
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
AMINOMETHYLPHOSPHONIC ACID (AMPA)
First draft prepared by
E. van Apeldoorn and P.H. van Hoeven
National Institute of Public Health and Environment,
Bilthoven, The Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: Dermal and ocular irritation
Toxicological profiles of AMPA and glyphosate
Comments
Toxicological evaluation
References
Explanation
Glyphosate was evaluated toxicologically by the 1986 JMPR, which
allocated an ADI of 0-0.3 mg/kg bw (Annex I, reference 47). The
primary degradation product of glyphosate in plants, soil, and water
is aminomethylphosphonic acid (AMPA), whose chemical structure is very
similar to that of glyphosate (Figure 1). AMPA itself has no
commercial use.
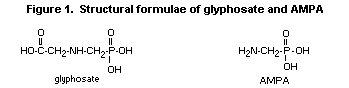 On the basis of the low residual levels of AMPA in crops which
are susceptible to glyphosate, the 1986 Joint Meeting concluded that
AMPA could be omitted from the residue in recommendations for MRLs,
but recent supervised trials on the application of glyphosate to
genetically modified crops have shown AMPA to be the main residue. As
residues of AMPA may therefore be of toxicological concern, the
compound was evaluated by the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Eight male Wistar rats weighing about 150 g were fasted for 4 h
and then given a single oral dose of 6.7 mg/kg bw 14C-AMPA by gavage.
Urine, faeces, and expired carbon dioxide (in 1 N NaOH) were analysed
for radiolabel 12, 24, 48, 72, 96, and 120 h after treatment (see
Table 1). After 120 h, a blood sample was taken and the animals were
killed; cage washes and tissues (see Table 2) were then analysed for
radiolabel. AMPA was moderately absorbed from the gut, as demonstrated
by excretion of 13% of the radiolabel in urine within 12 h, 18% within
24 h, and 20% within 120 h. Consequently, the major route of excretion
is the faeces: > 50% of the administered radiolabel was excreted
within 24 h and 74% by 120 h. In tissues, 0.06% of the radiolabel was
recovered after 120 h; < 0.1% was recovered as expired CO2 within 24
h. Minor residues were found in liver, kidneys, and muscle (Colvin et
al., 1973). Bone, bone-marrow, and eyes, which contain relatively high
residues of radiolabel (0.2-0.6% of the dose in comparison with
0.02-0.05% in other tissues) after oral administration of
14C-glyphosate to rats (Howe et al., 1988; Ridley & Mirly, 1988),
were not analysed in the study of Colvin et al. (1973).
(b) Biotransformation
Two groups of five Wistar rats of each sex received 10 mg/kg bw
14C-glyphosate orally, one group after preconditioning with
unlabelled glyphosate. Only 0.2-0.4% of the radiolabel recovered in
the excreta appeared to be AMPA (Howe et al., 1988).
Table 1. Recovery of radiolabel after a single oral dose of 6.7 mg/kg bw 14C-AMPA
Matrix Time after treatment (h)
0-12 12-24 24-48 48-72 72-96 96-120 0-120
Urine 13 4.5 1.5 0.66 02.3 0.05 20
Faeces 5.9 47 19 0.91 0.12 0.06 73
CO2 0.06 0.01 0 0 0 0 0.07
Cage wash 0.13
Tissues 0.06
Total 19 52 21 1.6 0.35 0.11 94
Table 2. Radiolabelled AMPA in tissues 120 h after a single oral
dose of 6.7 mg/kg bw
Tissue % of dose AMPA equivalent
(µg/kg fresh weight)
Liver 0.01 6
Kidney <0.01 6
Muscle 0.02 3
Fat 0.01 4
Gut 0.02 8
Spleen <0.01 4
Heart <0.01 4
Brain <0.01 1
Testis <0.01 3
Blood <0.01 3
Colvin et al. (1973) showed that most orally absorbed 14C-APMA
is excreted as unchanged compound in the urine of rats, as measured by
thin-layer and gas-liquid chromatography and nuclear magnetic
resonance and mass spectral analyses. The fact that < 0.1% of the
administered activity was expired as carbon dioxide also demonstrated
that only very small amounts of the absorbed material were
metabolized.
2. Toxicological studies
(a) Acute toxicity
Groups of two male and three female Sprague-Dawley rats were
given AMPA (purity unspecified) as a 40% solution in corn oil at one
of four doses by gavage and observed for seven days. Deaths occurred
at a dose of 8300 mg/kg bw after one to two days. Reduced appetite and
activity, increasing weakness, slight diarrhoea, collapse, and death
were observed. At autopsy, slight liver discolouration and acute
gastrointestinal inflammation were seen; the viscera appeared normal
macroscopically (Birch, 1973).
(b) Short-term toxicity
Rats
In a 14-day range-finding study available only in summary, groups
of five male and five female rats received AMPA at 0, 1000, 2000, or
4000 mg/kg bw per day in the diet. Moderately reduced body-weight gain
and food consumption (no further details) were seen at 4000 mg/kg bw
per day. No other effects were observed. The NOAEL was 2000 mg/kg bw
per day (Goldenthal, 1978).
In a 13-week study of toxicity, groups of 20 male and 20 female
weanling Charles River CD rats (aged four weeks) received AMPA
(purity, 99.9%) at 0, 400, 1200, or 4800 mg/kg bw per day in the diet.
The animals were observed for death and signs of overt toxicity twice
a day on seven days a week. Body weights and food consumption were
monitored weekly. Haematological, biochemical, and urinary parameters
were measured in 10 rats of each sex per group after 45 and 88 days;
the baseline values for these parameters were measured in an
additional 10 male and 10 female rats that were sacrificed for this
purpose. At the end of the study, the absolute and relative (to body
weight) weights of the liver, kidneys, testes/ovaries, heart, and
brain were determined in each animal, and macroscopy was performed.
About 30 tissues from controls and rats at the highest dose and liver,
kidneys, heart, urinary bladder, and tissues with gross lesions from
all rats at 400 and 1200 mg/kg bw per day were examined
microscopically.
One male and five female rats at 4800 mg/kg bw per day were found
dead after blood collection on day 45, and one female rat in the
control group and two female rats at 1200 mg/kg bw per day were dead
after blood collection on day 88. One male control rat found moribund
after observation of soft stools, distended abdomen, and morbidity was
killed at week 12. Body-weight gain was significantly decreased in
males and females at 4800 mg/kg bw per day (difference for females at
termination, < 10%) and in males at 1200 mg/kg bw per day
(difference, < 10%). The food consumption of treated males was lower
than that of control males, but the differences were not statistically
signifcant. No treatment-related changes in haematological parameters
were observed after 45 or 88 days. Biochemical analyses showed
increased lactic dehydrogenase activity in males and females at 4800
mg/kg bw per day, which was statistically significant for males after
45 and 88 days and for females after 88 days only. Males at 1200 mg/kg
bw per day also showed statistically significant increases in lactic
dehydrogenase activity after 88 days; females at 400 or 1200 mg/kg bw
per day showed significant increases in the activity of this enzyme
after 88 days, but the biological significance of these observations
is questionable in view of the magnitude of the increases and the low
control values in females. Glucose levels were significantly decreased
in males and females at 4800 mg/kg bw per day after 88 days in
comparison with control values, but the levels in animals at 400 or
1200 mg/kg bw per day were significantly increased after 45 days. At
4800 mg/kg bw per day, males and females showed increased activity of
aspartate aminotransferase, which was significant in females after 45
days and in both males and females after 88 days, and males showed
increased serum cholesterol levels, which were significant after both
45 and 88 days. The occasionally significant changes in other
biochemical parameters (albumin and bilirubin) did not show a
dose-response relationship and were considered not to be biologically
significant. Urinalysis revealed significant decreases in pH and
increased calcium oxalate crystals in males at 4800 mg/kg bw per day
after 88 days and in females after 45 and 88 days. The weights of
several organs showed significant changes from control values, but the
absence of any dose-response relationship or of morphological changes
in these organs make their biological significance questionable.
Macroscopy revealed no treatment-related changes. Microscopy showed
dose-related increases in the incidence and severity of irritation of
the mucosal and submucosal layers of the urinary tract in males and
females at 1200 or 4800 mg/kg bw per day, corresponding to hyperplasia
of the urinary bladder, which was more marked in males than in
females. Epithelial hyperplasia in the pelvic section was seen in the
kidneys of several rats at 4800 mg/kg bw per day, and some of the
hyperplastic epithelial cells contained a hyaline-like cytoplasmic
material. The NOAEL was 400 mg/kg bw per day (Estes et al., 1979).
Dogs
In a one-month range-finding study, groups of two male and two
female beagle dogs (aged about six months and weighing 7.3-9.8 kg for
males and 5.9-8.4 kg for females) received AMPA (purity, 94.4%) at 0,
10, 30, 100, 300, or 1000 mg/kg bw per day orally in gelatin capsules.
The animals were observed for mortality, moribundity, and signs of
toxicity twice daily and for detailed signs of toxicity once weekly.
Body weight and food consumption were determined weekly.
Haematological and biochemical analyses were carried out in all dogs
at termination; no urinalysis was performed. The absolute and relative
weights of the liver, kidneys, spleen, heart, brain, thyroid,
adrenals, and testes of all dogs were determined. Macroscopy was
performed on all animals, but no microscopy was done.
No unscheduled death occurred. Diarrhoea was seen in both males
and one female dog at 1000 mg/kg bw per day (total, 18 incidents), and
emesis was observed in both females and one male dog at the same dose
(four incidents). No effects on body-weight gain or food consumption
were seen. Males and females at 1000 mg/kg bw per day had decreased
haemoglobin and haematocrit values (significant in females), decreased
erythrocyte counts (significant in males and females), and increased
reticulocyte counts (significant in females). In addition, an increase
in mean corpuscular haemoglobin concentration was seen in males at
1000 mg/kg bw per day (significant) and 300 mg/kg bw per day, but with
no dose-response relationship. Females at 300 mg/kg bw per day had
significantly decreased haemoglobin and haematocrit values and a
nonsignificant decrease in reticulocyte count. Activated partial
prothrombin times were significantly decreased in females at all
doses, but these decreases were considered not to be biologically
significant because of their small size and the lack of a clear
dose-response relationship. No effects on biochemical parameters were
seen. Organ weights were unchanged, and macroscopy revealed no
treatment-related abnormalities. No adverse effects were seen at
100 mg/kg bw per day. There was no NOAEL owing to the limited number
of animals. The authors stated that no adverse effects were observed
at 300 mg/kg bw per day in males and 100 mg/kg bw per day in females
(Stout, 1991).
Groups of five male and five female beagle dogs (males weighing
8.1-12 kg and females, 6.8-11 kg) received AMPA (purity, 87.8%) at
doses of 0, 10, 30 100, or 300 mg/kg bw per day orally in gelatin
capsules for 91-92 days. The dogs were observed twice daily for
mortality, moribundity, and overt signs of toxicity. Ophthalmoscopy
was conducted on all dogs before the study and at week 12. Body
weights were recorded weekly and food consumption daily.
Haematological, biochemical, and urinary analyses were carried out in
all dogs before the study and during weeks 6 and 13. The absolute and
relative weights of the liver, kidneys, brain, thyroid, adrenals, and
ovaries/testes of all dogs were determined. All dogs were examined
macroscopically, and about 40 tissues and all gross lesions were
examined microscopically.
Treatment had no effect on survival, and ophthalmoscopy revealed
no abnormalities. Several clinical signs were seen at all doses and in
controls, but there was generally no clear dose-response relationship.
Only the incidence of scabbed, reddened, and/or swollen ears in males
at 300 mg/kg bw per day was increased; in females at this dose, the
incidence of these symptoms was below that in controls, the latter
being the same as that in males at 300 mg/kg bw per day. The incidence
of soft stools varied from group to group, with considerable
individual variability; no dose-response relationship was seen. No
significant treatment-related effects on body-weight gain or food
consumption were observed. Haematology showed occasionally significant
decreases in erythrocyte count, haemoglobin and haematocrit values,
mean corpuscular volume, mean corpuscular haemoglobin, or mean
corpuscular haemoglobin concentration at low doses. No relationship
with dose or time was seen, and these changes were considered not to
be biologically significant. No treatment-related effects on
biochemical or urinary parameters or organ weights were seen, and
there were no macroscopic or microscopic changes. The NOAEL was 300
mg/kg bw per day, the highest dose tested (Tompkins, 1991).
(c) Long-term toxicity and carcinogenicity
No long-term toxicity studies have been performed with AMPA
itself, but two-year toxicity studies are available in mice and rats
treated with technical-grade glyphosate contaminated at a low level
with AMPA (WHO, 1994).
In a two-year study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
mice at dietary levels of 0, 0.1, 0.5, or 3%, no treatment-related
increase in tumour incidence was seen. The NOAEL was 0.5%, equal to
810 mg/kg bw per day (Bio/Dynamics Inc., 1983).
In a 26-month study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
rats at dietary levels of 0.006, 0.02, or 0.06%, equal to about 3, 10,
or 32 mg/kg bw per day, no treatment-related increase in tumour
incidence was seen. The NOAEL was 32 mg/kg bw per day, the highest
dose tested (Bio/Dynamics Inc., 1981a).
In a two-year study of toxicity and carcinogenicity, rats were
given technical-grade glyphosate containing 0.68% AMPAin the diet to
provide doses of 0.2, 0.8, or 2% glyphosate, equivalent to 100, 400,
or 1000 mg/kg bw per day. The diets thus contained 14, 54, and 135 mg
of AMPA per kg, corresponding to 0.69, 2.8, and 7.2 mg/kg bw per day.
No increase in tumour incidence was seen. At the highest dose, female
body-weight gain was reduced, and cataracts were found in males. The
NOAEL was 0.8% in the diet, equivalent to 400 mg/kg bw per day
glyphosate and 2.8 mg/kg bw per day AMPA (Monsanto 1990a; Stout &
Ruecker, 1990).
(d) Genotoxicity
The results of tests for the genotoxicity of AMPA are shown in
Table 3. In the test for micronucleus formation, groups of 15 male and
15 female mice were used. Five animals of each sex per dose were
killed 24, 48, and 72 h after treatment. Toxicity was observed at 500
and 1000 mg/kg bw, as demonstrated by weight loss and listlessness. No
significant decrease in mean polychromatic erythrocytes:total
erythrocytes was seen at any dose. At 100 mg/kg bw, a significant
increase in mean nucleated polychromatic erythrocyte frequency was
seen after 72 h in females only; however, the increased frequency was
within the range in historical vehicle controls in the same
laboratory, and the frequency was not increased in males at 100 mg/kg
bw or in males or females at higher doses. The increase in mean
nucleated polychromatic erythrocyte frequency in females at 100 mg/kg
bw was thus considered not to be related to treatment (Kier &
Stegeman, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
No multigeneration studies of reproductive toxicity have been
performed with AMPA itself, but two studies were reported in rats
treated with technical-grade glyphosate contaminated at low levels
with AMPA (WHO, 1994).
In a three-generation study of reproductive toxicity, rats were
given diets providing technical-grade glyphosate (AMPA content not
specified) at doses of 0, 3, 10, or 30 mg/kg bw per day. The only
effect was an increased incidence of unilateral renal tubular
dilatation in male F3b pups at 30 mg/kg bw per day (6/10 versus 0/10
in controls); F1 and F2 pups were not examined (Bio/Dynamics Inc.,
1981b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Sprague-Dawley rats received diets containing
technical-grade glyphosate with 0.61% AMPA (Reyna, 1990), providing 0,
0.2, 1, or 3% glyphosate and 0, 12, 61, or 180 ppm AMPA. Parental
animals receiving 3% glyphosate had soft stools, decreased body
weights, and slightly decreased litter sizes; pup weights were
decreased on days 14 and 21 of lactation. Parental and pup body
weights were also slightly decreased after treatment with 1.0%. The
NOAEL was 1.0% in the diet, corresponding to 740 mg/kg bw per day
glyphosate and 3 mg/kg bw per day AMPA (Monsanto, 1990b). The renal
changes seen in the three-generation study were thus not reproduced;
however, the numbers of pups examined histologically was limited in
both studies.
(ii) Developmental toxicity
In a range-finding study, groups of eight pregnant Sprague-Dawley
rats received AMPA (purity, 94.4%) in corn oil at doses of 0, 125,
250, 500, 750, or 1000 mg/kg bw by gavage daily on days 6-15 of
gestation. All animals were killed on day 20 of gestation. Clinical
observations, body weights, net body-weight changes, and gravid
uterine weights of the dams were recorded, and the weights of the
liver, kidney, and spleen were determined. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
Table 3. Results of tests for the genotoxicity of AMPA
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 99 Negativeb Shirasu et al. (1980)
TA98, TA100, in distilled water
TA1535, TA1537,
TA1538, and
E. coli WP2
DNA repairc B. subtilis H17, 20-2000 µg/disc 99 Negatived Shirasu et al. (1980)
M45 (rec+/-) in distilled water
Unscheduled DNA Primary hepatocytes 5-5000 µg/ml in 94.4 Negative Bakke (1991)
synthesise from male Fischer culture medium
344 rats
In vivo
Micronucleus CD-1 mice Single intraperitoneal 94.4 Negative Kier & Stegeman
formulationf injection of 100, 500, (1993)
or 1000 mg/kg bw in
corn oil
a No independent duplicate study but duplicate plates at each concentration; distilled water used as solvent; solvent and
positive controls included
b With and without metabolic activation
c No independent duplicate study; distilled water used as solvent; solvent and positive controls included
d Only without metabolic activation
e Cytotoxicity at 3800 and 5000 µg/ml
f No characterization of test or control substances or their concentrations, homogeneity in carrier, or stability of test
and control substances neat and after mixing with carrier
weighed, sexed, and examined externally for developmental
abnormalities and variations. No effect on survival or body or organ
weights was seen in the dams. Greyish faeces were observed
infrequently in animals at doses > 125 mg/kg bw per day. The
predominant findings 1 h after treatment were red staining around the
nose in one or two animals per treated group and a low incidence of
salivation in those at 250, 500, 750, or 1000 mg/kg bw per day. In the
absence of a clear dose-response relationship and the limited
findings, they did not appear to be treatment-related. Fetuses had no
treatment-related effects (Holson, 1991a).
