
TRIAZOPHOS
EXPLANATION
Triazophos was evaluated for acceptable daily intake by the 1982
Joint Meeting (Annex 1, FAO/WHO, 1983a), at which time a temporary ADI
of 0 - 0.0002 mg/kg b.w. was established. A toxicological monograph
was published after that Meeting (Annex 1, FAO/WHO, 1983b). Additional
studies required to complete the toxicological data base were a
carcinogenicity study, a teratogenicity study in at least 1 mammalian
species, metabolism studies in additional mammalian species (to
explain species differences in acute toxicity studies, and additional
mutagenicity studies. The results of the additional studies that were
submitted to the present Meeting are summarized in this monograph
addendum.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Rats
The metabolic fate of triazophos was studied in Wistar WIDK f SPF
rats. 14C-Radiolabelled triazophos (labelled at the 3-position of
the triazophos ring; 98% radiochemical purity) was administered by
single oral gavage at about 5 mg/kg b.w. Pooled urine and faecal
samples were collected after 24, 48, and 96 hours. Blood samples
were also taken at 0, 0.5, 2, 4, 6, 8, 24, and 48 hours after
administration. Maximal blood concentrations were attained after
about 4 hours. The recovery rate of 98% after 96 hours indicates
that excretion was relatively complete. The predominant route of
excretion of radioactivity was urinary, with greater than 90% of the
administered radioactivity excreted in 48 hours. Faecal elimination of
administered radioactivity amounted to 4.6% after 48 hours. Residual
tissue radioactivity concentrations were highest in the kidney and
liver but were, nevertheless, relatively low at less than 0.004 ppm.
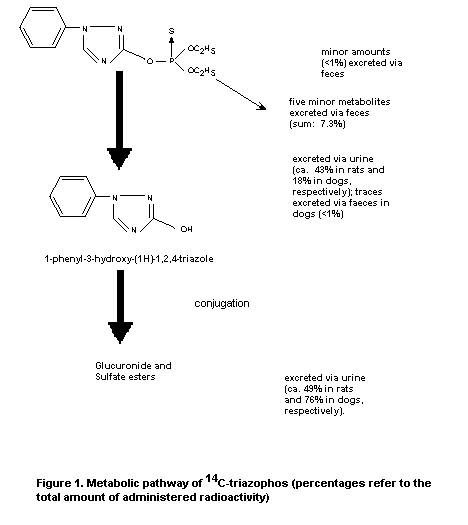
Pooled rat urine contained 3 identifiable metabolites, as shown
in Figure 1, 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (43%) and its
glucuronide (36%) and sulfate conjugates (13%). Unchanged triazophos
was not detected in the urine or faeces (Schwalbe-Fehl et al.,
1986).
Dogs
The metabolic fate of triazophos was also examined in beagle dogs
using the same treatment and sampling regime as for rats. Two female
dogs were treated by gavage with 14C-radiolabelled triazophos
(5 mg/kg b.w.) in sesame oil. Urinary excretion predominated,
averaging 85% of administered dose after 24 hours and 92% after
48 hours. Faecal elimination accounted for 0.3% and 7.5% of the
administered dose after 24 and 48 hours, respectively. Maximal blood
concentrations were attained after 2 hours; after 48 hours,
radioactivity was not detectable in the blood.
Residual tissue levels in the dogs were not determined.
Qualitatively, the metabolic fate of triazophos in dogs was similar to
that established in rats (see Figure 1). The urine contained the same
three metabolites as in rats and in comparable amounts, namely
1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (49%) and its glucuronide (39%)
and sulfate (5%) conjugates. Unchanged triazophos was not detected. In
each case, the glucuronide metabolite underwent spontaneous conversion
to the intermediate trazole metabolite (Schwalbe-Fehl et al., 1986).
 Toxicological studies
Special studies on mutagenicity
Triazophos was without mutagenic activity in a number of
in vitro and in vivo assays (see Table 1).
Special study on acute toxicity
The acute oral toxicity of the principal metabolite of
triazophos, 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole, was greater than
5000 mg/kg b.w. (Diehl & Leist, 1986).
