
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
EDIFENPHOS
Explanation
Edifenphos was evaluated by the 1976 and 1979 Joint Meetings.* A
temporary ADI was allocated in 1976 and extended in 1979. Temporary
MRLs recommended by the 1976 Meeting were increased in 1979.
Further studies were required on hepatic involvement observed in
several animal species and the results of a carcinogenicity study was
also required. Information was also desired on humans relative to
occupational exposure and on residues of edifenphos and its p-hydroxy
metabolite in food animals arising from the use of rice straw and bran
in animal feeds.
Data were received on the carcinogenicity study in mice, as well
as additional data on residue levels in rice and in animal products,
and are evaluated in this monograph addendum.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Rat and mouse - distribution and excretion
Comparative studies on the metabolic fate of 35S-labelled
edifenphos in rats and mice have been reported (Ueyama et al. 1978).
Dose levels were 10 mg/kg in female rats and 20 mg/kg in mice and male
rats. Edifenphos was rapidly absorbed and metabolized by both species
following oral administration. The major metabolic pathway was the
cleavage of the P-S bond, accompanied by the release of benzene thiol
(Fukami et al. 1969; Ueyama and Takase 1976, Ueyama et al 1978).
Only 15 to 30% of the administered radioactivity was detected in
digestive organs 6 h after administration. At 72 h, only a trace
amount of radioactivity was found over the whole body of the animals.
The major part of the radioactivity was excreted in urine (75-90%) and
faeces (5-20%). The main metabolite in the rat was ethyl hydrogen-S-
phenyl phosphorothiolate (54-59%) and that in mice was dihydrogen-S-
phenyl. Diphenyl disulphide was found in faeces in both species, but
metabolites such methyl phenyl sulphide and its derivatives were
absent.
* See Annex II for FAO and WHO documentation.
Poultry - distribution and excretion
Five groups of 4 chickens each were fed Hinosan at levels of 0,
1.5, 4.5, 15 and 45 ppm in the diet for 28 days. Only slight weight
losses were noted in the two highest feeding levels. Only two giblet
samples from the highest feeding level (45.0 ppm) showed any
measurable residues (0.08 mg/kg). All other tissue samples had gross
residues <0.01 mg/kg; 26-and 28-day egg samples from all the feeding
studies indicated less than 0.001 mg/kg residue levels. No adverse
effects could be detected on egg production for any of the treated
groups compared to the control birds (Morris 1976).
In vitro hepatic subcellular metabolism
The metabolism of 35S-edifenphos by hepatic subcellular
fractions of five mammalian species was studied in vitro in order to
investigate the effect of inhibitors against drug metabolism enzymes
and to observe metabolism and degradation in hepatic microsomes that
were induced by administration with phenobarbital. Additionally,
animal species-related differences of metabolic patterns, degradation
and metabolism of edifenphos in five different species of mammalian
hepatic sub cellular fractions, including humans was examined by the
comparative method (Ueyama et al 1976).
The salient results were as follows: (a) enzymatic
degradation of edifenphos occurred in both microsomal and soluble
(105,000 g-supernatant) fractions; (b) degradation of edifenphos by
microsomes was accelerated by addition of NADPH and inhibited by SKF
525-A or piperonyl butoxide; (c) 35S-edifenphos was more rapidly
metabolized by rat hepatic microsomes that were previously induced by
administration with phenobarbital; (d) the above mentioned results
suggested that a part of enzymatic degradation of edifenphos was
accomplished by drug metabolizing enzymes; (e) the degradation of
35S-edifenphos by the soluble fraction was not increased by addition
of glutathione; (f) the difference of metabolic patterns of edifenphos
by hepatic microsomes of five mammalian species implied that the
metabolic activity for edifenphos was as follows: guinea pig > rabbit
> mouse > human > rat; (g) in the water soluble fractions of these
mammalian microsomes, ethyl hydrogen S-phenyl phosphorothiolate was
found for human and mouse, and dihydrogen S-phenyl phosphorothiolate
was also found for mouse and rabbit.
Antiesterase activity in vitro and in vivo
The in vitro anticholinesterase action of Hinosan was measured
by addition of the compound at several concentrations to homogenized
rat brain in the cholinesterase system. Hinosan was a potent direct
inhibitor of cholinesterase in vitro producing 50% inhibition of
cholinesterase at a concentration of 1.05 × 10-6M (Chen et al
1972).
The effect of Hinosan on cholinesterase activity in vivo was
studied by giving 5/8 of the acute i.p. LD50 to male and female rats.
Because of the sex difference in susceptibility, equitoxic doses
amount to 16 mg/kg for females and 41 mg/kg for males. Hinosan is a
very effective inhibitor in vivo, which gains access to and inhibits
the cholinesterase activity of both the central nervous system and
peripheral tissues and hence produces a generalized cholinergic
action. The rapid onset of action and the extremely slow reversal of
its inhibitory action on cholinesterase are two distinguishing
features of this compound (Chen et al. 1972).
Aliesterase inhibition
Data obtained by feeding Holtzman rats various levels of Hinosan
in the diet (0.75, 5, 12.5, 25, 50, 75 and 100 ppm) for 1 week
demonstrated that Hinosan was a strong inhibitor of aliesterases. The
inhibitory effect of Hinosan on the enzymes that were examined was in
the order of tributyrinase (the most susceptible), diethylsuccinase
(the next most sensitive) and cholinesterase (the most resistant)
(Chen et al 1972). (A similar order of susceptibility was obtained
with several insecticidal organophosphorus compounds, Su et al 1971)
The dietary levels producing 50% inhibition of enzyme activity
were obtained by plotting percent inhibition of the enzyme against the
logarithm of the dietary levels. From this plot of data, 50%
inhibition of hydrolysis of diethylsuccinate by liver and serum were
found to occur at the levels of 11.5 and 7.4 ppm respectively for
female rats, and 18.4 and 10.2 ppm respectively for male rats. The
levels that produced 50% inhibition of tributyrin hydrolysis by liver
and serum were found to be 5.4 and 9.5 ppm respectively for female
rats, and 4.9 and 8.4 ppm respectively for male rats.
In a few experiments, Hinosan was fed at a dietary level of 5 ppm
to young rats (28 days old) for 1 week. There was 60% inhibition of
diethylsuccinate hydrolysis and 70% inhibition of the liver
tributyrinase in young rats as compared with 50% and 50% of the
diethylsuccinase and tributyrinase, respectively, of adult rat liver
at this same dietary level. However, the greater enzyme induction in
the livers of young rats might be due to higher dietary intake,
relative to body weight, than in adults (Chen et al. 1972).
Effects of pre-treatment with microsomal enzyme inducers
Pre-treatment of animals with microsomal enzyme inducers has
been shown to protect against the toxicity of various organophosphorus
compounds. Pre-treatment of rats with phenobarbital, DDT,
3-methylcholanthrene or testosterone markedly reduced the
anticholinesterase action of Hinosan (Chen et al 1972). The amount
of inhibition of cholinesterase of brain and submaxillary gland of
female rats was somewhat less than in male rats after treatment with
the inducing agents. Phenobarbital and DDT were more effective in
protecting against Hinosan toxicity than 3-methylcholanthrene and
testosterone.
Pesticide potentiation
To ascertain the degree of potentiation of malathion toxicity by
Hinosan, groups each containing 4 Holtzman rats were given a sublethal
dose of malathion (400 mg/kg)i.p. after they had been fed for 1 week
with various dietary levels of Hinosan. After feeding dietary levels
of 0, 5 and 25 ppm, the mortality resulting from 400 mg/kg of
malathion was 0, 25 and 100% respectively. Thus, Hinosan was shown
to increase markedly the susceptibility of rats to malathion (Chen
et al 1972).
TOXICOLOGICAL STUDIES
Acute toxicity
Edifenphos is intermediate in toxicity among the organophosphorus
insecticides. A sex difference in the acute toxicity (oral and i.p.)
to rats was observed, with male rats exhibiting greater resistance
than females (see Table 1).
