions.
Half-life of compound
Conditions I II III Ref.
(ethiofencarb) (ethiofenoarb (ethiofencarb
sulphoxide) sulphone)
pH: 9.0 6.67 hours 2.03 hours 2.13 hours Dorough, 1976d
9.5 2.67 hours 0.47 hours 0.72 hours
10.0 1.17 hours 0.23 hours 0.40 hours
10.5 0.43 hours 0.08 hours 0.12 hours
11.0 0.09 hours 0.02 hours 0.03 hours
25 °C: 10 ppm; pH 3 stable Kurtz and
pH 6 324 days Gronberg, 1976
pH 9 3.5 days
25 °C; 100 ppm; pH 3 stable
pH 6 stable
pH 9 2.5 days
35 °C; 10 ppm; pH 3 stable
pH 6 68 days
pH 9 1.5 days
35 °C; 100 ppm; pH 3 stable
pH 6 68 days
pH 9 2.0 days
45 °C; 10 ppm; pH 3 stable
pH 6 22 days
pH 9 0.7 days
45 °C; 100 ppm; pH 3 stable
pH 6 20 days
pH 9 0.3 days
TABLE 10A. Photodegradation of 14c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound1) after indicated time
1 hour 3 hours
Conditions
O I II III IV V VI xn O I II III IV V VI xn
artifical light, 1.0 51.4 44.9 3.5 2.3 1.8 16.3 70.3 4.8 2.9
TLC plates
germicidal light, 14.2 6.6 22.7 24.1 0.6 15.6 9.5 20.1 1.3 10.8 23.9 1.0 14.6 10.0
TLC
plates
natural
light, 0.6 42.1 62.0 0.7 1.9 0.8 1.2 2.9 91.4 2.2 2.7 1.2
TLC plates
artifical light, 1.6 1.0 84.2 4.3 7.1 0.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
germicidal light, 1.6 1.8 80.1 44.4 10.4 1.9 1.9 1.4 79.1 5.3 1.7 9.0 1.0
soil plates
natural light, 1.4 1.3 80.5 5.9 9.5 1.5 1.2 1.9 82.7 6.3 0.5 6.6 0.9
soil plates
natural light, 1.1 0.5 46.1 5.1 1.7 2.3 0.6 36.1 7.7 1.6
glass plates
natural light, 1.9 6.5 78.2 6.5 1.0 4.6 1.6 4.2 2.9 70.1 22.5 6.5 5.3
aquous
solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
TABLE 10B. Photodegradation of 14 c-ring labelled ethiofencarb (Dorough, 1976e)
% of applied 14C recovered as indicated Compound 1) after indicated time
6 hours
Conditions
O I II III IV V VI xn
artifical light, 2.4 5.2 78.6 6.3 3.5
TLC plates
germicidal light, 21.2 4.3 8.8 24.3 1.0 14.0 9.7
TLC plates
natural light, 1.4 0.8 90.5 2.7 2.9 1.2
TLC plates
artifical light, 2.1 1.0 83.6 5.9 5.1 1.6
soil plates
germicidal light, 1.9 2.6 76.3 6.5 1.0 9.1 1.3
soil plates
natural light, 1.0 1.1 87.8 5.7 0.8 2.6 1.1
soil plates
natural light, 4.2 0.3 26.0 13.1 0.9 2.3
glass plates
natural light, 5.8 0.4 65.6 11.7 1.8 8.4 3.2
aquous solution
1) 0 = polar material remaining at origin of TLC plate; I-VI are identified in Figure 1;
xn = total unidentified radioactivity not remaining at TLC origin.
Studies to investigate the stability of the sulphoxide and sulphone
metabolites in potatoes revealed that the content of both compounds
remained constant on storage for 11 months at -20°C (Chemagro, 1976b).
When bovine and poultry tissues, milk and eggs were fortified with
14C-labelled ethiofencarb and stored under frozen conditions for 7
months, 84-98% of the residue was found as intact carbamate (Chemagro,
1976c).
Poultry rations fortified with ethiofencarb for use in various animal
feeding studies were stored for 5 weeks at -10°C; no degradation of
the ethiofencarb was noted (Chemagro, 1975).
