
TRIFORINE JMPR 1977
IDENTITY
Chemical name
1,4-bis(2,2,2-trichloro-1-formamidoethyl)piperazine
1,1'-piperazine-1,4-diyldi-(N-(2,2,2-trichloroethyl)formamide)
Synonyms
Cela W 524, W 524, Saprol(R), Flanginex
Structural formula
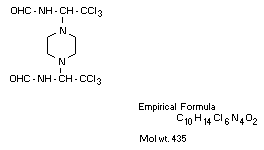 Other information on identity and properties
State: colorless and odorless, crystalline
Melting point: 155°C
Triforine is hydrolysed rapidly in aqueous solutions at 21°C, about
50% being degraded in the first two clays with the formation of
chloride ions and, through several intermediates,
1-(dihydroxyacetyl)piperazine and piperazine. On exposure to sunlight,
about half of the triforine was degraded in 150 hours.
Photolysis by ultraviolet light in the absence of water leads
preferentially to the removal of one side chain, the second side chain
being attacked more slowly.
In aqueous solution, triforine is rapidly decomposed by ultraviolet
light. N-(2,2dichlorovinyl)formamide was isolated as an intermediate
photodecomposition product (Darda et al., 1977),
After 3 hours irradiation with u.v. light or 30 hours exposure to
sunlight, 50% of triforine in aqueous solution was inactivated
(Buchenanery 1975).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, metabolism and excretion
Male Wistar rats, weighing approximately 200 g, were dosed orally with
2.3 3H)-triforine (ring labelled) or 3.75 mg 14C-triforine (side
chains labelled.
After oral administration to 9 animals the blood level curve passed
its maximum (1.3% of the administered dose) 4 hours after dosage,
followed by a more rapid decrease in the 4-10 hours interval and a
subsequent slower fall. At the end of this blood level experiment
(96 hours), an average of 0.3% of the administered radioactivity was
present in the total blood compartment of the rat. The main portion of
the total radioactivity, 74.3% of the (3H)-triforine and 52.5% of the
(14C)-labelled triforine, was excreted reneally within the first
24 hours. In this period the faecal excretion amounted to 16.5% of the
(3H)-labelled and 39.5% of the (14C)-labelled triforine. The average
total triforine eliminated by the rat within one day of dosing was
90.8% (3H) and 92.0% (14C).
During a period of 30 hours after dosing, 19% of the orally
administered (3H) dose was 15% of the (14C) dose was excreted via
the bile. The elimination in the urine following increased dose of
triforine (25, 50, 100 and 200 mg/kg for 8 animals) followed the same
metabolic pattern: 70% of the (3H)-labelled and 50% of the
(14C)-labelled substance was excreted after 24 hr. The main
metabolite in the urine was identified as
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide (Darda, 1977).
COMMENTS
In rats, the compound was rapidly absorbed and excreted, mainly in the
urine. The main metabolite was
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide.
Full toxicological data are required before a recommendation of the
ADI for humans can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
No data were available for consideration.
FURTHER WORK OR INFORMATION
REQUIRED by 30 June 1978 and before an acceptable daily intake for
humans (ADI) can be established and maximum residue limits (MRL) can
be recommended.
1. Submission of full data relating to toxicological and residue
studies.
REFERENCES
Buchanauer, H. (1975) Inactivation of triforine by u.v. and sunlight
on glass and on leaves of bean plant. Pestic. Sci. 6, 553-559.
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D. Ost, W. and Wotschokowsky, M.
(1977) Hyrdolysis and photolysis of the fungicide triforine. Pestic.
Sci 8, 183-192.
Other information on identity and properties
State: colorless and odorless, crystalline
Melting point: 155°C
Triforine is hydrolysed rapidly in aqueous solutions at 21°C, about
50% being degraded in the first two clays with the formation of
chloride ions and, through several intermediates,
1-(dihydroxyacetyl)piperazine and piperazine. On exposure to sunlight,
about half of the triforine was degraded in 150 hours.
Photolysis by ultraviolet light in the absence of water leads
preferentially to the removal of one side chain, the second side chain
being attacked more slowly.
