
ETHOXYQUIN JMPR 1998
First draft prepared by
I. Dewhurst
Pesticides Safety Directorate, Ministry of Agriculture,
Fisheries and Food,
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical
parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Ethoxyquin was previously evaluated by the Joint Meeting in 1969
(Annex 1, reference 12), when an ADI of 0-0.06 mg/kg bw was
established on the basis of the NOAELs in a long-term feeding study in
dogs and a study of reproductive toxicity in rats. The compound was
reviewed at the present meeting within the CCPR periodic review
programme. This monograph summarizes new data and data not previously
reviewed on ethoxyquin and relevant data from the previous monograph
(Annex 1, reference 13).
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Data from the 1950s were summarized briefly in the 1969 JMPR
monograph (Annex 1, reference 13). In pretreated rats, equal amounts
of a 1.5-mg dose were excreted in urine and faeces, with a total of
64% excreted in 48 h; about 1% of the administered radiolabel was
exhaled as carbon dioxide. The liver, kidneys, fat, and skeletal
muscle contained the highest concentrations of residues (1-5 mg/kg).
Newborn rats had tissue concentrations of 0.12-0.21 mg/kg, indicating
some placental transfer; similar concentrations were found in rat
milk, but maternal tissue concentrations were not available for
comparison. In dogs, excretion occurred primarily in the urine as
unidentified metabolites. In chickens, 99% of the radiolabel from an
oral dose of ethoxyquin was excreted as metabolites within 48 h; after
12 weeks of administration of about 130 ppm in the diet, the tissue
concentrations were low (0.1 mg/kg) and declined rapidly after
withdrawal of the treated diet.
The absorption, distribution, and excretion of ethoxyquin were
investigated in groups of three male Fischer 344 rats and B6C3F1
mice, approximately eight weeks old, which received single doses of
ethoxyquin (purity, 90%) containing [3-14C]ethoxyquin (purity, 96%)
by gavage at 2.5 (rats only), 25, or 250 mg/kg bw or single
intravenous doses of 25 mg/kg bw. The substance was administered in a
1:1:8 mixture of ethanol:emulphor EL620:water at a volume of 1 ml/kg
bw, equivalent to 25-50 µCi/kg. Urine, faeces, and air were sampled at
various times between 4 and 72 h. The concentrations in liver, kidney,
blood, muscle, skin, and adipose tissue were determined by sequential
kills between 0.25 and 72 h. Additional groups of four rats were used
to determine blood and plasma concentrations in the jugular vein
0.08-24 h after a dose of 25 mg/kg bw and to determine tissue, urine,
and faecal concentrations after six doses of 25 or 250 mg/kg bw.
Radiolabel was determined by liquid scintillation counting; faecal
samples were first powdered and combusted. The concentrations of
parent ethoxyquin in the samples were determined by high-performance
liquid chromatography (HPLC).
The disposition of ethoxyquin was similar when administered
orally and intravenously. It was rapidly absorbed, with peak blood and
tissue concentrations within 1 h. Excretion of the oral doses of 2.5
and 25 mg/kg bw was extensive (> 85% within 24 h) and approximately
1.5-fold greater via urine than faeces. The tissue concentrations at
24 h were < 2% of the administered dose (Table 1). Little difference
was seen with dose in rats, although the higher dose was excreted more
slowly than the lower doses, attributed by the authors to delayed
gastric emptying, and there was evidence of significant adipose
deposition. The results after three or four repeated doses of 250
mg/kg bw per day were reported to be similar to those after repeated
and single dosing at 25 mg/kg bw, indicating induction of metabolizing
enzymes and/or a return to normal gastric emptying (data not presented
in the published report). The rate of excretion by mice was slightly
more rapid than in rats. As parent ethoxyquin was not detectable in
plasma at most times, the authors did not calculate its overall
bioavailability. About 60% of the radiolabel in blood was in the
plasma, and 8% was associated with precipitating plasma proteins.
Repeated administration at 25 mg/kg bw per day and, to a lesser
extent, 250 mg/kg bw per day to rats was followed by some evidence of
bioaccumulation (data not presented) but not in muscle.
Table 1. Tissue distribution at 24 h and excretion over 0-24 h as percent of 14C-ethoxyquin
administered orally or intravenously (i.v.)
Species Dose Blood Liver Kidney Muscle Skin Adipose Urine Faeces
(mg/kg bw) tissue
Rat 2.5 (oral) 0.7 1.4 0.3 0.4 0.3 0.9 57 31
25 (oral) 1 1.3 0.2 0.7 0.4 1.7 64 26
250 (oral) 0.9 1.6 0.2 1.8 1.2 12 41 11
25 (i.v.) 1 1.5 0.2 1 0.7 6.4 57 23
Mouse 2.5 (oral) 0.4 1.2 0.1 0.4 0.7 0.6 60 42
250 (oral) 0.3 1 0.2 1.2 1.2 2.2 43 16
25 (i.v.) 0.5 1.1 0.2 0.9 1.2 0.9 58 33
From Sanders et al. (1996)
Means of three to six animals
After intravenous administration, the highest initial tissue
concentrations were found in liver and kidney, although in mice a
transiently high concentration was seen in adipose tissue at about 2 h
(Table 2). A significant proportion (> 20%) of the intravenous dose
was excreted in the faeces in both species (Table 1), and 40% of the
administered dose was found in the bile of bile-cannulated rats,
indicating that biliary excretion and enterohepatic circulation play a
significant role in the toxicokinetics of ethoxyquin. Parent
ethoxyquin was not detected in urine and was present in only trace
amounts in faeces, liver, kidney, and adipose tissue. The elimination
half-life in plasma for parent ethoxyquin was calculated to be 23 min
(Sanders et al., 1996).
Table 2. Tissue concentrations of 14C at different times after an intravenous
dose of 14C-ethoxyquin at 25 mg/kg bw as microgram equivalent per gram
Species Time (h) Blood Liver Kidney Muscle Skin Adipose
tissue
Rat 0.25 6 66 51 9 15 29
2 5 27 21 2 10 29
12 2 12 11 < 1 3 24
24 3 9 10 < 1 1 15
Mouse 0.25 10 45 40 11 27 40
2 4 27 17 3 16 67
12 2 9 8 < 1 3 22
24 2 5 3 < 1 2 2
From Sanders et al. (1996)
Means for three animals
(b) Biotransformation
Data summarized in the 1969 monograph (Annex 1, reference 13)
indicate that the metabolism of ethoxyquin is extensive in rats, dogs,
and chickens, although the metabolites were not identified.
Samples of urine, faeces, and tissues were obtained from rats and
mice given [3-14C]ethoxyquin at 25 or 250 mg/kg bw orally or 25 mg/kg
bw intravenously in the study of Sanders et al. (1996), described
above. Urine and bile samples were stored frozen, defrosted, and
centrifuged before separation by HPLC; liver, kidney, and faecal
samples were extracted three times with 1:1:1 water:methanol:ethyl
acetate, and the supernatants were separated by HPLC. Plasma samples
were mixed 1:1 with acetonitrile prior to centrifugation and
investigation by HPLC. The effects of incubation with glucuronidase
containing arylsulfatase activity were also studied. Metabolites were
investigated by HPLC, 1H-nuclear magnetic resonance spectroscopy, and
three types of mass spectroscopy, including comparison with results
for synthesized reference compounds.
Eight metabolites were detected in urine, although only four were
characterized (Table 3, Figure 1); no parent ethoxyquin was reported.
The main metabolic pathway in both rats and mice seems to involve
O-deethylation at C-6 followed by conjugation at C-6 with sulfate
(metabolite G) or glucuronide (metabolite F). Subsidiary pathways of
hydroxylation and glucuronidation at C-8 (metabolite H) or
O-deethylation at C-6 and epoxidation between C-3,4 with sulfation
at C-6 are also indicated. The major difference between rats and mice
was the higher levels of glucuronidation in the latter. No significant
difference was reported in the metabolite profiles of rats dosed with
25 mg/kg bw orally or intravenously. Administration of ethoxyquin at
250 mg/kg bw resulted in a greater proportion of radiolabel on the C-6
sulfate (metabolite G) than after dosing with 25 mg/kg bw (Table 3).
Six doses of 25 mg/kg bw resulted in a urinary metabolite profile
similar to that after a single dose. Six doses of 250 mg/kg bw
resulted in greater proportions of the glucuronide metabolites F and H
and smaller proportions of metabolites G and E than after a single
dose, indicating that sulfation may have been saturated or
glucuronidation reactions induced. In the kidney and liver, the major
metabolite was G. Faecal samples could not be satisfactorily extracted
(< 30% recovery), and no reliable conclusions could be drawn. In the
bile, three glutathione conjugates were detected, and < 5% of the
radiolabel was present as the parent; this finding is cited as
contrasting with the results of other workers, who had reported that
most of the biliary radiolabel was present as ethoxyquin. The authors
proposed a reaction scheme for the biliary metabolites (Figure 1)
which involves production of reactive electrophilic intermediates
(epoxides) (Burka et al., 1996).
(c) Effects on enzymes and other biochemical parameters
Administration of ethoxyquin (purity unspecified) to male
Sprague-Dawley rats at 5000 ppm in the diet for three days
significantly induced both phase-1 and phase-2 xenobiotic metabolizing
enzymes. Northern blotting of liver preparations for mRNA of
cytochrome P450 (CYP) isozymes showed increasing amounts of CYP2B1 >
2B2 > 3A2 > 1A2; assays for enzyme activity showed a twofold
increase in the specific activity of CYP1A2 and a 10-fold increase in
that of the CYP2B family. Blotting with probes for glutathione
S-transferase mRNA showed that ethoxyquin increased Ya1, Ya2, and
Yb1, with an approximate doubling of the activity of cytosolic
glutathione S-transferase. Enzyme assays and mRNA blotting also
showed increases in NADPH-quinone oxidoreductase, gamma-
glutamylcysteine synthetase, and UDP-glucuronosyl transferase
activities. Ethoxyquin did not alter cellular glutathione
concentrations or induce CYP1A1 (Buetler et al., 1995).
Table 3. Metabolic profile of 14C-ethoxyquin administered by oral
gavage to rats, as percent of total radioactivity in 24-h urine sample
Metabolitea Dose (mg/kg bw)
1 × 25 6 × 25 1 × 250 6 × 250
A 6 7 4 9
B 6 5 4 7
C 9 8 5 3
D 7 6 2 < 1
E 17 12 10 6
F 5 6 3 15
G 34 42 59 30
H 3 4 4 14
Parent < 1 < 1 < 1 < 1
From Burka et al. (1996)
a See Figure 1 for structures
Administration of diets containing ethoxyquin (purity
unspecified) at 50, 100, 500, 2000, or 5000 ppm to male Sprague-Dawley
rats for 14 days induced a range of effects on xenobiotic metabolizing
systems. The liver:body weight ratios were increased at 5000 ppm: and
total cytochromes P450 and b5 concentrations were increased by 30% at
2000 and 5000 ppm. Analysis of the CO-reduced microsomal ultraviolet
spectra showed that ethoxyquin-treated animals had a lambdamax of
449.5 nm, indicating a phenobarbital-type induction pattern rather
than a methylcholanthrene-type (lambdamax, 448 nm). Monooxygenase
activity in microsomes from rats receiving ethoxyquin in the diet at
5000 ppm was increased by 1.5 to 2-fold and epoxide hydratase activity
by threefold when styrene oxide was the substrate, but the activities
were slightly lower when benzo [a]pyrene was used as the substrate.
Assays in vitro with microsomes from animals induced with
phenobarbital or methylcholanthrene showed that ethoxyquin inhibited
arylhydrocarbon hydroxylase activity at concentrations of 5 µmol/L and
higher. Animals receiving both ethoxyquin-treated diet (5000 ppm) and
methylcholanthrene (three intraperitoneal injections of 20 mg/kg bw)
showed no evidence of additive induction of drug metabolizing systems.
The NOAEL for changes in xenobiotic metabolizing enzyme systems was
500 ppm, equivalent to 25 mg/kg bw per day (Kahl & Netter, 1977).
2. Toxicological studies
(a) Acute toxicity
Ethoxyquin had little acute toxicity, except when administered
parenterally (Table 4). The clinical signs of toxicity after exposure
to ethoxyquin were tremors, ataxia, hypoactivity, hypothermia, and
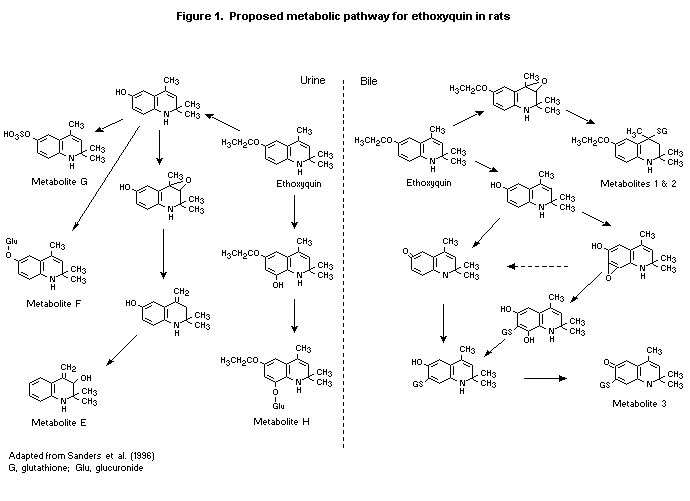 Table 4. Acute toxicity of ethoxyquin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Rat Oral gavage 1700 97.6 Varsho (1995a)
Rat Dermal (24 h) > 2000 97.6 Varsho (1995b)
Rat Inhalation, whole body > 2.0 97.6 Ulrich (1996)
Mouse Intraperitoneal ~ 900 Wilson & DeEds (1959)a
Mouse Intravenous ~ 180 Wilson & DeEds (1959)a
a Cited in 1969 JMPR monograph
red-yellow staining of the fur. Gross and histopathological changes
indicated an irritant effect on the gastrointestinal tract.
Ethoxyquin produced transient, slight erythema when applied to
rabbit skin for 4 h under semi-occlusive conditions. There was no
oedema, but desquamation was present seven days after exposure
(Varsho, 1995c). This result is consistent with the findings of a
study summarized in the 1969 monograph (Kelly, 1960; cited in 1969
JMPR monograph; Annex 1, reference 13).
Ethoxyquin produced transient, slight-to-mild conjunctival
redness and chemosis in rabbits. All of the effects had fully
regressed within four days (Varsho, 1995d).
In a sensitization study in six guinea-pigs of each sex,
ethoxyquin was at most a very weak skin sensitizer. Induction was at
100%, with challenge applications at 50% in acetone. Very weak
erythematous responses were seen in both treated and control groups
after challenge and re-challenge, one test animal producing a weak
response to both challenge and re-challenge (Varsho, 1995e).
(b) Short-term studies of toxicity
Rats
Two studies of dietary administration of ethoxyquin to rats for
200 days were summarized in the 1969 monograph. Kidney lesions
(unspecified) were reported at 500 ppm and higher in males, with
increased kidney:body weight ratios in males at 250 ppm and higher.
The frequency of cytoplasmic inclusions in hepatocytes was increased
at 2000 ppm (Wilson & DeEds, 1959; Cox, 1953; cited in 1969 JMPR
monograph; Annex 1, reference 13).
In a study described in more detail below of the effects of
ethoxyquin on induction of liver tumours by N-nitrosodiethylamine, a
control group received ethoxyquin only. Thus, 15 male Fischer 344
rats, six weeks old, received an intraperiotneal injection of 0.9%
saline, were placed on a diet containing 8000 ppm ethoxyquin (purity
unspecified), equivalent to 500 mg/kg bw per day in young rats, in
week 2, and were partially (66%) hepatectomized in week 3. When the
animals were killed at week 8, a background level of gamma-glutamyl
transpeptidase (gamma-GT)-positive foci was found in the liver (Ito et
al., 1985).
Groups of five male and five female Sprague-Dawley rats received
ethoxyquin (purity, 97.6%) by gavage in corn oil for 28 days at doses
of 0, 50, 250, 500, or 1000 mg/kg bw per day. Histopathological
examination was limited to the liver, lung, kidney, stomach, and gross
lesions in animals at 50, 250, and 1000 mg/kg bw per day. All of the
animals at 1000 mg/kg bw per day had died by day 3 with multiple organ
involvement; the cause of death in two animals was considered to be
necrosis and ulceration of the forestomach. The prevalences of
salivation, stained fur, and brown urine were increased at 250 mg/kg
bw per day and higher. Initial body-weight gain was reduced by 50% in
males receiving 500 mg/kg bw per day. Erythrocyte count, haematocrit,
and haemoglobin concentration were decreased by about 10% in females
at 250 mg/kg bw per day and in animals of each sex at 500 mg/kg bw per
day. Alterations in serum clinical chemical parameters were seen in
both males and females, but were more frequent in males at 250 and
500 mg/kg bw per day; they included increased quantities of protein,
total bilirubin, cholesterol, inorganic phosphorus, potassium, and
calcium, and gamma-GT activity, while the concentration of glucose was
decreased. Increased absolute and relative liver weights (> 40%)
were seen in animals of each sex at 250 mg/kg bw per day and higher,
and the relative kidney weights were increased (< 10%) in a
dose-related fashion. There were no gross lesions at doses < 1000
mg/kg bw per day. Histopathological investigation showed kidney
lesions (interstitial infiltration, tubular epithelial regeneration,
and tubular dilatation) in males receiving 50 and 250 mg/kg bw per day
and in animals of each sex at 500 mg/kg bw per day. The incidences of
haemorrhage and oedema of the lung and hepatocellular swelling were
increased at 500 mg/kg bw per day. A NOAEL was not identified (Naas,
1997).
Groups of 10 Sprague-Dawley rats of each sex, six weeks old at
the beginning of the study, received ethoxyquin (purity, 97.6%) by
gavage in corn oil at 0, 20, 40, 200, or 400 mg/kg bw per day for 13
weeks. Minor overdosing (2-14%) of the group at 200 mg/kg bw per day
on day 67 is considered not to have compromised the study.
Ophthalmoscopy was performed before treatment and during week 12. A
full post-mortem examination was performed on all animals, and samples
of lung, liver, kidney, and gross lesions from all animals were
examined histologically, as were more than 30 tissues from controls
and from animals at the highest dose.
There were no deaths during the study. Clinical signs were seen
in animals of each sex, but more often in females, at 200 and 400
mg/kg bw per day, including staining of various body parts and
particularly the anogenital area, salivation, and brown urine.
Body-weight gain was clearly reduced in males at 200 and 400 mg/kg bw
per day, with a slight effect (10%) at 40 mg/kg bw per day; food
consumption was similar in test and control groups. Haematological and
clinical chemical parameters were altered in animals of each sex at
400 mg/kg bw per day, and many were also significant at 200 mg/kg bw
per day. These included increased reticulocyte counts, total
bilirubin, blood urea nitrogen, gamma-GT activity, cholesterol, and
thyroid-stimulating hormone; and decreased erythrocyte and leukocyte
counts, prothrombin time, and glucose. Urine was more deeply coloured
at 200 and 400 mg/kg bw per day, and the volume was increased in
animals at the highest dose, with no change in specific gravity. There
were no treatment-related effects on the eyes.
The main gross finding was reddened thyroids in animals of each
sex at 200 and 400 mg/kg bw per day. The absolute weights of the liver
and that relative to body weight were increased in a dose-related
fashion (by 15-70%), and those of the kidneys increased by 4-20% in
animals of each sex at 200 and 400 mg/kg bw per day; changes in the
body-weight ratios of brain and testes are considered to be secondary
to the reduced body weights. Histological examination identified the
kidney as the main target organ in animals of each sex, with increased
incidences of tubular mineralization, papillary necrosis, and
cytoplasmic vacuolation in males at the high dose; and increased
incidences of mineralization, papillary necrosis, and nephropathy in
females at the high dose. The incidence of nephropathy was also
increased in females at 200 mg/kg bw per day. The incidence of
ultimobranchial cysts of the thyroid was increased in males receiving
200 and 400 mg/kg bw per day and females receiving 200 mg/kg bw per
day. Increased incidences of cytoplasmic vacuolation of the adrenals,
suppurative inflammation of the epididymides, non-suppurative
inflammation of the prostrate, mineralization of the lung, and
alveolar histiocytosis were also seen in males at the high dose, and
the incidences of inflammation of the oesophagus and epithelial
hyperplasia of the thymus were increased in females at this dose. It
should be noted that only gross lesions, liver, lung, and kidney were
examined from groups at lower doses. As the decrease in body-weight
gain in males at 40 mg/kg bw per day was part of a dose-response
relationship and was not associated with reduced food consumption, the
NOAEL for this study was 20 mg/kg bw per day (Naas, 1996a).
Dogs
A one-year study in three dogs given ethoxyquin by gavage was
summarized in the 1969 monograph; the NOAEL was 3 mg/kg bw per day.
The effects reported at the next highest dose (10 mg/kg bw per day)
included renal nephrosis, increased bromosulphthalein retention
indicating liver dysfunction, and abdominal tenderness (Hanzal, 1955;
cited in 1969 JMPR monograph; Annex 1, reference 13).
Groups of one male and one female beagles received ethoxyquin
(purity, 97.6%) by capsule at a dose of 0, 25, 50, 100, or 200 mg/kg
bw per day for 28 days. All of the animals at 100 or 200 mg/kg bw per
day group died or were sacrificed by day 17 or day 7, respectively;
one female at 50 mg/kg bw per day was sacrificed on day 21. The signs
seen in dogs that died and in survivors included hypoactivity, reduced
defaecation, brown urine, and pale gums. Given the small initial group
sizes and the deaths, only major, consistent changes are summarized
here. Reduced body-weight gain and food consumption were seen at all
doses. The serum activities of enzymes indicative of liver damage were
increased at four weeks in all groups in which they were measured (25
and 50 mg/kg bw per day): alkaline phosphatase by fivefold, aspartate
aminotransferase by threefold, alanine aminotransferase by 20-fold,
and gamma-GT by threefold; there were indications of reduced activated
partial thromboplastin times. The ratios of liver and kidney weights
to body weight were increased at 25 and 50 mg/kg bw per day. Common
post-mortem findings included redness of the gastrointestinal tract
and darkened livers. Histological examination showed pigmentation of
the liver in all treated animals but not in controls. A NOAEL was not
identified (Naas, 1996b).
In a 90-day study, groups of five beagles of each sex were given
ethoxyquin (purity, 97.6%) by capsule at 0, 2, 4, 20, or 40 mg/kg bw
per day. Clear signs of toxicity were seen during the first seven
weeks of the study at 40 mg/kg bw per day, including reduced body
weight, staining of the body surface, brown urine, brown sclera, dark
mucoid faeces, and emesis, and these groups received only empty
capsules for the final six weeks of the study, effectively becoming
reversibility groups. Investigations of clinical chemistry (including
thyroid hormones), haematology, and ophthalmoscopy were performed
before treatment and at weeks 4 and 12 or 13. Post-mortem
investigations included microscopic examination of a wide range of
tissues from all animals and special stains for pigment
identification.
One female at the highest dose was sacrificed in extremis on
day 13. Other findings were similar in males and females. Clinical
signs including brown staining of the abdomen and urogenital area,
brown urine, decreased faeces, and emesis were seen regularly at 20
and 40 mg/kg bw per day and occasionally during the 4 h after dosing
at 4 mg/kg bw per day; these signs were still present between weeks 7
and 13 ('recovery') in animals at the highest dose. Body-weight loss
occurred at 40 mg/kg bw per day in weeks 1-7, which reversed when
dosing stopped, but females had a lower (12%) mean body weight than
controls at termination. At 20 mg/kg bw per day, body-weight gain was
reduced (60%) throughout the study. Food consumption was reduced at 20
(20%) and 40 mg/kg bw per day (up to 50%). The only notable change in
haematological parameters was a dose-related decrease in activated
partial thromboplastin times in males at 4 mg/kg bw per day and higher
and in females at the highest dose. Marked increases in total
bilirubin concentration and in alkaline phosphatase, alanine
aminotransferase, aspartate aminotransferase, and gamma-GT activities
in serum, indicative of liver dysfunction, were seen at 20 mg/kg bw
per day in weeks 4 and 12 or 13 and at 40 mg/kg bw per day in week 4
only; alanine aminotransferase and, to a lesser extent, alkaline
phosphatase activities were also increased at 4 mg/kg bw per day. By
week 13, the serum values in animals at 40 mg/kg bw per day
(seven-week treatment, six-week recovery) had returned approximately
to control values. There were no significant changes in absolute or
relative organ weights. Treatment-related gross and microscopic
findings were limited to the liver. At 20 and 40 mg/kg bw per day, a
darkened appearance was associated microscopically with increased
pigment deposition, hepatocellular necrosis, cytoplasmic vacuolation,
and bile-duct hyperplasia; at 4 mg/kg bw per day, occasional findings
of mild or moderate pigmentation, minimal hepatocellular necrosis, and
vacuolation were recorded. The pigments were found to be porphyrin and
bilirubin in most cases, some sections also staining for haemosiderin.
The NOAEL was 2 mg/kg bw per day (Naas, 1996c).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Solutions of ethoxyquin (as Santoquin(R)) at 10 or 50 mg/ml
were administered subcutaneously to neonatal Swiss ICR/Ha mice on days
1 and 7 (0.1 ml) and 14 and 21 (0.2 ml) of age or as a single dose of
100 mg/ml (0.1 ml on day 1). Each dose was equal to 500, 2500, and
5000 mg/kg bw on day 1 and 250 and 1250 mg/kg bw on day 21. The groups
consisted of 57 mice at the low dose, 53 at the intermediate dose, and
28 at the high dose. By the time the mice were weaned, 100% at the
high dose, 74% at the intermediate dose, and 2% at the low dose had
died; 15% of controls had died at this time. Small groups of mice were
sacrificed at various times up to termination at week 53. A limited
range of tissues and lesions were examined primarily for tumours. The
incidences of pulmonary tumours and hepatomas were similar in the
treated and control groups; a slight increase in the incidence of
malignant lymphoma in four females at the low dose and two at the
intermediate dose, with none in controls, was considered equivocal by
the authors. The results indicates that four subcutaneous
administrations of ethoxyquin at near-lethal doses to neonatal mice
did not significantly increase the incidence of tumours in mice up to
one year of age (Epstein et al., 1970).
Rats
A two-year study of ethoxyquin in the diet of rats was summarized
in the 1969 monograph. Groups of approximately 10 male and 10 female
rats were fed diets containing ethoxyquin at concentrations of 0, 62,
125, 250, 500, 1000, 2000, or 4000 ppm for up to two years. The
animals were sacrificed for autopsy after 200, 400, 600, or 715 days.
The mortality rates were not significantly different from those of
controls. Significantly reduced body-weight gain was seen at 2000 ppm
after 225 days in males and after 21 days in females. After 200 days,
increased relative liver and kidney weights were found in males at 250
ppm and in females at 1000 ppm. The haemoglobin values for animals of
each sex were normal 100 and 300 days after the start of the
experiment in rats fed 2000 and 4000 ppm. Histological changes in the
renal cortex were clearly evident after 200 days in male rats
receiving 2000 or 4000 ppm but not in females. All other organs were
normal in animals of each sex after 200 days. After 400 days, lesions
in the kidneys (pyelonephritis), liver, and thyroid were seen in males
only. Similar lesions were seen for periods up to 717 days in animals
of each sex, although they were more marked in males. Occasional
tumours were found after 700 days, but the incidence was unrelated to
dose, and they were also seen in controls. No clearly defined effects
were evident after feeding 62 ppm, but minute lesions were present in
the kidneys of two males receiving 500 ppm. It was difficult to
distinguish the abnormalities in the group examined after 700 days
from the pathological manifestations of senility after that time
(Wilson & DeEds, 1959; cited in 1969 JMPR monograph; Annex 1,
reference 13). The 1969 JMPR concluded that the NOAEL was 125 ppm,
equivalent to 6 mg/kg bw per day. The small groups used in this study
limit its sensitivity for detecting changes in rare events such as
tumours with low background rates; however, the wide spread of doses
and sequential sampling times provides a degree of assurance in the
reported findings.
