
First draft prepared by
P. V. Shah
United States Environmental Protection Agency
Office of Pesticide Programs
Washington DC, USA
Cyprodinil is the ISO approved name for (4-cyclopropyl-6-methyl-pyrimidin-2-yl)-phenyl-amine, a systemic fungicide that acts by inhibiting the biosynthesis of methionine. Cyprodinil has not been evaluated previously by the JMPR.
Rats
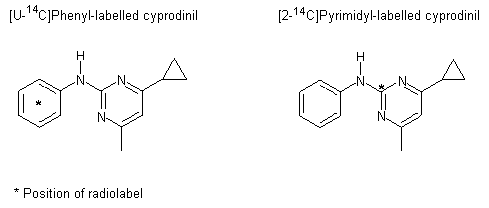
Groups of Tif RAIf (SPF) rats were given a single oral dose of radiolabelled cyprodinil (purity, >98%) at 0.5 or 100 mg/kg bw. Cyprodinil was labelled either in the phenyl ring or on the pyrimidyl group; the positions of the radiolabel, 14C, are shown in Figure 1. One group (C1) was given unlabelled cyprodinil (purity, >99%) at a dose of 0.5 mg/kg bw for 2 weeks before being treated with the radiolabelled compound. Groups of five male and female rats were given a suspension of radiolabelled cyprodinil in ethanol/polyethyleneglycol 200/water (1:2:1, v/v) by gavage. The design of the study is described in Table 1. The absorption, distribution and elimination of cyprodinil administered intravenously were not evaluated, owing to the limited solubility of the test substance in aqueous media.
Table 1. Design of a study of absorption, distribution and excretion of radiolabelled cyprodinil in rats treated by gavage
|
Group |
No. of animals and sex |
Dose |
Times at which samples were collected |
|
|
(mg/kg bw) |
(kBq) |
|||
|
Bl |
5 males and 5 females |
0.5a |
185 |
Urine: 0-8, 8-24, and every 24 h thereafter until 168 h |
|
Faeces: 0-24, and every 24 h thereafter until 168 h |
||||
|
Tissues: 168 h |
||||
|
C1c |
5 males |
0.45a |
185 |
Urine: 0-8, 8-24, and every 24 h thereafter until 168 h |
|
5 females |
0.52a |
185 |
Faeces: 0-24, and every 24 h thereafter until 168 h |
|
|
Tissues: 168 h |
||||
|
Dl |
5 males |
94.3a |
7247 |
Urine: 0-8, 8-24, and every 24 h thereafter until 168 h |
|
5 females |
93.7a |
7247 |
Faeces: 0-24, and every 24 h thereafter until 168 h |
|
|
Expired air: 0-24, 24-48 h |
||||
|
Tissues: 168 h |
||||
|
D2 |
5 males |
102.2b |
7313 |
Urine: 0-8, 8-24 and every 24 h thereafter until 168 h |
|
5 females |
100.4b |
7313 |
Faeces: 0-24, and every 24 h thereafter until 168 h |
|
|
Expired air: 0-24, 24-48 h |
||||
|
Tissues: 168 h |
||||
|
El |
3 males |
0.51a |
183 |
Blood: 0.25, 0.5, 1, 2, 4, 8, 12, 24, 32, 48h |
|
F14 |
4 × 3 males |
0.54a |
188 |
Tissues: 0.25, 1.25, 11, 17 h |
|
4 × 1 males |
— |
— |
||
|
Gl |
5 males |
106.5a |
9406 |
Urine: 0-24, 24-48 h |
|
Faeces: 0-24, 24-48 h |
||||
|
Bile: 0-0.5, 0.5-1, 1-2, 2-4, 4-8, 8-18, 18-24, 24-42, 42-48h |
||||
From Thanei (1992)
a [U-14C]Phenyl label
b [2-14C]Pyrimidyl label
c Pretreated with unlabelled cyprodinil, 14 × 0.5 mg/kg bw per day
The absorption of cyprodinil was estimated from the total amount of radiolabel excreted in the bile and urine in animals with bile cannulae and receiving a higher dose (group G1). In the 48h after dosing, 39% (range, 27.5-43.4%) of the administered dose was eliminated in the bile and 35.4% (range, 27.8-44.2%) was eliminated in the urine. Therefore, the total estimated absorption of cyprodinil was 75.5% (range, 71-85%) of the administered dose within 48 h.
The blood kinetics of radiolabelled cyprodinil, measured in animals in group E1, indicated an initial rapid absorption, with a peak concentration of cyprodinil equivalents (Cmax = 0.083 ± 0.036 mg/kg) being reached 15 min after administration. The concentration of cyprodinil equivalents in the blood declined rapidly to one-half of the maximum by 1.25 h after administration. A second prolonged phase of absorption showed a peak (0.029 ± 0.019 mg/kg) at about 8 h after administration; this was probably caused by reabsorption of radiolabel that was excreted in the bile. At 48 h, the mean concentration of cyprodinil equivalents in the blood was 0.0017 ± 0.0001 mg/kg.

Figure 1. Position of radiolabel on cyprodinil used in a study of absorption, distribution and excretion in rats
Seven days (168 h) after the administration of single oral doses at 0.5 mg/kg bw (group B1), the mean tissue concentration of cyprodinil equivalents was below the limit of determination or at the limit of detection of radioactivity, except in liver, kidney, spleen (B1 females only), whole blood (B1 males or females) and remaining carcass (B1 females only). Concentrations of cyprodinil equivalents in selected tissues of the B1 males and females were as follows: liver, 0.0046-0.0048 mg/kg; kidneys, 0.0031-0.0061 mg/kg; spleen, 0-0.0015 mg/kg; blood, 0.001 1-0.0012 mg/kg and remaining carcass, 0-0.0017 mg/kg. Residues in the tissues of animals that had received repeated doses at 0.45 and 0.52 mg/kg bw for males and females, respectively (group C1; pretreated with unlabelled cyprodinil) were of the same order of magnitude as those in animals that had received a single dose (group B1). Tissue residues were low in the groups that were given a high single dose (groups D1 and D2); the concentration of cyprodinil equivalents did not exceed 0.2 mg/kg, except in kidneys, liver, lungs, spleen (females only), thyroid, whole blood, and remaining carcass (< 1.5 mg/kg). The distribution and concentrations of the two different radiolabelled compounds were similar (groups D1 and D2). Tissue residues in females were 0.5-2.7-fold greater than in males, and total tissue residues for animals in both treated groups accounted for 0.15-0.6% of the administered dose. In rats with bile cannulae (group G2), 1.13% of the administered dose remained in the carcass (excluding the gastrointestinal contents) at sacrifice, 48 h after dosing.
The pattern of elimination of orally absorbed [U-14C]phenyl- and [2-14C]pyrimidyl-cyprodinil is shown in Table 2. The pattern of excretion was independent of sex, dose, pre-treatment, and position of the radiolabel. Elimination of the radiolabel was essentially complete within 48 h (92-97% of the administered dose), with 96-98% being eliminated by day 7 (168 h) after dosing. Less than 0.1% of the radiolabel was detected in exhaled air. Excretion was rapid in animals with cannulated bile ducts (group G1), with 39%, 35%, and 14% of the administered dose being excreted in the bile, urine and faeces, respectively, within 48 h. The amount of radiolabel excreted via the urine and faeces was significantly reduced in animals with cannulated bile ducts, indicating that a portion of the material excreted in the bile is reabsorbed from the intestinal tract and is eliminated via the kidneys (enterohepatic circulation) in animals without bile cannulae (Thanei, 1992).
Table 2. Elimination of [U-14C]phenyl-labelled cyprodinil and [2-14C]pyrimidyl-labelled cyprodinil in rats (% of the administered dose)
|
Group |
B1 |
C1a |
D1 |
D2 |
G1 |
||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
|
|
Dose (mg/kg bw) |
0.50 |
0.50 |
0.45 |
0.52 |
94.3 |
93.7 |
102.2 |
100.4 |
106.5 |
|
Urine |
|||||||||
|
0-8 h |
32.2 |
26.3 |
25.2 |
28.9 |
25.0 |
23.1 |
14.8 |
19.3 |
24.0 |
|
8-24 h |
18.3 |
27.6 |
24.6 |
16.7 |
26.1 |
33.2 |
39.6 |
43.0 |
11.4 |
|
24-48 h |
1.6 |
2.7 |
1.6 |
2.0 |
1.9 |
2.7 |
5.2 |
4.2 |
— |
|
48-168 h |
0.6 |
1.4 |
0.4 |
0.7 |
0.6 |
0.7 |
1.0 |
1.2 |
— |
|
Subtotal |
52.7 |
58.0 |
51.8 |
48.3 |
53.6 |
59.7 |
60.6 |
67.7 |
35.4 |
|
Bile |
|||||||||
|
0-8 h |
— |
— |
— |
— |
— |
— |
— |
— |
14.0 |
|
8-24 h |
— |
— |
— |
— |
— |
— |
— |
— |
11.3 |
|
24-48 h |
— |
— |
— |
— |
— |
— |
— |
— |
13.6 |
|
0-24 h |
— |
— |
— |
— |
— |
— |
— |
— |
25.3 |
|
24-48 h |
— |
— |
— |
— |
— |
— |
— |
— |
13.6 |
|
Subtotal |
38.9 |
||||||||
|
Faeces |
|||||||||
|
0-24 h |
40.2 |
27.5 |
38.6 |
34.2 |
37.2 |
27.5 |
26.1 |
17.5 |
6.3 |
|
24-48 h |
4.4 |
8.0 |
5.7 |
11.1 |
5.5 |
8.3 |
9.3 |
9.2 |
7.6 |
|
48-168 h |
0.7 |
2.0 |
0.5 |
1.6 |
0.8 |
1.6 |
1.5 |
2.1 |
— |
|
Subtotal |
45.3 |
37.5 |
44.8 |
46.9 |
43.5 |
37.4 |
36.9 |
28.8 |
13.9 |
|
Expired air |
|||||||||
|
0-24 h |
ND |
ND |
ND |
ND |
<0.1 |
<0.1 |
<0.1 |
<0.1 |
— |
|
24-48 h |
ND |
ND |
ND |
ND |
<0.1 |
<0.1 |
<0.1 |
<0.1 |
— |
|
Subtotal |
ND |
ND |
ND |
ND |
<0.1 |
<0.1 |
<0.1 |
<0.1 |
— |
|
Cage wash |
|||||||||
|
0-168 h |
0.2 |
1.2 |
0.1 |
0.5 |
0.2 |
0.2 |
0.3 |
0.3 |
1.7 |
|
Total excretion |
|||||||||
|
0-48 h |
96.7 |
92.1 |
95.6 |
92.8 |
95.7 |
94.7 |
95.0 |
93.1 |
90.0 |
|
0-168 h |
98.2 |
96.7 |
96.6 |
95.7 |
97.3 |
97.1 |
97.8 |
96.8 |
— |
|
Tissue |
0.21 |
0.37 |
0.15 |
0.33 |
0.35 |
0.40 |
0.40 |
0.60 |
1.13 |
From Thanei (1992)
a Pretreated with unlabelled cyprodinil, 14 × 0.5 mg/kg bw per day
ND, Not detected
—, Not measured
In a separate study of absorption, distribution and depletion kinetics, male and female Tif RAIf (SPF) rats were given [U-14C]phenyl-labelled cyprodinil in polyethylene glycol 200/ethanol/water, 2:1:1 (v/v), as a single oral dose at about 0.5 mg/kg bw or about 100 mg/kg bw. The experimental design is shown in Table 3.
Table 3. Design of a study of absorption, distribution and depletion kinetics of [U-14C]phenyl-labelled cyprodinil in rats
|
Group |
No. of animals and sex |
Dose |
Samples collected |
|
|
(mg/kg bw) |
(kBq) |
|||
|
El |
3 males |
0.54 |
196 |
Blood: 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48 h |
|
3 females |
0.56 |
|||
|
E2 |
3 males |
100.1 |
2650 |
Blood: 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48 h |
|
3 females |
108.5 |
|||
|
F14 |
4 × 3 males |
0.52 |
186 |
Tissues: 0.5 (tcmax), 2 (tcmax/2), 10.5, 24 h |
|
F2 |
3 × 3 males |
93.3 |
2427 |
Tissues: 12 (tcmax), 19 (tcmax/2), 30 h |
|
F3 |
4 × 3 females |
0.52 |
185 |
Tissues: 1 (tcmax), 2.5 (tcmax/2), 12, 40 h |
|
F4 |
3 × 3 females |
101.3 |
2428 |
Tissues: 8 (tcmax), 40 (tcmax/2), 72 h |
From Muller (1996)
At the lower dose, the maximum concentration of radiolabel in the blood was reached at 0.5 h in males and at 1 h in females (group E1). In females, the first peak concentration in blood (cyprodinil equivalents, 0.47 mg/kg) was six-fold higher than that in males (cyprodinil equivalents, 0.08 mg/kg). Elimination from blood followed biphasic first-order kinetics. In females, a second maximum concentration was reached at 8-12 h, indicating enterohepatic recycling. In males, the second maximum concentration was not as pronounced as in females. After the first peak, the concentration of radiolabel in blood declined rapidly; with time at which maximum concentration was reached, tcmax/2, equal to 1 and 2 h at the lower dose for males and females, respectively. At the higher dose (group E2), the second maximum concentration was higher than the first and tcmax/2 was 19 h and 36 h for males and females, respectively. At the lower dose (group E1), the area under the curve (AUC) was about 10-fold greater in females than in males. The residues found in the blood of animals used for investigation of tissue distribution of radiolabelled cyprodinil at the lower dose (groups F1 and F3) at various time-points were compared with the blood residues found in animals used for experiments on blood kinetics (group E1). Similar concentrations of radiolabel were found in the blood of males in group F1 and E1, while the concentrations of radiolabel in the blood of females in group E1 were approximately 17 times higher than those of females in group F3. The concentrations of radiolabel in the blood of F1 males and F3 females were similar in magnitude. Therefore, the differences in blood concentrations and the rate of elimination from the blood observed in females at the lower dose may be regarded as a fortuitous effect.
Concentrations of tissue residues were measured at tcmax and tcmax/2, and at one or two other time-points. Generally, the depletion of residues in tissues followed biphasic first-order kinetics. At the lower dose, concentrations of tissue residues initially decreased very rapidly with a half-life of <2h, followed by a prolonged depletion phase (5-18 h). At the higher dose, the half-lives of both phases were longer in females than in males. Particularly in the slow depletion phase, the delayed depletion of blood residues affected the half-lives of residues in well-perfused organs, i.e. kidneys (248 h), lung (232 h) and spleen (182h) (Muller, 1996).
Rats
In a study of dermal absorption in rats in vivo, groups of 12 male Tif RAIf (SPF) rats received a dermal application of [2-14C]phenyl-labelled cyprodinil, formulated as SWITCH®62.5 WG, at a concentration of 6 or 870 µg/cm2, for a 6 h period of exposure. Four rats per dose were sacrificed immediately after 6 h, and an additional four rats per dose were sacrificed at 24 and 48 h after exposure. Skin at the application site was washed after 6 h of exposure. The lower dose (6 µg/cm2) reflected a typical concentration recommended for use in the field (final diluted spray containing 450 g of active ingredient/800 l and to be applied per hectare). The higher dose (rat skin, 1016 µg/cm2; human skin, 1376 µg/cm2) represented the highest concentration that could feasibly be applied homogeneously to the skin. [2-14C]Phenyl-labelled cyprodinil (purity, >98%) and unlabelled cyprodinil (purity, 99.9%) were mixed with the formulation ingredients (blank formulation did not contain cyprodinil). Distilled water was used as a vehicle for dermal application. On the basis of the dosing procedure described in the study, it appeared that all animals in the group receiving the lower dose had received cyprodinil at the same dose. Although there may have been small differences in the applied dose, these differences are not expected to affect overall values for dermal absorption at the lower dose, or the conclusions of the study. The application area was confined by a double "O" ring, which was glued with a cyanoacrylate adhesive to a 10 cm2 previously clipped dorsal area. The test solution (100 µl) was uniformly spread over an area of 10 cm2. The "O" ring was covered with permeable tape. A collar was placed over the neck area of the animal to prevent ingestion of the test substance.
Treated animals were placed in glass metabolism cages for collection urine, faeces, CO2 and volatile substances. After the 6 h period of exposure, the cover of the "O" ring was removed and retained for analysis. The unabsorbed material was removed from the application site by washing at least three times with a mild soap solution, using cotton swabs. Finally, the moist skin was dried with cotton swabs and a fresh cover was applied to the "O"-ring for those animals to be sacrificed at 24 h and 48 h after exposure. At study termination, blood, the treated area of skin, non-treated skin, carcass, urine, faeces, skin wash, and cage wash were analysed for radiolabel.
Analysis of the skin wash showed that >98% of the radiolabel was present as parent compound at 6 h. The total recovery of radiolabel ranged from 93% to 101.5% of the applied dose. At the lower dose, the concentration of radiolabel in the blood reached a maximum (cyprodinil equivalents, 0.0037 mg/kg) at 2 h after application. Blood concentrations remained fairly constant until the end of exposure, at 6 h. Thereafter, concentrations of residue in the blood decreased to 0.0025 mg/kg at 8 h and were below the limit of determination by 24 h after application. At the higher dose, the concentration of radiolabel in the blood reached a maximum of (cyprodinil equivalents, 0.093 mg/kg) after 1 h of exposure and decreased rapidly thereafter, to below the limit of determination by 4 h.
Percutaneous absorption and excretion of cyprodinil is described in Table 4. At the lower dose, 17.3%, 21.7% and 16.6% of the applied dose was absorbed through the skin during the periods 0-6, 0-24 and 0-48 h after dosing, respectively. The amount of radiolabel in the skin wash (i.e. the dislodged dose) was 62.79%, 59.90% and 66.48% at 6, 24 and 48 h, respectively. The slightly higher dermal absorption at 24 h compared with that at 48 h may be due to poor efficacy in skin washing at 48 h. No 14CO2was detected in exhaled air. Only 0.02% of the applied dose was recovered in the volatile trap. The systemically absorbed dose was rapidly excreted in the urine and faeces; at 24 and 48 h, the absorbed radiolabel was excreted in the urine (9.7% and 9.0%, of the applied dose, respectively) and the faeces (8.1% and 5.9%, of the applied dose, respectively).
Table 4. Dermal absorption and excretion of cyprodinil in rats (% of applied dose)
|
Time-point (subgroup) |
Dose (µg/cm2) |
||||||
|
Lower dose |
Higher dose |
||||||
|
5.9 |
5.9 |
6.9 |
871.1 |
||||
|
t1 (6 h) |
t2 (24 h) |
t3 (48 h) |
t1 (6h) |
t2 (24 h) |
t3 (48 h) |
||
|
Urine |
|||||||
|
0-6 h |
3.69 |
2.35 |
2.51 |
0.07 |
0.05 |
0.06 |
|
|
6-24 h |
— |
7.33 |
4.21 |
— |
0.68 |
0.74 |
|
|
24-48 h |
— |
— |
2.28 |
— |
— |
0.81 |
|
|
Subtotal |
3.69 |
9.68 |
9.00 |
0.07 |
0.73 |
1.62 |
|
|
Faeces |
|||||||
|
0-6 h |
0.03 |
0.12 |
0.08 |
<0.01 |
<0.01 |
<0.01 |
|
|
6-24 h |
— |
7.97 |
3.21 |
— |
0.30 |
0.35 |
|
|
24-48 h |
— |
— |
2.56 |
— |
— |
0.60 |
|
|
Subtotal |
0.03 |
8.09 |
5.85 |
<0.01 |
0.30 |
0.96 |
|
|
Expired air |
ND |
ND |
0.02 |
ND |
ND |
ND |
|
|
Cage wash |
1.12 |
1.30 |
0.91 |
0.02 |
0.18 |
0.15 |
|
|
Total excretion |
4.84 |
19.08 |
15.78 |
0.10 |
1.21 |
2.73 |
|
|
Residues |
|||||||
|
Whole blood |
0.09 |
0.03 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
|
|
Untreated skin |
0.03 |
0.03 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
|
|
Remaining carcass |
12.34 |
2.58 |
0.80 |
0.38 |
0.65 |
0.46 |
|
|
Subtotal |
12.47 |
2.63 |
0.81 |
0.39 |
0.66 |
0.47 |
|
|
Systemic absorption |
17.31 |
21.71 |
16.59 |
0.49 |
1.86 |
3.20 |
|
|
Treated skin |
15.97 |
12.07 |
9.97 |
4.88 |
3.02 |
2.21 |
|
|
Dislodged dose |
62.79 |
59.90 |
66.48 |
959.906 |
96.50 |
96.13 |
|
|
Total recovery |
96.08 |
93.68 |
93.04 |
100.43 |
101.38 |
101.54 |
|
From Mewes (1999a)
Figures may not add up exactly, due to rounding
ND, not detected
At the higher dose, 0.5%, 1.9% and 3.2% of the applied dose was absorbed through the rat skin during the periods 0-6, 0-24 and 0-48 h after dosing, respectively. No 14CO2 was detected in exhaled air. The systemically absorbed dose was rapidly excreted in the urine and faeces; at 24 and 48 h, the absorbed radioactivity was excreted in the urine (0.7% and 1.6% of the applied dose, respectively) and in the faeces (0.3% and 1.0%, respectively). The amount of radiolabel remaining at the site of application (treated skin) after skin washing at 6 h was 16.0% and 4.9% of the applied dose, at the lower and higher dose, respectively. The radiolabel remaining in the treated skin appears to be available for systemic absorption; at the lower dose, the amount of radiolabel at the site of application decreased to 12.1% at 24 h and to 10.0% at 48 h, compared with 16% at 6 h.
