
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
CAPTAN
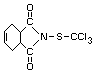 Explanation
This fungicide was evaluated by the Joint Meetings in 1965, 1969,
1973, 1974, 1977 and 1978 (FAO/WHO 1965b, 1970b, 1974b, 1975b, 1978b
and 1979).1/ A full ADI of 0-0.1 mg/kg body weight was established on
the basis of the no-effect levels partially taken from data reported
by Industrial Bio-Test Laboratories (IBT). The latter include
long-term/carcinogenicity studies in mice and rats, a three-generation
reproduction in rats and teratology studies in rabbits, dogs and
monkeys.
New studies have become available and are summarized and
discussed in this monograph addendum. In addition, the major concerns
raised in a Rebuttable Presumption Against Registration (RPAR) issued
in the U.S. for captan in August 1980 relating to oncogenic and
mutagenic effects have been considered in this review of new data.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
In normal, sham-operated and partially hepatectomized male rats,
the rate of elimination of a single dose or three daily doses of
(35S)-captan, at 6 mg/kg body weight given intraperitoneally, was
similar. Approximately 77-89% of the total administered radioactivity
was eliminated within 72 hours, of the single or multiple dosing
primarily in the urine but also in the faeces. The pattern of
distribution of 35S-residues in tissues from these rats, sacrificed
75 hours after the single dose or 24 hours after the third daily dose,
was also essentially the same with the highest concentration being
detected in the blood and the lowest in brain. Additionally, nuclei
isolated from liver of rats after treatment with three daily
intraperitoneal doses of (35S)-captan contained appreciable amounts
of radioactivity. There were no differences in normal, sham-operated
or partially hepatectomized animals (Couch et al 1977).
1/ See Annex 2 for WHO and FAO documentation.
Effects on Other Biochemical Parameters
Incubation of isolated liver nuclei from normal rats with captan
for 10 minutes at 37°C resulted in changes in distribution of various
protein fractions. One of the major changes was extensive aggregation
of nuclear sap proteins and deoxyribonucleoprotein. Binding studies
with isolated rat liver nuclei indicated evolution as gas(es) of a
large percentage of the total radioactivity from (35S)-captan,
suggesting occurrence of an interaction of captan with thiol groups in
the nucleus with subsequent release of the SCCl3 moiety. Following
incubation with 35S-captan, the rate of 35S binding and the
percentage of 35S-labelled gas evolved were slightly higher in
isolated liver nuclei from normal rats than from partially
hepatectomized rats (Couch et al 1977).
In adult Swiss Webster female mice fed technical captan in their
diet at levels ranging from 4 000 to 16 000 ppm for up to 35 days,
soluble thiol levels in the duodenum from the proximal end were
elevated throughout the feeding period. The increase, not strictly
dose-dependent, was already evident on day 1 and peaked between 14 and
21 days following initiation of the study. The liver from these
animals exhibited normal or slightly depressed levels of soluble
thiols. Results from this experiment were confirmed by those of a
second test with groups of female mice of the same strain fed dietary
levels of captan ranging from 500 to 16 000 ppm for up to 213 days. In
the latter experiment, a dose-response relationship was noted with
respect to levels of duodeneal soluble thiols (Miaullis et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of 15 male and 30 female rats (Charles River COBS CD
strain, 31 days old) were fed technical captan in their diet at the
equivalent of 0, 25, 100, 250 or 500 mg/kg body weight/day for 102
days and then mated to initiate a three-generation reproduction study.
The procedure normally used in a standard three-generation (two
litters/generation) study was followed but an additional litter, F2c,
was produced from F1 parents for teratological evaluation. Pups of
the second litters (F1b and F2b) became parents of the next
generation. After the third mating trial, F1 dams were sacrificed on
gestation day 19 and the F2c foetuses were removed by caesarean
section for examination of external, skeletal and soft tissue
abnormalities. In the parental generations, no compound-related effect
on mortality, appearance and behaviour was apparent. Dose-related
growth depression occurred in both sexes at and above 100 mg/kg body
weight/day throughout all three generations and in F0 and F2 males
even at 25 mg/kg body weight/day. Food consumption was decreased at
all dosage levels in a dose-dependent trend in all three generations.
A reduction in pregnancy rate was observed in F0 generation (first
mating trial) at 500 mg/kg body weight/day and possibly also at
250 mg/kg body weight/day. In the progeny generations litter size at
birth was reduced in all treated groups in F1a and F1b litters and at
100 mg/kg body weight/day and above in F2a and F2b litters. Pup
survival was adversely affected at 250 mg/kg body weight/day in F1a,
F2a and F3a litters throughout lactation day 4 and at 500 mg/kg body
weight practically throughout the lactation period in all progeny
generations. Dose-related depression in pup weight was seen at and
above 100 mg/kg body weight/day in litters of all three generations
and at 25 mg/kg body weight/day in most litters. Weanling pups in each
generation exhibited no compound-related abnormalities at necropsy.
Teratological evaluation of the F2c litter revealed no effect
attributable to treatment with respect to fertility, number of non-
viable foetuses, early and late resorptions and post-implantation loss
per dam, sex ratio and incidence of foetuses or litters with
malformations. Foetal body weight was depressed at 500 mg/kg body
weight/day. There was a slight decrease in the mean number of viable
foetuses per dam and a corresponding decrease in total implantations
at 250 mg/kg body weight/day and above. The number of corpora lutea
per dam was reduced at 500 mg/kg body weight/day.
The study failed to demonstrate a no-effect level on
reproduction, although teratogenic effect was not evident from data on
the F2c litter (International Research and Development 1982a).
A preliminary report of a one-generation, one litter reproduction
study in rats (Charles River CD) with groups of 15 males and 30
females fed captan-treated diets for 100 days prior to mating
indicated that "minor" effects on litter weight, noted at 25 mg/kg
body weight/day, were not apparent at 6 or 12.5 mg/kg body weight/day.
No other reproductive effects were reportedly noted (International
Research and Development 1982b).
Special Studies on Teratogenicity
Mouse
According to results reported in an abstract, pregnant CD-1
mice exposed via inhalation to captan (whole body exposure) at
approximately 488 mg/hour/m3 (average particle size 5 µm), 4
hours/day from day 6 through 13 of gestation produced no malformed
foetuses. Similarly, captan was not teratogenic in pregnant CD-1 mice
treated with 100 mg/kg body weight/day of the compound orally or
subcutaneously from day 6 through 15 of gestation. Reduction of
maternal body weight and a slight increase in foetal mortality were
noted in the experiments (Courtney et al 1978).
Hamster
In a pilot (range-finding) teratology study with mated female
(Golden Syrian) hamsters given oral doses of technical captan at 50,
100, 200, 300 or 400 mg/kg body weight/day on day 5 through 10 of
gestation, there was no maternal death or treatment-related effect on
appearance and behaviour, number of corpora lutea, implantations or
resorptions and litter size. The only adverse effect observed was a
dose-related decrease in maternal weight gain at 300 mg/kg body
weight/day and above (Goldenthal 1978).
Groups of 30 mated female Golden Syrian hamsters were given
technical captan in 1% carboxy methylcellulose by gavage at 0, 50, 200
or 400 mg/kg body weight/day on days 5 through 10 of gestation. The
females were sacrificed on day 14 of gestation and foetuses were
removed by caesarean section for external, soft tissue and skeletal
examination. Four females at 400 mg/kg body weight/day and one female
at 200 mg/kg body weight/day died of undetermined cause(s). Stained
and matted anogenital fur and vaginal discharge were noted at
400 mg/kg body weight/day. Other adverse effects at 400 mg/kg body
weight/ day included maternal weight loss, a reduction in litter size
and foetal weight and an increase in early and late resorptions.
Additionally, increased post-implantation loss and a significant
difference in sex (M/F) ratio occurred in this dosage group. Maternal
weight gain and foetal weight were also depressed at 200 mg/kg body
weight/day. In general the incidence of malformations appeared to be
comparable in all groups. However, the incidence of non-specified rib
anomalies in foetuses was 0.3, 1.4, 1.8, 2.9%, respectively, at 0, 50,
200 and 400 mg/kg body weight/day and in litters was 3.6, 15.4, 15.4
and 8.7% (Goldenthal et al 1979).
Rabbit
Groups of 15 mated female New Zealand White rabbits were
intubated with technical captan (89% pure) in 0.5% sodium
carboxymethyl cellulose at 0, 6, 12, 25 or 60 mg/kg body weight/day on
days 6-28 inclusive of pregnancy. Does were sacrificed on day 29 of
pregnancy and uterine contents were examined. Foetuses were evaluated
for gross, skeletal and visceral abnormalities. Pregnancy rate at
60 mg/kg body weight/day and maternal body weight at and above
12 mg/kg body weight/day were adversely affected. Incidence of non-
specific signs (anorexia, reduced faecal output and water consumption)
was increased at 60 mg/kg body weight/day and possibly also at
25 mg/kg body weight/day. Litter and foetal weights as well as foetal
crown/rump length were depressed at 60 mg/kg body weight/ day. There
were no compound-related effects on the other tested parameters, i.e.
mortality, abortion rate, total resorptions, pre-implantation loss and
post-implantation loss including stillbirths), total litter loss in
pregnant animals surviving to autopsy, litter size, sex ratio and
incidence of major malformations or minor anomalies (Palmer
et al 1981).
Special Studies for Carcinogenicity
Mouse
Groups of 50 male and 50 female mice (B6C 3F1 hybrid; 35-day old)
were fed captan of unspecified purity in their diet at 8 000 or
16 000 ppm for 80 weeks and then maintained on a control diet for 11
weeks. The control group comprised 10 mice of each sex. The animals
were placed in the same room as groups of mice receiving aldrin or
photodieldrin and their controls. Pooled controls, used for
statistical comparisons, consisted of a total of 80 male and 80 female
mice of the same strain from seven similar bio-assays (including the
one with captan). Survival was good and no dose-related effect on
mortality was evident. Growth was depressed at 16 000 ppm throughout
most of the study. The total incidence of tumours was low in control
and treated groups. However, adenomatous polyp of the duodenum was
noted in 0/9 males and 1/9 female controls, 2/43 males and 1/49
females at 8 000 ppm and 2/46 males and 0/49 females at 16 000 ppm. In
addition, polypoid adenocarcinoma of the duodenum occurred in 1/43
males and 0/49 females at 8 000 ppm and 3/46 males and 3/49 females at
16 000 ppm, but in none of the concurrent controls (hyperplasia of the
gastric mucosa was present in 3/46 males at 16 000 ppm). In males, the
incidence of either adenomatous polyps or polypoid adenocarcinoma
alone was not statistically significant, but when the two lesions,
usually rare in occurrence, were considered together, the incidence at
16 000 ppm was statistically significant, as compared to the pooled
control group. In females, incidence of the duodenal lesions in
question, considered alone or together, was not statistically
significant. There was, however, a statistically significant dose-
related trend with respect to incidences of both duodenal lesions in
males and females. The conclusion was made in the report that, under
the conditions of the bioassay, tumours in the duodenum were
associated with treatment with captan (NCI 1977).
Rat
Groups of 50 rats (Osborne-Mendel, 35 days old) of each sex were
fed a diet containing captan (purity not specified) at time-weighted
average doses of 2 525 or 6 050 ppm. The low dosage groups was
actually given 4 000 ppm for 21 weeks, 2 000 ppm for 59 weeks and
control diet for 33 weeks. In the case of the high dosage group,
animals were fed 8 000 ppm for 41 weeks, 4 000 ppm for 39 weeks and
control diet for 33 weeks. (After 18 weeks, 7 males and 1 female in
this group had died and were replaced by healthy animals from a
16 000 ppm-group, on which studies were terminated after 18 weeks
because of high toxicity.) Pooled control groups, of 75 males and 75
females (including the 10 male and 10 female matched controls from the
present study) from similar bioassays of 7 test chemicals, were used
for statistical comparisons. No dose-related trend pertaining to
mortality was apparent. At least 60% females of control and treated
groups were still alive at termination. Survival was slightly lower in
the males, especially in the high dosage group, with only 46% of
animals in the latter group surviving the duration of the study.
Growth retardation occurred in both treated groups throughout the
study. Tumours were observed in animals of all groups, including the
controls, and were of types not uncommonly seen in the strain. There
was no indication of a significant increase in incidence of any
particular type of tumour. Under the conditions of the bioassay, there
was no evidence suggestive of carcinogenic activity of captan (NCI
1977).
Special Studies on Mutagenicity
In vitro microbial assays with tester strains of Salmonella
typhimurium indicated captan to be mutagenic, in the presence of
metabolic activation, to strains TA 1535, 1537, G46 (Herbold and
Buselmaier 1976), TA 100, TA 1537 and TA 98 (McCann et al 1975) and
to strain TA 1535 without metabolic activation (Seiler 1975).
Captan was found to be mutagenic in rec-assay with H17 Rec+
and M45 Rec- strains of Bacillus subtilis at 20 µg/disc and in
reversion assays with Escherichia coli (WP2) and S. typhimurium
tester strains TA 1535 at 50 µg/plate without inclusion of a metabolic
activation preparation in the test systems. At this concentration, no
positive mutagenic response was obtained with S. typhimurium tester
strains TA 1536, TA 1537 and TA 1538 (Shirasu et al 1976).
Captan was evaluated for its ability to induce forward mutations
to Colin E2- resistance in repair deficient strains of E. coli
(E-Coli JC 411/CoLE 2+ was used as the colicin-producing strain).
Positive results were obtained with strains WP2 and WP2 uvr A. No
mutagenic activity was detected in strains CM561 and CD611, which were
more sensitive to the toxicity of captan (Bridges et al 1973).
In a spot test with selected repair-deficient strains of
E. coli, filter paper impregnated with captan was placed on an agar
plate or on the lid of an inverted petri dish to test any volatile
component of captan. Mutagenic response was obtained in each case with
all of the E. coli strains tested, i.e. WP2, WP2 UvrA, WP2 ROC A,
WP2 ExrA and WP2 UvrA ExrA. The high mutation rates noted in UvrA
strains, as compared to other tester strains, indicated that much of
the mutagenic activity probably involved production of a DNA lesion
detectable and repairable by excision repair. There were indications
that a large part of the mutagenic activity of captan was caused by a
short-lived, volatile fraction inducing DNA damage of an excisable
nature. A second diffusable mutagenic component causing excisable DNA
damage might also occur (Bridges et al 1972).
