
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
AMINOCARB
Explanation
Owing to insufficiency of data the 1978 Meeting was only able to
undertake a preliminary review of information concerning this
compound. Toxicological and residue data, made available to the
current meeting, are reviewed and summarized in this monograph
addendum.
IDENTITY
Chemical Name: 4-dimethylamino-3-methylphenyl N-
methylcarbamate 4-dimethylamino-m-tolyl N-methylcarbamate
Synonyms: Matacil (R), Bayer 44,646, A 353, Matacil (R)
Chemical Structure:
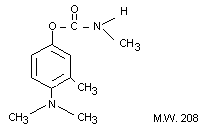 Empirical Formula: C11H16N2O2
Other Information on Identity and Properties:
Description: White, crystalline solid
M.P. 93-94°C
V.P. Non-volatile
Solubility: Slightly soluble in water; moderately soluble
in aromatic solvents; soluble in polar organic solvents
Stability: Unstable in alkaline media.
Empirical Formula: C11H16N2O2
Other Information on Identity and Properties:
Description: White, crystalline solid
M.P. 93-94°C
V.P. Non-volatile
Solubility: Slightly soluble in water; moderately soluble
in aromatic solvents; soluble in polar organic solvents
Stability: Unstable in alkaline media.
 The half-life for the hydrolysis of aminocarb in pH 9.3 buffer was
reported to be 4 hours. On glass surfaces under fluorescent light at
25°C the rate of loss was approximately linear for the first few hours
with a half-life of 1.6 hours. In the period of 4 to 12 hours, after
application, there was a decrease in slope, indicative of conversion
to less volatile products on exposure to air and light (Abdel-Wahab
et al., 1966).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Miniature swine (Sus scrofa, 1 male and 1 female) received a
single oral dose of 0.5 mg/kg body weight 14C labelled aminocarb.
The compound was rapidly absorbed. The 14C peak value in blood was
reached within one hour. Excretion via the faeces did not exceed 2
percent, while 96% was excreted in the urine within 48 hours.
Elimination was rapid with approximately 75 percent observed in urine
within 6-24 hours.
The major urinary metabolites consisted of
conjugated-4-dimethylamino-, 4-methylamino-, and 4-
amino-3-methyl-phenol. Additionally, slight amounts (1-4%) of
aminocarb, its 4-formylamino-3-methyl phenol, and demethylation
products were observed. The same animals received 0.26 mg/kg body
weight of 14C-labelled aminocarb for five days and were sacrificed 45
minutes after the last dose. More than 90% of the daily dose was
excreted in the urine and with less than 2% in the faeces. Remarkable
amounts of 14C occurred in the kidney (0.7 ppm, expressed as
aminocarb) and liver (0.2 ppm) with conjugated
4-dimethyl-amino-3-methyl-phenol characterized as the main metabolite.
A similar amount of unidentified metabolite(s) was also observed.
Additionally, slight amounts (1-5%) of aminocarb, its demethylation
products, the formylamino derivative, and conjugated phenol-analogues
were determined. Slight quantities of 14C (0.02-0.09 ppm) were found
in other tissues (skin, brain, heart, muscles, and fat) with higher
values noted in the skin and fat (Shaw, 1978).
Bioaccumulation
Quail were fed 0, 10 and 50 ppm of 14C ring labelled aminocarb. The
14C content of liver, breast muscle, and mesenteric fat continuously
increased during the 14 days of treatment followed by continuous
elimination during a 14-day withdrawal period (Lamb, et al., 1976).
In channel catfish (Ictalurus punctatus) exposed to 10 ppb 14C
ring-labelled aminocarb, accumulation reached an equilibrium level
after one day. Elimination was rapid. A 50% reduction of residues
was noted in one day following a 28-day exposure. 14C was equally
distributed in the edible and non-edible parts of the fish (Lamb and
Roney, 1976).
A group of 9 male albino rats received a single dose of 25 mg/kg
aminocarb by gavage. The cholinesterase activity in the erythrocytes
was reduced by 50% after 1,3 and 5 hours. Recovery was rapid, being
complete within 24 hours (Kimmerle, 1961).
Special Studies on Cholinesterase Inhibition
Rat
Groups of male and female (Sprague-Dawley) rats (3 animals/group)
received oral doses of 0, 0.5, 2.0 and 8.0 mg/kg aminocarb by gavage
once a week for three weeks. Reduced cholinesterase activity was
noted at the highest dose (8.0 mg/kg) 1 and 3 hours after treatment
(approximately 10 to 20% in plasma and 30 to 40% in erythrocytes). In
the 2 mg/kg group, reduced erythrocyte cholinesterase activity was
observed after one hour. Twenty-four hours after dosing, all
inhibitory effects had disappeared in the blood. There was no
inhibition of brain cholinesterase activity noted in this study
(Nelson, 1978b).
A group of male albino rats which received 5 mg aminocarb/kg by gavage
for four weeks showed a 20-30% inhibition of erythrocyte
cholinesterase activity. There were no clinical signs of poisoning
associated with this dose level.
Groups of young adult female Sprague-Dawley rats (5 animals/group)
were administered aminocarb by intraperitoneal injection at daily dose
levels of 0, 2, 4, 8 and 10 mg/kg for 60 days. At dosage levels above
2 mg/kg, a decrease in body weight was noted. The animals
administered 4 and 8 mg/kg developed acute signs of poisoning which
persisted for one or two hours after dosing. All animals at the
highest dose level and one animal of the 8 mg/kg level failed to
survive the 60-day trial. One day after the last dose, cholinesterase
activity of brain, submaxillary gland and serum was measured and
inhibition data are represented in the following table:
% of inhibition at:
2 mg/kg 4mg/kg 8mg/kg
Brain 0 0 0
Submaxillary gland 11 24 34
Serum 29 44 53
(Dubois and Kinoshita, 1762).
TOXICOLOGICAL STUDIES
Special Studies or Reproduction
Four groups of 10 male and 20 female (Sprague Dawley) rats were fed
aminocarb in the diet at doses of 0, 100, 200 and 800 ppm. Treatment
started when the parents were approximately 75 days old and was
continued through a standard three generation reproduction study. All
generations showed a light hypersensitivity at 800 ppm. With the
exception of females of the F1b generation, retarded weight gain was
noted in all rats at 800 ppm. Weight gain was also retarded in the
females of the F0 and in males of the F1 and F2 generation at 200
ppm. Food consumption at the two higher dose levels was depressed in
a dose dependent manner. At birth, a reduced litter size and weight
was noted in the first mating of the F0, F1 and F2 generations at
800 ppm. At weaning, a reduced litter weight and mean pup weight for
all generations was seen at 800 ppm. At 200 ppm, a reduced pup weight
was observed at the first mating of the F0 generation. No
abnormalities were found as a result of aminocarb in the diet. The
young animals from the second litter of the third generation of rats
treated with 800 ppm did not show significant alteration in tissue
morphology associated with treatment. A no-effect level in this study
was 100 ppm (Palmer and Fletcher, 1966).
Special Studies on Neurotoxicity
In 20 hens over 9 months of age, which were orally administered
aminocarb (74 mg/kg) on day 0 and day 30, no signs of delayed
neurotoxicity were noted over the observation period of 30 days or in
the 30-day observation period following the second dosing.
Histopathologically, no alterations were observed in the nervous
tissue (Kruckenberg, 1978a).
In groups of 18-20 month old hens (3 animals/group) which were fed 0,
250, 500, 1000 and 2000 ppm aminocarb in the diet for 30 days and
thereafter observed for another four weeks, no delayed neurotoxic
effects were found. At doses of 500, 1000, and 2000 ppm, mortality
was observed as 2, 6 and 7 animals, respectively, died. At all dose
levels, blood cholinesterase was significantly inhibited and body
weight was reduced (Kimmerle, 1965a).
Acute Toxicity
Table 1. Acute-Toxicity of Aminocarb
Route of LD50
Species Sex administration (mg/kg) Reference
Mouse M IP 8 Dubois & Raymund, 1962a
Mouse F IP 9 Dubois & Raymund, 1962a
Mouse F IP 4.7 Abdel-Wahab & Casida,
1967
Mouse F Dermal 31 " "
Mouse F 1 hr exposure
2 um size) 4 mg/L Dilley & Doull, 1962
Rat M&F Oral 30 Dubois & Raymund, 1962a
Rat F Oral 22-27 Nelson, 1978a
Rat M Oral ca.50 Kimmerle, 1961
Table 1. Continued...
Route of LD50
Species Sex administration (mg/kg) Reference
Rat M IP 13 Dubois & Raymund, 1962a
Rat M IP 21 Kimmerle, 1961
Rat F IP 13.5 Dubois & Raymund, 1962a
Rat F Dermal 275 " "
Rat M Dermal <1000 Kimmerle, 1961
Rat F Inhalation
(1 hr exposure) 6 mg/L Dilley & Doull, 1962
Rat M&F Inhalation
(4 hr exposure) 0.2 mg/L Kimmerle, 1961; 1966
Guinea pig M Oral 60 Dubois de Raymund,1962a
Guinea pig M IP 30 " "
Chicken F Oral 74 Kruckenberg, 1978b
Chicken F Oral 75 Dubois, 1962
Chicken F Oral 50-100 Kimmerle, 1965b
Chicken F IP 25-50 Kimmerle, 1965b
In an acute inhalation toxicity test, groups of rats (20 males/group)
were exposed using a dynamic flow chamber into which aerosols at
concentrations of 0.1 and 1 mg/L aminocarb were continuously dispersed
for four hours. All animals at 1 mg/L and 4/20 animals at 0.1 mg/L
died within eight hours after exposure (Kimmerle, 1961; 1966).
In a subacute inhalation toxicity test with 20 male albino rats using
a dynamic flow chamber into which an aerosol with a concentration of
0.04 mg/L was dispersed eight hours per day for five consecutive days,
no specific signs of poisoning were observed. One animal died four
days after the end of the test (Kimmerle, 1961).
Signs of poisoning
Acutely toxic doses of aminocarb produce cholinergic effects typical
of cholinesterase inhibitors. The signs of poisoning appeared rapidly
after oral or intraperitoneal administration and were similar in all
species examined. After lethal doses, death occurred within two
hours, and usually within the first 30 minutes. After sublethal
doses, the symptoms subsided rapidly and apparent complete recovery
was usually noted within three hours (Dubois and Raymund, 1962a).
Special Studies on Potentiation
Potentiation of the acute toxicity of aminocarb was absent in female
(Sprague Dawley) rats when aminocarb was given by intraperitoneal
injection alone and in combination with 15 other anticholinesterase
insecticides (Dubois and Raymund, 1962b).
Short-Term Studies
Cat
In a cat which received 5 mg/kg aminocarb by gavage for ten
consecutive days, salivation, loss of appetite, and weight loss was
observed. Signs of cholinesterase inhibition were noted after two
days in 2 cats which received doses of 10 mg/kg; these animals died
after 4 days of treatment (Kimmerle, 1961).
Quail and Duck
Aminocarb and four of its major metabolites (THS 1013, THS 1003, THS
0995 and THS 1029) were fed to 15-day old bobwhite quail and 12-day
old mallard ducks at a dose of 1000 ppm for a period of 5 days.
Except for a reduction in feed consumption and reduced weight gain of
the mallard ducks, no toxic signs were observed (Lamb and Jones,
1975).
Rat
Groups of weanling (Sprague Dawley) rats (12 male and 12 female
rats/group) were fed 0, 5, 10 and 50 ppm aminocarb in the diet for 16
weeks. No effects on cholinesterase activity, growth, food
consumption, gross organ weight, or on histological examination of
tissues and organs were found in this study. A similar study was
started with dietary levels of 0, 100 and 200 ppm. As these levels
also failed to induce a significant inhibition of cholinesterase in
the first 3 weeks, the dietary levels were increased to 400 and 800
ppm, and were maintained for a further 19 weeks. The duration of this
study was 22 weeks. Over the course of the study, many of the animals
appeared to be hyperexcited and irritable. At the end of the study, a
decrease in growth rate was noted in males (14%) and females (13%) fed
800 ppm and in males (13%) and females (10%) fed 400 ppm.
Cholinesterase activity was measured on five animals/sex/group. Data
at the conclusion of the study revealed an inhibition in the serum of
males (16%) and females (42%) fed 800 ppm, and in the serum of females
(31%) fed 400 ppm. No inhibition was found in the brain, submaxillary
glands and erythrocytes. No effects were observed with respect to
organ weights and histopathological examination of tissues and organs
(Root, et al., 1963).
Table 2. Acute Toxicity of Aminocarb Metabolites
LD50
Compound Species Sex Route (mg/kg) Reference
4-(N'formyl-N'-methylamino- Mouse F IP 21 Abdel-Wahab & Casida, 1967
3-methylphenyl-N-methylcarbamate Mouse F Dermal >500 " "
4-(methylamino)-3-methyl
phenyl-N-methylcarbamate Mouse F IP 3.0 " "
Mouse F Dermal 52 " "
Rat F Oral 27.8(22.9-33.7) Kimmerle, 1974
4-formylamino-3-methylPhenyl- Mouse F IP 13 Abdel-Wahab & Casida, 1967
N-methylcarbamate
Mouse F Dermal 500 " "
4-amino-3-methylphenyl-N- Mouse F IP 1.6 " "
methylcarbamate Mouse F Dermal 17 " "
Rat F Oral 18.3(16.2-20.7) Kimmerle, 1974
4-(N'-methoxy-N'-methylamino)- Rat F Oral 102.8(88.5-119.4) Kimmerle, 1974
3-methylphenyl-N-methyl
carbamate
4-methoxyamino-3-methylphenyl- Rat F oral 40.1(33.7-47.5) Kimmerle, 1974
N-methylcarbamate
Dog
Groups of two male and two female beagle dogs were fed 0, 200, 400 and
800 ppm aminocarb in the diet for 12 weeks. At the end of this
feeding period, the dogs were returned to the control diet for an
additional 4-week period. Weight loss was observed in the animals of
the 800 ppm and 400 ppm groups. Signs of poisoning (e.g. vomiting,
retching, ataxia and incoordination) were dose-related and were found
in all dose groups, especially in the female animals. Signs of
poisoning were particularly prevalent during and just after
consumption of the aminocarb diet and were minimal or absent during
the remaining part of the day. There were no signs of poisoning noted
in the 4-week recovery period. Blood samples, taken prior to feeding
when symptoms were minimal or absent showed no inhibition of the
cholinesterase activity, of the serum or erythrocytes (Doull and Root,
1963).
Groups of beagle dogs (4 male and 4 female dogs/group) were orally
dosed by gavage at dosage levels of 0, 2, 4 and 10 mg/kg/day (0, 1, 2
and 5 mg/kg twice a day) for two years. After 12 weeks, one dog in
the highest dose group died. Moderately severe signs of poisoning,
(e.g., general excitability, salivation, pupil constriction and fine
visible trembling of muscles) were seen in all dogs in the high dose
group. Less severe signs of poisoning were noted in dogs at 4 and 2
mg/kg/day. The highest dose group showed a slight reduction in body
weight and a reduced rate of growth. No inhibition of cholinesterase
activity in serum and erythrocytes was noted, nor were changes in
hematology and blood chemistry found, except with the one dog that
died. Brain cholinesterase activity was not measured. Samples for
serum and erythrocyte cholinesterase were taken prior to daily dosing
in the first 20 months of the study and in the last 4 months of the
study, within 45 minutes of dosing.
At the conclusion of the study, adrenal weight, as a percentage of
body weight only, was increased in the high dose level group. Other
organ and organ weight ratios were within normal limits.
Histopathologically, macrophages containing lipofucsin materials were
seen throughout the liver of all groups, possibly more frequently in
the high dose animals. In the other tissues, no compound-related
effects were seen histologically (Noel, et al., 1966).
Long-Term Studies
Rat
Groups of weanling Sprague-Dawley rats (24 males and 24 females/group)
were fed 0, 100, 200, 400 and 800 ppm aminocarb in the diet for 20
months. Growth was reduced in males and females at 800 ppm, and to a
lesser extent, at all lower dose levels. The median survival time and
food consumption were not affected by aminocarb. Final examinations
of 5 animals/sex/group did not reveal significant effects on
cholinesterase activity in the brain, serum, and erythrocytes, whereas
a slight inhibition (approximately 20%) was noted in the submaxillary
glands of males and females at 800 ppm. The relative weight of the
heart was slightly increased in all dose groups and in microscopic
examination showed cardiac lesions in both males and females at 800
ppm and in males at 400 ppm. Reduced spermato-genesis was observed in
some animals of the two highest dose groups. Liver changes were also
noted at dose levels of 200 ppm and above (Doull, et al., 1967).
COMMENTS
Aminocarb, an N-methyl carbamate ester, is rapidly absorbed and
metabolized by demethylation and/or hydroxylation or ester hydrolysis.
Those metabolites containing an intact carbamate ester structure show
acute toxicity similar to or greater than the parent compound.
Aminocarb and its metabolites are rapidly excreted and there is no
evidence of accumulation. In acute toxicity studies, aminocarb was
not potentiated by other cholinesterase inhibitors, nor did it induce
a delayed neurotoxic response in hens.
In several short- and long-term studies in the rat, aminocarb induced
a reversible cholinesterase inhibition. In a 2-year dog study,
clinical symptoms of poisoning were seen in all dose levels tested.
In a 3-generation study with rats, no effects were observed at dose
levels of 100 ppm or below.
The meeting concluded that the short- and long-term rat studies were
not carried out in accordance with currently acceptable protocols. As
there was growth depression and an increased relative weight of the
heart at all dose levels tested in the long-term rat study, a
no-effect level in the rat could not be determined. An acceptable
daily intake for man was not allocated. The meeting recommended that
aminocarb be re-examined when the ongoing long-term study in rodents
becomes available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Aminocarb has been used mainly for the control of lepidopterous
defoliators of Canadian forests where it has been found notably
effective against the spruce budworm, Choristoneura fumiferana
(Clemens). About 3.7 million hectares of Canadian forest have been
treated by aircraft at an average dosage of 0.07 kg ai/ha. 167 g/l
oil-soluble concentrate is used.
Aminocarb is also effective for the control of lepidopterous larvae
and other biting insects in cotton, tomatoes, tobacco and fruit crops,
but usage in these crops appears to be low. For these purposes, 50
and 75% wettable formulations are used. In New Zealand, the
insecticide was, but is no longer used widely against the coddling
moth, leaf roller and mealy bug. It is applied at 0.75-1.12 kg ai/100
L. In stone fruit and citrus, it is effective against the leaf
roller, mealy bug and scales, where it is applied at 75-94 g ai/100 L.
Aminocarb is also effective against caterpillars in vegetables and is
applied at 0.75-1.5 kg ai/ha (New Zealand, 1978).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Very few residue data on food crops are available because of the
limited usage of aminocarb in these crops. The manufacturer provided
the data available for apples and pears.
Studies carried out in Australia with apples involved the application
of aminocarb at 0.075, 0.1 and 0.125% solutions, equivalent to 3/4, 1,
and 1 1/4 lbs./100 gals, respectively, to give a spray coverage of
13-18 L/tree. The recommended preharvest interval of three days
justify a limit of 4 mg/kg (Table 3). The data for pears (Table 4)
also adequately support a 4 mg/kg limit at the three days preharvest
interval.
Table 3. Aminocarb Residues in Apples
Application rates Residue levels (mg/kg)
(% Solution) Period in days
3 10 17 24
0.075 2.8 1.3 1.6 0.6
0.1 2.2 1.0 1.6 1.4
0.125 2.6 1.4 1.8 1.0
Table 4. Aminocarb Residues in Pears
Residue levels (mg/kg)
Application rate Period in days
(% solution) 0 7 13 15 21
(23-27 L/tree)
0.1 1 0.8 n.d. - ND
0.1 3.0 1.0 0.4 - 0.4
0.1 1.3 - - n.d. 0.4
(23-27 L/tree)
0.1 1.4 - - n.d. 0.4
(23-27 L/tree
0.125 1.9 - - 0.4 0.4
(23-27 L/tree)
0.075 1.4 - - n.d. n.d.
FATE OF RESIDUES
General Comments
Most of the data on aminocarb were generated in connection with its
use in the control of forest pests. Aminocarb was degraded rapidly in
soil, water, plants, animals and by ultraviolet irradiation (Chemagro,
1976). The major route of breakdown in the presence of light or on
surfaces was oxidation of the dimethylamino portion of the molecule to
produce traces of 4-(formamidomethylamino)-, 4-(formamidoamino)- and
4-amino-3-methylphenol. These products are not detected in animals or
within plant tissues. In plants, hydroxylation followed by
conjugation is the main degradation pathway. In soil, N-demethylation
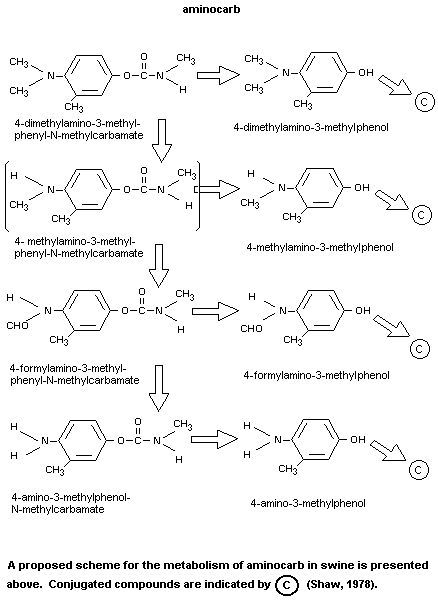
is predominant. In swine, conjugates of aminocarb phenol and the
methylamino analogue of aminocarb phenol were the major metabolites
found in males and females respectively. The principal tissue
constituent was conjugated aminocarb phenol.
Under normal use conditions, aminocarb was found at about 0.01 mg/kg
in soil and water, and about 2 mg/kg on foliage. Half-lives under
normal use conditions ranged from less than one day in soil to about
six days on spruce foliage. Maguire (1973) reviewed the chemistry of
aminocarb and the effect on the environment has been summarized
(Chemagro, 1976, 1979). The metabolism of aminocarb in different
substrates is shown in Figure 1.
