
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
CYPERMETHRIN
IDENTITY
Common Name: Cypermethrin (BSI).
Chemical Name (IUPAC): [S,R]-alpha-cyano-3-phenoxybenzyl-2,2-dimethyl
[1R,1S, cis, trans]-3-(2,2-dichlorovinyl)
cyclopropanecarboxylate.
Synonyms: WL 43467 (Shell), PP383 (ICI), CCN52(ICI), NRDG
149, RIPCORD(R) (Shell), CYMBUSH (R) (SHELL),
CYMBUSH (R) (ICI)
Empirical Formula: C22H19O3NCl2
Structural Formula: 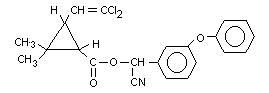 Molecular Weight: 416
Technical Material: Contains not less than 90% w/w cypermethrin
which is a racemic mixture of the 8 isomeric
forms with a cis: trans isomer ratio of
approximately 40:60
Physical Form Technical material is a viscous yellow liquid.
Density: 1.12 g/ml at 22°C.
Thermal Stability: Thermal analysis has shown that at a
temperature of 259°C slow weight losses occur
accompanied by negligible heat of reaction
indicating high thermal stability.
Solubility: Water 0.009 mg/l. Hexane 103 g/l. Acetone,
cyclohexanone, ethanol xylene and chloroform
450 g/l.
Volatility: relatively non-volatile
Vapour pressure: 5 × 10-6 N/m2 at 70°C
Flash point: 80°C
Formulations: Currently available in emulsifiable
concentrates ranging from 25-400 a.i. g/l.
Several formulations for ultra low volume
applications containing 8-75 g/l are also
available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Studies were performed in vivo on a wide range of mammalian species
and in vitro on isolated cell fractions to define the
pharmacokinetics of cypermethrin. In general, a wide range of studies
have shown that cypermethrin is rapidly absorbed, distributed to a
variety of tissues and organs, metabolised and rapidly excreted from
the body. Studies have been performed to evaluate the
pharmacokinetics in mice, rats, dogs, sheep and cows.
Mice
Groups of male mice were orally administered the cis or trans isomers
of cypermethrin dissolved in methoxytriglycol at dose levels of 7
mg/kg with the cyclopropyl-14C isomer and 8 mg/kg with the benzyl-14C
isomer. Results suggest that the proportion of cypermethrin which was
readily absorbed following oral dosing was rapidly eliminated in the
urine of mice. Absorption from the gastrointestinal tract was more
rapid with the trans isomer than with the cis as evidenced by high
radioactivity levels of the cis isomers in the faeces within the first
few days of the study. The rate of urinary excretion of cypermethrin
isomers labelled in acid and alcohol portions of the molecule was very
similar with the major quantity of radioactivity being eliminated from
the body within three days. Tissue residues after three days were
low. Adipose tissue was found to retain small quantities of the
radioactivity in concentrations higher than that noted for most other
tissues (Hutson, 1978a).
To evaluate the elimination of cypermethrin and its metabolites from
animal tissues following oral administration, groups of male mice were
administered cypermethrin at dose levels approximating 9 mg/kg body
weight (14C-benzyl, cypermethrin). In contrast to data from other
tissues, the rate of elimination of cypermethrin from adipose tissue
of mice was relatively slow with a half-life of approximately 10-20
days. Unchanged cis-cypermethrin was the only chemical residue
identified in adipose tissue. There was little change in the residue
during the last half of the 42-day trial following the relatively
rapid elimination during the first three weeks suggesting possible
long term storage and buildup of residue in adipose tissue (Crawford
and Hutson, 1978a).
Rat
Groups of male and female Wistar rats were orally administered
cypermethrin (14C benzyl, cis-isomer) at the dose level of 2.4 mg/kg
for females and 1.8 mg/kg for males. Cypermethrin was administered in
corn oil solution and animals and tissues were monitored over a period
of eight days. Excretion was rapid with both sexes. There was a
significant sex difference in the rate of excretion with male rats
excreting a substantially larger portion of the radioactivity in urine
than females over the first 24 hour interval. However, there were no
substantial differences in the overall rate of elimination by both
males and females when data were evaluated over the total 8-day period
of the test. The residues of radioactivity in the tissue of animals
of both males and females sacrificed at various periods over the 8-day
interval showed a small residue in a variety of tissues. While the
tissue residue in blood, liver, kidney, etc. was rapidly depleted over
the test interval, concentrations of cypermethrin in adipose tissue
were relatively stable over the 8-day trial (Crawford, 1976a).
Groups of male and female rats were orally administered cypermethrin
14C-benzyl, trans-isomer) at dose rates of 2.4 mg/kg for males and
3.0 mg/kg for females. Again, excretion was rapid with both sexes
with approximately 95% of the administered dose excreted within 48
hours. In contrast to that noted with the cis-isomer, there appeared
to be no sex differences in the elimination of the trans-isomer.
Residues in adipose tissue of females was somewhat higher than noted
in adipose tissue of males. The observations are consistent with the
suggestion that the trans-isomer is metabolised faster than the
cis-isomer (Crawford, 1976b).
Groups of rats were administered cypermethrin (1:1 cis: trans-isomer
ratio, 14C-cyclopropyl) at dose rate of 1 mg/kg for males and 2 mg/kg
for females. Rapid elimination of cypermethrin was observed with a
substantial difference noted in the 24-hour urinary excretion in males
and females. Females excreted considerably more radioactivity in
urine over the first 24 hours. At the end of 72 hours urinary and
faecal excretion in both sexes was approximately the same. Small
quantities of radioactivity were expired as 14CO2 which suggested
some metabolic breakdown of the cyclopropyl ring. At the end of three
days, tissue concentrations in liver, kidney muscle, brain, blood,
skin and remaining carcass were relatively low. Intestinal content
was somewhat higher in males than in females ranging from 3 to 9% of
the administered dose. Low residue concentrations, which never
exceeded 1 ppm, were observed in fat (Crawford, 1977).
The elimination pattern of cyclopropyl- and benzyl-labelled
cypermethrin appears to be the owns in both males and females.
Females absorb and metabolise the trans-isomer faster than males and
males absorb and metabolise the cis-isomer faster than females
(Crawford, 1976b). In a further study to resolve the residual nature
of the residues of cypermethrin in adipose tissue of rat, Crawford and
Hutson (1978b) again found that the cis-isomer was relatively stable
in adipose tissue. Administration of 14C-benzyl cypermethrin
(cis-isomer) at a dose of approximately 2 mg/kg to female rats
resulted in a residue of approximately 0.3 ppm in the fat eight days
after a single oral dose. Further studies through 42 days following
dosing were performed to evaluate the half-life of the cis-isomer in
fat and the total elimination pattern. At the end of 42 days,
residues were observed in fat which were approximately 10% of those
concentrations noted at eight days. There was a 90% loss of the
material from fat over the 8-42 day interval during which time samples
were taken. From these data with rats, a half-life of approximately
20-25 days was estimated with respect to removal of residues from fat
following a single oral dose. These half-life values are somewhat
longer than those noted with the mouse.
Dogs
Groups of male beagle dogs were administered 14C-benzyl cypermethrin
and the individual 14C-benzyl labelled cis- and trans-isomers of
cypermethrin, orally, at a dose of 2 mg/kg. Elimination of
radioactivity from all animals was rapid although differences in data
from individual dogs precluded a complete evaluation of the rate of
elimination. Differences in the rates of elimination of the
individual dogs in the study may have been due to differences in
absorption as cypermethrin was given orally in a capsule with no
solvent. Tissue residues observed 4 days after oral administration of
cypermethrin were extremely low. The vast majority of the excreted
material was observed in faeces (80%) with urine containing only 11%
of the administered dose. As with other species, small residues of
cypermethrin were observed in fat, approximating 2% of the
administered dose (this residue was estimated to be 0.3 ppm based upon
total adipose tissue of the dog) (Crawford, 1979b).
Sheep
Sheep were administered cypermethrin dermally (962 mg/animal
administered in acetone) or orally (without solvent) in gelatin
capsule at a dose of 4 mg/kg body weight. Following dermal
administration, cypermethrin was not readily absorbed (less than 0.5%
of the administered dose was observed in urine within 24 hours and 2%
over the six day test interval). Little radioactivity was found in
the liver and kidney of dermally-treated sheep. Tissue residues in
renal and subcutaneous fat was found to be similar to those noted with
other animal species and was qualitatively identified as cypermethrin.
Elimination of cypermethrin from the orally-dosed sheep was rapid with
61% of the administered dose being eliminated within 48 hours (41% of
the dose in urine and 20% of the dose in faeces). The tissue residue
pattern in sheep following oral administration was similar to that
noted with other mammalian species. Low levels of cypermethrin were
noted in various tissues, including renal and subcutaneous fat
(Crawford and Rutson, 1977a).
Cows
Cypermethrin (1:1, cis:trans-isomer, 14C-benzyl-label) was orally
administered to lactating cows over a period of three weeks at a
dietary dosage of 0.2 ppm. Urine, faeces and milk residues were
examined over the course of the study and at the conclusion of the
three week dietary interval, animals were sacrificed and tissue
residues examined. Elimination of radioactivity in urine and faeces
rapidly reached an equilibrium within 2-3 days. Radioactivity was
eliminated in the urine and faeces predominantly and accounted for the
major quantity of cypermethrin residues. Small quantities of
radioactivity were noted in milk with a total radioactivity residue
approximating 0.5% of the administered radioactive cypermethrin.
Tissue residues were extremely low with notable residues in bile and
fat as well as in the liver and kidney. There were no detectable
residues in muscle, blood or brain. Analysis of the milk fat showed
that a high percentage of the radioactivity was present in this
portion of the milk. Residues in the milk fat or cream sample were
reflective of the lipoidal nature of cypermethrin, with residues
concentrating in fatty tissues (Hutson and Stoydin, 1976).
In a further study to obtain information on the metabolic distribution
of cypermethrin in cows, lactating cows were treated for 7 days at a
dietary concentration of 5 ppm. Urine, faeces, and milk were analysed
and at the conclusion of the feeding trial, all animals were
sacrificed and tissue residues were determined. As in the previous
study, elimination occurred rapidly after the onset of dosing with the
major route of excretion being via urine and faeces. Tissue residues
were extremely low with measurable amounts observed in blood, liver,
kidney, bile and adipose tissue. Total milk residues amounted to
approximately 0.2% of the applied cypermethrin, with the largest
proportion of the residue again found in milk fat (Crawford, 1978).
Metabolism
The metabolic fate of cypermethrin was investigated in a variety of
mammalian species and was determined to be relatively similar in the
different species. The major metabolites are derived by cleavage of
the ester linkage followed by subsequent metabolism of both the acid
and alcohol fragments and conjugation and excretion of the fragments.
Studies on the metabolic fate of both cis- and trans-cypermethrin
administered orally to rats have shown that cypermethrin is rapidly
cleaved at the ester bond to yield the cyclopropanecarboxylic acid and
the 3-phenoxybenzyl alcohol moiety. The latter is rapidly oxidised to
3-phenoxybenzoic acid and conjugated prior to elimination. Major
reactions occurring with the cyclopropanecarboxycyclic acid include,
in part, oxidation at the methyl groups and apparent lactone
rearrangement prior to conjugation and elimination. Hydroxylation of
the phenoxybenzoyl moiety has been noted to occur in at least two
positions prior to conjugation and elimination. Rats and mice
metabolised cypermethrin in a similar fashion. In mice, a substantial
portion of the phenoxybenzoic acid was conjugated with taurine as well
as with glucuronic acid (Hutson, 1977). Oxidation has been noted to
occur in the 4'- position of phenoxybenzoic acid. Following
oxidation, the molecule is conjugated as a sulfate and excreted. A
portion of unconjugated hydroxylated phenoxybenzoic acid was also
excreted. Hydroxylated cypermethrin was observed in mouse faeces
suggesting that hydroxylation may occur prior to ester cleavage
(Hutson, 1978b). Shono, Ohsawa, and Casida (1978), using mouse liver
microsomal preparations, showed that hydroxylation of the
phenoxybenzyl moiety at positions other than the 4'- position can
occur. Small amounts of the 5- and 6-hydroxy phenoxybenzyl derivatives
were also observed. Both cis- and trans-isomers were rapidly
metabolised by cleavage of the ester bond and aromatic hydroxylation
at the 4'-position of the 3-phenoxybenzyl alcohol moiety. In rats,
approximately half of the administered cypermethrin was excreted as
sulfate conjugates of the hydroxylated phenoxybenzoic acid.
Phenoxybenzoic acid was also excreted free and conjugated with glycine
in contrast to the conjugate pattern noted in mice (Crawford and
Hutson, 1977b, 1978c). In studies with cyclopropanecarboxylic
acid-labelled cypermethrin, hydroxylation of the methyl groups on the
cyclopropane ring occurred to a limited extent. Oxidation was
believed to have occurred following ester cleavage. While most of the
radioactive metabolites were found in the urinal a small quantity of
hydroxylated metabolites were eliminated via the
biliary-intestinal-faecal route suggesting absorption and
re-distribution through the bile to the faeces.
In both rats and mice as well as other species, ester cleavage is
rapid. There is quantitative and qualitative evidence to suggest that
the cis-isomer is more stable yielding a larger variety of
hydroxylated products prior to ester cleavage while the trans-isomer
yields a wider variety of hydrolytic products which in part are
oxidised following the ester cleavage. The acid fragment is
conjugated predominantly with glucuronic acid an a ß-glucuronide.
Conjugated cyclopropanecarboxylic acid derivatives in urine were
identified as glucuronides have a relative resistance to the action of
ß-glucuronidase. ß-Glucuronides formed from these acids are generally
of the ester type readily cleaved by mild acid hydrolysis. Thus,
treatment of urine with methanol and sulfuric acid will convert both
the free acid and the acid glucuronide into a methyl ester. The
methyl ester of the carboxylic acid has been shown to be volatile and
analytical losses may occur with this compound. The alcohol fragment,
the alpha-cyano-3-phenoxybenzyl alcohol, is readily oxidised
metabolically to the benzoic acid, conjugated and excreted. Alcohol
conjugates with glucuronic acid are generally ether derivatives,
stable to acid and enzymatically hydrolysable. The phenoxybenzyl
moiety of cypermethrin has been found to form both ester and ether
ß-glucuronides.
Thus, the results of the studies with both rats and mice suggest that
most of the excreted metabolites of cypermethrin are hydrolysis
products, although hydroxylation of the intact ester has been
reported. Hydroxylation of the methyl groups attached to the
cyclopropane ring has also been reported. There was no evidence of
metabolism at the 2,2-dichlorovinyl moiety. Evidence exists that the
cis-isomer is somewhat more resistant than the trans-isomer to
hydrolysis. In all cases, only small amounts of hydroxylated
metabolites with the intact ester bond were observed with the
cypermethrin.
The metabolic fate of cypermethrin in dogs was qualitatively similar
to that observed with other species with the exception being
conjugation reactions of several metabolites. As with other species,
cypermethrin is rapidly metabolised by cleavage of the ester bond and
by hydroxylation of the phenoxybenzyl moiety at the 4' position.
Sulfate conjugation of the hydroxylated benzoic acid was reported.
Additionally, a major urinary metabolite was identified as the
3-phenoxybenzyl glycine. Studies on the metabolic fate of
3-phenoxybenzoic acid in dogs revealed a metabolic pattern of
oxidation and conjugation similar to that noted with cypermethrin
(Crawford, 1979a and 1979c).
In cows, the major urinary metabolite of cypermethrin ( 14C-benzyl)
was the glutamic acid conjugate of the 3-phenoxybenzoic acid
(Crawford, 1978). The cyclopropanecarboxylic acid moiety was
hydroxylated and/or conjugated with glucuronic acid prior to
excretion.
3-Phenoxybenzoic acid has been observed as a major metabolite of
cypermethrin in all species studied. Residues of cypermethrin in rat
skin (<3% of the dose) were noted after oral administration. Further
studies to define the skin residue were performed using
14C-3-phenoxybenzoic acid administered orally to rats as seven
consecutive daily doses. The radioactive metabolites in skin were
identified as predominately 3-phenoxybenzoic acid and a small quantity
of glyceryl dipalmitate esters of 3-phenoxybenzoic acid (Crawford and
Hutson, 1979).
Further studies were performed in rats of the metabolism of
3-phenoxybenzoic acid and a glucoside conjugate isolated from plant
tissue. 3-Phenoxybenzoic acid was oxidised at the 4' position of both
aromatic moieties and to a minor extent at the 6' position of the
benzoic acid moiety. Excretion occurred as conjugates, predominantly
the 4-hydroxy-3-phenoxybenzoic acid-O-sulfate and 3-phenoxybenzoic
acid. Additionally, other hydroxylated derivatives and conjugates of
these oxidation products were observed. The excretion pattern of the
glycoside conjugates of 3-phenoxybenzoic acid was qualitatively and
quantitatively similar to that observed with 3-phenoxybenzoic acid
suggesting rapid hydrolysis and availability of plant metabolites
following oral administration (Crawford, 1978).
Effects on Enzymes and Other Biochemical Parameters
Preliminary evidence suggested that an increase in the activity of
certain lysosomal enzymes in peripheral nerves and deficits in
behavioural functioning tests could serve as indicators of peripheral
nerve damage. During Wallerian degeneration, the activity of such
enzymes as ß-glucuronidase and ß-galactosidase as well as other
enzymes in nerve preparations were shown to be increased significantly
(Dewar, 1977a).
Cypermethrin (1:1 cis:trans) was administered to male and female rats
at dose levels ranging from 25 to 200 mg/kg/day for five consecutive
days by oral intubation as a 10% W/V solution in DMSO. A dose related
functional deficit was observed when the mean slip angle test and the
landing foot spread test were applied to the animals. The deficit was
maximal from 6 to 14 days after the beginning of treatment and
complete functional recovery occurred within four weeks. Substantial
variation in data from the landing foot spread test was noted. Data
were inconsistent over the course of the study. ß-glucuronidase
activity was increased in a dose-dependent fashion in both males and
females. The results suggest that cypermethrin produced a primary
axonal degeneration, readily measurable 28 days after treatment as an
increase in ß-glucuronidase activity and in deficits in specific
behavioural-function testing of rats (Dewar, 1977b).
When further studies were performed on the biochemical indices of
nerve degeneration to compare results with known degenerating
compounds (methyl mercury), it was observed that the changes in
ß-glucuronidase and ß-glucuronidase activity were considerably smaller
and less reproducible than those obtained following methyl mercury
poisoning (7.5 mg/kg/day for 7 days). In comparison of central versus
peripheral nerve damage, there was no evidence that the trigeminal
nerve was more sensitive to the effects of cypermethrin than the
sciatic and posterior tibial nerves. At near lethal doses of
cypermethrin biochemical changes in the trigeminal nerve were
consistent with those of Wallerian degeneration. The changes were
similar qualitatively to those seen with methyl mercury, but were
quantitatively much less intense (Dewar and Moffett 1978a).
Electrophysiological studies were performed to determine whether acute
or subacute intoxication with cypermethrin produced changes in the
conduction velocity of slower fibres in peripheral nerves or
alterations in the maximal motor conduction velocity. There was no
evidence to suggest that cypermethrin, at doses that induced severe
clinical signs of intoxication, including ataxia, had any effect on
maximal motor conduction velocity or conduction velocity of the slower
motor fibers in peripheral nerves. Doses used in the study ranged
from a single dose of 200 mg/kg to 7 consecutive doses of 150 mg/kg
followed by 2 doses of 400 mg/kg. At near-lethal doses there were no
effects noted on conduction velocity in the slower motor fibers of the
sciatic nerve and tail or on the maximal conduction velocity, even in
the presence of clinical signs of acute intoxication and at dose
levels where previous studies had shown functional degeneration.
These electrophysiological findings are reflective of motor function
which would suggest that the physiological and functional deficits
observed as a result of acute intoxication may be primarily sensory in
nature (Dewar and Deacon, 1977).
SPECIAL STUDIES ON REPRODUCTION
Dominant Lethal Studies
Mice
Groups of male mice (12 mice/group, 36 mice were used as controls)
were administered a single dose of cypermethrin dissolved in dimethyl
sulfoxide at dosage levels of 0, 6.25, 12.5 and 25 mg/kg body weight
or 5 successive daily oral doses of 0, 2.5 and 5.0 mg/kg. Following
dosing, each male was caged with 3 virgin females for 7 days. The
mating procedure was repeated weekly over an interval of 8 weeks in a
standard dominant lethal test.
Females mated to males treated with 5 daily oral doses of 2.5 mg/kg
and those mated to males receiving a single dose of 12.5 mg/kg showed
a significant reduction in the incidence of pregnancy during the
second and third week respectively of the onset of treatment. This
did not occur with other either higher or lower dosed groups or at
other intervals. In females, mated to males treated daily with doses
of cypermethrin a significant reduction in foetal implants was
observed during the second week of mating. Early foetal deaths were
increased in the second week at 5 mg/kg. No such increases occurred
in any other weekly interval or any other dosed group. Thus, multiple
administration of cypermethrin on five successive days induced a
significant reduction in foetal implants during the second week of
mating and a marginal increase in early foetal deaths at the same time
interval.
To evaluate the potential (noted above) for a dominant lethal effect,
a second experiment was performed. Groups of male mice (12
mice/group, a control group consisted of 36 mice) were administered
five daily oral doses of cypermethrin at levels of 0, 2.5, 5.0, 7.5
and 10 mg/kg (in dimethyl sulfoxide). Following dosing each male was
mated with three virgin females for four days and subsequently
provided with virgin females every four days for a period of three
weeks. Female mice were examined for evidence of dominant lethality
13 days after mating. In addition, 40 males of proven fertility were
dosed for five successive days at the same dosage levels, 0, 2, 5,
5.0, 7.5 and 10 mg/kg. These animals were also mated with four virgin
females on four successive days for a period of three weeks. Four
animals from each group were sacrificed for histological examination
of the testes and epididymis on days 1 and 7 after the final dosing.
In contrast to the previous trial, no reduction in foetal implants was
noted in any of the animals mated with cypermethrin-treated males. The
number of early foetal deaths was marginally increased at the highest
dose level in the 12-16 day interval after dosing and in the first 4
day period after treatment with 7.5 mg/kg males.
In the groups of animals examined histologically, no abnormalities
were detected in the testes and epididymis and there were no
observable histological differences between any of the test groups and
the controls (Dean et al., 1977).
Rat
Groups of rats (30 male and 30 female rats/group) were fed
cypermethrin in the diet at concentrations of 0, 10, 100 and 500 ppm
for five weeks after which they were mated to initiate a standard
three generation, 2-litter per generation, reproduction study. After
the selection of the second litter as parents for the following
generation, the original parents were sacrificed and subjected to
gross and microscopic examination. At the conclusion of the study,
ten animals of each sex at the age of 21 days were also examined
histologically. Calculations were made of the indices of fertility,
gestation, viability and lactation. There was no mortality over the
course of the study, and behaviour was not abnormal. Reduced body
weight and food intake was seen at various intervals in both males and
females fed 500 ppm in the diet. Reduced total weight was observed at
500 ppm in the F1A litters and reduced total weight and litter size
were noted at 100 and 500 ppm in the F1B. No compound related gross
or microscopic pathological findings were noted in any rats (Hend et
al., 1978).
SPECIAL STUDIES ON TERATOGENICITY
Rat
Groups of 25 pregnant rats were administered orally from day 6 to day
15 of gestation at dose levels of 0, 17.5, 35.0 and 70.0 mg/kg/day in
a standard teratology bioassay. On day 21 of gestation, the animals
were sacrificed and gross examination of foetuses were made, including
skeletal and somatic examinations. Pre-implantation losses were
evaluated based on corpora lutes, counts and implantation sites and
post-implantation losses were evaluated based upon implantation sites
and viable foetuses. Foetal somatic and skeletal examinations on day
21 of gestation showed no teratogenic changes that could be
attributable to the treatment. There were no indications of
embryotoxic or teratogenic events in the study (Tesh et al., 1978).
Rabbit
Groups of pregnant rabbits (20 rabbits/group, 30 rabbits were used as
an additional control group) were administered cypermethrin dissolved
in corn oil at dose levels of 0, 3, 10 and 30 mg/kg body weight orally
from day 6 to day 18 of gestation. On day 28 of gestation the rabbits
were sacrificed and examination made of live foetuses, dead foetuses,
resorption sites and corpora lutea. Live foetuses were maintained for
24 hours to assess viability. Foetuses were also examined for gross
somatic and skeletal deformities.
There was no significant mortality or differences in weight gain
during the period of gestation. There were no significant differences
between control and test groups with respect to pregnancy, foetal
death and survival. Although a wide range of skeletal and visceral
abnormalities were found in the course of the study, there were no
differences between control and test groups with respect to
abnormalities. It was concluded that oral dosing at up to 30 mg/kg
during the major period of organogenesis resulted in no teratogenic
effects in the offspring (Dix, 1978).
SPECIAL STUDIES FOR MUTAGENICITY
Mice Host-Mediated Assay
Groups of male mice (2-3 mice per group) were administered
cypermethrin, orally, at dose levels of 0, 25 and 50 mg/kg in
dimethylsulfoxide. The animals were immediately injected
interperitoneally with a suspension of S. cerevisiae in a standard
host mediated assay. After five hours, the mutagenic conversion rates
in the yeast cells recovered from the treated animals were comparable
with those of control animals suggesting that, under the terms of this
assay, there was no evidence to suggest a mutagenic potential (Brooks,
1976).
Microorganisms
The mutagenic potential of cypermethrin on various microorganism
species including: S. cerevisiae, E. coli WP2 uvr A, and S.
typhimurium TA-1538 (with and without the use of a rat activation
system) was examined. No increase in the mitotic gene conversion was
recorded in S. cerevisiae, either in the presence or absence of
microsomal oxidation. Cypermethrin, at concentrations up to 500
micrograms per plate, did not induce an increased mutation rate with
E. coli or S. typhimurium TA-1538, in vitro either in the
presence or in the absence of the microsomal oxidation system (Brooks,
1976).
Cypermethrin did not increase the number of revertant colonies of S.
typhymurium (TA-1535, TA-1537, TA-1538, TA-98 and TA-100) in the
presence or absence of a mouse liver subcellular activation
preparation obtained from 6 strains of PCB-treated mice. Cypermethrin
was tested at dose levels up to 1 mg/plate (Suzuki, 1977).
Hamster - Chromosomes
Groups of Chinese Hamsters (12 males and 12 females/group) were
administered orally on each of two successive days at a dose rate of
0, 20 or 40 mg/kg cypermethrin dissolved in dimethylsulfoxide.
Positive and negative controls consisted of cyclophosphamide (100
mg/kg) and dimethylsulfoxide alone. In chromosome preparations of the
control and cypermethrin-treated hamsters, there were no indications
of abnormalities. The cylophosphamide treatment induced a significant
degree of chromosomal damage (Dean, 1977).
SPECIAL STUDIES ON NEUROTOXICITY
Rats
Cypermethrin, orally administered to rats at high acute dosage levels,
produced severe clinical signs of poisoning which was accompanied by
histological evidence of sciatic nerve damage. Within one day
following the acute poisoning, neuropathy was evident as axonal breaks
in the sciatic nerve (Carter and Butterworth, 1976).
Groups of rats (10 males per group) were utilised in a paired feeding
study to examine the neurotoxicological effects of high dietary
levels. Rats were fed dietary levels of cypermethrin (45:55,
cis:trans-isomer ratio) for 14 days at dosage levels of 0, 1250, 2500,
and 5000 ppm. Growth was monitored and clinical evaluations for
adverse behaviour were recorded during the course of the study. Gross
pathology on tissues and organs and microscopic examination of the
sciatic nerve were performed at the conclusion of the study.
At 5000 ppm, mortality was observed with all rats either dying or
sacrificed in a moribund condition within the first week. At 2500
ppm, 6 of 10 rats died before the conclusion of the study. There was
no mortality in the low dose group. Clinical signs of neurotoxicity
were characterised by an impaired ability to walk and splayed hind
limbs. In extreme cases, clinical signs of ataxia and paralysis were
reported. Other clinical signs included: hypersensitivity to external
stimuli, gross disorientation and convulsions, the latter generally
seen at high dose levels. The neurotoxic signs of poisoning observed
in the 1250 ppm group after day three were spontaneously reversed by
day nine when all surviving animals that were initially affected
appeared to be normal. Remission of ataxia at the 2500 ppm group was
also noted within 11 days of treatment. Growth reduction was observed
in all animals in the study. At the lowest dose level, the rate of
growth was delayed for the first few days after which time the rate
was consistent with that of controls for the remainder of the study.
The absolute body weight of the treated animals was, however,
significantly lower than the controls at the conclusion of the
two-week study. Reduction in body weight was consistent with
reduction in food consumption. Ultrastructural changes in the sciatic
nerve were observed in the two highest dose levels, although the
number of animals examined was small. There was some evidence of
axonal damage in the myelinated nerves primarily at the two highest
dose groups. Changes in unmyelinated axons were not observed. In
general, the histological and ultrastructural changes in the sciatic
nerve, accompanied by clinical signs of poisoning, are not readily
apparent at low doses. Thus, cypermethrin, at acutely toxic dietary
levels,induces damage to sciatic nerves, the clinical signs of which
may be reversible (Glaister et al., 1977a).
Hamsters
Groups of male and female Syrian hamsters were orally administered
doses exceeding the LD50 in an attempt to define clinical signs of
poisoning and to evaluate the histological damage to the sciatic
nerve. At doses of 794 mg/kg and above, all animals showed clinical
signs of poisoning including tremors, abnormal, irregular movements,
and an unusual gait. As noted with rats, axon and myelin degeneration
was noted in all groups treated. The lesions included swelling and
breaks in the axons and clumping of myelin (Butterworth and Clark,
1977).
A series of experiments were performed to further evaluate the
neurotoxic potential following subacute, oral administration to
Chinese hamsters. Clinical examination, functional testing and enzyme
determinations using ß-galactosidase and ß-glucuronidase were
performed. The functional test consists of measuring the mean slip
angle where the animal is maintained on an inclined plane that
steadily increases its angle until the animal can no longer maintain a
stationary position. (The average angle of five replicate trials
constituted the mean slip angle test.)
Groups of 20 male and 20 female Chinese hamsters were orally
administered at a dose of 40 mg/kg followed by a dose of 20 mg/kg for
the following four days. Fifteen animals in each sex served as
controls. There was extensive weight loss in all dosed groups and
some mortality was observed primarily as a result of the initial
administration of 40 mg/kg. There was a loss of fur and a dermal
ulceration observed in the early parts of the study. This dermal
occurrence was transient, disappearing rapidly after the treatments
were concluded. There was significant weight loss in the initial
phase of the study. However, after the last dose, the surviving
animals rapidly gained weight at a rate consistent with the control
animals. There were no effects noted on the mean slip angle
experiment and a marginal increase in ß-galactosidase was observed in
peripheral nerve.
A further experiment utilising five male and five female Chinese
hamsters administered cypermethrin daily for five days at dose levels
of 0, 5, 10 and 20 mg/kg showed no mortality over the course of the
study. There was a slower growth of the animals treated with 20 mg/kg
which again was reversed at the conclusion of the treatment period.
Hyperexcitability was noted in one female at the high dose level.
There were no notable differences in behaviour in any of the animals.
There was a significant deficit in the mean slip angle test with
females showing an earlier dose-related deficit than noted in males.
The females recovered from this deficit earlier than males who showed
an erratic pattern of recovery. ß-galactosidase activity was
increased at all dose levels three weeks after the onset of the
experiment. This increase was statistically significant at the two
highest dose levels. Dermal irritation and fur loss was not noted in
this experiment.
Sixteen male and sixteen female Chinese hamsters were administered
five daily doses of 30 mg per kg of body weight or a control of
dimethyl sulfoxide. Additionally eight animals of each sex were
administered five daily doses of methyl mercury (7.5 mg/kg body
weight) as a positive control. With cypermethrin, there was no
mortality and the rate of weight gain was consistent with that of the
controls. There was a transient dermal irritation in the majority of
the animals accompanied by skin ulceration. This condition
disappeared at the conclusion of the treatment interval. One male
administered cypermethrin had an unusual gait. There was a slight
deficit in the inclined plane test which was noted in the early parts
of the experiment but was absent by the end of the third week.
Increases in both ß-glucuronidase and ß-galactosidase were evident in
peripheral nerve tissue. It was concluded that cypermethrin when
administered at high subacute doses, produced changes in the sciatic
nerve consistent with Wallerian degeneration. Biochemical changes
were evident as increases in ß-glucuronidase and ß-galactosidase
activity and clinically functional deficits were noted (Dewar and
Moffett, 1978b).
Hen
Groups of adult hens were fed cypermethrin at a dosage rate of 1 mg/kg
for five successive days. After 3 weeks, the dosing regimen was
repeated. Positive (TOCP) and negative control groups were included
in the study. There were no signs of delayed neurotoxicity (as
generally noted with certain organophosphate esters) seen following
cypermethrin treatment. TOCP induced the standard clinical and
histological signs of axon and myelin disruption (Owen and
Butterworth, 1977).
Acute Toxicity
Table 1 contains a summary of the findings of various studies on acute
toxicities to a number of animals.
Table 2 illustrates that the cis isomer is more toxic than the trans.
Table 1. Acute Toxicity of Cypermethrin to Various Animals
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Mouse M,F Oral Corn oil 82 Coombes, Carter et al., 1976
Mouse M,F Oral Dimethylsulphoxide 138 Coombes, Carter et al., 1976
Mouse M,F Oral Aq.suspension 779b Jaggers, 1979
Mouse M,F I.P. corn oil 485 Combs, Carter et al., 1976
Rat M,F Oral Corn oil 251-992 Combs, Carter et al., 1976
Rat M,F Oral Dimethylsulphoxide 303 Combs, Carter et al., 1976
Rat M Oral Glycerol formal 200-400 Combs, Carter, et al.,1976
Rat F oral Glycerol formal app. 200 Combs, Carter et al., 1976
Rat M Oral Aq.suspension 400-800 Combs, Carter et al., 1976
Rat F Oral Aq.suspension app. 400 Combs, Carter et al., 1976
Rat F Oral Aq.suspension 4123b Jaggers, 1979
Rat M Oral Aq.suspension 3000c Jaggers, 1979
Rat (3wks) M,F Oral Dimethylsulphoxide 163 Rose and Dewar, 1978
(6wks) M,F Oral Dimethylsulphoxide 322 Rose and Dewar, 1978
(12 wks) M,F Oral Dimethylsulphoxide 526 Rose and Dewar, 1978
Rat F I.P. Aq.suspension >500b Jaggers, 1979
Rat M,F I.P. Propylene glycol 1000-2000b Jaggers, 1979
Rat F Dermal Undiluted >4800b Jaggers, 1979
Rat M,F Dermal Xylene >1600 Combs, Carter et al., 1976
Syrian
hamster M,F Oral Corn oil >400 Combs, Carter et al., 1976
Chinese
Hamster M,F Oral Corn oil 203 Combs, Carter et al., 1976
Guinea pig M Oral Corn oil app. 500 Combs, Carter et al., 1976
Guinea pig F Oral Corn oil >1000 Combs, Carter et al., 1976
Guinea pig M Oral Aq.suspension >4000b Jaggers, 1979
Table 1. Continued...
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Rabbit F Oral Undiluted >2400b Jaggers, 1979
Rabbit F Dermal Undiluted >2400b Jaggers, 1979
Dom. fowl M,F Oral Dimethylsulphoxide >2000 Combs, Carter et al., 1976
Partridge M,F Oral Dimethylsulphoxide >3000 Combs, Carter et al., 1976
a) Unless specified, all values refer to data generated using 50:50 cis:trans material.
b) Data generated using 40:60 cis:trans material.
c) Data generated using 53:46 cis:trans material.
Table 2. Effect of Cis:Trans Ratio on Acute Toxicity of Cypermethrin
LD50
Species Sex Vehicle Cis:trans ratio (mg/kg) Reference
Rat M,F Dimethylsulphoxide cis only 160-300 Brown, 1979a
M,F Dimethylsulphoxide trans only >2000 Brown, 1979b
F Corn oil cis:trans 90:10 367 Jaggers, 1979
F Corn oil cis:trans 40:60 891 Jaggers, 1979
Cypermethrin was administered dermally to rats by a single application
(5000 mg/kg) or 5 consecutive daily doses (2500 or 5000 mg/kg/day).
Low mortality was observed in the multiple dose groups (20-30%).
Toxic signs of poisoning were similar to those described below.
Axonal lesions of the sciatic nerve were noted in microscopic
examination (Okuno et al., 1976).
The toxic signs noted following acute poisoning with cypermethrin were
similar in most mammalian species. In rats this consisted of
sedation, ataxia, a splayed or ataxic gait and occasional tremors or
convulsions. The onset of toxic signs of poisoning were rapid and
disappeared within several days in survivors. In dogs, signs of acute
intoxication included nervousness, inappetance, diarrhea, vomiting,
tremors and exaggerated ataxia while walking were noted at extremely
high levels. A single application of undiluted cypermethrin to rabbit
eyes produced a mild transient conjunctivitis lasting two days.
Groups of ten male and ten female guinea pigs were used to assess the
skin sensitizing potential of cypermethrin. Two guinea pigs out of
the 20 exposed to cypermethrin showed a positive skin reaction
indicating that cypermethrin may have a weak skin-sensitizing
potential. Groups of four male and four female rabbits were
administered cypermethrin dermally in a 24-hour skin test. A single
application of cypermethrin was observed to be a moderate irritant to
rabbit skin (Coombs et al., 1976).
SHORT TERM STUDIES
Rat
Groups of rats (20 male and 20 female rats per group) were fed
cypermethrin in the diet at concentrations of 0, 75, 150 and 1500 ppm
for 90 days. Cypermethrin used in the study was a 44:56
(cis:trans-ratio) with a purity of 92%. Random samples were
chemically analysed periodically during the course of the study. At
the conclusion of the study, four animals of each sex were maintained
on control diets for a one-week recovery period.
There was no mortality over the course of the study although both
males and females at 1500 ppm had reduced body weight and food
consumption in the first month of the study. After the first month,
the growth rate was similar to that of controls although the animals
never achieved the weight noted in the other groups. At the
conclusion of the study, hematology, clinical chemistry, urinalysis
and histopathology were performed on all animals. With the exception
of a slight increase in M/E ratio in the bone marrow of female rats
fed 1500 ppm in the diet, there were no hematological or urinalysis
effects. An increased microsomal (smooth endoplasmic reticulum)
oxidative activity was noted as an increased hepatic aminopyrine
demethylase activity in both males and females at 1500 ppm and in
males at 150 ppm. These adaptive changes in the subcellular portions
of the liver were substantially reversed within the four week recovery
period. Increased liver weight was not noted on gross examination at
the conclusion of the study. A slight reduction in pituitary weight
of males, while statistically significant, was not dose-related. A
slight decrease in female kidney weight was dose-dependent and
significantly different from control values at the highest level. The
decrease in kidney weight in males was slight and not significantly
different from controls in any of the dietary levels. Gross and
microscopic (including electron microscopic) examinations of tissues
and organs showed no significant differences from the observations
noted in controls. Examination of the sciatic nerve from animals in
the control and 1500 ppm dietary group showed no changes which could
be directly attributable to the presence of cypermethrin in the diet.
Seven of the sixteen males fed 1500 ppm and two of twelve male
controls showed slight changes in myelin which may or may not have
been brought about by histological fixation of the material prior to
examination. The condition of the sciatic nerve of females was
similar to that noted in control females. There were no effects noted
in unmyelinated axons in both males and females. Interim histological
examination at 30 days, during which time clinical signs of poisoning
were noted, was not performed in this study (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats/group, 14 of each sex were
used as controls) were fed trans-isomer for five weeks at dietary
concentrations of 0, 30, 100, 300, 1000 and 3000 ppm. There was no
mortality observed over the course of the study. Food intake and
growth were reduced in the high dosage group. Alkaline phosphatase
activity was increased at the two highest dose levels. Minimal
changes were observed at the two highest dose levels including several
hematological parameters (red blood cell count and hemoglobin
concentration). Liver, spleen and kidney weights were increased at
1000 ppm and above with the spleen and at the 3000 ppm level with the
kidney and liver. Gross and microscopic histopathologic examination
of several tissues and organs revealed no cypermethrin-related
injuries. Special reference was given to the examination of the
sciatic nerve which showed no damage (Hend and Butterworth, 1977a).
Groups of rats (6 male and 6 female rats/group, 10 of each sex were
used as controls) were fed dietary concentrations of cis-isomer at
concentrations of 0, 30, 100, 300, 750 and 1500 ppm for five weeks
following a protocol similar to that described above. Mortality was
observed at the 1500 ppm dose occurring at intervals from 4 to 17 days
from the start of the experiment with all animals displaying
neurotoxic signs of poisoning. At the next lower dose group (750 ppm)
almost all of the animals showed gross neurotoxic signs of poisoning,
abnormal sensitivity to sound and touch and slight cases of ataxia.
There was no compound-related mortality at dose levels of 750 ppm or
below. Growth was reduced at the 750 ppm over the course of the
study. Significant reductions in food intake were also noted at 300
ppm and above at the initial phase of the study. Gross examination of
tissues and organs again revealed a reduction in heart, liver, spleen
and kidney weights. Adjustment of the data for terminal body weight
revealed a statistically significant change in kidney weight at 300
ppm and above while liver weight was increased at 750 ppm. Plasma
protein levels were decreased and urea and potassium concentrations
were increased in male rats at 750 ppm. Gross and microscopic
examinations of tissues and organs showed substantial degeneration at
1500 ppm and above in both the liver and sciatic nerve. Liver lesions
consisted of a coagulative necrosis of hepatocytes occurring in both
males and females. The sciatic nerve from both sexes showed swelling
and breaks in axons with concomitant myelin degeneration and
vacuolation. There were no lesions observed in the brain or the
spinal cord (Hend and Butterworth, 1977b).
Groups of rats (12 male and 12 female rats/group, 24 of each sex were
controls) were fed cypermethrin in the diet at dosage levels of 0, 25,
100, 400, and 1600 ppm for 13 weeks. After two weeks exposure to 1600
ppm, both males and females exhibited hypersensitivity and varying
signs of ataxia. Mortality was observed with male rats up to the
fifth week of feeding after which the surviving animals improved
clinically and appeared normal at the end of the study. Reductions in
body weight, growth and food intake were also noted at the 1600 ppm
dose level. At the conclusion of the study, small increases in plasma
urea were noted in both sexes. Males had a slight increase in plasma
potassium while females had a slight increase in alkaline phosphatase
and plasma protein levels at the 1600 ppm dosage group. Hematological
abnormalities noted at 1600 ppm at the end of 13 weeks included a
reduction of hemoglobin, packed cell volume and red blood cell count
in females and a reduction in kaolin-cephalin clotting time in males.
There were no effects at dose levels below 1600 ppm.
Gross and microscopic pathology performed at the conclusion of the
study showed axonal breaks and vacuolation in the sciatic nerve of
animals fed 1600 ppm in the diet. This was especially noted with
those animals that showed clinical signs of ataxia and died during the
course of the study. At the conclusion of the 13-week trial, sciatic
nerve lesions were not noted in any surviving animals. An increased
kidney weight in males fed 400 ppm was observed which was not
associated with any clinical or pathological signs of abnormality
(Hend and Butterworth, 1976).
Dogs
Groups of beagle dogs (4 male and 4 female dogs/group) were fed
cypermethrin (1:1 cis:trans-isomeric mixture) in the diet at
concentrations of 0, 5, 50, 500 and 1500 ppm for 13 weeks. There was
no mortality over the course of the study. However, there were
significant signs of poisoning observed at the 1500 ppm level which
included diarrhea, anorexia and tremors as well as ataxia,
incoordination and hyperesthesia. On account of these clinical signs,
two male and two female dogs at the high dose level had to be
sacrificed before the end of the experiment. Minor variations were
observed at various hematological parameters with the kaolin-cephalin
clotting time observed to be consistently lower throughout the study
in female dogs at 500 ppm. There were no other significant
differences with respect to standard hematological or clinical
chemistry parameters. Gross examination of kidneys and organs showed
no effect on organ weights attributable to the diet. Microscopic
examination of tissues and organs revealed a nonspecific focal
bronchopneumonia in the lungs of the animals surviving 1500 ppm.
There were no compound-related changes and no histological
abnormalities in other tissues examined with the exception of a
variation in the intensity of the pink colour of the optic disc noted
on ophthalmologic examination at the conclusion of the study.
Because of the pink colouration observed in the optic disc, a further
experiment was undertaken to determine the cause of this occurrence.
Cypermethrin was fed to two groups of three male dogs at
concentrations of 0 and 500 ppm for 13 weeks. Specific ophthalmologic
examination was made to evaluate the degree of colouration of the
optic disc. At the end of 13 weeks, there were no consistent
differences between the colour of the optic discs of the treated dogs
and controls. A no-effect level of 50 ppm was observed in this
short-term study (Buckwell and Butterworth, 1977).
LONG-TERM STUDIES
Rat
Groups of rats (48 male and 48 female rats/group, 96 of each sex were
used as controls) were maintained under SPF conditions and fed 1:1
cis:trans in the diet for 2 years at dosage levels of 0, 1, 10, 100
and 1000 ppm. Chemical analyses were made confirming stability of
cypermethrin in the diet over the test interval.
There was no mortality over the course of the study attributable to
cypermethrin in the diet. General health and behaviour of the
cypermethrin-fed animals was not different from that of the control
animals. Body weight and food consumption data showed a reduction in
dietary intake and growth in both males and females in those groups
fed 1000 ppm in the diet.
Gross morphological changes were noted periodically over the course of
the study. At six months, males fed 1000 ppm had a slightly reduced
testes weight which was not seen thereafter. Slight changes in the
gross kidney weight at periodic intervals in the study were not
accompanied by notable changes in clinical measurements or on
microscopic examinations. These changes in kidney weight were
believed to be unrelated to the presence in the diet. Liver weight
increases were noted at 18 months again in those animals maintained at
the high dose level. The only clinical chemistry changes noted over
the course of the study was a slight decrease in the activity level of
alkaline phosphatase in males at the 2 year interval. The decrease in
alkaline phosphatase activity was not dose-related and was not noted
in females. Minor fluctuations were seen in various hematological
parameters over the course of the study but these, too, were not
compound related nor statistically significant. These changes are not
believed to be related to the presence in the diet. Gross and
microscopic examination of tissues and organs periodically over the
course of the study did not suggest adverse somatic effects.
The kidneys of most animals after 12 months showed chronic nephrosis,
histologically characterized as tubular dilatations, interstitial
chronic inflammatory infiltration and glomerular scarring. In the
liver, varying degrees of chronic inflammatory infiltration,
hepatocyte vacuolation and bile duct proliferation were observed.
These changes were characteristic of ageing processes and were not
attributable to cypermethrin. Dermal ulceration was present in a
number of animals and over the course of the study.
A portion of the sciatic nerve from 12 animals fed 1000 ppm for one
year and 12 control animals was histologically examined for signs of
neurotoxicity. There were no differences in the incidence of abnormal
fibres in the control and animals fed for cypermethrin for 12 months
(Trigg et al., 1977). Microscopic examination of the sciatic nerve
from animals sacrificed at the conclusion of the study showed a small
number of nerve fibers exhibiting slight Wallerian degeneration
changes. These degenerative changes increased with age but did not
appear to be dose-related with respect to severity. Thus,
cypermethrin did not induce neuropathy clinically nor did it induce
histological evidence of nerve degeneration.
There did not appear to be a significant increase in the incidence of
tumor formation in either males or females over the course of the
study. A large number of pituitary adenomas were reported
predominantly in females of all dose groups including controls. This
occurrence did not appear to be dose-related as similar events were
recorded in all dose levels. Based on the reduced body weight
observed at 1000 ppm in the diet of both males and females, the
no-effect level is 100 ppm (McAusland et al., 1978).
Observations in Humans
Urine obtained from operators spraying cypermethrin in experimental
trials was analysed for the presence of the chlorinated cyclopropane
carboxylic acid metabolite. Using standard gas liquid chromatography,
the carboxylic acid metabolite was observed in urine of exposed
workers at levels up to 0.4 µg/ml (the limit of detection was
estimated to be 0.05 µg/ml) (Baldwin, 1978). Studies were carried out
in the Ivory Coast, Africa where operators sprayed with a hand-held
ULV apparatus either cypermethrin or a control UW formulation. The
cypermethrin sprayers were found to have residues on the exposed parts
of their bodies. Medical and electrophysiological examinations showed
no adverse effects from the exposure. The rate of dermal exposure of
the operators during spraying ranged from 1.5 to 46.1 mg/hour. There
was a reasonable relationship between the total cypermethrin deposited
dermally and the excretion in urine. The levels of the cyclopropane
carboxylic and acid metabolite in the 24 hour urine were between
<0.05 and 0.32 mg. This, together with the finding of 0.6 mg of this
metabolite in 72 hour urine from one man led to the estimation that
approximately 3% of the total dermal dose was absorbed and rapidly
excreted by the operators (Prinsen and van Sittert, 1978).
COMMENTS
Cypermethrin is an insecticidally active synthetic pyrethroid
currently being developed for use in agriculture. The acute toxicity
has been studied in a variety of species and the toxic response in all
species is very similar. Cypermethrin has a moderately acute oral
toxicity. The acute signs of poisoning, in addition to a generalized
nervous system response, include a neurological involvement in rodents
where reversible clinical signs of ataxia was accompanied by
histological and biochemical evidence of peripheral (and possibly
central) nerve disruption. Myelin and axon degeneration in the rat
sciatic nerve was noted following high dose level administration.
There was no information on the relative sensitivity of humans to the
nervous system disruption noted in rodent species (see Report Section
3.5).
Cypermethrin is readily absorbed, distributed, and metabolised in
mammals. Because of the chemical and especially the isomeric
complexity of the molecule, the metabolic profile with respect to all
of its isomers is extremely complex. Cypermethrin is readily cleaved
at the ester linkage and subjected to oxidative degradation and
conjugation of the metabolic products. Elimination from the body
following acute and subacute administration is rapid. However, the
clearance rate from adipose tissue is slow and a half-life in rats and
mice may range from 10 to 30 days. The data suggested a potential for
bioaccumulation in the body following continuous exposure.
In a wide variety of studies, there was no carcinogenic or mutagenic
potential as evidenced by short-term bioassays or a long-term chronic
study. Reproduction was unaffected and no signs of teratogenic
potential were noted in two species examined. Two dominant lethal
tests were performed, and while data were basically negative, these
studies did not give full assurance of the lack of a dominant lethal
effect on male reproduction. In short-term tests, only younger
animals were susceptible to the inductive effects of cypermethrin on
liver metabolizing enzymes.
The extensive toxicological data base already in existence lent
assurance to the estimation of an ADI for man. The lack of data on
tissue residue storage and release and on the limited human experience
with cypermethrin were instrumental in making this temporary, pending
further information.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 100 ppm in the diet equivalent to 5.0 mg/kg body weight
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.006 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cypermethrin is a recently introduced insecticide recommended as a
foliar spray for the control of a wide range of insect pests. In line
with other members of the pyrethroid group of compounds, it is used at
lower rates than most other types of chemical insecticides.
Application rates seldom exceed 250 g/ha. In most instances a
considerably lower rate is required. Several applications, up to two
per month and up to 7 days before harvesting, may be made during a
growing season.
Current recommendations for use on various food crops are summarised
in Table 3.
Table 3. Current dosage rates for use on various crops
Recommendations
Crop (in active ingredients)
Alfalfa 25 g/ha
Apples 0.003%-0.0125%
Brassica leafy vegetables 30-200 g/ha
Cereals (barley, wheat) 25-200 g/ha
Cherries 0.0075%-0.0125%
Citrus 0.0025%-0.01%
Cotton 50 - 100 g/ha
Grapes 30-50 g/ha
Kiwifruit 0.01%
Legume vegetables 25-200 g/ha
Lettuce 50-150 g/ha
Maize 25-200 g/ha
Pasture 100 g/ha
Peaches 0.002%-0.01%
Pears 0.002%-0.0125%
Plums 0.05%-0.0125%
Potatoes 20-150 g/ha
Rape 25-75 g/ha
Raspberries 0.0075%-0.015%
Soya 50-250 g/ha
Strawberries 0.0075%-0.015%
Sugar beet 25-125 g/ha
Tomatoes 40-150 g/ha
Post-harvest uses
Although cypermethrin may be introduced to control pests of stored
commodities and for animal health purposes, no data were available for
evaluation at this meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials carried out on a
world-wide basis and on the main crops treated. Some data from
excessive treatments rates are included where they provide useful
information, e.g. on the effect of processing. Treatments were
generally made using E.C. formulations which are representative of
current application practice.
MAIN CROPS
Apples
Residue data have been obtained from trials in several countries. In
these experiments, dosages covered recommended and higher rates and
were in the range of 0.0025% - 0.02% (25-200 g/ha), based on a spray
volume of 1000 l/ha. Treatments were made on up to 12 occasions.
Results and details of the trials are summarised in Table 4.
Residues in whole fruit one week after treatment at recommended rates
up to 0.02% were usually below 1 mg/kg except when six or more
applications were made. Apart from one instance, however, these were
all less than 1.5 mg/kg. In the one case, where 2.7 mg/kg was
reported, the result was considered to be atypical.
Examination of fruit pulp and peel separately showed that residues
were located mainly in the peel, the average weight of which was
10-18% of the whole fruit. After peeling, levels in 78 samples were
generally less than 10% of those in whole fruit.
Pears
Samples of fruit were analyzed from trials carried out in Europe,
Canada, Australia and South Africa. In these experiments, treatment
rates were between 0.0008% and 0.04% (or 30-400 g/ha). Between one
and six treatments were made and fruit harvested at various intervals
after treatment. The results obtained were similar to those with
apples. Residues in whole fruit, one week after treatment at rates up
to 0.02% (slightly above the recommended rate of 0.015%), were all
below 1 mg/kg. As with apples, analysis of peeled fruit showed that
cypermethrin residues were mainly in the peel. In 32 samples,
residues in the pulp did not exceed 30% of those in whole fruit.
Table 4. Residues Following Supervised Trials with Apples (1976-1978)
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Granny Smith Australia 3 0.02% (to
run off) 382 0.95 0.45 0.45 1
MacIntosh Canada 3 0.18 2 0.57 0.38 2
Lobo 3 0.18 2 0.47 0.32
Northern spy 8 0.07 9-15
days 0.13-0.3 3
MacIntosh 8 0.07 9-15
days 0.1-0.251
Northern Spy 8 0.05 " 0.2-0.26
MacIntosh 8 0.15 " 0.2-2.25
Richared France 1 0.15 0.25 0.19 0.6 0.25 4
Golden
Delicious 7 0.05 2-3 0.05 5
Golden
Delicious FRG 6 0.2 2,3,2,3 3.1 1.0 0.6 0.3 0.5 6
and 1 3.2 2.7 1.9 1.7 0.81
James Grieve 6 0.02% 1,1,1´
4 & 1´ 1.3 0.96 0.82 0.8 0.67 7
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Cox's Orange 6 0.02% 1,3,4
4 & 2 0.97 0.75 0.6 0.84 0.31
Golden
Delicious 6 0.02% 1,6,6,
2 & 2 1.7 1.3 0.93 0.9 0.9
6 0.1 1-3 1.2 1.1 1.0 0.9 0.8 8
6 0.1 1-3 0.8 0.7 0.7 0.95 0.5
Italy 1 0.037 - 0.3-0.5 11
Strumer N. Zealand 12 0.008% 2-3 1.1 0.6 0.7 0.53 12
Reinette Portugal 5 0.005% 3 0.19 0.2 0.14 13
5 0.0075% 3 0.32 0.1
5 0.01 3 0.6 0.35 0.45
Granny Smith S. Africa 1 0.005% - 0.42 0.36 0.28 0.25 14
Spain 1 0.024 - 0.06 0.05 0.04 10
1 0.048 - 0.1 0.1 0.05 0.06 10
U.K. 3 0.005% 1-2 0.29 0.45 0.27
3 0.01% 1-2 0.99 0.91 0.52
3 0.005% 1´ 0.6 0.81 0.64
3 0.01 1´ 1.1 0.98 0.82
1 0.01 - 1.3 1.2 0.83
Worcester
Pearmain U.K. 2 0.015% 0.01 15
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Chivers
Delight 2 0.0025% 4´ 0.02 16
0.005% 4´ 0.05
2 0.01% 6´ 0.01
Laxton Superb 2 0.01% 6´ 0.01
Spartan 1 0.012% - 0.36 0.15 0.07 0.05 17
0.01 - 0.36 0.12 0.03 <0.01
Lobo Sweden 3 2´-4 0.1422 Sweden
Ribston 3 2´-4 0.25
1 Minimum-maximum residues in three samples.
2 90 days after treatment.
Peaches
Residues in whole fruit, less stone, from trees sprayed with
cypermethrin at recommended rate (0.005%-0.01%) and harvested one week
after treatment were generally 1 mg/kg or less although in one trial
in Germany a residue of 1.8 mg/kg was reported in fruit under these
conditions. Analysing 43 samples of fruit after peeling and removal
of the stone showed that levels in the pulp were less than 10% of
levels found in whole fruit.
Details of the trials are given in Table 5, which contains data
relating to some treatments at rates higher than recommended.
Citrus
Experiments were carried out in South Africa (oranges), Spain (lemons)
and Italy (oranges) in which applications of cypermethrin at
recommended rates up to 200 g/ha were made on 1 or 2 occasions. Fruit
was harvested 1 to 39 weeks after treatment, depending on the trial,
and analysed for residues of cypermethrin. The results are shown in
Table 6.
Residues up to 1.3 mg/kg were found in whole fruit taken within six
weeks of treatment. Analysis of peel and pulp showed that the
residues were largely (90%) percent in the peel and that residues in
the pulp were normally below the limit of determination, although in
one series of experiments residue levels up to 0.16 mg/kg were
reported (4 weeks after treatment at 0.02%). In some experiments
samples of orange juice were obtained from treated fruit but residues
were all less than 0.01 mg/kg.
Cotton
Samples of seed were obtained at harvest from trials in several
countries in which up to 12 applications were made at recommended
rates (up to 150 g/ha) and also at treatment rates up to 240 g/ha. The
intervals between the last treatment and harvest varied between 1 day
and 13 weeks. The data obtained are given in Table 7 which also
summarises the trial details.
The seed from these trials was ginned after harvest and was separated
in the laboratory into kernels and hulls. The latter also contained
adhering linters.
In 8 samples no residue of cypermethrin was found in the kernels
(limit of determination of 0.01 mg/kg). Analysis of hulls, which in
most instances had adhering linters, showed that any residues present
were located entirely on the seed surface and its associated fibre.
Table 5. Residues Following Supervised Trials on Peaches (1976-1978)
Application
rate Intervals Residues in mg/kg, at intervals (days) after application1 (Shell)
Country No. kg a.i./ha /week/ 0-1 6-7 12-14 18-21 28-30 40-43 54 Ref.
or %
Australia 3 0.02% 3 & 2 1.0 33
Canada 3 0.006% 4 & 5 0.07 34
3 0.006% 0.34 0.15 0.06 35
0.1 0.052
France 1 0.005% - <0.02 36
0.0075% <0.02
0.0125% - <0.01
0.0187% <0.01
0.015% 0.5 0.4 0.1 0.15
Federal Republic
of Germany 4 0.02% 2 3.2 1.6 1.1 0.65 38
4 0.01% 3,3 & 2 0.85 0.7 0.5 0.18 39
4 0.01% 3,3 & 2 3.0 1.8 1.6 0.75
5 0.01% 2,3,3 & 2 2.0 1.0 0.85 0.3
Italy 1 0.01% - 0.1 40
1 0.02% 0.5
Portugal 1 0.005% 0.08 0.08 0.08 41
0.0075% 0.19 0.11 0.09
0.01% 0.32 0.21 0.14
Spain 1 0.15 0.15 42
2 0.195 3 0.03
2 0.195 1 0.04
1 Results refer to whole fruit without stone.
2 Average of ten samples taken from the same site (min.-.max. values: 0.02-0.11 mg/kg).
Table 6. Residues of Cypermethrin in Citrus (1976 - 1977)
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
LEMONS 150 g/ha 2 27 7 <0.01 2.1 1.1 43
Verna 14 <0.01 2.0 1.0
21 <0.01 2.1 1.1
(Spain) 58 <0.01 1.1 0.55
JUICE
ORANGES 0.01% 1 - 60 <0.01 0.22 0.14 44
Moro 0.02% 1 - 60 <0.01 0.44 0.21
(Italy)
PULP
ORANGES
Moro 0.005% 1 - 67 0.02 0.91 0.30 45
0.01% 1 - 67 0.02 1.2 0.39
0.005 1 - 91 0.01 0.69 0.19
0.01% 1 - 91 0.02 1.3 0.35
0.01% 1 - 27 0.06 1.0 0.36 46
43 0.05 1.5 0.48
56 0.05 1.3 0.40
0.02% 1 - 27 0.16 3.0 1.2
43 0.13 3.7 1.3
56 0.09 1.9 0.73
JUICE
0.005% 1 - 1 <0.01 0.50 0.13 47
4 <0.01 1.2 0.20
8 <0.01 1.0 0.16
18 <0.01 0.81 0.21
39 <0.01 1.1 0.28
68 <0.01 1.3 0.39
(S. Africa) 96 <0.01 1.3 0.38
Table 6. Continued...