Captan was evaluated for its potential to induce point mutations
to 8-azaquanine resistance with Aspergillus nidulans haploid strain
35 (pa ba A1, anA1, yA2, meth GI, nic A2, nic B8) and mitotic non-
disjunction as well as crossing-over with diploid strain P (first
chromosome: suad E20, ribo A1, faA1, pro A1, paba A1, yA2, ad E20,
bi A1). The test material was found in the spot test to induce point
mutation and mitotic crossing over but not non-disjunction (Bignami
et al 1977).
A significant dose-related increase in the frequency of sister
chromatid exchanges and chromosomal aberrations was observed in
cultured Chinese hamster cells treated with captan at concentrations
of 6 × 10-6 M and above (Tezuka et al 1980).
The frequency of sister chromatid exchanges in human lymphocytes
exposed to captan in vitro at a concentration of 3 × 10-5 M was
significantly elevated. Higher concentrations of captan were too toxic
to test for induction of sister chromatid exchanges (Vigfusson and
Vyse 1980).
The mutagenicity of captan, as a suspension in buffer and as a
source of a volatile component, was tested for the induction of
8-azaguanine and ouabain-resistant mutants in V79-4 Chinese hamster
lung cell cultures. In cells exposed to the captan suspension, there
was no increased incidence of 8-azaguanine resistant colonies. An
increase in the absolute number of ouabain resistant variants,
however, occurred at low incidence, the frequency being dose-
dependent. The volatile component from filter paper impregnated with
captan induced an increase in the number of azaguanine and ouabain
resistant colonies (Arlett et al 1975).
Unscheduled DNA synthesis was induced in cultured SV-40
transformed human fibroblast cells (VA-4) treated with captan at
concentrations of 1.0 to 1 000 µM. Metabolic activation employing S-9
mix from rat liver did not significantly modify the activity of the
compound (Ahmed et al 1977).
The transforming potential of technical captan was evaluated
using the 1-1 subclone of clone A-31 of BALB/3T3 mouse cells or the
subclone C-14 of clone C1-13. No increase in morphologically
transformed foci was induced when cells were exposed to the test
material at concentrations up to 0.1 µg/ml for 4 hours in serum-free
medium or at concentrations ranging from 0.04 to 0.2 µg/ml for 3 days
in foetal bovine serum supplemented medium. Under the conditions of
the assay, technical captan did not induce morphological
transformations (Environmental Health Center 1981).
Captan was among a total of 174 chemicals tests for mutagenicity
in ICR/Ha Swiss mice using a modified dominant lethal assay. Acute
intraperitoneal doses of 9 to 30 mg/kg body weight, single oral dose
at 500 or 800 mg/kg body weight or 5 successive daily oral doses of 25
or 50 mg/kg body weight/day were given to male mice (5-11 per group)
prior to mating. Untreated virgin females were mated weekly with
treated males for 3 or 8 consecutive weeks (i.p. dosing) or for 8
consecutive weeks (oral treatment). It was indicated that captan was
among the "agents producing early foetal deaths and pre-implantation
losses within control limits". Acute intraperitoneal doses of 15 or
30 mg/kg body weight were fatal to 90-100% of the treated males
(Epstein et al 1972).
In a dominant lethal assay, groups of male Charles River mice
were fed technical captan in their diet at 0, 500, 3 000 or 7 000 ppm
for 8 weeks and then mated with untreated female mice weekly for 8
consecutive weeks. Total weight gain over the 8-week period was
reduced in males at 7 000 ppm. There was no treatment-related effect
with respect to fertility rate, the number of implantations, early and
late resorption or viable foetuses (Salamon et al 1977).
Dominant lethal assays were conducted in groups of 15 male mice
or rats treated with technical captan orally at 0, 50, 100 or
200 mg/kg body weight/day or intraperitoneally at 0, 2.5, 5.0 or
10 mg/kg body weight/day for 5 days. The animals were then mated
weekly with virgin females of the respective species consecutively for
12 weeks (mice) or 10 weeks (rats). The females were sacrificed on day
12 or 13 of pregnancy, respectively, for the examination of uterine
contents.
In neither species were compound-related effects seen on
mortality, pregnancy rate and mean total implantations per pregnancy
after oral or intraperitoneal exposure. The mean number of early
deaths per pregnancy was elevated after intraperitoneal treatment at
10 mg/kg body weight/day for the first 7 weeks in both mice and rats
and after oral dosing at and above 100 mg/kg body weight/day for the
first 2 weeks in mice and at all treated levels in the rat. The
increase in the latter species was indicated to be statistically
significant at 100 mg/kg body weight/day during week 4 and at
200 mg/kg body weight/day during weeks 1, 2 and 5. A statistically
significant linear trend in incidence of litters with two or more
early deaths was noted in mice practically through the first 7 weeks
after the intraperitoneal doses and for the first 5 weeks after oral
treatment, and in rats for weeks 4 and 5 and for weeks 1 and 2
following intraperitoneal and oral administration, respectively
(Collins 1972).
Groups of pregnant mice were fed captan in their diet at 0, 100,
1 000 or 5 000 ppm on days 6 through 12 of pregnancy in a specific-
locus somatic cell mutation assay. ("The assay involves exposing
embryos heterozygous for a number of recessive coat-colour markers to
the test article and later looking for clones of mutant cells which
appear as coat colour mosaic patches on otherwise black mice".) The
newborn pups were scored for spots on day 12 and at weaning. Body
weight and food consumption were depressed at 5 000 ppm. No treatment-
related effects were seen on pregnancy rate, litter size, pup survival
to 12 days or gross abnormalities. There was no increased frequency of
recessive spots in the treated groups, suggesting no captan-induced
somatic mutations in the treated mice (Nguyen and Brusick 1980).
Observations in Humans
A study was conducted, using a retrospective cohort design, in a
total of 134 male workers, who had been occupationally exposed to
technical captan for periods ranging from 3 months to over 20 years.
All of these individuals worked in a chemical plant producing only
captan and an analogue of captan. Two-thirds of the study population
were exposed to captan for less than 5 years. There were 18 deaths in
the cohort as compared to 11 expected. Two of the deaths were caused
by tumour of the pancreas and the mediastinum (thymomas),
respectively. The individual with the mediastinum tumour was one of
the 16 men employed for 20 or more years. The excess mortality rate
was primarily attributable to cardiovascular diseases and external
causes. Neither duodenal cancer nor increased incidence of cancers was
observed among the study population. Medical records for those
employees still alive were not available (Palshaw 1980).
COMMENTS
A three-generation reproduction study in rats, as a replacement
study for the one previously conducted by IBT, did not adequately
demonstrate a no-effect level on reproduction, although data on F2c
litters revealed no evidence of teratogenicity. Teratology studies in
rabbits treated orally and in mice exposed to captan orally,
subcutaneously or by inhalation were negative. (The latter studies in
mice were available in the form of an abstract.) In a hamster
teratology study, while incidence of malformations was probably not
affected by captan, conclusions with respect to teratogenicity in this
respect cannot be made with confidence until details of the non-
specified rib anomalies are made available. If the rib anomalies
comprised abnormalities of minimal concern, then 50 mg/kg body weight
might be considered as a no-effect level for the study. A
carcinogenicity study in Osborne-Mendel rats gave no indication of
carcinogenic activity. However, an increased incidence of duodenal
tumours was observed in a bioassay with B6C 3F1 hybrid mice fed
dietary levels of 8 000 or 16 000 ppm for 80 weeks. This particular
finding was reportedly confirmed in another 2-year mouse study at
dietary levels of captan ranging from 6 000 to 16 000 ppm (Stauffer
1980). It is understood that an additional 2-year study in mice using
dietary levels of 100 to 6 000 ppm is currently underway (Stauffer
1980). A 12-month interim report of a new 2-year study (International
Research and Development 1980) in rats was received, but a complete
evaluation of the study would depend on availability of the final
report. Terminal survivors from this study exhibited no compound-
related lesions in the duodenum, according to a preliminary report of
histopathological findings in the particular tissue (Arceo 1981).
A variety of mutagenicity studies were submitted, which provided
further evidence that captan was mutagenic to most microbial or other
non-mammalian systems in vitro and to isolated mammalian cell
systems. Captan did not induce morphological transformation of
BALB/3T3 cells in vitro or somatic cell mutation in mice in vivo.
Results of dominant lethal assays appeared to be equivocal.
The data provided on the retrospective study in humans, while
indicating no occurrence of duodenal cancer or increased incidence of
cancers among the cohort, were of limited value since the majority in
the study population were exposed to captan for less than five years.
In view of the concerns with respect to carcinogenicity in mice,
the inability to demonstrate a no-effect level in the 3-generation
reproduction study in rats and the finding of the long term/
carcinogenicity studies in both mice and rats conducted by IBT being
invalid, the Meeting decided that the full ADI be modified to a
temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Final report of the new two-year study in rats.
2. Complete report of the two-year study in mice understood to have
been completed.
3. Full data of a new two-year oral carcinogenicity study in mice,
known to be in progress.
4. A two-generation (two litters/generation) reproduction study in
rats to define a no-effect level on reproduction.
Desirable
1. Details of unspecified rib anomalies in the hamster teratology
study.
2. Further observations in humans.
3. Full details of the one-generation (one litter) reproduction
study in rats.
REFERENCES
Ahmed, F.E., Hart, R. and Lewis, N.J. Pesticide induced DNA damage
1977 and its repair in cultured human cells. Mutat. Res.
42:f161-174.
Arceo, R.J. Gross and microscopic examination of the duodenum.
1981 Report from International Research and Development Corp.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
Arlett, C.F., Turnbull, D., Harcourt, B.A., Lehmann, A.R. and
1975 Colella, C.M. A comparison of the 8-azaguanine and ouabain-
resistance systems for the selection of induced mutant
Chinese hamster cells. Mutat. Res. 33:261-278.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. and Morpurgo, G.
1977 Mutagenic and recombinogence action of pesticides in
Aspergillus nidulans. Mutat. Res. 46:395-402.
Bridges, B.A., Mottershead, R.P., Rothwell, M.H. and Green, M.H.L.
1972 Repair-deficient bacterial strains unsuitable for
mutagenicity screening: Test with the fungicide captan.
Chem. Biol. Interact. 5:77-84.
Bridges, B.A., Mottershead, R.P. and Colella, C. Induction of
1973 forward mutations to colicin E2 resistance in repair
deficient strains of Escherichia coli: Experiments with
ultraviolet light and captan. Mutat. Res. 23:303-313.
Collins, T.F.X. Dominant lethal assay. I. Captan. Food Cosmet.
1972 Toxicol. 10:353-361.
Couch, R.C., Siegel, M.R. and Dorough, H.W. Fate of captan and
1977 folpet in rats and their effects on isolated liver nuclei.
Pestic. Biochem. and Physiol. 7:547-558.
Courtney, K.D., Andres, J.E. and Stevens, J.T. Inhalation teratology
1978 studies with captan and folpet. Toxicol. appl. Pharmacol.
45(1):292.
Environmental Health Center. Captan technical (Lot No. 5093-25-1).
1981 Morphological transformation of BALB/3T3 cells. Report from
Environmental Health Center, Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Epstein, S.S., Arnold, E., Andrea, J., Bacs, W. and Bishop, Y.
1972 Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol. 23:288-325.
Goldenthal, E.I. Pilot teratology study in hamster. Report from
International Research and Development Corp. submitted to
the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Goldenthal, E.I., Jessop, D.C. and Rodwell, D.E. Captan teratology
study in hamsters. Report from International Research and
Development Corp., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Herbold, B. and Buselmaier, W. Induction of point mutations by
1976 different chemical mechanisms in the liver microsomal assay.
Mutation Research, 40:73-84.
International Research and Development. Captan technical, SX 944
1980 2-year oral toxicity/carcinogenicity study of captan in rats
(12-month interim). Report from International Research and
Development Corp. submitted to the World Health Organization
by Stauffer Chemical Co. (Unpublished)
1982a Captan three generation reproduction study in rats. Report
from International Research and Development Corp. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
1982b One-generation reproduction study in rats with captan.
Status report from International Research and Development
Corp. submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
McCann, J., Choi, E., Yamasaki, E. and Ames, B.N. Detection of
1975 carcinogens as mutagens in the salmonella/microsome test:
Assay of 300 chemicals. Proc. Nat. Acad. Sci.
72(12):5135-5139.
Miaullis, J.B., Quale, D.C., Vispetto, A.R. and DeBaun, J.R. Effect
1980 of dietary captan on soluble thiol content of liver and
duodenal tissues. Report from Stauffer Chemical Co.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
NCI. Bioassay of captan for possible carcinogenicity. National
1977 Cancer Institute. Carcinogenesis Technical Report Series No.
15. D.H.E.W. Publication No. (NIH) 77-815.
Nguyen, T.D. and Brusick, D.J. Mutagenic evaluation of captan in the
1980 somatic cell mutation assay. Final report from Litton
Bionetics, Inc., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palmer, A.K., Barton, S.J. and Clark, R. Effect of technical captan
1981 on pregnancy of the New Zealand White rabbit. Huntingdon
Research Centre, England, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palshaw, M.W. An epidemiologic study of mortality within a cohort of
captan workers. Report from Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Salamon, C., Smith, S., Kennedy, G.L., Kineshita, F.K. and
1977 Keplinger, M.L. Dominant lethal study with captan technical
in albino mice exposed for 8 weeks to the chemical in the
diet. Report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Stauffer
Chemical Co. (The study was recently validated by the
Canadian Government.) (Unpublished)
Seiler, J.P. Evaluation of some pesticides for mutagenicity. Proc.
1975 of the Eur. Soc. Toxicol. 17:398-404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutat. Res. 40:19-30.
Stauffer, Captan RPAR risk rebuttal and summaries of benefits. Pages
5 and 19, Volume 1. Document prepared by Stauffer Chemical
Co. November 26, 1980, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Tezuka, H., Ando, N., Suzuki, R., Terahata, M., Moriya, M. and
1980 Shirasu, Y. Sister-chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with
pesticides positive in microbial reversion assays. Mutat.
Res. 78:177-191.
Vigfusson, N.V., Vyse, E.R. The effect of the pesticides, dexon,
1980 captan and round-up on sister-chromatid exchanges in human
lymphocytes in vitro. Mutat. Res. 79:53-57.