In animals
(See Biochemical Aspects). In an ascorbic acid system which simulates
biological oxidation processes, Balba and Saha (1974) found at least
12 products formed from aminocarb. At least 10 of these products
contained either one or both the N-14CH3 groups. The ascorbic acid
system caused demethylation, hydroxylation of the aromatic ring,
oxidation of the -NHCH3, group to -NHCH20N, and cleavage of the
carbamate to the phenol. The 4-methylamino derivative was the major
degradation product.
In Plants
Abdel-Wahab et al., (1966) found that aminocarb was degraded with
the carbamate moiety intact when the carbamate was applied to glass or
silica gel surfaces or the leaves of growing bean plants, or injected
into the stems of bean plants. The methylcarbamate derivatives formed
included the 4-methylamino, 4-amino, 4-methylformamido, and
4-formamido analogues. The C14-carbonyl activity was found in the
unextractable portion six days after injection into bean plants. The
water-soluble metabolites formed following injection into the bean
plants result in part from hydroxylation of the carbamate on the
N-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates, mainly as glycosides (Kuhr
and Casida, 1967). These glycosides were quite persistent.
Dorough (1964) determined the stability of aminocarb applied as a leaf
surface treatment to cotton, garden snap beans, broccoli and tomatoes.
The metabolic fates of directly injected and surface-applied
carbonyl-C14 aminocarb appeared to be the same. The original
carbamate was converted into water-soluble metabolites which were
probably conjugates. Conversion was almost quantitative and the
metabolites were persistent. Leaf surface residues appear to be
degraded via hydrolysis at the carbamate group before or during
penetration. Penetration of aminocarb through the leaf surface of
beans and cotton was about eight percent after eight hours, although
50% of the residues was already lost from the surface (Dorough, 1979).
On the other hand, broccoli plants did not contain any internal
residues. Aminocarb was metabolized to persistent water-soluble
metabolites in these plants.
The fate and persistence of aminocarb in spruce foliage was
investigated by Sundaram and Hopewell (1977a) using a simulated spray
at the rate of 3.4 L/ha containing 57 g ai. The initial concentration
in foliage was about 10 mg/kg and dropped to less than 0.2 mg/kg
within 47 days. The half-life was six days. Aminocarb was found to
be labile and was dissipated rapidly under normal weathering
conditions.
In a similar study, Nagel et al. (1978) was not able to detect
volatilization of radioactivity with ring -1-C14 aminocarb.
Aminocarb residues declined from an initial level of 6.7 mg/kg to 3.3
mg/kg in 28 days. They attributed the decline to growth dilution.
The methylamino analogue was the only identified metabolite. Although
several unidentified metabolites were detected, no single component
contributed more than 10% of the total radioactivity.
The actual situations where aminocarb was applied serially at 70 g
ai/ha, the concentration in spruce foliage at 0.6 days was 0.7 mg/kg,
increased to a maximum of 2.2 mg/kg after four days and thereafter
decreases exponentially with a half-life of 5.6 days (Sundaram et
al., 1976). No residues could be detected after 64 days, presumably
owing to physical factors.
The penetration, translocation and fate of C14 aminocarb in spruce
trees was also investigated using trunk implantation (TIT), foliar
painting (FP), and basal bark painting (BBP) (Sundaram and Hopewell
1977b). Aminocarb appears to be weakly systemic. The aminocarb
absorbed after trunk implantation was gradually lost after 64 days,
probably owing to hydroxylation to water-soluble metabolites, some of
which were incorporated into the cellular structure of the foliage.
The major route of translocation was from the old to the
newly-developing foliage. In the case of foliar painting, basal bark
painting techniques, the mechanism of dissipation appeared to be
physical rather than biochemical.
In soil
In a comparative study of the persistence of aminocarb in silt loam
and sandy loam soil, Murphy et al, (1975) using
carbonyl-C14-aminocarb, found that breakdown was slower and more
soil-bound radioactivity was detected in the latter. In silt loam,
less than 15 percent of the applied insecticide was still present 24
hours after application. Microbial activity was essential for rapid
metabolism and C14102 was the principal product. Some of the
liberated C14102 was assimilated into the normal, insoluble soil
constituents. Minor (1978) also found that the insecticide was
degraded through removal of the carbamate portion by hydrolysis
followed by soil binding.
Aminocarb could be readily absorbed from aqueous solutions by loam
soil (Atwell, 1978). Although it is ranked as intermediate in soil
mobility using the soil thin layer system (Thornton et al, 1976)
leaching studies with sandy loam soil columns showed aminocarb to be
essentially retained in the upper 2.5 cm of soil. Less than 0.1% of
the radiolabel used was found in the leaching water after passing
through the soil column.
When aminocarb was applied to sandy loam soil at 1 mg/kg, the
half-life was less than 24 hours (Minor, 1978). The only identified
compounds were aminocarb and its N-demethylated analogue. Within 24
hours, 75% of the radioactivity was bound. Microbial activity was
necessary for degradation. In a pond water/soil study, aminocarb had
a half-life of 3.5 days and the metabolites identified were the
N-demethylated analogues and the phenolic hydrolysis product of
aminocarb. Formation of soil-bound residues was observed.
Sundaram and Hopewell (1977a) found that with simulated aerial
application, the initial residue in soil was 7 mg/kg. The half-life
was two days and residues could no longer be detected after 27 days.
In Water
In buffered solutions, aminocarb stability increased as the
temperature and pH decreased. At pH 4, its half-life was >127 days,
at pH 7, it varied between five and eleven days, while at pH 9, it was
<1 day (Tessier et al., 1978). Murphy et al. (1975) observed a
half-life of 28.5 days at pH 7 and 28.5 days in pond water under
normal environmental conditions. The principal metabolite appeared to
be the phenol although the methylamino and formylamino analogues were
also observed. (Tessier et al., 1978; Murphy et al., 1975; Minor,
1978).
Forest aerial application at 70 g ai/ha resulted in 2.1 and 1.9 mg/kg
residues in pond and stream water respectively (Sundaram et al.,
1976). Half-lives were 4.4 and 8.7 days, respectively, in the above
substrates. Residues were below the 0.1 mg/kg limits of analytical
sensitivity within 32 days.
In a pilot study, application of the insecticide at 2.4 lb ai/acre
resulted in residues ranging from <0.1 to 0.2 mg/kg 48 hours after
application. These levels were generally higher than found after 24
hours (Chemagro, 1976).
Photodecomposition
Irradiation of aminocarb in aerated and degassed ethanol and
cyclohexane at >300 pm gave the phenol as the major product (Addison
et al., 1974). Trace quantities of other products were also
observed. On the other hand, Abdel-Wahab and Casida (1967) found that
in bean foliage, photolysis involved extensive oxidation of the
dimethylamino moiety but not of other groups. There was a stepwise
demethylation of the dimethylamino moiety and one of the methyl
radicals was oxidized to the formamido group. The same result was
obtained earlier by Abdel-Wahab et al (1967). Aminocarb was not
readily decomposed when exposed as spots on the thin layer plates in
the dark, in fluorescent light or in long-wavelength UV light. Short
wavelength UV-light or sunlight resulted in considerable degradation.
Crosby et al. (1965) observed at least two compounds with
acetylcholinesterase inhibitory effects of many decomposition products
upon exposure to sunlight and ultraviolet radiation.
In aqueous buffer at pH 4.9, aminocarb had a half-life of 10 days when
irradiated with a mercury lamp. The methylamino analogue and two
unknown products amounting to 10% and 12% of the total radioactivity,
were detected in the solution after 30 days exposure. In an
acetone-sensitized solution, the half-life was reduced to 1.5 days
(Mulkey et al., 1978).
When ring -C14 aminocarb applied to a soil surface was subjected to
light from a high intensity mercury lamp, the calculated half-life was
4.6 hours (Augenstein, 1978). Approximately 33% of the applied
radioactivity was volatilized and 50% was bound to the soil during 192
hours of exposure to the light. The methylamino, methylformylamino,
formylamino and amino analogues of aminocarb as well as the
formylamino analogue of the phenol were detected. Unidentified
products accounted for <10% of the applied radioactivity.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
In New Zealand, six samples of apples in 1971 showed residues ranging
from 0.06 to 2.1 mg/kg with a mean of 0.86 mg/kg.
METHODS OF RESIDUE ANALYSIS
The analysis of carbamate insecticides by the popular GLC technique
has been an on-going problem for two reasons: 1) the thermal
instability of some compounds and 2) the lack of a sensitive detector
for the underivatized material. Several GLC derivatization procedures
using the sensitive electron capture detector have been tried. For
aminocarb, Sundaram et al. (1976) initially tried forming the
N-hepta-fluorobutyryl derivative. The procedure had an unsatisfactory
minimum detection limit (MDL) of 0.5 mg/kg in foliar extracts because
of impurities with retention times similar to aminocarb. Stanley and
Delphia (1978) subsequently used the 2,4-dinitrophenyl ether
derivatization technique of Holden (1973) and obtained a MDL of <0.01
mg/kg for spruce needles, soil and fish and <0.001 mg/kg for water.
GLC analysis of aminocarb as the intact molecule detected by such
nitrogen-"specific" detectors as the alkali flame ionization (AFID)
and the Hall microelectrolytic conductivity detectors appear
promising. The direct GLC procedures generally require only minimum
cleanup because they are less subject to sample interferences. Bayer
(1975) used the AFID to detect 0.1 mg/kg aminocarb on plant materials
and 0.02 mg/kg in water. Sundaram and Hopewell (1977) using the Hall
detector, found an MDL of 0.2 mg/kg for spruce foliage and soil. In
water, Sundaram et al. (1978) achieved an MDL of 1 × 10-4 mg/kg for
both aminocarb and its phenol.
High-pressure liquid chromatography (HPLC) has been applied by
Laurence (1977) to the detection of aminocarb in cabbage, corn, potato
and wheat. A UV detector was used at 254 nm. As little as 0.8 ng of
insecticide can be detected in the above crops.
Olson (1964) described a colorimetric procedure for residue
determination in soil, after the reaction of the phenol with
4-aminoantipyrine. The procedure can detect as little as 0.1 mg/kg
aminocarb and recovery was 90% for soils. It is also applicable to
crops.
In effect, the determination of the intact carbamate offers
possibilities as a regulatory method, especially with improvements in
the detection limits of the nitrogen specific detectors. The Hall
detector is particularly useful. If greater sensitivity is needed,
the 2,4-dinitrophenyl ether derivative can be used. Adoption of HPLC
for regulatory purpose would have to await further improvements in the
detection system and widespread use of the technique. The
4-aminoantipyrine procedure can be used for spectrophotometry.
NATIONAL MRLs REPORTED TO THE MEETING
According to the information supplied to the meeting, only the
following countries have established limits for aminocarb in food:
Limit Preharvest
Country Commodity established Interval
(mg/kg) (days)
Australia apples, pears 4 3
cottonseed 1
vegetables, fruit
(except apples, pears) 1
Germany pome fruit 1
(FRG)
APPRAISAL
Aminocarb is mainly used for the control of lepidopterous defoliators
in conifer forests where it is applied by air. There is only limited
usage in other crops. For aerial application, a 1.4 lb ai/US gal.
oil-soluble concentrate formulation is used. For general usage, 50
and 75% wettable powder formulations are available.
In view of the limited usage in food crops, very few residue trials
have been done to establish MRLs. Data on apples and pears adequately
support MRLs of 5 and 2 mg/kg, respectively.
Aminocarb is degraded rapidly in soil, water, plants, animals and when
exposed to short-wave ultraviolet radiation. In plants, metabolism
involves hydroxylation of the molecule at the carbamate on the
1,1-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates mainly as glycosides.
Conjugates of aminocarb phenol and the methylamino analogue of
aminocarb phenol were the major metabolites in swine. The principal
tissues constituent was conjugated aminocarb phenol. In soil,
N-demethylation predominate. Under normal use conditions, half-lives
of aminocarb ranged from less than one day in soil to about six days
on spruce foliage. Detection by gas chromatography as the intact
carbamate or detection as the 2,4-dinitrophenyl ether derivative
appear adequate as regulatory methods; the latter can detect as low as
0.01 mg/kg aminocarb in spruce needles, soil and fish and 0.001
aminocarb in water.
RECOMMENDATIONS
Since the main use of aminocarb is in forest pest control, the
likelihood of its occurrence in food items for international trade is
generally low. However, the setting of MRLs are considered necessary
in some cases. In the absence of an ADI, the following guideline
levels are recorded for aminocarb:
Commodity GL
mg/kg
apples 5
pears 2
FURTHER WORK OR INFORMATION
If the usage of aminocarb in food items should increase, additional
information should be provided.
REFERENCES
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. - Fate of
C14-carbonyl-labelled aryl methylcarbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14., 290-298.
Abdel-Waheb, A.M. and Casida, J.E. - Photo-oxidation of Two
4-Dimethylaminoaryl methylcarbamate Insecticides (Zectram and Matacil)
on Bean Foliage and of Alkylaminophenyl Methylcarbamates on Silica Gel
Chromatoplates. J. Agr. Food Chem. 15: 479-487.
Addison, J.B., Silk, P.J. and Unger, I. - The photochemical reactions
of carbamates II. The solution photo chemistry of Matacil
(4-dimethylamino m-tolyl-N-methyl (carbamate) and Landrin
(3,4,5-trimethylphenyl-N-methyl carbamate). Bull. Environmental
Contam. Toxicol. 11, 250-255.
Atwell, S.M. Soil absorption and desorption of C14-Natacil. Chemagro
Report No. 55424 July 12 (1973), Unpublished.
Augenstein, L.L., McPhaul, L. and Wargo, J.P., Jr. - Photodegradation
of 14C Matacil on a soil surface. Cemagro Report No. 50446, October
20 (1973), Unpublished.
Balba, M.M. and Saha, J.G. - Degradation of Matacil by the ascorbic
acid oxidation system. Bull. Environ. Contam. Toxicol. 11, 193-200.
Bayer, A.G. Gas Chromatographische bestimmung von
aminocarbruckstanden in boden und wasser. Oct. 28 (1975), Unpublished.
Bayer, A. G. Data submitted to the 1979 JMPR
Chemagro. Matacil - The effect on the environment. Chemagro Report,
April 17 (1976), Unpublished.
Coburn, J.A., Ripley, B.D. and Chau, A.S.Y. - Analysis of Pesticide
residues by chemical derivatization. II. N-methylcarbamates in natural
water and soils. J. Assoc. Off. Anal. Chem. 59, 188-196.
Crosby, D.G., Leitis, E and. Winterlin, W. L. - Photodecomposition of
carbamate insecticides. J. Agr. Food Chem. 13, 204-207.
Dilley, J. and Doull, J. - The Acute Inhalation Toxicity of Bayer
44646 to Rats and Mice. (1962) Unpublished report from the University
of Chicago, submitted by Bayer, AG.
Dorough, H.W. Fate of Bayer 44646 in several plant species. Progress
Report, Texas A & M University, Sept. 1 (1964), Unpublished.
Fate of Matacil in several plant species. Progress Report, Texas A &
M University, July (1970), Unpublished.
Dorough, H.W. and Thorstenson, J.H. - Analysis of carbamate
insecticides and metabolites J. Chromatogr. Sci. 13, 212-224.
Doull, J. and Root, M. - Subacute Oral Toxicity of Bayer 44646 in Male
and Female Dogs. (1963) Unpublished report from the University of
Chicago, submitted by Bayer AG.
Doull, J., Root, M. and Keskauskas, J. - Chronic Oral Toxicity of
Bayer 44646 to Rat. (Addendum: Hibbs, C., and Nelson, D.L. Microscopic
Findings in Tissues of Male and Female Rats Fed Bayer 44646 for Two
years). (1967) Unpublished report from the University of Chicago,
submitted by Bayer AG.
Dubois, K.P. The Acute Oral Toxicity of Bayer 44646 to Chickens.
(1962) Unpublished report from the University of Chicago, submitted by
Bayer AG.
Dubois, K.P. and Raymund, A.B. - Acute Toxicity of Bayer 44646 to
Mammals. (1962a) Unpublished report, from the University of Chicago,
submitted by Bayer AG.
The Acute Toxicity of Bayer 44646 in Combination with other
Anticholinesterase Insecticides. (1962b) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Dubois, K.P. and Kinoshita, F. - The Subacute Parenteral Toxicity of
Bayer 44646 to Female Rats. (1962) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Holden, E.R. Gas Chromatographic determination of residues of
methylcarbamate insecticides in crops as their 2,4-dinitrophenyl ether
derivatives. J. Assoc. Off. Anal. Chem. 56, 713-717.
Kimmerle, G., Wirkstoff Dr. Heib A. 363 (E 44646). (1961) Unpublished
report from Bayer AG, Tox. Gew. Hyg. Labor, submitted by Bayer AG.
Neurotoxische Untersuchungen mit A 363-Wirkstoff. (1965a) Unpublished
report from the Institut für Toxikologie, submitted by Bayer AG.
Wirkstoff A 363 als Formulierung F1 604/149 Acute
Neurotoxizitätsprüfung bei Hühnern. (1965b) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Matacil-Wirkstoff. (1966) Unpublished report from the Institut für
Toxikologie, submitted by Bayer AG.
3-Methyl-4-methoxymethylaminophenyl-N-monomethy9lcarbamat,
3-methyl-4-monomethoxyaminophenyl-N-monomethylcarbamat,
3-methyl-4-monomethyl-aminophenyl-N-monomethyl-carbamat, und
3-methyl-4-aminophenyl-N monomethylcarbamat. Akate Akute Toxizität bei
Ratten. (1974) Unpublished report from the Institut Für Toxikologie,
submitted by Bayer AG.
Kuhr, R.J. and Casida, J.E. - Persistent glycosides of metabolites of
methylcarbamate insecticide chemicals formed by hydroxylation in bean
plants. J. Agr. Food Chem. 15, 814-824.
Kruckenberg, S.M. - Delayed Neurotoxicity Study of Matacil in Hens.
(1978a) Unpublished report from Kansas State University, by Bayer AG.
Acute Oral Toxicity of Matacil in Chickens. (1978b) Unpublished
report from Kansas State University, submitted by Bayer AG.
Lamb, D. W. and Jones, R.E. - Dietary Toxicity of Matacil Technical
and 4-Matacil Metabolites to Bobwhite Quail and Mallard Ducks. (1975)
Unpublished report from Chemagro Agr. Div., Mobay Chemical Corp.,
submitted by Bayer AG.
Lamb, D.W. and Roney, D.J. - Accumulation and Persistence of Residues
in Channel Catfish exposed to Matacil-14C. (1976) Unpublished report
from Mobay Chemical Corp., submitted by Bayer AG.
Lamb, D.W. et al. Accumulation and Elimination of Residues in
Bobwhite Quail Exposed to Matacil-14C. (1976) Unpublished report from
Mobay Chemical Corp., submitted by Bayer AG.
Lawrence, J.F. Direct analysis of some carbamate pesticides in food
by high pressure liquid chromatography. J. Agr. Food Chem. 25,
211-212.
Maguire, R.J. Aminocarb: A review of its chemistry. Manuscript
submitted for publication by the Canadian Forestry Service. (1978).
Minor, R.G. Metabolism of Matacil in soil and in an aquatic
environment. Chemagro Report No. 50427, October 15, 1978, Unpublished.
Mobay, Matacil - the effect on the environment, Addition no. 1 to
Chemagro Report 1976, January 14 (Unpublished).
Mulkey, N.S., McPhaul, L., Augenstein, L.L. and Wargo, J.P. Jr.
Photodegradation of 14C Matacil in aqueous solution. Chemagro report
No. 50447, October 18 (1978), Unpublished.
Murphy, J.J., Jacobs, K. and Minor, R.G. - Stability of Matacil in
aqueous systems. Chemagro Report No. 43450. January 20 (1975a),
Unpublished.
Murphy, J.J., Minor, R.G., Jacobs, K. and Shaif II, H.R. - Persistence
of Matacil in soil. Chemagro Report No. 43444, January 31 (1975b),
Unpublished.
Nagel, C.D., Tessier, J.F., McPhaul, L., Augenstein, L.L. and Wargo,
Jr. J.P. - Spruce foliage metabolism study with 14C Matacil. Chemagro
report No. 50445, October 16 (1978), Unpublished.
Nelson, D.L. - Acute Oral Toxicity of Matacil Technical and Matacil
Analytical Grade to Female Rats. (1973a) Unpublished report from
Chemagro Agr. Div., Mobay Chemical Corp., submitted by Bayer AG.
Acute Rat Cholinesterase No-effect Study with Matacil Technical.
(1978b) Unpublished report from the Chemagro Agr. Div., Mobay Chemical
Corp., Submitted by Bayer AG.
New Zealand Report of the Codex Contact Point. (1978).
Noel, R.B., Mawdesley-Thomas, L.E. Chesterman, H., Clarke, E., and
Street, A.E. Bayer 44646 Chronic Oral Toxicity Study in Dogs. Final
Report. (1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Obrist, J.J. - Leaching characteristics of aged Matacil soil residues.
Chemagro report No. 65857, April 28 (1978), Unpublished.
Palmer, K.A. and Fletcher, M.A. - Effect of Bayer 44646 Upon
Reproduction of Multiple Rat Generations. Addendurn Histopathology.
(1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Root, M., Cowan, J. and Doull, J. - Subacute Oral Toxicity of Bayer
44646 to Male and Female Rats. (1963) Unpublished report from the
University of Chicago, submitted by Bayer AG.
Shaw, H. R. - Metabolism of Matacil in Swine. (1978) Unpublished
report from Mobay Chemical Corp., submitted by Bayer AG.
Silk, P.J., Semeluk, G.P. and Unger, I. - The photoreactions of
carbamate in insecticides. Phytoparasitica, 4, 51-63.
Stanley, C.W. and Delphia, L.M. - Gas-liquid chromatographic method
for detecting residues of Matacil in spruce needles, fish, soil and
water. Chemagro Report No. 66512, Sept. 15 (1978), Unpublished.