Assuming that penetration of cyprodinil through the rat skin was constant for the first 6 h of treatment, the mean penetration rate (flux) was calculated to be 0.17 and 0.71 µg/cm2 per h for the lower and higher doses, respectively (Mewes, 1999a).
In a study of dermal absorption in vitro, epidermal membranes from rat skin and from human cadaver skin were treated with the cyprodinil formulation SWITCH®62.5 WG at a dose of 5, 1016 (rat skin) or 1376 (human skin) µg/cm2. The lowest dose (5 µg/cm2) approximately reflected the typical concentration recommended for use in the field (final diluted spray containing 450g of active ingredient/800 l and applied per hectare). The higher dose (870 µg/cm2) represented the highest concentration that could feasibly be applied homogeneously to the skin. [2-14C]Phenyl-labelled cyprodinil (purity, >98%) and unlabelled cyprodinil (purity, 99.9%) were mixed with the formulation ingredients and distilled water. Rat epidermal skin came from male Tif RAIf (SPF) rats and human cadaver skin came from male and female Caucasian donors. The epidermis and dermis from the frozen rat skin were separated by soaking in 2 mol/l aqueous sodium bromide solution containing 0.01% sodium azide for approximately 18 h. The epidermis and dermis were separated from the frozen human skin by hot-water treatment. The samples of epidermis from the rat and human skin were mounted on flow-through cells between donor and receptor chambers. The diffusion cells were placed on manifolds and connected to a peristaltic pump. The integrity of the epidermal membranes was checked using tritiated water. Rat epidermis with a permeability coefficient (Kp) value of >3.5 × 10-3/cm per h and human epidermis with a Kp value of >2.5 × 10-3/cm per h were rejected. Those cells containing membranes of acceptable Kp, according to the integrity test, received 6 ml of the formulation; the dosing suspension was spread uniformly over a surface area of 0.64 cm2 on the donor side. The donor chamber was kept open to the air. The receptor fluid was pumped through the receptor chamber. The receptor fluid used was saline during integrity testing with tritiated water and ethanol/water (1:1 v/v) during the exposure to cyprodinil. Perfusates were collected at various intervals for up to 48 h and analysed for radioactivity. At termination, the epidermal surface was rinsed with ethanol/water (1:1 w/v). The treated skin was analysed for radioactivity. The cells were disassembled and washed with ethanol/water (1:1 w/v) and the contents were analysed for radioactivity.
The nature of the radiolabelled compounds contained in the skin rinse of rat and human epidermis was analysed by the thin-layer chromatography (TLC), which indicated that >94% of the radiolabel remained as unchanged cyprodinil after 48 h. The mean recovery of radiolabel ranged from 94.1% to 108.5%. At the lower dose, 61.9%, 68.7%, and 70.3% of the applied dose penetrated the rat epidermis in 12, 24 and 48 h, respectively. At the higher dose, 3.3%, 7.6% and 16.7% of the applied dose penetrated the rat epidermis in 12, 24 and 48 h, respectively. The flux, which reflects the absorption rate under steady-state conditions, was 0.58 mg/cm2 per h for rat epidermis (steady-state conditions from about 1 to 5 h) at the lower dose. At the higher dose, the flux for rat epidermis was 4.5 mg/cm2 per h (steady-state conditions from about 4 to 48 h).
At the lower dose, 8.4%, 15.2%, and 26.5% of the applied dose penetrated the human epidermis in 12, 24 and 48 h, respectively. At the higher dose, 0.3%, 0.7%, and 1.8% of the applied dose penetrated the human epidermis in 12, 24 and 48 h, respectively. The flux, which reflects the absorption rate under steady-state conditions, was 0.03 µg/cm2 per h for human epidermis (steady-state conditions from about 2 to 30 h) at the lower dose. At the higher dose, the flux for the human epidermis was 0.60 µg/cm2 per h (steady-state conditions from about 8 to 48 h).
In conclusion, the dermal penetration of cyprodinil formulated as SWITCH® 62.5 WG was very much lower through human epidermis than through rat epidermis. The ratios of human to rat flux constants were 1:19 at a concentration of 5 µg/cm2, and 1:7.5 at the higher concentration (1016 and 1376 µg/cm2 for rat and human skin, respectively) (Mewes, 1999b).
Rats
The proposed metabolic pathways of cyprodinil in rats, goats and hens are shown in Figure 2. High-performance liquid chromatography (HPLC) analysis of rat urine identified eight different fractions (U1-U8), each accounting for 1-35% of the administered dose (Table 5). There were no qualitative differences related to sex, doses, treatment regimen, presence of bile cannulae, or type of radiolabel, but a quantitative difference between males and females was observed for metabolite fractions U2 and U6 (Table 5). The quantitative difference in metabolites in the group of animals with bile cannulae compared with other groups was caused by lower renal excretion. The similar pattern of metabolites in urine and faeces for both types of radiolabel, and no radiolabel-dependent differences in the excretion and residue data indicated that the C-N-C bridge between the phenyl and pyrimidyl rings was not cleaved. No metabolite fraction corresponded to unchanged test material.
Table 5. Quantitative pattern of urinary metabolites in rats treated orally with radiolabelled cyprodinil (% of applied dose in metabolite fraction)
|
Urinary metabolite fraction |
Group |
||||||||
|
B1 |
C1 |
D1 |
D2 |
G1 |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
|
|
U1 |
3.3 |
2.3 |
ND |
ND |
1.9 |
2.5 |
1.3 |
2.5 |
1.4 |
|
U2 |
12.8 |
0.5 |
13.7 |
ND |
13.6 |
0.6 |
13.8 |
0.8 |
11.5 |
|
U3 |
3.3 |
2.0 |
2.7 |
2.6 |
2.5 |
2.4 |
2.7 |
3.7 |
|
|
U4 |
5.9 |
8.3 |
6.9 |
7.4 |
5.5 |
6.7 |
4.8 |
7.4 |
2.7 |
|
U5 |
0.8 |
0.5 |
0.6 |
0.7 |
1.2 |
0.7 |
1.3 |
1.2 |
1.3 |
|
U6 |
17.2 |
34.7 |
19.0 |
30.0 |
17.0 |
31.0 |
19.8 |
34.3 |
9.5 |
|
U7 |
3.6 |
2.3 |
3.4 |
2.7 |
5.4 |
5.9 |
8.4 |
8.3 |
6.7 |
|
U8 |
5.1 |
5.9 |
5.1 |
4.3 |
4.0 |
6.4 |
7.6 |
8.2 |
2.4 |
|
Total identified |
52.0 |
56.25 |
51.4 |
47.7 |
51.1 |
56.2 |
59.7 |
66.4 |
35.5 |
From Thanei (1992)
ND, not detected
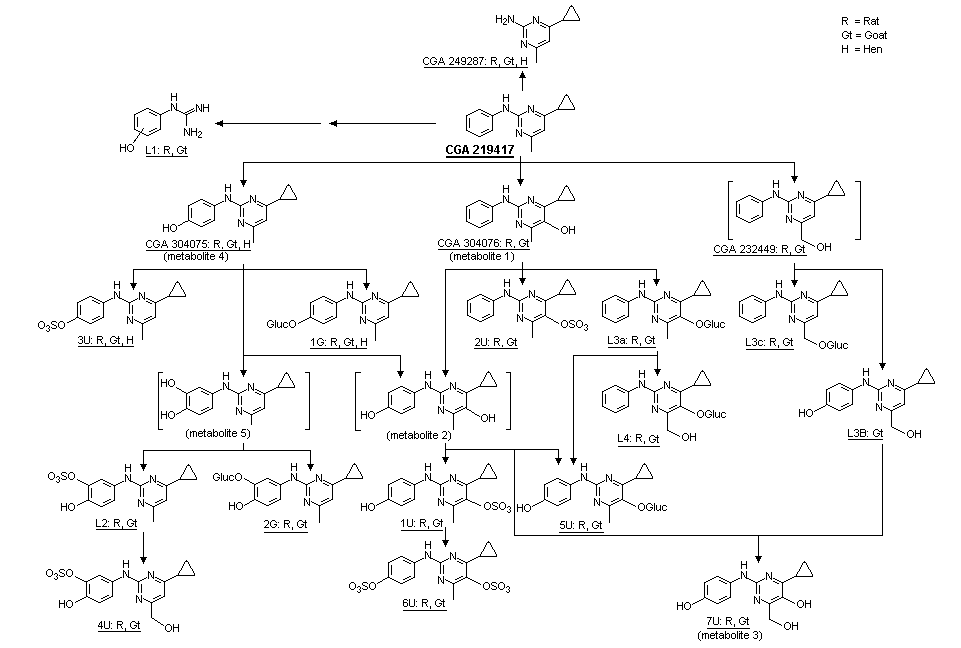
Figure 2. Proposed metabolic pathways for cyprodinil
HPLC analysis of faecal extracts from animals receiving the higher dose revealed eight metabolite fractions in extract 1. Fraction F8 corresponded to the parent compound (3-8% of the administered dose), and F6 corresponded to CGA 304076 (4-cyclopropyl-6-hydroxy-methyl-N-phenyl-2-pyrimidinamine). The pattern of metabolites was essentially independent of sex, dose, treatment regimen and type of radiolabel, but showed quantitative differences between the two doses (Table 6). The pattern of faecal metabolites for rats with bile cannulae (group G1) was less complex, with >90% of the administered dose corresponding to unchanged parent compound.
Table 6. Quantitative pattern of faecal metabolites in rats treated orally with radiolabelled cyprodinil (% of applied dose in metabolite fraction)
|
Faecal |
Group |
Assignment to reference compounds |
||||||||
|
metabolite fraction |
B1 |
C1 |
D1 |
D2 |
G1 |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
||
|
F1 |
ND |
ND |
ND |
ND |
0.9 |
0.6 |
0.5 |
0.6 |
0.2 |
|
|
F2 |
4.7 |
3.1 |
ND |
Traces |
1.5 |
0.4 |
0.2 |
0.5 |
0.3 |
|
|
F3 |
5.9 |
ND |
2.3 |
2.7 |
3.6 |
2.4 |
2.3 |
1.8 |
0.3 |
|
|
F4 |
ND |
ND |
ND |
ND |
2.8 |
2.1 |
2.3 |
1.5 |
ND |
|
|
F5 |
3.0 |
2.9 |
2.7 |
3.4 |
4.5 |
3.4 |
4.3 |
3.9 |
ND |
|
|
F6 |
6.4 |
5.2 |
11.4 |
11.3 |
11.2 |
10.4 |
8.1 |
4.8 |
ND |
CGA 304076a (CGA 232449)b |
|
F7 |
3.9 |
3.2 |
1.1 |
2.2 |
1.3 |
1.4 |
0.8 |
0.7 |
ND |
|
|
F8 |
4.2 |
4.9 |
8.1 |
5.4 |
4.2 |
2.7 |
4.5 |
2.6 |
12.6 |
Cyprodinil |
|
S Extract 1c |
28.1 |
19.3 |
25.6 |
25.0 |
30.0 |
23.4 |
23.0 |
16.4 |
13.4 |
|
|
Extract 2d |
2.8 |
3.0 |
3.8 |
3.5 |
3.2 |
2.4 |
3.4 |
2.6 |
0.1 |
|
|
Residue |
13.7 |
13.2 |
14.7 |
16.8 |
9.6 |
10.0 |
9.1 |
7.6 |
0.4 |
|
|
Total |
44.6 |
35.5 |
44.1 |
45.3 |
42.8 |
35.8 |
35.5 |
26.6 |
13.9 |
|
From Thanei (1992)
ND, not detected
aCGA 304076 is 4-cyclopropyl-6-methyl-2-phenylamino-pyrimidine-5-ol
bCGA 232449 is (6-cyclopropyl-2-phenylamino-pyrimidine-4-yl)-methanol
cS Extract 1 = sum of radioactivity of faecal metabolites F1-F8, from faecal samples extracted in methanol: water 4:1 (v/v)
d Extract 2 = radioactivity in faecal sample after a second extraction of extract 1 with methanol: water 4:1 (v/v) in a soxhlet apparatus for about 20 h
HPLC analysis of bile identified nine metabolite fractions, each accounting for 1-11% of the administered dose (total, 39.0%). None of the fractions corresponded to parent material, but most fractions showed similar chromatographic characteristics to those of the urine metabolite fractions (Thanei, 1992).
In another study, excreta (group D1 and D2) and bile (group G1) fromTif RAIf (SPF) rats treated with radiolabelled cyprodinil in a previous study by Thanei (1992) were used to characterize, isolate and identify metabolites of cyprodinil. The design of the study is described in Table 1. Identification of metabolites in the urine and bile and their relative proportions are shown in Table 7; identification of faecal metabolites and their relative proportions are shown in Table 8. Eleven metabolites were isolated from urine, faeces and bile; proposed metabolic pathways in the rat are described in Figure 2. HPLC analysis of the samples of urine revealed the presence of eight metabolite fractions designated U1-U8. HPLC analysis of the samples of faeces revealed the presence of eight metabolite fractions designated F1-F8. All urinary and biliary metabolites (with the exception of 7U) were conjugated with glucuronic acid or sulfonated, and excreted. Cyprodinil was almost completely metabolized by hydroxylation of the phenyl ring (position 4) or the pyrimidine ring (position 5), followed by conjugation. An alternative pathway involved oxidation of the phenyl ring, followed by conjugation with glucuronic acid. A quantitative difference between the sexes was observed with respect to sulfonation of the major metabolite that formed 6U. The monosulfate metabolite (1U) was predominant in females, whereas equal amounts of mono-and disulfate (6U) conjugates were noted in males. Most of the significant metabolites in faeces were exocons of biliary metabolites (2U, 3U, 1G). These were assumed to be decon-jugated in the intestines, partially reabsorbed into the general circulation, conjugated again, and eliminated renally. Faecal metabolite 1F is the exocon of metabolite 2U. Faecal metabolite 2F is the exocon of metabolite 3U and metabolite 1G. The major metabolic pathways of cyprodinil were not significantly influenced by dose, treatment regimen, or sex of the animal (Muller, 1992).
Table 7. Identification of metabolites in the urine and bile and assignment to quantitative profilesa (% of the administered dose in metabolite fraction)
|
Urine fraction |
Assignment of metabolites |
Corresponding bile metabolite fraction |
Group |
||||
|
D1b |
D2c |
G1d |
|||||
|
Male |
Female |
Male |
Female |
Male |
|||
|
U1 |
7U |
G1 |
1.9 |
2.5 |
1.3 |
2.5 |
1.4 |
|
U2 |
6U |
G2 |
13.6 |
0.6 |
13.8 |
0.8 ^ |
|
|
U3 |
5U |
G3 |
2.5 |
2.4 |
2.7 |
3.7 } |
|
|
U4 |
4U |
G4 |
5.5 |
6.7 |
4.8 |
7.4 |
2.7 |
|
U5 |
— |
— |
1.2 |
0.7 |
1.3 |
1.2 |
1.3 |
|
U6 |
1U |
G5 |
17.0 |
31.0 |
19.8 |
34.2 |
9.5 |
|
U7 |
3U |
G7 |
5.4 |
5.9 |
8.4 |
8.3 |
6.7 |
|
U8 |
2U |
G8 |
4.0 |
6.4 |
7.6 |
8.2 |
2.4 |
|
G6 (= 1G [80%], 2G [20%]) |
— |
— |
— |
— |
— |
||
|
G9 |
— |
— |
— |
— |
— |
||
|
% of radioactivity identified |
51.1 |
56.2 |
59.6 |
66.4 |
35.4 |
||
From Muller (1992)
a See Figure 2 for identification of metabolites and metabolic pathway
b Animals in group D1 were treated with [U-14C]phenyl-labelled cyprodinil as a single oral dose at 100 mg/kg bw and excreta were collected up to 168 h
cAnimals in group D2 were treated with [2-14C]pyrimidyl-labelled cyprodinil as a single oral dose at 100 mg/kg bw and excreta were collected up to 168 h
dAnimals in group G1 had cannulated bile ducts and were treated with [U-14C]phenyl-labelled cyprodinil as a single oral dose at 100 mg/kg bw and bile was collected up to 48 h. The value represents the amount of radiolabel in bile as a % of the administered dose
Table 8. Identification of faecal metabolites (% of the administered dose)
|
Faecal extract fraction |
Assignment of metabolites |
Group |
||||
|
D1 |
D2 |
G1 |
||||
|
Male |
Female |
Male |
Female |
Male |
||
|
F1 |
— |
0.9 |
0.6 |
0.5 |
0.6 |
0.2 |
|
F2 |
— |
1.5 |
0.4 |
0.2 |
0.5 |
0.3 |
|
F3 |
— |
3.6 |
2.4 |
2.3 |
1.8 |
0.3 |
|
F4 |
— |
2.8 |
2.1 |
2.3 |
1.5 |
ND |
|
F5 |
2F |
4.5 |
3.4 |
4.3 |
3.9 |
ND |
|
F6 |
1F |
11.2 |
10.4 |
8.1 |
4.8 |
ND |
|
F7 |
— |
1.3 |
1.4 |
0.8 |
0.7 |
ND |
|
F8a |
CGA 219417 |
4.2 |
2.7 |
4.5 |
2.6 |
12.6 |
|
Sigma Extract 1b |
— |
30.0 |
23.4 |
22.9 |
16.4 |
13.4 |
|
Sigma Extracts & residuec |
— |
42.7 |
35.8 |
35.4 |
26.7 |
13.9 |
From Muller (1992)
a Corresponds to parent compound
b Sigma Extract 1 = sum of radioactivity in fractions F1-F8, the pooled faeces from 0-48 h were extracted with 4.7 ml of methanol/g for 30 min at room temperature, with agitation. After filtration, this procedure was repeated twice with methanol/water (4:1 v/v), and all extracts were combined
c Sigma Extracts & residue = total radioactivity in extract 1 and residue (residual radiolabel in solid after extraction, i.e. nonextractable radioactivity)
In a separate study, three male Tif RAIf (SPF) rats were given [2-14C]pyrimidine-labelled cyprodinil as a single dose at 100 mg/kg bw by gavage. The animals were sacrificed 12 h after administration of the test substance and blood, urine, faeces, liver, kidneys and residual carcass were collected. The amount of radiolabel in various tissues and excreta was determined. The amounts of metabolites in selected tissues were also determined.
The patern of metabolites in urine was similar to that described in the previous study by Muller (1992), apart from the detection of additional metabolites CGA 249287 (4-cyclopropyl-6-methyl-pyrimidine-2-ylamine) and L1 (hydroxylated N-phenyl-guanidine; a breakdown product of the pyrimidyl ring) in the liver. Cyprodinil was found as a minor residue in liver (2.9%) and kidneys (1%). Hydroxylation in position 4 of the phenyl ring produced CGA 304075, i.e. 4-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-phenol; this compound was found in the liver (5.9%) and kidneys (2%), together with its sulfate (3U) and glucuronide conjugate (1G), which were also present in the urine.
CGA 304076, i.e. 4-cyclopropyl-6-methyl-2-phenylamino-pyrimidine-5-ol, was the product of hydroxylation in position 5 of the pyrimidine ring. Its sulfate conjugate (2U) and glucuronide conjugate (L3a) were found in the liver, kidney and urine. Additional hydroxylation of the glucuronide at the methyl group resulted in the minor metabolite (L4), i.e. the glucuronide of 4-cyclopropyl-6-hydroxymethyl-2-phenylamino-pyrimidine-5-ol.