Explanation
This fungicide was evaluated by the Joint Meetings in 1965, 1969,
1973, 1974, 1977 and 1978 (FAO/WHO 1965b, 1970b, 1974b, 1975b, 1978b
and 1979).1/ A full ADI of 0-0.1 mg/kg body weight was established on
the basis of the no-effect levels partially taken from data reported
by Industrial Bio-Test Laboratories (IBT). The latter include
long-term/carcinogenicity studies in mice and rats, a three-generation
reproduction in rats and teratology studies in rabbits, dogs and
monkeys.
New studies have become available and are summarized and
discussed in this monograph addendum. In addition, the major concerns
raised in a Rebuttable Presumption Against Registration (RPAR) issued
in the U.S. for captan in August 1980 relating to oncogenic and
mutagenic effects have been considered in this review of new data.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
In normal, sham-operated and partially hepatectomized male rats,
the rate of elimination of a single dose or three daily doses of
(35S)-captan, at 6 mg/kg body weight given intraperitoneally, was
similar. Approximately 77-89% of the total administered radioactivity
was eliminated within 72 hours, of the single or multiple dosing
primarily in the urine but also in the faeces. The pattern of
distribution of 35S-residues in tissues from these rats, sacrificed
75 hours after the single dose or 24 hours after the third daily dose,
was also essentially the same with the highest concentration being
detected in the blood and the lowest in brain. Additionally, nuclei
isolated from liver of rats after treatment with three daily
intraperitoneal doses of (35S)-captan contained appreciable amounts
of radioactivity. There were no differences in normal, sham-operated
or partially hepatectomized animals (Couch et al 1977).
1/ See Annex 2 for WHO and FAO documentation.
Effects on Other Biochemical Parameters
Incubation of isolated liver nuclei from normal rats with captan
for 10 minutes at 37°C resulted in changes in distribution of various
protein fractions. One of the major changes was extensive aggregation
of nuclear sap proteins and deoxyribonucleoprotein. Binding studies
with isolated rat liver nuclei indicated evolution as gas(es) of a
large percentage of the total radioactivity from (35S)-captan,
suggesting occurrence of an interaction of captan with thiol groups in
the nucleus with subsequent release of the SCCl3 moiety. Following
incubation with 35S-captan, the rate of 35S binding and the
percentage of 35S-labelled gas evolved were slightly higher in
isolated liver nuclei from normal rats than from partially
hepatectomized rats (Couch et al 1977).
In adult Swiss Webster female mice fed technical captan in their
diet at levels ranging from 4 000 to 16 000 ppm for up to 35 days,
soluble thiol levels in the duodenum from the proximal end were
elevated throughout the feeding period. The increase, not strictly
dose-dependent, was already evident on day 1 and peaked between 14 and
21 days following initiation of the study. The liver from these
animals exhibited normal or slightly depressed levels of soluble
thiols. Results from this experiment were confirmed by those of a
second test with groups of female mice of the same strain fed dietary
levels of captan ranging from 500 to 16 000 ppm for up to 213 days. In
the latter experiment, a dose-response relationship was noted with
respect to levels of duodeneal soluble thiols (Miaullis et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of 15 male and 30 female rats (Charles River COBS CD
strain, 31 days old) were fed technical captan in their diet at the
equivalent of 0, 25, 100, 250 or 500 mg/kg body weight/day for 102
days and then mated to initiate a three-generation reproduction study.
The procedure normally used in a standard three-generation (two
litters/generation) study was followed but an additional litter, F2c,
was produced from F1 parents for teratological evaluation. Pups of
the second litters (F1b and F2b) became parents of the next
generation. After the third mating trial, F1 dams were sacrificed on
gestation day 19 and the F2c foetuses were removed by caesarean
section for examination of external, skeletal and soft tissue
abnormalities. In the parental generations, no compound-related effect
on mortality, appearance and behaviour was apparent. Dose-related
growth depression occurred in both sexes at and above 100 mg/kg body
weight/day throughout all three generations and in F0 and F2 males
even at 25 mg/kg body weight/day. Food consumption was decreased at
all dosage levels in a dose-dependent trend in all three generations.
A reduction in pregnancy rate was observed in F0 generation (first
mating trial) at 500 mg/kg body weight/day and possibly also at
250 mg/kg body weight/day. In the progeny generations litter size at
birth was reduced in all treated groups in F1a and F1b litters and at
100 mg/kg body weight/day and above in F2a and F2b litters. Pup
survival was adversely affected at 250 mg/kg body weight/day in F1a,
F2a and F3a litters throughout lactation day 4 and at 500 mg/kg body
weight practically throughout the lactation period in all progeny
generations. Dose-related depression in pup weight was seen at and
above 100 mg/kg body weight/day in litters of all three generations
and at 25 mg/kg body weight/day in most litters. Weanling pups in each
generation exhibited no compound-related abnormalities at necropsy.
Teratological evaluation of the F2c litter revealed no effect
attributable to treatment with respect to fertility, number of non-
viable foetuses, early and late resorptions and post-implantation loss
per dam, sex ratio and incidence of foetuses or litters with
malformations. Foetal body weight was depressed at 500 mg/kg body
weight/day. There was a slight decrease in the mean number of viable
foetuses per dam and a corresponding decrease in total implantations
at 250 mg/kg body weight/day and above. The number of corpora lutea
per dam was reduced at 500 mg/kg body weight/day.
The study failed to demonstrate a no-effect level on
reproduction, although teratogenic effect was not evident from data on
the F2c litter (International Research and Development 1982a).
A preliminary report of a one-generation, one litter reproduction
study in rats (Charles River CD) with groups of 15 males and 30
females fed captan-treated diets for 100 days prior to mating
indicated that "minor" effects on litter weight, noted at 25 mg/kg
body weight/day, were not apparent at 6 or 12.5 mg/kg body weight/day.
No other reproductive effects were reportedly noted (International
Research and Development 1982b).
Special Studies on Teratogenicity
Mouse
According to results reported in an abstract, pregnant CD-1
mice exposed via inhalation to captan (whole body exposure) at
approximately 488 mg/hour/m3 (average particle size 5 µm), 4
hours/day from day 6 through 13 of gestation produced no malformed
foetuses. Similarly, captan was not teratogenic in pregnant CD-1 mice
treated with 100 mg/kg body weight/day of the compound orally or
subcutaneously from day 6 through 15 of gestation. Reduction of
maternal body weight and a slight increase in foetal mortality were
noted in the experiments (Courtney et al 1978).
Hamster
In a pilot (range-finding) teratology study with mated female
(Golden Syrian) hamsters given oral doses of technical captan at 50,
100, 200, 300 or 400 mg/kg body weight/day on day 5 through 10 of
gestation, there was no maternal death or treatment-related effect on
appearance and behaviour, number of corpora lutea, implantations or
resorptions and litter size. The only adverse effect observed was a
dose-related decrease in maternal weight gain at 300 mg/kg body
weight/day and above (Goldenthal 1978).
Groups of 30 mated female Golden Syrian hamsters were given
technical captan in 1% carboxy methylcellulose by gavage at 0, 50, 200
or 400 mg/kg body weight/day on days 5 through 10 of gestation. The
females were sacrificed on day 14 of gestation and foetuses were
removed by caesarean section for external, soft tissue and skeletal
examination. Four females at 400 mg/kg body weight/day and one female
at 200 mg/kg body weight/day died of undetermined cause(s). Stained
and matted anogenital fur and vaginal discharge were noted at
400 mg/kg body weight/day. Other adverse effects at 400 mg/kg body
weight/ day included maternal weight loss, a reduction in litter size
and foetal weight and an increase in early and late resorptions.
Additionally, increased post-implantation loss and a significant
difference in sex (M/F) ratio occurred in this dosage group. Maternal
weight gain and foetal weight were also depressed at 200 mg/kg body
weight/day. In general the incidence of malformations appeared to be
comparable in all groups. However, the incidence of non-specified rib
anomalies in foetuses was 0.3, 1.4, 1.8, 2.9%, respectively, at 0, 50,
200 and 400 mg/kg body weight/day and in litters was 3.6, 15.4, 15.4
and 8.7% (Goldenthal et al 1979).
Rabbit
Groups of 15 mated female New Zealand White rabbits were
intubated with technical captan (89% pure) in 0.5% sodium
carboxymethyl cellulose at 0, 6, 12, 25 or 60 mg/kg body weight/day on
days 6-28 inclusive of pregnancy. Does were sacrificed on day 29 of
pregnancy and uterine contents were examined. Foetuses were evaluated
for gross, skeletal and visceral abnormalities. Pregnancy rate at
60 mg/kg body weight/day and maternal body weight at and above
12 mg/kg body weight/day were adversely affected. Incidence of non-
specific signs (anorexia, reduced faecal output and water consumption)
was increased at 60 mg/kg body weight/day and possibly also at
25 mg/kg body weight/day. Litter and foetal weights as well as foetal
crown/rump length were depressed at 60 mg/kg body weight/ day. There
were no compound-related effects on the other tested parameters, i.e.
mortality, abortion rate, total resorptions, pre-implantation loss and
post-implantation loss including stillbirths), total litter loss in
pregnant animals surviving to autopsy, litter size, sex ratio and
incidence of major malformations or minor anomalies (Palmer
et al 1981).
Special Studies for Carcinogenicity
Mouse
Groups of 50 male and 50 female mice (B6C 3F1 hybrid; 35-day old)
were fed captan of unspecified purity in their diet at 8 000 or
16 000 ppm for 80 weeks and then maintained on a control diet for 11
weeks. The control group comprised 10 mice of each sex. The animals
were placed in the same room as groups of mice receiving aldrin or
photodieldrin and their controls. Pooled controls, used for
statistical comparisons, consisted of a total of 80 male and 80 female
mice of the same strain from seven similar bio-assays (including the
one with captan). Survival was good and no dose-related effect on
mortality was evident. Growth was depressed at 16 000 ppm throughout
most of the study. The total incidence of tumours was low in control
and treated groups. However, adenomatous polyp of the duodenum was
noted in 0/9 males and 1/9 female controls, 2/43 males and 1/49
females at 8 000 ppm and 2/46 males and 0/49 females at 16 000 ppm. In
addition, polypoid adenocarcinoma of the duodenum occurred in 1/43
males and 0/49 females at 8 000 ppm and 3/46 males and 3/49 females at
16 000 ppm, but in none of the concurrent controls (hyperplasia of the
gastric mucosa was present in 3/46 males at 16 000 ppm). In males, the
incidence of either adenomatous polyps or polypoid adenocarcinoma
alone was not statistically significant, but when the two lesions,
usually rare in occurrence, were considered together, the incidence at
16 000 ppm was statistically significant, as compared to the pooled
control group. In females, incidence of the duodenal lesions in
question, considered alone or together, was not statistically
significant. There was, however, a statistically significant dose-
related trend with respect to incidences of both duodenal lesions in
males and females. The conclusion was made in the report that, under
the conditions of the bioassay, tumours in the duodenum were
associated with treatment with captan (NCI 1977).
Rat
Groups of 50 rats (Osborne-Mendel, 35 days old) of each sex were
fed a diet containing captan (purity not specified) at time-weighted
average doses of 2 525 or 6 050 ppm. The low dosage groups was
actually given 4 000 ppm for 21 weeks, 2 000 ppm for 59 weeks and
control diet for 33 weeks. In the case of the high dosage group,
animals were fed 8 000 ppm for 41 weeks, 4 000 ppm for 39 weeks and
control diet for 33 weeks. (After 18 weeks, 7 males and 1 female in
this group had died and were replaced by healthy animals from a
16 000 ppm-group, on which studies were terminated after 18 weeks
because of high toxicity.) Pooled control groups, of 75 males and 75
females (including the 10 male and 10 female matched controls from the
present study) from similar bioassays of 7 test chemicals, were used
for statistical comparisons. No dose-related trend pertaining to
mortality was apparent. At least 60% females of control and treated
groups were still alive at termination. Survival was slightly lower in
the males, especially in the high dosage group, with only 46% of
animals in the latter group surviving the duration of the study.
Growth retardation occurred in both treated groups throughout the
study. Tumours were observed in animals of all groups, including the
controls, and were of types not uncommonly seen in the strain. There
was no indication of a significant increase in incidence of any
particular type of tumour. Under the conditions of the bioassay, there
was no evidence suggestive of carcinogenic activity of captan (NCI
1977).
Special Studies on Mutagenicity
In vitro microbial assays with tester strains of Salmonella
typhimurium indicated captan to be mutagenic, in the presence of
metabolic activation, to strains TA 1535, 1537, G46 (Herbold and
Buselmaier 1976), TA 100, TA 1537 and TA 98 (McCann et al 1975) and
to strain TA 1535 without metabolic activation (Seiler 1975).
Captan was found to be mutagenic in rec-assay with H17 Rec+
and M45 Rec- strains of Bacillus subtilis at 20 µg/disc and in
reversion assays with Escherichia coli (WP2) and S. typhimurium
tester strains TA 1535 at 50 µg/plate without inclusion of a metabolic
activation preparation in the test systems. At this concentration, no
positive mutagenic response was obtained with S. typhimurium tester
strains TA 1536, TA 1537 and TA 1538 (Shirasu et al 1976).
Captan was evaluated for its ability to induce forward mutations
to Colin E2- resistance in repair deficient strains of E. coli
(E-Coli JC 411/CoLE 2+ was used as the colicin-producing strain).
Positive results were obtained with strains WP2 and WP2 uvr A. No
mutagenic activity was detected in strains CM561 and CD611, which were
more sensitive to the toxicity of captan (Bridges et al 1973).
In a spot test with selected repair-deficient strains of
E. coli, filter paper impregnated with captan was placed on an agar
plate or on the lid of an inverted petri dish to test any volatile
component of captan. Mutagenic response was obtained in each case with
all of the E. coli strains tested, i.e. WP2, WP2 UvrA, WP2 ROC A,
WP2 ExrA and WP2 UvrA ExrA. The high mutation rates noted in UvrA
strains, as compared to other tester strains, indicated that much of
the mutagenic activity probably involved production of a DNA lesion
detectable and repairable by excision repair. There were indications
that a large part of the mutagenic activity of captan was caused by a
short-lived, volatile fraction inducing DNA damage of an excisable
nature. A second diffusable mutagenic component causing excisable DNA
damage might also occur (Bridges et al 1972).