As part of an investigation of kidney and liver tumours induced
by N-nitrosoethyl- N-hydroxyethylamine, one control group received
only ethoxyquin. This group of 25 male Fischer 344 rats received a
diet containing 8000 ppm ethoxyquin from nine weeks of age to
termination at 41 weeks of age. Sections of liver, kidney, and gross
lesions were investigated histologically. No gamma-GT-positive foci,
hyperplastic nodules, or hepatocellular carcinoma were found in the
liver; no data were presented on kidney lesions. A control group of 25
male Fischer 344 rats received a diet containing 8000 ppm ethoxyquin
as part of a parallel investigation of urinary bladder carcinogenesis
induced by N-nitrosobutyl- N-hydroxybutylamine. After 32 weeks,
there were higher incidences of simple hyperplasia and papillary or
nodular hyperplasia of the urinary bladder than in groups receiving
ascorbic acid or sodium erythorbate, the incidence of simple
hyperplasia being greater than that induced by
N-nitrosobutyl- N-hydroxybutylamine alone; no untreated controls
were used. No urinary bladder papillomas or carcinomas were seen in
the group receiving ethoxyquin only (Ito et al., 1985).
An almost identical study of urinary bladder carcinogenesis
showed that 24 weeks' exposure to a diet containing 8000 ppm
ethoxyquin, considered to be equivalent to 400 mg/kg bw per day, did
not induce papillary or nodal hyperplasia or papilloma of the urinary
bladder in a group of 15 male Fischer 344 rats (Miyata et al., 1985).
The dependence of the renal lesions produced by ethoxyquin on age
and sex was investigated in Fischer 344 rats. Groups of four to eight
male rats received diets containing ethoxyquin (purity, 90%) at 5000
ppm from three or eight weeks of age for 20, 26, or 30 weeks. Eight
female rats received a diet containing 5000 ppm ethoxyquin for 30
weeks from eight weeks of age. Histopathological examination of the
kidney comprised bromodeoxyuridine (BrdU) labelling, gamma-GT
histochemistry, haematoxylin and eosin staining, Alizarin red
staining, and immunoblotting of urine samples for albumin and alpha2u
globulin. Body-weight gain was reduced by 10-15% in treated animals.
In males, the absolute kidney weights were increased by 5-50%, with a
consequent increase in the relative weights; females showed a 12%
increase in kidney:body weight ratios. Renal cortical changes were
seen in all treated males, consisting of eosinic cytoplasmic
inclusions in tubular epithelial cells and protein accumulation in the
lamina of the tubules. In males dosed from three weeks of age,
papillary necrosis, slight calcium deposition, and hyperplasia of the
transitional epithelium of the renal pelvis were seen. The
histological appearance of the kidneys of treated females was similar
to that of controls, except for high concentrations of lipofuscin
deposition. BrdU labelling was increased in males at 30 weeks, but not
at 20 weeks, in both regenerating basophilic tubules and those
staining normally with haematoxylin and eosin; BrdU labelling in
females was not described. The concentrations of alpha2u globulin in
urine were slightly lower in treated males, but those of albumin were
significantly increased. The time of first exposure can thus
significantly alter the pattern of renal lesions in rats consuming
diets containing ethoxyquin at 5000 ppm, equivalent to 250 mg/kg bw
per day, exposure from three weeks of age resulting in papillary
necrosis in addition to the cortical lesions seen in animals exposed
from eight weeks of age (Manson et al., 1992).
Groups of 6-19 Fischer 344 rats of each sex, three weeks of age
at the start of the study, received diets containing ethoxyquin
(purity unspecified) at 0 or 5000 ppm for up to 18 months; one group
received ethoxyquin in the diet for 24 weeks followed by 34 weeks on
control diet. The study was designed to investigate the progression of
renal lesions and involved interim sacrifices at 4, 12 or 14, 24, 58,
and 78 weeks. The body-weight gain of treated females was reduced in
weeks 1-5 and that of males from week 3 onwards; food consumption was
reduced in animals of each sex during the first four weeks.
Investigations of renal pathology showed a clear difference between
males and females. Males had significant interstitial degeneration of
the papilla at weeks 4 and 14, which progressed to necrosis with
pyelonephritis of the cortex and urothelial hyperplasia of the renal
pelvis by week 24. In females, interstitial degeneration of the
papilla was only slight at week 14 and did not progress consistently.
The chronic progressive nephropathy commonly seen in Fischer 344 rats
was accelerated in animals receiving ethoxyquin. The authors reported
that this was more marked in males, but the data presented do not
substantiate that statement. A golden-brown pigmentation, found to be
lipofuscin by Schmorl's stain, was noted in the proximal tubules of
treated rats, particularly females. The lesions present at 24 weeks
showed no evidence of reversibility after 34 weeks on control diets.
The authors considered that there was no evidence for preneoplastic
proliferative lesions. This study showed that ethoxyquin at 5000 ppm
in the diet, equivalent to 250 mg/kg bw per day, is a potent
nephrotoxin in young male Fischer 344 rats (Hard & Neal, 1992).
A number of reports have been published of the results of
investigations into the effects of antioxidants, including ethoxyquin,
on the induction of neoplasia and preneoplastic lesions by known
carcinogens. The most comprehensive series of studies with ethoxyquin
is probably that of Ito and co-workers (Ito et al., 1985; Miyata et
al., 1985; Masui et al., 1986), who used Fischer 344 rats to
investigate moderation of effects on the liver induced by
N-nitrosodiethylamine, effects on the kidney and liver produced by
N-nitrosoethyl- N-hydroxyethylamine, and effects on the bladder
produced by N-nitrosobutyl- N-(4-hydroxybutyl)amine.
Groups of 18 six-week-old male Fischer 344 rats received an
intraperitoneal injection of 200 mg/kg bw N-nitrosodiethylamine in
0.9% saline; two weeks later, they were transferred to a diet
containing 0 or 8000 ppm ethoxyquin (purity unspecified) and underwent
a partial (60%) hepatectomy one week later. The rats were sacrificed
at week 8, and liver sections were stained with haematoxylin and eosin
and a histochemical stain for gamma-GT-positive foci. The rats
receiving ethoxyquin had a significant (p < 0.001) decrease in the
number of foci (0.9 versus 3.3/cm2) and in the area of the foci (0.06
versus 0.19 mm2/cm2) (Ito et al., 1985).
Groups of 23 or 27 six-week-old male Fischer 344 rats received
drinking-water containing 0.1% N-nitrosoethyl- N-hydroxyethylamine
for two weeks; between weeks 3 and sacrifice at week 32, they received
diets containing 0 or 8000 ppm ethoxyquin (purity unspecified). All
rats had gamma-GT-positive foci, but the ethoxyquin-treated group had
fewer (1 versus 21/cm2) and smaller (10 versus 22 mm2/cm2) foci.
The group given ethoxyquin also had a reduced area of hyperplastic
nodules (2.3 versus 7 mm2/cm2), and fewer animals had hepatocellular
carcinomas (3/27 versus 11/23). Conversely, the kidneys of the
ethoxyquin-treated group had higher frequencies of atypical-cell foci
(0.9 versus 0.2/cm2) and adenomas (5.6 versus 0.8/cm2), and there
were more animals with foci (26/27 versus 12/23) and adenomas (17/27
versus 5/23) and larger foci (5.6 versus 0.9 × 10-2 mm2/cm2) and
adenomas (24 versus 8 × 10-2 mm2/cm2) (Ito et al., 1985).
Groups of 25 six-week-old male Fischer 344 rats received
drinking-water containing 0.05%
N-nitrosobutyl- N-(4-hydroxybutyl)amine for four weeks; between
weeks 4 and sacrifice at week 36, they received diets containing 0 or
8000 ppm ethoxyquin (purity unspecified). Examination of the urinary
bladders showed that ethoxyquin had increased the incidence of animals
with simple hyperplasia (25/25 versus 14/24), the incidence and extent
of papillary or nodular hyperplasia (25/25 versus 8/24 and 9.4 versus
0.48/10 cm of basement membrane), the incidence and extent of
papillomas (17/25 versus 5/24 and 1.1 versus 0.19/10 cm), and the
incidence and extent of carcinomas (4/25 versus 1/24 and 0.17 versus
0.04/10 cm) (Ito et al., 1985). A similar study involving two weeks'
dosing with N-nitrosobutyl- N-(4-hydroxybutyl)amine (0.05% in
water) and sacrifice at 24 weeks also showed that dietary
administration of ethoxyquin at 8000 ppm (purity unspecified)
increased the incidence and extent of papillary or nodular hyperplasia
but not of papillomas of the urinary bladder (Miyata et al., 1985).
This group of studies shows that ethoxyquin markedly reduces the
preneoplastic effects of N-nitrosodiethylamine and
N-nitrosoethyl- N-hydroxyethylamine on the liver, possibly by a
combination of antioxidant effects and induction of detoxification
mechanisms. The increase produced by ethoxyquin in the incidence of
neoplastic and preneoplastic kidney lesions induced by
N-nitrosoethyl- N-hydroxyethylamine may be secondary to the direct
toxic effects of ethoxyquin on the kidney. The mechanism of the
effects of ethoxyquin on
N-nitrosobutyl- N-(4-hydroxy-butyl)amine-induced urinary bladder
neoplasia is unknown.
Dogs
Ethoxyquin was fed to two groups of 14 dogs and bitches at a
dietary concentration of 0 or 300 ppm for five years. No effects were
observed on haematological, urinary, or clinical chemical end-points
(aspartate aminotransferase activity, blood urea nitrogen, and
bromosulphthalein retention), organ weights, organ:body weight ratios,
body weight, or gross or histopathological appearance (Monsanto, 1966;
cited in 1969 JMPR monograph; Annex 1, reference 13). The 1969 JMPR
concluded that the NOAEL in this study was 300 ppm, equivalent to 7.5
mg/kg bw per day.
(d) Genotoxicity
A number of published papers indicate that ethoxyquin is not
genotoxic in bacterial systems (Table 5); however, these reports could
not be validated as only minimal details were available. The results
of assays in eukaryotic systems have not been reported.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
After 40 days on a diet slightly deficient in tocopherol and
containing 0, 250, 500, or 1000 ppm ethoxyquin, rats were bred to
produce three consecutive litters. The offspring of the first litter
were used to produce a second-generation litter. The highest dose was
discarded after production of one litter (reason unknown). No effects
on reproduction, as reflected in fertility, litter size, or survival
of offspring, were observed. The animals receiving the experimental
diet produced young and raised them more successfully than controls,
the 500 ppm diet being more effective than the 250 ppm diet (Wilson,
1956; Wilson & DeEds, 1959, cited in 1969 JMPR monograph; Annex 1,
reference 13). The short dosing period before mating and the unknown
purity and group size compromise this report to some extent, but it
Table 5. Results of assays for the genotoxicity of ethoxuquin
End-point Test system Concentration Purity Result Reference
(%)
Reverse mutation S. typhimurium 10-1000 µg/plate 'Pure' Negativea Joner (1977)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium > 5000 µg/plate NR Negativea Ohta et al. (1980)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr trp
Reverse mutation NR NR NR Negative Zeiger (1993)
Gene mutation B. subtitlis H17 0.2 ml NR Negative Ohta et al. (1980)
rec+ and M45 rec-
a With and without metabolic activation
can be concluded that ethoxyquin in the diet at 500 ppm, equivalent to
25 mg/kg bw per day, has no marked effect on reproductive outcome.
Another study cited in the 1969 JMPR monograph had some results
contrary to those described above. Groups of eight or nine female rats
were placed on diets containing 0, 125, 375, or 1125 ppm ethoxyquin on
the day of mating. The length of gestation was comparable in all
groups, but the litter size was slightly depressed at doses of 375 ppm
and higher, and at 1125 ppm the incidence of stillbirths was increased
and survival to weaning was decreased. The NOAEL in this unusual study
was 125 ppm, equivalent to 6 mg/kg bw per day. In a separate part of
the same study, no effects were found on litter size, the number of
stillbirths, survival to weaning, or weanling weight in rats receiving
up to 1125 ppm ethoxyquin in the diet starting between days 1 and 10
of gestation (Derse, 1956; cited in 1969 JMPR monograph; Annex 1,
reference 13).
Dogs
The effects of ethoxyquin on reproduction over two generations
were studied in groups of beagles. Dogs were chosen as ethoxyquin is
added to commercial dog food to help prevent oxidative deterioration.
In the first mating (F0), groups of five males and 10 females
received diets containing ethoxyquin (Santoquin(R)) at a mean
analytical concentration of 0, 100, or 225 ppm for a minimum of 82
days before pairing. The eight male and 13 female pups used
subsequently for the F1 matings received diets containing 0, 100, or
225 ppm ethoxyquin from weaning until breeding at 10-30 months (2nd
estrus cycle in females). Semen samples were taken during the first
week of treatment and at around the time of breeding in order to
determine the volume, sperm count, motility, speed, and morphology.
Animals were observed and underwent extensive physical examinations
routinely; if possible, they were also observed during labour. Mating,
whelping, and lactation indices were determined. Urine samples and
blood samples were taken for haematology and clinical chemistry from
fasted adults before treatment and at the end of the F0 phase; at
weeks 10, 23, 36, 49, and 62 and at termination in the F1 growth
phase; and at termination of the F1 mating phase. Ophthalmological
examinations were performed at the beginning and end of the F1 growth
and mating phases. All F1 adults and pups that showed signs of
toxicity were necropsied. A range of tissues from controls and F1
adults at the high dose were examined histologically, with selected
tissues from F2 pups that showed clinical signs; the livers and
gall-bladders from F1 adults at the low dose and the adrenals and
spleens from F1 adult females at the low dose were also examined.
Macroscopic and microscopic examinations were performed only on F0
and F1 animals that died or were sacrificed prematurely.
In the F0 mating, there was considerable intra-group variation
in body weights, but F0 adults receiving 225 ppm ethoxyquin showed a
trend for reduced body weight from the initiation of dosing to week 17
and during the latter stages of gestation. Males thad reduced food
consumption during most of the study. Two females at the high dose
that were confirmed to be pregnant did not give birth. There were no
other differences between the groups in mating performance, labour,
birth, or weaning indices, semen parameters, or clinical signs. Litter
size, pup survival, and pup weight and growth were similar in all
groups. At 225 ppm, there was an increased number of pups of each sex
with a raw or red anus, dehydration, nasal discharge, and excessive
lachrymation; the incidence of the last two signs was also increased
at 100 ppm. Statistically significant increases in serum alkaline
phosphatase activity were seen in male parents at the high dose and in
female parents at the low and high dose; there was also an indication
of reductions in monocytes and partial thromboplastin times in animals
of each sex at the high dose, although all of the values were claimed
to be within the normal ranges. There were no effects on urinary
parameters. Remating of three female controls and two at 225 ppm from
this phase which failed to mate during the initial phase was
successful.
Among F1 animals, one male at the low dose and two females at
the high dose died or were sacrificed in extremis. The male was
sacrificed because of suspected neurological signs; one of the females
died of suspected heart disease, and the other was sacrificed because
of pneumonia. The clinical signs included excessive lachrymation,
dehydration, thinness, and pale gums and showed a dose-related
increase in both the number of animals of each sex with a particular
sign and the number of occasions on which it was observed. Males at
the high dose had a lower mean body weight than controls up to week 48
of the study. Initially, animals at the high dose consumed more food
than controls, but food consumption was consistently lower in weeks
8-18 in males and in weeks 8-30 in females. Considerable variations in
haematological end-points were seen throughout the study in both
treated and control animals. There was evidence of treatment-related
effects on erythrocyte count, haematocrit, and haemoglobin, which were
reduced by up to 11% relative to controls in treated males and females
at weeks 10 and 23, and on partial thromboplastin times, which were
reduced in females at the high dose in weeks 23 and 36 and in females
at the low dose in weeks 23 and 62 and at the final analysis.
Increased serum activities of alkaline phosphatase, gamma-GT, and
alanine aminotransferase and reduced albumin:globulin ratios were
found in animals at the high dose in weeks 10, 23, and 36, with
evidence of lesser perturbations at the lower dose. These changes are
indicative of impaired liver function. The results of urinary analysis
were unremarkable.
In the F1 mating, there were no clear differences in semen
analyses or mating, gestation, whelping, or weaning indices between
control and ethoxyquin-treated animals. In adults, the only
treatment-related clinical sign was excessive lachrymation, which
occurred more frequently in males at the low and high doses than in
controls. Haematological end-points were similar in all groups.
Dose-related changes were seen in a number of clinical chemical
parameters in females, which attained statistical significance
(p < 0.05) at the high dose. These comprised reductions in glucose,
cholesterol, protein, albumin, and albumin:globulin ratio, and
increases in total bilirubin concentration and in gamma-GT, alkaline
phosphatase, and alanine aminotransferase activities. In males, the
dose-related increases in alkaline phosphatase, gamma-GT, and alanine
aminotransferase activities did not attain statistical significance.
Macroscopic examination showed dark-plum-coloured livers in one male
and two females at the high dose and cervical lymph node haemorrhages
in two females at the low and high doses; these lesions were possibly
related to treatment as they were not present in control animals.
Increases in the absolute weights of the spleen and testes and in the
weights of these organs relative to the brain weight were seen in
treated males, giving statistically significant increases in relation
to body weight. In females, increases in the absolute and relative
weights of the liver (10%), kidneys (10%), and spleen (40%) were
reported but were not statistically significant. Histopathological
examination showed that the liver, pituitary, and spleen were the
target organs. The macroscopic finding of increased cervical lymph
node haemorrhage in females was not confirmed. A dark-reddish-brown
pigment, subsequently identified as protoporphyrin IX, was not found
in the livers of controls or males at the low dose but was present in
the livers of 7/13 females at the low dose, 2/7 males at the high
dose, and 10/11 females at the high dose, with a dose-related increase
in severity. The frequencies of fibrosis and haemorrhage of the spleen
were increased in females at the high dose (3/11 versus 0/13 in
controls), and the incidence of pituitary cysts was increased in
animals at the high dose when compared with controls (2/6 versus 0/8
in males and 4/10 versus 2/12 in females)
Treated male pups had increased incidences of grey or pale gums,
excessive lachrymation, and dehydration, and female pups had an
increased incidence of dehydration. The pup weights at birth and to
week 6 of gestation were slightly reduced (< 10%), with a
dose-related effect in female pups. An increased mortality rate in
pups at the low dose was not seen at the high dose and was probably
related to the larger litter sizes in this group; the rates of
mortality were 7/62 controls, 24/91 at the low dose, and 10/77 at the
high dose.
During the study, four males and one female at 100 ppm and two
females at 225 ppm showed signs of neuropathy: The animals had
impaired hindlimb function, inability to stand, and unsteadiness of
the head and body which was found to be associated with myelin
degeneration. Examination of clinically normal littermates showed no
neurological deficits. The breeding records showed that all of the
affected animals had a common male ancestor which was not in the
breeding line of any of the control animals. When the parents of some
of the affected pups were removed from the treated diets and mated,
the incidence of neurologically affected animals was 17% in one litter
and 25% in the other. The evidence from this part of the study is
strongly indicative of a genetic etiology.
Estimation of the actual intakes of ethoxyquin in this study was
confounded by up to twofold increased consumption during lactation and
the fact that 180 and 360 ppm had to be added to obtain nominal
concentrations of 150 and 300 ppm, however, the analysis showed mean
initial values of 100 and 225 ppm. Although the actual amounts of food
consumed varied during the study, a value of 25 g/kg bw per day was
considered to be a representative mean, which resulted in intakes of
2.5 mg/kg bw per day ethxyquin at 100 ppm and 6 mg/kg bw per day at
225 ppm.
The results of this study show that ethoxyquin in the diet at
concentrations up to 225 ppm did not affect reproductive performance
or outcome in beagles. There was no clear overall NOAEL because of
increased incidences of clinical signs such as excess lachrymation and
dehydration, clinical chemical changes and pigment deposition in the
liver. The lowest dose, 100 ppm, equal to 2.5 mg/kg bw per day, was
considered to be the minimal effect level (Gilman & Voss, 1995).
(ii) Developmental toxicity
Rats
In a range-finding study for teratogenicity in Sprague-Dawley
rats, groups of eight mated females received ethoxyquin (purity,
97.6%) by gavage in corn oil at 0, 62, 125, 250, 500, or 1000 mg/kg bw
per day on days 6-19 of gestation. All animals given 1000 mg/kg bw per
day died or were sacrificed by day 9, and three animals given 500
mg/kg bw per day died between days 10 and 11 of gestation; post-mortem
examinations did not show any adverse effects. Clinical signs of
reduced defaecation, dark urine, and brown staining of fur were
dose-related and affected all treated groups. Reduced food consumption
and body-weight loss were seen at doses > 125 mg/kgbw per day at
the beginning of dosing; from day 9 onwards, body-weight gain was
similar in all groups up to 500 mg/kg bw per day, and these animals
had a 20% body-weight deficit by day 20 when compared with controls.
Fetal weights were reduced at 500 mg/kg bw per day, but examination
for external malformations, sex ratio, and crown-rump length showed no
effects of treatment (Nemec, 1996a).
In the main study, groups of 25 mated female Sprague-Dawley rats
received ethoxyquin (purity, 97.6%) by gavage in corn oil at 0, 50,
150 or 350 mg/kg bw per day on days 6-19 of gestation. The dams were
sacrificed on day 20, their uteri and ovaries were examined, and all
fetuses were investigated for weight, sex, and external and visceral
malformations. The heads of one-half of the fetuses were examined by
Wilson sectioning and the other half by mid-coronal section. All
fetuses were stained with Alizarin Red S for skeletal investigation.
There were no deaths during the study. Urogenital staining was seen in
dams after treatment at the highest dose, and staining of other areas
was also seen in these and in some animals receiving 150 mg/kg bw per
day. At 350 mg/kg bw per day, dams lost weight on days 6-7, and a 13%
reduction in body-weight gain compared with controls was evident on
days 6-20; 150 mg/kg bw per day resulted in a 5% reduction in
body-weight gain on days 6-20. Food consumption was reduced by 9% at
150 mg/kg bw per day and by 13% at 350 mg/kg bw per day. There were no
significant findings in dams post mortem: uterine weights, litter
size, resorptions, pre- and post-implantation losses, sex ratios, and
fetal weights were similar in all groups. Isolated findings of
malformations and anomalies were within the range in historical
controls and showed no relationship to treatment. The overall
incidence of variations was highest in controls, with no significant
increase in any individual variation. The overall NOAEL for this study
was 50 mg/kg bw per day on the basis of clinical signs (fur staining)
and reduced maternal body weight at higher doses. The NOAEL for
fetotoxicity was 350 mg/kg bw per day, the highest dose tested (Nemec,
1996b).
3. Observations in humans
Cases of dermatitis have been reported in workers handling fruit
treated with ethoxyquin. A study with patch tests, cited in the 1969
monograph, showed that the cause was a sensitization reaction rather
than direct irritation (Wood, 1965).
A number of reports (Burrows, 1975; van Hecke, 1977; Zachariae,
1978; Brandao, 1983) have indicated that ethoxyquin is the probable
cause of an often severe dermatitis seen in workers who handle animal
feed containing ethoxyquin. Positive results in patch tests have been
recorded in affected workers given challenge concentrations of as
little as 0.01% ethoxyquin in petrolatum (Zachariae, 1978). The
authors of some of the reports indicated that airborne contamination
and light sensitivity are implicated.
A study cited in the 1969 monograph indicated that no evidence of
skin irritation or sensitivity had been reported in 20 years of
ethoxyquin production (Kelly, 1960).
Comments
Published studies show that ethoxyquin is rapidly absorbed from
the gastrointestinal tract of rats and mice, with peak blood levels
within 1 h. Liver, kidney, and adipose tissue have the highest tissue
concentrations. Excretion occurs predominantly via the urine and is
rapid, with more than 85% of doses up to 25 mg/kg bw being excreted
within 24 h. At 250 mg/kg bw, absorption and excretion are slowed,
which is attributed to reduced gastric emptying, and only 50% of the
dose is excreted within 24 h. Repeated oral doses of 25 mg/kg bw per
day resulted in an excretion profile similar to that for single doses,
but repeated administration of 250 mg/kg bw per day was reported to
result in a profile similar to that for lower doses, indicating
induction of metabolism, transport, and/or a return to normal gastric
emptying. Biliary excretion and enterohepatic recirculation play a
significant role in the toxicokinetics of ethoxyquin, more than 40% of
an intravenous dose of 25 mg/kg bw being detected in the bile of
bile-duct-cannulated rats. The metabolism of ethoxyquin involves
O-deethylation or hydroxylation followed by conjugation as the
sulfate or glucuronide. A proposed reaction scheme for the production
of biliary metabolites involves epoxidation and the generation of
reactive, electrophilic intermediates. No information on plant
metabolites was available.
Ethoxyquin has low acute toxicity when administered orally (LD50
= 1700 mg/kg bw), dermally, or by inhalation. It is slightly
irritating to the eyes and skin and had only very weak sensitizing
potential when administered topically to guinea-pigs. Exposure to
ethoxyquin in the workplace has been linked to allergic contact
dermatitis, and the substance should be considered as a sensitizer in
humans.
WHO has not classified ethoxyquin for acute toxicity.
The main target organ after repeated administration of ethoxyquin
to rats for 28 days or more at doses of 50-1000 mg/kg bw per day was
the kidney. Mechanistic studies with dietary concentrations equivalent
to 250 mg/kg bw per day showed that the precise effects were dependent
on the age at first exposure, were progressive, more severe in males
than in females, and not reversible after 24 weeks of exposure. Other
effects seen in rats exposed to ethoxyquin for 28 or 90 days at doses
> 200 mg/kg bw per day were stained fur, brown urine, changes to
haematological parameters, increased liver weights, and changes in
clinical chemical parameters consistent with altered liver function.
The overall NOAEL in the short-term studies in rats was 20 mg/kg bw
per day.
In dogs given capsules containing ethoxyquin for 90 days at 0, 2,
4, 20, or 40 mg/kg bw per day, the liver was the primary target.
Alterations in haematological parameters and clinical chemical changes
indicative of altered liver function were seen at doses > 4 mg/kg bw
per day, together with hepatocellular necrosis, vacuolation, and
pigment deposition. Although staining indicated that the pigment was
haemosiderin, a specific investigation showed it to be protoporphyrin
IX. The overall NOAEL in short-term studies in dogs was 2 mg/kg bw per
day. This is consistent with the results of an older, one-year study
in dogs in which a NOAEL of 3 mg/kg bw per day was established on the
basis of findings suggestive of effects on the kidney and liver at 10
mg/kg bw per day.
No modern long-term studies of toxicity or carcinogenicity have
been performed. In studies summarized by the 1969 Meeting in which
ethoxyquin was administered to dogs at 0 or 300 ppm in the diet for 5
years or at 0, 3, 10, 50, or 100 mg/kg bw per day by gavage for one
year, effects were observed in the liver and kidneys at doses of 10
mg/kg bw per day and above. The NOAEL was 300 ppm, equivalent to 7.5
mg/kg bw per day. A two-year study in rats that received dietary
concentrations of 0, 62, 125, 250, 500, 1000, 2000, or 4000 ppm,
published in 1959, gave no indication of carcinogenicity, with an
overall NOAEL of 125 ppm, equivalent to 6 mg/kg bw per day; lesions in
the kidney, liver, and thyroid gland were seen at higher doses.
Mechanistic studies on tumour induction and promotion show that
ethoxyquin induces both phase-I and phase-II xenobiotic metabolism.
Although its incorporation into the diet at 8000 ppm after treatment
with an N-nitrosamine reduced the formation of preneoplastic foci in
the liver, it increased the incidence of preneoplastic and neoplastic
events in the kidney and urinary bladder. No significant increase in
tumour incidence was seen after one year in mice that received four
subcutaneous, near-lethal doses of ethoxyquin.
Published reports of studies of bacterial mutagenicity indicate
that ethoxyquin is not mutagenic in prokaryotic systems, but only
limited details of the protocols and results were provided. No data
were available on other genotoxic end-points.
No modern study of reproductive toxicity has been performed in
rodents. Three studies in which rats received 0, 125, 250, 375, 500,
1000, or 1125 ppm in the diet, all with non-standard protocols, which
were summarized by the 1969 Meeting, gave slightly contradictory
results. Two of the studies, including the most extensive, apparently
showed no effects on the aspects of reproduction investigated at doses
up to 1125 ppm in the diet (equivalent to 56 mg/kg bw per day), while
the other showed an increased incidence of stillbirths at 1125 ppm and
decreased litter size at 375 ppm, with a NOAEL of 125 ppm (equivalent
to 6 mg/kg bw per day).