Special studies on teratogenicity
Rats
Triazophos was fed in the diet to groups of 20 - 23 pregnant
Hoe:WISK(SPF71) Wistar rats from day 6 to day 15 of gestation at
approximate daily doses of 0.87, 4.2, or 22 mg/kg b.w. Dams were
sacrificed at day 21 and the fetuses were examined for developmental
abnormalities. There were no treatment-related effects on food
consumption or body-weight gain. The number of fetal resorptions and
viable fetuses, fetal weights, crown-rump lengths, and sex ratios did
not differ significantly between treated and control groups. Placentas
and placental weights were unaffected by treatment. No visceral or
skeletal abnormalities were associated with treatment. No significant
macroscopic changes were observed in the viscera of the dams at post
mortem. Accordingly, the no-observed-effect level in this study
exceeded 22 mg/kg b.w./day (Baeder et al., 1976).
Rabbits
The effects of repeated oral administration of triazophos on the
progress and outcome of pregnancy were studied in the rabbit. As a
previously conducted dose-ranging study had demonstrated that
triazophos produced maternal toxicity at daily doses above 10 mg/kg
b.w./day (Tesh et al., 1985a), groups of 18 artificially inseminated
New Zealand white rabbits received 0, 2, 4, or 8 mg/kg b.w./day
triazophos (92.1% pure) in sesame oil by gavage from day 6 to day 19
of treatment, inclusively. The animals were sacrificed on day 19 and
the uterine contents were removed and necropsied. Only the females
receiving the highest dose exhibited a significant reduction of food
intake and body-weight gain during the first third of the treatment
period, although there was a tendency for the body-weight gain of all
treated groups to lag behind that of controls. There were no
dose-related influences of treatment on the numbers of early or late
resorptions or on pre- or post-implantation losses. The number of
viable young, their sex ratios, and fetal and placental weights were
also unaffected. One dam in the highest-treatment group aborted, while
another exhibited total litter resorption. No treatment-related
effects on the morphogenesis of fetal viscera or skeletons were
detected at necropsy of the viable young. Accordingly, the
no-observed-effect level in this study was 4 mg/kg b.w./day
(Tesh et al., 1985b).
Table 1: Results of mutagenicity studies on triazophos
Test system Test object Concentration of Results Reference
triazophos
Ames test S. typhimurium 0, 0.2, 20, Negative Gerick & Wagner,
TA98, TA100, 50, & 500 1977
TA1535, TA15371 µ/plate
Gene conversion Saccharomyces 1000, 2000, Negative Mondino, 1980a
cerevisiae D41 & 4000 µg/1
Forward mutation Schizosacchromyces 1000, 2000, Negative Mondino, 1980b
pombe1 & 4000 µg/1
Chromosome aberration Human lymphocytes1 0.05, 0.5, Negative Mondino, 1981
5, & 50 µg/1
Micronucleus test NMRI mice 0, 0.2, 2, Negative Mayer, et al.,
& 20 mg/kg 1980
1 ± metabolic activation (S-9)
COMMENTS
Comparative metabolism studies of triazophos in female rats and
dogs did not reveal any significant qualitative differences. In each
case the only significant urinary metabolites were 14C-1-phenyl-
3-hydroxy-(1H)-1,2,4-triazole and its glucuronide and sulfate
conjugates. This finding contrasts with the previous metabolism study
in rats in which 14C-urea was the predominant urinary metabolite.
The similarity observed in the present study of the metabolic fate
and the excretion profiles in rats and dogs suggests that the relative
differences in acute toxicity in these species noted by the 1982 Joint
Meeting may have been due to other factors, such as the nature of the
test vehicle employed in acute toxicity testing (viz., starch
suspension and sesame oil), or to differences in species suscep-
tibility to inhibition of acetylcholinesterase.
Triazophos was apparently not embryotoxic or teratogenic in rats
or in rabbits. It provided no evidence of mutagenicity in a series of
short-term assays.
Owing to the continuing absence of a carcinogenicity study, the
Meeting extended the temporary ADI.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 10 ppm in the diet, equivalent to 0.5 /kg b.w./day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE,
TO BE SUBMITTED BY 1990:
Results of the ongoing carcinogenicity study in mice. [Note: This
is a correction of the 1986 Joint Meeting report, in which this was
incorrectly listed as a rat study.]
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. An appropriate delayed neurotoxicity study in hens.
