
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
ISOFENPHOS
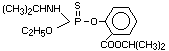 Explanation
Isofenphos was evaluated by the Joint Meeting in 1981 (FAO/WHO
1982)1, and a temporary ADI was allocated. The absence of an
appropriate neurotoxicity study in hens was the basis for the
temporary nature of the ADI. Such a study was required for a full
evaluation of the insecticide.
The required study has not been provided, although another acute
delayed neurotoxicity study in hens, a teratology study in rats and a
mutagenicity study have been made available. These new studies are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies in Teratogenicity
Rat
Groups of 25 mated rats (Wistar KFM-HAN outbred strain) were
intubated with isofenphos (91.8% pure) as a suspension in
carboxymethylcellulose at 0, 0.3, 1.0 or 3 mg/kg bw/day from day 6
through day 15 of pregnancy (day 0 = day positive vaginal smear or
vaginal plug observed). The dams were sacrificed on day 21 of
pregnancy and foetuses were removed by caesarean section for external,
skeletal and visceral examination. There were no mortalities or signs
of toxicity. Maternal body weight and food consumption from day 0 to
day 21 of pregnancy, the number of embryonal deaths, foetal deaths,
dead foetuses and live foetuses as well as foetal weight were not
1 See Annex 2 for WHO and FAO documentation.
significantly different between control and treated groups. A
significant difference from the concurrent control group in the sex
ratio of foetuses was noted at 3 mg/kg bw/day. This was unlikely to be
related to treatment, since a similar sex ratio (approximately 44%
males) reportedly occurred in historical untreated controls. No foetal
external malformations and no treatment-related increase in incidence
of skeletal anomalies were evident. Visceral examination revealed
bilateral hydrocephalus internus in one control foetus, medial
dystopia of an undescended testis and bilateral hydrocephalus
internus, respectively, in 2 foetuses (from 2 litters) at 0.3 mg/kg
bw/day, ventral dystopia of the kidney and unilateral hydrocephalus
internus respectively in 2 foetuses (from 2 litters) at 1 mg/kg bw/day
and dilated aqueductus cerebre silvii and anasarca respectively in 2
foetuses (from 2 litters) at 3 mg/kg body weight/day. The author of
the report stated that such malformations occurred spontaneously in
untreated rats of this strain. Indeed, in view of their low and
isolated incidences, it would appear unlikely that the observed
malformations were induced by isofenphos (Becker 1981).
Special Studies on Mutagenicity
Mouse
In a micronucleus test, three groups of 5 male and 5 female mice
(Bor: NMRI (SPF Han)) strain were treated orally with a 0, 15 or
30 mg/kg bw dose of isofenphos (91.8% pure) on two occasions,
separated by 24 hours. Six hours after the second dose, the animals
were sacrificed and femoral bone marrow smears were prepared. There
were no mortalities or clinical signs of toxicity. The incidence of
micronucleated polychromatic erythrocytes or of micronucleated cells
in normochromatic erythrocytes was not significantly different between
controls and treated groups. Under the conditions of the test,
isofenphos was not mutagenic (Herbold 1981).
Special Studies on Neurotoxicity
Hen
Thirty White Leghorn laying hens, approximately 12 1/2 months
old, were intubated with a single dose of 32 mg isofenphos (91.9%
pure)/kg bw. (The oral LD50 of the compound was not determined in the
study. It was found to be 21 mg/kg body weight in an earlier study by
another laboratory, see FAO/WHO 1982). These birds were also treated
with 50 mg/kg bw of atropine i.p. at the time isofenphos was given.
Seventeen of the treated hens died within 24 hours of dosing.
Locomotor ataxia and paralysis were observed in all treated hens
beginning on day 1 or 2 but these signs were not seen beyond day 6.
Histopathological examination of the brain, spinal cord and sciatic
nerve from the 13 surviving hens sacrificed at the end of a 21-day
observation period revealed minimal changes that were similar in
incidence and severity to those noted in concurrent controls (5 hens
given water by gavage as dosing controls, and 5 hens as untreated
controls). Positive controls, treated orally with tri-o-tolyl
phosphate (TOTP) at 500 mg/kg bw, displayed clinical signs and
histopathological lesions of the nervous tissue typical of delayed
neurotoxicity (Hixson 1982).
COMMENTS
The available teratogenicity study in rats and micronucleus test
in mice were both negative. The single-dose delayed neurotoxicity
study in hens, while indicating no delayed neurotoxic potential of the
insecticide, could be considered to be only a screen, as was a similar
study previously evaluated (FAO/WHO 1982). Similarly, the suitability
of a single litter per generation study in the multigeneration study
was questioned by the Meeting. Because of the absence of an
appropriate delayed neurotoxicity study and the deliberations
regarding the multigeneration studies, the Meeting recommended an
extension of the temporary ADI estimated in 1981.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.15 mg/kg bw
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0005 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1986)
1. An appropriate delayed neurotoxicity study.
2. Two-generation (two litters/generation) reproduction study.
Desirable
1. In vitro biochemical studies on purified isofenphos with
respect to anticholinesterase activity.
2. Further observations in humans,
REFERENCES
Becker, H. Embryotoxicity and teratogenicity study in rats, Report
1981 from RCC Research and Consulting Company Ltd., Switzerland,
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Herbold, B. Micronucleus test on mouse to evaluate SRA 12869
1981 (isofenphos: active ingredient of Oftanol) for mutagenic
potential. Report from Bayer AG Institut Fur Toxikologie
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Hixson, E.J. Acute delayed neurotoxicity of technical isofenphos in
1982 hens. Report from Mobay Chemical Corporation, U.S.A.
submitted to the World Health Organization by Bayer AG.
(Unpublished)
ISOFENPHOS
Explanation
Isofenphos was evaluated by the Joint Meeting in 1981 (FAO/WHO
1982)1, and a temporary ADI was allocated. The absence of an
appropriate neurotoxicity study in hens was the basis for the
temporary nature of the ADI. Such a study was required for a full
evaluation of the insecticide.
The required study has not been provided, although another acute
delayed neurotoxicity study in hens, a teratology study in rats and a
mutagenicity study have been made available. These new studies are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies in Teratogenicity
Rat
Groups of 25 mated rats (Wistar KFM-HAN outbred strain) were
intubated with isofenphos (91.8% pure) as a suspension in
carboxymethylcellulose at 0, 0.3, 1.0 or 3 mg/kg bw/day from day 6
through day 15 of pregnancy (day 0 = day positive vaginal smear or
vaginal plug observed). The dams were sacrificed on day 21 of
pregnancy and foetuses were removed by caesarean section for external,
skeletal and visceral examination. There were no mortalities or signs
of toxicity. Maternal body weight and food consumption from day 0 to
day 21 of pregnancy, the number of embryonal deaths, foetal deaths,
dead foetuses and live foetuses as well as foetal weight were not
1 See Annex 2 for WHO and FAO documentation.
significantly different between control and treated groups. A
significant difference from the concurrent control group in the sex
ratio of foetuses was noted at 3 mg/kg bw/day. This was unlikely to be
related to treatment, since a similar sex ratio (approximately 44%
males) reportedly occurred in historical untreated controls. No foetal
external malformations and no treatment-related increase in incidence
of skeletal anomalies were evident. Visceral examination revealed
bilateral hydrocephalus internus in one control foetus, medial
dystopia of an undescended testis and bilateral hydrocephalus
internus, respectively, in 2 foetuses (from 2 litters) at 0.3 mg/kg
bw/day, ventral dystopia of the kidney and unilateral hydrocephalus
internus respectively in 2 foetuses (from 2 litters) at 1 mg/kg bw/day
and dilated aqueductus cerebre silvii and anasarca respectively in 2
foetuses (from 2 litters) at 3 mg/kg body weight/day. The author of
the report stated that such malformations occurred spontaneously in
untreated rats of this strain. Indeed, in view of their low and
isolated incidences, it would appear unlikely that the observed
malformations were induced by isofenphos (Becker 1981).
Special Studies on Mutagenicity
Mouse
In a micronucleus test, three groups of 5 male and 5 female mice
(Bor: NMRI (SPF Han)) strain were treated orally with a 0, 15 or
30 mg/kg bw dose of isofenphos (91.8% pure) on two occasions,
separated by 24 hours. Six hours after the second dose, the animals
were sacrificed and femoral bone marrow smears were prepared. There
were no mortalities or clinical signs of toxicity. The incidence of
micronucleated polychromatic erythrocytes or of micronucleated cells
in normochromatic erythrocytes was not significantly different between
controls and treated groups. Under the conditions of the test,
isofenphos was not mutagenic (Herbold 1981).
Special Studies on Neurotoxicity
Hen
Thirty White Leghorn laying hens, approximately 12 1/2 months
old, were intubated with a single dose of 32 mg isofenphos (91.9%
pure)/kg bw. (The oral LD50 of the compound was not determined in the
study. It was found to be 21 mg/kg body weight in an earlier study by
another laboratory, see FAO/WHO 1982). These birds were also treated
with 50 mg/kg bw of atropine i.p. at the time isofenphos was given.
Seventeen of the treated hens died within 24 hours of dosing.
Locomotor ataxia and paralysis were observed in all treated hens
beginning on day 1 or 2 but these signs were not seen beyond day 6.
Histopathological examination of the brain, spinal cord and sciatic
nerve from the 13 surviving hens sacrificed at the end of a 21-day
observation period revealed minimal changes that were similar in
incidence and severity to those noted in concurrent controls (5 hens
given water by gavage as dosing controls, and 5 hens as untreated
controls). Positive controls, treated orally with tri-o-tolyl
phosphate (TOTP) at 500 mg/kg bw, displayed clinical signs and
histopathological lesions of the nervous tissue typical of delayed
neurotoxicity (Hixson 1982).
COMMENTS
The available teratogenicity study in rats and micronucleus test
in mice were both negative. The single-dose delayed neurotoxicity
study in hens, while indicating no delayed neurotoxic potential of the
insecticide, could be considered to be only a screen, as was a similar
study previously evaluated (FAO/WHO 1982). Similarly, the suitability
of a single litter per generation study in the multigeneration study
was questioned by the Meeting. Because of the absence of an
appropriate delayed neurotoxicity study and the deliberations
regarding the multigeneration studies, the Meeting recommended an
extension of the temporary ADI estimated in 1981.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.15 mg/kg bw
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0005 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1986)
1. An appropriate delayed neurotoxicity study.
2. Two-generation (two litters/generation) reproduction study.
Desirable
1. In vitro biochemical studies on purified isofenphos with
respect to anticholinesterase activity.
2. Further observations in humans,
REFERENCES
Becker, H. Embryotoxicity and teratogenicity study in rats, Report
1981 from RCC Research and Consulting Company Ltd., Switzerland,
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Herbold, B. Micronucleus test on mouse to evaluate SRA 12869
1981 (isofenphos: active ingredient of Oftanol) for mutagenic
potential. Report from Bayer AG Institut Fur Toxikologie
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Hixson, E.J. Acute delayed neurotoxicity of technical isofenphos in
1982 hens. Report from Mobay Chemical Corporation, U.S.A.
submitted to the World Health Organization by Bayer AG.