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
ORANGES 0.01% 1 - 1 <0.01 1.0 0.30 47
Moro 4 <0.01 0.55 0.22
8 <0.01 0.19 0.16
18 <0.01 0.83 0.25
39 <0.01 0.60 0.16
68 <0.01 0.45 0.13
96 <0.01 0.45 0.13
0.015% 1 - 1 <0.01 2.6 0.58
4 <0.01 1.2 0.30
8 <0.01 1.0 0.24
18 <0.01 1.3 0.25
39 <0.01 1.2 0.21
68 <0.01 1.8 0.40
96 <0.01 0.95 0.22
0.01% 1 - 0 <0.01 1.6 0.52
1 <0.01 2.9 0.85
2 <0.01 2.2 0.65
4 <0.01 2.8 0.55
9 <0.01 1.8 0.58
16 <0.01 1.9 0.72
0.005% 2 6-weeks 69 <0.01 <0.01 <0.01
114 <0.01 <0.01 <0.01
237 <0.01 <0.01 <0.01
0.01% 1 - 110 <0.01 <0.01 <0.01
155 <0.01 <0.01 <0.01
(S. Africa) 278 <0.01 <0.01 <0.01
Table 7. Residues Resulting from Supervised Trials in Cotton Seed (1975-1977)
Application Residues in whole seed (mg/kg) at Intervals (days) after app. lication Ref.
Country rate kg Intervals (Shell)
No. a.i./ha or /Week) 1 7-8 13-15 19-20 28-37 44-55 70-91
Australia 1 0.0015% - 0.01 0.01 10
1 0.003% 0.05 0.01
Brazil 4 0.12 1 0.01 51
4 0.24 1 0.01
4 0.12 1 <0.01 52
0.24 1 0.01
Columbia 1 0.1 0.09 0.03 0.01 54
5 0.1 3 to 8 days <0.01 55
2 0.075 12 days <0.02 56
South Africa 12 0.2 1 to 2 0.01 58
11 0.2 1 to 2 0.02
1 0.1 - <0.01 10
0.2 <0.01
Spain 5 0.1 2,2, 1´ & 2´ 0.04 59
4 0.1 2,2 & 1´ 0.03
5 0.2 2,2, 1´ & 2´ 0.03
4 0.2 2,2,1´ 0.06
3 0.1 2 0.03
3 0.2 2 0.04
3 0.2 0.02
The presence of occasional small residues on the fibre and the outside
of the seed case is to be expected, particularly where applications
were made after the bolls had opened. However, the results indicate
that migration through the seed case to the kernel did not occur even
after treatment at excessive rates, and that measurable residues in
kernels are unlikely to be found in practice.
As a whole, the data suggest that, following treatment according to
recommendations, seed taken 1 week after a series of applications is
unlikely to contain more than 0.1 mg/kg cypermethrin on the whole
seed.
Leafy Vegetables (Brassicae)
Trials have been carried out in a number of countries in order to
obtain residue data on cabbages - including savoy, broccoli, Brussel
sprouts, cauliflower and kale. These are described in Table 8.
The treatments were made according to recommendations (up to 200 g
a.i./ha or 0.02%) or at slightly higher dose rates. In most cabbage
samples as expected, the residue was located mainly in the outer
leaves, which are normally removed in the field, and the majority of
the samples examined were received without the "discard" leaves. In
one experiment however (New Zealand) whole unstripped cabbages were
received. Residues in these samples reaches 3.2 mg/kg but were found
to be reduced to below 0.1 mg/kg on removal of the outer leaves in the
laboratory.
With most of the other brassicae examined, including cauliflower
heads, residue levels were similar to those in cabbage without discard
leaves. But residues in kale from experiments in Germany tended to be
somewhat higher.
OTHER CROPS
Supervised trials were carried out with a number of other crops in
various locations. Residue data obtained in these experiments are
summarized in Table 9 and the following comments.
Alfalfa
A single application at a rate equivalent to 0.1 kg a.i/ha was used to
establish a residue decay curve.
Beans
Trials were carried out in South America on soya and on phaseolus
bean. Residues in beans (soya and phaseolus) removed from their pods
were below measurable levels (0.01 mg/kg) four weeks after treatment,
even where excessive rates (up to 300 g/ha) were used.
Table 8. Residues Resulting from Supervised Trials with Various Vegetables (1976/79)
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha (week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
BROCCOLI 3 0.16 3 & 2 0.38 0.28 0.16 0.06 69
1 0.0015 - 0.11 0.03 <0.01 10
(Canada) 1 0.003% - 0.3 0.03 <0.01
BRUSSELS SPROUTS 3 0.15 1,5 0.55 0.25 0.25 70
7 0.07 3,3,2,2 0.14 0.1 71
2 & 3 0.12 0.09
0.23 0.13
0.18 0.18
6 0.07 3,3 & 2 0.06
0.09
0.1
(Canada) 0.08
CABBAGES 3 0.07 1,5 0.22 0.11 0.11 60
0.22 0.12 0.06
0.16 0.27 0.17
0.22 0.27 0.05
3 0.15 1,5 0.43 0.03 <0.01 61
(Canada) 3 0.16 2 0.15 0.1 0.02 <0.01 62
CABBAGES 4 0.06 2 1 0.4 0.11 <0.01 <0.01 63
(Germany) 4 0.06 2 0.1 0.02 <0.01 <0.01 <0.01
CABBAGES 1 0.25 0.161a Hungary
0.1
(Hungary) 0.2
0.2
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
CABBAGES 5 0.002% 2 0.03 <0.01 0.01
5 0.006 2 0.05 0.02 0.02 66
1 0.002% - 0.07 0.02 0.02
(South Africa) 1 0.006% - 0.08 0.03 0.02
CABBAGES 6 0.14 2 2,11 1,71 1.41 67
0.061b 0.011b 0.011b
6 0.14 0.76 0.6 0.57 0.47 N.Z.
(New Zealand) 6 0.28 1.13 0.82 0.68 0.42
CABBAGES 1 0.008% - 0.51 68
(U.K.)
CABBAGES
(Savoy) 4 0.06 1,1 & 2 0.55 0.4 0.35 0.15 <0.01 64
(Germany) 4 0.06 2 0.3 0.2 0.1 0.05 <0.01
1 0.005% 0.02 65
1 )0.015% 0.02
(Italy) 1 0.03% 0.02
CAULIFLOWERS 3 0.16 3 & 2 0.17 0.09 0.01 <0.01 72
3 0.07 1,5 0.23 0.21 <0.01 73
0.26 0.30 0.01
0.34 0.24 0.08
(Canada) 0.3 0.08 0.11
4 0.06 2 0.03 <0.01 <0.01 <0.01
(Germany) 3 0.06 2 0.05 <0.01 <0.01 <0.01 <0.01
KALE 4 0.06 2 1,6 0.85 0.4 0.3 0.25 75
(Germany) 4 0.06 2 1,4 0.9 0.7 0.5
2 0.075+0.04 6.5 1.1 0.76 0.47 0.13 76
(U.K.) 2 0.225+0.08 6.5 1.5 0.95 0.60 0.23
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
KALE
(China) 6 0.005% 4-5 days 0.85
(Thailand)
NOTE: The cabbages were analysed without the outer leaves with some exceptions:
1 whole cabbage
1a whole cabbages as specified by CC/PR/153/
1b heart only.
Cherries and Plums
Samples of plums and cherries have been obtained from field
experiments in Germany in which cypermethrin was applied on several
occasions (three in the case of cherries, five for plums) at
approximately the recommended rate and double the recommended rate
(0.01% and 0.02% respectively). Under similar conditions, residues in
plums were found to be somewhat lower than in cherries.
Grapes
At recommended rates of up to 80 g/ha and treatments on up to 4
occasions, residues in grapes harvested one week after the last
application were at or below 1 mg/kg (Table 9). In practice, the
material is generally applied an a prophylactic treatment at 50 g/ha
and harvest is likely to be more than one week after treatment.
Lettuce
Residue data are available from trials carried out in Canada, France
and Germany at rates generally in the range of 50-70 g/ha (0.005% to
0.007%, based on 1000 l/ha spray volume). Residue levels were 1.6
mg/kg cypermethrin or less in lettuce taken one week after treatment.
From the glasshouse data available, there was no evidence that levels
in glasshouse lettuce were substantially different from levels in
outdoor crops.
Kiwi Fruit
Supervised trials were carried out in New Zealand at the recommended
dose rate and at double rate (Table 9). The pulp contained 5-10% of
that which was found in whole fruit.
Maize
Residue experiments have been carried out in several countries in
which cypermethrin has been applied to maize at recommended rates up
to 150 g/ha. Green maize (whole plants) were sampled at intervals
following treatment, and in some instances grain was also sampled at
the sweet corn stage or when fully mature.
In green maize following treatments at rates up to 150 g/ha, residues
were below 1 mg/kg two weeks after treatment and below the limit of
determination (0.01 mg/kg) after eight weeks. In grain, no residues
were found in either sweet corn or mature grain, even in samples taken
three days after treatment at this rate.
Pasture
Supervised trials were carried out in New Zealand in which
cypermethrin was applied at several times higher than the recommended
rate. The residues on the grass increased roughly in proportion to
the increases in dosage (Table 8).
Peas
Trials were carried out on peas in the U.K., Hungary and South Africa.
No residues were found at dose rates of 15 to 100 g/ha in the peas
themselves which were sampled between one day and four weeks after
treatment (limit of determination 0.01 mg/kg). However, an excessive
dose rate of 200 g/ha resulted in residues between 0.04-0.07 mg/kg in
the peas (Table 9).
Potatoes
Samples were obtained from residue trials carried out in Europe,
Cyprus and Canada, with from 1-9 treatments at dosage rates in the
range of 10 g/ha to 200 g/ha, and intervals between last treatment and
harvest from 0-8 weeks. Detectable residues were absent from all
samples of whole or peeled tubers, even 3-9 days after treatment.
However, samples from one trial in Italy, obtained three weeks after
treatment were peeled. Residues up to 0.05 mg/kg were found in the
peel, but none was detectable in the peeled potatoes.
Rape
Data from supervised trials in Canada, Germany and the U.K. show that,
where treatments at recommended rates were made more than six weeks
before harvest, residues in the whole seed were below the limit of
determination of 0.01 mg/kg. In one of the replicated Canadian
experiments, however, residues were found in samples taken six weeks
after treatment; residues between replicates varied from 0.01 mg/kg to
0.12 mg/kg.
Raspberries and Strawberries
Trials were carried out in Canada and the U.K. in which raspberry
canes were treated with cypermethrin at rates between 0.005% and
0.015% (the maximum recommended rate). Residues in fruit taken 12 or
more days after treatment were all below 0.5 mg/kg even where multiple
applications were made. Residues in strawberries from Canadian
experiments using cypermethrin at rates of 0.006% or 0.01% on two
occasions at an interval of 8 or 9 days were also below 0.5 mg/kg when
harvested three weeks after treatment (Table 9).
Sugar Beet
In experiments carried out in France, Germany and the U.K., 1 to 5
treatments were carried out at rates between 60 g/ha and 200 g/ha
cypermethrin. Samples of both roots and tops were taken for analysis
at intervals up to 17 weeks from the time of treatment.
Apart from one sample taken two weeks after treatment at 60 g/ha,
which contained a small residue of 0.02 mg/kg, no residues of
cypermethrin were found in the root samples.
In leaves, residues were between 6 and 13 mg/kg immediately after the
last of 5 applications made at two weekly intervals. Subsequently
these declined, reaching 0.02-0.13 mg/kg six weeks after treatment.
After 11 weeks residues were 0.01 mg/kg or less (Table 9).
Tomatoes
In outdoor experiments carried out in Southern Europe, Australia,
Canada, S. Africa, the Canary Islands and Mexico, tomatoes were given
foliar treatments at rates between 50 g/ha and 300 g/ha. Applications
were made on up to 9 occasions at intervals from 5 days to 3 weeks
depending on the experiment. Samples were taken at intervals ranging
from the day of treatment to six weeks later. Although some of the
tomatoes received were green, they were close to ripening
(approximately 5 cm diameter) and in the condition at which they would
be picked for export. The residues (Table 9) did not exceed 0.5 mg/kg
in any of the samples taken shortly after treatment at the recommended
rates (up to 0.0% or 100 g/ha assuming use at 1000 l/ha).
Wheat
Residue trials have been carried out on wheat in Canada and Brazil,
with application rates up to 150 g/ha. In the grain residues did not
exceed 0.1 mg/kg at harvest two weeks after application. Residues in
straw were higher, and the few data obtained suggest that under these
conditions they may approach 10 mg/kg (Table 9).
Table 9. Residues Resulting from Supervised Trials with Various Fruits and Other Crops (1974/79)
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
ALFALFA Cyprus 1 0.1 12 5.6 4.7 10
BEANS
(Dried) Colombia 3 0.15 1 <0.011 87
CHERRIES Germany 3 0.02% 1-2 4.5 3.3 0.65 0.6 18
to run off
3 0.02% 1-2 2.7 1.6 1.0 0.39
to run off
3 0.02%
to run off 1-2 7.4 5.4 1.5 1.2
3 0.01% 1.5-2 0.59 0.47 0.27 0.25 0.2 19
3 1.5-2 1.0 0.6 0.3 0.1 0.1
GRAPES Canada
(Delaware) 1 0.08 - 0.7 0.32 20
21
(Elvira) 4 0.07 4,5 & 3 0.24
(0.1 to
0.37)
(Friedamia) 1 0.006%
to run off 0.75 0.41 0.25
(Sauvignon) France 4 0.075 1 0.08 0.03 0.02 <0.01 22
(Alicante)
(Bouschet) 1 0.075 - 0.4 0.28 0.23 0.12 23
2 0.075 3 0.37
(Riesling) Germany 4 0.08 5,4 & 2 0.5 0.2 0.11 0.09 0.05 24
4 0.08 5,4 & 2 0.8 0.5 0.1 0.05 0.05
(Barbera) Italy 1 0.01% 0.1 25
S. Africa 1 0.0375 - 0.2 0.1 0.18 <0.05 <0.05 S. Africa
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
GRAPES
(Waltham
Cross) 2 0.0375 2 0.24 0.2 0.19 0.07 <0.05
(Chemin
Blane) 1 0.0375 - 0.1 0.12 <0.05 <0.05
2 0.0375 2 0.12 0.07 <0.05 <0.05 <0.05
KIWI FRUIT N. Zealand 6 0.01% 2.2 1.9 1.7 1.3 N.Zealand
6 0.02% 6.2 3.9 3.4 2.8
LETTUCE Canada 6 0.07 1 <0.012 78
0.02
France 1 0.15 0.52 79
0.3 1.1
France 1 0.05 1.2 0.27 0.123 80
1 0.05 1.1 0.3 0.06 81
1 0.05 2.2 1.6 0.35
1 0.05 1.8 0.5 0.02
1 0.054 2.4 1.0 0.85 82
Germany 3 0.06 2 4.0 0.25 0.04 0.02 <0.01 83
3 0.06 2 1.2 0.06 0.01 <0.01
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
MAIZE
Cobs Australia 1 0.0015% <0.01 10
Cobs 0.003% <0.01
Sweet corn Canada 5 0.07 5 days <0.01 84
Sweet corn 3 0.075 1 <0.01 <0.01
Sweet corn 6 0.064 5 days <0.01 <0.01 <0.01 85
Germany 1 0.1 1.9 0.04 86
1 0.1 2.7 2.2 0.51 0.05 <0.015
1 0.1 2.9 1.1 0.15 0.05 0.025
<0.01
Leaves S. Africa 1 0.075 5.2 3.86 0.4 10
Leaves 1 0.15 17 106 2.6
Stalks 1 0.075 <0.01 0.086 <0.01
Stalks 1 0.15 0.05 0.02 0.06
PEAS U.K. 2 0.05 2 <0.01 88
w/o pods 1 0.025 - <0.01 89
Peas Hungary 1 0.1 - <0.017
pods (only) - 0.07
Peas 1 0.2 0.04
0.077
Sweden 2 0.08 2 <0.01 165
S. Africa 1 0.015 - <0.01 <0.016 <0.01 10
1 0.03 - <0.01 <0.016 <0.01
PLUMS Germany 5 0.02% 1-3 1.0 0.65 0.5 90
5 0.02% 1-3 0.9 0.75 0.4 0.25
5 0.02% 1-3 1.3 1.1 0.7
5 0.01% 3,2,3 & 1 0.3 0.29 0.29 0.25 0.11 91
5 0.01% 3,2,3 & 1 0.24 0.15 0.12 0.1
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
POTATOES Canada 4 0.075 2 <0.01 92
4 0.15 2 <0.01
9 0.072 1 <0.01
9 0.145 1 <0.01
4 0.150 2 <0.017 93
9 0.15 1 <0.01 94
Cyprus 1 0.1 <0.01 <0.01 10
France 1 0.15 <0.01 95
Germany 4 0.090 2 <0.01 <0.01 <0.01 96
4 0.2 2 <0.01 <0.01 <0.01 97
4 0.2 2 <0.01 <0.0l <0.01
4 0.2 2 <0.01 <0.01 <0.01
Italy 1 0.04 - 0.058 98
1 0.12 - 0.038
SOYA BEANS Brazil 2 0.24 2 <0.01 99
3 0.24 2 <0.01
dried Colombia 4 0.3 1,1 week
& 4 days <0.01 100
SUGARBEET France 1 0.1 - <0.01
Leaves after 101
17 wks
1 0.15 <0.01
after 102
17 wks
Germany 5 0.06 )2,2,2 1.3 0.52 0.07 0.05 103
5 0.06 )& 1 2.2 0.1 0.03 <0.01
5 0.2 2 12 4.0 2.3 0.02 104
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
SUGARBEET Germany 5 0.2 2 6.2 2.3 0.18 0.05
Leaves 5 0.2 2 13 0.73 0.23 0.13
U.K. 1 0.2 <0.01
after
15 weeks 105
RASPBERRIES Canada 1 0.225 - 0.2110 114
3 0.18 2 0.4510 115
3 0.135 2 0.410 116
U.K. 1 0.005% 1 0.1311 117
0.015% 1 0.2511
STRAWBERRIES Canada 2 0.23 9 days 0.07 118
2 0.18 90 0.04 119
2 0.18 8 days 0.45 120
TOMATOES Australia 9 O.01% 5-7 days 0.3 0.3 0.24 0.14 106
9 0.02% 5-7 days 0.57 0.57 0.44 0.53
1 0.0015% - 0.02 0.03 10
Canada 1 0.15 - 0.13 0.07 107
2 0.15 1 0.2 0.05
France 1 0.0075% - 0.09 0.06 0.04 108
Italy 1 0.005% - 0.02 109
0.015% 0.08
0.03 0.17
Mexico 1 0.0075 - 0.02 0.01 10
1 0.015 - 0.05 0.04
1 0.03 - 0.08 0.13
Portugal 3 0.075% 11 & 7 0.06 0.032 110
days
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
TOMATOES S. Africa 4 0.0075% 3,2,2,2 0.17 0.1 0.05 111
4 0.01% 3,2,2,2 0.27 0.15 0.09
4 0 02% 312,2,2 0.27 0.14 0.08
4 0:03% 3,2,2,2 0.29 0.2 0.6
Spain 7 0.03% 3,2 & 2 0.08 112
1 0.075 - 0.16 0.07 0.14 10
0.16 0.06 0.10
2 0.2 1 0.1 0.09 0.06 113
3 0.2 1 0.25 0.2 0.05
Canary Is. 2 0.1 1 0.25 0.15 0.01 113
3 0.1 1 0.2 0.15 0.06 0.03
2 0.15 1 0.4 0.3 0.15
3 0.15 1 0.55 0.4 0.2 0.6
WHEAT Canada 1 0.14 - 0.1 0.07 0.05 121
1 0.15 - 0.08 0.08 0.08 0.04 122
0.07 0.05 0.06 0.03
0.07 0.05 0.05 0.04
0.09 0.09 0.06 0.04
Brazil 1 0.09 <0.01 <0.01 123
2 0.09 12 days 0.04
2 0.09 31 days 0.03
2 0.09 19 days 0.01
3 0.09 19 & 12
days 0.04
1 +4 week storage, beans were analysed without pods
2 In 3 samples
3 10 days after treatment
4 In glasshouse
Table 9. Continued...
5 The grain contained <0.01 mg/kg
6 8 days after treatment
7 In 9 samples
8 In the peel
9 24 days after treatment
10 12-13 days after treatment
11 19 days after treatment
FATE OF RESIDUES
GENERAL OBSERVATIONS
Following applications to crops cypermethrin may degrade to a variety
of hydrolysis and oxidation products. The most likely degradation
products present in crops at harvest following normal agricultural use
of cypermethrin are the derived amide (compound B), 3-phenoxybenzoic
acid and 2-(2',2'-dichloro vinyl)-3,3 dimethyl cyclopropane carboxylic
acid (compound C), the structures of which are shown in Figure 1. The
latter two compounds are found in the free state as well as in
conjugated forms. However, the evidence indicates that the major
component of any residue present at harvest will be cypermethrin
itself. A number of crop samples obtained from supervised trials
discussed previously were analysed also to determine compounds B, C
and phenoxybenzoic acid. The results of these examinations involving
some 20 crops showed no residues of Compound B, of Compound C or of
3-phenoxy benzoic acid in excess of 0.05 mg/kg.
Following use on animal feed crops, residue may be present in feed at
levels depending on the crop. However, since the product is readily
metabolized by animals, these amounts are unlikely to give rise to
more than traces of foods of animal origin.
IN ANIMALS
Cattle Feeding Studies
Studies were undertaken to investigate the fate of cypermethrin in
cattle and whether residues in meat or milk could arise from the use
of cattle feed containing products made from treated crops. Two
experiments were undertaken, both using radiolabelled cypermethrin.
a. Low Dietary Intake (0.2 mg/kg)
Two lactating cows were given feed concentrate containing
radio-labelled cypermethrin twice daily for a three-week period with a
feeding level equivalent to 0.2 mg/kg on total daily feed. The cows
were milked twice daily and the amount of radioactivity determined at
each milking. The total radioactivity present in whole milk was in
the range of 0.0002 mg/1 to 0.0012 mg/1 in terms of cypermethrin
equivalents and 60-70% of the amount of radioactivity in the milk was
present in the cream fraction. The total radioactivity found in the
milk amounted to only 0.5% of the radioactivity fed to the animals.
The remainder of the radioactivity was excreted in the urine (54%) and
faeces (43%). The elimination occurred rapidly after dosing and the
rate of elimination of radioactivity reached its peak (near 100% of
applied dose) after three days.
Molecular Weight: 416
Technical Material: Contains not less than 90% w/w cypermethrin
which is a racemic mixture of the 8 isomeric
forms with a cis: trans isomer ratio of
approximately 40:60
Physical Form Technical material is a viscous yellow liquid.
Density: 1.12 g/ml at 22°C.
Thermal Stability: Thermal analysis has shown that at a
temperature of 259°C slow weight losses occur
accompanied by negligible heat of reaction
indicating high thermal stability.
Solubility: Water 0.009 mg/l. Hexane 103 g/l. Acetone,
cyclohexanone, ethanol xylene and chloroform
450 g/l.
Volatility: relatively non-volatile
Vapour pressure: 5 × 10-6 N/m2 at 70°C
Flash point: 80°C
Formulations: Currently available in emulsifiable
concentrates ranging from 25-400 a.i. g/l.
Several formulations for ultra low volume
applications containing 8-75 g/l are also
available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Studies were performed in vivo on a wide range of mammalian species
and in vitro on isolated cell fractions to define the
pharmacokinetics of cypermethrin. In general, a wide range of studies
have shown that cypermethrin is rapidly absorbed, distributed to a
variety of tissues and organs, metabolised and rapidly excreted from
the body. Studies have been performed to evaluate the
pharmacokinetics in mice, rats, dogs, sheep and cows.
Mice
Groups of male mice were orally administered the cis or trans isomers
of cypermethrin dissolved in methoxytriglycol at dose levels of 7
mg/kg with the cyclopropyl-14C isomer and 8 mg/kg with the benzyl-14C
isomer. Results suggest that the proportion of cypermethrin which was
readily absorbed following oral dosing was rapidly eliminated in the
urine of mice. Absorption from the gastrointestinal tract was more
rapid with the trans isomer than with the cis as evidenced by high
radioactivity levels of the cis isomers in the faeces within the first
few days of the study. The rate of urinary excretion of cypermethrin
isomers labelled in acid and alcohol portions of the molecule was very
similar with the major quantity of radioactivity being eliminated from
the body within three days. Tissue residues after three days were
low. Adipose tissue was found to retain small quantities of the
radioactivity in concentrations higher than that noted for most other
tissues (Hutson, 1978a).
To evaluate the elimination of cypermethrin and its metabolites from
animal tissues following oral administration, groups of male mice were
administered cypermethrin at dose levels approximating 9 mg/kg body
weight (14C-benzyl, cypermethrin). In contrast to data from other
tissues, the rate of elimination of cypermethrin from adipose tissue
of mice was relatively slow with a half-life of approximately 10-20
days. Unchanged cis-cypermethrin was the only chemical residue
identified in adipose tissue. There was little change in the residue
during the last half of the 42-day trial following the relatively
rapid elimination during the first three weeks suggesting possible
long term storage and buildup of residue in adipose tissue (Crawford
and Hutson, 1978a).
Rat
Groups of male and female Wistar rats were orally administered
cypermethrin (14C benzyl, cis-isomer) at the dose level of 2.4 mg/kg
for females and 1.8 mg/kg for males. Cypermethrin was administered in
corn oil solution and animals and tissues were monitored over a period
of eight days. Excretion was rapid with both sexes. There was a
significant sex difference in the rate of excretion with male rats
excreting a substantially larger portion of the radioactivity in urine
than females over the first 24 hour interval. However, there were no
substantial differences in the overall rate of elimination by both
males and females when data were evaluated over the total 8-day period
of the test. The residues of radioactivity in the tissue of animals
of both males and females sacrificed at various periods over the 8-day
interval showed a small residue in a variety of tissues. While the
tissue residue in blood, liver, kidney, etc. was rapidly depleted over
the test interval, concentrations of cypermethrin in adipose tissue
were relatively stable over the 8-day trial (Crawford, 1976a).
Groups of male and female rats were orally administered cypermethrin
14C-benzyl, trans-isomer) at dose rates of 2.4 mg/kg for males and
3.0 mg/kg for females. Again, excretion was rapid with both sexes
with approximately 95% of the administered dose excreted within 48
hours. In contrast to that noted with the cis-isomer, there appeared
to be no sex differences in the elimination of the trans-isomer.
Residues in adipose tissue of females was somewhat higher than noted
in adipose tissue of males. The observations are consistent with the
suggestion that the trans-isomer is metabolised faster than the
cis-isomer (Crawford, 1976b).
Groups of rats were administered cypermethrin (1:1 cis: trans-isomer
ratio, 14C-cyclopropyl) at dose rate of 1 mg/kg for males and 2 mg/kg
for females. Rapid elimination of cypermethrin was observed with a
substantial difference noted in the 24-hour urinary excretion in males
and females. Females excreted considerably more radioactivity in
urine over the first 24 hours. At the end of 72 hours urinary and
faecal excretion in both sexes was approximately the same. Small
quantities of radioactivity were expired as 14CO2 which suggested
some metabolic breakdown of the cyclopropyl ring. At the end of three
days, tissue concentrations in liver, kidney muscle, brain, blood,
skin and remaining carcass were relatively low. Intestinal content
was somewhat higher in males than in females ranging from 3 to 9% of
the administered dose. Low residue concentrations, which never
exceeded 1 ppm, were observed in fat (Crawford, 1977).
The elimination pattern of cyclopropyl- and benzyl-labelled
cypermethrin appears to be the owns in both males and females.
Females absorb and metabolise the trans-isomer faster than males and
males absorb and metabolise the cis-isomer faster than females
(Crawford, 1976b). In a further study to resolve the residual nature
of the residues of cypermethrin in adipose tissue of rat, Crawford and
Hutson (1978b) again found that the cis-isomer was relatively stable
in adipose tissue. Administration of 14C-benzyl cypermethrin
(cis-isomer) at a dose of approximately 2 mg/kg to female rats
resulted in a residue of approximately 0.3 ppm in the fat eight days
after a single oral dose. Further studies through 42 days following
dosing were performed to evaluate the half-life of the cis-isomer in
fat and the total elimination pattern. At the end of 42 days,
residues were observed in fat which were approximately 10% of those
concentrations noted at eight days. There was a 90% loss of the
material from fat over the 8-42 day interval during which time samples
were taken. From these data with rats, a half-life of approximately
20-25 days was estimated with respect to removal of residues from fat
following a single oral dose. These half-life values are somewhat
longer than those noted with the mouse.
Dogs
Groups of male beagle dogs were administered 14C-benzyl cypermethrin
and the individual 14C-benzyl labelled cis- and trans-isomers of
cypermethrin, orally, at a dose of 2 mg/kg. Elimination of
radioactivity from all animals was rapid although differences in data
from individual dogs precluded a complete evaluation of the rate of
elimination. Differences in the rates of elimination of the
individual dogs in the study may have been due to differences in
absorption as cypermethrin was given orally in a capsule with no
solvent. Tissue residues observed 4 days after oral administration of
cypermethrin were extremely low. The vast majority of the excreted
material was observed in faeces (80%) with urine containing only 11%
of the administered dose. As with other species, small residues of
cypermethrin were observed in fat, approximating 2% of the
administered dose (this residue was estimated to be 0.3 ppm based upon
total adipose tissue of the dog) (Crawford, 1979b).
Sheep
Sheep were administered cypermethrin dermally (962 mg/animal
administered in acetone) or orally (without solvent) in gelatin
capsule at a dose of 4 mg/kg body weight. Following dermal
administration, cypermethrin was not readily absorbed (less than 0.5%
of the administered dose was observed in urine within 24 hours and 2%
over the six day test interval). Little radioactivity was found in
the liver and kidney of dermally-treated sheep. Tissue residues in
renal and subcutaneous fat was found to be similar to those noted with
other animal species and was qualitatively identified as cypermethrin.
Elimination of cypermethrin from the orally-dosed sheep was rapid with
61% of the administered dose being eliminated within 48 hours (41% of
the dose in urine and 20% of the dose in faeces). The tissue residue
pattern in sheep following oral administration was similar to that
noted with other mammalian species. Low levels of cypermethrin were
noted in various tissues, including renal and subcutaneous fat
(Crawford and Rutson, 1977a).
Cows
Cypermethrin (1:1, cis:trans-isomer, 14C-benzyl-label) was orally
administered to lactating cows over a period of three weeks at a
dietary dosage of 0.2 ppm. Urine, faeces and milk residues were
examined over the course of the study and at the conclusion of the
three week dietary interval, animals were sacrificed and tissue
residues examined. Elimination of radioactivity in urine and faeces
rapidly reached an equilibrium within 2-3 days. Radioactivity was
eliminated in the urine and faeces predominantly and accounted for the
major quantity of cypermethrin residues. Small quantities of
radioactivity were noted in milk with a total radioactivity residue
approximating 0.5% of the administered radioactive cypermethrin.
Tissue residues were extremely low with notable residues in bile and
fat as well as in the liver and kidney. There were no detectable
residues in muscle, blood or brain. Analysis of the milk fat showed
that a high percentage of the radioactivity was present in this
portion of the milk. Residues in the milk fat or cream sample were
reflective of the lipoidal nature of cypermethrin, with residues
concentrating in fatty tissues (Hutson and Stoydin, 1976).
In a further study to obtain information on the metabolic distribution
of cypermethrin in cows, lactating cows were treated for 7 days at a
dietary concentration of 5 ppm. Urine, faeces, and milk were analysed
and at the conclusion of the feeding trial, all animals were
sacrificed and tissue residues were determined. As in the previous
study, elimination occurred rapidly after the onset of dosing with the
major route of excretion being via urine and faeces. Tissue residues
were extremely low with measurable amounts observed in blood, liver,
kidney, bile and adipose tissue. Total milk residues amounted to
approximately 0.2% of the applied cypermethrin, with the largest
proportion of the residue again found in milk fat (Crawford, 1978).
Metabolism
The metabolic fate of cypermethrin was investigated in a variety of
mammalian species and was determined to be relatively similar in the
different species. The major metabolites are derived by cleavage of
the ester linkage followed by subsequent metabolism of both the acid
and alcohol fragments and conjugation and excretion of the fragments.
Studies on the metabolic fate of both cis- and trans-cypermethrin
administered orally to rats have shown that cypermethrin is rapidly
cleaved at the ester bond to yield the cyclopropanecarboxylic acid and
the 3-phenoxybenzyl alcohol moiety. The latter is rapidly oxidised to
3-phenoxybenzoic acid and conjugated prior to elimination. Major
reactions occurring with the cyclopropanecarboxycyclic acid include,
in part, oxidation at the methyl groups and apparent lactone
rearrangement prior to conjugation and elimination. Hydroxylation of
the phenoxybenzoyl moiety has been noted to occur in at least two
positions prior to conjugation and elimination. Rats and mice
metabolised cypermethrin in a similar fashion. In mice, a substantial
portion of the phenoxybenzoic acid was conjugated with taurine as well
as with glucuronic acid (Hutson, 1977). Oxidation has been noted to
occur in the 4'- position of phenoxybenzoic acid. Following
oxidation, the molecule is conjugated as a sulfate and excreted. A
portion of unconjugated hydroxylated phenoxybenzoic acid was also
excreted. Hydroxylated cypermethrin was observed in mouse faeces
suggesting that hydroxylation may occur prior to ester cleavage
(Hutson, 1978b). Shono, Ohsawa, and Casida (1978), using mouse liver
microsomal preparations, showed that hydroxylation of the
phenoxybenzyl moiety at positions other than the 4'- position can
occur. Small amounts of the 5- and 6-hydroxy phenoxybenzyl derivatives
were also observed. Both cis- and trans-isomers were rapidly
metabolised by cleavage of the ester bond and aromatic hydroxylation
at the 4'-position of the 3-phenoxybenzyl alcohol moiety. In rats,
approximately half of the administered cypermethrin was excreted as
sulfate conjugates of the hydroxylated phenoxybenzoic acid.
Phenoxybenzoic acid was also excreted free and conjugated with glycine
in contrast to the conjugate pattern noted in mice (Crawford and
Hutson, 1977b, 1978c). In studies with cyclopropanecarboxylic
acid-labelled cypermethrin, hydroxylation of the methyl groups on the
cyclopropane ring occurred to a limited extent. Oxidation was
believed to have occurred following ester cleavage. While most of the
radioactive metabolites were found in the urinal a small quantity of
hydroxylated metabolites were eliminated via the
biliary-intestinal-faecal route suggesting absorption and
re-distribution through the bile to the faeces.
In both rats and mice as well as other species, ester cleavage is
rapid. There is quantitative and qualitative evidence to suggest that
the cis-isomer is more stable yielding a larger variety of
hydroxylated products prior to ester cleavage while the trans-isomer
yields a wider variety of hydrolytic products which in part are
oxidised following the ester cleavage. The acid fragment is
conjugated predominantly with glucuronic acid an a ß-glucuronide.
Conjugated cyclopropanecarboxylic acid derivatives in urine were
identified as glucuronides have a relative resistance to the action of
ß-glucuronidase. ß-Glucuronides formed from these acids are generally
of the ester type readily cleaved by mild acid hydrolysis. Thus,
treatment of urine with methanol and sulfuric acid will convert both
the free acid and the acid glucuronide into a methyl ester. The
methyl ester of the carboxylic acid has been shown to be volatile and
analytical losses may occur with this compound. The alcohol fragment,
the alpha-cyano-3-phenoxybenzyl alcohol, is readily oxidised
metabolically to the benzoic acid, conjugated and excreted. Alcohol
conjugates with glucuronic acid are generally ether derivatives,
stable to acid and enzymatically hydrolysable. The phenoxybenzyl
moiety of cypermethrin has been found to form both ester and ether
ß-glucuronides.
Thus, the results of the studies with both rats and mice suggest that
most of the excreted metabolites of cypermethrin are hydrolysis
products, although hydroxylation of the intact ester has been
reported. Hydroxylation of the methyl groups attached to the
cyclopropane ring has also been reported. There was no evidence of
metabolism at the 2,2-dichlorovinyl moiety. Evidence exists that the
cis-isomer is somewhat more resistant than the trans-isomer to
hydrolysis. In all cases, only small amounts of hydroxylated
metabolites with the intact ester bond were observed with the
cypermethrin.
The metabolic fate of cypermethrin in dogs was qualitatively similar
to that observed with other species with the exception being
conjugation reactions of several metabolites. As with other species,
cypermethrin is rapidly metabolised by cleavage of the ester bond and
by hydroxylation of the phenoxybenzyl moiety at the 4' position.
Sulfate conjugation of the hydroxylated benzoic acid was reported.
Additionally, a major urinary metabolite was identified as the
3-phenoxybenzyl glycine. Studies on the metabolic fate of
3-phenoxybenzoic acid in dogs revealed a metabolic pattern of
oxidation and conjugation similar to that noted with cypermethrin
(Crawford, 1979a and 1979c).
In cows, the major urinary metabolite of cypermethrin ( 14C-benzyl)
was the glutamic acid conjugate of the 3-phenoxybenzoic acid
(Crawford, 1978). The cyclopropanecarboxylic acid moiety was
hydroxylated and/or conjugated with glucuronic acid prior to
excretion.
3-Phenoxybenzoic acid has been observed as a major metabolite of
cypermethrin in all species studied. Residues of cypermethrin in rat
skin (<3% of the dose) were noted after oral administration. Further
studies to define the skin residue were performed using
14C-3-phenoxybenzoic acid administered orally to rats as seven
consecutive daily doses. The radioactive metabolites in skin were
identified as predominately 3-phenoxybenzoic acid and a small quantity
of glyceryl dipalmitate esters of 3-phenoxybenzoic acid (Crawford and
Hutson, 1979).
Further studies were performed in rats of the metabolism of
3-phenoxybenzoic acid and a glucoside conjugate isolated from plant
tissue. 3-Phenoxybenzoic acid was oxidised at the 4' position of both
aromatic moieties and to a minor extent at the 6' position of the
benzoic acid moiety. Excretion occurred as conjugates, predominantly
the 4-hydroxy-3-phenoxybenzoic acid-O-sulfate and 3-phenoxybenzoic
acid. Additionally, other hydroxylated derivatives and conjugates of
these oxidation products were observed. The excretion pattern of the
glycoside conjugates of 3-phenoxybenzoic acid was qualitatively and
quantitatively similar to that observed with 3-phenoxybenzoic acid
suggesting rapid hydrolysis and availability of plant metabolites
following oral administration (Crawford, 1978).
Effects on Enzymes and Other Biochemical Parameters
Preliminary evidence suggested that an increase in the activity of
certain lysosomal enzymes in peripheral nerves and deficits in
behavioural functioning tests could serve as indicators of peripheral
nerve damage. During Wallerian degeneration, the activity of such
enzymes as ß-glucuronidase and ß-galactosidase as well as other
enzymes in nerve preparations were shown to be increased significantly
(Dewar, 1977a).
Cypermethrin (1:1 cis:trans) was administered to male and female rats
at dose levels ranging from 25 to 200 mg/kg/day for five consecutive
days by oral intubation as a 10% W/V solution in DMSO. A dose related
functional deficit was observed when the mean slip angle test and the
landing foot spread test were applied to the animals. The deficit was
maximal from 6 to 14 days after the beginning of treatment and
complete functional recovery occurred within four weeks. Substantial
variation in data from the landing foot spread test was noted. Data
were inconsistent over the course of the study. ß-glucuronidase
activity was increased in a dose-dependent fashion in both males and
females. The results suggest that cypermethrin produced a primary
axonal degeneration, readily measurable 28 days after treatment as an
increase in ß-glucuronidase activity and in deficits in specific
behavioural-function testing of rats (Dewar, 1977b).
When further studies were performed on the biochemical indices of
nerve degeneration to compare results with known degenerating
compounds (methyl mercury), it was observed that the changes in
ß-glucuronidase and ß-glucuronidase activity were considerably smaller
and less reproducible than those obtained following methyl mercury
poisoning (7.5 mg/kg/day for 7 days). In comparison of central versus
peripheral nerve damage, there was no evidence that the trigeminal
nerve was more sensitive to the effects of cypermethrin than the
sciatic and posterior tibial nerves. At near lethal doses of
cypermethrin biochemical changes in the trigeminal nerve were
consistent with those of Wallerian degeneration. The changes were
similar qualitatively to those seen with methyl mercury, but were
quantitatively much less intense (Dewar and Moffett 1978a).
Electrophysiological studies were performed to determine whether acute
or subacute intoxication with cypermethrin produced changes in the
conduction velocity of slower fibres in peripheral nerves or
alterations in the maximal motor conduction velocity. There was no
evidence to suggest that cypermethrin, at doses that induced severe
clinical signs of intoxication, including ataxia, had any effect on
maximal motor conduction velocity or conduction velocity of the slower
motor fibers in peripheral nerves. Doses used in the study ranged
from a single dose of 200 mg/kg to 7 consecutive doses of 150 mg/kg
followed by 2 doses of 400 mg/kg. At near-lethal doses there were no
effects noted on conduction velocity in the slower motor fibers of the
sciatic nerve and tail or on the maximal conduction velocity, even in
the presence of clinical signs of acute intoxication and at dose
levels where previous studies had shown functional degeneration.
These electrophysiological findings are reflective of motor function
which would suggest that the physiological and functional deficits
observed as a result of acute intoxication may be primarily sensory in
nature (Dewar and Deacon, 1977).
SPECIAL STUDIES ON REPRODUCTION
Dominant Lethal Studies
Mice
Groups of male mice (12 mice/group, 36 mice were used as controls)
were administered a single dose of cypermethrin dissolved in dimethyl
sulfoxide at dosage levels of 0, 6.25, 12.5 and 25 mg/kg body weight
or 5 successive daily oral doses of 0, 2.5 and 5.0 mg/kg. Following
dosing, each male was caged with 3 virgin females for 7 days. The
mating procedure was repeated weekly over an interval of 8 weeks in a
standard dominant lethal test.
Females mated to males treated with 5 daily oral doses of 2.5 mg/kg
and those mated to males receiving a single dose of 12.5 mg/kg showed
a significant reduction in the incidence of pregnancy during the
second and third week respectively of the onset of treatment. This
did not occur with other either higher or lower dosed groups or at
other intervals. In females, mated to males treated daily with doses
of cypermethrin a significant reduction in foetal implants was
observed during the second week of mating. Early foetal deaths were
increased in the second week at 5 mg/kg. No such increases occurred
in any other weekly interval or any other dosed group. Thus, multiple
administration of cypermethrin on five successive days induced a
significant reduction in foetal implants during the second week of
mating and a marginal increase in early foetal deaths at the same time
interval.
To evaluate the potential (noted above) for a dominant lethal effect,
a second experiment was performed. Groups of male mice (12
mice/group, a control group consisted of 36 mice) were administered
five daily oral doses of cypermethrin at levels of 0, 2.5, 5.0, 7.5
and 10 mg/kg (in dimethyl sulfoxide). Following dosing each male was
mated with three virgin females for four days and subsequently
provided with virgin females every four days for a period of three
weeks. Female mice were examined for evidence of dominant lethality
13 days after mating. In addition, 40 males of proven fertility were
dosed for five successive days at the same dosage levels, 0, 2, 5,
5.0, 7.5 and 10 mg/kg. These animals were also mated with four virgin
females on four successive days for a period of three weeks. Four
animals from each group were sacrificed for histological examination
of the testes and epididymis on days 1 and 7 after the final dosing.
In contrast to the previous trial, no reduction in foetal implants was
noted in any of the animals mated with cypermethrin-treated males. The
number of early foetal deaths was marginally increased at the highest
dose level in the 12-16 day interval after dosing and in the first 4
day period after treatment with 7.5 mg/kg males.
In the groups of animals examined histologically, no abnormalities
were detected in the testes and epididymis and there were no
observable histological differences between any of the test groups and
the controls (Dean et al., 1977).
Rat
Groups of rats (30 male and 30 female rats/group) were fed
cypermethrin in the diet at concentrations of 0, 10, 100 and 500 ppm
for five weeks after which they were mated to initiate a standard
three generation, 2-litter per generation, reproduction study. After
the selection of the second litter as parents for the following
generation, the original parents were sacrificed and subjected to
gross and microscopic examination. At the conclusion of the study,
ten animals of each sex at the age of 21 days were also examined
histologically. Calculations were made of the indices of fertility,
gestation, viability and lactation. There was no mortality over the
course of the study, and behaviour was not abnormal. Reduced body
weight and food intake was seen at various intervals in both males and
females fed 500 ppm in the diet. Reduced total weight was observed at
500 ppm in the F1A litters and reduced total weight and litter size
were noted at 100 and 500 ppm in the F1B. No compound related gross
or microscopic pathological findings were noted in any rats (Hend et
al., 1978).
SPECIAL STUDIES ON TERATOGENICITY
Rat
Groups of 25 pregnant rats were administered orally from day 6 to day
15 of gestation at dose levels of 0, 17.5, 35.0 and 70.0 mg/kg/day in
a standard teratology bioassay. On day 21 of gestation, the animals
were sacrificed and gross examination of foetuses were made, including
skeletal and somatic examinations. Pre-implantation losses were
evaluated based on corpora lutes, counts and implantation sites and
post-implantation losses were evaluated based upon implantation sites
and viable foetuses. Foetal somatic and skeletal examinations on day
21 of gestation showed no teratogenic changes that could be
attributable to the treatment. There were no indications of
embryotoxic or teratogenic events in the study (Tesh et al., 1978).
Rabbit
Groups of pregnant rabbits (20 rabbits/group, 30 rabbits were used as
an additional control group) were administered cypermethrin dissolved
in corn oil at dose levels of 0, 3, 10 and 30 mg/kg body weight orally
from day 6 to day 18 of gestation. On day 28 of gestation the rabbits
were sacrificed and examination made of live foetuses, dead foetuses,
resorption sites and corpora lutea. Live foetuses were maintained for
24 hours to assess viability. Foetuses were also examined for gross
somatic and skeletal deformities.
There was no significant mortality or differences in weight gain
during the period of gestation. There were no significant differences
between control and test groups with respect to pregnancy, foetal
death and survival. Although a wide range of skeletal and visceral
abnormalities were found in the course of the study, there were no
differences between control and test groups with respect to
abnormalities. It was concluded that oral dosing at up to 30 mg/kg
during the major period of organogenesis resulted in no teratogenic
effects in the offspring (Dix, 1978).
SPECIAL STUDIES FOR MUTAGENICITY
Mice Host-Mediated Assay
Groups of male mice (2-3 mice per group) were administered
cypermethrin, orally, at dose levels of 0, 25 and 50 mg/kg in
dimethylsulfoxide. The animals were immediately injected
interperitoneally with a suspension of S. cerevisiae in a standard
host mediated assay. After five hours, the mutagenic conversion rates
in the yeast cells recovered from the treated animals were comparable
with those of control animals suggesting that, under the terms of this
assay, there was no evidence to suggest a mutagenic potential (Brooks,
1976).
Microorganisms
The mutagenic potential of cypermethrin on various microorganism
species including: S. cerevisiae, E. coli WP2 uvr A, and S.
typhimurium TA-1538 (with and without the use of a rat activation
system) was examined. No increase in the mitotic gene conversion was
recorded in S. cerevisiae, either in the presence or absence of
microsomal oxidation. Cypermethrin, at concentrations up to 500
micrograms per plate, did not induce an increased mutation rate with
E. coli or S. typhimurium TA-1538, in vitro either in the
presence or in the absence of the microsomal oxidation system (Brooks,
1976).
Cypermethrin did not increase the number of revertant colonies of S.
typhymurium (TA-1535, TA-1537, TA-1538, TA-98 and TA-100) in the
presence or absence of a mouse liver subcellular activation
preparation obtained from 6 strains of PCB-treated mice. Cypermethrin
was tested at dose levels up to 1 mg/plate (Suzuki, 1977).
Hamster - Chromosomes
Groups of Chinese Hamsters (12 males and 12 females/group) were
administered orally on each of two successive days at a dose rate of
0, 20 or 40 mg/kg cypermethrin dissolved in dimethylsulfoxide.
Positive and negative controls consisted of cyclophosphamide (100
mg/kg) and dimethylsulfoxide alone. In chromosome preparations of the
control and cypermethrin-treated hamsters, there were no indications
of abnormalities. The cylophosphamide treatment induced a significant
degree of chromosomal damage (Dean, 1977).
SPECIAL STUDIES ON NEUROTOXICITY
Rats
Cypermethrin, orally administered to rats at high acute dosage levels,
produced severe clinical signs of poisoning which was accompanied by
histological evidence of sciatic nerve damage. Within one day
following the acute poisoning, neuropathy was evident as axonal breaks
in the sciatic nerve (Carter and Butterworth, 1976).
Groups of rats (10 males per group) were utilised in a paired feeding
study to examine the neurotoxicological effects of high dietary
levels. Rats were fed dietary levels of cypermethrin (45:55,
cis:trans-isomer ratio) for 14 days at dosage levels of 0, 1250, 2500,
and 5000 ppm. Growth was monitored and clinical evaluations for
adverse behaviour were recorded during the course of the study. Gross
pathology on tissues and organs and microscopic examination of the
sciatic nerve were performed at the conclusion of the study.
At 5000 ppm, mortality was observed with all rats either dying or
sacrificed in a moribund condition within the first week. At 2500
ppm, 6 of 10 rats died before the conclusion of the study. There was
no mortality in the low dose group. Clinical signs of neurotoxicity
were characterised by an impaired ability to walk and splayed hind
limbs. In extreme cases, clinical signs of ataxia and paralysis were
reported. Other clinical signs included: hypersensitivity to external
stimuli, gross disorientation and convulsions, the latter generally
seen at high dose levels. The neurotoxic signs of poisoning observed
in the 1250 ppm group after day three were spontaneously reversed by
day nine when all surviving animals that were initially affected
appeared to be normal. Remission of ataxia at the 2500 ppm group was
also noted within 11 days of treatment. Growth reduction was observed
in all animals in the study. At the lowest dose level, the rate of
growth was delayed for the first few days after which time the rate
was consistent with that of controls for the remainder of the study.
The absolute body weight of the treated animals was, however,
significantly lower than the controls at the conclusion of the
two-week study. Reduction in body weight was consistent with
reduction in food consumption. Ultrastructural changes in the sciatic
nerve were observed in the two highest dose levels, although the
number of animals examined was small. There was some evidence of
axonal damage in the myelinated nerves primarily at the two highest
dose groups. Changes in unmyelinated axons were not observed. In
general, the histological and ultrastructural changes in the sciatic
nerve, accompanied by clinical signs of poisoning, are not readily
apparent at low doses. Thus, cypermethrin, at acutely toxic dietary
levels,induces damage to sciatic nerves, the clinical signs of which
may be reversible (Glaister et al., 1977a).
Hamsters
Groups of male and female Syrian hamsters were orally administered
doses exceeding the LD50 in an attempt to define clinical signs of
poisoning and to evaluate the histological damage to the sciatic
nerve. At doses of 794 mg/kg and above, all animals showed clinical
signs of poisoning including tremors, abnormal, irregular movements,
and an unusual gait. As noted with rats, axon and myelin degeneration
was noted in all groups treated. The lesions included swelling and
breaks in the axons and clumping of myelin (Butterworth and Clark,
1977).
A series of experiments were performed to further evaluate the
neurotoxic potential following subacute, oral administration to
Chinese hamsters. Clinical examination, functional testing and enzyme
determinations using ß-galactosidase and ß-glucuronidase were
performed. The functional test consists of measuring the mean slip
angle where the animal is maintained on an inclined plane that
steadily increases its angle until the animal can no longer maintain a
stationary position. (The average angle of five replicate trials
constituted the mean slip angle test.)
Groups of 20 male and 20 female Chinese hamsters were orally
administered at a dose of 40 mg/kg followed by a dose of 20 mg/kg for
the following four days. Fifteen animals in each sex served as
controls. There was extensive weight loss in all dosed groups and
some mortality was observed primarily as a result of the initial
administration of 40 mg/kg. There was a loss of fur and a dermal
ulceration observed in the early parts of the study. This dermal
occurrence was transient, disappearing rapidly after the treatments
were concluded. There was significant weight loss in the initial
phase of the study. However, after the last dose, the surviving
animals rapidly gained weight at a rate consistent with the control
animals. There were no effects noted on the mean slip angle
experiment and a marginal increase in ß-galactosidase was observed in
peripheral nerve.
A further experiment utilising five male and five female Chinese
hamsters administered cypermethrin daily for five days at dose levels
of 0, 5, 10 and 20 mg/kg showed no mortality over the course of the
study. There was a slower growth of the animals treated with 20 mg/kg
which again was reversed at the conclusion of the treatment period.
Hyperexcitability was noted in one female at the high dose level.
There were no notable differences in behaviour in any of the animals.
There was a significant deficit in the mean slip angle test with
females showing an earlier dose-related deficit than noted in males.
The females recovered from this deficit earlier than males who showed
an erratic pattern of recovery. ß-galactosidase activity was
increased at all dose levels three weeks after the onset of the
experiment. This increase was statistically significant at the two
highest dose levels. Dermal irritation and fur loss was not noted in
this experiment.
Sixteen male and sixteen female Chinese hamsters were administered
five daily doses of 30 mg per kg of body weight or a control of
dimethyl sulfoxide. Additionally eight animals of each sex were
administered five daily doses of methyl mercury (7.5 mg/kg body
weight) as a positive control. With cypermethrin, there was no
mortality and the rate of weight gain was consistent with that of the
controls. There was a transient dermal irritation in the majority of
the animals accompanied by skin ulceration. This condition
disappeared at the conclusion of the treatment interval. One male
administered cypermethrin had an unusual gait. There was a slight
deficit in the inclined plane test which was noted in the early parts
of the experiment but was absent by the end of the third week.
Increases in both ß-glucuronidase and ß-galactosidase were evident in
peripheral nerve tissue. It was concluded that cypermethrin when
administered at high subacute doses, produced changes in the sciatic
nerve consistent with Wallerian degeneration. Biochemical changes
were evident as increases in ß-glucuronidase and ß-galactosidase
activity and clinically functional deficits were noted (Dewar and
Moffett, 1978b).
Hen
Groups of adult hens were fed cypermethrin at a dosage rate of 1 mg/kg
for five successive days. After 3 weeks, the dosing regimen was
repeated. Positive (TOCP) and negative control groups were included
in the study. There were no signs of delayed neurotoxicity (as
generally noted with certain organophosphate esters) seen following
cypermethrin treatment. TOCP induced the standard clinical and
histological signs of axon and myelin disruption (Owen and
Butterworth, 1977).
Acute Toxicity
Table 1 contains a summary of the findings of various studies on acute
toxicities to a number of animals.
Table 2 illustrates that the cis isomer is more toxic than the trans.
Table 1. Acute Toxicity of Cypermethrin to Various Animals
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Mouse M,F Oral Corn oil 82 Coombes, Carter et al., 1976
Mouse M,F Oral Dimethylsulphoxide 138 Coombes, Carter et al., 1976
Mouse M,F Oral Aq.suspension 779b Jaggers, 1979
Mouse M,F I.P. corn oil 485 Combs, Carter et al., 1976
Rat M,F Oral Corn oil 251-992 Combs, Carter et al., 1976
Rat M,F Oral Dimethylsulphoxide 303 Combs, Carter et al., 1976
Rat M Oral Glycerol formal 200-400 Combs, Carter, et al.,1976
Rat F oral Glycerol formal app. 200 Combs, Carter et al., 1976
Rat M Oral Aq.suspension 400-800 Combs, Carter et al., 1976
Rat F Oral Aq.suspension app. 400 Combs, Carter et al., 1976
Rat F Oral Aq.suspension 4123b Jaggers, 1979
Rat M Oral Aq.suspension 3000c Jaggers, 1979
Rat (3wks) M,F Oral Dimethylsulphoxide 163 Rose and Dewar, 1978
(6wks) M,F Oral Dimethylsulphoxide 322 Rose and Dewar, 1978
(12 wks) M,F Oral Dimethylsulphoxide 526 Rose and Dewar, 1978
Rat F I.P. Aq.suspension >500b Jaggers, 1979
Rat M,F I.P. Propylene glycol 1000-2000b Jaggers, 1979
Rat F Dermal Undiluted >4800b Jaggers, 1979
Rat M,F Dermal Xylene >1600 Combs, Carter et al., 1976
Syrian
hamster M,F Oral Corn oil >400 Combs, Carter et al., 1976
Chinese
Hamster M,F Oral Corn oil 203 Combs, Carter et al., 1976
Guinea pig M Oral Corn oil app. 500 Combs, Carter et al., 1976
Guinea pig F Oral Corn oil >1000 Combs, Carter et al., 1976
Guinea pig M Oral Aq.suspension >4000b Jaggers, 1979
Table 1. Continued...
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Rabbit F Oral Undiluted >2400b Jaggers, 1979
Rabbit F Dermal Undiluted >2400b Jaggers, 1979
Dom. fowl M,F Oral Dimethylsulphoxide >2000 Combs, Carter et al., 1976
Partridge M,F Oral Dimethylsulphoxide >3000 Combs, Carter et al., 1976
a) Unless specified, all values refer to data generated using 50:50 cis:trans material.
b) Data generated using 40:60 cis:trans material.
c) Data generated using 53:46 cis:trans material.
Table 2. Effect of Cis:Trans Ratio on Acute Toxicity of Cypermethrin
LD50
Species Sex Vehicle Cis:trans ratio (mg/kg) Reference
Rat M,F Dimethylsulphoxide cis only 160-300 Brown, 1979a
M,F Dimethylsulphoxide trans only >2000 Brown, 1979b
F Corn oil cis:trans 90:10 367 Jaggers, 1979
F Corn oil cis:trans 40:60 891 Jaggers, 1979
Cypermethrin was administered dermally to rats by a single application
(5000 mg/kg) or 5 consecutive daily doses (2500 or 5000 mg/kg/day).
Low mortality was observed in the multiple dose groups (20-30%).
Toxic signs of poisoning were similar to those described below.
Axonal lesions of the sciatic nerve were noted in microscopic
examination (Okuno et al., 1976).
The toxic signs noted following acute poisoning with cypermethrin were
similar in most mammalian species. In rats this consisted of
sedation, ataxia, a splayed or ataxic gait and occasional tremors or
convulsions. The onset of toxic signs of poisoning were rapid and
disappeared within several days in survivors. In dogs, signs of acute
intoxication included nervousness, inappetance, diarrhea, vomiting,
tremors and exaggerated ataxia while walking were noted at extremely
high levels. A single application of undiluted cypermethrin to rabbit
eyes produced a mild transient conjunctivitis lasting two days.
Groups of ten male and ten female guinea pigs were used to assess the
skin sensitizing potential of cypermethrin. Two guinea pigs out of
the 20 exposed to cypermethrin showed a positive skin reaction
indicating that cypermethrin may have a weak skin-sensitizing
potential. Groups of four male and four female rabbits were
administered cypermethrin dermally in a 24-hour skin test. A single
application of cypermethrin was observed to be a moderate irritant to
rabbit skin (Coombs et al., 1976).
SHORT TERM STUDIES
Rat
Groups of rats (20 male and 20 female rats per group) were fed
cypermethrin in the diet at concentrations of 0, 75, 150 and 1500 ppm
for 90 days. Cypermethrin used in the study was a 44:56
(cis:trans-ratio) with a purity of 92%. Random samples were
chemically analysed periodically during the course of the study. At
the conclusion of the study, four animals of each sex were maintained
on control diets for a one-week recovery period.
There was no mortality over the course of the study although both
males and females at 1500 ppm had reduced body weight and food
consumption in the first month of the study. After the first month,
the growth rate was similar to that of controls although the animals
never achieved the weight noted in the other groups. At the
conclusion of the study, hematology, clinical chemistry, urinalysis
and histopathology were performed on all animals. With the exception
of a slight increase in M/E ratio in the bone marrow of female rats
fed 1500 ppm in the diet, there were no hematological or urinalysis
effects. An increased microsomal (smooth endoplasmic reticulum)
oxidative activity was noted as an increased hepatic aminopyrine
demethylase activity in both males and females at 1500 ppm and in
males at 150 ppm. These adaptive changes in the subcellular portions
of the liver were substantially reversed within the four week recovery
period. Increased liver weight was not noted on gross examination at
the conclusion of the study. A slight reduction in pituitary weight
of males, while statistically significant, was not dose-related. A
slight decrease in female kidney weight was dose-dependent and
significantly different from control values at the highest level. The
decrease in kidney weight in males was slight and not significantly
different from controls in any of the dietary levels. Gross and
microscopic (including electron microscopic) examinations of tissues
and organs showed no significant differences from the observations
noted in controls. Examination of the sciatic nerve from animals in
the control and 1500 ppm dietary group showed no changes which could
be directly attributable to the presence of cypermethrin in the diet.
Seven of the sixteen males fed 1500 ppm and two of twelve male
controls showed slight changes in myelin which may or may not have
been brought about by histological fixation of the material prior to
examination. The condition of the sciatic nerve of females was
similar to that noted in control females. There were no effects noted
in unmyelinated axons in both males and females. Interim histological
examination at 30 days, during which time clinical signs of poisoning
were noted, was not performed in this study (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats/group, 14 of each sex were
used as controls) were fed trans-isomer for five weeks at dietary
concentrations of 0, 30, 100, 300, 1000 and 3000 ppm. There was no
mortality observed over the course of the study. Food intake and
growth were reduced in the high dosage group. Alkaline phosphatase
activity was increased at the two highest dose levels. Minimal
changes were observed at the two highest dose levels including several
hematological parameters (red blood cell count and hemoglobin
concentration). Liver, spleen and kidney weights were increased at
1000 ppm and above with the spleen and at the 3000 ppm level with the
kidney and liver. Gross and microscopic histopathologic examination
of several tissues and organs revealed no cypermethrin-related
injuries. Special reference was given to the examination of the
sciatic nerve which showed no damage (Hend and Butterworth, 1977a).