Groups of 25 pregnant Sprague-Dawley rats received AMPA (purity,
94.4%) in corn oil at doses of 0, 150, 400, or 1000 mg/kg bw by
gavage, daily on days 6-15 of gestation. All animals were killed on
day 20 of gestation. The dams were observed twice daily for
moribundity and mortality, and signs of toxicity about 1 h after
treatment and clinical observations on days 0-20 of gestation were
recorded. The body weights and food consumption of the dams were
determined on gestation days 0, 6, 9, 12, 16, and 20. Gravid uterine
weights and net body-weight changes were recorded, and the liver,
kidney, and spleen of all dams were weighed. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
abnormalities or variations.
Maternal survival was not affected. Dams at 400 or 1000 mg/kg bw
per day had dose-related increased incidences of mucoid faeces, hair
loss, and soft stools. Dams at 150 mg/kg bw per day had only an
increased incidence of soft stools. The total occurrence of soft
stools/no. of animals was 49/15 in controls, 89/19 in dams at 150
mg/kg bw per day, 102/22 at 400 mg/kg bw per day, and 135/23 at 1000
mg/kg bw per day. At the highest dose, the dams had short periods of
slightly decreased body-weight gain and food consumption. No effect
was seen on the weights of the liver, kidney, spleen, or gravid
uterus. Fetal survival was not affected. A slight but significant
decrease in mean fetal body weight was seen at 1000 mg/kg bw per day.
No further developmental effect was seen, and there were no
teratogenic effects. Because the increased incidence of soft stools at
150 mg/kg bw per day was not accompanied by possibly associated
effects such as hair loss and mucoid faeces, this dose is the NOAEL
for maternal toxicity. The NOAEL for developmental toxicity was 400
mg/kg bw per day, as the authors stated that the only possible
maternal responses at this dose were mucoid faeces, soft stools, and
hair loss (Holson, 1991b).
(f) Special studies: Dermal and ocular irritation
Two male and one female albino rabbits (strain not specified)
received an application of 0.5 g finely ground AMPA moistened with
water on the clipped intact skin (6.5 cm2), which was kept under
cover for 24 h. Observations were made after 1, 24, 48, 72, 120, and
168 h. No skin irritation was seen (Birch, 1973). Only a limited
report was available.
One male and two female albino rabbits (strain not specified)
received an application of 0.1 g finely ground AMPA into one eye. Both
the control and the treated eye were rinsed with warm isotonic saline
after 24 h. Observations were made after 1, 24, 48, 72, 120, and 168 h
and scored according to Draize. Slight erythema, very slight oedema,
and copious discharge were seen after 10 min and after 1 h, and
slight-to-moderate erythema, a moderate discharge containing a white
exudate, and no oedema were seen after 24 h. After 72 h, slight
erythema was seen in one animal, but without oedema or discharge.
After 120 and 168 h, the treated eyes were normal. The average scores
were 10 out of 110 after 1 h and 7.3, 2.6, and 0.6 after 24, 48, and
72 h, respectively. AMPA was considered by the author to be a slight
ocular irritant; however, the average score after 24, 48, and 72 h was
3.5, indicating that AMPA is not an ocular irritant (Birch, 1973). In
the limited report, separate scores were not given for conjunctivae,
iris, and cornea.
3. Toxicological profiles of AMPA and glyphosate
The results of toxicological studies of the metabolite AMPA and of its
parent compound glyphosate, as previously evaluated (Annex 1,
reference 47; WHO, 1994), are compared in Table 4. AMPA was no more
toxic than glyphosate. Similar effects were often found,e xcept for
the lesions in the salivary gland seen in the 90-day studies of
toxicity and carcinogenicity with glyphosate in mice nad rats from the
US national Toxicology Program and the cataracts induced by glyphosate
in a two-year toxicity study in rats. Such effects were not seen in
other studies with glyphosate or AMPA.
Comments
After oral administration of AMPA to rats, 20% of the dose was
absorbed and excreted unmetabolized in the urine within 120 h (17% of
the dose within 24 h), and 73% of the dose was eliminated in the
faeces. Only 0.07% of the dose was excreted as expired carbon dioxide
within 24 h, and 0.06% of the dose was recovered from tissues after
120 h. Minor amounts (1-6 µg/kg) were found in tissues after 120 h.
AMPA is slightly hazardous to rats given a single oral dose, with
an LD50 of 8300 mg/kg bw (WHO, 1996).
In a 90-day study of toxicity, rats received AMPA in the diet at
0, 400, 1200, or 4800 mg/kg bw per day. A significant, dose-related
decrease in body-weight gain was seen in males at the two highest
doses and in females at the highest dose. The two highest doses also
resulted in significantly increased lactate dehydrogenase activity,
whereas aspartate aminotransferase activity and cholesterol levels
were significantly increased only at the highest dose. Urinalysis
showed a significant decrease in urinary pH and increased amounts of
calcium oxalate crystals in the urine of animals at the highest dose.
Table 4. Toxicity of AMPA and technical-grade glyphosate
Type of study AMPA Glyphosatea
Metabolism 20% absorption after oral exposure; excreted 36% absorption after oral exposure;
unchanged in urine; only 0.07% expired as essentially no biotransformation (0.2-
CO2; minor residues in tissues 0.4% AMPA)
Acute oral toxicity LD50 = 8300 mg/kg bw LD50 > 5000 mg/kg bw
90-day toxicity, diet, mice NOAEL = 10 000 ppm, equal to 1890
me/kg bw. Effects at LOAEL of 50 000
ppm: decreased growth, increased brain,
heart, and kidney weights
90-day toxicity, diet, mice NOAEL = 3125 ppm, equal to 505 mg/
kg bw. Effects at LOAEL of 6250 ppm:
lesions of salivary gland
90-day toxicity, diet, rats NOAEL = 400 mg/kg bw. Effects at LOAEL No effect at highest dose of 20 000 ppm,
of 1200 mg.kg bw: biochemical changes, equal to 1300 mg/kg bw
changes in urinary bladder. At 4800 mg/kg bw,
also renal changes
90-day toxicity, diet, rats NOAEL < 3100 ppm, equal to < 200 mg/
kg bw; lesions of salivary gland
90-day toxicity, diet, rats No effect at highest dose of 12 500 ppm,
equal to 1300 mg/kg bw
90-day toxicity, gelatin capsule,
dogs No effect at highest dose of 300 mg/kg bw
52-week toxicity, gelatin capsule,
dogs No effect at highest dose of 500 mg/kg bw
Table 4. (continued)
Type of study AMPA Glyphosatea
Two-year toxicity and carcinogenicity, NOAEL = 5000 ppm, equal to 810 mg/
diet, mice kg bw. Effects at LOAEL of 30 000
ppm: decreased growth, changes in
urinary bladder and kidneys
Two-year toxicity and carcinogenicity, No effect at highest dose of 600 ppm,
diet, rats equal to 32 mg/kg bw; slight decrease in
growth considered not to be relevant
Two-year toxicity and carcinogenicity, NOAEL = 8000 ppm, equivalent to 400
diet, rats mg/kg bw. Effects at LOAEL of 20 000
ppm: decreased growth and cataracts.
Technical-grade glyphosate containing
0.68% AMPA
Three-generation reproductive Increased incidence (6/10 versus 1/10 in
toxicity, diet, rats controls) of unilateral renal tubular
dilatation in F3b pups at highest dose of
30 mg/kg bw; not seen in more recent
study (below)
Two-generation reproductive NOAEL = 10 000 ppm, equivalent to
toxicity, diet, rats 500 mg/kg bw. Effects at LOAEL of
30 000 ppm: soft stools, decreased
weights of parental animals and pups.
Technical-grade glyphosate containing
0.61% AMPA
Developmental toxicity, rats NOAEL for maternal toxicity = 150 mg/kg NOAEL = 1000 mg/kg bw. Effects at
bw; NOAEL for developmental toxicity = 400 LOAEL of 3500 mg/kg bw: deaths,
mg/kg bw. At 400 mg/kg bw, increased clinical signs, decreased growth of dams,
incidences of soft stools, mucoid faeces, and resorptions, decreased implantations and
hair loss in dams. At 1000 mg/kg bw, slight visible fetuses, decreased ossification of
decrease in fetal body weight fetal sternebrae; no fetal malformations
Table 4. (continued)
Type of study AMPA Glyphosatea
Developmental toxicity, rabbits NOAEL = 175 mg/kg bw. Effects at
LOAEL of 350 mg/kg bw: diarrhoea,
soft stools, and nasal discharge in dams
Genotoxicity
Reverse mutation, S. typhimurium Negative Negative
Reverse mutation, E. coli Negative Negative
DNA repair, B. subtilis rec Negative Negative
Gene mutation, Chinese hamster ovary Negative
cells
Unscheduled DNA synthesis, rat Negative Negative
hepatocytes
Chromosomal aberration in vivo, rat Negative
bone marrow
Micronucleus formation in vivo, Negative Negative
mouse bone marrow
Dominant lethal mutation in vivo, Negative
mice
Recessive sex-linked lethal mutation, Negative
Drosophila melanogaster
a From WHO (1994) and Annex I, reference 47
Dose-related irritation of the mucosal and submucosal layers of the
urinary tract, corresponding to hyperplasia of the urinary bladder,
was seen in rats at 1200 and 4800 mg/kg bw per day, the effect being
more marked in males than in females. In addition, epithelial
hyperplasia in the renal pelvis was observed at the highest dose. The
NOAEL was 400 mg/kg bw per day.
In a 90-day study of toxicity in dogs receiving AMPA at 0, 10,
30, 100, or 300 mg/kg bw per day in gelatin capsules, no statistically
significant treatment-related changes were observed. The NOAEL was
thus the highest dose, 300 mg/kg bw per day. It should be noted that
in a one-month range-finding study with groups of only two male and
two female dogs, changes in some haematological parameters (e.g.
decreased haemoglobin, packed cell volume, and erythrocyte counts)
were seen in animals at 300 or 1000 mg/kg bw per day. These effects
were not reproduced in the 90-day study.
No indication of genotoxic activity was seen in studies of gene
mutation in bacteria, of DNA repair in bacteria and mammalian cells
in vitro, or of micronucleus formation in vivo. No assays for gene
mutation were performed in mammalian cells in vitro, but the
structural similarity of AMPA to glyphosate and the lack of
genotoxicity of glyphosate, including in an assay for gene mutation in
mammalian cells in vitro, indicate that such an assay with AMPA
would be redundant.
In a study of developmental toxicity, rats received AMPA at 0,
150, 400, or 1000 mg/kg bw per day in corn oil by gavage. Dose-related
increases in the incidences of soft stools, mucoid faeces, and hair
loss were seen in dams at the two higher doses. Dams at the highest
dose also had short periods of decreased body-weight gain and food
consumption. Fetal body weight was decreased at 1000 mg/kg bw per day.
No teratogenic effects were observed. Dams at 150 mg/kg bw per day
also had an increased incidence of soft stools; however, in the
absence of any associated effects, such as hair loss or mucoid faeces,
the Meeting considered this dose to be the NOAEL for maternal
toxicity. The NOAEL for developmental toxicity was 400 mg/kg bw per
day.
AMPA did not induce dermal or ocular irritation in rabbits.
No long-term study of the toxicity or carcinogenicity of AMPA has
been carried out, but in the more recent of two such studies with
technical-grade glyphosate in rats at dietary levels of 0.2, 0.8, or
2%, the AMPA content of the test compound was given, namely 0.68%. At
the highest dose of 2% glyphosate in the diet, females showed
decreased body-weight gain and males showed an increased incidence of
degenerative lenticular changes. The NOAEL for technical-grade
glyphosate was 0.8% in the diet, corresponding to 400 mg/kg bw per day
for glyphosate and 2.7 mg/kg bw per day for AMPA. No increase in
tumour incidence was seen in this study.
No multigeneration study of the reproductive toxicity of AMPA has
been reported, but in a recent two-generation study in rats with
technical-grade glyphosate at dietary levels of 0.2, 1, or 3%, the
test compound contained 0.61% AMPA. At the highest dose, soft stools,
decreased parental body weights, slightly decreased litter sizes, and
decreased pup weights were observed. The NOAEL was 1% in the diet,
corresponding to 740 mg/kg bw per day glyphosate and 4.5 mg/kg bw per
day AMPA.
Glyphosate and AMPA have very similar chemical structures.
Studies of the metabolism of glyphosate in experimental animals
indicate that essentially none is biotransformed into AMPA.
Toxicological data on the metabolite are therefore essential for risk
assessment. The Meeting compared the toxicity profile of AMPA with
that of glyphosate and concluded that the major targets of the
toxicity of AMPA had been investigated. The results showed little
toxicity. The Meeting concluded that the two compounds have similar
toxicological profiles and concluded that a full database on AMPA is
unnecessary. AMPA was considered to be of no greater toxicological
concern than its parent compound. The Meeting established a group ADI
for AMPA alone or in combination with glyphosate of 0-0.3 mg/kg bw on
the basis of the 26-month study of toxicity in rats fed
technical-grade glyphosate, using a safety factor of 100.
Since the last JMPR evaluation fof glyphosate or toxicity in
1986, new data have become available, some of which are evaluated in
WHO (1994). The Meeting recommended that glyphosate be re-evaluated by
the JMPR.
Toxicological evaluation
Levels that cause no toxic effect
AMPA
Rat: 400 mg/kg bw per day (90-day study of toxicity)
150 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
400 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Dog: 300 mg/kg bw per day (highest dose in 90-day study of
toxicity)
Glyphosate (from Annex I, reference 47):
Mouse: 0.5% in the diet, equal to 810 mg/kg bw per day (two-
year study of toxicity and carcinogenicity)
Rat: 31 mg/kg bw per day (26-month study of toxicity and
carcinogenicity)
Dog: 500 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.3 mg/kg bw (sum of glyphosate and AMPA)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to aminomethylphosphonic acid (AMPA)
Human exposure Relevant route, study type, species Results/remarks
Short-term Oral toxicity, rat LD50 = 8300 mg/kg bw
(1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Not irritating
Skin sensitization No data
Medium-term Repeated oral, 90 days, toxicity, rat NOAEL = 400 mg/kg bw per day: urinary tract changes
(1-26 weeks) Repeated oral, developmental toxicity, rat NOAEL = 150 mg/kg bw per day: maternal toxicity
NOAEL = 400 mg/kg bw per day: developmental
toxicity
Repeated oral, reproductive toxicity No data
Long-term Repeated oral, toxicity No data
(> 1 year)
References
Bakke, J.P. (1991) Evaluation of the potential of AMPA to induce
unscheduled DNA synthesis in the in vitro hepatocyte DNA repair
assay using the male F-344 rat. Unpublished study No. 2495-V01-91 from
SRI International, Menlo Park, California, USA (SRI Project LSC-2495;
Monsanto study No. SR-91-234, dated 4 December 1991). Submitted to WHO
by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project N0. 410/77 [BDN-77-416]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1981b) A three generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]). Final report.
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1983) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Birch, M.D. (1973) Toxicological investigation of CP 50435 Lot:
XHD-16. Unpublished report from Younger Laboratories Inc., St Louis,
Missouri, USA, dated 7 March 1973. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Colvin, L.B., Moran, S.J. & Miller, J.A. (1973) Final Report on CP
67573 residue and metabolism. Part 11: The metabolism of
aminomethylphosphonic acid-14C (CP 50435-14C) in the laboratory rat.
Job No. 9-23-760.06-7863. Unpublished report from Monsanto Commercial
Products Co., Agricultural Research Report No. 303 dated August 1973.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Estes, F.L., Jefferson, N.D., Blair, M. & Goldenthal, E.I. (1979)
90-Day subacute rat toxicity study (IRD-78-174). Test article: CP
50435. Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, dated 15 August 1979. Submitted
to WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Goldenthal, E.I. (1978) CP 50435 (aminomethyl phosphonic acid)
fourteen day rat feeding study. Unpublished report No. IRD-77-319 from
Monsanto. Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Holson, J.F. (1991a) A dose range-finding developmental toxicity study
of AMPA in rats. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. WIL Project No.: WIL-50146.
Sponsor No.: WI-90-247, dated 21 May 1991. Submitted to WHO by
Monsanto, Agricultural Group, St Louis, Missouri, USA.
Holson, J.F. (1991b) A developmental toxicity study of AMPA in rats.
Final report. Unpublished report from WIL Research Laboratories Inc.,
Ashland, Ohio, USA. WIL Project No.: WIL-50159. Sponsor No.:
WI-90-266, dated 6 August 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Howe, R.K., Chott, R.C. & McClanahan, R.H. (1988) Volume 2: Metabolism
of glyphosate in Sprague-Dawley rats. Part II. Identification,
characterization, and quantitation of glyphosate and its metabolites
after intravenous and oral administration. Unpublished report from
Monsanto Co., St Louis, Missouri, USA. Laboratory project no.
MSL-7206, dated February 1988. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Kier, L.D. & Stegeman, S.D. (1993) Final report. Mouse micronucleus
study of AMPA. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. EHL Study Number: 90170.
Sponsor Project Number: ML-90-404. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Monsanto (1990a) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). Monsanto Environmental Health
Laboratory, St Louis, Missouri, USA. Unpublished report submitted
to WHO by Monsanto, St Louis, Missouri, USA.
Monsanto (1990b) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). Monsanto
Environmental Health Laboratory, St Louis, Missouri, USA. Unpublished
report submitted to WHO by Monsanto, St Louis, Missouri, USA.
Reyna, M.S. (1990) Two generation reproduction study with glyphosate
in Sprague-Dawley rats. Unpublished Report no. ML-88-106. Submitted to
WHO by Monsanto, St Louis, Missouri, USA.
Ridley, W.P. & Mirly (1988) Volume 1: The metabolism of glyphosate in
Sprague-Dawley rats. Part I. Excretion and tissue distribution of
glyphosate and its metabolites following intravenous and oral
administration. Unpublished report MSL-7215 from Monsanto Co.,
Environmental Health Laboratory, St Louis, Missouri, USA. Submitted to
WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) CP 50435: Microbial
mutagenicity study. Unpublished report from the Institute of
Environmental Toxicology Kodaira, Tokyo, Japan, dated November 1980.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Stout, L.D. (1991) One month study of AMPA administered by capsule to
beagle dogs. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. Study Number 90074. Project
Number: ML-90-186. Submitted to WHO by Monsanto, Agricultural Group,
St Louis, Missouri, USA.
Stout, L.D. & Ruecker, F.A. (1990) Chronic study of glyphosate
administered in feed to albino rats. Unpublished report no. ML-90-186.
Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Tompkins, E.C. (1991) 90-Day oral (capsule) toxicity study in dogs
with AMPA. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. Project No.: WIL-50173. Sponsor
No.: WI-90-354, dated 16 July 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
WHO (1994) Environmental Health Criteria 159. Glyphosate. Geneva,
International Programme on Chemical Safety.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
On the basis of the low residual levels of AMPA in crops which
are susceptible to glyphosate, the 1986 Joint Meeting concluded that
AMPA could be omitted from the residue in recommendations for MRLs,
but recent supervised trials on the application of glyphosate to
genetically modified crops have shown AMPA to be the main residue. As
residues of AMPA may therefore be of toxicological concern, the
compound was evaluated by the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Eight male Wistar rats weighing about 150 g were fasted for 4 h
and then given a single oral dose of 6.7 mg/kg bw 14C-AMPA by gavage.
Urine, faeces, and expired carbon dioxide (in 1 N NaOH) were analysed
for radiolabel 12, 24, 48, 72, 96, and 120 h after treatment (see
Table 1). After 120 h, a blood sample was taken and the animals were
killed; cage washes and tissues (see Table 2) were then analysed for
radiolabel. AMPA was moderately absorbed from the gut, as demonstrated
by excretion of 13% of the radiolabel in urine within 12 h, 18% within
24 h, and 20% within 120 h. Consequently, the major route of excretion
is the faeces: > 50% of the administered radiolabel was excreted
within 24 h and 74% by 120 h. In tissues, 0.06% of the radiolabel was
recovered after 120 h; < 0.1% was recovered as expired CO2 within 24
h. Minor residues were found in liver, kidneys, and muscle (Colvin et
al., 1973). Bone, bone-marrow, and eyes, which contain relatively high
residues of radiolabel (0.2-0.6% of the dose in comparison with
0.02-0.05% in other tissues) after oral administration of
14C-glyphosate to rats (Howe et al., 1988; Ridley & Mirly, 1988),
were not analysed in the study of Colvin et al. (1973).
(b) Biotransformation
Two groups of five Wistar rats of each sex received 10 mg/kg bw
14C-glyphosate orally, one group after preconditioning with
unlabelled glyphosate. Only 0.2-0.4% of the radiolabel recovered in
the excreta appeared to be AMPA (Howe et al., 1988).
Table 1. Recovery of radiolabel after a single oral dose of 6.7 mg/kg bw 14C-AMPA
Matrix Time after treatment (h)
0-12 12-24 24-48 48-72 72-96 96-120 0-120
Urine 13 4.5 1.5 0.66 02.3 0.05 20
Faeces 5.9 47 19 0.91 0.12 0.06 73
CO2 0.06 0.01 0 0 0 0 0.07
Cage wash 0.13
Tissues 0.06
Total 19 52 21 1.6 0.35 0.11 94
Table 2. Radiolabelled AMPA in tissues 120 h after a single oral
dose of 6.7 mg/kg bw
Tissue % of dose AMPA equivalent
(µg/kg fresh weight)
Liver 0.01 6
Kidney <0.01 6
Muscle 0.02 3
Fat 0.01 4
Gut 0.02 8
Spleen <0.01 4
Heart <0.01 4
Brain <0.01 1
Testis <0.01 3
Blood <0.01 3
Colvin et al. (1973) showed that most orally absorbed 14C-APMA
is excreted as unchanged compound in the urine of rats, as measured by
thin-layer and gas-liquid chromatography and nuclear magnetic
resonance and mass spectral analyses. The fact that < 0.1% of the
administered activity was expired as carbon dioxide also demonstrated
that only very small amounts of the absorbed material were
metabolized.
2. Toxicological studies
(a) Acute toxicity
Groups of two male and three female Sprague-Dawley rats were
given AMPA (purity unspecified) as a 40% solution in corn oil at one
of four doses by gavage and observed for seven days. Deaths occurred
at a dose of 8300 mg/kg bw after one to two days. Reduced appetite and
activity, increasing weakness, slight diarrhoea, collapse, and death
were observed. At autopsy, slight liver discolouration and acute
gastrointestinal inflammation were seen; the viscera appeared normal
macroscopically (Birch, 1973).
(b) Short-term toxicity
Rats
In a 14-day range-finding study available only in summary, groups
of five male and five female rats received AMPA at 0, 1000, 2000, or
4000 mg/kg bw per day in the diet. Moderately reduced body-weight gain
and food consumption (no further details) were seen at 4000 mg/kg bw
per day. No other effects were observed. The NOAEL was 2000 mg/kg bw
per day (Goldenthal, 1978).
In a 13-week study of toxicity, groups of 20 male and 20 female
weanling Charles River CD rats (aged four weeks) received AMPA
(purity, 99.9%) at 0, 400, 1200, or 4800 mg/kg bw per day in the diet.
The animals were observed for death and signs of overt toxicity twice
a day on seven days a week. Body weights and food consumption were
monitored weekly. Haematological, biochemical, and urinary parameters
were measured in 10 rats of each sex per group after 45 and 88 days;
the baseline values for these parameters were measured in an
additional 10 male and 10 female rats that were sacrificed for this
purpose. At the end of the study, the absolute and relative (to body
weight) weights of the liver, kidneys, testes/ovaries, heart, and
brain were determined in each animal, and macroscopy was performed.
About 30 tissues from controls and rats at the highest dose and liver,
kidneys, heart, urinary bladder, and tissues with gross lesions from
all rats at 400 and 1200 mg/kg bw per day were examined
microscopically.
One male and five female rats at 4800 mg/kg bw per day were found
dead after blood collection on day 45, and one female rat in the
control group and two female rats at 1200 mg/kg bw per day were dead
after blood collection on day 88. One male control rat found moribund
after observation of soft stools, distended abdomen, and morbidity was
killed at week 12. Body-weight gain was significantly decreased in
males and females at 4800 mg/kg bw per day (difference for females at
termination, < 10%) and in males at 1200 mg/kg bw per day
(difference, < 10%). The food consumption of treated males was lower
than that of control males, but the differences were not statistically
signifcant. No treatment-related changes in haematological parameters
were observed after 45 or 88 days. Biochemical analyses showed
increased lactic dehydrogenase activity in males and females at 4800
mg/kg bw per day, which was statistically significant for males after
45 and 88 days and for females after 88 days only. Males at 1200 mg/kg
bw per day also showed statistically significant increases in lactic
dehydrogenase activity after 88 days; females at 400 or 1200 mg/kg bw
per day showed significant increases in the activity of this enzyme
after 88 days, but the biological significance of these observations
is questionable in view of the magnitude of the increases and the low
control values in females. Glucose levels were significantly decreased
in males and females at 4800 mg/kg bw per day after 88 days in
comparison with control values, but the levels in animals at 400 or
1200 mg/kg bw per day were significantly increased after 45 days. At
4800 mg/kg bw per day, males and females showed increased activity of
aspartate aminotransferase, which was significant in females after 45
days and in both males and females after 88 days, and males showed
increased serum cholesterol levels, which were significant after both
45 and 88 days. The occasionally significant changes in other
biochemical parameters (albumin and bilirubin) did not show a
dose-response relationship and were considered not to be biologically
significant. Urinalysis revealed significant decreases in pH and
increased calcium oxalate crystals in males at 4800 mg/kg bw per day
after 88 days and in females after 45 and 88 days. The weights of
several organs showed significant changes from control values, but the
absence of any dose-response relationship or of morphological changes
in these organs make their biological significance questionable.
Macroscopy revealed no treatment-related changes. Microscopy showed
dose-related increases in the incidence and severity of irritation of
the mucosal and submucosal layers of the urinary tract in males and
females at 1200 or 4800 mg/kg bw per day, corresponding to hyperplasia
of the urinary bladder, which was more marked in males than in
females. Epithelial hyperplasia in the pelvic section was seen in the
kidneys of several rats at 4800 mg/kg bw per day, and some of the
hyperplastic epithelial cells contained a hyaline-like cytoplasmic
material. The NOAEL was 400 mg/kg bw per day (Estes et al., 1979).
Dogs
In a one-month range-finding study, groups of two male and two
female beagle dogs (aged about six months and weighing 7.3-9.8 kg for
males and 5.9-8.4 kg for females) received AMPA (purity, 94.4%) at 0,
10, 30, 100, 300, or 1000 mg/kg bw per day orally in gelatin capsules.
The animals were observed for mortality, moribundity, and signs of
toxicity twice daily and for detailed signs of toxicity once weekly.
Body weight and food consumption were determined weekly.
Haematological and biochemical analyses were carried out in all dogs
at termination; no urinalysis was performed. The absolute and relative
weights of the liver, kidneys, spleen, heart, brain, thyroid,
adrenals, and testes of all dogs were determined. Macroscopy was
performed on all animals, but no microscopy was done.
No unscheduled death occurred. Diarrhoea was seen in both males
and one female dog at 1000 mg/kg bw per day (total, 18 incidents), and
emesis was observed in both females and one male dog at the same dose
(four incidents). No effects on body-weight gain or food consumption
were seen. Males and females at 1000 mg/kg bw per day had decreased
haemoglobin and haematocrit values (significant in females), decreased
erythrocyte counts (significant in males and females), and increased
reticulocyte counts (significant in females). In addition, an increase
in mean corpuscular haemoglobin concentration was seen in males at
1000 mg/kg bw per day (significant) and 300 mg/kg bw per day, but with
no dose-response relationship. Females at 300 mg/kg bw per day had
significantly decreased haemoglobin and haematocrit values and a
nonsignificant decrease in reticulocyte count. Activated partial
prothrombin times were significantly decreased in females at all
doses, but these decreases were considered not to be biologically
significant because of their small size and the lack of a clear
dose-response relationship. No effects on biochemical parameters were
seen. Organ weights were unchanged, and macroscopy revealed no
treatment-related abnormalities. No adverse effects were seen at
100 mg/kg bw per day. There was no NOAEL owing to the limited number
of animals. The authors stated that no adverse effects were observed
at 300 mg/kg bw per day in males and 100 mg/kg bw per day in females
(Stout, 1991).
Groups of five male and five female beagle dogs (males weighing
8.1-12 kg and females, 6.8-11 kg) received AMPA (purity, 87.8%) at
doses of 0, 10, 30 100, or 300 mg/kg bw per day orally in gelatin
capsules for 91-92 days. The dogs were observed twice daily for
mortality, moribundity, and overt signs of toxicity. Ophthalmoscopy
was conducted on all dogs before the study and at week 12. Body
weights were recorded weekly and food consumption daily.
Haematological, biochemical, and urinary analyses were carried out in
all dogs before the study and during weeks 6 and 13. The absolute and
relative weights of the liver, kidneys, brain, thyroid, adrenals, and
ovaries/testes of all dogs were determined. All dogs were examined
macroscopically, and about 40 tissues and all gross lesions were
examined microscopically.
Treatment had no effect on survival, and ophthalmoscopy revealed
no abnormalities. Several clinical signs were seen at all doses and in
controls, but there was generally no clear dose-response relationship.
Only the incidence of scabbed, reddened, and/or swollen ears in males
at 300 mg/kg bw per day was increased; in females at this dose, the
incidence of these symptoms was below that in controls, the latter
being the same as that in males at 300 mg/kg bw per day. The incidence
of soft stools varied from group to group, with considerable
individual variability; no dose-response relationship was seen. No
significant treatment-related effects on body-weight gain or food
consumption were observed. Haematology showed occasionally significant
decreases in erythrocyte count, haemoglobin and haematocrit values,
mean corpuscular volume, mean corpuscular haemoglobin, or mean
corpuscular haemoglobin concentration at low doses. No relationship
with dose or time was seen, and these changes were considered not to
be biologically significant. No treatment-related effects on
biochemical or urinary parameters or organ weights were seen, and
there were no macroscopic or microscopic changes. The NOAEL was 300
mg/kg bw per day, the highest dose tested (Tompkins, 1991).
(c) Long-term toxicity and carcinogenicity
No long-term toxicity studies have been performed with AMPA
itself, but two-year toxicity studies are available in mice and rats
treated with technical-grade glyphosate contaminated at a low level
with AMPA (WHO, 1994).
In a two-year study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
mice at dietary levels of 0, 0.1, 0.5, or 3%, no treatment-related
increase in tumour incidence was seen. The NOAEL was 0.5%, equal to
810 mg/kg bw per day (Bio/Dynamics Inc., 1983).
In a 26-month study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
rats at dietary levels of 0.006, 0.02, or 0.06%, equal to about 3, 10,
or 32 mg/kg bw per day, no treatment-related increase in tumour
incidence was seen. The NOAEL was 32 mg/kg bw per day, the highest
dose tested (Bio/Dynamics Inc., 1981a).
In a two-year study of toxicity and carcinogenicity, rats were
given technical-grade glyphosate containing 0.68% AMPAin the diet to
provide doses of 0.2, 0.8, or 2% glyphosate, equivalent to 100, 400,
or 1000 mg/kg bw per day. The diets thus contained 14, 54, and 135 mg
of AMPA per kg, corresponding to 0.69, 2.8, and 7.2 mg/kg bw per day.
No increase in tumour incidence was seen. At the highest dose, female
body-weight gain was reduced, and cataracts were found in males. The
NOAEL was 0.8% in the diet, equivalent to 400 mg/kg bw per day
glyphosate and 2.8 mg/kg bw per day AMPA (Monsanto 1990a; Stout &
Ruecker, 1990).
(d) Genotoxicity
The results of tests for the genotoxicity of AMPA are shown in
Table 3. In the test for micronucleus formation, groups of 15 male and
15 female mice were used. Five animals of each sex per dose were
killed 24, 48, and 72 h after treatment. Toxicity was observed at 500
and 1000 mg/kg bw, as demonstrated by weight loss and listlessness. No
significant decrease in mean polychromatic erythrocytes:total
erythrocytes was seen at any dose. At 100 mg/kg bw, a significant
increase in mean nucleated polychromatic erythrocyte frequency was
seen after 72 h in females only; however, the increased frequency was
within the range in historical vehicle controls in the same
laboratory, and the frequency was not increased in males at 100 mg/kg
bw or in males or females at higher doses. The increase in mean
nucleated polychromatic erythrocyte frequency in females at 100 mg/kg
bw was thus considered not to be related to treatment (Kier &
Stegeman, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
No multigeneration studies of reproductive toxicity have been
performed with AMPA itself, but two studies were reported in rats
treated with technical-grade glyphosate contaminated at low levels
with AMPA (WHO, 1994).
In a three-generation study of reproductive toxicity, rats were
given diets providing technical-grade glyphosate (AMPA content not
specified) at doses of 0, 3, 10, or 30 mg/kg bw per day. The only
effect was an increased incidence of unilateral renal tubular
dilatation in male F3b pups at 30 mg/kg bw per day (6/10 versus 0/10
in controls); F1 and F2 pups were not examined (Bio/Dynamics Inc.,
1981b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Sprague-Dawley rats received diets containing
technical-grade glyphosate with 0.61% AMPA (Reyna, 1990), providing 0,
0.2, 1, or 3% glyphosate and 0, 12, 61, or 180 ppm AMPA. Parental
animals receiving 3% glyphosate had soft stools, decreased body
weights, and slightly decreased litter sizes; pup weights were
decreased on days 14 and 21 of lactation. Parental and pup body
weights were also slightly decreased after treatment with 1.0%. The
NOAEL was 1.0% in the diet, corresponding to 740 mg/kg bw per day
glyphosate and 3 mg/kg bw per day AMPA (Monsanto, 1990b). The renal
changes seen in the three-generation study were thus not reproduced;
however, the numbers of pups examined histologically was limited in
both studies.
(ii) Developmental toxicity
In a range-finding study, groups of eight pregnant Sprague-Dawley
rats received AMPA (purity, 94.4%) in corn oil at doses of 0, 125,
250, 500, 750, or 1000 mg/kg bw by gavage daily on days 6-15 of
gestation. All animals were killed on day 20 of gestation. Clinical
observations, body weights, net body-weight changes, and gravid
uterine weights of the dams were recorded, and the weights of the
liver, kidney, and spleen were determined. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
Table 3. Results of tests for the genotoxicity of AMPA
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 99 Negativeb Shirasu et al. (1980)
TA98, TA100, in distilled water
TA1535, TA1537,
TA1538, and
E. coli WP2
DNA repairc B. subtilis H17, 20-2000 µg/disc 99 Negatived Shirasu et al. (1980)
M45 (rec+/-) in distilled water
Unscheduled DNA Primary hepatocytes 5-5000 µg/ml in 94.4 Negative Bakke (1991)
synthesise from male Fischer culture medium
344 rats
In vivo
Micronucleus CD-1 mice Single intraperitoneal 94.4 Negative Kier & Stegeman
formulationf injection of 100, 500, (1993)
or 1000 mg/kg bw in
corn oil
a No independent duplicate study but duplicate plates at each concentration; distilled water used as solvent; solvent and
positive controls included
b With and without metabolic activation
c No independent duplicate study; distilled water used as solvent; solvent and positive controls included
d Only without metabolic activation
e Cytotoxicity at 3800 and 5000 µg/ml
f No characterization of test or control substances or their concentrations, homogeneity in carrier, or stability of test
and control substances neat and after mixing with carrier
weighed, sexed, and examined externally for developmental
abnormalities and variations. No effect on survival or body or organ
weights was seen in the dams. Greyish faeces were observed
infrequently in animals at doses > 125 mg/kg bw per day. The
predominant findings 1 h after treatment were red staining around the
nose in one or two animals per treated group and a low incidence of
salivation in those at 250, 500, 750, or 1000 mg/kg bw per day. In the
absence of a clear dose-response relationship and the limited
findings, they did not appear to be treatment-related. Fetuses had no
treatment-related effects (Holson, 1991a).
Groups of 25 pregnant Sprague-Dawley rats received AMPA (purity,
94.4%) in corn oil at doses of 0, 150, 400, or 1000 mg/kg bw by
gavage, daily on days 6-15 of gestation. All animals were killed on
day 20 of gestation. The dams were observed twice daily for
moribundity and mortality, and signs of toxicity about 1 h after
treatment and clinical observations on days 0-20 of gestation were
recorded. The body weights and food consumption of the dams were
determined on gestation days 0, 6, 9, 12, 16, and 20. Gravid uterine
weights and net body-weight changes were recorded, and the liver,
kidney, and spleen of all dams were weighed. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
abnormalities or variations.
