
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
GUAZATINE
First draft prepared by
I. Dewhurst
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and
Food
York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: Dermal and ocular irritation and
dermal sensitization
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Guazatine was evaluated by the Joint Meeting in 1978 (Annex 1,
reference 30), when an ADI of 0-0.03 mg/kg bw was established on the
basis of an NOAEL of 200 ppm (equivalent to 3 mg/kg bw per day) in a
two-year dietary study in dogs. The compound was reviewed at the
present Meeting within the CCPR periodic review programme. New data on
its absorption and metabolism, the toxicity of repeated doses in mice
and dogs, its long-term toxicity in rats and mice, genotoxicity,
reproductive toxicity, and developmental toxicity were assessed. Data
on the pesticide 1,1-imino-di(octamethylene)diguanidine (common name,
iminoctadine), which constitutes about 1.5% of the guazatine mixture,
were also considered.
Evaluation for acceptable daily intake
Guazatine is a preparation of the triacetates of dimeric and trimeric
guanidated 1,8-diamino-octane which also contains a range of oligomers
and reaction products. Pure guazatine reportedly cannot be produced
industrially; all of the oligomers are necessary for its biological
activity and are considered together as active ingredients. A coding
system is used for the compounds that make up guazatine, in which N
represents any amino group and G represents any guanidated amino
group; NN represents H2N-(CH2)8-NH2 and GG represents
H2N-C(NH)NH-(CH2)8-NH-C(NH)-NH2. The stated purity of the
guazatine tested in toxicology studies was usually about 70%, i.e.
that of the marketed technical-grade product. The percent purity is
based on the results of titrimetric analyses, which are normalized to
one of the constituents (1,1'-iminodi(octamethylene)diguanidine, GNG).
There are no controls on the levels of individual components. All
doses and concentrations expressed on a w/w basis in this monograph
were corrected on the basis of dietary analyses; however, it is not
clear whether the results are for the technical-grade material or for
the free base. In some published Japanese studies, the term
'guazatine' is used synonymously with 'iminoctadine' (> 99% pure
GNG), which represents about 1.5% of the technical-grade material
1. Biochemical aspects
(a) Absorption, distribution, and excretion
In one male Wistar rat that received an unspecified dose of
14C-guanidino- and 3H-octyl-labelled guazatine, the recoveries were
83% 14C and 93% 3H; no volatile compounds were trapped. Over 72 h,
faecal excretion accounted for 64% and urinary excretion for 15% of
the administered 14C; 39% of the administered 3H was excreted in
faeces and 42% in urine. The carcass and tissues contained
approximately 3% of the 14C and 12% of the 3H. It is not clear
whether these differences in isotope ratios are due to metabolism or
tritium exchange, as thin-layer chromatography of the faecal and
urinary extracts showed only spots similar to those for the
administered material (Leegwater, 1975).
Two male rats weighing about 200 g received about 10 mg/kg bw of
[14C- guanidino]-labelled guazatine by gavage as a 0.25% solution of
Panoctine (purity unspecified; specific activity, 3.8 µCi/mg), and
faeces and urine were collected over 120 h. Mean recovery was > 90%,
the majority (about 60%) being found in the urine and about 30% in
faeces. About 90% of the urinary excretion and 50% of the faecal
excretion occurred during the first 24 h. The carcass and tissues
contained 2.5% of the administered dose, with mean values of 0.6% in
the liver, 0.4% in blood, and 0.08% in kidney. Qualitative thin-layer
chromatography of faecal and urinary extracts showed one major and
several minor components, which also appeared in the parent compound.
The reasons for the apparent difference in the main route of excretion
in this study and others are unknown (Leegwater, 1980).
Groups of CD rats received [14C-octyl]-labelled guazatine (purity,
78%; specific activity not given) by garage in distilled water in a
variety of protocols, outlined in Table 1. Faeces, urine, expired air,
and cage wash samples were collected for animals in the first four
groups. At termination, residues were determined in the carcass and
selected tissues from animals in groups 1, 2, and 5. The results of
high-performance liquid chromatography analyses reported in a
supplementary document (Prout, 1996), showed that the profile of
radiolabelled guazatine was qualitatively similar to that of
commercial guazatine (GTA70), although the ratios of the constituents
were different.
Mean recoveries were all within the range 95-105%. The results for
animals in groups 1, 2, and 3 were similar, faecal excretion at 24 h
accounting for 85-94% and urinary excretion for 3-6% of the
administered dose, with no sex difference. Less than 1% of the
administered dose was detected in exhaled air. In groups 1 and 2,
residues in the carcass and tissues at 96 h accounted for < 2% of the
administered dose; peak levels, proportionately similar in both
groups, were seen in the liver (2 and 0.2 g equivalents per g) and
kidneys (1 and 0.1 g equivalents per g). In bile duct-cannutated
animals (group 4), urinary excretion at 24 h was similar to that in
other groups (about 6%), but faecal excretion was reduced to about 55%
of the administered dose. The levels in bile were low, representing
less than 0.25% of the administered dose over 24 h. In these animals,
the residues in the gut accounted for 40% of the administered dose in
males and 24% in females, and those in the carcass represented 3.5% in
males and 11% in females. In animals given 14 × 2 mg/kg bw of
guazatine, there was evidence of accumulation in liver, kidney, and
fat during treatment, with partial clearance over the next 14 days
(Table 2).
Guazatine thus appears to be poorly absorbed after oral administration
at 2 or 20 mg/kg bw, and faecal excretion apparently represents
unabsorbed compound. The repeated dosing schedule indicates a limited
potential for bio-accumulation of guazatine, as clearance was not
completed 14 days after the last dose (Cameron et al., 1989).
Subsequent analysis of samples from this study indicated that two
components, the fully guanidated diamine GG and triamine GGG, are
absorbed preferentially (Prout, 1996), but the data to support this
assumption are not considered to be conclusive.
An extensive investigation of the absorption, distribution, and
excretion of iminoctadine (used synonymously for guazatine) was
reported by Kato et al. (1985). Groups of four male Wistar-Imamichi
rats received [14C-guanidine] - labelled iminoctadine triacetate
(purity, 99.7%) in saline at 3 or 30 mg/kg bw by gavage. Urine and
faeces were collected for up to seven days; blood samples were
collected periodically from animals that received 30 mg/kg bw. Tissues
were removed from animals killed on day 7.
The excretion patterns were unaltered by dose; overall excretion of
the administered dose was 4.6% in urine and 89-91% in faeces,
primarily during the first 96 h. About 1% of the administered dose was
found in the carcass after seven days. Peak blood levels (0.13 g
equivalents per g) were recorded 10 min after treatment, with an
elimination phase half-life of 27 h and the area under the
concentration-time curve representing 3.7 g equivalents-h per g. The
tissue concentrations at seven days were proportional to the
administered dose, the highest concentrations in animals at 30 mg/kg
Table 1. Treatment regimens of rats receiving 14C-guazatine
Group No. of Dose Sampling times Comment
animals (mg/kg bw)
1 5/sex 1 × 20 0-96 h
2 5/sex 1 × 2 0-96 h
3 5 males 1 × 2 0-96 h Fasted
4 1/sex 1 × 2 0-24 h Bile cannulated
5 15/sex; 1-14 × 2, daily 1, 3, 7, 14, 15, 16, 17, All doses labelled with
3 animals/ 20, 23, and 27 days 14C; sequential sacrifices
sacrifice after first dose
From Cameron et al. (1989)
Table 2. Residues (g equivalents per g tissue) of guazatine in
three rats receiving up to 14 daily doses of 2 mg/kg bw by gavage
Time Liver Kidney Fat
24 h after dose 1 0.33-0.43 0.08-0.10 0.01-0.02
24 h after dose 7 1.32-1.97 0.63-0.68 0.04-0.06
24 h after dose 14 1.75-2.02 1.11-1.36 0.14-0.19
72 h after dose 14 0.58-1.07 1.05-1.18 0.10-0.12
168 h after dose 14 0.28-0.50 0.72-0.96 0.06-0.11
336 h after dose 14 0.11-0.14 0.45-0.64 0.05-0.06
From Cameron et al. (1989)
bw being found in kidney (6.2 g equivalents per g), bone marrow (1.1 g
equivalents per g), liver, spleen, thyroid, salivary gland, and
pituitary (all 0.47-0.74 g equivalents per g). The finding of high
residues in kidney is not entirely consistent with the results of
Cameron et al. (1989), who found the highest levels in liver; they may
be due to differences in the constituents of the administered
compounds.
The distribution of guazatine (purity, 71.7%) containing [14C-octyl]-
labelled guazatine (specific activity, 6.6 µCi/mg) was investigated in
one Fresian cow given a single intraruminal injection through the body
wall and three cows given repeated (21 doses over 10.5 days)
injections at 0.1 or 1 mg/kg bw per day. The treated animals also
received the repeated doses. Samples of urine, faeces, expired air,
milk, blood, and saliva were obtained during the study; at termination
6 h after the last dose, a range of tissues were removed for analysis
of residue levels. After single or repeated administration of
guazatine at 0.1 mg/kg bw per day, the plasma concentrations were at
or below the level of reliable determination (30 dpm; about 2 ng
equivalents per ml). The single administration of 1 mg/kg bw per day
resulted in a peak plasma level of 2.6 ng equivalents per ml at 6-24
h, whereas the plasma levels after repeated treatment rose to 17.7 ng
equivalents per ml with time, indicating potential accumulation.
Guazatine was secreted into milk, with a peak residue of 28 ng
equivalents per ml after nine days of administration at 1 mg/kg bw per
day. The relative concentrations in milk fat, curd, and whey were
about 4:3:1. More than 92% of the single doses were excreted in faeces
over seven days, with a half-life of 36 h; urinary excretion accounted
for < 2% of the administered dose. The tissue residues in cows given
the low dose were near the level of reliable determination; at the
high dose, the levels of residues were higher than in plasma, with the
highest levels in liver and kidney (mean concentration, 80 ng
equivalents per g) but with considerable inter-animal variation, and
< 15 ng equivalents per g in muscle. These results indicate that
guazatine may concentrate preferentially in tissues rather than in
plasma (Cameron & Philips, 1986).
The fates of 14C-guazatine (as iminoctadine) photo-products and
14C-iminoctadine residues in apples were investigated in male
Wistar-Imamichi rats. The photo-products were produced in vitro by
irradiation of [14C-guanidino]-labelled iminoctadine triacetate for
seven weeks with sunlight bulbs (lambdamax, 515 nm). The resulting
mixture contained 39% unchanged iminoctadine, 35 % of the photo-
product 4- or 6-methyl-5-oxo-9-azaheptadecane-1,17-diguanadine, and
eight other photo-products. Three rats received the mixture of photo-
as an oral dose of 3 mg equivalents of iminoctadine per kg bw in
water. Two rats received homogenized apples that had been cultivated
and treated with an aqueous solution of 14C-iminoctadine (specific
activity, 0.132 µCi/kg) by gavage. The residues in the apples
consisted of 81% iminoctadine, 4% 4- or 6-methyl-5-oxo-9-
azaheptadecane-1,17-diguanadine, 7% minor photo-products, and 8% other
constituents; the administered dose (given as five doses of 10 g
homogenate per kg bw) was equal to 0.2 mg equivalents of iminoctadine
per kg bw. Three further rats received 14C-iminoctadine at 3 mg/kg bw
with apple homogenate at 5.3 g/kg bw. Urine and faeces were sampled
daily, and tissue samples were taken on day 7 for analysis of
radiolabel. The photo-product was more readily absorbed (26%) than
iminoctadine (about 10%) and tended to concentrate in the liver rather
than the kidney. The residue in apples was less bioavailable than
iminoctadine administered with apple homogenate, although a
significant concentration of radiolabel was reported in the kidney
(Sato et al., 1986).
Groups of four male Wistar-Imamichi rats with or without bile-duct
cannulae received 14C-iminoctadine triacetate (purity, 99.7%),
labelled at either the methylene or the guanidine carbon, at 3 mg/kg
bw by intravenous injection in saline. Blood, urine, and faeces were
collected for up to seven days, when tissue samples were removed for
analysis. The route of excretion varied with the position of the
radiolabel (Table 3), indicating cleavage of the parent molecule, with
some retention of the octylamine moieties.
The relatively high level of faecal excretion with only low levels of
radiolabel in the bile was attributed to secretion of iminoctadine or
metabolites by the salivary glands and glands of the stomach (pars
proventricularis and pars glandularis), which showed high
concentrations of radiolabel in whole-body autoradiographs.
Measurements of 14C-guanidine radiolabel in plasma showed an area
under the curve of 2.7 g equivalents-h per g and a half-life of 33 h,
whereas the half life of the 14C-methylene radiolabel was 69 h. After
seven days, the kidney contained the highest levels of radiolabel
(10-17 g equivalents per g; about 27% of the body burden for both
labels); salivary, pituitary, and thyroid glands had higher
concentrations (1.2-3.6 g equivalents per g) than the liver (0.8-1.6
g-equivalents per g). The radiolabel concentrations in blood and
Table 3. Excretion of 14C-iminoctadine triacetate after intravenous
administration to male rats at 3 mg/kg bw (as % of administered dose)
Group Route of excretion Time (h) Position of 14C label
Guanidine Methylene
Intact Urine 0-96 46 28
Urine 0-168 56 38
Faeces 0-96 22 24
Faeces 0-168 25 29
Carcass 168 21 34
Bile cannulated Bile 0-3 0.3 0.4
Bile 0-24 0.6 1.3
Urine 0-24 20 5
Faeces 24 10 9
plasma were very low (< 0.01 g equivalents per g) at day 7.
Autoradiography of the kidneys showed that the radiolabel was mainly
in the malpighian corpuscles (Kato et al., 1985).
(b) Biotransformation
Pooled samples of urine, faeces, liver, and kidneys from groups 1, 2,
and 5 of the study by Cameron et al. (1989), described above, were
extracted with methanolic acetate systems and investigated by
radio-high-performance liquid chromatography. The extraction
efficiencies were > 80% for urine, liver, and kidney, but only 44%
for faeces. The findings were similar in all treated animals. In urine
samples, three peaks were detected, which were tentatively attributed
to 1,8-diaminooctylacetic acid (NN), 1,8-diaminooctylacetic acid dimer
(NNN), and diguanidated diaminooctylacetic acid dimer (GNG/GGN). No
quantitative data were presented, but the largest peak was attributed
to NNN. Co-chromatography with standards did not confirm the proposed
identities, and no additional techniques were used to characterize the
components. Kidney and liver samples contained one major peak,
attributed to monoguanidated diaminooctylacetic acid dimer (GNN/NGN).
As NN and NNN are reported to be present at only low levels in the
parent compound, the results indicate extensive deamidination or
deguanidation. Given the ill-defined composition of the parent
guazatine and the lack of definitive confirmation of the metabolites,
the Meeting decided that no firm conclusions could be drawn about the
metabolism of guazatine from this study.
Samples of urine and faeces obtained over 96 h from male
Wistar-Imamichi rats given 14C-methylene- or guanidino-labelled
guazatine (as iminoctadine triacetate; purity, 99.7%) intravenously at
3 mg/kg bw were analysed for metabolites. Most of the urinary
metabolites were not characterized, but monodeamidino-iminoctadine was
found to represent 5% of the radiolabel from the guanidine-labelled
material and 16% from the 14C-methylene compound. No guazatine,
dideamidino-iminoctadine, or creatinine was found in urine. In faeces,
the major component was unchanged iminoctadine (78%);
monodeamidino-iminoctadine represented 3% of the radiolabel, and 15%
was due to a compound with identical chromatographic properties to the
main photo-product (4- or 6-methyl-5-oxo-9-azaheptadecane-1,
17-diguanadine), although its identity was not confirmed by mass
spectrometry.
Analyses of kidney samples from rats given the labelled compound
intravenously at 3 mg/kg bw, orally at 30 mg/kg bw, intraperitoneally
at 15 mg/kg bw, or intraperitoneally at 4 × 10 mg/kg bw per day showed
16 metabolites, which were reported to be independent of route of
administration. The three main constituents characterized were
iminoctadine, monodeamidino-iminoctadine, and
dideamidino-iminoctadine; two key metabolites were not identified. The
proportion of iminoctadine decreased between days 1 and 7 after
treatment, and the amounts of the four main metabolites increased over
time. Analyses of liver samples identified a similar pattern to that
seen in kidney. The results indicate that deamidination is a
significant step in the metabolism of iminoctadine (Kato et al.,
1985).
In the study of Cameron & Philips (1986), described above, samples of
liver, kidney, urine, and faeces from cows given 21 doses of guazatine
at 1 mg/kg bw per day for 10.5 days showed that much of the residue
was similar to some components of guazatine. There was evidence of
selective absorption, as the peak ratios in the chromatogram of faeces
differed from those in that compound, which was not present in the
parent compound. The liver residue contained two polar metabolites in
addition to guazatine components. Milk samples were not analysed for
metabolites. Despite the availability of a range of standards,
individual peaks were not characterized.
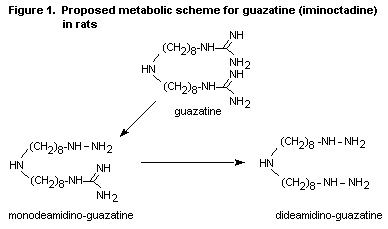
A scheme for the metabolic pathway of guazatine in rats is shown in
Figure 1. Other components of guazatine would be expected to undergo
similar deamidination.
 2. Toxicological studies
(a) Acute toxicity
Guazatine is of moderate toxicity when given orally, of low toxicity
when applied dermally, but of moderately high toxicity when
administered by inhalation or intraperitoneally. The results of
studies of the acute toxicity of guazatine are summarized in Table 4.
The clinical signs of toxicity after treatment were lethargy or
sedation, hypothermia, coma, and local irritation. The gross and
histopathological changes were consistent with a response to a local
irritant; them was no clear systemic toxicity.
(b) Short-term toxicity
Mice
In a range-finding study, groups of 10 male and 10 female CD-1 mice
received guazatine (purity, 70.6%) in the diet at 0, 10, 50, 200, or
500 ppm for 13 weeks. Samples were taken for limited blood and
clinical chemical analyses at week 13. A limited range of tissues was
removed, weighed, and examined macroscopically; only the liver was
examined histologically. Two male controls, one male at 500 ppm, and
one at 50 ppm died during the study. Marked reductions in body-weight
gain (> 25%) were seen in animals of each sex at 200 or 500 ppm. In
females at the highest dose, aspartate aminotransferase activity was
increased. The relative liver weights were increased in animals of
each sex at 500 ppm; reductions in the absolute weights of several
organs appeared to be secondary to reduced body-weight gain. Altered
centrilobular hepatocytes were seen in 80% of animals at 200 or 500
ppm. Given the limited extent of the investigations, no NOAEL was
identified (Atkinson et al., 1990).
Table 4. Acute toxicity of guazatine and formulations
Test material Species Route Purity LD50/LC50 Reference
(%) (mg/kg bw or
mg/m3)
Guazatine GTA Rat Oral 69.2 280 Spanjers & Til (1980)
Panoctine 42 Cat Oral NR 0.38 ml/kg bw De Grout (1976a)
Guazatine GTA70 Rat Dermal 70.9 1050 Cuthbert & D'Arcy-Burt (1986)
Panoctine 42 Rabbit Dermal NR 2.8 ml/kg bw van Beck et al. (1976)
Panoctine Plus 300020 Rabbit Dermal NR 2.8-5.6 ml/kg bw van Beck (1980)
Paaoctine 42 Rat Intraperitoneal NR 0.053 ml/kg bw De Groot (1976b)
Guazatine GTA Rat Inhalation 69.2 225 Appelman (1980)
Panoctine 42 Rat Inhalation NR 11 Kruysse & Immel (1976)
Rats
Groups of 10 male and 10 female Wistar-derived rats received guazatine
(54.8% w/w) in the diet at 0, 60, or 200 ppm for 14 weeks.
Haematological and urinary parameters were measured in samples taken
at week 13, and serum enzymes and total protein were measured in
samples taken at termination at week 14. There were no deaths or
adverse clinical signs. Body weight and food conversion efficiency
were unaltered by treatment. A slight, dose-related decrease in
leukocyte count was seen in animals of each sex (up to 13% in males
and 6% in females), but this was not statistically significant, and
there was no marked change in differential counts. Serum alkaline
phosphatase activity was decreased in treated males, by 22% at 200 ppm
and 7% at 60 ppm. Urinary parameters were unaffected by treatment, but
the volume and specific gravity were not given. There were no
significant findings at gross or microscopic examination. The findings
in this study are of minimal toxicological significance; the NOAEL was
200 ppm, equivalent to 10 mg/kg bw per day (Sinkeldam & van der
Heijden, 1974).
Groups of 10 Wistar-derived rats of each sex received guazatine (54.8%
w/w) in the diet at 800 ppm for six weeks and then 1200 ppm for eight
weeks or control diet for 14 weeks. Haematological and urinary
parameters were measured in samples taken at week 13, and serum
enzymes and total protein were measured in samples taken at
termination at week 14. There were no deaths or adverse clinical
signs. Body-weight gain was reduced in animals of each sex by
approximately 6% at week 6 and by 8-10% at termination. Food
efficiency was unaffected in males but reduced in females.
Haematological parameters were unaltered by treatment. In males, serum
aspartate aminotransferase activity was increased and alkaline
phosphatase activity decreased. Females had an increased urine volume
with an associated decrease in specific gravity; the report does not
indicate whether samples were collected under fasting conditions.
Increases in relative adrenal, testis, and heart weights were seen in
treated animals, without abnormal pathological findings. As data on
individual animals and absolute organ weights were not presented, the
significance of these findings is uncertain. In treated females, high
levels of 'iron' deposition in the spleen were reported. The thyroids
of two females given guazatine were increased in weight and contained
small follicles lined with large epithelial cells that had small
apical nuclei. A re-evaluation of salivary gland tissues from this
study, reported very briefly by Til & Hendricksen (1976), described
hyperplasia in the epithelial lining of the excretory ducts of the
parotid salivary glands, with associated mononuclear cell infiltrates
in some treated animals. No NOAEL was identified (Til & Feron, 1975).
Panoctine 42 (purity unspecified) was administered to groups of 10
Wistar-derived rats of each sex at 0 or 1500/2000 ppm in the diet; the
level was increased from 1500 to 2000 ppm after week 4. Samples for
examination by haematology, limited clinical chemistry, and urinalysis
were taken during week 13. Animals were killed at week 14 and examined
grossly and histologically. There were no deaths or adverse clinical
signs. Body-weight gain was reduced by about 10% from week 2 in males
and by about 8% from week 6 in females. Food efficiency was reduced in
males for the first 12 weeks of the study. Serum proteins and alkaline
phosphatase activity were reduced in both treated groups. In treated
females, the leukocyte counts were increased (by 30%), and
differential counts indicated an increase in neutrophils and a
decrease in eosinophils. Increased relative weights of the liver (by
8%) and kidney (by 14%) were seen in treated males, without associated
histological findings. In females, an increased relative weight of the
heart (by 17%) and decreased thyroid weights (by 8%) were reported,
again without associated histological findings. Urinary aspartate
aminotransferase activity, proposed as a measure of renal toxicity,
was increased in treated animals, and urine volume was increased and
specific gravity decreased in animals of each sex. These findings
taken together are indicative of renal toxicity, though no associated
findings were reported at pathological examination. The only
histopathological finding of note was hyperplasia of the epithelial
lining of the excretory ducts of the parotid salivary glands with
associated mononuclear cell infiltrates in six males and six females
in the treated groups and in none of the controls (rho < 0.01). No
NOAEL was identified (Til & Hendricksen, 1976).
Dogs
In a range-finding study in beagle dogs, guazatine (purity, 67.9%)
given at 9.4 mg/kg bw per day by gavage for four days induced
body-weight loss in one of four animals. Subsequent administration of
14 mg/kg bw per day for four days induced marked body-weight loss and
reduced food consumption in all animals. The pathological findings
indicated local irritation of the gastrointestinal tract.
Administration of 374 ppm guazatine in the diet (12-15 mg/kg bw per
day) resulted in reduced food consumption, reduced body-weight gain,
and increased activities of aspartate aminotransferase, alanine
aminotransferase, and lactate dehydrogenase in serum after two weeks
(Goburdhun & Carter, 1989).
Groups of four male and four female beagle dogs received guazatine
(purity, 54.8%) in the diet at levels of 0, 60, 200, or 300/600 ppm
for two years; the level of 300 ppm was increased to 600 ppm after 26
weeks. Extensive observations were made, including haematological,
clinical chemical, and urinary determinations at 12/13, 26, 52, 78,
and 104 weeks, and tests for liver function (bromosulfthalein) and
kidney function (phenol red excretion) at 26, 52, and 104 weeks. Gross
and histopathological examinations were performed at termination at
week 104, although the results of histopathology in individual animals
were not reported. One control male died due to urolithiasis. There
was no evidence of treatment-related changes in clinical signs.
Body-weight gain and food consumption were unaffected by exposure. A
decreased leukocyte count (by 12-25 %) was present in animals at the
high dose, particularly females, from week 13 onwards, achieving
statistical significance at week 52, although there were no marked
alterations in differential counts. Sporadic changes in other
haematological, clinical chemical, and urinary parameters were not
consistent or of statistical significance. The results of liver and
kidney function tests were similar for control and treated groups.
Increases in absolute and relative ovarian weights were seen in
females at the high dose, although the significance of these changes
is unclear as there was considerable inter-animal variation and no
obviously associated histological findings. Given the uncertainties
about the findings at the high dose, the NOAEL was 200 ppm, equivalent
to 5 mg/kg bw per day (Reuzel et al., 1976).
Groups of four beagle dogs of each sex received guazatine (purity,
70.6%) in the diet for 52 weeks. Animals were given 400 g of diet per
day containing 0, 25, 75, or 250 ppm guazatine; the achieved intakes
were about 0, 0.8, 2.5, or 8 mg/kg bw per day. Extensive observations
included ophthalmoscopy at 0, 13, 26, and 51 weeks and haematology,
clinical chemistry, and urinalysis at 0, 6, 13, 26, and 51 weeks.
Animals were killed at week 52 and examined grossly; 13 tissues were
weighed, and more than 40 tissues were investigated by histopathology.
Owing to poor weight gain, one control and two males at the high dose
received extra food, which reversed the condition. In females, there
was a clearly dose-related reduction in body-weight gain starting at
week 10 and continuing throughout the study, with reductions of 30% in
comparison with controls in the group at the high dose, 20% in those
at the intermediate dose, and 12% in those at the low dose. Food
consumption was reduced in females receiving 75 or 250 ppm, with
occasional reductions in those at 25 ppm. Haematology showed no
consistent time- or dose-related reactions to treatment. Marked
increases (up to sixfold) in serum alanine aminotransferase activity
were seen throughout the study in males and females receiving 250 ppm.
Serum aspartate aminotransferase activity was increased in males at
the high dose throughout the study; an apparent increase in alkaline
phosphatase activity in males appears to be related to differences in
pretreatment values. A slight increase in liver weight was noted in
females at the high dose. Reduced prostate weights in males at the low
dose, increased ovarian weights in all treated females, and increased
thyroid weights in females at the intermediate dose did not show
dose-response relationships and were not clearly related to treatment,
although effects on ovarian weights were also see in the study of
Reuzel et al. (1976). Gross and microscopic examination showed no
changes. The reduced body-weight gain of females appeared to be due
only in part to unpalatability, as food conversion efficiency was also
reduced. The NOAEL was 25 ppm, equivalent to 0.8 mg/kg bw per day, on
the basis of marked reductions in body-weight gain in females at 75
and 250 ppm and clear increases in serum enzyme activities, indicative
of effects on the liver, in animals of each sex at 250 ppm (Oshodi &
Thompson, 1993).