Groups of rats (6 male and 6 female rats/group, 10 of each sex were
used as controls) were fed dietary concentrations of cis-isomer at
concentrations of 0, 30, 100, 300, 750 and 1500 ppm for five weeks
following a protocol similar to that described above. Mortality was
observed at the 1500 ppm dose occurring at intervals from 4 to 17 days
from the start of the experiment with all animals displaying
neurotoxic signs of poisoning. At the next lower dose group (750 ppm)
almost all of the animals showed gross neurotoxic signs of poisoning,
abnormal sensitivity to sound and touch and slight cases of ataxia.
There was no compound-related mortality at dose levels of 750 ppm or
below. Growth was reduced at the 750 ppm over the course of the
study. Significant reductions in food intake were also noted at 300
ppm and above at the initial phase of the study. Gross examination of
tissues and organs again revealed a reduction in heart, liver, spleen
and kidney weights. Adjustment of the data for terminal body weight
revealed a statistically significant change in kidney weight at 300
ppm and above while liver weight was increased at 750 ppm. Plasma
protein levels were decreased and urea and potassium concentrations
were increased in male rats at 750 ppm. Gross and microscopic
examinations of tissues and organs showed substantial degeneration at
1500 ppm and above in both the liver and sciatic nerve. Liver lesions
consisted of a coagulative necrosis of hepatocytes occurring in both
males and females. The sciatic nerve from both sexes showed swelling
and breaks in axons with concomitant myelin degeneration and
vacuolation. There were no lesions observed in the brain or the
spinal cord (Hend and Butterworth, 1977b).
Groups of rats (12 male and 12 female rats/group, 24 of each sex were
controls) were fed cypermethrin in the diet at dosage levels of 0, 25,
100, 400, and 1600 ppm for 13 weeks. After two weeks exposure to 1600
ppm, both males and females exhibited hypersensitivity and varying
signs of ataxia. Mortality was observed with male rats up to the
fifth week of feeding after which the surviving animals improved
clinically and appeared normal at the end of the study. Reductions in
body weight, growth and food intake were also noted at the 1600 ppm
dose level. At the conclusion of the study, small increases in plasma
urea were noted in both sexes. Males had a slight increase in plasma
potassium while females had a slight increase in alkaline phosphatase
and plasma protein levels at the 1600 ppm dosage group. Hematological
abnormalities noted at 1600 ppm at the end of 13 weeks included a
reduction of hemoglobin, packed cell volume and red blood cell count
in females and a reduction in kaolin-cephalin clotting time in males.
There were no effects at dose levels below 1600 ppm.
Gross and microscopic pathology performed at the conclusion of the
study showed axonal breaks and vacuolation in the sciatic nerve of
animals fed 1600 ppm in the diet. This was especially noted with
those animals that showed clinical signs of ataxia and died during the
course of the study. At the conclusion of the 13-week trial, sciatic
nerve lesions were not noted in any surviving animals. An increased
kidney weight in males fed 400 ppm was observed which was not
associated with any clinical or pathological signs of abnormality
(Hend and Butterworth, 1976).
Dogs
Groups of beagle dogs (4 male and 4 female dogs/group) were fed
cypermethrin (1:1 cis:trans-isomeric mixture) in the diet at
concentrations of 0, 5, 50, 500 and 1500 ppm for 13 weeks. There was
no mortality over the course of the study. However, there were
significant signs of poisoning observed at the 1500 ppm level which
included diarrhea, anorexia and tremors as well as ataxia,
incoordination and hyperesthesia. On account of these clinical signs,
two male and two female dogs at the high dose level had to be
sacrificed before the end of the experiment. Minor variations were
observed at various hematological parameters with the kaolin-cephalin
clotting time observed to be consistently lower throughout the study
in female dogs at 500 ppm. There were no other significant
differences with respect to standard hematological or clinical
chemistry parameters. Gross examination of kidneys and organs showed
no effect on organ weights attributable to the diet. Microscopic
examination of tissues and organs revealed a nonspecific focal
bronchopneumonia in the lungs of the animals surviving 1500 ppm.
There were no compound-related changes and no histological
abnormalities in other tissues examined with the exception of a
variation in the intensity of the pink colour of the optic disc noted
on ophthalmologic examination at the conclusion of the study.
Because of the pink colouration observed in the optic disc, a further
experiment was undertaken to determine the cause of this occurrence.
Cypermethrin was fed to two groups of three male dogs at
concentrations of 0 and 500 ppm for 13 weeks. Specific ophthalmologic
examination was made to evaluate the degree of colouration of the
optic disc. At the end of 13 weeks, there were no consistent
differences between the colour of the optic discs of the treated dogs
and controls. A no-effect level of 50 ppm was observed in this
short-term study (Buckwell and Butterworth, 1977).
LONG-TERM STUDIES
Rat
Groups of rats (48 male and 48 female rats/group, 96 of each sex were
used as controls) were maintained under SPF conditions and fed 1:1
cis:trans in the diet for 2 years at dosage levels of 0, 1, 10, 100
and 1000 ppm. Chemical analyses were made confirming stability of
cypermethrin in the diet over the test interval.
There was no mortality over the course of the study attributable to
cypermethrin in the diet. General health and behaviour of the
cypermethrin-fed animals was not different from that of the control
animals. Body weight and food consumption data showed a reduction in
dietary intake and growth in both males and females in those groups
fed 1000 ppm in the diet.
Gross morphological changes were noted periodically over the course of
the study. At six months, males fed 1000 ppm had a slightly reduced
testes weight which was not seen thereafter. Slight changes in the
gross kidney weight at periodic intervals in the study were not
accompanied by notable changes in clinical measurements or on
microscopic examinations. These changes in kidney weight were
believed to be unrelated to the presence in the diet. Liver weight
increases were noted at 18 months again in those animals maintained at
the high dose level. The only clinical chemistry changes noted over
the course of the study was a slight decrease in the activity level of
alkaline phosphatase in males at the 2 year interval. The decrease in
alkaline phosphatase activity was not dose-related and was not noted
in females. Minor fluctuations were seen in various hematological
parameters over the course of the study but these, too, were not
compound related nor statistically significant. These changes are not
believed to be related to the presence in the diet. Gross and
microscopic examination of tissues and organs periodically over the
course of the study did not suggest adverse somatic effects.
The kidneys of most animals after 12 months showed chronic nephrosis,
histologically characterized as tubular dilatations, interstitial
chronic inflammatory infiltration and glomerular scarring. In the
liver, varying degrees of chronic inflammatory infiltration,
hepatocyte vacuolation and bile duct proliferation were observed.
These changes were characteristic of ageing processes and were not
attributable to cypermethrin. Dermal ulceration was present in a
number of animals and over the course of the study.
A portion of the sciatic nerve from 12 animals fed 1000 ppm for one
year and 12 control animals was histologically examined for signs of
neurotoxicity. There were no differences in the incidence of abnormal
fibres in the control and animals fed for cypermethrin for 12 months
(Trigg et al., 1977). Microscopic examination of the sciatic nerve
from animals sacrificed at the conclusion of the study showed a small
number of nerve fibers exhibiting slight Wallerian degeneration
changes. These degenerative changes increased with age but did not
appear to be dose-related with respect to severity. Thus,
cypermethrin did not induce neuropathy clinically nor did it induce
histological evidence of nerve degeneration.
There did not appear to be a significant increase in the incidence of
tumor formation in either males or females over the course of the
study. A large number of pituitary adenomas were reported
predominantly in females of all dose groups including controls. This
occurrence did not appear to be dose-related as similar events were
recorded in all dose levels. Based on the reduced body weight
observed at 1000 ppm in the diet of both males and females, the
no-effect level is 100 ppm (McAusland et al., 1978).
Observations in Humans
Urine obtained from operators spraying cypermethrin in experimental
trials was analysed for the presence of the chlorinated cyclopropane
carboxylic acid metabolite. Using standard gas liquid chromatography,
the carboxylic acid metabolite was observed in urine of exposed
workers at levels up to 0.4 µg/ml (the limit of detection was
estimated to be 0.05 µg/ml) (Baldwin, 1978). Studies were carried out
in the Ivory Coast, Africa where operators sprayed with a hand-held
ULV apparatus either cypermethrin or a control UW formulation. The
cypermethrin sprayers were found to have residues on the exposed parts
of their bodies. Medical and electrophysiological examinations showed
no adverse effects from the exposure. The rate of dermal exposure of
the operators during spraying ranged from 1.5 to 46.1 mg/hour. There
was a reasonable relationship between the total cypermethrin deposited
dermally and the excretion in urine. The levels of the cyclopropane
carboxylic and acid metabolite in the 24 hour urine were between
<0.05 and 0.32 mg. This, together with the finding of 0.6 mg of this
metabolite in 72 hour urine from one man led to the estimation that
approximately 3% of the total dermal dose was absorbed and rapidly
excreted by the operators (Prinsen and van Sittert, 1978).
COMMENTS
Cypermethrin is an insecticidally active synthetic pyrethroid
currently being developed for use in agriculture. The acute toxicity
has been studied in a variety of species and the toxic response in all
species is very similar. Cypermethrin has a moderately acute oral
toxicity. The acute signs of poisoning, in addition to a generalized
nervous system response, include a neurological involvement in rodents
where reversible clinical signs of ataxia was accompanied by
histological and biochemical evidence of peripheral (and possibly
central) nerve disruption. Myelin and axon degeneration in the rat
sciatic nerve was noted following high dose level administration.
There was no information on the relative sensitivity of humans to the
nervous system disruption noted in rodent species (see Report Section
3.5).
Cypermethrin is readily absorbed, distributed, and metabolised in
mammals. Because of the chemical and especially the isomeric
complexity of the molecule, the metabolic profile with respect to all
of its isomers is extremely complex. Cypermethrin is readily cleaved
at the ester linkage and subjected to oxidative degradation and
conjugation of the metabolic products. Elimination from the body
following acute and subacute administration is rapid. However, the
clearance rate from adipose tissue is slow and a half-life in rats and
mice may range from 10 to 30 days. The data suggested a potential for
bioaccumulation in the body following continuous exposure.
In a wide variety of studies, there was no carcinogenic or mutagenic
potential as evidenced by short-term bioassays or a long-term chronic
study. Reproduction was unaffected and no signs of teratogenic
potential were noted in two species examined. Two dominant lethal
tests were performed, and while data were basically negative, these
studies did not give full assurance of the lack of a dominant lethal
effect on male reproduction. In short-term tests, only younger
animals were susceptible to the inductive effects of cypermethrin on
liver metabolizing enzymes.
The extensive toxicological data base already in existence lent
assurance to the estimation of an ADI for man. The lack of data on
tissue residue storage and release and on the limited human experience
with cypermethrin were instrumental in making this temporary, pending
further information.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 100 ppm in the diet equivalent to 5.0 mg/kg body weight
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.006 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cypermethrin is a recently introduced insecticide recommended as a
foliar spray for the control of a wide range of insect pests. In line
with other members of the pyrethroid group of compounds, it is used at
lower rates than most other types of chemical insecticides.
Application rates seldom exceed 250 g/ha. In most instances a
considerably lower rate is required. Several applications, up to two
per month and up to 7 days before harvesting, may be made during a
growing season.
Current recommendations for use on various food crops are summarised
in Table 3.
Table 3. Current dosage rates for use on various crops
Recommendations
Crop (in active ingredients)
Alfalfa 25 g/ha
Apples 0.003%-0.0125%
Brassica leafy vegetables 30-200 g/ha
Cereals (barley, wheat) 25-200 g/ha
Cherries 0.0075%-0.0125%
Citrus 0.0025%-0.01%
Cotton 50 - 100 g/ha
Grapes 30-50 g/ha
Kiwifruit 0.01%
Legume vegetables 25-200 g/ha
Lettuce 50-150 g/ha
Maize 25-200 g/ha
Pasture 100 g/ha
Peaches 0.002%-0.01%
Pears 0.002%-0.0125%
Plums 0.05%-0.0125%
Potatoes 20-150 g/ha
Rape 25-75 g/ha
Raspberries 0.0075%-0.015%
Soya 50-250 g/ha
Strawberries 0.0075%-0.015%
Sugar beet 25-125 g/ha
Tomatoes 40-150 g/ha
Post-harvest uses
Although cypermethrin may be introduced to control pests of stored
commodities and for animal health purposes, no data were available for
evaluation at this meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials carried out on a
world-wide basis and on the main crops treated. Some data from
excessive treatments rates are included where they provide useful
information, e.g. on the effect of processing. Treatments were
generally made using E.C. formulations which are representative of
current application practice.
MAIN CROPS
Apples
Residue data have been obtained from trials in several countries. In
these experiments, dosages covered recommended and higher rates and
were in the range of 0.0025% - 0.02% (25-200 g/ha), based on a spray
volume of 1000 l/ha. Treatments were made on up to 12 occasions.
Results and details of the trials are summarised in Table 4.
Residues in whole fruit one week after treatment at recommended rates
up to 0.02% were usually below 1 mg/kg except when six or more
applications were made. Apart from one instance, however, these were
all less than 1.5 mg/kg. In the one case, where 2.7 mg/kg was
reported, the result was considered to be atypical.
Examination of fruit pulp and peel separately showed that residues
were located mainly in the peel, the average weight of which was
10-18% of the whole fruit. After peeling, levels in 78 samples were
generally less than 10% of those in whole fruit.
Pears
Samples of fruit were analyzed from trials carried out in Europe,
Canada, Australia and South Africa. In these experiments, treatment
rates were between 0.0008% and 0.04% (or 30-400 g/ha). Between one
and six treatments were made and fruit harvested at various intervals
after treatment. The results obtained were similar to those with
apples. Residues in whole fruit, one week after treatment at rates up
to 0.02% (slightly above the recommended rate of 0.015%), were all
below 1 mg/kg. As with apples, analysis of peeled fruit showed that
cypermethrin residues were mainly in the peel. In 32 samples,
residues in the pulp did not exceed 30% of those in whole fruit.
Table 4. Residues Following Supervised Trials with Apples (1976-1978)
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Granny Smith Australia 3 0.02% (to
run off) 382 0.95 0.45 0.45 1
MacIntosh Canada 3 0.18 2 0.57 0.38 2
Lobo 3 0.18 2 0.47 0.32
Northern spy 8 0.07 9-15
days 0.13-0.3 3
MacIntosh 8 0.07 9-15
days 0.1-0.251
Northern Spy 8 0.05 " 0.2-0.26
MacIntosh 8 0.15 " 0.2-2.25
Richared France 1 0.15 0.25 0.19 0.6 0.25 4
Golden
Delicious 7 0.05 2-3 0.05 5
Golden
Delicious FRG 6 0.2 2,3,2,3 3.1 1.0 0.6 0.3 0.5 6
and 1 3.2 2.7 1.9 1.7 0.81
James Grieve 6 0.02% 1,1,1´
4 & 1´ 1.3 0.96 0.82 0.8 0.67 7
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Cox's Orange 6 0.02% 1,3,4
4 & 2 0.97 0.75 0.6 0.84 0.31
Golden
Delicious 6 0.02% 1,6,6,
2 & 2 1.7 1.3 0.93 0.9 0.9
6 0.1 1-3 1.2 1.1 1.0 0.9 0.8 8
6 0.1 1-3 0.8 0.7 0.7 0.95 0.5
Italy 1 0.037 - 0.3-0.5 11
Strumer N. Zealand 12 0.008% 2-3 1.1 0.6 0.7 0.53 12
Reinette Portugal 5 0.005% 3 0.19 0.2 0.14 13
5 0.0075% 3 0.32 0.1
5 0.01 3 0.6 0.35 0.45
Granny Smith S. Africa 1 0.005% - 0.42 0.36 0.28 0.25 14
Spain 1 0.024 - 0.06 0.05 0.04 10
1 0.048 - 0.1 0.1 0.05 0.06 10
U.K. 3 0.005% 1-2 0.29 0.45 0.27
3 0.01% 1-2 0.99 0.91 0.52
3 0.005% 1´ 0.6 0.81 0.64
3 0.01 1´ 1.1 0.98 0.82
1 0.01 - 1.3 1.2 0.83
Worcester
Pearmain U.K. 2 0.015% 0.01 15
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Chivers
Delight 2 0.0025% 4´ 0.02 16
0.005% 4´ 0.05
2 0.01% 6´ 0.01
Laxton Superb 2 0.01% 6´ 0.01
Spartan 1 0.012% - 0.36 0.15 0.07 0.05 17
0.01 - 0.36 0.12 0.03 <0.01
Lobo Sweden 3 2´-4 0.1422 Sweden
Ribston 3 2´-4 0.25
1 Minimum-maximum residues in three samples.
2 90 days after treatment.
Peaches
Residues in whole fruit, less stone, from trees sprayed with
cypermethrin at recommended rate (0.005%-0.01%) and harvested one week
after treatment were generally 1 mg/kg or less although in one trial
in Germany a residue of 1.8 mg/kg was reported in fruit under these
conditions. Analysing 43 samples of fruit after peeling and removal
of the stone showed that levels in the pulp were less than 10% of
levels found in whole fruit.
Details of the trials are given in Table 5, which contains data
relating to some treatments at rates higher than recommended.
Citrus
Experiments were carried out in South Africa (oranges), Spain (lemons)
and Italy (oranges) in which applications of cypermethrin at
recommended rates up to 200 g/ha were made on 1 or 2 occasions. Fruit
was harvested 1 to 39 weeks after treatment, depending on the trial,
and analysed for residues of cypermethrin. The results are shown in
Table 6.
Residues up to 1.3 mg/kg were found in whole fruit taken within six
weeks of treatment. Analysis of peel and pulp showed that the
residues were largely (90%) percent in the peel and that residues in
the pulp were normally below the limit of determination, although in
one series of experiments residue levels up to 0.16 mg/kg were
reported (4 weeks after treatment at 0.02%). In some experiments
samples of orange juice were obtained from treated fruit but residues
were all less than 0.01 mg/kg.
Cotton
Samples of seed were obtained at harvest from trials in several
countries in which up to 12 applications were made at recommended
rates (up to 150 g/ha) and also at treatment rates up to 240 g/ha. The
intervals between the last treatment and harvest varied between 1 day
and 13 weeks. The data obtained are given in Table 7 which also
summarises the trial details.
The seed from these trials was ginned after harvest and was separated
in the laboratory into kernels and hulls. The latter also contained
adhering linters.
In 8 samples no residue of cypermethrin was found in the kernels
(limit of determination of 0.01 mg/kg). Analysis of hulls, which in
most instances had adhering linters, showed that any residues present
were located entirely on the seed surface and its associated fibre.
Table 5. Residues Following Supervised Trials on Peaches (1976-1978)
Application
rate Intervals Residues in mg/kg, at intervals (days) after application1 (Shell)
Country No. kg a.i./ha /week/ 0-1 6-7 12-14 18-21 28-30 40-43 54 Ref.
or %
Australia 3 0.02% 3 & 2 1.0 33
Canada 3 0.006% 4 & 5 0.07 34
3 0.006% 0.34 0.15 0.06 35
0.1 0.052
France 1 0.005% - <0.02 36
0.0075% <0.02
0.0125% - <0.01
0.0187% <0.01
0.015% 0.5 0.4 0.1 0.15
Federal Republic
of Germany 4 0.02% 2 3.2 1.6 1.1 0.65 38
4 0.01% 3,3 & 2 0.85 0.7 0.5 0.18 39
4 0.01% 3,3 & 2 3.0 1.8 1.6 0.75
5 0.01% 2,3,3 & 2 2.0 1.0 0.85 0.3
Italy 1 0.01% - 0.1 40
1 0.02% 0.5
Portugal 1 0.005% 0.08 0.08 0.08 41
0.0075% 0.19 0.11 0.09
0.01% 0.32 0.21 0.14
Spain 1 0.15 0.15 42
2 0.195 3 0.03
2 0.195 1 0.04
1 Results refer to whole fruit without stone.
2 Average of ten samples taken from the same site (min.-.max. values: 0.02-0.11 mg/kg).
Table 6. Residues of Cypermethrin in Citrus (1976 - 1977)
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
LEMONS 150 g/ha 2 27 7 <0.01 2.1 1.1 43
Verna 14 <0.01 2.0 1.0
21 <0.01 2.1 1.1
(Spain) 58 <0.01 1.1 0.55
JUICE
ORANGES 0.01% 1 - 60 <0.01 0.22 0.14 44
Moro 0.02% 1 - 60 <0.01 0.44 0.21
(Italy)
PULP
ORANGES
Moro 0.005% 1 - 67 0.02 0.91 0.30 45
0.01% 1 - 67 0.02 1.2 0.39
0.005 1 - 91 0.01 0.69 0.19
0.01% 1 - 91 0.02 1.3 0.35
0.01% 1 - 27 0.06 1.0 0.36 46
43 0.05 1.5 0.48
56 0.05 1.3 0.40
0.02% 1 - 27 0.16 3.0 1.2
43 0.13 3.7 1.3
56 0.09 1.9 0.73
JUICE
0.005% 1 - 1 <0.01 0.50 0.13 47
4 <0.01 1.2 0.20
8 <0.01 1.0 0.16
18 <0.01 0.81 0.21
39 <0.01 1.1 0.28
68 <0.01 1.3 0.39
(S. Africa) 96 <0.01 1.3 0.38
Table 6. Continued...
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
ORANGES 0.01% 1 - 1 <0.01 1.0 0.30 47
Moro 4 <0.01 0.55 0.22
8 <0.01 0.19 0.16
18 <0.01 0.83 0.25
39 <0.01 0.60 0.16
68 <0.01 0.45 0.13
96 <0.01 0.45 0.13
0.015% 1 - 1 <0.01 2.6 0.58
4 <0.01 1.2 0.30
8 <0.01 1.0 0.24
18 <0.01 1.3 0.25
39 <0.01 1.2 0.21
68 <0.01 1.8 0.40
96 <0.01 0.95 0.22
0.01% 1 - 0 <0.01 1.6 0.52
1 <0.01 2.9 0.85
2 <0.01 2.2 0.65
4 <0.01 2.8 0.55
9 <0.01 1.8 0.58
16 <0.01 1.9 0.72
0.005% 2 6-weeks 69 <0.01 <0.01 <0.01
114 <0.01 <0.01 <0.01
237 <0.01 <0.01 <0.01
0.01% 1 - 110 <0.01 <0.01 <0.01
155 <0.01 <0.01 <0.01
(S. Africa) 278 <0.01 <0.01 <0.01
Table 7. Residues Resulting from Supervised Trials in Cotton Seed (1975-1977)
Application Residues in whole seed (mg/kg) at Intervals (days) after app. lication Ref.
Country rate kg Intervals (Shell)
No. a.i./ha or /Week) 1 7-8 13-15 19-20 28-37 44-55 70-91
Australia 1 0.0015% - 0.01 0.01 10
1 0.003% 0.05 0.01
Brazil 4 0.12 1 0.01 51
4 0.24 1 0.01
4 0.12 1 <0.01 52
0.24 1 0.01
Columbia 1 0.1 0.09 0.03 0.01 54
5 0.1 3 to 8 days <0.01 55
2 0.075 12 days <0.02 56
South Africa 12 0.2 1 to 2 0.01 58
11 0.2 1 to 2 0.02
1 0.1 - <0.01 10
0.2 <0.01
Spain 5 0.1 2,2, 1´ & 2´ 0.04 59
4 0.1 2,2 & 1´ 0.03
5 0.2 2,2, 1´ & 2´ 0.03
4 0.2 2,2,1´ 0.06
3 0.1 2 0.03
3 0.2 2 0.04
3 0.2 0.02
The presence of occasional small residues on the fibre and the outside
of the seed case is to be expected, particularly where applications
were made after the bolls had opened. However, the results indicate
that migration through the seed case to the kernel did not occur even
after treatment at excessive rates, and that measurable residues in
kernels are unlikely to be found in practice.
As a whole, the data suggest that, following treatment according to
recommendations, seed taken 1 week after a series of applications is
unlikely to contain more than 0.1 mg/kg cypermethrin on the whole
seed.
Leafy Vegetables (Brassicae)
Trials have been carried out in a number of countries in order to
obtain residue data on cabbages - including savoy, broccoli, Brussel
sprouts, cauliflower and kale. These are described in Table 8.
The treatments were made according to recommendations (up to 200 g
a.i./ha or 0.02%) or at slightly higher dose rates. In most cabbage
samples as expected, the residue was located mainly in the outer
leaves, which are normally removed in the field, and the majority of
the samples examined were received without the "discard" leaves. In
one experiment however (New Zealand) whole unstripped cabbages were
received. Residues in these samples reaches 3.2 mg/kg but were found
to be reduced to below 0.1 mg/kg on removal of the outer leaves in the
laboratory.
With most of the other brassicae examined, including cauliflower
heads, residue levels were similar to those in cabbage without discard
leaves. But residues in kale from experiments in Germany tended to be
somewhat higher.
OTHER CROPS
Supervised trials were carried out with a number of other crops in
various locations. Residue data obtained in these experiments are
summarized in Table 9 and the following comments.
Alfalfa
A single application at a rate equivalent to 0.1 kg a.i/ha was used to
establish a residue decay curve.
Beans
Trials were carried out in South America on soya and on phaseolus
bean. Residues in beans (soya and phaseolus) removed from their pods
were below measurable levels (0.01 mg/kg) four weeks after treatment,
even where excessive rates (up to 300 g/ha) were used.
Table 8. Residues Resulting from Supervised Trials with Various Vegetables (1976/79)
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha (week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
BROCCOLI 3 0.16 3 & 2 0.38 0.28 0.16 0.06 69
1 0.0015 - 0.11 0.03 <0.01 10
(Canada) 1 0.003% - 0.3 0.03 <0.01
BRUSSELS SPROUTS 3 0.15 1,5 0.55 0.25 0.25 70
7 0.07 3,3,2,2 0.14 0.1 71
2 & 3 0.12 0.09
0.23 0.13
0.18 0.18
6 0.07 3,3 & 2 0.06
0.09
0.1
(Canada) 0.08
CABBAGES 3 0.07 1,5 0.22 0.11 0.11 60
0.22 0.12 0.06
0.16 0.27 0.17
0.22 0.27 0.05
3 0.15 1,5 0.43 0.03 <0.01 61
(Canada) 3 0.16 2 0.15 0.1 0.02 <0.01 62
CABBAGES 4 0.06 2 1 0.4 0.11 <0.01 <0.01 63
(Germany) 4 0.06 2 0.1 0.02 <0.01 <0.01 <0.01
CABBAGES 1 0.25 0.161a Hungary
0.1
(Hungary) 0.2
0.2
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
CABBAGES 5 0.002% 2 0.03 <0.01 0.01
5 0.006 2 0.05 0.02 0.02 66
1 0.002% - 0.07 0.02 0.02
(South Africa) 1 0.006% - 0.08 0.03 0.02
CABBAGES 6 0.14 2 2,11 1,71 1.41 67
0.061b 0.011b 0.011b
6 0.14 0.76 0.6 0.57 0.47 N.Z.
(New Zealand) 6 0.28 1.13 0.82 0.68 0.42
CABBAGES 1 0.008% - 0.51 68
(U.K.)
CABBAGES
(Savoy) 4 0.06 1,1 & 2 0.55 0.4 0.35 0.15 <0.01 64
(Germany) 4 0.06 2 0.3 0.2 0.1 0.05 <0.01
1 0.005% 0.02 65
1 )0.015% 0.02
(Italy) 1 0.03% 0.02
CAULIFLOWERS 3 0.16 3 & 2 0.17 0.09 0.01 <0.01 72
3 0.07 1,5 0.23 0.21 <0.01 73
0.26 0.30 0.01
0.34 0.24 0.08
(Canada) 0.3 0.08 0.11
4 0.06 2 0.03 <0.01 <0.01 <0.01
(Germany) 3 0.06 2 0.05 <0.01 <0.01 <0.01 <0.01
KALE 4 0.06 2 1,6 0.85 0.4 0.3 0.25 75
(Germany) 4 0.06 2 1,4 0.9 0.7 0.5
2 0.075+0.04 6.5 1.1 0.76 0.47 0.13 76
(U.K.) 2 0.225+0.08 6.5 1.5 0.95 0.60 0.23
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
KALE
(China) 6 0.005% 4-5 days 0.85
(Thailand)
NOTE: The cabbages were analysed without the outer leaves with some exceptions:
1 whole cabbage
1a whole cabbages as specified by CC/PR/153/
1b heart only.
Cherries and Plums
Samples of plums and cherries have been obtained from field
experiments in Germany in which cypermethrin was applied on several
occasions (three in the case of cherries, five for plums) at
approximately the recommended rate and double the recommended rate
(0.01% and 0.02% respectively). Under similar conditions, residues in
plums were found to be somewhat lower than in cherries.
Grapes
At recommended rates of up to 80 g/ha and treatments on up to 4
occasions, residues in grapes harvested one week after the last
application were at or below 1 mg/kg (Table 9). In practice, the
material is generally applied an a prophylactic treatment at 50 g/ha
and harvest is likely to be more than one week after treatment.
Lettuce
Residue data are available from trials carried out in Canada, France
and Germany at rates generally in the range of 50-70 g/ha (0.005% to
0.007%, based on 1000 l/ha spray volume). Residue levels were 1.6
mg/kg cypermethrin or less in lettuce taken one week after treatment.
From the glasshouse data available, there was no evidence that levels
in glasshouse lettuce were substantially different from levels in
outdoor crops.
Kiwi Fruit
Supervised trials were carried out in New Zealand at the recommended
dose rate and at double rate (Table 9). The pulp contained 5-10% of
that which was found in whole fruit.
Maize
Residue experiments have been carried out in several countries in
which cypermethrin has been applied to maize at recommended rates up
to 150 g/ha. Green maize (whole plants) were sampled at intervals
following treatment, and in some instances grain was also sampled at
the sweet corn stage or when fully mature.
In green maize following treatments at rates up to 150 g/ha, residues
were below 1 mg/kg two weeks after treatment and below the limit of
determination (0.01 mg/kg) after eight weeks. In grain, no residues
were found in either sweet corn or mature grain, even in samples taken
three days after treatment at this rate.
Pasture
Supervised trials were carried out in New Zealand in which
cypermethrin was applied at several times higher than the recommended
rate. The residues on the grass increased roughly in proportion to
the increases in dosage (Table 8).
Peas
Trials were carried out on peas in the U.K., Hungary and South Africa.
No residues were found at dose rates of 15 to 100 g/ha in the peas
themselves which were sampled between one day and four weeks after
treatment (limit of determination 0.01 mg/kg). However, an excessive
dose rate of 200 g/ha resulted in residues between 0.04-0.07 mg/kg in
the peas (Table 9).
Potatoes
Samples were obtained from residue trials carried out in Europe,
Cyprus and Canada, with from 1-9 treatments at dosage rates in the
range of 10 g/ha to 200 g/ha, and intervals between last treatment and
harvest from 0-8 weeks. Detectable residues were absent from all
samples of whole or peeled tubers, even 3-9 days after treatment.
However, samples from one trial in Italy, obtained three weeks after
treatment were peeled. Residues up to 0.05 mg/kg were found in the
peel, but none was detectable in the peeled potatoes.
Rape
Data from supervised trials in Canada, Germany and the U.K. show that,
where treatments at recommended rates were made more than six weeks
before harvest, residues in the whole seed were below the limit of
determination of 0.01 mg/kg. In one of the replicated Canadian
experiments, however, residues were found in samples taken six weeks
after treatment; residues between replicates varied from 0.01 mg/kg to
0.12 mg/kg.
Raspberries and Strawberries
Trials were carried out in Canada and the U.K. in which raspberry
canes were treated with cypermethrin at rates between 0.005% and
0.015% (the maximum recommended rate). Residues in fruit taken 12 or
more days after treatment were all below 0.5 mg/kg even where multiple
applications were made. Residues in strawberries from Canadian
experiments using cypermethrin at rates of 0.006% or 0.01% on two
occasions at an interval of 8 or 9 days were also below 0.5 mg/kg when
harvested three weeks after treatment (Table 9).
Sugar Beet
In experiments carried out in France, Germany and the U.K., 1 to 5
treatments were carried out at rates between 60 g/ha and 200 g/ha
cypermethrin. Samples of both roots and tops were taken for analysis
at intervals up to 17 weeks from the time of treatment.
Apart from one sample taken two weeks after treatment at 60 g/ha,
which contained a small residue of 0.02 mg/kg, no residues of
cypermethrin were found in the root samples.
In leaves, residues were between 6 and 13 mg/kg immediately after the
last of 5 applications made at two weekly intervals. Subsequently
these declined, reaching 0.02-0.13 mg/kg six weeks after treatment.
After 11 weeks residues were 0.01 mg/kg or less (Table 9).
Tomatoes
In outdoor experiments carried out in Southern Europe, Australia,
Canada, S. Africa, the Canary Islands and Mexico, tomatoes were given
foliar treatments at rates between 50 g/ha and 300 g/ha. Applications
were made on up to 9 occasions at intervals from 5 days to 3 weeks
depending on the experiment. Samples were taken at intervals ranging
from the day of treatment to six weeks later. Although some of the
tomatoes received were green, they were close to ripening
(approximately 5 cm diameter) and in the condition at which they would
be picked for export. The residues (Table 9) did not exceed 0.5 mg/kg
in any of the samples taken shortly after treatment at the recommended
rates (up to 0.0% or 100 g/ha assuming use at 1000 l/ha).
Wheat
Residue trials have been carried out on wheat in Canada and Brazil,
with application rates up to 150 g/ha. In the grain residues did not
exceed 0.1 mg/kg at harvest two weeks after application. Residues in
straw were higher, and the few data obtained suggest that under these
conditions they may approach 10 mg/kg (Table 9).
Table 9. Residues Resulting from Supervised Trials with Various Fruits and Other Crops (1974/79)
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
ALFALFA Cyprus 1 0.1 12 5.6 4.7 10
BEANS
(Dried) Colombia 3 0.15 1 <0.011 87
CHERRIES Germany 3 0.02% 1-2 4.5 3.3 0.65 0.6 18
to run off
3 0.02% 1-2 2.7 1.6 1.0 0.39
to run off
3 0.02%
to run off 1-2 7.4 5.4 1.5 1.2
3 0.01% 1.5-2 0.59 0.47 0.27 0.25 0.2 19
3 1.5-2 1.0 0.6 0.3 0.1 0.1
GRAPES Canada
(Delaware) 1 0.08 - 0.7 0.32 20
21
(Elvira) 4 0.07 4,5 & 3 0.24
(0.1 to
0.37)
(Friedamia) 1 0.006%
to run off 0.75 0.41 0.25
(Sauvignon) France 4 0.075 1 0.08 0.03 0.02 <0.01 22
(Alicante)
(Bouschet) 1 0.075 - 0.4 0.28 0.23 0.12 23
2 0.075 3 0.37
(Riesling) Germany 4 0.08 5,4 & 2 0.5 0.2 0.11 0.09 0.05 24
4 0.08 5,4 & 2 0.8 0.5 0.1 0.05 0.05
(Barbera) Italy 1 0.01% 0.1 25
S. Africa 1 0.0375 - 0.2 0.1 0.18 <0.05 <0.05 S. Africa
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
GRAPES
(Waltham
Cross) 2 0.0375 2 0.24 0.2 0.19 0.07 <0.05
(Chemin
Blane) 1 0.0375 - 0.1 0.12 <0.05 <0.05
2 0.0375 2 0.12 0.07 <0.05 <0.05 <0.05
KIWI FRUIT N. Zealand 6 0.01% 2.2 1.9 1.7 1.3 N.Zealand
6 0.02% 6.2 3.9 3.4 2.8
LETTUCE Canada 6 0.07 1 <0.012 78
0.02
France 1 0.15 0.52 79
0.3 1.1
France 1 0.05 1.2 0.27 0.123 80
1 0.05 1.1 0.3 0.06 81
1 0.05 2.2 1.6 0.35
1 0.05 1.8 0.5 0.02
1 0.054 2.4 1.0 0.85 82
Germany 3 0.06 2 4.0 0.25 0.04 0.02 <0.01 83
3 0.06 2 1.2 0.06 0.01 <0.01
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
MAIZE
Cobs Australia 1 0.0015% <0.01 10
Cobs 0.003% <0.01
Sweet corn Canada 5 0.07 5 days <0.01 84
Sweet corn 3 0.075 1 <0.01 <0.01
Sweet corn 6 0.064 5 days <0.01 <0.01 <0.01 85
Germany 1 0.1 1.9 0.04 86
1 0.1 2.7 2.2 0.51 0.05 <0.015
1 0.1 2.9 1.1 0.15 0.05 0.025
<0.01
Leaves S. Africa 1 0.075 5.2 3.86 0.4 10
Leaves 1 0.15 17 106 2.6
Stalks 1 0.075 <0.01 0.086 <0.01
Stalks 1 0.15 0.05 0.02 0.06
PEAS U.K. 2 0.05 2 <0.01 88
w/o pods 1 0.025 - <0.01 89
Peas Hungary 1 0.1 - <0.017
pods (only) - 0.07
Peas 1 0.2 0.04
0.077
Sweden 2 0.08 2 <0.01 165
S. Africa 1 0.015 - <0.01 <0.016 <0.01 10
1 0.03 - <0.01 <0.016 <0.01
PLUMS Germany 5 0.02% 1-3 1.0 0.65 0.5 90
5 0.02% 1-3 0.9 0.75 0.4 0.25
5 0.02% 1-3 1.3 1.1 0.7
5 0.01% 3,2,3 & 1 0.3 0.29 0.29 0.25 0.11 91
5 0.01% 3,2,3 & 1 0.24 0.15 0.12 0.1
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
POTATOES Canada 4 0.075 2 <0.01 92
4 0.15 2 <0.01
9 0.072 1 <0.01
9 0.145 1 <0.01
4 0.150 2 <0.017 93
9 0.15 1 <0.01 94
Cyprus 1 0.1 <0.01 <0.01 10
France 1 0.15 <0.01 95
Germany 4 0.090 2 <0.01 <0.01 <0.01 96
4 0.2 2 <0.01 <0.01 <0.01 97
4 0.2 2 <0.01 <0.0l <0.01
4 0.2 2 <0.01 <0.01 <0.01
Italy 1 0.04 - 0.058 98
1 0.12 - 0.038
SOYA BEANS Brazil 2 0.24 2 <0.01 99
3 0.24 2 <0.01
dried Colombia 4 0.3 1,1 week
& 4 days <0.01 100
SUGARBEET France 1 0.1 - <0.01
Leaves after 101
17 wks
1 0.15 <0.01
after 102
17 wks
Germany 5 0.06 )2,2,2 1.3 0.52 0.07 0.05 103
5 0.06 )& 1 2.2 0.1 0.03 <0.01
5 0.2 2 12 4.0 2.3 0.02 104
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
SUGARBEET Germany 5 0.2 2 6.2 2.3 0.18 0.05
Leaves 5 0.2 2 13 0.73 0.23 0.13
U.K. 1 0.2 <0.01
after
15 weeks 105
RASPBERRIES Canada 1 0.225 - 0.2110 114
3 0.18 2 0.4510 115
3 0.135 2 0.410 116
U.K. 1 0.005% 1 0.1311 117
0.015% 1 0.2511
STRAWBERRIES Canada 2 0.23 9 days 0.07 118
2 0.18 90 0.04 119
2 0.18 8 days 0.45 120
TOMATOES Australia 9 O.01% 5-7 days 0.3 0.3 0.24 0.14 106
9 0.02% 5-7 days 0.57 0.57 0.44 0.53
1 0.0015% - 0.02 0.03 10
Canada 1 0.15 - 0.13 0.07 107
2 0.15 1 0.2 0.05
France 1 0.0075% - 0.09 0.06 0.04 108
Italy 1 0.005% - 0.02 109
0.015% 0.08
0.03 0.17
Mexico 1 0.0075 - 0.02 0.01 10
1 0.015 - 0.05 0.04
1 0.03 - 0.08 0.13
Portugal 3 0.075% 11 & 7 0.06 0.032 110
days
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
TOMATOES S. Africa 4 0.0075% 3,2,2,2 0.17 0.1 0.05 111
4 0.01% 3,2,2,2 0.27 0.15 0.09
4 0 02% 312,2,2 0.27 0.14 0.08
4 0:03% 3,2,2,2 0.29 0.2 0.6
Spain 7 0.03% 3,2 & 2 0.08 112
1 0.075 - 0.16 0.07 0.14 10
0.16 0.06 0.10
2 0.2 1 0.1 0.09 0.06 113
3 0.2 1 0.25 0.2 0.05
Canary Is. 2 0.1 1 0.25 0.15 0.01 113
3 0.1 1 0.2 0.15 0.06 0.03
2 0.15 1 0.4 0.3 0.15
3 0.15 1 0.55 0.4 0.2 0.6
WHEAT Canada 1 0.14 - 0.1 0.07 0.05 121
1 0.15 - 0.08 0.08 0.08 0.04 122
0.07 0.05 0.06 0.03
0.07 0.05 0.05 0.04
0.09 0.09 0.06 0.04
Brazil 1 0.09 <0.01 <0.01 123
2 0.09 12 days 0.04
2 0.09 31 days 0.03
2 0.09 19 days 0.01
3 0.09 19 & 12
days 0.04
1 +4 week storage, beans were analysed without pods
2 In 3 samples
3 10 days after treatment
4 In glasshouse
Table 9. Continued...
5 The grain contained <0.01 mg/kg
6 8 days after treatment
7 In 9 samples
8 In the peel
9 24 days after treatment
10 12-13 days after treatment
11 19 days after treatment
FATE OF RESIDUES
GENERAL OBSERVATIONS
Following applications to crops cypermethrin may degrade to a variety
of hydrolysis and oxidation products. The most likely degradation
products present in crops at harvest following normal agricultural use
of cypermethrin are the derived amide (compound B), 3-phenoxybenzoic
acid and 2-(2',2'-dichloro vinyl)-3,3 dimethyl cyclopropane carboxylic
acid (compound C), the structures of which are shown in Figure 1. The
latter two compounds are found in the free state as well as in
conjugated forms. However, the evidence indicates that the major
component of any residue present at harvest will be cypermethrin
itself. A number of crop samples obtained from supervised trials
discussed previously were analysed also to determine compounds B, C
and phenoxybenzoic acid. The results of these examinations involving
some 20 crops showed no residues of Compound B, of Compound C or of
3-phenoxy benzoic acid in excess of 0.05 mg/kg.
Following use on animal feed crops, residue may be present in feed at
levels depending on the crop. However, since the product is readily
metabolized by animals, these amounts are unlikely to give rise to
more than traces of foods of animal origin.
IN ANIMALS
Cattle Feeding Studies
Studies were undertaken to investigate the fate of cypermethrin in
cattle and whether residues in meat or milk could arise from the use
of cattle feed containing products made from treated crops. Two
experiments were undertaken, both using radiolabelled cypermethrin.
a. Low Dietary Intake (0.2 mg/kg)
Two lactating cows were given feed concentrate containing
radio-labelled cypermethrin twice daily for a three-week period with a
feeding level equivalent to 0.2 mg/kg on total daily feed. The cows
were milked twice daily and the amount of radioactivity determined at
each milking. The total radioactivity present in whole milk was in
the range of 0.0002 mg/1 to 0.0012 mg/1 in terms of cypermethrin
equivalents and 60-70% of the amount of radioactivity in the milk was
present in the cream fraction. The total radioactivity found in the
milk amounted to only 0.5% of the radioactivity fed to the animals.
The remainder of the radioactivity was excreted in the urine (54%) and
faeces (43%). The elimination occurred rapidly after dosing and the
rate of elimination of radioactivity reached its peak (near 100% of
applied dose) after three days.
 In the urine 3(4-hydroxyphenoxy) benzoic acid-O-sulphate and the
glutamic acid conjugate of 3-phenoxybenzoic acid were detected in the
ratio of 1:4. The free acids were not detectable. The faeces
contained the parent compound amounting to approximately 85% of the
faecal 14C, that is, about 36% of the amount ingested. At the end of
the three-week feeding period the animals were slaughtered and samples
of tissues examined for radioactivity. Levels were below 0.001 mg/kg,
in cypermethrin equivalents, in blood, muscle and brain. In
subcutaneous and renal fats, liver and kidney samples, residues were
at or below the equivalent of 0.012 mg/kg cypermethrin (Shell R. 124).
b. High Dietary Intake (5 mg/kg)
Radiolabelled cypermethrin was fed in the feed concentrate twice daily
and was given in amount equivalent to 4 mg/kg on total diet over a
period of 7 days.
The radioactivity in the milk, urine and faeces was monitored
throughout the feeding period and showed that the major excretory
route was via the kidneys. All the cows were found to be pregnant at
necropsy and the milk yields throughout the experiment were poor.
Equilibrium between intake and excretion was reached 3-4 days after
commencement of feeding, after which levels in whole milk ranged
between 0.009 mg/l and 0.013 mg/l cypermethrin equivalent to
radioactivity present. The cream fraction of the milk contained
85-90% of the total radioactivity. Results of experiments carried out
with 14C-benzyl and 14C-cyclopropyl labels indicate that the residue
in milk is an ester and contains both the acidic and alcoholic
functions of the parent compound.
The major urinary metabolites in cows were identified as the glutamic
acid conjugate of 3-phenoxybenzoic acid (68%), 3-phenoxybenzylglycine
(16%), 3-phenoxybenzoic acid (9%). 3(4-hydroxy-phenoxy) enzoic-acid
and its O-sulphate conjugate appeared to be present in only small
amounts (1%).
At the end of the test feeding period the animals were slaughtered and
tissues were taken for measurement, with the following results,
radioactivity being expressed as cypermethrin equivalents.
Muscle 0.04 mg/kg
Fat 0.01-0.10 mg/kg
Liver and Kidney 0.05-0.13 mg/kg
These results indicate that the cypermethrin does not accumulate in
the tissues of the animals. Even at a high intake, residues are
mostly in the fat, liver and kidney. Cereals and components of feed
(e.g. cotton seed) treated with the highest recommended dose rates
therefore, are unlikely to result in measurable residues in meat or
milk of cattle (Shell R. 125).
IN PLANTS
Degradation on cotton, lettuce and apples has been studied using
14C-radiolabelled material with the label in various positions in the
cypermethrin molecule. Structures of compounds mentioned are given in
Figure 1.
a. Cotton
An experiment in which the cis- and trans-isomers of cypermethrin
were applied in acetone/water solution to cotton leaves using a small
syringe was carried out on plants grown in pots in the greenhouse.
Each of the two isomers, which in themselves consisted of a racemic
mixture of two enantiomeric pairs of stereo isomers, was labelled in
the cyclopropyl and benzyl rings in separate experiments. In each
experiment the rate of application was 10-20 µg/g of leaf. Leaf
samples were taken for examination immediately after treatment and
again 42 days later. The samples were extracted with acetone and the
examined by chromatographic and radiochemical techniques.
Forty-two days after treatment 75-85% of the original total applied
radioactivity was still present in cotton leaves and 90% of this was
acetone extractable. Thin-layer chromatography showed that at least
six components were present in the extracts. Products identified and
the approximate proportion present in the extracts were cypermethrin
(40-50%), 4-hydroxy-cypermethrin (compound A/ 5-8%), 3-phenoxybenzoic
acid (15%), and an amide (compound B/ 8-20%). In this preliminary
study unidentified material accounted for the remaining 20-30% and was
mainly polar material. No evidence of an appreciable difference in
the rate of loss and breakdown of the two isomers was obtained.
A second experiment was carried out on a larger scale, in which
cypermethrin was 14C radio-labelled in the benzyl ring and applied at
the rate of 4 µg/g leaf. Two applications were made 15 days apart and
leaf samples were taken 5 weeks after the second treatment. Results
were qualitatively similar to the first experiment, except that
smaller amounts of metabolites were found and, in addition, some
3-(4-hydroxyphenoxy)-benzoic acid was also reported to be present. Due
to the larger scale of the experiment it was possible to examine the
polar fraction in more detail. This was found to consist of at least
8 compounds which were mainly conjugated forms of 3-phenoxybenzoic
acids 3-(4-hydroxyphenoxy)-benzoic acid and 3-phenoxybenzyl alcohol.
When the results from experiments using cypermethrin, separately
labelled in the nitrile, cyclopropyl and benzyl groups, were compared,
the properties of the polar products formed differed according to the
label position. Thus it was again concluded that, following the
primary step of hydrolysis of the ester link, further degradation of
the component parts, including loss of nitrile, oxidation and
conjugation, took place. In this work, the cyclopropane carboxylic
acid (compound C) was identified as a metabolite.
In a third experiment the cotton was grown in boxes outdoors in
Seville, Spain. Cypermethrin, labelled either in the cyclopropane or
benzyl rings, was applied to separate plants. The cis and trans
isomers, in each case, were also applied in separate experiments.
Three applications were made at rates equivalent to 300 g/ha and
samples were taken at harvest 15 weeks after the final treatment.
Seed, fibre, boll cases and leaves were examined separately. All the
kernel samples separated from the seed cases contained small amounts
of radioactivity equivalent to 0.07-0.24 mg/kg cypermethrin. Due to
the oily nature of the extracts and the small size of the samples, it
was not possible to fully identify the products present, although it
was shown the radioactivity was not present in the form of
cypermethrin itself. Seed cases freed from lint also contained
radioactivity equivalent to up to 0.17 mg/kg cypermethrin, which again
was shown not to be parent insecticide.
The lint samples contained rather more variable amounts, between 0.08
and 0.62 mg/kg cypermethrin equivalents, probably depending on the
degree of opening of the bolls at the time of spraying. Cypermethrin
itself was the major compound present (between 50 and 90% of the
total) but the remainder consisted of polar products. Foliage and
boll cases were found to contain the same products as those reported
from glasshouse experiments. These included cypermethrin,
3-phenoxybenzoic acid in free form, the cyclopropane carboxylic acid
(free) and the amide, Compound A. The latter in particular was
present, however, in considerably smaller amounts than found in the
glasshouse experiment.
Polar materials were also found in appreciable amounts. These could
be hydrolysed by acid to give 3-phenoxybenzoic acid, 3-phenoxybenzyl
alcohol and 3-phenoxybenzaldehyde. Unidentified materials in foliage
amounted to only a small proportion of the total radio-label present.
In another experiment, abscised cotton leaves were placed with their
stems immersed in an aqueous solution containing ring
14C-radiolabelled Compound C for 72 hours. At the end of this period
90% of the radioactive material originally present in the solution had
been taken up by the leaves. The major proportion (78%) of this
material was found to be present as sugar conjugates of Compound C.
Experiments have also been carried out with the degradation products
3-phenoxybenzoic acid (14C ring-labelled) and
cis-2-(2',2'-dichlorovinyl)-3,3-dimethyl cyclopropane carboxylic
acid (14C-C1-label). In some of these the stems of cotton leaves
were immersed in aqueous solutions. There was a rapid uptake of both
compounds and a rapid conversion to polar products. These products
were subsequently shown to be derivatives with various mono- or
di-saccharide sugar products in the case of the cyclopropane
carboxylic acid and mainly glucose in the case of 3-phenoxybenzoic
acid. Leaves to which 3-phenoxybenzoic acid was applied topically on
the intact plant showed similar conversions to a mixture of sugar
conjugates although at a much slower rate than in the case of petiole
uptake. Similar experiments with 3-phenoxybenzoic acid have been
undertaken on vine, tomato, soybeans, pea and broad bean leaves and
with similar findings, (Shell R. 126, 127, 128, 158, 159 and 160)
b. Lettuce
Experiments were carried out on plants grown in pots in the glasshouse
and treated with solutions of either the cis- or trans-isomer of
cypermethrin. Each isomer was also labelled in either the benzyl or
cyclopropyl rings. Details were similar to the first cotton
experiment, described above, except that samples were taken
immediately after treatment and 18 days later, and the results were
qualitatively similar. Just over 70% of the original applied
radioactivity was still present 18 days after application and 90% of
this was extractable with acetone. But a higher proportion (50-70%)
of the extracted radioactivity was present as cypermethrin than with
cotton. The proportions of other products found were: compound A,
5-10%; 3-phenoxybenzoic acid, 10-15%; and compound B, 12-15%. An
unidentified 11-15% consisted of several minor metabolites.
An outdoor experiment was also performed on lettuce using cypermethrin
labelled in the benzyl and cyclopropyl rings. The two labelled
materials were applied to different plants as an overall foliar spray
of a diluted E.C. formulation at a rate equivalent to 300 g/ha.
Treatments were made on two occasions with an interval of about two
weeks, and the plants were harvested approximately three weeks after
the second treatment. The major part of the residue, which amounted
to 0.8-1 mg/kg(radioactivity equivalent to cypermethrin) was located
in the outer leaves and only 10-20% was present in the hearts. In the
benzyl labelled experiment, 50% of the radioactivity was present as
cypermethrin itself and most of the remainder as polar materials which
individually were in too small amounts for detailed study. Only 4%
was not extracted. In the cyclopropyl labelled experiment, 30% of the
residue consisted of cypermethrin and 40% as conjugates of Compound C
of which a glucose ester was identified (Shell R. 126, 129).
c. Apples
Apple trees growing in an outdoor wire-covered enclosure were treated
with cis-cypermethrin, separately labelled in the benzyl or
cyclopropyl rings, or with trans-cypermethrin, labelled in the
benzyl ring. Leaves were treated on three occasions at intervals of
25 and 37 days, and harvested four weeks after the last treatment.
Fruit was treated twice at a 24-day interval and harvested three weeks
after the second application.
Examination of the leaves showed that 32-46% of the residue consisted
of the parent isomer applied. In addition, 7-15% of the radioactivity
present was identifiable as compounds A and C, 3-phenoxybenzyl
alcohol, 3-phenoxybenzaldehyde and 3-phenoxybenzoic acid. Polar
compounds made up most of the remaining residue. Sugar conjugates of
3-phenoxybenzoic acid and the corresponding alcohol, as well as of
3-(4-hydroxyphenoxy)-benzoic acid, were identified.
In fruit, results were similar although the quantities present were
much lower. Examinations of peel and pulp separately showed that less
than 2% of the total radioactivity in the fruit was present in the
pulp. On the peel 50-77% was present in the form of the parent isomer
applied. During the experiment 15% of the cis isomer was converted
into the trans isomer. The amounts of metabolites were somewhat
less than in leaves and accounted, as free compounds, for 3-7% of the
radioactivity present. Polar materials from the fruit were present in
much smaller amounts then in leaves and were not present in sufficient
quantities to be studied in detail.
In the experiments with the cis isomer some conversion (30%) into
the trans isomer occurred, but the reverse change was not observed.
In some routine examinations of field samples for residues of
cypermethrin the glc analytical method used results in separation of
the cis and trans isomers on the chromatograph trace. No change
in the relative sizes of the two peaks with different treatment to
harvest intervals, or between unresolved standard material and the
test samples, has been observed. It would, therefore, seem that the
conversion of cis isomer into trans compensates for the faster
degradation of the trans isomer (Shell R. 130).
IN SOIL
Degradation on soils was studied by adding 14C-labelled cypermethrin
to three different soils - a clay and a sandy clay from Spain, and a
sandy loam from the U.K. Separate experiments were carried out with
the molecule labelled in the benzyl or the cyclopropyl moiety. In
addition, the cis and trans isomers were studied individually
(Roberts and Standen, 1977).
The cis isomer was found to degrade at an initial rate equivalent to
a half life of between 2 and 4 weeks in sandy clay and sandy loam, and
about 10 weeks in the clay soil. The trans isomer degraded more
rapidly than the cis isomer in all three soils, with initial
half-lives between about one and three weeks. Degradation was shown
to proceed by hydrolysis of the ester link and cyano group with loss
of carbon dioxide to give 3-phenoxybenzoic acid and Compound C, which
were both present as free compounds. Subsequent degradation of both
compounds was shown to occur, since 14 C-radiolabelled carbon dioxide
was evolved over 22 weeks in amounts equivalent to 24% and 38%,
respectively, of the total radioactivity initially present as ring
label. As indicated by the presence of small amounts of Compounds A
and 3-(4-hydroxyphenoxy)-benzoic acid, some hydroxylation of the
aromatic rings also took place.
Up to 36% of the original radioactivity was found to be difficult to
extract 16 weeks after the commencement of the experiment. A study of
this fraction showed it to be composed of several materials bound to
soil components. Examination of the acid hydrolysed material showed
that 30% or more of the radioactivity was in the form of Compound C.
Smaller amounts of 3-phenoxybenzoic acid and
3-(4-hydroxyphenoxy)-benzoic acid were also found. After 52 weeks
unchanged parent material accounts for 1.4-10.7% of the radioactivity
originally applied to the soils. In one sample of a Spanish soil
(Brenes) a trace amount of Compound B was also present and in the same
soil 5.8% of the original radioactivity was present as a cyclopropane
dicarboxylic acid, Compound D. In a separate experiment, this latter
compound was also found, in amounts of 3-13% of the applied
radioactivity, 8 weeks after application to soils stored in the
laboratory. Under waterlogged and anaerobic conditions hydrolytic
breakdown was found to be somewhat slower than under aeroboic
conditions. Breakdown of 3-phenoxybenzoic acid was also slower.
Compound C has also been shown to be formed from permethrin in soils
and to be degraded by hydroxylation at the gem-dimethyl groups.
Subsequent further breakdown has been shown in further studies,
leading to extensive evolution of CO2 derived from ring and
chloromethylene carbons (ICI, 132, 133, 134, 135). These data
substantially confirm the results obtained with cypermethrin, showing
that Compound C is unlikely to accumulate in soils following treatment
with the pesticide.
In addition to the degradation studies referred to above, studies have
also been made to determine the rate of loss of cypermethrin from
soils from field experiments in which very high rates of application
had been made. The compound was applied as a diluted 40% EC and was
partly incorporated to the soil (3-5 cm) after treatment. Samples
were generally taken from 0-15 cm depth. The data obtained (Shell C
and R. 136 to 140), show that for the same rate applied, the amount of
residue initially present after treatment varied widely from one trial
to the other, and that cypermethrin degrades fairly rapidly in soils.
Only 1-5% of initial residue, if any, could be detected 4-8 months
after treatment. The leaching studies with labelled cypermethrin in a
column containing sandy loam soil show that less than 2% of applied
radioactivity had been eluted with water at a rate of 2 ml/hr from the
column over the 45-day period (Hungary, 1979), and that 90% of the
initial activity was present in the top 2 cm of the soil. The
findings from laboratory experiments are in agreement with the results
of field trials.
From the above findings, it may be concluded that, even when
applications have been made to crops at recommended rates, residues of
cypermethrin itself are unlikely to be present at detectable levels in
soils at the beginning of the season following that in which
treatments were made. It may also be concluded that crops grown in
plots where treatments with cypermethrin have been made in a previous
season are unlikely to contain detectable residues.
IN PROCESSING
Cypermethrin is a moderately stable and water-insoluble compound. Data
relating to the effect on residue levels of various treatments given
to harvested crops are as follows:
a. Peeling
Numerous data show that the residue present in a crop is largely on
the surface. Analyses of pulp and peel after peeling apples, pears,
peaches and citrus fruits (Table 6) show that levels in the pulp were
below 30%, and in most instances below 10%, of those in whole fruit.
b. Juice extraction - citrus
Data for citrus have shown that residues of cypermethrin in juice
extracted from treated fruits contained no measurable residues of the
pesticide (see Table 6).
C. Wine making
Wine has been manufactured from grapes treated with cypermethrin and
containing up to 0.15 mg/kg pesticide. No residues (limit of
determination 0.01 mg/l) were found in the juice after fermentation
(Shell C., 142).
d. Cooking
Studies were made on the effect of boiling on residue levels in plums,
and cabbage. Levels were not substantially reduced by boiling plums
for 30 minutes or cabbage for 45 minutes. Residues in the cooked
commodities were 75-90% of the initial levels and only very small
amounts were found in the cooking water (Shell C., 143).
e. Oil seed processing
An experiment was carried out with a cotton seed sample deliberately
treated at the high rate of 300 g/ha and harvested one day after
treatment. It contained 0.12 mg/kg cypermethrin on whole seed, with
adhering linters. The sample was processed by simulating commercial
practice in a laboratory specialising in the techniques. Residues
were found to be transferred to kernels, which originally did not
contain any detectable residue in the seed, during the commercial
mechanical separation process (Shell R., 144). The residues of
cypermethrin in the extracted oil at various stages were as follows:
Crude oil 0.10 mg/l, neutralised oil 0.07 mg/l, bleached oil 0.08 mg/l
and deodorised oil 0.05 mg/l. Both the alkali wash and deodorisation
steps contribute to some losses. The results from this experiment
suggest that the two processes together may be expected to remove
about half of the residue. Hence, it is possible that residues may
occasionally occur in oil obtained from seed, treated under practical
conditions, at levels approaching those in whole seed.
Photodecomposition
The photodecomposition of cypermethrin was studied in methanol
solution under UV light and in the solid phase (3 mg/cm2 on glass)
under sunlight. 55% of cypermethrin was recovered from the methanol
solution after two days. In sunlight there was no detectable loss of
cypermethrin after 30 hrs. In both experiments cypermethrin proved to
be more stable than decamethrin and its behaviour was comparable to
permethrin (Ruzo et al, 1976, 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
At present no data deriving from national monitoring studies or market
control are available.
METHODS OF RESIDUES ANALYSIS
Several methods have been developed for the analysis of agricultural
commodities for residues. These are all based on gas-liquid
chromatography procedures using equipment commonly found in modern
analytical laboratories. Limits of determination of 0.01 mg/kg are
usually attainable.