2. Acute oral toxicity data on any metabolites of triazophos
that are found in food crops.
REFERENCES
Baeder, C., Weigand, W., & Kramer, M.. Report on the embryotoxicity
1976 study of triazophos in Wistar rats after oral administration
in the feed. Unpublished Hoechst report No. 43676. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Diehl & Leist. Hoe 014622 - Technical substance (Code Hoe 014622
1986 Ut 2C99 0001): Test of the acute oral toxicity in male and
female Wistar rats. Unpublished Hoechst Report No. 860771.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Gericke, D. & Wagner, H.H. Test for mutagenicity in bacteria strains
1977 in the absence and presence of a liver preparation.
Unpublished Hoechst report No. 12/77. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Mayer, D., Kramer, M., & Weigan, W. Test report on the mutagenicity of
1980 HOE 02960 - active ingredient (Code - Hoe 02960 OI AS204)
after oral administration to NMRI - Mice micronucleus test.
Unpublished Hoechst Report No. 381/80. Submitted to WHO by
Hoechst AG, Frankfurt, FRG
Mondino, A. Study of the mutagenic activity of the compound HOE 02960
1980a with Saccharomyces cerevisiae. Unpublished report from
Instituto Di Richerche Biomediche "Antoine Marxer" S.P.A.,
Ivrea, Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Mondino, A. Study of the mutagenic activity in vitro of the compound
1980b HOE 02960 with Schizosaccharomyces pombe. Unpublished
report from Instituto Di Richerche Biomediche "Antoine
Marxer" S.P.A., Ivrea, Italy. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Mondino, A. In vitro study of chromosome aberration induced by the
1981 test article HOE 02960 OF AS 204 in cultured human
lymphocytes. Unpublished report from Instituto Di Richerche
Biomediche "Antoine Marxer" S.P.A., Ivrea, Italy. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Schwalbe-Fehl, M., Schmidt, E., Kellner, H.M., & Eckert, H.H.
1986 HOE 02960-14C, Triazophos, comparative metabolism
study in rats and dogs. Unpublished Hoechst Analytical and
Radiochemical Laboratories Report No. CM0481/85. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W. & Wightman, T.J. Triazophos. Active ingredient
1985a technical (Code: HOE 02960 OI ZD 002): Embryotoxicity study
in rabbits, range-finding study. Unpublished report
No. 84/HAG091/303 from Life Science Research, Eye,
Suffolk, UK. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W., Wightman, T.J., & Wilby, O.K. Triazophos.
1985b Active ingredient technical (Code; HOE 02960 OI ZD 002):
Embryotoxicity in rabbits. Unpublished report No. 84/HAG092/
549 from Life Science Research, Eye, Suffolk, UK. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Toxicological studies
Special studies on mutagenicity
Triazophos was without mutagenic activity in a number of
in vitro and in vivo assays (see Table 1).
Special study on acute toxicity
The acute oral toxicity of the principal metabolite of
triazophos, 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole, was greater than
5000 mg/kg b.w. (Diehl & Leist, 1986).
Special studies on teratogenicity
Rats
Triazophos was fed in the diet to groups of 20 - 23 pregnant
Hoe:WISK(SPF71) Wistar rats from day 6 to day 15 of gestation at
approximate daily doses of 0.87, 4.2, or 22 mg/kg b.w. Dams were
sacrificed at day 21 and the fetuses were examined for developmental
abnormalities. There were no treatment-related effects on food
consumption or body-weight gain. The number of fetal resorptions and
viable fetuses, fetal weights, crown-rump lengths, and sex ratios did
not differ significantly between treated and control groups. Placentas
and placental weights were unaffected by treatment. No visceral or
skeletal abnormalities were associated with treatment. No significant
macroscopic changes were observed in the viscera of the dams at post
mortem. Accordingly, the no-observed-effect level in this study
exceeded 22 mg/kg b.w./day (Baeder et al., 1976).
