
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
CARBOSULFAN
IDENTITY
Chemical Name: 2,3-dihydro-2,2-dimethyl-7-benzofuranyl
[(dibutylamino) thio] methylcarbamate
Synonyms: FMC 35001, Marshal(R), Advantage(c)
Empirical Formula: C20H32N2O3S
Structural Formula:
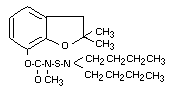 Molecular Weight: 380.5
Technical Material: Approximately 93 percent a.i.;
Manufacturing Use Product (MUP) composition
limits range between 86.0 and 91.0 percent
(w/w) a.i.
Physical Form: Viscous brown liquid
Specific Gravity: 1.056 g/ml at 20°C
Stability: Stable for one year at 22°C, 30 weeks at
50°C, undergoes decomposition at 80°C under
0.1 Torr pressure.
Solubility: Water (0.3 ppm), Completely miscible in
Xylene, Hexane, Chloroform, Methylene
Chloride, Methanol, Acetone.
Volatility: Relatively non-volatile
Vapor Pressure: 0.31 × 10-6 Torr at 25°C
Flash Point: 96°C - closed-cup method
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of ring-14C-Carbosulfan was determined in male and
female rats by examining excreta and selected tissue for activity
following oral administration in corn oil. Animals were fasted for
18 hours prior to oral dosing of 30 and 3.8 mg/kg body weight (b.w.).
A separate group of rats were administered a single dose of 3.3 mg/kg
b.w. unlabelled compound followed 14 days later with the same amount
of labelled compound. There were ten rats (five male and five female)
in each group. Males and females demonstrated the same pattern of
preferential excretion via the urine. Within 24 hours, urinary
excretion in female and male rats was 65.9 and 69.4 percent,
respectively, following a single high dose; 80.08 and 71.04 percent
following a single low dose; and 85.28 and 87.5 percent following the
multiple dose protocol. Elimination in faeces accounted for an
additional 5.9 percent of the administered dose. The majority of the
dose was recovered within the first 24 hours. Tissue activity in males
and females was minimal with <0.01 percent of the dose present in
liver, kidney and skin following the high dose of 30 mg/kg, but at
3 mg/kg 14C activity was generally less then background activity.
Skin accounted for the highest ng equivalents/gram of tissue analyzed.
The urinary metabolites were primarily conjugated, with
3-keto-7 phenol the most abundant, and 3-hydroxy-carbofuran, 7-phenol
and 3-hydroxy-7-phenol also present. Carbofuran, 3-keto-carbofuran,
and N-hydroxymethyl-3-hydroxy carbofuran were minor metabolites
identified. The primary faecal metabolites were unchanged carbosulfan
and 3-hydroxy-carbofuran. Carbofuran was also present in measurable
quantities followed by 3-keto-7-phenol, 3-hydroxy-7-phenol, 7-phenol,
and N-hydroxymethyl-3-hydroxy carbofuran (Wu, 1982a).
In a similar evaluation to the preceding, carbosulfan, labelled
with 14C in the C-1 position of the dibutylamino (DBA) moiety, was
orally gavaged in corn oil to Sprague-Dawley rats. The low dose was
equivalent to 4 mg/kg, administered to five male and five female rats;
the high dose was approximately 27 mg/kg administered to five male
rats, and the multiple dose protocol used five male rats administered
3.6 mg/kg b.w. The majority of radioactivity was excreted in the first
24 hours, with 78-83 percent in the urine and 7-10 percent recovered
in faeces. There were no apparent sex differences identified.
Unlike the ring-labelled carbosulfan study, rats excreted 3-8
percent less DBA-14C carbosulfan radioactivity in the excreta with 1.1
to 2.6 percent tissue and carcase retention. Liver, skin and adipose
accounted for the highest levels in tissues analyzed. This suggests
possible incorporation of the DBA side-chain in the form of fatty
acids, amino acids, sugars or other neutral products. Dibutylamine was
the major metabolite in urine and carbosulfan and 3-keto-carbosulfan
the major metabolites in faeces (Wu, 1982b).
Whole-body autoradiography and physiologic disposition studies
were conducted with ring-14C and DBA-14C carbosulfan. A single oral
dose of both products was absorbed and distributed within 30 minutes
after administration. DBA-14C carbosulfan was distributed more widely
and achieved higher tissue concentrations which persisted longer
than the ring-labelled compound. Tissue sites where DBA-derived
radioactivity was found included: gastrointestinal tract,
epididymides, CNS, glandular tissue, muscle, lung, blood, bone marrow,
liver and ethmoturbinate epithelium, as well as faeces and urine. The
ring-14C labelled compound radioactivity was found in the
gastrointestinal tract, liver, kidney and epididymides. Both compounds
achieved peak concentrations in the tissues at six hours. By 24 hours,
remaining ring-labelled 14C-equivalents, not excreted in the urine
and faeces, were in the gastrointestinal tract, liver and kidney
(Liss, et al., 1981).
Metabolism
Rat
The metabolism of carbosulfan was investigated in female
Sprague-Dawley rats administered 30 mg/kg of aromatic ring-14C,
carbonyl carbamate-14C and DBA-14C labelled chemical in corn oil.
Urine, faeces and carbon dioxide were monitored for four days, after
which the rats were sacrificed and tissues were examined for
radioactivity. Excretion was rapid, with 65-80 percent of the
administered dose being eliminated in respired air, urine and faecal
products during the first 24 hours after treatment. Urinary excretion
was the major route of elimination for both the 14C ring-labelled
(>80 percent) and DBA-labelled (>50 percent) carbosulfan,
carbonyl-labelled carbosulfan preferentially eliminated via expired
carbon dioxide (38 percent). Faecal elimination was minor, except for
the DBA-labelled compound. Radioactive residues remaining in the rat
tissues were low and levels of radioactivity varied with labelling
position. The highest levels of radioactivity were found in the blood,
liver, kidney, lung, heart and spleen, and ranged from 0.1-1.5 mg/kg
(ppm) (carbosulfan equivalents) in ring-14C and DBA-14C
carbosulfan-treated rats after 96 hours. Levels of 0.6-9.6 mg/kg
(carbosulfan equivalents) were noted in tissues of carbonyl-14C
carbosulfan-treated animals after 48 hours. Results support previous
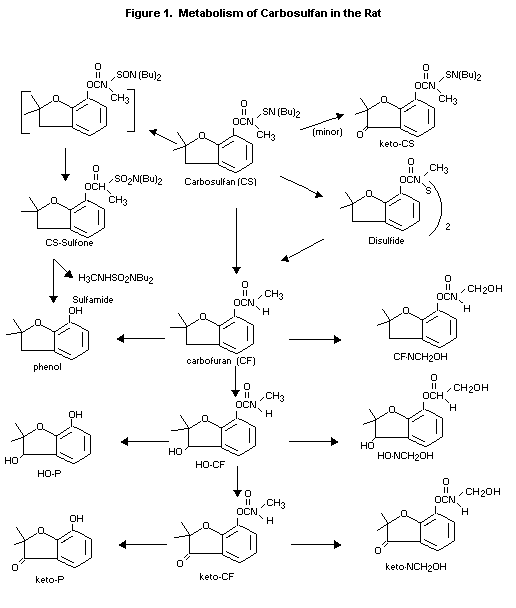
findings and suggest that Carbosulfan is metabolized by two primary
pathways: (1) oxidation of the sulfur to yield CS-sulfone and
sulfamide, and (2) N-S bond cleavage to yield Carbofuran. The first
step represents a significant detoxification mechanism. The scheme in
Figure 1. is proposed for the metabolic pathway in rats.
Molecular Weight: 380.5
Technical Material: Approximately 93 percent a.i.;
Manufacturing Use Product (MUP) composition
limits range between 86.0 and 91.0 percent
(w/w) a.i.
Physical Form: Viscous brown liquid
Specific Gravity: 1.056 g/ml at 20°C
Stability: Stable for one year at 22°C, 30 weeks at
50°C, undergoes decomposition at 80°C under
0.1 Torr pressure.
Solubility: Water (0.3 ppm), Completely miscible in
Xylene, Hexane, Chloroform, Methylene
Chloride, Methanol, Acetone.
Volatility: Relatively non-volatile
Vapor Pressure: 0.31 × 10-6 Torr at 25°C
Flash Point: 96°C - closed-cup method
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of ring-14C-Carbosulfan was determined in male and
female rats by examining excreta and selected tissue for activity
following oral administration in corn oil. Animals were fasted for
18 hours prior to oral dosing of 30 and 3.8 mg/kg body weight (b.w.).
A separate group of rats were administered a single dose of 3.3 mg/kg
b.w. unlabelled compound followed 14 days later with the same amount
of labelled compound. There were ten rats (five male and five female)
in each group. Males and females demonstrated the same pattern of
preferential excretion via the urine. Within 24 hours, urinary
excretion in female and male rats was 65.9 and 69.4 percent,
respectively, following a single high dose; 80.08 and 71.04 percent
following a single low dose; and 85.28 and 87.5 percent following the
multiple dose protocol. Elimination in faeces accounted for an
additional 5.9 percent of the administered dose. The majority of the
dose was recovered within the first 24 hours. Tissue activity in males
and females was minimal with <0.01 percent of the dose present in
liver, kidney and skin following the high dose of 30 mg/kg, but at
3 mg/kg 14C activity was generally less then background activity.
Skin accounted for the highest ng equivalents/gram of tissue analyzed.
The urinary metabolites were primarily conjugated, with
3-keto-7 phenol the most abundant, and 3-hydroxy-carbofuran, 7-phenol
and 3-hydroxy-7-phenol also present. Carbofuran, 3-keto-carbofuran,
and N-hydroxymethyl-3-hydroxy carbofuran were minor metabolites
identified. The primary faecal metabolites were unchanged carbosulfan
and 3-hydroxy-carbofuran. Carbofuran was also present in measurable
quantities followed by 3-keto-7-phenol, 3-hydroxy-7-phenol, 7-phenol,
and N-hydroxymethyl-3-hydroxy carbofuran (Wu, 1982a).
In a similar evaluation to the preceding, carbosulfan, labelled
with 14C in the C-1 position of the dibutylamino (DBA) moiety, was
orally gavaged in corn oil to Sprague-Dawley rats. The low dose was
equivalent to 4 mg/kg, administered to five male and five female rats;
the high dose was approximately 27 mg/kg administered to five male
rats, and the multiple dose protocol used five male rats administered
3.6 mg/kg b.w. The majority of radioactivity was excreted in the first
24 hours, with 78-83 percent in the urine and 7-10 percent recovered
in faeces. There were no apparent sex differences identified.
Unlike the ring-labelled carbosulfan study, rats excreted 3-8
percent less DBA-14C carbosulfan radioactivity in the excreta with 1.1
to 2.6 percent tissue and carcase retention. Liver, skin and adipose
accounted for the highest levels in tissues analyzed. This suggests
possible incorporation of the DBA side-chain in the form of fatty
acids, amino acids, sugars or other neutral products. Dibutylamine was
the major metabolite in urine and carbosulfan and 3-keto-carbosulfan
the major metabolites in faeces (Wu, 1982b).
Whole-body autoradiography and physiologic disposition studies
were conducted with ring-14C and DBA-14C carbosulfan. A single oral
dose of both products was absorbed and distributed within 30 minutes
after administration. DBA-14C carbosulfan was distributed more widely
and achieved higher tissue concentrations which persisted longer
than the ring-labelled compound. Tissue sites where DBA-derived
radioactivity was found included: gastrointestinal tract,
epididymides, CNS, glandular tissue, muscle, lung, blood, bone marrow,
liver and ethmoturbinate epithelium, as well as faeces and urine. The
ring-14C labelled compound radioactivity was found in the
gastrointestinal tract, liver, kidney and epididymides. Both compounds
achieved peak concentrations in the tissues at six hours. By 24 hours,
remaining ring-labelled 14C-equivalents, not excreted in the urine
and faeces, were in the gastrointestinal tract, liver and kidney
(Liss, et al., 1981).
Metabolism
Rat
The metabolism of carbosulfan was investigated in female
Sprague-Dawley rats administered 30 mg/kg of aromatic ring-14C,
carbonyl carbamate-14C and DBA-14C labelled chemical in corn oil.
Urine, faeces and carbon dioxide were monitored for four days, after
which the rats were sacrificed and tissues were examined for
radioactivity. Excretion was rapid, with 65-80 percent of the
administered dose being eliminated in respired air, urine and faecal
products during the first 24 hours after treatment. Urinary excretion
was the major route of elimination for both the 14C ring-labelled
(>80 percent) and DBA-labelled (>50 percent) carbosulfan,
carbonyl-labelled carbosulfan preferentially eliminated via expired
carbon dioxide (38 percent). Faecal elimination was minor, except for
the DBA-labelled compound. Radioactive residues remaining in the rat
tissues were low and levels of radioactivity varied with labelling
position. The highest levels of radioactivity were found in the blood,
liver, kidney, lung, heart and spleen, and ranged from 0.1-1.5 mg/kg
(ppm) (carbosulfan equivalents) in ring-14C and DBA-14C
carbosulfan-treated rats after 96 hours. Levels of 0.6-9.6 mg/kg
(carbosulfan equivalents) were noted in tissues of carbonyl-14C
carbosulfan-treated animals after 48 hours. Results support previous
findings and suggest that Carbosulfan is metabolized by two primary
pathways: (1) oxidation of the sulfur to yield CS-sulfone and
sulfamide, and (2) N-S bond cleavage to yield Carbofuran. The first
step represents a significant detoxification mechanism. The scheme in
Figure 1. is proposed for the metabolic pathway in rats.
 A female rat received a single dose (30 mg/kg) of ring-14C
Carbosulfan via oral gavage. Analyses indicated that levels of
unchanged parent chemical persisted in the blood for three hours after
dosing. Approximately 72 percent of the radiocarbon detected in blood
was attributed to parent chemical (Marsden, Kuwawo & Fukuto, 1982). In
a separate study, female Sprague-Dawley rats were administered
30 mg/kg of carbonyl-14C Carbosulfan. The rats were sacrificed at 15,
35 and 80 minutes after dosing and the stomach contents were assayed
for radioactivity. Approximately 50 percent of the recovered
radioactivity was Carbosulfan after 80 minutes (Umetsu & Fukuto,
1982).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Carbosulfan was administered to groups of Charles River CD rats
(15 males and 30 females/group) at dietary levels of 0, 10, 20 and
250 ppm for three generations. Two successive litters were reared from
each female. General condition and behavior were routinely observed
and individual body weights were recorded throughout the study. The
number of pups in each litter were examined externally and culled to
10/litter at four days of age. Individual pup weights were measured at
days 0, 4, 7, 14 and 21. Ten male and ten female F1b, F2b, and F3b
weanlings were randomly selected for gross necropsy and tissue
collection. The F1a and F2a litters were discarded at weaning, and
the F1b and F2b litters were used to produce succeeding generations.
Weanlings not selected for continuation on the study (F1a, F2a,
F1b, F2b, F3a and F3b) were subjected to gross external
examination, sacrificed and discarded.
Body weights of F0 males and females were decreased initially at
20 and 250 ppm. These decreases were associated with decreased food
consumption, and both recovered to normal from week 4 to sacrifice.
Body weights of parental males (F1 and F2) receiving the 250 ppm
diet were consistently lower than those in the corresponding control
group. A similar effect was observed in the 250 ppm group females
during portions of gestation and lactation, but not during the growth
phases. Mating index, gestation index and number of viable foetuses
were essentially normal throughout, except for F2b dams at the
250 ppm level, which were decreased.
Litter size, pup weights and/or pup weight gains for all litter
sets in the 250 ppm group were significantly lower at most age
intervals between birth and weaning when compared to concurrent
control animals. Neonatal survival among the 250 ppm group was also
significantly lower for the first four litters (F1a, F1b, F2a and
F2b).
There were no treatment-related gross or histologic changes
observed among the F0, F1b and F2b adult groups and the F1b,
F2b and F3b weanling groups. Carbosulfan did not have a demonstrated
adverse effect on reproductive performance, except for the effect on
pup weight, litter size, and pup survival at 250 ppm. The NOEL is
20 ppm (Kehoe & MacKenzie, 1982).
Special Studies on Teratogenicity
Rat
Carbosulfan was administered orally by gavage to groups of 25
Charles River CD rats during gestation days 6 through 19 at dosages of
0, 2, 10 and 20 mg/kg/day. Surviving females were necropsied on day 20
and foetuses delivered by hysterotomy. The number and position of
viable/non-viable foetuses, early/late resorptions, mean number of
corpora lutea and total number of implantations were recorded.
External, internal and skeletal examinations of foetuses were
performed for evidence of abnormalities and anomalies. One third of
the foetuses were evaluated for soft tissue anomalies and the
remaining two thirds for skeletal effects.
There was no dose-related mortality in any treatment group. Body
tremors and clear oral discharge were reported in the 20 mg/kg/day
group after administration of each dose. Mean maternal body weight
gains were slightly reduced at 10 mg/kg/day and significantly reduced
at the 20 mg/kg level during the gestation period. There were no
differences in number of pregnancies, early/late resorptions, viable
foetuses, post-implantation loss or sex distribution. The number of
corpora lutea per dam was increased in all treated groups, while the
mean foetal body weight was significantly reduced at 10 and
20 mg/kg/day. There was also an increase in the number of litters with
developmental variations at 20 mg/kg/day which included reduced
ossification of the skull, hyoid body unossified, sternebrae 5 and 6
unossified, and renal papilla not developed and/or distended ureters.
There were no reported effects on the number or percentage of foetuses
or litters with external, internal or skeletal malformations or
anomalies at any dose level. Carbosulfan was not demonstrated to be
teratogenic in rats at doses up to and including 20 mg/kg/day (Janes,
Rodwell & Blair, 1980a).
Rabbit
Groups of 16 New Zealand Albino Rabbits received carbosulfan by
gavage at dosages of 0, 2, 5, and 10 mg/kg b.w./day on days 6 through
28 of gestation. Pups were delivered by caesarean section on day 29
and the number, location, and distribution of viable/non-viable
foetuses, corpora lutea, early/late resorptions and total
implantations were recorded. All foetuses were examined grossly,
sectioned for visceral anomalies and stained for skeletal anomalies.
There were three deaths at 10 mg/kg and one each in the control
and low-dose groups. The number of dams with viable foetuses were 12,
10, 15 and 11 for the control, 2, 5 and 10 mg/kg groups, respectively.
There were no compound-related effects on the number of viable
foetuses, corpora lutea, foetal sex distribution or total
implantations/dam. There were compound-related effects (e.g. increase)
on post implantation losses and early resorption rate at all doses,
but most notably at 5 and 10 mg/kg. Also, the mean foetal body weight
was decreased in the high dose group. There was a single occurrence of
scoliosis in each of the three treated groups, but none were reported
in the controls. There were no compound-related effects on skeletal
variations, such as delayed ossification. Major vessel variations,
identified primarily as left carotid arising from the innominate, were
observed in 16.7, 100, 46.7 and 72.7 percent of the litters at 0, 2,
5, and 10 mg/kg/day, respectively. The percentage of foetuses
presenting this defect were 4.9, 44, 12.8 and 20.8 percent,
respectively. Although this foetotoxic response was evident and
increased at all dose levels there was no evidence of teratogenicity.
The NOEL for teratogenicity is 10 mg/kg/day (Janes, Rodwell & Blair,
1980b).
Special Studies on Mutagenicity
Carbosulfan was negative in a wide battery of mutagenicity
studies conducted. See Table 1 for a summary of the studies
considered.
Special Studies on Carcinogenicity (See also long-term studies)
Mice
Groups of Charles River CD-1 Mice (100 males and 100 females per
group) were administered Carbosulfan (purity, 94.5 to 95.6 percent
with 0.6 to 2.4 percent carbofuran) in the diet at dose levels of 0,
10, 20, 500 and 2500 ppm for 24 months. All animals were observed
daily for signs of toxicity, moribundity and mortality. Body weight
and food consumption values were recorded weekly for the first 14
weeks and bi-weekly thereafter. Water consumption was determined
monthly. Haematological and biochemical measurements and urinalyses
were performed on ten unfasted mice/sex/group at the 6, 12, 18 and 24
month sacrifices. Ophthalmoscopic evaluations were performed in all
survivors at 24 months. Selected organs were weighed, gross necropsies
conducted and a complete list of tissues and organs examined
microscopically.
There were no measurable effects on mortality or survivability
related to treatment. Mean body weights were reduced throughout the
study at 500 and 2500 ppm for males and 2500 for females. For females
body weight changes at other doses were sporadic and not related to
treatment. However, 20 ppm males demonstrated decreased body weight
gains from week 42 to terminal sacrifice at 104 weeks. Food
consumption was depressed at 2500 ppm for males and females, but only
sporadic decreases were noted at other doses. The actual measured
compound ingested per dose was 0, 1.3, 2.5, 61.5, and 319.5 mg/kg b.w.
for males, and 0, 1.5, 3.1, 71.9 and 337.2 mg/kg b.w. for females for
0, 10, 20, 500 and 2500 ppm, respectively.