Direct hydroxylation at the methyl group of cyprodinil also took place, as shown by the presence of the glucuronic acid conjugate (L3c, (6-cyclopropyl-2-phenylamino-pyrimidine-4-yl)-methanol) in the liver and urine. Hydroxylation and subsequent sulfate conjugation in position 3 of the phenol ring occurred in the dihydroxylated metabolite L2 (3-sulfate of 4-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-benzene-1,2-diol) and in the trihydroxylated metabolite 4U (3-sulfate of 4-(4-cyclopropyl-6-hydroxymethyl-pyrimidine-2-ylamino)-benzene-1,2-diol). These two metabolites were found in the kidney and urine.
Other dihydroxylated metabolites, dihydroxylated in positions 4 and 5 of the phenol and pyrimidine ring, respectively, were found as the sulfate (1U), bis-sulfate (6U) and glucuronide conjugate (5U) in the liver, kidney and urine. The trihydroxylated metabolite 7U (4-cyclopropyl-6-hydroxymethyl-2-(4-hydroxy-phenylamino)-pyrimidine-5-ol) was found in the liver, kidneys, and urine (Rumbeli, 1996).
Absorption, distribution, metabolism and excretion of cyprodinil administered as a single dose at 0.5 or 100 mg/kg bw by gavage was studied in male and female Tif RAIf (SPF) rats by Thanei (1992) and Muller (1992). Cyprodinil was rapidly excreted (92-97% within 48 h), irrespective of dose or sex. Approximately 53-60% and 37-45% of the administered dose was excreted within 168 h into the urine and faeces, respectively. The pattern of metabolites in urine exhibited a significant sex-related difference with respect to the major metabolite. In the urine of males, both the metabolites 1U and 6U were detected in amounts of 13-20% of the dose, while metabolite 6U was found in females only in marginal amounts (0-0.8%) (see Figure 2 for identification of metabolites). The sum of radio-label in 1U and 6U was in the same range in both sexes (30-34% and 30-35% of the administered dose for males and females, respectively). Cyprodinil is metabolized by sequential oxidation of phenyl and pyrimidinyl rings. Hydroxylation of the phenyl or the pyrimidinyl ring yields the 4-hydroxyphenyl (metabolite 4) or 5-hydroxypyrimidinyl metabolites (metabolite 1), respectively, which are excreted as sulfate conjugates (2U and 3U, respectively). A minor pathway is the further oxidation of the 4-hydroxy moiety to a 3,4-dihydroxyphenyl metabolite (metabolite 5), which is sulfated at the 3-hydroxy group (L2).
The major pathway involves hydroxylation on both rings to form a 4-hydroxyphenyl-5-hydroxypyrimidinyl metabolite (metabolite 2). Before excretion, metabolite 2 is conjugated with sulfate and, to a lesser extent, with glucuronic acid. Sulfation and glucuronidation of the 5-hydroxypyrimidyl group, yielding 1U and 5U respectively, occurs to the same extent in males and females. However, in males 1U undergoes further sulfation at the 4-hydroxyphenyl group, yielding the disulfate 6U, which is not found in females. Small amounts of a trihydroxy metabolite (7U) are excreted unconjugated in males and females.
Disulfate conjugates are rarely observed in the metabolism of xenobiotics, but they are well known in the metabolism of endogenous dihydroxysteroids and dihydroxy-diarylamines, which is catalysed by steroid and phenol sulfotransferases, respectively. A disulfate conjugate was found to be a major metabolite of diphenylamine in rat urine (metabolite 2). In the sulfation of metabolite 2 in males, two distinct sulfotransferases appear to be involved. The first sulfotransferase mediates the transfer of sulfonate to the 5-hydroxy-pyrimidyl site. As the polarity and hydrophilicity of 1U are increased markedly in comparison to metabolite 2, the sulfation of the 4-hydroxyphenyl group is most likely to be catalysed by a second sulfotransferase having a different specificity. Since only males formed 6U, the activity of the second sulfotransferase is sex-dependent (Muller et al., 1999).
No information was available.
Data on the acute toxicity of cyprodinil are summarized in Table 9.
Table 9. Acute toxicity of cyprodinila
|
Species |
Strain |
Route |
LD50 (mg/kg bw) or LC50 (mg/l of air) |
Reference |
|
Rat |
Tif RAIf (SPF) |
Oral |
>2000 mg/kg bw |
Hartmann (1990b) |
|
Mouse |
Tif MAG (SPF) |
>5000 mg/kg bw |
Winkler (1995) |
|
|
Rat |
Tif RAIf (SPF) |
Dermal |
>2000 mg/kg bw |
Hartmann (1990a) |
|
Rat |
Tif RAIf (SPF) |
Inhalation |
>1200mg/m3 |
Hartmann (1991) |
|
Rabbit |
New Zealand white |
Dermal irritation |
Not an irritant |
Schneider (1990a) |
|
Rabbit |
New Zealand white |
Ocular irritation |
Not an irritant |
Schneider (1990b) |
|
Guinea-pig |
Pirbright white |
Skin sensitization: maximization test |
Sensitizer |
Winkler (1996a) |
a Technical grade cyprodinil of >99% purity was used in all studies
Groups of five male and five female Tif RAIf (SPF) rats were given cyprodinil (purity, 99.5%) in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) as a single dose at 2000 mg/kg bw by gavage. Gross necropsy was performed at the end of a 14-day period of observation. This study complied with good laboratory practice (GLP). Rats treated with cyprodinil exhibited clinical signs such as piloerection, hunched posture, dyspnoea and reduced locomotor activity (on the day of administration in males only) after a single dose of 2000 mg/kg bw, and all treated animals recovered within 5 days. There was no mortality or significant gross pathological findings (Hartmann, 1990a).
Groups of five male and five female Tif MAG (SPF) mice were given cyprodinil (purity, 99.2%) in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) as a single dose at 5000 mg/kg bw by gavage. Gross necropsy was performed at the end of a 14-day period of observation. This study complied with GLP. Mice treated with cyprodinil exhibited clinical signs such as piloerection and hunched posture, which occurred only in the first 5 h after dosing. These signs were not considered to be compound-related effects. No mortality occurred and no gross abnormalities were observed at necropsy (Winkler, 1995).
Groups of five male and five female young adult Tif RAIf (SPF) albino rats received a dermal application of cyprodinil (purity, 99.5%) at a dose of 2000 mg/kg bw (limit dose) for 24 h. The test substance, suspended in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v), was applied to approximately 10% of the total surface area of the body. Animals were observed for clinical signs and mortality for up to 14 days after dosing. This study complied with GLP. Rats treated with cyprodinil exhibited clinical signs such as piloerection, abnormal body position, and dyspnoea after a single dermal application of 2000 mg/kg bw; all treated animals recovered within 5 days. No mortality was observed in the 14-day period of observation. No gross abnormalities were observed at necropsy (Hartman, 1990b).
Five male and five female young adult Tif RAIf (SPF) rats were exposed by nose-only inhalation to cyprodinil (purity, 99.5%) at a dose of 1.20 mg/l (the highest attainable concentration) for 4 h. A control group of five males and five females were exposed to filtered humidified air only. Animals were observed for clinical signs and mortality for up to 14 days after exposure. This study complied with GLP. Rats exposed to cyprodinil via nose-only inhalation exhibited clinical signs such as piloerection and dyspenia after a single exposure of 1.2 mg/l for 4 h; all treated animals recovered within 5 days. No mortality was observed. No gross abnormalities were observed at necropsy (Hartmann, 1991).
(d) Dermal and ocular irritation
In a study of primary dermal irritation, three female Chbb:NWZ rabbits received an application of 0.5 g of cyprodinil (purity, 99.5%), moistened with carboxymethylcellulose (0.5% w/v) in aqueous polysorbate 80 (0.1% w/v), for 4 h. A gauze patch (surface area, approximately 12-16 cm2) containing the test article was applied to the right flank of the each animal. A control gauze patch was applied to the contralateral flank. This study complied with GLP. No skin reactions were observed at 24 and 72 h after application. No mortality was observed (Schneider, 1990a).
In a study of primary ocular irritation, three male Chbb:NWZ rabbits received an instillation of 0.1 ml (40 mg) of undiluted cyprodinil (purity, 99.5%) into the conjuctival sac of the left eye. The eyes were observed for 72 h after instillation and scored for irritation at 1, 24, 48, and 72 h according to the OECD scoring system. This study complied with GLP. Slight to moderate conjuctival redness and mild chemosis were observed at 1 h, and mild redness in one animal after 24 h. All treated eyes recovered within 48 h after instillation (Schneider, 1990b).
In a study of dermal sensitization using the maximization method of Magnusson and Kligman, cyprodinil (purity, 99.2%) was given to 10 male and 10 female 10-week-old Tif DHP (Pirbright white) guinea-pigs. An additional five animals of each sex were used as controls and received adjuvant, vehicle, or challenge treatment only. After the challenge, 9 out of 20 treated animals (45%) exhibited very slight or well-defined erythema (Draize score of 1 or 2) without oedema 24 h after Hilltop chamber and dressing removal. At 48 h, very slight erythema without oedema persisted in 6 out of 20 treated animals (30%). Two of these animals (both females) had scaling erythema. No other treated animal and no control animal exhibited any dermal irritation casued by the challenge application. Data from positive controls indicated an appropriate positive response. This study complied with GLP (Winkler, 1996a).
Mice
In a 3-month study of toxicity, groups of 10 male and 10 female Tif MAGf (SPF) albino mice were fed diets containing cyprodinil (purity, 99.5%) at a concentration of 0, 500, 2000 or 6000 mg/kg (equal to 0, 73.32, 257.3 or 848.6 mg/kg bw per day in males and 0, 102.5, 349.2 or 1121.0 mg/kg per day in females). Animals were examined at least once daily for morbidity and mortality, and body weights and food consumption were recorded weekly. Haematological parameters were measured for all surviving animals at each dose at study termination. All animals that survived to study termination were subjected to detailed necropsy. Organ weights were recorded and histological examinations were performed. This study complied with GLP.
There were no treatment-related effects on mortality, clinical signs or haematological parameters. There was no clear treatment-related effect on body weight, and there were no consistent differences in body-weight gains between control and treated animals at various times throughout the study. However, a slight trend towards reduced body-weight gain was observed at the highest dose. There was no treatment-related effect on food consumption or food consumption efficiency. Values for overall food consumption in treated animals were within 5% of those for controls.
There were dose-related increases in mean absolute liver weights, mean liver to body weight ratios, and mean liver to brain weight ratios in males at the intermediate and highest doses, relative to controls; these were statistically significant at the highest dose. Mean absolute and relative spleen weights were significantly greater in females at the intermediate and highest doses, and mean absolute spleen weight was significantly greater in males at the highest dose. However, there was no dose-response relationship for absolute spleen weights and no haematological or histopathological findings to support a toxicologically significant effect. Absolute and relative thyroid weights showed a dose-related decrease in males; however, values for these parameters were slightly increased in females at the highest dose. In the absence of histopathological findings, thyroid changes were not considered to be toxicologically significant.
There were no macroscopic findings in control or treated animals. Moderate multifocal single cell hepatocyte necrosis was observed throughout the liver parenchyma in 3 out of 10 males in each group receiving the intermediate or highest dose, and glycogen depletion was noted in 7 and 10 out of 10 females at the intermediate and highest dose, respectively. No other findings were considered to be related to treatment.
The no-observed-effect level (NOEL) was 500 mg/kg (equal to 73.3 mg/kg bw per day in males, 103 mg/kg bw per day in females) on the basis of multifocal single cell hepatocyte necrosis at 2000 and 6000 mg/kg in males and glycogen depletion at 2000 and 6000 mg/kg in females. Single cell necrosis was moderate and multifocal in all affected animals, the lesion being observed throughout the liver parenchyma. It should, however, be noted that no dose-response relationship was observed in this study, and there was no clear treatment-related effect on liver necrosis in the 18-month study in mice. As stated in the study report, glycogen depletion was indicative of physiological stress, starvation, or an underlying pathological condition, particularly in young animals, in which glycogen in the cytoplasm is essential for liver metabolism (Fankhauser, 1991a).
Rats
In a 28-day range-finding study, groups of 10 male and 10 female Tif RAIf (SPF) rats were given cyprodinil (purity, 99.5%) in carboxymethylcellulose (0.5% w/v)/Tween 80 (0.1% w/v) in distilled water, at a dose of 0, 10, 100 or 1000 mg/kg bw per day by gavage, once daily for 28 days. Animals were observed twice daily on working days and once at weekends for mortality, and daily for clinical signs of toxicity. Individual body weight, food and water consumption were recorded weekly. Ophthalmoscopic examinations of animals in the control groups and the groups receiving a dose of 1000 mg/kg bw per day were conducted 5 days before and 23 days after the initiation of treatment. Haematological and clinical chemistry tests and necropsy were performed at the end of the study period. Urine analysis was not conducted in this study. At the end of the study, gross necropsies were performed and selected organs were removed, weighed and histopathological examination performed. This study complied with GLP.
No treatment-related clinical signs of toxicity or deaths occurred during the study. Mean body-weight gains for male rats at 1000 mg/kg bw were 22% lower (p < 0.05) than those for controls after 2 weeks of treatment. Between weeks 2 and 4, these treated rats gained more weight than the controls, so the total body-weight gain for male rats at 1000 mg/kg bw at the termination of the study was only 9% lower than that of the controls. Body-weight gains for female rats at 1000 mg/kg bw were 19% and 15% lower than those of the controls at weeks 2 and 4, respectively; these differences were not statistically significant. The body weights and body-weight gains of males and females at 10 and 100 mg/kg bw were not significantly different from those of the controls.
During the first week of treatment, food consumption (g of food/kg bw per day) in males and females at 1000 mg/kg bw was 30% lower than food consumption in the same animals before initiation of treatment and 8-10% lower than that in the control groups during the first week of treatment. During the remainder of the study, food consumption (g of food/kg bw per day) in animals in the groups receiving the highest dose was similar to that in the controls. Food consumption at 10 or 100 mg/kg was similar to that in the controls.
Water consumption (g of water/animal) at 100 (males only) and 1000 mg/kg bw was slightly higher than that in the controls.
No treatment-related ophthalmological abnormalities were noted during the study. Urine analysis was not conducted in this study.
Rats in the groups receiving a dose of 100 and 1000 mg/kg had higher leukocyte counts and lower mean corpuscular haemoglobin (MCH) values compared with those of the controls. At 1000 mg/kg, prothrombin times were had significantly faster (up to 25-28%) than those of the controls. The slight increase in leukocyte count and minimal decrease in MCH values at 100 mg/kg bw per day were within the range of values for historical controls and normal biological variation and were considered not to be toxicologically relevant.
Rats treated with a dose of 1000 mg/kg bw had higher concentrations of blood protein (11% higher), albumin (7-10% higher), globulin (12-18% higher), total bilirubin (21-29% higher), cholesterol (56-71% higher), and phospholipid (41-51% higher) than the control animals. At the highest dose, slightly elevated serum activities were observed for alanine aminotransferase and alkaline phosphatase. No other treatment-related differences existed between the treated and the control rats; all other observed differences in blood chemistry remained within the expected biological ranges for this strain of rat.
Increased absolute and relative liver weights were observed at 100 mg/kg bw per day (10-17% greater than controls) and 1000 mg/kg bw per day (27-54% greater than controls); the increases were statistically significant for both sexes at 1000 mg/kg bw per day. Increased absolute thyroid weights (7-16%) and relative thyroid weights (12-23%) were observed at 1000 mg/kg bw per day. These differences were not statistically significant.
No toxicological relevance was attributed to slight variations in thymus weight since the decrease was not dose-related, and was statistically significant only for treated females.
Enlargement of the liver was noted in one female at 100 mg/kg bw per day, and in nearly all animals at 1000 mg/kg bw per day. "Minimal" hypertrophy of liver hepatocytes was noted at 100 (females only) and 1000 mg/kg bw per day. Slightly increased liver weight, associated with a minimal hepatocellular hypertrophy, was considered to be an adaptive response in the liver. Minimal to moderate hypertrophy of the follicular epithelium of the thyroid gland was noted in almost all animals at 1000 mg/kg bw per day. No abnormalities were observed in the thymus of the treated rats. All other abnormalities occurred randomly and sporadically in all groups. No neoplastic tissue was observed in rats in the treated or control groups. Although the NOEL identified by the study author was 10 mg/kg bw per day, this reviewer considered that the no-observed-adverse-effect level (NOAEL) was 100 mg/kg bw per day. The lowest-observed-adverse-effect level (LOAEL) was 1000 mg/kg bw per day for rats, on the basis of slightly decreased body weight, decreased food consumption, increased liver weights, changes in haematological and clinical chemistry parameters, abnormalities in liver morphology and hypertrophy of the follicular epithelium of the thyroid glands (Fankhauser, 1991c).
In a 28-day study of dermal toxicity, groups of five male and five female Tif RAIf (SPF) rats received repeated dermal applications of cyprodinil (purity, 99.5%) at a dose of 0, 5, 25, 125 or 1000 mg/kg bw per day, for 6 h per day, 5 days per week over 28 days. The fur of each animal was clipped from the dorsal area of the trunk over an area of at least 10% of the body surface shortly before the first application and weekly thereafter. The test substance was suspended in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v). The test article/vehicle suspension was applied to the clipped area and held in place under an occlusive dressing. Rats in the control group were exposed to the vehicle only, using the same procedure. Animals were observed daily for mortality, signs of toxicity, and the presence of dermal irritation. The animals were also examined for signs of local skin irritation approximately 17 h after removing the gauze patches and were evaluated according to the method of Draize. Animals were weighed before the start of treatment, then weekly, to ensure accurate dosing. Body weights were recorded weekly. Food consumption for each cage of animals (five rats per cage) was determined weekly. Haematological and clinical chemistry parameters were measured for surviving animals at study termination. All animals were sacrificed on schedule and subjected to gross pathological examination. Selected tissues were collected for weighing and histological examination. This study complied with GLP.
No animals died or had to be killed during the course of the study. Clinical signs were observed in females at > 25 mg/kg bw per day and in males at 1000 mg/kg bw per day. Piloerection was seen in: 2 out of 5 females at 25 mg/kg bw per day primarily during week 4; four out of five females at 125 mg/kg bw per day during weeks 3 and 4; and in males (four out of five) and females (five out of five) at 1000 mg/kg bw per day during weeks 2-4. Hunched posture was seen in two out of five females at 125 mg/kg bw per day during weeks 3 and 4, and in four out of five females at 1000 mg/kg bw per day during week 4. Dyspnoea was observed in two out of five males and two out of five females at 1000 mg/kg bw per day during weeks 2 and/or 3. No local skin irritation or other differences in clinical signs were observed between the treated and control groups.
Body weights or body-weight gains for both sexes in all of the treated groups were not significantly different from those of the controls. Reduced food consumption (g/animal per week) in males at 1000 mg/kg bw per day during week 4 was statistically significant (p < 0.05) compared with that in the controls, and may have been treatment-related. No other variations in food consumption appeared to be treatment-related for any of the treated groups. Reduced food consumption in males at 5, 25, and 125 mg/kg bw per day during weeks 1, 3, and 4 was not concentration-related, although it was statistically significant (p < 0.05) for males at 5 and 25mg/kg during week 3 compared with that in the controls. Decreased food consumption ratios for males at 5, 25, and 1000 mg/kg bw during weeks 3 and 4 compared with that for the controls did not appear to be concentration-related, since food consumption ratios for males at 125 mg/kg bw per day were comparable with those of the controls. Food consumption by females in all treated groups was comparable to that of the controls. Food consumption ratios for females in the treated and control groups varied throughout the study.
One female from the group receiving a dose of 1000 mg/kg bw per day exhibited anaemia associated with an increased number of reticulocytes. The toxicological relevance of this finding is considered to be equivocal, since only one animal was affected at this, the highest, dose.
No other differences in haematology were observed between any of the treated groups and the control groups. No differences in clinical blood chemistry parameters attributable to treatment were observed for any of the treated groups. Differences in clinical chemistry parameters were observed, but they were within the normal biological ranges for this strain of rat and were attributed to normal variation, although they were statistically significant (p < 0.05) compared with those for the controls.