(Unpublished)
ISOFENPHOS
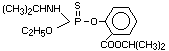 Explanation
Isofenphos was evaluated by the 1981 Joint Meeting. 1 A
temporary acceptable daily intake for man and maximum residue levels
for several commodities were recommended. Additional data from
supervised trials on potatoes and onions were considered desirable.
New information on use pattern, residues resulting from supervised
trials and on the fate of residues were made available for evaluation
and they are discussed in this addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In addition to the use pattern included in the 1981 Monograph,
the following new recommendations were submitted.
Crop Application rate Formulation No. of treatments
(a.i.)
Citrus fruit 0.05% EC, WP 2
Bananas 2 g/plant GR 3
Pears 0.05-0.075% EC 3
RESIDUES RESULTING FROM SUPERVISED TRIALS
Supervised trials were carried out on various crops in Finland,
France, Germany, Italy and South Africa. Analyses were done for
isofenphos and isofenphos oxygen analogue (IOA).
1 See Annex 2 for FAO and WHO documentation.
Bananas
Five supervised trials were carried out in various countries.
Oftanol GR was applied at rates of 1.2-4 g a.i./plant two or three
times, three to five months apart. Samples were taken at intervals
from 3 to 154 days after last application. Isofenphos and isofenphos
oxon were analysed in the peel and whole fruit. The residue was below
the limit of determination (0.01 mg/kg) in every case.
Citrus Fruit
Navel orange and lemon trees were treated with Oftanol 50 EC at
the normal (0.05% a.i.) and at double (0.1% a.i.) application rate in
South Africa. Samples taken at various intervals were analysed for
isofenphos and its oxygen analogue applying a method having a
0.05 mg/kg limit of determination for both compounds. The results are
summarized in Table 1 (South Africa, 1981).
Pear
In supervised trials, Oftanol 500 EC was applied one to four
times in a solution containing 0.05-0.075% a.i. for the treatment of
'Alexander' and 'Williams' pear trees in Italy and once in France.
Details and results of experiments are summarized in Table 2. The
residue declined moderately during the first 4 weeks after treatment;
there was little difference in the residue range and in the average
residue level thereafter.
Brassica Leafy Vegetables
Narrow-stem kale seed was treated at the recommended rate
(16 g a.i./kg seed) with Oftanol C. The plants were sampled 133-169
days after sowing. No residue could be detected over the limit of
determination (0.01 mg/kg).
Supervised trials were carried out in Norway applying OftanolR
at the approved rates and time. Residues (the parent compound and its
oxon) in broccoli, Chinese cabbage and kohlrabi were generally below
0.02 mg/kg and did not exceed 0.1 mg/kg.
Oilseed Rape
Seed dressing was carried out at a rate of 16 g a.i./kg seed with
Oftanol C or Oftanol T in four experiments. Samples of mature seed
taken 125-305 days after sowing contained only non-detectable
residues.
Table 1. Residues resulting from supervised trials in citrus fruit, 1980
Application Residues 1 (mg/kg) at intervals (days) after application
Crop No. Rate Compound
(% a.i.) 1 7 14 21 28 35 42
Lemon 1 0.05 I 1.3 1.1 0.8 0.53 0.75 0.82 0.56
IOA 0.13 0.26 0.23 0.15 0.22 0.19 0.17
Lemon 1 0.1 I 2.9 1.8 1.1 1 0.8 0.63 0.54
IOA 0.25 0.42 0.36 0.31 0.28 0.29 0.23
Orange 1 0.05 I 2.7 1.7 1.1 1 0.87 0.76 0.67
IOA 0.11 0.17 0.12 0.12 0.12 0.1 0.1
Orange 1 0.1 I 3.4 2 1.6 1.4 1.3 0.85 0.78
IOA 0.13 0.2 0.18 0.15 0.19 0.14 0.12
1 Results are the average of two replicates
Table 2. Residues resulting from supervised trials in pears 1
Application Residues (mg/kg) at intervals (days)
Variety Country Year No. Rate after application
(kg a.i./ha Interval
and %) (weeks) 0 14 21 28 35 40-43 60
Alexander France 1977 2 0.6(0.06) 2 0.12
Alexander Italy 1974 1 1.5(0.075) 2.24 0.71 0.71 0.41
Alexander Italy 1976 4 1.5(0.05) 4 0.27 0.16
Alexander Italy 1976 4 1.5(0.05) 4 0.47 0.11
Alexander Italy 1976 3 1.5(0.05) 4 0.2 0.29
Alexander Italy 1976 3 1.5(0.05) 4 0.17 0.39
Williams Italy 1976 1 1.75(0.05) 0.97 0.62 0.47 0.47 0.27 0.12
Alexander Italy 1975 1 1.75(0.05) 2.54 0.84 0.32 0.23 0.22
Alexander Italy 1975 1 1.75(0.05) 5.21 0.86 0.35 0.32 0.25
Williams Italy 1975 1 1.75(0.05) 1.12 0.46 0.38 0.44 0.44
Alexander Italy 1976 1 2.25(0.075) 0.42 0.34
Alexander Italy 1976 4 2.25(0.075) 4 0.63 0.33
Alexander Italy 1976 3 2.25(0.075) 4 0.32 0.42
Alexander Italy 1976 3 2.25(0.075) 4 0.44 0.75
Williams Italy 1975 1 63 (0.0075) 1.06 0.84 0.69 0.64 0.34
Alexander Italy 1975 1 2.63(0.075) 0.86 0.88 0.53 0.4 0.28
Alexander Italy 1975 1 2.63(0.075) 5.35 0.75 0.39 0.3 0.27
Williams Italy 1975 1 2.63(0.075) 0.99 0.61 0.67 0.72 0.34
1 Bayer AG 1982.
Swedes
Swedes were treated at a rate of 16 g a.i,/kg seed with Oftanol C
in the Federal Republic of Germany (FRG). No residue was detected
either in the leaves or in the roots 140-169 days after treatment. In
supervised trials carried out in Finland, no residue was detectable in
washed plants grown after seed dressing, while 0.008-0.07 mg/kg
isofenphos and 0.015-0.01 mg/kg IOA were detected following soil
treatment at planting at a rate of 0.25 g a.i./m2.
Turnip
Oftanol 500 EC was used at a rate of 0.075 g a.i./m for the
treatment of the plots in 10 cm band at planting. Residues detected in
the leaves and roots are summarized in Table 3.
Table 3. Residues in turnips after soil treatment with isofenphos
Residues (mg/kg)at intervals (days) after application
42 60 80
root leaf root leaf root leaf
0.05 0.03 0.04 0.01 <0.01 <0.01
0.15 0.08 0.02 0.01 0.05 0.01
0.2 0.05 0.02 <0.01 0.01 0.01
Turnip plants grown from seeds treated with isofenphos at a
dosage rate of 16 g a.i./kg seed did not contain detectable residues
in trials carried out in FRG and Finland.
Other Crops
Supervised trials were carried out in Finland in various
vegetables. Carrot and radish were treated with 0.5 g a.i./m2 and
onion seeds were dipped in a solution containing 0.025% a.i. The crops
were washed before analysis. The sum of isofenphos and IOA found in
different crops at various intervals after treatment was the
following: carrot-2 mg/kg (22 days), 0.4 mg/kg (84 days), radish:
1.2 mg/kg (6 days), 1.25 mg/kg (13 days), onion 0.024 mg/kg (62 days)
(Finland undated).
FATE OF RESIDUES
In Plants
Ethoxy-1-3H, ring UL-14C isofenphos, formulated as 6E, was
applied to cabbage seeds in furrow at planting at a rate of
0.13 g a.i./m. At transplanting, 28 days after planting, some of the
cabbage plants were transferred to untreated soil. The remaining
cabbage plants received a second application of labelled isofenphos as
a direct spray at the base of the plants at a rate of 1.68 kg a.i./ha
(Strankowski and Murphy 1980). At various sampling intervals the
radioactivity was released from the cabbage tissues by methanol-water
extraction, enzyme, acid and basic hydrolyses. In plants receiving one
and two treatments, 80-98% and 89-96% of the radioactivity were
identified respectively.
The major degradation pathway for isofenphos in cabbage involved
oxidation to its oxon analogue (IOA) and subsequent hydrolysis to
isopropyl salicylate (IPS) and salicylic acid (SA). Further breakdown
then produced 2,3-dihydroxy benzoic acid (2,3-DHBA) and benzoic acid
(BA). Des-N-isopropyl isofenphos oxygen analogue (des IOA) was also
identified. The IPS, 2,3-DHBA and BA were found in free and conjugated
form while 2,3-DHBA appeared only in conjugated form.
In Norway the results of supervised trials indicated that the
residues were below 0.05 mg/kg in celeriac potted out in soil
containing 225 g isofenphos/m3. Residues were not detectable in
carrots, onion and winter radish following seed dressing.
The radioactive residues (0.001-0.006 mg/kg) identified in mature
samples were characterized as being very polar hydrolysis products.
The amount of organosoluble residue and the metabolites identified in
maize and onion [FAO/WHO 1981) and in cabbage were very similar. The
main difference between the metabolism of isofenphos in cabbage and
maize or onion was that no IPS was found in the latter two crops.
The uptake of radioactivity in cabbage seedlings appeared to be
continuous. Each successive new leaf contained radioactivity, but in
decreasing concentration. The concentration of total radioactivity in
cabbage plants decreased with time, and 189 days after planting the
total residue was equivalent to <0.012 mg/kg and 0.057 mg/kg
isofenphos in mature cabbage heads receiving one or two treatments
respectively. The overall distribution of radioactivity at various
intervals after applications is summarized in Table 4.
Table 4. Distribution of isofenphos and its metabolites in cabbage
Total radioactivity (%) at intervals (days) after
one and two applications
Days/ 7 14 28 42 56 189
No. of applications 1 1 1 1 2 1 2 1 2
Total residue 1 14.2 5.28 2.36 0.17 0.61 0.06 0.3 <0.012 0.057
Isofenphos 79 53 37 15 33 5 29 <1 0
IOA 14 34 43 24 28 19 32 4 13
Des IOA <1 1 2 2 1 <1 1 1 1
IPS 1 3 4 24 16 30 13 12 29
SA 1 2 2 12 10 21 12 31 19
BA <1 1 3 1 <1 2 <1 3 2
2,3 DHBA <1 1 1 2 2 3 1 3 6
1 Expressed in mg/kg isofenphos equivalent.
In Soil
The mobility of isofenphos was studied in German standard soils
2.1, 2.2 and 2.3. Equivalents of 2-4 kg a.i./ha of two formulations
were applied to the soil and leached for two days. Neither isofenphos
nor its oxon derivative was detectable in the leachates (limit of
determination 0.0001 mg/kg) (Bayer AG 1982).