(c) Long term toxicity and carcinogenicity
Mice
Groups of 10 male and 10 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for one year; the
achieved doses were 0, 7.6, 19, or 55 mg/kg bw per day in males and 0,
10, 25, or 67 mg/kg bw per day in females. All animals were examined
macroscopically. A wide range of tissues from animals receiving 0 or
300 ppm and those that died before week 52 were examined
histologically; the gall-bladder, kidney, liver, lung, salivary gland,
and grossly abnormal organs from all other animals were also examined
histologically. One male and one female at 120 ppm and two females at
300 ppm died during the study. There were no clinical signs associated
with treatment. Body-weight gain was reduced from week 4 in animals of
each sex at 300 ppm; by week 52, the weight gain of males was 72% that
of controls and that of females 66% of control values. Food
consumption was similar in treated and control animals. The relative
liver weights were increased by about 10% in all treated males. The
only notable finding at gross necropsy was an increased incidence of
ovarian cysts in females receiving 120 or 300 ppm (9/10 versus 6/10 in
controls). Microscopic examination showed increased incidences of
lymphocytic infiltration of the kidney and ovarian cysts in females at
the high dose (the ovaries of those at the intermediate dose were not
examined), of hepatocellular adenoma in males at the high dose (2/10
versus 0/10 in controls), and a decreased incidence of lymphoid foci
of the mandibular salivary gland in females at the intermediate and
high doses. The finding of hepatocellular adenomas may indicate that
guazatine reduces the time to onset of the liver turnouts seen
commonly in mice. Given the small group size, limited examination of
tissues from animals at low and intermediate doses, and clear effects
at 300 ppm, there was no NOAEL (Heath et al., 1995).
In the part of the same study designed to test for carcinogenicity,
groups of 50 male and 50 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for two years; the
achieved doses were 0, 6.8, 17,or 47 mg/kg bw per day in males and 0,
8.7, 21, or 57 mg/kg bw per day in females. All animals were examined
macroscopically, and a wide range of tissues from animals receiving 0
or 300 ppm or that died before week 104 were examined histologically;
only the gall-bladder, kidney, liver, lung, salivary glands, and
grossly abnormal organs from animals in other groups were examined.
Blood samples for differential leukocyte counts were taken from all
surviving controls and animals at 300 ppm during weeks 52, 78, and
103.
The pattern of deaths was similar in treated and control groups, with
> 50% survival in all groups until week 100 in males and week gg in
females. Reduced body-weight gain was seen at the high dose from week
2 onwards, the deficit being approximately 20% at termination. During
the second year, males at the intermediate dose lost more weight than
controls, while females at this dose gained more weight than controls.
Food consumption, differential leukocyte counts, and findings at gross
necropsy were unaffected by treatment. Increased absolute and relative
weights of the kidney and liver were seen in females at the
intermediate but not the high dose.
The incidences of a range of neoplastic and non-neoplastic lesions
showed evidence of treatment-related effects (Table 5). The incidences
of haemangiosarcoma of the liver in males at the intermediate and high
doses and of the spleen in males at the high dose were higher than
those in historical controls, which were 04% in males and 0-3% in
females for liver and 0-2% in males and 045% in females for spleen.
The high incidence of haemangiosarcoma in females at the low dose is
considered to be a chance finding, as them was no dose-response
relationship. The incidence of hepatocellular carcinoma in females at
the high dose was greater than that in historical controls (0-4%). The
incidences of renal adenoma and carcinoma in male historical controls
were 0-4% and 0-2%, respectively, indicating that the incidence of
renal adenoma in males at the high dose is a significant finding.
The presence of rare tumours, including malignant ones, at multiple
sites was considered by the Meeting to be of concern. The mechanism by
which these tumours are induced is not evident from the available
data. Guazatine is not genotoxic (see below), indicating the mechanism
is probably nongenotoxic, although haemangiosarcomas are generally
associated with a genotoxic mechanism. As them was no evidence of
marked toxic or hyperplastic responses in the affected tissues and
them was evidence of a dose-response relationship, it was not clear
that these tumours are a direct result of disrupted cell function
associated with exposure to doses above the maximum tolerated dose.
Although only a limited range of tissues was examined from animals at
the low and intermediate doses, it included those with the most
significant effects at the high dose. The NOAEL was 50 ppm, equal to
6.8 mg/kg bw per day, on the basis of effects on body-weight gain and
the occurrence of haemangiosarcomas in males at 120 ppm, marked
effects on body-weight gain, and a range of neoplastic and
non-neoplastic changes at 300 ppm (Heath et al., 1995).
The findings of another two-year study with guazatine in mice were
summarized in abstract form only. Guazatine (as iminoctadine) was
given to groups of 80 male and 80 female specific-pathogen-free
ICR-Crj mice at dietary levels of 0,10, 100, or 300 ppm. Eight animals
of each sex at each dose were killed at weeks 26 and 52 for
examination. At 300 ppm (26 mg/kg bw per day in males, 30 mg/kg bw per
day in females), males and females showed a remarkable depression in
body-weight gain, decreased food efficiency, and anaemia at weeks 26
and/or 52, increased plasma urea nitrogen level (only in females),
increased alkaline phosphatase level (at week 26 in animals of each
sex and also at week 52 in females), and increased weights of the
kidney (absolute and relative) and liver (relative). Histological
examination revealed swelling of hepatocytes and proximal tubular
cells in animals of each sex. There were increased incidences of
subcutaneous oedema in females and hydrothorax in males. The
incidences of splenic atrophy in animals of each sex, atrophy and/or
brown pigment deposition of the ovary, and glandular epithelial
atrophy of the Harderian gland in males were significantly increased.
The incidence of renal epithelial tumours increased slightly in males.
No toxic changes were seen at the other doses, except for slightly
Table 5. Lesions (as % of samples examined) seen in CD1 mice exposed to guazatine in the diet
for two years
Lesion Guazatine (ppm)
Males Females
0 50 120 300 0 50 120 300
Brain mineralization 22 26 33 20 8 16 10 22*
Caecal carcinoma 0 0 0 0 0 0 0 2
Renal tubular carcinoma 0 0 0 2 0 0 0 0
Renal tubular adenoma 2 2 2 8 0 0 2 0
Renal glomerular amyloid 12 8 16 10 24 8 8 0*
Hepatocellular carcinoma 22 24 22 22 2 2 2 10
Liver haemangiosarcoma
Multifocal 0 0 2 4 0 0 2 0
Other 2 2 6 6 0 6 2 0
Increase in bronchus-associated
lymphoid tissue 10 12 12 20 4 14 16 28*
Lung lymphoid foci 0 0 2 0 0 0 4 10*
Spleen haemangiosarcoraa 0 0 2 4 2 0 2 4
Vaginal keratinized epithelium - - - - 15 - - 43
-, not determined
* One-tailed p < 0.05, Fisher exact test
increased incidences of swelling of proximal tubular cells in animals
of each sex at 100 ppm (Maita et al., 1985).
Rats
Groups of 60 male and 60 female Wistar-derived rats, in two parallel
series initiated two months apart, received diets containing guazatine
(54% solution) at 0, 20, 60, or 200 ppm for two years. A group
receiving 6 ppm was terminated after a few months, as the results of
other studies indicated that 20 ppm was probably a NOAEL. Blood and
urine samples were taken from 10 fasted animals of each sex at each
dose at various times during the study for haematological, limited
clinical chemical, and urinary analyses. All animals were examined
grossly post mortem. A wide range of organs from surviving animals
in the first series (11-20) were weighed, but only kidney, spleen, and
adrenals from rats in the second series were weighed. A full
histological examination was performed on tissues from 20 rats of each
sex in the control and high-dose groups in the first series, and gross
lesions, tumours, adrenals, thyroid, and pituitaries from all animals
were examined.
Behaviour and clinical signs were reported to be unaffected by
treatment. There were more early deaths (before week 72) among treated
males, but overall survival to week 104 was satisfactory and similar
in all groups. Body weights were similar in all groups until week 88,
when all treated females showed a deficit in comparison with controls,
which lasted until termination. Food consumption and conversion
efficiency were similar in all groups over the first four weeks.
Leukocyte counts were decreased (by about 11%) in males at the high
dose from week 26, with no consistent change in differential counts;
females showed sporadic changes in leukocyte counts with no consistent
pattern. Urinalysis gave similar findings in all groups. Reduced
relative testicular weights (by 11%) were seen in males at the high
dose, although the absolute weights were similar since the mean body
weight was 8% higher. In treated females, the relative weights of the
spleen, brain, and kidney were increased; however, as the absolute
weights were decreased and there was no evidence of a dose-response
relationship, this finding is probably secondary to the effects on
body weight. Occasional increases in the incidences of non-neoplastic
lesions were reported, but the only statistically significant
(p < 0.05) increases were for chronic respiratory disease and mammary
gland inflammation or dilatation in females at the high dose. The
number of tumour-bearing animals and the total numbers of tumours were
similar in all groups. The incidence of monocytic leukaemia was
increased in males and females at the high dose, occurring in four
males and three females, with none in male and in one female controls.
As the total numbers of animals examined is unclear, this may be a
chance finding. Although the study indicates that guazatine has no
marked toxicity, the limited, investigations performed and the unusual
design make interpretation of the findings difficult. No NOAEL was
identified (Til et al., 1976a).
Groups of 20 male and 20 female Sprague Dawley rats received guazatine
(purity, 70.6%) in the diet at 0, 50, 150, or 350 ppm for one year.
The achieved intakes of guazatine were 0, 2.5, 7, or 19 mg/kg bw per
day for males and 0, 3, 9, or 22 mg/kg bw per day for females. Samples
for haematological, clinical chemical, and urinary analyses were
obtained from 10 animals of each sex in each group at weeks 26 and 50
or 51. At week 52, the animals were killed and examined grossly.
Selected tissues were weighed, and samples from a wide range of organs
from controls and animals at the high dose were examined
histologically; gross lesions, kidneys, liver, lung, and salivary
glands from animals at the low and intermediate doses were also
examined histologically.
Behaviour, clinical signs, body-weight gain, food consumption, urinary
parameters, and the findings at gross necropsy were similar in all
groups. None of the controls but one male at the low dose, two each at
the intermediate and high doses, and one female at 150 ppm died. Total
leukocyte and lymphocyte counts were reduced in males at 350 ppm at
both 26 and 51 weeks, but increases were seen in treated females.
Serum alanine aminotransferase activity was decreased markedly (by
>40%) at both sampling times in males and females receiving 350
ppm, but there were occasional deficits in other serum enzyme
activities (particularly aspartate aminotransferase); total protein
was similar in all groups. Dose-related decreases in absolute and
relative prostate weights were significant (rho < 0.05) at both 150
and 350 ppm and were associated with a low incidence of hyperplasia at
350 ppm. Females had dose-related increases in the relative and
absolute weights of the adrenals (by < 10%) and ovary by
(< 50%). Five males at the high dose but none of the controls had
testicular atrophy. Mononuclear-cell infiltration of the parotid
salivary gland was increased in males (6/20 versus 1/20) and females
(5/20 versus 1/20) receiving 350 ppm. The NOAEL was 150 ppm, equal to
7 mg/kg bw per day, on the basis of decreased leukocyte count,
prostatic hyperplasia, decreased alanine aminotransferase activity,
and effects on the salivary gland at 350 ppm. The minimal changes seen
at 150 ppm were not consistent or of sufficient magnitude to be
considered adverse (Heath et al., 1994).
In the phase of the study that addressed carcinogenicity, groups of 50
Sprague Dawley rots of each sex received guazatine (purity, 70.6%) in
the diet at 0, 50, 150, or 350 ppm for two years. The achieved intakes
were about 0, 2.5, 7, and 19 mg/kg bw per day for males and 0, 3, 9,
and 22 mg/kg bw per day for females. Samples for haematological,
clinical chemical, and urinary analyses were obtained from 10 rats of
each sex per group at weeks 53 (for haematology only), 78, and 101 or
103. At week 104, the animals were killed and underwent a full gross
examination. Selected tissues were weighed, and samples from a wide
range of organs from controls and animals at the high dose were
examined histologically; gross lesions, kidneys, liver, lung, and
salivary glands from animals at the low and intermediate doses were
also examined histologically.
Behaviour, clinical signs, and organ weights were similar in all
groups. Survival was acceptable (50% at week 94) and similar in all
groups. Low body weights were seen consistently in females at the high
dose from week 9 and in males at this dose from week 20; in the latter
quarter of the study, females at the low and intermediate doses had
reduced body-weight gain in comparison with controls. Males at the
high dose showed consistent reductions in leukocyte and platelet
counts but increased erythrocyte counts. In females at 350 ppm,
erythrocyte counts were reduced; an increase in leukocyte counts at
termination was due to a neurilemmoma in one animal. Reduced
activities of serum alanine and aspartate aminotransferases (by
< 50%) were seen consistently in animals of each sex receiving 350
ppm. Reduced urinary pH was seen at the high dose in animals of each
sex throughout the study. A slight excess of abnormal findings in
lymph nodes was seen in females at this dose, but no individual effect
was significant when compared with the very low background incidence.
The incidences and severity of a range of lesions in salivary glands,
lymph nodes, ovaries, spleen, and pituitary were increased in females
receiving 350 ppm. In males, the only notable finding was increased
severity and incidence of testicular germinal epithelial degeneration
at 350 ppm. A rare malignant oligodendroglioma was found in the brain
of a single female at the high dose, but there was no evidence of
treatment-related carcinogenicity in any other animal. The NOAEL was
150 ppm, equal to 7 mg/kg bw per day, on the basis of reduced
body-weight gain and a range of clinical chemical and histological
findings at 350 ppm (Heath et al., 1994).
The findings of a two-year study of guazatine in rats were summarized
in abstract form only. Guazatine (as iminoctadine) was presented to
groups of 80 male and 80 female Fischer 344 rats at dietary levels of
0, 10, 100, or 300 ppm for two years. At 6 and 12 months, eight
animals of each sex from each group were killed for examination.
Animals of each sex at 300 ppm (11 mg/kg bw per day for males, 14
mg/kg bw per day for females) had remarkably depressed body-weight
gain, decreased food efficiency, higher mortality rates, a tendency to
anaemia, decreased total protein, and increased kidney and adrenal
weights; decreased potassium and albumin and increased calcium,
aspartate aminotransferase and gamma-glutamyltransferase activities,
and spleen weight were also observed. Histologically, swelling,
degeneration, and necrosis of renal tubular cells and metaplasia in
the glandular stomach were observed in animals of each sex. The
incidence of sperm granuloma in the deferent duct and/or epididymides
was significantly increased, and the incidences of leukaemia in males
and of adrenal phaeochromocytoma in animals of each sex were slightly
increased. At 100 ppm, males had a slightly higher mortality rate, and
females had depressed body-weight gain; decreased potassium and
increased calcium and gamma-glutamyltransferase activity were
occasionally observed in animals of either sex. The kidney and spleen
weights were increased, and the incidences of intestinal metaplasia
and sperm granuloma were significantly higher than in controls. At 10
ppm, no toxic change attributable to guazatine were seen (Hirano et
al., 1985).
(d) Genotoxicity
Guazatine has been tested for its potential to induce gene mutations,
sister chromatid exchange, and chromosomal aberrations in vitro and
micronuclei in vivo. Negative results were obtained in all studies.
The data are summarized in Table 6.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female Wistar-derived rats received diets
containing 0, 60, or 200 ppm guazatine (purity, 54.8%) over four
generations. The reporting of the study lacks the results for
individual animals, dietary analyses, time to mating, length of
gestation, absolute organ weights, and other data. F0 animals
received the test diets for 12 weeks before the first mating to
produce the F1a generation; the second mating eight weeks later
produced the F1b generation. Litters were culled to eight pups on day
1 after birth, and the F1a litters were discarded at weaning. Animals
were selected from the F1b litters at weaning to produce the F2
generation, with mating identical to that for F0 animals, and
similarly for the F3 generation from the F2b litters. Groups of 10
animals of each sex from the F3b litters were selected at weaning and
given test diets for four weeks; they were then killed and subjected
to gross and histopathological examinations.
Treatment had no effect on survival, clinical signs, litter size, sex
ratio, or resorption rates. Minor variations in the body weights of
pups appeared to be secondary to variations in litter size. F3b
animals receiving 60 or 200 ppm had increased, relative kidney
weights (by 8% at 60 ppm and 12-17% at 200 ppm), and increased
relative thymus weights (by 19-34%) were seen in all treated groups. A
dose-related reduction in relative testicular weights (8%) was also
seen. Histological examination gave no evidence of treatment-related
effects in any organ. In a summarily described extension of the study,
it was reported that males in F4b litters given test diets for four
weeks after weaning had reduced relative weights of the testis (9%)
and increased relative weights of the thymus (25%) at both doses, and
females at the high dose had increased relative kidney weights (12%).
Again, no treatment-related histological findings were reported. This
study shows that guazatine does not adversely affect reproductive
outcome in rats. The finding of increased thymus weights may indicate
that it affects the control of thymic growth or involution; such
effects may not be detectable in older animals when thymic involution
is well advanced. Given potential concern about effects on the thymus
at both 60 and 200 ppm, no NOAEL was identified (Til et al., 1976b).
Table 6. Results of genotoxicity assays on guazatine
End-point Test system Concentration/ Purity Results Reference
dose (%)
In vitro
Reverse mutation S. typhimurium 0.6-50 µg/plate 73 Negativea Wilmer (1983a)
TA98, TA 100. Cytotoxic
TA1535. TA1537. at 50 g/plate
TA1538
Reverse mutation S. typhimurium To cytotoxic levels NR Negative Moriya et al.
TA98, TA100, TA1535, (numbers not given) (1983)
TA1537, TA1538, and
E. coli WP2 hcr
Gene mutation Chinese hamster 25-100 nl/ml -S9 NR, Negativea Davis (1983a)
ovary cells (line K1), 50-200 nl/ml +S9 approx. Cytotoxic at
hprt locus 70% highest
concentrations
Sister chromatid Chinese hamster 5-30 nl/ml NR, Negativea Davis (1983b)
exchange ovary cells (line K1) approx. Cytotoxic
70% at > 35 nl/ml
Chromosomal Human 3.7-100 g/ml -S9 73 Negativea Wilmer (1983b)
aberration lymphocytes 14.8-400 g/ml +S9 Cytotoxic
at highest
concentrations
In vivo
Micronucleus Mouse (5/sex at 150 mg/kg bw by 73 Negative Willems (1983)
formation each time) bone gavage in saline No effect on
marrow (approx. 50% of P:N ratio
LD50); 24, 48, and
72h
Table 6 (continued)
NR, not reported; S9, exogenous metabolic activation system from Aroclor 1254-induced rat liver preparations;
P:N, polychromatic:normochromatic erythrocytes
a With and without metabolic activation
The reproductive effects of guazatine (purity, 70.6%) were
investigated in a two-generation (one litter per generation) study in
which groups of 28 young adult CD rats received diets containing
guazatine at 0, 50, 150, or 350 ppm, equal to a minimal intake of 0,
3, 10, or 22 mg/kg bw per day in males and 0, 4, 11, or 25 mg/kg bw
per day in females. Animals were given the treated diet for 10 weeks
before F0 mating (1:1) and continuously until sacrifice. Groups of 24
male and 24 female F1 animals were mated at about 17 weeks; they were
then killed and examined grossly, and reproductive organs and
pituitary, liver, adrenals, and salivary glands were weighed and
prepared for histological examination. Pups that died before weaning
were examined grossly. On day 21, one pup of each sex per litter was
examined grossly, and the liver and salivary glands were removed for
histological examination. All other pups were examined externally.
Treatment had no effect on clinical signs, survival, time to mating,
fertility indices, gestation length, litter size, gestation indices,
or lactation indices. Slight reductions in body-weight gain (5-10%)
were closely related to reductions in food consumption (4-9%). Slight
reductions in survival to day 21 (81-83% versus 87% in controls) and
litter weight at day 21 (about 8%) were seen in F1 pups in all
treated groups; as there was no dose-response relationship and the
effects were of small magnitude and were not seen in the F2
generation, they were considered not to be of biological significance.
Post-mortem examination showed no treatment-related effects; however,
the thymus was not weighed or investigated specifically. The NOAEL was
350 ppm, equal to 22 mg/kg bw per day, the highest dose tested
(Barton, 1993).
(ii) Developmental toxicity
Rats
The developmental effects of guazatine were investigated in animals
selected from the F2b and F3b litters of the study of Til et al
(1976b), described above Groups of five males and 15 females were
selected at weaning and maintained on test diets (0, 60, or 200 ppm
guazatine) until mating (1:3) at week 12; it was not clear whether the
test diets were given during gestation. On day 21 of gestation, the
dams were killed, their reproductive tracts investigated, and pups
examined for gross abnormalities and skeletal defects (with Alizarin
Red S staining); effects on soft tissues were studied only in controls
and rats at 200 ppm (by Wilson sectioning). F3b animals showed a
dose-related reduction in litter size, with 10.7 pups in controls, 10
at 60 ppm, and 8.9 at 200 ppm; at 200 ppm, this reduction was
associated with increased pre- and postimplantational losses in two
dams. Mean fetal weight, and hence litter weight, was reduced at 200
ppm in the F3b-derived group There were no notable visceral effects;
although there was an indication of reduced ossification in treated
animals, this was not consistent with regard to dose, generation, or
site. Reduced litter size was seen mainly in two animals and is not
consistent with the results of the main study. The NOAEL was 200 ppm,
equal to 12 mg/kg bw per day, as guazatine did not directly affect the
developing fetus or the dam (Til et al., 1976b).
In a range-finding study, pregnant Sprague Dawley rats died after
receiving guazatine (purity, 70.9%) at levels of 40, 80, or 120 mg/kg
bw per day by gavage on days 6-16 of gestation. At 20 mg/kg bw per
day, there was no evidence of toxicity. Necropsy of animals at doses
> 40 mg/kg bw per day showed marked irritation of the
gastrointestinal tract (Hazelden, 1987).
Four groups of 27 timed-mated Sprague Dawley rats received guazatine
(purity, 70.9%) in distilled water by garage on days 6-16 of presumed
gestation at doses of 0, 5, 10, or 20 mg/kg bw per day, on the basis
of the findings in the range-finding study. An adequate range of
examinations was performed, and animals were necropsied on day 20
after nitrogen asphyxiation. About two-thirds of the fetuses from each
litter were examined for gross external and visceral abnormalities
before staining with Alizarin Red S for observation of skeletal
abnormalities and variants. The remainder were examined by free-hand
dissection (Wilson technique) for soft-tissue abnormalities. One
control and two animals at the high dose died during the study due to
dosing accidents. There were no signs of maternal toxicity;
body-weight gain was similar in all groups, although food consumption
was decreased marginally (by 1-2%) in rats at 10 and 20 mg/kg bw.
There were no effects on litter size or fetal weight, or on soft
tissues or viscera. There was an indication of slightly retarded
development of some bones (e.g. scapula and pectoral girdle) at 10 and
20 mg/kg bw, but the findings were not significant, and the overall
degree of skeletal ossification was unaffected by treatment. The lack
of maternal toxicity at the high dose may be considered to have
compromised the study, but use of 20 mg/kg bw per day is considered
acceptable in view of the 40% mortality rate at 40 mg/kg bw per day in
the range-finding study. The NOAEL for maternal toxicity,
fetotoxicity, and teratogenicity was 20 mg/kg bw per day, the highest
dose tested (Hazelden & Wilson, 1986).
Rabbits
Groups of 15 timed-mated New Zealand white rabbits received guazatine
(purity, 67.9%) in distilled water by gavage on days 6-18 of presumed
gestation at doses of 0, 2.8. 5.6, or 11 mg/kg bw per day. An adequate
range of examinations was performed, and necropsy was carried out on
day 29. About two-thirds of fetuses from each litter were examined for
gross external and visceral abnormalities before staining with
Alizarin Red S for observation of skeletal abnormalities and variants.
The remainder were examined by free-hand dissection for soft-tissue
abnormalities. After marked weight loss, one animal at each dose was
killed during the study. One animal at the high dose aborted. Reduced
body-weight gain (76% of control weight) and reduced food consumption
(87% of control) were seen during treatment with 11 mg/kg bw per day.
During days 12-18 of gestation, reduced body-weight gain (87% of
controls) was seen at 5.6 mg/kg bw per day; them was no associated
effect on food consumption. Body-weight gain was similar in all groups
between days 18 and 29. Slight reductions in fetal weight were evident
at 5.6 mg/kg bw per day (by 3%) and 11 mg/kg bw per day (5%), but
these findings may have been secondary to the reduced body-weight
gains of the dams. There were no effects on litter size, fetal
viability, external abnormalities, skeletal abnormalities or variants,
extent of ossification, or soft-tissue abnormalities. The overall
NOAEL was 5.6 mg/kg bw per day on the basis of the markedly decreased
body-weight gain in dams at 11 mg/kg bw per day. There was no evidence
that guazatine is fetotoxic or teratogenic to rabbits (Barton &
Wilson, 1988).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
Guazatine GTA70 (purity unspecified) was severely irritating when
applied to rabbit skin for 4 h under occlusive conditions. The lesions
became more pronounced with time, and severe erythema and moderate
oedema were seen at the end of the study on day 7 (Cuthbert & Carr,
1989).
A 40% guazatine formulation induced ocular lesions that worsened with
time. A 10% solution of the formulation was only slightly irritating
to rabbit eyes (van Beek, 1974).
In a Magnusson and Kligmann maximization protocol, guazatine GTA70
(purity, 69.2%) did not have sensitizing potential in guinea-pigs at
induction concentrations of 0.5% intradermally or 1% topically and a
challenge concentration of 0.5% (Til & Keizer, 1980).
3. Observations in humans
Koyama et al. (1993) reported a case of attempted suicide with a
formulation containing 25% iminoctadine and 5%
polyoxyethylenealkylether (Befran). The patient was admitted with
severe cyanosis and in a stuporous state. Blood pressure and pulse
could not be measured (the radial arteries were not palpable) until
noradrenaline (1 mg) was administered.
[The authors cited a similar case and some experimental work involving
intravenous administration to dogs, which indicated that severe
hypotension was produced by iminoctadine. Subsequent work (Koyama et
al., 1994) on anaesthetized rats showed that iminoctadine administered
intravenously induced marked (> 10%) tachycardia and hypotension at
doses > 0.05 mg/kg bw in vivo. Experiments on isolated rat aorta
and atria in vitro showed that the primary effect of iminoctadine
was vasodilatory.]
Comments
Guazatine is a preparation of the triacetates of dimeric and trimeric
guanidated 1,8-diamino-octane, which also contains a range of
oligomers and reaction products. The Meeting was concerned that the
production controls and specifications for guazatine were inadequate.
The quoted purity of 70% is based on normalization to a component
which comprises approximately 1.5 % of the mixture and provides no
control over the levels of the other components. Some data were
provided to show that the composition of the batch used in the key
toxicity studies was similar to that of other batches produced at the
same time, 1990-1991; however, there were no data to confirm that the
batches used in the studies of toxicity were representative of those
currently produced.
Some components of guazatine were absorbed by rats to a limited extent
after oral administration of 14C-labelled compound and then excreted
rapidly. Within 24 h, faecal elimination represented 85-94% of the
dose, with 3-6% in the urine and < 1% in exhaled air. The highest
levels of radiolabel were found in the kidney and liver; there was
evidence that the salivary, pituitary, and thyroid glands may also
contain significant amounts of residue. A study involving treatment
with 14 doses of 2 mg/kg bw showed limited potential accumulation in
the liver and kidney. The results of a study by intravenous injection
showed that some components of guazatine may be secreted back into the
gastrointestinal tract via the stomach and salivary glands. The
metabolism of guazatine has not been fully characterized, but
monodeamidination and dideamidination play significant roles
in vivo.
Guazatine produces severe local irritation, and single oral doses are
of moderate toxicity, with an oral LD50 value in rats of 280 mg/kg
bw. WHO has classified guazatine as moderately hazardous (WHO, 1996).