Rabbits
The effects of repeated oral administration of triazophos on the
progress and outcome of pregnancy were studied in the rabbit. As a
previously conducted dose-ranging study had demonstrated that
triazophos produced maternal toxicity at daily doses above 10 mg/kg
b.w./day (Tesh et al., 1985a), groups of 18 artificially inseminated
New Zealand white rabbits received 0, 2, 4, or 8 mg/kg b.w./day
triazophos (92.1% pure) in sesame oil by gavage from day 6 to day 19
of treatment, inclusively. The animals were sacrificed on day 19 and
the uterine contents were removed and necropsied. Only the females
receiving the highest dose exhibited a significant reduction of food
intake and body-weight gain during the first third of the treatment
period, although there was a tendency for the body-weight gain of all
treated groups to lag behind that of controls. There were no
dose-related influences of treatment on the numbers of early or late
resorptions or on pre- or post-implantation losses. The number of
viable young, their sex ratios, and fetal and placental weights were
also unaffected. One dam in the highest-treatment group aborted, while
another exhibited total litter resorption. No treatment-related
effects on the morphogenesis of fetal viscera or skeletons were
detected at necropsy of the viable young. Accordingly, the
no-observed-effect level in this study was 4 mg/kg b.w./day
(Tesh et al., 1985b).
Table 1: Results of mutagenicity studies on triazophos
Test system Test object Concentration of Results Reference
triazophos
Ames test S. typhimurium 0, 0.2, 20, Negative Gerick & Wagner,
TA98, TA100, 50, & 500 1977
TA1535, TA15371 µ/plate
Gene conversion Saccharomyces 1000, 2000, Negative Mondino, 1980a
cerevisiae D41 & 4000 µg/1
Forward mutation Schizosacchromyces 1000, 2000, Negative Mondino, 1980b
pombe1 & 4000 µg/1
Chromosome aberration Human lymphocytes1 0.05, 0.5, Negative Mondino, 1981
5, & 50 µg/1
Micronucleus test NMRI mice 0, 0.2, 2, Negative Mayer, et al.,
& 20 mg/kg 1980
1 ± metabolic activation (S-9)
COMMENTS
Comparative metabolism studies of triazophos in female rats and
dogs did not reveal any significant qualitative differences. In each
case the only significant urinary metabolites were 14C-1-phenyl-
3-hydroxy-(1H)-1,2,4-triazole and its glucuronide and sulfate
conjugates. This finding contrasts with the previous metabolism study
in rats in which 14C-urea was the predominant urinary metabolite.
The similarity observed in the present study of the metabolic fate
and the excretion profiles in rats and dogs suggests that the relative
differences in acute toxicity in these species noted by the 1982 Joint
Meeting may have been due to other factors, such as the nature of the
test vehicle employed in acute toxicity testing (viz., starch
suspension and sesame oil), or to differences in species suscep-
tibility to inhibition of acetylcholinesterase.
Triazophos was apparently not embryotoxic or teratogenic in rats
or in rabbits. It provided no evidence of mutagenicity in a series of
short-term assays.
Owing to the continuing absence of a carcinogenicity study, the
Meeting extended the temporary ADI.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 10 ppm in the diet, equivalent to 0.5 /kg b.w./day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE,
TO BE SUBMITTED BY 1990:
Results of the ongoing carcinogenicity study in mice. [Note: This
is a correction of the 1986 Joint Meeting report, in which this was
incorrectly listed as a rat study.]
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. An appropriate delayed neurotoxicity study in hens.
2. Acute oral toxicity data on any metabolites of triazophos
that are found in food crops.
REFERENCES
Baeder, C., Weigand, W., & Kramer, M.. Report on the embryotoxicity
1976 study of triazophos in Wistar rats after oral administration
in the feed. Unpublished Hoechst report No. 43676. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Diehl & Leist. Hoe 014622 - Technical substance (Code Hoe 014622
1986 Ut 2C99 0001): Test of the acute oral toxicity in male and
female Wistar rats. Unpublished Hoechst Report No. 860771.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Gericke, D. & Wagner, H.H. Test for mutagenicity in bacteria strains
1977 in the absence and presence of a liver preparation.
Unpublished Hoechst report No. 12/77. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Mayer, D., Kramer, M., & Weigan, W. Test report on the mutagenicity of
1980 HOE 02960 - active ingredient (Code - Hoe 02960 OI AS204)
after oral administration to NMRI - Mice micronucleus test.