There were no measurable differences regarding general appearance
and behavior, except for increased eye irregularities at 2500 ppm.
These included corneal opacity, eccentric pupil, and white, cloudy
eyes.
There were no treatment-related effects or haematological changes
except for a tendency toward slightly increased segmented neutrophils
and decreased lymphocyte counts in males at 2500 ppm. There were no
demonstrated effects on glucose, BUN, SGPT or Alk phosphatase in
either sex at any level.
Cholinesterase values for plasma, erythrocytes and brain tissue
were significantly depressed in both males and females at 500 and
2500 ppm, at all time periods measured.
Ophthalmoscopic examinations indicated an increase in punctuate
opacities of the iris at 500 and 2500 ppm. Pathological changes were
indicative of ageing mice and many were stated to be masked by use of
short-acting mydriatic solutions. However, two separate pathological
opinions concerning the incidence of cataracts in treated mice
indicated that there were compound-related increases in male mice at
500 and 20 ppm, respectively. Females were apparently unaffected by
treatment, although the incidence reached 84 to 100 percent in most
groups. The occurrence overall in control and treated groups indicate
that the effect is possibly exacerbated by the anticholinergic effect,
similarly demonstrated in the rat study. Special evaluation and
concern for the iris, because of compound-related effects in the rat,
did not indicate similar effects in mice.
Absolute organ weight changes were variably affected in both
males and females except for decreased spleen weight in females at 500
and 2500 ppm. Relative spleen weights were also significantly
depressed in females at 500 and 2500 ppm. Relative brain weights were
also significantly increased for both sexes throughout the study at
2500 ppm. This is considered a reflection of the significant effects
on body weight at the higher doses.
Gross and histopathological examination were essentially
unremarkable. The most common findings reported were malignant
lymphoma and bronchio-alveolar adenoma which were equally distributed
among all groups, except for low-dose females which demonstrated a
significant increase in the number of metastatic malignant lymphomas
of mediastinal and mesenteric lymph nodes, as well as thymus and
spleen. Generally, control and low-dose females presented the highest
incidence of malignant lymphomas, in comparison to 20-2500 ppm groups.
The results of the histopathologic examination indicated that the type
and incidence of non-neoplastic and neoplastic lesions were usual
findings in the mouse and unrelated to treatment. Carbosulfan was not
carcinogenic in mice at dietary levels up to and including 2500 ppm.
TABLE 1. Special Studies on Mutagenicity
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Ames' Test S. typhimurium 0.1, 0.5, 2.5, 5 93% Negative Haworth et al.,
(both with and TA 98 and 10 ul/plate 1980
without metabolic TA 100 disolved in DMSO
activation) TA 1535
TA 1537
TA 1538
Bacterial DNA E. coli 0.025, 0.05, 93% Negative. Haworth et al.,
Damage/Repair strains WP2 0.1 and 0.2 ml Preferential kill 1981
Suspension Assay uvr A+ exr A+ per plate of repair deficient
(both with and and CM611 E. coli CM611
without metabolic uvr A- exr A-; without rat liver
activation) S typhimurium microscrees, at
strains TA 1978 0.025 to 0.2 ml
uvr B+ and per plate.
TA 1538 uvr B-
Mouse Lymphoma Mouse Nonactivated: 93% Negative. Kirby et al.,
Forward Mutation L5178Y TK+/- 0.0024, 0.0032, 0.0042, Positive controls 1981
Assay phenotype cells 0.0056, 0.0075, 0.010, (EMS 1 ml/ml; DMAA
(both with and 0.013, 0.018, 0.024 and 7.5 mg/ml) gave
without metabolic 0.032 ml/ml expected response.
activation)
Activated:
0.0056, 0.0075, 0.010,
0.013, 0.018, 0.024,
0.032, 0.042, 0.056
and 0.075 ml/ml
TABLE 1. (continued)
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Microrucleus Mouse, bone Orally, 43.5 and 93% Negative. Kirkhart, Jones
marrow 87 mg/kg (174 mg/kg Positive control & Skinner, 1979
gave excessive mortality (trimethyl phosphate)
and was excluded) yielded expected positive
response at 2 x 1000 mg/kg
i.p.
In Vivo Rat Orally, single doses 93% Carbosulfan was not Putnam and
Cytogenetic of 0, 5, 12 and 30 clastogenic. Positive Schectman, 1981
Assay mg/kg on 5 consecutive control (triethylene-
days melamine) gave expected
response at 0.25 mg/kg
Dominant Mouse Orally, single doses 93% No effect on any Preache, Shefner
Lethal of 0, 7, 20 and 60 reproduction parameter & Reed, 1981
mg/kg for 5 consecutive evaluated (i.e.
days fertility index, number
of implantations, number of
implantation deaths, percent
post-implantation deaths).
Positive control (TEM) gave
expected response at 0.3 mg/kg i.p.
The NOEL is 10 ppm (equal to 1.3 mg/kg b.w.) based on body weight
depression and cataracts in males at 20 pm (DeProspo, Norvell &
Fletcher, 1982b).
Special Study on Neurotoxicity
Groups of adult hens (16 months old) were administered a single
oral dose of Carbosulfan at levels of 0 and 500 mg/kg b.w. A separate
positive control group received 750 mg/kg b.w. of TOCP (a dose seven
times the effective dose needed to produce delayed neuropathy). There
were ten hens in the vehicle and positive control groups, and 40 hens
in the Carbosulfan group. Atropine sulfate (25 mg/kg b.w.) was given
to all birds prior to administration of Carbosulfan. Preliminary range
finding and LD50 evaluations demonstrated the LD50 and LD70 to be
371 and 500 mg/kg b.w., respectively. Body weight and food consumption
were determined weekly. Throughout the study each bird was evaluated
for neurologic effects. At the conclusion of the study (21 days), all
surviving hens were sacrificed and subjected to gross and microscopic
examinations following intravascular perfusion. Axon and myelin
degeneration were assessed in prepared sections of sciatic nerve and
spinal cord.
There were four deaths in the Carbosulfan group within five days
after dosing. No other deaths occurred. Food consumption and body
weight were depressed in the TOCP group. Although food consumption in
the Carbosulfan group was marginally depressed during the first 15
days, there were no differences in body weight gain compared to
vehicle controls. There were no differences in neurologic scores
between vehicle control and the Carbosulfan-treated birds. The TOCP
group gave the expected response, starting on day 13, which consisted
of unsteadiness in walking, inability to walk, staggering, difficulty
in standing and advanced neurotoxic signs. Histologic examination
revealed no evidence of neurotoxic effects in either the Carbosulfan
or vehicle control groups. The TOCP group demonstrated a marked
increase in the number of swollen axons in the cervical, thoracic and
lumbar sections of spinal cord. There were no axonal changes in the
sciatic nerve. The report included only cage-side observations for
locomotor impairment and the number of swollen axons per section of
spinal cord as indicators for evidence of delayed neurotoxicity.
Furthermore, the LD70 dose is considered too low and should actually
have been four to eight times the LD50 (under appropriate protection)
in order to evaluate the delayed neurotoxic potential. The absence of
effects in this study, although partially the result of study design,
support the conclusion that monomethyl carbamates do not cause delayed
neuropathy (Gephart, Becci & Parent, 1979).
Special Studies on Acute Toxicity
The acute toxicity of technical Carbosulfan via various routes of
administration has been evaluated in a variety of mammalian species. A
summary of the acute toxicity data is presented in Table 2. Signs of
toxicity observed in these studies were those commonly associated with
compounds which inhibit acetylcholinesterase including: salivation,
lacrimation, diarrhoea, ataxia, piloerection, urination, miosis,
labored breathing, bloody tears, exophthalmia, cyanosis, tremors,
convulsions, and death.
Carbosulfan (0.1 ml) was instilled into the everted eyelid of
nine rabbits (Mehta, 1981). Three of the nine eyes were washed with
water 30 seconds after instillation. All animals had slightly
constricted pupils at one hour. Moderate conjunctivitis was observed
after one hour while only mild conjunctivitis was observed after 24
hours. All eyes appeared normal within 72 hours which indicated that
Carbosulfan is minimally irritating to the eye.
A primary dermal irritation study was conducted with undiluted
Carbosulfan (Mehta, 1982). Slight erythema and oedema were observed
within 24 hours after the application of 0.5 ml Carbosulfan to two
intact and two abraded skin sites on each of six rabbits. Oedema
subsided within four days and all sites returned to normal within six
days. One rabbit died, which is considered to be compound-related. The
primary irritation index was 1.36, classifying Carbosulfan as slightly
irritating to the skin.
The potential for Carbosulfan to cause dermal sensitization was
assessed using a patch technique, similar to the Buehler and Open
epicutaneous tests. A group of ten guinea-pigs was administered 0.5 ml
of Carbosulfan as a 0.1 percent (w/v) corn oil suspension. A
concurrent positive control group received 0.5 ml of a 0.05 percent
(w/v) solution of 2,4-dinitrochlorobenzene in ethanol. Carbosulfan was
determined to be a dermal sensitizer when slight to moderate
irritation was observed after the challenge application. (Cannelongo,
1981a).
Short-term Studies
Rat
Groups of Charles River CD Rats (25 males and 25 females per
group) were administered Carbosulfan (technical grade) in the diet at
dose levels of 0, 10, 20 and 500 ppm for 90 days. All animals were
observed for overt toxicity and behavioral changes twice daily while
body weight and food consumption were recorded weekly. An
ophthalmologic examination was conducted in all animals prior to
treatment and during the last week of the study. Haematologic,
clinical chemistry and urinalysis determinations were performed on ten
rats/sex/group prior to study initiation and after 30 and 90 days of
study. Erythrocyte and plasma cholinesterase activities were
determined between 8 and 10 a.m. and at the same intervals designated
for the other blood parameters; brain cholinesterase activity was
determined after 90 days. Detailed gross and histopathological
examinations were performed on all animals at the termination of the
study, and selected organs weighed.
TABLE 2. A Summary of the Acute Toxicity of Carbosulfan Technical
LD50
Species Sex Route Vehicle (mg/kg) Reference
Rat Ma Oral Undiluted 250 Cannelongo, 1979a
Fa Oral Undiluted 185 Cannelongo, 1979a
M&F Oral Undiluted 209 Cannelongo, 1979a
Ma Oral Corn oil1 182 Cannelongo, 1979b
Fa Oral Corn oil1 90.5 Cannelongo, 1979b
M&F Oral Corn oil1 138 Cannelongo, 1979b
Mb IP Undiluted 397 Cannelongo, 1979c
Fb IP Undiluted 458 Cannelongo, 1979c
M&F IP Undiluted 422 Cannelongo, 1979c
Ma Inhal. Undiluted 1.53* Cavender & Casorso, 1979
Fa Inhal. Undiluted 0.61* Cavender & Casorso, 1979
Mouse Ma Oral Corn Oil2 32.7 Cannelongo, 1979d
Fa Oral Corn Oil2 81.5 Cannelongo, 1979d
M&F Oral Corn Oil2 46.1 Cannelongo, 1979d
Ma Oral Corn Oil1 124 Cannelongo, 1979e
Fa Oral Corn Oil1 123 Cannelongo, 1979e
M&F Oral Corn Oil1 124 Cannelongo, 1979e
Rabbit Mb Oral Undiluted 42.0 Cannelongo, 1979f
Fb Oral Undiluted 45.8 Cannelongo, 1979f
M&F Oral Undiluted 43.9 Cannelongo, 1979f
Ma Oral Corn Oil1 36.7 Cannelongo, 1979g
Fa Oral Corn Oil1 52.7 Cannelongo, 1979g
M&F Oral Corn oil1 42.7 Cannelongo, 1979g
Mb Dermal Undiluted >2000 Sabol, 1979
Fb Dermal Undiluted >2000 Sabol, 1979
M&F Dermal Undiluted >2000 Sabol, 1979
1 Administered at a constant volume
2 Administered at a constant concentration
* Units in mg/l of air; time-weighted average (analytical).
a. 10M and 10F per dose
b. 5M and 5F per dose
There were no demonstrated effects on body weight, food
consumption, general appearance, mortality, urinalysis or
ophthalmologic examination. Haematology evaluations were essentially
normal except for increased leucocyte counts for treated males at 90
days and increased reticulocyte count for treated females at 90 days.
Clinical chemistry determinations were within normal biological
variation, except for cholinesterase. Treatment-related reductions in
plasma, erythrocyte and brain cholinesterase activity values were
observed among animals receiving the 500 ppm diet; animals receiving
the 10 or 20 ppm diets were not apparently affected. Gross and
histopathological examinations demonstrated no compound-related
effects. Relative kidney and brain weight increases were evident in
high dose males, as well as increased ovarian weights for mid- and
high-dose females. These organ weight changes were not explained by
clinical chemistry or pathological data and, therefore, not considered
compound-related. The NOEL was 20 ppm, based on plasma, RBC, and brain
cholinesterase depression at 500 ppm (Marshall, et al., 1979).
Dog
Groups of 6-month-old beagle dogs (six males and six females per
group) were administered Carbosulfan in the diet for 26 weeks at
dosage levels of 0, 50, 500, and 1000 ppm. A pre-selected high-dose
level of 2000 ppm was reduced to 1000 ppm when one female died during
the first week of treatment. Animals were observed daily for mortality
and signs of toxicity. Body weight and food consumption data were
determined weekly. Each animal was subjected to an ophthalmoscopic
examination prior to initiation and termination. Haematologic and
clinical chemistry parameters were evaluated monthly while urinalyses
were performed at 2, 4 and 6 months. Plasma and erythrocyte
cholinesterase values determined between 8 and 10 a.m. were measured
at 1, 3 and 6 months while brain cholinesterase values were determined
at termination. Animals were allowed free access to treated diet at
all times. At the conclusion of the study all animals were killed,
selected organs weighed and complete gross and histopathological
examinations performed.
There was no mortality associated with doses up to and including
1000 ppm. Diarrhoea/soft stools and emesis were routinely observed in
all groups. Food consumption for 1000 ppm females was decreased
throughout the study, which was reflected in depressed body weight
gain for this group. The 500 ppm females showed occasional decreases
for both food consumption and body weight gain. Food consumption for
males was also only occasionally depressed at the high dose, with
slightly decreased body weight gains for both 500 and 1000 ppm groups,
beginning at weeks 13/14.
Initially, beginning at month 2, females in all treated groups
demonstrated significant decrease in erythrocyte count, haemoglobin
and haematocrit. These effects recovered to normal at month 4. MCHC,
MCH and MCV were comparable to control females throughout. Males in
the 500 and 1000 ppm groups demonstrated the same dose-related effects
at month 4, which continued to termination of the study. MCHC values
were also significantly decreased in mid- and high-dose males at
termination.
Male and female dogs in the mid- and high-dose groups
demonstrated decreased albumin, total protein, globulin, and increased
cholesterol levels in comparison to controls. Effects were more
noticeable in the high-dose group, except for cholesterol, which was
uniformly increased in all treated male dogs throughout the study.
Significant depression of plasma cholinesterase was evident in
500 ppm females and males at 3 months only, while 1000 ppm males and
females demonstrated depression at 1, 3 and 6 months. Erythrocyte
cholinesterase was significantly depressed in 1000 ppm animals at 1
month only, although at 3 and 6 months levels were less than control.
Brain cholinesterase was significantly depressed in 1000 ppm females
only, although 1000 ppm males also showed less than controls.
Urinalysis and ophthalmoscopic examinations were unremarkable.
Absolute spleen weights for male and female dogs in the high-dose
group were significantly decreased, as were the relative spleen
weights for 500 and 1000 ppm males. Other organ weights were not
significantly different from controls. Gross necropsy and
histopathological examinations were unremarkable and did not
demonstrate any compound-related effects. A NOEL was demonstrated at
50 ppm (Nye, 1981).
Long-term Studies (See Also Special Studies on Carcinogenicity)
Rat
Groups of Charles River CD Rats (90 males and 90 females/group)
were administered Carbosulfan (94.5 - 95.6% purity) in the diet for
104 weeks at dosage levels of 0, 10, 20, 500 and 2500 ppm. Carbofuran
was present in the technical material at concentrations of 0.6 to 2.4
percent. Growth was observed by body weight changes and food
consumption data which were recorded weekly for the first 14 weeks and
bi-weekly thereafter. Daily observations were made with respect to
behavioural changes and mortality. At periodic intervals throughout
the study, haematologic, biochemical and cholinesterase analyses were
performed on unfasted animals. Urinalysis was conducted on fasted
animals. Eyes were examined at 12, 18 and 24 months. At 6, 12 and 18
months of the study, ten males and ten females per group were
sacrificed and necropsied. At the conclusion of the study all
surviving animals were sacrificed and gross pathological and
microscopic examinations of tissues and organs were made. Selected
organs were weighed.
Tremors, labored breathing and eye-related changes were more
frequently observed in animals receiving the 500 and 2500 ppm diets.
Mean body weight and food consumption values in the 500 and 2500 ppm
groups were significantly lower than control values throughout the
study, except for female food consumption, which was comparable to
controls. This is reflected in the actual test material consumption
which was determined to be 0, 0.5, 1.0, 26.8 and 152.8 mg/kg b.w. for
males and 0, 0.6, 1.2, 34.7 and 213.3 mg/kg b.w. for females in the
control, 10, 20, 500 and 2500 ppm groups, respectively. Survival was
not apparently affected by treatment.
Haematology measurements at 6, 12, 18 and 24 months demonstrated
a compound-related effect at 18 months in the 2500 ppm males and
females for significantly increased leucocyte count (primarily
segmented neutrophils), and platelet count, slightly increased
reticulocyte count and significantly decreased lymphocyte count.
Haemoglobin, haematocrit, MCV and MCH were all decreased, although not
significantly, in high-dose males compared to controls at 24 months.
Urinalysis determinations in high-dose males and females demonstrated
an increase in ketone levels over controls at month 18. At terminal
sacrifice there was an increase in mid- and high-dose animals of
mononuclear leucocyte infiltrates in the kidney and an increase of
pigmentation (haemosiderin) in the mediastinal lymph nodes of treated
females. All of these data are indicative of leucocytosis and toxic
neutrophilia. During early phases of neutrophilia there is a tendency
toward acidosis, which was demonstrated by the presence of ketone
bodies in the urine. The elevated platelet count is also a reflection
of hyperactivity of the bone marrow. Histopathology of bone marrow,
spleen and liver were otherwise unremarkable, however.
Biochemical analyses were generally comparable to controls,
except in high-dose males and females, where decreased albumin, total
protein and globulin were reported. Cholinesterase determinations in
males and females demonstrated significant depression of plasma,
erythrocyte and brain cholinesterases at doses of 500 and 2500 ppm.
Significantly increased relative brain, heart, liver and kidney
weights in mid- and high-dose males and females were attributed to
lower body weights. Absolute spleen, adrenal and thyroid weights were
uniformly depressed in 500 and 2500 ppm dose groups but were not
different from control on an organ-to-body weight basis. Gross and
histopathologic examinations of all tissues, except the eye, revealed
no compound-related effects on the incidence or type of neoplastic or
non-neoplastic changes.
Carbosulfan produced compound-related effects on the eye
described pathologically as focal iris atrophy, iris coloboma and
absence of iris tissues in the 500 and 2500 ppm males and females, as
well as degenerative retinopathy in 2500 ppm females. The atrophy of
the iris was attributed, in part, to an extensive anticholinesterase
effect. There were no treatment-related effects on the eye at 10 or
20 ppm. A NOEL was demonstrated at 20 ppm based on pathology of the
eye and cholinesterase inhibition. Carbosulfan was not carcinogenic to
rats at dietary doses up to and including 2500 ppm (DeProspo, Norvell
& Fletcher, 1982a).
Comments
Carbosulfan is an anticholinesterase methylcarbamate currently in
use as a soil and foliar insecticide.
Absorption, distribution and excretion data in rats demonstrate
the preferential excretion of Carbosulfan, conjugated metabolites and
Carbofuran in the urine, with the majority (>90%) of the dose
recovered within the first 24 hours. Rapid absorption occurs within
the first 30 minutes after oral administration, reaching peak
concentrations in liver, G.I. tract, blood and skin at 6 hours.
Carbosulfan is metabolized by two primary pathways: (1) oxidation of
the sulphur to yield Carbosulfan sulphone and sulphamide, and (2)
N-S bond cleavage to yield Carbofuran.
Carbosulfan was demonstrated to be moderately toxic acutely when
administered to a variety of test animals via various routes of
exposure. It did not induce delayed neurotoxicity in atropinized adult
Leghorn hens at 500 mg/kg b.w.
Carbosulfan demonstrated adverse effects on pup weight, litter
size and pup survival at 250 ppm in rats. No other adverse effects
were apparent and a NOEL was demonstrated at 20 ppm. Similarly, in a
teratogenic study in rats, Carbosulfan was not teratogenic at doses up
to and including 20 mg/kg/day.
Carbosulfan, when administered in the diet to rats and dogs for
90 days and 6 months respectively, demonstrated dose-related effects
on cholinesterases. The NOEL for rats was 20 ppm, based on depression
of plasma, erythrocyte and brain cholinesterase activity. In dogs, the
NOEL for these enzymes was 50 ppm.