In a number of short-term studies in rats, guazatine was administered
at doses of 0, 60, 200, 800/1200, or 1500/2000 ppm in the diet for 14
weeks. At doses of 60 and 200 ppm, the activity of serum alkaline
phosphatase was slightly decreased, but no significant changes were
seen in body-weight gain or in the results of pathological,
haematological, or urinary examinations. At doses of 800 ppm and
above, decreased body-weight gain, increased activities of alanine and
aspartate aminotranferases, and decreased activity of alkaline
phosphatase were found, together with pathological changes such as
local irritation of the gut and hyperplasia of the epithelia of the
excretory ducts of the parotid gland with mononuclear-cell
infiltration. Increased weights of the kidney, liver, and heart were
seen without associated histopathological changes. The overall NOAEL
was 200 ppm, equivalent to 10 mg/kg bw per day.
In a 13-week range-finding study, mice received guazatine at 0, 10,
50, 200, or 500 ppm. Significantly reduced body-weight gain was seen
in animals of each sex at 200 ppm and above. Increased liver weights
and alterations in centrilobular hepatocytes were seen in both males
and females at 500 ppm. Alterations in erythrocyte parameters were
seen in animals at doses of 200 ppm and above. Although no significant
effects were reported at 10 or 50 ppm, in view of limited histological
investigations in the study there was no NOAEL.
In a one-year study in dogs, guazatine was administered at 0, 25, 75,
or 250 ppm. Reduced body-weight gain in females, increased alanine
aminotransferase activity in animals of each sex, and increased
aspartate aminotransferase activity in males were observed at a
dietary concentration of 250 ppm. In females at 75 ppm, body-weight
gain was reduced. The NOAEL was 25 ppm, equal to 0.8 mg/kg bw per day.
Guazatine was not carcinogenic in two two-year studies in rats given
doses of 0, 20, 60, or 200 ppm or 0, 50, 150, or 350 ppm. The
non-neoplastic findings included reduced serum alanine and aspartate
aminotranferases activities, salivary gland hyperplasia, and
testicular atrophy at 350 ppm. In a two-year study of iminoctadine
administered at 0, 10, 100, or 300 ppm, there was no reported increase
in tumour incidence. The overall NOAEL was 150 ppm, equal to 7 mg/kg
bw per day.
In a study of carcinogenicity in mice, the animals received 0, 50,
120, or 300 ppm guazatine. The incidences of malignant tumours were
increased at 120 and 300 ppm: haemangiosarcoma of the liver and spleen
was seen in males at 120 and 300 ppm and hepatocellular carcinoma in
females at 300 ppm. The incidence of renal-tubular tumours (adenoma
and carcinoma) was increased in males receiving 300 ppm. These are
rare tumour types in the mouse strain that was used, normally being
seen in only 0-6% of animals. Although the absolute incidences of
these tumours in guazatine-treated animals were low and not
statistically significant, they were clearly greater than those in
historical controls. No convincing information was available on the
underlying mechanism of tumour production. The non-neoplastic effects
seen in this study were increased incidences of lymphoid foci in the
lung, bronchiole-associated lymphoid tissue, keratinized vaginal
epithelium, and brain mineralization in females receiving 300 ppm.
Body-weight gain was reduced by approximately 20% in animals of each
sex receiving 300 ppm. In addition, an abstract describing a study on
iminoctadine (at 0, 10, 100, or 300 ppm) reported a slight increase in
the incidence of renal epithelial tumours in male mice receiving 300
ppm. The Meeting considered that the production of rare malignant
tumours by an unknown mechanism is of great concern. The overall NOAEL
for long-term administration to mice was 50 ppm, equal to 6.8 mg/kg bw
per day, on the basis of increases in the incidence of
haemangiosarcoma in males at 120 ppm, equal to 17 mg/kg bw day.
Guazatine has been tested in an adequate battery of assays for
genotoxicity. The Meeting concluded that it is not genotoxic.
In a multigeneration study of reproductive toxicity in rats receiving
guazatine at 0, 60, or 200 ppm, no significant effects were seen at
the highest dose, equivalent to 12 mg/kg bw per day. In a
two-generation study of reproductive toxicity in rats, guazatine
administered at 0, 50, 150, or 350 ppm did not affect reproductive
performance at the highest dose, equal to 22 mg/kg bw per day.
In a study of developmental toxicity in rats, guazatine was
administered at 0, 5, 10, or 20 mg/kg bw per day. The NOAEL for
maternal toxicity, teratogenicity, and fetotoxicity was 20 mg/kg bw
per day, the highest dose tested. In a range-finding study,
significant mortality was seen at 40 mg/kg bw per day.
In a study of developmental toxicity in rabbits, guazatine was
administered at 0, 2.8, 5.6, or 11 mg/kg bw per day. There were no
signs of fetotoxicity or teratogenicity at the highest dose. The NOAEL
was 5.6 mg/kg bw per day on the basis of marked decreases in maternal
body-weight gain.
The Meeting considered that it could not establish an ADI for
guazatine owing to the inadequate information on its composition and
concerns about the production of rare malignant tumours in mice.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 150 ppm, equal to 7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
350 ppm, equal to 22 mg/kg bw per day (highest dose
tested in a two-generation study of reproductive
toxicity)
20 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Dog: 25 ppm, equal to 0.8 mg/kg bw per day (one-year study
of toxicity)
Studies that would provide information necessary for continued
evaluation of the compound
1. Data on the levels of individual components in batches of
guazatine from recent production runs
2. Investigation of the mechanism of tumour production in mice
3. Clarification of the extent of absorption, excretion, and
metabolism of all components of guazatine
4. Clarification as to whether the stated doses used in the studies
of toxicity were expressed as free base or triacetate
References
Atkinson, C., Perry, C.J., Hudson, P. & Robb, D.T (1990) Guazatine 13
week dietary dose range finding study in mice. Unpublished study from
Inveresk Research International (report No. 7552). Submitted to WHO by
Rhône Poulenc Secteur Agro, Lyon, France.
Appelman, L.M. (1980) Acute inhalation toxicity of guazatine
triacetate in rats. Unpublished study from CIVO-TNO (report No. R
6636). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon France
Barton, S.J. (1993) Guazatine: Two-generation reproduction study in
rats. Unpublished study from Inveresk Research International (report
No. 7854). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Barton, S.J. & Wilson, I.A. (1988) Guazatine: Teratugenicity study in
rabbits. Unpublished study from Inveresk Research International
(report No. 5344). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
van Beek, L. (1974) Eye irritation test with Panoctine 40 in albino
rabbits. Unpublished study from CIVO-TNO (report No. R4363). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L. (1980) Acute dermal toxicity study with Panoctine plus
300020 in albino rabbits. Unpublished study from CIVO-TNO (report No.
R6703). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L., van Oostrum, E.C.M. & Immel, H.R. (1976) Acute dermal
toxicity of Panoctine 42 in albino rabbits. Unpublished study from
CIVO-TNO (report No. R4921). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Cameron, B.D. & Phillips, M.W.A. (1986) The disposition of guazatine
in the lactating cow. Unpublished study from Inveresk Research
International (report No. 4141 ). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cameron, B.D., Mutch, P.J. & Scott, G. (1989) The metabolism of
[14C]-guazatine in the rat. Unpublished study from Inveresk Research
International (report No. 4826). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & Cart, S.M.A. (1989) Guazatine: Acute dermal
irritation test in rabbits. Unpublished study from Inveresk Research
International (report No. 5505). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & D'Arcy-Burt, K.J. (1986) Guazatine: Acute dermal
toxicity in rats (limit test) Unpublished study from Inveresk Research
International (report No. 3461). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Davis, P.B. (1983a) An investigation into the possible induction of
point mutations at the HGPRT locus of Chinese hamster ovary cells by
GTA70 (guazatine triacetate) Unpublished study from CIVO-TNO (report
No. R83/86). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Davis, P.B. (1983b) An investigation into the possible induction of
sister chromatid exchanges in Chinese hamster ovary cells by GTA 70
(guazatine triacetate). Unpublished study from CIVO-TNO (report No.
R83/85). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976a) Determination of the acute oral toxicity of
Panoctine 42 in cats. Unpublished study from CIVO-TNO. Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976b) Determination of the intraperitoneal toxicity
of Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
21-1-76). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Goburdhun, R. & Carter, P.B. (1989) Guazatine: Oral maximum tolerated
dose study in dogs. Unpublished study from Inveresk Research
International (report No. 5424). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Hazelden, K. (1987) Guazatine: Dose range finding study in rats,
preliminary to teratogenicity study. Unpublished study from Inveresk
Research International (report No. 3441). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Hazelden, K.E & Wilson, J.A. (1986) Guazatine: Teratogenicity study in
rats. Unpublished study from Inveresk Research International (report
No. 3540). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Heath, J., Perry, C.J., Hudson, R, Dobb, D. & Millar, P (1994)
Guazatine 104 week dietary study in rats with 52 week interim kill.
Unpublished study from Inveresk Research International (report No.
11006). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Heath, J., Perry, C.J., Hudson, E & Aitken, R. (1995) Guazatine 104
week dietary study in mice with 52 week interim kill. Unpublished
study from Inveresk Research International (report No. 11084).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Hirano, M., Maita, K., Mitsumori, K. & Shirasu, Y. (1985) 24 Month
chronic toxicity study with guazatine in rats. J. Toxicol. Sci., 10,
266.
Kato, Y., Sato, K., Maki, S., Matano, O. & Goto, K. (1985) Metabolic
fate including deamidination of guazatine triacetate in male rats.
J. Pestic. Sci., 10, 661-675.
Koyama, K., Yamashita, M., Miyauchi, T. & Goto, K. (1993) A fungicide
containing iminoctadine causes circulatory failure in acute oral
poisoning. Vet. Hum. Toxicol., 35, 512.
Koyama, K., Goto, K. & Yamashita, M. (1994) Circulatory failure caused
by a fungicide containing iminoctadine and a surfactant: A
pharmacological analysis in rats. Toxicol Appl. Pharmacol., 126,
197-201.
Kruysse, A. & Immel, H.R. (1976) Acute inhalation toxicity study with
Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
R4965). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Leegwater, D.C. (1975) Study on the fate of [14C, 3H guazatine
preparation in the rat and in sandy loam. Unpublished study from
CIVO-TNO (report No. R4823). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Leegwater, D.C. (1980) Study on the metabolic fate of [14C] Panoctine
preparation in the rat. Unpublished study from CIVO-TNO (report No.
R6571). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Maita, K., Mitsumori, K., Hirano, M. & Shirasu, Y. (1985) 24-Month
chronic toxicity study with guazatine in mice. J. Toxicol. Sci., 10,
267.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Oshodi, R.O. & Thompson, D.C. (1993) Guazatine: 52 Week dietary study
in dogs. Unpublished study from Inveresk Research International
(report No. 7903). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Prout, M.S. (1996) The metabolism of [14C]-guazatine in the rat.
Unpublished study from Inveresk Research International (Supplement 1
to report No. 4826: Cameron et al., 1989). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Reuzel, P.G.J, Til, H.P. & Kollen, C.H. (1976) Long term (two year)
toxicity study with guazatine in beagle dogs. Unpublished study from
CIVO-TNO (report No. R4983). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Sato, K., Kato, Y., Maki, S., Matano, O. & Goto, S. (1986) Metabolic
fate of plant guazatine residues in male rats. J. Pestic. Sci., 11,
267-270.
Sinkeldam, E. H. & van der Heijden, C.A. (1974) Sub-chronic (90-day)
toxicity study with guazatine in albino rats (final report).
Unpublished study from CIVO-TNO (report No. R4354). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Spanjers, M.T & Til, H.P. (1980) Determination of the acute oral
toxicity of acetates of guanidated amines (GTA) in rats. Unpublished
study from CIVO-TNO. Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, H.P. & Feron, V.J. (1975) Feeding study with guazatine in rats
for 13 weeks. Unpublished study from CIVO-TNO (report No. R4870).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P & Hendriksen, C.F.M. (1976) Toxicity study with Panoctine 42
(guazatine) in rats for 13 weeks. Unpublished study from CIVO-TNO
(report No. R5062). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, M.P. & Keizer, A.M.M. (1980) Maximisation test with GTA in guinea
pigs. Unpublished study from CIVO-TNO (report No. R6522). Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Hendriksen, C.F.M. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with guazatine in rats.
Unpublished study from CIVO-TNO (report No. R4985). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Koeter, H.B.W.M., Immel, H.R. & van der Heijden, C.A.
(1976b) Multigeneration study with guazatine in rats. Unpublished
study from CIVO-TNO (report No. R5078). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Willems, M.I. (1983) Examination of GTA in the micronucleus test.
Unpublished study from CIVO-TNO (report No. V83.118/230201). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Wilmer, J.W.G.M. (1983a) Examination of GTA for mutagenic activity in
the Ames test. Unpublished study from CIVO-TNO (report No. V83.
122/230064). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Wilmer, J.W.G.M. (1983b) Chromosome analysis of cultured human
lymphocytes treated in vitro with GTA. Unpublished study from CIVO-TNO
(report No. V83. 235.230573). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
2. Toxicological studies
(a) Acute toxicity
Guazatine is of moderate toxicity when given orally, of low toxicity
when applied dermally, but of moderately high toxicity when
administered by inhalation or intraperitoneally. The results of
studies of the acute toxicity of guazatine are summarized in Table 4.
The clinical signs of toxicity after treatment were lethargy or
sedation, hypothermia, coma, and local irritation. The gross and
histopathological changes were consistent with a response to a local
irritant; them was no clear systemic toxicity.
(b) Short-term toxicity
Mice
In a range-finding study, groups of 10 male and 10 female CD-1 mice
received guazatine (purity, 70.6%) in the diet at 0, 10, 50, 200, or
500 ppm for 13 weeks. Samples were taken for limited blood and
clinical chemical analyses at week 13. A limited range of tissues was
removed, weighed, and examined macroscopically; only the liver was
examined histologically. Two male controls, one male at 500 ppm, and
one at 50 ppm died during the study. Marked reductions in body-weight
gain (> 25%) were seen in animals of each sex at 200 or 500 ppm. In
females at the highest dose, aspartate aminotransferase activity was
increased. The relative liver weights were increased in animals of
each sex at 500 ppm; reductions in the absolute weights of several
organs appeared to be secondary to reduced body-weight gain. Altered
centrilobular hepatocytes were seen in 80% of animals at 200 or 500
ppm. Given the limited extent of the investigations, no NOAEL was
identified (Atkinson et al., 1990).
Table 4. Acute toxicity of guazatine and formulations
Test material Species Route Purity LD50/LC50 Reference
(%) (mg/kg bw or
mg/m3)
Guazatine GTA Rat Oral 69.2 280 Spanjers & Til (1980)
Panoctine 42 Cat Oral NR 0.38 ml/kg bw De Grout (1976a)
Guazatine GTA70 Rat Dermal 70.9 1050 Cuthbert & D'Arcy-Burt (1986)
Panoctine 42 Rabbit Dermal NR 2.8 ml/kg bw van Beck et al. (1976)
Panoctine Plus 300020 Rabbit Dermal NR 2.8-5.6 ml/kg bw van Beck (1980)
Paaoctine 42 Rat Intraperitoneal NR 0.053 ml/kg bw De Groot (1976b)
Guazatine GTA Rat Inhalation 69.2 225 Appelman (1980)
Panoctine 42 Rat Inhalation NR 11 Kruysse & Immel (1976)
Rats
Groups of 10 male and 10 female Wistar-derived rats received guazatine
(54.8% w/w) in the diet at 0, 60, or 200 ppm for 14 weeks.
Haematological and urinary parameters were measured in samples taken
at week 13, and serum enzymes and total protein were measured in
samples taken at termination at week 14. There were no deaths or
adverse clinical signs. Body weight and food conversion efficiency
were unaltered by treatment. A slight, dose-related decrease in
leukocyte count was seen in animals of each sex (up to 13% in males
and 6% in females), but this was not statistically significant, and
there was no marked change in differential counts. Serum alkaline
phosphatase activity was decreased in treated males, by 22% at 200 ppm
and 7% at 60 ppm. Urinary parameters were unaffected by treatment, but
the volume and specific gravity were not given. There were no
significant findings at gross or microscopic examination. The findings
in this study are of minimal toxicological significance; the NOAEL was
200 ppm, equivalent to 10 mg/kg bw per day (Sinkeldam & van der
Heijden, 1974).
Groups of 10 Wistar-derived rats of each sex received guazatine (54.8%
w/w) in the diet at 800 ppm for six weeks and then 1200 ppm for eight
weeks or control diet for 14 weeks. Haematological and urinary
parameters were measured in samples taken at week 13, and serum
enzymes and total protein were measured in samples taken at
termination at week 14. There were no deaths or adverse clinical
signs. Body-weight gain was reduced in animals of each sex by
approximately 6% at week 6 and by 8-10% at termination. Food
efficiency was unaffected in males but reduced in females.
Haematological parameters were unaltered by treatment. In males, serum
aspartate aminotransferase activity was increased and alkaline
phosphatase activity decreased. Females had an increased urine volume
with an associated decrease in specific gravity; the report does not
indicate whether samples were collected under fasting conditions.
Increases in relative adrenal, testis, and heart weights were seen in
treated animals, without abnormal pathological findings. As data on
individual animals and absolute organ weights were not presented, the
significance of these findings is uncertain. In treated females, high
levels of 'iron' deposition in the spleen were reported. The thyroids
of two females given guazatine were increased in weight and contained
small follicles lined with large epithelial cells that had small
apical nuclei. A re-evaluation of salivary gland tissues from this
study, reported very briefly by Til & Hendricksen (1976), described
hyperplasia in the epithelial lining of the excretory ducts of the
parotid salivary glands, with associated mononuclear cell infiltrates
in some treated animals. No NOAEL was identified (Til & Feron, 1975).
Panoctine 42 (purity unspecified) was administered to groups of 10
Wistar-derived rats of each sex at 0 or 1500/2000 ppm in the diet; the
level was increased from 1500 to 2000 ppm after week 4. Samples for
examination by haematology, limited clinical chemistry, and urinalysis
were taken during week 13. Animals were killed at week 14 and examined
grossly and histologically. There were no deaths or adverse clinical
signs. Body-weight gain was reduced by about 10% from week 2 in males
and by about 8% from week 6 in females. Food efficiency was reduced in
males for the first 12 weeks of the study. Serum proteins and alkaline
phosphatase activity were reduced in both treated groups. In treated
females, the leukocyte counts were increased (by 30%), and
differential counts indicated an increase in neutrophils and a
decrease in eosinophils. Increased relative weights of the liver (by
8%) and kidney (by 14%) were seen in treated males, without associated
histological findings. In females, an increased relative weight of the
heart (by 17%) and decreased thyroid weights (by 8%) were reported,
again without associated histological findings. Urinary aspartate
aminotransferase activity, proposed as a measure of renal toxicity,
was increased in treated animals, and urine volume was increased and
specific gravity decreased in animals of each sex. These findings
taken together are indicative of renal toxicity, though no associated
findings were reported at pathological examination. The only
histopathological finding of note was hyperplasia of the epithelial
lining of the excretory ducts of the parotid salivary glands with
associated mononuclear cell infiltrates in six males and six females
in the treated groups and in none of the controls (rho < 0.01). No
NOAEL was identified (Til & Hendricksen, 1976).
Dogs
In a range-finding study in beagle dogs, guazatine (purity, 67.9%)
given at 9.4 mg/kg bw per day by gavage for four days induced
body-weight loss in one of four animals. Subsequent administration of
14 mg/kg bw per day for four days induced marked body-weight loss and
reduced food consumption in all animals. The pathological findings
indicated local irritation of the gastrointestinal tract.
Administration of 374 ppm guazatine in the diet (12-15 mg/kg bw per
day) resulted in reduced food consumption, reduced body-weight gain,
and increased activities of aspartate aminotransferase, alanine
aminotransferase, and lactate dehydrogenase in serum after two weeks
(Goburdhun & Carter, 1989).
Groups of four male and four female beagle dogs received guazatine
(purity, 54.8%) in the diet at levels of 0, 60, 200, or 300/600 ppm
for two years; the level of 300 ppm was increased to 600 ppm after 26
weeks. Extensive observations were made, including haematological,
clinical chemical, and urinary determinations at 12/13, 26, 52, 78,
and 104 weeks, and tests for liver function (bromosulfthalein) and
kidney function (phenol red excretion) at 26, 52, and 104 weeks. Gross
and histopathological examinations were performed at termination at
week 104, although the results of histopathology in individual animals
were not reported. One control male died due to urolithiasis. There
was no evidence of treatment-related changes in clinical signs.
Body-weight gain and food consumption were unaffected by exposure. A
decreased leukocyte count (by 12-25 %) was present in animals at the
high dose, particularly females, from week 13 onwards, achieving
statistical significance at week 52, although there were no marked
alterations in differential counts. Sporadic changes in other
haematological, clinical chemical, and urinary parameters were not
consistent or of statistical significance. The results of liver and
kidney function tests were similar for control and treated groups.
Increases in absolute and relative ovarian weights were seen in
females at the high dose, although the significance of these changes
is unclear as there was considerable inter-animal variation and no
obviously associated histological findings. Given the uncertainties
about the findings at the high dose, the NOAEL was 200 ppm, equivalent
to 5 mg/kg bw per day (Reuzel et al., 1976).
Groups of four beagle dogs of each sex received guazatine (purity,
70.6%) in the diet for 52 weeks. Animals were given 400 g of diet per
day containing 0, 25, 75, or 250 ppm guazatine; the achieved intakes
were about 0, 0.8, 2.5, or 8 mg/kg bw per day. Extensive observations
included ophthalmoscopy at 0, 13, 26, and 51 weeks and haematology,
clinical chemistry, and urinalysis at 0, 6, 13, 26, and 51 weeks.
Animals were killed at week 52 and examined grossly; 13 tissues were
weighed, and more than 40 tissues were investigated by histopathology.
Owing to poor weight gain, one control and two males at the high dose
received extra food, which reversed the condition. In females, there
was a clearly dose-related reduction in body-weight gain starting at
week 10 and continuing throughout the study, with reductions of 30% in
comparison with controls in the group at the high dose, 20% in those
at the intermediate dose, and 12% in those at the low dose. Food
consumption was reduced in females receiving 75 or 250 ppm, with
occasional reductions in those at 25 ppm. Haematology showed no
consistent time- or dose-related reactions to treatment. Marked
increases (up to sixfold) in serum alanine aminotransferase activity
were seen throughout the study in males and females receiving 250 ppm.
Serum aspartate aminotransferase activity was increased in males at
the high dose throughout the study; an apparent increase in alkaline
phosphatase activity in males appears to be related to differences in
pretreatment values. A slight increase in liver weight was noted in
females at the high dose. Reduced prostate weights in males at the low
dose, increased ovarian weights in all treated females, and increased
thyroid weights in females at the intermediate dose did not show
dose-response relationships and were not clearly related to treatment,
although effects on ovarian weights were also see in the study of
Reuzel et al. (1976). Gross and microscopic examination showed no
changes. The reduced body-weight gain of females appeared to be due
only in part to unpalatability, as food conversion efficiency was also
reduced. The NOAEL was 25 ppm, equivalent to 0.8 mg/kg bw per day, on
the basis of marked reductions in body-weight gain in females at 75
and 250 ppm and clear increases in serum enzyme activities, indicative
of effects on the liver, in animals of each sex at 250 ppm (Oshodi &
Thompson, 1993).
(c) Long term toxicity and carcinogenicity
Mice
Groups of 10 male and 10 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for one year; the
achieved doses were 0, 7.6, 19, or 55 mg/kg bw per day in males and 0,
10, 25, or 67 mg/kg bw per day in females. All animals were examined
macroscopically. A wide range of tissues from animals receiving 0 or
300 ppm and those that died before week 52 were examined
histologically; the gall-bladder, kidney, liver, lung, salivary gland,
and grossly abnormal organs from all other animals were also examined
histologically. One male and one female at 120 ppm and two females at
300 ppm died during the study. There were no clinical signs associated
with treatment. Body-weight gain was reduced from week 4 in animals of
each sex at 300 ppm; by week 52, the weight gain of males was 72% that
of controls and that of females 66% of control values. Food
consumption was similar in treated and control animals. The relative
liver weights were increased by about 10% in all treated males. The
only notable finding at gross necropsy was an increased incidence of
ovarian cysts in females receiving 120 or 300 ppm (9/10 versus 6/10 in
controls). Microscopic examination showed increased incidences of
lymphocytic infiltration of the kidney and ovarian cysts in females at
the high dose (the ovaries of those at the intermediate dose were not
examined), of hepatocellular adenoma in males at the high dose (2/10
versus 0/10 in controls), and a decreased incidence of lymphoid foci
of the mandibular salivary gland in females at the intermediate and
high doses. The finding of hepatocellular adenomas may indicate that
guazatine reduces the time to onset of the liver turnouts seen
commonly in mice. Given the small group size, limited examination of
tissues from animals at low and intermediate doses, and clear effects
at 300 ppm, there was no NOAEL (Heath et al., 1995).
In the part of the same study designed to test for carcinogenicity,
groups of 50 male and 50 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for two years; the
achieved doses were 0, 6.8, 17,or 47 mg/kg bw per day in males and 0,
8.7, 21, or 57 mg/kg bw per day in females. All animals were examined
macroscopically, and a wide range of tissues from animals receiving 0
or 300 ppm or that died before week 104 were examined histologically;
only the gall-bladder, kidney, liver, lung, salivary glands, and
grossly abnormal organs from animals in other groups were examined.
Blood samples for differential leukocyte counts were taken from all
surviving controls and animals at 300 ppm during weeks 52, 78, and
103.
The pattern of deaths was similar in treated and control groups, with
> 50% survival in all groups until week 100 in males and week gg in
females. Reduced body-weight gain was seen at the high dose from week
2 onwards, the deficit being approximately 20% at termination. During
the second year, males at the intermediate dose lost more weight than
controls, while females at this dose gained more weight than controls.
Food consumption, differential leukocyte counts, and findings at gross
necropsy were unaffected by treatment. Increased absolute and relative
weights of the kidney and liver were seen in females at the
intermediate but not the high dose.
The incidences of a range of neoplastic and non-neoplastic lesions
showed evidence of treatment-related effects (Table 5). The incidences
of haemangiosarcoma of the liver in males at the intermediate and high
doses and of the spleen in males at the high dose were higher than
those in historical controls, which were 04% in males and 0-3% in
females for liver and 0-2% in males and 045% in females for spleen.
The high incidence of haemangiosarcoma in females at the low dose is
considered to be a chance finding, as them was no dose-response
relationship. The incidence of hepatocellular carcinoma in females at
the high dose was greater than that in historical controls (0-4%). The
incidences of renal adenoma and carcinoma in male historical controls
were 0-4% and 0-2%, respectively, indicating that the incidence of
renal adenoma in males at the high dose is a significant finding.
The presence of rare tumours, including malignant ones, at multiple
sites was considered by the Meeting to be of concern. The mechanism by
which these tumours are induced is not evident from the available
data. Guazatine is not genotoxic (see below), indicating the mechanism
is probably nongenotoxic, although haemangiosarcomas are generally
associated with a genotoxic mechanism. As them was no evidence of
marked toxic or hyperplastic responses in the affected tissues and
them was evidence of a dose-response relationship, it was not clear
that these tumours are a direct result of disrupted cell function
associated with exposure to doses above the maximum tolerated dose.
Although only a limited range of tissues was examined from animals at
the low and intermediate doses, it included those with the most
significant effects at the high dose. The NOAEL was 50 ppm, equal to
6.8 mg/kg bw per day, on the basis of effects on body-weight gain and
the occurrence of haemangiosarcomas in males at 120 ppm, marked
effects on body-weight gain, and a range of neoplastic and
non-neoplastic changes at 300 ppm (Heath et al., 1995).