Unpublished Hoechst Report No. 381/80. Submitted to WHO by
Hoechst AG, Frankfurt, FRG
Mondino, A. Study of the mutagenic activity of the compound HOE 02960
1980a with Saccharomyces cerevisiae. Unpublished report from
Instituto Di Richerche Biomediche "Antoine Marxer" S.P.A.,
Ivrea, Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Mondino, A. Study of the mutagenic activity in vitro of the compound
1980b HOE 02960 with Schizosaccharomyces pombe. Unpublished
report from Instituto Di Richerche Biomediche "Antoine
Marxer" S.P.A., Ivrea, Italy. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Mondino, A. In vitro study of chromosome aberration induced by the
1981 test article HOE 02960 OF AS 204 in cultured human
lymphocytes. Unpublished report from Instituto Di Richerche
Biomediche "Antoine Marxer" S.P.A., Ivrea, Italy. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Schwalbe-Fehl, M., Schmidt, E., Kellner, H.M., & Eckert, H.H.
1986 HOE 02960-14C, Triazophos, comparative metabolism
study in rats and dogs. Unpublished Hoechst Analytical and
Radiochemical Laboratories Report No. CM0481/85. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W. & Wightman, T.J. Triazophos. Active ingredient
1985a technical (Code: HOE 02960 OI ZD 002): Embryotoxicity study
in rabbits, range-finding study. Unpublished report
No. 84/HAG091/303 from Life Science Research, Eye,
Suffolk, UK. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W., Wightman, T.J., & Wilby, O.K. Triazophos.
1985b Active ingredient technical (Code; HOE 02960 OI ZD 002):
Embryotoxicity in rabbits. Unpublished report No. 84/HAG092/
549 from Life Science Research, Eye, Suffolk, UK. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
See Also:
Toxicological Abbreviations
Triazophos (JMPR Evaluations 2002 Part II Toxicological)
Triazophos (Pesticide residues in food: 1982 evaluations)
Triazophos (Pesticide residues in food: 1983 evaluations)
Triazophos (Pesticide residues in food: 1991 evaluations Part II Toxicology)
Triazophos (Pesticide residues in food: 1993 evaluations Part II Toxicology)
 Toxicological studies
Special studies on mutagenicity
Triazophos was without mutagenic activity in a number of
in vitro and in vivo assays (see Table 1).
Special study on acute toxicity
The acute oral toxicity of the principal metabolite of
triazophos, 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole, was greater than
5000 mg/kg b.w. (Diehl & Leist, 1986).
Special studies on teratogenicity
Rats
Triazophos was fed in the diet to groups of 20 - 23 pregnant
Hoe:WISK(SPF71) Wistar rats from day 6 to day 15 of gestation at
approximate daily doses of 0.87, 4.2, or 22 mg/kg b.w. Dams were
sacrificed at day 21 and the fetuses were examined for developmental
abnormalities. There were no treatment-related effects on food
consumption or body-weight gain. The number of fetal resorptions and
viable fetuses, fetal weights, crown-rump lengths, and sex ratios did
not differ significantly between treated and control groups. Placentas
and placental weights were unaffected by treatment. No visceral or
skeletal abnormalities were associated with treatment. No significant
macroscopic changes were observed in the viscera of the dams at post
mortem. Accordingly, the no-observed-effect level in this study
exceeded 22 mg/kg b.w./day (Baeder et al., 1976).
Rabbits
The effects of repeated oral administration of triazophos on the
progress and outcome of pregnancy were studied in the rabbit. As a
previously conducted dose-ranging study had demonstrated that
triazophos produced maternal toxicity at daily doses above 10 mg/kg
b.w./day (Tesh et al., 1985a), groups of 18 artificially inseminated
New Zealand white rabbits received 0, 2, 4, or 8 mg/kg b.w./day
triazophos (92.1% pure) in sesame oil by gavage from day 6 to day 19
of treatment, inclusively. The animals were sacrificed on day 19 and
the uterine contents were removed and necropsied. Only the females
receiving the highest dose exhibited a significant reduction of food
intake and body-weight gain during the first third of the treatment
period, although there was a tendency for the body-weight gain of all
treated groups to lag behind that of controls. There were no
dose-related influences of treatment on the numbers of early or late
resorptions or on pre- or post-implantation losses. The number of
viable young, their sex ratios, and fetal and placental weights were
also unaffected. One dam in the highest-treatment group aborted, while
another exhibited total litter resorption. No treatment-related
effects on the morphogenesis of fetal viscera or skeletons were
detected at necropsy of the viable young. Accordingly, the
no-observed-effect level in this study was 4 mg/kg b.w./day
(Tesh et al., 1985b).