No differences in absolute or relative organ weights were observed between any of the treated and control groups, except for a statistically significant (p < 0.05) increase in mean relative testes weight for males at 5 mg/kg bw per day. No differences in gross pathology were observed between any of the treatment and control groups. Comparable numbers of isolated macroscopic findings occurred in all treated and control groups, and were similar to those occurring spontaneously in the colony from which the animals were selected. No neoplastic alterations attributable to treatment occurred in animals in any of the treated groups. The incidence, distribution, and morphological appearance of microscopic alterations that occurred at the dermal test site were similar in the control and treated rats, and were considered to be caused by the application procedure. All other changes were reported to be common to the colony from which the test animals were selected. No neoplastic tissue was observed in the treated or control rats.
The pattern of toxicity seen in this study appears to be more severe than that observed in the 28-day study of rats treated by gavage. Moreover, there appear to be more severe effects in females than in males; this was not apparent in other toxicological studies. The reason for these differences is not clear on consideration of the available data on dermal absorption, and other relevant data. Therefore, the results of this study should be used with caution in any risk assessment. The NOEL was given as 5 mg/kg bw per day by the study author. This reviewer considered the NOAEL to be 25 mg/kg bw per day for females and 125 mg/kg bw per day for males. The LOAEL was 125 mg/kg bw per day for females and 1000 mg/kg bw per day for males, on the basis of alterations in clinical signs (hunched posture and/or piloerection) (Hagemann, 1991).
In a short-term study of toxicity, groups of 20 male and 20 female Tif RAIf (SPF) rats were given diets containing cyprodinil at a concentration of 0 or 12 000 mg/kg (equal to 0 or 810 mg/kg bw per day for males and 0 or 803 mg/kg bw per day for females), and groups of 10 male and 10 female rats were given diets containing cyprodinil at a cocentra-tion of 50, 300, or 2000 mg/kg (equal to 3.14, 19.0, or 134 mg/kg bw per day, respectively, for males, and 3.24, 19.3, or 137 mg/kg bw per day for females) for 90 days. All animals were sacrificed after 90 days, except for 10 rats of each sex in the control group and in the group receiving cyprodinil at 12 000 mg/kg, which were sacrificed after a 4-week period of recovery.
Animals were inspected at least once each day for signs of toxicity and mortality. Animals were weighed before the start of treatment, then once per week for the duration of the study. Food and water consumption for each cage of animals (five rats per cage) was determined weekly. Mean food consumption was reported as g of food/animal per day and the food consumption ratio (g of food/kg bw per day) was calculated as an indicator of the food consumption efficiency. Food conversion efficiency was not determined. Ophthalmoscopic examinations of animals in the control group and the group receiving cyprodinil at 12 000 mg/kg were conducted 4 days before and 88 days after the start of treatment, and at the end of the 4-week period of recovery (day 115). For evaluation of clinical chemistry and haematological parameters, blood was collected from the orbital sinus of all animals surviving to termination of the study on the morning following overnight fasting. Urine analysis was performed on samples collected from fasted animals held overnight in special metabolism cages at the termination of the study. All animals that died and those that were sacrificed on schedule were subjected to gross pathological examination; selected organs were weighed and tissues were collected for histological examination. This study complied with GLP.
There were no treatment-related effects on mortality, clinical signs, urine analysis or upon ophthalmological examination.
At 12 000 mg/kg, mean body weights of male and female rats were 13-17% lower (p < 0.01) than those of controls each week during the treatment period, and body-weight gains were 20-26% lower than those of controls at termination of the study. At the end of the recovery period, the treated rats weighed only 11-12% less than the controls, indicating some recovery of body weights. Body weights and body-weight gains of animals in other treated groups were affected to a minimal degree.
The food consumption ratio (g of food/kg bw per day) for males and females at 12 000 mg/kg was 20-28% lower than that for the controls and other treated groups during the first week of the study only, indicating a palatability problem. During weeks 2-13 of treatment, food consumption by the control group and all treated groups was similar. During the first week of the 4-week period of recovery (for rats at 0 and 12 000 mg/kg only), male and female rats at 12 000 mg/kg consumed 36-37% more food than did the controls. For the entire 4-week period following treatment, treated rats consumed an average of 25-29% more food than the controls.
Water consumption (g of water/animal per week) for males and females at 12 000 mg/kg was lower than that for the controls and other treated groups during the study.
All differences in haematology parameters that were observed between the control and treatment groups remained within the expected biological ranges for this strain of rat. Differences that carried statistical significance and may have been treatment-related included increases in haemoglobin concentration, erythrocyte volume fraction, and leukocyte count. At 12 000 mg/kg, male rats had lower haemoglobin and erythrocyte volume fraction values than the controls, and male and female rats had leukocyte counts that were 19% and 56% higher, respectively, than those of the controls. At 300, 2000, or 12 000 mg/kg, male rats also had prolonged prothrombin times in comparison with those of controls. In contrast, while females at 12 000 mg/kg had prolonged prothrombin times compared with those of the controls, females at 300 mg/kg or 2000 mg/kg had reduced prothrombin times compared with the controls. The prolongation in prothrombin time seen at 300 mg/kg and 2000 mg/kg was small compared with that in concurrent controls and no clear trend was seen in either sex. Therefore, no toxicological significance is attributed to the variations in prothrombin time at 300 mg/kg and 2000 mg/kg. All haematological differences observed between the control group and and the group treated with cyprodinil at 12 000 mg/kg disappeared by the end of the 4-week period of recovery.
Cyprodinil had a significant (p < 0.01 or 0.05) effect on the clinical blood chemistry of male rats at 2000 or 12 000 mg/kg, and of female rats in all treated groups. Cholesterol concentrations were higher in females at 50 (18%) and 300 (20%) mg/kg, and in males and females at 2000 (37-48%) and 12 000 (85-105%) mg/kg. Phospholipid concentrations were higher in females at 50 (18%) and 300 (23%) mg/kg, and in males and females at 2000 (36%) and 12 000 (71-90%) mg/kg. The slightly higher cholesterol and phospholipid concentrations measured in females at 50 and 300 mg/kg were not considered to be toxicologically relevant due to the marginal degree of increase (1.2-fold), and because values were mainly within the range of values for the concurrent control group. In addition, the 2-year study of carcinogenicity in rats did not show a treatment-related increase in blood cholesterol and phospholipid concentrations at a dose of up to 2000 mg/kg (at week 105, terminal measurements). Protein and globulin concentrations were each up to 7% higher in males at 2000 and 12 000 mg/kg. In males at 12 000 mg/kg, albumin concentrations were 6% higher and glucose concentrations were 11% lower than those of controls.
All other differences in blood chemistry that were observed remained within the expected biological ranges for this strain of rat. Statistically significant treatment-related increases were observed in alkaline phosphatase activity in males at 2000 mg/kg and in males and female at 12 000 mg/kg, and in gamma-glutamyl transpeptidase activity in males and females at 12 000 mg/kg. Other parameters that were statistically different from controls but that did not appear to be related to treatment were aspartate aminotransferase activity (increased in males at 50 and 300 mg/kg, decreased in females at 12 000 mg/kg), alanine aminotransferase activity (increased in males at 300, 2000, and 12 000 mg/kg), blood urea (decreased in males at 2000 mg/kg and in females at 12 000 mg/kg), creatinine (increased in females at 300 and 2000 mg/kg), total bilirubin (increased in males at 12 000 mg/kg), triglycerides (decreased in males at 12 000 mg/kg and increased in females at 300 and 2000 mg/kg), calcium (increased in males at 2000 and 12 000 mg/kg, and in females at 50 and 12 000 mg/kg), and phosphate (increased in males at 12 000 mg/kg).
After 4 weeks of recovery, the blood chemistry parameters that had been affected by treatment at 12 000 mg/kg were similar to those of the control group, with the exception of alkaline phosphatase activity in males and cholesterol and phospholipid concentrations in females. Alkaline phosphatase activity in males at 12 000 mg/kg remained 24% greater than that in the control group after treatment. At 12 000 mg/kg, cholesterol concentrations in females decreased from 85% greater than the control group (after treatment) to 22% greater (after recovery), and phospholipid concentrations decreased from 71% to 15% greater during the same period.
Cyprodinil affected the relative weights of the liver and thyroid of rats at 2000 and 12 000 mg/kg, and the relative weights of the kidneys of rats at 12 000 mg/kg. Relative liver weights were 13-15% and 27-37% higher at 2000 and 12 000 mg/kg, respectively; the increases were statistically significant for males at 12 000 mg/kg and for females at 2000 and 12 000 mg/kg. The relative weights of the thyroid glands of male rats at 2000 and 12 000 mg/kg were 22% and 38% higher, respectively, than those of the controls. The relative weights of the thyroid glands of female rats at 12 000 mg/kg were 42% higher than those of the controls. The relative weights of the adrenals of males at 12 000 mg/kg were 22% heavier than those of controls (0.201 g versus 0.164 g); however, this difference was not statistically significant. The relative weights of the kidneys of male rats at 12 000 mg/kg were 14% higher than those of rats in the control group. Decreases in the absolute mean weight of the hearts, adrenals, and ovaries of female rats at 12 000 mg/kg, compared with that of the controls, were statistically significant, but of no apparent biological significance. No other differences were observed between rats in the treated and control groups.
After 4 weeks of recovery, the relative weights of the liver and thyroid glands of males at 12 000 mg/kg were 9% and 63% greater, respectively, than those of the control group. The relative weights of organs in females at 12 000 mg/kg and in the control groups were similar after recovery.
Tissue discoloration and all other abnormalities occurred randomly and sporadically in all groups. Histopathological changes were observed in the liver, pituitary gland, thyroid, adrenal cortex, and kidneys of treated males, and in the liver, pituitary gland, and kidneys of treated females (Table 10). Liver abnormalities, including hepatocyte hypertrophy, hepatocyte necrosis, and the presence of "sharply demarcated membraneous structures containing a vacuolated eosinophilic material" ("cytoplasmic inclusion bodies"), were observed in both sexes at 2000 and 12 000 mg/kg. "Minimal" to "moderate" hypertrophy of hepatocytes was noted in 4/10 males at 300 mg/kg, 9/10 males and 6/10 females at 2000 mg/kg, and 10/10 males and 8/10 females at 12 000 mg/kg. Monocellular foci of necrotic hepatocytes were observed in 2/10 males at 300 mg/kg, 10/10 males and 4/10 females at 2000 mg/kg, and in 8/10 males and 7/10 females at 12 000 mg/kg. The study author stated that, "1/10 male animals in both groups 4 and 5 (2000 mg/kg and 12 000 mg/kg) showed a recent necrosis of a distinct group of hepatocytes." Cytoplasmic inclusion bodies, usually present in groups and located primarily in the hypertrophic cells attached to the periportal tract of the liver lobules, were noted in 3/10 males and 3/10 females at 2 000 mg/kg, and 9/10 males and 1/10 females at 12 000 mg/kg.
Table 10. Incidence of pathological findings in a 90-day study of toxicity in rats given diets containing cyprodinil
|
Dietary concentration (mg/kg) |
||||||||||
|
0 |
50 |
300 |
2 000 |
12 000 |
||||||
|
Male |
Female |
Male |
Female |
Male |
Female |
male |
Female |
Male |
Female |
|
|
Liver hepatocytes |
||||||||||
|
Hypertrophy |
||||||||||
|
S1 |
1/10 |
0 |
1/10 |
0 |
4/10 |
0 |
9/10 |
6 |
10/10 |
8/10 |
|
S2 |
0 |
0 |
— |
— |
— |
— |
— |
— |
2/10 |
0/10 |
|
Necrosis |
||||||||||
|
S1 |
0 |
10/10 |
10/10 |
0 |
2/10 |
0 |
1/10 |
4 |
8/10 |
7/10 |
|
S2 |
1/10 |
0 |
— |
— |
— |
— |
— |
— |
1/10 |
0 |
|
Inclusion bodies |
||||||||||
|
S1 |
0 |
1/10 |
0 |
0 |
0 |
0 |
3/10 |
3/10 |
9/10 |
1/10 |
|
S2 |
0 |
0 |
— |
— |
— |
— |
— |
— |
2/10 |
0 |
|
Kidney |
||||||||||
|
Chronic tubular lesion |
||||||||||
|
S1 |
0 |
2/10 |
0 |
2/10 |
3/10 |
0 |
5/10 |
5/10 |
8/10 |
8/10 |
|
S2 |
2/10 |
0 |
— |
— |
— |
— |
— |
— |
8/10 |
6/10 |
|
Nephrocalcinosis |
||||||||||
|
S1 |
0 |
10/10 |
0 |
9/10 |
10/10 |
9/10 |
0 |
10/10 |
5/10 |
10/10 |
|
S2 |
0 |
7/10 |
— |
— |
— |
— |
— |
— |
1/10 |
7/10 |
|
Thyroid follicular epithelium |
||||||||||
|
Hypertrophy |
||||||||||
|
S1 |
0 |
0 |
1/10 |
0 |
4/10 |
0 |
109/10 |
1/10 |
9/10 |
6/10 |
|
S2 |
1/10 |
0 |
— |
— |
— |
— |
— |
— |
0 |
0 |
|
Pituitary cell |
||||||||||
|
Hypertrophy |
||||||||||
|
S1 |
0 |
0 |
0 |
0 |
4/10 |
0 |
108/10 |
2/10 |
8/10 |
4/10 |
|
S2 |
0 |
0 |
— |
— |
— |
— |
— |
— |
3/10 |
0 |
S1 = rats sacrificed at week 14; S2 = rats sacrificed at week 18 (after 4-week period of recovery)—only rats at 0 and 12 000 mg/kg
Enlarged (hypertrophic) pituitary cells in the adenohypophysis were observed in 4/10 males at 300 mg/kg, 10/10 males and 2/10 females at 2000 mg/kg, and 8/10 males and 4/10 females at 12 000 mg/kg. This abnormality was observed in both single and small clusters of cells.
"Minimal" to "moderate" hypertrophy of the follicular epithelia of the thyroid was noted in 4/10 males at 300 mg/kg, 10/10 at 2000 mg/kg, and 9/10 at 12 000 mg/kg. No thyroid abnormalities were observed in female rats in any treated group.
"Minimal" increases in the number of fat vesicles in the adrenal cortex were noted in 6 out of 10 males at 12 000 mg/kg.
Kidney abnormalities were observed in male and female rats at 300, 2000, and 12 000 mg/kg. Nephrocalcinosis was noted in 1/10 males at 300 mg/kg and 5/10 males at 12 000 mg/kg, but not at 2000 mg/kg; in contrast, nephrocalcinosis was noted in the majority (90-100%) of females in the control group and in all treated groups. Vacuolization of the epithelium of the proximal convoluted tubules was observed in 1/10, 1/10, and 2/10 male rats at 300, 2000, and 12 000 mg/kg, respectively. Chronic tubular lesions were increased in male rats at 300, 2000, and 12 000 mg/kg (0, 0, 30, 50, 80%, in controls, 50, 300, or 2000 and 12 000 mg/kg, respectively) and in females only at 2000 and 12 000 mg/kg (20%, 20%, 0%, 50% and 80% in control, 50, 300, 2000 and 12 000 mg/kg, respectively).
All other abnormalities occurred randomly and sporadically in all groups after treatment.
With the exception of chronic tubular lesions in the kidneys, the prevalence of abnormalities was reduced after a 4-week period of recovery in rats that had received cyprodinil at a concentration of 12 000 mg/kg in the diet. After the period of recovery in males at 12 000 mg/kg, the following observations were made: hepatocyte hypertrophy, 2/10 animals; hepatocyte necrosis, 1/10 animals; cytoplasmic inclusion bodies in liver cells, 2/10 animals; hypertrophy of pituitary cells, 3/10 animals; and chronic tubular lesions in the kidneys, 8/10 animals. No abnormalities were observed in the thyroid, while the adrenal cortex was not examined. After recovery, females receiving cyprodinil at 12 000 mg/kg were examined only for chronic tubular lesions in the kidneys; these abnormalities were observed in 6/10 animals.
No neoplastic changes were observed in rats in treated or control groups. At 50 mg/kg, changes were not considered to be toxicologically significant and a LOAEL was not identified. At 300 mg/kg, increased incidences of hepatocyte hypertrophy, necrosis, cytoplamic inclusion bodies, chronic tubular lesions in the kidneys, nephrocalcinosis, cytoplamic vacuolization in proximal tubules, thyroid epithelium hypertrophy and pituitary hypertrophy were seen in males. A slightly increased incidence of minimal hypertrophy of liver hepatocytes, pituitary cells, and thyroid follicular epithelium was considered to be treatment related. However, these effects were not seen in the same strain of rats in the 90-day study of neurotoxicity at a concentration of up to 800 mg/kg, nor in the 2-year study of carcinogenicity at a concentration of up to 1000 mg/kg. Therefore, the LOAEL was set at 2000 mg/kg (equal to 134 and 137 mg/kg bw per day in males and females respectively) for rats, on the basis of effects on clinical chemistry, increased relative liver weight (females), increased absolute and relative thyroid weight (males), and histopathological findings in liver, kidneys, thyroid and pituitary. The NOAEL was 300 mg/kg (equal to 19 and 19.4 mg/kg bw per day in males and females respectively). The NOEL was 50 mg/kg, equal to a mean daily intake of 3.14 mg/kg bw per day (Fankhauser, 1991b).
Dogs
In a 90-day study of toxicity, groups of four male and four female beagle dogs were fed diets containing cyprodinil (purity, 99.5%) at a concentration of 0, 200, 1 500, 7000 or 20 000 mg/kg (equal to 0, 6.07, 45.87, 210.33 or 559.66 mg/kg bw per day for males and 0, 6.79, 52.75, 231.93 or 580.95 mg/kg bw per day for females). Animals were examined at least once daily for signs of morbidity and mortality, body weights were recorded weekly, and food consumption was recorded daily and reported as a weekly mean. Eye examination (external inspection; lens, iris, and fundus examination; pupillary reflex; third eyelid inspection) was performed before the start of treatment and at termination (week 13). Haematology, blood chemistry, and urine analysis were carried out on all animals before the start of treatment, at week 7, and at week 13. This study complied with GLP.
There were no effects on mortality, ophthalmology, or clinical chemistry parameters. Treatment-related effects included vomiting in all females receiving the highest dose during the first 3 days of treatment. No other relevant clinical symptoms were noted.
Mean body-weight gains were consistently lower in males and females receiving the highest dose relative to those of controls throughout the study period. All animals receiving the highest dose exhibited weight loss during the first 2-3 weeks of the study. At the highest dose, both sexes showed clearly depressed body-weight gains, with values being 36% (males) and 24% (females) those of control animals. A slight weight reduction was also noted in females at 7000 mg/kg after the first week of treatment; however, no differences relative to controls were noted in this group or in groups at lower doses throughout the remainder of the study.
Mean food consumption was consistently lower in animals at the highest dose, relative to controls, throughout the period of treatment; this was considered to be treatment-related. The most prominent reduction in food consumption occurred after the first week of treatment. The only difference in haematological parameters measured at weeks 7 and 13 was an increase in the mean platelet count of females at the highest dose relative to that of the controls. In the absence of any associated histopathological findings, this was not considered to be toxicologically significant.
Examination of data on mean and individual organ weights gave no clear indication of any treatment-related effect. Slight increases in relative spleen, liver, kidney, and adrenal weights were observed, but in the absence of any histopathological observations or a clear dose-response relationship, these findings were not considered to be toxicologically significant. Histopathological examinations did not reveal any treatment-related changes.
The NOAEL was 7000 mg/kg (210 mg/kg bw per day in males, 232 mg/kg bw per day in females) on the basis of lower body-weight gains and decreased food consumption in males and females at the highest dose. Lower body-weight gain and reduced food consumption in females at 7000 mg/kg was observed after the first week of treatment only, after which an adjustment was made to the palatibility of the food and these effects were, therefore, not considered to be adverse. The LOAEL was 20 000 mg/kg (560 and 581 mg/kg bw per day in males and females respectively). The NOEL was 1 500 mg/kg, equal to 45.9 mg/kg bw per day for males and 52.8 mg/kg bw per day for females (Altmann, 1991).
In a one-year study of toxicity, groups of four male and four female beagle dogs were fed diets containing cyprodinil (purity, 99.6%) at a concentration of 0, 25, 250, 2 500 or 15 000 mg/kg (equal to 0, 0.72, 6.87, 65.63 or 449.25 for males and 0, 0.76, 6.80, 67.99 or 446.37 mg/kg bw per day for females). Animals were examined at least once daily for signs of morbidity and mortality, body weights were recorded weekly, and food consumption was recorded daily and reported as a weekly mean. Eye examination (external inspection; lens, iris, and fundus examination; pupillary reflex; third eyelid inspection) was performed before the start of treatment and at termination (week 52). Haematology, blood chemistry, and urine analysis were carried out on all animals before the start of treatment, and at weeks 13, 26 and 52. All animals survived to study termination and were subjected to detailed necropsy. Organ weights were recorded and full histological examinations were performed. This study complied with GLP.