The findings of another two-year study with guazatine in mice were
summarized in abstract form only. Guazatine (as iminoctadine) was
given to groups of 80 male and 80 female specific-pathogen-free
ICR-Crj mice at dietary levels of 0,10, 100, or 300 ppm. Eight animals
of each sex at each dose were killed at weeks 26 and 52 for
examination. At 300 ppm (26 mg/kg bw per day in males, 30 mg/kg bw per
day in females), males and females showed a remarkable depression in
body-weight gain, decreased food efficiency, and anaemia at weeks 26
and/or 52, increased plasma urea nitrogen level (only in females),
increased alkaline phosphatase level (at week 26 in animals of each
sex and also at week 52 in females), and increased weights of the
kidney (absolute and relative) and liver (relative). Histological
examination revealed swelling of hepatocytes and proximal tubular
cells in animals of each sex. There were increased incidences of
subcutaneous oedema in females and hydrothorax in males. The
incidences of splenic atrophy in animals of each sex, atrophy and/or
brown pigment deposition of the ovary, and glandular epithelial
atrophy of the Harderian gland in males were significantly increased.
The incidence of renal epithelial tumours increased slightly in males.
No toxic changes were seen at the other doses, except for slightly
Table 5. Lesions (as % of samples examined) seen in CD1 mice exposed to guazatine in the diet
for two years
Lesion Guazatine (ppm)
Males Females
0 50 120 300 0 50 120 300
Brain mineralization 22 26 33 20 8 16 10 22*
Caecal carcinoma 0 0 0 0 0 0 0 2
Renal tubular carcinoma 0 0 0 2 0 0 0 0
Renal tubular adenoma 2 2 2 8 0 0 2 0
Renal glomerular amyloid 12 8 16 10 24 8 8 0*
Hepatocellular carcinoma 22 24 22 22 2 2 2 10
Liver haemangiosarcoma
Multifocal 0 0 2 4 0 0 2 0
Other 2 2 6 6 0 6 2 0
Increase in bronchus-associated
lymphoid tissue 10 12 12 20 4 14 16 28*
Lung lymphoid foci 0 0 2 0 0 0 4 10*
Spleen haemangiosarcoraa 0 0 2 4 2 0 2 4
Vaginal keratinized epithelium - - - - 15 - - 43
-, not determined
* One-tailed p < 0.05, Fisher exact test
increased incidences of swelling of proximal tubular cells in animals
of each sex at 100 ppm (Maita et al., 1985).
Rats
Groups of 60 male and 60 female Wistar-derived rats, in two parallel
series initiated two months apart, received diets containing guazatine
(54% solution) at 0, 20, 60, or 200 ppm for two years. A group
receiving 6 ppm was terminated after a few months, as the results of
other studies indicated that 20 ppm was probably a NOAEL. Blood and
urine samples were taken from 10 fasted animals of each sex at each
dose at various times during the study for haematological, limited
clinical chemical, and urinary analyses. All animals were examined
grossly post mortem. A wide range of organs from surviving animals
in the first series (11-20) were weighed, but only kidney, spleen, and
adrenals from rats in the second series were weighed. A full
histological examination was performed on tissues from 20 rats of each
sex in the control and high-dose groups in the first series, and gross
lesions, tumours, adrenals, thyroid, and pituitaries from all animals
were examined.
Behaviour and clinical signs were reported to be unaffected by
treatment. There were more early deaths (before week 72) among treated
males, but overall survival to week 104 was satisfactory and similar
in all groups. Body weights were similar in all groups until week 88,
when all treated females showed a deficit in comparison with controls,
which lasted until termination. Food consumption and conversion
efficiency were similar in all groups over the first four weeks.
Leukocyte counts were decreased (by about 11%) in males at the high
dose from week 26, with no consistent change in differential counts;
females showed sporadic changes in leukocyte counts with no consistent
pattern. Urinalysis gave similar findings in all groups. Reduced
relative testicular weights (by 11%) were seen in males at the high
dose, although the absolute weights were similar since the mean body
weight was 8% higher. In treated females, the relative weights of the
spleen, brain, and kidney were increased; however, as the absolute
weights were decreased and there was no evidence of a dose-response
relationship, this finding is probably secondary to the effects on
body weight. Occasional increases in the incidences of non-neoplastic
lesions were reported, but the only statistically significant
(p < 0.05) increases were for chronic respiratory disease and mammary
gland inflammation or dilatation in females at the high dose. The
number of tumour-bearing animals and the total numbers of tumours were
similar in all groups. The incidence of monocytic leukaemia was
increased in males and females at the high dose, occurring in four
males and three females, with none in male and in one female controls.
As the total numbers of animals examined is unclear, this may be a
chance finding. Although the study indicates that guazatine has no
marked toxicity, the limited, investigations performed and the unusual
design make interpretation of the findings difficult. No NOAEL was
identified (Til et al., 1976a).
Groups of 20 male and 20 female Sprague Dawley rats received guazatine
(purity, 70.6%) in the diet at 0, 50, 150, or 350 ppm for one year.
The achieved intakes of guazatine were 0, 2.5, 7, or 19 mg/kg bw per
day for males and 0, 3, 9, or 22 mg/kg bw per day for females. Samples
for haematological, clinical chemical, and urinary analyses were
obtained from 10 animals of each sex in each group at weeks 26 and 50
or 51. At week 52, the animals were killed and examined grossly.
Selected tissues were weighed, and samples from a wide range of organs
from controls and animals at the high dose were examined
histologically; gross lesions, kidneys, liver, lung, and salivary
glands from animals at the low and intermediate doses were also
examined histologically.
Behaviour, clinical signs, body-weight gain, food consumption, urinary
parameters, and the findings at gross necropsy were similar in all
groups. None of the controls but one male at the low dose, two each at
the intermediate and high doses, and one female at 150 ppm died. Total
leukocyte and lymphocyte counts were reduced in males at 350 ppm at
both 26 and 51 weeks, but increases were seen in treated females.
Serum alanine aminotransferase activity was decreased markedly (by
>40%) at both sampling times in males and females receiving 350
ppm, but there were occasional deficits in other serum enzyme
activities (particularly aspartate aminotransferase); total protein
was similar in all groups. Dose-related decreases in absolute and
relative prostate weights were significant (rho < 0.05) at both 150
and 350 ppm and were associated with a low incidence of hyperplasia at
350 ppm. Females had dose-related increases in the relative and
absolute weights of the adrenals (by < 10%) and ovary by
(< 50%). Five males at the high dose but none of the controls had
testicular atrophy. Mononuclear-cell infiltration of the parotid
salivary gland was increased in males (6/20 versus 1/20) and females
(5/20 versus 1/20) receiving 350 ppm. The NOAEL was 150 ppm, equal to
7 mg/kg bw per day, on the basis of decreased leukocyte count,
prostatic hyperplasia, decreased alanine aminotransferase activity,
and effects on the salivary gland at 350 ppm. The minimal changes seen
at 150 ppm were not consistent or of sufficient magnitude to be
considered adverse (Heath et al., 1994).
In the phase of the study that addressed carcinogenicity, groups of 50
Sprague Dawley rots of each sex received guazatine (purity, 70.6%) in
the diet at 0, 50, 150, or 350 ppm for two years. The achieved intakes
were about 0, 2.5, 7, and 19 mg/kg bw per day for males and 0, 3, 9,
and 22 mg/kg bw per day for females. Samples for haematological,
clinical chemical, and urinary analyses were obtained from 10 rats of
each sex per group at weeks 53 (for haematology only), 78, and 101 or
103. At week 104, the animals were killed and underwent a full gross
examination. Selected tissues were weighed, and samples from a wide
range of organs from controls and animals at the high dose were
examined histologically; gross lesions, kidneys, liver, lung, and
salivary glands from animals at the low and intermediate doses were
also examined histologically.
Behaviour, clinical signs, and organ weights were similar in all
groups. Survival was acceptable (50% at week 94) and similar in all
groups. Low body weights were seen consistently in females at the high
dose from week 9 and in males at this dose from week 20; in the latter
quarter of the study, females at the low and intermediate doses had
reduced body-weight gain in comparison with controls. Males at the
high dose showed consistent reductions in leukocyte and platelet
counts but increased erythrocyte counts. In females at 350 ppm,
erythrocyte counts were reduced; an increase in leukocyte counts at
termination was due to a neurilemmoma in one animal. Reduced
activities of serum alanine and aspartate aminotransferases (by
< 50%) were seen consistently in animals of each sex receiving 350
ppm. Reduced urinary pH was seen at the high dose in animals of each
sex throughout the study. A slight excess of abnormal findings in
lymph nodes was seen in females at this dose, but no individual effect
was significant when compared with the very low background incidence.
The incidences and severity of a range of lesions in salivary glands,
lymph nodes, ovaries, spleen, and pituitary were increased in females
receiving 350 ppm. In males, the only notable finding was increased
severity and incidence of testicular germinal epithelial degeneration
at 350 ppm. A rare malignant oligodendroglioma was found in the brain
of a single female at the high dose, but there was no evidence of
treatment-related carcinogenicity in any other animal. The NOAEL was
150 ppm, equal to 7 mg/kg bw per day, on the basis of reduced
body-weight gain and a range of clinical chemical and histological
findings at 350 ppm (Heath et al., 1994).
The findings of a two-year study of guazatine in rats were summarized
in abstract form only. Guazatine (as iminoctadine) was presented to
groups of 80 male and 80 female Fischer 344 rats at dietary levels of
0, 10, 100, or 300 ppm for two years. At 6 and 12 months, eight
animals of each sex from each group were killed for examination.
Animals of each sex at 300 ppm (11 mg/kg bw per day for males, 14
mg/kg bw per day for females) had remarkably depressed body-weight
gain, decreased food efficiency, higher mortality rates, a tendency to
anaemia, decreased total protein, and increased kidney and adrenal
weights; decreased potassium and albumin and increased calcium,
aspartate aminotransferase and gamma-glutamyltransferase activities,
and spleen weight were also observed. Histologically, swelling,
degeneration, and necrosis of renal tubular cells and metaplasia in
the glandular stomach were observed in animals of each sex. The
incidence of sperm granuloma in the deferent duct and/or epididymides
was significantly increased, and the incidences of leukaemia in males
and of adrenal phaeochromocytoma in animals of each sex were slightly
increased. At 100 ppm, males had a slightly higher mortality rate, and
females had depressed body-weight gain; decreased potassium and
increased calcium and gamma-glutamyltransferase activity were
occasionally observed in animals of either sex. The kidney and spleen
weights were increased, and the incidences of intestinal metaplasia
and sperm granuloma were significantly higher than in controls. At 10
ppm, no toxic change attributable to guazatine were seen (Hirano et
al., 1985).
(d) Genotoxicity
Guazatine has been tested for its potential to induce gene mutations,
sister chromatid exchange, and chromosomal aberrations in vitro and
micronuclei in vivo. Negative results were obtained in all studies.
The data are summarized in Table 6.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female Wistar-derived rats received diets
containing 0, 60, or 200 ppm guazatine (purity, 54.8%) over four
generations. The reporting of the study lacks the results for
individual animals, dietary analyses, time to mating, length of
gestation, absolute organ weights, and other data. F0 animals
received the test diets for 12 weeks before the first mating to
produce the F1a generation; the second mating eight weeks later
produced the F1b generation. Litters were culled to eight pups on day
1 after birth, and the F1a litters were discarded at weaning. Animals
were selected from the F1b litters at weaning to produce the F2
generation, with mating identical to that for F0 animals, and
similarly for the F3 generation from the F2b litters. Groups of 10
animals of each sex from the F3b litters were selected at weaning and
given test diets for four weeks; they were then killed and subjected
to gross and histopathological examinations.
Treatment had no effect on survival, clinical signs, litter size, sex
ratio, or resorption rates. Minor variations in the body weights of
pups appeared to be secondary to variations in litter size. F3b
animals receiving 60 or 200 ppm had increased, relative kidney
weights (by 8% at 60 ppm and 12-17% at 200 ppm), and increased
relative thymus weights (by 19-34%) were seen in all treated groups. A
dose-related reduction in relative testicular weights (8%) was also
seen. Histological examination gave no evidence of treatment-related
effects in any organ. In a summarily described extension of the study,
it was reported that males in F4b litters given test diets for four
weeks after weaning had reduced relative weights of the testis (9%)
and increased relative weights of the thymus (25%) at both doses, and
females at the high dose had increased relative kidney weights (12%).
Again, no treatment-related histological findings were reported. This
study shows that guazatine does not adversely affect reproductive
outcome in rats. The finding of increased thymus weights may indicate
that it affects the control of thymic growth or involution; such
effects may not be detectable in older animals when thymic involution
is well advanced. Given potential concern about effects on the thymus
at both 60 and 200 ppm, no NOAEL was identified (Til et al., 1976b).
Table 6. Results of genotoxicity assays on guazatine
End-point Test system Concentration/ Purity Results Reference
dose (%)
In vitro
Reverse mutation S. typhimurium 0.6-50 µg/plate 73 Negativea Wilmer (1983a)
TA98, TA 100. Cytotoxic
TA1535. TA1537. at 50 g/plate
TA1538
Reverse mutation S. typhimurium To cytotoxic levels NR Negative Moriya et al.
TA98, TA100, TA1535, (numbers not given) (1983)
TA1537, TA1538, and
E. coli WP2 hcr
Gene mutation Chinese hamster 25-100 nl/ml -S9 NR, Negativea Davis (1983a)
ovary cells (line K1), 50-200 nl/ml +S9 approx. Cytotoxic at
hprt locus 70% highest
concentrations
Sister chromatid Chinese hamster 5-30 nl/ml NR, Negativea Davis (1983b)
exchange ovary cells (line K1) approx. Cytotoxic
70% at > 35 nl/ml
Chromosomal Human 3.7-100 g/ml -S9 73 Negativea Wilmer (1983b)
aberration lymphocytes 14.8-400 g/ml +S9 Cytotoxic
at highest
concentrations
In vivo
Micronucleus Mouse (5/sex at 150 mg/kg bw by 73 Negative Willems (1983)
formation each time) bone gavage in saline No effect on
marrow (approx. 50% of P:N ratio
LD50); 24, 48, and
72h
Table 6 (continued)
NR, not reported; S9, exogenous metabolic activation system from Aroclor 1254-induced rat liver preparations;
P:N, polychromatic:normochromatic erythrocytes
a With and without metabolic activation
The reproductive effects of guazatine (purity, 70.6%) were
investigated in a two-generation (one litter per generation) study in
which groups of 28 young adult CD rats received diets containing
guazatine at 0, 50, 150, or 350 ppm, equal to a minimal intake of 0,
3, 10, or 22 mg/kg bw per day in males and 0, 4, 11, or 25 mg/kg bw
per day in females. Animals were given the treated diet for 10 weeks
before F0 mating (1:1) and continuously until sacrifice. Groups of 24
male and 24 female F1 animals were mated at about 17 weeks; they were
then killed and examined grossly, and reproductive organs and
pituitary, liver, adrenals, and salivary glands were weighed and
prepared for histological examination. Pups that died before weaning
were examined grossly. On day 21, one pup of each sex per litter was
examined grossly, and the liver and salivary glands were removed for
histological examination. All other pups were examined externally.
Treatment had no effect on clinical signs, survival, time to mating,
fertility indices, gestation length, litter size, gestation indices,
or lactation indices. Slight reductions in body-weight gain (5-10%)
were closely related to reductions in food consumption (4-9%). Slight
reductions in survival to day 21 (81-83% versus 87% in controls) and
litter weight at day 21 (about 8%) were seen in F1 pups in all
treated groups; as there was no dose-response relationship and the
effects were of small magnitude and were not seen in the F2
generation, they were considered not to be of biological significance.
Post-mortem examination showed no treatment-related effects; however,
the thymus was not weighed or investigated specifically. The NOAEL was
350 ppm, equal to 22 mg/kg bw per day, the highest dose tested
(Barton, 1993).
(ii) Developmental toxicity
Rats
The developmental effects of guazatine were investigated in animals
selected from the F2b and F3b litters of the study of Til et al
(1976b), described above Groups of five males and 15 females were
selected at weaning and maintained on test diets (0, 60, or 200 ppm
guazatine) until mating (1:3) at week 12; it was not clear whether the
test diets were given during gestation. On day 21 of gestation, the
dams were killed, their reproductive tracts investigated, and pups
examined for gross abnormalities and skeletal defects (with Alizarin
Red S staining); effects on soft tissues were studied only in controls
and rats at 200 ppm (by Wilson sectioning). F3b animals showed a
dose-related reduction in litter size, with 10.7 pups in controls, 10
at 60 ppm, and 8.9 at 200 ppm; at 200 ppm, this reduction was
associated with increased pre- and postimplantational losses in two
dams. Mean fetal weight, and hence litter weight, was reduced at 200
ppm in the F3b-derived group There were no notable visceral effects;
although there was an indication of reduced ossification in treated
animals, this was not consistent with regard to dose, generation, or
site. Reduced litter size was seen mainly in two animals and is not
consistent with the results of the main study. The NOAEL was 200 ppm,
equal to 12 mg/kg bw per day, as guazatine did not directly affect the
developing fetus or the dam (Til et al., 1976b).
In a range-finding study, pregnant Sprague Dawley rats died after
receiving guazatine (purity, 70.9%) at levels of 40, 80, or 120 mg/kg
bw per day by gavage on days 6-16 of gestation. At 20 mg/kg bw per
day, there was no evidence of toxicity. Necropsy of animals at doses
> 40 mg/kg bw per day showed marked irritation of the
gastrointestinal tract (Hazelden, 1987).
Four groups of 27 timed-mated Sprague Dawley rats received guazatine
(purity, 70.9%) in distilled water by garage on days 6-16 of presumed
gestation at doses of 0, 5, 10, or 20 mg/kg bw per day, on the basis
of the findings in the range-finding study. An adequate range of
examinations was performed, and animals were necropsied on day 20
after nitrogen asphyxiation. About two-thirds of the fetuses from each
litter were examined for gross external and visceral abnormalities
before staining with Alizarin Red S for observation of skeletal
abnormalities and variants. The remainder were examined by free-hand
dissection (Wilson technique) for soft-tissue abnormalities. One
control and two animals at the high dose died during the study due to
dosing accidents. There were no signs of maternal toxicity;
body-weight gain was similar in all groups, although food consumption
was decreased marginally (by 1-2%) in rats at 10 and 20 mg/kg bw.
There were no effects on litter size or fetal weight, or on soft
tissues or viscera. There was an indication of slightly retarded
development of some bones (e.g. scapula and pectoral girdle) at 10 and
20 mg/kg bw, but the findings were not significant, and the overall
degree of skeletal ossification was unaffected by treatment. The lack
of maternal toxicity at the high dose may be considered to have
compromised the study, but use of 20 mg/kg bw per day is considered
acceptable in view of the 40% mortality rate at 40 mg/kg bw per day in
the range-finding study. The NOAEL for maternal toxicity,
fetotoxicity, and teratogenicity was 20 mg/kg bw per day, the highest
dose tested (Hazelden & Wilson, 1986).
Rabbits
Groups of 15 timed-mated New Zealand white rabbits received guazatine
(purity, 67.9%) in distilled water by gavage on days 6-18 of presumed
gestation at doses of 0, 2.8. 5.6, or 11 mg/kg bw per day. An adequate
range of examinations was performed, and necropsy was carried out on
day 29. About two-thirds of fetuses from each litter were examined for
gross external and visceral abnormalities before staining with
Alizarin Red S for observation of skeletal abnormalities and variants.
The remainder were examined by free-hand dissection for soft-tissue
abnormalities. After marked weight loss, one animal at each dose was
killed during the study. One animal at the high dose aborted. Reduced
body-weight gain (76% of control weight) and reduced food consumption
(87% of control) were seen during treatment with 11 mg/kg bw per day.
During days 12-18 of gestation, reduced body-weight gain (87% of
controls) was seen at 5.6 mg/kg bw per day; them was no associated
effect on food consumption. Body-weight gain was similar in all groups
between days 18 and 29. Slight reductions in fetal weight were evident
at 5.6 mg/kg bw per day (by 3%) and 11 mg/kg bw per day (5%), but
these findings may have been secondary to the reduced body-weight
gains of the dams. There were no effects on litter size, fetal
viability, external abnormalities, skeletal abnormalities or variants,
extent of ossification, or soft-tissue abnormalities. The overall
NOAEL was 5.6 mg/kg bw per day on the basis of the markedly decreased
body-weight gain in dams at 11 mg/kg bw per day. There was no evidence
that guazatine is fetotoxic or teratogenic to rabbits (Barton &
Wilson, 1988).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
Guazatine GTA70 (purity unspecified) was severely irritating when
applied to rabbit skin for 4 h under occlusive conditions. The lesions
became more pronounced with time, and severe erythema and moderate
oedema were seen at the end of the study on day 7 (Cuthbert & Carr,
1989).
A 40% guazatine formulation induced ocular lesions that worsened with
time. A 10% solution of the formulation was only slightly irritating
to rabbit eyes (van Beek, 1974).
In a Magnusson and Kligmann maximization protocol, guazatine GTA70
(purity, 69.2%) did not have sensitizing potential in guinea-pigs at
induction concentrations of 0.5% intradermally or 1% topically and a
challenge concentration of 0.5% (Til & Keizer, 1980).
3. Observations in humans
Koyama et al. (1993) reported a case of attempted suicide with a
formulation containing 25% iminoctadine and 5%
polyoxyethylenealkylether (Befran). The patient was admitted with
severe cyanosis and in a stuporous state. Blood pressure and pulse
could not be measured (the radial arteries were not palpable) until
noradrenaline (1 mg) was administered.
[The authors cited a similar case and some experimental work involving
intravenous administration to dogs, which indicated that severe
hypotension was produced by iminoctadine. Subsequent work (Koyama et
al., 1994) on anaesthetized rats showed that iminoctadine administered
intravenously induced marked (> 10%) tachycardia and hypotension at
doses > 0.05 mg/kg bw in vivo. Experiments on isolated rat aorta
and atria in vitro showed that the primary effect of iminoctadine
was vasodilatory.]
Comments
Guazatine is a preparation of the triacetates of dimeric and trimeric
guanidated 1,8-diamino-octane, which also contains a range of
oligomers and reaction products. The Meeting was concerned that the
production controls and specifications for guazatine were inadequate.
The quoted purity of 70% is based on normalization to a component
which comprises approximately 1.5 % of the mixture and provides no
control over the levels of the other components. Some data were
provided to show that the composition of the batch used in the key
toxicity studies was similar to that of other batches produced at the
same time, 1990-1991; however, there were no data to confirm that the
batches used in the studies of toxicity were representative of those
currently produced.
Some components of guazatine were absorbed by rats to a limited extent
after oral administration of 14C-labelled compound and then excreted
rapidly. Within 24 h, faecal elimination represented 85-94% of the
dose, with 3-6% in the urine and < 1% in exhaled air. The highest
levels of radiolabel were found in the kidney and liver; there was
evidence that the salivary, pituitary, and thyroid glands may also
contain significant amounts of residue. A study involving treatment
with 14 doses of 2 mg/kg bw showed limited potential accumulation in
the liver and kidney. The results of a study by intravenous injection
showed that some components of guazatine may be secreted back into the
gastrointestinal tract via the stomach and salivary glands. The
metabolism of guazatine has not been fully characterized, but
monodeamidination and dideamidination play significant roles
in vivo.
Guazatine produces severe local irritation, and single oral doses are
of moderate toxicity, with an oral LD50 value in rats of 280 mg/kg
bw. WHO has classified guazatine as moderately hazardous (WHO, 1996).
In a number of short-term studies in rats, guazatine was administered
at doses of 0, 60, 200, 800/1200, or 1500/2000 ppm in the diet for 14
weeks. At doses of 60 and 200 ppm, the activity of serum alkaline
phosphatase was slightly decreased, but no significant changes were
seen in body-weight gain or in the results of pathological,
haematological, or urinary examinations. At doses of 800 ppm and
above, decreased body-weight gain, increased activities of alanine and
aspartate aminotranferases, and decreased activity of alkaline
phosphatase were found, together with pathological changes such as
local irritation of the gut and hyperplasia of the epithelia of the
excretory ducts of the parotid gland with mononuclear-cell
infiltration. Increased weights of the kidney, liver, and heart were
seen without associated histopathological changes. The overall NOAEL
was 200 ppm, equivalent to 10 mg/kg bw per day.
In a 13-week range-finding study, mice received guazatine at 0, 10,
50, 200, or 500 ppm. Significantly reduced body-weight gain was seen
in animals of each sex at 200 ppm and above. Increased liver weights
and alterations in centrilobular hepatocytes were seen in both males
and females at 500 ppm. Alterations in erythrocyte parameters were
seen in animals at doses of 200 ppm and above. Although no significant
effects were reported at 10 or 50 ppm, in view of limited histological
investigations in the study there was no NOAEL.
In a one-year study in dogs, guazatine was administered at 0, 25, 75,
or 250 ppm. Reduced body-weight gain in females, increased alanine
aminotransferase activity in animals of each sex, and increased
aspartate aminotransferase activity in males were observed at a
dietary concentration of 250 ppm. In females at 75 ppm, body-weight
gain was reduced. The NOAEL was 25 ppm, equal to 0.8 mg/kg bw per day.
Guazatine was not carcinogenic in two two-year studies in rats given
doses of 0, 20, 60, or 200 ppm or 0, 50, 150, or 350 ppm. The
non-neoplastic findings included reduced serum alanine and aspartate
aminotranferases activities, salivary gland hyperplasia, and
testicular atrophy at 350 ppm. In a two-year study of iminoctadine
administered at 0, 10, 100, or 300 ppm, there was no reported increase
in tumour incidence. The overall NOAEL was 150 ppm, equal to 7 mg/kg
bw per day.
In a study of carcinogenicity in mice, the animals received 0, 50,
120, or 300 ppm guazatine. The incidences of malignant tumours were
increased at 120 and 300 ppm: haemangiosarcoma of the liver and spleen
was seen in males at 120 and 300 ppm and hepatocellular carcinoma in
females at 300 ppm. The incidence of renal-tubular tumours (adenoma
and carcinoma) was increased in males receiving 300 ppm. These are
rare tumour types in the mouse strain that was used, normally being
seen in only 0-6% of animals. Although the absolute incidences of
these tumours in guazatine-treated animals were low and not
statistically significant, they were clearly greater than those in
historical controls. No convincing information was available on the
underlying mechanism of tumour production. The non-neoplastic effects
seen in this study were increased incidences of lymphoid foci in the
lung, bronchiole-associated lymphoid tissue, keratinized vaginal
epithelium, and brain mineralization in females receiving 300 ppm.
Body-weight gain was reduced by approximately 20% in animals of each
sex receiving 300 ppm. In addition, an abstract describing a study on
iminoctadine (at 0, 10, 100, or 300 ppm) reported a slight increase in
the incidence of renal epithelial tumours in male mice receiving 300
ppm. The Meeting considered that the production of rare malignant
tumours by an unknown mechanism is of great concern. The overall NOAEL
for long-term administration to mice was 50 ppm, equal to 6.8 mg/kg bw
per day, on the basis of increases in the incidence of
haemangiosarcoma in males at 120 ppm, equal to 17 mg/kg bw day.
Guazatine has been tested in an adequate battery of assays for
genotoxicity. The Meeting concluded that it is not genotoxic.
In a multigeneration study of reproductive toxicity in rats receiving
guazatine at 0, 60, or 200 ppm, no significant effects were seen at
the highest dose, equivalent to 12 mg/kg bw per day. In a
two-generation study of reproductive toxicity in rats, guazatine
administered at 0, 50, 150, or 350 ppm did not affect reproductive
performance at the highest dose, equal to 22 mg/kg bw per day.
In a study of developmental toxicity in rats, guazatine was
administered at 0, 5, 10, or 20 mg/kg bw per day. The NOAEL for
maternal toxicity, teratogenicity, and fetotoxicity was 20 mg/kg bw
per day, the highest dose tested. In a range-finding study,
significant mortality was seen at 40 mg/kg bw per day.
In a study of developmental toxicity in rabbits, guazatine was
administered at 0, 2.8, 5.6, or 11 mg/kg bw per day. There were no
signs of fetotoxicity or teratogenicity at the highest dose. The NOAEL
was 5.6 mg/kg bw per day on the basis of marked decreases in maternal
body-weight gain.