Table 1: Results of mutagenicity studies on triazophos
Test system Test object Concentration of Results Reference
triazophos
Ames test S. typhimurium 0, 0.2, 20, Negative Gerick & Wagner,
TA98, TA100, 50, & 500 1977
TA1535, TA15371 µ/plate
Gene conversion Saccharomyces 1000, 2000, Negative Mondino, 1980a
cerevisiae D41 & 4000 µg/1
Forward mutation Schizosacchromyces 1000, 2000, Negative Mondino, 1980b
pombe1 & 4000 µg/1
Chromosome aberration Human lymphocytes1 0.05, 0.5, Negative Mondino, 1981
5, & 50 µg/1
Micronucleus test NMRI mice 0, 0.2, 2, Negative Mayer, et al.,
& 20 mg/kg 1980
1 ± metabolic activation (S-9)
COMMENTS
Comparative metabolism studies of triazophos in female rats and
dogs did not reveal any significant qualitative differences. In each
case the only significant urinary metabolites were 14C-1-phenyl-
3-hydroxy-(1H)-1,2,4-triazole and its glucuronide and sulfate
conjugates. This finding contrasts with the previous metabolism study
in rats in which 14C-urea was the predominant urinary metabolite.
The similarity observed in the present study of the metabolic fate
and the excretion profiles in rats and dogs suggests that the relative
differences in acute toxicity in these species noted by the 1982 Joint
Meeting may have been due to other factors, such as the nature of the
test vehicle employed in acute toxicity testing (viz., starch
suspension and sesame oil), or to differences in species suscep-
tibility to inhibition of acetylcholinesterase.
Triazophos was apparently not embryotoxic or teratogenic in rats
or in rabbits. It provided no evidence of mutagenicity in a series of
short-term assays.
Owing to the continuing absence of a carcinogenicity study, the
Meeting extended the temporary ADI.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 10 ppm in the diet, equivalent to 0.5 /kg b.w./day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE,
TO BE SUBMITTED BY 1990:
Results of the ongoing carcinogenicity study in mice. [Note: This
is a correction of the 1986 Joint Meeting report, in which this was
incorrectly listed as a rat study.]
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. An appropriate delayed neurotoxicity study in hens.
2. Acute oral toxicity data on any metabolites of triazophos
that are found in food crops.
REFERENCES
Baeder, C., Weigand, W., & Kramer, M.. Report on the embryotoxicity
1976 study of triazophos in Wistar rats after oral administration
in the feed. Unpublished Hoechst report No. 43676. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Diehl & Leist. Hoe 014622 - Technical substance (Code Hoe 014622
1986 Ut 2C99 0001): Test of the acute oral toxicity in male and
female Wistar rats. Unpublished Hoechst Report No. 860771.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Gericke, D. & Wagner, H.H. Test for mutagenicity in bacteria strains
1977 in the absence and presence of a liver preparation.
Unpublished Hoechst report No. 12/77. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Mayer, D., Kramer, M., & Weigan, W. Test report on the mutagenicity of
1980 HOE 02960 - active ingredient (Code - Hoe 02960 OI AS204)
after oral administration to NMRI - Mice micronucleus test.
Unpublished Hoechst Report No. 381/80. Submitted to WHO by
Hoechst AG, Frankfurt, FRG
Mondino, A. Study of the mutagenic activity of the compound HOE 02960
1980a with Saccharomyces cerevisiae. Unpublished report from
Instituto Di Richerche Biomediche "Antoine Marxer" S.P.A.,
Ivrea, Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Mondino, A. Study of the mutagenic activity in vitro of the compound
1980b HOE 02960 with Schizosaccharomyces pombe. Unpublished
report from Instituto Di Richerche Biomediche "Antoine
Marxer" S.P.A., Ivrea, Italy. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Mondino, A. In vitro study of chromosome aberration induced by the
1981 test article HOE 02960 OF AS 204 in cultured human
lymphocytes. Unpublished report from Instituto Di Richerche
Biomediche "Antoine Marxer" S.P.A., Ivrea, Italy. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Schwalbe-Fehl, M., Schmidt, E., Kellner, H.M., & Eckert, H.H.