Sundaram, K.M.S. and Hopewell, W.W. - Fate and persistence of
aminocarb in conifer foliage and forest soil after simulated aerial
spray application. Canada Forest Pest Management Institute Report No.
FPM-x-6), October (1977a), Unpublished.
Penetration, translocation and fate of C-14 aminocarb in spruce
trees. Canadian Forestry Service Report CC-x-140 February (1977b),
Unpublished.
Sundaram, K.M.S., Volpe, Y. Smith G.G. and Duffy J.R. - A preliminary
study on the persistence and distribution of Matacil in a forest
environment. Canadian Forestry Service Report No. CC-X-116, January
(1976), Unpublished.
Sundaram, K.M.S., and Hindle, R. - Isolation and analysis of aminocarb
and its phenol from environmental waters. Canadian Forest Pest
Management Institute report. FPM-X-18 June (1978), Unpublished.
Tessier, J.F., Mulkey, W.S., Augenstein, L.L. and Wargo jr, J.P. -
14C Matacil buffer hydrolysis study. Chemagro Report No. 50444,
October 5 (1978), Unpublished.
Thornton, J.S., Hurley, J.B. and Obrist, J.J. -Soil thin-layer
mobility of twenty four pesticide chemicals. (Chemagro Report No.
57016, Dec. 15 (1976), Unpublished.
The half-life for the hydrolysis of aminocarb in pH 9.3 buffer was
reported to be 4 hours. On glass surfaces under fluorescent light at
25°C the rate of loss was approximately linear for the first few hours
with a half-life of 1.6 hours. In the period of 4 to 12 hours, after
application, there was a decrease in slope, indicative of conversion
to less volatile products on exposure to air and light (Abdel-Wahab
et al., 1966).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Miniature swine (Sus scrofa, 1 male and 1 female) received a
single oral dose of 0.5 mg/kg body weight 14C labelled aminocarb.
The compound was rapidly absorbed. The 14C peak value in blood was
reached within one hour. Excretion via the faeces did not exceed 2
percent, while 96% was excreted in the urine within 48 hours.
Elimination was rapid with approximately 75 percent observed in urine
within 6-24 hours.
The major urinary metabolites consisted of
conjugated-4-dimethylamino-, 4-methylamino-, and 4-
amino-3-methyl-phenol. Additionally, slight amounts (1-4%) of
aminocarb, its 4-formylamino-3-methyl phenol, and demethylation
products were observed. The same animals received 0.26 mg/kg body
weight of 14C-labelled aminocarb for five days and were sacrificed 45
minutes after the last dose. More than 90% of the daily dose was
excreted in the urine and with less than 2% in the faeces. Remarkable
amounts of 14C occurred in the kidney (0.7 ppm, expressed as
aminocarb) and liver (0.2 ppm) with conjugated
4-dimethyl-amino-3-methyl-phenol characterized as the main metabolite.
A similar amount of unidentified metabolite(s) was also observed.
Additionally, slight amounts (1-5%) of aminocarb, its demethylation
products, the formylamino derivative, and conjugated phenol-analogues
were determined. Slight quantities of 14C (0.02-0.09 ppm) were found
in other tissues (skin, brain, heart, muscles, and fat) with higher
values noted in the skin and fat (Shaw, 1978).
Bioaccumulation
Quail were fed 0, 10 and 50 ppm of 14C ring labelled aminocarb. The
14C content of liver, breast muscle, and mesenteric fat continuously
increased during the 14 days of treatment followed by continuous
elimination during a 14-day withdrawal period (Lamb, et al., 1976).
In channel catfish (Ictalurus punctatus) exposed to 10 ppb 14C
ring-labelled aminocarb, accumulation reached an equilibrium level
after one day. Elimination was rapid. A 50% reduction of residues
was noted in one day following a 28-day exposure. 14C was equally
distributed in the edible and non-edible parts of the fish (Lamb and
Roney, 1976).
A group of 9 male albino rats received a single dose of 25 mg/kg
aminocarb by gavage. The cholinesterase activity in the erythrocytes
was reduced by 50% after 1,3 and 5 hours. Recovery was rapid, being
complete within 24 hours (Kimmerle, 1961).
Special Studies on Cholinesterase Inhibition
Rat
Groups of male and female (Sprague-Dawley) rats (3 animals/group)
received oral doses of 0, 0.5, 2.0 and 8.0 mg/kg aminocarb by gavage
once a week for three weeks. Reduced cholinesterase activity was
noted at the highest dose (8.0 mg/kg) 1 and 3 hours after treatment
(approximately 10 to 20% in plasma and 30 to 40% in erythrocytes). In
the 2 mg/kg group, reduced erythrocyte cholinesterase activity was
observed after one hour. Twenty-four hours after dosing, all
inhibitory effects had disappeared in the blood. There was no
inhibition of brain cholinesterase activity noted in this study
(Nelson, 1978b).
A group of male albino rats which received 5 mg aminocarb/kg by gavage
for four weeks showed a 20-30% inhibition of erythrocyte
cholinesterase activity. There were no clinical signs of poisoning
associated with this dose level.
Groups of young adult female Sprague-Dawley rats (5 animals/group)
were administered aminocarb by intraperitoneal injection at daily dose
levels of 0, 2, 4, 8 and 10 mg/kg for 60 days. At dosage levels above
2 mg/kg, a decrease in body weight was noted. The animals
administered 4 and 8 mg/kg developed acute signs of poisoning which
persisted for one or two hours after dosing. All animals at the
highest dose level and one animal of the 8 mg/kg level failed to
survive the 60-day trial. One day after the last dose, cholinesterase
activity of brain, submaxillary gland and serum was measured and
inhibition data are represented in the following table:
% of inhibition at:
2 mg/kg 4mg/kg 8mg/kg
Brain 0 0 0
Submaxillary gland 11 24 34
Serum 29 44 53
(Dubois and Kinoshita, 1762).
TOXICOLOGICAL STUDIES
Special Studies or Reproduction
Four groups of 10 male and 20 female (Sprague Dawley) rats were fed
aminocarb in the diet at doses of 0, 100, 200 and 800 ppm. Treatment
started when the parents were approximately 75 days old and was
continued through a standard three generation reproduction study. All
generations showed a light hypersensitivity at 800 ppm. With the
exception of females of the F1b generation, retarded weight gain was
noted in all rats at 800 ppm. Weight gain was also retarded in the
females of the F0 and in males of the F1 and F2 generation at 200
ppm. Food consumption at the two higher dose levels was depressed in
a dose dependent manner. At birth, a reduced litter size and weight
was noted in the first mating of the F0, F1 and F2 generations at
800 ppm. At weaning, a reduced litter weight and mean pup weight for
all generations was seen at 800 ppm. At 200 ppm, a reduced pup weight
was observed at the first mating of the F0 generation. No
abnormalities were found as a result of aminocarb in the diet. The
young animals from the second litter of the third generation of rats
treated with 800 ppm did not show significant alteration in tissue
morphology associated with treatment. A no-effect level in this study
was 100 ppm (Palmer and Fletcher, 1966).
Special Studies on Neurotoxicity
In 20 hens over 9 months of age, which were orally administered
aminocarb (74 mg/kg) on day 0 and day 30, no signs of delayed
neurotoxicity were noted over the observation period of 30 days or in
the 30-day observation period following the second dosing.
Histopathologically, no alterations were observed in the nervous
tissue (Kruckenberg, 1978a).
In groups of 18-20 month old hens (3 animals/group) which were fed 0,
250, 500, 1000 and 2000 ppm aminocarb in the diet for 30 days and
thereafter observed for another four weeks, no delayed neurotoxic
effects were found. At doses of 500, 1000, and 2000 ppm, mortality
was observed as 2, 6 and 7 animals, respectively, died. At all dose
levels, blood cholinesterase was significantly inhibited and body
weight was reduced (Kimmerle, 1965a).
Acute Toxicity
Table 1. Acute-Toxicity of Aminocarb
Route of LD50
Species Sex administration (mg/kg) Reference
Mouse M IP 8 Dubois & Raymund, 1962a
Mouse F IP 9 Dubois & Raymund, 1962a
Mouse F IP 4.7 Abdel-Wahab & Casida,
1967
Mouse F Dermal 31 " "
Mouse F 1 hr exposure
2 um size) 4 mg/L Dilley & Doull, 1962
Rat M&F Oral 30 Dubois & Raymund, 1962a
Rat F Oral 22-27 Nelson, 1978a
Rat M Oral ca.50 Kimmerle, 1961
Table 1. Continued...
Route of LD50
Species Sex administration (mg/kg) Reference
Rat M IP 13 Dubois & Raymund, 1962a
Rat M IP 21 Kimmerle, 1961
Rat F IP 13.5 Dubois & Raymund, 1962a
Rat F Dermal 275 " "
Rat M Dermal <1000 Kimmerle, 1961
Rat F Inhalation
(1 hr exposure) 6 mg/L Dilley & Doull, 1962
Rat M&F Inhalation
(4 hr exposure) 0.2 mg/L Kimmerle, 1961; 1966
Guinea pig M Oral 60 Dubois de Raymund,1962a
Guinea pig M IP 30 " "
Chicken F Oral 74 Kruckenberg, 1978b
Chicken F Oral 75 Dubois, 1962
Chicken F Oral 50-100 Kimmerle, 1965b
Chicken F IP 25-50 Kimmerle, 1965b
In an acute inhalation toxicity test, groups of rats (20 males/group)
were exposed using a dynamic flow chamber into which aerosols at
concentrations of 0.1 and 1 mg/L aminocarb were continuously dispersed
for four hours. All animals at 1 mg/L and 4/20 animals at 0.1 mg/L
died within eight hours after exposure (Kimmerle, 1961; 1966).
In a subacute inhalation toxicity test with 20 male albino rats using
a dynamic flow chamber into which an aerosol with a concentration of
0.04 mg/L was dispersed eight hours per day for five consecutive days,
no specific signs of poisoning were observed. One animal died four
days after the end of the test (Kimmerle, 1961).
Signs of poisoning
Acutely toxic doses of aminocarb produce cholinergic effects typical
of cholinesterase inhibitors. The signs of poisoning appeared rapidly
after oral or intraperitoneal administration and were similar in all
species examined. After lethal doses, death occurred within two
hours, and usually within the first 30 minutes. After sublethal
doses, the symptoms subsided rapidly and apparent complete recovery
was usually noted within three hours (Dubois and Raymund, 1962a).
Special Studies on Potentiation
Potentiation of the acute toxicity of aminocarb was absent in female
(Sprague Dawley) rats when aminocarb was given by intraperitoneal
injection alone and in combination with 15 other anticholinesterase
insecticides (Dubois and Raymund, 1962b).
Short-Term Studies
Cat
In a cat which received 5 mg/kg aminocarb by gavage for ten
consecutive days, salivation, loss of appetite, and weight loss was
observed. Signs of cholinesterase inhibition were noted after two
days in 2 cats which received doses of 10 mg/kg; these animals died
after 4 days of treatment (Kimmerle, 1961).
Quail and Duck
Aminocarb and four of its major metabolites (THS 1013, THS 1003, THS
0995 and THS 1029) were fed to 15-day old bobwhite quail and 12-day
old mallard ducks at a dose of 1000 ppm for a period of 5 days.
Except for a reduction in feed consumption and reduced weight gain of
the mallard ducks, no toxic signs were observed (Lamb and Jones,
1975).
Rat
Groups of weanling (Sprague Dawley) rats (12 male and 12 female
rats/group) were fed 0, 5, 10 and 50 ppm aminocarb in the diet for 16
weeks. No effects on cholinesterase activity, growth, food
consumption, gross organ weight, or on histological examination of
tissues and organs were found in this study. A similar study was
started with dietary levels of 0, 100 and 200 ppm. As these levels
also failed to induce a significant inhibition of cholinesterase in
the first 3 weeks, the dietary levels were increased to 400 and 800
ppm, and were maintained for a further 19 weeks. The duration of this
study was 22 weeks. Over the course of the study, many of the animals
appeared to be hyperexcited and irritable. At the end of the study, a
decrease in growth rate was noted in males (14%) and females (13%) fed
800 ppm and in males (13%) and females (10%) fed 400 ppm.
Cholinesterase activity was measured on five animals/sex/group. Data
at the conclusion of the study revealed an inhibition in the serum of
males (16%) and females (42%) fed 800 ppm, and in the serum of females
(31%) fed 400 ppm. No inhibition was found in the brain, submaxillary
glands and erythrocytes. No effects were observed with respect to
organ weights and histopathological examination of tissues and organs
(Root, et al., 1963).
Table 2. Acute Toxicity of Aminocarb Metabolites
LD50
Compound Species Sex Route (mg/kg) Reference
4-(N'formyl-N'-methylamino- Mouse F IP 21 Abdel-Wahab & Casida, 1967
3-methylphenyl-N-methylcarbamate Mouse F Dermal >500 " "
4-(methylamino)-3-methyl
phenyl-N-methylcarbamate Mouse F IP 3.0 " "
Mouse F Dermal 52 " "
Rat F Oral 27.8(22.9-33.7) Kimmerle, 1974
4-formylamino-3-methylPhenyl- Mouse F IP 13 Abdel-Wahab & Casida, 1967
N-methylcarbamate
Mouse F Dermal 500 " "
4-amino-3-methylphenyl-N- Mouse F IP 1.6 " "
methylcarbamate Mouse F Dermal 17 " "
Rat F Oral 18.3(16.2-20.7) Kimmerle, 1974
4-(N'-methoxy-N'-methylamino)- Rat F Oral 102.8(88.5-119.4) Kimmerle, 1974
3-methylphenyl-N-methyl
carbamate
4-methoxyamino-3-methylphenyl- Rat F oral 40.1(33.7-47.5) Kimmerle, 1974
N-methylcarbamate
Dog
Groups of two male and two female beagle dogs were fed 0, 200, 400 and
800 ppm aminocarb in the diet for 12 weeks. At the end of this
feeding period, the dogs were returned to the control diet for an
additional 4-week period. Weight loss was observed in the animals of
the 800 ppm and 400 ppm groups. Signs of poisoning (e.g. vomiting,
retching, ataxia and incoordination) were dose-related and were found
in all dose groups, especially in the female animals. Signs of
poisoning were particularly prevalent during and just after
consumption of the aminocarb diet and were minimal or absent during
the remaining part of the day. There were no signs of poisoning noted
in the 4-week recovery period. Blood samples, taken prior to feeding
when symptoms were minimal or absent showed no inhibition of the
cholinesterase activity, of the serum or erythrocytes (Doull and Root,
1963).
Groups of beagle dogs (4 male and 4 female dogs/group) were orally
dosed by gavage at dosage levels of 0, 2, 4 and 10 mg/kg/day (0, 1, 2
and 5 mg/kg twice a day) for two years. After 12 weeks, one dog in
the highest dose group died. Moderately severe signs of poisoning,
(e.g., general excitability, salivation, pupil constriction and fine
visible trembling of muscles) were seen in all dogs in the high dose
group. Less severe signs of poisoning were noted in dogs at 4 and 2
mg/kg/day. The highest dose group showed a slight reduction in body
weight and a reduced rate of growth. No inhibition of cholinesterase
activity in serum and erythrocytes was noted, nor were changes in
hematology and blood chemistry found, except with the one dog that
died. Brain cholinesterase activity was not measured. Samples for
serum and erythrocyte cholinesterase were taken prior to daily dosing
in the first 20 months of the study and in the last 4 months of the
study, within 45 minutes of dosing.
At the conclusion of the study, adrenal weight, as a percentage of
body weight only, was increased in the high dose level group. Other
organ and organ weight ratios were within normal limits.
Histopathologically, macrophages containing lipofucsin materials were
seen throughout the liver of all groups, possibly more frequently in
the high dose animals. In the other tissues, no compound-related
effects were seen histologically (Noel, et al., 1966).
Long-Term Studies
Rat
Groups of weanling Sprague-Dawley rats (24 males and 24 females/group)
were fed 0, 100, 200, 400 and 800 ppm aminocarb in the diet for 20
months. Growth was reduced in males and females at 800 ppm, and to a
lesser extent, at all lower dose levels. The median survival time and
food consumption were not affected by aminocarb. Final examinations
of 5 animals/sex/group did not reveal significant effects on
cholinesterase activity in the brain, serum, and erythrocytes, whereas
a slight inhibition (approximately 20%) was noted in the submaxillary
glands of males and females at 800 ppm. The relative weight of the
heart was slightly increased in all dose groups and in microscopic
examination showed cardiac lesions in both males and females at 800
ppm and in males at 400 ppm. Reduced spermato-genesis was observed in
some animals of the two highest dose groups. Liver changes were also
noted at dose levels of 200 ppm and above (Doull, et al., 1967).
COMMENTS
Aminocarb, an N-methyl carbamate ester, is rapidly absorbed and
metabolized by demethylation and/or hydroxylation or ester hydrolysis.
Those metabolites containing an intact carbamate ester structure show
acute toxicity similar to or greater than the parent compound.
Aminocarb and its metabolites are rapidly excreted and there is no
evidence of accumulation. In acute toxicity studies, aminocarb was
not potentiated by other cholinesterase inhibitors, nor did it induce
a delayed neurotoxic response in hens.
In several short- and long-term studies in the rat, aminocarb induced
a reversible cholinesterase inhibition. In a 2-year dog study,
clinical symptoms of poisoning were seen in all dose levels tested.
In a 3-generation study with rats, no effects were observed at dose
levels of 100 ppm or below.
The meeting concluded that the short- and long-term rat studies were
not carried out in accordance with currently acceptable protocols. As
there was growth depression and an increased relative weight of the
heart at all dose levels tested in the long-term rat study, a
no-effect level in the rat could not be determined. An acceptable
daily intake for man was not allocated. The meeting recommended that
aminocarb be re-examined when the ongoing long-term study in rodents
becomes available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Aminocarb has been used mainly for the control of lepidopterous
defoliators of Canadian forests where it has been found notably
effective against the spruce budworm, Choristoneura fumiferana
(Clemens). About 3.7 million hectares of Canadian forest have been
treated by aircraft at an average dosage of 0.07 kg ai/ha. 167 g/l
oil-soluble concentrate is used.
Aminocarb is also effective for the control of lepidopterous larvae
and other biting insects in cotton, tomatoes, tobacco and fruit crops,
but usage in these crops appears to be low. For these purposes, 50
and 75% wettable formulations are used. In New Zealand, the
insecticide was, but is no longer used widely against the coddling
moth, leaf roller and mealy bug. It is applied at 0.75-1.12 kg ai/100
L. In stone fruit and citrus, it is effective against the leaf
roller, mealy bug and scales, where it is applied at 75-94 g ai/100 L.
Aminocarb is also effective against caterpillars in vegetables and is
applied at 0.75-1.5 kg ai/ha (New Zealand, 1978).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Very few residue data on food crops are available because of the
limited usage of aminocarb in these crops. The manufacturer provided
the data available for apples and pears.
Studies carried out in Australia with apples involved the application
of aminocarb at 0.075, 0.1 and 0.125% solutions, equivalent to 3/4, 1,
and 1 1/4 lbs./100 gals, respectively, to give a spray coverage of
13-18 L/tree. The recommended preharvest interval of three days
justify a limit of 4 mg/kg (Table 3). The data for pears (Table 4)
also adequately support a 4 mg/kg limit at the three days preharvest
interval.
Table 3. Aminocarb Residues in Apples
Application rates Residue levels (mg/kg)
(% Solution) Period in days
3 10 17 24
0.075 2.8 1.3 1.6 0.6
0.1 2.2 1.0 1.6 1.4
0.125 2.6 1.4 1.8 1.0
Table 4. Aminocarb Residues in Pears
Residue levels (mg/kg)
Application rate Period in days
(% solution) 0 7 13 15 21
(23-27 L/tree)
0.1 1 0.8 n.d. - ND
0.1 3.0 1.0 0.4 - 0.4
0.1 1.3 - - n.d. 0.4
(23-27 L/tree)
0.1 1.4 - - n.d. 0.4
(23-27 L/tree
0.125 1.9 - - 0.4 0.4
(23-27 L/tree)
0.075 1.4 - - n.d. n.d.
FATE OF RESIDUES
General Comments
Most of the data on aminocarb were generated in connection with its
use in the control of forest pests. Aminocarb was degraded rapidly in
soil, water, plants, animals and by ultraviolet irradiation (Chemagro,
1976). The major route of breakdown in the presence of light or on
surfaces was oxidation of the dimethylamino portion of the molecule to
produce traces of 4-(formamidomethylamino)-, 4-(formamidoamino)- and
4-amino-3-methylphenol. These products are not detected in animals or
within plant tissues. In plants, hydroxylation followed by
conjugation is the main degradation pathway. In soil, N-demethylation
is predominant. In swine, conjugates of aminocarb phenol and the
methylamino analogue of aminocarb phenol were the major metabolites
found in males and females respectively. The principal tissue
constituent was conjugated aminocarb phenol.
Under normal use conditions, aminocarb was found at about 0.01 mg/kg
in soil and water, and about 2 mg/kg on foliage. Half-lives under
normal use conditions ranged from less than one day in soil to about
six days on spruce foliage. Maguire (1973) reviewed the chemistry of
aminocarb and the effect on the environment has been summarized
(Chemagro, 1976, 1979). The metabolism of aminocarb in different
substrates is shown in Figure 1.
In animals
(See Biochemical Aspects). In an ascorbic acid system which simulates
biological oxidation processes, Balba and Saha (1974) found at least
12 products formed from aminocarb. At least 10 of these products
contained either one or both the N-14CH3 groups. The ascorbic acid
system caused demethylation, hydroxylation of the aromatic ring,
oxidation of the -NHCH3, group to -NHCH20N, and cleavage of the
carbamate to the phenol. The 4-methylamino derivative was the major
degradation product.