The racemic cypermethrin contains eight possible steric and optical
isomers which can be partially resolved in the GLC column depending on
its polarity. On analysis of the mixture of isomers on OV-225 phase
three partially resolved peaks with a characteristic pattern are
obtained. However with apolar or moderately polar packings (OV-101 or
OV-17) no resolution of isomers occurs which facilitates the
quantitation of the residue. Four methods have been developed by
Shell. For cotton and other oil-seed crops, the sample is extracted
with an acetone/petroleum mixture and the extract subjected to
clean-up by solvent partition followed by absorption chromatography on
Florisil. The appropriate fractions of the eluate are then examined
by gas-liquid chromatography using electron capture (63Ni) detection
(Shell R. 147). For other crops the samples are ground with anhydrous
sodium sulphate and cypermethrin extracted with acetone and petroleum
spirit. The extract is washed with water and the resultant petroleum
spirit layer separated and cleaned-up by chromatography on a Florisil
column. Cypermethrin is eluted with ether/petrol and determined using
gas-liquid chromatography and a (63Ni) electron-capture detector
(Shell R. 148).
Residues in animal tissues are determined by extracting the tissue
with mixed acetone:hexane, and fat removed by partitioning between
hexane and acetonitrile. The extract is cleaned-up by absorption
chromatography on a column of Florisil and cypermethrin determined in
the appropriate eluate fractions, by glc using electron-capture
detection (Shell R. 149).
Soil samples are mixed with anhydrous sodium sulphate and extracted
with a mixture of acetone and petroleum spirit. The extract, which
contains any cypermethrin present in the soil, is washed with water to
remove acetone and cleaned up by adsorption chromatography on
Florisil. Residues of cypermethrin are determined using GLC with an
electron-capture detector (Shell R. 150).
Confirmation of a residue in either soil or crop may be obtained by
TLC (thin-layer chromatography) on Silica Gel plate eluted with
toluene without previous saturation followed by further GLC. ICI have
also developed basically similar analytical methods for application to
crop samples. In their method the crop samples are extracted with 20%
acetone in hexane and, where necessary, co-extractives removed by
solvent partition. Further clean-up is carried out by adsorption
column chromatography on Silica Gel or Florisil and cypermethrin
determined by GLC in conjunction with an 63Ni E.C. detector (ICI
151).
Recovery experiments have shown that all the methods cited are capable
of measuring 80% or more of any cypermethrin residue present and that
they are suitable for regulatory purposes.
Chapman reported on the applicability of various adsorbent-solvent
systems for the cleanup of some plant extracts containing
cypermethrin, fenpropanate, permethrin, and fenvalerate. Analytical
methods suitable for the determination of metabolites of cypermethrin
in crop samples obtained following practical applications in the field
have been developed using HPLC. Under normal circumstances the limit
of determination is 0.05 mg/kg.(Shell R. 161)
The amide, Compound B, is extracted with a mixture of water and
acetonitrile from the sample of which the water content is known.
Clean-up of the extract is performed by solvent partition and
reversed-phase partition chromatography followed by reversed phase
HPLC. Final analysis is carried out with normal HPLC using a UV
detector (Shell R. 162).
3-phenoxybenzoic acid may be determined in a similar manner, although
partition between dilute aqueous alkaline acetonitrile and hexane in
used to remove parent cypermethrin from free 3-phenoxybenzoic acid.
Conjugated material is hydrolysed by treatment of the concentrated
aqueous extract with hydrochloric acid. The 3-phenoxybenzoic acid is
converted into the methyl ester which may then be determined directly
on HPLC. Alternatively, if further clean-up is required, HPLC may be
used for this purpose and gas chromatography/mass spectrometry with
multiple ion monitoring used for the determination (Shell R. 163).
Compound C may also be extracted from crop samples with a mixture of
water and acetonitrile and separated from unwanted material by solvent
partition. Conjugated Compound C is however retained in the aqueous
phase which may be concentrated and hydrolysed with hydrochloric acid.
The liberated compound C may then be extracted with an ether/petroleum
spirit mixture. Clean-up of the extracts is performed by reversed
phase HPLC and Compound C determined by normal phase HPLC. If
extracts are insufficiently clean for HPLC they may be treated with
alpha-cyano-3-phenoxy-benzyl bromide, and the cypermethrin formed
determined by gas chromatography with electron capture detection
(Shell R. 164).
Other experiments have shown that the great majority of samples may be
stored for long periods in the deep freeze without appreciable loss of
residues. Residue levels were determined in crop and soil samples at
intervals following addition of known quantities of cypermethrin in
the range of 0.2 mg/kg to 1 mg/kg. The samples were stored at -18°C
between treatment and extraction for analysis, for periods between 1
and 54 weeks for crops and 4-49 weeks for soil. Recoveries of
cypermethrin added to crops (21 samples) were 85-110%, apart from one
sample of tobacco which yielded 45% after 6 months storage. In the
case of soils (5 samples) recoveries were all between 90 and 110%
(Shell R. 152).
Studies have also been carried out on the stability in storage at
-18°C of residues of 3-phenoxybenzoic acid and Compounds B and C.
Over three months there was no evidence of loss of 3-phenoxybenzoic
acid, since the total amounts recovered from the 6 crops used
(lettuce, potatoes, cabbage, apples, pears and maize grain) were in
the range of 75-100% and comparable to recovery values of the method
itself. With Compound B experiments were carried over three months
with sweet corn and over five months with apples and cabbage.
Recoveries were 70%, 75% and 75% respectively. Cabbage and apples
treated with Compound C were also stored at -18°C for five months.
Recoveries were 90-95% respectively. These data, therefore, indicate
that loss of any of the three degradation compounds mentioned were
negligible over periods of 3-5 months at 18°C.
NATIONAL MRLs REPORTED TO THE MEETING
National MRLs and pre-harvest intervals have been established in
several countries based on local requirements. The following were
presented at the meeting:
Country Commodity MRL Pre-harvest int. (days)
Argentina Apples 21
Cotton 20
Peaches 25
Tomatoes 7
Soya, Sorghum 40
Sunflower 30
Colombia Cotton 35
Costa Rica Cotton, Vegetables 15
Country Commodity MRL Pre-harvest int. (days)
Cyprus Vegetables 14
Top Fruit
Fed. Rep.
of Germany Leafy vegetables 14
Potatoes 14
Maize 49
France Apples 0.5 14
Peaches 0.5 14
Vines 0.5 14
New Zealand Kiwi fruit 2.0 14
Pome fruit 1.0 14
Brassicas 1.0 7
Peru Cotton 15
Tomatoes 15
Potatoes 15
Spain Cotton 21
Potatoes 21
Tomatoes 21
Citrus 21
South Africa Cotton 0.05 28
Grapes 0.05 28
Maise 1.5 28
Peas 0.1 7
Tomatoes 0.2 4
Syria Top fruit 7-10
Vegetables
Experimental or wider use of cypermethrin in further 43 countries was
reported where neither preharvest intervals nor MRL's have been yet
established.
APPRAISAL
Cypermethrin is active against a wide range of insects which attack
crops and can be used at a relatively low dose rate in the range of
0.02-0.25 kg a.i./ha. It is a moderately stable and water insoluble
compound. The technical material contains not less than 90% w/w
cypermethrin, which is a mixture of optical isomers, with a cis:trans
isomer ratio of approximately 40:60. The maximum concentration of
residues in/on the treated crops in the range of 0.05-2 mg/kg and
decreases slowly.
The majority of residues are in the peel or outer leaves, and in most
instances the pulp or hearts contain below 10% of those in the whole
crop. The trans isomers of cypermethrin degrades slightly faster
than the cis, which is compensated by the conversion of cis isomer
into trans during degradation, thus the isomers remain in a constant
ratio at different times after application in plants. Degradation in
crops occurs mainly by hydrolysis of the ester bond followed by
further hydrolytic and oxidative processes to give a variety of
products. Less rapid processes observed were hydrolysis of the
nitrile group to amide and hydroxylation of the phenoxy ring. The
compounds formed were, in turn, also hydrolysed at the ester link.
However, metabolites have not been detected in crop commodities from
supervised trials. At least 90% of the total residue present in plant
material is extractable with acetone. Processing of treated crops
after harvest usually reduces the residue significantly.
Cattle consuming feed items treated with cypermethrin eliminate the
residue rapidly. Equilibrium between intake and excretion is reached
in 3-4 days. The total radioactivity found in the milk amounted to
only 0.5% of the radioactivity fed to the animals and 60-90 of this
residue was present in the cream fraction. The residues in the milk
are esters and contain both acidic and alcoholic moieties of the
parent compound. The vast majority of residue in the feed is excreted
in the urine and faeces in roughly similar proportions. The main
metabolite in urine is 3-phenoxybenzoic acid which is present as
glutamic acid conjugate and glycine derivatives. The faeces mainly
contain the intact molecule.
Cypermethrin does not accumulate in the meat. Even when fed at a high
dose rate residues are mostly in the fat, liver and kidney. The data
indicate that the feeding of crops treated with cypermethrin following
the recommended use patterns does not result in measurable residues in
meat or milk of cattle. Since the compound may be used for direct
treatment of animals and data deriving from the latter use are not
available, recommendations of MRLs for animal products cannot be made
at present.
In soils, spray deposits remain in the surface layer. The rate of
degradation is dependent on the type of soil and proceeds by
hydrolysis of the ester link and cyano group finally resulting in
acids from both parts of the parent compound. Some hydroxylation of
the phenoxy aromatic ring occurs. Subsequent degradation also takes
place with the breakdown of the carbon rings. The trans isomer
degrades more rapidly than the cis. The results indicate that no
detectable residue of cypermethrin itself will be present in soil 8-12
months after treatment and that crops grown in the next season will
not contain measurable residues of cypermethrin.
Analytical methods available for the determination of residues in
various commodities are suitable for regulatory purposes. The limit
of determination is typically 0.01-0.02 mg/kg.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended based
on the pre-harvest intervals indicated below. The limits refer to the
sum of isomers of the parent compound in the portion of sample to be
analysed as described by CC/PR.
Pre-harvest interval on
Commodity Temporary MRL mg/kg which recommendation is
based (days)
Citrus fruits 2 14
Peaches 2 7
Pome fruits 2 7
Cherries 1 7
Grapes 1 7
Brassica leafy
vegetables 1 7
Lettuce 2 7
Plums 1 7
Raspberries 0.5 14
Strawberries 0.5 21
Tomatoes 0.5 3
Rapeseed 0.2 42
Wheat 0.2 14
Cottonseed 0.1 7
Cottonseed oil
(finished) 0.2
Kidney beans, peas,
soybeans
(without pods) 0.05 7
Maize 0.05 7
Potatoes 0.05 7
Sugar beet (roots) 0.05 14
Sweet corn 0.05 7
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
cypermethrin and/or metabolites in adipose tissue.
2. Observations in man, especially those with high level of
occupational exposure, to evaluate the potential susceptibility of
man to the neurotoxic syndrome observed in rodents.
Desirable
1. A dominant lethal bioassay.
2. Further residue data on kiwi fruit from supervised trials.
3. Selective surveys of residues in crops known to have been treated
under practical circumstances.
4. Use patterns for animal health use and residues in animal products
deriving from the recommended application.
REFERENCES
Baldwin, M.K. The analysis of a Metabolite of WL 43467 in Human Urine
as an Index of Exposure to that Compound. (1978) Unpublished Report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
Brooks, T.M. Toxicity Studies with WL 43467. Mutagenicity Studies
with WL43467 in the Host-Mediated Assay and in Micro-Organisms In
Vitro. (1976) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Brown, V.K. Toxicology of WL 43467 Isomers: Acute Toxicity of WL
43481 in DMSO to rats. (1979a) Unpublished report submitted by Shell
International Chemical Company.
Toxicology of WL43467 Isomers: Acute Toxicity of WL42641 in DMSO to
Rats. (1979b) Unpublished report submitted by Shell International
Chemical Company.
Buckwell, A.C. and Butterworth, S.T.G. Toxicity Studies on the
Pyrethroid Insecticide WL 43467. A 13-Week Feeding Study in Dogs.
(1977) Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Butterworth, S.T.G. and Clark, D.G. Toxicity Studies on the
Insecticide WL43467: Acute Oral Toxicity and Neuropathological
Effects in Syrian Hamsters. (1977) Unpublished report from Shell
Research Ltd., Submitted by Shell International Chemical Company.
Carter, B.I. and Butterworth, S.T.G. Toxicity of Insecticides. The
Acute Oral Toxicity and Neuropathological Effects of WL 43467 to Rats.
(1976) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Chapman, R.A., and Harris, C.R. Extraction and Liquid-Solid
Chromatography Cleanup Procedures for the Direct Analysis of 4
Pyrethroid insecticides in Crops by Gas Liquid Chromatography. J. of
Chromatography 166. 513-518.
Combs A.D., Carter, B.I. Hend, R.W., Butterworth, S.G. and Backwell,
A.C. Toxicity Studies on the Insecticide WL-43467: (1976) Unpublished
summary of results of preliminary experiments from Shell Research
Ltd., submitted by Shell International Chemical Company.
Crawford, M. The Metabolism of WL 43467 in Mammals (1). The fate of
a Single Oral Dose of [14C-Benzyl] WL 43481 (cis-WL 43467) in the
Rat. 1976a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of WL 43467 in Mammals. The Fate of a Single Oral
Dose of [14C] WL-42641 (trans-WL43467), in the Rat. (1976b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, N.J. The Metabolism of WL 43467 in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] WL 43467 in the Rat. (1977)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Excretion and Residues of Radioactivity in Cows Treated Orally
with 14C-Labelled WL 43467. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 434671) in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] Cypermethrin in the Dog.
(1979a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 43461) in Mammals. The Fate of
Single Oral Doses of cis- and trans- [14C-benzyl] Cypermethrin in
the Dog. (1979b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
The Metabolic Fate of the cis- and Trans-isomers of WL 43467
(Cypermethrin) and of 3-Phenoxybenzoic Acid in Dogs. (1979c)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.H. The Elimination and Retention of WL
43467 when administered Dermally or Orally to Sheep. (1977a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.R. The Metabolic Fate of the cis- and
trans- isomers of WL 43467 (Cypermethrin). Metabolism and
Elimination of 14C-labelled cis- and trans-isomers in Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Mice Following the Oral
Administration of [14C-benzyl-] WL 43481 (cis-WL43467). (1978a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Rats Following the Oral
Administration of [14C-benzyl] WL 43481 (cis-WL 43467). (1978b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Metabolic Fate of the cis-and trans-isomers of Cypermethrin
in the Rat. Metabolites Derived from the 14C-Labelled Cyclopropyl
Ring. (1978c) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Crawford, J.V. A Study of the Metabolism of 3-Phenoxybenzoic Acid and
its Glucoside Conjugate in Rats. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Crawford, J.V. and Hutson, D.H. The identification of Metabolites in
the Tissues of Rats Treated Orally with 3-Phenoxybenzoic Acid. (1979)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dean, B.J. Toxicity Studies with WL 43467: Chromosome Studies on Bone
Marrow Cells of Chinese Hamsters After Two Daily Oral Doses of WL
43467. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Dean, B.J., van der Paux. C.L. and Butterworth S.T.G. Toxicity
Studies with WL 43467: Dominant Lethal Assay in Male Mice After Single
Oral Doses of WL 43467. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Dewar, A.J. The Use of Lysosomal Enzyme Measurements as an Indicator
of Chemically-Induced Peripheral Neuropathy. (1977a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Co.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Functional Studies on the Neurotoxicity of WL 43467 to Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dewar, A.J. and Deacon, P.A. Toxicity Studies on the Insecticide WL
43467: Electrophysiological Studies on the Neurotoxicity of WL 43467
to Rats. I. - The Effect on Motor Conduction Velocity in the Sciatic
and Tail Nerves. (1977) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Dewar, A.J. and Moffett, B.J. Toxicity Studies on the insecticide WL
43467: Biochemical studies on the effect of WL 43467 on the rat
trigeminal nerve and ganglion. (1978a) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Function Studies on the Neurotoxicity of WL 43467 to Chinese Hamsters.
(1978b) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
Dix, K.M. Toxicity of WL 43467: Teratological Studies in Rabbits
given WL 43467 Orally. (1978) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Glaister, J.R., Pratt, I. and Richards, D. PP 383: Effects of High
Dietary Levels on Clinical Behaviour and Structure of Sciatic Nerves
in the Rat. (1977a) Unpublished report from ICI Central Toxicology
Laboratory submitted by ICI Ltd.
Glaister, J.R., Gare, C.W., Marsat, G.J., Phillips, C. and Pratt, I.
PP 383: 90-Day Feeding Study in Rats. (1977b) Unpublished report
from ICI Central Toxicology Laboratory submitted by ICI Ltd.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies on the
Insecticide WL 43467: A Three-month Feeding Study in Rats. (1976)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Co.
Toxicity Studies on the Insecticide WL 42641: a Five-Week Feeding
Study in Rats. (1977a) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43481: A five-Week Feeding
Study in Rats. (1977b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Hend, R.W., Hendy, R. and Fleming, D.J. Toxicity Studies on the
Insecticide WL 43467: A Three-Generation Reproduction Study in Rats.
(1978) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Hungary. Information supplied by country delegation to Codex
Committee on Pesticide Residues on compounds on priority list. (1979).
Hutson, D.H. Taurine Conjugation in the Metabolism of
3-Phenoxybenzoic Acid and the Pyrethroid Insecticide Cypermethrin (WL
43467). (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company. (Published in Xenobiotica, 8
(1978) 565-571).
The Elimination of Radioactivity by Mice Following Oral Dosing with
14C-cis and 14C-trans-WL 43467 (Cypermethrin). (1978a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Company.
The Metabolites of cis- and trans-Cypermethrin (WL 43467) in
Mice. (1978b) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Hutson, D.H. and Stoydin, G. The Excretion of Radioactivity from Cows
fed with Radioactively Labelled WL 43467. (1976) Unpublished report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
ICI. (1979) Unpublished reports available to meeting (referred to in
text by ICI and code numbers 10, 132 to 135 and 151).
Jaggers, S. Cypermethrin: Summary and Review of Acute Toxicities in
Laboratory Species. (1979) Unpublished report submitted by ICI, Ltd.
McAusland, H.E., Butterworth, S.T.G. and Hunt, P.F. Toxicity Studies
on the Insecticide WL 43467: A Two-year Feeding Study in Rats. (1978)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
New Zealand. Information supplied by CCPR country delegation on
compounds on priority list. (1979)
Okuno Y., Kohda, H. and Kadota, T. Neurotoxic Effects of S-5602 and
NRDC 149 by Dermal Application in Rats. (1976) Unpublished report
from Sumitomo Chemical Company submitted by Shell International
Chemical Co.
Owen, D.E. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticides: Investigation of the Neurotoxic Potential of WL 43467 to
Adult Domestic Hens. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Prinsen, G.H. and Van Sittert, W.J. Exposure and Medical Monitoring
Study of the Pyrethroid WL 43467 After Single Application on Cotton in
Ivory Coast. (1978) Unpublished report from Shell International
Research Maatschappij, B.V., submitted by Shell International Chemical
Co.
Roberts, T.R. and Stande, M.E. Degradation of the Pyrethroid
Cypermethrin NRDC 149 (± alpha-cyano-3-phenoxybenzyl) ± (cis trans
3(2,2-dichlorovinyl) 2,2-dimethycyclopropane carboxylate and
Respective cis (NRDC 160) and trans (NRDC 159) Isomers in Soils.
Poetic. Sci. 8, 305-319 (1977).
Rose, G.P. and Dewar, A.J. Toxicity Studies on the Insecticide WL
43467: The Effect of Age on the Neurotoxicity of WL 43467 to Rats.
(1978) Unpublished report from Shell Research Ltd.,
Ruzo, L.O., Holmstead, R.L., Casida, J.E. Solution photochemistry of
the potent pyrethroid insecticides alpha-cyano-3-phenoxybenzyl cis
2,2-dimethyl-3-(2,2dibromovinyl) cyclopropane carboxylate.
Tetrahedron Letters 35, 3045/1976.
Ruzo, L.O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin J. Agr. Food Chem 25, 1385 (1977).
Shell Chemie. France. Unpublished Reports. Referred to by "Shell C"
and numbers in the text: 2-9, 11, 12, 14, 17-25, 27-30, 34,40, 44, 47,
55, 56, 60-64, 67-75, 77-86, 90-97, 101-105, 107-109, 112, 114-116,
118-122 136, 137, 142, 143, 152, 154, 155.
Shell Research Ltd., England - Unpublished reports referred to by
"Shell R." and numbers in the text: 1, 13, 15, 16, 26, 31-33, 41-43,
45, 46, 48-54, 57-59, 65, 66, 76, 87-89, 98-100, 106, 110, 111, 113,
117 123-130, 138-140, 144, 147-150, 152, 156, 158-160, 162-164.
Shono, T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-Cypermethrin, and Decamethrin by Microsomal Enzymes. J. Agric.
Food Chem., 27 (2), 316-325.
South Africa. Information on compounds on priority list. (1979)
Sweden. Information on compounds on priority list. (1979)
Suzuki, H. Studies on the Mutagenicity of Some Pyrethroids on
Salmonella Strains in the Presence of Mouse Hepatic S9 Fraction.
(1977) Unpublished report from Sumitomo Chemical Co., Ltd., submitted
by Sumitomo Chemical Co., Ltd.
Tesh, J.M., Tesh, S.A. and Davies, W. WL 43467: Effects Upon the
Progress and Outcome of Pregnancy in Rat. (1978) Unpublished report
from Life Science submitted by Shell International Chemical Company.
Trigg, C.E., Butterworth, S. and Hunt, P.F. Neurotoxicity of
Pyrethroids: A Study of Teased Nerves from Rats Fed WL 43467 for 12
Months. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
In the urine 3(4-hydroxyphenoxy) benzoic acid-O-sulphate and the
glutamic acid conjugate of 3-phenoxybenzoic acid were detected in the
ratio of 1:4. The free acids were not detectable. The faeces
contained the parent compound amounting to approximately 85% of the
faecal 14C, that is, about 36% of the amount ingested. At the end of
the three-week feeding period the animals were slaughtered and samples
of tissues examined for radioactivity. Levels were below 0.001 mg/kg,
in cypermethrin equivalents, in blood, muscle and brain. In
subcutaneous and renal fats, liver and kidney samples, residues were
at or below the equivalent of 0.012 mg/kg cypermethrin (Shell R. 124).
b. High Dietary Intake (5 mg/kg)
Radiolabelled cypermethrin was fed in the feed concentrate twice daily
and was given in amount equivalent to 4 mg/kg on total diet over a
period of 7 days.
The radioactivity in the milk, urine and faeces was monitored
throughout the feeding period and showed that the major excretory
route was via the kidneys. All the cows were found to be pregnant at
necropsy and the milk yields throughout the experiment were poor.
Equilibrium between intake and excretion was reached 3-4 days after
commencement of feeding, after which levels in whole milk ranged
between 0.009 mg/l and 0.013 mg/l cypermethrin equivalent to
radioactivity present. The cream fraction of the milk contained
85-90% of the total radioactivity. Results of experiments carried out
with 14C-benzyl and 14C-cyclopropyl labels indicate that the residue
in milk is an ester and contains both the acidic and alcoholic
functions of the parent compound.
The major urinary metabolites in cows were identified as the glutamic
acid conjugate of 3-phenoxybenzoic acid (68%), 3-phenoxybenzylglycine
(16%), 3-phenoxybenzoic acid (9%). 3(4-hydroxy-phenoxy) enzoic-acid
and its O-sulphate conjugate appeared to be present in only small
amounts (1%).
At the end of the test feeding period the animals were slaughtered and
tissues were taken for measurement, with the following results,
radioactivity being expressed as cypermethrin equivalents.
Muscle 0.04 mg/kg
Fat 0.01-0.10 mg/kg
Liver and Kidney 0.05-0.13 mg/kg
These results indicate that the cypermethrin does not accumulate in
the tissues of the animals. Even at a high intake, residues are
mostly in the fat, liver and kidney. Cereals and components of feed
(e.g. cotton seed) treated with the highest recommended dose rates
therefore, are unlikely to result in measurable residues in meat or
milk of cattle (Shell R. 125).
IN PLANTS
Degradation on cotton, lettuce and apples has been studied using
14C-radiolabelled material with the label in various positions in the
cypermethrin molecule. Structures of compounds mentioned are given in
Figure 1.
a. Cotton
An experiment in which the cis- and trans-isomers of cypermethrin
were applied in acetone/water solution to cotton leaves using a small
syringe was carried out on plants grown in pots in the greenhouse.
Each of the two isomers, which in themselves consisted of a racemic
mixture of two enantiomeric pairs of stereo isomers, was labelled in
the cyclopropyl and benzyl rings in separate experiments. In each
experiment the rate of application was 10-20 µg/g of leaf. Leaf
samples were taken for examination immediately after treatment and
again 42 days later. The samples were extracted with acetone and the
examined by chromatographic and radiochemical techniques.
Forty-two days after treatment 75-85% of the original total applied
radioactivity was still present in cotton leaves and 90% of this was
acetone extractable. Thin-layer chromatography showed that at least
six components were present in the extracts. Products identified and
the approximate proportion present in the extracts were cypermethrin
(40-50%), 4-hydroxy-cypermethrin (compound A/ 5-8%), 3-phenoxybenzoic
acid (15%), and an amide (compound B/ 8-20%). In this preliminary
study unidentified material accounted for the remaining 20-30% and was
mainly polar material. No evidence of an appreciable difference in
the rate of loss and breakdown of the two isomers was obtained.
A second experiment was carried out on a larger scale, in which
cypermethrin was 14C radio-labelled in the benzyl ring and applied at
the rate of 4 µg/g leaf. Two applications were made 15 days apart and
leaf samples were taken 5 weeks after the second treatment. Results
were qualitatively similar to the first experiment, except that
smaller amounts of metabolites were found and, in addition, some
3-(4-hydroxyphenoxy)-benzoic acid was also reported to be present. Due
to the larger scale of the experiment it was possible to examine the
polar fraction in more detail. This was found to consist of at least
8 compounds which were mainly conjugated forms of 3-phenoxybenzoic
acids 3-(4-hydroxyphenoxy)-benzoic acid and 3-phenoxybenzyl alcohol.
When the results from experiments using cypermethrin, separately
labelled in the nitrile, cyclopropyl and benzyl groups, were compared,
the properties of the polar products formed differed according to the
label position. Thus it was again concluded that, following the
primary step of hydrolysis of the ester link, further degradation of
the component parts, including loss of nitrile, oxidation and
conjugation, took place. In this work, the cyclopropane carboxylic
acid (compound C) was identified as a metabolite.
In a third experiment the cotton was grown in boxes outdoors in
Seville, Spain. Cypermethrin, labelled either in the cyclopropane or
benzyl rings, was applied to separate plants. The cis and trans
isomers, in each case, were also applied in separate experiments.
Three applications were made at rates equivalent to 300 g/ha and
samples were taken at harvest 15 weeks after the final treatment.
Seed, fibre, boll cases and leaves were examined separately. All the
kernel samples separated from the seed cases contained small amounts
of radioactivity equivalent to 0.07-0.24 mg/kg cypermethrin. Due to
the oily nature of the extracts and the small size of the samples, it
was not possible to fully identify the products present, although it
was shown the radioactivity was not present in the form of
cypermethrin itself. Seed cases freed from lint also contained
radioactivity equivalent to up to 0.17 mg/kg cypermethrin, which again
was shown not to be parent insecticide.
The lint samples contained rather more variable amounts, between 0.08
and 0.62 mg/kg cypermethrin equivalents, probably depending on the
degree of opening of the bolls at the time of spraying. Cypermethrin
itself was the major compound present (between 50 and 90% of the
total) but the remainder consisted of polar products. Foliage and
boll cases were found to contain the same products as those reported
from glasshouse experiments. These included cypermethrin,
3-phenoxybenzoic acid in free form, the cyclopropane carboxylic acid
(free) and the amide, Compound A. The latter in particular was
present, however, in considerably smaller amounts than found in the
glasshouse experiment.
Polar materials were also found in appreciable amounts. These could
be hydrolysed by acid to give 3-phenoxybenzoic acid, 3-phenoxybenzyl
alcohol and 3-phenoxybenzaldehyde. Unidentified materials in foliage
amounted to only a small proportion of the total radio-label present.
In another experiment, abscised cotton leaves were placed with their
stems immersed in an aqueous solution containing ring
14C-radiolabelled Compound C for 72 hours. At the end of this period
90% of the radioactive material originally present in the solution had
been taken up by the leaves. The major proportion (78%) of this
material was found to be present as sugar conjugates of Compound C.
Experiments have also been carried out with the degradation products
3-phenoxybenzoic acid (14C ring-labelled) and
cis-2-(2',2'-dichlorovinyl)-3,3-dimethyl cyclopropane carboxylic
acid (14C-C1-label). In some of these the stems of cotton leaves
were immersed in aqueous solutions. There was a rapid uptake of both
compounds and a rapid conversion to polar products. These products
were subsequently shown to be derivatives with various mono- or
di-saccharide sugar products in the case of the cyclopropane
carboxylic acid and mainly glucose in the case of 3-phenoxybenzoic
acid. Leaves to which 3-phenoxybenzoic acid was applied topically on
the intact plant showed similar conversions to a mixture of sugar
conjugates although at a much slower rate than in the case of petiole
uptake. Similar experiments with 3-phenoxybenzoic acid have been
undertaken on vine, tomato, soybeans, pea and broad bean leaves and
with similar findings, (Shell R. 126, 127, 128, 158, 159 and 160)
b. Lettuce
Experiments were carried out on plants grown in pots in the glasshouse
and treated with solutions of either the cis- or trans-isomer of
cypermethrin. Each isomer was also labelled in either the benzyl or
cyclopropyl rings. Details were similar to the first cotton
experiment, described above, except that samples were taken
immediately after treatment and 18 days later, and the results were
qualitatively similar. Just over 70% of the original applied
radioactivity was still present 18 days after application and 90% of
this was extractable with acetone. But a higher proportion (50-70%)
of the extracted radioactivity was present as cypermethrin than with
cotton. The proportions of other products found were: compound A,
5-10%; 3-phenoxybenzoic acid, 10-15%; and compound B, 12-15%. An
unidentified 11-15% consisted of several minor metabolites.
An outdoor experiment was also performed on lettuce using cypermethrin
labelled in the benzyl and cyclopropyl rings. The two labelled
materials were applied to different plants as an overall foliar spray
of a diluted E.C. formulation at a rate equivalent to 300 g/ha.
Treatments were made on two occasions with an interval of about two
weeks, and the plants were harvested approximately three weeks after
the second treatment. The major part of the residue, which amounted
to 0.8-1 mg/kg(radioactivity equivalent to cypermethrin) was located
in the outer leaves and only 10-20% was present in the hearts. In the
benzyl labelled experiment, 50% of the radioactivity was present as
cypermethrin itself and most of the remainder as polar materials which
individually were in too small amounts for detailed study. Only 4%
was not extracted. In the cyclopropyl labelled experiment, 30% of the
residue consisted of cypermethrin and 40% as conjugates of Compound C
of which a glucose ester was identified (Shell R. 126, 129).
c. Apples
Apple trees growing in an outdoor wire-covered enclosure were treated
with cis-cypermethrin, separately labelled in the benzyl or
cyclopropyl rings, or with trans-cypermethrin, labelled in the
benzyl ring. Leaves were treated on three occasions at intervals of
25 and 37 days, and harvested four weeks after the last treatment.
Fruit was treated twice at a 24-day interval and harvested three weeks
after the second application.
Examination of the leaves showed that 32-46% of the residue consisted
of the parent isomer applied. In addition, 7-15% of the radioactivity
present was identifiable as compounds A and C, 3-phenoxybenzyl
alcohol, 3-phenoxybenzaldehyde and 3-phenoxybenzoic acid. Polar
compounds made up most of the remaining residue. Sugar conjugates of
3-phenoxybenzoic acid and the corresponding alcohol, as well as of
3-(4-hydroxyphenoxy)-benzoic acid, were identified.
In fruit, results were similar although the quantities present were
much lower. Examinations of peel and pulp separately showed that less
than 2% of the total radioactivity in the fruit was present in the
pulp. On the peel 50-77% was present in the form of the parent isomer
applied. During the experiment 15% of the cis isomer was converted
into the trans isomer. The amounts of metabolites were somewhat
less than in leaves and accounted, as free compounds, for 3-7% of the
radioactivity present. Polar materials from the fruit were present in
much smaller amounts then in leaves and were not present in sufficient
quantities to be studied in detail.
In the experiments with the cis isomer some conversion (30%) into
the trans isomer occurred, but the reverse change was not observed.
In some routine examinations of field samples for residues of
cypermethrin the glc analytical method used results in separation of
the cis and trans isomers on the chromatograph trace. No change
in the relative sizes of the two peaks with different treatment to
harvest intervals, or between unresolved standard material and the
test samples, has been observed. It would, therefore, seem that the
conversion of cis isomer into trans compensates for the faster
degradation of the trans isomer (Shell R. 130).
IN SOIL
Degradation on soils was studied by adding 14C-labelled cypermethrin
to three different soils - a clay and a sandy clay from Spain, and a
sandy loam from the U.K. Separate experiments were carried out with
the molecule labelled in the benzyl or the cyclopropyl moiety. In
addition, the cis and trans isomers were studied individually
(Roberts and Standen, 1977).
The cis isomer was found to degrade at an initial rate equivalent to
a half life of between 2 and 4 weeks in sandy clay and sandy loam, and
about 10 weeks in the clay soil. The trans isomer degraded more
rapidly than the cis isomer in all three soils, with initial
half-lives between about one and three weeks. Degradation was shown
to proceed by hydrolysis of the ester link and cyano group with loss
of carbon dioxide to give 3-phenoxybenzoic acid and Compound C, which
were both present as free compounds. Subsequent degradation of both
compounds was shown to occur, since 14 C-radiolabelled carbon dioxide
was evolved over 22 weeks in amounts equivalent to 24% and 38%,
respectively, of the total radioactivity initially present as ring
label. As indicated by the presence of small amounts of Compounds A
and 3-(4-hydroxyphenoxy)-benzoic acid, some hydroxylation of the
aromatic rings also took place.
Up to 36% of the original radioactivity was found to be difficult to
extract 16 weeks after the commencement of the experiment. A study of
this fraction showed it to be composed of several materials bound to
soil components. Examination of the acid hydrolysed material showed
that 30% or more of the radioactivity was in the form of Compound C.
Smaller amounts of 3-phenoxybenzoic acid and
3-(4-hydroxyphenoxy)-benzoic acid were also found. After 52 weeks
unchanged parent material accounts for 1.4-10.7% of the radioactivity
originally applied to the soils. In one sample of a Spanish soil
(Brenes) a trace amount of Compound B was also present and in the same
soil 5.8% of the original radioactivity was present as a cyclopropane
dicarboxylic acid, Compound D. In a separate experiment, this latter
compound was also found, in amounts of 3-13% of the applied
radioactivity, 8 weeks after application to soils stored in the
laboratory. Under waterlogged and anaerobic conditions hydrolytic
breakdown was found to be somewhat slower than under aeroboic
conditions. Breakdown of 3-phenoxybenzoic acid was also slower.
Compound C has also been shown to be formed from permethrin in soils
and to be degraded by hydroxylation at the gem-dimethyl groups.
Subsequent further breakdown has been shown in further studies,
leading to extensive evolution of CO2 derived from ring and
chloromethylene carbons (ICI, 132, 133, 134, 135). These data
substantially confirm the results obtained with cypermethrin, showing
that Compound C is unlikely to accumulate in soils following treatment
with the pesticide.
In addition to the degradation studies referred to above, studies have
also been made to determine the rate of loss of cypermethrin from
soils from field experiments in which very high rates of application
had been made. The compound was applied as a diluted 40% EC and was
partly incorporated to the soil (3-5 cm) after treatment. Samples
were generally taken from 0-15 cm depth. The data obtained (Shell C
and R. 136 to 140), show that for the same rate applied, the amount of
residue initially present after treatment varied widely from one trial
to the other, and that cypermethrin degrades fairly rapidly in soils.
Only 1-5% of initial residue, if any, could be detected 4-8 months
after treatment. The leaching studies with labelled cypermethrin in a
column containing sandy loam soil show that less than 2% of applied
radioactivity had been eluted with water at a rate of 2 ml/hr from the
column over the 45-day period (Hungary, 1979), and that 90% of the
initial activity was present in the top 2 cm of the soil. The
findings from laboratory experiments are in agreement with the results
of field trials.
From the above findings, it may be concluded that, even when
applications have been made to crops at recommended rates, residues of
cypermethrin itself are unlikely to be present at detectable levels in
soils at the beginning of the season following that in which
treatments were made. It may also be concluded that crops grown in
plots where treatments with cypermethrin have been made in a previous
season are unlikely to contain detectable residues.
IN PROCESSING
Cypermethrin is a moderately stable and water-insoluble compound. Data
relating to the effect on residue levels of various treatments given
to harvested crops are as follows:
a. Peeling
Numerous data show that the residue present in a crop is largely on
the surface. Analyses of pulp and peel after peeling apples, pears,
peaches and citrus fruits (Table 6) show that levels in the pulp were
below 30%, and in most instances below 10%, of those in whole fruit.
b. Juice extraction - citrus
Data for citrus have shown that residues of cypermethrin in juice
extracted from treated fruits contained no measurable residues of the
pesticide (see Table 6).
C. Wine making
Wine has been manufactured from grapes treated with cypermethrin and
containing up to 0.15 mg/kg pesticide. No residues (limit of
determination 0.01 mg/l) were found in the juice after fermentation
(Shell C., 142).
d. Cooking
Studies were made on the effect of boiling on residue levels in plums,
and cabbage. Levels were not substantially reduced by boiling plums
for 30 minutes or cabbage for 45 minutes. Residues in the cooked
commodities were 75-90% of the initial levels and only very small
amounts were found in the cooking water (Shell C., 143).
e. Oil seed processing
An experiment was carried out with a cotton seed sample deliberately
treated at the high rate of 300 g/ha and harvested one day after
treatment. It contained 0.12 mg/kg cypermethrin on whole seed, with
adhering linters. The sample was processed by simulating commercial
practice in a laboratory specialising in the techniques. Residues
were found to be transferred to kernels, which originally did not
contain any detectable residue in the seed, during the commercial
mechanical separation process (Shell R., 144). The residues of
cypermethrin in the extracted oil at various stages were as follows:
Crude oil 0.10 mg/l, neutralised oil 0.07 mg/l, bleached oil 0.08 mg/l
and deodorised oil 0.05 mg/l. Both the alkali wash and deodorisation
steps contribute to some losses. The results from this experiment
suggest that the two processes together may be expected to remove
about half of the residue. Hence, it is possible that residues may
occasionally occur in oil obtained from seed, treated under practical
conditions, at levels approaching those in whole seed.
Photodecomposition
The photodecomposition of cypermethrin was studied in methanol
solution under UV light and in the solid phase (3 mg/cm2 on glass)
under sunlight. 55% of cypermethrin was recovered from the methanol
solution after two days. In sunlight there was no detectable loss of
cypermethrin after 30 hrs. In both experiments cypermethrin proved to
be more stable than decamethrin and its behaviour was comparable to
permethrin (Ruzo et al, 1976, 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
At present no data deriving from national monitoring studies or market
control are available.
METHODS OF RESIDUES ANALYSIS
Several methods have been developed for the analysis of agricultural
commodities for residues. These are all based on gas-liquid
chromatography procedures using equipment commonly found in modern
analytical laboratories. Limits of determination of 0.01 mg/kg are
usually attainable.
The racemic cypermethrin contains eight possible steric and optical
isomers which can be partially resolved in the GLC column depending on
its polarity. On analysis of the mixture of isomers on OV-225 phase
three partially resolved peaks with a characteristic pattern are
obtained. However with apolar or moderately polar packings (OV-101 or
OV-17) no resolution of isomers occurs which facilitates the
quantitation of the residue. Four methods have been developed by
Shell. For cotton and other oil-seed crops, the sample is extracted
with an acetone/petroleum mixture and the extract subjected to
clean-up by solvent partition followed by absorption chromatography on
Florisil. The appropriate fractions of the eluate are then examined
by gas-liquid chromatography using electron capture (63Ni) detection
(Shell R. 147). For other crops the samples are ground with anhydrous
sodium sulphate and cypermethrin extracted with acetone and petroleum
spirit. The extract is washed with water and the resultant petroleum
spirit layer separated and cleaned-up by chromatography on a Florisil
column. Cypermethrin is eluted with ether/petrol and determined using
gas-liquid chromatography and a (63Ni) electron-capture detector
(Shell R. 148).
Residues in animal tissues are determined by extracting the tissue
with mixed acetone:hexane, and fat removed by partitioning between
hexane and acetonitrile. The extract is cleaned-up by absorption
chromatography on a column of Florisil and cypermethrin determined in
the appropriate eluate fractions, by glc using electron-capture
detection (Shell R. 149).
Soil samples are mixed with anhydrous sodium sulphate and extracted
with a mixture of acetone and petroleum spirit. The extract, which
contains any cypermethrin present in the soil, is washed with water to
remove acetone and cleaned up by adsorption chromatography on
Florisil. Residues of cypermethrin are determined using GLC with an
electron-capture detector (Shell R. 150).
Confirmation of a residue in either soil or crop may be obtained by
TLC (thin-layer chromatography) on Silica Gel plate eluted with
toluene without previous saturation followed by further GLC. ICI have
also developed basically similar analytical methods for application to
crop samples. In their method the crop samples are extracted with 20%
acetone in hexane and, where necessary, co-extractives removed by
solvent partition. Further clean-up is carried out by adsorption
column chromatography on Silica Gel or Florisil and cypermethrin
determined by GLC in conjunction with an 63Ni E.C. detector (ICI
151).
Recovery experiments have shown that all the methods cited are capable
of measuring 80% or more of any cypermethrin residue present and that
they are suitable for regulatory purposes.
Chapman reported on the applicability of various adsorbent-solvent
systems for the cleanup of some plant extracts containing
cypermethrin, fenpropanate, permethrin, and fenvalerate. Analytical
methods suitable for the determination of metabolites of cypermethrin
in crop samples obtained following practical applications in the field
have been developed using HPLC. Under normal circumstances the limit
of determination is 0.05 mg/kg.(Shell R. 161)
The amide, Compound B, is extracted with a mixture of water and
acetonitrile from the sample of which the water content is known.
Clean-up of the extract is performed by solvent partition and
reversed-phase partition chromatography followed by reversed phase
HPLC. Final analysis is carried out with normal HPLC using a UV
detector (Shell R. 162).
3-phenoxybenzoic acid may be determined in a similar manner, although
partition between dilute aqueous alkaline acetonitrile and hexane in
used to remove parent cypermethrin from free 3-phenoxybenzoic acid.
Conjugated material is hydrolysed by treatment of the concentrated
aqueous extract with hydrochloric acid. The 3-phenoxybenzoic acid is
converted into the methyl ester which may then be determined directly
on HPLC. Alternatively, if further clean-up is required, HPLC may be
used for this purpose and gas chromatography/mass spectrometry with
multiple ion monitoring used for the determination (Shell R. 163).
Compound C may also be extracted from crop samples with a mixture of
water and acetonitrile and separated from unwanted material by solvent
partition. Conjugated Compound C is however retained in the aqueous
phase which may be concentrated and hydrolysed with hydrochloric acid.
The liberated compound C may then be extracted with an ether/petroleum
spirit mixture. Clean-up of the extracts is performed by reversed
phase HPLC and Compound C determined by normal phase HPLC. If
extracts are insufficiently clean for HPLC they may be treated with
alpha-cyano-3-phenoxy-benzyl bromide, and the cypermethrin formed
determined by gas chromatography with electron capture detection
(Shell R. 164).
Other experiments have shown that the great majority of samples may be
stored for long periods in the deep freeze without appreciable loss of
residues. Residue levels were determined in crop and soil samples at
intervals following addition of known quantities of cypermethrin in
the range of 0.2 mg/kg to 1 mg/kg. The samples were stored at -18°C
between treatment and extraction for analysis, for periods between 1
and 54 weeks for crops and 4-49 weeks for soil. Recoveries of
cypermethrin added to crops (21 samples) were 85-110%, apart from one
sample of tobacco which yielded 45% after 6 months storage. In the
case of soils (5 samples) recoveries were all between 90 and 110%
(Shell R. 152).
Studies have also been carried out on the stability in storage at
-18°C of residues of 3-phenoxybenzoic acid and Compounds B and C.
Over three months there was no evidence of loss of 3-phenoxybenzoic
acid, since the total amounts recovered from the 6 crops used
(lettuce, potatoes, cabbage, apples, pears and maize grain) were in
the range of 75-100% and comparable to recovery values of the method
itself. With Compound B experiments were carried over three months
with sweet corn and over five months with apples and cabbage.
Recoveries were 70%, 75% and 75% respectively. Cabbage and apples
treated with Compound C were also stored at -18°C for five months.
Recoveries were 90-95% respectively. These data, therefore, indicate
that loss of any of the three degradation compounds mentioned were
negligible over periods of 3-5 months at 18°C.
NATIONAL MRLs REPORTED TO THE MEETING
National MRLs and pre-harvest intervals have been established in
several countries based on local requirements. The following were
presented at the meeting:
Country Commodity MRL Pre-harvest int. (days)
Argentina Apples 21
Cotton 20
Peaches 25
Tomatoes 7
Soya, Sorghum 40
Sunflower 30
Colombia Cotton 35
Costa Rica Cotton, Vegetables 15
Country Commodity MRL Pre-harvest int. (days)
Cyprus Vegetables 14
Top Fruit
Fed. Rep.
of Germany Leafy vegetables 14
Potatoes 14
Maize 49
France Apples 0.5 14
Peaches 0.5 14
Vines 0.5 14
New Zealand Kiwi fruit 2.0 14
Pome fruit 1.0 14
Brassicas 1.0 7
Peru Cotton 15
Tomatoes 15
Potatoes 15
Spain Cotton 21
Potatoes 21
Tomatoes 21
Citrus 21
South Africa Cotton 0.05 28
Grapes 0.05 28
Maise 1.5 28
Peas 0.1 7
Tomatoes 0.2 4
Syria Top fruit 7-10
Vegetables
Experimental or wider use of cypermethrin in further 43 countries was
reported where neither preharvest intervals nor MRL's have been yet
established.
APPRAISAL
Cypermethrin is active against a wide range of insects which attack
crops and can be used at a relatively low dose rate in the range of
0.02-0.25 kg a.i./ha. It is a moderately stable and water insoluble
compound. The technical material contains not less than 90% w/w
cypermethrin, which is a mixture of optical isomers, with a cis:trans
isomer ratio of approximately 40:60. The maximum concentration of
residues in/on the treated crops in the range of 0.05-2 mg/kg and
decreases slowly.
The majority of residues are in the peel or outer leaves, and in most
instances the pulp or hearts contain below 10% of those in the whole
crop. The trans isomers of cypermethrin degrades slightly faster
than the cis, which is compensated by the conversion of cis isomer
into trans during degradation, thus the isomers remain in a constant
ratio at different times after application in plants. Degradation in
crops occurs mainly by hydrolysis of the ester bond followed by
further hydrolytic and oxidative processes to give a variety of
products. Less rapid processes observed were hydrolysis of the
nitrile group to amide and hydroxylation of the phenoxy ring. The
compounds formed were, in turn, also hydrolysed at the ester link.
However, metabolites have not been detected in crop commodities from
supervised trials. At least 90% of the total residue present in plant
material is extractable with acetone. Processing of treated crops
after harvest usually reduces the residue significantly.
Cattle consuming feed items treated with cypermethrin eliminate the
residue rapidly. Equilibrium between intake and excretion is reached
in 3-4 days. The total radioactivity found in the milk amounted to
only 0.5% of the radioactivity fed to the animals and 60-90 of this
residue was present in the cream fraction. The residues in the milk
are esters and contain both acidic and alcoholic moieties of the
parent compound. The vast majority of residue in the feed is excreted
in the urine and faeces in roughly similar proportions. The main
metabolite in urine is 3-phenoxybenzoic acid which is present as
glutamic acid conjugate and glycine derivatives. The faeces mainly
contain the intact molecule.
Cypermethrin does not accumulate in the meat. Even when fed at a high
dose rate residues are mostly in the fat, liver and kidney. The data
indicate that the feeding of crops treated with cypermethrin following
the recommended use patterns does not result in measurable residues in
meat or milk of cattle. Since the compound may be used for direct
treatment of animals and data deriving from the latter use are not
available, recommendations of MRLs for animal products cannot be made
at present.
In soils, spray deposits remain in the surface layer. The rate of
degradation is dependent on the type of soil and proceeds by
hydrolysis of the ester link and cyano group finally resulting in
acids from both parts of the parent compound. Some hydroxylation of
the phenoxy aromatic ring occurs. Subsequent degradation also takes
place with the breakdown of the carbon rings. The trans isomer
degrades more rapidly than the cis. The results indicate that no
detectable residue of cypermethrin itself will be present in soil 8-12
months after treatment and that crops grown in the next season will
not contain measurable residues of cypermethrin.
Analytical methods available for the determination of residues in
various commodities are suitable for regulatory purposes. The limit
of determination is typically 0.01-0.02 mg/kg.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended based
on the pre-harvest intervals indicated below. The limits refer to the
sum of isomers of the parent compound in the portion of sample to be
analysed as described by CC/PR.
Pre-harvest interval on
Commodity Temporary MRL mg/kg which recommendation is
based (days)
Citrus fruits 2 14
Peaches 2 7
Pome fruits 2 7
Cherries 1 7
Grapes 1 7
Brassica leafy
vegetables 1 7
Lettuce 2 7
Plums 1 7
Raspberries 0.5 14
Strawberries 0.5 21
Tomatoes 0.5 3
Rapeseed 0.2 42
Wheat 0.2 14
Cottonseed 0.1 7
Cottonseed oil
(finished) 0.2
Kidney beans, peas,
soybeans
(without pods) 0.05 7
Maize 0.05 7
Potatoes 0.05 7
Sugar beet (roots) 0.05 14
Sweet corn 0.05 7
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
cypermethrin and/or metabolites in adipose tissue.
2. Observations in man, especially those with high level of
occupational exposure, to evaluate the potential susceptibility of
man to the neurotoxic syndrome observed in rodents.
Desirable
1. A dominant lethal bioassay.
2. Further residue data on kiwi fruit from supervised trials.
3. Selective surveys of residues in crops known to have been treated
under practical circumstances.
4. Use patterns for animal health use and residues in animal products
deriving from the recommended application.
REFERENCES
Baldwin, M.K. The analysis of a Metabolite of WL 43467 in Human Urine
as an Index of Exposure to that Compound. (1978) Unpublished Report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
Brooks, T.M. Toxicity Studies with WL 43467. Mutagenicity Studies
with WL43467 in the Host-Mediated Assay and in Micro-Organisms In
Vitro. (1976) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Brown, V.K. Toxicology of WL 43467 Isomers: Acute Toxicity of WL
43481 in DMSO to rats. (1979a) Unpublished report submitted by Shell
International Chemical Company.
Toxicology of WL43467 Isomers: Acute Toxicity of WL42641 in DMSO to
Rats. (1979b) Unpublished report submitted by Shell International
Chemical Company.
Buckwell, A.C. and Butterworth, S.T.G. Toxicity Studies on the
Pyrethroid Insecticide WL 43467. A 13-Week Feeding Study in Dogs.
(1977) Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Butterworth, S.T.G. and Clark, D.G. Toxicity Studies on the
Insecticide WL43467: Acute Oral Toxicity and Neuropathological
Effects in Syrian Hamsters. (1977) Unpublished report from Shell
Research Ltd., Submitted by Shell International Chemical Company.
Carter, B.I. and Butterworth, S.T.G. Toxicity of Insecticides. The
Acute Oral Toxicity and Neuropathological Effects of WL 43467 to Rats.
(1976) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Chapman, R.A., and Harris, C.R. Extraction and Liquid-Solid
Chromatography Cleanup Procedures for the Direct Analysis of 4
Pyrethroid insecticides in Crops by Gas Liquid Chromatography. J. of
Chromatography 166. 513-518.
Combs A.D., Carter, B.I. Hend, R.W., Butterworth, S.G. and Backwell,
A.C. Toxicity Studies on the Insecticide WL-43467: (1976) Unpublished
summary of results of preliminary experiments from Shell Research
Ltd., submitted by Shell International Chemical Company.
Crawford, M. The Metabolism of WL 43467 in Mammals (1). The fate of
a Single Oral Dose of [14C-Benzyl] WL 43481 (cis-WL 43467) in the
Rat. 1976a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of WL 43467 in Mammals. The Fate of a Single Oral
Dose of [14C] WL-42641 (trans-WL43467), in the Rat. (1976b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, N.J. The Metabolism of WL 43467 in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] WL 43467 in the Rat. (1977)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Excretion and Residues of Radioactivity in Cows Treated Orally
with 14C-Labelled WL 43467. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 434671) in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] Cypermethrin in the Dog.
(1979a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 43461) in Mammals. The Fate of
Single Oral Doses of cis- and trans- [14C-benzyl] Cypermethrin in
the Dog. (1979b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
The Metabolic Fate of the cis- and Trans-isomers of WL 43467
(Cypermethrin) and of 3-Phenoxybenzoic Acid in Dogs. (1979c)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.H. The Elimination and Retention of WL
43467 when administered Dermally or Orally to Sheep. (1977a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.R. The Metabolic Fate of the cis- and
trans- isomers of WL 43467 (Cypermethrin). Metabolism and
Elimination of 14C-labelled cis- and trans-isomers in Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Mice Following the Oral
Administration of [14C-benzyl-] WL 43481 (cis-WL43467). (1978a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Rats Following the Oral
Administration of [14C-benzyl] WL 43481 (cis-WL 43467). (1978b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Metabolic Fate of the cis-and trans-isomers of Cypermethrin
in the Rat. Metabolites Derived from the 14C-Labelled Cyclopropyl
Ring. (1978c) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Crawford, J.V. A Study of the Metabolism of 3-Phenoxybenzoic Acid and
its Glucoside Conjugate in Rats. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Crawford, J.V. and Hutson, D.H. The identification of Metabolites in
the Tissues of Rats Treated Orally with 3-Phenoxybenzoic Acid. (1979)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dean, B.J. Toxicity Studies with WL 43467: Chromosome Studies on Bone
Marrow Cells of Chinese Hamsters After Two Daily Oral Doses of WL
43467. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Dean, B.J., van der Paux. C.L. and Butterworth S.T.G. Toxicity
Studies with WL 43467: Dominant Lethal Assay in Male Mice After Single
Oral Doses of WL 43467. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Dewar, A.J. The Use of Lysosomal Enzyme Measurements as an Indicator
of Chemically-Induced Peripheral Neuropathy. (1977a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Co.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Functional Studies on the Neurotoxicity of WL 43467 to Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dewar, A.J. and Deacon, P.A. Toxicity Studies on the Insecticide WL
43467: Electrophysiological Studies on the Neurotoxicity of WL 43467
to Rats. I. - The Effect on Motor Conduction Velocity in the Sciatic
and Tail Nerves. (1977) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Dewar, A.J. and Moffett, B.J. Toxicity Studies on the insecticide WL
43467: Biochemical studies on the effect of WL 43467 on the rat
trigeminal nerve and ganglion. (1978a) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Function Studies on the Neurotoxicity of WL 43467 to Chinese Hamsters.
(1978b) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
Dix, K.M. Toxicity of WL 43467: Teratological Studies in Rabbits
given WL 43467 Orally. (1978) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Glaister, J.R., Pratt, I. and Richards, D. PP 383: Effects of High
Dietary Levels on Clinical Behaviour and Structure of Sciatic Nerves
in the Rat. (1977a) Unpublished report from ICI Central Toxicology
Laboratory submitted by ICI Ltd.
Glaister, J.R., Gare, C.W., Marsat, G.J., Phillips, C. and Pratt, I.
PP 383: 90-Day Feeding Study in Rats. (1977b) Unpublished report
from ICI Central Toxicology Laboratory submitted by ICI Ltd.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies on the
Insecticide WL 43467: A Three-month Feeding Study in Rats. (1976)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Co.
Toxicity Studies on the Insecticide WL 42641: a Five-Week Feeding
Study in Rats. (1977a) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43481: A five-Week Feeding
Study in Rats. (1977b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Hend, R.W., Hendy, R. and Fleming, D.J. Toxicity Studies on the
Insecticide WL 43467: A Three-Generation Reproduction Study in Rats.
(1978) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Hungary. Information supplied by country delegation to Codex
Committee on Pesticide Residues on compounds on priority list. (1979).
Hutson, D.H. Taurine Conjugation in the Metabolism of
3-Phenoxybenzoic Acid and the Pyrethroid Insecticide Cypermethrin (WL
43467). (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company. (Published in Xenobiotica, 8
(1978) 565-571).
The Elimination of Radioactivity by Mice Following Oral Dosing with
14C-cis and 14C-trans-WL 43467 (Cypermethrin). (1978a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Company.
The Metabolites of cis- and trans-Cypermethrin (WL 43467) in
Mice. (1978b) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Hutson, D.H. and Stoydin, G. The Excretion of Radioactivity from Cows
fed with Radioactively Labelled WL 43467. (1976) Unpublished report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
ICI. (1979) Unpublished reports available to meeting (referred to in
text by ICI and code numbers 10, 132 to 135 and 151).
Jaggers, S. Cypermethrin: Summary and Review of Acute Toxicities in
Laboratory Species. (1979) Unpublished report submitted by ICI, Ltd.
McAusland, H.E., Butterworth, S.T.G. and Hunt, P.F. Toxicity Studies
on the Insecticide WL 43467: A Two-year Feeding Study in Rats. (1978)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
New Zealand. Information supplied by CCPR country delegation on
compounds on priority list. (1979)
Okuno Y., Kohda, H. and Kadota, T. Neurotoxic Effects of S-5602 and
NRDC 149 by Dermal Application in Rats. (1976) Unpublished report
from Sumitomo Chemical Company submitted by Shell International
Chemical Co.
Owen, D.E. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticides: Investigation of the Neurotoxic Potential of WL 43467 to
Adult Domestic Hens. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Prinsen, G.H. and Van Sittert, W.J. Exposure and Medical Monitoring
Study of the Pyrethroid WL 43467 After Single Application on Cotton in
Ivory Coast. (1978) Unpublished report from Shell International
Research Maatschappij, B.V., submitted by Shell International Chemical
Co.
Roberts, T.R. and Stande, M.E. Degradation of the Pyrethroid
Cypermethrin NRDC 149 (± alpha-cyano-3-phenoxybenzyl) ± (cis trans
3(2,2-dichlorovinyl) 2,2-dimethycyclopropane carboxylate and
Respective cis (NRDC 160) and trans (NRDC 159) Isomers in Soils.
Poetic. Sci. 8, 305-319 (1977).
Rose, G.P. and Dewar, A.J. Toxicity Studies on the Insecticide WL
43467: The Effect of Age on the Neurotoxicity of WL 43467 to Rats.
(1978) Unpublished report from Shell Research Ltd.,
Ruzo, L.O., Holmstead, R.L., Casida, J.E. Solution photochemistry of
the potent pyrethroid insecticides alpha-cyano-3-phenoxybenzyl cis
2,2-dimethyl-3-(2,2dibromovinyl) cyclopropane carboxylate.
Tetrahedron Letters 35, 3045/1976.
Ruzo, L.O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin J. Agr. Food Chem 25, 1385 (1977).
Shell Chemie. France. Unpublished Reports. Referred to by "Shell C"
and numbers in the text: 2-9, 11, 12, 14, 17-25, 27-30, 34,40, 44, 47,
55, 56, 60-64, 67-75, 77-86, 90-97, 101-105, 107-109, 112, 114-116,
118-122 136, 137, 142, 143, 152, 154, 155.
Shell Research Ltd., England - Unpublished reports referred to by
"Shell R." and numbers in the text: 1, 13, 15, 16, 26, 31-33, 41-43,
45, 46, 48-54, 57-59, 65, 66, 76, 87-89, 98-100, 106, 110, 111, 113,
117 123-130, 138-140, 144, 147-150, 152, 156, 158-160, 162-164.
Shono, T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-Cypermethrin, and Decamethrin by Microsomal Enzymes. J. Agric.
Food Chem., 27 (2), 316-325.
South Africa. Information on compounds on priority list. (1979)
Sweden. Information on compounds on priority list. (1979)
Suzuki, H. Studies on the Mutagenicity of Some Pyrethroids on
Salmonella Strains in the Presence of Mouse Hepatic S9 Fraction.
(1977) Unpublished report from Sumitomo Chemical Co., Ltd., submitted
by Sumitomo Chemical Co., Ltd.
Tesh, J.M., Tesh, S.A. and Davies, W. WL 43467: Effects Upon the
Progress and Outcome of Pregnancy in Rat. (1978) Unpublished report
from Life Science submitted by Shell International Chemical Company.
Trigg, C.E., Butterworth, S. and Hunt, P.F. Neurotoxicity of
Pyrethroids: A Study of Teased Nerves from Rats Fed WL 43467 for 12
Months. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
See Also:
Toxicological Abbreviations
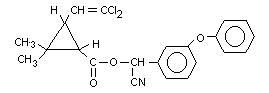 Molecular Weight: 416
Technical Material: Contains not less than 90% w/w cypermethrin
which is a racemic mixture of the 8 isomeric
forms with a cis: trans isomer ratio of
approximately 40:60
Physical Form Technical material is a viscous yellow liquid.
Density: 1.12 g/ml at 22°C.
Thermal Stability: Thermal analysis has shown that at a
temperature of 259°C slow weight losses occur
accompanied by negligible heat of reaction
indicating high thermal stability.
Solubility: Water 0.009 mg/l. Hexane 103 g/l. Acetone,
cyclohexanone, ethanol xylene and chloroform
450 g/l.