NATIONAL MAXIMUM RESIDUE LIMITS
Available information on national MRLs reported to the Meeting is
given in Table 5.
Table 5. National Maximum Residue Limits and preharvest intervals
Preharvest
Country Crop interval MRL
(days) (mg/kg)
Federal Republic
of Germany Leafy and other
sprouting vegetables 0.1
Root vegetables,
rape seed 0.05
Italy Artichokes 0.1
Brassicas 0.1
Eggplant 0.1
Fruit 0.1
Garlic 0.1
Onion 0.1
Pear 42 0.1
Pepper 0.1
Sugarbeet 42 0.1
Tomato 0.1
Netherlands Cabbage 56 0.1
Brussels sprouts 56 0.05
Cauliflower 56 0.05
Colerisc 0.05
Celery 0.05
Leek 0.1
Onion 0.1
Norway Root vegetables 90
Stem vegetables 90
South Africa Citrus 42 0.2
Spain Garlic 21
Onion 21
Table 5. (con't)
Preharvest
Country Crop interval MRL
(days) (mg/kg)
U.S. Maize (fodder) 75 1.0
Maize (forage) 75 1.0
Maize (fresh, incl.
sweet corn) 75 0.1
Maize (grain) 75 0.1
Eggs 0.02
Meat, fat, meat by-
products of cattle,
goats, pigs, horses,
sheep and poultry 0.1
Milk 0.02
APPRAISAL
Since isofenphos was last evaluated by the 1981 JMPR, additional
data have been made available for further consideration.
The use of isofenphos is recommended in banana, citrus fruit and
pear. It is applied as a spray containing 0.05% and 0.05-0.075% a.i. 2
and 3 times in citrus fruit and pear respectively. Banana trees can be
treated with 2 g a.i./plant up to 3 times annually.
Supervised trials were carried out on various crops and the
residues of isofenphos (I) and its oxygen analogue (IOA) were
analysed. The limit of determination was 0.01-0.05 mg/kg for both
compounds. Bananas did not contain detectable residues after three
treatments at the recommended rate. Residue levels in lemons and
oranges are approximately similar 42 days after treatment, and
isofenphos amounts to 70-87% of the total residues. In pear, the
initial residues declined moderately in the first month after
application while there was little difference in range and average
level of residue in samples taken in the second month.
The treatment of soil at planting or sowing resulted in residues
in carrots, onions, swedes and turnips; however, no measurable
residues were found in subsequent crops after seed dressing.
Isofenphos residues are readily taken up by cabbage plants from
soil. The major degradation pathway for isofenphos in cabbage involved
oxidation (IOA) and subsequent hydrolysis to isopropyl salicylate
(IPS) and salicylic acid (SA). The parent compound and its three
metabolites formed the major part of the total residue in the plant.
The proportion of these compounds varied with time. At maturity IOA,
IPS and SA were the only residues present while intact isofenphos was
not detectable. The total radioactivity, expressed in isofenphos
equivalents, was 0.057 mg/kg in mature cabbage treated at planting and
one month later. There was no difference in the degradation pathway
for isofenphos in cabbage receiving one or two treatments. The amounts
of organosoluble residues in cabbage were similar to those found in
maize and onions in previous experiments (FAO/WHO 1981). The
degradation products were also similar with the exception of IPS,
which was found only in cabbage.
Further leaching experiments confirmed the low mobility of
isofenphos in different soils, indicating that the contamination of
underground water with isofenphos residues used for soil treatment is
unlikely.
RECOMMENDATIONS
The Meeting examined residue data from new supervised trials and
was able to estimate maximum residue levels for the following
commodities in addition to those previously recommended. Results of
other experiments supported the recommendations of the 1981 meeting,
and no amendments were necessary. The levels refer to the sum of
isofenphos and isofenphos oxygen analogue.
Temporary Maximum Preharvest
Residue Level Interval
(mg/kg) (weeks)
Citrus fruit 1 6
Pears 0.5 6
Bananas 0.02 1 8
1 Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Further information on the residue in different varieties of
citrus treated at the recommended rate with isofenphos and
on the distribution of residues in the peel and pulp of
citrus fruits.
2. Additional data from supervised trials on potatoes and
onions, including information on soil residues and soil
moisture content.
REFERENCES
Bayer AG. Pflanzenschutzmittle-Rückstände: Bananas: Report Nos. 4042-
1982 4043/78, 4000/80, 4001/I/80, 4001/II/80, Narrow-stem kale:
Report Nos. 4010-4012/78; Pears: Report Nos. 4019/77,
4055/74, 4019/B/76, 4019/A/76, 4017/B/76, 4017/A/76,
4009/75, 4049/75, 4043/75, 4011/75, 4020/B/76, 4020/A/76,
4018/B/76, 4018/A/76, 4010/75, 4046/75, 4044/75, 4041/75;
Oilseed Rape; Report Nos, 4007-4009/78, 4019/78;
Swedes:Report Nos. 4016/78, 4018/78; Turnips: Report Nos
4014-4015/78, 4006-4008/77; Water: Report Nos, 4046-4048/74,
4008-4010/81, (Unpublished)
Finland. Information on pesticides included in the priority list.
undated
South Africa, Determination of isofenphos residues in citrus fruit,
1981 Bureau of Standards, Report No. 311/88005/U9. (Unpublished)
Strankowsky, K.J. and Murphy, J.J. Metabolism of AMAZE in cabbage,
1980 Mobay Report No. 69 174. (Unpublished)
Explanation
Isofenphos was evaluated by the 1981 Joint Meeting. 1 A
temporary acceptable daily intake for man and maximum residue levels
for several commodities were recommended. Additional data from
supervised trials on potatoes and onions were considered desirable.
New information on use pattern, residues resulting from supervised
trials and on the fate of residues were made available for evaluation
and they are discussed in this addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In addition to the use pattern included in the 1981 Monograph,
the following new recommendations were submitted.
Crop Application rate Formulation No. of treatments
(a.i.)
Citrus fruit 0.05% EC, WP 2
Bananas 2 g/plant GR 3
Pears 0.05-0.075% EC 3
RESIDUES RESULTING FROM SUPERVISED TRIALS
Supervised trials were carried out on various crops in Finland,
France, Germany, Italy and South Africa. Analyses were done for
isofenphos and isofenphos oxygen analogue (IOA).
1 See Annex 2 for FAO and WHO documentation.
Bananas
Five supervised trials were carried out in various countries.
Oftanol GR was applied at rates of 1.2-4 g a.i./plant two or three
times, three to five months apart. Samples were taken at intervals
from 3 to 154 days after last application. Isofenphos and isofenphos
oxon were analysed in the peel and whole fruit. The residue was below
the limit of determination (0.01 mg/kg) in every case.
Citrus Fruit
Navel orange and lemon trees were treated with Oftanol 50 EC at
the normal (0.05% a.i.) and at double (0.1% a.i.) application rate in
South Africa. Samples taken at various intervals were analysed for
isofenphos and its oxygen analogue applying a method having a
0.05 mg/kg limit of determination for both compounds. The results are
summarized in Table 1 (South Africa, 1981).
Pear
In supervised trials, Oftanol 500 EC was applied one to four
times in a solution containing 0.05-0.075% a.i. for the treatment of
'Alexander' and 'Williams' pear trees in Italy and once in France.
Details and results of experiments are summarized in Table 2. The
residue declined moderately during the first 4 weeks after treatment;
there was little difference in the residue range and in the average
residue level thereafter.
Brassica Leafy Vegetables
Narrow-stem kale seed was treated at the recommended rate
(16 g a.i./kg seed) with Oftanol C. The plants were sampled 133-169
days after sowing. No residue could be detected over the limit of
determination (0.01 mg/kg).
Supervised trials were carried out in Norway applying OftanolR
at the approved rates and time. Residues (the parent compound and its
oxon) in broccoli, Chinese cabbage and kohlrabi were generally below
0.02 mg/kg and did not exceed 0.1 mg/kg.
Oilseed Rape
Seed dressing was carried out at a rate of 16 g a.i./kg seed with
Oftanol C or Oftanol T in four experiments. Samples of mature seed
taken 125-305 days after sowing contained only non-detectable
residues.
Table 1. Residues resulting from supervised trials in citrus fruit, 1980
Application Residues 1 (mg/kg) at intervals (days) after application
Crop No. Rate Compound
(% a.i.) 1 7 14 21 28 35 42
Lemon 1 0.05 I 1.3 1.1 0.8 0.53 0.75 0.82 0.56
IOA 0.13 0.26 0.23 0.15 0.22 0.19 0.17
Lemon 1 0.1 I 2.9 1.8 1.1 1 0.8 0.63 0.54
IOA 0.25 0.42 0.36 0.31 0.28 0.29 0.23
Orange 1 0.05 I 2.7 1.7 1.1 1 0.87 0.76 0.67
IOA 0.11 0.17 0.12 0.12 0.12 0.1 0.1
Orange 1 0.1 I 3.4 2 1.6 1.4 1.3 0.85 0.78
IOA 0.13 0.2 0.18 0.15 0.19 0.14 0.12
1 Results are the average of two replicates
Table 2. Residues resulting from supervised trials in pears 1
Application Residues (mg/kg) at intervals (days)
Variety Country Year No. Rate after application
(kg a.i./ha Interval
and %) (weeks) 0 14 21 28 35 40-43 60
Alexander France 1977 2 0.6(0.06) 2 0.12
Alexander Italy 1974 1 1.5(0.075) 2.24 0.71 0.71 0.41
Alexander Italy 1976 4 1.5(0.05) 4 0.27 0.16
Alexander Italy 1976 4 1.5(0.05) 4 0.47 0.11
Alexander Italy 1976 3 1.5(0.05) 4 0.2 0.29
Alexander Italy 1976 3 1.5(0.05) 4 0.17 0.39
Williams Italy 1976 1 1.75(0.05) 0.97 0.62 0.47 0.47 0.27 0.12
Alexander Italy 1975 1 1.75(0.05) 2.54 0.84 0.32 0.23 0.22
Alexander Italy 1975 1 1.75(0.05) 5.21 0.86 0.35 0.32 0.25
Williams Italy 1975 1 1.75(0.05) 1.12 0.46 0.38 0.44 0.44
Alexander Italy 1976 1 2.25(0.075) 0.42 0.34
Alexander Italy 1976 4 2.25(0.075) 4 0.63 0.33
Alexander Italy 1976 3 2.25(0.075) 4 0.32 0.42
Alexander Italy 1976 3 2.25(0.075) 4 0.44 0.75
Williams Italy 1975 1 63 (0.0075) 1.06 0.84 0.69 0.64 0.34
Alexander Italy 1975 1 2.63(0.075) 0.86 0.88 0.53 0.4 0.28
Alexander Italy 1975 1 2.63(0.075) 5.35 0.75 0.39 0.3 0.27
Williams Italy 1975 1 2.63(0.075) 0.99 0.61 0.67 0.72 0.34
1 Bayer AG 1982.
Swedes
Swedes were treated at a rate of 16 g a.i,/kg seed with Oftanol C
in the Federal Republic of Germany (FRG). No residue was detected
either in the leaves or in the roots 140-169 days after treatment. In
supervised trials carried out in Finland, no residue was detectable in
washed plants grown after seed dressing, while 0.008-0.07 mg/kg
isofenphos and 0.015-0.01 mg/kg IOA were detected following soil
treatment at planting at a rate of 0.25 g a.i./m2.