The Meeting considered that it could not establish an ADI for
guazatine owing to the inadequate information on its composition and
concerns about the production of rare malignant tumours in mice.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 150 ppm, equal to 7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
350 ppm, equal to 22 mg/kg bw per day (highest dose
tested in a two-generation study of reproductive
toxicity)
20 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Dog: 25 ppm, equal to 0.8 mg/kg bw per day (one-year study
of toxicity)
Studies that would provide information necessary for continued
evaluation of the compound
1. Data on the levels of individual components in batches of
guazatine from recent production runs
2. Investigation of the mechanism of tumour production in mice
3. Clarification of the extent of absorption, excretion, and
metabolism of all components of guazatine
4. Clarification as to whether the stated doses used in the studies
of toxicity were expressed as free base or triacetate
References
Atkinson, C., Perry, C.J., Hudson, P. & Robb, D.T (1990) Guazatine 13
week dietary dose range finding study in mice. Unpublished study from
Inveresk Research International (report No. 7552). Submitted to WHO by
Rhône Poulenc Secteur Agro, Lyon, France.
Appelman, L.M. (1980) Acute inhalation toxicity of guazatine
triacetate in rats. Unpublished study from CIVO-TNO (report No. R
6636). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon France
Barton, S.J. (1993) Guazatine: Two-generation reproduction study in
rats. Unpublished study from Inveresk Research International (report
No. 7854). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Barton, S.J. & Wilson, I.A. (1988) Guazatine: Teratugenicity study in
rabbits. Unpublished study from Inveresk Research International
(report No. 5344). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
van Beek, L. (1974) Eye irritation test with Panoctine 40 in albino
rabbits. Unpublished study from CIVO-TNO (report No. R4363). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L. (1980) Acute dermal toxicity study with Panoctine plus
300020 in albino rabbits. Unpublished study from CIVO-TNO (report No.
R6703). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L., van Oostrum, E.C.M. & Immel, H.R. (1976) Acute dermal
toxicity of Panoctine 42 in albino rabbits. Unpublished study from
CIVO-TNO (report No. R4921). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Cameron, B.D. & Phillips, M.W.A. (1986) The disposition of guazatine
in the lactating cow. Unpublished study from Inveresk Research
International (report No. 4141 ). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cameron, B.D., Mutch, P.J. & Scott, G. (1989) The metabolism of
[14C]-guazatine in the rat. Unpublished study from Inveresk Research
International (report No. 4826). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & Cart, S.M.A. (1989) Guazatine: Acute dermal
irritation test in rabbits. Unpublished study from Inveresk Research
International (report No. 5505). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & D'Arcy-Burt, K.J. (1986) Guazatine: Acute dermal
toxicity in rats (limit test) Unpublished study from Inveresk Research
International (report No. 3461). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Davis, P.B. (1983a) An investigation into the possible induction of
point mutations at the HGPRT locus of Chinese hamster ovary cells by
GTA70 (guazatine triacetate) Unpublished study from CIVO-TNO (report
No. R83/86). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Davis, P.B. (1983b) An investigation into the possible induction of
sister chromatid exchanges in Chinese hamster ovary cells by GTA 70
(guazatine triacetate). Unpublished study from CIVO-TNO (report No.
R83/85). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976a) Determination of the acute oral toxicity of
Panoctine 42 in cats. Unpublished study from CIVO-TNO. Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976b) Determination of the intraperitoneal toxicity
of Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
21-1-76). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Goburdhun, R. & Carter, P.B. (1989) Guazatine: Oral maximum tolerated
dose study in dogs. Unpublished study from Inveresk Research
International (report No. 5424). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Hazelden, K. (1987) Guazatine: Dose range finding study in rats,
preliminary to teratogenicity study. Unpublished study from Inveresk
Research International (report No. 3441). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Hazelden, K.E & Wilson, J.A. (1986) Guazatine: Teratogenicity study in
rats. Unpublished study from Inveresk Research International (report
No. 3540). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Heath, J., Perry, C.J., Hudson, R, Dobb, D. & Millar, P (1994)
Guazatine 104 week dietary study in rats with 52 week interim kill.
Unpublished study from Inveresk Research International (report No.
11006). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Heath, J., Perry, C.J., Hudson, E & Aitken, R. (1995) Guazatine 104
week dietary study in mice with 52 week interim kill. Unpublished
study from Inveresk Research International (report No. 11084).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Hirano, M., Maita, K., Mitsumori, K. & Shirasu, Y. (1985) 24 Month
chronic toxicity study with guazatine in rats. J. Toxicol. Sci., 10,
266.
Kato, Y., Sato, K., Maki, S., Matano, O. & Goto, K. (1985) Metabolic
fate including deamidination of guazatine triacetate in male rats.
J. Pestic. Sci., 10, 661-675.
Koyama, K., Yamashita, M., Miyauchi, T. & Goto, K. (1993) A fungicide
containing iminoctadine causes circulatory failure in acute oral
poisoning. Vet. Hum. Toxicol., 35, 512.
Koyama, K., Goto, K. & Yamashita, M. (1994) Circulatory failure caused
by a fungicide containing iminoctadine and a surfactant: A
pharmacological analysis in rats. Toxicol Appl. Pharmacol., 126,
197-201.
Kruysse, A. & Immel, H.R. (1976) Acute inhalation toxicity study with
Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
R4965). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Leegwater, D.C. (1975) Study on the fate of [14C, 3H guazatine
preparation in the rat and in sandy loam. Unpublished study from
CIVO-TNO (report No. R4823). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Leegwater, D.C. (1980) Study on the metabolic fate of [14C] Panoctine
preparation in the rat. Unpublished study from CIVO-TNO (report No.
R6571). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Maita, K., Mitsumori, K., Hirano, M. & Shirasu, Y. (1985) 24-Month
chronic toxicity study with guazatine in mice. J. Toxicol. Sci., 10,
267.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Oshodi, R.O. & Thompson, D.C. (1993) Guazatine: 52 Week dietary study
in dogs. Unpublished study from Inveresk Research International
(report No. 7903). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Prout, M.S. (1996) The metabolism of [14C]-guazatine in the rat.
Unpublished study from Inveresk Research International (Supplement 1
to report No. 4826: Cameron et al., 1989). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Reuzel, P.G.J, Til, H.P. & Kollen, C.H. (1976) Long term (two year)
toxicity study with guazatine in beagle dogs. Unpublished study from
CIVO-TNO (report No. R4983). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Sato, K., Kato, Y., Maki, S., Matano, O. & Goto, S. (1986) Metabolic
fate of plant guazatine residues in male rats. J. Pestic. Sci., 11,
267-270.
Sinkeldam, E. H. & van der Heijden, C.A. (1974) Sub-chronic (90-day)
toxicity study with guazatine in albino rats (final report).
Unpublished study from CIVO-TNO (report No. R4354). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Spanjers, M.T & Til, H.P. (1980) Determination of the acute oral
toxicity of acetates of guanidated amines (GTA) in rats. Unpublished
study from CIVO-TNO. Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, H.P. & Feron, V.J. (1975) Feeding study with guazatine in rats
for 13 weeks. Unpublished study from CIVO-TNO (report No. R4870).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P & Hendriksen, C.F.M. (1976) Toxicity study with Panoctine 42
(guazatine) in rats for 13 weeks. Unpublished study from CIVO-TNO
(report No. R5062). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, M.P. & Keizer, A.M.M. (1980) Maximisation test with GTA in guinea
pigs. Unpublished study from CIVO-TNO (report No. R6522). Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Hendriksen, C.F.M. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with guazatine in rats.
Unpublished study from CIVO-TNO (report No. R4985). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Koeter, H.B.W.M., Immel, H.R. & van der Heijden, C.A.
(1976b) Multigeneration study with guazatine in rats. Unpublished
study from CIVO-TNO (report No. R5078). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Willems, M.I. (1983) Examination of GTA in the micronucleus test.
Unpublished study from CIVO-TNO (report No. V83.118/230201). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Wilmer, J.W.G.M. (1983a) Examination of GTA for mutagenic activity in
the Ames test. Unpublished study from CIVO-TNO (report No. V83.
122/230064). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Wilmer, J.W.G.M. (1983b) Chromosome analysis of cultured human
lymphocytes treated in vitro with GTA. Unpublished study from CIVO-TNO
(report No. V83. 235.230573). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
See Also:
Toxicological Abbreviations
Guazatine (Pesticide residues in food: 1978 evaluations)
Guazatine (Pesticide residues in food: 1980 evaluations)
 2. Toxicological studies
(a) Acute toxicity
Guazatine is of moderate toxicity when given orally, of low toxicity
when applied dermally, but of moderately high toxicity when
administered by inhalation or intraperitoneally. The results of
studies of the acute toxicity of guazatine are summarized in Table 4.
The clinical signs of toxicity after treatment were lethargy or
sedation, hypothermia, coma, and local irritation. The gross and
histopathological changes were consistent with a response to a local
irritant; them was no clear systemic toxicity.
(b) Short-term toxicity
Mice
In a range-finding study, groups of 10 male and 10 female CD-1 mice
received guazatine (purity, 70.6%) in the diet at 0, 10, 50, 200, or
500 ppm for 13 weeks. Samples were taken for limited blood and
clinical chemical analyses at week 13. A limited range of tissues was
removed, weighed, and examined macroscopically; only the liver was
examined histologically. Two male controls, one male at 500 ppm, and
one at 50 ppm died during the study. Marked reductions in body-weight
gain (> 25%) were seen in animals of each sex at 200 or 500 ppm. In
females at the highest dose, aspartate aminotransferase activity was
increased. The relative liver weights were increased in animals of
each sex at 500 ppm; reductions in the absolute weights of several
organs appeared to be secondary to reduced body-weight gain. Altered
centrilobular hepatocytes were seen in 80% of animals at 200 or 500
ppm. Given the limited extent of the investigations, no NOAEL was
identified (Atkinson et al., 1990).
Table 4. Acute toxicity of guazatine and formulations
Test material Species Route Purity LD50/LC50 Reference
(%) (mg/kg bw or
mg/m3)
Guazatine GTA Rat Oral 69.2 280 Spanjers & Til (1980)
Panoctine 42 Cat Oral NR 0.38 ml/kg bw De Grout (1976a)
Guazatine GTA70 Rat Dermal 70.9 1050 Cuthbert & D'Arcy-Burt (1986)
Panoctine 42 Rabbit Dermal NR 2.8 ml/kg bw van Beck et al. (1976)
Panoctine Plus 300020 Rabbit Dermal NR 2.8-5.6 ml/kg bw van Beck (1980)
Paaoctine 42 Rat Intraperitoneal NR 0.053 ml/kg bw De Groot (1976b)
Guazatine GTA Rat Inhalation 69.2 225 Appelman (1980)
Panoctine 42 Rat Inhalation NR 11 Kruysse & Immel (1976)
Rats
Groups of 10 male and 10 female Wistar-derived rats received guazatine
(54.8% w/w) in the diet at 0, 60, or 200 ppm for 14 weeks.
Haematological and urinary parameters were measured in samples taken
at week 13, and serum enzymes and total protein were measured in
samples taken at termination at week 14. There were no deaths or
adverse clinical signs. Body weight and food conversion efficiency
were unaltered by treatment. A slight, dose-related decrease in
leukocyte count was seen in animals of each sex (up to 13% in males
and 6% in females), but this was not statistically significant, and
there was no marked change in differential counts. Serum alkaline
phosphatase activity was decreased in treated males, by 22% at 200 ppm
and 7% at 60 ppm. Urinary parameters were unaffected by treatment, but
the volume and specific gravity were not given. There were no
significant findings at gross or microscopic examination. The findings
in this study are of minimal toxicological significance; the NOAEL was
200 ppm, equivalent to 10 mg/kg bw per day (Sinkeldam & van der
Heijden, 1974).
Groups of 10 Wistar-derived rats of each sex received guazatine (54.8%
w/w) in the diet at 800 ppm for six weeks and then 1200 ppm for eight
weeks or control diet for 14 weeks. Haematological and urinary
parameters were measured in samples taken at week 13, and serum
enzymes and total protein were measured in samples taken at
termination at week 14. There were no deaths or adverse clinical
signs. Body-weight gain was reduced in animals of each sex by
approximately 6% at week 6 and by 8-10% at termination. Food
efficiency was unaffected in males but reduced in females.
Haematological parameters were unaltered by treatment. In males, serum
aspartate aminotransferase activity was increased and alkaline
phosphatase activity decreased. Females had an increased urine volume
with an associated decrease in specific gravity; the report does not
indicate whether samples were collected under fasting conditions.
Increases in relative adrenal, testis, and heart weights were seen in
treated animals, without abnormal pathological findings. As data on
individual animals and absolute organ weights were not presented, the
significance of these findings is uncertain. In treated females, high
levels of 'iron' deposition in the spleen were reported. The thyroids
of two females given guazatine were increased in weight and contained
small follicles lined with large epithelial cells that had small
apical nuclei. A re-evaluation of salivary gland tissues from this
study, reported very briefly by Til & Hendricksen (1976), described
hyperplasia in the epithelial lining of the excretory ducts of the
parotid salivary glands, with associated mononuclear cell infiltrates
in some treated animals. No NOAEL was identified (Til & Feron, 1975).
Panoctine 42 (purity unspecified) was administered to groups of 10
Wistar-derived rats of each sex at 0 or 1500/2000 ppm in the diet; the
level was increased from 1500 to 2000 ppm after week 4. Samples for
examination by haematology, limited clinical chemistry, and urinalysis
were taken during week 13. Animals were killed at week 14 and examined
grossly and histologically. There were no deaths or adverse clinical
signs. Body-weight gain was reduced by about 10% from week 2 in males
and by about 8% from week 6 in females. Food efficiency was reduced in
males for the first 12 weeks of the study. Serum proteins and alkaline
phosphatase activity were reduced in both treated groups. In treated
females, the leukocyte counts were increased (by 30%), and
differential counts indicated an increase in neutrophils and a
decrease in eosinophils. Increased relative weights of the liver (by
8%) and kidney (by 14%) were seen in treated males, without associated
histological findings. In females, an increased relative weight of the
heart (by 17%) and decreased thyroid weights (by 8%) were reported,
again without associated histological findings. Urinary aspartate
aminotransferase activity, proposed as a measure of renal toxicity,
was increased in treated animals, and urine volume was increased and
specific gravity decreased in animals of each sex. These findings
taken together are indicative of renal toxicity, though no associated
findings were reported at pathological examination. The only
histopathological finding of note was hyperplasia of the epithelial
lining of the excretory ducts of the parotid salivary glands with
associated mononuclear cell infiltrates in six males and six females
in the treated groups and in none of the controls (rho < 0.01). No
NOAEL was identified (Til & Hendricksen, 1976).
Dogs
In a range-finding study in beagle dogs, guazatine (purity, 67.9%)
given at 9.4 mg/kg bw per day by gavage for four days induced
body-weight loss in one of four animals. Subsequent administration of
14 mg/kg bw per day for four days induced marked body-weight loss and
reduced food consumption in all animals. The pathological findings
indicated local irritation of the gastrointestinal tract.
Administration of 374 ppm guazatine in the diet (12-15 mg/kg bw per
day) resulted in reduced food consumption, reduced body-weight gain,
and increased activities of aspartate aminotransferase, alanine
aminotransferase, and lactate dehydrogenase in serum after two weeks
(Goburdhun & Carter, 1989).
Groups of four male and four female beagle dogs received guazatine
(purity, 54.8%) in the diet at levels of 0, 60, 200, or 300/600 ppm
for two years; the level of 300 ppm was increased to 600 ppm after 26
weeks. Extensive observations were made, including haematological,
clinical chemical, and urinary determinations at 12/13, 26, 52, 78,
and 104 weeks, and tests for liver function (bromosulfthalein) and
kidney function (phenol red excretion) at 26, 52, and 104 weeks. Gross
and histopathological examinations were performed at termination at
week 104, although the results of histopathology in individual animals
were not reported. One control male died due to urolithiasis. There
was no evidence of treatment-related changes in clinical signs.
Body-weight gain and food consumption were unaffected by exposure. A
decreased leukocyte count (by 12-25 %) was present in animals at the
high dose, particularly females, from week 13 onwards, achieving
statistical significance at week 52, although there were no marked
alterations in differential counts. Sporadic changes in other
haematological, clinical chemical, and urinary parameters were not
consistent or of statistical significance. The results of liver and
kidney function tests were similar for control and treated groups.
Increases in absolute and relative ovarian weights were seen in
females at the high dose, although the significance of these changes
is unclear as there was considerable inter-animal variation and no
obviously associated histological findings. Given the uncertainties
about the findings at the high dose, the NOAEL was 200 ppm, equivalent
to 5 mg/kg bw per day (Reuzel et al., 1976).
Groups of four beagle dogs of each sex received guazatine (purity,
70.6%) in the diet for 52 weeks. Animals were given 400 g of diet per
day containing 0, 25, 75, or 250 ppm guazatine; the achieved intakes
were about 0, 0.8, 2.5, or 8 mg/kg bw per day. Extensive observations
included ophthalmoscopy at 0, 13, 26, and 51 weeks and haematology,
clinical chemistry, and urinalysis at 0, 6, 13, 26, and 51 weeks.
Animals were killed at week 52 and examined grossly; 13 tissues were
weighed, and more than 40 tissues were investigated by histopathology.
Owing to poor weight gain, one control and two males at the high dose
received extra food, which reversed the condition. In females, there
was a clearly dose-related reduction in body-weight gain starting at
week 10 and continuing throughout the study, with reductions of 30% in
comparison with controls in the group at the high dose, 20% in those
at the intermediate dose, and 12% in those at the low dose. Food
consumption was reduced in females receiving 75 or 250 ppm, with
occasional reductions in those at 25 ppm. Haematology showed no
consistent time- or dose-related reactions to treatment. Marked
increases (up to sixfold) in serum alanine aminotransferase activity
were seen throughout the study in males and females receiving 250 ppm.
Serum aspartate aminotransferase activity was increased in males at
the high dose throughout the study; an apparent increase in alkaline
phosphatase activity in males appears to be related to differences in
pretreatment values. A slight increase in liver weight was noted in
females at the high dose. Reduced prostate weights in males at the low
dose, increased ovarian weights in all treated females, and increased
thyroid weights in females at the intermediate dose did not show
dose-response relationships and were not clearly related to treatment,
although effects on ovarian weights were also see in the study of
Reuzel et al. (1976). Gross and microscopic examination showed no
changes. The reduced body-weight gain of females appeared to be due
only in part to unpalatability, as food conversion efficiency was also
reduced. The NOAEL was 25 ppm, equivalent to 0.8 mg/kg bw per day, on
the basis of marked reductions in body-weight gain in females at 75
and 250 ppm and clear increases in serum enzyme activities, indicative
of effects on the liver, in animals of each sex at 250 ppm (Oshodi &
Thompson, 1993).
(c) Long term toxicity and carcinogenicity
Mice
Groups of 10 male and 10 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for one year; the
achieved doses were 0, 7.6, 19, or 55 mg/kg bw per day in males and 0,
10, 25, or 67 mg/kg bw per day in females. All animals were examined
macroscopically. A wide range of tissues from animals receiving 0 or
300 ppm and those that died before week 52 were examined
histologically; the gall-bladder, kidney, liver, lung, salivary gland,
and grossly abnormal organs from all other animals were also examined
histologically. One male and one female at 120 ppm and two females at
300 ppm died during the study. There were no clinical signs associated
with treatment. Body-weight gain was reduced from week 4 in animals of
each sex at 300 ppm; by week 52, the weight gain of males was 72% that
of controls and that of females 66% of control values. Food
consumption was similar in treated and control animals. The relative
liver weights were increased by about 10% in all treated males. The
only notable finding at gross necropsy was an increased incidence of
ovarian cysts in females receiving 120 or 300 ppm (9/10 versus 6/10 in
controls). Microscopic examination showed increased incidences of
lymphocytic infiltration of the kidney and ovarian cysts in females at
the high dose (the ovaries of those at the intermediate dose were not
examined), of hepatocellular adenoma in males at the high dose (2/10
versus 0/10 in controls), and a decreased incidence of lymphoid foci
of the mandibular salivary gland in females at the intermediate and
high doses. The finding of hepatocellular adenomas may indicate that
guazatine reduces the time to onset of the liver turnouts seen
commonly in mice. Given the small group size, limited examination of
tissues from animals at low and intermediate doses, and clear effects
at 300 ppm, there was no NOAEL (Heath et al., 1995).
In the part of the same study designed to test for carcinogenicity,
groups of 50 male and 50 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for two years; the
achieved doses were 0, 6.8, 17,or 47 mg/kg bw per day in males and 0,
8.7, 21, or 57 mg/kg bw per day in females. All animals were examined
macroscopically, and a wide range of tissues from animals receiving 0
or 300 ppm or that died before week 104 were examined histologically;
only the gall-bladder, kidney, liver, lung, salivary glands, and
grossly abnormal organs from animals in other groups were examined.
Blood samples for differential leukocyte counts were taken from all
surviving controls and animals at 300 ppm during weeks 52, 78, and
103.
The pattern of deaths was similar in treated and control groups, with
> 50% survival in all groups until week 100 in males and week gg in
females. Reduced body-weight gain was seen at the high dose from week
2 onwards, the deficit being approximately 20% at termination. During
the second year, males at the intermediate dose lost more weight than
controls, while females at this dose gained more weight than controls.
Food consumption, differential leukocyte counts, and findings at gross
necropsy were unaffected by treatment. Increased absolute and relative
weights of the kidney and liver were seen in females at the
intermediate but not the high dose.
The incidences of a range of neoplastic and non-neoplastic lesions
showed evidence of treatment-related effects (Table 5). The incidences
of haemangiosarcoma of the liver in males at the intermediate and high
doses and of the spleen in males at the high dose were higher than
those in historical controls, which were 04% in males and 0-3% in
females for liver and 0-2% in males and 045% in females for spleen.
The high incidence of haemangiosarcoma in females at the low dose is
considered to be a chance finding, as them was no dose-response
relationship. The incidence of hepatocellular carcinoma in females at
the high dose was greater than that in historical controls (0-4%). The
incidences of renal adenoma and carcinoma in male historical controls
were 0-4% and 0-2%, respectively, indicating that the incidence of
renal adenoma in males at the high dose is a significant finding.
The presence of rare tumours, including malignant ones, at multiple
sites was considered by the Meeting to be of concern. The mechanism by
which these tumours are induced is not evident from the available
data. Guazatine is not genotoxic (see below), indicating the mechanism
is probably nongenotoxic, although haemangiosarcomas are generally
associated with a genotoxic mechanism. As them was no evidence of
marked toxic or hyperplastic responses in the affected tissues and
them was evidence of a dose-response relationship, it was not clear
that these tumours are a direct result of disrupted cell function
associated with exposure to doses above the maximum tolerated dose.
Although only a limited range of tissues was examined from animals at
the low and intermediate doses, it included those with the most
significant effects at the high dose. The NOAEL was 50 ppm, equal to
6.8 mg/kg bw per day, on the basis of effects on body-weight gain and
the occurrence of haemangiosarcomas in males at 120 ppm, marked
effects on body-weight gain, and a range of neoplastic and
non-neoplastic changes at 300 ppm (Heath et al., 1995).
The findings of another two-year study with guazatine in mice were
summarized in abstract form only. Guazatine (as iminoctadine) was
given to groups of 80 male and 80 female specific-pathogen-free
ICR-Crj mice at dietary levels of 0,10, 100, or 300 ppm. Eight animals
of each sex at each dose were killed at weeks 26 and 52 for
examination. At 300 ppm (26 mg/kg bw per day in males, 30 mg/kg bw per
day in females), males and females showed a remarkable depression in
body-weight gain, decreased food efficiency, and anaemia at weeks 26
and/or 52, increased plasma urea nitrogen level (only in females),
increased alkaline phosphatase level (at week 26 in animals of each
sex and also at week 52 in females), and increased weights of the
kidney (absolute and relative) and liver (relative). Histological
examination revealed swelling of hepatocytes and proximal tubular
cells in animals of each sex. There were increased incidences of
subcutaneous oedema in females and hydrothorax in males. The
incidences of splenic atrophy in animals of each sex, atrophy and/or
brown pigment deposition of the ovary, and glandular epithelial
atrophy of the Harderian gland in males were significantly increased.
The incidence of renal epithelial tumours increased slightly in males.
No toxic changes were seen at the other doses, except for slightly
Table 5. Lesions (as % of samples examined) seen in CD1 mice exposed to guazatine in the diet
for two years
Lesion Guazatine (ppm)
Males Females
0 50 120 300 0 50 120 300
Brain mineralization 22 26 33 20 8 16 10 22*
Caecal carcinoma 0 0 0 0 0 0 0 2
Renal tubular carcinoma 0 0 0 2 0 0 0 0
Renal tubular adenoma 2 2 2 8 0 0 2 0
Renal glomerular amyloid 12 8 16 10 24 8 8 0*
Hepatocellular carcinoma 22 24 22 22 2 2 2 10
Liver haemangiosarcoma
Multifocal 0 0 2 4 0 0 2 0
Other 2 2 6 6 0 6 2 0
Increase in bronchus-associated
lymphoid tissue 10 12 12 20 4 14 16 28*
Lung lymphoid foci 0 0 2 0 0 0 4 10*
Spleen haemangiosarcoraa 0 0 2 4 2 0 2 4
Vaginal keratinized epithelium - - - - 15 - - 43
-, not determined
* One-tailed p < 0.05, Fisher exact test
increased incidences of swelling of proximal tubular cells in animals
of each sex at 100 ppm (Maita et al., 1985).
Rats
Groups of 60 male and 60 female Wistar-derived rats, in two parallel
series initiated two months apart, received diets containing guazatine
(54% solution) at 0, 20, 60, or 200 ppm for two years. A group
receiving 6 ppm was terminated after a few months, as the results of
other studies indicated that 20 ppm was probably a NOAEL. Blood and
urine samples were taken from 10 fasted animals of each sex at each
dose at various times during the study for haematological, limited
clinical chemical, and urinary analyses. All animals were examined
grossly post mortem. A wide range of organs from surviving animals
in the first series (11-20) were weighed, but only kidney, spleen, and
adrenals from rats in the second series were weighed. A full
histological examination was performed on tissues from 20 rats of each
sex in the control and high-dose groups in the first series, and gross
lesions, tumours, adrenals, thyroid, and pituitaries from all animals
were examined.
Behaviour and clinical signs were reported to be unaffected by
treatment. There were more early deaths (before week 72) among treated
males, but overall survival to week 104 was satisfactory and similar
in all groups. Body weights were similar in all groups until week 88,
when all treated females showed a deficit in comparison with controls,
which lasted until termination. Food consumption and conversion
efficiency were similar in all groups over the first four weeks.
Leukocyte counts were decreased (by about 11%) in males at the high
dose from week 26, with no consistent change in differential counts;
females showed sporadic changes in leukocyte counts with no consistent
pattern. Urinalysis gave similar findings in all groups. Reduced
relative testicular weights (by 11%) were seen in males at the high
dose, although the absolute weights were similar since the mean body
weight was 8% higher. In treated females, the relative weights of the
spleen, brain, and kidney were increased; however, as the absolute
weights were decreased and there was no evidence of a dose-response
relationship, this finding is probably secondary to the effects on
body weight. Occasional increases in the incidences of non-neoplastic
lesions were reported, but the only statistically significant
(p < 0.05) increases were for chronic respiratory disease and mammary
gland inflammation or dilatation in females at the high dose. The
number of tumour-bearing animals and the total numbers of tumours were
similar in all groups. The incidence of monocytic leukaemia was
increased in males and females at the high dose, occurring in four
males and three females, with none in male and in one female controls.
As the total numbers of animals examined is unclear, this may be a
chance finding. Although the study indicates that guazatine has no
marked toxicity, the limited, investigations performed and the unusual
design make interpretation of the findings difficult. No NOAEL was
identified (Til et al., 1976a).
Groups of 20 male and 20 female Sprague Dawley rats received guazatine
(purity, 70.6%) in the diet at 0, 50, 150, or 350 ppm for one year.