Available mutagenicity studies in vitro and in vivo were
negative.
In a long-term feeding study in rats, Carbosulfan at 500 and
2500 ppm in the diet produced compound-related effects on the eye,
which included focal iris atrophy and degenerative retinopathy. There
was also evidence of leucocytosis and toxic neutrophilia at 2500 ppm.
Plasma, erythrocyte and brain cholinesterases were inhibited at both
doses. There was no evidence of oncogenicity at either dose. The NOEL
for the study was 20 ppm.
Mice, exposed to dietary doses of 10, 20, 500 and 2500 ppm for
two years demonstrated inhibition of erythrocyte, plasma and brain
cholinesterases at >500 ppm, but no evidence of oncogenicity at any
dose. There was evidence of a cataractogenic effect at >20 ppm but
the ophthalmological interpretations require further clarification.
The NOEL was 10 ppm, based on compound-related body weight depression
in males at >20 ppm.
On the basis of all available data, no-effect levels in certain
mammalian species have been determined, but because additional data
are needed to clarify the cataractogenic response in the two-year
mouse feeding study, a temporary ADI was allocated.
Level causing no toxicological effect
Mouse: 10 ppm in the diet, equal to 1.3 mg/kg b.w.
Rat: 20 ppm in the diet, equal to 1.0 mg/kg b.w.
Dog: 50 ppm in the diet, equal to 1.25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans:
0 - 0.005 mg/kg b.w.
FURTHER WORK OR INFORMATION
Required (by 1986):
Additional information to clarify the different pathological
interpretations of the ophthalmological data in the two-year mouse
study and to demonstrate the NOEL for the cataract effects.
Desirable:
Metabolic studies in non-rodent species.
Observations in humans, particularly concerning effects on the eye.
REFERENCES
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979a FMC Report No. ACT 281.01. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979b FMC Report No. ACT 281.01A. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute intraperitoneal toxicity study of FMC 35001
1979c technical in rats. FMC Report No. ACT 281.05. Stillmeadow,
Inc. Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979d mice. FMC Report No. ACT 281.02. Stillmeadow, Inc. Submitted
by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979e mice. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979f rabbits. FMC Report No. ACT 281.02. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979g rabbits. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Dermal sensitization study of FMC 35001 technical in
1981 guinea-pigs. FMC Report No. A79-327. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cavender, F. & Casorso, D.R. Acute inhalation toxicity study of FMC
1979 35001 technical in rats. FMC Report No. ACT 291.04.
ToxiGenics, Inc. Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982a dietary toxicity/oncogenicity study in rats with FMC 35001.
FMC Report No. A79-333. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982b dietary toxicity/oncogenicity study in mice with FMC 35001.
FMC Report No. A79-334. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Gephart, L., Becci, P.J. & Parent, R.A. delayed neurotoxicity
1979 evaluation of FMC 35001 in adult leghorn hens. FMC Report
No.: A79-335. Food and Drug Research Laboratories, Inc.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1980 Simmons, R.T. & Reichard, G.L., Salmonella/mammalian-
microsome plate incorporation mutagenesis assay: FMC 35001.
FMC Report No.: A79-396. EG&G Mason Research Institute.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1981 Simmons, R.T. Hans, L.J. & Reichard, G.L., DNA damage/repair
suspension assay: FMC 35001. FMC Report No.: A80-397. EG&G
Mason Research Institute. Submitted by FMC Corp. to WHO.
Janes, J.M., D.E., & Blair, M. Teratology study in rats: FMC 35001
1980a technical. FMC Report No.: ACT 265.33. International
Research and Development Corp. Submitted by FMC Corp. to
WHO.
Janes, J.M., Rodwell, D.E. & Blair, M. Teratology study in rabbits:
1980b FMC 35001 technical. FMC Report No.: ACT 265.33.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Kehoe, D.F. & MacKenzie, K.M. Three-generation rat reproduction study
1982 with two litters in each generation: FMC 35001. FMC Report
No.: A80-401. Hazleton Raltech, Inc. Submitted by FMC Corp.
to WHO.
Kirby, P.E., Pizzarello, R.F., Brauninger, R.M. Vega, R.A., Cohen, A.,
1981 Reichard, G.L., Williams, P.E., Wattam, R.E., Johnson, J.L.
& Clarke, J.J. Evaluation of test article FMC 35001 (MRI#
556) for mutagenic potential employing the L51 78Y TK+/-
mutagenesis assay. FMC Report No.: A80-426. EG&G Mason
Research Institute. Submitted by FMC Corp. to WHO.
Kirkhart, B., Jones, D.C.L. & Skinner, W.A. Micronucleus test on FMC
1979 35001. FMC Report No.: A79-366. S.R.I. International.
Submitted by FMC Corp. to WHO.
Liss, R.H., Chadwick, M. Schepis, J.P. & Hayes, D. Whole-body
1981 radioautography and physiologic disposition studies on
differentially-labelled FMC 35001. Contract No.: 85600.
Arthur D. Little, Inc. Submitted by FMC Corp. to WHO.
Marsden, P.J., Kuwano., E. & Fukuto, T.R., Metabolism and toxicity of
1982 [2,3-Dihydro-2,2-dimenthylbenzofuran-7-yl (di-n-
butylaminothio)-methylcarbamate] in the rat and house fly.
Pestic. Biochem. Physiol. 18:38.
Marshall, P., Jefferson, N.D., Blair, M. & Goldenthal, E.I. 90-day
1979 subchronic study in rats. FMC Report No.: ACT 258.34.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Mehta, C.S. Eye irritation study of FMC 35001 technical in rabbits.
1981 FMC Report No. A79-325. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Mehta, C.S. Dermal irritation study of FMC 35001 technical in rabbits.
1982 FMC Report No. A80-447. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Nye, D.E. Six-month dietary toxicity study in dogs - FMC 35001. FMC
1981 Report No.: ACT 271.36. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Preache, M.M., Shefner, A.M. & Reed, J.M. Dominant lethal assay of FMC
1981 35001 in mice. FMC Report No. A80-424. IIT Research
Institute. Submitted by FMC Corp. to WHO.
Putnam, D.L. & Schechtman, L.M. Activity of T1638 (FMC 35001) in the
1981 in vivo cytogenetics assay in rodents. FMC Report No.:
A80-425. Microbiological Associates. Submitted by FMC Corp.
to WHO.
Sabol, R.J. Acute dermal toxicity of FMC 35001 technical in rabbits.
1979 FMC Report No. ACT 281.03. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Umetsu, N. & Fukuto, R.T. Alteration of Carbosulfan [2,3-Dihydro-2,2-
1982 dimethyl-7-benzofuranyl (Di-n-butylaminosulfenyl)
methylcarbamate] in the rat stomach. J. Agric. Food Chem.
30:555.
Wu, J. Metabolism of ring-14C Carbosulfan in rats. FMC Report No.:
1982a M-4833. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
Wu, J. Metabolism of DBA-14C Carbosulfan in rats. FMC Report No.:
1982b MA-4864. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
A female rat received a single dose (30 mg/kg) of ring-14C
Carbosulfan via oral gavage. Analyses indicated that levels of
unchanged parent chemical persisted in the blood for three hours after
dosing. Approximately 72 percent of the radiocarbon detected in blood
was attributed to parent chemical (Marsden, Kuwawo & Fukuto, 1982). In
a separate study, female Sprague-Dawley rats were administered
30 mg/kg of carbonyl-14C Carbosulfan. The rats were sacrificed at 15,
35 and 80 minutes after dosing and the stomach contents were assayed
for radioactivity. Approximately 50 percent of the recovered
radioactivity was Carbosulfan after 80 minutes (Umetsu & Fukuto,
1982).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Carbosulfan was administered to groups of Charles River CD rats
(15 males and 30 females/group) at dietary levels of 0, 10, 20 and
250 ppm for three generations. Two successive litters were reared from
each female. General condition and behavior were routinely observed
and individual body weights were recorded throughout the study. The
number of pups in each litter were examined externally and culled to
10/litter at four days of age. Individual pup weights were measured at
days 0, 4, 7, 14 and 21. Ten male and ten female F1b, F2b, and F3b
weanlings were randomly selected for gross necropsy and tissue
collection. The F1a and F2a litters were discarded at weaning, and
the F1b and F2b litters were used to produce succeeding generations.
Weanlings not selected for continuation on the study (F1a, F2a,
F1b, F2b, F3a and F3b) were subjected to gross external
examination, sacrificed and discarded.
Body weights of F0 males and females were decreased initially at
20 and 250 ppm. These decreases were associated with decreased food
consumption, and both recovered to normal from week 4 to sacrifice.
Body weights of parental males (F1 and F2) receiving the 250 ppm
diet were consistently lower than those in the corresponding control
group. A similar effect was observed in the 250 ppm group females
during portions of gestation and lactation, but not during the growth
phases. Mating index, gestation index and number of viable foetuses
were essentially normal throughout, except for F2b dams at the
250 ppm level, which were decreased.
Litter size, pup weights and/or pup weight gains for all litter
sets in the 250 ppm group were significantly lower at most age
intervals between birth and weaning when compared to concurrent
control animals. Neonatal survival among the 250 ppm group was also
significantly lower for the first four litters (F1a, F1b, F2a and
F2b).
There were no treatment-related gross or histologic changes
observed among the F0, F1b and F2b adult groups and the F1b,
F2b and F3b weanling groups. Carbosulfan did not have a demonstrated
adverse effect on reproductive performance, except for the effect on
pup weight, litter size, and pup survival at 250 ppm. The NOEL is
20 ppm (Kehoe & MacKenzie, 1982).
Special Studies on Teratogenicity
Rat
Carbosulfan was administered orally by gavage to groups of 25
Charles River CD rats during gestation days 6 through 19 at dosages of
0, 2, 10 and 20 mg/kg/day. Surviving females were necropsied on day 20
and foetuses delivered by hysterotomy. The number and position of
viable/non-viable foetuses, early/late resorptions, mean number of
corpora lutea and total number of implantations were recorded.
External, internal and skeletal examinations of foetuses were
performed for evidence of abnormalities and anomalies. One third of
the foetuses were evaluated for soft tissue anomalies and the
remaining two thirds for skeletal effects.
There was no dose-related mortality in any treatment group. Body
tremors and clear oral discharge were reported in the 20 mg/kg/day
group after administration of each dose. Mean maternal body weight
gains were slightly reduced at 10 mg/kg/day and significantly reduced
at the 20 mg/kg level during the gestation period. There were no
differences in number of pregnancies, early/late resorptions, viable
foetuses, post-implantation loss or sex distribution. The number of
corpora lutea per dam was increased in all treated groups, while the
mean foetal body weight was significantly reduced at 10 and
20 mg/kg/day. There was also an increase in the number of litters with
developmental variations at 20 mg/kg/day which included reduced
ossification of the skull, hyoid body unossified, sternebrae 5 and 6
unossified, and renal papilla not developed and/or distended ureters.
There were no reported effects on the number or percentage of foetuses
or litters with external, internal or skeletal malformations or
anomalies at any dose level. Carbosulfan was not demonstrated to be
teratogenic in rats at doses up to and including 20 mg/kg/day (Janes,
Rodwell & Blair, 1980a).
Rabbit
Groups of 16 New Zealand Albino Rabbits received carbosulfan by
gavage at dosages of 0, 2, 5, and 10 mg/kg b.w./day on days 6 through
28 of gestation. Pups were delivered by caesarean section on day 29
and the number, location, and distribution of viable/non-viable
foetuses, corpora lutea, early/late resorptions and total
implantations were recorded. All foetuses were examined grossly,
sectioned for visceral anomalies and stained for skeletal anomalies.
There were three deaths at 10 mg/kg and one each in the control
and low-dose groups. The number of dams with viable foetuses were 12,
10, 15 and 11 for the control, 2, 5 and 10 mg/kg groups, respectively.
There were no compound-related effects on the number of viable
foetuses, corpora lutea, foetal sex distribution or total
implantations/dam. There were compound-related effects (e.g. increase)
on post implantation losses and early resorption rate at all doses,
but most notably at 5 and 10 mg/kg. Also, the mean foetal body weight
was decreased in the high dose group. There was a single occurrence of
scoliosis in each of the three treated groups, but none were reported
in the controls. There were no compound-related effects on skeletal
variations, such as delayed ossification. Major vessel variations,
identified primarily as left carotid arising from the innominate, were
observed in 16.7, 100, 46.7 and 72.7 percent of the litters at 0, 2,
5, and 10 mg/kg/day, respectively. The percentage of foetuses
presenting this defect were 4.9, 44, 12.8 and 20.8 percent,
respectively. Although this foetotoxic response was evident and
increased at all dose levels there was no evidence of teratogenicity.
The NOEL for teratogenicity is 10 mg/kg/day (Janes, Rodwell & Blair,
1980b).
Special Studies on Mutagenicity
Carbosulfan was negative in a wide battery of mutagenicity
studies conducted. See Table 1 for a summary of the studies
considered.
Special Studies on Carcinogenicity (See also long-term studies)
Mice
Groups of Charles River CD-1 Mice (100 males and 100 females per
group) were administered Carbosulfan (purity, 94.5 to 95.6 percent
with 0.6 to 2.4 percent carbofuran) in the diet at dose levels of 0,
10, 20, 500 and 2500 ppm for 24 months. All animals were observed
daily for signs of toxicity, moribundity and mortality. Body weight
and food consumption values were recorded weekly for the first 14
weeks and bi-weekly thereafter. Water consumption was determined
monthly. Haematological and biochemical measurements and urinalyses
were performed on ten unfasted mice/sex/group at the 6, 12, 18 and 24
month sacrifices. Ophthalmoscopic evaluations were performed in all
survivors at 24 months. Selected organs were weighed, gross necropsies
conducted and a complete list of tissues and organs examined
microscopically.
There were no measurable effects on mortality or survivability
related to treatment. Mean body weights were reduced throughout the
study at 500 and 2500 ppm for males and 2500 for females. For females
body weight changes at other doses were sporadic and not related to
treatment. However, 20 ppm males demonstrated decreased body weight
gains from week 42 to terminal sacrifice at 104 weeks. Food
consumption was depressed at 2500 ppm for males and females, but only
sporadic decreases were noted at other doses. The actual measured
compound ingested per dose was 0, 1.3, 2.5, 61.5, and 319.5 mg/kg b.w.
for males, and 0, 1.5, 3.1, 71.9 and 337.2 mg/kg b.w. for females for
0, 10, 20, 500 and 2500 ppm, respectively.
There were no measurable differences regarding general appearance
and behavior, except for increased eye irregularities at 2500 ppm.
These included corneal opacity, eccentric pupil, and white, cloudy
eyes.
There were no treatment-related effects or haematological changes
except for a tendency toward slightly increased segmented neutrophils
and decreased lymphocyte counts in males at 2500 ppm. There were no
demonstrated effects on glucose, BUN, SGPT or Alk phosphatase in
either sex at any level.
Cholinesterase values for plasma, erythrocytes and brain tissue
were significantly depressed in both males and females at 500 and
2500 ppm, at all time periods measured.
Ophthalmoscopic examinations indicated an increase in punctuate
opacities of the iris at 500 and 2500 ppm. Pathological changes were
indicative of ageing mice and many were stated to be masked by use of
short-acting mydriatic solutions. However, two separate pathological
opinions concerning the incidence of cataracts in treated mice
indicated that there were compound-related increases in male mice at
500 and 20 ppm, respectively. Females were apparently unaffected by
treatment, although the incidence reached 84 to 100 percent in most
groups. The occurrence overall in control and treated groups indicate
that the effect is possibly exacerbated by the anticholinergic effect,
similarly demonstrated in the rat study. Special evaluation and
concern for the iris, because of compound-related effects in the rat,
did not indicate similar effects in mice.
Absolute organ weight changes were variably affected in both
males and females except for decreased spleen weight in females at 500
and 2500 ppm. Relative spleen weights were also significantly
depressed in females at 500 and 2500 ppm. Relative brain weights were
also significantly increased for both sexes throughout the study at
2500 ppm. This is considered a reflection of the significant effects
on body weight at the higher doses.
Gross and histopathological examination were essentially
unremarkable. The most common findings reported were malignant
lymphoma and bronchio-alveolar adenoma which were equally distributed
among all groups, except for low-dose females which demonstrated a
significant increase in the number of metastatic malignant lymphomas
of mediastinal and mesenteric lymph nodes, as well as thymus and
spleen. Generally, control and low-dose females presented the highest
incidence of malignant lymphomas, in comparison to 20-2500 ppm groups.
The results of the histopathologic examination indicated that the type
and incidence of non-neoplastic and neoplastic lesions were usual
findings in the mouse and unrelated to treatment. Carbosulfan was not
carcinogenic in mice at dietary levels up to and including 2500 ppm.
TABLE 1. Special Studies on Mutagenicity
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Ames' Test S. typhimurium 0.1, 0.5, 2.5, 5 93% Negative Haworth et al.,
(both with and TA 98 and 10 ul/plate 1980
without metabolic TA 100 disolved in DMSO
activation) TA 1535
TA 1537
TA 1538
Bacterial DNA E. coli 0.025, 0.05, 93% Negative. Haworth et al.,
Damage/Repair strains WP2 0.1 and 0.2 ml Preferential kill 1981
Suspension Assay uvr A+ exr A+ per plate of repair deficient
(both with and and CM611 E. coli CM611
without metabolic uvr A- exr A-; without rat liver
activation) S typhimurium microscrees, at
strains TA 1978 0.025 to 0.2 ml
uvr B+ and per plate.
TA 1538 uvr B-
Mouse Lymphoma Mouse Nonactivated: 93% Negative. Kirby et al.,
Forward Mutation L5178Y TK+/- 0.0024, 0.0032, 0.0042, Positive controls 1981
Assay phenotype cells 0.0056, 0.0075, 0.010, (EMS 1 ml/ml; DMAA
(both with and 0.013, 0.018, 0.024 and 7.5 mg/ml) gave
without metabolic 0.032 ml/ml expected response.
activation)
Activated:
0.0056, 0.0075, 0.010,
0.013, 0.018, 0.024,
0.032, 0.042, 0.056
and 0.075 ml/ml
TABLE 1. (continued)
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Microrucleus Mouse, bone Orally, 43.5 and 93% Negative. Kirkhart, Jones
marrow 87 mg/kg (174 mg/kg Positive control & Skinner, 1979
gave excessive mortality (trimethyl phosphate)
and was excluded) yielded expected positive
response at 2 x 1000 mg/kg
i.p.
In Vivo Rat Orally, single doses 93% Carbosulfan was not Putnam and
Cytogenetic of 0, 5, 12 and 30 clastogenic. Positive Schectman, 1981
Assay mg/kg on 5 consecutive control (triethylene-
days melamine) gave expected
response at 0.25 mg/kg
Dominant Mouse Orally, single doses 93% No effect on any Preache, Shefner
Lethal of 0, 7, 20 and 60 reproduction parameter & Reed, 1981
mg/kg for 5 consecutive evaluated (i.e.
days fertility index, number
of implantations, number of
implantation deaths, percent
post-implantation deaths).
Positive control (TEM) gave
expected response at 0.3 mg/kg i.p.
The NOEL is 10 ppm (equal to 1.3 mg/kg b.w.) based on body weight
depression and cataracts in males at 20 pm (DeProspo, Norvell &
Fletcher, 1982b).
Special Study on Neurotoxicity
Groups of adult hens (16 months old) were administered a single
oral dose of Carbosulfan at levels of 0 and 500 mg/kg b.w. A separate
positive control group received 750 mg/kg b.w. of TOCP (a dose seven
times the effective dose needed to produce delayed neuropathy). There
were ten hens in the vehicle and positive control groups, and 40 hens
in the Carbosulfan group. Atropine sulfate (25 mg/kg b.w.) was given
to all birds prior to administration of Carbosulfan. Preliminary range
finding and LD50 evaluations demonstrated the LD50 and LD70 to be
371 and 500 mg/kg b.w., respectively. Body weight and food consumption
were determined weekly. Throughout the study each bird was evaluated
for neurologic effects. At the conclusion of the study (21 days), all
surviving hens were sacrificed and subjected to gross and microscopic
examinations following intravascular perfusion. Axon and myelin
degeneration were assessed in prepared sections of sciatic nerve and
spinal cord.
There were four deaths in the Carbosulfan group within five days
after dosing. No other deaths occurred. Food consumption and body
weight were depressed in the TOCP group. Although food consumption in
the Carbosulfan group was marginally depressed during the first 15
days, there were no differences in body weight gain compared to
vehicle controls. There were no differences in neurologic scores
between vehicle control and the Carbosulfan-treated birds. The TOCP
group gave the expected response, starting on day 13, which consisted
of unsteadiness in walking, inability to walk, staggering, difficulty
in standing and advanced neurotoxic signs. Histologic examination
revealed no evidence of neurotoxic effects in either the Carbosulfan
or vehicle control groups. The TOCP group demonstrated a marked
increase in the number of swollen axons in the cervical, thoracic and
lumbar sections of spinal cord. There were no axonal changes in the
sciatic nerve. The report included only cage-side observations for
locomotor impairment and the number of swollen axons per section of
spinal cord as indicators for evidence of delayed neurotoxicity.