Turnip
Oftanol 500 EC was used at a rate of 0.075 g a.i./m for the
treatment of the plots in 10 cm band at planting. Residues detected in
the leaves and roots are summarized in Table 3.
Table 3. Residues in turnips after soil treatment with isofenphos
Residues (mg/kg)at intervals (days) after application
42 60 80
root leaf root leaf root leaf
0.05 0.03 0.04 0.01 <0.01 <0.01
0.15 0.08 0.02 0.01 0.05 0.01
0.2 0.05 0.02 <0.01 0.01 0.01
Turnip plants grown from seeds treated with isofenphos at a
dosage rate of 16 g a.i./kg seed did not contain detectable residues
in trials carried out in FRG and Finland.
Other Crops
Supervised trials were carried out in Finland in various
vegetables. Carrot and radish were treated with 0.5 g a.i./m2 and
onion seeds were dipped in a solution containing 0.025% a.i. The crops
were washed before analysis. The sum of isofenphos and IOA found in
different crops at various intervals after treatment was the
following: carrot-2 mg/kg (22 days), 0.4 mg/kg (84 days), radish:
1.2 mg/kg (6 days), 1.25 mg/kg (13 days), onion 0.024 mg/kg (62 days)
(Finland undated).
FATE OF RESIDUES
In Plants
Ethoxy-1-3H, ring UL-14C isofenphos, formulated as 6E, was
applied to cabbage seeds in furrow at planting at a rate of
0.13 g a.i./m. At transplanting, 28 days after planting, some of the
cabbage plants were transferred to untreated soil. The remaining
cabbage plants received a second application of labelled isofenphos as
a direct spray at the base of the plants at a rate of 1.68 kg a.i./ha
(Strankowski and Murphy 1980). At various sampling intervals the
radioactivity was released from the cabbage tissues by methanol-water
extraction, enzyme, acid and basic hydrolyses. In plants receiving one
and two treatments, 80-98% and 89-96% of the radioactivity were
identified respectively.
The major degradation pathway for isofenphos in cabbage involved
oxidation to its oxon analogue (IOA) and subsequent hydrolysis to
isopropyl salicylate (IPS) and salicylic acid (SA). Further breakdown
then produced 2,3-dihydroxy benzoic acid (2,3-DHBA) and benzoic acid
(BA). Des-N-isopropyl isofenphos oxygen analogue (des IOA) was also
identified. The IPS, 2,3-DHBA and BA were found in free and conjugated
form while 2,3-DHBA appeared only in conjugated form.
In Norway the results of supervised trials indicated that the
residues were below 0.05 mg/kg in celeriac potted out in soil
containing 225 g isofenphos/m3. Residues were not detectable in
carrots, onion and winter radish following seed dressing.
The radioactive residues (0.001-0.006 mg/kg) identified in mature
samples were characterized as being very polar hydrolysis products.
The amount of organosoluble residue and the metabolites identified in
maize and onion [FAO/WHO 1981) and in cabbage were very similar. The
main difference between the metabolism of isofenphos in cabbage and
maize or onion was that no IPS was found in the latter two crops.
The uptake of radioactivity in cabbage seedlings appeared to be
continuous. Each successive new leaf contained radioactivity, but in
decreasing concentration. The concentration of total radioactivity in
cabbage plants decreased with time, and 189 days after planting the
total residue was equivalent to <0.012 mg/kg and 0.057 mg/kg
isofenphos in mature cabbage heads receiving one or two treatments
respectively. The overall distribution of radioactivity at various
intervals after applications is summarized in Table 4.
Table 4. Distribution of isofenphos and its metabolites in cabbage
Total radioactivity (%) at intervals (days) after
one and two applications
Days/ 7 14 28 42 56 189
No. of applications 1 1 1 1 2 1 2 1 2
Total residue 1 14.2 5.28 2.36 0.17 0.61 0.06 0.3 <0.012 0.057
Isofenphos 79 53 37 15 33 5 29 <1 0
IOA 14 34 43 24 28 19 32 4 13
Des IOA <1 1 2 2 1 <1 1 1 1
IPS 1 3 4 24 16 30 13 12 29
SA 1 2 2 12 10 21 12 31 19
BA <1 1 3 1 <1 2 <1 3 2
2,3 DHBA <1 1 1 2 2 3 1 3 6
1 Expressed in mg/kg isofenphos equivalent.
In Soil
The mobility of isofenphos was studied in German standard soils
2.1, 2.2 and 2.3. Equivalents of 2-4 kg a.i./ha of two formulations
were applied to the soil and leached for two days. Neither isofenphos
nor its oxon derivative was detectable in the leachates (limit of
determination 0.0001 mg/kg) (Bayer AG 1982).
NATIONAL MAXIMUM RESIDUE LIMITS
Available information on national MRLs reported to the Meeting is
given in Table 5.
Table 5. National Maximum Residue Limits and preharvest intervals
Preharvest
Country Crop interval MRL
(days) (mg/kg)
Federal Republic
of Germany Leafy and other
sprouting vegetables 0.1
Root vegetables,
rape seed 0.05
Italy Artichokes 0.1
Brassicas 0.1
Eggplant 0.1
Fruit 0.1
Garlic 0.1
Onion 0.1
Pear 42 0.1
Pepper 0.1
Sugarbeet 42 0.1
Tomato 0.1
Netherlands Cabbage 56 0.1
Brussels sprouts 56 0.05
Cauliflower 56 0.05
Colerisc 0.05
Celery 0.05
Leek 0.1
Onion 0.1
Norway Root vegetables 90
Stem vegetables 90
South Africa Citrus 42 0.2
Spain Garlic 21
Onion 21
Table 5. (con't)
Preharvest
Country Crop interval MRL
(days) (mg/kg)
U.S. Maize (fodder) 75 1.0
Maize (forage) 75 1.0
Maize (fresh, incl.
sweet corn) 75 0.1
Maize (grain) 75 0.1
Eggs 0.02
Meat, fat, meat by-
products of cattle,
goats, pigs, horses,
sheep and poultry 0.1
Milk 0.02
APPRAISAL
Since isofenphos was last evaluated by the 1981 JMPR, additional
data have been made available for further consideration.
The use of isofenphos is recommended in banana, citrus fruit and
pear. It is applied as a spray containing 0.05% and 0.05-0.075% a.i. 2
and 3 times in citrus fruit and pear respectively. Banana trees can be
treated with 2 g a.i./plant up to 3 times annually.
Supervised trials were carried out on various crops and the
residues of isofenphos (I) and its oxygen analogue (IOA) were
analysed. The limit of determination was 0.01-0.05 mg/kg for both
compounds. Bananas did not contain detectable residues after three
treatments at the recommended rate. Residue levels in lemons and
oranges are approximately similar 42 days after treatment, and
isofenphos amounts to 70-87% of the total residues. In pear, the
initial residues declined moderately in the first month after
application while there was little difference in range and average
level of residue in samples taken in the second month.
The treatment of soil at planting or sowing resulted in residues
in carrots, onions, swedes and turnips; however, no measurable
residues were found in subsequent crops after seed dressing.
Isofenphos residues are readily taken up by cabbage plants from
soil. The major degradation pathway for isofenphos in cabbage involved
oxidation (IOA) and subsequent hydrolysis to isopropyl salicylate
(IPS) and salicylic acid (SA). The parent compound and its three
metabolites formed the major part of the total residue in the plant.
The proportion of these compounds varied with time. At maturity IOA,
IPS and SA were the only residues present while intact isofenphos was
not detectable. The total radioactivity, expressed in isofenphos
equivalents, was 0.057 mg/kg in mature cabbage treated at planting and
one month later. There was no difference in the degradation pathway
for isofenphos in cabbage receiving one or two treatments. The amounts
of organosoluble residues in cabbage were similar to those found in
maize and onions in previous experiments (FAO/WHO 1981). The
degradation products were also similar with the exception of IPS,
which was found only in cabbage.
Further leaching experiments confirmed the low mobility of
isofenphos in different soils, indicating that the contamination of
underground water with isofenphos residues used for soil treatment is
unlikely.
RECOMMENDATIONS
The Meeting examined residue data from new supervised trials and
was able to estimate maximum residue levels for the following
commodities in addition to those previously recommended. Results of
other experiments supported the recommendations of the 1981 meeting,
and no amendments were necessary. The levels refer to the sum of
isofenphos and isofenphos oxygen analogue.
Temporary Maximum Preharvest
Residue Level Interval
(mg/kg) (weeks)
Citrus fruit 1 6
Pears 0.5 6
Bananas 0.02 1 8
1 Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Further information on the residue in different varieties of
citrus treated at the recommended rate with isofenphos and
on the distribution of residues in the peel and pulp of
citrus fruits.
2. Additional data from supervised trials on potatoes and
onions, including information on soil residues and soil
moisture content.
REFERENCES
Bayer AG. Pflanzenschutzmittle-Rückstände: Bananas: Report Nos. 4042-
1982 4043/78, 4000/80, 4001/I/80, 4001/II/80, Narrow-stem kale:
Report Nos. 4010-4012/78; Pears: Report Nos. 4019/77,
4055/74, 4019/B/76, 4019/A/76, 4017/B/76, 4017/A/76,
4009/75, 4049/75, 4043/75, 4011/75, 4020/B/76, 4020/A/76,
4018/B/76, 4018/A/76, 4010/75, 4046/75, 4044/75, 4041/75;
Oilseed Rape; Report Nos, 4007-4009/78, 4019/78;
Swedes:Report Nos. 4016/78, 4018/78; Turnips: Report Nos
4014-4015/78, 4006-4008/77; Water: Report Nos, 4046-4048/74,
4008-4010/81, (Unpublished)
Finland. Information on pesticides included in the priority list.
undated
South Africa, Determination of isofenphos residues in citrus fruit,
1981 Bureau of Standards, Report No. 311/88005/U9. (Unpublished)
Strankowsky, K.J. and Murphy, J.J. Metabolism of AMAZE in cabbage,
1980 Mobay Report No. 69 174. (Unpublished)
See Also:
Toxicological Abbreviations
Isofenphos (Pesticide residues in food: 1981 evaluations)
Isofenphos (Pesticide residues in food: 1984 evaluations)
Isofenphos (Pesticide residues in food: 1986 evaluations Part II Toxicology)
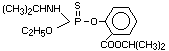 Explanation
Isofenphos was evaluated by the Joint Meeting in 1981 (FAO/WHO
1982)1, and a temporary ADI was allocated. The absence of an
appropriate neurotoxicity study in hens was the basis for the
temporary nature of the ADI. Such a study was required for a full
evaluation of the insecticide.