In acute toxicity studies of orally administered edifenphos
conducted on buffalo calves, it was shown that doses of 30, 45 and
60 mg/kg resulted in maximal inhibition of blood cholinesterase to the
extent of 59.3, 71.4 and 73.9% respectively after 12 to 24h of
edifenphos administration (Malik et al 1978a). The onset of severe
toxic symptoms with higher doses (45 and 60 mg/kg) correlated well
with the maximum inhibition of cholinesterase activity. The inhibited
enzyme recovered slowly after 4 weeks, returning to 86.5 and 81.6% of
the pre-treatment level with 30 and 45 mg/kg of edifenphos
respectively. Doses of 45 and 60 mg/kg increased the serum glutamic-
pyruvic transaminase levels (SGPT) while elevation in levels of serum
glutamic-oxalacetic transaminase (SGOT) was found with all the doses,
suggesting that edifenphos can induce internal tissue damage. Doses of
30 and 45 mg/kg resulted in a gradual rise of the SGOT level, which
approached its peak at the second week and began recovering
afterwards, but remained elevated 4 weeks after administration of
edifenphos. No change in serum alkaline phosphatase activity was
observed (Malik et al 1978a).
TABLE 1. Acute toxicity of edifenphos in four animal species
Species Sex Route LD50(mg/kg) Reference
Rat M oral 340 Martin 1972
M oral 212 Bayer 1976
F oral 150 Martin 1972
F oral 100 Bayer 1976
M i.p. 66.5 ± 7.7 Chen et al 1972
F i.p. 25.5 ± 0.8 ibid.
i.p. 26 Melnikov 1971
Mouse M oral 214 Bayer 1976
F oral 218 Martin 1972
Guinea pig oral 3501 Bayer 1976;
Wisswesser 1976
Rabbit oral 3502 ibid; ibid.
1 Technical product in ethanol/propylene glycol
2 Technical product in Lutrol (polyethylene glycol 400).
Short-term studies
Buffalo
Edifenphos was administered orally in daily doses of 4 and
8 mg/kg bw for 28 days to male buffalo calves (Bubalus bubalis). The
lower dose did not produce any apparent toxic manifestation. With the
higher dose all the animals died within 13 to 17 days. A gradual and
significant inhibition of blood cholinesterase was noted with both the
doses (p <0.01). The extent of inhibition of blood cholinesterase was
not related to the severity of toxicosis. No significant change in
levels of blood glucose, blood lactic acid, serum total cholesterol
and serum creatinine was observed after the administration of the drug
(Malik et al 1978a, b).
Edifenphos given at 4 mg/kg/day did not produce toxic symptoms
until 28 days had passed, while doses of 8 mg/kg brought on toxic
symptoms between days 11 and 14. The toxic symptoms included anorexia,
depression, increased salivation, lachrymation and diarrhoea.
Diarrhoea was more pronounced in the later stages and was followed by
weakness of the hind legs and paralysis. Animals receiving 8 mg/kg of
edifenphos showed maximum increases in SGOT levels of 141.73 and
167.77% on day 28 and 12 after the start of the study. The SGOT
elevation was both dose and time dependent. SGPT levels were
increased, but not significantly. No significant changes were noted in
the serum alkaline phosphatase activity throughout the period (Malik
et al 1978c).
Rat
Groups of male and female rats were treated daily for 28 days
with the following dosage regimens: (a) Hinosan, 20 mg/kg; (b)
Hinosan/thiophenol (20:1), 5 and 20 mg/kg and (c) thiophenol, 1 and
10 mg/kg.
Hinosan 20 mg/kg bw as well as Hinosan/thiophenol (20:1), 5 and
20 mg/kg caused a reduction in cholinesterase activity. Doses of 5 mg
of Hinosan/thiophenol 20:1 should be considered a no-effect dosage for
both plasma cholinesterease as well as erythrocyte cholinesterase.
The liver metabolized all three of the above samples similarly,
with no apparent differences. The effects found were: (a) increases in
liver enzymes and liver weight (the effect was more pronounced in male
than in female rats); (b) in the investigated dose levels no liver
damage could be observed. All 3 samples induced definite kidney
damage. In male rats, no minimal safe dose could be established for
Hinosan or Hinosan/thiophenol (20:1 mixture) and thiophenol. A
borderline dose for male rats with thiophenol is 1 mg/kg bw. Female
rats tolerated 1 mg/kg bw thiophenol during the 28-day treatment
without any damage. As a borderline dose for female rats, the
following were considered: Hinosan/thiophenol (20:1 mixture)-5 mg/kg
bw; thiophenol-10 mg/kg bw. The results indicate that the 3 test
samples using the above mentioned dosages could induce kidney damage
and somatic changes in the liver (Thyssen and Schilde 1978).
Long-term studies
Groups of 40 male and 40 female NMRI mice were maintained for 108
weeks on a diet containing SRA 7847 (supplied as a 50% premix) at
dietary concentrations of 2, 15 and 100 ppm, respectively. The control
group also consisted of 40 males and 40 females. Subgroups consisting
of 20 male and 20 female mice each were additionally formed for
haematological and clinical chemical tests. Physical appearance,
behavioural patterns and survival rate of both males and females were
not affected by administration of SRA 7847 even at the highest dietary
level. No differences were seen between treated mice and controls with
respect to growth rate and food consumption. The haematological and
urinalysis data recorded for the treated groups and the controls were
within the physiological range.
Glutamate-pyruvate transaminase (GPT) and alkaline phosphatase
(AP) activities did not show any variations from normal in any of the
groups. Blood plasma and erythrocyte cholinesterase activities in mice
of the 15 and 100 ppm groups showed nonphysiological depression (more
than 20%).
Statistically significantly increased thyroid weights (100 ppm
group) and statistically significantly increased brain weights (15 and
100 ppm groups) were recorded, but only in male mice. A statistically
significantly lower heart weight was additionally noted in the 100 ppm
group.
The number of tumours noted in all groups corresponded to the
known spontaneous tumour rate for the animal strain used in the study.
There was no indication of a dose/incidence relationship. Under the
described experimental conditions SRA 7847 was not carcinogenic.
Although benign pituitary tumours were observed in males of the 2 ppm
group, the incidence was comparable to that in the control group.
Taking account of the cholinesterase activities, the no-effect
dose in the chosen experimental design was stated to be 2 ppm SRA 7847
(Mohr 1980).
Special studies on acute oral toxicity of Hinosan metabolites
Four metabolites, e.g., S-36 [O-ethyl-S-phenyl-S-
(4-hydroxyphenyl)-phosphorodithioate]; S-37 [S,S-diphenyl
phosphorodithioate]; S-39 [O-ethyl-S-phenyl-phosphorothioate] and
S-64 [O-ethyl-S-phenyl-S-(3-hydroxyphenyl)-phosphorodithioate] were
administered in a diluent of Lutrol (polyethylene glycol 400) to
Sprague-Dawley rats fasted for 18 to 20 h. Dose volumes were given at
0.5% body weight. Lethargy, diarrhoea and ataxia were symptoms for
S-39 treated animals. S-64 treated animals showed symptoms of
lethargy, diarrhoea, muscular fasciculations, salivation and
lachrymation. Occurrence and severity of symptoms were dose related.
Although minor lung lesions occurred in a few animals on each
compound, these lesions did not appear to be compound-induced. No
other lesions were noted (Lamb and Matzkanin 1976). Table 2
illustrates the acute toxicity of the 4 metabolites in terms of the
structures of S-36, S-37, S-39 and S-64; doses administered to rats of
both sexes; observations (deaths) symptoms/number of animals exposed;
time of beginning and end of symptoms, time and death and LD50's.