Processing of plant material by baking and boiling results in the
reduction of ethiofencarb residues. Broad beans which had a residue
content of 0.13 mg/kg before boiling were found to contain < 0.02 mg
I + II + III/kg after being boiled for 15 minutes. In the green parts
(stems and leaves), the residues were reduced by boiling to between 3
and 7% of the original content of 14-30 mg/kg; 0.4-3.4% of the
original residue (I + II + III) was found in the water used for
boiling (Bayer, 1975).
Raw potatoes fortified with 14C-labelled ethiofencarb (1 mg/kg) were
processed by pan frying, french frying and baking. Both pan and french
frying processes reduced the residue (I + II + III) with an average
loss of 52 and 58%, respectively. Leaching of the residue (2-20%) into
the frying oils and volatilization were observed. The baking process
showed a loss of about 2% of the 14C activity. Of the remaining
activity, ethiofencarb accounted for 66%, ethiofencarb sulphoxide for
14%, and ethiofencarb phenol for 20% (Chemagro Report, 1976d).
METHODS OF RESIDUE ANALYSIS
Residues of ethiofencarb can be determined by gas chromatography using
a sulphur-specific flame photometric detector. The methods available
for the analysis of plant samples# animal tissue samples and soil
samples simultaneously determine metabolites with an intact carbamate
structure, i.e. ethiofencarb sulphoxide and ethiofencarb sulphone.
Oxidation with potassium permanganate converts ethiofencarb and
ethiofencarb sulphoxide to the sulphone. For gas-chromatographic
determination, the total carbamate residue is derivatized by reaction
with bix-trimethylsilytrifluoroacetamide to the thermostable, volatile
2-ethylsulfonylmethylphenyl trimethylsilyl ether.
For the extraction step, acetone (Dräger, 1974) or methanol/water
(Morris and Gronberg, 1976; Dorough, 1976a) were used for plant and
soil samples. Soil samples can also be extracted with a mixture of
acetonitrile and 0.1 × hydrochloric acid (Morris and Gronberg, 1977)
or by reflux with a methanol/chloroform mixture (Morris, 1976a).
Water-containing animal tissues and eggs are extracted with
acetonitrile; prior to extraction, fat is dissolved in hexane and
acetone is added to milk to separate protein (Dorough, 1976b).
Isolation of the residues from the extracts is achieved by shaking
with chloroform, and separation of co-extractives is accomplished by
precipitating with a solution of ammonium chloride/phosphoric acid.
After oxidation of the residues with potassium permanganate to
ethiofencarb sulphone, silylation is carried out in acetone or
benzene.
With some types of sample, further chromatographic clean-up steps are
needed before silylation, in order to separate sulphur-containing
plant co-extractives which interfere with the gas-chromatographic
determination. With brassica extracts clean-up was by thinlayer
chromatography and with hop extracts by column chromatography (Dräger,
1974). In the analysis of animal tissues, eggs and milk, the residue
was hydrolyzed to the corresponding phenol sulphone before silylation
in order to increase reproducibility (Dorough, 1976b).
For the gas-chromatographic determination, columns packed with 5%
DC-200 on Gaschrom Q (Dräger, 1974) and with -3% OV-1 on Chromosorb W
(Morris and Gronberg, 1977) have proved suitable. For a confirmatory
procedure, a column packed with the polar phase Carbowax 20-M on
Chromosorb W (Morris,1976b) was used. The lower limit of determination
is generally 0.02-0.05 mg/kg for plant and soil samples. 0.01 mg/kg
for fat, and 0.005 mg/kg for eggs and milk.
The methods were shown to be specific in a study in which pesticides
registered in the U.S.A. for use on potatoes, sorghum, animal tissues,
milk and eggs were checked for possible interference: none of the 50
chemicals tested interfered at their maximum registered level
(Dorough, 1976c).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The only tolerances reported to the Meeting were on fruit and
vegetables in Switzerland, but pre-harvest intervals have been
specified in several other countries as shown below (Table 11).
APPRAISAL
Ethiofencarb is a systemic insecticide used especially against sacking
insects an vegetables, fruit, field crops and tobacco, It is effective
against some organophosphorasresistant strains. It is formulated an
granules and emulsifiable concentrates. The usual dose is 0.3-2 kg
a.i./ha in spray, or approximately 0.1 g a.i. per linear metre as
granules at sowing or planting. Ethiofencarb is used in various
European countries, Chile and Israel.