A modern two-generation study of reproductive toxicity in dogs
given diets containing 0, 100, or 225 ppm showed that ethoxyquin had
no effects on reproductive parameters at 225 ppm (equivalent to 5.6
mg/kg bw per day), the highest dose tested. The clinical signs
observed included dehydration, excess lachrymation, and evidence of
hepatic toxicity, especially in bitches. The effects were seen at both
doses and were consistent with the results of the short-term studies
in dogs. The findings in bitches may have been related to increased
consumption during gestation and lactation. The lowest dose tested,
100 ppm, equivalent to 2.5 mg/kg bw per day, was considered to be a
minimal effect level.
A study of developmental toxicity in rats at 0, 50, 150, or 350
mg/kg bw per day showed that ethoxyquin is not fetotoxic or
teratogenic at doses up to 350 mg/kg bw per day. Maternal toxicity,
stained fur, and reduced body-weight gain were seen at 150 and 350
mg/kg bw per day. No studies of developmental toxicity have been
performed in other species.
An ADI of 0-0.005 mg/kg bw per day was established on the basis
of the minimal-effect level of 2.5 mg/kg bw per day in the
multigeneration study in dogs and a 500-fold safety factor to account
for the lack of a NOAEL in this study and for the incompleteness of
the database. The multigeneration study of reproductive toxicity was
of longer duration and more recent than a 90-day study in dogs treated
by gavage with a NOAEL of 2 mg/kg bw per day.
An acute RfD was not allocated because ethoxyquin is of low acute
toxicity. The Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 125 ppm, equivalent to 6 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equivalent to 25 mg/kg bw per day
(two-generation study of reproductive toxicity)
50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
350 mg/kg bw per day (developmental toxicity)
Dog: 2 mg/kg bw per day (general toxicity in a 90-day study
of toxicity)
3 mg/kg bw per day (one-year study of toxicity)
300 ppm, equivalent to 7.5 mg/kg bw per day (five-year
study of toxicity)
2.5 mg/kg bw per day (minimal effect level for general
toxicity in a two-generation study of reproductive
toxicity)
5 mg/kg bw per day (reproductive performance; highest
dose tested)
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of genotoxicity in mammalian systems
2. A long-term study of toxicity and carcinogenicity in rats
that complies with modern guidelines
3. Observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid, > 50%
Dermal absorption No relevant information
Distribution Widely distributed; liver, kidney, adipose tissue
Potential for accumulation Slight evidence of bioaccumulation
Rate and extent of excretion > 85% eliminated within 24 h
Metabolism in animals Extensive; no parent compound detected in urine
Toxicologically significant compounds Metabolites considered of equivalent toxicity to parent
(animals, plants and environment) compound
Acute toxicity
Rat :LD50 oral 1700 mg/kg bw
Rat: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation > 2.0 mg/L (whole-body exposure)
Skin irritation Slightly irritating
Eye irritation Slightly irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity in multigeneration study
Lowest relevant oral NOAEL Dog: < 2.5 mg/kg bw per day (reproductive toxicity)
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity No evidence of genotoxicity, but testing inadequate
Long-term toxicity and carcinogenicity
Target/critical effect: Inadequate data
Lowest relevant NOAEL Inadequate data
Carcinogenicity No evidence of carcinogenicity, but testing inadequate
Reproductive toxicity
Reproduction target / critical effect No adverse effect on reproduction
Lowest relevant reproductive NOAEL Dog: 5 mg/kg per day, multigeneration study
Developmental target /critical effect No adverse effect on development
Lowest relevant developmental NOAEL Rat: 350 mg/kg/bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern from other studies
Other toxicological studies Not an initiator or promoter of liver tumours in
rats
Possible increase in urinary bladder preneoplastic
and neoplastic changes
Medical data Contact allergic dermatitis reported in food
handlers
Summary Value Study Safety factor
ADI 0-0.005 mg/kg bw Dog, multigeneration 500
study of reproductive
toxicity
Acute reference dose Not allocated
(unnecessary)
References
Brandao, F.M. (1983) Contact dermatitis to ethoxyquin. Contact
Derm., 9, 240.
Buetler, T.M., Gallagher, E.P., Wang, C., Stahl, D.L., Hayes, J.D. &
Eaton, D.L. (1995) Induction of phase I and phase II drug metabolising
enzyme mRNA, protein and activity by BHA, ethoxyquin and Oltipraz.
Toxicol. Appl. Pharmacol., 135, 45-57.
Burka, L.T., Sanders, J.M. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. II.
Metabolism. Xenobiotica, 26, 597-611.
Burrows, D. (1975) Contact dermatitis in animal feed mill workers.
Br. J. Dermatol., 92, 167-170.
Cox, A.J. (1953) 6-Ethoxy-2,2,4-trimethyl-1,2-dihydroquinone (EMHQ).
Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Co., cited in 1969 JMPR monograph, Annex 1, reference 13.
Derse, P. (1956) Assay report. Unpublished report from Wisconsin
Alumni Research Foundation. Submitted to WHO by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Epstein, S.S., Fujii, K., Andrea, J. & Mantel, N. (1970)
Carcinogenicity testing of selected food additives by parenteral
administration to infant Swiss mice. Toxicol. Appl. Pharmacol., 16,
321-334.
Gilman, M.R. & Voss, W.R (1995) Reproduction/chronic toxicology study
of ethoxyquin with beagle dogs. Unpublished report HRP-MI #203-1.1
from HRP Inc for Monsanto, St Louis, Missouri, USA. Submitted to WHO
by the Oregon, Washington and California Pear Association.
Hanzal, R.F. (1955) Final report and addendum. Chronic oral
administration--dogs--metabolic studies. Unpublished report from
Hazleton Laboratories. Submitted by Monsanto Chemical Co., cited in
1969 JMPR monograph, Annex 1, reference 13.
Hard, G.C. & Neal, G.E. (1992) Sequential study of the chronic
nephrotoxicity induced by dietary administration of ethoxyquin in
Fisher 344 rats Fundam. Appl. Toxicol., 18, 278-287.
van Hecke, E. (1977) Contact dermatitis to ethoxyquin in animal feeds.
Contact Derm., 3, 341-352.
Ito, N., Fukushima, S. & Tsuda, H. (1985) Carcinogenicity and
modification of the carcinogenic response by BHA, BHT and other
antioxidants. CRC Crit. Rev. Toxicol., 15, 109-150.
Joner, P.E. (1977) Butylhydroxyanisol (BHA), butylhyroxytoluene (BHT)
and ethoxyquin (EMQ) tested for mutagenicity. Acta Vet. Scand., 18,
187-193.
Kahl, R. & Netter, K.J. (1977) Ethoxyquin as an inducer and inhibitor
of phenobarbital-type cytochrome-P450 in rat liver microsomes.
Toxicol. Appl. Pharmacol., 40, 473-483.
Kelly, R.E. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Co.; cited in 1969 JMPR
monograph; Annex 1, reference 13.
Manson, M.M., Green, J.A., Wright, B.J. & Carthew, P. (1992) Degree of
ethoxyquin-induced nephrotoxicity in rat is dependent on age and sex.
Arch. Toxicol., 66, 51-56.
Masui, T., Tsuda, H., Inoue, K., Ogiso, T. & Ito, N. (1986) Inhibitory
effects of ethoxyquin, 4,4'-diaminophenylmethane and acetaminophen on
rat hepatocarcinogenesis. Jpn. J. Cancer Res. (Gann), 77, 231-237.
Miyata, Y., Fukushima, S., Hirose, M., Masui, T. & Ito, N. (1985)
Short-term screening of promoters of bladder carcinogenesis in
N-butyl-n-(4-hydroxy)-nitrosamine-initiated, unilaterally
ureter-ligated rats. Jpn. J. Cancer Res. (Gann), 76, 828-834.
Monsanto (1966) Five year Santoquin feeding study in dogs. Unpublished
collection of reports prepared and submitted by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Naas, D.J. (1996a) A 90-day oral (gavage) toxicity study of ethoxyquin
in rats, Unpublished report No. WIL-273011 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Naas. D.J. (1996b) A 28-day oral (capsule) dose range-finding study of
ethoxyquin in dogs. Unpublished report No. WIL-273012 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas. D.J. (1996c) A 90-day oral (capsule) toxicity study of
ethoxyquin in dogs. Unpublished report No. WIL-273013 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas, D.J. (1997) A 28-day oral (gavage) toxicity study of ethoxyquin
in rats. Unpublished report No. WIL-273010 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Nemec, M.D. (1996a) A dose range-finding developmental toxicity study
of ethoxyquin in rats. Unpublished report No. WIL-273008 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Nemec, M.D. (1996b) A developmental toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273009 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Ohta, T., Moriya, M., Kaneda, K., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Sanders, J.M., Burka, L.T. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. I.
Disposition. Xenobiotica, 26, 583-595.
Ulrich, C.E. (1996) Acute inhalation toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273006 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995a) Acute oral toxicity study of ethoxyquin in albino
rats. Unpublished report No. WIL-273001 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995b) Acute dermal toxicity study of ethoxyquin in
albino rats. Unpublished report No. WIL-273002 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995c) Primary eye irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273004 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995d) Primary dermal irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273003 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995e) Skin sensitization study of ethoxyquin in albino
guinea pigs. Unpublished report No. WIL-273005 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Wilson, R.H. (1956) Final report: Reproduction study in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, US Department of Agriculture. Submitted to WHO by Monsanto
Chemical Co.; cited in 1969 JMPR monograph; Annex 1, reference 13.
Wilson, R.H. & DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agric. Food
Chem., 7, 203-206.
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report submitted by Monsanto Chemical Co., cited
in 1969 JMPR monograph; Annex 1, reference 13.
Zachariae, H. (1978) Ethoxyquin dermatitis. Contact Derm., 4,
117-118.
Zeiger, E. (1993) Mutagenicity of chemicals added to foods. Mutat.
Res., 290, 53-61.
Table 4. Acute toxicity of ethoxyquin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Rat Oral gavage 1700 97.6 Varsho (1995a)
Rat Dermal (24 h) > 2000 97.6 Varsho (1995b)
Rat Inhalation, whole body > 2.0 97.6 Ulrich (1996)
Mouse Intraperitoneal ~ 900 Wilson & DeEds (1959)a
Mouse Intravenous ~ 180 Wilson & DeEds (1959)a
a Cited in 1969 JMPR monograph
red-yellow staining of the fur. Gross and histopathological changes
indicated an irritant effect on the gastrointestinal tract.
Ethoxyquin produced transient, slight erythema when applied to
rabbit skin for 4 h under semi-occlusive conditions. There was no
oedema, but desquamation was present seven days after exposure
(Varsho, 1995c). This result is consistent with the findings of a
study summarized in the 1969 monograph (Kelly, 1960; cited in 1969
JMPR monograph; Annex 1, reference 13).
Ethoxyquin produced transient, slight-to-mild conjunctival
redness and chemosis in rabbits. All of the effects had fully
regressed within four days (Varsho, 1995d).
In a sensitization study in six guinea-pigs of each sex,
ethoxyquin was at most a very weak skin sensitizer. Induction was at
100%, with challenge applications at 50% in acetone. Very weak
erythematous responses were seen in both treated and control groups
after challenge and re-challenge, one test animal producing a weak
response to both challenge and re-challenge (Varsho, 1995e).
(b) Short-term studies of toxicity
Rats
Two studies of dietary administration of ethoxyquin to rats for
200 days were summarized in the 1969 monograph. Kidney lesions
(unspecified) were reported at 500 ppm and higher in males, with
increased kidney:body weight ratios in males at 250 ppm and higher.
The frequency of cytoplasmic inclusions in hepatocytes was increased
at 2000 ppm (Wilson & DeEds, 1959; Cox, 1953; cited in 1969 JMPR
monograph; Annex 1, reference 13).
In a study described in more detail below of the effects of
ethoxyquin on induction of liver tumours by N-nitrosodiethylamine, a
control group received ethoxyquin only. Thus, 15 male Fischer 344
rats, six weeks old, received an intraperiotneal injection of 0.9%
saline, were placed on a diet containing 8000 ppm ethoxyquin (purity
unspecified), equivalent to 500 mg/kg bw per day in young rats, in
week 2, and were partially (66%) hepatectomized in week 3. When the
animals were killed at week 8, a background level of gamma-glutamyl
transpeptidase (gamma-GT)-positive foci was found in the liver (Ito et
al., 1985).
Groups of five male and five female Sprague-Dawley rats received
ethoxyquin (purity, 97.6%) by gavage in corn oil for 28 days at doses
of 0, 50, 250, 500, or 1000 mg/kg bw per day. Histopathological
examination was limited to the liver, lung, kidney, stomach, and gross
lesions in animals at 50, 250, and 1000 mg/kg bw per day. All of the
animals at 1000 mg/kg bw per day had died by day 3 with multiple organ
involvement; the cause of death in two animals was considered to be
necrosis and ulceration of the forestomach. The prevalences of
salivation, stained fur, and brown urine were increased at 250 mg/kg
bw per day and higher. Initial body-weight gain was reduced by 50% in
males receiving 500 mg/kg bw per day. Erythrocyte count, haematocrit,
and haemoglobin concentration were decreased by about 10% in females
at 250 mg/kg bw per day and in animals of each sex at 500 mg/kg bw per
day. Alterations in serum clinical chemical parameters were seen in
both males and females, but were more frequent in males at 250 and
500 mg/kg bw per day; they included increased quantities of protein,
total bilirubin, cholesterol, inorganic phosphorus, potassium, and
calcium, and gamma-GT activity, while the concentration of glucose was
decreased. Increased absolute and relative liver weights (> 40%)
were seen in animals of each sex at 250 mg/kg bw per day and higher,
and the relative kidney weights were increased (< 10%) in a
dose-related fashion. There were no gross lesions at doses < 1000
mg/kg bw per day. Histopathological investigation showed kidney
lesions (interstitial infiltration, tubular epithelial regeneration,
and tubular dilatation) in males receiving 50 and 250 mg/kg bw per day
and in animals of each sex at 500 mg/kg bw per day. The incidences of
haemorrhage and oedema of the lung and hepatocellular swelling were
increased at 500 mg/kg bw per day. A NOAEL was not identified (Naas,
1997).
Groups of 10 Sprague-Dawley rats of each sex, six weeks old at
the beginning of the study, received ethoxyquin (purity, 97.6%) by
gavage in corn oil at 0, 20, 40, 200, or 400 mg/kg bw per day for 13
weeks. Minor overdosing (2-14%) of the group at 200 mg/kg bw per day
on day 67 is considered not to have compromised the study.
Ophthalmoscopy was performed before treatment and during week 12. A
full post-mortem examination was performed on all animals, and samples
of lung, liver, kidney, and gross lesions from all animals were
examined histologically, as were more than 30 tissues from controls
and from animals at the highest dose.
There were no deaths during the study. Clinical signs were seen
in animals of each sex, but more often in females, at 200 and 400
mg/kg bw per day, including staining of various body parts and
particularly the anogenital area, salivation, and brown urine.
Body-weight gain was clearly reduced in males at 200 and 400 mg/kg bw
per day, with a slight effect (10%) at 40 mg/kg bw per day; food
consumption was similar in test and control groups. Haematological and
clinical chemical parameters were altered in animals of each sex at
400 mg/kg bw per day, and many were also significant at 200 mg/kg bw
per day. These included increased reticulocyte counts, total
bilirubin, blood urea nitrogen, gamma-GT activity, cholesterol, and
thyroid-stimulating hormone; and decreased erythrocyte and leukocyte
counts, prothrombin time, and glucose. Urine was more deeply coloured
at 200 and 400 mg/kg bw per day, and the volume was increased in
animals at the highest dose, with no change in specific gravity. There
were no treatment-related effects on the eyes.
The main gross finding was reddened thyroids in animals of each
sex at 200 and 400 mg/kg bw per day. The absolute weights of the liver
and that relative to body weight were increased in a dose-related
fashion (by 15-70%), and those of the kidneys increased by 4-20% in
animals of each sex at 200 and 400 mg/kg bw per day; changes in the
body-weight ratios of brain and testes are considered to be secondary
to the reduced body weights. Histological examination identified the
kidney as the main target organ in animals of each sex, with increased
incidences of tubular mineralization, papillary necrosis, and
cytoplasmic vacuolation in males at the high dose; and increased
incidences of mineralization, papillary necrosis, and nephropathy in
females at the high dose. The incidence of nephropathy was also
increased in females at 200 mg/kg bw per day. The incidence of
ultimobranchial cysts of the thyroid was increased in males receiving
200 and 400 mg/kg bw per day and females receiving 200 mg/kg bw per
day. Increased incidences of cytoplasmic vacuolation of the adrenals,
suppurative inflammation of the epididymides, non-suppurative
inflammation of the prostrate, mineralization of the lung, and
alveolar histiocytosis were also seen in males at the high dose, and
the incidences of inflammation of the oesophagus and epithelial
hyperplasia of the thymus were increased in females at this dose. It
should be noted that only gross lesions, liver, lung, and kidney were
examined from groups at lower doses. As the decrease in body-weight
gain in males at 40 mg/kg bw per day was part of a dose-response
relationship and was not associated with reduced food consumption, the
NOAEL for this study was 20 mg/kg bw per day (Naas, 1996a).
Dogs
A one-year study in three dogs given ethoxyquin by gavage was
summarized in the 1969 monograph; the NOAEL was 3 mg/kg bw per day.
The effects reported at the next highest dose (10 mg/kg bw per day)
included renal nephrosis, increased bromosulphthalein retention
indicating liver dysfunction, and abdominal tenderness (Hanzal, 1955;
cited in 1969 JMPR monograph; Annex 1, reference 13).
Groups of one male and one female beagles received ethoxyquin
(purity, 97.6%) by capsule at a dose of 0, 25, 50, 100, or 200 mg/kg
bw per day for 28 days. All of the animals at 100 or 200 mg/kg bw per
day group died or were sacrificed by day 17 or day 7, respectively;
one female at 50 mg/kg bw per day was sacrificed on day 21. The signs
seen in dogs that died and in survivors included hypoactivity, reduced
defaecation, brown urine, and pale gums. Given the small initial group
sizes and the deaths, only major, consistent changes are summarized
here. Reduced body-weight gain and food consumption were seen at all
doses. The serum activities of enzymes indicative of liver damage were
increased at four weeks in all groups in which they were measured (25
and 50 mg/kg bw per day): alkaline phosphatase by fivefold, aspartate
aminotransferase by threefold, alanine aminotransferase by 20-fold,
and gamma-GT by threefold; there were indications of reduced activated
partial thromboplastin times. The ratios of liver and kidney weights
to body weight were increased at 25 and 50 mg/kg bw per day. Common
post-mortem findings included redness of the gastrointestinal tract
and darkened livers. Histological examination showed pigmentation of
the liver in all treated animals but not in controls. A NOAEL was not
identified (Naas, 1996b).
In a 90-day study, groups of five beagles of each sex were given
ethoxyquin (purity, 97.6%) by capsule at 0, 2, 4, 20, or 40 mg/kg bw
per day. Clear signs of toxicity were seen during the first seven
weeks of the study at 40 mg/kg bw per day, including reduced body
weight, staining of the body surface, brown urine, brown sclera, dark
mucoid faeces, and emesis, and these groups received only empty
capsules for the final six weeks of the study, effectively becoming
reversibility groups. Investigations of clinical chemistry (including
thyroid hormones), haematology, and ophthalmoscopy were performed
before treatment and at weeks 4 and 12 or 13. Post-mortem
investigations included microscopic examination of a wide range of
tissues from all animals and special stains for pigment
identification.
One female at the highest dose was sacrificed in extremis on
day 13. Other findings were similar in males and females. Clinical
signs including brown staining of the abdomen and urogenital area,
brown urine, decreased faeces, and emesis were seen regularly at 20
and 40 mg/kg bw per day and occasionally during the 4 h after dosing
at 4 mg/kg bw per day; these signs were still present between weeks 7
and 13 ('recovery') in animals at the highest dose. Body-weight loss
occurred at 40 mg/kg bw per day in weeks 1-7, which reversed when
dosing stopped, but females had a lower (12%) mean body weight than
controls at termination. At 20 mg/kg bw per day, body-weight gain was
reduced (60%) throughout the study. Food consumption was reduced at 20
(20%) and 40 mg/kg bw per day (up to 50%). The only notable change in
haematological parameters was a dose-related decrease in activated
partial thromboplastin times in males at 4 mg/kg bw per day and higher
and in females at the highest dose. Marked increases in total
bilirubin concentration and in alkaline phosphatase, alanine
aminotransferase, aspartate aminotransferase, and gamma-GT activities
in serum, indicative of liver dysfunction, were seen at 20 mg/kg bw
per day in weeks 4 and 12 or 13 and at 40 mg/kg bw per day in week 4
only; alanine aminotransferase and, to a lesser extent, alkaline
phosphatase activities were also increased at 4 mg/kg bw per day. By
week 13, the serum values in animals at 40 mg/kg bw per day
(seven-week treatment, six-week recovery) had returned approximately
to control values. There were no significant changes in absolute or
relative organ weights. Treatment-related gross and microscopic
findings were limited to the liver. At 20 and 40 mg/kg bw per day, a
darkened appearance was associated microscopically with increased
pigment deposition, hepatocellular necrosis, cytoplasmic vacuolation,
and bile-duct hyperplasia; at 4 mg/kg bw per day, occasional findings
of mild or moderate pigmentation, minimal hepatocellular necrosis, and
vacuolation were recorded. The pigments were found to be porphyrin and
bilirubin in most cases, some sections also staining for haemosiderin.
The NOAEL was 2 mg/kg bw per day (Naas, 1996c).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Solutions of ethoxyquin (as Santoquin(R)) at 10 or 50 mg/ml
were administered subcutaneously to neonatal Swiss ICR/Ha mice on days
1 and 7 (0.1 ml) and 14 and 21 (0.2 ml) of age or as a single dose of
100 mg/ml (0.1 ml on day 1). Each dose was equal to 500, 2500, and
5000 mg/kg bw on day 1 and 250 and 1250 mg/kg bw on day 21. The groups
consisted of 57 mice at the low dose, 53 at the intermediate dose, and
28 at the high dose. By the time the mice were weaned, 100% at the
high dose, 74% at the intermediate dose, and 2% at the low dose had
died; 15% of controls had died at this time. Small groups of mice were
sacrificed at various times up to termination at week 53. A limited
range of tissues and lesions were examined primarily for tumours. The
incidences of pulmonary tumours and hepatomas were similar in the
treated and control groups; a slight increase in the incidence of
malignant lymphoma in four females at the low dose and two at the
intermediate dose, with none in controls, was considered equivocal by
the authors. The results indicates that four subcutaneous
administrations of ethoxyquin at near-lethal doses to neonatal mice
did not significantly increase the incidence of tumours in mice up to
one year of age (Epstein et al., 1970).
Rats
A two-year study of ethoxyquin in the diet of rats was summarized
in the 1969 monograph. Groups of approximately 10 male and 10 female
rats were fed diets containing ethoxyquin at concentrations of 0, 62,
125, 250, 500, 1000, 2000, or 4000 ppm for up to two years. The
animals were sacrificed for autopsy after 200, 400, 600, or 715 days.
The mortality rates were not significantly different from those of
controls. Significantly reduced body-weight gain was seen at 2000 ppm
after 225 days in males and after 21 days in females. After 200 days,
increased relative liver and kidney weights were found in males at 250
ppm and in females at 1000 ppm. The haemoglobin values for animals of
each sex were normal 100 and 300 days after the start of the
experiment in rats fed 2000 and 4000 ppm. Histological changes in the
renal cortex were clearly evident after 200 days in male rats
receiving 2000 or 4000 ppm but not in females. All other organs were
normal in animals of each sex after 200 days. After 400 days, lesions
in the kidneys (pyelonephritis), liver, and thyroid were seen in males
only. Similar lesions were seen for periods up to 717 days in animals
of each sex, although they were more marked in males. Occasional
tumours were found after 700 days, but the incidence was unrelated to
dose, and they were also seen in controls. No clearly defined effects
were evident after feeding 62 ppm, but minute lesions were present in
the kidneys of two males receiving 500 ppm. It was difficult to
distinguish the abnormalities in the group examined after 700 days
from the pathological manifestations of senility after that time
(Wilson & DeEds, 1959; cited in 1969 JMPR monograph; Annex 1,
reference 13). The 1969 JMPR concluded that the NOAEL was 125 ppm,
equivalent to 6 mg/kg bw per day. The small groups used in this study
limit its sensitivity for detecting changes in rare events such as
tumours with low background rates; however, the wide spread of doses
and sequential sampling times provides a degree of assurance in the
reported findings.
As part of an investigation of kidney and liver tumours induced
by N-nitrosoethyl- N-hydroxyethylamine, one control group received
only ethoxyquin. This group of 25 male Fischer 344 rats received a
diet containing 8000 ppm ethoxyquin from nine weeks of age to
termination at 41 weeks of age. Sections of liver, kidney, and gross
lesions were investigated histologically. No gamma-GT-positive foci,
hyperplastic nodules, or hepatocellular carcinoma were found in the
liver; no data were presented on kidney lesions. A control group of 25
male Fischer 344 rats received a diet containing 8000 ppm ethoxyquin
as part of a parallel investigation of urinary bladder carcinogenesis
induced by N-nitrosobutyl- N-hydroxybutylamine. After 32 weeks,
there were higher incidences of simple hyperplasia and papillary or
nodular hyperplasia of the urinary bladder than in groups receiving
ascorbic acid or sodium erythorbate, the incidence of simple
hyperplasia being greater than that induced by
N-nitrosobutyl- N-hydroxybutylamine alone; no untreated controls
were used. No urinary bladder papillomas or carcinomas were seen in
the group receiving ethoxyquin only (Ito et al., 1985).
An almost identical study of urinary bladder carcinogenesis
showed that 24 weeks' exposure to a diet containing 8000 ppm
ethoxyquin, considered to be equivalent to 400 mg/kg bw per day, did
not induce papillary or nodal hyperplasia or papilloma of the urinary
bladder in a group of 15 male Fischer 344 rats (Miyata et al., 1985).
The dependence of the renal lesions produced by ethoxyquin on age
and sex was investigated in Fischer 344 rats. Groups of four to eight
male rats received diets containing ethoxyquin (purity, 90%) at 5000
ppm from three or eight weeks of age for 20, 26, or 30 weeks. Eight
female rats received a diet containing 5000 ppm ethoxyquin for 30
weeks from eight weeks of age. Histopathological examination of the
kidney comprised bromodeoxyuridine (BrdU) labelling, gamma-GT
histochemistry, haematoxylin and eosin staining, Alizarin red
staining, and immunoblotting of urine samples for albumin and alpha2u
globulin. Body-weight gain was reduced by 10-15% in treated animals.
In males, the absolute kidney weights were increased by 5-50%, with a
consequent increase in the relative weights; females showed a 12%
increase in kidney:body weight ratios. Renal cortical changes were
seen in all treated males, consisting of eosinic cytoplasmic
inclusions in tubular epithelial cells and protein accumulation in the
lamina of the tubules. In males dosed from three weeks of age,
papillary necrosis, slight calcium deposition, and hyperplasia of the
transitional epithelium of the renal pelvis were seen. The
histological appearance of the kidneys of treated females was similar
to that of controls, except for high concentrations of lipofuscin
deposition. BrdU labelling was increased in males at 30 weeks, but not
at 20 weeks, in both regenerating basophilic tubules and those
staining normally with haematoxylin and eosin; BrdU labelling in
females was not described. The concentrations of alpha2u globulin in
urine were slightly lower in treated males, but those of albumin were
significantly increased. The time of first exposure can thus
significantly alter the pattern of renal lesions in rats consuming
diets containing ethoxyquin at 5000 ppm, equivalent to 250 mg/kg bw
per day, exposure from three weeks of age resulting in papillary
necrosis in addition to the cortical lesions seen in animals exposed
from eight weeks of age (Manson et al., 1992).
Groups of 6-19 Fischer 344 rats of each sex, three weeks of age
at the start of the study, received diets containing ethoxyquin
(purity unspecified) at 0 or 5000 ppm for up to 18 months; one group
received ethoxyquin in the diet for 24 weeks followed by 34 weeks on
control diet. The study was designed to investigate the progression of
renal lesions and involved interim sacrifices at 4, 12 or 14, 24, 58,
and 78 weeks. The body-weight gain of treated females was reduced in
weeks 1-5 and that of males from week 3 onwards; food consumption was
reduced in animals of each sex during the first four weeks.