There were no treatment-related effects on mortality, clinical signs, ophthalmology, urine analysis, haematology or clinical chemistry parameters. Any findings that were statistically significant were sporadic.
Mean body-weight gain was reduced in both sexes at 15 000 mg/kg (69% and 62% of that of the controls, for males and females, respectively). Terminal body weights were approximately 10% lower in the animals at the highest dose than in the controls.
During the first half of the treatment period, mean food consumption was lower in males and females at the highest dose, relative to controls; this was considered to be treatment-related. Mean ratios of food consumption to body-weight gain were up to about 1.2-fold higher than control ratios in animals at the highest dose during the latter half of the study, indicating reduced food conversion efficiency. This was reflected in the lower body-weight gains in this group.
There was a dose-related increase in the mean absolute and relative spleen weights in males receiving cyprodinil at concentrations of 25-15 000 mg/kg in the diet, compared with controls. On histopathological examination, acute congestion of a moderate degree was noted in these animals. The study author cited insufficient exsanguinations as a probable cause of this finding, since there were no haematological or histopathological changes indicative of peripheral or central cardiovascular disorders. There was an increase in mean relative liver weights in males at 2 500 and 15 000 mg/kg and in females at 250-15 000 mg/kg, although there was no dose-response relationship. There was also an increase in mean absolute and relative testes weights in all treated groups relative to the controls, (this was statistically significant for relative testicular weights in males at the highest dose), and absolute and relative thymus weights were significantly increased in all groups of treated females, although there was no dose-response relationship. In the absence of histopathological findings in any tissues, these observations were not considered to be toxicologically relevant.
One female at the lowest dose and three females at the highest dose had mottled lungs, which was reported to be of similar incidence as that observed in the colony. These changes correlated with foreign body granuloma in the animal at the lowest dose, and interstitial pneumonia in two animals at the highest dose. Therefore, these changes were not considered to be treatment-related.
Slight hepatocyte pigmentation, which was reported to be presumably of lipofuscin origin, was noted in three out of four males at the highest dose. Acute congestion of the spleen was noted in one male from each group at 250 and 2 500 mg/kg, in two males at the highest dose, and in two and one females at 2 500 and 15 000 mg/kg, respectively, which, as noted above, was considered to result from insufficient exsanguinations. No other findings were considered treatment-related. The NOAEL was considered to be 2 500 mg/kg (66 mg/kg bw per day in males, 68 mg/kg bw per day in females) on the basis of decreased body-weight gains, decreased food consumption, and reduced food efficiency in males and females at the LOAEL of 15 000 mg/kg (449.25 mg/kg bw per day in males, 446.37 mg/kg bw per day in females). The NOEL was 2 500 mg/kg, equal to 66 mg/kg bw per day for males and 68 mg/kg bw per day for females (Altmann, 1992).
Mice
In an 18-month study of carcinogenicity, groups of 70 male and 70 female Tif MAGf (SPF) albino mice were fed diets containing cyprodinil (purity, 99.6%) at a concentration of 0, 10, 150, 2000 or 5000 mg/kg (equal to 0, 1.15, 16.1, 212.4 or 630 mg/kg bw per day for males and 0, 1.08, 14.7, 196.3 or 558.1 mg/kg bw per day for females). Animals were examined at least once daily for signs of morbidity and mortality. Body-weight and food consumption were recorded weekly for the first 3 months, then monthly thereafter. Blood was collected from the orbital sinus of animals that had been fasted overnight (10 of each sex per dose per collection), under ether anaesthesia, at weeks 53 and 78 to assess haema-tology parameters. All animals that died during the study and those that were sacrificed moribund and at study termination were subjected to a detailed necropsy. A full histological examination was performed on all animals participating in the carcinogenicity part of the study, including those that died or were sacrificed before study termination. All organs from 10% of the animals of both sexes (selected at random), all target organs, all tumours, and all unusual lesions were re-evaluated by the reviewing pathologist. This study complied with GLP.
There was no effect on mortality, haematology parameters, and no clinical signs of toxicity. Survival was similar or greater in treated groups than in control groups, with males and females at the highest dose having the greatest survival rate of any of the groups (90-95%) at termination (Table 11). Mean body weights were significantly lower in males at the highest dose from week 8 until study termination, with an overall body-weight gain that was 10% lower than that in the control group. At the highest dose, there was a more progressive effect on body weight and body-weight gain in females than in males; body weights were significantly lower in this group compared with those in the control group from week 21 onward, with an overall body-weight gain that was 25% lower than that in the control group. This was considered to be treatment-related. Food consumption was difficult to assess in males, due to the high incidence of food spillage, particularly in the group receiving the highest dose. According to the study author, recalculating the group mean food consumption by excluding the values recorded when food wastage occurred resulted in similar values for treated and control groups. Although there appeared to be no differences in food consumption, progressively higher food consumption ratios (g of food/kg bw per day) were noted in males and females at the highest dose throughout the study period, which indicated reduced food conversion efficiency in these groups, and was considered to be treatment-related.
Table 11. Number of survivors at termination of an 18-month study of carcinogenicity in mice fed diets containing cyprodinil
|
Dietary concentration (mg/kg) |
Number of survivors |
|
|
Male |
Female |
|
|
0 (control) |
36/50 |
41/50 |
|
10 |
33/50 |
40/50 |
|
150 |
39/50 |
46/50 |
|
2000 |
39/50 |
43/50 |
|
5000 |
45/50* |
48/50 |
From Fankhauser (1994a, 1994b)
*p = 0.028 in comparison with control group
There was a non-significant increase (8.7%) in the absolute mean liver weight in males at the highest dose and a significant increase (approximately 16%) in the mean liver to body weight ratios in males and females at the highest dose at termination, relative to controls. Relative mean kidney weight was also greater in females at the highest dose, although absolute kidney weights did not reveal any dose-response relationship.
There was a dose-related increase in the incidence of enlarged livers in males at 2000 and 5000 mg/kg. However, there was only a slight increase in the incidence of hepatic hypertrophy in the males at the highest dose relative to the controls, with no other liver histopathology. Furthermore, there was no clear treatment-related effect on liver necrosis in males, and the incidence of liver necrosis in females at the highest dose was, in fact, below that of the controls. No other macroscopic findings were treatment-related.
There was a dose-related increase in the incidence of minimal to marked focal and multifocal hyperplasia in pancreatic acinar cells in males at 2000 and 5000 mg/kg, which was statistically significant at 5000 mg/kg. In male mice, hyperplasia in pancreatic acinar cells was observed in 4/50, 4/50, 5/50, 8/50 and 14/50 animals at 0, 10, 150, 2000 and 5000 mg/kg, respectively. In female mice, hyperplasia in pancreatic acinar cells was observed in 3/50, 6/50, 2/50, 4/50 and 6/50 animals at 0, 10, 150, 2000 and 5000 mg/kg, respectively. In male mice, hyperplasia in pancreatic acinar cells was statistically significant only at the highest dose. It should be noted that incidences in all treated groups were higher than values for historical controls (range, 0-1.7% for both 18- and 24-month studies; data from 3 and 10 studies, respectively) provided in the study report. Additional data on historical controls provided by the sponsor show a range of 0-3.7% for incidence of hyperplasia in pancreatic acinar cells. Therefore, this was considered to be a treatment-related effect only in males at 5000 mg/kg. Incidences of pancreatic hyperplasia in all groups of females were also noted as being above those for historical controls (0% in 3 18-month studies, 0-3.9% in 10 24-month studies), but in the absence of a dose-response relationship, this was not considered to be treatment-related. The increased incidence of Harderian gland inflammation, observed in males only, was attributed to post-traumatic changes related to blood sampling, on the basis of morphological appearance and unilateral occurrence, and was considered to be an incidental finding. At 5000 mg/kg, females exhibited a significantly increased incidence of cystic kidneys and splenic haemosiderosis, and a nonsignificant increase in lymphohistiocytic infiltration of the kidney and splenic extramedullary haematopoiesis. The incidence and severity of haemosiderosis (Dunn, 1954; Hirouchi et al., 1994) and splenic extramedullary haematopoiesis generally increases with age, with a greater incidence of enhanced eythropoiesis in females than in males of certain mouse strains (Frith & Wiley, 1981). The study author suggested that there was a relationship between the increased incidence of haemosiderin pigment accumulation and the increased longevity of the animals at the highest dose, and further indicated that the lack of any indication of increased erythrocyte turnover supported this as an incidental finding, which was considered to be a valid conclusion. It was also indicated that the other changes noted were common occurrences in the colony, although data on historical controls were not provided to validate this statement.
There was no indication of carcinogenic potential at any dose. The NOAEL was 2000 mg/kg (212 and 196 mg/kg bw per day in males and females, respectively). The LOAEL was 5000 mg/kg (630 and 558 mg/kg bw per day in males and females, respectively) on the basis of the increase in the incidence of focal and multifocal hyperplasia of the exocrine pancreas in males, reduced body weights in males and females, increased relative kidney weights in females, and increased in relative liver weights in males and females. The NOEL was 2000 mg/kg (equal to 212 mg/kg bw per day for males and 196 mg/kg bw per day for females) (Fankhauser, 1994a, 1994b).
Rats
In a 24-month study of toxicity and carcinogenicity, groups of 50 male and 50 female Tif RAIf rats (plus groups of 20 males and 20 females that were used for laboratory investigations) were fed diets containing cyprodinil (active ingredient, 99.2-99.6%) at a concentration of 0, 5, 75, 1000 or 2000 mg/kg (equal to 0, 0.177, 2.7, 35.6 or 73.6 mg/kg bw per day for males, respectively, and 0, 0.204, 3.22, 41.2 or 87.1 mg/kg bw per day for females, respectively) for 24 months. Additional groups of 10 males and 10 females received the test diets for 12 months (interim sacrifice). Animals were examined at least once daily for signs of morbidity and mortality. Body weights and food consumption were recorded weekly for the first 3 months, then monthly thereafter. Water consumption was recorded monthly for the first 6 months; measurement was suspended thereafter, since no differences were found. Ophthalmological examinations were performed on all animals in the control groups and at the highest dose (the group being monitored for carcinogenicity) before the start of treatment and at 6, 12, 18, and 24 months. In addition, the eyes of all females were examined at termination. Haematological and clinical chemistry parameters were evaluated at weeks 13, 27, 53, 78, and 105. Urine analysis was performed at weeks 13, 27, 53, 78 and 105. All animals that died during the study and those that were sacrificed moribund and at study termination were subjected to gross necropsy. A full histological examination was performed on all animals in the group monitored for carcinogenicity and the group that was sacrificed in the interim only, including (when possible) those that died or were sacrificed before study termination. This study complied with GLP.
Survival was similar in treated and control groups, with approximately 50% of males and approximately 60% of females surviving in all groups, except in the group of males receiving the highest dose, which had the lowest survival rate of 46%. None of the clinical signs observed appeared to be treatment-related. Treated females in all groups showed an increase in frequency of cloudy or opaque eyes relative to controls, however, there was no dose-response relationship, no increase observed in males, and the observations were unilateral in 50% of affected females. Thus, this finding was considered to be incidental. There were no differences in body weight or body-weight gain between control and treated animals at any point during the 24-month study. There were no differences in food consumption or food conversion efficiency (g of food/kgbw per day) between control and treated groups over the entire study period. Urine analysis did not detect any treatment-related findings.
The most notable change in haematological parameters was a significant prolongation in prothrombin time in males at 2000 mg/kg at week 27 and in males at 1000 and 2000 mg/kg at week 53, although these values were within the upper physiological range provided in the company report. No differences were noted between control and treated groups at later time-points, and therefore the change in prothrombin time was of no toxicological concern. Any other changes in haematological parameters were sporadic, lacked a dose-response relationship, and were noted at earlier time-points but not at termination, and therefore were not of concern.
At termination, plasma urea concentrations (4.67, 5.88, 5.60, 7.88, 8.70 nmol/l at 0, 5, 75, 1000, and 2000 mg/kg, respectively) were greater than the upper physiological limit (given as 6.94 nmol/l in the study report) in males at 1000 and 2000 mg/kg, although these values were not statistically significant, relative to controls. Cholesterol and phospholipid concentrations were significantly greater than controls in all groups of treated females at week 27 (with the exception of phospholipid concentrations in females at the lowest dose), although these were still within the physiological range. However, there were no significant or dose-related differences in these values at any other time over the 24-month study. Therefore, changes in these parameters were unlikely to be of toxicological significance. Any other changes in clinical chemistry parameters, in either males or females, were sporadic with no dose-response relationship, or were transient, and fell within the normal physiological range provided in the study report.
The only apparent treatment-related effect on organ weight was a significant increase (11%) in absolute and relative liver weights in males at the highest dose, compared with controls, at termination. Increased relative mean kidney weights males and females at the highest dose at the interim sacrifice and lower mean exsanguinated body weight in females at the highest dose were not observed in animals at termination. At the highest dose, absolute and relative kidney and adrenal weights were increased relative to controls in males, and the mean ovarian weight was increased in females at study termination; however, these differences were attributed to grossly enlarged organs in one or two animals for each tissue type, and were not considered to be treatment-related.
At gross necropsy, females at the highest dose exhibited an increased incidence of mottled lungs, which correlated with an accumulation of foam cells in lung alveoli, and an increase in ovarian cysts (all at terminal sacrifice), which was often noted in conjunction with ovarian atrophy (Table 12). These lesions are common occurrences in ageing animals and were not considered to be toxicologically significant. The incidence of ovarian atrophy in concurrent controls was 45%.
Table 12. Selected observations at gross necropsy of female rats given diets containing cyprodinil in a 24-month study of toxicity/carcinogenicity
|
Lesion |
Dietary concentration (mg/kg) |
||||
|
0 |
5 |
75 |
1000 |
2000 |
|
|
Mottled lungs |
6/80 (8.0%) |
7/80 (9%) |
8/80 (10%) |
6/80 (8%) |
16/79 (20%) |
|
Cystic ovaries |
2/80 (3%) |
1/80 (1%) |
2/80 (3%) |
0/80 (0%) |
9/80(11%) |
From Fankhauser (1994c, 1994d)
Selected non-neoplastic findings on microscopic histopathological examination are presented in Table 13. There was a dose-related increase in the incidence of spongiosis hepatis (sinusoidal cystic dilation) in the liver of males at 1000 and 2000 mg/kg, which was considered to be toxicologically significant. This is a degenerative, multilobular, cystic liver change that affects primarily perisinusoidal cells of the liver. Excessive amounts of acid mucopolysaccarides and/or proteinaceous material are produced, leading to the development of large cavities characteristic of this lesion (Banasch et al., 1985, Eustis et al., 1990).
Table 13. Incidence of selected non-neoplastic lesions in a 24-month study of toxicity/carcinogenicity in rats given diets containing cyprodinil
|
Lesion |
Sex |
Dietary concentration (mg/kg) |
||||
|
0 |
5 |
75 |
1000 |
2000 |
||
|
Spongiosis hepatitis |
Male |
3/60 |
3/60 |
2/60 |
10/60*## |
14/60**## |
|
(5%) |
(5%) |
(3%) |
(17%) |
(23%) |
||
|
Liver necrosis [interim sacrifice; main group] |
Male |
9/60 (15%) |
9/60 (15%) |
6/60 (10%) |
9/60 (15%) |
16/60 (27%) |
|
[2/10; 7/50] |
[5/10; 4/50] |
[1/10; 5/50] |
[2/10; 7/50] |
[5/10; 11/50#] |
||
|
Progressive nephropathy |
Male |
39/60 (65%) |
40/60 (67%) |
37/60 (62%) |
39/60 (65%) |
49/60*## (82%) |
|
Female |
19/60 (32%) |
22/60 (37%) |
21/60 (35%) |
16/60 (27%) |
24/60 (40%) |
|
|
Foam cells—lung |
Female |
18/60 (30%) |
15/60 (25%) |
16/60 (27%) |
26/60# (43%) |
26/60## (43%) |
From Fankhauser (1994c, 1994d)
*p < 0.05; **p < 0.01 (pairwise comparison); #p < 0.05, ##p < 0.01 (positive trend)
An increased incidence of liver necrosis was noted in males at the highest dose, relative to the controls. However, this was attributed to a high incidence of recent necrosis, a peri-mortal artifact that was noted at the highest dose in 10 out of 11 males that were found dead. Therefore, this finding was not directly related to treatment. Progressive nephropathy was observed at a higher incidence in males at the highest dose, compared with the controls. However, the range for incidence of progressive nephropathy in historical controls of this strain of male rats was 25-88%, which excluded this lesion from being considered as a treatment-related finding. Although there was a higher incidence of lung alveolar foam cells in females at 1000 and 2000 mg/kg, relative to controls, this fell within the range of 5-54% for historical controls, and was not considered to be toxicologically significant.
Table 14 lists the incidence of selected neoplastic findings. Although the occurrence of hepatocellular carcinoma in males at the highest dose showed a significant positive trend (p < 0.05), the low incidence (2 out of 60 versus 0 out of 60 in controls) did not support this finding as a treatment-related effect (Table 17). There was an increased incidence of mammary gland fibroadenomas in females at the highest dose; the data indicated that the frequency fell within the range of 11-57% for historical controls (overall mean, 41%, from studies initiated in 1984-1990 and 40% for studies initiated in 1988-1990; in 5 of these 10 studies, the incidence of mammary gland fibroadenomas was > 44% or above). In studies initiated within 3 years of the present study (1988-1990), incidences of 44-57% were observed in 4 out of 10 studies. It should be noted that incidences in the concurrent control group and in the three groups receiving cyprodinil at 5, 75 or 1000 mg/kg, were lower than those for historical controls in 9 out of 10 studies. In view of the data on historical controls, and the lack of any such findings in the study of carcinogenicity in mice, the mammary fibroadenomas were not considered to be treatment-related.
Table 14. Selected neoplastic findings in rats given diets containing cyprodinil in a 24-month study of toxicity/carcinogenicity
|
Organ/tumour |
Sex |
Dietary concentration (mg/kg) |
Historical controls |
||||
|
0 |
5 |
75 |
1000 |
2000 |
|||
|
Liver |
Male |
||||||
|
Adenomas |
1/60 |
3/60 |
3/60 |
1/60 |
2/60 |
— |
|
|
Carcinomas |
0 |
0 |
1/60 |
1/60 |
2/60# |
— |
|
|
Mammary gland |
Female |
||||||
|
Fibroadenomaa |
15/50 |
18/50 |
15/50 |
18/50 |
27/50**## |
11-57%b |
|
From Fankhauser (1994c, 1994d)
a Number of animals examined does not include interim sacrifice group
b Ten studies, conducted over 1984-1990
*p < 0.05, **p < 0.01 (pairwise comparison); #p < 0.05, ##p < 0.01 (trend)
The NOAEL for toxicity was 75 mg/kg (2.7 mg/kg bw per day) on the basis of a dose-related increase in the incidence of spongiosis hepatitis in the liver of males at 1000 and 2000 mg/kg. There was no evidence of significant chronic toxic effects in females. The NOEL was 75 mg/kg, equal to 2.7 mg/kg bw per day for males and 3.22 mg/kg bw per day for females (Fankhauser, 1994c, 1994d).
Cyprodinil was tested in various assays for for genotoxicity in vivo and in vitro (Table 15). These studies complied with GLP. There was no evidence for gene mutations in assays for bacterial reverse mutation in vitro (in S. typhimurium and E. coli), or for gene mutation in Chinese hamster lung V79 cells or Chinese hamster ovary cells, either in the presence or absence of metabolic activation. Cyprodinil gave negative results in a test for mammalian cell DNA repair in vitro and in a test for micronucleus formation in mouse cells in vivo.