Captan was evaluated for its potential to induce point mutations
to 8-azaquanine resistance with Aspergillus nidulans haploid strain
35 (pa ba A1, anA1, yA2, meth GI, nic A2, nic B8) and mitotic non-
disjunction as well as crossing-over with diploid strain P (first
chromosome: suad E20, ribo A1, faA1, pro A1, paba A1, yA2, ad E20,
bi A1). The test material was found in the spot test to induce point
mutation and mitotic crossing over but not non-disjunction (Bignami
et al 1977).
A significant dose-related increase in the frequency of sister
chromatid exchanges and chromosomal aberrations was observed in
cultured Chinese hamster cells treated with captan at concentrations
of 6 × 10-6 M and above (Tezuka et al 1980).
The frequency of sister chromatid exchanges in human lymphocytes
exposed to captan in vitro at a concentration of 3 × 10-5 M was
significantly elevated. Higher concentrations of captan were too toxic
to test for induction of sister chromatid exchanges (Vigfusson and
Vyse 1980).
The mutagenicity of captan, as a suspension in buffer and as a
source of a volatile component, was tested for the induction of
8-azaguanine and ouabain-resistant mutants in V79-4 Chinese hamster
lung cell cultures. In cells exposed to the captan suspension, there
was no increased incidence of 8-azaguanine resistant colonies. An
increase in the absolute number of ouabain resistant variants,
however, occurred at low incidence, the frequency being dose-
dependent. The volatile component from filter paper impregnated with
captan induced an increase in the number of azaguanine and ouabain
resistant colonies (Arlett et al 1975).
Unscheduled DNA synthesis was induced in cultured SV-40
transformed human fibroblast cells (VA-4) treated with captan at
concentrations of 1.0 to 1 000 µM. Metabolic activation employing S-9
mix from rat liver did not significantly modify the activity of the
compound (Ahmed et al 1977).
The transforming potential of technical captan was evaluated
using the 1-1 subclone of clone A-31 of BALB/3T3 mouse cells or the
subclone C-14 of clone C1-13. No increase in morphologically
transformed foci was induced when cells were exposed to the test
material at concentrations up to 0.1 µg/ml for 4 hours in serum-free
medium or at concentrations ranging from 0.04 to 0.2 µg/ml for 3 days
in foetal bovine serum supplemented medium. Under the conditions of
the assay, technical captan did not induce morphological
transformations (Environmental Health Center 1981).
Captan was among a total of 174 chemicals tests for mutagenicity
in ICR/Ha Swiss mice using a modified dominant lethal assay. Acute
intraperitoneal doses of 9 to 30 mg/kg body weight, single oral dose
at 500 or 800 mg/kg body weight or 5 successive daily oral doses of 25
or 50 mg/kg body weight/day were given to male mice (5-11 per group)
prior to mating. Untreated virgin females were mated weekly with
treated males for 3 or 8 consecutive weeks (i.p. dosing) or for 8
consecutive weeks (oral treatment). It was indicated that captan was
among the "agents producing early foetal deaths and pre-implantation
losses within control limits". Acute intraperitoneal doses of 15 or
30 mg/kg body weight were fatal to 90-100% of the treated males
(Epstein et al 1972).
In a dominant lethal assay, groups of male Charles River mice
were fed technical captan in their diet at 0, 500, 3 000 or 7 000 ppm
for 8 weeks and then mated with untreated female mice weekly for 8
consecutive weeks. Total weight gain over the 8-week period was
reduced in males at 7 000 ppm. There was no treatment-related effect
with respect to fertility rate, the number of implantations, early and
late resorption or viable foetuses (Salamon et al 1977).
Dominant lethal assays were conducted in groups of 15 male mice
or rats treated with technical captan orally at 0, 50, 100 or
200 mg/kg body weight/day or intraperitoneally at 0, 2.5, 5.0 or
10 mg/kg body weight/day for 5 days. The animals were then mated
weekly with virgin females of the respective species consecutively for
12 weeks (mice) or 10 weeks (rats). The females were sacrificed on day
12 or 13 of pregnancy, respectively, for the examination of uterine
contents.
In neither species were compound-related effects seen on
mortality, pregnancy rate and mean total implantations per pregnancy
after oral or intraperitoneal exposure. The mean number of early
deaths per pregnancy was elevated after intraperitoneal treatment at
10 mg/kg body weight/day for the first 7 weeks in both mice and rats
and after oral dosing at and above 100 mg/kg body weight/day for the
first 2 weeks in mice and at all treated levels in the rat. The
increase in the latter species was indicated to be statistically
significant at 100 mg/kg body weight/day during week 4 and at
200 mg/kg body weight/day during weeks 1, 2 and 5. A statistically
significant linear trend in incidence of litters with two or more
early deaths was noted in mice practically through the first 7 weeks
after the intraperitoneal doses and for the first 5 weeks after oral
treatment, and in rats for weeks 4 and 5 and for weeks 1 and 2
following intraperitoneal and oral administration, respectively
(Collins 1972).
Groups of pregnant mice were fed captan in their diet at 0, 100,
1 000 or 5 000 ppm on days 6 through 12 of pregnancy in a specific-
locus somatic cell mutation assay. ("The assay involves exposing
embryos heterozygous for a number of recessive coat-colour markers to
the test article and later looking for clones of mutant cells which
appear as coat colour mosaic patches on otherwise black mice".) The
newborn pups were scored for spots on day 12 and at weaning. Body
weight and food consumption were depressed at 5 000 ppm. No treatment-
related effects were seen on pregnancy rate, litter size, pup survival
to 12 days or gross abnormalities. There was no increased frequency of
recessive spots in the treated groups, suggesting no captan-induced
somatic mutations in the treated mice (Nguyen and Brusick 1980).
Observations in Humans
A study was conducted, using a retrospective cohort design, in a
total of 134 male workers, who had been occupationally exposed to
technical captan for periods ranging from 3 months to over 20 years.
All of these individuals worked in a chemical plant producing only
captan and an analogue of captan. Two-thirds of the study population
were exposed to captan for less than 5 years. There were 18 deaths in
the cohort as compared to 11 expected. Two of the deaths were caused
by tumour of the pancreas and the mediastinum (thymomas),
respectively. The individual with the mediastinum tumour was one of
the 16 men employed for 20 or more years. The excess mortality rate
was primarily attributable to cardiovascular diseases and external
causes. Neither duodenal cancer nor increased incidence of cancers was
observed among the study population. Medical records for those
employees still alive were not available (Palshaw 1980).
COMMENTS
A three-generation reproduction study in rats, as a replacement
study for the one previously conducted by IBT, did not adequately
demonstrate a no-effect level on reproduction, although data on F2c
litters revealed no evidence of teratogenicity. Teratology studies in
rabbits treated orally and in mice exposed to captan orally,
subcutaneously or by inhalation were negative. (The latter studies in
mice were available in the form of an abstract.) In a hamster
teratology study, while incidence of malformations was probably not
affected by captan, conclusions with respect to teratogenicity in this
respect cannot be made with confidence until details of the non-
specified rib anomalies are made available. If the rib anomalies
comprised abnormalities of minimal concern, then 50 mg/kg body weight
might be considered as a no-effect level for the study. A
carcinogenicity study in Osborne-Mendel rats gave no indication of
carcinogenic activity. However, an increased incidence of duodenal
tumours was observed in a bioassay with B6C 3F1 hybrid mice fed
dietary levels of 8 000 or 16 000 ppm for 80 weeks. This particular
finding was reportedly confirmed in another 2-year mouse study at
dietary levels of captan ranging from 6 000 to 16 000 ppm (Stauffer
1980). It is understood that an additional 2-year study in mice using
dietary levels of 100 to 6 000 ppm is currently underway (Stauffer
1980). A 12-month interim report of a new 2-year study (International
Research and Development 1980) in rats was received, but a complete
evaluation of the study would depend on availability of the final
report. Terminal survivors from this study exhibited no compound-
related lesions in the duodenum, according to a preliminary report of
histopathological findings in the particular tissue (Arceo 1981).
A variety of mutagenicity studies were submitted, which provided
further evidence that captan was mutagenic to most microbial or other
non-mammalian systems in vitro and to isolated mammalian cell
systems. Captan did not induce morphological transformation of
BALB/3T3 cells in vitro or somatic cell mutation in mice in vivo.
Results of dominant lethal assays appeared to be equivocal.
The data provided on the retrospective study in humans, while
indicating no occurrence of duodenal cancer or increased incidence of
cancers among the cohort, were of limited value since the majority in
the study population were exposed to captan for less than five years.
In view of the concerns with respect to carcinogenicity in mice,
the inability to demonstrate a no-effect level in the 3-generation
reproduction study in rats and the finding of the long term/
carcinogenicity studies in both mice and rats conducted by IBT being
invalid, the Meeting decided that the full ADI be modified to a
temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Final report of the new two-year study in rats.
2. Complete report of the two-year study in mice understood to have
been completed.
3. Full data of a new two-year oral carcinogenicity study in mice,
known to be in progress.
4. A two-generation (two litters/generation) reproduction study in
rats to define a no-effect level on reproduction.
Desirable
1. Details of unspecified rib anomalies in the hamster teratology
study.
2. Further observations in humans.
3. Full details of the one-generation (one litter) reproduction
study in rats.
REFERENCES
Ahmed, F.E., Hart, R. and Lewis, N.J. Pesticide induced DNA damage
1977 and its repair in cultured human cells. Mutat. Res.
42:f161-174.
Arceo, R.J. Gross and microscopic examination of the duodenum.
1981 Report from International Research and Development Corp.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
Arlett, C.F., Turnbull, D., Harcourt, B.A., Lehmann, A.R. and
1975 Colella, C.M. A comparison of the 8-azaguanine and ouabain-
resistance systems for the selection of induced mutant
Chinese hamster cells. Mutat. Res. 33:261-278.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. and Morpurgo, G.
1977 Mutagenic and recombinogence action of pesticides in
Aspergillus nidulans. Mutat. Res. 46:395-402.
Bridges, B.A., Mottershead, R.P., Rothwell, M.H. and Green, M.H.L.
1972 Repair-deficient bacterial strains unsuitable for
mutagenicity screening: Test with the fungicide captan.
Chem. Biol. Interact. 5:77-84.
Bridges, B.A., Mottershead, R.P. and Colella, C. Induction of
1973 forward mutations to colicin E2 resistance in repair
deficient strains of Escherichia coli: Experiments with
ultraviolet light and captan. Mutat. Res. 23:303-313.
Collins, T.F.X. Dominant lethal assay. I. Captan. Food Cosmet.
1972 Toxicol. 10:353-361.
Couch, R.C., Siegel, M.R. and Dorough, H.W. Fate of captan and
1977 folpet in rats and their effects on isolated liver nuclei.
Pestic. Biochem. and Physiol. 7:547-558.
Courtney, K.D., Andres, J.E. and Stevens, J.T. Inhalation teratology
1978 studies with captan and folpet. Toxicol. appl. Pharmacol.
45(1):292.
Environmental Health Center. Captan technical (Lot No. 5093-25-1).
1981 Morphological transformation of BALB/3T3 cells. Report from
Environmental Health Center, Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Epstein, S.S., Arnold, E., Andrea, J., Bacs, W. and Bishop, Y.
1972 Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol. 23:288-325.
Goldenthal, E.I. Pilot teratology study in hamster. Report from
International Research and Development Corp. submitted to
the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Goldenthal, E.I., Jessop, D.C. and Rodwell, D.E. Captan teratology
study in hamsters. Report from International Research and
Development Corp., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Herbold, B. and Buselmaier, W. Induction of point mutations by
1976 different chemical mechanisms in the liver microsomal assay.
Mutation Research, 40:73-84.
International Research and Development. Captan technical, SX 944
1980 2-year oral toxicity/carcinogenicity study of captan in rats
(12-month interim). Report from International Research and
Development Corp. submitted to the World Health Organization
by Stauffer Chemical Co. (Unpublished)
1982a Captan three generation reproduction study in rats. Report
from International Research and Development Corp. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
1982b One-generation reproduction study in rats with captan.
Status report from International Research and Development
Corp. submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
McCann, J., Choi, E., Yamasaki, E. and Ames, B.N. Detection of
1975 carcinogens as mutagens in the salmonella/microsome test:
Assay of 300 chemicals. Proc. Nat. Acad. Sci.
72(12):5135-5139.
Miaullis, J.B., Quale, D.C., Vispetto, A.R. and DeBaun, J.R. Effect
1980 of dietary captan on soluble thiol content of liver and
duodenal tissues. Report from Stauffer Chemical Co.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
NCI. Bioassay of captan for possible carcinogenicity. National
1977 Cancer Institute. Carcinogenesis Technical Report Series No.
15. D.H.E.W. Publication No. (NIH) 77-815.
Nguyen, T.D. and Brusick, D.J. Mutagenic evaluation of captan in the
1980 somatic cell mutation assay. Final report from Litton
Bionetics, Inc., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palmer, A.K., Barton, S.J. and Clark, R. Effect of technical captan
1981 on pregnancy of the New Zealand White rabbit. Huntingdon
Research Centre, England, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palshaw, M.W. An epidemiologic study of mortality within a cohort of
captan workers. Report from Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Salamon, C., Smith, S., Kennedy, G.L., Kineshita, F.K. and
1977 Keplinger, M.L. Dominant lethal study with captan technical
in albino mice exposed for 8 weeks to the chemical in the
diet. Report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Stauffer
Chemical Co. (The study was recently validated by the
Canadian Government.) (Unpublished)
Seiler, J.P. Evaluation of some pesticides for mutagenicity. Proc.
1975 of the Eur. Soc. Toxicol. 17:398-404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutat. Res. 40:19-30.
Stauffer, Captan RPAR risk rebuttal and summaries of benefits. Pages
5 and 19, Volume 1. Document prepared by Stauffer Chemical
Co. November 26, 1980, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Tezuka, H., Ando, N., Suzuki, R., Terahata, M., Moriya, M. and
1980 Shirasu, Y. Sister-chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with
pesticides positive in microbial reversion assays. Mutat.
Res. 78:177-191.
Vigfusson, N.V., Vyse, E.R. The effect of the pesticides, dexon,
1980 captan and round-up on sister-chromatid exchanges in human
lymphocytes in vitro. Mutat. Res. 79:53-57.