In Plants
Abdel-Wahab et al., (1966) found that aminocarb was degraded with
the carbamate moiety intact when the carbamate was applied to glass or
silica gel surfaces or the leaves of growing bean plants, or injected
into the stems of bean plants. The methylcarbamate derivatives formed
included the 4-methylamino, 4-amino, 4-methylformamido, and
4-formamido analogues. The C14-carbonyl activity was found in the
unextractable portion six days after injection into bean plants. The
water-soluble metabolites formed following injection into the bean
plants result in part from hydroxylation of the carbamate on the
N-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates, mainly as glycosides (Kuhr
and Casida, 1967). These glycosides were quite persistent.
Dorough (1964) determined the stability of aminocarb applied as a leaf
surface treatment to cotton, garden snap beans, broccoli and tomatoes.
The metabolic fates of directly injected and surface-applied
carbonyl-C14 aminocarb appeared to be the same. The original
carbamate was converted into water-soluble metabolites which were
probably conjugates. Conversion was almost quantitative and the
metabolites were persistent. Leaf surface residues appear to be
degraded via hydrolysis at the carbamate group before or during
penetration. Penetration of aminocarb through the leaf surface of
beans and cotton was about eight percent after eight hours, although
50% of the residues was already lost from the surface (Dorough, 1979).
On the other hand, broccoli plants did not contain any internal
residues. Aminocarb was metabolized to persistent water-soluble
metabolites in these plants.
The fate and persistence of aminocarb in spruce foliage was
investigated by Sundaram and Hopewell (1977a) using a simulated spray
at the rate of 3.4 L/ha containing 57 g ai. The initial concentration
in foliage was about 10 mg/kg and dropped to less than 0.2 mg/kg
within 47 days. The half-life was six days. Aminocarb was found to
be labile and was dissipated rapidly under normal weathering
conditions.
In a similar study, Nagel et al. (1978) was not able to detect
volatilization of radioactivity with ring -1-C14 aminocarb.
Aminocarb residues declined from an initial level of 6.7 mg/kg to 3.3
mg/kg in 28 days. They attributed the decline to growth dilution.
The methylamino analogue was the only identified metabolite. Although
several unidentified metabolites were detected, no single component
contributed more than 10% of the total radioactivity.
The actual situations where aminocarb was applied serially at 70 g
ai/ha, the concentration in spruce foliage at 0.6 days was 0.7 mg/kg,
increased to a maximum of 2.2 mg/kg after four days and thereafter
decreases exponentially with a half-life of 5.6 days (Sundaram et
al., 1976). No residues could be detected after 64 days, presumably
owing to physical factors.
The penetration, translocation and fate of C14 aminocarb in spruce
trees was also investigated using trunk implantation (TIT), foliar
painting (FP), and basal bark painting (BBP) (Sundaram and Hopewell
1977b). Aminocarb appears to be weakly systemic. The aminocarb
absorbed after trunk implantation was gradually lost after 64 days,
probably owing to hydroxylation to water-soluble metabolites, some of
which were incorporated into the cellular structure of the foliage.
The major route of translocation was from the old to the
newly-developing foliage. In the case of foliar painting, basal bark
painting techniques, the mechanism of dissipation appeared to be
physical rather than biochemical.
In soil
In a comparative study of the persistence of aminocarb in silt loam
and sandy loam soil, Murphy et al, (1975) using
carbonyl-C14-aminocarb, found that breakdown was slower and more
soil-bound radioactivity was detected in the latter. In silt loam,
less than 15 percent of the applied insecticide was still present 24
hours after application. Microbial activity was essential for rapid
metabolism and C14102 was the principal product. Some of the
liberated C14102 was assimilated into the normal, insoluble soil
constituents. Minor (1978) also found that the insecticide was
degraded through removal of the carbamate portion by hydrolysis
followed by soil binding.
Aminocarb could be readily absorbed from aqueous solutions by loam
soil (Atwell, 1978). Although it is ranked as intermediate in soil
mobility using the soil thin layer system (Thornton et al, 1976)
leaching studies with sandy loam soil columns showed aminocarb to be
essentially retained in the upper 2.5 cm of soil. Less than 0.1% of
the radiolabel used was found in the leaching water after passing
through the soil column.
When aminocarb was applied to sandy loam soil at 1 mg/kg, the
half-life was less than 24 hours (Minor, 1978). The only identified
compounds were aminocarb and its N-demethylated analogue. Within 24
hours, 75% of the radioactivity was bound. Microbial activity was
necessary for degradation. In a pond water/soil study, aminocarb had
a half-life of 3.5 days and the metabolites identified were the
N-demethylated analogues and the phenolic hydrolysis product of
aminocarb. Formation of soil-bound residues was observed.
Sundaram and Hopewell (1977a) found that with simulated aerial
application, the initial residue in soil was 7 mg/kg. The half-life
was two days and residues could no longer be detected after 27 days.
In Water
In buffered solutions, aminocarb stability increased as the
temperature and pH decreased. At pH 4, its half-life was >127 days,
at pH 7, it varied between five and eleven days, while at pH 9, it was
<1 day (Tessier et al., 1978). Murphy et al. (1975) observed a
half-life of 28.5 days at pH 7 and 28.5 days in pond water under
normal environmental conditions. The principal metabolite appeared to
be the phenol although the methylamino and formylamino analogues were
also observed. (Tessier et al., 1978; Murphy et al., 1975; Minor,
1978).
Forest aerial application at 70 g ai/ha resulted in 2.1 and 1.9 mg/kg
residues in pond and stream water respectively (Sundaram et al.,
1976). Half-lives were 4.4 and 8.7 days, respectively, in the above
substrates. Residues were below the 0.1 mg/kg limits of analytical
sensitivity within 32 days.
In a pilot study, application of the insecticide at 2.4 lb ai/acre
resulted in residues ranging from <0.1 to 0.2 mg/kg 48 hours after
application. These levels were generally higher than found after 24
hours (Chemagro, 1976).
Photodecomposition
Irradiation of aminocarb in aerated and degassed ethanol and
cyclohexane at >300 pm gave the phenol as the major product (Addison
et al., 1974). Trace quantities of other products were also
observed. On the other hand, Abdel-Wahab and Casida (1967) found that
in bean foliage, photolysis involved extensive oxidation of the
dimethylamino moiety but not of other groups. There was a stepwise
demethylation of the dimethylamino moiety and one of the methyl
radicals was oxidized to the formamido group. The same result was
obtained earlier by Abdel-Wahab et al (1967). Aminocarb was not
readily decomposed when exposed as spots on the thin layer plates in
the dark, in fluorescent light or in long-wavelength UV light. Short
wavelength UV-light or sunlight resulted in considerable degradation.
Crosby et al. (1965) observed at least two compounds with
acetylcholinesterase inhibitory effects of many decomposition products
upon exposure to sunlight and ultraviolet radiation.
In aqueous buffer at pH 4.9, aminocarb had a half-life of 10 days when
irradiated with a mercury lamp. The methylamino analogue and two
unknown products amounting to 10% and 12% of the total radioactivity,
were detected in the solution after 30 days exposure. In an
acetone-sensitized solution, the half-life was reduced to 1.5 days
(Mulkey et al., 1978).
When ring -C14 aminocarb applied to a soil surface was subjected to
light from a high intensity mercury lamp, the calculated half-life was
4.6 hours (Augenstein, 1978). Approximately 33% of the applied
radioactivity was volatilized and 50% was bound to the soil during 192
hours of exposure to the light. The methylamino, methylformylamino,
formylamino and amino analogues of aminocarb as well as the
formylamino analogue of the phenol were detected. Unidentified
products accounted for <10% of the applied radioactivity.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
In New Zealand, six samples of apples in 1971 showed residues ranging
from 0.06 to 2.1 mg/kg with a mean of 0.86 mg/kg.
METHODS OF RESIDUE ANALYSIS
The analysis of carbamate insecticides by the popular GLC technique
has been an on-going problem for two reasons: 1) the thermal
instability of some compounds and 2) the lack of a sensitive detector
for the underivatized material. Several GLC derivatization procedures
using the sensitive electron capture detector have been tried. For
aminocarb, Sundaram et al. (1976) initially tried forming the
N-hepta-fluorobutyryl derivative. The procedure had an unsatisfactory
minimum detection limit (MDL) of 0.5 mg/kg in foliar extracts because
of impurities with retention times similar to aminocarb. Stanley and
Delphia (1978) subsequently used the 2,4-dinitrophenyl ether
derivatization technique of Holden (1973) and obtained a MDL of <0.01
mg/kg for spruce needles, soil and fish and <0.001 mg/kg for water.
GLC analysis of aminocarb as the intact molecule detected by such
nitrogen-"specific" detectors as the alkali flame ionization (AFID)
and the Hall microelectrolytic conductivity detectors appear
promising. The direct GLC procedures generally require only minimum
cleanup because they are less subject to sample interferences. Bayer
(1975) used the AFID to detect 0.1 mg/kg aminocarb on plant materials
and 0.02 mg/kg in water. Sundaram and Hopewell (1977) using the Hall
detector, found an MDL of 0.2 mg/kg for spruce foliage and soil. In
water, Sundaram et al. (1978) achieved an MDL of 1 × 10-4 mg/kg for
both aminocarb and its phenol.
High-pressure liquid chromatography (HPLC) has been applied by
Laurence (1977) to the detection of aminocarb in cabbage, corn, potato
and wheat. A UV detector was used at 254 nm. As little as 0.8 ng of
insecticide can be detected in the above crops.
Olson (1964) described a colorimetric procedure for residue
determination in soil, after the reaction of the phenol with
4-aminoantipyrine. The procedure can detect as little as 0.1 mg/kg
aminocarb and recovery was 90% for soils. It is also applicable to
crops.
In effect, the determination of the intact carbamate offers
possibilities as a regulatory method, especially with improvements in
the detection limits of the nitrogen specific detectors. The Hall
detector is particularly useful. If greater sensitivity is needed,
the 2,4-dinitrophenyl ether derivative can be used. Adoption of HPLC
for regulatory purpose would have to await further improvements in the
detection system and widespread use of the technique. The
4-aminoantipyrine procedure can be used for spectrophotometry.
NATIONAL MRLs REPORTED TO THE MEETING
According to the information supplied to the meeting, only the
following countries have established limits for aminocarb in food:
Limit Preharvest
Country Commodity established Interval
(mg/kg) (days)
Australia apples, pears 4 3
cottonseed 1
vegetables, fruit
(except apples, pears) 1
Germany pome fruit 1
(FRG)
APPRAISAL
Aminocarb is mainly used for the control of lepidopterous defoliators
in conifer forests where it is applied by air. There is only limited
usage in other crops. For aerial application, a 1.4 lb ai/US gal.
oil-soluble concentrate formulation is used. For general usage, 50
and 75% wettable powder formulations are available.
In view of the limited usage in food crops, very few residue trials
have been done to establish MRLs. Data on apples and pears adequately
support MRLs of 5 and 2 mg/kg, respectively.
Aminocarb is degraded rapidly in soil, water, plants, animals and when
exposed to short-wave ultraviolet radiation. In plants, metabolism
involves hydroxylation of the molecule at the carbamate on the
1,1-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates mainly as glycosides.
Conjugates of aminocarb phenol and the methylamino analogue of
aminocarb phenol were the major metabolites in swine. The principal
tissues constituent was conjugated aminocarb phenol. In soil,
N-demethylation predominate. Under normal use conditions, half-lives
of aminocarb ranged from less than one day in soil to about six days
on spruce foliage. Detection by gas chromatography as the intact
carbamate or detection as the 2,4-dinitrophenyl ether derivative
appear adequate as regulatory methods; the latter can detect as low as
0.01 mg/kg aminocarb in spruce needles, soil and fish and 0.001
aminocarb in water.
RECOMMENDATIONS
Since the main use of aminocarb is in forest pest control, the
likelihood of its occurrence in food items for international trade is
generally low. However, the setting of MRLs are considered necessary
in some cases. In the absence of an ADI, the following guideline
levels are recorded for aminocarb:
Commodity GL
mg/kg
apples 5
pears 2
FURTHER WORK OR INFORMATION
If the usage of aminocarb in food items should increase, additional
information should be provided.
REFERENCES
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. - Fate of
C14-carbonyl-labelled aryl methylcarbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14., 290-298.
Abdel-Waheb, A.M. and Casida, J.E. - Photo-oxidation of Two
4-Dimethylaminoaryl methylcarbamate Insecticides (Zectram and Matacil)
on Bean Foliage and of Alkylaminophenyl Methylcarbamates on Silica Gel
Chromatoplates. J. Agr. Food Chem. 15: 479-487.
Addison, J.B., Silk, P.J. and Unger, I. - The photochemical reactions
of carbamates II. The solution photo chemistry of Matacil
(4-dimethylamino m-tolyl-N-methyl (carbamate) and Landrin
(3,4,5-trimethylphenyl-N-methyl carbamate). Bull. Environmental
Contam. Toxicol. 11, 250-255.
Atwell, S.M. Soil absorption and desorption of C14-Natacil. Chemagro
Report No. 55424 July 12 (1973), Unpublished.
Augenstein, L.L., McPhaul, L. and Wargo, J.P., Jr. - Photodegradation
of 14C Matacil on a soil surface. Cemagro Report No. 50446, October
20 (1973), Unpublished.
Balba, M.M. and Saha, J.G. - Degradation of Matacil by the ascorbic
acid oxidation system. Bull. Environ. Contam. Toxicol. 11, 193-200.
Bayer, A.G. Gas Chromatographische bestimmung von
aminocarbruckstanden in boden und wasser. Oct. 28 (1975), Unpublished.
Bayer, A. G. Data submitted to the 1979 JMPR
Chemagro. Matacil - The effect on the environment. Chemagro Report,
April 17 (1976), Unpublished.
Coburn, J.A., Ripley, B.D. and Chau, A.S.Y. - Analysis of Pesticide
residues by chemical derivatization. II. N-methylcarbamates in natural
water and soils. J. Assoc. Off. Anal. Chem. 59, 188-196.
Crosby, D.G., Leitis, E and. Winterlin, W. L. - Photodecomposition of
carbamate insecticides. J. Agr. Food Chem. 13, 204-207.
Dilley, J. and Doull, J. - The Acute Inhalation Toxicity of Bayer
44646 to Rats and Mice. (1962) Unpublished report from the University
of Chicago, submitted by Bayer, AG.
Dorough, H.W. Fate of Bayer 44646 in several plant species. Progress
Report, Texas A & M University, Sept. 1 (1964), Unpublished.
Fate of Matacil in several plant species. Progress Report, Texas A &
M University, July (1970), Unpublished.
Dorough, H.W. and Thorstenson, J.H. - Analysis of carbamate
insecticides and metabolites J. Chromatogr. Sci. 13, 212-224.
Doull, J. and Root, M. - Subacute Oral Toxicity of Bayer 44646 in Male
and Female Dogs. (1963) Unpublished report from the University of
Chicago, submitted by Bayer AG.
Doull, J., Root, M. and Keskauskas, J. - Chronic Oral Toxicity of
Bayer 44646 to Rat. (Addendum: Hibbs, C., and Nelson, D.L. Microscopic
Findings in Tissues of Male and Female Rats Fed Bayer 44646 for Two
years). (1967) Unpublished report from the University of Chicago,
submitted by Bayer AG.
Dubois, K.P. The Acute Oral Toxicity of Bayer 44646 to Chickens.
(1962) Unpublished report from the University of Chicago, submitted by
Bayer AG.
Dubois, K.P. and Raymund, A.B. - Acute Toxicity of Bayer 44646 to
Mammals. (1962a) Unpublished report, from the University of Chicago,
submitted by Bayer AG.
The Acute Toxicity of Bayer 44646 in Combination with other
Anticholinesterase Insecticides. (1962b) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Dubois, K.P. and Kinoshita, F. - The Subacute Parenteral Toxicity of
Bayer 44646 to Female Rats. (1962) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Holden, E.R. Gas Chromatographic determination of residues of
methylcarbamate insecticides in crops as their 2,4-dinitrophenyl ether
derivatives. J. Assoc. Off. Anal. Chem. 56, 713-717.
Kimmerle, G., Wirkstoff Dr. Heib A. 363 (E 44646). (1961) Unpublished
report from Bayer AG, Tox. Gew. Hyg. Labor, submitted by Bayer AG.
Neurotoxische Untersuchungen mit A 363-Wirkstoff. (1965a) Unpublished
report from the Institut für Toxikologie, submitted by Bayer AG.
Wirkstoff A 363 als Formulierung F1 604/149 Acute
Neurotoxizitätsprüfung bei Hühnern. (1965b) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Matacil-Wirkstoff. (1966) Unpublished report from the Institut für
Toxikologie, submitted by Bayer AG.
3-Methyl-4-methoxymethylaminophenyl-N-monomethy9lcarbamat,
3-methyl-4-monomethoxyaminophenyl-N-monomethylcarbamat,
3-methyl-4-monomethyl-aminophenyl-N-monomethyl-carbamat, und
3-methyl-4-aminophenyl-N monomethylcarbamat. Akate Akute Toxizität bei
Ratten. (1974) Unpublished report from the Institut Für Toxikologie,
submitted by Bayer AG.
Kuhr, R.J. and Casida, J.E. - Persistent glycosides of metabolites of
methylcarbamate insecticide chemicals formed by hydroxylation in bean
plants. J. Agr. Food Chem. 15, 814-824.
Kruckenberg, S.M. - Delayed Neurotoxicity Study of Matacil in Hens.
(1978a) Unpublished report from Kansas State University, by Bayer AG.
Acute Oral Toxicity of Matacil in Chickens. (1978b) Unpublished
report from Kansas State University, submitted by Bayer AG.
Lamb, D. W. and Jones, R.E. - Dietary Toxicity of Matacil Technical
and 4-Matacil Metabolites to Bobwhite Quail and Mallard Ducks. (1975)
Unpublished report from Chemagro Agr. Div., Mobay Chemical Corp.,
submitted by Bayer AG.
Lamb, D.W. and Roney, D.J. - Accumulation and Persistence of Residues
in Channel Catfish exposed to Matacil-14C. (1976) Unpublished report
from Mobay Chemical Corp., submitted by Bayer AG.
Lamb, D.W. et al. Accumulation and Elimination of Residues in
Bobwhite Quail Exposed to Matacil-14C. (1976) Unpublished report from
Mobay Chemical Corp., submitted by Bayer AG.
Lawrence, J.F. Direct analysis of some carbamate pesticides in food
by high pressure liquid chromatography. J. Agr. Food Chem. 25,
211-212.
Maguire, R.J. Aminocarb: A review of its chemistry. Manuscript
submitted for publication by the Canadian Forestry Service. (1978).
Minor, R.G. Metabolism of Matacil in soil and in an aquatic
environment. Chemagro Report No. 50427, October 15, 1978, Unpublished.
Mobay, Matacil - the effect on the environment, Addition no. 1 to
Chemagro Report 1976, January 14 (Unpublished).
Mulkey, N.S., McPhaul, L., Augenstein, L.L. and Wargo, J.P. Jr.
Photodegradation of 14C Matacil in aqueous solution. Chemagro report
No. 50447, October 18 (1978), Unpublished.
Murphy, J.J., Jacobs, K. and Minor, R.G. - Stability of Matacil in
aqueous systems. Chemagro Report No. 43450. January 20 (1975a),
Unpublished.
Murphy, J.J., Minor, R.G., Jacobs, K. and Shaif II, H.R. - Persistence
of Matacil in soil. Chemagro Report No. 43444, January 31 (1975b),
Unpublished.
Nagel, C.D., Tessier, J.F., McPhaul, L., Augenstein, L.L. and Wargo,
Jr. J.P. - Spruce foliage metabolism study with 14C Matacil. Chemagro
report No. 50445, October 16 (1978), Unpublished.
Nelson, D.L. - Acute Oral Toxicity of Matacil Technical and Matacil
Analytical Grade to Female Rats. (1973a) Unpublished report from
Chemagro Agr. Div., Mobay Chemical Corp., submitted by Bayer AG.
Acute Rat Cholinesterase No-effect Study with Matacil Technical.
(1978b) Unpublished report from the Chemagro Agr. Div., Mobay Chemical
Corp., Submitted by Bayer AG.
New Zealand Report of the Codex Contact Point. (1978).
Noel, R.B., Mawdesley-Thomas, L.E. Chesterman, H., Clarke, E., and
Street, A.E. Bayer 44646 Chronic Oral Toxicity Study in Dogs. Final
Report. (1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Obrist, J.J. - Leaching characteristics of aged Matacil soil residues.
Chemagro report No. 65857, April 28 (1978), Unpublished.
Palmer, K.A. and Fletcher, M.A. - Effect of Bayer 44646 Upon
Reproduction of Multiple Rat Generations. Addendurn Histopathology.
(1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Root, M., Cowan, J. and Doull, J. - Subacute Oral Toxicity of Bayer
44646 to Male and Female Rats. (1963) Unpublished report from the
University of Chicago, submitted by Bayer AG.
Shaw, H. R. - Metabolism of Matacil in Swine. (1978) Unpublished
report from Mobay Chemical Corp., submitted by Bayer AG.
Silk, P.J., Semeluk, G.P. and Unger, I. - The photoreactions of
carbamate in insecticides. Phytoparasitica, 4, 51-63.
Stanley, C.W. and Delphia, L.M. - Gas-liquid chromatographic method
for detecting residues of Matacil in spruce needles, fish, soil and
water. Chemagro Report No. 66512, Sept. 15 (1978), Unpublished.
Sundaram, K.M.S. and Hopewell, W.W. - Fate and persistence of
aminocarb in conifer foliage and forest soil after simulated aerial
spray application. Canada Forest Pest Management Institute Report No.
FPM-x-6), October (1977a), Unpublished.
Penetration, translocation and fate of C-14 aminocarb in spruce
trees. Canadian Forestry Service Report CC-x-140 February (1977b),
Unpublished.
Sundaram, K.M.S., Volpe, Y. Smith G.G. and Duffy J.R. - A preliminary
study on the persistence and distribution of Matacil in a forest
environment. Canadian Forestry Service Report No. CC-X-116, January
(1976), Unpublished.