Maternal survival was not affected. Dams at 400 or 1000 mg/kg bw
per day had dose-related increased incidences of mucoid faeces, hair
loss, and soft stools. Dams at 150 mg/kg bw per day had only an
increased incidence of soft stools. The total occurrence of soft
stools/no. of animals was 49/15 in controls, 89/19 in dams at 150
mg/kg bw per day, 102/22 at 400 mg/kg bw per day, and 135/23 at 1000
mg/kg bw per day. At the highest dose, the dams had short periods of
slightly decreased body-weight gain and food consumption. No effect
was seen on the weights of the liver, kidney, spleen, or gravid
uterus. Fetal survival was not affected. A slight but significant
decrease in mean fetal body weight was seen at 1000 mg/kg bw per day.
No further developmental effect was seen, and there were no
teratogenic effects. Because the increased incidence of soft stools at
150 mg/kg bw per day was not accompanied by possibly associated
effects such as hair loss and mucoid faeces, this dose is the NOAEL
for maternal toxicity. The NOAEL for developmental toxicity was 400
mg/kg bw per day, as the authors stated that the only possible
maternal responses at this dose were mucoid faeces, soft stools, and
hair loss (Holson, 1991b).
(f) Special studies: Dermal and ocular irritation
Two male and one female albino rabbits (strain not specified)
received an application of 0.5 g finely ground AMPA moistened with
water on the clipped intact skin (6.5 cm2), which was kept under
cover for 24 h. Observations were made after 1, 24, 48, 72, 120, and
168 h. No skin irritation was seen (Birch, 1973). Only a limited
report was available.
One male and two female albino rabbits (strain not specified)
received an application of 0.1 g finely ground AMPA into one eye. Both
the control and the treated eye were rinsed with warm isotonic saline
after 24 h. Observations were made after 1, 24, 48, 72, 120, and 168 h
and scored according to Draize. Slight erythema, very slight oedema,
and copious discharge were seen after 10 min and after 1 h, and
slight-to-moderate erythema, a moderate discharge containing a white
exudate, and no oedema were seen after 24 h. After 72 h, slight
erythema was seen in one animal, but without oedema or discharge.
After 120 and 168 h, the treated eyes were normal. The average scores
were 10 out of 110 after 1 h and 7.3, 2.6, and 0.6 after 24, 48, and
72 h, respectively. AMPA was considered by the author to be a slight
ocular irritant; however, the average score after 24, 48, and 72 h was
3.5, indicating that AMPA is not an ocular irritant (Birch, 1973). In
the limited report, separate scores were not given for conjunctivae,
iris, and cornea.
3. Toxicological profiles of AMPA and glyphosate
The results of toxicological studies of the metabolite AMPA and of its
parent compound glyphosate, as previously evaluated (Annex 1,
reference 47; WHO, 1994), are compared in Table 4. AMPA was no more
toxic than glyphosate. Similar effects were often found,e xcept for
the lesions in the salivary gland seen in the 90-day studies of
toxicity and carcinogenicity with glyphosate in mice nad rats from the
US national Toxicology Program and the cataracts induced by glyphosate
in a two-year toxicity study in rats. Such effects were not seen in
other studies with glyphosate or AMPA.
Comments
After oral administration of AMPA to rats, 20% of the dose was
absorbed and excreted unmetabolized in the urine within 120 h (17% of
the dose within 24 h), and 73% of the dose was eliminated in the
faeces. Only 0.07% of the dose was excreted as expired carbon dioxide
within 24 h, and 0.06% of the dose was recovered from tissues after
120 h. Minor amounts (1-6 µg/kg) were found in tissues after 120 h.
AMPA is slightly hazardous to rats given a single oral dose, with
an LD50 of 8300 mg/kg bw (WHO, 1996).
In a 90-day study of toxicity, rats received AMPA in the diet at
0, 400, 1200, or 4800 mg/kg bw per day. A significant, dose-related
decrease in body-weight gain was seen in males at the two highest
doses and in females at the highest dose. The two highest doses also
resulted in significantly increased lactate dehydrogenase activity,
whereas aspartate aminotransferase activity and cholesterol levels
were significantly increased only at the highest dose. Urinalysis
showed a significant decrease in urinary pH and increased amounts of
calcium oxalate crystals in the urine of animals at the highest dose.
Table 4. Toxicity of AMPA and technical-grade glyphosate
Type of study AMPA Glyphosatea
Metabolism 20% absorption after oral exposure; excreted 36% absorption after oral exposure;
unchanged in urine; only 0.07% expired as essentially no biotransformation (0.2-
CO2; minor residues in tissues 0.4% AMPA)
Acute oral toxicity LD50 = 8300 mg/kg bw LD50 > 5000 mg/kg bw
90-day toxicity, diet, mice NOAEL = 10 000 ppm, equal to 1890
me/kg bw. Effects at LOAEL of 50 000
ppm: decreased growth, increased brain,
heart, and kidney weights
90-day toxicity, diet, mice NOAEL = 3125 ppm, equal to 505 mg/
kg bw. Effects at LOAEL of 6250 ppm:
lesions of salivary gland
90-day toxicity, diet, rats NOAEL = 400 mg/kg bw. Effects at LOAEL No effect at highest dose of 20 000 ppm,
of 1200 mg.kg bw: biochemical changes, equal to 1300 mg/kg bw
changes in urinary bladder. At 4800 mg/kg bw,
also renal changes
90-day toxicity, diet, rats NOAEL < 3100 ppm, equal to < 200 mg/
kg bw; lesions of salivary gland
90-day toxicity, diet, rats No effect at highest dose of 12 500 ppm,
equal to 1300 mg/kg bw
90-day toxicity, gelatin capsule,
dogs No effect at highest dose of 300 mg/kg bw
52-week toxicity, gelatin capsule,
dogs No effect at highest dose of 500 mg/kg bw
Table 4. (continued)
Type of study AMPA Glyphosatea
Two-year toxicity and carcinogenicity, NOAEL = 5000 ppm, equal to 810 mg/
diet, mice kg bw. Effects at LOAEL of 30 000
ppm: decreased growth, changes in
urinary bladder and kidneys
Two-year toxicity and carcinogenicity, No effect at highest dose of 600 ppm,
diet, rats equal to 32 mg/kg bw; slight decrease in
growth considered not to be relevant
Two-year toxicity and carcinogenicity, NOAEL = 8000 ppm, equivalent to 400
diet, rats mg/kg bw. Effects at LOAEL of 20 000
ppm: decreased growth and cataracts.
Technical-grade glyphosate containing
0.68% AMPA
Three-generation reproductive Increased incidence (6/10 versus 1/10 in
toxicity, diet, rats controls) of unilateral renal tubular
dilatation in F3b pups at highest dose of
30 mg/kg bw; not seen in more recent
study (below)
Two-generation reproductive NOAEL = 10 000 ppm, equivalent to
toxicity, diet, rats 500 mg/kg bw. Effects at LOAEL of
30 000 ppm: soft stools, decreased
weights of parental animals and pups.
Technical-grade glyphosate containing
0.61% AMPA
Developmental toxicity, rats NOAEL for maternal toxicity = 150 mg/kg NOAEL = 1000 mg/kg bw. Effects at
bw; NOAEL for developmental toxicity = 400 LOAEL of 3500 mg/kg bw: deaths,
mg/kg bw. At 400 mg/kg bw, increased clinical signs, decreased growth of dams,
incidences of soft stools, mucoid faeces, and resorptions, decreased implantations and
hair loss in dams. At 1000 mg/kg bw, slight visible fetuses, decreased ossification of
decrease in fetal body weight fetal sternebrae; no fetal malformations
Table 4. (continued)
Type of study AMPA Glyphosatea
Developmental toxicity, rabbits NOAEL = 175 mg/kg bw. Effects at
LOAEL of 350 mg/kg bw: diarrhoea,
soft stools, and nasal discharge in dams
Genotoxicity
Reverse mutation, S. typhimurium Negative Negative
Reverse mutation, E. coli Negative Negative
DNA repair, B. subtilis rec Negative Negative
Gene mutation, Chinese hamster ovary Negative
cells
Unscheduled DNA synthesis, rat Negative Negative
hepatocytes
Chromosomal aberration in vivo, rat Negative
bone marrow
Micronucleus formation in vivo, Negative Negative
mouse bone marrow
Dominant lethal mutation in vivo, Negative
mice
Recessive sex-linked lethal mutation, Negative
Drosophila melanogaster
a From WHO (1994) and Annex I, reference 47
Dose-related irritation of the mucosal and submucosal layers of the
urinary tract, corresponding to hyperplasia of the urinary bladder,
was seen in rats at 1200 and 4800 mg/kg bw per day, the effect being
more marked in males than in females. In addition, epithelial
hyperplasia in the renal pelvis was observed at the highest dose. The
NOAEL was 400 mg/kg bw per day.
In a 90-day study of toxicity in dogs receiving AMPA at 0, 10,
30, 100, or 300 mg/kg bw per day in gelatin capsules, no statistically
significant treatment-related changes were observed. The NOAEL was
thus the highest dose, 300 mg/kg bw per day. It should be noted that
in a one-month range-finding study with groups of only two male and
two female dogs, changes in some haematological parameters (e.g.
decreased haemoglobin, packed cell volume, and erythrocyte counts)
were seen in animals at 300 or 1000 mg/kg bw per day. These effects
were not reproduced in the 90-day study.
No indication of genotoxic activity was seen in studies of gene
mutation in bacteria, of DNA repair in bacteria and mammalian cells
in vitro, or of micronucleus formation in vivo. No assays for gene
mutation were performed in mammalian cells in vitro, but the
structural similarity of AMPA to glyphosate and the lack of
genotoxicity of glyphosate, including in an assay for gene mutation in
mammalian cells in vitro, indicate that such an assay with AMPA
would be redundant.
In a study of developmental toxicity, rats received AMPA at 0,
150, 400, or 1000 mg/kg bw per day in corn oil by gavage. Dose-related
increases in the incidences of soft stools, mucoid faeces, and hair
loss were seen in dams at the two higher doses. Dams at the highest
dose also had short periods of decreased body-weight gain and food
consumption. Fetal body weight was decreased at 1000 mg/kg bw per day.
No teratogenic effects were observed. Dams at 150 mg/kg bw per day
also had an increased incidence of soft stools; however, in the
absence of any associated effects, such as hair loss or mucoid faeces,
the Meeting considered this dose to be the NOAEL for maternal
toxicity. The NOAEL for developmental toxicity was 400 mg/kg bw per
day.
AMPA did not induce dermal or ocular irritation in rabbits.
No long-term study of the toxicity or carcinogenicity of AMPA has
been carried out, but in the more recent of two such studies with
technical-grade glyphosate in rats at dietary levels of 0.2, 0.8, or
2%, the AMPA content of the test compound was given, namely 0.68%. At
the highest dose of 2% glyphosate in the diet, females showed
decreased body-weight gain and males showed an increased incidence of
degenerative lenticular changes. The NOAEL for technical-grade
glyphosate was 0.8% in the diet, corresponding to 400 mg/kg bw per day
for glyphosate and 2.7 mg/kg bw per day for AMPA. No increase in
tumour incidence was seen in this study.
No multigeneration study of the reproductive toxicity of AMPA has
been reported, but in a recent two-generation study in rats with
technical-grade glyphosate at dietary levels of 0.2, 1, or 3%, the
test compound contained 0.61% AMPA. At the highest dose, soft stools,
decreased parental body weights, slightly decreased litter sizes, and
decreased pup weights were observed. The NOAEL was 1% in the diet,
corresponding to 740 mg/kg bw per day glyphosate and 4.5 mg/kg bw per
day AMPA.
Glyphosate and AMPA have very similar chemical structures.
Studies of the metabolism of glyphosate in experimental animals
indicate that essentially none is biotransformed into AMPA.
Toxicological data on the metabolite are therefore essential for risk
assessment. The Meeting compared the toxicity profile of AMPA with
that of glyphosate and concluded that the major targets of the
toxicity of AMPA had been investigated. The results showed little
toxicity. The Meeting concluded that the two compounds have similar
toxicological profiles and concluded that a full database on AMPA is
unnecessary. AMPA was considered to be of no greater toxicological
concern than its parent compound. The Meeting established a group ADI
for AMPA alone or in combination with glyphosate of 0-0.3 mg/kg bw on
the basis of the 26-month study of toxicity in rats fed
technical-grade glyphosate, using a safety factor of 100.
Since the last JMPR evaluation fof glyphosate or toxicity in
1986, new data have become available, some of which are evaluated in
WHO (1994). The Meeting recommended that glyphosate be re-evaluated by
the JMPR.
Toxicological evaluation
Levels that cause no toxic effect
AMPA
Rat: 400 mg/kg bw per day (90-day study of toxicity)
150 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
400 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Dog: 300 mg/kg bw per day (highest dose in 90-day study of
toxicity)
Glyphosate (from Annex I, reference 47):
Mouse: 0.5% in the diet, equal to 810 mg/kg bw per day (two-
year study of toxicity and carcinogenicity)
Rat: 31 mg/kg bw per day (26-month study of toxicity and
carcinogenicity)
Dog: 500 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.3 mg/kg bw (sum of glyphosate and AMPA)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to aminomethylphosphonic acid (AMPA)
Human exposure Relevant route, study type, species Results/remarks
Short-term Oral toxicity, rat LD50 = 8300 mg/kg bw
(1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Not irritating
Skin sensitization No data
Medium-term Repeated oral, 90 days, toxicity, rat NOAEL = 400 mg/kg bw per day: urinary tract changes
(1-26 weeks) Repeated oral, developmental toxicity, rat NOAEL = 150 mg/kg bw per day: maternal toxicity
NOAEL = 400 mg/kg bw per day: developmental
toxicity
Repeated oral, reproductive toxicity No data
Long-term Repeated oral, toxicity No data
(> 1 year)
References
Bakke, J.P. (1991) Evaluation of the potential of AMPA to induce
unscheduled DNA synthesis in the in vitro hepatocyte DNA repair
assay using the male F-344 rat. Unpublished study No. 2495-V01-91 from
SRI International, Menlo Park, California, USA (SRI Project LSC-2495;
Monsanto study No. SR-91-234, dated 4 December 1991). Submitted to WHO
by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project N0. 410/77 [BDN-77-416]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1981b) A three generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]). Final report.
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1983) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Birch, M.D. (1973) Toxicological investigation of CP 50435 Lot:
XHD-16. Unpublished report from Younger Laboratories Inc., St Louis,
Missouri, USA, dated 7 March 1973. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Colvin, L.B., Moran, S.J. & Miller, J.A. (1973) Final Report on CP
67573 residue and metabolism. Part 11: The metabolism of
aminomethylphosphonic acid-14C (CP 50435-14C) in the laboratory rat.
Job No. 9-23-760.06-7863. Unpublished report from Monsanto Commercial
Products Co., Agricultural Research Report No. 303 dated August 1973.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Estes, F.L., Jefferson, N.D., Blair, M. & Goldenthal, E.I. (1979)
90-Day subacute rat toxicity study (IRD-78-174). Test article: CP
50435. Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, dated 15 August 1979. Submitted
to WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Goldenthal, E.I. (1978) CP 50435 (aminomethyl phosphonic acid)
fourteen day rat feeding study. Unpublished report No. IRD-77-319 from
Monsanto. Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Holson, J.F. (1991a) A dose range-finding developmental toxicity study
of AMPA in rats. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. WIL Project No.: WIL-50146.
Sponsor No.: WI-90-247, dated 21 May 1991. Submitted to WHO by
Monsanto, Agricultural Group, St Louis, Missouri, USA.
Holson, J.F. (1991b) A developmental toxicity study of AMPA in rats.
Final report. Unpublished report from WIL Research Laboratories Inc.,
Ashland, Ohio, USA. WIL Project No.: WIL-50159. Sponsor No.:
WI-90-266, dated 6 August 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Howe, R.K., Chott, R.C. & McClanahan, R.H. (1988) Volume 2: Metabolism
of glyphosate in Sprague-Dawley rats. Part II. Identification,
characterization, and quantitation of glyphosate and its metabolites
after intravenous and oral administration. Unpublished report from
Monsanto Co., St Louis, Missouri, USA. Laboratory project no.
MSL-7206, dated February 1988. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Kier, L.D. & Stegeman, S.D. (1993) Final report. Mouse micronucleus
study of AMPA. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. EHL Study Number: 90170.
Sponsor Project Number: ML-90-404. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Monsanto (1990a) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). Monsanto Environmental Health
Laboratory, St Louis, Missouri, USA. Unpublished report submitted
to WHO by Monsanto, St Louis, Missouri, USA.
Monsanto (1990b) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). Monsanto
Environmental Health Laboratory, St Louis, Missouri, USA. Unpublished
report submitted to WHO by Monsanto, St Louis, Missouri, USA.
Reyna, M.S. (1990) Two generation reproduction study with glyphosate
in Sprague-Dawley rats. Unpublished Report no. ML-88-106. Submitted to
WHO by Monsanto, St Louis, Missouri, USA.
Ridley, W.P. & Mirly (1988) Volume 1: The metabolism of glyphosate in
Sprague-Dawley rats. Part I. Excretion and tissue distribution of
glyphosate and its metabolites following intravenous and oral
administration. Unpublished report MSL-7215 from Monsanto Co.,
Environmental Health Laboratory, St Louis, Missouri, USA. Submitted to
WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) CP 50435: Microbial
mutagenicity study. Unpublished report from the Institute of
Environmental Toxicology Kodaira, Tokyo, Japan, dated November 1980.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Stout, L.D. (1991) One month study of AMPA administered by capsule to
beagle dogs. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. Study Number 90074. Project
Number: ML-90-186. Submitted to WHO by Monsanto, Agricultural Group,
St Louis, Missouri, USA.
Stout, L.D. & Ruecker, F.A. (1990) Chronic study of glyphosate
administered in feed to albino rats. Unpublished report no. ML-90-186.
Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Tompkins, E.C. (1991) 90-Day oral (capsule) toxicity study in dogs
with AMPA. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. Project No.: WIL-50173. Sponsor
No.: WI-90-354, dated 16 July 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
WHO (1994) Environmental Health Criteria 159. Glyphosate. Geneva,
International Programme on Chemical Safety.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
See Also:
Toxicological Abbreviations
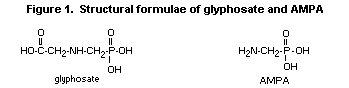 On the basis of the low residual levels of AMPA in crops which
are susceptible to glyphosate, the 1986 Joint Meeting concluded that
AMPA could be omitted from the residue in recommendations for MRLs,
but recent supervised trials on the application of glyphosate to
genetically modified crops have shown AMPA to be the main residue. As
residues of AMPA may therefore be of toxicological concern, the
compound was evaluated by the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Eight male Wistar rats weighing about 150 g were fasted for 4 h
and then given a single oral dose of 6.7 mg/kg bw 14C-AMPA by gavage.
Urine, faeces, and expired carbon dioxide (in 1 N NaOH) were analysed
for radiolabel 12, 24, 48, 72, 96, and 120 h after treatment (see
Table 1). After 120 h, a blood sample was taken and the animals were
killed; cage washes and tissues (see Table 2) were then analysed for
radiolabel. AMPA was moderately absorbed from the gut, as demonstrated
by excretion of 13% of the radiolabel in urine within 12 h, 18% within
24 h, and 20% within 120 h. Consequently, the major route of excretion
is the faeces: > 50% of the administered radiolabel was excreted
within 24 h and 74% by 120 h. In tissues, 0.06% of the radiolabel was
recovered after 120 h; < 0.1% was recovered as expired CO2 within 24
h. Minor residues were found in liver, kidneys, and muscle (Colvin et
al., 1973). Bone, bone-marrow, and eyes, which contain relatively high
residues of radiolabel (0.2-0.6% of the dose in comparison with
0.02-0.05% in other tissues) after oral administration of
14C-glyphosate to rats (Howe et al., 1988; Ridley & Mirly, 1988),
were not analysed in the study of Colvin et al. (1973).
(b) Biotransformation
Two groups of five Wistar rats of each sex received 10 mg/kg bw
14C-glyphosate orally, one group after preconditioning with
unlabelled glyphosate. Only 0.2-0.4% of the radiolabel recovered in
the excreta appeared to be AMPA (Howe et al., 1988).
Table 1. Recovery of radiolabel after a single oral dose of 6.7 mg/kg bw 14C-AMPA
Matrix Time after treatment (h)
0-12 12-24 24-48 48-72 72-96 96-120 0-120
Urine 13 4.5 1.5 0.66 02.3 0.05 20
Faeces 5.9 47 19 0.91 0.12 0.06 73
CO2 0.06 0.01 0 0 0 0 0.07
Cage wash 0.13
Tissues 0.06
Total 19 52 21 1.6 0.35 0.11 94
Table 2. Radiolabelled AMPA in tissues 120 h after a single oral
dose of 6.7 mg/kg bw
Tissue % of dose AMPA equivalent
(µg/kg fresh weight)
Liver 0.01 6
Kidney <0.01 6
Muscle 0.02 3
Fat 0.01 4
Gut 0.02 8
Spleen <0.01 4
Heart <0.01 4
Brain <0.01 1
Testis <0.01 3
Blood <0.01 3
Colvin et al. (1973) showed that most orally absorbed 14C-APMA
is excreted as unchanged compound in the urine of rats, as measured by
thin-layer and gas-liquid chromatography and nuclear magnetic
resonance and mass spectral analyses. The fact that < 0.1% of the
administered activity was expired as carbon dioxide also demonstrated
that only very small amounts of the absorbed material were
metabolized.
2. Toxicological studies
(a) Acute toxicity
Groups of two male and three female Sprague-Dawley rats were
given AMPA (purity unspecified) as a 40% solution in corn oil at one
of four doses by gavage and observed for seven days. Deaths occurred
at a dose of 8300 mg/kg bw after one to two days. Reduced appetite and
activity, increasing weakness, slight diarrhoea, collapse, and death
were observed. At autopsy, slight liver discolouration and acute
gastrointestinal inflammation were seen; the viscera appeared normal
macroscopically (Birch, 1973).
(b) Short-term toxicity
Rats
In a 14-day range-finding study available only in summary, groups
of five male and five female rats received AMPA at 0, 1000, 2000, or
4000 mg/kg bw per day in the diet. Moderately reduced body-weight gain
and food consumption (no further details) were seen at 4000 mg/kg bw
per day. No other effects were observed. The NOAEL was 2000 mg/kg bw
per day (Goldenthal, 1978).
In a 13-week study of toxicity, groups of 20 male and 20 female
weanling Charles River CD rats (aged four weeks) received AMPA
(purity, 99.9%) at 0, 400, 1200, or 4800 mg/kg bw per day in the diet.
The animals were observed for death and signs of overt toxicity twice
a day on seven days a week. Body weights and food consumption were
monitored weekly. Haematological, biochemical, and urinary parameters
were measured in 10 rats of each sex per group after 45 and 88 days;
the baseline values for these parameters were measured in an
additional 10 male and 10 female rats that were sacrificed for this
purpose. At the end of the study, the absolute and relative (to body
weight) weights of the liver, kidneys, testes/ovaries, heart, and
brain were determined in each animal, and macroscopy was performed.
About 30 tissues from controls and rats at the highest dose and liver,
kidneys, heart, urinary bladder, and tissues with gross lesions from
all rats at 400 and 1200 mg/kg bw per day were examined
microscopically.
One male and five female rats at 4800 mg/kg bw per day were found
dead after blood collection on day 45, and one female rat in the
control group and two female rats at 1200 mg/kg bw per day were dead
after blood collection on day 88. One male control rat found moribund
after observation of soft stools, distended abdomen, and morbidity was
killed at week 12. Body-weight gain was significantly decreased in
males and females at 4800 mg/kg bw per day (difference for females at
termination, < 10%) and in males at 1200 mg/kg bw per day
(difference, < 10%). The food consumption of treated males was lower
than that of control males, but the differences were not statistically
signifcant. No treatment-related changes in haematological parameters
were observed after 45 or 88 days. Biochemical analyses showed
increased lactic dehydrogenase activity in males and females at 4800
mg/kg bw per day, which was statistically significant for males after
45 and 88 days and for females after 88 days only. Males at 1200 mg/kg
bw per day also showed statistically significant increases in lactic
dehydrogenase activity after 88 days; females at 400 or 1200 mg/kg bw
per day showed significant increases in the activity of this enzyme
after 88 days, but the biological significance of these observations
is questionable in view of the magnitude of the increases and the low
control values in females. Glucose levels were significantly decreased
in males and females at 4800 mg/kg bw per day after 88 days in
comparison with control values, but the levels in animals at 400 or
1200 mg/kg bw per day were significantly increased after 45 days. At
4800 mg/kg bw per day, males and females showed increased activity of
aspartate aminotransferase, which was significant in females after 45
days and in both males and females after 88 days, and males showed
increased serum cholesterol levels, which were significant after both
45 and 88 days. The occasionally significant changes in other
biochemical parameters (albumin and bilirubin) did not show a
dose-response relationship and were considered not to be biologically
significant. Urinalysis revealed significant decreases in pH and
increased calcium oxalate crystals in males at 4800 mg/kg bw per day
after 88 days and in females after 45 and 88 days. The weights of
several organs showed significant changes from control values, but the
absence of any dose-response relationship or of morphological changes
in these organs make their biological significance questionable.
Macroscopy revealed no treatment-related changes. Microscopy showed
dose-related increases in the incidence and severity of irritation of
the mucosal and submucosal layers of the urinary tract in males and
females at 1200 or 4800 mg/kg bw per day, corresponding to hyperplasia
of the urinary bladder, which was more marked in males than in
females. Epithelial hyperplasia in the pelvic section was seen in the
kidneys of several rats at 4800 mg/kg bw per day, and some of the
hyperplastic epithelial cells contained a hyaline-like cytoplasmic
material. The NOAEL was 400 mg/kg bw per day (Estes et al., 1979).
Dogs
In a one-month range-finding study, groups of two male and two
female beagle dogs (aged about six months and weighing 7.3-9.8 kg for
males and 5.9-8.4 kg for females) received AMPA (purity, 94.4%) at 0,
10, 30, 100, 300, or 1000 mg/kg bw per day orally in gelatin capsules.
The animals were observed for mortality, moribundity, and signs of
toxicity twice daily and for detailed signs of toxicity once weekly.
Body weight and food consumption were determined weekly.
Haematological and biochemical analyses were carried out in all dogs
at termination; no urinalysis was performed. The absolute and relative
weights of the liver, kidneys, spleen, heart, brain, thyroid,
adrenals, and testes of all dogs were determined. Macroscopy was
performed on all animals, but no microscopy was done.
No unscheduled death occurred. Diarrhoea was seen in both males
and one female dog at 1000 mg/kg bw per day (total, 18 incidents), and
emesis was observed in both females and one male dog at the same dose
(four incidents). No effects on body-weight gain or food consumption
were seen. Males and females at 1000 mg/kg bw per day had decreased
haemoglobin and haematocrit values (significant in females), decreased
erythrocyte counts (significant in males and females), and increased
reticulocyte counts (significant in females). In addition, an increase
in mean corpuscular haemoglobin concentration was seen in males at
1000 mg/kg bw per day (significant) and 300 mg/kg bw per day, but with
no dose-response relationship. Females at 300 mg/kg bw per day had
significantly decreased haemoglobin and haematocrit values and a
nonsignificant decrease in reticulocyte count. Activated partial
prothrombin times were significantly decreased in females at all
doses, but these decreases were considered not to be biologically
significant because of their small size and the lack of a clear
dose-response relationship. No effects on biochemical parameters were
seen. Organ weights were unchanged, and macroscopy revealed no
treatment-related abnormalities. No adverse effects were seen at
100 mg/kg bw per day. There was no NOAEL owing to the limited number
of animals. The authors stated that no adverse effects were observed
at 300 mg/kg bw per day in males and 100 mg/kg bw per day in females
(Stout, 1991).
Groups of five male and five female beagle dogs (males weighing
8.1-12 kg and females, 6.8-11 kg) received AMPA (purity, 87.8%) at
doses of 0, 10, 30 100, or 300 mg/kg bw per day orally in gelatin
capsules for 91-92 days. The dogs were observed twice daily for
mortality, moribundity, and overt signs of toxicity. Ophthalmoscopy
was conducted on all dogs before the study and at week 12. Body
weights were recorded weekly and food consumption daily.
Haematological, biochemical, and urinary analyses were carried out in
all dogs before the study and during weeks 6 and 13. The absolute and
relative weights of the liver, kidneys, brain, thyroid, adrenals, and
ovaries/testes of all dogs were determined. All dogs were examined
macroscopically, and about 40 tissues and all gross lesions were
examined microscopically.
Treatment had no effect on survival, and ophthalmoscopy revealed
no abnormalities. Several clinical signs were seen at all doses and in
controls, but there was generally no clear dose-response relationship.
Only the incidence of scabbed, reddened, and/or swollen ears in males
at 300 mg/kg bw per day was increased; in females at this dose, the
incidence of these symptoms was below that in controls, the latter
being the same as that in males at 300 mg/kg bw per day. The incidence
of soft stools varied from group to group, with considerable
individual variability; no dose-response relationship was seen. No
significant treatment-related effects on body-weight gain or food
consumption were observed. Haematology showed occasionally significant
decreases in erythrocyte count, haemoglobin and haematocrit values,
mean corpuscular volume, mean corpuscular haemoglobin, or mean
corpuscular haemoglobin concentration at low doses. No relationship
with dose or time was seen, and these changes were considered not to
be biologically significant. No treatment-related effects on
biochemical or urinary parameters or organ weights were seen, and
there were no macroscopic or microscopic changes. The NOAEL was 300
mg/kg bw per day, the highest dose tested (Tompkins, 1991).
(c) Long-term toxicity and carcinogenicity
No long-term toxicity studies have been performed with AMPA
itself, but two-year toxicity studies are available in mice and rats
treated with technical-grade glyphosate contaminated at a low level
with AMPA (WHO, 1994).
In a two-year study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
mice at dietary levels of 0, 0.1, 0.5, or 3%, no treatment-related
increase in tumour incidence was seen. The NOAEL was 0.5%, equal to
810 mg/kg bw per day (Bio/Dynamics Inc., 1983).
In a 26-month study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
rats at dietary levels of 0.006, 0.02, or 0.06%, equal to about 3, 10,
or 32 mg/kg bw per day, no treatment-related increase in tumour
incidence was seen. The NOAEL was 32 mg/kg bw per day, the highest
dose tested (Bio/Dynamics Inc., 1981a).
In a two-year study of toxicity and carcinogenicity, rats were
given technical-grade glyphosate containing 0.68% AMPAin the diet to
provide doses of 0.2, 0.8, or 2% glyphosate, equivalent to 100, 400,
or 1000 mg/kg bw per day. The diets thus contained 14, 54, and 135 mg
of AMPA per kg, corresponding to 0.69, 2.8, and 7.2 mg/kg bw per day.
No increase in tumour incidence was seen. At the highest dose, female
body-weight gain was reduced, and cataracts were found in males. The
NOAEL was 0.8% in the diet, equivalent to 400 mg/kg bw per day
glyphosate and 2.8 mg/kg bw per day AMPA (Monsanto 1990a; Stout &
Ruecker, 1990).
(d) Genotoxicity
The results of tests for the genotoxicity of AMPA are shown in
Table 3. In the test for micronucleus formation, groups of 15 male and
15 female mice were used. Five animals of each sex per dose were
killed 24, 48, and 72 h after treatment. Toxicity was observed at 500
and 1000 mg/kg bw, as demonstrated by weight loss and listlessness. No
significant decrease in mean polychromatic erythrocytes:total
erythrocytes was seen at any dose. At 100 mg/kg bw, a significant
increase in mean nucleated polychromatic erythrocyte frequency was
seen after 72 h in females only; however, the increased frequency was
within the range in historical vehicle controls in the same
laboratory, and the frequency was not increased in males at 100 mg/kg
bw or in males or females at higher doses. The increase in mean
nucleated polychromatic erythrocyte frequency in females at 100 mg/kg
bw was thus considered not to be related to treatment (Kier &
Stegeman, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
No multigeneration studies of reproductive toxicity have been
performed with AMPA itself, but two studies were reported in rats
treated with technical-grade glyphosate contaminated at low levels
with AMPA (WHO, 1994).
In a three-generation study of reproductive toxicity, rats were
given diets providing technical-grade glyphosate (AMPA content not
specified) at doses of 0, 3, 10, or 30 mg/kg bw per day. The only
effect was an increased incidence of unilateral renal tubular
dilatation in male F3b pups at 30 mg/kg bw per day (6/10 versus 0/10
in controls); F1 and F2 pups were not examined (Bio/Dynamics Inc.,
1981b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Sprague-Dawley rats received diets containing
technical-grade glyphosate with 0.61% AMPA (Reyna, 1990), providing 0,
0.2, 1, or 3% glyphosate and 0, 12, 61, or 180 ppm AMPA. Parental
animals receiving 3% glyphosate had soft stools, decreased body
weights, and slightly decreased litter sizes; pup weights were
decreased on days 14 and 21 of lactation. Parental and pup body
weights were also slightly decreased after treatment with 1.0%. The
NOAEL was 1.0% in the diet, corresponding to 740 mg/kg bw per day
glyphosate and 3 mg/kg bw per day AMPA (Monsanto, 1990b). The renal
changes seen in the three-generation study were thus not reproduced;
however, the numbers of pups examined histologically was limited in
both studies.
(ii) Developmental toxicity
In a range-finding study, groups of eight pregnant Sprague-Dawley
rats received AMPA (purity, 94.4%) in corn oil at doses of 0, 125,
250, 500, 750, or 1000 mg/kg bw by gavage daily on days 6-15 of
gestation. All animals were killed on day 20 of gestation. Clinical
observations, body weights, net body-weight changes, and gravid
uterine weights of the dams were recorded, and the weights of the
liver, kidney, and spleen were determined. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
Table 3. Results of tests for the genotoxicity of AMPA
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 99 Negativeb Shirasu et al. (1980)
TA98, TA100, in distilled water
TA1535, TA1537,
TA1538, and
E. coli WP2
DNA repairc B. subtilis H17, 20-2000 µg/disc 99 Negatived Shirasu et al. (1980)
M45 (rec+/-) in distilled water
Unscheduled DNA Primary hepatocytes 5-5000 µg/ml in 94.4 Negative Bakke (1991)
synthesise from male Fischer culture medium
344 rats
In vivo
Micronucleus CD-1 mice Single intraperitoneal 94.4 Negative Kier & Stegeman
formulationf injection of 100, 500, (1993)
or 1000 mg/kg bw in
corn oil
a No independent duplicate study but duplicate plates at each concentration; distilled water used as solvent; solvent and
positive controls included
b With and without metabolic activation
c No independent duplicate study; distilled water used as solvent; solvent and positive controls included
d Only without metabolic activation
e Cytotoxicity at 3800 and 5000 µg/ml
f No characterization of test or control substances or their concentrations, homogeneity in carrier, or stability of test
and control substances neat and after mixing with carrier
weighed, sexed, and examined externally for developmental
abnormalities and variations. No effect on survival or body or organ
weights was seen in the dams. Greyish faeces were observed
infrequently in animals at doses > 125 mg/kg bw per day. The
predominant findings 1 h after treatment were red staining around the
nose in one or two animals per treated group and a low incidence of
salivation in those at 250, 500, 750, or 1000 mg/kg bw per day. In the
absence of a clear dose-response relationship and the limited
findings, they did not appear to be treatment-related. Fetuses had no
treatment-related effects (Holson, 1991a).
Groups of 25 pregnant Sprague-Dawley rats received AMPA (purity,
94.4%) in corn oil at doses of 0, 150, 400, or 1000 mg/kg bw by
gavage, daily on days 6-15 of gestation. All animals were killed on
day 20 of gestation. The dams were observed twice daily for
moribundity and mortality, and signs of toxicity about 1 h after
treatment and clinical observations on days 0-20 of gestation were
recorded. The body weights and food consumption of the dams were
determined on gestation days 0, 6, 9, 12, 16, and 20. Gravid uterine
weights and net body-weight changes were recorded, and the liver,
kidney, and spleen of all dams were weighed. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
abnormalities or variations.