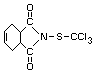 Explanation
This fungicide was evaluated by the Joint Meetings in 1965, 1969,
1973, 1974, 1977 and 1978 (FAO/WHO 1965b, 1970b, 1974b, 1975b, 1978b
and 1979).1/ A full ADI of 0-0.1 mg/kg body weight was established on
the basis of the no-effect levels partially taken from data reported
by Industrial Bio-Test Laboratories (IBT). The latter include
long-term/carcinogenicity studies in mice and rats, a three-generation
reproduction in rats and teratology studies in rabbits, dogs and
monkeys.
New studies have become available and are summarized and
discussed in this monograph addendum. In addition, the major concerns
raised in a Rebuttable Presumption Against Registration (RPAR) issued
in the U.S. for captan in August 1980 relating to oncogenic and
mutagenic effects have been considered in this review of new data.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
In normal, sham-operated and partially hepatectomized male rats,
the rate of elimination of a single dose or three daily doses of
(35S)-captan, at 6 mg/kg body weight given intraperitoneally, was
similar. Approximately 77-89% of the total administered radioactivity
was eliminated within 72 hours, of the single or multiple dosing
primarily in the urine but also in the faeces. The pattern of
distribution of 35S-residues in tissues from these rats, sacrificed
75 hours after the single dose or 24 hours after the third daily dose,
was also essentially the same with the highest concentration being
detected in the blood and the lowest in brain. Additionally, nuclei
isolated from liver of rats after treatment with three daily
intraperitoneal doses of (35S)-captan contained appreciable amounts
of radioactivity. There were no differences in normal, sham-operated
or partially hepatectomized animals (Couch et al 1977).
1/ See Annex 2 for WHO and FAO documentation.
Effects on Other Biochemical Parameters
Incubation of isolated liver nuclei from normal rats with captan
for 10 minutes at 37°C resulted in changes in distribution of various
protein fractions. One of the major changes was extensive aggregation
of nuclear sap proteins and deoxyribonucleoprotein. Binding studies
with isolated rat liver nuclei indicated evolution as gas(es) of a
large percentage of the total radioactivity from (35S)-captan,
suggesting occurrence of an interaction of captan with thiol groups in
the nucleus with subsequent release of the SCCl3 moiety. Following
incubation with 35S-captan, the rate of 35S binding and the
percentage of 35S-labelled gas evolved were slightly higher in
isolated liver nuclei from normal rats than from partially
hepatectomized rats (Couch et al 1977).
In adult Swiss Webster female mice fed technical captan in their
diet at levels ranging from 4 000 to 16 000 ppm for up to 35 days,
soluble thiol levels in the duodenum from the proximal end were
elevated throughout the feeding period. The increase, not strictly
dose-dependent, was already evident on day 1 and peaked between 14 and
21 days following initiation of the study. The liver from these
animals exhibited normal or slightly depressed levels of soluble
thiols. Results from this experiment were confirmed by those of a
second test with groups of female mice of the same strain fed dietary
levels of captan ranging from 500 to 16 000 ppm for up to 213 days. In
the latter experiment, a dose-response relationship was noted with
respect to levels of duodeneal soluble thiols (Miaullis et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of 15 male and 30 female rats (Charles River COBS CD
strain, 31 days old) were fed technical captan in their diet at the
equivalent of 0, 25, 100, 250 or 500 mg/kg body weight/day for 102
days and then mated to initiate a three-generation reproduction study.
The procedure normally used in a standard three-generation (two
litters/generation) study was followed but an additional litter, F2c,
was produced from F1 parents for teratological evaluation. Pups of
the second litters (F1b and F2b) became parents of the next
generation. After the third mating trial, F1 dams were sacrificed on
gestation day 19 and the F2c foetuses were removed by caesarean
section for examination of external, skeletal and soft tissue
abnormalities. In the parental generations, no compound-related effect
on mortality, appearance and behaviour was apparent. Dose-related
growth depression occurred in both sexes at and above 100 mg/kg body
weight/day throughout all three generations and in F0 and F2 males
even at 25 mg/kg body weight/day. Food consumption was decreased at
all dosage levels in a dose-dependent trend in all three generations.
A reduction in pregnancy rate was observed in F0 generation (first
mating trial) at 500 mg/kg body weight/day and possibly also at
250 mg/kg body weight/day. In the progeny generations litter size at
birth was reduced in all treated groups in F1a and F1b litters and at
100 mg/kg body weight/day and above in F2a and F2b litters. Pup
survival was adversely affected at 250 mg/kg body weight/day in F1a,
F2a and F3a litters throughout lactation day 4 and at 500 mg/kg body
weight practically throughout the lactation period in all progeny
generations. Dose-related depression in pup weight was seen at and
above 100 mg/kg body weight/day in litters of all three generations
and at 25 mg/kg body weight/day in most litters. Weanling pups in each
generation exhibited no compound-related abnormalities at necropsy.
Teratological evaluation of the F2c litter revealed no effect
attributable to treatment with respect to fertility, number of non-
viable foetuses, early and late resorptions and post-implantation loss
per dam, sex ratio and incidence of foetuses or litters with
malformations. Foetal body weight was depressed at 500 mg/kg body
weight/day. There was a slight decrease in the mean number of viable
foetuses per dam and a corresponding decrease in total implantations
at 250 mg/kg body weight/day and above. The number of corpora lutea
per dam was reduced at 500 mg/kg body weight/day.
The study failed to demonstrate a no-effect level on
reproduction, although teratogenic effect was not evident from data on
the F2c litter (International Research and Development 1982a).
A preliminary report of a one-generation, one litter reproduction
study in rats (Charles River CD) with groups of 15 males and 30
females fed captan-treated diets for 100 days prior to mating
indicated that "minor" effects on litter weight, noted at 25 mg/kg
body weight/day, were not apparent at 6 or 12.5 mg/kg body weight/day.
No other reproductive effects were reportedly noted (International
Research and Development 1982b).
Special Studies on Teratogenicity
Mouse
According to results reported in an abstract, pregnant CD-1
mice exposed via inhalation to captan (whole body exposure) at
approximately 488 mg/hour/m3 (average particle size 5 µm), 4
hours/day from day 6 through 13 of gestation produced no malformed
foetuses. Similarly, captan was not teratogenic in pregnant CD-1 mice
treated with 100 mg/kg body weight/day of the compound orally or
subcutaneously from day 6 through 15 of gestation. Reduction of
maternal body weight and a slight increase in foetal mortality were
noted in the experiments (Courtney et al 1978).
Hamster
In a pilot (range-finding) teratology study with mated female
(Golden Syrian) hamsters given oral doses of technical captan at 50,
100, 200, 300 or 400 mg/kg body weight/day on day 5 through 10 of
gestation, there was no maternal death or treatment-related effect on
appearance and behaviour, number of corpora lutea, implantations or
resorptions and litter size. The only adverse effect observed was a
dose-related decrease in maternal weight gain at 300 mg/kg body
weight/day and above (Goldenthal 1978).
Groups of 30 mated female Golden Syrian hamsters were given
technical captan in 1% carboxy methylcellulose by gavage at 0, 50, 200
or 400 mg/kg body weight/day on days 5 through 10 of gestation. The
females were sacrificed on day 14 of gestation and foetuses were
removed by caesarean section for external, soft tissue and skeletal
examination. Four females at 400 mg/kg body weight/day and one female
at 200 mg/kg body weight/day died of undetermined cause(s). Stained
and matted anogenital fur and vaginal discharge were noted at
400 mg/kg body weight/day. Other adverse effects at 400 mg/kg body
weight/ day included maternal weight loss, a reduction in litter size
and foetal weight and an increase in early and late resorptions.
Additionally, increased post-implantation loss and a significant
difference in sex (M/F) ratio occurred in this dosage group. Maternal
weight gain and foetal weight were also depressed at 200 mg/kg body
weight/day. In general the incidence of malformations appeared to be
comparable in all groups. However, the incidence of non-specified rib
anomalies in foetuses was 0.3, 1.4, 1.8, 2.9%, respectively, at 0, 50,
200 and 400 mg/kg body weight/day and in litters was 3.6, 15.4, 15.4
and 8.7% (Goldenthal et al 1979).
Rabbit
Groups of 15 mated female New Zealand White rabbits were
intubated with technical captan (89% pure) in 0.5% sodium
carboxymethyl cellulose at 0, 6, 12, 25 or 60 mg/kg body weight/day on
days 6-28 inclusive of pregnancy. Does were sacrificed on day 29 of
pregnancy and uterine contents were examined. Foetuses were evaluated
for gross, skeletal and visceral abnormalities. Pregnancy rate at
60 mg/kg body weight/day and maternal body weight at and above
12 mg/kg body weight/day were adversely affected. Incidence of non-
specific signs (anorexia, reduced faecal output and water consumption)
was increased at 60 mg/kg body weight/day and possibly also at
25 mg/kg body weight/day. Litter and foetal weights as well as foetal
crown/rump length were depressed at 60 mg/kg body weight/ day. There
were no compound-related effects on the other tested parameters, i.e.
mortality, abortion rate, total resorptions, pre-implantation loss and
post-implantation loss including stillbirths), total litter loss in
pregnant animals surviving to autopsy, litter size, sex ratio and
incidence of major malformations or minor anomalies (Palmer
et al 1981).
Special Studies for Carcinogenicity
Mouse
Groups of 50 male and 50 female mice (B6C 3F1 hybrid; 35-day old)
were fed captan of unspecified purity in their diet at 8 000 or
16 000 ppm for 80 weeks and then maintained on a control diet for 11
weeks. The control group comprised 10 mice of each sex. The animals
were placed in the same room as groups of mice receiving aldrin or
photodieldrin and their controls. Pooled controls, used for
statistical comparisons, consisted of a total of 80 male and 80 female
mice of the same strain from seven similar bio-assays (including the
one with captan). Survival was good and no dose-related effect on
mortality was evident. Growth was depressed at 16 000 ppm throughout
most of the study. The total incidence of tumours was low in control
and treated groups. However, adenomatous polyp of the duodenum was
noted in 0/9 males and 1/9 female controls, 2/43 males and 1/49
females at 8 000 ppm and 2/46 males and 0/49 females at 16 000 ppm. In
addition, polypoid adenocarcinoma of the duodenum occurred in 1/43
males and 0/49 females at 8 000 ppm and 3/46 males and 3/49 females at
16 000 ppm, but in none of the concurrent controls (hyperplasia of the
gastric mucosa was present in 3/46 males at 16 000 ppm). In males, the
incidence of either adenomatous polyps or polypoid adenocarcinoma
alone was not statistically significant, but when the two lesions,
usually rare in occurrence, were considered together, the incidence at
16 000 ppm was statistically significant, as compared to the pooled
control group. In females, incidence of the duodenal lesions in
question, considered alone or together, was not statistically
significant. There was, however, a statistically significant dose-
related trend with respect to incidences of both duodenal lesions in
males and females. The conclusion was made in the report that, under
the conditions of the bioassay, tumours in the duodenum were
associated with treatment with captan (NCI 1977).
Rat
Groups of 50 rats (Osborne-Mendel, 35 days old) of each sex were
fed a diet containing captan (purity not specified) at time-weighted
average doses of 2 525 or 6 050 ppm. The low dosage groups was
actually given 4 000 ppm for 21 weeks, 2 000 ppm for 59 weeks and
control diet for 33 weeks. In the case of the high dosage group,
animals were fed 8 000 ppm for 41 weeks, 4 000 ppm for 39 weeks and
control diet for 33 weeks. (After 18 weeks, 7 males and 1 female in
this group had died and were replaced by healthy animals from a
16 000 ppm-group, on which studies were terminated after 18 weeks
because of high toxicity.) Pooled control groups, of 75 males and 75
females (including the 10 male and 10 female matched controls from the
present study) from similar bioassays of 7 test chemicals, were used
for statistical comparisons. No dose-related trend pertaining to
mortality was apparent. At least 60% females of control and treated
groups were still alive at termination. Survival was slightly lower in
the males, especially in the high dosage group, with only 46% of
animals in the latter group surviving the duration of the study.
Growth retardation occurred in both treated groups throughout the
study. Tumours were observed in animals of all groups, including the
controls, and were of types not uncommonly seen in the strain. There
was no indication of a significant increase in incidence of any
particular type of tumour. Under the conditions of the bioassay, there
was no evidence suggestive of carcinogenic activity of captan (NCI
1977).
Special Studies on Mutagenicity
In vitro microbial assays with tester strains of Salmonella
typhimurium indicated captan to be mutagenic, in the presence of
metabolic activation, to strains TA 1535, 1537, G46 (Herbold and
Buselmaier 1976), TA 100, TA 1537 and TA 98 (McCann et al 1975) and
to strain TA 1535 without metabolic activation (Seiler 1975).
Captan was found to be mutagenic in rec-assay with H17 Rec+
and M45 Rec- strains of Bacillus subtilis at 20 µg/disc and in
reversion assays with Escherichia coli (WP2) and S. typhimurium
tester strains TA 1535 at 50 µg/plate without inclusion of a metabolic
activation preparation in the test systems. At this concentration, no
positive mutagenic response was obtained with S. typhimurium tester
strains TA 1536, TA 1537 and TA 1538 (Shirasu et al 1976).
Captan was evaluated for its ability to induce forward mutations
to Colin E2- resistance in repair deficient strains of E. coli
(E-Coli JC 411/CoLE 2+ was used as the colicin-producing strain).
Positive results were obtained with strains WP2 and WP2 uvr A. No
mutagenic activity was detected in strains CM561 and CD611, which were
more sensitive to the toxicity of captan (Bridges et al 1973).
In a spot test with selected repair-deficient strains of
E. coli, filter paper impregnated with captan was placed on an agar
plate or on the lid of an inverted petri dish to test any volatile
component of captan. Mutagenic response was obtained in each case with
all of the E. coli strains tested, i.e. WP2, WP2 UvrA, WP2 ROC A,
WP2 ExrA and WP2 UvrA ExrA. The high mutation rates noted in UvrA
strains, as compared to other tester strains, indicated that much of
the mutagenic activity probably involved production of a DNA lesion
detectable and repairable by excision repair. There were indications
that a large part of the mutagenic activity of captan was caused by a
short-lived, volatile fraction inducing DNA damage of an excisable
nature. A second diffusable mutagenic component causing excisable DNA
damage might also occur (Bridges et al 1972).