Sundaram, K.M.S., and Hindle, R. - Isolation and analysis of aminocarb
and its phenol from environmental waters. Canadian Forest Pest
Management Institute report. FPM-X-18 June (1978), Unpublished.
Tessier, J.F., Mulkey, W.S., Augenstein, L.L. and Wargo jr, J.P. -
14C Matacil buffer hydrolysis study. Chemagro Report No. 50444,
October 5 (1978), Unpublished.
Thornton, J.S., Hurley, J.B. and Obrist, J.J. -Soil thin-layer
mobility of twenty four pesticide chemicals. (Chemagro Report No.
57016, Dec. 15 (1976), Unpublished.
See Also:
Toxicological Abbreviations
Aminocarb (ICSC)
Aminocarb (Pesticide residues in food: 1978 evaluations)
 Empirical Formula: C11H16N2O2
Other Information on Identity and Properties:
Description: White, crystalline solid
M.P. 93-94°C
V.P. Non-volatile
Solubility: Slightly soluble in water; moderately soluble
in aromatic solvents; soluble in polar organic solvents
Stability: Unstable in alkaline media.
Empirical Formula: C11H16N2O2
Other Information on Identity and Properties:
Description: White, crystalline solid
M.P. 93-94°C
V.P. Non-volatile
Solubility: Slightly soluble in water; moderately soluble
in aromatic solvents; soluble in polar organic solvents
Stability: Unstable in alkaline media.
 The half-life for the hydrolysis of aminocarb in pH 9.3 buffer was
reported to be 4 hours. On glass surfaces under fluorescent light at
25°C the rate of loss was approximately linear for the first few hours
with a half-life of 1.6 hours. In the period of 4 to 12 hours, after
application, there was a decrease in slope, indicative of conversion
to less volatile products on exposure to air and light (Abdel-Wahab
et al., 1966).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Miniature swine (Sus scrofa, 1 male and 1 female) received a
single oral dose of 0.5 mg/kg body weight 14C labelled aminocarb.
The compound was rapidly absorbed. The 14C peak value in blood was
reached within one hour. Excretion via the faeces did not exceed 2
percent, while 96% was excreted in the urine within 48 hours.
Elimination was rapid with approximately 75 percent observed in urine
within 6-24 hours.
The major urinary metabolites consisted of
conjugated-4-dimethylamino-, 4-methylamino-, and 4-
amino-3-methyl-phenol. Additionally, slight amounts (1-4%) of
aminocarb, its 4-formylamino-3-methyl phenol, and demethylation
products were observed. The same animals received 0.26 mg/kg body
weight of 14C-labelled aminocarb for five days and were sacrificed 45
minutes after the last dose. More than 90% of the daily dose was
excreted in the urine and with less than 2% in the faeces. Remarkable
amounts of 14C occurred in the kidney (0.7 ppm, expressed as
aminocarb) and liver (0.2 ppm) with conjugated
4-dimethyl-amino-3-methyl-phenol characterized as the main metabolite.
A similar amount of unidentified metabolite(s) was also observed.
Additionally, slight amounts (1-5%) of aminocarb, its demethylation
products, the formylamino derivative, and conjugated phenol-analogues
were determined. Slight quantities of 14C (0.02-0.09 ppm) were found
in other tissues (skin, brain, heart, muscles, and fat) with higher
values noted in the skin and fat (Shaw, 1978).
Bioaccumulation
Quail were fed 0, 10 and 50 ppm of 14C ring labelled aminocarb. The
14C content of liver, breast muscle, and mesenteric fat continuously
increased during the 14 days of treatment followed by continuous
elimination during a 14-day withdrawal period (Lamb, et al., 1976).
In channel catfish (Ictalurus punctatus) exposed to 10 ppb 14C
ring-labelled aminocarb, accumulation reached an equilibrium level
after one day. Elimination was rapid. A 50% reduction of residues
was noted in one day following a 28-day exposure. 14C was equally
distributed in the edible and non-edible parts of the fish (Lamb and
Roney, 1976).
A group of 9 male albino rats received a single dose of 25 mg/kg
aminocarb by gavage. The cholinesterase activity in the erythrocytes
was reduced by 50% after 1,3 and 5 hours. Recovery was rapid, being
complete within 24 hours (Kimmerle, 1961).
Special Studies on Cholinesterase Inhibition
Rat
Groups of male and female (Sprague-Dawley) rats (3 animals/group)
received oral doses of 0, 0.5, 2.0 and 8.0 mg/kg aminocarb by gavage
once a week for three weeks. Reduced cholinesterase activity was
noted at the highest dose (8.0 mg/kg) 1 and 3 hours after treatment
(approximately 10 to 20% in plasma and 30 to 40% in erythrocytes). In
the 2 mg/kg group, reduced erythrocyte cholinesterase activity was
observed after one hour. Twenty-four hours after dosing, all
inhibitory effects had disappeared in the blood. There was no
inhibition of brain cholinesterase activity noted in this study
(Nelson, 1978b).
A group of male albino rats which received 5 mg aminocarb/kg by gavage
for four weeks showed a 20-30% inhibition of erythrocyte
cholinesterase activity. There were no clinical signs of poisoning
associated with this dose level.
Groups of young adult female Sprague-Dawley rats (5 animals/group)
were administered aminocarb by intraperitoneal injection at daily dose
levels of 0, 2, 4, 8 and 10 mg/kg for 60 days. At dosage levels above
2 mg/kg, a decrease in body weight was noted. The animals
administered 4 and 8 mg/kg developed acute signs of poisoning which
persisted for one or two hours after dosing. All animals at the
highest dose level and one animal of the 8 mg/kg level failed to
survive the 60-day trial. One day after the last dose, cholinesterase
activity of brain, submaxillary gland and serum was measured and
inhibition data are represented in the following table:
% of inhibition at:
2 mg/kg 4mg/kg 8mg/kg
Brain 0 0 0
Submaxillary gland 11 24 34
Serum 29 44 53
(Dubois and Kinoshita, 1762).
TOXICOLOGICAL STUDIES
Special Studies or Reproduction
Four groups of 10 male and 20 female (Sprague Dawley) rats were fed
aminocarb in the diet at doses of 0, 100, 200 and 800 ppm. Treatment
started when the parents were approximately 75 days old and was
continued through a standard three generation reproduction study. All
generations showed a light hypersensitivity at 800 ppm. With the
exception of females of the F1b generation, retarded weight gain was
noted in all rats at 800 ppm. Weight gain was also retarded in the
females of the F0 and in males of the F1 and F2 generation at 200
ppm. Food consumption at the two higher dose levels was depressed in
a dose dependent manner. At birth, a reduced litter size and weight
was noted in the first mating of the F0, F1 and F2 generations at
800 ppm. At weaning, a reduced litter weight and mean pup weight for
all generations was seen at 800 ppm. At 200 ppm, a reduced pup weight
was observed at the first mating of the F0 generation. No
abnormalities were found as a result of aminocarb in the diet. The
young animals from the second litter of the third generation of rats
treated with 800 ppm did not show significant alteration in tissue
morphology associated with treatment. A no-effect level in this study
was 100 ppm (Palmer and Fletcher, 1966).
Special Studies on Neurotoxicity
In 20 hens over 9 months of age, which were orally administered
aminocarb (74 mg/kg) on day 0 and day 30, no signs of delayed
neurotoxicity were noted over the observation period of 30 days or in
the 30-day observation period following the second dosing.
Histopathologically, no alterations were observed in the nervous
tissue (Kruckenberg, 1978a).
In groups of 18-20 month old hens (3 animals/group) which were fed 0,
250, 500, 1000 and 2000 ppm aminocarb in the diet for 30 days and
thereafter observed for another four weeks, no delayed neurotoxic
effects were found. At doses of 500, 1000, and 2000 ppm, mortality
was observed as 2, 6 and 7 animals, respectively, died. At all dose
levels, blood cholinesterase was significantly inhibited and body
weight was reduced (Kimmerle, 1965a).
Acute Toxicity
Table 1. Acute-Toxicity of Aminocarb
Route of LD50
Species Sex administration (mg/kg) Reference
Mouse M IP 8 Dubois & Raymund, 1962a
Mouse F IP 9 Dubois & Raymund, 1962a
Mouse F IP 4.7 Abdel-Wahab & Casida,
1967
Mouse F Dermal 31 " "
Mouse F 1 hr exposure
2 um size) 4 mg/L Dilley & Doull, 1962
Rat M&F Oral 30 Dubois & Raymund, 1962a
Rat F Oral 22-27 Nelson, 1978a
Rat M Oral ca.50 Kimmerle, 1961
Table 1. Continued...
Route of LD50
Species Sex administration (mg/kg) Reference
Rat M IP 13 Dubois & Raymund, 1962a
Rat M IP 21 Kimmerle, 1961
Rat F IP 13.5 Dubois & Raymund, 1962a
Rat F Dermal 275 " "
Rat M Dermal <1000 Kimmerle, 1961
Rat F Inhalation
(1 hr exposure) 6 mg/L Dilley & Doull, 1962
Rat M&F Inhalation
(4 hr exposure) 0.2 mg/L Kimmerle, 1961; 1966
Guinea pig M Oral 60 Dubois de Raymund,1962a
Guinea pig M IP 30 " "
Chicken F Oral 74 Kruckenberg, 1978b
Chicken F Oral 75 Dubois, 1962
Chicken F Oral 50-100 Kimmerle, 1965b
Chicken F IP 25-50 Kimmerle, 1965b
In an acute inhalation toxicity test, groups of rats (20 males/group)
were exposed using a dynamic flow chamber into which aerosols at
concentrations of 0.1 and 1 mg/L aminocarb were continuously dispersed
for four hours. All animals at 1 mg/L and 4/20 animals at 0.1 mg/L
died within eight hours after exposure (Kimmerle, 1961; 1966).
In a subacute inhalation toxicity test with 20 male albino rats using
a dynamic flow chamber into which an aerosol with a concentration of
0.04 mg/L was dispersed eight hours per day for five consecutive days,
no specific signs of poisoning were observed. One animal died four
days after the end of the test (Kimmerle, 1961).
Signs of poisoning
Acutely toxic doses of aminocarb produce cholinergic effects typical
of cholinesterase inhibitors. The signs of poisoning appeared rapidly
after oral or intraperitoneal administration and were similar in all
species examined. After lethal doses, death occurred within two
hours, and usually within the first 30 minutes. After sublethal
doses, the symptoms subsided rapidly and apparent complete recovery
was usually noted within three hours (Dubois and Raymund, 1962a).
Special Studies on Potentiation
Potentiation of the acute toxicity of aminocarb was absent in female
(Sprague Dawley) rats when aminocarb was given by intraperitoneal
injection alone and in combination with 15 other anticholinesterase
insecticides (Dubois and Raymund, 1962b).
Short-Term Studies
Cat
In a cat which received 5 mg/kg aminocarb by gavage for ten
consecutive days, salivation, loss of appetite, and weight loss was
observed. Signs of cholinesterase inhibition were noted after two
days in 2 cats which received doses of 10 mg/kg; these animals died
after 4 days of treatment (Kimmerle, 1961).
Quail and Duck
Aminocarb and four of its major metabolites (THS 1013, THS 1003, THS
0995 and THS 1029) were fed to 15-day old bobwhite quail and 12-day
old mallard ducks at a dose of 1000 ppm for a period of 5 days.
Except for a reduction in feed consumption and reduced weight gain of
the mallard ducks, no toxic signs were observed (Lamb and Jones,
1975).
Rat
Groups of weanling (Sprague Dawley) rats (12 male and 12 female
rats/group) were fed 0, 5, 10 and 50 ppm aminocarb in the diet for 16
weeks. No effects on cholinesterase activity, growth, food
consumption, gross organ weight, or on histological examination of
tissues and organs were found in this study. A similar study was
started with dietary levels of 0, 100 and 200 ppm. As these levels
also failed to induce a significant inhibition of cholinesterase in
the first 3 weeks, the dietary levels were increased to 400 and 800
ppm, and were maintained for a further 19 weeks. The duration of this
study was 22 weeks. Over the course of the study, many of the animals
appeared to be hyperexcited and irritable. At the end of the study, a
decrease in growth rate was noted in males (14%) and females (13%) fed
800 ppm and in males (13%) and females (10%) fed 400 ppm.
Cholinesterase activity was measured on five animals/sex/group. Data
at the conclusion of the study revealed an inhibition in the serum of
males (16%) and females (42%) fed 800 ppm, and in the serum of females
(31%) fed 400 ppm. No inhibition was found in the brain, submaxillary
glands and erythrocytes. No effects were observed with respect to
organ weights and histopathological examination of tissues and organs
(Root, et al., 1963).
Table 2. Acute Toxicity of Aminocarb Metabolites
LD50
Compound Species Sex Route (mg/kg) Reference
4-(N'formyl-N'-methylamino- Mouse F IP 21 Abdel-Wahab & Casida, 1967
3-methylphenyl-N-methylcarbamate Mouse F Dermal >500 " "
4-(methylamino)-3-methyl
phenyl-N-methylcarbamate Mouse F IP 3.0 " "
Mouse F Dermal 52 " "
Rat F Oral 27.8(22.9-33.7) Kimmerle, 1974
4-formylamino-3-methylPhenyl- Mouse F IP 13 Abdel-Wahab & Casida, 1967
N-methylcarbamate
Mouse F Dermal 500 " "
4-amino-3-methylphenyl-N- Mouse F IP 1.6 " "
methylcarbamate Mouse F Dermal 17 " "
Rat F Oral 18.3(16.2-20.7) Kimmerle, 1974
4-(N'-methoxy-N'-methylamino)- Rat F Oral 102.8(88.5-119.4) Kimmerle, 1974
3-methylphenyl-N-methyl
carbamate
4-methoxyamino-3-methylphenyl- Rat F oral 40.1(33.7-47.5) Kimmerle, 1974
N-methylcarbamate
Dog
Groups of two male and two female beagle dogs were fed 0, 200, 400 and
800 ppm aminocarb in the diet for 12 weeks. At the end of this
feeding period, the dogs were returned to the control diet for an
additional 4-week period. Weight loss was observed in the animals of
the 800 ppm and 400 ppm groups. Signs of poisoning (e.g. vomiting,
retching, ataxia and incoordination) were dose-related and were found
in all dose groups, especially in the female animals. Signs of
poisoning were particularly prevalent during and just after
consumption of the aminocarb diet and were minimal or absent during
the remaining part of the day. There were no signs of poisoning noted
in the 4-week recovery period. Blood samples, taken prior to feeding
when symptoms were minimal or absent showed no inhibition of the
cholinesterase activity, of the serum or erythrocytes (Doull and Root,
1963).
Groups of beagle dogs (4 male and 4 female dogs/group) were orally
dosed by gavage at dosage levels of 0, 2, 4 and 10 mg/kg/day (0, 1, 2
and 5 mg/kg twice a day) for two years. After 12 weeks, one dog in
the highest dose group died. Moderately severe signs of poisoning,
(e.g., general excitability, salivation, pupil constriction and fine
visible trembling of muscles) were seen in all dogs in the high dose
group. Less severe signs of poisoning were noted in dogs at 4 and 2
mg/kg/day. The highest dose group showed a slight reduction in body
weight and a reduced rate of growth. No inhibition of cholinesterase
activity in serum and erythrocytes was noted, nor were changes in
hematology and blood chemistry found, except with the one dog that
died. Brain cholinesterase activity was not measured. Samples for
serum and erythrocyte cholinesterase were taken prior to daily dosing
in the first 20 months of the study and in the last 4 months of the
study, within 45 minutes of dosing.
At the conclusion of the study, adrenal weight, as a percentage of
body weight only, was increased in the high dose level group. Other
organ and organ weight ratios were within normal limits.
Histopathologically, macrophages containing lipofucsin materials were
seen throughout the liver of all groups, possibly more frequently in
the high dose animals. In the other tissues, no compound-related
effects were seen histologically (Noel, et al., 1966).
Long-Term Studies
Rat
Groups of weanling Sprague-Dawley rats (24 males and 24 females/group)
were fed 0, 100, 200, 400 and 800 ppm aminocarb in the diet for 20
months. Growth was reduced in males and females at 800 ppm, and to a
lesser extent, at all lower dose levels. The median survival time and
food consumption were not affected by aminocarb. Final examinations
of 5 animals/sex/group did not reveal significant effects on
cholinesterase activity in the brain, serum, and erythrocytes, whereas
a slight inhibition (approximately 20%) was noted in the submaxillary
glands of males and females at 800 ppm. The relative weight of the
heart was slightly increased in all dose groups and in microscopic
examination showed cardiac lesions in both males and females at 800
ppm and in males at 400 ppm. Reduced spermato-genesis was observed in
some animals of the two highest dose groups. Liver changes were also
noted at dose levels of 200 ppm and above (Doull, et al., 1967).
COMMENTS
Aminocarb, an N-methyl carbamate ester, is rapidly absorbed and
metabolized by demethylation and/or hydroxylation or ester hydrolysis.
Those metabolites containing an intact carbamate ester structure show
acute toxicity similar to or greater than the parent compound.
Aminocarb and its metabolites are rapidly excreted and there is no
evidence of accumulation. In acute toxicity studies, aminocarb was
not potentiated by other cholinesterase inhibitors, nor did it induce
a delayed neurotoxic response in hens.
In several short- and long-term studies in the rat, aminocarb induced
a reversible cholinesterase inhibition. In a 2-year dog study,
clinical symptoms of poisoning were seen in all dose levels tested.
In a 3-generation study with rats, no effects were observed at dose
levels of 100 ppm or below.
The meeting concluded that the short- and long-term rat studies were
not carried out in accordance with currently acceptable protocols. As
there was growth depression and an increased relative weight of the
heart at all dose levels tested in the long-term rat study, a
no-effect level in the rat could not be determined. An acceptable
daily intake for man was not allocated. The meeting recommended that
aminocarb be re-examined when the ongoing long-term study in rodents
becomes available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Aminocarb has been used mainly for the control of lepidopterous
defoliators of Canadian forests where it has been found notably
effective against the spruce budworm, Choristoneura fumiferana
(Clemens). About 3.7 million hectares of Canadian forest have been
treated by aircraft at an average dosage of 0.07 kg ai/ha. 167 g/l
oil-soluble concentrate is used.
Aminocarb is also effective for the control of lepidopterous larvae
and other biting insects in cotton, tomatoes, tobacco and fruit crops,
but usage in these crops appears to be low. For these purposes, 50
and 75% wettable formulations are used. In New Zealand, the
insecticide was, but is no longer used widely against the coddling
moth, leaf roller and mealy bug. It is applied at 0.75-1.12 kg ai/100
L. In stone fruit and citrus, it is effective against the leaf
roller, mealy bug and scales, where it is applied at 75-94 g ai/100 L.
Aminocarb is also effective against caterpillars in vegetables and is
applied at 0.75-1.5 kg ai/ha (New Zealand, 1978).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Very few residue data on food crops are available because of the
limited usage of aminocarb in these crops. The manufacturer provided
the data available for apples and pears.
Studies carried out in Australia with apples involved the application
of aminocarb at 0.075, 0.1 and 0.125% solutions, equivalent to 3/4, 1,
and 1 1/4 lbs./100 gals, respectively, to give a spray coverage of
13-18 L/tree. The recommended preharvest interval of three days
justify a limit of 4 mg/kg (Table 3). The data for pears (Table 4)
also adequately support a 4 mg/kg limit at the three days preharvest
interval.
Table 3. Aminocarb Residues in Apples
Application rates Residue levels (mg/kg)
(% Solution) Period in days
3 10 17 24
0.075 2.8 1.3 1.6 0.6
0.1 2.2 1.0 1.6 1.4
0.125 2.6 1.4 1.8 1.0
Table 4. Aminocarb Residues in Pears
Residue levels (mg/kg)
Application rate Period in days
(% solution) 0 7 13 15 21
(23-27 L/tree)
0.1 1 0.8 n.d. - ND
0.1 3.0 1.0 0.4 - 0.4
0.1 1.3 - - n.d. 0.4
(23-27 L/tree)
0.1 1.4 - - n.d. 0.4
(23-27 L/tree
0.125 1.9 - - 0.4 0.4
(23-27 L/tree)
0.075 1.4 - - n.d. n.d.
FATE OF RESIDUES
General Comments
Most of the data on aminocarb were generated in connection with its
use in the control of forest pests. Aminocarb was degraded rapidly in
soil, water, plants, animals and by ultraviolet irradiation (Chemagro,
1976). The major route of breakdown in the presence of light or on
surfaces was oxidation of the dimethylamino portion of the molecule to
produce traces of 4-(formamidomethylamino)-, 4-(formamidoamino)- and
4-amino-3-methylphenol. These products are not detected in animals or
within plant tissues. In plants, hydroxylation followed by
conjugation is the main degradation pathway. In soil, N-demethylation
is predominant. In swine, conjugates of aminocarb phenol and the
methylamino analogue of aminocarb phenol were the major metabolites
found in males and females respectively. The principal tissue
constituent was conjugated aminocarb phenol.
Under normal use conditions, aminocarb was found at about 0.01 mg/kg
in soil and water, and about 2 mg/kg on foliage. Half-lives under
normal use conditions ranged from less than one day in soil to about
six days on spruce foliage. Maguire (1973) reviewed the chemistry of
aminocarb and the effect on the environment has been summarized
(Chemagro, 1976, 1979). The metabolism of aminocarb in different
substrates is shown in Figure 1.
In animals
(See Biochemical Aspects). In an ascorbic acid system which simulates
biological oxidation processes, Balba and Saha (1974) found at least
12 products formed from aminocarb. At least 10 of these products
contained either one or both the N-14CH3 groups. The ascorbic acid
system caused demethylation, hydroxylation of the aromatic ring,
oxidation of the -NHCH3, group to -NHCH20N, and cleavage of the
carbamate to the phenol. The 4-methylamino derivative was the major
degradation product.