Investigations of renal pathology showed a clear difference between
males and females. Males had significant interstitial degeneration of
the papilla at weeks 4 and 14, which progressed to necrosis with
pyelonephritis of the cortex and urothelial hyperplasia of the renal
pelvis by week 24. In females, interstitial degeneration of the
papilla was only slight at week 14 and did not progress consistently.
The chronic progressive nephropathy commonly seen in Fischer 344 rats
was accelerated in animals receiving ethoxyquin. The authors reported
that this was more marked in males, but the data presented do not
substantiate that statement. A golden-brown pigmentation, found to be
lipofuscin by Schmorl's stain, was noted in the proximal tubules of
treated rats, particularly females. The lesions present at 24 weeks
showed no evidence of reversibility after 34 weeks on control diets.
The authors considered that there was no evidence for preneoplastic
proliferative lesions. This study showed that ethoxyquin at 5000 ppm
in the diet, equivalent to 250 mg/kg bw per day, is a potent
nephrotoxin in young male Fischer 344 rats (Hard & Neal, 1992).
A number of reports have been published of the results of
investigations into the effects of antioxidants, including ethoxyquin,
on the induction of neoplasia and preneoplastic lesions by known
carcinogens. The most comprehensive series of studies with ethoxyquin
is probably that of Ito and co-workers (Ito et al., 1985; Miyata et
al., 1985; Masui et al., 1986), who used Fischer 344 rats to
investigate moderation of effects on the liver induced by
N-nitrosodiethylamine, effects on the kidney and liver produced by
N-nitrosoethyl- N-hydroxyethylamine, and effects on the bladder
produced by N-nitrosobutyl- N-(4-hydroxybutyl)amine.
Groups of 18 six-week-old male Fischer 344 rats received an
intraperitoneal injection of 200 mg/kg bw N-nitrosodiethylamine in
0.9% saline; two weeks later, they were transferred to a diet
containing 0 or 8000 ppm ethoxyquin (purity unspecified) and underwent
a partial (60%) hepatectomy one week later. The rats were sacrificed
at week 8, and liver sections were stained with haematoxylin and eosin
and a histochemical stain for gamma-GT-positive foci. The rats
receiving ethoxyquin had a significant (p < 0.001) decrease in the
number of foci (0.9 versus 3.3/cm2) and in the area of the foci (0.06
versus 0.19 mm2/cm2) (Ito et al., 1985).
Groups of 23 or 27 six-week-old male Fischer 344 rats received
drinking-water containing 0.1% N-nitrosoethyl- N-hydroxyethylamine
for two weeks; between weeks 3 and sacrifice at week 32, they received
diets containing 0 or 8000 ppm ethoxyquin (purity unspecified). All
rats had gamma-GT-positive foci, but the ethoxyquin-treated group had
fewer (1 versus 21/cm2) and smaller (10 versus 22 mm2/cm2) foci.
The group given ethoxyquin also had a reduced area of hyperplastic
nodules (2.3 versus 7 mm2/cm2), and fewer animals had hepatocellular
carcinomas (3/27 versus 11/23). Conversely, the kidneys of the
ethoxyquin-treated group had higher frequencies of atypical-cell foci
(0.9 versus 0.2/cm2) and adenomas (5.6 versus 0.8/cm2), and there
were more animals with foci (26/27 versus 12/23) and adenomas (17/27
versus 5/23) and larger foci (5.6 versus 0.9 × 10-2 mm2/cm2) and
adenomas (24 versus 8 × 10-2 mm2/cm2) (Ito et al., 1985).
Groups of 25 six-week-old male Fischer 344 rats received
drinking-water containing 0.05%
N-nitrosobutyl- N-(4-hydroxybutyl)amine for four weeks; between
weeks 4 and sacrifice at week 36, they received diets containing 0 or
8000 ppm ethoxyquin (purity unspecified). Examination of the urinary
bladders showed that ethoxyquin had increased the incidence of animals
with simple hyperplasia (25/25 versus 14/24), the incidence and extent
of papillary or nodular hyperplasia (25/25 versus 8/24 and 9.4 versus
0.48/10 cm of basement membrane), the incidence and extent of
papillomas (17/25 versus 5/24 and 1.1 versus 0.19/10 cm), and the
incidence and extent of carcinomas (4/25 versus 1/24 and 0.17 versus
0.04/10 cm) (Ito et al., 1985). A similar study involving two weeks'
dosing with N-nitrosobutyl- N-(4-hydroxybutyl)amine (0.05% in
water) and sacrifice at 24 weeks also showed that dietary
administration of ethoxyquin at 8000 ppm (purity unspecified)
increased the incidence and extent of papillary or nodular hyperplasia
but not of papillomas of the urinary bladder (Miyata et al., 1985).
This group of studies shows that ethoxyquin markedly reduces the
preneoplastic effects of N-nitrosodiethylamine and
N-nitrosoethyl- N-hydroxyethylamine on the liver, possibly by a
combination of antioxidant effects and induction of detoxification
mechanisms. The increase produced by ethoxyquin in the incidence of
neoplastic and preneoplastic kidney lesions induced by
N-nitrosoethyl- N-hydroxyethylamine may be secondary to the direct
toxic effects of ethoxyquin on the kidney. The mechanism of the
effects of ethoxyquin on
N-nitrosobutyl- N-(4-hydroxy-butyl)amine-induced urinary bladder
neoplasia is unknown.
Dogs
Ethoxyquin was fed to two groups of 14 dogs and bitches at a
dietary concentration of 0 or 300 ppm for five years. No effects were
observed on haematological, urinary, or clinical chemical end-points
(aspartate aminotransferase activity, blood urea nitrogen, and
bromosulphthalein retention), organ weights, organ:body weight ratios,
body weight, or gross or histopathological appearance (Monsanto, 1966;
cited in 1969 JMPR monograph; Annex 1, reference 13). The 1969 JMPR
concluded that the NOAEL in this study was 300 ppm, equivalent to 7.5
mg/kg bw per day.
(d) Genotoxicity
A number of published papers indicate that ethoxyquin is not
genotoxic in bacterial systems (Table 5); however, these reports could
not be validated as only minimal details were available. The results
of assays in eukaryotic systems have not been reported.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
After 40 days on a diet slightly deficient in tocopherol and
containing 0, 250, 500, or 1000 ppm ethoxyquin, rats were bred to
produce three consecutive litters. The offspring of the first litter
were used to produce a second-generation litter. The highest dose was
discarded after production of one litter (reason unknown). No effects
on reproduction, as reflected in fertility, litter size, or survival
of offspring, were observed. The animals receiving the experimental
diet produced young and raised them more successfully than controls,
the 500 ppm diet being more effective than the 250 ppm diet (Wilson,
1956; Wilson & DeEds, 1959, cited in 1969 JMPR monograph; Annex 1,
reference 13). The short dosing period before mating and the unknown
purity and group size compromise this report to some extent, but it
Table 5. Results of assays for the genotoxicity of ethoxuquin
End-point Test system Concentration Purity Result Reference
(%)
Reverse mutation S. typhimurium 10-1000 µg/plate 'Pure' Negativea Joner (1977)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium > 5000 µg/plate NR Negativea Ohta et al. (1980)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr trp
Reverse mutation NR NR NR Negative Zeiger (1993)
Gene mutation B. subtitlis H17 0.2 ml NR Negative Ohta et al. (1980)
rec+ and M45 rec-
a With and without metabolic activation
can be concluded that ethoxyquin in the diet at 500 ppm, equivalent to
25 mg/kg bw per day, has no marked effect on reproductive outcome.
Another study cited in the 1969 JMPR monograph had some results
contrary to those described above. Groups of eight or nine female rats
were placed on diets containing 0, 125, 375, or 1125 ppm ethoxyquin on
the day of mating. The length of gestation was comparable in all
groups, but the litter size was slightly depressed at doses of 375 ppm
and higher, and at 1125 ppm the incidence of stillbirths was increased
and survival to weaning was decreased. The NOAEL in this unusual study
was 125 ppm, equivalent to 6 mg/kg bw per day. In a separate part of
the same study, no effects were found on litter size, the number of
stillbirths, survival to weaning, or weanling weight in rats receiving
up to 1125 ppm ethoxyquin in the diet starting between days 1 and 10
of gestation (Derse, 1956; cited in 1969 JMPR monograph; Annex 1,
reference 13).
Dogs
The effects of ethoxyquin on reproduction over two generations
were studied in groups of beagles. Dogs were chosen as ethoxyquin is
added to commercial dog food to help prevent oxidative deterioration.
In the first mating (F0), groups of five males and 10 females
received diets containing ethoxyquin (Santoquin(R)) at a mean
analytical concentration of 0, 100, or 225 ppm for a minimum of 82
days before pairing. The eight male and 13 female pups used
subsequently for the F1 matings received diets containing 0, 100, or
225 ppm ethoxyquin from weaning until breeding at 10-30 months (2nd
estrus cycle in females). Semen samples were taken during the first
week of treatment and at around the time of breeding in order to
determine the volume, sperm count, motility, speed, and morphology.
Animals were observed and underwent extensive physical examinations
routinely; if possible, they were also observed during labour. Mating,
whelping, and lactation indices were determined. Urine samples and
blood samples were taken for haematology and clinical chemistry from
fasted adults before treatment and at the end of the F0 phase; at
weeks 10, 23, 36, 49, and 62 and at termination in the F1 growth
phase; and at termination of the F1 mating phase. Ophthalmological
examinations were performed at the beginning and end of the F1 growth
and mating phases. All F1 adults and pups that showed signs of
toxicity were necropsied. A range of tissues from controls and F1
adults at the high dose were examined histologically, with selected
tissues from F2 pups that showed clinical signs; the livers and
gall-bladders from F1 adults at the low dose and the adrenals and
spleens from F1 adult females at the low dose were also examined.
Macroscopic and microscopic examinations were performed only on F0
and F1 animals that died or were sacrificed prematurely.
In the F0 mating, there was considerable intra-group variation
in body weights, but F0 adults receiving 225 ppm ethoxyquin showed a
trend for reduced body weight from the initiation of dosing to week 17
and during the latter stages of gestation. Males thad reduced food
consumption during most of the study. Two females at the high dose
that were confirmed to be pregnant did not give birth. There were no
other differences between the groups in mating performance, labour,
birth, or weaning indices, semen parameters, or clinical signs. Litter
size, pup survival, and pup weight and growth were similar in all
groups. At 225 ppm, there was an increased number of pups of each sex
with a raw or red anus, dehydration, nasal discharge, and excessive
lachrymation; the incidence of the last two signs was also increased
at 100 ppm. Statistically significant increases in serum alkaline
phosphatase activity were seen in male parents at the high dose and in
female parents at the low and high dose; there was also an indication
of reductions in monocytes and partial thromboplastin times in animals
of each sex at the high dose, although all of the values were claimed
to be within the normal ranges. There were no effects on urinary
parameters. Remating of three female controls and two at 225 ppm from
this phase which failed to mate during the initial phase was
successful.
Among F1 animals, one male at the low dose and two females at
the high dose died or were sacrificed in extremis. The male was
sacrificed because of suspected neurological signs; one of the females
died of suspected heart disease, and the other was sacrificed because
of pneumonia. The clinical signs included excessive lachrymation,
dehydration, thinness, and pale gums and showed a dose-related
increase in both the number of animals of each sex with a particular
sign and the number of occasions on which it was observed. Males at
the high dose had a lower mean body weight than controls up to week 48
of the study. Initially, animals at the high dose consumed more food
than controls, but food consumption was consistently lower in weeks
8-18 in males and in weeks 8-30 in females. Considerable variations in
haematological end-points were seen throughout the study in both
treated and control animals. There was evidence of treatment-related
effects on erythrocyte count, haematocrit, and haemoglobin, which were
reduced by up to 11% relative to controls in treated males and females
at weeks 10 and 23, and on partial thromboplastin times, which were
reduced in females at the high dose in weeks 23 and 36 and in females
at the low dose in weeks 23 and 62 and at the final analysis.
Increased serum activities of alkaline phosphatase, gamma-GT, and
alanine aminotransferase and reduced albumin:globulin ratios were
found in animals at the high dose in weeks 10, 23, and 36, with
evidence of lesser perturbations at the lower dose. These changes are
indicative of impaired liver function. The results of urinary analysis
were unremarkable.
In the F1 mating, there were no clear differences in semen
analyses or mating, gestation, whelping, or weaning indices between
control and ethoxyquin-treated animals. In adults, the only
treatment-related clinical sign was excessive lachrymation, which
occurred more frequently in males at the low and high doses than in
controls. Haematological end-points were similar in all groups.
Dose-related changes were seen in a number of clinical chemical
parameters in females, which attained statistical significance
(p < 0.05) at the high dose. These comprised reductions in glucose,
cholesterol, protein, albumin, and albumin:globulin ratio, and
increases in total bilirubin concentration and in gamma-GT, alkaline
phosphatase, and alanine aminotransferase activities. In males, the
dose-related increases in alkaline phosphatase, gamma-GT, and alanine
aminotransferase activities did not attain statistical significance.
Macroscopic examination showed dark-plum-coloured livers in one male
and two females at the high dose and cervical lymph node haemorrhages
in two females at the low and high doses; these lesions were possibly
related to treatment as they were not present in control animals.
Increases in the absolute weights of the spleen and testes and in the
weights of these organs relative to the brain weight were seen in
treated males, giving statistically significant increases in relation
to body weight. In females, increases in the absolute and relative
weights of the liver (10%), kidneys (10%), and spleen (40%) were
reported but were not statistically significant. Histopathological
examination showed that the liver, pituitary, and spleen were the
target organs. The macroscopic finding of increased cervical lymph
node haemorrhage in females was not confirmed. A dark-reddish-brown
pigment, subsequently identified as protoporphyrin IX, was not found
in the livers of controls or males at the low dose but was present in
the livers of 7/13 females at the low dose, 2/7 males at the high
dose, and 10/11 females at the high dose, with a dose-related increase
in severity. The frequencies of fibrosis and haemorrhage of the spleen
were increased in females at the high dose (3/11 versus 0/13 in
controls), and the incidence of pituitary cysts was increased in
animals at the high dose when compared with controls (2/6 versus 0/8
in males and 4/10 versus 2/12 in females)
Treated male pups had increased incidences of grey or pale gums,
excessive lachrymation, and dehydration, and female pups had an
increased incidence of dehydration. The pup weights at birth and to
week 6 of gestation were slightly reduced (< 10%), with a
dose-related effect in female pups. An increased mortality rate in
pups at the low dose was not seen at the high dose and was probably
related to the larger litter sizes in this group; the rates of
mortality were 7/62 controls, 24/91 at the low dose, and 10/77 at the
high dose.
During the study, four males and one female at 100 ppm and two
females at 225 ppm showed signs of neuropathy: The animals had
impaired hindlimb function, inability to stand, and unsteadiness of
the head and body which was found to be associated with myelin
degeneration. Examination of clinically normal littermates showed no
neurological deficits. The breeding records showed that all of the
affected animals had a common male ancestor which was not in the
breeding line of any of the control animals. When the parents of some
of the affected pups were removed from the treated diets and mated,
the incidence of neurologically affected animals was 17% in one litter
and 25% in the other. The evidence from this part of the study is
strongly indicative of a genetic etiology.
Estimation of the actual intakes of ethoxyquin in this study was
confounded by up to twofold increased consumption during lactation and
the fact that 180 and 360 ppm had to be added to obtain nominal
concentrations of 150 and 300 ppm, however, the analysis showed mean
initial values of 100 and 225 ppm. Although the actual amounts of food
consumed varied during the study, a value of 25 g/kg bw per day was
considered to be a representative mean, which resulted in intakes of
2.5 mg/kg bw per day ethxyquin at 100 ppm and 6 mg/kg bw per day at
225 ppm.
The results of this study show that ethoxyquin in the diet at
concentrations up to 225 ppm did not affect reproductive performance
or outcome in beagles. There was no clear overall NOAEL because of
increased incidences of clinical signs such as excess lachrymation and
dehydration, clinical chemical changes and pigment deposition in the
liver. The lowest dose, 100 ppm, equal to 2.5 mg/kg bw per day, was
considered to be the minimal effect level (Gilman & Voss, 1995).
(ii) Developmental toxicity
Rats
In a range-finding study for teratogenicity in Sprague-Dawley
rats, groups of eight mated females received ethoxyquin (purity,
97.6%) by gavage in corn oil at 0, 62, 125, 250, 500, or 1000 mg/kg bw
per day on days 6-19 of gestation. All animals given 1000 mg/kg bw per
day died or were sacrificed by day 9, and three animals given 500
mg/kg bw per day died between days 10 and 11 of gestation; post-mortem
examinations did not show any adverse effects. Clinical signs of
reduced defaecation, dark urine, and brown staining of fur were
dose-related and affected all treated groups. Reduced food consumption
and body-weight loss were seen at doses > 125 mg/kgbw per day at
the beginning of dosing; from day 9 onwards, body-weight gain was
similar in all groups up to 500 mg/kg bw per day, and these animals
had a 20% body-weight deficit by day 20 when compared with controls.
Fetal weights were reduced at 500 mg/kg bw per day, but examination
for external malformations, sex ratio, and crown-rump length showed no
effects of treatment (Nemec, 1996a).
In the main study, groups of 25 mated female Sprague-Dawley rats
received ethoxyquin (purity, 97.6%) by gavage in corn oil at 0, 50,
150 or 350 mg/kg bw per day on days 6-19 of gestation. The dams were
sacrificed on day 20, their uteri and ovaries were examined, and all
fetuses were investigated for weight, sex, and external and visceral
malformations. The heads of one-half of the fetuses were examined by
Wilson sectioning and the other half by mid-coronal section. All
fetuses were stained with Alizarin Red S for skeletal investigation.
There were no deaths during the study. Urogenital staining was seen in
dams after treatment at the highest dose, and staining of other areas
was also seen in these and in some animals receiving 150 mg/kg bw per
day. At 350 mg/kg bw per day, dams lost weight on days 6-7, and a 13%
reduction in body-weight gain compared with controls was evident on
days 6-20; 150 mg/kg bw per day resulted in a 5% reduction in
body-weight gain on days 6-20. Food consumption was reduced by 9% at
150 mg/kg bw per day and by 13% at 350 mg/kg bw per day. There were no
significant findings in dams post mortem: uterine weights, litter
size, resorptions, pre- and post-implantation losses, sex ratios, and
fetal weights were similar in all groups. Isolated findings of
malformations and anomalies were within the range in historical
controls and showed no relationship to treatment. The overall
incidence of variations was highest in controls, with no significant
increase in any individual variation. The overall NOAEL for this study
was 50 mg/kg bw per day on the basis of clinical signs (fur staining)
and reduced maternal body weight at higher doses. The NOAEL for
fetotoxicity was 350 mg/kg bw per day, the highest dose tested (Nemec,
1996b).
3. Observations in humans
Cases of dermatitis have been reported in workers handling fruit
treated with ethoxyquin. A study with patch tests, cited in the 1969
monograph, showed that the cause was a sensitization reaction rather
than direct irritation (Wood, 1965).
A number of reports (Burrows, 1975; van Hecke, 1977; Zachariae,
1978; Brandao, 1983) have indicated that ethoxyquin is the probable
cause of an often severe dermatitis seen in workers who handle animal
feed containing ethoxyquin. Positive results in patch tests have been
recorded in affected workers given challenge concentrations of as
little as 0.01% ethoxyquin in petrolatum (Zachariae, 1978). The
authors of some of the reports indicated that airborne contamination
and light sensitivity are implicated.
A study cited in the 1969 monograph indicated that no evidence of
skin irritation or sensitivity had been reported in 20 years of
ethoxyquin production (Kelly, 1960).
Comments
Published studies show that ethoxyquin is rapidly absorbed from
the gastrointestinal tract of rats and mice, with peak blood levels
within 1 h. Liver, kidney, and adipose tissue have the highest tissue
concentrations. Excretion occurs predominantly via the urine and is
rapid, with more than 85% of doses up to 25 mg/kg bw being excreted
within 24 h. At 250 mg/kg bw, absorption and excretion are slowed,
which is attributed to reduced gastric emptying, and only 50% of the
dose is excreted within 24 h. Repeated oral doses of 25 mg/kg bw per
day resulted in an excretion profile similar to that for single doses,
but repeated administration of 250 mg/kg bw per day was reported to
result in a profile similar to that for lower doses, indicating
induction of metabolism, transport, and/or a return to normal gastric
emptying. Biliary excretion and enterohepatic recirculation play a
significant role in the toxicokinetics of ethoxyquin, more than 40% of
an intravenous dose of 25 mg/kg bw being detected in the bile of
bile-duct-cannulated rats. The metabolism of ethoxyquin involves
O-deethylation or hydroxylation followed by conjugation as the
sulfate or glucuronide. A proposed reaction scheme for the production
of biliary metabolites involves epoxidation and the generation of
reactive, electrophilic intermediates. No information on plant
metabolites was available.
Ethoxyquin has low acute toxicity when administered orally (LD50
= 1700 mg/kg bw), dermally, or by inhalation. It is slightly
irritating to the eyes and skin and had only very weak sensitizing
potential when administered topically to guinea-pigs. Exposure to
ethoxyquin in the workplace has been linked to allergic contact
dermatitis, and the substance should be considered as a sensitizer in
humans.
WHO has not classified ethoxyquin for acute toxicity.
The main target organ after repeated administration of ethoxyquin
to rats for 28 days or more at doses of 50-1000 mg/kg bw per day was
the kidney. Mechanistic studies with dietary concentrations equivalent
to 250 mg/kg bw per day showed that the precise effects were dependent
on the age at first exposure, were progressive, more severe in males
than in females, and not reversible after 24 weeks of exposure. Other
effects seen in rats exposed to ethoxyquin for 28 or 90 days at doses
> 200 mg/kg bw per day were stained fur, brown urine, changes to
haematological parameters, increased liver weights, and changes in
clinical chemical parameters consistent with altered liver function.
The overall NOAEL in the short-term studies in rats was 20 mg/kg bw
per day.
In dogs given capsules containing ethoxyquin for 90 days at 0, 2,
4, 20, or 40 mg/kg bw per day, the liver was the primary target.
Alterations in haematological parameters and clinical chemical changes
indicative of altered liver function were seen at doses > 4 mg/kg bw
per day, together with hepatocellular necrosis, vacuolation, and
pigment deposition. Although staining indicated that the pigment was
haemosiderin, a specific investigation showed it to be protoporphyrin
IX. The overall NOAEL in short-term studies in dogs was 2 mg/kg bw per
day. This is consistent with the results of an older, one-year study
in dogs in which a NOAEL of 3 mg/kg bw per day was established on the
basis of findings suggestive of effects on the kidney and liver at 10
mg/kg bw per day.
No modern long-term studies of toxicity or carcinogenicity have
been performed. In studies summarized by the 1969 Meeting in which
ethoxyquin was administered to dogs at 0 or 300 ppm in the diet for 5
years or at 0, 3, 10, 50, or 100 mg/kg bw per day by gavage for one
year, effects were observed in the liver and kidneys at doses of 10
mg/kg bw per day and above. The NOAEL was 300 ppm, equivalent to 7.5
mg/kg bw per day. A two-year study in rats that received dietary
concentrations of 0, 62, 125, 250, 500, 1000, 2000, or 4000 ppm,
published in 1959, gave no indication of carcinogenicity, with an
overall NOAEL of 125 ppm, equivalent to 6 mg/kg bw per day; lesions in
the kidney, liver, and thyroid gland were seen at higher doses.
Mechanistic studies on tumour induction and promotion show that
ethoxyquin induces both phase-I and phase-II xenobiotic metabolism.
Although its incorporation into the diet at 8000 ppm after treatment
with an N-nitrosamine reduced the formation of preneoplastic foci in
the liver, it increased the incidence of preneoplastic and neoplastic
events in the kidney and urinary bladder. No significant increase in
tumour incidence was seen after one year in mice that received four
subcutaneous, near-lethal doses of ethoxyquin.
Published reports of studies of bacterial mutagenicity indicate
that ethoxyquin is not mutagenic in prokaryotic systems, but only
limited details of the protocols and results were provided. No data
were available on other genotoxic end-points.
No modern study of reproductive toxicity has been performed in
rodents. Three studies in which rats received 0, 125, 250, 375, 500,
1000, or 1125 ppm in the diet, all with non-standard protocols, which
were summarized by the 1969 Meeting, gave slightly contradictory
results. Two of the studies, including the most extensive, apparently
showed no effects on the aspects of reproduction investigated at doses
up to 1125 ppm in the diet (equivalent to 56 mg/kg bw per day), while
the other showed an increased incidence of stillbirths at 1125 ppm and
decreased litter size at 375 ppm, with a NOAEL of 125 ppm (equivalent
to 6 mg/kg bw per day).
A modern two-generation study of reproductive toxicity in dogs
given diets containing 0, 100, or 225 ppm showed that ethoxyquin had
no effects on reproductive parameters at 225 ppm (equivalent to 5.6
mg/kg bw per day), the highest dose tested. The clinical signs
observed included dehydration, excess lachrymation, and evidence of
hepatic toxicity, especially in bitches. The effects were seen at both
doses and were consistent with the results of the short-term studies
in dogs. The findings in bitches may have been related to increased
consumption during gestation and lactation. The lowest dose tested,
100 ppm, equivalent to 2.5 mg/kg bw per day, was considered to be a
minimal effect level.
A study of developmental toxicity in rats at 0, 50, 150, or 350
mg/kg bw per day showed that ethoxyquin is not fetotoxic or
teratogenic at doses up to 350 mg/kg bw per day. Maternal toxicity,
stained fur, and reduced body-weight gain were seen at 150 and 350
mg/kg bw per day. No studies of developmental toxicity have been
performed in other species.
An ADI of 0-0.005 mg/kg bw per day was established on the basis
of the minimal-effect level of 2.5 mg/kg bw per day in the
multigeneration study in dogs and a 500-fold safety factor to account
for the lack of a NOAEL in this study and for the incompleteness of
the database. The multigeneration study of reproductive toxicity was
of longer duration and more recent than a 90-day study in dogs treated
by gavage with a NOAEL of 2 mg/kg bw per day.
An acute RfD was not allocated because ethoxyquin is of low acute
toxicity. The Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 125 ppm, equivalent to 6 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equivalent to 25 mg/kg bw per day
(two-generation study of reproductive toxicity)
50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
350 mg/kg bw per day (developmental toxicity)
Dog: 2 mg/kg bw per day (general toxicity in a 90-day study
of toxicity)
3 mg/kg bw per day (one-year study of toxicity)
300 ppm, equivalent to 7.5 mg/kg bw per day (five-year
study of toxicity)
2.5 mg/kg bw per day (minimal effect level for general
toxicity in a two-generation study of reproductive
toxicity)
5 mg/kg bw per day (reproductive performance; highest
dose tested)
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of genotoxicity in mammalian systems
2. A long-term study of toxicity and carcinogenicity in rats
that complies with modern guidelines
3. Observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid, > 50%
Dermal absorption No relevant information
Distribution Widely distributed; liver, kidney, adipose tissue
Potential for accumulation Slight evidence of bioaccumulation
Rate and extent of excretion > 85% eliminated within 24 h
Metabolism in animals Extensive; no parent compound detected in urine
Toxicologically significant compounds Metabolites considered of equivalent toxicity to parent
(animals, plants and environment) compound
Acute toxicity
Rat :LD50 oral 1700 mg/kg bw
Rat: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation > 2.0 mg/L (whole-body exposure)
Skin irritation Slightly irritating
Eye irritation Slightly irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity in multigeneration study
Lowest relevant oral NOAEL Dog: < 2.5 mg/kg bw per day (reproductive toxicity)
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity No evidence of genotoxicity, but testing inadequate
Long-term toxicity and carcinogenicity
Target/critical effect: Inadequate data
Lowest relevant NOAEL Inadequate data
Carcinogenicity No evidence of carcinogenicity, but testing inadequate
Reproductive toxicity
Reproduction target / critical effect No adverse effect on reproduction
Lowest relevant reproductive NOAEL Dog: 5 mg/kg per day, multigeneration study
Developmental target /critical effect No adverse effect on development
Lowest relevant developmental NOAEL Rat: 350 mg/kg/bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern from other studies
Other toxicological studies Not an initiator or promoter of liver tumours in
rats
Possible increase in urinary bladder preneoplastic
and neoplastic changes
Medical data Contact allergic dermatitis reported in food
handlers
Summary Value Study Safety factor
ADI 0-0.005 mg/kg bw Dog, multigeneration 500
study of reproductive
toxicity
Acute reference dose Not allocated
(unnecessary)
References
Brandao, F.M. (1983) Contact dermatitis to ethoxyquin. Contact
Derm., 9, 240.