In plants and soil ethiofencarb (I) is oxidized to its sulphoxide (II)
and sulphone (III) which are also hydrolysed. In plants and soil the
main intact carbamates are II and III. Little or no ethiofencarb is
usually detectable at harvest. In animals, the corresponding phenols
and their conjugates are the main metabolites.
TABLE 11. National tolerances and pre-harvest intervals reported to the Meeting.
Country Crop Tolerances Pre-harvest
in mg/kg interval in
days
Germany Beets, sugar beets 28
(FRG) Head lettuce 4
Bush beans, pole beans,
broad beans 7
Potatoes 14
Israel Peppers 5
Potatoes 4
Cauliflower, drumhead
cabbage 7
Apples 7
Italy Vegetables 7
Fruit crops 14
Beets 30
Spain Fruit crops incl. citrus,
winter cereals, maize,
potatoes, beets, sugarcane,
cotton, oilseed crops 21
Switzerland Pome, stone and small
fruit 0.2
Vegetables excl. spinach 1.0 14
Fruit crops, peas for
harvesting dry (dried,
threshed) 21
Sugar beet, field beans 42
Half-lives of I + II + III in soil vary between 2 and 12 weeks in the
field.
On the basis of feeding a diet containing 50 ppm of I to cows, pigs
and hens, it can be estimated that residues of I + II + III are
unlikely to exceed 0.02 mg/kg in their meat or in eggs or milk.
Boiling broad beans reduced carbamate residues of 0.13 mg/kg to below
0.02 mg/kg. Cooking the green parts of beans reduced the residues to
3-7% of the original content of I + II + III (14-30 mg/kg). 0.4-3.4%
of these residues was found in the water used for boiling. Deep and
shallow frying of potatoes fortified with 1 mg ethiofencarb/kg
decreased the total carbamate residue by about 50%. Baking potatoes
caused no loss of carbamate residues.
Gas-chromatographic procedures for the determination of residues of I
+ II + III, suitable for regulatory purposes, in various crops, eggs,
milk and soil have been elaborated.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended. They
refer to the am of ethiofencarb, its sulphoxide and its sulphone,
expressed as ethiofencarb.
Commodity Limit, Pre-harvest interval (days)
mg/kg on which recommendations
are based
Cherries, lettuce 10 4-7
Apples, pears, apricots 4-7
Artichokes, beans with pod, 4-7
beet tops,
sugar beet tops (leaves), 14
brassicas peaches plums 5 4-7
Currants (black, red),
eggplants, 4-7
wheat straw 2 14
Cucumbers 1 4
Potatoes, 14
Radishes 0.5 4
Beans without pods
soybeans without pod 0.2 4-7
Fodder beets,
sugar beets (roots) 0.1 14
Raw grain
(barley, oats, rye, wheat) 0.05 30
Meat of cattle, pigs
and poultry, eggs, milk 0.02*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by July 1978)
1. Submission of the results of the reproduction study now in
progress.
2. Further residue data from supervised trials on apricots,
artichokes, various brassicas, cherries, cucumbers (including those
grown under glass), eggplants, plums, radishes, soybeans.
DESIRABLE
1. Further residue data from supervised trials on other crops on which
ethiofencarb is known to be used.
REFERENCES
Bayer, A.G. (1972/77) Ethiofencarb; Pflanzensohutzmittel-Rückstände,
Dräger, Bayer-Leverkusen, unpublished.
Bayer, A.G. (1974) Report RA 301; (unpublished).
Bayer, A.G. (1975) Report RA 655; 20 Nov. 1975.
Bothard, E. and Löser, E. (1976) HOX 1901 Chronic Toxicity Study on
Rats. Nov. 30, Rep. No. 6493 from Bayer AG, Inst. f. Tox., unpublished
report submitted by Bayer AG. Addendum., Histopathological Examination
by J.P. Finn, Hazleton Laboratories, Inc.
Chemagro Report (1975) 45 176; 12 Sept, 1975, R & D Dep. of Chemagro,
Agricult. Div., Mobay Chemical Corp., Kansas City, No. 64 1209 USA.
Chemagro Report (1976a) Chemagro Agricult. Div.; Groneton; Potatoes
(Kats; Russet Burbank), Rep. No. 49 735/49 751; 17 Nov. 1976
(unpublished).