TABLE 2. Acute oral toxicity of Hinosan metabolites to rats1
Observations Symptoms Time of
Dose Deaths/ Begin End Death
Sample Weight (g) Sex Symptoms/No. within within within LD50
(mg/kg) Exposed (h) (h) (h) (mg Samples/kg)
S36: O-ethyl-S-phenyl-S- 270-320 M 500 0/0/5 --- --- --- >500
(4-hydroxyphenyl)-
phosphorodithioate
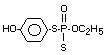 178-240 F 500 0/0/5 --- --- ---
1000 0/0/5 --- --- --- >1000
S37: S,S-diphenyl. 222-377 M 500 0/0/5 --- --- ---
phosphorodithioate 1000 0/5/5 1.5 24 --- >1000; <2000
2000 4/5/5 0.5 48 16
178-240 F 500 0/0/5 --- --- ---
1000 0/0/5 --- --- --- >1000
S37: S,S-diphenyl. 222-377 M 500 0/0/5 --- --- ---
phosphorodithioate 1000 0/5/5 1.5 24 --- >1000; <2000
2000 4/5/5 0.5 48 16
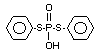 180-253 F 500 0/0/5 --- --- ---
1000 1/5/5 1 24 1.5 >1000; <2000
2000 5/5/5 0.5 --- 2
TABLE 2. (con't)
Observations Symptoms Time of
Dose Deaths/ Begin End Death
Sample Weight (g) Sex Symptoms/No. within within within LD50
(mg/kg) Exposed (h) (h) (h) (mg Samples/kg)
S39: O-ethyl-S-phenyl- 230-364 M 500 0/5/5 1.5 24 ---
phosphorothioate 1000 1/5/5 1.5 24 2 >1000; <2000
2000 4/5/5 0.5 48 2.5
180-253 F 500 0/0/5 --- --- ---
1000 1/5/5 1 24 1.5 >1000; <2000
2000 5/5/5 0.5 --- 2
TABLE 2. (con't)
Observations Symptoms Time of
Dose Deaths/ Begin End Death
Sample Weight (g) Sex Symptoms/No. within within within LD50
(mg/kg) Exposed (h) (h) (h) (mg Samples/kg)
S39: O-ethyl-S-phenyl- 230-364 M 500 0/5/5 1.5 24 ---
phosphorothioate 1000 1/5/5 1.5 24 2 >1000; <2000
2000 4/5/5 0.5 48 2.5
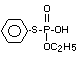 180-242 F 250 0/0/5 --- --- ---
500 2/5/5 1.5 24 2.5 616 (397 to 954)2
1000 4/5/5 1 24 3
2000 5/5/5 0.5 --- 1
S64: O-ethyl-S-phenyl-S- 156-266 M 500 0/5/5 2 24 ---
(3-hydroxyphenyl)- 1000 2/5/5 1 72 16 approx. 1000
phosphorodithioate
180-242 F 250 0/0/5 --- --- ---
500 2/5/5 1.5 24 2.5 616 (397 to 954)2
1000 4/5/5 1 24 3
2000 5/5/5 0.5 --- 1
S64: O-ethyl-S-phenyl-S- 156-266 M 500 0/5/5 2 24 ---
(3-hydroxyphenyl)- 1000 2/5/5 1 72 16 approx. 1000
phosphorodithioate
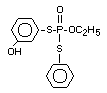 168-198 F 250 0/0/5 --- --- ---
500 2/5/5 2 48 3 approx. 500
1000 4/5/5 2 7 days 24
1 From Lamb and Matzkanin 1976; 2 95% confidence limits.
Special studies on mutagenicity
Edifenphos, tested at a concentration of 1 mg/ml in DMSO with
screening methods consisting of the rec-assay procedure (a sensitivity
test utilizing H17Rec+ and M45Rec- strains of Bacillus subtilis)
as well as the reversion assays on plates utilizing auxotrophic
strains of Escherichia coli (WP 2) and Salmonella typhimurium
strains TA1535 (reversible by base change type mutagens) and TA 1536,
1537 and 1538 (reversible by frameshift mutagens), was found to be
nonmutagenic (Shirasu et al 1976).
In the dominant lethal test, no mutagenic effects were noted when
edifenphos was administered to male mice in an acute oral dose of
100 mg/kg bw (Herbold 1980a).
A micronucleus test was performed with edifenphos according to
the procedure of Schmid. The doses of edifenphos were 2 × 40 mg/kg and
2 × 80 mg/kg bw and for the positive control (Endoxan) 2 × 660 mg/kg
orally administered to mice. No mutagenic effects were noted for
edifenphos at the doses tested (Herbold 1980b).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Rice
Supervised trials were carried out at 3 locations in the USA in
1976. Hinosan 4.5 EC was applied one to four times at rates of 0.63
and 1.26 kg a.i./ha, which are in the recommended dose rate interval
(0.3 to 0.8 kg a.i./ha) or somewhat higher (Mobay 1976). Hinosan DF
and EC formulations were used at supervised trials in Japan. In 7
experiments edifenphos was applied three to four times at rates of
0.45 to 1 kg/ha (Nihon 1979).
Samples were taken 21 to 30 days after the last treatment, with
two exceptions when samples were taken 60 days after the last
application. Rice in husk, hulled rice, polished rice, rice bran,
straw and hulls were analysed separately in most of the experiments.
The limits of determination of analytical methods applied were
0.005 mg/kg in the Japanese experiments and 0.02 mg/kg in the USA
ones.
The intact edifenphos was detected alone in all of the
experiments. The results of these experiments, summarized in Table 3,
showed no difference in the distribution of residues in samples taken
21 to 31 days after the last treatment, independently of the number of
applications or dose rates. Measurable residue (0.005 to 0.16 mg/kg)
was detected in polished rice and the residues in rice in husk were
significantly higher than those found in previous experiments
evaluated by the 1976 Joint Meeting.
TABLE 3. Residues of edifenphos in rice
Sample Dosage Number of samples in residue ranges (mg/kg)
(a.i. kg/ha) <0.02 <0.05 <0.1 <0.2 <0.5 <1 <2 <5 <10
Rice in husk 0.45-0.63 1 1 1 1 1 1
1-1.26 2 2
Rice (bulled) 0.45-0.63 2 1 3
1-1.26 7 1 1 1
Rice (polished) 0.45-0.63 9
1-1.26 10
Rice bran 0.45-0.63 4 1 4 1
1-1.26 3 1 2 2 1
Straw 0.45-0.63 2 3
1-1.26 1 2 2 1 1
Hulls 0.45-0.63 1 2 2 2 1
1-1.26 2 1 1 2 2 1
Soil samples, taken from depths of 0 to 15 and 15 to 30 cm
in the rice field at the same time as the rice samples, contained no
detectable residue (<0.01 mg/kg) in all cases.
FATE OF RESIDUES
In animals
Lactating dairy cows were fed, via bolus, with technical
edifenphos twice daily in equal portions for 28 consecutive days.
Dosages administered to animals were 0.9 mg/kg and 5.7 mg/kg on a dry
feed basis, which were approximately equivalent to 0.03 and 0.17 mg/kg
bw/day respectively.
Residues in milk samples taken 26 to 28 days after the first
treatment were below the limit of determination (<0.001 mg/kg) in all
cases.
In another experiment, edifenphos was mixed in alfalfa meal and
pressed into pellets to provide 5, 15 and 50 mg/kg concentrations in
the feed. The pellets were fed to lactating cows for 28 days (Mobay
1976b). The animals were sacrificed 28 days after first application
and brain, heart, liver, kidney, muscle and fat samples were analysed
for edifenphos. No residue (<0.01 mg/kg) was detectable in any
of the samples with the exception of liver, in which 0.02, 0.07 and
0.13 mg/kg edifenphos was found at the 50 mg/kg feeding level.
Laying hens kept on a diet containing 1.5, 4.5, 15 and 45 mg/kg
technical edifenphos were sacrificed 28 days after consecutive
feeding. Eggs were collected on the 26th and 28th days. Giblet,
muscle, fat, skin and eggs were sampled and analysed for edifenphos.
Two of the three giblet samples contained 0.08 mg/kg residue, but
none was detected in any tissues or in eggs above the limit of
determination, i.e. 0.01 mg/kg in tissues and 0.001 mg/kg in eggs.
EVALUATION
COMMENTS AND APPRAISAL
Satisfactory data were received detailing the results, required
by the 1979 JMPR, of the carcinogenicity study in mice. These
alleviated concern with respect to this aspect of toxicology.
The concerns regarding effects on the liver in experimental
animals previously reported were reconsidered. They were deemed to be
of doubtful significance since they appeared to result from liver
microsomal enzyme induction. Hence an ADI was allocated.
Results of supervised trials, carried out in the USA and Japan,
indicated no difference in the distribution or levels of residues in
samples taken 21 to 31 days post-treatment, independently of the
number of applications or dose rates. These results support the
changes proposed by the 1979 JMPR for rice, in husk, rice, hulled and
rice, polished.
The residue of edifenphos was below the limit of determination in
various tissues (<0.01 mg/kg), milk or eggs (<0.001 mg/kg) of
lactating dairy cows and laying hens fed with feed containing
edifenphos up to 15 mg/kg.
The liver of cattle and poultry giblets were the only samples
containing detectable residues up to 0.13 and 0.08 mg/kg at a diet of
50 and 45 mg edifenphos per kg, respectively, for 28 days. The results
of feeding studies indicate that no detectable residue can be expected
in animal products deriving from animals fed with straw and bran of
rice treated with edifenphos according to good agriculture practice.
Level causing no toxicological effect
Mouse : 10 ppm in the diet equivalent to 1.53 mg/kg bw/day
Rat : 5 ppm in the diet equivalent to 0.25 mg/kg bw/day
Dog : 20 ppm in the diet equivalent to 0.58 mg/kg bw/day
Estimate of acceptable daily intake for man
0 - 0.003 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of the new data the Meeting recommends the following
additional temporary maximum residue limits. The levels refer to the
parent compound only.