Buetler, T.M., Gallagher, E.P., Wang, C., Stahl, D.L., Hayes, J.D. &
Eaton, D.L. (1995) Induction of phase I and phase II drug metabolising
enzyme mRNA, protein and activity by BHA, ethoxyquin and Oltipraz.
Toxicol. Appl. Pharmacol., 135, 45-57.
Burka, L.T., Sanders, J.M. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. II.
Metabolism. Xenobiotica, 26, 597-611.
Burrows, D. (1975) Contact dermatitis in animal feed mill workers.
Br. J. Dermatol., 92, 167-170.
Cox, A.J. (1953) 6-Ethoxy-2,2,4-trimethyl-1,2-dihydroquinone (EMHQ).
Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Co., cited in 1969 JMPR monograph, Annex 1, reference 13.
Derse, P. (1956) Assay report. Unpublished report from Wisconsin
Alumni Research Foundation. Submitted to WHO by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Epstein, S.S., Fujii, K., Andrea, J. & Mantel, N. (1970)
Carcinogenicity testing of selected food additives by parenteral
administration to infant Swiss mice. Toxicol. Appl. Pharmacol., 16,
321-334.
Gilman, M.R. & Voss, W.R (1995) Reproduction/chronic toxicology study
of ethoxyquin with beagle dogs. Unpublished report HRP-MI #203-1.1
from HRP Inc for Monsanto, St Louis, Missouri, USA. Submitted to WHO
by the Oregon, Washington and California Pear Association.
Hanzal, R.F. (1955) Final report and addendum. Chronic oral
administration--dogs--metabolic studies. Unpublished report from
Hazleton Laboratories. Submitted by Monsanto Chemical Co., cited in
1969 JMPR monograph, Annex 1, reference 13.
Hard, G.C. & Neal, G.E. (1992) Sequential study of the chronic
nephrotoxicity induced by dietary administration of ethoxyquin in
Fisher 344 rats Fundam. Appl. Toxicol., 18, 278-287.
van Hecke, E. (1977) Contact dermatitis to ethoxyquin in animal feeds.
Contact Derm., 3, 341-352.
Ito, N., Fukushima, S. & Tsuda, H. (1985) Carcinogenicity and
modification of the carcinogenic response by BHA, BHT and other
antioxidants. CRC Crit. Rev. Toxicol., 15, 109-150.
Joner, P.E. (1977) Butylhydroxyanisol (BHA), butylhyroxytoluene (BHT)
and ethoxyquin (EMQ) tested for mutagenicity. Acta Vet. Scand., 18,
187-193.
Kahl, R. & Netter, K.J. (1977) Ethoxyquin as an inducer and inhibitor
of phenobarbital-type cytochrome-P450 in rat liver microsomes.
Toxicol. Appl. Pharmacol., 40, 473-483.
Kelly, R.E. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Co.; cited in 1969 JMPR
monograph; Annex 1, reference 13.
Manson, M.M., Green, J.A., Wright, B.J. & Carthew, P. (1992) Degree of
ethoxyquin-induced nephrotoxicity in rat is dependent on age and sex.
Arch. Toxicol., 66, 51-56.
Masui, T., Tsuda, H., Inoue, K., Ogiso, T. & Ito, N. (1986) Inhibitory
effects of ethoxyquin, 4,4'-diaminophenylmethane and acetaminophen on
rat hepatocarcinogenesis. Jpn. J. Cancer Res. (Gann), 77, 231-237.
Miyata, Y., Fukushima, S., Hirose, M., Masui, T. & Ito, N. (1985)
Short-term screening of promoters of bladder carcinogenesis in
N-butyl-n-(4-hydroxy)-nitrosamine-initiated, unilaterally
ureter-ligated rats. Jpn. J. Cancer Res. (Gann), 76, 828-834.
Monsanto (1966) Five year Santoquin feeding study in dogs. Unpublished
collection of reports prepared and submitted by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Naas, D.J. (1996a) A 90-day oral (gavage) toxicity study of ethoxyquin
in rats, Unpublished report No. WIL-273011 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Naas. D.J. (1996b) A 28-day oral (capsule) dose range-finding study of
ethoxyquin in dogs. Unpublished report No. WIL-273012 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas. D.J. (1996c) A 90-day oral (capsule) toxicity study of
ethoxyquin in dogs. Unpublished report No. WIL-273013 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas, D.J. (1997) A 28-day oral (gavage) toxicity study of ethoxyquin
in rats. Unpublished report No. WIL-273010 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Nemec, M.D. (1996a) A dose range-finding developmental toxicity study
of ethoxyquin in rats. Unpublished report No. WIL-273008 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Nemec, M.D. (1996b) A developmental toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273009 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Ohta, T., Moriya, M., Kaneda, K., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Sanders, J.M., Burka, L.T. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. I.
Disposition. Xenobiotica, 26, 583-595.
Ulrich, C.E. (1996) Acute inhalation toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273006 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995a) Acute oral toxicity study of ethoxyquin in albino
rats. Unpublished report No. WIL-273001 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995b) Acute dermal toxicity study of ethoxyquin in
albino rats. Unpublished report No. WIL-273002 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995c) Primary eye irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273004 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995d) Primary dermal irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273003 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995e) Skin sensitization study of ethoxyquin in albino
guinea pigs. Unpublished report No. WIL-273005 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Wilson, R.H. (1956) Final report: Reproduction study in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, US Department of Agriculture. Submitted to WHO by Monsanto
Chemical Co.; cited in 1969 JMPR monograph; Annex 1, reference 13.
Wilson, R.H. & DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agric. Food
Chem., 7, 203-206.
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report submitted by Monsanto Chemical Co., cited
in 1969 JMPR monograph; Annex 1, reference 13.
Zachariae, H. (1978) Ethoxyquin dermatitis. Contact Derm., 4,
117-118.
Zeiger, E. (1993) Mutagenicity of chemicals added to foods. Mutat.
Res., 290, 53-61.
See Also:
Toxicological Abbreviations
Ethoxyquin (FAO/PL:1969/M/17/1)
Ethoxyquin (JMPR Evaluations 2005 Part II Toxicological)
 Table 4. Acute toxicity of ethoxyquin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Rat Oral gavage 1700 97.6 Varsho (1995a)
Rat Dermal (24 h) > 2000 97.6 Varsho (1995b)
Rat Inhalation, whole body > 2.0 97.6 Ulrich (1996)
Mouse Intraperitoneal ~ 900 Wilson & DeEds (1959)a
Mouse Intravenous ~ 180 Wilson & DeEds (1959)a
a Cited in 1969 JMPR monograph
red-yellow staining of the fur. Gross and histopathological changes
indicated an irritant effect on the gastrointestinal tract.
Ethoxyquin produced transient, slight erythema when applied to
rabbit skin for 4 h under semi-occlusive conditions. There was no
oedema, but desquamation was present seven days after exposure
(Varsho, 1995c). This result is consistent with the findings of a
study summarized in the 1969 monograph (Kelly, 1960; cited in 1969
JMPR monograph; Annex 1, reference 13).
Ethoxyquin produced transient, slight-to-mild conjunctival
redness and chemosis in rabbits. All of the effects had fully
regressed within four days (Varsho, 1995d).
In a sensitization study in six guinea-pigs of each sex,
ethoxyquin was at most a very weak skin sensitizer. Induction was at
100%, with challenge applications at 50% in acetone. Very weak
erythematous responses were seen in both treated and control groups
after challenge and re-challenge, one test animal producing a weak
response to both challenge and re-challenge (Varsho, 1995e).
(b) Short-term studies of toxicity
Rats
Two studies of dietary administration of ethoxyquin to rats for
200 days were summarized in the 1969 monograph. Kidney lesions
(unspecified) were reported at 500 ppm and higher in males, with
increased kidney:body weight ratios in males at 250 ppm and higher.
The frequency of cytoplasmic inclusions in hepatocytes was increased
at 2000 ppm (Wilson & DeEds, 1959; Cox, 1953; cited in 1969 JMPR
monograph; Annex 1, reference 13).
In a study described in more detail below of the effects of
ethoxyquin on induction of liver tumours by N-nitrosodiethylamine, a
control group received ethoxyquin only. Thus, 15 male Fischer 344
rats, six weeks old, received an intraperiotneal injection of 0.9%
saline, were placed on a diet containing 8000 ppm ethoxyquin (purity
unspecified), equivalent to 500 mg/kg bw per day in young rats, in
week 2, and were partially (66%) hepatectomized in week 3. When the
animals were killed at week 8, a background level of gamma-glutamyl
transpeptidase (gamma-GT)-positive foci was found in the liver (Ito et
al., 1985).
Groups of five male and five female Sprague-Dawley rats received
ethoxyquin (purity, 97.6%) by gavage in corn oil for 28 days at doses
of 0, 50, 250, 500, or 1000 mg/kg bw per day. Histopathological
examination was limited to the liver, lung, kidney, stomach, and gross
lesions in animals at 50, 250, and 1000 mg/kg bw per day. All of the
animals at 1000 mg/kg bw per day had died by day 3 with multiple organ
involvement; the cause of death in two animals was considered to be
necrosis and ulceration of the forestomach. The prevalences of
salivation, stained fur, and brown urine were increased at 250 mg/kg
bw per day and higher. Initial body-weight gain was reduced by 50% in
males receiving 500 mg/kg bw per day. Erythrocyte count, haematocrit,
and haemoglobin concentration were decreased by about 10% in females
at 250 mg/kg bw per day and in animals of each sex at 500 mg/kg bw per
day. Alterations in serum clinical chemical parameters were seen in
both males and females, but were more frequent in males at 250 and
500 mg/kg bw per day; they included increased quantities of protein,
total bilirubin, cholesterol, inorganic phosphorus, potassium, and
calcium, and gamma-GT activity, while the concentration of glucose was
decreased. Increased absolute and relative liver weights (> 40%)
were seen in animals of each sex at 250 mg/kg bw per day and higher,
and the relative kidney weights were increased (< 10%) in a
dose-related fashion. There were no gross lesions at doses < 1000
mg/kg bw per day. Histopathological investigation showed kidney
lesions (interstitial infiltration, tubular epithelial regeneration,
and tubular dilatation) in males receiving 50 and 250 mg/kg bw per day
and in animals of each sex at 500 mg/kg bw per day. The incidences of
haemorrhage and oedema of the lung and hepatocellular swelling were
increased at 500 mg/kg bw per day. A NOAEL was not identified (Naas,
1997).
Groups of 10 Sprague-Dawley rats of each sex, six weeks old at
the beginning of the study, received ethoxyquin (purity, 97.6%) by
gavage in corn oil at 0, 20, 40, 200, or 400 mg/kg bw per day for 13
weeks. Minor overdosing (2-14%) of the group at 200 mg/kg bw per day
on day 67 is considered not to have compromised the study.
Ophthalmoscopy was performed before treatment and during week 12. A
full post-mortem examination was performed on all animals, and samples
of lung, liver, kidney, and gross lesions from all animals were
examined histologically, as were more than 30 tissues from controls
and from animals at the highest dose.
There were no deaths during the study. Clinical signs were seen
in animals of each sex, but more often in females, at 200 and 400
mg/kg bw per day, including staining of various body parts and
particularly the anogenital area, salivation, and brown urine.
Body-weight gain was clearly reduced in males at 200 and 400 mg/kg bw
per day, with a slight effect (10%) at 40 mg/kg bw per day; food
consumption was similar in test and control groups. Haematological and
clinical chemical parameters were altered in animals of each sex at
400 mg/kg bw per day, and many were also significant at 200 mg/kg bw
per day. These included increased reticulocyte counts, total
bilirubin, blood urea nitrogen, gamma-GT activity, cholesterol, and
thyroid-stimulating hormone; and decreased erythrocyte and leukocyte
counts, prothrombin time, and glucose. Urine was more deeply coloured
at 200 and 400 mg/kg bw per day, and the volume was increased in
animals at the highest dose, with no change in specific gravity. There
were no treatment-related effects on the eyes.
The main gross finding was reddened thyroids in animals of each
sex at 200 and 400 mg/kg bw per day. The absolute weights of the liver
and that relative to body weight were increased in a dose-related
fashion (by 15-70%), and those of the kidneys increased by 4-20% in
animals of each sex at 200 and 400 mg/kg bw per day; changes in the
body-weight ratios of brain and testes are considered to be secondary
to the reduced body weights. Histological examination identified the
kidney as the main target organ in animals of each sex, with increased
incidences of tubular mineralization, papillary necrosis, and
cytoplasmic vacuolation in males at the high dose; and increased
incidences of mineralization, papillary necrosis, and nephropathy in
females at the high dose. The incidence of nephropathy was also
increased in females at 200 mg/kg bw per day. The incidence of
ultimobranchial cysts of the thyroid was increased in males receiving
200 and 400 mg/kg bw per day and females receiving 200 mg/kg bw per
day. Increased incidences of cytoplasmic vacuolation of the adrenals,
suppurative inflammation of the epididymides, non-suppurative
inflammation of the prostrate, mineralization of the lung, and
alveolar histiocytosis were also seen in males at the high dose, and
the incidences of inflammation of the oesophagus and epithelial
hyperplasia of the thymus were increased in females at this dose. It
should be noted that only gross lesions, liver, lung, and kidney were
examined from groups at lower doses. As the decrease in body-weight
gain in males at 40 mg/kg bw per day was part of a dose-response
relationship and was not associated with reduced food consumption, the
NOAEL for this study was 20 mg/kg bw per day (Naas, 1996a).
Dogs
A one-year study in three dogs given ethoxyquin by gavage was
summarized in the 1969 monograph; the NOAEL was 3 mg/kg bw per day.
The effects reported at the next highest dose (10 mg/kg bw per day)
included renal nephrosis, increased bromosulphthalein retention
indicating liver dysfunction, and abdominal tenderness (Hanzal, 1955;
cited in 1969 JMPR monograph; Annex 1, reference 13).
Groups of one male and one female beagles received ethoxyquin
(purity, 97.6%) by capsule at a dose of 0, 25, 50, 100, or 200 mg/kg
bw per day for 28 days. All of the animals at 100 or 200 mg/kg bw per
day group died or were sacrificed by day 17 or day 7, respectively;
one female at 50 mg/kg bw per day was sacrificed on day 21. The signs
seen in dogs that died and in survivors included hypoactivity, reduced
defaecation, brown urine, and pale gums. Given the small initial group
sizes and the deaths, only major, consistent changes are summarized
here. Reduced body-weight gain and food consumption were seen at all
doses. The serum activities of enzymes indicative of liver damage were
increased at four weeks in all groups in which they were measured (25
and 50 mg/kg bw per day): alkaline phosphatase by fivefold, aspartate
aminotransferase by threefold, alanine aminotransferase by 20-fold,
and gamma-GT by threefold; there were indications of reduced activated
partial thromboplastin times. The ratios of liver and kidney weights
to body weight were increased at 25 and 50 mg/kg bw per day. Common
post-mortem findings included redness of the gastrointestinal tract
and darkened livers. Histological examination showed pigmentation of
the liver in all treated animals but not in controls. A NOAEL was not
identified (Naas, 1996b).
In a 90-day study, groups of five beagles of each sex were given
ethoxyquin (purity, 97.6%) by capsule at 0, 2, 4, 20, or 40 mg/kg bw
per day. Clear signs of toxicity were seen during the first seven
weeks of the study at 40 mg/kg bw per day, including reduced body
weight, staining of the body surface, brown urine, brown sclera, dark
mucoid faeces, and emesis, and these groups received only empty
capsules for the final six weeks of the study, effectively becoming
reversibility groups. Investigations of clinical chemistry (including
thyroid hormones), haematology, and ophthalmoscopy were performed
before treatment and at weeks 4 and 12 or 13. Post-mortem
investigations included microscopic examination of a wide range of
tissues from all animals and special stains for pigment
identification.
One female at the highest dose was sacrificed in extremis on
day 13. Other findings were similar in males and females. Clinical
signs including brown staining of the abdomen and urogenital area,
brown urine, decreased faeces, and emesis were seen regularly at 20
and 40 mg/kg bw per day and occasionally during the 4 h after dosing
at 4 mg/kg bw per day; these signs were still present between weeks 7
and 13 ('recovery') in animals at the highest dose. Body-weight loss
occurred at 40 mg/kg bw per day in weeks 1-7, which reversed when
dosing stopped, but females had a lower (12%) mean body weight than
controls at termination. At 20 mg/kg bw per day, body-weight gain was
reduced (60%) throughout the study. Food consumption was reduced at 20
(20%) and 40 mg/kg bw per day (up to 50%). The only notable change in
haematological parameters was a dose-related decrease in activated
partial thromboplastin times in males at 4 mg/kg bw per day and higher
and in females at the highest dose. Marked increases in total
bilirubin concentration and in alkaline phosphatase, alanine
aminotransferase, aspartate aminotransferase, and gamma-GT activities
in serum, indicative of liver dysfunction, were seen at 20 mg/kg bw
per day in weeks 4 and 12 or 13 and at 40 mg/kg bw per day in week 4
only; alanine aminotransferase and, to a lesser extent, alkaline
phosphatase activities were also increased at 4 mg/kg bw per day. By
week 13, the serum values in animals at 40 mg/kg bw per day
(seven-week treatment, six-week recovery) had returned approximately
to control values. There were no significant changes in absolute or
relative organ weights. Treatment-related gross and microscopic
findings were limited to the liver. At 20 and 40 mg/kg bw per day, a
darkened appearance was associated microscopically with increased
pigment deposition, hepatocellular necrosis, cytoplasmic vacuolation,
and bile-duct hyperplasia; at 4 mg/kg bw per day, occasional findings
of mild or moderate pigmentation, minimal hepatocellular necrosis, and
vacuolation were recorded. The pigments were found to be porphyrin and
bilirubin in most cases, some sections also staining for haemosiderin.
The NOAEL was 2 mg/kg bw per day (Naas, 1996c).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Solutions of ethoxyquin (as Santoquin(R)) at 10 or 50 mg/ml
were administered subcutaneously to neonatal Swiss ICR/Ha mice on days
1 and 7 (0.1 ml) and 14 and 21 (0.2 ml) of age or as a single dose of
100 mg/ml (0.1 ml on day 1). Each dose was equal to 500, 2500, and
5000 mg/kg bw on day 1 and 250 and 1250 mg/kg bw on day 21. The groups
consisted of 57 mice at the low dose, 53 at the intermediate dose, and
28 at the high dose. By the time the mice were weaned, 100% at the
high dose, 74% at the intermediate dose, and 2% at the low dose had
died; 15% of controls had died at this time. Small groups of mice were
sacrificed at various times up to termination at week 53. A limited
range of tissues and lesions were examined primarily for tumours. The
incidences of pulmonary tumours and hepatomas were similar in the
treated and control groups; a slight increase in the incidence of
malignant lymphoma in four females at the low dose and two at the
intermediate dose, with none in controls, was considered equivocal by
the authors. The results indicates that four subcutaneous
administrations of ethoxyquin at near-lethal doses to neonatal mice
did not significantly increase the incidence of tumours in mice up to
one year of age (Epstein et al., 1970).
Rats
A two-year study of ethoxyquin in the diet of rats was summarized
in the 1969 monograph. Groups of approximately 10 male and 10 female
rats were fed diets containing ethoxyquin at concentrations of 0, 62,
125, 250, 500, 1000, 2000, or 4000 ppm for up to two years. The
animals were sacrificed for autopsy after 200, 400, 600, or 715 days.
The mortality rates were not significantly different from those of
controls. Significantly reduced body-weight gain was seen at 2000 ppm
after 225 days in males and after 21 days in females. After 200 days,
increased relative liver and kidney weights were found in males at 250
ppm and in females at 1000 ppm. The haemoglobin values for animals of
each sex were normal 100 and 300 days after the start of the
experiment in rats fed 2000 and 4000 ppm. Histological changes in the
renal cortex were clearly evident after 200 days in male rats
receiving 2000 or 4000 ppm but not in females. All other organs were
normal in animals of each sex after 200 days. After 400 days, lesions
in the kidneys (pyelonephritis), liver, and thyroid were seen in males
only. Similar lesions were seen for periods up to 717 days in animals
of each sex, although they were more marked in males. Occasional
tumours were found after 700 days, but the incidence was unrelated to
dose, and they were also seen in controls. No clearly defined effects
were evident after feeding 62 ppm, but minute lesions were present in
the kidneys of two males receiving 500 ppm. It was difficult to
distinguish the abnormalities in the group examined after 700 days
from the pathological manifestations of senility after that time
(Wilson & DeEds, 1959; cited in 1969 JMPR monograph; Annex 1,
reference 13). The 1969 JMPR concluded that the NOAEL was 125 ppm,
equivalent to 6 mg/kg bw per day. The small groups used in this study
limit its sensitivity for detecting changes in rare events such as
tumours with low background rates; however, the wide spread of doses
and sequential sampling times provides a degree of assurance in the
reported findings.
As part of an investigation of kidney and liver tumours induced
by N-nitrosoethyl- N-hydroxyethylamine, one control group received
only ethoxyquin. This group of 25 male Fischer 344 rats received a
diet containing 8000 ppm ethoxyquin from nine weeks of age to
termination at 41 weeks of age. Sections of liver, kidney, and gross
lesions were investigated histologically. No gamma-GT-positive foci,
hyperplastic nodules, or hepatocellular carcinoma were found in the
liver; no data were presented on kidney lesions. A control group of 25
male Fischer 344 rats received a diet containing 8000 ppm ethoxyquin
as part of a parallel investigation of urinary bladder carcinogenesis
induced by N-nitrosobutyl- N-hydroxybutylamine. After 32 weeks,
there were higher incidences of simple hyperplasia and papillary or
nodular hyperplasia of the urinary bladder than in groups receiving
ascorbic acid or sodium erythorbate, the incidence of simple
hyperplasia being greater than that induced by
N-nitrosobutyl- N-hydroxybutylamine alone; no untreated controls
were used. No urinary bladder papillomas or carcinomas were seen in
the group receiving ethoxyquin only (Ito et al., 1985).
An almost identical study of urinary bladder carcinogenesis
showed that 24 weeks' exposure to a diet containing 8000 ppm
ethoxyquin, considered to be equivalent to 400 mg/kg bw per day, did
not induce papillary or nodal hyperplasia or papilloma of the urinary
bladder in a group of 15 male Fischer 344 rats (Miyata et al., 1985).
The dependence of the renal lesions produced by ethoxyquin on age
and sex was investigated in Fischer 344 rats. Groups of four to eight
male rats received diets containing ethoxyquin (purity, 90%) at 5000
ppm from three or eight weeks of age for 20, 26, or 30 weeks. Eight
female rats received a diet containing 5000 ppm ethoxyquin for 30
weeks from eight weeks of age. Histopathological examination of the
kidney comprised bromodeoxyuridine (BrdU) labelling, gamma-GT
histochemistry, haematoxylin and eosin staining, Alizarin red
staining, and immunoblotting of urine samples for albumin and alpha2u
globulin. Body-weight gain was reduced by 10-15% in treated animals.
In males, the absolute kidney weights were increased by 5-50%, with a
consequent increase in the relative weights; females showed a 12%
increase in kidney:body weight ratios. Renal cortical changes were
seen in all treated males, consisting of eosinic cytoplasmic
inclusions in tubular epithelial cells and protein accumulation in the
lamina of the tubules. In males dosed from three weeks of age,
papillary necrosis, slight calcium deposition, and hyperplasia of the
transitional epithelium of the renal pelvis were seen. The
histological appearance of the kidneys of treated females was similar
to that of controls, except for high concentrations of lipofuscin
deposition. BrdU labelling was increased in males at 30 weeks, but not
at 20 weeks, in both regenerating basophilic tubules and those
staining normally with haematoxylin and eosin; BrdU labelling in
females was not described. The concentrations of alpha2u globulin in
urine were slightly lower in treated males, but those of albumin were
significantly increased. The time of first exposure can thus
significantly alter the pattern of renal lesions in rats consuming
diets containing ethoxyquin at 5000 ppm, equivalent to 250 mg/kg bw
per day, exposure from three weeks of age resulting in papillary
necrosis in addition to the cortical lesions seen in animals exposed
from eight weeks of age (Manson et al., 1992).
Groups of 6-19 Fischer 344 rats of each sex, three weeks of age
at the start of the study, received diets containing ethoxyquin
(purity unspecified) at 0 or 5000 ppm for up to 18 months; one group
received ethoxyquin in the diet for 24 weeks followed by 34 weeks on
control diet. The study was designed to investigate the progression of
renal lesions and involved interim sacrifices at 4, 12 or 14, 24, 58,
and 78 weeks. The body-weight gain of treated females was reduced in
weeks 1-5 and that of males from week 3 onwards; food consumption was
reduced in animals of each sex during the first four weeks.
Investigations of renal pathology showed a clear difference between
males and females. Males had significant interstitial degeneration of
the papilla at weeks 4 and 14, which progressed to necrosis with
pyelonephritis of the cortex and urothelial hyperplasia of the renal
pelvis by week 24. In females, interstitial degeneration of the
papilla was only slight at week 14 and did not progress consistently.
The chronic progressive nephropathy commonly seen in Fischer 344 rats
was accelerated in animals receiving ethoxyquin. The authors reported
that this was more marked in males, but the data presented do not
substantiate that statement. A golden-brown pigmentation, found to be
lipofuscin by Schmorl's stain, was noted in the proximal tubules of
treated rats, particularly females. The lesions present at 24 weeks
showed no evidence of reversibility after 34 weeks on control diets.
The authors considered that there was no evidence for preneoplastic
proliferative lesions. This study showed that ethoxyquin at 5000 ppm
in the diet, equivalent to 250 mg/kg bw per day, is a potent
nephrotoxin in young male Fischer 344 rats (Hard & Neal, 1992).
A number of reports have been published of the results of
investigations into the effects of antioxidants, including ethoxyquin,
on the induction of neoplasia and preneoplastic lesions by known
carcinogens. The most comprehensive series of studies with ethoxyquin
is probably that of Ito and co-workers (Ito et al., 1985; Miyata et
al., 1985; Masui et al., 1986), who used Fischer 344 rats to
investigate moderation of effects on the liver induced by
N-nitrosodiethylamine, effects on the kidney and liver produced by
N-nitrosoethyl- N-hydroxyethylamine, and effects on the bladder
produced by N-nitrosobutyl- N-(4-hydroxybutyl)amine.
Groups of 18 six-week-old male Fischer 344 rats received an
intraperitoneal injection of 200 mg/kg bw N-nitrosodiethylamine in
0.9% saline; two weeks later, they were transferred to a diet
containing 0 or 8000 ppm ethoxyquin (purity unspecified) and underwent
a partial (60%) hepatectomy one week later. The rats were sacrificed
at week 8, and liver sections were stained with haematoxylin and eosin
and a histochemical stain for gamma-GT-positive foci. The rats
receiving ethoxyquin had a significant (p < 0.001) decrease in the
number of foci (0.9 versus 3.3/cm2) and in the area of the foci (0.06
versus 0.19 mm2/cm2) (Ito et al., 1985).
Groups of 23 or 27 six-week-old male Fischer 344 rats received
drinking-water containing 0.1% N-nitrosoethyl- N-hydroxyethylamine
for two weeks; between weeks 3 and sacrifice at week 32, they received
diets containing 0 or 8000 ppm ethoxyquin (purity unspecified). All
rats had gamma-GT-positive foci, but the ethoxyquin-treated group had
fewer (1 versus 21/cm2) and smaller (10 versus 22 mm2/cm2) foci.
The group given ethoxyquin also had a reduced area of hyperplastic
nodules (2.3 versus 7 mm2/cm2), and fewer animals had hepatocellular
carcinomas (3/27 versus 11/23). Conversely, the kidneys of the
ethoxyquin-treated group had higher frequencies of atypical-cell foci
(0.9 versus 0.2/cm2) and adenomas (5.6 versus 0.8/cm2), and there
were more animals with foci (26/27 versus 12/23) and adenomas (17/27
versus 5/23) and larger foci (5.6 versus 0.9 × 10-2 mm2/cm2) and
adenomas (24 versus 8 × 10-2 mm2/cm2) (Ito et al., 1985).