Furthermore, the LD70 dose is considered too low and should actually
have been four to eight times the LD50 (under appropriate protection)
in order to evaluate the delayed neurotoxic potential. The absence of
effects in this study, although partially the result of study design,
support the conclusion that monomethyl carbamates do not cause delayed
neuropathy (Gephart, Becci & Parent, 1979).
Special Studies on Acute Toxicity
The acute toxicity of technical Carbosulfan via various routes of
administration has been evaluated in a variety of mammalian species. A
summary of the acute toxicity data is presented in Table 2. Signs of
toxicity observed in these studies were those commonly associated with
compounds which inhibit acetylcholinesterase including: salivation,
lacrimation, diarrhoea, ataxia, piloerection, urination, miosis,
labored breathing, bloody tears, exophthalmia, cyanosis, tremors,
convulsions, and death.
Carbosulfan (0.1 ml) was instilled into the everted eyelid of
nine rabbits (Mehta, 1981). Three of the nine eyes were washed with
water 30 seconds after instillation. All animals had slightly
constricted pupils at one hour. Moderate conjunctivitis was observed
after one hour while only mild conjunctivitis was observed after 24
hours. All eyes appeared normal within 72 hours which indicated that
Carbosulfan is minimally irritating to the eye.
A primary dermal irritation study was conducted with undiluted
Carbosulfan (Mehta, 1982). Slight erythema and oedema were observed
within 24 hours after the application of 0.5 ml Carbosulfan to two
intact and two abraded skin sites on each of six rabbits. Oedema
subsided within four days and all sites returned to normal within six
days. One rabbit died, which is considered to be compound-related. The
primary irritation index was 1.36, classifying Carbosulfan as slightly
irritating to the skin.
The potential for Carbosulfan to cause dermal sensitization was
assessed using a patch technique, similar to the Buehler and Open
epicutaneous tests. A group of ten guinea-pigs was administered 0.5 ml
of Carbosulfan as a 0.1 percent (w/v) corn oil suspension. A
concurrent positive control group received 0.5 ml of a 0.05 percent
(w/v) solution of 2,4-dinitrochlorobenzene in ethanol. Carbosulfan was
determined to be a dermal sensitizer when slight to moderate
irritation was observed after the challenge application. (Cannelongo,
1981a).
Short-term Studies
Rat
Groups of Charles River CD Rats (25 males and 25 females per
group) were administered Carbosulfan (technical grade) in the diet at
dose levels of 0, 10, 20 and 500 ppm for 90 days. All animals were
observed for overt toxicity and behavioral changes twice daily while
body weight and food consumption were recorded weekly. An
ophthalmologic examination was conducted in all animals prior to
treatment and during the last week of the study. Haematologic,
clinical chemistry and urinalysis determinations were performed on ten
rats/sex/group prior to study initiation and after 30 and 90 days of
study. Erythrocyte and plasma cholinesterase activities were
determined between 8 and 10 a.m. and at the same intervals designated
for the other blood parameters; brain cholinesterase activity was
determined after 90 days. Detailed gross and histopathological
examinations were performed on all animals at the termination of the
study, and selected organs weighed.
TABLE 2. A Summary of the Acute Toxicity of Carbosulfan Technical
LD50
Species Sex Route Vehicle (mg/kg) Reference
Rat Ma Oral Undiluted 250 Cannelongo, 1979a
Fa Oral Undiluted 185 Cannelongo, 1979a
M&F Oral Undiluted 209 Cannelongo, 1979a
Ma Oral Corn oil1 182 Cannelongo, 1979b
Fa Oral Corn oil1 90.5 Cannelongo, 1979b
M&F Oral Corn oil1 138 Cannelongo, 1979b
Mb IP Undiluted 397 Cannelongo, 1979c
Fb IP Undiluted 458 Cannelongo, 1979c
M&F IP Undiluted 422 Cannelongo, 1979c
Ma Inhal. Undiluted 1.53* Cavender & Casorso, 1979
Fa Inhal. Undiluted 0.61* Cavender & Casorso, 1979
Mouse Ma Oral Corn Oil2 32.7 Cannelongo, 1979d
Fa Oral Corn Oil2 81.5 Cannelongo, 1979d
M&F Oral Corn Oil2 46.1 Cannelongo, 1979d
Ma Oral Corn Oil1 124 Cannelongo, 1979e
Fa Oral Corn Oil1 123 Cannelongo, 1979e
M&F Oral Corn Oil1 124 Cannelongo, 1979e
Rabbit Mb Oral Undiluted 42.0 Cannelongo, 1979f
Fb Oral Undiluted 45.8 Cannelongo, 1979f
M&F Oral Undiluted 43.9 Cannelongo, 1979f
Ma Oral Corn Oil1 36.7 Cannelongo, 1979g
Fa Oral Corn Oil1 52.7 Cannelongo, 1979g
M&F Oral Corn oil1 42.7 Cannelongo, 1979g
Mb Dermal Undiluted >2000 Sabol, 1979
Fb Dermal Undiluted >2000 Sabol, 1979
M&F Dermal Undiluted >2000 Sabol, 1979
1 Administered at a constant volume
2 Administered at a constant concentration
* Units in mg/l of air; time-weighted average (analytical).
a. 10M and 10F per dose
b. 5M and 5F per dose
There were no demonstrated effects on body weight, food
consumption, general appearance, mortality, urinalysis or
ophthalmologic examination. Haematology evaluations were essentially
normal except for increased leucocyte counts for treated males at 90
days and increased reticulocyte count for treated females at 90 days.
Clinical chemistry determinations were within normal biological
variation, except for cholinesterase. Treatment-related reductions in
plasma, erythrocyte and brain cholinesterase activity values were
observed among animals receiving the 500 ppm diet; animals receiving
the 10 or 20 ppm diets were not apparently affected. Gross and
histopathological examinations demonstrated no compound-related
effects. Relative kidney and brain weight increases were evident in
high dose males, as well as increased ovarian weights for mid- and
high-dose females. These organ weight changes were not explained by
clinical chemistry or pathological data and, therefore, not considered
compound-related. The NOEL was 20 ppm, based on plasma, RBC, and brain
cholinesterase depression at 500 ppm (Marshall, et al., 1979).
Dog
Groups of 6-month-old beagle dogs (six males and six females per
group) were administered Carbosulfan in the diet for 26 weeks at
dosage levels of 0, 50, 500, and 1000 ppm. A pre-selected high-dose
level of 2000 ppm was reduced to 1000 ppm when one female died during
the first week of treatment. Animals were observed daily for mortality
and signs of toxicity. Body weight and food consumption data were
determined weekly. Each animal was subjected to an ophthalmoscopic
examination prior to initiation and termination. Haematologic and
clinical chemistry parameters were evaluated monthly while urinalyses
were performed at 2, 4 and 6 months. Plasma and erythrocyte
cholinesterase values determined between 8 and 10 a.m. were measured
at 1, 3 and 6 months while brain cholinesterase values were determined
at termination. Animals were allowed free access to treated diet at
all times. At the conclusion of the study all animals were killed,
selected organs weighed and complete gross and histopathological
examinations performed.
There was no mortality associated with doses up to and including
1000 ppm. Diarrhoea/soft stools and emesis were routinely observed in
all groups. Food consumption for 1000 ppm females was decreased
throughout the study, which was reflected in depressed body weight
gain for this group. The 500 ppm females showed occasional decreases
for both food consumption and body weight gain. Food consumption for
males was also only occasionally depressed at the high dose, with
slightly decreased body weight gains for both 500 and 1000 ppm groups,
beginning at weeks 13/14.
Initially, beginning at month 2, females in all treated groups
demonstrated significant decrease in erythrocyte count, haemoglobin
and haematocrit. These effects recovered to normal at month 4. MCHC,
MCH and MCV were comparable to control females throughout. Males in
the 500 and 1000 ppm groups demonstrated the same dose-related effects
at month 4, which continued to termination of the study. MCHC values
were also significantly decreased in mid- and high-dose males at
termination.
Male and female dogs in the mid- and high-dose groups
demonstrated decreased albumin, total protein, globulin, and increased
cholesterol levels in comparison to controls. Effects were more
noticeable in the high-dose group, except for cholesterol, which was
uniformly increased in all treated male dogs throughout the study.
Significant depression of plasma cholinesterase was evident in
500 ppm females and males at 3 months only, while 1000 ppm males and
females demonstrated depression at 1, 3 and 6 months. Erythrocyte
cholinesterase was significantly depressed in 1000 ppm animals at 1
month only, although at 3 and 6 months levels were less than control.
Brain cholinesterase was significantly depressed in 1000 ppm females
only, although 1000 ppm males also showed less than controls.
Urinalysis and ophthalmoscopic examinations were unremarkable.
Absolute spleen weights for male and female dogs in the high-dose
group were significantly decreased, as were the relative spleen
weights for 500 and 1000 ppm males. Other organ weights were not
significantly different from controls. Gross necropsy and
histopathological examinations were unremarkable and did not
demonstrate any compound-related effects. A NOEL was demonstrated at
50 ppm (Nye, 1981).
Long-term Studies (See Also Special Studies on Carcinogenicity)
Rat
Groups of Charles River CD Rats (90 males and 90 females/group)
were administered Carbosulfan (94.5 - 95.6% purity) in the diet for
104 weeks at dosage levels of 0, 10, 20, 500 and 2500 ppm. Carbofuran
was present in the technical material at concentrations of 0.6 to 2.4
percent. Growth was observed by body weight changes and food
consumption data which were recorded weekly for the first 14 weeks and
bi-weekly thereafter. Daily observations were made with respect to
behavioural changes and mortality. At periodic intervals throughout
the study, haematologic, biochemical and cholinesterase analyses were
performed on unfasted animals. Urinalysis was conducted on fasted
animals. Eyes were examined at 12, 18 and 24 months. At 6, 12 and 18
months of the study, ten males and ten females per group were
sacrificed and necropsied. At the conclusion of the study all
surviving animals were sacrificed and gross pathological and
microscopic examinations of tissues and organs were made. Selected
organs were weighed.
Tremors, labored breathing and eye-related changes were more
frequently observed in animals receiving the 500 and 2500 ppm diets.
Mean body weight and food consumption values in the 500 and 2500 ppm
groups were significantly lower than control values throughout the
study, except for female food consumption, which was comparable to
controls. This is reflected in the actual test material consumption
which was determined to be 0, 0.5, 1.0, 26.8 and 152.8 mg/kg b.w. for
males and 0, 0.6, 1.2, 34.7 and 213.3 mg/kg b.w. for females in the
control, 10, 20, 500 and 2500 ppm groups, respectively. Survival was
not apparently affected by treatment.
Haematology measurements at 6, 12, 18 and 24 months demonstrated
a compound-related effect at 18 months in the 2500 ppm males and
females for significantly increased leucocyte count (primarily
segmented neutrophils), and platelet count, slightly increased
reticulocyte count and significantly decreased lymphocyte count.
Haemoglobin, haematocrit, MCV and MCH were all decreased, although not
significantly, in high-dose males compared to controls at 24 months.
Urinalysis determinations in high-dose males and females demonstrated
an increase in ketone levels over controls at month 18. At terminal
sacrifice there was an increase in mid- and high-dose animals of
mononuclear leucocyte infiltrates in the kidney and an increase of
pigmentation (haemosiderin) in the mediastinal lymph nodes of treated
females. All of these data are indicative of leucocytosis and toxic
neutrophilia. During early phases of neutrophilia there is a tendency
toward acidosis, which was demonstrated by the presence of ketone
bodies in the urine. The elevated platelet count is also a reflection
of hyperactivity of the bone marrow. Histopathology of bone marrow,
spleen and liver were otherwise unremarkable, however.
Biochemical analyses were generally comparable to controls,
except in high-dose males and females, where decreased albumin, total
protein and globulin were reported. Cholinesterase determinations in
males and females demonstrated significant depression of plasma,
erythrocyte and brain cholinesterases at doses of 500 and 2500 ppm.
Significantly increased relative brain, heart, liver and kidney
weights in mid- and high-dose males and females were attributed to
lower body weights. Absolute spleen, adrenal and thyroid weights were
uniformly depressed in 500 and 2500 ppm dose groups but were not
different from control on an organ-to-body weight basis. Gross and
histopathologic examinations of all tissues, except the eye, revealed
no compound-related effects on the incidence or type of neoplastic or
non-neoplastic changes.
Carbosulfan produced compound-related effects on the eye
described pathologically as focal iris atrophy, iris coloboma and
absence of iris tissues in the 500 and 2500 ppm males and females, as
well as degenerative retinopathy in 2500 ppm females. The atrophy of
the iris was attributed, in part, to an extensive anticholinesterase
effect. There were no treatment-related effects on the eye at 10 or
20 ppm. A NOEL was demonstrated at 20 ppm based on pathology of the
eye and cholinesterase inhibition. Carbosulfan was not carcinogenic to
rats at dietary doses up to and including 2500 ppm (DeProspo, Norvell
& Fletcher, 1982a).
Comments
Carbosulfan is an anticholinesterase methylcarbamate currently in
use as a soil and foliar insecticide.
Absorption, distribution and excretion data in rats demonstrate
the preferential excretion of Carbosulfan, conjugated metabolites and
Carbofuran in the urine, with the majority (>90%) of the dose
recovered within the first 24 hours. Rapid absorption occurs within
the first 30 minutes after oral administration, reaching peak
concentrations in liver, G.I. tract, blood and skin at 6 hours.
Carbosulfan is metabolized by two primary pathways: (1) oxidation of
the sulphur to yield Carbosulfan sulphone and sulphamide, and (2)
N-S bond cleavage to yield Carbofuran.
Carbosulfan was demonstrated to be moderately toxic acutely when
administered to a variety of test animals via various routes of
exposure. It did not induce delayed neurotoxicity in atropinized adult
Leghorn hens at 500 mg/kg b.w.
Carbosulfan demonstrated adverse effects on pup weight, litter
size and pup survival at 250 ppm in rats. No other adverse effects
were apparent and a NOEL was demonstrated at 20 ppm. Similarly, in a
teratogenic study in rats, Carbosulfan was not teratogenic at doses up
to and including 20 mg/kg/day.
Carbosulfan, when administered in the diet to rats and dogs for
90 days and 6 months respectively, demonstrated dose-related effects
on cholinesterases. The NOEL for rats was 20 ppm, based on depression
of plasma, erythrocyte and brain cholinesterase activity. In dogs, the
NOEL for these enzymes was 50 ppm.
Available mutagenicity studies in vitro and in vivo were
negative.
In a long-term feeding study in rats, Carbosulfan at 500 and
2500 ppm in the diet produced compound-related effects on the eye,
which included focal iris atrophy and degenerative retinopathy. There
was also evidence of leucocytosis and toxic neutrophilia at 2500 ppm.
Plasma, erythrocyte and brain cholinesterases were inhibited at both
doses. There was no evidence of oncogenicity at either dose. The NOEL
for the study was 20 ppm.
Mice, exposed to dietary doses of 10, 20, 500 and 2500 ppm for
two years demonstrated inhibition of erythrocyte, plasma and brain
cholinesterases at >500 ppm, but no evidence of oncogenicity at any
dose. There was evidence of a cataractogenic effect at >20 ppm but
the ophthalmological interpretations require further clarification.
The NOEL was 10 ppm, based on compound-related body weight depression
in males at >20 ppm.
On the basis of all available data, no-effect levels in certain
mammalian species have been determined, but because additional data
are needed to clarify the cataractogenic response in the two-year
mouse feeding study, a temporary ADI was allocated.
Level causing no toxicological effect
Mouse: 10 ppm in the diet, equal to 1.3 mg/kg b.w.
Rat: 20 ppm in the diet, equal to 1.0 mg/kg b.w.
Dog: 50 ppm in the diet, equal to 1.25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans:
0 - 0.005 mg/kg b.w.
FURTHER WORK OR INFORMATION
Required (by 1986):
Additional information to clarify the different pathological
interpretations of the ophthalmological data in the two-year mouse
study and to demonstrate the NOEL for the cataract effects.
Desirable:
Metabolic studies in non-rodent species.
Observations in humans, particularly concerning effects on the eye.
REFERENCES
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979a FMC Report No. ACT 281.01. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979b FMC Report No. ACT 281.01A. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute intraperitoneal toxicity study of FMC 35001
1979c technical in rats. FMC Report No. ACT 281.05. Stillmeadow,
Inc. Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979d mice. FMC Report No. ACT 281.02. Stillmeadow, Inc. Submitted
by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979e mice. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979f rabbits. FMC Report No. ACT 281.02. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979g rabbits. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Dermal sensitization study of FMC 35001 technical in
1981 guinea-pigs. FMC Report No. A79-327. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cavender, F. & Casorso, D.R. Acute inhalation toxicity study of FMC
1979 35001 technical in rats. FMC Report No. ACT 291.04.
ToxiGenics, Inc. Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982a dietary toxicity/oncogenicity study in rats with FMC 35001.
FMC Report No. A79-333. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982b dietary toxicity/oncogenicity study in mice with FMC 35001.
FMC Report No. A79-334. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Gephart, L., Becci, P.J. & Parent, R.A. delayed neurotoxicity
1979 evaluation of FMC 35001 in adult leghorn hens. FMC Report
No.: A79-335. Food and Drug Research Laboratories, Inc.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1980 Simmons, R.T. & Reichard, G.L., Salmonella/mammalian-
microsome plate incorporation mutagenesis assay: FMC 35001.
FMC Report No.: A79-396. EG&G Mason Research Institute.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1981 Simmons, R.T. Hans, L.J. & Reichard, G.L., DNA damage/repair
suspension assay: FMC 35001. FMC Report No.: A80-397. EG&G
Mason Research Institute. Submitted by FMC Corp. to WHO.
Janes, J.M., D.E., & Blair, M. Teratology study in rats: FMC 35001
1980a technical. FMC Report No.: ACT 265.33. International
Research and Development Corp. Submitted by FMC Corp. to
WHO.
Janes, J.M., Rodwell, D.E. & Blair, M. Teratology study in rabbits:
1980b FMC 35001 technical. FMC Report No.: ACT 265.33.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Kehoe, D.F. & MacKenzie, K.M. Three-generation rat reproduction study
1982 with two litters in each generation: FMC 35001. FMC Report
No.: A80-401. Hazleton Raltech, Inc. Submitted by FMC Corp.
to WHO.
Kirby, P.E., Pizzarello, R.F., Brauninger, R.M. Vega, R.A., Cohen, A.,
1981 Reichard, G.L., Williams, P.E., Wattam, R.E., Johnson, J.L.
& Clarke, J.J. Evaluation of test article FMC 35001 (MRI#
556) for mutagenic potential employing the L51 78Y TK+/-
mutagenesis assay. FMC Report No.: A80-426. EG&G Mason
Research Institute. Submitted by FMC Corp. to WHO.
Kirkhart, B., Jones, D.C.L. & Skinner, W.A. Micronucleus test on FMC
1979 35001. FMC Report No.: A79-366. S.R.I. International.
Submitted by FMC Corp. to WHO.
Liss, R.H., Chadwick, M. Schepis, J.P. & Hayes, D. Whole-body
1981 radioautography and physiologic disposition studies on
differentially-labelled FMC 35001. Contract No.: 85600.
Arthur D. Little, Inc. Submitted by FMC Corp. to WHO.
Marsden, P.J., Kuwano., E. & Fukuto, T.R., Metabolism and toxicity of
1982 [2,3-Dihydro-2,2-dimenthylbenzofuran-7-yl (di-n-
butylaminothio)-methylcarbamate] in the rat and house fly.
Pestic. Biochem. Physiol. 18:38.
Marshall, P., Jefferson, N.D., Blair, M. & Goldenthal, E.I. 90-day
1979 subchronic study in rats. FMC Report No.: ACT 258.34.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Mehta, C.S. Eye irritation study of FMC 35001 technical in rabbits.
1981 FMC Report No. A79-325. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Mehta, C.S. Dermal irritation study of FMC 35001 technical in rabbits.
1982 FMC Report No. A80-447. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Nye, D.E. Six-month dietary toxicity study in dogs - FMC 35001. FMC
1981 Report No.: ACT 271.36. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Preache, M.M., Shefner, A.M. & Reed, J.M. Dominant lethal assay of FMC
1981 35001 in mice. FMC Report No. A80-424. IIT Research
Institute. Submitted by FMC Corp. to WHO.
Putnam, D.L. & Schechtman, L.M. Activity of T1638 (FMC 35001) in the
1981 in vivo cytogenetics assay in rodents. FMC Report No.:
A80-425. Microbiological Associates. Submitted by FMC Corp.
to WHO.
Sabol, R.J. Acute dermal toxicity of FMC 35001 technical in rabbits.
1979 FMC Report No. ACT 281.03. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Umetsu, N. & Fukuto, R.T. Alteration of Carbosulfan [2,3-Dihydro-2,2-
1982 dimethyl-7-benzofuranyl (Di-n-butylaminosulfenyl)
methylcarbamate] in the rat stomach. J. Agric. Food Chem.
30:555.