Captan was evaluated for its potential to induce point mutations
to 8-azaquanine resistance with Aspergillus nidulans haploid strain
35 (pa ba A1, anA1, yA2, meth GI, nic A2, nic B8) and mitotic non-
disjunction as well as crossing-over with diploid strain P (first
chromosome: suad E20, ribo A1, faA1, pro A1, paba A1, yA2, ad E20,
bi A1). The test material was found in the spot test to induce point
mutation and mitotic crossing over but not non-disjunction (Bignami
et al 1977).
A significant dose-related increase in the frequency of sister
chromatid exchanges and chromosomal aberrations was observed in
cultured Chinese hamster cells treated with captan at concentrations
of 6 × 10-6 M and above (Tezuka et al 1980).
The frequency of sister chromatid exchanges in human lymphocytes
exposed to captan in vitro at a concentration of 3 × 10-5 M was
significantly elevated. Higher concentrations of captan were too toxic
to test for induction of sister chromatid exchanges (Vigfusson and
Vyse 1980).
The mutagenicity of captan, as a suspension in buffer and as a
source of a volatile component, was tested for the induction of
8-azaguanine and ouabain-resistant mutants in V79-4 Chinese hamster
lung cell cultures. In cells exposed to the captan suspension, there
was no increased incidence of 8-azaguanine resistant colonies. An
increase in the absolute number of ouabain resistant variants,
however, occurred at low incidence, the frequency being dose-
dependent. The volatile component from filter paper impregnated with
captan induced an increase in the number of azaguanine and ouabain
resistant colonies (Arlett et al 1975).
Unscheduled DNA synthesis was induced in cultured SV-40
transformed human fibroblast cells (VA-4) treated with captan at
concentrations of 1.0 to 1 000 µM. Metabolic activation employing S-9
mix from rat liver did not significantly modify the activity of the
compound (Ahmed et al 1977).
The transforming potential of technical captan was evaluated
using the 1-1 subclone of clone A-31 of BALB/3T3 mouse cells or the
subclone C-14 of clone C1-13. No increase in morphologically
transformed foci was induced when cells were exposed to the test
material at concentrations up to 0.1 µg/ml for 4 hours in serum-free
medium or at concentrations ranging from 0.04 to 0.2 µg/ml for 3 days
in foetal bovine serum supplemented medium. Under the conditions of
the assay, technical captan did not induce morphological
transformations (Environmental Health Center 1981).
Captan was among a total of 174 chemicals tests for mutagenicity
in ICR/Ha Swiss mice using a modified dominant lethal assay. Acute
intraperitoneal doses of 9 to 30 mg/kg body weight, single oral dose
at 500 or 800 mg/kg body weight or 5 successive daily oral doses of 25
or 50 mg/kg body weight/day were given to male mice (5-11 per group)
prior to mating. Untreated virgin females were mated weekly with
treated males for 3 or 8 consecutive weeks (i.p. dosing) or for 8
consecutive weeks (oral treatment). It was indicated that captan was
among the "agents producing early foetal deaths and pre-implantation
losses within control limits". Acute intraperitoneal doses of 15 or
30 mg/kg body weight were fatal to 90-100% of the treated males
(Epstein et al 1972).
In a dominant lethal assay, groups of male Charles River mice
were fed technical captan in their diet at 0, 500, 3 000 or 7 000 ppm
for 8 weeks and then mated with untreated female mice weekly for 8
consecutive weeks. Total weight gain over the 8-week period was
reduced in males at 7 000 ppm. There was no treatment-related effect
with respect to fertility rate, the number of implantations, early and
late resorption or viable foetuses (Salamon et al 1977).
Dominant lethal assays were conducted in groups of 15 male mice
or rats treated with technical captan orally at 0, 50, 100 or
200 mg/kg body weight/day or intraperitoneally at 0, 2.5, 5.0 or
10 mg/kg body weight/day for 5 days. The animals were then mated
weekly with virgin females of the respective species consecutively for
12 weeks (mice) or 10 weeks (rats). The females were sacrificed on day
12 or 13 of pregnancy, respectively, for the examination of uterine
contents.
In neither species were compound-related effects seen on
mortality, pregnancy rate and mean total implantations per pregnancy
after oral or intraperitoneal exposure. The mean number of early
deaths per pregnancy was elevated after intraperitoneal treatment at
10 mg/kg body weight/day for the first 7 weeks in both mice and rats
and after oral dosing at and above 100 mg/kg body weight/day for the
first 2 weeks in mice and at all treated levels in the rat. The
increase in the latter species was indicated to be statistically
significant at 100 mg/kg body weight/day during week 4 and at
200 mg/kg body weight/day during weeks 1, 2 and 5. A statistically
significant linear trend in incidence of litters with two or more
early deaths was noted in mice practically through the first 7 weeks
after the intraperitoneal doses and for the first 5 weeks after oral
treatment, and in rats for weeks 4 and 5 and for weeks 1 and 2
following intraperitoneal and oral administration, respectively
(Collins 1972).
Groups of pregnant mice were fed captan in their diet at 0, 100,
1 000 or 5 000 ppm on days 6 through 12 of pregnancy in a specific-
locus somatic cell mutation assay. ("The assay involves exposing
embryos heterozygous for a number of recessive coat-colour markers to
the test article and later looking for clones of mutant cells which
appear as coat colour mosaic patches on otherwise black mice".) The
newborn pups were scored for spots on day 12 and at weaning. Body
weight and food consumption were depressed at 5 000 ppm. No treatment-
related effects were seen on pregnancy rate, litter size, pup survival
to 12 days or gross abnormalities. There was no increased frequency of
recessive spots in the treated groups, suggesting no captan-induced
somatic mutations in the treated mice (Nguyen and Brusick 1980).
Observations in Humans
A study was conducted, using a retrospective cohort design, in a
total of 134 male workers, who had been occupationally exposed to
technical captan for periods ranging from 3 months to over 20 years.
All of these individuals worked in a chemical plant producing only
captan and an analogue of captan. Two-thirds of the study population
were exposed to captan for less than 5 years. There were 18 deaths in
the cohort as compared to 11 expected. Two of the deaths were caused
by tumour of the pancreas and the mediastinum (thymomas),
respectively. The individual with the mediastinum tumour was one of
the 16 men employed for 20 or more years. The excess mortality rate
was primarily attributable to cardiovascular diseases and external
causes. Neither duodenal cancer nor increased incidence of cancers was
observed among the study population. Medical records for those
employees still alive were not available (Palshaw 1980).
COMMENTS
A three-generation reproduction study in rats, as a replacement
study for the one previously conducted by IBT, did not adequately
demonstrate a no-effect level on reproduction, although data on F2c
litters revealed no evidence of teratogenicity. Teratology studies in
rabbits treated orally and in mice exposed to captan orally,
subcutaneously or by inhalation were negative. (The latter studies in
mice were available in the form of an abstract.) In a hamster
teratology study, while incidence of malformations was probably not
affected by captan, conclusions with respect to teratogenicity in this
respect cannot be made with confidence until details of the non-
specified rib anomalies are made available. If the rib anomalies
comprised abnormalities of minimal concern, then 50 mg/kg body weight
might be considered as a no-effect level for the study. A
carcinogenicity study in Osborne-Mendel rats gave no indication of
carcinogenic activity. However, an increased incidence of duodenal
tumours was observed in a bioassay with B6C 3F1 hybrid mice fed
dietary levels of 8 000 or 16 000 ppm for 80 weeks. This particular
finding was reportedly confirmed in another 2-year mouse study at
dietary levels of captan ranging from 6 000 to 16 000 ppm (Stauffer
1980). It is understood that an additional 2-year study in mice using
dietary levels of 100 to 6 000 ppm is currently underway (Stauffer
1980). A 12-month interim report of a new 2-year study (International
Research and Development 1980) in rats was received, but a complete
evaluation of the study would depend on availability of the final
report. Terminal survivors from this study exhibited no compound-
related lesions in the duodenum, according to a preliminary report of
histopathological findings in the particular tissue (Arceo 1981).
A variety of mutagenicity studies were submitted, which provided
further evidence that captan was mutagenic to most microbial or other
non-mammalian systems in vitro and to isolated mammalian cell
systems. Captan did not induce morphological transformation of
BALB/3T3 cells in vitro or somatic cell mutation in mice in vivo.
Results of dominant lethal assays appeared to be equivocal.
The data provided on the retrospective study in humans, while
indicating no occurrence of duodenal cancer or increased incidence of
cancers among the cohort, were of limited value since the majority in
the study population were exposed to captan for less than five years.
In view of the concerns with respect to carcinogenicity in mice,
the inability to demonstrate a no-effect level in the 3-generation
reproduction study in rats and the finding of the long term/
carcinogenicity studies in both mice and rats conducted by IBT being
invalid, the Meeting decided that the full ADI be modified to a
temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Final report of the new two-year study in rats.
2. Complete report of the two-year study in mice understood to have
been completed.
3. Full data of a new two-year oral carcinogenicity study in mice,
known to be in progress.
4. A two-generation (two litters/generation) reproduction study in
rats to define a no-effect level on reproduction.
Desirable
1. Details of unspecified rib anomalies in the hamster teratology
study.
2. Further observations in humans.
3. Full details of the one-generation (one litter) reproduction
study in rats.
REFERENCES
Ahmed, F.E., Hart, R. and Lewis, N.J. Pesticide induced DNA damage
1977 and its repair in cultured human cells. Mutat. Res.
42:f161-174.
Arceo, R.J. Gross and microscopic examination of the duodenum.
1981 Report from International Research and Development Corp.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
Arlett, C.F., Turnbull, D., Harcourt, B.A., Lehmann, A.R. and
1975 Colella, C.M. A comparison of the 8-azaguanine and ouabain-
resistance systems for the selection of induced mutant
Chinese hamster cells. Mutat. Res. 33:261-278.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. and Morpurgo, G.
1977 Mutagenic and recombinogence action of pesticides in
Aspergillus nidulans. Mutat. Res. 46:395-402.
Bridges, B.A., Mottershead, R.P., Rothwell, M.H. and Green, M.H.L.
1972 Repair-deficient bacterial strains unsuitable for
mutagenicity screening: Test with the fungicide captan.
Chem. Biol. Interact. 5:77-84.
Bridges, B.A., Mottershead, R.P. and Colella, C. Induction of
1973 forward mutations to colicin E2 resistance in repair
deficient strains of Escherichia coli: Experiments with
ultraviolet light and captan. Mutat. Res. 23:303-313.
Collins, T.F.X. Dominant lethal assay. I. Captan. Food Cosmet.
1972 Toxicol. 10:353-361.
Couch, R.C., Siegel, M.R. and Dorough, H.W. Fate of captan and
1977 folpet in rats and their effects on isolated liver nuclei.
Pestic. Biochem. and Physiol. 7:547-558.
Courtney, K.D., Andres, J.E. and Stevens, J.T. Inhalation teratology
1978 studies with captan and folpet. Toxicol. appl. Pharmacol.
45(1):292.
Environmental Health Center. Captan technical (Lot No. 5093-25-1).
1981 Morphological transformation of BALB/3T3 cells. Report from
Environmental Health Center, Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Epstein, S.S., Arnold, E., Andrea, J., Bacs, W. and Bishop, Y.
1972 Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol. 23:288-325.
Goldenthal, E.I. Pilot teratology study in hamster. Report from
International Research and Development Corp. submitted to
the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Goldenthal, E.I., Jessop, D.C. and Rodwell, D.E. Captan teratology
study in hamsters. Report from International Research and
Development Corp., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Herbold, B. and Buselmaier, W. Induction of point mutations by
1976 different chemical mechanisms in the liver microsomal assay.
Mutation Research, 40:73-84.
International Research and Development. Captan technical, SX 944
1980 2-year oral toxicity/carcinogenicity study of captan in rats
(12-month interim). Report from International Research and
Development Corp. submitted to the World Health Organization
by Stauffer Chemical Co. (Unpublished)
1982a Captan three generation reproduction study in rats. Report
from International Research and Development Corp. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
1982b One-generation reproduction study in rats with captan.
Status report from International Research and Development
Corp. submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
McCann, J., Choi, E., Yamasaki, E. and Ames, B.N. Detection of
1975 carcinogens as mutagens in the salmonella/microsome test:
Assay of 300 chemicals. Proc. Nat. Acad. Sci.
72(12):5135-5139.
Miaullis, J.B., Quale, D.C., Vispetto, A.R. and DeBaun, J.R. Effect
1980 of dietary captan on soluble thiol content of liver and
duodenal tissues. Report from Stauffer Chemical Co.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
NCI. Bioassay of captan for possible carcinogenicity. National
1977 Cancer Institute. Carcinogenesis Technical Report Series No.
15. D.H.E.W. Publication No. (NIH) 77-815.
Nguyen, T.D. and Brusick, D.J. Mutagenic evaluation of captan in the
1980 somatic cell mutation assay. Final report from Litton
Bionetics, Inc., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palmer, A.K., Barton, S.J. and Clark, R. Effect of technical captan
1981 on pregnancy of the New Zealand White rabbit. Huntingdon
Research Centre, England, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palshaw, M.W. An epidemiologic study of mortality within a cohort of
captan workers. Report from Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Salamon, C., Smith, S., Kennedy, G.L., Kineshita, F.K. and
1977 Keplinger, M.L. Dominant lethal study with captan technical
in albino mice exposed for 8 weeks to the chemical in the
diet. Report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Stauffer
Chemical Co. (The study was recently validated by the
Canadian Government.) (Unpublished)
Seiler, J.P. Evaluation of some pesticides for mutagenicity. Proc.
1975 of the Eur. Soc. Toxicol. 17:398-404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutat. Res. 40:19-30.
Stauffer, Captan RPAR risk rebuttal and summaries of benefits. Pages
5 and 19, Volume 1. Document prepared by Stauffer Chemical
Co. November 26, 1980, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Tezuka, H., Ando, N., Suzuki, R., Terahata, M., Moriya, M. and
1980 Shirasu, Y. Sister-chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with
pesticides positive in microbial reversion assays. Mutat.
Res. 78:177-191.
Vigfusson, N.V., Vyse, E.R. The effect of the pesticides, dexon,
1980 captan and round-up on sister-chromatid exchanges in human
lymphocytes in vitro. Mutat. Res. 79:53-57.