Maternal survival was not affected. Dams at 400 or 1000 mg/kg bw
per day had dose-related increased incidences of mucoid faeces, hair
loss, and soft stools. Dams at 150 mg/kg bw per day had only an
increased incidence of soft stools. The total occurrence of soft
stools/no. of animals was 49/15 in controls, 89/19 in dams at 150
mg/kg bw per day, 102/22 at 400 mg/kg bw per day, and 135/23 at 1000
mg/kg bw per day. At the highest dose, the dams had short periods of
slightly decreased body-weight gain and food consumption. No effect
was seen on the weights of the liver, kidney, spleen, or gravid
uterus. Fetal survival was not affected. A slight but significant
decrease in mean fetal body weight was seen at 1000 mg/kg bw per day.
No further developmental effect was seen, and there were no
teratogenic effects. Because the increased incidence of soft stools at
150 mg/kg bw per day was not accompanied by possibly associated
effects such as hair loss and mucoid faeces, this dose is the NOAEL
for maternal toxicity. The NOAEL for developmental toxicity was 400
mg/kg bw per day, as the authors stated that the only possible
maternal responses at this dose were mucoid faeces, soft stools, and
hair loss (Holson, 1991b).
(f) Special studies: Dermal and ocular irritation
Two male and one female albino rabbits (strain not specified)
received an application of 0.5 g finely ground AMPA moistened with
water on the clipped intact skin (6.5 cm2), which was kept under
cover for 24 h. Observations were made after 1, 24, 48, 72, 120, and
168 h. No skin irritation was seen (Birch, 1973). Only a limited
report was available.
One male and two female albino rabbits (strain not specified)
received an application of 0.1 g finely ground AMPA into one eye. Both
the control and the treated eye were rinsed with warm isotonic saline
after 24 h. Observations were made after 1, 24, 48, 72, 120, and 168 h
and scored according to Draize. Slight erythema, very slight oedema,
and copious discharge were seen after 10 min and after 1 h, and
slight-to-moderate erythema, a moderate discharge containing a white
exudate, and no oedema were seen after 24 h. After 72 h, slight
erythema was seen in one animal, but without oedema or discharge.
After 120 and 168 h, the treated eyes were normal. The average scores
were 10 out of 110 after 1 h and 7.3, 2.6, and 0.6 after 24, 48, and
72 h, respectively. AMPA was considered by the author to be a slight
ocular irritant; however, the average score after 24, 48, and 72 h was
3.5, indicating that AMPA is not an ocular irritant (Birch, 1973). In
the limited report, separate scores were not given for conjunctivae,
iris, and cornea.
3. Toxicological profiles of AMPA and glyphosate
The results of toxicological studies of the metabolite AMPA and of its
parent compound glyphosate, as previously evaluated (Annex 1,
reference 47; WHO, 1994), are compared in Table 4. AMPA was no more
toxic than glyphosate. Similar effects were often found,e xcept for
the lesions in the salivary gland seen in the 90-day studies of
toxicity and carcinogenicity with glyphosate in mice nad rats from the
US national Toxicology Program and the cataracts induced by glyphosate
in a two-year toxicity study in rats. Such effects were not seen in
other studies with glyphosate or AMPA.
Comments
After oral administration of AMPA to rats, 20% of the dose was
absorbed and excreted unmetabolized in the urine within 120 h (17% of
the dose within 24 h), and 73% of the dose was eliminated in the
faeces. Only 0.07% of the dose was excreted as expired carbon dioxide
within 24 h, and 0.06% of the dose was recovered from tissues after
120 h. Minor amounts (1-6 µg/kg) were found in tissues after 120 h.
AMPA is slightly hazardous to rats given a single oral dose, with
an LD50 of 8300 mg/kg bw (WHO, 1996).
In a 90-day study of toxicity, rats received AMPA in the diet at
0, 400, 1200, or 4800 mg/kg bw per day. A significant, dose-related
decrease in body-weight gain was seen in males at the two highest
doses and in females at the highest dose. The two highest doses also
resulted in significantly increased lactate dehydrogenase activity,
whereas aspartate aminotransferase activity and cholesterol levels
were significantly increased only at the highest dose. Urinalysis
showed a significant decrease in urinary pH and increased amounts of
calcium oxalate crystals in the urine of animals at the highest dose.
Table 4. Toxicity of AMPA and technical-grade glyphosate
Type of study AMPA Glyphosatea
Metabolism 20% absorption after oral exposure; excreted 36% absorption after oral exposure;
unchanged in urine; only 0.07% expired as essentially no biotransformation (0.2-
CO2; minor residues in tissues 0.4% AMPA)
Acute oral toxicity LD50 = 8300 mg/kg bw LD50 > 5000 mg/kg bw
90-day toxicity, diet, mice NOAEL = 10 000 ppm, equal to 1890
me/kg bw. Effects at LOAEL of 50 000
ppm: decreased growth, increased brain,
heart, and kidney weights
90-day toxicity, diet, mice NOAEL = 3125 ppm, equal to 505 mg/
kg bw. Effects at LOAEL of 6250 ppm:
lesions of salivary gland
90-day toxicity, diet, rats NOAEL = 400 mg/kg bw. Effects at LOAEL No effect at highest dose of 20 000 ppm,
of 1200 mg.kg bw: biochemical changes, equal to 1300 mg/kg bw
changes in urinary bladder. At 4800 mg/kg bw,
also renal changes
90-day toxicity, diet, rats NOAEL < 3100 ppm, equal to < 200 mg/
kg bw; lesions of salivary gland
90-day toxicity, diet, rats No effect at highest dose of 12 500 ppm,
equal to 1300 mg/kg bw
90-day toxicity, gelatin capsule,
dogs No effect at highest dose of 300 mg/kg bw
52-week toxicity, gelatin capsule,
dogs No effect at highest dose of 500 mg/kg bw
Table 4. (continued)
Type of study AMPA Glyphosatea
Two-year toxicity and carcinogenicity, NOAEL = 5000 ppm, equal to 810 mg/
diet, mice kg bw. Effects at LOAEL of 30 000
ppm: decreased growth, changes in
urinary bladder and kidneys
Two-year toxicity and carcinogenicity, No effect at highest dose of 600 ppm,
diet, rats equal to 32 mg/kg bw; slight decrease in
growth considered not to be relevant
Two-year toxicity and carcinogenicity, NOAEL = 8000 ppm, equivalent to 400
diet, rats mg/kg bw. Effects at LOAEL of 20 000
ppm: decreased growth and cataracts.
Technical-grade glyphosate containing
0.68% AMPA
Three-generation reproductive Increased incidence (6/10 versus 1/10 in
toxicity, diet, rats controls) of unilateral renal tubular
dilatation in F3b pups at highest dose of
30 mg/kg bw; not seen in more recent
study (below)
Two-generation reproductive NOAEL = 10 000 ppm, equivalent to
toxicity, diet, rats 500 mg/kg bw. Effects at LOAEL of
30 000 ppm: soft stools, decreased
weights of parental animals and pups.
Technical-grade glyphosate containing
0.61% AMPA
Developmental toxicity, rats NOAEL for maternal toxicity = 150 mg/kg NOAEL = 1000 mg/kg bw. Effects at
bw; NOAEL for developmental toxicity = 400 LOAEL of 3500 mg/kg bw: deaths,
mg/kg bw. At 400 mg/kg bw, increased clinical signs, decreased growth of dams,
incidences of soft stools, mucoid faeces, and resorptions, decreased implantations and
hair loss in dams. At 1000 mg/kg bw, slight visible fetuses, decreased ossification of
decrease in fetal body weight fetal sternebrae; no fetal malformations
Table 4. (continued)
Type of study AMPA Glyphosatea
Developmental toxicity, rabbits NOAEL = 175 mg/kg bw. Effects at
LOAEL of 350 mg/kg bw: diarrhoea,
soft stools, and nasal discharge in dams
Genotoxicity
Reverse mutation, S. typhimurium Negative Negative
Reverse mutation, E. coli Negative Negative
DNA repair, B. subtilis rec Negative Negative
Gene mutation, Chinese hamster ovary Negative
cells
Unscheduled DNA synthesis, rat Negative Negative
hepatocytes
Chromosomal aberration in vivo, rat Negative
bone marrow
Micronucleus formation in vivo, Negative Negative
mouse bone marrow
Dominant lethal mutation in vivo, Negative
mice
Recessive sex-linked lethal mutation, Negative
Drosophila melanogaster
a From WHO (1994) and Annex I, reference 47
Dose-related irritation of the mucosal and submucosal layers of the
urinary tract, corresponding to hyperplasia of the urinary bladder,
was seen in rats at 1200 and 4800 mg/kg bw per day, the effect being
more marked in males than in females. In addition, epithelial
hyperplasia in the renal pelvis was observed at the highest dose. The
NOAEL was 400 mg/kg bw per day.
In a 90-day study of toxicity in dogs receiving AMPA at 0, 10,
30, 100, or 300 mg/kg bw per day in gelatin capsules, no statistically
significant treatment-related changes were observed. The NOAEL was
thus the highest dose, 300 mg/kg bw per day. It should be noted that
in a one-month range-finding study with groups of only two male and
two female dogs, changes in some haematological parameters (e.g.
decreased haemoglobin, packed cell volume, and erythrocyte counts)
were seen in animals at 300 or 1000 mg/kg bw per day. These effects
were not reproduced in the 90-day study.
No indication of genotoxic activity was seen in studies of gene
mutation in bacteria, of DNA repair in bacteria and mammalian cells
in vitro, or of micronucleus formation in vivo. No assays for gene
mutation were performed in mammalian cells in vitro, but the
structural similarity of AMPA to glyphosate and the lack of
genotoxicity of glyphosate, including in an assay for gene mutation in
mammalian cells in vitro, indicate that such an assay with AMPA
would be redundant.
In a study of developmental toxicity, rats received AMPA at 0,
150, 400, or 1000 mg/kg bw per day in corn oil by gavage. Dose-related
increases in the incidences of soft stools, mucoid faeces, and hair
loss were seen in dams at the two higher doses. Dams at the highest
dose also had short periods of decreased body-weight gain and food
consumption. Fetal body weight was decreased at 1000 mg/kg bw per day.
No teratogenic effects were observed. Dams at 150 mg/kg bw per day
also had an increased incidence of soft stools; however, in the
absence of any associated effects, such as hair loss or mucoid faeces,
the Meeting considered this dose to be the NOAEL for maternal
toxicity. The NOAEL for developmental toxicity was 400 mg/kg bw per
day.
AMPA did not induce dermal or ocular irritation in rabbits.
No long-term study of the toxicity or carcinogenicity of AMPA has
been carried out, but in the more recent of two such studies with
technical-grade glyphosate in rats at dietary levels of 0.2, 0.8, or
2%, the AMPA content of the test compound was given, namely 0.68%. At
the highest dose of 2% glyphosate in the diet, females showed
decreased body-weight gain and males showed an increased incidence of
degenerative lenticular changes. The NOAEL for technical-grade
glyphosate was 0.8% in the diet, corresponding to 400 mg/kg bw per day
for glyphosate and 2.7 mg/kg bw per day for AMPA. No increase in
tumour incidence was seen in this study.
No multigeneration study of the reproductive toxicity of AMPA has
been reported, but in a recent two-generation study in rats with
technical-grade glyphosate at dietary levels of 0.2, 1, or 3%, the
test compound contained 0.61% AMPA. At the highest dose, soft stools,
decreased parental body weights, slightly decreased litter sizes, and
decreased pup weights were observed. The NOAEL was 1% in the diet,
corresponding to 740 mg/kg bw per day glyphosate and 4.5 mg/kg bw per
day AMPA.
Glyphosate and AMPA have very similar chemical structures.
Studies of the metabolism of glyphosate in experimental animals
indicate that essentially none is biotransformed into AMPA.
Toxicological data on the metabolite are therefore essential for risk
assessment. The Meeting compared the toxicity profile of AMPA with
that of glyphosate and concluded that the major targets of the
toxicity of AMPA had been investigated. The results showed little
toxicity. The Meeting concluded that the two compounds have similar
toxicological profiles and concluded that a full database on AMPA is
unnecessary. AMPA was considered to be of no greater toxicological
concern than its parent compound. The Meeting established a group ADI
for AMPA alone or in combination with glyphosate of 0-0.3 mg/kg bw on
the basis of the 26-month study of toxicity in rats fed
technical-grade glyphosate, using a safety factor of 100.
Since the last JMPR evaluation fof glyphosate or toxicity in
1986, new data have become available, some of which are evaluated in
WHO (1994). The Meeting recommended that glyphosate be re-evaluated by
the JMPR.
Toxicological evaluation
Levels that cause no toxic effect
AMPA
Rat: 400 mg/kg bw per day (90-day study of toxicity)
150 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
400 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Dog: 300 mg/kg bw per day (highest dose in 90-day study of
toxicity)
Glyphosate (from Annex I, reference 47):
Mouse: 0.5% in the diet, equal to 810 mg/kg bw per day (two-
year study of toxicity and carcinogenicity)
Rat: 31 mg/kg bw per day (26-month study of toxicity and
carcinogenicity)
Dog: 500 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.3 mg/kg bw (sum of glyphosate and AMPA)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to aminomethylphosphonic acid (AMPA)
Human exposure Relevant route, study type, species Results/remarks
Short-term Oral toxicity, rat LD50 = 8300 mg/kg bw
(1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Not irritating
Skin sensitization No data
Medium-term Repeated oral, 90 days, toxicity, rat NOAEL = 400 mg/kg bw per day: urinary tract changes
(1-26 weeks) Repeated oral, developmental toxicity, rat NOAEL = 150 mg/kg bw per day: maternal toxicity
NOAEL = 400 mg/kg bw per day: developmental
toxicity
Repeated oral, reproductive toxicity No data
Long-term Repeated oral, toxicity No data
(> 1 year)
References
Bakke, J.P. (1991) Evaluation of the potential of AMPA to induce
unscheduled DNA synthesis in the in vitro hepatocyte DNA repair
assay using the male F-344 rat. Unpublished study No. 2495-V01-91 from
SRI International, Menlo Park, California, USA (SRI Project LSC-2495;
Monsanto study No. SR-91-234, dated 4 December 1991). Submitted to WHO
by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project N0. 410/77 [BDN-77-416]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1981b) A three generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]). Final report.
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1983) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Birch, M.D. (1973) Toxicological investigation of CP 50435 Lot:
XHD-16. Unpublished report from Younger Laboratories Inc., St Louis,
Missouri, USA, dated 7 March 1973. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Colvin, L.B., Moran, S.J. & Miller, J.A. (1973) Final Report on CP
67573 residue and metabolism. Part 11: The metabolism of
aminomethylphosphonic acid-14C (CP 50435-14C) in the laboratory rat.
Job No. 9-23-760.06-7863. Unpublished report from Monsanto Commercial
Products Co., Agricultural Research Report No. 303 dated August 1973.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Estes, F.L., Jefferson, N.D., Blair, M. & Goldenthal, E.I. (1979)
90-Day subacute rat toxicity study (IRD-78-174). Test article: CP
50435. Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, dated 15 August 1979. Submitted
to WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Goldenthal, E.I. (1978) CP 50435 (aminomethyl phosphonic acid)
fourteen day rat feeding study. Unpublished report No. IRD-77-319 from
Monsanto. Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Holson, J.F. (1991a) A dose range-finding developmental toxicity study
of AMPA in rats. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. WIL Project No.: WIL-50146.
Sponsor No.: WI-90-247, dated 21 May 1991. Submitted to WHO by
Monsanto, Agricultural Group, St Louis, Missouri, USA.
Holson, J.F. (1991b) A developmental toxicity study of AMPA in rats.
Final report. Unpublished report from WIL Research Laboratories Inc.,
Ashland, Ohio, USA. WIL Project No.: WIL-50159. Sponsor No.:
WI-90-266, dated 6 August 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Howe, R.K., Chott, R.C. & McClanahan, R.H. (1988) Volume 2: Metabolism
of glyphosate in Sprague-Dawley rats. Part II. Identification,
characterization, and quantitation of glyphosate and its metabolites
after intravenous and oral administration. Unpublished report from
Monsanto Co., St Louis, Missouri, USA. Laboratory project no.
MSL-7206, dated February 1988. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Kier, L.D. & Stegeman, S.D. (1993) Final report. Mouse micronucleus
study of AMPA. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. EHL Study Number: 90170.
Sponsor Project Number: ML-90-404. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Monsanto (1990a) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). Monsanto Environmental Health
Laboratory, St Louis, Missouri, USA. Unpublished report submitted
to WHO by Monsanto, St Louis, Missouri, USA.
Monsanto (1990b) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). Monsanto
Environmental Health Laboratory, St Louis, Missouri, USA. Unpublished
report submitted to WHO by Monsanto, St Louis, Missouri, USA.
Reyna, M.S. (1990) Two generation reproduction study with glyphosate
in Sprague-Dawley rats. Unpublished Report no. ML-88-106. Submitted to
WHO by Monsanto, St Louis, Missouri, USA.
Ridley, W.P. & Mirly (1988) Volume 1: The metabolism of glyphosate in
Sprague-Dawley rats. Part I. Excretion and tissue distribution of
glyphosate and its metabolites following intravenous and oral
administration. Unpublished report MSL-7215 from Monsanto Co.,
Environmental Health Laboratory, St Louis, Missouri, USA. Submitted to
WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) CP 50435: Microbial
mutagenicity study. Unpublished report from the Institute of
Environmental Toxicology Kodaira, Tokyo, Japan, dated November 1980.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Stout, L.D. (1991) One month study of AMPA administered by capsule to
beagle dogs. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. Study Number 90074. Project
Number: ML-90-186. Submitted to WHO by Monsanto, Agricultural Group,
St Louis, Missouri, USA.
Stout, L.D. & Ruecker, F.A. (1990) Chronic study of glyphosate
administered in feed to albino rats. Unpublished report no. ML-90-186.
Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Tompkins, E.C. (1991) 90-Day oral (capsule) toxicity study in dogs
with AMPA. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. Project No.: WIL-50173. Sponsor
No.: WI-90-354, dated 16 July 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
WHO (1994) Environmental Health Criteria 159. Glyphosate. Geneva,
International Programme on Chemical Safety.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
On the basis of the low residual levels of AMPA in crops which
are susceptible to glyphosate, the 1986 Joint Meeting concluded that
AMPA could be omitted from the residue in recommendations for MRLs,
but recent supervised trials on the application of glyphosate to
genetically modified crops have shown AMPA to be the main residue. As
residues of AMPA may therefore be of toxicological concern, the
compound was evaluated by the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Eight male Wistar rats weighing about 150 g were fasted for 4 h
and then given a single oral dose of 6.7 mg/kg bw 14C-AMPA by gavage.