Volatility: relatively non-volatile
Vapour pressure: 5 × 10-6 N/m2 at 70°C
Flash point: 80°C
Formulations: Currently available in emulsifiable
concentrates ranging from 25-400 a.i. g/l.
Several formulations for ultra low volume
applications containing 8-75 g/l are also
available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Studies were performed in vivo on a wide range of mammalian species
and in vitro on isolated cell fractions to define the
pharmacokinetics of cypermethrin. In general, a wide range of studies
have shown that cypermethrin is rapidly absorbed, distributed to a
variety of tissues and organs, metabolised and rapidly excreted from
the body. Studies have been performed to evaluate the
pharmacokinetics in mice, rats, dogs, sheep and cows.
Mice
Groups of male mice were orally administered the cis or trans isomers
of cypermethrin dissolved in methoxytriglycol at dose levels of 7
mg/kg with the cyclopropyl-14C isomer and 8 mg/kg with the benzyl-14C
isomer. Results suggest that the proportion of cypermethrin which was
readily absorbed following oral dosing was rapidly eliminated in the
urine of mice. Absorption from the gastrointestinal tract was more
rapid with the trans isomer than with the cis as evidenced by high
radioactivity levels of the cis isomers in the faeces within the first
few days of the study. The rate of urinary excretion of cypermethrin
isomers labelled in acid and alcohol portions of the molecule was very
similar with the major quantity of radioactivity being eliminated from
the body within three days. Tissue residues after three days were
low. Adipose tissue was found to retain small quantities of the
radioactivity in concentrations higher than that noted for most other
tissues (Hutson, 1978a).
To evaluate the elimination of cypermethrin and its metabolites from
animal tissues following oral administration, groups of male mice were
administered cypermethrin at dose levels approximating 9 mg/kg body
weight (14C-benzyl, cypermethrin). In contrast to data from other
tissues, the rate of elimination of cypermethrin from adipose tissue
of mice was relatively slow with a half-life of approximately 10-20
days. Unchanged cis-cypermethrin was the only chemical residue
identified in adipose tissue. There was little change in the residue
during the last half of the 42-day trial following the relatively
rapid elimination during the first three weeks suggesting possible
long term storage and buildup of residue in adipose tissue (Crawford
and Hutson, 1978a).
Rat
Groups of male and female Wistar rats were orally administered
cypermethrin (14C benzyl, cis-isomer) at the dose level of 2.4 mg/kg
for females and 1.8 mg/kg for males. Cypermethrin was administered in
corn oil solution and animals and tissues were monitored over a period
of eight days. Excretion was rapid with both sexes. There was a
significant sex difference in the rate of excretion with male rats
excreting a substantially larger portion of the radioactivity in urine
than females over the first 24 hour interval. However, there were no
substantial differences in the overall rate of elimination by both
males and females when data were evaluated over the total 8-day period
of the test. The residues of radioactivity in the tissue of animals
of both males and females sacrificed at various periods over the 8-day
interval showed a small residue in a variety of tissues. While the
tissue residue in blood, liver, kidney, etc. was rapidly depleted over
the test interval, concentrations of cypermethrin in adipose tissue
were relatively stable over the 8-day trial (Crawford, 1976a).
Groups of male and female rats were orally administered cypermethrin
14C-benzyl, trans-isomer) at dose rates of 2.4 mg/kg for males and
3.0 mg/kg for females. Again, excretion was rapid with both sexes
with approximately 95% of the administered dose excreted within 48
hours. In contrast to that noted with the cis-isomer, there appeared
to be no sex differences in the elimination of the trans-isomer.
Residues in adipose tissue of females was somewhat higher than noted
in adipose tissue of males. The observations are consistent with the
suggestion that the trans-isomer is metabolised faster than the
cis-isomer (Crawford, 1976b).
Groups of rats were administered cypermethrin (1:1 cis: trans-isomer
ratio, 14C-cyclopropyl) at dose rate of 1 mg/kg for males and 2 mg/kg
for females. Rapid elimination of cypermethrin was observed with a
substantial difference noted in the 24-hour urinary excretion in males
and females. Females excreted considerably more radioactivity in
urine over the first 24 hours. At the end of 72 hours urinary and
faecal excretion in both sexes was approximately the same. Small
quantities of radioactivity were expired as 14CO2 which suggested
some metabolic breakdown of the cyclopropyl ring. At the end of three
days, tissue concentrations in liver, kidney muscle, brain, blood,
skin and remaining carcass were relatively low. Intestinal content
was somewhat higher in males than in females ranging from 3 to 9% of
the administered dose. Low residue concentrations, which never
exceeded 1 ppm, were observed in fat (Crawford, 1977).
The elimination pattern of cyclopropyl- and benzyl-labelled
cypermethrin appears to be the owns in both males and females.
Females absorb and metabolise the trans-isomer faster than males and
males absorb and metabolise the cis-isomer faster than females
(Crawford, 1976b). In a further study to resolve the residual nature
of the residues of cypermethrin in adipose tissue of rat, Crawford and
Hutson (1978b) again found that the cis-isomer was relatively stable
in adipose tissue. Administration of 14C-benzyl cypermethrin
(cis-isomer) at a dose of approximately 2 mg/kg to female rats
resulted in a residue of approximately 0.3 ppm in the fat eight days
after a single oral dose. Further studies through 42 days following
dosing were performed to evaluate the half-life of the cis-isomer in
fat and the total elimination pattern. At the end of 42 days,
residues were observed in fat which were approximately 10% of those
concentrations noted at eight days. There was a 90% loss of the
material from fat over the 8-42 day interval during which time samples
were taken. From these data with rats, a half-life of approximately
20-25 days was estimated with respect to removal of residues from fat
following a single oral dose. These half-life values are somewhat
longer than those noted with the mouse.
Dogs
Groups of male beagle dogs were administered 14C-benzyl cypermethrin
and the individual 14C-benzyl labelled cis- and trans-isomers of
cypermethrin, orally, at a dose of 2 mg/kg. Elimination of
radioactivity from all animals was rapid although differences in data
from individual dogs precluded a complete evaluation of the rate of
elimination. Differences in the rates of elimination of the
individual dogs in the study may have been due to differences in
absorption as cypermethrin was given orally in a capsule with no
solvent. Tissue residues observed 4 days after oral administration of
cypermethrin were extremely low. The vast majority of the excreted
material was observed in faeces (80%) with urine containing only 11%
of the administered dose. As with other species, small residues of
cypermethrin were observed in fat, approximating 2% of the
administered dose (this residue was estimated to be 0.3 ppm based upon
total adipose tissue of the dog) (Crawford, 1979b).
Sheep
Sheep were administered cypermethrin dermally (962 mg/animal
administered in acetone) or orally (without solvent) in gelatin
capsule at a dose of 4 mg/kg body weight. Following dermal
administration, cypermethrin was not readily absorbed (less than 0.5%
of the administered dose was observed in urine within 24 hours and 2%
over the six day test interval). Little radioactivity was found in
the liver and kidney of dermally-treated sheep. Tissue residues in
renal and subcutaneous fat was found to be similar to those noted with
other animal species and was qualitatively identified as cypermethrin.
Elimination of cypermethrin from the orally-dosed sheep was rapid with
61% of the administered dose being eliminated within 48 hours (41% of
the dose in urine and 20% of the dose in faeces). The tissue residue
pattern in sheep following oral administration was similar to that
noted with other mammalian species. Low levels of cypermethrin were
noted in various tissues, including renal and subcutaneous fat
(Crawford and Rutson, 1977a).
Cows
Cypermethrin (1:1, cis:trans-isomer, 14C-benzyl-label) was orally
administered to lactating cows over a period of three weeks at a
dietary dosage of 0.2 ppm. Urine, faeces and milk residues were
examined over the course of the study and at the conclusion of the
three week dietary interval, animals were sacrificed and tissue
residues examined. Elimination of radioactivity in urine and faeces
rapidly reached an equilibrium within 2-3 days. Radioactivity was
eliminated in the urine and faeces predominantly and accounted for the
major quantity of cypermethrin residues. Small quantities of
radioactivity were noted in milk with a total radioactivity residue
approximating 0.5% of the administered radioactive cypermethrin.
Tissue residues were extremely low with notable residues in bile and
fat as well as in the liver and kidney. There were no detectable
residues in muscle, blood or brain. Analysis of the milk fat showed
that a high percentage of the radioactivity was present in this
portion of the milk. Residues in the milk fat or cream sample were
reflective of the lipoidal nature of cypermethrin, with residues
concentrating in fatty tissues (Hutson and Stoydin, 1976).
In a further study to obtain information on the metabolic distribution
of cypermethrin in cows, lactating cows were treated for 7 days at a
dietary concentration of 5 ppm. Urine, faeces, and milk were analysed
and at the conclusion of the feeding trial, all animals were
sacrificed and tissue residues were determined. As in the previous
study, elimination occurred rapidly after the onset of dosing with the
major route of excretion being via urine and faeces. Tissue residues
were extremely low with measurable amounts observed in blood, liver,
kidney, bile and adipose tissue. Total milk residues amounted to
approximately 0.2% of the applied cypermethrin, with the largest
proportion of the residue again found in milk fat (Crawford, 1978).
Metabolism
The metabolic fate of cypermethrin was investigated in a variety of
mammalian species and was determined to be relatively similar in the
different species. The major metabolites are derived by cleavage of
the ester linkage followed by subsequent metabolism of both the acid
and alcohol fragments and conjugation and excretion of the fragments.
Studies on the metabolic fate of both cis- and trans-cypermethrin
administered orally to rats have shown that cypermethrin is rapidly
cleaved at the ester bond to yield the cyclopropanecarboxylic acid and
the 3-phenoxybenzyl alcohol moiety. The latter is rapidly oxidised to
3-phenoxybenzoic acid and conjugated prior to elimination. Major
reactions occurring with the cyclopropanecarboxycyclic acid include,
in part, oxidation at the methyl groups and apparent lactone
rearrangement prior to conjugation and elimination. Hydroxylation of
the phenoxybenzoyl moiety has been noted to occur in at least two
positions prior to conjugation and elimination. Rats and mice
metabolised cypermethrin in a similar fashion. In mice, a substantial
portion of the phenoxybenzoic acid was conjugated with taurine as well
as with glucuronic acid (Hutson, 1977). Oxidation has been noted to
occur in the 4'- position of phenoxybenzoic acid. Following
oxidation, the molecule is conjugated as a sulfate and excreted. A
portion of unconjugated hydroxylated phenoxybenzoic acid was also
excreted. Hydroxylated cypermethrin was observed in mouse faeces
suggesting that hydroxylation may occur prior to ester cleavage
(Hutson, 1978b). Shono, Ohsawa, and Casida (1978), using mouse liver
microsomal preparations, showed that hydroxylation of the
phenoxybenzyl moiety at positions other than the 4'- position can
occur. Small amounts of the 5- and 6-hydroxy phenoxybenzyl derivatives
were also observed. Both cis- and trans-isomers were rapidly
metabolised by cleavage of the ester bond and aromatic hydroxylation
at the 4'-position of the 3-phenoxybenzyl alcohol moiety. In rats,
approximately half of the administered cypermethrin was excreted as
sulfate conjugates of the hydroxylated phenoxybenzoic acid.
Phenoxybenzoic acid was also excreted free and conjugated with glycine
in contrast to the conjugate pattern noted in mice (Crawford and
Hutson, 1977b, 1978c). In studies with cyclopropanecarboxylic
acid-labelled cypermethrin, hydroxylation of the methyl groups on the
cyclopropane ring occurred to a limited extent. Oxidation was
believed to have occurred following ester cleavage. While most of the
radioactive metabolites were found in the urinal a small quantity of
hydroxylated metabolites were eliminated via the
biliary-intestinal-faecal route suggesting absorption and
re-distribution through the bile to the faeces.
In both rats and mice as well as other species, ester cleavage is
rapid. There is quantitative and qualitative evidence to suggest that
the cis-isomer is more stable yielding a larger variety of
hydroxylated products prior to ester cleavage while the trans-isomer
yields a wider variety of hydrolytic products which in part are
oxidised following the ester cleavage. The acid fragment is
conjugated predominantly with glucuronic acid an a ß-glucuronide.
Conjugated cyclopropanecarboxylic acid derivatives in urine were
identified as glucuronides have a relative resistance to the action of
ß-glucuronidase. ß-Glucuronides formed from these acids are generally
of the ester type readily cleaved by mild acid hydrolysis. Thus,
treatment of urine with methanol and sulfuric acid will convert both
the free acid and the acid glucuronide into a methyl ester. The
methyl ester of the carboxylic acid has been shown to be volatile and
analytical losses may occur with this compound. The alcohol fragment,
the alpha-cyano-3-phenoxybenzyl alcohol, is readily oxidised
metabolically to the benzoic acid, conjugated and excreted. Alcohol
conjugates with glucuronic acid are generally ether derivatives,
stable to acid and enzymatically hydrolysable. The phenoxybenzyl
moiety of cypermethrin has been found to form both ester and ether
ß-glucuronides.
Thus, the results of the studies with both rats and mice suggest that
most of the excreted metabolites of cypermethrin are hydrolysis
products, although hydroxylation of the intact ester has been
reported. Hydroxylation of the methyl groups attached to the
cyclopropane ring has also been reported. There was no evidence of
metabolism at the 2,2-dichlorovinyl moiety. Evidence exists that the
cis-isomer is somewhat more resistant than the trans-isomer to
hydrolysis. In all cases, only small amounts of hydroxylated
metabolites with the intact ester bond were observed with the
cypermethrin.
The metabolic fate of cypermethrin in dogs was qualitatively similar
to that observed with other species with the exception being
conjugation reactions of several metabolites. As with other species,
cypermethrin is rapidly metabolised by cleavage of the ester bond and
by hydroxylation of the phenoxybenzyl moiety at the 4' position.
Sulfate conjugation of the hydroxylated benzoic acid was reported.
Additionally, a major urinary metabolite was identified as the
3-phenoxybenzyl glycine. Studies on the metabolic fate of
3-phenoxybenzoic acid in dogs revealed a metabolic pattern of
oxidation and conjugation similar to that noted with cypermethrin
(Crawford, 1979a and 1979c).
In cows, the major urinary metabolite of cypermethrin ( 14C-benzyl)
was the glutamic acid conjugate of the 3-phenoxybenzoic acid
(Crawford, 1978). The cyclopropanecarboxylic acid moiety was
hydroxylated and/or conjugated with glucuronic acid prior to
excretion.
3-Phenoxybenzoic acid has been observed as a major metabolite of
cypermethrin in all species studied. Residues of cypermethrin in rat
skin (<3% of the dose) were noted after oral administration. Further
studies to define the skin residue were performed using
14C-3-phenoxybenzoic acid administered orally to rats as seven
consecutive daily doses. The radioactive metabolites in skin were
identified as predominately 3-phenoxybenzoic acid and a small quantity
of glyceryl dipalmitate esters of 3-phenoxybenzoic acid (Crawford and
Hutson, 1979).
Further studies were performed in rats of the metabolism of
3-phenoxybenzoic acid and a glucoside conjugate isolated from plant
tissue. 3-Phenoxybenzoic acid was oxidised at the 4' position of both
aromatic moieties and to a minor extent at the 6' position of the
benzoic acid moiety. Excretion occurred as conjugates, predominantly
the 4-hydroxy-3-phenoxybenzoic acid-O-sulfate and 3-phenoxybenzoic
acid. Additionally, other hydroxylated derivatives and conjugates of
these oxidation products were observed. The excretion pattern of the
glycoside conjugates of 3-phenoxybenzoic acid was qualitatively and
quantitatively similar to that observed with 3-phenoxybenzoic acid
suggesting rapid hydrolysis and availability of plant metabolites
following oral administration (Crawford, 1978).
Effects on Enzymes and Other Biochemical Parameters
Preliminary evidence suggested that an increase in the activity of
certain lysosomal enzymes in peripheral nerves and deficits in
behavioural functioning tests could serve as indicators of peripheral
nerve damage. During Wallerian degeneration, the activity of such
enzymes as ß-glucuronidase and ß-galactosidase as well as other
enzymes in nerve preparations were shown to be increased significantly
(Dewar, 1977a).
Cypermethrin (1:1 cis:trans) was administered to male and female rats
at dose levels ranging from 25 to 200 mg/kg/day for five consecutive
days by oral intubation as a 10% W/V solution in DMSO. A dose related
functional deficit was observed when the mean slip angle test and the
landing foot spread test were applied to the animals. The deficit was
maximal from 6 to 14 days after the beginning of treatment and
complete functional recovery occurred within four weeks. Substantial
variation in data from the landing foot spread test was noted. Data
were inconsistent over the course of the study. ß-glucuronidase
activity was increased in a dose-dependent fashion in both males and
females. The results suggest that cypermethrin produced a primary
axonal degeneration, readily measurable 28 days after treatment as an
increase in ß-glucuronidase activity and in deficits in specific
behavioural-function testing of rats (Dewar, 1977b).
When further studies were performed on the biochemical indices of
nerve degeneration to compare results with known degenerating
compounds (methyl mercury), it was observed that the changes in
ß-glucuronidase and ß-glucuronidase activity were considerably smaller
and less reproducible than those obtained following methyl mercury
poisoning (7.5 mg/kg/day for 7 days). In comparison of central versus
peripheral nerve damage, there was no evidence that the trigeminal
nerve was more sensitive to the effects of cypermethrin than the
sciatic and posterior tibial nerves. At near lethal doses of
cypermethrin biochemical changes in the trigeminal nerve were
consistent with those of Wallerian degeneration. The changes were
similar qualitatively to those seen with methyl mercury, but were
quantitatively much less intense (Dewar and Moffett 1978a).
Electrophysiological studies were performed to determine whether acute
or subacute intoxication with cypermethrin produced changes in the
conduction velocity of slower fibres in peripheral nerves or
alterations in the maximal motor conduction velocity. There was no
evidence to suggest that cypermethrin, at doses that induced severe
clinical signs of intoxication, including ataxia, had any effect on
maximal motor conduction velocity or conduction velocity of the slower
motor fibers in peripheral nerves. Doses used in the study ranged
from a single dose of 200 mg/kg to 7 consecutive doses of 150 mg/kg
followed by 2 doses of 400 mg/kg. At near-lethal doses there were no
effects noted on conduction velocity in the slower motor fibers of the
sciatic nerve and tail or on the maximal conduction velocity, even in
the presence of clinical signs of acute intoxication and at dose
levels where previous studies had shown functional degeneration.
These electrophysiological findings are reflective of motor function
which would suggest that the physiological and functional deficits
observed as a result of acute intoxication may be primarily sensory in
nature (Dewar and Deacon, 1977).
SPECIAL STUDIES ON REPRODUCTION
Dominant Lethal Studies
Mice
Groups of male mice (12 mice/group, 36 mice were used as controls)
were administered a single dose of cypermethrin dissolved in dimethyl
sulfoxide at dosage levels of 0, 6.25, 12.5 and 25 mg/kg body weight
or 5 successive daily oral doses of 0, 2.5 and 5.0 mg/kg. Following
dosing, each male was caged with 3 virgin females for 7 days. The
mating procedure was repeated weekly over an interval of 8 weeks in a
standard dominant lethal test.
Females mated to males treated with 5 daily oral doses of 2.5 mg/kg
and those mated to males receiving a single dose of 12.5 mg/kg showed
a significant reduction in the incidence of pregnancy during the
second and third week respectively of the onset of treatment. This
did not occur with other either higher or lower dosed groups or at
other intervals. In females, mated to males treated daily with doses
of cypermethrin a significant reduction in foetal implants was
observed during the second week of mating. Early foetal deaths were
increased in the second week at 5 mg/kg. No such increases occurred
in any other weekly interval or any other dosed group. Thus, multiple
administration of cypermethrin on five successive days induced a
significant reduction in foetal implants during the second week of
mating and a marginal increase in early foetal deaths at the same time
interval.
To evaluate the potential (noted above) for a dominant lethal effect,
a second experiment was performed. Groups of male mice (12
mice/group, a control group consisted of 36 mice) were administered
five daily oral doses of cypermethrin at levels of 0, 2.5, 5.0, 7.5
and 10 mg/kg (in dimethyl sulfoxide). Following dosing each male was
mated with three virgin females for four days and subsequently
provided with virgin females every four days for a period of three
weeks. Female mice were examined for evidence of dominant lethality
13 days after mating. In addition, 40 males of proven fertility were
dosed for five successive days at the same dosage levels, 0, 2, 5,
5.0, 7.5 and 10 mg/kg. These animals were also mated with four virgin
females on four successive days for a period of three weeks. Four
animals from each group were sacrificed for histological examination
of the testes and epididymis on days 1 and 7 after the final dosing.
In contrast to the previous trial, no reduction in foetal implants was
noted in any of the animals mated with cypermethrin-treated males. The
number of early foetal deaths was marginally increased at the highest
dose level in the 12-16 day interval after dosing and in the first 4
day period after treatment with 7.5 mg/kg males.
In the groups of animals examined histologically, no abnormalities
were detected in the testes and epididymis and there were no
observable histological differences between any of the test groups and
the controls (Dean et al., 1977).
Rat
Groups of rats (30 male and 30 female rats/group) were fed
cypermethrin in the diet at concentrations of 0, 10, 100 and 500 ppm
for five weeks after which they were mated to initiate a standard
three generation, 2-litter per generation, reproduction study. After
the selection of the second litter as parents for the following
generation, the original parents were sacrificed and subjected to
gross and microscopic examination. At the conclusion of the study,
ten animals of each sex at the age of 21 days were also examined
histologically. Calculations were made of the indices of fertility,
gestation, viability and lactation. There was no mortality over the
course of the study, and behaviour was not abnormal. Reduced body
weight and food intake was seen at various intervals in both males and
females fed 500 ppm in the diet. Reduced total weight was observed at
500 ppm in the F1A litters and reduced total weight and litter size
were noted at 100 and 500 ppm in the F1B. No compound related gross
or microscopic pathological findings were noted in any rats (Hend et
al., 1978).
SPECIAL STUDIES ON TERATOGENICITY
Rat
Groups of 25 pregnant rats were administered orally from day 6 to day
15 of gestation at dose levels of 0, 17.5, 35.0 and 70.0 mg/kg/day in
a standard teratology bioassay. On day 21 of gestation, the animals
were sacrificed and gross examination of foetuses were made, including
skeletal and somatic examinations. Pre-implantation losses were
evaluated based on corpora lutes, counts and implantation sites and
post-implantation losses were evaluated based upon implantation sites
and viable foetuses. Foetal somatic and skeletal examinations on day
21 of gestation showed no teratogenic changes that could be
attributable to the treatment. There were no indications of
embryotoxic or teratogenic events in the study (Tesh et al., 1978).
Rabbit
Groups of pregnant rabbits (20 rabbits/group, 30 rabbits were used as
an additional control group) were administered cypermethrin dissolved
in corn oil at dose levels of 0, 3, 10 and 30 mg/kg body weight orally
from day 6 to day 18 of gestation. On day 28 of gestation the rabbits
were sacrificed and examination made of live foetuses, dead foetuses,
resorption sites and corpora lutea. Live foetuses were maintained for
24 hours to assess viability. Foetuses were also examined for gross
somatic and skeletal deformities.
There was no significant mortality or differences in weight gain
during the period of gestation. There were no significant differences
between control and test groups with respect to pregnancy, foetal
death and survival. Although a wide range of skeletal and visceral
abnormalities were found in the course of the study, there were no
differences between control and test groups with respect to
abnormalities. It was concluded that oral dosing at up to 30 mg/kg
during the major period of organogenesis resulted in no teratogenic
effects in the offspring (Dix, 1978).
SPECIAL STUDIES FOR MUTAGENICITY
Mice Host-Mediated Assay
Groups of male mice (2-3 mice per group) were administered
cypermethrin, orally, at dose levels of 0, 25 and 50 mg/kg in
dimethylsulfoxide. The animals were immediately injected
interperitoneally with a suspension of S. cerevisiae in a standard
host mediated assay. After five hours, the mutagenic conversion rates
in the yeast cells recovered from the treated animals were comparable
with those of control animals suggesting that, under the terms of this
assay, there was no evidence to suggest a mutagenic potential (Brooks,
1976).
Microorganisms
The mutagenic potential of cypermethrin on various microorganism
species including: S. cerevisiae, E. coli WP2 uvr A, and S.
typhimurium TA-1538 (with and without the use of a rat activation
system) was examined. No increase in the mitotic gene conversion was
recorded in S. cerevisiae, either in the presence or absence of
microsomal oxidation. Cypermethrin, at concentrations up to 500
micrograms per plate, did not induce an increased mutation rate with
E. coli or S. typhimurium TA-1538, in vitro either in the
presence or in the absence of the microsomal oxidation system (Brooks,
1976).
Cypermethrin did not increase the number of revertant colonies of S.
typhymurium (TA-1535, TA-1537, TA-1538, TA-98 and TA-100) in the
presence or absence of a mouse liver subcellular activation
preparation obtained from 6 strains of PCB-treated mice. Cypermethrin
was tested at dose levels up to 1 mg/plate (Suzuki, 1977).
Hamster - Chromosomes
Groups of Chinese Hamsters (12 males and 12 females/group) were
administered orally on each of two successive days at a dose rate of
0, 20 or 40 mg/kg cypermethrin dissolved in dimethylsulfoxide.
Positive and negative controls consisted of cyclophosphamide (100
mg/kg) and dimethylsulfoxide alone. In chromosome preparations of the
control and cypermethrin-treated hamsters, there were no indications
of abnormalities. The cylophosphamide treatment induced a significant
degree of chromosomal damage (Dean, 1977).
SPECIAL STUDIES ON NEUROTOXICITY
Rats
Cypermethrin, orally administered to rats at high acute dosage levels,
produced severe clinical signs of poisoning which was accompanied by
histological evidence of sciatic nerve damage. Within one day
following the acute poisoning, neuropathy was evident as axonal breaks
in the sciatic nerve (Carter and Butterworth, 1976).
Groups of rats (10 males per group) were utilised in a paired feeding
study to examine the neurotoxicological effects of high dietary
levels. Rats were fed dietary levels of cypermethrin (45:55,
cis:trans-isomer ratio) for 14 days at dosage levels of 0, 1250, 2500,
and 5000 ppm. Growth was monitored and clinical evaluations for
adverse behaviour were recorded during the course of the study. Gross
pathology on tissues and organs and microscopic examination of the
sciatic nerve were performed at the conclusion of the study.
At 5000 ppm, mortality was observed with all rats either dying or
sacrificed in a moribund condition within the first week. At 2500
ppm, 6 of 10 rats died before the conclusion of the study. There was
no mortality in the low dose group. Clinical signs of neurotoxicity
were characterised by an impaired ability to walk and splayed hind
limbs. In extreme cases, clinical signs of ataxia and paralysis were
reported. Other clinical signs included: hypersensitivity to external
stimuli, gross disorientation and convulsions, the latter generally
seen at high dose levels. The neurotoxic signs of poisoning observed
in the 1250 ppm group after day three were spontaneously reversed by
day nine when all surviving animals that were initially affected
appeared to be normal. Remission of ataxia at the 2500 ppm group was
also noted within 11 days of treatment. Growth reduction was observed
in all animals in the study. At the lowest dose level, the rate of
growth was delayed for the first few days after which time the rate
was consistent with that of controls for the remainder of the study.
The absolute body weight of the treated animals was, however,
significantly lower than the controls at the conclusion of the
two-week study. Reduction in body weight was consistent with
reduction in food consumption. Ultrastructural changes in the sciatic
nerve were observed in the two highest dose levels, although the
number of animals examined was small. There was some evidence of
axonal damage in the myelinated nerves primarily at the two highest
dose groups. Changes in unmyelinated axons were not observed. In
general, the histological and ultrastructural changes in the sciatic
nerve, accompanied by clinical signs of poisoning, are not readily
apparent at low doses. Thus, cypermethrin, at acutely toxic dietary
levels,induces damage to sciatic nerves, the clinical signs of which
may be reversible (Glaister et al., 1977a).
Hamsters
Groups of male and female Syrian hamsters were orally administered
doses exceeding the LD50 in an attempt to define clinical signs of
poisoning and to evaluate the histological damage to the sciatic
nerve. At doses of 794 mg/kg and above, all animals showed clinical
signs of poisoning including tremors, abnormal, irregular movements,
and an unusual gait. As noted with rats, axon and myelin degeneration
was noted in all groups treated. The lesions included swelling and
breaks in the axons and clumping of myelin (Butterworth and Clark,
1977).
A series of experiments were performed to further evaluate the
neurotoxic potential following subacute, oral administration to
Chinese hamsters. Clinical examination, functional testing and enzyme
determinations using ß-galactosidase and ß-glucuronidase were
performed. The functional test consists of measuring the mean slip
angle where the animal is maintained on an inclined plane that
steadily increases its angle until the animal can no longer maintain a
stationary position. (The average angle of five replicate trials
constituted the mean slip angle test.)
Groups of 20 male and 20 female Chinese hamsters were orally
administered at a dose of 40 mg/kg followed by a dose of 20 mg/kg for
the following four days. Fifteen animals in each sex served as
controls. There was extensive weight loss in all dosed groups and
some mortality was observed primarily as a result of the initial
administration of 40 mg/kg. There was a loss of fur and a dermal
ulceration observed in the early parts of the study. This dermal
occurrence was transient, disappearing rapidly after the treatments
were concluded. There was significant weight loss in the initial
phase of the study. However, after the last dose, the surviving
animals rapidly gained weight at a rate consistent with the control
animals. There were no effects noted on the mean slip angle
experiment and a marginal increase in ß-galactosidase was observed in
peripheral nerve.
A further experiment utilising five male and five female Chinese
hamsters administered cypermethrin daily for five days at dose levels
of 0, 5, 10 and 20 mg/kg showed no mortality over the course of the
study. There was a slower growth of the animals treated with 20 mg/kg
which again was reversed at the conclusion of the treatment period.
Hyperexcitability was noted in one female at the high dose level.
There were no notable differences in behaviour in any of the animals.
There was a significant deficit in the mean slip angle test with
females showing an earlier dose-related deficit than noted in males.
The females recovered from this deficit earlier than males who showed
an erratic pattern of recovery. ß-galactosidase activity was
increased at all dose levels three weeks after the onset of the
experiment. This increase was statistically significant at the two
highest dose levels. Dermal irritation and fur loss was not noted in
this experiment.
Sixteen male and sixteen female Chinese hamsters were administered
five daily doses of 30 mg per kg of body weight or a control of
dimethyl sulfoxide. Additionally eight animals of each sex were
administered five daily doses of methyl mercury (7.5 mg/kg body
weight) as a positive control. With cypermethrin, there was no
mortality and the rate of weight gain was consistent with that of the
controls. There was a transient dermal irritation in the majority of
the animals accompanied by skin ulceration. This condition
disappeared at the conclusion of the treatment interval. One male
administered cypermethrin had an unusual gait. There was a slight
deficit in the inclined plane test which was noted in the early parts
of the experiment but was absent by the end of the third week.
Increases in both ß-glucuronidase and ß-galactosidase were evident in
peripheral nerve tissue. It was concluded that cypermethrin when
administered at high subacute doses, produced changes in the sciatic
nerve consistent with Wallerian degeneration. Biochemical changes
were evident as increases in ß-glucuronidase and ß-galactosidase
activity and clinically functional deficits were noted (Dewar and
Moffett, 1978b).
Hen
Groups of adult hens were fed cypermethrin at a dosage rate of 1 mg/kg
for five successive days. After 3 weeks, the dosing regimen was
repeated. Positive (TOCP) and negative control groups were included
in the study. There were no signs of delayed neurotoxicity (as
generally noted with certain organophosphate esters) seen following
cypermethrin treatment. TOCP induced the standard clinical and
histological signs of axon and myelin disruption (Owen and
Butterworth, 1977).
Acute Toxicity
Table 1 contains a summary of the findings of various studies on acute
toxicities to a number of animals.
Table 2 illustrates that the cis isomer is more toxic than the trans.
Table 1. Acute Toxicity of Cypermethrin to Various Animals
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Mouse M,F Oral Corn oil 82 Coombes, Carter et al., 1976
Mouse M,F Oral Dimethylsulphoxide 138 Coombes, Carter et al., 1976
Mouse M,F Oral Aq.suspension 779b Jaggers, 1979
Mouse M,F I.P. corn oil 485 Combs, Carter et al., 1976
Rat M,F Oral Corn oil 251-992 Combs, Carter et al., 1976
Rat M,F Oral Dimethylsulphoxide 303 Combs, Carter et al., 1976
Rat M Oral Glycerol formal 200-400 Combs, Carter, et al.,1976
Rat F oral Glycerol formal app. 200 Combs, Carter et al., 1976
Rat M Oral Aq.suspension 400-800 Combs, Carter et al., 1976
Rat F Oral Aq.suspension app. 400 Combs, Carter et al., 1976
Rat F Oral Aq.suspension 4123b Jaggers, 1979
Rat M Oral Aq.suspension 3000c Jaggers, 1979
Rat (3wks) M,F Oral Dimethylsulphoxide 163 Rose and Dewar, 1978
(6wks) M,F Oral Dimethylsulphoxide 322 Rose and Dewar, 1978
(12 wks) M,F Oral Dimethylsulphoxide 526 Rose and Dewar, 1978
Rat F I.P. Aq.suspension >500b Jaggers, 1979
Rat M,F I.P. Propylene glycol 1000-2000b Jaggers, 1979
Rat F Dermal Undiluted >4800b Jaggers, 1979
Rat M,F Dermal Xylene >1600 Combs, Carter et al., 1976
Syrian
hamster M,F Oral Corn oil >400 Combs, Carter et al., 1976
Chinese
Hamster M,F Oral Corn oil 203 Combs, Carter et al., 1976
Guinea pig M Oral Corn oil app. 500 Combs, Carter et al., 1976
Guinea pig F Oral Corn oil >1000 Combs, Carter et al., 1976
Guinea pig M Oral Aq.suspension >4000b Jaggers, 1979
Table 1. Continued...
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Rabbit F Oral Undiluted >2400b Jaggers, 1979
Rabbit F Dermal Undiluted >2400b Jaggers, 1979
Dom. fowl M,F Oral Dimethylsulphoxide >2000 Combs, Carter et al., 1976
Partridge M,F Oral Dimethylsulphoxide >3000 Combs, Carter et al., 1976
a) Unless specified, all values refer to data generated using 50:50 cis:trans material.
b) Data generated using 40:60 cis:trans material.
c) Data generated using 53:46 cis:trans material.
Table 2. Effect of Cis:Trans Ratio on Acute Toxicity of Cypermethrin
LD50
Species Sex Vehicle Cis:trans ratio (mg/kg) Reference
Rat M,F Dimethylsulphoxide cis only 160-300 Brown, 1979a
M,F Dimethylsulphoxide trans only >2000 Brown, 1979b
F Corn oil cis:trans 90:10 367 Jaggers, 1979
F Corn oil cis:trans 40:60 891 Jaggers, 1979
Cypermethrin was administered dermally to rats by a single application
(5000 mg/kg) or 5 consecutive daily doses (2500 or 5000 mg/kg/day).
Low mortality was observed in the multiple dose groups (20-30%).
Toxic signs of poisoning were similar to those described below.
Axonal lesions of the sciatic nerve were noted in microscopic
examination (Okuno et al., 1976).
The toxic signs noted following acute poisoning with cypermethrin were
similar in most mammalian species. In rats this consisted of
sedation, ataxia, a splayed or ataxic gait and occasional tremors or
convulsions. The onset of toxic signs of poisoning were rapid and
disappeared within several days in survivors. In dogs, signs of acute
intoxication included nervousness, inappetance, diarrhea, vomiting,
tremors and exaggerated ataxia while walking were noted at extremely
high levels. A single application of undiluted cypermethrin to rabbit
eyes produced a mild transient conjunctivitis lasting two days.
Groups of ten male and ten female guinea pigs were used to assess the
skin sensitizing potential of cypermethrin. Two guinea pigs out of
the 20 exposed to cypermethrin showed a positive skin reaction
indicating that cypermethrin may have a weak skin-sensitizing
potential. Groups of four male and four female rabbits were
administered cypermethrin dermally in a 24-hour skin test. A single
application of cypermethrin was observed to be a moderate irritant to
rabbit skin (Coombs et al., 1976).
SHORT TERM STUDIES
Rat
Groups of rats (20 male and 20 female rats per group) were fed
cypermethrin in the diet at concentrations of 0, 75, 150 and 1500 ppm
for 90 days. Cypermethrin used in the study was a 44:56
(cis:trans-ratio) with a purity of 92%. Random samples were
chemically analysed periodically during the course of the study. At
the conclusion of the study, four animals of each sex were maintained
on control diets for a one-week recovery period.
There was no mortality over the course of the study although both
males and females at 1500 ppm had reduced body weight and food
consumption in the first month of the study. After the first month,
the growth rate was similar to that of controls although the animals
never achieved the weight noted in the other groups. At the
conclusion of the study, hematology, clinical chemistry, urinalysis
and histopathology were performed on all animals. With the exception
of a slight increase in M/E ratio in the bone marrow of female rats
fed 1500 ppm in the diet, there were no hematological or urinalysis
effects. An increased microsomal (smooth endoplasmic reticulum)
oxidative activity was noted as an increased hepatic aminopyrine
demethylase activity in both males and females at 1500 ppm and in
males at 150 ppm. These adaptive changes in the subcellular portions
of the liver were substantially reversed within the four week recovery
period. Increased liver weight was not noted on gross examination at
the conclusion of the study. A slight reduction in pituitary weight
of males, while statistically significant, was not dose-related. A
slight decrease in female kidney weight was dose-dependent and
significantly different from control values at the highest level. The
decrease in kidney weight in males was slight and not significantly
different from controls in any of the dietary levels. Gross and
microscopic (including electron microscopic) examinations of tissues
and organs showed no significant differences from the observations
noted in controls. Examination of the sciatic nerve from animals in
the control and 1500 ppm dietary group showed no changes which could
be directly attributable to the presence of cypermethrin in the diet.
Seven of the sixteen males fed 1500 ppm and two of twelve male
controls showed slight changes in myelin which may or may not have
been brought about by histological fixation of the material prior to
examination. The condition of the sciatic nerve of females was
similar to that noted in control females. There were no effects noted
in unmyelinated axons in both males and females. Interim histological
examination at 30 days, during which time clinical signs of poisoning
were noted, was not performed in this study (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats/group, 14 of each sex were
used as controls) were fed trans-isomer for five weeks at dietary
concentrations of 0, 30, 100, 300, 1000 and 3000 ppm. There was no
mortality observed over the course of the study. Food intake and
growth were reduced in the high dosage group. Alkaline phosphatase
activity was increased at the two highest dose levels. Minimal
changes were observed at the two highest dose levels including several
hematological parameters (red blood cell count and hemoglobin
concentration). Liver, spleen and kidney weights were increased at
1000 ppm and above with the spleen and at the 3000 ppm level with the
kidney and liver. Gross and microscopic histopathologic examination
of several tissues and organs revealed no cypermethrin-related
injuries. Special reference was given to the examination of the
sciatic nerve which showed no damage (Hend and Butterworth, 1977a).
Groups of rats (6 male and 6 female rats/group, 10 of each sex were
used as controls) were fed dietary concentrations of cis-isomer at
concentrations of 0, 30, 100, 300, 750 and 1500 ppm for five weeks
following a protocol similar to that described above. Mortality was
observed at the 1500 ppm dose occurring at intervals from 4 to 17 days
from the start of the experiment with all animals displaying
neurotoxic signs of poisoning. At the next lower dose group (750 ppm)
almost all of the animals showed gross neurotoxic signs of poisoning,
abnormal sensitivity to sound and touch and slight cases of ataxia.
There was no compound-related mortality at dose levels of 750 ppm or
below. Growth was reduced at the 750 ppm over the course of the
study. Significant reductions in food intake were also noted at 300
ppm and above at the initial phase of the study. Gross examination of
tissues and organs again revealed a reduction in heart, liver, spleen
and kidney weights. Adjustment of the data for terminal body weight
revealed a statistically significant change in kidney weight at 300
ppm and above while liver weight was increased at 750 ppm. Plasma
protein levels were decreased and urea and potassium concentrations
were increased in male rats at 750 ppm. Gross and microscopic
examinations of tissues and organs showed substantial degeneration at
1500 ppm and above in both the liver and sciatic nerve. Liver lesions
consisted of a coagulative necrosis of hepatocytes occurring in both
males and females. The sciatic nerve from both sexes showed swelling
and breaks in axons with concomitant myelin degeneration and
vacuolation. There were no lesions observed in the brain or the
spinal cord (Hend and Butterworth, 1977b).
Groups of rats (12 male and 12 female rats/group, 24 of each sex were
controls) were fed cypermethrin in the diet at dosage levels of 0, 25,
100, 400, and 1600 ppm for 13 weeks. After two weeks exposure to 1600
ppm, both males and females exhibited hypersensitivity and varying
signs of ataxia. Mortality was observed with male rats up to the
fifth week of feeding after which the surviving animals improved
clinically and appeared normal at the end of the study. Reductions in
body weight, growth and food intake were also noted at the 1600 ppm
dose level. At the conclusion of the study, small increases in plasma
urea were noted in both sexes. Males had a slight increase in plasma
potassium while females had a slight increase in alkaline phosphatase
and plasma protein levels at the 1600 ppm dosage group. Hematological
abnormalities noted at 1600 ppm at the end of 13 weeks included a
reduction of hemoglobin, packed cell volume and red blood cell count
in females and a reduction in kaolin-cephalin clotting time in males.
There were no effects at dose levels below 1600 ppm.
Gross and microscopic pathology performed at the conclusion of the
study showed axonal breaks and vacuolation in the sciatic nerve of
animals fed 1600 ppm in the diet. This was especially noted with
those animals that showed clinical signs of ataxia and died during the
course of the study. At the conclusion of the 13-week trial, sciatic
nerve lesions were not noted in any surviving animals. An increased
kidney weight in males fed 400 ppm was observed which was not
associated with any clinical or pathological signs of abnormality
(Hend and Butterworth, 1976).
Dogs
Groups of beagle dogs (4 male and 4 female dogs/group) were fed
cypermethrin (1:1 cis:trans-isomeric mixture) in the diet at
concentrations of 0, 5, 50, 500 and 1500 ppm for 13 weeks. There was
no mortality over the course of the study. However, there were
significant signs of poisoning observed at the 1500 ppm level which
included diarrhea, anorexia and tremors as well as ataxia,
incoordination and hyperesthesia. On account of these clinical signs,
two male and two female dogs at the high dose level had to be
sacrificed before the end of the experiment. Minor variations were
observed at various hematological parameters with the kaolin-cephalin
clotting time observed to be consistently lower throughout the study
in female dogs at 500 ppm. There were no other significant
differences with respect to standard hematological or clinical
chemistry parameters. Gross examination of kidneys and organs showed
no effect on organ weights attributable to the diet. Microscopic
examination of tissues and organs revealed a nonspecific focal
bronchopneumonia in the lungs of the animals surviving 1500 ppm.
There were no compound-related changes and no histological
abnormalities in other tissues examined with the exception of a
variation in the intensity of the pink colour of the optic disc noted
on ophthalmologic examination at the conclusion of the study.
Because of the pink colouration observed in the optic disc, a further
experiment was undertaken to determine the cause of this occurrence.
Cypermethrin was fed to two groups of three male dogs at
concentrations of 0 and 500 ppm for 13 weeks. Specific ophthalmologic
examination was made to evaluate the degree of colouration of the
optic disc. At the end of 13 weeks, there were no consistent
differences between the colour of the optic discs of the treated dogs
and controls. A no-effect level of 50 ppm was observed in this
short-term study (Buckwell and Butterworth, 1977).
LONG-TERM STUDIES
Rat
Groups of rats (48 male and 48 female rats/group, 96 of each sex were
used as controls) were maintained under SPF conditions and fed 1:1
cis:trans in the diet for 2 years at dosage levels of 0, 1, 10, 100
and 1000 ppm. Chemical analyses were made confirming stability of
cypermethrin in the diet over the test interval.
There was no mortality over the course of the study attributable to
cypermethrin in the diet. General health and behaviour of the
cypermethrin-fed animals was not different from that of the control
animals. Body weight and food consumption data showed a reduction in
dietary intake and growth in both males and females in those groups
fed 1000 ppm in the diet.
Gross morphological changes were noted periodically over the course of
the study. At six months, males fed 1000 ppm had a slightly reduced
testes weight which was not seen thereafter. Slight changes in the
gross kidney weight at periodic intervals in the study were not
accompanied by notable changes in clinical measurements or on
microscopic examinations. These changes in kidney weight were
believed to be unrelated to the presence in the diet. Liver weight
increases were noted at 18 months again in those animals maintained at
the high dose level. The only clinical chemistry changes noted over
the course of the study was a slight decrease in the activity level of
alkaline phosphatase in males at the 2 year interval. The decrease in
alkaline phosphatase activity was not dose-related and was not noted
in females. Minor fluctuations were seen in various hematological
parameters over the course of the study but these, too, were not
compound related nor statistically significant. These changes are not
believed to be related to the presence in the diet. Gross and
microscopic examination of tissues and organs periodically over the
course of the study did not suggest adverse somatic effects.
The kidneys of most animals after 12 months showed chronic nephrosis,
histologically characterized as tubular dilatations, interstitial
chronic inflammatory infiltration and glomerular scarring. In the
liver, varying degrees of chronic inflammatory infiltration,
hepatocyte vacuolation and bile duct proliferation were observed.
These changes were characteristic of ageing processes and were not
attributable to cypermethrin. Dermal ulceration was present in a
number of animals and over the course of the study.
A portion of the sciatic nerve from 12 animals fed 1000 ppm for one
year and 12 control animals was histologically examined for signs of
neurotoxicity. There were no differences in the incidence of abnormal
fibres in the control and animals fed for cypermethrin for 12 months
(Trigg et al., 1977). Microscopic examination of the sciatic nerve
from animals sacrificed at the conclusion of the study showed a small
number of nerve fibers exhibiting slight Wallerian degeneration
changes. These degenerative changes increased with age but did not
appear to be dose-related with respect to severity. Thus,
cypermethrin did not induce neuropathy clinically nor did it induce
histological evidence of nerve degeneration.
There did not appear to be a significant increase in the incidence of
tumor formation in either males or females over the course of the
study. A large number of pituitary adenomas were reported
predominantly in females of all dose groups including controls. This
occurrence did not appear to be dose-related as similar events were
recorded in all dose levels. Based on the reduced body weight
observed at 1000 ppm in the diet of both males and females, the
no-effect level is 100 ppm (McAusland et al., 1978).
Observations in Humans
Urine obtained from operators spraying cypermethrin in experimental
trials was analysed for the presence of the chlorinated cyclopropane
carboxylic acid metabolite. Using standard gas liquid chromatography,
the carboxylic acid metabolite was observed in urine of exposed
workers at levels up to 0.4 µg/ml (the limit of detection was
estimated to be 0.05 µg/ml) (Baldwin, 1978). Studies were carried out
in the Ivory Coast, Africa where operators sprayed with a hand-held
ULV apparatus either cypermethrin or a control UW formulation. The
cypermethrin sprayers were found to have residues on the exposed parts
of their bodies. Medical and electrophysiological examinations showed
no adverse effects from the exposure. The rate of dermal exposure of
the operators during spraying ranged from 1.5 to 46.1 mg/hour. There
was a reasonable relationship between the total cypermethrin deposited
dermally and the excretion in urine. The levels of the cyclopropane
carboxylic and acid metabolite in the 24 hour urine were between
<0.05 and 0.32 mg. This, together with the finding of 0.6 mg of this
metabolite in 72 hour urine from one man led to the estimation that
approximately 3% of the total dermal dose was absorbed and rapidly
excreted by the operators (Prinsen and van Sittert, 1978).
COMMENTS
Cypermethrin is an insecticidally active synthetic pyrethroid
currently being developed for use in agriculture. The acute toxicity
has been studied in a variety of species and the toxic response in all
species is very similar. Cypermethrin has a moderately acute oral
toxicity. The acute signs of poisoning, in addition to a generalized
nervous system response, include a neurological involvement in rodents
where reversible clinical signs of ataxia was accompanied by
histological and biochemical evidence of peripheral (and possibly
central) nerve disruption. Myelin and axon degeneration in the rat
sciatic nerve was noted following high dose level administration.
There was no information on the relative sensitivity of humans to the
nervous system disruption noted in rodent species (see Report Section
3.5).
Cypermethrin is readily absorbed, distributed, and metabolised in
mammals. Because of the chemical and especially the isomeric
complexity of the molecule, the metabolic profile with respect to all
of its isomers is extremely complex. Cypermethrin is readily cleaved
at the ester linkage and subjected to oxidative degradation and
conjugation of the metabolic products. Elimination from the body
following acute and subacute administration is rapid. However, the
clearance rate from adipose tissue is slow and a half-life in rats and
mice may range from 10 to 30 days. The data suggested a potential for
bioaccumulation in the body following continuous exposure.
In a wide variety of studies, there was no carcinogenic or mutagenic
potential as evidenced by short-term bioassays or a long-term chronic
study. Reproduction was unaffected and no signs of teratogenic
potential were noted in two species examined. Two dominant lethal
tests were performed, and while data were basically negative, these
studies did not give full assurance of the lack of a dominant lethal
effect on male reproduction. In short-term tests, only younger
animals were susceptible to the inductive effects of cypermethrin on
liver metabolizing enzymes.
The extensive toxicological data base already in existence lent
assurance to the estimation of an ADI for man. The lack of data on
tissue residue storage and release and on the limited human experience
with cypermethrin were instrumental in making this temporary, pending
further information.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 100 ppm in the diet equivalent to 5.0 mg/kg body weight
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.006 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cypermethrin is a recently introduced insecticide recommended as a
foliar spray for the control of a wide range of insect pests. In line
with other members of the pyrethroid group of compounds, it is used at
lower rates than most other types of chemical insecticides.
Application rates seldom exceed 250 g/ha. In most instances a
considerably lower rate is required. Several applications, up to two
per month and up to 7 days before harvesting, may be made during a
growing season.
Current recommendations for use on various food crops are summarised
in Table 3.
Table 3. Current dosage rates for use on various crops
Recommendations
Crop (in active ingredients)
Alfalfa 25 g/ha
Apples 0.003%-0.0125%
Brassica leafy vegetables 30-200 g/ha
Cereals (barley, wheat) 25-200 g/ha
Cherries 0.0075%-0.0125%
Citrus 0.0025%-0.01%
Cotton 50 - 100 g/ha
Grapes 30-50 g/ha
Kiwifruit 0.01%
Legume vegetables 25-200 g/ha
Lettuce 50-150 g/ha
Maize 25-200 g/ha
Pasture 100 g/ha
Peaches 0.002%-0.01%
Pears 0.002%-0.0125%
Plums 0.05%-0.0125%
Potatoes 20-150 g/ha
Rape 25-75 g/ha
Raspberries 0.0075%-0.015%
Soya 50-250 g/ha
Strawberries 0.0075%-0.015%
Sugar beet 25-125 g/ha
Tomatoes 40-150 g/ha
Post-harvest uses
Although cypermethrin may be introduced to control pests of stored
commodities and for animal health purposes, no data were available for
evaluation at this meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials carried out on a
world-wide basis and on the main crops treated. Some data from
excessive treatments rates are included where they provide useful
information, e.g. on the effect of processing. Treatments were
generally made using E.C. formulations which are representative of
current application practice.
MAIN CROPS
Apples
Residue data have been obtained from trials in several countries. In
these experiments, dosages covered recommended and higher rates and
were in the range of 0.0025% - 0.02% (25-200 g/ha), based on a spray
volume of 1000 l/ha. Treatments were made on up to 12 occasions.
Results and details of the trials are summarised in Table 4.
Residues in whole fruit one week after treatment at recommended rates
up to 0.02% were usually below 1 mg/kg except when six or more
applications were made. Apart from one instance, however, these were
all less than 1.5 mg/kg. In the one case, where 2.7 mg/kg was
reported, the result was considered to be atypical.
Examination of fruit pulp and peel separately showed that residues
were located mainly in the peel, the average weight of which was
10-18% of the whole fruit. After peeling, levels in 78 samples were
generally less than 10% of those in whole fruit.
Pears
Samples of fruit were analyzed from trials carried out in Europe,
Canada, Australia and South Africa. In these experiments, treatment
rates were between 0.0008% and 0.04% (or 30-400 g/ha). Between one
and six treatments were made and fruit harvested at various intervals
after treatment. The results obtained were similar to those with
apples. Residues in whole fruit, one week after treatment at rates up
to 0.02% (slightly above the recommended rate of 0.015%), were all
below 1 mg/kg. As with apples, analysis of peeled fruit showed that
cypermethrin residues were mainly in the peel. In 32 samples,
residues in the pulp did not exceed 30% of those in whole fruit.
Table 4. Residues Following Supervised Trials with Apples (1976-1978)
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Granny Smith Australia 3 0.02% (to
run off) 382 0.95 0.45 0.45 1
MacIntosh Canada 3 0.18 2 0.57 0.38 2
Lobo 3 0.18 2 0.47 0.32
Northern spy 8 0.07 9-15
days 0.13-0.3 3
MacIntosh 8 0.07 9-15
days 0.1-0.251
Northern Spy 8 0.05 " 0.2-0.26
MacIntosh 8 0.15 " 0.2-2.25
Richared France 1 0.15 0.25 0.19 0.6 0.25 4
Golden
Delicious 7 0.05 2-3 0.05 5
Golden
Delicious FRG 6 0.2 2,3,2,3 3.1 1.0 0.6 0.3 0.5 6
and 1 3.2 2.7 1.9 1.7 0.81
James Grieve 6 0.02% 1,1,1´
4 & 1´ 1.3 0.96 0.82 0.8 0.67 7
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Cox's Orange 6 0.02% 1,3,4
4 & 2 0.97 0.75 0.6 0.84 0.31
Golden
Delicious 6 0.02% 1,6,6,
2 & 2 1.7 1.3 0.93 0.9 0.9
6 0.1 1-3 1.2 1.1 1.0 0.9 0.8 8
6 0.1 1-3 0.8 0.7 0.7 0.95 0.5
Italy 1 0.037 - 0.3-0.5 11
Strumer N. Zealand 12 0.008% 2-3 1.1 0.6 0.7 0.53 12
Reinette Portugal 5 0.005% 3 0.19 0.2 0.14 13
5 0.0075% 3 0.32 0.1
5 0.01 3 0.6 0.35 0.45
Granny Smith S. Africa 1 0.005% - 0.42 0.36 0.28 0.25 14
Spain 1 0.024 - 0.06 0.05 0.04 10
1 0.048 - 0.1 0.1 0.05 0.06 10
U.K. 3 0.005% 1-2 0.29 0.45 0.27
3 0.01% 1-2 0.99 0.91 0.52
3 0.005% 1´ 0.6 0.81 0.64
3 0.01 1´ 1.1 0.98 0.82
1 0.01 - 1.3 1.2 0.83
Worcester
Pearmain U.K. 2 0.015% 0.01 15
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Chivers
Delight 2 0.0025% 4´ 0.02 16
0.005% 4´ 0.05
2 0.01% 6´ 0.01
Laxton Superb 2 0.01% 6´ 0.01
Spartan 1 0.012% - 0.36 0.15 0.07 0.05 17
0.01 - 0.36 0.12 0.03 <0.01
Lobo Sweden 3 2´-4 0.1422 Sweden
Ribston 3 2´-4 0.25
1 Minimum-maximum residues in three samples.
2 90 days after treatment.
Peaches
Residues in whole fruit, less stone, from trees sprayed with
cypermethrin at recommended rate (0.005%-0.01%) and harvested one week
after treatment were generally 1 mg/kg or less although in one trial
in Germany a residue of 1.8 mg/kg was reported in fruit under these
conditions. Analysing 43 samples of fruit after peeling and removal
of the stone showed that levels in the pulp were less than 10% of
levels found in whole fruit.
Details of the trials are given in Table 5, which contains data
relating to some treatments at rates higher than recommended.
Citrus
Experiments were carried out in South Africa (oranges), Spain (lemons)
and Italy (oranges) in which applications of cypermethrin at
recommended rates up to 200 g/ha were made on 1 or 2 occasions. Fruit
was harvested 1 to 39 weeks after treatment, depending on the trial,
and analysed for residues of cypermethrin. The results are shown in
Table 6.
Residues up to 1.3 mg/kg were found in whole fruit taken within six
weeks of treatment. Analysis of peel and pulp showed that the
residues were largely (90%) percent in the peel and that residues in
the pulp were normally below the limit of determination, although in
one series of experiments residue levels up to 0.16 mg/kg were
reported (4 weeks after treatment at 0.02%). In some experiments
samples of orange juice were obtained from treated fruit but residues
were all less than 0.01 mg/kg.
Cotton
Samples of seed were obtained at harvest from trials in several
countries in which up to 12 applications were made at recommended
rates (up to 150 g/ha) and also at treatment rates up to 240 g/ha. The
intervals between the last treatment and harvest varied between 1 day
and 13 weeks. The data obtained are given in Table 7 which also
summarises the trial details.
The seed from these trials was ginned after harvest and was separated
in the laboratory into kernels and hulls. The latter also contained
adhering linters.
In 8 samples no residue of cypermethrin was found in the kernels
(limit of determination of 0.01 mg/kg). Analysis of hulls, which in
most instances had adhering linters, showed that any residues present
were located entirely on the seed surface and its associated fibre.
Table 5. Residues Following Supervised Trials on Peaches (1976-1978)
Application
rate Intervals Residues in mg/kg, at intervals (days) after application1 (Shell)
Country No. kg a.i./ha /week/ 0-1 6-7 12-14 18-21 28-30 40-43 54 Ref.
or %
Australia 3 0.02% 3 & 2 1.0 33
Canada 3 0.006% 4 & 5 0.07 34
3 0.006% 0.34 0.15 0.06 35
0.1 0.052
France 1 0.005% - <0.02 36
0.0075% <0.02
0.0125% - <0.01
0.0187% <0.01
0.015% 0.5 0.4 0.1 0.15
Federal Republic
of Germany 4 0.02% 2 3.2 1.6 1.1 0.65 38
4 0.01% 3,3 & 2 0.85 0.7 0.5 0.18 39
4 0.01% 3,3 & 2 3.0 1.8 1.6 0.75
5 0.01% 2,3,3 & 2 2.0 1.0 0.85 0.3
Italy 1 0.01% - 0.1 40
1 0.02% 0.5
Portugal 1 0.005% 0.08 0.08 0.08 41
0.0075% 0.19 0.11 0.09
0.01% 0.32 0.21 0.14
Spain 1 0.15 0.15 42
2 0.195 3 0.03
2 0.195 1 0.04
1 Results refer to whole fruit without stone.
2 Average of ten samples taken from the same site (min.-.max. values: 0.02-0.11 mg/kg).
Table 6. Residues of Cypermethrin in Citrus (1976 - 1977)
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
LEMONS 150 g/ha 2 27 7 <0.01 2.1 1.1 43
Verna 14 <0.01 2.0 1.0
21 <0.01 2.1 1.1
(Spain) 58 <0.01 1.1 0.55
JUICE
ORANGES 0.01% 1 - 60 <0.01 0.22 0.14 44
Moro 0.02% 1 - 60 <0.01 0.44 0.21
(Italy)
PULP
ORANGES
Moro 0.005% 1 - 67 0.02 0.91 0.30 45
0.01% 1 - 67 0.02 1.2 0.39
0.005 1 - 91 0.01 0.69 0.19
0.01% 1 - 91 0.02 1.3 0.35
0.01% 1 - 27 0.06 1.0 0.36 46
43 0.05 1.5 0.48
56 0.05 1.3 0.40
0.02% 1 - 27 0.16 3.0 1.2
43 0.13 3.7 1.3
56 0.09 1.9 0.73
JUICE
0.005% 1 - 1 <0.01 0.50 0.13 47
4 <0.01 1.2 0.20
8 <0.01 1.0 0.16
18 <0.01 0.81 0.21
39 <0.01 1.1 0.28
68 <0.01 1.3 0.39
(S. Africa) 96 <0.01 1.3 0.38
Table 6. Continued...
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
ORANGES 0.01% 1 - 1 <0.01 1.0 0.30 47
Moro 4 <0.01 0.55 0.22
8 <0.01 0.19 0.16
18 <0.01 0.83 0.25
39 <0.01 0.60 0.16
68 <0.01 0.45 0.13
96 <0.01 0.45 0.13
0.015% 1 - 1 <0.01 2.6 0.58
4 <0.01 1.2 0.30
8 <0.01 1.0 0.24
18 <0.01 1.3 0.25
39 <0.01 1.2 0.21
68 <0.01 1.8 0.40
96 <0.01 0.95 0.22
0.01% 1 - 0 <0.01 1.6 0.52
1 <0.01 2.9 0.85
2 <0.01 2.2 0.65
4 <0.01 2.8 0.55
9 <0.01 1.8 0.58
16 <0.01 1.9 0.72
0.005% 2 6-weeks 69 <0.01 <0.01 <0.01
114 <0.01 <0.01 <0.01
237 <0.01 <0.01 <0.01
0.01% 1 - 110 <0.01 <0.01 <0.01
155 <0.01 <0.01 <0.01
(S. Africa) 278 <0.01 <0.01 <0.01
Table 7. Residues Resulting from Supervised Trials in Cotton Seed (1975-1977)
Application Residues in whole seed (mg/kg) at Intervals (days) after app. lication Ref.