Commodity MRL (mg/kg)
Rice bran 1
Milk 0.01 *
Carcass meat of cattle 0.02 *
Cattle meat by-product 0.02 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
Eggs 0.01 *
* Limit of determination.
REFERENCES
Chen, T.S., Kinoshita, F.K. and DuBois, K.P. Acute toxicity and
1972 antiesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan). Toxicology and Applied
Pharmacology, 23:529-527
Fukami, J., Shisido, T. and Fukunaga, F. Abstracts of papers. Annual
1969 Meeting of Agricultural Chemical Society, Tokyo, Japan, p.
149.
Herbold, B. SRA 7847 - Edifenphos. Hinosan-Wirkstoff Dominant-Lethal
1980a Test an der Männ-lichen Maus zur Prüfung auf mutagene
Wirkung, 26. November, Bericht Nr.9575, Bayer AG, Institut
für Toxikologie. Report submitted by Bayer AG to WHO.
(Unpublished)
1980b SRA 7847 - Edifenphos - Hinosan-Wirkstoff Mikronucleus-Test
an der Maus zur Prüfung auf mutagene Wirkung, 26. November,
Bericht Nr. 9576, Bayer AG, Institut für Toxikologie. Report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Matzkanin, C.W. The acute oral toxicity of Hinosan
1976 metabolites to rats, Report No. 48840. Mobay Chemical Corp.,
Agr. Div. Report submitted by Bayer AG to WHO. (Unpublished)
Malik, J.K., Verma, S.P., Gars, B.D. and Ahmad, A. Studies on blood
1978a enzymes during acute toxicity of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan) in buffalo calves, Indian
Journal of Pharmacology, 10: 7-14.
Malik, J.K, Garg, B.D., and Ahmad, A. Experimental subacute toxicity
1978b and anticholinesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate in buffalo calves. Indian Journal of
Animal Science, 48 (5): 358-361.
Malik, J.C., Garg, B.D., Verma, S.P. and Ahmad A. Serum transaminases
1978c and alkaline phosphatase activities during subacute toxicity
of Hinosan in Bubalus bubalis. Indian Journal of
Experimental Biology, 16(4): 497-499.
Martin, H. Pesticide Manual, 3rd Edition, British Crop Protection
1972 Council, Worcester, UK.
Melnikov, N.N. Chemistry of Pesticides, Springer-Verlag, p.375
1971
Mobay. Residue Reports Nos. 48851-48863. (Unpublished)
1976a
Mobay. Residue Reports Nos. 48843. (Unpublished)
1976b
Mobay. Residue Reports Nos. 32357, 32360. (Unpublished)
1972
Mohr, U. Chronic toxicity study on NMRI mice (108-week feeding
1980 experiment) Report No. R1754. Faculty of Medicine, Hannover
University. Report submitted by Bayer AG to WHO.
(Unpublished)
Morris, R.A. Effects of feeding Hinosan to poultry. Report No. 48756.
Mobay Chemical Corp., Agr. Division. Report submitted by
Bayer AG to WHO. (Unpublished)
Nihon. Residue Reports Nos. 699-703, 709, 711. (Unpublished)
1979
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutation Research, 40: 19-30.
Su, M.Q., Konoshita, F.K., Frawley, J.P. and DuBois, K.P. Comparative
1971 inhibition of abesterases and cholinsterase in rats fed
eighteen organophosphorus insecticides. Toxicology and
Applied Pharmacology, 20: 241-249.
Thyssen, J. and Schilde, B. Subakuter oraler Kumulationsversuch an
1978 Ratten mit Hinosan-Workstoff, Hinosan-Workstoff/Thiophenol
(20:11)-Gemisch Bzw. mit Thiophenol. Report No. 7997, Bayer
Institut fur Toxikologie. Report of Bayer AG submitted to
WHO. (Unpublished)
Ueyama, I. and Takase, I. Nitokumo residue analysis report No. 1046
1976 (RA). Report of Nikon Tokusha Noyaku Seizo Co., cited in
reference Ueyama et al 1976. (Unpublished)
Ueyama, I., and Takase, I., Nakazato, Y., and Tomizawa, C. 35S
1976 Edifenphos (ethyl,S,S-diphenylphosphorodithiolate,
HinosanR) Metabolism in five species of mammalian hepatic
subcellular fractions. Report submitted by Nikon Tokushu
Noyaka Seizo Co. to Bayer AG. (Unpublished)
Ueyama, I., Takase, I., and Tomizawa, C. Metabolism of edifenphos
1978 (O-ethyldiphenylphosphorodithiolate) in mouse and rat.
Agricultural and Biological Chemistry, 42(4): 885-887.
Wisswesser, W.J. Pesticide Index, 5th Edition. The Entomological
1976 Society of America, College Park, MD, p. 91
168-198 F 250 0/0/5 --- --- ---
500 2/5/5 2 48 3 approx. 500
1000 4/5/5 2 7 days 24
1 From Lamb and Matzkanin 1976; 2 95% confidence limits.
Special studies on mutagenicity
Edifenphos, tested at a concentration of 1 mg/ml in DMSO with
screening methods consisting of the rec-assay procedure (a sensitivity
test utilizing H17Rec+ and M45Rec- strains of Bacillus subtilis)
as well as the reversion assays on plates utilizing auxotrophic
strains of Escherichia coli (WP 2) and Salmonella typhimurium
strains TA1535 (reversible by base change type mutagens) and TA 1536,
1537 and 1538 (reversible by frameshift mutagens), was found to be
nonmutagenic (Shirasu et al 1976).
In the dominant lethal test, no mutagenic effects were noted when
edifenphos was administered to male mice in an acute oral dose of
100 mg/kg bw (Herbold 1980a).
A micronucleus test was performed with edifenphos according to
the procedure of Schmid. The doses of edifenphos were 2 × 40 mg/kg and
2 × 80 mg/kg bw and for the positive control (Endoxan) 2 × 660 mg/kg
orally administered to mice. No mutagenic effects were noted for
edifenphos at the doses tested (Herbold 1980b).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Rice
Supervised trials were carried out at 3 locations in the USA in
1976. Hinosan 4.5 EC was applied one to four times at rates of 0.63
and 1.26 kg a.i./ha, which are in the recommended dose rate interval
(0.3 to 0.8 kg a.i./ha) or somewhat higher (Mobay 1976). Hinosan DF
and EC formulations were used at supervised trials in Japan. In 7
experiments edifenphos was applied three to four times at rates of
0.45 to 1 kg/ha (Nihon 1979).
Samples were taken 21 to 30 days after the last treatment, with
two exceptions when samples were taken 60 days after the last
application. Rice in husk, hulled rice, polished rice, rice bran,
straw and hulls were analysed separately in most of the experiments.
The limits of determination of analytical methods applied were
0.005 mg/kg in the Japanese experiments and 0.02 mg/kg in the USA
ones.
The intact edifenphos was detected alone in all of the
experiments. The results of these experiments, summarized in Table 3,
showed no difference in the distribution of residues in samples taken
21 to 31 days after the last treatment, independently of the number of
applications or dose rates. Measurable residue (0.005 to 0.16 mg/kg)
was detected in polished rice and the residues in rice in husk were
significantly higher than those found in previous experiments
evaluated by the 1976 Joint Meeting.
TABLE 3. Residues of edifenphos in rice
Sample Dosage Number of samples in residue ranges (mg/kg)
(a.i. kg/ha) <0.02 <0.05 <0.1 <0.2 <0.5 <1 <2 <5 <10
Rice in husk 0.45-0.63 1 1 1 1 1 1
1-1.26 2 2
Rice (bulled) 0.45-0.63 2 1 3
1-1.26 7 1 1 1
Rice (polished) 0.45-0.63 9
1-1.26 10
Rice bran 0.45-0.63 4 1 4 1
1-1.26 3 1 2 2 1
Straw 0.45-0.63 2 3
1-1.26 1 2 2 1 1
Hulls 0.45-0.63 1 2 2 2 1
1-1.26 2 1 1 2 2 1
Soil samples, taken from depths of 0 to 15 and 15 to 30 cm
in the rice field at the same time as the rice samples, contained no
detectable residue (<0.01 mg/kg) in all cases.
FATE OF RESIDUES
In animals
Lactating dairy cows were fed, via bolus, with technical
edifenphos twice daily in equal portions for 28 consecutive days.