Groups of 25 six-week-old male Fischer 344 rats received
drinking-water containing 0.05%
N-nitrosobutyl- N-(4-hydroxybutyl)amine for four weeks; between
weeks 4 and sacrifice at week 36, they received diets containing 0 or
8000 ppm ethoxyquin (purity unspecified). Examination of the urinary
bladders showed that ethoxyquin had increased the incidence of animals
with simple hyperplasia (25/25 versus 14/24), the incidence and extent
of papillary or nodular hyperplasia (25/25 versus 8/24 and 9.4 versus
0.48/10 cm of basement membrane), the incidence and extent of
papillomas (17/25 versus 5/24 and 1.1 versus 0.19/10 cm), and the
incidence and extent of carcinomas (4/25 versus 1/24 and 0.17 versus
0.04/10 cm) (Ito et al., 1985). A similar study involving two weeks'
dosing with N-nitrosobutyl- N-(4-hydroxybutyl)amine (0.05% in
water) and sacrifice at 24 weeks also showed that dietary
administration of ethoxyquin at 8000 ppm (purity unspecified)
increased the incidence and extent of papillary or nodular hyperplasia
but not of papillomas of the urinary bladder (Miyata et al., 1985).
This group of studies shows that ethoxyquin markedly reduces the
preneoplastic effects of N-nitrosodiethylamine and
N-nitrosoethyl- N-hydroxyethylamine on the liver, possibly by a
combination of antioxidant effects and induction of detoxification
mechanisms. The increase produced by ethoxyquin in the incidence of
neoplastic and preneoplastic kidney lesions induced by
N-nitrosoethyl- N-hydroxyethylamine may be secondary to the direct
toxic effects of ethoxyquin on the kidney. The mechanism of the
effects of ethoxyquin on
N-nitrosobutyl- N-(4-hydroxy-butyl)amine-induced urinary bladder
neoplasia is unknown.
Dogs
Ethoxyquin was fed to two groups of 14 dogs and bitches at a
dietary concentration of 0 or 300 ppm for five years. No effects were
observed on haematological, urinary, or clinical chemical end-points
(aspartate aminotransferase activity, blood urea nitrogen, and
bromosulphthalein retention), organ weights, organ:body weight ratios,
body weight, or gross or histopathological appearance (Monsanto, 1966;
cited in 1969 JMPR monograph; Annex 1, reference 13). The 1969 JMPR
concluded that the NOAEL in this study was 300 ppm, equivalent to 7.5
mg/kg bw per day.
(d) Genotoxicity
A number of published papers indicate that ethoxyquin is not
genotoxic in bacterial systems (Table 5); however, these reports could
not be validated as only minimal details were available. The results
of assays in eukaryotic systems have not been reported.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
After 40 days on a diet slightly deficient in tocopherol and
containing 0, 250, 500, or 1000 ppm ethoxyquin, rats were bred to
produce three consecutive litters. The offspring of the first litter
were used to produce a second-generation litter. The highest dose was
discarded after production of one litter (reason unknown). No effects
on reproduction, as reflected in fertility, litter size, or survival
of offspring, were observed. The animals receiving the experimental
diet produced young and raised them more successfully than controls,
the 500 ppm diet being more effective than the 250 ppm diet (Wilson,
1956; Wilson & DeEds, 1959, cited in 1969 JMPR monograph; Annex 1,
reference 13). The short dosing period before mating and the unknown
purity and group size compromise this report to some extent, but it
Table 5. Results of assays for the genotoxicity of ethoxuquin
End-point Test system Concentration Purity Result Reference
(%)
Reverse mutation S. typhimurium 10-1000 µg/plate 'Pure' Negativea Joner (1977)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium > 5000 µg/plate NR Negativea Ohta et al. (1980)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr trp
Reverse mutation NR NR NR Negative Zeiger (1993)
Gene mutation B. subtitlis H17 0.2 ml NR Negative Ohta et al. (1980)
rec+ and M45 rec-
a With and without metabolic activation
can be concluded that ethoxyquin in the diet at 500 ppm, equivalent to
25 mg/kg bw per day, has no marked effect on reproductive outcome.
Another study cited in the 1969 JMPR monograph had some results
contrary to those described above. Groups of eight or nine female rats
were placed on diets containing 0, 125, 375, or 1125 ppm ethoxyquin on
the day of mating. The length of gestation was comparable in all
groups, but the litter size was slightly depressed at doses of 375 ppm
and higher, and at 1125 ppm the incidence of stillbirths was increased
and survival to weaning was decreased. The NOAEL in this unusual study
was 125 ppm, equivalent to 6 mg/kg bw per day. In a separate part of
the same study, no effects were found on litter size, the number of
stillbirths, survival to weaning, or weanling weight in rats receiving
up to 1125 ppm ethoxyquin in the diet starting between days 1 and 10
of gestation (Derse, 1956; cited in 1969 JMPR monograph; Annex 1,
reference 13).
Dogs
The effects of ethoxyquin on reproduction over two generations
were studied in groups of beagles. Dogs were chosen as ethoxyquin is
added to commercial dog food to help prevent oxidative deterioration.
In the first mating (F0), groups of five males and 10 females
received diets containing ethoxyquin (Santoquin(R)) at a mean
analytical concentration of 0, 100, or 225 ppm for a minimum of 82
days before pairing. The eight male and 13 female pups used
subsequently for the F1 matings received diets containing 0, 100, or
225 ppm ethoxyquin from weaning until breeding at 10-30 months (2nd
estrus cycle in females). Semen samples were taken during the first
week of treatment and at around the time of breeding in order to
determine the volume, sperm count, motility, speed, and morphology.
Animals were observed and underwent extensive physical examinations
routinely; if possible, they were also observed during labour. Mating,
whelping, and lactation indices were determined. Urine samples and
blood samples were taken for haematology and clinical chemistry from
fasted adults before treatment and at the end of the F0 phase; at
weeks 10, 23, 36, 49, and 62 and at termination in the F1 growth
phase; and at termination of the F1 mating phase. Ophthalmological
examinations were performed at the beginning and end of the F1 growth
and mating phases. All F1 adults and pups that showed signs of
toxicity were necropsied. A range of tissues from controls and F1
adults at the high dose were examined histologically, with selected
tissues from F2 pups that showed clinical signs; the livers and
gall-bladders from F1 adults at the low dose and the adrenals and
spleens from F1 adult females at the low dose were also examined.
Macroscopic and microscopic examinations were performed only on F0
and F1 animals that died or were sacrificed prematurely.
In the F0 mating, there was considerable intra-group variation
in body weights, but F0 adults receiving 225 ppm ethoxyquin showed a
trend for reduced body weight from the initiation of dosing to week 17
and during the latter stages of gestation. Males thad reduced food
consumption during most of the study. Two females at the high dose
that were confirmed to be pregnant did not give birth. There were no
other differences between the groups in mating performance, labour,
birth, or weaning indices, semen parameters, or clinical signs. Litter
size, pup survival, and pup weight and growth were similar in all
groups. At 225 ppm, there was an increased number of pups of each sex
with a raw or red anus, dehydration, nasal discharge, and excessive
lachrymation; the incidence of the last two signs was also increased
at 100 ppm. Statistically significant increases in serum alkaline
phosphatase activity were seen in male parents at the high dose and in
female parents at the low and high dose; there was also an indication
of reductions in monocytes and partial thromboplastin times in animals
of each sex at the high dose, although all of the values were claimed
to be within the normal ranges. There were no effects on urinary
parameters. Remating of three female controls and two at 225 ppm from
this phase which failed to mate during the initial phase was
successful.
Among F1 animals, one male at the low dose and two females at
the high dose died or were sacrificed in extremis. The male was
sacrificed because of suspected neurological signs; one of the females
died of suspected heart disease, and the other was sacrificed because
of pneumonia. The clinical signs included excessive lachrymation,
dehydration, thinness, and pale gums and showed a dose-related
increase in both the number of animals of each sex with a particular
sign and the number of occasions on which it was observed. Males at
the high dose had a lower mean body weight than controls up to week 48
of the study. Initially, animals at the high dose consumed more food
than controls, but food consumption was consistently lower in weeks
8-18 in males and in weeks 8-30 in females. Considerable variations in
haematological end-points were seen throughout the study in both
treated and control animals. There was evidence of treatment-related
effects on erythrocyte count, haematocrit, and haemoglobin, which were
reduced by up to 11% relative to controls in treated males and females
at weeks 10 and 23, and on partial thromboplastin times, which were
reduced in females at the high dose in weeks 23 and 36 and in females
at the low dose in weeks 23 and 62 and at the final analysis.
Increased serum activities of alkaline phosphatase, gamma-GT, and
alanine aminotransferase and reduced albumin:globulin ratios were
found in animals at the high dose in weeks 10, 23, and 36, with
evidence of lesser perturbations at the lower dose. These changes are
indicative of impaired liver function. The results of urinary analysis
were unremarkable.
In the F1 mating, there were no clear differences in semen
analyses or mating, gestation, whelping, or weaning indices between
control and ethoxyquin-treated animals. In adults, the only
treatment-related clinical sign was excessive lachrymation, which
occurred more frequently in males at the low and high doses than in
controls. Haematological end-points were similar in all groups.
Dose-related changes were seen in a number of clinical chemical
parameters in females, which attained statistical significance
(p < 0.05) at the high dose. These comprised reductions in glucose,
cholesterol, protein, albumin, and albumin:globulin ratio, and
increases in total bilirubin concentration and in gamma-GT, alkaline
phosphatase, and alanine aminotransferase activities. In males, the
dose-related increases in alkaline phosphatase, gamma-GT, and alanine
aminotransferase activities did not attain statistical significance.
Macroscopic examination showed dark-plum-coloured livers in one male
and two females at the high dose and cervical lymph node haemorrhages
in two females at the low and high doses; these lesions were possibly
related to treatment as they were not present in control animals.
Increases in the absolute weights of the spleen and testes and in the
weights of these organs relative to the brain weight were seen in
treated males, giving statistically significant increases in relation
to body weight. In females, increases in the absolute and relative
weights of the liver (10%), kidneys (10%), and spleen (40%) were
reported but were not statistically significant. Histopathological
examination showed that the liver, pituitary, and spleen were the
target organs. The macroscopic finding of increased cervical lymph
node haemorrhage in females was not confirmed. A dark-reddish-brown
pigment, subsequently identified as protoporphyrin IX, was not found
in the livers of controls or males at the low dose but was present in
the livers of 7/13 females at the low dose, 2/7 males at the high
dose, and 10/11 females at the high dose, with a dose-related increase
in severity. The frequencies of fibrosis and haemorrhage of the spleen
were increased in females at the high dose (3/11 versus 0/13 in
controls), and the incidence of pituitary cysts was increased in
animals at the high dose when compared with controls (2/6 versus 0/8
in males and 4/10 versus 2/12 in females)
Treated male pups had increased incidences of grey or pale gums,
excessive lachrymation, and dehydration, and female pups had an
increased incidence of dehydration. The pup weights at birth and to
week 6 of gestation were slightly reduced (< 10%), with a
dose-related effect in female pups. An increased mortality rate in
pups at the low dose was not seen at the high dose and was probably
related to the larger litter sizes in this group; the rates of
mortality were 7/62 controls, 24/91 at the low dose, and 10/77 at the
high dose.
During the study, four males and one female at 100 ppm and two
females at 225 ppm showed signs of neuropathy: The animals had
impaired hindlimb function, inability to stand, and unsteadiness of
the head and body which was found to be associated with myelin
degeneration. Examination of clinically normal littermates showed no
neurological deficits. The breeding records showed that all of the
affected animals had a common male ancestor which was not in the
breeding line of any of the control animals. When the parents of some
of the affected pups were removed from the treated diets and mated,
the incidence of neurologically affected animals was 17% in one litter
and 25% in the other. The evidence from this part of the study is
strongly indicative of a genetic etiology.
Estimation of the actual intakes of ethoxyquin in this study was
confounded by up to twofold increased consumption during lactation and
the fact that 180 and 360 ppm had to be added to obtain nominal
concentrations of 150 and 300 ppm, however, the analysis showed mean
initial values of 100 and 225 ppm. Although the actual amounts of food
consumed varied during the study, a value of 25 g/kg bw per day was
considered to be a representative mean, which resulted in intakes of
2.5 mg/kg bw per day ethxyquin at 100 ppm and 6 mg/kg bw per day at
225 ppm.
The results of this study show that ethoxyquin in the diet at
concentrations up to 225 ppm did not affect reproductive performance
or outcome in beagles. There was no clear overall NOAEL because of
increased incidences of clinical signs such as excess lachrymation and
dehydration, clinical chemical changes and pigment deposition in the
liver. The lowest dose, 100 ppm, equal to 2.5 mg/kg bw per day, was
considered to be the minimal effect level (Gilman & Voss, 1995).
(ii) Developmental toxicity
Rats
In a range-finding study for teratogenicity in Sprague-Dawley
rats, groups of eight mated females received ethoxyquin (purity,
97.6%) by gavage in corn oil at 0, 62, 125, 250, 500, or 1000 mg/kg bw
per day on days 6-19 of gestation. All animals given 1000 mg/kg bw per
day died or were sacrificed by day 9, and three animals given 500
mg/kg bw per day died between days 10 and 11 of gestation; post-mortem
examinations did not show any adverse effects. Clinical signs of
reduced defaecation, dark urine, and brown staining of fur were
dose-related and affected all treated groups. Reduced food consumption
and body-weight loss were seen at doses > 125 mg/kgbw per day at
the beginning of dosing; from day 9 onwards, body-weight gain was
similar in all groups up to 500 mg/kg bw per day, and these animals
had a 20% body-weight deficit by day 20 when compared with controls.
Fetal weights were reduced at 500 mg/kg bw per day, but examination
for external malformations, sex ratio, and crown-rump length showed no
effects of treatment (Nemec, 1996a).
In the main study, groups of 25 mated female Sprague-Dawley rats
received ethoxyquin (purity, 97.6%) by gavage in corn oil at 0, 50,
150 or 350 mg/kg bw per day on days 6-19 of gestation. The dams were
sacrificed on day 20, their uteri and ovaries were examined, and all
fetuses were investigated for weight, sex, and external and visceral
malformations. The heads of one-half of the fetuses were examined by
Wilson sectioning and the other half by mid-coronal section. All
fetuses were stained with Alizarin Red S for skeletal investigation.
There were no deaths during the study. Urogenital staining was seen in
dams after treatment at the highest dose, and staining of other areas
was also seen in these and in some animals receiving 150 mg/kg bw per
day. At 350 mg/kg bw per day, dams lost weight on days 6-7, and a 13%
reduction in body-weight gain compared with controls was evident on
days 6-20; 150 mg/kg bw per day resulted in a 5% reduction in
body-weight gain on days 6-20. Food consumption was reduced by 9% at
150 mg/kg bw per day and by 13% at 350 mg/kg bw per day. There were no
significant findings in dams post mortem: uterine weights, litter
size, resorptions, pre- and post-implantation losses, sex ratios, and
fetal weights were similar in all groups. Isolated findings of
malformations and anomalies were within the range in historical
controls and showed no relationship to treatment. The overall
incidence of variations was highest in controls, with no significant
increase in any individual variation. The overall NOAEL for this study
was 50 mg/kg bw per day on the basis of clinical signs (fur staining)
and reduced maternal body weight at higher doses. The NOAEL for
fetotoxicity was 350 mg/kg bw per day, the highest dose tested (Nemec,
1996b).
3. Observations in humans
Cases of dermatitis have been reported in workers handling fruit
treated with ethoxyquin. A study with patch tests, cited in the 1969
monograph, showed that the cause was a sensitization reaction rather
than direct irritation (Wood, 1965).
A number of reports (Burrows, 1975; van Hecke, 1977; Zachariae,
1978; Brandao, 1983) have indicated that ethoxyquin is the probable
cause of an often severe dermatitis seen in workers who handle animal
feed containing ethoxyquin. Positive results in patch tests have been
recorded in affected workers given challenge concentrations of as
little as 0.01% ethoxyquin in petrolatum (Zachariae, 1978). The
authors of some of the reports indicated that airborne contamination
and light sensitivity are implicated.
A study cited in the 1969 monograph indicated that no evidence of
skin irritation or sensitivity had been reported in 20 years of
ethoxyquin production (Kelly, 1960).
Comments
Published studies show that ethoxyquin is rapidly absorbed from
the gastrointestinal tract of rats and mice, with peak blood levels
within 1 h. Liver, kidney, and adipose tissue have the highest tissue
concentrations. Excretion occurs predominantly via the urine and is
rapid, with more than 85% of doses up to 25 mg/kg bw being excreted
within 24 h. At 250 mg/kg bw, absorption and excretion are slowed,
which is attributed to reduced gastric emptying, and only 50% of the
dose is excreted within 24 h. Repeated oral doses of 25 mg/kg bw per
day resulted in an excretion profile similar to that for single doses,
but repeated administration of 250 mg/kg bw per day was reported to
result in a profile similar to that for lower doses, indicating
induction of metabolism, transport, and/or a return to normal gastric
emptying. Biliary excretion and enterohepatic recirculation play a
significant role in the toxicokinetics of ethoxyquin, more than 40% of
an intravenous dose of 25 mg/kg bw being detected in the bile of
bile-duct-cannulated rats. The metabolism of ethoxyquin involves
O-deethylation or hydroxylation followed by conjugation as the
sulfate or glucuronide. A proposed reaction scheme for the production
of biliary metabolites involves epoxidation and the generation of
reactive, electrophilic intermediates. No information on plant
metabolites was available.
Ethoxyquin has low acute toxicity when administered orally (LD50
= 1700 mg/kg bw), dermally, or by inhalation. It is slightly
irritating to the eyes and skin and had only very weak sensitizing
potential when administered topically to guinea-pigs. Exposure to
ethoxyquin in the workplace has been linked to allergic contact
dermatitis, and the substance should be considered as a sensitizer in
humans.
WHO has not classified ethoxyquin for acute toxicity.
The main target organ after repeated administration of ethoxyquin
to rats for 28 days or more at doses of 50-1000 mg/kg bw per day was
the kidney. Mechanistic studies with dietary concentrations equivalent
to 250 mg/kg bw per day showed that the precise effects were dependent
on the age at first exposure, were progressive, more severe in males
than in females, and not reversible after 24 weeks of exposure. Other
effects seen in rats exposed to ethoxyquin for 28 or 90 days at doses
> 200 mg/kg bw per day were stained fur, brown urine, changes to
haematological parameters, increased liver weights, and changes in
clinical chemical parameters consistent with altered liver function.
The overall NOAEL in the short-term studies in rats was 20 mg/kg bw
per day.
In dogs given capsules containing ethoxyquin for 90 days at 0, 2,
4, 20, or 40 mg/kg bw per day, the liver was the primary target.
Alterations in haematological parameters and clinical chemical changes
indicative of altered liver function were seen at doses > 4 mg/kg bw
per day, together with hepatocellular necrosis, vacuolation, and
pigment deposition. Although staining indicated that the pigment was
haemosiderin, a specific investigation showed it to be protoporphyrin
IX. The overall NOAEL in short-term studies in dogs was 2 mg/kg bw per
day. This is consistent with the results of an older, one-year study
in dogs in which a NOAEL of 3 mg/kg bw per day was established on the
basis of findings suggestive of effects on the kidney and liver at 10
mg/kg bw per day.
No modern long-term studies of toxicity or carcinogenicity have
been performed. In studies summarized by the 1969 Meeting in which
ethoxyquin was administered to dogs at 0 or 300 ppm in the diet for 5
years or at 0, 3, 10, 50, or 100 mg/kg bw per day by gavage for one
year, effects were observed in the liver and kidneys at doses of 10
mg/kg bw per day and above. The NOAEL was 300 ppm, equivalent to 7.5
mg/kg bw per day. A two-year study in rats that received dietary
concentrations of 0, 62, 125, 250, 500, 1000, 2000, or 4000 ppm,
published in 1959, gave no indication of carcinogenicity, with an
overall NOAEL of 125 ppm, equivalent to 6 mg/kg bw per day; lesions in
the kidney, liver, and thyroid gland were seen at higher doses.
Mechanistic studies on tumour induction and promotion show that
ethoxyquin induces both phase-I and phase-II xenobiotic metabolism.
Although its incorporation into the diet at 8000 ppm after treatment
with an N-nitrosamine reduced the formation of preneoplastic foci in
the liver, it increased the incidence of preneoplastic and neoplastic
events in the kidney and urinary bladder. No significant increase in
tumour incidence was seen after one year in mice that received four
subcutaneous, near-lethal doses of ethoxyquin.
Published reports of studies of bacterial mutagenicity indicate
that ethoxyquin is not mutagenic in prokaryotic systems, but only
limited details of the protocols and results were provided. No data
were available on other genotoxic end-points.
No modern study of reproductive toxicity has been performed in
rodents. Three studies in which rats received 0, 125, 250, 375, 500,
1000, or 1125 ppm in the diet, all with non-standard protocols, which
were summarized by the 1969 Meeting, gave slightly contradictory
results. Two of the studies, including the most extensive, apparently
showed no effects on the aspects of reproduction investigated at doses
up to 1125 ppm in the diet (equivalent to 56 mg/kg bw per day), while
the other showed an increased incidence of stillbirths at 1125 ppm and
decreased litter size at 375 ppm, with a NOAEL of 125 ppm (equivalent
to 6 mg/kg bw per day).
A modern two-generation study of reproductive toxicity in dogs
given diets containing 0, 100, or 225 ppm showed that ethoxyquin had
no effects on reproductive parameters at 225 ppm (equivalent to 5.6
mg/kg bw per day), the highest dose tested. The clinical signs
observed included dehydration, excess lachrymation, and evidence of
hepatic toxicity, especially in bitches. The effects were seen at both
doses and were consistent with the results of the short-term studies
in dogs. The findings in bitches may have been related to increased
consumption during gestation and lactation. The lowest dose tested,
100 ppm, equivalent to 2.5 mg/kg bw per day, was considered to be a
minimal effect level.
A study of developmental toxicity in rats at 0, 50, 150, or 350
mg/kg bw per day showed that ethoxyquin is not fetotoxic or
teratogenic at doses up to 350 mg/kg bw per day. Maternal toxicity,
stained fur, and reduced body-weight gain were seen at 150 and 350
mg/kg bw per day. No studies of developmental toxicity have been
performed in other species.
An ADI of 0-0.005 mg/kg bw per day was established on the basis
of the minimal-effect level of 2.5 mg/kg bw per day in the
multigeneration study in dogs and a 500-fold safety factor to account
for the lack of a NOAEL in this study and for the incompleteness of
the database. The multigeneration study of reproductive toxicity was
of longer duration and more recent than a 90-day study in dogs treated
by gavage with a NOAEL of 2 mg/kg bw per day.
An acute RfD was not allocated because ethoxyquin is of low acute
toxicity. The Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 125 ppm, equivalent to 6 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equivalent to 25 mg/kg bw per day
(two-generation study of reproductive toxicity)
50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
350 mg/kg bw per day (developmental toxicity)
Dog: 2 mg/kg bw per day (general toxicity in a 90-day study
of toxicity)
3 mg/kg bw per day (one-year study of toxicity)
300 ppm, equivalent to 7.5 mg/kg bw per day (five-year
study of toxicity)
2.5 mg/kg bw per day (minimal effect level for general
toxicity in a two-generation study of reproductive
toxicity)
5 mg/kg bw per day (reproductive performance; highest
dose tested)
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of genotoxicity in mammalian systems
2. A long-term study of toxicity and carcinogenicity in rats
that complies with modern guidelines
3. Observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid, > 50%
Dermal absorption No relevant information
Distribution Widely distributed; liver, kidney, adipose tissue
Potential for accumulation Slight evidence of bioaccumulation
Rate and extent of excretion > 85% eliminated within 24 h
Metabolism in animals Extensive; no parent compound detected in urine
Toxicologically significant compounds Metabolites considered of equivalent toxicity to parent
(animals, plants and environment) compound
Acute toxicity
Rat :LD50 oral 1700 mg/kg bw
Rat: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation > 2.0 mg/L (whole-body exposure)
Skin irritation Slightly irritating
Eye irritation Slightly irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity in multigeneration study
Lowest relevant oral NOAEL Dog: < 2.5 mg/kg bw per day (reproductive toxicity)
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity No evidence of genotoxicity, but testing inadequate
Long-term toxicity and carcinogenicity
Target/critical effect: Inadequate data
Lowest relevant NOAEL Inadequate data
Carcinogenicity No evidence of carcinogenicity, but testing inadequate
Reproductive toxicity
Reproduction target / critical effect No adverse effect on reproduction
Lowest relevant reproductive NOAEL Dog: 5 mg/kg per day, multigeneration study
Developmental target /critical effect No adverse effect on development
Lowest relevant developmental NOAEL Rat: 350 mg/kg/bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern from other studies
Other toxicological studies Not an initiator or promoter of liver tumours in
rats
Possible increase in urinary bladder preneoplastic
and neoplastic changes
Medical data Contact allergic dermatitis reported in food
handlers
Summary Value Study Safety factor
ADI 0-0.005 mg/kg bw Dog, multigeneration 500
study of reproductive
toxicity
Acute reference dose Not allocated
(unnecessary)
References
Brandao, F.M. (1983) Contact dermatitis to ethoxyquin. Contact
Derm., 9, 240.
Buetler, T.M., Gallagher, E.P., Wang, C., Stahl, D.L., Hayes, J.D. &
Eaton, D.L. (1995) Induction of phase I and phase II drug metabolising
enzyme mRNA, protein and activity by BHA, ethoxyquin and Oltipraz.
Toxicol. Appl. Pharmacol., 135, 45-57.
Burka, L.T., Sanders, J.M. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. II.
Metabolism. Xenobiotica, 26, 597-611.
Burrows, D. (1975) Contact dermatitis in animal feed mill workers.
Br. J. Dermatol., 92, 167-170.
Cox, A.J. (1953) 6-Ethoxy-2,2,4-trimethyl-1,2-dihydroquinone (EMHQ).
Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Co., cited in 1969 JMPR monograph, Annex 1, reference 13.
Derse, P. (1956) Assay report. Unpublished report from Wisconsin
Alumni Research Foundation. Submitted to WHO by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Epstein, S.S., Fujii, K., Andrea, J. & Mantel, N. (1970)
Carcinogenicity testing of selected food additives by parenteral
administration to infant Swiss mice. Toxicol. Appl. Pharmacol., 16,
321-334.
Gilman, M.R. & Voss, W.R (1995) Reproduction/chronic toxicology study
of ethoxyquin with beagle dogs. Unpublished report HRP-MI #203-1.1
from HRP Inc for Monsanto, St Louis, Missouri, USA. Submitted to WHO
by the Oregon, Washington and California Pear Association.
Hanzal, R.F. (1955) Final report and addendum. Chronic oral
administration--dogs--metabolic studies. Unpublished report from
Hazleton Laboratories. Submitted by Monsanto Chemical Co., cited in
1969 JMPR monograph, Annex 1, reference 13.
Hard, G.C. & Neal, G.E. (1992) Sequential study of the chronic
nephrotoxicity induced by dietary administration of ethoxyquin in
Fisher 344 rats Fundam. Appl. Toxicol., 18, 278-287.
van Hecke, E. (1977) Contact dermatitis to ethoxyquin in animal feeds.
Contact Derm., 3, 341-352.
Ito, N., Fukushima, S. & Tsuda, H. (1985) Carcinogenicity and
modification of the carcinogenic response by BHA, BHT and other
antioxidants. CRC Crit. Rev. Toxicol., 15, 109-150.
Joner, P.E. (1977) Butylhydroxyanisol (BHA), butylhyroxytoluene (BHT)
and ethoxyquin (EMQ) tested for mutagenicity. Acta Vet. Scand., 18,
187-193.
Kahl, R. & Netter, K.J. (1977) Ethoxyquin as an inducer and inhibitor
of phenobarbital-type cytochrome-P450 in rat liver microsomes.
Toxicol. Appl. Pharmacol., 40, 473-483.
Kelly, R.E. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Co.; cited in 1969 JMPR
monograph; Annex 1, reference 13.
Manson, M.M., Green, J.A., Wright, B.J. & Carthew, P. (1992) Degree of
ethoxyquin-induced nephrotoxicity in rat is dependent on age and sex.