1986 HOE 02960-14C, Triazophos, comparative metabolism
study in rats and dogs. Unpublished Hoechst Analytical and
Radiochemical Laboratories Report No. CM0481/85. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W. & Wightman, T.J. Triazophos. Active ingredient
1985a technical (Code: HOE 02960 OI ZD 002): Embryotoxicity study
in rabbits, range-finding study. Unpublished report
No. 84/HAG091/303 from Life Science Research, Eye,
Suffolk, UK. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W., Wightman, T.J., & Wilby, O.K. Triazophos.
1985b Active ingredient technical (Code; HOE 02960 OI ZD 002):
Embryotoxicity in rabbits. Unpublished report No. 84/HAG092/
549 from Life Science Research, Eye, Suffolk, UK. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Toxicological studies
Special studies on mutagenicity
Triazophos was without mutagenic activity in a number of
in vitro and in vivo assays (see Table 1).
Special study on acute toxicity
The acute oral toxicity of the principal metabolite of
triazophos, 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole, was greater than
5000 mg/kg b.w. (Diehl & Leist, 1986).
Special studies on teratogenicity
Rats
Triazophos was fed in the diet to groups of 20 - 23 pregnant
Hoe:WISK(SPF71) Wistar rats from day 6 to day 15 of gestation at
approximate daily doses of 0.87, 4.2, or 22 mg/kg b.w. Dams were
sacrificed at day 21 and the fetuses were examined for developmental
abnormalities. There were no treatment-related effects on food
consumption or body-weight gain. The number of fetal resorptions and
viable fetuses, fetal weights, crown-rump lengths, and sex ratios did
not differ significantly between treated and control groups. Placentas
and placental weights were unaffected by treatment. No visceral or
skeletal abnormalities were associated with treatment. No significant
macroscopic changes were observed in the viscera of the dams at post
mortem. Accordingly, the no-observed-effect level in this study
exceeded 22 mg/kg b.w./day (Baeder et al., 1976).
Rabbits
The effects of repeated oral administration of triazophos on the
progress and outcome of pregnancy were studied in the rabbit. As a
previously conducted dose-ranging study had demonstrated that
triazophos produced maternal toxicity at daily doses above 10 mg/kg
b.w./day (Tesh et al., 1985a), groups of 18 artificially inseminated
New Zealand white rabbits received 0, 2, 4, or 8 mg/kg b.w./day
triazophos (92.1% pure) in sesame oil by gavage from day 6 to day 19
of treatment, inclusively. The animals were sacrificed on day 19 and
the uterine contents were removed and necropsied. Only the females
receiving the highest dose exhibited a significant reduction of food
intake and body-weight gain during the first third of the treatment
period, although there was a tendency for the body-weight gain of all
treated groups to lag behind that of controls. There were no
dose-related influences of treatment on the numbers of early or late
resorptions or on pre- or post-implantation losses. The number of
viable young, their sex ratios, and fetal and placental weights were
also unaffected. One dam in the highest-treatment group aborted, while
another exhibited total litter resorption. No treatment-related
effects on the morphogenesis of fetal viscera or skeletons were
detected at necropsy of the viable young. Accordingly, the
no-observed-effect level in this study was 4 mg/kg b.w./day
(Tesh et al., 1985b).
Table 1: Results of mutagenicity studies on triazophos
Test system Test object Concentration of Results Reference
triazophos
Ames test S. typhimurium 0, 0.2, 20, Negative Gerick & Wagner,
TA98, TA100, 50, & 500 1977
TA1535, TA15371 µ/plate
Gene conversion Saccharomyces 1000, 2000, Negative Mondino, 1980a
cerevisiae D41 & 4000 µg/1
Forward mutation Schizosacchromyces 1000, 2000, Negative Mondino, 1980b
pombe1 & 4000 µg/1
Chromosome aberration Human lymphocytes1 0.05, 0.5, Negative Mondino, 1981
5, & 50 µg/1
Micronucleus test NMRI mice 0, 0.2, 2, Negative Mayer, et al.,
& 20 mg/kg 1980
1 ± metabolic activation (S-9)
COMMENTS
Comparative metabolism studies of triazophos in female rats and
dogs did not reveal any significant qualitative differences. In each
case the only significant urinary metabolites were 14C-1-phenyl-
3-hydroxy-(1H)-1,2,4-triazole and its glucuronide and sulfate
conjugates. This finding contrasts with the previous metabolism study
in rats in which 14C-urea was the predominant urinary metabolite.