Dosages administered to animals were 0.9 mg/kg and 5.7 mg/kg on a dry
feed basis, which were approximately equivalent to 0.03 and 0.17 mg/kg
bw/day respectively.
Residues in milk samples taken 26 to 28 days after the first
treatment were below the limit of determination (<0.001 mg/kg) in all
cases.
In another experiment, edifenphos was mixed in alfalfa meal and
pressed into pellets to provide 5, 15 and 50 mg/kg concentrations in
the feed. The pellets were fed to lactating cows for 28 days (Mobay
1976b). The animals were sacrificed 28 days after first application
and brain, heart, liver, kidney, muscle and fat samples were analysed
for edifenphos. No residue (<0.01 mg/kg) was detectable in any
of the samples with the exception of liver, in which 0.02, 0.07 and
0.13 mg/kg edifenphos was found at the 50 mg/kg feeding level.
Laying hens kept on a diet containing 1.5, 4.5, 15 and 45 mg/kg
technical edifenphos were sacrificed 28 days after consecutive
feeding. Eggs were collected on the 26th and 28th days. Giblet,
muscle, fat, skin and eggs were sampled and analysed for edifenphos.
Two of the three giblet samples contained 0.08 mg/kg residue, but
none was detected in any tissues or in eggs above the limit of
determination, i.e. 0.01 mg/kg in tissues and 0.001 mg/kg in eggs.
EVALUATION
COMMENTS AND APPRAISAL
Satisfactory data were received detailing the results, required
by the 1979 JMPR, of the carcinogenicity study in mice. These
alleviated concern with respect to this aspect of toxicology.
The concerns regarding effects on the liver in experimental
animals previously reported were reconsidered. They were deemed to be
of doubtful significance since they appeared to result from liver
microsomal enzyme induction. Hence an ADI was allocated.
Results of supervised trials, carried out in the USA and Japan,
indicated no difference in the distribution or levels of residues in
samples taken 21 to 31 days post-treatment, independently of the
number of applications or dose rates. These results support the
changes proposed by the 1979 JMPR for rice, in husk, rice, hulled and
rice, polished.
The residue of edifenphos was below the limit of determination in
various tissues (<0.01 mg/kg), milk or eggs (<0.001 mg/kg) of
lactating dairy cows and laying hens fed with feed containing
edifenphos up to 15 mg/kg.
The liver of cattle and poultry giblets were the only samples
containing detectable residues up to 0.13 and 0.08 mg/kg at a diet of
50 and 45 mg edifenphos per kg, respectively, for 28 days. The results
of feeding studies indicate that no detectable residue can be expected
in animal products deriving from animals fed with straw and bran of
rice treated with edifenphos according to good agriculture practice.
Level causing no toxicological effect
Mouse : 10 ppm in the diet equivalent to 1.53 mg/kg bw/day
Rat : 5 ppm in the diet equivalent to 0.25 mg/kg bw/day
Dog : 20 ppm in the diet equivalent to 0.58 mg/kg bw/day
Estimate of acceptable daily intake for man
0 - 0.003 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of the new data the Meeting recommends the following
additional temporary maximum residue limits. The levels refer to the
parent compound only.
Commodity MRL (mg/kg)
Rice bran 1
Milk 0.01 *
Carcass meat of cattle 0.02 *
Cattle meat by-product 0.02 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
Eggs 0.01 *
* Limit of determination.
REFERENCES
Chen, T.S., Kinoshita, F.K. and DuBois, K.P. Acute toxicity and
1972 antiesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan). Toxicology and Applied
Pharmacology, 23:529-527
Fukami, J., Shisido, T. and Fukunaga, F. Abstracts of papers. Annual
1969 Meeting of Agricultural Chemical Society, Tokyo, Japan, p.
149.
Herbold, B. SRA 7847 - Edifenphos. Hinosan-Wirkstoff Dominant-Lethal
1980a Test an der Männ-lichen Maus zur Prüfung auf mutagene
Wirkung, 26. November, Bericht Nr.9575, Bayer AG, Institut
für Toxikologie. Report submitted by Bayer AG to WHO.
(Unpublished)
1980b SRA 7847 - Edifenphos - Hinosan-Wirkstoff Mikronucleus-Test
an der Maus zur Prüfung auf mutagene Wirkung, 26. November,
Bericht Nr. 9576, Bayer AG, Institut für Toxikologie. Report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Matzkanin, C.W. The acute oral toxicity of Hinosan
1976 metabolites to rats, Report No. 48840. Mobay Chemical Corp.,
Agr. Div. Report submitted by Bayer AG to WHO. (Unpublished)
Malik, J.K., Verma, S.P., Gars, B.D. and Ahmad, A. Studies on blood
1978a enzymes during acute toxicity of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan) in buffalo calves, Indian
Journal of Pharmacology, 10: 7-14.
Malik, J.K, Garg, B.D., and Ahmad, A. Experimental subacute toxicity
1978b and anticholinesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate in buffalo calves. Indian Journal of
Animal Science, 48 (5): 358-361.
Malik, J.C., Garg, B.D., Verma, S.P. and Ahmad A. Serum transaminases
1978c and alkaline phosphatase activities during subacute toxicity
of Hinosan in Bubalus bubalis. Indian Journal of
Experimental Biology, 16(4): 497-499.
Martin, H. Pesticide Manual, 3rd Edition, British Crop Protection
1972 Council, Worcester, UK.
Melnikov, N.N. Chemistry of Pesticides, Springer-Verlag, p.375
1971
Mobay. Residue Reports Nos. 48851-48863. (Unpublished)
1976a
Mobay. Residue Reports Nos. 48843. (Unpublished)
1976b
Mobay. Residue Reports Nos. 32357, 32360. (Unpublished)
1972
Mohr, U. Chronic toxicity study on NMRI mice (108-week feeding
1980 experiment) Report No. R1754. Faculty of Medicine, Hannover
University. Report submitted by Bayer AG to WHO.
(Unpublished)
Morris, R.A. Effects of feeding Hinosan to poultry. Report No. 48756.
Mobay Chemical Corp., Agr. Division. Report submitted by
Bayer AG to WHO. (Unpublished)
Nihon. Residue Reports Nos. 699-703, 709, 711. (Unpublished)
1979
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutation Research, 40: 19-30.
Su, M.Q., Konoshita, F.K., Frawley, J.P. and DuBois, K.P. Comparative
1971 inhibition of abesterases and cholinsterase in rats fed
eighteen organophosphorus insecticides. Toxicology and
Applied Pharmacology, 20: 241-249.
Thyssen, J. and Schilde, B. Subakuter oraler Kumulationsversuch an
1978 Ratten mit Hinosan-Workstoff, Hinosan-Workstoff/Thiophenol
(20:11)-Gemisch Bzw. mit Thiophenol. Report No. 7997, Bayer
Institut fur Toxikologie. Report of Bayer AG submitted to
WHO. (Unpublished)
Ueyama, I. and Takase, I. Nitokumo residue analysis report No. 1046
1976 (RA). Report of Nikon Tokusha Noyaku Seizo Co., cited in
reference Ueyama et al 1976. (Unpublished)
Ueyama, I., and Takase, I., Nakazato, Y., and Tomizawa, C. 35S
1976 Edifenphos (ethyl,S,S-diphenylphosphorodithiolate,
HinosanR) Metabolism in five species of mammalian hepatic
subcellular fractions. Report submitted by Nikon Tokushu
Noyaka Seizo Co. to Bayer AG. (Unpublished)
Ueyama, I., Takase, I., and Tomizawa, C. Metabolism of edifenphos
1978 (O-ethyldiphenylphosphorodithiolate) in mouse and rat.
Agricultural and Biological Chemistry, 42(4): 885-887.