Chemagro Report (1976b) No. 51 062; 30 Dec. 1976 (unpublished).
Chemagro Report (1976c) No. 49 896; 27 Dec. 1976.
Chemagro Report (1976d) No. 46995; 11 Feb. 1976.
Dorough H.W. (1976a) A Gas Chromatographic Method for the
Determination of Croneton Residues in Various Animal Tissues, Crops,
and Soil. CR 49 879, 1.12.1976.
Dorough H.W. (1976b) A Gas Chromatographic Method for the
Determination of Croneton Residues in Bovine Tissues and Milk and
Poultry Tissues and Eggs. CR 49 880; 18.11.1976.
Dorough H.W. (1976c) An Interference Study for the Residue Method for
Croneton and Metabolites in Various Crops and Animal Tissuee, Milk and
Eggs. CR 49 893, 17.12.1976.
Dorough H.W. (1976d) Rate of Hydrolysis of CRONETON TM GRONETON
Sulphoxide, and CRONSTON Sulphone in Aqueous Solutions. Chemagro
Report No. 49 890 (1976).
Dorough H.W. (1976e) Photodcoomposition of CRONETON TM on Various
Substrates. Chemagro Report No. 49 891 (1976).
Dorough H.W. (1976f) The Metabolism and Residue in Potatoes and
Sorghum Treated Under Field Conditions Using CRONETON
TM-ring-UL-14C. Chemagro Report No. 49 894 (1976).
Dorough H.W. (1976g) The Metabolism of CRONETON TM in Plants.
Chemagro Report No. 49 895 (1976).
Dorough H.W. (1976h) Metabolism of CRONETON TM in a Lactating
Holstein Cow and a Male Yorkshire Swine. Chemagro Report No. 49 897
(unpublished).
Dorough H.W. (1976i) The Metabolism of CRONETON TM Poultry. Chemagro
Report No. 49 898 (unpublished).
Dorough, H.W. (1976j) Correlation of Blood and Tissue Radiocarbon
Levels Following a Single Oral Dose of CRONETON TM-ring-14C to Rats.
Chemagro Report No. 49 887 (1976).
Dräger, G. (1974) Method for gas-chromatographic determination of
Croneton residues in plants and soil. Pflanzenschutz-Nachrichten Bayer
91, 144-155 (1974).
Dräger, G. (1976a) Study to investigate uptake and metabolism of
Croneton in beans (Vioia faba) after application in granular form.
Bayer AG, Pflanzensohutz-Anwendungstechnik, unpublished Report RA-79,
January 19, 1976.
Dräger, G. (1976b) Studying the Metabolism of Croneton in Water. Bayer
AG Pflanzenschutz-Anwenclungstcohnik, unpublished Report RA-438v June
9, 1976.
Dräger, G. (1976c) Studies on the Metabolism of CRONETON in Potatoes
and Soil and Identification of Bound Residues in the Potato Tuber.
Bayer AG, Pflanzenschut Anwendungstechnik, unpublished Report RA-7989
October 89 1976.
Dräger, G. (1976d) Untersuchunger zum Versickerungsverhalten von
CronetonR (Makro-Säulen-Versuch). Bayer AG,
Pflanzenschutz-Anwenclungeteohnik, unpublished Report RA-961.
Dräger, G. (1977a) Untersuchung des Metabolismus von Groneton in Boden
und Wasser. Pflanzenschutz-Nachrichten Bayer 30, 18-27 (1977).
Dräger, G. (1977b) Weiterer Abbau, von gealterten Croneton-Rückständen
im Boden unter Laborbedingungen Bayer AG Pflanzenschutz-
Anwendungsteohnik, unpublished report RA-133, February 16, 1977
Dräger, G. (1977c) Versickerungsverhplten von gealterten
Groneton-Rlickstgnden in Boden unter Laborbedingunger, Bayer AG,
Pflanzenschutz-Anwendungsteohnik, unpublished Report RA-346.
Gronberg, R.R., Dorough, H.W., (1976) Effect of Feeding CRONTEONTM
to Poultry. Chemagro Report No. 49 899 (unpublished).
Gronberg, R.R., Dorough, H.W., Hemken, R.W. (1976) Effect of Feeding
CRONETONTM to Dairy Cattle. Chemagro Report No. 50 500 (1976)
(unpublished).