In aqueous solution, triforine is rapidly decomposed by ultraviolet
light. N-(2,2dichlorovinyl)formamide was isolated as an intermediate
photodecomposition product (Darda et al., 1977),
After 3 hours irradiation with u.v. light or 30 hours exposure to
sunlight, 50% of triforine in aqueous solution was inactivated
(Buchenanery 1975).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, metabolism and excretion
Male Wistar rats, weighing approximately 200 g, were dosed orally with
2.3 3H)-triforine (ring labelled) or 3.75 mg 14C-triforine (side
chains labelled.
After oral administration to 9 animals the blood level curve passed
its maximum (1.3% of the administered dose) 4 hours after dosage,
followed by a more rapid decrease in the 4-10 hours interval and a
subsequent slower fall. At the end of this blood level experiment
(96 hours), an average of 0.3% of the administered radioactivity was
present in the total blood compartment of the rat. The main portion of
the total radioactivity, 74.3% of the (3H)-triforine and 52.5% of the
(14C)-labelled triforine, was excreted reneally within the first
24 hours. In this period the faecal excretion amounted to 16.5% of the
(3H)-labelled and 39.5% of the (14C)-labelled triforine. The average
total triforine eliminated by the rat within one day of dosing was
90.8% (3H) and 92.0% (14C).
During a period of 30 hours after dosing, 19% of the orally
administered (3H) dose was 15% of the (14C) dose was excreted via
the bile. The elimination in the urine following increased dose of
triforine (25, 50, 100 and 200 mg/kg for 8 animals) followed the same
metabolic pattern: 70% of the (3H)-labelled and 50% of the
(14C)-labelled substance was excreted after 24 hr. The main
metabolite in the urine was identified as
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide (Darda, 1977).
COMMENTS
In rats, the compound was rapidly absorbed and excreted, mainly in the
urine. The main metabolite was
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide.
Full toxicological data are required before a recommendation of the
ADI for humans can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
No data were available for consideration.
FURTHER WORK OR INFORMATION
REQUIRED by 30 June 1978 and before an acceptable daily intake for
humans (ADI) can be established and maximum residue limits (MRL) can
be recommended.
1. Submission of full data relating to toxicological and residue
studies.
REFERENCES
Buchanauer, H. (1975) Inactivation of triforine by u.v. and sunlight
on glass and on leaves of bean plant. Pestic. Sci. 6, 553-559.
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D. Ost, W. and Wotschokowsky, M.
(1977) Hyrdolysis and photolysis of the fungicide triforine. Pestic.
Sci 8, 183-192.
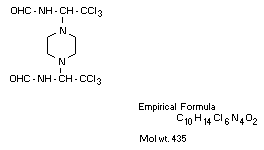 Other information on identity and properties
State: colorless and odorless, crystalline
Melting point: 155°C
Triforine is hydrolysed rapidly in aqueous solutions at 21°C, about
50% being degraded in the first two clays with the formation of
chloride ions and, through several intermediates,
1-(dihydroxyacetyl)piperazine and piperazine. On exposure to sunlight,
about half of the triforine was degraded in 150 hours.
Photolysis by ultraviolet light in the absence of water leads
preferentially to the removal of one side chain, the second side chain
being attacked more slowly.
In aqueous solution, triforine is rapidly decomposed by ultraviolet
light. N-(2,2dichlorovinyl)formamide was isolated as an intermediate
photodecomposition product (Darda et al., 1977),
After 3 hours irradiation with u.v. light or 30 hours exposure to
sunlight, 50% of triforine in aqueous solution was inactivated
(Buchenanery 1975).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, metabolism and excretion
Male Wistar rats, weighing approximately 200 g, were dosed orally with
2.3 3H)-triforine (ring labelled) or 3.75 mg 14C-triforine (side
chains labelled.
After oral administration to 9 animals the blood level curve passed
its maximum (1.3% of the administered dose) 4 hours after dosage,
followed by a more rapid decrease in the 4-10 hours interval and a
subsequent slower fall. At the end of this blood level experiment
(96 hours), an average of 0.3% of the administered radioactivity was
present in the total blood compartment of the rat. The main portion of
the total radioactivity, 74.3% of the (3H)-triforine and 52.5% of the
(14C)-labelled triforine, was excreted reneally within the first
24 hours. In this period the faecal excretion amounted to 16.5% of the
(3H)-labelled and 39.5% of the (14C)-labelled triforine. The average
total triforine eliminated by the rat within one day of dosing was
90.8% (3H) and 92.0% (14C).