Table 15. Results of studies of genotoxicity with cyprodinil
|
End-point |
Test object |
Concentration/dose |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
S. typhimurium |
|||||
|
Reverse mutation |
TA98, TA100, TA1535 and TA1537; E. coli WP2 urvA |
20.0-5000 µg/plate in DMSO, ± S9 |
99.5 |
Negative |
Ogorek (1990) |
|
Gene mutation |
V79 Chinese hamster lung fibroblast cells; Hgprt locus |
6-150 µg/ml in DMSO, +S9 |
99.5 |
Negative |
Geleick (1990) |
|
Chromosomal aberrations |
Chinese hamster ovary cells |
6.25-50 µg/ml in DMSO, +S |
99.5 |
Negative |
Strasser(1991) |
|
DNA repair |
Rat hepatocytes |
0.74-80 µg/ml in DMSO |
99.5 |
Negative |
Geleick (1991) |
|
In vivo |
|||||
|
Micronucleus formation |
Mouse bone-marrow cells |
1250-5000 mg/kg bw, single dose by gavage |
99.5 |
Negative |
Ceresa (1990) |
S9, 9000 × g supernatant fraction of Aroclor 1254-induced rat liver microsomes; DMSO, dimethylsulfoxide
Rats
A two-generation study of reproduction was carried out in Tif RAIf rats (one litter per generation). Groups of 30 males and 30 females received diets containing cyprodinil (purity, 99.5%) at a concentration of 0, 10, 100, 1000 or 4000 mg/kg (equal to 0, 0.7, 7.4, 74.4 or 294.7 mg/kg bw per day for males, respectively, and 0, 0.8, 8.2, 81.3 or 326.4 mg/kg bw per day for females, respectively; dietary concentrations in mg/kg bw per day are based on food consumption during the pre-mating period for F0 animals) for two generations. Animals were fed test diets during the 10-week pre-mating period in both generations, then randomly allocated to mating pairs (1:1) for a maximum of 19 days. Upon detection of vaginal sperm or a copulating plug, which was considered to be day 0 of gestation, females were moved to individual cages and males were returned to their original cages. Precautions were taken to avoid matings between siblings. F1 litters were culled to four males and four females, if possible, on postnatal day 4. Animals were observed daily for clinical signs and mortality. Body weight and food consumption were recorded weekly. Necropsy was performed at termination. Selected organs were removed, weighed and histopathological examination was performed. This study complied with GLP.
No treatment-related clinical signs or mortalities were observed. Signs of toxicity at 4000 mg/kg consisted of a statistically significant decrease in body-weight gain (12.5%) in the F0 females after the pre-mating period. Mean food consumption was decreased in F0 females during the initial pre-mating phase. There were no treatment related effects on the reproductive parameters evaluated. The increased liver weight in males and females at 1000 (marginal) and 4000 mg/kg was not supported by histopathological findings; although these changes were treatment-related, they were not considered to be adverse. Mean absolute and relative kidney weights were significantly greater in F0 males at 100-4000 mg/kg than those in controls, as were relative kidney weights in F0 females at 1000 and 4000 mg/kg and in F1 males at 4000 mg/kg. There were no histopathological findings in the kidney. However, subsequent histopathological examination revealed a marginal increase in the incidence and severity of renal tubular basophilia in Fo males at the highest dose. Treatment with cyprodinil had no effect on proliferative activity in basophilic tubules and in unaffected cortical and medullary renal tubules according to assays for proliferating cell nuclear antigen (PCNA). Effects in kidneys were considered to be treatment-related; however, in the absence of significant histopathological findings (marginal renal tubule basophilia at the highest dose) or effect on proliferative activity, the increased organ weights were not considered to be toxicologically significant. At necropsy, liver enlargement was noted in males at the highest dose; other findings were considered to be incidental. The LOAEL for parental systemic toxicity was 4000 mg/kg (295 mg/kg bw per day) on the basis of decreased body-weight gain in the F0 females during the premating period. The NOAEL for parental systemic toxicity was 1000 mg/kg (74 mg/kg bw per day).
Signs of offspring toxicity included significantly lower weights for F1 and F2 pups at the highest dose during lactation; these continued to be lower than those of controls after weaning and after the pre-mating period (examined in F1 generation only). The LOAEL for offspring toxicity was 4000 mg/kg (295 mg/kg bw per day) on the basis of decreased pup body weights (F1 and F2 generations) during lactation and continuing into adulthood for F1 rats. The NOAEL for offspring toxicity was 1000 mg/kg (74 mg/kg bw per day). The NOEL was 100 mg/kg, equal to 7.4 mg/kg bw per day for males and 8.2 mg/kg bw per day for females (Khalil, 1993).
Rats
In a study of developmental toxicity, groups of 20-23 Tif RAIf (SPF) female albino rats were given cyprodinil (purity, 99.5%) at a dose of 0, 20, 200 or 1000 mg/kg bw per day in aqueous corn starch suspension (3%) by oral gavage on days 6-15 of gestation. Animals were observed daily for signs of morbidity, mortality, and abortion. Body weights were recorded daily. Food consumption was determined on days 0-6, 6-11, 11-16 and 16-21 of gestation and recorded as daily means. Females were sacrificed on day 21 of gestation and subjected to gross examination. Fetuses were delivered by caesarian section, weighed, sexed, and examined for possible external, visceral and skeletal anomalies. This study complied with GLP.
One female in the group receiving the highest dose showed reduced locomotor activity on day 16 of gestation, which was considered to be treatment-related. No other clinical signs of toxicity were observed.
Significantly lower mean body weights were observed in females at the highest dose, beginning 1 day after initiation of treatment (day 7 of gestation) and continuing daily throughout the remainder of the study. Mean body-weight gains were significantly lower in females at the highest dose than in controls throughout the period of treatment.
Food consumption was significantly lower (58-68% of that in controls) in females at the highest dose throughout the entire dosing period; this correlated with the decrease in body-weight gain. Food consumption was comparable in control and females at the highest dose from days 16-21 of gestation. Food consumption was significantly lower (88% of that of the control group) in the group receiving a dose of 200 mg/kg bw during the first half of the period of treatment (days 6-11 of gestation); however, this did not appear to influence body weight in this group.
There were no other treatment-related observations. There were no differences in rates of pregnancy, mean numbers of corpora lutea, or implantation sites between groups. The LOAEL for maternal toxicity was 1000 mg/kg bw per day on the basis of lower body weight, body-weight gain and reduced food consumption. The NOAEL for maternal toxicity was 200 mg/kg bw per day.
There were non-significant increases in the number of early resorptions at the two higher doses compared with the controls (Table 16). The data on means for historical controls for early resorptions was reported to range from 2.9% to 10.5%. Although there appeared to be a dose-response relationship for early resorptions, the mean litter size was only slightly lower at the highest dose relative to the controls. Thus, a clear treatment-related effect could not be established.
Table 16. Summary of litter data in rats given cyprodinil by gavage
|
Parameter |
Dose (mg/kg bw per day) |
|||
|
0 |
20 |
200 |
1000 |
|
|
No. of live litters |
23 |
20 |
22 |
20 |
|
No. of live fetuses, male/female |
168/164 |
129/153 |
167/142 |
167/142 |
|
No. of resorptions |
13 |
13 |
20 |
23 |
|
Early resorptions (%) |
10 (2.8) |
13 (4.4) |
20 (6.1) |
22 (7.4) |
|
Late resorptions (%) |
3 (0.8) |
0 |
0 |
1 (0.3) |
|
Mean no. of resorptions per litter |
0.6 ±0.7 |
0.6 ±0.9 |
0.9 ± 1.4 |
1.1 ± 1.3 |
|
Mean no. of pups per litter |
14.4 ± 4.2 |
14.1 ±2.6 |
14.0 ± 3.5 |
13.7 ±2.8 |
|
Mean % of male pups |
50.6 |
45.7 |
54.0 |
51.6 |
|
Mean fetal weight |
5.3 ±0.3 |
5.3 ±0.3 |
5.5 ±0.4 |
4.7 ±0.5* |
From Marty (1991a)
*p < 0.01
One fetus (out of a total of 273 fetuses) in the group receiving the highest dose had omphalocele, which was not considered to be treatment-related. There were no visceral abnormalities. There were significantly lower mean fetal weights (11%) at the highest dose compared with controls, as well as a significant increase in skeletal anomalies in this group relative to the controls, due to abnormal ossification (4 out of 20 litters in the group receiving the highest dose compared with 0 in any other group), primarily as absent or reduced ossification of metacarpal 5. These skeletal anomalies/variations were considered to represent a transient developmental delay that occurs secondary to the maternal toxicity observed at the highest dose. The LOAEL for developmental toxicity was 1000 mg/kg bw per day on the basis of lower mean fetal weights and an increased incidence of delayed ossification. The NOAEL for developmental toxicity was 200 mg/kg bw per day. There was no evidence of teratogenicity in this study at a dose of up to 1000 mg/kg bw per day, given during the period of major organogenesis. The NOEL was 200 mg/kg bw per day (Marty, 1991a).
Rabbits
In a study of developmental toxicity, 19 inseminated female rabbits (Russian Chbb:HM (SPF)) were given cyprodinil (purity, 99.5%) at a dose of 0, 5, 30, 150, or 400 mg/kg bw per day in aqueous corn starch suspension (3%) by gavage from days 7 to 19 of gestation. The animals were checked daily for mortality or clinical signs. Body-weight data were recorded daily. Food consumption was determined for days 0-4, 4-7, 7-12, 12-16, 16-20, 20-24, and 24-29 of gestation and recorded as daily means. Dams were sacrificed on day 29 of gestation. Macroscopic pathological examinations of the main organs were carried out at sacrifice. Fetuses were removed, weighed, sexed, and examined for external, visceral, and skeletal anomalies. This study complied with GLP.
All rabbits survived to termination on day 29 of gestation. No treatment-related clinical observations were reported. The mean maternal body weight for dams at 400 mg/kg bw per day was comparable to that for controls on day 7 of gestation, but decreased slightly (not statistically significant) after dosing began. Mean body-weight gain in dams at 400 mg/kg bw per day was -56 g between days 7 and 13 of gestation compared with -9g in the controls (statistically significant,p < 0.01), and was 58% less than that in controls (controls, 40 g; high dose, 17 g) between days 13 and 19 (not statistically significant). The mean body-weight gain in this group for the entire treatment period (days 7-19) was significantly depressed (p < 0.01). On days 19-29 after treatment, the mean body-weight gain in the dams at 400 mg/kg bw per day was 35% greater than in controls (not significant). Mean gravid uterine weights were comparable across all groups. Food consumption was 34% less than that in controls (not statistically significant) on days 7-12 after insemination. This decrease parallelled the decreased body-weight gain for the first week of treatment. No treatment-related gross pathological findings were observed. No effects were noted on pregnancy parameters.
The LOAEL for maternal toxicity was 400 mg/kg bw per day on the basis of decreased body-weight gain. The NOAEL for maternal toxicity was 150 mg/kg bw per day.
At 400 mg/kg bw per day, fetuses exhibited indications of an increased incidence (not statistically significant) of a thirteenth rib. Although there were indications of developmental delays in fetal skeletons, such as poor or absent ossification of the sternebrae and metacarpals at all doses, only the development of the extra rib is likely to be compound-related. Uni- or bilateral hyperflexion of the carpal joint at 400 and 150 mg/kg bw per day (not statistically significant) was not associated with the skeletal changes. At the highest dose tested, a slight increase in the number of litters in which pups were born with an extra (thirteenth) rib was observed in rabbits in the presence of maternal toxicity, an effect that was not considered to be toxicologically relevant. Therefore, the NOAEL for fetal development was 400 mg/kg bw per day, the highest dose tested. Under the study conditions used, cyprodinil was not teratogenic to rabbits at a dose of up to 400 mg/kg bw per day. The NOAEL for maternal toxicity was 150 mg/kg bw per day and the NOAEL for developmental toxicity was 400 mg/kg bw per day, the highest dose tested (Marty, 1991b).
Increased absolute and relative kidney weights were observed in F0 males given cyprodinil at a dietary concentration of 1000 and 4000 mg/kg in the two-generation study of reproduction in rats. In addition, a small increase in the incidence and severity of tubular basophilia was observed at 4000 mg/kg. In the study described below, formalin-fixed renal tissues from F0 males in the two-generation study of reproduction were investigated by immunohistochemical/morphological analysis of PCNA. PCNA-stained sections were also used for determining proliferative activity in basophilic tubules. This study complied with GLP.
Basophilic tubules, mainly found in the cortex and the outer stripe of outer medulla, were characterized by one or more of the following features: cytoplasmic basophilia, pale and sometimes crowded nuclei, thickening of the basement membrane, infiltration with inflammatory cells (lymphocytes) and associated tubular dilatation with eosinophilic contents. Basophilic tubules as observed in this study therefore correspond to those spontaneous, age-related lesions in the laboratory rat that have mainly been interpreted as degenerative/regenerative tubules or early forms of progressive nephropathy. All other histopathological findings in the kidney were devoid of any treatment-related effect and there was no indication of the occurrence of putative pre-neoplastic kidney lesions in any group.
No effect of treatment was observed on the numerical density of tubular cell nuclei (number of tubular cell nuclei per mm2) in cortical and medullary compartments. In addition, the relationship between numerical density of tubular cell nuclei in the cortex and in the medulla was unchanged.
Treatment with cyprodinil did not influence the number of tubular cell nuclei that stained postive for PCNA in unaffected cortex or medulla (outer stripe of outer medulla). In addition, no difference was noted in the numerical density of PCNA-positive nuclei in the cortex and in the medulla between treated and control animals. In all animals, the number of PCNA-positive nuclei was significantly higher in cortical than in medullary renal tubules.
Sections from those animals with basophilic tubules (identified by staining with haematoxylin and eosin) were stained for PCNA and morphometrically examined to determine the PCNA-labelling index in basophilic and in adjacent unchanged tubules. Generally, the labelling index was more than 10-fold higher in basophilic tubules than in adjacent unchanged tubules. No treatment-related effect on the labelling index of basophilic and adjacent unchanged tubules was noted. Additionally, the relationship between the PCNA-labelling index in basophilic and in adjacent unchanged tubules remained uninfluenced by treatment with cyprodinil. Therefore, cyprodinil at a dietary concentration of up to 4000 mg/kg did not increase proliferative activity (as measured by PCNA-labelling index) in basophilic and adjacent unchanged tubules.
Under the study conditions used, treatment with cyprodinil resulted in a slight increase in the severity of tubular basophilia in F0 males at the highest dose. These basophilic tubules mainly correspond to degenerative/regenerative or chronic progressive nephropathic lesions, in terms of histopathology and proliferative activity. Treatment with cyprodinil had no effect on proliferative activity in basophilic tubules or in unaffected cortical and medullary renal tubules (Weber, 1997).
Rats
In a range-finding study of acute oral neurotoxicity, groups of three male and female Tif RAIf (SPF) rats were given a single dose of cyprodinil (purity, 99.2%) in car-boxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) at 0, 300, 1000 or 2000 mg/kg bw, administered by gavage in a volume of 10 ml/kgbw. Animals were examined daily for clinical signs and mortality. Body weights were recorded weekly and food consumption was recorded twice per week. All animals were subjected to a functional observational battery (FOB), and motor activity evaluations were conducted before the treatment and on days 1 (1, 2, 4, 6, and 8 h after administration), 2, 5, and 8. Necropsies were not performed. This study complied with GLP.
Mortality, body weight, and body-weight gains were unaffected by the test substance. Food consumption was transiently reduced in animals at the highest dose (2000 mg/kg bw). At this dose, reduced activity, decreased muscle tone, lowered responsivity, hunched posture/gait, and piloerection and in some animals, palpebral closure and slight gait abnormalities, were observed in FOB parameters. These effects appeared first at 1 h after dosing, peaked at about 2 h after dosing, and disappeared on day 2. The same clinical findings were recorded for one female at 1000 mg/kg bw. The gait anomalies noted in two females at the highest dose were attributed to non-specific systemic toxicity as they were observed soon after dosing and were not present later, at the time of peak effects. The signs and symptoms observed at the limit dose in this study were attributed to the systemic toxicity of cyprodinil and were not considered to be an indication of any specific neurotoxic effects of cyprodinil. The LOAEL was 1000 mg/kg bw on the basis of systemic toxicity observed in one female. The NOAEL was 300 mg/kg bw and the NOEL was 300 mg/kg bw (Classen, 1996).
In a study of acute neurotoxicity, groups of 10 male and 10 female Tif RAIf (SPF) rats were given a single dose of cyprodinil (purity, 99.2%) in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) at 0, 200, 600, or 2000 mg/kg bw, administered by gavage in a volume of 10 ml/kg bw. Animals were examined daily for clinical signs and mortality. Body weights were recorded weekly and food consumption twice per week. All animals were subjected to a FOB and motor activity evaluations were conducted before treatment and on days 1 (2 h after dosing), 8, and 15. Upon study termination, neuropathological examination of perfusion-fixed tissues from the central and peripheral nervous system was conducted on five rats of each sex from the control group and the group receiving the highest dose. Data on positive controls were not provided. This study complied with GLP.
One male and one female at 600 mg/kg bw died on day 2 and day 18, respectively. No other mortalities were observed. The presence of clear fluid in the thoracic cavity of the dead male rat is indicative of intubation error. Hunched posture was seen in all females at 600 and 2000 mg/kg bw, which was accompanied by piloerection and reduced activity in most animals. In addition, hunched posture was seen in five females at 200 mg/kg bw from day 1 to day 3, which is not considered as an adverse finding since no clinical signs were noted in two other studies of neurotoxicity (Classen, 1996, 1998) at comparable doses. All signs disappeared in 3 to 4 days. Body weight, body-weight gain and food consumption were comparable in animals in treated groups and controls.
At the time of peak effects, reduced activity, hunched posture, piloerection, and increased responsiveness to sensory stimuli were observed in females at 600 and 2000 mg/kg bw and persisted until day 4. A dose-related decrease in body temperature was noted in all treated groups. The decrease in body temperature was statistically significant for all treated males and for females at 600 and 2000 mg/kg bw. The decrease in body temperature at 200 mg/kg bw was not considered to be biologically relevant since it was minimal, and not seen in the other study of neurotoxicity at a similar dose (Classen, 1998). No treatment-related neuropathic changes were observed. The signs and symptoms observed at 600 and 2000 mg/kg bw (the limit dose) in this study were attributed to the systemic toxicity of cyprodinil and were not indicative of neurotoxic effects of cyprodinil.
The LOAEL was 600 mg/kg bw on the basis of reduced activity, hunched posture, piloerection, increased responsiveness to sensory stimuli, and hypothermia. The NOAEL was 200 mg/kg bw and the NOEL was 200 mg/kg bw (Classen, 1997a).
A study of acute neurotoxicity in rats was conducted to verify the occurrence of minimal hypothermia and hunched posture seen at 200 mg/kg bw in previous studies and to identify a clear NOEL for these findings. In this study, groups of 10 male and 10 female Tif RAIf (SPF) rats were given a single dose of cyprodinil (purity, 99.2%) in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) at 0, 20, 60, or 200 mg/kg bw, administered by gavage in a volume of 10ml/kgbw. Animals were examined daily for clinical signs and mortality. Body weights were recorded weekly and food consumption was recorded twice per week. All animals were subjected to a FOB and motor activity evaluations were conducted before treatment and on days 1 (2 h after dosing) and 8. The study was terminated on day 10 because there were no treatment-related effects. Gross necropsy and histopathology were not performed. Data on positive controls were not provided. This study complied with GLP.
Mortality, clinical signs, body weights, body-weight gains, food consumption, FOB parameters, and motor activity were unaffected by the test substance. A statistically significant reduction in body temperature was observed on day 8 in males at the highest dose (-0.2°C). This effect was considered to be incidental and toxicologically irrelevant because it was minimal and was not seen at the time of peak effects. No evidence of neurotoxicity was observed at any dose tested. The NOEL was 200 mg/kg bw, the highest dose tested (Classen, 1998).
In a study of short-term neurotoxicity/toxicity, groups of 10 male and 10 female Tif RAIf (SPF) rats were given diets containing cyprodinil (purity, 99.2%) at a concentration of 0, 80, 800, or 8000 mg/kg (equivalent to 0, 5.8, 54.5, and 601 mg/kg bw per day, for males, respectively, and 0, 6.3, 58.7 and 631 mg/kg bw per day for females, respectively) for 13 weeks. Animals were observed daily for clinical signs and mortality. Body weight and food consumption were recorded weekly. All animals were evaluated for FOB and motor activity at weeks -1, 4, 8, and 13. Upon study termination, neuropathological examination of perfusion-fixed central and peripheral nervous tissues was conducted on five rats of each sex from the control group and from the group receiving cyprodinil at 8000 mg/kg. Pituitary glands were also examined in five rats of each sex from the control group and from the group receiving cyprodinil at 8000 mg/kg. Data on positive controls were not provided. This study complied with GLP.