Country rate kg Intervals (Shell)
No. a.i./ha or /Week) 1 7-8 13-15 19-20 28-37 44-55 70-91
Australia 1 0.0015% - 0.01 0.01 10
1 0.003% 0.05 0.01
Brazil 4 0.12 1 0.01 51
4 0.24 1 0.01
4 0.12 1 <0.01 52
0.24 1 0.01
Columbia 1 0.1 0.09 0.03 0.01 54
5 0.1 3 to 8 days <0.01 55
2 0.075 12 days <0.02 56
South Africa 12 0.2 1 to 2 0.01 58
11 0.2 1 to 2 0.02
1 0.1 - <0.01 10
0.2 <0.01
Spain 5 0.1 2,2, 1´ & 2´ 0.04 59
4 0.1 2,2 & 1´ 0.03
5 0.2 2,2, 1´ & 2´ 0.03
4 0.2 2,2,1´ 0.06
3 0.1 2 0.03
3 0.2 2 0.04
3 0.2 0.02
The presence of occasional small residues on the fibre and the outside
of the seed case is to be expected, particularly where applications
were made after the bolls had opened. However, the results indicate
that migration through the seed case to the kernel did not occur even
after treatment at excessive rates, and that measurable residues in
kernels are unlikely to be found in practice.
As a whole, the data suggest that, following treatment according to
recommendations, seed taken 1 week after a series of applications is
unlikely to contain more than 0.1 mg/kg cypermethrin on the whole
seed.
Leafy Vegetables (Brassicae)
Trials have been carried out in a number of countries in order to
obtain residue data on cabbages - including savoy, broccoli, Brussel
sprouts, cauliflower and kale. These are described in Table 8.
The treatments were made according to recommendations (up to 200 g
a.i./ha or 0.02%) or at slightly higher dose rates. In most cabbage
samples as expected, the residue was located mainly in the outer
leaves, which are normally removed in the field, and the majority of
the samples examined were received without the "discard" leaves. In
one experiment however (New Zealand) whole unstripped cabbages were
received. Residues in these samples reaches 3.2 mg/kg but were found
to be reduced to below 0.1 mg/kg on removal of the outer leaves in the
laboratory.
With most of the other brassicae examined, including cauliflower
heads, residue levels were similar to those in cabbage without discard
leaves. But residues in kale from experiments in Germany tended to be
somewhat higher.
OTHER CROPS
Supervised trials were carried out with a number of other crops in
various locations. Residue data obtained in these experiments are
summarized in Table 9 and the following comments.
Alfalfa
A single application at a rate equivalent to 0.1 kg a.i/ha was used to
establish a residue decay curve.
Beans
Trials were carried out in South America on soya and on phaseolus
bean. Residues in beans (soya and phaseolus) removed from their pods
were below measurable levels (0.01 mg/kg) four weeks after treatment,
even where excessive rates (up to 300 g/ha) were used.
Table 8. Residues Resulting from Supervised Trials with Various Vegetables (1976/79)
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha (week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
BROCCOLI 3 0.16 3 & 2 0.38 0.28 0.16 0.06 69
1 0.0015 - 0.11 0.03 <0.01 10
(Canada) 1 0.003% - 0.3 0.03 <0.01
BRUSSELS SPROUTS 3 0.15 1,5 0.55 0.25 0.25 70
7 0.07 3,3,2,2 0.14 0.1 71
2 & 3 0.12 0.09
0.23 0.13
0.18 0.18
6 0.07 3,3 & 2 0.06
0.09
0.1
(Canada) 0.08
CABBAGES 3 0.07 1,5 0.22 0.11 0.11 60
0.22 0.12 0.06
0.16 0.27 0.17
0.22 0.27 0.05
3 0.15 1,5 0.43 0.03 <0.01 61
(Canada) 3 0.16 2 0.15 0.1 0.02 <0.01 62
CABBAGES 4 0.06 2 1 0.4 0.11 <0.01 <0.01 63
(Germany) 4 0.06 2 0.1 0.02 <0.01 <0.01 <0.01
CABBAGES 1 0.25 0.161a Hungary
0.1
(Hungary) 0.2
0.2
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
CABBAGES 5 0.002% 2 0.03 <0.01 0.01
5 0.006 2 0.05 0.02 0.02 66
1 0.002% - 0.07 0.02 0.02
(South Africa) 1 0.006% - 0.08 0.03 0.02
CABBAGES 6 0.14 2 2,11 1,71 1.41 67
0.061b 0.011b 0.011b
6 0.14 0.76 0.6 0.57 0.47 N.Z.
(New Zealand) 6 0.28 1.13 0.82 0.68 0.42
CABBAGES 1 0.008% - 0.51 68
(U.K.)
CABBAGES
(Savoy) 4 0.06 1,1 & 2 0.55 0.4 0.35 0.15 <0.01 64
(Germany) 4 0.06 2 0.3 0.2 0.1 0.05 <0.01
1 0.005% 0.02 65
1 )0.015% 0.02
(Italy) 1 0.03% 0.02
CAULIFLOWERS 3 0.16 3 & 2 0.17 0.09 0.01 <0.01 72
3 0.07 1,5 0.23 0.21 <0.01 73
0.26 0.30 0.01
0.34 0.24 0.08
(Canada) 0.3 0.08 0.11
4 0.06 2 0.03 <0.01 <0.01 <0.01
(Germany) 3 0.06 2 0.05 <0.01 <0.01 <0.01 <0.01
KALE 4 0.06 2 1,6 0.85 0.4 0.3 0.25 75
(Germany) 4 0.06 2 1,4 0.9 0.7 0.5
2 0.075+0.04 6.5 1.1 0.76 0.47 0.13 76
(U.K.) 2 0.225+0.08 6.5 1.5 0.95 0.60 0.23
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
KALE
(China) 6 0.005% 4-5 days 0.85
(Thailand)
NOTE: The cabbages were analysed without the outer leaves with some exceptions:
1 whole cabbage
1a whole cabbages as specified by CC/PR/153/
1b heart only.
Cherries and Plums
Samples of plums and cherries have been obtained from field
experiments in Germany in which cypermethrin was applied on several
occasions (three in the case of cherries, five for plums) at
approximately the recommended rate and double the recommended rate
(0.01% and 0.02% respectively). Under similar conditions, residues in
plums were found to be somewhat lower than in cherries.
Grapes
At recommended rates of up to 80 g/ha and treatments on up to 4
occasions, residues in grapes harvested one week after the last
application were at or below 1 mg/kg (Table 9). In practice, the
material is generally applied an a prophylactic treatment at 50 g/ha
and harvest is likely to be more than one week after treatment.
Lettuce
Residue data are available from trials carried out in Canada, France
and Germany at rates generally in the range of 50-70 g/ha (0.005% to
0.007%, based on 1000 l/ha spray volume). Residue levels were 1.6
mg/kg cypermethrin or less in lettuce taken one week after treatment.
From the glasshouse data available, there was no evidence that levels
in glasshouse lettuce were substantially different from levels in
outdoor crops.
Kiwi Fruit
Supervised trials were carried out in New Zealand at the recommended
dose rate and at double rate (Table 9). The pulp contained 5-10% of
that which was found in whole fruit.
Maize
Residue experiments have been carried out in several countries in
which cypermethrin has been applied to maize at recommended rates up
to 150 g/ha. Green maize (whole plants) were sampled at intervals
following treatment, and in some instances grain was also sampled at
the sweet corn stage or when fully mature.
In green maize following treatments at rates up to 150 g/ha, residues
were below 1 mg/kg two weeks after treatment and below the limit of
determination (0.01 mg/kg) after eight weeks. In grain, no residues
were found in either sweet corn or mature grain, even in samples taken
three days after treatment at this rate.
Pasture
Supervised trials were carried out in New Zealand in which
cypermethrin was applied at several times higher than the recommended
rate. The residues on the grass increased roughly in proportion to
the increases in dosage (Table 8).
Peas
Trials were carried out on peas in the U.K., Hungary and South Africa.
No residues were found at dose rates of 15 to 100 g/ha in the peas
themselves which were sampled between one day and four weeks after
treatment (limit of determination 0.01 mg/kg). However, an excessive
dose rate of 200 g/ha resulted in residues between 0.04-0.07 mg/kg in
the peas (Table 9).
Potatoes
Samples were obtained from residue trials carried out in Europe,
Cyprus and Canada, with from 1-9 treatments at dosage rates in the
range of 10 g/ha to 200 g/ha, and intervals between last treatment and
harvest from 0-8 weeks. Detectable residues were absent from all
samples of whole or peeled tubers, even 3-9 days after treatment.
However, samples from one trial in Italy, obtained three weeks after
treatment were peeled. Residues up to 0.05 mg/kg were found in the
peel, but none was detectable in the peeled potatoes.
Rape
Data from supervised trials in Canada, Germany and the U.K. show that,
where treatments at recommended rates were made more than six weeks
before harvest, residues in the whole seed were below the limit of
determination of 0.01 mg/kg. In one of the replicated Canadian
experiments, however, residues were found in samples taken six weeks
after treatment; residues between replicates varied from 0.01 mg/kg to
0.12 mg/kg.
Raspberries and Strawberries
Trials were carried out in Canada and the U.K. in which raspberry
canes were treated with cypermethrin at rates between 0.005% and
0.015% (the maximum recommended rate). Residues in fruit taken 12 or
more days after treatment were all below 0.5 mg/kg even where multiple
applications were made. Residues in strawberries from Canadian
experiments using cypermethrin at rates of 0.006% or 0.01% on two
occasions at an interval of 8 or 9 days were also below 0.5 mg/kg when
harvested three weeks after treatment (Table 9).
Sugar Beet
In experiments carried out in France, Germany and the U.K., 1 to 5
treatments were carried out at rates between 60 g/ha and 200 g/ha
cypermethrin. Samples of both roots and tops were taken for analysis
at intervals up to 17 weeks from the time of treatment.
Apart from one sample taken two weeks after treatment at 60 g/ha,
which contained a small residue of 0.02 mg/kg, no residues of
cypermethrin were found in the root samples.
In leaves, residues were between 6 and 13 mg/kg immediately after the
last of 5 applications made at two weekly intervals. Subsequently
these declined, reaching 0.02-0.13 mg/kg six weeks after treatment.
After 11 weeks residues were 0.01 mg/kg or less (Table 9).
Tomatoes
In outdoor experiments carried out in Southern Europe, Australia,
Canada, S. Africa, the Canary Islands and Mexico, tomatoes were given
foliar treatments at rates between 50 g/ha and 300 g/ha. Applications
were made on up to 9 occasions at intervals from 5 days to 3 weeks
depending on the experiment. Samples were taken at intervals ranging
from the day of treatment to six weeks later. Although some of the
tomatoes received were green, they were close to ripening
(approximately 5 cm diameter) and in the condition at which they would
be picked for export. The residues (Table 9) did not exceed 0.5 mg/kg
in any of the samples taken shortly after treatment at the recommended
rates (up to 0.0% or 100 g/ha assuming use at 1000 l/ha).
Wheat
Residue trials have been carried out on wheat in Canada and Brazil,
with application rates up to 150 g/ha. In the grain residues did not
exceed 0.1 mg/kg at harvest two weeks after application. Residues in
straw were higher, and the few data obtained suggest that under these
conditions they may approach 10 mg/kg (Table 9).
Table 9. Residues Resulting from Supervised Trials with Various Fruits and Other Crops (1974/79)
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
ALFALFA Cyprus 1 0.1 12 5.6 4.7 10
BEANS
(Dried) Colombia 3 0.15 1 <0.011 87
CHERRIES Germany 3 0.02% 1-2 4.5 3.3 0.65 0.6 18
to run off
3 0.02% 1-2 2.7 1.6 1.0 0.39
to run off
3 0.02%
to run off 1-2 7.4 5.4 1.5 1.2
3 0.01% 1.5-2 0.59 0.47 0.27 0.25 0.2 19
3 1.5-2 1.0 0.6 0.3 0.1 0.1
GRAPES Canada
(Delaware) 1 0.08 - 0.7 0.32 20
21
(Elvira) 4 0.07 4,5 & 3 0.24
(0.1 to
0.37)
(Friedamia) 1 0.006%
to run off 0.75 0.41 0.25
(Sauvignon) France 4 0.075 1 0.08 0.03 0.02 <0.01 22
(Alicante)
(Bouschet) 1 0.075 - 0.4 0.28 0.23 0.12 23
2 0.075 3 0.37
(Riesling) Germany 4 0.08 5,4 & 2 0.5 0.2 0.11 0.09 0.05 24
4 0.08 5,4 & 2 0.8 0.5 0.1 0.05 0.05
(Barbera) Italy 1 0.01% 0.1 25
S. Africa 1 0.0375 - 0.2 0.1 0.18 <0.05 <0.05 S. Africa
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
GRAPES
(Waltham
Cross) 2 0.0375 2 0.24 0.2 0.19 0.07 <0.05
(Chemin
Blane) 1 0.0375 - 0.1 0.12 <0.05 <0.05
2 0.0375 2 0.12 0.07 <0.05 <0.05 <0.05
KIWI FRUIT N. Zealand 6 0.01% 2.2 1.9 1.7 1.3 N.Zealand
6 0.02% 6.2 3.9 3.4 2.8
LETTUCE Canada 6 0.07 1 <0.012 78
0.02
France 1 0.15 0.52 79
0.3 1.1
France 1 0.05 1.2 0.27 0.123 80
1 0.05 1.1 0.3 0.06 81
1 0.05 2.2 1.6 0.35
1 0.05 1.8 0.5 0.02
1 0.054 2.4 1.0 0.85 82
Germany 3 0.06 2 4.0 0.25 0.04 0.02 <0.01 83
3 0.06 2 1.2 0.06 0.01 <0.01
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
MAIZE
Cobs Australia 1 0.0015% <0.01 10
Cobs 0.003% <0.01
Sweet corn Canada 5 0.07 5 days <0.01 84
Sweet corn 3 0.075 1 <0.01 <0.01
Sweet corn 6 0.064 5 days <0.01 <0.01 <0.01 85
Germany 1 0.1 1.9 0.04 86
1 0.1 2.7 2.2 0.51 0.05 <0.015
1 0.1 2.9 1.1 0.15 0.05 0.025
<0.01
Leaves S. Africa 1 0.075 5.2 3.86 0.4 10
Leaves 1 0.15 17 106 2.6
Stalks 1 0.075 <0.01 0.086 <0.01
Stalks 1 0.15 0.05 0.02 0.06
PEAS U.K. 2 0.05 2 <0.01 88
w/o pods 1 0.025 - <0.01 89
Peas Hungary 1 0.1 - <0.017
pods (only) - 0.07
Peas 1 0.2 0.04
0.077
Sweden 2 0.08 2 <0.01 165
S. Africa 1 0.015 - <0.01 <0.016 <0.01 10
1 0.03 - <0.01 <0.016 <0.01
PLUMS Germany 5 0.02% 1-3 1.0 0.65 0.5 90
5 0.02% 1-3 0.9 0.75 0.4 0.25
5 0.02% 1-3 1.3 1.1 0.7
5 0.01% 3,2,3 & 1 0.3 0.29 0.29 0.25 0.11 91
5 0.01% 3,2,3 & 1 0.24 0.15 0.12 0.1
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
POTATOES Canada 4 0.075 2 <0.01 92
4 0.15 2 <0.01
9 0.072 1 <0.01
9 0.145 1 <0.01
4 0.150 2 <0.017 93
9 0.15 1 <0.01 94
Cyprus 1 0.1 <0.01 <0.01 10
France 1 0.15 <0.01 95
Germany 4 0.090 2 <0.01 <0.01 <0.01 96
4 0.2 2 <0.01 <0.01 <0.01 97
4 0.2 2 <0.01 <0.0l <0.01
4 0.2 2 <0.01 <0.01 <0.01
Italy 1 0.04 - 0.058 98
1 0.12 - 0.038
SOYA BEANS Brazil 2 0.24 2 <0.01 99
3 0.24 2 <0.01
dried Colombia 4 0.3 1,1 week
& 4 days <0.01 100
SUGARBEET France 1 0.1 - <0.01
Leaves after 101
17 wks
1 0.15 <0.01
after 102
17 wks
Germany 5 0.06 )2,2,2 1.3 0.52 0.07 0.05 103
5 0.06 )& 1 2.2 0.1 0.03 <0.01
5 0.2 2 12 4.0 2.3 0.02 104
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
SUGARBEET Germany 5 0.2 2 6.2 2.3 0.18 0.05
Leaves 5 0.2 2 13 0.73 0.23 0.13
U.K. 1 0.2 <0.01
after
15 weeks 105
RASPBERRIES Canada 1 0.225 - 0.2110 114
3 0.18 2 0.4510 115
3 0.135 2 0.410 116
U.K. 1 0.005% 1 0.1311 117
0.015% 1 0.2511
STRAWBERRIES Canada 2 0.23 9 days 0.07 118
2 0.18 90 0.04 119
2 0.18 8 days 0.45 120
TOMATOES Australia 9 O.01% 5-7 days 0.3 0.3 0.24 0.14 106
9 0.02% 5-7 days 0.57 0.57 0.44 0.53
1 0.0015% - 0.02 0.03 10
Canada 1 0.15 - 0.13 0.07 107
2 0.15 1 0.2 0.05
France 1 0.0075% - 0.09 0.06 0.04 108
Italy 1 0.005% - 0.02 109
0.015% 0.08
0.03 0.17
Mexico 1 0.0075 - 0.02 0.01 10
1 0.015 - 0.05 0.04
1 0.03 - 0.08 0.13
Portugal 3 0.075% 11 & 7 0.06 0.032 110
days
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
TOMATOES S. Africa 4 0.0075% 3,2,2,2 0.17 0.1 0.05 111
4 0.01% 3,2,2,2 0.27 0.15 0.09
4 0 02% 312,2,2 0.27 0.14 0.08
4 0:03% 3,2,2,2 0.29 0.2 0.6
Spain 7 0.03% 3,2 & 2 0.08 112
1 0.075 - 0.16 0.07 0.14 10
0.16 0.06 0.10
2 0.2 1 0.1 0.09 0.06 113
3 0.2 1 0.25 0.2 0.05
Canary Is. 2 0.1 1 0.25 0.15 0.01 113
3 0.1 1 0.2 0.15 0.06 0.03
2 0.15 1 0.4 0.3 0.15
3 0.15 1 0.55 0.4 0.2 0.6
WHEAT Canada 1 0.14 - 0.1 0.07 0.05 121
1 0.15 - 0.08 0.08 0.08 0.04 122
0.07 0.05 0.06 0.03
0.07 0.05 0.05 0.04
0.09 0.09 0.06 0.04
Brazil 1 0.09 <0.01 <0.01 123
2 0.09 12 days 0.04
2 0.09 31 days 0.03
2 0.09 19 days 0.01
3 0.09 19 & 12
days 0.04
1 +4 week storage, beans were analysed without pods
2 In 3 samples
3 10 days after treatment
4 In glasshouse
Table 9. Continued...
5 The grain contained <0.01 mg/kg
6 8 days after treatment
7 In 9 samples
8 In the peel
9 24 days after treatment
10 12-13 days after treatment
11 19 days after treatment
FATE OF RESIDUES
GENERAL OBSERVATIONS
Following applications to crops cypermethrin may degrade to a variety
of hydrolysis and oxidation products. The most likely degradation
products present in crops at harvest following normal agricultural use
of cypermethrin are the derived amide (compound B), 3-phenoxybenzoic
acid and 2-(2',2'-dichloro vinyl)-3,3 dimethyl cyclopropane carboxylic
acid (compound C), the structures of which are shown in Figure 1. The
latter two compounds are found in the free state as well as in
conjugated forms. However, the evidence indicates that the major
component of any residue present at harvest will be cypermethrin
itself. A number of crop samples obtained from supervised trials
discussed previously were analysed also to determine compounds B, C
and phenoxybenzoic acid. The results of these examinations involving
some 20 crops showed no residues of Compound B, of Compound C or of
3-phenoxy benzoic acid in excess of 0.05 mg/kg.
Following use on animal feed crops, residue may be present in feed at
levels depending on the crop. However, since the product is readily
metabolized by animals, these amounts are unlikely to give rise to
more than traces of foods of animal origin.
IN ANIMALS
Cattle Feeding Studies
Studies were undertaken to investigate the fate of cypermethrin in
cattle and whether residues in meat or milk could arise from the use
of cattle feed containing products made from treated crops. Two
experiments were undertaken, both using radiolabelled cypermethrin.
a. Low Dietary Intake (0.2 mg/kg)
Two lactating cows were given feed concentrate containing
radio-labelled cypermethrin twice daily for a three-week period with a
feeding level equivalent to 0.2 mg/kg on total daily feed. The cows
were milked twice daily and the amount of radioactivity determined at
each milking. The total radioactivity present in whole milk was in
the range of 0.0002 mg/1 to 0.0012 mg/1 in terms of cypermethrin
equivalents and 60-70% of the amount of radioactivity in the milk was
present in the cream fraction. The total radioactivity found in the
milk amounted to only 0.5% of the radioactivity fed to the animals.
The remainder of the radioactivity was excreted in the urine (54%) and
faeces (43%). The elimination occurred rapidly after dosing and the
rate of elimination of radioactivity reached its peak (near 100% of
applied dose) after three days.
Molecular Weight: 416
Technical Material: Contains not less than 90% w/w cypermethrin
which is a racemic mixture of the 8 isomeric
forms with a cis: trans isomer ratio of
approximately 40:60
Physical Form Technical material is a viscous yellow liquid.
Density: 1.12 g/ml at 22°C.
Thermal Stability: Thermal analysis has shown that at a
temperature of 259°C slow weight losses occur
accompanied by negligible heat of reaction
indicating high thermal stability.
Solubility: Water 0.009 mg/l. Hexane 103 g/l. Acetone,
cyclohexanone, ethanol xylene and chloroform
450 g/l.
Volatility: relatively non-volatile
Vapour pressure: 5 × 10-6 N/m2 at 70°C
Flash point: 80°C
Formulations: Currently available in emulsifiable
concentrates ranging from 25-400 a.i. g/l.
Several formulations for ultra low volume
applications containing 8-75 g/l are also
available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Studies were performed in vivo on a wide range of mammalian species
and in vitro on isolated cell fractions to define the
pharmacokinetics of cypermethrin. In general, a wide range of studies
have shown that cypermethrin is rapidly absorbed, distributed to a
variety of tissues and organs, metabolised and rapidly excreted from
the body. Studies have been performed to evaluate the
pharmacokinetics in mice, rats, dogs, sheep and cows.
Mice
Groups of male mice were orally administered the cis or trans isomers
of cypermethrin dissolved in methoxytriglycol at dose levels of 7
mg/kg with the cyclopropyl-14C isomer and 8 mg/kg with the benzyl-14C
isomer. Results suggest that the proportion of cypermethrin which was
readily absorbed following oral dosing was rapidly eliminated in the
urine of mice. Absorption from the gastrointestinal tract was more
rapid with the trans isomer than with the cis as evidenced by high
radioactivity levels of the cis isomers in the faeces within the first
few days of the study. The rate of urinary excretion of cypermethrin
isomers labelled in acid and alcohol portions of the molecule was very
similar with the major quantity of radioactivity being eliminated from
the body within three days. Tissue residues after three days were
low. Adipose tissue was found to retain small quantities of the
radioactivity in concentrations higher than that noted for most other
tissues (Hutson, 1978a).
To evaluate the elimination of cypermethrin and its metabolites from
animal tissues following oral administration, groups of male mice were
administered cypermethrin at dose levels approximating 9 mg/kg body
weight (14C-benzyl, cypermethrin). In contrast to data from other
tissues, the rate of elimination of cypermethrin from adipose tissue
of mice was relatively slow with a half-life of approximately 10-20
days. Unchanged cis-cypermethrin was the only chemical residue
identified in adipose tissue. There was little change in the residue
during the last half of the 42-day trial following the relatively
rapid elimination during the first three weeks suggesting possible
long term storage and buildup of residue in adipose tissue (Crawford
and Hutson, 1978a).
Rat
Groups of male and female Wistar rats were orally administered
cypermethrin (14C benzyl, cis-isomer) at the dose level of 2.4 mg/kg
for females and 1.8 mg/kg for males. Cypermethrin was administered in
corn oil solution and animals and tissues were monitored over a period
of eight days. Excretion was rapid with both sexes. There was a
significant sex difference in the rate of excretion with male rats
excreting a substantially larger portion of the radioactivity in urine
than females over the first 24 hour interval. However, there were no
substantial differences in the overall rate of elimination by both
males and females when data were evaluated over the total 8-day period
of the test. The residues of radioactivity in the tissue of animals
of both males and females sacrificed at various periods over the 8-day
interval showed a small residue in a variety of tissues. While the
tissue residue in blood, liver, kidney, etc. was rapidly depleted over
the test interval, concentrations of cypermethrin in adipose tissue
were relatively stable over the 8-day trial (Crawford, 1976a).
Groups of male and female rats were orally administered cypermethrin
14C-benzyl, trans-isomer) at dose rates of 2.4 mg/kg for males and
3.0 mg/kg for females. Again, excretion was rapid with both sexes
with approximately 95% of the administered dose excreted within 48
hours. In contrast to that noted with the cis-isomer, there appeared
to be no sex differences in the elimination of the trans-isomer.
Residues in adipose tissue of females was somewhat higher than noted
in adipose tissue of males. The observations are consistent with the
suggestion that the trans-isomer is metabolised faster than the
cis-isomer (Crawford, 1976b).
Groups of rats were administered cypermethrin (1:1 cis: trans-isomer
ratio, 14C-cyclopropyl) at dose rate of 1 mg/kg for males and 2 mg/kg
for females. Rapid elimination of cypermethrin was observed with a
substantial difference noted in the 24-hour urinary excretion in males
and females. Females excreted considerably more radioactivity in
urine over the first 24 hours. At the end of 72 hours urinary and
faecal excretion in both sexes was approximately the same. Small
quantities of radioactivity were expired as 14CO2 which suggested
some metabolic breakdown of the cyclopropyl ring. At the end of three
days, tissue concentrations in liver, kidney muscle, brain, blood,
skin and remaining carcass were relatively low. Intestinal content
was somewhat higher in males than in females ranging from 3 to 9% of
the administered dose. Low residue concentrations, which never
exceeded 1 ppm, were observed in fat (Crawford, 1977).
The elimination pattern of cyclopropyl- and benzyl-labelled
cypermethrin appears to be the owns in both males and females.
Females absorb and metabolise the trans-isomer faster than males and
males absorb and metabolise the cis-isomer faster than females
(Crawford, 1976b). In a further study to resolve the residual nature
of the residues of cypermethrin in adipose tissue of rat, Crawford and
Hutson (1978b) again found that the cis-isomer was relatively stable
in adipose tissue. Administration of 14C-benzyl cypermethrin
(cis-isomer) at a dose of approximately 2 mg/kg to female rats
resulted in a residue of approximately 0.3 ppm in the fat eight days
after a single oral dose. Further studies through 42 days following
dosing were performed to evaluate the half-life of the cis-isomer in
fat and the total elimination pattern. At the end of 42 days,
residues were observed in fat which were approximately 10% of those
concentrations noted at eight days. There was a 90% loss of the
material from fat over the 8-42 day interval during which time samples
were taken. From these data with rats, a half-life of approximately
20-25 days was estimated with respect to removal of residues from fat
following a single oral dose. These half-life values are somewhat
longer than those noted with the mouse.
Dogs
Groups of male beagle dogs were administered 14C-benzyl cypermethrin
and the individual 14C-benzyl labelled cis- and trans-isomers of
cypermethrin, orally, at a dose of 2 mg/kg. Elimination of
radioactivity from all animals was rapid although differences in data
from individual dogs precluded a complete evaluation of the rate of
elimination. Differences in the rates of elimination of the
individual dogs in the study may have been due to differences in
absorption as cypermethrin was given orally in a capsule with no
solvent. Tissue residues observed 4 days after oral administration of
cypermethrin were extremely low. The vast majority of the excreted
material was observed in faeces (80%) with urine containing only 11%
of the administered dose. As with other species, small residues of
cypermethrin were observed in fat, approximating 2% of the
administered dose (this residue was estimated to be 0.3 ppm based upon
total adipose tissue of the dog) (Crawford, 1979b).
Sheep
Sheep were administered cypermethrin dermally (962 mg/animal
administered in acetone) or orally (without solvent) in gelatin
capsule at a dose of 4 mg/kg body weight. Following dermal
administration, cypermethrin was not readily absorbed (less than 0.5%
of the administered dose was observed in urine within 24 hours and 2%
over the six day test interval). Little radioactivity was found in
the liver and kidney of dermally-treated sheep. Tissue residues in
renal and subcutaneous fat was found to be similar to those noted with
other animal species and was qualitatively identified as cypermethrin.
Elimination of cypermethrin from the orally-dosed sheep was rapid with
61% of the administered dose being eliminated within 48 hours (41% of
the dose in urine and 20% of the dose in faeces). The tissue residue
pattern in sheep following oral administration was similar to that
noted with other mammalian species. Low levels of cypermethrin were
noted in various tissues, including renal and subcutaneous fat
(Crawford and Rutson, 1977a).
Cows
Cypermethrin (1:1, cis:trans-isomer, 14C-benzyl-label) was orally
administered to lactating cows over a period of three weeks at a
dietary dosage of 0.2 ppm. Urine, faeces and milk residues were
examined over the course of the study and at the conclusion of the
three week dietary interval, animals were sacrificed and tissue
residues examined. Elimination of radioactivity in urine and faeces
rapidly reached an equilibrium within 2-3 days. Radioactivity was
eliminated in the urine and faeces predominantly and accounted for the
major quantity of cypermethrin residues. Small quantities of
radioactivity were noted in milk with a total radioactivity residue
approximating 0.5% of the administered radioactive cypermethrin.
Tissue residues were extremely low with notable residues in bile and
fat as well as in the liver and kidney. There were no detectable
residues in muscle, blood or brain. Analysis of the milk fat showed
that a high percentage of the radioactivity was present in this
portion of the milk. Residues in the milk fat or cream sample were
reflective of the lipoidal nature of cypermethrin, with residues
concentrating in fatty tissues (Hutson and Stoydin, 1976).
In a further study to obtain information on the metabolic distribution
of cypermethrin in cows, lactating cows were treated for 7 days at a
dietary concentration of 5 ppm. Urine, faeces, and milk were analysed
and at the conclusion of the feeding trial, all animals were
sacrificed and tissue residues were determined. As in the previous
study, elimination occurred rapidly after the onset of dosing with the
major route of excretion being via urine and faeces. Tissue residues
were extremely low with measurable amounts observed in blood, liver,
kidney, bile and adipose tissue. Total milk residues amounted to
approximately 0.2% of the applied cypermethrin, with the largest
proportion of the residue again found in milk fat (Crawford, 1978).
Metabolism
The metabolic fate of cypermethrin was investigated in a variety of
mammalian species and was determined to be relatively similar in the
different species. The major metabolites are derived by cleavage of
the ester linkage followed by subsequent metabolism of both the acid
and alcohol fragments and conjugation and excretion of the fragments.
Studies on the metabolic fate of both cis- and trans-cypermethrin
administered orally to rats have shown that cypermethrin is rapidly
cleaved at the ester bond to yield the cyclopropanecarboxylic acid and
the 3-phenoxybenzyl alcohol moiety. The latter is rapidly oxidised to
3-phenoxybenzoic acid and conjugated prior to elimination. Major
reactions occurring with the cyclopropanecarboxycyclic acid include,
in part, oxidation at the methyl groups and apparent lactone
rearrangement prior to conjugation and elimination. Hydroxylation of
the phenoxybenzoyl moiety has been noted to occur in at least two
positions prior to conjugation and elimination. Rats and mice
metabolised cypermethrin in a similar fashion. In mice, a substantial
portion of the phenoxybenzoic acid was conjugated with taurine as well
as with glucuronic acid (Hutson, 1977). Oxidation has been noted to
occur in the 4'- position of phenoxybenzoic acid. Following
oxidation, the molecule is conjugated as a sulfate and excreted. A
portion of unconjugated hydroxylated phenoxybenzoic acid was also
excreted. Hydroxylated cypermethrin was observed in mouse faeces
suggesting that hydroxylation may occur prior to ester cleavage
(Hutson, 1978b). Shono, Ohsawa, and Casida (1978), using mouse liver
microsomal preparations, showed that hydroxylation of the
phenoxybenzyl moiety at positions other than the 4'- position can
occur. Small amounts of the 5- and 6-hydroxy phenoxybenzyl derivatives
were also observed. Both cis- and trans-isomers were rapidly
metabolised by cleavage of the ester bond and aromatic hydroxylation
at the 4'-position of the 3-phenoxybenzyl alcohol moiety. In rats,
approximately half of the administered cypermethrin was excreted as
sulfate conjugates of the hydroxylated phenoxybenzoic acid.
Phenoxybenzoic acid was also excreted free and conjugated with glycine
in contrast to the conjugate pattern noted in mice (Crawford and
Hutson, 1977b, 1978c). In studies with cyclopropanecarboxylic
acid-labelled cypermethrin, hydroxylation of the methyl groups on the
cyclopropane ring occurred to a limited extent. Oxidation was
believed to have occurred following ester cleavage. While most of the
radioactive metabolites were found in the urinal a small quantity of
hydroxylated metabolites were eliminated via the
biliary-intestinal-faecal route suggesting absorption and
re-distribution through the bile to the faeces.
In both rats and mice as well as other species, ester cleavage is
rapid. There is quantitative and qualitative evidence to suggest that
the cis-isomer is more stable yielding a larger variety of
hydroxylated products prior to ester cleavage while the trans-isomer
yields a wider variety of hydrolytic products which in part are
oxidised following the ester cleavage. The acid fragment is
conjugated predominantly with glucuronic acid an a ß-glucuronide.
Conjugated cyclopropanecarboxylic acid derivatives in urine were
identified as glucuronides have a relative resistance to the action of
ß-glucuronidase. ß-Glucuronides formed from these acids are generally
of the ester type readily cleaved by mild acid hydrolysis. Thus,
treatment of urine with methanol and sulfuric acid will convert both
the free acid and the acid glucuronide into a methyl ester. The
methyl ester of the carboxylic acid has been shown to be volatile and
analytical losses may occur with this compound. The alcohol fragment,
the alpha-cyano-3-phenoxybenzyl alcohol, is readily oxidised
metabolically to the benzoic acid, conjugated and excreted. Alcohol
conjugates with glucuronic acid are generally ether derivatives,
stable to acid and enzymatically hydrolysable. The phenoxybenzyl
moiety of cypermethrin has been found to form both ester and ether
ß-glucuronides.
Thus, the results of the studies with both rats and mice suggest that
most of the excreted metabolites of cypermethrin are hydrolysis
products, although hydroxylation of the intact ester has been
reported. Hydroxylation of the methyl groups attached to the
cyclopropane ring has also been reported. There was no evidence of
metabolism at the 2,2-dichlorovinyl moiety. Evidence exists that the
cis-isomer is somewhat more resistant than the trans-isomer to
hydrolysis. In all cases, only small amounts of hydroxylated
metabolites with the intact ester bond were observed with the
cypermethrin.
The metabolic fate of cypermethrin in dogs was qualitatively similar
to that observed with other species with the exception being
conjugation reactions of several metabolites. As with other species,
cypermethrin is rapidly metabolised by cleavage of the ester bond and
by hydroxylation of the phenoxybenzyl moiety at the 4' position.
Sulfate conjugation of the hydroxylated benzoic acid was reported.
Additionally, a major urinary metabolite was identified as the
3-phenoxybenzyl glycine. Studies on the metabolic fate of
3-phenoxybenzoic acid in dogs revealed a metabolic pattern of
oxidation and conjugation similar to that noted with cypermethrin
(Crawford, 1979a and 1979c).
In cows, the major urinary metabolite of cypermethrin ( 14C-benzyl)
was the glutamic acid conjugate of the 3-phenoxybenzoic acid
(Crawford, 1978). The cyclopropanecarboxylic acid moiety was
hydroxylated and/or conjugated with glucuronic acid prior to
excretion.
3-Phenoxybenzoic acid has been observed as a major metabolite of
cypermethrin in all species studied. Residues of cypermethrin in rat
skin (<3% of the dose) were noted after oral administration. Further
studies to define the skin residue were performed using
14C-3-phenoxybenzoic acid administered orally to rats as seven
consecutive daily doses. The radioactive metabolites in skin were
identified as predominately 3-phenoxybenzoic acid and a small quantity
of glyceryl dipalmitate esters of 3-phenoxybenzoic acid (Crawford and
Hutson, 1979).
Further studies were performed in rats of the metabolism of
3-phenoxybenzoic acid and a glucoside conjugate isolated from plant
tissue. 3-Phenoxybenzoic acid was oxidised at the 4' position of both
aromatic moieties and to a minor extent at the 6' position of the
benzoic acid moiety. Excretion occurred as conjugates, predominantly
the 4-hydroxy-3-phenoxybenzoic acid-O-sulfate and 3-phenoxybenzoic
acid. Additionally, other hydroxylated derivatives and conjugates of
these oxidation products were observed. The excretion pattern of the
glycoside conjugates of 3-phenoxybenzoic acid was qualitatively and
quantitatively similar to that observed with 3-phenoxybenzoic acid
suggesting rapid hydrolysis and availability of plant metabolites
following oral administration (Crawford, 1978).
Effects on Enzymes and Other Biochemical Parameters
Preliminary evidence suggested that an increase in the activity of
certain lysosomal enzymes in peripheral nerves and deficits in
behavioural functioning tests could serve as indicators of peripheral
nerve damage. During Wallerian degeneration, the activity of such
enzymes as ß-glucuronidase and ß-galactosidase as well as other
enzymes in nerve preparations were shown to be increased significantly
(Dewar, 1977a).
Cypermethrin (1:1 cis:trans) was administered to male and female rats
at dose levels ranging from 25 to 200 mg/kg/day for five consecutive
days by oral intubation as a 10% W/V solution in DMSO. A dose related
functional deficit was observed when the mean slip angle test and the
landing foot spread test were applied to the animals. The deficit was
maximal from 6 to 14 days after the beginning of treatment and
complete functional recovery occurred within four weeks. Substantial
variation in data from the landing foot spread test was noted. Data
were inconsistent over the course of the study. ß-glucuronidase
activity was increased in a dose-dependent fashion in both males and
females. The results suggest that cypermethrin produced a primary
axonal degeneration, readily measurable 28 days after treatment as an
increase in ß-glucuronidase activity and in deficits in specific
behavioural-function testing of rats (Dewar, 1977b).
When further studies were performed on the biochemical indices of
nerve degeneration to compare results with known degenerating
compounds (methyl mercury), it was observed that the changes in
ß-glucuronidase and ß-glucuronidase activity were considerably smaller
and less reproducible than those obtained following methyl mercury
poisoning (7.5 mg/kg/day for 7 days). In comparison of central versus
peripheral nerve damage, there was no evidence that the trigeminal
nerve was more sensitive to the effects of cypermethrin than the
sciatic and posterior tibial nerves. At near lethal doses of
cypermethrin biochemical changes in the trigeminal nerve were
consistent with those of Wallerian degeneration. The changes were
similar qualitatively to those seen with methyl mercury, but were
quantitatively much less intense (Dewar and Moffett 1978a).
Electrophysiological studies were performed to determine whether acute
or subacute intoxication with cypermethrin produced changes in the
conduction velocity of slower fibres in peripheral nerves or
alterations in the maximal motor conduction velocity. There was no
evidence to suggest that cypermethrin, at doses that induced severe
clinical signs of intoxication, including ataxia, had any effect on
maximal motor conduction velocity or conduction velocity of the slower
motor fibers in peripheral nerves. Doses used in the study ranged
from a single dose of 200 mg/kg to 7 consecutive doses of 150 mg/kg
followed by 2 doses of 400 mg/kg. At near-lethal doses there were no
effects noted on conduction velocity in the slower motor fibers of the
sciatic nerve and tail or on the maximal conduction velocity, even in
the presence of clinical signs of acute intoxication and at dose
levels where previous studies had shown functional degeneration.
These electrophysiological findings are reflective of motor function
which would suggest that the physiological and functional deficits
observed as a result of acute intoxication may be primarily sensory in
nature (Dewar and Deacon, 1977).
SPECIAL STUDIES ON REPRODUCTION
Dominant Lethal Studies
Mice
Groups of male mice (12 mice/group, 36 mice were used as controls)
were administered a single dose of cypermethrin dissolved in dimethyl
sulfoxide at dosage levels of 0, 6.25, 12.5 and 25 mg/kg body weight
or 5 successive daily oral doses of 0, 2.5 and 5.0 mg/kg. Following
dosing, each male was caged with 3 virgin females for 7 days. The
mating procedure was repeated weekly over an interval of 8 weeks in a
standard dominant lethal test.
Females mated to males treated with 5 daily oral doses of 2.5 mg/kg
and those mated to males receiving a single dose of 12.5 mg/kg showed
a significant reduction in the incidence of pregnancy during the
second and third week respectively of the onset of treatment. This
did not occur with other either higher or lower dosed groups or at
other intervals. In females, mated to males treated daily with doses
of cypermethrin a significant reduction in foetal implants was
observed during the second week of mating. Early foetal deaths were
increased in the second week at 5 mg/kg. No such increases occurred
in any other weekly interval or any other dosed group. Thus, multiple
administration of cypermethrin on five successive days induced a
significant reduction in foetal implants during the second week of
mating and a marginal increase in early foetal deaths at the same time
interval.
To evaluate the potential (noted above) for a dominant lethal effect,
a second experiment was performed. Groups of male mice (12
mice/group, a control group consisted of 36 mice) were administered
five daily oral doses of cypermethrin at levels of 0, 2.5, 5.0, 7.5
and 10 mg/kg (in dimethyl sulfoxide). Following dosing each male was
mated with three virgin females for four days and subsequently
provided with virgin females every four days for a period of three
weeks. Female mice were examined for evidence of dominant lethality
13 days after mating. In addition, 40 males of proven fertility were
dosed for five successive days at the same dosage levels, 0, 2, 5,
5.0, 7.5 and 10 mg/kg. These animals were also mated with four virgin
females on four successive days for a period of three weeks. Four
animals from each group were sacrificed for histological examination
of the testes and epididymis on days 1 and 7 after the final dosing.
In contrast to the previous trial, no reduction in foetal implants was
noted in any of the animals mated with cypermethrin-treated males. The
number of early foetal deaths was marginally increased at the highest
dose level in the 12-16 day interval after dosing and in the first 4
day period after treatment with 7.5 mg/kg males.
In the groups of animals examined histologically, no abnormalities
were detected in the testes and epididymis and there were no
observable histological differences between any of the test groups and
the controls (Dean et al., 1977).
Rat
Groups of rats (30 male and 30 female rats/group) were fed
cypermethrin in the diet at concentrations of 0, 10, 100 and 500 ppm
for five weeks after which they were mated to initiate a standard
three generation, 2-litter per generation, reproduction study. After
the selection of the second litter as parents for the following
generation, the original parents were sacrificed and subjected to
gross and microscopic examination. At the conclusion of the study,
ten animals of each sex at the age of 21 days were also examined
histologically. Calculations were made of the indices of fertility,
gestation, viability and lactation. There was no mortality over the
course of the study, and behaviour was not abnormal. Reduced body
weight and food intake was seen at various intervals in both males and
females fed 500 ppm in the diet. Reduced total weight was observed at
500 ppm in the F1A litters and reduced total weight and litter size
were noted at 100 and 500 ppm in the F1B. No compound related gross
or microscopic pathological findings were noted in any rats (Hend et
al., 1978).
SPECIAL STUDIES ON TERATOGENICITY
Rat
Groups of 25 pregnant rats were administered orally from day 6 to day
15 of gestation at dose levels of 0, 17.5, 35.0 and 70.0 mg/kg/day in
a standard teratology bioassay. On day 21 of gestation, the animals
were sacrificed and gross examination of foetuses were made, including
skeletal and somatic examinations. Pre-implantation losses were
evaluated based on corpora lutes, counts and implantation sites and
post-implantation losses were evaluated based upon implantation sites
and viable foetuses. Foetal somatic and skeletal examinations on day
21 of gestation showed no teratogenic changes that could be
attributable to the treatment. There were no indications of
embryotoxic or teratogenic events in the study (Tesh et al., 1978).
Rabbit
Groups of pregnant rabbits (20 rabbits/group, 30 rabbits were used as
an additional control group) were administered cypermethrin dissolved
in corn oil at dose levels of 0, 3, 10 and 30 mg/kg body weight orally
from day 6 to day 18 of gestation. On day 28 of gestation the rabbits
were sacrificed and examination made of live foetuses, dead foetuses,
resorption sites and corpora lutea. Live foetuses were maintained for
24 hours to assess viability. Foetuses were also examined for gross
somatic and skeletal deformities.
There was no significant mortality or differences in weight gain
during the period of gestation. There were no significant differences
between control and test groups with respect to pregnancy, foetal
death and survival. Although a wide range of skeletal and visceral
abnormalities were found in the course of the study, there were no
differences between control and test groups with respect to
abnormalities. It was concluded that oral dosing at up to 30 mg/kg
during the major period of organogenesis resulted in no teratogenic
effects in the offspring (Dix, 1978).
SPECIAL STUDIES FOR MUTAGENICITY
Mice Host-Mediated Assay
Groups of male mice (2-3 mice per group) were administered
cypermethrin, orally, at dose levels of 0, 25 and 50 mg/kg in
dimethylsulfoxide. The animals were immediately injected
interperitoneally with a suspension of S. cerevisiae in a standard
host mediated assay. After five hours, the mutagenic conversion rates
in the yeast cells recovered from the treated animals were comparable
with those of control animals suggesting that, under the terms of this
assay, there was no evidence to suggest a mutagenic potential (Brooks,
1976).
Microorganisms
The mutagenic potential of cypermethrin on various microorganism
species including: S. cerevisiae, E. coli WP2 uvr A, and S.
typhimurium TA-1538 (with and without the use of a rat activation
system) was examined. No increase in the mitotic gene conversion was
recorded in S. cerevisiae, either in the presence or absence of
microsomal oxidation. Cypermethrin, at concentrations up to 500
micrograms per plate, did not induce an increased mutation rate with
E. coli or S. typhimurium TA-1538, in vitro either in the
presence or in the absence of the microsomal oxidation system (Brooks,
1976).
Cypermethrin did not increase the number of revertant colonies of S.
typhymurium (TA-1535, TA-1537, TA-1538, TA-98 and TA-100) in the
presence or absence of a mouse liver subcellular activation
preparation obtained from 6 strains of PCB-treated mice. Cypermethrin
was tested at dose levels up to 1 mg/plate (Suzuki, 1977).
Hamster - Chromosomes
Groups of Chinese Hamsters (12 males and 12 females/group) were
administered orally on each of two successive days at a dose rate of
0, 20 or 40 mg/kg cypermethrin dissolved in dimethylsulfoxide.
Positive and negative controls consisted of cyclophosphamide (100
mg/kg) and dimethylsulfoxide alone. In chromosome preparations of the
control and cypermethrin-treated hamsters, there were no indications
of abnormalities. The cylophosphamide treatment induced a significant
degree of chromosomal damage (Dean, 1977).
SPECIAL STUDIES ON NEUROTOXICITY
Rats
Cypermethrin, orally administered to rats at high acute dosage levels,
produced severe clinical signs of poisoning which was accompanied by
histological evidence of sciatic nerve damage. Within one day
following the acute poisoning, neuropathy was evident as axonal breaks
in the sciatic nerve (Carter and Butterworth, 1976).
Groups of rats (10 males per group) were utilised in a paired feeding
study to examine the neurotoxicological effects of high dietary
levels. Rats were fed dietary levels of cypermethrin (45:55,
cis:trans-isomer ratio) for 14 days at dosage levels of 0, 1250, 2500,
and 5000 ppm. Growth was monitored and clinical evaluations for
adverse behaviour were recorded during the course of the study. Gross
pathology on tissues and organs and microscopic examination of the
sciatic nerve were performed at the conclusion of the study.
At 5000 ppm, mortality was observed with all rats either dying or
sacrificed in a moribund condition within the first week. At 2500
ppm, 6 of 10 rats died before the conclusion of the study. There was
no mortality in the low dose group. Clinical signs of neurotoxicity
were characterised by an impaired ability to walk and splayed hind
limbs. In extreme cases, clinical signs of ataxia and paralysis were
reported. Other clinical signs included: hypersensitivity to external
stimuli, gross disorientation and convulsions, the latter generally
seen at high dose levels. The neurotoxic signs of poisoning observed
in the 1250 ppm group after day three were spontaneously reversed by
day nine when all surviving animals that were initially affected
appeared to be normal. Remission of ataxia at the 2500 ppm group was
also noted within 11 days of treatment. Growth reduction was observed
in all animals in the study. At the lowest dose level, the rate of
growth was delayed for the first few days after which time the rate
was consistent with that of controls for the remainder of the study.
The absolute body weight of the treated animals was, however,
significantly lower than the controls at the conclusion of the
two-week study. Reduction in body weight was consistent with
reduction in food consumption. Ultrastructural changes in the sciatic
nerve were observed in the two highest dose levels, although the
number of animals examined was small. There was some evidence of
axonal damage in the myelinated nerves primarily at the two highest
dose groups. Changes in unmyelinated axons were not observed. In
general, the histological and ultrastructural changes in the sciatic
nerve, accompanied by clinical signs of poisoning, are not readily
apparent at low doses. Thus, cypermethrin, at acutely toxic dietary
levels,induces damage to sciatic nerves, the clinical signs of which
may be reversible (Glaister et al., 1977a).
Hamsters
Groups of male and female Syrian hamsters were orally administered
doses exceeding the LD50 in an attempt to define clinical signs of
poisoning and to evaluate the histological damage to the sciatic
nerve. At doses of 794 mg/kg and above, all animals showed clinical
signs of poisoning including tremors, abnormal, irregular movements,
and an unusual gait. As noted with rats, axon and myelin degeneration
was noted in all groups treated. The lesions included swelling and
breaks in the axons and clumping of myelin (Butterworth and Clark,
1977).
A series of experiments were performed to further evaluate the
neurotoxic potential following subacute, oral administration to
Chinese hamsters. Clinical examination, functional testing and enzyme
determinations using ß-galactosidase and ß-glucuronidase were
performed. The functional test consists of measuring the mean slip
angle where the animal is maintained on an inclined plane that
steadily increases its angle until the animal can no longer maintain a
stationary position. (The average angle of five replicate trials
constituted the mean slip angle test.)
Groups of 20 male and 20 female Chinese hamsters were orally
administered at a dose of 40 mg/kg followed by a dose of 20 mg/kg for
the following four days. Fifteen animals in each sex served as
controls. There was extensive weight loss in all dosed groups and
some mortality was observed primarily as a result of the initial
administration of 40 mg/kg. There was a loss of fur and a dermal
ulceration observed in the early parts of the study. This dermal
occurrence was transient, disappearing rapidly after the treatments
were concluded. There was significant weight loss in the initial
phase of the study. However, after the last dose, the surviving
animals rapidly gained weight at a rate consistent with the control
animals. There were no effects noted on the mean slip angle
experiment and a marginal increase in ß-galactosidase was observed in
peripheral nerve.
A further experiment utilising five male and five female Chinese
hamsters administered cypermethrin daily for five days at dose levels
of 0, 5, 10 and 20 mg/kg showed no mortality over the course of the
study. There was a slower growth of the animals treated with 20 mg/kg
which again was reversed at the conclusion of the treatment period.
Hyperexcitability was noted in one female at the high dose level.
There were no notable differences in behaviour in any of the animals.
There was a significant deficit in the mean slip angle test with
females showing an earlier dose-related deficit than noted in males.
The females recovered from this deficit earlier than males who showed
an erratic pattern of recovery. ß-galactosidase activity was
increased at all dose levels three weeks after the onset of the
experiment. This increase was statistically significant at the two
highest dose levels. Dermal irritation and fur loss was not noted in
this experiment.
Sixteen male and sixteen female Chinese hamsters were administered
five daily doses of 30 mg per kg of body weight or a control of
dimethyl sulfoxide. Additionally eight animals of each sex were
administered five daily doses of methyl mercury (7.5 mg/kg body
weight) as a positive control. With cypermethrin, there was no
mortality and the rate of weight gain was consistent with that of the
controls. There was a transient dermal irritation in the majority of
the animals accompanied by skin ulceration. This condition
disappeared at the conclusion of the treatment interval. One male
administered cypermethrin had an unusual gait. There was a slight
deficit in the inclined plane test which was noted in the early parts
of the experiment but was absent by the end of the third week.
Increases in both ß-glucuronidase and ß-galactosidase were evident in
peripheral nerve tissue. It was concluded that cypermethrin when
administered at high subacute doses, produced changes in the sciatic
nerve consistent with Wallerian degeneration. Biochemical changes
were evident as increases in ß-glucuronidase and ß-galactosidase
activity and clinically functional deficits were noted (Dewar and
Moffett, 1978b).
Hen
Groups of adult hens were fed cypermethrin at a dosage rate of 1 mg/kg
for five successive days. After 3 weeks, the dosing regimen was
repeated. Positive (TOCP) and negative control groups were included
in the study. There were no signs of delayed neurotoxicity (as
generally noted with certain organophosphate esters) seen following
cypermethrin treatment. TOCP induced the standard clinical and
histological signs of axon and myelin disruption (Owen and
Butterworth, 1977).
Acute Toxicity
Table 1 contains a summary of the findings of various studies on acute
toxicities to a number of animals.
Table 2 illustrates that the cis isomer is more toxic than the trans.
Table 1. Acute Toxicity of Cypermethrin to Various Animals
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Mouse M,F Oral Corn oil 82 Coombes, Carter et al., 1976
Mouse M,F Oral Dimethylsulphoxide 138 Coombes, Carter et al., 1976
Mouse M,F Oral Aq.suspension 779b Jaggers, 1979
Mouse M,F I.P. corn oil 485 Combs, Carter et al., 1976
Rat M,F Oral Corn oil 251-992 Combs, Carter et al., 1976
Rat M,F Oral Dimethylsulphoxide 303 Combs, Carter et al., 1976
Rat M Oral Glycerol formal 200-400 Combs, Carter, et al.,1976
Rat F oral Glycerol formal app. 200 Combs, Carter et al., 1976
Rat M Oral Aq.suspension 400-800 Combs, Carter et al., 1976
Rat F Oral Aq.suspension app. 400 Combs, Carter et al., 1976
Rat F Oral Aq.suspension 4123b Jaggers, 1979
Rat M Oral Aq.suspension 3000c Jaggers, 1979
Rat (3wks) M,F Oral Dimethylsulphoxide 163 Rose and Dewar, 1978
(6wks) M,F Oral Dimethylsulphoxide 322 Rose and Dewar, 1978
(12 wks) M,F Oral Dimethylsulphoxide 526 Rose and Dewar, 1978
Rat F I.P. Aq.suspension >500b Jaggers, 1979
Rat M,F I.P. Propylene glycol 1000-2000b Jaggers, 1979
Rat F Dermal Undiluted >4800b Jaggers, 1979
Rat M,F Dermal Xylene >1600 Combs, Carter et al., 1976
Syrian
hamster M,F Oral Corn oil >400 Combs, Carter et al., 1976
Chinese
Hamster M,F Oral Corn oil 203 Combs, Carter et al., 1976
Guinea pig M Oral Corn oil app. 500 Combs, Carter et al., 1976
Guinea pig F Oral Corn oil >1000 Combs, Carter et al., 1976
Guinea pig M Oral Aq.suspension >4000b Jaggers, 1979
Table 1. Continued...
Species Sex Route Vehicle LD50 Reference
(mg/kg)
Rabbit F Oral Undiluted >2400b Jaggers, 1979
Rabbit F Dermal Undiluted >2400b Jaggers, 1979
Dom. fowl M,F Oral Dimethylsulphoxide >2000 Combs, Carter et al., 1976
Partridge M,F Oral Dimethylsulphoxide >3000 Combs, Carter et al., 1976
a) Unless specified, all values refer to data generated using 50:50 cis:trans material.
b) Data generated using 40:60 cis:trans material.
c) Data generated using 53:46 cis:trans material.
Table 2. Effect of Cis:Trans Ratio on Acute Toxicity of Cypermethrin
LD50
Species Sex Vehicle Cis:trans ratio (mg/kg) Reference
Rat M,F Dimethylsulphoxide cis only 160-300 Brown, 1979a
M,F Dimethylsulphoxide trans only >2000 Brown, 1979b
F Corn oil cis:trans 90:10 367 Jaggers, 1979
F Corn oil cis:trans 40:60 891 Jaggers, 1979
Cypermethrin was administered dermally to rats by a single application
(5000 mg/kg) or 5 consecutive daily doses (2500 or 5000 mg/kg/day).
Low mortality was observed in the multiple dose groups (20-30%).
Toxic signs of poisoning were similar to those described below.
Axonal lesions of the sciatic nerve were noted in microscopic
examination (Okuno et al., 1976).
The toxic signs noted following acute poisoning with cypermethrin were
similar in most mammalian species. In rats this consisted of
sedation, ataxia, a splayed or ataxic gait and occasional tremors or
convulsions. The onset of toxic signs of poisoning were rapid and
disappeared within several days in survivors. In dogs, signs of acute
intoxication included nervousness, inappetance, diarrhea, vomiting,
tremors and exaggerated ataxia while walking were noted at extremely
high levels. A single application of undiluted cypermethrin to rabbit
eyes produced a mild transient conjunctivitis lasting two days.
Groups of ten male and ten female guinea pigs were used to assess the
skin sensitizing potential of cypermethrin. Two guinea pigs out of
the 20 exposed to cypermethrin showed a positive skin reaction
indicating that cypermethrin may have a weak skin-sensitizing
potential. Groups of four male and four female rabbits were
administered cypermethrin dermally in a 24-hour skin test. A single
application of cypermethrin was observed to be a moderate irritant to
rabbit skin (Coombs et al., 1976).
SHORT TERM STUDIES
Rat
Groups of rats (20 male and 20 female rats per group) were fed
cypermethrin in the diet at concentrations of 0, 75, 150 and 1500 ppm
for 90 days. Cypermethrin used in the study was a 44:56
(cis:trans-ratio) with a purity of 92%. Random samples were
chemically analysed periodically during the course of the study. At
the conclusion of the study, four animals of each sex were maintained
on control diets for a one-week recovery period.
There was no mortality over the course of the study although both
males and females at 1500 ppm had reduced body weight and food
consumption in the first month of the study. After the first month,
the growth rate was similar to that of controls although the animals
never achieved the weight noted in the other groups. At the
conclusion of the study, hematology, clinical chemistry, urinalysis
and histopathology were performed on all animals. With the exception
of a slight increase in M/E ratio in the bone marrow of female rats
fed 1500 ppm in the diet, there were no hematological or urinalysis
effects. An increased microsomal (smooth endoplasmic reticulum)
oxidative activity was noted as an increased hepatic aminopyrine
demethylase activity in both males and females at 1500 ppm and in
males at 150 ppm. These adaptive changes in the subcellular portions
of the liver were substantially reversed within the four week recovery
period. Increased liver weight was not noted on gross examination at
the conclusion of the study. A slight reduction in pituitary weight
of males, while statistically significant, was not dose-related. A
slight decrease in female kidney weight was dose-dependent and
significantly different from control values at the highest level. The
decrease in kidney weight in males was slight and not significantly
different from controls in any of the dietary levels. Gross and
microscopic (including electron microscopic) examinations of tissues
and organs showed no significant differences from the observations
noted in controls. Examination of the sciatic nerve from animals in
the control and 1500 ppm dietary group showed no changes which could
be directly attributable to the presence of cypermethrin in the diet.
Seven of the sixteen males fed 1500 ppm and two of twelve male
controls showed slight changes in myelin which may or may not have
been brought about by histological fixation of the material prior to
examination. The condition of the sciatic nerve of females was
similar to that noted in control females. There were no effects noted
in unmyelinated axons in both males and females. Interim histological
examination at 30 days, during which time clinical signs of poisoning
were noted, was not performed in this study (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats/group, 14 of each sex were
used as controls) were fed trans-isomer for five weeks at dietary
concentrations of 0, 30, 100, 300, 1000 and 3000 ppm. There was no
mortality observed over the course of the study. Food intake and
growth were reduced in the high dosage group. Alkaline phosphatase
activity was increased at the two highest dose levels. Minimal
changes were observed at the two highest dose levels including several
hematological parameters (red blood cell count and hemoglobin
concentration). Liver, spleen and kidney weights were increased at
1000 ppm and above with the spleen and at the 3000 ppm level with the
kidney and liver. Gross and microscopic histopathologic examination
of several tissues and organs revealed no cypermethrin-related
injuries. Special reference was given to the examination of the
sciatic nerve which showed no damage (Hend and Butterworth, 1977a).
Groups of rats (6 male and 6 female rats/group, 10 of each sex were
used as controls) were fed dietary concentrations of cis-isomer at
concentrations of 0, 30, 100, 300, 750 and 1500 ppm for five weeks
following a protocol similar to that described above. Mortality was
observed at the 1500 ppm dose occurring at intervals from 4 to 17 days
from the start of the experiment with all animals displaying
neurotoxic signs of poisoning. At the next lower dose group (750 ppm)
almost all of the animals showed gross neurotoxic signs of poisoning,
abnormal sensitivity to sound and touch and slight cases of ataxia.
There was no compound-related mortality at dose levels of 750 ppm or
below. Growth was reduced at the 750 ppm over the course of the
study. Significant reductions in food intake were also noted at 300
ppm and above at the initial phase of the study. Gross examination of
tissues and organs again revealed a reduction in heart, liver, spleen
and kidney weights. Adjustment of the data for terminal body weight
revealed a statistically significant change in kidney weight at 300
ppm and above while liver weight was increased at 750 ppm. Plasma
protein levels were decreased and urea and potassium concentrations
were increased in male rats at 750 ppm. Gross and microscopic
examinations of tissues and organs showed substantial degeneration at
1500 ppm and above in both the liver and sciatic nerve. Liver lesions
consisted of a coagulative necrosis of hepatocytes occurring in both
males and females. The sciatic nerve from both sexes showed swelling
and breaks in axons with concomitant myelin degeneration and
vacuolation. There were no lesions observed in the brain or the
spinal cord (Hend and Butterworth, 1977b).