Urine, faeces, and expired carbon dioxide (in 1 N NaOH) were analysed
for radiolabel 12, 24, 48, 72, 96, and 120 h after treatment (see
Table 1). After 120 h, a blood sample was taken and the animals were
killed; cage washes and tissues (see Table 2) were then analysed for
radiolabel. AMPA was moderately absorbed from the gut, as demonstrated
by excretion of 13% of the radiolabel in urine within 12 h, 18% within
24 h, and 20% within 120 h. Consequently, the major route of excretion
is the faeces: > 50% of the administered radiolabel was excreted
within 24 h and 74% by 120 h. In tissues, 0.06% of the radiolabel was
recovered after 120 h; < 0.1% was recovered as expired CO2 within 24
h. Minor residues were found in liver, kidneys, and muscle (Colvin et
al., 1973). Bone, bone-marrow, and eyes, which contain relatively high
residues of radiolabel (0.2-0.6% of the dose in comparison with
0.02-0.05% in other tissues) after oral administration of
14C-glyphosate to rats (Howe et al., 1988; Ridley & Mirly, 1988),
were not analysed in the study of Colvin et al. (1973).
(b) Biotransformation
Two groups of five Wistar rats of each sex received 10 mg/kg bw
14C-glyphosate orally, one group after preconditioning with
unlabelled glyphosate. Only 0.2-0.4% of the radiolabel recovered in
the excreta appeared to be AMPA (Howe et al., 1988).
Table 1. Recovery of radiolabel after a single oral dose of 6.7 mg/kg bw 14C-AMPA
Matrix Time after treatment (h)
0-12 12-24 24-48 48-72 72-96 96-120 0-120
Urine 13 4.5 1.5 0.66 02.3 0.05 20
Faeces 5.9 47 19 0.91 0.12 0.06 73
CO2 0.06 0.01 0 0 0 0 0.07
Cage wash 0.13
Tissues 0.06
Total 19 52 21 1.6 0.35 0.11 94
Table 2. Radiolabelled AMPA in tissues 120 h after a single oral
dose of 6.7 mg/kg bw
Tissue % of dose AMPA equivalent
(µg/kg fresh weight)
Liver 0.01 6
Kidney <0.01 6
Muscle 0.02 3
Fat 0.01 4
Gut 0.02 8
Spleen <0.01 4
Heart <0.01 4
Brain <0.01 1
Testis <0.01 3
Blood <0.01 3
Colvin et al. (1973) showed that most orally absorbed 14C-APMA
is excreted as unchanged compound in the urine of rats, as measured by
thin-layer and gas-liquid chromatography and nuclear magnetic
resonance and mass spectral analyses. The fact that < 0.1% of the
administered activity was expired as carbon dioxide also demonstrated
that only very small amounts of the absorbed material were
metabolized.
2. Toxicological studies
(a) Acute toxicity
Groups of two male and three female Sprague-Dawley rats were
given AMPA (purity unspecified) as a 40% solution in corn oil at one
of four doses by gavage and observed for seven days. Deaths occurred
at a dose of 8300 mg/kg bw after one to two days. Reduced appetite and
activity, increasing weakness, slight diarrhoea, collapse, and death
were observed. At autopsy, slight liver discolouration and acute
gastrointestinal inflammation were seen; the viscera appeared normal
macroscopically (Birch, 1973).
(b) Short-term toxicity
Rats
In a 14-day range-finding study available only in summary, groups
of five male and five female rats received AMPA at 0, 1000, 2000, or
4000 mg/kg bw per day in the diet. Moderately reduced body-weight gain
and food consumption (no further details) were seen at 4000 mg/kg bw
per day. No other effects were observed. The NOAEL was 2000 mg/kg bw
per day (Goldenthal, 1978).
In a 13-week study of toxicity, groups of 20 male and 20 female
weanling Charles River CD rats (aged four weeks) received AMPA
(purity, 99.9%) at 0, 400, 1200, or 4800 mg/kg bw per day in the diet.
The animals were observed for death and signs of overt toxicity twice
a day on seven days a week. Body weights and food consumption were
monitored weekly. Haematological, biochemical, and urinary parameters
were measured in 10 rats of each sex per group after 45 and 88 days;
the baseline values for these parameters were measured in an
additional 10 male and 10 female rats that were sacrificed for this
purpose. At the end of the study, the absolute and relative (to body
weight) weights of the liver, kidneys, testes/ovaries, heart, and
brain were determined in each animal, and macroscopy was performed.
About 30 tissues from controls and rats at the highest dose and liver,
kidneys, heart, urinary bladder, and tissues with gross lesions from
all rats at 400 and 1200 mg/kg bw per day were examined
microscopically.
One male and five female rats at 4800 mg/kg bw per day were found
dead after blood collection on day 45, and one female rat in the
control group and two female rats at 1200 mg/kg bw per day were dead
after blood collection on day 88. One male control rat found moribund
after observation of soft stools, distended abdomen, and morbidity was
killed at week 12. Body-weight gain was significantly decreased in
males and females at 4800 mg/kg bw per day (difference for females at
termination, < 10%) and in males at 1200 mg/kg bw per day
(difference, < 10%). The food consumption of treated males was lower
than that of control males, but the differences were not statistically
signifcant. No treatment-related changes in haematological parameters
were observed after 45 or 88 days. Biochemical analyses showed
increased lactic dehydrogenase activity in males and females at 4800
mg/kg bw per day, which was statistically significant for males after
45 and 88 days and for females after 88 days only. Males at 1200 mg/kg
bw per day also showed statistically significant increases in lactic
dehydrogenase activity after 88 days; females at 400 or 1200 mg/kg bw
per day showed significant increases in the activity of this enzyme
after 88 days, but the biological significance of these observations
is questionable in view of the magnitude of the increases and the low
control values in females. Glucose levels were significantly decreased
in males and females at 4800 mg/kg bw per day after 88 days in
comparison with control values, but the levels in animals at 400 or
1200 mg/kg bw per day were significantly increased after 45 days. At
4800 mg/kg bw per day, males and females showed increased activity of
aspartate aminotransferase, which was significant in females after 45
days and in both males and females after 88 days, and males showed
increased serum cholesterol levels, which were significant after both
45 and 88 days. The occasionally significant changes in other
biochemical parameters (albumin and bilirubin) did not show a
dose-response relationship and were considered not to be biologically
significant. Urinalysis revealed significant decreases in pH and
increased calcium oxalate crystals in males at 4800 mg/kg bw per day
after 88 days and in females after 45 and 88 days. The weights of
several organs showed significant changes from control values, but the
absence of any dose-response relationship or of morphological changes
in these organs make their biological significance questionable.
Macroscopy revealed no treatment-related changes. Microscopy showed
dose-related increases in the incidence and severity of irritation of
the mucosal and submucosal layers of the urinary tract in males and
females at 1200 or 4800 mg/kg bw per day, corresponding to hyperplasia
of the urinary bladder, which was more marked in males than in
females. Epithelial hyperplasia in the pelvic section was seen in the
kidneys of several rats at 4800 mg/kg bw per day, and some of the
hyperplastic epithelial cells contained a hyaline-like cytoplasmic
material. The NOAEL was 400 mg/kg bw per day (Estes et al., 1979).
Dogs
In a one-month range-finding study, groups of two male and two
female beagle dogs (aged about six months and weighing 7.3-9.8 kg for
males and 5.9-8.4 kg for females) received AMPA (purity, 94.4%) at 0,
10, 30, 100, 300, or 1000 mg/kg bw per day orally in gelatin capsules.
The animals were observed for mortality, moribundity, and signs of
toxicity twice daily and for detailed signs of toxicity once weekly.
Body weight and food consumption were determined weekly.
Haematological and biochemical analyses were carried out in all dogs
at termination; no urinalysis was performed. The absolute and relative
weights of the liver, kidneys, spleen, heart, brain, thyroid,
adrenals, and testes of all dogs were determined. Macroscopy was
performed on all animals, but no microscopy was done.
No unscheduled death occurred. Diarrhoea was seen in both males
and one female dog at 1000 mg/kg bw per day (total, 18 incidents), and
emesis was observed in both females and one male dog at the same dose
(four incidents). No effects on body-weight gain or food consumption
were seen. Males and females at 1000 mg/kg bw per day had decreased
haemoglobin and haematocrit values (significant in females), decreased
erythrocyte counts (significant in males and females), and increased
reticulocyte counts (significant in females). In addition, an increase
in mean corpuscular haemoglobin concentration was seen in males at
1000 mg/kg bw per day (significant) and 300 mg/kg bw per day, but with
no dose-response relationship. Females at 300 mg/kg bw per day had
significantly decreased haemoglobin and haematocrit values and a
nonsignificant decrease in reticulocyte count. Activated partial
prothrombin times were significantly decreased in females at all
doses, but these decreases were considered not to be biologically
significant because of their small size and the lack of a clear
dose-response relationship. No effects on biochemical parameters were
seen. Organ weights were unchanged, and macroscopy revealed no
treatment-related abnormalities. No adverse effects were seen at
100 mg/kg bw per day. There was no NOAEL owing to the limited number
of animals. The authors stated that no adverse effects were observed
at 300 mg/kg bw per day in males and 100 mg/kg bw per day in females
(Stout, 1991).
Groups of five male and five female beagle dogs (males weighing
8.1-12 kg and females, 6.8-11 kg) received AMPA (purity, 87.8%) at
doses of 0, 10, 30 100, or 300 mg/kg bw per day orally in gelatin
capsules for 91-92 days. The dogs were observed twice daily for
mortality, moribundity, and overt signs of toxicity. Ophthalmoscopy
was conducted on all dogs before the study and at week 12. Body
weights were recorded weekly and food consumption daily.
Haematological, biochemical, and urinary analyses were carried out in
all dogs before the study and during weeks 6 and 13. The absolute and
relative weights of the liver, kidneys, brain, thyroid, adrenals, and
ovaries/testes of all dogs were determined. All dogs were examined
macroscopically, and about 40 tissues and all gross lesions were
examined microscopically.
Treatment had no effect on survival, and ophthalmoscopy revealed
no abnormalities. Several clinical signs were seen at all doses and in
controls, but there was generally no clear dose-response relationship.
Only the incidence of scabbed, reddened, and/or swollen ears in males
at 300 mg/kg bw per day was increased; in females at this dose, the
incidence of these symptoms was below that in controls, the latter
being the same as that in males at 300 mg/kg bw per day. The incidence
of soft stools varied from group to group, with considerable
individual variability; no dose-response relationship was seen. No
significant treatment-related effects on body-weight gain or food
consumption were observed. Haematology showed occasionally significant
decreases in erythrocyte count, haemoglobin and haematocrit values,
mean corpuscular volume, mean corpuscular haemoglobin, or mean
corpuscular haemoglobin concentration at low doses. No relationship
with dose or time was seen, and these changes were considered not to
be biologically significant. No treatment-related effects on
biochemical or urinary parameters or organ weights were seen, and
there were no macroscopic or microscopic changes. The NOAEL was 300
mg/kg bw per day, the highest dose tested (Tompkins, 1991).
(c) Long-term toxicity and carcinogenicity
No long-term toxicity studies have been performed with AMPA
itself, but two-year toxicity studies are available in mice and rats
treated with technical-grade glyphosate contaminated at a low level
with AMPA (WHO, 1994).
In a two-year study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
mice at dietary levels of 0, 0.1, 0.5, or 3%, no treatment-related
increase in tumour incidence was seen. The NOAEL was 0.5%, equal to
810 mg/kg bw per day (Bio/Dynamics Inc., 1983).
In a 26-month study of toxicity and carcinogenicity in which
technical-grade glyphosate (AMPA content not specified) was given to
rats at dietary levels of 0.006, 0.02, or 0.06%, equal to about 3, 10,
or 32 mg/kg bw per day, no treatment-related increase in tumour
incidence was seen. The NOAEL was 32 mg/kg bw per day, the highest
dose tested (Bio/Dynamics Inc., 1981a).
In a two-year study of toxicity and carcinogenicity, rats were
given technical-grade glyphosate containing 0.68% AMPAin the diet to
provide doses of 0.2, 0.8, or 2% glyphosate, equivalent to 100, 400,
or 1000 mg/kg bw per day. The diets thus contained 14, 54, and 135 mg
of AMPA per kg, corresponding to 0.69, 2.8, and 7.2 mg/kg bw per day.
No increase in tumour incidence was seen. At the highest dose, female
body-weight gain was reduced, and cataracts were found in males. The
NOAEL was 0.8% in the diet, equivalent to 400 mg/kg bw per day
glyphosate and 2.8 mg/kg bw per day AMPA (Monsanto 1990a; Stout &
Ruecker, 1990).
(d) Genotoxicity
The results of tests for the genotoxicity of AMPA are shown in
Table 3. In the test for micronucleus formation, groups of 15 male and
15 female mice were used. Five animals of each sex per dose were
killed 24, 48, and 72 h after treatment. Toxicity was observed at 500
and 1000 mg/kg bw, as demonstrated by weight loss and listlessness. No
significant decrease in mean polychromatic erythrocytes:total
erythrocytes was seen at any dose. At 100 mg/kg bw, a significant
increase in mean nucleated polychromatic erythrocyte frequency was
seen after 72 h in females only; however, the increased frequency was
within the range in historical vehicle controls in the same
laboratory, and the frequency was not increased in males at 100 mg/kg
bw or in males or females at higher doses. The increase in mean
nucleated polychromatic erythrocyte frequency in females at 100 mg/kg
bw was thus considered not to be related to treatment (Kier &
Stegeman, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
No multigeneration studies of reproductive toxicity have been
performed with AMPA itself, but two studies were reported in rats
treated with technical-grade glyphosate contaminated at low levels
with AMPA (WHO, 1994).
In a three-generation study of reproductive toxicity, rats were
given diets providing technical-grade glyphosate (AMPA content not
specified) at doses of 0, 3, 10, or 30 mg/kg bw per day. The only
effect was an increased incidence of unilateral renal tubular
dilatation in male F3b pups at 30 mg/kg bw per day (6/10 versus 0/10
in controls); F1 and F2 pups were not examined (Bio/Dynamics Inc.,
1981b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Sprague-Dawley rats received diets containing
technical-grade glyphosate with 0.61% AMPA (Reyna, 1990), providing 0,
0.2, 1, or 3% glyphosate and 0, 12, 61, or 180 ppm AMPA. Parental
animals receiving 3% glyphosate had soft stools, decreased body
weights, and slightly decreased litter sizes; pup weights were
decreased on days 14 and 21 of lactation. Parental and pup body
weights were also slightly decreased after treatment with 1.0%. The
NOAEL was 1.0% in the diet, corresponding to 740 mg/kg bw per day
glyphosate and 3 mg/kg bw per day AMPA (Monsanto, 1990b). The renal
changes seen in the three-generation study were thus not reproduced;
however, the numbers of pups examined histologically was limited in
both studies.
(ii) Developmental toxicity
In a range-finding study, groups of eight pregnant Sprague-Dawley
rats received AMPA (purity, 94.4%) in corn oil at doses of 0, 125,
250, 500, 750, or 1000 mg/kg bw by gavage daily on days 6-15 of
gestation. All animals were killed on day 20 of gestation. Clinical
observations, body weights, net body-weight changes, and gravid
uterine weights of the dams were recorded, and the weights of the
liver, kidney, and spleen were determined. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
Table 3. Results of tests for the genotoxicity of AMPA
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 99 Negativeb Shirasu et al. (1980)
TA98, TA100, in distilled water
TA1535, TA1537,
TA1538, and
E. coli WP2
DNA repairc B. subtilis H17, 20-2000 µg/disc 99 Negatived Shirasu et al. (1980)
M45 (rec+/-) in distilled water
Unscheduled DNA Primary hepatocytes 5-5000 µg/ml in 94.4 Negative Bakke (1991)
synthesise from male Fischer culture medium
344 rats
In vivo
Micronucleus CD-1 mice Single intraperitoneal 94.4 Negative Kier & Stegeman
formulationf injection of 100, 500, (1993)
or 1000 mg/kg bw in
corn oil
a No independent duplicate study but duplicate plates at each concentration; distilled water used as solvent; solvent and
positive controls included
b With and without metabolic activation
c No independent duplicate study; distilled water used as solvent; solvent and positive controls included
d Only without metabolic activation
e Cytotoxicity at 3800 and 5000 µg/ml
f No characterization of test or control substances or their concentrations, homogeneity in carrier, or stability of test
and control substances neat and after mixing with carrier
weighed, sexed, and examined externally for developmental
abnormalities and variations. No effect on survival or body or organ
weights was seen in the dams. Greyish faeces were observed
infrequently in animals at doses > 125 mg/kg bw per day. The
predominant findings 1 h after treatment were red staining around the
nose in one or two animals per treated group and a low incidence of
salivation in those at 250, 500, 750, or 1000 mg/kg bw per day. In the
absence of a clear dose-response relationship and the limited
findings, they did not appear to be treatment-related. Fetuses had no
treatment-related effects (Holson, 1991a).
Groups of 25 pregnant Sprague-Dawley rats received AMPA (purity,
94.4%) in corn oil at doses of 0, 150, 400, or 1000 mg/kg bw by
gavage, daily on days 6-15 of gestation. All animals were killed on
day 20 of gestation. The dams were observed twice daily for
moribundity and mortality, and signs of toxicity about 1 h after
treatment and clinical observations on days 0-20 of gestation were
recorded. The body weights and food consumption of the dams were
determined on gestation days 0, 6, 9, 12, 16, and 20. Gravid uterine
weights and net body-weight changes were recorded, and the liver,
kidney, and spleen of all dams were weighed. Uteri and ovaries were
examined, and the numbers of fetuses, early and late resorptions,
implantation sites, and corpora lutea were recorded. Fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
abnormalities or variations.
Maternal survival was not affected. Dams at 400 or 1000 mg/kg bw
per day had dose-related increased incidences of mucoid faeces, hair
loss, and soft stools. Dams at 150 mg/kg bw per day had only an
increased incidence of soft stools. The total occurrence of soft
stools/no. of animals was 49/15 in controls, 89/19 in dams at 150
mg/kg bw per day, 102/22 at 400 mg/kg bw per day, and 135/23 at 1000
mg/kg bw per day. At the highest dose, the dams had short periods of
slightly decreased body-weight gain and food consumption. No effect
was seen on the weights of the liver, kidney, spleen, or gravid
uterus. Fetal survival was not affected. A slight but significant
decrease in mean fetal body weight was seen at 1000 mg/kg bw per day.