The required study has not been provided, although another acute
delayed neurotoxicity study in hens, a teratology study in rats and a
mutagenicity study have been made available. These new studies are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies in Teratogenicity
Rat
Groups of 25 mated rats (Wistar KFM-HAN outbred strain) were
intubated with isofenphos (91.8% pure) as a suspension in
carboxymethylcellulose at 0, 0.3, 1.0 or 3 mg/kg bw/day from day 6
through day 15 of pregnancy (day 0 = day positive vaginal smear or
vaginal plug observed). The dams were sacrificed on day 21 of
pregnancy and foetuses were removed by caesarean section for external,
skeletal and visceral examination. There were no mortalities or signs
of toxicity. Maternal body weight and food consumption from day 0 to
day 21 of pregnancy, the number of embryonal deaths, foetal deaths,
dead foetuses and live foetuses as well as foetal weight were not
1 See Annex 2 for WHO and FAO documentation.
significantly different between control and treated groups. A
significant difference from the concurrent control group in the sex
ratio of foetuses was noted at 3 mg/kg bw/day. This was unlikely to be
related to treatment, since a similar sex ratio (approximately 44%
males) reportedly occurred in historical untreated controls. No foetal
external malformations and no treatment-related increase in incidence
of skeletal anomalies were evident. Visceral examination revealed
bilateral hydrocephalus internus in one control foetus, medial
dystopia of an undescended testis and bilateral hydrocephalus
internus, respectively, in 2 foetuses (from 2 litters) at 0.3 mg/kg
bw/day, ventral dystopia of the kidney and unilateral hydrocephalus
internus respectively in 2 foetuses (from 2 litters) at 1 mg/kg bw/day
and dilated aqueductus cerebre silvii and anasarca respectively in 2
foetuses (from 2 litters) at 3 mg/kg body weight/day. The author of
the report stated that such malformations occurred spontaneously in
untreated rats of this strain. Indeed, in view of their low and
isolated incidences, it would appear unlikely that the observed
malformations were induced by isofenphos (Becker 1981).
Special Studies on Mutagenicity
Mouse
In a micronucleus test, three groups of 5 male and 5 female mice
(Bor: NMRI (SPF Han)) strain were treated orally with a 0, 15 or
30 mg/kg bw dose of isofenphos (91.8% pure) on two occasions,
separated by 24 hours. Six hours after the second dose, the animals
were sacrificed and femoral bone marrow smears were prepared. There
were no mortalities or clinical signs of toxicity. The incidence of
micronucleated polychromatic erythrocytes or of micronucleated cells
in normochromatic erythrocytes was not significantly different between
controls and treated groups. Under the conditions of the test,
isofenphos was not mutagenic (Herbold 1981).
Special Studies on Neurotoxicity
Hen
Thirty White Leghorn laying hens, approximately 12 1/2 months
old, were intubated with a single dose of 32 mg isofenphos (91.9%
pure)/kg bw. (The oral LD50 of the compound was not determined in the
study. It was found to be 21 mg/kg body weight in an earlier study by
another laboratory, see FAO/WHO 1982). These birds were also treated
with 50 mg/kg bw of atropine i.p. at the time isofenphos was given.
Seventeen of the treated hens died within 24 hours of dosing.
Locomotor ataxia and paralysis were observed in all treated hens
beginning on day 1 or 2 but these signs were not seen beyond day 6.
Histopathological examination of the brain, spinal cord and sciatic
nerve from the 13 surviving hens sacrificed at the end of a 21-day
observation period revealed minimal changes that were similar in
incidence and severity to those noted in concurrent controls (5 hens
given water by gavage as dosing controls, and 5 hens as untreated
controls). Positive controls, treated orally with tri-o-tolyl
phosphate (TOTP) at 500 mg/kg bw, displayed clinical signs and
histopathological lesions of the nervous tissue typical of delayed
neurotoxicity (Hixson 1982).
COMMENTS
The available teratogenicity study in rats and micronucleus test
in mice were both negative. The single-dose delayed neurotoxicity
study in hens, while indicating no delayed neurotoxic potential of the
insecticide, could be considered to be only a screen, as was a similar
study previously evaluated (FAO/WHO 1982). Similarly, the suitability
of a single litter per generation study in the multigeneration study
was questioned by the Meeting. Because of the absence of an
appropriate delayed neurotoxicity study and the deliberations
regarding the multigeneration studies, the Meeting recommended an
extension of the temporary ADI estimated in 1981.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.15 mg/kg bw
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0005 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1986)
1. An appropriate delayed neurotoxicity study.
2. Two-generation (two litters/generation) reproduction study.
Desirable
1. In vitro biochemical studies on purified isofenphos with
respect to anticholinesterase activity.
2. Further observations in humans,
REFERENCES
Becker, H. Embryotoxicity and teratogenicity study in rats, Report
1981 from RCC Research and Consulting Company Ltd., Switzerland,
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Herbold, B. Micronucleus test on mouse to evaluate SRA 12869
1981 (isofenphos: active ingredient of Oftanol) for mutagenic
potential. Report from Bayer AG Institut Fur Toxikologie
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Hixson, E.J. Acute delayed neurotoxicity of technical isofenphos in
1982 hens. Report from Mobay Chemical Corporation, U.S.A.
submitted to the World Health Organization by Bayer AG.
(Unpublished)
ISOFENPHOS
Explanation
Isofenphos was evaluated by the Joint Meeting in 1981 (FAO/WHO
1982)1, and a temporary ADI was allocated. The absence of an
appropriate neurotoxicity study in hens was the basis for the
temporary nature of the ADI. Such a study was required for a full
evaluation of the insecticide.
The required study has not been provided, although another acute
delayed neurotoxicity study in hens, a teratology study in rats and a
mutagenicity study have been made available. These new studies are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies in Teratogenicity
Rat
Groups of 25 mated rats (Wistar KFM-HAN outbred strain) were
intubated with isofenphos (91.8% pure) as a suspension in
carboxymethylcellulose at 0, 0.3, 1.0 or 3 mg/kg bw/day from day 6
through day 15 of pregnancy (day 0 = day positive vaginal smear or
vaginal plug observed). The dams were sacrificed on day 21 of
pregnancy and foetuses were removed by caesarean section for external,
skeletal and visceral examination. There were no mortalities or signs
of toxicity. Maternal body weight and food consumption from day 0 to
day 21 of pregnancy, the number of embryonal deaths, foetal deaths,
dead foetuses and live foetuses as well as foetal weight were not
1 See Annex 2 for WHO and FAO documentation.
significantly different between control and treated groups. A
significant difference from the concurrent control group in the sex
ratio of foetuses was noted at 3 mg/kg bw/day. This was unlikely to be
related to treatment, since a similar sex ratio (approximately 44%
males) reportedly occurred in historical untreated controls. No foetal
external malformations and no treatment-related increase in incidence
of skeletal anomalies were evident. Visceral examination revealed
bilateral hydrocephalus internus in one control foetus, medial
dystopia of an undescended testis and bilateral hydrocephalus
internus, respectively, in 2 foetuses (from 2 litters) at 0.3 mg/kg
bw/day, ventral dystopia of the kidney and unilateral hydrocephalus
internus respectively in 2 foetuses (from 2 litters) at 1 mg/kg bw/day
and dilated aqueductus cerebre silvii and anasarca respectively in 2
foetuses (from 2 litters) at 3 mg/kg body weight/day. The author of
the report stated that such malformations occurred spontaneously in
untreated rats of this strain. Indeed, in view of their low and
isolated incidences, it would appear unlikely that the observed
malformations were induced by isofenphos (Becker 1981).
Special Studies on Mutagenicity
Mouse
In a micronucleus test, three groups of 5 male and 5 female mice
(Bor: NMRI (SPF Han)) strain were treated orally with a 0, 15 or
30 mg/kg bw dose of isofenphos (91.8% pure) on two occasions,
separated by 24 hours. Six hours after the second dose, the animals
were sacrificed and femoral bone marrow smears were prepared. There
were no mortalities or clinical signs of toxicity. The incidence of
micronucleated polychromatic erythrocytes or of micronucleated cells
in normochromatic erythrocytes was not significantly different between
controls and treated groups. Under the conditions of the test,
isofenphos was not mutagenic (Herbold 1981).
Special Studies on Neurotoxicity
Hen
Thirty White Leghorn laying hens, approximately 12 1/2 months
old, were intubated with a single dose of 32 mg isofenphos (91.9%
pure)/kg bw. (The oral LD50 of the compound was not determined in the
study. It was found to be 21 mg/kg body weight in an earlier study by
another laboratory, see FAO/WHO 1982). These birds were also treated
with 50 mg/kg bw of atropine i.p. at the time isofenphos was given.
Seventeen of the treated hens died within 24 hours of dosing.
Locomotor ataxia and paralysis were observed in all treated hens
beginning on day 1 or 2 but these signs were not seen beyond day 6.
Histopathological examination of the brain, spinal cord and sciatic
nerve from the 13 surviving hens sacrificed at the end of a 21-day
observation period revealed minimal changes that were similar in
incidence and severity to those noted in concurrent controls (5 hens
given water by gavage as dosing controls, and 5 hens as untreated
controls). Positive controls, treated orally with tri-o-tolyl
phosphate (TOTP) at 500 mg/kg bw, displayed clinical signs and
histopathological lesions of the nervous tissue typical of delayed
neurotoxicity (Hixson 1982).
COMMENTS
The available teratogenicity study in rats and micronucleus test
in mice were both negative. The single-dose delayed neurotoxicity
study in hens, while indicating no delayed neurotoxic potential of the
insecticide, could be considered to be only a screen, as was a similar
study previously evaluated (FAO/WHO 1982). Similarly, the suitability
of a single litter per generation study in the multigeneration study
was questioned by the Meeting. Because of the absence of an
appropriate delayed neurotoxicity study and the deliberations
regarding the multigeneration studies, the Meeting recommended an
extension of the temporary ADI estimated in 1981.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.15 mg/kg bw
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0005 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1986)
1. An appropriate delayed neurotoxicity study.
2. Two-generation (two litters/generation) reproduction study.
Desirable
1. In vitro biochemical studies on purified isofenphos with
respect to anticholinesterase activity.