Groups of rats (12 male and 12 female rats/group, 24 of each sex were
controls) were fed cypermethrin in the diet at dosage levels of 0, 25,
100, 400, and 1600 ppm for 13 weeks. After two weeks exposure to 1600
ppm, both males and females exhibited hypersensitivity and varying
signs of ataxia. Mortality was observed with male rats up to the
fifth week of feeding after which the surviving animals improved
clinically and appeared normal at the end of the study. Reductions in
body weight, growth and food intake were also noted at the 1600 ppm
dose level. At the conclusion of the study, small increases in plasma
urea were noted in both sexes. Males had a slight increase in plasma
potassium while females had a slight increase in alkaline phosphatase
and plasma protein levels at the 1600 ppm dosage group. Hematological
abnormalities noted at 1600 ppm at the end of 13 weeks included a
reduction of hemoglobin, packed cell volume and red blood cell count
in females and a reduction in kaolin-cephalin clotting time in males.
There were no effects at dose levels below 1600 ppm.
Gross and microscopic pathology performed at the conclusion of the
study showed axonal breaks and vacuolation in the sciatic nerve of
animals fed 1600 ppm in the diet. This was especially noted with
those animals that showed clinical signs of ataxia and died during the
course of the study. At the conclusion of the 13-week trial, sciatic
nerve lesions were not noted in any surviving animals. An increased
kidney weight in males fed 400 ppm was observed which was not
associated with any clinical or pathological signs of abnormality
(Hend and Butterworth, 1976).
Dogs
Groups of beagle dogs (4 male and 4 female dogs/group) were fed
cypermethrin (1:1 cis:trans-isomeric mixture) in the diet at
concentrations of 0, 5, 50, 500 and 1500 ppm for 13 weeks. There was
no mortality over the course of the study. However, there were
significant signs of poisoning observed at the 1500 ppm level which
included diarrhea, anorexia and tremors as well as ataxia,
incoordination and hyperesthesia. On account of these clinical signs,
two male and two female dogs at the high dose level had to be
sacrificed before the end of the experiment. Minor variations were
observed at various hematological parameters with the kaolin-cephalin
clotting time observed to be consistently lower throughout the study
in female dogs at 500 ppm. There were no other significant
differences with respect to standard hematological or clinical
chemistry parameters. Gross examination of kidneys and organs showed
no effect on organ weights attributable to the diet. Microscopic
examination of tissues and organs revealed a nonspecific focal
bronchopneumonia in the lungs of the animals surviving 1500 ppm.
There were no compound-related changes and no histological
abnormalities in other tissues examined with the exception of a
variation in the intensity of the pink colour of the optic disc noted
on ophthalmologic examination at the conclusion of the study.
Because of the pink colouration observed in the optic disc, a further
experiment was undertaken to determine the cause of this occurrence.
Cypermethrin was fed to two groups of three male dogs at
concentrations of 0 and 500 ppm for 13 weeks. Specific ophthalmologic
examination was made to evaluate the degree of colouration of the
optic disc. At the end of 13 weeks, there were no consistent
differences between the colour of the optic discs of the treated dogs
and controls. A no-effect level of 50 ppm was observed in this
short-term study (Buckwell and Butterworth, 1977).
LONG-TERM STUDIES
Rat
Groups of rats (48 male and 48 female rats/group, 96 of each sex were
used as controls) were maintained under SPF conditions and fed 1:1
cis:trans in the diet for 2 years at dosage levels of 0, 1, 10, 100
and 1000 ppm. Chemical analyses were made confirming stability of
cypermethrin in the diet over the test interval.
There was no mortality over the course of the study attributable to
cypermethrin in the diet. General health and behaviour of the
cypermethrin-fed animals was not different from that of the control
animals. Body weight and food consumption data showed a reduction in
dietary intake and growth in both males and females in those groups
fed 1000 ppm in the diet.
Gross morphological changes were noted periodically over the course of
the study. At six months, males fed 1000 ppm had a slightly reduced
testes weight which was not seen thereafter. Slight changes in the
gross kidney weight at periodic intervals in the study were not
accompanied by notable changes in clinical measurements or on
microscopic examinations. These changes in kidney weight were
believed to be unrelated to the presence in the diet. Liver weight
increases were noted at 18 months again in those animals maintained at
the high dose level. The only clinical chemistry changes noted over
the course of the study was a slight decrease in the activity level of
alkaline phosphatase in males at the 2 year interval. The decrease in
alkaline phosphatase activity was not dose-related and was not noted
in females. Minor fluctuations were seen in various hematological
parameters over the course of the study but these, too, were not
compound related nor statistically significant. These changes are not
believed to be related to the presence in the diet. Gross and
microscopic examination of tissues and organs periodically over the
course of the study did not suggest adverse somatic effects.
The kidneys of most animals after 12 months showed chronic nephrosis,
histologically characterized as tubular dilatations, interstitial
chronic inflammatory infiltration and glomerular scarring. In the
liver, varying degrees of chronic inflammatory infiltration,
hepatocyte vacuolation and bile duct proliferation were observed.
These changes were characteristic of ageing processes and were not
attributable to cypermethrin. Dermal ulceration was present in a
number of animals and over the course of the study.
A portion of the sciatic nerve from 12 animals fed 1000 ppm for one
year and 12 control animals was histologically examined for signs of
neurotoxicity. There were no differences in the incidence of abnormal
fibres in the control and animals fed for cypermethrin for 12 months
(Trigg et al., 1977). Microscopic examination of the sciatic nerve
from animals sacrificed at the conclusion of the study showed a small
number of nerve fibers exhibiting slight Wallerian degeneration
changes. These degenerative changes increased with age but did not
appear to be dose-related with respect to severity. Thus,
cypermethrin did not induce neuropathy clinically nor did it induce
histological evidence of nerve degeneration.
There did not appear to be a significant increase in the incidence of
tumor formation in either males or females over the course of the
study. A large number of pituitary adenomas were reported
predominantly in females of all dose groups including controls. This
occurrence did not appear to be dose-related as similar events were
recorded in all dose levels. Based on the reduced body weight
observed at 1000 ppm in the diet of both males and females, the
no-effect level is 100 ppm (McAusland et al., 1978).
Observations in Humans
Urine obtained from operators spraying cypermethrin in experimental
trials was analysed for the presence of the chlorinated cyclopropane
carboxylic acid metabolite. Using standard gas liquid chromatography,
the carboxylic acid metabolite was observed in urine of exposed
workers at levels up to 0.4 µg/ml (the limit of detection was
estimated to be 0.05 µg/ml) (Baldwin, 1978). Studies were carried out
in the Ivory Coast, Africa where operators sprayed with a hand-held
ULV apparatus either cypermethrin or a control UW formulation. The
cypermethrin sprayers were found to have residues on the exposed parts
of their bodies. Medical and electrophysiological examinations showed
no adverse effects from the exposure. The rate of dermal exposure of
the operators during spraying ranged from 1.5 to 46.1 mg/hour. There
was a reasonable relationship between the total cypermethrin deposited
dermally and the excretion in urine. The levels of the cyclopropane
carboxylic and acid metabolite in the 24 hour urine were between
<0.05 and 0.32 mg. This, together with the finding of 0.6 mg of this
metabolite in 72 hour urine from one man led to the estimation that
approximately 3% of the total dermal dose was absorbed and rapidly
excreted by the operators (Prinsen and van Sittert, 1978).
COMMENTS
Cypermethrin is an insecticidally active synthetic pyrethroid
currently being developed for use in agriculture. The acute toxicity
has been studied in a variety of species and the toxic response in all
species is very similar. Cypermethrin has a moderately acute oral
toxicity. The acute signs of poisoning, in addition to a generalized
nervous system response, include a neurological involvement in rodents
where reversible clinical signs of ataxia was accompanied by
histological and biochemical evidence of peripheral (and possibly
central) nerve disruption. Myelin and axon degeneration in the rat
sciatic nerve was noted following high dose level administration.
There was no information on the relative sensitivity of humans to the
nervous system disruption noted in rodent species (see Report Section
3.5).
Cypermethrin is readily absorbed, distributed, and metabolised in
mammals. Because of the chemical and especially the isomeric
complexity of the molecule, the metabolic profile with respect to all
of its isomers is extremely complex. Cypermethrin is readily cleaved
at the ester linkage and subjected to oxidative degradation and
conjugation of the metabolic products. Elimination from the body
following acute and subacute administration is rapid. However, the
clearance rate from adipose tissue is slow and a half-life in rats and
mice may range from 10 to 30 days. The data suggested a potential for
bioaccumulation in the body following continuous exposure.
In a wide variety of studies, there was no carcinogenic or mutagenic
potential as evidenced by short-term bioassays or a long-term chronic
study. Reproduction was unaffected and no signs of teratogenic
potential were noted in two species examined. Two dominant lethal
tests were performed, and while data were basically negative, these
studies did not give full assurance of the lack of a dominant lethal
effect on male reproduction. In short-term tests, only younger
animals were susceptible to the inductive effects of cypermethrin on
liver metabolizing enzymes.
The extensive toxicological data base already in existence lent
assurance to the estimation of an ADI for man. The lack of data on
tissue residue storage and release and on the limited human experience
with cypermethrin were instrumental in making this temporary, pending
further information.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 100 ppm in the diet equivalent to 5.0 mg/kg body weight
Dog: 50 ppm in the diet equivalent to 1.25 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.006 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cypermethrin is a recently introduced insecticide recommended as a
foliar spray for the control of a wide range of insect pests. In line
with other members of the pyrethroid group of compounds, it is used at
lower rates than most other types of chemical insecticides.
Application rates seldom exceed 250 g/ha. In most instances a
considerably lower rate is required. Several applications, up to two
per month and up to 7 days before harvesting, may be made during a
growing season.
Current recommendations for use on various food crops are summarised
in Table 3.
Table 3. Current dosage rates for use on various crops
Recommendations
Crop (in active ingredients)
Alfalfa 25 g/ha
Apples 0.003%-0.0125%
Brassica leafy vegetables 30-200 g/ha
Cereals (barley, wheat) 25-200 g/ha
Cherries 0.0075%-0.0125%
Citrus 0.0025%-0.01%
Cotton 50 - 100 g/ha
Grapes 30-50 g/ha
Kiwifruit 0.01%
Legume vegetables 25-200 g/ha
Lettuce 50-150 g/ha
Maize 25-200 g/ha
Pasture 100 g/ha
Peaches 0.002%-0.01%
Pears 0.002%-0.0125%
Plums 0.05%-0.0125%
Potatoes 20-150 g/ha
Rape 25-75 g/ha
Raspberries 0.0075%-0.015%
Soya 50-250 g/ha
Strawberries 0.0075%-0.015%
Sugar beet 25-125 g/ha
Tomatoes 40-150 g/ha
Post-harvest uses
Although cypermethrin may be introduced to control pests of stored
commodities and for animal health purposes, no data were available for
evaluation at this meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials carried out on a
world-wide basis and on the main crops treated. Some data from
excessive treatments rates are included where they provide useful
information, e.g. on the effect of processing. Treatments were
generally made using E.C. formulations which are representative of
current application practice.
MAIN CROPS
Apples
Residue data have been obtained from trials in several countries. In
these experiments, dosages covered recommended and higher rates and
were in the range of 0.0025% - 0.02% (25-200 g/ha), based on a spray
volume of 1000 l/ha. Treatments were made on up to 12 occasions.
Results and details of the trials are summarised in Table 4.
Residues in whole fruit one week after treatment at recommended rates
up to 0.02% were usually below 1 mg/kg except when six or more
applications were made. Apart from one instance, however, these were
all less than 1.5 mg/kg. In the one case, where 2.7 mg/kg was
reported, the result was considered to be atypical.
Examination of fruit pulp and peel separately showed that residues
were located mainly in the peel, the average weight of which was
10-18% of the whole fruit. After peeling, levels in 78 samples were
generally less than 10% of those in whole fruit.
Pears
Samples of fruit were analyzed from trials carried out in Europe,
Canada, Australia and South Africa. In these experiments, treatment
rates were between 0.0008% and 0.04% (or 30-400 g/ha). Between one
and six treatments were made and fruit harvested at various intervals
after treatment. The results obtained were similar to those with
apples. Residues in whole fruit, one week after treatment at rates up
to 0.02% (slightly above the recommended rate of 0.015%), were all
below 1 mg/kg. As with apples, analysis of peeled fruit showed that
cypermethrin residues were mainly in the peel. In 32 samples,
residues in the pulp did not exceed 30% of those in whole fruit.
Table 4. Residues Following Supervised Trials with Apples (1976-1978)
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Granny Smith Australia 3 0.02% (to
run off) 382 0.95 0.45 0.45 1
MacIntosh Canada 3 0.18 2 0.57 0.38 2
Lobo 3 0.18 2 0.47 0.32
Northern spy 8 0.07 9-15
days 0.13-0.3 3
MacIntosh 8 0.07 9-15
days 0.1-0.251
Northern Spy 8 0.05 " 0.2-0.26
MacIntosh 8 0.15 " 0.2-2.25
Richared France 1 0.15 0.25 0.19 0.6 0.25 4
Golden
Delicious 7 0.05 2-3 0.05 5
Golden
Delicious FRG 6 0.2 2,3,2,3 3.1 1.0 0.6 0.3 0.5 6
and 1 3.2 2.7 1.9 1.7 0.81
James Grieve 6 0.02% 1,1,1´
4 & 1´ 1.3 0.96 0.82 0.8 0.67 7
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Cox's Orange 6 0.02% 1,3,4
4 & 2 0.97 0.75 0.6 0.84 0.31
Golden
Delicious 6 0.02% 1,6,6,
2 & 2 1.7 1.3 0.93 0.9 0.9
6 0.1 1-3 1.2 1.1 1.0 0.9 0.8 8
6 0.1 1-3 0.8 0.7 0.7 0.95 0.5
Italy 1 0.037 - 0.3-0.5 11
Strumer N. Zealand 12 0.008% 2-3 1.1 0.6 0.7 0.53 12
Reinette Portugal 5 0.005% 3 0.19 0.2 0.14 13
5 0.0075% 3 0.32 0.1
5 0.01 3 0.6 0.35 0.45
Granny Smith S. Africa 1 0.005% - 0.42 0.36 0.28 0.25 14
Spain 1 0.024 - 0.06 0.05 0.04 10
1 0.048 - 0.1 0.1 0.05 0.06 10
U.K. 3 0.005% 1-2 0.29 0.45 0.27
3 0.01% 1-2 0.99 0.91 0.52
3 0.005% 1´ 0.6 0.81 0.64
3 0.01 1´ 1.1 0.98 0.82
1 0.01 - 1.3 1.2 0.83
Worcester
Pearmain U.K. 2 0.015% 0.01 15
Table 4. Continued...
Application Interval
between Ref.
Varieties Country No. rate Appl.s Residues in mg/kg, at intervals (days) after application (Shell)
kg a.i./ha (week)
or % 0 7 12-17 21 28-30 35-42 56
Chivers
Delight 2 0.0025% 4´ 0.02 16
0.005% 4´ 0.05
2 0.01% 6´ 0.01
Laxton Superb 2 0.01% 6´ 0.01
Spartan 1 0.012% - 0.36 0.15 0.07 0.05 17
0.01 - 0.36 0.12 0.03 <0.01
Lobo Sweden 3 2´-4 0.1422 Sweden
Ribston 3 2´-4 0.25
1 Minimum-maximum residues in three samples.
2 90 days after treatment.
Peaches
Residues in whole fruit, less stone, from trees sprayed with
cypermethrin at recommended rate (0.005%-0.01%) and harvested one week
after treatment were generally 1 mg/kg or less although in one trial
in Germany a residue of 1.8 mg/kg was reported in fruit under these
conditions. Analysing 43 samples of fruit after peeling and removal
of the stone showed that levels in the pulp were less than 10% of
levels found in whole fruit.
Details of the trials are given in Table 5, which contains data
relating to some treatments at rates higher than recommended.
Citrus
Experiments were carried out in South Africa (oranges), Spain (lemons)
and Italy (oranges) in which applications of cypermethrin at
recommended rates up to 200 g/ha were made on 1 or 2 occasions. Fruit
was harvested 1 to 39 weeks after treatment, depending on the trial,
and analysed for residues of cypermethrin. The results are shown in
Table 6.
Residues up to 1.3 mg/kg were found in whole fruit taken within six
weeks of treatment. Analysis of peel and pulp showed that the
residues were largely (90%) percent in the peel and that residues in
the pulp were normally below the limit of determination, although in
one series of experiments residue levels up to 0.16 mg/kg were
reported (4 weeks after treatment at 0.02%). In some experiments
samples of orange juice were obtained from treated fruit but residues
were all less than 0.01 mg/kg.
Cotton
Samples of seed were obtained at harvest from trials in several
countries in which up to 12 applications were made at recommended
rates (up to 150 g/ha) and also at treatment rates up to 240 g/ha. The
intervals between the last treatment and harvest varied between 1 day
and 13 weeks. The data obtained are given in Table 7 which also
summarises the trial details.
The seed from these trials was ginned after harvest and was separated
in the laboratory into kernels and hulls. The latter also contained
adhering linters.
In 8 samples no residue of cypermethrin was found in the kernels
(limit of determination of 0.01 mg/kg). Analysis of hulls, which in
most instances had adhering linters, showed that any residues present
were located entirely on the seed surface and its associated fibre.
Table 5. Residues Following Supervised Trials on Peaches (1976-1978)
Application
rate Intervals Residues in mg/kg, at intervals (days) after application1 (Shell)
Country No. kg a.i./ha /week/ 0-1 6-7 12-14 18-21 28-30 40-43 54 Ref.
or %
Australia 3 0.02% 3 & 2 1.0 33
Canada 3 0.006% 4 & 5 0.07 34
3 0.006% 0.34 0.15 0.06 35
0.1 0.052
France 1 0.005% - <0.02 36
0.0075% <0.02
0.0125% - <0.01
0.0187% <0.01
0.015% 0.5 0.4 0.1 0.15
Federal Republic
of Germany 4 0.02% 2 3.2 1.6 1.1 0.65 38
4 0.01% 3,3 & 2 0.85 0.7 0.5 0.18 39
4 0.01% 3,3 & 2 3.0 1.8 1.6 0.75
5 0.01% 2,3,3 & 2 2.0 1.0 0.85 0.3
Italy 1 0.01% - 0.1 40
1 0.02% 0.5
Portugal 1 0.005% 0.08 0.08 0.08 41
0.0075% 0.19 0.11 0.09
0.01% 0.32 0.21 0.14
Spain 1 0.15 0.15 42
2 0.195 3 0.03
2 0.195 1 0.04
1 Results refer to whole fruit without stone.
2 Average of ten samples taken from the same site (min.-.max. values: 0.02-0.11 mg/kg).
Table 6. Residues of Cypermethrin in Citrus (1976 - 1977)
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
LEMONS 150 g/ha 2 27 7 <0.01 2.1 1.1 43
Verna 14 <0.01 2.0 1.0
21 <0.01 2.1 1.1
(Spain) 58 <0.01 1.1 0.55
JUICE
ORANGES 0.01% 1 - 60 <0.01 0.22 0.14 44
Moro 0.02% 1 - 60 <0.01 0.44 0.21
(Italy)
PULP
ORANGES
Moro 0.005% 1 - 67 0.02 0.91 0.30 45
0.01% 1 - 67 0.02 1.2 0.39
0.005 1 - 91 0.01 0.69 0.19
0.01% 1 - 91 0.02 1.3 0.35
0.01% 1 - 27 0.06 1.0 0.36 46
43 0.05 1.5 0.48
56 0.05 1.3 0.40
0.02% 1 - 27 0.16 3.0 1.2
43 0.13 3.7 1.3
56 0.09 1.9 0.73
JUICE
0.005% 1 - 1 <0.01 0.50 0.13 47
4 <0.01 1.2 0.20
8 <0.01 1.0 0.16
18 <0.01 0.81 0.21
39 <0.01 1.1 0.28
68 <0.01 1.3 0.39
(S. Africa) 96 <0.01 1.3 0.38
Table 6. Continued...
Interval Cypermethrin
Crop Dose Rate No. of between Residue (mg/kg) Ref.
(Country) (a.i.) Applications applications Intervals WHOLE (Shell)
(days) (days) PULP PEEL FRUIT
ORANGES 0.01% 1 - 1 <0.01 1.0 0.30 47
Moro 4 <0.01 0.55 0.22
8 <0.01 0.19 0.16
18 <0.01 0.83 0.25
39 <0.01 0.60 0.16
68 <0.01 0.45 0.13
96 <0.01 0.45 0.13
0.015% 1 - 1 <0.01 2.6 0.58
4 <0.01 1.2 0.30
8 <0.01 1.0 0.24
18 <0.01 1.3 0.25
39 <0.01 1.2 0.21
68 <0.01 1.8 0.40
96 <0.01 0.95 0.22
0.01% 1 - 0 <0.01 1.6 0.52
1 <0.01 2.9 0.85
2 <0.01 2.2 0.65
4 <0.01 2.8 0.55
9 <0.01 1.8 0.58
16 <0.01 1.9 0.72
0.005% 2 6-weeks 69 <0.01 <0.01 <0.01
114 <0.01 <0.01 <0.01
237 <0.01 <0.01 <0.01
0.01% 1 - 110 <0.01 <0.01 <0.01
155 <0.01 <0.01 <0.01
(S. Africa) 278 <0.01 <0.01 <0.01
Table 7. Residues Resulting from Supervised Trials in Cotton Seed (1975-1977)
Application Residues in whole seed (mg/kg) at Intervals (days) after app. lication Ref.
Country rate kg Intervals (Shell)
No. a.i./ha or /Week) 1 7-8 13-15 19-20 28-37 44-55 70-91
Australia 1 0.0015% - 0.01 0.01 10
1 0.003% 0.05 0.01
Brazil 4 0.12 1 0.01 51
4 0.24 1 0.01
4 0.12 1 <0.01 52
0.24 1 0.01
Columbia 1 0.1 0.09 0.03 0.01 54
5 0.1 3 to 8 days <0.01 55
2 0.075 12 days <0.02 56
South Africa 12 0.2 1 to 2 0.01 58
11 0.2 1 to 2 0.02
1 0.1 - <0.01 10
0.2 <0.01
Spain 5 0.1 2,2, 1´ & 2´ 0.04 59
4 0.1 2,2 & 1´ 0.03
5 0.2 2,2, 1´ & 2´ 0.03
4 0.2 2,2,1´ 0.06
3 0.1 2 0.03
3 0.2 2 0.04
3 0.2 0.02
The presence of occasional small residues on the fibre and the outside
of the seed case is to be expected, particularly where applications
were made after the bolls had opened. However, the results indicate
that migration through the seed case to the kernel did not occur even
after treatment at excessive rates, and that measurable residues in
kernels are unlikely to be found in practice.
As a whole, the data suggest that, following treatment according to
recommendations, seed taken 1 week after a series of applications is
unlikely to contain more than 0.1 mg/kg cypermethrin on the whole
seed.
Leafy Vegetables (Brassicae)
Trials have been carried out in a number of countries in order to
obtain residue data on cabbages - including savoy, broccoli, Brussel
sprouts, cauliflower and kale. These are described in Table 8.
The treatments were made according to recommendations (up to 200 g
a.i./ha or 0.02%) or at slightly higher dose rates. In most cabbage
samples as expected, the residue was located mainly in the outer
leaves, which are normally removed in the field, and the majority of
the samples examined were received without the "discard" leaves. In
one experiment however (New Zealand) whole unstripped cabbages were
received. Residues in these samples reaches 3.2 mg/kg but were found
to be reduced to below 0.1 mg/kg on removal of the outer leaves in the
laboratory.
With most of the other brassicae examined, including cauliflower
heads, residue levels were similar to those in cabbage without discard
leaves. But residues in kale from experiments in Germany tended to be
somewhat higher.
OTHER CROPS
Supervised trials were carried out with a number of other crops in
various locations. Residue data obtained in these experiments are
summarized in Table 9 and the following comments.
Alfalfa
A single application at a rate equivalent to 0.1 kg a.i/ha was used to
establish a residue decay curve.
Beans
Trials were carried out in South America on soya and on phaseolus
bean. Residues in beans (soya and phaseolus) removed from their pods
were below measurable levels (0.01 mg/kg) four weeks after treatment,
even where excessive rates (up to 300 g/ha) were used.
Table 8. Residues Resulting from Supervised Trials with Various Vegetables (1976/79)
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha (week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
BROCCOLI 3 0.16 3 & 2 0.38 0.28 0.16 0.06 69
1 0.0015 - 0.11 0.03 <0.01 10
(Canada) 1 0.003% - 0.3 0.03 <0.01
BRUSSELS SPROUTS 3 0.15 1,5 0.55 0.25 0.25 70
7 0.07 3,3,2,2 0.14 0.1 71
2 & 3 0.12 0.09
0.23 0.13
0.18 0.18
6 0.07 3,3 & 2 0.06
0.09
0.1
(Canada) 0.08
CABBAGES 3 0.07 1,5 0.22 0.11 0.11 60
0.22 0.12 0.06
0.16 0.27 0.17
0.22 0.27 0.05
3 0.15 1,5 0.43 0.03 <0.01 61
(Canada) 3 0.16 2 0.15 0.1 0.02 <0.01 62
CABBAGES 4 0.06 2 1 0.4 0.11 <0.01 <0.01 63
(Germany) 4 0.06 2 0.1 0.02 <0.01 <0.01 <0.01
CABBAGES 1 0.25 0.161a Hungary
0.1
(Hungary) 0.2
0.2
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
CABBAGES 5 0.002% 2 0.03 <0.01 0.01
5 0.006 2 0.05 0.02 0.02 66
1 0.002% - 0.07 0.02 0.02
(South Africa) 1 0.006% - 0.08 0.03 0.02
CABBAGES 6 0.14 2 2,11 1,71 1.41 67
0.061b 0.011b 0.011b
6 0.14 0.76 0.6 0.57 0.47 N.Z.
(New Zealand) 6 0.28 1.13 0.82 0.68 0.42
CABBAGES 1 0.008% - 0.51 68
(U.K.)
CABBAGES
(Savoy) 4 0.06 1,1 & 2 0.55 0.4 0.35 0.15 <0.01 64
(Germany) 4 0.06 2 0.3 0.2 0.1 0.05 <0.01
1 0.005% 0.02 65
1 )0.015% 0.02
(Italy) 1 0.03% 0.02
CAULIFLOWERS 3 0.16 3 & 2 0.17 0.09 0.01 <0.01 72
3 0.07 1,5 0.23 0.21 <0.01 73
0.26 0.30 0.01
0.34 0.24 0.08
(Canada) 0.3 0.08 0.11
4 0.06 2 0.03 <0.01 <0.01 <0.01
(Germany) 3 0.06 2 0.05 <0.01 <0.01 <0.01 <0.01
KALE 4 0.06 2 1,6 0.85 0.4 0.3 0.25 75
(Germany) 4 0.06 2 1,4 0.9 0.7 0.5
2 0.075+0.04 6.5 1.1 0.76 0.47 0.13 76
(U.K.) 2 0.225+0.08 6.5 1.5 0.95 0.60 0.23
Table 8. Continued...
Crop Application Intervals
(Country) rate between appl.s Residues in mg/kg, at intervals/days after application Ref.
No. kg a.i./ha /week/ 0-1 3-4 7-8 10 14-16 21 28 (Shell)
KALE
(China) 6 0.005% 4-5 days 0.85
(Thailand)
NOTE: The cabbages were analysed without the outer leaves with some exceptions:
1 whole cabbage
1a whole cabbages as specified by CC/PR/153/
1b heart only.
Cherries and Plums
Samples of plums and cherries have been obtained from field
experiments in Germany in which cypermethrin was applied on several
occasions (three in the case of cherries, five for plums) at
approximately the recommended rate and double the recommended rate
(0.01% and 0.02% respectively). Under similar conditions, residues in
plums were found to be somewhat lower than in cherries.
Grapes
At recommended rates of up to 80 g/ha and treatments on up to 4
occasions, residues in grapes harvested one week after the last
application were at or below 1 mg/kg (Table 9). In practice, the
material is generally applied an a prophylactic treatment at 50 g/ha
and harvest is likely to be more than one week after treatment.
Lettuce
Residue data are available from trials carried out in Canada, France
and Germany at rates generally in the range of 50-70 g/ha (0.005% to
0.007%, based on 1000 l/ha spray volume). Residue levels were 1.6
mg/kg cypermethrin or less in lettuce taken one week after treatment.
From the glasshouse data available, there was no evidence that levels
in glasshouse lettuce were substantially different from levels in
outdoor crops.
Kiwi Fruit
Supervised trials were carried out in New Zealand at the recommended
dose rate and at double rate (Table 9). The pulp contained 5-10% of
that which was found in whole fruit.
Maize
Residue experiments have been carried out in several countries in
which cypermethrin has been applied to maize at recommended rates up
to 150 g/ha. Green maize (whole plants) were sampled at intervals
following treatment, and in some instances grain was also sampled at
the sweet corn stage or when fully mature.
In green maize following treatments at rates up to 150 g/ha, residues
were below 1 mg/kg two weeks after treatment and below the limit of
determination (0.01 mg/kg) after eight weeks. In grain, no residues
were found in either sweet corn or mature grain, even in samples taken
three days after treatment at this rate.
Pasture
Supervised trials were carried out in New Zealand in which
cypermethrin was applied at several times higher than the recommended
rate. The residues on the grass increased roughly in proportion to
the increases in dosage (Table 8).
Peas
Trials were carried out on peas in the U.K., Hungary and South Africa.
No residues were found at dose rates of 15 to 100 g/ha in the peas
themselves which were sampled between one day and four weeks after
treatment (limit of determination 0.01 mg/kg). However, an excessive
dose rate of 200 g/ha resulted in residues between 0.04-0.07 mg/kg in
the peas (Table 9).
Potatoes
Samples were obtained from residue trials carried out in Europe,
Cyprus and Canada, with from 1-9 treatments at dosage rates in the
range of 10 g/ha to 200 g/ha, and intervals between last treatment and
harvest from 0-8 weeks. Detectable residues were absent from all
samples of whole or peeled tubers, even 3-9 days after treatment.
However, samples from one trial in Italy, obtained three weeks after
treatment were peeled. Residues up to 0.05 mg/kg were found in the
peel, but none was detectable in the peeled potatoes.
Rape
Data from supervised trials in Canada, Germany and the U.K. show that,
where treatments at recommended rates were made more than six weeks
before harvest, residues in the whole seed were below the limit of
determination of 0.01 mg/kg. In one of the replicated Canadian
experiments, however, residues were found in samples taken six weeks
after treatment; residues between replicates varied from 0.01 mg/kg to
0.12 mg/kg.
Raspberries and Strawberries
Trials were carried out in Canada and the U.K. in which raspberry
canes were treated with cypermethrin at rates between 0.005% and
0.015% (the maximum recommended rate). Residues in fruit taken 12 or
more days after treatment were all below 0.5 mg/kg even where multiple
applications were made. Residues in strawberries from Canadian
experiments using cypermethrin at rates of 0.006% or 0.01% on two
occasions at an interval of 8 or 9 days were also below 0.5 mg/kg when
harvested three weeks after treatment (Table 9).
Sugar Beet
In experiments carried out in France, Germany and the U.K., 1 to 5
treatments were carried out at rates between 60 g/ha and 200 g/ha
cypermethrin. Samples of both roots and tops were taken for analysis
at intervals up to 17 weeks from the time of treatment.
Apart from one sample taken two weeks after treatment at 60 g/ha,
which contained a small residue of 0.02 mg/kg, no residues of
cypermethrin were found in the root samples.
In leaves, residues were between 6 and 13 mg/kg immediately after the
last of 5 applications made at two weekly intervals. Subsequently
these declined, reaching 0.02-0.13 mg/kg six weeks after treatment.
After 11 weeks residues were 0.01 mg/kg or less (Table 9).
Tomatoes
In outdoor experiments carried out in Southern Europe, Australia,
Canada, S. Africa, the Canary Islands and Mexico, tomatoes were given
foliar treatments at rates between 50 g/ha and 300 g/ha. Applications
were made on up to 9 occasions at intervals from 5 days to 3 weeks
depending on the experiment. Samples were taken at intervals ranging
from the day of treatment to six weeks later. Although some of the
tomatoes received were green, they were close to ripening
(approximately 5 cm diameter) and in the condition at which they would
be picked for export. The residues (Table 9) did not exceed 0.5 mg/kg
in any of the samples taken shortly after treatment at the recommended
rates (up to 0.0% or 100 g/ha assuming use at 1000 l/ha).
Wheat
Residue trials have been carried out on wheat in Canada and Brazil,
with application rates up to 150 g/ha. In the grain residues did not
exceed 0.1 mg/kg at harvest two weeks after application. Residues in
straw were higher, and the few data obtained suggest that under these
conditions they may approach 10 mg/kg (Table 9).
Table 9. Residues Resulting from Supervised Trials with Various Fruits and Other Crops (1974/79)
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
ALFALFA Cyprus 1 0.1 12 5.6 4.7 10
BEANS
(Dried) Colombia 3 0.15 1 <0.011 87
CHERRIES Germany 3 0.02% 1-2 4.5 3.3 0.65 0.6 18
to run off
3 0.02% 1-2 2.7 1.6 1.0 0.39
to run off
3 0.02%
to run off 1-2 7.4 5.4 1.5 1.2
3 0.01% 1.5-2 0.59 0.47 0.27 0.25 0.2 19
3 1.5-2 1.0 0.6 0.3 0.1 0.1
GRAPES Canada
(Delaware) 1 0.08 - 0.7 0.32 20
21
(Elvira) 4 0.07 4,5 & 3 0.24
(0.1 to
0.37)
(Friedamia) 1 0.006%
to run off 0.75 0.41 0.25
(Sauvignon) France 4 0.075 1 0.08 0.03 0.02 <0.01 22
(Alicante)
(Bouschet) 1 0.075 - 0.4 0.28 0.23 0.12 23
2 0.075 3 0.37
(Riesling) Germany 4 0.08 5,4 & 2 0.5 0.2 0.11 0.09 0.05 24
4 0.08 5,4 & 2 0.8 0.5 0.1 0.05 0.05
(Barbera) Italy 1 0.01% 0.1 25
S. Africa 1 0.0375 - 0.2 0.1 0.18 <0.05 <0.05 S. Africa
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 (Shell)
GRAPES
(Waltham
Cross) 2 0.0375 2 0.24 0.2 0.19 0.07 <0.05
(Chemin
Blane) 1 0.0375 - 0.1 0.12 <0.05 <0.05
2 0.0375 2 0.12 0.07 <0.05 <0.05 <0.05
KIWI FRUIT N. Zealand 6 0.01% 2.2 1.9 1.7 1.3 N.Zealand
6 0.02% 6.2 3.9 3.4 2.8
LETTUCE Canada 6 0.07 1 <0.012 78
0.02
France 1 0.15 0.52 79
0.3 1.1
France 1 0.05 1.2 0.27 0.123 80
1 0.05 1.1 0.3 0.06 81
1 0.05 2.2 1.6 0.35
1 0.05 1.8 0.5 0.02
1 0.054 2.4 1.0 0.85 82
Germany 3 0.06 2 4.0 0.25 0.04 0.02 <0.01 83
3 0.06 2 1.2 0.06 0.01 <0.01
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
MAIZE
Cobs Australia 1 0.0015% <0.01 10
Cobs 0.003% <0.01
Sweet corn Canada 5 0.07 5 days <0.01 84
Sweet corn 3 0.075 1 <0.01 <0.01
Sweet corn 6 0.064 5 days <0.01 <0.01 <0.01 85
Germany 1 0.1 1.9 0.04 86
1 0.1 2.7 2.2 0.51 0.05 <0.015
1 0.1 2.9 1.1 0.15 0.05 0.025
<0.01
Leaves S. Africa 1 0.075 5.2 3.86 0.4 10
Leaves 1 0.15 17 106 2.6
Stalks 1 0.075 <0.01 0.086 <0.01
Stalks 1 0.15 0.05 0.02 0.06
PEAS U.K. 2 0.05 2 <0.01 88
w/o pods 1 0.025 - <0.01 89
Peas Hungary 1 0.1 - <0.017
pods (only) - 0.07
Peas 1 0.2 0.04
0.077
Sweden 2 0.08 2 <0.01 165
S. Africa 1 0.015 - <0.01 <0.016 <0.01 10
1 0.03 - <0.01 <0.016 <0.01
PLUMS Germany 5 0.02% 1-3 1.0 0.65 0.5 90
5 0.02% 1-3 0.9 0.75 0.4 0.25
5 0.02% 1-3 1.3 1.1 0.7
5 0.01% 3,2,3 & 1 0.3 0.29 0.29 0.25 0.11 91
5 0.01% 3,2,3 & 1 0.24 0.15 0.12 0.1
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
POTATOES Canada 4 0.075 2 <0.01 92
4 0.15 2 <0.01
9 0.072 1 <0.01
9 0.145 1 <0.01
4 0.150 2 <0.017 93
9 0.15 1 <0.01 94
Cyprus 1 0.1 <0.01 <0.01 10
France 1 0.15 <0.01 95
Germany 4 0.090 2 <0.01 <0.01 <0.01 96
4 0.2 2 <0.01 <0.01 <0.01 97
4 0.2 2 <0.01 <0.0l <0.01
4 0.2 2 <0.01 <0.01 <0.01
Italy 1 0.04 - 0.058 98
1 0.12 - 0.038
SOYA BEANS Brazil 2 0.24 2 <0.01 99
3 0.24 2 <0.01
dried Colombia 4 0.3 1,1 week
& 4 days <0.01 100
SUGARBEET France 1 0.1 - <0.01
Leaves after 101
17 wks
1 0.15 <0.01
after 102
17 wks
Germany 5 0.06 )2,2,2 1.3 0.52 0.07 0.05 103
5 0.06 )& 1 2.2 0.1 0.03 <0.01
5 0.2 2 12 4.0 2.3 0.02 104
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
SUGARBEET Germany 5 0.2 2 6.2 2.3 0.18 0.05
Leaves 5 0.2 2 13 0.73 0.23 0.13
U.K. 1 0.2 <0.01
after
15 weeks 105
RASPBERRIES Canada 1 0.225 - 0.2110 114
3 0.18 2 0.4510 115
3 0.135 2 0.410 116
U.K. 1 0.005% 1 0.1311 117
0.015% 1 0.2511
STRAWBERRIES Canada 2 0.23 9 days 0.07 118
2 0.18 90 0.04 119
2 0.18 8 days 0.45 120
TOMATOES Australia 9 O.01% 5-7 days 0.3 0.3 0.24 0.14 106
9 0.02% 5-7 days 0.57 0.57 0.44 0.53
1 0.0015% - 0.02 0.03 10
Canada 1 0.15 - 0.13 0.07 107
2 0.15 1 0.2 0.05
France 1 0.0075% - 0.09 0.06 0.04 108
Italy 1 0.005% - 0.02 109
0.015% 0.08
0.03 0.17
Mexico 1 0.0075 - 0.02 0.01 10
1 0.015 - 0.05 0.04
1 0.03 - 0.08 0.13
Portugal 3 0.075% 11 & 7 0.06 0.032 110
days
Table 9. Continued...
Crop Country Application Interval
(Variety) rate between Residues in mg/kg,at intervals (days) after application
No. kg a.i./ha, appl.s Ref.
or % (week) 0 2-3 6-7 13-17 21 28-30 42-56 (Shell)
TOMATOES S. Africa 4 0.0075% 3,2,2,2 0.17 0.1 0.05 111
4 0.01% 3,2,2,2 0.27 0.15 0.09
4 0 02% 312,2,2 0.27 0.14 0.08
4 0:03% 3,2,2,2 0.29 0.2 0.6
Spain 7 0.03% 3,2 & 2 0.08 112
1 0.075 - 0.16 0.07 0.14 10
0.16 0.06 0.10
2 0.2 1 0.1 0.09 0.06 113
3 0.2 1 0.25 0.2 0.05
Canary Is. 2 0.1 1 0.25 0.15 0.01 113
3 0.1 1 0.2 0.15 0.06 0.03
2 0.15 1 0.4 0.3 0.15
3 0.15 1 0.55 0.4 0.2 0.6
WHEAT Canada 1 0.14 - 0.1 0.07 0.05 121
1 0.15 - 0.08 0.08 0.08 0.04 122
0.07 0.05 0.06 0.03
0.07 0.05 0.05 0.04
0.09 0.09 0.06 0.04
Brazil 1 0.09 <0.01 <0.01 123
2 0.09 12 days 0.04
2 0.09 31 days 0.03
2 0.09 19 days 0.01
3 0.09 19 & 12
days 0.04
1 +4 week storage, beans were analysed without pods
2 In 3 samples
3 10 days after treatment
4 In glasshouse
Table 9. Continued...
5 The grain contained <0.01 mg/kg
6 8 days after treatment
7 In 9 samples
8 In the peel
9 24 days after treatment
10 12-13 days after treatment
11 19 days after treatment
FATE OF RESIDUES
GENERAL OBSERVATIONS
Following applications to crops cypermethrin may degrade to a variety
of hydrolysis and oxidation products. The most likely degradation
products present in crops at harvest following normal agricultural use
of cypermethrin are the derived amide (compound B), 3-phenoxybenzoic
acid and 2-(2',2'-dichloro vinyl)-3,3 dimethyl cyclopropane carboxylic
acid (compound C), the structures of which are shown in Figure 1. The
latter two compounds are found in the free state as well as in
conjugated forms. However, the evidence indicates that the major
component of any residue present at harvest will be cypermethrin
itself. A number of crop samples obtained from supervised trials
discussed previously were analysed also to determine compounds B, C
and phenoxybenzoic acid. The results of these examinations involving
some 20 crops showed no residues of Compound B, of Compound C or of
3-phenoxy benzoic acid in excess of 0.05 mg/kg.
Following use on animal feed crops, residue may be present in feed at
levels depending on the crop. However, since the product is readily
metabolized by animals, these amounts are unlikely to give rise to
more than traces of foods of animal origin.
IN ANIMALS
Cattle Feeding Studies
Studies were undertaken to investigate the fate of cypermethrin in
cattle and whether residues in meat or milk could arise from the use
of cattle feed containing products made from treated crops. Two
experiments were undertaken, both using radiolabelled cypermethrin.
a. Low Dietary Intake (0.2 mg/kg)
Two lactating cows were given feed concentrate containing
radio-labelled cypermethrin twice daily for a three-week period with a
feeding level equivalent to 0.2 mg/kg on total daily feed. The cows
were milked twice daily and the amount of radioactivity determined at
each milking. The total radioactivity present in whole milk was in
the range of 0.0002 mg/1 to 0.0012 mg/1 in terms of cypermethrin
equivalents and 60-70% of the amount of radioactivity in the milk was
present in the cream fraction. The total radioactivity found in the
milk amounted to only 0.5% of the radioactivity fed to the animals.
The remainder of the radioactivity was excreted in the urine (54%) and
faeces (43%). The elimination occurred rapidly after dosing and the
rate of elimination of radioactivity reached its peak (near 100% of
applied dose) after three days.
 In the urine 3(4-hydroxyphenoxy) benzoic acid-O-sulphate and the
glutamic acid conjugate of 3-phenoxybenzoic acid were detected in the
ratio of 1:4. The free acids were not detectable. The faeces
contained the parent compound amounting to approximately 85% of the
faecal 14C, that is, about 36% of the amount ingested. At the end of
the three-week feeding period the animals were slaughtered and samples
of tissues examined for radioactivity. Levels were below 0.001 mg/kg,
in cypermethrin equivalents, in blood, muscle and brain. In
subcutaneous and renal fats, liver and kidney samples, residues were
at or below the equivalent of 0.012 mg/kg cypermethrin (Shell R. 124).
b. High Dietary Intake (5 mg/kg)
Radiolabelled cypermethrin was fed in the feed concentrate twice daily
and was given in amount equivalent to 4 mg/kg on total diet over a
period of 7 days.
The radioactivity in the milk, urine and faeces was monitored
throughout the feeding period and showed that the major excretory
route was via the kidneys. All the cows were found to be pregnant at
necropsy and the milk yields throughout the experiment were poor.
Equilibrium between intake and excretion was reached 3-4 days after
commencement of feeding, after which levels in whole milk ranged
between 0.009 mg/l and 0.013 mg/l cypermethrin equivalent to
radioactivity present. The cream fraction of the milk contained
85-90% of the total radioactivity. Results of experiments carried out
with 14C-benzyl and 14C-cyclopropyl labels indicate that the residue
in milk is an ester and contains both the acidic and alcoholic
functions of the parent compound.
The major urinary metabolites in cows were identified as the glutamic
acid conjugate of 3-phenoxybenzoic acid (68%), 3-phenoxybenzylglycine
(16%), 3-phenoxybenzoic acid (9%). 3(4-hydroxy-phenoxy) enzoic-acid
and its O-sulphate conjugate appeared to be present in only small
amounts (1%).
At the end of the test feeding period the animals were slaughtered and
tissues were taken for measurement, with the following results,
radioactivity being expressed as cypermethrin equivalents.
Muscle 0.04 mg/kg
Fat 0.01-0.10 mg/kg
Liver and Kidney 0.05-0.13 mg/kg
These results indicate that the cypermethrin does not accumulate in
the tissues of the animals. Even at a high intake, residues are
mostly in the fat, liver and kidney. Cereals and components of feed
(e.g. cotton seed) treated with the highest recommended dose rates
therefore, are unlikely to result in measurable residues in meat or
milk of cattle (Shell R. 125).
IN PLANTS
Degradation on cotton, lettuce and apples has been studied using
14C-radiolabelled material with the label in various positions in the
cypermethrin molecule. Structures of compounds mentioned are given in
Figure 1.
a. Cotton
An experiment in which the cis- and trans-isomers of cypermethrin
were applied in acetone/water solution to cotton leaves using a small
syringe was carried out on plants grown in pots in the greenhouse.
Each of the two isomers, which in themselves consisted of a racemic
mixture of two enantiomeric pairs of stereo isomers, was labelled in
the cyclopropyl and benzyl rings in separate experiments. In each
experiment the rate of application was 10-20 µg/g of leaf. Leaf
samples were taken for examination immediately after treatment and
again 42 days later. The samples were extracted with acetone and the
examined by chromatographic and radiochemical techniques.
Forty-two days after treatment 75-85% of the original total applied
radioactivity was still present in cotton leaves and 90% of this was
acetone extractable. Thin-layer chromatography showed that at least
six components were present in the extracts. Products identified and
the approximate proportion present in the extracts were cypermethrin
(40-50%), 4-hydroxy-cypermethrin (compound A/ 5-8%), 3-phenoxybenzoic
acid (15%), and an amide (compound B/ 8-20%). In this preliminary
study unidentified material accounted for the remaining 20-30% and was
mainly polar material. No evidence of an appreciable difference in
the rate of loss and breakdown of the two isomers was obtained.
A second experiment was carried out on a larger scale, in which
cypermethrin was 14C radio-labelled in the benzyl ring and applied at
the rate of 4 µg/g leaf. Two applications were made 15 days apart and
leaf samples were taken 5 weeks after the second treatment. Results
were qualitatively similar to the first experiment, except that
smaller amounts of metabolites were found and, in addition, some
3-(4-hydroxyphenoxy)-benzoic acid was also reported to be present. Due
to the larger scale of the experiment it was possible to examine the
polar fraction in more detail. This was found to consist of at least
8 compounds which were mainly conjugated forms of 3-phenoxybenzoic
acids 3-(4-hydroxyphenoxy)-benzoic acid and 3-phenoxybenzyl alcohol.
When the results from experiments using cypermethrin, separately
labelled in the nitrile, cyclopropyl and benzyl groups, were compared,
the properties of the polar products formed differed according to the
label position. Thus it was again concluded that, following the
primary step of hydrolysis of the ester link, further degradation of
the component parts, including loss of nitrile, oxidation and
conjugation, took place. In this work, the cyclopropane carboxylic
acid (compound C) was identified as a metabolite.
In a third experiment the cotton was grown in boxes outdoors in
Seville, Spain. Cypermethrin, labelled either in the cyclopropane or
benzyl rings, was applied to separate plants. The cis and trans
isomers, in each case, were also applied in separate experiments.
Three applications were made at rates equivalent to 300 g/ha and
samples were taken at harvest 15 weeks after the final treatment.
Seed, fibre, boll cases and leaves were examined separately. All the
kernel samples separated from the seed cases contained small amounts
of radioactivity equivalent to 0.07-0.24 mg/kg cypermethrin. Due to
the oily nature of the extracts and the small size of the samples, it
was not possible to fully identify the products present, although it
was shown the radioactivity was not present in the form of
cypermethrin itself. Seed cases freed from lint also contained
radioactivity equivalent to up to 0.17 mg/kg cypermethrin, which again
was shown not to be parent insecticide.
The lint samples contained rather more variable amounts, between 0.08
and 0.62 mg/kg cypermethrin equivalents, probably depending on the
degree of opening of the bolls at the time of spraying. Cypermethrin
itself was the major compound present (between 50 and 90% of the
total) but the remainder consisted of polar products. Foliage and
boll cases were found to contain the same products as those reported
from glasshouse experiments. These included cypermethrin,
3-phenoxybenzoic acid in free form, the cyclopropane carboxylic acid
(free) and the amide, Compound A. The latter in particular was
present, however, in considerably smaller amounts than found in the
glasshouse experiment.
Polar materials were also found in appreciable amounts. These could
be hydrolysed by acid to give 3-phenoxybenzoic acid, 3-phenoxybenzyl
alcohol and 3-phenoxybenzaldehyde. Unidentified materials in foliage
amounted to only a small proportion of the total radio-label present.
In another experiment, abscised cotton leaves were placed with their
stems immersed in an aqueous solution containing ring
14C-radiolabelled Compound C for 72 hours. At the end of this period
90% of the radioactive material originally present in the solution had
been taken up by the leaves. The major proportion (78%) of this
material was found to be present as sugar conjugates of Compound C.
Experiments have also been carried out with the degradation products
3-phenoxybenzoic acid (14C ring-labelled) and
cis-2-(2',2'-dichlorovinyl)-3,3-dimethyl cyclopropane carboxylic
acid (14C-C1-label). In some of these the stems of cotton leaves
were immersed in aqueous solutions. There was a rapid uptake of both
compounds and a rapid conversion to polar products. These products
were subsequently shown to be derivatives with various mono- or
di-saccharide sugar products in the case of the cyclopropane
carboxylic acid and mainly glucose in the case of 3-phenoxybenzoic
acid. Leaves to which 3-phenoxybenzoic acid was applied topically on
the intact plant showed similar conversions to a mixture of sugar
conjugates although at a much slower rate than in the case of petiole
uptake. Similar experiments with 3-phenoxybenzoic acid have been
undertaken on vine, tomato, soybeans, pea and broad bean leaves and
with similar findings, (Shell R. 126, 127, 128, 158, 159 and 160)
b. Lettuce
Experiments were carried out on plants grown in pots in the glasshouse
and treated with solutions of either the cis- or trans-isomer of
cypermethrin. Each isomer was also labelled in either the benzyl or
cyclopropyl rings. Details were similar to the first cotton
experiment, described above, except that samples were taken
immediately after treatment and 18 days later, and the results were
qualitatively similar. Just over 70% of the original applied
radioactivity was still present 18 days after application and 90% of
this was extractable with acetone. But a higher proportion (50-70%)
of the extracted radioactivity was present as cypermethrin than with
cotton. The proportions of other products found were: compound A,
5-10%; 3-phenoxybenzoic acid, 10-15%; and compound B, 12-15%. An
unidentified 11-15% consisted of several minor metabolites.
An outdoor experiment was also performed on lettuce using cypermethrin
labelled in the benzyl and cyclopropyl rings. The two labelled
materials were applied to different plants as an overall foliar spray
of a diluted E.C. formulation at a rate equivalent to 300 g/ha.
Treatments were made on two occasions with an interval of about two
weeks, and the plants were harvested approximately three weeks after
the second treatment. The major part of the residue, which amounted
to 0.8-1 mg/kg(radioactivity equivalent to cypermethrin) was located
in the outer leaves and only 10-20% was present in the hearts. In the
benzyl labelled experiment, 50% of the radioactivity was present as
cypermethrin itself and most of the remainder as polar materials which
individually were in too small amounts for detailed study. Only 4%
was not extracted. In the cyclopropyl labelled experiment, 30% of the
residue consisted of cypermethrin and 40% as conjugates of Compound C
of which a glucose ester was identified (Shell R. 126, 129).
c. Apples
Apple trees growing in an outdoor wire-covered enclosure were treated
with cis-cypermethrin, separately labelled in the benzyl or
cyclopropyl rings, or with trans-cypermethrin, labelled in the
benzyl ring. Leaves were treated on three occasions at intervals of
25 and 37 days, and harvested four weeks after the last treatment.
Fruit was treated twice at a 24-day interval and harvested three weeks
after the second application.
Examination of the leaves showed that 32-46% of the residue consisted
of the parent isomer applied. In addition, 7-15% of the radioactivity
present was identifiable as compounds A and C, 3-phenoxybenzyl
alcohol, 3-phenoxybenzaldehyde and 3-phenoxybenzoic acid. Polar
compounds made up most of the remaining residue. Sugar conjugates of
3-phenoxybenzoic acid and the corresponding alcohol, as well as of
3-(4-hydroxyphenoxy)-benzoic acid, were identified.
In fruit, results were similar although the quantities present were
much lower. Examinations of peel and pulp separately showed that less
than 2% of the total radioactivity in the fruit was present in the
pulp. On the peel 50-77% was present in the form of the parent isomer
applied. During the experiment 15% of the cis isomer was converted
into the trans isomer. The amounts of metabolites were somewhat
less than in leaves and accounted, as free compounds, for 3-7% of the
radioactivity present. Polar materials from the fruit were present in
much smaller amounts then in leaves and were not present in sufficient
quantities to be studied in detail.
In the experiments with the cis isomer some conversion (30%) into
the trans isomer occurred, but the reverse change was not observed.
In some routine examinations of field samples for residues of
cypermethrin the glc analytical method used results in separation of
the cis and trans isomers on the chromatograph trace. No change
in the relative sizes of the two peaks with different treatment to
harvest intervals, or between unresolved standard material and the
test samples, has been observed. It would, therefore, seem that the
conversion of cis isomer into trans compensates for the faster
degradation of the trans isomer (Shell R. 130).
IN SOIL
Degradation on soils was studied by adding 14C-labelled cypermethrin
to three different soils - a clay and a sandy clay from Spain, and a
sandy loam from the U.K. Separate experiments were carried out with
the molecule labelled in the benzyl or the cyclopropyl moiety. In
addition, the cis and trans isomers were studied individually
(Roberts and Standen, 1977).
The cis isomer was found to degrade at an initial rate equivalent to
a half life of between 2 and 4 weeks in sandy clay and sandy loam, and
about 10 weeks in the clay soil. The trans isomer degraded more
rapidly than the cis isomer in all three soils, with initial
half-lives between about one and three weeks. Degradation was shown
to proceed by hydrolysis of the ester link and cyano group with loss
of carbon dioxide to give 3-phenoxybenzoic acid and Compound C, which
were both present as free compounds. Subsequent degradation of both
compounds was shown to occur, since 14 C-radiolabelled carbon dioxide
was evolved over 22 weeks in amounts equivalent to 24% and 38%,
respectively, of the total radioactivity initially present as ring
label. As indicated by the presence of small amounts of Compounds A
and 3-(4-hydroxyphenoxy)-benzoic acid, some hydroxylation of the
aromatic rings also took place.
Up to 36% of the original radioactivity was found to be difficult to
extract 16 weeks after the commencement of the experiment. A study of
this fraction showed it to be composed of several materials bound to
soil components. Examination of the acid hydrolysed material showed
that 30% or more of the radioactivity was in the form of Compound C.
Smaller amounts of 3-phenoxybenzoic acid and
3-(4-hydroxyphenoxy)-benzoic acid were also found. After 52 weeks
unchanged parent material accounts for 1.4-10.7% of the radioactivity
originally applied to the soils. In one sample of a Spanish soil
(Brenes) a trace amount of Compound B was also present and in the same
soil 5.8% of the original radioactivity was present as a cyclopropane
dicarboxylic acid, Compound D. In a separate experiment, this latter
compound was also found, in amounts of 3-13% of the applied
radioactivity, 8 weeks after application to soils stored in the
laboratory. Under waterlogged and anaerobic conditions hydrolytic
breakdown was found to be somewhat slower than under aeroboic
conditions. Breakdown of 3-phenoxybenzoic acid was also slower.
Compound C has also been shown to be formed from permethrin in soils
and to be degraded by hydroxylation at the gem-dimethyl groups.
Subsequent further breakdown has been shown in further studies,
leading to extensive evolution of CO2 derived from ring and
chloromethylene carbons (ICI, 132, 133, 134, 135). These data
substantially confirm the results obtained with cypermethrin, showing
that Compound C is unlikely to accumulate in soils following treatment
with the pesticide.
In addition to the degradation studies referred to above, studies have
also been made to determine the rate of loss of cypermethrin from
soils from field experiments in which very high rates of application
had been made. The compound was applied as a diluted 40% EC and was
partly incorporated to the soil (3-5 cm) after treatment. Samples
were generally taken from 0-15 cm depth. The data obtained (Shell C
and R. 136 to 140), show that for the same rate applied, the amount of
residue initially present after treatment varied widely from one trial
to the other, and that cypermethrin degrades fairly rapidly in soils.
Only 1-5% of initial residue, if any, could be detected 4-8 months
after treatment. The leaching studies with labelled cypermethrin in a
column containing sandy loam soil show that less than 2% of applied
radioactivity had been eluted with water at a rate of 2 ml/hr from the
column over the 45-day period (Hungary, 1979), and that 90% of the
initial activity was present in the top 2 cm of the soil. The
findings from laboratory experiments are in agreement with the results
of field trials.
From the above findings, it may be concluded that, even when
applications have been made to crops at recommended rates, residues of
cypermethrin itself are unlikely to be present at detectable levels in
soils at the beginning of the season following that in which
treatments were made. It may also be concluded that crops grown in
plots where treatments with cypermethrin have been made in a previous
season are unlikely to contain detectable residues.
IN PROCESSING
Cypermethrin is a moderately stable and water-insoluble compound. Data
relating to the effect on residue levels of various treatments given
to harvested crops are as follows:
a. Peeling
Numerous data show that the residue present in a crop is largely on
the surface. Analyses of pulp and peel after peeling apples, pears,
peaches and citrus fruits (Table 6) show that levels in the pulp were
below 30%, and in most instances below 10%, of those in whole fruit.
b. Juice extraction - citrus
Data for citrus have shown that residues of cypermethrin in juice
extracted from treated fruits contained no measurable residues of the
pesticide (see Table 6).
C. Wine making
Wine has been manufactured from grapes treated with cypermethrin and
containing up to 0.15 mg/kg pesticide. No residues (limit of
determination 0.01 mg/l) were found in the juice after fermentation
(Shell C., 142).
d. Cooking
Studies were made on the effect of boiling on residue levels in plums,
and cabbage. Levels were not substantially reduced by boiling plums
for 30 minutes or cabbage for 45 minutes. Residues in the cooked
commodities were 75-90% of the initial levels and only very small
amounts were found in the cooking water (Shell C., 143).
e. Oil seed processing
An experiment was carried out with a cotton seed sample deliberately
treated at the high rate of 300 g/ha and harvested one day after
treatment. It contained 0.12 mg/kg cypermethrin on whole seed, with
adhering linters. The sample was processed by simulating commercial
practice in a laboratory specialising in the techniques. Residues
were found to be transferred to kernels, which originally did not
contain any detectable residue in the seed, during the commercial
mechanical separation process (Shell R., 144). The residues of
cypermethrin in the extracted oil at various stages were as follows:
Crude oil 0.10 mg/l, neutralised oil 0.07 mg/l, bleached oil 0.08 mg/l
and deodorised oil 0.05 mg/l. Both the alkali wash and deodorisation
steps contribute to some losses. The results from this experiment
suggest that the two processes together may be expected to remove
about half of the residue. Hence, it is possible that residues may
occasionally occur in oil obtained from seed, treated under practical
conditions, at levels approaching those in whole seed.
Photodecomposition
The photodecomposition of cypermethrin was studied in methanol
solution under UV light and in the solid phase (3 mg/cm2 on glass)
under sunlight. 55% of cypermethrin was recovered from the methanol
solution after two days. In sunlight there was no detectable loss of
cypermethrin after 30 hrs. In both experiments cypermethrin proved to
be more stable than decamethrin and its behaviour was comparable to
permethrin (Ruzo et al, 1976, 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
At present no data deriving from national monitoring studies or market
control are available.
METHODS OF RESIDUES ANALYSIS
Several methods have been developed for the analysis of agricultural
commodities for residues. These are all based on gas-liquid
chromatography procedures using equipment commonly found in modern
analytical laboratories. Limits of determination of 0.01 mg/kg are
usually attainable.
The racemic cypermethrin contains eight possible steric and optical
isomers which can be partially resolved in the GLC column depending on
its polarity. On analysis of the mixture of isomers on OV-225 phase
three partially resolved peaks with a characteristic pattern are
obtained. However with apolar or moderately polar packings (OV-101 or
OV-17) no resolution of isomers occurs which facilitates the
quantitation of the residue. Four methods have been developed by
Shell. For cotton and other oil-seed crops, the sample is extracted
with an acetone/petroleum mixture and the extract subjected to
clean-up by solvent partition followed by absorption chromatography on
Florisil. The appropriate fractions of the eluate are then examined
by gas-liquid chromatography using electron capture (63Ni) detection
(Shell R. 147). For other crops the samples are ground with anhydrous
sodium sulphate and cypermethrin extracted with acetone and petroleum
spirit. The extract is washed with water and the resultant petroleum
spirit layer separated and cleaned-up by chromatography on a Florisil
column. Cypermethrin is eluted with ether/petrol and determined using
gas-liquid chromatography and a (63Ni) electron-capture detector
(Shell R. 148).