Hoffmann, K. and Weischer, C.H. (1976) 1901/Chronio Study on Dogs. May
26, Rep. No. 6120 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Homeyer, B. (1976) Croneton als wurzelaystemieches Aphizid.
Ptlanzenschutz-Nachrichten Bayer 29 255-266 (1976).
Hurst, H., Dorough, H.W. (1976) The Metabolism of CRONETON TM-
N-methyl-14C in Rats-A Progress Report. Chemagro Report No. 49 886
(1976).
Kimmerle, G. (1972b) HOX 1901/Acute Toxicity Studies. July 10, Rep.
No. 3557 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. (1972c) HOX 1901/Acute orale Toxizität an männlichen
Kaninchen. Nov. 13, report from from Bayer AG, Inst. f. Tox.,
unpublished report submitted by Bayer AG.
Kimmerle, G. (1972d) Antidotal Effect of Atropine. July 10, report
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Kimmerle, G. (1972e) HOX 1901/Subacute Toxicity Studies. July 10, Rep.
No. 3558 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Kimmerle, G. and Eben, A. (1974) HOX 1901/Effect of Acute and Subacute
Oral Administration and Subacute Inhalation on Plasma, Erythrocyte and
Brain Acetylcholinesterase Activity in Rats and Dogs. Aug. 8, Rep. No.
4843 from Bayer AG, Inst. f. Tox.p unpublished report submitted by
Bayer AG.
Klimmer, O.R. (1973) Study Report / Bayer HOX 1901/Subchronic
Toxicological Studies on Rats. Febr. 27, Rep. No. R 739 from
Pharmakologisches Instit der Rhein. Friedr.-Wilh.-Univ., Bonn,
unpublished report submitted by Bayer, AG.
Kurtz, S.K., Gronberg, R.R. (1976) The Stability of CRONETON TM in
Aqueous Systems. Chemagro Report No. 50 798 (1976).
Lamb, D.W., Roncy, D.J. (1976) Accumulation and Persistence of
Residues in Channel Catfish Exposed to CRONETON-14C. Chemagro Report
No. 49 128 (unpublished).
Lamb, D.W. and Matzkanin, G.S. (1977a) The Acute Oral Toxicity of
CRONETON Sulphoxide. Febr. 18, Rep. No. 51 716 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lambt D.W. and Matzkanin, G.S. (1977b) The Acute Oral Toxicity of
CRONETON Sulphone. Febr. 21, Rep. No. 51 717 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, G.S. (1977c) The Acute Oral Toxicity of
CRONETON Phenol. Febr. 17, Rep. 51 719 from Mobay Chem. Corp.,
unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkaning G.S. (1977d) The Acute Oral Toxicity of
CRONETON Phenol Sulphoxide. Febr. 17, Rep. No. 51 718 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977e) The Acute Oral Toxicity of
CRONETON Phenol Sulphone. Febr. 17, Rep. No. 57 715 from Mobay Chem.
Corp., unpublished report submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977f) The Acute Oral Toxicity of
CRONETON (Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroxy). April
26, Rep. No. 52 878 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977g) Acute Oral Toxicity of CRONETON
(Formerly BAY HOX 1901) Metabolite N-Methyl (Hydroyy) Sulphoxide.
April 29, Rep. No. 52 879 from Mobay Chem. Corp., unpublished report
submitted by Bayer AG.
Lamb, D.W. and Matzkanin, C.S. (1977h) The Acute Oral Toxicity of
CRONETON Metabolite N-Methyl (Hydroxy) Sulphone. April 29, Rep. No. 52
880 from Mobay Chem. Corp., unpublished report submitted by Bayer AG.
Machemer, L. (1973a) HOX 1901/Dominant Lethal Test on the Male Mouse
to Test for Mutagenic Effect. Dec. 14, Rep. No. 4339 from Bayer AG,
Inst. f. Tox., unpublished report submitted by Bayer AG.
Machemer, L. (1973b) HOX 1901/Studies for Embryotoxic and Teratogenic
Effects on Rats Following Oral Administration. July 25, Rep. No. 4174
from Bayer AG, Inst. f. Tox., unpublished report submitted by Bayer
AG.
Machemer, L. (1975) HOX 1901/Studies of Embryotoxic and Teratogenic
Effect on Rabbits Following Oral Administration. July 24, Rep. No.