The achieved intakes of guazatine were 0, 2.5, 7, or 19 mg/kg bw per
day for males and 0, 3, 9, or 22 mg/kg bw per day for females. Samples
for haematological, clinical chemical, and urinary analyses were
obtained from 10 animals of each sex in each group at weeks 26 and 50
or 51. At week 52, the animals were killed and examined grossly.
Selected tissues were weighed, and samples from a wide range of organs
from controls and animals at the high dose were examined
histologically; gross lesions, kidneys, liver, lung, and salivary
glands from animals at the low and intermediate doses were also
examined histologically.
Behaviour, clinical signs, body-weight gain, food consumption, urinary
parameters, and the findings at gross necropsy were similar in all
groups. None of the controls but one male at the low dose, two each at
the intermediate and high doses, and one female at 150 ppm died. Total
leukocyte and lymphocyte counts were reduced in males at 350 ppm at
both 26 and 51 weeks, but increases were seen in treated females.
Serum alanine aminotransferase activity was decreased markedly (by
>40%) at both sampling times in males and females receiving 350
ppm, but there were occasional deficits in other serum enzyme
activities (particularly aspartate aminotransferase); total protein
was similar in all groups. Dose-related decreases in absolute and
relative prostate weights were significant (rho < 0.05) at both 150
and 350 ppm and were associated with a low incidence of hyperplasia at
350 ppm. Females had dose-related increases in the relative and
absolute weights of the adrenals (by < 10%) and ovary by
(< 50%). Five males at the high dose but none of the controls had
testicular atrophy. Mononuclear-cell infiltration of the parotid
salivary gland was increased in males (6/20 versus 1/20) and females
(5/20 versus 1/20) receiving 350 ppm. The NOAEL was 150 ppm, equal to
7 mg/kg bw per day, on the basis of decreased leukocyte count,
prostatic hyperplasia, decreased alanine aminotransferase activity,
and effects on the salivary gland at 350 ppm. The minimal changes seen
at 150 ppm were not consistent or of sufficient magnitude to be
considered adverse (Heath et al., 1994).
In the phase of the study that addressed carcinogenicity, groups of 50
Sprague Dawley rots of each sex received guazatine (purity, 70.6%) in
the diet at 0, 50, 150, or 350 ppm for two years. The achieved intakes
were about 0, 2.5, 7, and 19 mg/kg bw per day for males and 0, 3, 9,
and 22 mg/kg bw per day for females. Samples for haematological,
clinical chemical, and urinary analyses were obtained from 10 rats of
each sex per group at weeks 53 (for haematology only), 78, and 101 or
103. At week 104, the animals were killed and underwent a full gross
examination. Selected tissues were weighed, and samples from a wide
range of organs from controls and animals at the high dose were
examined histologically; gross lesions, kidneys, liver, lung, and
salivary glands from animals at the low and intermediate doses were
also examined histologically.
Behaviour, clinical signs, and organ weights were similar in all
groups. Survival was acceptable (50% at week 94) and similar in all
groups. Low body weights were seen consistently in females at the high
dose from week 9 and in males at this dose from week 20; in the latter
quarter of the study, females at the low and intermediate doses had
reduced body-weight gain in comparison with controls. Males at the
high dose showed consistent reductions in leukocyte and platelet
counts but increased erythrocyte counts. In females at 350 ppm,
erythrocyte counts were reduced; an increase in leukocyte counts at
termination was due to a neurilemmoma in one animal. Reduced
activities of serum alanine and aspartate aminotransferases (by
< 50%) were seen consistently in animals of each sex receiving 350
ppm. Reduced urinary pH was seen at the high dose in animals of each
sex throughout the study. A slight excess of abnormal findings in
lymph nodes was seen in females at this dose, but no individual effect
was significant when compared with the very low background incidence.
The incidences and severity of a range of lesions in salivary glands,
lymph nodes, ovaries, spleen, and pituitary were increased in females
receiving 350 ppm. In males, the only notable finding was increased
severity and incidence of testicular germinal epithelial degeneration
at 350 ppm. A rare malignant oligodendroglioma was found in the brain
of a single female at the high dose, but there was no evidence of
treatment-related carcinogenicity in any other animal. The NOAEL was
150 ppm, equal to 7 mg/kg bw per day, on the basis of reduced
body-weight gain and a range of clinical chemical and histological
findings at 350 ppm (Heath et al., 1994).
The findings of a two-year study of guazatine in rats were summarized
in abstract form only. Guazatine (as iminoctadine) was presented to
groups of 80 male and 80 female Fischer 344 rats at dietary levels of
0, 10, 100, or 300 ppm for two years. At 6 and 12 months, eight
animals of each sex from each group were killed for examination.
Animals of each sex at 300 ppm (11 mg/kg bw per day for males, 14
mg/kg bw per day for females) had remarkably depressed body-weight
gain, decreased food efficiency, higher mortality rates, a tendency to
anaemia, decreased total protein, and increased kidney and adrenal
weights; decreased potassium and albumin and increased calcium,
aspartate aminotransferase and gamma-glutamyltransferase activities,
and spleen weight were also observed. Histologically, swelling,
degeneration, and necrosis of renal tubular cells and metaplasia in
the glandular stomach were observed in animals of each sex. The
incidence of sperm granuloma in the deferent duct and/or epididymides
was significantly increased, and the incidences of leukaemia in males
and of adrenal phaeochromocytoma in animals of each sex were slightly
increased. At 100 ppm, males had a slightly higher mortality rate, and
females had depressed body-weight gain; decreased potassium and
increased calcium and gamma-glutamyltransferase activity were
occasionally observed in animals of either sex. The kidney and spleen
weights were increased, and the incidences of intestinal metaplasia
and sperm granuloma were significantly higher than in controls. At 10
ppm, no toxic change attributable to guazatine were seen (Hirano et
al., 1985).
(d) Genotoxicity
Guazatine has been tested for its potential to induce gene mutations,
sister chromatid exchange, and chromosomal aberrations in vitro and
micronuclei in vivo. Negative results were obtained in all studies.
The data are summarized in Table 6.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female Wistar-derived rats received diets
containing 0, 60, or 200 ppm guazatine (purity, 54.8%) over four
generations. The reporting of the study lacks the results for
individual animals, dietary analyses, time to mating, length of
gestation, absolute organ weights, and other data. F0 animals
received the test diets for 12 weeks before the first mating to
produce the F1a generation; the second mating eight weeks later
produced the F1b generation. Litters were culled to eight pups on day
1 after birth, and the F1a litters were discarded at weaning. Animals
were selected from the F1b litters at weaning to produce the F2
generation, with mating identical to that for F0 animals, and
similarly for the F3 generation from the F2b litters. Groups of 10
animals of each sex from the F3b litters were selected at weaning and
given test diets for four weeks; they were then killed and subjected
to gross and histopathological examinations.
Treatment had no effect on survival, clinical signs, litter size, sex
ratio, or resorption rates. Minor variations in the body weights of
pups appeared to be secondary to variations in litter size. F3b
animals receiving 60 or 200 ppm had increased, relative kidney
weights (by 8% at 60 ppm and 12-17% at 200 ppm), and increased
relative thymus weights (by 19-34%) were seen in all treated groups. A
dose-related reduction in relative testicular weights (8%) was also
seen. Histological examination gave no evidence of treatment-related
effects in any organ. In a summarily described extension of the study,
it was reported that males in F4b litters given test diets for four
weeks after weaning had reduced relative weights of the testis (9%)
and increased relative weights of the thymus (25%) at both doses, and
females at the high dose had increased relative kidney weights (12%).
Again, no treatment-related histological findings were reported. This
study shows that guazatine does not adversely affect reproductive
outcome in rats. The finding of increased thymus weights may indicate
that it affects the control of thymic growth or involution; such
effects may not be detectable in older animals when thymic involution
is well advanced. Given potential concern about effects on the thymus
at both 60 and 200 ppm, no NOAEL was identified (Til et al., 1976b).
Table 6. Results of genotoxicity assays on guazatine
End-point Test system Concentration/ Purity Results Reference
dose (%)
In vitro
Reverse mutation S. typhimurium 0.6-50 µg/plate 73 Negativea Wilmer (1983a)
TA98, TA 100. Cytotoxic
TA1535. TA1537. at 50 g/plate
TA1538
Reverse mutation S. typhimurium To cytotoxic levels NR Negative Moriya et al.
TA98, TA100, TA1535, (numbers not given) (1983)
TA1537, TA1538, and
E. coli WP2 hcr
Gene mutation Chinese hamster 25-100 nl/ml -S9 NR, Negativea Davis (1983a)
ovary cells (line K1), 50-200 nl/ml +S9 approx. Cytotoxic at
hprt locus 70% highest
concentrations
Sister chromatid Chinese hamster 5-30 nl/ml NR, Negativea Davis (1983b)
exchange ovary cells (line K1) approx. Cytotoxic
70% at > 35 nl/ml
Chromosomal Human 3.7-100 g/ml -S9 73 Negativea Wilmer (1983b)
aberration lymphocytes 14.8-400 g/ml +S9 Cytotoxic
at highest
concentrations
In vivo
Micronucleus Mouse (5/sex at 150 mg/kg bw by 73 Negative Willems (1983)
formation each time) bone gavage in saline No effect on
marrow (approx. 50% of P:N ratio
LD50); 24, 48, and
72h
Table 6 (continued)
NR, not reported; S9, exogenous metabolic activation system from Aroclor 1254-induced rat liver preparations;
P:N, polychromatic:normochromatic erythrocytes
a With and without metabolic activation
The reproductive effects of guazatine (purity, 70.6%) were
investigated in a two-generation (one litter per generation) study in
which groups of 28 young adult CD rats received diets containing
guazatine at 0, 50, 150, or 350 ppm, equal to a minimal intake of 0,
3, 10, or 22 mg/kg bw per day in males and 0, 4, 11, or 25 mg/kg bw
per day in females. Animals were given the treated diet for 10 weeks
before F0 mating (1:1) and continuously until sacrifice. Groups of 24
male and 24 female F1 animals were mated at about 17 weeks; they were
then killed and examined grossly, and reproductive organs and
pituitary, liver, adrenals, and salivary glands were weighed and
prepared for histological examination. Pups that died before weaning
were examined grossly. On day 21, one pup of each sex per litter was
examined grossly, and the liver and salivary glands were removed for
histological examination. All other pups were examined externally.
Treatment had no effect on clinical signs, survival, time to mating,
fertility indices, gestation length, litter size, gestation indices,
or lactation indices. Slight reductions in body-weight gain (5-10%)
were closely related to reductions in food consumption (4-9%). Slight
reductions in survival to day 21 (81-83% versus 87% in controls) and
litter weight at day 21 (about 8%) were seen in F1 pups in all
treated groups; as there was no dose-response relationship and the
effects were of small magnitude and were not seen in the F2
generation, they were considered not to be of biological significance.
Post-mortem examination showed no treatment-related effects; however,
the thymus was not weighed or investigated specifically. The NOAEL was
350 ppm, equal to 22 mg/kg bw per day, the highest dose tested
(Barton, 1993).
(ii) Developmental toxicity
Rats
The developmental effects of guazatine were investigated in animals
selected from the F2b and F3b litters of the study of Til et al
(1976b), described above Groups of five males and 15 females were
selected at weaning and maintained on test diets (0, 60, or 200 ppm
guazatine) until mating (1:3) at week 12; it was not clear whether the
test diets were given during gestation. On day 21 of gestation, the
dams were killed, their reproductive tracts investigated, and pups
examined for gross abnormalities and skeletal defects (with Alizarin
Red S staining); effects on soft tissues were studied only in controls
and rats at 200 ppm (by Wilson sectioning). F3b animals showed a
dose-related reduction in litter size, with 10.7 pups in controls, 10
at 60 ppm, and 8.9 at 200 ppm; at 200 ppm, this reduction was
associated with increased pre- and postimplantational losses in two
dams. Mean fetal weight, and hence litter weight, was reduced at 200
ppm in the F3b-derived group There were no notable visceral effects;
although there was an indication of reduced ossification in treated
animals, this was not consistent with regard to dose, generation, or
site. Reduced litter size was seen mainly in two animals and is not
consistent with the results of the main study. The NOAEL was 200 ppm,
equal to 12 mg/kg bw per day, as guazatine did not directly affect the
developing fetus or the dam (Til et al., 1976b).
In a range-finding study, pregnant Sprague Dawley rats died after
receiving guazatine (purity, 70.9%) at levels of 40, 80, or 120 mg/kg
bw per day by gavage on days 6-16 of gestation. At 20 mg/kg bw per
day, there was no evidence of toxicity. Necropsy of animals at doses
> 40 mg/kg bw per day showed marked irritation of the
gastrointestinal tract (Hazelden, 1987).
Four groups of 27 timed-mated Sprague Dawley rats received guazatine
(purity, 70.9%) in distilled water by garage on days 6-16 of presumed
gestation at doses of 0, 5, 10, or 20 mg/kg bw per day, on the basis
of the findings in the range-finding study. An adequate range of
examinations was performed, and animals were necropsied on day 20
after nitrogen asphyxiation. About two-thirds of the fetuses from each
litter were examined for gross external and visceral abnormalities
before staining with Alizarin Red S for observation of skeletal
abnormalities and variants. The remainder were examined by free-hand
dissection (Wilson technique) for soft-tissue abnormalities. One
control and two animals at the high dose died during the study due to
dosing accidents. There were no signs of maternal toxicity;
body-weight gain was similar in all groups, although food consumption
was decreased marginally (by 1-2%) in rats at 10 and 20 mg/kg bw.
There were no effects on litter size or fetal weight, or on soft
tissues or viscera. There was an indication of slightly retarded
development of some bones (e.g. scapula and pectoral girdle) at 10 and
20 mg/kg bw, but the findings were not significant, and the overall
degree of skeletal ossification was unaffected by treatment. The lack
of maternal toxicity at the high dose may be considered to have
compromised the study, but use of 20 mg/kg bw per day is considered
acceptable in view of the 40% mortality rate at 40 mg/kg bw per day in
the range-finding study. The NOAEL for maternal toxicity,
fetotoxicity, and teratogenicity was 20 mg/kg bw per day, the highest
dose tested (Hazelden & Wilson, 1986).
Rabbits
Groups of 15 timed-mated New Zealand white rabbits received guazatine
(purity, 67.9%) in distilled water by gavage on days 6-18 of presumed
gestation at doses of 0, 2.8. 5.6, or 11 mg/kg bw per day. An adequate
range of examinations was performed, and necropsy was carried out on
day 29. About two-thirds of fetuses from each litter were examined for
gross external and visceral abnormalities before staining with
Alizarin Red S for observation of skeletal abnormalities and variants.
The remainder were examined by free-hand dissection for soft-tissue
abnormalities. After marked weight loss, one animal at each dose was
killed during the study. One animal at the high dose aborted. Reduced
body-weight gain (76% of control weight) and reduced food consumption
(87% of control) were seen during treatment with 11 mg/kg bw per day.
During days 12-18 of gestation, reduced body-weight gain (87% of
controls) was seen at 5.6 mg/kg bw per day; them was no associated
effect on food consumption. Body-weight gain was similar in all groups
between days 18 and 29. Slight reductions in fetal weight were evident
at 5.6 mg/kg bw per day (by 3%) and 11 mg/kg bw per day (5%), but
these findings may have been secondary to the reduced body-weight
gains of the dams. There were no effects on litter size, fetal
viability, external abnormalities, skeletal abnormalities or variants,
extent of ossification, or soft-tissue abnormalities. The overall
NOAEL was 5.6 mg/kg bw per day on the basis of the markedly decreased
body-weight gain in dams at 11 mg/kg bw per day. There was no evidence
that guazatine is fetotoxic or teratogenic to rabbits (Barton &
Wilson, 1988).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
Guazatine GTA70 (purity unspecified) was severely irritating when
applied to rabbit skin for 4 h under occlusive conditions. The lesions
became more pronounced with time, and severe erythema and moderate
oedema were seen at the end of the study on day 7 (Cuthbert & Carr,
1989).
A 40% guazatine formulation induced ocular lesions that worsened with
time. A 10% solution of the formulation was only slightly irritating
to rabbit eyes (van Beek, 1974).
In a Magnusson and Kligmann maximization protocol, guazatine GTA70
(purity, 69.2%) did not have sensitizing potential in guinea-pigs at
induction concentrations of 0.5% intradermally or 1% topically and a
challenge concentration of 0.5% (Til & Keizer, 1980).
3. Observations in humans
Koyama et al. (1993) reported a case of attempted suicide with a
formulation containing 25% iminoctadine and 5%
polyoxyethylenealkylether (Befran). The patient was admitted with
severe cyanosis and in a stuporous state. Blood pressure and pulse
could not be measured (the radial arteries were not palpable) until
noradrenaline (1 mg) was administered.
[The authors cited a similar case and some experimental work involving
intravenous administration to dogs, which indicated that severe
hypotension was produced by iminoctadine. Subsequent work (Koyama et
al., 1994) on anaesthetized rats showed that iminoctadine administered
intravenously induced marked (> 10%) tachycardia and hypotension at
doses > 0.05 mg/kg bw in vivo. Experiments on isolated rat aorta
and atria in vitro showed that the primary effect of iminoctadine
was vasodilatory.]
Comments
Guazatine is a preparation of the triacetates of dimeric and trimeric
guanidated 1,8-diamino-octane, which also contains a range of
oligomers and reaction products. The Meeting was concerned that the
production controls and specifications for guazatine were inadequate.
The quoted purity of 70% is based on normalization to a component
which comprises approximately 1.5 % of the mixture and provides no
control over the levels of the other components. Some data were
provided to show that the composition of the batch used in the key
toxicity studies was similar to that of other batches produced at the
same time, 1990-1991; however, there were no data to confirm that the
batches used in the studies of toxicity were representative of those
currently produced.
Some components of guazatine were absorbed by rats to a limited extent
after oral administration of 14C-labelled compound and then excreted
rapidly. Within 24 h, faecal elimination represented 85-94% of the
dose, with 3-6% in the urine and < 1% in exhaled air. The highest
levels of radiolabel were found in the kidney and liver; there was
evidence that the salivary, pituitary, and thyroid glands may also
contain significant amounts of residue. A study involving treatment
with 14 doses of 2 mg/kg bw showed limited potential accumulation in
the liver and kidney. The results of a study by intravenous injection
showed that some components of guazatine may be secreted back into the
gastrointestinal tract via the stomach and salivary glands. The
metabolism of guazatine has not been fully characterized, but
monodeamidination and dideamidination play significant roles
in vivo.
Guazatine produces severe local irritation, and single oral doses are
of moderate toxicity, with an oral LD50 value in rats of 280 mg/kg
bw. WHO has classified guazatine as moderately hazardous (WHO, 1996).
In a number of short-term studies in rats, guazatine was administered
at doses of 0, 60, 200, 800/1200, or 1500/2000 ppm in the diet for 14
weeks. At doses of 60 and 200 ppm, the activity of serum alkaline
phosphatase was slightly decreased, but no significant changes were
seen in body-weight gain or in the results of pathological,
haematological, or urinary examinations. At doses of 800 ppm and
above, decreased body-weight gain, increased activities of alanine and
aspartate aminotranferases, and decreased activity of alkaline
phosphatase were found, together with pathological changes such as
local irritation of the gut and hyperplasia of the epithelia of the
excretory ducts of the parotid gland with mononuclear-cell
infiltration. Increased weights of the kidney, liver, and heart were
seen without associated histopathological changes. The overall NOAEL
was 200 ppm, equivalent to 10 mg/kg bw per day.
In a 13-week range-finding study, mice received guazatine at 0, 10,
50, 200, or 500 ppm. Significantly reduced body-weight gain was seen
in animals of each sex at 200 ppm and above. Increased liver weights
and alterations in centrilobular hepatocytes were seen in both males
and females at 500 ppm. Alterations in erythrocyte parameters were
seen in animals at doses of 200 ppm and above. Although no significant
effects were reported at 10 or 50 ppm, in view of limited histological
investigations in the study there was no NOAEL.
In a one-year study in dogs, guazatine was administered at 0, 25, 75,
or 250 ppm. Reduced body-weight gain in females, increased alanine
aminotransferase activity in animals of each sex, and increased
aspartate aminotransferase activity in males were observed at a
dietary concentration of 250 ppm. In females at 75 ppm, body-weight
gain was reduced. The NOAEL was 25 ppm, equal to 0.8 mg/kg bw per day.
Guazatine was not carcinogenic in two two-year studies in rats given
doses of 0, 20, 60, or 200 ppm or 0, 50, 150, or 350 ppm. The
non-neoplastic findings included reduced serum alanine and aspartate
aminotranferases activities, salivary gland hyperplasia, and
testicular atrophy at 350 ppm. In a two-year study of iminoctadine
administered at 0, 10, 100, or 300 ppm, there was no reported increase
in tumour incidence. The overall NOAEL was 150 ppm, equal to 7 mg/kg
bw per day.
In a study of carcinogenicity in mice, the animals received 0, 50,
120, or 300 ppm guazatine. The incidences of malignant tumours were
increased at 120 and 300 ppm: haemangiosarcoma of the liver and spleen
was seen in males at 120 and 300 ppm and hepatocellular carcinoma in
females at 300 ppm. The incidence of renal-tubular tumours (adenoma
and carcinoma) was increased in males receiving 300 ppm. These are
rare tumour types in the mouse strain that was used, normally being
seen in only 0-6% of animals. Although the absolute incidences of
these tumours in guazatine-treated animals were low and not
statistically significant, they were clearly greater than those in
historical controls. No convincing information was available on the
underlying mechanism of tumour production. The non-neoplastic effects
seen in this study were increased incidences of lymphoid foci in the
lung, bronchiole-associated lymphoid tissue, keratinized vaginal
epithelium, and brain mineralization in females receiving 300 ppm.
Body-weight gain was reduced by approximately 20% in animals of each
sex receiving 300 ppm. In addition, an abstract describing a study on
iminoctadine (at 0, 10, 100, or 300 ppm) reported a slight increase in
the incidence of renal epithelial tumours in male mice receiving 300
ppm. The Meeting considered that the production of rare malignant
tumours by an unknown mechanism is of great concern. The overall NOAEL
for long-term administration to mice was 50 ppm, equal to 6.8 mg/kg bw
per day, on the basis of increases in the incidence of
haemangiosarcoma in males at 120 ppm, equal to 17 mg/kg bw day.
Guazatine has been tested in an adequate battery of assays for
genotoxicity. The Meeting concluded that it is not genotoxic.
In a multigeneration study of reproductive toxicity in rats receiving
guazatine at 0, 60, or 200 ppm, no significant effects were seen at
the highest dose, equivalent to 12 mg/kg bw per day. In a
two-generation study of reproductive toxicity in rats, guazatine
administered at 0, 50, 150, or 350 ppm did not affect reproductive
performance at the highest dose, equal to 22 mg/kg bw per day.
In a study of developmental toxicity in rats, guazatine was
administered at 0, 5, 10, or 20 mg/kg bw per day. The NOAEL for
maternal toxicity, teratogenicity, and fetotoxicity was 20 mg/kg bw
per day, the highest dose tested. In a range-finding study,
significant mortality was seen at 40 mg/kg bw per day.
In a study of developmental toxicity in rabbits, guazatine was
administered at 0, 2.8, 5.6, or 11 mg/kg bw per day. There were no
signs of fetotoxicity or teratogenicity at the highest dose. The NOAEL
was 5.6 mg/kg bw per day on the basis of marked decreases in maternal
body-weight gain.
The Meeting considered that it could not establish an ADI for
guazatine owing to the inadequate information on its composition and
concerns about the production of rare malignant tumours in mice.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 150 ppm, equal to 7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
350 ppm, equal to 22 mg/kg bw per day (highest dose
tested in a two-generation study of reproductive
toxicity)
20 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Dog: 25 ppm, equal to 0.8 mg/kg bw per day (one-year study
of toxicity)
Studies that would provide information necessary for continued
evaluation of the compound
1. Data on the levels of individual components in batches of
guazatine from recent production runs
2. Investigation of the mechanism of tumour production in mice
3. Clarification of the extent of absorption, excretion, and
metabolism of all components of guazatine
4. Clarification as to whether the stated doses used in the studies
of toxicity were expressed as free base or triacetate
References
Atkinson, C., Perry, C.J., Hudson, P. & Robb, D.T (1990) Guazatine 13
week dietary dose range finding study in mice. Unpublished study from
Inveresk Research International (report No. 7552). Submitted to WHO by
Rhône Poulenc Secteur Agro, Lyon, France.
Appelman, L.M. (1980) Acute inhalation toxicity of guazatine
triacetate in rats. Unpublished study from CIVO-TNO (report No. R
6636). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon France
Barton, S.J. (1993) Guazatine: Two-generation reproduction study in
rats. Unpublished study from Inveresk Research International (report
No. 7854). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Barton, S.J. & Wilson, I.A. (1988) Guazatine: Teratugenicity study in
rabbits. Unpublished study from Inveresk Research International
(report No. 5344). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
van Beek, L. (1974) Eye irritation test with Panoctine 40 in albino
rabbits. Unpublished study from CIVO-TNO (report No. R4363). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L. (1980) Acute dermal toxicity study with Panoctine plus
300020 in albino rabbits. Unpublished study from CIVO-TNO (report No.
R6703). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L., van Oostrum, E.C.M. & Immel, H.R. (1976) Acute dermal
toxicity of Panoctine 42 in albino rabbits. Unpublished study from
CIVO-TNO (report No. R4921). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Cameron, B.D. & Phillips, M.W.A. (1986) The disposition of guazatine
in the lactating cow. Unpublished study from Inveresk Research
International (report No. 4141 ). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cameron, B.D., Mutch, P.J. & Scott, G. (1989) The metabolism of
[14C]-guazatine in the rat. Unpublished study from Inveresk Research
International (report No. 4826). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & Cart, S.M.A. (1989) Guazatine: Acute dermal
irritation test in rabbits. Unpublished study from Inveresk Research
International (report No. 5505). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & D'Arcy-Burt, K.J. (1986) Guazatine: Acute dermal
toxicity in rats (limit test) Unpublished study from Inveresk Research
International (report No. 3461). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Davis, P.B. (1983a) An investigation into the possible induction of
point mutations at the HGPRT locus of Chinese hamster ovary cells by
GTA70 (guazatine triacetate) Unpublished study from CIVO-TNO (report
No. R83/86). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Davis, P.B. (1983b) An investigation into the possible induction of
sister chromatid exchanges in Chinese hamster ovary cells by GTA 70
(guazatine triacetate). Unpublished study from CIVO-TNO (report No.
R83/85). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976a) Determination of the acute oral toxicity of
Panoctine 42 in cats. Unpublished study from CIVO-TNO. Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976b) Determination of the intraperitoneal toxicity
of Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
21-1-76). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Goburdhun, R. & Carter, P.B. (1989) Guazatine: Oral maximum tolerated
dose study in dogs. Unpublished study from Inveresk Research
International (report No. 5424). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Hazelden, K. (1987) Guazatine: Dose range finding study in rats,
preliminary to teratogenicity study. Unpublished study from Inveresk
Research International (report No. 3441). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Hazelden, K.E & Wilson, J.A. (1986) Guazatine: Teratogenicity study in
rats. Unpublished study from Inveresk Research International (report
No. 3540). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Heath, J., Perry, C.J., Hudson, R, Dobb, D. & Millar, P (1994)
Guazatine 104 week dietary study in rats with 52 week interim kill.
Unpublished study from Inveresk Research International (report No.
11006). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Heath, J., Perry, C.J., Hudson, E & Aitken, R. (1995) Guazatine 104
week dietary study in mice with 52 week interim kill. Unpublished
study from Inveresk Research International (report No. 11084).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Hirano, M., Maita, K., Mitsumori, K. & Shirasu, Y. (1985) 24 Month
chronic toxicity study with guazatine in rats. J. Toxicol. Sci., 10,
266.