Arch. Toxicol., 66, 51-56.
Masui, T., Tsuda, H., Inoue, K., Ogiso, T. & Ito, N. (1986) Inhibitory
effects of ethoxyquin, 4,4'-diaminophenylmethane and acetaminophen on
rat hepatocarcinogenesis. Jpn. J. Cancer Res. (Gann), 77, 231-237.
Miyata, Y., Fukushima, S., Hirose, M., Masui, T. & Ito, N. (1985)
Short-term screening of promoters of bladder carcinogenesis in
N-butyl-n-(4-hydroxy)-nitrosamine-initiated, unilaterally
ureter-ligated rats. Jpn. J. Cancer Res. (Gann), 76, 828-834.
Monsanto (1966) Five year Santoquin feeding study in dogs. Unpublished
collection of reports prepared and submitted by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Naas, D.J. (1996a) A 90-day oral (gavage) toxicity study of ethoxyquin
in rats, Unpublished report No. WIL-273011 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Naas. D.J. (1996b) A 28-day oral (capsule) dose range-finding study of
ethoxyquin in dogs. Unpublished report No. WIL-273012 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas. D.J. (1996c) A 90-day oral (capsule) toxicity study of
ethoxyquin in dogs. Unpublished report No. WIL-273013 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas, D.J. (1997) A 28-day oral (gavage) toxicity study of ethoxyquin
in rats. Unpublished report No. WIL-273010 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Nemec, M.D. (1996a) A dose range-finding developmental toxicity study
of ethoxyquin in rats. Unpublished report No. WIL-273008 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Nemec, M.D. (1996b) A developmental toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273009 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Ohta, T., Moriya, M., Kaneda, K., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Sanders, J.M., Burka, L.T. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. I.
Disposition. Xenobiotica, 26, 583-595.
Ulrich, C.E. (1996) Acute inhalation toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273006 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995a) Acute oral toxicity study of ethoxyquin in albino
rats. Unpublished report No. WIL-273001 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995b) Acute dermal toxicity study of ethoxyquin in
albino rats. Unpublished report No. WIL-273002 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995c) Primary eye irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273004 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995d) Primary dermal irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273003 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995e) Skin sensitization study of ethoxyquin in albino
guinea pigs. Unpublished report No. WIL-273005 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Wilson, R.H. (1956) Final report: Reproduction study in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, US Department of Agriculture. Submitted to WHO by Monsanto
Chemical Co.; cited in 1969 JMPR monograph; Annex 1, reference 13.
Wilson, R.H. & DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agric. Food
Chem., 7, 203-206.
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report submitted by Monsanto Chemical Co., cited
in 1969 JMPR monograph; Annex 1, reference 13.
Zachariae, H. (1978) Ethoxyquin dermatitis. Contact Derm., 4,
117-118.
Zeiger, E. (1993) Mutagenicity of chemicals added to foods. Mutat.
Res., 290, 53-61.
Table 4. Acute toxicity of ethoxyquin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Rat Oral gavage 1700 97.6 Varsho (1995a)
Rat Dermal (24 h) > 2000 97.6 Varsho (1995b)
Rat Inhalation, whole body > 2.0 97.6 Ulrich (1996)
Mouse Intraperitoneal ~ 900 Wilson & DeEds (1959)a
Mouse Intravenous ~ 180 Wilson & DeEds (1959)a
a Cited in 1969 JMPR monograph
red-yellow staining of the fur. Gross and histopathological changes
indicated an irritant effect on the gastrointestinal tract.
Ethoxyquin produced transient, slight erythema when applied to
rabbit skin for 4 h under semi-occlusive conditions. There was no
oedema, but desquamation was present seven days after exposure
(Varsho, 1995c). This result is consistent with the findings of a
study summarized in the 1969 monograph (Kelly, 1960; cited in 1969
JMPR monograph; Annex 1, reference 13).
Ethoxyquin produced transient, slight-to-mild conjunctival
redness and chemosis in rabbits. All of the effects had fully
regressed within four days (Varsho, 1995d).
In a sensitization study in six guinea-pigs of each sex,
ethoxyquin was at most a very weak skin sensitizer. Induction was at
100%, with challenge applications at 50% in acetone. Very weak
erythematous responses were seen in both treated and control groups
after challenge and re-challenge, one test animal producing a weak
response to both challenge and re-challenge (Varsho, 1995e).
(b) Short-term studies of toxicity
Rats
Two studies of dietary administration of ethoxyquin to rats for
200 days were summarized in the 1969 monograph. Kidney lesions
(unspecified) were reported at 500 ppm and higher in males, with
increased kidney:body weight ratios in males at 250 ppm and higher.
The frequency of cytoplasmic inclusions in hepatocytes was increased
at 2000 ppm (Wilson & DeEds, 1959; Cox, 1953; cited in 1969 JMPR
monograph; Annex 1, reference 13).
In a study described in more detail below of the effects of
ethoxyquin on induction of liver tumours by N-nitrosodiethylamine, a
control group received ethoxyquin only. Thus, 15 male Fischer 344
rats, six weeks old, received an intraperiotneal injection of 0.9%
saline, were placed on a diet containing 8000 ppm ethoxyquin (purity
unspecified), equivalent to 500 mg/kg bw per day in young rats, in
week 2, and were partially (66%) hepatectomized in week 3. When the
animals were killed at week 8, a background level of gamma-glutamyl
transpeptidase (gamma-GT)-positive foci was found in the liver (Ito et
al., 1985).
Groups of five male and five female Sprague-Dawley rats received
ethoxyquin (purity, 97.6%) by gavage in corn oil for 28 days at doses
of 0, 50, 250, 500, or 1000 mg/kg bw per day. Histopathological
examination was limited to the liver, lung, kidney, stomach, and gross
lesions in animals at 50, 250, and 1000 mg/kg bw per day. All of the
animals at 1000 mg/kg bw per day had died by day 3 with multiple organ
involvement; the cause of death in two animals was considered to be
necrosis and ulceration of the forestomach. The prevalences of
salivation, stained fur, and brown urine were increased at 250 mg/kg
bw per day and higher. Initial body-weight gain was reduced by 50% in
males receiving 500 mg/kg bw per day. Erythrocyte count, haematocrit,
and haemoglobin concentration were decreased by about 10% in females
at 250 mg/kg bw per day and in animals of each sex at 500 mg/kg bw per
day. Alterations in serum clinical chemical parameters were seen in
both males and females, but were more frequent in males at 250 and
500 mg/kg bw per day; they included increased quantities of protein,
total bilirubin, cholesterol, inorganic phosphorus, potassium, and
calcium, and gamma-GT activity, while the concentration of glucose was
decreased. Increased absolute and relative liver weights (> 40%)
were seen in animals of each sex at 250 mg/kg bw per day and higher,
and the relative kidney weights were increased (< 10%) in a
dose-related fashion. There were no gross lesions at doses < 1000
mg/kg bw per day. Histopathological investigation showed kidney
lesions (interstitial infiltration, tubular epithelial regeneration,
and tubular dilatation) in males receiving 50 and 250 mg/kg bw per day
and in animals of each sex at 500 mg/kg bw per day. The incidences of
haemorrhage and oedema of the lung and hepatocellular swelling were
increased at 500 mg/kg bw per day. A NOAEL was not identified (Naas,
1997).
Groups of 10 Sprague-Dawley rats of each sex, six weeks old at
the beginning of the study, received ethoxyquin (purity, 97.6%) by
gavage in corn oil at 0, 20, 40, 200, or 400 mg/kg bw per day for 13
weeks. Minor overdosing (2-14%) of the group at 200 mg/kg bw per day
on day 67 is considered not to have compromised the study.
Ophthalmoscopy was performed before treatment and during week 12. A
full post-mortem examination was performed on all animals, and samples
of lung, liver, kidney, and gross lesions from all animals were
examined histologically, as were more than 30 tissues from controls
and from animals at the highest dose.
There were no deaths during the study. Clinical signs were seen
in animals of each sex, but more often in females, at 200 and 400
mg/kg bw per day, including staining of various body parts and
particularly the anogenital area, salivation, and brown urine.
Body-weight gain was clearly reduced in males at 200 and 400 mg/kg bw
per day, with a slight effect (10%) at 40 mg/kg bw per day; food
consumption was similar in test and control groups. Haematological and
clinical chemical parameters were altered in animals of each sex at
400 mg/kg bw per day, and many were also significant at 200 mg/kg bw
per day. These included increased reticulocyte counts, total
bilirubin, blood urea nitrogen, gamma-GT activity, cholesterol, and
thyroid-stimulating hormone; and decreased erythrocyte and leukocyte
counts, prothrombin time, and glucose. Urine was more deeply coloured
at 200 and 400 mg/kg bw per day, and the volume was increased in
animals at the highest dose, with no change in specific gravity. There
were no treatment-related effects on the eyes.
The main gross finding was reddened thyroids in animals of each
sex at 200 and 400 mg/kg bw per day. The absolute weights of the liver
and that relative to body weight were increased in a dose-related
fashion (by 15-70%), and those of the kidneys increased by 4-20% in
animals of each sex at 200 and 400 mg/kg bw per day; changes in the
body-weight ratios of brain and testes are considered to be secondary
to the reduced body weights. Histological examination identified the
kidney as the main target organ in animals of each sex, with increased
incidences of tubular mineralization, papillary necrosis, and
cytoplasmic vacuolation in males at the high dose; and increased
incidences of mineralization, papillary necrosis, and nephropathy in
females at the high dose. The incidence of nephropathy was also
increased in females at 200 mg/kg bw per day. The incidence of
ultimobranchial cysts of the thyroid was increased in males receiving
200 and 400 mg/kg bw per day and females receiving 200 mg/kg bw per
day. Increased incidences of cytoplasmic vacuolation of the adrenals,
suppurative inflammation of the epididymides, non-suppurative
inflammation of the prostrate, mineralization of the lung, and
alveolar histiocytosis were also seen in males at the high dose, and
the incidences of inflammation of the oesophagus and epithelial
hyperplasia of the thymus were increased in females at this dose. It
should be noted that only gross lesions, liver, lung, and kidney were
examined from groups at lower doses. As the decrease in body-weight
gain in males at 40 mg/kg bw per day was part of a dose-response
relationship and was not associated with reduced food consumption, the
NOAEL for this study was 20 mg/kg bw per day (Naas, 1996a).
Dogs
A one-year study in three dogs given ethoxyquin by gavage was
summarized in the 1969 monograph; the NOAEL was 3 mg/kg bw per day.
The effects reported at the next highest dose (10 mg/kg bw per day)
included renal nephrosis, increased bromosulphthalein retention
indicating liver dysfunction, and abdominal tenderness (Hanzal, 1955;
cited in 1969 JMPR monograph; Annex 1, reference 13).
Groups of one male and one female beagles received ethoxyquin
(purity, 97.6%) by capsule at a dose of 0, 25, 50, 100, or 200 mg/kg
bw per day for 28 days. All of the animals at 100 or 200 mg/kg bw per
day group died or were sacrificed by day 17 or day 7, respectively;
one female at 50 mg/kg bw per day was sacrificed on day 21. The signs
seen in dogs that died and in survivors included hypoactivity, reduced
defaecation, brown urine, and pale gums. Given the small initial group
sizes and the deaths, only major, consistent changes are summarized
here. Reduced body-weight gain and food consumption were seen at all
doses. The serum activities of enzymes indicative of liver damage were
increased at four weeks in all groups in which they were measured (25
and 50 mg/kg bw per day): alkaline phosphatase by fivefold, aspartate
aminotransferase by threefold, alanine aminotransferase by 20-fold,
and gamma-GT by threefold; there were indications of reduced activated
partial thromboplastin times. The ratios of liver and kidney weights
to body weight were increased at 25 and 50 mg/kg bw per day. Common
post-mortem findings included redness of the gastrointestinal tract
and darkened livers. Histological examination showed pigmentation of
the liver in all treated animals but not in controls. A NOAEL was not
identified (Naas, 1996b).
In a 90-day study, groups of five beagles of each sex were given
ethoxyquin (purity, 97.6%) by capsule at 0, 2, 4, 20, or 40 mg/kg bw
per day. Clear signs of toxicity were seen during the first seven
weeks of the study at 40 mg/kg bw per day, including reduced body
weight, staining of the body surface, brown urine, brown sclera, dark
mucoid faeces, and emesis, and these groups received only empty
capsules for the final six weeks of the study, effectively becoming
reversibility groups. Investigations of clinical chemistry (including
thyroid hormones), haematology, and ophthalmoscopy were performed
before treatment and at weeks 4 and 12 or 13. Post-mortem
investigations included microscopic examination of a wide range of
tissues from all animals and special stains for pigment
identification.
One female at the highest dose was sacrificed in extremis on
day 13. Other findings were similar in males and females. Clinical
signs including brown staining of the abdomen and urogenital area,
brown urine, decreased faeces, and emesis were seen regularly at 20
and 40 mg/kg bw per day and occasionally during the 4 h after dosing
at 4 mg/kg bw per day; these signs were still present between weeks 7
and 13 ('recovery') in animals at the highest dose. Body-weight loss
occurred at 40 mg/kg bw per day in weeks 1-7, which reversed when
dosing stopped, but females had a lower (12%) mean body weight than
controls at termination. At 20 mg/kg bw per day, body-weight gain was
reduced (60%) throughout the study. Food consumption was reduced at 20
(20%) and 40 mg/kg bw per day (up to 50%). The only notable change in
haematological parameters was a dose-related decrease in activated
partial thromboplastin times in males at 4 mg/kg bw per day and higher
and in females at the highest dose. Marked increases in total
bilirubin concentration and in alkaline phosphatase, alanine
aminotransferase, aspartate aminotransferase, and gamma-GT activities
in serum, indicative of liver dysfunction, were seen at 20 mg/kg bw
per day in weeks 4 and 12 or 13 and at 40 mg/kg bw per day in week 4
only; alanine aminotransferase and, to a lesser extent, alkaline
phosphatase activities were also increased at 4 mg/kg bw per day. By
week 13, the serum values in animals at 40 mg/kg bw per day
(seven-week treatment, six-week recovery) had returned approximately
to control values. There were no significant changes in absolute or
relative organ weights. Treatment-related gross and microscopic
findings were limited to the liver. At 20 and 40 mg/kg bw per day, a
darkened appearance was associated microscopically with increased
pigment deposition, hepatocellular necrosis, cytoplasmic vacuolation,
and bile-duct hyperplasia; at 4 mg/kg bw per day, occasional findings
of mild or moderate pigmentation, minimal hepatocellular necrosis, and
vacuolation were recorded. The pigments were found to be porphyrin and
bilirubin in most cases, some sections also staining for haemosiderin.
The NOAEL was 2 mg/kg bw per day (Naas, 1996c).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Solutions of ethoxyquin (as Santoquin(R)) at 10 or 50 mg/ml
were administered subcutaneously to neonatal Swiss ICR/Ha mice on days
1 and 7 (0.1 ml) and 14 and 21 (0.2 ml) of age or as a single dose of
100 mg/ml (0.1 ml on day 1). Each dose was equal to 500, 2500, and
5000 mg/kg bw on day 1 and 250 and 1250 mg/kg bw on day 21. The groups
consisted of 57 mice at the low dose, 53 at the intermediate dose, and
28 at the high dose. By the time the mice were weaned, 100% at the
high dose, 74% at the intermediate dose, and 2% at the low dose had
died; 15% of controls had died at this time. Small groups of mice were
sacrificed at various times up to termination at week 53. A limited
range of tissues and lesions were examined primarily for tumours. The
incidences of pulmonary tumours and hepatomas were similar in the
treated and control groups; a slight increase in the incidence of
malignant lymphoma in four females at the low dose and two at the
intermediate dose, with none in controls, was considered equivocal by
the authors. The results indicates that four subcutaneous
administrations of ethoxyquin at near-lethal doses to neonatal mice
did not significantly increase the incidence of tumours in mice up to
one year of age (Epstein et al., 1970).
Rats
A two-year study of ethoxyquin in the diet of rats was summarized
in the 1969 monograph. Groups of approximately 10 male and 10 female
rats were fed diets containing ethoxyquin at concentrations of 0, 62,
125, 250, 500, 1000, 2000, or 4000 ppm for up to two years. The
animals were sacrificed for autopsy after 200, 400, 600, or 715 days.
The mortality rates were not significantly different from those of
controls. Significantly reduced body-weight gain was seen at 2000 ppm
after 225 days in males and after 21 days in females. After 200 days,
increased relative liver and kidney weights were found in males at 250
ppm and in females at 1000 ppm. The haemoglobin values for animals of
each sex were normal 100 and 300 days after the start of the
experiment in rats fed 2000 and 4000 ppm. Histological changes in the
renal cortex were clearly evident after 200 days in male rats
receiving 2000 or 4000 ppm but not in females. All other organs were
normal in animals of each sex after 200 days. After 400 days, lesions
in the kidneys (pyelonephritis), liver, and thyroid were seen in males
only. Similar lesions were seen for periods up to 717 days in animals
of each sex, although they were more marked in males. Occasional
tumours were found after 700 days, but the incidence was unrelated to
dose, and they were also seen in controls. No clearly defined effects
were evident after feeding 62 ppm, but minute lesions were present in
the kidneys of two males receiving 500 ppm. It was difficult to
distinguish the abnormalities in the group examined after 700 days
from the pathological manifestations of senility after that time
(Wilson & DeEds, 1959; cited in 1969 JMPR monograph; Annex 1,
reference 13). The 1969 JMPR concluded that the NOAEL was 125 ppm,
equivalent to 6 mg/kg bw per day. The small groups used in this study
limit its sensitivity for detecting changes in rare events such as
tumours with low background rates; however, the wide spread of doses
and sequential sampling times provides a degree of assurance in the
reported findings.
As part of an investigation of kidney and liver tumours induced
by N-nitrosoethyl- N-hydroxyethylamine, one control group received
only ethoxyquin. This group of 25 male Fischer 344 rats received a
diet containing 8000 ppm ethoxyquin from nine weeks of age to
termination at 41 weeks of age. Sections of liver, kidney, and gross
lesions were investigated histologically. No gamma-GT-positive foci,
hyperplastic nodules, or hepatocellular carcinoma were found in the
liver; no data were presented on kidney lesions. A control group of 25
male Fischer 344 rats received a diet containing 8000 ppm ethoxyquin
as part of a parallel investigation of urinary bladder carcinogenesis
induced by N-nitrosobutyl- N-hydroxybutylamine. After 32 weeks,
there were higher incidences of simple hyperplasia and papillary or
nodular hyperplasia of the urinary bladder than in groups receiving
ascorbic acid or sodium erythorbate, the incidence of simple
hyperplasia being greater than that induced by
N-nitrosobutyl- N-hydroxybutylamine alone; no untreated controls
were used. No urinary bladder papillomas or carcinomas were seen in
the group receiving ethoxyquin only (Ito et al., 1985).
An almost identical study of urinary bladder carcinogenesis
showed that 24 weeks' exposure to a diet containing 8000 ppm
ethoxyquin, considered to be equivalent to 400 mg/kg bw per day, did
not induce papillary or nodal hyperplasia or papilloma of the urinary
bladder in a group of 15 male Fischer 344 rats (Miyata et al., 1985).
The dependence of the renal lesions produced by ethoxyquin on age
and sex was investigated in Fischer 344 rats. Groups of four to eight
male rats received diets containing ethoxyquin (purity, 90%) at 5000
ppm from three or eight weeks of age for 20, 26, or 30 weeks. Eight
female rats received a diet containing 5000 ppm ethoxyquin for 30
weeks from eight weeks of age. Histopathological examination of the
kidney comprised bromodeoxyuridine (BrdU) labelling, gamma-GT
histochemistry, haematoxylin and eosin staining, Alizarin red
staining, and immunoblotting of urine samples for albumin and alpha2u
globulin. Body-weight gain was reduced by 10-15% in treated animals.
In males, the absolute kidney weights were increased by 5-50%, with a
consequent increase in the relative weights; females showed a 12%
increase in kidney:body weight ratios. Renal cortical changes were
seen in all treated males, consisting of eosinic cytoplasmic
inclusions in tubular epithelial cells and protein accumulation in the
lamina of the tubules. In males dosed from three weeks of age,
papillary necrosis, slight calcium deposition, and hyperplasia of the
transitional epithelium of the renal pelvis were seen. The
histological appearance of the kidneys of treated females was similar
to that of controls, except for high concentrations of lipofuscin
deposition. BrdU labelling was increased in males at 30 weeks, but not
at 20 weeks, in both regenerating basophilic tubules and those
staining normally with haematoxylin and eosin; BrdU labelling in
females was not described. The concentrations of alpha2u globulin in
urine were slightly lower in treated males, but those of albumin were
significantly increased. The time of first exposure can thus
significantly alter the pattern of renal lesions in rats consuming
diets containing ethoxyquin at 5000 ppm, equivalent to 250 mg/kg bw
per day, exposure from three weeks of age resulting in papillary
necrosis in addition to the cortical lesions seen in animals exposed
from eight weeks of age (Manson et al., 1992).
Groups of 6-19 Fischer 344 rats of each sex, three weeks of age
at the start of the study, received diets containing ethoxyquin
(purity unspecified) at 0 or 5000 ppm for up to 18 months; one group
received ethoxyquin in the diet for 24 weeks followed by 34 weeks on
control diet. The study was designed to investigate the progression of
renal lesions and involved interim sacrifices at 4, 12 or 14, 24, 58,
and 78 weeks. The body-weight gain of treated females was reduced in
weeks 1-5 and that of males from week 3 onwards; food consumption was
reduced in animals of each sex during the first four weeks.
Investigations of renal pathology showed a clear difference between
males and females. Males had significant interstitial degeneration of
the papilla at weeks 4 and 14, which progressed to necrosis with
pyelonephritis of the cortex and urothelial hyperplasia of the renal
pelvis by week 24. In females, interstitial degeneration of the
papilla was only slight at week 14 and did not progress consistently.
The chronic progressive nephropathy commonly seen in Fischer 344 rats
was accelerated in animals receiving ethoxyquin. The authors reported
that this was more marked in males, but the data presented do not
substantiate that statement. A golden-brown pigmentation, found to be
lipofuscin by Schmorl's stain, was noted in the proximal tubules of
treated rats, particularly females. The lesions present at 24 weeks
showed no evidence of reversibility after 34 weeks on control diets.
The authors considered that there was no evidence for preneoplastic
proliferative lesions. This study showed that ethoxyquin at 5000 ppm
in the diet, equivalent to 250 mg/kg bw per day, is a potent
nephrotoxin in young male Fischer 344 rats (Hard & Neal, 1992).
A number of reports have been published of the results of
investigations into the effects of antioxidants, including ethoxyquin,
on the induction of neoplasia and preneoplastic lesions by known
carcinogens. The most comprehensive series of studies with ethoxyquin
is probably that of Ito and co-workers (Ito et al., 1985; Miyata et
al., 1985; Masui et al., 1986), who used Fischer 344 rats to
investigate moderation of effects on the liver induced by
N-nitrosodiethylamine, effects on the kidney and liver produced by
N-nitrosoethyl- N-hydroxyethylamine, and effects on the bladder
produced by N-nitrosobutyl- N-(4-hydroxybutyl)amine.
Groups of 18 six-week-old male Fischer 344 rats received an
intraperitoneal injection of 200 mg/kg bw N-nitrosodiethylamine in
0.9% saline; two weeks later, they were transferred to a diet
containing 0 or 8000 ppm ethoxyquin (purity unspecified) and underwent
a partial (60%) hepatectomy one week later. The rats were sacrificed
at week 8, and liver sections were stained with haematoxylin and eosin
and a histochemical stain for gamma-GT-positive foci. The rats
receiving ethoxyquin had a significant (p < 0.001) decrease in the
number of foci (0.9 versus 3.3/cm2) and in the area of the foci (0.06
versus 0.19 mm2/cm2) (Ito et al., 1985).
Groups of 23 or 27 six-week-old male Fischer 344 rats received
drinking-water containing 0.1% N-nitrosoethyl- N-hydroxyethylamine
for two weeks; between weeks 3 and sacrifice at week 32, they received
diets containing 0 or 8000 ppm ethoxyquin (purity unspecified). All
rats had gamma-GT-positive foci, but the ethoxyquin-treated group had
fewer (1 versus 21/cm2) and smaller (10 versus 22 mm2/cm2) foci.
The group given ethoxyquin also had a reduced area of hyperplastic
nodules (2.3 versus 7 mm2/cm2), and fewer animals had hepatocellular
carcinomas (3/27 versus 11/23). Conversely, the kidneys of the
ethoxyquin-treated group had higher frequencies of atypical-cell foci
(0.9 versus 0.2/cm2) and adenomas (5.6 versus 0.8/cm2), and there
were more animals with foci (26/27 versus 12/23) and adenomas (17/27
versus 5/23) and larger foci (5.6 versus 0.9 × 10-2 mm2/cm2) and
adenomas (24 versus 8 × 10-2 mm2/cm2) (Ito et al., 1985).
Groups of 25 six-week-old male Fischer 344 rats received
drinking-water containing 0.05%
N-nitrosobutyl- N-(4-hydroxybutyl)amine for four weeks; between
weeks 4 and sacrifice at week 36, they received diets containing 0 or
8000 ppm ethoxyquin (purity unspecified). Examination of the urinary
bladders showed that ethoxyquin had increased the incidence of animals
with simple hyperplasia (25/25 versus 14/24), the incidence and extent
of papillary or nodular hyperplasia (25/25 versus 8/24 and 9.4 versus
0.48/10 cm of basement membrane), the incidence and extent of
papillomas (17/25 versus 5/24 and 1.1 versus 0.19/10 cm), and the
incidence and extent of carcinomas (4/25 versus 1/24 and 0.17 versus
0.04/10 cm) (Ito et al., 1985). A similar study involving two weeks'
dosing with N-nitrosobutyl- N-(4-hydroxybutyl)amine (0.05% in
water) and sacrifice at 24 weeks also showed that dietary
administration of ethoxyquin at 8000 ppm (purity unspecified)
increased the incidence and extent of papillary or nodular hyperplasia
but not of papillomas of the urinary bladder (Miyata et al., 1985).
This group of studies shows that ethoxyquin markedly reduces the
preneoplastic effects of N-nitrosodiethylamine and
N-nitrosoethyl- N-hydroxyethylamine on the liver, possibly by a
combination of antioxidant effects and induction of detoxification
mechanisms. The increase produced by ethoxyquin in the incidence of
neoplastic and preneoplastic kidney lesions induced by
N-nitrosoethyl- N-hydroxyethylamine may be secondary to the direct
toxic effects of ethoxyquin on the kidney. The mechanism of the
effects of ethoxyquin on
N-nitrosobutyl- N-(4-hydroxy-butyl)amine-induced urinary bladder
neoplasia is unknown.
Dogs
Ethoxyquin was fed to two groups of 14 dogs and bitches at a
dietary concentration of 0 or 300 ppm for five years. No effects were
observed on haematological, urinary, or clinical chemical end-points
(aspartate aminotransferase activity, blood urea nitrogen, and
bromosulphthalein retention), organ weights, organ:body weight ratios,
body weight, or gross or histopathological appearance (Monsanto, 1966;
cited in 1969 JMPR monograph; Annex 1, reference 13). The 1969 JMPR
concluded that the NOAEL in this study was 300 ppm, equivalent to 7.5
mg/kg bw per day.
(d) Genotoxicity
A number of published papers indicate that ethoxyquin is not
genotoxic in bacterial systems (Table 5); however, these reports could
not be validated as only minimal details were available. The results
of assays in eukaryotic systems have not been reported.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
After 40 days on a diet slightly deficient in tocopherol and
containing 0, 250, 500, or 1000 ppm ethoxyquin, rats were bred to
produce three consecutive litters. The offspring of the first litter
were used to produce a second-generation litter. The highest dose was
discarded after production of one litter (reason unknown). No effects
on reproduction, as reflected in fertility, litter size, or survival
of offspring, were observed. The animals receiving the experimental
diet produced young and raised them more successfully than controls,
the 500 ppm diet being more effective than the 250 ppm diet (Wilson,
1956; Wilson & DeEds, 1959, cited in 1969 JMPR monograph; Annex 1,
reference 13). The short dosing period before mating and the unknown
purity and group size compromise this report to some extent, but it
Table 5. Results of assays for the genotoxicity of ethoxuquin
End-point Test system Concentration Purity Result Reference
(%)
Reverse mutation S. typhimurium 10-1000 µg/plate 'Pure' Negativea Joner (1977)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium > 5000 µg/plate NR Negativea Ohta et al. (1980)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr trp
Reverse mutation NR NR NR Negative Zeiger (1993)
Gene mutation B. subtitlis H17 0.2 ml NR Negative Ohta et al. (1980)
rec+ and M45 rec-
a With and without metabolic activation
can be concluded that ethoxyquin in the diet at 500 ppm, equivalent to
25 mg/kg bw per day, has no marked effect on reproductive outcome.