Wisswesser, W.J. Pesticide Index, 5th Edition. The Entomological
1976 Society of America, College Park, MD, p. 91
See Also:
Toxicological Abbreviations
Edifenphos (Pesticide residues in food: 1976 evaluations)
Edifenphos (Pesticide residues in food: 1979 evaluations)
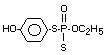 178-240 F 500 0/0/5 --- --- ---
1000 0/0/5 --- --- --- >1000
S37: S,S-diphenyl. 222-377 M 500 0/0/5 --- --- ---
phosphorodithioate 1000 0/5/5 1.5 24 --- >1000; <2000
2000 4/5/5 0.5 48 16
178-240 F 500 0/0/5 --- --- ---
1000 0/0/5 --- --- --- >1000
S37: S,S-diphenyl. 222-377 M 500 0/0/5 --- --- ---
phosphorodithioate 1000 0/5/5 1.5 24 --- >1000; <2000
2000 4/5/5 0.5 48 16
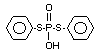 180-253 F 500 0/0/5 --- --- ---
1000 1/5/5 1 24 1.5 >1000; <2000
2000 5/5/5 0.5 --- 2
TABLE 2. (con't)
Observations Symptoms Time of
Dose Deaths/ Begin End Death
Sample Weight (g) Sex Symptoms/No. within within within LD50
(mg/kg) Exposed (h) (h) (h) (mg Samples/kg)
S39: O-ethyl-S-phenyl- 230-364 M 500 0/5/5 1.5 24 ---
phosphorothioate 1000 1/5/5 1.5 24 2 >1000; <2000
2000 4/5/5 0.5 48 2.5
180-253 F 500 0/0/5 --- --- ---
1000 1/5/5 1 24 1.5 >1000; <2000
2000 5/5/5 0.5 --- 2
TABLE 2. (con't)
Observations Symptoms Time of
Dose Deaths/ Begin End Death
Sample Weight (g) Sex Symptoms/No. within within within LD50
(mg/kg) Exposed (h) (h) (h) (mg Samples/kg)
S39: O-ethyl-S-phenyl- 230-364 M 500 0/5/5 1.5 24 ---
phosphorothioate 1000 1/5/5 1.5 24 2 >1000; <2000
2000 4/5/5 0.5 48 2.5
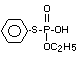 180-242 F 250 0/0/5 --- --- ---
500 2/5/5 1.5 24 2.5 616 (397 to 954)2
1000 4/5/5 1 24 3
2000 5/5/5 0.5 --- 1
S64: O-ethyl-S-phenyl-S- 156-266 M 500 0/5/5 2 24 ---
(3-hydroxyphenyl)- 1000 2/5/5 1 72 16 approx. 1000
phosphorodithioate
180-242 F 250 0/0/5 --- --- ---
500 2/5/5 1.5 24 2.5 616 (397 to 954)2
1000 4/5/5 1 24 3
2000 5/5/5 0.5 --- 1
S64: O-ethyl-S-phenyl-S- 156-266 M 500 0/5/5 2 24 ---
(3-hydroxyphenyl)- 1000 2/5/5 1 72 16 approx. 1000
phosphorodithioate
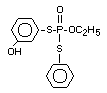 168-198 F 250 0/0/5 --- --- ---
500 2/5/5 2 48 3 approx. 500
1000 4/5/5 2 7 days 24
1 From Lamb and Matzkanin 1976; 2 95% confidence limits.
Special studies on mutagenicity
Edifenphos, tested at a concentration of 1 mg/ml in DMSO with
screening methods consisting of the rec-assay procedure (a sensitivity
test utilizing H17Rec+ and M45Rec- strains of Bacillus subtilis)
as well as the reversion assays on plates utilizing auxotrophic
strains of Escherichia coli (WP 2) and Salmonella typhimurium
strains TA1535 (reversible by base change type mutagens) and TA 1536,
1537 and 1538 (reversible by frameshift mutagens), was found to be
nonmutagenic (Shirasu et al 1976).
In the dominant lethal test, no mutagenic effects were noted when
edifenphos was administered to male mice in an acute oral dose of
100 mg/kg bw (Herbold 1980a).
A micronucleus test was performed with edifenphos according to
the procedure of Schmid. The doses of edifenphos were 2 × 40 mg/kg and
2 × 80 mg/kg bw and for the positive control (Endoxan) 2 × 660 mg/kg
orally administered to mice. No mutagenic effects were noted for
edifenphos at the doses tested (Herbold 1980b).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Rice
Supervised trials were carried out at 3 locations in the USA in
1976. Hinosan 4.5 EC was applied one to four times at rates of 0.63
and 1.26 kg a.i./ha, which are in the recommended dose rate interval
(0.3 to 0.8 kg a.i./ha) or somewhat higher (Mobay 1976). Hinosan DF
and EC formulations were used at supervised trials in Japan. In 7
experiments edifenphos was applied three to four times at rates of
0.45 to 1 kg/ha (Nihon 1979).
Samples were taken 21 to 30 days after the last treatment, with
two exceptions when samples were taken 60 days after the last
application. Rice in husk, hulled rice, polished rice, rice bran,
straw and hulls were analysed separately in most of the experiments.
The limits of determination of analytical methods applied were
0.005 mg/kg in the Japanese experiments and 0.02 mg/kg in the USA
ones.
The intact edifenphos was detected alone in all of the
experiments. The results of these experiments, summarized in Table 3,
showed no difference in the distribution of residues in samples taken
21 to 31 days after the last treatment, independently of the number of
applications or dose rates. Measurable residue (0.005 to 0.16 mg/kg)
was detected in polished rice and the residues in rice in husk were
significantly higher than those found in previous experiments
evaluated by the 1976 Joint Meeting.
TABLE 3. Residues of edifenphos in rice
Sample Dosage Number of samples in residue ranges (mg/kg)
(a.i. kg/ha) <0.02 <0.05 <0.1 <0.2 <0.5 <1 <2 <5 <10
Rice in husk 0.45-0.63 1 1 1 1 1 1
1-1.26 2 2
Rice (bulled) 0.45-0.63 2 1 3
1-1.26 7 1 1 1
Rice (polished) 0.45-0.63 9
1-1.26 10
Rice bran 0.45-0.63 4 1 4 1
1-1.26 3 1 2 2 1
Straw 0.45-0.63 2 3
1-1.26 1 2 2 1 1
Hulls 0.45-0.63 1 2 2 2 1
1-1.26 2 1 1 2 2 1
Soil samples, taken from depths of 0 to 15 and 15 to 30 cm
in the rice field at the same time as the rice samples, contained no
detectable residue (<0.01 mg/kg) in all cases.
FATE OF RESIDUES
In animals
Lactating dairy cows were fed, via bolus, with technical
edifenphos twice daily in equal portions for 28 consecutive days.
Dosages administered to animals were 0.9 mg/kg and 5.7 mg/kg on a dry
feed basis, which were approximately equivalent to 0.03 and 0.17 mg/kg
bw/day respectively.
Residues in milk samples taken 26 to 28 days after the first
treatment were below the limit of determination (<0.001 mg/kg) in all
cases.
In another experiment, edifenphos was mixed in alfalfa meal and
pressed into pellets to provide 5, 15 and 50 mg/kg concentrations in
the feed. The pellets were fed to lactating cows for 28 days (Mobay
1976b). The animals were sacrificed 28 days after first application
and brain, heart, liver, kidney, muscle and fat samples were analysed
for edifenphos. No residue (<0.01 mg/kg) was detectable in any
of the samples with the exception of liver, in which 0.02, 0.07 and
0.13 mg/kg edifenphos was found at the 50 mg/kg feeding level.
Laying hens kept on a diet containing 1.5, 4.5, 15 and 45 mg/kg
technical edifenphos were sacrificed 28 days after consecutive
feeding. Eggs were collected on the 26th and 28th days. Giblet,
muscle, fat, skin and eggs were sampled and analysed for edifenphos.
Two of the three giblet samples contained 0.08 mg/kg residue, but
none was detected in any tissues or in eggs above the limit of
determination, i.e. 0.01 mg/kg in tissues and 0.001 mg/kg in eggs.
EVALUATION
COMMENTS AND APPRAISAL
Satisfactory data were received detailing the results, required
by the 1979 JMPR, of the carcinogenicity study in mice. These
alleviated concern with respect to this aspect of toxicology.
The concerns regarding effects on the liver in experimental
animals previously reported were reconsidered. They were deemed to be
of doubtful significance since they appeared to result from liver
microsomal enzyme induction. Hence an ADI was allocated.
Results of supervised trials, carried out in the USA and Japan,
indicated no difference in the distribution or levels of residues in
samples taken 21 to 31 days post-treatment, independently of the
number of applications or dose rates. These results support the
changes proposed by the 1979 JMPR for rice, in husk, rice, hulled and
rice, polished.
The residue of edifenphos was below the limit of determination in
various tissues (<0.01 mg/kg), milk or eggs (<0.001 mg/kg) of
lactating dairy cows and laying hens fed with feed containing
edifenphos up to 15 mg/kg.