Explanation
This fungicide was evaluated by the Joint Meetings in 1965, 1969,
1973, 1974, 1977 and 1978 (FAO/WHO 1965b, 1970b, 1974b, 1975b, 1978b
and 1979).1/ A full ADI of 0-0.1 mg/kg body weight was established on
the basis of the no-effect levels partially taken from data reported
by Industrial Bio-Test Laboratories (IBT). The latter include
long-term/carcinogenicity studies in mice and rats, a three-generation
reproduction in rats and teratology studies in rabbits, dogs and
monkeys.
New studies have become available and are summarized and
discussed in this monograph addendum. In addition, the major concerns
raised in a Rebuttable Presumption Against Registration (RPAR) issued
in the U.S. for captan in August 1980 relating to oncogenic and
mutagenic effects have been considered in this review of new data.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
In normal, sham-operated and partially hepatectomized male rats,
the rate of elimination of a single dose or three daily doses of
(35S)-captan, at 6 mg/kg body weight given intraperitoneally, was
similar. Approximately 77-89% of the total administered radioactivity
was eliminated within 72 hours, of the single or multiple dosing
primarily in the urine but also in the faeces. The pattern of
distribution of 35S-residues in tissues from these rats, sacrificed
75 hours after the single dose or 24 hours after the third daily dose,
was also essentially the same with the highest concentration being
detected in the blood and the lowest in brain. Additionally, nuclei
isolated from liver of rats after treatment with three daily
intraperitoneal doses of (35S)-captan contained appreciable amounts
of radioactivity. There were no differences in normal, sham-operated
or partially hepatectomized animals (Couch et al 1977).
1/ See Annex 2 for WHO and FAO documentation.
Effects on Other Biochemical Parameters
Incubation of isolated liver nuclei from normal rats with captan
for 10 minutes at 37°C resulted in changes in distribution of various
protein fractions. One of the major changes was extensive aggregation
of nuclear sap proteins and deoxyribonucleoprotein. Binding studies
with isolated rat liver nuclei indicated evolution as gas(es) of a
large percentage of the total radioactivity from (35S)-captan,
suggesting occurrence of an interaction of captan with thiol groups in
the nucleus with subsequent release of the SCCl3 moiety. Following
incubation with 35S-captan, the rate of 35S binding and the
percentage of 35S-labelled gas evolved were slightly higher in
isolated liver nuclei from normal rats than from partially
hepatectomized rats (Couch et al 1977).
In adult Swiss Webster female mice fed technical captan in their
diet at levels ranging from 4 000 to 16 000 ppm for up to 35 days,
soluble thiol levels in the duodenum from the proximal end were
elevated throughout the feeding period. The increase, not strictly
dose-dependent, was already evident on day 1 and peaked between 14 and
21 days following initiation of the study. The liver from these
animals exhibited normal or slightly depressed levels of soluble
thiols. Results from this experiment were confirmed by those of a
second test with groups of female mice of the same strain fed dietary
levels of captan ranging from 500 to 16 000 ppm for up to 213 days. In
the latter experiment, a dose-response relationship was noted with
respect to levels of duodeneal soluble thiols (Miaullis et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of 15 male and 30 female rats (Charles River COBS CD
strain, 31 days old) were fed technical captan in their diet at the
equivalent of 0, 25, 100, 250 or 500 mg/kg body weight/day for 102
days and then mated to initiate a three-generation reproduction study.
The procedure normally used in a standard three-generation (two
litters/generation) study was followed but an additional litter, F2c,
was produced from F1 parents for teratological evaluation. Pups of
the second litters (F1b and F2b) became parents of the next
generation. After the third mating trial, F1 dams were sacrificed on
gestation day 19 and the F2c foetuses were removed by caesarean
section for examination of external, skeletal and soft tissue
abnormalities. In the parental generations, no compound-related effect
on mortality, appearance and behaviour was apparent. Dose-related
growth depression occurred in both sexes at and above 100 mg/kg body
weight/day throughout all three generations and in F0 and F2 males
even at 25 mg/kg body weight/day. Food consumption was decreased at
all dosage levels in a dose-dependent trend in all three generations.
A reduction in pregnancy rate was observed in F0 generation (first
mating trial) at 500 mg/kg body weight/day and possibly also at
250 mg/kg body weight/day. In the progeny generations litter size at
birth was reduced in all treated groups in F1a and F1b litters and at
100 mg/kg body weight/day and above in F2a and F2b litters. Pup
survival was adversely affected at 250 mg/kg body weight/day in F1a,
F2a and F3a litters throughout lactation day 4 and at 500 mg/kg body
weight practically throughout the lactation period in all progeny
generations. Dose-related depression in pup weight was seen at and
above 100 mg/kg body weight/day in litters of all three generations
and at 25 mg/kg body weight/day in most litters. Weanling pups in each
generation exhibited no compound-related abnormalities at necropsy.
Teratological evaluation of the F2c litter revealed no effect
attributable to treatment with respect to fertility, number of non-
viable foetuses, early and late resorptions and post-implantation loss
per dam, sex ratio and incidence of foetuses or litters with
malformations. Foetal body weight was depressed at 500 mg/kg body
weight/day. There was a slight decrease in the mean number of viable
foetuses per dam and a corresponding decrease in total implantations
at 250 mg/kg body weight/day and above. The number of corpora lutea
per dam was reduced at 500 mg/kg body weight/day.
The study failed to demonstrate a no-effect level on
reproduction, although teratogenic effect was not evident from data on
the F2c litter (International Research and Development 1982a).
A preliminary report of a one-generation, one litter reproduction
study in rats (Charles River CD) with groups of 15 males and 30
females fed captan-treated diets for 100 days prior to mating
indicated that "minor" effects on litter weight, noted at 25 mg/kg
body weight/day, were not apparent at 6 or 12.5 mg/kg body weight/day.
No other reproductive effects were reportedly noted (International
Research and Development 1982b).
Special Studies on Teratogenicity
Mouse
According to results reported in an abstract, pregnant CD-1
mice exposed via inhalation to captan (whole body exposure) at
approximately 488 mg/hour/m3 (average particle size 5 µm), 4
hours/day from day 6 through 13 of gestation produced no malformed
foetuses. Similarly, captan was not teratogenic in pregnant CD-1 mice
treated with 100 mg/kg body weight/day of the compound orally or
subcutaneously from day 6 through 15 of gestation. Reduction of
maternal body weight and a slight increase in foetal mortality were
noted in the experiments (Courtney et al 1978).
Hamster
In a pilot (range-finding) teratology study with mated female
(Golden Syrian) hamsters given oral doses of technical captan at 50,
100, 200, 300 or 400 mg/kg body weight/day on day 5 through 10 of
gestation, there was no maternal death or treatment-related effect on
appearance and behaviour, number of corpora lutea, implantations or
resorptions and litter size. The only adverse effect observed was a
dose-related decrease in maternal weight gain at 300 mg/kg body
weight/day and above (Goldenthal 1978).
Groups of 30 mated female Golden Syrian hamsters were given
technical captan in 1% carboxy methylcellulose by gavage at 0, 50, 200
or 400 mg/kg body weight/day on days 5 through 10 of gestation. The
females were sacrificed on day 14 of gestation and foetuses were
removed by caesarean section for external, soft tissue and skeletal
examination. Four females at 400 mg/kg body weight/day and one female
at 200 mg/kg body weight/day died of undetermined cause(s). Stained
and matted anogenital fur and vaginal discharge were noted at
400 mg/kg body weight/day. Other adverse effects at 400 mg/kg body
weight/ day included maternal weight loss, a reduction in litter size
and foetal weight and an increase in early and late resorptions.
Additionally, increased post-implantation loss and a significant
difference in sex (M/F) ratio occurred in this dosage group. Maternal
weight gain and foetal weight were also depressed at 200 mg/kg body
weight/day. In general the incidence of malformations appeared to be
comparable in all groups. However, the incidence of non-specified rib
anomalies in foetuses was 0.3, 1.4, 1.8, 2.9%, respectively, at 0, 50,
200 and 400 mg/kg body weight/day and in litters was 3.6, 15.4, 15.4
and 8.7% (Goldenthal et al 1979).
Rabbit
Groups of 15 mated female New Zealand White rabbits were
intubated with technical captan (89% pure) in 0.5% sodium
carboxymethyl cellulose at 0, 6, 12, 25 or 60 mg/kg body weight/day on
days 6-28 inclusive of pregnancy. Does were sacrificed on day 29 of
pregnancy and uterine contents were examined. Foetuses were evaluated
for gross, skeletal and visceral abnormalities. Pregnancy rate at
60 mg/kg body weight/day and maternal body weight at and above
12 mg/kg body weight/day were adversely affected. Incidence of non-
specific signs (anorexia, reduced faecal output and water consumption)
was increased at 60 mg/kg body weight/day and possibly also at
25 mg/kg body weight/day. Litter and foetal weights as well as foetal
crown/rump length were depressed at 60 mg/kg body weight/ day. There
were no compound-related effects on the other tested parameters, i.e.
mortality, abortion rate, total resorptions, pre-implantation loss and
post-implantation loss including stillbirths), total litter loss in
pregnant animals surviving to autopsy, litter size, sex ratio and
incidence of major malformations or minor anomalies (Palmer
et al 1981).
Special Studies for Carcinogenicity
Mouse
Groups of 50 male and 50 female mice (B6C 3F1 hybrid; 35-day old)
were fed captan of unspecified purity in their diet at 8 000 or
16 000 ppm for 80 weeks and then maintained on a control diet for 11
weeks. The control group comprised 10 mice of each sex. The animals
were placed in the same room as groups of mice receiving aldrin or
photodieldrin and their controls. Pooled controls, used for
statistical comparisons, consisted of a total of 80 male and 80 female
mice of the same strain from seven similar bio-assays (including the
one with captan). Survival was good and no dose-related effect on
mortality was evident. Growth was depressed at 16 000 ppm throughout
most of the study. The total incidence of tumours was low in control
and treated groups. However, adenomatous polyp of the duodenum was
noted in 0/9 males and 1/9 female controls, 2/43 males and 1/49
females at 8 000 ppm and 2/46 males and 0/49 females at 16 000 ppm. In
addition, polypoid adenocarcinoma of the duodenum occurred in 1/43
males and 0/49 females at 8 000 ppm and 3/46 males and 3/49 females at
16 000 ppm, but in none of the concurrent controls (hyperplasia of the
gastric mucosa was present in 3/46 males at 16 000 ppm). In males, the
incidence of either adenomatous polyps or polypoid adenocarcinoma
alone was not statistically significant, but when the two lesions,
usually rare in occurrence, were considered together, the incidence at
16 000 ppm was statistically significant, as compared to the pooled
control group. In females, incidence of the duodenal lesions in
question, considered alone or together, was not statistically
significant. There was, however, a statistically significant dose-
related trend with respect to incidences of both duodenal lesions in
males and females. The conclusion was made in the report that, under
the conditions of the bioassay, tumours in the duodenum were
associated with treatment with captan (NCI 1977).
Rat
Groups of 50 rats (Osborne-Mendel, 35 days old) of each sex were
fed a diet containing captan (purity not specified) at time-weighted
average doses of 2 525 or 6 050 ppm. The low dosage groups was
actually given 4 000 ppm for 21 weeks, 2 000 ppm for 59 weeks and
control diet for 33 weeks. In the case of the high dosage group,
animals were fed 8 000 ppm for 41 weeks, 4 000 ppm for 39 weeks and
control diet for 33 weeks. (After 18 weeks, 7 males and 1 female in
this group had died and were replaced by healthy animals from a
16 000 ppm-group, on which studies were terminated after 18 weeks
because of high toxicity.) Pooled control groups, of 75 males and 75
females (including the 10 male and 10 female matched controls from the
present study) from similar bioassays of 7 test chemicals, were used
for statistical comparisons. No dose-related trend pertaining to
mortality was apparent. At least 60% females of control and treated
groups were still alive at termination. Survival was slightly lower in
the males, especially in the high dosage group, with only 46% of
animals in the latter group surviving the duration of the study.
Growth retardation occurred in both treated groups throughout the
study. Tumours were observed in animals of all groups, including the
controls, and were of types not uncommonly seen in the strain. There
was no indication of a significant increase in incidence of any
particular type of tumour. Under the conditions of the bioassay, there
was no evidence suggestive of carcinogenic activity of captan (NCI
1977).
Special Studies on Mutagenicity
In vitro microbial assays with tester strains of Salmonella
typhimurium indicated captan to be mutagenic, in the presence of
metabolic activation, to strains TA 1535, 1537, G46 (Herbold and
Buselmaier 1976), TA 100, TA 1537 and TA 98 (McCann et al 1975) and
to strain TA 1535 without metabolic activation (Seiler 1975).
Captan was found to be mutagenic in rec-assay with H17 Rec+
and M45 Rec- strains of Bacillus subtilis at 20 µg/disc and in
reversion assays with Escherichia coli (WP2) and S. typhimurium
tester strains TA 1535 at 50 µg/plate without inclusion of a metabolic
activation preparation in the test systems. At this concentration, no
positive mutagenic response was obtained with S. typhimurium tester
strains TA 1536, TA 1537 and TA 1538 (Shirasu et al 1976).
Captan was evaluated for its ability to induce forward mutations
to Colin E2- resistance in repair deficient strains of E. coli
(E-Coli JC 411/CoLE 2+ was used as the colicin-producing strain).
Positive results were obtained with strains WP2 and WP2 uvr A. No
mutagenic activity was detected in strains CM561 and CD611, which were
more sensitive to the toxicity of captan (Bridges et al 1973).
In a spot test with selected repair-deficient strains of
E. coli, filter paper impregnated with captan was placed on an agar
plate or on the lid of an inverted petri dish to test any volatile
component of captan. Mutagenic response was obtained in each case with
all of the E. coli strains tested, i.e. WP2, WP2 UvrA, WP2 ROC A,
WP2 ExrA and WP2 UvrA ExrA. The high mutation rates noted in UvrA
strains, as compared to other tester strains, indicated that much of
the mutagenic activity probably involved production of a DNA lesion
detectable and repairable by excision repair. There were indications
that a large part of the mutagenic activity of captan was caused by a
short-lived, volatile fraction inducing DNA damage of an excisable
nature. A second diffusable mutagenic component causing excisable DNA
damage might also occur (Bridges et al 1972).
Captan was evaluated for its potential to induce point mutations
to 8-azaquanine resistance with Aspergillus nidulans haploid strain
35 (pa ba A1, anA1, yA2, meth GI, nic A2, nic B8) and mitotic non-
disjunction as well as crossing-over with diploid strain P (first
chromosome: suad E20, ribo A1, faA1, pro A1, paba A1, yA2, ad E20,
bi A1). The test material was found in the spot test to induce point
mutation and mitotic crossing over but not non-disjunction (Bignami
et al 1977).