Wu, J. Metabolism of ring-14C Carbosulfan in rats. FMC Report No.:
1982a M-4833. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
Wu, J. Metabolism of DBA-14C Carbosulfan in rats. FMC Report No.:
1982b MA-4864. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
See Also:
Toxicological Abbreviations
Carbosulfan (JMPR Evaluations 2003 Part II Toxicological)
Carbosulfan (Pesticide residues in food: 1984 evaluations)
Carbosulfan (Pesticide residues in food: 1986 evaluations Part II Toxicology)
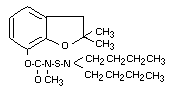 Molecular Weight: 380.5
Technical Material: Approximately 93 percent a.i.;
Manufacturing Use Product (MUP) composition
limits range between 86.0 and 91.0 percent
(w/w) a.i.
Physical Form: Viscous brown liquid
Specific Gravity: 1.056 g/ml at 20°C
Stability: Stable for one year at 22°C, 30 weeks at
50°C, undergoes decomposition at 80°C under
0.1 Torr pressure.
Solubility: Water (0.3 ppm), Completely miscible in
Xylene, Hexane, Chloroform, Methylene
Chloride, Methanol, Acetone.
Volatility: Relatively non-volatile
Vapor Pressure: 0.31 × 10-6 Torr at 25°C
Flash Point: 96°C - closed-cup method
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of ring-14C-Carbosulfan was determined in male and
female rats by examining excreta and selected tissue for activity
following oral administration in corn oil. Animals were fasted for
18 hours prior to oral dosing of 30 and 3.8 mg/kg body weight (b.w.).
A separate group of rats were administered a single dose of 3.3 mg/kg
b.w. unlabelled compound followed 14 days later with the same amount
of labelled compound. There were ten rats (five male and five female)
in each group. Males and females demonstrated the same pattern of
preferential excretion via the urine. Within 24 hours, urinary
excretion in female and male rats was 65.9 and 69.4 percent,
respectively, following a single high dose; 80.08 and 71.04 percent
following a single low dose; and 85.28 and 87.5 percent following the
multiple dose protocol. Elimination in faeces accounted for an
additional 5.9 percent of the administered dose. The majority of the
dose was recovered within the first 24 hours. Tissue activity in males
and females was minimal with <0.01 percent of the dose present in
liver, kidney and skin following the high dose of 30 mg/kg, but at
3 mg/kg 14C activity was generally less then background activity.
Skin accounted for the highest ng equivalents/gram of tissue analyzed.
The urinary metabolites were primarily conjugated, with
3-keto-7 phenol the most abundant, and 3-hydroxy-carbofuran, 7-phenol
and 3-hydroxy-7-phenol also present. Carbofuran, 3-keto-carbofuran,
and N-hydroxymethyl-3-hydroxy carbofuran were minor metabolites
identified. The primary faecal metabolites were unchanged carbosulfan
and 3-hydroxy-carbofuran. Carbofuran was also present in measurable
quantities followed by 3-keto-7-phenol, 3-hydroxy-7-phenol, 7-phenol,
and N-hydroxymethyl-3-hydroxy carbofuran (Wu, 1982a).
In a similar evaluation to the preceding, carbosulfan, labelled
with 14C in the C-1 position of the dibutylamino (DBA) moiety, was
orally gavaged in corn oil to Sprague-Dawley rats. The low dose was
equivalent to 4 mg/kg, administered to five male and five female rats;
the high dose was approximately 27 mg/kg administered to five male
rats, and the multiple dose protocol used five male rats administered
3.6 mg/kg b.w. The majority of radioactivity was excreted in the first
24 hours, with 78-83 percent in the urine and 7-10 percent recovered
in faeces. There were no apparent sex differences identified.
Unlike the ring-labelled carbosulfan study, rats excreted 3-8
percent less DBA-14C carbosulfan radioactivity in the excreta with 1.1
to 2.6 percent tissue and carcase retention. Liver, skin and adipose
accounted for the highest levels in tissues analyzed. This suggests
possible incorporation of the DBA side-chain in the form of fatty
acids, amino acids, sugars or other neutral products. Dibutylamine was
the major metabolite in urine and carbosulfan and 3-keto-carbosulfan
the major metabolites in faeces (Wu, 1982b).
Whole-body autoradiography and physiologic disposition studies
were conducted with ring-14C and DBA-14C carbosulfan. A single oral
dose of both products was absorbed and distributed within 30 minutes
after administration. DBA-14C carbosulfan was distributed more widely
and achieved higher tissue concentrations which persisted longer
than the ring-labelled compound. Tissue sites where DBA-derived
radioactivity was found included: gastrointestinal tract,
epididymides, CNS, glandular tissue, muscle, lung, blood, bone marrow,
liver and ethmoturbinate epithelium, as well as faeces and urine. The
ring-14C labelled compound radioactivity was found in the
gastrointestinal tract, liver, kidney and epididymides. Both compounds
achieved peak concentrations in the tissues at six hours. By 24 hours,
remaining ring-labelled 14C-equivalents, not excreted in the urine
and faeces, were in the gastrointestinal tract, liver and kidney
(Liss, et al., 1981).
Metabolism
Rat
The metabolism of carbosulfan was investigated in female
Sprague-Dawley rats administered 30 mg/kg of aromatic ring-14C,
carbonyl carbamate-14C and DBA-14C labelled chemical in corn oil.
Urine, faeces and carbon dioxide were monitored for four days, after
which the rats were sacrificed and tissues were examined for
radioactivity. Excretion was rapid, with 65-80 percent of the
administered dose being eliminated in respired air, urine and faecal
products during the first 24 hours after treatment. Urinary excretion
was the major route of elimination for both the 14C ring-labelled
(>80 percent) and DBA-labelled (>50 percent) carbosulfan,
carbonyl-labelled carbosulfan preferentially eliminated via expired
carbon dioxide (38 percent). Faecal elimination was minor, except for
the DBA-labelled compound. Radioactive residues remaining in the rat
tissues were low and levels of radioactivity varied with labelling
position. The highest levels of radioactivity were found in the blood,
liver, kidney, lung, heart and spleen, and ranged from 0.1-1.5 mg/kg
(ppm) (carbosulfan equivalents) in ring-14C and DBA-14C
carbosulfan-treated rats after 96 hours. Levels of 0.6-9.6 mg/kg
(carbosulfan equivalents) were noted in tissues of carbonyl-14C
carbosulfan-treated animals after 48 hours. Results support previous
findings and suggest that Carbosulfan is metabolized by two primary
pathways: (1) oxidation of the sulfur to yield CS-sulfone and
sulfamide, and (2) N-S bond cleavage to yield Carbofuran. The first
step represents a significant detoxification mechanism. The scheme in
Figure 1. is proposed for the metabolic pathway in rats.
Molecular Weight: 380.5
Technical Material: Approximately 93 percent a.i.;
Manufacturing Use Product (MUP) composition
limits range between 86.0 and 91.0 percent
(w/w) a.i.
Physical Form: Viscous brown liquid
Specific Gravity: 1.056 g/ml at 20°C
Stability: Stable for one year at 22°C, 30 weeks at
50°C, undergoes decomposition at 80°C under
0.1 Torr pressure.
Solubility: Water (0.3 ppm), Completely miscible in
Xylene, Hexane, Chloroform, Methylene
Chloride, Methanol, Acetone.
Volatility: Relatively non-volatile
Vapor Pressure: 0.31 × 10-6 Torr at 25°C
Flash Point: 96°C - closed-cup method
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of ring-14C-Carbosulfan was determined in male and
female rats by examining excreta and selected tissue for activity
following oral administration in corn oil. Animals were fasted for
18 hours prior to oral dosing of 30 and 3.8 mg/kg body weight (b.w.).
A separate group of rats were administered a single dose of 3.3 mg/kg
b.w. unlabelled compound followed 14 days later with the same amount
of labelled compound. There were ten rats (five male and five female)
in each group. Males and females demonstrated the same pattern of
preferential excretion via the urine. Within 24 hours, urinary
excretion in female and male rats was 65.9 and 69.4 percent,
respectively, following a single high dose; 80.08 and 71.04 percent
following a single low dose; and 85.28 and 87.5 percent following the
multiple dose protocol. Elimination in faeces accounted for an
additional 5.9 percent of the administered dose. The majority of the
dose was recovered within the first 24 hours. Tissue activity in males
and females was minimal with <0.01 percent of the dose present in
liver, kidney and skin following the high dose of 30 mg/kg, but at
3 mg/kg 14C activity was generally less then background activity.
Skin accounted for the highest ng equivalents/gram of tissue analyzed.
The urinary metabolites were primarily conjugated, with
3-keto-7 phenol the most abundant, and 3-hydroxy-carbofuran, 7-phenol
and 3-hydroxy-7-phenol also present. Carbofuran, 3-keto-carbofuran,
and N-hydroxymethyl-3-hydroxy carbofuran were minor metabolites
identified. The primary faecal metabolites were unchanged carbosulfan
and 3-hydroxy-carbofuran. Carbofuran was also present in measurable
quantities followed by 3-keto-7-phenol, 3-hydroxy-7-phenol, 7-phenol,
and N-hydroxymethyl-3-hydroxy carbofuran (Wu, 1982a).
In a similar evaluation to the preceding, carbosulfan, labelled
with 14C in the C-1 position of the dibutylamino (DBA) moiety, was
orally gavaged in corn oil to Sprague-Dawley rats. The low dose was
equivalent to 4 mg/kg, administered to five male and five female rats;
the high dose was approximately 27 mg/kg administered to five male
rats, and the multiple dose protocol used five male rats administered
3.6 mg/kg b.w. The majority of radioactivity was excreted in the first
24 hours, with 78-83 percent in the urine and 7-10 percent recovered
in faeces. There were no apparent sex differences identified.
Unlike the ring-labelled carbosulfan study, rats excreted 3-8
percent less DBA-14C carbosulfan radioactivity in the excreta with 1.1
to 2.6 percent tissue and carcase retention. Liver, skin and adipose
accounted for the highest levels in tissues analyzed. This suggests
possible incorporation of the DBA side-chain in the form of fatty
acids, amino acids, sugars or other neutral products. Dibutylamine was
the major metabolite in urine and carbosulfan and 3-keto-carbosulfan
the major metabolites in faeces (Wu, 1982b).
Whole-body autoradiography and physiologic disposition studies
were conducted with ring-14C and DBA-14C carbosulfan. A single oral
dose of both products was absorbed and distributed within 30 minutes
after administration. DBA-14C carbosulfan was distributed more widely
and achieved higher tissue concentrations which persisted longer
than the ring-labelled compound. Tissue sites where DBA-derived
radioactivity was found included: gastrointestinal tract,
epididymides, CNS, glandular tissue, muscle, lung, blood, bone marrow,
liver and ethmoturbinate epithelium, as well as faeces and urine. The
ring-14C labelled compound radioactivity was found in the
gastrointestinal tract, liver, kidney and epididymides. Both compounds
achieved peak concentrations in the tissues at six hours. By 24 hours,
remaining ring-labelled 14C-equivalents, not excreted in the urine
and faeces, were in the gastrointestinal tract, liver and kidney
(Liss, et al., 1981).
Metabolism
Rat
The metabolism of carbosulfan was investigated in female
Sprague-Dawley rats administered 30 mg/kg of aromatic ring-14C,
carbonyl carbamate-14C and DBA-14C labelled chemical in corn oil.
Urine, faeces and carbon dioxide were monitored for four days, after
which the rats were sacrificed and tissues were examined for
radioactivity. Excretion was rapid, with 65-80 percent of the
administered dose being eliminated in respired air, urine and faecal
products during the first 24 hours after treatment. Urinary excretion
was the major route of elimination for both the 14C ring-labelled
(>80 percent) and DBA-labelled (>50 percent) carbosulfan,
carbonyl-labelled carbosulfan preferentially eliminated via expired
carbon dioxide (38 percent). Faecal elimination was minor, except for
the DBA-labelled compound. Radioactive residues remaining in the rat
tissues were low and levels of radioactivity varied with labelling
position. The highest levels of radioactivity were found in the blood,
liver, kidney, lung, heart and spleen, and ranged from 0.1-1.5 mg/kg
(ppm) (carbosulfan equivalents) in ring-14C and DBA-14C
carbosulfan-treated rats after 96 hours. Levels of 0.6-9.6 mg/kg
(carbosulfan equivalents) were noted in tissues of carbonyl-14C
carbosulfan-treated animals after 48 hours. Results support previous
findings and suggest that Carbosulfan is metabolized by two primary
pathways: (1) oxidation of the sulfur to yield CS-sulfone and
sulfamide, and (2) N-S bond cleavage to yield Carbofuran. The first
step represents a significant detoxification mechanism. The scheme in
Figure 1. is proposed for the metabolic pathway in rats.
 A female rat received a single dose (30 mg/kg) of ring-14C
Carbosulfan via oral gavage. Analyses indicated that levels of
unchanged parent chemical persisted in the blood for three hours after
dosing. Approximately 72 percent of the radiocarbon detected in blood
was attributed to parent chemical (Marsden, Kuwawo & Fukuto, 1982). In
a separate study, female Sprague-Dawley rats were administered
30 mg/kg of carbonyl-14C Carbosulfan. The rats were sacrificed at 15,
35 and 80 minutes after dosing and the stomach contents were assayed
for radioactivity. Approximately 50 percent of the recovered
radioactivity was Carbosulfan after 80 minutes (Umetsu & Fukuto,
1982).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Carbosulfan was administered to groups of Charles River CD rats
(15 males and 30 females/group) at dietary levels of 0, 10, 20 and
250 ppm for three generations. Two successive litters were reared from
each female. General condition and behavior were routinely observed
and individual body weights were recorded throughout the study. The
number of pups in each litter were examined externally and culled to
10/litter at four days of age. Individual pup weights were measured at
days 0, 4, 7, 14 and 21. Ten male and ten female F1b, F2b, and F3b
weanlings were randomly selected for gross necropsy and tissue
collection. The F1a and F2a litters were discarded at weaning, and
the F1b and F2b litters were used to produce succeeding generations.
Weanlings not selected for continuation on the study (F1a, F2a,
F1b, F2b, F3a and F3b) were subjected to gross external
examination, sacrificed and discarded.
Body weights of F0 males and females were decreased initially at
20 and 250 ppm. These decreases were associated with decreased food
consumption, and both recovered to normal from week 4 to sacrifice.
Body weights of parental males (F1 and F2) receiving the 250 ppm
diet were consistently lower than those in the corresponding control
group. A similar effect was observed in the 250 ppm group females
during portions of gestation and lactation, but not during the growth
phases. Mating index, gestation index and number of viable foetuses
were essentially normal throughout, except for F2b dams at the
250 ppm level, which were decreased.
Litter size, pup weights and/or pup weight gains for all litter
sets in the 250 ppm group were significantly lower at most age
intervals between birth and weaning when compared to concurrent
control animals. Neonatal survival among the 250 ppm group was also
significantly lower for the first four litters (F1a, F1b, F2a and
F2b).
There were no treatment-related gross or histologic changes
observed among the F0, F1b and F2b adult groups and the F1b,
F2b and F3b weanling groups. Carbosulfan did not have a demonstrated
adverse effect on reproductive performance, except for the effect on
pup weight, litter size, and pup survival at 250 ppm. The NOEL is
20 ppm (Kehoe & MacKenzie, 1982).
Special Studies on Teratogenicity
Rat
Carbosulfan was administered orally by gavage to groups of 25
Charles River CD rats during gestation days 6 through 19 at dosages of
0, 2, 10 and 20 mg/kg/day. Surviving females were necropsied on day 20
and foetuses delivered by hysterotomy. The number and position of
viable/non-viable foetuses, early/late resorptions, mean number of
corpora lutea and total number of implantations were recorded.
External, internal and skeletal examinations of foetuses were
performed for evidence of abnormalities and anomalies. One third of
the foetuses were evaluated for soft tissue anomalies and the
remaining two thirds for skeletal effects.
There was no dose-related mortality in any treatment group. Body
tremors and clear oral discharge were reported in the 20 mg/kg/day
group after administration of each dose. Mean maternal body weight
gains were slightly reduced at 10 mg/kg/day and significantly reduced
at the 20 mg/kg level during the gestation period. There were no
differences in number of pregnancies, early/late resorptions, viable
foetuses, post-implantation loss or sex distribution. The number of
corpora lutea per dam was increased in all treated groups, while the
mean foetal body weight was significantly reduced at 10 and
20 mg/kg/day. There was also an increase in the number of litters with
developmental variations at 20 mg/kg/day which included reduced
ossification of the skull, hyoid body unossified, sternebrae 5 and 6
unossified, and renal papilla not developed and/or distended ureters.
There were no reported effects on the number or percentage of foetuses
or litters with external, internal or skeletal malformations or
anomalies at any dose level. Carbosulfan was not demonstrated to be
teratogenic in rats at doses up to and including 20 mg/kg/day (Janes,
Rodwell & Blair, 1980a).
Rabbit
Groups of 16 New Zealand Albino Rabbits received carbosulfan by
gavage at dosages of 0, 2, 5, and 10 mg/kg b.w./day on days 6 through
28 of gestation. Pups were delivered by caesarean section on day 29
and the number, location, and distribution of viable/non-viable
foetuses, corpora lutea, early/late resorptions and total
implantations were recorded. All foetuses were examined grossly,
sectioned for visceral anomalies and stained for skeletal anomalies.
There were three deaths at 10 mg/kg and one each in the control
and low-dose groups. The number of dams with viable foetuses were 12,
10, 15 and 11 for the control, 2, 5 and 10 mg/kg groups, respectively.
There were no compound-related effects on the number of viable
foetuses, corpora lutea, foetal sex distribution or total
implantations/dam. There were compound-related effects (e.g. increase)
on post implantation losses and early resorption rate at all doses,
but most notably at 5 and 10 mg/kg. Also, the mean foetal body weight
was decreased in the high dose group. There was a single occurrence of
scoliosis in each of the three treated groups, but none were reported
in the controls. There were no compound-related effects on skeletal
variations, such as delayed ossification. Major vessel variations,
identified primarily as left carotid arising from the innominate, were
observed in 16.7, 100, 46.7 and 72.7 percent of the litters at 0, 2,
5, and 10 mg/kg/day, respectively. The percentage of foetuses
presenting this defect were 4.9, 44, 12.8 and 20.8 percent,
respectively. Although this foetotoxic response was evident and
increased at all dose levels there was no evidence of teratogenicity.
The NOEL for teratogenicity is 10 mg/kg/day (Janes, Rodwell & Blair,
1980b).
Special Studies on Mutagenicity
Carbosulfan was negative in a wide battery of mutagenicity
studies conducted. See Table 1 for a summary of the studies
considered.
Special Studies on Carcinogenicity (See also long-term studies)
Mice
Groups of Charles River CD-1 Mice (100 males and 100 females per
group) were administered Carbosulfan (purity, 94.5 to 95.6 percent
with 0.6 to 2.4 percent carbofuran) in the diet at dose levels of 0,
10, 20, 500 and 2500 ppm for 24 months. All animals were observed
daily for signs of toxicity, moribundity and mortality. Body weight
and food consumption values were recorded weekly for the first 14
weeks and bi-weekly thereafter. Water consumption was determined
monthly. Haematological and biochemical measurements and urinalyses
were performed on ten unfasted mice/sex/group at the 6, 12, 18 and 24
month sacrifices. Ophthalmoscopic evaluations were performed in all
survivors at 24 months. Selected organs were weighed, gross necropsies
conducted and a complete list of tissues and organs examined
microscopically.
There were no measurable effects on mortality or survivability
related to treatment. Mean body weights were reduced throughout the
study at 500 and 2500 ppm for males and 2500 for females. For females
body weight changes at other doses were sporadic and not related to
treatment. However, 20 ppm males demonstrated decreased body weight
gains from week 42 to terminal sacrifice at 104 weeks. Food
consumption was depressed at 2500 ppm for males and females, but only
sporadic decreases were noted at other doses. The actual measured
compound ingested per dose was 0, 1.3, 2.5, 61.5, and 319.5 mg/kg b.w.
for males, and 0, 1.5, 3.1, 71.9 and 337.2 mg/kg b.w. for females for
0, 10, 20, 500 and 2500 ppm, respectively.
There were no measurable differences regarding general appearance
and behavior, except for increased eye irregularities at 2500 ppm.
These included corneal opacity, eccentric pupil, and white, cloudy
eyes.
There were no treatment-related effects or haematological changes
except for a tendency toward slightly increased segmented neutrophils
and decreased lymphocyte counts in males at 2500 ppm. There were no
demonstrated effects on glucose, BUN, SGPT or Alk phosphatase in
either sex at any level.
Cholinesterase values for plasma, erythrocytes and brain tissue
were significantly depressed in both males and females at 500 and
2500 ppm, at all time periods measured.
Ophthalmoscopic examinations indicated an increase in punctuate
opacities of the iris at 500 and 2500 ppm. Pathological changes were
indicative of ageing mice and many were stated to be masked by use of
short-acting mydriatic solutions. However, two separate pathological
opinions concerning the incidence of cataracts in treated mice
indicated that there were compound-related increases in male mice at
500 and 20 ppm, respectively. Females were apparently unaffected by
treatment, although the incidence reached 84 to 100 percent in most
groups. The occurrence overall in control and treated groups indicate
that the effect is possibly exacerbated by the anticholinergic effect,
similarly demonstrated in the rat study. Special evaluation and
concern for the iris, because of compound-related effects in the rat,
did not indicate similar effects in mice.