Another study cited in the 1969 JMPR monograph had some results
contrary to those described above. Groups of eight or nine female rats
were placed on diets containing 0, 125, 375, or 1125 ppm ethoxyquin on
the day of mating. The length of gestation was comparable in all
groups, but the litter size was slightly depressed at doses of 375 ppm
and higher, and at 1125 ppm the incidence of stillbirths was increased
and survival to weaning was decreased. The NOAEL in this unusual study
was 125 ppm, equivalent to 6 mg/kg bw per day. In a separate part of
the same study, no effects were found on litter size, the number of
stillbirths, survival to weaning, or weanling weight in rats receiving
up to 1125 ppm ethoxyquin in the diet starting between days 1 and 10
of gestation (Derse, 1956; cited in 1969 JMPR monograph; Annex 1,
reference 13).
Dogs
The effects of ethoxyquin on reproduction over two generations
were studied in groups of beagles. Dogs were chosen as ethoxyquin is
added to commercial dog food to help prevent oxidative deterioration.
In the first mating (F0), groups of five males and 10 females
received diets containing ethoxyquin (Santoquin(R)) at a mean
analytical concentration of 0, 100, or 225 ppm for a minimum of 82
days before pairing. The eight male and 13 female pups used
subsequently for the F1 matings received diets containing 0, 100, or
225 ppm ethoxyquin from weaning until breeding at 10-30 months (2nd
estrus cycle in females). Semen samples were taken during the first
week of treatment and at around the time of breeding in order to
determine the volume, sperm count, motility, speed, and morphology.
Animals were observed and underwent extensive physical examinations
routinely; if possible, they were also observed during labour. Mating,
whelping, and lactation indices were determined. Urine samples and
blood samples were taken for haematology and clinical chemistry from
fasted adults before treatment and at the end of the F0 phase; at
weeks 10, 23, 36, 49, and 62 and at termination in the F1 growth
phase; and at termination of the F1 mating phase. Ophthalmological
examinations were performed at the beginning and end of the F1 growth
and mating phases. All F1 adults and pups that showed signs of
toxicity were necropsied. A range of tissues from controls and F1
adults at the high dose were examined histologically, with selected
tissues from F2 pups that showed clinical signs; the livers and
gall-bladders from F1 adults at the low dose and the adrenals and
spleens from F1 adult females at the low dose were also examined.
Macroscopic and microscopic examinations were performed only on F0
and F1 animals that died or were sacrificed prematurely.
In the F0 mating, there was considerable intra-group variation
in body weights, but F0 adults receiving 225 ppm ethoxyquin showed a
trend for reduced body weight from the initiation of dosing to week 17
and during the latter stages of gestation. Males thad reduced food
consumption during most of the study. Two females at the high dose
that were confirmed to be pregnant did not give birth. There were no
other differences between the groups in mating performance, labour,
birth, or weaning indices, semen parameters, or clinical signs. Litter
size, pup survival, and pup weight and growth were similar in all
groups. At 225 ppm, there was an increased number of pups of each sex
with a raw or red anus, dehydration, nasal discharge, and excessive
lachrymation; the incidence of the last two signs was also increased
at 100 ppm. Statistically significant increases in serum alkaline
phosphatase activity were seen in male parents at the high dose and in
female parents at the low and high dose; there was also an indication
of reductions in monocytes and partial thromboplastin times in animals
of each sex at the high dose, although all of the values were claimed
to be within the normal ranges. There were no effects on urinary
parameters. Remating of three female controls and two at 225 ppm from
this phase which failed to mate during the initial phase was
successful.
Among F1 animals, one male at the low dose and two females at
the high dose died or were sacrificed in extremis. The male was
sacrificed because of suspected neurological signs; one of the females
died of suspected heart disease, and the other was sacrificed because
of pneumonia. The clinical signs included excessive lachrymation,
dehydration, thinness, and pale gums and showed a dose-related
increase in both the number of animals of each sex with a particular
sign and the number of occasions on which it was observed. Males at
the high dose had a lower mean body weight than controls up to week 48
of the study. Initially, animals at the high dose consumed more food
than controls, but food consumption was consistently lower in weeks
8-18 in males and in weeks 8-30 in females. Considerable variations in
haematological end-points were seen throughout the study in both
treated and control animals. There was evidence of treatment-related
effects on erythrocyte count, haematocrit, and haemoglobin, which were
reduced by up to 11% relative to controls in treated males and females
at weeks 10 and 23, and on partial thromboplastin times, which were
reduced in females at the high dose in weeks 23 and 36 and in females
at the low dose in weeks 23 and 62 and at the final analysis.
Increased serum activities of alkaline phosphatase, gamma-GT, and
alanine aminotransferase and reduced albumin:globulin ratios were
found in animals at the high dose in weeks 10, 23, and 36, with
evidence of lesser perturbations at the lower dose. These changes are
indicative of impaired liver function. The results of urinary analysis
were unremarkable.
In the F1 mating, there were no clear differences in semen
analyses or mating, gestation, whelping, or weaning indices between
control and ethoxyquin-treated animals. In adults, the only
treatment-related clinical sign was excessive lachrymation, which
occurred more frequently in males at the low and high doses than in
controls. Haematological end-points were similar in all groups.
Dose-related changes were seen in a number of clinical chemical
parameters in females, which attained statistical significance
(p < 0.05) at the high dose. These comprised reductions in glucose,
cholesterol, protein, albumin, and albumin:globulin ratio, and
increases in total bilirubin concentration and in gamma-GT, alkaline
phosphatase, and alanine aminotransferase activities. In males, the
dose-related increases in alkaline phosphatase, gamma-GT, and alanine
aminotransferase activities did not attain statistical significance.
Macroscopic examination showed dark-plum-coloured livers in one male
and two females at the high dose and cervical lymph node haemorrhages
in two females at the low and high doses; these lesions were possibly
related to treatment as they were not present in control animals.
Increases in the absolute weights of the spleen and testes and in the
weights of these organs relative to the brain weight were seen in
treated males, giving statistically significant increases in relation
to body weight. In females, increases in the absolute and relative
weights of the liver (10%), kidneys (10%), and spleen (40%) were
reported but were not statistically significant. Histopathological
examination showed that the liver, pituitary, and spleen were the
target organs. The macroscopic finding of increased cervical lymph
node haemorrhage in females was not confirmed. A dark-reddish-brown
pigment, subsequently identified as protoporphyrin IX, was not found
in the livers of controls or males at the low dose but was present in
the livers of 7/13 females at the low dose, 2/7 males at the high
dose, and 10/11 females at the high dose, with a dose-related increase
in severity. The frequencies of fibrosis and haemorrhage of the spleen
were increased in females at the high dose (3/11 versus 0/13 in
controls), and the incidence of pituitary cysts was increased in
animals at the high dose when compared with controls (2/6 versus 0/8
in males and 4/10 versus 2/12 in females)
Treated male pups had increased incidences of grey or pale gums,
excessive lachrymation, and dehydration, and female pups had an
increased incidence of dehydration. The pup weights at birth and to
week 6 of gestation were slightly reduced (< 10%), with a
dose-related effect in female pups. An increased mortality rate in
pups at the low dose was not seen at the high dose and was probably
related to the larger litter sizes in this group; the rates of
mortality were 7/62 controls, 24/91 at the low dose, and 10/77 at the
high dose.
During the study, four males and one female at 100 ppm and two
females at 225 ppm showed signs of neuropathy: The animals had
impaired hindlimb function, inability to stand, and unsteadiness of
the head and body which was found to be associated with myelin
degeneration. Examination of clinically normal littermates showed no
neurological deficits. The breeding records showed that all of the
affected animals had a common male ancestor which was not in the
breeding line of any of the control animals. When the parents of some
of the affected pups were removed from the treated diets and mated,
the incidence of neurologically affected animals was 17% in one litter
and 25% in the other. The evidence from this part of the study is
strongly indicative of a genetic etiology.
Estimation of the actual intakes of ethoxyquin in this study was
confounded by up to twofold increased consumption during lactation and
the fact that 180 and 360 ppm had to be added to obtain nominal
concentrations of 150 and 300 ppm, however, the analysis showed mean
initial values of 100 and 225 ppm. Although the actual amounts of food
consumed varied during the study, a value of 25 g/kg bw per day was
considered to be a representative mean, which resulted in intakes of
2.5 mg/kg bw per day ethxyquin at 100 ppm and 6 mg/kg bw per day at
225 ppm.
The results of this study show that ethoxyquin in the diet at
concentrations up to 225 ppm did not affect reproductive performance
or outcome in beagles. There was no clear overall NOAEL because of
increased incidences of clinical signs such as excess lachrymation and
dehydration, clinical chemical changes and pigment deposition in the
liver. The lowest dose, 100 ppm, equal to 2.5 mg/kg bw per day, was
considered to be the minimal effect level (Gilman & Voss, 1995).
(ii) Developmental toxicity
Rats
In a range-finding study for teratogenicity in Sprague-Dawley
rats, groups of eight mated females received ethoxyquin (purity,
97.6%) by gavage in corn oil at 0, 62, 125, 250, 500, or 1000 mg/kg bw
per day on days 6-19 of gestation. All animals given 1000 mg/kg bw per
day died or were sacrificed by day 9, and three animals given 500
mg/kg bw per day died between days 10 and 11 of gestation; post-mortem
examinations did not show any adverse effects. Clinical signs of
reduced defaecation, dark urine, and brown staining of fur were
dose-related and affected all treated groups. Reduced food consumption
and body-weight loss were seen at doses > 125 mg/kgbw per day at
the beginning of dosing; from day 9 onwards, body-weight gain was
similar in all groups up to 500 mg/kg bw per day, and these animals
had a 20% body-weight deficit by day 20 when compared with controls.
Fetal weights were reduced at 500 mg/kg bw per day, but examination
for external malformations, sex ratio, and crown-rump length showed no
effects of treatment (Nemec, 1996a).
In the main study, groups of 25 mated female Sprague-Dawley rats
received ethoxyquin (purity, 97.6%) by gavage in corn oil at 0, 50,
150 or 350 mg/kg bw per day on days 6-19 of gestation. The dams were
sacrificed on day 20, their uteri and ovaries were examined, and all
fetuses were investigated for weight, sex, and external and visceral
malformations. The heads of one-half of the fetuses were examined by
Wilson sectioning and the other half by mid-coronal section. All
fetuses were stained with Alizarin Red S for skeletal investigation.
There were no deaths during the study. Urogenital staining was seen in
dams after treatment at the highest dose, and staining of other areas
was also seen in these and in some animals receiving 150 mg/kg bw per
day. At 350 mg/kg bw per day, dams lost weight on days 6-7, and a 13%
reduction in body-weight gain compared with controls was evident on
days 6-20; 150 mg/kg bw per day resulted in a 5% reduction in
body-weight gain on days 6-20. Food consumption was reduced by 9% at
150 mg/kg bw per day and by 13% at 350 mg/kg bw per day. There were no
significant findings in dams post mortem: uterine weights, litter
size, resorptions, pre- and post-implantation losses, sex ratios, and
fetal weights were similar in all groups. Isolated findings of
malformations and anomalies were within the range in historical
controls and showed no relationship to treatment. The overall
incidence of variations was highest in controls, with no significant
increase in any individual variation. The overall NOAEL for this study
was 50 mg/kg bw per day on the basis of clinical signs (fur staining)
and reduced maternal body weight at higher doses. The NOAEL for
fetotoxicity was 350 mg/kg bw per day, the highest dose tested (Nemec,
1996b).
3. Observations in humans
Cases of dermatitis have been reported in workers handling fruit
treated with ethoxyquin. A study with patch tests, cited in the 1969
monograph, showed that the cause was a sensitization reaction rather
than direct irritation (Wood, 1965).
A number of reports (Burrows, 1975; van Hecke, 1977; Zachariae,
1978; Brandao, 1983) have indicated that ethoxyquin is the probable
cause of an often severe dermatitis seen in workers who handle animal
feed containing ethoxyquin. Positive results in patch tests have been
recorded in affected workers given challenge concentrations of as
little as 0.01% ethoxyquin in petrolatum (Zachariae, 1978). The
authors of some of the reports indicated that airborne contamination
and light sensitivity are implicated.
A study cited in the 1969 monograph indicated that no evidence of
skin irritation or sensitivity had been reported in 20 years of
ethoxyquin production (Kelly, 1960).
Comments
Published studies show that ethoxyquin is rapidly absorbed from
the gastrointestinal tract of rats and mice, with peak blood levels
within 1 h. Liver, kidney, and adipose tissue have the highest tissue
concentrations. Excretion occurs predominantly via the urine and is
rapid, with more than 85% of doses up to 25 mg/kg bw being excreted
within 24 h. At 250 mg/kg bw, absorption and excretion are slowed,
which is attributed to reduced gastric emptying, and only 50% of the
dose is excreted within 24 h. Repeated oral doses of 25 mg/kg bw per
day resulted in an excretion profile similar to that for single doses,
but repeated administration of 250 mg/kg bw per day was reported to
result in a profile similar to that for lower doses, indicating
induction of metabolism, transport, and/or a return to normal gastric
emptying. Biliary excretion and enterohepatic recirculation play a
significant role in the toxicokinetics of ethoxyquin, more than 40% of
an intravenous dose of 25 mg/kg bw being detected in the bile of
bile-duct-cannulated rats. The metabolism of ethoxyquin involves
O-deethylation or hydroxylation followed by conjugation as the
sulfate or glucuronide. A proposed reaction scheme for the production
of biliary metabolites involves epoxidation and the generation of
reactive, electrophilic intermediates. No information on plant
metabolites was available.
Ethoxyquin has low acute toxicity when administered orally (LD50
= 1700 mg/kg bw), dermally, or by inhalation. It is slightly
irritating to the eyes and skin and had only very weak sensitizing
potential when administered topically to guinea-pigs. Exposure to
ethoxyquin in the workplace has been linked to allergic contact
dermatitis, and the substance should be considered as a sensitizer in
humans.
WHO has not classified ethoxyquin for acute toxicity.
The main target organ after repeated administration of ethoxyquin
to rats for 28 days or more at doses of 50-1000 mg/kg bw per day was
the kidney. Mechanistic studies with dietary concentrations equivalent
to 250 mg/kg bw per day showed that the precise effects were dependent
on the age at first exposure, were progressive, more severe in males
than in females, and not reversible after 24 weeks of exposure. Other
effects seen in rats exposed to ethoxyquin for 28 or 90 days at doses
> 200 mg/kg bw per day were stained fur, brown urine, changes to
haematological parameters, increased liver weights, and changes in
clinical chemical parameters consistent with altered liver function.
The overall NOAEL in the short-term studies in rats was 20 mg/kg bw
per day.
In dogs given capsules containing ethoxyquin for 90 days at 0, 2,
4, 20, or 40 mg/kg bw per day, the liver was the primary target.
Alterations in haematological parameters and clinical chemical changes
indicative of altered liver function were seen at doses > 4 mg/kg bw
per day, together with hepatocellular necrosis, vacuolation, and
pigment deposition. Although staining indicated that the pigment was
haemosiderin, a specific investigation showed it to be protoporphyrin
IX. The overall NOAEL in short-term studies in dogs was 2 mg/kg bw per
day. This is consistent with the results of an older, one-year study
in dogs in which a NOAEL of 3 mg/kg bw per day was established on the
basis of findings suggestive of effects on the kidney and liver at 10
mg/kg bw per day.
No modern long-term studies of toxicity or carcinogenicity have
been performed. In studies summarized by the 1969 Meeting in which
ethoxyquin was administered to dogs at 0 or 300 ppm in the diet for 5
years or at 0, 3, 10, 50, or 100 mg/kg bw per day by gavage for one
year, effects were observed in the liver and kidneys at doses of 10
mg/kg bw per day and above. The NOAEL was 300 ppm, equivalent to 7.5
mg/kg bw per day. A two-year study in rats that received dietary
concentrations of 0, 62, 125, 250, 500, 1000, 2000, or 4000 ppm,
published in 1959, gave no indication of carcinogenicity, with an
overall NOAEL of 125 ppm, equivalent to 6 mg/kg bw per day; lesions in
the kidney, liver, and thyroid gland were seen at higher doses.
Mechanistic studies on tumour induction and promotion show that
ethoxyquin induces both phase-I and phase-II xenobiotic metabolism.
Although its incorporation into the diet at 8000 ppm after treatment
with an N-nitrosamine reduced the formation of preneoplastic foci in
the liver, it increased the incidence of preneoplastic and neoplastic
events in the kidney and urinary bladder. No significant increase in
tumour incidence was seen after one year in mice that received four
subcutaneous, near-lethal doses of ethoxyquin.
Published reports of studies of bacterial mutagenicity indicate
that ethoxyquin is not mutagenic in prokaryotic systems, but only
limited details of the protocols and results were provided. No data
were available on other genotoxic end-points.
No modern study of reproductive toxicity has been performed in
rodents. Three studies in which rats received 0, 125, 250, 375, 500,
1000, or 1125 ppm in the diet, all with non-standard protocols, which
were summarized by the 1969 Meeting, gave slightly contradictory
results. Two of the studies, including the most extensive, apparently
showed no effects on the aspects of reproduction investigated at doses
up to 1125 ppm in the diet (equivalent to 56 mg/kg bw per day), while
the other showed an increased incidence of stillbirths at 1125 ppm and
decreased litter size at 375 ppm, with a NOAEL of 125 ppm (equivalent
to 6 mg/kg bw per day).
A modern two-generation study of reproductive toxicity in dogs
given diets containing 0, 100, or 225 ppm showed that ethoxyquin had
no effects on reproductive parameters at 225 ppm (equivalent to 5.6
mg/kg bw per day), the highest dose tested. The clinical signs
observed included dehydration, excess lachrymation, and evidence of
hepatic toxicity, especially in bitches. The effects were seen at both
doses and were consistent with the results of the short-term studies
in dogs. The findings in bitches may have been related to increased
consumption during gestation and lactation. The lowest dose tested,
100 ppm, equivalent to 2.5 mg/kg bw per day, was considered to be a
minimal effect level.
A study of developmental toxicity in rats at 0, 50, 150, or 350
mg/kg bw per day showed that ethoxyquin is not fetotoxic or
teratogenic at doses up to 350 mg/kg bw per day. Maternal toxicity,
stained fur, and reduced body-weight gain were seen at 150 and 350
mg/kg bw per day. No studies of developmental toxicity have been
performed in other species.
An ADI of 0-0.005 mg/kg bw per day was established on the basis
of the minimal-effect level of 2.5 mg/kg bw per day in the
multigeneration study in dogs and a 500-fold safety factor to account
for the lack of a NOAEL in this study and for the incompleteness of
the database. The multigeneration study of reproductive toxicity was
of longer duration and more recent than a 90-day study in dogs treated
by gavage with a NOAEL of 2 mg/kg bw per day.
An acute RfD was not allocated because ethoxyquin is of low acute
toxicity. The Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 125 ppm, equivalent to 6 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equivalent to 25 mg/kg bw per day
(two-generation study of reproductive toxicity)
50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
350 mg/kg bw per day (developmental toxicity)
Dog: 2 mg/kg bw per day (general toxicity in a 90-day study
of toxicity)
3 mg/kg bw per day (one-year study of toxicity)
300 ppm, equivalent to 7.5 mg/kg bw per day (five-year
study of toxicity)
2.5 mg/kg bw per day (minimal effect level for general
toxicity in a two-generation study of reproductive
toxicity)
5 mg/kg bw per day (reproductive performance; highest
dose tested)
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of genotoxicity in mammalian systems
2. A long-term study of toxicity and carcinogenicity in rats
that complies with modern guidelines
3. Observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid, > 50%
Dermal absorption No relevant information
Distribution Widely distributed; liver, kidney, adipose tissue
Potential for accumulation Slight evidence of bioaccumulation
Rate and extent of excretion > 85% eliminated within 24 h
Metabolism in animals Extensive; no parent compound detected in urine
Toxicologically significant compounds Metabolites considered of equivalent toxicity to parent
(animals, plants and environment) compound
Acute toxicity
Rat :LD50 oral 1700 mg/kg bw
Rat: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation > 2.0 mg/L (whole-body exposure)
Skin irritation Slightly irritating
Eye irritation Slightly irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity in multigeneration study
Lowest relevant oral NOAEL Dog: < 2.5 mg/kg bw per day (reproductive toxicity)
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity No evidence of genotoxicity, but testing inadequate
Long-term toxicity and carcinogenicity
Target/critical effect: Inadequate data
Lowest relevant NOAEL Inadequate data
Carcinogenicity No evidence of carcinogenicity, but testing inadequate
Reproductive toxicity
Reproduction target / critical effect No adverse effect on reproduction
Lowest relevant reproductive NOAEL Dog: 5 mg/kg per day, multigeneration study
Developmental target /critical effect No adverse effect on development
Lowest relevant developmental NOAEL Rat: 350 mg/kg/bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern from other studies
Other toxicological studies Not an initiator or promoter of liver tumours in
rats
Possible increase in urinary bladder preneoplastic
and neoplastic changes
Medical data Contact allergic dermatitis reported in food
handlers
Summary Value Study Safety factor
ADI 0-0.005 mg/kg bw Dog, multigeneration 500
study of reproductive
toxicity
Acute reference dose Not allocated
(unnecessary)
References
Brandao, F.M. (1983) Contact dermatitis to ethoxyquin. Contact
Derm., 9, 240.
Buetler, T.M., Gallagher, E.P., Wang, C., Stahl, D.L., Hayes, J.D. &
Eaton, D.L. (1995) Induction of phase I and phase II drug metabolising
enzyme mRNA, protein and activity by BHA, ethoxyquin and Oltipraz.
Toxicol. Appl. Pharmacol., 135, 45-57.
Burka, L.T., Sanders, J.M. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. II.
Metabolism. Xenobiotica, 26, 597-611.
Burrows, D. (1975) Contact dermatitis in animal feed mill workers.
Br. J. Dermatol., 92, 167-170.
Cox, A.J. (1953) 6-Ethoxy-2,2,4-trimethyl-1,2-dihydroquinone (EMHQ).
Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Co., cited in 1969 JMPR monograph, Annex 1, reference 13.
Derse, P. (1956) Assay report. Unpublished report from Wisconsin
Alumni Research Foundation. Submitted to WHO by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Epstein, S.S., Fujii, K., Andrea, J. & Mantel, N. (1970)
Carcinogenicity testing of selected food additives by parenteral
administration to infant Swiss mice. Toxicol. Appl. Pharmacol., 16,
321-334.
Gilman, M.R. & Voss, W.R (1995) Reproduction/chronic toxicology study
of ethoxyquin with beagle dogs. Unpublished report HRP-MI #203-1.1
from HRP Inc for Monsanto, St Louis, Missouri, USA. Submitted to WHO
by the Oregon, Washington and California Pear Association.
Hanzal, R.F. (1955) Final report and addendum. Chronic oral
administration--dogs--metabolic studies. Unpublished report from
Hazleton Laboratories. Submitted by Monsanto Chemical Co., cited in
1969 JMPR monograph, Annex 1, reference 13.
Hard, G.C. & Neal, G.E. (1992) Sequential study of the chronic
nephrotoxicity induced by dietary administration of ethoxyquin in
Fisher 344 rats Fundam. Appl. Toxicol., 18, 278-287.
van Hecke, E. (1977) Contact dermatitis to ethoxyquin in animal feeds.
Contact Derm., 3, 341-352.
Ito, N., Fukushima, S. & Tsuda, H. (1985) Carcinogenicity and
modification of the carcinogenic response by BHA, BHT and other
antioxidants. CRC Crit. Rev. Toxicol., 15, 109-150.
Joner, P.E. (1977) Butylhydroxyanisol (BHA), butylhyroxytoluene (BHT)
and ethoxyquin (EMQ) tested for mutagenicity. Acta Vet. Scand., 18,
187-193.
Kahl, R. & Netter, K.J. (1977) Ethoxyquin as an inducer and inhibitor
of phenobarbital-type cytochrome-P450 in rat liver microsomes.
Toxicol. Appl. Pharmacol., 40, 473-483.
Kelly, R.E. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Co.; cited in 1969 JMPR
monograph; Annex 1, reference 13.
Manson, M.M., Green, J.A., Wright, B.J. & Carthew, P. (1992) Degree of
ethoxyquin-induced nephrotoxicity in rat is dependent on age and sex.
Arch. Toxicol., 66, 51-56.
Masui, T., Tsuda, H., Inoue, K., Ogiso, T. & Ito, N. (1986) Inhibitory
effects of ethoxyquin, 4,4'-diaminophenylmethane and acetaminophen on
rat hepatocarcinogenesis. Jpn. J. Cancer Res. (Gann), 77, 231-237.
Miyata, Y., Fukushima, S., Hirose, M., Masui, T. & Ito, N. (1985)
Short-term screening of promoters of bladder carcinogenesis in
N-butyl-n-(4-hydroxy)-nitrosamine-initiated, unilaterally
ureter-ligated rats. Jpn. J. Cancer Res. (Gann), 76, 828-834.
Monsanto (1966) Five year Santoquin feeding study in dogs. Unpublished
collection of reports prepared and submitted by Monsanto Chemical Co.,
cited in 1969 JMPR monograph; Annex 1, reference 13.
Naas, D.J. (1996a) A 90-day oral (gavage) toxicity study of ethoxyquin
in rats, Unpublished report No. WIL-273011 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Naas. D.J. (1996b) A 28-day oral (capsule) dose range-finding study of
ethoxyquin in dogs. Unpublished report No. WIL-273012 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas. D.J. (1996c) A 90-day oral (capsule) toxicity study of
ethoxyquin in dogs. Unpublished report No. WIL-273013 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Naas, D.J. (1997) A 28-day oral (gavage) toxicity study of ethoxyquin
in rats. Unpublished report No. WIL-273010 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association,
Nemec, M.D. (1996a) A dose range-finding developmental toxicity study
of ethoxyquin in rats. Unpublished report No. WIL-273008 from WIL
Research Laboratories, Inc., Ohio, USA. Submitted to WHO by the
Oregon, Washington and California Pear Association.
Nemec, M.D. (1996b) A developmental toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273009 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Ohta, T., Moriya, M., Kaneda, K., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Sanders, J.M., Burka, L.T. & Matthews, H.B. (1996) Comparative
metabolism and disposition of ethoxyquin in rat and mouse. I.
Disposition. Xenobiotica, 26, 583-595.
Ulrich, C.E. (1996) Acute inhalation toxicity study of ethoxyquin in
rats. Unpublished report No. WIL-273006 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995a) Acute oral toxicity study of ethoxyquin in albino
rats. Unpublished report No. WIL-273001 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995b) Acute dermal toxicity study of ethoxyquin in
albino rats. Unpublished report No. WIL-273002 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995c) Primary eye irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273004 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995d) Primary dermal irritation study of ethoxyquin in
albino rabbits. Unpublished report No. WIL-273003 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Varsho, B.J. (1995e) Skin sensitization study of ethoxyquin in albino
guinea pigs. Unpublished report No. WIL-273005 from WIL Research
Laboratories, Inc., Ohio, USA. Submitted to WHO by the Oregon,
Washington and California Pear Association.
Wilson, R.H. (1956) Final report: Reproduction study in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, US Department of Agriculture. Submitted to WHO by Monsanto
Chemical Co.; cited in 1969 JMPR monograph; Annex 1, reference 13.
Wilson, R.H. & DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agric. Food
Chem., 7, 203-206.
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report submitted by Monsanto Chemical Co., cited
in 1969 JMPR monograph; Annex 1, reference 13.
Zachariae, H. (1978) Ethoxyquin dermatitis. Contact Derm., 4,
117-118.
Zeiger, E. (1993) Mutagenicity of chemicals added to foods. Mutat.
Res., 290, 53-61.