2. Further observations in humans,
REFERENCES
Becker, H. Embryotoxicity and teratogenicity study in rats, Report
1981 from RCC Research and Consulting Company Ltd., Switzerland,
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Herbold, B. Micronucleus test on mouse to evaluate SRA 12869
1981 (isofenphos: active ingredient of Oftanol) for mutagenic
potential. Report from Bayer AG Institut Fur Toxikologie
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Hixson, E.J. Acute delayed neurotoxicity of technical isofenphos in
1982 hens. Report from Mobay Chemical Corporation, U.S.A.
submitted to the World Health Organization by Bayer AG.
(Unpublished)
ISOFENPHOS
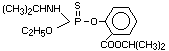 Explanation
Isofenphos was evaluated by the 1981 Joint Meeting. 1 A
temporary acceptable daily intake for man and maximum residue levels
for several commodities were recommended. Additional data from
supervised trials on potatoes and onions were considered desirable.
New information on use pattern, residues resulting from supervised
trials and on the fate of residues were made available for evaluation
and they are discussed in this addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In addition to the use pattern included in the 1981 Monograph,
the following new recommendations were submitted.
Crop Application rate Formulation No. of treatments
(a.i.)
Citrus fruit 0.05% EC, WP 2
Bananas 2 g/plant GR 3
Pears 0.05-0.075% EC 3
RESIDUES RESULTING FROM SUPERVISED TRIALS
Supervised trials were carried out on various crops in Finland,
France, Germany, Italy and South Africa. Analyses were done for
isofenphos and isofenphos oxygen analogue (IOA).
1 See Annex 2 for FAO and WHO documentation.
Bananas
Five supervised trials were carried out in various countries.
Oftanol GR was applied at rates of 1.2-4 g a.i./plant two or three
times, three to five months apart. Samples were taken at intervals
from 3 to 154 days after last application. Isofenphos and isofenphos
oxon were analysed in the peel and whole fruit. The residue was below
the limit of determination (0.01 mg/kg) in every case.
Citrus Fruit
Navel orange and lemon trees were treated with Oftanol 50 EC at
the normal (0.05% a.i.) and at double (0.1% a.i.) application rate in
South Africa. Samples taken at various intervals were analysed for
isofenphos and its oxygen analogue applying a method having a
0.05 mg/kg limit of determination for both compounds. The results are
summarized in Table 1 (South Africa, 1981).
Pear
In supervised trials, Oftanol 500 EC was applied one to four
times in a solution containing 0.05-0.075% a.i. for the treatment of
'Alexander' and 'Williams' pear trees in Italy and once in France.
Details and results of experiments are summarized in Table 2. The
residue declined moderately during the first 4 weeks after treatment;
there was little difference in the residue range and in the average
residue level thereafter.
Brassica Leafy Vegetables
Narrow-stem kale seed was treated at the recommended rate
(16 g a.i./kg seed) with Oftanol C. The plants were sampled 133-169
days after sowing. No residue could be detected over the limit of
determination (0.01 mg/kg).
Supervised trials were carried out in Norway applying OftanolR
at the approved rates and time. Residues (the parent compound and its
oxon) in broccoli, Chinese cabbage and kohlrabi were generally below
0.02 mg/kg and did not exceed 0.1 mg/kg.
Oilseed Rape
Seed dressing was carried out at a rate of 16 g a.i./kg seed with
Oftanol C or Oftanol T in four experiments. Samples of mature seed
taken 125-305 days after sowing contained only non-detectable
residues.
Table 1. Residues resulting from supervised trials in citrus fruit, 1980
Application Residues 1 (mg/kg) at intervals (days) after application
Crop No. Rate Compound
(% a.i.) 1 7 14 21 28 35 42
Lemon 1 0.05 I 1.3 1.1 0.8 0.53 0.75 0.82 0.56
IOA 0.13 0.26 0.23 0.15 0.22 0.19 0.17
Lemon 1 0.1 I 2.9 1.8 1.1 1 0.8 0.63 0.54
IOA 0.25 0.42 0.36 0.31 0.28 0.29 0.23
Orange 1 0.05 I 2.7 1.7 1.1 1 0.87 0.76 0.67
IOA 0.11 0.17 0.12 0.12 0.12 0.1 0.1
Orange 1 0.1 I 3.4 2 1.6 1.4 1.3 0.85 0.78
IOA 0.13 0.2 0.18 0.15 0.19 0.14 0.12
1 Results are the average of two replicates
Table 2. Residues resulting from supervised trials in pears 1
Application Residues (mg/kg) at intervals (days)
Variety Country Year No. Rate after application
(kg a.i./ha Interval
and %) (weeks) 0 14 21 28 35 40-43 60
Alexander France 1977 2 0.6(0.06) 2 0.12
Alexander Italy 1974 1 1.5(0.075) 2.24 0.71 0.71 0.41
Alexander Italy 1976 4 1.5(0.05) 4 0.27 0.16
Alexander Italy 1976 4 1.5(0.05) 4 0.47 0.11
Alexander Italy 1976 3 1.5(0.05) 4 0.2 0.29
Alexander Italy 1976 3 1.5(0.05) 4 0.17 0.39
Williams Italy 1976 1 1.75(0.05) 0.97 0.62 0.47 0.47 0.27 0.12
Alexander Italy 1975 1 1.75(0.05) 2.54 0.84 0.32 0.23 0.22
Alexander Italy 1975 1 1.75(0.05) 5.21 0.86 0.35 0.32 0.25
Williams Italy 1975 1 1.75(0.05) 1.12 0.46 0.38 0.44 0.44
Alexander Italy 1976 1 2.25(0.075) 0.42 0.34
Alexander Italy 1976 4 2.25(0.075) 4 0.63 0.33
Alexander Italy 1976 3 2.25(0.075) 4 0.32 0.42
Alexander Italy 1976 3 2.25(0.075) 4 0.44 0.75
Williams Italy 1975 1 63 (0.0075) 1.06 0.84 0.69 0.64 0.34
Alexander Italy 1975 1 2.63(0.075) 0.86 0.88 0.53 0.4 0.28
Alexander Italy 1975 1 2.63(0.075) 5.35 0.75 0.39 0.3 0.27
Williams Italy 1975 1 2.63(0.075) 0.99 0.61 0.67 0.72 0.34
1 Bayer AG 1982.
Swedes
Swedes were treated at a rate of 16 g a.i,/kg seed with Oftanol C
in the Federal Republic of Germany (FRG). No residue was detected
either in the leaves or in the roots 140-169 days after treatment. In
supervised trials carried out in Finland, no residue was detectable in
washed plants grown after seed dressing, while 0.008-0.07 mg/kg
isofenphos and 0.015-0.01 mg/kg IOA were detected following soil
treatment at planting at a rate of 0.25 g a.i./m2.
Turnip
Oftanol 500 EC was used at a rate of 0.075 g a.i./m for the
treatment of the plots in 10 cm band at planting. Residues detected in
the leaves and roots are summarized in Table 3.
Table 3. Residues in turnips after soil treatment with isofenphos
Residues (mg/kg)at intervals (days) after application
42 60 80
root leaf root leaf root leaf
0.05 0.03 0.04 0.01 <0.01 <0.01
0.15 0.08 0.02 0.01 0.05 0.01
0.2 0.05 0.02 <0.01 0.01 0.01
Turnip plants grown from seeds treated with isofenphos at a
dosage rate of 16 g a.i./kg seed did not contain detectable residues
in trials carried out in FRG and Finland.
Other Crops
Supervised trials were carried out in Finland in various
vegetables. Carrot and radish were treated with 0.5 g a.i./m2 and
onion seeds were dipped in a solution containing 0.025% a.i. The crops
were washed before analysis. The sum of isofenphos and IOA found in
different crops at various intervals after treatment was the
following: carrot-2 mg/kg (22 days), 0.4 mg/kg (84 days), radish:
1.2 mg/kg (6 days), 1.25 mg/kg (13 days), onion 0.024 mg/kg (62 days)
(Finland undated).
FATE OF RESIDUES
In Plants
Ethoxy-1-3H, ring UL-14C isofenphos, formulated as 6E, was
applied to cabbage seeds in furrow at planting at a rate of
0.13 g a.i./m. At transplanting, 28 days after planting, some of the
cabbage plants were transferred to untreated soil. The remaining
cabbage plants received a second application of labelled isofenphos as
a direct spray at the base of the plants at a rate of 1.68 kg a.i./ha
(Strankowski and Murphy 1980). At various sampling intervals the
radioactivity was released from the cabbage tissues by methanol-water
extraction, enzyme, acid and basic hydrolyses. In plants receiving one
and two treatments, 80-98% and 89-96% of the radioactivity were
identified respectively.
The major degradation pathway for isofenphos in cabbage involved
oxidation to its oxon analogue (IOA) and subsequent hydrolysis to
isopropyl salicylate (IPS) and salicylic acid (SA). Further breakdown
then produced 2,3-dihydroxy benzoic acid (2,3-DHBA) and benzoic acid
(BA). Des-N-isopropyl isofenphos oxygen analogue (des IOA) was also
identified. The IPS, 2,3-DHBA and BA were found in free and conjugated
form while 2,3-DHBA appeared only in conjugated form.
In Norway the results of supervised trials indicated that the
residues were below 0.05 mg/kg in celeriac potted out in soil
containing 225 g isofenphos/m3. Residues were not detectable in
carrots, onion and winter radish following seed dressing.
The radioactive residues (0.001-0.006 mg/kg) identified in mature
samples were characterized as being very polar hydrolysis products.
The amount of organosoluble residue and the metabolites identified in
maize and onion [FAO/WHO 1981) and in cabbage were very similar. The
main difference between the metabolism of isofenphos in cabbage and
maize or onion was that no IPS was found in the latter two crops.
The uptake of radioactivity in cabbage seedlings appeared to be
continuous. Each successive new leaf contained radioactivity, but in
decreasing concentration. The concentration of total radioactivity in
cabbage plants decreased with time, and 189 days after planting the
total residue was equivalent to <0.012 mg/kg and 0.057 mg/kg
isofenphos in mature cabbage heads receiving one or two treatments
respectively. The overall distribution of radioactivity at various
intervals after applications is summarized in Table 4.
Table 4. Distribution of isofenphos and its metabolites in cabbage
Total radioactivity (%) at intervals (days) after
one and two applications
Days/ 7 14 28 42 56 189
No. of applications 1 1 1 1 2 1 2 1 2
Total residue 1 14.2 5.28 2.36 0.17 0.61 0.06 0.3 <0.012 0.057
Isofenphos 79 53 37 15 33 5 29 <1 0
IOA 14 34 43 24 28 19 32 4 13
Des IOA <1 1 2 2 1 <1 1 1 1
IPS 1 3 4 24 16 30 13 12 29
SA 1 2 2 12 10 21 12 31 19
BA <1 1 3 1 <1 2 <1 3 2
2,3 DHBA <1 1 1 2 2 3 1 3 6
1 Expressed in mg/kg isofenphos equivalent.
In Soil
The mobility of isofenphos was studied in German standard soils
2.1, 2.2 and 2.3. Equivalents of 2-4 kg a.i./ha of two formulations
were applied to the soil and leached for two days. Neither isofenphos
nor its oxon derivative was detectable in the leachates (limit of
determination 0.0001 mg/kg) (Bayer AG 1982).