The liver of cattle and poultry giblets were the only samples
containing detectable residues up to 0.13 and 0.08 mg/kg at a diet of
50 and 45 mg edifenphos per kg, respectively, for 28 days. The results
of feeding studies indicate that no detectable residue can be expected
in animal products deriving from animals fed with straw and bran of
rice treated with edifenphos according to good agriculture practice.
Level causing no toxicological effect
Mouse : 10 ppm in the diet equivalent to 1.53 mg/kg bw/day
Rat : 5 ppm in the diet equivalent to 0.25 mg/kg bw/day
Dog : 20 ppm in the diet equivalent to 0.58 mg/kg bw/day
Estimate of acceptable daily intake for man
0 - 0.003 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of the new data the Meeting recommends the following
additional temporary maximum residue limits. The levels refer to the
parent compound only.
Commodity MRL (mg/kg)
Rice bran 1
Milk 0.01 *
Carcass meat of cattle 0.02 *
Cattle meat by-product 0.02 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
Eggs 0.01 *
* Limit of determination.
REFERENCES
Chen, T.S., Kinoshita, F.K. and DuBois, K.P. Acute toxicity and
1972 antiesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan). Toxicology and Applied
Pharmacology, 23:529-527
Fukami, J., Shisido, T. and Fukunaga, F. Abstracts of papers. Annual
1969 Meeting of Agricultural Chemical Society, Tokyo, Japan, p.
149.
Herbold, B. SRA 7847 - Edifenphos. Hinosan-Wirkstoff Dominant-Lethal
1980a Test an der Männ-lichen Maus zur Prüfung auf mutagene
Wirkung, 26. November, Bericht Nr.9575, Bayer AG, Institut
für Toxikologie. Report submitted by Bayer AG to WHO.
(Unpublished)
1980b SRA 7847 - Edifenphos - Hinosan-Wirkstoff Mikronucleus-Test
an der Maus zur Prüfung auf mutagene Wirkung, 26. November,
Bericht Nr. 9576, Bayer AG, Institut für Toxikologie. Report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Matzkanin, C.W. The acute oral toxicity of Hinosan
1976 metabolites to rats, Report No. 48840. Mobay Chemical Corp.,
Agr. Div. Report submitted by Bayer AG to WHO. (Unpublished)
Malik, J.K., Verma, S.P., Gars, B.D. and Ahmad, A. Studies on blood
1978a enzymes during acute toxicity of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan) in buffalo calves, Indian
Journal of Pharmacology, 10: 7-14.
Malik, J.K, Garg, B.D., and Ahmad, A. Experimental subacute toxicity
1978b and anticholinesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate in buffalo calves. Indian Journal of
Animal Science, 48 (5): 358-361.
Malik, J.C., Garg, B.D., Verma, S.P. and Ahmad A. Serum transaminases
1978c and alkaline phosphatase activities during subacute toxicity
of Hinosan in Bubalus bubalis. Indian Journal of
Experimental Biology, 16(4): 497-499.
Martin, H. Pesticide Manual, 3rd Edition, British Crop Protection
1972 Council, Worcester, UK.
Melnikov, N.N. Chemistry of Pesticides, Springer-Verlag, p.375
1971
Mobay. Residue Reports Nos. 48851-48863. (Unpublished)
1976a
Mobay. Residue Reports Nos. 48843. (Unpublished)
1976b
Mobay. Residue Reports Nos. 32357, 32360. (Unpublished)
1972
Mohr, U. Chronic toxicity study on NMRI mice (108-week feeding
1980 experiment) Report No. R1754. Faculty of Medicine, Hannover
University. Report submitted by Bayer AG to WHO.
(Unpublished)
Morris, R.A. Effects of feeding Hinosan to poultry. Report No. 48756.
Mobay Chemical Corp., Agr. Division. Report submitted by
Bayer AG to WHO. (Unpublished)
Nihon. Residue Reports Nos. 699-703, 709, 711. (Unpublished)
1979
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutation Research, 40: 19-30.
Su, M.Q., Konoshita, F.K., Frawley, J.P. and DuBois, K.P. Comparative
1971 inhibition of abesterases and cholinsterase in rats fed
eighteen organophosphorus insecticides. Toxicology and
Applied Pharmacology, 20: 241-249.
Thyssen, J. and Schilde, B. Subakuter oraler Kumulationsversuch an
1978 Ratten mit Hinosan-Workstoff, Hinosan-Workstoff/Thiophenol
(20:11)-Gemisch Bzw. mit Thiophenol. Report No. 7997, Bayer
Institut fur Toxikologie. Report of Bayer AG submitted to
WHO. (Unpublished)
Ueyama, I. and Takase, I. Nitokumo residue analysis report No. 1046
1976 (RA). Report of Nikon Tokusha Noyaku Seizo Co., cited in
reference Ueyama et al 1976. (Unpublished)
Ueyama, I., and Takase, I., Nakazato, Y., and Tomizawa, C. 35S
1976 Edifenphos (ethyl,S,S-diphenylphosphorodithiolate,
HinosanR) Metabolism in five species of mammalian hepatic
subcellular fractions. Report submitted by Nikon Tokushu
Noyaka Seizo Co. to Bayer AG. (Unpublished)
Ueyama, I., Takase, I., and Tomizawa, C. Metabolism of edifenphos
1978 (O-ethyldiphenylphosphorodithiolate) in mouse and rat.
Agricultural and Biological Chemistry, 42(4): 885-887.
Wisswesser, W.J. Pesticide Index, 5th Edition. The Entomological
1976 Society of America, College Park, MD, p. 91
168-198 F 250 0/0/5 --- --- ---
500 2/5/5 2 48 3 approx. 500
1000 4/5/5 2 7 days 24
1 From Lamb and Matzkanin 1976; 2 95% confidence limits.
Special studies on mutagenicity
Edifenphos, tested at a concentration of 1 mg/ml in DMSO with
screening methods consisting of the rec-assay procedure (a sensitivity
test utilizing H17Rec+ and M45Rec- strains of Bacillus subtilis)
as well as the reversion assays on plates utilizing auxotrophic
strains of Escherichia coli (WP 2) and Salmonella typhimurium
strains TA1535 (reversible by base change type mutagens) and TA 1536,
1537 and 1538 (reversible by frameshift mutagens), was found to be
nonmutagenic (Shirasu et al 1976).
In the dominant lethal test, no mutagenic effects were noted when
edifenphos was administered to male mice in an acute oral dose of
100 mg/kg bw (Herbold 1980a).
A micronucleus test was performed with edifenphos according to
the procedure of Schmid. The doses of edifenphos were 2 × 40 mg/kg and
2 × 80 mg/kg bw and for the positive control (Endoxan) 2 × 660 mg/kg
orally administered to mice. No mutagenic effects were noted for
edifenphos at the doses tested (Herbold 1980b).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Rice
Supervised trials were carried out at 3 locations in the USA in
1976. Hinosan 4.5 EC was applied one to four times at rates of 0.63
and 1.26 kg a.i./ha, which are in the recommended dose rate interval
(0.3 to 0.8 kg a.i./ha) or somewhat higher (Mobay 1976). Hinosan DF
and EC formulations were used at supervised trials in Japan. In 7
experiments edifenphos was applied three to four times at rates of
0.45 to 1 kg/ha (Nihon 1979).
Samples were taken 21 to 30 days after the last treatment, with
two exceptions when samples were taken 60 days after the last
application. Rice in husk, hulled rice, polished rice, rice bran,
straw and hulls were analysed separately in most of the experiments.
The limits of determination of analytical methods applied were
0.005 mg/kg in the Japanese experiments and 0.02 mg/kg in the USA
ones.
The intact edifenphos was detected alone in all of the
experiments. The results of these experiments, summarized in Table 3,
showed no difference in the distribution of residues in samples taken
21 to 31 days after the last treatment, independently of the number of
applications or dose rates. Measurable residue (0.005 to 0.16 mg/kg)
was detected in polished rice and the residues in rice in husk were
significantly higher than those found in previous experiments
evaluated by the 1976 Joint Meeting.
TABLE 3. Residues of edifenphos in rice
Sample Dosage Number of samples in residue ranges (mg/kg)
(a.i. kg/ha) <0.02 <0.05 <0.1 <0.2 <0.5 <1 <2 <5 <10
Rice in husk 0.45-0.63 1 1 1 1 1 1
1-1.26 2 2
Rice (bulled) 0.45-0.63 2 1 3
1-1.26 7 1 1 1
Rice (polished) 0.45-0.63 9
1-1.26 10
Rice bran 0.45-0.63 4 1 4 1
1-1.26 3 1 2 2 1
Straw 0.45-0.63 2 3
1-1.26 1 2 2 1 1
Hulls 0.45-0.63 1 2 2 2 1
1-1.26 2 1 1 2 2 1
Soil samples, taken from depths of 0 to 15 and 15 to 30 cm
in the rice field at the same time as the rice samples, contained no
detectable residue (<0.01 mg/kg) in all cases.