In Plants
Abdel-Wahab et al., (1966) found that aminocarb was degraded with
the carbamate moiety intact when the carbamate was applied to glass or
silica gel surfaces or the leaves of growing bean plants, or injected
into the stems of bean plants. The methylcarbamate derivatives formed
included the 4-methylamino, 4-amino, 4-methylformamido, and
4-formamido analogues. The C14-carbonyl activity was found in the
unextractable portion six days after injection into bean plants. The
water-soluble metabolites formed following injection into the bean
plants result in part from hydroxylation of the carbamate on the
N-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates, mainly as glycosides (Kuhr
and Casida, 1967). These glycosides were quite persistent.
Dorough (1964) determined the stability of aminocarb applied as a leaf
surface treatment to cotton, garden snap beans, broccoli and tomatoes.
The metabolic fates of directly injected and surface-applied
carbonyl-C14 aminocarb appeared to be the same. The original
carbamate was converted into water-soluble metabolites which were
probably conjugates. Conversion was almost quantitative and the
metabolites were persistent. Leaf surface residues appear to be
degraded via hydrolysis at the carbamate group before or during
penetration. Penetration of aminocarb through the leaf surface of
beans and cotton was about eight percent after eight hours, although
50% of the residues was already lost from the surface (Dorough, 1979).
On the other hand, broccoli plants did not contain any internal
residues. Aminocarb was metabolized to persistent water-soluble
metabolites in these plants.
The fate and persistence of aminocarb in spruce foliage was
investigated by Sundaram and Hopewell (1977a) using a simulated spray
at the rate of 3.4 L/ha containing 57 g ai. The initial concentration
in foliage was about 10 mg/kg and dropped to less than 0.2 mg/kg
within 47 days. The half-life was six days. Aminocarb was found to
be labile and was dissipated rapidly under normal weathering
conditions.
In a similar study, Nagel et al. (1978) was not able to detect
volatilization of radioactivity with ring -1-C14 aminocarb.
Aminocarb residues declined from an initial level of 6.7 mg/kg to 3.3
mg/kg in 28 days. They attributed the decline to growth dilution.
The methylamino analogue was the only identified metabolite. Although
several unidentified metabolites were detected, no single component
contributed more than 10% of the total radioactivity.
The actual situations where aminocarb was applied serially at 70 g
ai/ha, the concentration in spruce foliage at 0.6 days was 0.7 mg/kg,
increased to a maximum of 2.2 mg/kg after four days and thereafter
decreases exponentially with a half-life of 5.6 days (Sundaram et
al., 1976). No residues could be detected after 64 days, presumably
owing to physical factors.
The penetration, translocation and fate of C14 aminocarb in spruce
trees was also investigated using trunk implantation (TIT), foliar
painting (FP), and basal bark painting (BBP) (Sundaram and Hopewell
1977b). Aminocarb appears to be weakly systemic. The aminocarb
absorbed after trunk implantation was gradually lost after 64 days,
probably owing to hydroxylation to water-soluble metabolites, some of
which were incorporated into the cellular structure of the foliage.
The major route of translocation was from the old to the
newly-developing foliage. In the case of foliar painting, basal bark
painting techniques, the mechanism of dissipation appeared to be
physical rather than biochemical.
In soil
In a comparative study of the persistence of aminocarb in silt loam
and sandy loam soil, Murphy et al, (1975) using
carbonyl-C14-aminocarb, found that breakdown was slower and more
soil-bound radioactivity was detected in the latter. In silt loam,
less than 15 percent of the applied insecticide was still present 24
hours after application. Microbial activity was essential for rapid
metabolism and C14102 was the principal product. Some of the
liberated C14102 was assimilated into the normal, insoluble soil
constituents. Minor (1978) also found that the insecticide was
degraded through removal of the carbamate portion by hydrolysis
followed by soil binding.
Aminocarb could be readily absorbed from aqueous solutions by loam
soil (Atwell, 1978). Although it is ranked as intermediate in soil
mobility using the soil thin layer system (Thornton et al, 1976)
leaching studies with sandy loam soil columns showed aminocarb to be
essentially retained in the upper 2.5 cm of soil. Less than 0.1% of
the radiolabel used was found in the leaching water after passing
through the soil column.
When aminocarb was applied to sandy loam soil at 1 mg/kg, the
half-life was less than 24 hours (Minor, 1978). The only identified
compounds were aminocarb and its N-demethylated analogue. Within 24
hours, 75% of the radioactivity was bound. Microbial activity was
necessary for degradation. In a pond water/soil study, aminocarb had
a half-life of 3.5 days and the metabolites identified were the
N-demethylated analogues and the phenolic hydrolysis product of
aminocarb. Formation of soil-bound residues was observed.
Sundaram and Hopewell (1977a) found that with simulated aerial
application, the initial residue in soil was 7 mg/kg. The half-life
was two days and residues could no longer be detected after 27 days.
In Water
In buffered solutions, aminocarb stability increased as the
temperature and pH decreased. At pH 4, its half-life was >127 days,
at pH 7, it varied between five and eleven days, while at pH 9, it was
<1 day (Tessier et al., 1978). Murphy et al. (1975) observed a
half-life of 28.5 days at pH 7 and 28.5 days in pond water under
normal environmental conditions. The principal metabolite appeared to
be the phenol although the methylamino and formylamino analogues were
also observed. (Tessier et al., 1978; Murphy et al., 1975; Minor,
1978).
Forest aerial application at 70 g ai/ha resulted in 2.1 and 1.9 mg/kg
residues in pond and stream water respectively (Sundaram et al.,
1976). Half-lives were 4.4 and 8.7 days, respectively, in the above
substrates. Residues were below the 0.1 mg/kg limits of analytical
sensitivity within 32 days.
In a pilot study, application of the insecticide at 2.4 lb ai/acre
resulted in residues ranging from <0.1 to 0.2 mg/kg 48 hours after
application. These levels were generally higher than found after 24
hours (Chemagro, 1976).
Photodecomposition
Irradiation of aminocarb in aerated and degassed ethanol and
cyclohexane at >300 pm gave the phenol as the major product (Addison
et al., 1974). Trace quantities of other products were also
observed. On the other hand, Abdel-Wahab and Casida (1967) found that
in bean foliage, photolysis involved extensive oxidation of the
dimethylamino moiety but not of other groups. There was a stepwise
demethylation of the dimethylamino moiety and one of the methyl
radicals was oxidized to the formamido group. The same result was
obtained earlier by Abdel-Wahab et al (1967). Aminocarb was not
readily decomposed when exposed as spots on the thin layer plates in
the dark, in fluorescent light or in long-wavelength UV light. Short
wavelength UV-light or sunlight resulted in considerable degradation.
Crosby et al. (1965) observed at least two compounds with
acetylcholinesterase inhibitory effects of many decomposition products
upon exposure to sunlight and ultraviolet radiation.
In aqueous buffer at pH 4.9, aminocarb had a half-life of 10 days when
irradiated with a mercury lamp. The methylamino analogue and two
unknown products amounting to 10% and 12% of the total radioactivity,
were detected in the solution after 30 days exposure. In an
acetone-sensitized solution, the half-life was reduced to 1.5 days
(Mulkey et al., 1978).
When ring -C14 aminocarb applied to a soil surface was subjected to
light from a high intensity mercury lamp, the calculated half-life was
4.6 hours (Augenstein, 1978). Approximately 33% of the applied
radioactivity was volatilized and 50% was bound to the soil during 192
hours of exposure to the light. The methylamino, methylformylamino,
formylamino and amino analogues of aminocarb as well as the
formylamino analogue of the phenol were detected. Unidentified
products accounted for <10% of the applied radioactivity.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
In New Zealand, six samples of apples in 1971 showed residues ranging
from 0.06 to 2.1 mg/kg with a mean of 0.86 mg/kg.
METHODS OF RESIDUE ANALYSIS
The analysis of carbamate insecticides by the popular GLC technique
has been an on-going problem for two reasons: 1) the thermal
instability of some compounds and 2) the lack of a sensitive detector
for the underivatized material. Several GLC derivatization procedures
using the sensitive electron capture detector have been tried. For
aminocarb, Sundaram et al. (1976) initially tried forming the
N-hepta-fluorobutyryl derivative. The procedure had an unsatisfactory
minimum detection limit (MDL) of 0.5 mg/kg in foliar extracts because
of impurities with retention times similar to aminocarb. Stanley and
Delphia (1978) subsequently used the 2,4-dinitrophenyl ether
derivatization technique of Holden (1973) and obtained a MDL of <0.01
mg/kg for spruce needles, soil and fish and <0.001 mg/kg for water.
GLC analysis of aminocarb as the intact molecule detected by such
nitrogen-"specific" detectors as the alkali flame ionization (AFID)
and the Hall microelectrolytic conductivity detectors appear
promising. The direct GLC procedures generally require only minimum
cleanup because they are less subject to sample interferences. Bayer
(1975) used the AFID to detect 0.1 mg/kg aminocarb on plant materials
and 0.02 mg/kg in water. Sundaram and Hopewell (1977) using the Hall
detector, found an MDL of 0.2 mg/kg for spruce foliage and soil. In
water, Sundaram et al. (1978) achieved an MDL of 1 × 10-4 mg/kg for
both aminocarb and its phenol.
High-pressure liquid chromatography (HPLC) has been applied by
Laurence (1977) to the detection of aminocarb in cabbage, corn, potato
and wheat. A UV detector was used at 254 nm. As little as 0.8 ng of
insecticide can be detected in the above crops.
Olson (1964) described a colorimetric procedure for residue
determination in soil, after the reaction of the phenol with
4-aminoantipyrine. The procedure can detect as little as 0.1 mg/kg
aminocarb and recovery was 90% for soils. It is also applicable to
crops.
In effect, the determination of the intact carbamate offers
possibilities as a regulatory method, especially with improvements in
the detection limits of the nitrogen specific detectors. The Hall
detector is particularly useful. If greater sensitivity is needed,
the 2,4-dinitrophenyl ether derivative can be used. Adoption of HPLC
for regulatory purpose would have to await further improvements in the
detection system and widespread use of the technique. The
4-aminoantipyrine procedure can be used for spectrophotometry.
NATIONAL MRLs REPORTED TO THE MEETING
According to the information supplied to the meeting, only the
following countries have established limits for aminocarb in food:
Limit Preharvest
Country Commodity established Interval
(mg/kg) (days)
Australia apples, pears 4 3
cottonseed 1
vegetables, fruit
(except apples, pears) 1
Germany pome fruit 1
(FRG)
APPRAISAL
Aminocarb is mainly used for the control of lepidopterous defoliators
in conifer forests where it is applied by air. There is only limited
usage in other crops. For aerial application, a 1.4 lb ai/US gal.
oil-soluble concentrate formulation is used. For general usage, 50
and 75% wettable powder formulations are available.
In view of the limited usage in food crops, very few residue trials
have been done to establish MRLs. Data on apples and pears adequately
support MRLs of 5 and 2 mg/kg, respectively.
Aminocarb is degraded rapidly in soil, water, plants, animals and when
exposed to short-wave ultraviolet radiation. In plants, metabolism
involves hydroxylation of the molecule at the carbamate on the
1,1-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates mainly as glycosides.
Conjugates of aminocarb phenol and the methylamino analogue of
aminocarb phenol were the major metabolites in swine. The principal
tissues constituent was conjugated aminocarb phenol. In soil,
N-demethylation predominate. Under normal use conditions, half-lives
of aminocarb ranged from less than one day in soil to about six days
on spruce foliage. Detection by gas chromatography as the intact
carbamate or detection as the 2,4-dinitrophenyl ether derivative
appear adequate as regulatory methods; the latter can detect as low as
0.01 mg/kg aminocarb in spruce needles, soil and fish and 0.001
aminocarb in water.
RECOMMENDATIONS
Since the main use of aminocarb is in forest pest control, the
likelihood of its occurrence in food items for international trade is
generally low. However, the setting of MRLs are considered necessary
in some cases. In the absence of an ADI, the following guideline
levels are recorded for aminocarb:
Commodity GL
mg/kg
apples 5
pears 2
FURTHER WORK OR INFORMATION
If the usage of aminocarb in food items should increase, additional
information should be provided.
REFERENCES
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. - Fate of
C14-carbonyl-labelled aryl methylcarbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14., 290-298.
Abdel-Waheb, A.M. and Casida, J.E. - Photo-oxidation of Two
4-Dimethylaminoaryl methylcarbamate Insecticides (Zectram and Matacil)
on Bean Foliage and of Alkylaminophenyl Methylcarbamates on Silica Gel
Chromatoplates. J. Agr. Food Chem. 15: 479-487.
Addison, J.B., Silk, P.J. and Unger, I. - The photochemical reactions
of carbamates II. The solution photo chemistry of Matacil
(4-dimethylamino m-tolyl-N-methyl (carbamate) and Landrin
(3,4,5-trimethylphenyl-N-methyl carbamate). Bull. Environmental
Contam. Toxicol. 11, 250-255.
Atwell, S.M. Soil absorption and desorption of C14-Natacil. Chemagro
Report No. 55424 July 12 (1973), Unpublished.
Augenstein, L.L., McPhaul, L. and Wargo, J.P., Jr. - Photodegradation
of 14C Matacil on a soil surface. Cemagro Report No. 50446, October
20 (1973), Unpublished.
Balba, M.M. and Saha, J.G. - Degradation of Matacil by the ascorbic
acid oxidation system. Bull. Environ. Contam. Toxicol. 11, 193-200.
Bayer, A.G. Gas Chromatographische bestimmung von
aminocarbruckstanden in boden und wasser. Oct. 28 (1975), Unpublished.
Bayer, A. G. Data submitted to the 1979 JMPR
Chemagro. Matacil - The effect on the environment. Chemagro Report,
April 17 (1976), Unpublished.
Coburn, J.A., Ripley, B.D. and Chau, A.S.Y. - Analysis of Pesticide
residues by chemical derivatization. II. N-methylcarbamates in natural
water and soils. J. Assoc. Off. Anal. Chem. 59, 188-196.
Crosby, D.G., Leitis, E and. Winterlin, W. L. - Photodecomposition of
carbamate insecticides. J. Agr. Food Chem. 13, 204-207.
Dilley, J. and Doull, J. - The Acute Inhalation Toxicity of Bayer
44646 to Rats and Mice. (1962) Unpublished report from the University
of Chicago, submitted by Bayer, AG.
Dorough, H.W. Fate of Bayer 44646 in several plant species. Progress
Report, Texas A & M University, Sept. 1 (1964), Unpublished.
Fate of Matacil in several plant species. Progress Report, Texas A &
M University, July (1970), Unpublished.
Dorough, H.W. and Thorstenson, J.H. - Analysis of carbamate
insecticides and metabolites J. Chromatogr. Sci. 13, 212-224.
Doull, J. and Root, M. - Subacute Oral Toxicity of Bayer 44646 in Male
and Female Dogs. (1963) Unpublished report from the University of
Chicago, submitted by Bayer AG.
Doull, J., Root, M. and Keskauskas, J. - Chronic Oral Toxicity of
Bayer 44646 to Rat. (Addendum: Hibbs, C., and Nelson, D.L. Microscopic
Findings in Tissues of Male and Female Rats Fed Bayer 44646 for Two
years). (1967) Unpublished report from the University of Chicago,
submitted by Bayer AG.
Dubois, K.P. The Acute Oral Toxicity of Bayer 44646 to Chickens.
(1962) Unpublished report from the University of Chicago, submitted by
Bayer AG.
Dubois, K.P. and Raymund, A.B. - Acute Toxicity of Bayer 44646 to
Mammals. (1962a) Unpublished report, from the University of Chicago,
submitted by Bayer AG.
The Acute Toxicity of Bayer 44646 in Combination with other
Anticholinesterase Insecticides. (1962b) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Dubois, K.P. and Kinoshita, F. - The Subacute Parenteral Toxicity of
Bayer 44646 to Female Rats. (1962) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Holden, E.R. Gas Chromatographic determination of residues of
methylcarbamate insecticides in crops as their 2,4-dinitrophenyl ether
derivatives. J. Assoc. Off. Anal. Chem. 56, 713-717.
Kimmerle, G., Wirkstoff Dr. Heib A. 363 (E 44646). (1961) Unpublished
report from Bayer AG, Tox. Gew. Hyg. Labor, submitted by Bayer AG.
Neurotoxische Untersuchungen mit A 363-Wirkstoff. (1965a) Unpublished
report from the Institut für Toxikologie, submitted by Bayer AG.
Wirkstoff A 363 als Formulierung F1 604/149 Acute
Neurotoxizitätsprüfung bei Hühnern. (1965b) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Matacil-Wirkstoff. (1966) Unpublished report from the Institut für
Toxikologie, submitted by Bayer AG.
3-Methyl-4-methoxymethylaminophenyl-N-monomethy9lcarbamat,
3-methyl-4-monomethoxyaminophenyl-N-monomethylcarbamat,
3-methyl-4-monomethyl-aminophenyl-N-monomethyl-carbamat, und
3-methyl-4-aminophenyl-N monomethylcarbamat. Akate Akute Toxizität bei
Ratten. (1974) Unpublished report from the Institut Für Toxikologie,
submitted by Bayer AG.
Kuhr, R.J. and Casida, J.E. - Persistent glycosides of metabolites of
methylcarbamate insecticide chemicals formed by hydroxylation in bean
plants. J. Agr. Food Chem. 15, 814-824.
Kruckenberg, S.M. - Delayed Neurotoxicity Study of Matacil in Hens.
(1978a) Unpublished report from Kansas State University, by Bayer AG.
Acute Oral Toxicity of Matacil in Chickens. (1978b) Unpublished
report from Kansas State University, submitted by Bayer AG.
Lamb, D. W. and Jones, R.E. - Dietary Toxicity of Matacil Technical
and 4-Matacil Metabolites to Bobwhite Quail and Mallard Ducks. (1975)
Unpublished report from Chemagro Agr. Div., Mobay Chemical Corp.,
submitted by Bayer AG.
Lamb, D.W. and Roney, D.J. - Accumulation and Persistence of Residues
in Channel Catfish exposed to Matacil-14C. (1976) Unpublished report
from Mobay Chemical Corp., submitted by Bayer AG.
Lamb, D.W. et al. Accumulation and Elimination of Residues in
Bobwhite Quail Exposed to Matacil-14C. (1976) Unpublished report from
Mobay Chemical Corp., submitted by Bayer AG.
Lawrence, J.F. Direct analysis of some carbamate pesticides in food
by high pressure liquid chromatography. J. Agr. Food Chem. 25,
211-212.
Maguire, R.J. Aminocarb: A review of its chemistry. Manuscript
submitted for publication by the Canadian Forestry Service. (1978).
Minor, R.G. Metabolism of Matacil in soil and in an aquatic
environment. Chemagro Report No. 50427, October 15, 1978, Unpublished.
Mobay, Matacil - the effect on the environment, Addition no. 1 to
Chemagro Report 1976, January 14 (Unpublished).
Mulkey, N.S., McPhaul, L., Augenstein, L.L. and Wargo, J.P. Jr.
Photodegradation of 14C Matacil in aqueous solution. Chemagro report
No. 50447, October 18 (1978), Unpublished.
Murphy, J.J., Jacobs, K. and Minor, R.G. - Stability of Matacil in
aqueous systems. Chemagro Report No. 43450. January 20 (1975a),
Unpublished.
Murphy, J.J., Minor, R.G., Jacobs, K. and Shaif II, H.R. - Persistence
of Matacil in soil. Chemagro Report No. 43444, January 31 (1975b),
Unpublished.
Nagel, C.D., Tessier, J.F., McPhaul, L., Augenstein, L.L. and Wargo,
Jr. J.P. - Spruce foliage metabolism study with 14C Matacil. Chemagro
report No. 50445, October 16 (1978), Unpublished.
Nelson, D.L. - Acute Oral Toxicity of Matacil Technical and Matacil
Analytical Grade to Female Rats. (1973a) Unpublished report from
Chemagro Agr. Div., Mobay Chemical Corp., submitted by Bayer AG.
Acute Rat Cholinesterase No-effect Study with Matacil Technical.
(1978b) Unpublished report from the Chemagro Agr. Div., Mobay Chemical
Corp., Submitted by Bayer AG.
New Zealand Report of the Codex Contact Point. (1978).
Noel, R.B., Mawdesley-Thomas, L.E. Chesterman, H., Clarke, E., and
Street, A.E. Bayer 44646 Chronic Oral Toxicity Study in Dogs. Final
Report. (1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Obrist, J.J. - Leaching characteristics of aged Matacil soil residues.
Chemagro report No. 65857, April 28 (1978), Unpublished.
Palmer, K.A. and Fletcher, M.A. - Effect of Bayer 44646 Upon
Reproduction of Multiple Rat Generations. Addendurn Histopathology.
(1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Root, M., Cowan, J. and Doull, J. - Subacute Oral Toxicity of Bayer
44646 to Male and Female Rats. (1963) Unpublished report from the
University of Chicago, submitted by Bayer AG.
Shaw, H. R. - Metabolism of Matacil in Swine. (1978) Unpublished
report from Mobay Chemical Corp., submitted by Bayer AG.
Silk, P.J., Semeluk, G.P. and Unger, I. - The photoreactions of
carbamate in insecticides. Phytoparasitica, 4, 51-63.
Stanley, C.W. and Delphia, L.M. - Gas-liquid chromatographic method
for detecting residues of Matacil in spruce needles, fish, soil and
water. Chemagro Report No. 66512, Sept. 15 (1978), Unpublished.
Sundaram, K.M.S. and Hopewell, W.W. - Fate and persistence of
aminocarb in conifer foliage and forest soil after simulated aerial
spray application. Canada Forest Pest Management Institute Report No.
FPM-x-6), October (1977a), Unpublished.
Penetration, translocation and fate of C-14 aminocarb in spruce
trees. Canadian Forestry Service Report CC-x-140 February (1977b),
Unpublished.
Sundaram, K.M.S., Volpe, Y. Smith G.G. and Duffy J.R. - A preliminary
study on the persistence and distribution of Matacil in a forest
environment. Canadian Forestry Service Report No. CC-X-116, January
(1976), Unpublished.
Sundaram, K.M.S., and Hindle, R. - Isolation and analysis of aminocarb
and its phenol from environmental waters. Canadian Forest Pest
Management Institute report. FPM-X-18 June (1978), Unpublished.