NATIONAL MAXIMUM RESIDUE LIMITS
Available information on national MRLs reported to the Meeting is
given in Table 5.
Table 5. National Maximum Residue Limits and preharvest intervals
Preharvest
Country Crop interval MRL
(days) (mg/kg)
Federal Republic
of Germany Leafy and other
sprouting vegetables 0.1
Root vegetables,
rape seed 0.05
Italy Artichokes 0.1
Brassicas 0.1
Eggplant 0.1
Fruit 0.1
Garlic 0.1
Onion 0.1
Pear 42 0.1
Pepper 0.1
Sugarbeet 42 0.1
Tomato 0.1
Netherlands Cabbage 56 0.1
Brussels sprouts 56 0.05
Cauliflower 56 0.05
Colerisc 0.05
Celery 0.05
Leek 0.1
Onion 0.1
Norway Root vegetables 90
Stem vegetables 90
South Africa Citrus 42 0.2
Spain Garlic 21
Onion 21
Table 5. (con't)
Preharvest
Country Crop interval MRL
(days) (mg/kg)
U.S. Maize (fodder) 75 1.0
Maize (forage) 75 1.0
Maize (fresh, incl.
sweet corn) 75 0.1
Maize (grain) 75 0.1
Eggs 0.02
Meat, fat, meat by-
products of cattle,
goats, pigs, horses,
sheep and poultry 0.1
Milk 0.02
APPRAISAL
Since isofenphos was last evaluated by the 1981 JMPR, additional
data have been made available for further consideration.
The use of isofenphos is recommended in banana, citrus fruit and
pear. It is applied as a spray containing 0.05% and 0.05-0.075% a.i. 2
and 3 times in citrus fruit and pear respectively. Banana trees can be
treated with 2 g a.i./plant up to 3 times annually.
Supervised trials were carried out on various crops and the
residues of isofenphos (I) and its oxygen analogue (IOA) were
analysed. The limit of determination was 0.01-0.05 mg/kg for both
compounds. Bananas did not contain detectable residues after three
treatments at the recommended rate. Residue levels in lemons and
oranges are approximately similar 42 days after treatment, and
isofenphos amounts to 70-87% of the total residues. In pear, the
initial residues declined moderately in the first month after
application while there was little difference in range and average
level of residue in samples taken in the second month.
The treatment of soil at planting or sowing resulted in residues
in carrots, onions, swedes and turnips; however, no measurable
residues were found in subsequent crops after seed dressing.
Isofenphos residues are readily taken up by cabbage plants from
soil. The major degradation pathway for isofenphos in cabbage involved
oxidation (IOA) and subsequent hydrolysis to isopropyl salicylate
(IPS) and salicylic acid (SA). The parent compound and its three
metabolites formed the major part of the total residue in the plant.
The proportion of these compounds varied with time. At maturity IOA,
IPS and SA were the only residues present while intact isofenphos was
not detectable. The total radioactivity, expressed in isofenphos
equivalents, was 0.057 mg/kg in mature cabbage treated at planting and
one month later. There was no difference in the degradation pathway
for isofenphos in cabbage receiving one or two treatments. The amounts
of organosoluble residues in cabbage were similar to those found in
maize and onions in previous experiments (FAO/WHO 1981). The
degradation products were also similar with the exception of IPS,
which was found only in cabbage.
Further leaching experiments confirmed the low mobility of
isofenphos in different soils, indicating that the contamination of
underground water with isofenphos residues used for soil treatment is
unlikely.
RECOMMENDATIONS
The Meeting examined residue data from new supervised trials and
was able to estimate maximum residue levels for the following
commodities in addition to those previously recommended. Results of
other experiments supported the recommendations of the 1981 meeting,
and no amendments were necessary. The levels refer to the sum of
isofenphos and isofenphos oxygen analogue.
Temporary Maximum Preharvest
Residue Level Interval
(mg/kg) (weeks)
Citrus fruit 1 6
Pears 0.5 6
Bananas 0.02 1 8
1 Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Further information on the residue in different varieties of
citrus treated at the recommended rate with isofenphos and
on the distribution of residues in the peel and pulp of
citrus fruits.
2. Additional data from supervised trials on potatoes and
onions, including information on soil residues and soil
moisture content.
REFERENCES
Bayer AG. Pflanzenschutzmittle-Rückstände: Bananas: Report Nos. 4042-
1982 4043/78, 4000/80, 4001/I/80, 4001/II/80, Narrow-stem kale:
Report Nos. 4010-4012/78; Pears: Report Nos. 4019/77,
4055/74, 4019/B/76, 4019/A/76, 4017/B/76, 4017/A/76,
4009/75, 4049/75, 4043/75, 4011/75, 4020/B/76, 4020/A/76,
4018/B/76, 4018/A/76, 4010/75, 4046/75, 4044/75, 4041/75;
Oilseed Rape; Report Nos, 4007-4009/78, 4019/78;
Swedes:Report Nos. 4016/78, 4018/78; Turnips: Report Nos
4014-4015/78, 4006-4008/77; Water: Report Nos, 4046-4048/74,
4008-4010/81, (Unpublished)
Finland. Information on pesticides included in the priority list.
undated
South Africa, Determination of isofenphos residues in citrus fruit,
1981 Bureau of Standards, Report No. 311/88005/U9. (Unpublished)
Strankowsky, K.J. and Murphy, J.J. Metabolism of AMAZE in cabbage,
1980 Mobay Report No. 69 174. (Unpublished)
Explanation
Isofenphos was evaluated by the 1981 Joint Meeting. 1 A
temporary acceptable daily intake for man and maximum residue levels
for several commodities were recommended. Additional data from
supervised trials on potatoes and onions were considered desirable.
New information on use pattern, residues resulting from supervised
trials and on the fate of residues were made available for evaluation
and they are discussed in this addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In addition to the use pattern included in the 1981 Monograph,
the following new recommendations were submitted.
Crop Application rate Formulation No. of treatments
(a.i.)
Citrus fruit 0.05% EC, WP 2
Bananas 2 g/plant GR 3
Pears 0.05-0.075% EC 3
RESIDUES RESULTING FROM SUPERVISED TRIALS
Supervised trials were carried out on various crops in Finland,
France, Germany, Italy and South Africa. Analyses were done for
isofenphos and isofenphos oxygen analogue (IOA).
1 See Annex 2 for FAO and WHO documentation.
Bananas
Five supervised trials were carried out in various countries.
Oftanol GR was applied at rates of 1.2-4 g a.i./plant two or three
times, three to five months apart. Samples were taken at intervals
from 3 to 154 days after last application. Isofenphos and isofenphos
oxon were analysed in the peel and whole fruit. The residue was below
the limit of determination (0.01 mg/kg) in every case.
Citrus Fruit
Navel orange and lemon trees were treated with Oftanol 50 EC at
the normal (0.05% a.i.) and at double (0.1% a.i.) application rate in
South Africa. Samples taken at various intervals were analysed for
isofenphos and its oxygen analogue applying a method having a
0.05 mg/kg limit of determination for both compounds. The results are
summarized in Table 1 (South Africa, 1981).
Pear
In supervised trials, Oftanol 500 EC was applied one to four
times in a solution containing 0.05-0.075% a.i. for the treatment of
'Alexander' and 'Williams' pear trees in Italy and once in France.
Details and results of experiments are summarized in Table 2. The
residue declined moderately during the first 4 weeks after treatment;
there was little difference in the residue range and in the average
residue level thereafter.
Brassica Leafy Vegetables
Narrow-stem kale seed was treated at the recommended rate
(16 g a.i./kg seed) with Oftanol C. The plants were sampled 133-169
days after sowing. No residue could be detected over the limit of
determination (0.01 mg/kg).
Supervised trials were carried out in Norway applying OftanolR
at the approved rates and time. Residues (the parent compound and its
oxon) in broccoli, Chinese cabbage and kohlrabi were generally below
0.02 mg/kg and did not exceed 0.1 mg/kg.
Oilseed Rape
Seed dressing was carried out at a rate of 16 g a.i./kg seed with
Oftanol C or Oftanol T in four experiments. Samples of mature seed
taken 125-305 days after sowing contained only non-detectable
residues.
Table 1. Residues resulting from supervised trials in citrus fruit, 1980
Application Residues 1 (mg/kg) at intervals (days) after application
Crop No. Rate Compound
(% a.i.) 1 7 14 21 28 35 42
Lemon 1 0.05 I 1.3 1.1 0.8 0.53 0.75 0.82 0.56
IOA 0.13 0.26 0.23 0.15 0.22 0.19 0.17
Lemon 1 0.1 I 2.9 1.8 1.1 1 0.8 0.63 0.54
IOA 0.25 0.42 0.36 0.31 0.28 0.29 0.23
Orange 1 0.05 I 2.7 1.7 1.1 1 0.87 0.76 0.67
IOA 0.11 0.17 0.12 0.12 0.12 0.1 0.1
Orange 1 0.1 I 3.4 2 1.6 1.4 1.3 0.85 0.78
IOA 0.13 0.2 0.18 0.15 0.19 0.14 0.12
1 Results are the average of two replicates
Table 2. Residues resulting from supervised trials in pears 1
Application Residues (mg/kg) at intervals (days)
Variety Country Year No. Rate after application
(kg a.i./ha Interval
and %) (weeks) 0 14 21 28 35 40-43 60
Alexander France 1977 2 0.6(0.06) 2 0.12
Alexander Italy 1974 1 1.5(0.075) 2.24 0.71 0.71 0.41
Alexander Italy 1976 4 1.5(0.05) 4 0.27 0.16
Alexander Italy 1976 4 1.5(0.05) 4 0.47 0.11
Alexander Italy 1976 3 1.5(0.05) 4 0.2 0.29
Alexander Italy 1976 3 1.5(0.05) 4 0.17 0.39
Williams Italy 1976 1 1.75(0.05) 0.97 0.62 0.47 0.47 0.27 0.12
Alexander Italy 1975 1 1.75(0.05) 2.54 0.84 0.32 0.23 0.22
Alexander Italy 1975 1 1.75(0.05) 5.21 0.86 0.35 0.32 0.25
Williams Italy 1975 1 1.75(0.05) 1.12 0.46 0.38 0.44 0.44
Alexander Italy 1976 1 2.25(0.075) 0.42 0.34
Alexander Italy 1976 4 2.25(0.075) 4 0.63 0.33
Alexander Italy 1976 3 2.25(0.075) 4 0.32 0.42
Alexander Italy 1976 3 2.25(0.075) 4 0.44 0.75
Williams Italy 1975 1 63 (0.0075) 1.06 0.84 0.69 0.64 0.34
Alexander Italy 1975 1 2.63(0.075) 0.86 0.88 0.53 0.4 0.28
Alexander Italy 1975 1 2.63(0.075) 5.35 0.75 0.39 0.3 0.27
Williams Italy 1975 1 2.63(0.075) 0.99 0.61 0.67 0.72 0.34
1 Bayer AG 1982.
Swedes
Swedes were treated at a rate of 16 g a.i,/kg seed with Oftanol C
in the Federal Republic of Germany (FRG). No residue was detected
either in the leaves or in the roots 140-169 days after treatment. In
supervised trials carried out in Finland, no residue was detectable in
washed plants grown after seed dressing, while 0.008-0.07 mg/kg
isofenphos and 0.015-0.01 mg/kg IOA were detected following soil
treatment at planting at a rate of 0.25 g a.i./m2.