Absolute organ weight changes were variably affected in both
males and females except for decreased spleen weight in females at 500
and 2500 ppm. Relative spleen weights were also significantly
depressed in females at 500 and 2500 ppm. Relative brain weights were
also significantly increased for both sexes throughout the study at
2500 ppm. This is considered a reflection of the significant effects
on body weight at the higher doses.
Gross and histopathological examination were essentially
unremarkable. The most common findings reported were malignant
lymphoma and bronchio-alveolar adenoma which were equally distributed
among all groups, except for low-dose females which demonstrated a
significant increase in the number of metastatic malignant lymphomas
of mediastinal and mesenteric lymph nodes, as well as thymus and
spleen. Generally, control and low-dose females presented the highest
incidence of malignant lymphomas, in comparison to 20-2500 ppm groups.
The results of the histopathologic examination indicated that the type
and incidence of non-neoplastic and neoplastic lesions were usual
findings in the mouse and unrelated to treatment. Carbosulfan was not
carcinogenic in mice at dietary levels up to and including 2500 ppm.
TABLE 1. Special Studies on Mutagenicity
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Ames' Test S. typhimurium 0.1, 0.5, 2.5, 5 93% Negative Haworth et al.,
(both with and TA 98 and 10 ul/plate 1980
without metabolic TA 100 disolved in DMSO
activation) TA 1535
TA 1537
TA 1538
Bacterial DNA E. coli 0.025, 0.05, 93% Negative. Haworth et al.,
Damage/Repair strains WP2 0.1 and 0.2 ml Preferential kill 1981
Suspension Assay uvr A+ exr A+ per plate of repair deficient
(both with and and CM611 E. coli CM611
without metabolic uvr A- exr A-; without rat liver
activation) S typhimurium microscrees, at
strains TA 1978 0.025 to 0.2 ml
uvr B+ and per plate.
TA 1538 uvr B-
Mouse Lymphoma Mouse Nonactivated: 93% Negative. Kirby et al.,
Forward Mutation L5178Y TK+/- 0.0024, 0.0032, 0.0042, Positive controls 1981
Assay phenotype cells 0.0056, 0.0075, 0.010, (EMS 1 ml/ml; DMAA
(both with and 0.013, 0.018, 0.024 and 7.5 mg/ml) gave
without metabolic 0.032 ml/ml expected response.
activation)
Activated:
0.0056, 0.0075, 0.010,
0.013, 0.018, 0.024,
0.032, 0.042, 0.056
and 0.075 ml/ml
TABLE 1. (continued)
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Microrucleus Mouse, bone Orally, 43.5 and 93% Negative. Kirkhart, Jones
marrow 87 mg/kg (174 mg/kg Positive control & Skinner, 1979
gave excessive mortality (trimethyl phosphate)
and was excluded) yielded expected positive
response at 2 x 1000 mg/kg
i.p.
In Vivo Rat Orally, single doses 93% Carbosulfan was not Putnam and
Cytogenetic of 0, 5, 12 and 30 clastogenic. Positive Schectman, 1981
Assay mg/kg on 5 consecutive control (triethylene-
days melamine) gave expected
response at 0.25 mg/kg
Dominant Mouse Orally, single doses 93% No effect on any Preache, Shefner
Lethal of 0, 7, 20 and 60 reproduction parameter & Reed, 1981
mg/kg for 5 consecutive evaluated (i.e.
days fertility index, number
of implantations, number of
implantation deaths, percent
post-implantation deaths).
Positive control (TEM) gave
expected response at 0.3 mg/kg i.p.
The NOEL is 10 ppm (equal to 1.3 mg/kg b.w.) based on body weight
depression and cataracts in males at 20 pm (DeProspo, Norvell &
Fletcher, 1982b).
Special Study on Neurotoxicity
Groups of adult hens (16 months old) were administered a single
oral dose of Carbosulfan at levels of 0 and 500 mg/kg b.w. A separate
positive control group received 750 mg/kg b.w. of TOCP (a dose seven
times the effective dose needed to produce delayed neuropathy). There
were ten hens in the vehicle and positive control groups, and 40 hens
in the Carbosulfan group. Atropine sulfate (25 mg/kg b.w.) was given
to all birds prior to administration of Carbosulfan. Preliminary range
finding and LD50 evaluations demonstrated the LD50 and LD70 to be
371 and 500 mg/kg b.w., respectively. Body weight and food consumption
were determined weekly. Throughout the study each bird was evaluated
for neurologic effects. At the conclusion of the study (21 days), all
surviving hens were sacrificed and subjected to gross and microscopic
examinations following intravascular perfusion. Axon and myelin
degeneration were assessed in prepared sections of sciatic nerve and
spinal cord.
There were four deaths in the Carbosulfan group within five days
after dosing. No other deaths occurred. Food consumption and body
weight were depressed in the TOCP group. Although food consumption in
the Carbosulfan group was marginally depressed during the first 15
days, there were no differences in body weight gain compared to
vehicle controls. There were no differences in neurologic scores
between vehicle control and the Carbosulfan-treated birds. The TOCP
group gave the expected response, starting on day 13, which consisted
of unsteadiness in walking, inability to walk, staggering, difficulty
in standing and advanced neurotoxic signs. Histologic examination
revealed no evidence of neurotoxic effects in either the Carbosulfan
or vehicle control groups. The TOCP group demonstrated a marked
increase in the number of swollen axons in the cervical, thoracic and
lumbar sections of spinal cord. There were no axonal changes in the
sciatic nerve. The report included only cage-side observations for
locomotor impairment and the number of swollen axons per section of
spinal cord as indicators for evidence of delayed neurotoxicity.
Furthermore, the LD70 dose is considered too low and should actually
have been four to eight times the LD50 (under appropriate protection)
in order to evaluate the delayed neurotoxic potential. The absence of
effects in this study, although partially the result of study design,
support the conclusion that monomethyl carbamates do not cause delayed
neuropathy (Gephart, Becci & Parent, 1979).
Special Studies on Acute Toxicity
The acute toxicity of technical Carbosulfan via various routes of
administration has been evaluated in a variety of mammalian species. A
summary of the acute toxicity data is presented in Table 2. Signs of
toxicity observed in these studies were those commonly associated with
compounds which inhibit acetylcholinesterase including: salivation,
lacrimation, diarrhoea, ataxia, piloerection, urination, miosis,
labored breathing, bloody tears, exophthalmia, cyanosis, tremors,
convulsions, and death.
Carbosulfan (0.1 ml) was instilled into the everted eyelid of
nine rabbits (Mehta, 1981). Three of the nine eyes were washed with
water 30 seconds after instillation. All animals had slightly
constricted pupils at one hour. Moderate conjunctivitis was observed
after one hour while only mild conjunctivitis was observed after 24
hours. All eyes appeared normal within 72 hours which indicated that
Carbosulfan is minimally irritating to the eye.
A primary dermal irritation study was conducted with undiluted
Carbosulfan (Mehta, 1982). Slight erythema and oedema were observed
within 24 hours after the application of 0.5 ml Carbosulfan to two
intact and two abraded skin sites on each of six rabbits. Oedema
subsided within four days and all sites returned to normal within six
days. One rabbit died, which is considered to be compound-related. The
primary irritation index was 1.36, classifying Carbosulfan as slightly
irritating to the skin.
The potential for Carbosulfan to cause dermal sensitization was
assessed using a patch technique, similar to the Buehler and Open
epicutaneous tests. A group of ten guinea-pigs was administered 0.5 ml
of Carbosulfan as a 0.1 percent (w/v) corn oil suspension. A
concurrent positive control group received 0.5 ml of a 0.05 percent
(w/v) solution of 2,4-dinitrochlorobenzene in ethanol. Carbosulfan was
determined to be a dermal sensitizer when slight to moderate
irritation was observed after the challenge application. (Cannelongo,
1981a).
Short-term Studies
Rat
Groups of Charles River CD Rats (25 males and 25 females per
group) were administered Carbosulfan (technical grade) in the diet at
dose levels of 0, 10, 20 and 500 ppm for 90 days. All animals were
observed for overt toxicity and behavioral changes twice daily while
body weight and food consumption were recorded weekly. An
ophthalmologic examination was conducted in all animals prior to
treatment and during the last week of the study. Haematologic,
clinical chemistry and urinalysis determinations were performed on ten
rats/sex/group prior to study initiation and after 30 and 90 days of
study. Erythrocyte and plasma cholinesterase activities were
determined between 8 and 10 a.m. and at the same intervals designated
for the other blood parameters; brain cholinesterase activity was
determined after 90 days. Detailed gross and histopathological
examinations were performed on all animals at the termination of the
study, and selected organs weighed.
TABLE 2. A Summary of the Acute Toxicity of Carbosulfan Technical
LD50
Species Sex Route Vehicle (mg/kg) Reference
Rat Ma Oral Undiluted 250 Cannelongo, 1979a
Fa Oral Undiluted 185 Cannelongo, 1979a
M&F Oral Undiluted 209 Cannelongo, 1979a
Ma Oral Corn oil1 182 Cannelongo, 1979b
Fa Oral Corn oil1 90.5 Cannelongo, 1979b
M&F Oral Corn oil1 138 Cannelongo, 1979b
Mb IP Undiluted 397 Cannelongo, 1979c
Fb IP Undiluted 458 Cannelongo, 1979c
M&F IP Undiluted 422 Cannelongo, 1979c
Ma Inhal. Undiluted 1.53* Cavender & Casorso, 1979
Fa Inhal. Undiluted 0.61* Cavender & Casorso, 1979
Mouse Ma Oral Corn Oil2 32.7 Cannelongo, 1979d
Fa Oral Corn Oil2 81.5 Cannelongo, 1979d
M&F Oral Corn Oil2 46.1 Cannelongo, 1979d
Ma Oral Corn Oil1 124 Cannelongo, 1979e
Fa Oral Corn Oil1 123 Cannelongo, 1979e
M&F Oral Corn Oil1 124 Cannelongo, 1979e
Rabbit Mb Oral Undiluted 42.0 Cannelongo, 1979f
Fb Oral Undiluted 45.8 Cannelongo, 1979f
M&F Oral Undiluted 43.9 Cannelongo, 1979f
Ma Oral Corn Oil1 36.7 Cannelongo, 1979g
Fa Oral Corn Oil1 52.7 Cannelongo, 1979g
M&F Oral Corn oil1 42.7 Cannelongo, 1979g
Mb Dermal Undiluted >2000 Sabol, 1979
Fb Dermal Undiluted >2000 Sabol, 1979
M&F Dermal Undiluted >2000 Sabol, 1979
1 Administered at a constant volume
2 Administered at a constant concentration
* Units in mg/l of air; time-weighted average (analytical).
a. 10M and 10F per dose
b. 5M and 5F per dose
There were no demonstrated effects on body weight, food
consumption, general appearance, mortality, urinalysis or
ophthalmologic examination. Haematology evaluations were essentially
normal except for increased leucocyte counts for treated males at 90
days and increased reticulocyte count for treated females at 90 days.
Clinical chemistry determinations were within normal biological
variation, except for cholinesterase. Treatment-related reductions in
plasma, erythrocyte and brain cholinesterase activity values were
observed among animals receiving the 500 ppm diet; animals receiving
the 10 or 20 ppm diets were not apparently affected. Gross and
histopathological examinations demonstrated no compound-related
effects. Relative kidney and brain weight increases were evident in
high dose males, as well as increased ovarian weights for mid- and
high-dose females. These organ weight changes were not explained by
clinical chemistry or pathological data and, therefore, not considered
compound-related. The NOEL was 20 ppm, based on plasma, RBC, and brain
cholinesterase depression at 500 ppm (Marshall, et al., 1979).
Dog
Groups of 6-month-old beagle dogs (six males and six females per
group) were administered Carbosulfan in the diet for 26 weeks at
dosage levels of 0, 50, 500, and 1000 ppm. A pre-selected high-dose
level of 2000 ppm was reduced to 1000 ppm when one female died during
the first week of treatment. Animals were observed daily for mortality
and signs of toxicity. Body weight and food consumption data were
determined weekly. Each animal was subjected to an ophthalmoscopic
examination prior to initiation and termination. Haematologic and
clinical chemistry parameters were evaluated monthly while urinalyses
were performed at 2, 4 and 6 months. Plasma and erythrocyte
cholinesterase values determined between 8 and 10 a.m. were measured
at 1, 3 and 6 months while brain cholinesterase values were determined
at termination. Animals were allowed free access to treated diet at
all times. At the conclusion of the study all animals were killed,
selected organs weighed and complete gross and histopathological
examinations performed.
There was no mortality associated with doses up to and including
1000 ppm. Diarrhoea/soft stools and emesis were routinely observed in
all groups. Food consumption for 1000 ppm females was decreased
throughout the study, which was reflected in depressed body weight
gain for this group. The 500 ppm females showed occasional decreases
for both food consumption and body weight gain. Food consumption for
males was also only occasionally depressed at the high dose, with
slightly decreased body weight gains for both 500 and 1000 ppm groups,
beginning at weeks 13/14.
Initially, beginning at month 2, females in all treated groups
demonstrated significant decrease in erythrocyte count, haemoglobin
and haematocrit. These effects recovered to normal at month 4. MCHC,
MCH and MCV were comparable to control females throughout. Males in
the 500 and 1000 ppm groups demonstrated the same dose-related effects
at month 4, which continued to termination of the study. MCHC values
were also significantly decreased in mid- and high-dose males at
termination.
Male and female dogs in the mid- and high-dose groups
demonstrated decreased albumin, total protein, globulin, and increased
cholesterol levels in comparison to controls. Effects were more
noticeable in the high-dose group, except for cholesterol, which was
uniformly increased in all treated male dogs throughout the study.
Significant depression of plasma cholinesterase was evident in
500 ppm females and males at 3 months only, while 1000 ppm males and
females demonstrated depression at 1, 3 and 6 months. Erythrocyte
cholinesterase was significantly depressed in 1000 ppm animals at 1
month only, although at 3 and 6 months levels were less than control.
Brain cholinesterase was significantly depressed in 1000 ppm females
only, although 1000 ppm males also showed less than controls.
Urinalysis and ophthalmoscopic examinations were unremarkable.
Absolute spleen weights for male and female dogs in the high-dose
group were significantly decreased, as were the relative spleen
weights for 500 and 1000 ppm males. Other organ weights were not
significantly different from controls. Gross necropsy and
histopathological examinations were unremarkable and did not
demonstrate any compound-related effects. A NOEL was demonstrated at
50 ppm (Nye, 1981).
Long-term Studies (See Also Special Studies on Carcinogenicity)
Rat
Groups of Charles River CD Rats (90 males and 90 females/group)
were administered Carbosulfan (94.5 - 95.6% purity) in the diet for
104 weeks at dosage levels of 0, 10, 20, 500 and 2500 ppm. Carbofuran
was present in the technical material at concentrations of 0.6 to 2.4
percent. Growth was observed by body weight changes and food
consumption data which were recorded weekly for the first 14 weeks and
bi-weekly thereafter. Daily observations were made with respect to
behavioural changes and mortality. At periodic intervals throughout
the study, haematologic, biochemical and cholinesterase analyses were
performed on unfasted animals. Urinalysis was conducted on fasted
animals. Eyes were examined at 12, 18 and 24 months. At 6, 12 and 18
months of the study, ten males and ten females per group were
sacrificed and necropsied. At the conclusion of the study all
surviving animals were sacrificed and gross pathological and
microscopic examinations of tissues and organs were made. Selected
organs were weighed.
Tremors, labored breathing and eye-related changes were more
frequently observed in animals receiving the 500 and 2500 ppm diets.
Mean body weight and food consumption values in the 500 and 2500 ppm
groups were significantly lower than control values throughout the
study, except for female food consumption, which was comparable to
controls. This is reflected in the actual test material consumption
which was determined to be 0, 0.5, 1.0, 26.8 and 152.8 mg/kg b.w. for
males and 0, 0.6, 1.2, 34.7 and 213.3 mg/kg b.w. for females in the
control, 10, 20, 500 and 2500 ppm groups, respectively. Survival was
not apparently affected by treatment.
Haematology measurements at 6, 12, 18 and 24 months demonstrated
a compound-related effect at 18 months in the 2500 ppm males and
females for significantly increased leucocyte count (primarily
segmented neutrophils), and platelet count, slightly increased
reticulocyte count and significantly decreased lymphocyte count.
Haemoglobin, haematocrit, MCV and MCH were all decreased, although not
significantly, in high-dose males compared to controls at 24 months.
Urinalysis determinations in high-dose males and females demonstrated
an increase in ketone levels over controls at month 18. At terminal
sacrifice there was an increase in mid- and high-dose animals of
mononuclear leucocyte infiltrates in the kidney and an increase of
pigmentation (haemosiderin) in the mediastinal lymph nodes of treated
females. All of these data are indicative of leucocytosis and toxic
neutrophilia. During early phases of neutrophilia there is a tendency
toward acidosis, which was demonstrated by the presence of ketone
bodies in the urine. The elevated platelet count is also a reflection
of hyperactivity of the bone marrow. Histopathology of bone marrow,
spleen and liver were otherwise unremarkable, however.
Biochemical analyses were generally comparable to controls,
except in high-dose males and females, where decreased albumin, total
protein and globulin were reported. Cholinesterase determinations in
males and females demonstrated significant depression of plasma,
erythrocyte and brain cholinesterases at doses of 500 and 2500 ppm.
Significantly increased relative brain, heart, liver and kidney
weights in mid- and high-dose males and females were attributed to
lower body weights. Absolute spleen, adrenal and thyroid weights were
uniformly depressed in 500 and 2500 ppm dose groups but were not
different from control on an organ-to-body weight basis. Gross and
histopathologic examinations of all tissues, except the eye, revealed
no compound-related effects on the incidence or type of neoplastic or
non-neoplastic changes.
Carbosulfan produced compound-related effects on the eye
described pathologically as focal iris atrophy, iris coloboma and
absence of iris tissues in the 500 and 2500 ppm males and females, as
well as degenerative retinopathy in 2500 ppm females. The atrophy of
the iris was attributed, in part, to an extensive anticholinesterase
effect. There were no treatment-related effects on the eye at 10 or
20 ppm. A NOEL was demonstrated at 20 ppm based on pathology of the
eye and cholinesterase inhibition. Carbosulfan was not carcinogenic to
rats at dietary doses up to and including 2500 ppm (DeProspo, Norvell
& Fletcher, 1982a).
Comments
Carbosulfan is an anticholinesterase methylcarbamate currently in
use as a soil and foliar insecticide.
Absorption, distribution and excretion data in rats demonstrate
the preferential excretion of Carbosulfan, conjugated metabolites and
Carbofuran in the urine, with the majority (>90%) of the dose
recovered within the first 24 hours. Rapid absorption occurs within
the first 30 minutes after oral administration, reaching peak
concentrations in liver, G.I. tract, blood and skin at 6 hours.
Carbosulfan is metabolized by two primary pathways: (1) oxidation of
the sulphur to yield Carbosulfan sulphone and sulphamide, and (2)
N-S bond cleavage to yield Carbofuran.
Carbosulfan was demonstrated to be moderately toxic acutely when
administered to a variety of test animals via various routes of
exposure. It did not induce delayed neurotoxicity in atropinized adult
Leghorn hens at 500 mg/kg b.w.
Carbosulfan demonstrated adverse effects on pup weight, litter
size and pup survival at 250 ppm in rats. No other adverse effects
were apparent and a NOEL was demonstrated at 20 ppm. Similarly, in a
teratogenic study in rats, Carbosulfan was not teratogenic at doses up
to and including 20 mg/kg/day.
Carbosulfan, when administered in the diet to rats and dogs for
90 days and 6 months respectively, demonstrated dose-related effects
on cholinesterases. The NOEL for rats was 20 ppm, based on depression
of plasma, erythrocyte and brain cholinesterase activity. In dogs, the
NOEL for these enzymes was 50 ppm.
Available mutagenicity studies in vitro and in vivo were
negative.
In a long-term feeding study in rats, Carbosulfan at 500 and
2500 ppm in the diet produced compound-related effects on the eye,
which included focal iris atrophy and degenerative retinopathy. There
was also evidence of leucocytosis and toxic neutrophilia at 2500 ppm.
Plasma, erythrocyte and brain cholinesterases were inhibited at both
doses. There was no evidence of oncogenicity at either dose. The NOEL
for the study was 20 ppm.
Mice, exposed to dietary doses of 10, 20, 500 and 2500 ppm for
two years demonstrated inhibition of erythrocyte, plasma and brain
cholinesterases at >500 ppm, but no evidence of oncogenicity at any
dose. There was evidence of a cataractogenic effect at >20 ppm but
the ophthalmological interpretations require further clarification.
The NOEL was 10 ppm, based on compound-related body weight depression
in males at >20 ppm.