FATE OF RESIDUES
In animals
Lactating dairy cows were fed, via bolus, with technical
edifenphos twice daily in equal portions for 28 consecutive days.
Dosages administered to animals were 0.9 mg/kg and 5.7 mg/kg on a dry
feed basis, which were approximately equivalent to 0.03 and 0.17 mg/kg
bw/day respectively.
Residues in milk samples taken 26 to 28 days after the first
treatment were below the limit of determination (<0.001 mg/kg) in all
cases.
In another experiment, edifenphos was mixed in alfalfa meal and
pressed into pellets to provide 5, 15 and 50 mg/kg concentrations in
the feed. The pellets were fed to lactating cows for 28 days (Mobay
1976b). The animals were sacrificed 28 days after first application
and brain, heart, liver, kidney, muscle and fat samples were analysed
for edifenphos. No residue (<0.01 mg/kg) was detectable in any
of the samples with the exception of liver, in which 0.02, 0.07 and
0.13 mg/kg edifenphos was found at the 50 mg/kg feeding level.
Laying hens kept on a diet containing 1.5, 4.5, 15 and 45 mg/kg
technical edifenphos were sacrificed 28 days after consecutive
feeding. Eggs were collected on the 26th and 28th days. Giblet,
muscle, fat, skin and eggs were sampled and analysed for edifenphos.
Two of the three giblet samples contained 0.08 mg/kg residue, but
none was detected in any tissues or in eggs above the limit of
determination, i.e. 0.01 mg/kg in tissues and 0.001 mg/kg in eggs.
EVALUATION
COMMENTS AND APPRAISAL
Satisfactory data were received detailing the results, required
by the 1979 JMPR, of the carcinogenicity study in mice. These
alleviated concern with respect to this aspect of toxicology.
The concerns regarding effects on the liver in experimental
animals previously reported were reconsidered. They were deemed to be
of doubtful significance since they appeared to result from liver
microsomal enzyme induction. Hence an ADI was allocated.
Results of supervised trials, carried out in the USA and Japan,
indicated no difference in the distribution or levels of residues in
samples taken 21 to 31 days post-treatment, independently of the
number of applications or dose rates. These results support the
changes proposed by the 1979 JMPR for rice, in husk, rice, hulled and
rice, polished.
The residue of edifenphos was below the limit of determination in
various tissues (<0.01 mg/kg), milk or eggs (<0.001 mg/kg) of
lactating dairy cows and laying hens fed with feed containing
edifenphos up to 15 mg/kg.
The liver of cattle and poultry giblets were the only samples
containing detectable residues up to 0.13 and 0.08 mg/kg at a diet of
50 and 45 mg edifenphos per kg, respectively, for 28 days. The results
of feeding studies indicate that no detectable residue can be expected
in animal products deriving from animals fed with straw and bran of
rice treated with edifenphos according to good agriculture practice.
Level causing no toxicological effect
Mouse : 10 ppm in the diet equivalent to 1.53 mg/kg bw/day
Rat : 5 ppm in the diet equivalent to 0.25 mg/kg bw/day
Dog : 20 ppm in the diet equivalent to 0.58 mg/kg bw/day
Estimate of acceptable daily intake for man
0 - 0.003 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of the new data the Meeting recommends the following
additional temporary maximum residue limits. The levels refer to the
parent compound only.
Commodity MRL (mg/kg)
Rice bran 1
Milk 0.01 *
Carcass meat of cattle 0.02 *
Cattle meat by-product 0.02 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
Eggs 0.01 *
* Limit of determination.
REFERENCES
Chen, T.S., Kinoshita, F.K. and DuBois, K.P. Acute toxicity and
1972 antiesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan). Toxicology and Applied
Pharmacology, 23:529-527
Fukami, J., Shisido, T. and Fukunaga, F. Abstracts of papers. Annual
1969 Meeting of Agricultural Chemical Society, Tokyo, Japan, p.
149.
Herbold, B. SRA 7847 - Edifenphos. Hinosan-Wirkstoff Dominant-Lethal
1980a Test an der Männ-lichen Maus zur Prüfung auf mutagene
Wirkung, 26. November, Bericht Nr.9575, Bayer AG, Institut
für Toxikologie. Report submitted by Bayer AG to WHO.
(Unpublished)
1980b SRA 7847 - Edifenphos - Hinosan-Wirkstoff Mikronucleus-Test
an der Maus zur Prüfung auf mutagene Wirkung, 26. November,
Bericht Nr. 9576, Bayer AG, Institut für Toxikologie. Report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Matzkanin, C.W. The acute oral toxicity of Hinosan
1976 metabolites to rats, Report No. 48840. Mobay Chemical Corp.,
Agr. Div. Report submitted by Bayer AG to WHO. (Unpublished)
Malik, J.K., Verma, S.P., Gars, B.D. and Ahmad, A. Studies on blood
1978a enzymes during acute toxicity of O-ethyl-S,S-diphenyl
phosphorodithioate (Hinosan) in buffalo calves, Indian
Journal of Pharmacology, 10: 7-14.
Malik, J.K, Garg, B.D., and Ahmad, A. Experimental subacute toxicity
1978b and anticholinesterase action of O-ethyl-S,S-diphenyl
phosphorodithioate in buffalo calves. Indian Journal of
Animal Science, 48 (5): 358-361.
Malik, J.C., Garg, B.D., Verma, S.P. and Ahmad A. Serum transaminases
1978c and alkaline phosphatase activities during subacute toxicity
of Hinosan in Bubalus bubalis. Indian Journal of
Experimental Biology, 16(4): 497-499.
Martin, H. Pesticide Manual, 3rd Edition, British Crop Protection
1972 Council, Worcester, UK.
Melnikov, N.N. Chemistry of Pesticides, Springer-Verlag, p.375
1971
Mobay. Residue Reports Nos. 48851-48863. (Unpublished)
1976a
Mobay. Residue Reports Nos. 48843. (Unpublished)
1976b
Mobay. Residue Reports Nos. 32357, 32360. (Unpublished)
1972
Mohr, U. Chronic toxicity study on NMRI mice (108-week feeding
1980 experiment) Report No. R1754. Faculty of Medicine, Hannover
University. Report submitted by Bayer AG to WHO.
(Unpublished)
Morris, R.A. Effects of feeding Hinosan to poultry. Report No. 48756.
Mobay Chemical Corp., Agr. Division. Report submitted by
Bayer AG to WHO. (Unpublished)
Nihon. Residue Reports Nos. 699-703, 709, 711. (Unpublished)
1979
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutation Research, 40: 19-30.
Su, M.Q., Konoshita, F.K., Frawley, J.P. and DuBois, K.P. Comparative
1971 inhibition of abesterases and cholinsterase in rats fed
eighteen organophosphorus insecticides. Toxicology and
Applied Pharmacology, 20: 241-249.
Thyssen, J. and Schilde, B. Subakuter oraler Kumulationsversuch an
1978 Ratten mit Hinosan-Workstoff, Hinosan-Workstoff/Thiophenol
(20:11)-Gemisch Bzw. mit Thiophenol. Report No. 7997, Bayer
Institut fur Toxikologie. Report of Bayer AG submitted to
WHO. (Unpublished)
Ueyama, I. and Takase, I. Nitokumo residue analysis report No. 1046
1976 (RA). Report of Nikon Tokusha Noyaku Seizo Co., cited in
reference Ueyama et al 1976. (Unpublished)
Ueyama, I., and Takase, I., Nakazato, Y., and Tomizawa, C. 35S
1976 Edifenphos (ethyl,S,S-diphenylphosphorodithiolate,
HinosanR) Metabolism in five species of mammalian hepatic
subcellular fractions. Report submitted by Nikon Tokushu
Noyaka Seizo Co. to Bayer AG. (Unpublished)
Ueyama, I., Takase, I., and Tomizawa, C. Metabolism of edifenphos
1978 (O-ethyldiphenylphosphorodithiolate) in mouse and rat.
Agricultural and Biological Chemistry, 42(4): 885-887.
Wisswesser, W.J. Pesticide Index, 5th Edition. The Entomological
1976 Society of America, College Park, MD, p. 91