The similarity observed in the present study of the metabolic fate
and the excretion profiles in rats and dogs suggests that the relative
differences in acute toxicity in these species noted by the 1982 Joint
Meeting may have been due to other factors, such as the nature of the
test vehicle employed in acute toxicity testing (viz., starch
suspension and sesame oil), or to differences in species suscep-
tibility to inhibition of acetylcholinesterase.
Triazophos was apparently not embryotoxic or teratogenic in rats
or in rabbits. It provided no evidence of mutagenicity in a series of
short-term assays.
Owing to the continuing absence of a carcinogenicity study, the
Meeting extended the temporary ADI.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 10 ppm in the diet, equivalent to 0.5 /kg b.w./day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE,
TO BE SUBMITTED BY 1990:
Results of the ongoing carcinogenicity study in mice. [Note: This
is a correction of the 1986 Joint Meeting report, in which this was
incorrectly listed as a rat study.]
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. An appropriate delayed neurotoxicity study in hens.
2. Acute oral toxicity data on any metabolites of triazophos
that are found in food crops.
REFERENCES
Baeder, C., Weigand, W., & Kramer, M.. Report on the embryotoxicity
1976 study of triazophos in Wistar rats after oral administration
in the feed. Unpublished Hoechst report No. 43676. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Diehl & Leist. Hoe 014622 - Technical substance (Code Hoe 014622
1986 Ut 2C99 0001): Test of the acute oral toxicity in male and
female Wistar rats. Unpublished Hoechst Report No. 860771.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Gericke, D. & Wagner, H.H. Test for mutagenicity in bacteria strains
1977 in the absence and presence of a liver preparation.
Unpublished Hoechst report No. 12/77. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Mayer, D., Kramer, M., & Weigan, W. Test report on the mutagenicity of
1980 HOE 02960 - active ingredient (Code - Hoe 02960 OI AS204)
after oral administration to NMRI - Mice micronucleus test.
Unpublished Hoechst Report No. 381/80. Submitted to WHO by
Hoechst AG, Frankfurt, FRG
Mondino, A. Study of the mutagenic activity of the compound HOE 02960
1980a with Saccharomyces cerevisiae. Unpublished report from
Instituto Di Richerche Biomediche "Antoine Marxer" S.P.A.,
Ivrea, Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Mondino, A. Study of the mutagenic activity in vitro of the compound
1980b HOE 02960 with Schizosaccharomyces pombe. Unpublished
report from Instituto Di Richerche Biomediche "Antoine
Marxer" S.P.A., Ivrea, Italy. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Mondino, A. In vitro study of chromosome aberration induced by the
1981 test article HOE 02960 OF AS 204 in cultured human
lymphocytes. Unpublished report from Instituto Di Richerche
Biomediche "Antoine Marxer" S.P.A., Ivrea, Italy. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Schwalbe-Fehl, M., Schmidt, E., Kellner, H.M., & Eckert, H.H.
1986 HOE 02960-14C, Triazophos, comparative metabolism
study in rats and dogs. Unpublished Hoechst Analytical and
Radiochemical Laboratories Report No. CM0481/85. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W. & Wightman, T.J. Triazophos. Active ingredient
1985a technical (Code: HOE 02960 OI ZD 002): Embryotoxicity study
in rabbits, range-finding study. Unpublished report
No. 84/HAG091/303 from Life Science Research, Eye,
Suffolk, UK. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Tesh, J.M., Ross, F.W., Wightman, T.J., & Wilby, O.K. Triazophos.
1985b Active ingredient technical (Code; HOE 02960 OI ZD 002):
Embryotoxicity in rabbits. Unpublished report No. 84/HAG092/
549 from Life Science Research, Eye, Suffolk, UK. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