On the basis of all available data, no-effect levels in certain
mammalian species have been determined, but because additional data
are needed to clarify the cataractogenic response in the two-year
mouse feeding study, a temporary ADI was allocated.
Level causing no toxicological effect
Mouse: 10 ppm in the diet, equal to 1.3 mg/kg b.w.
Rat: 20 ppm in the diet, equal to 1.0 mg/kg b.w.
Dog: 50 ppm in the diet, equal to 1.25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans:
0 - 0.005 mg/kg b.w.
FURTHER WORK OR INFORMATION
Required (by 1986):
Additional information to clarify the different pathological
interpretations of the ophthalmological data in the two-year mouse
study and to demonstrate the NOEL for the cataract effects.
Desirable:
Metabolic studies in non-rodent species.
Observations in humans, particularly concerning effects on the eye.
REFERENCES
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979a FMC Report No. ACT 281.01. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979b FMC Report No. ACT 281.01A. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute intraperitoneal toxicity study of FMC 35001
1979c technical in rats. FMC Report No. ACT 281.05. Stillmeadow,
Inc. Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979d mice. FMC Report No. ACT 281.02. Stillmeadow, Inc. Submitted
by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979e mice. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979f rabbits. FMC Report No. ACT 281.02. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979g rabbits. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Dermal sensitization study of FMC 35001 technical in
1981 guinea-pigs. FMC Report No. A79-327. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cavender, F. & Casorso, D.R. Acute inhalation toxicity study of FMC
1979 35001 technical in rats. FMC Report No. ACT 291.04.
ToxiGenics, Inc. Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982a dietary toxicity/oncogenicity study in rats with FMC 35001.
FMC Report No. A79-333. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982b dietary toxicity/oncogenicity study in mice with FMC 35001.
FMC Report No. A79-334. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Gephart, L., Becci, P.J. & Parent, R.A. delayed neurotoxicity
1979 evaluation of FMC 35001 in adult leghorn hens. FMC Report
No.: A79-335. Food and Drug Research Laboratories, Inc.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1980 Simmons, R.T. & Reichard, G.L., Salmonella/mammalian-
microsome plate incorporation mutagenesis assay: FMC 35001.
FMC Report No.: A79-396. EG&G Mason Research Institute.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1981 Simmons, R.T. Hans, L.J. & Reichard, G.L., DNA damage/repair
suspension assay: FMC 35001. FMC Report No.: A80-397. EG&G
Mason Research Institute. Submitted by FMC Corp. to WHO.
Janes, J.M., D.E., & Blair, M. Teratology study in rats: FMC 35001
1980a technical. FMC Report No.: ACT 265.33. International
Research and Development Corp. Submitted by FMC Corp. to
WHO.
Janes, J.M., Rodwell, D.E. & Blair, M. Teratology study in rabbits:
1980b FMC 35001 technical. FMC Report No.: ACT 265.33.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Kehoe, D.F. & MacKenzie, K.M. Three-generation rat reproduction study
1982 with two litters in each generation: FMC 35001. FMC Report
No.: A80-401. Hazleton Raltech, Inc. Submitted by FMC Corp.
to WHO.
Kirby, P.E., Pizzarello, R.F., Brauninger, R.M. Vega, R.A., Cohen, A.,
1981 Reichard, G.L., Williams, P.E., Wattam, R.E., Johnson, J.L.
& Clarke, J.J. Evaluation of test article FMC 35001 (MRI#
556) for mutagenic potential employing the L51 78Y TK+/-
mutagenesis assay. FMC Report No.: A80-426. EG&G Mason
Research Institute. Submitted by FMC Corp. to WHO.
Kirkhart, B., Jones, D.C.L. & Skinner, W.A. Micronucleus test on FMC
1979 35001. FMC Report No.: A79-366. S.R.I. International.
Submitted by FMC Corp. to WHO.
Liss, R.H., Chadwick, M. Schepis, J.P. & Hayes, D. Whole-body
1981 radioautography and physiologic disposition studies on
differentially-labelled FMC 35001. Contract No.: 85600.
Arthur D. Little, Inc. Submitted by FMC Corp. to WHO.
Marsden, P.J., Kuwano., E. & Fukuto, T.R., Metabolism and toxicity of
1982 [2,3-Dihydro-2,2-dimenthylbenzofuran-7-yl (di-n-
butylaminothio)-methylcarbamate] in the rat and house fly.
Pestic. Biochem. Physiol. 18:38.
Marshall, P., Jefferson, N.D., Blair, M. & Goldenthal, E.I. 90-day
1979 subchronic study in rats. FMC Report No.: ACT 258.34.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Mehta, C.S. Eye irritation study of FMC 35001 technical in rabbits.
1981 FMC Report No. A79-325. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Mehta, C.S. Dermal irritation study of FMC 35001 technical in rabbits.
1982 FMC Report No. A80-447. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Nye, D.E. Six-month dietary toxicity study in dogs - FMC 35001. FMC
1981 Report No.: ACT 271.36. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Preache, M.M., Shefner, A.M. & Reed, J.M. Dominant lethal assay of FMC
1981 35001 in mice. FMC Report No. A80-424. IIT Research
Institute. Submitted by FMC Corp. to WHO.
Putnam, D.L. & Schechtman, L.M. Activity of T1638 (FMC 35001) in the
1981 in vivo cytogenetics assay in rodents. FMC Report No.:
A80-425. Microbiological Associates. Submitted by FMC Corp.
to WHO.
Sabol, R.J. Acute dermal toxicity of FMC 35001 technical in rabbits.
1979 FMC Report No. ACT 281.03. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Umetsu, N. & Fukuto, R.T. Alteration of Carbosulfan [2,3-Dihydro-2,2-
1982 dimethyl-7-benzofuranyl (Di-n-butylaminosulfenyl)
methylcarbamate] in the rat stomach. J. Agric. Food Chem.
30:555.
Wu, J. Metabolism of ring-14C Carbosulfan in rats. FMC Report No.:
1982a M-4833. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
Wu, J. Metabolism of DBA-14C Carbosulfan in rats. FMC Report No.:
1982b MA-4864. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
A female rat received a single dose (30 mg/kg) of ring-14C
Carbosulfan via oral gavage. Analyses indicated that levels of
unchanged parent chemical persisted in the blood for three hours after
dosing. Approximately 72 percent of the radiocarbon detected in blood
was attributed to parent chemical (Marsden, Kuwawo & Fukuto, 1982). In
a separate study, female Sprague-Dawley rats were administered
30 mg/kg of carbonyl-14C Carbosulfan. The rats were sacrificed at 15,
35 and 80 minutes after dosing and the stomach contents were assayed
for radioactivity. Approximately 50 percent of the recovered
radioactivity was Carbosulfan after 80 minutes (Umetsu & Fukuto,
1982).
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Carbosulfan was administered to groups of Charles River CD rats
(15 males and 30 females/group) at dietary levels of 0, 10, 20 and
250 ppm for three generations. Two successive litters were reared from
each female. General condition and behavior were routinely observed
and individual body weights were recorded throughout the study. The
number of pups in each litter were examined externally and culled to
10/litter at four days of age. Individual pup weights were measured at
days 0, 4, 7, 14 and 21. Ten male and ten female F1b, F2b, and F3b
weanlings were randomly selected for gross necropsy and tissue
collection. The F1a and F2a litters were discarded at weaning, and
the F1b and F2b litters were used to produce succeeding generations.
Weanlings not selected for continuation on the study (F1a, F2a,
F1b, F2b, F3a and F3b) were subjected to gross external
examination, sacrificed and discarded.
Body weights of F0 males and females were decreased initially at
20 and 250 ppm. These decreases were associated with decreased food
consumption, and both recovered to normal from week 4 to sacrifice.
Body weights of parental males (F1 and F2) receiving the 250 ppm
diet were consistently lower than those in the corresponding control
group. A similar effect was observed in the 250 ppm group females
during portions of gestation and lactation, but not during the growth
phases. Mating index, gestation index and number of viable foetuses
were essentially normal throughout, except for F2b dams at the
250 ppm level, which were decreased.
Litter size, pup weights and/or pup weight gains for all litter
sets in the 250 ppm group were significantly lower at most age
intervals between birth and weaning when compared to concurrent
control animals. Neonatal survival among the 250 ppm group was also
significantly lower for the first four litters (F1a, F1b, F2a and
F2b).
There were no treatment-related gross or histologic changes
observed among the F0, F1b and F2b adult groups and the F1b,
F2b and F3b weanling groups. Carbosulfan did not have a demonstrated
adverse effect on reproductive performance, except for the effect on
pup weight, litter size, and pup survival at 250 ppm. The NOEL is
20 ppm (Kehoe & MacKenzie, 1982).
Special Studies on Teratogenicity
Rat
Carbosulfan was administered orally by gavage to groups of 25
Charles River CD rats during gestation days 6 through 19 at dosages of
0, 2, 10 and 20 mg/kg/day. Surviving females were necropsied on day 20
and foetuses delivered by hysterotomy. The number and position of
viable/non-viable foetuses, early/late resorptions, mean number of
corpora lutea and total number of implantations were recorded.
External, internal and skeletal examinations of foetuses were
performed for evidence of abnormalities and anomalies. One third of
the foetuses were evaluated for soft tissue anomalies and the
remaining two thirds for skeletal effects.
There was no dose-related mortality in any treatment group. Body
tremors and clear oral discharge were reported in the 20 mg/kg/day
group after administration of each dose. Mean maternal body weight
gains were slightly reduced at 10 mg/kg/day and significantly reduced
at the 20 mg/kg level during the gestation period. There were no
differences in number of pregnancies, early/late resorptions, viable
foetuses, post-implantation loss or sex distribution. The number of
corpora lutea per dam was increased in all treated groups, while the
mean foetal body weight was significantly reduced at 10 and
20 mg/kg/day. There was also an increase in the number of litters with
developmental variations at 20 mg/kg/day which included reduced
ossification of the skull, hyoid body unossified, sternebrae 5 and 6
unossified, and renal papilla not developed and/or distended ureters.
There were no reported effects on the number or percentage of foetuses
or litters with external, internal or skeletal malformations or
anomalies at any dose level. Carbosulfan was not demonstrated to be
teratogenic in rats at doses up to and including 20 mg/kg/day (Janes,
Rodwell & Blair, 1980a).
Rabbit
Groups of 16 New Zealand Albino Rabbits received carbosulfan by
gavage at dosages of 0, 2, 5, and 10 mg/kg b.w./day on days 6 through
28 of gestation. Pups were delivered by caesarean section on day 29
and the number, location, and distribution of viable/non-viable
foetuses, corpora lutea, early/late resorptions and total
implantations were recorded. All foetuses were examined grossly,
sectioned for visceral anomalies and stained for skeletal anomalies.
There were three deaths at 10 mg/kg and one each in the control
and low-dose groups. The number of dams with viable foetuses were 12,
10, 15 and 11 for the control, 2, 5 and 10 mg/kg groups, respectively.
There were no compound-related effects on the number of viable
foetuses, corpora lutea, foetal sex distribution or total
implantations/dam. There were compound-related effects (e.g. increase)
on post implantation losses and early resorption rate at all doses,
but most notably at 5 and 10 mg/kg. Also, the mean foetal body weight
was decreased in the high dose group. There was a single occurrence of
scoliosis in each of the three treated groups, but none were reported
in the controls. There were no compound-related effects on skeletal
variations, such as delayed ossification. Major vessel variations,
identified primarily as left carotid arising from the innominate, were
observed in 16.7, 100, 46.7 and 72.7 percent of the litters at 0, 2,
5, and 10 mg/kg/day, respectively. The percentage of foetuses
presenting this defect were 4.9, 44, 12.8 and 20.8 percent,
respectively. Although this foetotoxic response was evident and
increased at all dose levels there was no evidence of teratogenicity.
The NOEL for teratogenicity is 10 mg/kg/day (Janes, Rodwell & Blair,
1980b).
Special Studies on Mutagenicity
Carbosulfan was negative in a wide battery of mutagenicity
studies conducted. See Table 1 for a summary of the studies
considered.
Special Studies on Carcinogenicity (See also long-term studies)
Mice
Groups of Charles River CD-1 Mice (100 males and 100 females per
group) were administered Carbosulfan (purity, 94.5 to 95.6 percent
with 0.6 to 2.4 percent carbofuran) in the diet at dose levels of 0,
10, 20, 500 and 2500 ppm for 24 months. All animals were observed
daily for signs of toxicity, moribundity and mortality. Body weight
and food consumption values were recorded weekly for the first 14
weeks and bi-weekly thereafter. Water consumption was determined
monthly. Haematological and biochemical measurements and urinalyses
were performed on ten unfasted mice/sex/group at the 6, 12, 18 and 24
month sacrifices. Ophthalmoscopic evaluations were performed in all
survivors at 24 months. Selected organs were weighed, gross necropsies
conducted and a complete list of tissues and organs examined
microscopically.
There were no measurable effects on mortality or survivability
related to treatment. Mean body weights were reduced throughout the
study at 500 and 2500 ppm for males and 2500 for females. For females
body weight changes at other doses were sporadic and not related to
treatment. However, 20 ppm males demonstrated decreased body weight
gains from week 42 to terminal sacrifice at 104 weeks. Food
consumption was depressed at 2500 ppm for males and females, but only
sporadic decreases were noted at other doses. The actual measured
compound ingested per dose was 0, 1.3, 2.5, 61.5, and 319.5 mg/kg b.w.
for males, and 0, 1.5, 3.1, 71.9 and 337.2 mg/kg b.w. for females for
0, 10, 20, 500 and 2500 ppm, respectively.
There were no measurable differences regarding general appearance
and behavior, except for increased eye irregularities at 2500 ppm.
These included corneal opacity, eccentric pupil, and white, cloudy
eyes.
There were no treatment-related effects or haematological changes
except for a tendency toward slightly increased segmented neutrophils
and decreased lymphocyte counts in males at 2500 ppm. There were no
demonstrated effects on glucose, BUN, SGPT or Alk phosphatase in
either sex at any level.
Cholinesterase values for plasma, erythrocytes and brain tissue
were significantly depressed in both males and females at 500 and
2500 ppm, at all time periods measured.
Ophthalmoscopic examinations indicated an increase in punctuate
opacities of the iris at 500 and 2500 ppm. Pathological changes were
indicative of ageing mice and many were stated to be masked by use of
short-acting mydriatic solutions. However, two separate pathological
opinions concerning the incidence of cataracts in treated mice
indicated that there were compound-related increases in male mice at
500 and 20 ppm, respectively. Females were apparently unaffected by
treatment, although the incidence reached 84 to 100 percent in most
groups. The occurrence overall in control and treated groups indicate
that the effect is possibly exacerbated by the anticholinergic effect,
similarly demonstrated in the rat study. Special evaluation and
concern for the iris, because of compound-related effects in the rat,
did not indicate similar effects in mice.
Absolute organ weight changes were variably affected in both
males and females except for decreased spleen weight in females at 500
and 2500 ppm. Relative spleen weights were also significantly
depressed in females at 500 and 2500 ppm. Relative brain weights were
also significantly increased for both sexes throughout the study at
2500 ppm. This is considered a reflection of the significant effects
on body weight at the higher doses.
Gross and histopathological examination were essentially
unremarkable. The most common findings reported were malignant
lymphoma and bronchio-alveolar adenoma which were equally distributed
among all groups, except for low-dose females which demonstrated a
significant increase in the number of metastatic malignant lymphomas
of mediastinal and mesenteric lymph nodes, as well as thymus and
spleen. Generally, control and low-dose females presented the highest
incidence of malignant lymphomas, in comparison to 20-2500 ppm groups.
The results of the histopathologic examination indicated that the type
and incidence of non-neoplastic and neoplastic lesions were usual
findings in the mouse and unrelated to treatment. Carbosulfan was not
carcinogenic in mice at dietary levels up to and including 2500 ppm.
TABLE 1. Special Studies on Mutagenicity
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Ames' Test S. typhimurium 0.1, 0.5, 2.5, 5 93% Negative Haworth et al.,
(both with and TA 98 and 10 ul/plate 1980
without metabolic TA 100 disolved in DMSO
activation) TA 1535
TA 1537
TA 1538
Bacterial DNA E. coli 0.025, 0.05, 93% Negative. Haworth et al.,
Damage/Repair strains WP2 0.1 and 0.2 ml Preferential kill 1981
Suspension Assay uvr A+ exr A+ per plate of repair deficient
(both with and and CM611 E. coli CM611
without metabolic uvr A- exr A-; without rat liver
activation) S typhimurium microscrees, at
strains TA 1978 0.025 to 0.2 ml
uvr B+ and per plate.
TA 1538 uvr B-
Mouse Lymphoma Mouse Nonactivated: 93% Negative. Kirby et al.,
Forward Mutation L5178Y TK+/- 0.0024, 0.0032, 0.0042, Positive controls 1981
Assay phenotype cells 0.0056, 0.0075, 0.010, (EMS 1 ml/ml; DMAA
(both with and 0.013, 0.018, 0.024 and 7.5 mg/ml) gave
without metabolic 0.032 ml/ml expected response.
activation)
Activated:
0.0056, 0.0075, 0.010,
0.013, 0.018, 0.024,
0.032, 0.042, 0.056
and 0.075 ml/ml
TABLE 1. (continued)
Test System Test Object Concerntrations of Purity Results Reference
carbosulfan Used
Microrucleus Mouse, bone Orally, 43.5 and 93% Negative. Kirkhart, Jones
marrow 87 mg/kg (174 mg/kg Positive control & Skinner, 1979
gave excessive mortality (trimethyl phosphate)
and was excluded) yielded expected positive
response at 2 x 1000 mg/kg
i.p.
In Vivo Rat Orally, single doses 93% Carbosulfan was not Putnam and
Cytogenetic of 0, 5, 12 and 30 clastogenic. Positive Schectman, 1981
Assay mg/kg on 5 consecutive control (triethylene-
days melamine) gave expected
response at 0.25 mg/kg
Dominant Mouse Orally, single doses 93% No effect on any Preache, Shefner
Lethal of 0, 7, 20 and 60 reproduction parameter & Reed, 1981
mg/kg for 5 consecutive evaluated (i.e.
days fertility index, number
of implantations, number of
implantation deaths, percent
post-implantation deaths).
Positive control (TEM) gave
expected response at 0.3 mg/kg i.p.
The NOEL is 10 ppm (equal to 1.3 mg/kg b.w.) based on body weight
depression and cataracts in males at 20 pm (DeProspo, Norvell &
Fletcher, 1982b).
Special Study on Neurotoxicity
Groups of adult hens (16 months old) were administered a single
oral dose of Carbosulfan at levels of 0 and 500 mg/kg b.w. A separate
positive control group received 750 mg/kg b.w. of TOCP (a dose seven
times the effective dose needed to produce delayed neuropathy). There
were ten hens in the vehicle and positive control groups, and 40 hens
in the Carbosulfan group. Atropine sulfate (25 mg/kg b.w.) was given
to all birds prior to administration of Carbosulfan. Preliminary range
finding and LD50 evaluations demonstrated the LD50 and LD70 to be
371 and 500 mg/kg b.w., respectively. Body weight and food consumption
were determined weekly. Throughout the study each bird was evaluated
for neurologic effects. At the conclusion of the study (21 days), all
surviving hens were sacrificed and subjected to gross and microscopic
examinations following intravascular perfusion. Axon and myelin
degeneration were assessed in prepared sections of sciatic nerve and
spinal cord.
There were four deaths in the Carbosulfan group within five days
after dosing. No other deaths occurred. Food consumption and body
weight were depressed in the TOCP group. Although food consumption in
the Carbosulfan group was marginally depressed during the first 15
days, there were no differences in body weight gain compared to
vehicle controls. There were no differences in neurologic scores
between vehicle control and the Carbosulfan-treated birds. The TOCP
group gave the expected response, starting on day 13, which consisted
of unsteadiness in walking, inability to walk, staggering, difficulty
in standing and advanced neurotoxic signs. Histologic examination
revealed no evidence of neurotoxic effects in either the Carbosulfan
or vehicle control groups. The TOCP group demonstrated a marked
increase in the number of swollen axons in the cervical, thoracic and
lumbar sections of spinal cord. There were no axonal changes in the
sciatic nerve. The report included only cage-side observations for
locomotor impairment and the number of swollen axons per section of
spinal cord as indicators for evidence of delayed neurotoxicity.
Furthermore, the LD70 dose is considered too low and should actually
have been four to eight times the LD50 (under appropriate protection)
in order to evaluate the delayed neurotoxic potential. The absence of
effects in this study, although partially the result of study design,
support the conclusion that monomethyl carbamates do not cause delayed
neuropathy (Gephart, Becci & Parent, 1979).
Special Studies on Acute Toxicity
The acute toxicity of technical Carbosulfan via various routes of
administration has been evaluated in a variety of mammalian species. A
summary of the acute toxicity data is presented in Table 2. Signs of
toxicity observed in these studies were those commonly associated with
compounds which inhibit acetylcholinesterase including: salivation,
lacrimation, diarrhoea, ataxia, piloerection, urination, miosis,
labored breathing, bloody tears, exophthalmia, cyanosis, tremors,
convulsions, and death.