Tessier, J.F., Mulkey, W.S., Augenstein, L.L. and Wargo jr, J.P. -
14C Matacil buffer hydrolysis study. Chemagro Report No. 50444,
October 5 (1978), Unpublished.
Thornton, J.S., Hurley, J.B. and Obrist, J.J. -Soil thin-layer
mobility of twenty four pesticide chemicals. (Chemagro Report No.
57016, Dec. 15 (1976), Unpublished.
The half-life for the hydrolysis of aminocarb in pH 9.3 buffer was
reported to be 4 hours. On glass surfaces under fluorescent light at
25°C the rate of loss was approximately linear for the first few hours
with a half-life of 1.6 hours. In the period of 4 to 12 hours, after
application, there was a decrease in slope, indicative of conversion
to less volatile products on exposure to air and light (Abdel-Wahab
et al., 1966).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Miniature swine (Sus scrofa, 1 male and 1 female) received a
single oral dose of 0.5 mg/kg body weight 14C labelled aminocarb.
The compound was rapidly absorbed. The 14C peak value in blood was
reached within one hour. Excretion via the faeces did not exceed 2
percent, while 96% was excreted in the urine within 48 hours.
Elimination was rapid with approximately 75 percent observed in urine
within 6-24 hours.
The major urinary metabolites consisted of
conjugated-4-dimethylamino-, 4-methylamino-, and 4-
amino-3-methyl-phenol. Additionally, slight amounts (1-4%) of
aminocarb, its 4-formylamino-3-methyl phenol, and demethylation
products were observed. The same animals received 0.26 mg/kg body
weight of 14C-labelled aminocarb for five days and were sacrificed 45
minutes after the last dose. More than 90% of the daily dose was
excreted in the urine and with less than 2% in the faeces. Remarkable
amounts of 14C occurred in the kidney (0.7 ppm, expressed as
aminocarb) and liver (0.2 ppm) with conjugated
4-dimethyl-amino-3-methyl-phenol characterized as the main metabolite.
A similar amount of unidentified metabolite(s) was also observed.
Additionally, slight amounts (1-5%) of aminocarb, its demethylation
products, the formylamino derivative, and conjugated phenol-analogues
were determined. Slight quantities of 14C (0.02-0.09 ppm) were found
in other tissues (skin, brain, heart, muscles, and fat) with higher
values noted in the skin and fat (Shaw, 1978).
Bioaccumulation
Quail were fed 0, 10 and 50 ppm of 14C ring labelled aminocarb. The
14C content of liver, breast muscle, and mesenteric fat continuously
increased during the 14 days of treatment followed by continuous
elimination during a 14-day withdrawal period (Lamb, et al., 1976).
In channel catfish (Ictalurus punctatus) exposed to 10 ppb 14C
ring-labelled aminocarb, accumulation reached an equilibrium level
after one day. Elimination was rapid. A 50% reduction of residues
was noted in one day following a 28-day exposure. 14C was equally
distributed in the edible and non-edible parts of the fish (Lamb and
Roney, 1976).
A group of 9 male albino rats received a single dose of 25 mg/kg
aminocarb by gavage. The cholinesterase activity in the erythrocytes
was reduced by 50% after 1,3 and 5 hours. Recovery was rapid, being
complete within 24 hours (Kimmerle, 1961).
Special Studies on Cholinesterase Inhibition
Rat
Groups of male and female (Sprague-Dawley) rats (3 animals/group)
received oral doses of 0, 0.5, 2.0 and 8.0 mg/kg aminocarb by gavage
once a week for three weeks. Reduced cholinesterase activity was
noted at the highest dose (8.0 mg/kg) 1 and 3 hours after treatment
(approximately 10 to 20% in plasma and 30 to 40% in erythrocytes). In
the 2 mg/kg group, reduced erythrocyte cholinesterase activity was
observed after one hour. Twenty-four hours after dosing, all
inhibitory effects had disappeared in the blood. There was no
inhibition of brain cholinesterase activity noted in this study
(Nelson, 1978b).
A group of male albino rats which received 5 mg aminocarb/kg by gavage
for four weeks showed a 20-30% inhibition of erythrocyte
cholinesterase activity. There were no clinical signs of poisoning
associated with this dose level.
Groups of young adult female Sprague-Dawley rats (5 animals/group)
were administered aminocarb by intraperitoneal injection at daily dose
levels of 0, 2, 4, 8 and 10 mg/kg for 60 days. At dosage levels above
2 mg/kg, a decrease in body weight was noted. The animals
administered 4 and 8 mg/kg developed acute signs of poisoning which
persisted for one or two hours after dosing. All animals at the
highest dose level and one animal of the 8 mg/kg level failed to
survive the 60-day trial. One day after the last dose, cholinesterase
activity of brain, submaxillary gland and serum was measured and
inhibition data are represented in the following table:
% of inhibition at:
2 mg/kg 4mg/kg 8mg/kg
Brain 0 0 0
Submaxillary gland 11 24 34
Serum 29 44 53
(Dubois and Kinoshita, 1762).
TOXICOLOGICAL STUDIES
Special Studies or Reproduction
Four groups of 10 male and 20 female (Sprague Dawley) rats were fed
aminocarb in the diet at doses of 0, 100, 200 and 800 ppm. Treatment
started when the parents were approximately 75 days old and was
continued through a standard three generation reproduction study. All
generations showed a light hypersensitivity at 800 ppm. With the
exception of females of the F1b generation, retarded weight gain was
noted in all rats at 800 ppm. Weight gain was also retarded in the
females of the F0 and in males of the F1 and F2 generation at 200
ppm. Food consumption at the two higher dose levels was depressed in
a dose dependent manner. At birth, a reduced litter size and weight
was noted in the first mating of the F0, F1 and F2 generations at
800 ppm. At weaning, a reduced litter weight and mean pup weight for
all generations was seen at 800 ppm. At 200 ppm, a reduced pup weight
was observed at the first mating of the F0 generation. No
abnormalities were found as a result of aminocarb in the diet. The
young animals from the second litter of the third generation of rats
treated with 800 ppm did not show significant alteration in tissue
morphology associated with treatment. A no-effect level in this study
was 100 ppm (Palmer and Fletcher, 1966).
Special Studies on Neurotoxicity
In 20 hens over 9 months of age, which were orally administered
aminocarb (74 mg/kg) on day 0 and day 30, no signs of delayed
neurotoxicity were noted over the observation period of 30 days or in
the 30-day observation period following the second dosing.
Histopathologically, no alterations were observed in the nervous
tissue (Kruckenberg, 1978a).
In groups of 18-20 month old hens (3 animals/group) which were fed 0,
250, 500, 1000 and 2000 ppm aminocarb in the diet for 30 days and
thereafter observed for another four weeks, no delayed neurotoxic
effects were found. At doses of 500, 1000, and 2000 ppm, mortality
was observed as 2, 6 and 7 animals, respectively, died. At all dose
levels, blood cholinesterase was significantly inhibited and body
weight was reduced (Kimmerle, 1965a).
Acute Toxicity
Table 1. Acute-Toxicity of Aminocarb
Route of LD50
Species Sex administration (mg/kg) Reference
Mouse M IP 8 Dubois & Raymund, 1962a
Mouse F IP 9 Dubois & Raymund, 1962a
Mouse F IP 4.7 Abdel-Wahab & Casida,
1967
Mouse F Dermal 31 " "
Mouse F 1 hr exposure
2 um size) 4 mg/L Dilley & Doull, 1962
Rat M&F Oral 30 Dubois & Raymund, 1962a
Rat F Oral 22-27 Nelson, 1978a
Rat M Oral ca.50 Kimmerle, 1961
Table 1. Continued...
Route of LD50
Species Sex administration (mg/kg) Reference
Rat M IP 13 Dubois & Raymund, 1962a
Rat M IP 21 Kimmerle, 1961
Rat F IP 13.5 Dubois & Raymund, 1962a
Rat F Dermal 275 " "
Rat M Dermal <1000 Kimmerle, 1961
Rat F Inhalation
(1 hr exposure) 6 mg/L Dilley & Doull, 1962
Rat M&F Inhalation
(4 hr exposure) 0.2 mg/L Kimmerle, 1961; 1966
Guinea pig M Oral 60 Dubois de Raymund,1962a
Guinea pig M IP 30 " "
Chicken F Oral 74 Kruckenberg, 1978b
Chicken F Oral 75 Dubois, 1962
Chicken F Oral 50-100 Kimmerle, 1965b
Chicken F IP 25-50 Kimmerle, 1965b
In an acute inhalation toxicity test, groups of rats (20 males/group)
were exposed using a dynamic flow chamber into which aerosols at
concentrations of 0.1 and 1 mg/L aminocarb were continuously dispersed
for four hours. All animals at 1 mg/L and 4/20 animals at 0.1 mg/L
died within eight hours after exposure (Kimmerle, 1961; 1966).
In a subacute inhalation toxicity test with 20 male albino rats using
a dynamic flow chamber into which an aerosol with a concentration of
0.04 mg/L was dispersed eight hours per day for five consecutive days,
no specific signs of poisoning were observed. One animal died four
days after the end of the test (Kimmerle, 1961).
Signs of poisoning
Acutely toxic doses of aminocarb produce cholinergic effects typical
of cholinesterase inhibitors. The signs of poisoning appeared rapidly
after oral or intraperitoneal administration and were similar in all
species examined. After lethal doses, death occurred within two
hours, and usually within the first 30 minutes. After sublethal
doses, the symptoms subsided rapidly and apparent complete recovery
was usually noted within three hours (Dubois and Raymund, 1962a).
Special Studies on Potentiation
Potentiation of the acute toxicity of aminocarb was absent in female
(Sprague Dawley) rats when aminocarb was given by intraperitoneal
injection alone and in combination with 15 other anticholinesterase
insecticides (Dubois and Raymund, 1962b).
Short-Term Studies
Cat
In a cat which received 5 mg/kg aminocarb by gavage for ten
consecutive days, salivation, loss of appetite, and weight loss was
observed. Signs of cholinesterase inhibition were noted after two
days in 2 cats which received doses of 10 mg/kg; these animals died
after 4 days of treatment (Kimmerle, 1961).
Quail and Duck
Aminocarb and four of its major metabolites (THS 1013, THS 1003, THS
0995 and THS 1029) were fed to 15-day old bobwhite quail and 12-day
old mallard ducks at a dose of 1000 ppm for a period of 5 days.
Except for a reduction in feed consumption and reduced weight gain of
the mallard ducks, no toxic signs were observed (Lamb and Jones,
1975).
Rat
Groups of weanling (Sprague Dawley) rats (12 male and 12 female
rats/group) were fed 0, 5, 10 and 50 ppm aminocarb in the diet for 16
weeks. No effects on cholinesterase activity, growth, food
consumption, gross organ weight, or on histological examination of
tissues and organs were found in this study. A similar study was
started with dietary levels of 0, 100 and 200 ppm. As these levels
also failed to induce a significant inhibition of cholinesterase in
the first 3 weeks, the dietary levels were increased to 400 and 800
ppm, and were maintained for a further 19 weeks. The duration of this
study was 22 weeks. Over the course of the study, many of the animals
appeared to be hyperexcited and irritable. At the end of the study, a
decrease in growth rate was noted in males (14%) and females (13%) fed
800 ppm and in males (13%) and females (10%) fed 400 ppm.
Cholinesterase activity was measured on five animals/sex/group. Data
at the conclusion of the study revealed an inhibition in the serum of
males (16%) and females (42%) fed 800 ppm, and in the serum of females
(31%) fed 400 ppm. No inhibition was found in the brain, submaxillary
glands and erythrocytes. No effects were observed with respect to
organ weights and histopathological examination of tissues and organs
(Root, et al., 1963).
Table 2. Acute Toxicity of Aminocarb Metabolites
LD50
Compound Species Sex Route (mg/kg) Reference
4-(N'formyl-N'-methylamino- Mouse F IP 21 Abdel-Wahab & Casida, 1967
3-methylphenyl-N-methylcarbamate Mouse F Dermal >500 " "
4-(methylamino)-3-methyl
phenyl-N-methylcarbamate Mouse F IP 3.0 " "
Mouse F Dermal 52 " "
Rat F Oral 27.8(22.9-33.7) Kimmerle, 1974
4-formylamino-3-methylPhenyl- Mouse F IP 13 Abdel-Wahab & Casida, 1967
N-methylcarbamate
Mouse F Dermal 500 " "
4-amino-3-methylphenyl-N- Mouse F IP 1.6 " "
methylcarbamate Mouse F Dermal 17 " "
Rat F Oral 18.3(16.2-20.7) Kimmerle, 1974
4-(N'-methoxy-N'-methylamino)- Rat F Oral 102.8(88.5-119.4) Kimmerle, 1974
3-methylphenyl-N-methyl
carbamate
4-methoxyamino-3-methylphenyl- Rat F oral 40.1(33.7-47.5) Kimmerle, 1974
N-methylcarbamate
Dog
Groups of two male and two female beagle dogs were fed 0, 200, 400 and
800 ppm aminocarb in the diet for 12 weeks. At the end of this
feeding period, the dogs were returned to the control diet for an
additional 4-week period. Weight loss was observed in the animals of
the 800 ppm and 400 ppm groups. Signs of poisoning (e.g. vomiting,
retching, ataxia and incoordination) were dose-related and were found
in all dose groups, especially in the female animals. Signs of
poisoning were particularly prevalent during and just after
consumption of the aminocarb diet and were minimal or absent during
the remaining part of the day. There were no signs of poisoning noted
in the 4-week recovery period. Blood samples, taken prior to feeding
when symptoms were minimal or absent showed no inhibition of the
cholinesterase activity, of the serum or erythrocytes (Doull and Root,
1963).
Groups of beagle dogs (4 male and 4 female dogs/group) were orally
dosed by gavage at dosage levels of 0, 2, 4 and 10 mg/kg/day (0, 1, 2
and 5 mg/kg twice a day) for two years. After 12 weeks, one dog in
the highest dose group died. Moderately severe signs of poisoning,
(e.g., general excitability, salivation, pupil constriction and fine
visible trembling of muscles) were seen in all dogs in the high dose
group. Less severe signs of poisoning were noted in dogs at 4 and 2
mg/kg/day. The highest dose group showed a slight reduction in body
weight and a reduced rate of growth. No inhibition of cholinesterase
activity in serum and erythrocytes was noted, nor were changes in
hematology and blood chemistry found, except with the one dog that
died. Brain cholinesterase activity was not measured. Samples for
serum and erythrocyte cholinesterase were taken prior to daily dosing
in the first 20 months of the study and in the last 4 months of the
study, within 45 minutes of dosing.
At the conclusion of the study, adrenal weight, as a percentage of
body weight only, was increased in the high dose level group. Other
organ and organ weight ratios were within normal limits.
Histopathologically, macrophages containing lipofucsin materials were
seen throughout the liver of all groups, possibly more frequently in
the high dose animals. In the other tissues, no compound-related
effects were seen histologically (Noel, et al., 1966).
Long-Term Studies
Rat
Groups of weanling Sprague-Dawley rats (24 males and 24 females/group)
were fed 0, 100, 200, 400 and 800 ppm aminocarb in the diet for 20
months. Growth was reduced in males and females at 800 ppm, and to a
lesser extent, at all lower dose levels. The median survival time and
food consumption were not affected by aminocarb. Final examinations
of 5 animals/sex/group did not reveal significant effects on
cholinesterase activity in the brain, serum, and erythrocytes, whereas
a slight inhibition (approximately 20%) was noted in the submaxillary
glands of males and females at 800 ppm. The relative weight of the
heart was slightly increased in all dose groups and in microscopic
examination showed cardiac lesions in both males and females at 800
ppm and in males at 400 ppm. Reduced spermato-genesis was observed in
some animals of the two highest dose groups. Liver changes were also
noted at dose levels of 200 ppm and above (Doull, et al., 1967).
COMMENTS
Aminocarb, an N-methyl carbamate ester, is rapidly absorbed and
metabolized by demethylation and/or hydroxylation or ester hydrolysis.
Those metabolites containing an intact carbamate ester structure show
acute toxicity similar to or greater than the parent compound.
Aminocarb and its metabolites are rapidly excreted and there is no
evidence of accumulation. In acute toxicity studies, aminocarb was
not potentiated by other cholinesterase inhibitors, nor did it induce
a delayed neurotoxic response in hens.
In several short- and long-term studies in the rat, aminocarb induced
a reversible cholinesterase inhibition. In a 2-year dog study,
clinical symptoms of poisoning were seen in all dose levels tested.
In a 3-generation study with rats, no effects were observed at dose
levels of 100 ppm or below.
The meeting concluded that the short- and long-term rat studies were
not carried out in accordance with currently acceptable protocols. As
there was growth depression and an increased relative weight of the
heart at all dose levels tested in the long-term rat study, a
no-effect level in the rat could not be determined. An acceptable
daily intake for man was not allocated. The meeting recommended that
aminocarb be re-examined when the ongoing long-term study in rodents
becomes available.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Aminocarb has been used mainly for the control of lepidopterous
defoliators of Canadian forests where it has been found notably
effective against the spruce budworm, Choristoneura fumiferana
(Clemens). About 3.7 million hectares of Canadian forest have been
treated by aircraft at an average dosage of 0.07 kg ai/ha. 167 g/l
oil-soluble concentrate is used.
Aminocarb is also effective for the control of lepidopterous larvae
and other biting insects in cotton, tomatoes, tobacco and fruit crops,
but usage in these crops appears to be low. For these purposes, 50
and 75% wettable formulations are used. In New Zealand, the
insecticide was, but is no longer used widely against the coddling
moth, leaf roller and mealy bug. It is applied at 0.75-1.12 kg ai/100
L. In stone fruit and citrus, it is effective against the leaf
roller, mealy bug and scales, where it is applied at 75-94 g ai/100 L.
Aminocarb is also effective against caterpillars in vegetables and is
applied at 0.75-1.5 kg ai/ha (New Zealand, 1978).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Very few residue data on food crops are available because of the
limited usage of aminocarb in these crops. The manufacturer provided
the data available for apples and pears.
Studies carried out in Australia with apples involved the application
of aminocarb at 0.075, 0.1 and 0.125% solutions, equivalent to 3/4, 1,
and 1 1/4 lbs./100 gals, respectively, to give a spray coverage of
13-18 L/tree. The recommended preharvest interval of three days
justify a limit of 4 mg/kg (Table 3). The data for pears (Table 4)
also adequately support a 4 mg/kg limit at the three days preharvest
interval.
Table 3. Aminocarb Residues in Apples
Application rates Residue levels (mg/kg)
(% Solution) Period in days
3 10 17 24
0.075 2.8 1.3 1.6 0.6
0.1 2.2 1.0 1.6 1.4
0.125 2.6 1.4 1.8 1.0
Table 4. Aminocarb Residues in Pears
Residue levels (mg/kg)
Application rate Period in days
(% solution) 0 7 13 15 21
(23-27 L/tree)
0.1 1 0.8 n.d. - ND
0.1 3.0 1.0 0.4 - 0.4
0.1 1.3 - - n.d. 0.4
(23-27 L/tree)
0.1 1.4 - - n.d. 0.4
(23-27 L/tree
0.125 1.9 - - 0.4 0.4
(23-27 L/tree)
0.075 1.4 - - n.d. n.d.
FATE OF RESIDUES
General Comments
Most of the data on aminocarb were generated in connection with its
use in the control of forest pests. Aminocarb was degraded rapidly in
soil, water, plants, animals and by ultraviolet irradiation (Chemagro,
1976). The major route of breakdown in the presence of light or on
surfaces was oxidation of the dimethylamino portion of the molecule to
produce traces of 4-(formamidomethylamino)-, 4-(formamidoamino)- and
4-amino-3-methylphenol. These products are not detected in animals or
within plant tissues. In plants, hydroxylation followed by
conjugation is the main degradation pathway. In soil, N-demethylation
is predominant. In swine, conjugates of aminocarb phenol and the
methylamino analogue of aminocarb phenol were the major metabolites
found in males and females respectively. The principal tissue
constituent was conjugated aminocarb phenol.
Under normal use conditions, aminocarb was found at about 0.01 mg/kg
in soil and water, and about 2 mg/kg on foliage. Half-lives under
normal use conditions ranged from less than one day in soil to about
six days on spruce foliage. Maguire (1973) reviewed the chemistry of
aminocarb and the effect on the environment has been summarized
(Chemagro, 1976, 1979). The metabolism of aminocarb in different
substrates is shown in Figure 1.
In animals
(See Biochemical Aspects). In an ascorbic acid system which simulates
biological oxidation processes, Balba and Saha (1974) found at least
12 products formed from aminocarb. At least 10 of these products
contained either one or both the N-14CH3 groups. The ascorbic acid
system caused demethylation, hydroxylation of the aromatic ring,
oxidation of the -NHCH3, group to -NHCH20N, and cleavage of the
carbamate to the phenol. The 4-methylamino derivative was the major
degradation product.
In Plants
Abdel-Wahab et al., (1966) found that aminocarb was degraded with
the carbamate moiety intact when the carbamate was applied to glass or
silica gel surfaces or the leaves of growing bean plants, or injected
into the stems of bean plants. The methylcarbamate derivatives formed
included the 4-methylamino, 4-amino, 4-methylformamido, and
4-formamido analogues. The C14-carbonyl activity was found in the
unextractable portion six days after injection into bean plants. The
water-soluble metabolites formed following injection into the bean
plants result in part from hydroxylation of the carbamate on the
N-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates, mainly as glycosides (Kuhr
and Casida, 1967). These glycosides were quite persistent.
Dorough (1964) determined the stability of aminocarb applied as a leaf
surface treatment to cotton, garden snap beans, broccoli and tomatoes.