There were no deaths. No toxicologically relevant clinical signs or changes in behaviour were observed during the study. The body-weight gain of the males and females at the highest dose was reduced compared with that of the controls. A transient decrease in food consumption was observed in the animals at the highest dose in the first week of treatment. Observational and functional tests indicated no toxicologically relevant deviation from the controls. At week 13, mean landing foot splay was increased in male rats at 800 mg/kg dose, however, this finding was considered to be incidental in view of the absence of any other effects and the fact that there was no dose-response relationship. No treatment-related effects on the various motor activity parameters were detected. The absolute and relative liver weights were increased in animals of both sexes at the highest dose and absolute and relative kidney weights were increased in females at the highest dose.
At necropsy, there were no macroscopic findings that indicated adverse treatment-related effects. An increased incidence of hepatocellular hypertrophy was observed in males and females at the highest dose. An increased incidence of chronic tubular lesions, classified as slight with respect to severity, was observed at the highest dose. In addition, an increased incidence of tubular casts of a slight degree of severity were observed at the highest dose. There was also an increased incidence of slight hypertrophy of follicular epithelial cells in the thyroid gland in animals at the highest dose. All five males examined in the control group and the group receiving the highest dose showed hypertrophy of the pars distalis, of moderate to marked severity. In females, two out of five rats at the highest dose showed thyroid hypertrophy, classified as being of minimal severity. All hypertrophic cells were positive for thyroid-stimulating hormone (TSH), but in all animals examined, additional TSH-positive cells existed that were not hypertrophic. No relevant differences in the number of TSH-positive cells in control and treated groups were found, while males generally had more TSH-positive and hypertrophic cells than females. Histopathological examination did not reveal any additional treatment-related abnormalities.
No evidence for neurotoxicity was observed at any dose tested. The LOAEL for this study was 8000 mg/kg (equal to 601 and 631 mg/kg bw per day for males and females, respectively) on the basis of liver, kidney and thyroid histopathology, reduced body-weight gain and food consumption, and increased absolute and relative liver and kidney weights (females only). The NOAEL for this study was 800 mg/kg (equal to 54.5 and 58.7 mg/kg bw per day for males and females, respectively). The NOEL for neurotoxicity was 8000 mg/kg, (equal to 601 mg/kg bw per day for males and 631 mg/kg bw per day for females) (Classen, 1997b).
(i) Acute toxicity
CGA 249287 (4-cyclopropyl-6-methyl-pyrimidine-2-ylamine)
CGA 249287 is formed as a result of the cleavage of the phenyl group from the active substance, cyprodinil. It was identified in rats and also detected in studies of photolysis in soil and water, in plants such as tomato, wheat, apple, potato, peaches, and in goats and hens. In rat liver, approximately 0.014% of the administered dose of cyprodinil was recovered as CGA 249287.
In a study of acute oral toxicity, five male and five female young adult Tif RAIf (SPF) rats that had been fasted overnight were given CGA 249287 technical (purity, 99.8%), at a dose of 2000 mg/kg bw by oral administration. The test material was suspended in car-boxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) and administered in a volume of 10 ml/kg bw. Animals were observed daily for clinical signs and mortality. Body weights were recorded at the start of the study and weekly thereafter. Treated animals were observed for 14 days. This study complied with GLP.
One male was found dead 1 day after dosing. There were no other mortalities. Most animals showed the following signs: piloerection, hunched posture, dyspnoea, reduced locomotor activity and chromodacryorrhoea, with some of these symptoms appearing as soon as 1 h after dosing. Symptoms were no longer evident by day 6 (females) or day 7 (males). All survivors gained weight during days 0-7, and again during days 7-14.
The only finding at gross necropsy performed on the rat that died was a spotted thymus. No abnormalities were found in the other animals at necropsy.
The oral LD50 for male and female rats was >2000 mg/kg bw (Hartmann, 1992).
CGA 275535 3-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-phenol
CGA 275535 is formed as a result of hydroxylation at position 3 of the phenyl ring of cyprodinil. It was found in studies of soil degradation, and in plants such as wheat and potato (amounts not considered toxicologically relevant).
In a study of acute oral toxicity, five male and five female young adult Hanlbm:WIST (SPF) rats that had been fasted overnight were given CGA 275535 technical (metabolite of cyprodinil; purity, 99.0%), at a dose of 2000 mg/kg bw by oral administration. The test material was suspended in carboxymethylcellulose (0.5% w/v) and aqueous polysorbate 80 (0.1% w/v) and administered in a volume of 10 ml/kg bw. Animals were observed daily for clinical signs and mortality. Body weights were recorded at the start of the study and weekly thereafter. Treated animals were observed for 14 days. This study complied with GLP.
There were no clinical signs of toxicity or mortality. No effects on body weights or body-weight gains were observed. Gross necropsy did not reveal any abnormalities. The acute oral LD50 of CGA 275535 was >2000 mg/kg bw (Sommer, 2000a).
NOA 422054 (4-cyclopropyl-6-hydroxymethyl-pyrimidine-2-ylamine)
NOA 422054 is formed as a result of hydroxylation at the pyrimidine-methyl group and cleavage of the phenyl ring of cyprodinil. NOA 422054 was detected in studies of crop rotation and is considered to be a hydroxy-methyl derivative of the plant, soil and rat metabolite, CGA 249287.
In a study of acute oral toxicity, five male and five female young adult Hanlbm: WIST (SPF) rats that had been fasted overnight were given NOA 422054 technical (metabolite of cyprodinil; purity, 99.0%) at a dose of 2000 mg/kg bw by oral administration. The test material was suspended in carboxymethylcellulose (0.5% w/v) in aqueous polysorbate 80 (0.1% w/v) and administered in a volume of 10 ml/kg bw. Animals were observed daily for clinical signs and mortality. Body weights were recorded at the start of the study and weekly thereafter. Treated animals were observed for 14 days. This study complied with GLP.
One female died on day 1 after treatment with a dose of 2000 mg/kg bw. There were no other mortalities. At 2000 mg/kg bw, hunched posture and ventral recumbency were observed in both male and female rats. Increased dyspnoea, and hypoactivity were also observed in male and female rats in this group. One female was seen pushing her head through bedding. All surviving animals appeared to be normal by day 2. No effects on body weights or body-weight gains were observed. Gross necropsy did not reveal any abnormalities, except fluid in the small intestine and the stomach in one female. The acute oral LD50 of NOA 422054 was >2000 mg/kg bw (Sommer, 2000b).
CGA 321915 4-cyclopropyl-6-methyl-pyrimidin-2-ol
CGA 321915 is formed as a result of hydroxylation and cleavage of the aminophenyl moiety of cyprodinil. It was found in studies of soil degradation and photolysis and in plants such as wheat.
In a study of acute oral toxicity, five male and five female young adult Tif RAIf (SPF) rats that had been fasted overnight were given CGA 321915 technical (metabolite of cyprodinil; purity, 94.0%), at 2000 mg/kg bw by oral administration. The test material was suspended in distilled water and administered in a volume of 10 ml/kg bw. Animals were observed daily for clinical signs and mortality. Body weights were recorded at the start of the study and weekly thereafter. Treated animals were observed for 14 days. This study complied with GLP.
There were no mortalities. At 2000 mg/kg bw, piloerection and hunched posture was reported in all animals; this subsided within 1 day. No effects on body weights or body-weight gains were observed. Gross necropsy did not reveal any abnormalities. The acute oral LD50 of CGA 321915 was >2000 mg/kg bw (Winkler, 1996b).
(ii) Short-term studies of toxicity
CGA 249287 (4-cyclopropyl-6-methyl-pyrimidine-2-ylamine)
In a 90-day study of toxicity, groups of 12 male and 12 female Alpk:ApfSD (Wistar-derived) rats were given diets containing CGA 249287 (metabolite of cyprodinil, purity, 100%) at a concentration of 0, 300, 1000, or 4000 mg/kg (equal to 0, 23.9, 79.5, and 304.8 mg/kg bw per day for males and 0,27.2, 90.5, and 342.6 mg/kg bw per day for females) for 90 days. Animals were observed daily for clinical signs and mortality. Body weights and food consumptions were recorded weekly. All animals were evaluated for FOB and motor activity during week 13. Ophthalmoscopic examinations were performed on all animals before treatment and on controls and animals in the group receiving the highest dose before sacrifice. Haematological, clinical chemistry and urine analysis were performed at termination. All treated animals were sacrificed, selected organs were removed and weighed, and histopathological examination was performed. This study complied with GLP.
No mortalities were observed. No treatment-related effects on clinical signs of toxicity, ophthalmoscopic examination, haematology parameters, clinical chemistry, clinical pathology or urine analysis were observed. There were no treatment-related effects on FOB, landing foot splay measurements, grip strength, time to tail flick or motor activity. At the highest dose, a slight increase in the value for forelimb grip was noted in males, which was considered to be incidential and unrelated to treatment because no effects were seen in females, no effects were seen on hindlimb splay in either sex, and the values for males were within the range for historical controls.
At 4000 mg/kg, body weights were reduced by 16% for males and 7% for females, compared with those for concurrent controls. Similarly, body-weight gains for male and female rats at 4000 mg/kg were reduced by 26% for males and 17% for females, compared with those for controls. Food consumption of animals was also reduced by 15-17% at 4000 mg/kg, compared with controls. There were no effects on body weight, body-weight gain or food consumption in other treated groups. There was a reduction in the severity of intratubular microlithiasis in the kidneys of females at 4000 mg/kg, which was considered to caused by reduced food consumption and not directly related to treatment.
The LOAEL was 4000 mg/kg (304.8 and 342.6 mg/kg bw per day for males and females, respectively) on the basis of reduced body weight, body-weight gain and food consumption. The NOAEL was 1000 mg/kg (79.5 and 90.5 mg/kg bw per day for males and females, respectively). The NOEL was 1000 mg/kg, equal to 79.5 mg/kg bw per day for males and 90.5 mg/kg bw per day for females (Milburn, 2001).
(iii) Genotoxicity
CGA 249287 (4-cyclopropyl-6-methyl-pyrimidine-2-ylamine)
In assays for reverse gene mutation in bacteria, cultures of four Salmonella typhimurium histidine-deficient (his-) strains (TA1535, TA1537, TA98 and TA100) and one tryptophan-deficient (try-) strain of Escherichia coli (WP2 uvrA) were exposed to CGA 249287 (active ingredient, 99.5%; in dimethylsulfoxide, DMSO) for 48 h at 37 ± 1.5°C to CGA 249287 at five concentrations ranging from 312.5 to 5000 µg/plate, in the presence and absence of metabolic activation from S9 (9000 × g supernatant of Aroclor 1254-induced rat liver microsomes). While some cultures were exposed to the vehicle (DMSO) alone, other cultures were treated with known mutagens and served as positive controls. At harvest, the frequency of reversion to prototrophy (his+, try+) in test cultures was compared to that in vehicle controls. This study complied with GLP.
Slight cytotoxicity was observed in E. coli WP2 uvrA at the highest concentration in the absence of metabolic stimulation, but at no concentration of test substance in either assay was the frequency of revertant (his+ and try+) colonies greater than that for the negative control (DMSO) in either the presence or the absence of metabolic activation. All positive controls reacted with marked increases in the frequency of revertants.
Thus, CGA 249287 (a metabolite of cyprodinil) was negative for induction of reverse mutation in these bacterial cultures, under the given experimental conditions (Hertner, 1992).
In two independent assays for mammalian cell gene mutation in vitro, L5178Y Tk+/ mouse lymphoma cells were exposed to CGA 249287 (purity, 100%) in DMSO at a dose of up to 1492 µg/ml, with and without exogenous metabolic activation from S9 (the 9000 × g supernatant of Aroclor-induced liver microsomes from male Sprague Dawley rats). This study complied with GLP.
No reproducible increases in frequency of mutation at the Tk locus were observed in cultures treated with CGA 249287 technical, with or without metabolic activation. The positive controls, ethyl methanesulphonate and benzo[a]pyrene, induced a marked and significant increase in the frequency of mutation in both assays (Clay, 2001).
In an assay for chromosomal aberrations, Chinese hamster ovary CHO-CCL 61 cells were exposed to CGA 249287 technical (metabolite of cyprodinil; purity, 100%) in DMSO in two independent assays. In the initial assay, cells were exposed to CGA 249287 at a concentration of 0, 350, 700 or 1 400 µg/ml for 3 h, with or without metabolic activation, followed by a further incubation of 18 h. In the confirmatory assay, cells were exposed to CGA 249287 at a concentration of 0, 175, 350 or 700 µg/ml for 21 h in the absence of metabolic activation and harvested immediately after exposure; or cells were exposed to CGA 249287 at a concentration of 0, 350, 700 or 1 400 µg/ml for 3 h in the presence of metabolic activation, followed by a recovery period of 18 h. Metabolic activation was provided by the S9 fraction from Aroclor 1254-induced male HanIbm:WIST (SPF) rat livers. This study complied with GLP.
CGA 249287 technical was tested at concentrations up to those that were cytotoxic. In the initial assay, a statistically significant increase in the number of metaphases with aberrations was observed at 1 400 µg/ml in the absence of metabolic activation. This increase in aberrations was within the range for historical controls and did not fulfil the criteria for a positive response; it is therefore not considered to be treatment-related. No statistically significant increase in the number of metaphases with specific aberrations was observed in the confirmatory assay in the absence of metabolic activation. No statistically significant increase in the number of metaphases with specific aberrations was observed in either the initial or the confirmatory assay, in the presence of metabolic activation. The positive controls resulted in a clearly increased number of abberant metaphases in all experiments. There was no evidence that the test material increased the incidence of chromosome aberrations compared with controls (Ogorek, 2001).
CGA 275535 3-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-phenol
In repeat (initial and confirmatory) assays for reverse gene mutation in bacteria, cultures of five histidine-deficient (his- ) strains of S. typhimurium (TA98, TA100, TA102, TA1535 andTA1537) and the tryptophan-deficient (try-) strain (WP2 uvrA) of E. coli were exposed to CGA 275535 technical (metabolite of cyprodinil; purity, 99%, in DMSO) for 48 h at 37 ± 1.5°C at concentrations in the range of 62.5-5000 µg/plate in the presence of metabolic activation (provided by S9, the 9000 × g supernatant of Aroclor 1254-induced rat liver), and in the range of 15.6-1000 µg/plate in the absence of metabolic activation by both the standard plate assay (initial trial, ±S9; confirmatory experiment, -S9), and with preincubation (confirmatory experiment, +S9) were used. Cultures exposed to the solvent alone (DMSO) served as negative controls, while cultures treated with known mutagens served as positive controls. At harvest, numbers of revertant colonies (his+, try+) were compared with those for controls treated with DMSO. This study complied with GLP.
Reproducible inhibition of growth occurred with or without metabolic activation in WP2 uvrA cultures treated with CGA 275535 at the highest concentration (2000 µg/plate), as shown by a reduction in the number of revertant (try+) colonies. In neither experiment was there any increase in revertant (his+ or try+) colonies at any concentration, in either the presence or absence of metabolic activation. In contrast, all positive controls exhibited marked increases in numbers of revertants.
Therefore, neither CGA 275535 technical nor its metabolites induced gene mutations in the bacterial strains assayed (Deparade, 2001).
NOA 422054 (4-cyclopropyl-6-hydroxymethyl-pyrimidine-2-ylamine)
In repeat (initial and confirmatory) assays for reverse gene mutation in bacteria, cultures of five histidine-deficient (his-) strains of S. typhimurium (TA98, TA100, TA102, TA1535 andTA1537) and the tryptophan-deficient (try-) strain (WP2 uvrA) of E. coli were exposed to NOA 422054 technical (metabolite of cyprodinil; purity, 99.0%; in DMSO) for 48h at 37 ± 1.5°C at five concentrations ranging from 312.5 to 5000 µg/plate, in the presence and absence of metabolic activation from S9 (the 9000 × g supernatant of Aroclor 1254-induced rat liver) in both the standard plate assay (initial trial, ±S9; confirmatory experiment, -S9), and with preincubation (confirmatory experiment, +S9). Cultures exposed to the solvent alone (DMSO) served as negative controls, while cultures treated with known mutagens served as positive controls. At harvest, the numbers of revertant colonies (his+, try+) were compared with those for controls treated with DMSO. This study complied with GLP.
Reproducible growth inhibition occurred both in the presence and absence of metabolic activation in WP2 uvrA cultures at the highest concentration, 5000 µg/plate), as shown by a reduction in the number of revertant (try+) colonies. No precipitation was found in any test culture at any concentration. In neither experiment was there any increase in revertant (his+ or try+) colonies at any concentration, in either the presence or absence of metabolic activation. In contrast, all positive control cultures exhibited marked increases in number of revertants.
Therefore, neither NOA 422054 technical nor its metabolites induced gene mutations in the bacterial strains assayed (Deparade, 2000).
CGA 321915 (4-cyclopropyl-6-methyl-pyrimidin-2-ol)
In repeat (initial and confirmatory) assays for reverse gene mutation in bacteria, cultures of five histidine-deficient (his-) strains of S. typhimurium (TA98, TA100, TA102, TA1535 and TA1537) and the tryptophan-deficient (try-) strain (WP2 uvrA) of E. coli were exposed to CGA 321915 technical (metabolite of cyprodinil; purity, 94.0%; in distilled water) for 48 h at 37 ± 1.5°C at five concentrations ranging from 312.5 to 5000 µg/plate in the presence and absence of metabolic activation from S9 (9000 × g supernatant of Aroclor 1254-induced rat liver) by both the standard plate assay (initial trial, ±S9; confirmatory experiment, -S9), and with preincubation (confirmatory experiment, +S9). This study complied with GLP.
Reproducible growth inhibition occurred in both activated and nonactivated WP2 uvrA cultures treated at the highest concentration (5000 µg/plate), as shown by a reduction in the number of revertant (try+) colonies. No precipitation was found in any test culture at any concentration. In neither experiment was there any increase in revertant (his+ or try+) colonies at any concentration, in either the presence or absence of metabolic activation. In contrast, all positive controls exhibited marked increases in number of revertants.
Therefore, neither CGA 321915 technical nor its metabolites induced gene mutations in the bacterial strains assayed (Ogorek, 1996).
Available information on medical surveillance of workers employed in a pilot plant indicated that three employees (the total number of employees potentially exposed was not reported) in the laboratory area showed symptoms of flush, sensation of warmth and swelling of eyelids when weighing this substance. It was considered that these symptoms were caused by exposure to cyprodinil, which has the potential to cause slight eye irritation and skin sensitization, as reported in studies in animals. In a plant carrying out large-scale production of cyprodinil, one case of accidental exposure to the eye was reported. The affected person suffered slight conjunctivitis. After medical treatment, the exposed worker returned to work (Lorez & Schulze-Rosario, 2001).
In a published study using the alkaline comet assay, DNA damage was determined for four groups of farmers before and after one day of spraying of pesticides. The farmers were handling chlorothalonil, isoproturon, and triazoles in this study and exposure to cyprodinil occurred only during mixing. There was no evidence for DNA damage in the group of farmers exposed to cyprodinil and other pesticides.
No epidemiological studies were available in the published literature (Lebailly et al., 1998).
In studies of metabolism in rats, radiolabelled cyprodinil administered by gavage as a single dose of 0.5 or 100 mg/kg bw, or as repeated doses of 0.5 mg/kg bw per day for 14 days, was rapidly absorbed from the gastrointestinal tract and excreted. Approximately 75% (range, 71-85%) of an orally administered dose was absorbed over 48 h. At a dose of 0.5 and 100 mg/kg bw, two plasma level maxima of radioactivity were observed at approximately 0.5-1 h and 8-12 h, probably caused by reabsorption of material excreted in the bile. Approximately 92-97% of the administered dose was eliminated within 48 h in the urine (48-68%), faeces (29-47%), and bile (accounting for up to 35.4% of the dose in cannulated rats), with elimination being almost complete by day 7. Seven days after single or repeated oral administration at the lower dose, total tissue residues accounted for 0.15-0.60% of the administered dose. Cyprodinil was primarily metabolized by hydroxylation of the phenyl and pyrimidine rings and methyl group, and excreted mainly as glucuronide or sulfate conjugates in urine, faeces and bile. Approximately 3-8% of the parent compound was detected in the faeces. Excretion, distribution and metabolite profiles were essentially independent of dose, pretreatment and site of radiolabel, although there were some quantitative sex-dependent differences in urinary metabolites.
Cyprodinil has low toxicity when administered by the oral, dermal or inhalation routes. LD50 values after oral administration were >2000 and >5000 mg/kg bw in rats and mice, respectively. The LD50 in rats treated dermally was >2000 mg/kg bw. The LC50 in rats treated by inhalation was >1.20 mg/l (the highest attainable concentration) after an exposure of 4 h. Clinical signs of toxicity such as piloerection, hunched posture, dyspnoea, and reduced locomotor activity were seen. Cyprodinil was not a dermal or ocular irritant, but was a skin sensitizer.