No further developmental effect was seen, and there were no
teratogenic effects. Because the increased incidence of soft stools at
150 mg/kg bw per day was not accompanied by possibly associated
effects such as hair loss and mucoid faeces, this dose is the NOAEL
for maternal toxicity. The NOAEL for developmental toxicity was 400
mg/kg bw per day, as the authors stated that the only possible
maternal responses at this dose were mucoid faeces, soft stools, and
hair loss (Holson, 1991b).
(f) Special studies: Dermal and ocular irritation
Two male and one female albino rabbits (strain not specified)
received an application of 0.5 g finely ground AMPA moistened with
water on the clipped intact skin (6.5 cm2), which was kept under
cover for 24 h. Observations were made after 1, 24, 48, 72, 120, and
168 h. No skin irritation was seen (Birch, 1973). Only a limited
report was available.
One male and two female albino rabbits (strain not specified)
received an application of 0.1 g finely ground AMPA into one eye. Both
the control and the treated eye were rinsed with warm isotonic saline
after 24 h. Observations were made after 1, 24, 48, 72, 120, and 168 h
and scored according to Draize. Slight erythema, very slight oedema,
and copious discharge were seen after 10 min and after 1 h, and
slight-to-moderate erythema, a moderate discharge containing a white
exudate, and no oedema were seen after 24 h. After 72 h, slight
erythema was seen in one animal, but without oedema or discharge.
After 120 and 168 h, the treated eyes were normal. The average scores
were 10 out of 110 after 1 h and 7.3, 2.6, and 0.6 after 24, 48, and
72 h, respectively. AMPA was considered by the author to be a slight
ocular irritant; however, the average score after 24, 48, and 72 h was
3.5, indicating that AMPA is not an ocular irritant (Birch, 1973). In
the limited report, separate scores were not given for conjunctivae,
iris, and cornea.
3. Toxicological profiles of AMPA and glyphosate
The results of toxicological studies of the metabolite AMPA and of its
parent compound glyphosate, as previously evaluated (Annex 1,
reference 47; WHO, 1994), are compared in Table 4. AMPA was no more
toxic than glyphosate. Similar effects were often found,e xcept for
the lesions in the salivary gland seen in the 90-day studies of
toxicity and carcinogenicity with glyphosate in mice nad rats from the
US national Toxicology Program and the cataracts induced by glyphosate
in a two-year toxicity study in rats. Such effects were not seen in
other studies with glyphosate or AMPA.
Comments
After oral administration of AMPA to rats, 20% of the dose was
absorbed and excreted unmetabolized in the urine within 120 h (17% of
the dose within 24 h), and 73% of the dose was eliminated in the
faeces. Only 0.07% of the dose was excreted as expired carbon dioxide
within 24 h, and 0.06% of the dose was recovered from tissues after
120 h. Minor amounts (1-6 µg/kg) were found in tissues after 120 h.
AMPA is slightly hazardous to rats given a single oral dose, with
an LD50 of 8300 mg/kg bw (WHO, 1996).
In a 90-day study of toxicity, rats received AMPA in the diet at
0, 400, 1200, or 4800 mg/kg bw per day. A significant, dose-related
decrease in body-weight gain was seen in males at the two highest
doses and in females at the highest dose. The two highest doses also
resulted in significantly increased lactate dehydrogenase activity,
whereas aspartate aminotransferase activity and cholesterol levels
were significantly increased only at the highest dose. Urinalysis
showed a significant decrease in urinary pH and increased amounts of
calcium oxalate crystals in the urine of animals at the highest dose.
Table 4. Toxicity of AMPA and technical-grade glyphosate
Type of study AMPA Glyphosatea
Metabolism 20% absorption after oral exposure; excreted 36% absorption after oral exposure;
unchanged in urine; only 0.07% expired as essentially no biotransformation (0.2-
CO2; minor residues in tissues 0.4% AMPA)
Acute oral toxicity LD50 = 8300 mg/kg bw LD50 > 5000 mg/kg bw
90-day toxicity, diet, mice NOAEL = 10 000 ppm, equal to 1890
me/kg bw. Effects at LOAEL of 50 000
ppm: decreased growth, increased brain,
heart, and kidney weights
90-day toxicity, diet, mice NOAEL = 3125 ppm, equal to 505 mg/
kg bw. Effects at LOAEL of 6250 ppm:
lesions of salivary gland
90-day toxicity, diet, rats NOAEL = 400 mg/kg bw. Effects at LOAEL No effect at highest dose of 20 000 ppm,
of 1200 mg.kg bw: biochemical changes, equal to 1300 mg/kg bw
changes in urinary bladder. At 4800 mg/kg bw,
also renal changes
90-day toxicity, diet, rats NOAEL < 3100 ppm, equal to < 200 mg/
kg bw; lesions of salivary gland
90-day toxicity, diet, rats No effect at highest dose of 12 500 ppm,
equal to 1300 mg/kg bw
90-day toxicity, gelatin capsule,
dogs No effect at highest dose of 300 mg/kg bw
52-week toxicity, gelatin capsule,
dogs No effect at highest dose of 500 mg/kg bw
Table 4. (continued)
Type of study AMPA Glyphosatea
Two-year toxicity and carcinogenicity, NOAEL = 5000 ppm, equal to 810 mg/
diet, mice kg bw. Effects at LOAEL of 30 000
ppm: decreased growth, changes in
urinary bladder and kidneys
Two-year toxicity and carcinogenicity, No effect at highest dose of 600 ppm,
diet, rats equal to 32 mg/kg bw; slight decrease in
growth considered not to be relevant
Two-year toxicity and carcinogenicity, NOAEL = 8000 ppm, equivalent to 400
diet, rats mg/kg bw. Effects at LOAEL of 20 000
ppm: decreased growth and cataracts.
Technical-grade glyphosate containing
0.68% AMPA
Three-generation reproductive Increased incidence (6/10 versus 1/10 in
toxicity, diet, rats controls) of unilateral renal tubular
dilatation in F3b pups at highest dose of
30 mg/kg bw; not seen in more recent
study (below)
Two-generation reproductive NOAEL = 10 000 ppm, equivalent to
toxicity, diet, rats 500 mg/kg bw. Effects at LOAEL of
30 000 ppm: soft stools, decreased
weights of parental animals and pups.
Technical-grade glyphosate containing
0.61% AMPA
Developmental toxicity, rats NOAEL for maternal toxicity = 150 mg/kg NOAEL = 1000 mg/kg bw. Effects at
bw; NOAEL for developmental toxicity = 400 LOAEL of 3500 mg/kg bw: deaths,
mg/kg bw. At 400 mg/kg bw, increased clinical signs, decreased growth of dams,
incidences of soft stools, mucoid faeces, and resorptions, decreased implantations and
hair loss in dams. At 1000 mg/kg bw, slight visible fetuses, decreased ossification of
decrease in fetal body weight fetal sternebrae; no fetal malformations
Table 4. (continued)
Type of study AMPA Glyphosatea
Developmental toxicity, rabbits NOAEL = 175 mg/kg bw. Effects at
LOAEL of 350 mg/kg bw: diarrhoea,
soft stools, and nasal discharge in dams
Genotoxicity
Reverse mutation, S. typhimurium Negative Negative
Reverse mutation, E. coli Negative Negative
DNA repair, B. subtilis rec Negative Negative
Gene mutation, Chinese hamster ovary Negative
cells
Unscheduled DNA synthesis, rat Negative Negative
hepatocytes
Chromosomal aberration in vivo, rat Negative
bone marrow
Micronucleus formation in vivo, Negative Negative
mouse bone marrow
Dominant lethal mutation in vivo, Negative
mice
Recessive sex-linked lethal mutation, Negative
Drosophila melanogaster
a From WHO (1994) and Annex I, reference 47
Dose-related irritation of the mucosal and submucosal layers of the
urinary tract, corresponding to hyperplasia of the urinary bladder,
was seen in rats at 1200 and 4800 mg/kg bw per day, the effect being
more marked in males than in females. In addition, epithelial
hyperplasia in the renal pelvis was observed at the highest dose. The
NOAEL was 400 mg/kg bw per day.
In a 90-day study of toxicity in dogs receiving AMPA at 0, 10,
30, 100, or 300 mg/kg bw per day in gelatin capsules, no statistically
significant treatment-related changes were observed. The NOAEL was
thus the highest dose, 300 mg/kg bw per day. It should be noted that
in a one-month range-finding study with groups of only two male and
two female dogs, changes in some haematological parameters (e.g.
decreased haemoglobin, packed cell volume, and erythrocyte counts)
were seen in animals at 300 or 1000 mg/kg bw per day. These effects
were not reproduced in the 90-day study.
No indication of genotoxic activity was seen in studies of gene
mutation in bacteria, of DNA repair in bacteria and mammalian cells
in vitro, or of micronucleus formation in vivo. No assays for gene
mutation were performed in mammalian cells in vitro, but the
structural similarity of AMPA to glyphosate and the lack of
genotoxicity of glyphosate, including in an assay for gene mutation in
mammalian cells in vitro, indicate that such an assay with AMPA
would be redundant.
In a study of developmental toxicity, rats received AMPA at 0,
150, 400, or 1000 mg/kg bw per day in corn oil by gavage. Dose-related
increases in the incidences of soft stools, mucoid faeces, and hair
loss were seen in dams at the two higher doses. Dams at the highest
dose also had short periods of decreased body-weight gain and food
consumption. Fetal body weight was decreased at 1000 mg/kg bw per day.
No teratogenic effects were observed. Dams at 150 mg/kg bw per day
also had an increased incidence of soft stools; however, in the
absence of any associated effects, such as hair loss or mucoid faeces,
the Meeting considered this dose to be the NOAEL for maternal
toxicity. The NOAEL for developmental toxicity was 400 mg/kg bw per
day.
AMPA did not induce dermal or ocular irritation in rabbits.
No long-term study of the toxicity or carcinogenicity of AMPA has
been carried out, but in the more recent of two such studies with
technical-grade glyphosate in rats at dietary levels of 0.2, 0.8, or
2%, the AMPA content of the test compound was given, namely 0.68%. At
the highest dose of 2% glyphosate in the diet, females showed
decreased body-weight gain and males showed an increased incidence of
degenerative lenticular changes. The NOAEL for technical-grade
glyphosate was 0.8% in the diet, corresponding to 400 mg/kg bw per day
for glyphosate and 2.7 mg/kg bw per day for AMPA. No increase in
tumour incidence was seen in this study.
No multigeneration study of the reproductive toxicity of AMPA has
been reported, but in a recent two-generation study in rats with
technical-grade glyphosate at dietary levels of 0.2, 1, or 3%, the
test compound contained 0.61% AMPA. At the highest dose, soft stools,
decreased parental body weights, slightly decreased litter sizes, and
decreased pup weights were observed. The NOAEL was 1% in the diet,
corresponding to 740 mg/kg bw per day glyphosate and 4.5 mg/kg bw per
day AMPA.
Glyphosate and AMPA have very similar chemical structures.
Studies of the metabolism of glyphosate in experimental animals
indicate that essentially none is biotransformed into AMPA.
Toxicological data on the metabolite are therefore essential for risk
assessment. The Meeting compared the toxicity profile of AMPA with
that of glyphosate and concluded that the major targets of the
toxicity of AMPA had been investigated. The results showed little
toxicity. The Meeting concluded that the two compounds have similar
toxicological profiles and concluded that a full database on AMPA is
unnecessary. AMPA was considered to be of no greater toxicological
concern than its parent compound. The Meeting established a group ADI
for AMPA alone or in combination with glyphosate of 0-0.3 mg/kg bw on
the basis of the 26-month study of toxicity in rats fed
technical-grade glyphosate, using a safety factor of 100.
Since the last JMPR evaluation fof glyphosate or toxicity in
1986, new data have become available, some of which are evaluated in
WHO (1994). The Meeting recommended that glyphosate be re-evaluated by
the JMPR.
Toxicological evaluation
Levels that cause no toxic effect
AMPA
Rat: 400 mg/kg bw per day (90-day study of toxicity)
150 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
400 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Dog: 300 mg/kg bw per day (highest dose in 90-day study of
toxicity)
Glyphosate (from Annex I, reference 47):
Mouse: 0.5% in the diet, equal to 810 mg/kg bw per day (two-
year study of toxicity and carcinogenicity)
Rat: 31 mg/kg bw per day (26-month study of toxicity and
carcinogenicity)
Dog: 500 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.3 mg/kg bw (sum of glyphosate and AMPA)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to aminomethylphosphonic acid (AMPA)
Human exposure Relevant route, study type, species Results/remarks
Short-term Oral toxicity, rat LD50 = 8300 mg/kg bw
(1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Not irritating
Skin sensitization No data
Medium-term Repeated oral, 90 days, toxicity, rat NOAEL = 400 mg/kg bw per day: urinary tract changes
(1-26 weeks) Repeated oral, developmental toxicity, rat NOAEL = 150 mg/kg bw per day: maternal toxicity
NOAEL = 400 mg/kg bw per day: developmental
toxicity
Repeated oral, reproductive toxicity No data
Long-term Repeated oral, toxicity No data
(> 1 year)
References
Bakke, J.P. (1991) Evaluation of the potential of AMPA to induce
unscheduled DNA synthesis in the in vitro hepatocyte DNA repair
assay using the male F-344 rat. Unpublished study No. 2495-V01-91 from
SRI International, Menlo Park, California, USA (SRI Project LSC-2495;
Monsanto study No. SR-91-234, dated 4 December 1991). Submitted to WHO
by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project N0. 410/77 [BDN-77-416]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1981b) A three generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]). Final report.
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Bio/Dynamics Inc. (1983) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]).
Bio/Dynamics Inc., Division of Biology and Safety Evaluation, East
Millstone, New Jersey, USA. Submitted to WHO by Monsanto, St Louis,
Missouri, USA.
Birch, M.D. (1973) Toxicological investigation of CP 50435 Lot:
XHD-16. Unpublished report from Younger Laboratories Inc., St Louis,
Missouri, USA, dated 7 March 1973. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Colvin, L.B., Moran, S.J. & Miller, J.A. (1973) Final Report on CP
67573 residue and metabolism. Part 11: The metabolism of
aminomethylphosphonic acid-14C (CP 50435-14C) in the laboratory rat.
Job No. 9-23-760.06-7863. Unpublished report from Monsanto Commercial
Products Co., Agricultural Research Report No. 303 dated August 1973.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Estes, F.L., Jefferson, N.D., Blair, M. & Goldenthal, E.I. (1979)
90-Day subacute rat toxicity study (IRD-78-174). Test article: CP
50435. Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, dated 15 August 1979. Submitted
to WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Goldenthal, E.I. (1978) CP 50435 (aminomethyl phosphonic acid)
fourteen day rat feeding study. Unpublished report No. IRD-77-319 from
Monsanto. Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Holson, J.F. (1991a) A dose range-finding developmental toxicity study
of AMPA in rats. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. WIL Project No.: WIL-50146.
Sponsor No.: WI-90-247, dated 21 May 1991. Submitted to WHO by
Monsanto, Agricultural Group, St Louis, Missouri, USA.
Holson, J.F. (1991b) A developmental toxicity study of AMPA in rats.
Final report. Unpublished report from WIL Research Laboratories Inc.,
Ashland, Ohio, USA. WIL Project No.: WIL-50159. Sponsor No.:
WI-90-266, dated 6 August 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Howe, R.K., Chott, R.C. & McClanahan, R.H. (1988) Volume 2: Metabolism
of glyphosate in Sprague-Dawley rats. Part II. Identification,
characterization, and quantitation of glyphosate and its metabolites
after intravenous and oral administration. Unpublished report from
Monsanto Co., St Louis, Missouri, USA. Laboratory project no.
MSL-7206, dated February 1988. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Kier, L.D. & Stegeman, S.D. (1993) Final report. Mouse micronucleus
study of AMPA. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. EHL Study Number: 90170.
Sponsor Project Number: ML-90-404. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
Monsanto (1990a) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). Monsanto Environmental Health
Laboratory, St Louis, Missouri, USA. Unpublished report submitted
to WHO by Monsanto, St Louis, Missouri, USA.
Monsanto (1990b) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). Monsanto
Environmental Health Laboratory, St Louis, Missouri, USA. Unpublished
report submitted to WHO by Monsanto, St Louis, Missouri, USA.
Reyna, M.S. (1990) Two generation reproduction study with glyphosate
in Sprague-Dawley rats. Unpublished Report no. ML-88-106. Submitted to
WHO by Monsanto, St Louis, Missouri, USA.
Ridley, W.P. & Mirly (1988) Volume 1: The metabolism of glyphosate in
Sprague-Dawley rats. Part I. Excretion and tissue distribution of
glyphosate and its metabolites following intravenous and oral
administration. Unpublished report MSL-7215 from Monsanto Co.,
Environmental Health Laboratory, St Louis, Missouri, USA. Submitted to
WHO by Monsanto, Agricultural Group, St Louis, Missouri, USA.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) CP 50435: Microbial
mutagenicity study. Unpublished report from the Institute of
Environmental Toxicology Kodaira, Tokyo, Japan, dated November 1980.
Submitted to WHO by Monsanto, Agricultural Group, St Louis, Missouri,
USA.
Stout, L.D. (1991) One month study of AMPA administered by capsule to
beagle dogs. Unpublished report from Environmental Health Laboratory
of Monsanto Co., St Louis, Missouri, USA. Study Number 90074. Project
Number: ML-90-186. Submitted to WHO by Monsanto, Agricultural Group,
St Louis, Missouri, USA.
Stout, L.D. & Ruecker, F.A. (1990) Chronic study of glyphosate
administered in feed to albino rats. Unpublished report no. ML-90-186.
Submitted to WHO by Monsanto, St Louis, Missouri, USA.
Tompkins, E.C. (1991) 90-Day oral (capsule) toxicity study in dogs
with AMPA. Final report. Unpublished report from WIL Research
Laboratories Inc., Ashland, Ohio, USA. Project No.: WIL-50173. Sponsor
No.: WI-90-354, dated 16 July 1991. Submitted to WHO by Monsanto,
Agricultural Group, St Louis, Missouri, USA.
WHO (1994) Environmental Health Criteria 159. Glyphosate. Geneva,
International Programme on Chemical Safety.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