The metabolic fates of directly injected and surface-applied
carbonyl-C14 aminocarb appeared to be the same. The original
carbamate was converted into water-soluble metabolites which were
probably conjugates. Conversion was almost quantitative and the
metabolites were persistent. Leaf surface residues appear to be
degraded via hydrolysis at the carbamate group before or during
penetration. Penetration of aminocarb through the leaf surface of
beans and cotton was about eight percent after eight hours, although
50% of the residues was already lost from the surface (Dorough, 1979).
On the other hand, broccoli plants did not contain any internal
residues. Aminocarb was metabolized to persistent water-soluble
metabolites in these plants.
The fate and persistence of aminocarb in spruce foliage was
investigated by Sundaram and Hopewell (1977a) using a simulated spray
at the rate of 3.4 L/ha containing 57 g ai. The initial concentration
in foliage was about 10 mg/kg and dropped to less than 0.2 mg/kg
within 47 days. The half-life was six days. Aminocarb was found to
be labile and was dissipated rapidly under normal weathering
conditions.
In a similar study, Nagel et al. (1978) was not able to detect
volatilization of radioactivity with ring -1-C14 aminocarb.
Aminocarb residues declined from an initial level of 6.7 mg/kg to 3.3
mg/kg in 28 days. They attributed the decline to growth dilution.
The methylamino analogue was the only identified metabolite. Although
several unidentified metabolites were detected, no single component
contributed more than 10% of the total radioactivity.
The actual situations where aminocarb was applied serially at 70 g
ai/ha, the concentration in spruce foliage at 0.6 days was 0.7 mg/kg,
increased to a maximum of 2.2 mg/kg after four days and thereafter
decreases exponentially with a half-life of 5.6 days (Sundaram et
al., 1976). No residues could be detected after 64 days, presumably
owing to physical factors.
The penetration, translocation and fate of C14 aminocarb in spruce
trees was also investigated using trunk implantation (TIT), foliar
painting (FP), and basal bark painting (BBP) (Sundaram and Hopewell
1977b). Aminocarb appears to be weakly systemic. The aminocarb
absorbed after trunk implantation was gradually lost after 64 days,
probably owing to hydroxylation to water-soluble metabolites, some of
which were incorporated into the cellular structure of the foliage.
The major route of translocation was from the old to the
newly-developing foliage. In the case of foliar painting, basal bark
painting techniques, the mechanism of dissipation appeared to be
physical rather than biochemical.
In soil
In a comparative study of the persistence of aminocarb in silt loam
and sandy loam soil, Murphy et al, (1975) using
carbonyl-C14-aminocarb, found that breakdown was slower and more
soil-bound radioactivity was detected in the latter. In silt loam,
less than 15 percent of the applied insecticide was still present 24
hours after application. Microbial activity was essential for rapid
metabolism and C14102 was the principal product. Some of the
liberated C14102 was assimilated into the normal, insoluble soil
constituents. Minor (1978) also found that the insecticide was
degraded through removal of the carbamate portion by hydrolysis
followed by soil binding.
Aminocarb could be readily absorbed from aqueous solutions by loam
soil (Atwell, 1978). Although it is ranked as intermediate in soil
mobility using the soil thin layer system (Thornton et al, 1976)
leaching studies with sandy loam soil columns showed aminocarb to be
essentially retained in the upper 2.5 cm of soil. Less than 0.1% of
the radiolabel used was found in the leaching water after passing
through the soil column.
When aminocarb was applied to sandy loam soil at 1 mg/kg, the
half-life was less than 24 hours (Minor, 1978). The only identified
compounds were aminocarb and its N-demethylated analogue. Within 24
hours, 75% of the radioactivity was bound. Microbial activity was
necessary for degradation. In a pond water/soil study, aminocarb had
a half-life of 3.5 days and the metabolites identified were the
N-demethylated analogues and the phenolic hydrolysis product of
aminocarb. Formation of soil-bound residues was observed.
Sundaram and Hopewell (1977a) found that with simulated aerial
application, the initial residue in soil was 7 mg/kg. The half-life
was two days and residues could no longer be detected after 27 days.
In Water
In buffered solutions, aminocarb stability increased as the
temperature and pH decreased. At pH 4, its half-life was >127 days,
at pH 7, it varied between five and eleven days, while at pH 9, it was
<1 day (Tessier et al., 1978). Murphy et al. (1975) observed a
half-life of 28.5 days at pH 7 and 28.5 days in pond water under
normal environmental conditions. The principal metabolite appeared to
be the phenol although the methylamino and formylamino analogues were
also observed. (Tessier et al., 1978; Murphy et al., 1975; Minor,
1978).
Forest aerial application at 70 g ai/ha resulted in 2.1 and 1.9 mg/kg
residues in pond and stream water respectively (Sundaram et al.,
1976). Half-lives were 4.4 and 8.7 days, respectively, in the above
substrates. Residues were below the 0.1 mg/kg limits of analytical
sensitivity within 32 days.
In a pilot study, application of the insecticide at 2.4 lb ai/acre
resulted in residues ranging from <0.1 to 0.2 mg/kg 48 hours after
application. These levels were generally higher than found after 24
hours (Chemagro, 1976).
Photodecomposition
Irradiation of aminocarb in aerated and degassed ethanol and
cyclohexane at >300 pm gave the phenol as the major product (Addison
et al., 1974). Trace quantities of other products were also
observed. On the other hand, Abdel-Wahab and Casida (1967) found that
in bean foliage, photolysis involved extensive oxidation of the
dimethylamino moiety but not of other groups. There was a stepwise
demethylation of the dimethylamino moiety and one of the methyl
radicals was oxidized to the formamido group. The same result was
obtained earlier by Abdel-Wahab et al (1967). Aminocarb was not
readily decomposed when exposed as spots on the thin layer plates in
the dark, in fluorescent light or in long-wavelength UV light. Short
wavelength UV-light or sunlight resulted in considerable degradation.
Crosby et al. (1965) observed at least two compounds with
acetylcholinesterase inhibitory effects of many decomposition products
upon exposure to sunlight and ultraviolet radiation.
In aqueous buffer at pH 4.9, aminocarb had a half-life of 10 days when
irradiated with a mercury lamp. The methylamino analogue and two
unknown products amounting to 10% and 12% of the total radioactivity,
were detected in the solution after 30 days exposure. In an
acetone-sensitized solution, the half-life was reduced to 1.5 days
(Mulkey et al., 1978).
When ring -C14 aminocarb applied to a soil surface was subjected to
light from a high intensity mercury lamp, the calculated half-life was
4.6 hours (Augenstein, 1978). Approximately 33% of the applied
radioactivity was volatilized and 50% was bound to the soil during 192
hours of exposure to the light. The methylamino, methylformylamino,
formylamino and amino analogues of aminocarb as well as the
formylamino analogue of the phenol were detected. Unidentified
products accounted for <10% of the applied radioactivity.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
In New Zealand, six samples of apples in 1971 showed residues ranging
from 0.06 to 2.1 mg/kg with a mean of 0.86 mg/kg.
METHODS OF RESIDUE ANALYSIS
The analysis of carbamate insecticides by the popular GLC technique
has been an on-going problem for two reasons: 1) the thermal
instability of some compounds and 2) the lack of a sensitive detector
for the underivatized material. Several GLC derivatization procedures
using the sensitive electron capture detector have been tried. For
aminocarb, Sundaram et al. (1976) initially tried forming the
N-hepta-fluorobutyryl derivative. The procedure had an unsatisfactory
minimum detection limit (MDL) of 0.5 mg/kg in foliar extracts because
of impurities with retention times similar to aminocarb. Stanley and
Delphia (1978) subsequently used the 2,4-dinitrophenyl ether
derivatization technique of Holden (1973) and obtained a MDL of <0.01
mg/kg for spruce needles, soil and fish and <0.001 mg/kg for water.
GLC analysis of aminocarb as the intact molecule detected by such
nitrogen-"specific" detectors as the alkali flame ionization (AFID)
and the Hall microelectrolytic conductivity detectors appear
promising. The direct GLC procedures generally require only minimum
cleanup because they are less subject to sample interferences. Bayer
(1975) used the AFID to detect 0.1 mg/kg aminocarb on plant materials
and 0.02 mg/kg in water. Sundaram and Hopewell (1977) using the Hall
detector, found an MDL of 0.2 mg/kg for spruce foliage and soil. In
water, Sundaram et al. (1978) achieved an MDL of 1 × 10-4 mg/kg for
both aminocarb and its phenol.
High-pressure liquid chromatography (HPLC) has been applied by
Laurence (1977) to the detection of aminocarb in cabbage, corn, potato
and wheat. A UV detector was used at 254 nm. As little as 0.8 ng of
insecticide can be detected in the above crops.
Olson (1964) described a colorimetric procedure for residue
determination in soil, after the reaction of the phenol with
4-aminoantipyrine. The procedure can detect as little as 0.1 mg/kg
aminocarb and recovery was 90% for soils. It is also applicable to
crops.
In effect, the determination of the intact carbamate offers
possibilities as a regulatory method, especially with improvements in
the detection limits of the nitrogen specific detectors. The Hall
detector is particularly useful. If greater sensitivity is needed,
the 2,4-dinitrophenyl ether derivative can be used. Adoption of HPLC
for regulatory purpose would have to await further improvements in the
detection system and widespread use of the technique. The
4-aminoantipyrine procedure can be used for spectrophotometry.
NATIONAL MRLs REPORTED TO THE MEETING
According to the information supplied to the meeting, only the
following countries have established limits for aminocarb in food:
Limit Preharvest
Country Commodity established Interval
(mg/kg) (days)
Australia apples, pears 4 3
cottonseed 1
vegetables, fruit
(except apples, pears) 1
Germany pome fruit 1
(FRG)
APPRAISAL
Aminocarb is mainly used for the control of lepidopterous defoliators
in conifer forests where it is applied by air. There is only limited
usage in other crops. For aerial application, a 1.4 lb ai/US gal.
oil-soluble concentrate formulation is used. For general usage, 50
and 75% wettable powder formulations are available.
In view of the limited usage in food crops, very few residue trials
have been done to establish MRLs. Data on apples and pears adequately
support MRLs of 5 and 2 mg/kg, respectively.
Aminocarb is degraded rapidly in soil, water, plants, animals and when
exposed to short-wave ultraviolet radiation. In plants, metabolism
involves hydroxylation of the molecule at the carbamate on the
1,1-methyl group, on the ring, or on a ring substituent, followed by
conjugation of the hydroxylated carbamates mainly as glycosides.
Conjugates of aminocarb phenol and the methylamino analogue of
aminocarb phenol were the major metabolites in swine. The principal
tissues constituent was conjugated aminocarb phenol. In soil,
N-demethylation predominate. Under normal use conditions, half-lives
of aminocarb ranged from less than one day in soil to about six days
on spruce foliage. Detection by gas chromatography as the intact
carbamate or detection as the 2,4-dinitrophenyl ether derivative
appear adequate as regulatory methods; the latter can detect as low as
0.01 mg/kg aminocarb in spruce needles, soil and fish and 0.001
aminocarb in water.
RECOMMENDATIONS
Since the main use of aminocarb is in forest pest control, the
likelihood of its occurrence in food items for international trade is
generally low. However, the setting of MRLs are considered necessary
in some cases. In the absence of an ADI, the following guideline
levels are recorded for aminocarb:
Commodity GL
mg/kg
apples 5
pears 2
FURTHER WORK OR INFORMATION
If the usage of aminocarb in food items should increase, additional
information should be provided.
REFERENCES
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. - Fate of
C14-carbonyl-labelled aryl methylcarbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14., 290-298.
Abdel-Waheb, A.M. and Casida, J.E. - Photo-oxidation of Two
4-Dimethylaminoaryl methylcarbamate Insecticides (Zectram and Matacil)
on Bean Foliage and of Alkylaminophenyl Methylcarbamates on Silica Gel
Chromatoplates. J. Agr. Food Chem. 15: 479-487.
Addison, J.B., Silk, P.J. and Unger, I. - The photochemical reactions
of carbamates II. The solution photo chemistry of Matacil
(4-dimethylamino m-tolyl-N-methyl (carbamate) and Landrin
(3,4,5-trimethylphenyl-N-methyl carbamate). Bull. Environmental
Contam. Toxicol. 11, 250-255.
Atwell, S.M. Soil absorption and desorption of C14-Natacil. Chemagro
Report No. 55424 July 12 (1973), Unpublished.
Augenstein, L.L., McPhaul, L. and Wargo, J.P., Jr. - Photodegradation
of 14C Matacil on a soil surface. Cemagro Report No. 50446, October
20 (1973), Unpublished.
Balba, M.M. and Saha, J.G. - Degradation of Matacil by the ascorbic
acid oxidation system. Bull. Environ. Contam. Toxicol. 11, 193-200.
Bayer, A.G. Gas Chromatographische bestimmung von
aminocarbruckstanden in boden und wasser. Oct. 28 (1975), Unpublished.
Bayer, A. G. Data submitted to the 1979 JMPR
Chemagro. Matacil - The effect on the environment. Chemagro Report,
April 17 (1976), Unpublished.
Coburn, J.A., Ripley, B.D. and Chau, A.S.Y. - Analysis of Pesticide
residues by chemical derivatization. II. N-methylcarbamates in natural
water and soils. J. Assoc. Off. Anal. Chem. 59, 188-196.
Crosby, D.G., Leitis, E and. Winterlin, W. L. - Photodecomposition of
carbamate insecticides. J. Agr. Food Chem. 13, 204-207.
Dilley, J. and Doull, J. - The Acute Inhalation Toxicity of Bayer
44646 to Rats and Mice. (1962) Unpublished report from the University
of Chicago, submitted by Bayer, AG.
Dorough, H.W. Fate of Bayer 44646 in several plant species. Progress
Report, Texas A & M University, Sept. 1 (1964), Unpublished.
Fate of Matacil in several plant species. Progress Report, Texas A &
M University, July (1970), Unpublished.
Dorough, H.W. and Thorstenson, J.H. - Analysis of carbamate
insecticides and metabolites J. Chromatogr. Sci. 13, 212-224.
Doull, J. and Root, M. - Subacute Oral Toxicity of Bayer 44646 in Male
and Female Dogs. (1963) Unpublished report from the University of
Chicago, submitted by Bayer AG.
Doull, J., Root, M. and Keskauskas, J. - Chronic Oral Toxicity of
Bayer 44646 to Rat. (Addendum: Hibbs, C., and Nelson, D.L. Microscopic
Findings in Tissues of Male and Female Rats Fed Bayer 44646 for Two
years). (1967) Unpublished report from the University of Chicago,
submitted by Bayer AG.
Dubois, K.P. The Acute Oral Toxicity of Bayer 44646 to Chickens.
(1962) Unpublished report from the University of Chicago, submitted by
Bayer AG.
Dubois, K.P. and Raymund, A.B. - Acute Toxicity of Bayer 44646 to
Mammals. (1962a) Unpublished report, from the University of Chicago,
submitted by Bayer AG.
The Acute Toxicity of Bayer 44646 in Combination with other
Anticholinesterase Insecticides. (1962b) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Dubois, K.P. and Kinoshita, F. - The Subacute Parenteral Toxicity of
Bayer 44646 to Female Rats. (1962) Unpublished report, from the
University of Chicago, submitted by Bayer AG.
Holden, E.R. Gas Chromatographic determination of residues of
methylcarbamate insecticides in crops as their 2,4-dinitrophenyl ether
derivatives. J. Assoc. Off. Anal. Chem. 56, 713-717.
Kimmerle, G., Wirkstoff Dr. Heib A. 363 (E 44646). (1961) Unpublished
report from Bayer AG, Tox. Gew. Hyg. Labor, submitted by Bayer AG.
Neurotoxische Untersuchungen mit A 363-Wirkstoff. (1965a) Unpublished
report from the Institut für Toxikologie, submitted by Bayer AG.
Wirkstoff A 363 als Formulierung F1 604/149 Acute
Neurotoxizitätsprüfung bei Hühnern. (1965b) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Matacil-Wirkstoff. (1966) Unpublished report from the Institut für
Toxikologie, submitted by Bayer AG.
3-Methyl-4-methoxymethylaminophenyl-N-monomethy9lcarbamat,
3-methyl-4-monomethoxyaminophenyl-N-monomethylcarbamat,
3-methyl-4-monomethyl-aminophenyl-N-monomethyl-carbamat, und
3-methyl-4-aminophenyl-N monomethylcarbamat. Akate Akute Toxizität bei
Ratten. (1974) Unpublished report from the Institut Für Toxikologie,
submitted by Bayer AG.
Kuhr, R.J. and Casida, J.E. - Persistent glycosides of metabolites of
methylcarbamate insecticide chemicals formed by hydroxylation in bean
plants. J. Agr. Food Chem. 15, 814-824.
Kruckenberg, S.M. - Delayed Neurotoxicity Study of Matacil in Hens.
(1978a) Unpublished report from Kansas State University, by Bayer AG.
Acute Oral Toxicity of Matacil in Chickens. (1978b) Unpublished
report from Kansas State University, submitted by Bayer AG.
Lamb, D. W. and Jones, R.E. - Dietary Toxicity of Matacil Technical
and 4-Matacil Metabolites to Bobwhite Quail and Mallard Ducks. (1975)
Unpublished report from Chemagro Agr. Div., Mobay Chemical Corp.,
submitted by Bayer AG.
Lamb, D.W. and Roney, D.J. - Accumulation and Persistence of Residues
in Channel Catfish exposed to Matacil-14C. (1976) Unpublished report
from Mobay Chemical Corp., submitted by Bayer AG.
Lamb, D.W. et al. Accumulation and Elimination of Residues in
Bobwhite Quail Exposed to Matacil-14C. (1976) Unpublished report from
Mobay Chemical Corp., submitted by Bayer AG.
Lawrence, J.F. Direct analysis of some carbamate pesticides in food
by high pressure liquid chromatography. J. Agr. Food Chem. 25,
211-212.
Maguire, R.J. Aminocarb: A review of its chemistry. Manuscript
submitted for publication by the Canadian Forestry Service. (1978).
Minor, R.G. Metabolism of Matacil in soil and in an aquatic
environment. Chemagro Report No. 50427, October 15, 1978, Unpublished.
Mobay, Matacil - the effect on the environment, Addition no. 1 to
Chemagro Report 1976, January 14 (Unpublished).
Mulkey, N.S., McPhaul, L., Augenstein, L.L. and Wargo, J.P. Jr.
Photodegradation of 14C Matacil in aqueous solution. Chemagro report
No. 50447, October 18 (1978), Unpublished.
Murphy, J.J., Jacobs, K. and Minor, R.G. - Stability of Matacil in
aqueous systems. Chemagro Report No. 43450. January 20 (1975a),
Unpublished.
Murphy, J.J., Minor, R.G., Jacobs, K. and Shaif II, H.R. - Persistence
of Matacil in soil. Chemagro Report No. 43444, January 31 (1975b),
Unpublished.
Nagel, C.D., Tessier, J.F., McPhaul, L., Augenstein, L.L. and Wargo,
Jr. J.P. - Spruce foliage metabolism study with 14C Matacil. Chemagro
report No. 50445, October 16 (1978), Unpublished.
Nelson, D.L. - Acute Oral Toxicity of Matacil Technical and Matacil
Analytical Grade to Female Rats. (1973a) Unpublished report from
Chemagro Agr. Div., Mobay Chemical Corp., submitted by Bayer AG.
Acute Rat Cholinesterase No-effect Study with Matacil Technical.
(1978b) Unpublished report from the Chemagro Agr. Div., Mobay Chemical
Corp., Submitted by Bayer AG.
New Zealand Report of the Codex Contact Point. (1978).
Noel, R.B., Mawdesley-Thomas, L.E. Chesterman, H., Clarke, E., and
Street, A.E. Bayer 44646 Chronic Oral Toxicity Study in Dogs. Final
Report. (1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Obrist, J.J. - Leaching characteristics of aged Matacil soil residues.
Chemagro report No. 65857, April 28 (1978), Unpublished.
Palmer, K.A. and Fletcher, M.A. - Effect of Bayer 44646 Upon
Reproduction of Multiple Rat Generations. Addendurn Histopathology.
(1966) Unpublished report from the Huntingdon Research Centre
submitted by Bayer AG.
Root, M., Cowan, J. and Doull, J. - Subacute Oral Toxicity of Bayer
44646 to Male and Female Rats. (1963) Unpublished report from the
University of Chicago, submitted by Bayer AG.
Shaw, H. R. - Metabolism of Matacil in Swine. (1978) Unpublished
report from Mobay Chemical Corp., submitted by Bayer AG.
Silk, P.J., Semeluk, G.P. and Unger, I. - The photoreactions of
carbamate in insecticides. Phytoparasitica, 4, 51-63.
Stanley, C.W. and Delphia, L.M. - Gas-liquid chromatographic method
for detecting residues of Matacil in spruce needles, fish, soil and
water. Chemagro Report No. 66512, Sept. 15 (1978), Unpublished.
Sundaram, K.M.S. and Hopewell, W.W. - Fate and persistence of
aminocarb in conifer foliage and forest soil after simulated aerial
spray application. Canada Forest Pest Management Institute Report No.
FPM-x-6), October (1977a), Unpublished.
Penetration, translocation and fate of C-14 aminocarb in spruce
trees. Canadian Forestry Service Report CC-x-140 February (1977b),
Unpublished.
Sundaram, K.M.S., Volpe, Y. Smith G.G. and Duffy J.R. - A preliminary
study on the persistence and distribution of Matacil in a forest
environment. Canadian Forestry Service Report No. CC-X-116, January
(1976), Unpublished.
Sundaram, K.M.S., and Hindle, R. - Isolation and analysis of aminocarb
and its phenol from environmental waters. Canadian Forest Pest
Management Institute report. FPM-X-18 June (1978), Unpublished.
Tessier, J.F., Mulkey, W.S., Augenstein, L.L. and Wargo jr, J.P. -
14C Matacil buffer hydrolysis study. Chemagro Report No. 50444,
October 5 (1978), Unpublished.
Thornton, J.S., Hurley, J.B. and Obrist, J.J. -Soil thin-layer
mobility of twenty four pesticide chemicals. (Chemagro Report No.
57016, Dec. 15 (1976), Unpublished.