Kato, Y., Sato, K., Maki, S., Matano, O. & Goto, K. (1985) Metabolic
fate including deamidination of guazatine triacetate in male rats.
J. Pestic. Sci., 10, 661-675.
Koyama, K., Yamashita, M., Miyauchi, T. & Goto, K. (1993) A fungicide
containing iminoctadine causes circulatory failure in acute oral
poisoning. Vet. Hum. Toxicol., 35, 512.
Koyama, K., Goto, K. & Yamashita, M. (1994) Circulatory failure caused
by a fungicide containing iminoctadine and a surfactant: A
pharmacological analysis in rats. Toxicol Appl. Pharmacol., 126,
197-201.
Kruysse, A. & Immel, H.R. (1976) Acute inhalation toxicity study with
Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
R4965). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Leegwater, D.C. (1975) Study on the fate of [14C, 3H guazatine
preparation in the rat and in sandy loam. Unpublished study from
CIVO-TNO (report No. R4823). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Leegwater, D.C. (1980) Study on the metabolic fate of [14C] Panoctine
preparation in the rat. Unpublished study from CIVO-TNO (report No.
R6571). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Maita, K., Mitsumori, K., Hirano, M. & Shirasu, Y. (1985) 24-Month
chronic toxicity study with guazatine in mice. J. Toxicol. Sci., 10,
267.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Oshodi, R.O. & Thompson, D.C. (1993) Guazatine: 52 Week dietary study
in dogs. Unpublished study from Inveresk Research International
(report No. 7903). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Prout, M.S. (1996) The metabolism of [14C]-guazatine in the rat.
Unpublished study from Inveresk Research International (Supplement 1
to report No. 4826: Cameron et al., 1989). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Reuzel, P.G.J, Til, H.P. & Kollen, C.H. (1976) Long term (two year)
toxicity study with guazatine in beagle dogs. Unpublished study from
CIVO-TNO (report No. R4983). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Sato, K., Kato, Y., Maki, S., Matano, O. & Goto, S. (1986) Metabolic
fate of plant guazatine residues in male rats. J. Pestic. Sci., 11,
267-270.
Sinkeldam, E. H. & van der Heijden, C.A. (1974) Sub-chronic (90-day)
toxicity study with guazatine in albino rats (final report).
Unpublished study from CIVO-TNO (report No. R4354). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Spanjers, M.T & Til, H.P. (1980) Determination of the acute oral
toxicity of acetates of guanidated amines (GTA) in rats. Unpublished
study from CIVO-TNO. Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, H.P. & Feron, V.J. (1975) Feeding study with guazatine in rats
for 13 weeks. Unpublished study from CIVO-TNO (report No. R4870).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P & Hendriksen, C.F.M. (1976) Toxicity study with Panoctine 42
(guazatine) in rats for 13 weeks. Unpublished study from CIVO-TNO
(report No. R5062). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, M.P. & Keizer, A.M.M. (1980) Maximisation test with GTA in guinea
pigs. Unpublished study from CIVO-TNO (report No. R6522). Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Hendriksen, C.F.M. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with guazatine in rats.
Unpublished study from CIVO-TNO (report No. R4985). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Koeter, H.B.W.M., Immel, H.R. & van der Heijden, C.A.
(1976b) Multigeneration study with guazatine in rats. Unpublished
study from CIVO-TNO (report No. R5078). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Willems, M.I. (1983) Examination of GTA in the micronucleus test.
Unpublished study from CIVO-TNO (report No. V83.118/230201). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Wilmer, J.W.G.M. (1983a) Examination of GTA for mutagenic activity in
the Ames test. Unpublished study from CIVO-TNO (report No. V83.
122/230064). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Wilmer, J.W.G.M. (1983b) Chromosome analysis of cultured human
lymphocytes treated in vitro with GTA. Unpublished study from CIVO-TNO
(report No. V83. 235.230573). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
2. Toxicological studies
(a) Acute toxicity
Guazatine is of moderate toxicity when given orally, of low toxicity
when applied dermally, but of moderately high toxicity when
administered by inhalation or intraperitoneally. The results of
studies of the acute toxicity of guazatine are summarized in Table 4.
The clinical signs of toxicity after treatment were lethargy or
sedation, hypothermia, coma, and local irritation. The gross and
histopathological changes were consistent with a response to a local
irritant; them was no clear systemic toxicity.
(b) Short-term toxicity
Mice
In a range-finding study, groups of 10 male and 10 female CD-1 mice
received guazatine (purity, 70.6%) in the diet at 0, 10, 50, 200, or
500 ppm for 13 weeks. Samples were taken for limited blood and
clinical chemical analyses at week 13. A limited range of tissues was
removed, weighed, and examined macroscopically; only the liver was
examined histologically. Two male controls, one male at 500 ppm, and
one at 50 ppm died during the study. Marked reductions in body-weight
gain (> 25%) were seen in animals of each sex at 200 or 500 ppm. In
females at the highest dose, aspartate aminotransferase activity was
increased. The relative liver weights were increased in animals of
each sex at 500 ppm; reductions in the absolute weights of several
organs appeared to be secondary to reduced body-weight gain. Altered
centrilobular hepatocytes were seen in 80% of animals at 200 or 500
ppm. Given the limited extent of the investigations, no NOAEL was
identified (Atkinson et al., 1990).
Table 4. Acute toxicity of guazatine and formulations
Test material Species Route Purity LD50/LC50 Reference
(%) (mg/kg bw or
mg/m3)
Guazatine GTA Rat Oral 69.2 280 Spanjers & Til (1980)
Panoctine 42 Cat Oral NR 0.38 ml/kg bw De Grout (1976a)
Guazatine GTA70 Rat Dermal 70.9 1050 Cuthbert & D'Arcy-Burt (1986)
Panoctine 42 Rabbit Dermal NR 2.8 ml/kg bw van Beck et al. (1976)
Panoctine Plus 300020 Rabbit Dermal NR 2.8-5.6 ml/kg bw van Beck (1980)
Paaoctine 42 Rat Intraperitoneal NR 0.053 ml/kg bw De Groot (1976b)
Guazatine GTA Rat Inhalation 69.2 225 Appelman (1980)
Panoctine 42 Rat Inhalation NR 11 Kruysse & Immel (1976)
Rats
Groups of 10 male and 10 female Wistar-derived rats received guazatine
(54.8% w/w) in the diet at 0, 60, or 200 ppm for 14 weeks.
Haematological and urinary parameters were measured in samples taken
at week 13, and serum enzymes and total protein were measured in
samples taken at termination at week 14. There were no deaths or
adverse clinical signs. Body weight and food conversion efficiency
were unaltered by treatment. A slight, dose-related decrease in
leukocyte count was seen in animals of each sex (up to 13% in males
and 6% in females), but this was not statistically significant, and
there was no marked change in differential counts. Serum alkaline
phosphatase activity was decreased in treated males, by 22% at 200 ppm
and 7% at 60 ppm. Urinary parameters were unaffected by treatment, but
the volume and specific gravity were not given. There were no
significant findings at gross or microscopic examination. The findings
in this study are of minimal toxicological significance; the NOAEL was
200 ppm, equivalent to 10 mg/kg bw per day (Sinkeldam & van der
Heijden, 1974).
Groups of 10 Wistar-derived rats of each sex received guazatine (54.8%
w/w) in the diet at 800 ppm for six weeks and then 1200 ppm for eight
weeks or control diet for 14 weeks. Haematological and urinary
parameters were measured in samples taken at week 13, and serum
enzymes and total protein were measured in samples taken at
termination at week 14. There were no deaths or adverse clinical
signs. Body-weight gain was reduced in animals of each sex by
approximately 6% at week 6 and by 8-10% at termination. Food
efficiency was unaffected in males but reduced in females.
Haematological parameters were unaltered by treatment. In males, serum
aspartate aminotransferase activity was increased and alkaline
phosphatase activity decreased. Females had an increased urine volume
with an associated decrease in specific gravity; the report does not
indicate whether samples were collected under fasting conditions.
Increases in relative adrenal, testis, and heart weights were seen in
treated animals, without abnormal pathological findings. As data on
individual animals and absolute organ weights were not presented, the
significance of these findings is uncertain. In treated females, high
levels of 'iron' deposition in the spleen were reported. The thyroids
of two females given guazatine were increased in weight and contained
small follicles lined with large epithelial cells that had small
apical nuclei. A re-evaluation of salivary gland tissues from this
study, reported very briefly by Til & Hendricksen (1976), described
hyperplasia in the epithelial lining of the excretory ducts of the
parotid salivary glands, with associated mononuclear cell infiltrates
in some treated animals. No NOAEL was identified (Til & Feron, 1975).
Panoctine 42 (purity unspecified) was administered to groups of 10
Wistar-derived rats of each sex at 0 or 1500/2000 ppm in the diet; the
level was increased from 1500 to 2000 ppm after week 4. Samples for
examination by haematology, limited clinical chemistry, and urinalysis
were taken during week 13. Animals were killed at week 14 and examined
grossly and histologically. There were no deaths or adverse clinical
signs. Body-weight gain was reduced by about 10% from week 2 in males
and by about 8% from week 6 in females. Food efficiency was reduced in
males for the first 12 weeks of the study. Serum proteins and alkaline
phosphatase activity were reduced in both treated groups. In treated
females, the leukocyte counts were increased (by 30%), and
differential counts indicated an increase in neutrophils and a
decrease in eosinophils. Increased relative weights of the liver (by
8%) and kidney (by 14%) were seen in treated males, without associated
histological findings. In females, an increased relative weight of the
heart (by 17%) and decreased thyroid weights (by 8%) were reported,
again without associated histological findings. Urinary aspartate
aminotransferase activity, proposed as a measure of renal toxicity,
was increased in treated animals, and urine volume was increased and
specific gravity decreased in animals of each sex. These findings
taken together are indicative of renal toxicity, though no associated
findings were reported at pathological examination. The only
histopathological finding of note was hyperplasia of the epithelial
lining of the excretory ducts of the parotid salivary glands with
associated mononuclear cell infiltrates in six males and six females
in the treated groups and in none of the controls (rho < 0.01). No
NOAEL was identified (Til & Hendricksen, 1976).
Dogs
In a range-finding study in beagle dogs, guazatine (purity, 67.9%)
given at 9.4 mg/kg bw per day by gavage for four days induced
body-weight loss in one of four animals. Subsequent administration of
14 mg/kg bw per day for four days induced marked body-weight loss and
reduced food consumption in all animals. The pathological findings
indicated local irritation of the gastrointestinal tract.
Administration of 374 ppm guazatine in the diet (12-15 mg/kg bw per
day) resulted in reduced food consumption, reduced body-weight gain,
and increased activities of aspartate aminotransferase, alanine
aminotransferase, and lactate dehydrogenase in serum after two weeks
(Goburdhun & Carter, 1989).
Groups of four male and four female beagle dogs received guazatine
(purity, 54.8%) in the diet at levels of 0, 60, 200, or 300/600 ppm
for two years; the level of 300 ppm was increased to 600 ppm after 26
weeks. Extensive observations were made, including haematological,
clinical chemical, and urinary determinations at 12/13, 26, 52, 78,
and 104 weeks, and tests for liver function (bromosulfthalein) and
kidney function (phenol red excretion) at 26, 52, and 104 weeks. Gross
and histopathological examinations were performed at termination at
week 104, although the results of histopathology in individual animals
were not reported. One control male died due to urolithiasis. There
was no evidence of treatment-related changes in clinical signs.
Body-weight gain and food consumption were unaffected by exposure. A
decreased leukocyte count (by 12-25 %) was present in animals at the
high dose, particularly females, from week 13 onwards, achieving
statistical significance at week 52, although there were no marked
alterations in differential counts. Sporadic changes in other
haematological, clinical chemical, and urinary parameters were not
consistent or of statistical significance. The results of liver and
kidney function tests were similar for control and treated groups.
Increases in absolute and relative ovarian weights were seen in
females at the high dose, although the significance of these changes
is unclear as there was considerable inter-animal variation and no
obviously associated histological findings. Given the uncertainties
about the findings at the high dose, the NOAEL was 200 ppm, equivalent
to 5 mg/kg bw per day (Reuzel et al., 1976).
Groups of four beagle dogs of each sex received guazatine (purity,
70.6%) in the diet for 52 weeks. Animals were given 400 g of diet per
day containing 0, 25, 75, or 250 ppm guazatine; the achieved intakes
were about 0, 0.8, 2.5, or 8 mg/kg bw per day. Extensive observations
included ophthalmoscopy at 0, 13, 26, and 51 weeks and haematology,
clinical chemistry, and urinalysis at 0, 6, 13, 26, and 51 weeks.
Animals were killed at week 52 and examined grossly; 13 tissues were
weighed, and more than 40 tissues were investigated by histopathology.
Owing to poor weight gain, one control and two males at the high dose
received extra food, which reversed the condition. In females, there
was a clearly dose-related reduction in body-weight gain starting at
week 10 and continuing throughout the study, with reductions of 30% in
comparison with controls in the group at the high dose, 20% in those
at the intermediate dose, and 12% in those at the low dose. Food
consumption was reduced in females receiving 75 or 250 ppm, with
occasional reductions in those at 25 ppm. Haematology showed no
consistent time- or dose-related reactions to treatment. Marked
increases (up to sixfold) in serum alanine aminotransferase activity
were seen throughout the study in males and females receiving 250 ppm.
Serum aspartate aminotransferase activity was increased in males at
the high dose throughout the study; an apparent increase in alkaline
phosphatase activity in males appears to be related to differences in
pretreatment values. A slight increase in liver weight was noted in
females at the high dose. Reduced prostate weights in males at the low
dose, increased ovarian weights in all treated females, and increased
thyroid weights in females at the intermediate dose did not show
dose-response relationships and were not clearly related to treatment,
although effects on ovarian weights were also see in the study of
Reuzel et al. (1976). Gross and microscopic examination showed no
changes. The reduced body-weight gain of females appeared to be due
only in part to unpalatability, as food conversion efficiency was also
reduced. The NOAEL was 25 ppm, equivalent to 0.8 mg/kg bw per day, on
the basis of marked reductions in body-weight gain in females at 75
and 250 ppm and clear increases in serum enzyme activities, indicative
of effects on the liver, in animals of each sex at 250 ppm (Oshodi &
Thompson, 1993).
(c) Long term toxicity and carcinogenicity
Mice
Groups of 10 male and 10 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for one year; the
achieved doses were 0, 7.6, 19, or 55 mg/kg bw per day in males and 0,
10, 25, or 67 mg/kg bw per day in females. All animals were examined
macroscopically. A wide range of tissues from animals receiving 0 or
300 ppm and those that died before week 52 were examined
histologically; the gall-bladder, kidney, liver, lung, salivary gland,
and grossly abnormal organs from all other animals were also examined
histologically. One male and one female at 120 ppm and two females at
300 ppm died during the study. There were no clinical signs associated
with treatment. Body-weight gain was reduced from week 4 in animals of
each sex at 300 ppm; by week 52, the weight gain of males was 72% that
of controls and that of females 66% of control values. Food
consumption was similar in treated and control animals. The relative
liver weights were increased by about 10% in all treated males. The
only notable finding at gross necropsy was an increased incidence of
ovarian cysts in females receiving 120 or 300 ppm (9/10 versus 6/10 in
controls). Microscopic examination showed increased incidences of
lymphocytic infiltration of the kidney and ovarian cysts in females at
the high dose (the ovaries of those at the intermediate dose were not
examined), of hepatocellular adenoma in males at the high dose (2/10
versus 0/10 in controls), and a decreased incidence of lymphoid foci
of the mandibular salivary gland in females at the intermediate and
high doses. The finding of hepatocellular adenomas may indicate that
guazatine reduces the time to onset of the liver turnouts seen
commonly in mice. Given the small group size, limited examination of
tissues from animals at low and intermediate doses, and clear effects
at 300 ppm, there was no NOAEL (Heath et al., 1995).
In the part of the same study designed to test for carcinogenicity,
groups of 50 male and 50 female CD-1 mice received guazatine (purity,
70.6%) in the diet at 0, 50, 120, or 300 ppm for two years; the
achieved doses were 0, 6.8, 17,or 47 mg/kg bw per day in males and 0,
8.7, 21, or 57 mg/kg bw per day in females. All animals were examined
macroscopically, and a wide range of tissues from animals receiving 0
or 300 ppm or that died before week 104 were examined histologically;
only the gall-bladder, kidney, liver, lung, salivary glands, and
grossly abnormal organs from animals in other groups were examined.
Blood samples for differential leukocyte counts were taken from all
surviving controls and animals at 300 ppm during weeks 52, 78, and
103.
The pattern of deaths was similar in treated and control groups, with
> 50% survival in all groups until week 100 in males and week gg in
females. Reduced body-weight gain was seen at the high dose from week
2 onwards, the deficit being approximately 20% at termination. During
the second year, males at the intermediate dose lost more weight than
controls, while females at this dose gained more weight than controls.
Food consumption, differential leukocyte counts, and findings at gross
necropsy were unaffected by treatment. Increased absolute and relative
weights of the kidney and liver were seen in females at the
intermediate but not the high dose.
The incidences of a range of neoplastic and non-neoplastic lesions
showed evidence of treatment-related effects (Table 5). The incidences
of haemangiosarcoma of the liver in males at the intermediate and high
doses and of the spleen in males at the high dose were higher than
those in historical controls, which were 04% in males and 0-3% in
females for liver and 0-2% in males and 045% in females for spleen.
The high incidence of haemangiosarcoma in females at the low dose is
considered to be a chance finding, as them was no dose-response
relationship. The incidence of hepatocellular carcinoma in females at
the high dose was greater than that in historical controls (0-4%). The
incidences of renal adenoma and carcinoma in male historical controls
were 0-4% and 0-2%, respectively, indicating that the incidence of
renal adenoma in males at the high dose is a significant finding.
The presence of rare tumours, including malignant ones, at multiple
sites was considered by the Meeting to be of concern. The mechanism by
which these tumours are induced is not evident from the available
data. Guazatine is not genotoxic (see below), indicating the mechanism
is probably nongenotoxic, although haemangiosarcomas are generally
associated with a genotoxic mechanism. As them was no evidence of
marked toxic or hyperplastic responses in the affected tissues and
them was evidence of a dose-response relationship, it was not clear
that these tumours are a direct result of disrupted cell function
associated with exposure to doses above the maximum tolerated dose.
Although only a limited range of tissues was examined from animals at
the low and intermediate doses, it included those with the most
significant effects at the high dose. The NOAEL was 50 ppm, equal to
6.8 mg/kg bw per day, on the basis of effects on body-weight gain and
the occurrence of haemangiosarcomas in males at 120 ppm, marked
effects on body-weight gain, and a range of neoplastic and
non-neoplastic changes at 300 ppm (Heath et al., 1995).
The findings of another two-year study with guazatine in mice were
summarized in abstract form only. Guazatine (as iminoctadine) was
given to groups of 80 male and 80 female specific-pathogen-free
ICR-Crj mice at dietary levels of 0,10, 100, or 300 ppm. Eight animals
of each sex at each dose were killed at weeks 26 and 52 for
examination. At 300 ppm (26 mg/kg bw per day in males, 30 mg/kg bw per
day in females), males and females showed a remarkable depression in
body-weight gain, decreased food efficiency, and anaemia at weeks 26
and/or 52, increased plasma urea nitrogen level (only in females),
increased alkaline phosphatase level (at week 26 in animals of each
sex and also at week 52 in females), and increased weights of the
kidney (absolute and relative) and liver (relative). Histological
examination revealed swelling of hepatocytes and proximal tubular
cells in animals of each sex. There were increased incidences of
subcutaneous oedema in females and hydrothorax in males. The
incidences of splenic atrophy in animals of each sex, atrophy and/or
brown pigment deposition of the ovary, and glandular epithelial
atrophy of the Harderian gland in males were significantly increased.
The incidence of renal epithelial tumours increased slightly in males.
No toxic changes were seen at the other doses, except for slightly
Table 5. Lesions (as % of samples examined) seen in CD1 mice exposed to guazatine in the diet
for two years
Lesion Guazatine (ppm)
Males Females
0 50 120 300 0 50 120 300
Brain mineralization 22 26 33 20 8 16 10 22*
Caecal carcinoma 0 0 0 0 0 0 0 2
Renal tubular carcinoma 0 0 0 2 0 0 0 0
Renal tubular adenoma 2 2 2 8 0 0 2 0
Renal glomerular amyloid 12 8 16 10 24 8 8 0*
Hepatocellular carcinoma 22 24 22 22 2 2 2 10
Liver haemangiosarcoma
Multifocal 0 0 2 4 0 0 2 0
Other 2 2 6 6 0 6 2 0
Increase in bronchus-associated
lymphoid tissue 10 12 12 20 4 14 16 28*
Lung lymphoid foci 0 0 2 0 0 0 4 10*
Spleen haemangiosarcoraa 0 0 2 4 2 0 2 4
Vaginal keratinized epithelium - - - - 15 - - 43
-, not determined
* One-tailed p < 0.05, Fisher exact test
increased incidences of swelling of proximal tubular cells in animals
of each sex at 100 ppm (Maita et al., 1985).
Rats
Groups of 60 male and 60 female Wistar-derived rats, in two parallel
series initiated two months apart, received diets containing guazatine
(54% solution) at 0, 20, 60, or 200 ppm for two years. A group
receiving 6 ppm was terminated after a few months, as the results of
other studies indicated that 20 ppm was probably a NOAEL. Blood and
urine samples were taken from 10 fasted animals of each sex at each
dose at various times during the study for haematological, limited
clinical chemical, and urinary analyses. All animals were examined
grossly post mortem. A wide range of organs from surviving animals
in the first series (11-20) were weighed, but only kidney, spleen, and
adrenals from rats in the second series were weighed. A full
histological examination was performed on tissues from 20 rats of each
sex in the control and high-dose groups in the first series, and gross
lesions, tumours, adrenals, thyroid, and pituitaries from all animals
were examined.
Behaviour and clinical signs were reported to be unaffected by
treatment. There were more early deaths (before week 72) among treated
males, but overall survival to week 104 was satisfactory and similar
in all groups. Body weights were similar in all groups until week 88,
when all treated females showed a deficit in comparison with controls,
which lasted until termination. Food consumption and conversion
efficiency were similar in all groups over the first four weeks.
Leukocyte counts were decreased (by about 11%) in males at the high
dose from week 26, with no consistent change in differential counts;
females showed sporadic changes in leukocyte counts with no consistent
pattern. Urinalysis gave similar findings in all groups. Reduced
relative testicular weights (by 11%) were seen in males at the high
dose, although the absolute weights were similar since the mean body
weight was 8% higher. In treated females, the relative weights of the
spleen, brain, and kidney were increased; however, as the absolute
weights were decreased and there was no evidence of a dose-response
relationship, this finding is probably secondary to the effects on
body weight. Occasional increases in the incidences of non-neoplastic
lesions were reported, but the only statistically significant
(p < 0.05) increases were for chronic respiratory disease and mammary
gland inflammation or dilatation in females at the high dose. The
number of tumour-bearing animals and the total numbers of tumours were
similar in all groups. The incidence of monocytic leukaemia was
increased in males and females at the high dose, occurring in four
males and three females, with none in male and in one female controls.
As the total numbers of animals examined is unclear, this may be a
chance finding. Although the study indicates that guazatine has no
marked toxicity, the limited, investigations performed and the unusual
design make interpretation of the findings difficult. No NOAEL was
identified (Til et al., 1976a).
Groups of 20 male and 20 female Sprague Dawley rats received guazatine
(purity, 70.6%) in the diet at 0, 50, 150, or 350 ppm for one year.
The achieved intakes of guazatine were 0, 2.5, 7, or 19 mg/kg bw per
day for males and 0, 3, 9, or 22 mg/kg bw per day for females. Samples
for haematological, clinical chemical, and urinary analyses were
obtained from 10 animals of each sex in each group at weeks 26 and 50
or 51. At week 52, the animals were killed and examined grossly.
Selected tissues were weighed, and samples from a wide range of organs
from controls and animals at the high dose were examined
histologically; gross lesions, kidneys, liver, lung, and salivary
glands from animals at the low and intermediate doses were also
examined histologically.
Behaviour, clinical signs, body-weight gain, food consumption, urinary
parameters, and the findings at gross necropsy were similar in all
groups. None of the controls but one male at the low dose, two each at
the intermediate and high doses, and one female at 150 ppm died. Total
leukocyte and lymphocyte counts were reduced in males at 350 ppm at
both 26 and 51 weeks, but increases were seen in treated females.
Serum alanine aminotransferase activity was decreased markedly (by
>40%) at both sampling times in males and females receiving 350
ppm, but there were occasional deficits in other serum enzyme
activities (particularly aspartate aminotransferase); total protein
was similar in all groups. Dose-related decreases in absolute and
relative prostate weights were significant (rho < 0.05) at both 150
and 350 ppm and were associated with a low incidence of hyperplasia at
350 ppm. Females had dose-related increases in the relative and
absolute weights of the adrenals (by < 10%) and ovary by
(< 50%). Five males at the high dose but none of the controls had
testicular atrophy. Mononuclear-cell infiltration of the parotid
salivary gland was increased in males (6/20 versus 1/20) and females
(5/20 versus 1/20) receiving 350 ppm. The NOAEL was 150 ppm, equal to
7 mg/kg bw per day, on the basis of decreased leukocyte count,
prostatic hyperplasia, decreased alanine aminotransferase activity,
and effects on the salivary gland at 350 ppm. The minimal changes seen
at 150 ppm were not consistent or of sufficient magnitude to be
considered adverse (Heath et al., 1994).
In the phase of the study that addressed carcinogenicity, groups of 50
Sprague Dawley rots of each sex received guazatine (purity, 70.6%) in
the diet at 0, 50, 150, or 350 ppm for two years. The achieved intakes
were about 0, 2.5, 7, and 19 mg/kg bw per day for males and 0, 3, 9,
and 22 mg/kg bw per day for females. Samples for haematological,
clinical chemical, and urinary analyses were obtained from 10 rats of
each sex per group at weeks 53 (for haematology only), 78, and 101 or
103. At week 104, the animals were killed and underwent a full gross
examination. Selected tissues were weighed, and samples from a wide
range of organs from controls and animals at the high dose were
examined histologically; gross lesions, kidneys, liver, lung, and
salivary glands from animals at the low and intermediate doses were
also examined histologically.
Behaviour, clinical signs, and organ weights were similar in all
groups. Survival was acceptable (50% at week 94) and similar in all
groups. Low body weights were seen consistently in females at the high
dose from week 9 and in males at this dose from week 20; in the latter
quarter of the study, females at the low and intermediate doses had
reduced body-weight gain in comparison with controls. Males at the
high dose showed consistent reductions in leukocyte and platelet
counts but increased erythrocyte counts. In females at 350 ppm,
erythrocyte counts were reduced; an increase in leukocyte counts at
termination was due to a neurilemmoma in one animal. Reduced
activities of serum alanine and aspartate aminotransferases (by
< 50%) were seen consistently in animals of each sex receiving 350
ppm. Reduced urinary pH was seen at the high dose in animals of each
sex throughout the study. A slight excess of abnormal findings in
lymph nodes was seen in females at this dose, but no individual effect
was significant when compared with the very low background incidence.
The incidences and severity of a range of lesions in salivary glands,
lymph nodes, ovaries, spleen, and pituitary were increased in females
receiving 350 ppm. In males, the only notable finding was increased
severity and incidence of testicular germinal epithelial degeneration
at 350 ppm. A rare malignant oligodendroglioma was found in the brain
of a single female at the high dose, but there was no evidence of
treatment-related carcinogenicity in any other animal. The NOAEL was
150 ppm, equal to 7 mg/kg bw per day, on the basis of reduced
body-weight gain and a range of clinical chemical and histological
findings at 350 ppm (Heath et al., 1994).
The findings of a two-year study of guazatine in rats were summarized
in abstract form only. Guazatine (as iminoctadine) was presented to
groups of 80 male and 80 female Fischer 344 rats at dietary levels of
0, 10, 100, or 300 ppm for two years. At 6 and 12 months, eight
animals of each sex from each group were killed for examination.