Carbosulfan (0.1 ml) was instilled into the everted eyelid of
nine rabbits (Mehta, 1981). Three of the nine eyes were washed with
water 30 seconds after instillation. All animals had slightly
constricted pupils at one hour. Moderate conjunctivitis was observed
after one hour while only mild conjunctivitis was observed after 24
hours. All eyes appeared normal within 72 hours which indicated that
Carbosulfan is minimally irritating to the eye.
A primary dermal irritation study was conducted with undiluted
Carbosulfan (Mehta, 1982). Slight erythema and oedema were observed
within 24 hours after the application of 0.5 ml Carbosulfan to two
intact and two abraded skin sites on each of six rabbits. Oedema
subsided within four days and all sites returned to normal within six
days. One rabbit died, which is considered to be compound-related. The
primary irritation index was 1.36, classifying Carbosulfan as slightly
irritating to the skin.
The potential for Carbosulfan to cause dermal sensitization was
assessed using a patch technique, similar to the Buehler and Open
epicutaneous tests. A group of ten guinea-pigs was administered 0.5 ml
of Carbosulfan as a 0.1 percent (w/v) corn oil suspension. A
concurrent positive control group received 0.5 ml of a 0.05 percent
(w/v) solution of 2,4-dinitrochlorobenzene in ethanol. Carbosulfan was
determined to be a dermal sensitizer when slight to moderate
irritation was observed after the challenge application. (Cannelongo,
1981a).
Short-term Studies
Rat
Groups of Charles River CD Rats (25 males and 25 females per
group) were administered Carbosulfan (technical grade) in the diet at
dose levels of 0, 10, 20 and 500 ppm for 90 days. All animals were
observed for overt toxicity and behavioral changes twice daily while
body weight and food consumption were recorded weekly. An
ophthalmologic examination was conducted in all animals prior to
treatment and during the last week of the study. Haematologic,
clinical chemistry and urinalysis determinations were performed on ten
rats/sex/group prior to study initiation and after 30 and 90 days of
study. Erythrocyte and plasma cholinesterase activities were
determined between 8 and 10 a.m. and at the same intervals designated
for the other blood parameters; brain cholinesterase activity was
determined after 90 days. Detailed gross and histopathological
examinations were performed on all animals at the termination of the
study, and selected organs weighed.
TABLE 2. A Summary of the Acute Toxicity of Carbosulfan Technical
LD50
Species Sex Route Vehicle (mg/kg) Reference
Rat Ma Oral Undiluted 250 Cannelongo, 1979a
Fa Oral Undiluted 185 Cannelongo, 1979a
M&F Oral Undiluted 209 Cannelongo, 1979a
Ma Oral Corn oil1 182 Cannelongo, 1979b
Fa Oral Corn oil1 90.5 Cannelongo, 1979b
M&F Oral Corn oil1 138 Cannelongo, 1979b
Mb IP Undiluted 397 Cannelongo, 1979c
Fb IP Undiluted 458 Cannelongo, 1979c
M&F IP Undiluted 422 Cannelongo, 1979c
Ma Inhal. Undiluted 1.53* Cavender & Casorso, 1979
Fa Inhal. Undiluted 0.61* Cavender & Casorso, 1979
Mouse Ma Oral Corn Oil2 32.7 Cannelongo, 1979d
Fa Oral Corn Oil2 81.5 Cannelongo, 1979d
M&F Oral Corn Oil2 46.1 Cannelongo, 1979d
Ma Oral Corn Oil1 124 Cannelongo, 1979e
Fa Oral Corn Oil1 123 Cannelongo, 1979e
M&F Oral Corn Oil1 124 Cannelongo, 1979e
Rabbit Mb Oral Undiluted 42.0 Cannelongo, 1979f
Fb Oral Undiluted 45.8 Cannelongo, 1979f
M&F Oral Undiluted 43.9 Cannelongo, 1979f
Ma Oral Corn Oil1 36.7 Cannelongo, 1979g
Fa Oral Corn Oil1 52.7 Cannelongo, 1979g
M&F Oral Corn oil1 42.7 Cannelongo, 1979g
Mb Dermal Undiluted >2000 Sabol, 1979
Fb Dermal Undiluted >2000 Sabol, 1979
M&F Dermal Undiluted >2000 Sabol, 1979
1 Administered at a constant volume
2 Administered at a constant concentration
* Units in mg/l of air; time-weighted average (analytical).
a. 10M and 10F per dose
b. 5M and 5F per dose
There were no demonstrated effects on body weight, food
consumption, general appearance, mortality, urinalysis or
ophthalmologic examination. Haematology evaluations were essentially
normal except for increased leucocyte counts for treated males at 90
days and increased reticulocyte count for treated females at 90 days.
Clinical chemistry determinations were within normal biological
variation, except for cholinesterase. Treatment-related reductions in
plasma, erythrocyte and brain cholinesterase activity values were
observed among animals receiving the 500 ppm diet; animals receiving
the 10 or 20 ppm diets were not apparently affected. Gross and
histopathological examinations demonstrated no compound-related
effects. Relative kidney and brain weight increases were evident in
high dose males, as well as increased ovarian weights for mid- and
high-dose females. These organ weight changes were not explained by
clinical chemistry or pathological data and, therefore, not considered
compound-related. The NOEL was 20 ppm, based on plasma, RBC, and brain
cholinesterase depression at 500 ppm (Marshall, et al., 1979).
Dog
Groups of 6-month-old beagle dogs (six males and six females per
group) were administered Carbosulfan in the diet for 26 weeks at
dosage levels of 0, 50, 500, and 1000 ppm. A pre-selected high-dose
level of 2000 ppm was reduced to 1000 ppm when one female died during
the first week of treatment. Animals were observed daily for mortality
and signs of toxicity. Body weight and food consumption data were
determined weekly. Each animal was subjected to an ophthalmoscopic
examination prior to initiation and termination. Haematologic and
clinical chemistry parameters were evaluated monthly while urinalyses
were performed at 2, 4 and 6 months. Plasma and erythrocyte
cholinesterase values determined between 8 and 10 a.m. were measured
at 1, 3 and 6 months while brain cholinesterase values were determined
at termination. Animals were allowed free access to treated diet at
all times. At the conclusion of the study all animals were killed,
selected organs weighed and complete gross and histopathological
examinations performed.
There was no mortality associated with doses up to and including
1000 ppm. Diarrhoea/soft stools and emesis were routinely observed in
all groups. Food consumption for 1000 ppm females was decreased
throughout the study, which was reflected in depressed body weight
gain for this group. The 500 ppm females showed occasional decreases
for both food consumption and body weight gain. Food consumption for
males was also only occasionally depressed at the high dose, with
slightly decreased body weight gains for both 500 and 1000 ppm groups,
beginning at weeks 13/14.
Initially, beginning at month 2, females in all treated groups
demonstrated significant decrease in erythrocyte count, haemoglobin
and haematocrit. These effects recovered to normal at month 4. MCHC,
MCH and MCV were comparable to control females throughout. Males in
the 500 and 1000 ppm groups demonstrated the same dose-related effects
at month 4, which continued to termination of the study. MCHC values
were also significantly decreased in mid- and high-dose males at
termination.
Male and female dogs in the mid- and high-dose groups
demonstrated decreased albumin, total protein, globulin, and increased
cholesterol levels in comparison to controls. Effects were more
noticeable in the high-dose group, except for cholesterol, which was
uniformly increased in all treated male dogs throughout the study.
Significant depression of plasma cholinesterase was evident in
500 ppm females and males at 3 months only, while 1000 ppm males and
females demonstrated depression at 1, 3 and 6 months. Erythrocyte
cholinesterase was significantly depressed in 1000 ppm animals at 1
month only, although at 3 and 6 months levels were less than control.
Brain cholinesterase was significantly depressed in 1000 ppm females
only, although 1000 ppm males also showed less than controls.
Urinalysis and ophthalmoscopic examinations were unremarkable.
Absolute spleen weights for male and female dogs in the high-dose
group were significantly decreased, as were the relative spleen
weights for 500 and 1000 ppm males. Other organ weights were not
significantly different from controls. Gross necropsy and
histopathological examinations were unremarkable and did not
demonstrate any compound-related effects. A NOEL was demonstrated at
50 ppm (Nye, 1981).
Long-term Studies (See Also Special Studies on Carcinogenicity)
Rat
Groups of Charles River CD Rats (90 males and 90 females/group)
were administered Carbosulfan (94.5 - 95.6% purity) in the diet for
104 weeks at dosage levels of 0, 10, 20, 500 and 2500 ppm. Carbofuran
was present in the technical material at concentrations of 0.6 to 2.4
percent. Growth was observed by body weight changes and food
consumption data which were recorded weekly for the first 14 weeks and
bi-weekly thereafter. Daily observations were made with respect to
behavioural changes and mortality. At periodic intervals throughout
the study, haematologic, biochemical and cholinesterase analyses were
performed on unfasted animals. Urinalysis was conducted on fasted
animals. Eyes were examined at 12, 18 and 24 months. At 6, 12 and 18
months of the study, ten males and ten females per group were
sacrificed and necropsied. At the conclusion of the study all
surviving animals were sacrificed and gross pathological and
microscopic examinations of tissues and organs were made. Selected
organs were weighed.
Tremors, labored breathing and eye-related changes were more
frequently observed in animals receiving the 500 and 2500 ppm diets.
Mean body weight and food consumption values in the 500 and 2500 ppm
groups were significantly lower than control values throughout the
study, except for female food consumption, which was comparable to
controls. This is reflected in the actual test material consumption
which was determined to be 0, 0.5, 1.0, 26.8 and 152.8 mg/kg b.w. for
males and 0, 0.6, 1.2, 34.7 and 213.3 mg/kg b.w. for females in the
control, 10, 20, 500 and 2500 ppm groups, respectively. Survival was
not apparently affected by treatment.
Haematology measurements at 6, 12, 18 and 24 months demonstrated
a compound-related effect at 18 months in the 2500 ppm males and
females for significantly increased leucocyte count (primarily
segmented neutrophils), and platelet count, slightly increased
reticulocyte count and significantly decreased lymphocyte count.
Haemoglobin, haematocrit, MCV and MCH were all decreased, although not
significantly, in high-dose males compared to controls at 24 months.
Urinalysis determinations in high-dose males and females demonstrated
an increase in ketone levels over controls at month 18. At terminal
sacrifice there was an increase in mid- and high-dose animals of
mononuclear leucocyte infiltrates in the kidney and an increase of
pigmentation (haemosiderin) in the mediastinal lymph nodes of treated
females. All of these data are indicative of leucocytosis and toxic
neutrophilia. During early phases of neutrophilia there is a tendency
toward acidosis, which was demonstrated by the presence of ketone
bodies in the urine. The elevated platelet count is also a reflection
of hyperactivity of the bone marrow. Histopathology of bone marrow,
spleen and liver were otherwise unremarkable, however.
Biochemical analyses were generally comparable to controls,
except in high-dose males and females, where decreased albumin, total
protein and globulin were reported. Cholinesterase determinations in
males and females demonstrated significant depression of plasma,
erythrocyte and brain cholinesterases at doses of 500 and 2500 ppm.
Significantly increased relative brain, heart, liver and kidney
weights in mid- and high-dose males and females were attributed to
lower body weights. Absolute spleen, adrenal and thyroid weights were
uniformly depressed in 500 and 2500 ppm dose groups but were not
different from control on an organ-to-body weight basis. Gross and
histopathologic examinations of all tissues, except the eye, revealed
no compound-related effects on the incidence or type of neoplastic or
non-neoplastic changes.
Carbosulfan produced compound-related effects on the eye
described pathologically as focal iris atrophy, iris coloboma and
absence of iris tissues in the 500 and 2500 ppm males and females, as
well as degenerative retinopathy in 2500 ppm females. The atrophy of
the iris was attributed, in part, to an extensive anticholinesterase
effect. There were no treatment-related effects on the eye at 10 or
20 ppm. A NOEL was demonstrated at 20 ppm based on pathology of the
eye and cholinesterase inhibition. Carbosulfan was not carcinogenic to
rats at dietary doses up to and including 2500 ppm (DeProspo, Norvell
& Fletcher, 1982a).
Comments
Carbosulfan is an anticholinesterase methylcarbamate currently in
use as a soil and foliar insecticide.
Absorption, distribution and excretion data in rats demonstrate
the preferential excretion of Carbosulfan, conjugated metabolites and
Carbofuran in the urine, with the majority (>90%) of the dose
recovered within the first 24 hours. Rapid absorption occurs within
the first 30 minutes after oral administration, reaching peak
concentrations in liver, G.I. tract, blood and skin at 6 hours.
Carbosulfan is metabolized by two primary pathways: (1) oxidation of
the sulphur to yield Carbosulfan sulphone and sulphamide, and (2)
N-S bond cleavage to yield Carbofuran.
Carbosulfan was demonstrated to be moderately toxic acutely when
administered to a variety of test animals via various routes of
exposure. It did not induce delayed neurotoxicity in atropinized adult
Leghorn hens at 500 mg/kg b.w.
Carbosulfan demonstrated adverse effects on pup weight, litter
size and pup survival at 250 ppm in rats. No other adverse effects
were apparent and a NOEL was demonstrated at 20 ppm. Similarly, in a
teratogenic study in rats, Carbosulfan was not teratogenic at doses up
to and including 20 mg/kg/day.
Carbosulfan, when administered in the diet to rats and dogs for
90 days and 6 months respectively, demonstrated dose-related effects
on cholinesterases. The NOEL for rats was 20 ppm, based on depression
of plasma, erythrocyte and brain cholinesterase activity. In dogs, the
NOEL for these enzymes was 50 ppm.
Available mutagenicity studies in vitro and in vivo were
negative.
In a long-term feeding study in rats, Carbosulfan at 500 and
2500 ppm in the diet produced compound-related effects on the eye,
which included focal iris atrophy and degenerative retinopathy. There
was also evidence of leucocytosis and toxic neutrophilia at 2500 ppm.
Plasma, erythrocyte and brain cholinesterases were inhibited at both
doses. There was no evidence of oncogenicity at either dose. The NOEL
for the study was 20 ppm.
Mice, exposed to dietary doses of 10, 20, 500 and 2500 ppm for
two years demonstrated inhibition of erythrocyte, plasma and brain
cholinesterases at >500 ppm, but no evidence of oncogenicity at any
dose. There was evidence of a cataractogenic effect at >20 ppm but
the ophthalmological interpretations require further clarification.
The NOEL was 10 ppm, based on compound-related body weight depression
in males at >20 ppm.
On the basis of all available data, no-effect levels in certain
mammalian species have been determined, but because additional data
are needed to clarify the cataractogenic response in the two-year
mouse feeding study, a temporary ADI was allocated.
Level causing no toxicological effect
Mouse: 10 ppm in the diet, equal to 1.3 mg/kg b.w.
Rat: 20 ppm in the diet, equal to 1.0 mg/kg b.w.
Dog: 50 ppm in the diet, equal to 1.25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans:
0 - 0.005 mg/kg b.w.
FURTHER WORK OR INFORMATION
Required (by 1986):
Additional information to clarify the different pathological
interpretations of the ophthalmological data in the two-year mouse
study and to demonstrate the NOEL for the cataract effects.
Desirable:
Metabolic studies in non-rodent species.
Observations in humans, particularly concerning effects on the eye.
REFERENCES
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979a FMC Report No. ACT 281.01. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity of FMC 35001 technical in rats.
1979b FMC Report No. ACT 281.01A. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Cannelongo, B.F. Acute intraperitoneal toxicity study of FMC 35001
1979c technical in rats. FMC Report No. ACT 281.05. Stillmeadow,
Inc. Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979d mice. FMC Report No. ACT 281.02. Stillmeadow, Inc. Submitted
by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979e mice. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979f rabbits. FMC Report No. ACT 281.02. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Acute oral toxicity study of FMC 35001 technical in
1979g rabbits. FMC Report No. ACT 281.02A. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cannelongo, B.F. Dermal sensitization study of FMC 35001 technical in
1981 guinea-pigs. FMC Report No. A79-327. Stillmeadow, Inc.
Submitted by FMC Corp. to WHO.
Cavender, F. & Casorso, D.R. Acute inhalation toxicity study of FMC
1979 35001 technical in rats. FMC Report No. ACT 291.04.
ToxiGenics, Inc. Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982a dietary toxicity/oncogenicity study in rats with FMC 35001.
FMC Report No. A79-333. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. Twenty-four month
1982b dietary toxicity/oncogenicity study in mice with FMC 35001.
FMC Report No. A79-334. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Gephart, L., Becci, P.J. & Parent, R.A. delayed neurotoxicity
1979 evaluation of FMC 35001 in adult leghorn hens. FMC Report
No.: A79-335. Food and Drug Research Laboratories, Inc.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1980 Simmons, R.T. & Reichard, G.L., Salmonella/mammalian-
microsome plate incorporation mutagenesis assay: FMC 35001.
FMC Report No.: A79-396. EG&G Mason Research Institute.
Submitted by FMC Corp. to WHO.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J.,
1981 Simmons, R.T. Hans, L.J. & Reichard, G.L., DNA damage/repair
suspension assay: FMC 35001. FMC Report No.: A80-397. EG&G
Mason Research Institute. Submitted by FMC Corp. to WHO.
Janes, J.M., D.E., & Blair, M. Teratology study in rats: FMC 35001
1980a technical. FMC Report No.: ACT 265.33. International
Research and Development Corp. Submitted by FMC Corp. to
WHO.
Janes, J.M., Rodwell, D.E. & Blair, M. Teratology study in rabbits:
1980b FMC 35001 technical. FMC Report No.: ACT 265.33.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Kehoe, D.F. & MacKenzie, K.M. Three-generation rat reproduction study
1982 with two litters in each generation: FMC 35001. FMC Report
No.: A80-401. Hazleton Raltech, Inc. Submitted by FMC Corp.
to WHO.
Kirby, P.E., Pizzarello, R.F., Brauninger, R.M. Vega, R.A., Cohen, A.,
1981 Reichard, G.L., Williams, P.E., Wattam, R.E., Johnson, J.L.
& Clarke, J.J. Evaluation of test article FMC 35001 (MRI#
556) for mutagenic potential employing the L51 78Y TK+/-
mutagenesis assay. FMC Report No.: A80-426. EG&G Mason
Research Institute. Submitted by FMC Corp. to WHO.
Kirkhart, B., Jones, D.C.L. & Skinner, W.A. Micronucleus test on FMC
1979 35001. FMC Report No.: A79-366. S.R.I. International.
Submitted by FMC Corp. to WHO.
Liss, R.H., Chadwick, M. Schepis, J.P. & Hayes, D. Whole-body
1981 radioautography and physiologic disposition studies on
differentially-labelled FMC 35001. Contract No.: 85600.
Arthur D. Little, Inc. Submitted by FMC Corp. to WHO.
Marsden, P.J., Kuwano., E. & Fukuto, T.R., Metabolism and toxicity of
1982 [2,3-Dihydro-2,2-dimenthylbenzofuran-7-yl (di-n-
butylaminothio)-methylcarbamate] in the rat and house fly.
Pestic. Biochem. Physiol. 18:38.
Marshall, P., Jefferson, N.D., Blair, M. & Goldenthal, E.I. 90-day
1979 subchronic study in rats. FMC Report No.: ACT 258.34.
International Research and Development Corp. Submitted by
FMC Corp. to WHO.
Mehta, C.S. Eye irritation study of FMC 35001 technical in rabbits.
1981 FMC Report No. A79-325. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Mehta, C.S. Dermal irritation study of FMC 35001 technical in rabbits.
1982 FMC Report No. A80-447. Stillmeadow, Inc. Submitted by FMC
Corp. to WHO.
Nye, D.E. Six-month dietary toxicity study in dogs - FMC 35001. FMC
1981 Report No.: ACT 271.36. FMC Corporate Toxicology Department.
Submitted by FMC Corp. to WHO.
Preache, M.M., Shefner, A.M. & Reed, J.M. Dominant lethal assay of FMC
1981 35001 in mice. FMC Report No. A80-424. IIT Research
Institute. Submitted by FMC Corp. to WHO.
Putnam, D.L. & Schechtman, L.M. Activity of T1638 (FMC 35001) in the
1981 in vivo cytogenetics assay in rodents. FMC Report No.:
A80-425. Microbiological Associates. Submitted by FMC Corp.
to WHO.
Sabol, R.J. Acute dermal toxicity of FMC 35001 technical in rabbits.
1979 FMC Report No. ACT 281.03. Stillmeadow, Inc. Submitted by
FMC Corp. to WHO.
Umetsu, N. & Fukuto, R.T. Alteration of Carbosulfan [2,3-Dihydro-2,2-
1982 dimethyl-7-benzofuranyl (Di-n-butylaminosulfenyl)
methylcarbamate] in the rat stomach. J. Agric. Food Chem.
30:555.
Wu, J. Metabolism of ring-14C Carbosulfan in rats. FMC Report No.:
1982a M-4833. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
Wu, J. Metabolism of DBA-14C Carbosulfan in rats. FMC Report No.:
1982b MA-4864. FMC Agricultural Chemical Group. Submitted by FMC
Corp. to WHO.