A significant dose-related increase in the frequency of sister
chromatid exchanges and chromosomal aberrations was observed in
cultured Chinese hamster cells treated with captan at concentrations
of 6 × 10-6 M and above (Tezuka et al 1980).
The frequency of sister chromatid exchanges in human lymphocytes
exposed to captan in vitro at a concentration of 3 × 10-5 M was
significantly elevated. Higher concentrations of captan were too toxic
to test for induction of sister chromatid exchanges (Vigfusson and
Vyse 1980).
The mutagenicity of captan, as a suspension in buffer and as a
source of a volatile component, was tested for the induction of
8-azaguanine and ouabain-resistant mutants in V79-4 Chinese hamster
lung cell cultures. In cells exposed to the captan suspension, there
was no increased incidence of 8-azaguanine resistant colonies. An
increase in the absolute number of ouabain resistant variants,
however, occurred at low incidence, the frequency being dose-
dependent. The volatile component from filter paper impregnated with
captan induced an increase in the number of azaguanine and ouabain
resistant colonies (Arlett et al 1975).
Unscheduled DNA synthesis was induced in cultured SV-40
transformed human fibroblast cells (VA-4) treated with captan at
concentrations of 1.0 to 1 000 µM. Metabolic activation employing S-9
mix from rat liver did not significantly modify the activity of the
compound (Ahmed et al 1977).
The transforming potential of technical captan was evaluated
using the 1-1 subclone of clone A-31 of BALB/3T3 mouse cells or the
subclone C-14 of clone C1-13. No increase in morphologically
transformed foci was induced when cells were exposed to the test
material at concentrations up to 0.1 µg/ml for 4 hours in serum-free
medium or at concentrations ranging from 0.04 to 0.2 µg/ml for 3 days
in foetal bovine serum supplemented medium. Under the conditions of
the assay, technical captan did not induce morphological
transformations (Environmental Health Center 1981).
Captan was among a total of 174 chemicals tests for mutagenicity
in ICR/Ha Swiss mice using a modified dominant lethal assay. Acute
intraperitoneal doses of 9 to 30 mg/kg body weight, single oral dose
at 500 or 800 mg/kg body weight or 5 successive daily oral doses of 25
or 50 mg/kg body weight/day were given to male mice (5-11 per group)
prior to mating. Untreated virgin females were mated weekly with
treated males for 3 or 8 consecutive weeks (i.p. dosing) or for 8
consecutive weeks (oral treatment). It was indicated that captan was
among the "agents producing early foetal deaths and pre-implantation
losses within control limits". Acute intraperitoneal doses of 15 or
30 mg/kg body weight were fatal to 90-100% of the treated males
(Epstein et al 1972).
In a dominant lethal assay, groups of male Charles River mice
were fed technical captan in their diet at 0, 500, 3 000 or 7 000 ppm
for 8 weeks and then mated with untreated female mice weekly for 8
consecutive weeks. Total weight gain over the 8-week period was
reduced in males at 7 000 ppm. There was no treatment-related effect
with respect to fertility rate, the number of implantations, early and
late resorption or viable foetuses (Salamon et al 1977).
Dominant lethal assays were conducted in groups of 15 male mice
or rats treated with technical captan orally at 0, 50, 100 or
200 mg/kg body weight/day or intraperitoneally at 0, 2.5, 5.0 or
10 mg/kg body weight/day for 5 days. The animals were then mated
weekly with virgin females of the respective species consecutively for
12 weeks (mice) or 10 weeks (rats). The females were sacrificed on day
12 or 13 of pregnancy, respectively, for the examination of uterine
contents.
In neither species were compound-related effects seen on
mortality, pregnancy rate and mean total implantations per pregnancy
after oral or intraperitoneal exposure. The mean number of early
deaths per pregnancy was elevated after intraperitoneal treatment at
10 mg/kg body weight/day for the first 7 weeks in both mice and rats
and after oral dosing at and above 100 mg/kg body weight/day for the
first 2 weeks in mice and at all treated levels in the rat. The
increase in the latter species was indicated to be statistically
significant at 100 mg/kg body weight/day during week 4 and at
200 mg/kg body weight/day during weeks 1, 2 and 5. A statistically
significant linear trend in incidence of litters with two or more
early deaths was noted in mice practically through the first 7 weeks
after the intraperitoneal doses and for the first 5 weeks after oral
treatment, and in rats for weeks 4 and 5 and for weeks 1 and 2
following intraperitoneal and oral administration, respectively
(Collins 1972).
Groups of pregnant mice were fed captan in their diet at 0, 100,
1 000 or 5 000 ppm on days 6 through 12 of pregnancy in a specific-
locus somatic cell mutation assay. ("The assay involves exposing
embryos heterozygous for a number of recessive coat-colour markers to
the test article and later looking for clones of mutant cells which
appear as coat colour mosaic patches on otherwise black mice".) The
newborn pups were scored for spots on day 12 and at weaning. Body
weight and food consumption were depressed at 5 000 ppm. No treatment-
related effects were seen on pregnancy rate, litter size, pup survival
to 12 days or gross abnormalities. There was no increased frequency of
recessive spots in the treated groups, suggesting no captan-induced
somatic mutations in the treated mice (Nguyen and Brusick 1980).
Observations in Humans
A study was conducted, using a retrospective cohort design, in a
total of 134 male workers, who had been occupationally exposed to
technical captan for periods ranging from 3 months to over 20 years.
All of these individuals worked in a chemical plant producing only
captan and an analogue of captan. Two-thirds of the study population
were exposed to captan for less than 5 years. There were 18 deaths in
the cohort as compared to 11 expected. Two of the deaths were caused
by tumour of the pancreas and the mediastinum (thymomas),
respectively. The individual with the mediastinum tumour was one of
the 16 men employed for 20 or more years. The excess mortality rate
was primarily attributable to cardiovascular diseases and external
causes. Neither duodenal cancer nor increased incidence of cancers was
observed among the study population. Medical records for those
employees still alive were not available (Palshaw 1980).
COMMENTS
A three-generation reproduction study in rats, as a replacement
study for the one previously conducted by IBT, did not adequately
demonstrate a no-effect level on reproduction, although data on F2c
litters revealed no evidence of teratogenicity. Teratology studies in
rabbits treated orally and in mice exposed to captan orally,
subcutaneously or by inhalation were negative. (The latter studies in
mice were available in the form of an abstract.) In a hamster
teratology study, while incidence of malformations was probably not
affected by captan, conclusions with respect to teratogenicity in this
respect cannot be made with confidence until details of the non-
specified rib anomalies are made available. If the rib anomalies
comprised abnormalities of minimal concern, then 50 mg/kg body weight
might be considered as a no-effect level for the study. A
carcinogenicity study in Osborne-Mendel rats gave no indication of
carcinogenic activity. However, an increased incidence of duodenal
tumours was observed in a bioassay with B6C 3F1 hybrid mice fed
dietary levels of 8 000 or 16 000 ppm for 80 weeks. This particular
finding was reportedly confirmed in another 2-year mouse study at
dietary levels of captan ranging from 6 000 to 16 000 ppm (Stauffer
1980). It is understood that an additional 2-year study in mice using
dietary levels of 100 to 6 000 ppm is currently underway (Stauffer
1980). A 12-month interim report of a new 2-year study (International
Research and Development 1980) in rats was received, but a complete
evaluation of the study would depend on availability of the final
report. Terminal survivors from this study exhibited no compound-
related lesions in the duodenum, according to a preliminary report of
histopathological findings in the particular tissue (Arceo 1981).
A variety of mutagenicity studies were submitted, which provided
further evidence that captan was mutagenic to most microbial or other
non-mammalian systems in vitro and to isolated mammalian cell
systems. Captan did not induce morphological transformation of
BALB/3T3 cells in vitro or somatic cell mutation in mice in vivo.
Results of dominant lethal assays appeared to be equivocal.
The data provided on the retrospective study in humans, while
indicating no occurrence of duodenal cancer or increased incidence of
cancers among the cohort, were of limited value since the majority in
the study population were exposed to captan for less than five years.
In view of the concerns with respect to carcinogenicity in mice,
the inability to demonstrate a no-effect level in the 3-generation
reproduction study in rats and the finding of the long term/
carcinogenicity studies in both mice and rats conducted by IBT being
invalid, the Meeting decided that the full ADI be modified to a
temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Final report of the new two-year study in rats.
2. Complete report of the two-year study in mice understood to have
been completed.
3. Full data of a new two-year oral carcinogenicity study in mice,
known to be in progress.
4. A two-generation (two litters/generation) reproduction study in
rats to define a no-effect level on reproduction.
Desirable
1. Details of unspecified rib anomalies in the hamster teratology
study.
2. Further observations in humans.
3. Full details of the one-generation (one litter) reproduction
study in rats.
REFERENCES
Ahmed, F.E., Hart, R. and Lewis, N.J. Pesticide induced DNA damage
1977 and its repair in cultured human cells. Mutat. Res.
42:f161-174.
Arceo, R.J. Gross and microscopic examination of the duodenum.
1981 Report from International Research and Development Corp.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
Arlett, C.F., Turnbull, D., Harcourt, B.A., Lehmann, A.R. and
1975 Colella, C.M. A comparison of the 8-azaguanine and ouabain-
resistance systems for the selection of induced mutant
Chinese hamster cells. Mutat. Res. 33:261-278.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. and Morpurgo, G.
1977 Mutagenic and recombinogence action of pesticides in
Aspergillus nidulans. Mutat. Res. 46:395-402.
Bridges, B.A., Mottershead, R.P., Rothwell, M.H. and Green, M.H.L.
1972 Repair-deficient bacterial strains unsuitable for
mutagenicity screening: Test with the fungicide captan.
Chem. Biol. Interact. 5:77-84.
Bridges, B.A., Mottershead, R.P. and Colella, C. Induction of
1973 forward mutations to colicin E2 resistance in repair
deficient strains of Escherichia coli: Experiments with
ultraviolet light and captan. Mutat. Res. 23:303-313.
Collins, T.F.X. Dominant lethal assay. I. Captan. Food Cosmet.
1972 Toxicol. 10:353-361.
Couch, R.C., Siegel, M.R. and Dorough, H.W. Fate of captan and
1977 folpet in rats and their effects on isolated liver nuclei.
Pestic. Biochem. and Physiol. 7:547-558.
Courtney, K.D., Andres, J.E. and Stevens, J.T. Inhalation teratology
1978 studies with captan and folpet. Toxicol. appl. Pharmacol.
45(1):292.
Environmental Health Center. Captan technical (Lot No. 5093-25-1).
1981 Morphological transformation of BALB/3T3 cells. Report from
Environmental Health Center, Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Epstein, S.S., Arnold, E., Andrea, J., Bacs, W. and Bishop, Y.
1972 Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol. 23:288-325.
Goldenthal, E.I. Pilot teratology study in hamster. Report from
International Research and Development Corp. submitted to
the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Goldenthal, E.I., Jessop, D.C. and Rodwell, D.E. Captan teratology
study in hamsters. Report from International Research and
Development Corp., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Herbold, B. and Buselmaier, W. Induction of point mutations by
1976 different chemical mechanisms in the liver microsomal assay.
Mutation Research, 40:73-84.
International Research and Development. Captan technical, SX 944
1980 2-year oral toxicity/carcinogenicity study of captan in rats
(12-month interim). Report from International Research and
Development Corp. submitted to the World Health Organization
by Stauffer Chemical Co. (Unpublished)
1982a Captan three generation reproduction study in rats. Report
from International Research and Development Corp. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
1982b One-generation reproduction study in rats with captan.
Status report from International Research and Development
Corp. submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
McCann, J., Choi, E., Yamasaki, E. and Ames, B.N. Detection of
1975 carcinogens as mutagens in the salmonella/microsome test:
Assay of 300 chemicals. Proc. Nat. Acad. Sci.
72(12):5135-5139.
Miaullis, J.B., Quale, D.C., Vispetto, A.R. and DeBaun, J.R. Effect
1980 of dietary captan on soluble thiol content of liver and
duodenal tissues. Report from Stauffer Chemical Co.
submitted to the World Health Organization by Stauffer
Chemical Co. (Unpublished)
NCI. Bioassay of captan for possible carcinogenicity. National
1977 Cancer Institute. Carcinogenesis Technical Report Series No.
15. D.H.E.W. Publication No. (NIH) 77-815.
Nguyen, T.D. and Brusick, D.J. Mutagenic evaluation of captan in the
1980 somatic cell mutation assay. Final report from Litton
Bionetics, Inc., U.S.A. submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palmer, A.K., Barton, S.J. and Clark, R. Effect of technical captan
1981 on pregnancy of the New Zealand White rabbit. Huntingdon
Research Centre, England, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Palshaw, M.W. An epidemiologic study of mortality within a cohort of
captan workers. Report from Stauffer Chemical Co. submitted
to the World Health Organization by Stauffer Chemical Co.
(Unpublished)
Salamon, C., Smith, S., Kennedy, G.L., Kineshita, F.K. and
1977 Keplinger, M.L. Dominant lethal study with captan technical
in albino mice exposed for 8 weeks to the chemical in the
diet. Report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Stauffer
Chemical Co. (The study was recently validated by the
Canadian Government.) (Unpublished)
Seiler, J.P. Evaluation of some pesticides for mutagenicity. Proc.
1975 of the Eur. Soc. Toxicol. 17:398-404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. and Kada, T.
1976 Mutagenicity screening of pesticides in the microbial
system. Mutat. Res. 40:19-30.
Stauffer, Captan RPAR risk rebuttal and summaries of benefits. Pages
5 and 19, Volume 1. Document prepared by Stauffer Chemical
Co. November 26, 1980, submitted to the World Health
Organization by Stauffer Chemical Co. (Unpublished)
Tezuka, H., Ando, N., Suzuki, R., Terahata, M., Moriya, M. and
1980 Shirasu, Y. Sister-chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with
pesticides positive in microbial reversion assays. Mutat.
Res. 78:177-191.
Vigfusson, N.V., Vyse, E.R. The effect of the pesticides, dexon,
1980 captan and round-up on sister-chromatid exchanges in human
lymphocytes in vitro. Mutat. Res. 79:53-57.