Animals of each sex at 300 ppm (11 mg/kg bw per day for males, 14
mg/kg bw per day for females) had remarkably depressed body-weight
gain, decreased food efficiency, higher mortality rates, a tendency to
anaemia, decreased total protein, and increased kidney and adrenal
weights; decreased potassium and albumin and increased calcium,
aspartate aminotransferase and gamma-glutamyltransferase activities,
and spleen weight were also observed. Histologically, swelling,
degeneration, and necrosis of renal tubular cells and metaplasia in
the glandular stomach were observed in animals of each sex. The
incidence of sperm granuloma in the deferent duct and/or epididymides
was significantly increased, and the incidences of leukaemia in males
and of adrenal phaeochromocytoma in animals of each sex were slightly
increased. At 100 ppm, males had a slightly higher mortality rate, and
females had depressed body-weight gain; decreased potassium and
increased calcium and gamma-glutamyltransferase activity were
occasionally observed in animals of either sex. The kidney and spleen
weights were increased, and the incidences of intestinal metaplasia
and sperm granuloma were significantly higher than in controls. At 10
ppm, no toxic change attributable to guazatine were seen (Hirano et
al., 1985).
(d) Genotoxicity
Guazatine has been tested for its potential to induce gene mutations,
sister chromatid exchange, and chromosomal aberrations in vitro and
micronuclei in vivo. Negative results were obtained in all studies.
The data are summarized in Table 6.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female Wistar-derived rats received diets
containing 0, 60, or 200 ppm guazatine (purity, 54.8%) over four
generations. The reporting of the study lacks the results for
individual animals, dietary analyses, time to mating, length of
gestation, absolute organ weights, and other data. F0 animals
received the test diets for 12 weeks before the first mating to
produce the F1a generation; the second mating eight weeks later
produced the F1b generation. Litters were culled to eight pups on day
1 after birth, and the F1a litters were discarded at weaning. Animals
were selected from the F1b litters at weaning to produce the F2
generation, with mating identical to that for F0 animals, and
similarly for the F3 generation from the F2b litters. Groups of 10
animals of each sex from the F3b litters were selected at weaning and
given test diets for four weeks; they were then killed and subjected
to gross and histopathological examinations.
Treatment had no effect on survival, clinical signs, litter size, sex
ratio, or resorption rates. Minor variations in the body weights of
pups appeared to be secondary to variations in litter size. F3b
animals receiving 60 or 200 ppm had increased, relative kidney
weights (by 8% at 60 ppm and 12-17% at 200 ppm), and increased
relative thymus weights (by 19-34%) were seen in all treated groups. A
dose-related reduction in relative testicular weights (8%) was also
seen. Histological examination gave no evidence of treatment-related
effects in any organ. In a summarily described extension of the study,
it was reported that males in F4b litters given test diets for four
weeks after weaning had reduced relative weights of the testis (9%)
and increased relative weights of the thymus (25%) at both doses, and
females at the high dose had increased relative kidney weights (12%).
Again, no treatment-related histological findings were reported. This
study shows that guazatine does not adversely affect reproductive
outcome in rats. The finding of increased thymus weights may indicate
that it affects the control of thymic growth or involution; such
effects may not be detectable in older animals when thymic involution
is well advanced. Given potential concern about effects on the thymus
at both 60 and 200 ppm, no NOAEL was identified (Til et al., 1976b).
Table 6. Results of genotoxicity assays on guazatine
End-point Test system Concentration/ Purity Results Reference
dose (%)
In vitro
Reverse mutation S. typhimurium 0.6-50 µg/plate 73 Negativea Wilmer (1983a)
TA98, TA 100. Cytotoxic
TA1535. TA1537. at 50 g/plate
TA1538
Reverse mutation S. typhimurium To cytotoxic levels NR Negative Moriya et al.
TA98, TA100, TA1535, (numbers not given) (1983)
TA1537, TA1538, and
E. coli WP2 hcr
Gene mutation Chinese hamster 25-100 nl/ml -S9 NR, Negativea Davis (1983a)
ovary cells (line K1), 50-200 nl/ml +S9 approx. Cytotoxic at
hprt locus 70% highest
concentrations
Sister chromatid Chinese hamster 5-30 nl/ml NR, Negativea Davis (1983b)
exchange ovary cells (line K1) approx. Cytotoxic
70% at > 35 nl/ml
Chromosomal Human 3.7-100 g/ml -S9 73 Negativea Wilmer (1983b)
aberration lymphocytes 14.8-400 g/ml +S9 Cytotoxic
at highest
concentrations
In vivo
Micronucleus Mouse (5/sex at 150 mg/kg bw by 73 Negative Willems (1983)
formation each time) bone gavage in saline No effect on
marrow (approx. 50% of P:N ratio
LD50); 24, 48, and
72h
Table 6 (continued)
NR, not reported; S9, exogenous metabolic activation system from Aroclor 1254-induced rat liver preparations;
P:N, polychromatic:normochromatic erythrocytes
a With and without metabolic activation
The reproductive effects of guazatine (purity, 70.6%) were
investigated in a two-generation (one litter per generation) study in
which groups of 28 young adult CD rats received diets containing
guazatine at 0, 50, 150, or 350 ppm, equal to a minimal intake of 0,
3, 10, or 22 mg/kg bw per day in males and 0, 4, 11, or 25 mg/kg bw
per day in females. Animals were given the treated diet for 10 weeks
before F0 mating (1:1) and continuously until sacrifice. Groups of 24
male and 24 female F1 animals were mated at about 17 weeks; they were
then killed and examined grossly, and reproductive organs and
pituitary, liver, adrenals, and salivary glands were weighed and
prepared for histological examination. Pups that died before weaning
were examined grossly. On day 21, one pup of each sex per litter was
examined grossly, and the liver and salivary glands were removed for
histological examination. All other pups were examined externally.
Treatment had no effect on clinical signs, survival, time to mating,
fertility indices, gestation length, litter size, gestation indices,
or lactation indices. Slight reductions in body-weight gain (5-10%)
were closely related to reductions in food consumption (4-9%). Slight
reductions in survival to day 21 (81-83% versus 87% in controls) and
litter weight at day 21 (about 8%) were seen in F1 pups in all
treated groups; as there was no dose-response relationship and the
effects were of small magnitude and were not seen in the F2
generation, they were considered not to be of biological significance.
Post-mortem examination showed no treatment-related effects; however,
the thymus was not weighed or investigated specifically. The NOAEL was
350 ppm, equal to 22 mg/kg bw per day, the highest dose tested
(Barton, 1993).
(ii) Developmental toxicity
Rats
The developmental effects of guazatine were investigated in animals
selected from the F2b and F3b litters of the study of Til et al
(1976b), described above Groups of five males and 15 females were
selected at weaning and maintained on test diets (0, 60, or 200 ppm
guazatine) until mating (1:3) at week 12; it was not clear whether the
test diets were given during gestation. On day 21 of gestation, the
dams were killed, their reproductive tracts investigated, and pups
examined for gross abnormalities and skeletal defects (with Alizarin
Red S staining); effects on soft tissues were studied only in controls
and rats at 200 ppm (by Wilson sectioning). F3b animals showed a
dose-related reduction in litter size, with 10.7 pups in controls, 10
at 60 ppm, and 8.9 at 200 ppm; at 200 ppm, this reduction was
associated with increased pre- and postimplantational losses in two
dams. Mean fetal weight, and hence litter weight, was reduced at 200
ppm in the F3b-derived group There were no notable visceral effects;
although there was an indication of reduced ossification in treated
animals, this was not consistent with regard to dose, generation, or
site. Reduced litter size was seen mainly in two animals and is not
consistent with the results of the main study. The NOAEL was 200 ppm,
equal to 12 mg/kg bw per day, as guazatine did not directly affect the
developing fetus or the dam (Til et al., 1976b).
In a range-finding study, pregnant Sprague Dawley rats died after
receiving guazatine (purity, 70.9%) at levels of 40, 80, or 120 mg/kg
bw per day by gavage on days 6-16 of gestation. At 20 mg/kg bw per
day, there was no evidence of toxicity. Necropsy of animals at doses
> 40 mg/kg bw per day showed marked irritation of the
gastrointestinal tract (Hazelden, 1987).
Four groups of 27 timed-mated Sprague Dawley rats received guazatine
(purity, 70.9%) in distilled water by garage on days 6-16 of presumed
gestation at doses of 0, 5, 10, or 20 mg/kg bw per day, on the basis
of the findings in the range-finding study. An adequate range of
examinations was performed, and animals were necropsied on day 20
after nitrogen asphyxiation. About two-thirds of the fetuses from each
litter were examined for gross external and visceral abnormalities
before staining with Alizarin Red S for observation of skeletal
abnormalities and variants. The remainder were examined by free-hand
dissection (Wilson technique) for soft-tissue abnormalities. One
control and two animals at the high dose died during the study due to
dosing accidents. There were no signs of maternal toxicity;
body-weight gain was similar in all groups, although food consumption
was decreased marginally (by 1-2%) in rats at 10 and 20 mg/kg bw.
There were no effects on litter size or fetal weight, or on soft
tissues or viscera. There was an indication of slightly retarded
development of some bones (e.g. scapula and pectoral girdle) at 10 and
20 mg/kg bw, but the findings were not significant, and the overall
degree of skeletal ossification was unaffected by treatment. The lack
of maternal toxicity at the high dose may be considered to have
compromised the study, but use of 20 mg/kg bw per day is considered
acceptable in view of the 40% mortality rate at 40 mg/kg bw per day in
the range-finding study. The NOAEL for maternal toxicity,
fetotoxicity, and teratogenicity was 20 mg/kg bw per day, the highest
dose tested (Hazelden & Wilson, 1986).
Rabbits
Groups of 15 timed-mated New Zealand white rabbits received guazatine
(purity, 67.9%) in distilled water by gavage on days 6-18 of presumed
gestation at doses of 0, 2.8. 5.6, or 11 mg/kg bw per day. An adequate
range of examinations was performed, and necropsy was carried out on
day 29. About two-thirds of fetuses from each litter were examined for
gross external and visceral abnormalities before staining with
Alizarin Red S for observation of skeletal abnormalities and variants.
The remainder were examined by free-hand dissection for soft-tissue
abnormalities. After marked weight loss, one animal at each dose was
killed during the study. One animal at the high dose aborted. Reduced
body-weight gain (76% of control weight) and reduced food consumption
(87% of control) were seen during treatment with 11 mg/kg bw per day.
During days 12-18 of gestation, reduced body-weight gain (87% of
controls) was seen at 5.6 mg/kg bw per day; them was no associated
effect on food consumption. Body-weight gain was similar in all groups
between days 18 and 29. Slight reductions in fetal weight were evident
at 5.6 mg/kg bw per day (by 3%) and 11 mg/kg bw per day (5%), but
these findings may have been secondary to the reduced body-weight
gains of the dams. There were no effects on litter size, fetal
viability, external abnormalities, skeletal abnormalities or variants,
extent of ossification, or soft-tissue abnormalities. The overall
NOAEL was 5.6 mg/kg bw per day on the basis of the markedly decreased
body-weight gain in dams at 11 mg/kg bw per day. There was no evidence
that guazatine is fetotoxic or teratogenic to rabbits (Barton &
Wilson, 1988).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
Guazatine GTA70 (purity unspecified) was severely irritating when
applied to rabbit skin for 4 h under occlusive conditions. The lesions
became more pronounced with time, and severe erythema and moderate
oedema were seen at the end of the study on day 7 (Cuthbert & Carr,
1989).
A 40% guazatine formulation induced ocular lesions that worsened with
time. A 10% solution of the formulation was only slightly irritating
to rabbit eyes (van Beek, 1974).
In a Magnusson and Kligmann maximization protocol, guazatine GTA70
(purity, 69.2%) did not have sensitizing potential in guinea-pigs at
induction concentrations of 0.5% intradermally or 1% topically and a
challenge concentration of 0.5% (Til & Keizer, 1980).
3. Observations in humans
Koyama et al. (1993) reported a case of attempted suicide with a
formulation containing 25% iminoctadine and 5%
polyoxyethylenealkylether (Befran). The patient was admitted with
severe cyanosis and in a stuporous state. Blood pressure and pulse
could not be measured (the radial arteries were not palpable) until
noradrenaline (1 mg) was administered.
[The authors cited a similar case and some experimental work involving
intravenous administration to dogs, which indicated that severe
hypotension was produced by iminoctadine. Subsequent work (Koyama et
al., 1994) on anaesthetized rats showed that iminoctadine administered
intravenously induced marked (> 10%) tachycardia and hypotension at
doses > 0.05 mg/kg bw in vivo. Experiments on isolated rat aorta
and atria in vitro showed that the primary effect of iminoctadine
was vasodilatory.]
Comments
Guazatine is a preparation of the triacetates of dimeric and trimeric
guanidated 1,8-diamino-octane, which also contains a range of
oligomers and reaction products. The Meeting was concerned that the
production controls and specifications for guazatine were inadequate.
The quoted purity of 70% is based on normalization to a component
which comprises approximately 1.5 % of the mixture and provides no
control over the levels of the other components. Some data were
provided to show that the composition of the batch used in the key
toxicity studies was similar to that of other batches produced at the
same time, 1990-1991; however, there were no data to confirm that the
batches used in the studies of toxicity were representative of those
currently produced.
Some components of guazatine were absorbed by rats to a limited extent
after oral administration of 14C-labelled compound and then excreted
rapidly. Within 24 h, faecal elimination represented 85-94% of the
dose, with 3-6% in the urine and < 1% in exhaled air. The highest
levels of radiolabel were found in the kidney and liver; there was
evidence that the salivary, pituitary, and thyroid glands may also
contain significant amounts of residue. A study involving treatment
with 14 doses of 2 mg/kg bw showed limited potential accumulation in
the liver and kidney. The results of a study by intravenous injection
showed that some components of guazatine may be secreted back into the
gastrointestinal tract via the stomach and salivary glands. The
metabolism of guazatine has not been fully characterized, but
monodeamidination and dideamidination play significant roles
in vivo.
Guazatine produces severe local irritation, and single oral doses are
of moderate toxicity, with an oral LD50 value in rats of 280 mg/kg
bw. WHO has classified guazatine as moderately hazardous (WHO, 1996).
In a number of short-term studies in rats, guazatine was administered
at doses of 0, 60, 200, 800/1200, or 1500/2000 ppm in the diet for 14
weeks. At doses of 60 and 200 ppm, the activity of serum alkaline
phosphatase was slightly decreased, but no significant changes were
seen in body-weight gain or in the results of pathological,
haematological, or urinary examinations. At doses of 800 ppm and
above, decreased body-weight gain, increased activities of alanine and
aspartate aminotranferases, and decreased activity of alkaline
phosphatase were found, together with pathological changes such as
local irritation of the gut and hyperplasia of the epithelia of the
excretory ducts of the parotid gland with mononuclear-cell
infiltration. Increased weights of the kidney, liver, and heart were
seen without associated histopathological changes. The overall NOAEL
was 200 ppm, equivalent to 10 mg/kg bw per day.
In a 13-week range-finding study, mice received guazatine at 0, 10,
50, 200, or 500 ppm. Significantly reduced body-weight gain was seen
in animals of each sex at 200 ppm and above. Increased liver weights
and alterations in centrilobular hepatocytes were seen in both males
and females at 500 ppm. Alterations in erythrocyte parameters were
seen in animals at doses of 200 ppm and above. Although no significant
effects were reported at 10 or 50 ppm, in view of limited histological
investigations in the study there was no NOAEL.
In a one-year study in dogs, guazatine was administered at 0, 25, 75,
or 250 ppm. Reduced body-weight gain in females, increased alanine
aminotransferase activity in animals of each sex, and increased
aspartate aminotransferase activity in males were observed at a
dietary concentration of 250 ppm. In females at 75 ppm, body-weight
gain was reduced. The NOAEL was 25 ppm, equal to 0.8 mg/kg bw per day.
Guazatine was not carcinogenic in two two-year studies in rats given
doses of 0, 20, 60, or 200 ppm or 0, 50, 150, or 350 ppm. The
non-neoplastic findings included reduced serum alanine and aspartate
aminotranferases activities, salivary gland hyperplasia, and
testicular atrophy at 350 ppm. In a two-year study of iminoctadine
administered at 0, 10, 100, or 300 ppm, there was no reported increase
in tumour incidence. The overall NOAEL was 150 ppm, equal to 7 mg/kg
bw per day.
In a study of carcinogenicity in mice, the animals received 0, 50,
120, or 300 ppm guazatine. The incidences of malignant tumours were
increased at 120 and 300 ppm: haemangiosarcoma of the liver and spleen
was seen in males at 120 and 300 ppm and hepatocellular carcinoma in
females at 300 ppm. The incidence of renal-tubular tumours (adenoma
and carcinoma) was increased in males receiving 300 ppm. These are
rare tumour types in the mouse strain that was used, normally being
seen in only 0-6% of animals. Although the absolute incidences of
these tumours in guazatine-treated animals were low and not
statistically significant, they were clearly greater than those in
historical controls. No convincing information was available on the
underlying mechanism of tumour production. The non-neoplastic effects
seen in this study were increased incidences of lymphoid foci in the
lung, bronchiole-associated lymphoid tissue, keratinized vaginal
epithelium, and brain mineralization in females receiving 300 ppm.
Body-weight gain was reduced by approximately 20% in animals of each
sex receiving 300 ppm. In addition, an abstract describing a study on
iminoctadine (at 0, 10, 100, or 300 ppm) reported a slight increase in
the incidence of renal epithelial tumours in male mice receiving 300
ppm. The Meeting considered that the production of rare malignant
tumours by an unknown mechanism is of great concern. The overall NOAEL
for long-term administration to mice was 50 ppm, equal to 6.8 mg/kg bw
per day, on the basis of increases in the incidence of
haemangiosarcoma in males at 120 ppm, equal to 17 mg/kg bw day.
Guazatine has been tested in an adequate battery of assays for
genotoxicity. The Meeting concluded that it is not genotoxic.
In a multigeneration study of reproductive toxicity in rats receiving
guazatine at 0, 60, or 200 ppm, no significant effects were seen at
the highest dose, equivalent to 12 mg/kg bw per day. In a
two-generation study of reproductive toxicity in rats, guazatine
administered at 0, 50, 150, or 350 ppm did not affect reproductive
performance at the highest dose, equal to 22 mg/kg bw per day.
In a study of developmental toxicity in rats, guazatine was
administered at 0, 5, 10, or 20 mg/kg bw per day. The NOAEL for
maternal toxicity, teratogenicity, and fetotoxicity was 20 mg/kg bw
per day, the highest dose tested. In a range-finding study,
significant mortality was seen at 40 mg/kg bw per day.
In a study of developmental toxicity in rabbits, guazatine was
administered at 0, 2.8, 5.6, or 11 mg/kg bw per day. There were no
signs of fetotoxicity or teratogenicity at the highest dose. The NOAEL
was 5.6 mg/kg bw per day on the basis of marked decreases in maternal
body-weight gain.
The Meeting considered that it could not establish an ADI for
guazatine owing to the inadequate information on its composition and
concerns about the production of rare malignant tumours in mice.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 150 ppm, equal to 7 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
350 ppm, equal to 22 mg/kg bw per day (highest dose
tested in a two-generation study of reproductive
toxicity)
20 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Dog: 25 ppm, equal to 0.8 mg/kg bw per day (one-year study
of toxicity)
Studies that would provide information necessary for continued
evaluation of the compound
1. Data on the levels of individual components in batches of
guazatine from recent production runs
2. Investigation of the mechanism of tumour production in mice
3. Clarification of the extent of absorption, excretion, and
metabolism of all components of guazatine
4. Clarification as to whether the stated doses used in the studies
of toxicity were expressed as free base or triacetate
References
Atkinson, C., Perry, C.J., Hudson, P. & Robb, D.T (1990) Guazatine 13
week dietary dose range finding study in mice. Unpublished study from
Inveresk Research International (report No. 7552). Submitted to WHO by
Rhône Poulenc Secteur Agro, Lyon, France.
Appelman, L.M. (1980) Acute inhalation toxicity of guazatine
triacetate in rats. Unpublished study from CIVO-TNO (report No. R
6636). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon France
Barton, S.J. (1993) Guazatine: Two-generation reproduction study in
rats. Unpublished study from Inveresk Research International (report
No. 7854). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Barton, S.J. & Wilson, I.A. (1988) Guazatine: Teratugenicity study in
rabbits. Unpublished study from Inveresk Research International
(report No. 5344). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
van Beek, L. (1974) Eye irritation test with Panoctine 40 in albino
rabbits. Unpublished study from CIVO-TNO (report No. R4363). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L. (1980) Acute dermal toxicity study with Panoctine plus
300020 in albino rabbits. Unpublished study from CIVO-TNO (report No.
R6703). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
van Beek, L., van Oostrum, E.C.M. & Immel, H.R. (1976) Acute dermal
toxicity of Panoctine 42 in albino rabbits. Unpublished study from
CIVO-TNO (report No. R4921). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Cameron, B.D. & Phillips, M.W.A. (1986) The disposition of guazatine
in the lactating cow. Unpublished study from Inveresk Research
International (report No. 4141 ). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cameron, B.D., Mutch, P.J. & Scott, G. (1989) The metabolism of
[14C]-guazatine in the rat. Unpublished study from Inveresk Research
International (report No. 4826). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & Cart, S.M.A. (1989) Guazatine: Acute dermal
irritation test in rabbits. Unpublished study from Inveresk Research
International (report No. 5505). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Cuthbert, J.A. & D'Arcy-Burt, K.J. (1986) Guazatine: Acute dermal
toxicity in rats (limit test) Unpublished study from Inveresk Research
International (report No. 3461). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Davis, P.B. (1983a) An investigation into the possible induction of
point mutations at the HGPRT locus of Chinese hamster ovary cells by
GTA70 (guazatine triacetate) Unpublished study from CIVO-TNO (report
No. R83/86). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Davis, P.B. (1983b) An investigation into the possible induction of
sister chromatid exchanges in Chinese hamster ovary cells by GTA 70
(guazatine triacetate). Unpublished study from CIVO-TNO (report No.
R83/85). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976a) Determination of the acute oral toxicity of
Panoctine 42 in cats. Unpublished study from CIVO-TNO. Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
De Groot, A.P. (1976b) Determination of the intraperitoneal toxicity
of Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
21-1-76). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Goburdhun, R. & Carter, P.B. (1989) Guazatine: Oral maximum tolerated
dose study in dogs. Unpublished study from Inveresk Research
International (report No. 5424). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
Hazelden, K. (1987) Guazatine: Dose range finding study in rats,
preliminary to teratogenicity study. Unpublished study from Inveresk
Research International (report No. 3441). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Hazelden, K.E & Wilson, J.A. (1986) Guazatine: Teratogenicity study in
rats. Unpublished study from Inveresk Research International (report
No. 3540). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Heath, J., Perry, C.J., Hudson, R, Dobb, D. & Millar, P (1994)
Guazatine 104 week dietary study in rats with 52 week interim kill.
Unpublished study from Inveresk Research International (report No.
11006). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Heath, J., Perry, C.J., Hudson, E & Aitken, R. (1995) Guazatine 104
week dietary study in mice with 52 week interim kill. Unpublished
study from Inveresk Research International (report No. 11084).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Hirano, M., Maita, K., Mitsumori, K. & Shirasu, Y. (1985) 24 Month
chronic toxicity study with guazatine in rats. J. Toxicol. Sci., 10,
266.
Kato, Y., Sato, K., Maki, S., Matano, O. & Goto, K. (1985) Metabolic
fate including deamidination of guazatine triacetate in male rats.
J. Pestic. Sci., 10, 661-675.
Koyama, K., Yamashita, M., Miyauchi, T. & Goto, K. (1993) A fungicide
containing iminoctadine causes circulatory failure in acute oral
poisoning. Vet. Hum. Toxicol., 35, 512.
Koyama, K., Goto, K. & Yamashita, M. (1994) Circulatory failure caused
by a fungicide containing iminoctadine and a surfactant: A
pharmacological analysis in rats. Toxicol Appl. Pharmacol., 126,
197-201.
Kruysse, A. & Immel, H.R. (1976) Acute inhalation toxicity study with
Panoctine 42 in rats. Unpublished study from CIVO-TNO (report No.
R4965). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Leegwater, D.C. (1975) Study on the fate of [14C, 3H guazatine
preparation in the rat and in sandy loam. Unpublished study from
CIVO-TNO (report No. R4823). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Leegwater, D.C. (1980) Study on the metabolic fate of [14C] Panoctine
preparation in the rat. Unpublished study from CIVO-TNO (report No.
R6571). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Maita, K., Mitsumori, K., Hirano, M. & Shirasu, Y. (1985) 24-Month
chronic toxicity study with guazatine in mice. J. Toxicol. Sci., 10,
267.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Oshodi, R.O. & Thompson, D.C. (1993) Guazatine: 52 Week dietary study
in dogs. Unpublished study from Inveresk Research International
(report No. 7903). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Prout, M.S. (1996) The metabolism of [14C]-guazatine in the rat.
Unpublished study from Inveresk Research International (Supplement 1
to report No. 4826: Cameron et al., 1989). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
Reuzel, P.G.J, Til, H.P. & Kollen, C.H. (1976) Long term (two year)
toxicity study with guazatine in beagle dogs. Unpublished study from
CIVO-TNO (report No. R4983). Submitted to WHO by Rhône Poulenc Secteur
Agro, Lyon, France.
Sato, K., Kato, Y., Maki, S., Matano, O. & Goto, S. (1986) Metabolic
fate of plant guazatine residues in male rats. J. Pestic. Sci., 11,
267-270.
Sinkeldam, E. H. & van der Heijden, C.A. (1974) Sub-chronic (90-day)
toxicity study with guazatine in albino rats (final report).
Unpublished study from CIVO-TNO (report No. R4354). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Spanjers, M.T & Til, H.P. (1980) Determination of the acute oral
toxicity of acetates of guanidated amines (GTA) in rats. Unpublished
study from CIVO-TNO. Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, H.P. & Feron, V.J. (1975) Feeding study with guazatine in rats
for 13 weeks. Unpublished study from CIVO-TNO (report No. R4870).
Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P & Hendriksen, C.F.M. (1976) Toxicity study with Panoctine 42
(guazatine) in rats for 13 weeks. Unpublished study from CIVO-TNO
(report No. R5062). Submitted to WHO by Rhône Poulenc Secteur Agro,
Lyon, France.
Til, M.P. & Keizer, A.M.M. (1980) Maximisation test with GTA in guinea
pigs. Unpublished study from CIVO-TNO (report No. R6522). Submitted to
WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Hendriksen, C.F.M. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with guazatine in rats.
Unpublished study from CIVO-TNO (report No. R4985). Submitted to WHO
by Rhône Poulenc Secteur Agro, Lyon, France.
Til, H.P., Koeter, H.B.W.M., Immel, H.R. & van der Heijden, C.A.
(1976b) Multigeneration study with guazatine in rats. Unpublished
study from CIVO-TNO (report No. R5078). Submitted to WHO by Rhône
Poulenc Secteur Agro, Lyon, France.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Willems, M.I. (1983) Examination of GTA in the micronucleus test.
Unpublished study from CIVO-TNO (report No. V83.118/230201). Submitted
to WHO by Rhône Poulenc Secteur Agro, Lyon, France.
Wilmer, J.W.G.M. (1983a) Examination of GTA for mutagenic activity in
the Ames test. Unpublished study from CIVO-TNO (report No. V83.
122/230064). Submitted to WHO by Rhône Poulenc Secteur Agro, Lyon,
France.
Wilmer, J.W.G.M. (1983b) Chromosome analysis of cultured human
lymphocytes treated in vitro with GTA. Unpublished study from CIVO-TNO
(report No. V83. 235.230573). Submitted to WHO by Rhône Poulenc
Secteur Agro, Lyon, France.