5545 from Bayer AG, Inst. f. Tox., unpublished report submitted by
Bayer AG.
Marshall, T., Dorough, H.W. (1976) Fate of Water Soluble CRONETON TM
Plant Metabolites in Rats (Progress Report). Chemagro Report No. 49
892 (1976).
Marshall, T.C., Dorough, H.W. (1977) Bioavailability in Rate of Bound
and Conjugated Plant Carbamate Insecticide Residues. Chemagro Report
No. 52 737 (1977).
Morris, R.A. (1976a) Determination of Baygon, Baytex, Bolster,
Croneton, Dasanit, Di-Syston, Dylox, Guthion, Hinosan, Mesurol,
Meyasystox-R, Monitor, Morestan, Nemacur, Systox Residues in Soil. CR
49 675, 15.9.1976.
Morris, R.A. (1976b) A Confirmatory Procedure for the Analytical
Residue Method for Croneton in Potatoes, Plant Forage and Cereal
Grains. CR 51 0639 30.12.1976.
Morris, R.A. Gronberg, R.R. (1976) A Gas Chromatographic Method for
the Determination of Residues of Croneton (Bay BOX 1901) and
Metabolites in Potatoes, Plant Forage, and Cereal Grains. CR 49 666#
23.8.1976.
Morris R.A., Gronberg, R.R. (1977) A Gas Chromatographic Procedure for
the Determination of Croneton (Bay HOX 1901) in Soil. CR 49 6659
10.2.1977.
Mürmann, P. (1973) HOX 1901/Subohronic Toxicity Study on Dogs. June
28, Rep. No. 4143 from Bayer AG, Inst. f. Tox., (unpublished).
Nitokuno Inst., (1975a) Report No. 3599 unpublished.
Nitokuno Inst., (1975b) Reports No. 335 and 336, unpublished.
Nitokuno Inst., (1975/76a) Reports No. 333, 334 and 390, unpublished.
Nitokuno Inst., (1975/76b) Reports No. 360-362 and 395-396,
unpublished.
Nitokuno Inst., (1976a) Report No. 379, unpublished.
Nitokuno Inst. (1976b) Reports No. 391-1949 unpublished.
Nitokuno Inst. (1976c) Reports No. 397-4009 unpublished.
Nitokuno Inst. (1976d) NIHON TOKUSHU NOYAKU SEIZO K.K. Residual
Analysis Results of HOX 1901 (ethiofencarb) in Upland Soil. Report No.
1042 (RA), January, 28, 1976.
Nye, D.E., Hurst, H.E., Dorough, H.W. (1976) Fate of Croneton
(2-Ethylthiomethylphenyl N-Methyl-carbamate) in Rats. J. Agric. Food
Chem. 24, 371-377.
Rieck, C.E. (1976a) Leaching characteristics of CRONETON TM in
various soils. Chemagro Report No. 50 783.
Rieck, C.E. (1976b) Leaching characteristics of CRONETON TM on aged
soil. Chemagro Report No. 51 028.
Thorton, J.S., Hurley, J.B. and Obrist, J.J. (1976) Soil thin-layer
mobility of twenty four pesticides chemicals. Chernagro Report No. 51
016.
Thyssen, J. and Kimmerle G. (1975) Präparat 6757/Untersuchungen zur
Anwendungstoxikologie. Nov. 27, Rep. No. 5751 from Bayer AG, Inst. f.
Tox., unpublished report submitted by Bayer AG.
Thyseen, J. (1975a) HOX 1901/Neurotoxicity Studies on Hens. Nov. 18,
Rep. No. 5735 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG. Appendix: ROX 1901 Neurotoxicity Studies on
Hens/Histopathological Finding by Prof. Dr. U. Mohr, Med. Hoohsohule
Hannover.
Thyssen, J. (1975b) Studies to Investigate Acute Oral Toxicity of HOX
1901 when Administered Simultaneously with Malathion or EPN, Nov. 18,
Rep. No. 5732 from Bayer AG, Inst. f. Tox., unpublished report
submitted by Bayer AG.
Thyssen, J. (1976) HOX 1901-Metaboliten. Sept. 1, report from Bayer
AG, Inst. f. Tox., unpublished report submitted by Bayer AG.