In short-term studies in mice, rats and dogs, administration of cyprodinil in the diet or by gavage resulted in reduced body-weight gain and reduced food consumption at a dose of 6000 mg/kg (equal to 849 mg/kg bw per day), 1000 mg/kg bw per day, and >15 000 mg/kg (equal to 446 mg/kg bw per day) in mice, rats and dogs, respectively. The major target organs were the liver, kidney, and thyroid in rats, and the liver in mice and dogs. Increases in liver weights were observed in mice at 6000 mg/kg (equal to 849 mg/kg bw per day) and in rats at >2000 mg/kg (equal to 134 mg/kg bw per day). Increases in thyroid weight were observed in rats at >2000 mg/kg (equal to 134 mg/kg bw per day). Some mild to moderate histopathological changes in the liver, such as hepatocyte hypertrophy and multifocal single cell hepatocyte necrosis, were seen in mice and rats at >2000 mg/kg (equal to 25 mg/kg bw per day in mice and equal to 134 mg/kg bw per day in rats). In the kidneys, adverse effects were manifested in rats as chronic tubular lesions and chronic kidney inflammation at >2000 mg/kg (equal to 134 mg/kg bw per day). The NOAEL in a 90-day study of toxicity in mice was 500 mg/kg (equal to 73.3 mg/kg bw per day). The NOAEL in a 28-day study in rats treated by gavage was 100 mg/kg bw per day. The NOAEL in a 90-day study of toxicity in rats was 300 mg/kg (equal to 19 mg/kg bw per day). The NOAELs in a 90-day and a 1-year study of toxicity in dogs were 7000 mg/kg (equal to 210 mg/kg bw per day) and 2 500 mg/kg (equal to 66 mg/kg bw per day), respectively.
In long-term studies of toxicity and carcinogenicity in mice and rats, there were no treatment-related neoplastic findings. In mice, a slightly increased incidence of hyperplasia in acinar cells of the exocrine pancreas in males was observed at the highest dose tested, 5000 mg/kg (equal to 558 mg/kg bw per day). The NOAEL for systemic toxicity in mice was 2000 mg/kg (equal to 196 mg/kg bw per day), on tha basis of an increase in the incidence of focal and multifocal hyperplasia of the exocrine pancreas in males, reduced body weights in males and females, increased relative kidney weights in females, and increased relative liver weights in males and females, seen at the highest dose tested. In rats, histopathological changes in the liver (spongiosis hepatitis) and increased liver weights were observed at > 1000 mg/kg (equal to 3 5.6 mg/kg bw per day). The NOAEL for systemic toxicity in rats was 75 mg/kg (equal to 2.7 mg/kg bw per day), on the basis of a dose-related increase in the incidence of spongiosis hepatitis in males at 1000 and 2000 mg/kg. There was no evidence for significant long-term toxicity in females. Cyprodinil was not carcinogenic in mice or rats.
Cyprodinil gave negative results in a battery of studies of genotoxicity in bacteria and cultured mammalian cells in vitro, and in a test for micronuclei formation in mice in vivo.
The Meeting concluded that cyprodinil is unlikely to be genotoxic.
In view of the lack of genotoxicity and the absence of carcinogenicity in rats and mice, the Meeting concluded that cyprodinil is unlikely to pose a carcinogenic risk to humans.
In a two-generation study of reproduction in rats, reproductive parameters were not affected at the highest dose tested (4000 mg/kg, equal to 295 mg/kg bw per day). The NOAEL for parental systemic toxicity was 1000 mg/kg (equal to 74 mg/kg bw per day) on the basis of decreased body-weight gain in F0 females at the highest dose tested, 4000 mg/kg (equal to 295 mg/kg bw per day). The NOAEL for offspring toxicity was 1000 mg/kg (equal to 74 mg/kg bw per day) on the basis of decreased body weights for F1 and F2 pups at the highest dose tested. Cyprodinil was not teratogenic in rats and rabbits at a dose of up to 1000 and 400 mg/kg bw per day in rats and rabbits, respectively. In a study of developmental toxicity in rats, lower fetal body weights and an increased incidence of delayed ossification at a dose of 1000 mg/kg bw per day were considered to be secondary to maternal toxicity. At the highest dose tested, a slight increase in the number of litters in which pups were born with an extra (thirteenth) rib was observed in rabbits in the presence of maternal toxicity, an effect that was not considered to be toxicologically relevant.
In a study of an acute neurotoxicity in rats, cyprodinil at a dose of 600 or 2000 mg/kg bw caused reduced activity, hunched posture, piloerection, increased responsiveness to stimuli, and hypothermia; the NOAEL was 200 mg/kg bw. In a study of short-term neurotoxicity, no signs of neurotoxicity were observed in a FOB, on evaluation of motor activity, or on neuropathological examination, in rats receiving cyprodinil in the diet at a concentration of up to 8000 mg/kg (equal to 601 mg/kg bw per day), the highest dose tested. The NOAEL was 800 mg/kg (equal to 54.5 mg/kg bw per day) on the basis of liver, kidney and thyroid histopathology, and reduced body-weight gain seen at the highest dose tested (8000 mg/kg, equal to 601 mg/kg bw per day).
Several soil and plant metabolites of cyprodinil were investigated using the Ames test and for acute oral toxicity at the highest dose (2000 mg/kg bw). The LD50 for each of these metabolites was >2000 mg/kg bw and no mutagenic potential was detected.
The Meeting concluded that the existing data were adequate to characterize the potential hazard to fetuses, infants and children.
The Meeting established an acceptable daily intake (ADI) of 0-0.03 mg/kg bw based on a NOAEL of 2.7 mg/kg bw per day in a 24-month study in rats fed with cyprodinil, on the basis of liver effects (spongiosis hepatitis) seen in males at higher doses, and a 100-fold safety factor.
The Meeting concluded that the establishment of an acute RfD for cyprodinil was not necessary, on the basis of its low acute toxicity, the absence of developmental toxicity in rats and rabbits, the lack of neurotoxicity after a single exposure, and absence of any other toxicological end-point that would be elicited by a single dose.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
18-month study of toxicity and carcinogenicitya |
Toxicity |
2000 mg/kg, equal to |
5000 mg/kg, equal to |
|
Carcinogenicity |
5000 mg/kg, equal to |
— |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
75 mg/kg, equal to |
1000 mg/kg, equal to |
|
Carcinogenicity |
2000 mg/kg, equal to |
|||
|
Multi-generation study of reproductive toxicitya |
Parental toxicity/offspring toxicity |
1000 mg/kg, equal to |
4000 mg/kg, equal to |
|
|
Study of developmental toxicityb |
Maternal toxicity |
200 mg/kg bw per day |
1000 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
200 mg/kg bw per day |
1000 mg/kg bw per day |
||
|
Study of acute neurotoxicityb |
Neurotoxicity |
200 mg/kg bw per day |
600 mg/kg bw per day |
|
|
Rabbit |
Study of developmental toxicityb |
Maternal toxicity |
150 mg/kg bw per day |
400 mg/kg bw per day |
|
Embryo- and fetotoxicity |
400 mg/kg bw per day |
— |
||
|
Dog |
1 -year study of toxicitya |
Toxicity |
2500 mg/kg, equal to |
15 000 mg/kg, equal to |
a Diet
b Gavage
c Highest dose tested
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for the continued evaluation of the compound
Further observations in humans.
Summary of critical end-points for cyprodinil
|
Absorption, distribution, excretion, and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid; maximum reached in blood by 0.15-1.0 h; about 71-85% absorbed after 48 h |
|
Dermal absorption |
At 6 µg/cm2, absorption in rats in vivo was 21.7% in 0-24 h; at 870 µg/cm2, absorption in vivo was 1.9% in 0-24 h |
|
Distribution |
Extensive; highest concentrations in liver, kidney, spleen, and blood |
|
Potential for accumulation |
No evidence of significant accumulation; about 0.2-0.6% of the total dose was found in tissues after 168 h |
|
Rate and extent of excretion |
Excretion was rapid; >90% excreted into urine (48-67%) and faeces (27^15%) within 48 h |
|
Metabolism in animals |
Very extensive; metabolic pathways include hydroxylation of the phenyl and pyrimidyl rings and conjugation with sulfate or glucuronic acid; limited cleavage of bond between phenyl and pyrimidyl rings; about 3-8% unchanged cyprodinil in faeces |
|
Toxicologically significant compounds |
Cyprodinil |
|
Acute toxicity |
|
|
Mouse, LD50, oral |
>5000 mg/kg bw |
|
Rat, LD50, oral |
>2000 mg/kg bw |
|
Rat, LD50, dermal |
>2000 mg/kg bw |
|
Rat, LC50, inhalation |
>1.2mg/l (maximum attainable concentration, 4-h exposure, nose only) |
|
Rabbit, dermal irritation |
Not an irritant |
|
Rabbit, ocular irritation |
Not an irritant |
|
Skin sensitization |
Sensitizing (maximization test) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Histopathological findings in liver, kidneys and thyroid |
|
Lowest relevant oral NOAEL |
19 mg/kg bw per day (90-day study in rats) |
|
Lowest relevant dermal NOAEL |
No suitable study is available |
|
Lowest relevant inhalation NOAEC |
No studies are available |
|
Genotoxicity |
No genotoxic potential |
|
Long-term studies of toxicity and carcinoggenicity |
|
|
Target/critical effect |
Degenerative liver lesions (spongiosis hepatitis) in males, in rats |
|
Lowest relevant NOAEL |
2.7mg/kgbw per day (2-year study in rats) |
|
Carcinogenicity |
No carcinogenicity in mice and rats |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
Reduced pup body weight |
|
Lowest relevant reproductive NOAEL |
74 mg/kg per day (rats) |
|
Developmental target/critical effect |
No toxicologically relevant effects were observed |
|
Lowest relevant developmental NOAEL |
400 mg/kg per day (rabbits) |
|
Neurotoxicity/delayed neurotoxicity |
|
|
Acute neurotoxicity |
No evidence of neuropathology at doses of up to 2000 mg/kgbw in rats; NOAEL was 200 mg/kgbw, on the basis of clinical signs |
|
90-day study of neurotoxicity |
No evidence of neurotoxicity or neuropathology; NOAEL was 54.5 mg/kg bw per day on the basis of liver, kidney and thyroid histopathology |
|
Other toxicological studies |
|
|
Metabolites: study of acute toxicity |
LD50 of >2000 mg/kg bw for four metabolitesa |
|
Metabolite: 90-day study, in diet |
NOAEL of 79.5 mg/kg bw per day for CGA 249287 |
|
Metabolites: genotoxicity |
No genotoxic potential for four metabolitesa |
|
Medical data |
Limited data; slight eye irritation and sensitization reported in workers |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0-0.03 mg/kgbw |
2-year study of toxicity and carcinogenicity |
100 |
|
Acute RfD |
Not allocated (unnecessary) |
Not applicable |
Not applicable |
a CGA 249287: (4-cyclopropyl-6-methyl-pyrimidine-2-ylamine)
CGA 275535: 3-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-phenol
NOA 422054: (4-cyclopropyl-6-hydroxymethyl-pyrimidine-2-ylamine)
CGA 321915: 4-cyclopropyl-6-methyl-pyrimidin-2-ol
Altmann, B. (1991) 3-Month subchronic oral toxicity study in beagle dogs: CGA 219417 Technical. Unpublished report No. 891323 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Altmann, B. (1992) 12-Month chronic dietary toxicity study in beagle dogs: CGA 219417 Technical. Unpublished report No. 891324 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Banasch, ? et al. (1985) Digestive system. In: ILSI Monographs on pathology of laboratory animals. ed. Jones et al., Springer-Verlag.
Ceresa, C. (1990) Micronucleus test, mouse, in vivo study: CGA 219417 Technical. Unpublished report No. 901082 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Classen, W. (1996) Acute oral rangefinding neurotoxicity study in rats: CGA 219417 Technical. Unpublished report No. 963030 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Classen, W. (1997a) Acute oral neurotoxicity study in rats: CGA 219417 Technical. Unpublished report No. 963032 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Classen, W. (1997b) 90-Day subchronic neurotoxicity study in rats: CGA 219417 Technical. Unpublished report No. 963031 from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Classen, W. (1998) Temperature and neurobehavioral assessment in rats: CGA 219417 Technical. Unpublished report No. 983001 from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Clay, P. (2001) CGA 249287 Tech: L5178Y TK+/- mouse lymphoma mutation assay. Unpublished report No. 20003071 (CTL study No. VV0260, document No. CTL/VV0260/REG/REPT) from Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Deparade, E. (2000) Salmonella and Escherichia/mammalian-microsome mutagenicity test: NOA 422054 technical (metabolite of CGA 219417). Unpublished report No. 20003021 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Deparade, E. (2001) Salmonella and Escherichia/mammalian-microsome mutagenicity test: CGA 275535 technical (metabolite of CGA 219417). Unpublished report No. 20003070 from Syngenta Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Dunn, T.B. (1954) Normal and pathologic anatomy of the reticular tissue in laboratory mice. J. Natl. Cancer Inst., 14, 1281-1432.
Eustis, ? et al. (1990) Liver. In: Boorman et al., ed., Pathology of the Fischer rat. Academic Press.
Fankhauser, H. (1991a) 3-Month range finding toxicity study in mice (administration in food): CGA 219417 technical. Unpublished report No. 891320 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Stein, Switzerland.
Fankhauser, H. (1991b) 3-Month oral toxicity study in rats (administration in food): CGA 219417 technical. Unpublished report No. 891321 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Fankhauser, H. (1991c) 28-Day oral cumulative toxicity study in rats (gavage): CGA 219417 technical. Unpublished report No. 891322 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Fankhauser, H. (1994a) 18-Month carcinogenicity study in mice: CGA 219417 technical. Unpublished report No. 891325 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Fankhauser, H. (1994b) First addendum to the final report: 18-month carcinogenicity study in mice: CGA 219417 technical. Unpublished report No. 891325 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Fankhauser, H. (1994c) 24-months carcinogenicity and chronic toxicity study in rats: CGA 219417 technical. Unpublished report No. 891326 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Fankhauser, H. (1994d) First addendum to the final report: 24-month carcinogenicity and chronic toxicity study in rats: CGA219417 technical. Unpublished report No. 891326 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Frith, C. & Wiley, L. (1981) Morphologic classification and correlation of incidence of hyperplastic and neoplastic haematopoietic lesions in mice with age. J. Gerontol., 36, 534-545.
Geleick, D. (1990) Gene mutation test with Chinese hamster cells V79, in vitro: CGA 219417 technical. Unpublished report No. 901086 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Geleick, D. (1991) Autoradiographic DNA repair test on rat hepatocytes, in vitro: CGA 219417 technical. Unpublished report No. 901083 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hagemann, C. (1991) 28-Day repeated dose dermal toxicity study in the rat: CGA 219417 technical. Unpublished report No. 901078 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hartmann, H.R. (1990a) Acute oral toxicity in the rat: CGA 219417 technical. Unpublished report No. 901075 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hartmann, H.R. (1990b) Acute dermal toxicity in the rat: CGA 219417 technical. Unpublished report No. 901079 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hartmann, H.R. (1991) Acute inhalation toxicity in the rat: CGA 219417 technical. Unpublished report No. 901081 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hartmann, H.R. (1992) Acute oral toxicity in the rat. CGA 249287 technical. Unpublished report No. 923090 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hertner, T. (1992) Salmonella and Escherichia/Liver-microsome test. CGA 249287 technical. Unpublished report No. 923089 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Hirouchi, Y. et al. (1994) Historical data of neoplastic and non-neoplastic lesions in B6C3F1 mice. J. Toxicol. Pathol., 7, 153-177.
Khalil, S. (1993) Two-generation reproduction toxicity study in rats with CGA 219417 technical (dietary administration). Unpublished report No. 891328 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Lebailly, P., Vigreux, C., Lechevrel, C., Ledemeney, D., Godard, T., Sichel, F., LeTalaer, J.Y., Henry-Amar, M., & Gauduchon, P. (1998) DNA damage in mononuclear leukocytes of farmers measured using the alkaline comet assay: modification of DNA damage levels after a one-day field spraying period with selected pesticides. Cancer Epidemiol., Biomarkers Prev., 7, 929-940.
Lorez, C. & Schulze-Rosario, C. (2001) CGA 219417 Cyprodinil (ISO draft). Medical data. Unpublished report from Syngenta Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Marty, J.H. (1991a) Developmental toxicity (teratogenicity) study with CGA 219417 technical in rats: final report. Unpublished report No. 891327 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Marty, J.H. (1991b) Developmental toxicity (teratogenicity) study in rabbits with CGA 219417 Technical (oral administration). Unpublished report No. 891329 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Mewes, K.E. (1999a) Dermal absorption of [phenyl-U-14C] CGA 219417 formulated as switch 62.5 WG (A-9219 B) in the rat. Unpublished report No. 028AM04 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Mewes, K.E. (1999b) The in vitro percutaneous absorption of [phenyl-U-14C] CGA 219417 formulated as switch 62.5 WG (A-9219 B) through rat and human epidermis. Unpublished report No. 028AM05 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Milburn, G.M. (2001) CGA 249287 Tech: 90-day dietary toxicity study in rats. Unpublished study No. PR1218, document No. CTL/PR1218/REG/REPT from Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Muller, T. (1992) The metabolism of [U-14C] phenyl CGA 219417 and [2-14C] pyrimidyl CGA 219417 in the rat. Unpublished report No. 13/92, project No. 03TM01 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Muller, T. (1996) Absorption, distribution, depletion kinetics of [U-14C] phenyl CGA 219417 in the rat after oral administration. Unpublished report No. 3/96, project No. 028AM02 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Muller, T., Thanei, P., Mucke, W., Kriemler, H.P. & Winkler, T. (1999) The sex-specific sulfation of the major metabolite of the novel fungicide cyprodinil in the rat. Pestic. Sci., 55, 566-614.
Ogorek, B. (1990) Salmonella and Escherichia/liver-microsome test: CGA 219417 technical. Unpublished report No. 901084 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Ogorek, B. (1996) Salmonella and Escherichia/mammalian-microsome mutagenicity test: CGA 321915 technical (metabolite of CGA 219417). Unpublished report No. 963139 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Ogorek, B. (2001) Cytogenetic test on Chinese hamster cells in vitro. CGA 249287 technical (metabolite of CGA 219417). Unpublished report No. 20003072 from Syngenta Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Rumbeli, R. (1996) The nature of residues in liver and kidneys of rats after single oral administration of [pyrimidine-2-14C] CGA 219417. Unpublished report No. 16/96, project No. 028AM03 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Schneider, M. (1990a) Acute dermal irritation/corrosion study in the rabbit: CGA 219417 technical. Unpublished report No. 901077 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Schneider, M. (1990b) Acute eye irritation/corrosion study in the rabbit: CGA 219417 technical. Unpublished report No. 901076 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Sommer, E. (2000a) Acute oral toxicity in the rat (limit test): CGA 275535 technical (metabolite of CGA 219417): final report. Unpublished report No. 20003069 from Syngenta Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Sommer, E. (2000b) Acute oral toxicity in the rat (limit test): NOA 422054 technical (metabolite of CGA 219417): final report. Unpublished report No. 20003020 from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Strasser, F. (1991) Cytogenetic test on Chinese hamster cells in vitro: CGA 219417 technical. Unpublished report No. 901085 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Thanei, P. (1992) Absorption, distribution, metabolism, and excretion of [U-14C] phenyl and [2-14C] pyrimidyl CGA 219417 in the rat. Unpublished report No. 1/92, project Nos 08PT01/08PT02/08PT03 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Weber, E. (1997) CGA 219417: Assessment of renal tubular cell proliferation in the course of a two-generation reproduction toxicity study in rats. Unpublished report CB 97/52 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Winkler, G. (1995) Acute oral toxicity in the mouse (limit test): CGA 219417 technical. Unpublished report No. 953025 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Winkler, G. (1996a) Skin sensitisation test in the guinea pig maximisation test: CGA 219417 technical. Unpublished report No. 963087 from Ciba-Geigy Ltd., Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland.
Winkler, G. (1996b) Acute oral toxicity in the rat (limit test): CGA 321915 (metabolite of CGA 219417). Unpublished report No. 963138 from Ciba-Geigy Ltd., Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Stein, Switzerland.
See Also:
Toxicological Abbreviations