Residues in animal tissues are determined by extracting the tissue
with mixed acetone:hexane, and fat removed by partitioning between
hexane and acetonitrile. The extract is cleaned-up by absorption
chromatography on a column of Florisil and cypermethrin determined in
the appropriate eluate fractions, by glc using electron-capture
detection (Shell R. 149).
Soil samples are mixed with anhydrous sodium sulphate and extracted
with a mixture of acetone and petroleum spirit. The extract, which
contains any cypermethrin present in the soil, is washed with water to
remove acetone and cleaned up by adsorption chromatography on
Florisil. Residues of cypermethrin are determined using GLC with an
electron-capture detector (Shell R. 150).
Confirmation of a residue in either soil or crop may be obtained by
TLC (thin-layer chromatography) on Silica Gel plate eluted with
toluene without previous saturation followed by further GLC. ICI have
also developed basically similar analytical methods for application to
crop samples. In their method the crop samples are extracted with 20%
acetone in hexane and, where necessary, co-extractives removed by
solvent partition. Further clean-up is carried out by adsorption
column chromatography on Silica Gel or Florisil and cypermethrin
determined by GLC in conjunction with an 63Ni E.C. detector (ICI
151).
Recovery experiments have shown that all the methods cited are capable
of measuring 80% or more of any cypermethrin residue present and that
they are suitable for regulatory purposes.
Chapman reported on the applicability of various adsorbent-solvent
systems for the cleanup of some plant extracts containing
cypermethrin, fenpropanate, permethrin, and fenvalerate. Analytical
methods suitable for the determination of metabolites of cypermethrin
in crop samples obtained following practical applications in the field
have been developed using HPLC. Under normal circumstances the limit
of determination is 0.05 mg/kg.(Shell R. 161)
The amide, Compound B, is extracted with a mixture of water and
acetonitrile from the sample of which the water content is known.
Clean-up of the extract is performed by solvent partition and
reversed-phase partition chromatography followed by reversed phase
HPLC. Final analysis is carried out with normal HPLC using a UV
detector (Shell R. 162).
3-phenoxybenzoic acid may be determined in a similar manner, although
partition between dilute aqueous alkaline acetonitrile and hexane in
used to remove parent cypermethrin from free 3-phenoxybenzoic acid.
Conjugated material is hydrolysed by treatment of the concentrated
aqueous extract with hydrochloric acid. The 3-phenoxybenzoic acid is
converted into the methyl ester which may then be determined directly
on HPLC. Alternatively, if further clean-up is required, HPLC may be
used for this purpose and gas chromatography/mass spectrometry with
multiple ion monitoring used for the determination (Shell R. 163).
Compound C may also be extracted from crop samples with a mixture of
water and acetonitrile and separated from unwanted material by solvent
partition. Conjugated Compound C is however retained in the aqueous
phase which may be concentrated and hydrolysed with hydrochloric acid.
The liberated compound C may then be extracted with an ether/petroleum
spirit mixture. Clean-up of the extracts is performed by reversed
phase HPLC and Compound C determined by normal phase HPLC. If
extracts are insufficiently clean for HPLC they may be treated with
alpha-cyano-3-phenoxy-benzyl bromide, and the cypermethrin formed
determined by gas chromatography with electron capture detection
(Shell R. 164).
Other experiments have shown that the great majority of samples may be
stored for long periods in the deep freeze without appreciable loss of
residues. Residue levels were determined in crop and soil samples at
intervals following addition of known quantities of cypermethrin in
the range of 0.2 mg/kg to 1 mg/kg. The samples were stored at -18°C
between treatment and extraction for analysis, for periods between 1
and 54 weeks for crops and 4-49 weeks for soil. Recoveries of
cypermethrin added to crops (21 samples) were 85-110%, apart from one
sample of tobacco which yielded 45% after 6 months storage. In the
case of soils (5 samples) recoveries were all between 90 and 110%
(Shell R. 152).
Studies have also been carried out on the stability in storage at
-18°C of residues of 3-phenoxybenzoic acid and Compounds B and C.
Over three months there was no evidence of loss of 3-phenoxybenzoic
acid, since the total amounts recovered from the 6 crops used
(lettuce, potatoes, cabbage, apples, pears and maize grain) were in
the range of 75-100% and comparable to recovery values of the method
itself. With Compound B experiments were carried over three months
with sweet corn and over five months with apples and cabbage.
Recoveries were 70%, 75% and 75% respectively. Cabbage and apples
treated with Compound C were also stored at -18°C for five months.
Recoveries were 90-95% respectively. These data, therefore, indicate
that loss of any of the three degradation compounds mentioned were
negligible over periods of 3-5 months at 18°C.
NATIONAL MRLs REPORTED TO THE MEETING
National MRLs and pre-harvest intervals have been established in
several countries based on local requirements. The following were
presented at the meeting:
Country Commodity MRL Pre-harvest int. (days)
Argentina Apples 21
Cotton 20
Peaches 25
Tomatoes 7
Soya, Sorghum 40
Sunflower 30
Colombia Cotton 35
Costa Rica Cotton, Vegetables 15
Country Commodity MRL Pre-harvest int. (days)
Cyprus Vegetables 14
Top Fruit
Fed. Rep.
of Germany Leafy vegetables 14
Potatoes 14
Maize 49
France Apples 0.5 14
Peaches 0.5 14
Vines 0.5 14
New Zealand Kiwi fruit 2.0 14
Pome fruit 1.0 14
Brassicas 1.0 7
Peru Cotton 15
Tomatoes 15
Potatoes 15
Spain Cotton 21
Potatoes 21
Tomatoes 21
Citrus 21
South Africa Cotton 0.05 28
Grapes 0.05 28
Maise 1.5 28
Peas 0.1 7
Tomatoes 0.2 4
Syria Top fruit 7-10
Vegetables
Experimental or wider use of cypermethrin in further 43 countries was
reported where neither preharvest intervals nor MRL's have been yet
established.
APPRAISAL
Cypermethrin is active against a wide range of insects which attack
crops and can be used at a relatively low dose rate in the range of
0.02-0.25 kg a.i./ha. It is a moderately stable and water insoluble
compound. The technical material contains not less than 90% w/w
cypermethrin, which is a mixture of optical isomers, with a cis:trans
isomer ratio of approximately 40:60. The maximum concentration of
residues in/on the treated crops in the range of 0.05-2 mg/kg and
decreases slowly.
The majority of residues are in the peel or outer leaves, and in most
instances the pulp or hearts contain below 10% of those in the whole
crop. The trans isomers of cypermethrin degrades slightly faster
than the cis, which is compensated by the conversion of cis isomer
into trans during degradation, thus the isomers remain in a constant
ratio at different times after application in plants. Degradation in
crops occurs mainly by hydrolysis of the ester bond followed by
further hydrolytic and oxidative processes to give a variety of
products. Less rapid processes observed were hydrolysis of the
nitrile group to amide and hydroxylation of the phenoxy ring. The
compounds formed were, in turn, also hydrolysed at the ester link.
However, metabolites have not been detected in crop commodities from
supervised trials. At least 90% of the total residue present in plant
material is extractable with acetone. Processing of treated crops
after harvest usually reduces the residue significantly.
Cattle consuming feed items treated with cypermethrin eliminate the
residue rapidly. Equilibrium between intake and excretion is reached
in 3-4 days. The total radioactivity found in the milk amounted to
only 0.5% of the radioactivity fed to the animals and 60-90 of this
residue was present in the cream fraction. The residues in the milk
are esters and contain both acidic and alcoholic moieties of the
parent compound. The vast majority of residue in the feed is excreted
in the urine and faeces in roughly similar proportions. The main
metabolite in urine is 3-phenoxybenzoic acid which is present as
glutamic acid conjugate and glycine derivatives. The faeces mainly
contain the intact molecule.
Cypermethrin does not accumulate in the meat. Even when fed at a high
dose rate residues are mostly in the fat, liver and kidney. The data
indicate that the feeding of crops treated with cypermethrin following
the recommended use patterns does not result in measurable residues in
meat or milk of cattle. Since the compound may be used for direct
treatment of animals and data deriving from the latter use are not
available, recommendations of MRLs for animal products cannot be made
at present.
In soils, spray deposits remain in the surface layer. The rate of
degradation is dependent on the type of soil and proceeds by
hydrolysis of the ester link and cyano group finally resulting in
acids from both parts of the parent compound. Some hydroxylation of
the phenoxy aromatic ring occurs. Subsequent degradation also takes
place with the breakdown of the carbon rings. The trans isomer
degrades more rapidly than the cis. The results indicate that no
detectable residue of cypermethrin itself will be present in soil 8-12
months after treatment and that crops grown in the next season will
not contain measurable residues of cypermethrin.
Analytical methods available for the determination of residues in
various commodities are suitable for regulatory purposes. The limit
of determination is typically 0.01-0.02 mg/kg.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended based
on the pre-harvest intervals indicated below. The limits refer to the
sum of isomers of the parent compound in the portion of sample to be
analysed as described by CC/PR.
Pre-harvest interval on
Commodity Temporary MRL mg/kg which recommendation is
based (days)
Citrus fruits 2 14
Peaches 2 7
Pome fruits 2 7
Cherries 1 7
Grapes 1 7
Brassica leafy
vegetables 1 7
Lettuce 2 7
Plums 1 7
Raspberries 0.5 14
Strawberries 0.5 21
Tomatoes 0.5 3
Rapeseed 0.2 42
Wheat 0.2 14
Cottonseed 0.1 7
Cottonseed oil
(finished) 0.2
Kidney beans, peas,
soybeans
(without pods) 0.05 7
Maize 0.05 7
Potatoes 0.05 7
Sugar beet (roots) 0.05 14
Sweet corn 0.05 7
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
cypermethrin and/or metabolites in adipose tissue.
2. Observations in man, especially those with high level of
occupational exposure, to evaluate the potential susceptibility of
man to the neurotoxic syndrome observed in rodents.
Desirable
1. A dominant lethal bioassay.
2. Further residue data on kiwi fruit from supervised trials.
3. Selective surveys of residues in crops known to have been treated
under practical circumstances.
4. Use patterns for animal health use and residues in animal products
deriving from the recommended application.
REFERENCES
Baldwin, M.K. The analysis of a Metabolite of WL 43467 in Human Urine
as an Index of Exposure to that Compound. (1978) Unpublished Report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
Brooks, T.M. Toxicity Studies with WL 43467. Mutagenicity Studies
with WL43467 in the Host-Mediated Assay and in Micro-Organisms In
Vitro. (1976) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Brown, V.K. Toxicology of WL 43467 Isomers: Acute Toxicity of WL
43481 in DMSO to rats. (1979a) Unpublished report submitted by Shell
International Chemical Company.
Toxicology of WL43467 Isomers: Acute Toxicity of WL42641 in DMSO to
Rats. (1979b) Unpublished report submitted by Shell International
Chemical Company.
Buckwell, A.C. and Butterworth, S.T.G. Toxicity Studies on the
Pyrethroid Insecticide WL 43467. A 13-Week Feeding Study in Dogs.
(1977) Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Butterworth, S.T.G. and Clark, D.G. Toxicity Studies on the
Insecticide WL43467: Acute Oral Toxicity and Neuropathological
Effects in Syrian Hamsters. (1977) Unpublished report from Shell
Research Ltd., Submitted by Shell International Chemical Company.
Carter, B.I. and Butterworth, S.T.G. Toxicity of Insecticides. The
Acute Oral Toxicity and Neuropathological Effects of WL 43467 to Rats.
(1976) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Chapman, R.A., and Harris, C.R. Extraction and Liquid-Solid
Chromatography Cleanup Procedures for the Direct Analysis of 4
Pyrethroid insecticides in Crops by Gas Liquid Chromatography. J. of
Chromatography 166. 513-518.
Combs A.D., Carter, B.I. Hend, R.W., Butterworth, S.G. and Backwell,
A.C. Toxicity Studies on the Insecticide WL-43467: (1976) Unpublished
summary of results of preliminary experiments from Shell Research
Ltd., submitted by Shell International Chemical Company.
Crawford, M. The Metabolism of WL 43467 in Mammals (1). The fate of
a Single Oral Dose of [14C-Benzyl] WL 43481 (cis-WL 43467) in the
Rat. 1976a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of WL 43467 in Mammals. The Fate of a Single Oral
Dose of [14C] WL-42641 (trans-WL43467), in the Rat. (1976b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, N.J. The Metabolism of WL 43467 in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] WL 43467 in the Rat. (1977)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Excretion and Residues of Radioactivity in Cows Treated Orally
with 14C-Labelled WL 43467. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 434671) in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] Cypermethrin in the Dog.
(1979a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 43461) in Mammals. The Fate of
Single Oral Doses of cis- and trans- [14C-benzyl] Cypermethrin in
the Dog. (1979b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
The Metabolic Fate of the cis- and Trans-isomers of WL 43467
(Cypermethrin) and of 3-Phenoxybenzoic Acid in Dogs. (1979c)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.H. The Elimination and Retention of WL
43467 when administered Dermally or Orally to Sheep. (1977a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.R. The Metabolic Fate of the cis- and
trans- isomers of WL 43467 (Cypermethrin). Metabolism and
Elimination of 14C-labelled cis- and trans-isomers in Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Mice Following the Oral
Administration of [14C-benzyl-] WL 43481 (cis-WL43467). (1978a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Rats Following the Oral
Administration of [14C-benzyl] WL 43481 (cis-WL 43467). (1978b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Metabolic Fate of the cis-and trans-isomers of Cypermethrin
in the Rat. Metabolites Derived from the 14C-Labelled Cyclopropyl
Ring. (1978c) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Crawford, J.V. A Study of the Metabolism of 3-Phenoxybenzoic Acid and
its Glucoside Conjugate in Rats. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Crawford, J.V. and Hutson, D.H. The identification of Metabolites in
the Tissues of Rats Treated Orally with 3-Phenoxybenzoic Acid. (1979)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dean, B.J. Toxicity Studies with WL 43467: Chromosome Studies on Bone
Marrow Cells of Chinese Hamsters After Two Daily Oral Doses of WL
43467. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Dean, B.J., van der Paux. C.L. and Butterworth S.T.G. Toxicity
Studies with WL 43467: Dominant Lethal Assay in Male Mice After Single
Oral Doses of WL 43467. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Dewar, A.J. The Use of Lysosomal Enzyme Measurements as an Indicator
of Chemically-Induced Peripheral Neuropathy. (1977a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Co.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Functional Studies on the Neurotoxicity of WL 43467 to Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dewar, A.J. and Deacon, P.A. Toxicity Studies on the Insecticide WL
43467: Electrophysiological Studies on the Neurotoxicity of WL 43467
to Rats. I. - The Effect on Motor Conduction Velocity in the Sciatic
and Tail Nerves. (1977) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Dewar, A.J. and Moffett, B.J. Toxicity Studies on the insecticide WL
43467: Biochemical studies on the effect of WL 43467 on the rat
trigeminal nerve and ganglion. (1978a) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Function Studies on the Neurotoxicity of WL 43467 to Chinese Hamsters.
(1978b) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
Dix, K.M. Toxicity of WL 43467: Teratological Studies in Rabbits
given WL 43467 Orally. (1978) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Glaister, J.R., Pratt, I. and Richards, D. PP 383: Effects of High
Dietary Levels on Clinical Behaviour and Structure of Sciatic Nerves
in the Rat. (1977a) Unpublished report from ICI Central Toxicology
Laboratory submitted by ICI Ltd.
Glaister, J.R., Gare, C.W., Marsat, G.J., Phillips, C. and Pratt, I.
PP 383: 90-Day Feeding Study in Rats. (1977b) Unpublished report
from ICI Central Toxicology Laboratory submitted by ICI Ltd.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies on the
Insecticide WL 43467: A Three-month Feeding Study in Rats. (1976)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Co.
Toxicity Studies on the Insecticide WL 42641: a Five-Week Feeding
Study in Rats. (1977a) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43481: A five-Week Feeding
Study in Rats. (1977b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Hend, R.W., Hendy, R. and Fleming, D.J. Toxicity Studies on the
Insecticide WL 43467: A Three-Generation Reproduction Study in Rats.
(1978) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Hungary. Information supplied by country delegation to Codex
Committee on Pesticide Residues on compounds on priority list. (1979).
Hutson, D.H. Taurine Conjugation in the Metabolism of
3-Phenoxybenzoic Acid and the Pyrethroid Insecticide Cypermethrin (WL
43467). (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company. (Published in Xenobiotica, 8
(1978) 565-571).
The Elimination of Radioactivity by Mice Following Oral Dosing with
14C-cis and 14C-trans-WL 43467 (Cypermethrin). (1978a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Company.
The Metabolites of cis- and trans-Cypermethrin (WL 43467) in
Mice. (1978b) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Hutson, D.H. and Stoydin, G. The Excretion of Radioactivity from Cows
fed with Radioactively Labelled WL 43467. (1976) Unpublished report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
ICI. (1979) Unpublished reports available to meeting (referred to in
text by ICI and code numbers 10, 132 to 135 and 151).
Jaggers, S. Cypermethrin: Summary and Review of Acute Toxicities in
Laboratory Species. (1979) Unpublished report submitted by ICI, Ltd.
McAusland, H.E., Butterworth, S.T.G. and Hunt, P.F. Toxicity Studies
on the Insecticide WL 43467: A Two-year Feeding Study in Rats. (1978)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
New Zealand. Information supplied by CCPR country delegation on
compounds on priority list. (1979)
Okuno Y., Kohda, H. and Kadota, T. Neurotoxic Effects of S-5602 and
NRDC 149 by Dermal Application in Rats. (1976) Unpublished report
from Sumitomo Chemical Company submitted by Shell International
Chemical Co.
Owen, D.E. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticides: Investigation of the Neurotoxic Potential of WL 43467 to
Adult Domestic Hens. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Prinsen, G.H. and Van Sittert, W.J. Exposure and Medical Monitoring
Study of the Pyrethroid WL 43467 After Single Application on Cotton in
Ivory Coast. (1978) Unpublished report from Shell International
Research Maatschappij, B.V., submitted by Shell International Chemical
Co.
Roberts, T.R. and Stande, M.E. Degradation of the Pyrethroid
Cypermethrin NRDC 149 (± alpha-cyano-3-phenoxybenzyl) ± (cis trans
3(2,2-dichlorovinyl) 2,2-dimethycyclopropane carboxylate and
Respective cis (NRDC 160) and trans (NRDC 159) Isomers in Soils.
Poetic. Sci. 8, 305-319 (1977).
Rose, G.P. and Dewar, A.J. Toxicity Studies on the Insecticide WL
43467: The Effect of Age on the Neurotoxicity of WL 43467 to Rats.
(1978) Unpublished report from Shell Research Ltd.,
Ruzo, L.O., Holmstead, R.L., Casida, J.E. Solution photochemistry of
the potent pyrethroid insecticides alpha-cyano-3-phenoxybenzyl cis
2,2-dimethyl-3-(2,2dibromovinyl) cyclopropane carboxylate.
Tetrahedron Letters 35, 3045/1976.
Ruzo, L.O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin J. Agr. Food Chem 25, 1385 (1977).
Shell Chemie. France. Unpublished Reports. Referred to by "Shell C"
and numbers in the text: 2-9, 11, 12, 14, 17-25, 27-30, 34,40, 44, 47,
55, 56, 60-64, 67-75, 77-86, 90-97, 101-105, 107-109, 112, 114-116,
118-122 136, 137, 142, 143, 152, 154, 155.
Shell Research Ltd., England - Unpublished reports referred to by
"Shell R." and numbers in the text: 1, 13, 15, 16, 26, 31-33, 41-43,
45, 46, 48-54, 57-59, 65, 66, 76, 87-89, 98-100, 106, 110, 111, 113,
117 123-130, 138-140, 144, 147-150, 152, 156, 158-160, 162-164.
Shono, T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-Cypermethrin, and Decamethrin by Microsomal Enzymes. J. Agric.
Food Chem., 27 (2), 316-325.
South Africa. Information on compounds on priority list. (1979)
Sweden. Information on compounds on priority list. (1979)
Suzuki, H. Studies on the Mutagenicity of Some Pyrethroids on
Salmonella Strains in the Presence of Mouse Hepatic S9 Fraction.
(1977) Unpublished report from Sumitomo Chemical Co., Ltd., submitted
by Sumitomo Chemical Co., Ltd.
Tesh, J.M., Tesh, S.A. and Davies, W. WL 43467: Effects Upon the
Progress and Outcome of Pregnancy in Rat. (1978) Unpublished report
from Life Science submitted by Shell International Chemical Company.
Trigg, C.E., Butterworth, S. and Hunt, P.F. Neurotoxicity of
Pyrethroids: A Study of Teased Nerves from Rats Fed WL 43467 for 12
Months. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
In the urine 3(4-hydroxyphenoxy) benzoic acid-O-sulphate and the
glutamic acid conjugate of 3-phenoxybenzoic acid were detected in the
ratio of 1:4. The free acids were not detectable. The faeces
contained the parent compound amounting to approximately 85% of the
faecal 14C, that is, about 36% of the amount ingested. At the end of
the three-week feeding period the animals were slaughtered and samples
of tissues examined for radioactivity. Levels were below 0.001 mg/kg,
in cypermethrin equivalents, in blood, muscle and brain. In
subcutaneous and renal fats, liver and kidney samples, residues were
at or below the equivalent of 0.012 mg/kg cypermethrin (Shell R. 124).
b. High Dietary Intake (5 mg/kg)
Radiolabelled cypermethrin was fed in the feed concentrate twice daily
and was given in amount equivalent to 4 mg/kg on total diet over a
period of 7 days.
The radioactivity in the milk, urine and faeces was monitored
throughout the feeding period and showed that the major excretory
route was via the kidneys. All the cows were found to be pregnant at
necropsy and the milk yields throughout the experiment were poor.
Equilibrium between intake and excretion was reached 3-4 days after
commencement of feeding, after which levels in whole milk ranged
between 0.009 mg/l and 0.013 mg/l cypermethrin equivalent to
radioactivity present. The cream fraction of the milk contained
85-90% of the total radioactivity. Results of experiments carried out
with 14C-benzyl and 14C-cyclopropyl labels indicate that the residue
in milk is an ester and contains both the acidic and alcoholic
functions of the parent compound.
The major urinary metabolites in cows were identified as the glutamic
acid conjugate of 3-phenoxybenzoic acid (68%), 3-phenoxybenzylglycine
(16%), 3-phenoxybenzoic acid (9%). 3(4-hydroxy-phenoxy) enzoic-acid
and its O-sulphate conjugate appeared to be present in only small
amounts (1%).
At the end of the test feeding period the animals were slaughtered and
tissues were taken for measurement, with the following results,
radioactivity being expressed as cypermethrin equivalents.
Muscle 0.04 mg/kg
Fat 0.01-0.10 mg/kg
Liver and Kidney 0.05-0.13 mg/kg
These results indicate that the cypermethrin does not accumulate in
the tissues of the animals. Even at a high intake, residues are
mostly in the fat, liver and kidney. Cereals and components of feed
(e.g. cotton seed) treated with the highest recommended dose rates
therefore, are unlikely to result in measurable residues in meat or
milk of cattle (Shell R. 125).
IN PLANTS
Degradation on cotton, lettuce and apples has been studied using
14C-radiolabelled material with the label in various positions in the
cypermethrin molecule. Structures of compounds mentioned are given in
Figure 1.
a. Cotton
An experiment in which the cis- and trans-isomers of cypermethrin
were applied in acetone/water solution to cotton leaves using a small
syringe was carried out on plants grown in pots in the greenhouse.
Each of the two isomers, which in themselves consisted of a racemic
mixture of two enantiomeric pairs of stereo isomers, was labelled in
the cyclopropyl and benzyl rings in separate experiments. In each
experiment the rate of application was 10-20 µg/g of leaf. Leaf
samples were taken for examination immediately after treatment and
again 42 days later. The samples were extracted with acetone and the
examined by chromatographic and radiochemical techniques.
Forty-two days after treatment 75-85% of the original total applied
radioactivity was still present in cotton leaves and 90% of this was
acetone extractable. Thin-layer chromatography showed that at least
six components were present in the extracts. Products identified and
the approximate proportion present in the extracts were cypermethrin
(40-50%), 4-hydroxy-cypermethrin (compound A/ 5-8%), 3-phenoxybenzoic
acid (15%), and an amide (compound B/ 8-20%). In this preliminary
study unidentified material accounted for the remaining 20-30% and was
mainly polar material. No evidence of an appreciable difference in
the rate of loss and breakdown of the two isomers was obtained.
A second experiment was carried out on a larger scale, in which
cypermethrin was 14C radio-labelled in the benzyl ring and applied at
the rate of 4 µg/g leaf. Two applications were made 15 days apart and
leaf samples were taken 5 weeks after the second treatment. Results
were qualitatively similar to the first experiment, except that
smaller amounts of metabolites were found and, in addition, some
3-(4-hydroxyphenoxy)-benzoic acid was also reported to be present. Due
to the larger scale of the experiment it was possible to examine the
polar fraction in more detail. This was found to consist of at least
8 compounds which were mainly conjugated forms of 3-phenoxybenzoic
acids 3-(4-hydroxyphenoxy)-benzoic acid and 3-phenoxybenzyl alcohol.
When the results from experiments using cypermethrin, separately
labelled in the nitrile, cyclopropyl and benzyl groups, were compared,
the properties of the polar products formed differed according to the
label position. Thus it was again concluded that, following the
primary step of hydrolysis of the ester link, further degradation of
the component parts, including loss of nitrile, oxidation and
conjugation, took place. In this work, the cyclopropane carboxylic
acid (compound C) was identified as a metabolite.
In a third experiment the cotton was grown in boxes outdoors in
Seville, Spain. Cypermethrin, labelled either in the cyclopropane or
benzyl rings, was applied to separate plants. The cis and trans
isomers, in each case, were also applied in separate experiments.
Three applications were made at rates equivalent to 300 g/ha and
samples were taken at harvest 15 weeks after the final treatment.
Seed, fibre, boll cases and leaves were examined separately. All the
kernel samples separated from the seed cases contained small amounts
of radioactivity equivalent to 0.07-0.24 mg/kg cypermethrin. Due to
the oily nature of the extracts and the small size of the samples, it
was not possible to fully identify the products present, although it
was shown the radioactivity was not present in the form of
cypermethrin itself. Seed cases freed from lint also contained
radioactivity equivalent to up to 0.17 mg/kg cypermethrin, which again
was shown not to be parent insecticide.
The lint samples contained rather more variable amounts, between 0.08
and 0.62 mg/kg cypermethrin equivalents, probably depending on the
degree of opening of the bolls at the time of spraying. Cypermethrin
itself was the major compound present (between 50 and 90% of the
total) but the remainder consisted of polar products. Foliage and
boll cases were found to contain the same products as those reported
from glasshouse experiments. These included cypermethrin,
3-phenoxybenzoic acid in free form, the cyclopropane carboxylic acid
(free) and the amide, Compound A. The latter in particular was
present, however, in considerably smaller amounts than found in the
glasshouse experiment.
Polar materials were also found in appreciable amounts. These could
be hydrolysed by acid to give 3-phenoxybenzoic acid, 3-phenoxybenzyl
alcohol and 3-phenoxybenzaldehyde. Unidentified materials in foliage
amounted to only a small proportion of the total radio-label present.
In another experiment, abscised cotton leaves were placed with their
stems immersed in an aqueous solution containing ring
14C-radiolabelled Compound C for 72 hours. At the end of this period
90% of the radioactive material originally present in the solution had
been taken up by the leaves. The major proportion (78%) of this
material was found to be present as sugar conjugates of Compound C.
Experiments have also been carried out with the degradation products
3-phenoxybenzoic acid (14C ring-labelled) and
cis-2-(2',2'-dichlorovinyl)-3,3-dimethyl cyclopropane carboxylic
acid (14C-C1-label). In some of these the stems of cotton leaves
were immersed in aqueous solutions. There was a rapid uptake of both
compounds and a rapid conversion to polar products. These products
were subsequently shown to be derivatives with various mono- or
di-saccharide sugar products in the case of the cyclopropane
carboxylic acid and mainly glucose in the case of 3-phenoxybenzoic
acid. Leaves to which 3-phenoxybenzoic acid was applied topically on
the intact plant showed similar conversions to a mixture of sugar
conjugates although at a much slower rate than in the case of petiole
uptake. Similar experiments with 3-phenoxybenzoic acid have been
undertaken on vine, tomato, soybeans, pea and broad bean leaves and
with similar findings, (Shell R. 126, 127, 128, 158, 159 and 160)
b. Lettuce
Experiments were carried out on plants grown in pots in the glasshouse
and treated with solutions of either the cis- or trans-isomer of
cypermethrin. Each isomer was also labelled in either the benzyl or
cyclopropyl rings. Details were similar to the first cotton
experiment, described above, except that samples were taken
immediately after treatment and 18 days later, and the results were
qualitatively similar. Just over 70% of the original applied
radioactivity was still present 18 days after application and 90% of
this was extractable with acetone. But a higher proportion (50-70%)
of the extracted radioactivity was present as cypermethrin than with
cotton. The proportions of other products found were: compound A,
5-10%; 3-phenoxybenzoic acid, 10-15%; and compound B, 12-15%. An
unidentified 11-15% consisted of several minor metabolites.
An outdoor experiment was also performed on lettuce using cypermethrin
labelled in the benzyl and cyclopropyl rings. The two labelled
materials were applied to different plants as an overall foliar spray
of a diluted E.C. formulation at a rate equivalent to 300 g/ha.
Treatments were made on two occasions with an interval of about two
weeks, and the plants were harvested approximately three weeks after
the second treatment. The major part of the residue, which amounted
to 0.8-1 mg/kg(radioactivity equivalent to cypermethrin) was located
in the outer leaves and only 10-20% was present in the hearts. In the
benzyl labelled experiment, 50% of the radioactivity was present as
cypermethrin itself and most of the remainder as polar materials which
individually were in too small amounts for detailed study. Only 4%
was not extracted. In the cyclopropyl labelled experiment, 30% of the
residue consisted of cypermethrin and 40% as conjugates of Compound C
of which a glucose ester was identified (Shell R. 126, 129).
c. Apples
Apple trees growing in an outdoor wire-covered enclosure were treated
with cis-cypermethrin, separately labelled in the benzyl or
cyclopropyl rings, or with trans-cypermethrin, labelled in the
benzyl ring. Leaves were treated on three occasions at intervals of
25 and 37 days, and harvested four weeks after the last treatment.
Fruit was treated twice at a 24-day interval and harvested three weeks
after the second application.
Examination of the leaves showed that 32-46% of the residue consisted
of the parent isomer applied. In addition, 7-15% of the radioactivity
present was identifiable as compounds A and C, 3-phenoxybenzyl
alcohol, 3-phenoxybenzaldehyde and 3-phenoxybenzoic acid. Polar
compounds made up most of the remaining residue. Sugar conjugates of
3-phenoxybenzoic acid and the corresponding alcohol, as well as of
3-(4-hydroxyphenoxy)-benzoic acid, were identified.
In fruit, results were similar although the quantities present were
much lower. Examinations of peel and pulp separately showed that less
than 2% of the total radioactivity in the fruit was present in the
pulp. On the peel 50-77% was present in the form of the parent isomer
applied. During the experiment 15% of the cis isomer was converted
into the trans isomer. The amounts of metabolites were somewhat
less than in leaves and accounted, as free compounds, for 3-7% of the
radioactivity present. Polar materials from the fruit were present in
much smaller amounts then in leaves and were not present in sufficient
quantities to be studied in detail.
In the experiments with the cis isomer some conversion (30%) into
the trans isomer occurred, but the reverse change was not observed.
In some routine examinations of field samples for residues of
cypermethrin the glc analytical method used results in separation of
the cis and trans isomers on the chromatograph trace. No change
in the relative sizes of the two peaks with different treatment to
harvest intervals, or between unresolved standard material and the
test samples, has been observed. It would, therefore, seem that the
conversion of cis isomer into trans compensates for the faster
degradation of the trans isomer (Shell R. 130).
IN SOIL
Degradation on soils was studied by adding 14C-labelled cypermethrin
to three different soils - a clay and a sandy clay from Spain, and a
sandy loam from the U.K. Separate experiments were carried out with
the molecule labelled in the benzyl or the cyclopropyl moiety. In
addition, the cis and trans isomers were studied individually
(Roberts and Standen, 1977).
The cis isomer was found to degrade at an initial rate equivalent to
a half life of between 2 and 4 weeks in sandy clay and sandy loam, and
about 10 weeks in the clay soil. The trans isomer degraded more
rapidly than the cis isomer in all three soils, with initial
half-lives between about one and three weeks. Degradation was shown
to proceed by hydrolysis of the ester link and cyano group with loss
of carbon dioxide to give 3-phenoxybenzoic acid and Compound C, which
were both present as free compounds. Subsequent degradation of both
compounds was shown to occur, since 14 C-radiolabelled carbon dioxide
was evolved over 22 weeks in amounts equivalent to 24% and 38%,
respectively, of the total radioactivity initially present as ring
label. As indicated by the presence of small amounts of Compounds A
and 3-(4-hydroxyphenoxy)-benzoic acid, some hydroxylation of the
aromatic rings also took place.
Up to 36% of the original radioactivity was found to be difficult to
extract 16 weeks after the commencement of the experiment. A study of
this fraction showed it to be composed of several materials bound to
soil components. Examination of the acid hydrolysed material showed
that 30% or more of the radioactivity was in the form of Compound C.
Smaller amounts of 3-phenoxybenzoic acid and
3-(4-hydroxyphenoxy)-benzoic acid were also found. After 52 weeks
unchanged parent material accounts for 1.4-10.7% of the radioactivity
originally applied to the soils. In one sample of a Spanish soil
(Brenes) a trace amount of Compound B was also present and in the same
soil 5.8% of the original radioactivity was present as a cyclopropane
dicarboxylic acid, Compound D. In a separate experiment, this latter
compound was also found, in amounts of 3-13% of the applied
radioactivity, 8 weeks after application to soils stored in the
laboratory. Under waterlogged and anaerobic conditions hydrolytic
breakdown was found to be somewhat slower than under aeroboic
conditions. Breakdown of 3-phenoxybenzoic acid was also slower.
Compound C has also been shown to be formed from permethrin in soils
and to be degraded by hydroxylation at the gem-dimethyl groups.
Subsequent further breakdown has been shown in further studies,
leading to extensive evolution of CO2 derived from ring and
chloromethylene carbons (ICI, 132, 133, 134, 135). These data
substantially confirm the results obtained with cypermethrin, showing
that Compound C is unlikely to accumulate in soils following treatment
with the pesticide.
In addition to the degradation studies referred to above, studies have
also been made to determine the rate of loss of cypermethrin from
soils from field experiments in which very high rates of application
had been made. The compound was applied as a diluted 40% EC and was
partly incorporated to the soil (3-5 cm) after treatment. Samples
were generally taken from 0-15 cm depth. The data obtained (Shell C
and R. 136 to 140), show that for the same rate applied, the amount of
residue initially present after treatment varied widely from one trial
to the other, and that cypermethrin degrades fairly rapidly in soils.
Only 1-5% of initial residue, if any, could be detected 4-8 months
after treatment. The leaching studies with labelled cypermethrin in a
column containing sandy loam soil show that less than 2% of applied
radioactivity had been eluted with water at a rate of 2 ml/hr from the
column over the 45-day period (Hungary, 1979), and that 90% of the
initial activity was present in the top 2 cm of the soil. The
findings from laboratory experiments are in agreement with the results
of field trials.
From the above findings, it may be concluded that, even when
applications have been made to crops at recommended rates, residues of
cypermethrin itself are unlikely to be present at detectable levels in
soils at the beginning of the season following that in which
treatments were made. It may also be concluded that crops grown in
plots where treatments with cypermethrin have been made in a previous
season are unlikely to contain detectable residues.
IN PROCESSING
Cypermethrin is a moderately stable and water-insoluble compound. Data
relating to the effect on residue levels of various treatments given
to harvested crops are as follows:
a. Peeling
Numerous data show that the residue present in a crop is largely on
the surface. Analyses of pulp and peel after peeling apples, pears,
peaches and citrus fruits (Table 6) show that levels in the pulp were
below 30%, and in most instances below 10%, of those in whole fruit.
b. Juice extraction - citrus
Data for citrus have shown that residues of cypermethrin in juice
extracted from treated fruits contained no measurable residues of the
pesticide (see Table 6).
C. Wine making
Wine has been manufactured from grapes treated with cypermethrin and
containing up to 0.15 mg/kg pesticide. No residues (limit of
determination 0.01 mg/l) were found in the juice after fermentation
(Shell C., 142).
d. Cooking
Studies were made on the effect of boiling on residue levels in plums,
and cabbage. Levels were not substantially reduced by boiling plums
for 30 minutes or cabbage for 45 minutes. Residues in the cooked
commodities were 75-90% of the initial levels and only very small
amounts were found in the cooking water (Shell C., 143).
e. Oil seed processing
An experiment was carried out with a cotton seed sample deliberately
treated at the high rate of 300 g/ha and harvested one day after
treatment. It contained 0.12 mg/kg cypermethrin on whole seed, with
adhering linters. The sample was processed by simulating commercial
practice in a laboratory specialising in the techniques. Residues
were found to be transferred to kernels, which originally did not
contain any detectable residue in the seed, during the commercial
mechanical separation process (Shell R., 144). The residues of
cypermethrin in the extracted oil at various stages were as follows:
Crude oil 0.10 mg/l, neutralised oil 0.07 mg/l, bleached oil 0.08 mg/l
and deodorised oil 0.05 mg/l. Both the alkali wash and deodorisation
steps contribute to some losses. The results from this experiment
suggest that the two processes together may be expected to remove
about half of the residue. Hence, it is possible that residues may
occasionally occur in oil obtained from seed, treated under practical
conditions, at levels approaching those in whole seed.
Photodecomposition
The photodecomposition of cypermethrin was studied in methanol
solution under UV light and in the solid phase (3 mg/cm2 on glass)
under sunlight. 55% of cypermethrin was recovered from the methanol
solution after two days. In sunlight there was no detectable loss of
cypermethrin after 30 hrs. In both experiments cypermethrin proved to
be more stable than decamethrin and its behaviour was comparable to
permethrin (Ruzo et al, 1976, 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
At present no data deriving from national monitoring studies or market
control are available.
METHODS OF RESIDUES ANALYSIS
Several methods have been developed for the analysis of agricultural
commodities for residues. These are all based on gas-liquid
chromatography procedures using equipment commonly found in modern
analytical laboratories. Limits of determination of 0.01 mg/kg are
usually attainable.
The racemic cypermethrin contains eight possible steric and optical
isomers which can be partially resolved in the GLC column depending on
its polarity. On analysis of the mixture of isomers on OV-225 phase
three partially resolved peaks with a characteristic pattern are
obtained. However with apolar or moderately polar packings (OV-101 or
OV-17) no resolution of isomers occurs which facilitates the
quantitation of the residue. Four methods have been developed by
Shell. For cotton and other oil-seed crops, the sample is extracted
with an acetone/petroleum mixture and the extract subjected to
clean-up by solvent partition followed by absorption chromatography on
Florisil. The appropriate fractions of the eluate are then examined
by gas-liquid chromatography using electron capture (63Ni) detection
(Shell R. 147). For other crops the samples are ground with anhydrous
sodium sulphate and cypermethrin extracted with acetone and petroleum
spirit. The extract is washed with water and the resultant petroleum
spirit layer separated and cleaned-up by chromatography on a Florisil
column. Cypermethrin is eluted with ether/petrol and determined using
gas-liquid chromatography and a (63Ni) electron-capture detector
(Shell R. 148).
Residues in animal tissues are determined by extracting the tissue
with mixed acetone:hexane, and fat removed by partitioning between
hexane and acetonitrile. The extract is cleaned-up by absorption
chromatography on a column of Florisil and cypermethrin determined in
the appropriate eluate fractions, by glc using electron-capture
detection (Shell R. 149).
Soil samples are mixed with anhydrous sodium sulphate and extracted
with a mixture of acetone and petroleum spirit. The extract, which
contains any cypermethrin present in the soil, is washed with water to
remove acetone and cleaned up by adsorption chromatography on
Florisil. Residues of cypermethrin are determined using GLC with an
electron-capture detector (Shell R. 150).
Confirmation of a residue in either soil or crop may be obtained by
TLC (thin-layer chromatography) on Silica Gel plate eluted with
toluene without previous saturation followed by further GLC. ICI have
also developed basically similar analytical methods for application to
crop samples. In their method the crop samples are extracted with 20%
acetone in hexane and, where necessary, co-extractives removed by
solvent partition. Further clean-up is carried out by adsorption
column chromatography on Silica Gel or Florisil and cypermethrin
determined by GLC in conjunction with an 63Ni E.C. detector (ICI
151).
Recovery experiments have shown that all the methods cited are capable
of measuring 80% or more of any cypermethrin residue present and that
they are suitable for regulatory purposes.
Chapman reported on the applicability of various adsorbent-solvent
systems for the cleanup of some plant extracts containing
cypermethrin, fenpropanate, permethrin, and fenvalerate. Analytical
methods suitable for the determination of metabolites of cypermethrin
in crop samples obtained following practical applications in the field
have been developed using HPLC. Under normal circumstances the limit
of determination is 0.05 mg/kg.(Shell R. 161)
The amide, Compound B, is extracted with a mixture of water and
acetonitrile from the sample of which the water content is known.
Clean-up of the extract is performed by solvent partition and
reversed-phase partition chromatography followed by reversed phase
HPLC. Final analysis is carried out with normal HPLC using a UV
detector (Shell R. 162).
3-phenoxybenzoic acid may be determined in a similar manner, although
partition between dilute aqueous alkaline acetonitrile and hexane in
used to remove parent cypermethrin from free 3-phenoxybenzoic acid.
Conjugated material is hydrolysed by treatment of the concentrated
aqueous extract with hydrochloric acid. The 3-phenoxybenzoic acid is
converted into the methyl ester which may then be determined directly
on HPLC. Alternatively, if further clean-up is required, HPLC may be
used for this purpose and gas chromatography/mass spectrometry with
multiple ion monitoring used for the determination (Shell R. 163).
Compound C may also be extracted from crop samples with a mixture of
water and acetonitrile and separated from unwanted material by solvent
partition. Conjugated Compound C is however retained in the aqueous
phase which may be concentrated and hydrolysed with hydrochloric acid.
The liberated compound C may then be extracted with an ether/petroleum
spirit mixture. Clean-up of the extracts is performed by reversed
phase HPLC and Compound C determined by normal phase HPLC. If
extracts are insufficiently clean for HPLC they may be treated with
alpha-cyano-3-phenoxy-benzyl bromide, and the cypermethrin formed
determined by gas chromatography with electron capture detection
(Shell R. 164).
Other experiments have shown that the great majority of samples may be
stored for long periods in the deep freeze without appreciable loss of
residues. Residue levels were determined in crop and soil samples at
intervals following addition of known quantities of cypermethrin in
the range of 0.2 mg/kg to 1 mg/kg. The samples were stored at -18°C
between treatment and extraction for analysis, for periods between 1
and 54 weeks for crops and 4-49 weeks for soil. Recoveries of
cypermethrin added to crops (21 samples) were 85-110%, apart from one
sample of tobacco which yielded 45% after 6 months storage. In the
case of soils (5 samples) recoveries were all between 90 and 110%
(Shell R. 152).
Studies have also been carried out on the stability in storage at
-18°C of residues of 3-phenoxybenzoic acid and Compounds B and C.
Over three months there was no evidence of loss of 3-phenoxybenzoic
acid, since the total amounts recovered from the 6 crops used
(lettuce, potatoes, cabbage, apples, pears and maize grain) were in
the range of 75-100% and comparable to recovery values of the method
itself. With Compound B experiments were carried over three months
with sweet corn and over five months with apples and cabbage.
Recoveries were 70%, 75% and 75% respectively. Cabbage and apples
treated with Compound C were also stored at -18°C for five months.
Recoveries were 90-95% respectively. These data, therefore, indicate
that loss of any of the three degradation compounds mentioned were
negligible over periods of 3-5 months at 18°C.
NATIONAL MRLs REPORTED TO THE MEETING
National MRLs and pre-harvest intervals have been established in
several countries based on local requirements. The following were
presented at the meeting:
Country Commodity MRL Pre-harvest int. (days)
Argentina Apples 21
Cotton 20
Peaches 25
Tomatoes 7
Soya, Sorghum 40
Sunflower 30
Colombia Cotton 35
Costa Rica Cotton, Vegetables 15
Country Commodity MRL Pre-harvest int. (days)
Cyprus Vegetables 14
Top Fruit
Fed. Rep.
of Germany Leafy vegetables 14
Potatoes 14
Maize 49
France Apples 0.5 14
Peaches 0.5 14
Vines 0.5 14
New Zealand Kiwi fruit 2.0 14
Pome fruit 1.0 14
Brassicas 1.0 7
Peru Cotton 15
Tomatoes 15
Potatoes 15
Spain Cotton 21
Potatoes 21
Tomatoes 21
Citrus 21
South Africa Cotton 0.05 28
Grapes 0.05 28
Maise 1.5 28
Peas 0.1 7
Tomatoes 0.2 4
Syria Top fruit 7-10
Vegetables
Experimental or wider use of cypermethrin in further 43 countries was
reported where neither preharvest intervals nor MRL's have been yet
established.
APPRAISAL
Cypermethrin is active against a wide range of insects which attack
crops and can be used at a relatively low dose rate in the range of
0.02-0.25 kg a.i./ha. It is a moderately stable and water insoluble
compound. The technical material contains not less than 90% w/w
cypermethrin, which is a mixture of optical isomers, with a cis:trans
isomer ratio of approximately 40:60. The maximum concentration of
residues in/on the treated crops in the range of 0.05-2 mg/kg and
decreases slowly.
The majority of residues are in the peel or outer leaves, and in most
instances the pulp or hearts contain below 10% of those in the whole
crop. The trans isomers of cypermethrin degrades slightly faster
than the cis, which is compensated by the conversion of cis isomer
into trans during degradation, thus the isomers remain in a constant
ratio at different times after application in plants. Degradation in
crops occurs mainly by hydrolysis of the ester bond followed by
further hydrolytic and oxidative processes to give a variety of
products. Less rapid processes observed were hydrolysis of the
nitrile group to amide and hydroxylation of the phenoxy ring. The
compounds formed were, in turn, also hydrolysed at the ester link.
However, metabolites have not been detected in crop commodities from
supervised trials. At least 90% of the total residue present in plant
material is extractable with acetone. Processing of treated crops
after harvest usually reduces the residue significantly.
Cattle consuming feed items treated with cypermethrin eliminate the
residue rapidly. Equilibrium between intake and excretion is reached
in 3-4 days. The total radioactivity found in the milk amounted to
only 0.5% of the radioactivity fed to the animals and 60-90 of this
residue was present in the cream fraction. The residues in the milk
are esters and contain both acidic and alcoholic moieties of the
parent compound. The vast majority of residue in the feed is excreted
in the urine and faeces in roughly similar proportions. The main
metabolite in urine is 3-phenoxybenzoic acid which is present as
glutamic acid conjugate and glycine derivatives. The faeces mainly
contain the intact molecule.
Cypermethrin does not accumulate in the meat. Even when fed at a high
dose rate residues are mostly in the fat, liver and kidney. The data
indicate that the feeding of crops treated with cypermethrin following
the recommended use patterns does not result in measurable residues in
meat or milk of cattle. Since the compound may be used for direct
treatment of animals and data deriving from the latter use are not
available, recommendations of MRLs for animal products cannot be made
at present.
In soils, spray deposits remain in the surface layer. The rate of
degradation is dependent on the type of soil and proceeds by
hydrolysis of the ester link and cyano group finally resulting in
acids from both parts of the parent compound. Some hydroxylation of
the phenoxy aromatic ring occurs. Subsequent degradation also takes
place with the breakdown of the carbon rings. The trans isomer
degrades more rapidly than the cis. The results indicate that no
detectable residue of cypermethrin itself will be present in soil 8-12
months after treatment and that crops grown in the next season will
not contain measurable residues of cypermethrin.
Analytical methods available for the determination of residues in
various commodities are suitable for regulatory purposes. The limit
of determination is typically 0.01-0.02 mg/kg.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended based
on the pre-harvest intervals indicated below. The limits refer to the
sum of isomers of the parent compound in the portion of sample to be
analysed as described by CC/PR.
Pre-harvest interval on
Commodity Temporary MRL mg/kg which recommendation is
based (days)
Citrus fruits 2 14
Peaches 2 7
Pome fruits 2 7
Cherries 1 7
Grapes 1 7
Brassica leafy
vegetables 1 7
Lettuce 2 7
Plums 1 7
Raspberries 0.5 14
Strawberries 0.5 21
Tomatoes 0.5 3
Rapeseed 0.2 42
Wheat 0.2 14
Cottonseed 0.1 7
Cottonseed oil
(finished) 0.2
Kidney beans, peas,
soybeans
(without pods) 0.05 7
Maize 0.05 7
Potatoes 0.05 7
Sugar beet (roots) 0.05 14
Sweet corn 0.05 7
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
cypermethrin and/or metabolites in adipose tissue.
2. Observations in man, especially those with high level of
occupational exposure, to evaluate the potential susceptibility of
man to the neurotoxic syndrome observed in rodents.
Desirable
1. A dominant lethal bioassay.
2. Further residue data on kiwi fruit from supervised trials.
3. Selective surveys of residues in crops known to have been treated
under practical circumstances.
4. Use patterns for animal health use and residues in animal products
deriving from the recommended application.
REFERENCES
Baldwin, M.K. The analysis of a Metabolite of WL 43467 in Human Urine
as an Index of Exposure to that Compound. (1978) Unpublished Report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
Brooks, T.M. Toxicity Studies with WL 43467. Mutagenicity Studies
with WL43467 in the Host-Mediated Assay and in Micro-Organisms In
Vitro. (1976) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Brown, V.K. Toxicology of WL 43467 Isomers: Acute Toxicity of WL
43481 in DMSO to rats. (1979a) Unpublished report submitted by Shell
International Chemical Company.
Toxicology of WL43467 Isomers: Acute Toxicity of WL42641 in DMSO to
Rats. (1979b) Unpublished report submitted by Shell International
Chemical Company.
Buckwell, A.C. and Butterworth, S.T.G. Toxicity Studies on the
Pyrethroid Insecticide WL 43467. A 13-Week Feeding Study in Dogs.
(1977) Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Butterworth, S.T.G. and Clark, D.G. Toxicity Studies on the
Insecticide WL43467: Acute Oral Toxicity and Neuropathological
Effects in Syrian Hamsters. (1977) Unpublished report from Shell
Research Ltd., Submitted by Shell International Chemical Company.
Carter, B.I. and Butterworth, S.T.G. Toxicity of Insecticides. The
Acute Oral Toxicity and Neuropathological Effects of WL 43467 to Rats.
(1976) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Chapman, R.A., and Harris, C.R. Extraction and Liquid-Solid
Chromatography Cleanup Procedures for the Direct Analysis of 4
Pyrethroid insecticides in Crops by Gas Liquid Chromatography. J. of
Chromatography 166. 513-518.
Combs A.D., Carter, B.I. Hend, R.W., Butterworth, S.G. and Backwell,
A.C. Toxicity Studies on the Insecticide WL-43467: (1976) Unpublished
summary of results of preliminary experiments from Shell Research
Ltd., submitted by Shell International Chemical Company.
Crawford, M. The Metabolism of WL 43467 in Mammals (1). The fate of
a Single Oral Dose of [14C-Benzyl] WL 43481 (cis-WL 43467) in the
Rat. 1976a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of WL 43467 in Mammals. The Fate of a Single Oral
Dose of [14C] WL-42641 (trans-WL43467), in the Rat. (1976b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, N.J. The Metabolism of WL 43467 in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] WL 43467 in the Rat. (1977)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Excretion and Residues of Radioactivity in Cows Treated Orally
with 14C-Labelled WL 43467. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 434671) in Mammals. The Fate of a
Single Oral Dose of [14C-cyclopropyl] Cypermethrin in the Dog.
(1979a) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
The Metabolism of Cypermethrin (WL 43461) in Mammals. The Fate of
Single Oral Doses of cis- and trans- [14C-benzyl] Cypermethrin in
the Dog. (1979b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
The Metabolic Fate of the cis- and Trans-isomers of WL 43467
(Cypermethrin) and of 3-Phenoxybenzoic Acid in Dogs. (1979c)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.H. The Elimination and Retention of WL
43467 when administered Dermally or Orally to Sheep. (1977a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Crawford, M.J. and Hutson, D.R. The Metabolic Fate of the cis- and
trans- isomers of WL 43467 (Cypermethrin). Metabolism and
Elimination of 14C-labelled cis- and trans-isomers in Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Mice Following the Oral
Administration of [14C-benzyl-] WL 43481 (cis-WL43467). (1978a)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Elimination of Residues from the Fat of Rats Following the Oral
Administration of [14C-benzyl] WL 43481 (cis-WL 43467). (1978b)
Unpublished Report from Shell Research Ltd., submitted by Shell
International Chemical Company.
The Metabolic Fate of the cis-and trans-isomers of Cypermethrin
in the Rat. Metabolites Derived from the 14C-Labelled Cyclopropyl
Ring. (1978c) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Crawford, J.V. A Study of the Metabolism of 3-Phenoxybenzoic Acid and
its Glucoside Conjugate in Rats. (1978) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Crawford, J.V. and Hutson, D.H. The identification of Metabolites in
the Tissues of Rats Treated Orally with 3-Phenoxybenzoic Acid. (1979)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dean, B.J. Toxicity Studies with WL 43467: Chromosome Studies on Bone
Marrow Cells of Chinese Hamsters After Two Daily Oral Doses of WL
43467. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Dean, B.J., van der Paux. C.L. and Butterworth S.T.G. Toxicity
Studies with WL 43467: Dominant Lethal Assay in Male Mice After Single
Oral Doses of WL 43467. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Dewar, A.J. The Use of Lysosomal Enzyme Measurements as an Indicator
of Chemically-Induced Peripheral Neuropathy. (1977a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Co.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Functional Studies on the Neurotoxicity of WL 43467 to Rats. (1977b)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Dewar, A.J. and Deacon, P.A. Toxicity Studies on the Insecticide WL
43467: Electrophysiological Studies on the Neurotoxicity of WL 43467
to Rats. I. - The Effect on Motor Conduction Velocity in the Sciatic
and Tail Nerves. (1977) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Dewar, A.J. and Moffett, B.J. Toxicity Studies on the insecticide WL
43467: Biochemical studies on the effect of WL 43467 on the rat
trigeminal nerve and ganglion. (1978a) Unpublished report from Shell
Research Ltd., submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43467: Biochemical and
Function Studies on the Neurotoxicity of WL 43467 to Chinese Hamsters.
(1978b) Unpublished report from Shell Research Ltd., submitted by
Shell International Chemical Company.
Dix, K.M. Toxicity of WL 43467: Teratological Studies in Rabbits
given WL 43467 Orally. (1978) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Glaister, J.R., Pratt, I. and Richards, D. PP 383: Effects of High
Dietary Levels on Clinical Behaviour and Structure of Sciatic Nerves
in the Rat. (1977a) Unpublished report from ICI Central Toxicology
Laboratory submitted by ICI Ltd.
Glaister, J.R., Gare, C.W., Marsat, G.J., Phillips, C. and Pratt, I.
PP 383: 90-Day Feeding Study in Rats. (1977b) Unpublished report
from ICI Central Toxicology Laboratory submitted by ICI Ltd.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies on the
Insecticide WL 43467: A Three-month Feeding Study in Rats. (1976)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Co.
Toxicity Studies on the Insecticide WL 42641: a Five-Week Feeding
Study in Rats. (1977a) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Toxicity Studies on the Insecticide WL 43481: A five-Week Feeding
Study in Rats. (1977b) Unpublished report from Shell Research Ltd.,
submitted by Shell International Chemical Company.
Hend, R.W., Hendy, R. and Fleming, D.J. Toxicity Studies on the
Insecticide WL 43467: A Three-Generation Reproduction Study in Rats.
(1978) Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
Hungary. Information supplied by country delegation to Codex
Committee on Pesticide Residues on compounds on priority list. (1979).
Hutson, D.H. Taurine Conjugation in the Metabolism of
3-Phenoxybenzoic Acid and the Pyrethroid Insecticide Cypermethrin (WL
43467). (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company. (Published in Xenobiotica, 8
(1978) 565-571).
The Elimination of Radioactivity by Mice Following Oral Dosing with
14C-cis and 14C-trans-WL 43467 (Cypermethrin). (1978a) Unpublished
report from Shell Research Ltd., submitted by Shell International
Chemical Company.
The Metabolites of cis- and trans-Cypermethrin (WL 43467) in
Mice. (1978b) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
Hutson, D.H. and Stoydin, G. The Excretion of Radioactivity from Cows
fed with Radioactively Labelled WL 43467. (1976) Unpublished report
from Shell Research Ltd., submitted by Shell International Chemical
Co.
ICI. (1979) Unpublished reports available to meeting (referred to in
text by ICI and code numbers 10, 132 to 135 and 151).
Jaggers, S. Cypermethrin: Summary and Review of Acute Toxicities in
Laboratory Species. (1979) Unpublished report submitted by ICI, Ltd.
McAusland, H.E., Butterworth, S.T.G. and Hunt, P.F. Toxicity Studies
on the Insecticide WL 43467: A Two-year Feeding Study in Rats. (1978)
Unpublished report from Shell Research Ltd., submitted by Shell
International Chemical Company.
New Zealand. Information supplied by CCPR country delegation on
compounds on priority list. (1979)
Okuno Y., Kohda, H. and Kadota, T. Neurotoxic Effects of S-5602 and
NRDC 149 by Dermal Application in Rats. (1976) Unpublished report
from Sumitomo Chemical Company submitted by Shell International
Chemical Co.
Owen, D.E. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticides: Investigation of the Neurotoxic Potential of WL 43467 to
Adult Domestic Hens. (1977) Unpublished report from Shell Research
Ltd., submitted by Shell International Chemical Company.
Prinsen, G.H. and Van Sittert, W.J. Exposure and Medical Monitoring
Study of the Pyrethroid WL 43467 After Single Application on Cotton in
Ivory Coast. (1978) Unpublished report from Shell International
Research Maatschappij, B.V., submitted by Shell International Chemical
Co.
Roberts, T.R. and Stande, M.E. Degradation of the Pyrethroid
Cypermethrin NRDC 149 (± alpha-cyano-3-phenoxybenzyl) ± (cis trans
3(2,2-dichlorovinyl) 2,2-dimethycyclopropane carboxylate and
Respective cis (NRDC 160) and trans (NRDC 159) Isomers in Soils.
Poetic. Sci. 8, 305-319 (1977).
Rose, G.P. and Dewar, A.J. Toxicity Studies on the Insecticide WL
43467: The Effect of Age on the Neurotoxicity of WL 43467 to Rats.
(1978) Unpublished report from Shell Research Ltd.,
Ruzo, L.O., Holmstead, R.L., Casida, J.E. Solution photochemistry of
the potent pyrethroid insecticides alpha-cyano-3-phenoxybenzyl cis
2,2-dimethyl-3-(2,2dibromovinyl) cyclopropane carboxylate.
Tetrahedron Letters 35, 3045/1976.
Ruzo, L.O., Holmstead, R.L. and Casida, J.E. Pyrethroid
Photochemistry: Decamethrin J. Agr. Food Chem 25, 1385 (1977).
Shell Chemie. France. Unpublished Reports. Referred to by "Shell C"
and numbers in the text: 2-9, 11, 12, 14, 17-25, 27-30, 34,40, 44, 47,
55, 56, 60-64, 67-75, 77-86, 90-97, 101-105, 107-109, 112, 114-116,
118-122 136, 137, 142, 143, 152, 154, 155.
Shell Research Ltd., England - Unpublished reports referred to by
"Shell R." and numbers in the text: 1, 13, 15, 16, 26, 31-33, 41-43,
45, 46, 48-54, 57-59, 65, 66, 76, 87-89, 98-100, 106, 110, 111, 113,
117 123-130, 138-140, 144, 147-150, 152, 156, 158-160, 162-164.
Shono, T., Ohsawa, K. and Casida, J.E. Metabolism of trans- and
cis-Cypermethrin, and Decamethrin by Microsomal Enzymes. J. Agric.
Food Chem., 27 (2), 316-325.
South Africa. Information on compounds on priority list. (1979)
Sweden. Information on compounds on priority list. (1979)
Suzuki, H. Studies on the Mutagenicity of Some Pyrethroids on
Salmonella Strains in the Presence of Mouse Hepatic S9 Fraction.
(1977) Unpublished report from Sumitomo Chemical Co., Ltd., submitted
by Sumitomo Chemical Co., Ltd.
Tesh, J.M., Tesh, S.A. and Davies, W. WL 43467: Effects Upon the
Progress and Outcome of Pregnancy in Rat. (1978) Unpublished report
from Life Science submitted by Shell International Chemical Company.
Trigg, C.E., Butterworth, S. and Hunt, P.F. Neurotoxicity of
Pyrethroids: A Study of Teased Nerves from Rats Fed WL 43467 for 12
Months. (1977) Unpublished report from Shell Research Ltd., submitted
by Shell International Chemical Company.