Turnip
Oftanol 500 EC was used at a rate of 0.075 g a.i./m for the
treatment of the plots in 10 cm band at planting. Residues detected in
the leaves and roots are summarized in Table 3.
Table 3. Residues in turnips after soil treatment with isofenphos
Residues (mg/kg)at intervals (days) after application
42 60 80
root leaf root leaf root leaf
0.05 0.03 0.04 0.01 <0.01 <0.01
0.15 0.08 0.02 0.01 0.05 0.01
0.2 0.05 0.02 <0.01 0.01 0.01
Turnip plants grown from seeds treated with isofenphos at a
dosage rate of 16 g a.i./kg seed did not contain detectable residues
in trials carried out in FRG and Finland.
Other Crops
Supervised trials were carried out in Finland in various
vegetables. Carrot and radish were treated with 0.5 g a.i./m2 and
onion seeds were dipped in a solution containing 0.025% a.i. The crops
were washed before analysis. The sum of isofenphos and IOA found in
different crops at various intervals after treatment was the
following: carrot-2 mg/kg (22 days), 0.4 mg/kg (84 days), radish:
1.2 mg/kg (6 days), 1.25 mg/kg (13 days), onion 0.024 mg/kg (62 days)
(Finland undated).
FATE OF RESIDUES
In Plants
Ethoxy-1-3H, ring UL-14C isofenphos, formulated as 6E, was
applied to cabbage seeds in furrow at planting at a rate of
0.13 g a.i./m. At transplanting, 28 days after planting, some of the
cabbage plants were transferred to untreated soil. The remaining
cabbage plants received a second application of labelled isofenphos as
a direct spray at the base of the plants at a rate of 1.68 kg a.i./ha
(Strankowski and Murphy 1980). At various sampling intervals the
radioactivity was released from the cabbage tissues by methanol-water
extraction, enzyme, acid and basic hydrolyses. In plants receiving one
and two treatments, 80-98% and 89-96% of the radioactivity were
identified respectively.
The major degradation pathway for isofenphos in cabbage involved
oxidation to its oxon analogue (IOA) and subsequent hydrolysis to
isopropyl salicylate (IPS) and salicylic acid (SA). Further breakdown
then produced 2,3-dihydroxy benzoic acid (2,3-DHBA) and benzoic acid
(BA). Des-N-isopropyl isofenphos oxygen analogue (des IOA) was also
identified. The IPS, 2,3-DHBA and BA were found in free and conjugated
form while 2,3-DHBA appeared only in conjugated form.
In Norway the results of supervised trials indicated that the
residues were below 0.05 mg/kg in celeriac potted out in soil
containing 225 g isofenphos/m3. Residues were not detectable in
carrots, onion and winter radish following seed dressing.
The radioactive residues (0.001-0.006 mg/kg) identified in mature
samples were characterized as being very polar hydrolysis products.
The amount of organosoluble residue and the metabolites identified in
maize and onion [FAO/WHO 1981) and in cabbage were very similar. The
main difference between the metabolism of isofenphos in cabbage and
maize or onion was that no IPS was found in the latter two crops.
The uptake of radioactivity in cabbage seedlings appeared to be
continuous. Each successive new leaf contained radioactivity, but in
decreasing concentration. The concentration of total radioactivity in
cabbage plants decreased with time, and 189 days after planting the
total residue was equivalent to <0.012 mg/kg and 0.057 mg/kg
isofenphos in mature cabbage heads receiving one or two treatments
respectively. The overall distribution of radioactivity at various
intervals after applications is summarized in Table 4.
Table 4. Distribution of isofenphos and its metabolites in cabbage
Total radioactivity (%) at intervals (days) after
one and two applications
Days/ 7 14 28 42 56 189
No. of applications 1 1 1 1 2 1 2 1 2
Total residue 1 14.2 5.28 2.36 0.17 0.61 0.06 0.3 <0.012 0.057
Isofenphos 79 53 37 15 33 5 29 <1 0
IOA 14 34 43 24 28 19 32 4 13
Des IOA <1 1 2 2 1 <1 1 1 1
IPS 1 3 4 24 16 30 13 12 29
SA 1 2 2 12 10 21 12 31 19
BA <1 1 3 1 <1 2 <1 3 2
2,3 DHBA <1 1 1 2 2 3 1 3 6
1 Expressed in mg/kg isofenphos equivalent.
In Soil
The mobility of isofenphos was studied in German standard soils
2.1, 2.2 and 2.3. Equivalents of 2-4 kg a.i./ha of two formulations
were applied to the soil and leached for two days. Neither isofenphos
nor its oxon derivative was detectable in the leachates (limit of
determination 0.0001 mg/kg) (Bayer AG 1982).
NATIONAL MAXIMUM RESIDUE LIMITS
Available information on national MRLs reported to the Meeting is
given in Table 5.
Table 5. National Maximum Residue Limits and preharvest intervals
Preharvest
Country Crop interval MRL
(days) (mg/kg)
Federal Republic
of Germany Leafy and other
sprouting vegetables 0.1
Root vegetables,
rape seed 0.05
Italy Artichokes 0.1
Brassicas 0.1
Eggplant 0.1
Fruit 0.1
Garlic 0.1
Onion 0.1
Pear 42 0.1
Pepper 0.1
Sugarbeet 42 0.1
Tomato 0.1
Netherlands Cabbage 56 0.1
Brussels sprouts 56 0.05
Cauliflower 56 0.05
Colerisc 0.05
Celery 0.05
Leek 0.1
Onion 0.1
Norway Root vegetables 90
Stem vegetables 90
South Africa Citrus 42 0.2
Spain Garlic 21
Onion 21
Table 5. (con't)
Preharvest
Country Crop interval MRL
(days) (mg/kg)
U.S. Maize (fodder) 75 1.0
Maize (forage) 75 1.0
Maize (fresh, incl.
sweet corn) 75 0.1
Maize (grain) 75 0.1
Eggs 0.02
Meat, fat, meat by-
products of cattle,
goats, pigs, horses,
sheep and poultry 0.1
Milk 0.02
APPRAISAL
Since isofenphos was last evaluated by the 1981 JMPR, additional
data have been made available for further consideration.
The use of isofenphos is recommended in banana, citrus fruit and
pear. It is applied as a spray containing 0.05% and 0.05-0.075% a.i. 2
and 3 times in citrus fruit and pear respectively. Banana trees can be
treated with 2 g a.i./plant up to 3 times annually.
Supervised trials were carried out on various crops and the
residues of isofenphos (I) and its oxygen analogue (IOA) were
analysed. The limit of determination was 0.01-0.05 mg/kg for both
compounds. Bananas did not contain detectable residues after three
treatments at the recommended rate. Residue levels in lemons and
oranges are approximately similar 42 days after treatment, and
isofenphos amounts to 70-87% of the total residues. In pear, the
initial residues declined moderately in the first month after
application while there was little difference in range and average
level of residue in samples taken in the second month.
The treatment of soil at planting or sowing resulted in residues
in carrots, onions, swedes and turnips; however, no measurable
residues were found in subsequent crops after seed dressing.
Isofenphos residues are readily taken up by cabbage plants from
soil. The major degradation pathway for isofenphos in cabbage involved
oxidation (IOA) and subsequent hydrolysis to isopropyl salicylate
(IPS) and salicylic acid (SA). The parent compound and its three
metabolites formed the major part of the total residue in the plant.
The proportion of these compounds varied with time. At maturity IOA,
IPS and SA were the only residues present while intact isofenphos was
not detectable. The total radioactivity, expressed in isofenphos
equivalents, was 0.057 mg/kg in mature cabbage treated at planting and
one month later. There was no difference in the degradation pathway
for isofenphos in cabbage receiving one or two treatments. The amounts
of organosoluble residues in cabbage were similar to those found in
maize and onions in previous experiments (FAO/WHO 1981). The
degradation products were also similar with the exception of IPS,
which was found only in cabbage.
Further leaching experiments confirmed the low mobility of
isofenphos in different soils, indicating that the contamination of
underground water with isofenphos residues used for soil treatment is
unlikely.
RECOMMENDATIONS
The Meeting examined residue data from new supervised trials and
was able to estimate maximum residue levels for the following
commodities in addition to those previously recommended. Results of
other experiments supported the recommendations of the 1981 meeting,
and no amendments were necessary. The levels refer to the sum of
isofenphos and isofenphos oxygen analogue.
Temporary Maximum Preharvest
Residue Level Interval
(mg/kg) (weeks)
Citrus fruit 1 6
Pears 0.5 6
Bananas 0.02 1 8
1 Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Further information on the residue in different varieties of
citrus treated at the recommended rate with isofenphos and
on the distribution of residues in the peel and pulp of
citrus fruits.
2. Additional data from supervised trials on potatoes and
onions, including information on soil residues and soil
moisture content.
REFERENCES
Bayer AG. Pflanzenschutzmittle-Rückstände: Bananas: Report Nos. 4042-
1982 4043/78, 4000/80, 4001/I/80, 4001/II/80, Narrow-stem kale:
Report Nos. 4010-4012/78; Pears: Report Nos. 4019/77,
4055/74, 4019/B/76, 4019/A/76, 4017/B/76, 4017/A/76,
4009/75, 4049/75, 4043/75, 4011/75, 4020/B/76, 4020/A/76,
4018/B/76, 4018/A/76, 4010/75, 4046/75, 4044/75, 4041/75;
Oilseed Rape; Report Nos, 4007-4009/78, 4019/78;
Swedes:Report Nos. 4016/78, 4018/78; Turnips: Report Nos
4014-4015/78, 4006-4008/77; Water: Report Nos, 4046-4048/74,
4008-4010/81, (Unpublished)
Finland. Information on pesticides included in the priority list.
undated
South Africa, Determination of isofenphos residues in citrus fruit,
1981 Bureau of Standards, Report No. 311/88005/U9. (Unpublished)
Strankowsky, K.J. and Murphy, J.J. Metabolism of AMAZE in cabbage,
1980 Mobay Report No. 69 174. (Unpublished)