During a period of 30 hours after dosing, 19% of the orally
administered (3H) dose was 15% of the (14C) dose was excreted via
the bile. The elimination in the urine following increased dose of
triforine (25, 50, 100 and 200 mg/kg for 8 animals) followed the same
metabolic pattern: 70% of the (3H)-labelled and 50% of the
(14C)-labelled substance was excreted after 24 hr. The main
metabolite in the urine was identified as
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide (Darda, 1977).
COMMENTS
In rats, the compound was rapidly absorbed and excreted, mainly in the
urine. The main metabolite was
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide.
Full toxicological data are required before a recommendation of the
ADI for humans can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
No data were available for consideration.
FURTHER WORK OR INFORMATION
REQUIRED by 30 June 1978 and before an acceptable daily intake for
humans (ADI) can be established and maximum residue limits (MRL) can
be recommended.
1. Submission of full data relating to toxicological and residue
studies.
REFERENCES
Buchanauer, H. (1975) Inactivation of triforine by u.v. and sunlight
on glass and on leaves of bean plant. Pestic. Sci. 6, 553-559.
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D. Ost, W. and Wotschokowsky, M.
(1977) Hyrdolysis and photolysis of the fungicide triforine. Pestic.
Sci 8, 183-192.
Other information on identity and properties
State: colorless and odorless, crystalline
Melting point: 155°C
Triforine is hydrolysed rapidly in aqueous solutions at 21°C, about
50% being degraded in the first two clays with the formation of
chloride ions and, through several intermediates,
1-(dihydroxyacetyl)piperazine and piperazine. On exposure to sunlight,
about half of the triforine was degraded in 150 hours.
Photolysis by ultraviolet light in the absence of water leads
preferentially to the removal of one side chain, the second side chain
being attacked more slowly.
In aqueous solution, triforine is rapidly decomposed by ultraviolet
light. N-(2,2dichlorovinyl)formamide was isolated as an intermediate
photodecomposition product (Darda et al., 1977),
After 3 hours irradiation with u.v. light or 30 hours exposure to
sunlight, 50% of triforine in aqueous solution was inactivated
(Buchenanery 1975).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, metabolism and excretion
Male Wistar rats, weighing approximately 200 g, were dosed orally with
2.3 3H)-triforine (ring labelled) or 3.75 mg 14C-triforine (side
chains labelled.
After oral administration to 9 animals the blood level curve passed
its maximum (1.3% of the administered dose) 4 hours after dosage,
followed by a more rapid decrease in the 4-10 hours interval and a
subsequent slower fall. At the end of this blood level experiment
(96 hours), an average of 0.3% of the administered radioactivity was
present in the total blood compartment of the rat. The main portion of
the total radioactivity, 74.3% of the (3H)-triforine and 52.5% of the
(14C)-labelled triforine, was excreted reneally within the first
24 hours. In this period the faecal excretion amounted to 16.5% of the
(3H)-labelled and 39.5% of the (14C)-labelled triforine. The average
total triforine eliminated by the rat within one day of dosing was
90.8% (3H) and 92.0% (14C).
During a period of 30 hours after dosing, 19% of the orally
administered (3H) dose was 15% of the (14C) dose was excreted via
the bile. The elimination in the urine following increased dose of
triforine (25, 50, 100 and 200 mg/kg for 8 animals) followed the same
metabolic pattern: 70% of the (3H)-labelled and 50% of the
(14C)-labelled substance was excreted after 24 hr. The main
metabolite in the urine was identified as
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide (Darda, 1977).
COMMENTS
In rats, the compound was rapidly absorbed and excreted, mainly in the
urine. The main metabolite was
N-(2,2,2-trichloro-1-(piperazin-1-yl)ethyl)formaide.
Full toxicological data are required before a recommendation of the
ADI for humans can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
No data were available for consideration.
FURTHER WORK OR INFORMATION
REQUIRED by 30 June 1978 and before an acceptable daily intake for
humans (ADI) can be established and maximum residue limits (MRL) can
be recommended.
1. Submission of full data relating to toxicological and residue
studies.
REFERENCES
Buchanauer, H. (1975) Inactivation of triforine by u.v. and sunlight
on glass and on leaves of bean plant. Pestic. Sci. 6, 553-559.
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D. Ost, W. and Wotschokowsky, M.
(1977) Hyrdolysis and photolysis of the fungicide triforine. Pestic.
Sci 8, 183-192.
